Ethical care for research animals

WHY ANIMAL RESEARCH?
The use of animals in some forms of biomedical research remains essential to the discovery of the causes, diagnoses, and treatment of disease and suffering in humans and in animals., stanford shares the public's concern for laboratory research animals..
Many people have questions about animal testing ethics and the animal testing debate. We take our responsibility for the ethical treatment of animals in medical research very seriously. At Stanford, we emphasize that the humane care of laboratory animals is essential, both ethically and scientifically. Poor animal care is not good science. If animals are not well-treated, the science and knowledge they produce is not trustworthy and cannot be replicated, an important hallmark of the scientific method .
There are several reasons why the use of animals is critical for biomedical research:
• Animals are biologically very similar to humans. In fact, mice share more than 98% DNA with us!
• Animals are susceptible to many of the same health problems as humans – cancer, diabetes, heart disease, etc.
• With a shorter life cycle than humans, animal models can be studied throughout their whole life span and across several generations, a critical element in understanding how a disease processes and how it interacts with a whole, living biological system.
The ethics of animal experimentation
Nothing so far has been discovered that can be a substitute for the complex functions of a living, breathing, whole-organ system with pulmonary and circulatory structures like those in humans. Until such a discovery, animals must continue to play a critical role in helping researchers test potential new drugs and medical treatments for effectiveness and safety, and in identifying any undesired or dangerous side effects, such as infertility, birth defects, liver damage, toxicity, or cancer-causing potential.
U.S. federal laws require that non-human animal research occur to show the safety and efficacy of new treatments before any human research will be allowed to be conducted. Not only do we humans benefit from this research and testing, but hundreds of drugs and treatments developed for human use are now routinely used in veterinary clinics as well, helping animals live longer, healthier lives.
It is important to stress that 95% of all animals necessary for biomedical research in the United States are rodents – rats and mice especially bred for laboratory use – and that animals are only one part of the larger process of biomedical research.
Our researchers are strong supporters of animal welfare and view their work with animals in biomedical research as a privilege.
Stanford researchers are obligated to ensure the well-being of all animals in their care..
Stanford researchers are obligated to ensure the well-being of animals in their care, in strict adherence to the highest standards, and in accordance with federal and state laws, regulatory guidelines, and humane principles. They are also obligated to continuously update their animal-care practices based on the newest information and findings in the fields of laboratory animal care and husbandry.
Researchers requesting use of animal models at Stanford must have their research proposals reviewed by a federally mandated committee that includes two independent community members. It is only with this committee’s approval that research can begin. We at Stanford are dedicated to refining, reducing, and replacing animals in research whenever possible, and to using alternative methods (cell and tissue cultures, computer simulations, etc.) instead of or before animal studies are ever conducted.

Organizations and Resources
There are many outreach and advocacy organizations in the field of biomedical research.
- Learn more about outreach and advocacy organizations

Stanford Discoveries
What are the benefits of using animals in research? Stanford researchers have made many important human and animal life-saving discoveries through their work.
- Learn more about research discoveries at Stanford


Search form
You are here, why is animal testing good benefits of animal testing.
The unstoppable advance of medicine and the pharmaceutical industry has increased the volume of animal experimentation. Animal testing is crucial in Drug Discovery and Development, bridging in vitro research and human clinical trials.
Regulatory agencies demand data obtained from animal experiments to advance from the preclinical phase to the clinical phase one. Animal testing is the gold standard to guarantee the safety and efficacy of potential future lifesaving medications. However, ethical and other concerns are always present, and regulatory agencies set legislation on animals in science centers-based part on the 3R Principle : Reduction, Replacement, and Refinement, which first appeared in 1959.
What are the benefits of animal testing?
Although questioning why animal testing is good is a reasonable doubt, we must not forget that animal research has enabled significant breakthroughs in developing modern medications, vaccines, and various medical procedures . Animal testing has saved millions of human lives. This in vivo testing approach has aided scientists in discovering treatments and preventive measures for multiple conditions, including high blood pressure, diabetes, tuberculosis, polio, muscular dystrophy, and Parkinson's disease.
One of the advantages of animal testing is that they provide a complete, whole-organism context that allows for studying the interactions between different biological systems. In vitro systems do not fully recapitulate the complexity of the human biochemical process. Besides, animal testing offers the convenience of shorter lifespans for animals, facilitating the acquisition of a complete view of their life cycle and shortening experimental times .
Moreover, many animals, especially mammals like mice and rats, share significant genetic and physiological similarities with humans . Almost every gene in humans has been found in mice, sharing a genetic homology of 98%. This similarity is one of the reasons why rodent testing is an ally in research since it helps to infer how a new drug or product might affect humans by observing its effects on these animals. But we have to take into account they are nocturnal animals, while humans are diurnals, which can create many inconveniences in the experimental designs and interpretation. Using animal testing for research allows the establishment of a safe dose for human clinical trials and outlines the potential side effects that should be monitored .
While the ethical debate around animal testing is complex, animal models are often preferable to directly testing new drugs and chemicals on humans without prior evidence of safety. Using animals may help to prevent human harm.

Animal testing pros and cons
While animal testing benefits have been exposed from the scientific point of view, animal testing presents some cons that need to be stated. Laboratory procedures and conditions vary across laboratories and can cause distress and abnormal behaviors, preventing species' behavior, which makes it difficult to draw conclusions. Interspecies issues regarding physiology, behavior, and pharmacokinetics can limit the reliability of the data obtained from animal studies.
One of the main disadvantages of animal research is its ethical implications , especially regarding the treatment of animals. It involves subjecting animals to procedures that may cause them discomfort, distress, or death, which many people find morally unacceptable.
Since January 2013, a new EU directive, 2010/63/EU , aiming to harmonize the European internal market, has stated that “wherever possible, a scientifically satisfactory method or testing strategy not entailing the use of live animals shall be used.”
The development of New Alternative Models or Methodologies (NAMs) has emerged as a solution to overcome ethical concerns in animal testing. NAMs are innovative approaches developed to reduce, refine, and replace the use of animals in research . These models aim to offer more ethical, efficient, and sometimes more accurate methods for studying biological processes, disease mechanisms, and the safety and efficacy of new treatments. Some examples include organ-on-a-chip approaches, advanced 3D cell cultures such as organoids, or alternative animal models such as Zebrafish.
Zebrafish can be considered NAMs because the larvae under 5-6 days post fertilization (dpf) are not yet classified as animals under the European legislation (EU Directive 2010/63/EU) because they are not feeding and swimming independently.
Zebrafish are a popular alternative model because they share many similarities with the human genome . Zebrafish presents a high genetic homology with humans (>70%) and a high genetic homology of genes implicated in human diseases (>80%). Also, alternative models like Zebrafish are more cost-effective than traditional animal models and allow for higher content screening of compounds, combining the advantages of in vitro models while presenting many advantages of in vivo models.
With the emergence of NAMs, an increasing number of governments, including the European Union, are developing the political determination to move away from viewing animal testing as the definitive standard for ensuring the safety and efficacy of medications. A shift towards a globalized use of NAMs and reducing animal testing is possible with promising alternatives such as the Zebrafish.

Akhtar A. The flaws and human harms of animal experimentation. Camb Q Healthc Ethics. 2015 Oct;24(4):407-19.
Cassar S, Adatto I, Freeman JL, Gamse JT, Iturria I, Lawrence C, Muriana A, Peterson RT, Van Cruchten S, Zon LI. Use of Zebrafish in Drug Discovery Toxicology. Chem Res Toxicol. 2020 Jan 21;33(1):95-118.
Swaters D, van Veen A, van Meurs W, Turner JE, Ritskes-Hoitinga M. A History of Regulatory Animal Testing: What Can We Learn? Altern Lab Anim. 2022 Sep;50(5):322-329.
Why mouse matters. National Human Genome Research Institute. [Internet]. [updated 2010 July 23]; [cited 2024 Feb 22]. Available from : https://www.genome.gov/10001345/importance-of-mouse-genome

- biobide's blog
- The Importance of Animal Research
- BIOMEDICAL RESEARCH

Research Advancing Health
The goal of biomedical research is to translate discoveries and observations in the laboratory or clinic into new therapies. Biomedical research methods range from predictive studies to those that involve whole living systems. Areas of study may include (1) gross populations, (2) individual human subjects, (3) nonhuman animals, (4) in vitro techniques using cells and tissues from humans, animals or even plants, (5) microorganisms including bacteria, yeast or viruses, and even (6) molecular analyses of genes, proteins and other biomolecules. Animal models are utilized in biomedical research when questions require a study of whole organisms that cannot be carried out in humans.

Typically, animal studies are essential for research that seeks to understand complex questions of disease progression, genetics, lifetime risk or other biological mechanisms of a whole living system that would be unethical, morally unacceptable or technically unfeasible or too difficult to perform in human subjects. The most common laboratory animal in biomedical research are purpose bred rats and transgenic mice. In fact, approximately 95% of all warm-blooded laboratory animals are rodents. The contributions made by these animals and other species help researchers answer questions of biological uncertainty and are necessary and critical to the advancement of both human and animal health.
Other very important aids include mathematical modeling, database analysis, computer simulations and in vitro models, such as cell and tissue cultures. These computational methods are utilized to analyze large volumes of historical experimental data in order to highlight biological trends and high priority research objectives, as well as to compile large volumes of experimental data into virtual biological systems and networks that, within the bounds of current knowledge, are capable of making predictive assessments of research questions.
The focus of biomedical researchers are diverse, but all seek to answer questions relevant to human and animal health that may one day translate into clinical practice. Research programs can be found in public health, epidemiology, preventive medicine, epigenetics, cancer, aging, endocrinology, neuroendocrinology, diabetes, cellular biology, molecular biology, pharmacology, psychopharmacology, neuroscience, genetics, virology and much more.
Role of Animal Research in Medical Advances
Virtually every major medical advance of the last century has depended upon research with animals. Animals have served as surrogates in the investigation of human diseases and have yielded valuable data in the process of discovering new ways to treat, cure or prevent them. From immunizations to cancer therapy, our ability to manage the health of animals has also improved because of animal research and the application of medical breakthroughs in veterinary medicine.
While a majority of the American public supports the necessary use of animals in biomedical research, they are also concerned about the care and treatment of laboratory animals. NABR, along with the scientific community, is committed to ensuring that all research conducted is ethical, responsible and humane.
The close relationship between dogs and people may pre-date recorded history. Over millennia, dogs have become our most beloved pets and also our hardest working partners. They guide those with special needs; help police, fire and rescue personnel; and even assist in herding other animals. One of the most significant results of our partnership with dogs has been their contribution to our understanding of disease, and how to prevent and cure it. In fact, dogs and people get many of the same diseases, from heart disease to cancer. What we can glean from studying dogs in medical and scientific research often yields treatments that help not only people, but also dogs themselves.

Mice and Rats
Rodents play an invaluable role in biomedical research. Approximately 95% of all laboratory animals are mice and rats. Reducing reliance on higher-order species, rodents have become the animal model of choice for biomedical researchers because their physiology and genetic makeup closely resembles that of people. Despite certain differences between people and rodents, the similarities are strong enough to give researchers an enormously powerful and versatile mammalian system in which to investigate human disease.

Non-Human Primates
Medical advances are usually built on a foundation of basic biomedical research and the application of newly found knowledge is often proved feasible in non-human primate (NHPs) models. Although irreplaceable in many types of research, only about 1/4 of 1% of animals used in research in the U.S. are NHPs and most of these animals are species of monkeys, not chimpanzees or other great apes. Historically, the polio vaccine, blood transfusions and organ transplantation among many other advances could not have been possible without NHP research.
Read here how non-human primates are helping in curing diseases

Medical Progress and Biomedical Research The Nobel Laureates
Every Nobel Prize in Medicine awarded in the last three decades was dependent on data from animal models. Overall, 83% of the Nobel Prizes awarded for outstanding contributions to medicine have involved animal research since the program was founded in 1901, more than 100 years ago. To learn more about the lab animals that have made important contributions in nearly every Nobel Prize in medicine, click here to find out more from the Foundation of Biomedical Research website.

THE ANIMAL RESEARCH BEHIND THE TOP 25 MOST PRESCRIBED DRUGS
Animal research and testing were needed for every prescription medicine available today. The U.S. Food and Drug Administration (FDA) requires animal testing to ensure the safety of many drugs and devices.
Please click here to find out about the Top 25 Drugs from Foundation of Biomedical Research website.

Using animals for scientific research is still indispensable for society as we know it
Senior Advisor Animal Ethics and Outreach, Donders Centre for Neuroscience, Radboud University
Professor, Radboud University
Associate Professor in Neuroinformatics, Radboud University
Disclosure statement
The authors do not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.
View all partners
Kenya’s national airline – Kenya Airways – made headlines when it announced it would stop transporting monkeys for animal research. This followed an accidental highway crash in Pennsylvania , in the US, which involved a truck transporting monkeys that had been bred in Mauritius for laboratory experiments in the US.
Following the accident, the People for the Ethical Treatment of Animals (PETA) US, an animal rights group, contacted Kenya Airways urging them to reconsider transporting the animals, putting forward their view that animal experimentation is a cruel industry.
Read more: The macaque monkeys of Mauritius: an invasive alien species, and a major export for research
Such an incident is indeed tragic. But if we consider the number of people who would have died without the existence of medication and novel medical technologies developed thanks to animal research, then ending animal research could lead to a more tragic outcome in the longer term.
Most countries do animal research, perhaps not very tiny countries or very poor countries. There is a nationwide ban on animal testing for cosmetics throughout the European Union, Israel, Norway, as well as in India. But animal testing for other reasons is still widely accepted.
Most of the animals used come from commercial breeders – one is Jackson Laboratory in the US. Other sources include specialist breeders and large breeding centres which can provide genetically modified animals for specific research. The animal testing facilities themselves may also rear animals.
In general, all over the world, policymakers do aim to move towards animal-free methods of scientific research and have introduced very strict regulations for animal research.
Scientists and policymakers share the long-term goal of reducing animal use in scientific research and where possible eventually even stopping it. It’s an ambitious goal. For this to happen, animal-free methods need to be developed and validated before they can become a new standard.
Animal-free innovations have been developed for some areas of biomedical research, such as toxicology . However, most parties recognise that at present, not all research questions can be answered using only animal-free methods.
Based on decades of doing research on the human brain, which involves using animals, to us it’s clear that – for the foreseeable future – there remains a crucial need for animal models to understand health and disease and to develop medicines.
Unique knowledge
It is animal research that provides researchers with unique knowledge about how humans and animals function. Perhaps more than in any other field of biomedical research, complete living animals are needed to understand brain function, behaviour and cognition.
Behaviour and cognition, the final outputs of a brain organ, cannot be mimicked using any existing animal-free technologies. We currently simply do not understand the brain well enough to make animal-free solutions.
Another striking, very recent example that showed the current need for animal research is the COVID-19 pandemic . The way out of the pandemic required the development of a functioning vaccine. Researchers amazed the world when they made targeted vaccines available within one year. This, however, has relied greatly on the use of animals for testing the efficacy and safety of the vaccine.
A key fact that remains often invisible is that the rules and regulations for conducting animal research are, in comparison, perhaps even stricter and more regulated, by for example the Animal Welfare act in the US and the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes in Europe. Than, for example, in the food and entertainment industry, although regulations are in place here too such as governmental rules for the treatment of animals in order to protect their health and wellbeing.
Should it be banned?
In the world as we know it today, animal research is still generally accepted as part of society. There are many important reasons why laboratory animal research is still needed:
To learn about biological processes in animals and humans.
To learn about the cause of diseases.
To develop new treatments and vaccines and evaluate their effects.
To develop methods that can prevent disease both in animals and humans.
To develop methods for the management of animals such as pests but also for the conservation of endangered species.
Of course many, animal researchers included, are hopeful that one day animal experiments will no longer be necessary to achieve the much needed scientific outcomes. However, the situation is that for many research questions related to human and animal health we still need animals.
As long as we cannot replace animals, there should be more focus on transparency and animal welfare, to benefit the animals as well as science. Awareness and financial support of this at the governmental level is key to enable animal researchers to always strive for the highest level of animal welfare possible.
- Scientific research
- Science and innovation for development

Economics Editor

Deputy Vice-Chancellor (Indigenous Strategy and Services)

Director and Chief of Staff, Indigenous Portfolio

Chief People & Culture Officer
Lecturer / senior lecturer in construction and project management.
- Investigations
- Science & Tech
- Food & Drink
- Fashion & Beauty
‘The greater good’: The ethics of animal testing in scientific research
By Jacklin Kwan
Article Summary
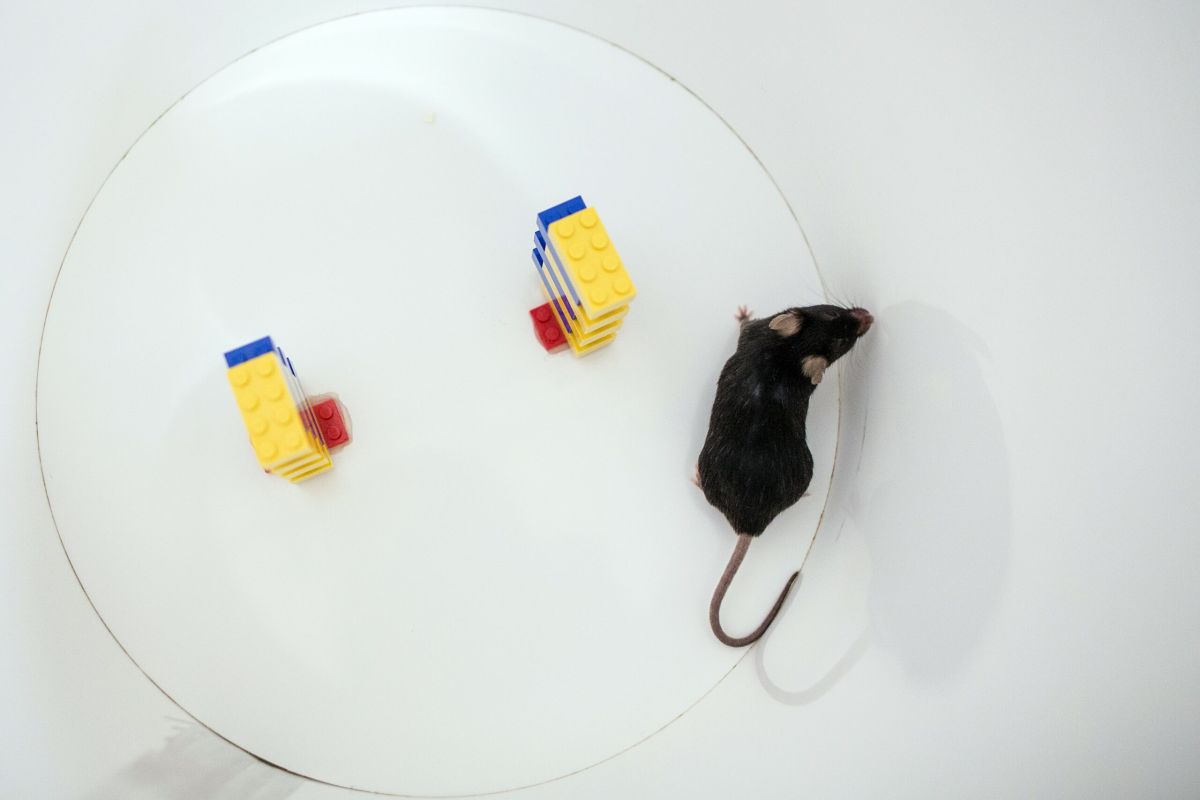
Animal testing is often a controversial and emotionally-charged subject. It’s a mental space that people almost never encounter in their day-to-day lives: that animals, which are sentient and can feel pain, are being instrumentalised at a scale of approximately 4 million animals per annum in the UK alone. This mental space is made even more alien when it’s shaped by the extreme images of animal suffering that we interact with on social media or activism campaigns.
In this light, it is difficult to understand or appreciate what, if any, research would be worth inflicting this much prolonged suffering onto another living being. It is hard to reconcile with the moral thinking that govern our everyday lives.
Because the concepts are so foreign – even for those who do science degrees – it’s sometime easy to take a polarised stance such as “let’s completely banning animal research”. However, before taking a position, we should explore the animal testing that goes on closest to us first.
If you walk behind keycard-secured doors in the Biological Science Facility in the Stopford Building , you’ll arrive at their animal testing unit. After putting on scrubs, I was toured around their various divisions by Graham Morrissey, the Direstor of the Biological Science Facility. He guided me through the many rooms that houses zebrafish, dogfish, sheep, rats, mice, frogs, and lizards.
Each animal is used for specific traits that makes them indispensable to different areas of research: Dogfish are used to study how water temperature affects their development and behaviour; sheep are used to study heart disease; and rats and mice are used to study processes like brain surgery, the activity of parasites, and mental disorders such as schizophrenia.
All these animals are used by licensed researchers who were able to prove that animal testing could not be substituted by in-vitro (in the glass) methods or computer simulations. They also had to show that the number of animals they would use were minimised, and that steps would be taken to mitigate their suffering (e.g. with the provision of painkillers). These requirements are known as the 3R’s : Replacement, reduction, and refinement. Their application was be approved by a committee of scientists, statisticians, animal technicians, and laymen that would assess its strength for scientific and ethical rigour.
However, it should be noted that much debate still remains regarding the adequacy of the 3R’s, and whether they fully capture important ethical concepts. Throughout the tour, I experienced a feeling of discord that I cannot fully articulate. Graham would show me elements in the animals’ enclosures that would ‘enrich’ their lives. I saw sheep with shaved chests and mechanical hearts that would kill them, scratching against a wooden pole; mice with stitches still visible on their heads, running through toilet rolls; pigs squinting after major eye surgery, squealing in delight as they take a bath.
Retrospectively, those pieces of enrichment seemed like sticking plasters over a more uncomfortable truth: That animals, unlike human beings, are secondary to the scientific question. And perhaps, it is for this reason that there has been yearly increases of lab animals being used despite the slow development of other alternative methods.
The necessity of such techniques was advocated by a senior researcher at the facility. The studies that were being produced could one day save hundreds upon thousands of human lives, and even benefit animals outside of cages through an increased understanding of their behaviour and how can be impacted by phenomena like climate change. A consistent theme was that the complexity of a live organism is needed for accurate testing, that computer simulations or in-vitro methods will never be as intricate as the dynamic systems of an organic body.
At times, the tone with which the researcher approached the subject was radically different. He even seemed exasperated with the UK’s sometimes obstructive regulations that delay responsive research, but it’d be too simple to assume that the interests of scientists and the animals they work with are always diametrically opposed.
Dr Robert Kirk , a historian at the University, has written on how the increased standards of sanitation in animal testing produced more resilient and healthy test subjects: “There is a balance to be struck between the needs of a given scientific procedure and the welfare of animals used within that procedure. One could see the needs of science and those of animal welfare as being in tension in the sense that they are connected and need to be managed. However, they are not necessarily in opposition or in conflict.”
The tension between scientific inquiry and animal welfare is not an easy one to mediate. However, Kirk says it is important: “Managing this tension appears as a core driver of change responsible for improvements in experimental technique as well as improvements in animal welfare… As a lay person, as a member of the public, I feel I should be as informed as I need to be in order to critically understand the role animals have played in making the world I inhabit.”
Our attitudes towards animals are always fluid as we learn more about their internal lives and our interactions with them in an ecosystem much larger than our own. In these dynamic ethical boundaries, it is critical that the public always be aware and reflect upon what constitutes humane behaviour, and what trade-offs we are willing to make.
If you would like to learn more about animal testing in Stopford, visit their website where you can take a virtual tour of their facilities.
- animal testing
- stopford building
Jacklin Kwan
More coverage.

Circadian rhythms of health: Why syncing with the environment is vital to wellbeing

Ice, Ice, Maybe? The art of remembering and forgetting, from a roundworm’s ice bath

What Game Theory reveals about the science of cooperation

Celebrating 70 years of science at CERN
Popular articles.

Shōgun episode 1 review: Culture clash

Malena and the male gaze

Who’s ‘packing’ in the primates?

Sonny Angels: The art history behind your new best friend

Should Animals Be Used for Scientific or Commercial Testing?
- History of Animal Testing
Animals are used to develop medical treatments, determine the toxicity of medications, check the safety of products destined for human use, and other biomedical , commercial, and health care uses. Research on living animals has been practiced since at least 500 BC.
Descriptions of the dissection of live animals have been found in ancient Greek writings from as early as circa 500 BC. Physician-scientists such as Aristotle , Herophilus , and Erasistratus performed the experiments to discover the functions of living organisms. Vivisection (dissection of a living organism) was practiced on human criminals in ancient Rome and Alexandria, but prohibitions against mutilation of the human body in ancient Greece led to a reliance on animal subjects. Aristotle believed that animals lacked intelligence, and so the notions of justice and injustice did not apply to them. Theophrastus , a successor to Aristotle, disagreed, objecting to the vivisection of animals on the grounds that, like humans, they can feel pain, and causing pain to animals was an affront to the gods. Read more background…
Pro & Con Arguments
Pro 1 Animal testing contributes to life-saving cures and treatments for humans and animals alike. Nearly every medical breakthrough in the last 100 years has resulted directly from research using animals, according to the California Biomedical Research Association. To name just a few examples, animal research has contributed to major advances in treating conditions including breast cancer, brain injury, childhood leukemia, cystic fibrosis, multiple sclerosis, and tuberculosis. Testing on animals was also instrumental in the development of pacemakers, cardiac valve substitutes, and anesthetics. [ 9 ] [ 10 ] [ 11 ] [ 12 ] [ 13 ] Scientists racing to develop a vaccine for coronavirus during the 2020 global pandemic needed to test on genetically modified mice to ensure that the vaccine did not make the virus worse. Nikolai Petrovsky, professor in the College of Medicine and Public Health at Flinders University in Australia, said testing a coronavirus vaccine on animals is “absolutely essential” and skipping that step would be “fraught with difficulty and danger.” [ 119 ] [ 133 ] Researchers have to test extensively to prevent “vaccine enhancement,” a situation in which a vaccine actually makes the disease worse in some people. “The way you reduce that risk is first you show it does not occur in laboratory animals,” explains Peter Hotez, Dean for the National School of Tropical Medicine at Baylor College. [ 119 ] [ 141 ] Further, animals themselves benefit from the results of animal testing. Vaccines tested on animals have saved millions of animals that would otherwise have died from rabies, distemper, feline leukemia, infectious hepatitis virus, tetanus, anthrax, and canine parvo virus. Treatments for animals developed using animal testing also include pacemakers for heart disease and remedies for glaucoma and hip dysplasia. [ 9 ] [ 21 ] Animal testing has also been instrumental in saving endangered species from extinction, including the black-footed ferret, the California condor and the tamarins of Brazil. The American Veterinary Medical Association (AVMA) endorses animal testing to develop safe drugs, vaccines, and medical devices. [ 9 ] [ 13 ] [ 23 ] Read More
Pro 2 Animals are appropriate research subjects because they are similar to human beings in many ways. Chimpanzees share 99% of their DNA with humans, and mice are 98% genetically similar to humans. All mammals, including humans, are descended from common ancestors, and all have the same set of organs (heart, kidneys, lungs, etc.) that function in essentially the same way with the help of a bloodstream and central nervous system. Because animals and humans are so biologically similar, they are susceptible to many of the same conditions and illnesses, including heart disease, cancer, and diabetes. [ 9 ] [ 17 ] [ 18 ] Animals often make better research subjects than humans because of their shorter life cycles. Laboratory mice, for example, live for only two to three years, so researchers can study the effects of treatments or genetic manipulation over a whole lifespan, or across several generations, which would be infeasible using human subjects. Mice and rats are particularly well-suited to long-term cancer research, partly because of their short lifespans. [ 9 ] [ 29 ] [ 30 ] Further, animals must be used in cases when ethical considerations prevent the use of human subjects. When testing medicines for potential toxicity, the lives of human volunteers should not be put in danger unnecessarily. It would be unethical to perform invasive experimental procedures on human beings before the methods have been tested on animals, and some experiments involve genetic manipulation that would be unacceptable to impose on human subjects before animal testing. The World Medical Association Declaration of Helsinki states that human trials should be preceded by tests on animals. [ 19 ] [ 20 ] A poll of 3,748 scientists by the Pew Research Center found that 89% favored the use of animals in scientific research. The American Cancer Society, American Physiological Society, National Association for Biomedical Research, American Heart Association, and the Society of Toxicology all advocate the use of animals in scientific research. [ 36 ] [ 37 ] [ 38 ] [ 39 ] [ 40 ] [ 120 ] Read More
Pro 3 Animal research is highly regulated, with laws in place to protect animals from mistreatment. In addition to local and state laws and guidelines, animal research has been regulated by the federal Animal Welfare Act (AWA) since 1966. As well as stipulating minimum housing standards for research animals (enclosure size, temperature, access to clean food and water, and others), the AWA also requires regular inspections by veterinarians. [ 3 ] All proposals to use animals for research must be approved by an Institutional Animal Care and Use Committee (IACUC) set up by each research facility. Most major research institutions’ programs are voluntarily reviewed for humane practices by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). [ 24 ] [ 25 ] Animal researchers treat animals humanely, both for the animals’ sake and to ensure reliable test results. Research animals are cared for by veterinarians, husbandry specialists, and animal health technicians to ensure their well-being and more accurate findings. Rachel Rubino, attending veterinarian and director of the animal facility at Cold Springs Harbor Laboratory, says, “Most people who work with research animals love those animals…. We want to give them the best lives possible, treat them humanely.” At Cedars-Sinai Medical Center’s animal research facility, dogs are given exercise breaks twice daily to socialize with their caretakers and other dogs, and a “toy rotation program” provides opportunities for play. [ 28 ] [ 32 ] Read More
Con 1 Animal testing is cruel and inhumane. Animals used in experiments are commonly subjected to force feeding, food and water deprivation, the infliction of burns and other wounds to study the healing process, the infliction of pain to study its effects and remedies, and “killing by carbon dioxide asphyxiation, neck-breaking, decapitation, or other means,” according to Humane Society International. The US Department of Agriculture reported in Jan. 2020 that research facilities used over 300,000 animals in activities involving pain in just one year. [ 47 ] [ 102 ] Plus, most experiments involving animals are flawed, wasting the lives of the animal subjects. A peer-reviewed study found serious flaws in the majority of publicly funded US and UK animal studies using rodents and primates: “only 59% of the studies stated the hypothesis or objective of the study and the number and characteristics of the animals used.” A 2017 study found further flaws in animal studies, including “incorrect data interpretation, unforeseen technical issues, incorrectly constituted (or absent) control groups, selective data reporting, inadequate or varying software systems, and blatant fraud.” [ 64 ] [ 128 ] Only 5% of animals used in experiments are protected by US law. The Animal Welfare Act (AWA) does not apply to rats, mice, fish, and birds, which account for 95% of the animals used in research. The types of animals covered by the AWA account for fewer than one million animals used in research facilities each year, which leaves around 25 million other animals without protection from mistreatment. The US Department of Agriculture, which inspects facilities for AWA compliance, compiles annual statistics on animal testing but they only include data on the small percentage of animals subject to the Act. [ 1 ] [ 2 ] [ 26 ] [ 28 ] [ 135 ] Even the animals protected by the AWA are mistreated. Violations of the Animal Welfare Act at the federally funded New Iberia Research Center (NIRC) in Louisiana included maltreatment of primates who were suffering such severe psychological stress that they engaged in self-mutilation, infant primates awake and alert during painful experiments, and chimpanzees being intimidated and shot with a dart gun. [ 68 ] Read More
Con 2 Animal tests do not reliably predict results in human beings. 94% of drugs that pass animal tests fail in human clinical trials. Over 100 stroke drugs and over 85 HIV vaccines failed in humans after succeeding in animal trials. Nearly 150 clinical trials (human tests) of treatments to reduce inflammation in critically ill patients have been undertaken, and all of them failed, despite being successful in animal tests. [ 57 ] [ 58 ] [ 59 ] Drugs that pass animal tests are not necessarily safe. The 1950s sleeping pill thalidomide, which caused 10,000 babies to be born with severe deformities, was tested on animals prior to its commercial release. Later tests on pregnant mice, rats, guinea pigs, cats, and hamsters did not result in birth defects unless the drug was administered at extremely high doses. Animal tests on the arthritis drug Vioxx showed that it had a protective effect on the hearts of mice, yet the drug went on to cause more than 27,000 heart attacks and sudden cardiac deaths before being pulled from the market. [ 5 ] [ 55 ] [ 56 ] [ 109 ] [ 110 ] Plus, animal tests may mislead researchers into ignoring potential cures and treatments. Some chemicals that are ineffective on (or harmful to) animals prove valuable when used by humans. Aspirin, for example, is dangerous for some animal species. Intravenous vitamin C has shown to be effective in treating sepsis in humans, but makes no difference to mice. Fk-506 (tacrolimus), used to lower the risk of organ transplant rejection, was “almost shelved” because of animal test results, according to neurologist Aysha Akhtar. A report on Slate.com stated that a “source of human suffering may be the dozens of promising drugs that get shelved when they cause problems in animals that may not be relevant for humans.” [ 105 ] [ 106 ] [ 127 ] Read More
Con 3 Alternative testing methods now exist that can replace the need for animals. Other research methods such as in vitro testing (tests done on human cells or tissue in a petri dish) offer opportunities to reduce or replace animal testing. Technological advancements in 3D printing allow the possibility for tissue bioprinting: a French company is working to bioprint a liver that can test the toxicity of a drug. Artificial human skin, such as the commercially available products EpiDerm and ThinCert, can be made from sheets of human skin cells grown in test tubes or plastic wells and may produce more useful results than testing chemicals on animal skin. [ 15 ] [ 16 ] [ 50 ] [ 51 ] Michael Bachelor, Senior Scientist and Product Manager at biotech company MatTek, stated, “We can now create a model from human skin cells — keratinocytes — and produce normal skin or even a model that mimics a skin disease like psoriasis. Or we can use human pigment-producing cells — melanocytes — to create a pigmented skin model that is similar to human skin from different ethnicities. You can’t do that on a mouse or a rabbit.” The Environmental Protection Agency is so confident in alternatives that the agency intends to reduce chemical testing on mammals 30% by 2025 and end it altogether by 2035. [ 61 ] [ 134 ] [ 140 ] Scientists are also able to test vaccines on humans volunteers. Unlike animals used for research, humans are able to give consent to be used in testing and are a viable option when the need arises. The COVID-19 (coronavirus) global pandemic demonstrated that researchers can skip animal testing and go straight to observing how vaccines work in humans. One company working on a COVID-19 vaccine, Moderna Therapeutics, worked on developing a vaccine using new technology: instead of being based on a weakened form of the virus, it was developed using a synthetic copy of the COVID-19 genetic code. [ 142 ] [ 143 ] Read More
| Did You Know? |
|---|
| 1. 95% of animals used in experiments are not protected by the federal Animal Welfare Act (AWA), which excludes birds, rats and mice bred for research, and cold-blooded animals such as reptiles and most fish. [ ] [ ] [ ] |
| 2. 89% of scientists surveyed by the Pew Research Center were in favor of animal testing for scientific research. [ ] |
| 3. Chimpanzees share 99% of their DNA with humans, and mice are 98% genetically similar to humans. The US National Institutes of Health announced it would retire its remaining 50 research chimpanzees to the Federal Chimpanzee Sanctuary System in 2015, leaving Gabon as the only country to still experiment on chimps. [ ] [ ] |
| 4. A Jan. 2020 report from the USDA showed that in one year of research, California used more cats (1,682) for testing than any other state. Ohio used the most guinea pigs (35,206), and Massachusetts used the most dogs (6,771) and primates (11,795). [ ] |
| 5. Researchers Joseph and Charles Vacanti grew a human "ear" seeded from implanted cow cartilage cells on the back of a living mouse to explore the possibility of fabricating body parts for plastic and reconstructive surgery. [ ] |

| More Animal Pros and Cons |
|---|
| Proponents say zoos educate the public about animals. Opponents say wild animals should never be kept captive. |
| Proponents say dissecting real animals is a better learning experience. Opponents say the practice is bad for the environment. |
| Proponents say CBD is helpful for pets' anxiety and other conditions. Opponents say the products aren't regulated. |
Our Latest Updates (archived after 30 days)
ProCon/Encyclopaedia Britannica, Inc. 325 N. LaSalle Street, Suite 200 Chicago, Illinois 60654 USA
Natalie Leppard Managing Editor [email protected]
© 2023 Encyclopaedia Britannica, Inc. All rights reserved
- Animal Testing – Pros & Cons
- Pro & Con Quotes
- Did You Know?
- Glossary: Animals Used in Animal Testing
- Number of Animals Used for Testing
- Cite this Page
- Artificial Intelligence
- Private Prisons
- Space Colonization
- Social Media
- Death Penalty
- School Uniforms
- Video Games
- Animal Testing
- Gun Control
- Banned Books
- Teachers’ Corner
Cite This Page
ProCon.org is the institutional or organization author for all ProCon.org pages. Proper citation depends on your preferred or required style manual. Below are the proper citations for this page according to four style manuals (in alphabetical order): the Modern Language Association Style Manual (MLA), the Chicago Manual of Style (Chicago), the Publication Manual of the American Psychological Association (APA), and Kate Turabian's A Manual for Writers of Term Papers, Theses, and Dissertations (Turabian). Here are the proper bibliographic citations for this page according to four style manuals (in alphabetical order):
[Editor's Note: The APA citation style requires double spacing within entries.]
[Editor’s Note: The MLA citation style requires double spacing within entries.]
Is animal research ethical?
Posted: by John Meredith on 16/02/22
More on these Topics:

How can it be right to use an animal for research where we could consider it unethical to use a human being? This is a fundamental question that confronts anybody who benefits from research using animals. If we claim that causing harm to animals is sometimes justifiable where it would be unacceptable to inflict a similar harm or risk on a person, then it seems we are assuming that animals must, in some sense, have less moral value. But is that a justifiable assumption, or is it just a self-serving prejudice? Are there solid rational arguments for treating humans differently from other animals, or are we simply falling back on outmoded habits of thought, a smokescreen that helps us avoid looking the ugly truth of our actions in the eye?
Moral status of animals
In the past, the moral status of animals did not merit a great deal of consideration; raising questions about whether humans were entitled to exploit animals would have struck most people as quaint or absurd. The great moral philosopher Rene Descartes, for example, the man famous for the phrase cogito ergo sum - ‘I think therefore I am’ - believed that animals had no inner life at all, that they were essentially as lifeless as clockwork dolls, incapable of emotion, self-awareness, or even feeling pain.
Such ideas seem laughable to us now. We take it for granted that most animals experience pain and many have complex emotional lives that can depend on relationships with other animals and which can deliver feelings of pleasure and satisfaction. Since Descartes’ day, the growing study of animal behaviour makes this seem obvious, and cleverly designed experiments have confirmed what has been learned from observation, forcing us to acknowledge that sentience – inner life – exists in a great number of other species and sometimes at a very high level.
But what implications does all this have for the moral consideration of animals? How should it affect the way we treat them? Philosopher Peter Singer, whose book Animal Liberation transformed the public debate on animal welfare, believes it should have deep and wide-reaching consequences. Singer argues that it is wrong to inflict harm on a person not because of any cosmic or biblical law about harm but because it is against that person’s interests as they themselves understand them. Considering moral questions in that light, he argues, explodes any idea that we can justify distinctions between individuals based on their sex or race, distinctions that have been passionately defended over many centuries. There are many differences between people of all kinds including, of course, both sexes, but they all have interests that are alike: an interest in avoiding pain or hunger for example. There is no rational basis for preferring the interests of any particular individual, or people of one race or sex class over those of another, that is simply racism and sexism. This is an idea has become widely accepted, if only recently, and it doesn’t seem particularly radical to us today, but Singer takes the idea a step further.
If there is no non-arbitrary reason to prefer the interests of one human animal over another, how can there be any good reason to prefer the interests of a human animal over a non-human animal? Claims that humans are of special moral interest because of their intelligence or capacity for language or any of the many other things that have been suggested cut no ice. A less intelligent human has as much interest in avoiding pain as a mathematical genius does, and the same goes for a dog, or a mouse, or a fish. To deny this, says Singer is to make a moral mistake akin to sexism or racism and he calls this way of thinking speciesism .
One objection to the argument from speciesism is that it implies that there can never be a reason to prefer the welfare of a human being over any other animal where considerations of interest are the same. This strikes most people as counter-intuitive to say the least. Jean Kazez, philosopher and animal rights activist, suggests a thought experiment. Imagine a dedicated vegan responsible for the care of ten young children. It so happens that famine strikes and the children are all in danger of starvation except that our vegan carer owns a cow. Would it be morally acceptable for the vegan to stick by her principles and refuse to slaughter the cow to save the children? If the answer is no, then there seems to be some problem with the speciesist position. It would probably not be considered acceptable to slaughter one of the children to feed the others, after all. So, our intuition is that there must be some foundation for our moral preference for a human over an animal, at least in some extreme conditions. Perhaps the intuition is that there is moral value in feelings of kinship because this is a necessary feeling in order to be a fully healthy human, to flourish as a human being. If that is the case, then, kinship, for humans, is a kind of interest in the Singer sense and one that overrides other interests. That may be why we don’t find it reprehensible when a mother prefers the welfare of her child over that of another.
The moral value of ‘kinship’ overrides speciesism
If kinship carries moral weight, then the speciesist argument loses ground and a possible justification for preferring animals over human beings in research emerges. Medical research is an attempt to save human lives and reduce human suffering (it has similar benefits for animal as well, of course, but we can set that aside for now, for the sake of simplicity). If, as scientists argue, this can only be achieved with the use of an animal model, then we are morally entitled to prefer the use of a non-human animal, so long as kinship has the moral value we are claiming for it and the suffering and distress of the animals is minimised as much as possible.
But what if this is all just a complicated exercise in justifying what we want to do anyway, what if our moral intuitions are just wrong? It is easy to imagine a Singerian arguing, in the case of our starving children and vegan nanny, that the cow has as much moral standing as any of the others: it has the same interest in living and not suffering the pain of hunger as the others and, what’s more, it may be better able to survive the famine given its ability to eat vegetation that cannot sustain humans. In that case, it seems the advocate of speciesism must argue that they all should starve together in the interests of admirable intellectual rigour, even if it feels a little hard on the children.

Using utility to resolve moral conflicts
As usual, though, the situation is more complicated. Peter Singer and his followers recognise that there is often a conflict of moral interests and so we need a framework for finding a resolution. This framework should not be ad hoc or arbitrary or based on scripture or any other culturally specific text or tradition but should be rational. Within Singer’s argument the rational moral grounding is provided by utilitarianism the ethical doctrine first proposed by Jeremy Bentham in the 19th century. Utilitarianism argues that when two actions are in conflict, the morally correct one is the one that delivers the most happiness for the largest number (Bentham called this ‘utility’ for obscure reasons). In other words, the morality of an action is decided by its consequences, not by the intentions of the actor or anything else. Applied to the problem of our starving infants and their increasingly paranoid cow, a utilitarian might argue that killing the cow is justified despite it having a similar interest in living to the children because the slaughter would maximise future happiness (utility). If they all die, happiness would be at zero, and if a child was sacrificed to save the others, that would reduce overall happiness because of the distress of the survivors at their loss, the suffering endured by the child selected to die, and the indifference of the cow.
How do you measure happiness?
Problems with utilitarian ways of thinking immediately suggest themselves: how can happiness be measured? How can the ‘happiness’ of a mouse, for example, be weighed against a person, or any other animal? Must we consider a well-intentioned action that has bad outcomes immoral instead of just unfortunate? The literature goes into all these problems and more at great depth, but for our purposes, it is at least clear that a utilitarian moral framework allows for the use of research animals in some circumstances. The human happiness delivered by a successful medical treatment can be great and long lasting while any pain or distress caused to the experimental animals is kept to a minimum and is of very limited duration. In the utilitarian scales, this tips firmly towards an ethical justification of animal research. It is a surprise to many people that Peter Singer, the father of the modern animal rights movement, comes to the same conclusion, although he argues for stricter controls and more work to reduce and mitigate the use of animals. Even without appealing to concepts such as kinship, in other words, the concept of speciesism, perhaps the most formidable intellectual weapon aimed against animal research by protest groups, does not carry the day. It is perfectly possible to allow the moral value of an animal’s interests and still justify its use in research – even if that research causes the animal harm or distress – so long as the future outcomes maximise happiness.
Animal rights arguments
The only significant ethical argument against animal research that remains is based on the idea of rights. Just as humans have inalienable rights, the argument goes, so do animals. According to this view, the use of animals for research can never be justified for exactly the same reasons that we cannot justify using humans. But argument from rights has many more problems than argument from interests: from where are rights derived? What specific rights do animals have? Should rights be protected even when this is damaging to the welfare of the animal? This last point is perhaps the most salient. If we allow an animal has a right to its freedom, say, not to be kept in captivity (one of the key rights usually claimed by activists), then we are not only committed to ending all ownership of animals, but to the immediate release of all domestic animals into the wild even if that were to the detriment of the animals’ welfare as it surely would be. The problems mount at every step. How can it be possible to reconcile a vole’s right to life with a falcon’s right to eat? What possible mechanism could be constructed to resolve such conflicts and how much irreparable harm to natural ecosystems would follow if we built one? Without answers to questions like this it is hard to see animal rights arguments as much more than rhetoric.
Maximising future happiness and minimising present suffering is enough for an ethical justification of animal research
The case for ethical animal research, then, does not need as much building as it might at first appear. None of the major philosophical arguments for animal welfare exclude the possibility of ethical animal research. The harm that is done to animals in well-regulated research environments serves a higher moral purpose: the reduction of death and suffering by disease and other disorders. Of course, this is only true if pain, suffering and distress, are minimised – as they are through animal welfare regulations in the UK and EU for example. These regulations also require the application of the principles of the 3Rs – but it is quite obvious, all other things being equal, that the use of a mouse in an investigation into cancer development, for example, will create less suffering than using a person for the same purposes.
So, a utilitarian calculation of maximising future happiness and minimising present suffering is enough for an ethical justification of animal research even for tough minded opponents of animal exploitation such as Professor Singer. But maybe justification is the wrong word.
Are we not morally obliged to use animals in research?
If, as the biological sciences are almost unanimous in claiming, we cannot have new medicines without some animal research, and if there are hundreds of devastating human illnesses that will continue to cause misery, pain, and heartache without those new treatments, should we not think of animal research as a moral obligation instead? It is difficult science to do, both technically and emotionally, but if we choose not to carry it out, we are effectively choosing to allow human suffering to continue in the future that our efforts today have the potential to reduce or eliminate. We don’t know which suffering we will be successful in mitigating when, but we can be certain that progress is being made. Remove animal research and we don’t not remove suffering, we simply transfer it from the animals now (where it is carefully controlled and minimised, very often to nothing) to future humans. That is the heart of the ethical case for animal research and one that needs to be better addressed by those who oppose it.
Last edited: 7 April 2022 12:16
Back to News
Related articles

The ethics of animal research

Animal rights activism and extremism

Animal Rights Extremism
Subscribe to our newsletter.
Get the latest articles and news from Understanding Animal Research in your email inbox every month. For more information, please see our privacy policy .
Home — Essay Samples — Social Issues — Animal Testing
Argumentative Essays on Animal Testing
Hook examples for animal testing essays, the ethical dilemma hook.
Begin your essay by presenting the ethical dilemma surrounding animal testing. Explore the moral questions it raises and the conflicting viewpoints of proponents and opponents.
The Historical Perspective Hook
Take your readers on a journey through the history of animal testing. Discuss its origins, evolution, and its role in scientific and medical advancements over time.
The Scientific Advancements Hook
Highlight the scientific breakthroughs and discoveries that have resulted from animal testing. Discuss how it has contributed to medical treatments, vaccines, and the understanding of diseases.
The Alternatives and Innovations Hook
Explore alternative methods and innovations in research that aim to replace or reduce the use of animals in testing. Discuss advancements like in vitro testing and computer modeling.
The Animal Welfare Hook
Focus on the welfare and ethical treatment of animals used in testing. Discuss regulations, guidelines, and efforts to minimize harm and suffering in research.
The Legal and Regulatory Landscape Hook
Examine the legal and regulatory framework surrounding animal testing in different countries. Discuss laws, restrictions, and their enforcement.
The Public Opinion and Activism Hook
Discuss public perceptions of animal testing and the role of animal rights activists in advocating for change. Highlight notable campaigns and their impact.
The Unintended Consequences Hook
Explore unintended consequences or risks associated with animal testing, such as potential harm to humans due to species differences or the limitations of animal models.
The Future of Research Hook
Discuss the future of scientific research and the possibilities for reducing or eliminating animal testing. Explore emerging technologies and trends in biomedical research.
The Personal Story Hook
Share a personal or anecdotal story related to animal testing, such as the experiences of a researcher, activist, or someone affected by medical advancements achieved through animal testing.
Arguments Aganist Using Animals in Experiments and Testing
Reasons to stop animal testing, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
The Reasons Why Animal Testing Should Be Stopped
Discussion whether animals testing is necessary, an argument favoring the use of animals in testing and the benefits it has brought, animal testing in modern world, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Pros and Cons of Animal Testing: The Conflicting Debate
The ethics of animal testing: an argument against its practice, discussion: should animals be used for scientific research, the arguments concerning animal testing, get a personalized essay in under 3 hours.
Expert-written essays crafted with your exact needs in mind
Why Animal Testing Should Be Viewed as Beneficial
Saving the animals: alternative ways to test products, discussion on whether scientists should be allowed to test products on animals, the arguments why we should not test on animals, reasons why animal testing should be forbidden, why we should not continue test on animals, arguments for the reduction of animal testing, the problem of human cruelty to animals, how animal testing benefits us from diseases, stop the cruel and unnecessary animal testing, animal testing and alternatives for developing cruelty-free makeup, animals should not be a part of scientific research, worldwide problem of animal testing, analysis of the perspectives in support for animal testing and against it, animal testing in the united states, animal testing in the world, arguments for eliminating the use of animal testing, humane cosmetics: abolishing animal testing on cosmetics in america, review on the animal testing, animal experimentation in the world.
Animal testing, referred to as animal experimentation, animal research, or in vivo testing, involves the utilization of animals other than humans in scientific experiments aimed at manipulating the factors influencing the behavior or biological processes being investigated.
Throughout history, the practice of animal testing has deep roots dating back centuries. The earliest recorded instances can be traced back to ancient civilizations, where animals were used for various scientific and medical purposes. The Greek physician Galen, during the second century AD, conducted experiments on animals to understand human anatomy and physiology. However, the formal establishment of animal testing as a systematic approach began to take shape during the 19th century with the emergence of modern medical research. In the late 1800s, advances in scientific knowledge and technology led to an increased demand for animal testing in various fields, including medicine, toxicology, and physiology. The development of anesthesia further facilitated the experimentation on animals by reducing pain and discomfort. Throughout the 20th century, animal testing became more widespread and institutionalized, particularly in the pharmaceutical industry.
Public opinion on animal testing is a complex and diverse topic, with viewpoints spanning a wide spectrum. While there are those who support the use of animals in scientific research for the advancement of human knowledge and medical breakthroughs, others express strong opposition due to ethical concerns and the perceived mistreatment of animals. Some people argue that animal testing is necessary for the development of life-saving treatments and the improvement of human health. They believe that animals provide valuable insights into human biology and the effectiveness of potential therapies. On the other hand, opponents of animal testing argue that it is cruel and unnecessary, advocating for alternative methods such as in vitro testing, computer modeling, and human cell-based assays. Public opinion on animal testing often hinges on the balance between scientific progress and animal welfare. The growing awareness of animal rights and ethical considerations has fueled debates and discussions surrounding the topic. As society becomes more conscious of animal welfare, there is an increasing demand for alternative testing methods and greater transparency in the treatment of animals involved in research. Ultimately, public opinion plays a crucial role in shaping policies and regulations surrounding animal testing.
1. Scientific advancement. 2. Human health and safety. 3. Understanding diseases. 4. Regulatory requirements. 5. Animal welfare improvements.
1. Ethical concerns. 2. Inadequate human relevance. 3. Availability of alternatives. 4. Animal welfare. 5. Speciesism and moral status.
One example of media representation is the documentary "Earthlings" directed by Shaun Monson. This influential film explores different aspects of animal exploitation, including animal testing, and highlights the ethical concerns surrounding the practice. It has garnered widespread attention and prompted discussions about the treatment of animals in scientific research. Social media platforms have also become powerful tools for activists and organizations to share information and advocate for alternatives to animal testing. Hashtags like #StopAnimalTesting and #CrueltyFree have gained traction, raising awareness and encouraging conversations on the topic.
The topic of animal testing is important due to its ethical, scientific, and societal implications. From an ethical standpoint, it raises profound questions about the treatment of sentient beings and the moral responsibility we have towards animals. It prompts us to consider the balance between scientific progress and animal welfare, urging us to explore alternative methods that minimize harm. Scientifically, animal testing has been instrumental in advancing medical knowledge and developing treatments for various diseases. However, it is essential to continually evaluate its effectiveness, limitations, and potential alternatives to ensure both human and animal well-being. Furthermore, the issue of animal testing has societal implications as it reflects our values and priorities as a society. It prompts discussions about our relationship with animals, the extent of their rights, and the importance of promoting more humane practices.
The topic of animal testing is worth writing an essay about due to its complex nature and the multitude of perspectives it encompasses. It is a subject that elicits strong emotions and raises critical ethical, scientific, and social questions. Writing an essay on animal testing allows for an in-depth exploration of these issues and encourages critical thinking and analysis. By delving into the topic, one can examine the ethical considerations surrounding the use of animals in experiments, weighing the potential benefits against the moral implications. Additionally, it provides an opportunity to evaluate the scientific validity and reliability of animal testing as a method for understanding human biology and developing medical treatments. Furthermore, an essay on animal testing opens avenues for discussing alternative approaches and advancements in technology that can reduce or replace animal experimentation. It allows for an exploration of the societal impact of animal testing, including public opinion, legislation, and the influence of media.
1. Each year, millions of animals are used in scientific experiments worldwide. According to estimates, over 100 million animals, including rabbits, mice, rats, dogs, and primates, are subjected to testing for various purposes, such as biomedical research, drug development, and toxicity testing. 2. Animal testing is not always reliable in predicting human outcomes. Studies have shown that there can be significant differences between animals and humans in terms of anatomy, physiology, and drug metabolism. This raises concerns about the validity and relevance of using animal models for understanding human diseases and developing treatments. 3. Alternatives to animal testing are emerging and gaining traction. Scientists and researchers are actively exploring innovative methods, such as in vitro cell cultures, computer modeling, and organ-on-a-chip technology, to simulate human biology and predict human responses more accurately. These alternative approaches aim to reduce or eliminate the need for animal testing while still ensuring the safety and efficacy of new products and treatments.
1. Abbott, A. (2005). Animal testing: more than a cosmetic change. Nature, 438(7065), 144-147. (https://go.gale.com/ps/i.do?id=GALE%7CA185466349&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=00280836&p=AONE&sw=w&userGroupName=anon%7E513ffe31) 2. Doke, S. K., & Dhawale, S. C. (2015). Alternatives to animal testing: A review. https://www.sciencedirect.com/science/article/pii/S1319016413001096 Saudi Pharmaceutical Journal, 23(3), 223-229. 3. Hajar, R. (2011). Animal testing and medicine. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3123518/ Heart views: the official journal of the Gulf Heart Association, 12(1), 42. 4. Bottini, A. A., & Hartung, T. (2009). Food for thought… on the economics of animal testing. ALTEX-Alternatives to animal experimentation, 26(1), 3-16. (https://www.altex.org/index.php/altex/article/view/633) 5. Valappil, S. P., Misra, S. K., Boccaccini, A. R., & Roy, I. (2006). Biomedical applications of polyhydroxyalkanoates, an overview of animal testing and in vivo responses. Expert Review of Medical Devices, 3(6), 853-868. (https://www.tandfonline.com/doi/abs/10.1586/17434440.3.6.853) 6. File, S. E., Lippa, A. S., Beer, B., & Lippa, M. T. (2004). Animal tests of anxiety. Current protocols in neuroscience, 26(1), 8-3. (https://currentprotocols.onlinelibrary.wiley.com/doi/abs/10.1002/0471142301.ns0803s26) 7. Madden, J. C., Enoch, S. J., Paini, A., & Cronin, M. T. (2020). A review of in silico tools as alternatives to animal testing: principles, resources and applications. Alternatives to Laboratory Animals, 48(4), 146-172. (https://journals.sagepub.com/doi/pdf/10.1177/0261192920965977) 8. Donnellan, L. (2006). Animal testing in cosmetics: recent developments in the European Union and the United States. Animal L., 13, 251. (https://heinonline.org/HOL/LandingPage?handle=hein.journals/anim13&div=18&id=&page=)
Relevant topics
- Gun Violence
- Human Trafficking
- Gun Control
- Pro Choice (Abortion)
- Civil Disobedience
- Freedom of Speech
- Gender Equality
- Black Lives Matter
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Bibliography
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cambridge Open

The Flaws and Human Harms of Animal Experimentation
Nonhuman animal (“animal”) experimentation is typically defended by arguments that it is reliable, that animals provide sufficiently good models of human biology and diseases to yield relevant information, and that, consequently, its use provides major human health benefits. I demonstrate that a growing body of scientific literature critically assessing the validity of animal experimentation generally (and animal modeling specifically) raises important concerns about its reliability and predictive value for human outcomes and for understanding human physiology. The unreliability of animal experimentation across a wide range of areas undermines scientific arguments in favor of the practice. Additionally, I show how animal experimentation often significantly harms humans through misleading safety studies, potential abandonment of effective therapeutics, and direction of resources away from more effective testing methods. The resulting evidence suggests that the collective harms and costs to humans from animal experimentation outweigh potential benefits and that resources would be better invested in developing human-based testing methods.
Introduction
Annually, more than 115 million animals are used worldwide in experimentation or to supply the biomedical industry. 1 Nonhuman animal (hereafter “animal”) experimentation falls under two categories: basic (i.e., investigation of basic biology and human disease) and applied (i.e., drug research and development and toxicity and safety testing). Regardless of its categorization, animal experimentation is intended to inform human biology and health sciences and to promote the safety and efficacy of potential treatments. Despite its use of immense resources, the animal suffering involved, and its impact on human health, the question of animal experimentation’s efficacy has been subjected to little systematic scrutiny. 2
Although it is widely accepted that medicine should be evidence based , animal experimentation as a means of informing human health has generally not been held, in practice, to this standard. This fact makes it surprising that animal experimentation is typically viewed as the default and gold standard of preclinical testing and is generally supported without critical examination of its validity. A survey published in 2008 of anecdotal cases and statements given in support of animal experimentation demonstrates how it has not and could not be validated as a necessary step in biomedical research, and the survey casts doubt on its predictive value. 3 I show that animal experimentation is poorly predictive of human outcomes, 4 that it is unreliable across a wide category of disease areas, 5 and that existing literature demonstrates the unreliability of animal experimentation, thereby undermining scientific arguments in its favor. I further show that the collective harms that result from an unreliable practice tip the ethical scale of harms and benefits against continuation in much, if not all, of experimentation involving animals. 6
Problems of Successful Translation to Humans of Data from Animal Experimentation
Although the unreliability and limitations of animal experimentation have increasingly been acknowledged, there remains a general confidence within much of the biomedical community that they can be overcome. 7 However, three major conditions undermine this confidence and explain why animal experimentation, regardless of the disease category studied, fails to reliably inform human health: (1) the effects of the laboratory environment and other variables on study outcomes, (2) disparities between animal models of disease and human diseases, and (3) species differences in physiology and genetics. I argue for the critical importance of each of these conditions.
The Influence of Laboratory Procedures and Environments on Experimental Results
Laboratory procedures and conditions exert influences on animals’ physiology and behaviors that are difficult to control and that can ultimately impact research outcomes. Animals in laboratories are involuntarily placed in artificial environments, usually in windowless rooms, for the duration of their lives. Captivity and the common features of biomedical laboratories—such as artificial lighting, human-produced noises, and restricted housing environments—can prevent species-typical behaviors, causing distress and abnormal behaviors among animals. 8 Among the types of laboratory-generated distress is the phenomenon of contagious anxiety. 9 Cortisone levels rise in monkeys watching other monkeys being restrained for blood collection. 10 Blood pressure and heart rates elevate in rats watching other rats being decapitated. 11 Routine laboratory procedures, such as catching an animal and removing him or her from the cage, in addition to the experimental procedures, cause significant and prolonged elevations in animals’ stress markers. 12 These stress-related changes in physiological parameters caused by the laboratory procedures and environments can have significant effects on test results. 13 Stressed rats, for example, develop chronic inflammatory conditions and intestinal leakage, which add variables that can confound data. 14
A variety of conditions in the laboratory cause changes in neurochemistry, genetic expression, and nerve regeneration. 15 In one study, for example, mice were genetically altered to develop aortic defects. Yet, when the mice were housed in larger cages, those defects almost completely disappeared. 16 Providing further examples, typical noise levels in laboratories can damage blood vessels in animals, and even the type of flooring on which animals are tested in spinal cord injury experiments can affect whether a drug shows a benefit. 17
In order to control for potential confounders, some investigators have called for standardization of laboratory settings and procedures. 18 One notable effort was made by Crabbe et al. in their investigation of the potential confounding influences of the laboratory environment on six mouse behaviors that are commonly studied in neurobehavioral experiments. Despite their “extraordinary lengths to equate test apparatus, testing protocols, and all possible features of animal husbandry” across three laboratories, there were systematic differences in test results in these labs. 19 Additionally, different mouse strains varied markedly in all behavioral tests, and for some tests the magnitude of genetic differences depended on the specific testing laboratory. The results suggest that there are important influences of environmental conditions and procedures specific to individual laboratories that can be difficult—perhaps even impossible—to eliminate. These influences can confound research results and impede extrapolation to humans.
The Discordance between Human Diseases and Animal Models of Diseases
The lack of sufficient congruence between animal models and human diseases is another significant obstacle to translational reliability. Human diseases are typically artificially induced in animals, but the enormous difficulty of reproducing anything approaching the complexity of human diseases in animal models limits their usefulness. 20 Even if the design and conduct of an animal experiment are sound and standardized, the translation of its results to the clinic may fail because of disparities between the animal experimental model and the human condition. 21
Stroke research presents one salient example of the difficulties in modeling human diseases in animals. Stroke is relatively well understood in its underlying pathology. Yet accurately modeling the disease in animals has proven to be an exercise in futility. To address the inability to replicate human stroke in animals, many assert the need to use more standardized animal study design protocols. This includes the use of animals who represent both genders and wide age ranges, who have comorbidities and preexisting conditions that occur naturally in humans, and who are consequently given medications that are indicated for human patients. 22 In fact, a set of guidelines, named STAIR, was implemented by a stroke roundtable in 1999 (and updated in 2009) to standardize protocols, limit the discrepancies, and improve the applicability of animal stroke experiments to humans. 23 One of the most promising stroke treatments later to emerge was NXY-059, which proved effective in animal experiments. However, the drug failed in clinical trials, despite the fact that the set of animal experiments on this drug was considered the poster child for the new experimental standards. 24 Despite such vigorous efforts, the development of STAIR and other criteria has yet to make a recognizable impact in clinical translation. 25
Under closer scrutiny, it is not difficult to surmise why animal stroke experiments fail to successfully translate to humans even with new guidelines. Standard stroke medications will likely affect different species differently. There is little evidence to suggest that a female rat, dog, or monkey sufficiently reproduces the physiology of a human female. Perhaps most importantly, reproducing the preexisting conditions of stroke in animals proves just as difficult as reproducing stroke pathology and outcomes. For example, most animals don’t naturally develop significant atherosclerosis, a leading contributor to ischemic stroke. In order to reproduce the effects of atherosclerosis in animals, researchers clamp their blood vessels or artificially insert blood clots. These interventions, however, do not replicate the elaborate pathology of atherosclerosis and its underlying causes. Reproducing human diseases in animals requires reproducing the predisposing diseases, also a formidable challenge. The inability to reproduce the disease in animals so that it is congruent in relevant respects with human stroke has contributed to a high failure rate in drug development. More than 114 potential therapies initially tested in animals failed in human trials. 26
Further examples of repeated failures based on animal models include drug development in cancer, amyotrophic lateral sclerosis (ALS), traumatic brain injury (TBI), Alzheimer’s disease (AD), and inflammatory conditions. Animal cancer models in which tumors are artificially induced have been the basic translational model used to study key physiological and biochemical properties in cancer onset and propagation and to evaluate novel treatments. Nevertheless, significant limitations exist in the models’ ability to faithfully mirror the complex process of human carcinogenesis. 27 These limitations are evidenced by the high (among the highest of any disease category) clinical failure rate of cancer drugs. 28 Analyses of common mice ALS models demonstrate significant differences from human ALS. 29 The inability of animal ALS models to predict beneficial effects in humans with ALS is recognized. 30 More than twenty drugs have failed in clinical trials, and the only U.S. Food and Drug Administration (FDA)–approved drug to treat ALS is Riluzole, which shows notably marginal benefit on patient survival. 31 Animal models have also been unable to reproduce the complexities of human TBI. 32 In 2010, Maas et al. reported on 27 large Phase 3 clinical trials and 6 unpublished trials in TBI that all failed to show human benefit after showing benefit in animals. 33 Additionally, even after success in animals, around 172 and 150 drug development failures have been identified in the treatment of human AD 34 and inflammatory diseases, 35 respectively.
The high clinical failure rate in drug development across all disease categories is based, at least in part, on the inability to adequately model human diseases in animals and the poor predictability of animal models. 36 A notable systematic review, published in 2007, compared animal experimentation results with clinical trial findings across interventions aimed at the treatment of head injury, respiratory distress syndrome, osteoporosis, stroke, and hemorrhage. 37 The study found that the human and animal results were in accordance only half of the time. In other words, the animal experiments were no more likely than a flip of the coin to predict whether those interventions would benefit humans.
In 2004, the FDA estimated that 92 percent of drugs that pass preclinical tests, including “pivotal” animal tests, fail to proceed to the market. 38 More recent analysis suggests that, despite efforts to improve the predictability of animal testing, the failure rate has actually increased and is now closer to 96 percent. 39 The main causes of failure are lack of effectiveness and safety problems that were not predicted by animal tests. 40
Usually, when an animal model is found wanting, various reasons are proffered to explain what went wrong—poor methodology, publication bias, lack of preexisting disease and medications, wrong gender or age, and so on. These factors certainly require consideration, and recognition of each potential difference between the animal model and the human disease motivates renewed efforts to eliminate these differences. As a result, scientific progress is sometimes made by such efforts. However, the high failure rate in drug testing and development, despite attempts to improve animal testing, suggests that these efforts remain insufficient to overcome the obstacles to successful translation that are inherent to the use of animals. Too often ignored is the well-substantiated idea that these models are, for reasons summarized here, intrinsically lacking in relevance to, and thus highly unlikely to yield useful information about, human diseases. 41
Interspecies Differences in Physiology and Genetics
Ultimately, even if considerable congruence were shown between an animal model and its corresponding human disease, interspecies differences in physiology, behavior, pharmacokinetics, and genetics would significantly limit the reliability of animal studies, even after a substantial investment to improve such studies. In spinal cord injury, for example, drug testing results vary according to which species and even which strain within a species is used, because of numerous interspecies and interstrain differences in neurophysiology, anatomy, and behavior. 42 The micropathology of spinal cord injury, injury repair mechanisms, and recovery from injury varies greatly among different strains of rats and mice. A systematic review found that even among the most standardized and methodologically superior animal experiments, testing results assessing the effectiveness of methylprednisolone for spinal cord injury treatment varied considerably among species. 43 This suggests that factors inherent to the use of animals account for some of the major differences in results.
Even rats from the same strain but purchased from different suppliers produce different test results. 44 In one study, responses to 12 different behavioral measures of pain sensitivity, which are important markers of spinal cord injury, varied among 11 strains of mice, with no clear-cut patterns that allowed prediction of how each strain would respond. 45 These differences influenced how the animals responded to the injury and to experimental therapies. A drug might be shown to help one strain of mice recover but not another. Despite decades of using animal models, not a single neuroprotective agent that ameliorated spinal cord injury in animal tests has proven efficacious in clinical trials to date. 46
Further exemplifying the importance of physiological differences among species, a 2013 study reported that the mouse models used extensively to study human inflammatory diseases (in sepsis, burns, infection, and trauma) have been misleading. The study found that mice differ greatly from humans in their responses to inflammatory conditions. Mice differed from humans in what genes were turned on and off and in the timing and duration of gene expression. The mouse models even differed from one another in their responses. The investigators concluded that “our study supports higher priority to focus on the more complex human conditions rather than relying on mouse models to study human inflammatory disease.” 47 The different genetic responses between mice and humans are likely responsible, at least in part, for the high drug failure rate. The authors stated that every one of almost 150 clinical trials that tested candidate agents’ ability to block inflammatory responses in critically ill patients failed.
Wide differences have also become apparent in the regulation of the same genes, a point that is readily seen when observing differences between human and mouse livers. 48 Consistent phenotypes (observable physical or biochemical characteristics) are rarely obtained by modification of the same gene, even among different strains of mice. 49 Gene regulation can substantially differ among species and may be as important as the presence or absence of a specific gene. Despite the high degree of genome conservation, there are critical differences in the order and function of genes among species. To use an analogy: as pianos have the same keys, humans and other animals share (largely) the same genes. Where we mostly differ is in the way the genes or keys are expressed. For example, if we play the keys in a certain order, we hear Chopin; in a different order, we hear Ray Charles; and in yet a different order, it’s Jerry Lee Lewis. In other words, the same keys or genes are expressed, but their different orders result in markedly different outcomes.
Recognizing the inherent genetic differences among species as a barrier to translation, researches have expressed considerable enthusiasm for genetically modified (GM) animals, including transgenic mice models, wherein human genes are inserted into the mouse genome. However, if a human gene is expressed in mice, it will likely function differently from the way it functions in humans, being affected by physiological mechanisms that are unique in mice. For example, a crucial protein that controls blood sugar in humans is missing in mice. 50 When the human gene that makes this protein was expressed in genetically altered mice, it had the opposite effect from that in humans: it caused loss of blood sugar control in mice. Use of GM mice has failed to successfully model human diseases and to translate into clinical benefit across many disease categories. 51 Perhaps the primary reason why GM animals are unlikely to be much more successful than other animal models in translational medicine is the fact that the “humanized” or altered genes are still in nonhuman animals.
In many instances, nonhuman primates (NHPs) are used instead of mice or other animals, with the expectation that NHPs will better mimic human results. However, there have been sufficient failures in translation to undermine this optimism. For example, NHP models have failed to reproduce key features of Parkinson’s disease, both in function and in pathology. 52 Several therapies that appeared promising in both NHPs and rat models of Parkinson’s disease showed disappointing results in humans. 53 The campaign to prescribe hormone replacement therapy (HRT) in millions of women to prevent cardiovascular disease was based in large part on experiments on NHPs. HRT is now known to increase the risk of these diseases in women. 54
HIV/AIDS vaccine research using NHPs represents one of the most notable failures in animal experimentation translation. Immense resources and decades of time have been devoted to creating NHP (including chimpanzee) models of HIV. Yet all of about 90 HIV vaccines that succeeded in animals failed in humans. 55 After HIV vaccine gp120 failed in clinical trials, despite positive outcomes in chimpanzees, a BMJ article commented that important differences between NHPs and humans with HIV misled researchers, taking them down unproductive experimental paths. 56 Gp120 failed to neutralize HIV grown and tested in cell culture. However, because the serum protected chimpanzees from HIV infection, two Phase 3 clinical trials were undertaken 57 —a clear example of how expectations that NHP data are more predictive than data from other (in this case, cell culture) testing methods are unproductive and harmful. Despite the repeated failures, NHPs (though not chimpanzees or other great apes) remain widely used for HIV research.
The implicit assumption that NHP (and indeed any animal) data are reliable has also led to significant and unjustifiable human suffering. For example, clinical trial volunteers for gp120 were placed at unnecessary risk of harm because of unfounded confidence in NHP experiments. Two landmark studies involving thousands of menopausal women being treated with HRT were terminated early because of increased stroke and breast cancer risk. 58 In 2003, Elan Pharmaceuticals was forced to prematurely terminate a Phase 2 clinical trial when an investigational AD vaccine was found to cause brain swelling in human subjects. No significant adverse effects were detected in GM mice or NHPs. 59
In another example of human suffering resulting from animal experimentation, six human volunteers were injected with an immunomodulatory drug, TGN 1412, in 2006. 60 Within minutes of receiving the experimental drug, all volunteers suffered a severe adverse reaction resulting from a life-threatening cytokine storm that led to catastrophic systemic organ failure. The compound was designed to dampen the immune system, but it had the opposite effect in humans. Prior to this first human trial, TGN 1412 was tested in mice, rabbits, rats, and NHPs with no ill effects. NHPs also underwent repeat-dose toxicity studies and were given 500 times the human dose for at least four consecutive weeks. 61 None of the NHPs manifested the ill effects that humans showed almost immediately after receiving minute amounts of the test drug. Cynomolgus and rhesus monkeys were specifically chosen because their CD28 receptors demonstrated similar affinity to TGN 1412 as human CD28 receptors. Based on such data as these, it was confidently concluded that results obtained from these NHPs would most reliably predict drug responses in humans—a conclusion that proved devastatingly wrong.
As exemplified by the study of HIV/AIDS, TGN 1412, and other experiences, 62 experiments with NHPs are not necessarily any more predictive of human responses than experiments with other animals. The repeated failures in translation from studies with NHPs belie arguments favoring use of any nonhuman species to study human physiology and diseases and to test potential treatments. If experimentation using chimpanzees and other NHPs, our closest genetic cousins, are unreliable, how can we expect research using other animals to be reliable? The bottom line is that animal experiments, no matter the species used or the type of disease research undertaken, are highly unreliable—and they have too little predictive value to justify the resultant risks of harms for humans, for reasons I now explain.
The Collective Harms That Result from Misleading Animal Experiments
As medical research has explored the complexities and subtle nuances of biological systems, problems have arisen because the differences among species along these subtler biological dimensions far outweigh the similarities , as a growing body of evidence attests. These profoundly important—and often undetected—differences are likely one of the main reasons human clinical trials fail. 63
“Appreciation of differences” and “caution” about extrapolating results from animals to humans are now almost universally recommended. But, in practice, how does one take into account differences in drug metabolism, genetics, expression of diseases, anatomy, influences of laboratory environments, and species- and strain-specific physiologic mechanisms—and, in view of these differences, discern what is applicable to humans and what is not? If we cannot determine which physiological mechanisms in which species and strains of species are applicable to humans (even setting aside the complicating factors of different caging systems and types of flooring), the usefulness of the experiments must be questioned.
It has been argued that some information obtained from animal experiments is better than no information. 64 This thesis neglects how misleading information can be worse than no information from animal tests. The use of nonpredictive animal experiments can cause human suffering in at least two ways: (1) by producing misleading safety and efficacy data and (2) by causing potential abandonment of useful medical treatments and misdirecting resources away from more effective testing methods.
Humans are harmed because of misleading animal testing results. Imprecise results from animal experiments may result in clinical trials of biologically faulty or even harmful substances, thereby exposing patients to unnecessary risk and wasting scarce research resources. 65 Animal toxicity studies are poor predictors of toxic effects of drugs in humans. 66 As seen in some of the preceding examples (in particular, stroke, HRT, and TGN1412), humans have been significantly harmed because investigators were misled by the safety and efficacy profile of a new drug based on animal experiments. 67 Clinical trial volunteers are thus provided with raised hopes and a false sense of security because of a misguided confidence in efficacy and safety testing using animals.
An equal if indirect source of human suffering is the opportunity cost of abandoning promising drugs because of misleading animal tests. 68 As candidate drugs generally proceed down the development pipeline and to human testing based largely on successful results in animals 69 (i.e., positive efficacy and negative adverse effects), drugs are sometimes not further developed due to unsuccessful results in animals (i.e., negative efficacy and/or positive adverse effects). Because much pharmaceutical company preclinical data are proprietary and thus publicly unavailable, it is difficult to know the number of missed opportunities due to misleading animal experiments. However, of every 5,000–10,000 potential drugs investigated, only about 5 proceed to Phase 1 clinical trials. 70 Potential therapeutics may be abandoned because of results in animal tests that do not apply to humans. 71 Treatments that fail to work or show some adverse effect in animals because of species-specific influences may be abandoned in preclinical testing even if they may have proved effective and safe in humans if allowed to continue through the drug development pipeline.
An editorial in Nature Reviews Drug Discovery describes cases involving two drugs in which animal test results from species-specific influences could have derailed their development. In particular, it describes how tamoxifen, one of the most effective drugs for certain types of breast cancer, “would most certainly have been withdrawn from the pipeline” if its propensity to cause liver tumor in rats had been discovered in preclinical testing rather than after the drug had been on the market for years. 72 Gleevec provides another example of effective drugs that could have been abandoned based on misleading animal tests: this drug, which is used to treat chronic myelogenous leukemia (CML), showed serious adverse effects in at least five species tested, including severe liver damage in dogs. However, liver toxicity was not detected in human cell assays, and clinical trials proceeded, which confirmed the absence of significant liver toxicity in humans. 73 Fortunately for CML patients, Gleevec is a success story of predictive human-based testing. Many useful drugs that have safely been used by humans for decades, such as aspirin and penicillin, may not have been available today if the current animal testing regulatory requirements were in practice during their development. 74
A further example of near-missed opportunities is provided by experiments on animals that delayed the acceptance of cyclosporine, a drug widely and successfully used to treat autoimmune disorders and prevent organ transplant rejection. 75 Its immunosuppressive effects differed so markedly among species that researchers judged that the animal results limited any direct inferences that could be made to humans. Providing further examples, PharmaInformatic released a report describing how several blockbuster drugs, including aripiprazole (Abilify) and esomeprazole (Nexium), showed low oral bioavailability in animals. They would likely not be available on the market today if animal tests were solely relied on. Understanding the implications of its findings for drug development in general, PharmaInformatic asked, “Which other blockbuster drugs would be on the market today, if animal trials would have not been used to preselect compounds and drug-candidates for further development?” 76 These near-missed opportunities and the overall 96 percent failure rate in clinical drug testing strongly suggest the unsoundness of animal testing as a precondition of human clinical trials and provide powerful evidence for the need for a new, human-based paradigm in medical research and drug development.
In addition to potentially causing abandonment of useful treatments, use of an invalid animal disease model can lead researchers and the industry in the wrong research direction, wasting time and significant investment. 77 Repeatedly, researchers have been lured down the wrong line of investigation because of information gleaned from animal experiments that later proved to be inaccurate, irrelevant, or discordant with human biology. Some claim that we do not know which benefits animal experiments, particularly in basic research, may provide down the road. Yet human lives remain in the balance, waiting for effective therapies. Funding must be strategically invested in the research areas that offer the most promise.
The opportunity costs of continuing to fund unreliable animal tests may impede development of more accurate testing methods. Human organs grown in the lab, human organs on a chip, cognitive computing technologies, 3D printing of human living tissues, and the Human Toxome Project are examples of new human-based technologies that are garnering widespread enthusiasm. The benefit of using these testing methods in the preclinical setting over animal experiments is that they are based on human biology. Thus their use eliminates much of the guesswork required when attempting to extrapolate physiological data from other species to humans. Additionally, these tests offer whole-systems biology, in contrast to traditional in vitro techniques. Although they are gaining momentum, these human-based tests are still in their relative infancy, and funding must be prioritized for their further development. The recent advancements made in the development of more predictive, human-based systems and biological approaches in chemical toxicological testing are an example of how newer and improved tests have been developed because of a shift in prioritization. 78 Apart from toxicology, though, financial investment in the development of human-based technologies generally falls far short of investment in animal experimentation. 79
The unreliability of applying animal experimental results to human biology and diseases is increasingly recognized. Animals are in many respects biologically and psychologically similar to humans, perhaps most notably in the shared characteristics of pain, fear, and suffering. 80 In contrast, evidence demonstrates that critically important physiological and genetic differences between humans and other animals can invalidate the use of animals to study human diseases, treatments, pharmaceuticals, and the like. In significant measure, animal models specifically, and animal experimentation generally, are inadequate bases for predicting clinical outcomes in human beings in the great bulk of biomedical science. As a result, humans can be subject to significant and avoidable harm.
The data showing the unreliability of animal experimentation and the resultant harms to humans (and nonhumans) undermine long-standing claims that animal experimentation is necessary to enhance human health and therefore ethically justified. Rather, they demonstrate that animal experimentation poses significant costs and harms to human beings. It is possible—as I have argued elsewhere—that animal research is more costly and harmful, on the whole, than it is beneficial to human health. 81 When considering the ethical justifiability of animal experiments, we should ask if it is ethically acceptable to deprive humans of resources, opportunity, hope, and even their lives by seeking answers in what may be the wrong place. In my view, it would be better to direct resources away from animal experimentation and into developing more accurate, human-based technologies.
Aysha Akhtar , M.D., M.P.H., is a neurologist and preventive medicine specialist and Fellow at the Oxford Centre for Animal Ethics, Oxford, United Kingdom.
1. Taylor K, Gordon N, Langley G, Higgins W. Estimates for worldwide laboratory animal use in 2005 . Alternatives to Laboratory Animals 2008; 36 :327–42. [ PubMed ] [ Google Scholar ]
2. Systematic reviews that have been conducted generally reveal the unreliability and poor predictability of animal tests. See Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, et al. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMJ 2007;334:197. See also Pound P, Bracken MB. Is animal research sufficiently evidence based to be a cornerstone of biomedical research? BMJ 2014;348:g3387. See Godlee F. How predictive and productive is animal research? BMJ 2014;348:g3719. See Benatar M. Lost in translation: Treatment trials in the SOD 1 mouse and in human ALS. Neurobiology Disease 2007; 26 :1–13 [ PubMed ] [ Google Scholar ] . And see Akhtar AZ, Pippin JJ, Sandusky CB. Animal studies in spinal cord injury: A systematic review of methylprednisolone . Alternatives to Laboratory Animals 2009; 37 :43–62. [ PubMed ] [ Google Scholar ]
3. Mathews RAJ. Medical progress depends on animal models—doesn’t it? Journal of the Royal Society of Medicine 2008; 101 :95–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
4. See Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philosophy, Ethics, and Humanities in Medicine 2009; 4 :2 [ PMC free article ] [ PubMed ] [ Google Scholar ] . See also Wall RJ, Shani M. Are animal models as good as we think? Theriogenology 2008; 69 :2–9. [ PubMed ] [ Google Scholar ]
5. See note 3, Mathews 2008. See also Hartung T, Zurlo J. Food for thought… alternative approaches for medical countermeasures to biological and chemical terrorism and warfare . ALTEX 2012; 29 :251–60 [ PubMed ] [ Google Scholar ] . See Leist M, Hartung T. Inflammatory findings on species extrapolations: Humans are definitely no 70-kg mice . Archives in Toxicology 2013; 87 :563–7 [ PMC free article ] [ PubMed ] [ Google Scholar ] . See Mak IWY, Evaniew N, Ghert M. Lost in translation: Animal models and clinical trials in cancer treatment . American Journal in Translational Research 2014; 6 :114–18 [ PMC free article ] [ PubMed ] [ Google Scholar ] . And see Pippin J. Animal research in medical sciences: Seeking a convergence of science, medicine, and animal law . South Texas Law Review 2013; 54 :469–511. [ Google Scholar ]
6. For an overview of the harms-versus-benefits argument, see LaFollette H. Animal experimentation in biomedical research In: Beauchamp TL, Frey RG, eds. The Oxford Handbook of Animal Ethics . Oxford: Oxford University Press; 2011:812–18. [ Google Scholar ]
7. See Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases . Nature Medicine 2010; 16 :1210–14 [ PubMed ] [ Google Scholar ] . See Institute of Medicine. Improving the Utility and Translation of Animal Models for Nervous System Disorders: Workshop Summary. Washington, DC: The National Academies Press; 2013. And see Degryse AL, Lawson WE. Progress towards improving animal models for IPF . American Journal of Medical Science 2011; 341 :444–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
8. See Morgan KN, Tromborg CT. Sources of stress in captivity . Applied Animal Behaviour Science 2007; 102 :262–302 [ Google Scholar ] . See Hart PC, Bergner CL, Dufour BD, Smolinsky AN, Egan RJ, LaPorte L, et al. Analysis of abnormal repetitive behaviors in experimental animal models In Warrick JE, Kauleff AV, eds. Translational Neuroscience and Its Advancement of Animal Research Ethics . New York: Nova Science; 2009:71–82 [ Google Scholar ] . See Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience . American Journal of Primatology 2003; 60 :1–15 [ PubMed ] [ Google Scholar ] . And see Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress . Contemporary Topics in Laboratory Animal Science 2004; 43 :42–51. [ PubMed ] [ Google Scholar ]
9. Suckow MA, Weisbroth SH, Franklin CL. The Laboratory Rat . 2nd ed. Burlington, MA: Elsevier Academic Press; 2006, at 323.
10. Flow BL, Jaques JT. Effect of room arrangement and blood sample collection sequence on serum thyroid hormone and cortisol concentrations in cynomolgus macaques ( Macaca fascicularis ). Contemporary Topics in Laboratory Animal Science 1997;36:65–8.
11. See note 8, Balcombe et al. 2004.
12. See note 8, Balcombe et al. 2004.
13. Baldwin A, Bekoff M. Too stressed to work. New Scientist 2007;194:24.
14. See note 13, Baldwin, Bekoff 2007.
15. Akhtar A, Pippin JJ, Sandusky CB. Animal models in spinal cord injury: A review . Reviews in the Neurosciences 2008; 19 :47–60. [ PubMed ] [ Google Scholar ]
16. See note 13, Baldwin, Bekoff 2007.
17. See note 15, Akhtar et al. 2008.
18. See Macleod MR, O’Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias . Stroke 2004; 35 :1203–8 [ PubMed ] [ Google Scholar ] . See also O’ Neil BJ, Kline JA, Burkhart K, Younger J. Research fundamentals: V. The use of laboratory animal models in research. Academic Emergency Medicine 1999;6:75–82.
19. Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment . Science 1999; 284 :1670–2, at 1670. [ PubMed ] [ Google Scholar ]
20. See Curry SH. Why have so many drugs with stellar results in laboratory stroke models failed in clinical trials? A theory based on allometric relationships. Annals of the New York Academy of Sciences 2003;993:69–74. See also Dirnagl U. Bench to bedside: The quest for quality in experimental stroke research . Journal of Cerebral Blood Flow & Metabolism 2006; 26 :1465–78 [ PubMed ] [ Google Scholar ] .
21. van der Worp HB, Howells DW, Sena ES, Poritt MJ, Rewell S, O’Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Medicine 2010; 7 :e1000245. [ PMC free article ] [ PubMed ] [ Google Scholar ] .
22. See note 20, Dirnagl 2006. See also Sena E, van der Worp B, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends in Neurosciences 2007; 30 :433–9. [ PubMed ] [ Google Scholar ]
23. See Gawrylewski A. The trouble with animal models: Why did human trials fail? The Scientist 2007;21:44. See also Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations . Stroke 2009; 40 :2244–50. [ PMC free article ] [ PubMed ] [ Google Scholar ]
24. See note 23, Gawrylewski 2007. There is some dispute as to how vigorously investigators adhered to the suggested criteria. Nevertheless, NXY-059 animal studies were considered an example of preclinical studies that most faithfully adhered to the STAIR criteria. For further discussion see also Wang MM, Guohua X, Keep RF. Should the STAIR criteria be modified for preconditioning studies? Translational Stroke Research 2013; 4 :3–14 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
25. See note 24, Wang et al. 2013.
26. O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke . Annals of Neurology 2006; 59 :467–7 [ PubMed ] [ Google Scholar ] .
27. See note 5, Mak et al. 2014.
28. See note 5, Mak et al. 2014.
29. See Perrin S. Preclinical research: Make mouse studies work . Nature 2014; 507 :423–5 [ PubMed ] [ Google Scholar ] . See also, generally, Wilkins HM, Bouchard RJ, Lorenzon NM, Linseman DA. Poor correlation between drug efficacies in the mutant SOD1 mouse mode versus clinical trials of ALS necessitates the development of novel animal models for sporadic motor neuron disease. In: Costa A, Villalba E, eds. Horizons in Neuroscience Research. Vol. 5. Hauppauge, NY: Nova Science; 2011:1–39.
30. Traynor BJ, Bruijn L, Conwit R, Beal F, O’Neill G, Fagan SC, et al. Neuroprotective agents for clinical trials in ALS: A systematic assessment . Neurology 2006; 67 :20–7 [ PubMed ] [ Google Scholar ] .
31. Sinha G. Another blow for ALS . Nature Biotechnology 2013; 31 :185 [ Google Scholar ] . See also note 30, Traynor et al. 2006.
32. See Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, et al. Experimental models of traumatic brain injury: Do we really need a better mousetrap? Neuroscience 2005; 136 :971–89 [ PubMed ] [ Google Scholar ] . See also Xiong YE, Mahmood A, Chopp M. Animal models of traumatic brain injury . Nature Reviews Neuroscience 2013; 14 :128–42 [ PMC free article ] [ PubMed ] [ Google Scholar ] . And see commentary by Farber: Farber N. Drug development in brain injury. International Brain Injury Association ; available at http://www.internationalbrain.org/articles/drug-development-in-traumatic-brain-injury/ (last accessed 7 Dec 2014).
33. Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: Past experience and current developments . Neurotherapeutics 2010; 7 :115–26. [ PMC free article ] [ PubMed ] [ Google Scholar ]
34. Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. Clinical trials and late-stage drug development in Alzheimer’s disease: An appraisal from 1984 to 2014 . Journal of Internal Medicine 2014; 275 :251–83 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
35. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases . Proceedings of the National Academy of Sciences USA 2013; 110 :3507–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
36. Palfreyman MG, Charles V, Blander J. The importance of using human-based models in gene and drug discovery . Drug Discovery World 2002. Fall:33–40 [ Google Scholar ] .
37. See note 2, Perel et al. 2007.
38. Harding A. More compounds failing phase I. The Scientist 2004 Sept 13; available at http://www.the-scientist.com/?articles.view/articleNo/23003/title/More-compounds-failing-Phase-I/ (last accessed 2 June 2014).
39. See note 5, Pippin 2013.
40. See note 5, Hartung, Zurlo 2012.
41. Wiebers DO, Adams HP, Whisnant JP. Animal models of stroke: Are they relevant to human disease? Stroke 1990; 21 :1–3. [ PubMed ] [ Google Scholar ]
42. See note 15, Akhtar et al. 2008.
43. See note 2, Akhtar et al. 2009.
44. Lonjon N, Prieto M, Haton H, Brøchner CB, Bauchet L, Costalat V, et al. Minimum information about animal experiments: Supplier is also important . Journal of Neuroscience Research 2009; 87 :403–7. [ PubMed ] [ Google Scholar ]
45. Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, et al. Heritability of nociception I: Responses of 11 inbred mouse strains on 12 measures of nociception . Pain 1999; 80 :67–82. [ PubMed ] [ Google Scholar ]
46. Tator H, Hashimoto R, Raich A, Norvell D, Fehling MG, Harrop JS, et al. Translational potential of preclinical trials of neuroprotection through pharmacotherapy for spinal cord injury . Journal of Neurosurgery: Spine 2012; 17 :157–229. [ PubMed ] [ Google Scholar ]
47. See note 35, Seok et al. 2013, at 3507.
48. Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, et al. Tissue-specific transcriptional regulation has diverged significantly between human and mouse . Nature Genetics 2007; 39 :730–2 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
49. Horrobin DF. Modern biomedical research: An internally self-consistent universe with little contact with medical reality? Nature Reviews Drug Discovery 2003; 2 :151–4. [ PubMed ] [ Google Scholar ]
50. Vassilopoulous S, Esk C, Hoshino S, Funke BH, Chen CY, Plocik AM, et al. A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism . Science 2009; 324 :1192–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
51. See Guttman-Yassky E, Krueger JG. Psoriasis: Evolution of pathogenic concepts and new therapies through phases of translational research . British Journal of Dermatology 2007; 157 :1103–15 [ PubMed ] [ Google Scholar ] . See also The mouse model: Less than perfect, still invaluable. Johns Hopkins Medicine ; available at http://www.hopkinsmedicine.org/institute_basic_biomedical_sciences/news_events/articles_and_stories/model_organisms/201010_mouse_model.html (last accessed 10 Dec 2014). See note 23, Gawrylewski 2007. See note 2, Benatar 2007. See note 29, Perrin 2014 and Wilkins et al. 2011. See Cavanaugh S, Pippin J, Barnard N. Animal models of Alzheimer disease: Historical pitfalls and a path forward. ALTEX online first; 2014 Apr 10. And see Woodroofe A, Coleman RA. ServiceNote: Human tissue research for drug discovery . Genetic Engineering and Biotechnology News 2007; 27 :18 [ Google Scholar ] .
52. Lane E, Dunnett S. Animal models of Parkinson’s disease and L-dopa induced dyskinesia: How close are we to the clinic? Psychopharmacology 2008; 199 :303–12. [ PubMed ] [ Google Scholar ]
53. See note 52, Lane, Dunnett 2008.
54. See note 5, Pippin 2013.
55. Bailey J. An assessment of the role of chimpanzees in AIDS vaccine research . Alternatives to Laboratory Animals 2008; 36 :381–428. [ PubMed ] [ Google Scholar ]
56. Tonks A. The quest for the AIDs vaccine . BMJ 2007; 334 :1346–8 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
57. Johnston MI, Fauci AS. An HIV vaccine—evolving concepts . New England Journal of Medicine 2007; 356 :2073–81. [ PubMed ] [ Google Scholar ]
58. See Rossouw JE, Andersen GL, Prentice RL, LaCroix AZ, Kooperberf C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy menopausal women: Principle results from the Women’s Health Initiative randomized controlled trial . JAMA 2002; 288 :321–33 [ PubMed ] [ Google Scholar ] . See also Andersen GL, Limacher A, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial . JAMA 2004; 291 :1701–12 [ PubMed ] [ Google Scholar ] .
59. Lemere CA. Developing novel immunogens for a safe and effective Alzheimer’s disease vaccine . Progress in Brain Research 2009; 175 :83. [ PMC free article ] [ PubMed ] [ Google Scholar ] .
60. Allen A. Of mice and men: The problems with animal testing. Slate 2006 June 1; available at http://www.slate.com/articles/health_and_science/medical_examiner/2006/06/of_mice_or_men.html (last accessed 10 Dec 2014).
61. Attarwala H. TGN1412: From discovery to disaster . Journal of Young Pharmacists 2010; 2 :332–6 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
62. See Hogan RJ. Are nonhuman primates good models for SARS? PLoS Medicine 2006; 3 :1656–7 [ PMC free article ] [ PubMed ] [ Google Scholar ] . See also Bailey J. Non-human primates in medical research and drug development: A critical review . Biogenic Amines 2005; 19 :235–55. [ Google Scholar ]
63. See note 4, Wall, Shani 2008.
64. Lemon R, Dunnett SB. Surveying the literature from animal experiments: Critical reviews may be helpful—not systematic ones . BMJ 2005; 330 :977–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
65. Roberts I, Kwan I, Evans P, Haig S. Does animal experimentation inform human health care? Observations from a systematic review of international animal experiments on fluid resuscitation . BMJ 2002; 324 :474–6 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
66. See note 60,Allen 2006. See also Heywood R. Target organ toxicity . Toxicology Letters 1981; 8 :349–58 [ PubMed ] [ Google Scholar ] . See Fletcher AP. Drug safety tests and subsequent clinical experience . Journal of the Royal Society of Medicine 1978; 71 :693–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
67. See note 60, Allen 2006. See note 5, Pippin 2013. See also Greek R, Greek J. Animal research and human disease . JAMA 2000; 283 :743–4 [ PubMed ] [ Google Scholar ] .
68. See note 60, Allen 2006. See also note 5, Leist, Hartung 2013.
69. Food and Drug Administration. Development & approval process (drugs); available at http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ (last accessed 7 Dec 2014). See also http://www.fda.gov/drugs/resourcesforyou/consumers/ucm143534.htm (last accessed 7 Dec 2014).
70. Drug discovery pipeline. IRSF; available at http://www.rettsyndrome.org/research-programs/programmatic-overview/drug-discovery-pipeline (last accessed 24 Sept 2014).
71. See note 60, Allen 2006.
72. Follow the yellow brick road. Nature Reviews Drug Discovery 2003;2:167, at 167.
73. See note 5, Pippin 2013.
74. For data on aspirin, see Hartung T. Per aspirin as astra … Alternatives to Laboratory Animals 2009; 37 (Suppl 2 ):45–7 [ PubMed ] [ Google Scholar ] . See also note 5, Pippin 2013. For data on penicillin, see Koppanyi T, Avery MA. Species differences and the clinical trial of new drugs: A review . Clinical Pharmacology and Therapeutics 1966; 7 :250–70 [ PubMed ] [ Google Scholar ] . See also Schneierson SS, Perlman E. Toxicity of penicillin for the Syrian hamster . Proceedings of the Society for Experimental Biology and Medicine 1956; 91 :229–30. [ PubMed ] [ Google Scholar ]
75. See note 67, Greek, Greek 2000.
76. Oral bioavailability of blockbuster drugs in humans and animals. PharmaInformatic . available at http://www.pharmainformatic.com/html/blockbuster_drugs.html (last accessed 19 Sept 2014).
77. Sams-Dodd F. Strategies to optimize the validity of disease models in the drug discovery process . Drug Discovery Today 2006; 11 :355–63 [ PubMed ] [ Google Scholar ] .
78. Zurlo J. No animals harmed: Toward a paradigm shift in toxicity testing . Hastings Center Report 2012;42. Suppl:s23–6 [ PubMed ] [ Google Scholar ] .
79. There is no direct analysis of the amount of money spent on animal testing versus alternatives across all categories; however, in 2008 the Chronicle of Higher Education reported that funding of research involving animals (under basic research) of the National Institute of Health (NIH) remained steady at about 42 percent since 1990. See Monastersky R. Protesters fail to slow animal research. Chronicle of Higher Education 2008:54. In 2012, NIH director Francis Collins noted that the NIH’s support for basic research has held steady at 54 percent of the agency’s budget for decades. The remainder of the NIH’s budget is heavily funded toward clinical research, suggesting that preclinical human-based testing methods are much less funded. See also Wadman M. NIH director grilled over translational research centre. Nature News Blog 2012 Mar 20. Available at http://blogs.nature.com/news/2012/03/nih-director-grilled-over-translational-research-center.html (last accessed 5 Mar 2015). There is no data that suggests that the NIH’s funding of animal experimentation has decreased. A 2010 analysis estimates that at least 50 percent of the NIH’s extramural funding is directed into animal research; see Greek R, Greek J. Is the use of sentient animals in basic research justifiable? Philosophy, Ethics, and Humanities in Medicine 2010; 5 :14 [ PMC free article ] [ PubMed ] [ Google Scholar ] .
80. For a helpful discussion on animal pain, fear, and suffering, see DeGrazia D. Taking Animals Seriously: Mental Lives and Moral Status . New York: Cambridge University Press; 1996:116–23. [ Google Scholar ]
81. See Akhtar A. Animals and Public Health: Why Treating Animals Better Is Critical to Human Welfare . Hampshire, UK: Palgrave Macmillan; 2012:chap. 5.
- Share full article
Advertisement
Supported by
student opinion
Is Animal Testing Ever Justified?
The E.P.A. recently said it would move away from requiring the testing of potentially harmful chemicals on animals. Do you support the decision?

By Natalie Proulx
Find all our Student Opinion questions here.
On Sept. 10, the Environmental Protection Agency said it would move away from requiring the testing of potentially harmful chemicals on animals, a decision that was hailed by animal rights groups but criticized by environmentalists and researchers who said the practice was necessary to rigorously safeguard human health.
What are your thoughts on animal testing? Do you think it is ever justified? Why or why not?
In “ E.P.A. Says It Will Drastically Reduce Animal Testing ,” Mihir Zaveri, Mariel Padilla and Jaclyn Peiser write about the decision:
The E.P.A. Administrator Andrew Wheeler said the agency plans to reduce the amount of studies that involve mammal testing by 30 percent by 2025, and to eliminate the studies entirely by 2035, though some may still be approved on a case-by-case basis. The agency said it would also invest $4.25 million in projects at four universities and a medical center that are developing alternate ways of testing chemicals that do not involve animals. “We can protect human health and the environment by using cutting-edge, ethically sound science in our decision-making that efficiently and cost-effectively evaluates potential effects without animal testing,” Mr. Wheeler said in a memo announcing the changes. The E.P.A. has for decades required testing on a variety of animals — including rats, dogs, birds and fish — to gauge their toxicity before the chemicals can be bought, sold or used in the environment.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Animal Testing: History and Arguments Essay
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
In general, animal testing is allowed all over the world. Some countries impose certain restrictions on that matter, some – do not introduce any restrictions at all. However, even those countries that have certain laws prohibiting tests on animals do not take into account the fact that animals are living creatures and must not suffer for the sake of an experiment. Moreover, in most facilities and laboratories, animals are kept in cages, thereby having absolutely no freedom. Most of the experiments performed on animals bring them suffering, lead to disability, and even death. This inhumane treatment of animals does not justify any cause (Haugen, 2000). Thus, the main reason why these experiments must be stopped is that, according to the statistics, the majority of them are ineffective and inaccurate.
History of Animal Testing
Animal testing has a long history. Considering the fact that animals are living creatures, medical experiments on them were already conducted at least three thousand years ago. The first records mentioning the experiments on animals date back to the fourth century BCE in Ancient Greece (Murnaghan, 2017). Thus, in ancient times, it was a widely adopted practice to perform dissections of animals in order to understand how to make surgical operations on humans.
Since the 18 th century, with the development of medicine, the frequency of animal testing has significantly increased. Moreover, if a couple of centuries ago, there were only single experiments that were performed by separate scientists, now, it has developed into the large industry that catches animals in the wild and uses them as guinea pigs (Scutti, 2013). Thus, although there are many innovative technologies that can serve as better alternatives to animal testing, people are still reluctant to change the current state of affairs.
Despite animal testing being rather an old practice, ethical considerations on that matter also occurred quite a long time ago (Scutti, 2013). For example, in the 17 th century, a psychologist Edmund O’Meara stated that animal testing was unnecessary, as it often gave inaccurate results. In this respect, he provided an example regarding vivisection that, as he claimed, placed the body of an animal in an unnatural state, in which it endured a lot of pain that was both cruel and gave false results.
The first animal protection law was established in Great Britain in 1822. A significant milestone in the history of animal protection legislation was the introduction of the Cruelty to Animals Act in 1876 in Great Britain. This law was promoted by Charles Darwin who, despite being a biologist and a scientist, was against vivisection. In the 1860s, the movements against animal testing occurred in the USA. As a result, Henry Bergh established the American Society for the Prevention of Cruelty to Animals (ASPCA) in 1866. After that, the American Anti-Vivisection Society (AAVS) was founded in 1883 (Haugen, 2000). Thus, the end of the 19 th century was the time when many articles were written, and campaigns were started calling for terminating the experiments on animals.
However, at the beginning of the 20 th century, the tendency of releasing laws about animal protection changed. Unfortunately, the efforts of antivivisectionists to promote their campaigns to make the US government to ban animal testing failed due to the overall support of such experiments by the public, which was assured by the organizations who performed these experiments that animals were kept in good conditions, bred well, and injected with anaesthetics in those operations that could cause them much pain. Therefore, only in the 1960s, the efforts of antivivisectionists were partially justified, with the release of the Laboratory Animal Welfare Act in 1966 (Haugen, 2000). Nevertheless, that law was more focused on the welfare of animals in laboratories rather than on the prohibition of animal testing.
Nowadays, there are a great number of organizations that advocate for stopping using animals in the experiments. Although the overall effectiveness of their campaigns is quite low, they have managed to achieve some positive results concerning the problem of animal testing (Murnaghan, 2017). Additionally, considering the current tendency of the active development of various technologies that can easily substitute experiments on animals, there is hope that soon the animal testing industry will cease to exist.
Animal Testing Is Cruel
The first argument against animal testing is that it is simply cruel. People must understand that animals are the same living creature as them and can feel both psychological and physical pain in the same way as humans. Thus, in the case of experimenting on animals, the ethical and humane aspects of the issue must prevail and give people a stimulus to seek for other ways of studying diseases that can be much better.
Animal Testing and Its Types
First of all, it is necessary to describe the types of animal testing in order to understand the degree of the cruelty of these experiments. In general, animal testing is the process of experimenting on animals where they usually undergo various medical procedures which cause them suffering or even death. These experiments are usually aimed at finding a cure to some disease that humans and certain animals have in common or at exploring how a biological organism works. During the experiments, scientists usually keep animals in cages and use them in laboratories where they harm them on purpose (“What is animal testing,” 2016). Moreover, there are certain kinds of experiments that cause animals a lot of pain, and, in many of them, animals die.
The most common type of an experiment on animals is feeding them with certain substances and injecting them with experimental medications. After the procedure is completed, scientists observe the effects that these substances have caused. In fact, the result is often unpredictable, and animals can die a horrible death with much pain. Another type of experiments is exposing animals to toxic substances and radiation. These experiments are primarily aimed at discovering the effects that radiation and certain chemicals can have on a biological body. Similarly, such experiments make animals suffer (“What is animal testing,” 2016). Moreover, if animals survive after such experiments, the damage that radiation and chemicals have caused to them is often permanent, and they will live the rest of their lives suffering.
One more type of experiments on animals is dissecting animals while they are still alive. Certainly, during this operation, they are under anesthetics, but it does not justify the result that they get after the procedure is completed. The main reason for these experiments is to find out how the internal parts of the biological body work. This operation usually involves removing internal organs, pumping out blood, and excising parts of tissues, which makes animals cripples afterwards. Additionally, there is one more type of an experiment that is usually practiced in laboratories. This is a psychological experiment that involves placing animals in situations and conditions which cause them to feel fear, anxiety, or depression. Such experiments are usually aimed at identifying the principles of animals’ behavior and comparing it to that of humans (“The five worst animal experiments,” 2014). Nevertheless, after these experiments, animals usually become very aggressive and cannot normally function in their animal “society”.
Laws and Animal Testing
According to most religious laws, animal testing is forbidden, as they are defined as the same creatures as humans. Certainly, animals are not as smart as humans, and their perception of reality is different, but they have similar bodies and experience similar feelings. Therefore, before making horrible experiments on animals, humans must think what it would be like if they were experimented on (“The Muslim view on animal,” 2017). Thus, animals have the same right to live their full lives as humans.
Although human laws impose a certain restriction regarding the experiments on animals, they are not enough, as they still allow people to torture them in the experiments. According to European legislation, all vertebrate animals including reptiles, fish, birds, and mammals and only some invertebrates such as octopuses are considered “animals”, on which it is prohibited to experiment (“Treatment of animals,” 2016). In the USA, the situation is worse and such creatures as mice, amphibians, birds, fish, and rats are not defined as “animals”, and scientists can freely perform any experiments on them that they want.
The system of experimenting on animals has grown into a multi-million dollar industry that has many facilities and laboratories around the world. They also have special facilities aimed at breeding animals specifically for testing. In these facilities, animals usually live in bad conditions being imprisoned and forcibly fed. Using wild-caught animals is prohibited in Europe and in some other countries, but it is allowed in other countries of the world. It is usually forbidden to use such domestic animals as dogs and cats in experiments, but, unfortunately, not in all countries (McKay, 2016). Even monkeys that resemble humans the most are often used in experiments.
In terms of animal suffering, The EU even introduced a scale which measures the degree of suffering experienced by animals in a particular experiment. Thus, they distinguish between “minor”, “moderate”, and “severe” suffering inflicted on animals. For example, in 2012, in the UK, more than 60% of permissions were granted by the British government allowing animals to be undergone from moderate to severe suffering. Reportedly, approximately 75% of the experiments were performed without injecting the animals with anesthetics. Moreover, quite a big percentage of those experiments required animals to die (Scheler, 2017). For instance, the tests for various vaccines and chemicals resulted in the death of more than 50% of the animals involved in these experiments.
Animal Testing Is Ineffective
The second argument against animal testing is that it is often ineffective, as the results received from the experiments can be inaccurate. There are many reasons for this, but, the most important point is that in such science as medicine, the information must be reliable; otherwise, there is always a risk that a particular medicine will cause unpleasant effects in humans or even be life-threatening.
Examples of the Ineffectiveness of Animal Testing
In addition to being cruel and inhumane, the experiments on animals often turn out to be ineffective. The main reason for this is that the animal organism either responds differently to many life-threatening diseases that humans suffer from or is completely immune to them. For example, animals do not suffer from most heart diseases, some types of cancer and HIV, they do not have Parkinson’s disease and the majority of psychiatric diseases such as schizophrenia. However, some of these diseases can be artificially induced in them for the sake of an experiment that allegedly shows how these diseases can be cured in humans. Thus, the most important argument is that in these experiments, people usually do not take into consideration other factors that are inherent only in humans and affect the behavior of diseases (“Cruelty to animals,” 2017). These factors include socio-economic conditions, genetics, psychological issues, and personal experience.
Indeed, according to the statistics, quite a great number of experiments on animals, that were promising in terms of finding a cure to some diseases, turned out to be ineffective for humans. In this respect, the end does not justify the means, as animals suffered for nothing. As a result, animals’ lives along with the time and money were wasted, and no effective treatment was developed (“Arguments against animal,” 2016). In addition, as it can be seen, after the decades of animal testing aimed at finding a cure for Alzheimer’s disease, stroke, AIDS, Parkinson’s disease, diabetes, and cancer, there is still no reliable cure and effective treatment for them.
Thus, according to the statistics, the majority of experiments on animals that show promising results, turn out to be ineffective when it comes to humans. Moreover, the experimenting on smaller animals such as rabbits, mice, and rats showed an even lower rate of success, primarily because their organisms differ from that of a human (Scheler, 2017). Additionally, statistics show that only 20% of experimental drugs used on animals are effective in humans. In terms of testing the safety of drugs, only 45% of experiments work for humans.
According to the overall results of the experiments on animals conducted all over the world, approximately 120 million animals are used in them, and only about 30 new medications are approved every year, which is far from being efficient. The investment of the U.S. drug industry in the experiments equals $50 billion each year, but the approval rate has not changed since the 1960s. Among those drugs that are approved, not all of them are completely effective for everyone due to different individual reactions (McKay, 2016). Overall, for the last 20 years, only five percent of experiments performed on animals resulted in a successful approval of treatments.
Sometimes, animal testing can be dangerous even for humans. A vivid example is a drug called Vioxx that was used for arthritis. After successful experiments on monkeys and on some other mammals, this drug was approved for human usage. However, Vioxx turned out to be dangerous for humans causing more than 300,000 heart attacks all over the world, almost half of which resulted in the lethal outcome. Another example is fialuridine, a Hepatitis B drug that was prohibited for having caused liver damage resulting in five deaths. However, this drug had been several times tested on animals before. One more illustrative example is a monoclonal antibody treatment (TGN1412) that was tested on human volunteers. As a result, it caused an allergic reaction, after which the volunteers were hospitalized (Haugen, 2000). However, this drug had been used on monkeys several hundred times before, and no side effects were identified.
Alternatives to Animal Testing
Banning animal testing does not necessarily mean that the development of medications that can provide treatment for incurable diseases will stop, as there are always alternatives, which can improve progress in medicine and add humaneness to the science. Thus, with technological developments in the sphere of science, the number of alternatives to animal testing is increasing. In this respect, the main problem is that most people are reluctant to use new technologies (“Animal testing 101,” 2016). Instead, they tend to stick to more conservative and traditional methods that certainly involve animal testing.
Another obstacle in the process of adoption of these new methods is bureaucracy. There are a lot of organizations and charities that advocate for the prohibition of animal testing, and they can accelerate the process of implementation of these innovations.
In terms of the alternatives, there are several of them that are very effective. The first alternative is growing cells and other organic material in laboratories. Nowadays, almost any type of a human cell can be created in a laboratory. These cells are used in the creation of special devices that are called “organs-on-chips”. These devices can be used for experiments instead of animals. There were already several successful experiments conducted on these devices that involved observing the behavior of diseases and the effects of drugs (“Alternatives to animal,” 2016). Additionally, cell cultures are now the primary focus regarding the development of treatment to such diseases as cancer, AIDS, kidney diseases, and sepsis.
Another alternative to animal testing, which is not new though, is human tissues. Human tissues that can be provided by volunteers or extracted from dead bodies can be used in some kinds of experiments. Moreover, there are many operations such as cosmetic surgery, biopsy, and transplants that can serve as a reliable source of human tissues. Using brain tissues from dead bodies has also lead to a better understanding of such diseases as Parkinson’s disease or multiple sclerosis.
One more alternative to animal testing, the importance of which has been increasingly growing for the past several decades, is computer models. Indeed, the most powerful contemporary computers in the world are able to simulate many processes that would occur in a human body after taking a particular experimental medication. These virtual experiments are primarily based on the already existing data about a particular disease and its behavior in the human body and on mathematical, chemical, and physical laws integrated into this program of simulation (“Alternatives to animal,” 2016). Certainly, now, computer sphere is not powerful enough for complex virtual experiments, but taking into account the rate of its growth, it will be soon.
Arguments for Animal Testing
Despite all the evidence listed above and showing that animal testing is both a cruel and ineffective practice, it still has its defenders. For example, the IQ Consortium DruSafe argues that nonclinical animal testing is crucial when it comes to assessing the risks of developing new drugs (Mangipudy, 2014). They believe that there are no in vitro or in silico systems developed enough to accurately emulate all of the complexities of the human organism (Mangipudy, 2014). Further, even with the eventual development of these surrogate systems, their ability to predict all of the negative effects from unique toxicities is still under question. In general, many people, while denouncing animal testing for cosmetic purposes, still insist that it is an unavoidable necessity for improving human health. Just as well, many people consider animal testing to be a necessary evil – they agree about it being cruel while saying that without it there would be no treatments like insulin, vaccines, HIV drugs and so on and that it helped us get better understanding of diseases like malaria or hemophilia (Murnaghan, 2017).
While it is true that animal testing has proven to be useful in the past, it does not mean that we need to stop developing new methods of testing and keep it around animals. If we consider ourselves to be a truly evolved species, we need to abandon any sort of animal cruelty completely and find ways to benefit ourselves without causing harm to anyone or anything. Furthermore, the effectiveness of alternative methods of testing is not to be underestimated, considering that more and more of them are being researched and improved with each passing year. These alternatives, both with their quantity and their quality, clearly highlight the obsolescence of animal testing and the need for replacing it with more humane and harmless methods.
Thus, as it can be seen from the statistics, animal testing is cruel and in most cases, not effective. Therefore, it must be banned, especially now, when there are many innovative technologies that can be used as alternatives. Moreover, these alternatives have already shown great promises in being much more efficient than animal testing. Fortunately, the current tendency shows that these alternatives will be adopted in the near future, thereby bringing the end to violent experiments on animals.
Alternatives to animal testing . (2016). Web.
Animal testing 101 . (2016). Web.
Arguments against animal testing . (2016). Web.
Cruelty to animals in laboratories . (2017). Web.
The five worst animal experiments happening right now . (2014). Web.
Harm and suffering . (2017). Web.
Haugen, D. M. (2000). Animal experimentation . San Diego, CA: Greenhaven Press.
McKay, M. (2016). The cruelty of lab animal testing. Web.
Murnaghan, I. (2017). Background and history of animal testing. Web.
The Muslim view on animal testing . (2017). Web.
Scheler, S. (2017). Everything you need to know about animal testing. Web.
Scutti, S. (2013). Animal testing: A long, unpretty history. Web.
Treatment of animals . (2016). Web.
What is animal testing? (2016). Web.
Mangipudy, R., Burkhardt, J., & Kadambi, V. J. (2014). Use of animals for toxicology testing is necessary to ensure patient safety in pharmaceutical development. Regulatory Toxicology and Pharmacology , 70 (2), 439-441.
Murnaghan, I. (2017). Using Animals for Testing: Pros Versus Cons. Web.
- Rayon and Its Impact on Health and Environment
- Unemployment as the Consequence of the Development of New Technologies. What Should Private Firms and Government Do?
- Animal Testing as an Unnecessary and Atrocious Practice
- Negative Impacts of Animal Testing
- Animal Testing: History and Ethics
- Solid Waste in the Arabian Gulf Reduction
- California and Water Shortage
- Ways of Producing and Consuming Material Resources
- Environmental Regulation of Oil and Gas
- Chemicals on Birds’ Populations Effects
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, January 19). Animal Testing: History and Arguments. https://ivypanda.com/essays/animal-testing-history-and-arguments/
"Animal Testing: History and Arguments." IvyPanda , 19 Jan. 2022, ivypanda.com/essays/animal-testing-history-and-arguments/.
IvyPanda . (2022) 'Animal Testing: History and Arguments'. 19 January.
IvyPanda . 2022. "Animal Testing: History and Arguments." January 19, 2022. https://ivypanda.com/essays/animal-testing-history-and-arguments/.
1. IvyPanda . "Animal Testing: History and Arguments." January 19, 2022. https://ivypanda.com/essays/animal-testing-history-and-arguments/.
Bibliography
IvyPanda . "Animal Testing: History and Arguments." January 19, 2022. https://ivypanda.com/essays/animal-testing-history-and-arguments/.
IvyPanda uses cookies and similar technologies to enhance your experience, enabling functionalities such as:
- Basic site functions
- Ensuring secure, safe transactions
- Secure account login
- Remembering account, browser, and regional preferences
- Remembering privacy and security settings
- Analyzing site traffic and usage
- Personalized search, content, and recommendations
- Displaying relevant, targeted ads on and off IvyPanda
Please refer to IvyPanda's Cookies Policy and Privacy Policy for detailed information.
Certain technologies we use are essential for critical functions such as security and site integrity, account authentication, security and privacy preferences, internal site usage and maintenance data, and ensuring the site operates correctly for browsing and transactions.
Cookies and similar technologies are used to enhance your experience by:
- Remembering general and regional preferences
- Personalizing content, search, recommendations, and offers
Some functions, such as personalized recommendations, account preferences, or localization, may not work correctly without these technologies. For more details, please refer to IvyPanda's Cookies Policy .
To enable personalized advertising (such as interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. These partners may have their own information collected about you. Turning off the personalized advertising setting won't stop you from seeing IvyPanda ads, but it may make the ads you see less relevant or more repetitive.
Personalized advertising may be considered a "sale" or "sharing" of the information under California and other state privacy laws, and you may have the right to opt out. Turning off personalized advertising allows you to exercise your right to opt out. Learn more in IvyPanda's Cookies Policy and Privacy Policy .

IMAGES
VIDEO
COMMENTS
Many people have questions about animal testing ethics and the animal testing debate. We take our responsibility for the ethical treatment of animals in medical research very seriously. At Stanford, we emphasize that the humane care of laboratory animals is essential, both ethically and scientifically. Poor animal care is not good science.
Benefits of animal testing. The unstoppable advance of medicine and the pharmaceutical industry has increased the volume of animal experimentation. Animal testing is crucial in Drug Discovery and Development, bridging in vitro research and human clinical trials. Regulatory agencies demand data obtained from animal experiments to advance from ...
For some types of research, animals must be engineered to have or lack certain genes (or the proteins made by these genes) in order to determine what role a gene and its protein might play in disease development. This is not possible to do in humans for legal, ethical, and scientific reasons. Researchers often recreate many serious diseases ...
Animal Testing: Conclusion. Animal testing is a helpful phenomenon in biological, medical, and other scientific investigations demanding its incorporation. The phenomenon is helpful, viable, and should be embraced despite the opposing opinions. Animal testing helps in developing effective, safe, viable, qualitative, and less toxic drugs.
Genzel et al. , in particular, take issue with the proposal for a European ban on animal testing. Finally, there is a danger in bypassing animal research in developing new vaccines for diseases such as COVID-19 . The purpose of this paper is to show that, while animal research is necessary for the health of both humans and animals, there is a ...
Here are the examples of animal testing essay topics you can choose from: The question of animal intelligence from the perspective of animal testing. Animal testing should (not) be banned. How animal testing affects endangered species. The history and consequences of animal testing.
Animal models are utilized in biomedical research when questions require a study of whole organisms that cannot be carried out in humans. Typically, animal studies are essential for research that seeks to understand complex questions of disease progression, genetics, lifetime risk or other biological mechanisms of a whole living system that ...
Animal Testing and Medicine. "The greatness of a nation and its moral progress can be judged by the way its animals are treated.". - Mahatma Gandhi. Animals have been used repeatedly throughout the history of biomedical research. Early Greek physician-scientists, such as Aristotle, (384 - 322 BC) and Erasistratus, (304 - 258 BC ...
In the world as we know it today, animal research is still generally accepted as part of society. There are many important reasons why laboratory animal research is still needed: To learn about ...
Animal testing is often a controversial and emotionally-charged subject. It's a mental space that people almost never encounter in their day-to-day lives: that animals, which are sentient and can feel pain, are being instrumentalised at a scale of approximately 4 million animals per annum in the UK alone. This mental space is made even more ...
According to the UK Home Office (12), in the year 2016, 48.6% of the animal tests in medical research were conducted for genetically oriented studies. Moreover, 28.5% of the medical research involving animal testing was for basic biological research, 13.5% was for regulatory. testing, 8.6% was for translating research from animals to humans ...
1. 95% of animals used in experiments are not protected by the federal Animal Welfare Act (AWA), which excludes birds, rats and mice bred for research, and cold-blooded animals such as reptiles and most fish. [1] [2] [3] 3. Chimpanzees share 99% of their DNA with humans, and mice are 98% genetically similar to humans.
The Debate on Animal Testing Essay. Animal testing is described as a procedure involving vivisection and/or In vivo testing of animals for experimentation or research. In the pursuit of what is known as scientific progress, animals have fallen victims of distress in the process. Throughout history, human has employed animals in carrying out ...
1. Animal Testing and Medicine. Written by a cardiologist, this article provides a brief overview of the history of animal testing but ultimately argues that animal testing is necessary and beneficial. (If you're writing an argument of your own, check out How to Write a Winning Argument Essay.) MLA 8 Citation.
Animal rights arguments. The only significant ethical argument against animal research that remains is based on the idea of rights. Just as humans have inalienable rights, the argument goes, so do animals. According to this view, the use of animals for research can never be justified for exactly the same reasons that we cannot justify using humans.
Animal testing should be viewed as beneficial. There are so many benefits to animal testing. Research on living animals has been going on ever since at least 500 BC. Many scientific breakthroughs couldn't have happened without animal testing. Testing on animals can be connected to almost every medical breakthrough in the past one hundred years.
Animal testing involves using non-human animals for scientific experiments to gain insights into human diseases, develop new pharmaceuticals, and test the safety of various products. The central question is whether the benefits derived from animal testing justify the ethical costs. This essay argues that animal testing should be banned due to ...
Discussion Whether Animals Testing is Necessary. Essay grade: Good. 4 pages / 1634 words. Introduction: Animal testing is a debated issue over the previous decades. Animal testing in simple words is the use of animals in researches in order to determine the safety of various products such as foods, drugs and cosmetics.
Abstract: Nonhuman animal ("animal") experimentation is typically defended by arguments that it is reliable, that animals provide sufficiently good models of human biology and diseases to yield relevant information, and that, consequently, its use provides major human health benefits. I demonstrate that a growing body of scientific ...
Find all our Student Opinion questions here. On Sept. 10, the Environmental Protection Agency said it would move away from requiring the testing of potentially harmful chemicals on animals, a ...
Animal testing, otherwise known as vivisection has been around prior to the 19th century, in fact, in 1973 there was already a total of over 1,500,000 animals being experimented on. Britain was the first to stand up against animal testing, stating that it was cruel in 1876 with the Cruelty to Animals Act. America on the opposing hand, launched ...
The first animal protection law was established in Great Britain in 1822. A significant milestone in the history of animal protection legislation was the introduction of the Cruelty to Animals Act in 1876 in Great Britain. This law was promoted by Charles Darwin who, despite being a biologist and a scientist, was against vivisection.
It's now easy to see why animal testing is wrong: it violates basic principles of ethical research: it is maleficent, or harmful to the research subjects; it is not beneficial to them; it is forced on them since they don't consent; and it is unjust in that animals are burdened with problems not their own. Research - at least with animals ...