- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Questions and answers /

Coronavirus disease (COVID-19): Vaccine research and development
Reviewed and current on 10 August 2021.
WHO and its partners are committed to accelerating the development of COVID-19 vaccines while maintaining the highest standards on safety.
Vaccines go through various phases of development and testing – there are usually three phases to clinical trials, with the last one designed to assess the ability of the product to protect against disease, which is called efficacy. All phases assess safety. The last phase, phase III, are usually conducted in a large number of people, often 10’s of thousands. After that, the vaccine needs to go through a review by the national regulatory authority, who will decide if the vaccine is safe and effective enough to be put on the market, and a policy committee, who will decide how the vaccine should be used.
In the past, vaccines have been developed through a series of consecutive steps that can take many years. Now, given the urgent need for COVID-19 vaccines, unprecedented financial investments and scientific collaborations are changing how vaccines are developed. This means that some of the steps in the research and development process have been happening in parallel, while still maintaining strict clinical and safety standards. For example, some clinical trials are evaluating multiple vaccines at the same time. It is the scale of the financial and political commitments to the development of a vaccine that has allowed this accelerated development to take place. However, this does not make the studies any less rigorous.
The more vaccines in development the more opportunities there are for success.
Any longer-term safety assessment will be conducted through continued follow up of the clinical trial participants, as well as through specific studies and general pharmacovigilance of those being vaccinated in the roll out. This represents standard practise for all newly authorized vaccines.
In a regular vaccine study, one group of volunteers at risk for a disease is given an experimental vaccine, and another group is not; researchers monitor both groups over time and compare outcomes to see if the vaccine is safe and effective.
In a human challenge vaccine study, healthy volunteers are given an experimental vaccine, and then deliberately exposed to the organism causing the disease to see if the vaccine works. Some scientists believe that this approach could accelerate COVID-19 vaccine development, in part because it would require far fewer volunteers than a typical study.
However, there are important ethical considerations that must be addressed – particularly for a new disease like COVID-19, which we do not yet fully understand and are still learning how to treat; it may be difficult for the medical community and potential volunteers to properly estimate the potential risks of participating in a COVID-19 human challenge study. For more information, see this WHO publication on the ethics of COVID-19 human challenge studies .
Small (phase I) safety studies of COVID-19 vaccines should enroll healthy adult volunteers. Larger (phase II and III) studies should include volunteers that reflect the populations for whom the vaccines are intended. This means enrolling people from diverse geographic areas, racial and ethnic backgrounds, genders, and ages, as well as those with underlying health conditions that put them at higher risk for COVID-19. Including these groups in clinical trials is the only way to make sure that a vaccine will be safe and effective for everyone who needs it.
Opportunities to volunteer for a COVID-19 vaccine trial vary from country to country. If you are interested in volunteering, check with local health officials or research institutions or email [email protected] for more information about vaccine trials.
The push for a COVID-19 vaccine
Vaccines explained
Related Q&As:
Vaccines and immunization: What is vaccination?
Coronavirus disease (COVID-19): Vaccines
Coronavirus disease (COVID-19): COVID-19 Vaccine access and allocation
- Contact Tracing
- Pandemic Data Initiative
- Events & News
- Primer on COVID-19 Vaccine
- COVID-19 Vaccine Matters
JHU has stopped collecting data as of
After three years of around-the-clock tracking of COVID-19 data from...
With dozens of COVID-19 vaccines now in clinical trials, it is important to understand the accelerated timelines for development, the different types of vaccines available, and the facts related to vaccine safety and efficacy. Additionally, as vaccines are approved, we will track data on vaccination efforts.
VACCINE TRACKER
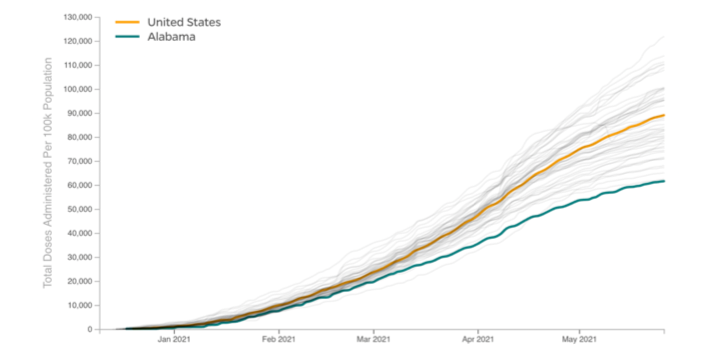
U.s. vaccination efforts.
Explore Vaccination Progress by U.S. State
International Vaccination Efforts
Explore Vaccination Progress by Country
Vaccine Story
Explore how U.S. states delivered their supplies of COVID-19 vaccines to their residents at vastly different speeds that led to vastly different results.
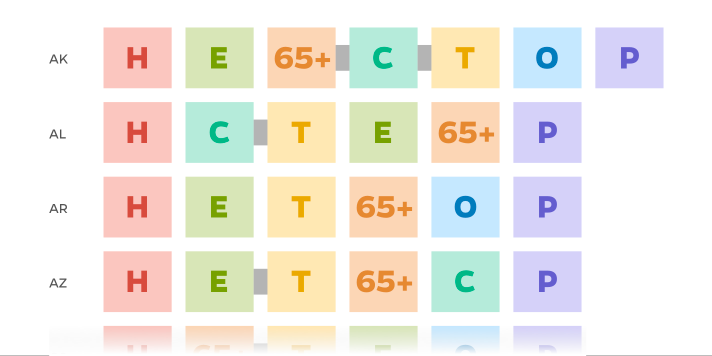
Vaccine State Plans
Compare the various strategies U.S. states used for rolling out vaccinations. While states all agreed health care workers should be first in line for vaccines, the states quickly diverged on who came next.
Vaccine Insights

INTERACTIVE
Developing vaccines quickly and safely
An interactive tool from our vaccine experts that explores how a vaccine is developed and the differences between a typical timeline and an accelerated timeline.

UNDERSTANDING COVID-19
Johns Hopkins International Vaccine Access Center
VIEW-hub is a publicly available interactive tool that displays up-to-date information on vaccine characteristics, and vaccine introduction and use globally. Vaccines include COVID-19 as well as many childhood vaccines in routine immunization programs.

Understanding COVID-19
COMIT: COVID-19 Maternal Immunization Tracker
COMIT, the COVID-19 Maternal Immunization Tracker, provides a global picture of national public health policies that influence access to COVID-19 vaccines for pregnant and lactating people.
Questions about Vaccines
Vaccines Q & A
'Vaccine Ambassador’ Course Aims to Arm Parents with Credible Information
The Johns Hopkins International Vaccine Access Center provides free, online training to equip people with knowledge to debunk false claims about vaccinations.
Q&A: Youth Vaccines Still Face Hurdles
Emergency use authorization for Pfizer/BioNTech’s vaccine for U.S. children is unlikely to lead to widespread vaccinations among the nation’s 28 million 5-to-11-year-olds.
As Africa goes unvaccinated, U.S. remains awash in vaccines
Nearly 60% of the U.S. population is fully vaccinated. All of Africa: just 6%. Yet the United States is expanding boosters and authorizing vaccines for children age 5 to 11 years old – and hoarding and wasting additional doses.
New A.I. ChatBot Helps Thousands Learn Vaccine Truths
An artificial intelligence tool helps vaccine hesitant people find facts about vaccines.
Report Series: A PRIMER ON COVID-19 VACCINE DEVELOPMENT, ALLOCATION AND DEPLOYMENT
This series of brief reports will shed light on COVID-19 vaccine development, allocation and deployment in the United States and globally. Topics will include ensuring the safety and efficacy of Covid-19 vaccines, principles for vaccine allocation, strategies for deployment and delivery of Covid-19 vaccines, vaccine confidence and demand, and the economics of Covid-19 vaccines.
REPORT | May 26, 2021
Q&A: 80 Million Donated Vaccine Doses: Too Little, Too Late?
By William Moss
REPORT | March 18, 2021
Building trust in vaccination
By Molly Sauer, Rupali Limaye
REPORT | March 1, 2021
Experts Explore the Ethics of Covid-19 Vaccines and Pregnancy
By Carleigh Krubiner, Chizoba Wonodi, Elana Jaffe, Ruth Faden
REPORT | December 23, 2020
Economics of COVID-19 Vaccines
By Deborah Odihi, Elizabeth Watts, Bryan Patenaude, Salin Sriudomporn, Cristina Garcia, Gatien de Broucker
COVID-19 VACCINE MATTERS
COVID-19 VACCINE MATTERS: A blog series discussing the evolving science and policy of COVID-19 vaccines, led by internationally renowned experts in vaccine development, Dr. Larry Corey of the Univeristy of Washington, and Dr. Chris Beyrer of Johns Hopkins University.

Report | February 25, 2022
The Omicron Story: The Winter of Our Discontent, Part 3
By Larry Corey
Report | February 22, 2022
The Omicron Story: The Winter of Our Discontent, Part 2
Report | February 17, 2022
The Omicron Story: The Winter of Our Discontent, Part 1
Jhu-uw vaccine symposium.
Johns Hopkins University and the University of Washington hosted a high-level symposium, “The Scientific Integrity of COVID-19 Vaccine Efficacy Trials: From Clinical Trials to Public Allocation,” that explored complex issues, brought together leading voices in the field, and put forward a concise plan for protecting the scientific integrity of these ongoing efforts.
webcasts | October 6, 2020
Full Video: JHU-UW Vaccine Symposium
Part 1: the science and structure of the u.s. government’s covid-19 vaccine trial program, part 2: protecting scientific integrity in the design & conduct of covid-19 vaccine efficacy trials, part 3: regulatory integrity and the assessment of vaccine safety and efficacy, latest news & resources.
news | June 30, 2022
The New York Times: As vaccines for younger U.S. children roll out, the effects on day care centers may be muted.
American children ages 6 months through 4 years recently became eligible to receive coronavirus vaccinations, after federal regulators and health officials cleared vaccines from Moderna and Pfizer-BioNTech for very young children.
news | June 27, 2022
Associated Press: US grapples with whether to modify COVID vaccine for fall
U.S. health authorities are facing a critical decision: whether to offer new COVID-19 booster shots this fall that are modified to better match recent changes of the shape-shifting coronavirus.
news | June 16, 2022
Fast Company: As the coronavirus evolves, how should your next vaccine dose change?
When the Food and Drug Administration (FDA) meets at the end of June to discuss whether the vaccine should be modified before the likely rollout of more booster shots in the fall, it will have to make decisions without knowing how the virus will continue to change.
news | May 23, 2022
Associated Press: Pfizer says 3 COVID shots protect children under 5
Three doses of Pfizer’s COVID-19 vaccine offer strong protection for children younger than 5, the company announced Monday, another step toward shots for the littlest kids possibly beginning in early summer.
news | May 9, 2022
FiveThirtyEight: Why even a less effective COVID-19 vaccine is worth getting
Since the Omicron wave crested in January, multiple studies and datasets have demonstrated that the mRNA vaccines are not nearly as effective against this variant as they were against earlier variants or the original virus.
news | April 18, 2022
Verywell Health: Which booster should you get if you received a Johnson & Johnson vaccine?
According to the CDC, Pfizer-BioNTech or Moderna COVID-19 vaccine boosters are preferred for individuals who received the single-dose Johnson & Johnson shot.
news | April 1, 2022
AARP: Second booster shots: Who should get one and when?
Adults 50 and older can now roll up their sleeves for a second COVID-19 booster, as can younger individuals with certain immune-compromising conditions.
news | March 31, 2022
USA Today: Those who got J&J’s COVID vaccine should seriously consider a Pfizer or Moderna booster, experts say
More than 16 million Americans rolled up their sleeves last year to get Johnson & Johnson's COVID-19 vaccine because it promised to be a "one and done" shot, but newer information and booster authorizations suggest they should consider a third dose.
news | March 29, 2022
AARP: FDA OKs second COVID-19 booster for 50+
The Centers for Disease Control and Prevention (CDC) agreed with the FDA and has updated its vaccine recommendations to include a second booster.
CNN: Who’s eligible for second COVID-19 booster shots – and when to get them
On Tuesday, the FDA announced that it has expanded the emergency use authorization for the two vaccines to allow adults 50 and older to get a second booster as early as fourth months after their first booster dose.
experts | March 29, 2022
Associated Press: US opens second COVID boosters to 50 and up, others at risk
The Food and Drug Administration on Tuesday authorized an extra dose of the Pfizer or Moderna vaccine for Americans 50 and older and for certain younger people with severely weakened immune systems.
news | March 18, 2022
The Washington Post: Vaccines remained highly effective at preventing serious illness and death during Omicron surge, CDC report says
The coronavirus vaccines most widely used in the United States remained highly effective at preventing the worst outcomes from infections even in the face of the highly transmissible Omicron variant in January, a report released Friday by federal disease trackers shows.
- Biochemistry and Molecular Biology
- Biostatistics
- Environmental Health and Engineering
- Epidemiology
- Health Policy and Management
- Health, Behavior and Society
- International Health
- Mental Health
- Molecular Microbiology and Immunology
- Population, Family and Reproductive Health
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
More Questions and Answers About COVID-19 Vaccines
Interview by Stephanie Desmond
How is it possible that COVID-19 vaccines prevent serious illness and death but may not prevent mild infection? How effective are vaccines at preventing long-haul COVID? How soon might we see flu mRNA vaccines and would those have to go through clinical trials?
Josh Sharfstein answers a list of important questions about COVID-19 vaccines.
Most COVID vaccine information is focused on how effective they are at preventing serious disease, hospitalization, and death. How is it possible that the vaccine is more effective at preventing serious illness and death than it is preventing a mild infection?
It’s actually very common for vaccines to be much better at preventing serious illness and death than preventing infection or mild infection. For example, with the flu vaccine, people can still often get the flu, but they are much less likely to get seriously ill or die if they get the flu vaccine.
The question is why. It partly depends on how the immune system responds to vaccines. Any infection whatsoever is a certain type of immune response, and very few vaccines give what people call a “sterilizing immune response.”
What vaccines do cause is an immune response that is strong and multifaceted inside your body. So, even if you knew that the virus can replicate a bit for a mild infection, it can’t cause that huge overwhelming infection that really puts people at risk.
Early on in the pandemic, before we even had vaccines, some vaccine experts were saying the most important thing is going to be [preventing] serious illness and death, and [vaccines will] probably will be much better for that than for mild illness, just like almost every other vaccine out there. Sure enough, that proved to be the case.
How effective are vaccines at preventing long-haul COVID?
We don’t know. It’s a good question, because people can get these long-term symptoms from relatively mild infection.
There are some studies being set up to assess this, but we don’t know for sure. The safe bet would be that the chance of getting a long-haul infection is going to be much lower [for] someone who’s vaccinated compared to someone who’s not, just because that person is much less likely to get infected at all.
There’s also this related question of whether people with long-term symptoms from COVID actually might benefit from getting vaccinated. Somebody who had an infection and has been suffering some of those symptoms like fatigue and brain fog—does it get better if you get vaccinated? There’s no answer to that; however, at multiple clinical sites, some of the doctors are hearing from their patients that they’re feeling somewhat better. I think that the real answer to that, though, is going to depend on studies that will be completed, to see whether it makes a difference.
If I have no symptoms at all after receiving the Pfizer or Moderna vaccines, does this indicate that if I had gotten COVID, I would have been asymptomatic or had mild symptoms?
I do not think it means that.
What determines how sick you are from COVID-19 is a complex set of things that include how much virus your body actually took in. That’s one reason why people who get exposed to lower levels of virus are more likely to have an infection without symptoms, for example.
It also relates to different aspects of people’s immune system and probably some other factors we haven’t figured out, so I would not assume that the response to the vaccine is the same as the response to the actual virus.
Is this the first time mRNA technology has been used in a vaccine?
It is not, actually. There are several vaccines that are in development with mRNA technology. They’ve completed safety studies for them, and that includes influenza—so there could be an mRNA flu vaccine in the future—cytomegalovirus, Zika virus, and the rabies virus.
[These vaccine trials] haven’t made it all the way to the end [because] those were going through the regular vaccine process where you go one step at a time. Those companies aren’t going to invest in a big, next trial until they’ve really analyzed the data from the previous study.
In the case of [COVID-19 vaccines], we had a lot of urgency and all the money was put up, up front. The companies didn’t have to find the money for each stage—they were just able to just proceed from the safety study to the effectiveness study very quickly. This let the coronavirus vaccines go to the front of the line because of the urgency.
This is a technology that’s been well studied, not just for vaccines, but also for therapeutics.
Do you think that having successful mRNA COVID vaccines will pave the way for these other vaccines?
It’s going to be great for people’s comfort level with the vaccine, both at a level of understanding—like, “Wow, that’s going to be like the coronavirus vaccine, and it was so successful!”—and also scientifically, I think there’ll be a greater understanding of mRNA vaccines, and that will help with the development and the review of other mRNA vaccines for different different viruses.
Having said that, just because an mRNA vaccine works for coronavirus doesn’t mean it’s necessarily going to work for a rabies or influenza virus. They’re going to have to do studies to find out.
Do we know yet how soon flu vaccines may be made as mRNA vaccines, and will they have to go through clinical trials as a new vaccine?
I would expect that they would go to clinical trials … but I do know that some studies have already been done, and hopefully this will proceed and we’ll get another great vaccine.
One of the long-held goals for flu vaccination is a vaccine that lasts more than one year, and maybe a vaccine that doesn’t require a strain change every year. The mRNA vaccines may be a way to get to that goal, but there obviously has to be a lot more research.
Why are mRNA vaccines so encouraging for the future?
This is a platform that has certain advantages, among them, that you can stand it up so quickly. It doesn’t require a lot of different ingredients—it’s a very, very small number of things that go into the vaccine—and it can be updated, very quickly, so if you need to change the strain, it’s very possible to do that.
I think we’ll look back and think that mRNA kind of had its coming out party with coronavirus, but [was] around beforehand, and it will hopefully lead to some other important advances in medicine.
How are side effects from COVID-19 vaccines being monitored?
They’re being monitored in multiple ways. One thing that people who have gotten vaccinated know is that you have an opportunity to get texted about the potential side effects you’re experiencing. The Centers for Disease Control is looking at that from millions of people who are getting vaccinated to understand the profile of side effects. People also submit reports to the manufacturers and to the FDA about potential side effects, and there are studies that are done in large insurance databases or clinical databases where you can look at the people who got the vaccine compared to people who didn’t get the vaccine to see whether there’s any difference in case there’s a question about whether or not a particular side effect might be caused by the vaccine.
On a regular basis, there is a big group that comes together and looks at data from all these different sources to see what the safety profile is and, so far, it’s been very, very strong.
I was just looking at a 60-page document that’s posted on the CDC website where they went through all these different sources and they have a huge analysis of allergic reactions. I think the Pfizer vaccine had five serious allergic reactions per million doses given, and per 2.8 million for the Moderna vaccine. Almost always, those allergic reactions are in the first dose. Not always, but almost always.
It also talks about the evidence of the mild side effects people get. Seventy percent of the people get a sore arm; I think about a third got a headache, a third got fatigue, but then of course they feel better in just a couple of days.
They’ve been even doing studies in these insurance databases to compare people who are vaccinated and people who aren’t vaccinated just for things that people think “Well, maybe, could it possibly relate to this [vaccine]?” and they have not found any serious red flags coming up.
So, there’s a lot of analysis of safety data and there will continue to be. It’s a very important part of vaccination and the vaccination program to look at safety and not just in one way, but in multiple ways.
Does someone who recovered from COVID and then gets vaccinated have a higher immunity than someone who hasn’t had COVID and also gets vaccinated?
In general, people who have had COVID have some immune reaction to COVID when they recover. But it’s variable—some people may have a pretty mild immune reaction, and some people may have a very protective immune reaction—and right now, we don’t have an easy way to tell the difference between them.
That’s why vaccination is recommended for everyone, even if you’ve had COVID before. There will be studies of different types of people, their vaccination status and when they got vaccinated, and hopefully we’ll get a picture and some markers like a blood test that you could take to find out how protected you are. We have that for certain infectious diseases. You can, for example, for hepatitis B, see whether you have antibodies.
One of the things we’ll learn from some of these studies is, is there a way to test people for their ability to withstand a coronavirus infection? When we have that, I think that might be more important than these general questions because probably it will depend on the individual and having some way to test to figure that out over time is what will be helpful to people.
If I’ve had COVID, how long should I wait to get vaccinated? Is it okay to get my first dose if I no longer have symptoms?
The basic standard requirements are that if you are in that period where you’re sick and could be spreading COVID to stay home until you get better, which I think is around 10 days and no symptoms—then it’s fine to get vaccinated.
[Some] people have said you’re probably relatively protected from another infection for a couple months after that infection and, if you want to wait a couple of months to get vaccinated, you can do that. But there’s no requirement to do that. It’s perfectly fine to get vaccinated.
There are people who may get COVID right after their first shot, before there’s any protection, and they could get vaccinated for their second shot on time if they want, with one exception: If they’ve been treated for that COVID infection with antibody treatment, then there’s a recommendation to wait 90 days so that that antibody treatment doesn’t interfere with the vaccination.
What will happen if everyone gets vaccinated? Won’t the variants get tougher as their source of food gets eliminated?
The virus is constantly mutating and every time that it replicates, there’s a chance that you could develop a variant. If the virus can’t replicate, the virus can’t develop a variant. If the virus is replicating a lot, then you’re more likely to get variants.
The goal of a vaccination campaign now is to reduce the spread of the virus, which reduces the replication of the virus, which will reduce the chance that there will be more variants.
With less virus, fewer people are dying. And with less virus, fewer variants.
The CDC recently released guidance for what vaccinated people can do safely. What do you think of this?
One important principle is that vaccination is important to people both directly and indirectly.
Directly, it’s important if you’re protected, and there may be some things that are different, like you can meet up in small groups with people who are vaccinated.
There’s also the indirect benefit, which is the more people get vaccinated, the less coronavirus is spreading out there. The less coronavirus spreading out there, the easier it is to open things up again. That’s the indirect benefit, and that may not happen the day you get vaccinated or the day you’re protected from your vaccine. But, the more people in your community get vaccinated, the more likely the benefit is going to come help you.
This is exciting because we can see what the end of the pandemic might look like, but we just have to get there. We can’t trip on our way running too fast to the end of the pandemic.
Meanwhile, states like Texas and Mississippi have both rescinded their mask mandates. Is this getting a little too far ahead?
We have to push COVID as far into the end zone as it can go through good mask wearing, social distancing, and vaccination until we really are able to open things with competence. The risk of doing it too soon is that the virus keeps spreading, you get mutations, you get potential variants spreading, and we wind up taking a step back. That takes longer, in the end, to get to the place that we all want to go.
I’m also concerned about the mixed messaging. Mask wearing really does reduce infection, and we still have a lot of infections in the United States, even though it has come down. Just to hear from one level of government “Do this,” and another level of government “Do that,” it just stirs the pot again and makes it harder for people just to stick with the program long enough to put coronavirus back in a box, which I think is within reach.
Now, will what the governors do really upend that? We don’t know. But will it increase the risk of a problem? It might, and I think that’s why you hear so many people saying, “We’re headed toward the end zone, don’t blow it.”
Joshua Sharfstein, MD , is the vice dean for Public Health Practice and Community Engagement and a professor in Health Policy and Management . He is also the director of the Bloomberg American Health Initiative and a host of the Public Health On Call podcast.
Stephanie Desmon is the co-host of the Public Health On Call podcast. She is the director of public relations and marketing for the Johns Hopkins Center for Communication Programs , the largest center at the Bloomberg School of Public Health.
RELATED CONTENT
- Monica Gandhi and Vaccine Optimism (Podcast)
- Vaccine Week Finale: Q&A with Dr. Josh Sharfstein (podcast)
- For mRNA Vaccines, COVID-19 Is Just the Beginning
Facebook Live Q&A
This conversation is excerpted from a March 5 Facebook Live.
See More Like This
Related Content

Why We’re Still Waiting for a Pandemic Treaty

Why COVID Surges in the Summer
The Problem with Pulse Oximeters: A Long History of Racial Bias

What to Know About COVID FLiRT Variants

Rotavirus the Leading Cause of Diarrheal Deaths Among Children Under 5, New Analysis Finds
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Vaccines articles from across Nature Portfolio
Vaccines are a clinical product that is composed of live or dead material from an infectious agent – bacterium, virus, fungus or parasite – that elicit protective immunity against the pathogen when administered. These substances are used to prevent the spread of infectious diseases.
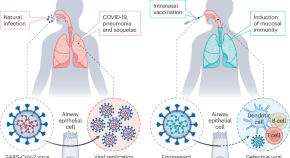
The evolving landscape of live attenuated COVID-19 vaccines
A genetic approach based on the introduction of premature termination codons can attenuate SARS-CoV-2 and induce protective mucosal immunity.
- Simone Pecetta
- Rino Rappuoli
Related Subjects
- Cell vaccines
- Conjugate vaccines
- DNA vaccines
- Inactivated vaccines
- Live attenuated vaccines
- Peptide vaccines
- Protein vaccines
- RNA vaccines
Latest Research and Reviews
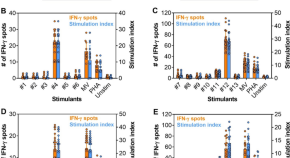
Memory B cell responses induced by pneumococcal conjugate vaccine schedules with fewer doses: analysis of a randomised-controlled trial in Viet Nam
As part of a randomized controlled trial in Viet Nam, this study finds that pneumococcal-specific memory B cells (B mem ) are higher following a 1 + 1 compared to a 0 + 1 pneumococcal conjugate vaccine (PCV) schedule and higher for PCV13 compared to PCV10. B mem did not wane as rapidly as IgG by 24 months of age.
- Darren Suryawijaya Ong
- Thanh V. Phan
- Paul Vincent Licciardi
Lack of detectable HPV18 antibodies in 14% of quadrivalent vaccinees in a longitudinal cohort study
- Penelope Gray
- Filipe Colaço Mariz
- Matti Lehtinen
Immunogenicity of a peptide-based vaccine for measles: a pilot evaluation in a mouse model
- Huy Quang Quach
- Tamar Ratishvili
- Richard B. Kennedy
A real-world observation of patients with glioblastoma treated with a personalized peptide vaccine
Despite new treatment options, prognosis for patients with glioblastoma (GBM) remains poor. Here the authors report the clinical course of patients with GBM treated with a personalized neoantigen-derived peptide vaccine treated within the scope of an individual healing attempt.
- Pauline Latzer
- Henning Zelba
- Saskia Biskup
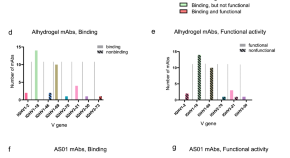
Antibody gene features associated with binding and functional activity in malaria vaccine-derived human mAbs
- Camila H. Coelho
- Susanna Marquez
- Patrick E. Duffy
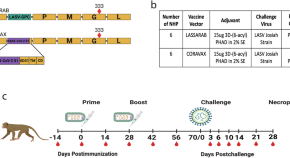
Inactivated rabies-based Lassa fever virus vaccine candidate LASSARAB protects nonhuman primates from lethal disease
- Gabrielle Scher
- Catherine Yankowski
- Matthias J. Schnell
News and Comment

Targeting the spread of antimicrobial resistance plasmids
A recent study demonstrates targeting plasmid-encoded bacterial proteins containing immunoglobulin-like domains to prevent the conjugation and spread of antimicrobial resistance plasmids.
- Ashley York
Growing mpox outbreak triggers Africa’s first health emergency — and fears of wider spread
The Africa Centres for Disease Control and Prevention issues a historic declaration as several countries see their first cases of the disease caused by the monkeypox virus.
The pathogens that could spark the next pandemic
The World Health Organization has updated its list of most dangerous viruses and bacteria.
- Smriti Mallapaty

Your nose has its own army of immune cells — here’s how it protects you
Detailed profile of the immune cells in the upper airway could help to improve nasal vaccines.

Elixir for DCs
Age-associated defects in dendritic cells can be corrected by hyperactivating adjuvants containing an oxidized phospholipid to induce effective antitumour responses in mice.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Better Than Traditional Shots: New Nasal Vaccine Halts COVID-19 Transmission
Research on hamsters suggests that vaccines aimed at the nose and mouth could be crucial in curbing the transmission of respiratory infections.
The rapid development of COVID-19 vaccines within months of the virus ’s emergence was a remarkable achievement of modern science, saving millions of lives. But for all the good they did in reducing illnesses and deaths, the shots were unable to end the pandemic because of one notable weakness: They couldn’t stop the spread of the virus.
A new study by researchers at Washington University School of Medicine in St. Louis indicates that next-generation vaccines that target the virus’s points of entry — the nose and mouth — may be able to do what traditional shots cannot: contain the spread of respiratory infections and prevent transmission. Using a nasal COVID-19 vaccine based on Washington University technology, approved for use in India and licensed to Ocugen for further development in the U.S., the researchers showed that vaccinated hamsters that developed infections did not pass the virus on to others, breaking the cycle of transmission. In contrast, an approved COVID-19 vaccine that is injected failed to prevent the spread of the virus.
Efficacy of Mucosal Vaccines
The findings, published July 31 in Science Advances , provide further evidence that so-called mucosal vaccines sprayed into the nose or dropped into the mouth may be the key to controlling respiratory infections such as influenza and COVID-19 that continue to circulate and cause significant illness and death.
“To prevent transmission, you need to keep the amount of virus in the upper airways low,” said senior author Jacco Boon, PhD, a professor of medicine, of molecular microbiology and of pathology & immunology. “The less virus that is there to begin with, the less likely you are to infect someone else if you cough or sneeze or even just breathe on them. This study shows that mucosal vaccines are superior to injected vaccines in terms of limiting viral replication in the upper airways and preventing the spread to the next individual. In an epidemic or pandemic situation, this is the kind of vaccine you’re going to want.”
Developing vaccines that can control virus levels in the nose has proven challenging. Viruses such as influenza virus, SARS-CoV-2 (the virus that causes COVID-19), and respiratory syncytial virus (RSV) multiply rapidly in the nose and spread from person to person within a few days of initial exposure. Traditional injectable vaccines generate immune responses that can take a week to build to full strength and are much less potent in the nose than in the bloodstream, leaving the nose relatively unprotected against a fast-multiplying, fast-spreading virus.
In principle, a vaccine sprayed or dropped directly into the nose or mouth could limit viral reproduction and thereby reduce transmission by eliciting an immune response right where it’s needed most. But gathering evidence that mucosal vaccines actually do reduce transmission has proven tricky. Animal models of transmission are not well-established, and tracking person-to-person transmission is fiendishly complicated, given the number and variety of encounters a typical person has on any given day.
For this study, Boon and colleagues developed and validated a model for community transmission using hamsters and then used it to assess the effect of mucosal vaccination on the spread of SARS-CoV-2. (Unlike mice, hamsters are naturally susceptible to infection with SARS-CoV-2, making them the ideal laboratory animals for a transmission study.)
Study Methodology and Findings
The researchers immunized groups of hamsters with laboratory versions of approved COVID-19 vaccines: the nasal iNCOVACC used in India or the injected Pfizer vaccine. For comparison, some hamsters were not immunized. After giving the vaccinated hamsters a few weeks for their immune responses to fully mature, the researchers infected other hamsters with SARS-CoV-2 and then placed the immunized hamsters with the infected hamsters for eight hours. This first step of the experiment mimics the experience of vaccinated people who are exposed to a person with COVID-19.
After spending eight hours rubbing shoulders with infected hamsters, most of the vaccinated animals became infected. Virus was found in the noses and lungs of 12 of 14 (86%) hamsters that had received the nasal vaccine, and 15 of 16 (94%) hamsters that had received the injected vaccine. Importantly, while most animals in both groups were infected, they weren’t infected to the same degree. Hamsters that had been nasally immunized had virus levels in the airways 100 to 100,000 times lower than those that had received the shot or had not been vaccinated. The study did not assess the animals’ health, but previous studies have shown that both vaccines reduce the likelihood of severe illness and death from COVID-19.
The second step of the experiment yielded even more striking results. The researchers took vaccinated hamsters that subsequently developed infections and placed them with healthy vaccinated and unvaccinated hamsters for eight hours to model transmission of virus from a vaccinated person to others.
None of the hamsters that were exposed to nasally vaccinated hamsters became infected, regardless of whether the recipient hamster had been vaccinated or not. In contrast, roughly half of the hamsters that were exposed to hamsters vaccinated by injection became infected — again, regardless of the recipient’s immunization status. In other words, vaccination through the nose — but not by injection — broke the cycle of transmission.
These data, Boon said, could be important as the world prepares for the possibility that avian influenza, currently causing an outbreak in dairy cows, might adapt to humans and trigger a flu epidemic. An injectable vaccine for avian influenza already exists, and a team of researchers at Washington University is working toward a nasal vaccine for avian influenza. That team includes Boon and co-author Michael S. Diamond, MD, PhD, the Herbert S. Gasser Professor of Medicine, and one of the inventors of the nasal vaccine technology used in this paper.
“Mucosal vaccines are the future of vaccines for respiratory infections,” Boon said. “Historically, developing such vaccines has been challenging. There’s still so much we don’t know about the kind of immune response we need and how to elicit it. I think we’re going to see a lot of very exciting research in the next few years that could lead to big improvements in vaccines for respiratory infections.”
Reference: “Mucosal immunization with ChAd-SARS-CoV-2-S prevents sequential transmission of SARS-CoV-2 to unvaccinated hamsters” by Tamarand L. Darling, Houda H. Harastani, Astha Joshi, Traci L. Bricker, Nadia Soudani, Kuljeet Seehra, Ahmed O. Hassan, Michael S. Diamond and Adrianus C. M. Boon, 31 July 2024, Science Advances . DOI: 10.1126/sciadv.adp1290
The study was funded by the National Institute of Allergy and Infectious Diseases.
Disclosures: M.S.D. is a consultant for inbios, vir Biotechnology, Ocugen, topspin therapeutics, GlaxoSmithKline, Allen & Overy llP, Moderna and immunome. the Diamond laboratory has received unrelated funding support in sponsored research agreements from vir Biotechnology, emergent BioSolutions and Moderna. M.S.D. and A.O.h. are inventors of the chAd-SARS-cov-2 technology, which Washington University has licensed to Bharat Biotech and Ocugen inc. for commercial development. the Boon laboratory has received unrelated funding support in sponsored research agreements from Greenlight Biosciences inc. the Boon laboratory has received funding support from Abbvie inc. for the commercial development of SARS-cov-2 mAb and Moderna for unrelated work. All other authors declare that they have no competing interests.
Related Posts
Antidepressant fluvoxamine may prevent serious illness in covid-19 patients, “silent” covid-19 infection may be far more common than thought – high rate of false negative test results, evidence shows cloth masks may help against covid-19 – especially those with several layers of cotton cloth, scientists test best fabric choices for making a homemade covid mask, vitamin d determines severity in covid-19: researchers urge government to change advice, how covid-19 kills: new study explains the mechanisms of the new coronavirus, vitamin d linked to low coronavirus death rate, how effective are cloth masks against coronavirus [video], key insights on how coronavirus spreads from chinese megacity of shenzhen.
Perhaps conventional vaccines did not stop the spread of Covid-19 because of the refusal of enough people to wear masks and accept vaccination, because vaccinations were restricted by governments to certain cohorts because of the expense of the vaccines and because governments failed to control quarantine at ports of entry. Apart from being too slow on the uptake in regard to how pandemics develop rapidly.
from the opening paragraph of this article:
“But for all the good they did in reducing illnesses and deaths, the shots were unable to end the pandemic because of one notable weakness: They couldn’t stop the spread of the virus.”
no, vaccines, alone, could not stop the spread of the virus because vaccines are not developed to, alone, stop the spread of the virus by the same method outlined in the 2nd paragraph of this article stating, “… next-generation vaccines that target the virus’s points of entry”. next-generation… not currently available vaccines.
CDC defines vaccines, vaccination and inoculation as:
* Vaccine: A preparation that is used to stimulate the body’s immune response against diseases.
* Vaccination: The act of introducing a vaccine into the body to produce protection from a specific disease.
* Immunization: A process by which a person becomes protected against a disease through vaccination.
in addition to vaccination / immunization, these, and other, preventative measures were recommended: * the use of PPE (personal protective equipment) * physical distancing * hand hygiene * routine cleaning& disinfection * appropriate air handling & ventilation * universal masking * stay-at-home orders
all measures, including vaccines, are focused, both now and in the past, on prevention and many of these measures, including being vaccinated, were / are dependent on human compliance, and there was, and continues to be, human non-compliance.
links to support the information in this reply are available upon request.
Save my name, email, and website in this browser for the next time I comment.
Type above and press Enter to search. Press Esc to cancel.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Assessment of Risk for Sudden Cardiac Death Among Adolescents and Young Adults After Receipt of COVID-19 Vaccine — Oregon, June 2021–December 2022
Weekly / April 11, 2024 / 73(14);317–320
Juventila Liko, MD 1 ; Paul R. Cieslak, MD 1 ( View author affiliations )
What is already known about this topic?
In April 2021, cases of myocarditis after COVID-19 vaccination, particularly among young male vaccine recipients, were reported to the Vaccine Adverse Event Reporting System.
What is added by this report?
To determine risk for sudden cardiac death among adolescents and young adults after COVID-19 vaccination, investigators examined June 2021–December 2022 Oregon death certificate data for decedents aged 16–30 years. Of 40 deaths that occurred among persons who had received an mRNA COVID-19 vaccine dose, three occurred ≤100 days after vaccination. Among these, two occurred in persons with underlying illness, and one decedent had an undetermined cause of death.
What are the implications for public health practice?
The data do not support an association of COVID-19 vaccination with sudden cardiac death among previously healthy young persons. COVID-19 vaccination is recommended for all persons aged ≥6 months to prevent COVID-19 and complications, including death.
- Article PDF
- Full Issue PDF
COVID-19 vaccination has been associated with myocarditis in adolescents and young adults, and concerns have been raised about possible vaccine-related cardiac fatalities in this age group. In April 2021, cases of myocarditis after COVID-19 vaccination, particularly among young male vaccine recipients, were reported to the Vaccine Adverse Event Reporting System. To assess this possibility, investigators searched death certificates for Oregon residents aged 16–30 years who died during June 2021–December 2022 for cardiac or undetermined causes of death. For identified decedents, records in Oregon’s immunization information system were reviewed for documentation of mRNA COVID-19 vaccination received ≤100 days before death. Among 1,292 identified deaths, COVID-19 was cited as the cause for 30. For 101 others, a cardiac cause of death could not be excluded; among these decedents, immunization information system records were available for 88, three of whom had received an mRNA COVID-19 vaccination within 100 days of death. Of 40 deaths that occurred among persons who had received an mRNA COVID-19 vaccine dose, three occurred ≤100 days after vaccination. Two of these deaths were attributed to chronic underlying conditions; the cause was undetermined for one. No death certificate attributed death to vaccination. These data do not support an association between receipt of mRNA COVID-19 vaccine and sudden cardiac death among previously healthy young persons. COVID-19 vaccination is recommended for all persons aged ≥6 months to prevent COVID-19 and complications, including death.
Introduction
In December 2020, the Food and Drug Administration authorized two COVID-19 mRNA vaccines for use in the United States. Early vaccine supplies were prioritized for health care personnel and long-term care facility residents, with phased vaccination of other persons, beginning with those who were older or had high-risk medical conditions, and concluding with healthy younger persons ( 1 ). In Oregon, healthy persons aged ≥16 years became eligible for COVID-19 vaccination on April 19, 2021. In April 2021, reports of myocarditis after COVID-19 vaccination, particularly among young male vaccine recipients, began to appear.* , † Investigators in Israel estimated that the risk for myocarditis associated with receipt of mRNA COVID-19 vaccine was 2.13 per 100,000 among vaccine recipients, and was highest among adolescents and young adult males (10.69 per 100,000) ( 2 ). Published accounts suggest that postvaccination myocarditis is typically mild and associated with good outcomes after brief hospitalization ( 3 , 4 ). As of July 17, 2023, no fatal cases of myocarditis in Oregon had been reported to the federal Vaccine Adverse Event Reporting System (VAERS); however, because VAERS is a passive reporting system, adverse events after vaccination are likely underestimated. In late 2022, reports of sudden deaths among previously healthy young athletes, with suggested attribution to COVID-19 vaccination, appeared in the lay press § and then in the medical literature ( 5 , 6 ). To ascertain whether young persons in Oregon might be dying from cardiac causes shortly after having received a COVID-19 vaccine dose, Oregon death certificate data were reviewed.
Data Sources
Oregon law requires that a certificate of death be completed for each death in Oregon. Oregon’s vital records system abides by CDC’s National Center for Health Statistics’ data-quality standards ¶ , including extensive quality-assurance review. An independent source of data for assessing the completeness of death certificate reporting is not available. Data on Oregon resident deaths occurring outside the state are also collected through interstate exchange agreements. The ALERT Immunization Information System (IIS) is Oregon’s statewide and lifespan immunization registry. During the COVID-19 pandemic, reporting of all COVID-19 vaccinations to ALERT IIS was mandated in Oregon.
Data Analysis
To ascertain the occurrence of sudden cardiac deaths among adolescents or young adults that might plausibly be attributed to recent COVID-19 vaccination, investigators searched the Oregon death certificate database to identify persons aged 16–30 years who died during June 1, 2021–December 31, 2022 with “sudden death,” “arrhythmia,” “dysrhythmia,” “asystole,” “cardiac arrest,” “myocarditis,” “congestive heart failure,” “unknown,” “undetermined,” or “pending” cited among the immediate or four possible entries for underlying causes of death and other significant conditions contributing to death. Among the subset of decedents for whom death from a cardiac cause could not be ruled out by accompanying information in the death certificate database, records of mRNA COVID-19 vaccination within 100 days ( 7 ) before the date of death were retrieved from ALERT(IIS. Findings were stratified by sex. This activity was reviewed by the Oregon Health Authority, deemed not research, and was conducted consistent with applicable federal law and Oregon Health Authority policy.**
In Oregon, during June 2021–December 2022, a total of 1,292 deaths among persons aged 16–30 years were identified. These decedents included 925 (72%) males and 367 (28%) females ( Figure ).
Male Decedents
Among the 925 male decedents, no death certificate listed vaccination either as the immediate or as a contributing cause of death. Overall, 17 (2%) deaths among males were attributed to COVID-19. Death certificates cited noncardiac causes of death or other conditions contributing to death for 842 (91%) of the male decedents. Among the remaining 66 (7%) male decedents, excluding a cardiac cause of death based on the death certificate was not possible. Among these 66 decedents, IIS vaccination records were available for 58 (88%); receipt of at least one mRNA COVID-19 vaccination was recorded for 24 (41%).
Among the 24 male decedents with an mRNA COVID-19 vaccination record in IIS, two (8%) died within 100 days of having received the vaccine. The first death was recorded as having occurred in a natural manner 21 days after COVID-19 vaccination. The immediate cause of death noted on the death certificate was congestive heart failure attributed to hypertension; other significant conditions included morbid obesity, type 2 diabetes, and obstructive sleep apnea. The second decedent had received a COVID-19 vaccine dose 45 days before the date of death; the cause of death was recorded as “undetermined natural cause.” Toxicology results were negative for alcohol, cannabinoids, methamphetamine, and opiates; aripiprazole, ritalinic acid, and trazodone were detected. Follow-up with the medical examiner could neither confirm nor exclude a vaccine-associated adverse event as a cause of death for this decedent.
Female Decedents
Among the 367 female decedents, no death certificate listed vaccination as either the immediate or a contributing cause of death. Thirteen (4%) deaths were attributed to COVID-19. Noncardiac causes were recorded on the death certificates for 319 (87%) decedents. Among the remaining 35 (10%) female decedents, IIS records for 30 (86%) were identified, 16 (53%) of whom had documentation of receipt of at least 1 mRNA COVID-19 vaccine dose. Only one of these deaths occurred within 100 days of having received an mRNA COVID-19 vaccine dose; the decedent died 4 days after COVID-19 vaccination. The manner of death was recorded as natural, and the immediate cause was listed as undetermined but as a consequence of chronic respiratory failure with hypoxia attributed to mitral stenosis.
Electronic health record data from 40 U.S. health care systems during January 2021–January 2022, showed that the risk for cardiac complications was significantly higher after COVID-19 infection than after mRNA COVID-19 vaccination among persons aged ≥5 years ( 8 ). Data from CDC’s National Center for Health Statistics show a background mortality rate from diseases of the heart among Oregonians aged 15–34 years of 2.9 and 4.1 deaths per 100,000, during 2019 and 2021, respectively. Although the rate was higher during the pandemic year of 2021, myocarditis remained an infrequent cause of death among persons in this age group. †† Detection of a small difference in mortality rate from myocarditis would require a larger sample size.
In this study of 1,292 deaths among Oregon residents aged 16–30 years during June 2021–December 2022, none could definitively be attributed to cardiac causes within 100 days of receipt of an mRNA COVID-19 vaccine dose; one male died from undetermined causes 45 days after receipt of a COVID-19 vaccine. During May 1, 2021–December 31, 2022, a total of 979,289 doses of COVID-19 vaccines were administered to Oregonians aged 16–30 years (unpublished data, ALERT IIS, 2024.)
During the same period, COVID-19 was cited as the cause of death for 30 Oregon residents in this age group. Among these 30 decedents, ALERT IIS had records for 22 (73%), only three of whom had received any COVID-19 vaccination. Studies have shown significant reductions in COVID-19–related mortality among vaccinated persons; during the first 2 years of COVID-19 vaccine availability in the United States, vaccination prevented an estimated 18.5 million hospitalizations and 3.2 million deaths ( 9 ).
Limitations
The findings in this report are subject to at least two limitations. First, this report cannot exclude the possibility of vaccine-associated cardiac deaths >100 days after COVID-19 vaccine administration. However, published data indicate that potential adverse events associated with vaccinations tend to occur within 42 days of vaccine receipt ( 10 ). Second, small population size made it less likely that Oregon would see a rare event such as sudden cardiac death among adolescents and young adults.

Implications for Public Health Practice
These data do not support an association between receipt of mRNA COVID-19 vaccine and sudden cardiac death among previously healthy young persons. COVID-19 vaccination is recommended for all persons aged ≥6 months to prevent COVID-19 and complications, including death.
Acknowledgments
Michael Day, Tasha Martin, Anne Vancuren, Center for Health Statistics, Oregon Public Health Division; Rebecca Millius, Office of the Chief Medical Examiner, Medical Examiner Division, Oregon State Police.
Corresponding author: Juventila Liko, [email protected] .
1 Public Health Division, Oregon Health Authority, Portland, Oregon
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
* www.cdc.gov/vaccines/acip/work-groups-vast/report-2021-05-17.html
† https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/04-COVID-Lee-508.pdf
§ https://www.nytimes.com/2022/01/28/technology/covid-vaccines-misinformation.html
¶ https://www.oregon.gov/oha/PH/BirthDeathCertificates/VitalStatistics/death/Pages/index.aspx
** 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
†† https://wonder.cdc.gov/ucd-icd10-expanded.html (Accessed February 12, 2024).
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1657–60. https://doi.org/10.15585/mmwr.mm695152e2 PMID:33382671
- Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med 2021;385:2132–9. https://doi.org/10.1056/NEJMoa2110737 PMID:34614329
- Power JR, Keyt LK, Adler ED. Myocarditis following COVID-19 vaccination: incidence, mechanisms, and clinical considerations. Expert Rev Cardiovasc Ther 2022;20:241–51. https://doi.org/10.1080/14779072.2022.2066522 PMID:35414326
- Behers BJ, Patrick GA, Jones JM, et al. Myocarditis following COVID-19 vaccination: a systematic review of case reports. Yale J Biol Med 2022;95:237–47. PMID:35782472
- Polykretis P, McCullough PA. Rational harm-benefit assessments by age group are required for continued COVID-19 vaccination. Scand J Immunol 2022;98:e13242. https://doi.org/10.1111/sji.13242 PMID:38441161
- Sun CLF, Jaffe E, Levi R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Sci Rep 2022;12:6978. https://doi.org/10.1038/s41598-022-10928-z PMID:35484304
- Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2022;40:1499–511. https://doi.org/10.1016/j.vaccine.2021.11.074 PMID:35105494
- Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:517–23. https://doi.org/10.15585/mmwr.mm7114e1 PMID:35389977
- Fitzpatrick M, Moghadas S, Pandey A, Galvani A. Two years of U.S. COVID-19 vaccines have prevented millions of hospitalizations and deaths. New York, NY: The Commonwealth Fund; 2022. https://www.commonwealthfund.org/blog/2022/two-years-covid-vaccines-prevented-millions-deaths-hospitalizations https://doi.org/10.26099/whsf-fp90
- CDC. Update: vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1996;45(No. RR-12):1–35. PMID:8801442
FIGURE . Deaths* among persons aged 16–30 years, by sex, cause of death, † and mRNA COVID-19 vaccination status §,¶, ** (N = 1,292) — Oregon, June 2021–December 2022
* Coded on the death certificate as sudden death, arrhythmia, dysrhythmia, asystole, cardiac arrest, myocarditis, congestive heart failure, unknown, undetermined, or pending.
† Cardiac versus noncardiac.
§ Six of the 34 males who did not receive mRNA COVID-19 vaccine received Janssen (Johnson & Johnson) vaccine.
¶ An alternative plausible cause of death was identified for one of the males who had been vaccinated ≤100 days before death. After review of death certificate and medical examiner findings, an adverse event from COVID‐19 vaccination could neither be confirmed nor excluded as the cause for the other decedent.
** The only female decedent vaccinated ≤100 days before death was vaccinated 4 days before death. The manner of death was recorded as natural, and the immediate cause was “undetermined” as a consequence of chronic respiratory failure with hypoxia due to mitral stenosis.
Suggested citation for this article: Liko J, Cieslak PR. Assessment of Risk for Sudden Cardiac Death Among Adolescents and Young Adults After Receipt of COVID-19 Vaccine — Oregon, June 2021–December 2022. MMWR Morb Mortal Wkly Rep 2024;73:317–320. DOI: http://dx.doi.org/10.15585/mmwr.mm7314a5 .
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version ( https://www.cdc.gov/mmwr ) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- All Summits
- STATUS List
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
Better safety studies could restore America’s confidence in vaccines
By Gregory A. Poland Aug. 14, 2024

I n February 2021, I received my second Covid-19 shot — the newly developed vaccine that would eventually save millions of lives worldwide — with great anticipation. It proved to be a life-changing event: Two hours later as I was driving home, the shock of a sudden loud and high-pitched whistling nearly caused me to veer off the road. It was as if an audible dog whistle began blaring right next to me. But it wasn’t a dog whistle. It was the acute onset of tinnitus , a ringing in the ear with no external source.
For several years I had lived with minimal, intermittent tinnitus, but never anything like this, so loud and unrelenting.
advertisement
Could the Covid-19 vaccine have amplified my tinnitus, or was this just a coincidence? I was suspicious, but at that time no data had demonstrated any relationship between the mRNA Covid-19 vaccines and tinnitus.
When I got my third dose in November 2021, the sound became even louder and more bothersome. This new noise level has continued to this day, nonstop, often keeping me from sleep and sometimes bringing me to tears.
Related: Covid-19 vaccine safety and the public trust: lessons from Paul Meier and polio
As someone who has studied vaccines for 40 years, I know that the mRNA Covid-19 vaccines, and other vaccines, have saved millions upon millions of lives. For the vast majority of people, the benefits of getting vaccinated far exceed the risks.
But some people, like me, have unexpected effects from vaccines that affect our health and well-being. I say “some” because no one really knows how often vaccine-related injuries occur. Understanding that would be a first step toward reducing these rare risks even further. Are such adverse events predictable, and can they be prevented by identifying risk factors for the onset of a vaccine injury? I believe the answer is yes, but it won’t be achievable without increased funding for vaccine safety research.
Americans’ trust in vaccines has been slowly eroding. It accelerated with the advent of the World Wide Web in the 1990s and has been declining ever since. The Covid-19 outbreak temporarily obscured the problem, as many expressed the desire for a vaccine against this plague and millions got it once it became available.
Sign up for Morning Rounds
Understand how science, health policy, and medicine shape the world every day
Covid-19 also obscured the problem another way: lockdowns and sheltering in place reduced people’s normal activities and movements, and with it the spread of all viruses, not just SARS-CoV-2. But as people once again gathered and shopped and worshipped together, as well as traveled from country to country, measles — one of the most infectious viruses — has returned. In the first three months of 2024, the United States has had 17 times more measles cases than during the same time period for the past three years. The United States is now on the verge of losing its status as a nation in which measles has been eliminated.
Increasing vaccine hesitancy and rejection has contributed to ongoing outbreaks of measles in the U.S. Last year, the number of parents in the U.S. seeking exemptions to vaccinate their children increased in 41 states , and similar trends are being seen with the flu, pneumonia, and HPV vaccines.
Although the Covid-19 vaccines were a huge public health success, politicization and opposition to them became weaponized through disinformation for political gain. Whether or not to receive a Covid-19 vaccine became an expression of opinion, rather than an informed health decision, and this has had a trickle-down effect to other vaccines.
A renewed and well-funded focus on the study of vaccine safety is among the critical measures needed to stop the return of vaccine-preventable infectious diseases like measles and to reverse the loss of public confidence in vaccines. While the system to evaluate vaccine safety is extensive, it must be strengthened. Researchers and public health experts need to know which events are caused by vaccines, who is at increased risk for them, and why. With that information in hand they can move forward to reduce such risk.
Related: Empathy should be the first response to people with vaccine injury, fears
To be sure, systems are in place for vaccine safety monitoring. The national Vaccine Adverse Event Reporting System (VAERS) collects reports of possible side effects after vaccinations. It’s an important tool for spotting potential safety issues, but it has limitations. VAERS mainly captures problems that are obvious, lead to doctor visits, or show up on medical tests. This means it might not catch all types of side effects, especially those that are less visible or don’t prompt immediate medical attention.
Large and ongoing studies using real-world data are also needed to fully understand vaccine safety. Once vaccine-related injuries have been identified, federal entities overseeing vaccine safety must take them seriously and investigate thoroughly, publishing the method and data used.
Myocarditis following mRNA Covid-19 vaccination is an example of a rare vaccine adverse reaction that can be detected only after the vaccine has been administered to millions of people — it would almost never show up in clinical trials testing the vaccine. Once a rare adverse reaction is discovered, understanding its biological mechanism is necessary to prevent it. This hasn’t yet been done for vaccine-induced myocarditis, which will require a significant scientific investment.
Compensating people who have been injured by vaccines is another dimension of the issue. The National Vaccine Injury Compensation Program has been in place since the 1980s. Through it, individuals who file a petition and are found to have been injured by a covered vaccine can receive financial compensation.
Vaccine injury reporting and compensation have worked adequately for routine vaccines. For vaccines provided under emergency use authorization, however, such as vaccines against H1N1 in 2009 and now Covid-19, safety science and compensation programs have been chronically underfunded. While the Centers for Disease Control and Prevention has an annual vaccine budget of up to $5 billion to purchase and promote vaccines, the study of vaccine safety at the CDC has been limited to $20 million per year since the time when Dr. Louis Cooper, former president of the American Academy of Pediatrics, warned of the vaccine confidence crisis more than 20 years ago. Since then, many new vaccines have been developed, including some intended for children, pregnant people, and older Americans, who can require different preparations and protocols.
Related: How will people act after getting vaccinated? The complex psychology of safety
While federal budgets are under intense scrutiny, there is a budget-neutral solution for vaccine safety science. The law that created the National Vaccine Injury Compensation Program provided liability protection to pharmaceutical companies and no-fault compensation to people who are injured by a vaccine. The law also focused on vaccine safety monitoring and the prevention of adverse reactions. Funding was created from a separate tax law by adding a 75-cent excise tax to childhood vaccines for any disease the vaccine was intended to prevent. This law, however, allowed the excise tax money to be used only for compensation. Vaccine safety science and prevention of vaccine injuries, as intended by the initial law, were not allowed.
As recently published in The New England Journal of Medicine , the compensation program currently has an excess of more than $4 billion. Congress should amend this tax law to allow these revenues — a budget-neutral approach — to fund vaccine safety science and prevent rare vaccine injuries.
Americans’ confidence in vaccines will improve only if the vaccine safety system is trusted, transparent, and credible. The independent National Academies of Sciences, Engineering, and Medicine should be asked to review the current vaccine safety system and develop an optimal structure and governance for an adequately funded vaccine safety and compensation system.
Because of the difficulty of eradicating infectious diseases, vaccines will always be needed. The United States needs a robust, sustainable, and well-funded vaccine safety system to help overcome the crisis in vaccine confidence. For too many Americans, confidence in vaccines is truly a matter of life and death.
Gregory A. Poland, M.D., is a virologist who studies the immunogenetics of vaccine responses in adults and children, the editor of the academic journal Vaccine , and president of the Atria Academy of Science and Medicine . He reports being chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories, has been a consultant for various vaccine and pharmaceutical companies, and is an adviser to the White House on Covid-19 vaccines and the World Health Organization on monkeypox.
LETTER TO THE EDITOR
Have an opinion on this essay submit a letter to the editor here ., about the author reprints, gregory a. poland.
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

Ultra-processed foods: the tobacco of the 21st century?

Aspirin after a broken bone: health equity in a $5 bottle

STAT Plus: Health Care's Colossus: How UnitedHealth turned a questionable artery-screening program into a gold mine

STAT Plus: Health Care's Colossus: How UnitedHealth harnesses its physician empire to squeeze profits out of patients

STAT Plus: The race to build better CRISPR delivery vehicles is heating up
Study: COVID vaccines saved 1.6 million lives in Europe
Simen Stromme / iStock
New estimates published in The Lancet Respiratory Medicine show that at least 1.6 million European lives have been directly saved by COVID-19 vaccinations, with 60% of those lives saved when Omicron became the dominant strain of the virus—and those numbers may be an undercount.
As of March 2023, 2.2 million COVID-19–related deaths have been reported across Europe.
The data come from The World Health Organization European Respiratory Surveillance Network, which analyzed vaccination efforts from December 2020 to March 2023, a period that encompasses both initial vaccine rollouts in the European region and booster doses. A total of 34 of 54 countries in the region were included in the study.
In the 34 countries, total vaccine overage in all adults aged 25 years or older was 87% for the primary vaccine series, 82% for the second dose, 71% for the first booster, 24% for the second booster, and 5% for the third booster by March of 2023.
The study looked at deaths divided by age-groups (25 to 49 years, 50 to 59, 60 and older, 60 to 69, 70 to 79, and 80 and older). Countries that reported weekly data for both COVID-19 vaccination and mortality by age-group for 90% or more of study weeks or more were included.
First booster dose saved most lives
Each week was also associated with a variant of concern (VOC), which accounted for 50% or more sequences per week.
In 29 countries there were 1,064,165 COVID-19–related deaths in people aged 25 years or older; of these deaths, 454,131 (43%) were in people aged 80 years or older, the authors said. By contrast, 40,788 (4%) and 19,831 (2%) of COVID-19–related deaths were in people aged 50 to 59 years and 25 to 49 years, respectively.
Most reported deaths were during the Omicron period.
"When considering each VOC mortality per variant month (PVM), regardless of age, most reported deaths were during the Omicron period (390,358 deaths); however, the Delta period had the highest number of reported deaths PVM (33,234 deaths)," the authors said.
Overall, those aged 60 years or older accounted for 96% of the total lives saved; whereas, people aged 80 years or older represented 52% of the total lives saved.
In temporal analysis, the first booster saved the most lives (51% of all lives saved) aligning with 60% of lives saved during the Omicron period. The administration of the first booster doses started around week 30 of 2021 in Europe.
In an editorial on the study, Oliver Watson, PhD, and Alexandra Hogan, PhD, of Imperial College London, write that the number of lives saved as reported by the authors is probably an underestimation.
"This study does not consider the additional herd effects of COVID-19 vaccination, whereby the population-level reduction in transmission indirectly reduces exposures, and therefore deaths, in the unvaccinated population," they write.
"Second, the estimate of averted deaths is based on the reported COVID-19 mortality by each country, which is known to underestimate the true burden of COVID-19."
Related news
Routine lab tests can't reliably distinguish long covid from other illnesses, nih study suggests.

New studies estimate long-COVID rates, identify risk factors

COVID drops to 10th leading cause of death in US

MIS-C tied to rare autoimmune overreaction in some children

US COVID markers continue steady rise

Study spotlights psychiatric, cognitive problems years after severe COVID-19

Risk of heart attack, stroke drops after COVID vaccination, data show

Americans' trust in doctors, hospitals plunged during pandemic, survey suggests

This week's top reads
In the latest variant update, the CDC said the proportion of KP.3.1.1 jumped from 14.4% to 27.8% over the last 2 weeks.

US COVID activity continues to pick up
Wastewater detections have now jumped to the "very high" level.
CDC updates mpox alert amid expansion in African outbreaks
Though the risk remains very low, the CDC and state partners continue to look for the clade, including in wastewater samples.
About 34% of infected postmenopausal women had symptoms for 8 weeks or more, while 61% of survivors in a second study had symptoms at 2 years.

WHO considers public health emergency as mpox cases mount in Africa
Over the weekend South Africa said it now has 22 cases of the virus.
USDA confirms more H5N1 in dairy cows, wild birds, and small mammals
The latest confirmations also include more wild birds and small mammals from Weld County, a Colorado hit hard by dairy cow and poultry outbreaks.
Clinicians detail H5N1 infections in 2 Michigan farm workers
One patient had conjunctivitis in one eye, and the other had longer-lasting flulike symptoms.

Total vaccine coverage in all adults aged 25 years or older was 87% for the primary vaccine series.
Deaths from COVID-19 dropped by 69% in 1 year.

Colorado's bulk-tank testing IDs more avian flu in dairy herds
In related developments, Colorado's governor recently extended an emergency declaration that frees up more resources to battle the outbreaks.

Our underwriters
Unrestricted financial support provided by.

- Antimicrobial Resistance
- Chronic Wasting Disease
- All Topics A-Z
- Resilient Drug Supply
- Influenza Vaccines Roadmap
- CIDRAP Leadership Forum
- Roadmap Development
- Coronavirus Vaccines Roadmap
- Antimicrobial Stewardship
- Osterholm Update
- Newsletters
- About CIDRAP
- CIDRAP in the News
- Our Director
- Osterholm in the Press
- Shop Merchandise
Along with Stanford news and stories, show me:
- Student information
- Faculty/Staff information
We want to provide announcements, events, leadership messages and resources that are relevant to you. Your selection is stored in a browser cookie which you can remove at any time using “Clear all personalization” below.
Researchers at Stanford Engineering have developed a nanoparticle platform that could make existing vaccines more effective, including those for influenza, COVID-19, and HIV. In addition to helping vaccine candidates produce stronger, longer-lasting immune responses, the platform will allow researchers to elicit and test different types of immune responses to determine what is most effective for protecting against specific pathogens.
“These nanoparticles elicit stronger, more robust immune responses, and the breadth of our platform allows us to readily tune the type of immune response in a way that just was not feasible with previous technologies,” said Eric Appel , an associate professor of materials science and engineering and senior author on the paper published Aug. 7 in Science Advances . “This can be a tool to understand how different types of immune responses give rise to better or worse protection – it was impossible to even ask that question before.”
A better adjuvant
Most modern vaccines teach our immune systems to recognize and fight off infections by introducing only a piece of a pathogen – such as the coronavirus’s now-infamous spike protein – instead of the whole virus. On their own, these fragments may not cause much of a reaction, so vaccines also contain adjuvants – additives that help stimulate and shape the body’s immune response. But there are currently only a handful of adjuvants available for clinical use and their effectiveness can vary widely.
“We wanted to create as potent of an adjuvant as possible,” said Ben Ou, a doctoral student in Appel’s lab and first author on the paper. “We combined two different adjuvant technologies to create a nanoparticle platform that will activate different immune pathways and improve vaccine responses.”
The researchers determined that they could attach molecules called toll-like receptor agonists, or TLR agonists, which interact with receptors on our innate immune cells, to a base nanoparticle made of saponin molecules, which have been used as effective adjuvants for decades, including in the Novavax COVID-19 vaccine. The result was an adjuvant that acted through multiple immune pathways, producing a broad, strong, long-lasting response.
Ou, Appel, and their colleagues tested their adjuvants, collectively called TLRa-SNP adjuvants, with both COVID-19 and HIV vaccine candidates. In both cases, the adjuvants greatly improved the effectiveness of the vaccines. In comparison to versions paired with an existing adjuvant, the vaccines were more potent and lasted longer. They also created immune responses that could detect and neutralize multiple versions of the pathogens – with the TLRa-SNP adjuvants, the COVID-19 vaccine candidate was effective against the original virus as well as Delta, Omicron, and other variants.
Finding the right immune response
There are multiple types of TLR agonists, each of which binds to a different immune receptor. The researchers created five different versions of their adjuvants using the saponin nanoparticle as a base platform and swapping the attached TLR agonists. While all the adjuvants were effective, each version created a slightly different type of immune response, activating different signaling proteins and prompting different actions from immune cells.
“All of our adjuvants improve overall vaccine responses, but the specific types of improvements are different,” Ou said. “If we know that a specific type of immune activation will confer better protection, we now have a platform that will allow you to pick the specific formulation that will drive that distinct response.”
With existing adjuvants, researchers can test which one creates the strongest immune response when paired with a particular vaccine, but the adjuvants are too different to allow investigations into which type of immune response would be most effective at protecting against infection for a given pathogen. The interchangeability of the TLR agonists in the TLRa-SNP adjuvants would allow researchers to tweak the nature of the immune response while maintaining the strong immune activation created by the saponin nanoparticle base.
There are other TLR agonists that could be paired with this platform, beyond the five that they tested in this paper, Ou said. He is interested in investigating others, as well as investigating the effects of using more than one type of TLR agonist at a time – the researchers have already shown that this is possible and hope to make additional bespoke nanoparticle adjuvants in the future, with the goal of developing the most effective adjuvants possible.
“This platform approach will open up opportunities for people in the field to ask more probing questions about what immunology works better in different contexts,” Appel said. “And it’s also making significantly better adjuvants.”
Related story
Drug delivery system could reduce daily diabetes shots to just three a year
Dietary management drugs have transformed Type 2 diabetes care, but daily injection routines are challenging for some patients. A new hydrogel could mean shots just three times a year.
For more information
Appel is a senior fellow of the Stanford Woods Institute for the Environment ; a member of Stanford Bio-X , the Stanford Cardiovascular Institute , the Wu Tsai Human Performance Alliance , the Maternal & Child Health Research Institute , the Stanford Cancer Institute , and the Wu Tsai Neurosciences Institute ; and a faculty fellow of Stanford Sarafan ChEM-H .
Additional Stanford co-authors of this research include Bali Pulendran , the Violetta L. Horton Professor in the School of Medicine; postdoctoral researcher Julie Baillet; and graduate students Maria V. Filsinger Interrante, Julia Z. Adamska, Xueting Zou, Olivia M. Saouaf, Jerry Yan, John H. Klich, Carolyn K. Jons, and Emily L. Meany.
Other co-authors on this work are from the University of Washington.
This work was funded by the Bill & Melinda Gates Foundation, the National Institute of Allergy and Infectious Disease, the Eastman Kodak Fellowship, the National Institutes of Health, the National Science Foundation, the Stanford Graduate Fellowship in Science and Engineering, and Sarafan ChEM-H.
Media contact: Jill Wu, School of Engineering: [email protected]
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
COVID-19 & Science
Americans’ largely positive views of childhood vaccines hold steady.
About nine-in-ten (88%) Americans say, overall, the benefits of childhood vaccines for measles, mumps and rubella outweigh the risks, identical to the share who said this before the coronavirus outbreak. U.S. adults are less confident in COVID-19 vaccines: Fewer than half rate them as having high health benefits and a low risk of side effects.
Lack of Preparedness Among Top Reactions Americans Have to Public Health Officials’ COVID-19 Response
Overall, 46% of Americans say the statement “public health officials were unprepared for the outbreak” describes their views extremely or very well, including similar shares of Republicans and Democrats.
Partisan differences are common in the lessons Americans take away from COVID-19
Here’s what Americans said they learned about the development of vaccines and medical treatments and their advice for handling a future outbreak.
Sign up for our weekly newsletter
Fresh data delivery Saturday mornings
How Americans View the Coronavirus, COVID-19 Vaccines Amid Declining Levels of Concern
Just 20% of the public views the coronavirus as a major threat to the health of the U.S. population and only 10% are very concerned about getting a serious case themselves. In addition, a relatively small share of U.S. adults (28%) say they’ve received an updated COVID-19 vaccine since last fall.
Americans’ Trust in Scientists, Positive Views of Science Continue to Decline
The share of Americans who say science has had a mostly positive impact on society has fallen 16 percentage points since before the start of the coronavirus outbreak, from 73% in January 2019 to 57% today.
About half of recent online daters in U.S. say it’s important to see COVID-19 vaccination status on profiles
Online dating users who are Democrats are far more likely their Republican counterparts to say someone’s vaccination status is important for them to see.
Many Americans say they have shifted their priorities around health and social activities during COVID-19
We asked respondents to describe in their own words what rose and fell in importance to them during the pandemic. Here are some of the key themes that emerged.
Americans Reflect on Nation’s COVID-19 Response
Americans offer a lackluster evaluation of how the country has balanced priorities during the coronavirus outbreak. Fewer than half say the country has given the right amount of priority to the needs of K-12 students, public health or quality of life.
57% of Americans say masks should be required on airplanes and public transportation
As has often been the case on policy questions about how to deal with the pandemic, partisans are far apart in their views on mask mandates.
Black Americans’ Views of and Engagement With Science
Black Americans hold multifaceted views when it comes to trust in medical research scientists: Majorities hold largely positive views of their competence, but express concern about the potential for misconduct.
REFINE YOUR SELECTION
Research teams.
901 E St. NW, Suite 300 Washington, DC 20004 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
© 2024 Pew Research Center
ORIGINAL RESEARCH article
Investigating the influencing factors of vaccination decisions for newly-developed and established vaccines: a comparative study based on latent class logit models in china highlights.

- Nanjing University, Nanjing, China
The final, formatted version of the article will be published soon.
Select one of your emails
You have multiple emails registered with Frontiers:
Notify me on publication
Please enter your email address:
If you already have an account, please login
You don't have a Frontiers account ? You can register here
Abstract Background: The factors influencing vaccination decision-making for newly-developed vaccines may be both similar to and different from those for established vaccines. Understanding these underlying differences and similarities is crucial for designing targeted measures to promote new vaccines against potential novel viruses. Objective: This study aims to compare public vaccination decisions for newly-developed and established vaccines and to identify the differences and similarities in the influencing factors. Method: A discrete choice experiment (DCE) was conducted on 1509 representatives of the general population in China to collect data on preferences for the COVID-19 and influenza vaccines, representing the newly-developed and established vaccines, respectively. The latent class logit model was employed to identify latent classes within the sample, allowing for an analysis of the factors distinctly influencing choices for both types of vaccines. Result: Participants valued similar attributes for both vaccines. However, concerns about sequelae were more significant for the newly-developed vaccine, while effectiveness was prioritized for the established vaccine. Class membership analysis revealed these differences and similarities were significantly correlated with age, health, yearly household income, acquaintances' vaccination status and risk perception. Conclusion: The study highlights the need for tailored communication strategies and targeted vaccination interventions. For the new-developed vaccines, addressing concerns about side effects is more crucial. For the long-standing vaccines, emphasizing its effectiveness can enhance uptake more significantly. Engaging healthcare providers and community influencers is essential for both vaccines to increase public confidence and vaccination rates. Clear communication and community engagement are critical strategies for addressing public concerns and misinformation, particularly during periods of heightened concern.
Keywords: COVID-19 vaccines, Influenza Vaccines, Latent class logit model, choice experiment, vaccine preferences
Received: 27 Jun 2024; Accepted: 09 Aug 2024.
Copyright: © 2024 Chang, Biao, Xi and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Shiyun Chang, Nanjing University, Nanjing, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection
- PMC10026222

The top 100 most cited articles on COVID-19 vaccine: a bibliometric analysis
Weigang wang.
1 Department of Epidemiology, School of Public Health, Shanxi Medical University, 56 Xinjian South Road, Taiyuan, 030001 Shanxi China
2 Shanxi Province Cancer Hospital, Taiyuan, China
3 Center of Clinical Epidemiology and Evidence Based Medicine, Shanxi Medical University, Taiyuan, China
4 First Hospital/First Clinical Medical College of Shanxi Medical University, Taiyuan, China
Yongliang Feng
Suping wang, associated data.
All data generated or analyzed during this study are included in this published article.
This study aimed to uncover the current major topics regarding COVID-19 vaccine, and systematically evaluate the development trends for future research. The top 100 most cited original articles on COVID-19 vaccine from January 2020 to October 2022 were identified from Web of Science Core Collection database. CiteSpace (v6.1.R3) was adopted for bibliometric analysis with statistical and visual analysis. The number of citations ranged from 206 to 5881, with a median of 349.5. The USA ( n = 56), England ( n = 33), and China ( n = 16) ranked the top three countries/regions in terms of the number of publications. Harvard Medical School (centrality = 0.71), Boston Children’s Hospital (centrality = 0.67), and Public Health England (centrality = 0.57) were the top three institutions leading the way on COVID-19 vaccine research. The New England of medicine journal dominated with 22 articles in the 32 high-quality journals. The three most frequent keywords were immunization (centrality = 0.25), influenza vaccination (centrality = 0.21), and coronavirus (centrality = 0.18). Cluster analysis of keywords showed that the top four categories were protection efficacy, vaccine hesitancy, spike protein, and second vaccine dose ( Q value = 0.535, S value = 0.879). Cluster analysis of cited references showed that top eight largest categories were Cov-2 variant, clinical trial, large integrated health system, COV-2 rhesus macaque, mRNA vaccine, vaccination intent, phase II study, and Cov-2 omicron variant ( Q value = 0.672, S value = 0.794). The research on COVID-19 vaccine is currently the hottest topic in academic community. At present, COVID-19 vaccines researches have focused on vaccine efficacy, vaccine hesitancy, and the efficacy of current vaccines on omicron variants. However, how to increase vaccine uptake, focus on mutations in the spike protein, evaluate of the efficacy of booster vaccine, and how effective new vaccines under pre- and clinical development against omicron will be spotlight in the future.
Introduction
Coronavirus disease 2019 (COVID-19) is a contagious respiratory disease caused by the SARS-Cov-2 virus, which was first reported in Wuhan, Hubei province, China, in December 2019, and then spread rapidly around the world. On March 11, 2020, World Health Organization (WHO) declared the outbreak of the COVID-19 as a global pandemic [ 1 ]. To date, COVID-19 pandemic has caused incalculable disaster on the global medical, economy, and public health. Unfortunately, the epidemic trends of COVID-19 remain uncertain in the foreseeable future. As of October 7, 2022, exceeding 610 million confirmed cases of COVID-19 and more than 6 million deaths from the disease have been reported worldwide [ 2 ], the situation of epidemic prevention and control is still not optimistic.
Compared with drugs and other treatments, safe and effective vaccines have become the most economical way to prevent and slow down transmission of COVID-19 infection [ 3 ]. Vaccination not only decreases the disease prevalence, but also protect the unimmunized individuals of the community [ 4 ]. Vaccination, in combination with non-pharmaceutical interventions including properly wear mask, teleworking, social distancing, and isolations, was regarded as the best way to control the pandemic [ 5 – 7 ]. With the outbreak of the pandemic, governments and researchers immediately put the invention of COVID-19 vaccine at the top of their agenda. The wonderful news was that as of October 7, 2022, 11 vaccines were listed for emergency use by WHO, and 172 vaccines were in clinical development and 199 were in preclinical development [ 8 ]. Moreover, more than 12 billion vaccine doses have been administered globally as of October 3, 2022 [ 2 ].
Bibliometric analysis is a quantitative analysis method combining mathematics and statistics [ 3 , 9 ]. It can analyze and visualize the authors, institutions, countries, keywords, and other key information of published literatures, which could quantitatively present the research highlights and development trends. Consequently, bibliometric analysis is almost extensively used in the medical research nowadays [ 10 – 12 ], which can promote researchers clarify ideas for scientific studies, and provide a reference for research cooperation [ 13 , 14 ].
Citations are created whenever the article reference another peer-reviewed publication [ 15 ]. Therefore, the number of citations of an article can quantitatively reflect its impact on the scientific community [ 14 ]. Bibliometric analysis of high-quality and value publications can map the burgeoning research hotspots and future research tendency.
There were fewer studies using bibliometric analysis to outline the status and prospect of COVID-19 vaccine research [ 3 , 4 , 16 ]. However, to our knowledge, there is no bibliometric analysis to focus on the top 100 most cited articles on COVID-19 vaccine. This study analyzed the top 100 most cited original articles on COVID-19 vaccine research using bibliometric analysis, with the aim of revealing the current hotspots, then systematically predict the development direction for follow-up work.
Materials and methods
Search strategy.
Web of Science (WOS) represents a comprehensive, multidisciplinary database, which contains more than 12,000 authoritative and high-impact academic journals [ 17 ].What is more, it can provide critical information of all publications, including authors, authors’ affiliated institution, keywords, the number of citations, and publishers, etc. With its powerful functions and citation reports, it can quickly target highly impactful and representative researches, thus track down the research interests concerned to domestic and international authorities, as well as capture the growing trends of the discipline [ 18 ]. Hence, WOS database has been used extensively in bibliometric analysis [ 4 , 19 , 20 ].
The article search was conducted on the Web of Science Core Collection (WOSCC) database. The article search strategies were TI = (corona* OR 2019-nCoV OR nCoV-19 OR SARS-CoV2 OR SARS-CoV-2 OR COVID*) AND TI = (vacc* OR immuniz*). The number of citations was provided by WOSCC database. Two investigators independently evaluate the article by reading the abstract or full text of the article to confirm whether it was related to the COVID-19 vaccine. If there was disagreement on the article, it will be discussed until reach a consensus. The articles search was completed on October 18, 2022. Documents were downloaded in plain text format.
Inclusion and exclusion criteria
Inclusion criteria:
- The document type was original article;
- The articles were published by English;
- The time span was limited to January 2020 to October 2022.
Exclusion criteria:
- The document type was review, letter, editorial material, early access, etc.;
- The articles were published by non-English.
Statistical analysis and visualization
Statistical and visual analysis was performed by CiteSpace 6.1.R3 (64-bit). CiteSpace is a visualization software for bibliometric analysis based on Java platform developed by Professor Chaomei Chen [ 21 ]. The features of visualization map were described by three variables: node size, distance, and color. Each node in the map represents a country, author, institution, or keyword. The size of nodes indicates the frequency of occurrence or citation, and color of nodes denoted different years. The connection lines between nodes were regarded as the co-occurrence or co-citation relationship, the lines’ thickness represented the strength of the relationship [ 22 , 23 ]. The centrality was also named betweenness centrality, which measures the percentage of shortest paths in the network to which a given node belongs [ 24 ]. Nodes with high centrality (> 0.1) were usually deemed turning points or pivotal points in this domain [ 23 ]. The threshold represents the number of occurrences. The number of variable labels displayed in the visualization map can control by adjusting the threshold. In the cluster analysis, the modularity (Q value) and the mean silhouette scores ( S value) were employed to evaluate the overall structural characteristic of the network. Q value > 0.3 indicated an overall significant structure. The clustering was regarded as reasonable when S value > 0.5 and convincing when S value > 0.7 [ 25 ]. We imported the “download_*.txt” file into CiteSpace and selected “Data” to remove duplicated literatures. The CiteSpace parameters were set as follows: time-slicing was chosen from January 2020 to October 2022, the time slice is one year, and all options in the term source were selected, node types were selected one at a time, selection criteria ( g -index, g 2 ≤ kΣ i ≤ g c i , k ∈ Z + , k = 25), and set the others as default values. The impact factor and quartile of the journals were obtained from Journal Citation Reports 2021.The citations of the top 10 most cited articles were analyzed and plotted using GraphPad prism v9.2.0.
Characteristics of the included articles
A total of 12,088 articles related to COVID-19 vaccine were retrieved from WOSCC database, while the top 100 most cited articles were determined by ranking of citations in descending order. The years of publication ranged from 2020 to 2022, with 41 articles in 2020, 57 articles in 2021, and two articles in 2022. The top 100 most cited articles on COVID-19 vaccine have been cumulatively cited 55,255 times. The median number of citations was 349.5, with a range of 206 to 5881. Only two studies were cited more than 3000 times, nine articles were cited more than 1000 times, and nearly a quarter of the articles ( n = 23) were cited between 500 and 1000 times. In all, 167 authors, 48 countries/regions, 32 journals, and 133 institutions were involved. Table Table1 1 and Fig. 1 were exhibited top 10 most cited articles on COVID-19 vaccine.
Top 10 most cited articles on COVID-19 vaccine
| Rank | Frist author | Article title | Journal | Publication years | Citations |
|---|---|---|---|---|---|
| 1 | Polack, FP | Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine | New England Journal of Medicine | 2020 | 5881 |
| 2 | Baden, LR | Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine | New England Journal of Medicine | 2021 | 3864 |
| 3 | Voysey, M | Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK | Lancet | 2021 | 1979 |
| 4 | Jackson, LA | An mRNA Vaccine against SARS-CoV-2-Preliminary Report | New England Journal of Medicine | 2020 | 1578 |
| 5 | Bernal, JL | Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant | New England Journal of Medicine | 2021 | 1322 |
| 6 | Folegatti, PM | Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial | Lancet | 2020 | 1190 |
| 7 | Walsh, EE | Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates | New England Journal of Medicine | 2020 | 1187 |
| 8 | Dagan, N | BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting | New England Journal of Medicine | 2021 | 1153 |
| 9 | Lazarus, JV | A global survey of potential acceptance of a COVID-19 vaccine | Nature Medicine | 2021 | 1081 |
| 10 | Sadoff, J | Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19 | New England Journal of Medicine | 2021 | 990 |

Analysis of countries/regions
Altogether, 48 countries/regions published articles were involved in COVID-19 vaccine in the top 100 most cited articles. As depicted in Fig. 2 , there were 48 nodes and 166 links in the network diagram of these countries/regions mapped by CiteSpace. The USA ( n = 56), England ( n = 33), and China ( n = 16) ranked the top three countries/regions in terms of the number of publications. The top three countries/regions in terms of citations were the USA ( n = 35,262), England ( n = 22,831), and Germany ( n = 13,034). Node centrality analysis demonstrated that the USA had the highest centrality (centrality = 0.63), which was relatively higher than other countries/regions, indicating that the USA play a prominent role in this field, as well as has extensive cooperation with other countries/regions. (Table (Table2 2 ).

Countries/regions of top 100 most cited articles on COVID-19 vaccine
Top 6 countries/regions of top 100 most cited articles on COVID-19 vaccine
| Rank | Country/region | Count | Centrality | Citations |
|---|---|---|---|---|
| 1 | The USA | 56 | 0.63 | 35,623 |
| 2 | England | 33 | 0.19 | 22,831 |
| 3 | Peoples R China | 16 | 0 | 6771 |
| 4 | Germany | 14 | 0 | 13,034 |
| 5 | Scotland | 10 | 0.13 | 4798 |
| 6 | South Africa | 10 | 0 | 11,966 |
Analysis of institutions
A total of 133 institutions published articles on COVID-19 vaccine in the top 100 most cited articles. The network diagram of these institutions demonstrated that there were 133 nodes and 445 links (Fig. 3 ). The top two institutions with the maximum number of published articles were University of Oxford ( n = 14) and Pfizer Pharmaceuticals Ltd ( n = 10). The top three institutions in terms of citations were Pfizer Pharmaceuticals Ltd ( n = 10,357), BioNTech ( n = 9698), and University of Maryland ( n = 9650). However, node centrality analysis manifested that Harvard Medical School had the highest centrality (centrality = 0.71), followed by Boston Children’s Hospital (centrality = 0.67) and Public Health England (centrality = 0.57), proving that these three institutions played greater role than other institutions in COVID-19 vaccine research (Table (Table3). 3 ). These finding demonstrated that institutions from the USA and the UK dominate the field of vaccine research.

Institutions of top 100 most cited articles on COVID-19 vaccine
Top 15 institutions of top 100 most cited articles on COVID-19 vaccine
| Rank | Institutions | Count | Centrality | Citations | Country |
|---|---|---|---|---|---|
| 1 | University of Oxford | 14 | 0.42 | 6318 | The UK |
| 2 | Pfizer Pharmaceuticals Ltd | 10 | 0 | 10,357 | The USA |
| 3 | Harvard Medical School | 9 | 0.71 | 4174 | The USA |
| 4 | London School of Hygiene & Tropical Medicine | 9 | 0.04 | 4616 | The UK |
| 5 | University College London | 9 | 0.03 | 2368 | The UK |
| 6 | Public Health England | 8 | 0.57 | 4471 | The UK |
| 7 | BioNTech | 8 | 0.08 | 9698 | Germany |
| 8 | Boston Children’s Hospital | 6 | 0.67 | 2807 | The USA |
| 9 | National Institute of Allergy and Infectious Disease | 6 | 0.22 | 8179 | The USA |
| 10 | National Institutes for Food and Drug Control | 6 | 0.06 | 3230 | China |
| 11 | University of Glasgow | 6 | 0 | 877 | The UK |
| 12 | University of North Carolina | 5 | 0.34 | 2544 | The USA |
| 13 | University of Maryland | 5 | 0.27 | 9650 | The USA |
| 14 | Cincinnati Children’s Hospital | 5 | 0.03 | 8519 | The USA |
| 15 | Imperial College London | 5 | 0.03 | 3552 | The UK |
Analysis of journals
The top 100 most cited articles were distributed across 32 journals. Table Table4 4 shows that The New England of medicine journal dominated with 22 articles, more than 20% of the all journals, followed by Lancet (fifteen articles), Nature (eight articles), and Cell (six articles). It was worth pointing out that the number of articles from these four journals accounted for 51% (51/100) of the top 100 most cited articles on COVID-19 vaccine. In addition, the top three journals in terms of citations were The New England of medicine journal ( n = 22,564), Lancet ( n = 9626), and Lancet ( n = 3531). Notably, seven of the top 10 most cited articles were from The New England of medicine journal (Table (Table1 1 ) . The above results indicated that these top three authoritative journals will be one of the main sources of information on the latest developments in further COVID-19 vaccine research.
Top 5 journals with most cited articles on COVID-19 vaccine
| Rank | Journal | Count | Citations | Impact factor (2021) | Quartile (2021) | Published Country/region |
|---|---|---|---|---|---|---|
| 1 | New England Journal of Medicine | 22 | 22,564 | 176.082 | Q1 | The USA |
| 2 | Lancet | 15 | 9626 | 202.731 | Q1 | England |
| 3 | Nature | 8 | 3531 | 69.504 | Q1 | England |
| 4 | Cell | 6 | 2121 | 66.850 | Q1 | The USA |
| 5 | Lancet Infectious Diseases | 5 | 1929 | 71.421 | Q1 | The USA |
| 6 | Vaccine | 5 | 1464 | 4.169 | Q3 | Netherlands |
Analysis of authors
There were altogether 167 authors published papers related to COVID-19 vaccine. The network diagram of authors revealed that it was consisted of 167 nodes and 566 links (Fig. 4 ). Professor L ambe.T., Gilbert. S., Dormitzer. P., and Swanson. K. produced the most top-cited articles in this bibliometric analysis ( n = nine, each). Professor L ambe.T. and Gilbert. S. from University of Oxford, and Professor Dormitzer. P. and Swanson. K. from Pfizer Pharmaceuticals Ltd. Significantly, these two institutions rank in the top two in the number of publications in this study (Table (Table3 3 ).

Authors of top 100 most cited articles on COVID-19 vaccine
Analysis of keywords
The top 100 most cited articles included in this study comprised 81 keywords. Node centrality analysis demonstrated that the top six keywords were immunization (centrality = 0.25), influenza vaccination (centrality = 0.21), coronavirus (centrality = 0.18), challenge (centrality = 0.18), infection (centrality = 0.15), and immunity (centrality = 0.15). The keywords of the network map were constructed by CiteSpace and consisted of 81 nodes and 239 links (Fig. 5 ). The results revealed that the immunological effect of vaccines was a primary concern for researchers.

Keywords of top 100 most cited articles on COVID-19 vaccine
A cluster analysis of the keywords displayed that 81 keywords were divided into four main clusters ( Q value = 0.535, S value = 0.879): # 0 protection efficacy (red), # 1 vaccine hesitancy (green), # 2 spike protein (blue), and # 3 second vaccine dose (pink) (Fig. 6 ). The efficacy of vaccine and booster vaccination were the focus of vaccine research. Vaccine hesitancy studies investigated the reasons why partial population unwilling to vaccinate, so as to take appropriate action, thus to increase vaccine uptake. Additionally, the studies of spike proteins make a valuable contribution to the studies of new variants and the development of new vaccines. Consequently, these were major concerns in the field of COVID-19 vaccine research.

The clustering of keywords of top 100 most cited articles on COVID-19 vaccine (Color figure online)
Analysis of cited references
A total of 208 references were cited by the top 100 most cited articles included in this study, while these references were cited by 862 times. “ Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine ” [ 26 ], published by Polack, F. P. was the most cited paper in the top 100 most cited article with 40 citations, followed by “ Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine ” [ 27 ] published by Baden LR. with 24 citations, both of them were published in The New England of medicine journal (Fig. 7 ). The whole citations of these two articles were ranked top two in this bibliometric analysis, with 5881 and 3864 citations, respectively (Table (Table1 1 ).

Cited references of top 100 most cited articles on COVID-19 vaccine
A cluster analysis of cited references demonstrated that 208 cited references were divided into eight largest clusters ( Q value = 0.672, S value = 0.794): #0 Cov-2 variant (red), #1 clinical trial (yellow), #2 large integrated health system (pale green), #3 COV-2 rhesus macaque (emerald green), #4 mRNA vaccine (cyan), #5 vaccination intent (sky blue), #6 phase II study (dark blue), and #7 Cov-2 omicron variant (pink) (Fig. 8 ). It was showed that variants of COVID-19, rhesus macaque animal studies, and clinical trials were the focus of early COVID-19 vaccine research.

The clustering of cited reference of top 100 most cited articles on COVID-19 vaccine (Color figure online)
Analysis of cited journals
Publications from 217 journals were cited by the top 100 most cited articles included in this research, and these journals were cited by 1479 times. The journal co-citation network was mapped by CiteSpace, and it consisted of 217 nodes and 857 links (Fig. 9 ). The most cited journal was The New England of medicine journal ( n = 82), followed by Lancet ( n = 65) and Science ( n = 60). While BMJ-British Medical Journal and Health Psychology had the highest centrality (0.17), followed by Medical Journal of Australia (0.16). To be sure, these top academic journals will continue to publish the latest major advances in COVID-19 vaccine research, which requires more attention from researchers in the future.

Cited journals of top 100 most cited articles on COVID-19 vaccine
There was an urgent need for safe and effective COVID-19 vaccine after the emergence of SARS-CoV-2. The increasing number of research teams, and pharmaceutical companies were committed to vaccines research and development, which has contributed to an explosion of literatures on COVID-19 vaccines published in the past three years [ 3 ]. We performed this first bibliometric analysis of the top 100 most cited articles on COVID-19 vaccine to unveil the most influential authors, institutions, and journals in this field, systematically describe the research focus in the last three years, and highlight the future research direction.
Our finding revealed that the median number of citations of the top 100 most cited articles on COVID-19 vaccine was 349.5. This was lower than some fields, such as vaccine [ 28 ], bladder cancer [ 29 ], while higher than others, such as anaphylaxis [ 30 ], triple‑negative breast cancer [ 12 ]. Of note, the average citations per year of this study was remarkably higher than the above domains, suggesting that this topic is presently one of the hottest research focuses. It was indisputable that the development pace of COVID-19 vaccine will not quit, as mutation in the SARS-COV-2 was a continuous process resulting in multiple variant introductions [ 31 ]. It will remain attract intensive attention of numerous scientific researchers until free of pandemic. Harvard Medical School, Boston Children’s Hospital, and Public Health England were the top three institutions leading the way in COVID-19 vaccine research. Institutions from the USA and the UK dominated this research field, as evidenced by seven of the top 15 institutions being from the USA, and six of them from the UK. The USA, England, and China ranked the top three countries/regions in accordance with the number of publications, which meant that these three countries/regions represented the highest level in COVID-19 vaccine research worldwide. Among that, more than half of the articles (56%) had participation from American scholars, because basic research conditions in the USA are superior to other countries, with advanced laboratory equipment, adequate funding for scientific research, and first-class research teams [ 18 ]. Although China ranked third in the number of publications, the centrality value was zero (Table (Table2), 2 ), indicating that China should strengthen international cooperation and carry out more in-depth research on COVID-19 vaccine research.
To curb this epidemic as soon as possible, the academic community lost no time in joining this “battlefield” to carried out vaccine research and development. Furthermore, medical journals with high influence such as The New England Journal of Medicine , Lancet , and British Medical Journal, have also opened special columns for COVID-19 [ 32 ], which promoted communication on the global COVID-19 research. The present study indicated that the highest-ranking journal in this field was The New England Journal of Medicine , with 22 publications and an impact factor (IF) of 176.079, and seven of the top 10 most cited articles were from this journal. It was outperformed all other journals in both quantity and quality of publications, signing its dominance on COVID-19 vaccine research . Thus, we predict that more influential and advanced researches in this field will still be published in this journal in the future.
Cluster analysis of keywords disclosed that the top four categories were protection efficacy, vaccine hesitancy, spike protein, and second vaccine dose, respectively. It demonstrated that the primary concerns of vaccine research including spike protein, protection, and vaccine hesitancy. Spike protein, one of the four major structural proteins encoded by SARS-CoV-2 [ 33 ], is a critical target for vaccine development [ 34 ]. Consequently, the studies of spike protein mutations play a pivotal role in the further COVID-19 vaccine development, because the spike protein has impact on the titers of IgG antibodies, which was correlation with vaccine efficacy [ 35 ]. Furthermore, it was proved useful to utilize the computational methods to predict the impact of the variants of concern (VOC) on the COVID-19 vaccines [ 36 , 37 ]. Previous studies have been revealed that a two-dose regimen of vaccine conferred 85–95% protection against COVID-19, and safety was similar to other virus vaccines [ 26 , 38 ]. Although emerging evidence indicated that the vaccines have high level of protection and sufficient safety, a segment of population remained unwilling to receive the COVID-19 vaccine [ 39 , 40 ]. In addition, COVID-19 vaccine acceptance was highly heterogeneous between countries and between groups with different sociodemographic characteristics [ 41 – 43 ]. Hence, how to cultivate vaccine confidence and eliminate the barriers to accessing vaccination so as to increase vaccine uptake may be future trends of COVID-19 vaccine research.
In the cluster analyses, the cited references were classified into eight largest categories: Cov-2 variant, clinical trial, large integrated health system, COV-2 rhesus macaque, mRNA vaccine, vaccination intent, phase II study, and Cov-2 omicron variant. More importantly, animal studies were essential to conclusively identify of vaccine-mediated protection. The novel experimental vaccine was tested in animal before entering clinical trials. Rhesus macaques, one of non-human primates, was involved in COVID-19 vaccine experiments, and developed protective immune responses to COVID-19 [ 44 , 45 ]. Given that emerging omicron subvariant, characterized by highly transmission, was the predominant variant worldwide, the protection of current COVID-19 vaccines against omicron variant has become a hot research topic. It was exciting that studies proved that the booster-dose vaccine could provide efficacy against the omicron variant [ 46 – 48 ]. Thus, the protection and efficacy of new vaccines under pre- and clinical development against omicron will be highlight research areas in the future. Additionally, side effects and adverse events after COVID-19 vaccination were attracted more attention for current researches [ 49 , 50 ]. The development of COVID-19 antiviral drugs is equally urgent. Notably, some new bioinformatics analysis methods, such as weighted gene co-expression network analysis, play a crucial role in identifying biomarkers and potential therapeutic targets in COVID-19 [ 51 ].
We searched the articles related to COVID-19 vaccines published in several high-impact journals from January 2022 to February 2023 based on WOSCC, and found that the researches mainly focused on the following aspects: first, studies on vaccine efficacy, safety and immunogenicity, including clinical trials of new vaccines, vaccine protection against omicron, and infection protection of special populations, etc.; second, evaluation of the efficacy of third and fourth dose of vaccine; third, the investigations of vaccination willingness and vaccine hesitancy in various populations; finally, basic research involve COVID-19 virus and vaccines. Of note, these were basically consistent with our findings.
However, there were some limitations in our study. First, only two articles published in 2022 were included in the present study. Some high-quality articles published in 2022 may not be included in our study due to short publication time frame and fewer citations, which may result in bias. Second, the WOSCC database was not fully inclusive of all literature. Hence, it might not provide truly representative citation counts. Third, the number of citations might not be fully representative of article quality. Low citation number may not mean low research value, and high citation number does not necessarily indicate important guidance to this field. Fourth, the statistical methods used by CiteSpace software may have some biases, which was an inherent weakness of software. Finally, a small number of non-English articles were not included in this study due to our inclusion criteria, leading to bias in literature selection. Despite these limitations, our study offers fresh insights into the present state and development direction for further study of COVID-19 vaccine.
In conclusion, a plethora of papers on COVID-19 vaccine have been published in the last three years, indicating that this theme is currently hottest topic in academic community. The USA, the UK, and China made many contributions to COVID-19 vaccine research globally. Harvard Medical School, Boston Children’s Hospital, and Public Health England were the top three institutions leading the way on COVID-19 vaccine research. The New England Journal of Medicine was dominant in publishing high-quality COVID-19 vaccine papers. At present, COVID-19 vaccines researches have focused on vaccine efficacy, vaccine hesitancy, and the efficacy of current vaccines on omicron variants. However, how to increase vaccine uptake, focus on mutations in the spike protein, evaluate of the efficacy of booster vaccine, and how effective new vaccines under pre- and clinical development against omicron will be spotlight in the future.
Author contributions
YF and SW designed this study, WW and HW collected the data, TY, YL, and JL performed data analysis and interpretation, WW, LY, and YG drafted the article, WW, JL, YF, and SW revised the article, YF and SW approved the final version to be published. All authors reviewed the manuscript.
The study was supported by the COVID-19 Project of Shanxi Provincial Finance, and the Project of Shanxi Provincial Key Laboratory for major infectious disease response.
Data availability
Declarations.
The authors declare that they have no competing interests.
All data were obtained and downloaded from a publicly available database and did not involve any ethical issues requiring approval.
Not applicable.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Weigang Wang, Email: nc.ude.umxs@gnagiewgnaw .
Hu Wang, Email: nc.ude.umxs@uhgnaw .
Tian Yao, Email: nc.ude.umxs@6201naitoay .
Yandi Li, Email: nc.ude.umxs.b@10601508102 .
Linzhu Yi, Email: nc.ude.umxs.d@73015009102rd .
Ying Gao, Email: nc.ude.umxs@gniyoag .
Jia Lian, Email: moc.ude.umxs@aijnail .
Yongliang Feng, Email: [email protected] .
Suping Wang, Email: nc.ude.umxs@gnawgnipus .
- Share full article
Advertisement
Supported by
Childhood Vaccines Aren’t Just Saving Lives. They’re Saving Money.
Over the past three decades, routine immunizations have prevented 1.1 million deaths and saved the United States $540 billion, the C.D.C. estimated.

By Teddy Rosenbluth
There’s no way to put a price on the pain and suffering prevented by childhood vaccines. But as it turns out, you can pinpoint the savings to the country.
For nearly three decades, childhood vaccines — including those that target measles, tetanus and diphtheria — have saved the United States $540 billion in health care costs, according to a new report from the Centers for Disease Control and Prevention.
Routine childhood vaccinations have prevented approximately 508 million cases of illness, 32 million hospitalizations and 1,129,000 deaths, the agency estimated on Thursday.
“These vaccine programs, when you create the right infrastructure to implement them, they pay for themselves right away,” said William Padula, a health economist at the University of Southern California who was not involved in the new research.
The savings estimate includes money that would have been spent on treating the initial infection and managing later, related conditions. The figure dwarfs the cost of developing the shots.
But vaccine-preventable illnesses can also cause indirect economic effects if children become permanently disabled from an infection, or parents miss work while caring for their sick children.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .

IMAGES
COMMENTS
1. A phase 1/2 randomized, placebo-controlled, multicentre study to evaluate the safety and immunogenicity of COVID-19 vaccine in healthy adults. RBD SARS-CoV-2 HBsAg VLP vaccine, administered at two dose amounts 5 mcg and 25 mcg, by intramuscular injection by investigators during an in-clinic visit. RBD-HBsAg VLPs.
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
As the research is rapidly expanding, we have looked at the association between pregnancy and COVID-19 vaccinations, in addition to the current reviews on the mixing of vaccines. ... The BNT162b2 COVID-19 vaccine developed by BioNTech and Pfizer is a lipid nanoparticle-formulated, ... Studies on these topics are rapidly being conducted and ...
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement ...
WHO and its partners are committed to accelerating the development of COVID-19 vaccines while maintaining the highest standards on safety. Vaccines go through various phases of development and testing - there are usually three phases to clinical trials, with the last one designed to assess the ability of the product to protect against disease, which is called efficacy.
The development and deployment of vaccines for COVID-19 is a remarkable science success story: within 16 months of the first vaccine trials, 2.8 billion vaccine doses have been administered.
COVID-19 Topics. For up-to-date information on topics related to COVID-19, visit these U.S. government resources. OPEN ALL. Treatments. Administration for Strategic Preparedness and Response (ASPR), HHS. COVID-19 Therapeutics Prioritized for Testing in Clinical Trials. National Institute of Allergy and Infectious Disease (NIAID)
The Moderna vaccine for COVID-19 may seem like it developed quickly, but it was built on decades of research by NIH's Dale and Betty Bumpers Vaccine Research Center (VRC). The scientists of the VRC have learned from experience by studying the immune system and working on vaccines for other infectious diseases that pose major threats to human ...
Johns Hopkins International Vaccine Access Center. VIEW-hub is a publicly available interactive tool that displays up-to-date information on vaccine characteristics, and vaccine introduction and use globally. Vaccines include COVID-19 as well as many childhood vaccines in routine immunization programs. Understanding COVID-19.
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
The coronavirus pandemic has claimed more than 670,000 lives in the United States as of Sept. 20, and the spread of the highly transmissible delta variant has added new urgency to the federal government's efforts to vaccinate all Americans against the virus. As the drive to inoculate more people continues, here are 10 facts about Americans and COVID-19 vaccines, based on an August Pew ...
In the case of [COVID-19 vaccines], we had a lot of urgency and all the money was put up, up front. The companies didn't have to find the money for each stage—they were just able to just proceed from the safety study to the effectiveness study very quickly. This let the coronavirus vaccines go to the front of the line because of the urgency.
Overall, 62% of U.S. adults say that the benefits of COVID-19 vaccines outweigh the risks, while a much smaller share think the risks outweigh the benefits (36%). Even so, when asked to rate the preventative health benefits of COVID-19 vaccines from very high to very low, fewer than half of Americans (45%) rate the benefits as high.
The evolving landscape of live attenuated COVID-19 vaccines. ... of circulating SARS-CoV-2 specific B cells following mRNA vaccination or COVID-19. ... Research Highlights 13 Aug 2024 Nature ...
The new national survey by Pew Research Center, conducted Sept. 8-13 among 10,093 U.S. adults, finds intent to get a COVID-19 vaccine has declined across all major political and demographic groups. However, sizable differences across groups remain. Democrats and those who lean to the Democratic Party are 14 percentage points more likely than ...
Yusen Zhai, Ph.D. New research published in Lancet Regional Health-Americas from the University of Alabama at Birmingham School of Education and Human Sciences, College of Arts and Sciences, and Heersink School of Medicine found that the rollout of the COVID-19 vaccine in the United States was associated with decreased anxiety and depression rates among adults.
Research on hamsters suggests that vaccines aimed at the nose and mouth could be crucial in curbing the transmission of respiratory infections. The rapid development of COVID-19 vaccines within months of the virus 's emergence was a remarkable achievement of modern science, saving millions of lives. But for all the good they did in reducing ...
Introduction. In December 2020, the Food and Drug Administration authorized two COVID-19 mRNA vaccines for use in the United States. Early vaccine supplies were prioritized for health care personnel and long-term care facility residents, with phased vaccination of other persons, beginning with those who were older or had high-risk medical conditions, and concluding with healthy younger persons ...
COVID-19 vaccines have been effective in reducing the spread of infection, severity of symptoms, and death [6], [7]. A high population uptake of vaccines can result in the achievement of a herd immunity threshold. A high uptake of effective vaccines, such as that for COVID-19, can lead to substantial reductions in infections [8], [9].
For vaccines provided under emergency use authorization, however, such as vaccines against H1N1 in 2009 and now Covid-19, safety science and compensation programs have been chronically underfunded.
New estimates published in The Lancet Respiratory Medicine show that at least 1.6 million European lives have been directly saved by COVID-19 vaccinations, with 60% of those lives saved when Omicron became the dominant strain of the virus—and those numbers may be an undercount.. As of March 2023, 2.2 million COVID-19-related deaths have been reported across Europe.
Kids ages 6 months to 4 years old need multiple doses to be up to date, and at least one of these doses should be the 2023-2024 formula COVID-19 vaccine. See the full vaccination schedule ...
As of August 2024, the availability of COVID-19 vaccines and the timing for boosters is an important topic, as health authorities prepare for the upcoming fall and winter seasons.
Researchers at Stanford Engineering have developed a nanoparticle platform that could make existing vaccines more effective, including those for influenza, COVID-19, and HIV.
About nine-in-ten (88%) Americans say, overall, the benefits of childhood vaccines for measles, mumps and rubella outweigh the risks, identical to the share who said this before the coronavirus outbreak. U.S. adults are less confident in COVID-19 vaccines: Fewer than half rate them as having high health benefits and a low risk of side effects.
Research shows recent COVID-19 vaccines are about 70% effective at preventing serious COVID-19 and hospitalization as well as long COVID. Taking Paxlovid or other therapies like remdesivir early in the course of infection can also reduce the risk of hospitalization even if you are unvaccinated.
However, the 2019 onset of the COVID-19 pandemic prompted a substantial reallocation of research resources towards COVID-19 vaccine development . This shift swiftly brought LNPs and mRNA technologies into sharp focus, introducing a new research direction across various nanovaccine domains . Furthermore, the rise of cancer immunotherapy has ...
This article is part of the Research Topic Vaccine Education and Promotion View all 10 articles. ... (DCE) was conducted on 1509 representatives of the general population in China to collect data on preferences for the COVID-19 and influenza vaccines, representing the newly-developed and established vaccines, respectively. The latent class ...
This study aimed to uncover the current major topics regarding COVID-19 vaccine, and systematically evaluate the development trends for future research. The top 100 most cited original articles on COVID-19 vaccine from January 2020 to October 2022 were identified from Web of Science Core Collection database. ... This study analyzed the top 100 ...
When people had concerns about Covid-19 vaccines, she said, few people had a doctor they trusted to answer questions. ... according to ongoing research by John Brownstein, an epidemiologist at ...