

Transition Words
As a "part of speech" transition words are used to link words, phrases or sentences. They help the reader to progress from one idea (expressed by the author) to the next idea. Thus, they help to build up coherent relationships within the text.
Transitional Words
This structured list of commonly used English transition words — approximately 200, can be considered as quasi complete. It can be used (by students and teachers alike) to find the right expression. English transition words are essential, since they not only connect ideas, but also can introduce a certain shift, contrast or opposition, emphasis or agreement, purpose, result or conclusion, etc. in the line of argument. The transition words and phrases have been assigned only once to somewhat artificial categories, although some words belong to more than one category.
There is some overlapping with prepositions and postpositions, but for the purpose of usage and completeness of this concise guide, I did not differentiate.
Linking & Connecting Words — Part 1/2
Agreement / Addition / Similarity
Opposition / limitation / contradiction, examples / support / emphasis, cause / condition / purpose, effect / consequence / result, conclusion / summary / restatement, time / chronology / sequence, space / location / place.
The transition words like also, in addition, and, likewise , add information , reinforce ideas , and express agreement with preceding material.
in the first place
not only ... but also
as a matter of fact
in like manner
in addition
coupled with
in the same fashion / way
first, second, third
in the light of
not to mention
to say nothing of
equally important
by the same token
identically
together with
comparatively
correspondingly
furthermore
additionally
Transition phrases like but , rather and or , express that there is evidence to the contrary or point out alternatives , and thus introduce a change the line of reasoning ( contrast ).
although this may be true
in contrast
different from
of course ..., but
on the other hand
on the contrary
at the same time
in spite of
even so / though
be that as it may
(and) still
even though
nevertheless
nonetheless
notwithstanding
These transitional phrases present specific conditions or intentions .
in the event that
granted (that)
as / so long as
on (the) condition (that)
for the purpose of
with this intention
with this in mind
in the hope that
to the end that
for fear that
in order to
seeing / being that
provided that
only / even if
inasmuch as
These transitional devices (like especially ) are used to introduce examples as support , to indicate importance or as an illustration so that an idea is cued to the reader.
in other words
to put it differently
for one thing
as an illustration
in this case
for this reason
to put it another way
that is to say
with attention to
by all means
important to realize
another key point
first thing to remember
most compelling evidence
must be remembered
point often overlooked
to point out
on the positive side
on the negative side
specifically
surprisingly
significantly
particularly
in particular
for example
for instance
to demonstrate
to emphasize
to enumerate
Some of these transition words ( thus, then, accordingly, consequently, therefore, henceforth ) are time words that are used to show that after a particular time there was a consequence or an effect .
Note that for and because are placed before the cause/reason. The other devices are placed before the consequences or effects.
as a result
under those circumstances
in that case
because the
consequently
accordingly
These transition words and phrases conclude , summarize and / or restate ideas, or indicate a final general statement . Also some words (like therefore ) from the Effect / Consequence category can be used to summarize.
as can be seen
generally speaking
in the final analysis
all things considered
as shown above
in the long run
given these points
as has been noted
for the most part
in conclusion
to summarize
by and large
on the whole
in any event
in either case
These transitional words (like finally ) have the function of limiting, restricting, and defining time . They can be used either alone or as part of adverbial expressions .
at the present time
from time to time
sooner or later
up to the present time
to begin with
in due time
in the meantime
in a moment
without delay
all of a sudden
at this instant
first, second
immediately
straightaway
by the time
occasionally
Many transition words in the time category ( consequently; first, second, third; further; hence; henceforth; since; then, when; and whenever ) have other uses.
Except for the numbers ( first, second, third ) and further they add a meaning of time in expressing conditions, qualifications, or reasons. The numbers are also used to add information or list examples . Further is also used to indicate added space as well as added time.
These transition words are often used as part of adverbial expressions and have the function to restrict, limit or qualify space . Quite a few of these are also found in the Time category and can be used to describe spatial order or spatial reference.
in the middle
to the left/right
in front of
on this side
in the distance
here and there
in the foreground
in the background
in the center of
adjacent to
opposite to
List of Transition Words

Transition Words are also sometimes called (or put in the category of) Connecting Words. Please feel free to download them via this link to the category page: Linking Words & Connecting Words as a PDF. It contains all the transition words listed on this site. The image to the left gives you an impression how it looks like.
Usage of Transition Words in Essays
Transition words and phrases are vital devices for essays , papers or other literary compositions. They improve the connections and transitions between sentences and paragraphs. They thus give the text a logical organization and structure (see also: a List of Synonyms ).
All English transition words and phrases (sometimes also called 'conjunctive adverbs') do the same work as coordinating conjunctions : they connect two words, phrases or clauses together and thus the text is easier to read and the coherence is improved.
Usage: transition words are used with a special rule for punctuation : a semicolon or a period is used after the first 'sentence', and a comma is almost always used to set off the transition word from the second 'sentence'.
Example 1: People use 43 muscles when they frown; however, they use only 28 muscles when they smile.
Example 2: however, transition words can also be placed at the beginning of a new paragraph or sentence - not only to indicate a step forward in the reasoning, but also to relate the new material to the preceding thoughts..
Use a semicolon to connect sentences, only if the group of words on either side of the semicolon is a complete sentence each (both must have a subject and a verb, and could thus stand alone as a complete thought).
Further helpful readings about expressions, writing and grammar: Compilation of Writing Tips How to write good ¦ Correct Spelling Study by an English University
Are you using WORD for writing professional texts and essays? There are many easy Windows Shortcuts available which work (almost) system-wide (e.g. in every programm you use).

Transitional Words and Phrases
One of your primary goals as a writer is to present ideas in a clear and understandable way. To help readers move through your complex ideas, you want to be intentional about how you structure your paper as a whole as well as how you form the individual paragraphs that comprise it. In order to think through the challenges of presenting your ideas articulately, logically, and in ways that seem natural to your readers, check out some of these resources: Developing a Thesis Statement , Paragraphing , and Developing Strategic Transitions: Writing that Establishes Relationships and Connections Between Ideas.
While clear writing is mostly achieved through the deliberate sequencing of your ideas across your entire paper, you can guide readers through the connections you’re making by using transitional words in individual sentences. Transitional words and phrases can create powerful links between your ideas and can help your reader understand your paper’s logic.
In what follows, we’ve included a list of frequently used transitional words and phrases that can help you establish how your various ideas relate to each other. We’ve divided these words and phrases into categories based on the common kinds of relationships writers establish between ideas.
Two recommendations: Use these transitions strategically by making sure that the word or phrase you’re choosing matches the logic of the relationship you’re emphasizing or the connection you’re making. All of these words and phrases have different meanings, nuances, and connotations, so before using a particular transitional word in your paper, be sure you understand its meaning and usage completely, and be sure that it’s the right match for your paper’s logic. Use these transitional words and phrases sparingly because if you use too many of them, your readers might feel like you are overexplaining connections that are already clear.
Categories of Transition Words and Phrases
Causation Chronology Combinations Contrast Example
Importance Location Similarity Clarification Concession
Conclusion Intensification Purpose Summary
Transitions to help establish some of the most common kinds of relationships
Causation– Connecting instigator(s) to consequence(s).
accordingly as a result and so because
consequently for that reason hence on account of
since therefore thus
Chronology– Connecting what issues in regard to when they occur.
after afterwards always at length during earlier following immediately in the meantime
later never next now once simultaneously so far sometimes
soon subsequently then this time until now when whenever while
Combinations Lists– Connecting numerous events. Part/Whole– Connecting numerous elements that make up something bigger.
additionally again also and, or, not as a result besides even more
finally first, firstly further furthermore in addition in the first place in the second place
last, lastly moreover next second, secondly, etc. too
Contrast– Connecting two things by focusing on their differences.
after all although and yet at the same time but
despite however in contrast nevertheless nonetheless notwithstanding
on the contrary on the other hand otherwise though yet
Example– Connecting a general idea to a particular instance of this idea.
as an illustration e.g., (from a Latin abbreviation for “for example”)
for example for instance specifically that is
to demonstrate to illustrate
Importance– Connecting what is critical to what is more inconsequential.
chiefly critically
foundationally most importantly
of less importance primarily
Location– Connecting elements according to where they are placed in relationship to each other.
above adjacent to below beyond
centrally here nearby neighboring on
opposite to peripherally there wherever
Similarity– Connecting to things by suggesting that they are in some way alike.
by the same token in like manner
in similar fashion here in the same way
likewise wherever
Other kinds of transitional words and phrases Clarification
i.e., (from a Latin abbreviation for “that is”) in other words
that is that is to say to clarify to explain
to put it another way to rephrase it
granted it is true
naturally of course
finally lastly
in conclusion in the end
to conclude
Intensification
in fact indeed no
of course surely to repeat
undoubtedly without doubt yes
for this purpose in order that
so that to that end
to this end
in brief in sum
in summary in short
to sum up to summarize

Improving Your Writing Style
This is an accordion element with a series of buttons that open and close related content panels.
Clear, Concise Sentences
Use the active voice
Put the action in the verb
Tidy up wordy phrases
Reduce wordy verbs
Reduce prepositional phrases
Reduce expletive constructions
Avoid using vague nouns
Avoid unneccessarily inflated words
Avoid noun strings
Connecting Ideas Through Transitions
Using Transitional Words and Phrases
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Writing Transitions

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
Good transitions can connect paragraphs and turn disconnected writing into a unified whole. Instead of treating paragraphs as separate ideas, transitions can help readers understand how paragraphs work together, reference one another, and build to a larger point. The key to producing good transitions is highlighting connections between corresponding paragraphs. By referencing in one paragraph the relevant material from previous paragraphs, writers can develop important points for their readers.
It is a good idea to continue one paragraph where another leaves off. (Instances where this is especially challenging may suggest that the paragraphs don't belong together at all.) Picking up key phrases from the previous paragraph and highlighting them in the next can create an obvious progression for readers. Many times, it only takes a few words to draw these connections. Instead of writing transitions that could connect any paragraph to any other paragraph, write a transition that could only connect one specific paragraph to another specific paragraph.
Some experts argue that focusing on individual actions to combat climate change takes the focus away from the collective action required to keep carbon levels from rising. Change will not be effected, say some others, unless individual actions raise the necessary awareness.
While a reader can see the connection between the sentences above, it’s not immediately clear that the second sentence is providing a counterargument to the first. In the example below, key “old information” is repeated in the second sentence to help readers quickly see the connection. This makes the sequence of ideas easier to follow.
Sentence pair #2: Effective Transition
Some experts argue that focusing on individual actions to combat climate change takes the focus away from the collective action required to keep carbon levels from rising. Other experts argue that individual actions are key to raising the awareness necessary to effect change.
You can use this same technique to create clear transitions between paragraphs. Here’s an example:
Some experts argue that focusing on individual actions to combat climate change takes the focus away from the collective action required to keep carbon levels from rising. Other experts argue that individual actions are key to raising the awareness necessary to effect change. According to Annie Lowery, individual actions are important to making social change because when individuals take action, they can change values, which can lead to more people becoming invested in fighting climate change. She writes, “Researchers believe that these kinds of household-led trends can help avert climate catastrophe, even if government and corporate actions are far more important” (Lowery).
So, what’s an individual household supposed to do?
The repetition of the word “household” in the new paragraph helps readers see the connection between what has come before (a discussion of whether household actions matter) and what is about to come (a proposal for what types of actions households can take to combat climate change).
Sometimes, transitional words can help readers see how ideas are connected. But it’s not enough to just include a “therefore,” “moreover,” “also,” or “in addition.” You should choose these words carefully to show your readers what kind of connection you are making between your ideas.
To decide which transitional word to use, start by identifying the relationship between your ideas. For example, you might be
- making a comparison or showing a contrast Transitional words that compare and contrast include also, in the same way, similarly, in contrast, yet, on the one hand, on the other hand. But before you signal comparison, ask these questions: Do your readers need another example of the same thing? Is there a new nuance in this next point that distinguishes it from the previous example? For those relationships between ideas, you might try this type of transition: While x may appear the same, it actually raises a new question in a slightly different way.
- expressing agreement or disagreement When you are making an argument, you need to signal to readers where you stand in relation to other scholars and critics. You may agree with another person’s claim, you may want to concede some part of the argument even if you don’t agree with everything, or you may disagree. Transitional words that signal agreement, concession, and disagreement include however, nevertheless, actually, still, despite, admittedly, still, on the contrary, nonetheless .
- showing cause and effect Transitional phrases that show cause and effect include therefore, hence, consequently, thus, so. Before you choose one of these words, make sure that what you are about to illustrate is really a causal link. Novice writers tend to add therefore and hence when they aren’t sure how to transition; you should reserve these words for when they accurately signal the progression of your ideas.
- explaining or elaborating Transitions can signal to readers that you are going to expand on a point that you have just made or explain something further. Transitional words that signal explanation or elaboration include in other words, for example, for instance, in particular, that is, to illustrate, moreover .
- drawing conclusions You can use transitions to signal to readers that you are moving from the body of your argument to your conclusions. Before you use transitional words to signal conclusions, consider whether you can write a stronger conclusion by creating a transition that shows the relationship between your ideas rather than by flagging the paragraph simply as a conclusion. Transitional words that signal a conclusion include in conclusion , as a result, ultimately, overall— but strong conclusions do not necessarily have to include those phrases.
If you’re not sure which transitional words to use—or whether to use one at all—see if you can explain the connection between your paragraphs or sentence either out loud or in the margins of your draft.
For example, if you write a paragraph in which you summarize physician Atul Gawande’s argument about the value of incremental care, and then you move on to a paragraph that challenges those ideas, you might write down something like this next to the first paragraph: “In this paragraph I summarize Gawande’s main claim.” Then, next to the second paragraph, you might write, “In this paragraph I present a challenge to Gawande’s main claim.” Now that you have identified the relationship between those two paragraphs, you can choose the most effective transition between them. Since the second paragraph in this example challenges the ideas in the first, you might begin with something like “but,” or “however,” to signal that shift for your readers.
- picture_as_pdf Transitions

Transitions
What this handout is about.
In this crazy, mixed-up world of ours, transitions glue our ideas and our essays together. This handout will introduce you to some useful transitional expressions and help you employ them effectively.
The function and importance of transitions
In both academic writing and professional writing, your goal is to convey information clearly and concisely, if not to convert the reader to your way of thinking. Transitions help you to achieve these goals by establishing logical connections between sentences, paragraphs, and sections of your papers. In other words, transitions tell readers what to do with the information you present to them. Whether single words, quick phrases, or full sentences, they function as signs that tell readers how to think about, organize, and react to old and new ideas as they read through what you have written.
Transitions signal relationships between ideas—relationships such as: “Another example coming up—stay alert!” or “Here’s an exception to my previous statement” or “Although this idea appears to be true, here’s the real story.” Basically, transitions provide the reader with directions for how to piece together your ideas into a logically coherent argument. Transitions are not just verbal decorations that embellish your paper by making it sound or read better. They are words with particular meanings that tell the reader to think and react in a particular way to your ideas. In providing the reader with these important cues, transitions help readers understand the logic of how your ideas fit together.
Signs that you might need to work on your transitions
How can you tell whether you need to work on your transitions? Here are some possible clues:
- Your instructor has written comments like “choppy,” “jumpy,” “abrupt,” “flow,” “need signposts,” or “how is this related?” on your papers.
- Your readers (instructors, friends, or classmates) tell you that they had trouble following your organization or train of thought.
- You tend to write the way you think—and your brain often jumps from one idea to another pretty quickly.
- You wrote your paper in several discrete “chunks” and then pasted them together.
- You are working on a group paper; the draft you are working on was created by pasting pieces of several people’s writing together.
Organization
Since the clarity and effectiveness of your transitions will depend greatly on how well you have organized your paper, you may want to evaluate your paper’s organization before you work on transitions. In the margins of your draft, summarize in a word or short phrase what each paragraph is about or how it fits into your analysis as a whole. This exercise should help you to see the order of and connection between your ideas more clearly.
If after doing this exercise you find that you still have difficulty linking your ideas together in a coherent fashion, your problem may not be with transitions but with organization. For help in this area (and a more thorough explanation of the “reverse outlining” technique described in the previous paragraph), please see the Writing Center’s handout on organization .
How transitions work
The organization of your written work includes two elements: (1) the order in which you have chosen to present the different parts of your discussion or argument, and (2) the relationships you construct between these parts. Transitions cannot substitute for good organization, but they can make your organization clearer and easier to follow. Take a look at the following example:
El Pais , a Latin American country, has a new democratic government after having been a dictatorship for many years. Assume that you want to argue that El Pais is not as democratic as the conventional view would have us believe.
One way to effectively organize your argument would be to present the conventional view and then to provide the reader with your critical response to this view. So, in Paragraph A you would enumerate all the reasons that someone might consider El Pais highly democratic, while in Paragraph B you would refute these points. The transition that would establish the logical connection between these two key elements of your argument would indicate to the reader that the information in paragraph B contradicts the information in paragraph A. As a result, you might organize your argument, including the transition that links paragraph A with paragraph B, in the following manner:
Paragraph A: points that support the view that El Pais’s new government is very democratic.
Transition: Despite the previous arguments, there are many reasons to think that El Pais’s new government is not as democratic as typically believed.
Paragraph B: points that contradict the view that El Pais’s new government is very democratic.
In this case, the transition words “Despite the previous arguments,” suggest that the reader should not believe paragraph A and instead should consider the writer’s reasons for viewing El Pais’s democracy as suspect.
As the example suggests, transitions can help reinforce the underlying logic of your paper’s organization by providing the reader with essential information regarding the relationship between your ideas. In this way, transitions act as the glue that binds the components of your argument or discussion into a unified, coherent, and persuasive whole.
Types of transitions
Now that you have a general idea of how to go about developing effective transitions in your writing, let us briefly discuss the types of transitions your writing will use.
The types of transitions available to you are as diverse as the circumstances in which you need to use them. A transition can be a single word, a phrase, a sentence, or an entire paragraph. In each case, it functions the same way: First, the transition either directly summarizes the content of a preceding sentence, paragraph, or section or implies such a summary (by reminding the reader of what has come before). Then, it helps the reader anticipate or comprehend the new information that you wish to present.
- Transitions between sections: Particularly in longer works, it may be necessary to include transitional paragraphs that summarize for the reader the information just covered and specify the relevance of this information to the discussion in the following section.
- Transitions between paragraphs: If you have done a good job of arranging paragraphs so that the content of one leads logically to the next, the transition will highlight a relationship that already exists by summarizing the previous paragraph and suggesting something of the content of the paragraph that follows. A transition between paragraphs can be a word or two (however, for example, similarly), a phrase, or a sentence. Transitions can be at the end of the first paragraph, at the beginning of the second paragraph, or in both places.
- Transitions within paragraphs: As with transitions between sections and paragraphs, transitions within paragraphs act as cues by helping readers to anticipate what is coming before they read it. Within paragraphs, transitions tend to be single words or short phrases.
Transitional expressions
Effectively constructing each transition often depends upon your ability to identify words or phrases that will indicate for the reader the kind of logical relationships you want to convey. The table below should make it easier for you to find these words or phrases. Whenever you have trouble finding a word, phrase, or sentence to serve as an effective transition, refer to the information in the table for assistance. Look in the left column of the table for the kind of logical relationship you are trying to express. Then look in the right column of the table for examples of words or phrases that express this logical relationship.
Keep in mind that each of these words or phrases may have a slightly different meaning. Consult a dictionary or writer’s handbook if you are unsure of the exact meaning of a word or phrase.
| also, in the same way, just as … so too, likewise, similarly | |
| but, however, in spite of, on the one hand … on the other hand, nevertheless, nonetheless, notwithstanding, in contrast, on the contrary, still, yet | |
| first, second, third, … next, then, finally | |
| after, afterward, at last, before, currently, during, earlier, immediately, later, meanwhile, now, recently, simultaneously, subsequently, then | |
| for example, for instance, namely, specifically, to illustrate | |
| even, indeed, in fact, of course, truly | |
| above, adjacent, below, beyond, here, in front, in back, nearby, there | |
| accordingly, consequently, hence, so, therefore, thus | |
| additionally, again, also, and, as well, besides, equally important, further, furthermore, in addition, moreover, then | |
| finally, in a word, in brief, briefly, in conclusion, in the end, in the final analysis, on the whole, thus, to conclude, to summarize, in sum, to sum up, in summary |
You may reproduce it for non-commercial use if you use the entire handout and attribute the source: The Writing Center, University of North Carolina at Chapel Hill
Make a Gift
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Transition sentences | Tips & examples for clear writing
Transition Sentences | Tips & Examples for Clear Writing
Published on June 9, 2020 by Jack Caulfield . Revised on July 23, 2023.
Clear transitions are crucial to clear writing: They show the reader how different parts of your essay, paper, or thesis are connected. Transition sentences can be used to structure your text and link together paragraphs or sections.
… In this case, the researchers concluded that the method was unreliable.
However , evidence from a more recent study points to a different conclusion . …
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
Transitioning between paragraphs, transitioning to a new section, transitions within a paragraph, other interesting articles.
When you start a new paragraph , the first sentence should clearly express:
- What this paragraph will discuss
- How it relates to the previous paragraph
The examples below show some examples of transition sentences between paragraphs and what they express.
| Transition sentence | This paragraph… |
|---|---|
| evidence in support of is provided by Smith (2019). | … the previous one, providing more support for . |
| , Patel’s arguments are on the matter. | … the previous one by presenting related to the previous discussion. |
| the relationship between these factors, to draw conclusions about the broader process. | …treats the preceding point as on which to more general arguments. |
Placement of transition sentences
The beginning of a new paragraph is generally the right place for a transition sentence. Each paragraph should focus on one topic, so avoid spending time at the end of a paragraph explaining the theme of the next one.
The first dissenter to consider is …
However, several scholars dissent from this consensus. The first one to consider is …
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
While transitions between paragraphs are generally a single sentence, when you start a new section in a longer text, you may need an entire transition paragraph. Transitioning to a new section involves summarizing the content of the previous section and expressing how the new one will build upon or depart from it.
For example, the following sentences might be an effective transition for a new section in a literary analysis essay.
Having established that the subjective experience of time is one of Mann’s key concerns in The Magic Mountain , it is now possible to explore how this theme facilitates the novel’s connection with World War I. The war itself is not narrated in the book, but rather hinted at as something awaiting Castorp beyond the final pages. In this way, Mann links his protagonist’s subjective experience of time to more than just his illness; it is also used to explore the period leading up to the outbreak of war.
As in academic writing generally, aim to be as concise as you can while maintaining clarity: If you can transition to a new section clearly with a single sentence, do so, but use more when necessary.
It’s also important to use effective transitions within each paragraph you write, leading the reader through your arguments efficiently and avoiding ambiguity.
The known-new contract
The order of information within each of your sentences is important to the cohesion of your text. The known-new contract , a useful writing concept, states that a new sentence should generally begin with some reference to information from the previous sentence, and then go on to connect it to new information.
In the following example, the second sentence doesn’t follow very clearly from the first. The connection only becomes clear when we reach the end.
By reordering the information in the second sentence so that it begins with a reference to the first, we can help the reader follow our argument more smoothly.
Note that the known-new contract is just a general guideline. Not every sentence needs to be structured this way, but it’s a useful technique if you’re struggling to make your sentences cohere.
Transition words and phrases
Using appropriate transition words helps show your reader connections within and between sentences. Transition words and phrases come in four main types:
- Additive transitions, which introduce new information or examples
- Adversative transitions, which signal a contrast or departure from the previous text
- Causal transitions, which are used to describe cause and effect
- Sequential transitions, which indicate a sequence
The table below gives a few examples for each type:
| Type | Example sentence | Transition words and phrases |
|---|---|---|
| Additive | We found that the mixture was effective. , it appeared to have additional effects we had not predicted. | furthermore, moreover, for example, in regard to x, similarly, in other words |
| Adversative | The novel does deal with the theme of family. , its central theme is more broadly political … | however, although, nevertheless, regardless, above all, (or) at least |
| Causal | Hitler failed to respond to the British ultimatum, France and the UK declared war on Germany. | because, therefore, consequently, if, provided that, so that, to |
| Sequential | This has historically had several consequences: , the conflict is not given the weight of other conflicts in historical narratives. , its causes are inadequately understood. , … | first, second, third, initially, subsequently, finally, lastly, to return/returning to x, as previously mentioned, in conclusion |
Grouping similar information
While transition words and phrases are essential, and every essay will contain at least some of them, it’s also important to avoid overusing them. One way to do this is by grouping similar information together so that fewer transitions are needed.
For example, the following text uses three transition words and jumps back and forth between ideas. This makes it repetitive and difficult to follow.
Rewriting it to group similar information allows us to use just one transition, making the text more concise and readable.
If you want to know more about AI tools , college essays , or fallacies make sure to check out some of our other articles with explanations and examples or go directly to our tools!
- Ad hominem fallacy
- Post hoc fallacy
- Appeal to authority fallacy
- False cause fallacy
- Sunk cost fallacy
College essays
- Choosing Essay Topic
- Write a College Essay
- Write a Diversity Essay
- College Essay Format & Structure
- Comparing and Contrasting in an Essay
(AI) Tools
- Grammar Checker
- Paraphrasing Tool
- Text Summarizer
- AI Detector
- Plagiarism Checker
- Citation Generator
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Caulfield, J. (2023, July 23). Transition Sentences | Tips & Examples for Clear Writing. Scribbr. Retrieved August 12, 2024, from https://www.scribbr.com/academic-essay/transition-sentences/
Is this article helpful?

Jack Caulfield
Other students also liked, transition words & phrases | list & examples, how to write topic sentences | 4 steps, examples & purpose, academic paragraph structure | step-by-step guide & examples, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
33 Transition Words and Phrases
Transitional terms give writers the opportunity to prepare readers for a new idea, connecting the previous sentence to the next one.
Many transitional words are nearly synonymous: words that broadly indicate that “this follows logically from the preceding” include accordingly, therefore, and consequently . Words that mean “in addition to” include moreover, besides, and further . Words that mean “contrary to what was just stated” include however, nevertheless , and nonetheless .
as a result : THEREFORE : CONSEQUENTLY
The executive’s flight was delayed and they accordingly arrived late.
in or by way of addition : FURTHERMORE
The mountain has many marked hiking trails; additionally, there are several unmarked trails that lead to the summit.
at a later or succeeding time : SUBSEQUENTLY, THEREAFTER
Afterward, she got a promotion.
even though : ALTHOUGH
She appeared as a guest star on the show, albeit briefly.
in spite of the fact that : even though —used when making a statement that differs from or contrasts with a statement you have just made
They are good friends, although they don't see each other very often.
in addition to what has been said : MOREOVER, FURTHERMORE
I can't go, and besides, I wouldn't go if I could.
as a result : in view of the foregoing : ACCORDINGLY
The words are often confused and are consequently misused.
in a contrasting or opposite way —used to introduce a statement that contrasts with a previous statement or presents a differing interpretation or possibility
Large objects appear to be closer. Conversely, small objects seem farther away.
used to introduce a statement that is somehow different from what has just been said
These problems are not as bad as they were. Even so, there is much more work to be done.
used as a stronger way to say "though" or "although"
I'm planning to go even though it may rain.
in addition : MOREOVER
I had some money to invest, and, further, I realized that the risk was small.
in addition to what precedes : BESIDES —used to introduce a statement that supports or adds to a previous statement
These findings seem plausible. Furthermore, several studies have confirmed them.
because of a preceding fact or premise : for this reason : THEREFORE
He was a newcomer and hence had no close friends here.
from this point on : starting now
She announced that henceforth she would be running the company.
in spite of that : on the other hand —used when you are saying something that is different from or contrasts with a previous statement
I'd like to go; however, I'd better not.
as something more : BESIDES —used for adding information to a statement
The city has the largest population in the country and in addition is a major shipping port.
all things considered : as a matter of fact —used when making a statement that adds to or strengthens a previous statement
He likes to have things his own way; indeed, he can be very stubborn.
for fear that —often used after an expression denoting fear or apprehension
He was concerned lest anyone think that he was guilty.
in addition : ALSO —often used to introduce a statement that adds to and is related to a previous statement
She is an acclaimed painter who is likewise a sculptor.
at or during the same time : in the meantime
You can set the table. Meanwhile, I'll start making dinner.
BESIDES, FURTHER : in addition to what has been said —used to introduce a statement that supports or adds to a previous statement
It probably wouldn't work. Moreover, it would be very expensive to try it.
in spite of that : HOWEVER
It was a predictable, but nevertheless funny, story.
in spite of what has just been said : NEVERTHELESS
The hike was difficult, but fun nonetheless.
without being prevented by (something) : despite—used to say that something happens or is true even though there is something that might prevent it from happening or being true
Notwithstanding their youth and inexperience, the team won the championship.
if not : or else
Finish your dinner. Otherwise, you won't get any dessert.
more correctly speaking —used to introduce a statement that corrects what you have just said
We can take the car, or rather, the van.
in spite of that —used to say that something happens or is true even though there is something that might prevent it from happening or being true
I tried again and still I failed.
by that : by that means
He signed the contract, thereby forfeiting his right to the property.
for that reason : because of that
This tablet is thin and light and therefore very convenient to carry around.
immediately after that
The committee reviewed the documents and thereupon decided to accept the proposal.
because of this or that : HENCE, CONSEQUENTLY
This detergent is highly concentrated and thus you will need to dilute it.
while on the contrary —used to make a statement that describes how two people, groups, etc., are different
Some of these species have flourished, whereas others have struggled.
NEVERTHELESS, HOWEVER —used to introduce a statement that adds something to a previous statement and usually contrasts with it in some way
It was pouring rain out, yet his clothes didn’t seem very wet.
Word of the Day
See Definitions and Examples »
Get Word of the Day daily email!
Games & Quizzes

Usage Notes
Prepositions, ending a sentence with, hypercorrections: are you making these 6 common mistakes, a comprehensive guide to forming compounds, can ‘criteria’ ever be singular, singular nonbinary ‘they’: is it ‘they are’ or ‘they is’, grammar & usage, more words you always have to look up, the difference between 'i.e.' and 'e.g.', more commonly misspelled words, plural and possessive names: a guide, commonly misspelled words, pilfer: how to play and win, 8 words with fascinating histories, flower etymologies for your spring garden, 8 words for lesser-known musical instruments, it's a scorcher words for the summer heat.
- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
- APA Citation Generator
- MLA Citation Generator
- Chicago Citation Generator
- Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources
Effective Transition Words for Research Papers
What are transition words in academic writing?
A transition is a change from one idea to another idea in writing or speaking and can be achieved using transition terms or phrases. These transitions are usually placed at the beginning of sentences, independent clauses, and paragraphs and thus establish a specific relationship between ideas or groups of ideas. Transitions are used to enhance cohesion in your paper and make its logical development clearer to readers.
Types of Transition Words
Transitions accomplish many different objectives. We can divide all transitions into four basic categories:
- Additive transitions signal to the reader that you are adding or referencing information
- Adversative transitions indicate conflict or disagreement between pieces of information
- Causal transitions point to consequences and show cause-and-effect relationships
- Sequential transitions clarify the order and sequence of information and the overall structure of the paper
Additive Transitions
These terms signal that new information is being added (between both sentences and paragraphs), introduce or highlight information, refer to something that was just mentioned, add a similar situation, or identify certain information as important.
| Adding Information | Also; Additionally; Furthermore; Moreover | In addition to; As well as; In fact; Not only…but also; As a matter of fact | “ , the data shows that X is a significant factor.”“ the above-mentioned study, Rogers also presents…” |
| Introducing/Highlighting | Particularly; Notably; Especially; Significantly | For example/instance; To illustrate; In particular; One example (of this is) | “ , only two species of this fish survive.”“ phenomenon is X.” |
| Referencing | Considering (this); Concerning (this); Regarding (this) | As for (this); The fact that; With regards to (this); On the subject of (this); Looking at (this information); With reference to (something) | “ the amount of research in this area, little evidence has been found.” “ the Blue Whale, its teeth are also the largest of any mammal.” |
| Showing Similarity | Similarly; Likewise; Equally; | By the same token; In the same way; In a similar way | “ the algorithm was applied to Y.”“ this principle can be applied to Z.” |
| Clarifying/Identifying Important Information | Specifically; Namely | That is (to say); In other words; (To) put (it) another way; What this means is; This means (that) | “There are two factors: , X and Y.”“ , the fall of the Empire was caused by over-expansion.” |
Adversative Transitions
These terms and phrases distinguish facts, arguments, and other information, whether by contrasting and showing differences; by conceding points or making counterarguments; by dismissing the importance of a fact or argument; or replacing and suggesting alternatives.
| Contrasting/ Showing conflict | But; Still; However; While; Whereas; Conversely; (and) yet | In contrast; On the contrary; On the other hand; …when in fact; By way of contrast | “ there is still more research needed.”“ the 1997 study does not recognize these outcomes.” |
| Distinguishing/ Emphasizing | Indeed; Besides; Significantly; Primarily | Even more; Above all; More/Most importantly | “ a placebo is essential to any pharmaceutical study.”“ the X enzyme increased.” |
| Conceding a point | Nevertheless; Nonetheless; Although; Despite (this); However; Regardless (of this); Admittedly | Even so; Even though; In spite of (this); Notwithstanding (this); Be that as it may | “ X is still an important factor.”“ New York still has a high standard of living.”“ this may be true, there are still other factors to consider.” |
| Dismissing an argument or assertion | Regardless (of) | Either way; In any case; In any event; Whatever happens; All the same; At any rate | “ of the result, this fact is true.”“ the effect is the same.”“ this will not change the public’s view.” |
| Replacing/ Indicating an Alternative | Instead (of); (or) rather; | (or) at least | “ using X, the scientists used Z.”“ why not implement a brand new policy?” |
Causal Transitions
These terms and phrases signal the reasons, conditions, purposes, circumstances, and cause-and-effect relationships. These transitions often come after an important point in the research paper has been established or to explore hypothetical relationships or circumstances.
| Showing Cause or Reason | Since; For; As; Because (of the fact that) | Due to (the fact that); For the reason that; Owing to (the fact); Inasmuch as | “ the original sample group was too small, researchers called for more participants.”“ funding will be cut in half.” |
| Explaining the Conditions | If…then; Unless; Granting (that); Granted (that); Provided (that) | In the event that; As/So long as; Only if | “ these conditions change, more will need to be done.”“ there is oxygen, there will be oxygenation.” |
| Showing the Effects/Results | Consequently; Therefore; Thus; Accordingly; Because (of this) | As a result (of this); For this reason; As a consequence; So much (so) that | “ we can conclude that this was an asymmetric catalysis.”“ many consumers began to demand safer products.” |
| Showing the Purpose | For the purpose(s) of; With (this fact) in mind; In the hope that; In order that/to; So as to | “ following standards, X rule was observed.”“ this study focused on preservation.” | |
| Highlighting the Importance of Circumstances | Otherwise | Under those circumstances; That being the case; In that case; If so; All else being equal | “ this effect will continue into the future.”“ the economic impact of this law seems positive.” |
Sequential Transitions
These transition terms and phrases organize your paper by numerical sequence; by showing continuation in thought or action; by referring to previously-mentioned information; by indicating digressions; and, finally, by concluding and summing up your paper. Sequential transitions are essential to creating structure and helping the reader understand the logical development through your paper’s methods, results, and analysis.
| Organizing by Number | Initially; Secondly; Thirdly; (First/Second/Third); Last | First of all; To start with; In the (first/second/third) place | “ subjects were asked to write their names.”“ dolphins are the smartest creatures in the sea.” |
| Showing Continuation | Subsequently; Previously; Afterwards; Eventually; Next; After (this) | “ subjects were taken to their rooms.”“ they were asked about their experiences.” | |
| Summarizing/ Repeating Information | (Once) again; Summarizing (this) | To repeat; As (was) stated before; As (was) mentioned earlier/above | “ this data, it becomes evident that there is a pattern.”“ pollution has become an increasing problem.” |
| Digression/Resumption | Incidentally; Coincidentally; Anyway | By the way; to resume; Returning to the subject; At any rate | “ the methods used in the two studies were similar.”“ this section will analyze the results.” |
| Concluding/ Summarizing | Thus; Hence; Ultimately; Finally; Therefore; Altogether; Overall; Consequently | To conclude; As a final point; In conclusion; Given these points; In summary; To sum up | “ these results will be valuable to the study of X.”“ there are three things to keep in mind—A, B, and C.” |
How to Choose Transitions in Academic Writing
Transitions are commonplace elements in writing, but they are also powerful tools that can be abused or misapplied if one isn’t careful. Here are some ways to ensure you are using transitions effectively.
- Check for overused, awkward, or absent transitions during the paper editing process. Don’t spend too much time trying to find the “perfect” transition while writing the paper.
- When you find a suitable place where a transition could connect ideas, establish relationships, and make it easier for the reader to understand your point, use the list to find a suitable transition term or phrase.
- Similarly, if you have repeated some terms again and again, find a substitute transition from the list and use that instead. This will help vary your writing and enhance the communication of ideas.
- Read the beginning of each paragraph. Did you include a transition? If not, look at the information in that paragraph and the preceding paragraph and ask yourself: “How does this information connect?” Then locate the best transition from the list.
- Check the structure of your paper—are your ideas clearly laid out in order? You should be able to locate sequence terms such as “first,” “second,” “following this,” “another,” “in addition,” “finally,” “in conclusion,” etc. These terms will help outline your paper for the reader.
For more helpful information on academic writing and the journal publication process, visit Wordvice’s Academic Resources Page. And be sure to check out Wordvice’s professional English editing services if you are looking for paper editing and proofreading after composing your academic document.
Wordvice Tools
- Wordvice APA Citation Generator
- Wordvice MLA Citation Generator
- Wordvice Chicago Citation Generator
- Wordvice Vancouver Citation Generator
- Wordvice Plagiarism Checker
- Editing & Proofreading Guide
Wordvice Resources
- How to Write the Best Journal Submissions Cover Letter
- 100+ Strong Verbs That Will Make Your Research Writing Amazing
- How to Write an Abstract
- Which Tense to Use in Your Abstract
- Active and Passive Voice in Research Papers
- Common Phrases Used in Academic Writing
Other Resources Around the Web
- MSU Writing Center. Transition Words.
- UW-Madison Writing Center. Transition Words and Phrases.
54 Best Transition Words for Paragraphs

Chris Drew (PhD)
Dr. Chris Drew is the founder of the Helpful Professor. He holds a PhD in education and has published over 20 articles in scholarly journals. He is the former editor of the Journal of Learning Development in Higher Education. [Image Descriptor: Photo of Chris]
Learn about our Editorial Process

Good transition words for starting a paragraph include addition phrases like ‘furthermore’, cause and effect words like ‘consequently’, and contradiction words like ‘however’. Scroll down for a full table of transition words.
Using transition words in your writing can help you improve the readability and flow of your paragraph to the next.
These words help your text flow seamlessly into the next idea, which shows your readers the relationship between paragraphs and phrases.
List of Transition Words for Starting a Paragraph
Transition words can fall into more than one category based on what type of transition in your paragraph you’re planning to make.
For example, you’d want a different transition word if your second paragraph contradicts your first than if it supports it. Take the following examples:
| Second body paragraph statement in the first body paragraph | Furthermore, What’s more, Similarly, Supporting evidence finds, Likewise. |
| Second body paragraph statement in the first body paragraph | However, Nevertheless, Contradictory evidence finds, Despite the above points. |
Here is a list of transition words and what category they fall under.
- Addition – A transition that combines two or more ideas and shows their relationship. Examples include, what’s more, equally important, again, also, and, furthermore, moreover, besides .
- Cause and Effect – When one idea triggers another. This lets the reader know that they are directly connected. Examples include, consequently, hence, therefore, thus, next, as a result .
- Clarification – This is to rephrase what was said to clarify a statement and provide emphasis. Examples include, in other words, that is to say, to clarify.
- Compare and Contrast – This shows a relationship between two ideas that are compared based on differences or similarities. Examples are, after all, although this may be true, in contrast, likewise, on the contrary, similarly, whereas, yet.
- Emphasis (Boosting) – This shows certainty. Examples include, emphatically, in fact, surprisingly, undeniably, in any case, indeed, never, without a doubt.
- Providing examples : For example, for instance, as illustrated by, take the following case in point.
- Exception or Contradiction – This happens when an action with a pre-conceived notion ends with a different action. Examples are, however, nevertheless, in spite of, of course, once in a while, despite.
- Summarize or conclude – This signals the reader that they are at the end of the paragraph. Examples are, as this essay has shown, as a result, In conclusion, therefore, thus, hence, in short, in brief.
- Sequential – This expresses a numerical sequence, conclusion, continuation, resumption, or summation. Examples are to change the topic, to conclude with, afterward, incidentally, by the way, initially.
List of Transition Words for New Paragraphs
| Emphatically, In fact, Surprisingly, Undeniably, Without a doubt, Indeed, Of course, Surely, Undoubtedly, Without a doubt. | |
| Furthermore, Moreover, Supporting the above points, Similar research has found, In fact ( ). | |
| To demonstrate, Evidence of this fact can be seen in, Proof of this point is found in, For instance, Compelling evidence shows, For a case in point, In fact, Notably, One study found, Supporting evidence shows. ( ). | |
| Consequently, Hence, Therefore, Thus, As a result, accordingly, The consequence is. | |
| In other words, That is to say, To clarify, For example, More evidence can be found, Furthermore. | |
| However, However, Conversely, Despite this, In spite of the above statements, Nonetheless, Nevertheless, A contradictory argument, Regardless. | |
| As this essay has shown, In conclusion, To summarize, The balance of evidence finds, The research compellingly indicates | |
| Firstly, Secondly, Thirdly, Subsequently, Next, Afterwards, Later, Consequently. |
Transition Words to Avoid
I recommend avoiding the following transition words:
| Your teacher may write: “If you mentioned this before, why are you saying it again?” | |
| This is a cliché transition word for beginning conclusion paragraphs. Instead, try using the callback method discussed in my . | |
| Too colloquial. Try using more formal language such as: “The weight of evidence finds…” | |
| Many teachers don’t like first person language in essays. Use third person language and back claims up with academic research rather than personal opinion (except if it’s a reflective piece). | |
| Teachers like to pick at you if you talk in generalizations. Instead, hedge your statements by saying “Sometimes”, “Often”, or “The majority of” and back this up with references. |
Examples in Sentences
The best way to understand transition words is to provide examples. Let’s look at this sentence:
“Amy did not study for her test. Therefore, she did not get a good result.”
When you see the word ‘therefore,’ the reader knows that this is a cause and effect. What happened in the first sentence caused a resulting action.
The transition word provided a seamless flow into the next sentence that describes this effect.
Using the transitional word, ‘therefore,’ shows that the two sentences are part of one idea/process. Even with skimming, the reader can guess what’s the resulting action. This is how transition words hold your ideas together. Without them, it’s like your piece is just a jumble of coherent words.
Transition words don’t have to be placed at the start of a sentence. Let’s look at this sentence:
“Many people came to the event. Cristine, Emily, and David, for instance.”
In this sentence, ‘for instance’ is at the end of the sentence. However, it still gives the reader the necessary information to see how the two sentences are linked.
What are Transition Words?
Transition words for beginning paragraphs help writers to introduce a shift, opposition, contrast, agreement, emphasis, purpose, result, or conclusion from what was previously written. They are essential in argumentative essays.
Transition words are like bridges between the different paragraphs in your pieces. They serve as the cues that help your reader understand your ideas. They carry your ideas from one sentence to the next and one paragraph to the next.
Transitional words and phrases link an idea from a sentence to the following paragraph, so your work is read smoothly without abrupt jumps or sudden breaks between concepts.
Why use Transition Words
Proper communication of your ideas through paragraphs is important in writing. In order for your reader to read your piece with a thorough understanding of each idea and point conveyed in the piece, you have to use transition words and phrases.
With the examples provided, you would see that transitions string together your ideas by establishing a clear connection between the sentences and paragraphs.
Without transition words, your work may seem daunting and stressful to read, and the reader will not understand the idea you’re trying to convey.
Transitional phrases are especially important when writing an essay or thesis statement , as each paragraph has to connect ideas effortlessly.
Therefore, when a paragraph ends, the next idea must have some link to the previous one, which is why transition words play an important role.
Where Else to use Transition Words in an Essay
Transition words are important English devices for essays and papers. They enhance the transitions and connections between the sentences and paragraphs, giving your essay a flowing structure and logical thought.
Transition terms may seem easy to remember; however, placing them in the incorrect manner can cause your essay to fall flat.
Here are some places where essays transition words may fit:
- To show a connection between evidence and the ending
- To flow into the next paragraph, use your closing statement at the conclusion of each one
- At the start of the first body paragraph
- At the start of the second body paragraph
- In some of the starting sections of your summary or introductory paragraphs
- In an overview of your opinions/solutions in the conclusion
When adding your transition words and phrases in your essay, make sure not to accidentally form an incomplete or fragmented sentence. This is common with transitions, such as, if, although, and since .
While transition words are important in any writing piece, you have to make sure that the word or phrase you choose matches the logic of the paragraph or point you’re making. Use these words and phrases in moderation, as too much of them can also heavily bring the quality of your work down.

- Chris Drew (PhD) https://helpfulprofessor.com/author/chris-drew-phd-2/ 25 Number Games for Kids (Free and Easy)
- Chris Drew (PhD) https://helpfulprofessor.com/author/chris-drew-phd-2/ 25 Word Games for Kids (Free and Easy)
- Chris Drew (PhD) https://helpfulprofessor.com/author/chris-drew-phd-2/ 25 Outdoor Games for Kids
- Chris Drew (PhD) https://helpfulprofessor.com/author/chris-drew-phd-2/ 50 Incentives to Give to Students
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *

Conclusion transition words: Phrases for summarizing and ending
Transition words help us structure our thoughts and guide the reader or listener through what we are saying. When it’s time to summarize your message or end a paragraph, conclusion transition words let you signal this closing.
It’s good to know some synonyms for ‘in conclusion’ and ‘to conclude’, because although these are good examples of concluding words, they can get repetitive.
Our comprehensive list of transition words for conclusion and summary should give you all the inspiration you need, whether you are writing an essay or speech, or just want to become more confident forming an argument. These signal words can also be helpful for restating ideas, drawing attention to key points as you conclude.
We have included plenty of examples of how you can use these transition words for concluding paragraphs or sentences, so by the end of this article, you should be clear on how to use them properly.

Conclusion transition words with examples
We have grouped these summarizing and concluding transition words according to how and where they can be used. For example, some should only be used when forming a final conclusion, whereas others can be used to summarize sections mid-way through your speech or writing.
First, let’s be clear about the difference between a summary and a conclusion .
Summary vs conclusion
A conclusion comes at the end of a speech, chapter, or piece of text, and it brings together all of the points mentioned. A summary, however, can be placed anywhere (even at the beginning). A summary gives a brief outline of the main points but is not as in-depth as a conclusion.
If you are giving a presentation or writing a blog, you may wish to summarize the main points in your introduction so that people know what you are going to cover. You could also summarize a section part-way through before moving on to another angle or topic.
In contrast, the conclusion always comes at the end, and you should only use specific conclusion transition words as you are drawing to a close.
Transition words for conclusion paragraphs
Let’s begin with some discourse markers that signal you are moving to the concluding paragraph in your presentation, speech, essay, or paper. These can all be used to start a conclusion paragraph.
- In conclusion
- To conclude
- We can conclude that
- Given these points
- In the final analysis
- As can be seen
- In the long run
- When all is said and done
- I’ll end by
- As we draw to a close
The last three on this list, the ‘closing’ transition words, would generally only be used in spoken discourse.
Some transition words for order and sequencing should also help with structuring what you want to say, including the ending.
Example conclusion sentences
The following sentences show how to use conclusion words correctly:
- In conclusion , we can say that plan A will be of greater benefit to the company.
- When all is said and done , it’s clear that we should steer clear of this investment strategy.
- Given these points , I believe the trial was a great success.
- I’ll end by reminding you all that this experiment was just the beginning of a much larger project.
- To wrap up , let’s look at how this learning can be applied.
- In the long run , we will make more profit by investing heavily in new machinery.
- Having analyzed seven of our competitors in detail, we can conclude that our content marketing strategy should be updated.
Transition words for summary
The following summary transition words may be used as part of a conclusion paragraph, but they are especially helpful for concisely drawing together several points.
- To summarize
- On the whole
- Generally speaking
- All things considered
- In a nutshell (informal)
- In any case
Note that although you can insert summary transition words anywhere, the specific phrases ‘In summary’, ‘To summarize’ and ‘To sum up’ are generally only used at the end, similar to conclusion phrases.

Example summary sentences
- In brief , this presentation is going to cover the pros and cons of the device and how we can apply this to our own product development.
- This new technology is, in a word , revolutionary.
- All things considered , we found that Berlin was a great city for a weekend break.
- To summarize , we can say that Shakespeare’s writing continues to have a global influence.
- We can say that the combustion engine was, on the whole , a good invention.
- In any case , we should put the necessary precautions in place.
- Generally speaking , girls are more thoughtful than boys.
Transition words to end a paragraph
You may wish to add ending transition words in the final sentence of a paragraph to conclude the ideas in that section of text, before moving on to another point.
Here are some transition words to conclude a paragraph:
- This means that
- With this in mind
- By and large
- For the most part
Note that some of these could equally be used to begin a new paragraph, so long as that paragraph is summarizing the points previously mentioned.
Cause and effect transition words could also be helpful in this context.
Examples of transition words for the end of a paragraph
- Jamie is a vegan and Sheryl has a lot of allergies. This means that we should be careful which restaurant we choose.
- The weather forecast said it would rain this afternoon. With this in mind , should we postpone our hike?
- Each of the students has their own opinion about where to go for the field trip. Ultimately , though, it’s the teacher who will decide.
Restating points as you conclude
Conclusion transition words can also signal that you are restating a point you mentioned earlier. This is common practice in both writing and speaking as it draws the reader or listener’s attention back to something you want them to keep in mind. These are, therefore, also examples of transition words for emphasizing a point .
Here are some helpful transition words for concluding or summarizing by restating points:
- As mentioned previously
- As stated earlier
- As has been noted
- As shown above
- As I have said
- As I have mentioned
- As we have seen
- As has been demonstrated
You may switch most of these between the passive and active voice, depending on which is most appropriate. For example, ‘As has been demonstrated’ could become ‘As I have demonstrated’ and ‘As shown above’ could become ‘As I have shown’.
Example sentences to restate a point in conclusion or summary
- As I stated earlier , the only way we can get meaningful results from this survey is by including at least a thousand people.
- As has been demonstrated throughout this conference, there are exciting things happening in the world of neuroscience.
- As shown by this study, the trials have been promising.
If you were researching these transition words for concluding an essay, you might find it helpful to read this guide to strong essay conclusions . Of course, there are many ways to use summary transition words beyond essays. They may be a little formal for casual conversation, but they certainly can be used in speech as part of a presentation, debate, or argument.
Can you think of any other concluding words or phrases that should be on this list? Leave a comment below to share them!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and site URL in my browser for next time I post a comment.
Sign me up for the newsletter!
50 Transitional Phrases for Conclusions(+ Examples You Can Use)
When writing a conclusion, you want to ensure that your final thoughts are clear and concise. Using transitional phrases can help you achieve this by linking your ideas together and making your writing flow smoothly.
There are various types of transitional phrases that can be used in a conclusion. Some examples include:
Transitional Phrases for Conclusions
When writing an essay or a speech, it is important to use transitional phrases to signal that you are reaching the end of your argument or presentation. These phrases help to summarize your main points and prepare your audience for the conclusion.
These phrases can be used to signal that you are about to wrap up your argument or presentation. They help to guide your audience to your final thoughts and summarize the main points you have made throughout your work.
In addition to these phrases, you can also use other techniques to signal the end of your work. For example, you can use a rhetorical question to provoke thought or a call to action to encourage your audience to take action based on your argument.
50 Transition Phrases for Conclusions(+Examples)
Transitional phrases for adding information.
When writing a conclusion, it is important to add information that supports your thesis statement. Transitional phrases can help you do this by linking your ideas together and making your writing more coherent. Here are some transitional phrases that you can use to add information to your conclusion:
Transitional Phrases for Comparing and Contrasting
Using transitional phrases can help you compare and contrast different ideas in a clear and concise manner. By using these phrases, you can guide the reader through your thought process and make your writing more engaging and informative.
Transitional Phrases for Cause and Effect
When writing an article or essay, it is important to use transitional phrases to link ideas and concepts. One of the most commonly used types of transitional phrases is the cause-and-effect transitional phrase. These phrases help to connect two events or actions and describe how one event or action led to another.
Another commonly used transitional phrase for cause and effect is “as a result.” For example, “The company experienced a loss of profits this quarter. As a result, they are considering cutting back on expenses.” Here, the effect of the loss of profits is the company’s decision to cut back on expenses.
In addition to “cause” and “result,” other transitional phrases that can be used to indicate cause and effect include “consequently,” “hence,” and “thus.” These transitional phrases are useful for indicating the relationship between two events or actions.
Transitional Phrases for Time and Sequence
Using these transitional phrases for time and sequence can help you structure your writing in a clear and organized way. For example, if you are writing a process essay, you can use these phrases to describe each step of the process in a logical order.
Transitional Phrases for Concluding Thoughts
When writing an essay or a speech, it is essential to conclude your thoughts in a clear and concise manner. Transitional phrases can help you achieve this by linking your ideas together and providing a smooth transition to your conclusion. Here are some transitional phrases that you can use for concluding thoughts:
Practical Examples of Transitional Phrases
When it comes to writing conclusions, transitional phrases can help you effectively summarize your main points and leave a lasting impression on your reader. Here are some practical examples of transitional phrases that you can use to make your writing more cohesive and engaging:
Related posts:
Home > Blog > Best Transition Words for Essays (With Examples)

Best Transition Words for Essays (With Examples)
- Smodin Editorial Team
- Updated: August 13, 2024
- General Guide About Content and Writing
Most essays require you to discuss more than one idea. However, transitioning into a new idea abruptly can be sudden and jarring for the reader. This is where transition words come in. Even if writing essays isn’t your strong suit , it’s pretty easy to learn how to use transition words and phrases.
So, what are transition words? They are words and phrases that show a link between two elements. They might show that you’re pivoting to a new topic, introducing new evidence, or summing up your points. They can even link paragraphs together with ease and improve your writing flow . Sprinkling these throughout your essay helps the reader understand your argument more clearly.
Essentially, transition words for essays are crucial to improve the flow of your writing. But you still need to know how to wield them effectively if you want top marks.

How to Use Transition Words in Essays
A transition word signals a change in your writing. You use them as part of transition sentences, which contain either two opposing topics or connect similar ideas.
You don’t need transition words in every sentence. Just re-read our introduction–not every sentence has one. Not even every paragraph has them! But including them throughout your work can really help the reader understand where they’re at in the text.
Consider this example:
- The experiment was successful. We decided to conduct further research.
- The experiment was successful. Therefore, we decided to conduct further research.
The second sentence highlights the connection between these two ideas. The connection could be contrasting ideas, similar ones, or a sequence. As a result, the reader understands a more logical flow within the text. Although the first sentence is still grammatically correct, it’s a little jarring.
And this is a crucial thing to note about transition words. Sometimes, the connection is obvious. Words and phrases like “however” and “for example” appear completely logically in the text. In the same way, sequential transition words like “first”, “next,” “last”, and so on are obvious choices. However, other transition words are less obvious–their primary goal is just to improve flow. They keep your writing organized.
Most transition words and phrases appear at the beginning of sentences, paragraphs, or clauses. Although, they can sometimes appear in other places, too.
It’s also worth noting that you need to choose the right transition word. Let’s explore some examples below.

Examples of Transition Words for Essays
Depending on the essay you’re writing, you might find that it calls for different transition words. Some transition words and phrases introduce new content ideas , while others highlight that two ideas are equally important.
In fact, you might need several of these types in any academic writing you do. Let’s explore eight types of transition words that you might need.
Transition Words for Contradictions
In many essays, you’ll need to weigh up an argument with a counter-argument. There are individual words and phrases that can highlight this contrast, moving from one idea to its opposing one.
Contradiction words include:
- On the other hand
- Nevertheless
- On the contrary
You can say something like:
- Conversely, an alternative method proved effective.
- However, this approach has its limitations.
This is great for analytical essays where you’re including a comparison.
Transition Words for Additions
Most essays require you to demonstrate your reasoning. As a result, you need to illustrate the evidence you have for a particular point. You might even need more than one point.
There are several transition words for this purpose:
- Additionally
- Furthermore
- In addition
You could say:
- Additionally, he brings a positive attitude alongside his skills.
- Furthermore, the research supports this conclusion
Both of these sentences can help you add onto your previous statement, hammering your idea home. Often, you can use these words to show that two ideas are equally important, too. It provides a simple addition, rather than making one seem more important than the other.
Transition Words for Introducing Examples
In the same way, there are other transition words that you can use specifically for examples. These are great (and easy) ways to introduce your evidence. They include:
- For example
- For instance
Your sentence might look like this:
- For example, the evidence illustrates that…
- Namely, adding elements like humor can engage readers.
It’s a good idea to write down a list of these that you can use throughout your essay. That way, you’re not using the same transition words throughout.
Transition Words for Conditions and Cause and Effect
Sometimes, a transition word can denote causality between two things. This is like the example we saw before, using “therefore.” These transition words are not always as obvious, but they can vastly improve the flow of your writing.
They include:
- Consequently
- As a result
If you’re writing a history essay or trying to show a consequence, these transition words are your best friends. Think of these example sentences:
- Therefore, it’s crucial to follow guidelines.
- Hence, the project was delayed.
Again, try writing these down to include throughout your essay.
Transition Words for Extra Clarification
The right transition word can also help you clarify your points or add emphasis. Often, it just takes a single word to change the meaning of your sentence and add some emphasis. For example:
- Undoubtedly
Often, these words and phrases are great for stressing the importance of a point you just made. For instance, you could say:
- Indeed, the findings are significant.
- Clearly, this approach is more effective.
Be careful how you use these transition words, though. Depending on how convincing your argument is, your reader might draw a different conclusion. So, don’t use words like “undoubtedly” unless you’re really sure!
Transition Words for Summarizing
At the end of your essay, you need to provide a summary of all your points. Launching straight into the conclusion can be sudden, which is why you need a transition word to announce the summary. Try these:
- In conclusion
- To summarize
- All things considered
- To conclude
All of these words are great for introducing the final paragraph. They show the reader that you’re about to recap your key points. You’ll often see sentences like this:
- In conclusion, the study supports the hypothesis.
- To summarize, the benefits outweigh the costs.
Adding these words or phrases is a great way to introduce your final analysis.
Transition Words for Time Relations
Whenever you read a recipe, you’ll find transitions that show time relationships. This is because recipes come in steps, where each point follows immediately after the previous. However, there are also other ways to use these transition words. First, let’s look at some of the options:
- Subsequently
Here are two examples of these words in use, one from a step-by-step and another from an essay:
- First, gather all the necessary materials.
- Subsequently, the researchers analyzed the data.
As you can see in the second example, you can use these transitions in analysis, too.
Transition Words for Series and Sequences
Sequential transition words are very similar to what we discussed previously. In fact, there’s some overlap. However, these words appear in an exact sequence:
- In the first place
- In the second place
And so on. You can use each one to illustrate where you are in a specific process. For example:
- First, we need to address the main issue.
- In the first place, the policy needs revision.
Again, you can use these for sequential events like recipes, but also to lead the reader through your argument. Remember, every essay should have a beginning, middle, and end. So, you can use transition words like this to signpost where you are in your argument.

How Smodin’s AI Writer Can Help with Transition Words
Still struggling with transition words and phrases? There’s a tool for that! Smodin’s AI writer can help you generate content in line with your ideas. It’s an advanced essay writing tool that writes academic papers to a high standard. And it seamlessly integrates transition words! By using a variety of single words and phrases, Smodin creates engaging text with a great flow.
Here’s an example:
- Without Smodin’s AI Writer: “Climate change is a pressing issue. Many countries are implementing green policies.”
- With Smodin’s AI Writer: “Climate change is a pressing issue; therefore, many countries are implementing green policies.”
And, Smodin’s AI writer meets rigorous academic standards. Compared to other generative AI tools, Smodin is far better for academic work. It has a much more specific database of high-quality academic work, allowing it to write texts of a similar grade.
This ensures that the transition words and phrases used are appropriate for formal writing. The tool understands the nuances of academic language, suggesting transitions that elevate the quality of your essay.
Key Features of Smodin’s AI Writer
Smodin has several key features that can help you create essays of high standard, such as:
- Enhanced Flow and Coherence: The AI suggests transition words that enhance the readability and logical flow of your essay.
- Contextual Suggestions: Based on the context of your writing, the AI provides suitable transition words and phrases.
- Tailored to Academic Standards: Smodin’s AI ensures that the transitions used are appropriate for academic writing, adhering to formal standards.
So what are you waiting for? See how Smodin’s AI writer can effortlessly enhance your essays through both structure and flow. With Smodin, you can get higher grades and a better understanding of your work.

Use the Best Transition Words for Essays
Using transition words in essays is crucial for creating a smooth and coherent flow of ideas. These words and phrases are the bridges that guide your readers through your arguments. Without them, it’s harder to understand the connections between different points. So, effective use of transition words not only improves the readability of your essay but also strengthens your overall argument.
Smodin’s AI Writer is an invaluable tool for incorporating transition words seamlessly into your essay. This advanced tool suggests contextually appropriate transitions, ensuring your essay flows logically and meets high academic standards.
Don’t let the challenge of finding the right transition words hinder your writing. Explore Smodin’s AI Writer to elevate your essay writing skills and produce top-quality academic papers. Perhaps you’re a student aiming for higher grades or a writer seeking to improve the flow of your work. Smodin’s AI Writer is here to help. Try Smodin today and experience the difference in your writing!
Transition Words Worksheets and Exercises with Answers
Transition words Worksheets are essential for improving writing skills. These resources help students understand how to use transition words effectively. By practicing with worksheets, learners can learn how to connect their ideas smoothly. Transition words are words or phrases that help readers understand the relationship between sentences and paragraphs. Using transition words makes writing clearer and easier to follow. These worksheets provide various exercises to practice using transition words in different contexts. Teachers find transition words Worksheets helpful for teaching students how to write better. With these worksheets, students can improve their writing by learning to use transition words correctly.
Table of Contents
Transition words Worksheets
Transition words worksheet 1.
Fill in the blanks using Transition Words.
- She wanted to go outside; ______ it was raining heavily.
- He enjoyed soccer; ______ he excelled in other sports.
- They planned a picnic; ______ it started to thunderstorm.
- Sarah loves mystery novels; ______ she often stays up late.
- The bakery had pastries; ______ we tried a few.
- The movie got mixed reviews; ______ it got awards.
- John wanted a car; ______ he couldn’t decide.
- He turned off the lights______ everyone could sleep peacefully.
- She enjoys outdoor activities; ______ , hiking, biking, etc.
- She took a nap; ______ heard a loud noise.
- Peter hikes; ______ he connects with nature.
- They missed the bus; ______ they walked to school.
- The road was closed; ______ we took another route.
- The instructions were clear; ______ we finished efficiently.
- The sun shone brightly; ______ we went swimming.
- The store closed; ______ we found another.
- She loves chocolate; ______ she carries a bar.
- They were exhausted; ______ they had far to go.
- He felt better; ______ he enjoyed his vacation.
- The cat scratched; ______ it needed a post.
Scroll down to See Answers

Transition words Worksheet 2
Choose the Right Option.
- Which transition word indicates a contrast between ideas:
a) Consequently b) However c) Furthermore d) Additionally
- To provide an example or illustration, one might use the transition word:
a) Nevertheless b) For instance c) In contrast d) Therefore
- ______, we can conclude that climate change is a pressing issue.
a) Nonetheless b) In addition c) Hence d) Although
- Sarah loves reading; ______, she often spends hours in the library.
a) Consequently b) Similarly c) Therefore d) Despite
- ______, the company’s profits have declined this quarter.
a) In summary b) Nonetheless c) Consequently d) Furthermore
- Alex enjoys swimming; _______, he dislikes diving.
a) In contrast b) Similarly c) Additionally d) Therefore
- Jane studied hard; _______, she aced her exams.
a) Nonetheless b) Hence c) Furthermore d) Consequently
- The weather was chilly; _______, we decided to stay indoors.
a) Therefore b) Consequently c) In contrast d) Nevertheless
- Tom is allergic to cats; ______, he owns three of them.
a) Nonetheless b) Additionally c) Hence d) In contrast
- ______ the rain, the picnic was still enjoyable.
a) Despite b) Furthermore c) Consequently d) Similarly
- Julia likes tea; _______, she prefers coffee.
a) Therefore b) Nevertheless c) Consequently d) In addition
- ______, it is important to consider the environmental impact.
a) Conversely b) Moreover c) Hence d) Despite
- Mark studied diligently; _______, he failed the exam.
a) Consequently b) Nevertheless c) Similarly d) Moreover
- The team worked hard; _______, they lost the match.
a) However b) Therefore c) In contrast d) Consequently
- ______, the book was insightful and thought-provoking.
a) Additionally b) Therefore c) Nonetheless d) Consequently
- ______, she arrived early for the meeting.
a) Furthermore b) Despite c) Consequently d) Nevertheless
- The results were inconclusive; _______, further research is needed.
a) Consequently b) Nevertheless c) Similarly d) Therefore
- Max loves hiking; _______, he dislikes camping.
a) Nevertheless b) Additionally c) Hence d) Furthermore
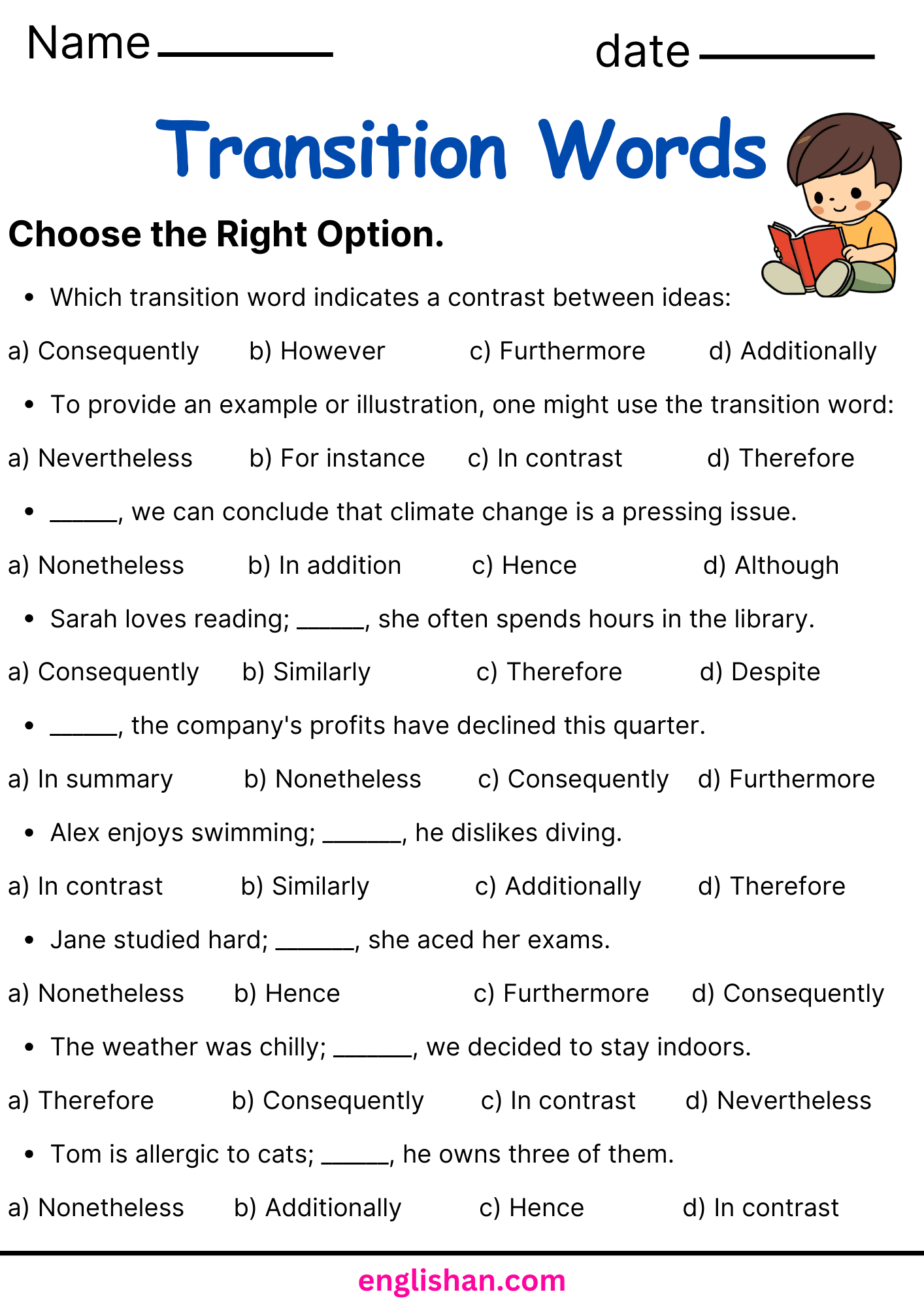
Transition words Worksheet 3
Underline the Transition Words.
- John wanted to go to the party; however, he had too much work.
- Sarah loves to travel; moreover, she enjoys exploring new cultures.
- Despite the forecast of rain, we postponed our outdoor picnic.
- The exam was challenging; nonetheless, I passed.
- The weather was beautiful, but the wind made it chilly.
- Mark studied hard; consequently, he achieved the highest score.
- The team worked tirelessly; hence, they celebrated their success.
- The restaurant had great food; however, the service lacked.
- Sarah wanted the latest smartphone; unfortunately, she couldn’t afford it.
- The movie received mixed reviews; nevertheless, it was a box office hit.
Scroll down to See Answers
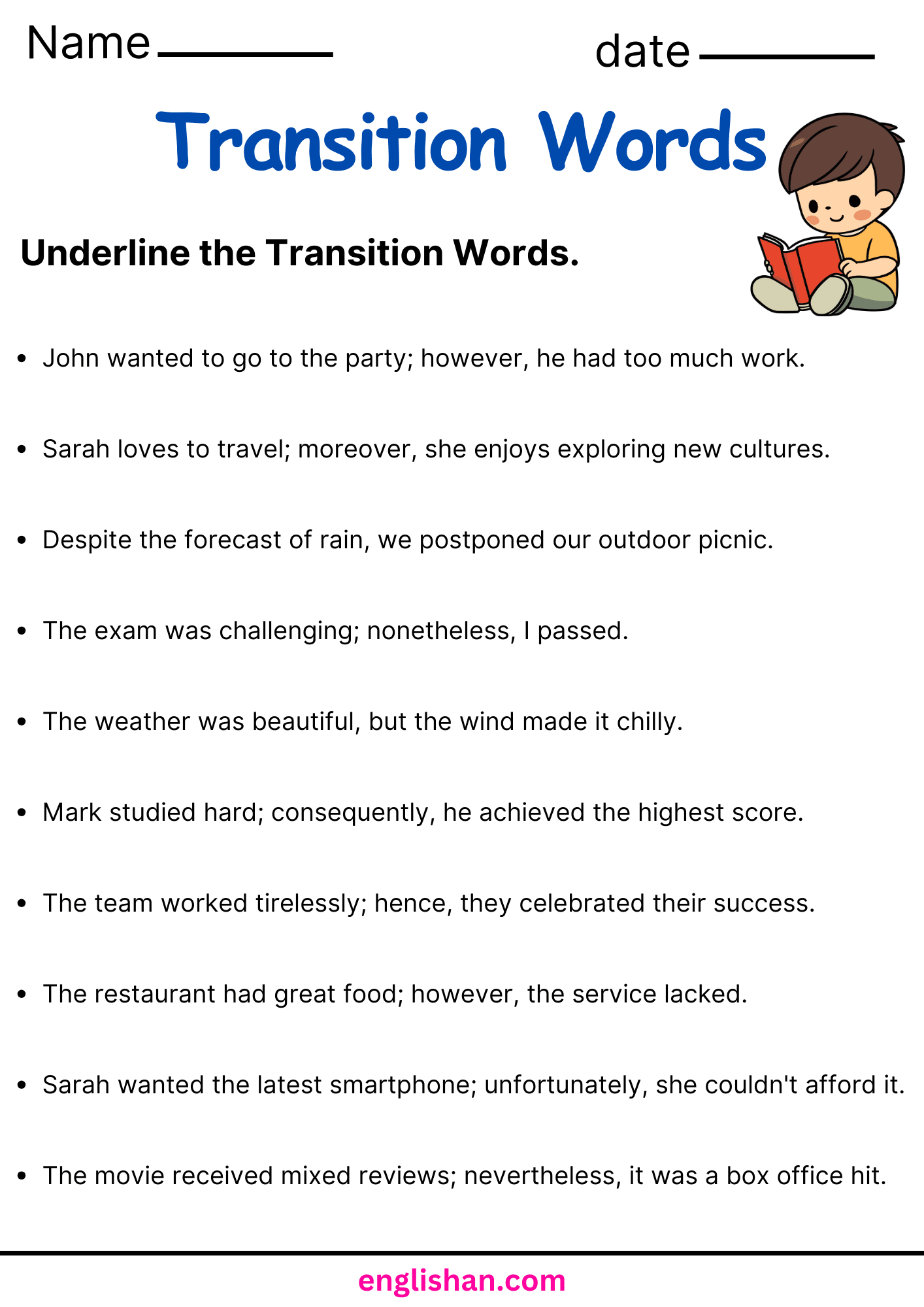
Answer of Worksheet 1:
- unfortunately
- consequently
- nonetheless
- for example
- nevertheless
- undoubtedly
Answer of Worksheet 2:
- b) For instance
- b) Similarly
- c) Consequently
- a) In contrast
- d) Consequently
- b) Consequently
- a) Nonetheless
- b) Nevertheless
- b) Moreover
- a) Consequently
- a) Additionally
- a) Furthermore
- a) Nevertheless
Answer of Worksheet 3:
- Consequently
- Nevertheless
You May Also Like:
- Transition Words for Essays with Examples
- Present Perfect Continuous Worksheets
- Future Perfect Continuous Worksheets
- Synonyms Worksheets and Exercises
- In On At Worksheets
25 Fill in the blanks using Transition Word Worksheets 25 Sentences using Transition Word Worksheets Identify the Transition Word Transition Word Mcqs Worksheets Transition Word Worksheets Transition Words Exercises Transition Words Exercises with Answers Transitional Words Worksheets Underline the Transition Words Worksheets for Transitional Word

Linking Verbs Worksheets and Exercises with Answers

Demonstrative Pronouns | Definition and Examples
Copyright © 2024 by englishan
Username or Email Address
Remember Me
Forgot password?
Enter your account data and we will send you a link to reset your password.
Your password reset link appears to be invalid or expired.
Privacy policy.
To use social login you have to agree with the storage and handling of your data by this website. %privacy_policy%
Add to Collection
Public collection title
Private collection title
No Collections
Here you'll find all collections you've created before.
More From Forbes
The future of multifunctional devices in our everyday lives.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Achin Bhowmik, Ph.D., Chief Technology Officer and Executive Vice President of Engineering at Starkey and adjunct professor at Stanford.
“The most profound technologies are those that disappear. They weave themselves into the fabric of everyday life until they are indistinguishable from it.”
This quote is from Mark Weiser , a pioneer proponent of ubiquitous computing. It perfectly captures the goal of technological advancements—to become a part of our lives to the point where we don’t notice them.
Take electricity, for example. Where there used to be visible infrastructure and numerous cables and wires throughout a building to provide power in the early days, now all you need to do is plug a cord into an outlet in the wall, and it’s on. The infrastructure disappeared in the background, as electricity became part of our everyday lives and completely changed civilization. We use it to light and heat our homes, cook, clean, work, manufacture, transport, and so much more. Can you imagine life without it?
Connectivity to the internet is a more recent example. Two decades ago, many of us were still using dial-up or ethernet cables. Today, you can access Wi-Fi in cafes, airports, hotel lobbies and even entire cities. The same is true for cellular connectivity, which has become even more pervasive. The accessibility to information has never been easier in human history. The technology infrastructure has expanded so rapidly that being able to call or text someone or look up information is becoming increasingly ubiquitous.
The Transition To Multifunctional Devices
The devices that we use to access and use this information are also having a greater impact as they become multifunctional while getting smaller to carry or wear and easier to use. From mainframe computers for scientific research, government or industrial work, to desktop computers for home recordkeeping, emails and financials, access to computing was limited to being tethered to a location in the early days. But as nearly every home grew to have a desktop computer, many individuals adopted personal, portable notebooks or laptop computers. As we connected to Wi-Fi, the realm of functions for our computers opened up to browsing the internet, streaming movies, processing photos and videos, performing work remotely and so much more.
Smartphones have become a supreme example of a multifunctional device. When Steve Jobs introduced the iPhone in 2007, he said he was introducing a widescreen iPod with touch controls, a revolutionary mobile phone and a breakthrough internet device. The catch, he said, “These are not three separate devices. This is one device.”
This revolutionary, multifunctional device changed how we communicate, access and utilize information.
The Future Of Multifunctional Devices
Following this trajectory of multifunctional devices, can we predict the future of technology in our everyday lives?
As Mark Wieser said, the best technology disappears. Imagine a person with glasses on, but they’re not just your typical glasses. They are augmented reality devices with retinal projection displays so the user can see a large screen virtually in the space in front of them. They could be watching a movie or browsing visual information through their glasses while listening to the sound through their hearing aids. The hearing aids they’re wearing can barely be seen. It’s in-ear technology that acts as a two-way audio communication device so they can interface with the vast world of information. The hearing aid is listening to the user and the outside world, understanding voice commands and translating them into actions, and at the same time clarifying the world of sound by suppressing the cacophony of noise and enhancing speech signals. The voice replaces the keyboard and the mouse, allowing natural and intuitive human interfaces to technology.
Modern hearing devices have become so advanced that the artificial intelligence technology being used in hearing aids mimics the sound processing capabilities of the brain while also streaming audio from devices, monitoring health, detecting if you fall, responding to your questions, translating and transcribing languages and more. They’re now multifunctional devices that can change the way we live so we’re healthier and more productive. Yes, all this sounds like science fiction depicted in movies, but it’s quickly becoming a reality, and this is just the beginning of what it can do.
Artificial intelligence enables us to solve problems more quickly by using machine learning techniques where a deep neural network model is trained to recognize patterns within vast amounts of data. The use of this in multifunctional devices is developing faster than ever before, and how it’s used has the opportunity to advance society in unprecedented ways.
Like any new potentially disruptive technology that moves quickly, however, you must also pause and make sure it’s being developed and used for the right reasons. We need to work diligently to translate the potential of AI technology into a positive impact by asking key questions: What is the purpose of these multifunctional, AI devices? Who will be impacted by the use of this technology? Are important oversights to ensure responsible use being put in place? How are we ensuring the privacy and security of the users?
We’ve already seen how multifunctional devices can be woven into our everyday lives with the internet, computers and smartphones, but there are still endless possibilities for what comes next. Despite the potential risks the rapid advancement of artificial intelligence in multifunctional devices opens up, the power of this technology is not only an exciting opportunity for those working in this field, but it would continue to positively impact society as a whole, enhancing the ways we live, work, connect and communicate.
Forbes Technology Council is an invitation-only community for world-class CIOs, CTOs and technology executives. Do I qualify?

- Editorial Standards
- Reprints & Permissions

An official website of the United States government
Here’s how you know
The .gov means it’s official.
Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure.
The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

Fact Sheets Final Notice — Transitional Coverage for Emerging Technologies (CMS-3421-FN)
The Centers for Medicare & Medicaid Services (CMS) is committed to making sure people with Medicare have access to medical advancements that improve health outcomes and enhance health quality. CMS’ Transitional Coverage for Emerging Technologies (TCET) Pathway helps people with Medicare access the latest medical advances, enables doctors and other clinicians to provide the best care for their patients, and benefits manufacturers who create innovative technologies. With support from policymakers, trust from patients and providers, and meaningful collaboration with manufacturers, CMS aims to improve the care and quality of life for people with Medicare while enhancing and encouraging innovation.
CMS issued a final procedural notice outlining a Medicare coverage pathway to achieve more timely and predictable access to certain new medical technologies for people with Medicare. The new TCET pathway for certain Food & Drug Administration (FDA)-designated Breakthrough Devices increases the number of NCDs that CMS will conduct per year and supports both improved patient care and innovation by providing a clear, transparent, and consistent coverage process while maintaining robust safeguards for the Medicare population. CMS anticipates accepting up to five TCET candidates per year and, for technologies accepted into and continuing in the TCET pathway, CMS’ goal is to finalize a national coverage determination (NCD) within six months after FDA market authorization.
TCET Pathway at a Glance
The TCET pathway is intended to balance multiple considerations when making Medicare coverage determinations: (1) facilitating early, predictable, and safer access for people with Medicare to new technologies; (2) reducing uncertainty about coverage by evaluating early the potential benefits and harms of technologies with manufacturers; and (3) encouraging evidence development if notable evidence gaps exist for coverage purposes.
How TCET Works
The TCET pathway uses current national coverage determination (NCD) and coverage with evidence development (CED) processes to expedite Medicare coverage of certain FDA-designated Breakthrough Devices. TCET balances providing people with Medicare access to the latest medical innovations while ensuring these new technologies are appropriate and beneficial to the Medicare population based on data and available evidence. In addition, TCET promotes patient-centered care by supporting evidence development so that people with Medicare, their caregivers, and their doctors will have reliable information about the latest breakthrough technologies to make informed health care decisions. Finally, TCET gives device manufacturers what they have long asked for: a more efficient and transparent Medicare coverage review process that allows for enhanced communications between industry and CMS, clear evidence requirements, and defined timelines for final coverage actions.
TCET is voluntary for manufacturers and aims to reduce uncertainty about coverage options through a pre-market evaluation of potential harms and benefits of technologies while identifying any important evidence gaps. Additionally, the TCET pathway includes an evidence development framework that provides manufacturers with opportunities for increased pre-market engagement with CMS and reduces manufacturer burden, with increased flexibility to address evidence gaps, to ultimately support Medicare coverage. Specifically, TCET allows for any evidence gaps to be addressed through fit-for-purpose studies with patients that reflect the Medicare population. A fit-for-purpose study is one where the study design, analysis plan, and study data are appropriate for the question the study aims to answer. Furthermore, the TCET pathway will help coordinate Medicare benefit category determination, coding, and payment reviews, further helping translate innovation into meaningful patient access.
When developing the pathway, CMS solicited extensive feedback from patient groups, medical professionals, device manufacturers, innovators, and other Federal agencies. This feedback included requests for CMS to utilize a more agile, iterative evidence review process that considers fit-for-purpose study designs, including those that make secondary use of real-world data. CMS partnered with the Agency for Healthcare Research and Quality (AHRQ) to develop a comprehensive approach that incorporates greater flexibility into the CED paradigm and allows fit-for-purpose study designs. As the pathway is implemented, CMS will continue to engage with interested parties to ensure Medicare promotes access to emerging medical technologies while maintaining appropriate safeguards and rigorous evidence standards essential to the health of people with Medicare.
Evidence Development Under TCET
Evidence development to support Medicare coverage through the TCET pathway will be based upon a more transparent and predictable evidence-generation framework. In addition to the TCET procedural notice, CMS has finalized updated criteria in the CED guidance document based on the November 2022 AHRQ Report [ https://effectivehealthcare.ahrq.gov/products/coverage-evidence-development/research-report ], and February 2023 Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) meeting [ https://www.cms.gov/medicare-coverage-database/view/medcac-meeting.aspx?medcacid=79 ], as well as final National Coverage Analysis Evidence Review guidance to more clearly allow for fit-for-purpose study designs. We expect to publish more detailed fit-for-purpose guidance in the future. Additionally, CMS finalized the first in a series of guidance documents that review health outcomes and their clinically meaningful differences within priority therapeutic areas, beginning with knee osteoarthritis. CMS’ guidance documents can be accessed here: https://www.cms.gov/medicare-coverage-database/reports/national-coverage-medicare-coverage-documents-report.aspx?docTypeId=1# .
Key components of the TCET pathway to facilitate evidence development include:
- An Evidence Preview , which is a focused literature review for a specific item or service, will provide early feedback on the strengths and weaknesses of the available evidence, including any evidence gaps. It is intended to inform CMS and manufacturers about the best available coverage options for an item or service and offers greater efficiency, predictability, and transparency to manufacturers and CMS on the state of the evidence and any notable evidence gaps. CMS intends for Evidence Previews to be conducted by a contractor using standardized evidence grading, risk of bias assessment, and applicability assessment. If an NCD is opened following the Evidence Preview, an evidence summary, including a disclosure of which contractor completed the review, will be posted with the tracking sheet on the CMS website for public comment.
- An Evidence Development Plan (EDP) will be developed by the manufacturer to address any evidence gaps identified in the Evidence Preview. EDPs may include traditional clinical study designs and/or fit-for-purpose study designs, including those that rely on secondary use of real-world data, consistent with applicable CMS guidance documents. The development of an EDP will include CMS-AHRQ collaboration to evaluate the EDP to ensure it meets established standards of scientific integrity and relevance to the Medicare population. Additionally, CMS will engage with the manufacturer to provide feedback and discuss any recommended refinements. Non-proprietary elements of the CMS and AHRQ-approved EDP will be made publicly available on the CMS website when a proposed NCD is posted.
Device Eligibility
Given the unique FDA criteria for Breakthrough designation status, the TCET pathway will apply to certain eligible FDA-designated Breakthrough Devices because this is the area with the most immediate need for a pathway like TCET.
Appropriate candidates for the TCET pathway would include those devices that are:
- FDA-designated Breakthrough Devices.
- Determined to be within a Medicare benefit category.
- Not already the subject of an existing Medicare NCD.
- Not otherwise excluded from coverage through law or regulation.
The Federal Food, Drug, and Cosmetic Act definition of medical device includes in vitro diagnostic (IVD) products, such as diagnostic laboratory tests. IVDs, including diagnostic laboratory tests, are a highly specific area of coverage policy development, and CMS has historically delegated review of many of these tests to specialized Medicare Administrative Contractors (MACs). CMS believes the majority of coverage determinations for IVDs granted FDA-designated Breakthrough Device status should continue to be made by the MACs through existing pathways and has prioritized the devices listed above for the TCET pathway given available resources.
Nominations for the TCET Pathway
Manufacturers of FDA-designated Breakthrough Devices may self-nominate to participate in the TCET pathway. Manufacturers may submit a non-binding letter of intent to nominate a potentially eligible device for the TCET pathway, approximately 18 to 24 months before anticipated FDA marketing authorization as determined by the manufacturer. The appropriate timeframe for manufacturers to submit TCET pathway nominations to CMS is approximately 12 months prior to when the manufacturer anticipates an FDA decision on a submission.
The notice outlines the information that manufacturers should include in the voluntary letter of intent and self-nomination packet. CMS will accept suitable TCET candidates quarterly. If a suitable nomination is not selected in the first review, it will be automatically considered in the subsequent quarter. Manufacturers will not need to resubmit to be considered in a subsequent quarter but can withdraw if they choose to do so. CMS’ consideration of nominations will include an initial meeting with the manufacturer to discuss the technology and answer any questions on the process. In addition, CMS will meet with FDA to help CMS gain a better understanding of the device and potential FDA review timing.As part of the nomination consideration process, the technology may undergo a benefit category review. Upon completion of CMS’ review of the nomination, CMS will notify the manufacturer by email whether the product is an appropriate candidate for the TCET pathway.
TCET and the NCD process
If a device accepted into the TCET pathway receives FDA marketing authorization, CMS will initiate the NCD process by posting a tracking sheet following FDA market authorization (that is, the date the technology receives premarket approval (PMA); 510(k) clearance; or the granting of a De Novo request) pending a CMS and AHRQ-approved EDP (in cases where there are evidence gaps as identified in the Evidence Preview). The process for Medicare coverage under the TCET pathway would follow the NCD statutory timeframes in section 1862(l) of the Social Security Act (the Act). CMS’ goal is to finalize a TCET NCD within six months after FDA market authorization.
Duration of Coverage Under the TCET Pathway
Coverage under the TCET NCD will continue only as long as needed to facilitate the timely generation of evidence that can inform patient and clinician decision making. The duration of transitional coverage through the TCET pathway will be tied to the CMS- and AHRQ-approved EDP. The review date specified in the EDP will provide one additional year of coverage after study completion to allow manufacturers to complete their analysis, draft one or more reports, and submit them for peer-reviewed publication. In general, we anticipate coverage under a TCET NCD may last for approximately five or more years as evidence is generated to address evidence gaps identified in the Evidence Preview and lead to a predictable, long-term Medicare coverage determination.
Transition to Post-TCET Coverage
CMS intends to conduct an updated evidence review within six calendar months of the review date specified in the EDP. Similar to the process used for the Evidence Preview, CMS intends to engage a third-party, neutral contractor to conduct the systematic literature review. The contractor will perform a qualitative evidence synthesis and compare those findings against the benchmarks for each outcome specified in the CMS and AHRQ-approved EDP that was developed to fulfill the original NCD requirements. CMS will conduct quality assurance on the contractor review. CMS will also review applicable practice guidelines and consensus statements and consider whether the conditions of coverage remain appropriate.
Based upon this assessment, when appropriate, CMS will open an NCD reconsideration by posting a decision that proposes one of the following outcomes: (1) an NCD without evidence development requirements; (2) an NCD with continued evidence development requirements; (3) a non-coverage NCD; or (4) rescinding the NCD, resulting in coverage decisions being made by MACs under section 1862(a)(1)(A) of the Act. Standard NCD processes and timelines will continue to apply, and following a 30-day public comment period, CMS will have 60 days to finalize the NCD reconsideration.
Coverage of Similar Devices
FDA market-authorized Breakthrough Devices are often followed by similar devices that other manufacturers develop. CMS believes it is important to let physicians and their patients make decisions about the best available treatment depending on the patient’s individual situation. NCDs are limited to particular items or services, but it is possible that more than one device could fall under the same NCD because it addresses the same indication. We recognize that some differences may exist for technologies in a class that may result in a distinct benefit/risk profile, and each will be evaluated on its own merit. In these instances, CMS will follow the existing NCD process detailed in section 1862(l) of the Act.
Changes from Proposed Notice to Final Notice
Based on CMS’ analysis of the topics raised during the public comment period, CMS made several changes between the proposed notice and final notice. A comprehensive list of changes is provided in the final notice. Notable changes include:
- We incorporate an opportunity for manufacturers to submit a non-binding letter of intent to nominate a potentially eligible device approximately 18 to 24 months before anticipated FDA marketing authorization.
- We clarify that when CMS is aware that manufacturers will likely pursue the TCET pathway for devices where appropriate clinical endpoints are uncertain, we may preemptively conduct a clinical endpoints’ review, and may convene a MEDCAC panel. We note that submission of a non-binding letter of intent may avoid delays in TCET reviews.
- We have revised the timeframe for reviewing TCET nominations. We will review nominations on a quarterly basis.
- We state that if an NCD is opened, an evidence summary, including a disclosure of which contractor completed the review, will be posted with the tracking sheet on the CMS website for public comment.
- We state that EDPs should incorporate interim reporting to ensure adequate progress and timely completion. Interim reports should also disclose any meaningful changes to prespecified study protocols, which are essential to transparency.
- We express our intent to release soon the proposed factors CMS will use to prioritize TCET nominations to provide greater transparency, consistency, and predictability. (In the meantime, we will prioritize TCET candidates based upon the language from the August 7, 2013, Federal Register notice (78 FR 48164) stating that in the event we have a large volume of NCD requests for simultaneous review, we prioritize these requests based on the magnitude of the potential impact on the Medicare program and its beneficiaries and staffing resources.)
- We indicate that some information on TCET devices will be added to the NCD Dashboard, including the number of devices in the TCET pathway, the date of nomination, the date of acceptance, and the date the NCD process was initiated. This dashboard will be updated on an ongoing basis.
Next Steps for Manufacturers Interested in the TCET Pathway
If interested in the TCET pathway, manufacturers simply need to notify CMS of their interest in TCET electronically via the Coverage Center Web site using the “Contact Us” link at http://www.cms.gov/Medicare/Coverage/InfoExchange/contactus.html . To be considered for the first quarterly review, nominations will need to be submitted by October 31, 2024. The deadlines for the next three quarterly review cycles are January 31, 2025, April 30, 2025, and July 31, 2025.
For more information on the TCET pathway, please visit: https://www.federalregister.gov/public-inspection/2024-17603/medicare-program-transitional-coverage-for-emerging-technologies
- Previous Newsroom article
- Next Newsroom article
CMS News and Media Group Catherine Howden, Director Media Inquiries Form 202-690-6145
CMS Provides New — But Limited — Pathway for Coverage of Medical Devices
- Thomas A. Gustafson, Ph.D.
- Monique Nolan, M.D., J.D.
- Amanda Cassidy, M.P.P.
- John McInnes, M.D., J.D.
- Bobby McMillin, J.D.
In August 2024, the Centers for Medicare & Medicaid Services (CMS) established the Transitional Coverage for Emerging Technologies (TCET) pathway, intended to “accelerate patient access to beneficial medical products while generating evidence” needed to assess long-term coverage. 1 This pathway is limited to certain devices that have been designed as Breakthrough devices by the Food and Drug Administration (FDA), and CMS expects that it will be able to accommodate only five devices a year. This Advisory summarizes the main points of the final notice implementing TCET, which is substantially similar to a proposed notice that CMS issued for public comment in June 2023. 2
The TCET pathway will fall within CMS’ existing National Coverage Determination (NCD) process and pathway for Coverage with Evidence Development (CED), with significant process refinements but without altering existing standards for these coverage mechanisms. One implication of this approach is that TCET coverage will be limited to Medicare participants in the studies specified for evidence development. 3
CMS’ design embodies several principles: limiting the pathway to certain candidates; conducting early evidence reviews that can identify gaps in the evidence needed for Medicare coverage; engaging with manufacturers to limit the burden on manufacturers, clinicians, and patients, while maintaining rigorous evidence requirements; ensuring any CED requirement will be time-limited; and permitting manufacturers of candidate devices to opt out of TCET at several points. The notice also describes how CMS anticipates collaborating with the FDA and the Agency for Healthcare Research and Quality (AHRQ).
What Devices Can Be Included?
The TCET pathway will be limited to certain qualifying Breakthrough devices, as nominated by their manufacturers, and eligibility will be conditional on timing.
CMS notes that TCET is designed to address those devices where more evidence is needed specifically to support coverage for the Medicare patient population; other routes for Medicare coverage remain available for other devices.
Appropriate candidates for TCET pathway are devices that are:
- FDA-designated Breakthrough devices that receive FDA’s market authorization for one or more indications for use covered by the Breakthrough designation
- Determined by CMS to be within a Medicare benefit category
- Not already the subject of an existing Medicare NCD
- Not otherwise excluded from coverage through law or regulation
In response to comments requesting clarification of whether diagnostic tests could be included, CMS states that the majority of coverage determinations for diagnostic laboratory tests granted Breakthrough designation should be made by Medicare Administrative Contractors (MACs). However, the agency acknowledges that in some instances a manufacturer of a diagnostic test and CMS may agree that an NCD is appropriate. CMS encourages manufacturers of diagnostic tests believing additional evidence generation may be needed to secure Medicare coverage to contact CMS to discuss options specifically.
Eligibility Tied to Approval Timing . CMS ties the initiation of TCET to FDA market authorization. For a device to be eligible for consideration, its manufacturer would have to submit a nomination to CMS 12 months in advance of anticipated FDA market authorization, as discussed below. CMS may not accept nominations that are submitted within six months of the expected FDA market authorization. CMS will consider nominations on a quarterly basis, and if a nomination is not accepted in one quarter it will be automatically held over for consideration in the next quarter.
CMS was asked to incorporate a look-back period that would permit eligibility of devices that had received FDA market authorization recently or were expected to do so soon; CMS declines to extend eligibility in that fashion, citing operational difficulties in the context of TCET.
Eligible Devices Must Fall into an Existing Medicare Benefit Category . The agency anticipates initiating a benefit category review if the matter is open to question, but it cautions that in some instances such a review could occasion a delay. CMS declines requests to add certain benefit categories (e.g., for artificial intelligence), indicating that benefit categories are statutory.
Number of Devices . CMS expects that approximately eight devices would be nominated for TCET each year and that, based on currently available resources, the agency would be able to accommodate not more than five devices each year. CMS expects that many Breakthrough devices either will be coverable without TCET (e.g., items subject to an existing NCD) or not indicated for the Medicare population (e.g., devices for pediatric patients). CMS declines to increase the number of devices that would be accepted each year (or to extend TCET to items and services that are not Breakthrough devices), citing constraints on its administrative resources. CMS suggests it may consider expansion in the future as experience with TCET is gained and resources are available.
How CMS would prioritize which nominated devices would be included remains somewhat unclear. CMS promises to issue shortly further guidance, open for public comment, on how it would set priorities. In the meantime, CMS intends to rely on a 2013 notice that indicates prioritization would be based on the “magnitude of potential impact on the Medicare program and its beneficiaries and staffing resources.”
Outline of the TCET Process
Starting the Process — Evidence Preview . Manufacturers would have the opportunity to submit a non-binding letter of intent generally 18 to 24 months in advance of the expected date of FDA market authorization. This letter would trigger an Evidence Preview, “a focused literature review that would provide early feedback on the strengths and weaknesses of the available evidence, including any evidence gaps.” The review would cover published studies, unpublished reports provided by the manufacturer of clinical studies supporting the FDA market authorization application, and evidence-based guidelines and consensus statements. CMS would employ a contractor, which would use standard evidence assessment tools and qualitatively synthesize the results. CMS declines to permit manufacturers to contact these contractors directly.
Next Steps — Nomination and Evidence Development Plan . Manufacturers wishing to proceed would initiate the TCET process through a formal letter to CMS, generally approximately 12 months in advance of anticipated FDA market authorization. If a nomination is accepted, then the manufacturer would be responsible for presenting an Evidence Development Plan (EDP) describing the planned studies to address identified evidence gaps. Upon approval of the EDP by CMS and AHRQ, CMS would open a CED NCD, and the studies could proceed under the CED authority. CMS looks to have a finalized EDP not later than 90 days after FDA market authorization.
Nominations for the first quarterly review are due October 31, 2024. The deadlines for the next three quarterly review cycles are January 31, 2025, April 30, 2025, and July 31, 2025. 4
Duration of TCET Coverage . CMS contemplates a transitional coverage period of approximately five years, including a year after study completion for analysis, report drafting, and submission for peer review. However, “the duration of transitional coverage should be tied to an EDP that sufficiently addresses the material evidence gaps identified in the EP, and we will work with manufacturers to define an appropriate NCD reconsideration window.”
Preventing Possible Gaps in Coverage Before NCD Revision . CMS calls on applicant manufacturers to provide for a “continued access study” that maintains market access after the primary EDP is complete and before a decision regarding post-TCET coverage is finalized.
Post-TCET Coverage . CMS will conduct an updated evidence review within six months of the review date specified in the EDP and also examine applicable practice guidelines and consensus statements, considering whether the conditions of coverage remain appropriate. 5 CMS would, as appropriate, open an NCD reconsideration aimed at either (1) an NCD without evidence development requirements; (2) an NCD with continued evidence development requirements; (3) a non-coverage NCD; or (4) coverage at the discretion of the MACs.
Other Issues
Safety . To address possible safety concerns, “… CMS retains the right to reconsider an NCD at any point in time.”
Availability of Evidence Summary and Evidence Gap Analysis . If a manufacturer withdraws from the TCET pathway, CMS will publish an evidence summary without the evidence gap analysis. Similarly, only a summary of the evidence would be posted with a tracking sheet if CMS opens a national coverage analysis.
Coverage of Similar Devices .
- In instances where NCDs would apply to multiple products for the same indication, CMS will follow the existing NCD process. CMS recognizes that some technologies in the same class may have distinct risk/benefit profiles, and each will be evaluated on its own merit.
- CMS deliberately does not define “similar devices” and apparently expects to address this question on a case-by-case basis in consultation with FDA and the manufacturers.
- CMS declines to provide coverage exclusivity for first-to-market devices.
Fit-For-Purpose Studies . CMS alludes to but does not address substantively the possible use of fit-for-purpose studies in EDPs. The agency plans to furnish guidance shortly on fit-for-purpose study designs and analysis methods, emphasizing secondary use of real-world data.
Considerations for Industry
The Scope of TCET Will Be Distinctly Limited . CMS anticipates accepting up to five TCET candidates annually for the foreseeable future. How the agency expects to determine which devices to include is unclear at present.
Role of CED . For devices in the TCET pathway, coverage will be afforded under the CED paradigm, given that CMS expects that devices eligible for TCET would not yet meet Medicare’s “reasonable and necessary” standard for coverage. A key element of CED is that coverage is limited to Medicare participants in studies specified to develop evidence.
TCET Timeline . The proposed timeline for initiating the TCET pathway may not be realistic — particularly the stages that require engagement with the agency over evidence. CMS implies that, in some cases involving novel items or conflicting evidence, the time needed for completing a benefit category review or an Evidence Preview may lengthen the schedule. On the back end, depending on the circumstances, completing the EDP may require more than the five years CMS generally expects.
Benefit Category, Coding, Payment . The final notice alludes to improved coordination efforts of benefit determination, coding, and payment reviews, but CMS does not provide detail about how this will be accomplished.
TCET in Context
TCET is CMS’ second attempt at addressing an accelerated coverage pathway for Breakthrough devices. CMS regards TCET as a substitute for the Medicare Coverage for Innovative Technology (MCIT) final rule , which would have afforded all Breakthrough devices immediate coverage following market authorization for four years, without any requirement for evidence development as a condition of coverage during this period. The present administration repealed the MCIT rule in November 2021, before it took effect, citing, among other reasons, beneficiary safety concerns. In the current notice, CMS declines to extend coverage to all Breakthrough devices.
The device industry has advocated for a change in law in reaction to CMS’ posture. A bill to require the agency to in essence reinstate MCIT, H.R. 1691 , was cosponsored by 87 members of the House of Representatives as of early August.
Last November, the House Energy & Commerce Health Subcommittee advanced this bill, though the full committee has yet to consider it. On June 27, 2024, the House Ways & Means Committee, responding to budget scoring concerns, reported a much more limited version of this bill. The House Ways & Means Committee version would provide for Medicare coverage for a “transitional period” of four years for certain Breakthrough devices — without invoking the CED apparatus.
- In the case of devices cleared under section 510(k) of the Federal Food, Drug, and Cosmetic Act, coverage would be limited to those cleared based on clinical trial information from trials that included Medicare beneficiaries.
- Coverage of off-label uses of included devices during the transitional period would not be covered.
- CMS could exclude a device upon determining, based on review of clinical data, that the device presents an “undue risk of harm” to Medicare beneficiaries.
- Deadlines on CMS actions: Determination of whether a device is subject to the provision would be required within six months of application from the manufacturer. CMS would be required to make a final decision on a request for a NCD prior to the end of the transitional period.
Despite bipartisan support, prospects for passage of either version remain uncertain given the bill’s cost. If sufficient offsets can be identified, we expect supporters of the legislation to push for its inclusion in a post-election funding package. Of course, if a bill along these lines were enacted, the TCET pathway would be changed materially.
If readers have further questions, Arnold & Porter professionals following these issues would be happy to fill in details and provide perspectives. Feel free to contact any of the authors listed above.
© Arnold & Porter Kaye Scholer LLP 2024 All Rights Reserved. This Advisory is intended to be a general summary of the law and does not constitute legal advice. You should consult with counsel to determine applicable legal requirements in a specific fact situation.
Share this with others:
- Legislative and Public Policy
- Life Sciences
- Virtual and Digital Health
Key Contacts

Related Services
Email disclaimer.
- Environment
- Science & Technology
- Business & Industry
- Health & Public Welfare
- Topics (CFR Indexing Terms)
- Public Inspection
- Presidential Documents
- Document Search
- Advanced Document Search
- Public Inspection Search
- Reader Aids Home
- Office of the Federal Register Announcements
- Using FederalRegister.Gov
- Understanding the Federal Register
- Recent Site Updates
- Federal Register & CFR Statistics
- Videos & Tutorials
- Developer Resources
- Government Policy and OFR Procedures
- Congressional Review
- My Clipboard
- My Comments
- My Subscriptions
- Sign In / Sign Up
- Site Feedback
- Search the Federal Register
This site displays a prototype of a “Web 2.0” version of the daily Federal Register. It is not an official legal edition of the Federal Register, and does not replace the official print version or the official electronic version on GPO’s govinfo.gov.
The documents posted on this site are XML renditions of published Federal Register documents. Each document posted on the site includes a link to the corresponding official PDF file on govinfo.gov. This prototype edition of the daily Federal Register on FederalRegister.gov will remain an unofficial informational resource until the Administrative Committee of the Federal Register (ACFR) issues a regulation granting it official legal status. For complete information about, and access to, our official publications and services, go to About the Federal Register on NARA's archives.gov.
The OFR/GPO partnership is committed to presenting accurate and reliable regulatory information on FederalRegister.gov with the objective of establishing the XML-based Federal Register as an ACFR-sanctioned publication in the future. While every effort has been made to ensure that the material on FederalRegister.gov is accurately displayed, consistent with the official SGML-based PDF version on govinfo.gov, those relying on it for legal research should verify their results against an official edition of the Federal Register. Until the ACFR grants it official status, the XML rendition of the daily Federal Register on FederalRegister.gov does not provide legal notice to the public or judicial notice to the courts.
Design Updates: As part of our ongoing effort to make FederalRegister.gov more accessible and easier to use we've enlarged the space available to the document content and moved all document related data into the utility bar on the left of the document. Read more in our feature announcement .
Medicare Program; Transitional Coverage for Emerging Technologies
A Notice by the Centers for Medicare & Medicaid Services on 08/12/2024
This document has been published in the Federal Register . Use the PDF linked in the document sidebar for the official electronic format.
- Document Details Published Content - Document Details Agencies Department of Health and Human Services Centers for Medicare & Medicaid Services Agency/Docket Number CMS-3421-FN Document Citation 89 FR 65724 Document Number 2024-17603 Document Type Notice Pages 65724-65754 (31 pages) Publication Date 08/12/2024 Published Content - Document Details
- View printed version (PDF)
- Document Dates Published Content - Document Dates Effective Date 08/12/2024 Dates Text This final notice is effective August 12, 2024. Published Content - Document Dates
This table of contents is a navigational tool, processed from the headings within the legal text of Federal Register documents. This repetition of headings to form internal navigation links has no substantive legal effect.
FOR FURTHER INFORMATION CONTACT:
Supplementary information:, i. background, a. current medicare coverage mechanisms, 1. claim-by-claim adjudication, 2. local coverage determinations (lcds), 3. national coverage determinations (ncds), b. differences between fda and cms review, c. fda breakthrough devices program, ii. summary of proposed provisions and cms responses to public comments on the proposed notice, a. overarching comments regarding cms' proposal to establish the tcet pathway, 1. general concerns, 2. tcet timelines, 3. limiting the tcet pathway to five candidates yearly, 4. operational issues, b. appropriate candidates, 1. scope of pathway and fda-designated breakthrough devices, 2. necessity of falling into an existing benefit category, 3. limitation for devices already the subject of an existing ncd, 4. diagnostic laboratory tests, c. nominations, d. coordination with fda, e. bcd reviews, f. evidence preview, g. manufacturer decision to continue or discontinue, h. evidence development plans, i. cms ncd review and timing, j. input from interested parties, j. coverage of similar devices, k. duration of coverage, l. transition to post-tcet coverage, m. tcet and parallel review, n. prioritizing requests, o. tcet transparency, p. miscellaneous comments, iii. provisions of the final notice, iv. collection of information requirements, i. addendum—process and procedures for the tcet pathway, a. tcet pathway—an opportunity to accelerate patient access to promising medical products while generating evidence, b. tcet general principles, c. appropriate candidates, d. procedures for the tcet pathway, 1. premarket, a. non-binding letter of intent for the tcet pathway, b. nominations for the tcet pathway, c. cms nomination cycles and consideration of nominations, d. intake meeting, e. coordination with fda, f. benefit category review, g. manufacturer notification, h. evidence preview (ep), i. evidence preview meeting, j. manufacturer's decision to continue or discontinue with the tcet pathway, k. evidence development plan (edp), l. edp submission timing, m. edp meeting and finalization of the edp, 2. coverage under the tcet pathway, a. cms ncd review and timing, b. request for specific input on the evidence base and conditions of coverage, c. coverage of similar devices, d. duration of coverage under the tcet pathway, 3. transition to post-tcet coverage, a. updated evidence review, b. ncd reconsideration, 1. manufacturer, f. tcet and parallel review, g. prioritizing requests, h. tcet transparency.
Comments are no longer being accepted. See DATES for details.
Additional information is not currently available for this document.
- Sharing Enhanced Content - Sharing Shorter Document URL https://www.federalregister.gov/d/2024-17603 Email Email this document to a friend Enhanced Content - Sharing
- Print this document
Document page views are updated periodically throughout the day and are cumulative counts for this document. Counts are subject to sampling, reprocessing and revision (up or down) throughout the day.
This document is also available in the following formats:
More information and documentation can be found in our developer tools pages .
This PDF is the current document as it appeared on Public Inspection on 08/07/2024 at 4:15 pm.
It was viewed 0 times while on Public Inspection.
If you are using public inspection listings for legal research, you should verify the contents of the documents against a final, official edition of the Federal Register. Only official editions of the Federal Register provide legal notice of publication to the public and judicial notice to the courts under 44 U.S.C. 1503 & 1507 . Learn more here .
Document headings vary by document type but may contain the following:
- the agency or agencies that issued and signed a document
- the number of the CFR title and the number of each part the document amends, proposes to amend, or is directly related to
- the agency docket number / agency internal file number
- the RIN which identifies each regulatory action listed in the Unified Agenda of Federal Regulatory and Deregulatory Actions
See the Document Drafting Handbook for more details.
Department of Health and Human Services
Centers for medicare & medicaid services.
- [CMS-3421-FN]
Centers for Medicare & Medicaid Services (CMS), Department of Health and Human Services (HHS).
Final notice.
This final notice finalizes the process and procedures for the Transitional Coverage for Emerging Technologies (TCET) pathway and provides our responses to the public comments received.
This final notice is effective August 12, 2024.
Lori Ashby, (410) 786-6322.
This notice describes the method we will use to provide transitional coverage for emerging technologies (TCET) through the national coverage determination (NCD) process. The TCET pathway is designed to deliver transparent, predictable, and expedited national coverage for certain eligible Breakthrough Devices that are Food and Drug Administration (FDA) market authorized. It builds upon CMS' experience with the Parallel Review program and the Coverage with Evidence Development (CED) pathway. Additionally, the TCET pathway reflects the feedback received from interested parties, including beneficiaries, patient groups, medical professionals and societies, medical device manufacturers, other Federal partners, and others involved in developing innovative medical devices. This feedback was obtained from informal and formal meetings, the comments we received as we conducted rulemaking for the Medicare Coverage of Innovative Technology (MCIT) pathway (referenced later in this section), and during the two listening sessions that were held following the repeal of the January 14, 2021 MCIT/“Reasonable and Necessary (R&N)” final rule ( 86 FR 2987 ). Additionally, feedback was obtained from public comments and one listening session following publication of the June 28, 2023, Federal Register notice ( 88 FR 41633 ) announcing the TCET pathway. The TCET pathway described in this notice is intended to balance multiple considerations when making coverage determinations: (1) facilitating early, predictable, and safer beneficiary access to new technologies; (2) reducing uncertainty about coverage by evaluating early the potential benefits and harms of technologies with manufacturers; and (3) encouraging evidence development if notable evidence gaps exist for coverage purposes.
The Medicare program serves over 66.7 million beneficiaries [ 1 ] and is the largest single healthcare purchaser in the U.S. Currently, approximately 51 percent of the total Medicare beneficiary population, or 34 million Medicare beneficiaries, receive coverage through Medicare fee-for-service (FFS). More than 1.1 billion Medicare FFS claims were processed in fiscal year (FY) 2023, comprised of approximately 192 million Part A claims (such as inpatient care in hospitals, skilled nursing facility care, hospice care, and home health care) and 950 million Part B claims (such as doctor and other health care services and outpatient care, durable medical equipment, and some preventive services), providing approximately $431.5 billion in Medicare FFS benefits. [ 2 ]
Medicare Part A and Part B cover a wide range of items and services but may not cover every item or service that a physician or healthcare practitioner prescribes or orders. In general, for an item or service to be covered under Medicare, it must meet the standard described in section 1862(a)(1)(A) of the Social Security Act (the Act)—that is, it must be reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. CMS makes reasonable and necessary coverage decisions through various pathways to facilitate expeditious beneficiary access to items and services that meet the statutory standard for coverage.
We believe that new approaches could help make coverage decisions on certain new items and services, such as medical devices, more quickly and provide expedited access to new and innovative medical technologies. On November 15, 2021 ( 86 FR 62944 ), CMS published a final rule that repealed the MCIT rule before it was legally effective and, thus, was never implemented. [ 3 ] As promised in the repeal, CMS provided additional opportunities to engage with the public. We have incorporated that input, along with input gathered in MCIT rulemaking, as we have developed the TCET pathway to make decisions on certain emerging technologies at the national level.
We believe that the TCET pathway balances the needs of beneficiaries, patient groups, medical professionals and societies, medical device manufacturers, and others involved in developing innovative medical devices.
Items and services, including medical devices, are currently covered under Part A or Part B in one of three ways, presented here for context. The TCET pathway described in this notice will leverage the existing NCD pathway, and CED in particular, to provide a streamlined coverage pathway for emerging technologies. We note that the TCET pathway does not alter the existing standards for these coverage mechanisms.
In the absence of an NCD or a local coverage determination (LCD), Medicare Administrative Contractors (MACs) make coverage decisions under section 1862(a)(1)(A) of the Act and may cover items and services on a claim-by-claim basis if the MAC determines them to be reasonable and necessary for individual patients. Though claims may be denied if they are not determined to be reasonable and necessary, the claim-by-claim adjudication pathway remains the fastest path to potential coverage. The majority of all Medicare Parts A and B claims have coverage determined through the claim-by-claim adjudication process.
MACs develop LCDs under section 1862(a)(1)(A) that apply only within their geographic jurisdictions (see sections 1862(l)(6)(B) and 1869(f)(2)(B) of the Act). LCDs govern only the issuing MAC's claims adjudication and are not controlling authorities for qualified independent contractors or administrative law judges in the claims adjudication process.
The MACs follow specific guidance for developing LCDs for Medicare coverage as outlined in the CMS Program Integrity Manual (PIM), Chapter 13. LCDs generally take 9 to 12 months to develop. MACs are expected to finalize proposed LCDs within 365 days from opening, per Chapter 13.5.1-Local Coverage of the PIM. [ 4 ] That chapter will continue to be used in making determinations under section 1862(a)(1)(A) of the Act for items and services at the local level.
The term “national coverage determination” is defined in section 1862(l)(6)(A) of the Act and means a determination by the Secretary of the Department of Health and Human Services (the Secretary) with respect to whether or not a particular item or service is covered nationally under Title XVIII of the Act. In general, NCDs are national policy statements published to identify the circumstances under which a particular item or service will be considered covered (or not covered) by Medicare. NCDs serve as generally applicable rules to ensure that similar claims for items or services are covered in the same manner. Often, an NCD is written in terms of defined clinical characteristics that identify a population that may or may not receive Medicare coverage for a particular item or service. Traditionally, CMS relies heavily on health outcomes data to make NCDs.
Most NCDs have involved determinations under section 1862(a)(1)(A) of the Act, but NCDs can be made based on other provisions of the Act, such as section 1862(a)(1)(E) of the Act. Under section 1862(a)(1)(E) of the Act, Medicare has provided coverage for certain promising technologies with a limited evidence base on the condition that they are furnished in the context of approved clinical studies or with the collection of additional clinical data. CMS has used section 1862(a)(1)(E) of the Act to support the “Coverage with Evidence Development” or “CED” policy since July 12, 2006, and the most recent CED policy is described in the 2024 guidance document. [ 5 ] In general, CED enables providers and suppliers to perform high-quality studies that we expect will produce evidence that may lead to positive national coverage determinations under section 1862(a)(1)(A) of the Act.
The Agency for Healthcare Research and Quality (AHRQ) reviews all CED NCDs established under section 1862(a)(1)(E) of the Act. Consistent with section 1142 of the Act, AHRQ collaborates with CMS to define standards for the clinical research studies to address the CED questions and support and endorse the general standards for CED studies ( https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development ).
NCDs also include a determination on whether the item or service under consideration has a Medicare benefit category under Part A or Part B, [ 6 ] such as inpatient hospital services, physicians' services, durable medical equipment, or others. All items and services coverable by Medicare must fall within the scope of a statutory benefit category, and many of these specific terms are defined under section 1861 of the Act and in implementing regulations. While benefit category determinations (BCDs) may often be completed within 3 months, in some cases BCDs may take considerably longer. While CMS is working to align the coverage and BCD review processes better, manufacturers should be aware that, in some cases, benefit category reviews may not be completed within the accelerated timeframes needed for the TCET pathway. In addition, to be covered, the item or service must not be excluded from coverage by statute or our regulations at 42 CFR part 411, subpart A . The NCD pathway, which has statutorily prescribed timeframes, generally takes 9 to 12 months to complete from the opening of the tracking sheet. [ 7 ]
In addition to these coverage pathways, CMS has established a Clinical Trial Policy (CTP) NCD 310.1. The CTP policy is applied when Medicare covers routine care items and services (but generally not the technology under investigation) in a clinical study that is supported by certain Federal agencies. The CTP coverage policy was developed in 2000. [ 8 ] We note that coverage under CED and the CTP may not occur simultaneously.
Lastly, CMS has established the Parallel Review program. In the September 17, 2010, Federal Register ( 75 FR 57045 ), FDA and CMS announced their intention to initiate a Parallel Review pilot program in an effort to increase the quality of patient health care by facilitating earlier access to innovative medical technologies for Medicare beneficiaries. In the October 24, 2016, Federal Register ( 81 FR 73113 ), FDA and CMS published a joint notice that announced and described the processes for the fully implemented Program for Parallel Review of Medical Devices.
Parallel Review is a mechanism for FDA and CMS to simultaneously review the clinical data submitted by a manufacturer about a medical device to help decrease the time between FDA's approval of an original or supplemental premarket approval (PMA) application or granting of a de novo classification request (De Novo request) and the subsequent CMS proposed NCD. Parallel Review has two stages: (1) FDA and CMS meet with the manufacturer to provide feedback on the proposed pivotal clinical trial, and (2) FDA and CMS concurrently review (“in parallel”) the clinical trial results submitted in the PMA application, or De Novo request. FDA and CMS independently review the data to determine whether it meets their respective Agency's standards and communicate with the manufacturer during their respective reviews. This program relies upon a technology having a comprehensive evidence base to support the clinical analysis for the NCD.
While FDA and CMS have a well-established history of collaboration in the review of evidence for emerging medical technologies, FDA and CMS must consider different legal authorities and apply different statutory standards when making marketing authorization and coverage decisions, respectively, for medical devices. Generally, FDA makes marketing authorization decisions based on whether the relevant statutory standard for safety and effectiveness is met, while CMS generally makes NCDs based on whether an item or service is reasonable and necessary for the diagnosis or treatment of an illness or injury for individuals in the Medicare population. These two reviews are separate and are conducted independently by the two agencies. The FDA review of devices does not require a focus specifically on the Medicare population.
Among other objectives, FDA conducts a premarket review of certain devices to evaluate their safety and effectiveness and determine if they meet the applicable standard to be marketed in the United States. FDA approval or clearance alone does not entitle that technology to Medicare coverage, given separate Medicare statutory coverage requirements. While FDA reviews devices to ensure they meet applicable safety and effectiveness standards, there is often limited evidence regarding whether the device is clinically beneficial for Medicare patients. Of note, individuals representative of the Medicare population are often excluded from the studies used to generate the evidence reviewed by FDA. This is an important consideration for manufacturers and other interested parties seeking the most appropriate coverage pathway under Medicare. Where there is limited evidence on the health outcomes for individuals in the Medicare population, there may be insufficient evidence to support a full coverage national coverage determination under section 1862(a)(1)(A) of the Act.
In general, as discussed, under section 1862(a)(1)(A) of the Act, Congress required CMS to determine whether items and services are reasonable and necessary to diagnose or treat an illness or injury or to improve the functioning of a malformed body member for an individual with Medicare. For CMS, the evidence base underlying FDA's decision to approve or clear a device for particular indications for use has often been crucial for determining Medicare coverage through the NCD process. CMS looks to the evidence supporting FDA market authorization and the device's approved or cleared indications for use for evidence generalizable to the Medicare population, data on improvement in health outcomes, and the durability of those outcomes. If there is no data on those elements in the Medicare population, it is difficult for CMS to make an evidence-based decision on whether the device is reasonable and necessary for the Medicare population.
CMS considers whether the evidence shows that the item or service will improve the health of Medicare patients recognizing that Medicare beneficiaries are often older, have multiple comorbidities, and are often underrepresented or not represented in many clinical studies. [ 9 ] According to a recent study, [ 10 11 ] approximately 50 percent of Medicare patients have two or more diseases. Clinical studies that are conducted to gain FDA market authorization are not necessarily required to include participants with similar demographics and characteristics of the Medicare population. To demonstrate the safety and effectiveness of a device as clearly as possible, many studies impose stringent exclusion criteria that disqualify individuals with characteristics that may make it harder to ascertain a device's effects, such as comorbidities and concomitant treatment. Consequently, a device's potential benefits and harms for older patients with more comorbidities may not be well understood at the time of FDA market authorization.
Under the TCET coverage pathway, CMS will coordinate with FDA and manufacturers of Breakthrough Devices as those devices move through the FDA premarket review processes to ensure timely Medicare coverage decisions following any FDA market authorization, as described in detail later in this section. The FDA Breakthrough Devices Program is an evolution of the Expedited Access Pathway Program and the Priority Review Program. See section 515B of the FD&C Act, 21 U.S.C. 360e-3 ; see also final guidance for industry entitled, “Breakthrough Devices Program.” [ 12 ]
FDA's Breakthrough Devices Program is not for all new medical devices; rather, it is only for those that FDA determines meet the standards for Breakthrough Device designation. In accordance with section 515B of the FD&C Act ( 21 U.S.C. 360e-3 ), the Breakthrough Devices Program is for medical devices and device-led combination products [ 13 ] that meet two criteria. The first criterion is that the device provides for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions. The second criterion is that the device must satisfy one of the following elements: It represents a breakthrough technology; no approved or cleared alternatives exist; it offers significant advantages over existing approved or cleared alternatives, including the potential, compared to existing approved alternatives, to reduce or eliminate the need for hospitalization, improve patient quality of life, facilitate patients' ability to manage their own care (such as through self-directed personal assistance), or establish long-term clinical efficiencies; or device availability is in the best interest of patients (see 21 U.S.C. 360e-3(b)(2) ). These criteria make Breakthrough designated devices unique. Devices meeting these criteria are also likely to be highly relevant to the needs of the Medicare population who may not have other treatment options.
FDA has explained in guidance that because decisions on requests for Breakthrough designation will be made prior to marketing authorization, FDA considers whether there is a “reasonable expectation that a device could provide for more effective treatment or diagnosis relative to the current standard of care (SOC) in the U.S” for purposes of the designation. This reasonable expectation can be supported by sources including “literature or preliminary data (bench, animal, or clinical)”. [ 14 ]
In the June 28, 2023, Federal Register ( 88 FR 41633 ), we published a proposed notice to establish the TCET pathway. We received approximately 150 timely pieces of correspondence in response to the publication of the June 28, 2023, proposed notice. Commenters included a broad range of interested parties, including physicians, professional societies, manufacturers, manufacturer associations, venture capital firms, health plans, and patient advocates. Some comments addressed issues or expressed concerns that were beyond the scope of our proposals in the proposed notice or were not relevant and will not be summarized and included in our responses below. Revisions made to the TCET pathway in response to specific comments are noted in the applicable response to comments, and a listing of changes from proposed to final is included in section III. of this final notice. Additionally, clarifying edits have been made, as appropriate. The following is a summary of the public comments that we received related to the proposed notice, and our responses to the public comments.
CMS proposed that the TCET pathway use the NCD and CED processes to expedite Medicare coverage of certain Breakthrough Devices. Our proposal noted that the TCET pathway would be voluntary and stated that the goal of the pathway is to reduce uncertainty about coverage options through a pre-market evaluation of potential harms and benefits of technologies while identifying any important evidence gaps. Additionally, CMS' proposal for the TCET pathway provided an evidence development framework to provide manufacturers with opportunities for increased pre-market engagement with CMS and, to reduce manufacturer burden, increased flexibility to address evidence gaps to support Medicare coverage. In the proposed notice, CMS stated that we anticipate accepting up to five TCET candidates annually.
Comment: Many commenters generally supported the TCET concept, expressing that it could result in faster access to newly FDA market-authorized technologies for Medicare beneficiaries. Commenters appreciated that TCET will bring closer collaboration between FDA and CMS. Those who were supportive also stated their belief that the proposal would promote innovation, decrease uncertainty and delays in coverage, and improve beneficiary access to cutting-edge treatments. The majority of commenters expressed support for the TCET proposal in principle, noting that it is a “good first step,” and provided suggested modifications to improve the pathway.
Response: We appreciate the comments supporting the TCET proposal. We also appreciate the suggestions provided by commenters to improve the pathway. The modifications suggested by commenters and CMS' responses to those suggestions are provided throughout this section.
Comment: Some commenters do not believe CMS' proposal goes far enough and refer to it as “flawed” and a “missed opportunity.” Several commenters expressed concerns that the TCET pathway is limited in scope in that it only applies to “certain FDA-designated Breakthrough Devices that fall within a Medicare benefit category.” Some of these commenters expressed support for automatic, immediate coverage upon FDA market authorization. A commenter expressing a preference for immediate or near-immediate coverage referred to TCET as a “partial solution” to providing timely access to innovative devices as the pathway will be further limited by CMS resource constraints.
Response: We appreciate the public comments but do not agree that the TCET proposal is flawed or was a missed opportunity to provide better access to Breakthrough Devices for Medicare beneficiaries. We also disagree that it is a “partial solution.”
While the FDA reviews devices to ensure they meet applicable safety and effectiveness standards, there is often limited evidence regarding whether the device is clinically beneficial to Medicare patients at the time of FDA market authorization. As such, we do not believe that it is appropriate to grant all FDA market authorized Breakthrough Devices automatic coverage solely based on their Breakthrough Designation. Furthermore, when there is a lack of evidence specific to the Medicare population, it makes it difficult for CMS to ensure that devices are not posing additional risks in the Medicare population. Continuing to develop evidence generalizable to the Medicare population is important not only to payers, but is critical for patients, their caregivers, and their treating clinicians to make the most informed decisions for their treatment. We believe that it is important to require manufacturers participating in any innovative coverage pathway, such as TCET, to produce evidence that demonstrates the health benefit of the device and the related services for patients with demographics similar to that of the Medicare population.
Our proposal centered on Breakthrough Devices because we believe this is the area with the most immediate need, particularly considering the unique FDA criteria for Breakthrough designation status. We agree with commenters about the importance of promoting innovation across all items and services covered under Medicare. However, because we have consistently heard from interested parties about the need for more rapid coverage for Breakthrough Devices, we are focusing on Breakthrough Devices in this final notice.
The TCET pathway will result in a more transparent, predictable, and efficient Medicare coverage pathway that balances multiple competing interests. Coverage under CED can expedite beneficiary access to innovative technologies (and result in improved health outcomes) by ensuring that systematic patient safeguards—including assurance that the technology is provided to clinically appropriate patients—are in place that reduce the risks inherent to new technologies, or to new applications of older technologies. In the absence of CED, technologies with limited evidence would likely not be covered. Further, TCET represents a substantial transformation of how CMS conducts coverage reviews and is responsive to extensive feedback from interested parties. The pathway has broad support from the vast majority of commenters and CMS views this as the best option to provide coverage for emerging technologies for which the available evidence is insufficient to support broad national coverage at the time of FDA market authorization. As we gain experience with the TCET pathway, we may consider expanding its application to other items and services.
Comment: A commenter questioned CMS' legal authority to use CED and expressed opposition to CMS' use of it. This commenter noted past communications from their organization, including some previously shared with CMS, that “have, among other things, questioned CMS's reliance upon uncertain legal grounds for utilizing CED.” One of the examples provided by this commenter is the white paper, “Façade of Evidence: How Medicare's Coverage with Evidence Development Paradigm Rations Care and Exacerbates Inequity” [ 15 ] which cites an Advisory Opinion from the former General Counsel for HHS that “support” is usually used to mean funding.
Response: We disagree with the commenter's assertion that CMS does not have statutory authority for CED. Advisory Opinion 21-03 was issued by the past administration on January 14, 2021. It has been removed from the HHS website. Advisory opinions do not grant rights or impose obligations and the opinions can be revised, modified, or eliminated as necessary to reflect changing circumstances.
Congress has established an exception in section 1862(a)(1)(E) of the Act that authorizes the Medicare program to pay for items and services in the case of research conducted pursuant to section 1142 of the Act, so long as the items or services are reasonable and necessary to carry out the purposes of that section. Section 1142(a)(1)(A) of the Act authorizes the Secretary, acting through the AHRQ Director, to “conduct and support research with respect to the outcomes, effectiveness, and appropriateness of health care services and procedures in order to identify the manner in which diseases, disorders, and other health conditions can most effectively and appropriately be prevented, diagnosed, treated, and managed clinically[.]” In this subsection the word “support” is not necessarily limited to financial backing. “Support” pursuant to this subsection may also take the form of an appropriate AHRQ endorsement.
Under CED, AHRQ has endorsed and supported research for various items and services that were of particular importance to the Medicare population, but where the existing medical evidence was not sufficient to permit coverage under section 1862(a)(1)(A) of the Act. AHRQ's endorsement has occurred when AHRQ officials have used staff resources to identify the general characteristics and attributes that are necessary for any Medicare sponsored clinical trial. The general AHRQ recommendations have been included in current and prior CED guidance documents. AHRQ officials have also reviewed each NCD where CED has been proposed or finalized, focusing on the specific methodological approach that would be necessary for coverage in each specific CED NCD. AHRQ's support has been documented and included in the record for each CED NCD. AHRQ's expertise has been essential to support CED under sections 1142 and 1862(a)(1)(E) of the Act.
Additionally, HHS has recognized that AHRQ's endorsement of standards for qualifying clinical trials under section 1142 of the Act can provide the statutory authority for Medicare coverage for items and services under the Medicare program in circumstances outside of the CED policy. The Medicare clinical trial policy, now established at section 310 of the Medicare National Coverage Determinations Manual, relies on the same statutory authority and has been effective since September 19, 2000.
Subsequently, CMS, then known as the Health Care Financing Administration, requested AHRQ convene a multi-agency Federal group to develop readily verifiable criteria by which to identify trials that meet an appropriate standard of quality. On October 20, 2000, AHRQ held a public meeting to gather pertinent information and views that would contribute to defining the qualifying criteria used to identify sound clinical trials appropriate for Medicare coverage. The qualifying criteria was developed under the authority to support health care research in section1142 of the Act.
Comment: A commenter stated that TCET is not genuinely voluntary and restricts access. This commenter asserts that “CMS downplays the reality that manufacturers who do not follow through with the TCET pathway and subject themselves to CED requirements are virtually excluded from Medicare coverage altogether without regard to the implications for beneficiaries resulting from lack of access.”
Response: We disagree. Coverage under CED can expedite beneficiary access to innovative technologies (and result in improved health outcomes) while ensuring that systematic patient safeguards—including assurance that the technology is provided to clinically appropriate patients—are in place that reduce the risks inherent to new technologies, or to new applications of older technologies. CMS may cover certain items and services under the CED pathway that would otherwise not satisfy the reasonable and necessary standard. In the absence of CED, technologies with limited evidence could be noncovered. Participation is voluntary for beneficiaries in CED studies. Receipt of an item or service under a CED NCD is voluntary.
Comment: A commenter asserted that CMS' use of the NCD process, and CED more specifically, to establish coverage under TCET interferes with the practice of medicine. This commenter cited section 1801 of the Act and stated that “CMS' attempt to supervise or control healthcare provider qualifications, healthcare settings, and recipients of healthcare services violates the statute.”
Response: We acknowledge that under section 1801 of the Act Federal officers and employees are not authorized to exercise any supervision or control over the practice of medicine or the manner in which medical services are provided, or over the selection, tenure, or compensation of any officer or employee of any institution, agency, or person providing health services; or to exercise any supervision or control over the administration or operation of any such institution, agency, or person. We disagree, however, with commenter's suggestion that NCDs, CED, and the TCET proposal interfere in the practice of medicine.
As noted previously, the Medicare statute includes a number of restrictions that limit payment for items and services under Part A and Part B of Title XVIII. Medicare does not cover every item or service just because it was recommended by a physician or healthcare practitioner. Moreover, NCDs do not restrict the practice of medicine, but do inform beneficiaries and practitioners in advance when particular items and services will be covered (or not covered) nationally under Title XVIII. NCDs are binding authorities for Medicare contractors and adjudicators, but not medical practitioners (see 42 CFR 405.1060 ). NCDs ensure that similar claims are processed and paid in a uniform manner. Physicians can still prescribe or order other services that will not be paid by Medicare, and the beneficiary may agree to pay for items or services that Medicare does not cover. CMS' role in making NCDs is consistent with the agency's statutory authority.
Comment: A commenter questioned if obtaining an NCD without CED would be possible under TCET.
Response: Yes, an NCD without CED is an option if there is sufficient evidence to support Medicare coverage under section 1862(a)(1)(A) of the Act.
Comment: A commenter claimed that CMS' proposal for the TCET pathway undermines FDA's Breakthrough Devices Program and postmarket requirements.
Response: We do not agree that CMS is undermining FDA's Breakthrough Devices Program and postmarket requirements. When we find that the medical evidence is insufficient to permit Medicare payment under section 1862(a)(1)(A) of the Act, we often consider whether an item or service may still be clinically beneficial to patients within the Medicare population. The limited coverage that we provide to those beneficiaries that elect to participate in clinical studies through CED does not interfere with FDA's role under that agency's separate statutory authority. Further, there is opportunity under TCET to leverage an FDA-required postmarket study, if any, to address specific evidence gaps for Medicare beneficiaries.
Comment: Some commenters expressed that CMS should have issued the proposal as a proposed rule rather than a notice to facilitate meaningful changes and address key issues that hinder beneficiary access.
Response: We do not agree that a proposed rule is required to establish a procedural rule. The TCET pathway establishes procedures for the effective and efficient operations of the agency designed to expedite national coverage of Breakthrough Devices. The notice does not establish or change a substantive legal standard but establishes a process to identify specific evidence gaps and creates a framework to fill those missing evidentiary gaps. Establishing TCET through a proposed procedural notice enabled CMS to consider public comments but also has the advantage that the procedures may be modified as necessary as the Agency, manufacturers, and the public gain experience using the process. The procedural notice is important to explain how the public and TCET sponsors can work with CMS with respect to coverage for certain Breakthrough Devices and addresses key issues that may have hindered beneficiary access in the past. The TCET pathway is intended to balance multiple considerations when making coverage determinations: (1) facilitating early, predictable, and safer beneficiary access to new technologies; (2) reducing uncertainty about coverage by evaluating early the potential benefits and harms of technologies with manufacturers; and (3) encouraging evidence development if evidence gaps exist. Further, the TCET pathway aims to coordinate benefit category determination, coding, and payment reviews and to allow any evidence gaps to be addressed through fit-for-purpose (FFP) studies. The anticipated result of the new coverage pathway would be faster access to technologies within a predictable coverage framework that generates clinical evidence for the Medicare population.
Comment: Several commenters urged CMS to finalize the TCET pathway quickly and commit to periodic refinements as needed as experience is gained.
Response: We appreciate these comments. CMS has moved as quickly as possible to review and respond to the 150 comments received on the proposed notice and issue a final notice. We acknowledge that refinements to the TCET pathway may be needed as CMS, manufacturers, and other interested parties gain more experience.
Comment: Several commenters noted CMS' ambitious timelines for the TCET pathway and questioned whether CMS' timelines are realistic. Some of these commenters encouraged CMS to be forthcoming with realistic timelines. A few commenters suggested that CMS provide coverage for Breakthrough Devices sooner than the timeline proposed. Some commenters requested that CMS provide more definitive timelines.
Response: We appreciate these comments. We agree that it is important to provide reasonable and realistic timelines. In general, we believe our timelines are reasonable and realistic based on our experience and input from interested parties. While we understand that some would like faster and more definitive timelines, we have also heard that we should provide as much flexibility as possible, given that all interested parties may experience unanticipated obstacles and delays as they gain experience with TCET. We are making one specific timeline update in the final notice to specify that we will consider TCET nominations on a quarterly basis, rather than acting upon them within 30 days of submission. This additional time provides a more realistic timeframe for CMS to coordinate with the manufacturer and, as appropriate, FDA on any outstanding issues and to begin internal discussions within CMS regarding operational issues. It will also allow CMS to prioritize between eligible devices and provide a fairer opportunity for participation in the TCET pathway, regardless of the anticipated timing of FDA's decision on market authorization. Additional information regarding this change is detailed below in this section under “C. Nominations.”
Comment: Some commenters questioned whether CMS could adhere to the timelines considering CMS' long-standing resource constraints. These commenters cited the potential for delays. A few of these commenters expressed that CMS should be held accountable for meeting all timelines indicated in the notice.
Response: CMS expects to adhere to the timelines outlined in the notice barring unexpected complications based on current resources, and we expect that manufacturers will do the same or at least provide as much notice as possible when complications are encountered. We have built in flexibility for all parties to help ensure the success of the new TCET pathway. We do not believe that imposing consequences on the Agency or manufacturers for missed deadlines would be helpful. As we gain more experience, we may modify aspects of the TCET pathway, including timelines, in the future.
Comment: Many commenters expressed concerns with the potential limit to five TCET candidates yearly. Some commenters contend that the limitation is arbitrary and would like CMS to clarify how this number was derived.
Response: We appreciate these comments. Based on our multiple periodic assessments of Breakthrough Devices, we anticipate that we will receive approximately eight nominations for the TCET pathway per year. Most Breakthrough Devices are not appropriate for TCET because they are not appropriate for Medicare beneficiaries (for example, pediatric technologies not indicated for use in children with ESRD). NCDs are limited to particular items or services but it is possible that more than one device could fall under the same NCD because it addresses the same indication. Based on current resources, we do not anticipate being able to accept more than five candidates into the TCET pathway per year. As we gain more experience with TCET, we will re-evaluate and adjust if we can do so within our available resources.
Comment: A commenter stated that CMS does not have statutory authority to limit the number of nominations. The commenter noted that they are unaware of any other coverage, coding or payment mechanisms that have instituted limits. The commenter also noted that MCIT had no limitation to the number of technologies to be approved or considered.
Response: There is no statute that establishes a fixed limit on the number of NCDs that CMS might issue per year; neither is there a statute that requires CMS to issue an unlimited number of NCDs. The anticipated number of TCET NCDs is based on current resources. As we gain more experience with TCET, we will re-evaluate and adjust as appropriate.
We note that MCIT was repealed before it became effective. As we noted in the November 2021 final rule, [ 16 ] MCIT had significant limitations. For example, the MCIT pathway did not require evidence development and did not include a mechanism to coordinate benefit category, coding, and payment reviews. Additionally, MCIT did not include beneficiary safeguards beyond limiting coverage to the FDA approved or cleared indication(s) for use.
There are a number of ways in which TCET is different, and in fact improves upon, MCIT. The TCET pathway is intended to balance multiple considerations when making coverage determinations: (1) facilitating early, predictable, and safer beneficiary access to new technologies; (2) reducing uncertainty about coverage by evaluating early the potential benefits and harms of technologies with manufacturers; and (3) encouraging evidence development if notable evidence gaps exist for coverage purposes. Further, the TCET pathway aims to coordinate benefit category determination, coding, and payment reviews and to allow any evidence gaps to be addressed through fit-for-purpose studies.
Comment: Commenters noted that the demand for TCET may outpace available resources. Numerous commenters expressed that resources should not hinder TCET and that all eligible devices should be accepted into the pathway. Several commenters stated that the limitation would constrain the pathway's potential and that TCET can be more impactful if additional technologies can be accommodated. A few of these commenters noted that CMS' limitation may create potential access issues for beneficiaries and market disruption. A commenter suggested CMS should consider how resources can be aligned to support a higher number accepted into TCET and reevaluate the number annually. Another commenter suggested that perhaps more devices can pursue TCET once efficiencies can be realized. A commenter suggested that if CMS needs to limit the number of technologies accepted into the pathway, CMS should wait 2 years before creating a cap.
Response: We anticipate no more than five per year because that is the largest number that we believe we can address within current resources. As we gain more experience with TCET, we will re-evaluate and adjust as appropriate based on available resources.
Comment: Numerous commenters expressed concerns with CMS' limited resources within and beyond the TCET pathway. Some commenters questioned how the NCD backlog would impact TCET. A commenter cautioned that an excessive emphasis on coverage review for TCET devices could delay consideration of important non-Breakthrough NCD requests.
Response: In addition to the TCET NCDs, we intend to continue issuing our typical number of non-TCET NCDs. As we gain more experience with TCET, we will re-evaluate and adjust our volumes as appropriate within our available resources.
Comment: Some commenters expressed concerns that AHRQ's resources are even more limited than CMS'. A commenter requested that AHRQ and CMS resources be assessed to support the manufacturer feedback needed for the Evidence Preview (EP) and Evidence Development Plan (EDP).
Response: A budget analysis for this activity is beyond the scope of this notice.
Comment: Several commenters stated that CMS should clarify how additional resources can expand the scope and breadth of the pathway.
Response: The proposal was based on current resources. As we gain experience with the process, including any efficiencies that may emerge over time, we will use that information to make adjustments as appropriate.
Comment: A commenter stated that CMS needs sufficient resources to ensure TCET is more utilized than Parallel Review has been to date.
Response: We continue to pursue the necessary resources to do our work.
Comment: A commenter expressed appreciation for CMS' acknowledgment of resource constraints and noted that TCET does not preclude coverage of Breakthrough Devices through existing coverage mechanisms such as claim-by-claim adjudication by MACs, LCDs, and the Parallel Review Program.
Response: We appreciate this comment and acknowledge that existing coverage mechanisms remain available for manufacturers of Breakthrough Devices to pursue Medicare coverage.
Comment: Numerous commenters expressed concerns that the proposed procedural notice did not adequately address the operational issues ( e.g., coding and payment issues) that could inhibit the successful implementation of the TCET pathway and would still need to be addressed. Commenters indicated that the goals of TCET cannot be achieved until these operational issues are resolved. Some commenters requested that CMS provide more specifics on the coding and payment processes. Numerous commenters cited the necessity of alignment among coverage, coding, and payment. They requested that CMS provide more specific information on how these processes will be coordinated under TCET and include timelines. A commenter encouraged CMS to collaborate internally to improve alignment among these processes.
Response: We appreciate these comments and agree that coordination of coverage, coding, and payment processes supporting the TCET pathway is important. We have established new internal collaborations to improve coordination going forward. CMS recently released the CMS Guide for Medical Technology Companies and Other Interested Parties website, which provides interested parties, including, but not limited to, medical device, pharmaceutical, and biotechnology companies, with information about Medicare's processes for determining coding, coverage, and payment as well as other key considerations. The Guide will be updated to include information related to TCET in the near future. This resource can be accessed here: https://www.cms.gov/medicare/coding-billing/guide-medical-technology-companies-other-interested-parties .
Comment: Some commenters suggested that CMS begin discussions regarding operational issues when a technology is accepted into TCET. A commenter recommended that CMS offer a system readiness meeting within 45 days of acceptance that discusses coverage, BCD, coding, and payment considerations to ensure overall alignment. A second system readiness meeting could be scheduled following the EP meeting and the manufacturer's decision to continue in the pathway. The timing for this meeting can be flexible depending on factors such as EDP development progress, FDA decision timing, and potential NCD opening date. The recommended meeting could also be an opportunity to discuss the EDP.
Response: While we appreciate the suggestion to incorporate a specific systems readiness meeting into the TCET process, we have not added it as a formal step at this time . Instead, we believe that a more informal approach will provide more flexibility and be less burdensome for manufacturers since each technology and manufacturer may have unique circumstances that could impact the timing of these discussions. We continue to explore opportunities to better align coverage, coding, and payment considerations for devices in the TCET pathway.
CMS proposed to limit the TCET pathway to certain eligible FDA-designated Breakthrough Devices, since we believe that this is the area with the most immediate need. In our proposal, we stated that appropriate candidates for the TCET pathway would include those devices that are—
- Certain FDA-designated Breakthrough Devices;
- Determined to be within a Medicare benefit category;
- Not already the subject of an existing Medicare NCD; and
- Not otherwise excluded from coverage through law or regulation.
CMS also indicated that the majority of coverage determinations for diagnostic laboratory tests granted Breakthrough designation status should continue to be determined by the MACs through existing pathways.
Comment: Commenters provided varied suggestions regarding which technologies should be eligible for the TCET pathway. Some commenters offering general support stated that the TCET pathway should be limited to a subset of technologies, specifically, as we proposed, to FDA-designated Breakthrough Devices. A few commenters stated that TCET should be limited to Breakthrough Devices to ensure it is unique from existing processes. Other commenters suggested that non-Breakthrough Devices should be eligible for TCET or similar coverage pathways because non-Breakthrough items and services also improve patient health outcomes. These commenters pointed out that there may be innovative technologies that they believe should be covered by Medicare that choose not to use FDA's Breakthrough Devices Program or may be an innovative technology that may not qualify for the designation. A few commenters provided recommendations for CMS to consider if the TCET pathway were to be expanded, including eligibility for FDA-designated Regenerative Medicine Advanced Therapy products and FDA's Safer Technologies for Medical Devices Program. They also recommended that CMS align the TCET pathway with the Cancer Moonshot.
Response: We appreciate these comments and the suggestions for expanding eligibility for the TCET pathway. Over the last several years, we have heard concerns that there is more uncertainty surrounding coverage of devices than for other items and services, such as drugs and biologics. For this reason, our proposal centered on Breakthrough Devices since we believed this was the area with the most immediate need, particularly considering the unique FDA criteria for Breakthrough designation status. We agree with commenters about the importance of promoting innovation across all items and services covered under Medicare. However, because we have consistently heard from interested parties about the need for more rapid coverage for Breakthrough Devices, we are focusing on Breakthrough Devices in this final notice. As the TCET pathway develops and proves successful, we may consider expanding its application to other items and services, contingent on sufficient available resources.
Comment: Some commenters expressed that Breakthrough Devices have very little evidence at the time of FDA market authorization to support Medicare coverage. A commenter encouraged caution in allocating Medicare resources for coverage of Breakthrough Devices under TCET, considering what the commenter described as the relatively low threshold of evidence required for Breakthrough Device designation. It was also noted that if Breakthrough Device coverage is expanded, coverage for other evidence-based and effective interventions could be reduced. Several commenters noted potential safety concerns with Breakthrough Devices. Multiple commenters recommended that CMS maintain rigorous evidence development standards. Commenters stressed the need to monitor the use and outcomes of these devices and build a mechanism to trigger an NCD reconsideration if FDA withdraws approval or there are postmarket safety concerns.
Response: We appreciate the comments. Please note that Medicare coverage of Breakthrough-designated devices would only occur if the device gains FDA marketing authorization. Breakthrough Devices are held to the same safety and effectiveness standards to receive FDA market authorization as other medical devices that do not have Breakthrough Device designation; Breakthrough Device designation does not represent a market authorization from FDA. Further, for CMS to provide coverage for Breakthrough Devices, there must be sufficient evidence to conclude that the evidence is promising, and that the device is potentially important for the Medicare population even if the available evidence is insufficient to satisfy the reasonable and necessary standard. Coverage in these circumstances would be contingent on further evidence generation under sections 1862(a)(1)(E) and 1142 of the Act. We believe the TCET evidence generation framework will facilitate the development of reliable evidence for patients and their physicians. It also provides safeguards to ensure that Medicare beneficiaries are protected and continue receiving high-quality care. Coverage under CED can expedite earlier beneficiary access to innovative technology while ensuring that systematic patient safeguards—including assurance that the technology is provided to clinically appropriate patients—are in place to reduce the potential risks associated with new technologies, or to new applications of older technologies.
We agree that CMS should reconsider an NCD for Breakthrough Devices if safety concerns arise. We noted in the proposed procedural notice and reiterate in this final notice that CMS retains the right to reconsider an NCD at any point in time. If an NCD is repealed, MACs could deny coverage for particular devices. CMS may also issue a national non-coverage NCD that would bar all coverage for the device.
Comment: CMS proposed that a Breakthrough Device must fall into an existing benefit category to be included under TCET. In general, commenters supported this proposal. However, several commenters recommended the inclusion of Breakthrough Devices that do not fall within an existing benefit category, for example, many digital health technologies. Several commenters requested CMS review and update current benefit category definitions to reflect technological advances. These commenters requested that CMS create new benefit categories or make a determination that an item or service (for example, software or other digital technologies) falls within a benefit category. Numerous commenters noted that CMS' current approach to benefit category determinations is limited and requested that CMS be more flexible in its approach, including modifying existing benefit categories to include these devices. A commenter requested that CMS provide clear direction on how TCET can support AI and software technologies for which no clear benefit category exists. A commenter suggested that CMS ensure prescription digital therapeutics (PDTs) are eligible for TCET even though there is no benefit category for them.
Response: CMS does not have authority to establish new Part B benefit categories; benefit categories are statutory and established by Congress. Consequently, some Breakthrough Devices will not fall within a Medicare benefit category and cannot be covered or paid by Medicare.
Comment: Many commenters requested that CMS eliminate the limitation for devices already the subject of an existing Medicare NCD. These commenters noted that there may be a situation where an NCD was broadly written, and the new product was not specifically mentioned. Commenters requested that CMS expand TCET eligibility criteria to include technologies with an existing NCD that receive Breakthrough designation from FDA for a novel indication that is non-covered under an existing NCD or unrelated to the existing NCD. A commenter provided an example of a device that received Breakthrough designation for what the commenter described as very different indications with different evidence and research needs.
Response: We appreciate these comments. However, we will maintain the limitation. If devices are subject to an existing NCD, a reconsideration of the NCD may be required to establish coverage.
Comment: Numerous commenters disagreed with CMS' proposal that coverage determinations for Breakthrough-designated diagnostic laboratory tests should continue to be made by Medicare Administrative Contractors under existing coverage mechanisms. Several commenters claimed that diagnostic laboratory tests are subject to the same coverage rules and regulations as other medical devices and are no more specific than other areas of medicine. These commenters further asserted that it may not be appropriate to defer coverage determinations to the MACs for these tests. A few commenters noted CMS' past precedent of issuing NCDs for diagnostic laboratory tests and cited examples. Some commenters stated that not providing Medicare coverage for some Breakthrough Devices, including diagnostics, under TCET may limit the options that a physician can recommend for a patient. A commenter claimed that the justifications CMS offers for its general exclusion of diagnostic laboratory tests from eligibility for the TCET coverage pathway do not adequately support exclusion from TCET eligibility and may delay Medicare beneficiary access to innovative tests. Some commenters requested that CMS permit diagnostic laboratory tests to be eligible for TCET or provide a similar pathway.
Response: We appreciate these comments and acknowledge that the Medicare coverage statute (section 1862(a)(1)(A) of the Act) applies to clinical diagnostic laboratory tests just like other items and services under Part A and Part B. While the TCET pathway is open to FDA Breakthrough-designated devices, CMS expects the majority of coverage determinations for Breakthrough-designated diagnostic laboratory tests will continue to be made by Medicare Administrative Contractors. We acknowledge there may be instances where manufacturers and CMS agree that an NCD is appropriate for a diagnostic laboratory test. In those instances where manufacturers believe that additional evidence generation may be needed to satisfy the Medicare coverage standard, we encourage manufacturers to contact CMS to discuss options for their specific technology.
Comment: Some commenters who disagreed with CMS' proposal noted that existing pathways, specifically the Molecular Diagnostic Services (MolDx) Program, [ 17 ] have limitations and cannot be viewed as an alternative mechanism to TCET to accelerate Medicare coverage. These commenters stated that MolDx is not a national program and that CMS' proposal to leave the majority of coverage decisions for diagnostic laboratory tests to the MACs through existing pathways would limit access to care in regions that do not participate in the program. In addition, some of these commenters noted that the MolDx program reviews only nucleic-acid (DNA or RNA)-based tests performed by clinical laboratories in 28 states within Palmetto's jurisdiction, so it is not relevant to non-molecular tests nor clinical laboratories in states located outside of the MolDx jurisdiction. Furthermore, a commenter noted that MolDx reviews only tests with existing local coverage determinations and is not authorized to impose CED requirements. A commenter noted that the current coverage pathway for diagnostic laboratory tests is fragmented and burdensome and results in unequal Medicare beneficiary access, particularly when the tests are provided by the manufacturers to laboratories nationwide.
Response: MolDx was not specifically mentioned in the proposed notice when we stated that we have historically delegated review of many diagnostic laboratory tests to specialized MACs and proposed that coverage should continue to be determined by the MACs through existing pathways.
Under MolDx, a program developed by the Palmetto MAC, the MAC determines coverage for molecular diagnostic tests and other molecular pathology services. Several other MACs have implemented the MolDx program as part of their operations. In the MolDx program, the MACs review all evidence that a manufacturer produces to determine whether an item or service meets the reasonable and necessary standard.
We note that Congress in section 1834A(g)(2) of the Act specifically granted the Secretary the authority to designate one or more MACs to establish coverage policies for clinical diagnostic laboratory tests and did not specify any exceptions for certain tests.
We acknowledge there may be instances where manufacturers and CMS agree that an NCD is appropriate for a diagnostic laboratory test. In those instances where manufacturers believe that additional evidence generation may be needed to satisfy the Medicare coverage standard, we encourage manufacturers to contact CMS to discuss options for their specific technology.
Comment: Several commenters requested that CMS clarify whether the TCET pathway excludes diagnostic laboratory tests and diagnostic tests generally. They also noted that CMS did not expressly reference in vitro diagnostic (IVD) products and seemingly omitted them from TCET.
Response: We appreciate these comments. The TCET pathway is limited to Breakthrough Devices meeting the eligibility criteria outlined in this notice. While we continue to believe that the majority of coverage determinations for diagnostic laboratory tests granted Breakthrough Designation should continue to be determined by the Medicare Administrative Contractors through existing pathways, we acknowledge there may be instances where manufacturers and CMS agree that an NCD is appropriate for a diagnostic laboratory test. In those instances where manufacturers believe that additional evidence generation may be needed to satisfy the Medicare coverage standard, we encourage manufacturers to contact CMS to discuss options for their specific technology.
In response to public comments seeking clarification regarding the scope of the references to diagnostic laboratory tests in the proposed notice, we have added language to clarify that we intend to refer to IVDs, including diagnostic laboratory tests, in the discussion of appropriate candidates. Other non-IVD diagnostic devices, such as diagnostic imaging devices, may be considered for TCET.
Comment: A few commenters noted that the scope of CMS' proposed exclusion of diagnostic laboratory tests in the TCET pathway is unclear. These commenters stated that CMS did not articulate criteria for determining which diagnostic laboratory tests would be eligible for TCET. They requested that CMS clearly define TCET eligibility criteria for certain diagnostic laboratory tests. A commenter stated that the existing process likely remains appropriate for most laboratory tests but requested that CMS confirm that it will consider nominations of diagnostic laboratory tests and other diagnostic technologies when the TCET pathway would ensure timely beneficiary access and support further evidence generation. This commenter also encouraged CMS to consider how collaboration with the specialized MACs could provide the expertise and resources needed to develop a CED NCD under the TCET pathway for a particular diagnostic technology.
Response: We appreciate these comments. We continue to believe that that the majority of coverage determinations for IVD products, including diagnostic laboratory tests, granted Breakthrough Designation should continue to be determined by the Medicare Administrative Contractors through existing pathways. We acknowledge there may be instances where manufacturers and CMS agree that an NCD is appropriate for an IVD product. In addition to TCET, if a manufacturer of a Breakthrough-designated IVD product wishes to seek national coverage, they may submit an NCD request as outlined in 78 FR 48164 . Other, non-IVD diagnostic devices may be considered for TCET, contingent on there being an applicable benefit category.
CMS proposed that the appropriate timeframe for manufacturers to submit TCET pathway nominations is approximately 12 months prior to the anticipated FDA decision on a submission as determined by the manufacturer. In the proposal, CMS stated that manufacturers of certain FDA-designated Breakthrough Devices may self-nominate to participate in the TCET pathway. The proposed notice outlined the information that manufacturers should include in the self-nomination packet. CMS' proposal explained how CMS intended to consider nominations, including a meeting with the manufacturer to discuss the technology and a CMS-FDA meeting to learn more information about the technology. The proposal also noted that a technology may undergo a benefit category review as part of the nomination review process.
Comment: Commenters generally agreed with our proposal that nominations be submitted approximately 12 months before anticipated FDA marketing authorization. Some noted that early engagement between CMS and manufacturers before FDA authorization can inform and enable a more efficient and effective evidence-generation strategy.
Response: We appreciate these supportive comments and agree that early engagement between CMS and manufacturers is important.
Comment: Commenters encouraged CMS to be flexible with the timeframe for submitting nominations and expressed various timeframes for CMS to consider. A commenter suggested a 6-month timeframe is more realistic for nominations to ensure manufacturers can provide CMS with robust data. Other commenters encouraged CMS to provide an earlier self-nomination timeframe. These commenters suggested that CMS build in additional time “to align trial design requirements” and establish BCD, coding, and payment amounts. Another commenter recommended that nominations be accepted “following FDA approval into the Breakthrough Device status program” to provide more time to obtain feedback to inform EDP development. Several commenters suggested that CMS align nomination timing to an FDA milestone.
Response: We appreciate these suggestions. We agree with commenters that providing some flexibility in terms of nomination timeframes is important. However, CMS believes that 12 months prior to anticipated FDA market authorization is the appropriate timeframe that allows for TCET procedural steps to be completed and for better coordination of coding and payment. In this final notice we have modified the TCET pathway procedures to include an opportunity for a manufacturer to submit a non-binding letter of intent to nominate a potentially eligible device approximately 18 to 24 months before the manufacturer anticipates FDA marketing authorization. While formal nominations will still be considered approximately 12 months prior to anticipated market authorization, the submission of a non-binding letter of intent will improve CMS' ability to track potential candidates, coordinate with FDA, and make operational adjustments.
Comment: A commenter recommended that CMS build a meeting at “the pivotal trial milestone” into the process, which the commenter stated often occurs earlier than 12 months before FDA market authorization.
Response: While we are not incorporating a meeting into the process at a milestone tied to initiation or completion of certain clinical trials, meetings may occur between CMS and manufacturers in instances where manufacturers have chosen to submit a non-binding letter of intent approximately 18 to 24 months prior to FDA market authorization.
Comment: A commenter asserted that CMS' proposed nomination timelines might be a significant burden on clinical teams building an evidence strategy that satisfies both FDA and CMS and requested that CMS consider ways to improve the nomination submission timelines to minimize burden for manufacturers.
Response: We disagree. We believe our proposal for nominations to be submitted approximately 12 months before anticipated FDA marketing authorization is minimally burdensome and provides adequate flexibility for manufacturers to: (1) provide supportive evidence for their technology; (2) develop an EDP to address material evidence gaps for CMS coverage; and (3) coordinate BCD, coding, and payment processes. There is opportunity under TCET to leverage FDA-required postmarket studies, if any, to address specific evidence gaps for Medicare beneficiaries.
Comment: Some commenters provided feedback regarding specific timeframes in the TCET nomination process. A few commenters supported CMS' proposal to respond to nominations within 30 days. Another commenter requested that CMS extend the nomination review period to 60 days rather than 30 to ensure rigorous evaluation and selection of the most promising technologies for the TCET pathway.
Response: We appreciate these comments. We agree it is important to provide timely feedback to manufacturers on whether their technology is a suitable candidate for TCET. CMS is clarifying that suitable candidates will be approved for the TCET pathway on a quarterly basis. Consideration of TCET nominations on a quarterly basis will allow CMS to prioritize the most promising devices, will facilitate TCET implementation, and will establish a fair opportunity for eligible devices to be considered, regardless of the timing of FDA market authorization.
Comment: Many commenters recommended that CMS provide a lookback period, meaning that Breakthrough Devices that are nearing an FDA decision on market authorization (that is, less than 12 months) or those recently achieving authorization would be eligible for the TCET pathway. Several commenters recommended that a 3-year lookback period would be appropriate.
Response: We disagree and did not include a lookback period in the proposed notice. While we appreciate the substantial interest in the TCET pathway, it is designed to expedite national coverage through extensive premarket engagement. Developing an evidence development plan (EDP) generally takes considerable time, and absent an adequate lead time during the pre-market period, devices already available in the market are more appropriate for an NCD outside of the TCET pathway or for MAC determinations under section 1862(a)(1)(A) of the Act.
Comment: A commenter requested that CMS provide specific nomination guidance.
Response: We appreciate this comment. CMS will consider releasing more specific nomination information in the future.
Comment: Some commenters requested that CMS provide additional guidance on how it will treat nominations equitably when it receives many more than anticipated and/or an irregular pattern of nominations. For example, a commenter questioned how CMS would proceed when five candidates have already been accepted for the year and additional nominations come in. A commenter suggested that CMS define a set number of application cycles per year to limit first-come-first-served application bias.
Response: We appreciate these comments and acknowledge the importance of providing more transparency to the public on how CMS will prioritize TCET nominations. In this final notice, we are clarifying that suitable candidates will be approved for the TCET pathway on a quarterly basis. If a nomination is not accepted into the pathway in one quarterly review cycle, it may be considered again in the following quarterly review cycle. Manufacturers will not need to resubmit a nomination for it to be considered in a subsequent quarter. Since TCET is forward-looking and extensive pre-market engagement is essential, nominations for Breakthrough devices anticipated to receive an FDA decision on market authorization within 6 months may not be accepted since CMS will be unable to reach a final NCD within the expedited timeframes.
To provide greater transparency, consistency, and predictability we intend to release proposed prioritization factors for TCET nominations in the near future. We look forward to communicating additional details on our planned approach in the near future and will provide an opportunity for public comment.
Comment: A commenter requested that in instances where CMS declines a nomination, it should provide a rationale and feedback mechanism for the manufacturer. Another commenter stated that applicants should be permitted to reapply.
Response: CMS will provide a justification and contact information for additional information if we decline a nomination. CMS is clarifying in this final notice that eligible nominations will be considered for the TCET pathway on a quarterly basis. If not accepted into the TCET pathway in one quarter, nominations may be considered again in the subsequent quarter. Manufacturers will not need to resubmit a nomination to be considered in a subsequent quarter. However, since TCET is forward-looking and extensive pre-market engagement is essential, nominations for Breakthrough Devices anticipated to receive an FDA decision on market authorization within 6 months may not be accepted since CMS will be unable to reach a final NCD within the expedited timeframes.
Comment: A commenter noted that it would be helpful if CMS could create a TCET submission web page like the IDE Category A and B submission web page and include instructions, application questions, and a TCET checklist.
Response: We appreciate this comment. We intend to create several web pages to support the TCET procedural pathway and provide important process-related information to interested parties.
Comment: Several commenters requested that CMS streamline the TCET nomination process by eliminating or combining some steps.
Response: We appreciate these comments. However, we believe all steps of the TCET nomination process are important to successfully implementing the pathway. However, as we gain experience with TCET, we will consider revising the process as appropriate.
Comment: A commenter recommended that CMS permit manufacturers to provide information on how their devices promote health equity.
Response: We welcome and strongly encourage any information manufacturers wish to provide regarding how their devices promote health equity.
Comment: Many commenters expressed their support for enhanced FDA-CMS collaboration to support the TCET pathway, and more specifically, to foster alignment between FDA and CMS evidence development needs to ensure CMS evidence development requirements are not duplicative or contradictory with FDA requirements. Further, commenters stated that FDA and CMS should provide early clarity about postmarket evidence generation requirements to minimize provider and product developer burden.
Response: We appreciate these comments. CMS and FDA have taken a number of concrete steps to enhance alignment that will support the TCET pathway. For example, CMS and FDA intend to collaborate in identifying therapeutic areas where CMS clinical endpoint guidance would be most impactful. Additionally, by CMS articulating material evidence gaps for CMS coverage prior to FDA market authorization of devices, manufacturers will be better positioned to efficiently satisfy FDA and CMS requirements.
Comment: Some commenters would like additional information on how and when CMS will collaborate with FDA specific to the TCET pathway. Some commenters sought clarity as to whether manufacturers would be permitted to participate in meetings between FDA and CMS. It was suggested that CMS should provide a transcript to manufacturers if they are not allowed to participate in the meetings with FDA. A few commenters recommended that manufacturers be able to participate in the first meeting with FDA and for subsequent meetings only when there are specific questions that need to be addressed that the manufacturer is better positioned to answer so as not to hold up timelines.
Response: We appreciate these comments. As we outlined in the proposed notice and consistent with the FDA-CMS MOU, CMS may meet with FDA when considering a TCET nomination submitted for CMS review so CMS can learn more about the technology, including potential timing considerations. Some of these meetings may be deliberative and not appropriate for manufacturers or any other non-governmental parties to participate. However, similar to meetings conducted for parallel review, there may be occasions where it will be helpful to have CMS, FDA, and manufacturers participate in a meeting, and CMS will consider these requests on a case-by-case basis.
Comment: Commenters requested additional clarification regarding the process and timeline for benefit category determination reviews. These commenters note that the lack of an integrated, transparent, expedited BCD process will limit TCET's impact. A commenter sought additional information on how CMS will determine the devices that will undergo a BCD review and whether there will be an appeal mechanism.
Response: We note that new products may fall within one or more benefit categories or no benefit category at all. As stated in the proposed notice, if CMS believes that the device, prior to a decision on market authorization by FDA, is likely to be payable through one or more benefit categories, the device may be accepted into the TCET pathway. This is an interim step that is subject to change upon FDA's decision regarding market authorization of the device. Acceptance into TCET should not be viewed as a final determination that a device fits within a benefit category.
When CMS issues the proposed NCD following approval or clearance of the Breakthrough Device by FDA, the proposed NCD will include one or more benefit categories to which CMS has determined the Breakthrough Device falls. CMS will review and consider public comment on the proposed NCD before reaching a final determination on the BCD(s).
Comment: A commenter recommended that CMS provide a benefit category review for any FDA-designated Breakthrough Device earlier in the process, possibly when FDA is meeting with manufacturers of these devices regarding the design of their clinical trials to support FDA marketing authorization.
Response: The BCD may rely on information generated during the process to obtain FDA market authorization, making an earlier BCD infeasible. Additionally, not all devices achieve marketing authorization, so conducting a BCD review earlier would be inefficient and potentially waste CMS resources.
Comment: A few commenters expressed that BCDs can be made quickly and should not delay access. A commenter indicated that CMS should commit to making BCD decisions no later than 30 days after the nomination submission.
Response: CMS aims to make all BCD decisions expeditiously. CMS is unable to commit to making all BCD decisions within 30 days of nomination submission because the BCD may rely on information generated during the process to obtain FDA market authorization making an earlier BCD infeasible.
Comment: A commenter stated that when there is an issue in determining the BCD, a meeting between CMS' Center for Medicare and the manufacturer should be scheduled immediately.
Response: We appreciate these comments and agree that it is important for CMS to provide timely communication to the manufacturer when there are issues in determining the BCD.
CMS' proposal introduced the Evidence Preview (EP) concept, which is a focused literature review that would provide early feedback on the strengths and weaknesses of the available evidence, including any evidence gaps, for a specific item or service. It is intended to inform judgments by CMS and manufacturers about the best available coverage options for an item or service and offers greater efficiency, predictability and transparency to manufacturers and CMS on the state of the evidence and any notable evidence gaps. In the proposed notice, CMS expressed the intent for EPs to be supported by a contractor using standardized evidence grading, risk of bias assessment, and applicability assessment. CMS proposed that the EP would be made publicly available on the CMS website when a tracking sheet is posted announcing the opening of the NCD. Additionally, CMS proposed to share the EP with the Medicare Administrative Contractors following a manufacturer's decision to withdraw from the TCET pathway.
Comment: Some commenters requested that CMS provide more transparency regarding the evidence review contractor. Specifically, these commenters requested that CMS provide more information on the processes used to select and monitor the evidence review contractor and information regarding qualifications, safeguards, conflicts of interest, and how the contractor will be evaluated. Some of these commenters requested that CMS publish a list of contractors used for conducting evidence reviews on its website.
Response: The Secretary has broad authority to contract out functions under Title XVIII. CMS, in collaboration with AHRQ, established rigorous review criteria that have undergone detailed testing during the past year and are reflected in the 2024 CMS National Coverage Analysis (NCA) Evidence Review guidance. [ 18 ] The contractor's role is to conduct a rapid systematic literature review and summarize the evidence based on a modified Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) methodology. CMS and the contractor have also begun recruiting and incorporating clinical subject matter experts into the review process. All external subject matter experts are carefully assessed for their expertise, screened for conflicts of interest, and bound by non-disclosure agreements. The contractor supports and accelerates CMS reviews, but CMS performs extensive quality assurance on contracted reviews, contributes substantial portions of the EP independently, and ultimately determines policy. If an NCD is opened, an evidence summary will be included with the tracking sheet for full public comment, including which contractor completed the review.
Comment: Several commenters sought clarity on how the contractor will perform evidence reviews under TCET, specifically the criteria that the contractor will use to do the evidence preview. Some commenters requested that CMS specify whether these standards are the same or different from current processes. Commenters also asked that CMS define the evidence grading system used and what kind of evidence review conclusions are possible.
Response: We appreciate these comments. When nominating devices for the TCET pathway, manufacturers should submit a comprehensive bibliography of published studies for their device. For some devices, studies will not yet be published in the peer-reviewed literature, and CMS will instead review unpublished reports of clinical studies intended to support the FDA marketing application provided by the manufacturer. The contractor will supplement these materials with a focused search of published peer-reviewed literature. The contractor will use standardized tables to summarize the characteristics of each study included in their focused literature review. These tables provide information about each study's design, quality, interventions assessed, target population, and outcomes assessed.
The methodological quality, or risk of bias, for randomized and nonrandomized individual study designs will be assessed using a components approach, considering each study for specific aspects of design or conduct (such as allocation concealment for randomized controlled trials (RCTs) or use of methods to address potential confounding), as detailed in AHRQ's Methods Guide. [ 19 ] Studies of different designs are graded within the context of their respective designs. Thus, RCTs are graded as good, fair, or poor, and observational studies are separately graded as good, fair, or poor.
The contractor will also assess the applicability of the included studies to the Medicare population. Specifically, we plan to use the PICOTS (population, intervention, comparator, outcomes, timing, and setting) format to organize information relevant to applicability. The most important issue concerning applicability is whether the outcomes are different across studies that recruited diverse populations (for example, age groups, exclusions for comorbidities) or used different methods to implement the interventions of interest; that is, important characteristics are those that affect baseline (control group) rates of events, intervention group rates of events, or both.
Lastly, the contractor will identify and list any relevant evidence-based guidelines, specialty society recommendations, consensus statements, or appropriate use criteria that apply to the item or service addressed by the Evidence Preview (EP). The reviewed evidence is then qualitatively synthesized by the contractor. There are strict non-disclosure agreements in place with the contractor to ensure the protection of proprietary information.
Comment: Some commenters expressed concerns that CMS was ceding decision-making to the evidence review contractor. These commenters noted that the evidence review contractor should be prohibited from making qualitative assessments of the literature and providing any statements regarding medical necessity. Further, these commenters stated that CMS should maintain ultimate decision-making responsibility and CMS staff should be fully engaged to ensure that feedback among all participants is transparent and timely.
Response: All decision-making resides with CMS. CMS does not delegate the Secretary's authority to establish NCDs to a contractor. The role of the evidence review contractor is to support the CMS review team by summarizing the available evidence in a standardized format. CMS staff specify the review requirements, supervise the contractor, and conduct extensive quality assurance of all reviews. Additionally, one of the benefits of utilizing an evidence review contractor is that it will enhance CMS' ability to recruit specialized clinical expertise. Lastly, CMS contributes substantial portions independently and maintains ultimate decision-making responsibility. Any formal determination regarding whether an item or service meets the reasonable and necessary statutory standard will be made by CMS and completed using the NCD process, which includes at least one public comment period.
Comment: Some commenters stated that manufacturers should be able to communicate directly with the evidence review contractor during the development of the EP. Several commenters suggested that CMS establish contact points to facilitate dialogue between the manufacturer and the contractor responsible for conducting the EP. Some commenters recommended that manufacturers should have an opportunity to provide feedback on the literature search strategy, correct errors, and/or discuss interpretations of data in the EP. Commenters encouraged CMS to hold meetings before the EP to ensure appropriate search terms were incorporated and to discuss results after the EP. A commenter stated that CMS should allow manufacturers 30 days to return comments on the EP.
Response: We disagree that manufacturers should be able to contact the contractors that the government has engaged to summarize the scientific evidence on its behalf. CMS notes that manufacturers must submit a full bibliography of published studies with their TCET nomination. Much of the EP is written directly by CMS staff, and manufacturers have an opportunity to provide feedback on a draft of the EP before it is finalized.
CMS will establish CMS-staff-level contact points to facilitate timely communication with manufacturers, but CMS disagrees with having manufacturers communicate directly with the evidence review contractor. The contractor is performing work on CMS' behalf, and CMS needs to be involved in all discussions. Manufacturers are encouraged to review and provide feedback to CMS on the EP as soon as possible, ideally within 30 days. CMS believes it is important to provide flexibility especially as manufacturers gain experience with the TCET pathway and is not incorporating a specific timeframe in the final notice for manufacturers to submit feedback on an EP.
Comment: A commenter noted that truly novel devices might have completed only one study when applying for TCET, and “in some cases, the only published or presented data will be from the first in man or preliminary FDA safety studies.” The commenter expressed the position that devices with minimal data should not be excluded from TCET, and for devices with little published data, CMS should focus on “FDA pivotal trial results” in the EP even if those results are not published.
Response: The EP is a focused literature review summarizing the strengths and weaknesses of the available clinical evidence supporting a review request. The EP is not a national coverage analysis (NCA) and is not a commitment to coverage. The EP is intended to inform decisions about the best available coverage options for the nominated device. Further, a broader range of studies may be included in a full national coverage analysis (NCA) if one is opened. The EP reflects the best available information at the time it is conducted, but multiple elements of the EP may evolve during the review process.
When developing a literature search, we will carefully review the bibliography that manufacturers provide in their nomination. CMS recognizes that the most crucial study data from pivotal trial(s) may not yet be published in the pre-market stage. If unpublished studies are included in the review, the evidence review in the NCA, if one is opened, will reflect the final labeling of the FDA market authorized device and supplemental analyses and/or published, peer-reviewed report of the clinical study.
Comment: Several commenters requested that CMS clarify that the EP is a summary of the published peer-reviewed literature in the relevant clinical space and an examination of the outcomes of interest to CMS, associated endpoints, and clinically meaningful differences for the target disease or condition. These commenters further noted that the EP should not extend to include a gap analysis for the specific nominated technology. Additionally, some commenters requested that the EP explicitly state that it is not a coverage determination and should not be interpreted as a reasonable and necessary determination. A commenter noted that the EP, as currently constructed, will provide limited insight into device performance. Some commenters requested that the EP meeting between the manufacturer and CMS avoid bias toward additional data collection, especially when a device has robust clinical evidence.
Response: The EP is a focused literature review that summarizes the strengths and weaknesses of the available clinical evidence, including any evidence gaps for CMS coverage for a specific item or service. It offers greater efficiency, predictability, and transparency to manufacturers and CMS on the state of the evidence and any notable evidence gaps. It is intended to inform judgments by CMS and manufacturers about the best available coverage options for an item or service.
We disagree with commenters that an evidence preview should not be specific to a particular technology. We believe it is important to understand any material evidence gaps for CMS coverage for a particular technology as early as possible so that manufacturers can develop a plan to address them so that the device might be eligible for future Medicare coverage under section 1862(a)(1)(A) of the Act.
Comment: A commenter suggested that CMS clarify circumstances where they can convene a Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) or otherwise solicit broad public input to align on evidence gaps in the evidence preview stage and explain how this might affect evidence review timelines.
Response: We appreciate this comment. If a MEDCAC is needed to clarify the appropriate clinical endpoints for a particular device, the TCET review timeframes could be substantially delayed. The need for MEDCACs during a TCET review may be mitigated by identifying potential TCET candidates earlier in the review cycle than the timeframe we proposed in the June 2023 notice. When CMS is aware that manufacturers intend to pursue the TCET pathway for devices and appropriate clinical endpoints are uncertain, we may preemptively conduct a clinical endpoints review and may convene a MEDCAC. A CMS decision to initiate a clinical endpoint review or a MEDCAC does not imply that a benefit category exists for a particular device or make a commitment to make a local or national coverage determination. We clarify in the final notice how a MEDCAC may affect evidence review timelines and how submitting a non-binding letter of intent can help alleviate potential delays if a clinical endpoints review and/or MEDCAC is needed.
Comment: A commenter suggested that CMS reconsider the evidence preview process, which they believe should also include other considerations, such as shifts in the market, health equity, population considerations, accessibility, usability, and other metrics.
Response: While we acknowledge those factors may be important, the purpose of the evidence preview is to provide early feedback on the strengths and weaknesses of the available clinical evidence, including any evidence gaps, for a specific item or service. It is intended to inform judgments by CMS and manufacturers about the best available coverage options for an item or service. It offers greater efficiency, predictability, and transparency to manufacturers and CMS by clarifying the state of the evidence and any notable evidence gaps.
Comment: Many commenters disagreed with CMS' proposal to share the Evidence Preview with the Medicare Administrative Contractors following a manufacturer's decision to withdraw from the TCET pathway. These commenters expressed concerns with how the MACs would use this information, specifically that it would lead to de facto noncoverage without going through the full national coverage process. While commenters were generally opposed to CMS sharing this information, they provided some recommendations. They suggested alternatives if CMS chose to proceed, including obtaining manufacturer consent before sharing with MACs, updating the Program Integrity Manual to describe the appropriate use of EPs as MACs make coverage decisions, as well as scaling back the evidence preview so it is not specific to a particular technology.
Response: As we noted in the proposed notice, the EP represents a substantial investment of public resources, and CMS wants to ensure we use taxpayer dollars wisely. The EP includes a summary of the available evidence for an FDA Breakthrough-designated device; manufacturers can correct any errors and provide feedback before finalization. While CMS believes the EP will be a fair reflection of the strength of the available evidence to support Medicare coverage, CMS acknowledges that manufacturers may withdraw from the TCET pathway for reasons unrelated to the evidence. Based on the previous considerations and in response to public comments, CMS will publish an evidence summary without the evidence gap analysis if a manufacturer withdraws from the TCET pathway. Similarly, only a summary of the evidence would be posted with a tracking sheet if a national coverage analysis is opened. We believe this approach offers transparency, makes judicious use of public resources, and does not signal a specific conclusion about whether an item or service satisfies the reasonable and necessary standard.
Comment: A few commenters supported CMS sharing the evidence preview with the MACs. A commenter recommended that not only should the MACs receive the EPs but that they should be shared with Medicare Advantage Organizations as well. Another commenter suggested that these EPs be shared publicly.
Response: We appreciate these comments. After considering all public comments received on this issue, CMS has decided to publish an evidence summary without the evidence gap analysis rather than sharing the full EP with the MACs as we proposed.
CMS' proposal stated that upon finalization of the EP, the manufacturer may decide to pursue national coverage under the TCET pathway or to withdraw from the pathway. CMS proposed that if the manufacturer decided to continue, the next step would include a manufacturer's submission of a formal NCD letter expressing the manufacturer's desire for CMS to open a TCET NCD analysis. We stated in the proposal that most, if not all, of the information needed to begin the TCET NCD would already be included in the TCET pathway nomination and the EP, but we invited the manufacturer to submit any additional materials the manufacturer believed would support the TCET NCD request.
Comment: A commenter stated that it is unclear whether the manufacturer or CMS would initiate an NCD.
Response: If a manufacturer decides to continue pursuing coverage under the TCET pathway upon finalization of the EP, the next step is for the manufacturer to provide a formal NCD letter to CMS expressing the manufacturer's desire for CMS to open an NCA. The manufacturer would initiate the NCD request.
Comment: A commenter requested confirmation that CMS will not issue a non-coverage NCD if a manufacturer withdraws from TCET.
Response: There could be rare instances where a non-coverage NCD would be in the best interest of Medicare beneficiaries, such as when the evidence points to potential serious beneficiary harm. CMS can conduct a national coverage analysis at any time to swiftly act in those circumstances.
CMS' proposal introduced the Evidence Development Plan (EDP) concept. CMS proposed that EDPs would be developed by the manufacturer to address any evidence gaps identified in the EP. In the proposal, CMS indicated that EDPs may include fit-for-purpose (FFP) study designs, including traditional clinical study designs and those that rely on secondary use of real-world data, provided that those study designs follow all applicable CMS guidance documents. CMS proposed that the development of an EDP would include CMS-AHRQ collaboration to evaluate the EDP to ensure that it meets established standards of scientific integrity and relevance to the Medicare population. Additionally, CMS proposed that the EDP process will include CMS engagement with the manufacturer to provide feedback and discuss any recommended refinements. The proposal stated that elements of the EDP, specifically the non-proprietary information, would be made publicly available on the CMS website when a proposed NCD is posted.
Comment: Many commenters supported TCET's evidence development framework, specifically CMS' inclusion of a more flexible approach that allows for FFP studies. However, some commenters noted that CMS should maintain rigorous evidence development standards. Commenters pointed out that any evidence development framework should be patient-centered, and high-quality evidence should be required to protect beneficiaries.
Response: We appreciate these comments and agree that including a more flexible approach that allows for FFP studies is important. We believe that the evidence development framework for TCET outlined in this notice accomplishes that goal while also being patient-centered and facilitating the generation of high-quality evidence to support Medicare coverage. CMS expects to propose FFP study guidance in the future, with a particular emphasis on study designs that make secondary use of real-world data.
Comment: Commenters generally supported CMS' proposal regarding evidence development plans. Some commenters encouraged CMS to work with manufacturers to develop a reasonable, mutually agreed upon data collection and review period in the EDP. A commenter suggested that CMS consider structuring evidence development around achievement of milestones rather than time. A commenter recommended that the EDP be updated annually. The commenter recommended that CMS assign dedicated staff to work collaboratively with the manufacturer when developing the EDP. Some commenters cited that considerable time may be required to develop and negotiate an EDP. Another commenter expressed that an NCD should not be opened until an EDP is approved.
Response: We appreciate the supportive comments. For devices where the evidence is promising but does not yet satisfy the reasonable and necessary standard for Medicare coverage, the EDP intends to articulate how material evidence gaps will be addressed during transitional coverage so that the evidence may show that the device satisfies the reasonable and necessary standard when a CED NCD is reconsidered. Manufacturers often plan multiple studies for devices that are newly in the market. For example, they may plan conventional clinical studies in non-US markets, or conventional studies that test modifications to an existing device. If generalizability to the Medicare population is an important limitation of the existing evidence base, manufacturers may submit an FFP study protocol that relies on secondary use of real-world data as a component of their EDP. The EDP will describe the overall portfolio of planned studies and identify the appropriate timing of a future CED NCD reconsideration. We agree that providing enhanced engagement and flexibility is important during EDP development, and we are exploring ways that CMS can support manufacturers in efficiently developing FFP protocols, but manufacturers are responsible for developing their own EDPs. CMS agrees that a CMS and AHRQ-approved EDP should be in place prior to opening an NCD. We note that prolonged delays by manufacturers in drafting EDPs may substantially delay the finalization of a CED NCD under the TCET pathway.
We are finalizing our proposal to have EDPs include a schedule of updates and interim analyses along with a projected NCD reconsideration window. CMS continues to believe that a core purpose of the EDP is to anticipate the appropriate timing of reconsideration, but we recognize that timelines may in some cases need to be revised. Particularly for EDPs that propose longer CED timeframes, CMS agrees that they should include plans for interim reporting to ensure adequate progress and timely completion. Interim reports should also disclose any meaningful changes to prespecified study protocols, which are essential to transparency. These updates are in the interest of CMS, manufacturers, and the public because they provide early feedback on the viability of planned studies that use real-world data and offer early feedback on real-world outcomes. Any changes to the anticipated NCD reconsideration window will be reflected on the CED website.
Comment: Commenters encouraged CMS to be transparent and recommended that as much of the EDP as possible be made publicly available. Some commenters asked that CMS clarify what parts of the EDP will be publicly posted. It was recommended that the technical information regarding a device remain confidential. It was also suggested that the status of CED studies under TCET be updated in a publicly available manner.
Response: We appreciate these comments and agree that providing as much transparency as possible for EDPs and the studies conducted as part of them is important. To that end, a summary of the EDP, a linkage to CMS-approved CED studies on clinicaltrials.gov, and the anticipated CED NCD reconsideration window will be posted on the CMS website. We also recognize that manufacturers want to preserve confidentiality around their evidence-generation plans and product development strategies. CMS is actively developing guidance on the level of detail necessary to establish that a proposed study is FFP; while manufacturers may be able to demonstrate that these elements establish the scientific validity of a proposed study, it may not be necessary to make all details public.
Comment: A commenter suggested that CMS clearly and rigorously define what benchmarks will be considered “clinically meaningful” to its beneficiary population. A commenter requested that CMS clarify the meaning and significance of a post-market FFP study's potential to “demonstrate[e] external validity” concerning an EDP submission and whether such potential is a criterion for the protocol.
Response: We generally look to the published literature when assessing which clinical endpoints are important. In some instances, there is limited published literature to address minimal clinically important differences (MCIDs). Where appropriate clinical endpoints and MCIDs are uncertain, CMS may refer a topic to the MEDCAC to help CMS define evidence expectations through an open and transparent process. We are also developing a series of Clinical Endpoint Guidance documents to improve the predictability and transparency of our reviews. [ 20 ] The Knee Osteoarthritis Clinical Endpoint Guidance document is the first in this series.
A concern about generalizability depends on whether a new technology's effectiveness would reasonably be expected to vary between the populations studied in clinical trials and Medicare recipients, who are often older and have more comorbidities. If an Evidence Preview identifies generalizability as a material evidence deficiency, postmarket FFP studies may be used to confirm that expected benefits and harms extend to the applicable Medicare population and the context in which they receive care.
Comment: A commenter questioned how CMS will determine that an EDP is “sufficient” and how that sufficiency standard is related to the reasonable and necessary standard.
Response: The development of an EDP will include CMS-AHRQ collaboration to evaluate the EDP to ensure that it meets established standards of scientific integrity and relevance to the Medicare population. As with all technologies seeking Medicare coverage, CMS evaluates the available evidence when assessing whether an item/service satisfies the reasonable and necessary standard for coverage through the open and transparent NCD process.
Supportive clinical evidence that a device is reasonable and necessary in the Medicare population, including evidence regarding the device's safety and effectiveness for the Medicare population, is crucial to approve coverage for a device under section 1862(a)(1)(A) of the Act. Such evidence is used to determine whether a new technology meets the appropriateness criteria of the longstanding Medicare Program Integrity Manual Chapter 13 definition of reasonable and necessary. [ 21 ] We believe it is important for manufacturers participating in TCET to produce evidence demonstrating the health benefits of the device and the related services for patients with demographics similar to that of the relevant Medicare population.
Comment: A commenter sought clarification on CMS' and AHRQ's specific roles in reviewing the EDP and which Agency has ultimate approval.
Response: As we noted in the proposed notice and this subsequent notice, “Since we anticipate that many of the NCDs conducted under the TCET pathway will result in CED decisions, AHRQ will continue to review all CED NCDs consistent with current practice. Additionally, AHRQ will collaborate with CMS as appropriate, to evaluate the EP and EDP and will have opportunities to offer feedback throughout the process that will be shared with manufacturers. AHRQ will partner with CMS as the EP and EDP are being developed, and approvals for these documents will be a joint CMS-AHRQ decision.”
Comment: Several commenters expressed that manufacturers should be required to ensure diversity in clinical trials across a wide range of patient characteristics. A commenter stated that guidance from CMS is necessary to assist manufacturers in clinical trial designs that address access issues (specifically guidance on trial design for diversity and equitable access). They noted that research has demonstrated a lack of equitable representation in clinical trials.
Response: When reviewing evidence to assess whether items/services are reasonable and necessary, CMS must have a basis to conclude that the available evidence is generalizable to the intended Medicare population(s). The NIH, FDA, and CMS have long stressed the broad inclusion of diverse patient groups in clinical studies. Despite these efforts, pre-market studies frequently lack adequate inclusion of important patient subgroups, which limits their generalizability to the intended Medicare population(s). CMS agrees that post-market FFP studies may be necessary to address this common limitation of pre-market RCTs. The final CED guidance clarifies that CED studies should include specific patient groups that are essential to ensure the study is representative of the Medicare population when there is good clinical or scientific reason to expect that the results observed in premarket studies might not be observed in older adults or subpopulations identified by other clinical or demographic factors.
Comment: A commenter recommended that CMS should conduct regular audits on EPs and EDPs.
Response: We appreciate this comment; however, it is beyond the scope of this document.
Comment: A commenter recommended that CMS clarify that when the available evidence is promising but is insufficient to satisfy the reasonable and necessary standard for the Medicare population, CMS may extend coverage under the TCET pathway conditioned on completion of an FFP study that may convincingly address an evidence deficiency identified in the EP. Additionally, some commenters recommended that CMS acknowledge the dynamic nature of FFP studies and adopt documentation best practices for study design and analysis changes to ensure transparent study conduct and rigorous evidence development.
Response: We appreciate these comments and have clarified in the final notice that EDPs must address material evidence deficiencies identified in the EP. FFP studies addressing specific evidence deficiencies identified in the EP may be proposed as part of a broader EDP. CMS agrees that FFP studies, especially those that make secondary use of real-world data, may require modifications to the pre-specified protocol for various reasons. Thus, CMS agrees that EDPs should incorporate interim reporting to ensure adequate progress and timely completion. Interim reports should also disclose any meaningful changes to prespecified study protocols, which are essential to transparency. These updates are in the interest of CMS, manufacturers, and the public because they provide early confirmation of the viability of planned studies that use real-world data and early feedback on real-world outcomes. CMS expects to publish detailed guidance on acceptable FFP studies in the coming months.
Comment: A commenter noted that FFP evidence generation will likely require new data sources and methods of data collection, which may be particularly problematic for some Breakthrough Devices where the primary benefit to Medicare beneficiaries may be improvements in a patient-reported outcome (PRO). This commenter further stated that a clear definition of acceptable alternative evidence-generation methods and sources would be important since PROs are difficult to ascertain from administrative claims data or electronic health records. The commenter encouraged CMS to consider the balance between collecting data on outcomes that are important to patients and caregivers and minimizing the increased burden on providers, ideally by prioritizing outcomes that address patient priorities and are easy to collect as a part of routine care. This commenter suggested that a system that allows CMS claims data to be linked with other data sources is important for TCET to work effectively. The commenter suggested that accessing and working with Medicare claims data is difficult and burdensome. They recommended that CMS facilitate linkages to Medicare claims to facilitate evidence generation under the TCET pathway. Another commenter noted that CMS should ensure postmarket study designs support efficient acquisition and linkage of data sources data so studies can be efficiently completed.
Response: We appreciate these recommendations. We agree that patient-reported outcomes are often unavailable from claims or electronic health records (EHR) data sources. The real-world data (RWD)/real world evidence (RWE) field is rapidly developing, and new mechanisms for efficiently collecting supplemental data like PROs may emerge. CMS agrees that easier linkages between multiple data sources would simplify conduct of studies using real-world data. While indirect matching strategies are available, they may be cumbersome to implement.
CMS expects to publish detailed guidance on acceptable FFP studies in the coming months. CMS is closely tracking developments in this emerging field.
CMS proposed that if a device that is accepted into the TCET pathway receives FDA marketing authorization, CMS will initiate the NCD process by posting a tracking sheet following FDA market authorization (that is, the date the device receives PMA approval; 510(k) clearance; or the granting of a De Novo request) pending a CMS and AHRQ-approved Evidence Development Plan (in cases where there are evidence gaps as identified in the Evidence Preview). In the proposal CMS stated that the goal is to have a finalized EDP no later than 90 business days after FDA market authorization.
CMS' proposal stated that the process for Medicare coverage under the TCET pathway would follow the NCD statutory timeframes in section 1862(l) of the Act. CMS would start the process by posting a tracking sheet and elements of the finalized Evidence Preview, specifically the non-proprietary information, which would initiate a 30-day public comment period. Following further CMS review and analysis of public comments, CMS would issue a proposed TCET NCD and EDP within 6 months of opening the NCD. There would be a 30-day public comment period on the proposed TCET NCD and EDP, and a final TCET NCD would be due within 90 days of the release of the proposed TCET NCD.
Comment: Some commenters requested that the proposed decision memo for an initial TCET NCD should be posted at the same time as a tracking sheet, similar to what has previously been done for Parallel Review NCDs. These commenters note that this would help streamline the process since there would be only one 30-day public comment period.
Response: While we appreciate the suggestions to streamline the TCET process by providing for only one public comment period, we believe posting a tracking sheet with a proposed NCD is operationally impractical for CMS and provides insufficient opportunity for public feedback on the coverage conditions that optimize patient outcomes. The evidence base for emerging technologies is often incomplete, and practice guidelines are not yet established, so we believe input from interested parties is critical to ensure that Medicare is providing appropriate coverage for new, innovative technologies that balance access with beneficiary safeguards.
Comment: Several commenters noted inconsistencies in the proposed TCET process timeline. They noted CMS' stated goal of finalizing an NCD within 6 months of FDA marketing authorization and pointed out that we also state that there would be a tracking sheet posted with a 30-day comment with a proposed NCD posted 6 months after that (~7 months) and a final NCD statutorily due a few months later. Another commenter noted that the Timeline Diagram has a stakeholder meeting and evidence preview meeting listed, but the stakeholder meeting is not described in the notice.
Response: We appreciate the feedback. CMS notes that if material evidence deficiencies for Medicare coverage are identified in an evidence preview, manufacturers must have an approved evidence development plan before CMS will initiate a national coverage analysis. Delays in drafting an approvable evidence development plan may make it impossible to achieve coverage within 6 months of FDA authorization. Nonetheless, the final notice clarifies that the initial 30-day comment period is concurrent with the national coverage analysis, and CMS aims to shorten the NCD review by initiating our evidence review in the premarket period. We have removed the “stakeholder meeting” from the Timeline Diagram in the final notice since it is synonymous with the evidence preview meeting in the notice.
CMS stated in its proposal that feedback from the relevant specialty societies and patient advocacy organizations, in particular, their expert input and recommended conditions of coverage (with special attention to appropriate beneficiary safeguards), is especially important for technologies covered through the TCET pathway. In the proposal, CMS strongly encourages these organizations to provide specific feedback on the state of the evidence and their recommended best practices for the emerging technologies under review upon opening a national coverage analysis. We noted that while we prefer to have this information during the initial public comment period upon opening the NCD, we realize that, in many cases, it may take longer for these organizations to provide their collective perspectives to CMS since these technologies will have only recently received FDA market authorization. Further, we stated that since CMS may consider any information provided in the public domain while undertaking an NCD, CMS encourages these organizations to publicly post any additional feedback, including relevant practice guidelines, within 90 days of CMS' opening of the NCD. We specified that information considered by CMS to develop the proposed TCET NCD will become part of the NCD record and will be reflected in the bibliography as is typical for NCDs.
Comment: Numerous commenters agreed that engagement with all interested parties, particularly specialty societies, is important. Some commenters encouraged CMS to maintain close relationships with specialty societies and engage them as soon as an NCD is open. A few commenters suggested that CMS be flexible regarding the timeframe for developing consensus documents, as these documents may take an extended time to develop. Several commenters recommended that CMS be transparent when specialty society feedback is received outside a public comment period and suggested that CMS acknowledge receipt of the information and notify the manufacturer, post the information on the CMS website, and provide an opportunity for manufacturer feedback. Another commenter requested that CMS have a vetting process to ensure these sources of information are legitimate. It was noted that more formal public engagement mechanisms, like those used by FDA, are needed. A commenter suggested that CMS establish a Network of Experts like FDA's Center for Devices and Radiological Health (CDRH) and Center for Drug Evaluation and Research (CDER).
Response: We agree that engagement with specialty societies is important, and we intend to maintain our collaborative relationships with them to facilitate timely coverage and provide appropriate beneficiary access to promising new technologies. We believe that TCET includes adequate flexibility for specialty societies to provide important input. As is current practice, information sources that inform an NCD are documented in the decision memo and the bibliography of the proposed and final NCD. CMS carefully evaluates evidence and public comment when proposing and finalizing NCDs. While establishing more formal public engagement mechanisms is beyond the scope of this notice, we appreciate the suggestions commenters offered.
Comment: Several commenters requested that CMS establish a formal and robust patient engagement process. A few commenters stated that patients and patient organizations should be consulted regarding how CED affects access, outcomes, and caregiver experiences. They also stated that patient groups should be consulted to discuss study protocols and clinical endpoints. A commenter stated that CMS should agree to timely meetings with all interested parties.
Response: We routinely meet with interested parties about coverage requests for new technologies and reconsiderations of NCDs for existing technologies. CMS also regularly attends public meetings to discuss new technologies and to gather input from multiple perspectives. Furthermore, most NCDs allow two opportunities for public comment: when a national coverage analysis is initiated and when an NCD is proposed. Therefore, we believe the current process allows the public to express their views. CMS notes that additional public engagement requirements will increase coverage review costs and slow the initiation and completion of NCDs. CMS also solicits patient input on clinical endpoints that are relevant for coverage decisions and their minimally clinically meaningful differences through the Clinical Endpoints Guidance series.
In our proposal we noted that FDA market-authorized Breakthrough Devices are often followed by similar devices that other manufacturers develop. Additionally, we expressed that we believe it is important to let physicians and their patients make decisions about the best available treatment depending upon the patient's individual situation. We proposed that to be eligible for coverage under a TCET NCD, similar devices would be subject to the same coverage conditions, including a requirement to propose an EDP. CMS sought public comment on whether similar devices to the Breakthrough Device should be addressed under a separate NCD or should be subject to the same coverage conditions as the Breakthrough Device, including a requirement to propose an EDP.
Comment: Commenters generally supported CMS' proposal to cover similar devices under NCDs. Some commenters noted that NCDs have generally covered a particular class of technologies and supported a similar approach in the TCET pathway.
Response: We appreciate these comments. NCDs are limited to particular items or services, but some NCDs apply to products for the same indication. In these instances, CMS will follow the existing NCD process detailed in section 1862(l) of the Act. We recognize that some differences may exist for technologies in a class that may result in a distinct benefit/risk profile, and each will be evaluated on its own merit.
Comment: A few commenters disagreed with CMS' proposal, citing concerns that covering similar devices undercuts the voluntary nature of TCET. These commenters stated that Breakthrough Devices are unique and should be individually addressed.
Response: We appreciate the commenter's concern. We believe that it is important for the TCET pathway to foster innovation and not limit access to competitive devices. In some cases, providing coverage using a class approach may be appropriate and could avoid delays in access that would occur if a separate NCD were required to ensure coverage and would also provide more treatment options for patients and their physicians. We recognize that some differences may exist for technologies in a class that may result in a distinct benefit/risk profile, and each will be evaluated on its own merit.
Comment: Several commenters requested that CMS define “similar devices.” For example, a commenter suggested that CMS define similar devices as either: (1) those with the same or similar intended use as the initial product; or (2) devices with the same FDA product code. A commenter noted that it may be unclear whether two devices are in “the same category.”
Response: To preserve flexibility for manufacturers and CMS, we have not defined “similar devices” in the final notice. If the similarity of two or more devices is uncertain, CMS will consult with FDA and the manufacturer(s), as appropriate, when determining whether a device could be considered individually or as part of a class of similar devices for coverage purposes.
Comment: Some commenters requested that CMS clarify how coverage for similar devices will be handled under TCET. Commenters expressed mixed opinions and offered various suggestions as to how CMS could provide coverage under TCET for follow-on devices. Many commenters indicated that follow-on devices should be subject to the same coverage conditions and evidence standards and should be required to develop a comparable EDP to the original device. Several commenters recommended that CMS set clear expectations for evidence development for second and third devices to market. Some commenters encouraged CMS to be flexible in its approach to providing coverage for similar devices under TCET. A commenter suggested that CMS should require one EDP for all devices in the class. A commenter recommended that CMS require the same nomination and evidence preview processes for follow-on devices as for the first device to ensure that sponsors and CMS are aware of the available evidence. Another commenter suggested that CMS should require follow-on device manufacturers to submit an EDP but noted that existing endpoint guidance and the available data standards and infrastructure may reduce the cost of CED studies for follow-on devices with similar evidentiary questions.
Response: We appreciate these comments and agree with commenters that providing flexibility regarding coverage for follow-on devices is important. We do not believe that a one-size-fits-all approach to evidence development is appropriate since evidence gaps are specific to each device, and therefore, the evidence development needed to meet the reasonable and necessary standard may differ. In some cases, second and third follow-on devices may have fewer or different material evidence deficiencies. We recognize that each technology in a class may have differences that result in a distinct benefit/risk profile, and each will be evaluated on its own merit.
Comment: Some commenters recommended that the first device to market should have privileged status, such as a 1-year coverage exclusivity period. These commenters suggested that CMS should balance rewarding the first-to-market device with granting coverage to follow-on devices.
Response: We do not believe that a coverage exclusivity period for the first-to-market device is necessary and note that it would considerably complicate TCET implementation. Further, we believe that granting privileged status to the first-to-market device could impede Medicare beneficiary access to the best available device for their circumstances.
Comment: A commenter requested clarification on whether NCD reconsiderations would be delayed if two devices have EDPs with different timelines. Another commenter suggested that when a positive NCD is issued at the conclusion of a TCET NCD, CMS and the manufacturer of the follow-on device should discuss whether the agreed-upon evidence development should continue. Another commenter noted that a post-TCET NCD should apply to follow-on devices.
Response: We appreciate these comments. We believe it is important to provide flexibility so that a post-TCET NCD can apply to follow-on devices under appropriate circumstances. We do not anticipate a situation where we would delay an NCD reconsideration when two devices have EDPs with different timelines. Some studies in an EDP may continue beyond the pre-specified NCD reconsideration date. In this case, CMS strongly encourages manufacturers to complete these studies even if further evidence development is voluntary. CMS will engage with manufacturers when these situations are encountered to ensure the least burdensome approach is utilized while ensuring adequate evidence is collected to support Medicare coverage.
Comment: Several commenters requested that CMS clarify whether the follow-on devices would be required to have FDA Breakthrough designation to seek coverage under a TCET NCD. Some commenters expressed that follow-on devices should be required to have Breakthrough designation to receive coverage under TCET.
Response: We appreciate these comments. We believe that it is important for the TCET pathway to foster innovation and not limit access to competitive devices. To apply for the TCET pathway, the device must have FDA Breakthrough designation. All NCDs, whether through the TCET pathway or other, must follow the statutory process detailed in section 1862(l) of the Act which includes a public comment period.
Comment: Many commenters supported coverage of similar devices but recommended that follow-on devices not count against the annual cap of devices accepted into the TCET pathway.
Response: The final notice clarifies that follow-on devices will not count against the limit on TCET reviews.
Comment: A commenter questioned how CMS would address a situation where one device covered under the NCD has a safety concern, and other devices are covered under that NCD.
Response: CMS action would depend on the specific situation. CMS could reconsider a TCET NCD if safety concerns arise. We noted in the proposed procedural notice and reiterate in this final notice that CMS retains the right to reconsider an NCD at any point in time. Any reconsideration undertaken by CMS would be informed by the relevant evidentiary and safety information available at the time.
Comment: A commenter recommended that CMS solicit further feedback regarding coverage of similar devices under TCET and reassess after a year.
Response: CMS may reassess our approach as we gain more experience with the TCET pathway.
CMS proposed that the duration of transitional coverage through the TCET pathway would be time-limited and be tied to the CMS- and AHRQ-approved Evidence Development Plan (EDP). The proposal stated that the review date specified in the EDP will provide 1 additional year after study completion to allow manufacturers to complete their analysis draft one or more reports and submit them for peer-reviewed publication. In the proposed notice, we stated that we anticipate the transitional coverage period would last for 3 to 5 years as evidence is generated to address evidence gaps identified in the Evidence Preview.
Comment: Many commenters supported CMS' proposal for time-limited coverage under TCET with the coverage period specified in the EDP. Some commenters suggested a 3- to 5-year timeframe may not be sufficient as it may take longer for some studies to be completed and published, and encouraged CMS to be flexible, especially if a manufacturer acted in good faith or extenuating circumstances occurred. They noted that 3 to 5 years may be insufficient to gather all the necessary data and to ensure safety. A commenter encouraged CMS to remain flexible since cancer studies often use 5-year outcomes. Some commenters stated that CMS should establish a series of touch points where CMS and manufacturer can discuss progress and adjust the EDP as needed.
Response: We appreciate the supportive comments. CMS agrees that the duration of transitional coverage should be tied to an EDP that sufficiently addresses the material evidence gaps identified in the EP, and we will work with manufacturers to define an appropriate NCD reconsideration window. Particularly where longer periods of transitional coverage are anticipated, CMS agrees that EDPs should incorporate interim reporting to ensure adequate progress, public transparency, and timely completion. These updates are in the interest of CMS, manufacturers, and the public because they provide early confirmation of the viability of planned studies that use real-world data and early feedback on real-world outcomes. As noted in the proposed and final notice, we will be flexible when working with manufacturers if unavoidable delays occur.
Comment: Commenters offered support for sufficient time and flexibility to ensure seamless coverage following the TCET coverage period. Many commenters encouraged CMS to stipulate there would be no gap in coverage upon study completion and the time to reconsider the NCD. Others requested clarification on the timeline for using “continued access” to better ensure clarity for manufacturers participating in TCET. Some commenters suggested that CMS allow less burdensome alternatives (for example, literature review, claims data analysis) for data collection for the continued access study.
Response: We appreciate these comments. The inclusion of a continued access study in the EDP is intended to provide seamless coverage. As we noted in the proposed notice and are finalizing in this notice, “Manufacturers should conceive a continued access study that maintains market access between the period when the primary EDP is complete, the evidence review is refreshed, and a decision regarding post-TCET coverage is finalized. The continued access study may rely on a claims analysis, focusing on device utilization, geographic variations in care, and access disparities for traditionally underserved populations.”
Comment: A commenter requested clarification regarding the purpose and requirements of the continued access study.
Response: Under CED NCDs, coverage is granted for items and services provided within a clinical study. Evidence development requirements remain in place until an NCD reconsideration that removes them is finalized. Continued access studies maintain market access during the period beginning when the last patient is enrolled in a CED study until an NCD reconsideration that removes CED requirements is finalized. A discussion of requirements for continued access studies are beyond the scope of this document.
Comment: A commenter expressed that data collection should continue until an NCD reconsideration is conducted.
Response: An NCD with CED requirements remains in place until an NCD reconsideration without CED requirements is finalized. CMS has published details of the NCD process at 78 FR 48164 .
Comment: A commenter suggested that CMS consider including in the original TCET NCD, when appropriate, automatic termination of CED evidence collection requirements and conversion of the policy to a regular NCD in situations where all endpoints are met, and there are no serious adverse events or other significant problems during the CED study.
Response: We appreciate the suggestion, but an NCD with CED requirements remains in place until an NCD reconsideration is finalized. We are unable to include an automatic termination provision in the original TCET NCD. CMS has published details of the NCD process at 78 FR 48164 .
Comment: Some commenters expressed that if a study has met the endpoints, a change in coverage status should proceed without delay, and peer-reviewed publication should not be required.
Response: We disagree with the commenter regarding peer-reviewed publication. CMS believes that rigorous and publicly available evidence is necessary to inform beneficiaries, the clinical community, and the public about the benefits and harms of available treatment options. CMS generally considers peer-reviewed evidence of higher quality and evidentiary value than study results that are not peer-reviewed. Published studies are also necessary for devices to be included in evidence-based guidelines, which feature heavily in CMS' assessment of accepted standards of medical practice. Therefore, it is essential that evidence is published in the peer-reviewed clinical literature, and CMS applies rigorous methodologic standards in evidence review supporting local or national coverage analyses. CMS may sometimes review pre-publication evidence to accelerate our reviews. Nonetheless, the national coverage analysis process is open and transparent, and the evidence considered must be in the public domain. To judge whether CMS' analysis is appropriate, the public must also have access to the information that CMS relied on to conduct its evidence review. The 2024 CED guidance document states, “If peer-reviewed publication is not possible, results may also be published in an online publicly accessible registry dedicated to the dissemination of clinical trial information such as ClinicalTrials.gov, or in journals willing to publish in abbreviated format (for example, for studies with incomplete results).”
Comment: A commenter suggested that CMS ensure that studies are published in well-respected journals. This commenter also recommended naming specific examples to ensure scientific rigor.
Response: We appreciate these comments, but CMS does not believe it would be appropriate to name specific examples. As previously described, it is in the manufacturer's best interest to have their studies published in peer-reviewed journals.
CMS proposed to conduct an updated evidence review within 6 calendar months of the review date specified in the EDP. Additionally, as part of this process, CMS would review applicable practice guidelines and consensus statements and consider whether the conditions of coverage remain appropriate. CMS proposed that based upon this assessment, when appropriate, CMS would open an NCD reconsideration by posting a proposed decision that includes one of the following outcomes: (1) an NCD without evidence development requirements; (2) an NCD with continued evidence development requirements; (3) a non-coverage NCD; or (4) Medicare Administrative Contractor (MAC) discretion.
Comment: Commenters generally supported CMS' proposal to conduct an updated evidence review and an NCD reconsideration to facilitate the transition to post-TCET coverage.
Response: We appreciate these comments.
Comment: A few commenters stated that 6 months may not be enough time to complete the updated evidence review, and one of these commenters recommended 12 months. As in other TCET phases, some commenters suggested that CMS maintain flexibility.
Response: We agree with commenters that flexibility is important in the NCD reconsideration phase of the TCET pathway. Projected timeframes for the completion of real-world studies are estimated, and defining a precise date for a future NCD reconsideration is impossible. In this final notice, CMS clarifies that we intend to initiate an updated evidence review within 6 calendar months of the date specified in the EDP.
Comment: Additionally, several commenters requested that CMS clarify the manufacturer's role in the updated evidence review process and allow manufacturers to have a dialogue with the evidence review contractor to provide feedback on the evidence review findings.
Response: We disagree that manufacturers should be able to contact the contractors that the government has engaged to conduct an unbiased and neutral review of the scientific evidence. CMS has established rigorous review criteria that were developed in collaboration with AHRQ, have undergone detailed testing during the past year, and are reflected in the CMS NCA Evidence Review guidance. The contractor's role is to conduct a systematic literature review and summarize the evidence based on a modified GRADE methodology. The contractor supports and accelerates CMS reviews, but CMS performs extensive quality assurance on contracted reviews, contributes substantial portions of the NCA independently, and ultimately determines policy. Further, we believe there are ample opportunities for manufacturers to provide feedback throughout the process.
Comment: A commenter recommended that CMS look for opportunities to streamline the reconsideration process to preserve resources so that more technologies can be considered under the TCET pathway. This commenter suggested that CMS could eliminate the initial 30-day comment period for the NCD reconsideration and post a proposed decision along with the tracking sheet.
Response: We appreciate this comment and note that we stated in the proposed notice and reiterate in this final notice that we would open a TCET NCD reconsideration with a proposed NCD.
Comment: A commenter encouraged CMS to remain transparent and consider comments from interested parties on the reconsidered NCDs.
Response: We agree and will consider comments from interested parties during the NCD reconsideration process.
In the proposed notice, CMS noted that other potential expedited coverage mechanisms, such as Parallel Review, remain available. CMS stated in the proposal that eligibility for the Parallel Review program is broader than for the TCET pathway and could facilitate expedited CMS review of non-Breakthrough Devices. Further, CMS' proposal expressed CMS' intent to work with FDA to consider updates to the Parallel Review program and other initiatives to align procedures, as appropriate.
Comment: Several commenters stated that Parallel Review has yielded few results and noted many features of TCET have wide overlap with the Parallel Review Program. A commenter recommended that more technologies be accepted into the Parallel Review Program. Another commenter supported expanding the Parallel Review Program to accommodate devices that are ineligible for TCET.
Response: We appreciate these comments and will consider them as we move forward in conjunction with FDA to consider updates to the Parallel Review Program.
Comment: A commenter requested that CMS clarify whether technologies already accepted into parallel review are eligible for TCET.
Response: Technologies accepted into the Parallel Review Program may be considered for TCET if they align with the criteria for the TCET pathway.
CMS proposed to respond to TCET nominations within 30 days. Additionally, CMS' proposal indicated our intent to accept up to five candidates into the pathway annually. (We note that our responses to comments received regarding TCET timeframes, as well as the annual number of candidates accepted into the TCET pathway, are addressed in section II.A.3. of the notice.) CMS stated in the proposed notice that we intend to prioritize innovative medical devices that, as determined by CMS, have the potential to benefit the greatest number of individuals with Medicare.
Comment: Several commenters recommended that CMS establish and make public the prioritization factors used to triage TCET nominations when there are many candidates at a given time.
Response: We appreciate these comments and acknowledge the importance of clarifying how we will prioritize TCET nominations. To provide greater transparency, consistency, and predictability, we intend to release proposed prioritization factors for TCET nominations in the near future. We look forward to public comment on our proposed approach.
Comment: Some commenters disagreed with CMS' intention of prioritizing TCET candidates based on the overall impact on the Medicare population. These commenters noted that prioritizing based on overall impact will not address issues with underserved populations with limited or no treatment options. Commenters suggested that CMS should consider using the following factors when prioritizing NCD requests: making significant improvements to patients' lives; underserved populations; augmenting population health management practices; advancing the Quadruple Aim; potentially lifesaving; utilizing a more novel approach than current options, serving an unmet need, having a significant impact on patients and caregivers, addressing orphan diseases, and contributing to advancing health equity, access and improved health outcomes. Additionally, commenters requested that CMS provide consideration for special patient populations, including the needs of people with disabilities under age 65. A commenter suggested that CMS consider implementing well-established measures of healthcare benefits (for example, Quality- Adjusted Life Years (QALYs) and Disability-Adjusted Life Years (DALYs)) alongside measures that account for innovative benefits not traditionally derived from current standards-of-care (for example, procedure efficiency, treatment invasiveness, and Patient-Reported Outcome Metrics (PROMs)). Several commenters stated that CMS should not prioritize those technologies with the largest evidence bases.
Response: We appreciate these suggestions and will consider them when we propose TCET prioritization factors in the future. In the meantime, we will prioritize TCET candidates based upon the language from the August 7, 2013, Federal Register notice ( 78 FR 48164 ) stating that in the event we have a large volume of NCD requests for simultaneous review, we prioritize these requests based on the magnitude of the potential impact on the Medicare program and its beneficiaries and staffing resources. We note that section 1182(e) of the Act prohibits the Secretary from using QALYs or similar measures to determine coverage, reimbursement, or incentive programs under Medicare.
Comment: Several commenters requested that CMS be transparent regarding devices in the TCET pathway. Suggestions for more transparency included publicly posting information such as the number of devices in the TCET pathway, the date of nomination, the date of acceptance, and the date the NCD process is initiated. A commenter also recommended that information regarding TCET NCDs be included in the annual Report to Congress on NCDs.
Response: We appreciate these comments and recognize the importance of transparency regarding the TCET pathway. In response to public comments, we agree that including the number of devices in the TCET pathway, the date of nomination, the date of acceptance, and the date the NCD process is initiated would be helpful and we will incorporate this information into future iterations of the NCD Dashboard (available here: https://www.cms.gov/Medicare/Coverage/DeterminationProcess ). We intend to update the NCD Dashboard every quarter. Since we will use the NCD process to provide coverage under the TCET pathway, TCET NCDs will be reflected in the annual Report to Congress.
Comment: Several commenters suggested that CMS create a similar but separate pathway for technologies that meet the reasonable and necessary standard, but with limited context on real-world use in the Medicare population. This suggested pathway would offer temporary transitional national coverage and would not rely on the CED statutory authority; instead, these technologies could be covered under section 1862(a)(1)(A) of the Act. These commenters noted that this “limited context” pathway would accelerate beneficiary access to these technologies and promote the collection and assessment of real-world evidence to support the development of a long-term national coverage policy.
Response: These comments are outside the scope of the TCET notice. However, we will consider them in the future as we consider providing additional coverage pathways. In general, CED is not required for items and services that meet the reasonable and necessary standard. CED is an important option when the evidence is promising but does not yet satisfy the reasonable and necessary standard. CED relies primarily on the statutory exception in section 1862(a)(1)(E) of the Act, which effectively permits Medicare payment in the case of research conducted pursuant to section 1142 of the Act for items and services that are reasonable and necessary to carry out that section.
In some cases, the available evidence may satisfy the reasonable and necessary standard only within a narrow context and be appropriate for coverage on an individual claim determination basis. However, broad local or national coverage requires evidence generalizable to the intended Medicare beneficiary population.
Comment: A commenter requested that Medicare Advantage plans should be required to cover TCET technologies without prior authorization.
Response: This comment is out of scope as we are unable to impose new requirements on Medicare Advantage plans in this notice.
Comment: Some commenters requested that CMS build a CMS Office of the Actuary (OACT) determination into whether a Breakthrough Device in the TCET pathway triggers the significant cost threshold as soon as possible after an NCD.
Response: We do not believe building an OACT significant cost threshold determination into the TCET pathway is necessary. Significant cost threshold determinations for TCET NCDs will be handled consistent with the existing process we use for all NCDs.
Comment: A commenter recommended that CMS explain how the Clinical Endpoints Guidance documents will interact with and facilitate the TCET pathway and clarify whether CMS will prioritize TCET candidates in disease areas for which Clinical Guidance Documents have already been developed. This commenter also noted that through the FFP and Clinical Endpoints guidance documents, CMS can provide recommendations on the type of data collection best suited for given therapeutic areas, including guidance on data sources and data infrastructures. This commenter further stated that CMS could support better evidence development infrastructure by aligning TCET activities with its overall strategy to advance the use of interoperable electronic health data.
Response: We appreciate these comments. We intend to develop clinical endpoint guidance documents in therapeutic areas with a great deal of active research and development or in areas with considerable uncertainty about appropriate outcomes. The decision to develop a Clinical Endpoints Guidance (CEG) document is unrelated to our evaluation of a specific TCET nomination, and we may develop CEGs unrelated to the TCET pathway. Publication of a CEG does not imply that CMS intends to open an NCD. The RWD/RWE field is rapidly evolving, and CMS is closely tracking developments. CMS appreciates the suggestions for improving CEG documents by incorporating recommendations for data sources and infrastructures. CMS expects to publish detailed guidance on acceptable FFP studies in the coming months.
Comment: Commenters generally supported CMS collaboration with other HHS Agencies and encouraged further collaboration with FDA, NIH, and ARPA-H.
Response: We appreciate these comments. CMS intends to continue its collaboration with our fellow HHS sister agencies.
Comment: A commenter requested that CMS clarify the following sentence from the proposed notice: “We note that many Breakthrough Devices are currently coverable without the TCET pathway because they are not separately payable (that is, the device may be furnished under a bundled payment, such as payment for a hospital stay) or they are addressed by an existing NCD.” The commenter stated that it seems a device always used in the inpatient hospital setting would never need a coverage determination at the national or local level since it is part of a bundled payment. The commenter requested confirmation of the assumption that CMS would cover new devices for existing inpatient-only procedures, such as transcatheter aortic valve replacement since it would eliminate the need for many devices paid as part of a bundled payment to request coverage through the TCET pathway.
Response: We acknowledge this sentence has caused unintended confusion. It was not intended to communicate a universal statement regarding Medicare coverage. We have deleted the sentence from the final notice.
Comment: A commenter expressed concerns about accelerating the integration of 510(k) devices into practice since, as the commenter stated, 510(k) devices must prove “substantial equivalence” to a device that is already on the market and are not designed to demonstrate “safety and effectiveness” like the “Pre-Market Authorization” process. This commenter requested clarification on how an EDP would address devices without clinical evidence before clearance.
Response: In general, for an item or service to be covered under Medicare, it must meet the standard described in section 1862(a)(1)(A) of the Act—that is, it must be reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. In contrast, CED relies primarily on the statutory exception in section 1862(a)(1)(E) of the Act, which effectively permits Medicare payment when that bar has not yet been met, in order to support research conducted pursuant to section 1142 of the Act for items and services that are reasonable and necessary to carry out that section. CED is an important option when the evidence is promising but does not yet satisfy the reasonable and necessary standard under 1862(a)(1)(A).
In general, clinical evidence relevant to the Medicare population is necessary to achieve a favorable Medicare coverage decision. We anticipate that most TCET nominations will be for Breakthrough Devices where robust Medicare beneficiary protections and evidence generation are important to achieving optimal health outcomes. Additionally, CMS anticipates that most devices considered for the TCET pathway will be devices reviewed under a De Novo request or PMA submission. However, we note that devices subject to the 510(k) clearance pathway may qualify for Breakthrough designation (see 21 U.S.C. 360e-3(c) ). Although the 510(k) review standard is substantial equivalence of a new device to a legally marketed device, the principles of safety and effectiveness underlie the substantial equivalence determination in every 510(k) review. [ 22 ]
Comment: A commenter requested clarification as to who would be responsible for maintaining the integrity of an evidence development plan, particularly for FFP designs using real-world data. This commenter also questioned if CMS would consider a support mechanism if registries were required.
Response: It is the manufacturer's responsibility to maintain the integrity of an EDP. In approving EDPs, CMS, in collaboration with AHRQ, has agreed that the proposed studies are likely to substantially address material evidence gaps identified in the EP if faithfully executed. CMS' 2024 CED guidance document states that changes to approved study protocols must be justified and publicly reported. It also states that sponsors/investigators commit to making study data publicly available by sharing data, methods, analytic code, and analytical output with CMS or with a CMS-approved third party. The ultimate value of approved CED studies will be assessed when CED studies are completed, and the results are known.
CMS intends to issue FFP study guidance soon. We believe the guidance will clarify CMS' expectations for FFP studies, particularly those that rely on the secondary use of real-world data.
A discussion of CMS payment for data submission into registries is beyond the scope of this document.
Comment: A commenter requested that CMS clarify how upcoming pilots will relate to the TCET pathway and timing.
Response: We are currently testing aspects of the TCET process, specifically, the EP and EDP concepts within the existing NCD review process. More information will be provided as these NCDs are opened. We cannot provide information on the timing for opening any of these pilots.
Final Decision: After review of the public comments received, we are finalizing the TCET pathway with the modifications and clarifications noted previously in our responses to public comments. The following section lists our changes between the proposed and final notice.
The final notice incorporates many of the provisions of the proposed notice and revises some of the provisions as proposed in response to issues raised by commenters. Based on CMS' analysis of the topics raised during the public comment period, CMS made several changes between the proposed notice and final notice, specifically the following:
- Nominations:
++ We incorporate an opportunity for manufacturers to submit a non-binding letter of intent to nominate a potentially eligible device approximately 18 to 24 months before they anticipate FDA market authorization.
++ We clarify that when CMS is aware that manufacturers will likely pursue the TCET pathway for devices where appropriate clinical endpoints are uncertain, we may preemptively conduct a clinical endpoints review and may convene a MEDCAC. We note that submission of a non-binding letter of intent may avoid delays in TCET reviews.
++ We have revised the timeframe for reviewing TCET nominations. We will review nominations on a quarterly basis.
++ CMS clarifies that nominations for devices that are already FDA market authorized or those anticipated to receive an FDA decision on market authorization within 6 months of nomination will not be accepted for TCET because TCET relies on extensive pre-market engagement to expedite coverage reviews. CMS notes that if the timelines for this pre-market engagement are shortened, it is unlikely that an NCD will be finalized within 6 months of FDA market authorization. We note that pursuing an NCD outside of TCET or MAC discretion is also available.
- Evidence Preview (EP):
++ We clarify that the evidence review contractor's role is to support the CAG staff by conducting a rapid systematic literature review and summarizing the evidence based on a modified GRADE methodology. We further clarify that the contractor's role is to support and accelerate CMS reviews, but we will perform extensive quality assurance on contracted reviews, independently complete substantial portions of the EP, and determine coverage policy.
++ We state that if an NCD is opened, an evidence summary, including a disclosure of which contractor completed the review, will be posted with the tracking sheet on the CMS website for public comment.
++ We have changed our position on sharing the full EP with the MACs if a manufacturer withdraws from the TCET pathway. While we believe that an EP will be a fair reflection of the strength of evidence available at that time to support Medicare coverage, we acknowledge that manufacturers may withdraw from the TCET pathway for reasons unrelated to the strength of evidence. Nonetheless, EPs represent a substantial investment of public resources, and we will publicly post an evidence summary for devices that are withdrawn from the TCET pathway without an evidence gap assessment.
- Evidence Development Plans (EDPs):
++ We state that EDPs should incorporate interim reporting to ensure adequate progress and timely completion. Interim reports should also disclose any meaningful changes to prespecified study protocols, which are essential to transparency.
++ We note that the forthcoming FFP guidance will provide information on study designs and analysis methods that are FFP. We expect TCET CED studies to be registered and listed on the clinicaltrials.gov website. Additionally, we specify that a summary of the EDPs and the anticipated CED NCD reconsideration window will be posted on the CMS website so the public can stay informed throughout the process.
- Coverage of Similar Devices:
++ We clarify that NCDs are limited to particular items or services, but note that some NCDs apply to products for the same indication. In these instances, we will follow the existing NCD process detailed in section 1862(l) of the Act. We recognize that some differences may exist for technologies in a class that may result in a distinct benefit/risk profile, and each will be evaluated on its own merit.
++ We clarify that any follow-on devices in the TCET pathway will not count toward CMS' annual limit.
- Prioritizing Requests—We express our intent to release proposed prioritization factors for TCET nominations soon to provide greater transparency, consistency, and predictability.
- TCET Transparency—We indicate that information on TCET devices will be added to the NCD Dashboard, including the number of devices in the TCET pathway, the date of nomination, the date of acceptance, and the date the NCD process was initiated.
All other provisions are being finalized as proposed. The Addendum that follows provides the updated process and procedures for the TCET pathway that reflect the changes made in response to public comments.
Based on our initial assessment of Breakthrough Devices applying the characteristics we list in section I.C. of the Addendum to this notice regarding appropriate candidates for the TCET pathway, we anticipate receiving approximately eight nominations for the TCET pathway per year. Based on current resources, we do not anticipate the TCET pathway will accept more than five candidates per year. Since we estimate fewer than 10 respondents, the information collection requirements are exempt in accordance with the implementing regulations of the PRA at 5 CFR 1320.3(c) . As we gain experience with the TCET pathway, we will provide an updated analysis if we receive a higher number of respondents than anticipated.
Chiquita Brooks-LaSure, Administrator of the Centers for Medicare & Medicaid Services, approved this document on August 2, 2024.
Xavier Becerra,
Secretary, Department of Health and Human Services.
We describe in this Addendum the process and procedures for how interested parties and the public may engage with CMS to facilitate the TCET pathway. The topics addressed in the notice include the following: (1) TCET general principles; (2) appropriate candidates for the TCET pathway; (3) procedures for the TCET pathway; and (4) general roles.
We continue to work with various sectors of the scientific and medical communities to develop and publish guidance documents on our website that describe our approach when analyzing scientific and clinical evidence when developing NCDs. In response to feedback from interested parties, the 2024 CED and Evidence Review guidance documents incorporate recommendations for when FFP studies may be used to close material evidence gaps. FFP studies are those where the study design, analysis plan, and study data can credibly answer the research question. Additionally, CMS intends to publish a series of guidance documents that review health outcomes and their clinically meaningful differences within priority therapeutic areas. The public will have an opportunity to provide comments on these guidance documents available on the CMS coverage website. The website may be accessed at http://www.cms.gov/Medicare/Coverage/CoverageGenInfo/index.html .
Since CMS started covering technology in the context of clinical studies almost two decades ago, the timing of evidence development and the stages of the technology development lifecycle have evolved. Over the past few years, innovative technologies have come on the market earlier in the technology development lifecycle and reached the market with limited or developing evidence for coverage purposes. CMS has received inquiries for coverage of new technologies that are early in the product lifecycle, which means the clinical evidence is just starting to accumulate. For new technologies, there is often insufficient clinical evidence to support broad national coverage at this point.
In general, CMS relies heavily on health outcomes data, especially as it relates to the Medicare population when proposing an NCD. Early in the product lifecycle, there is usually evidence about whether the product is safe and may produce the intended result: for example, a laboratory measurement, radiographic image, physical sign, or other measure that is believed to predict clinical benefit but is not itself a measure of clinical benefit. However, there is often little evidence in the early stages of the product lifecycle regarding health outcomes (for example, mortality, disease progression, or impact on a patient's quality of life). When premarket, pivotal clinical study data is collected to support an application to FDA for market authorization, it provides clinical evidence for a defined population enrolled in the study.
If there is health outcome evidence for a new technology, it may not be generalizable to the Medicare population if Medicare beneficiaries are insufficiently represented in pivotal clinical studies. [ 23 ] Medicare beneficiaries have been historically underrepresented in pivotal studies due to age, access, multiple comorbidities, and concurrent treatments. When there is little or limited evidence, CMS may not have enough information to make a favorable NCD due to gaps in research about health outcomes, including potential safety risks to the Medicare population.
While CMS has attempted to streamline the NCD process with the Parallel Review program, we recognize that most emerging technologies are likely to have limited or developing bodies of clinical evidence that may not have included the Medicare population (that is, individuals over age 65, people with disabilities, and those with end-stage renal disease). Many Medicare beneficiaries have comorbid medical conditions, and those factors may have limited their participation in certain clinical trials. Additionally, we recognize the importance that applicable clinical trials reflect the demographic and clinical diversity among the Medicare beneficiaries who are the intended users of the intervention. At a minimum, this requires the availability of data on, and attention to, the intended users' racial and ethnic backgrounds, sex and gender, age, disabilities, important comorbidities, and relevant social determinants of health. We believe that the TCET pathway can support manufacturers that are interested in working with CMS to generate additional evidence that is applicable to Medicare beneficiaries and that may demonstrate improved health outcomes in the Medicare population to support more expeditious national Medicare coverage. While we believe that leveraging the statutorily established NCD process will allow us to responsibly cover new, innovative technologies with limited or developing evidence, it is important that we provide an evidence generation framework that, when appropriate, not only develops reliable evidence for patients and their physicians but also provides safeguards to ensure that Medicare beneficiaries are protected and continue to receive high-quality care.
Specifically, CED has been used to support evidence development for certain innovative technologies that are likely to show benefit for the Medicare population when the available evidence is not sufficient to demonstrate that the technologies are reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member under section 1862(a)(1)(A) of the Act. In instances where there is limited evidence, CED may be an option for Medicare beneficiaries seeking earlier coverage for promising technologies. CED has been a pathway whereby, after a CMS and AHRQ review, Medicare covers items and services on the condition that they are furnished in the context of approved clinical studies or with the collection of additional clinical data. Participation in a CED trial is voluntary, but beneficiaries are protected by separate regulations, including those at 45 CFR part 46 related to the protection of human research subjects.
With respect to evidence generation, the TCET pathway will build upon CMS and AHRQ's ongoing collaboration on the CED NCD process. We anticipate that many NCDs conducted under the TCET pathway will result in CED decisions, and AHRQ will continue to review all CED NCDs consistent with current practice. Additionally, AHRQ will collaborate with CMS as appropriate on evidence development activities, such as the EP and EDP, conducted to support Medicare coverage under the TCET pathway and will have opportunities to offer feedback throughout the process that will be shared with manufacturers. Approvals related to evidence development will be a joint CMS-AHRQ decision.
With respect to beneficiary safeguards, the NCD process allows for coverage with appropriate safeguards for Medicare beneficiaries, including coverage criteria based on evidence regarding eligibility, frequency, provider experience, site of service, or availability of supporting services. Specifically, CMS develops clinician and institutional requirements after careful review of expert physicians' specialty society guidelines and clinical study results. These guidelines and recommendations are often part of NCDs. Unless these coverage criteria are established within coverage determinations, devices could be provided by unqualified individuals, offered at inappropriate facilities, and utilized by patients who may be unlikely to benefit or likely to experience adverse effects.
Coverage under a CED NCD can expedite earlier beneficiary access for individuals who volunteer to participate in the clinical studies of innovative technologies while ensuring that systematic patient safeguards, including assurance that the technology is provided to clinically appropriate patients, are in place to reduce the potential risks of new technologies, or to new applications of older technologies. CMS' 2024 CED guidance document includes specific patient protections under CED. [ 24 ] Because the TCET pathway described in this document would utilize the existing CED NCD process, all these safeguards would apply.
Input from interested parties is important to CMS, and we are particularly interested in engagement with patient advocacy organizations and medical specialty societies as they have valuable expertise and first-hand experience in the field that will help CMS develop Medicare coverage policies. Because the TCET pathway would utilize the current NCD process, these opportunities for engagement with interested parties are also available in TCET.
CMS is committed to ensuring Medicare beneficiaries have access to promising emerging, technologies. CMS' goal is to finalize an NCD for technologies accepted into and continuing in the TCET pathway, within 6 months after FDA market authorization. The TCET pathway builds on prior initiatives, including CED. The TCET pathway will meet the following principles:
- Medicare coverage under the TCET pathway is limited to certain Breakthrough Devices that receive market authorization for one or more indications for use covered by the Breakthrough Device designation when used according to those indications for use. Manufacturers of FDA-designated Breakthrough Devices that fall within a Medicare benefit category may self-nominate to participate in the TCET pathway on a voluntary basis.
- CMS may conduct an early evidence review (Evidence Preview, more details can be found in section. I.D.1.h. of this Addendum) before FDA decides on marketing authorization for the device and discuss with the manufacturer the best available coverage pathways depending on the strength of the evidence.
- Prior to FDA marketing authorization, CMS and manufacturers may discuss any evidence gaps for coverage purposes and the types of studies that may need to be completed to address the gaps, which could include the manufacturer developing an evidence development plan and confirming that there are appropriate safeguards for Medicare beneficiaries.
- If CMS determines that further evidence development (that is, CED) is the best coverage pathway, CMS will work with the manufacturers to reduce the burden on manufacturers, clinicians, and patients while maintaining rigorous evidence requirements. CMS will work to ensure we are not requiring duplicative or conflicting evidence development with any FDA postmarket requirements for the device.
- CMS does not believe that an NCD that requires CED as a condition of coverage should last indefinitely, including under the TCET pathway. If the evidence supports a favorable coverage decision under CED, coverage will be time-limited to facilitate the timely generation of sufficient evidence to inform patient and clinician decision making and to support a Medicare coverage determination under section 1862(a)(1)(A) of the Act.
- Manufacturers and CMS have the option to withdraw from the pathway up until CMS opens the NCD by posting a tracking sheet. CMS will not publicly disclose participation of a manufacturer in the TCET pathway prior to CMS' posting of an NCD tracking sheet unless the manufacturer consents or has already made this information public or disclosure is required by law. CMS requests that a manufacturer who wishes to withdraw from the TCET pathway notify CMS by email.
Appropriate candidates for the TCET pathway would include those devices that are—
- FDA-designated Breakthrough Devices;
- Determined to be within a Medicare benefit category; [ 25 ]
- Not otherwise excluded from coverage through law or regulation. [ 26 ]
In section 201(h)(1) of the Federal Food, Drug, and Cosmetic Act ( 21 U.S.C. 321(h)(1) ), the definition of device includes IVD products, such as diagnostic laboratory tests. See 21 CFR 809.3 . IVDs, including diagnostic laboratory tests, are a highly specific area of coverage policy development, and CMS has historically delegated review of many of these products to specialized MACs. We believe that the majority of coverage determinations for IVDs granted Breakthrough designation should continue to be determined by the MACs through existing pathways.
The TCET pathway has three stages: (1) premarket; (2) coverage under the TCET pathway; and (3) transition to post-TCET coverage.
Manufacturers may submit a non-binding letter of intent to nominate a potentially eligible device for the TCET pathway approximately 18 to 24 months before anticipated FDA marketing authorization as determined by the manufacturer.
The letter of intent to nominate a device for the TCET pathway may be submitted electronically via the Coverage Center Website using the “Contact Us” link at http://www.cms.gov/Medicare/Coverage/InfoExchange/contactus.html . The following information will assist CMS in processing and responding to letters of intent:
- Name of the manufacturer and relevant contact information (name of contact person, address, email, and telephone number).
- Name of the product.
- Succinct description of the technology and the disease or condition the device is intended to diagnose or treat.
- Date of FDA Breakthrough Device Designation.
- Expected regulatory pathway (for example, PMA, De Novo, 510(k)).
- Expected completion date for pivotal clinical study.
CMS will email the manufacturer to confirm that a submitted letter of intent has been received by CMS.
The appropriate timeframe for manufacturers to submit nominations to CMS is approximately 12 months prior to when the manufacturer anticipates an FDA decision on a submission. Manufacturers are generally aware of when they intend to submit their application, and the FDA has agreed to review time goals as part of its device user fee program. [ 27 ] CMS encourages manufacturers not to delay submitting nominations to facilitate alignment among CMS benefit category determination, and coverage, coding, and payment considerations. Additionally, when CMS is aware that manufacturers will likely pursue the TCET pathway for devices where appropriate clinical endpoints are uncertain, we may preemptively conduct a clinical endpoints review and may convene a MEDCAC at a later date. In these instances, there may be a delay of several months due to the logistics involved in conducting these activities so the submission of a non-binding letter of intent may avoid potential delays.
Under the TCET pathway, CMS will conduct extensive work in the pre-market period to shorten coverage review timeframes after devices are FDA market-authorized. Since TCET is forward-looking and extensive pre-market engagement is essential, CMS will not accept nominations for already FDA market authorized devices or those anticipated to receive an FDA decision market authorization within 6 months of nomination. CMS may be unable to reach a final NCD within the expedited timeframes for TCET nominations submitted or accepted less than 12 months before anticipated FDA market authorization. We note that the option to pursue an NCD or LCD outside of the TCET pathway is available for these technologies.
The manufacturer may submit a nomination for the TCET pathway electronically via the Coverage Center website using the “Contact Us” link at http://www.cms.gov/Medicare/Coverage/InfoExchange/contactus.html . CMS will acknowledge receipt of nominations by email. The following information will assist CMS in processing and responding to nominations:
- Succinct description of the technology and disease or condition the device is intended to diagnose or treat.
++ Description of the product, including components (for example, single-use catheter, power source, charger system, etc.)
++ Description of the use context (for example, inpatient, ASC, outpatient clinic, home)
++ Description of the disease or condition that the product is intended to treat or diagnose and mechanism of action for the product
- State of development of the technology (that is, in pre-clinical testing, in clinical trials, currently undergoing premarket review by FDA). The submission of a copy of FDA's letter granting Breakthrough Device Designation and the PMA application, De Novo request or premarket notification (510(k)) submission, if available, is preferred.
++ Date of FDA Breakthrough Device Designation
++ Expected regulatory pathway (for example, PMA, De Novo, 510(k))
++ Current development status (for example, pre-clinical testing, in clinical trials, under FDA premarket review, post-market)
- A brief statement explaining why the device is an appropriate candidate for the TCET pathway as described under section I.C. of this Addendum (“C. Appropriate Candidates”).
++ Rationale for Breakthrough Designation
++ Unmet need the product addresses
- A statement describing how the medical device addresses the health needs of the Medicare population.
++ Description of the condition with respect to the full US population (for example, incidence, prevalence, significance)
++ Description of the applicable Medicare population(s) (for example, estimated population size, other considerations specific to the Medicare beneficiary population)
++ Description of the magnitude of the expected benefit from the product
- A statement that the medical device is not excluded by statute from Part A or Part B Medicare coverage or both, and a list of Part A or Part B or both Medicare benefit categories, as applicable, into which the manufacturer believes the medical device falls. Additionally, manufacturers are encouraged to provide additional specific information to help facilitate benefit category determinations.
++ Product not excluded from Medicare coverage by statute
++ Most likely benefit categories (for example, inpatient, physician services, DME, etc.)
++ A comprehensive list of peer-reviewed, English-language publications that are relevant to the nominated Breakthrough Device as applicable/available.
++ Relevant background literature (for example, important publications CMS should review for context).
++ Relevant unpublished clinical studies regarding the safety/efficacy of the product, with the expected publication date for each.
++ Relevant published clinical studies regarding the safety/efficacy of the product.
Two good sources of information to facilitate the development of nomination submissions are the CMS Coverage website ( https://www.cms.gov/Center/Special-Topic/Medicare-Coverage-Center ) and the CMS Guide for Medical Technology Companies and Other Interested Parties ( https://www.cms.gov/cms-guide-medical-tech-companies-other-parties ), which provide information that may facilitate durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) BCDs, along with coverage, coding and payment processes and considerations.
CMS will email the manufacturer to confirm that a submitted nomination appears to be complete and is under review. This email will include the date that CMS initiated the review of the complete nomination. CMS will contact the manufacturer for more information if the nomination is incomplete.
CMS will accept suitable TCET candidates quarterly. If a suitable nomination is not selected in the first review, it will be automatically considered in the subsequent quarter. Manufacturers will not need to resubmit to be considered in a subsequent quarter. Since TCET is forward-looking and extensive pre-market engagement is essential, nominations for Breakthrough Devices anticipated to receive an FDA decision on market authorization within 6 months may not be accepted since CMS will be unable to reach a final NCD within the expedited timeframes. It is possible that a nominated device that is not accepted in a first review may be accepted during a subsequent review even though FDA's decision on market authorization is anticipated within 6 months. If this occurs, CMS will work with the manufacturer to expedite the review as practically achievable.
CMS may contact the manufacturer to request supplemental information to ensure a timely review of the nomination. Once CMS decides to provisionally accept or decline a nomination, CMS will communicate their decision to the manufacturer by email with their designated point of contacts. Acceptance into TCET should not be viewed as a final determination that a device fits within a benefit category. When CMS issues the proposed NCD for a Breakthrough Device that has received FDA marketing authorization, the proposed NCD will include one or more benefit categories to which CMS has determined the Breakthrough Device falls. CMS will review and consider public comment on the proposed NCD before reaching a final determination on the BCD(s).
Following the submission of a complete TCET nomination, CMS will offer an initial meeting with the manufacturer to review the nomination within 20 business days of receipt of a complete nomination. In this initial meeting, the manufacturer is expected to describe the device, its intended application, place of service, a high-level summary of the evidence supporting its use, and the anticipated timeframe for FDA review. CMS will answer any questions about the TCET process. CMS intends for these meetings to be held remotely to reduce travel burden on manufacturers and expeditiously meet these timeframes. These meetings will have a duration of 30 minutes. If a manufacturer declines to meet or if there is difficulty finding a mutually convenient time for the meeting, then CMS action on the nomination may be delayed.
After CMS initiates review of a complete, formal nomination, representatives from CMS will meet with their counterparts at FDA to learn more information about the technology in the nomination to the extent the Agencies have not already done so. These discussions may help CMS gain a better understanding of the device and potential FDA review timing.
As noted in the Memorandum of Understanding [ 28 ] between FDA and CMS, FDA and CMS recognize that the following types of information transmitted between them in any medium and from any source must be protected from unauthorized disclosure: (1) trade secret and other confidential commercial information that would be protected from public disclosure pursuant to Exemption 4 of the Freedom of Information Act (FOIA); (2) personal privacy information, such as the information that would be protected from public disclosure pursuant to Exemption 6 or 7(c) of the FOIA; or (3) information that is otherwise protected from public disclosure by Federal statutes and their implementing regulations (for example, the Trade Secrets Act ( 18 U.S.C. 1905 ), the Privacy Act ( 5 U.S.C. 552a ), the Freedom of Information Act ( 5 U.S.C. 552 ), the Federal Food, Drug, and Cosmetic Act ( 21 U.S.C. 301 et seq. ), and the Health Insurance Portability and Accountability Act (HIPAA), Pub. L. 104-191 ).
Following discussions with FDA, CMS may initiate a benefit category review if all other pathway criteria have been met. Emerging devices may fit within a Medicare benefit category, but that does not mean all medical devices will fall within a benefit category. If CMS believes that the device, before a decision on market authorization by FDA, is likely to be payable through one or more benefit categories, the device may be accepted into the TCET pathway. This is an interim step that is subject to change upon FDA's decision regarding market authorization of the device. Acceptance into TCET should not be viewed as a final determination that a device fits within a benefit category. However, if it appears that a device, before a decision on market authorization by FDA, will not fall under an existing benefit category, the TCET nomination will be denied, and the rationale will be discussed in the denial letter. CMS will likely not assess every submitted application for a benefit category review, as the TCET pathway is limited in size per the discussion in section I.G. of this Addendum.
As noted previously, upon completion of CMS' review of the nomination, including the initial meeting with the manufacturer, discussions with FDA, and benefit category determination, CMS will notify the manufacturer by email whether the product has been accepted into the TCET pathway. In instances where CMS does not accept a nomination, CMS will offer a virtual meeting with the manufacturer to answer any questions and discuss other potential coverage pathways.
Following acceptance into the TCET pathway, CMS will initiate an Evidence Preview, which is a systematic literature review that would provide early feedback on the strengths and weaknesses of the publicly available evidence for a specific item or service. The EP will be a focused, but not necessarily exhaustive, review that will help CMS to identify any material evidence shortfalls. We believe the review conducted for the Evidence Preview will offer greater predictability and transparency to manufacturers and CMS on the state of the evidence and any notable evidence gaps for coverage purposes. It is intended to efficiently inform judgments by CMS and manufacturers about the best available coverage options for an item or service. CMS intends for the EP to be supported by a contractor using established rigorous review criteria that were developed in collaboration with AHRQ, have undergone detailed testing during the past year, and are reflected in the CMS NCA Evidence Review guidance. The contractor's role is to conduct a rapid systematic literature review and summarize the evidence based on a modified GRADE methodology. The contractor supports and accelerates CMS reviews, but CMS performs extensive quality assurance on contracted reviews, independently contributes substantial portions of the EP, and ultimately determines appropriate coverage policy. To initiate an EP, CMS will request written permission from the manufacturer to share any confidential commercial information (CCI) included in the nomination submission with the contractor. CMS anticipates that the EP will take approximately 12 weeks to complete once the review is initiated, following acknowledgment of an accepted nomination in the TCET pathway. More time may be needed to complete the review in the event the product is novel, has conflicting evidence, or other unanticipated issues arise.
CMS will share the EP with the manufacturer via email and will offer a meeting to discuss it. The EP will have been previously shared with AHRQ and may also be shared with FDA to obtain their feedback, as relevant. Representatives from those Agencies may participate in the EP meeting at their discretion. Manufacturers will have an opportunity to propose corrections to any errors, contribute supplemental materials, and raise any important concerns with the EP before it is finalized.
CMS will review the manufacturer feedback on the EP and work with our contractor to revise the draft, as appropriate, prior to finalization. Upon finalizing the EP, manufacturers may request a meeting to discuss the strengths and weaknesses of the evidence and discuss the available coverage pathways (examples include an NCD, which could include CED, or seeking coverage decisions made by a MAC). These meetings to discuss the EP may be conducted virtually or in person and will be scheduled for 60 minutes. If an NCD is opened, an evidence summary, including a disclosure of which contractor completed the review, will be posted with the tracking sheet on the CMS website for public comment.
There will be no publicly posted tracking sheet for manufacturers who withdraw from the TCET pathway after the completion of an EP. While CMS believes the EP will be a fair reflection of the strength of evidence available at that time to support Medicare coverage, CMS acknowledges that manufacturers may withdraw from the TCET pathway for reasons unrelated to the strength of evidence. Since the development of an EP review represents a substantial investment of public resources in a thorough evidence review for pre-market devices, CMS will publicly post a summary of the evidence. This summary will not include an evidence gap assessment.
Upon finalization of the EP, the manufacturer may decide to pursue national coverage under the TCET pathway or to withdraw from the pathway. If the manufacturer decides to continue, the next step will include submitting a formal NCD request cover letter expressing the manufacturer's desire for CMS to open a TCET NCD analysis. Most, if not all, of the information needed to begin the TCET NCD would be included in the initial TCET pathway nomination and the EP. However, CMS invites the manufacturer to submit any additional materials the manufacturer believes would support the TCET NCD request.
If CMS and/or AHRQ identifies evidence gaps during the EP, the manufacturer should also submit an evidence development plan (EDP) to CMS that sufficiently addresses the evidence gaps identified in the EP. The EDP should be submitted to CMS simultaneously with the formal NCD request cover letter. The EDP may include fit-for-purpose (FFP) study designs including traditional clinical study designs and those that rely on secondary use of real-world data, provided that those study designs follow all applicable CMS guidance documents. Additional information can be found here: https://www.cms.gov/Medicare/Coverage/DeterminationProcess/Medicare-Coverage-Guidance-Documents- .
An FFP study is one where the study design, analysis plan, and study data are appropriate for the question the study aims to answer. FFP study designs, which include traditional clinical study designs as well as those that rely on secondary use of real-world data, align sample size, duration, study type, analytic methods, etc., based on the utilization and risk profile of the item or service. We believe that permitting FFP study designs will be less burdensome for manufacturers and address the public's concerns that CED should be time-limited to facilitate the timely generation of evidence that can inform patient and clinician decision making and lead to predictable Medicare coverage.
Postmarket FFP study proposals, particularly those that rely on real world data, have the potential to generate evidence that complements tightly controlled premarket traditional clinical trials by demonstrating external validity. Nonetheless, manufacturers should be aware that these studies require considerable planning in data validation, linkage, and transformation; specification of the study protocol and documentation of any changes; data analysis; and reporting. The study design, patient inclusion criteria, primary and secondary endpoints, treatment setting, analytic approaches, timing of outcome assessment, and data sources should be fully pre-specified in the submitted protocol. CMS notes that though FFP studies that use real-world data may be less burdensome in terms of data collection, they may take more time to complete due to lags in the availability of administrative claims needed for the analysis. When writing EDPs, manufacturers should propose clinically meaningful benchmarks for each study outcome and provide supporting evidence. FFP studies addressing specific evidence deficiencies identified in the EP may be proposed as part of a broader EDP.
Manufacturers should incorporate a continued access study into their EDP to maintain market access between the completion of the primary EDP, the refresh of the evidence review, and the finalization of a decision regarding post-TCET coverage. The continued access study may rely on a claims analysis, focusing on device utilization, geographic variations in care, and access disparities for traditionally underserved populations.
Because of the tight timeframes needed to effectuate CMS' goal of finalizing a TCET NCD within 6 months after FDA market authorization, manufacturers are strongly encouraged to begin developing a rigorous proposed EDP as soon as possible after receiving the finalized EP. To meet the goal of having a finalized EDP within approximately 90 business days after FDA market authorization, the manufacturer is encouraged to submit an EDP to CMS as soon as possible after FDA market authorization.
Once CMS receives the EDP from the manufacturer, CMS will have 30 business days to review the proposed EDP and provide written feedback to the manufacturer. During this time, CMS will collaborate with AHRQ to evaluate the EDP to ensure that it addresses the material evidence gaps identified in the EP and meets established standards of scientific integrity and relevance to the Medicare population. CMS will incorporate AHRQ's feedback on the EDP and will email the consolidated feedback to the manufacturer. Soon after providing written feedback, CMS will schedule a meeting with the manufacturer, which may also include AHRQ, to discuss any recommended refinements and address any questions.
In the EDP meetings, the manufacturer should be prepared to demonstrate: (1) a compelling rationale for its evidence development plan; (2) the study design, analysis plan, and data for any CED studies are all fit for purpose; and (3) any CED studies sufficiently addresses threats to internal validity. The EDP should include clear enrollment, follow-up, study completion dates for included studies, and the timing and content of scheduled updates to CMS on study progress. For FFP studies with expected completion timeframes longer than 5 years, EDPs should incorporate interim reporting to ensure adequate progress and timely completion. Interim reports should also disclose any meaningful changes to prespecified study protocols, which are essential to transparency. Manufacturers should present and justify their study outcomes and performance benchmarks.
Following the EDP meeting, the manufacturer and CMS will have another 60 business days to make any adjustments to the EDP. We recognize that, in some instances, manufacturers may require additional time to develop and refine their EDP. In these instances, CMS may provide additional time to manufacturers, but we note that delays in submitting and revising an EDP may substantially impact the overall timeline for providing coverage under the TCET pathway. Elements of the CMS and AHRQ approved EDPs, specifically the non-proprietary information, will be made publicly available on the CMS website upon posting of the proposed TCET NCD. In addition, the anticipated CED NCD reconsideration window will also be posted. The forthcoming FFP guidance will provide information on the level of detail necessary to establish that a proposed study is fit for purpose; while manufacturers should demonstrate all these elements to establish the scientific validity of a proposed study, not all details need to be public.
In instances where the manufacturer's EDP is insufficient to meet CMS' and AHRQ's established standards and cannot be approved, CMS may exercise its option to withdraw acceptance into the TCET pathway as noted in section I.B. of this Addendum. We anticipate this will be a rare occurrence as CMS will make every effort to provide flexibility and information to manufacturers to facilitate the development of EDPs.
CMS follows applicable statutory requirements when developing coverage policy at the national level, which includes an open and transparent process. Though some elements of coverage review can be accelerated, gathering and reviewing meaningful public comment takes time. When CMS undertakes an NCD, we draw upon our analysis of the available evidence to identify the specific beneficiaries and conditions of coverage that are appropriate for the item or service. CMS also strongly considers information from patient advocacy organizations, specialty society guidance, expert consensus and recommendations for beneficiary selection, provider training and certification requirements, and facility requirements.
If a device that is accepted into the TCET pathway receives FDA market authorization, CMS will initiate the NCD process by posting a tracking sheet following FDA market authorization (that is, the date the device receives PMA approval; 510(k) clearance; or the granting of a De Novo request) pending a CMS and AHRQ-approved Evidence Development Plan (in cases where there are evidence gaps as identified in the Evidence Preview). The manufacturer may also withdraw from the TCET pathway at this stage in the process, in which case CMS would not proceed with the NCD review described in this section. As previously noted, the goal is to have a finalized EDP no later than 90 business days after FDA market authorization.
The process for Medicare coverage under the TCET pathway would follow the NCD statutory timeframes in section 1862(l) of the Act. CMS would start the process by posting a tracking sheet and an evidence summary from the finalized Evidence Preview, specifically the non-proprietary information, which would initiate a 30-day public comment period. Following further CMS review and analysis of public comments, CMS would issue a proposed TCET NCD and EDP within 6 months of opening the NCD. There would be a 30-day public comment period on the proposed TCET NCD and EDP, and a final TCET NCD would be due within 90 days of the release of the proposed TCET NCD. Our goal is to release the proposed and final NCD before the statutory deadline that applies to all NCDs. More information on the NCD process is outlined in the August 7, 2013 Federal Register notice ( 78 FR 48164 ).
Since the evidence base for these emerging technologies will likely be incomplete and practice standards not yet established, we believe that feedback from the relevant specialty societies and patient advocacy organizations, in particular, their expert input and recommended conditions of coverage (with special attention to appropriate beneficiary safeguards), is especially important for technologies covered through the TCET pathway.
Upon opening an NCD analysis, CMS strongly encourages these organizations to provide specific feedback on the state of the evidence and their suggested approaches to best practices for the emerging technologies under review. While CMS prefers to have this information during the initial public comment period upon opening the NCD, we realize that in many cases, it may take longer for these organizations to provide their collective perspectives to CMS since these technologies will have only recently received FDA market authorization. Since CMS may consider any information provided in the public domain while undertaking an NCD, CMS encourages these organizations to publicly post any additional feedback, including relevant practice guidelines, within 90 days of CMS' opening of the NCD. These organizations are encouraged to notify CMS when recommendations have been posted. All information considered by CMS to develop the proposed TCET NCD will become part of the NCD record and will be reflected in the bibliography as is typical for NCDs.
FDA market-authorized Breakthrough Devices are often followed by similar devices that other manufacturers develop. We believe that it is important to let physicians and their patients make decisions about the best available treatment, depending upon each patient's situation. NCDs are limited to particular items or services but it is possible that more than one device could fall under the same NCD because it addresses the same indication. We recognize that some differences may exist for technologies in a class that may result in a distinct benefit/risk profile, and each will be evaluated on its own merit.
In these instances, CMS will follow the existing NCD process detailed in section 1862(l) of the Act.
The duration of transitional coverage through the TCET pathway will be tied to the CMS- and AHRQ-approved EDP. CMS expects that TCET CED studies will be listed on the clinicaltrials.gov website, and a summary of the EDPs as well as the anticipated CED NCD reconsideration window will be posted on the CMS website so the public can stay informed throughout the process. The review date specified in the EDP will provide 1 additional year after study completion to allow manufacturers to complete their analysis, draft one or more reports, and submit them for peer-reviewed publication. Given the short timeframes in the TCET pathway, an unpublished publication draft that a journal has accepted may also be acceptable. CMS will consider the minimum period of transitional coverage necessary to address the evidence gaps identified in the EP. In general, we anticipate this transitional coverage period may last for 5 or more years as evidence is generated to address evidence gaps. However, CMS retains the right to reconsider an NCD at any point in time.
TCET provides time-limited coverage for devices with the potential to deliver improved outcomes to the Medicare population but do not yet meet the reasonable and necessary standard for coverage under section 1862(a)(1)(A) of the Act. Consequently, TCET coverage is conditioned on further evidence development as agreed in a CMS- and AHRQ-approved EDP.
CMS intends to initiate an updated evidence review within 6 calendar months of the review date specified in the EDP. CMS intends to engage a third-party contractor to conduct a systematic literature review using detailed requirements that CMS developed in collaboration with AHRQ. The contractor will then perform a qualitative evidence synthesis and compare those findings against the benchmarks for each outcome specified in the original NCD. After conducting quality assurance on the contractor review, CMS will assess whether the evidence is sufficient to reach the reasonable and necessary standard. CMS will also review applicable practice guidelines and consensus statements and consider whether the conditions of coverage remain appropriate. CMS will collaborate with AHRQ and FDA as appropriate as the updated Evidence Review is conducted and will share the updated review with them.
Based upon the updated evidence review and consideration of any applicable practice guidelines, CMS, when appropriate, will open an NCD reconsideration by posting a proposed decision that proposes one of the following outcomes: (1) an NCD without evidence development requirements; (2) an NCD with continued evidence development requirements; (3) a non-coverage NCD; or (4) permitting local MAC discretion under section 1862(a)(1)(A) of the Act. Neither an FDA market authorization nor a CMS approval of an Evidence Development Plan guarantees a favorable coverage decision. Standard NCD processes and timelines will continue to apply, and following a 30-day public comment period, CMS will have 60 days to finalize the NCD reconsideration.
The steps previously described for the TCET process and for obtaining a CMS coverage determination are illustrated in the diagram:

CMS has outlined the general roles of each participant in the TCET pathway.
The manufacturer may voluntarily choose to email a non-binding letter of intent to CMS to express intent to nominate a device for the TCET pathway. The manufacturer initiates formal consideration for TCET by voluntarily submitting a complete nomination as outlined previously under “1. b. Nominations for the TCET Pathway,” of section I.D. of this Addendum titled “Procedures for the TCET Pathway.” In the interest of expediting CMS decision making, the manufacturer should be prepared to quickly and completely respond to all issues and requests for information raised by the CMS reviewers. If CMS does not receive information from manufacturers in a timely fashion, CMS review timelines will be lengthened, potentially significantly. Manufacturers are encouraged to submit any materials they plan to present during meetings with CMS at least 7 days in advance of the scheduled meeting. Manufacturers should be prepared with the resources and skills to successfully develop, conduct, and complete the studies included in the EDP.
CMS will provide a secure and confidential nomination and review process as outlined previously in section I.D. of this Addendum. CMS will initiate review of nominations for the TCET pathway by retrieving applications from the secure mailbox and communicating with FDA regarding Breakthrough Devices seeking coverage under the TCET pathway. CMS will also oversee the work of the contractor conducting evidence reviews and will perform extensive quality assurance on contracted reviews, independently contribute substantial portions of the EP, and ultimately determine appropriate coverage policy. Along with AHRQ, CMS will review and make decisions regarding EDPs. Throughout all stages of the TCET pathway, CMS intends to maintain open communication channels with FDA, AHRQ, and the relevant manufacturer and fulfill its statutory obligations concerning the NCD process.
FDA will keep open lines of communication with CMS on Breakthrough Devices seeking coverage under the TCET pathway as resources permit. Participation in the TCET pathway does not change the review standards for FDA market authorization of a device, which are separate and distinct from the standards governing a CMS NCD.
Currently, AHRQ reviews all CED NCDs established under section 1862(a)(1)(E) of the Act. Consistent with section 1142 of the Act, AHRQ collaborates with CMS to define standards for clinical research studies to address the CED questions and meet the general standards for CED studies ( https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development ). Since we anticipate that many NCDs conducted under the TCET pathway will result in CED decisions, AHRQ will continue to review all CED NCDs to ensure they are consistent with current practice. Additionally, AHRQ will collaborate with CMS as appropriate, to evaluate the EP and EDP and will have opportunities to offer feedback throughout the process that will be shared with manufacturers. AHRQ will partner with CMS as the Evidence Preview and EDP are being developed, and approvals for these documents will be a joint CMS-AHRQ decision.
While the TCET pathway will be limited to Breakthrough Devices, other potential expedited coverage mechanisms, such as Parallel Review, remain available. Eligibility for the Parallel Review program is broader than for the TCET pathway and could facilitate expedited CMS review of non-Breakthrough Devices. To achieve greater efficiency and to simplify the coverage process generally, CMS intends to work with FDA to consider updates to the Parallel Review program and other initiatives to align procedures, as appropriate.
CMS intends to review TCET pathway nominations on a quarterly basis. CMS anticipates accepting up to five TCET candidates annually based on current resources. Any follow-on devices in the TCET pathway will not count toward CMS' annual limit. To provide greater transparency, consistency, and predictability, we intend to release proposed prioritization factors in the near future. The public will have an opportunity to provide comment on CMS' proposed prioritization factors. In the interim, CMS intends to prioritize innovative medical devices that, as determined by CMS, have the potential to benefit the greatest number of individuals with Medicare.
While CMS will not divulge the identity of specific manufacturers or devices in the TCET pathway prior to the opening of an NCD, we believe it is important to provide transparency regarding the devices accepted into the pathway. Specifically, CMS will include information such as the number of devices in the TCET pathway, the date of nomination, the date of acceptance, and the date the NCD process is initiated into future iterations of the NCD Dashboard. We intend to update the NCD Dashboard quarterly.
1. https://www.cms.gov/oact/tr/2024 .
2. https://www.cms.gov/Medicare/Medicare-Contracting/Medicare-Administrative-Contractors/What-is-a-MAC .
3. https://www.govinfo.gov/content/pkg/FR-2021-11-15/pdf/2021-24916.pdf .
4. CMS Program Integrity Manual, Chapter 13 Local Coverage Determinations, available at https:// www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/pim83c13.pdf .
5. The most recent CED guidance document is available at https://www.cms.gov/medicare/coverage/evidence .
6. Note: Medicare does not develop NCDs for Part D.
7. Section 1862(l) of the Act.
8. CMS, National Coverage Determination for Routine Costs in Clinical Trials available at https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=1&fromdb=true .
9. Davide L Vetrano, MD, Katie Palmer, Ph.D., Alessandra Marengoni, MD, Ph.D., Emanuele Marzetti, MD, Ph.D., Fabrizia Lattanzio, MD, Ph.D., Regina Roller-Wirnsberger, MD, MME, Luz Lopez Samaniego, Ph.D., Leocadio Rodríguez-Mañas, MD, Ph.D., Roberto Bernabei, MD, Graziano Onder, MD, Ph.D., Frailty and Multimorbidity: A Systematic Review and Meta-analysis, The Journals of Gerontology: Series A, Volume 74, Issue 5, May 2019, Pages 659-666, https://doi.org/10.1093/gerona/gly110 .
10. Tan, Y.Y., Papez, V., Chang, W.H., Mueller, S.H., Denaxas, S., & Lai, A.G. (2022). Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. The Lancet Healthy Longevity, 3(10), e674-e689.
11. Varma T, Mello M, Ross JS, et al Metrics, baseline scores, and a tool to improve sponsor performance on clinical trial diversity: retrospective cross sectional study BMJ Medicine 2023;2:e000395. doi: 10.1136/bmjmed-2022-000395.
12. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/breakthrough-devices-program .
13. Information on device-led combination products can be accessed here: https://www.fda.gov/media/119958/download .
14. Food and Drug Administration, Breakthrough Devices Program Guidance for Industry and Food and Drug Administration Staff, available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/breakthrough-devices-program .
15. Alliance for Aging Research, FAÇADE OF EVIDENCE: HOW MEDICARE'S COVERAGE WITH EVIDENCE DEVELOPMENT PARADIGM RATIONS CARE AND EXACERBATES INEQUITY (Feb. 13, 2023), available at https://www.agingresearch.org/wp-content/uploads/2023/02/Facade-of-Evidence-CED-2-13-2023.pdf .
16. https://www.govinfo.gov/content/pkg/FR-2021-11-15/pdf/2021-24916.pdf .
17. https://www.palmettogba.com/moldx .
18. CMS' guidance documents can be accessed here: https://www.cms.gov/medicare-coverage-database/reports/national-coverage-medicare-coverage-documents-report.aspx?docTypeId=1 #.
19. https://effectivehealthcare.ahrq.gov/products/collections/cer-methods-guide .
20. The clinical endpoints guidance can be accessed here upon release: https://www.cms.gov/medicare-coverage-database/reports/national-coverage-medicare-coverage-documents-report.aspx?docTypeId=1 #.
21. CMS, Medicare Program Integrity Manual, Chapter 13, 13.5.4, available at https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/pim83c13.pdf .
22. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/510k-program-evaluating-substantial-equivalence-premarket-notifications-510k .
23. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/design-considerations-pivotal-clinical-investigations-medical-devices .
24. https://www.cms.gov/medicare-coverage-database/reports/national-coverage-medicare-coverage-documents-report.aspx?docTypeId=1&status=all .
25. For more information on benefit category determinations see the CMS Guide for Medical Technology Companies and Other Interested Parties: https://www.cms.gov/medicare/coding-billing/guide-medical-technology-companies-other-interested-parties .
26. Information on coverage exclusions can be accessed here: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c16.pdf .
27. For more information on the specific review time goals that apply to different types of device premarket submissions, see MDUFA Performance Goals and Procedures, Fiscal Years 2023 Through 2027 ( https://www.fda.gov/media/158308/download ).
28. https://www.fda.gov/about-fda/domestic-mous/mou-225-10-0010 .
[ FR Doc. 2024-17603 Filed 8-7-24; 4:15 pm]
BILLING CODE 4120-01-P
- Executive Orders
Reader Aids
Information.
- About This Site
- Legal Status
- Accessibility
- No Fear Act
- Continuity Information

Trump and Allies Forge Plans to Increase Presidential Power in 2025
The former president and his backers aim to strengthen the power of the White House and limit the independence of federal agencies.
Donald J. Trump intends to bring independent regulatory agencies under direct presidential control. Credit... Doug Mills/The New York Times
Supported by
- Share full article

By Jonathan Swan Charlie Savage and Maggie Haberman
- Published July 17, 2023 Updated July 18, 2023
Donald J. Trump and his allies are planning a sweeping expansion of presidential power over the machinery of government if voters return him to the White House in 2025, reshaping the structure of the executive branch to concentrate far greater authority directly in his hands.
Their plans to centralize more power in the Oval Office stretch far beyond the former president’s recent remarks that he would order a criminal investigation into his political rival, President Biden, signaling his intent to end the post-Watergate norm of Justice Department independence from White House political control.
Mr. Trump and his associates have a broader goal: to alter the balance of power by increasing the president’s authority over every part of the federal government that now operates, by either law or tradition, with any measure of independence from political interference by the White House, according to a review of his campaign policy proposals and interviews with people close to him.
Mr. Trump intends to bring independent agencies — like the Federal Communications Commission, which makes and enforces rules for television and internet companies, and the Federal Trade Commission, which enforces various antitrust and other consumer protection rules against businesses — under direct presidential control.
He wants to revive the practice of “impounding” funds, refusing to spend money Congress has appropriated for programs a president doesn’t like — a tactic that lawmakers banned under President Richard Nixon.
He intends to strip employment protections from tens of thousands of career civil servants, making it easier to replace them if they are deemed obstacles to his agenda. And he plans to scour the intelligence agencies, the State Department and the defense bureaucracies to remove officials he has vilified as “the sick political class that hates our country.”
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Advertisement
Maintenance work is planned from 21:00 BST on Sunday 18th August 2024 to 21:00 BST on Monday 19th August 2024, and on Thursday 29th August 2024 from 11:00 to 12:00 BST.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to log in or to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.

Chemical Society Reviews
Spin crossover iron complexes with spin transition near room temperature based on nitrogen ligands containing aromatic rings: from molecular design to functional devices.

* Corresponding authors
a Hubei Key Laboratory of Plasma Chemistry and Advanced Materials, School of Materials Science and Engineering, Wuhan Institute of Technology, Wuhan, China E-mail: [email protected]
b Instituto de Ciencia Molecular (ICMol), Universidad de Valencia, Catedrático José Beltrán 2, 46980 Paterna, Spain E-mail: [email protected]
c Key Laboratory of Optoelectronic Chemical Materials and Devices (Ministry of Education), Jianghan University, Wuhan, China
During last decades, significant advances have been made in iron-based spin crossover (SCO) complexes, with a particular emphasis on achieving reversible and reproducible thermal hysteresis at room temperature (RT). This pursuit represents a pivotal goal within the field of molecular magnetism, aiming to create molecular devices capable of operating in ambient conditions. Here, we summarize the recent progress of iron complexes with spin transition near RT based on nitrogen ligands containing aromatic rings from molecular design to functional devices. Specifically, we discuss the various factors, including supramolecular interactions, crystal packing, guest molecules and pressure effects, that could influence its cooperativity and the spin transition temperature. Furthermore, the most recent advances in their implementation as mechanical actuators, switching/memories, sensors, and other devices, have been introduced as well. Finally, we give a perspective on current challenges and future directions in SCO community.

Article information
Download citation, permissions.
Y. Zhang, R. Torres-Cavanillas, X. Yan, Y. Zeng, M. Jiang, M. Clemente-León, E. Coronado and S. Shi, Chem. Soc. Rev. , 2024, Advance Article , DOI: 10.1039/D3CS00688C
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements

COMMENTS
Transitional devices are like bridges between parts of your paper. They are cues that help the reader to interpret ideas a paper develops. Transitional devices are words or phrases that help carry a thought from one sentence to another, from one idea to another, or from one paragraph to another. And finally, transitional devices link sentences ...
Abruptly switching topics in essays can be jarring; however, transition words can smooth the change for the convenience of the reader.Moreover, you can use essay transition words to start a paragraph, sentence, or clause more naturally.Additionally, essay transition words can connect new information to the previous statement so you don't have to say everything at once.
Usage of Transition Words in Essays. Transition words and phrases are vital devices for essays, papers or other literary compositions. They improve the connections and transitions between sentences and paragraphs. They thus give the text a logical organization and structure (see also: a List of Synonyms).
Transitional words and phrases can create powerful links between ideas in your paper and can help your reader understand the logic of your paper. However, these words all have different meanings, nuances, and connotations. Before using a particular transitional word in your paper, be sure you understand its meaning and usage completely and be sure…
Example sentence. Transition words and phrases. Addition. We found that the mixture was effective. Moreover, it appeared to have additional effects we had not predicted. indeed, furthermore, moreover, additionally, and, also, both x and y, not only x but also y, besides x, in fact. Introduction.
Writing Transitions. Good transitions can connect paragraphs and turn disconnected writing into a unified whole. Instead of treating paragraphs as separate ideas, transitions can help readers understand how paragraphs work together, reference one another, and build to a larger point. The key to producing good transitions is highlighting ...
explaining or elaborating. Transitions can signal to readers that you are going to expand on a point that you have just made or explain something further. Transitional words that signal explanation or elaboration include in other words, for example, for instance, in particular, that is, to illustrate, moreover. drawing conclusions.
A transition between paragraphs can be a word or two (however, for example, similarly), a phrase, or a sentence. Transitions can be at the end of the first paragraph, at the beginning of the second paragraph, or in both places. Transitions within paragraphs: As with transitions between sections and paragraphs, transitions within paragraphs act ...
Transitional words and phrases are vital devices for essays, papers or other literary compositions. They improve the connections and transitions between sentences and paragraphs. They thus give the text a logical organization and structure. All English transitional words and phrases (sometimes also called 'conjunctive
Clear transitions are crucial to clear writing: They show the reader how different parts of your essay, paper, or thesis are connected. Transition sentences can be used to structure your text and link together paragraphs or sections. Example of a transition sentence for a new paragraph. In this case, the researchers concluded that the method ...
Transition words illustrate relationships between other words and phrases. Although students are generally taught to use transition words at the beginning of sentences, this isn't the only place they're used. Generally, a transition word is the crux of its sentence. This is the decisive point where the sentence's core message is communicated.
See why leading organizations rely on MasterClass for learning & development. Transition words are useful for all types of writers. Whether you're attempting academic writing, blogging, speech writing, or writing fiction, transition words can help refine your text and create a narrative flow.
Clear transitions are crucial to clear writing: they connect different parts of your essay and structure your text. This video will walk you through the use ...
33 Transition Words and Phrases. 'Besides,' 'furthermore,' 'although,' and other words to help you jump from one idea to the next. Transitional terms give writers the opportunity to prepare readers for a new idea, connecting the previous sentence to the next one. Many transitional words are nearly synonymous: words that broadly indicate that ...
Transitions are used to enhance cohesion in your paper and make its logical development clearer to readers. Types of Transition Words. Transitions accomplish many different objectives. We can divide all transitions into four basic categories: Additive transitions signal to the reader that you are adding or referencing information
Transition words are important English devices for essays and papers. They enhance the transitions and connections between the sentences and paragraphs, giving your essay a flowing structure and logical thought. Transition terms may seem easy to remember; however, placing them in the incorrect manner can cause your essay to fall flat.
The last thing you want is your transition words to feel trite and uninspired. Discover what these words are and a variety of examples for your writing here. ... to connect paragraphs and to connect whole sections of an essay. See how transition words and phrases work to connect sentences, paragraphs and sections through examples.
Transitional devices play a crucial role in creating cohesion by linking sentences and paragraphs together. Synonyms/Repetition: Using synonyms or repeating key words can also serve as transitional devices. They help reinforce connections between ideas by using familiar language throughout an essay. Chronological Order/Time Transitions: Time ...
Transitional Devices Without the use of transitional devices, the following paragraph appears disjointed and disorganized: The Writing Center is a valuable resource for students who have trouble writing papers. It is also a good resource for students who are skilled at writing papers. These students might simply want to improve their writing ...
Transitional Devices in Essay Writing. Transitional Devices are like bridges connecting one thought to the next. Get in the habit of using transitions when carrying over a thought from one sentence to another, from one idea to another or from one paragraph to another. Using transitions effectively creates a paper that flows smoothly rather than ...
Do you want to learn how to write effective summaries and conclusions for your essays, reports, or presentations? Then you need to know how to use conclusion transition words, which are words or phrases that connect your ideas and signal the end of your argument. In this webpage, you will find 42 summary and conclusion transition words, with clear definitions and examples, to help you improve ...
Transition words: These are words that connect two ideas together. Examples include "however," "therefore," and "moreover.". It is important to use transitional phrases appropriately and sparingly. Overusing them can make your writing appear choppy and disjointed. Additionally, not all conclusions require the use of transitional ...
Examples of Transition Words for Essays. Depending on the essay you're writing, you might find that it calls for different transition words. Some transition words and phrases introduce new content ideas, while others highlight that two ideas are equally important. In fact, you might need several of these types in any academic writing you do.
Transition words Worksheet 2. Choose the Right Option. a) Consequently b) However c) Furthermore d) Additionally. a) Nevertheless b) For instance c) In contrast d) Therefore. ______, we can conclude that climate change is a pressing issue. a) Nonetheless b) In addition c) Hence d) Although.
The Transition To Multifunctional Devices. The devices that we use to access and use this information are also having a greater impact as they become multifunctional while getting smaller to carry ...
Device Eligibility. Given the unique FDA criteria for Breakthrough designation status, the TCET pathway will apply to certain eligible FDA-designated Breakthrough Devices because this is the area with the most immediate need for a pathway like TCET. Appropriate candidates for the TCET pathway would include those devices that are:
In August 2024, the Centers for Medicare & Medicaid Services (CMS) established the Transitional Coverage for Emerging Technologies (TCET) pathway, intended to "accelerate patient access to beneficial medical products while generating evidence" needed to assess long-term coverage. 1 This pathway is limited to certain devices that have been designed as Breakthrough devices by the Food and ...
For devices where the evidence is promising but does not yet satisfy the reasonable and necessary standard for Medicare coverage, the EDP intends to articulate how material evidence gaps will be addressed during transitional coverage so that the evidence may show that the device satisfies the reasonable and necessary standard when a CED NCD is ...
Mr. Vought and Mr. McEntee are involved in Project 2025, a $22 million presidential transition operation that is preparing policies, personnel lists and transition plans to recommend to any ...
This pursuit represents a pivotal goal within the field of molecular magnetism, aiming to create molecular devices capable of operating in ambient conditions. Here, we summarize the recent progress of iron complexes with spin transition near RT based on nitrogen ligands containing aromatic rings from molecular design to functional devices.