Chapter 1: Literature Review
- In book: Molecular Characterization of Full Genome HBV sequences from an urban hospital cohort in Pretoria, South Africa [thesis] (pp.1-48)
- Edition: 1st
- Publisher: University of Pretoria

- French National Centre for Scientific Research

Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 17 May 2021
Immunobiology and pathogenesis of hepatitis B virus infection
- Matteo Iannacone ORCID: orcid.org/0000-0002-9370-2671 1 , 2 , 3 &
- Luca G. Guidotti ORCID: orcid.org/0000-0002-0205-2678 1 , 2
Nature Reviews Immunology volume 22 , pages 19–32 ( 2022 ) Cite this article
19k Accesses
234 Citations
51 Altmetric
Metrics details
- Hepatitis B
- Immunological surveillance
- Viral host response
- Viral pathogenesis
Hepatitis B virus (HBV) is a non-cytopathic, hepatotropic virus with the potential to cause a persistent infection, ultimately leading to cirrhosis and hepatocellular carcinoma. Over the past four decades, the basic principles of HBV gene expression and replication as well as the viral and host determinants governing infection outcome have been largely uncovered. Whereas HBV appears to induce little or no innate immune activation, the adaptive immune response mediates both viral clearance as well as liver disease. Here, we review our current knowledge on the immunobiology and pathogenesis of HBV infection, focusing in particular on the role of CD8 + T cells and on several recent breakthroughs that challenge current dogmas. For example, we now trust that HBV integration into the host genome often serves as a relevant source of hepatitis B surface antigen (HBsAg) expression during chronic infection, possibly triggering dysfunctional T cell responses and favouring detrimental immunopathology. Further, the unique haemodynamics and anatomy of the liver — and the changes they frequently endure during disease progression to liver fibrosis and cirrhosis — profoundly influence T cell priming, differentiation and function. We also discuss why therapeutic approaches that limit the intrahepatic inflammatory processes triggered by HBV-specific T cells might be surprisingly beneficial for patients with chronic infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Hepatitis E virus: from innate sensing to adaptive immune responses

Long-term hepatitis B virus infection of rhesus macaques requires suppression of host immunity
The evolution and clinical impact of hepatitis B virus genome diversity
Guidotti, L. G. & Chisari, F. V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. Mech. Dis. 1 , 23–61 (2006).
Article CAS Google Scholar
Locarnini, S., Hatzakis, A., Chen, D.-S. & Lok, A. Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs. J. Hepatol. 62 , S76–S86 (2015).
Article PubMed Google Scholar
Yuen, M.-F. et al. Hepatitis B virus infection. Nat. Rev. Dis. Primers 4 , 18035 (2018).
Revill, P. A. et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol. Hepatol. 4 , 545–558 (2019).
Article PubMed PubMed Central Google Scholar
Udompap, P. & Kim, W. R. Development of hepatocellular carcinoma in patients with suppressed viral replication: changes in risk over time. Clin. Liver Dis. 15 , 85–90 (2020).
Article Google Scholar
Levrero, M., Testoni, B. & Zoulim, F. HBV cure: why, how, when? Curr. Opin. Virol. 18 , 135–143 (2016).
Fanning, G. C., Zoulim, F., Hou, J. & Bertoletti, A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat. Rev. Drug Discov. 18 , 827–844 (2019).
Article CAS PubMed Google Scholar
Rehermann, B., Ferrari, C., Pasquinelli, C. & Chisari, F. V. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat. Med. 2 , 1104–1108 (1996).
Kim, C. Y. & Tilles, J. G. Purification and biophysical characterization of hepatitis B antigen. J. Clin. Invest. 52 , 1176–1186 (1973).
Article CAS PubMed PubMed Central Google Scholar
Seeger, C. & Mason, W. S. Molecular biology of hepatitis B virus infection. Virology 479 , 672–686 (2015).
Article PubMed CAS Google Scholar
Bertoletti, A. & Ferrari, C. Adaptive immunity in HBV infection. J. Hepatol. 64 , S71–S83 (2016).
Guidotti, L. G., Isogawa, M. & Chisari, F. V. Host–virus interactions in hepatitis B virus infection. Curr. Opin. Immunol. 36 , 61–66 (2015).
Tu, T. et al. Integration occurs early in the viral life cycle in an in vitro infection model via sodium taurocholate cotransporting polypeptide-dependent uptake of enveloped virus particles. J. Virol. 92 , e02007–e02017 (2018). This study shows that HBV DNA integration occurs early upon infection in an in vitro infection model .
Summers, J. et al. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc. Natl Acad. Sci. USA 100 , 11652–11659 (2003).
Yang, W. & Summers, J. Integration of hepadnavirus DNA in infected liver: evidence for a linear precursor. J. Virol. 73 , 9710–9717 (1999).
Wooddell, C. I. et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci. Transl Med. 9 , eaan0241 (2017). This paper reveals integrated HBV DNA as a relevant source of HBsAg in patients and chimpanzees with chronic infection .
Article PubMed PubMed Central CAS Google Scholar
Simon, T. G. et al. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N. Engl. J. Med. 382 , 1018–1028 (2020). This manuscript represents one of a large number of meta-analyses describing an association between low-dose aspirin treatment and reduced HCC incidence .
Sitia, G. et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc. Natl Acad. Sci. USA 109 , E2165–E2172 (2012). This preclinical study shows that anti-platelet therapy reduces liver fibrosis and prevents HCC in mouse models of CHB .
Iannacone, M., Sitia, G., Narvaiza, I., Ruggeri, Z. M. & Guidotti, L. G. Antiplatelet drug therapy moderates immune-mediated liver disease and inhibits viral clearance in mice infected with a replication-deficient adenovirus. Clin. Vaccine Immunol. 14 , 1532–1535 (2007).
Jilbert, A. R., Miller, D. S., Scougall, C. A., Turnbull, H. & Burrell, C. J. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology 226 , 338–345 (1996).
Asabe, S. et al. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J. Virol. 83 , 9652–9662 (2009).
Wisse, E., Jacobs, F., Topal, B., Frederik, P. & Geest, B. D. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 15 , 1193–1199 (2008).
Vollmar, B. & Menger, M. D. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 89 , 1269–1339 (2009).
Whalley, S. A. et al. Kinetics of acute hepatitis B virus infection in humans. J. Exp. Med. 193 , 847–854 (2001).
Guidotti, L. G., Matzke, B., Schaller, H. & Chisari, F. V. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69 , 6158–6169 (1995).
Guidotti, L. G. et al. Viral clearance without destruction of infected cells during acute HBV infection. Science 284 , 825–829 (1999).
Wieland, S. F., Spangenberg, H. C., Thimme, R., Purcell, R. H. & Chisari, F. V. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc. Natl Acad. Sci. USA 101 , 2129–2134 (2004).
Wieland, S., Thimme, R., Purcell, R. H. & Chisari, F. V. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl Acad. Sci. USA 101 , 6669–6674 (2004).
Suslov, A. et al. Virus does not interfere with innate immune responses in the human liver. Gastroenterology 154 , 1778–1790 (2018).
Tsui, L. V., Guidotti, L. G., Ishikawa, T. & Chisari, F. V. Posttranscriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc. Natl Acad. Sci. USA 92 , 12398–12402 (1995).
Heise, T., Guidotti, L. G., Cavanaugh, V. J. & Chisari, F. V. Hepatitis B virus RNA-binding proteins associated with cytokine-induced clearance of viral RNA from the liver of transgenic mice. J. Virol. 73 , 474–481 (1999).
Heise, T., Guidotti, L. G. & Chisari, F. V. La autoantigen specifically recognizes a predicted stem-loop in hepatitis B virus RNA. J. Virol. 73 , 5767–5776 (1999).
McClary, H., Koch, R., Chisari, F. V. & Guidotti, L. G. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 74 , 2255–2264 (2000).
Wieland, S. F., Guidotti, L. G. & Chisari, F. V. Intrahepatic induction of α/β interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J. Virol. 74 , 4165–4173 (2000).
Kimura, K., Kakimi, K., Wieland, S., Guidotti, L. G. & Chisari, F. V. Activated intrahepatic antigen-presenting cells inhibit hepatitis B virus replication in the liver of transgenic mice. J. Immunol. 169 , 5188–5195 (2002).
Vilarinho, S., Ogasawara, K., Nishimura, S., Lanier, L. L. & Baron, J. L. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc. Natl Acad. Sci. USA 104 , 18187–18192 (2007).
Isogawa, M., Robek, M. D., Furuichi, Y. & Chisari, F. V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 79 , 7269–7272 (2005).
Suslov, A., Wieland, S. & Menne, S. Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B. Curr. Opin. Virol. 30 , 9–17 (2018).
Iwasaki, A. A virological view of innate immune recognition. Annu. Rev. Microbiol. 66 , 177–196 (2012).
Webster, G. J. M. et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 32 , 1117–1124 (2000).
Thimme, R. et al. CD8 + T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77 , 68–76 (2003).
Hoofnagle, J. H., Gerety, R. J. & Barker, L. F. Antibody to hepatitis-B-virus core in man. Lancet 302 , 869–873 (1973).
Maini, M. K. & Burton, A. R. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat. Rev. Gastroenterol. Hepatol. 16 , 662–675 (2019).
Guidotti, L. G. et al. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl Acad. Sci. USA 91 , 3764–3768 (1994).
Guidotti, L. G. et al. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4 , 25–36 (1996).
Wong, Y. C., Tay, S. S., McCaughan, G. W., Bowen, D. G. & Bertolino, P. Immune outcomes in the liver: is CD8 T cell fate determined by the environment? J. Hepatol. 63 , 1005–1014 (2015).
Isogawa et al. CD40 activation rescues antiviral CD8 + T cells from PD-1-mediated exhaustion. PLoS Pathog. 9 , e1003490 (2013).
Bénéchet, A. P. et al. Dynamics and genomic landscape of CD8 + T cells undergoing hepatic priming. Nature 574 , 200–205 (2019). This paper reveals that hepatocellular priming leads to a T cell dysfunction that is refractory to checkpoint inhibition but responds to IL-2 .
Bertolino, P. et al. Death by neglect as a deletional mechanism of peripheral tolerance. Int. Immunol. 11 , 1225–1238 (1999).
Pol et al. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 217 , 2261 (2020).
Blattman, J. N. et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9 , 540–547 (2003).
West, E. E. et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Invest. 123 , 2604–2615 (2013).
Kuipery, A., Gehring, A. J. & Isogawa, M. Mechanisms of HBV immune evasion. Antivir. Res. 179 , 104816 (2020).
Kennedy, P. T. F. et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 143 , 637–645 (2012).
Shimizu, Y., Guidotti, L. G., Fowler, P. & Chisari, F. V. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J. Immunol. 161 , 4520–4529 (1998).
Kakimi, K., Isogawa, M., Chung, J., Sette, A. & Chisari, F. V. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J. Virol. 76 , 8609–8620 (2002).
Ishak, K. et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 22 , 696–699 (1995).
Fisicaro, P. et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 23 , 327–336 (2017). This article suggests a central role for reactive oxygen species in T cell exhaustion during CHB, thus providing novel potential therapeutic targets .
Wieland, S. F. The chimpanzee model for hepatitis B virus infection. CSH Perspect. Med. 5 , a021469 (2015).
Google Scholar
Chen, M. T. et al. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc. Natl Acad. Sci. USA 101 , 14913–14918 (2004).
Chen, M. et al. Immune tolerance split between hepatitis B virus precore and core proteins. J. Virol. 79 , 3016–3027 (2005).
Tian, Y., Kuo, C., Akbari, O. & Ou, J. J. Maternal-derived hepatitis B virus e antigen alters macrophage function in offspring to drive viral persistence after vertical transmission. Immunity 44 , 1204–1214 (2016).
Publicover, J. et al. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J. Clin. Invest. 123 , 3728–3739 (2013).
Brunetto, M. R. et al. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc. Natl Acad. Sci. USA 88 , 4186–4190 (1991).
Rivino, L. et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J. Clin. Invest. 128 , 668–681 (2018).
Schuch, A. et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8 + T cells in chronically HBV-infected patients with low viral load. Gut 68 , 905–915 (2019).
Fumagalli, V. et al. Serum HBsAg clearance has minimal impact on CD8 + T cell responses in mouse models of HBV infection. J. Exp. Med. 217 , e20200298 (2020). This study shows that circulating HBsAg clearance does not improve HBV-specific CD8 + T cell responses .
Bert, N. L. et al. Effects of hepatitis B surface antigen on virus-specific and global T cells in patients with chronic hepatitis B virus infection. Gastroenterology 159 , 652–664 (2020).
Li et al. A potent human neutralizing antibody Fc-dependently reduces established HBV infections. eLife 6 , e26738 (2017).
Zhang, T.-Y. et al. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen. Gut 65 , 658 (2015).
Neumann et al. Novel mechanism of antibodies to hepatitis B virus in blocking viral particle release from cells. Hepatology 52 , 875–885 (2010).
Galun, E. et al. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology 35 , 673–679 (2002).
Bertoletti, A. et al. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J. Exp. Med. 180 , 933–943 (1994).
Bertoletti, A. et al. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature 369 , 407–410 (1994).
Maini, M. K. et al. T cell receptor usage of virus-specific CD8 cells and recognition of viral mutations during acute and persistent hepatitis B virus infection. Eur. J. Immunol. 30 , 3067–3078 (2000).
Bertoletti, A. & Kennedy, P. T. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol. Immunol. 12 , 258–263 (2015).
Fisicaro, P. et al. Pathogenetic mechanisms of T cell dysfunction in chronic HBV infection and related therapeutic approaches. Front. Immunol. 11 , 849 (2020).
Burton, A. R. et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J. Clin. Invest. 128 , 4588–4603 (2018).
Salimzadeh, L. et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J. Clin. Invest. 128 , 4573–4587 (2018). Together with Burton et al. (2018), this paper detects and characterizes dysfunctional HBsAg-specific B cell responses in patients with chronic HBV infection .
Tian, C. et al. Use of ELISpot assay to study HBs-specific B cell responses in vaccinated and HBV infected humans. Emerg. Microbes Infec 7 , 16 (2018).
Xu, X. et al. Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell Mol. Immunol. 12 , 309–316 (2015).
Bert, N. L. et al. Comparative characterization of B cells specific for HBV nucleocapsid and envelope proteins in patients with chronic hepatitis B. J. Hepatol. 72 , 34–44 (2019).
Vanwolleghem, T. et al. Hepatitis B core-specific memory B cell responses associate with clinical parameters in patients with chronic HBV. J. Hepatol. 73 , 52–61 (2020).
Milich, D. & McLachlan, A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 234 , 1398–1401 (1986).
Guidotti, L. G. & Iannacone, M. Effector CD8 T cell trafficking within the liver. Mol. Immunol. 55 , 94–99 (2013).
Iannacone, M. Hepatic effector CD8 + T-cell dynamics. Cell Mol. Immunol. 12 , 269–272 (2015).
Inverso, D. & Iannacone, M. Spatiotemporal dynamics of effector CD8 + T cell responses within the liver. J. Leukoc. Biol. 99 , 51–55 (2016).
Benechet, A. P. & Iannacone, M. Determinants of hepatic effector CD8 + T cell dynamics. J. Hepatol. 66 , 228–233 (2017).
Guidotti, L. G. et al. Immunosurveillance of the liver by intravascular effector CD8 + T cells. Cell 161 , 486–500 (2015). This manuscript reports that effector CD8 + T cells can recognize and kill antigen-expressing hepatocytes without extravasating by extending cytoplasmic protrusions through endothelial fenestration .
Sironi, L. et al. In vivo flow mapping in complex vessel networks by single image correlation. Sci. Rep. 4 , 7341 (2014).
Warren, A. et al. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 44 , 1182–1190 (2006).
Guidotti, L. G. The role of cytotoxic T cells and cytokines in the control of hepatitis B virus infection. Vaccine 20 , A80–A82 (2002).
Fioravanti, J. et al. Effector CD8 + T cell-derived interleukin-10 enhances acute liver immunopathology. J. Hepatol. 67 , 543–548 (2017).
Iannacone, M. & Guidotti, L. G. Mouse models of hepatitis B virus pathogenesis. CSH Perspect. Med. 5 , a021477 (2015).
Guidotti, L. G., McClary, H., Loudis, J. M. & Chisari, F. V. Nitric oxide inhibits hepatitis b virus replication in the livers of transgenic mice. J. Exp. Med. 191 , 1247–1252 (2000).
Wieland, S. F., Eustaquio, A., Whitten-Bauer, C., Boyd, B. & Chisari, F. V. Interferon prevents formation of replication-competent hepatitis B virus RNA-containing nucleocapsids. Proc. Natl Acad. Sci. USA 102 , 9913–9917 (2005).
Robek, M. D., Wieland, S. F. & Chisari, F. V. Inhibition of hepatitis B virus replication by interferon requires proteasome activity. J. Virol. 76 , 3570–3574 (2002).
Xia, Y. et al. Interferon-γ and tumor necrosis factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology 150 , 194–205 (2016).
Michalak, T. I., Pasquinelli, C., Guilhot, S. & Chisari, F. V. Hepatitis B virus persistence after recovery from acute viral hepatitis. J. Clin. Invest. 93 , 230–239 (1994).
Pallett, L. J. et al. IL-2 high tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J. Exp. Med. 214 , 1567–1580 (2017). This paper characterizes tissue-resident memory T cells in the liver of patients chronically infected by HBV .
Ando, K. et al. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 152 , 3245–3253 (1994).
Nakamoto, Y., Guidotti, L. G., Pasquetto, V., Schreiber, R. D. & Chisari, F. V. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J. Immunol. 158 , 5692–5697 (1997).
Sitia, G. et al. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog. 7 , e1002061 (2011).
Sitia et al. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J. Leukoc. Biol. 81 , 100–107 (2007).
Sitia, G. et al. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc. Natl Acad. Sci. USA 99 , 13717–13722 (2002).
Sitia, G. et al. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J. Clin. Invest. 113 , 1158–1167 (2004).
Kakimi, K. et al. Blocking chemokine responsive to γ-2/interferon (IFN)-γ inducible protein and monokine induced by IFN-γ activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J. Exp. Med. 194 , 1755–1766 (2001).
Maini, M. K. et al. The role of virus-specific CD8 + cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191 , 1269–1280 (2000).
Reignat, S. et al. Escaping high viral load exhaustion CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J. Exp. Med. 195 , 1089–1101 (2002).
Webster, G. J. M. et al. Longitudinal analysis of CD8 + T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J. Virol. 78 , 5707–5719 (2004).
Boni, C. et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81 , 4215–4225 (2007).
Hoogeveen, R. C. et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut 68 , 893–904 (2018).
Nakamoto, Y., Guidotti, L. G., Kuhlen, C. V., Fowler, P. & Chisari, F. V. Immune pathogenesis of hepatocellular carcinoma. J. Exp. Med. 188 , 341–350 (1998).
Isogawa, M., Furuichi, Y. & Chisari, F. V. Oscillating CD8 + T cell effector functions after antigen recognition in the liver. Immunity 23 , 53–63 (2005).
Khakpoor, A. et al. Spatiotemporal differences in presentation of CD8 T cell epitopes during hepatitis B virus infection. J. Virol . 93 , e01457-18 (2018).
Nakamoto, Y., Suda, T., Momoi, T. & Kaneko, S. Different procarcinogenic potentials of lymphocyte subsets in a transgenic mouse model of chronic hepatitis B. Cancer Res. 64 , 3326–3333 (2004).
Tang, L. S. Y., Covert, E., Wilson, E. & Kottilil, S. Chronic hepatitis B infection: a review. JAMA 319 , 1802–1813 (2018).
Buendia, M.-A. & Neuveut, C. Hepatocellular carcinoma. CSH Perspect. Med. 5 , a021444 (2015).
Levrero, M. & Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 64 , S84–S101 (2016).
Bisceglie, A. M. D. Hepatitis B and hepatocellular carcinoma. Hepatology 49 , S56–S60 (2009).
Schuppan, D. & Afdhal, N. H. Liver cirrhosis. Lancet 371 , 838–851 (2008).
Bataller, R. & Brenner, D. A. Liver fibrosis. J. Clin. Invest. 115 , 209–218 (2005).
Friedman, S. L. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat. Clin. Pract. Gastr 1 , 98–105 (2004).
Iannacone, M. et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat. Med. 11 , 1167–1169 (2005). This study establishes platelets as critical mediators of liver damage through their capacity to promote liver homing of effector CD8 + T cells .
Ornelas, A. et al. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metast Rev. 36 , 289–303 (2017).
Haemmerle, M., Stone, R. L., Menter, D. G., Afshar-Kharghan, V. & Sood, A. K. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell 33 , 965–983 (2018).
Lee, P.-C. et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann. Surg. Oncol. 23 , 874–883 (2016).
Hwang, I. C., Chang, J., Kim, K. & Park, S. M. Aspirin use and risk of hepatocellular carcinoma in a national cohort study of Korean adults. Sci. Rep. 8 , 4968 (2018).
Simon, T. G. et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 4 , 1683 (2018).
Lee, T.-Y. et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern. Med. 179 , 633–640 (2019).
Wang, S. et al. Association of aspirin therapy with risk of hepatocellular carcinoma: a systematic review and dose–response analysis of cohort studies with 2.5 million participants. Pharmacol. Res. 151 , 104585 (2019).
Liao, Y.-H. et al. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: a nationwide cohort study. BMC Gastroenterol. 20 , 6 (2020).
Bosetti, C., Santucci, C., Gallus, S., Martinetti, M. & Vecchia, C. L. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann. Oncol. 31 , 558–568 (2020).
Hayashi, T. et al. Antiplatelet therapy improves the prognosis of patients with hepatocellular carcinoma. Cancers 12 , 3215 (2020).
Article CAS PubMed Central Google Scholar
Guidotti, L. G., Vecchia, C. L. & Colombo, M. Is it time to recommend low-dose aspirin treatment for the prevention of hepatocellular carcinoma? Gastroenterology 159 , 1988–1990 (2020).
Martinez, M. G., Villeret, F., Testoni, B. & Zoulim, F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int. 40 , 27–34 (2020).
Hillis, W. D. Viral hepatitis associated with sub-human primates. Transfusion 3 , 445–454 (1963).
Walter, E., Keist, R., Niederöst, B., Pult, I. & Blum, H. E. Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo. Hepatology 24 , 1–5 (1996).
CAS PubMed Google Scholar
Schulze, A., Gripon, P. & Urban, S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 46 , 1759–1768 (2007).
Sureau, C. & Salisse, J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus A-determinant. Hepatology 57 , 985–994 (2013).
Roskams, T. et al. Heparan sulfate proteoglycan expression in normal human liver. Hepatology 21 , 950–958 (1995).
Yan, H. et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1 , e00049 (2012).
Döring, B., Lütteke, T., Geyer, J. & Petzinger, E. The SLC10 carrier family: transport functions and molecular structure. Curr. Top. Membr. 70 , 105–168 (2012).
Hu, J. & Liu, K. Complete and incomplete hepatitis B virus particles: formation, function, and application. Viruses 9 , 56 (2017).
Article PubMed Central CAS Google Scholar
Seitz, S., Habjanič, J., Schütz, A. K. & Bartenschlager, R. The hepatitis B virus envelope proteins: molecular gymnastics throughout the viral life cycle. Ann. Rev. Virol. 7 , 1–26 (2020).
CAS Google Scholar
Wisse, E., de Zanger, R. B., Charels, K., Van Der Smissen, P. & McCuskey, R. S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of disse. Hepatology 5 , 683–692 (1985).
Ficht, X. & Iannacone, M. Immune surveillance of the liver by T cells. Sci. Immunol. 5 , eaba2351 (2020).
Iwakiri, Y. The lymphatic system: a new frontier in hepatology. Hepatology 64 , 706–707 (2016).
Jenne, C. N. & Kubes, P. Immune surveillance by the liver. Nat. Immunol. 14 , 996–1006 (2013).
Horst, A. K., Neumann, K., Diehl, L. & Tiegs, G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol. Immunol. 13 , 277–292 (2016).
Wong, Y. C., McCaughan, G. W., Bowen, D. G. & Bertolino, P. The CD8 T-cell response during tolerance induction in liver transplantation. Clin. Transl Immunol. 5 , e102 (2016).
Mason, W. S. et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology 151 , 986–998.e4 (2016).
Tu, T., Budzinska, M. A., Shackel, N. A. & Urban, S. HBV DNA integration: molecular mechanisms and clinical implications. Viruses 9 , 75 (2017).
Budzinska, M. A., Shackel, N. A., Urban, S. & Tu, T. Cellular genomic sites of hepatitis B virus DNA integration. Genes 9 , 365 (2018).
Huang, Z. M. & Yen, T. S. Dysregulated surface gene expression from disrupted hepatitis B virus genomes. J. Virol. 67 , 7032–7040 (1993).
Dienes, H. P. et al. Hepatic expression patterns of the large and middle hepatitis B virus surface proteins in viremic and nonviremic chronic hepatitis B. Gastroenterology 98 , 1017–1023 (1990).
Chisari, F. V. et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 59 , 1145–1156 (1989).
Su, I., Wang, H., Wu, H. & Huang, W. Ground glass hepatocytes contain pre-S mutants and represent preneoplastic lesions in chronic hepatitis B virus infection. J. Gastroen Hepatol. 23 , 1169–1174 (2008).
Hadziyannis, S., Gerber, M. A., Vissoulis, C. & Popper, H. Cytoplasmic hepatitis B antigen in “ground-glass” hepatocytes of carriers. Arch. Pathol. 96 , 327–330 (1973).
Tu, T. et al. Clonal expansion of hepatocytes with a selective advantage occurs during all stages of chronic hepatitis B virus infection. J. Viral Hepat. 22 , 737–753 (2015).
Download references
Acknowledgements
The authors thank M. Silva for secretarial assistance, F. Andreata for help with figure preparation and the members of the Iannacone and Guidotti laboratories for helpful discussions. They apologize to all authors whose work they could not cite due to space constraints. M.I. is supported by the European Research Council (ERC) Consolidator Grant 725038, ERC Proof of Concept Grant 957502, Italian Association for Cancer Research (AIRC) Grants 19891 and 22737, Italian Ministry of Health (MoH) Grants RF-2018-12365801 and COVID-2020-12371617, Lombardy Foundation for Biomedical Research (FRRB) Grant 2015-0010, the European Molecular Biology Organization Young Investigator Program and a Funded Research Agreement from Gilead Sciences. L.G.G. is supported by the AIRC Grant 22737, Lombardy Open Innovation Grant 229452, PRIN Grant 2017MPCWPY from the Italian Ministry of Education, University and Research, and Funded Research Agreements from Gilead Sciences, Avalia Therapeutics and CNCCS SCARL.
Author information
Authors and affiliations.
Division of Immunology, Transplantation and Infectious Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy
Matteo Iannacone & Luca G. Guidotti
Vita-Salute San Raffaele University, Milan, Italy
Experimental Imaging Center, IRCCS San Raffaele Scientific Institute, Milan, Italy
Matteo Iannacone
You can also search for this author in PubMed Google Scholar
Contributions
M.I. and L.G.G. contributed equally to this work.
Corresponding authors
Correspondence to Matteo Iannacone or Luca G. Guidotti .
Ethics declarations
Competing interests.
M.I. participates in advisory boards/consultancies for Gilead Sciences, Roche, Third Rock Ventures, Amgen, Asher Bio and Allovir. L.G.G is a member of the board of directors at Genenta Science and Epsilon Bio and participates in advisory boards/consultancies for Gilead Sciences, Roche and Arbutus Biopharma. M.I. and L.G.G. are inventors on patents filed, owned and managed by San Raffaele Scientific Institute, Vita-Salute San Raffaele University and Telethon Foundation on technology related to work discussed in this manuscript (WO2020/016434, WO2020/016427, WO2020/030781, WO2020/234483, EU patent applications n. 19211249.8 and n. 20156716.1, and UK patent application n. 1907493.9).
Additional information
Peer review information.
Nature Reviews Immunology thanks Anna Lok, Antonio Bertoletti and Mala Maini for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The final stage of fibrosis in which fibrous septa surrounding nodules of regenerating hepatocytes induce profound architectural distortion of the liver and functional insufficiency.
A functional outcome of cross-presentation (the presentation of extracellular antigens on MHC class I molecules), whereby antigen-specific naive CD8 + T cells are activated by antigen-presenting cells to become effector cells.
A form of cancer immunotherapy targeting immune checkpoints (for example, PD1, CTLA4).
T cell-induced cytokines such as IFNγ and TNF have been shown to induce the post-transcriptional downregulation of hepatitis B virus (HBV) RNAs in vivo. This process appears to rely on the degradation of the full-length SSB/La protein, which normally functions as a HBV RNA stabilizer in the nucleus of the hepatocyte.
The serum concentrations of the liver enzyme alanine aminotransferase. Commonly measured clinically as a biomarker for liver damage.
(Also referred to as perisinusoidal space). The space that lies between the hepatocytes and the sinusoids.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Iannacone, M., Guidotti, L.G. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol 22 , 19–32 (2022). https://doi.org/10.1038/s41577-021-00549-4
Download citation
Accepted : 01 April 2021
Published : 17 May 2021
Issue Date : January 2022
DOI : https://doi.org/10.1038/s41577-021-00549-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Loading metrics
Open Access
Peer-reviewed
Research Article
A systematic review of Hepatitis B virus (HBV) prevalence and genotypes in Kenya: Data to inform clinical care and health policy
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft
Affiliations Nuffield Department of Medicine, Medawar Building for Pathogen Research, University of Oxford, Oxford, United Kingdom, Department of Infectious Diseases and Microbiology, John Radcliffe Hospital, Headley Way, Oxford, United Kingdom
Roles Formal analysis, Methodology, Writing – review & editing
Affiliation Nuffield Department of Medicine, Medawar Building for Pathogen Research, University of Oxford, Oxford, United Kingdom
Roles Investigation, Writing – review & editing
Affiliation CA Medlynks Clinic and Laboratory, Nairobi, and Fountain Projects and Research Office, Fountain Health Care Hospital, Eldoret, Kenya
Affiliations KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya, Department of Biochemistry and Biotechnology, Pwani University, Kilifi, Kenya
Roles Formal analysis, Methodology, Supervision, Writing – review & editing
Contributed equally to this work with: Philippa C. Matthews, Anthony O. Etyang
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing
* E-mail: [email protected]
Affiliations Nuffield Department of Medicine, Medawar Building for Pathogen Research, University of Oxford, Oxford, United Kingdom, The Francis Crick Institute, London, United Kingdom, Division of Infection and Immunity, University College London, London, London, United Kingdom, Department of Infectious Diseases, University College London Hospital, London, London, United Kingdom
Roles Conceptualization, Investigation, Supervision, Writing – review & editing
Affiliation KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya
- Louise O. Downs,
- Cori Campbell,
- Paul Yonga,
- George Githinji,
- M. Azim Ansari,
- Philippa C. Matthews,
- Anthony O. Etyang

- Published: January 31, 2023
- https://doi.org/10.1371/journal.pgph.0001165
- See the preprint
- Peer Review
- Reader Comments
The aim of this systematic review and meta-analysis is to evaluate available prevalence and viral sequencing data representing chronic hepatitis B (CHB) infection in Kenya. More than 20% of the global disease burden from CHB is in Africa, however there is minimal high quality seroprevalence data from individual countries and little viral sequencing data available to represent the continent. We undertook a systematic review of the prevalence and genetic data available for hepatitis B virus (HBV) in Kenya using the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) 2020 checklist. We identified 23 studies reporting HBV prevalence and 8 studies that included HBV genetic data published in English between January 2000 and December 2021. We assessed study quality using the Joanna Briggs Institute critical appraisal checklist. Due to study heterogeneity, we divided the studies to represent low, moderate, high and very high-risk for HBV infection, identifying 8, 7, 5 and 3 studies in these groups, respectively. We calculated pooled HBV prevalence within each group and evaluated available sequencing data. Pooled HBV prevalence was 3.4% (95% CI 2.7–4.2%), 6.1% (95% CI 5.1–7.4%), 6.2% (95% CI 4.64–8.2) and 29.2% (95% CI 12.2–55.1), respectively. Study quality was overall low; only three studies detailed sample size calculation and 17/23 studies were cross sectional. Eight studies included genetic information on HBV, with two undertaking whole genome sequencing. Genotype A accounted for 92% of infections. Other genotypes included genotype D (6%), D/E recombinants (1%) or mixed populations (1%). Drug resistance mutations were reported by two studies. There is an urgent need for more high quality seroprevalence and genetic data to represent HBV in Kenya to underpin improved HBV screening, treatment and prevention in order to support progress towards elimination targets.
Citation: Downs LO, Campbell C, Yonga P, Githinji G, Ansari MA, Matthews PC, et al. (2023) A systematic review of Hepatitis B virus (HBV) prevalence and genotypes in Kenya: Data to inform clinical care and health policy. PLOS Glob Public Health 3(1): e0001165. https://doi.org/10.1371/journal.pgph.0001165
Editor: Abraham D. Flaxman, University of Washington, UNITED STATES
Received: May 31, 2022; Accepted: November 28, 2022; Published: January 31, 2023
Copyright: © 2023 Downs et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All the data pertinent to the submission are included in the paper and its citations.
Funding: LD is funded by a Wellcome Clinician PhD fellowship (Grant number BST00070). CC is funded by GlaxoSmithKline (GSK) and the University of Oxford Nuffield Department of Medicine. PCM is funded by Wellcome (ref 110110Z/15/Z), UCL/UCLH NIHR Biomedical Research Centre (BRC) and core funding from the Francis Crick Institute. MAA is supported by a Sir Henry Dale Fellowship jointly funded by the Royal Society and Wellcome (ref 220171/Z/20/Z). For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: I have read the journal’s policy and the authors of this manuscript have the following competing interests: CC is partially funded by GlaxoSmithKline. There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Introduction
Chronic hepatitis B (CHB) accounts for an estimated 90,000 deaths annually across West, East and Southern Africa, where most countries are of medium to high prevalence for CHB (prevalence ≥4%), accounting for around 20% of the worldwide burden of infection [ 1 ]. The World Health Organisation’s (WHO) point prevalence estimate of CHB for Africa is 6.1% (95% CI 4.6–8.5%), but this varies substantially between settings, and high-quality data for individual countries are scarce [ 1 ]. CHB meets many of the WHO criteria for a neglected tropical disease, including disproportionately affecting populations living in poverty, being associated with significant stigma and discrimination, and poor investment in clinical infrastructure and research [ 2 ]. Fewer than 10% of people have access to testing and treatment, leading to delayed diagnosis, with associated risks of advanced liver disease including hepatocellular carcinoma (HCC) [ 1 ].
The Global Health Sector Strategy (GHSS) for viral hepatitis aims to eliminate HBV as a public health threat by 2030 by reducing the incidence of new chronic infections by 90% and reducing mortality by 65% from the 2015 baseline to achieve the 2030 WHO Sustainable Development Goals [ 3 ]. These are ambitious targets, and current estimates indicate they will not be attained in most settings until beyond 2050 [ 4 ]. Detailed seroprevalence data are lacking, but are urgently needed to target testing, treatment, and prevention interventions to the highest risk groups, to allocate resources, and to inform policy.
In Kenya, there is limited information regarding HBV prevalence. Most studies focus on specific groups such as blood donors and those living with HIV, which may not be representative of the general population [ 5 – 7 ]. Other studies have stringent inclusion criteria, meaning important demographic subgroups remain uncharacterised [ 8 ]. HBV testing is not done routinely in Kenya, even in antenatal populations.
Triple HBV vaccine from the age of 6 weeks onwards is recommended by the Kenyan Ministry of Health as a component of the multivalent vaccines rolled out by GAVI within the WHO Expanded Programme for Immunization (EPI). Hep B birth-dose (BD) vaccine for all babies within 24 hours of birth is recommended by the WHO, but has not been adopted by many countries–including Kenya–due to economic and logistical challenges [ 9 ]. However, more data are needed to underpin evidence-based policy in this domain, and there is increasing focus on PMTCT as part of ‘triple elimination’ strategies for HBV/HIV/Syphilis [ 10 ].
HBV is divided into 9 genotypes (A-I) with a 10 th putative genotype J [ 11 , 12 ]; these tend to have distinct geographical locations and have been linked to different outcomes. Genotype A predominates in many African countries and has been associated with horizontal transmission, chronicity, early HBeAg seroconversion [ 13 ], cirrhosis and HCC development [ 14 ]. Genotype also affects response to treatment (including drug resistance), and thus may influence clinical recommendations [ 13 – 15 ], though is not yet widely undertaken in clinical practice in most settings. Most studies of the impact of HBV genotype have been in Asia and Europe. There is a paucity of data on circulating genotypes and subgenotypes in Africa, including Kenya. Whole genome sequencing (WGS) of HBV in Kenya could provide information on transmission networks, disease and treatment outcomes, drug resistance and vaccine escape.
We here assimilate data to describe the seroprevalence and molecular characteristics of HBV infection in Kenya to underpin an evidence-base for local strategies for intervention, and highlight knowledge gaps to inform research. High resolution local data will be essential for development of local clinical care pathways and public health policy, to underpin progress towards the 2030 elimination targets.
Ethics statement
No ethical approval was required for this study.
Search strategy
We set out to review literature on prevalence and genetic characteristics of HBV infection in Kenya, using the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) 2020 statement checklist ( S1 PRISMA Checklist ). We searched the online databases PubMed, Embase, African Journals Online (AJOL) and Scopus on 6 th December 2021 using the terms in Table 1 . We included studies published in English, from 2000 to December 2021 (from 2003 for AJOL) that investigated prevalence, genotype and sequencing of HBV infection in Kenya. We only included data for adults from studies for which the full text was available. There was no minimum number of participants for studies included. We initially screened using a thorough review of the title and abstract, and subsequently reviewed the full manuscripts of eligible articles. Articles that did not meet the inclusion criteria were excluded. Any uncertainty regarding the inclusion of papers was discussed with another reviewer and a consensus obtained.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgph.0001165.t001
From each study, we extracted:
- Total number of individuals tested for HBV.
- Number of individuals found to be infected with HBV (either HBsAg positive or HBV DNA positive)
- Study location (city or geographical region)
- Participant selection criteria
- Laboratory methods for confirmation of HBV infection
- Whether any viral sequencing was undertaken, methods used and results (including genotype, presence of vaccine escape and drug resistance mutations).
Study heterogeneity and HBV risk groups
On the grounds of significant heterogeneity in the populations represented, we divided studies a priori into four groups representing populations with differing risks of testing positive for HBV infection. The low-risk group included studies likely to be most representative of the general population (antenatal women, healthcare workers, blood donors and the national survey). The moderate risk group consisted of studies containing populations living with HIV. High-risk groups were defined as people with risk factors for acquisition of blood-borne virus infection, including people who inject drugs, men who have sex with men (MSM) and sex workers. Those presenting to hospital with hepatitis or jaundice were defined as a very high-risk group, as HBV infection is enriched in populations presenting with established liver disease, particularly if the background population has medium or high HBV prevalence. This risk stratification system is a pragmatic approach to a highly heterogenous literature and we have used these risk groups for ease of reference throughout this review.
Quality assessment of studies
A thorough assessment of the study quality was done using the PRISMA guidelines [ 16 ] and Joanna Briggs Institute critical appraisal checklist for prevalence studies ( S1 Table ) [ 17 ]. Any dispute surrounding study quality was discussed with another reviewer and a consensus reached.
Identifying and analysing full-length HBV sequences from Kenya
We downloaded all full genome HBV sequences from Kenya in GenBank on 1-December-2021 to assimilate a reference set of all whole genome sequences representing Kenya. Sequences were aligned with available HBV reference sequences for each genotype (11) using MAFFT [ 18 ]. A maximum likelihood phylogenetic tree with bootstrap replicates of 1000 was created using NGPhylogeny.fr [ 19 ].
Statistical analysis
Occult HBV infection
Occult HBV infection (OBI) is defined as detectable HBV DNA in the absence of HBsAg. Where studies reported both HBsAg positivity rates and OBI rates in those who were HBsAg negative, only prevalence data based on HBsAg positivity was included in the meta-analysis, in order to ensure datasets were comparable between studies.
(i) Identification of studies
We identified 272 published studies, of which 23 studies met the inclusion criteria for prevalence assessment, representing a total of 11,467 people ( Fig 1 and Table 2 ). Three of these studies also screened individuals for occult HBV infection (OBI) in a total of 666 people using HBV DNA polymerase chain reaction (PCR) in addition to testing for HBsAg seroprevalence. Two studies screened initially with HBsAg, then with HBV DNA PCR on those who were HBsAg negative [ 20 , 21 ]. A further study included two different populations: a) those attending a clinic for sex workers, whom they screened initially for HBsAg, then HBV PCR in those who were HBsAg negative and b) known HBsAg negative, jaundiced participants whom they screened with HBV DNA PCR to detect OBI [ 22 ].
(AJOL: African Journal Online). All eight studies included for genetic analysis contain information on HBV genotype. Figure created in Biorender.com with licence to publish.
https://doi.org/10.1371/journal.pgph.0001165.g001
https://doi.org/10.1371/journal.pgph.0001165.t002
We identified nine studies reporting HBV sequence data (full or partial genome), including seven studies from among the 23 seroprevalence studies described above ( Table 2 ), and an additional two studies that only included HBsAg positive participants so were not included in prevalence analysis [ 23 , 24 ]. One study did not clearly report how many HBV samples were sequenced or the genotyping results, and this study was excluded from further analysis [ 25 ]. Eight studies remained for analysis representing 247 individuals ( Table 2 ).
We identified eight studies reporting HBsAg prevalence in low-risk populations (total number of individuals = 6828), seven studies in people living with HIV (medium risk, total number of individuals = 1861), five studies in high-risk groups (total number of individuals = 2221) and three studies in people presenting to clinical services with established liver disease (defined here as very high-risk for HBV infection; total number of individuals = 492).
(ii) Geographical distribution of HBV seroprevalence data
Of the 23 studies included, 14 (61%) were in Nairobi or Mombasa, Kenya’s most populous cities ( Table 2 ), and all studies were done in the South of the country along the infrastructure routes between Mombasa, Nairobi and Kisumu. These are also the most densely populated Kenyan counties [ 48 ]. Kisumu was the city most represented in the studies by overall sample size ( Fig 2 ).
Data from a systematic review of papers reporting prevalence and genetic data for HBV in Kenya between 2000 and 2021. The size of the red circle indicates numbers screened in each location, studies in the same location are grouped together. n = number of individuals reported. Surrounding countries are marked in blue, Kenya’s four most populous cities are marked in black. Figure created using R version 4.2.0, packages ggmaps version 3.0.0, ggplot2 version 3.3.6 and sf version 1.0–7. The Kenyan county shapefiles were obtained from the Humanitarian Data Exchange, available open source from https://data.humdata.org/dataset/geoboundaries-admin-boundaries-for-kenya .
https://doi.org/10.1371/journal.pgph.0001165.g002
The mean cohort sample size was 599 participants (IQR 434). 14 studies recruited participants for cohort inclusion at outpatient clinics (8 in HIV clinics, 4 in blood donor clinics, 1 in a health clinic and 1 in antenatal clinic), one captured data through the blood donor registry, three undertook community outreach screening, three recruited hospital inpatients, one recruited healthcare workers and one was a national survey of urban and rural population groups ( Table 2 ).
(iii) Quality assessment of the literature
Overall the quality of studies investigating HBV prevalence in Kenya was low ( Fig 3 and S1 Table ). 17/23 studies were cross sectional, reporting HBV population prevalence at a single time point only. Most cohort sampling methods were non-randomised and only 4/21 studies detailed their sample size calculation [ 20 , 21 , 37 , 41 ]. Several studies sampled people only from small geographical locations or from a subset of the general population e.g. HIV negative individuals. 21/23 studies used either an enzyme linked immunosorbent assay or chemiluminescent enzyme immunoassay (ELISA or CLEIA) for HBsAg diagnosis. Two studies used reverse passive haemagglutination for diagnosis of CHB, a method previously demonstrated to have poor sensitivity [ 26 , 27 , 43 ] ( Table 2 ). 2/23 studies went on to screen the HBsAg negative population for HBV DNA via PCR [ 20 , 21 ] and one study included a known HBsAg negative population which they screened for HBV DNA [ 22 ].
This is stratified by number of participants, study design, sampling method, data collection and diagnostic methods. RCT: Randomised controlled trial; EIA: Chemiluminescent enzyme immunoassay; ELISA: Enzyme linked immunosorbent assay.
https://doi.org/10.1371/journal.pgph.0001165.g003
(v) HBV prevalence estimates in different risk groups
The pooled estimate for HBV prevalence using a random effects model in the low-risk group was 3.36% (95% CI 2.67–4.21%) compared with 6.14% in the moderate risk group (95% CI 5.08–7.41%), 6.18% (95% CI 4.6–8.19%) in the high-risk group and 29.19% (95% CI 12.15–55.14%) in the very high-risk group, however we note that the confidence interval of this estimate is very wide ( Fig 4 ). Heterogeneity was significant (I 2 > 50%) within each subgroup, and highest in the very high-risk sub-group (I 2 = 95%, p < 0.01).
Data generated through a systematic review reporting prevalence and genetic data for HBV in Kenya between 2000–2021. In each case, the size of the population included is represented by the size of the square. Point prevalence and 95% Confidence Interval (CI) is indicated for each study. Studies are ordered by HBV prevalence in each risk group.
https://doi.org/10.1371/journal.pgph.0001165.g004
Three studies screened for OBI using HBV DNA PCR. These were in populations known to be HBsAg negative and from different HBV risk groups: blood donors, those living with HIV and those presenting to hospital with jaundice. OBI prevalence estimates in these studies were 2.4%, 5.3% and 18.7% respectively [ 20 – 22 ].
(vi) Identification of HBV sequences
All eight studies including HBV genetic information used PCR of the HBV basal core promotor, Pol or S genes for amplification, followed by Sanger sequencing to determine genotype. Two studies looked for known drug resistance-associated mutations (RAMs) [ 23 , 24 ]. Two studies undertook whole genome HBV sequencing in a total of 22 patients [ 23 , 28 ]. 228/247 (92%) of participants were infected with HBV genotype A, 15/247 (6%) with genotype D infection, whilst the remaining were either mixed genotype populations (2/247) or genotype D/E recombinants (2/247) ( Table 3 ). Sub-genotype was determined in 146/247 (59%) participants. This was most commonly sub-genotype A1 (134/146, 92%) in keeping with previous regional data [ 44 ].
Data from 8 studies marked * in Table 2 .
https://doi.org/10.1371/journal.pgph.0001165.t003
To provide further background context for HBV sequences in Kenya, we identified 25 full length HBV sequences from GenBank ( Fig 5 ). These were generated from three studies, published in 2013, 2015 and 2016 [ 24 , 28 , 45 ]. They primarily represented individuals presenting to hospital with jaundice (21/25 sequences), infected with genotypes A1 and D.
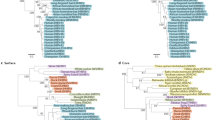
Kenyan sequences are those published in GenBank (downloaded 1 st Dec 2021) and are shown in red alongside genotype reference sequences in black (1000 bootstrap replicates were performed, and bootstrap support of ≥70% are indicated. Reference sequences from McNaughton et al. (2020) [ 11 ].
https://doi.org/10.1371/journal.pgph.0001165.g005
5/8 studies provided a detailed analysis of either amino acid or nucleotide substitutions found in the sequenced region of HBV [ 20 , 23 , 24 , 33 , 37 ]. 2/5 studies correlated these with known drug resistance mutations to lamivudine and other nucleoside analogues ( Table 4 ) [ 20 , 33 ]. One study reported the emergence of drug resistance mutations during lamivudine treatment associated with breakthrough HBV viraemia [ 33 ]. Multiple other mutations were described in the five studies, some of which were in the major hydrophilic region of the surface gene, and thus potentially important in influencing both natural and vaccine-mediated immunity [ 46 , 47 ].
https://doi.org/10.1371/journal.pgph.0001165.t004
(vi) HBV serology and HBV biomarkers
Exploring the prevalence of anti-HBs (vaccination or exposure) and anti-HBc (exposure to HBV) in HBsAg-negative populations is important to build up a full picture of population epidemiology. Among individuals testing HBsAg-positive, a panel of biomarkers is used to determine treatment eligibility, including HBeAg status, HBV DNA viral load, liver enzymes and imaging scores. These parameters are outside the primary scope of this study, but the data can be accessed as a supporting data file [ 48 ].
Enhanced efforts to characterise the epidemiology and disease burden of HBV are urgently required in Africa, as HBV is present at medium to high endemicity in many populations but has been neglected as a public health problem. Here we have reviewed the literature available on prevalence, genotypes and drug resistance data for CHB in Kenya. In our ‘low-risk’ category, intended to provide estimates most reflective of the general population, the pooled prevalence estimate for HBV infection was 3.4%. Point-prevalence estimates of ~6% were obtained for the groups we defined as medium and high risk, comprising people living with HIV infection and those with other identified risk factors for blood-borne virus infection. Similar prevalence estimates in the moderate- and high-risk groups was only evident after analysis. The number of studies was too low to allow for further subdivision into individual risk groups (e.g. comparing people who inject drugs, MSM, and sex workers). In the population presenting to healthcare facilities with established symptomatic liver disease (classified here as ‘very high risk’), the prevalence of HBV was 29.2% (although the underlying primary risk factor(s) for HBV acquisition in this group are not established).
In this very high-risk group, wide confidence intervals along with significant heterogeneity (I 2 = 95%) are notable. This population evidently has very different pre-test probabilities for HBV infection depending on underlying risk factors. In the absence of robust screening programmes, many people do not find out they have HBV infection until presenting to hospital with manifestations of liver disease. While the prevalence in this group evidently cannot be extrapolated to the general population, it is nevertheless an important observation that HBV in this setting accounts for such a high proportion of end-stage liver disease. Furthermore 2/3 studies in this very high-risk group used RPHA for HBsAg detection which is less sensitive than HBsAg, and therefore may underestimate true prevalence of HBV infection.
Most studies included in this review focussed on specific groups of people such as blood donors and those co-infected with HIV. Blood donation in Kenya is voluntary and often done by family members of those in need. There is no financial compensation for donation [ 49 ]. Routine screening for HBV through the Kenyan National Blood Transfusion Service (KNBTS) consists of ELISA for HBsAg only and there is no nucleic acid amplification testing (NAAT); some OBI may therefore go unidentified. Only one study in this review focussed on pregnant women [ 37 ] and one study enrolled healthcare workers [ 41 ]. These are accessible and important groups to screen for HBV infection given they are engaged with healthcare, likely to come for follow up visits, and interventions can have a significant impact on reducing transmission events. Treatment for pregnant mothers and healthcare workers would reduce onward transmission, and vaccination uninfected healthcare workers and babies at birth would decrease the overall burden of infection, reducing morbidity and mortality. One study was nationwide [ 38 ], but only included those who were HIV negative. More general population screening is lacking, and testing is not routinely done when presenting to healthcare facilities [ 50 ]. Some areas of Kenya have been more rigorous in their diagnostic approaches, but this is sporadic and may be increased only when there is a known outbreak of HBV in the local community, as has been the case in other African countries [ 51 , 52 ]. This may give a skewed view on population prevalence, but also leads to missed opportunities for diagnosis and intervention, particularly given the very high proportion of those presenting to hospital with jaundice or hepatitis found to be infected with HBV (pooled HBV prevalence 29.19% and 18.7% OBI prevalence).
It is notable that no studies were done in Northern Kenya, particularly along the borders with Somalia and South Sudan where the prevalence of HBV is likely to be substantially higher (for these two neighbouring countries, HBsAg prevalence is estimated at 19% and 12% respectively [ 53 , 54 ], however population density here is also very low [ 55 ].
Along with minimal population screening, there is very little sequencing of HBV in Kenya. Among the 25 papers we reviewed regarding HBV sequencing, only two reported whole genome sequencing, and none did next generation sequencing. We identified only 25 complete HBV genomes from Kenya in a GenBank search. Most available data is from single gene PCR and Sanger sequencing of S and P genes to determine genotype. Expanding these data will allow identification of recombinant genotypes, of which there is evidence in Kenya [ 28 , 31 ], but currently without good understanding of how these translate into clinical outcomes. Deep sequencing data will enable detection of minority variant mutations that may be relevant in emergence of vaccine escape and drug resistance, and also allow description of viral quasispecies, how this correlates with clinical phenotype and other biomarkers.
Three studies reviewed here screened for OBI using PCR. OBI prevalence was similar to estimated pooled HBsAg prevalence in the associated risk group (2.4%, 5.3% and 18.7% OBI prevalence in low, medium and high/very-risk groups compared with 3.36%, 6.14% and 6.18/29.19% pooled HBsAg positivity estimates in the equivalent groups). This indicates that many HBV cases are being missed due to the lack of appropriate screening tests, however the cost and poor availability of HBV DNA testing means it is not currently feasible to use as a universal screening test in Kenya. 20/23 studies solely reported HBsAg positivity diagnosed using other less sensitive tests. It is worth noting that of those presenting to hospital with jaundice who were HBsAg negative, nearly 20% were HBV DNA positive. It is not known whether the jaundice was due to acute HBV infection, or reactivation of chronic disease, but it seems to be an important indicator of HBV infection and screening of all those presenting to hospital with jaundice or hepatitis for OBI with HBV DNA PCR would be optimal. Few studies had characterised HBV exposure and vaccination status using anti-HBc and anti-HBs respectively. This highlights a broader issue around funding and access to laboratory tests needed for complete epidemiological assessment of populations.
HIV coinfection as a special case
The prevalence of HIV infection in adults in Kenya is 4.2% (95% CI 3.7–4.9%) [ 56 ]. Seven studies included in this analysis reported HBV prevalence in people living with HIV. The pooled HBV prevalence in this group was 6.14% (95% CI 5.08–7.41%). The HIV population is better represented than other groups at risk, as HBV screening is easier to offer to individuals already accessing healthcare for HIV monitoring and treatment. Through this established infrastructure for HIV (including clinics with staff, laboratory support, blood monitoring and drug distribution services), clinical care pathways for HBV could be incorporated. Although tenofovir is available free of charge in Kenya and is on the WHO list of essential medicines [ 57 ], it is only consistently available in combination with lamivudine or emtricitabine for HIV treatment, leaving the HBV monoinfected population unable to access licensed monotherapy.
Limitations
The HBV prevalence estimates we have generated here are wide and vary significantly between the risk groups (pooled risk group prevalence 3.36% - 29.19%). The very high-risk group also has a very wide confidence interval for prevalence estimates. Our risk groups were determined a priori based on existing understanding of the distribution of HBV infection, but data were insufficient to disaggregate into more specific groups, and we recognise that the prevalence of HBV infection in populations at risk varies substantially by setting. Other sources have different estimates of Kenyan HBV prevalence (e.g. 1% by the CDA Foundation [ 4 ]). The CDA data are from 2016, so may be out of date, but the varying estimates reflect difficulties with methods of data collection, varying data sources and data missingness. The overall quality of studies was low, with non-random sample selection common, no calculation of sample size in most studies and nearly all studies being cross sectional representing only a snapshot of HBV prevalence. Only selected populations are represented by the studies we identified, and even those studies seeming to represent the population more broadly are subject to bias. For example, the study of healthcare workers was primarily female nurses [ 41 ] and the nationwide survey only included HIV negative participants [ 38 ]. We considered only including those studies reaching a certain quality threshold in the prevalence meta-analysis, however this would have substantially restricted the available data. For example, including only those studies with random sampling methods and a documented sample size calculation would have left only three studies. One of the key findings of this systematic review is the lack of good quality seroprevalence data, and detailing this gives a good understanding of available literature.
There are no data for the northern part of Kenya, including the region around the border with South Sudan where there might be migration of high prevalence populations. It is likely that prevalence of HBV infection varies significantly by age, region of the country, and according to particular at-risk groups–thus targeted surveillance is important to provide an evidence-base for local and population-specific interventions.
No children were included in this review. In 2019 Kenya achieved an average coverage of 91% of 3 rd dose HBV childhood vaccination [ 58 ], but in future studies, screening children for HBsAg, anti-HBc and anti-HBs by birth cohort would be important to determine the impact of the vaccine campaign on infection, exposure and immunity, and to identify any populations being missed by vaccine coverage. There are increasing calls for the scale-up of BD HBV immunisation as part of a triple elimination campaign.
We highlight the poor representation of HBV in Kenya with sequencing data, identifying only two studies that undertook whole genome sequencing. 24/25 sequences available on GenBank were from two studies. This is clearly not representative of HBV in the general population, and work is required to determine circulating genotypes and to characterise polymorphisms that are relevant to outcomes of infection, treatment and vaccination.
Conclusions
We have assimilated epidemiological data for HBV in Kenya, together with genetic parameters where available, to provide the most refined picture possible to date. Our data suggest that Kenya falls into the ‘intermediate’ prevalence group (2–5%, as defined by the WHO). A sparse literature highlights the pressing need for clinical and research enterprise, to provide an evidence base for realistic and practical strategies that support country-specific scale-up of screening and treatment. Alongside continued efforts for three-dose vaccine coverage in infancy, enhanced interventions may include focus on HBV birth dose vaccine as part of the triple elimination initiative, with improved access to diagnostics, surveillance and treatment, to curtail the burden of disease in those currently infected, and reduce the incidence of new infections, moving Kenya towards 2030 elimination targets.
Supporting information
S1 checklist. preferred reporting items for systematic review and meta-analysis (prisma) 2020 statement checklist..
https://doi.org/10.1371/journal.pgph.0001165.s001
S1 Table. Joanna Briggs critical appraisal checklist.
https://doi.org/10.1371/journal.pgph.0001165.s002
Acknowledgments
This manuscript was written with the permission of the Director, KEMRI-CGMRC.
- 1. World Health Organisation. Global hepatitis report, 2017 [Internet]. 2017 [cited 2021 Nov 10]. https://www.who.int/publications/i/item/global-hepatitis-report-2017
- View Article
- PubMed/NCBI
- Google Scholar
- 3. WHO. Global health sector strategy on viral hepatitis 2016–2021. Global Hepatitis Programme Department of HIV/AIDS [Internet]. 2016 [cited 2021 Nov 9];(June):56. https://www.who.int/publications/i/item/WHO-HIV-2016.06
- 4. Countries Dashboard–CDA Foundation [Internet]. [cited 2022 Feb 7]. https://cdafound.org/polaris-countries-dashboard/
- 8. Kafeero HM, Ndagire D, Ocama P, Kudamba A, Walusansa A, Sendagire H. Prevalence and predictors of hepatitis B virus (HBV) infection in east Africa: evidence from a systematic review and meta-analysis of epidemiological studies published from 2005 to 2020. Archives of Public Health. 2021 Dec 1;79(1).
- 9. Ministry of Health. Kenya, National Policy Guidelines on Immunization 2013. 2013. 20 p.
- 10. Triple elimination initiative of mother-to-child transmission of HIV, syphilis and hepatitis B [Internet]. [cited 2022 Sep 12]. https://www.who.int/initiatives/triple-elimination-initiative-of-mother-to-child-transmission-of-hiv-syphilis-and-hepatitis-b
- 16. PRISMA 2020 Checklist Section and Topic Item # Checklist item Location where item is reported TITLE Title 1 Identify the report as a systematic review. [cited 2022 Feb 16]; http://www.prisma-statement.org/
- 17. critical-appraisal-tools—Critical Appraisal Tools | Joanna Briggs Institute [Internet]. [cited 2022 Feb 9]. https://jbi.global/critical-appraisal-tools
- 48. Downs L, Campbell C, Githinji G, Ansari A, Matthews P, Etyang AO. Treatment Eligibility Criteria Assessment in Hepatitis B Virus (HBV) Prevalence Studies in Kenya. 2022 Sep 12 [cited 2022 Sep 12]; /articles/journal_contribution/Treatment_Eligibility_Criteria_Assessment_in_Hepatitis_B_Virus_HBV_Prevalence_Studies_in_Kenya_/21063880/2
- 49. CDC Global Health—Kenya—Blog: Giving Blood, Giving Life [Internet]. [cited 2022 Feb 8]. https://www.cdc.gov/globalhealth/countries/kenya/blog/giving.htm
- 51. Nosocomial Outbreak of Hepatitis B Virus Infection in a Pediatric Hematology and Oncology Unit in South Africa: Epidemiological Investigation and Measures to Prevent Further Transmission. 2015;
- 55. 2019 Kenya Population and Housing Census Volume I: Population by County and Sub-County—Kenya National Bureau of Statistics [Internet]. [cited 2022 Mar 15]. https://www.knbs.or.ke/?wpdmpro=2019-kenya-population-and-housing-census-volume-i-population-by-county-and-sub-county
- 56. Kenya | UNAIDS [Internet]. [cited 2022 Feb 22]. https://www.unaids.org/en/regionscountries/countries/kenya
- 57. eEML—Electronic Essential Medicines List [Internet]. [cited 2022 Feb 22]. https://list.essentialmeds.org/?query=tenofovir%20disoproxil%20fumarate
- 58. Hepatitis B (HepB3) immunization coverage among 1-year-olds (%) [Internet]. [cited 2022 Feb 16]. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/hepatitis-b-(hepb3)-immunization-coverage-among-1-year-olds-(-)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Chronic Hepatitis B Infection: A Review
Affiliation.
- 1 Division of Clinical Care and Research, Institute of Human Virology, University of Maryland School of Medicine, Baltimore.
- PMID: 29715359
- DOI: 10.1001/jama.2018.3795
- Incorrect Expansion of a Term and Other Clarifications. [No authors listed] [No authors listed] JAMA. 2018 Sep 18;320(11):1202. doi: 10.1001/jama.2018.9637. JAMA. 2018. PMID: 30422281 No abstract available.
Importance: More than 240 million individuals worldwide are infected with chronic hepatitis B virus (HBV). Among individuals with chronic HBV infection who are untreated, 15% to 40% progress to cirrhosis, which may lead to liver failure and liver cancer.
Observations: Pegylated interferon and nucleos(t)ide analogues (lamivudine, adefovir, entecavir, tenofovir disoproxil, and tenofovir alafenamide) suppress HBV DNA replication and improve liver inflammation and fibrosis. Long-term viral suppression is associated with regression of liver fibrosis and reduced risk of hepatocellular carcinoma in cohort studies. The cure (defined as hepatitis B surface antigen loss with undetectable HBV DNA) rates after treatment remain low (3%-7% with pegylated interferon and 1%-12% with nucleos[t]ide analogue therapy). Pegylated interferon therapy can be completed in 48 weeks and is not associated with the development of resistance; however, its use is limited by poor tolerability and adverse effects such as bone marrow suppression and exacerbation of existing neuropsychiatric symptoms such as depression. Newer agents (entecavir, tenofovir disoproxil, and tenofovir alafenamide) may be associated with a significantly reduced risk of drug resistance compared with older agents (lamivudine and adefovir) and should be considered as the first-line treatment.
Conclusions and relevance: Antiviral treatment with either pegylated interferon or a nucleos(t)ide analogue (lamivudine, adefovir, entecavir, tenofovir disoproxil, or tenofovir alafenamide) should be offered to patients with chronic HBV infection and liver inflammation in an effort to reduce progression of liver disease. Nucleos(t)ide analogues should be considered as first-line therapy. Because cure rates are low, most patients will require therapy indefinitely.
PubMed Disclaimer
- Treatment of Chronic Hepatitis B Infection. Zhou YH. Zhou YH. JAMA. 2018 Sep 18;320(11):1201. doi: 10.1001/jama.2018.10007. JAMA. 2018. PMID: 30422294 No abstract available.
Similar articles
- Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Bourlière M, Rabiega P, Ganne-Carrie N, Serfaty L, Marcellin P, Barthe Y, Thabut D, Guyader D, Hezode C, Picon M, Causse X, Leroy V, Bronowicki JP, Carrieri P, Riachi G, Rosa I, Attali P, Molina JM, Bacq Y, Tran A, Grangé JD, Zoulim F, Fontaine H, Alric L, Bertucci I, Bouvier-Alias M, Carrat F; ANRS HB06 PEGAN Study Group. Bourlière M, et al. Lancet Gastroenterol Hepatol. 2017 Mar;2(3):177-188. doi: 10.1016/S2468-1253(16)30189-3. Epub 2017 Jan 20. Lancet Gastroenterol Hepatol. 2017. PMID: 28404133 Clinical Trial.
- Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Dittmer U, Krawczyk A, Vaillant A. Bazinet M, et al. Gastroenterology. 2020 Jun;158(8):2180-2194. doi: 10.1053/j.gastro.2020.02.058. Epub 2020 Mar 6. Gastroenterology. 2020. PMID: 32147484 Clinical Trial.
- Peginterferon for the treatment of chronic hepatitis B in the era of nucleos(t)ide analogues. Buster EH, Schalm SW, Janssen HL. Buster EH, et al. Best Pract Res Clin Gastroenterol. 2008;22(6):1093-108. doi: 10.1016/j.bpg.2008.11.007. Best Pract Res Clin Gastroenterol. 2008. PMID: 19187869 Review.
- Optimal therapy of chronic hepatitis B: how do I treat my HBeAg-negative patients? Viganò M, Invernizzi F, Lampertico P. Viganò M, et al. Liver Int. 2015 Jan;35 Suppl 1:107-13. doi: 10.1111/liv.12717. Liver Int. 2015. PMID: 25529095 Review.
- Peginterferon Is Superior to Nucleos(t)ide Analogues for Prevention of Hepatocellular Carcinoma in Chronic Hepatitis B. Liang KH, Hsu CW, Chang ML, Chen YC, Lai MW, Yeh CT. Liang KH, et al. J Infect Dis. 2016 Mar 15;213(6):966-74. doi: 10.1093/infdis/jiv547. Epub 2015 Nov 17. J Infect Dis. 2016. PMID: 26582959
- The impact of hepatitis B surface antigen seroconversion on the durability of functional cure induced by pegylated interferon alpha treatment. Zhang W, Chen J, Sun W, Xie N, Tian F, Ruan Q, Song J. Zhang W, et al. Virol J. 2024 Oct 3;21(1):243. doi: 10.1186/s12985-024-02522-8. Virol J. 2024. PMID: 39363288 Free PMC article.
- Characterizing Unique Clinical and Virological Profiles in Concurrent Chronic Hepatitis B and Metabolic Dysfunction-Associated Liver Disease: Insights from a Population-Based Cohort Study. Abu Baker F, Zeina AR, Taher R, Abu Mouch S, Israel A. Abu Baker F, et al. J Clin Med. 2024 Sep 21;13(18):5608. doi: 10.3390/jcm13185608. J Clin Med. 2024. PMID: 39337094 Free PMC article.
- Hepatitis B surface antigen impairs TLR4 signaling by upregulating A20 expression in monocytes. Wang C, Huang C, Li Y, Bai J, Zhao K, Fang Z, Chen J. Wang C, et al. Microbiol Spectr. 2024 Oct 3;12(10):e0090924. doi: 10.1128/spectrum.00909-24. Epub 2024 Sep 9. Microbiol Spectr. 2024. PMID: 39248482 Free PMC article.
- mTOR Signaling: Roles in Hepatitis B Virus Infection and Hepatocellular Carcinoma. Mei L, Sun H, Yan Y, Ji H, Su Q, Chang L, Wang L. Mei L, et al. Int J Biol Sci. 2024 Aug 1;20(11):4178-4189. doi: 10.7150/ijbs.95894. eCollection 2024. Int J Biol Sci. 2024. PMID: 39247820 Free PMC article. Review.
- Sex-specific associations of Notch signaling with chronic HBV infection: a study from Taiwan Biobank. Jen IA, Kuo TBJ, Liaw YP. Jen IA, et al. Biol Sex Differ. 2024 Sep 5;15(1):69. doi: 10.1186/s13293-024-00641-z. Biol Sex Differ. 2024. PMID: 39237981 Free PMC article.
Publication types
- Search in MeSH

Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- Silverchair Information Systems
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Chronic Hepatitis B Infection : A Review
- 1 Division of Clinical Care and Research, Institute of Human Virology, University of Maryland School of Medicine, Baltimore
- Comment & Response Treatment of Chronic Hepatitis B Infection Yi-Hua Zhou, MD, PhD JAMA
- Comment & Response Treatment of Chronic Hepatitis B Infection—Reply Lydia Tang, MBChB; Shyam Kottilil, MD, PhD JAMA
- Correction Incorrect Expansion of a Term and Other Clarifications JAMA
- JAMA Diagnostic Test Interpretation Serologic Testing for Hepatitis B Maroun M. Sfeir, MD, MPH, MS; Mary Snayd, MD JAMA
- Viewpoint Screening and Vaccination to Eradicate Hepatitis B Samuel So, MBBS; Norah Terrault, MD, MPH; Erin E. Conners, PhD, MPH JAMA
Importance More than 240 million individuals worldwide are infected with chronic hepatitis B virus (HBV). Among individuals with chronic HBV infection who are untreated, 15% to 40% progress to cirrhosis, which may lead to liver failure and liver cancer.
Observations Pegylated interferon and nucleos(t)ide analogues (lamivudine, adefovir, entecavir, tenofovir disoproxil, and tenofovir alafenamide) suppress HBV DNA replication and improve liver inflammation and fibrosis. Long-term viral suppression is associated with regression of liver fibrosis and reduced risk of hepatocellular carcinoma in cohort studies. The cure (defined as hepatitis B surface antigen loss with undetectable HBV DNA) rates after treatment remain low (3%-7% with pegylated interferon and 1%-12% with nucleos[t]ide analogue therapy). Pegylated interferon therapy can be completed in 48 weeks and is not associated with the development of resistance; however, its use is limited by poor tolerability and adverse effects such as bone marrow suppression and exacerbation of existing neuropsychiatric symptoms such as depression. Newer agents (entecavir, tenofovir disoproxil, and tenofovir alafenamide) may be associated with a significantly reduced risk of drug resistance compared with older agents (lamivudine and adefovir) and should be considered as the first-line treatment.
Conclusions and Relevance Antiviral treatment with either pegylated interferon or a nucleos(t)ide analogue (lamivudine, adefovir, entecavir, tenofovir disoproxil, or tenofovir alafenamide) should be offered to patients with chronic HBV infection and liver inflammation in an effort to reduce progression of liver disease. Nucleos(t)ide analogues should be considered as first-line therapy. Because cure rates are low, most patients will require therapy indefinitely.
Read More About
Tang LSY , Covert E , Wilson E , Kottilil S. Chronic Hepatitis B Infection : A Review . JAMA. 2018;319(17):1802–1813. doi:10.1001/jama.2018.3795
Manage citations:
© 2024
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Journal Proposal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Risk of hepatitis b virus reactivation in covid-19 patients receiving immunosuppressive treatment: a prospective study.

1. Introduction
2. materials and methods, 2.1. study design and participants, 2.2. data collection, 2.3. definitions, 2.4. statistical analysis, 3.1. seroprevalence of hbv infection in covid-19 patients, 3.2. general characteristics of study participants, 3.3. hepatitis b virus status of the study participants, 3.3.1. hbsag-positive patients, 3.3.2. hbsag-negative/anti-hbc-positive patients, 3.4. biochemical and hematological parameters of study participants, 3.5. immunosuppressive covid-19 treatment administered during hospitalization, 3.6. patients with hbvr.
- Patient no. 8, aged 56, with positive HBsAg and a baseline HBV DNA level of 179 (2.25 log) IU/mL, was treated with IV dexamethasone (8 mg/day for 5 days and then 4 mg/day for 2 days). The patient was initially re-evaluated 1 month after discharge, and at that time, HBV DNA was 1660 (3.22 log) IU/mL. At 3 months, a viral load of 17,378 (4.24 log) IU/mL was detected. FibroMax was also performed, which revealed F1/A0-A1/S3/N2/H0. According to the decision of her attending physician, treatment with entecavir 0.5 mg/day was then initiated.
- Patient no. 14, aged 75, was known to have chronic HBV infection. However, we found isolated anti-HBc and undetectable HBV DNA at the time of admission. He received a single dose (100 mg) of subcutaneous anakinra, a single dose (400 mg) of IV tocilizumab, and IV dexamethasone (8 mg/day for 3 days; then, 6 mg/day for 5 days, followed by 4 mg/day for 7 days). At follow-up, a detectable viral load was found (HBV DNA < 10 IU/mL).
- Patient no. 20, aged 63, with isolated anti-HBc and initially undetectable HBV DNA, developed a critical form of COVID-19 requiring admission to the Intensive Care Unit and non-invasive ventilation. During hospitalization, she received a single dose (800 mg) of IV tocilizumab, oral baricitinib (4 mg/day for 3 days), and IV dexamethasone in gradually decreasing doses (initially, 8 mg every 12 h for 1 day; then, 12 mg/day for 3 days; then, 8 mg/day for 7 days, 6 mg/day for 4 days, 4 mg/day for 1 day, and 2 mg/day for 2 days). At follow-up, a detectable viral load was found (HBV DNA < 10 IU/mL).
- Patient no. 28, aged 80, with isolated anti-HBc and initially undetectable HBV DNA, was treated with IV dexamethasone (6 mg/day for 7 days; then, 4 mg/day for 2 days). At follow-up, detectable HBV viral load (10 IU/mL) was found.
4. Discussion
4.1. seroprevalence of hbv infection in covid-19 patients, 4.2. definitions of hbvr, 4.3. risk of hbvr in patients receiving immunosuppressive treatment for covid-19, 4.4. risk of hbvr in patients receiving immunosuppressive treatment for non-covid-19 diseases, 4.5. management of hbv–sars-cov-2 co-infected patients receiving immunosuppressive treatment, 4.6. strengths and limitations of the study, 5. conclusions, supplementary materials, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 10 September 2024).
- Montazersaheb, S.; Khatibi, S.M.H.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Sorbeni, F.G.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022 , 19 , 92. [ Google Scholar ] [ CrossRef ]
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf (accessed on 8 August 2024).
- Liu, J.; Wang, T.; Cai, Q.; Sun, L.; Huang, D.; Zhou, G.; He, Q.; Wang, F.; Liu, L.; Chen, J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol. Res. 2020 , 50 , 1211–1221. [ Google Scholar ] [ CrossRef ]
- Rodríguez-Tajes, S.; Miralpeix, A.; Costa, J.; López-Suñé, E.; Laguno, M.; Pocurull, A.; Lens, S.; Mariño, Z.; Forns, X. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J. Viral Hepat. 2021 , 28 , 89–94. [ Google Scholar ] [ CrossRef ]
- Camarero, J.G.; Aranda, E.B.; Castro, R.Q.; Chumillas, R.M.S.; Vega, L.A.; Ruiz, S.D.; Manero, N.G.; Campo, R.V.; Plaza, F.J. Hepatitis B and C screening in hospitalized patients with SARS-CoV-2 infection. Gastroenterol. Hepatol. 2022 , 45 , 256–264. [ Google Scholar ] [ CrossRef ]
- Foo, H.; Phan, F.; Bagatella, M.; Petrovski, I.; Nagendra, V.; Acharya, P.; Levy, M.; Prakoso, E. Risk of hepatitis B reactivation following baricitinib or tocilizumab for treatment of COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2023 , 42 , 799–801. [ Google Scholar ] [ CrossRef ]
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B virus epidemiology. CSH Perspect. Med. 2015 , 5 , a021410. [ Google Scholar ] [ CrossRef ]
- Trickey, A.; Bivegete, S.; Duffell, E.; McNaughton, A.L.; Nerlander, L.; Walker, J.G.; Fraser, H.; Hickman, M.; Vickerman, P.; Brooks-Pollock, E.; et al. Estimating hepatitis B virus prevalence among key population groups for European Union and European Economic Area countries and the United Kingdom: A modelling study. BMC Infect. Dis. 2023 , 23 , 457. [ Google Scholar ] [ CrossRef ]
- Conners, E.E.; Panagiotakopoulos, L.; Hofmeister, M.G.; Spradling, P.R.; Hagan, L.M.; Harris, A.M.; Rogers-Brown, J.S.; Wester, C.; Nelson, N.P. Screening and testing for hepatitis B virus infection: CDC recommendations—United States, 2023. MMWR Recomm. Rep. 2023 , 72 , 1–25. [ Google Scholar ] [ CrossRef ]
- Perrillo, R.P.; Gish, R.; Falck-Ytter, Y.T. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015 , 148 , 221–244.e3. [ Google Scholar ] [ CrossRef ]
- Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin. Mol. Hepatol. 2019 , 25 , 93–159. [ Google Scholar ] [ CrossRef ]
- Lau, G.; Yu, M.-L.; Wong, G.; Thompson, A.; Ghazinian, H.; Hou, J.-L.; Piratvisuth, T.; Jia, J.-D.; Mizokami, M.; Cheng, G.; et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol. Int. 2021 , 15 , 1031–1048. [ Google Scholar ] [ CrossRef ]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018 , 67 , 1560–1599. [ Google Scholar ] [ CrossRef ]
- Jiménez, M.L.; Garrido, M.L.; Cano, M.C.F. Letter to the editor: Reactivation of HBV triggered by SARS-CoV-2 in a patient with cirrhosis. Hepatology 2022 , 75 , 765–766. [ Google Scholar ] [ CrossRef ]
- Giugliano, L.; Pinon, M.; Calvo, P.L. COVID-19 as a trigger of acute-on-chronic hepatitis B presenting with undetectable INR due to hypercoagulability in a 16-year-old girl. Pediatr. Infect. Dis. J. 2023 , 42 , 143–145. [ Google Scholar ] [ CrossRef ]
- Yang, S.; Wang, S.; Du, M.; Liu, M.; Liu, Y.; He, Y. Patients with COVID-19 and HBV coinfection are at risk of poor prognosis. Infect. Dis. Ther. 2022 , 11 , 1229–1242. [ Google Scholar ] [ CrossRef ]
- Mastroianni, A.; Greco, S.; Chidichimo, L.; Mauro, M.V.; Urso, F.; Vangeli, V. Antiviral prophylaxis for hepatitis B virus in COVID-19 patients treated with immunosuppressive drug therapy. Antivir. Ther. 2022 , 27 , 13596535211067602. [ Google Scholar ] [ CrossRef ]
- Wu, Y.-F.; Yu, W.-J.; Jiang, Y.-H.; Chen, Y.; Zhang, B.; Zhen, R.-B.; Zhang, J.-T.; Wang, Y.-P.; Li, Q.; Xu, F.; et al. COVID-19 or treatment associated immunosuppression may trigger hepatitis B virus reactivation: A case report. World J. Clin. Cases 2021 , 9 , 5266–5269. [ Google Scholar ] [ CrossRef ]
- Sagnelli, C.; Montella, L.; Grimaldi, P.; Pisaturo, M.; Alessio, L.; De Pascalis, S.; Sagnelli, E.; Coppola, N. COVID-19 as another trigger for HBV reactivation: Clinical case and review of literature. Pathogens 2022 , 11 , 816. [ Google Scholar ] [ CrossRef ]
- Braimakis, I.; Vasileiadi, S.; Trifylli, E.-M.; Papadopoulos, N.; Deutsch, M. Can hepatitis B virus (HBV) reactivation result from a mild COVID-19 infection? Livers 2023 , 3 , 347–353. [ Google Scholar ] [ CrossRef ]
- Figueredo, C.J.; Haider, T.; Guddati, H.; Massoumi, H. A case of hepatitis B reactivation associated hepatitis after tocilizumab therapy in a patient with SARS-CoV-2 infection. Am. J. Gastroenterol. 2021 , 116 , S1205–S1206. [ Google Scholar ] [ CrossRef ]
- Wong, G.L.; Yuen, B.W.; Chan, H.L.; Tse, Y.; Yip, T.C.; Lam, K.L.; Lui, G.C.; Wong, V.W. Impact of dose and duration of corticosteroid on the risk of hepatitis flare in patients with chronic hepatitis B. Liver Int. 2019 , 39 , 271–279. [ Google Scholar ] [ CrossRef ]
- Wong, G.L.-H.; Wong, V.W.-S.; Yuen, B.W.-Y.; Tse, Y.-K.; Yip, T.C.-F.; Luk, H.W.-S.; Lui, G.C.-Y.; Chan, H.L.-Y. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J. Hepatol. 2020 , 72 , 57–66. [ Google Scholar ] [ CrossRef ]
- Chen, L.-F.; Mo, Y.-Q.; Jing, J.; Ma, J.-D.; Zheng, D.-H.; Dai, L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: A prospective clinical observation. Int. J. Rheum. Dis. 2017 , 20 , 859–869. [ Google Scholar ] [ CrossRef ]
- Kuo, M.H.; Tseng, C.-W.; Lu, M.-C.; Tung, C.-H.; Tseng, K.-C.; Huang, K.-Y.; Lee, C.-H.; Lai, N.-S. Risk of hepatitis B virus reactivation in rheumatoid arthritis patients undergoing tocilizumab-containing treatment. Dig. Dis. Sci. 2021 , 66 , 4026–4234. [ Google Scholar ] [ CrossRef ]
- Chen, M.-H.; Liu, C.-Y.; Tsai, C.-Y.; Huang, D.-F.; Lin, H.-Y.; Lee, M.-H.; Huang, Y.-H. Hepatitis B virus reactivation in rheumatoid arthritis patients undergoing biologics treatment. J. Infect. Dis. 2016 , 215 , 566–573. [ Google Scholar ] [ CrossRef ]
- Sonneveld, M.J.; Murad, S.D.; van der Eijk, A.; de Man, R. Fulminant liver failure due to hepatitis B reactivation during treatment with tocilizumab. ACG Case Rep. J. 2019 , 6 , e00243. [ Google Scholar ] [ CrossRef ]
- Biehl, A.; Harinstein, L.; Brinker, A.; Glaser, R.; Muñoz, M.; Avigan, M. A case series analysis of serious exacerbations of viral hepatitis and non-viral hepatic injuries in tocilizumab-treated patients. Liver Int. 2021 , 41 , 515–528. [ Google Scholar ] [ CrossRef ]
- Campbell, C.; Andersson, M.I.; Ansari, M.A.; Moswela, O.; Misbah, S.A.; Klenerman, P.; Matthews, P.C. Risk of reactivation of hepatitis B virus (HBV) and tuberculosis (TB) and complications of hepatitis C virus (HCV) following tocilizumab therapy: A systematic review to inform risk assessment in the COVID-19 era. Front. Med. 2021 , 8 , 706482. [ Google Scholar ] [ CrossRef ]
- Yip, T.C.-F.; Gill, M.; Wong, G.L.-H.; Liu, K. Management of hepatitis B virus reactivation due to treatment of COVID-19. Hepatol. Int. 2022 , 16 , 257–268. [ Google Scholar ] [ CrossRef ]
- Harigai, M.; Winthrop, K.; Takeuchi, T.; Hsieh, T.-Y.; Chen, Y.-M.; Smolen, J.S.; Burmester, G.; Walls, C.; Wu, W.-S.; Dickson, C.; et al. Evaluation of hepatitis B virus in clinical trials of baricitinib in rheumatoid arthritis. RMD Open 2020 , 6 , e001095. [ Google Scholar ] [ CrossRef ]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury—Anakinra. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548615/ (accessed on 8 August 2024).
- Barone, M.; Notarnicola, A.; Lopalco, G.; Viggiani, M.T.; Sebastiani, F.; Covelli, M.; Iannone, F.; Avolio, A.W.; Di Leo, A.; Cantarini, L.; et al. Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology 2015 , 62 , 40–46. [ Google Scholar ] [ CrossRef ]
- Pérez, J.V.; Chinesta, J.R. Risk of hepatitis B reactivation associated with treatment against SARS-CoV-2 (COVID-19) with corticosteroids. Rev. Clin. Esp. 2020 , 220 , 535–536. [ Google Scholar ] [ CrossRef ]
- Spera, A.M. Hepatitis B virus infection reactivation in patients under immunosuppressive therapies: Pathogenesis, screening, prevention and treatment. World J. Virol. 2022 , 11 , 275–282. [ Google Scholar ] [ CrossRef ]
- Satsangi, S.; Gupta, N.; Kodan, P. Current and new drugs for COVID-19 treatment and its effects on the liver. J. Clin. Transl. Hepatol. 2021 , 9 , 436–446. [ Google Scholar ] [ CrossRef ]
- Wong, G.L.; Chan, H.L.; Yuen, B.W.; Tse, Y.; Luk, H.W.; Yip, T.C.; Hui, V.W.; Liang, L.Y.; Lee, H.; Lui, G.C.; et al. The safety of stopping nucleos(t)ide analogue treatment in patients with HBeAg-negative chronic hepatitis B. Liver Int. 2020 , 40 , 549–557. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Variables | All Patients (N = 32) |
|---|---|
| Age (years), median (IQR) | 67 (58.5–71.5) |
| Male, n (%) | 19 (59.4%) |
| BMI (kg/m ), median (IQR) | 28.5 (26.3–32.5) |
| Comorbidity, n (%) | |
| Hypertension | 20 (62.5%) |
| Obesity | 13 (40.6%) |
| Chronic pulmonary diseases | 8 (25%) |
| Diabetes mellitus | 7 (21.9%) |
| Alcohol use disorder | 3 (9.4%) |
| Other comorbidities | 4 (12.5%) |
| No comorbidities, n (%) | 4 (12.5%) |
| COVID-19 severity, n (%) | |
| Mild | 3 (9.4%) |
| Moderate | 18 (56.3%) |
| Severe | 9 (28.1%) |
| Critical | 2 (6.3%) |
| COVID-19 diagnosis during the Omicron variant circulation, n (%) | 18 (56.3%) |
| Immunosuppressive COVID-19 treatment administered during hospitalization, n (%) | |
| Systemic corticosteroids | 23 (71.9%) |
| Anakinra | 9 (28.1%) |
| Tocilizumab | 4 (12.5%) |
| Baricitinib | 4 (12.5%) |
| No immunosuppressive COVID-19 treatment, n (%) | 9 (28.1%) |
| Hepatitis B virus status, n (%) | |
| HBsAg-positive | 13 (40.6%) |
| HBsAg-negative/anti-HBc-positive | 19 (59.4%) |
| | | | ||||
|---|---|---|---|---|---|---|
| Patients who received tocilizumab, anakinra, and/or baricitinib | ||||||
| 1 | F | 58 | ANK—7 days, 1000 mg DEX—12 days, 82 mg | 164 | 362 | No |
| 2 | M | 43 | TCZ—1 dose, 400 mg ANK—5 days, 1400 mg DEX—10 days, 128 mg | 34 | 18 | No |
| 3 | F | 70 | ANK—7 days, 1000 mg DEX—7 days, 72 mg | <10 | <10 | No |
| 4 | F | 53 | ANK—7 days, 1000 mg DEX—9 days, 68 mg | 342 | 723 | No |
| 5 | M | 61 | ANK—4 days, 700 mg DEX—9 days, 76 mg | 18 | 66 | No |
| Patients who received systemic corticosteroids | ||||||
| 6 | M | 67 | DEX—16 days, 146 mg | 18 | <10 | No |
| 7 | F | 55 | DEX—2 days, 16 mg | U | 17 | No |
| 8 | F | 56 | DEX—7 days, 48 mg | 179 | 17,378 | Yes |
| Patients who did not receive any immunosuppressive treatment | ||||||
| 9 | M | 61 | - | 126 | 225 | No |
| 10 | M | 49 | - | 646 | 1190 | No |
| 11 | F | 34 | - | 77 | <10 | No |
| 12 | F | 77 | - | 11 | 118 | No |
| 13 | F | 67 | - | U | 16 | No |
| ( ) | | | ||||
| Patients who received tocilizumab, anakinra, and/or baricitinib | ||||||
| 14 | M | 75 | TCZ—1 dose, 400 mg ANK—1 day, 100 mg DEX—15 days, 82 mg | U | <10 | Yes |
| 15 | F | 73 | ANK—7 days, 1000 mg DEX—14 days, 152 mg | U | Positive anti-HBs | No |
| 16 | M | 66 | TCZ—1 dose, 400 mg ANK—6 days, 1200 mg DEX—33 days, 252 mg | U | U | No |
| 17 | M | 56 | ANK—1 day, 200 mg BARI—9 days, 36 mg DEX—9 days, 96 mg | - | U | No |
| 18 | M | 67 | BARI—14 days, 56 mg DEX—9 days, 98 mg | - | U | No |
| 19 | M | 75 | BARI—7 days, 28 mg DEX—7 days, 48 mg | - | U | No |
| 20 | F | 63 | TCZ—1 dose, 800 mg BARI—3 days, 12 mg DEX—18 days, 140 mg | U | <10 | Yes |
| Patients who received systemic corticosteroids | ||||||
| 21 | F | 59 | DEX—5 days, 30 mg | - | U | No |
| 22 | M | 68 | DEX—5 days, 30 mg | - | U | No |
| 23 | M | 62 | DEX—19 days, 60 mg | - | U | No |
| 24 | M | 83 | DEX—12 days, 104 mg | - | U | No |
| 25 | M | 67 | DEX—7 days, 38 mg | - | U | No |
| 26 | M | 62 | DEX—5 days, 36 mg | - | U | No |
| 27 | F | 68 | DEX—5 days, 30 mg | - | U | No |
| 28 | M | 80 | DEX—9 days, 50 mg | U | 10 | Yes |
| Patients who did not receive any immunosuppressive treatment | ||||||
| 29 | M | 75 | - | - | U | No |
| 30 | F | 69 | - | - | U | No |
| 31 | M | 74 | - | - | U | No |
| 32 | M | 70 | - | - | U | No |
| HBVr in HBsAg-Positive Patients | HBVr in HBsAg-Negative/Anti-HBc-Positive Patients | |
|---|---|---|
| KASL 2022 [ ] | ||
| APASL 2021 [ ] | ||
| AASLD 2018 [ ] | ||
| AGA 2015 [ ] |
| Medical Association | HBsAg-Positive Patients Who Experienced HBVr/ All Patients Followed, n/n | Study | HBsAg-Negative/ Anti-HBc-Positive Patients Who Experienced HBVr/ All Patients Followed, n/n | Study |
|---|---|---|---|---|
| KASL 2022 [ ] | 2/5 | Liu et al. [ ] | 3/15 | This work |
| 0/3 | Camarero et al. [ ] | |||
| 1/8 | This work | |||
| Pooled rate of HBVr, % | 18.75% (3/16) | 20% (3/15) | ||
| APASL 2021 [ ] | 2/5 | Liu et al. [ ] | 0/15 | This work |
| 0/3 | Camarero et al. [ ] | |||
| 1/8 | This work | |||
| Pooled rate of HBVr, % | 18.75% (3/16) | 0% | ||
| AASLD 2018 [ ] | 2/5 | Liu et al. [ ] | 2/6 | Tajez et al. [ ] |
| 0/3 | Camarero et al. [ ] | 3/15 | This work | |
| 1/8 | This work | |||
| Pooled rate of HBVr, % | 18.75% (3/16) | 23.8% (5/21) | ||
| AGA 2015 [ ] | 2/5 | Liu et al. [ ] | 3/15 | This work |
| 1/3 | Camarero et al. [ ] | |||
| 2/8 | This work | |||
| Pooled rate of HBVr, % | 31.25% (5/16) | 20% (3/15) | ||
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Mihai, N.; Olariu, M.C.; Ganea, O.-A.; Adamescu, A.-I.; Molagic, V.; Aramă, Ș.S.; Tilișcan, C.; Aramă, V. Risk of Hepatitis B Virus Reactivation in COVID-19 Patients Receiving Immunosuppressive Treatment: A Prospective Study. J. Clin. Med. 2024 , 13 , 6032. https://doi.org/10.3390/jcm13206032
Mihai N, Olariu MC, Ganea O-A, Adamescu A-I, Molagic V, Aramă ȘS, Tilișcan C, Aramă V. Risk of Hepatitis B Virus Reactivation in COVID-19 Patients Receiving Immunosuppressive Treatment: A Prospective Study. Journal of Clinical Medicine . 2024; 13(20):6032. https://doi.org/10.3390/jcm13206032
Mihai, Nicoleta, Mihaela Cristina Olariu, Oana-Alexandra Ganea, Aida-Isabela Adamescu, Violeta Molagic, Ștefan Sorin Aramă, Cătălin Tilișcan, and Victoria Aramă. 2024. "Risk of Hepatitis B Virus Reactivation in COVID-19 Patients Receiving Immunosuppressive Treatment: A Prospective Study" Journal of Clinical Medicine 13, no. 20: 6032. https://doi.org/10.3390/jcm13206032
Article Metrics
Supplementary material.
ZIP-Document (ZIP, 58 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

- {{subColumn.name}}
AIMS Biophysics
- {{newsColumn.name}}
- Share facebook twitter google linkedin

Hepatitis-B disease modelling of fractional order and parameter calibration using real data from the USA
- Mehmet Yavuz 1,2 , , ,
- Kübra Akyüz 1 ,
- Naime Büşra Bayraktar 1 ,
- Feyza Nur Özdemir 3
- 1. Department of Mathematics and Computer Sciences, Faculty of Science, Necmettin Erbakan University, Meram Yeniyol, 42090 Konya, Türkiye
- 2. Centre for Environmental Mathematics, Department of Earth and Environmental Sciences, University of Exeter-Penryn Campus, TR10 9FE, United Kingdom
- 3. Department of Computer Engineering, Institute of Science, Necmettin Erbakan University, Meram Köyceğiz, 42090 Konya, Türkiye
- Received: 20 July 2024 Revised: 20 September 2024 Accepted: 20 September 2024 Published: 29 September 2024
- Full Text(HTML)
- Download PDF
In this paper, a new mathematical model of Hepatitis B is studied to investigate the transmission dynamics of the Hepatitis B virus (HBV). Many diseases can start from the womb and find us humans throughout our lives. These diseases are specific abnormal conditions that negatively affect the structure or function of all or part of an organism and do not suddenly occur in any region due to external injury. In this study, we focus on HBV, and we state the graphics, interpretations, and detailed information about the disease and the newly established mathematical model of the disease. A fractional order differential equation system with a memory effect is used to model anomalous processes and to understand the effect of past infection events on the future spread dynamics of the system. In the model, susceptible, latent, acute, carrier, and recovered populations are taken into account by considering vertical transmission, which provides information about the inter-generational course of the disease. However, the migration effect is also used in the model due to the risk of disease transmission and increased migration in recent years. The course of the disease is examined using real data from the USA. Moreover, the model's positivity and boundedness are studied, and the equilibrium points are calculated. Additionally, the stability conditions for the disease-free equilibrium (DFE) are stated. A parameter calibration technique is used to determine the most accurate parameter values in the model. Finally, we provide numerical results and their biological interpretations to estimate the future course of the disease. The paper addresses the current migration problem with the migration parameter in the model. These differences from the literature can be regarded as important novelties of the paper.
- hepatitis-B (HBV) ,
- mathematical model of fractional-order ,
- migration factor ,
- real data ,
- vertical transmission
Citation: Mehmet Yavuz, Kübra Akyüz, Naime Büşra Bayraktar, Feyza Nur Özdemir. Hepatitis-B disease modelling of fractional order and parameter calibration using real data from the USA[J]. AIMS Biophysics, 2024, 11(3): 378-402. doi: 10.3934/biophy.2024021
Related Papers:
| [1] | Medical Park Hastaneler Grubu, 2021. Available from: |
| [2] | Sorrell MF, Belongia EA, Costa J, et al. (2009) National institutes of health consensus development conference statement: management of hepatitis B. 150: 104-110. |
| [3] | Kaya A, Erbey MF, Okur M, et al. (2011) Hepatitis B virus seropositivity and vaccination for children aged 0-18 in the Van region/Van yoresinde 0-18 yaslan arasindaki cocuklarda hepatit B virusu seropozitifligi ve asilanma durumu. 5: 132-136. |
| [4] | Hsu HY, Chang MH, Chen DS, et al. (1986) Baseline seroepidemiology of hepatitis B virus infection in children in Taipei, 1984: a study just before mass hepatitis B vaccination program in Taiwan. 18: 301-307. |
| [5] | Chang MH, Chen CJ, Lai MS, et al. (1997) Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. 336: 1855-1859. |
| [6] | Demir A, Kansu Tanca A (2023) Kronik hepatit B virüs enfeksiyonunda antiviral tedavi yönetimi. . Ankara: Türkiye Klinikleri Yayınevi 45-49. |
| [7] | Hethcote HW (2000) The mathematics of infectious diseases. 42: 599-653. |
| [8] | Işık N, Kaya A (2020) İnfeksiyöz Hastalıkların Yayılması ve Kontrolünde Matematiksel Modeller ve Sürü Bağışıklama. 15: 301-307. |
| [9] | Kermack WO, McKendrick AG (1927) A contribution to the mathematical theory of epidemics. 115: 700-721. |
| [10] | Kamyad AV, Akbari R, Heydari AA (2014) Mathematical modeling of transmission dynamics and optimal control of vaccination and treatment for hepatitis B virus. 2014: 475451. |
| [11] | Ciupe SM, Hews S (2012) Mathematical models of e-antigen mediated immune tolerance and activation following prenatal HBV infection. 7: e39591. |
| [12] | Guedj J, Rotman Y, Cotler SJ, et al. (2014) Understanding early serum hepatitis D virus and hepatitis B surface antigen kinetics during pegylated interferon-alpha therapy via mathematical modeling. 60: 1902-1910. |
| [13] | Wodajo FA, Mekonnen TT (2022) Effect of intervention of vaccination and treatment on the transmission dynamics of HBV disease: a mathematical model analysis. 9968832. |
| [14] | Goyal A, Ribeiro RM, Perelson AS (2017) The role of infected cell proliferation in the clearance of acute HBV infection in humans. 9: 350. |
| [15] | Carracedo Rodriguez A, Chung M, Ciupe SM (2017) Understanding the complex patterns observed during hepatitis B virus therapy. 9: 117. |
| [16] | Ciupe SM (2018) Modeling the dynamics of hepatitis B infection, immunity, and drug therapy. 285: 38-54. |
| [17] | Dahari H, Shudo E, Ribeiro RM, et al. (2009) Modeling complex decay profiles of hepatitis B virus during antiviral therapy. 49: 32-38. |
| [18] | Goyal A, Murray JM (2016) Modelling the impact of cell-to-cell transmission in hepatitis B virus. 11: e0161978. |
| [19] | Colombatto P, Civitano L, Bizzarri R, et al. (2006) A multiphase model of the dynamics of HBV infection in HBeAg-negative patients during pegylated interferon- 2a, lamivudine and combination therapy. 11: 197-212. |
| [20] | Cangelosi Q, Means SA, Ho H (2017) A multi-scale spatial model of hepatitis-B viral dynamics. 12: e0188209. |
| [21] | Khan MA, Islam S, Arif M, et al. (2013) Transmission model of hepatitis B virus with the migration effect. 2013: 150681. |
| [22] | Din A, Abidin MZ (2022) Analysis of fractional-order vaccinated Hepatitis-B epidemic model with Mittag-Leffler kernels. 2: 59-72. |
| [23] | Yavuz M, Özköse F, Susam M, et al. (2023) A new modeling of fractional-order and sensitivity analysis for hepatitis-b disease with real data. 7: 165. |
| [24] | Mustapha UT, Ahmad YU, Yusuf A, et al. (2023) Transmission dynamics of an age-structured Hepatitis-B infection with differential infectivity. 1: 124-152. |
| [25] | Wodajo FA, Gebru DM, Alemneh HT (2023) Mathematical model analysis of effective intervention strategies on transmission dynamics of hepatitis B virus. 13: 8737. |
| [26] | Xu C, Wang Y, Cheng K, et al. (2023) A mathematical model to study the potential hepatitis B virus infections and effects of vaccination strategies in China. 11: 1530. |
| [27] | Khatun Z, Islam MS, Ghosh U (2020) Mathematical modeling of hepatitis B virus infection incorporating immune responses. 1: 100017. |
| [28] | Aniji M, Kavitha N, Balamuralitharan S (2020) Mathematical modeling of hepatitis B virus infection for antiviral therapy using LHAM. 2020: 408. |
| [29] | Endashaw EE, Mekonnen TT (2022) Modeling the effect of vaccination and treatment on the transmission dynamics of hepatitis B virus and HIV/AIDS coinfection. 2022: 5246762. |
| [30] | Khan M, Khan T, Ahmad I, et al. (2022) Modeling of hepatitis B virus transmission with fractional analysis. 2022: 6202049. |
| [31] | Khan T, Jung IH, Zaman G (2019) A stochastic model for the transmission dynamics of hepatitis B virus. 13: 328-344. |
| [32] | Zhao S, Xu Z, Lu Y (2000) A mathematical model of hepatitis B virus transmission and its application for vaccination strategy in China. 29: 744-752. |
| [33] | Danane J, Allali K, Hammouch Z (2020) Mathematical analysis of a fractional differential model of HBV infection with antibody immune response. 136: 109787. |
| [34] | Goldstein ST, Zhou F, Hadler SC, et al. (2005) A mathematical model to estimate global hepatitis B disease burden and vaccination impact. 34: 1329-1339. |
| [35] | Liang P, Zu J, Zhuang G (2018) A literature review of mathematical models of hepatitis B virus transmission applied to immunization strategies from 1994 to 2015. 28: 221-229. |
| [36] | Li M, Zu J (2019) The review of differential equation models of HBV infection dynamics. 266: 103-113. |
| [37] | Min L, Su Y, Kuang Y (2008) Mathematical analysis of a basic virus infection model with application to HBV infection. 38: 1573-1585. |
| [38] | Goyal A, Liao LE, Perelson AS (2019) Within-host mathematical models of hepatitis B virus infection: Past, present, and future. 18: 27-35. |
| [39] | Zhang S, Zhou Y (2012) The analysis and application of an HBV model. 36: 1302-1312. |
| [40] | Naik PA, Yavuz M, Qureshi S, et al. (2024) Memory impacts in hepatitis C: a global analysis of a fractional-order model with an effective treatment. 254: 108306. |
| [41] | Mustapha UT, Maigoro YA, Yusuf A, et al. (2024) Mathematical modeling for the transmission dynamics of cholera with an optimal control strategy. 2: 1-20. |
| [42] | Kumar P, Erturk VS (2021) Dynamics of cholera disease by using two recent fractional numerical methods. 1: 102-111. |
| [43] | Ahmed I, Akgül A, Jarad F, et al. (2023) A Caputo-Fabrizio fractional-order cholera model and its sensitivity analysis. 3: 170-187. |
| [44] | Bolaji B, Onoja T, Agbata C, et al. (2024) Dynamical analysis of HIV-TB co-infection transmission model in the presence of treatment for TB. 2: 21-56. |
| [45] | Evirgen F, Uçar E, Uçar S, et al. (2023) Modelling influenza a disease dynamics under Caputo-Fabrizio fractional derivative with distinct contact rates. 3: 58-73. |
| [46] | Duru EC, Anyanwu MC (2023) Mathematical model for the transmission of mumps and its optimal control. 60: 77-95. |
| [47] | Raeisi E, Yavuz M, Khosravifarsani M, et al. (2024) Mathematical modeling of interactions between colon cancer and immune system with a deep learning algorithm. 139: 345. |
| [48] | Salih RI, Jawad S, Dehingia K, et al. (2024) The effect of a psychological scare on the dynamics of the tumor-immune interaction with optimal control strategy. 14: 276-293. |
| [49] | Joshi H, Yavuz M, Özdemir N (2024) Analysis of novel fractional order plastic waste model and its effects on air pollution with treatment mechanism. 14: 3078-3098. |
| [50] | Joshi H, Yavuz M (2024) Numerical analysis of compound biochemical calcium oscillations process in hepatocyte cells. 8: 2300647. |
| [51] | Munson A (2023) A harmonic oscillator model of atmospheric dynamics using the Newton-Kepler planetary approach. 3: 216-233. |
| [52] | Xu C, Farman M, Shehzad A (2023) Analysis and chaotic behavior of a fish farming model with singular and non-singular kernel. 2350105. |
| [53] | Yapışkan D, Eroğlu BBİ (2024) Fractional-order brucellosis transmission model between interspecies with a saturated incidence rate. 2: 114-132. |
| [54] | Naik PA, Eskandari Z, Yavuz M, et al. (2024) Bifurcation results and chaos in a two-dimensional predator-prey model incorporating Holling-type response function on the predator. . |
| [55] | Ghosh D, Santra PK, Mahapatra GS (2023) A three-component prey-predator system with interval number. 3: 1-16. |
| [56] | Wiratsudakul A, Suparit P, Modchang C (2018) Dynamics of Zika virus outbreaks: an overview of mathematical modeling approaches. 6: e4526. |
| [57] | Möhler L, Flockerzi D, Sann H, et al. (2005) Mathematical model of influenza A virus production in large-scale microcarrier culture. 90: 46-58. |
| [58] | Podlubny I (1999) Fractional Differential Equations.Academic Press. |
| [59] | Lin W (2007) Global existence theory and chaos control of fractional differential equations. 332: 709-726. |
| [60] | Naik PA, Yavuz M, Qureshi S, et al. (2020) Modeling and analysis of COVID-19 epidemics with treatment in fractional derivatives using real data from Pakistan. 135: 795. |
| [61] | Driessche P, Watmough J (2002) Reproduction numbers, and sub-threshold endemic equilibria for compartmental models of disease transmission. 180: 29-48. |
| [62] | Ahmed E, Elgazzar AS (2007) On fractional order differential equations model for nonlocal epidemics. 379: 607-614. |
| [63] | Matignon D Stability results for fractional differential equations with applications to control processing (1996)2: 963-968. |
| [64] | Danane J, Yavuz M, Yıldız M (2023) Stochastic modeling of three-species prey-predator model driven by Lévy jump with mixed holling-II and Beddington-DeAngelis functional responses. 7: 751. |
| [65] | Yavuz M, ur Rahman M, Yildiz M, et al. (2024) Mathematical modeling of middle east respiratory syndrome corona virus with bifurcation analysis. 5: 3997-4012. |
| [66] | Eskandari Z, Naik PA, Yavuz M (2024) Dynamical behaviors of a discrete-time prey-predator model with harvesting effect on the predator. 14: 283-297. |
| [67] | Statistics on deaths and causes of death, 2021. Available from: |
| [68] | Garrappa R (2010) On linear stability of predictor-corrector algorithms for fractional differential equations. 87: 2281-2290. |
| [69] | Garrappa R (2018) Numerical solution of fractional differential equations: a survey and a software tutorial. 6: 16. |
| [70] | Binbay NE, Gümgüm HB (2019) Bloch denklemlerinin, nümerik yöntemlerle çözümü 155-184. |
| [71] | Samko SG, Kilbas AA, Marichev OI (1993) Fractional integrals and derivatives. . Langhorne: Gordon and Breach Science Publishers. |
| [72] | Surveillance for Viral Hepatitis - United States, 2015. Available from: |
- This is an open access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0 ) -->
Supplements
Access History
- Corresponding author: * Correspondence: Email: [email protected] .;
Reader Comments
- © 2024 the Author(s), licensee AIMS Press. This is an open access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0 )
通讯作者: 陈斌, [email protected]
沈阳化工大学材料科学与工程学院 沈阳 110142

Article views( 60 ) PDF downloads( 8 ) Cited by( 0 )
Figures and Tables

Figures( 9 ) / Tables( 1 )

Associated material
Other articles by authors.
- Mehmet Yavuz
- Kübra Akyüz
- Naime Büşra Bayraktar
- Feyza Nur Özdemir
Related pages
- on Google Scholar
- Email to a friend
- Order reprints
Export File

- Figure 1. Schematic diagram of the SLACP model
- Figure 2. Time series of annual hepatitis-B cases for the USA from 1966 to 2021 and best-fitted curve associated with the proposed model
- Figure 3. Acute population dynamics for various variables of the transition rate
- Figure 4. Recovery rate of individuals in the carrier class
- Figure 5. Infected rate of mothers with HBV acute virus
- Figure 6. Population densities of the HBV model for the estimated values
- Figure 7. Status of acute and carrier populations relative to each other
- Figure 8. Effect of different fractional orders on the recovery rate of carrier class in the acute population
- Figure 9. Transition rate from acute to carrier class in carrier population of different fractional orders
- Temas populares
- All countries
- Región de África
- Región de las Américas
- Región de Europa
- Región del Mediterráneo oriental
- Región del Pacífico occidental
- Presencia de la OMS en los países
- Comunicados de prensa
- Declaraciones
- Campañas mundiales de salud pública de la OMS
- Comentarios
- Emergencias
- Noticias sobre brotes de enfermedades
- Informes de situación
- OMS en situaciones de emergencia
- Clasificaciones y terminologías
- Panel de control de COVID-19
- Aspectos destacados
- Asociaciones
- Grupos de Expertos
- Centros colaboradores de la OMS
- Quiénes somos
- Cómo se financia la OMS
- Asamblea Mundial de la Salud
- Consejo Ejecutivo
Infecciones de transmisión sexual
Las infecciones de transmisión sexual (ITS) se propagan predominantemente por contacto sexual sin protección. Algunas ITS también se pueden transmitir durante el embarazo y el parto y por medio de sangre o productos sanguíneos infectados.
Las ITS tienen repercusiones profundas en la salud. Si no se tratan pueden dar lugar a graves consecuencias, incluidas enfermedades neurológicas y cardiovasculares, infertilidad, embarazo ectópico, muerte prenatal y riesgo aumentado de contraer el virus de la inmunodeficiencia humana (VIH). Además, las ITS guardan relación con la estigmatización y la violencia doméstica, y afectan a la calidad de vida.
La mayoría de las ITS son asintomáticas. Cuando se manifiestan, los síntomas más comunes de ITS incluyen secreción vaginal o uretral, úlcera genital y dolor abdominal bajo.
Las ITS más comunes y curables son tricomoniasis, clamidiasis, blenorragia y sífilis. El rápido aumento de la resistencia a los antimicrobianos es una amenaza creciente en lo que respecta a la blenorragia no tratable.
Las ITS virales, entre ellas las causadas por el VIH, el virus del herpes simple de tipo 2, los virus de la hepatitis B y C, el virus del papiloma humano y el virus linfotrópico T humano de tipo 1, carecen de opciones terapéuticas o estas son muy limitadas. Existen vacunas contra el virus de la hepatitis B, que puede provocar cáncer de hígado, y el virus del papiloma humano que puede causar cáncer cervicouterino. Las infecciones por VIH, virus del herpes simple y virus linfotrópico T humano de tipo 1 perduran durante toda la vida: para el VIH y el virus del herpes simple existen tratamientos que permiten contener el virus, pero a día de hoy esas tres ITS no son curables.
Los preservativos utilizados de manera correcta y sistemática protegen eficazmente contra ITS y VIH. El cribado con diagnóstico precoz en personas con ITS y sus parejas sexuales ofrece la mejor oportunidad de tratamiento eficaz y previene las complicaciones y la ulterior transmisión.
Una persona puede tener una ITS sin síntomas evidentes de la enfermedad. Cuando se manifiestan, los síntomas más comunes de ITS incluyen secreción vaginal anormal, secreción uretral, úlceras y bultos genitales, y dolor abdominal bajo.
Síntomas de ITS específicas
Blenorragia y clamidiasis:
Estas ITS causan cervicitis en las mujeres, uretritis en los hombres e infecciones extragenitales que incluyen manifestaciones rectales y orofaríngeas. Los síntomas comunes pueden ser secreción vaginal o peneana y ardor al orinar. Los lactantes de madres infectadas pueden contraer conjuntivitis neonatal debida a la exposición a infecciones de transmisión sexual durante el parto vaginal. Las infecciones rectales y faríngeas pueden ser asintomáticas.
Sífilis
La sífilis primaria se presenta como una úlcera única e indolora. La sífilis secundaria se manifiesta en forma de lesiones generalizadas en la piel, mucosas y ganglios linfáticos, en particular una erupción característica en las palmas de las manos y las plantas de los pies. La sífilis latente es asintomática y se identifica mediante prueba serológica.
Tricomoniasis
Los síntomas más frecuentes incluyen secreción vaginal anormal con enrojecimiento de la vulva y relaciones sexuales con prurito y dolor.
Virus del herpes simple genital (HSV)
El HSV se presenta generalmente en forma de llagas, vesículas o ulceraciones de los genitales externos y la boca. El HSV genital sintomático es una enfermedad permanente que se puede caracterizar por recurrencias sintomáticas frecuentes.
Virus linfotrópico T humano de tipo 1 (HTLV-1)
Generalmente asintomático, la forma crónica del HTLV 1 puede causar enfermedad grave, incluida la leucemia/linfoma de células T adultas y un trastorno progresivo del sistema nervioso conocido como mielopatía o paraparesia espástica tropical asociada al HTLV 1.
En la actualidad se dispone de tratamiento eficaz para algunas ITS.
Tres ITS bacterianas (clamidiasis, blenorragia y sífilis) y una parasitaria (tricomoniasis) son generalmente curables con regímenes de antibióticos eficaces de una o más dosis.
En cuanto a las ITS virales (VIH, HSV y HTLV 1), los medicamentos más eficaces disponibles son fármacos antivíricos o antineoplásicos (en el caso del HTLV 1) que permiten atenuar el desarrollo de esas tres enfermedades, aunque no pueden curarlas.
La resistencia a los antimicrobianos relacionada con los antibióticos utilizados para tratar las ITS, en particular la blenorragia, ha aumentado rápidamente en los últimos años y ha limitado los resultados terapéuticos exitosos. Los resultados del Programa de Vigilancia Antimicrobiana Gonocócica (GASP) revelan tendencias hacia elevadas tasas de resistencia a la quinolona, creciente resistencia a la azitromicina y resistencia incipiente a las cefalosporinas de amplio espectro.
El desarrollo de una menor sensibilidad de N. gonorrhoeae a las cefalosporinas de amplio espectro, junto con los altos niveles detectados de resistencia a las penicilinas, sulfamidas, tetraciclinas, quinolonas y macrólidos, convierten a N. gonorrhoeae en un organismo polifarmacorresistente. Aunque menos común, la resistencia a los antimicrobianos existe también respecto de otras ITS, y por lo tanto la prevención y el tratamiento en fase temprana son aspectos críticos.
Para tratar debidamente las ITS es importante administrar los antimicrobianos adecuados, en dosis correctas y durante el periodo necesario específico para la ITS en cuestión, a fin de asegurar el tratamiento apropiado o la curación, y prevenir el desarrollo de resistencia a los antimicrobianos.
- Resistencia a los antimicrobianos
- Cáncer de cuello uterino
- Hepatitis B
- Virus del herpes simple
- Esterilidad
- Gonorrea multirresistente
- Infecciones de transmisión sexual (ITS)
- WHA A69/59 - Estrategias mundiales del sector de la salud contra el VIH, las hepatitis víricas y las infecciones de transmisión sexual para el periodo 2016-2021
Un nuevo informe señala un importante aumento de las infecciones de transmisión sexual, que se enmarca en los desafíos que plantean el VIH y las hepatitis
La OMS publica nuevas orientaciones para mejorar la detección y el diagnóstico de las infecciones de transmisión sexual
La OMS presenta datos científicos recientes y nuevas orientaciones sobre la supresión del VIH en la IAS 2023
Cada día, más de 1 millón de personas contraen una infección de transmisión sexual curable
Publicaciones
Contenidos multimedia

¿Cuáles son los resultados más importantes del trabajo scientifico que se esta haciendo en Mexico sobre la pollucion del aire?
- 22 (current)
Temas de salud conexos
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC11125943

Hepatitis B Virus Infection: A Mini Review
Diana asema asandem.
1 West African Center for Cell Biology of Infectious Pathogens, University of Ghana, Accra P.O. Box LG 52, Ghana; hg.ude.gu.ihcugon@mednasad
2 Department of Virology, Noguchi Memorial Institute for Medical Research, University of Ghana, Accra P.O. Box LG 581, Ghana
Selorm Philip Segbefia
3 Department of Immunology, Noguchi Memorial Noguchi Memorial Institute for Medical Research, University of Ghana, Accra P.O. Box LG 581, Ghana; hg.ude.gu.ihcugon@aifebgess (S.P.S.); hg.ude.gu.ihcugon@isuka (K.A.K.)
Kwadwo Asamoah Kusi
Joseph humphrey kofi bonney.
Hepatitis B and C viruses (HBV and HCV) are the leading causes of end-stage liver disease worldwide. Although there is a potent vaccine against HBV, many new infections are recorded annually, especially in poorly resourced places which have lax vaccination policies. Again, as HBV has no cure and chronic infection is lifelong, vaccines cannot help those already infected. Studies to thoroughly understand the HBV biology and pathogenesis are limited, leaving much yet to be understood about the genomic features and their role in establishing and maintaining infection. The current knowledge of the impact on disease progression and response to treatment, especially in hyperendemic regions, is inadequate. This calls for in-depth studies on viral biology, mainly for the purposes of coming up with better management strategies for infected people and more effective preventative measures for others. This information could also point us in the direction of a cure. Here, we discuss the progress made in understanding the genomic basis of viral activities leading to the complex interplay of the virus and the host, which determines the outcome of HBV infection as well as the impact of coinfections.
1. Introduction
The liver is an important multifunctional organ in the body and plays various metabolic and immunologic roles, making its malfunctioning critically fatal. Though there are varied therapeutic strategies, one of the best options for treatment in terminal liver infections is liver transplantation. This in itself is limited by restricted organ supply, the complexity of human leukocyte antigen (HLA) matching, immunologic side effects, and financial constraints [ 1 ]. Moreover, long-term survival with this option is not guaranteed.
Dysregulation of liver inflammation is a hallmark of chronic infection, autoimmunity, and malignancy, which is mediated by multiple overlapping pathways in different liver diseases. Immune dysregulation can also be targeted as a therapeutic strategy in liver disease. Certain liver diseases are characterised by dysregulated immune function, where the body’s attempt to eliminate the source of insult is either exacerbated beyond control or prolonged beyond containment [ 2 , 3 ]. Thus, a ‘collateral damage’ phenomenon ensues where the body’s aggravated defence mechanism tends to destroy self-cells. In some cases, the response may not necessarily be aggravated but rather prolonged and persistent, leading to destruction of hepatocytes and development of scar tissue. The body’s repair mechanisms may not be adequate to keep up with the constant damage of hepatocytes.
The term hepatitis is used to describe the totality of liver inflammation [ 4 ]. Although liver damage may be a result of over-exertion of the immune system, it could be triggered by a foreign agent commonly of viral aetiology [ 5 ]. Viral components and life processes such as replication activate an immune response that could result in an acute or chronic infection [ 6 ]. This is dependent on the infecting pathogen as well as the host and results in various outcomes.
The most common cause of viral hepatitis is Hepatitis B virus (HBV), which is a leading cause of end-stage liver disease worldwide [ 7 ]. Although there is an effective vaccine against HBV infection, new infections continue to occur due partly to limited vaccination coverage and the accessibility, availability, and cost of the vaccines in the highest-burdened regions [ 8 ]. Another avenue for new infections stems from viral breakthrough, which can manifest in up to 5% of infants, despite their timely receipt of the anti-HBV birth-dose vaccine [ 9 , 10 , 11 ]. In addition, vaccination cannot help those already infected. Eradication of infection requires complementing vaccination with the effective treatment of those already infected. The World Health Organization and its partners came up with the Global Health Sector Strategy (GHSS) on Viral Hepatitis, a key objective being to reduce new infections by 90% and mortality by 65% compared to the baseline year of 2015 [ 12 ]. To achieve this requires real-time information for focused action and timely intervention. This emphasizes the need for proactive case-search, enhanced clinical care, and expanded availability of HBV vaccination. Furthermore, a thorough understanding of the virus biology and its interactions with the host will guide the development of better treatment strategies. Our review outlines the existing knowledge on HBV biology, the complex interplay with other infections, and existing gaps in knowledge that could accelerate control efforts.
2. HBV Burden
About thirty million people are newly infected with HBV every year in the world and cumulatively 296 million persons currently live with chronic HBV. Of these, 2.7 million are coinfected with Human Immunodeficiency Virus (HIV) [ 13 ]. Although every year an estimated 1 million HBV related deaths occur worldwide, less than 10% of infected people are diagnosed and only about 1% are able to access treatment [ 14 ].
The disease burden is disproportionally distributed with Africa and Asia Pacific together contributing 68% of the global burden [ 15 ]. This could be attributed to the lax vaccination policies and the absence of continuous surveillance in the most burdened areas of Africa [ 16 ]. Within Africa alone, more than 60 million people are infected with hepatitis B, which amounts to over 60,000 deaths annually [ 17 ]. Most of these infections occur early in childhood, either through vertical transmission from a hepatitis B-infected mother to their child or by horizontal transmission during the first five years of life from contact with infected family members or close friends [ 18 ].
Candotti et al., in 2006 [ 19 ], reported that despite the high endemicity, little was known about the HBV-genotype distribution across Africa until recently. In Ghana, HBV genotype E is prevalent (87%) among blood donors while genotypes A and D constitute minor groups of strains accounting for 10% and 3%, respectively. The identification of one mixed infection of genotypes A and E confirmed the occurrence of HBV co-infection or superinfection with multiple strains. The predominance of genotype E is consistent with recent data obtained from neighbouring countries and most of Western Africa, from Senegal to Angola.
In Ghana, Abesig et al., in 2020, estimated the HBV burden with meta data from seroprevalence studies published between 2015 and 2019 to be 8.36% in adults, 14.30% in adolescents, and 0.55% in children under five years (10). In a recent multi-centre, cross-sectional study, which reviewed registers in 22 health centres across Ghana, a pooled HBV prevalence rate of 11.4% was estimated, making Ghana a hyper-endemic country [ 20 ]. The burden was disproportionately distributed within the country, with the savannah region having the highest (22.7%) and the Greater Accra region recording the least (6.4%) seroprevalence rates. A comprehensive surveillance system is needed to assess the community burden and accurately estimate the true burden of the disease in Ghana. Moreover, continuous active molecular surveillance is needed for early detection, tracking transmission, and monitoring viral evolution. This could be the basis for formulating policies, making a case for the implementation of birth-dose HBV vaccination as well as routine adult vaccination to strengthen control efforts within the country. Furthermore, it will aid clinicians in patient management through adopting better treatment measures.
3. Overview of Viral Biology
3.1. virus structure and genome.
The Hepatitis B virus (HBV) is the prototype virus of the Hepadnaviridae family of viruses [ 21 ]. Members of this family are hepatotropic DNA viruses known to infect birds (avihepadnavirus) and mammals (orthohepadnaviruses). Additionally, fish and amphibian Hepadnaviruses have been reported recently [ 22 , 23 ]. These viruses share similarities in their genome organisation as well as their replication approach, with up to 40% and 20% sequence diversity amongst orthohepadnaviruses and avihepadnaviruses, respectively [ 24 ].
Three types of HBV-virion particles are usually observed in the serum of infected persons. The infectious virion, also known as the Dane particle, is 42–45 nm in diameter, made up of HBsAg embedded in a lipid envelope, encasing the viral nucleocapsid containing a reverse transcriptase tethered to the nucleic-acid material [ 25 , 26 ]. The other two are subviral particles (22–24 nm), filamentous and spherical in shape, both comprising HBsAg embedded in a host-derived lipid membrane but lacking viral DNA [ 27 ]. Interestingly, the subviral particles outnumber the infectious particles by 100 to 100,000-fold in the blood and play immunomodulatory and immunoinhibitory roles. Their role has been extensively reviewed elsewhere [ 28 , 29 ].
The viral nucleic-acid material is a circular, partially double-stranded DNA of approximately 3.2 kb in size that is organized into four overlapping open reading frames (ORFs), each with its own promoter yet sharing a single polyadenylation sequence [ 26 ]. The ORFs encode the surface antigen (HBsAg), core antigen (HBcAg), polymerase, and X (HBxAg) proteins. Due to their small genome size, viral proteins are encoded in overlapping translational frames with regulatory elements lying within protein-coding sequences [ 30 ]. Additionally, the HBV virion has a highly error-prone polymerase that increases its mutation rate to about 2.0 × 10 −5 nucleotide substitutions per site per year [ 31 ]. Looking at the small genome size, the overlapping nature of ORFs, and the significance of each nucleotide position, such a high mutation rate could be detrimental to the survival of the virus. Remarkably, HBV can maintain replication competence and survive amidst the potential of genome rearrangement.
The virus is divided into four major serotypes (adr, adw, ayr, and ayw) based on antigenic epitopes presented on its envelope proteins, and into eight major genotypes (A–H). Differences between genotypes affect the disease severity and course, the likelihood of complications, the response to treatment, and possibly vaccination. There are at least 10 HBV genotypes, named A to J, classified based on a sequence divergence of more than 8%, as well as multiple sub-genotypes with sequences varying by 4–8% [ 32 ]. These exhibit a unique geographical distribution ( Table 1 ) and may also show distinct natural courses of infection [ 31 ]. Additionally, some distinct routes of infection have been observed to be common in areas with specific genotypes [ 32 , 33 , 34 ]. In natural infections with HBV, there is an excess of empty non-infectious subviral particles (SVP) that do not contain the viral capsid. They can contain all three forms of the HBV envelope proteins: L, M, and S. These share a common C terminus, with M containing the pre-S2 domain relative to S and L containing the pre-S1 domain relative to M (15). There is good evidence that, during infection, a domain within the pre-S1 of L is what interacts with an as-yet-unidentified host receptor(s).
The interplay between viral proteins and the innate responses remains central to the development of curative treatment strategies against HBV and HDV. Experimental infection systems and patient analyses support the notion that HBV avoids innate immune recognition but that the co-infection with HDV appears to cause profound changes in the infected liver. Dandri M. et al., in 2021 [ 35 ], observed higher production of chemokines and inflammatory cytokines, as well as the increased antigen-presentation capabilities determined in HBV/HDV infection, boosts the ability of immune cells to recognise infected cells, augments liver inflammation, and accelerates pathogenesis. Through research, the role of distinct HBV and HDV proteins in modulating the antiviral responses in infected hepatocytes has gained recognition and highlighted the importance of viral activity in counteracting the first line of host defences.
Geographical distribution of the various HBV genotypes is summarised in Table 1 . Nevertheless, there is still much to be understood about HBV genomic features and their role in establishing and maintaining infection. More studies on the molecular epidemiology of HBV are required to assess their impact on disease progression and response to treatment, especially in hyperendemic regions. For instance, in a recent article, Lemoine et al., in 2023 [ 36 ], maintains that the status quo in the management of hepatitis B in Africa is intolerable and they indicated that, to prevent new infections, Birth Dose Vaccine (BDV) must be made available for all new-borns in Africa. The authors reiterated that the current model that involves repeated laboratory assessments and complex criteria for the initiation of antiviral therapy, together with the cost of diagnostics, is an obstacle to those requiring therapy. However, they observed that there is a lack of education and awareness, poor access to relevant laboratory tests and imaging, and inconsistent access to appropriate medication. This is particularly pertinent in resource-constrained settings. Decentralisation, integration of services, and task-sharing, with adequate funding of the required infrastructure, will be key to upscaling delivery of care. However, new approaches such ‘Treat all’ or ‘Treat-all-except’ approaches do need further evidence from implementation-science research, including qualitative evaluation of understanding, attitudes, and acceptability of such approaches by both patients/carriers and policy makers.
Furthermore, there is a need for in-depth current studies on viral biology, mainly for the purposes of coming up with better management strategies for infected people and more effective preventative measures for the rest of the population. Intensified surveillance systems in endemic regions will also aid monitoring efforts to identify new genomic recombination events and their impact on control strategies.
3.2. Pathogenesis
HBV can be acquired through two main routes—perinatally, from infected mothers to their new-borns, which accounts for a majority of cases worldwide, and horizontal transmission through contact with an infected person’s body fluids, equipment for body piercing, tattoos, and injecting-drug use. Generally, the mechanisms by which HBV accesses and gains entry into hepatocytes is not fully understood. [ 37 ]. The heparan sulphate proteoglycans (HSG’s), sodium taurocholate co-transporting peptide (NTCP), and the epidermal growth factor receptor (EGFR) are some of the receptors that mediate this internalization although there are likely to be others [ 38 , 39 ]. Both the NTCP and HSG’s are hepatocyte-specific receptors, thus explaining the virus’ hepatotropic nature [ 40 ]. In-depth studies into these receptor interactions could significantly contribute to finding a cure to HBV through inhibition of viral spread within the liver. Recently, some compound leads have been shown to selectively inhibit the virus-receptor function of NTCP [ 41 ].
Following internalization, the virus uncoats and releases its nucleocapsid into the cytoplasm followed by release of the genome into the hepatocyte nucleus [ 42 ]. Here, HBV DNA, which is partially double stranded, is repaired by viral polymerase or host repair mechanisms, giving rise to a covalently closed circular DNA (cccDNA) that serves as the template for the synthesis of sub-genomic and pre-genomic RNA (sgRNA & pgRNA) [ 43 ]. A nucleocapsid encloses the viral DNA and a DNA polymerase that has reverse-transcriptase activity. The outer envelope contains embedded proteins that are involved in the viral binding of, and entry into, susceptible cells. The virus is one of the smallest enveloped animal viruses. The 42 nm virions, which are capable of infecting liver cells, are referred to as “Dane particles”. In addition to the Dane particles, filamentous and spherical bodies lacking a core can be found in the serum of infected individuals. These particles are not infectious and are composed of the lipid and protein that forms part of the surface of the virion, the surface antigens (HBsAg), and is produced in excess during the life cycle of the virus. The cccDNA is highly stable and can remain in hepatocytes indefinitely, becoming a permanent template for sg/pg RNA synthesis, thus promoting viral persistence in the cells. The sgRNA is translated into viral proteins whereas the pgRNA together with the polymerase is packaged to form the nucleocapsid where pgRNA is reverse transcribed into negative-stranded DNA, followed by the generation of rcDNA from plus-strand synthesis [ 44 ]. The nucleocapsids are either imported back into the nucleus for cccDNA synthesis or packaged and secreted through the endoplasmic reticulum (ER) to infect other hepatocytes.
As a result, HBV exits in the infected cells without causing cytocidal changes within the hepatocyte, although other cellular changes that occur tend to increase a person’s risk of liver injury and hepatocellular carcinoma [ 45 ]. Additionally, viral variants and components seem to play a role in the severity of damage that occurs in the liver [ 46 ]. The main culprit for liver damage in HBV infection is said to be the immune system, where the HBV-specific T-cell response initiated for viral clearance is dysregulated. Both innate and adaptive immune arms play significant roles in liver pathology and viral clearance. Not much is known about innate immune response in HBV infection and, previously, it was thought that HBV did not elicit an innate immune response [ 47 ]. However, some studies have suggested a role for polymorphonuclear cells in HBV-related liver inflammation, which may be achieved through cytokine production and recruitment of other immune cells. Furthermore, stress responses such as release of reactive oxygen species, endoplasmic reticulum (ER), and mitochondrial dysregulation may initiate cell-death signals in infected hepatocytes [ 48 , 49 ]. Gherlan GS, in 2022 observed that the factors that determine the outcome of occult hepatitis B infection (OBI) are now better understood, with host factors (immune or epigenetic) being identified as seemingly the main contributors. He stated that viral factors are important but account for only a minority of OBIs, but some external factors can contribute to its appearance by interfering either with the host immune system or with the lifecycle of HBV. Of these, HIV and HCV co-infections are notable.
In acute infection, viral clearance is achieved through the work of cytotoxic CD8+ T cells and helper CD4+ T cells, which help to eliminate infected cells and stimulate antibody production against viral antigens respectively [ 50 ]. Much remains to be studied about the role of these immune cells since, although we know their function is impaired in chronic infection, the mechanisms of impairment and the true significance is not clearly understood. A clear understanding of the HBV immune-response process and its role in cell damage can be exploited for management.
Overlap of HBV and HDV genogroups and associated outcomes of infection.
| HBV Genotype | Dominant Region | HDV Genotype Dominant in the Region | Reported Outcome of HDV Infection |
|---|---|---|---|
| A | North-western Europe Northern America Central Africa | HDV-1 | Varied severity of liver disease |
| B | Asia | HDV-2, HDV-4 | Comparatively less severe clinical manifestation |
| C | Asia | HDV-2, HDV-4 | Comparatively less severe clinical manifestation |
| D | Worldwide | HDV-1 | Varied severity of liver disease |
| E | Sub-Saharan Africa | HDV-5 to 8 | Yet to be clarified |
| F | South & Central America | HDV-3 | Most severe form of HDV |
| G | France & USA | HDV-3 | Severe fulminant Hepatitis |
| H | South America | HDV-3 | Severe fulminant Hepatitis |
| I | South America | HDV-3 | Severe fulminant Hepatitis |
| J | Japan | HDV-2, HDV-4 | Comparatively less severe clinical manifestation |
References: [ 28 , 30 , 44 ].
4. Treatment
Acute hepatitis B infection does not usually require treatment and most adults clear the infection spontaneously [ 51 ]. Early antiviral treatment may be required in fewer than 1% of people, that is the fulminant or the immunocompromised individuals. Treatment of chronically infected persons with persistently elevated serum alanine aminotransferase, a marker of liver damage, may be necessary to reduce the risk of cirrhosis and liver cancer [ 52 ]. Treatment lasts from six months to one year, depending on medication and genotype [ 53 ].
Although none of the available medications can clear the infection, they can stop the virus from replicating, thus minimising liver damage. The licensed medications for treatment include antiviral medications lamivudine, adefovir, tenofovir disoproxil, tenofovir alafenamide, telbivudine, entecavir, and the two immune system modulators interferon alpha-2a and PEGylated interferon alpha-2a. In 2015, the World Health Organization recommended tenofovir or entecavir as a first-line agent [ 54 ]. Those with current cirrhosis are in most need of treatment [ 54 ].
Ultimately, HBV elimination can be defined by complete suppression of HBV DNA levels, the loss of HBsAg, and seroconversion to anti-HBs antibodies after stopping antiviral therapy. Loss of HBsAg levels is critical since HBsAg levels are surrogate markers for levels of transcriptionally active covalently closed circular DNA (cccDNA), meaning that if HBsAg is eliminated, the virus is most likely inactivated [ 53 ]. In chronic-HBV individuals who are negative for HBeAg, the bulk of the HBsAg is produced from integrated HBV DNA. Although adaptive immunity is key to controlling and clearing HBV infection, the role of innate immunity cannot be ignored. Adaptive immunity depends on the activation signals and cytokines secreted by the innate immune system. HBsAb can bind to HBsAg to limit its spread and kill or phagocytose HBV-infected cells.
It seems unlikely that the disease will be eliminated by 2030, the goal set in 2016 by WHO. However, progress is being made in developing therapeutic treatments. In 2010, the Hepatitis B Foundation reported that three preclinical and 11 clinical-stage drugs were under development, based on largely similar mechanisms. In 2020, they reported that there were 17 preclinical- and 32 clinical-stage drugs under development, using diverse mechanisms [ 55 , 56 ].
Comorbidities with Other Liver Pathogens
Other microbes may co-infect the liver together with HBV, such as hepatitis A, C, D, and E viruses. Additionally, HBV can co-occur with non-communicable liver conditions including steatohepatitis and alcoholic liver disease. Although parasites such as liver flukes and biliary-tract bacterial infections also affect the liver, these are relatively less common [ 57 ]. Plasmodium species, the causative agent for malaria, asexually reproduces in the human liver as part of its life cycle. Its activities in the liver have not been linked to significant hepatocyte damage, yet immune response to this parasite in the liver may potentially influence that of HBV [ 58 , 59 ]. Moreover, the few studies on HBV-malaria coinfection have reported varied levels of interaction between the two pathogens [ 60 , 61 ]. Here, we examine the impact of other liver infections on HBV outcome.
Hepatitis Delta Virus (HDV): According to the WHO, about 5% of all HBV-infected people also have HDV [ 62 ], however Chen, Shen [ 63 ] estimate this to be 10.58% and this number is bound to increase with HBV incidence yearly. On its own, the risk of HBV-induced liver cirrhosis ranges from 6% in America to up to 38% in sub-Saharan Africa and 39% in East Asia [ 64 ]. However, compounded with HDV, the risk could be significantly higher.
The Hepatitis D virus, which was first discovered in 1977 among HBV patients with severe liver damage, is the smallest human-infecting RNA virus, with 36–40 nm diameter and roughly 1.7 kb single-stranded negative-sense circular RNA [ 65 , 66 ]. Known as defective because of its inability to establish an infection on its own, the virus is spherical and made up of an outer lipoprotein envelope, composed of HBsAg, which encloses a ribonucleoprotein. Not only does the HBsAg structure in HDV bear semblance to spherical SVPs more closely than filament SVPs and even Dane particles, their assembly and cell exit utilise the same pathways [ 28 , 67 ] The HDV ribonucleoprotein is unconventional, comprising genomic RNA complexed with 70–220 HDV-specific antigens known as the delta antigens (HDAg) [ 68 ]. The HDAg exists in two isoforms: L-HDAg, which has 19 additional amino acids at the C-terminal compared to S-HDAg [ 69 ]. These two isoforms have distinct roles in the HDV life cycle, with S-HDAg being essential for replication, while L-HDAg is crucial for assembly due to its C-terminal 19 amino acids, which contain the virus-assembly signal [ 70 , 71 ]. HDV infection could be classified either as a co-infection with HBV, where both viruses are acquired simultaneously, or as a superinfection, where a chronic HBV patient later acquires HDV [ 72 ].
The mechanism of viral entry into hepatocytes is like that of HBV. The virus gains entry into cells by interacting with the NTCP and HSGs on the hepatocyte surface [ 64 ]. Despite having an HBV-dependent entry pathway, HDV viral replication and assembly are distinct from HBV [ 73 ]. Additionally, HBV DNA suppression among superinfected persons has been observed with controversy on its association with faster progression to hepatocellular carcinoma (HCC). This could be due to the dominant HDV genotype in the region, some of which yield severe hepatitis than others ( Table 1 ). Since its identification as the cell surface receptor, NTCP, for HBV and HDV entry into hepatocytes, the search for molecules interfering with its binding led to the design of bulevirtide (BLV). This large polypeptide mimics a region of the pre-S1 HBsAg and blocks viral entry by inhibitory competition. BLV was initially tested in cell cultures, animal models, and, more recently, in Phase I–III human trials. As a monotherapy or in combination with peginterferon, BLV is well tolerated and exhibits potent antiviral activity. Plasma viremia significantly declines and/or becomes undetectable in more than 75% of patients treated for >24 weeks with BLV. However, serum HBsAg concentrations remain unchanged with BLV treatment even though plasma viremia drops. No selection of BLV resistance in HBV/HDV has been reported in vivo to date.
In the cell, HDV uncoats and its RNA genome is translocated to the cell’s nucleus, with the help of HDAg, where host RNA polymerase I and II are hijacked to make copies [ 74 ]. Looking at the virus structure, it is not surprising that HDV lacks the ability to replicate independently, relying on host RNA polymerase and ribosomes to make new copies of viral RNA and proteins respectively. Replication of HDV occurs by the rolling circle mechanism where multimeric liver transcripts complementary to the HDV genome, known as the antigenome RNA, are transcribed then self-cleaved by intrinsic ribozyme activity to separate monomers from multimeric transcripts. These are joined together to create a circular antigenomic template for the synthesis of the viral genomic strand [ 75 ]. This is then packaged into HBsAg envelopes and released in a clathrin-mediated manner [ 76 ]
Currently, there are at least eight HDV genotypes, named genotypes 1 to 8, and these show geographical restrictions just like in HBV ( Table 1 ). Genotype 1 has a global distribution and is most prevalent in Europe, North America, and parts of Asia, with a variable course of infection; whereas genotype 3, found in the Amazon Basin, is associated with early onset of HCC and acute liver failure [ 77 ]. Furthermore, genotype 2, found in Russia, Taiwan, and Japan, is associated with higher rates of remission than genotype 1 and genotype 4 in the same location and is associated with faster progression to cirrhosis [ 78 ]. Genotypes 5, 6, 7, and 8 are localised in Africa and African migrants in Europe, with limited data and the course of infection poorly classified [ 79 , 80 ]. The co-localization of these viral genotypes with specific HBV genotypes may explain the varying outcomes of infection and could be exploited to guide treatment strategies since the effectiveness of currently available treatment regimen is genotype dependent.
Large gaps in epidemiological data on HDV prevalence result in underestimated prevalence and incidence rates. For instance, in Ghana and many endemic developing regions, routine screening of HBV infected persons for HDV is lacking and prevalence estimates are based on sporadic screening of groups in various studies [ 81 , 82 ]. Additionally, since the HBV burden may be underestimated, it is likely that the HDV burden is also underestimated. Surveillance studies to accurately estimate prevalence, and studies to clearly define the role of genotypic differences in the severity of liver damage, are needed to guide control strategies. Global eradication of HDV is therefore directly linked with the eradication of HBV.
Plasmodium liver stage infection: Plasmodium is the protozoan parasite responsible for malaria infections; it caused 229 million cases in 2019 with about 400,000 deaths [ 82 ]. The majority of malaria cases occur in Africa and, in 2019, accounted for 95% of global cases. The parasite is spread through an infectious bite from a female Anopheles mosquito and, currently, there are five species known to infect man, including P. falciparum , P. vivax , P. ovale , P. malariae , and P. Knowlesi , of which P. falciparum is accountable for the majority of deaths [ 83 ].
The human parasite’s life cycle takes place within two hosts—the mosquito and man. The mosquito injects the parasite in its sporozoite form into the skin of a human host, where it finds its way into the bloodstream [ 84 ]. The parasite travels to the liver and develops into schizonts which in turn mature and rapture, releasing merozoites into the bloodstream to invade red blood cells and give rise to the clinical symptoms of malaria [ 85 ]. Although clinical symptoms are often not obvious at the liver stage, hepatic dysregulation has been reported in severe malaria characterized by hepatocyte necrosis, granulomatous lesions, Kupffer cell hyperplasia, and malarial pigmentation among others [ 86 ]. In asymptomatic infection, abnormal total bilirubin levels have been observed but resolved after a few days [ 87 ]. Elevated liver enzyme levels have also been reported in uncomplicated malaria at the time patients reported and was associated with parasite load, pointing to Plasmodium involvement rather than a drug-induced effect [ 88 ]. While the exact cause of these abnormalities is unclear, it could influence the immune response to other infections and their pathology.
In sub-Saharan Africa where HBV is endemic, Plasmodium infections are also endemic and may co-occur in individuals. Studies on HBV and Plasmodium coinfection are few and sporadic, making prevalence estimations challenging. However, a 6% pooled prevalence rate has been reported, with places like Gambia and Nigeria reporting 10% and 7%, respectively [ 57 ]. It has been established that areas endemic for malaria and HBV infection largely overlap geographically. A recent study has suggested the existence of an interaction between the two pathogens in symptomatic co-infected individuals on the South American continent. However, data presented by Freimanis GL et al., in 2012 [ 80 ] suggest that, in sub-Saharan Africa, asymptomatic co-infections with these two ubiquitous pathogens do not appear to significantly affect each other and evolve independently. Whereas HBV infections tend to be lifelong, Plasmodium infections are usually short-term and resolve with treatment in weeks. The Plasmodium liver phase is also short-lived, with the covert immune response characterised by the induction of immune inhibitory pathways shortly after the inflammatory response is mounted [ 89 , 90 ]. With the strong impact HBV and host diversity has on infection outcome, it is possible that immune response to Plasmodium infections and its impact on liver health could be modulated by the presence of HBV and vice versa. Also, the chronicity of HBV infection, and the potential of acquiring Plasmodium infections several times during HBV infection, may influence infection outcome of both diseases. This could also affect liver integrity in the long run, making it imperative to study and understand.
Hepatitis C virus infection : HCV is a single-stranded RNA virus which belongs to the Flaviviridae virus family and the Hepacivirus genus. An estimated 58 million people live with HCV worldwide, of which 3.2 million are adolescents and children [ 13 ]. Furthermore, 1.5 million new infections are reported each year with 290,000 deaths mainly through end-stage liver disease. Because of shared routes of transmission, HBV and HCV coinfection is common and can be seen in up to 30% of chronic-HBV-infected persons and about 10% of HCV-infected persons [ 91 , 92 ].
The HCV genome is 9.6 kb in length, organised into one continuous open reading frame (ORF) flanked by highly structured UTRs at both the 5′ and 3′ ends [ 93 ]. This ORF encodes a 3010 amino acid long polyprotein which goes through post translational modification to make three structural proteins, Core (C) and Envelope1 and 2 (E1 and E2); and 7 non-structural proteins, NS2, NS3, NS4A, NS4B, NS5A, NS5B, and p7 [ 94 ]. Like HBV, HCV replicates mainly in hepatocytes and, although their nucleic acid material differs, both at some point in replication yield an RNA intermediate that theoretically could interact [ 95 ]. There are conflicting reports on HBV/HCV interaction in vivo. Some studies suggest that both viruses can replicate in the same cell without restriction while others report a mutual suppression of each virus’ replication [ 96 , 97 , 98 ].
When HBV and HCV infections occur separately, they each contribute to liver damage by provoking an excessive inflammatory response against the respective viruses. Nevertheless, when both viruses infect an individual simultaneously, there are conflicting reports regarding the resulting liver disease outcomes. Some studies suggest the simultaneous suppression of both viruses, while others indicate the progression of one virus to a chronic state. In certain cases, fulminant hepatitis has been reported in dual infections [ 99 , 100 ]. These discrepancies underscore the potential role of host factors in shaping the divergent disease outcomes observed. Furthermore, certain studies have documented enduring epigenetic alterations in HCV, which remain present even after treatment and recovery, potentially increasing the risk of hepatocellular carcinoma (HCC) later in life [ 101 , 102 ]. HCV might become the first curable chronic disease due to the remarkable efficacy of the newly introduced direct-acting antiviral drugs (DAAs). Interferon-free regimens, based on combinations of DAAs with pan-genotypic activity, allow for shorter courses of treatment without severe side effects. However, the high cost of the DAAs precludes universal replacement of the suboptimal interferon-based therapy for chronic hepatitis C. Across the 9.6 kb genome of HCV, several regions have been extensively analysed in relation to treatment outcome, but the core region that is mostly used for HCV genotyping and classification has been reported to antagonize the antiviral response induced by IFN by interacting with the IFN-activating and -signalling pathways. Sultana C. et al., in 2016 [ 103 ] confirmed core substitutions are also found in Caucasian patients and, together with age and IL28B genotype, can be used as predictors of the outcome of interferon-based therapy. The study concluded that HCV core mutations can help distinguish between patients who can still benefit from the affordable IFN-based therapy and those who must be treated with DAAs to prevent the evolution towards end-stage liver disease [ 103 ]. While effective treatment can cure HCV, its lingering impacts may persist and potentially lead to cancer in the future. Thus, people who are cured of HCV could still experience the dual impact of HCV-HBV coinfection if they later acquire HBV.
Human Immunodeficiency Virus (HIV) : Human immunodeficiency virus (HIV), the causative agent of acquired immunodeficiency syndrome (AIDS), remains a significant cause for public-health concern worldwide. As at 2021, an estimated 38 million people live with HIV infection, over a million new cases were recorded, and 650,000 people died from it [ 97 ]. While a definitive cure for the disease remains elusive, antiretroviral drugs (ARVs) have played a crucial role in extending the life expectancy of individuals living with HIV, bringing it closer to that of uninfected individuals. ARVs employ diverse mechanisms to hinder viral replication, thereby alleviating the strain on the immune system and reducing the vulnerability to opportunistic infections. By virtue of their action, ARVs also contribute to a reduction in HIV transmission rates and a substantial enhancement of the overall quality of life for those affected by the virus.
Globally, 2.7 million people living with HIV (PLHIV) also have HBV (7.6% prevalence), with a majority of these in sub–Saharan Africa (1.9 million) where HBV is endemic [ 104 ]. Certainly, the ongoing lifelong management of HIV introduces the potential for hepatotoxicity, and when compounded with HBV infection, which is recognized for its liver-related complications, the consequences could be dire. Moreover, liver-related mortality amongst HIV-HBV-coinfected people is said to be 17x higher than in HBV-mono-infected people [ 97 ]. HIV infection has myriad effects on adipocyte biology that might co-ordinately impact liver disease. The most obvious connection is with the accumulation of additional liver fat (steatosis), which in some instances also is associated with disease (inflammation or fibrosis). Rosca A, et al., and Nguyen MH et al., both in 2020 [ 105 , 106 ], respectively wrote on the ‘Liver function in a cohort of young HIV-HBV co-infected patients on long-term combined antiretroviral therapy’ and ‘Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy’.
Several options exist for the treatment of hepatitis B including interferon, pegylated interferon, lamivudine, adefovir, entecavir, and telbivudine, as well as tenofovir, which has been licensed. The antivirals can be divided into “lamivudine-like” and “adefovir-like”, which clinically differ and their resistance profiles make them good combination partners, even in the absence of synergy in antiviral potency. The “adefovir-like” drugs best used in practice are adefovir in the HIV-infected patient in need of anti-HBV therapy while not yet needing anti-HIV therapy [ 104 ]. In other patients, tenofovir is to take over where adefovir is currently used, given its lower toxicity and higher activity. It is probable that all could be well combined with lamivudine, which will soon be off patent. Thus, it might be a cheap but potentially very active addition to any “adefovir-like” drug, given their different resistance profiles. However, in the case of tenofovir, this is not required, given its existence in combination with the lamivudine-like drug emtricitabine.
Aside from liver complications, secondary HIV infection in HBV-infected adults has been shown to increase the risk of HBV progressing to chronicity six-fold [ 98 ]. Moreover, the progression of HBV infection is notably affected, particularly in terms of changes in HBV antigen and antibody expression, along with an elevated susceptibility to HDV [ 99 ].
5. Control Strategies for HBV Infection
Presently, there is no cure for HBV infection and the treatment options available function mainly to reduce viral load so that liver damage can be slowed to the barest minimum. Thus, chronic HBV is a lifelong infection although, in acute cases, the immune system may adequately clear the infection completely. Fortunately, there exists a highly effective vaccine against HBV, which has played a pivotal role in the strategies of global control programs since its development.
The WHO’s Global Health Sector Strategy (GHSS) on viral hepatitis hopes to achieve elimination of viral hepatitis by 2030. This means reducing the annual disease incidence and mortality by 90% and 65%, respectively, using 2015 data as baseline [ 12 ]. To achieve this, control programmes have been encouraged to pursue HBV and HCV elimination simultaneously, although a country may prioritise based on their peculiar situation. With respect to reducing HBV incidence and mortality, vaccination must be complemented with a cure. The definition of a cure itself is a source of controversy, since, clinically, a cure means moving a chronic-HBV-infected person with risk of liver disease to the state of an uninfected person. Due to the latent persistence of HBV cccDNA in infected hepatocytes, this is difficult to achieve and may even require lifelong treatment. Thus, the realistic aim is to achieve a functional cure, meaning to maintain reduced viral load as well as other viral markers in the blood after therapy ceases.
The main goals of treating chronically infected HBV patients are to improve survivability by limiting progression to HCC, limiting mother-to-child transmission in pregnant women, and preventing extrahepatic complications. The possibility of achieving these goals depends on factors including the stage of infection and patient’s age when therapy is initiated. Currently, available treatment options for HBV are nucleo(s)tide analogs (NUCs) and interferon-based therapy. NUCs commonly approved for HBV treatment include Tenofovir disoproxil, Lamivudine, and Entecavir. These drugs function by inhibiting viral replication [ 107 ]. The activity of these NUCs can significantly reduce HBV DNA levels, but they are ineffective against cccDNA, which maintains the chronic HBV state [ 108 ]. It has been demonstrated that individuals with significant HBsAg decline have a commensurate loss of infected cells with transcriptionally active cccDNA, while individuals without HBsAg decline have stable or increasing numbers of cells producing HBsAg from invertebrate-derived DNA (iDNA). While NUC therapy may be effective at controlling cccDNA replication and transcription, innovative treatments are required to address iDNA transcription that sustains HBsAg production. Also, interferon-based therapies are more effective for certain HBV genotypes than others [ 109 ]. Up until 2020, no specific treatment existed for HDV even though drug discovery for HDV-specific antivirals is ongoing [ 110 ]. In 2020, Bulevertide (BLV) received approval from the European Medicines Agency (EMA) for the treatment of HDV. BLV impedes the attachment of HBsAg to NTCP and has demonstrated synergistic effects with Peg-IFNα, yielding superior outcomes compared to monotherapy with either drug [ 111 ]. Developing effective therapy for HDV and complementing HBV vaccination with effective management of chronic patients could significantly reduce the burden of infection and lead towards HBV elimination.
6. Conclusions
The observations we made indicate a need for prevention and control of, generally, serum hepatitis in hyperendemic and low-resourced countries, especially in the West African sub-region. There is the need for operative strategies which requires comprehensive investments to interrupt the transmission of serum hepatitis and reduce the consequential morbidity and mortality. The importance of expanding research in the field of HBV cannot be overstated. There is a pressing need to elevate efforts in HBV research to precisely assess prevalence rates, identify at-risk populations, establish treatment priorities, and deepen our comprehension of host-pathogen interactions that could ultimately lead to a cure. Such insights are essential not only for shaping control programs but also for informing the adoption of effective strategies and the development of treatment policies. Since the cure rates are minimal, therapies need to be accessible and affordable since they are required by patients indefinitely. Antiviral treatment with either pegylated interferon or a nucleos(t)ide analogue (lamivudine, adefovir, entecavir, tenofovir disoproxil, or tenofovir alafenamide) should be offered to patients with chronic HBV infection and liver inflammation in an effort to reduce the progression of liver disease. Nucleos(t)ide analogues should be considered as a first-line therapy. Considering the numerous co-infections that have the potential to complicate HBV pathogenesis and control, it is imperative to conduct thorough investigations into their impact on treatment and to formulate improved guidelines for their effective management.
Funding Statement
This research received no external funding.
Author Contributions
Conceptualization: D.A.A., K.A.K. and J.H.K.B.; Writing—Original Draft Preparation: D.A.A., S.P.S., K.A.K. and J.H.K.B.; Writing—Review and Editing: D.A.A., S.P.S., K.A.K. and J.H.K.B.; Supervision: K.A.K. and J.H.K.B. All authors have contributed substantially to the work. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.

COMMENTS
1. CHAPTER 1. LITERATURE REVIEW. 1.1 CLASSIFICATION. Hepatitis B Virus (HBV) is a DNA virus and was first identified in the 1960s. According to the ICTV classification, this virus belongs to the ...
Abstract. Hepatitis B infection is still a global concern progressing as acute-chronic hepatitis, severe liver failure, and death. The infection is most widely transmitted from the infected mother to a child, with infected blood and body fluids. Pregnant women, adolescents, and all adults at high risk of chronic infection are recommended to be ...
Hepatitis B. Wen-Juei Jeng, George V Papatheodoridis, Anna S F Lok. Hepatitis B virus (HBV) infection is a major public health problem, with an estimated 296 million people chronically infected and 820 000 deaths worldwide in 2019. Diagnosis of HBV infection requires serological testing for HBsAg and for acute infection additional testing for ...
Hepatitis B viral infection is a serious global healthcare problem. It is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). It is often transmitted via body fluids like blood, semen, and vaginal secretions. The majority (more than 95%) of immunocompetent adults infected with HBV can clear the infection spontaneously. Patients can present with acute ...
The core antibody to hepatitis B virus (anti-HBc) can be detected through immunoassays for total anti-HBc, which is able to detect both anti-HBc IgG and anti-HBc IgM. Anti-HBc IgM is the determinant of acute hepatitis B and is often the only sign that may be detected during the period of acute hepatitis B when HBsAg has become undetectable.
Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J Gen Virol 91:2080-2090.
Hepatitis B virus (HBV) is a hepatotropic virus that can establish a persistent and chronic infection in humans through immune anergy. Currently, 3.5% of the global population is chronically ...
prevalence) AND ('hepatitis b'/exp OR 'hepatitis b' OR 'hbv'/exp OR 'hbv')". Titles and abstracts were reviewed for relevance, and only studies that included HBsAg prevalence were included. We also included grey literature, ministry of health reports, conference presentations, local journals, and personal communi-
Hepatitis B virus (HBV) infection is a major public health problem, with an estimated 296 million people chronically infected and 820 000 deaths worldwide in 2019. Diagnosis of HBV infection requires serological testing for HBsAg and for acute infection additional testing for IgM hepatitis B core antibody (IgM anti-HBc, for the window period when neither HBsAg nor anti-HBs is detected).
This Review provides an overview of the global epidemiology and burden of hepatitis B virus (HBV) infection, identifying gaps in the HBV care cascade and proposing some solutions to help reach the ...
Hepatitis B virus (HBV), the prototypical member of the Hepadnaviridae family, is a non- cytopathic DNA virus that is transmitted by contacts with infected blood and body fluids and triggers ...
The aim of this systematic review and meta-analysis is to evaluate available prevalence and viral sequencing data representing chronic hepatitis B (CHB) infection in Kenya. More than 20% of the global disease burden from CHB is in Africa, however there is minimal high quality seroprevalence data from individual countries and little viral sequencing data available to represent the continent. We ...
Chronic hepatitis B (CHB) remains a major global health problem affecting more than 240 million people with high liver-related morbidity and mortality worldwide. This snapshot summarizes available HBV therapeutic strategies and highlights their corresponding stages of development from late preclinical to various clinical phases, which are indicated by different colors. The complete HBV life ...
1. Current Global Status. Almost 60 years after the discovery of Australian antigen (AuAg) and more than 30 years after the approval of the first vaccine, hepatitis B virus (HBV) infection remains the most common chronic infectious disease in humans corresponding to 292 million people globally [].According to the World Health Organization (WHO), at least 2 billion people live with serological ...
Abstract. Importance: More than 240 million individuals worldwide are infected with chronic hepatitis B virus (HBV). Among individuals with chronic HBV infection who are untreated, 15% to 40% progress to cirrhosis, which may lead to liver failure and liver cancer. Observations: Pegylated interferon and nucleos (t)ide analogues (lamivudine ...
Hepatitis B surface antigen (p.7) Chronic HBV infection* anti-HBs Test Hepatitis B surface antibody (p.7) anti-HBs Positive 1. Protect your patient (p.16) Give the hepatitis A vaccine Tell your patient to avoid alcohol Test family members and sex partners and vaccinate with hepatitis B vaccine if they are not protected 3. Screen for liver ...
INTRODUCTION. Hepatitis B virus (HBV) infection is a global public health problem. The World Health Organization estimated that, in 2019, there were 296 million HBV carriers, 1.5 million new infections per year, and an annual mortality of 820,000 individuals (mostly from complications of liver cirrhosis and hepatocellular carcinoma) [].The implementation of effective vaccination programs in ...
Chronic Hepatitis B Infection: A Review. ImportanceMore than 240 million individuals worldwide are infected with chronic hepatitis B virus (HBV). Among individuals with chronic HBV infection who are untreated, 15% to 40% progress to cirrhosis, which may lead to liver failure and liver cancer. ObservationsPegylated interferon and nucleos (t)ide ...
reviewed epidemiology and literature, directed an economic analysis, and deliberated upon recommendations. The ... HBV DNA hepatitis B virus deoxyribonucleic acid HCP health care personnel HCV hepatitis C virus ... the Work Group held a teleconference meeting to review results of an economic analysis of single-dose revaccination
INTRODUCTION. Hepatitis B virus (HBV) has infected humans for at least the past 40000 years[] and is the 10 th leading global cause of death[].HBV is the only DNA-based hepatotropic virus that exerts many adverse effects on the infected cells leading to necroinflammation, fibrosis, and carcinogenesis[].The world health organization (WHO), in 2015 has estimated 257 million people infected with ...
A plasma-derived Hepatitis B (HepB) vaccine was first licensed for use in the United States in 1981. The vaccine was safe and effective but was not well accepted, possibly because of unsubstantiated fears of transmission of live HBV and other blood-borne pathogens. Recombinant HepB vaccines replaced plasma-derived HepB vaccines beginning in 1986.
Objectives: This study aimed to evaluate the risk of hepatitis B virus reactivation (HBVr) in COVID-19 patients receiving immunosuppressive treatment, which has been insufficiently studied to date. Secondarily, we aimed to evaluate the seroprevalence of HBV infection in COVID-19 patients. Methods: We performed HBV screening on all Romanian adults hospitalized in four COVID-19 wards between ...
Hepatitis B Virus (HBV) infection is a lifelong dynamic disease that changes over time. Risk of end-stage liver disease and cancer increases with ongoing inflammation and HBV viremia in adults. Fibrosis can be reversible, and treatment can decrease fibrosis progression. At present, chronic HBV infection can be controlled but not cured.
In this paper, a new mathematical model of Hepatitis B is studied to investigate the transmission dynamics of the Hepatitis B virus (HBV). Many diseases can start from the womb and find us humans throughout our lives. These diseases are specific abnormal conditions that negatively affect the structure or function of all or part of an organism and do not suddenly occur in any region due to ...
Las ITS virales, como el VIH, el virus del herpes simple genital (VHS), la hepatitis B y C viral, el virus del papiloma humano (VPH) y el virus T-linfotrópico humano tipo 1 (HTLV-1) carecen o tienen opciones de tratamiento limitadas. Hay vacunas disponibles para la hepatitis B para prevenir infecciones que pueden provocar cáncer de hígado y ...
The Hepatitis B virus (HBV) is the prototype virus of the Hepadnaviridae family of viruses [21]. Members of this family are hepatotropic DNA viruses known to infect birds (avihepadnavirus) and mammals (orthohepadnaviruses). Additionally, fish and amphibian Hepadnaviruses have been reported recently [22, 23].