The Role of a Clinical Research Coordinator

Clinical research plays a crucial role in advancing medical knowledge and improving patient care. At the heart of every successful clinical research study is a Clinical Research Coordinator (CRC). As a CRC, you serve as the linchpin between researchers, study participants, and regulatory bodies.
In this comprehensive guide, we will explore the responsibilities, qualifications, challenges, and rewards of being a Clinical Research Coordinator. Whether you are considering a career in clinical research or already working in the field, this article provides valuable insights to help you succeed.

Responsibilities of a Clinical Research Coordinator
As a Clinical Research Coordinator, your responsibilities are diverse and demanding. You serve as the primary point of contact for study participants, ensuring their safety and well-being throughout the research process. You are responsible for recruiting and enrolling eligible participants, obtaining informed consent, and collecting accurate data. Additionally, you must adhere to strict regulatory guidelines and Good Clinical Practice ( GCP ) standards to ensure the integrity and validity of the study results.
Monitoring participants' progress, managing adverse events, and maintaining detailed records are also crucial aspects of your role as a CRC. To effectively carry out these responsibilities, strong organizational and communication skills are essential. You must be able to multitask, prioritize, and work well under pressure. Attention to detail is paramount, as any errors or oversights can compromise the validity of the study. As a CRC, you are also expected to stay updated on the latest research protocols and regulatory requirements to ensure compliance and contribute to the successful completion of the study.
Qualifications and Education Required to Become a Clinical Research Coordinator
While specific qualifications may vary depending on the institution or organization, a minimum educational requirement for most Clinical Research Coordinator positions is a bachelor's degree in a relevant field such as life sciences, nursing, or pharmacy. A solid foundation in biological sciences and research methodologies is crucial to understanding the complexities of clinical research. A master's degree in clinical research or a related field can further enhance your qualifications and open up opportunities for career advancement.
Apart from formal education, relevant work experience is highly valued in the field of clinical research. Prior experience in a research setting, such as working as a research assistant or in a healthcare role, can provide valuable insight into the research process and make you a more competitive candidate. Additionally, possessing knowledge of regulatory guidelines, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ( ICH-GCP ) guidelines, is advantageous.
Certification Options for Clinical Research Coordinators
While certification is not always mandatory, obtaining a certification as a Clinical Research Coordinator can enhance your professional credibility and increase your job prospects. Several organizations offer certification programs for CRCs, such as the Association of Clinical Research Professionals ( ACRP ) and the Society of Clinical Research Associates ( SoCRA ). To obtain certification, you typically need to meet certain eligibility criteria, which may include a combination of education, work experience, and passing a certification exam.
These certification programs cover a wide range of topics, including research ethics, study design, data management, and regulatory compliance. By obtaining certification, you demonstrate your commitment to maintaining high standards of practice and staying up-to-date with industry best practices.
Tips for Creating an Effective Clinical Research Coordinator Resume
In a competitive job market, a well-crafted resume can make all the difference in securing a Clinical Research Coordinator position. Here are some tips to help you create an effective resume that highlights your skills and qualifications:
1. Start with a compelling summary: Begin your resume with a concise summary that highlights your relevant experience, qualifications, and career goals. This section should grab the attention of potential employers and encourage them to read further.
2. Emphasize your research experience: Highlight your research experience, including any previous roles as a research assistant or involvement in clinical trials. Describe your responsibilities, methodologies used, and any noteworthy achievements.
3. Showcase your knowledge of regulations and guidelines: Demonstrate your familiarity with regulatory guidelines, such as ICH-GCP, and any additional certifications you have obtained. This shows your commitment to ethical research practices and compliance.
4. Highlight your organizational and communication skills: As a CRC, strong organizational and communication skills are crucial. Provide examples of how you have effectively managed multiple tasks, coordinated with various stakeholders, and maintained accurate documentation.
5. Include relevant technical skills: Depending on the specific requirements of the position, include any relevant technical skills such as proficiency in electronic data capture systems, statistical software, or data analysis tools. These skills can set you apart from other candidates.
Remember to tailor your resume to each specific job application, focusing on the skills and qualifications that are most relevant to the position. Proofread your resume carefully to ensure it is error-free and presents you in the best possible light.
Common Interview Questions for Clinical Research Coordinator Positions
Preparing for a job interview is essential to present yourself confidently and effectively. Here are some common interview questions for Clinical Research Coordinator positions, along with tips on how to answer them:
1. Tell us about your experience in clinical research: Be prepared to discuss your previous roles and responsibilities in clinical research, emphasizing your ability to manage study participants, collect accurate data, and ensure compliance with regulatory guidelines.
2. How do you handle challenges in clinical research?: Demonstrate your problem-solving skills by sharing examples of challenging situations you have encountered and how you successfully resolved them. Emphasize your ability to adapt to unexpected circumstances and maintain a high level of professionalism.
3. How do you ensure participant safety and informed consent?: Highlight your understanding of the importance of participant safety and informed consent in clinical research. Explain your approach to obtaining and documenting informed consent, as well as your strategies for monitoring participant well-being.
4. How do you manage time and prioritize tasks?: Showcase your organizational and time management skills by describing how you handle multiple tasks, prioritize responsibilities, and meet deadlines. Provide examples of how you have effectively managed your workload in previous roles.
5. What are your strategies for maintaining accurate and detailed documentation?: Stress the importance of accurate documentation in clinical research and describe your methods for ensuring meticulous record-keeping. Discuss your attention to detail and your ability to maintain confidentiality.
Remember to practice your responses to these questions beforehand, focusing on providing concise and well-thought-out answers. Also, prepare questions to ask the interviewer to demonstrate your interest in the role and organization.
Challenges and Rewards of Being a Clinical Research Coordinator
Working as a Clinical Research Coordinator comes with its own set of challenges and rewards. It is essential to be aware of both aspects to make an informed decision about pursuing a career in this field.
Challenges:
1. Time management: Balancing multiple tasks and deadlines can be challenging, especially when working on multiple studies simultaneously. Strong organizational skills and the ability to prioritize effectively are crucial.
2. Regulatory compliance: Adhering to strict regulatory guidelines and ensuring compliance with ethical standards can be complex. Staying updated on the latest regulations and guidelines is essential to avoid any non-compliance issues.
3. Participant recruitment: Recruiting and enrolling eligible participants can be challenging, particularly when dealing with specific inclusion and exclusion criteria. A proactive and strategic approach to participant recruitment is necessary.
Rewards:
1. Contribution to medical advancements: As a Clinical Research Coordinator, you play a vital role in advancing medical knowledge and improving patient care. The data and insights you collect contribute to the development of new treatments and therapies.
2. Personal and professional growth: Working in clinical research provides continuous opportunities for learning and professional development. You gain valuable experience in research methodologies, data management, and regulatory compliance.
3. Making a difference: By ensuring participant safety and well-being, you make a meaningful impact on the lives of study participants. Clinical research coordinators are instrumental in bringing new treatments and therapies to patients in need.
The challenges and rewards of being a Clinical Research Coordinator often go hand in hand. The satisfaction of overcoming challenges and contributing to medical advancements can be immensely rewarding and fulfilling.
Continuing Education and Professional Development Opportunities
Continuing education and professional development are crucial for Clinical Research Coordinators to stay updated on the latest research methodologies, regulations, and best practices. Here are some opportunities for ongoing learning and growth:
1. Workshops and conferences: Attend workshops and conferences related to clinical research to expand your knowledge, network with industry professionals, and stay informed about the latest advancements in the field.
2. Online courses and webinars: Take advantage of online courses and webinars offered by reputable organizations and institutions. These courses cover a wide range of topics, from research ethics to data analysis.
3. Association membership: Join professional associations such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates ( SoCRA ). These associations offer resources, networking opportunities, and certification programs.
4. Advanced degrees: Consider pursuing an advanced degree, such as a master's or doctoral degree, in clinical research or a related field. This can provide in-depth knowledge and open up opportunities for leadership roles in the field.
Continuing education not only enhances your skills and knowledge but also demonstrates your commitment to professional growth and maintaining high standards of practice.
Resources and Associations
As a Clinical Research Coordinator, it is essential to stay connected with the wider clinical research community and have access to valuable resources. Here are some notable associations and resources for CRCs:
1. Association of Clinical Research Professionals ( ACRP ): ACRP is a global membership association that provides educational resources, networking opportunities, and certification programs for clinical research professionals.
2. Society of Clinical Research Associates ( SoCRA ): SoCRA offers certification programs, training resources, and networking opportunities for clinical research professionals. They also publish a quarterly journal, "The Monitor," which provides valuable insights and updates in the field.
3. ClinicalTrials.gov : ClinicalTrials.gov is a public database maintained by the U.S. National Library of Medicine. It provides information on clinical trials worldwide, allowing CRCs to stay updated on ongoing and upcoming studies.
4. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ( ICH ): The ICH website provides access to guidelines and standards for the conduct of clinical research. Familiarize yourself with these guidelines to ensure compliance and ethical conduct.
By utilizing these resources and actively engaging with professional associations, you can stay informed about the latest industry developments, connect with peers, and access valuable tools and support.
The role of a Clinical Research Coordinator is diverse, demanding, and rewarding. As a CRC, you play a crucial role in advancing medical knowledge, ensuring participant safety, and contributing to the development of new treatments and therapies. By understanding the responsibilities, qualifications, and challenges of the role, you can position yourself for success in the field of clinical research.
Continuously seek opportunities for professional growth, stay updated on the latest regulations and best practices, and actively engage with the clinical research community. With dedication, passion, and a commitment to excellence, you can thrive as a Clinical Research Coordinator and make a significant impact in the field of clinical research.
Sign up for post alerts
Icon & you the potential of together..
Careers that improve the lives of patients, our clients and each other. Are you ready to make a difference?
Related jobs at ICON
Argentina, Buenos Aires
Buenos Aires
Remote Working
Hybrid: Office/Remote
Business Area
ICON Full Service & Corporate Support
Job Categories
Clinical Monitoring
Description
At ICON, it’s our people that set us apart. Our diverse teams enable us to become a better partner to our customers and help us to fulfil our mission to advance and improve patients’ lives. Our ‘Own I
Expiry date

Mexico, Mexico City
Full Service - Early Clinical and Bioanalytical Solutions
Mexico City
Office Based
Clinical Data Management
Clinical Data CoordinatorJR118138Site: Mexico At ICON, it’s our people that set us apart. Our diverse teams enable us to become a better partner to our customers and help us to fulfil our mission to a

Technical Project Management
Non-Clinical Project Management
ICON plc is a world-leading healthcare intelligence and clinical research organization. From molecule to medicine, we advance clinical research providing outsourced services to pharmaceutical, biotech

United States
ICON Strategic Solutions
ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and excellence, and we welcome you to join us
2024-109986

Related stories

Teaser label
Content type
Publish date
Deepak is a Clinical Data Management Project Manager at ICON Strategic Solutions, the largest global provider of Functional Service Provision (FSP). He works as a dedicated resource within one of o
Deepak shares his experience as Clinical Data Management Project Manager at ICON Strategic Solutions.
.png)
Overcoming Resume Gaps In an ideal world, resumes would neatly showcase an uninterrupted career progression. However, In today's dynamic job market, it's not uncommon for professionals to encoun
Periods of unemployment don't have to be a red flag. Learn proven strategies for addressing resume gaps.
.png)
The Art of Customisation: How to Tailor Your CV for Any Role or Industry In today's competitive job market, a one-size-fits-all CV often misses the mark. To truly stand out and position yourself a
Discover strategies to highlight your expertise, role-specific skills, and industry knowledge to help your CV stand out.
Recently viewed jobs
A better career. A better world. A better you.
Browse popular job categories below or search all jobs above
Clinical Researcher
Navigating a Career as a Clinical Research Professional: Where to Begin?
Clinical Researcher June 9, 2020

Clinical Researcher—June 2020 (Volume 34, Issue 6)
PEER REVIEWED
Bridget Kesling, MACPR; Carolynn Jones, DNP, MSPH, RN, FAAN; Jessica Fritter, MACPR; Marjorie V. Neidecker, PhD, MEng, RN, CCRP
Those seeking an initial career in clinical research often ask how they can “get a start” in the field. Some clinical research professionals may not have heard about clinical research careers until they landed that first job. Individuals sometimes report that they have entered the field “accidentally” and were not previously prepared. Those trying to enter the clinical research field lament that it is hard to “get your foot in the door,” even for entry-level jobs and even if you have clinical research education. An understanding of how individuals enter the field can be beneficial to newcomers who are targeting clinical research as a future career path, including those novices who are in an academic program for clinical research professionals.
We designed a survey to solicit information from students and alumni of an online academic clinical research graduate program offered by a large public university. The purpose of the survey was to gain information about how individuals have entered the field of clinical research; to identify facilitators and barriers of entering the field, including advice from seasoned practitioners; and to share the collected data with individuals who wanted to better understand employment prospects in clinical research.
Core competencies established and adopted for clinical research professionals in recent years have informed their training and education curricula and serve as a basis for evaluating and progressing in the major roles associated with the clinical research enterprise.{1,2} Further, entire academic programs have emerged to provide degree options for clinical research,{3,4} and academic research sites are focusing on standardized job descriptions.
For instance, Duke University re-structured its multiple clinical research job descriptions to streamline job titles and progression pathways using a competency-based, tiered approach. This led to advancement pathways and impacted institutional turnover rates in relevant research-related positions.{5,6} Other large clinical research sites or contract research organizations (CROs) have structured their onboarding and training according to clinical research core competencies. Indeed, major professional organizations and U.S. National Institutes of Health initiatives have adopted the Joint Task Force for Clinical Trial Competency as the gold standard approach to organizing training and certification.{7,8}
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as culprits in this turnover.{9} Further, CROs report a significant shortage of clinical research associate (CRA) personnel.{10} Therefore, addressing factors that would help novices gain initial jobs would address an important workforce gap.
This mixed-methods survey study was initiated by a student of a clinical research graduate program at a large Midwest university who wanted to know how to find her first job in clinical research. Current students and alumni of the graduate program were invited to participate in an internet-based survey in the fall semester of 2018 via e-mails sent through the program listservs of current and graduated students from the program’s lead faculty. After the initial e-mail, two reminders were sent to prospective participants.
The survey specifically targeted students or alumni who had worked in clinical research. We purposefully avoided those students with no previous clinical research work experience, since they would not be able to discuss their pathway into the field. We collected basic demographic information, student’s enrollment status, information about their first clinical research position (including how it was attained), and narrative information to describe their professional progression in clinical research. Additional information was solicited about professional organization membership and certification, and about the impact of graduate education on the acquisition of clinical research jobs and/or role progression.
The survey was designed so that all data gathered (from both objective responses and open-ended responses) were anonymous. The survey was designed using the internet survey instrument Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies. REDCap provides an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.{11}
Data were exported to Excel files and summary data were used to describe results. Three questions solicited open-ended responses about how individuals learned about clinical research career options, how they obtained their first job, and their advice to novices seeking their first job in clinical research. Qualitative methods were used to identify themes from text responses. The project was submitted to the university’s institutional review board and was classified as exempt from requiring board oversight.
A total of 215 survey invitations were sent out to 90 current students and 125 graduates. Five surveys were returned as undeliverable. A total of 48 surveys (22.9%) were completed. Because the survey was designed to collect information from those who were working or have worked in clinical research, those individuals (n=5) who reported (in the first question) that they had never worked in clinical research were eliminated. After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate).
The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry. The remaining respondents had worked in clinical research in the past. Collectively, participants’ clinical research experience ranged from less than one to 27 years.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. When comparing entry-level job titles of participants to their current job title, 28 (74%) respondents reported a higher level job title currently, compared to 10 (26%) who still had the same job title.
Twenty-four (65%) respondents were currently working at an academic medical center, with the remaining working with community medical centers or private practices (n=3); site management organizations or CROs (n=2); pharmaceutical or device companies (n=4); or the federal government (n=1).
Three respondents (8%) indicated that their employer used individualized development plans to aid in planning for professional advancement. We also asked if their current employer provided opportunities for professional growth and advancement. Among academic medical center respondents, 16 (67%) indicated in the affirmative. Respondents also affirmed growth opportunities in other employment settings, with the exception of one respondent working in government and one respondent working in a community medical center.
Twenty-five respondents indicated membership to a professional association, and of those, 60% reported being certified by either the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Open-Ended Responses
We asked three open-ended questions to gain personal perspectives of respondents about how they chose clinical research as a career, how they entered the field, and their advice for novices entering the profession. Participants typed narrative responses.
“Why did you decide to pursue a career in clinical research?”
This question was asked to find out how individuals made the decision to initially consider clinical research as a career. Only one person in the survey had exposure to clinical research as a career option in high school, and three learned about such career options as college undergraduates. One participant worked in clinical research as a transition to medical school, two as a transition to a doctoral degree program, and two with the desire to move from a bench (basic science) career to a clinical research career.
After college, individuals either happened across clinical research as a career “by accident” or through people they met. Some participants expressed that they found clinical research careers interesting (n=6) and provided an opportunity to contribute to patients or improvements in healthcare (n=7).
“How did you find out about your first job in clinical research?”
Qualitative responses were solicited to obtain information on how participants found their first jobs in clinical research. The major themes that were revealed are sorted in Figure 1.
Figure 1: How First Jobs in Clinical Research Were Found
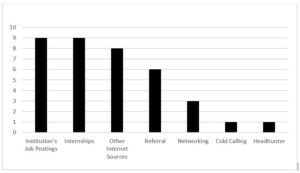
Some reported finding their initial job through an institution’s job posting.
“I worked in the hospital in the clinical lab. I heard of the opening after I earned my bachelor’s and applied.”
Others reported finding about their clinical research position through the internet. Several did not know about clinical research roles before exploring a job posting.
“In reviewing jobs online, I noticed my BS degree fit the criteria to apply for a job in clinical research. I knew nothing about the field.”
“My friend recommended I look into jobs with a CRO because I wanted to transition out of a production laboratory.”
“I responded to an ad. I didn’t really know that research could be a profession though. I didn’t know anything about the field, principles, or daily activities.”
Some of the respondents reported moving into a permanent position after a role as an intern.
“My first clinical job came from an internship I did in my undergrad in basic sleep research. I thought I wanted to get into patient therapies, so I was able to transfer to addiction clinical trials from a basic science lab. And the clinical data management I did as an undergrad turned into a job after a few months.”
“I obtained a job directly from my graduate school practicum.”
“My research assistant internship [as an] undergrad provided some patient enrollment and consenting experience and led to a CRO position.”
Networking and referrals were other themes that respondents indicated had a direct impact on them finding initial employment in clinical research.
“I received a job opportunity (notice of an opening) through my e-mail from the graduate program.”
“I was a medical secretary for a physician who did research and he needed a full-time coordinator for a new study.”
“I was recommended by my manager at the time.”
“A friend had a similar position at the time. I was interested in learning more about the clinical research coordinator position.”
“What advice do you have for students and new graduates trying to enter their first role in clinical research?”
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job.
Respondents stressed the importance of flexibility and persistence (general attitude/approach) when seeking jobs. Moreover, 16 respondents stressed the importance of learning as much as they could about clinical research and gaining as much experience as they could in their jobs, encouraging them to ask a lot of questions. They also stressed a broader understanding of the clinical research enterprise, the impact that clinical research professional roles have on study participants and future patients, and the global nature of the enterprise.
“Apply for all research positions that sound interesting to you. Even if you don’t meet all the requirements, still apply.”
“Be persistent and flexible. Be willing to learn new skills and take on new responsibilities. This will help develop your own niche within a group/organization while creating opportunities for advancement.”
“Be flexible with salary requirements earlier in your career and push yourself to learn more [about the industry’s] standards [on] a global scale.”
“Be ever ready to adapt and change along with your projects, science, and policy. Never forget the journey the patients are on and that we are here to advance and support it.”
“Learning the big picture, how everything intertwines and works together, will really help you progress in the field.”
In addition to learning as much as one can about roles, skills, and the enterprise as a whole, advice was given to shadow or intern whenever possible—formally or through networking—and to be willing to start with a smaller company or with a lower position. The respondents stressed that novices entering the field will advance in their careers as they continue to gain knowledge and experience, and as they broaden their network of colleagues.
“Take the best opportunity available to you and work your way up, regardless [if it is] at clinical trial site or in industry.”
“Getting as much experience as possible is important; and learning about different career paths is important (i.e., not everyone wants or needs to be a coordinator, not everyone goes to graduate school to get a PhD, etc.).”
“(A graduate) program is beneficial as it provides an opportunity to learn the basics that would otherwise accompany a few years of entry-level work experience.”
“Never let an opportunity pass you up. Reach out directly to decision-makers via e-mail or telephone—don’t just rely on a job application website. Be willing to start at the bottom. Absolutely, and I cannot stress this enough, [you should] get experience at the site level, even if it’s just an internship or [as a] volunteer. I honestly feel that you need the site perspective to have success at the CRO or pharma level.”
Several personal behaviors were also stressed by respondents, such as knowing how to set boundaries, understanding how to demonstrate what they know, and ability to advocate for their progression. Themes such as doing a good job, communicating well, being a good team player, and sharing your passion also emerged.
“Be a team player, ask questions, and have a good attitude.”
“Be eager to share your passion and drive. Although you may lack clinical research experience, your knowledge and ambition can impress potential employers.”
“[A] HUGE thing is learning to sell yourself. Many people I work with at my current CRO have such excellent experience, and they are in low-level positions because they didn’t know how to negotiate/advocate for themselves as an employee.”
This mixed-methods study used purposeful sampling of students in an academic clinical research program to gain an understanding of how novices to the field find their initial jobs in the clinical research enterprise; how to transition to a clinical research career; and how to find opportunities for career advancement. There are multiple clinical research careers and employers (see Figure 2) available to individuals working in the clinical research enterprise.
Figure 2: Employers and Sample Careers
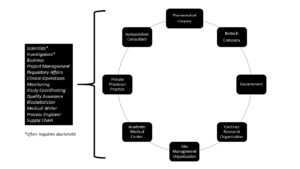
Despite the need for employees in the broad field of clinical research, finding a pathway to enter the field can be difficult for novices. The lack of knowledge about clinical research as a career option at the high school and college level points to an opportunity for broader inclusion of these careers in high school and undergraduate curricula, or as an option for guidance counselors to be aware of and share with students.
Because most clinical research jobs appear to require previous experience in order to gain entry, novices are often put into a “Catch-22” situation. However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
During COVID-19, there may be hiring freezes or furloughs of clinical research staff, but those personnel issues are predicted to be temporary. Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of individualized development planning revealed by our sample may indicate a unique workforce development need across roles of clinical research professionals.
This survey study is limited in that it is a small sample taken specifically from a narrow cohort of individuals who had obtained or were seeking a graduate degree in clinical research at a single institution. The study only surveyed those currently working in or who have a work history in clinical research. Moreover, the majority of respondents were employed at an academic medical center, which may not fully reflect the general population of clinical research professionals.
It was heartening to see the positive advancement in job titles for those individuals who had been employed in clinical research at program entry, compared to when they responded to the survey. However, the sample was too small to draw reliable correlations about job seeking or progression.
Although finding one’s first job in clinical research can be a lengthy and discouraging process, it is important to know that the opportunities are endless. Search in employment sites such as Indeed.com, but also search within job postings for targeted companies or research sites such as biopharmguy.com (see Table 1). Created a LinkedIn account and join groups and make connections. Participants in this study offered sound advice and tips for success in landing a job (see Figure 3).
Table 1: Sample Details from an Indeed.Com Job Search
Note: WCG = WIRB Copernicus Group
Figure 3: Twelve Tips for Finding Your First Job
- Seek out internships and volunteer opportunities
- Network, network, network
- Be flexible and persistent
- Learn as much as possible about clinical research
- Consider a degree in clinical research
- Ask a lot of questions of professionals working in the field
- Apply for all research positions that interest you, even if you think you are not qualified
- Be willing to learn new skills and take on new responsibilities
- Take the best opportunity available to you and work your way up
- Learn to sell yourself
- Sharpen communication (written and oral) and other soft skills
- Create an ePortfolio or LinkedIn account
Being willing to start at the ground level and working upwards was described as a positive approach because moving up does happen, and sometimes quickly. Also, learning soft skills in communication and networking were other suggested strategies. Gaining education in clinical research is one way to begin to acquire knowledge and applied skills and opportunities to network with experienced classmates who are currently working in the field.
Most individuals entering an academic program have found success in obtaining an initial job in clinical research, often before graduation. In fact, the student initiating the survey found a position in a CRO before graduation.
- Sonstein S, Seltzer J, Li R, Jones C, Silva H, Daemen E. 2014. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 28(3):17–23. doi:10.14524/CR-14-00002R1.1. https://acrpnet.org/crjune2014/
- Sonstein S, Brouwer RN, Gluck W, et al. 2018. Leveling the joint task force core competencies for clinical research professionals. Therap Innov Reg Sci .
- Jones CT, Benner J, Jelinek K, et al. 2016. Academic preparation in clinical research: experience from the field. Clinical Researcher 30(6):32–7. doi:10.14524/CR-16-0020. https://acrpnet.org/2016/12/01/academic-preparation-in-clinical-research-experience-from-the-field/
- Jones CT, Gladson B, Butler J. 2015. Academic programs that produce clinical research professionals. DIA Global Forum 7:16–9.
- Brouwer RN, Deeter C, Hannah D, et al. 2017. Using competencies to transform clinical research job classifications. J Res Admin 48:11–25.
- Stroo M, Ashfaw K, Deeter C, et al. 2020. Impact of implementing a competency-based job framework for clinical research professionals on employee turnover. J Clin Transl Sci.
- Calvin-Naylor N, Jones C, Wartak M, et al. 2017. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J Clin Transl Sci 1:16–25. doi:10.1017/cts.2016.2
- Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND). Center for Leading Innovation and Collaboration (CLIC). 2019. https://clic-ctsa.org/diamond
- Clinical Trials Talent Survey Report. 2018. http://www.appliedclinicaltrialsonline.com/node/351341/done?sid=15167
- Causey M. 2020. CRO workforce turnover hits new high. ACRP Blog . https://acrpnet.org/2020/01/08/cro-workforce-turnover-hits-new-high/
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81.
- Gwede CK, Johnson DJ, Roberts C, Cantor AB. 2005. Burnout in clinical research coordinators in the United States. Oncol Nursing Forum 32:1123–30.
A portion of this work was supported by the OSU CCTS, CTSA Grant #UL01TT002733.
Bridget Kesling, MACPR, ( [email protected] ) is a Project Management Analyst with IQVIA in Durham, N.C.
Carolynn Jones, DNP, MSPH, RN, FAAN, ( [email protected] ) is an Associate Professor of Clinical Nursing at The Ohio State University College of Nursing, Co-Director of Workforce Development for the university’s Center for Clinical and Translational Science, and Director of the university’s Master of Clinical Research program.
Jessica Fritter, MACPR, ( [email protected] ) is a Clinical Research Administration Manager at Nationwide Children’s Hospital and an Instructor for the Master of Clinical Research program at The Ohio State University.
Marjorie V. Neidecker, PhD, MEng, RN, CCRP, ( [email protected] ) is an Assistant Professor of Clinical Nursing at The Ohio State University Colleges of Nursing and Pharmacy.
Sorry, we couldn't find any jobs that match your criteria.

Barriers to Clinical Trial Enrollment: Focus on Underrepresented Populations

Using Simulation to Teach Research

An Approach to a Benefit-Risk Framework
You are using an outdated browser. Please upgrade your browser to improve your experience.
Health & Nursing
Courses and certificates.
- Bachelor's Degrees
- View all Business Bachelor's Degrees
- Business Management – B.S. Business Administration
- Healthcare Administration – B.S.
- Human Resource Management – B.S. Business Administration
- Information Technology Management – B.S. Business Administration
- Marketing – B.S. Business Administration
- Accounting – B.S. Business Administration
- Finance – B.S.
- Supply Chain and Operations Management – B.S.
- Accelerated Information Technology Bachelor's and Master's Degree (from the School of Technology)
- Health Information Management – B.S. (from the Leavitt School of Health)
Master's Degrees
- View all Business Master's Degrees
- Master of Business Administration (MBA)
- MBA Information Technology Management
- MBA Healthcare Management
- Management and Leadership – M.S.
- Accounting – M.S.
- Marketing – M.S.
- Human Resource Management – M.S.
- Master of Healthcare Administration (from the Leavitt School of Health)
- Data Analytics – M.S. (from the School of Technology)
- Information Technology Management – M.S. (from the School of Technology)
- Education Technology and Instructional Design – M.Ed. (from the School of Education)
Certificates
- Supply Chain
- Accounting Fundamentals
- View all Business Degrees
Bachelor's Preparing For Licensure
- View all Education Bachelor's Degrees
- Elementary Education – B.A.
- Special Education and Elementary Education (Dual Licensure) – B.A.
- Special Education (Mild-to-Moderate) – B.A.
- Mathematics Education (Middle Grades) – B.S.
- Mathematics Education (Secondary)– B.S.
- Science Education (Middle Grades) – B.S.
- Science Education (Secondary Chemistry) – B.S.
- Science Education (Secondary Physics) – B.S.
- Science Education (Secondary Biological Sciences) – B.S.
- Science Education (Secondary Earth Science)– B.S.
- View all Education Degrees
Bachelor of Arts in Education Degrees
- Educational Studies – B.A.
Master of Science in Education Degrees
- View all Education Master's Degrees
- Curriculum and Instruction – M.S.
- Educational Leadership – M.S.
- Education Technology and Instructional Design – M.Ed.
Master's Preparing for Licensure
- Teaching, Elementary Education – M.A.
- Teaching, English Education (Secondary) – M.A.
- Teaching, Mathematics Education (Middle Grades) – M.A.
- Teaching, Mathematics Education (Secondary) – M.A.
- Teaching, Science Education (Secondary) – M.A.
- Teaching, Special Education (K-12) – M.A.
Licensure Information
- State Teaching Licensure Information
Master's Degrees for Teachers
- Mathematics Education (K-6) – M.A.
- Mathematics Education (Middle Grade) – M.A.
- Mathematics Education (Secondary) – M.A.
- English Language Learning (PreK-12) – M.A.
- Endorsement Preparation Program, English Language Learning (PreK-12)
- Science Education (Middle Grades) – M.A.
- Science Education (Secondary Chemistry) – M.A.
- Science Education (Secondary Physics) – M.A.
- Science Education (Secondary Biological Sciences) – M.A.
- Science Education (Secondary Earth Science)– M.A.
- View all Technology Bachelor's Degrees
- Cloud Computing – B.S.
- Computer Science – B.S.
- Cybersecurity and Information Assurance – B.S.
- Data Analytics – B.S.
- Information Technology – B.S.
- Network Engineering and Security – B.S.
- Software Engineering – B.S.
- Accelerated Information Technology Bachelor's and Master's Degree
- Information Technology Management – B.S. Business Administration (from the School of Business)
- View all Technology Master's Degrees
- Cybersecurity and Information Assurance – M.S.
- Data Analytics – M.S.
- Information Technology Management – M.S.
- MBA Information Technology Management (from the School of Business)
- Full Stack Engineering
- Web Application Deployment and Support
- Front End Web Development
- Back End Web Development
3rd Party Certifications
- IT Certifications Included in WGU Degrees
- View all Technology Degrees
- View all Health & Nursing Bachelor's Degrees
- Nursing (RN-to-BSN online) – B.S.
- Nursing (Prelicensure) – B.S. (Available in select states)
- Health Information Management – B.S.
Health and Human Services – B.S.
- Psychology – B.S.
Health Science – B.S.
- Healthcare Administration – B.S. (from the School of Business)
- View all Nursing Post-Master's Certificates
- Nursing Education—Post-Master's Certificate
- Nursing Leadership and Management—Post-Master's Certificate
- Family Nurse Practitioner—Post-Master's Certificate
- Psychiatric Mental Health Nurse Practitioner —Post-Master's Certificate
- View all Health & Nursing Degrees
- View all Nursing & Health Master's Degrees
- Nursing – Education (BSN-to-MSN Program) – M.S.
- Nursing – Leadership and Management (BSN-to-MSN Program) – M.S.
- Nursing – Nursing Informatics (BSN-to-MSN Program) – M.S.
- Nursing – Family Nurse Practitioner (BSN-to-MSN Program) – M.S. (Available in select states)
- Nursing – Psychiatric Mental Health Nurse Practitioner (BSN-to-MSN Program) – M.S. (Available in select states)
- Nursing – Education (RN-to-MSN Program) – M.S.
- Nursing – Leadership and Management (RN-to-MSN Program) – M.S.
- Nursing – Nursing Informatics (RN-to-MSN Program) – M.S.
Master of Healthcare Administration
- MBA Healthcare Management (from the School of Business)
- Business Leadership (with the School of Business)
- Supply Chain (with the School of Business)
- Accounting Fundamentals (with the School of Business)
- Back End Web Development (with the School of Technology)
- Front End Web Development (with the School of Technology)
- Web Application Deployment and Support (with the School of Technology)
- Full Stack Engineering (with the School of Technology)
- Single Courses
- Course Bundles
Apply for Admission
Admission requirements.
- New Students
- WGU Returning Graduates
- WGU Readmission
- Enrollment Checklist
- Accessibility
- Accommodation Request
- School of Education Admission Requirements
- School of Business Admission Requirements
- School of Technology Admission Requirements
- Leavitt School of Health Admission Requirements
Additional Requirements
- Computer Requirements
- No Standardized Testing
- Clinical and Student Teaching Information
Transferring
- FAQs about Transferring
- Transfer to WGU
- Transferrable Certifications
- Request WGU Transcripts
- International Transfer Credit
- Tuition and Fees
- Financial Aid
- Scholarships
Other Ways to Pay for School
- Tuition—School of Business
- Tuition—School of Education
- Tuition—School of Technology
- Tuition—Leavitt School of Health
- Your Financial Obligations
- Tuition Comparison
- Applying for Financial Aid
- State Grants
- Consumer Information Guide
- Responsible Borrowing Initiative
- Higher Education Relief Fund
FAFSA Support
- Net Price Calculator
- FAFSA Simplification
- See All Scholarships
- Military Scholarships
- State Scholarships
- Scholarship FAQs
Payment Options
- Payment Plans
- Corporate Reimbursement
- Current Student Hardship Assistance
- Military Tuition Assistance
WGU Experience
- How You'll Learn
- Scheduling/Assessments
- Accreditation
- Student Support/Faculty
- Military Students
- Part-Time Options
- Virtual Military Education Resource Center
- Student Outcomes
- Return on Investment
- Students and Gradutes
- Career Growth
- Student Resources
- Communities
- Testimonials
- Career Guides
- Skills Guides
- Online Degrees
- All Degrees
- Explore Your Options
Admissions & Transfers
- Admissions Overview
Tuition & Financial Aid
Student Success
- Prospective Students
- Current Students
- Military and Veterans
- Commencement
- Careers at WGU
- Advancement & Giving
- Partnering with WGU
HEALTHCARE CAREER GUIDES
Clinical Research Coordinator Career
What is a clinical research coordinator.
Clinical research coordinators (CRCs) manage everything from participant recruitment to data collection. They’re responsible for directing the day-to-day activities involved in a diverse range of scientific inquiries, including drug trials, epidemiological investigations, genetic testing, and observational studies. CRCs help maintain a study's overall quality and integrity, ensuring that all systems and procedures adhere to informed consent laws, ethical standards, and federal regulations established by the Food and Drug Administration (FDA). Another central aspect of their work involves facilitating communication between the research team and the Institutional Review Board (IRB), an administrative body that safeguards participants’ rights. Before an organization can initiate a study, research coordinators must submit the study to the IRB for approval and make any requested modifications. A clinical research coordinator’s job is highly collaborative. Working closely with principal investigators, study sponsors, and regulatory agencies, these professionals promote goal alignment and foster a spirit of teamwork throughout the research process.
RESPONSIBILITIES
What Does a Clinical Research Coordinator Do?
Clinical research professionals’ work responsibilities can vary. A typical day often involves the following tasks:
- Recruiting patients to participate in clinical trials and research studies.
- Explaining the risks and potential benefits to participants so they can provide informed consent.
- Answering patients’ questions and addressing any of their concerns.
- Preparing and submitting reports detailing research practices to the IRB and FDA.
- Scheduling appointments and medical procedures.
- Ensuring that clinical studies comply with all relevant laws and regulations.
- Enabling smooth communication between the study subjects and the clinical staff.
- Conducting baseline assessments and patient interviews.
- Collecting and organizing data, including patient medical histories, study procedures, and test results.
- Maintaining updated documentation for regulatory authorities.
- Drafting reports about adverse events and protocol deviations.
- Managing the inventory of laboratory supplies and equipment.
- Assisting with grant applications, expense tracking, and participant reimbursement.

Where Does a Clinical Research Coordinator Work?
Clinical research coordinators play a crucial role in the healthcare industry. These professionals work in a variety of settings, including:
- Academic medical centers
- Universities
- Pharmaceutical companies
- Biotech companies
- Private research clinics
- Government agencies
- Nonprofit organizations
EDUCATION & BEST DEGREES
How do i become a clinical research coordinator .
The educational requirements for a clinical research coordinator position can differ based on the organization and job responsibilities. Employers typically seek candidates with at least a bachelor’s degree in a science or medical field. Many clinical research coordinators have educational backgrounds in public health, biology, health science , health and human services , biomedical technology, or healthcare administration . A graduate degree such as a master’s in public health may be required for senior positions or specialized roles. Individuals seeking to enhance their expertise and career prospects can also pursue professional certifications such as the Certified Clinical Research Professional (CCRP) and Certified Clinical Research Coordinator (CCRC) certifications.

Best Degrees for a Clinical Research Coordinator
An online health degree program for students who are committed to making a...
An online health degree program for students who are committed to making a difference for patients in a variety of ways.
- Time: 63% of students finish this program in 24 months
- Tuition: $4,085 per 6-month term
- Courses: 35 total courses in this program
Skills for your résumé that you will learn in this program:
- Epidemiology
- Community and Public Health
- Cultural Awareness
- Pathophysiology
- Healthcare Values and Ethics
- Substance Abuse Support
This degree allows you to work inside the healthcare industry, while also directly working with patients who need help.
An online health science program designed for students who want real-world...
An online health science program designed for students who want real-world skills for valuable health careers.
- Time: 63% of students finish similar programs in 24 months.
- Tuition: $4,210 per 6-month term
- Courses: 28 total courses in this program
- Disease prevention
- Behavioral health
- Substance abuse support
- Health research
- Medical technology
This degree prepares you with relevant industry skills and experience that will help you move forward in your healthcare career.
A master's focused on managing comprehensive, value-based care, directly...
A master's focused on managing comprehensive, value-based care, directly in line with innovations in health and healthcare.
- Time: 60% of grads finish within 21 months.
- Tuition: $4,755 per 6-month term.
- Courses: 12 total courses in this program.
- Collaborative Leadership
- Healthcare Models and Systems
- Healthcare Financial Management
- Enterprise Risk Management
- Healthcare Information Technology
Your rich experience in a health-related field can mean more when you bring a master's level of understanding to the problems that organizations need to solve.
Compare degrees
This program is not the only degree WGU offers designed to create leaders in the field of healthcare. Compare our health leadership degrees.

How Much Does a Clinical Research Coordinator Make?
According to Salary.com, the average annual salary for clinical research coordinators is $69,974 . Professionals in this field typically earn between $60,108 and $80,825 a year. However, the top 10% of earners can make more than $90,705. Salaries can vary depending on several factors, including geographic location, industry, work experience, certifications, and education. Clinical research coordinators with significant on-the-job experience can expect to earn higher wages than those just starting their careers.

What Is the Job Outlook for a Clinical Research Coordinator?
Advancements in technology and increased funding for scientific research have led to a growing demand for clinical research coordinators who can manage medical studies. From 2022 to 2032, the job growth for natural sciences managers, including research coordinators, is projected to increase by 5% . This is nearly twice the average growth rate for all occupations, meaning the job outlook for clinical research coordinators is favorable. According to the Bureau of Labor Statistics (BLS), there will be about 6,500 clinical research coordinator openings each year during this period.
What Skills Does a Clinical Research Coordinator Need?
Because the role involves a diverse set of responsibilities, clinical research coordinators need a combination of communication abilities, technical expertise, and managerial skills. Critical aptitudes for this job include:
- Technological proficiency. Research institutions use digital management systems, electronic investigator site files, electronic health record systems, and other technologies to automate tasks and organize information.
- Interpersonal communication. Clinical research coordinators utilize excellent communication skills to explain complex study protocols to subjects and to collaborate with interdisciplinary teams.
- Cultural sensitivity. Because they work with patients from diverse backgrounds, clinical research coordinators must understand cultural nuances and be respectful of differing beliefs.
- Medical knowledge. A basic understanding of medical terminology and healthcare practices helps facilitate smooth communication between research coordinators, investigators, and patients.
- Data management. Because they manage vital details about participants and procedures, clinical research coordinators should be adept at collecting and organizing data.
- Regulatory knowledge. To ensure compliance, clinical research coordinators need a comprehensive understanding of the laws, regulations, and standards involved in clinical research.
- Writing. Clinical research coordinators compose reports about research progress, adverse events, study outcomes, and compliance issues.
- Organization. Keeping orderly records of appointment schedules, research procedures, regulatory documentation, assessment data, and other information is essential to a research coordinator’s job.
- Time management. Clinical research coordinators often direct multiple studies simultaneously, so they must prioritize tasks and manage their time effectively.
- Adaptability . Study modifications, conflicts of interest, budgetary constraints, and other unexpected challenges are common, so research coordinators should be able to adapt to changing circumstances.
Our Online University Degree Programs Start on the First of Every Month, All Year Long
No need to wait for spring or fall semester. It's back-to-school time at WGU year-round. Get started by talking to an Enrollment Counselor today, and you'll be on your way to realizing your dream of a bachelor's or master's degree—sooner than you might think!
Next Start Date {{startdate}}
Interested in Becoming a Clinical Research Coordinator?
Learn more about degree programs that can prepare you for this meaningful career.
The University
For students.
- Student Portal
- Alumni Services
Most Visited Links
- Business Programs
- Student Experience
- Diversity, Equity, and Inclusion
- Student Communities
How to become a clinical research coordinator
Is becoming a clinical research coordinator right for me.
The first step to choosing a career is to make sure you are actually willing to commit to pursuing the career. You don’t want to waste your time doing something you don’t want to do. If you’re new here, you should read about:

Still unsure if becoming a clinical research coordinator is the right career path? Take the free CareerExplorer career test to find out if this career is right for you. Perhaps you are well-suited to become a clinical research coordinator or another similar career!
Described by our users as being “shockingly accurate”, you might discover careers you haven’t thought of before.
How to become a Clinical Research Coordinator
Becoming a clinical research coordinator involves a combination of education, relevant experience, and specific skills. Here is a guide on how to pursue a career as a CRC:
- Obtain a Relevant Bachelor's Degree: Most CRC positions require a bachelor's degree in a relevant field such as biology , chemistry , nursing , psychology , or healthcare administration . Some positions might prefer candidates with a degree in a specific area related to the type of clinical research they are involved in.
- Gain Clinical Experience: Having some clinical experience, such as working as a medical assistant, nurse, or laboratory technician, can be highly beneficial. This experience provides a foundation of medical knowledge and an understanding of healthcare practices, which are valuable in the field of clinical research.
- Pursue Additional Certifications (Optional): While not always mandatory, obtaining certifications related to clinical research can enhance your credentials. The Society of Clinical Research Associates (SoCRA) and the Association of Clinical Research Professionals (ACRP) offer certifications such as Certified Clinical Research Professional (CCRP) and Certified Clinical Research Coordinator (CCRC) respectively. These certifications demonstrate your expertise and commitment to the field (see below).
- Gain Entry-Level Experience: Entry-level positions in clinical research, such as research assistant or data entry clerk, can provide valuable experience and help you understand the intricacies of clinical trials. Networking within the clinical research community can also open doors to job opportunities.
- Pursue Advanced Education (Optional): Some CRCs choose to pursue master's degrees (such as Master of Public Health or Master of Clinical Research) to enhance their knowledge and career prospects. Advanced degrees can be particularly useful if you aspire to move into leadership or specialized roles within the clinical research field.
- Develop Relevant Skills: CRCs need a diverse skill set, including attention to detail, organizational skills, communication skills, and the ability to work in a team. Developing these skills, along with a good understanding of medical terminology and research methodologies, is crucial for success in this role.
- Apply for CRC Positions: Keep an eye on job listings from academic institutions, hospitals, research organizations, and pharmaceutical companies. Tailor your resume and cover letter to highlight your relevant experience and skills. Be prepared for interviews that might include questions about your understanding of clinical research protocols, ethical guidelines, and your ability to handle various responsibilities of a CRC.
- Continue Professional Development: Clinical research is a constantly evolving field. Stay updated with the latest developments, regulations, and technologies. Consider attending conferences, workshops, and training programs to enhance your knowledge and skills.
Certifications In the field of clinical research, certifications can enhance your credentials and demonstrate your expertise and commitment to prospective employers. Here are two widely recognized certifications:
- Certified Clinical Research Professional (CCRP) by the Society of Clinical Research Associates (SoCRA): SoCRA offers the Certified Clinical Research Professional (CCRP) certification, which is designed for individuals involved in the conduct and management of clinical trials. To be eligible for the CCRP exam, candidates typically need a combination of education and work experience in clinical research. The certification exam tests candidates on their knowledge of regulations, ethics, clinical trial conduct, and other essential topics. Obtaining CCRP certification demonstrates your expertise in clinical research practices and ethical standards. For more information about the CCRP certification, including eligibility requirements and exam details, you can visit the SoCRA website: SoCRA CCRP Certification.
- Certified Clinical Research Coordinator (CCRC) by the Association of Clinical Research Professionals (ACRP): ACRP offers the Certified Clinical Research Coordinator (CCRC) certification, which is aimed at professionals responsible for the coordination and administration of clinical trials. To be eligible for the CCRC exam, candidates need a combination of education and work experience in clinical research. The CCRC certification exam assesses candidates' knowledge of various aspects of clinical research, including protocol implementation, ethics, and participant safety. For more information about the CCRC certification, including eligibility requirements and exam details, you can visit the ACRP website: ACRP CCRC Certification.
What is a Research Coordinator?
Learn about the role of Research Coordinator, what they do on a daily basis, and what it's like to be one.
- What is a Research Coordinator
- How to Become
- Certifications
- Tools & Software
- LinkedIn Guide
- Interview Questions
- Work-Life Balance
- Professional Goals
- Resume Examples
- Cover Letter Examples
Start Your Research Coordinator Career with Teal
Definition of a Research Coordinator
What does a research coordinator do, key responsibilities of a research coordinator.
- Developing and implementing research protocols and standard operating procedures in collaboration with principal investigators
- Coordinating the recruitment, screening, and enrollment of study participants, ensuring informed consent is obtained and documented
- Managing the scheduling of study visits, tests, and procedures in accordance with research protocols
- Maintaining accurate and detailed records of study activities, including data collection and management
- Ensuring compliance with regulatory requirements, ethical standards, and institutional policies
- Monitoring study activities to safeguard the quality and integrity of research data
- Facilitating effective communication between the research team, participants, and external stakeholders
- Preparing and submitting documentation for institutional review board (IRB) approvals and renewals
- Assisting with the development and management of the study budget and financial records
- Training and supervising research support staff to ensure adherence to study protocols
- Coordinating with laboratories, vendors, and other external partners to manage study supplies and equipment
- Reporting adverse events and protocol deviations to appropriate regulatory bodies and committees
Day to Day Activities for Research Coordinator at Different Levels
Daily responsibilities for entry-level research coordinators.
- Assisting with participant recruitment and obtaining informed consent
- Collecting and managing research data with attention to confidentiality
- Coordinating appointments and maintaining participant follow-up
- Ensuring compliance with study protocols and ethical guidelines
- Handling administrative tasks such as organizing meetings and managing documentation
- Participating in team meetings and contributing to study discussions
Daily Responsibilities for Mid-Level Research Coordinators
- Overseeing day-to-day operations of research studies
- Developing study materials, such as questionnaires and information sheets
- Monitoring study budgets and resources
- Training and supervising junior staff and volunteers
- Ensuring data quality and integrity
- Contributing to the preparation of manuscripts and presentations
Daily Responsibilities for Senior Research Coordinators
- Designing research protocols and methodologies
- Securing funding through grant writing and maintaining relationships with funders
- Leading multidisciplinary research teams and collaborations
- Developing and implementing research strategies aligned with organizational goals
- Ensuring regulatory compliance and ethical conduct across all studies
- Mentoring and developing junior research staff
Types of Research Coordinators
Clinical research coordinator, social science research coordinator, biomedical research coordinator, grant-funded research coordinator, regulatory research coordinator, what's it like to be a research coordinator , research coordinator work environment, research coordinator working conditions, how hard is it to be a research coordinator, is a research coordinator a good career path, faqs about research coordinators, how do research coordinators collaborate with other teams within a company, what are some common challenges faced by research coordinators, what does the typical career progression look like for research coordinators.
How To Become a Research Coordinator in 2024
Related Career Paths
Uncovering insights through data, driving strategic decisions with informed analysis
Unearthing insights and data to drive decision-making, shaping the future of research
Unlocking business insights through data, driving strategic decisions with numbers
Harnessing data power to drive strategic decisions and optimize business performance
Unearthing insights from data, driving strategic decisions with predictive analytics
Transforming data into insights, driving strategic business decisions and growth
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Contemp Clin Trials Commun
- v.32; 2023 Apr
Clinical research coordinators: Key components of an efficient clinical trial unit
Vincenzina mora.
a Unità Coordinamento Trials (UCT) 2, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico A. Gemelli, IRCCS-Università Cattolica del Sacro Cuore, Rome, Italy
Stefania Colantuono
Caterina fanali, alessia leonetti, giulia wlderk, maria antonia pirro, francesca maria calà palmarino, roberta savini, gianluca ianiro.
b CEMAD Digestive Disease Center, Fondazione Policlinico Universitario A. Gemelli IRCCS, Dipartimento di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, Rome, Italy
Antonio Gasbarrini
Multicenter trials involve procedures, rules, and guidelines that require meticulous attention to details and strict adherence to compliance issues. It is widely accepted that the design, implementation, coordination and analysis of modern clinical trials require a multidisciplinary specialist approach [ 1 ]. At the same time, appropriate training and mastery of the competencies that characterize each role of the research process are essential to carry out high-quality clinical and translational research [ 2 , 3 ]. Usually research teams include the principal investigator (PI), sub-investigators (SI), clinical study nurses (CSNs), clinical research coordinators (CRC), also called study coordinators (SC) and other figure as data manager, statisticians, and clinical monitors also called clinical research associates (CRAs).
Although study coordinator figure exists for at least two decades, the CRCs position and their key role in the scenario of clinical trials, have only recently been defined [ [4] , [5] , [6] , [7] ]. While the responsibilities of investigators, sponsors and clinical monitors are well defined by national regulations of clinical trials and international guidelines on Good Clinical Practice, the importance of the CRC position, their responsibilities and main tasks, have not yet been completely described. Moreover, there is limited available evidence on this topic, since most papers mainly address the required skills of a CRC or describe their responsibilities within a specific topic. Moreover, the term data manager and study coordinator have often been used as synonyms for many years and this may have contributed to confusion and delay in CRC precise definition.
Data for this review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles, using the search terms “clinical research coordinator”, “study coordinator”, “research nurse”. Other search engines, as Google Scholar, have been considered in a second review of scientific literature, despite its limitations [ 8 ]. All found papers have been critically evaluated for review. Only article published in English were included; English abstract of other languages papers have been excluded from review when not sufficient to fully describe the study design.
The definition provided by the Association of Clinical Research Professionals states that " A Clinical Research coordinator, Study Site Research Nurse or Study Site Coordinator, works at a clinical research site under the immediate direction of a principal investigator, whose research activities are conducted under Good Clinical Practice regulations. Among other tasks, CRC's perform site preparation, patient screening and recruitment, patient enrolment, conduct and ensure the quality of case report forms, maintain source documents, and ensure site quality" [ 9 ].
This definition clearly separates the roles of CRCs and CSNs from those of data managers, whose activities represent only a part of the multiple tasks that belong to CRCs and nurses, as further described below and summarized in Table 1 .
CRC activities, divided in different areas, modified from Rico-Villademoros F et al. 2004 .
Even though recent literature has focused on the importance of these positions, the figure of CRCs is still likely to be neglected and undervalued in research practice. Moreover, it is possible to find an overlap of the professional figures, because many sites do not have the possibility to hire all the staff necessary to support the research. Therefore, it is frequent to find sub-inv and/or SN that fulfill the role and responsibilities of CRC and this may generate confusion between different roles.
In 2002 a group of American authors referred to CRCs as the invisible hand in clinical research [ 10 ]. They underlined that the CRCs are crucial for the success of clinical research due to their wide range of responsibilities, representing the connection between study sponsor and patients and clinical, regulatory and administrative staff. CRCs’ central position is highlighted in Fig. 1 that shows their specific coordinating role.

Figure shows the central position of CRC and the connections with all the subjects involved in the research machine .
In 2004, a Spanish group performed a survey to elucidate the standard tasks performed by CRCs in oncology clinical trials, to assess their job satisfaction and training needs [ 11 ]. They found some inaccuracies, starting from the definition of their role. More than two-thirds of the respondents were called "Data Managers'' while the title "Clinical Research Coordinator" (i.e. titles that included both terms "research" and "coordinator") appeared in only 3 job titles. As for educational level and training, they generally had a university degree (Medicine, Pharmacy, Biology and Nursing as the most frequent) and received specific training (Oncology, Clinical Research, Clinical Trials, GCP, Statistics, Database preparation & management in the majority of cases). As for the tasks, overall, CRCs seemed to be devoted to what we call "monitoring activities'', including patient registration/randomization, recruitment follow-up, case report form (CRF) completion, collaboration with the CRA, serious adverse events (SAE) reporting, investigator file handling, and preparing the site for and/or attending audits. It was not uncommon for CRCs participating in that survey to deal with the hospital pharmacy. They maintained the required records of study activity, including drug accountability logs, regulatory documents as well as financial records. To a lesser extent, they were also involved in some administrative activities (such as Ethic committee submission and scheduling patient's visits), and various clinical activities (inclusion/exclusion criteria assessment and completion of scales/questionnaires) ( Table 1 ). The CRC ensures that clinical trials run smoothly and preserve data quality, monitors study activity to guarantee protocol compliance and all relevant local, federal, and national regulatory and institutional policies. Although the PI is responsible for the overall conduct of the clinical trial, the CRC is responsible for the daily activities.
CRCs collaborate with the study nurse, but while the role of the clinical study nurse has been defined better during past years, CRCs’ role is still vague. The study nurse is essential in the management of everything related to research participants. The CRC is crucial in the management and coordination of the entire study protocol, from the early stages of the site selection to the close out visit.
Already many years ago, the addition of research nurses to clinical studies was found to have a positive effect on patient enrollment [ 12 ], showing that without a stable, knowledgeable research nurse workforce, the conduction of research is significantly affected. To address the need for educating clinical research nurses to support clinical trials, dedicated training programs were proposed [ 13 ], as the engagement of well-trained and motivated nurses in clinical trials is known to improve patient outcomes [ 14 ].
If the involvement of CSN in clinical trials is a crucial factor, studies have shown that adding a coordinator to a research team significantly improves subject recruitment numbers, subject retention and general study efficiency [ 11 ], increasing general quality in all study phases. A more active involvement of CRCs in the activities of clinical trials, including a more frequent participation to investigators meetings and a deeper contribution in protocol/case report form review and design, is advocated, since the CRC can provide a worthy perspective on the logistics of proposed study procedures. Finally, the involvement of CRCs in such activities will have a positive influence on their job satisfaction. A recent Italian survey, proposed by AIOM CRC Working Group and involving 319 oncology sites [ 15 ], confirmed a direct association between the number of clinical studies and the number of coordinators, whose contribution to the research activities was believed to be essential for trial conduction. More than 80% of participating sites associated the improvement of the quality of clinical research to the implementation of a coordinator as member of the team.
In 2018 a Japanese group examined the present status and the perspectives toward broader contribution of clinical research coordinators by a cross-sectional study [ 16 ]. More than 70% of the respondents were affiliated with medical institutions but were mainly involved in industry-initiated registration trials, mainly for funding issues. Half of the CRCs and other clinical research-related personnel viewed a broadening of CRCs’ involvement in research activities positively. Accordingly, a structured practical program aimed at encouraging such involvement may help expanding and strengthening their contribution into the future. Additionally, it is plausible that a greater involvement of CRCs in clinical research will help to ensure the reliability of investigator-initiated clinical research.
With the increase in regulatory oversight among clinical trials and upcoming novelties in regulatory procedures, the demands and expectations of CRCs have increased and therefore now require additional skills, training, and medical knowledge ( Table 2 ). Even if the role is often considered as administrative, CRCs have, as mentioned above, university education and are highly qualified. However, CRC is still considered a transient position, probably for the lack of professional identity for these roles, leading to unclear training requirements and job criteria.
Table summarizes the most important requested skills for a CRC.
In Italy in particular, the CRC professional profile is not formally recognized by specific certifications, and no formal CRC position exists in institutions’ staff, as required by national health care workforce regulations. This makes it difficult for institutions and policy makers to appraise the demand for CRCs and to respond by allocating the necessary resources [ 17 ].
Competency based training is critical to the task demands of CRCs. Nonetheless, staff turnover is high and opportunities for advancement limited, particularly at academic health centers. Although hiring a CRC represents a significant investment in time and training to properly carry out job responsibilities, turnover in this role is as much costly, mainly due to the time for education and development, marketing and recruitment, loss of productivity during orientation and training, and emotional costs of turnover on current staff. From this point of view, it is advantageous to retain individuals in this position for extended periods, positioning this role as a long-term career [ 18 , 19 ]. A recent paper by Buchanan DA and colleagues [ 20 ] focused on factors associated with job satisfaction and increased retention. These authors found that, although salary and compensation were a significant predictor of retention among this select population of clinical research staff, greater predictors of retention were related to the involvement and relationship with the PI, showing the importance to invest time in building genuine relationships with study staff.
The lack of a well-defined job position and scarce compensation on one hand, and the work overload generated by the continuous updating of regulatory procedures, without structured training and educational programs on the other, are probably the major causes of frustration and dissatisfaction reported from CRCs. In this point of view, some emerging evidences [ 21 ] indicated that the burnout phenomenon, that has been extensively investigated among health care professionals, particularly on physicians and nurses, does not even spare CRCs. Resilience, sleep dysfunction, stress, and incivility experienced from patients/family have been found significant predictors of burnout for oncology CRCs in a recent mixed-method American study [ 22 ].
1. Conclusion
Our review underlines that, being still not well defined, some crucial research roles as CRCs and study nurses are as heterogeneous as their tasks and job positions in different research contexts. As data management activities represent only a very small part of the multiple tasks usually performed by study coordinators, the term Clinical Research Coordinator is certainly more appropriate.
Emerging evidence [ 11 , 15 , 17 , 18 ] confirms that the establishment of a clear job description, the involvement as central positions in the complex research machine, and the standardization and regulation of their job titles are essential to avoid CRCs migration towards the private sector and guarantee academic successful and high-standard quality research.
No specific grant from any funding agency in the public, commercial, or not-for-profit sectors was received for this manuscript.
Author statement
All authors contributed to the concept of the manuscript. VM and SC conceptualized the idea, elaborated draft and revised versions of the manuscript. GW, MAP, RS and FMCP collaborated in bibliographic research and revised it critically, CF and AL edited and revised the manuscript and collaborated to built tables and figure, GI and AG coordinated and integrated work, providing their intellectual contribute to the manuscript. All authors approved this version to be published.
Declaration of competing interest
All authors declare no conflict of interest.
Acknowledgements
A special mention to Dr. D. Piacentini and Dr. R. Galluzzi (Direzione Risorse Umane, Fondazione Policlinico Universitario A. Gemelli, IRCCS) to contribute to define research profiles and job description within Fondazione and to Fondazione Roma (Via del Corso 239, 00187, Rome) for the relevant and continuous support to CEMAD in clinical research and its applications.
Contributor Information
SC&RN group 2 : Pirozzoli Maria Celeste , Giannone Luciana , Spataro Cristina , Graziani Cristina , Capodrossi Anna , Teberino Maria Anna , Tolusso Barbara , Di Ciurcio Marica , Verdirosi Diana , Rotunno Serena , Finotti Ludovica , Turchini Laura , Amatucci Valeria , Schiavoni Elisa , Napolitano Daniele , Durini Eleonora , Strazzeri Martina , Lombardi Maria Teresa , and Schifano Elisabetta
Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)

Thomas Boothby, MS, CCRP CRA II, Boston Scientific
Abstract : Research coordinators may transition to clinical research associates/monitors during their careers. This article provides an overview of how to determine whether it is the right time to make this transition, how to evaluate current competencies and gaps that must be filled in order to make this transition, and how to address needs during the on-boarding process. A roadmap in the form of a checklist is provided to help make the transition from research coordinator to clinical research associate (CRA) a smooth one.
Disclosure: The author has a relevant financial relationship with respect to this article with Boston Scientific, where he is employed as a monitoring CRA.
Introduction
A research coordinator is a person at the clinical research site who is involved in the daily tasks of enrollment, data entry, and all other aspects of clinical trials at the site level. A clinical research associate (CRA), or monitor, is the individual who visits clinical research sites to review their medical records and do the standard monitoring visits. Before the author was a CRA, he was a research coordinator for fourteen years. This article describes how the author made the transition from clinical research coordinator to CRA/clinical research monitor and includes some suggestions for those looking to make a similar career change.
When to Transition from Research Coordinator to CRA
While people naturally want to progress their careers as fast as possible, it is important to only make thetransition from research coordinator to CRA when the time is right. The grass is not always greener on the other side of the work fence.
The author knew that he was ready to make the transition from research coordinator to CRA because he felt that he had mastered all the tasks of a research coordinator. His job became stagnant, and he was looking for something better. Fatigue in the current work environment is another reason for why individuals may be looking to make this transition. Of all members of the clinical research team, research coordinators have the most difficult job. In the author’s opinion, they are often overworked and underpaid, and their contributions to the overall study are sometimes overlooked. Other reasons to make the transition from research coordinator to CRA include potential career progression and the opportunity to try something new. Some individuals may find that the travel component that goes along with being a monitor is a positive as well.
There are five stages of change according to a behavioral change model: pre-contemplation, contemplation, determination, action, and maintenance. In the pre-contemplation phase, people are not thinking about transitioning yet or may have obstacles in their daily lives that are preventing them from exploring new opportunities. When people are becoming serious about change, they are in the determination or action phases. During these phases, research coordinators who want to transition to CRAs might apply for new positions or become certified clinical research professionals (SOCRA CCRP®) as they try to gain new skills for the job market. When considering a transition from research coordinator to CRA, it is important to identify one’s place in the behavioral change model.
Qualifications and Background of CRAs
When the author was applying for CRA positions in 2015, he always saw a requirement for at least two years of experience as a monitor. This requirement is often a barrier to those looking to make this career transition. In 2010, ClinicalTrials.gov listed more than 100,000 clinical studies. By 2019, that number has increased to more than 300,000 clinical studies. The clinical research market has exploded over the last decade. More people are needed to monitor and to run clinical studies now than ever before. While some companies are less likely to require two years of monitoring experience now due to a depleted pool of candidates, these same companies may be more open to supplemental forms of experience such as certifications, course work, and on-the-job experience.
Thus, this is a great time to act on the decision to transition from research coordinator to CRA. From 2014 to 2024, the United States Bureau of Labor Statistics estimates that CRA positions will increase 14% annually. This increase in the job market, coupled with the high level of CRA turnover, could lead to a very strong job market in the future. At Boston Scientific, turnover among CRAs is fairly low due to the strong structure and principles. Many CRAs within Boston Scientific have been with the company for 10 to 20 years or longer.
Table 1 highlights the typical background of CRAs. Most CRAs are current or former nurses who have experience as a research coordinator or a research assistant. Many universities now offer bachelor’s, master’s, and certificate programs in clinical research as another form of training for these research related roles. In Michigan, where the author is from, Eastern Michigan University has a two-year master’s degree program in clinical research. Like the author, CRAs can often be a former research coordinator.
When the author was transitioning from research coordinator to CRA, he got his foot in the door by working closely with a monitor who still works for Boston Scientific. Relationships between research coordinators and CRAs can be contentious due to the nature of monitoring. Research coordinators should treat monitors and sponsor staff well and with respect, and they should treat monitoring visits as a learning opportunity and not a criticism of the coordinator’s work. These relationships do not need to be contentious. A good working relationship with a clinical research site’s CRAs can serve as a potential audition for a monitoring position.
CRAs typically have a clinical research certification, either SOCRA’s certified clinical research professional (CCRP®) or the Association of Clinical Research Professionals-Certified Professional (ACRP-CP). Some companies provide tuition reimbursement for programs and certifications such as these as a way of employee enhancement. Research coordinators can participate in enrichment programs such as these and obtain certifications to help boost their resume and become more marketable to CROs and sponsors. When researching these programs, individuals must do their due diligence to ensure that the program or certification is offered by a legitimate organization and is accredited. Hiring managers know where to find the gold standards in clinical research programs and certifications, and those that do not fit this standard can even be viewed as a negative on ones resume.
The author is a SOCRA CCRP®, Certified Clinical Research Professional, which is an excellent indicator of knowledge for a monitoring position. The test includes knowledge of the regulations and the role of the monitor. There are also some CRO-development programs such as SOCRA’s Clinical Research Monitoring Conference and one-year certificate programs such as the Harvard Medical School global clinical scholar’s research training program.
Networking through the clinical research site’s CRAs and professional forums and groups such as SOCRA is a great way to find CRA positions and interact with other research professionals. At conferences, CROs often have booths in the exhibit hall where research coordinators can meet CRO staff, learn more about opportunities, and leave their resume with CRO staff.
A Typical Day in the Life of a CRA
The life of a CRA has its positives and negatives (Table 2). There are many things that the author wishes he knew before he became a monitor. The author works from home a great deal of the time. If he is not on the road visiting a clinical research site, he is working at home either preparing for a visit, writing follow-up visit letters, or performing other administrative work. Visit preparation and follow up is a crucial part of the home office work. CRAs have very strict compliance guidelines for completing monitoring visits and monitoring reports in a timely manner. Since recently becoming a lead CRA, the author has also been doing a great deal of administrative and compliance work with more of a global view of a clinical trial.
Some clinical research organizations (CROs) and sponsors have onsite monitors who can do remote visits and activities. Whether visits are onsite or remote, monitors are constantly in contact with clinical research sites to follow up on action items from monitoring visits or to answer protocol specific questions the site coordinators may have.
At most companies, about 60-80% of the monitor’s time is spent traveling to sites. The author currently covers all of Michigan, and he has covered other areas, including Wisconsin, New York, Pennsylvania, and Ohio. CRAs are often away for several days at a time depending on the current workload. This can be difficult on families and personal relationships. While the author travels extensively, there are some times when he travels more than others. Sometimes he does back-to-back visits and may be gone for several days at a time. After that, he may be home for several days. The extensive travel required of CRAs is a key consideration when exploring this career transition.
Being a CRA takes a great deal of self-discipline. Monitoring offers a flexible work arrangement, so monitors can work later in the day or take time off during the normal workday. However, if the CRA does not accomplish what he/she should accomplish, this will be glaringly obvious. Management and co-workers will immediately know if the CRA does not show up to meetings or has difficulty answering questions about his/her monitoring activities or their monitor role in general.
Starting a Monitoring Job
Boston Scientific has a rigorous onboarding process comprised of four to six months of training. After the author was hired as a CRA, he spent months learning the work instructions and going out on preceptor visits. In the beginning, the new CRA observes a senior CRA. Over time, the new CRA does more of the monitoring. By the end of the training, the new CRA is doing the monitoring visit, and the senior CRA is observing and making suggestions to the new hire on how the new hire can improve.
There are various levels of monitors at Boston Scientific: CRA I (for new hires), CRA II, and senior CRA. More experienced CRAs often mentor new CRAs. It is extremely helpful to find CRAs who can serve as mentors and answer questions.
CROs and sponsors have many systems that CRAs must learn. At Boston Scientific, these systems include electronic data capture, clinical trial management, auxiliary programs to help remote employees, and cloud-based filing systems. Being a CRA might be very difficult for people who are resistant to change or have difficulty with technology.
There are several types of monitoring (Table 3). The author would be considered a traditional CRA or monitor. By this, he does traditional onsite monitoring via annual or semi-annual visits to clinical research sites based upon the study’s monitoring plan. At smaller organizations, monitors may travel more often or may have an expanded territory to cover. It is important to ask how much travel is involved and how many monitors are on the team during the interview process. If a company has fewer monitors, more travel will be involved.
Many Boston Scientific protocols require annual monitoring visits. The author visits his clinical research sites at minimum once a year but generally 2-3 times per year. Some of the more difficult sites, high enrollers, and those that are more likely to be inspected by the U.S. Food and Drug Administration are monitored more often. Many sites are participating in more than one Boston Scientific study. For example, the author monitors a site in New York that is conducting several studies. He will monitor two studies during one visit. This saves him time and travel and saves the company money by reducing travel costs. Boston Scientific also uses a risk-based monitoring strategy.
In-house regulatory CRAs at Boston Scientific, called trial management CRAs, interact with the sites on regulatory matters, study startup, and study closure. They work primarily by email and lean on traditional CRAs such as the monitor to be the face of the company with the research coordinators and help ensure that tasks are completed on time. Many hospitals also run their own clinical studies and may have in-house monitors.
Boston Scientific does use remote monitoring in certain studies and circumstances. Remote monitoring takes a great deal of work and technological experience at both the sponsor and site level. It involves a great deal of scanning and correspondence by the research coordinators, which can take a lot of their time and resources.
Sponsor CRAs generally deal with one indication, while CRO CRAs can work on studies for different indications or therapies. In one month, for example, CRO CRAs may be doing four indications at four sites for four sponsors. This requires understanding a great deal of information and being able to use different systems. Good organization is key when working as a CRA, whether for a sponsor or a CRO.
Recently, the author progressed from a CRA II to a senior CRA. As a senior CRA, the author has a larger leadership role and is expected to participate more in training and mentoring other CRAs. Boston Scientific has some centralized monitoring that will look at certain metrics and internal documents to guide monitors in their daily monitoring activities. Monitors are closely linked to the trial managers who actually run the studies. They also deal with safety and data managers as well as their CRA manager and the director of operations. Boston Scientific recently created an associate clinical trial manager position as a way to slowly transition some staff members into clinical trial managers, and the author is also transitioning into this role.
One common drawback about this transition process from research coordinator to CRA is that a CRA is one step removed from patient care. Working directly with patients as a research coordinator is something that the author misses. It is important to remember that CRAs help protect patients who are participating in clinical studies at more of an indirect level. This ideology helps prevent burnout, especially when monitors are swamped with the many reports that are necessary as part of the monitoring process.
Checklist for Transitioning from Research Coordinator to CRA
Table 4 has a checklist for determining whether one is ready to make the transition from research coordinator to CRA. Prior to applying for positions, the research coordinator must consider his/her stage in the behavior change model. Unless the research coordinator is ready to transition to a CRA position, he/she should not do it. Becoming a CRA can be difficult without two to five years of research experience in medical devices, pharma, or academia in some capacity. A research coordinator who wants to transition to a CRA should work closely with current CRAs who can provide mentoring and networking opportunities as well as exploring other networking avenues such as SOCRA and ACRP forums, LinkedIn, and also attending the annual events or local events put on by these organizations.
It is important for research coordinators to bolster their resumes by completing supplemental training or certifications. Resumes should be up-to-date and attractive to potential employers. This means including details about accomplishments along with basic information such as job titles and education.
The research coordinator must also consider the travel demands of a CRA position, the types of monitoring to pursue, and his/her stage in the behavior change model. Travel is a major part of a CRA position and should be a focal point of your conversation with a hiring representative. Finally, the types of monitoring including central monitoring, remote monitoring, and regional monitoring should be considered.
Monitoring is a great job. It allows a lot of freedom. However, CRAs also have a great deal of responsibility. CRAs must be driven, willing to put in the time, and have the necessary work ethic while maintaining vigilance and holding others accountable for good clinical practices.
Typical Background of a CRA
- Nursing degree with a clinical research background
- Bachelor’s or master’s degree in clinical research
- Former/current research coordinator
- Clinical experience (medical assistant, registered nurse, or nurse practitioner)
- Clinical research certified ( SOCRA CCRP ® or ACRP-CP)
- Research experience/background
- Science/academic research background
The Life of a CRA
- This requires being self-motivated and driven
- Sometimes performing a combination of onsite and remote monitoring
- Email, etc.
- At times, CRAs are gone for several days at a time depending on current workload
- Visit preparation and follow-up is a crucial part of work at home
Types of Monitoring
- Annual or semi-annual visits based upon the monitoring plan
- Risk-based monitoring/central monitoring
- Remote monitoring
- In-house CRAs and regulatory CRAs
- Sponsor CRA/monitor
- CRO CRA/monitor
Checklist for Transitioning from a Clinical Research Coordinator
to a Monitoring CRA (Clinical Research Associate)
- 2-5 years of research experience as a research coordinator or research assistant
- Able to work with current CRAs as part of a mentorship or network with CRAs
- Completion of supplemental training or certifications to support career goals and bolster resume
- Explore networking avenues
- Up-to-date resume that is attractive to potential employers
- Able to meet travel demands of a CRA position
- Consideration of types of monitoring to pursue
- Stage in the behavior change model
7 thoughts on “Career Progression in Clinical Research: Transitioning from a Clinical Research Coordinator to a Monitoring Clinical Research Associate (CRA)”
Your articles are always helpful and I always get something new to learn from them.
Research Update Organization
you are always giving something new. thank you for that.
Very clear and helpful article! The tables listing the different types of monitoring roles and the items to consider whether this is right transition are a great summary, too.
- Pingback: An overview of a career in clinical research: What jobs are available in this field? - Rebiz Zield
This is a very clear and collective article. The tables are the best helpful tips and resources for anyone interested for career advancement in clinical research. I really appreciate the author’s time and effort to sharing this article.
- Pingback: Career Growth Opportunities in Clinical Research
- Pingback: Introduction to Clinical Research: What Every Student Should Know - Drug Safety and Pharmacovigilance Course

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Mayo Clinic Careers
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Internal Medicine
- Lung Transplant
- Psychiatry & Psychology
- Nurse Practitioner & Physician Assistant
- Ambulance Service
- Clinical Labs
- Med Surg RN
- Radiology Imaging
Clinical Research Coordinator
- Respiratory Care
- Senior Care
- Surgical Services
- Travel Surgical Tech
- Practice Operations
- Administrative Fellowship Program
- Administrative Internship Program
- Career Exploration
- Nurse Residency and Training Program
- Nursing Intern/Extern Programs
- Residencies & Fellowships (Allied Health)
- Residencies & Fellowships (Medical)
- SkillBridge Internship Program
- Training Programs & Internships
- Diversity, Equity & Inclusion
- Employees with Disabilities
You're using Internet Explorer - therefore, some pages or features may not display properly. We recommend switching to a modern browser such as Chrome, Microsoft Edge, or Firefox for a smoother experience.
Search life-changing careers..
Search by Role or Keyword
Enter Location
- United States Applicants
- United Kingdom Applicants
- Current Employees
- Phoenix, AZ
Not ready to apply? Join our talent community
Receives direction from principal investigator, supervisor, or other staff involved in research protocol(s). Gives direction to and works cooperatively with other research staff. Collaborates with various departments within the institution. Works cooperatively with other investigators and personnel at all levels. Interacts with research participants, other research centers, and sponsoring companies to resolve problems and ensure efficient completion of research studies. Position Overview: (Major Functions and Non-Essential Functions): Independently coordinates complex (i.e. interventional, therapeutic greater than minimal risk) clinical research protocols with minimal direction from the principal investigator and/or supervisor in compliance with regulatory laws and institutional guidelines. Collaborates with research team to assess feasibility and management of research protocols. Ensures implementation of research protocols after IRB approval and provides information as appropriate for progress reports. Screens, enrolls, and recruits research participants. Coordinates schedules and monitors research activities and subject participation. Identifies, reviews, and reports adverse events, protocol deviations, and other unanticipated problems appropriately. Manages, monitors, and reports research data to maintain quality and compliance. Provides education/training for others within the department. Performs administrative and regulatory duties related to the study as appropriate. Some travel may be required. ADDENDUM (if applicable) Protocol Development and Maintenance Activities Responsibilities may include, but are not limited to: ongoing management of the protocol document and process through editing, amendments, proofing, coordination of study logistics (i.e. blood collection kits, data collection booklets, use of CRU, etc.), and verification of content to meet institutional and federal standards; communication with study sites and/or federal agencies regarding study status changes; Federal and Institutional Review Board (IRB) document preparation and submission; and provides consultative expertise regarding regulatory and policy requirements. Accurately applies investigators' scientific data into a cohesive format for the protocol document and associated procedures that are consistent with internal and external policies and regulatory requirements. Participates in other protocol development activities and executes other assignments as warranted and assigned.
*This position does not support visa sponsorship
Minimum Education and/or Experience Required: (Education Requirements and Experience): HS Diploma with at least 5 years of clinical research coordination/related experience OR Associate's degree/college Diploma/Certificate Program with at least 3 years of experience, Associate's in Clinical Research from an accredited academic institution without experience OR Bachelor's with at least 1 year of experience or completion of a Mayo Clinic-sponsored clinical research internship in lieu of 1 year of experience. Experience should be in the clinical setting or related experience. Additional Experience and/or Qualifications: (Has Achieved Competency in the Following Areas, Job Knowledge and Additional Considerations): Graduate or diploma from a study coordinator training program is preferred. One year of clinical research experience is preferred. Medical terminology course is preferred. Licensure/Certification Required: N/A
About our location
Phoenix, Arizona
We would love to connect with you.
Click the button for a list of our upcoming events.
Join Our Talent Community
Sign up, stay connected and get opportunities that match your skills sent right to your inbox
Email Address
Phone Number
Upload Resume/CV (Must be under 1MB) Remove
Job Category* Select One Advanced Practice Providers Business Education Engineering Executive Facilities Support Global Security Housekeeping Information Technology Internship Laboratory Nursing Office Support Patient Care - Other Pharmacy Phlebotomy Physician Post Doctoral Radiology Imaging Research Scientist Surgical Services Therapy
Location Select Location Albert Lea, Minnesota Arcadia, Wisconsin Austin, Minnesota Barron, Wisconsin Bloomer, Wisconsin Caledonia, Minnesota Cannon Falls, Minnesota Chippewa Falls, Wisconsin Decorah, Iowa Duluth, Minnesota Eau Claire, Wisconsin Fairmont, Minnesota Faribault, Minnesota Holmen, Wisconsin Jacksonville, Florida La Crosse, Wisconsin Lake City, Minnesota London, England Mankato, Minnesota Menomonie, Wisconsin Minneapolis-St. Paul-Bloomington, Minnesota New Prague, Minnesota Onalaska, Wisconsin Osseo, Wisconsin Owatonna, Minnesota Phoenix, Arizona Prairie du Chien, Wisconsin Red Wing, Minnesota Rice Lake, Wisconsin Rochester, Minnesota Saint Cloud, Minnesota Saint James, Minnesota Scottsdale, Arizona Sparta, Wisconsin Tomah, Wisconsin Waseca, Minnesota Zumbrota, Minnesota
Area of Interest Select One Nursing Research Radiology Laboratory Medicine & Pathology Facilities Licensed Practical Nurse (LPN) Neurology Pharmacy Cardiovascular Medicine Surgery Physical Medicine & Rehabilitation General Services Psychiatry & Psychology Respiratory Therapy Finance Environmental Services Mayo Collaborative Services Ambulance Services Mayo Clinic Laboratories Surgical Technician Anesthesiology & Perioperative Medicine Emergency Medicine Social Work Gastroenterology & Hepatology Information Technology Family Medicine International Hospital Internal Medicine Medical Oncology Orthopedics Radiation Oncology Global Security Housekeeping Patient Scheduling Cardiovascular Surgery Education Hematology Pediatrics Senior Care Transplant Administration General Internal Medicine Hospice & Palliative Care Obstetrics & Gynecology Ophthalmology Critical Care Office Support Pulmonary/Sleep Medicine Engineering Dermatology Urology Artificial Intelligence & Informatics Biochemistry & Molecular Biology Desk Operations Oncology Physiology & Biomedical Engineering Sports Medicine Clinical Genomics Community Internal Medicine Health Care Delivery Research Linen & Central Services Nephrology & Hypertension Surgical Assistant Digital Endocrinology Immunology Infectious Diseases Neurosciences Otolaryngology (ENT) Quality Regenerative Biotherapeutics Rheumatology Business Development Cancer Center Epidemiology Molecular Medicine Molecular Pharmacology & Experimental Therapeutics Neurologic Surgery Primary Care Clinical Trials & Biostatistics Development/Philanthropy Healthcare Technology Management Information Security Legal Marketing Pain Medicine Women's Health Allergic Diseases Bariatric Medicine Cancer Biology Center for Individualized Medicine Clinical Nutrition Communications Comparative Medicine Dental Specialities Geriatric Medicine & Gerontology Informatics Mayo Clinic Platform Occupational/Preventative Medicine Spine Center Spiritual Care Travel Volunteer Services
- Research, Phoenix, Arizona, United States Remove
Confirm Email
By submitting your information, you consent to receive email communication from Mayo Clinic.
Join our talent community.
Join our global talent community to receive alerts when new life-changing opportunities become available.

If you want to know what it's really like at Mayo Clinic, just ask. You'll find that our pride–in where we work, and in what we do–is a common trait. You will also find a lot of inspiring stories about lives changed for the better.
Nurse Residency Program
The Nurse Residency Program (NRP) is for all nurses with less than 12 months of experience, to be completed within the first year. NRP provides a framework for a successful transtion to a professional nurse by promoting educational and personal advancement.

As your career evolves, our compensation and benefits packages are designed to change with you — meeting needs now, and anticipating what comes next. We know that when Mayo Clinic takes care of you, you can take better care of our patients.
Equal opportunity
All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, gender identity, sexual orientation, national origin, protected veteran status, or disability status. Learn more about "EEO is the Law." Mayo Clinic participates in E-Verify and may provide the Social Security Administration and, if necessary, the Department of Homeland Security with information from each new employee's Form I-9 to confirm work authorization.
Reasonable accommodations
Mayo Clinic provides reasonable accommodations to individuals with disabilities to increase opportunities and eliminate barriers to employment. If you need a reasonable accommodation in the application process; to access job postings, to apply for a job, for a job interview, for pre-employment testing, or with the onboarding process, please contact HR Connect at 507-266-0440 or 888-266-0440.
Job offers are contingent upon successful completion of a post offer placement assessment including a urine drug screen, immunization review and tuberculin (TB) skin testing, if applicable.
Recruitment Fraud
Learn more about recruitment fraud and job scams
Advertising
Mayo Clinic is a not-for-profit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
Advertising and sponsorship policy | Advertising and sponsorship opportunities
Reprint permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
Terms and Conditions | Privacy Policy | Notice of Privacy Practices | Notice of Nondiscrimination
© 1998-2024 Mayo Foundation for Medical Education and Research (MFMER). All rights reserved.
Clinical Research Coordinator
Position Status: Full-Time, Exempt Reports to: Director, Clinical Research Location: New York, or Telecommute
Description
The Parkinson's Foundation (PF) makes life better for people with Parkinson’s disease by improving care and advancing research toward a cure. In everything we do, we build on the energy, experience, and passion of our global Parkinson's community.
A key focus of the Foundation is PD GENEration: Mapping the Future of Parkinson’s Disease. PD GENEration is a national initiative that offers genetic testing for clinically relevant Parkinson's-related genes and genetic counseling at no cost for people with Parkinson’s disease.
The Coordinator of Clinical Research will be an important member on the Clinical Research team, focusing on PD GENEration. This role will work with team members to further develop, implement, and coordinate the research, operational, and administrative activities for the successful management of PD GENEration and other clinically focused research programs sponsored by the Parkinson’s Foundation. Working within the Research Programs department, the Coordinator of Clinical Research will perform diverse duties requiring analysis, sound judgment, and a high level of knowledge of clinical research protocols.
Responsibilities
Responsibilities include, but are not limited to the following:
- Understand and interpret research protocols and procedures and man required documentation.
- Understand regulatory compliance and site management process in accordance with ICH Good Clinical Practices, FDA guidelines, local regulations, and Parkinson’s Foundation standard Operating procedures.
- Monitor the collection and recording of study participant and project intervention data performing regular checks to ensure accuracy of the data consistent with the study protocol.
- Develop and maintain collaborative relationships with investigational sites.
- Maintain an overview of relevant research findings.
- Contribute to conference presentations, posters, and scientific manuscripts.
- Participate in the publication of significant results.
- Interact with other departments within the Foundation.
- Prepare and write reports using Word, PowerPoint, and Excel.
- Perform other duties as assigned.
Experience/Skills Required
- Bachelor's degree in a science discipline.
- Minimum of two years of experience as a Research Associate or Research Coordinator.
- Strong collaboration in working with various professionals including researchers, physicians, nurses, and other clinical staff.
- Ability to handle and manage multiple collaborative projects of high complexity.
- Ability to complete assignments accurately and with attention to detail.
- Ability to analyze, organize and prioritize work.
- Ability to process and handle confidential information with discretion.
- Experience with Electronic Data Capture (EDC) systems.
- Experience with electronic databases (data entry, data cleaning, database development) is a plus.
- Ability to successfully work in an independent manner and/or in a collaborative team environment.
- Excellent communication skills.
- Able to follow verbal and written instructions.
- CITI and/or CCRP certification is a plus.
- Any appropriate combination of relevant education, experience and/or certifications may be considered.
- Demonstrated ability to collaborate, multi-task and work effectively in a fast-paced environment.
- Demonstrates the organizational values of excellence, teamwork, collaboration, integrity, positivity, dedication, and responsiveness.
Compensation
Range: $40,000-$50,000/year
Salary for this position depends on prior experience. In addition, a comprehensive benefits package is included.
How to Apply
Please email resume and cover letter to [email protected] . Applicant review will continue until the position is filled. Please indicate the job title in the subject line. Resumes without cover letters will not be considered. No phone calls please.
The Parkinson’s Foundation is an equal opportunity employer. We are committed to diversity, equity, and inclusion in our culture and in our work on behalf of people with Parkinson's disease.
All new hires are required to be fully vaccinated against the COVID-19 virus, subject to any legally required accommodations.
Parkinson's Connection
Personal information.

Role of Clinical Research Coordinator (CRC) in Clinical Trial
- Post author: pacifixresearch
- Post published: May 9, 2022
- Post category: Uncategorized
- Post comments: 7 Comments

We are writing about what clinical research coordinators do in their day to day work life. We want to go through their practical day to day work life experience as a clinical research coordinator but before that you should know about clinical research and What is the role of Clinical Research Coordinator
What is Clinical Research?
Clinical research is the study of health and illness in people. It is the way we learn how to prevent, diagnose and treat illness. Clinical research describes many different elements of scientific investigation. Simply put, it involves human participants and helps translate basic research done in labs into new treatments and information to benefit patients. Clinical trials as well as research in epidemiology, physiology and pathophysiology, health services, education, outcomes and mental health can all fall under the clinical research and also Clinical research refers to all research carried out on humans, healthy or sick people. It focuses on improving knowledge of diseases, developing diagnostic methods and new treatments or medical devices to ensure better patient care. It is very framed and respects a precise study protocol and is only realized under certain conditions
What is the role of Clinical Research Coordinator?
The clinical research coordinator career path is involved in supervising all successful drug trials and medical research. A clinical research coordinator is required to gather patients for medicinal trials by recruiting them and verifying them to be sure they fit the guidelines for trials. Additionally, he/she is also responsible for ensuring that the materials and supplies to be used in the trials are to be kept safe before and after the trials. Along with this, a clinical research coordinator is also responsible for maintaining proper documentation recorded during the trial period and also the clinical research coordinator reports primarily to the Principal Investigator with associated responsibilities to the department head, division administrator or program administrator. A coordinator is required to make sure that trials being conducted meet all the guidelines and regulations including those regarding safety, government rules and regulations, and company or hospital ethics and also is required to keep a track of patients in terms of health and progress in order to report them to the company. As a coordinator, one is also responsible for finding funds for their research, either through private funds or grants. Not only coordinators are required to apply for the grant, but also figure out how much grant or funding they require. Cost analysis comes as an integral part of a coordinator’s job. A coordinator is responsible for figuring out the budget that includes how much the research will cost, as well as payroll, travel, supplies, technology, pharmacy, and other costs that will affect the budget of the trial
What does a clinical research coordinator do in a day to day work life?
As you all know we will not talk about the theoretical part here we want to go through their practical day to day work life experience as a clinical research coordinator. Supposedly as a clinical research coordinator their day starts at 9.00 am and work is completely based on the phase of trial for example Initiation, recruitment, or closing of the trial. So we are writing this article for the recruitment phase trial, patient recruitment
The 1st work which they do is checking the emails. they might have received emails from CRO- CRA/CTA/Managers, sponsors, Principle Investigators or any other stakeholder and they check patient visits also if any. Considering the patient recruitment priority, he/she visits the hospital OPD to confirm the eligible patients where sub investigators prescreen the patients. They always prepare a plan for each patient visit as per the protocol requirement to avoid protocol deviations. If the patient is eligible, the PI process for ICF as per applicable guideline. As coordinator, their work is to coordinate all the patient processes. After completion of ICF consent, they connect the laboratory and other applicable departments e.g. X-ray, ECG, or any other department. During this process, they start documenting the ICF narrative. ICF narrative is a key element to document the entire process of ICF and screening. Once this coordination is complete, they schedule their work for eCRF data entry. As they are using the EDC system, hence they transcribe all the source data to eCRF. eCRF is an electronic case report form where they enter the subject data like screening visits for example laboratory values, vital signs, and trial indication details or, visit 1-N number of visit details, EOF (end of treatment). Apart from this they update screening and pre-screening logs, check if all the Case Report Forms, ICFs, and other questionnaires are complete and signed by PI. All the visits are completed by patients. Inform protocol deviation or protocol violation if any to PI and report to IEC, Maintain all trial related documents like Protocol Submission Report, ICFs, CRFs and protocols with updated versions, protocol approval letter and other essential documents in site master file. Review budget and update PI. Submission of continuing review application to IEC every 6 months. They also help PI for submitting Protocol submission forms and complete CTRI registration for new studies. Receive calls from patients, PIs, Co-Is and reply to their queries.Other activities include to attain meetings or trainings with PI. To check if stationary and copies of ICF, CRF and questionnaire are in stock. To courier documents to other sites. their day ends by 5.30 pm, most of the time days are hectic.
During this COVID-19 pandemic patients were not able to visit the hospital, patients called them and they answered their queries by discussing with PI. Also as a COVID-19 precautionary measure all the coordinators are assigned COVID-19 screening duties twice or thrice a month, with PPE kit worn. They measure the temperature of patients and their relatives or ask them Hospital’s standard COVID-19 questions, mark their answers before they enter the hospital or navigate patients inside the hospital. They stay in continuous contact with CRAs also for site monitoring, site close out, qualification or initiation visits. In a nutshell, as a CRC, their work is screening process, ICF narrative, source documentation, eCRF data entry, Shipment management, communication with CRO and site file management. This is only an overview of their work as clinical research coordinator. Apart from this you should know What is the work environment of a Clinical Research Coordinator like and their Employment Shifts or Employment Nature and what is the WorkPlace and Presence in Geographical Area and also you should know about Weekly Hours of Work and Overtime Details or Time Pressure
The work environment or Employment Nature of a Clinical Research Coordinator:
A career as a clinical research coordinator requires working in treatment rooms in hospitals and medical centers during different diagnosis and trials, and they also work in research laboratories. Individuals who opt for a career as a clinical research coordinator are expected to be up to date with the patient’s medical history and current health status. Before beginning the diagnosis and trial, the clinical research coordinator and the team determines which treatment equipment and techniques are to be used. This section contains in-depth details about the work environment of the clinical research coordinator. An individual in a clinical research coordinator career path involves employment on a permanent basis. Hence, the nature of his/her work is permanent. Medical centers or third-party medical companies employ clinical research coordinators for permanent posts.
Employment Shifts and Presence in Geographical Area:
Typically, clinical research coordinator jobs require working overtime 60-hour per week, and also might be required to be present on call on some nights, weekends, and holidays. Depending on the treatment, call responsibilities can get rigorous. In certain facilities that perform a large number of trials, a clinical research coordinator may work in shifts to ensure that a trained staff is available 24 hours a day.
Tier-1 cities such as New Delhi, Gurugram, Chennai, Kolkata, Hyderabad, Bengaluru, Pune are hubs for world class healthcare treatments making it a big hub for opportunities for healthcare workers working in several sectors. Clinical research coordinators in Chennai, Tamil Nadu earn an average of 10.6 percent more than the national average. The clinical research coordinators also find higher than average salaries in Bangalore, Karnataka (4.4 per cent more). The lowest salaries can be found in Mumbai, Maharashtra (11.5 percent less).
Weekly Hours of Work and Overtime Details or Time Pressure:
Usually, a clinical research coordinator is scheduled to work eight hours per day starting from 9 AM to 8 PM. However, depending on the requirements and the diagnosis and trial schedules, the clinical research coordinator might be required to work under certain shifts. Quite often, a clinical research coordinator is required to work overtime. Clinical research coordinators are required to work under various circumstances that involve long hours and weekends too. In a career as clinical research coordinator, individuals might also be required to report to medical emergencies. Hence working overtime is quite common in their profession. A clinical research coordinator is mainly required to work in treatment rooms in hospitals and medical centers during general diagnosis and trials, along with this, they also work in research laboratories. The clinical research coordinator jobs for clinical research coordinators vary greatly as they must answer the call of medical emergencies when needed. They might work overtime if the diagnosis and trial stretch to be too long.
Please Share This Share this content
- Opens in a new window
You Might Also Like
Top 10 clinical research organizations (cros) in the world – 2021, the ultimate guide to choosing the right medical coding course, artificial intelligence (ai) and machine learning (ml) in discrepancy management (clinical data management), this post has 7 comments.
Respected Sir/Ma’am
My name is Aliya Malik Khan looking for job opportunities in the field of Clinical Research. I completed my M.Sc in Clinical Research from the Institute of Clinical Research India, Pune in 2022.
Some of my personal qualities which you may find useful are:
– Ability to learn quickly coupled with innovative ideas for problem-solving.
– Ability to work in a team with strong communication skills.
– Hardworking and sincere towards work with an ability to take directions.
Please find my detailed CV for your consideration. If you need any more details, please do let me know. Thanking you for your time and looking forward to hearing from you.
Aliya Malik Khan [email protected] +91 8390038086
Itís difficult to find knowledgeable people in this particular subject, however, you seem like you know what youíre talking about! Thanks
I was very pleased to find this site. I wanted to thank you for your time for this particularly wonderful read!! I definitely savored every little bit of it and i also have you book-marked to see new stuff in your site.
There are some fascinating points in time in this article but I don?t know if I see all of them heart to heart. There is some validity but I will take maintain opinion till I look into it further. Good article , thanks and we wish extra! Added to FeedBurner as properly
I am so happy to read this. This is the kind of manual that needs to be given and not the random misinformation that is at the other blogs. Appreciate your sharing this best doc.
Oh my goodness! an incredible article dude. Thanks Nonetheless I am experiencing challenge with ur rss . Don’t know why Unable to subscribe to it. Is there anybody getting similar rss drawback? Anyone who is aware of kindly respond. Thnkx
Leave a Reply Cancel reply
Save my name, email, and website in this browser for the next time I comment.

Clinical Research Coordinator Associate
🔍 school of medicine, stanford, california, united states.
The Division of Cardiovascular Medicine , within Stanford University Department of Medicine, is a dynamic and innovative center dedicated to excellence in research, medical education, and clinical care. Our Division is driven by over 90 faculty members and a cadre of staff who are the pillar of strength in the Division's ongoing efforts into the prevention and treatment of cardiovascular disease.
We are seeking a Clinical Research Coordinator Associate (CRCA) who is passionate about clinical research and wants to deliver results. The CRCA will work with a robust clinical research team, hand in hand with Principal Investigators, Clinical Research Managers, Associates, and other stakeholders in support of cardiac patients.
The Clinical Research Coordinator Associate will be responsible for the overall implementation of an assigned set of research protocols assuring efficiency and regulatory compliance. Other responsibilities will include recruiting, screening, assisting in the informed consent process and enrolling subjects in accordance with good clinical practice guidelines as well as collecting, recording and maintaining complete data files using good clinical practice in accordance with HIPAA regulations. He/she will participate in data retrieval, reporting, and preparation of files and Case Report Forms for the various studies. Other duties may include processing of blood samples, reporting serious adverse events and maintenance of drug accountability, supplies and equipment.
CV Med Clinical Research is a growing, dynamic team who is dedicated to supporting translational medicine and contributing to Stanford Medicine’s mission. If you are eager to quickly achieve lasting results, we invite you to join our team.
Duties include:
- Serve as primary contact with research participants, sponsors, and regulatory agencies. Coordinate studies from start-up through close-out.
- Determine eligibility of and gather consent from study participants according to protocol. Assist in developing recruitment strategies.
- Coordinate collection of study specimens and processing.
- Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms.
- Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents. Prepare regulatory submissions, and ensure Institutional Review Board renewals are completed.
- Assemble study kits for study visits, monitor scheduling of procedures and charges, coordinate documents, and attend monitoring meetings with sponsors, acting as primary contact.
- Monitor expenditures and adherence to study budgets and resolve billing issues in collaboration with finance and/or management staff.
- Interact with the principal investigator regularly, ensuring patient safety and adherence to proper study conduct.
- Ensure essential documentation and recording of patient and research data in appropriate files per institutional and regulatory requirements.
- Participate in monitor visits and regulatory audits.
DESIRED QUALIFICATIONS:
- Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred.
EDUCATION & EXPERIENCE (REQUIRED):
- Two-year college degree and two years related work experience or a Bachelor's degree in a related field or an equivalent combination of related education and relevant experience.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
- Strong interpersonal skills.
- Proficiency with Microsoft Office.
- Knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
- Working toward certification(s) to perform basic patient measurements and tests, such as phlebotomy and EKG.
PHYSICAL REQUIREMENTS:
- Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
- Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
- Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
WORKING CONDITIONS:
- Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
- May require extended or unusual work hours based on research requirements and business needs.
- Occasional evening and weekend hours.
WORKING STANDARDS:
- Interpersonal Skills: Demonstrates the ability to work well with Stanford colleagues and clients and with external organizations.
- Promote Culture of Safety: Demonstrates commitment to personal responsibility and value for safety; communicates safety concerns; uses and promotes safe behaviors based on training and lessons learned.
- Subject to and expected to comply with all applicable University policies and procedures, including but not limited to the personnel policies and other policies found in the University’s Administrative Guide, http://adminguide.stanford.edu/ .
The expected pay range for this position is $31.73 - $36.54 hourly. Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs.
At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process.
Why Stanford is for You Imagine a world without search engines or social platforms. Consider lives saved through first-ever organ transplants and research to cure illnesses. Stanford University has revolutionized the way we live and enrich the world. Supporting this mission is our diverse and dedicated 17,000 staff. We seek talent driven to impact the future of our legacy. Our culture and unique perks empower you with:
- Freedom to grow. We offer career development programs, tuition reimbursement, or audit a course. Join a TedTalk, film screening, or listen to a renowned author or global leader speak.
- A caring culture. We provide superb retirement plans, generous time-off, and family care resources.
- A healthier you. Climb our rock wall, or choose from hundreds of health or fitness classes at our world-class exercise facilities. We also provide excellent health care benefits.
- Discovery and fun. Stroll through historic sculptures, trails, and museums.
- Enviable resources. Enjoy free commuter programs, ridesharing incentives, discounts and more.
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned.
Consistent with its obligations under the law, the University will provide reasonable accommodations to applicants and employees with disabilities. Applicants requiring a reasonable accommodation for any part of the application or hiring process should contact Stanford University Human Resources at [email protected]. For all other inquiries, please submit a contact form .
Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
- Schedule: Full-time
- Job Code: 1013
- Employee Status: Regular
- Requisition ID: 103289
- Work Arrangement : On Site
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Research
Post Date: May 07, 2024
Post Date: Jan 29, 2024
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs

Clinical Trial Research Coordinator Job Description [Updated for 2024]
In the fast-paced world of medical advancement, the role of clinical trial research coordinators has become increasingly crucial.
As medical research advances, the need for competent professionals who can manage, supervise, and ensure the smooth conduct of clinical trials is more necessary than ever.
But what exactly is expected from a clinical trial research coordinator?
Whether you are:
- A job seeker trying to understand the intricacies of this role,
- A hiring manager outlining the perfect candidate,
- Or simply curious about the nuts and bolts of clinical trial coordination,
You’ve come to the right place.
Today, we present a customizable Clinical Trial Research Coordinator job description template, crafted for effortless posting on job boards or career sites.
Let’s dive right into it.
Clinical Trial Research Coordinator Duties and Responsibilities
Clinical Trial Research Coordinators are responsible for managing and coordinating all aspects of conducting clinical trials.
They work closely with Principal Investigators, and other research staff to ensure the safety and well-being of the study participants as well as the integrity of the data collected.
They have the following duties and responsibilities:
- Assist in the planning and design of clinical trials
- Ensure that all clinical trials are conducted in accordance with the study protocol, standard operating procedures, good clinical practice and applicable regulatory requirements
- Identify and recruit potential study participants
- Conduct informed consent processes with the study participants
- Coordinate and perform routine and complex procedures such as blood draw, processing and shipping, and data collection
- Monitor patients’ health and side effects throughout the trial
- Record, manage and maintain data and source documents
- Prepare and submit status reports to the relevant bodies
- Ensure adequate supply of study materials by liaising with suppliers or sponsors
- Assist in the preparation, submission and maintenance of regulatory documents
- Provide education to patients and their families about the clinical trial
Clinical Trial Research Coordinator Job Description Template
We are seeking a dedicated Clinical Trial Research Coordinator to oversee all aspects of clinical trials.
Your responsibilities will include coordinating and supervising the execution of clinical trials, ensuring compliance with trial protocols, and overseeing data collection and reporting.
Our ideal candidate has a strong understanding of Good Clinical Practices (GCP), excellent organizational skills, and experience in a clinical research environment.
Ultimately, your role is to ensure our clinical trials contribute to our understanding and treatment of medical conditions.
Responsibilities
- Coordinate the day-to-day activities of clinical trials
- Ensure compliance with study protocols and overall clinical objectives
- Prepare and organize study documentation, including informed consent forms, case report forms (CRFs), and other materials
- Monitor patient safety and report adverse events
- Assist with recruitment and screening of study participants
- Coordinate and manage data collection, ensuring accuracy and confidentiality
- Liaise with investigators, study sponsors, and other healthcare professionals
- Prepare progress reports for the ethics committee or institutional review board
- Ensure all trial activities adhere to regulations and standards
Qualifications
- Proven experience as a Clinical Research Coordinator
- Knowledge of Good Clinical Practices (GCP) and relevant regulatory guidelines
- Excellent organizational and communication skills
- Strong numeric skills and ability to handle vast amounts of data
- Proficient in MS Office (especially Excel)
- Degree in nursing, life sciences or related field
- Certification as a Clinical Research Coordinator (e.g. ACRP or SOCRA) is a plus
- Health insurance
- Dental insurance
- Retirement plan
- Paid time off
- Professional development opportunities
Additional Information
- Job Title: Clinical Trial Research Coordinator
- Work Environment: Hospital or clinical research facility. May involve some travel to attend industry conferences or meetings.
- Reporting Structure: Reports to the Clinical Trial Manager or Principal Investigator.
- Salary: Salary is based upon candidate experience and qualifications, as well as market and business considerations.
- Pay Range: $60,000 minimum to $80,000 maximum
- Location: [City, State] (specify the location or indicate if remote)
- Employment Type: Full-time
- Equal Opportunity Statement: We are an equal opportunity employer and value diversity at our company. We do not discriminate on the basis of race, religion, color, national origin, gender, sexual orientation, age, marital status, veteran status, or disability status.
- Application Instructions: Please submit your resume and a cover letter outlining your qualifications and experience to [email address or application portal].
What Does a Clinical Trial Research Coordinator Do?
Clinical Trial Research Coordinators work predominantly in the healthcare and pharmaceutical sectors, specifically in areas involving human trials of new drugs and treatments.
They are primarily responsible for managing and coordinating clinical trials to ensure they are conducted safely, ethically, and in accordance with regulatory standards.
This involves the planning, execution, and supervision of clinical trials.
Their duties often include designing and implementing research protocols, developing data collection systems, and ensuring the correct collection, analysis, and reporting of data.
They liaise with the principal investigator and other members of the research team to ensure the study runs smoothly.
Clinical Trial Research Coordinators are also involved in the recruitment and screening of study participants, ensuring they meet the eligibility criteria.
They are responsible for ensuring informed consent is obtained from all participants and safeguarding their rights and wellbeing throughout the trial.
They are often the main point of contact for study participants, providing them with information about the trial, answering their questions, and addressing any concerns.
Furthermore, Clinical Trial Research Coordinators often have administrative duties such as managing budgets, procuring supplies, and maintaining documentation and records related to the trials.
They may also be required to prepare and submit reports to regulatory bodies.
In addition, they must stay updated with the latest research in their field and continually seek to improve the processes and procedures involved in conducting clinical trials.
Clinical Trial Research Coordinator Qualifications and Skills
A competent Clinical Trial Research Coordinator should possess the following skills and qualifications:
- Understanding of clinical trial principles, research methodologies, and medical terminologies to accurately conduct trials and interpret results.
- Strong organizational skills to effectively manage research data, patient records, and other documentation related to the trial.
- Exceptional communication skills to liaise with the trial participants, investigators, research personnel, and ethics committees, ensuring clear and accurate information flow.
- Attention to detail to ensure all protocols, procedures, and regulations are strictly adhered to during the trial process.
- Data analysis skills for interpreting and presenting research findings in an understandable manner.
- Proficient in using clinical research management systems and other computer software for data collection and analysis.
- Problem-solving skills to handle any issues that may arise during the trials, ensuring that the trials’ integrity is maintained.
- Ability to work well under pressure, demonstrating strong time management skills when meeting important deadlines.
- Empathy and interpersonal skills to interact with patients, making them feel comfortable and respected during the trial process.
Clinical Trial Research Coordinator Experience Requirements
Entry-level Clinical Trial Research Coordinators may have 1 to 2 years of experience, often gained through an internship or part-time role in clinical research.
These professionals can also gain hands-on experience in roles such as Clinical Research Associate, Data Analyst, or other health-related roles.
Candidates with 2-5 years of experience have often expanded their skills and knowledge in clinical research, patient recruitment, data management, and regulatory compliance.
These professionals are expected to have experience in protocol development, trial monitoring, and patient education.
Those with more than 5 years of experience may have additional responsibilities such as training junior staff, managing budgets, and overseeing multiple clinical trials concurrently.
They are also expected to have experience in dealing with ethical and legal issues related to clinical research.
This level of experience is often required for roles with greater responsibility, such as a Senior Clinical Trial Research Coordinator or Clinical Trial Manager.
In addition to these experience requirements, a Clinical Trial Research Coordinator should have a strong understanding of Good Clinical Practices, and may need specific certifications, depending on the area of research.
A bachelor’s degree in a science or health-related field is often a minimum requirement, and a master’s degree or higher can be advantageous.
Clinical Trial Research Coordinator Education and Training Requirements
Clinical Trial Research Coordinators typically hold a bachelor’s degree in a health science or related field, such as biology, nursing, or public health.
They are expected to have a strong foundation in research methodology, clinical procedures, and regulatory guidelines for clinical research.
Prior experience in clinical research or healthcare can be beneficial, as coordinators often oversee clinical trial procedures and ensure data collection is carried out accurately and ethically.
It’s highly recommended to obtain a certification from a recognized body such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SOCRA).
These certifications demonstrate the coordinator’s commitment and expertise in clinical research coordination.
Some roles may require a master’s degree in a related field, especially those in highly specialized or complex research trials.
In addition to formal education and certification, Clinical Trial Research Coordinators should also have excellent organizational, communication, and problem-solving skills.
They must be comfortable working with a variety of stakeholders, including patients, healthcare providers, and regulatory officials.
Ongoing professional development and keeping abreast of the latest advancements in clinical research are also crucial for success in this role.
Clinical Trial Research Coordinator Salary Expectations
The average salary for a Clinical Trial Research Coordinator is $59,604 (USD) per year.
The actual income can fluctuate based on factors such as experience in the field, location of work, and the organization that they are employed by.
Clinical Trial Research Coordinator Job Description FAQs
What skills does a clinical trial research coordinator need.
Clinical Trial Research Coordinators should have excellent organizational and administrative skills, as they often manage several aspects of a clinical trial.
They also require strong interpersonal and communication skills to interface with patients, researchers, and stakeholders.
It is also necessary for them to have a strong understanding of medical terminology, ethical guidelines, and regulatory requirements in clinical research.
Do Clinical Trial Research Coordinators need a degree?
Yes, Clinical Trial Research Coordinators usually require a bachelor’s degree in a related field such as nursing, life sciences, or public health.
Some roles may also require a master’s degree or further certification.
Additionally, they should have knowledge in research methodologies and Good Clinical Practice guidelines.
What should you look for in a Clinical Trial Research Coordinator’s resume?
In a Clinical Trial Research Coordinator’s resume, look for a background in scientific or health-related fields, research experience, and knowledge of clinical trial procedures.
Certifications such as Certified Clinical Research Professional (CCRP) are also a plus.
Strong skills in project management, communication, and data analysis should also be evident.
What qualities make a good Clinical Trial Research Coordinator?
A good Clinical Trial Research Coordinator should possess strong attention to detail, as they are responsible for maintaining accurate and detailed records.
They should have excellent organizational skills to manage different aspects of clinical trials and strong communication skills to liaise with patients, medical professionals, and other stakeholders.
They should also have strong ethical standards and a commitment to patient safety and confidentiality.
What are the daily duties of a Clinical Trial Research Coordinator?
A Clinical Trial Research Coordinator typically manages and oversees clinical trials’ day-to-day operations.
They liaise with researchers, patients, and other stakeholders, ensure that trials comply with ethical and regulatory standards, and maintain detailed records of trial data.
They may also be responsible for patient recruitment and consent, scheduling patient appointments, and submitting trial results for review.
And that’s a wrap.
Today, we’ve taken a close look at the intricate and crucial role of a clinical trial research coordinator .
It’s not just about conducting trials.
It’s about pioneering medical advancements, one study at a time.
With our comprehensive clinical trial research coordinator job description template and real-world examples, you’re ready to take the leap.
But why end here?
Plunge further with our job description generator . It’s your ideal guide for crafting meticulous job listings or honing your resume to perfection.
Always remember:
Every clinical trial is a stepping stone towards a healthier future.
Let’s pioneer that future. Together.
How to Become a Clinical Trial Research Coordinator (Complete Guide)
The Job Jeers: The Most Hated Jobs That No One Wants
Career Crowns: The Most Prestigious Jobs in the Business World
Weirdly Awesome: Unusual Jobs That Will Blow Your Mind
Bored of the Hustle? Try These Easy Jobs for a Stress-Free Payday!
The Editorial Team at InterviewGuy.com is composed of certified interview coaches, seasoned HR professionals, and industry insiders. With decades of collective expertise and access to an unparalleled database of interview questions, we are dedicated to empowering job seekers. Our content meets real-time industry demands, ensuring readers receive timely, accurate, and actionable advice. We value our readers' insights and encourage feedback, corrections, and questions to maintain the highest level of accuracy and relevance.
Similar Posts
![clinical research coordinator work environment Concert Hall Performer Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/05/concert-hall-performer-job-description-768x512.webp)
Concert Hall Performer Job Description [Updated for 2024]
![clinical research coordinator work environment Art Supplies Retailer Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/04/art-supplies-retailer-job-description-768x512.webp)
Art Supplies Retailer Job Description [Updated for 2024]
![clinical research coordinator work environment Child Welfare Services Assistant Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/05/child-welfare-services-assistant-job-description-768x512.webp)
Child Welfare Services Assistant Job Description [Updated for 2024]
![clinical research coordinator work environment Blockchain Security Engineer Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/04/blockchain-security-engineer-job-description-768x512.png)
Blockchain Security Engineer Job Description [Updated for 2024]
![clinical research coordinator work environment All-Terrain Crane Operator Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/04/all-terrain-crane-operator-job-description-768x512.webp)
All-Terrain Crane Operator Job Description [Updated for 2024]
![clinical research coordinator work environment Animation Voice Artist Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/04/animation-voice-artist-job-description-768x512.webp)
Animation Voice Artist Job Description [Updated for 2024]
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

Clinical Research Coordinator
- 1125908_RR00089993
- Research, Medicine-Pulmy+CCM Research (S2131), NYU Grossman School of Medicine
- Full-Time/Regular
- NYU Grossman School of Medicine (SOM)
- Monday – Friday 9:00 am – 5:00 pm
Apply Now -->
- Research at NYU Langone Health
- Similar Jobs
- Career Mobility
- Employee Stories
ABOUT NYU LANGONE HEALTH
NYU Langone Health is a world-class, patient-centered, integrated academic medical center. Ranked as the #1 Hospital for quality and patient safety by Vizient, Inc., and with 10 clinical specialties among the top 10 in the nation according to U.S. News & World Report , NYU Langone’s culture is rooted in excellence in patient care, education, and research. NYU Langone comprises more than 320 locations throughout the New York area and in Florida, including six inpatient locations , a children’s hospital , and four emergency rooms . Also part of NYU Langone Health is the Laura and Isaac Perlmutter Cancer Center , a National Cancer Institute designated comprehensive cancer center, NYU Grossman School of Medicine , and NYU Grossman Long Island School of Medicine . For more information, go to nyulangone.org , and interact with us on LinkedIn , Glassdoor , Indeed , Facebook , Twitter , YouTube , and Instagram .
Share this job
- Share on Facebook
- Share on Twitter
- Share on Linkedin
- Share by Mail
Working at NYU Langone Health
NYU Langone Health isn’t just a healthcare system; we’re also a research hospital with a mission to advance science and improve healthcare. We offer a collaborative approach to research that spans disciplines, pairing outstanding scientists with exceptional clinical researchers. This approach sparks new ideas and discoveries that lead to innovative advances in preventing, diagnosing, and treating disease. With access to state-of-the-art technologies and facilities, our scientists and researchers have pioneered groundbreaking medical treatments used around the world.
COVID-19 Vaccine Requirement: To protect the safety of our patients, staff, and the community at large effective August 16, 2021 NYU Langone Health requires COVID-19 vaccination of all faculty, staff, voluntary attending physicians, Howard Hughes Medical Institute employees, non-compensated faculty, students, clinical or academic observers, and volunteers, unless granted an approved exemption (in New York State, only medical exemptions shall be considered). --> Salaries shown on independent jobs related websites reflect market averages and do not represent information obtained directly from NYU Langone. We invite and encourage each candidate to discuss salary/hourly specifics during the application and hiring process.
Position Summary:
We have an exciting opportunity to join our team as a Clinical Research Coordinator.
The CRC will join the Critical Care and Resuscitation Research Group led by Dr. Sam Parnia within the Division of Pulmonary, Critical Care and Sleep Medicine. The Clinical Research Coordinator is an active participant in the coordination of critical care and resuscitation research studies from research planning to completion of studies. The Clinical Research Coordinator performs study coordination tasks under the direction of the investigator and works closely with the Senior Research Scientist to ensure overall compliance in the conduct of the study, and adherence to the approved study protocol.
Job Responsibilities:
- Additional responsibilities as needed including: Help in the development of SOPs, policy changes, education sessions, and quality improvement projects
- Work with licensed clinicians to develop tracking forms for all active trials, maintain tracking forms throughout the life cycle of the protocol
- Provide updates to study team members regarding changes to workflow or patient-related specific needs according to protocol modifications
- Schedule participants according to the approved protocol; coordinate with ancillary service providers to ensure patients remain adherent to the protocol
- Prepare for study visits: bio-specimen collection, research ticket preparation, lab and EKG orders, scheduling of biopsies and scans, facilitating, AE and Conmed form completion in conjunction with licensed professional and other tasks as needed.
- Act as primary point of contact for all bio-specimen collections
- Maintain follow up calendar ensuring all survival follow up assessments are completed per the protocol; performs assessments not requiring licensure
- Participates in Pre-Screening activities to identify patients that may be eligible for a clinical trial
- Participates in the feasibility and complexity assessment process for new protocols
- Assists patients in understanding the schedule of assessments according to the approved protocol, coordination of appointments, and ancillary services (under the supervision of a licensed clinician but clinician does not have to be physically present)
- Under the supervision of investigator, may take part in AE and Con Med documentation and reporting activities such as SAE and ECI reports (All documentation must be verified and signed by licensed clinicians before submission and all assessments must be made by a licensed clinician)
- Write research notes in EPIC (may not make assessments)
- Helps compile enrollment packet materials (must be reviewed and signed by investigator)
- Human Subjects’ Research – As applicable, oversee the submission of necessary documents required by the NYU Institutional Board (IRB), NYU Office of Clinical Trials and any other appropriate parties in order to obtain approval to conduct human subjects research (e.g., ensures the update and submission of necessary documents and/or forms to appropriate destination). Might prepare, audit and submit monthly enrollment statistics to the Office of Clinical Trials, and provides other information in timely manner, as necessary.
- Budget – Develops a preliminary draft budget and submit to the Director/Principal Investigator. Reviews sponsor-proposed budget for adequate coverage and recommend changes as appropriate. Assists in the preparation of funding reports to funding agencies and helps identify new potential sponsors/agents for trials and researches. Monitor budget throughout trial.
- Data Management – Responsible for collecting and auditing patient information for the research project(s). This may include abstraction of data from the patient chart (e.g., laboratory or diagnostic test results, surgical/radiation treatments delivered, adverse drug reactions, etc); abstraction of data for publications, or data collection from outside physicians offices. Audits and manages data from and into the database. Prepares forms and reports, compiles and analyses data, statistics, and other materials for reports. Conducts study visits, obtains and documents information within the time frame specified.
- Grants – Prepares and submit grant applications and other grants related an activity such as developing grants applications/proposals and fund raising activities if applicable. Collects and organizes required paperwork for submission if applicable. Follow up and coordinates resolution of all issues progress. Reports to the sponsors to fund medical research in the division.
Minimum Qualifications:
To qualify you must have a Bachelor’s Degree or equivalent combination of education and experience required. Computer literate with good interpersonal, writing and verbal communication skills. 2 years relevant experience required, Effective oral, written, communication, interpersonal skills. Must be able to work under the direction of supervision Ability to identify, analyze and solve problems. Time management skills and ability to work well under pressure. Proficiency in using various Microsoft Office applications such as World, Excel, Access, Power Point and Outlook. Familiar with Internet applications
Qualified candidates must be able to effectively communicate with all levels of the organization.
NYU Grossman School of Medicine provides its staff with far more than just a place to work. Rather, we are an institution you can be proud of, an institution where you’ll feel good about devoting your time and your talents.
NYU Grossman School of Medicine is an equal opportunity and affirmative action employer committed to diversity and inclusion in all aspects of recruiting and employment. All qualified individuals are encouraged to apply and will receive consideration without regard to race, color, gender, gender identity or expression, sex, sexual orientation, transgender status, gender dysphoria, national origin, age, religion, disability, military and veteran status, marital or parental status, citizenship status, genetic information or any other factor which cannot lawfully be used as a basis for an employment decision. We require applications to be completed online. If you wish to view NYU Grossman School of Medicine’s EEO policies, please click here . Please click here to view the Federal “EEO is the law” poster or visit https://www.dol.gov/ofccp/regs/compliance/posters/ofccpost.htm for more information.
NYU Langone Health provides a salary range to comply with the New York state Law on Salary Transparency in Job Advertisements. The salary range for the role is $62400.00 – $67,840 Annually. Actual salaries depend on a variety of factors, including experience, specialty, education, and hospital need. The salary range or contractual rate listed does not include bonuses/incentive, differential pay or other forms of compensation or benefits.
To view the Pay Transparency Notice, please click here
NYU Grossman School of Medicine is one of the nation’s top-ranked medical schools. For 175 years, NYU Grossman School of Medicine has trained thousands of physicians and scientists who have helped to shape the course of medical history and enrich the lives of countless people. An integral part of NYU Langone Health , the Grossman School of Medicine at its core is committed to improving the human condition through medical education, scientific research, and direct patient care. At NYU Langone Health, equity, diversity, and inclusion are fundamental values. We strive to be a place where our exceptionally talented faculty, staff, and students of all identities can thrive. We embrace diversity, inclusion, and individual skills, ideas, and knowledge. For more information, go to med.nyu.edu , and interact with us on LinkedIn , Glassdoor , Indeed , Facebook , Twitter and Instagram .
Other Jobs You May Like
How our employees grow.

- Oct 2017 Sr. Research Technician
- Nov 2019 Principal Research Technician
- June 2023 Data Analyst

- JUNE 2019 Project Associate
- SEPT 2021 Research Coordinator
- MAY 2023 Sr. Research Coordinator

- FEB 2015 Program Associate
- NOV 2015 FGP Medical Secretary
- OCT 2016 Administrative Assistant
- APRIL 2021 Program Coordinator

Ivelisse M.
- JUNE 2006 Cardiothoracic Surgery Intern
- SEPTEMBER 2011 FGP Assistant (Temp), Cardiology Associates
- JANUARY 2012 FGP Assistant, Cardiology Associates
- MARCH 2014 Administrator Coordinator, Department of Surgery
- NOVEMBER 2015 Residency/Fellowship Program Coordinator, Division of Hematology and Medical Oncology
- APRIL 2017 Senior Residency/Fellowship Program Coordinator, Division of Hematology and Medical Oncology
- MARCH 2020 Division Administrator, Hematology and Medical Oncology

- JAN 14, 1998 – OCT 11, 2012 Research Coordinator SM – Psychiatry – VA, Psychiatry MHADRP – Research
- OCT 12, 2012 – AUG 8, 2015 Research Coordinator, Psychiatry – Adult ADHD Programr
- AUG 9, 2015 – PRESENT Sr. Research Coordinator, Psychiatry – Mood & Anxiety Disorders

- JUNE 2015 ParentCorps Educator
- FEB 2018 ParentCorps Manager
- OCT 2020 Associate Director ParentCorps

- OCT 2014 Associate Research Coordinator
- JUNE 2017 Research Coordinator
- FEB 2019 Sr. Research Coordinator
- AUG 2019 Program Manager

- OCT 2017 FGP Secretary I – Intake/Scheduler
- MARCH 2019 Residency/Fellowship Program Coordinator
- NOV 2021 Senior Residency/Fellowship Program Coordinator
What Our Employees Say
- Why Work With Us
- Our Organization
- Career Opportunities
- Job Seeker FAQs
- Patient Care at NYU Langone Health
- Education & Research at NYU Langone Health
Need help accessing the site?
Diversity, equity, and inclusion statement, notice of non-discrimination, aedt bias audit results.
Clinical Research Coordinator I, Cancer Care
Research tech.
- 650 Albany Street, Boston, Massachusetts
Position: Clinical Research Coordinator, Cancer Care
Location: Boston, MA
Schedule: 40 hours per week, Hybrid
At Boston Medical Center (BMC), our diverse staff works together for one goal — to provide exceptional and equitable care to improve the health of the people of Boston. Our bold vision to transform health care is powered by our respect for our patients and our commitment to ensure everyone who comes through our doors has a positive experience.
You’ll find a supportive work environment at BMC, with rich opportunities throughout your career for training, development, and growth and where you’ll have the tools you need to take charge of your own practice environment.
POSITION SUMMARY:
The Clinical Research Coordinator (CRC) will perform research activities using approved techniques: Procures, processes and ships research specimens and conducts patient recruitment, administers questionnaires, abstracts medical records, maintains patient databases, enters data into sponsor-specific electronic data capture forms, performs administrative tasks, assists in grant preparation, and participates with the research team in preparation of data and other reports. Will also assist with audits.
JOB RESPONSIBILITIES:
- Evaluate and track the eligibility of all patients seen in the clinic for eligibility in research studies. Conduct telephone interviews. Schedule patients for study visits.
- Obtain informed consent (for non-treatment studies) and register patients to cohort studies and other protocols.
- Review and abstract the medical records for patients, including review of pathology reports.
- Access patient demographic and clinical information from the clinical systems. Enter information into the appropriate EMR and eCRF systems and departmental systems in an accurate manner.
- Perform clinical tests (Phlebotomy, EKGs). Assist with the coordination of the collection, processing, organization, and storage of biological specimens in the systems
- May be responsible for IRB and regulatory submissions and maintenance of regulatory files
- Maintain on-going communications with research managers and PIs for data collection needs.
- Other duties including send out mailings, take inventory/ordering supplies, monitor and set up equipment
The above statements in this job description are intended to depict the general nature and level of work assigned to the employee(s) in this job. The above is not intended to represent an exhaustive list of accountable duties and responsibilities required
JOB REQUIREMENTS
- A minimum of a Bachelor’s degree is required by time of hire.
EXPERIENCE:
- Prefer experience in clinical research (not to be confused with lab research).
- Previous experience with recruiting subjects, with an understanding of the ethical and technical conduct of research preferred
KNOWLEDGE AND SKILLS:
- Excellent English communication skills (oral and written).
- Cultural sensitivity and comfort with a wide range of social, racial and ethnic populations.
- Proficiency with Microsoft Office applications (i.e. MS Word, Excel, Access, PowerPoint, Outlook) and web browsers.
- Must be able to maintain strict confidentiality of all personal/health sensitive information.
JOB BENEFITS:
- Competitive pay
- Tuition reimbursement and tuition remission programs
- Highly subsidized medical, dental, and vision insurance options
- Career Advancement/Professional Development: Access a wealth of ongoing training and development opportunities that will not only enhance your skills but also expand your knowledge base especially for individuals pursuing careers in medicine or biomedical research.
- Pioneering Research: Engage in groundbreaking research projects that are driving the forefront of biomedical science.
ABOUT THE DEPARTMENT:
As the primary teaching hospital for Boston University Chobanian & Avedisian School of Medicine and BU schools of public health and dentistry, intellectual rigor shapes our inquiries. Our research is led by a belief that skin color, zip code, and financial circumstances shouldn’t dictate health.
Boston Medical Center is an Equal Opportunity/Affirmative Action Employer. If you need accommodation for any part of the application process because of a medical condition or disability, please send an e-mail to [email protected] or call 617-638-8582 to let us know the nature of your request.
Equal Opportunity Employer/Disabled/Veterans
Privacy policy
By clicking Submit, you acknowledge that you are willing to receive messages from Boston Medical Center relating to the status of your application or other recruitment related information to any mobile / cell phone number entered in the form above. You can easily opt-out of receiving messages by replying STOP to any messages you receive.
Not the right fit? Check out these other jobs
Executive director reimbursement & reporting, phlebotomy technician ii - south boston community health center, child life specialist, emergency department, 36 hours (day/evening), general cleaner, evenings and rotating weekends, recovery lpn, brockton behavioral health center, per diem, intern-facilities, refer a friend.
Your information:
Apply for this job now
EEO & Accommodation Statement Boston Medical Center is an equal employment/affirmative action employer. We ensure equal employment opportunities for all, without regard to race, color, religion, sex, national origin, age, disability, veteran status, sexual orientation, gender identity and/or expression or any other non-job-related characteristic. If you need accommodation for any part of the application process because of a medical condition or disability, please send an e-mail to [email protected] or call 617-638-8582 to let us know the nature of your request
E-Verify Program Boston Medical Center participates in the Electronic Employment Verification Program. As an E-Verify employer, prospective employees of BMC must complete a background check and receive medical clearance before beginning their employment at the hospital.
Federal Trade Commission Statement: According to the FTC, there has been a rise in employment offer scams. Our current job openings are listed on our website and applications are received only through our website. We do not ask or require downloads of any applications, or “apps” job offers are not extended over text messages or social media platforms. We do not ask individuals to purchase equipment for or prior to employment. To avoid becoming a victim of an employment offer scam, please follow these tips from the FTC: FTC Tips
Join the BMC Talent Community
Before you go, don't forget to join our talent community!

We use cookies to make your interactions with our website more meaningful. They help us better understand how our websites are used, so we can tailor content for you. For more information about the different cookies we are using, read the Privacy Statement . By continuing to navigate the site, you agree to the use of cookies on our behalf.
- Undergraduate Admission
- Graduate Admission
- Tuition & Financial Aid
- Communications
- Health Sciences and Human Performance
- Humanities and Sciences
- Music, Theatre, and Dance
- IC Resources
- Office of the President
- Ithaca College at a Glance
- Awards and Accolades
- Five-Year Strategic Plan
- Public Health
- Directories
- Course Catalog
- Undergraduate
Board of Trustees Recognizes Faculty Members
Congratulations to the 23 members of the faculty who were awarded promotions and/or tenure by the Ithaca College Board of Trustees at its May meetings.
The biographies of the faculty members were provided by their respective schools.
AWARDED PROMOTION FROM ASSOCIATE PROFESSOR TO PROFESSOR Department of Theatre and Dance Paula Murray Cole (M.F.A. Southern Methodist University) teaches acting, voice, and movement. Her professional work is centered on the development and dissemination of Rasaboxes, a suite of exercises originally devised by Richard Schechner. She co-authored and edited the first book dedicated to the exercises, “Inside the Performance Workshop: A Sourcebook for Rasaboxes and Other Exercises” (Routledge 2023), and co-authored “The Actor As Athlete of the Emotions: The Rasaboxes Exercise” for the book “Movement For Actors (2nd Edition, 2017), edited by Nicole Potter, Barabara Adrian, and Mary Fleischer. She has taught performance workshops at New York University, the University of Tennessee at Knoxville, the Dell’ Arte International School of Physical Theatre, Brown University, and Rose Bruford College and has presented Rasaboxes at conferences and workshops in Israel, Montreal, Turkey, Singapore, China, and Poland.
Department of Occupational Therapy Melinda Cozzolino (P.P.O.T.D. Creighton University) teaches courses in neuroscience, mental health, and research. She received the founding grant for the Center for Life Skills, an interdisciplinary program at Longview for adults with chronic neurological conditions. This program has operated for over 20 years and has provided experiential learning for thousands of students and therapeutic services for hundreds of community members. She is a prolific scholar in the areas of interprofessional education and supporting mental health and is an advocate for mental health at the local, regional, and national levels.
Department of Theatre Studies Chrystyna Dail (Ph.D. University of Maryland) serves as director of the Integrative Core Curriculum. Her area of specialization is theatre history, with research interests in U.S. social activist performance, labor theatre, 20th-century Ukrainian-American performance, and the representation of witches in performance. Her book, “Stage for Action: U.S. Social Activist Theatre in the 1940s,” is part of the Theater in the Americas series published through Southern Illinois University Press, and her chapter, “Driving Race Work: The UAW, Detroit, and Discrimination for Everybody!” is included in the edited collection “Working in the Wings: New Perspectives on Theatre History and Labor.” Additionally, her chapter on Margo Jones is included in the eight-volume book series The Great North American Stage Directors published through Methuen Drama. She is currently writing a book about theatrical stagings of the Salem witchcraft crisis by female-identifying artists, and is the book review editor of Theatre Survey, which is published through Cambridge University Press.
Department of Philosophy and Religion Serge Grigoriev (Ph.D. Temple University) imbues the array of courses that he teaches with his ready sense of humor and his gift for oratory. In his classes, laughter is a regular feature, allowing students to enjoy themselves intellectually as they grapple with complex material. His research focuses on pragmatism and the philosophy of history, and he has published prodigiously, producing original, philosophically significant, and refreshingly readable scholarly work. He has been a generous citizen of the college, bringing thoughtful insights to the H&S Faculty Senate, the C.P. Snow Lecture Series Committee, and the Faculty Grievance Committee, to name just three of his service endeavors.
Department of Management Narges Kasiri (Ph.D. Oklahoma State University) bridges theory and practice in her courses in operations management and business analytics. She has integrated cutting-edge technology, including generative AI, into the curriculum. Her collaborative projects with local businesses allow students to apply their skills in real-world settings, enhancing both their learning experience and IC’s engagement in the community. As a scholar, she has earned prestigious honors such as the Fulbright Innovation Award and a grant from HSBC’s Sustainability Office.
Department of Exercise and Athletic Training Patrick McKeon (Ph.D. University of Virginia) is best described as a teacher/servant/scholar. He teaches both graduate and undergraduate students to better understand research and its application to their clinical practice. He serves the department as the Athletic Training Clinical Education Coordinator, the college as chair of the Institutional Review Board and his profession as an editor of two prestigious professional journals. He is also a well-respected scholar, serving as an Executive Council member of the International Ankle Consortium and mentoring numerous students each year to present their own research at local, regional, and national conferences.
Department of Music Education James Mick (Ph.D. Florida State University) teaches undergraduate and graduate courses in string pedagogy, orchestral rehearsal techniques, instrumental conducting, and the psychology of music teaching and learning. In 2020 he was honored with Ithaca College’s Faculty Excellence Award. Recent all-state orchestra appearances include Alabama, Florida, Georgia, Kentucky, New York, Ohio, and Wyoming. Internationally, he has worked with student ensembles in the United Kingdom and Belgium. He served as music director and conductor of the Rochester Philharmonic Youth Orchestra from 2015 to 2023. During his tenure the RPYO held annual side-by-side performances with the Rochester Philharmonic Orchestra at Eastman Theatre’s Kodak Hall and performed at Carnegie Hall in New York City and the Kennedy Center in Washington D.C. A popular clinician, he has presented at numerous state, regional, and national conferences including the American String Teachers Association National Conference and the Midwest Clinic: An International Band & Orchestra Conference.
Department of Music Theory, History, and Composition Alexander Reed (Ph.D. University of Pittsburgh) is the author of the books “Assimilate: A Critical History of Industrial Music (2013 Oxford University Press) and “Laurie Anderson’s Big Science” (2021 Oxford University Press). He also co-wrote the volume on the They Might Be Giants album “Flood” (2014 Bloomsbury) for the 33 1/3 book series. He has published in the Journal of Popular Music Studies, Popular Music and Society, Perspectives of New Music, the Journal of Popular Music Education, ImageTexT, Music Theory Spectrum, Music Theory Online, and the Journal of Musicological Research. He is founder and former chair of the Popular Music Study Group of the American Musicological Society and has served on the board of the International Association for the Study of Popular Music’s U.S. branch. He has received awards, fellowships, and residencies at the Rock and Roll Hall of Fame, the Mellon Foundation, Contemporary Arts International, and the Association for Recorded Sound Collections. Active as a musician, he has toured internationally and released seven albums with his bands Seeming and ThouShaltNot. He has also produced dozens of records for others, and his work has aired on MTV and in popular television on series such as “Gossip Girl.”
Department of Music Performance Michael Titlebaum (M.M. Eastman School of Music) is a saxophonist/composer/arranger who serves as Director of Jazz Studies at Ithaca College, where he directs the Ithaca College Jazz Ensemble; coaches combos; and teaches jazz saxophone and courses in jazz standards, arranging, repertoire, and pedagogy. In 2010 he founded the Ithaca College Jazz Ensemble Composition Contest. He also teaches and coordinates the jazz area in the IC Summer Music Academy. He is the author of the book “Jazz Improvisation Using Simple Melodic Embellishment,” published by Routledge/Taylor and Francis in 2021. He has performed and given workshops and lectures at numerous state and national conferences, including the Jazz Education Network, the International Society for Improvised Music, the New York State School Music Association, the New York State Band Directors Association, and the Texas Music Educators Association.
Department of Computer Science Doug Turnbull (Ph.D. University of California) teaches across the computer science curriculum, exhibiting a persistent dedication to making his classes accessible and to providing research opportunities to the largest possible number of students. Students appreciate that he involves them in his research as genuine partners and grants them foundations for future careers. His scholarship has earned wide recognition in the form of NSF and NEA grants that have brought more than $600,000 to IC. He has published widely in the area of music information retrieval, and he recently delivered a keynote lecture at a conference in Singapore. In his service, he has continued his efforts to promote undergraduate research, and he serves on the H&S Faculty Senate. He also engages in service to the music information retrieval research community, nationally and internationally.
Department of Media Arts, Sciences, and Studies Andrew Utterson (Ph.D. Birkbeck College) has expertly taught courses across the Screen Studies curriculum including Film Aesthetics and Analysis, Hollywood and American History, and Fiction Film Theory as well as ICC courses and mini-courses for the Finger Lakes Environmental Film Festival, of which he is now co-director. The focus of his scholarship in film history, theory, and criticism is the intersection between film and new media as well as the changing nature of cinema from production to exhibition.
Department of Exercise and Athletic Training Justine Vosloo (Ph.D. West Virginia University) is a model for faculty within helping professions. She has spearheaded significant improvements to the department’s graduate Sport Psychology and Mental Performance programs. She is an outstanding mentor to students as they present their own research within professional journals and at national conferences and when they consult with student-athletes to improve their mental performance. Finally, she has grown to be a well-respected scholar within her profession as evidenced by her recent keynote lecture, “Reflections on cultural humility, inclusion, and belonging: Current trends and future challenges for the practice of sport psychology when considering the COVID-19 pandemic.”
Department of Music Education Baruch Whitehead (Ph.D. Capella University) is the founding director of the Dorothy Cotton Jubilee Singers, which is dedicated to the preservation of the Negro Spiritual. He also founded the Orff-Schulwerk certification program, a music education that views music as a basic system like language, at Ithaca College and Marshall University, and is the past director of the annual Orff Certification Training Course at Boston University. His other areas of expertise include diversity in music education, gospel music and its preservation within mainstream musical settings, African American music, and the music of the Civil Rights movement. He has been a featured speaker/workshop presenter at many state, national, and international conferences, including the International Arts and Humanities conference in Honolulu and MENC, NYSSMA, NJMEA, and the American Orff-Schulwerk Association national conference. He has taught at the World Music Village in Helsinki, Finland, and continues to present workshops on diversity in music education for state, national, and international conferences.
Department of Strategic Communication Cory Young (Ph.D. Bowling Green State University) regularly teaches Crisis Communication, and this topic is the focus of most of her research. She is an organizational communication scholar whose work also explores risk communication and projects on diversity and inclusion. She has served in many capacities, including administrative roles for her department and for the school’s graduate program as well as for the college as a whole, as director of the Honors Program, a member of All-College Tenure and Promotion Committee, and chair of the Faculty Handbook Committee.
AWARDED TENURE AND PROMOTION FROM ASSISTANT TO ASSOCIATE PROFESSOR Department of Music Performance Mike Truesdell (D.M.A. The Juilliard School) is a percussionist who has performed with numerous ensembles, including the New York City Ballet, International Contemporary Ensemble, and Lucerne Festival Ensemble conducted by Pierre Boulez, and with members of the New York Philharmonic, Metropolitan Opera, Chamber Music Society (New York), and Alarm Will Sound, among others. As an educator, he has previously been on the faculties of the University of Northern Colorado, Rutgers University, and Columbia University. Also engaged with mentoring the next generation, he has taught in the acclaimed Music Advancement Program at The Juilliard School, and founded Wildcat Percussion Camp, a summer percussion program to introduce aspiring percussionists to the spectrum of percussive sounds and techniques.
AWARDED TENURE AT RANK OF PROFESSOR Department of Media Arts, Sciences, and Studies James Rada (Ph.D. University of Georgia) expertly teaches budding journalists how to tell important stories in inventive ways in courses such as Documentary Journalism Workshop and Investigative Journalism. His creative activity includes producing and directing “With Infinite Hope: MLK and the Civil Rights Movement,” among other films he contributed to that tell the history of the movement and the Underground Railroad. He was awarded IC’s Faculty Excellence Award in 2020. He is an active reviewer and judge for several industry professional publications and organizations.
AWARDED TENURE AT RANK OF ASSOCIATE PROFESSOR Department of Media Arts, Sciences, and Studies Andy Watts (M.F.A. Columbia University) is an outstanding teacher who can successfully teach across the various film and television programs in the Roy H. Park School of Communications. His creative work as a screenwriter, director, and producer, combined with a 20-year career as a set lighting technician, directly contribute to his efficacy as an educator, mentor, and colleague. He has demonstrated an exemplary level of service to the department, the school, and the college, while maintaining ties to the industry.
AWARDED PROMOTION FROM ASSISTANT TO ASSOCIATE PROFESSOR Department of Biology Rebecca Brady (Ph.D. University of Texas at Austin) is renowned for her creative teaching of such classes as Human Genetics and Fundamentals of Biology, enlivening them with innovative techniques and placing a firm emphasis on students’ intellectual growth. Her scholarship is integrally connected to her teaching—she has contributed to the biology education literature through her published work in American Biology Teacher and is at work on a study of the flipped classroom. She has mentored student research projects that have resulted in public presentations, and her service contributions have students at their core. As examples, she has judged sessions for the Whalen Symposium and she was a member of the Innovation Scholars Program steering committee, helping to give birth to that vital new program in H&S.
Department of Music Performance Daniel Coakwell (D.M.A. Texas Tech University) teaches in the Voice area of the department, and students and peers alike commend his commitment to promoting a learning environment that prioritizes the mental health and well-being of his students. He also enjoys guest teaching artist residencies at institutions such as El Teatro Teresa Carreño in Venezuela, Yale University, and Dartmouth College. He specializes in the Evangelist and tenor roles of J.S. Bach, and he frequently performs the composer’s major oratorios—St. Matthew Passion, St. John Passion, Christmas Oratorio, and Mass in B-Minor—as well as many of Bach’s cantatas. Recent performances as a tenor soloist include G.T. Handel’s Messiah at the Myerson Symphony Center in Dallas, TX, and at the Steinmetz Hall in Orlando, FL, and as tenor soloist of J.S. Bach’s Mass in B Minor at the Judson Memorial Church in New York City and at St. Paul’s Episcopal Church in Salem, OR.
Department of the Environment Paula Turkon (Ph.D. Binghamton University) teaches generously not just in her own department but for programs across the college, including Anthropology and Innovation Scholars. She is known as an exuberant and imaginative instructor, and her students express gratitude for the lifelong impact she leaves on them, often helping them to forge careers in science. Her research in the areas of dendrochronology and aquaponics has resulted in three NSF grants as well as published scholarship. She has left an indelible imprint on H&S by leading a discussion that resulted in a new Innovation Scholars Program with sustainability at its core. Colleagues characterize her as an embodiment of the scholar-teacher ideal in the liberal arts.
Department of Writing Jaime Warburton (M.F.A. Sarah Lawrence College) offers courses at every level of the Writing curriculum, with a focus on first-year writing, poetics, creative writing, and gender. Faculty and students point to her welcoming and passionate approach to instruction, noting that she teaches with humor and vivacity, and she empowers students to interrogate their biases and preconceptions. She is a prolific author of creative nonfiction, poetry, and scholarship on the craft of writing. Reviewers call her work “gorgeous,” “self-aware,” and “self-deprecating.” She has been a generous citizen of IC, directing the Writing Center and the Ithaca Young Writers Institute, and chairing the Faculty Handbook Amendment Committee, among numerous activities.
AWARDED PROMOTION FROM CLINICAL ASSISTANT TO CLINICAL ASSOCIATE PROFESSOR Department of Physical Therapy Kayleigh Plumeau (D.P.T. Ithaca College) is a highly effective teacher and has exceptional clinical skills. She launched a novel mentoring program that directly addresses diversity, equity, and inclusion in clinical settings. She has had multiple presentations at national conferences including about the mentoring program, representation in clinical education, and growth mindset, with presentations and publications in interprofessional education and home exercise program for cancer survivors. She is the chair of the awards committee for the NY State Physical Therapy Association.
Department of Speech-Language Pathology and Audiology Jana Waller (M.S. Ithaca College) has been a clinical faculty member since 2011, serving as fieldwork coordinator, graduate co-chair, and interim chair. Since 2021 she has served as associate dean for the School of Health Sciences and Human Performance. She was selected for a prestigious HERS leadership development fellowship based on her leadership experience. She has conducted clinical research in autism, developing an innovative program for autistic adolescents and adults. More recently, her scholarly work has focused on interprofessional education in the health sciences.

IMAGES
VIDEO
COMMENTS
A clinical research coordinator reports to a principal investigator, who is responsible for the overall design and management of the study. In contrast, a clinical researcher organizes the day-to-day running of the trials. As a clinical research coordinator, you will also work closely with sponsors and staff within their department responsible ...
It's typically difficult to find a job as a clinical research coordinator. If you're curious about the numbers, check out the job market for clinical research coordinators. Info Do clinical research coordinators work full-time or part-time? 80% of clinical research coordinators work in full-time roles while 20% work part-time.
A clinical research coordinator (CRC) plays an important role in the field of clinical research, ensuring the smooth and efficient conduct of clinical trials and studies. These professionals act as a bridge between the research investigators, sponsors, and participants, overseeing various aspects of the research process. They collaborate closely with physicians, nurses, and other healthcare ...
Clinical research plays a crucial role in advancing medical knowledge and improving patient care. At the heart of every successful clinical research study is a Clinical Research Coordinator (CRC). As a CRC, you serve as the linchpin between researchers, study participants, and regulatory bodies. In this comprehensive guide, we will explore the ...
Clinical Research Coordinator: Earning this certification can help prove your ability to lead trial operations and uphold safe practices for all aspects of clinical research. Qualifications depend on your experience and educational background along with expertise in the six core disciplines outlined by the ACRP. ... Work environment for ...
However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
A Clinical Research Coordinator is responsible for a variety of duties including the following: Copy this section. Copied to clipboard Build a Job Description. Overseeing the trouble-free running of clinical trials. Collecting data obtained from research, coding and analyzing it. Managing budgets set aside for research.
The educational requirements for a clinical research coordinator position can differ based on the organization and job responsibilities. Employers typically seek candidates with at least a bachelor's degree in a science or medical field. Many clinical research coordinators have educational backgrounds in public health, biology, health science ...
Five significant predictors of retention. Agreement with: (A) work setting, (B) clinical research coordinator (CRC) level, (C) good salary, (D) understanding role of CRC, and (E) understood aspects of protocol development. Low retention (Low) defined as 0 to 5 years, and high retention (High) defined as 6 or more years.
As a Clinical Research Coordinator, you will have the opportunity to work with a variety of people, from patients to care teams, enabling you to be a part of the legacy that puts the needs of the patients first. In addition to driving innovation, the Research shield at Mayo Clinic is committed to creating a diverse environment that is open to ...
Here is a guide on how to pursue a career as a CRC: Obtain a Relevant Bachelor's Degree: Most CRC positions require a bachelor's degree in a relevant field such as biology, chemistry, nursing, psychology, or healthcare administration. Some positions might prefer candidates with a degree in a specific area related to the type of clinical ...
6,466 Clinical Research Coordinator jobs available on Indeed.com. Apply to Clinical Research Coordinator, Clinical Nurse Manager, Site Director and more! ... Hybrid work (352) Remote (106) Pay. $25.00+/hour (5,468) $27.50+/hour (4,933) ... 1 years' work experience in clinical research or pharmaceutical environment would be desirable.
Research Coordinator Work Environment The work environment for Research Coordinators can vary greatly depending on the setting, such as universities, hospitals, pharmaceutical companies, or private research firms. Typically, it involves a combination of office work and interaction with research participants or team members.
The definition provided by the Association of Clinical Research Professionals states that "A Clinical Research coordinator, Study Site Research Nurse or Study Site Coordinator, ... and the work overload generated by the continuous updating of regulatory procedures, without structured training and educational programs on the other, are probably ...
To identify factors associated with job satisfaction and retention, we surveyed a large cohort of clinical research coordinators (CRCs). In recent years, the clinical research coordinator has changed from a semi-permanent role to one that has a high turnover rate. The CRCs are integral to clinical research and instability in this role can cause patient stress and increase the burden on ...
A roadmap in the form of a checklist is provided to help make the transition from research coordinator to clinical research associate (CRA) a smooth one. ... Fatigue in the current work environment is another reason for why individuals may be looking to make this transition. Of all members of the clinical research team, research coordinators ...
Coordinates schedules and monitors research activities and subject participation. Identifies, reviews, and reports adverse events, protocol deviations, and other unanticipated problems appropriately. Manages, monitors, and reports research data to maintain quality and compliance. Provides education/training for others within the department.
The Coordinator of Clinical Research will be an important member on the Clinical Research team, focusing on PD GENEration. ... Demonstrated ability to collaborate, multi-task and work effectively in a fast-paced environment. Demonstrates the organizational values of excellence, teamwork, collaboration, integrity, positivity, dedication, and ...
The work environment or Employment Nature of a Clinical Research Coordinator: ... In certain facilities that perform a large number of trials, a clinical research coordinator may work in shifts to ensure that a trained staff is available 24 hours a day. Tier-1 cities such as New Delhi, Gurugram, Chennai, Kolkata, Hyderabad, Bengaluru, Pune are ...
The Division of Cardiovascular Medicine, within Stanford University Department of Medicine, is a dynamic and innovative center dedicated to excellence in research, medical education, and clinical care.Our Division is driven by over 90 faculty members and a cadre of staff who are the pillar of strength in the Division's ongoing efforts into the prevention and treatment of cardiovascular disease.
Clinical Trial Research Coordinator Salary Expectations. The average salary for a Clinical Trial Research Coordinator is $59,604 (USD) per year. The actual income can fluctuate based on factors such as experience in the field, location of work, and the organization that they are employed by. Clinical Trial Research Coordinator Job Description FAQs
The Clinical Research Coordinator performs study coordination tasks under the direction of the investigator and works closely with the Senior Research Scientist to ensure overall compliance in the conduct of the study, and adherence to the approved study protocol. ... If you are looking for a challenging work environment to leverage the skills ...
Position: Clinical Research Coordinator, Cancer CareLocation: Boston, MASchedule: 40 hours per week, HybridABOUT BMC:At Boston Medical Center (BMC), our diverse staff works together for one goal — to provide exceptional and equitable care to improve the health of the people of Boston. Our bold vision to transform health care is powered by our respect for our patients and our commitment to ...
He teaches both graduate and undergraduate students to better understand research and its application to their clinical practice. He serves the department as the Athletic Training Clinical Education Coordinator, the college as chair of the Institutional Review Board and his profession as an editor of two prestigious professional journals.