- Open access
- Published: 28 December 2022

Interventions to treat and prevent postpartum depression: a protocol for systematic review of the literature and parallel network meta-analyses
- David Thomas Monks ORCID: orcid.org/0000-0002-1677-5244 1 ,
- Basavaraj Ankalagi ORCID: orcid.org/0000-0001-9513-755X 2 ,
- Preet Mohinder Singh ORCID: orcid.org/0000-0001-7642-529X 1 ,
- Ebony Carter ORCID: orcid.org/0000-0002-7620-4929 3 ,
- Michelle Doering 4 ,
- Meg Guard 5 &
- Shannon Lenze ORCID: orcid.org/0000-0003-3410-3561 6
Systematic Reviews volume 11 , Article number: 282 ( 2022 ) Cite this article
4604 Accesses
Metrics details
Introduction
Postpartum depression has costly consequences for the mother, baby, and society. Numerous pharmacological and non-pharmacological interventions are available for the prevention and treatment of postpartum depression. To date, no attempt has been made to synthesize the evidence from comparisons of interventions both within and across these categories.
We will perform a systematic review of the literature and perform network meta-analysis of interventions to (a) prevent and (b) treat postpartum depression. This review will include studies of primiparous or multiparous women during pregnancy or within 12 months of delivery of their baby that assess either interventions initiated during pregnancy or within 1 year of childbirth. Comparators will be other eligible interventions or control conditions. The outcome of interests will be related to the antidepressant efficacy of the interventions as well as their acceptability. The published literature will be searched in Ovid MEDLINE 1946-, Embase.com 1947-, Scopus 1823-, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov. The search will use a combination of standardized terms and keywords for postpartum depression, a sensitive search filter to limit for randomized controlled trials, and a librarian-created “humans” filter. The search results will be uploaded to the Covidence online systematic review platform (Veritas Health Information Ltd., Victoria, Australia) where two review team members will independently screen articles. We will extract data to include year of publication, language, country, participants (number, demographic data, eligibility criteria, psychiatric symptoms, and co-morbidities), characteristics of the intervention and control conditions, and reported outcomes. Risk of bias for each study will be assessed independently by two review authors using the RoB 2: A revised Cochrane risk of bias tool for randomized trials. Network meta-analysis will be performed using a Bayesian hierarchical model supplemented with a Markov chain Monte Carlo approach.
Postpartum depression is a devastating disease with long-lasting consequences. Given the numerous available interventions to both prevent and treat postpartum depression and the great number of studies comparing them, it is imperative that clinicians and patients are provided with an assessment of their comparative efficacy and acceptability.
Systematic review registration
Prospero registration (CRD42022303247).
Peer Review reports
Postpartum depression (PPD) complicates 6–19% of pregnancies, leading to costly consequences for the mother, baby, and society. The effects of PPD are long-lasting, with 25% of women continuing to have symptoms of depression 1 year after diagnosis, and 12.5% continuing to have symptoms for 2 years. Furthermore, of those women whose postpartum depression goes into remission, there is a 40% relapse rate [ 1 , 2 ]. Unfortunately, suicidal thoughts are particularly common, affecting about 20% of women with PPD, and although still rare, suicide accounts for approximately 20% of postpartum deaths [ 3 ]. The societal burden is substantial: one study estimated an equivalent cost of $100,000 per case due to suicide, loss of work, morbidity, and infant malnutrition.
Numerous interventions are available for the prevention and treatment of postpartum depression. These include pharmacological, psychosocial, psychological, educational, and somatic therapies. To date, no attempt has been made to synthesize the evidence from comparisons of interventions both within and across these categories. Various systematic reviews of the literature have been published in the past decade, examining interventions to treat and prevent postpartum depression. These reviews have focused on specific interventions [ 4 ], groups of interventions [ 5 ] and specific patient groups [ 6 ] and have pooled data to perform pairwise meta-analysis or series of pairwise meta-analyses. For example, a 2021 Cochrane review [ 7 ] of antidepressant medications included 11 RCTs (1016 women) that compared antidepressants with placebo, treatment as usual, psychological interventions, psychosocial interventions, any other medicines, or another type of antidepressant; and complementary medicine (food supplements). They reported a series of meta-analyses between each of these and found that women treated with antidepressants might only experience a slightly better antidepressant benefit than women given a placebo. However, to our knowledge, there have been no network meta-analyses performed. Our planned analysis will attempt to compare all these interventions in a common network and utilize direct and indirect evidence to allow clinicians to compare their efficacy and safety. We aim to review the relevant literature and perform network meta-analysis of interventions to (a) prevent and (b) treat postpartum depression within 12 months of delivery. After reviewing our analysis, the reader should be able to assess the effectiveness of varying methods of preventing and treating PPD. Additionally, we will provide the reader with an understanding of the confidence in the evidence to support each intervention examined.
Methods/design
A systematic review of the relevant literature will be performed, employing methods outlined in the Cochrane Handbook, and data extracted to permit network meta-analysis [ 8 ]. Separate networks will be constructed for (a) preventative and (b) treatment interventions. The degree of connection between the networks that predominantly compare pharmacological and non-pharmacological interventions will determine whether separate networks are necessary to calculate network estimates. This protocol has been reported in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) guidelines [ 9 ]. The review will be reported in adherence with the PRISMA extension statement for incorporating network meta-analysis [ 10 ]. A completed PRISMA-P checklist for the current review protocol is provided in Supplementary Fig 1 . This protocol was registered with PROSPERO (CRD42022303247).
Eligibility criteria
Inclusion and exclusion criteria were defined according to the patient, intervention, comparator, outcomes, and study design format (PICOS).
This review will include studies of primiparous or multiparous women during pregnancy or within 12 months of delivery of their baby. As the initial search will include studies for each of the review questions related to (a) prevention and (b) treatment of postpartum depression, participants may or may not be depressed at baseline. We will use the individual study definition of the intervention, as either preventative or therapeutic.
Interventions
Studies of both pharmacological and non-pharmacological interventions initiated during pregnancy or within 1 year of childbirth will be included. Pharmacological interventions will be categorized by class and further sub-categorized by individual drug and dosing regimen as justified by the data. These subcategories will be created to define separate interventions on the condition that there are sufficient comparisons with other interventions to permit a cohesive network. Similarly, non-pharmacological interventions will be broadly categorized based on the nature (psychological, psychosocial, somatic, physical therapy, sensory therapy, etc.) and sub-categorized by clustering of their characteristics. Combinations of interventions will be evaluated according to a similar data-driven strategy. In all instances, the data collected on such characteristics will either inform sub-categorization or be noted as a potential effect modifier and considered a candidate for subsequent meta-regression. A detailed description of the process of node definition and network construction will be reported with the results of this analysis. Table 1 provides an anticipated list of potential categories and subcategories of interventions.
Comparators
In addition to the interventions described above, we anticipate a variety of control conditions which will be differentiated based on their broad categories (usual care, enhanced usual care, waiting-list, non-treatment, and placebo) and individual characteristics. We will, again, use this data and the integrity of potential networks to dictate the sub-division (or not) of these control conditions into separate nodes (interventions).
The outcome of interests will be related to the efficacy and acceptability of the interventions. Efficacy will be assessed, for preventative interventions, by difference in odds of developing PPD and, for therapeutic interventions, by odds of response. Response will be defined as the total number of patients who had a reduction of ≥ 50% of the total depression score, standardized from the validated depression scoring scale. Such rating scales include the Edinburgh Postpartum Depression Scale (EPDS), Hamilton Depression Rating Scale (HAMD), Beck Depression Inventory (BDI), Postpartum Depression Screening Scale, Patient Health Questionnaire (PHQ9), Self-Rating Depression Scale (SDS), Center for Epidemiologic Studies Depression Scale (CESD), Zung Self-Rating Depression Scale, Hospital anxiety and depression scale (HADS), and the Depression anxiety stress scale (DASS). Acceptability will be primarily assessed by modeling the odds of all-cause discontinuation of the interventions (the proportion of patients who withdrew for any reason). Additional outcomes will include related psychiatric symptoms and potential adverse effects of interventions such as anxiety, sedation, suicidal ideation, headache, nausea, dry mouth, insomnia, dizziness, diarrhea, constipation, sexual problems, fatigue, weight gain, tremors, and increased sweating. We will attempt to construct networks for any of these adverse effects that are consistently reported across enough studies and will report the rest in the table of study characteristics. Studies with crossover design will only be included if they give clear outcome data for each group (prior and after the cross over). Study groups evaluating mixed interventions will not be included in the evaluation unless the mixed intervention is considered as a separate node/intervention.
Study design
We will include all peer reviewed randomized and quasi-randomized clinical trials in our initial search. On appraisal of the available studies and the relative contribution of quasi-randomized comparisons, we will decide whether the benefit of including this data is outweighed by the consequent reduction in the confidence in the accumulated evidence.
Search strategy and information sources
The published literature will be searched using strategies created by a medical librarian for clinical trials on postpartum depression. The search will be implemented in Ovid MEDLINE 1946-, Embase.com 1947-, Scopus 1823-, Cochrane Central Register of Controlled Trials, and Clinicaltrials.gov. The search will use a combination of standardized terms and keywords for postpartum depression, a sensitive search filter to limit for randomized controlled trials, and a librarian-created “humans” filter. Conference abstracts will be excluded from the Embase and Scopus searches. Results will be imported into Endnote and duplicates will be identified and removed. The search of ClinicalTrials.gov aims to identify emerging studies nearing completion. We will attempt to contact authors of any pertinent unpublished studies to ensure complete data extraction; however, abstracts will be excluded if data remains incomplete. Non-English publications will be included provided an English-language abstract is available. A draft search strategy of Ovid MEDLINE is provided in Table 2 and the strategies for the remaining databases can be found in Supplementary Table 1 .
Study records
The search results will be uploaded to the Covidence online systematic review platform (Veritas Health Information Ltd., Victoria, Australia). Two review team members will screen articles independently using the predefined eligibility criteria. This will be done in two stages, the first by referring to title and abstracts only before confirming the eligibility decision by reference to the full text. In case of any disagreement, a third review team member will mediate consensus. Reasons for excluding full texts will be documented both in Covidence and an Excel spreadsheet. Study authors will be contacted if eligibility criteria remain unclear following article review. Final study inclusion will be presented in a PRISMA flow diagram.
Data extraction
We will use six randomly selected studies of preventative and therapeutic interventions, respectively, to guide the development of a data extraction spreadsheet. In the event of an essential data item being identified beyond the initial six studies, we will reformat the data extraction tool and go back to extract this missing item from all trials. We will extract data to include year of publication, language, country, participants (number, demographic data, eligibility criteria, psychiatric symptoms, and co-morbidities), characteristics of the intervention and control conditions, and reported outcomes. In the prevention network, we will attempt to categorize the population in each trial as “asymptomatic, at risk”, “subclinical symptomatology” or “mixed asymptomatic/subclinical” in accordance with the recommendations of the Institute of Medicine’s report on prevention research [ 11 ]. We will also collect study-level data to inform the definition of interventions, as described above, and other sources of heterogeneity and effect modification.
Assessment of quality of evidence
Risk of bias for each study will be assessed independently by two review authors using the RoB 2: A revised Cochrane risk of bias tool for randomized trials [ 12 ]. The overall risk of bias will be expressed as low risk, some concerns/uncertainty, or high risk. Publication bias will be assessed by visually inspecting a comparison-specific funnel plot (for the primary outcome). A funnel plot will be constructed for pairwise comparison for each of the treatment options included in our analysis. The Confidence in Network Meta-Analysis (CINeMA) approach will be used to evaluate the overall evidence quality. Trials will be individually assessed for the indirectness of evidence. Indirectness refers to the relevance of the included studies to the research question. It helps to establish how well the included studies address the research question for the present network meta-analysis. Included studies will be scored based on uniformity across three parameters: study participants, interventions, and outcome characteristics reported. The more divergence noted in these parameters, the more indirectness assumed. In addition, the GRADE tool will be employed to assess the certainty in the evidence for the pairwise comparison of each agent with a common comparator in the summary of findings table.
Summary of findings table
For the primary outcome, a summary of findings table will be constructed. Summary of findings tables summarize the results of NMA, provide absolute estimates of each interventions’ effect when compared to a common comparator, report probability ranking and an assessment of the certainty in the evidence (using the GRADE tool) in order to facilitate comprehensive interpretation of the results of NMA. Once the network has been constructed, we will identify the intervention that is compared to the most different interventions (i.e., maximally connected) in the largest number of trials (maximal direct evidence) and use this as the common comparator for the purpose of the summary of findings table.
Data synthesis
We will, initially, construct a network of the evidence to determine whether the data from comparisons of pharmacological and non-pharmacological interventions warrants separate analyses or whether one network can maximize the data available to inform network estimates. The resulting network meta-analysis/es will be conducted using Bayesian approach. The odds ratio and 95% CrI will be calculated for dichotomous outcomes. The mean difference (MD) and 95% credible intervals (CI) will be calculated for continuous outcomes. The geometry of each network will be reported in graphical form along with a league table of network estimates for each pairwise network estimates. We will calculate the cumulative probabilities for each intervention being at each possible rank and then use the surface under the cumulative ranking curve (SUCRA) to create a treatment hierarchy. SUCRA is a commonly used method to numerically summarize the cumulative rankings so that SUCRA is 1 when a treatment is certain to be the best and is 0 when a treatment is certain to be the worst [ 13 ]. Attempts will be made to locate and evaluate inconsistencies across the network using node-split modeling.
Statistical analysis
Analysis will be performed using a Bayesian hierarchical model (binomial modeling with logit link function) supplemented with Markov chain Monte Carlo (MCMC) approach. We will initially run 5000 adaptations, 20,000 iterations with a thinning factor of 10. These parameters will be adjusted as necessary to achieve a Potential Scale Reduction Factor (PSRF) of less than 1.05. The convergence diagnostics for the model will be reported in Gelman Rubin diagrams. The indirect estimates will be imputed using common comparators. The outcomes will be reported as credible intervals (CrI). Based on the distribution of credible intervals, rank probabilities (preferred order of therapeutic success) will be calculated for all the included treatment nodes. The statistical analysis will be performed by R assisted by package “gemtc” (Version 0.8-7, Github.com ), Netmeta (Version2.6-0, R-repository), Dmetar [ 14 ], and Bugsnet (bugsnetsoftware.github.io).
Exploration of model fitness, transitivity, and inconsistency
Model fitness will be evaluated using the Deviance Information Criterion (DIC) values and overall deviance for each parameter analyzed. The model with the lowest DIC values (in comparison to the data points) will be used for reporting the results. We intend to use the following network global evaluation metrics to evaluate transitivity: deviance information criterion, net heat plot and direct evidence plot (although not evaluating transitivity, per se, the greater the contribution of direct evidence to each network estimate, the lower the likelihood of inconsistency). For each comparison, we shall also look at the node split model, thus helping us to quantify comparison-specific inconsistency to estimate deficiencies in transitivity. We have identified potential effect modifiers (Table 3 ) from the literature [ 15 , 16 , 17 , 18 ] and will assess the distribution of effect modifiers to judge if the transitivity assumption holds. For those that prove to be a study-level parameter, then we plan to explore the impact of this on our network estimates by employing network meta-regression. We plan to use both Bayesian and Frequentist tools available to localize and quantify the inconsistencies in the network. For this, we will construct a node-spit model and a net-heat plot. We will evaluate the proportion of direct comparisons in the final outcome using the direct evidence plot. Using this approach, we will estimate the minimum number of independent paths in the network contributing to the effect estimate at an aggregated level. “Minimum parallelism” and the “mean path length” will allow estimation of the degree of indirectness in the reported pooled outcome.
Ethics and dissemination
Ethical approval was deemed unnecessary. We report here in a single manuscript, a broad and inclusive search strategy, designed to maximize the contribution of available evidence to answer two separate review questions: (a) what is the comparative effectiveness of available interventions to prevent postpartum depression? (b) what is the comparative effectiveness of available interventions to treat postpartum depression? We anticipate submitting these parallel network meta-analyses in separate manuscripts to recognized psychiatric journals.
Postpartum depression is a devastating disease with long-lasting consequences for patients, their families, and society. Given the numerous available interventions to both prevent and treat postpartum depression and the great number of studies comparing them, it is imperative that clinicians and patients are provided with an assessment of their comparative efficacy and acceptability along with a comprehensive appraisal of the quality of evidence that supports those assessments.
Availability of data and materials
Not applicable.
Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–83.
Article Google Scholar
Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315–23.
Jones I, Chandra PS, Dazzan P, Howard LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789–99.
Zeng M, Gong A, Wu Z. Paroxetine combined with traditional chinese medicine prescriptions in the treatment of postpartum depression: a systematic review of randomized controlled trials. Front Neuroendocrinol. 2022;67:101019.
Article CAS Google Scholar
Branquinho M, Rodriguez-Muñoz MF, Maia BR, Marques M, Matos M, Osma J, et al. Effectiveness of psychological interventions in the treatment of perinatal depression: a systematic review of systematic reviews and meta-analyses. J Affect Disord. 2021;291:294–306.
Shang J, Dolikun N, Tao X, Zhang P, Woodward M, Hackett ML, et al. The effectiveness of postpartum interventions aimed at improving women's mental health after medical complications of pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2022;22(1):809.
Brown JVE, Wilson CA, Ayre K, Robertson L, South E, Molyneaux E, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2021;2(2):CD013560.
Google Scholar
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. [updated March 2011] Available from https://training.cochrane.org/handbook .
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. https://doi.org/10.7326/M14-2385 .
Institute of Medicine (US). Committee on Prevention of Mental Disorders. In: Mrazek PJ, Haggerty RJ, editors. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. Washington (DC): National Academies Press (US); 1994.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. (Clinical research ed). https://doi.org/10.1136/bmj.l4898 .
Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71.
Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-Analysis with R: A Hands-On Guide. Boca Raton and London: Chapmann & Hall/CRC Press; 2021. ISBN 978-0-367-61007-4
Book Google Scholar
Alzahrani J, Al-Ghamdi S, Aldossari K, Al-Ajmi M, Al-Ajmi D, Alanazi F, et al. Postpartum depression prevalence and associated factors: an observational study in Saudi Arabia. Medicina (Kaunas). 2022;58(11):1595.
Yoo H, Ahn S, Park S, Kim J, Oh J, Koh M. Factors influencing prenatal and postpartum depression in Korea: a prospective cohort study. Korean J Women Health Nurs. 2021;27(4):326–36.
Munk-Olsen T, Liu X, Madsen KB, Kjeldsen MZ, Petersen LV, Bergink V, et al. Postpartum depression: a developed and validated model predicting individual risk in new mothers. Transl Psychiatry. 2022;12(1):419.
Silva BPD, Matijasevich A, Malta MB, Neves PAR, Mazzaia MC, Gabrielloni MC, et al. Common mental disorders in pregnancy and postnatal depressive symptoms in the MINA-Brazil study: occurrence and associated factors. Rev Saude Publica. 2022;56:83.
Download references
Acknowledgements
This research was not supported by any funding.
Author information
Authors and affiliations.
Department of Anesthesiology, Washington University School of Medicine, 660 S Euclid Avenue, St. Louis, USA
David Thomas Monks & Preet Mohinder Singh
Department of Anesthesiology, Toronto Western Hospital, University of Toronto, Toronto, Canada
Basavaraj Ankalagi
Department of Maternal and Fetal Medicine, Washington University School of Medicine, 660 S Euclid Avenue, St. Louis, USA
Ebony Carter
Becker Library, Washington University School of Medicine, 660 S Euclid Avenue, St. Louis, USA
Michelle Doering
Washington University School of Medicine, 660 S Euclid Avenue, St. Louis, USA
Perinatal Behavioral Health Service, Department of Psychiatry, Washington University School of Medicine, St. Louis, USA
Shannon Lenze
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the design of protocol and composition of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Correspondence to David Thomas Monks .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
PRISMA-P (Preferred Reporting Items for Systematic review and Meta-Analysis Protocols) 2015 checklist: recommended items to address in a systematic review protocol*.
Additional file 2: Supplemental Table 1.
Search strings.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Monks, D.T., Ankalagi, B., Singh, P.M. et al. Interventions to treat and prevent postpartum depression: a protocol for systematic review of the literature and parallel network meta-analyses. Syst Rev 11 , 282 (2022). https://doi.org/10.1186/s13643-022-02157-2
Download citation
Received : 10 March 2022
Accepted : 15 December 2022
Published : 28 December 2022
DOI : https://doi.org/10.1186/s13643-022-02157-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Postpartum depression: Proposal for prevention through an integrated care and support network

1997, Applied and Preventive Psychology
Related Papers
Issues in Mental Health Nursing
Linda Duffett-Leger
Jognn-journal of Obstetric Gynecologic and Neonatal Nursing
Marilyn Evans
Objective: To explore awareness of postpartum depression and its symptoms and available community resources for women with postpartum depression.Design: Cross-sectional surveillance research, using population-based data.Setting: Eight communities in southern and eastern Ontario, Canada.Participants: A random selection of adults 18 years of age and older with telephones.Method: Logistic regression and chi-square test were used to analyze awareness of postpartum depression and its symptoms, the baby blues, and sources of assistance for women with postpartum depression.Results: The vast majority of respondents were aware of postpartum depression (90.1%± 0.6% confidence interval) (n=8,750) as compared with the baby blues (62.5%± 1.1%). Awareness of postpartum depression, its symptoms, the baby blues, and sources of assistance varied according to the demographic profiles of the respondents (family structure, education, and language spoken at home).Conclusion: Awareness of the term postpartum depression does not necessarily imply awareness of its symptoms or sources of assistance. Public education is needed to address this fact in order to provide social support and encourage treatment for symptomatic women and their families. Education should target individuals with lower levels of education and non-English–speaking groups.
Archives of Women's Mental Health
Howard Leventhal , Amy Balbierz
Depressive symptoms and depression are a common complication of childbirth, and a growing body of literature suggests that there are modifiable factors associated with their occurrence. We developed a behavioral educational intervention targeting these factors and successfully reduced postpartum depressive symptoms in a randomized trial among low-income black and Latina women. We now report results of 540 predominantly white, high-income mothers in a second randomized trial. Mothers in the intervention arm received a two-step intervention that prepared and educated mothers about modifiable factors associated with postpartum depressive symptoms (e.g., physical symptoms, low self-efficacy), bolstered social support, and enhanced management skills. The control arm received enhanced usual care. Participants were surveyed prior to randomization, 3 weeks, 3 months, and 6 months postpartum. Depressive symptoms were assessed using the Edinburgh Postnatal Depression Scale (EPDS of 10 or greater). Prevalence of depressive symptoms postpartum was unexpectedly low precluding detection of difference in rates of depressive symptoms among intervention versus enhanced usual care posthospitalization: 3 weeks (6.0 vs. 5.6 %, p = 0.83), 3 months (5.1 vs. 6.5 %, p = 0.53), and 6 months (3.6 vs. 4.6 %, p = 0.53).
The Journal of Maternal-Fetal & Neonatal Medicine
Katarzyna Wszołek
The Israel Journal of Psychiatry and Related Sciences
Saralee Glasser
The current report presents an example of the path taken from identification of a public health problem at the primary health service level, to conducting research documenting the scope of the problem and nature of the risk factors, disseminating the findings, and fostering development and application of relevant policy. The example presented is the case of postpartum depression, an issue with bio-psycho-social implications. Public health nurses identified the problem, prompting epidemiological research. The findings encouraged the Ministry of Health (MOH) to conduct a pilot program for screening and early intervention among pregnant and postpartum women reporting depressive symptoms. Based on the results of the pilot program, the MOH is expanding the program to all Mother-Child Health (MCH) clinics. Israel?s largest Health Maintenance Organization has followed suit and is including this program in its own clinics. This Israeli experience may serve as an instructive example of a locally identified problem evolving into a national policy.
BMC Public Health
Ines Ben Messaoud Lamari
Journal of Maternity Care and Reproductive Health
Tetti Solehati
Mental health is a global problem including postpartum blues. Woman's perception of family’s support may increase postpartum blues symptoms. The purpose of this study was to describe the perceptions of women with postpartum blues towards family supports in Al Islam Hospital, Bandung, Indonesia. The design was descriptive and samples were chosen using the purposive sampling technique. The number of respondents was 35 women, and the data were gathered using two questioners. There were family support and modification of Kennerly and Gath's questioner. The results showed that most of respondent's perceived family provided emotional support 75%, instrument support 51%, reward support 54%, and informational support 54%. Based on the result, it is an opportunity for the hospital to provide counseling or health education to patient or family about the patient need on family support.Keywords: Family Support, Perception, Postpartum Blues
Social Work and Community Practice
Carol Kauppi
Psychotherapy and Psychosomatics
Iván Devosa
MCN: The American Journal of Maternal/Child Nursing
Stephan Arndt
RELATED PAPERS
Juan Martinez
Biblioteca de Babel: Revista de Filología Hispánica
Antonio Hidalgo-Navarro
Journal of Entrepreneurship
Muhammad Azam Roomi
Sharon Rosenkoetter
Mohammad Muhassin
William Badke
Muhammad Sholihin
stefan Jaronski
#Tear: Revista de Educação, Ciência e Tecnologia
Wasley Santos
fitik mideameliyati
COLEF/UNESCO
Alberto Hernández-Hernández
World Academy of Science, Engineering and Technology, International Journal of Computer, Electrical, Automation, Control and Information Engineering
Aissam Belghiat
Al-Usrah : Jurnal Al Ahwal As Syakhsiyah
Heri Firmansyah
Frontiers in Big Data
Qasem Abu Al-Haija
Ghana medical journal
Birch Saheeb
Contemporary Clinical Dentistry
Ipsita Maity
Research Square (Research Square)
Aisha Kamal
卡尔顿大学毕业证书 购买加拿大学历CU文凭学位证书成绩单
serge abramovici
Preeti Pandey
Heteroatom Chemistry
Nikolai Lukashev
The Journal of Infectious Diseases
Jo-Ann Passmore
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions
Postpartum Depression—New Screening Recommendations and Treatments
- 1 University of Massachusetts Chan Medical School, Worcester
- 2 UMass Memorial Health, Worcester, Massachusetts
- Medical News & Perspectives What to Know About the First Pill Approved for Postpartum Depression Rita Rubin, MA JAMA
- Comment & Response Screening Recommendations and Treatments for Postpartum Depression—Reply Tiffany A. Moore Simas, MD, MPH, MEd; Anna Whelan, MD; Nancy Byatt, DO, MS, MBA JAMA
- Comment & Response Screening Recommendations and Treatments for Postpartum Depression Itamar Nitzan, MD; Raylene Philips, MD; Robert D. White, MD JAMA
Perinatal mental health conditions are those that occur during pregnancy and the year following childbirth, whether onset of the condition(s) predates pregnancy or occurs in the perinatal period. Perinatal mental health conditions are the leading cause of overall and preventable maternal mortality and include a wide array of mental health conditions including anxiety, depression, and substance use disorders. 1 , 2 Perinatal depression specifically affects 1 in 7 perinatal individuals. 3 While commonly referred to as postpartum depression, it is more accurately called perinatal depression because its onset corresponds with prepregnancy (27%), pregnancy (33%), and postpartum (40%) time frames. 3
Read More About
Moore Simas TA , Whelan A , Byatt N. Postpartum Depression—New Screening Recommendations and Treatments. JAMA. 2023;330(23):2295–2296. doi:10.1001/jama.2023.21311
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 14 November 2023
Women’s experiences of psychological treatment and psychosocial interventions for postpartum depression: a qualitative systematic review and meta-synthesis
- Pamela Massoudi 1 , 2 ,
- Leif A. Strömwall 3 ,
- Johan Åhlen 4 ,
- Maja Kärrman Fredriksson 3 ,
- Anna Dencker 5 &
- Ewa Andersson 6
BMC Women's Health volume 23 , Article number: 604 ( 2023 ) Cite this article
2939 Accesses
1 Altmetric
Metrics details
To provide a comprehensive, systematic evaluation of the literature on experiences of psychological interventions for postpartum depression (PPD) in women. Depression is one of the most common postpartum mental disorders. Studies have identified that psychological interventions reduce depressive symptoms. However, less is known about the experiences of women who have received such treatments.
A systematic review of the literature was conducted by searching five databases (CINAHL, Cochrane Library, EMBASE, Medline, PsycINFO), in August 2022. Studies with qualitative methodology examining women’s experiences of professional treatment for PPD were included and checked for methodological quality. Eight studies (total N = 255) contributed to the findings, which were synthesized using thematic synthesis. Confidence in the synthesized evidence was assessed with GRADE CERQual.
The women had received cognitive behavioral therapy (5 studies) or supportive home visits (3 studies). Treatments were individual or group-based. Two main themes were identified: Circumstances and expectations, and Experiences of treatment, with six descriptive themes. Establishing a good relationship to their health professional was important for the women, regardless of treatment model. They also expressed that they wanted to be able to choose the type and format of treatment. The women were satisfied with the support and treatment received and expressed that their emotional well-being had been improved as well as the relationship to their infant.
The findings can be helpful to develop and tailor patient-centered care for women who are experiencing postnatal depression.
Peer Review reports
Pregnancy and the first year after childbirth involve significant changes in a woman’s life and can be associated with emotional distress of varying types and degrees. For some, worry and mood disturbances are natural and transient reactions to the challenges of a new life situation. For others, symptoms can persist and develop into a condition where support or treatment is needed. Depression is one of the most common postpartum mental disorders during this period. The prevalence of postpartum depression (PPD) has been estimated at 5–9% in high income countries, around 13% when self-report measures are used [ 1 , 2 ]. Women with previous mental health problems are more at risk, as well as women with previous or current stressful life experiences, especially being exposed to interpersonal violence, partner relationship problems, migration, and lack of support [ 3 , 4 ]. Associations between PPD and adverse outcomes on the child are most evident when depression is severe or recurrent, or when associated risk factors may explain a substantial part of the negative outcome on children [ 3 ].
In general, and across various cultures, mothers with PPD have been found to prefer talking therapies or supportive interventions over pharmacological treatments, in part due to fear of negative effects on the child by transmission to breastmilk [ 5 , 6 , 7 ]. A recent review highlighted how mothers put what they thought was best for their baby first when making decisions about treatment, including taking or not taking medication [ 8 ].
Systematic reviews have found that psychotherapy and psychosocial interventions for perinatal depression are generally effective [ 9 , 10 , 11 ]. Common treatments for PPD are cognitive behavior therapy (CBT), interpersonal psychotherapy (IPT), and non-directive supportive counseling, also called listening visits [ 9 ]. Treatments can use an individual or group format, take place as home visits, at a clinic, or be internet-based, and are often tailored for the postnatal period, sometimes including a parent-child interaction component.
Besides outcomes in terms of symptom reduction, it is also relevant to explore women’s experiences of treatment. A meta-synthesis focusing on experiences of seeking and receiving psychosocial interventions for postpartum depression found that women could experience several barriers to help-seeking, but that they were generally positive to the interventions they had received [ 12 ]. However, this meta-synthesis included low-quality studies. Barriers can be lack of time, stigma, childcare or transportation issues [ 5 , 6 , 7 ], and negative healthcare experiences [ 13 ]. Some women also have concerns about being judged as a “bad mother”, which may delay seeking help. Another meta-synthesis of studies concerning the experiences of perinatal women with a broader range of mental health problems, identified several unmet needs of information, collaborative integrated care, and post-treatment follow-up [ 14 ]. Some important components of treatment expressed by the women were the importance of the health professionals’ non-judgmental attitude as well as conveying hope.
The aim of the current review was to provide an updated and comprehensive understanding of women’s experiences of psychological interventions for postpartum depression, based on a systematic evaluation of the literature and a meta-synthesis of the findings, including an assessment of the reliability of the findings.
Search strategy
An information specialist (MKF) searched five databases: CINAHL (EBSCO), Cochrane Library (Wiley), EMBASE (Embase.com), Medline (Ovid), PsycINFO (EBSCO). Searches were run in November and December 2021, and updates in June 2022. A manual search of reference lists from the included articles was also undertaken to identify studies not captured by the electronic search.
The search strategy was developed by the information specialist in collaboration with the experts in the review team, and combined terms and phrases describing the population, interventions, patients’ experiences, and qualitative research methods. Another information specialist at the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU) reviewed the search strategy using the PRESS Checklist [ 15 ]. The search strategy and search terms used can be found in Appendix 1. The review used PRISMA Guidelines for reporting the search strategy [ 16 ].
Inclusion criteria
Studies were included if they satisfied the inclusion criteria, see Table 1 .
Study selection
The search process yielded 8804 unique studies. All titles and abstracts were screened for eligibility, 70 articles were assessed in full-text, and eight studies were included for data extraction and synthesis after assessing for quality (Fig. 1 ).

PRISMA Flow of study selection process
Quality assessment of primary studies
To assess the methodological quality and risk of bias, included studies were evaluated using the SBU Quality assessment tool for studies with a qualitative design [ 17 ]. This critical appraisal tool consists of five domains (adherence to epistemological position, recruitment and appropriateness of participants, appropriateness of data collection procedures, aspects of the data analysis, and the role of the researcher), each with signaling questions.
Three authors initially assessed each study (LS, PM, AD, EA, or JÅ), followed by a consensus discussion concerning the degree to which the methodological limitations impacted the findings, assessed as low, moderate, or high risk. For studies with a low or moderate risk of bias, data was extracted and compiled in tables while studies with high risk of bias were excluded from the further analyses.
Data extraction and synthesis
An inductive thematic synthesis was conducted using a three-stage procedure, largely in line with Thomas and Harden (2008) [ 18 ].
First, the included studies were read, in depth, to provide a full understanding. Three authors (EA, PM, and LS) also discussed their respective pre-understanding of the field, with both insider and outsider perspectives. PM (clinical psychologist) and EA (midwife) are both researchers in the field; PM also had experience of treating PPD. LS is a psychology professor, not experienced in this field, but in research methodology. These authors then independently extracted meaningful units from the included studies and translated them into codes. In stage 2, codes were grouped into descriptive themes, first individually, and then in a consensus procedure until everyone agreed.
The same three authors grouped the stage 2 themes, resulting in two overarching stage 3 themes. Thomas and Harden (2008) [ 18 ] have described this third step as generating analytical themes. In the current synthesis, however, the two main themes generated were descriptive, and will therefore be referred to as main themes. Throughout the process, the emerging results were reflected upon in relation to the results of the primary studies to ensure that the findings would be grounded in the data and interrelated with each other to form a systematic whole. Quotes illustrating the findings were selected by all authors together.
Assessment of the reliability of the combined findings
The reliability of the synthesis was assessed using GRADE-CERQual ( www.cerqual.org ), which consists of four domains: methodological limitations, coherence, adequacy of data, and relevance. Three authors (EA, PM, LS) conducted the assessments. First, two authors (LS and EA) assessed the synthesis individually and proposed a preliminary assessment which was then reviewed by a third author (PM), adding new perspectives. Finally, consensus was reached among the three authors to reach a reliability assessment for each descriptive theme in stage 2.
Characteristics of the included studies
The eight studies represented the experiences of 255 women from the UK, Australia, and Canada. See Table 2 for detailed information about the participants, the treatments, and the research methodologies.
- Meta-synthesis
The meta-synthesis resulted in two main themes: Circumstances and expectations; and Experiences of treatment (stage 3) with two and four (stage 2) descriptive themes, respectively. See Table 3 for certainty of evidence assessment and CERQual components grading for each descriptive theme.
Main theme 1: circumstances and expectations
Practical circumstances and social support were important for treatment to be feasible . Women in several studies described how important practical and social circumstances could be for them to take part in treatment.
Women talked about practical issues such as transportation [ 20 ] and childcare [ 19 , 20 ] as fundamental. The internet-based therapies were appreciated for being accessible outside of office hours, despite some women having limited time for the program [ 22 ]. Another aspect was that many participants felt a lack of support from family and friends [ 20 , 21 , 26 ], and treatment was their only opportunity to talk about how they were feeling. Other women experienced some support from their family and meant that this support was vital for treatment.
“I didn’t have anyone to talk to and no one actually knew about me being diagnosed with postnatal depression, my mum or anyone, no one knew, not even my partner. So it was quite nice just to offload on someone.” (HV listening visits [ 26 ] )
Expectations, previous experiences, and attitudes influenced how women experienced treatment.
Women in most of the studies reported on how previous experiences, expectations, motivation, and beliefs about PPD influenced their experience of treatment. The women’s expectations of treatment were generally positive, however, there were those who didn’t believe that treatment would help them, grounded in a sense of hopelessness [ 21 ], or because of low confidence in health services, e.g., fear of not being understood [ 25 , 26 ] or not being taken seriously [ 21 ]. Others talked about how their feelings of shame for being depressed, and thoughts about not being a good mother, affected how they believed treatment providers would perceive them [ 24 , 26 ]. Obstacles to seeking help could also be previous negative experiences of certain health professionals [ 24 , 25 ] or screening procedures [ 24 ], or fear of having their child removed if they revealed their depression [ 25 ].
“None of us have ever admitted to having postnatal depression…there is still a stigma it’s incredible.” (Online-CBT [ 21 ] )
There were women who had their own thoughts about why they were depressed, how it should be treated, and the potential of the treatments [ 19 , 24 , 25 ].
“All you want is someone to actually listen to what you’re saying, even if it is complete crap and it’s all coming out wrong. You just want someone to say: “it’s alright, sit down and I’ll listen to what you’ve got to say”. That would do you the world of good and I think it would actually stop people from developing worse symptoms because people just won’t talk about it.” (HV person-centered intervention [ 25 ] )
Some women worried that other participants in group sessions [ 20 ] or the health visitor [ 26 ] might disclose confidential information and chose therefore to not share all their thoughts and problems.
Main theme 2: experiences of treatment
Overall, the included studies showed that the women were satisfied with the treatments they had received. Contributing factors were the format and content of the treatments, as well as the clinician’s approach.
The received treatment’s modality was appreciated, but women had specific preferences concerning length, scope, and individual adaptations.
Most of the studies included women’s thoughts and experiences of the treatment formats. Women who had received group therapies generally expressed positive experiences. They appreciated hearing other women’s stories, and that they could support each other [ 19 , 20 ]. Objections towards the group format could be not feeling connected with others in the group, or that group therapy does not suit everyone [ 19 ]. Others would have liked more group sessions, and individual sessions as an adjunct to the group sessions [ 20 ].
Women who received home visits were satisfied to receive support in their own environment and with the continuity [ 23 , 25 , 26 ].
Some advantages mentioned by women who received internet-based CBT were accessibility and flexibility and to be able to work with the modules when they could fit it in [ 21 , 22 ]. Internet therapy was experienced as less scrutinizing than face-to-face therapy [ 22 ], and less stigmatizing [ 21 ]. In one treatment model, the internet format was individualized with a personal e-mail from the therapist, which was appreciated [ 22 ].
“When my maternal depression was really bad, there was no way I would have left my house to speak with a therapist — I was so weepy, shaky and terrified. …//… in those early weeks, the sort of anonymous nature of this program was a Godsend.” (Internet CBT [ 22 ] )
In another study, where the internet format did not include any personal contact with the therapist, there were more dropouts, and the women had several suggestions for improvement, e.g., a more needs-based and relevant content, a more interactive format, and more individual support [ 21 ].
Regardless of treatment format, there were women who would have liked more treatment sessions and more flexibility and tailoring [ 20 , 21 , 22 , 26 ]. Other women were happy with the number of sessions [ 22 ]. Ending therapy was described as a potentially anxiety provoking time [ 19 , 26 ]. When women experienced continued support from family, other group participants, or professionals, this did not have to be a problem [ 19 ]. When no other support was available, however, ending therapy could be experienced negatively [ 26 ].
“Just me thinking about it [the idea of no treatment after the visits] now makes me feel quite panicky… what would have been the point of ripping off the plaster and starting to abrade the wound, only to then just say, oh well.” (HV listening visits [ 26 ] )
The relationship with the clinician, and perceptions about her/his competencies influenced how treatment was experienced.
Women in all eight studies talked about how they experienced their relationship to their health professional and their competencies.
The relationship with the nurse or therapist was described as important, regardless of treatment model or format. A good relationship was associated with trust and being able to talk about their depression. Some specific aspects of the relationship mentioned were chemistry [ 19 , 22 ], credibility and broad competence [ 20 , 21 , 23 , 26 ], e.g., knowledge of both infant’s needs and postnatal depression [ 23 ], interpersonal skills [ 20 , 23 ], and intercultural and language competencies [ 20 ].
She [health visitor] was so understanding and easy to talk to and willing to listen, that I actually opened up, otherwise I wouldn’t have done. (HV listening visits [ 24 ] )
Sometimes, a good relationship was not established, or mothers did not feel confident that their therapist had the appropriate competence or necessary personal qualities [ 24 , 25 , 26 ], or was not flexible [ 26 ]. These experiences could lead women to decline further sessions [ 24 , 25 ]. Some mothers wondered about who the home visitor’s primary interest was, the mother or the baby [ 25 ].
Women expressed varying opinions about the treatments’ content, therapeutic approach, and the extent of their own expected contribution.
Most studies included views concerning the specific content and therapeutic approach of the received treatment, and how this impacted the women’s own contribution.
Women who received home visits had many thoughts about the health visitors’ approach [ 23 , 24 , 25 , 26 ]. Active listening with an empathetic and non-judgmental approach was appreciated by many women as helpful for feelings of guilt and inadequacy [ 23 ].
Homework between sessions could be perceived as burdensome while also helpful [ 19 , 20 ]. Some components were appreciated by many, for example, psychoeducation [ 22 , 25 ], challenging thoughts [ 19 ] and storytelling in group sessions [ 20 ].
We’ve analyzed all the reasons why I’ve been down and depressed, how to, sort of, challenge negative thoughts. (Individual CBT [ 25 ] )
In the older studies there were women who didn’t find the home visits meaningful [ 24 , 25 ], and these were sometimes described as too unstructured [ 24 ]. In the newer studies, however, the experiences of home visits were generally positive. Although the home visits were intended to be supportive, i.e., not giving advice, there were women who expressed a need for more clear and concrete advice from their home visitor [ 23 , 25 , 26 ].
Also, women who received CBT expressed positive experiences of their therapist’s personal approach [ 19 , 22 ].
[The internet therapist was] so helpful and thoughtful. She wasn’t hard on me like I am on myself and really made me stop and think about how I treat myself. (Individual CBT [ 22 ] )
Women described positive treatment outcomes, but a few did not experience any improvement.
In general, women experienced their received intervention as helpful, and positive for their confidence and self-esteem. Treatment was described having led to a better understanding of their own distress and to insights about depression [ 20 , 26 ], to acceptance and normalization, a generally more positive outlook on life and the future, and an increased sense of control [ 19 , 20 , 22 ].
Not dwelling on all the negatives that I might feel, and she really made me see the little things that actually were big things that I’d done in life, so yeah, I think it made me a very different, you know, person. (Individual CBT [ 19 ] )
A common experience following treatment was a better mother-infant relationship. Women described how they had gained knowledge about infants and about their own importance for their child’s development [ 23 ]. Many felt that their own improved mood had led to a better relationship with their child [ 19 , 22 ] and that they had become more relaxed, patient, and secure in their parental role [ 22 , 23 ].
By 12 months, I felt I had the tools within myself to continue with sureness that I was a capable, confident mother. (Supportive home visits [ 23 ] )
There were women who didn’t experience any improvement. In general, these women didn’t perceive supportive counselling as therapy [ 24 ], or as a sufficiently powerful intervention [ 26 ], and proceeded to seek other treatments instead. This was particularly notable in women with more chronic or recurrent depression [ 24 , 26 ].
This meta-synthesis was based on studies that explored women’s experiences of CBT or supportive home visits. Treatments were individual or group-based.
Overall, the women were satisfied with their treatment, although various practical and social circumstances, as well as their own expectations, had an impact on their participation in and experience of treatment. Some findings reported were increased confidence and sense of control, and a better mother-infant relationship. Similarly, in an earlier meta-synthesis of psychological and psychosocial interventions for PPD, almost all included studies reported that women found their interventions helpful, specifically concerning their distress, their parenting, and their relationships [ 12 ].
Reoccurring themes in the current and previous syntheses were women’s wishes of being involved in decisions concerning their treatment and the impact of their own expectations of treatment [ 12 , 14 , 27 ]. They wanted to be involved in the choice of treatment type and format, and for treatments to be individualized, e.g., the selection and order of modules to be tailored to their personal preferences and practical circumstances. It has been argued that therapeutic alliance as well as flexibility, i.e., tailoring psychological treatments to the individual’s needs and circumstances can be more important than fidelity to treatment protocols [ 28 ]. In meta-analyses exploring the effectiveness of PPD, CBT has consistently demonstrated a favorable impact, e.g., Sockol et al. (2015) and Huang et al., (2018) [ 29 , 30 ], with a relatively large number of studies confirming these results. Furthermore, this effect seems to be consistent for different formats (therapy delivered individually, in groups, or digitally) [ 31 ]. This is encouraging, suggesting that mothers’ preferences for various formats align with positive outcomes from an efficacy perspective, potentially instilling a sense of confidence in clinicians when considering the delivery of CBT in diverse forms. A recent synthesis investigating experiences of psychological treatment for depression in a broader context, excluding PPD [ 27 ], highlights how expectations concerning specific therapeutic approaches or formats can influence motivation and engagement in therapy.
The current synthesis identified some general expectations, e.g., positive previous experiences of care or expecting services to be under-resourced. There were also expectations, beliefs, and fears more specific to the perinatal period and related to being a new mother, in line with other syntheses in postpartum contexts [ 12 , 14 ], such as motivation to get better, or fear of not being understood or not taken seriously. Mothers also worried they were, or would be seen as a bad mother, sometimes to the extent of fear of having their child removed. Our synthesis, as well as the one by Hadfield et al. [ 12 ] also identified women’s uncertainty concerning the health visitor’s role and competence to assess and support the mental wellbeing of mothers, which could sometimes lead to discontinuing treatment.
Women who had received group therapy expressed mainly positive experiences, consistent with McPherson et al.’s (2020) synthesis of non-postpartum treatments, where the group format contributed to normalization when realizing that they shared similar experiences and were not alone [ 27 ]. A negative aspect of the group format identified by McPherson et al., but less evident in our synthesis, was not feeling safe disclosing feelings, thus censoring what they shared. Common findings regarding CBT approaches were finding homework burdening, and more evident in McPherson’s synthesis than in the current, that CBT-modules could be difficult to apply.
Another finding, in line with Hadfield and Wittkowski [ 12 ] and a review by Daehn et al. investigating help-seeking among perinatal women [ 7 ], was the role of support from the partner or other family members to seek and take part in treatment. Practical circumstances such as transportation and childcare issues were evident for depressed mothers in the current and Hadfield’s synthesis, providing one reason for home visits being appreciated. However, the review of treatments in non-postpartum populations by McPherson et al. also found that transportation could be a problem and that remote therapy was preferred by some patients [ 27 ].
The significance of establishing a good relationship to their health professional was emphasized by the women, regardless of the treatment’s format or theoretical basis, consistent with other syntheses [ 12 , 14 , 27 ]. An empathetic, supportive, and non-judgmental approach was essential for the women’s wish to follow through with the treatment, and for their recovery. This is understandable considering how depression during this period is associated with feelings of anxiety, guilt, and worthlessness [ 32 , 33 ]. In the synthesis by Megnin-Viggars et al. (2015) women emphasized continuity of care; for example, seeing the same nurse or therapist during the whole care period from assessment to treatment and follow-up, as important for being able to disclose symptoms of depression [ 14 ]. McPherson et al. (2020) emphasize patients’ descriptions of the therapeutic relationship as collaborative, and providing a space for sharing thoughts and feelings, and for receiving advice [ 27 ].
Methodological strengths and limitations
Eight studies with low and moderate methodological limitations were included in the synthesis, and the findings concerning the women’s experiences were concordant among the included studies. Most studies had relatively few participants, but the interviews generated rich data with detailed descriptions of experiences. Most of the studies contributed data to all six descriptive themes, which were assessed as reflecting the variation in the findings, including contradicting and differing views and the complexity in the participants’ experiences. Authors had used semi-structured interview schedules with similar topic guides, likely explaining the similar types of narratives found. All studies lacked information about the researchers’ competencies and experience, and relationship to the participants; thus, how the authors’ preunderstandings were taken into consideration is largely unknown.
Other limitations are that the included studies were from the UK, Australia, and Canada and only one study targeted ethnic minorities, limiting the generalizability of our findings. Also, four of the eight included studies were more than 10 years old. Considering that we found some differences between the older versus newer studies in our review, it is possible that the delivery and formats of these treatments, mainly listening visits by a nurse or health visitor, may have changed over time suggesting a need for more updated studies.
A treatment with perhaps even better effect on depression during the perinatal period is Interpersonal therapy (IPT) [ 34 ], although less studied. It has been suggested that IPT may be especially suitable for women with postpartum depression because it focuses on improving relationships and addressing social support, which can be critical during the challenging postpartum period. IPT has been found to help women navigate the interpersonal challenges and changes that often accompany motherhood [ 34 ]. Unfortunately, our current meta-synthesis did not include any IPT studies, and limited data on treatment experiences are available. However, one study by Grote et al. (2009) reported high treatment satisfaction among mothers treated for PPD with IPT, as assessed through a brief questionnaire [ 35 ].
Strengths of the study include our following of an established method for synthesizing qualitative findings. Furthermore, and unlike previous meta-syntheses, we used CERQual to assess confidence in these findings.
Conclusions
Most women described positive outcomes of the treatment they received, and findings suggested improved parent-related outcomes. The findings highlight the importance of involving women in decisions concerning treatment for postpartum depression so that support can be tailored to their circumstances and preferences. It is important for practitioners to take an interest in the women’s own thoughts about why they are depressed and their expectations of the treatment. Furthermore, the personal approach of the health professional; non-judgmental, sensitive, and able to convey hope is important during this vulnerable time. There is a need for updated research, including experiences of IPT.
Data availability
The search strategy and search terms used are available in Appendix 1. The data that support the findings of the current study is available in Swedish from the corresponding author upon reasonable request.
Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–83.
Article PubMed Google Scholar
Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86–92.
Article CAS PubMed Google Scholar
Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313–27.
Article PubMed PubMed Central Google Scholar
Howard LM et al. Non-psychotic mental disorders in the perinatal period. The Lancet. 2014;384(9956):1775–88.
Article Google Scholar
Dennis C-L, Chung-Lee L. Postpartum Depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth. 2006;33(4):323–31.
Goodman JH. Women’s attitudes, preferences, and perceived barriers to treatment for Perinatal Depression. Birth. 2009;36(1):60–9.
Daehn D, Rudolf S, Pawils S, Renneberg B. Perinatal mental health literacy: knowledge, attitudes, and help-seeking among perinatal women and the public – a systematic review. BMC Pregnancy Childbirth. 2022;22(1):574.
Westgate V, Manchanda T, Maxwell M. Women’s experiences of care and treatment preferences for perinatal depression: a systematic review. Arch Women Ment Health. 2023;26(3):311–9.
Cuijpers P et al. Psychological treatment of perinatal depression: a meta-analysis. Psychol Med. 2021;53(6):2596–608.
Dennis C-LE. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007(4):CD006116.
SBU. Psychological treatment for postpartum depression. A systematic review including health economic and ethical aspects in SBU assessment. Stockholm: Swedish Agency for Health Technology Assessment and Assessment of Social Services; 2022.
Hadfield H, Wittkowski A. Women’s experiences of seeking and receiving psychological and psychosocial interventions for postpartum depression: A systematic review and thematic synthesis of the qualitative literature. Journal of midwifery & women’s health; 2017; 62(6)723–36.
Byatt NDO et al. Patient’s views on depression care in obstetric settings: how do they compare to the views of perinatal health care professionals? Gen Hosp Psychiatry. 2013;35(6):598–604.
Megnin-Viggars O, Symington I, Howard LM, Pilling S. Experience of care for mental health problems in the antenatal or postnatal period for women in the UK: a systematic review and meta-synthesis of qualitative research. Arch Women Ment Health. 2015;18(6):745–59.
McGowan J, et al. PRESS peer review of electronic search strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement: e1000097 PLoS medicine, 2009;6(7).
SBU. Bedömning av studier med kvalitativ metodik [SBU Quality assessment tool for studies with a qualitative design] [In Swedish]. 2022. Available from: https://www.sbu.se/globalassets/ebm/bedomning_studier_kvalitativ_metodik.pdf .
Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008;8(1):45–5.
Hadfield H, Glendenning S, Bee P, Wittkowski A. Psychological Therapy for Postnatal Depression in UK Primary Care Mental Health Services: a qualitative investigation using Framework Analysis. J Child Fam stud. 2019;28(12):3519–32.
Masood Y et al. Group psychological intervention for postnatal depression: a nested qualitative study with British south Asian women. BMC Womens Health. 2015;15(1):109–9.
O’Mahen HA, et al. Women’s experiences of factors affecting treatment engagement and adherence in internet delivered behavioural activation for postnatal depression. Internet Interventions: The Application of Information Technology in Mental and Behavioural Health. 2015;2(1):84–90.
Pugh NE, Hadjistavropoulos HD, Hampton AJD, Bowen A, Williams J. Client experiences of guided internet cognitive behavior therapy for postpartum depression: a qualitative study. Arch Women Ment Health. 2015;18(2):209–19.
Rossiter C, Fowler C, McMahon C, Kowalenko N. Supporting depressed mothers at home: their views on an innovative relationship-based intervention. Contemp Nurse: J Australian Nurs Profession. 2012;41(1):90–100.
Shakespeare J, Blake F, Garcia J. How do women with postnatal depression experience listening visits in primary care? A qualitative interview study. J Reproductive Infant Psychol. 2006;24(2):149–62.
Slade P et al. Postnatal women’s experiences of management of depressive symptoms: a qualitative study. Br J Gen Pract. 2010;60(580):e440–8.
Turner KM, Chew-Graham C, Folkes L, Sharp D. Women’s experiences of health visitor delivered listening visits as a treatment for postnatal depression: a qualitative study. Patient Educ Couns. 2010;78(2):234–9.
McPherson S, Wicks C, Tercelli I. Patient experiences of psychological therapy for depression: a qualitative metasynthesis. BMC Psychiatry. 2020;20(1):313–3.
Fonagy P. Fidelity vs. flexibility in the implementation of psychotherapies: time to move on. World Psychiatry. 2019;18(3):270–1.
Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affect Disord. 2015;177:7–21.
Huang L, Zhao Y, Qiang C, Fan B. Is cognitive behavioral therapy a better choice for women with postnatal depression? A systematic review and meta-analysis. PLoS ONE. 2018;13(10):e0205243.
Li X, et al. Effectiveness of cognitive behavioral therapy for perinatal maternal depression, anxiety and stress: a systematic review and meta-analysis of randomized controlled trials. Clin Psychol Rev. 2022;92:102129.
Hoertel N, et al. Are symptom features of depression during pregnancy, the postpartum period and outside the peripartum period distinct? Results from a nationally representative sample using item response theory (IRT). Depress Anxiety. 2015;32(2):129–40.
Fox M, Sandman CA, Davis EP, Glynn LM. A longitudinal study of women’s depression symptom profiles during and after the postpartum phase. Depress Anxiety. 2018;35(4):292–304.
Jiang X et al. Efficacy of nondrug interventions in perinatal depression: a meta-analysis. Psychiatry Res. 2022;317:114916.
Grote NK et al. A randomized controlled trial of culturally relevant, brief interpersonal psychotherapy for Perinatal Depression. Psychiatric Serv. 2009;60(3):313–21.
Download references
Acknowledgements
Not applicable.
Open access funding provided by University of Gothenburg. Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU). SBU is a government agency under the Ministry of Health and Social Affairs in Sweden. This study was performed as part of a governmental research assignment in the area of women’s health.
Open access funding provided by University of Gothenburg.
Author information
Authors and affiliations.
Department of Psychology, University of Gothenburg, Gothenburg, Sweden
Pamela Massoudi
Department of Research and Development, Region Kronoberg, Växjö, Sweden
Swedish Agency for Health Technology Assessment and Assessment of Social Services, Stockholm, Sweden
Leif A. Strömwall & Maja Kärrman Fredriksson
Department of Global Public Health, Karolinska Institutet, Stockholm, Sweden
Johan Åhlen
Institute of Health and Care Sciences, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Anna Dencker
Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden
Ewa Andersson
You can also search for this author in PubMed Google Scholar
Contributions
This research is based on work conducted at The Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU), with author LE as project manager. All authors contributed to the study design and research questions. The search strategy was developed by information specialist, author MKF, in collaboration with all authors. MKF was responsible for the literature search. Authors PM, LE, JÅ, AD, and EA were involved in the initial assessments of each study. Three authors (PM, LS, and EA) were responsible for quality assessments of the included studies, for data extraction and synthesis, and for assessing the reliability of the synthesis (GRADE CERQual). The first draft of the manuscript was written by the first author (PM) and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Pamela Massoudi .
Ethics declarations
Consent for publication, role of funder.
The funder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Ethics approval
Consent to participate, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Massoudi, P., Strömwall, L.A., Åhlen, J. et al. Women’s experiences of psychological treatment and psychosocial interventions for postpartum depression: a qualitative systematic review and meta-synthesis. BMC Women's Health 23 , 604 (2023). https://doi.org/10.1186/s12905-023-02772-8
Download citation
Received : 12 July 2023
Accepted : 06 November 2023
Published : 14 November 2023
DOI : https://doi.org/10.1186/s12905-023-02772-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Perinatal mental health
- Postnatal depression
- Psychotherapy
- GRADE-CERQual
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Research article
- Open access
- Published: 30 March 2020
Prevalence of postpartum depression and associated factors among postnatal care attendees in Debre Berhan, Ethiopia, 2018
- Abate Dargie Wubetu 1 ,
- Nigus Alemnew Engidaw 1 &
- Kefyalew Dagne Gizachew 1
BMC Pregnancy and Childbirth volume 20 , Article number: 189 ( 2020 ) Cite this article
14k Accesses
22 Citations
3 Altmetric
Metrics details
Postpartum depression explains various groups of depressive symptoms and syndromes that can take place during the first 6 weeks following birth. The postpartum period is a critical time where both mild and severe mood disorders can occur. The familiar forms are baby blues and postpartum depression. Understanding the prevalence and associated factors of postpartum depression is mandatory for early detection and treatment.
Institution based cross-sectional study was conducted from 1st May to June 30, 2018. The study participants were eligible women who came to Debre Berhan referral hospital and health centers for postnatal care and vaccination service. The Edinburgh postnatal depression scale was used to assess postpartum depression. A systematic random sampling technique was used to collect the data after determining the skip fraction (k = 2). The collected data were coded and entered into Epi-info version 7 and transported to SPSS version 20 for analysis. Both bivariate and multivariate binary logistic regression were done to identify associated factors. During bivariate analysis, variables with p -value < 0.05 were included in multivariate analysis. Odds ratios and their 95% confidence intervals were computed and variables with p-value less than 0.05 were considered to declare significantly associated factors (multivariate analysis).
A total of 308 mothers who attended postpartum care we're included, which was a 100% response rate. The prevalence of postpartum depression was found to be 15.6% (95%CI = 11.7, 19.8). Being widowed/widower, having poor social support, having a current hospitalized child, and experienced a death of family member or close relative were significantly associated with postpartum depression.
Conclusions
The prevalence of postpartum depression was lower than most studies done in different areas. Major life events and traumas are associated with an increased risk of postpartum depression. Health professionals should be aware of the mother’s circumstances during the puerperium, they should initiate support to reduce the risk of depression in the postpartum period. Health care professionals working postpartum care clinics should give special attention to mothers who are widowed/widower, have poor social support, have a current hospitalized children, and experienced a death of family member or close relative.
Peer Review reports
Postpartum Depression (PPD) refers to non-psychotic depressive episodes that begin in or extend into the postpartum period [ 1 ]. According to The American Psychiatric Association (APA) postpartum depression is defined as the occurrence of a Major Depressive Episode (MDE) within 4 weeks after delivery [ 2 ].
About 14% of the worldwide burden of disease has been attributed to neuropsychiatric disorders, including those disorders that can occur during the postpartum period. Such estimates have drawn attention to the importance of mental disorders for public health [ 3 ]. The estimated lifetime prevalence of having one or more of the mental disorders varies widely across the world as shown by mental health surveys, from 12.1% in Nigeria to 47.4% in the United [ 4 ].
Postpartum depression is a non-psychotic depressive disorder that affects 13 to 19% of postpartum women and those women experience signs and symptoms like self-blaming thought, guilt about their inability to look after their new baby, low self-esteem, lack of interest in one’s environment, insecurity and suicidal thoughts. This condition begins in the postpartum period and persists up to a one-year duration after delivery. The treatment option for PPD women is behavioral counseling and anti-depressant therapy [ 2 , 5 , 6 , 7 ].
World Health Organization (WHO) reported that for women of reproductive age group depression becomes the leading cause of disease burden [ 8 ]. Postpartum nonpsychotic depression is a considerable public health problem and the most common complication of childbearing age that affect approximately 10–15% of postpartum women. In developing countries, the prevalence of postpartum depression almost doubled that of the developed world. The effect of postpartum depression on the mother, her marital relationship and her children make it an important condition to diagnose, treat and prevent [ 9 , 10 ]. Untreated postpartum depression can have a prolonged adverse effect on the mother and her children. Pregnant mothers’ ongoing depression can contribute to emotional, behavioral, cognitive and interpersonal problems [ 11 ].
Epidemiological studies conducted in China, Japan, India and New Dubai Hospital in Dubai, revealed that the overall prevalence of postpartum depression was 13.5, 17, 23, and 15.8% respectively [ 12 , 13 , 14 , 15 ]. Another quasi-experimental study conducted among 420 consenting pregnant women on the title of postpartum depression in peri-urban communities of Karachi, Pakistan, revealed that the overall prevalence of postpartum depression was 28.8% [ 16 ].The growth of the child is potentially affected in response to the potential decline in care by the mother experienced PPD. Determining the prevalence of postpartum depression, and identifying associated factors with it is important to show the magnitude of the problem. This study aimed to determine the prevalence of postpartum depression in the study area and to identify associated factors of postpartum depression.
Specific objectives
To determine the prevalence of postpartum depression in postnatal care attendees
To identify factors associated with postpartum depression in postnatal care attendees.
The study area, design and period
The study was conducted in Debre Berhan town which found in the North Shoa zone at Amhara regional state of Ethiopia. This town is found 130 km away from the capital city of Ethiopia; Addis Ababa. The cross-sectional study design was employed from 1st May to June 30, 2018. The study site had a total of one government-owned referral hospital, three health centers, five private clinics, and more than ten pharmacies. There were 613 mothers who gave birth and attended postpartum care and vaccination service during the study period.
Source Population: All women who came for postnatal care and vaccination services within 6 weeks after delivery in a referral hospital and health centers in Debre Berhan, Town Ethiopia.
Study Population: All women who came for postnatal care and vaccination service within 6 weeks after delivery during the data collection period.
Eligibility
Inclusion criteria.
All women who gave birth and who came for postnatal care and vaccination service within 6 weeks after delivery in health centers and referral hospital were included.
Exclusion Criteria
Women who had a verbal communication problem and complete loss of hearing were excluded.
Sample size calculation and sampling technique
The required sample size was determined by using a single population proportion formula with the following assumptions: (Z α/2) = value for the 95% CI, =1.96, the proportion of postpartum depression; similar study at Gondar, Ethiopia ( P = 23%) [ 17 ], d = margin of error taken as 5%; by adding 10% of study subjects as nonresponse rate, the final sample size became 308. The study subjects were interviewed by using systematic random sampling after determining the sampling fraction (k = 613/308 = 2) and the first participant was selected by using the lottery method. The total sample size ( n = 308) was allocated proportionally according to the total number of postpartum care and vaccination service attendees at each health center (district 04, district 07, district 08) and Debre Berhan referral hospital.
Study variables
Dependent variable.
Postpartum Depression (yes/no).
Independent variables
Socio-demographic factors: (age, educational status, economic, marital status, employment, monthly income, current residence).
Social factors: - social and husband support, emotional violence, physical violence, sexual violence.
Substance use: use of any substance during the puerperium period for a non-medical purpose (like Khat, alcohol, and cigarette).
Obstetrics factors: parity, pregnancy intention, currently a hospitalized child, mode of delivery, perinatal complication or illness, stressful life event during the puerperium period and undesired fetal sex.
Previous psychiatric history: A family history (first-degree relatives) of psychiatric problems.
Operational definitions
Poor social support.
Mothers who scored 3–8 on the (Oslo-3) social support scale during puerperium.
Moderate social support
Mothers who scored 9–11 on the (Oslo-3) social support scale during puerperium.
Strong social support
Mothers who scored 11–14 on the (Oslo-3) social support scale during puerperium.
Data collection tools and procedures
A structured interviewer-administered questionnaire was used to collecting information from study participants. Sociodemographic, clinical, and obstetric factors were assessed by predefined checklists. The social support level was assessed by using the Oslo social support scale, and the Edinburgh Postnatal Depression Scale (EPDS) was used to assess postpartum depression. Data were collected with an interviewer-administered questionnaire from mothers who came for postnatal care and vaccination service.
Data quality control and analysis
The data collection instrument was pre-tested on 5% of the sample size out of Debre Berhan town to improve language clarity and appropriateness of data collection tools. The estimated time required, and necessary amendments were made after the piloting of the questionnaire. Four fourth-year undergraduate nursing students collected the data. The data collectors were trained for 1 day on the techniques of data collection. The training also included the importance of disclosing the possible benefit and purpose of the study to the study participants before the start of data collection. The researcher checked completeness and consistency of questionnaires filled by the data collectors to ensure the quality of data and also visited the data collectors as many times as possible to check whether he/she collected the data appropriately. The collected data were entered into Epi-info version 7 and analysis was done after the data were imported to SPSS version 20. During bivariate analysis, variables with p -value < 0.05 were exported to multivariate analysis. Crude and adjusted odds ratios were analyzed using bivariate and multivariable binary logistic regression analysis and the level of significance of association was determined at p-value < 0.05.
Socio-demographic characteristics of postpartum mothers
There were 613 mothers who gave birth and attended postpartum care and vaccination service during the study period. Among them, 308 mothers were included in the study by using systematic random sampling technique, which was a 100% response rate. Among the study subjects, 286 (86%) were aged 25–45 years and almost 85% were married. The majority of the participants, 206(66.9%) had attended formal (modern) education. Regarding ethnicity, the majority of the study participants, 234(76%) were Amhara and 62(20.1%) were Oromo. Two hundred sixty-eight (87%) of the participants earn a monthly income greater than 2500 Ethiopian Birr. Almost 60 % of the participant’s religion, 191(62%) were orthodox Christian followers (Table 1 ).
Obstetric and clinical characteristic of postpartum mothers
From 308 study participants, the majority of respondents 254(82.5%) were multigravida (give birth > 1) and 54(17.5%) were primigravida (having a first child). Almost 80% of participants had two or more living children during the study period. Regarding termination of pregnancy, 53(17.2%) had experienced termination and 39(12.7%) had experienced the death of their child. Forty-eight (15.6%) participants reported that the recent pregnancy was unplanned. Moreover, the sex of the last baby 189(61.4%) were male and the rest were female. Regarding the desired sex of the last baby, 36(11.7%) of the respondents said that the sex of their infant was unwanted gender. Nearly 62% of participants, 190(61.7%) mode of delivery was a spontaneous vaginal delivery. Forty-seven, 47(15.3%) respondents had suffered from any diagnosed illness during their last pregnancy and 95(30.8%) study mothers reported their babies were admitted to the hospital at least once before (Table 2 ).
Psychosocial factors (in last 6 months) of postpartum mothers
From the total study participants, 62(20.1%) responded that their family or a close relative had died. Almost one fifth (19.5%), participants reported that there was a serious illness, injury or assault during the recent pregnancy. Almost 60, 59(19.2%) study participants had experienced parent or child death and 42(13.6%) participants reported that they were separated due to marital difficulty. In addition, 41(13.7%) study participants were unemployed / not been able to work in the last 6 months of the study period. Moreover, 40(13%) reported physical violence during the last pregnancy (Table 3 ).
Substance use among postpartum mothers
Overall, 31(10.1%) of study participants reported the use of any substance before pregnancy and of these the majority of use was alcohol-related; i.e. 21(67.7%). The remaining used only Khat at least once in a lifetime. Regarding substance used during the last pregnancy, 18(5.8%) respondents used any kind of substance, and all of them used alcohol.
History of known illness among postpartum mothers
Of the total study participants, 31(10.1%) had a known history of mental illness. In addition, 44(14.3%) study respondents had a family history of known mental illness and 28(9.1%) had diagnosed diabetes mellitus and hypertension.
Social support among postpartum mothers
Social support status was assessed by using the Oslo-3 social support scale. From the total study participants, the majority 137(44.5%) had moderate social support, 114(37%) had poor social support and the rest had strong social support. During pregnancy, 175(56.8%), 111(36%), and 22(7.1%) had strong, moderate, and poor husband support respectively. Thirty-six percent, 112(36.4%) study participants had no practical support from a family member during pregnancy (such as cooking, washing, cleaning or child-rearing), and during puerperium.
Prevalence of postpartum depression and its associated factors
According to the Edinburgh Postnatal Depression Scale (EDPS), study participants who scored ≥13 are considered as having postpartum depression. Hence, the prevalence of postpartum depression among mothers who have postnatal care follow up was 15.6% [95% CI = 11.7, 19.8].
Binary logistic regression was performed to assess the association of each independent variable with the outcome variable (postpartum depression). The variables that showed a significance level ( p < 0.05) during bivariate analysis were added to the multivariate regression model. Twenty-two independent variables were shown to be significantly associated during the bivariate analysis. The result of the multivariate analysis showed that only four variables were statistically significant. Being widowed/widower, having a current hospitalized child, having died family or close relative, having poor social support showed a significant association with postpartum depression.
The results showed that women who were widowed/widower had an association with postpartum depression; and were four times more likely to experience postpartum depression than those who were married [AOR = 4.17, 95% CI = 1.14, 15.20]. In addition, respondents who had poor social support were five times more likely to be depressed than those who had strong social support [AOR = 5.11, 95% CI = 1.00, 26.18]. Respondents who had a current hospitalized children were nearly 3 times more likely to be depressed as compared to respondents who does not have a current hospitalized child [AOR = 3.32, 95%CI = 1.39,7.93]. In a similar dimension, participants who had experienced a death of a family member or close relative in the last 6 months were three times more likely to be depressed than those who did not experience this [AOR = 2.92, 95%CI = 1.01,8.50], (Table 4 ).
Prevalence of postpartum depression
There were 613 mothers who gave birth and attended postpartum care and vaccination service during the study period. Among them, 308 mothers were included in the study by using systematic random sampling technique (k = 2), which was a 100% response rate. The overall prevalence of postpartum depression was 15.6%(95%CI = 11.7, 19.8).
This was almost similar to other studies that were conducted in Delhi and adjacent states of northern India, 15.8% [ 18 ], Egypt, 17.9% [ 19 ], and Uganda, 16.3% [ 20 ].
The prevalence rates were higher in our study when compared with Canadian, Denmark, and Uganda (Kampala), and Egypt study which was 1.6, 5.5 and 6.1%, 7.14% respectively [ 21 , 22 , 23 , 24 ]. The higher rate might be due to the use of different measurement tools, assessment period, social support level and economic status of the mothers.
On the other hand, this figure was lower when compared with other similar studies done in Lebanon, 21% [ 25 ], Cameroon, 23.4% [ 26 ], Nigeria, 23% [ 27 ]. The lower prevalence rate in our study might be due to difference in residency, and sample size difference. For instance, the study in Lebanon was conducted in a rural area by using a follow-up study with a sample size of 396 mothers. In addition, the study conducted in Cameroon used a case-control study design while our study used a cross-sectional study design. Similar studies in Ethiopia revealed that 22.1% [ 28 ], 22.4% [ 29 ], 31.5% [ 30 ], of mothers were depressed during puerperium. These studies had higher prevalence rates than our study. The higher prevalence report in these studies might be due to the screening tool, study design, and sample size. The study done in the Oromia region used a self-reporting questionnaire (SRQ) and a community-based cross-sectional study.
Factors associated with postpartum depression
Among the sociodemographic factors, study subjects who were widowed/widower had an association with postpartum depression: almost four times higher when compared with those who were married. In this cohort, the association was in agreement with the study done in Ethiopia [ 31 ]. The agreement might be due to the fact that being married is important for mental health; especially during the postpartum period.
In the social support dimension, respondents who had poor social support were more likely to be depressed than those who had strong social support. The association in estimation was in line with studies done in Malaysian and Pakistan [ 32 ], Cameroon; Yaoundé [ 26 ] and Hiwot Fana specialized University Hospital in Ethiopia [ 10 ]. In fact, having poor social support is one of the highest contributors to poor mental health [ 33 ].
The variables that were found to have an association with postpartum depression were having a hospitalized child during the postpartum period. Respondents who had a current hospitalized child were almost three times more likely to be depressed as compared to the respondent who had not a current hospitalized child. In a similar dimension, participants who had experienced a death of a family member or close relative in the last 6 months were three times more likely to have postpartum depression than those who does not experienced a death of a family member or close relative. The association was in agreement with the study done in Robe town; Bale Zone, Ethiopia [ 30 ]. The possible reason might be due to the fact that experiencing life-threatening events during the postpartum period became intolerable and may affect the mental wellness of the mothers.
Limitations
Postpartum women with persisting depression already acquired before/during pregnancy were not excluded and this may further increase the prevalence rates of postpartum depression. The study only included mothers who had postnatal care follow up in the urban area. Since we recruited multiple data collectors, there may be interviewer bias.
Though significant proportions of postnatal mothers had depression, the prevalence of postpartum depression was lower than most studies in different areas. Major life events and trauma are associated with an increased risk of postpartum depression. Health professionals should be aware of the mother’s circumstances during pregnancy. They should initiate support to reduce the risk of depression in the postpartum period. Health care professionals working in maternal and child health clinics should give special attention to pregnant mothers who are widowed/widower, have poor social support, have a current hospitalized child, and experienced a death of a family member or close relative.
Recommendations
It would be advisable if midwife professionals routinely screen postpartum depressive symptoms and link them to mental health services just like other reproductive health problems for mothers attending hospitals and health centers after delivery.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
O'Hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65(12):1258–69.
Article PubMed Google Scholar
Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®): American psychiatric pub; 2013.
Book Google Scholar
Johnson J, Weissman MM, Klerman GL. Service utilization and social morbidity associated with depressive symptoms in the community. Jama. 1992;267(11):1478–83.
Article CAS PubMed Google Scholar
Kessler RC, Angermeyer M, Anthony JC, De Graaf R, Demyttenaere K, Gasquet I, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's world mental health survey initiative. World Psychiatry. 2007;6(3):168.
PubMed PubMed Central Google Scholar
Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: a systematic review. Obstet Gynecol. 2004;103(4):698–709.
Evans J, Heron J, Patel RR, Wiles N. Depressive symptoms during pregnancy and low birth weight at term. Br J Psychiatry. 2007;191(1):84–5.
Health NCCfM. Depression in children and young people: identification and management in primary, community and secondary care. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]: Centre for Reviews and Dissemination (UK); 2005.
Organization WH. The global burden of disease: 2004 update. Geneva: WHO; 2008. p. 2017.
Google Scholar
Stewart DE, Robertson E, Dennis C-L, Grace SL, Wallington T. Postpartum depression: literature review of risk factors and interventions. Toronto: University Health Network Women’s Health Program for Toronto Public Health; 2003.
Shewangzaw A, Tadesse B, Ashani T, Misgana T, Shewasinad S. Prevalence of postpartum depression and associated factors among postnatal women attending at Hiwot Fana Specialized University hospital, Harar, East Ethiopia, 2015/2016. Open Access J Reproductive Syst Sexual Disorders. 2018;1(1):4–19.
Jacobsen T. Effects of postpartum disorders on parenting and on offspring. Miller J Perinatal Med. 1999;39(5):515–21.
Ghubash R, Abou-Saleh M. Postpartum psychiatric illness in Arab culture: prevalence and psychosocial correlates. Br J Psychiatry. 1997;171(1):65–8.
Lee DT, Yip AS, Chiu HF, Leung TY, Chung TK. A psychiatric epidemiological study of postpartum Chinese women. Am J Psychiatr. 2001;158(2):220–6.
Yoshida K, Yamashita H, Ueda M, Tashiro N. Postnatal depression in Japanese mothers and the reconsideration of ‘Satogaeri bunben’. Pediatr Int. 2001;43(2):189–93.
Patel V, Rodrigues M, DeSouza N. Gender, poverty, and postnatal depression: a study of mothers in Goa, India. Am J Psychiatr. 2002;159(1):43–7.
Ali NS, Ali BS, Azam IS. Post partum anxiety and depression in peri-urban communities of Karachi, Pakistan: a quasi-experimental study. BMC Public Health. 2009;9(1):384.
Article PubMed PubMed Central Google Scholar
Ayele TA, Azale T, Alemu K, Abdissa Z, Mulat H, Fekadu A. Prevalence and associated factors of antenatal depression among women attending antenatal care service at Gondar university hospital, Northwest Ethiopia. PLoS One. 2016;11(5):e0155125.
Article PubMed PubMed Central CAS Google Scholar
Gupta S, Kishore J, Mala Y, Ramji S, Aggarwal R. Postpartum depression in north Indian women: prevalence and risk factors. J Obstetrics Gynecol India. 2013;63(4):223–9.
Article Google Scholar
Saleh E-S, El-Bahei W, del El-Hadidy MA, Zayed A. Predictors of postpartum depression in a sample of Egyptian women. Neuropsychiatr Dis Treat. 2013;9:15.
Kakyo TA, Muliira JK, Mbalinda SN, Kizza IB, Muliira RS. Factors associated with depressive symptoms among postpartum mothers in a rural district in Uganda. Midwifery. 2012;28(3):374–9.
Eberhard-Gran M, Eskild A, Tambs K, Samuelsen S, Opjordsmoen S. Depression in postpartum and non-postpartum women: prevalence and risk factors. Acta Psychiatr Scand. 2002;106(6):426–33.
Nielsen D, Videbech P, Hedegaard M, Dalby J, Secher NJ. Postpartum depression: identification of women at risk. BJOG Int J Obstet Gynaecol. 2000;107(10):1210–7.
Nakku JN, Nakasi G, Mirembe F. Postpartum major depression at six weeks in primary health care: prevalence and associated factors. African Health Sci. 2006;6(4).
Salem MN, Thabet MN, Fouly H, Abbas AM. Factors affecting the occurrence of postpartum depression among puerperal women in Sohag city, Egypt. Proc Obstetrics Gynecol. 2017;7(1):1–10.
Chaaya M, Campbell O, El Kak F, Shaar D, Harb H, Kaddour A. Postpartum depression: prevalence and determinants in Lebanon. Archives Women’s Mental Health. 2002;5(2):65–72.
Article CAS Google Scholar
Adama ND, Foumane P, Olen JPK, Dohbit JS, Meka ENU, Mboudou E. Prevalence and risk factors of postpartum depression in Yaounde, Cameroon. Open J Obstetrics Gynecol. 2015;5(11):608.
Owoeye A, Aina O, Morakinyo O. Risk factors of postpartum depression and EPDS scores in a group of Nigerian women. Trop Dr. 2006;36(2):100–3.
Abebe A, Tesfaw G, Mulat H, Hibdye G. Postpartum depression and associated factors among mothers in Bahir Dar town, Northwest Ethiopia. Ann General Psychiatry. 2019;18(1):19.
Toru T, Chemir F, Anand S. Magnitude of postpartum depression and associated factors among women in Mizan Aman town, bench Maji zone, Southwest Ethiopia. BMC Pregnancy Childbirth. 2018;18(1):442.
Tefera TB, Erena AN, Kuti KA, Hussen MA. Perinatal depression and associated factors among reproductive aged group women at Goba and Robe Town of Bale Zone, Oromia Region, South East Ethiopia. Maternal Health Neonatol Perinatol. 2015;1(1):12.
Bitew T. Prevalence and risk factors of depression in Ethiopia: a review. Ethiop J Health Sci. 2014;24(2):161–9.
Klainin P, Arthur DG. Postpartum depression in Asian cultures: a literature review. Int J Nurs Stud. 2009;46(10):1355–73.
Kessler RC, McLeod JD. Social support and mental health in community samples. In S. Cohen & SL Sume (Eds). Social support and health. New York: Academic Press; 1985.
Download references
Acknowledgments
Our thanks dedicated to Debre Berhan University and AMARI project. Kefyalew Dagne was supported through AMARI (Africa mental health research initiative) which is funded through the DELTAS Africa initiative (DEL-15-01).
This study was not supported by any grant. Funding for data collection, entry, analysis and write-ups were provided by the authors.
Author information
Authors and affiliations.
Department of Psychiatry, College of Health Science and Medicine, Debre Berhan University, P.O. Box 445, Debre Berhan, Ethiopia
Abate Dargie Wubetu, Nigus Alemnew Engidaw & Kefyalew Dagne Gizachew
You can also search for this author in PubMed Google Scholar
Contributions
AD and NA: Analyzed the data and write up the thesis report and the manuscript. KD: selected the title and develop the proposal. All the authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Correspondence to Abate Dargie Wubetu .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance was obtained from the Debre Berhan University ethical review board (IRB). Permission letter to each study health institution was written and permission letter was taken.
Written informed consent was taken from each study participant.
Consent for publication
The manuscript did not contain individuals’ person detailed data in any form.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wubetu, A.D., Engidaw, N.A. & Gizachew, K.D. Prevalence of postpartum depression and associated factors among postnatal care attendees in Debre Berhan, Ethiopia, 2018. BMC Pregnancy Childbirth 20 , 189 (2020). https://doi.org/10.1186/s12884-020-02873-4
Download citation
Received : 10 September 2019
Accepted : 10 March 2020
Published : 30 March 2020
DOI : https://doi.org/10.1186/s12884-020-02873-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 14 May 2024
Exploring predictors and prevalence of postpartum depression among mothers: Multinational study
- Samar A. Amer ORCID: orcid.org/0000-0002-9475-6372 1 ,
- Nahla A. Zaitoun ORCID: orcid.org/0000-0002-5274-6061 2 ,
- Heba A. Abdelsalam 3 ,
- Abdallah Abbas ORCID: orcid.org/0000-0001-5101-5972 4 ,
- Mohamed Sh Ramadan 5 ,
- Hassan M. Ayal 6 ,
- Samaher Edhah Ahmed Ba-Gais 7 ,
- Nawal Mahboob Basha 8 ,
- Abdulrahman Allahham 9 ,
- Emmanuael Boateng Agyenim 10 &
- Walid Amin Al-Shroby 11
BMC Public Health volume 24 , Article number: 1308 ( 2024 ) Cite this article
119 Accesses
Metrics details
Postpartum depression (PPD) affects around 10% of women, or 1 in 7 women, after giving birth. Undiagnosed PPD was observed among 50% of mothers. PPD has an unfavorable relationship with women’s functioning, marital and personal relationships, the quality of the mother-infant connection, and the social, behavioral, and cognitive development of children. We aim to determine the frequency of PPD and explore associated determinants or predictors (demographic, obstetric, infant-related, and psychosocial factors) and coping strategies from June to August 2023 in six countries.
An analytical cross-sectional study included a total of 674 mothers who visited primary health care centers (PHCs) in Egypt, Yemen, Iraq, India, Ghana, and Syria. They were asked to complete self-administered assessments using the Edinburgh Postnatal Depression Scale (EPDS). The data underwent logistic regression analysis using SPSS-IBM 27 to list potential factors that could predict PPD.
The overall frequency of PPD in the total sample was 92(13.6%). It ranged from 2.3% in Syria to 26% in Ghana. Only 42 (6.2%) were diagnosed. Multiple logistic regression analysis revealed there were significant predictors of PPD. These factors included having unhealthy baby adjusted odds ratio (aOR) of 11.685, 95% CI: 1.405–97.139, p = 0.023), having a precious baby (aOR 7.717, 95% CI: 1.822–32.689, p = 0.006), who don’t receive support (aOR 9.784, 95% CI: 5.373–17.816, p = 0.001), and those who are suffering from PPD. However, being married and comfortable discussing mental health with family relatives are significant protective factors (aOR = 0.141 (95% CI: 0.04–0.494; p = 0.002) and (aOR = 0.369, 95% CI: 0.146–0.933, p = 0.035), respectively.
The frequency of PPD among the mothers varied significantly across different countries. PPD has many protective and potential factors. We recommend further research and screenings of PPD for all mothers to promote the well-being of the mothers and create a favorable environment for the newborn and all family members.
Peer Review reports

Introduction
Postpartum depression (PPD) is among the most prevalent mental health issues [ 1 ]. The onset of depressive episodes after childbirth occurs at a pivotal point in a woman’s life and can last for an extended period of 3 to 6 months; however, this varies based on several factors [ 2 ]. PPD can develop at any time within the first year after childbirth and last for years [ 2 ]. It refers to depressive symptoms that a mother experiences during the postpartum period, which are vastly different from “baby blues,” which many mothers experience within three to five days after the birth of their child [ 3 ].
Depressive episodes are twice as likely to occur during pregnancy compared to other times in a woman’s life, and they frequently go undetected and untreated [ 4 ]. According to estimates, almost 50% of mothers with PPD go undiagnosed [ 4 ]. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for PPD include mood instability, loss of interest, feelings of guilt, sleep disturbances, sleep disorders, and changes in appetite [ 5 ], as well as decreased libido, crying spells, anxiety, irritability, feelings of isolation, mental liability, thoughts of hurting oneself and/or the infant, and even suicidal ideation [ 6 ].
Approximately 1 in 10 women will experience PPD after giving birth, with some studies reporting 1 in 7 women [ 7 ]. Globally, the prevalence of PPD is estimated to be 17.22% (95% CI: 16.00–18.05) [ 4 ], with a prevalence of up to 15% in the previous year in eighty different countries or regions [ 1 ]. This estimate is lower than the 19% prevalence rate of PPD found in studies from low- and middle-income countries and higher than the 13% prevalence rate (95% CI: 12.3–13.4%) stated in a different meta-analysis of data from high-income countries [ 8 ].
The occurrence of postpartum depression is influenced by various factors, including social aspects like marital status, education level, lack of social support, violence, and financial difficulties, as well as other factors such as maternal age (particularly among younger women), obstetric stressors, parity, and unplanned pregnancy [ 4 ]. When a mother experiences depression, she may face challenges in forming a satisfying bond with her child, which can negatively affect both her partner and the emotional and cognitive development of infants and adolescents [ 4 ]. As a result, adverse effects may be observed in children during their toddlerhood, preschool years, and beyond [ 9 ].
Around one in seven women can develop PPD [ 7 ]. While women experiencing baby blues tend to recover quickly, PPD tends to last longer and severely affects women’s ability to return to normal function. PPD affects the mother and her relationship with the infant [ 7 ]. The prevalence of postpartum depression varies depending on the assessment method, timing of assessment, and cultural disparities among countries [ 7 ]. To address these aspects, we conducted a cross-sectional study focusing on mothers who gave birth within the previous 18 months. Objectives: to determine the frequency of PPD and explore associated determinants or predictors, including demographic, obstetric, infant-related, and psychosocial factors, and coping strategies from June to August 2023 in six countries.
Study design and participants
This is an analytical cross-sectional design and involved 674 mothers during the childbearing period (CBP) from six countries, based on the authors working settings, namely Egypt, Syria, Yemen, Ghana, India, and Iraq. It was conducted from June to August 2023. It involved all mothers who gave birth within the previous 18 months, citizens of one of the targeted countries, and those older than 18 years and less than 40 years. Women who visited for a routine postpartum follow-up visit and immunization of their newborns were surveyed.
Multiple pregnancies, illiteracy, or anyone deemed unfit to participate in accordance with healthcare authorities, mothers who couldn’t access or use the Internet, mothers who couldn’t read or speak Arabic or English and couldn’t deal with the online platform or smart devices, mothers whose babies were diagnosed with serious health problems, were stillborn, or experienced intrauterine fetal death, and participants with complicated medical, mental, or psychological disorders that interfered with completing the questionnaire were all exclusion criteria. There were no incentives offered to encourage participation.
Sample size and techniques
The sample size was estimated according to the following equation: n = Z 2 P (1-P)/d 2 . This calculation was based on the results of a systematic review and meta-analysis in 2020 of 17% as the worldwide prevalence of PPD and 12% as the worldwide incidence of PPD, as well as a 5% precision percentage, 80% power of the study, a 95% confidence level, and an 80% response rate [ 11 ]. The total calculated sample size is 675. The sample was diverse in terms of nationality, with the majority being Egyptian (16.3%), followed by Yemeni (24.3%) and Indian (19.1%), based on many factors discussed in the limitation section.
The sampling process for recruiting mothers utilized a multistage approach. Two governorates were randomly selected from each country. Moreover, we selected one rural and one urban area from each governorate. Through random selection, participants were chosen for the study. Popular and officially recognized online platforms, including websites and social media platforms such as Facebook, Twitter, WhatsApp groups, and registered emails across various health centers, were utilized for reaching out to participants. Furthermore, a community-based sample was obtained from different public locations, including well-baby clinics, PHCs, and family planning units.
Mothers completed the questionnaire using either tablets or cellphones provided by the data collectors or by scanning the QR code. All questions were mandatory to prevent incomplete forms. Once they provided their informed consent, they received the questionnaire, which they completed and submitted. To enhance the response rate, reminder messages and follow-up communications were employed until the desired sample size was achieved or until the end of August. To avoid seasonal affective disorders, the meteorological autumn season began on the 1st day of September, which may be associated with Autum depressive symptoms that may confound or affect our results.
Data collection tool
Questionnaire development and structure.
The questionnaire was developed and adapted based on data obtained from previous studies [ 7 , 8 , 9 , 10 , 11 , 12 ]. Initially, it was created in English and subsequently translated into Arabic. To ensure accuracy, a bilingual panel consisting of two healthcare experts and an externally qualified medical translator translated the English version into Arabic. Additionally, two English-speaking translators performed a back translation, and the original panel was consulted if any concerns arose.
Questionnaire validation
To collect the data, an online, self-administered questionnaire was utilized, designed in Arabic with a well-structured format. We conducted an assessment of the questionnaire’s reliability and validity to ensure a consistent interpretation of the questions. The questionnaire underwent validation by psychiatrists, obstetricians, and gynecologists. Furthermore, in a pilot study involving 20 women of CBA, the questionnaire’s clarity and comprehensibility were evaluated. It is important to note that the findings from the pilot study were not included in our main study.
The participants were asked to rate the questionnaire’s organization, clarity, and length, as well as provide a general opinion. Following that, certain questions were revised in light of their input. To check for reliability and reproducibility, the questionnaire was tested again on the same people one week later. The final data analysis will not include the data collected during the pilot test. We calculated a Cronbach’s alpha of 0.76 for the questionnaire.
The structure of the questionnaire
After giving their permission to take part in the study. The questionnaire consisted of the following sections:
Study information and electronic solicitation of informed consent.
Demographic and health-related factors: age, gender, place of residence, educational level, occupation, marital status, weight, height, and the fees of access to healthcare services.
Obstetric history: number of pregnancies, gravida, history of abortions, number of live children, history of dead children, inter-pregnancy space (y), current pregnancy status, type of the last delivery, weight gain during pregnancy (kg), baby age (months), premature labor, healthy baby, baby admitted to the NICU, Feeding difficulties, pregnancy problems, postnatal problems, and natal problems The nature of baby feeding.
Assessment of postpartum depression (PPD) levels using the Edinburgh 10-question scale: This scale is a simple and effective screening tool for identifying individuals at risk of perinatal depression. The EPDS (Edinburgh Postnatal Depression Scale) is a valuable instrument that helps identify the likelihood of a mother experiencing depressive symptoms of varying severity. A score exceeding 13 indicates an increased probability of a depressive illness. However, clinical discretion should not be disregarded when interpreting the EPDS score. This scale captures the mother’s feelings over the past week, and in cases of uncertainty, it may be beneficial to repeat the assessment after two weeks. It is important to note that this scale is not capable of identifying mothers with anxiety disorders, phobias, or personality disorders.
For Questions 1, 2, and 4 (without asterisks): Scores range from 0 to 3, with the top box assigned a score of 0 and the bottom box assigned a score of 3. For Questions 3 and 5–10 (with asterisks): Scores are reversed, with the top box assigned a score of 3 and the bottom box assigned a score of 0. The maximum score achievable is 30, and a probability of depression is considered when the score is 10 or higher. It is important to always consider item 10, which pertains to suicidal ideation [ 12 ].
Psychological and social characteristics: received support or treatment for PPD, awareness of symptoms and risk factors, experienced cultural stigma or judgment about PPD in the community, suffer from any disease or mental or psychiatric disorder, have you ever been diagnosed with PPD, problems with the husband, and financial problems.
Coping strategies and causes for not receiving the treatment and reactions to PPD, in descending order: social norms, cultural or traditional beliefs, personal barriers, 48.5% geographical or regional disparities in mental health resources, language or communication barriers, and financial constraints.
Statistical analysis
The collected data was computerized and statistically analyzed using the SPSS program (Statistical Package for Social Science), version 27. The data was tested for normal distribution using the Shapiro-Walk test. Qualitative data was represented as frequencies and relative percentages. Quantitative data was expressed as mean ± SD (standard deviation) if it was normally distributed; otherwise, median and interquartile range (IQR) were used. The Mann-Whitney test (MW) was used to calculate the difference between quantitative variables in two groups for non-parametric variables. Correlation analysis (using Spearman’s method) was used to assess the relationship between two nonparametric quantitative variables. All results were considered statistically significant when the significant probability was < 0.05. The chi-square test (χ 2 ) and Fisher exact were used to calculate the difference between qualitative variables.
The frequency of PPD among mothers (Fig. 1 )
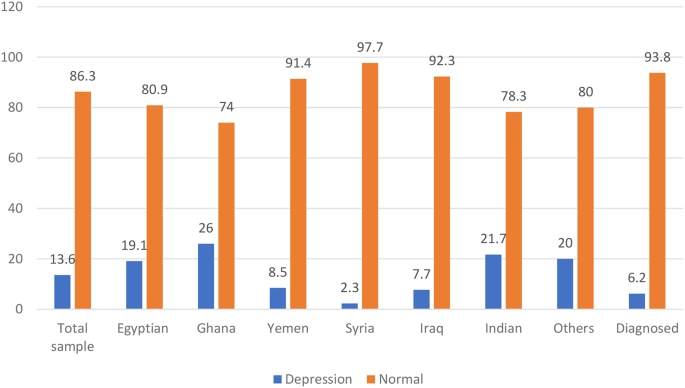
The frequency of PPD among the studied mothers
The frequency of PPD in the total sample using the Edinburgh 10-question scale was 13.5% (Table S1) and 92 (13.6%). Which significantly ( p = 0.001) varied across different countries, being highest among Ghana mothers 13 (26.0%) out of 50 and Indians 28 (21.7%) out of 129. Egyptian 21 (19.1) out of 110, Yemen 14 (8.5%) out of 164, Iraq 13 (7.7%) out of 168, and Syria 1 (2.3%) out of 43 in descending order. Nationality is also significantly associated with PPD ( p = 0.001).
Demographic, and health-related characteristics and their association with PPD (Table 1 )
The study included 674 participants. The median age was 27 years, with 407 (60.3%) of participants falling in the >25 to 40-year-old age group. The majority of participants were married, 650 (96.4%), had sufficient monthly income, 449 (66.6%), 498 (73.9%), had at least a preparatory or high school level of education, and were urban. Regarding health-related factors, 270 (40.01%) smoked, 645 (95.7%) smoked, 365 (54.2%) got the COVID-19 vaccine, and 297 (44.1%) got COVID-19. Moreover, 557 (82.6%) had no comorbidities, 623 (92.4%) had no psychiatric illness or family history, and they charged for health care services for themselves 494 (73.3%).
PPD is significant ( p < 0.05). Higher among single or widowed women 9 (56.3%) and mothers who had both medical, mental, or psychological problems 2 (66.7%), with ex-cigarette smoking 5 (35.7%) ( p = 0.033), alcohol consumption ( p = 0.022) and mothers were charged for the health care services for themselves 59 (11.9%).
Obstetric, current pregnancy, and infant-related characteristics and their association with PPD (Table 2 )
The majority of the studied mothers were on no hormonal treatment or contraceptive pills 411 (60.9%), the current pregnancy was unplanned and wanted 311 (46.1%), they gained 10 ≥ kg 463 (68.6%), 412 (61.1%) delivered vaginal, a healthy baby 613 (90.9%), and, on breastfeeding, only 325 (48.2%).
There was a significant ( P < 0.05) association observed between PPD, which was significantly higher among mothers on contraceptive methods, and those who had 1–2 live births (76.1%) and mothers who had interpregnancy space for less than 2 years. 86 (93.5%), and those who had a history of dead children. Moreover, among those who had postnatal problems (27.2%).
The psychosocial characteristics and their association with PPD (Table 3 )
Regarding the psychological and social characteristics of the mothers, the majority of mothers were unaware of the symptoms of PPD (75%), and only 236 (35.3%) experienced cultural stigma or judgment about PPD in the community. About 41 (6.1%) were diagnosed with PPD during the previous pregnancy, and only 42 (6.2%) were diagnosed and on medications.
A p -value of less than 0.001 demonstrates a highly statistically significant association with the presence of PPD. Mothers with PPD were significantly more likely to have a history of or be currently diagnosed with PPD, as well as financial and marital problems. Experienced cultural stigma or judgment about PPD and received more support.
Coping strategies and causes for not receiving the treatment and reaction to PPD (Table 3 ; Fig. 2 )
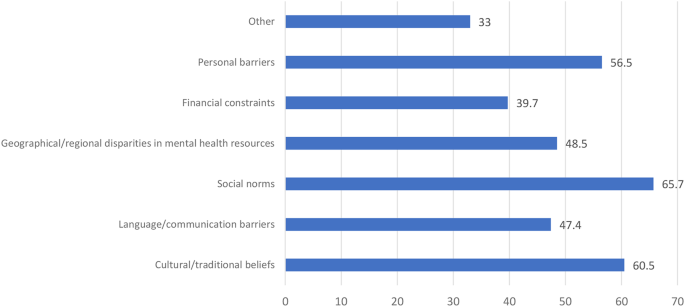
Causes for not receiving the treatment and reaction to PPD
Around half of the mothers didn’t feel comfortable discussing mental health: 292 (43.3%) with a physician, 307 (45.5%) with a husband, 326 (48.4%) with family, and 472 (70.0%) with the community. Moreover, mothers with PPD felt significantly more comfortable discussing mental health in descending order: 46 (50.0%) with a physician, 41 (44.6%) with a husband, and 39 (42.3%) with a family (Table 3 ).
There were different causes for not receiving the treatment and reactions to PPD, in descending order: 65.7% social norms, 60.5% cultural or traditional beliefs, 56.5% personal barriers, 48.5% geographical or regional disparities in mental health resources, 47.4% language or communication barriers, and 39.7% financial constraints.
Prediction of PPD (significant demographics, obstetric, current pregnancy, and infant-related, and psychosocial), and coping strategies derived from multiple logistic regression analysis (Table 4 ).
Significant demographic predictors of ppd.
Marital Status (Married or Single): The adjusted odds ratio (aOR) among PPD mothers who were married in comparison to their single counterparts was 0.141 (95% CI: 0.04–0.494; p -value = 0.002).
Nationality: For PPD Mothers of Yemeni nationality compared to those with Egyptian nationality, the aOR was 0.318 (95% CI: 0.123–0.821, p = 0.018). Similarly, for Syrian nationality in comparison to Egyptian nationality, the aOR was 0.111 (95% CI: 0.0139–0.887, p = 0.038), and for Iraqi nationality compared to Egyptian nationality, the aOR was 0.241 (95% CI: 0.0920–0.633, p = 0.004).
Significant obstetric, current pregnancy, and infant-related characteristics predictors of PPD
Current Pregnancy Status (Precious Baby—Planned): The aOR for the occurrence of PPD among women with a “precious baby” relative to those with a “planned” pregnancy was 7.717 (95% CI: 1.822–32.689, p = 0.006).
Healthy Baby (No-Yes): The aOR for the occurrence of PPD among women with unhealthy babies in comparison to those with healthy ones is 11.685 (95% CI: 1.405–97.139, p = 0.023).
Postnatal Problems (No–Yes): The aOR among PPD mothers reporting postnatal problems relative to those not reporting such problems was 0.234 (95% CI: 0.0785–0.696, p = 0.009).
Significant psychological and social predictors of PPD
Receiving support or treatment for PPD (No-Yes): The aOR among PPD mothers who were not receiving support or treatment relative to those receiving support or treatment was 9.784 (95% CI: 5.373–17.816, p = 0.001).
Awareness of symptoms and risk factors (No-Yes): The aOR among PPD mothers who lack awareness of symptoms and risk factors relative to those with awareness was 2.902 (95% CI: 1.633–5.154, p = 0.001).
Experienced cultural stigma or judgement about PPD in the community (No-Yes): The aOR among PPD mothers who had experienced cultural stigma or judgment in the community relative to those who have not was 4.406 (95% CI: 2.394–8.110, p < 0.001).
Suffering from any disease or mental or psychiatric disorder: For “Now I am suffering—not at all,” the aOR among PPD mothers was 12.871 (95% CI: 3.063–54.073, p = 0.001). Similarly, for “Had a past history but was treated—not at all,” the adjusted odds ratio was 16.6 (95% CI: 2.528–108.965, p = 0.003), and for “Had a family history—not at all,” the adjusted odds ratio was 3.551 (95% CI: 1.012–12.453, p = 0.048).
Significant coping predictors of PPD comfort: discussing mental health with family (maybe yes)
The aOR among PPD mothers who were maybe more comfortable discussing mental health with family relatives was 0.369 (95% CI: 0.146–0.933, p = 0.035).
PDD is a debilitating mental disorder that has many potential and protective risk factors that should be considered to promote the mental and psychological well-being of the mothers and to create a favorable environment for the newborn and all family members. This multinational cross-sectional survey was conducted in six different countries to determine the frequency of PDD using EPDS and to explore its predictors. It was found that PPD was a prevalent problem that varied across different nations.
The frequency of PPD across the studied countries
Using the widely used EPDS to determine the current PPD, we found that the overall frequency of PPD in the total sample was 92 (13.6%). Which significantly ( p = 0.001) varied across different countries, being highest among Ghana mothers 13 (26.0%) out of 50 and Indians 28 (21.7%) out of 129. Egyptian 21 (19.1) out of 110, Yemen 14 (8.5%) out of 164, Iraq 13 (7.7%) out of 169, and Syria 1 (2.3%) out of 43 in descending order. This prevalence was similar to that reported by Hairol et al. (2021) in Malaysia (14.3%) [ 13 ], Yusuff et al. (2010) in Malaysia (14.3%) [ 14 ], and Nakku et al. (2006) in New Delhi (12.75%) [ 15 ].
While the frequency of PPD varied greatly based on the timing, setting, and existence of many psychosocial and post-partum periods, for example, it was higher than that reported in Italy (2012), which was 4.7% [ 16 ], in Turkey (2017) was 9.1%/110 [ 17 ], 9.2% in Sudan [ 18 ], Eritrea (2020) was 7.4% [ 19 ], in the capital Kuala Lumpur (2001) was (3.9%) [ 20 ], in Malaysia (2002) was (9.8%) [ 21 ], and in European countries. (2021) was 13–19% [ 22 ].
Lower frequencies were than those reported; PPD is a predominant problem in Asia, e.g., in Pakistan, the three-month period after childbirth, ranging from 28.8% in 2003 to 36% in 2006 to 94% in 2007, while after 12 months after childbirth, it was 62% in 2021 [ 23 – 24 ]. While in 2022 Afghanistan 45% after their first labour [ 25 ] in Canada (2015) was 40% [ 26 ], in India, the systematic review in 2022 was 22% of Primipara [ 27 ], in Malaysia (2006) was 22.8% [ 28 ], in India (2019) was 21.5% [ 29 ], in the Tigray zone in Ethiopia (2017) was 19% [ 30 ], varied in Iran between 20.3% and 35% [ 31 – 32 ], and in China was 499 (27.37%) out of 1823 [ 33 ]. A possible explanation might be the differences in the study setting and the type of design utilized. Other differences should be considered, like different populations with different socioeconomic characteristics and the variation in the timing of post-partum follow-up. It is vital to consider the role of culture, the impact of patients’ beliefs, and the cultural support for receiving help for PPD.
Demographic and health-related associations, or predictors of PPD (Tables 1 and 4 )
Regarding age, our study found no significant difference between PPD and non-PPD mothers with regard to age. In agreement with our study [ 12 , 34 , 35 ], other studies [ 36 , 37 , 38 ] found an inverse association between women’s age and PPD, with an increased risk of PPD (increases EPDS scores) at a younger age significantly, as teenage mothers, being primiparous, encounter difficulty during the postpartum period due to their inability to cope with financial and emotional difficulties, as well as the challenge of motherhood. Cultural factors and social perspectives of young mothers in different countries could be a reason for this difference. [ 38 – 39 ] and Abdollahi et al. [ 36 ] reported that older mothers were a protective factor for PPD (OR = 0.88, 95% CI: 0.84–0.92].
Regarding marital status, after controlling for other variables, married mothers exhibited a significantly diminished likelihood of experiencing PPD in comparison to single women (0.141; 95% CI: 0.04–0.494; p = 0.002). Also, Gebregziabher et al. [ 19 ] reported that there were statistically significant differences in proportions between mothers’ PPD and marital status.
Regarding the mother’s education, in agreement with our study, Ahmed et al. [ 34 ] showed that there was no statistically significant difference between PPD and a mother’s education. While Agarwala et al. [ 29 ] showed that a higher level of mother’s education. increases the risk of PPD, Gebregziabher et al. [ 19 ] showed that the housewives were 0.24 times less likely to develop PPD as compared to the employed mothers (aOR = 0.24, 95% CI: 0.06–0.97; p = 0.046); those mothers who perceived their socioeconomic status (SES) as low were 13 times more likely to develop PPD as compared to the mothers who had good SES (aOR = 13.33, 95% CI: 2.66–66.78; p = 0.002).
Regarding the SES or monthly income, while other studies [ 18 , 40 ] found that there was a statistically significant association between PPD mothers and different domains of SES, 34% of depressed women were found to live under low SES conditions in comparison to only 15.4% who were found to live in high SES and experienced PPD. In disagreement with our study, Hairol et al. [ 12 ] demonstrated that the incidence of PPD was significantly p = 0.01 higher for participants from the low-income group (27.27%) who were 2.58 times more likely to have PDD symptoms (OR: 2.58, 95% CI: 1.23–5.19; p = 0.01 compared to those from the middle- and high-income groups (8.33%), and low household income (OR = 3.57 [95% CI: 1.49–8.5] increased the odds of PPD [ 41 ].
Adeyemo et al. (2020),and Al Nasr et al. (2020) revealed that there was no significant difference between the occurrence of PPD and socio-demographic characteristics. This difference may be due to a different sample size and ethnicity [ 42 , 43 ]. In agreement with our findings, Abdollahi et al. [ 36 ] demonstrated that after multiple logistic regression analyses, there were increased odds of PPD with a lower state of general health (OR = 1.08 [95% CI: 1.06–1.11]), gestational diabetes (OR = 2.93 [95% CI = 1.46–5.88]), and low household income (OR = 3.57 [95% CI: 1.49–8.5]). The odds of PPD decreased.
Regarding access to health care, in agreement with studies conducted at Gondar University Hospital, Ethiopia [ 18 ], North Carolina, Colorado [ 21 ], Khartoum, Sudan [ 44 ], Asaye et al. [ 45 ], the current study found that participants who did not have free access to the healthcare system were riskier for the development of PPD. the study results may be affected by the care given during the antenatal care (ANC) visits. This can be explained by the fact that PPD was four times higher than that of mothers who did not have ANC, where counseling and anticipatory guidance care are given that build maternal self-esteem and resiliency, along with knowledge about normal and problematic complications to discuss at care visits and their right to mental and physical wellness, including access to care. The increased access to care (including postpartum visits) will increase the diagnosis of PPD and provide guidance, reassurance, and appropriate referrals. Healthcare professionals have the ability to both educate and empower mothers as they care for their babies, their families, and themselves [ 46 ].
Regarding nationality, for PPD mothers of Yemeni nationality compared to those of Egyptian nationality, the aOR is 0.318 (95% CI: 0.123–0.821, p = 0.018). Similarly, for Syrian nationality in comparison to Egyptian nationality, the aOR is 0.111 (95% CI: 0.0139–0.887, p = 0.038), and for Iraqi nationality compared to Egyptian nationality, the aOR is 0.241 (95% CI: 0.0920–0.633, p = 0.004). These findings indicated that, while accounting for other covariates, individuals from the aforementioned nationalities were less predisposed to experiencing PPD than their Egyptian counterparts. These findings can be explained by the fact that, in Egypt, the younger age of marriage, especially in rural areas, poor mental health services, being illiterate, dropping out of school early, unemployment, and the stigma of psychiatric illnesses are cultural factors that hinder the diagnosis and treatment of PPD [ 40 ].
Obstetric, current pregnancy, and infant-related characteristics and their association or predictors of PPD (Tables 2 and 4 )
In the present study, the number of dead children was significantly associated with PPD. This report was supported by studies conducted with Gujarati postpartum women [ 41 ] and rural southern Ethiopia [ 43 ]. This might be because mothers who have dead children pose different psychosocial problems and might regret it for fear of complications developing during their pregnancy. Agarwala et al. [ 29 ] found that a history of previous abortions and having more than two children increased the risk of developing PPD due to a greater psychological burden. The inconsistencies in the findings of these studies indicate that the occurrence of postpartum depression is not solely determined by the number of childbirths.
In obstetric and current pregnancy , there was no significant difference regarding the baby’s age, number of miscarriages, type of last delivery, premature labour, healthy baby, baby admitted to the neonatal intensive care unit (NICU), or feeding difficulties. In agreement with Al Nasr et al. [ 42 ], inconsistent with Asaye et al. [ 45 ], they showed that concerning multivariable logistic regression analysis, abortion history, birth weight, and gestational age were significant associated factors of postpartum depression at a value of p < 0.05.
However, a close association was noted between the mode of delivery and the presence of PPD in mothers, with p = 0.107. There is a high tendency towards depression seen in mothers who have delivered more than three times (44%). In disagreement with what was reported by Adeyemo et al. [ 41 ], having more than five children ( p = 0.027), cesarean section delivery ( p = 0.002), and mothers’ poor state of health since delivery ( p < 0.001) are associated with an increase in the risk of PPD [ 47 ]. An increased risk of cesarean section as a mode of delivery was observed (OR = 1.958, p = 0.049) in a study by Al Nasr et al. [ 42 ].
We reported breastfeeding mothers had a lower, non-significant frequency of PPD compared to non-breast-feeding mothers (36.6% vs. 45%). In agreement with Ahmed et al. [ 34 ], they showed that with respect to breastfeeding and possible PPD, about 67.3% of women who depend on breastfeeding reported no PPD, while 32.7% only had PP. Inconsistency with Adeyemo et al. [ 41 ], who reported that unexclusive breastfeeding ( p = 0.003) was associated with PPD, while Shao et al. [ 40 ] reported that mothers who were exclusively formula feeding had a higher prevalence of PPD.
Regarding postnatal problems, our results revealed that postnatal problems display a significant association with PPD. In line with our results, Agarwala et al. [ 29 ] and Gebregziabher et al. [ 19 ] showed that mothers who experienced complications during childbirth, those who became ill after delivery, and those whose babies were unhealthy had a statistically significant higher proportion of PPD.
Hormone-related contraception methods were found to have a statistically significant association with PPD, consistent with the literature [ 46 ]; this can be explained by the hormones and neurotransmitters as biological factors that play significant roles in the onset of PPD. Estrogen hormones act as regulators of transcription from brain neurotransmitters and modulate the action of serotonin receptors. This hormone stimulates neurogenesis, the process of generating new neurons in the brain, and promotes the synthesis of neurotransmitters. In the hypothalamus, estrogen modulates neurotransmitters and governs sleep and temperature regulation. Variations in the levels of this hormone or its absence are linked to depression [ 19 ].
Participants whose last pregnancy was unplanned were 3.39 times more likely to have postpartum depression (aOR = 3.39, 95% CI: 1.24–9.28; p = 0.017). Mothers who experienced illness after delivery were more likely to develop PPD as compared to their counterparts (aOR = 7.42, 95% CI: 1.44–34.2; p = 0.016) [ 40 ]. In agreement with Asaye et al. [ 45 ] and Abdollahi et al. [ 36 ], unplanned pregnancy has been associated with the development of PPD (aOR = 2.02, 95% CI: 1.24, 3.31) and OR = 2.5 [95% CI: 1.69–3.7] than those of those who had planned, respectively.
The psychosocial characteristics and their association with PPD
Mothers with a family history of mental illness were significantly associated with PPD. This finding was in accordance with studies conducted in Istanbul, Turkey [ 47 ], and Bahrain [ 48 ]. Other studies also showed that women with PPD were most likely to have psychological symptoms during pregnancy [ 43 , 44 , 45 , 46 , 47 , 48 , 49 ]. A meta-analysis of 24,000 mothers concluded that having depression and anxiety during pregnancy and a previous history of psychiatric illness or a history of depression are strong risk factors for developing PPD [ 50 , 51 , 52 ]. Asaye et al. [ 45 ], mothers whose relatives had mental illness history were (aOR = 1.20, 95% CI: 1.09, 3.05 0) be depressed than those whose relatives did not have mental illness history.
This can be attributed to the links between genetic predisposition and mood disorders, considering both nature and nurture are important to address PDD. PPD may be seen as a “normal” condition for those who are acquainted with relatives with mood disorders, especially during the CBP. A family history of mental illness can be easily elicited in the ANC first visit history and requires special attention during the postnatal period. There are various risk factors for PPD, including stressful life events, low social support, the infant’s gender preference, and low income [ 53 ].
Concerning familial support and possible PPD, a statistically significant association was found between them. We reported that mothers who did not have social support (a partner or the father of the baby) had higher odds (aOR = 5.8, 95% CI: 1.33–25.29; p = 0.019) of experiencing PPD. Furthermore, Al Nasr et al. [ 42 ] revealed a significant association between the PPD and an unsupportive spouse ( P value = 0.023). while it was noted that 66.5% of women who received good familial support after giving birth had no depression, compared to 33.5% who only suffered from possible PPD [ 40 ]]. Also, Adeyemo et al. [ 41 ] showed that some psychosocial factors were significantly associated with having PPD: having an unsupportive partner ( p < 0.001), experiencing intimate partner violence ( p < 0.001), and not getting help in taking care of their baby ( p < 0.001). Al Nasr et al. (2020) revealed that the predictor of PPD was an unsupportive spouse (OR = 4.53, P = 0.049) [ 48 ].
Regarding the perceived stigma, in agreement with our study, Bina (2020) found that shame, stigma, the fear of being labeled mentally ill, and language and communication barriers were significant factors in women’s decisions to seek treatment or accept help [ 53 ]. Other mothers were hesitant about mental health services [ 54 ]. It is noteworthy that some PPD mothers refused to seek treatment due to perceived insufficient time and the inconvenience of attending appointments [ 55 ].
PPD was significantly higher among mothers with financial problems or problems with their husbands. This came in agreement with Ahmed et al. [ 34 ], who showed that, regarding stressful conditions and PPD, there was a statistically significant association with a higher percentage of PPD among mothers who had a history of stressful conditions (59.3%), compared to those with no history of stressful conditions (40.7%). Furthermore, Al Nasr et al. (2020) revealed that stressful life events contributed significantly ( P value = 0.003) to the development of PPD in the sample population. Al Nasr et al. stressful life events (OR = 2.677, p = 0.005) [ 42 ].
Coping strategies: causes of fearing and not seeking
Feeling at ease discussing mental health topics with one’s husband, family, community, and physician and experiencing cultural stigma or judgment regarding PPD within the community was significantly associated with the presence of PPD. In the current study, there were different reasons for not receiving the treatment, including cultural or traditional beliefs, language or communication barriers, social norms, and geographical or regional disparities in mental health resources. Haque and Malebranche [ 56 ] portrayed culture and the various conceptualizations of the maternal role as barriers to women seeking help and treatment.
In the present study, marital status, nationality, current pregnancy status, healthy baby, postnatal problems, receiving support or treatment for PPD, having awareness of symptoms and risk factors of PPD, suffering from any disease or mental or psychiatric disorder, comfort discussing mental health with family, and experiencing cultural stigma or judgment about PPD in the community were the significant predictors of PPD. In agreement with Ahmed et al. [ 34 ], the final logistic regression model contained seven predictors for PPD symptoms: SES, history of depression, history of PPD, history of stressful conditions, familial support, unwanted pregnancy, and male preference.
PPD has been recognized as a public health problem and may cause negative consequences for infants. It is estimated that 20 to 40% of women living in low-income countries experience depression during pregnancy or the postpartum period. The prevalence of PPD shows a wide variation, affecting 8–50% of postnatal mothers across countries [ 19 ].
Strengths and limitations
Strengths of our study include its multinational scope, which involved participants from six different countries, enhancing the generalizability of the findings. The study also boasted a large sample size of 674 participants, increasing the statistical power and reliability of the results. Standardized measures, such as the Edinburgh Postnatal Depression Scale (EPDS), were used for assessing postpartum depression, ensuring consistency and comparability across diverse settings. Additionally, the study explored a comprehensive range of predictors and associated factors of postpartum depression, including demographic, obstetric, health-related, and psychosocial characteristics. Rigorous analysis techniques, including multiple logistic regression analyses, were employed to identify significant predictors of postpartum depression, controlling for potential confounders and providing robust statistical evidence.
However, the study has several limitations that should be considered. Firstly, its cross-sectional design limits causal inference, as it does not allow for the determination of temporal relationships between variables. Secondly, the reliance on self-reported data, including information on postpartum depression symptoms and associated factors, may be subject to recall bias and social desirability bias. Thirdly, the use of convenience sampling methods may introduce selection bias and limit the generalizability of the findings to a broader population. Lastly, cultural differences in the perception and reporting of postpartum depression symptoms among participants from different countries could influence the results.
Moreover, the variation in sample size and response rates among countries can be attributed to two main variables. (1) The methodology showed that the sample size was determined by considering several parameters, such as allocating proportionately to the mothers who gave birth and fulfilling the selection criteria during the data collection period served by each health center. (2) The political turmoil in Syria affects how often and how well people can use the Internet, especially because the data was gathered using an online survey link, leading to a relatively low number of responses from those areas. (3) Language barrier in Ghana: as we used the Arabic and English-validated versions of the EPDS, Ghana is a multilingual country with approximately eighty languages spoken. Although English is considered an official language, the primarily spoken languages in the southern region are Akan, specifically the Akuapem Twi, Asante Twi, and Fante dialects. In the northern region, primarily spoken are the Mole-Dagbani ethnic languages, Dagaare and Dagbanli. Moreover, there are around seventy ethnic groups, each with its own unique language [ 57 ]. (4) At the end of the data collection period, to avoid seasonal affective disorders, the meteorological autumn season began on the 1st day of September, which may be associated with autumm depressive symptoms that may confound or affect our results. Furthermore, the sampling methods were not universal across all Arabic countries, potentially constraining the generalizability of our findings.
Recommendations
The antenatal programme should incorporate health education programmes about the symptoms of PPD. Health education programs about the symptoms of PPD should be included in the antenatal program.
Mass media awareness campaigns have a vital role in raising public awareness about PPD-related issues. Mass media.
The ANC first visit history should elicit a family history of mental illness, enabling early detection of risky mothers. Family history of mental illness can be easily elicited in the ANC first visit history.
For effective management of PPD, effective support (from husband, friends, and family) is an essential component. For effective management of PPD effectiveness of support.
The maternal (antenatal, natal, and postnatal) services should be provided for free and of high quality The maternal (antenatal, natal, postnatal) services should be provided free and of high quality.
It should be stressed that although numerous studies have been carried out on PPD, further investigation needs to be conducted on the global prevalence and incidence of depressive symptoms in pregnant women and related risk factors, especially in other populations.
Around 14% of the studied mothers had PPD, and the frequency varies across different countries and half of them do not know. Our study identified significant associations and predictors of postpartum depression (PPD) among mothers. Marital status was significantly associated with PPD, with married mothers having lower odds of experiencing PPD compared to single mothers. Nationality also emerged as a significant predictor, with Yemeni, Syrian, and Iraqi mothers showing lower odds of PPD compared to Egyptian mothers. Significant obstetric, current pregnancy, and infant-related predictors included the pregnancy status, the health status of the baby, and the presence of postnatal problems. Among psychological and social predictors, receiving support or treatment for PPD, awareness of symptoms and risk factors, experiencing cultural stigma or judgment about PPD, and suffering from any disease or mental disorder were significantly associated with PPD. Additionally, mothers who were maybe more comfortable discussing mental health with family relatives had lower odds of experiencing PPD.
These findings underscore the importance of considering various demographic, obstetric, psychosocial, and coping factors in the identification and management of PPD among mothers. Targeted interventions addressing these predictors could potentially mitigate the risk of PPD and improve maternal mental health outcomes.
Data availability
Yes, I have research data to declare.The data is available when requested from the corresponding author [email protected].
Abbreviations
Adjusted Odds Ratio
- Postpartum depression
Primary Health Care centers
Socioeconomic Status
program (Statistical Package for Social Science
The Edinburgh Postnatal Depression Scale
The Neonatal Intensive Care Unit
Sultan P, Ando K, Elkhateb R, George RB, Lim G, Carvalho B et al. (2022). Assessment of Patient-Reported Outcome Measures for Maternal Postpartum Depression Using the Consensus-Based Standards for the Selection of Health Measurement Instruments Guideline: A Systematic Review. JAMA Network Open; 1;5(6).
Crotty F, Sheehan J. Prevalence and detection of postnatal depression in an Irish community sample. Ir J Psychol Med. 2004;21:117–21.
Article PubMed Google Scholar
Goodman SH, Brand SR. Parental psychopathology and its relation to child psychopathology. In: Hersen M, Gross AM, editors. Handbook of clinical psychology vol 2: children and adolescents. Hoboken, NJ: Wiley; 2008. pp. 937–65.
Google Scholar
Wang Z, Liu J, Shuai H, Cai Z, Fu X, Liu Y, Xiao et al. (2021). Mapping global prevalence of depression among postpartum women. Transl Psychiatry. 2021;11(1):543. https://doi.org/10.1038/s41398-021-01663-6 . Erratum in: Transl Psychiatry; 20;11(1):640. PMID: 34671011IF: 6.8 Q1 B1; PMCID: PMC8528847IF: 6.8 Q1 B1.Lase accessed Jan 2024.
American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychiatric Association; Lase accessed October 2023.
Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–95.
Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes: Summary. AHRQ evidence report summaries; 2005. pp. 71–9.
O’hara MW, Swain AM. (1996). Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996; 8:37–54.
Goodman SH, Brand SR. (2008). Parental psychopathology and its relation to child psychopathology. In: Hersen M, Gross AM, editors. Handbook of clinical psychology Vol 2: Children and adolescents. Hoboken, NJ: John Wiley & Sons; 2008. pp. 937–65.
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150:782–6.
Article CAS PubMed Google Scholar
Martín-Gómez C, Moreno-Peral P, Bellón J, SC Cerón S, Campos-Paino H, Gómez-Gómez I, Rigabert A, Benítez I, Motrico E. Effectiveness of psychological, psychoeducational and psychosocial interventions to prevent postpartum depression in adolescent and adult mothers: study protocol for a systematic review and meta-analysis of randomised controlled trials. BMJ open. 2020;10(5):e034424. [accessed Mar 16 2024].
Article PubMed PubMed Central Google Scholar
Sehairi Z. (2020). Validation Of The Arabic Version Of The Edinburgh Postnatal Depression Scale And Prevalence Of Postnatal Depression On An Algerian Sample. https://api.semanticscholar.org/CorpusID:216391386 . Accessed August 2023.
Hairol MI, Ahmad SA, Sharanjeet-Kaur S et al. (2021). Incidence and predictors of postpartum depression among postpartum mothers in Kuala Lumpur, Malaysia: a cross-sectional study. PLoS ONE, 16(11), e0259782.
Yusuff AS, Tang L, Binns CW, Lee AH. Prevalence and risk factors for postnatal depression in Sabah, Malaysia: a cohort study. Women Birth. 2015;1(1):25–9. pmid:25466643
Article Google Scholar
Nakku JE, Nakasi G, Mirembe F. Postpartum major depression at six weeks in primary health care: prevalence and associated factors. Afr Health Sci. 2006;6(4):207–14. https://doi.org/10.5555/afhs.2006.6.4.207 . PMID: 17604509IF: 1.0 Q4 B4; PMCID: PMC1832062
Clavenna A, Seletti E, Cartabia M, Didoni A, Fortinguerra F, Sciascia T, et al. Postnatal depression screening in a paediatric primary care setting in Italy. BMC Psychiatry. 2017;17(1):42. pmid:28122520
Serhan N, Ege E, Ayrancı U, Kosgeroglu N. (2013). Prevalence of postpartum depression in mothers and fathers and its correlates. Journal of clinical nursing; 1;22(1–2):279–84. pmid:23216556
Deribachew H, Berhe D, Zaid T, et al. Assessment of prevalence and associated factors of postpartum depression among postpartum mothers in eastern zone of Tigray. Eur J Pharm Med Res. 2016;3(10):54–60.
Gebregziabher NK, Netsereab TB, Fessaha YG, et al. Prevalence and associated factors of postpartum depression among postpartum mothers in central region, Eritrea: a health facility based survey. BMC Public Health. 2020;20:1–10.
Grace J, Lee K, Ballard C, et al. The relationship between post-natal depression, somatization and behaviour in Malaysian women. Transcult Psychiatry. 2001;38(1):27–34.
Mahmud WMRW, Shariff S, Yaacob MJ. Postpartum depression: a survey of the incidence and associated risk factors among malay women in Beris Kubor Besar, Bachok, Kelantan. The Malaysian journal of medical sciences. Volume 9. MJMS; 2002. p. 41. 1.
Anna S. Postpartum depression and birthexperience in Russia. Psychol Russia: State Theart. 2021;14(1):28–38.
Yadav T, Shams R, Khan AF, Azam H, Anwar M et al. (2020).,. Postpartum Depression: Prevalence and Associated Risk Factors Among Women in Sindh, Pakistan. Cureus.22;12(12):e12216. https://doi.org/10.7759/cureus.12216 . PMID: 33489623IF: 1.2 NA NA; PMCID: PMC7815271IF: 1.2 NA NA.
Abdullah M, Ijaz S, Asad S. (2024). Postpartum depression-an exploratory mixed method study for developing an indigenous tool. BMC Pregnancy Childbirth 24, 49 (2024). https://doi.org/10.1186/s12884-023-06192-2 .
Upadhyay RP, Chowdhury R, Salehi A, Sarkar K, Singh SK, Sinha B et al. (2022). Postpartum depression in India: a systematic review and meta-analysis. Bull World Health Organ [Internet]. 2017 October 10 [cited 2022 October 6];95(10):706. https://doi.org/10.2471/BLT.17.192237/ .
Khalifa DS, Glavin K, Bjertness E et al. (2016). Determinants of postnatal depression in Sudanese women at 3 months postpartum: a cross-sectional study. BMJ open, 6(3), e00944327).
Khadija Sharifzade BK, Padhi S, Manna etal. (2022). Prevalence and associated factors of postpartum depression among Afghan women: a phase-wise cross-sectional study in Rezaie maternal hospital in Herat province.; Razi International Medical Journa2| 2|59| https://doi.org/10.56101/rimj.v2i2.59 .
Azidah A, Shaiful B, Rusli N, et al. Postnatal depression and socio-cultural practices among postnatal mothers in Kota Bahru, Kelantan, Malaysia. Med J Malay. 2006;61(1):76–83.
CAS Google Scholar
Agarwala A, Rao PA, Narayanan P. Prevalence and predictors of postpartum depression among mothers in the rural areas of Udupi Taluk, Karnataka, India: a cross-sectional study. Clin Epidemiol Global Health. 2019;7(3):342–5.
Arikan I, Korkut Y, Demir BK et al. (2017). The prevalence of postpartum depression and associated factors: a hospital-based descriptive study.
Azimi-Lolaty HMD, Hosaini SH, Khalilian A, et al. Prevalence and predictors of postpartum depression among pregnant women referred to mother-child health care clinics (MCH). Res J Biol Sci. 2007;2:285–90.
Najafi KFA, Nazifi F, Sabrkonandeh S. Prevalence of postpartum depression in Alzahra Hospital in Rasht in 2004. Guilan Univ Med Sci J. 2006;15:97–105. (In Persian.).
Deng AW, Xiong RB, Jiang TT, Luo YP, Chen WZ. (2014). Prevalence and risk factors of postpartum depression in a population-based sample of women in Tangxia Community, Guangzhou. Asian Pacific journal of tropical medicine; 1;7(3):244–9. pmid:24507649
Ahmed GK, Elbeh K, Shams RM, et al. Prevalence and predictors of postpartum depression in Upper Egypt: a multicenter primary health care study. J Affect Disord. 2021;290:211–8.
Cantilino A, Zambaldi CF, Albuquerque T, et al. Postpartum depression in Recife–Brazil: prevalence and association with bio-socio-demographic factors. J Bras Psiquiatr. 2010;59:1–9.
Abdollahi F, Zarghami M, Azhar MZ, et al. Predictors and incidence of post-partum depression: a longitudinal cohort study. J Obstet Gynecol Res. 2014;40(12):2191–200.
McCoy SJB, Beal JM, et al. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. JAOA. 2006;106:193–8.
PubMed Google Scholar
Sierra J. (2008). Risk Factors Related to Postpartum Depression in Low-Income Latina Mothers. Ann Arbor: ProQuest Information and Learning Company, 2008.
Çankaya S. The effect of psychosocial risk factors on postpartum depression in antenatal period: a prospective study. Arch Psychiatr Nurs. 2020;34(3):176–83.
Shao HH, Lee SC, Huang JP, et al. Prevalence of postpartum depression and associated predictors among Taiwanese women in a mother-child friendly hospital. Asia Pac J Public Health. 2021;33(4):411–7.
Adeyemo EO, Oluwole EO, Kanma-Okafor OJ, et al. Prevalence and predictors of postpartum depression among postnatal women in Lagos. Nigeria Afr Health Sci. 2020;20(4):1943–54.
Al Nasr RS, Altharwi K, Derbah MS et al. (2020). Prevalence and predictors of postpartum depression in Riyadh, Saudi Arabia: a cross sectional study. PLoS ONE, 15(2), e0228666.
Desai ND, Mehta RY, Ganjiwale J. Study of prevalence and risk factors of postpartum depression. Natl J Med Res. 2012;2(02):194–8.
Azale T, Fekadu A, Medhin G, et al. Coping strategies of women with postpartum depression symptoms in rural Ethiopia: a cross-sectional community study. BMC Psychiatry. 2018;18(1):1–13.
Asaye MM, Muche HA, Zelalem ED. (2020). Prevalence and predictors of postpartum depression: Northwest Ethiopia. Psychiatry journal, 2020.
Ayele TA, Azale T, Alemu K et al. (2016). Prevalence and associated factors of antenatal depression among women attending antenatal care service at Gondar University Hospital, Northwest Ethiopia. PLoS ONE, 11(5), e0155125.
Saraswat N, Wal P, Pal RS et al. (2021). A detailed Biological Approach on Hormonal Imbalance Causing Depression in critical periods (Postpartum, Postmenopausal and Perimenopausal Depression) in adult women. Open Biology J, 9.
Guida J, Sundaram S, Leiferman J. Antenatal physical activity: investigating the effects on postpartum depression. Health. 2012;4:1276–86.
Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–95.
Watanabe M, Wada K, Sakata Y, et al. Maternity blues as predictor of postpartum depression: a prospective cohort study among Japanese women. J Psychosom Obstet Gynecol. 2008;29:211–7.
Kirpinar I˙, Gözüm S, Pasinliog˘ lu T. Prospective study of post-partum depression in eastern Turkey prevalence, socio- demographic and obstetric correlates, prenatal anxiety and early awareness. J Clin Nurs. 2009;19:422–31.
Zhao XH, Zhang ZH. Risk factors for postpartum depression: an evidence-based systematic review of systematic reviews and meta-analyses. Asian J Psychiatry. 2020;53:102353.
Bina R. Predictors of postpartum depression service use: a theory-informed, integrative systematic review. Women Birth. 2020;33(1):e24–32.
Jannati N, Farokhzadian J, Ahmadian L. The experience of healthcare professionals providing mental health services to mothers with postpartum depression: a qualitative study. Sultan Qaboos Univ Med J. 2021;21(4):554.
Dennis CL, Chung-Lee L. Postpartum depression help‐seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth. 2006;33(4):323–31.
Haque S, Malebranche M. (2020). Impact of culture on refugee women’s conceptualization and experience of postpartum depression in high-income countries of resettlement: a scoping review. PLoS ONE, 15(9), e0238109.
https:// www.statista.com/statistics/1285335/population-in-ghana-by-languages-spoken/ .
Download references
Acknowledgements
We would like to express our deep thanks to Rovan Hossam Abdulnabi Ali for her role in completing this study and her unlimited support. Special thanks to Dr. Mohamed Liaquat Raza for his role in reviewing the questionnaire. Moreover, we would like to thank all the mothers who participated in this study.
No funding for this project.
Author information
Authors and affiliations.
Department of Public Health and Community Medicine, Faculty of Medicine, Zagazig University, Zagazig, Egypt
Samar A. Amer
Department of Family Medicine, Faculty of Medicine, Zagazig University, Zagazig, Egypt
Nahla A. Zaitoun
Department of Psychiatry, Faculty of Medicine, Zagazig University, Zagazig, Egypt
Heba A. Abdelsalam
Faculty of Medicine, Al-Azhar University, Damietta, Egypt
Abdallah Abbas
Department of Obstetrics and Gynecology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
Mohamed Sh Ramadan
Hammurabi Medical College, University of Babylon, Al-Diwaniyah, Iraq
Hassan M. Ayal
Hardamout University College of Medicine, Almukalla, Yemen
Samaher Edhah Ahmed Ba-Gais
Department of General Medicine, Shadan Institute of Medical Science, Hyderabad, India
Nawal Mahboob Basha
College of Medicine, Sulaiman Alrajhi University, Albukayriah, Al-Qassim, Saudi Arabia
Abdulrahman Allahham
Department of Virology, Noguchi Memorial Institute for Medical Research, University of Ghana Legon, Accra, Ghana
Emmanuael Boateng Agyenim
Department of Public Health and Community Medicine, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt
Walid Amin Al-Shroby
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: Samar A. Amer (SA); Methodology: SA, Nahal A. Zaitoun (NZ); Validation: Mohamed Ramadan Ali Shaaban (MR), Hassan Majid Abdulameer Aya (HM), Samaher Edhah Ahmed Ba-Gais (SG), Nawal Mahboob Basha (NB), Abdulrahman Allahham (AbAl), Emmanuael Boateng Agyenim (EB); Formal analysis: Abdallah Abbas (AA); Data curation: MR, HM, SG, NB, AbAl, NZ, and EB; Writing original draft preparation: SA, Heba Ahmed Abdelsalam (HAA), and NZ; Writing review and editing: MR, AA, Walid Amin Elshrowby (WE); Visualization: SA, AA; Supervision: SA; Project administration: AA. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Samar A. Amer .
Ethics declarations
Ethical approval and consent to participate.
All participants were provided with electronic informed consent after receiving clear explanations regarding the study’s objectives, data confidentiality, voluntary participation, and the right to withdraw. The questionnaire did not contain any sensitive questions, and data collection was performed anonymously. We affirm that all relevant ethical guidelines have been adhered to, and any necessary approvals from the ethics committee have been obtained. Approval was received from the ethical committee of the family medicine department, the faculty of medicine at Zagazig University, and from the patients included in the study. IRP#ZU-IRP#11079-8/10-2023.
Practicing ethical decision-making is crucial for providing clinical treatment. Such decisions are frequently made challenging due to a lack of knowledge and the mother’s ability to handle the associated complexities and uncertainties that affect the patient’s current level of functioning and ability to take care of her child. At the end of the survey, we raised concerns regarding the red flags, such as suicidal thoughts, and called for a revisit for the psychiatrist’s evaluation of the discussion of the risks, benefits, and alternatives to using medication.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Previous publication
We declare that this research paper has not been published elsewhere in any other academic journal or platform.
Generative AI in scientific writing
We declare that we have not used AI in writing any part of this manuscript.
Conflict of interest
No conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Amer, S.A., Zaitoun, N.A., Abdelsalam, H.A. et al. Exploring predictors and prevalence of postpartum depression among mothers: Multinational study. BMC Public Health 24 , 1308 (2024). https://doi.org/10.1186/s12889-024-18502-0
Download citation
Received : 06 February 2024
Accepted : 02 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s12889-024-18502-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- The Edinburgh postnatal depression scale (EPDS)
- Determinants
- Psychosocial
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Postpartum depression and anxiety: a community-based study on risk factors before, during and after pregnancy
Affiliations.
- 1 Department of Health Technology and Services Research, Technical Medical Centre, University of Twente, Enschede, the Netherlands. Electronic address: [email protected].
- 2 Department of Health Technology and Services Research, Technical Medical Centre, University of Twente, Enschede, the Netherlands.
- 3 Department of Health Sciences, University Medical Center Groningen, University of Groningen, the Netherlands.
- PMID: 33725615
- DOI: 10.1016/j.jad.2021.02.062
Background: Depression and anxiety occur frequently postpartum, calling for early detection and treatment. Evidence on risk factors may support early detection, but is inconclusive. Our aim was to identify risk factors for postpartum depression and anxiety, before, during and after pregnancy.
Methods: We used data from 1406 mothers of the intervention arm of the Post-Up study. Risk factors were collected at 3 weeks and 12 months postpartum. Depression and anxiety symptoms were measured in the first month postpartum by the Edinburgh Postnatal Depression Scale (EPDS) and 6-item State-Trait Anxiety Inventory (STAI-6), respectively. We used stepwise logistic regression to identify relevant risk factors.
Results: Of the mothers, 8.0% had EPDS-scores ≥9 and 14.7% STAI-6-scores ≥42. Factors associated with higher risk of depression were: foreign language spoken at home, history of depression, low maternal self-efficacy and poor current health of the mother. No initiation of breastfeeding was associated with lower risk of depression, no breastfeeding at 3 weeks postpartum increased the risk. Factors associated with higher risk of anxiety were: higher educational level, history of depression, preterm birth, negative experience of delivery and first week postpartum, excessive infant crying, low maternal self-efficacy, low partner support and poor current maternal health.
Limitations: Use of a self-report instrument, potential bias by postpartum mood status, and no inclusion of emerging depression cases after one month postpartum.
Conclusions: The shared and separate risk factors for postpartum depression and anxiety may help professionals in identifying mothers at increased risk and provide opportunities for preventive interventions and treatment.
Keywords: (postpartum) anxiety; Early detection; Postpartum depression; Risk factors.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Anxiety / epidemiology
- Anxiety Disorders / epidemiology
- Depression, Postpartum* / epidemiology
- Infant, Newborn
- Postpartum Period
- Premature Birth*
- Psychiatric Status Rating Scales
- Risk Factors
Sept. 1, 2022
NIH grant to examine universal postpartum depression prevention for moms
Maternal mental health is a critical public health component of perinatal care and maternal safety. Postpartum depression can have lasting consequences for the mother, child, and family. After each birth, 1 in 7 women will experience postpartum depression.
A team of researchers from Michigan State University, Care New England Health System and Henry Ford Health is collaborating on a $6.2 million National Institutes of Health mental health research grant, “The ROSE Scale-Up Study: Informing a decision about ROSE as universal postpartum depression prevention.”
The Reach Out, Stand Strong, Essentials for New Mothers program, funded by NIH through the end of 2022, has served low-income women at 98 prenatal clinics. Study findings show that ROSE prevents half of the cases of postpartum depression. Additionally, health care and community agencies find it is more feasible to provide ROSE as universal prevention for all women.
“Postpartum depression causes suffering for mothers and can slow child development,” said Jennifer Johnson , C.S. Mott Endowed Professor of Public Health at MSU's College of Human Medicine and one of two PIs on the grant.
“The newly funded program will be the first study to look at the effectiveness of postpartum depression among a general population of women and women screening negative for postpartum depression risk,” said Caron Zlotnick, one of two PIs on the grant and professor of Psychiatry and Human Behavior, OB/GYN and Internal Medicine at the Warren Alpert Medical School of Brown University and Director of Research for the Department of Medicine at Women and Infants Hospital. “If we find the intervention is effective, we can work to scale up the program, strengthen families while supporting moms, and reduce costs within the health care system.”
Michigan State University is partnering with Henry Ford Health to offer ROSE to all pregnant patients by adding ROSE to Childbirth Education offerings. The grant will support the expansion of the program for several years to come and help researchers determine if all pregnant women or only those at high risk should be given the opportunity to participate in ROSE, an evidence-based postpartum depression prevention intervention.
Amy Loree, Assistant Scientist at Henry Ford Health, will serve as study site co-investigator on the project.
“We’re very excited to be collaborating with the study team and to be able to offer this preventive service to our patients,” said Loree. “Improving perinatal mental health care through effective prevention approaches like ROSE is critical to improving health and well-being for birthing people and their families.”
“The U.S. Preventative Services Task Force recommends that women at risk for postpartum depression receive these preventive interventions. However, our experience implementing ROSE across the country suggests that a universal intervention may be better; easier for agencies, less stigmatizing for mothers, and no one is missed,” Johnson said.
Additionally, “Women who already face societal stigma can fear the consequences of seeking mental health support,” added Johnson. “Utilizing a universal prevention model will help to reduce that stigma, remove barriers to help, and make maternal mental health support available to every mom.”
The ROSE scale-up project will assess the effectiveness, cost-outcome, equity, and scalability of a universal (i.e., available to everyone) vs. selective (i.e., available to only moms determined to be at risk) postpartum depression prevention model using commonly available and existing screening tools.
Postpartum depression is a common and detrimental public health concern. The postpartum period places women at increased risk for a major depressive episode. No method of determining who is at risk is perfect. Offering ROSE to only women determined to be at risk may miss women who could benefit from ROSE.
“In the seven-and-a-half years since the Division of Public Health’s founding in Flint, faculty have been awarded more than $100 million in research funds,” said Aron Sousa, dean of the MSU College of Human Medicine. “This milestone is only made possible through collaboration, community partnership, and this latest grant from NIH. Scaling up this study across the United States will help to strengthen families at a critical time in an infant’s development.”
ROSE has been recommended by the U.S. Preventative Services Task Force for women at risk for postpartum depression. The intervention is freely available to agencies and providers. The curriculum is highly structured, easy to learn, and available in Spanish and English. It does not require a mental health professional. ROSE can be offered in various settings, including prenatal clinics, doula organizations, home visiting programs, and WIC agencies.
"Those who can work to reduce health disparities must accept that challenge as an imperative, especially when it comes to equity in maternal health," said Norman J. Beauchamp Jr., executive vice president for health sciences at MSU. “With these organizations, we have a shared commitment to diversity, equity and inclusion, which means addressing and eliminating historical inequities in health care across our state. This research and outreach program will bring health, hope and healing to more mothers and work to scale an important effort.”
By: Jill Vondrasek
Media Contacts
Jill Vondrasek
May 17, 2024
Walstrom family gift to MSU supports women’s health research, medical care
Ask the expert: how are mental health and wellness connected in the black community — and beyond.
May 16, 2024
New report reveals Michigan teacher salaries lag national averages, public supports increases
Msutoday weekly update.
The MSUToday Weekly Update email showcases how Spartans are making a difference through academic excellence, research impact and community outreach. Get inspired by these stories of innovation, collaboration and determination. Plus, enjoy photos and videos of campus and more MSU content to help keep you connected to the Spartan community.
Connect With Us

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Research Funded by NIMH
- Research Conducted at NIMH (Intramural Research Program)
- Priority Research Areas
- Research Resources

Postpartum Depression

Doctors at the National Institute of Mental Health (NIMH) in Bethesda, Maryland are trying to learn more about the causes of, treatments for, and genetic factors in postpartum depression (PPD). PPD develops around the time a woman gives birth. Women with PPD often struggle with anxiety, sadness, difficulty sleeping, or disturbing thoughts. Research suggests that it is triggered by changes in hormones and that women with PPD are sensitive to those changes. PPD occurs in approximately 15% of births.
You are invited to call the National Institute of Mental Health at 301-496-9576 for information on our research studies of Postpartum Depression (PPD). If you had PPD in the past, please call us. Together we can make a difference.
Back to the Participate in Research page
REVIEW article
Postpartum depression: current status and possible identification using biomarkers.

- 1 Central Laboratory, Yangjiang People's Hospital, Yangjiang, China
- 2 Center for Analyses and Measurements, College of Chemical Engineering, Zhejiang University of Technology, Hangzhou, China
- 3 Institute of Cell Biology, Zhejiang University, Hangzhou, China
Postpartum depression (PPD) is a serious health issue that can affect about 15% of the female population within after giving birth. It often conveys significant negative consequences to the offsprings. The symptoms and risk factors are somewhat similar to those found in non-postpartum depression. The main difference resides in the fact that PPD is triggered by postpartum specific factors, including especially biological changes in the hormone levels. Patients are usually diagnosed using a questionnaire onsite or in a clinic. Treatment of PPD often involves psychotherapy and antidepressant medications. In recent years, there have been more researches on the identification of biological markers for PPD. In this review, we will focus on the current research status of PPD, with an emphasis on the recent progress made on the identification of PPD biomarkers.
Introduction
Postpartum depression (PPD) has raised a major public health concern. It has been estimated that about 15% of women within 1 year after childbirth may suffer from PPD ( 1 ). Like major depression, PPD is a disabling disorder. Significant negative effects of PPD on children, even after they grow into adulthood, have been documented in the literature. PPD-related suicide has become the second-leading cause of death for women in the postpartum period ( 2 ). While antidepressants have been effective in treating PPD in many cases, possible side effects of antidepressant medication have been of great concern. Therefore, it is crucial to identify PPD at an early stage.
Although, known since 400 BC, PPD it did not catch wide attention until about half a century ago. Over the past decades, there have been many research efforts and reviews in the field of PPD. Grace et al. ( 3 ) reviewed the literature and found that PPD confers small effects on cognitive development such as language and IQ. Behavioral effects may persist up to 5 years post partum and beyond. Dennis and McQueen ( 4 ) reported that women with depressive symptoms at the early stage of the postpartum period were associated with increased risk for negative infant feeding outcomes. The review by Blum ( 5 ) focused on the psychodynamics of PPD, and found that a triad of three common, specific emotional conflicts (dependency conflicts, anger conflicts, and motherhood conflicts) was typical of many women who develop PPD. Yawn et al. ( 6 ) found that among those evaluated programs between 2000 and 2010, only four studies included patient outcomes, and only two reported success in improving outcomes. O'Hara and McCabe ( 7 ) found that the results of most studies did not seem to converge, and the majority had a small sample size or suffered from a lack of proper controls. Anderson and Maes ( 8 ) reviewed the biological aspects of PPD, and suggested that tryptophan catabolites, indoleamine 2,3-dioxygenase, serotonin, and autoimmunity play a powerful role in immuno-inflammation and oxidative and nitrosative stress. Furthermore, decreased level of endogenous anti-inflammatory compounds together with decreased ω-3 poly-unsaturated fatty acids (PUFA) in the post-partum period may be a central cause for PPD. Kim et al. ( 9 ) conducted a review on the role of oxytocin in the treatment of PPD, and found that the results was inconsistent. Yim et al. ( 10 ) conducted a review on researches published between 2000 and 2013 on the predictors for PPD and found that the biological and psychosocial literatures were largely disconnected, and integrative analyses were rare to find. They reported that the strongest biological predictors for PPD risk were hypothalamic-pituitary-adrenal (HPA) axis dysregulation, inflammatory processes, and genetic vulnerabilities, while the strongest psychosocial predictors were severe life events, chronic strain, poor relationship quality, and family support. There are many other reviews on PPD in the literature, which can be classified mainly into two distinct categories: biological vs. psychosocial approaches. The former addressed the endocrine system, the immune system, and genetic factors ( 11 – 14 ), while the latter addressed stressors and interpersonal relationships ( 5 , 15 – 17 ). Reviews that covered both categories ( 18 , 19 ) are relatively rare.
In the present review, we shall emphasize on the literature on PPD in the past several years. There has been an increasing number of researches on the screening and diagnosis of PPD using biological markers. Several biomarkers have been identified using the modern technology of multi-omics. The omics-based biomarkers can provide a more quantitative and objective criterion for the diagnosis of PPD, compared with the questionnaire-based diagnosis.
Diagnosis of PPD
There remains a controversy regarding the criterion for the onset time of PPD ( 1 ). The Diagnostic and Statistical Manual of Mental Disorders revision 5 (DSM-5) of the US encompasses episodes that sets in during pregnancy ( 20 ) and that begin within 6 months of delivery. In clinical practice and in various studies in the literature, the onset time for PPD has been generalized to up to 1 year post-partum.
A clinical interview can be used to diagnose PPD, such as the Structured Clinical Interview for the DSM-IV ( 21 ). Alternatively, easy-to-use self-report measures such as questionnaires have also been widely used for clinical assessment. The most prominent and widely used is the Edinburgh Postnatal Depression Scale (EPDS) ( 22 ), which has been reported to be reliable, well-validated, and often more practical and cost-effective in wide-scale screenings for PPD risk ( 23 ). EPDS lays an emphasis on psychic symptoms of depression, so as to reduce the weight of common symptoms of most new mothers. Other commonly used questionnaire-based screening tools include two-item Patient Health Questionnaire (PHQ-2) ( 24 ) and 9-item Patient Health Questionnaire (PHQ-9) ( 25 ). PHQ-2 contains the first two items of the PHQ-9. A typical EPDS or PHQ-9 score of 10 or above is used as the cutoff for being PPD positive. Brief subscales of EPDS have also been designed ( 26 ), such as 3-item, 7-item and 2-item subscales. Other screening tools include the Hamilton Rating Scale for Depredession (HAM-D) ( 27 ), which was not designed for PPD specifically. The reliability of HAM-D varies significantly in different evaluations, ranging from 0.46 to 0.98 ( 28 ). Other scales for diagnosing related mood disorders, such as the Bipolar Spectrum Diagnostic Scale (BSDS) ( 29 ) for bipolar disorders (BD), may also become relevant when such disorders occur during the perinatal period.
Etiological Models of PPD
The exact causes of PPD are still unknown. Models of PPD can mainly be divided into two categories: biological vs. psychological models. Integrated models of both are rare. However, many existing studies suffer from relatively small sample sizes or lack of control (or both), and none of the models mentioned here is conclusive.
Biological Models
It is well-known that childbirth is accompanied by a dramatic decreases in several hormones, such as estradiol, progesterone, and cortisol. In withdrawal models , reproductive hormones ( 30 ) and stress hormones ( 31 ) rise dramatically during pregnancy and then drop suddenly upon delivery, and thus leads to system dysregulation and hence PPD ( 32 ). These models cannot explain how the hormonal withdrawal acts to cause depression in women, nor can they explain those depressive symptoms that begin during pregnancy before parturition.
In depression models , PPD is associated with dysregulation of stress hormones , particularly cortisol ( 12 ). Several recent reviews suggested that dysregulation of the HPA axis plays a main role in the development of PPD ( 33 , 34 ). Diminished dopaminergic function may also play a role in PPD ( 35 ). Sudden estradiol withdrawal could lead to dysregulation in brain dopaminergic pathways and hence PPD. Multiple neuroendocrine changes caused by pregnancy may also play a role in PPD development, including dysfunctional gamma-aminobutyric acid (GABA) signaling ( 36 , 37 ). PPD has also been found to be associated with low allopregnanolone levels during pregnancy ( 38 ). The involvement of GABA and allopregnanolone in PPD has also been hypothesized in other models of PPD pathophysiology ( 39 ).
Psychological Models
Psychological models emphasize the deleterious role of psychological stressors and underlying cognitive vulnerabilities and the ameliorating role of psychosocial resources. In these theories, pregnancy, childbirth, and new parenthood are stressors that cause women to develop PPD symptoms. One can find consistent support for these models in the psychological literature ( 32 , 40 , 41 ).
Integrated Models
An integrated model may bridge the biological and psychological theories. For example, in the stress vulnerability model, stress can cause PPD symptoms in women that have genetic, hormonal, and cognitive vulnerabilities ( 32 ). The bio-psycho-social-cultural model of Halbreich ( 42 ) is a combination of the stress vulnerability model with biological and cultural factors. There exists limited evidence in the literature that supports these integrated models.
Evolutionary Models
Models from an evolutionary perspective regard PPD as a consequence of modern civilization, due to psychological adaptation during the human evolution. Hagen ( 43 ) proposed that PPD could cause parents to reduce or eliminate investment in infants that may have health and development problems, and it also may help them negotiate greater levels of investment from others. Recently, Hahn-Holbrook and Haselton ( 44 ) proposed a “mismatch hypothesis” of PPD that dramatic cultural changes that have occurred over the past century leads to significant divergences from the typical lifestyles throughout human evolutionary history, and gives rise to the current high incidence rate of PPD, suggesting that PPD may be a “disease of civilization.”
Treatment of PPD
Psychological treatment usually happens in the form of counseling (or psychotherapy), either one-on-one with a psychologist or in a group setting ( 45 ), and has been extremely beneficial for many women. Some women can effectively recover from their depression through counseling alone, while others may have to undergo counseling in conjunction with the use of antidepressants. Thus far, strong support can be found in the literature that a variety of psychological treatments of PPD can be effective.
Medical treatment for PPD includes pharmacotherapy with antidepressants. In fact, antidepressant medication is the most common treatment for PPD ( 46 ). There have been extensive investigations of a broad spectrum of antidepressants in the treatment of PPD, and many have been found to be associated with symptomatic improvement ( 46 ), and to be as effective as usual care plus counseling ( 47 ). Recently, an allopregnanolone-based treatment for PPD, brexanolone, now commercially called Zulresso®, has been approved by the Food and Drug Administration (FDA) in the United States as a fast-acting, long-lasting antidepressant ( 48 ). In double-blind, randomized, controlled clinical studies, brexanolone injection has been observed to be associated with a rapid reduction at 60 h in depressive symptoms compared to placebo, and the reduction could sustain no <30 days with broad responses ( 49 ).
Regarding the drug exposure of the infant through breastfeeding, a few reviews ( 50 – 53 ) concluded that nortriptyline, paroxetine, and sertraline have the strongest safety performance during lactation.
It should be noted that PPD may share some common genetic and biological risk factors and biomarkers with other mood disorders such as the major depressive disorder (MDD) and the bipolar disorder (BD). In this sense, PPD may be viewed as a forming part of a unique mood spectrum ( 54 , 55 ). Accordingly, these common features may cause misdiagnosis. Indeed, patients with BD may sometimes be misdiagnosed as having MDD, leading to inappropriate treatment with antidepressant medication ( 29 , 56 ). The misuse of antidepressants in patients with BD without mood-regulators may induce (hypo)mania or rapid cycling and may increase the risk of illness recurrence ( 57 – 60 ). Therefore, to ensure proper treatment, extra caution must be excised to ensure proper diagnosis.
Psychosocial Risks Factors for PPD
A very large body of literature has addressed risk factors for PPD based on cross-sectional and prospective studies ( 45 ). According to several meta-analyses of risk factors for PPD ( 15 , 16 ), risk factors can be categorized based on the strength of their association with PPD. Depression and anxiety during pregnancy, postpartum blues, history of depression, neuroticism, stressful life events, poor marital relationship, and poor social support, low self-esteem, as well as some cognitive emotion regulation strategies ( 61 ) have been found to have a strong or moderately strong association. A large-scale population based study ( 62 ), using data on more than 700,000 deliveries in Sweden between 1997 and 2008, reported that the risk of PPD for women with a depression history was over 20 times higher than women without. On the other hand, low socioeconomic status (SES), single marital status, unwanted pregnancy, obstetrical stressors, and difficult infant temperament ( 63 , 64 ) have been reported to exhibit a relatively weaker association. Maternal attitudes ( 65 ), women's experience of a various related complications such preterm birth, prenatal hospitalization, emergency cesarean section, pre-eclampsia, and poor infant health ( 66 ), can also cause an elevated risk of developing PPD ( 67 – 69 ). These risk factors are more closely related to the social and psychological aspects rather than biological aspects.
Biological Predictors and Biomarkers for PPD
In recent years, more development has been made to identify the biological predictors for PPD. Substantial biological changes can be associated with pregnancy. Such changes are necessary in order to maintain normal pregnancy and fetal development, as well as successful labor and lactation. Upon parturition, the intricate balance that has developed during gestation to sustain the maternal-placental-fetal unit is suddenly no longer needed. Furthermore, the maternal system has to undergo a dramatic biological changes into the lactation phase within a short time. It may days or even months to re-establish a new biological balance. It is conceivable that failure to re-establish the balance properly and promptly may cause maternal mental health issues in return.
Genetic and Epigenetic Studies
It is generally hoped that investment of possible genetic causes of a psychiatric disorder may help to reveal the underlying pathophysiological mechanism, which can help to find cures or improved treatments. Studies revealed that there may exist an underlying genetic cause for PPD ( 70 ). Viktorin et al. ( 71 ) found that the heritability of perinatal depression was estimated at 54 and 44%, respectively, in twin and sibling samples, which means that about half of the variability in perinatal depression can be explained by genetic factors. This is substantially higher than the heritability of non-perinatal depression at 32%. Forty et al. ( 72 ) and Murphy-Eberenz et al. ( 73 ) reported that PPD with onset within 4 weeks post-parturm exhibits familiality in families with MDD. These studies suggest that, while it also has its own unique features, the genetic basis for PPD may partially overlap with that for other mood disorders.
Unlike MDD, there have been relatively fewer studies addressing genetic contribution to PPD. A partial summary of genetic association studies of PPD was tabulated by Payne ( 70 ). In these studies, various genes were investigated for their roles in PPD symptoms, such as those associated with the regulation of the HPA axis, sex hormones, and the effects of stress on the prefrontal cortex ( 1 ). Mahon et al. ( 74 ) examined the genetic etiology of postpartum mood disorders using genome-wide data. They found that genetic variations on chromosomes 1 and 9 may increase susceptibility to postpartum mood symptoms, for women who had a history of pregnancy and any best-estimate mood disorder diagnosis. Specifically, the genes HMCN1 and METTL13 may contain polymorphisms that confer susceptibility to postpartum mood symptoms. Nevertheless, these associations were not significant enough to sustain statistical corrections from multiple testing. Alvim-Soares et al. ( 75 ) found that a HMCN1 polymorphism (rs2891230) is associated with PPD symptoms, and the heterozygosity for this single nucleotide polymorphism (SNP) was associated with an increased risk of PPD, in a sample of 110 randomly selected, unrelated Brazilian women of European descent, assessed at 8 weeks postpartum. Their result seems to support the finding of Mahon et al., however, future studies with a larger sample size are certainly needed. Costas et al. ( 76 ) reported a significant association (with p = 0.002) between the SNP rs11924390 between SNP at the transcriptional start site of kininogen 1 and PPD during the first 32 weeks after delivery. Clear signatures of gene expression of mononuclear cells were found in woman with PPD symptoms as compared with healthy controls ( 77 , 78 ). The usefulness of this study, however, suffered from its small sample size. Further studies with a larger sample size are warranted in order to confirm these associations. Nonetheless, while these results differ, they are not inconsistent with one another.
The serotonin transporter gene (SER T) has been one of the most widely studied candidate gene associated with PPD ( 70 ). It has two primary polymorphisms, 5-HTTLPR and STin2VNTR ( 10 ). The former contains a 44-bp deletion or insertion in the promoter region, corresponding to the short and long allele variants, respectively. The latter polymorphism involves a variable number of tandem repeats (VNTR) in the second intron, among which the longer VNTRs have been found to be associated with mental health issues and depressive disorders. So far, studies have shown mixed results on the role of SER T polymorphisms in PPD. Lesch and Mössner ( 79 ) found that the short allele may be associated with higher risk of developing PPD. However, the long allele variant of the 5-HTTLPR (serotonin transporter, i.e., 5-HTT, linked polymorphic region) was also reported to be associated with PPD symptoms at 6 weeks ( 80 , 81 ) or within 1 year post-partum ( 82 ).
A weak link between estrogen receptor gene (ESR1) and PPD has been reported ( 76 , 83 ), which, however, failed to remain statistically significant when corrections for multiple tests were taken into account. It was also suggested that the role for ESR1 in the etiology of PPD could possibly be mediated through the modulation of serotonin signaling ( 83 ). Mehta et al. ( 84 ) found that women with PPD (with onset within 7 weeks after delivery) displayed an increased sensitivity to estrogen signaling in comparison with controls. While these studies indeed support the idea that estrogen plays a role in the development of PPD, they are far from being conclusive, partly because of the relatively small sample size in these studies.
Catechol-Omethyltransferase (COMT) and monoamine oxidase-A (MAO-A) are allelic gene variations in the monoaminergic system. They have been shown to be related to MDD. The former is involved in dopamine and noradrenalin metabolism, while the latter plays a role in the degradation of serotonin and noradrenaline in the brain ( 85 ). A few studies revealed that MAO-A and COMT may be associated with PPDs that have an onset within 8 weeks postpartum ( 75 , 81 , 86 ). Sacher et al. ( 87 ) found an association between PPD and greater MAO-A V T (an index of MAO-A density) in the prefrontal and anterior cingulate cortex, compared to healthy controls.
Oxytocin has been found to plays an important role in physiological and genetic systems that permit the evolution of the human nervous system and allow the expression of contemporary human sociality, and stress reactivity. It acts to allow the facilitation of birth, lactation, and maternal behavior ( 88 , 89 ). Decrease in the oxytocin level in plasma has been associated with PPD ( 90 , 91 ). Possible roles in PPD have also been investigated for polymorphisms of the oxytocin receptor (OXTR) gene, the oxytocin peptide gene ( 92 ), the glucocorticoid receptor gene and the CRH receptor 1 gene ( 93 ). However, only some weak, suggestive links have been reported. Jonas et al. ( 94 ) found that polymorphisms in OXT rs2740210 interacted with early life adversity to predict PPD. Bell et al. ( 95 ) found that for women who do not show depression during pregnancy, but possess the rs53576_GG genotype and exhibit high levels of methylation in OXTR, the risk of developing PPD was nearly three times that for women of lower methylation levels. Kimmel et al. ( 96 ) found that the cytosine-guanines (CpGs) located on chr3 at positions 8810078 and 8810069 were associated with PPD scores significantly for a cohort of 240 women without a psychiatric history. They also found a PPD specific negative correlation between DNA methylation in the region and serum estradiol levels. In addition, estradiol levels and OXTR DNA methylation exhibited a significant interaction to associate with the ratio of allopregnanolone (ALLO) to progesterone. Recently, King et al. ( 97 ) found that mothers with persistent perinatal depression (depressive symptoms both prenatally and postpartum) exhibited significantly higher overall OXTR methylation at 16/22 individual CpG sites. While these studies seem to support the link between (the epigenetic DNA methylation of) OXTA and the risk of developing PPD, it should be noted that these findings are mostly derived from small sample sizes, with variable depression rating scales and a lack of prospective measures of DNA methylation, and thus should be interpreted with caution.
The brain-derived neurotrophic factor (BDNF) system is known to play an important role in many neuronal functions ( 15 ). Serum BDNF was found to decline considerably across pregnancy from 1st through 3rd trimesters ( p ≤ 0.008) and subsequently to increase at postpartum ( p < 0.001), and lower serum BDNF in late pregnancy was reported to be associated with higher depressive symptoms ( 98 ), although low BDNF levels were found to persist even 2 months after birth ( 99 ). Aydemir et al. ( 100 ) and Gazal et al. ( 101 ) found that the BDNF levels in serum of PPD patients were lower than in healthy control subjects. Serum BDNF levels in PPD patients that had a suicide risk were significantly lower than those of women that did not show a suicide risk ( 102 ). Recently, Fung et al. ( 103 ) found an association between lower levels of serum BDNF in early pregnancy and antepartum depression. However, Figueira et al. ( 104 ) and Comasco et al. ( 80 ) did not find an association between PPD and the BDNF polymorphism Val66Met. Overall, clear evidence for the association between BDNF and PPD symptoms is yet to be found ( 10 ). This may be partly due to the fact that the normal serum level of BDNF is a non-monotonic function of time in the perinatal period. Thus the precising timing and its dynamics may be important. The lack of consensus in the literature may partly reflect the difference in experiment design in terms of timing and sampling strategy, besides the often small sample sizes.
Katz et al. ( 105 ) collected maternal RNA longitudinally from preconception through the third trimester of pregnancy in 106 women with a lifetime history of mood or anxiety disorders. They reported that mRNA expression of a number of glucocorticoid receptor (GR)-complex regulating genes was up-regulated over pregnancy, and women with depressive symptoms showed significantly smaller increases in mRNA expression of four of these genes. They also found that GR sensitivity diminished with increasing maternal depressive symptoms. A prospective pregnancy cohort study of 56 healthy women with singleton term pregnancies found that altered placental genes expression involved in glucocorticoid and serotonin transfer may function as potential gestational-age-specific marker of PPD risk ( 106 ). Further studies with a larger sample size are needed to replicate these findings.
Recently, by RNA sequencing the whole transcriptomes of peripheral blood mononuclear cells, Pan et al. ( 107 ) found that PPD was positively correlated with multiple genes involved in energy metabolism, neurodegenerative diseases and immune response, while negatively correlated with multiple genes in mismatch repair and cancer-related pathways. In addition, genes associated with appetite regulation and nutrient response were differentially expressed between PPD ( n = 56) and control subjects ( n = 27).
Epigenetics refers to changes in gene function that do not alter the DNA sequence itself. The main focus in this area has been mostly on DNA methylation, which can be modified by medication and stress, as well as reproductive hormones. There has been some progress in the identification of biomarkers for DNA methylation. Recent studies have implicated epigenetic processes in the pathophysiology of MDD ( 108 ). Guintivano et al. ( 109 ) and Kaminsky and Payne ( 110 ) observed enhanced sensitivity to estrogen-based DNA methylation reprogramming in those at risk for PPD and identified two potential biomarker loci at the HP1BP3 and TTC9B genes that predicted PPD. Using blood drawn during pregnancy, DNA methylation at two genomic locations with an area under the receiver operator characteristic (ROC) curve [area under the curve (AUC)] of 0.87 in antenatally euthymic women and 0.12 in a replication sample of antenatally depressed women, along with complete blood count data, produced an AUC of 0.96 across both prepartum depressed and euthymic women ( 109 ). Osborne et al. ( 111 ) found that TTC9B and HP1BP3 DNA methylation in early antenatal stage showed moderate association with the change in estradiol and ALLO levels over the course of pregnancy, suggesting that epigenetic variation at these loci may be important for mediating hormonal sensitivity, and that PPD is mediated by differential gene expression and epigenetic sensitivity to pregnancy hormones and thus modeling proxies of this sensitivity may enable accurate prediction of PPD. Osborne et al. ( 38 ) further found an association between lower ALLO levels in the second trimester of pregnancy and an elevated risk of developing PPD, which seem to have been confirmed by latest studies ( 39 , 49 , 112 – 114 ), and can be traced back to the two genes identified above. Indeed, this seems to have gathered more support than many other biomarkers. Very recently, Payne et al. ( 115 ) found that antenatal TTC9B and HP1BP3 DNA methylation may be used to predict both antenatal and postpartum depression.
A prospective study ( 109 ) of 93 pregnant women with a history of either MDD or bipolar disorder found significant correlation between PPD risk and 17β-estradiol (E2)-induced DNA methylation change, suggesting that an enhanced sensitivity to estrogen-based DNA methylation reprogramming exists in women at risk for PPD. Estradiol increases the rate of transcription of the OXTR gene ( 116 ), resulting in elevations of oxytocin levels in the uterus ( 117 ) and in numerous brain regions ( 118 ) while heterozygosity for the OXTR rs2254298 polymorphism can interact with early life adversity to yield the highest levels of symptoms of depression, physical anxiety, and social anxiety ( 119 ). Association between higher DNA methylation of the OXTR gene and decreased expression of the gene was also observed ( 120 ). A case control study ( 95 ) on the OXTR gene DNA methylation at CpG site-934 and genotype rs53576 and rs2254298 found that women with GG genotype had higher risk of developing PPD with increasing methylation level. The finding of King et al. ( 97 ) regarding OXTR methylation seem to suggest that the onset timing of PPD is also an important factor.
Overall, the above findings suggest that polymorphic variations in candidate genes within the monoaminergic system can have an effect on the estrogen receptor, the oxytocin peptide, the glucocorticoid receptor, and the CRH receptor 1 genes, and may act as potential biomarkers for PPD. However, further investigation is needed in order to determine whether the short or long allele of the 5-HTTLPR is associated with PPD risk and under what conditions. Nevertheless, the lack of consensus in these data from the literature highlights the complex relationship between epigenetics and PPD related neuroendocrine changes.
Reproductive hormones
The association between the reproductive hormones and PPD has been studied and reviewed, in terms of estrogens, progesterone, prolactin, oxytocin, and testosterone. Bloch et al. ( 121 ) found evidence that the reproductive hormones estrogen and progesterone play a role in the development of PPD. However, the data by Klier et al. ( 122 ) did not support the hypothesis of a role of sex hormones in the etiology of PPD. A review of about 200 studies by Serati et al. ( 123 ) found little evidence that supports estrogen withdrawal theories, or suggests that progesterone in late pregnancy or postpartum period predicts PPD symptoms.
Recent studies seem to suggest strong association between the progesterone level and PPD. The levels of ALLO was found to increase progressively throughout gestation, and drop rapidly upon parturition ( 37 , 124 , 125 ). As a metabolite of progesterone, ALLO is a neuroactive steroid measurable in peripheral circulation. Therefore, its levels vary proportionally with progesterone levels throughout gestation and after the delivery, and an association between ALLO and PPD were found, suggesting that hormonal regulation plays an important role in the development of PPD ( 124 ). Previously, Bloch et al. ( 121 ) found that women with a PPD history are more sensitive to mood-destabilizing effects of gonadal steroids than healthy controls. Rather than progesterone withdrawal upon delivery, it has been found that a low ALLO level during pregnancy predicts PPD ( 38 , 112 , 114 ). Such an association was also found in earlier studies ( 126 , 127 ). Timing may be an important factor when assessing potential biomarkers. Epperson et al. ( 128 ) reported that cortex GABA levels and plasma ALLO concentrations were reduced in two different groups of postpartum women, regardless of PPD diagnosis at 9 weeks or 6 months postpartum, compared to healthy follicular phase women, and that no correlation was found between cortical GABA concentrations and estradiol, progesterone, or ALLO levels. Smith et al. ( 129 ) found that the effect of neuroactive steroids on inhibition, which influence anxiety state and seizure susceptibility, depends not only on the subunit composition of the receptor but also on the direction of Cl − current generated by these target receptors.
Prolactin has physiological functions that are especially relevant in the peripartum period, and may act as an attenuation for behavioral and neuroendocrine stress responses during both pregnancy and lactation ( 130 ). Despite mixed evidence from different studies, two studies with a big sample size indeed suggested an negative correlation between PPD and prolactin ( 131 , 132 ).
The oxytocin signaling network has been of great interest as it can play an important role in mother-infant bonding and interactions. Recently, there have been investigations on the trajectories of oxytocin throughout the gestation and lactation periods, especially how they respond to the onset and development of PPD symptoms. It has been suggested that lower levels of oxytocin in both the gestation and postpartum periods may imply an elevated risk for developing PPD ( 9 , 90 , 91 ). Jobst et al. ( 133 ) found that plasma oxytocin levels significantly increased from week 35 of gestation to 6 months postpartum in all women. However, levels decreased from the 38th week of gestation to 2 days after delivery in women with PPD, whereas, they increased continuously in the healthy control group. This suggests that the time evolution pattern of oxytocin may be a predictor of PPD in the immediate postpartum period (within 2 weeks). In comparison, Massey et al. ( 134 ) found that oxytocin level interacted with past MDD to predict PPD symptom severity in the third trimester; a higher oxytocin level predicted greater PPD symptom severity in women with past MDD, but not in women without. Thul et al. ( 135 ) reviewed the literature on the relationship between both endogenous and synthetic oxytocin and PPD, and found that out of the 12 studies that focused on endogenous oxytocin, eight studies suggested an inverse correlation between plasma oxytocin levels and depressive symptoms.
There is mixed evidence for the association between testosterone levels in late pregnancy and PPD symptoms in the early postpartum stage. Women with PPD symptoms were reported to have higher serum testosterone levels in the late third trimester ( 136 ) and around 24 h postpartum than healthy controls ( 137 ). However, other studies have failed to find such an association ( 138 ).
The role of thyroid hormone in the development of perinatal mood disorder has also been investigated ( 139 ). It has been suggested that timing may be critical in the correlation between thyroid hormones and PPD, since the function of thyroid is to respond to the constant changes in other hormones across gestation. Kuijpens et al. ( 140 ) reported that the presence of thyroperoxidase antibody (TPOAb) during gestation was associated with the occurrence of subsequent depression during the postpartum period and as such can be regarded as a marker for depression. Elevations in thyroid stimulating hormone (TSH) upon delivery have been proposed to be a predictor for PPD 6 month post-parturm ( 141 ). However, in an earlier large cohort study, Albacar et al. ( 142 ) examined 1,053 postpartum Spanish women without a previous history of depression, and concluded that thyroid function at 48 h after delivery does not predict PPD susceptibility. Groer and Vaughan ( 143 ) found that pregnant TPO-positive women were more likely to develop PPD 6 months after delivery. A review ( 144 ) suggested that TPOAb in early to mid-pregnancy was associated with concurrent depression and may be predictive of PPD. Recently, Wesseloo et al. ( 145 ) found that women with an increased TPOAb titer during early gestation were at increased risk for self-reported first-onset depression, which suggested an overlap in the etiology of first-onset PPD and autoimmune thyroid dysfunction. Li et al. ( 146 ) found that PPD patients showed elevated serum levels of triiodothyronine, thyroxine, free triiodothyronine, free thyroxine along with diminished estradiol, progesterone, and TSH levels. It was proposed that it may not just be thyroid hormones alone, but rather thyroid in conjunction with other factors such as estrogens ( 10 ) or trauma history ( 147 ), that are implicated in PPD etiology. A consensus regarding the role of thyroid hormones as a biomarker is yet to be reached.
While strong evidence seems to have been found to support that a low ALLO level during pregnancy predicts PPD, it can be seen, however, that for most reproductive hormones, a consensus regarding their association with PPD is yet to be reached. Many studies are limited by small sample sizes. Furthermore, the dynamical changes of reproductive hormones during the perinatal period should also be taken into account. This will require that the timing for sampling and depression assessment be arranged in a more systematic and consistent manner, hopefully across different studies.
Stress Hormones
Stress hormones, particularly those of the HPA axis, have been implicated in non-puerperal depression ( 148 ). It was suggested that the hyporesponsiveness of the HPA axis may persist for several months postpartum ( 149 ). There is strong evidence that the HPA axis plays an important role in perinatal depression, both during gestation and postpartum. Groer and Morgan ( 131 ) reported that depressed mothers had a down-regulated HPA axis, in that the salivary cortisol level was lower in PPD patients than in healthy controls. Similar to reproductive hormones, the levels of stress hormones also increase during pregnancy from the first to third trimester and then decrease abruptly upon parturition. In contrast, the neuropeptide corticotropin-releasing hormone (CRH) often increases exponentially in the process of pregnancy ( 150 ), mainly because CRH is also produced by the placenta ( 151 ). This also leads to an increased level of adrenocorticotropic hormone (ACTH) and cortisol over the course of pregnancy ( 152 ). The CRH level drops quickly upon parturition, when the placenta is discharged. Yim et al. ( 153 ) found that at a critical period in midpregnancy, placental CRH (pCRH) is a sensitive and specific early diagnostic test for PPD; at 25 weeks' GA, pCRH was a strong predictor of PPD, and the trajectories of pCRH in women with PPD are significantly accelerated from 23 to 26 weeks' GA. Hahn-Holbrook et al. ( 154 ) found that steeper increases in placental CRH from 29 to 37 weeks' gestation predicted more depressive symptoms postpartum. Iliadis et al. ( 155 ) reported an association between high CRH levels in gestational week 17 and the development of PPD symptoms, among women without depressive symptoms during pregnancy. On the contrary, Meltzer-Brody et al. ( 34 ) found that higher mid-pregnancy placental CRH was not associated with an increased risk of PPD. Glynn and Sandman ( 156 ) showed that depressive symptoms at 3 months postpartum were associated with elevated mid-gestational pCRH levels and also accelerated trajectories of pCRH, but pCRH was not predictive of PPD at 6 months postpartum. They concluded that elevated pCRH level during pregnancy may act as a marker of risk of developing PPD. Overall, the result on CRH as a viable biomarker is mixed so far, possibly due to limitations of small sample sizes and different timing for sampling and assessment of the PPD symptoms.
Alteration in the HPA axis is a robust biomarker of anxiety and depression, and significant mood symptoms in pregnancy was shown to be associated with altered diurnal cortisol in pregnancy ( 157 ). Labad et al. ( 158 ) found that women with postpartum thoughts of harming the infant had higher ACTH levels, when compared to those women without intrusive thoughts, and a dysregulation of the HPA axis may play a role in the etiology of postpartum thoughts of harming the infant. Jolley et al. ( 159 ) found higher ACTH and lower cortisol levels in women with PPD at 6 and 12 weeks postpartum when compared with controls. Women with PPD were found to have greater ACTH stress reactivity to cold pressor test (CPT), and a significantly elevated ACTH concentration level at 8 weeks postpartum in response to CPT ( 160 ), as well as a markedly blunted plasma ACTH response to serial ovine CRH tests at 3, 6, and 12 weeks postpartum ( 161 ), and it was suggested that the suppressed ACTH response to ovine CRH might serve as a biochemical marker of the postpartum “blues” or depression ( 161 ). Together, these findings again suggested that dysregulation of the HPA axis may be associated with PPD. However, a comparative study found no differences in the HPA axis reactivity in terms of the cortisol and ACTH response, e.g., the cortisol/ACTH ratio, to pharmacologic test and psychological challenges during the luteal phase between current euthymic postpartum women with a history of either PPD or MDD and controls ( 162 ). We note that the sample sizes for the last four studies were fairly small, with 12, 34, 17, and 15 × 3 (15 in each group), respectively. Further investigations with larger sample sizes and better designs are needed to reconcile these findings.
As for β-endorphin, Yim et al. ( 163 ) found that among women who were euthymic at 25 weeks' GA, those who developed PPD had higher β-endorphin levels throughout pregnancy than women without PPD symptoms, and suggested that β-endorphin may play an important role in the pathophysiology of PPD and may thus be a useful early predictor of PPD symptoms in women who show no depressive symptoms in mid-pregnancy. They also reported that around 25 weeks' gestation was a crucial time for assessing PPD symptoms. However, postpartum blood samples were taken only at 9 weeks for assessment of β-endorphin levels, and self-report was used to evaluate the depressive symptoms.
Parcells ( 164 ) found that cortisol levels directly correlated with maternal depression, anxiety, and stress. Recent studies found that women with PPD had higher salivary evening cortisol at 6 weeks postpartum ( 165 ), elevated hair cortisol levels in the first to third trimesters ( 166 ), or lower levels of evening cortisol in the immediate peripartum period ( 167 ), compared to healthy controls. Corwin et al. ( 168 ) found that cortisol levels, together with family history of depression and interleukin (IL)-8/IL-10 ratio, were significant predictors of PPD symptoms. However, a recent survey ( 169 ) showed that most studies reported no association between maternal cortisol and antenatal depression, and that among studies that reported an association, second-trimester and third-trimester cortisol assessments more consistently reported an association. The link between cortisol levels and postpartum or perinatal depressions is far from conclusive, and thus more future investigations are warranted.
Among stress hormones, it is less controversial that alteration of the HPA axis is a robust biomarker for PPD. However, results on CRH, ACTH and cortisol levels are rather mixed, and further studies are needed on these hormones as well as β-endorphin.
Immunological/Inflammatory Studies
The function of the immune system is to protect the body from foreign substances (as well as other pathogenic organisms) ( 10 ). However, the fetus should not be attacked during pregnancy even though it is necessarily genetically distinct and carries paternal antigens that are foreign to the maternal immune system. Thus this constitutes a big challenge for the maternal immune system. Although, it is not yet clear how it maintains the proper balance of proinflammatory cytokines [e.g., IL-6, IL-1β, tumor necrosis factor-alpha (TNF-α)] and anti-inflammatory cytokines (e.g., IL-10), it can be expected that the immune system will have more activities during the perinatal period. A growing body of literature suggests that inflammatory responses have an important role in the pathophysiology of depression ( 170 ). In addition, prolonged HPA axis hyperactivity activated by proinflammatory cytokines (IL-1, IL-6, and TNF-α) is one of the mechanisms underlying cytokine-induced depression ( 171 ), even in perinatal episodes ( 172 ).
It has been found that puerperal women usually have significantly higher levels of proinflammatory cytokines during the last trimester of pregnancy, and are also at higher risk for depression ( 173 ). Other stressors also cause proinflammatory cytokine levels to rise at such a time. Breastfeeding may attenuate stress and modulate the inflammatory response. Overall, the proinflammatory state during the late pregnancy and the early postpartum period plays an important role in the development of PPD ( 173 ). IL-6 is the most commonly studied cytokine, and has been consistently identified as being elevated in depression. An earlier study ( 174 ) found that the levels of serum IL-6 and its receptor (IL-6R) were significantly higher in the early puerperium than before delivery, and women who developed depressive symptoms in the early puerperium had significantly higher serum IL-6 and IL-6R concentrations than those without. Corwin et al. ( 175 ) reported an increase in IL-1β on Day 14 and Day 28 postpartum in women with PPD, compared to levels in euthymic women, suggesting an association between symptoms of PPD and elevated levels of IL-1β during the first month postpartum. Boufidou et al. ( 176 ) found that cytokine IL-6 and TNF-α levels in the cerebrospinal fluid (CSF) and TNF-α levels in serum were positively associated with depressive mood during the first 4 days postpartum and also at sixth week postpartum. Krause et al. ( 177 ) found that regulatory T cells in pregnancy strongly predicted PPD. Corwin et al. ( 168 ) found that family history of depression and cortisol AUC and the IL8/IL10 ratio both on day 14 were significant predictors of PPD. Liu et al. ( 178 ) found that elevated serum IL-6 at delivery was associated with development of PPD during the 6 months post partum. In a longitudinal study ( 179 ), the IL-6 and IL-10 levels measured in the third trimester were found to be a significant predictor of PPD. However, the evidence is mixed for the link between PPD and proinflammatory cytokines ( 10 ). For example, serum leptin levels at delivery were found earlier ( 180 ) to be negatively associated with self-reported depression during the first 6 months after delivery, but were significantly greater in women with PPD than in healthy controls at 3 months post-partum ( 181 ). However, a similar association was not found between serum IL-6 levels at delivery and later PPD symptoms.
Other proinflammatory cytokines (and their receptors) have also been investigated, as potential biomarkers for PPD. Groer and Morgan ( 131 ) found that women with PPD had lower serum levels of Interferon-gamma (IFN-γ) and a lower IFN-γ/IL-10 ratio in both serum and in whole blood stimulated cultures. Fransson et al. ( 182 ) found that mothers with depression had higher TGF-β2 concentrations in their breast milk than mothers without depression. They also found associations between maternal IL-6, IL-8 and cord IL-6, IL-8, IL-10, IL-13, and IL-18 levels and depressive symptoms in the first 5 days postpartum in women who delivered preterm. Clara Cell Protein (CC16), an endogenous anticytokine, may also be related to PPD. Maes et al. ( 183 ) found that parturients who developed PPD had significantly lower serum CC16 concentrations than women who did not. Bränn et al. ( 184 ) found that among 70 inflammatory markers, five were significantly elevated in women with PPD, including TNF ligand superfamily member (TRANCE), hepatocyte growth factor (HGF), IL-18, fibroblast growth factor 23 (FGF 23), and C-X-C motif chemokine 1 (CXCL1).
Mixed evidence has been found for the linkage between the C-reactive protein (CRP) and PPD. The review by Lambert and Gressier ( 185 ) suggested that the dosage of some inflammation biomarkers, including CRP, at the very end of pregnancy or immediately after delivery could predict PPD. Bränn et al. ( 186 ) found that the signal transducing adaptor molecule-binding protein (STAM-BP), axin-1, adenosine deaminase (ADA), sulfotransferase 1A1 (ST1A1), and IL-10 were lower in late pregnancy among women with PPD, and proposed a summary inflammation variable for predicting PPD. So far, further studies are needed to address inconsistencies in different results regarding the role of inflammatory processes in the development of PPD.
Overall, evidence for (anti-)proinflammatory cytokines as a diagnostic and predictive biomarker for PPD is mixed, which calls for more systematic studies in the future.
Biomarkers From Biochemical Studies
Identification of nutritional and biochemical markers for PPD diagnosis has gained attention lately. Wójcik et al. ( 187 ) revealed a correlation between the severity of depressive symptoms and decreased serum zinc concentration third day after delivery in PPD patients. Roomruangwong et al. ( 188 ) found that lower serum zinc (and higher CRP) levels strongly predicted prenatal depression and physio-somatic symptoms, which all together predicted postnatal depressive symptoms.
The role of vitamin D in the development of depression has been studied in recent years, because it has regulatory functions in the immune system, and thus may act effectively as a neurosteroid. Christesen et al. ( 189 ) conducted a review on the impact of vitamin D on pregnancy, and found that a decreased vitamin D level during pregnancy may lead to PPD in one study and preeclampsia in several studies. In an exploratory study ( 190 ), a significant relationship over time was found between low 25-hydroxyvitamin D (25(OH)D) levels and high EPDS scores. Brandenbarg et al. ( 191 ) found that low early-pregnancy vitamin D status was associated with elevated depressive symptoms in pregnancy. Gur et al. ( 192 ) found that lower maternal 25(OH)D3 levels were associated with higher levels of PPD at all time points, and thus may be a factor affecting the development of PPD. Similarly, Robinson et al. ( 193 ) also reported that low vitamin D during pregnancy is a risk factor for the development of PPD symptoms. A cross-sectional study ( 194 ) found that higher dietary vitamin D intake was significantly associated with a lower prevalence of depressive symptoms during pregnancy. A recent prospective study found an association between lower prenatal log 25(OH)D and significantly more severe PPD symptoms, among women with higher levels of inflammatory markers ( 195 ). In a controlled study, Fu et al. ( 196 ) found an association between lower serum 25(OH)D levels measured 24 h after delivery and PPD. Fortunately, these findings seem to be in agreement with each other.
In a prospective cohort study of 238 pregnant women, Teofilo et al. ( 197 ) found that HDL-cholesterol concentrations were inversely associated with EPDS scores during pregnancy. In a prospective study of 266 Dutch women, Van Dam et al. ( 198 ) did not find an association between rapid serum cholesterol decline and the risk of developing PPD.
The PUFA status in late pregnancy was studied in a large sample of women, and only a weak link between PUFA in late pregnancy and PPD risk was reported ( 199 ). In a community-based prospective cohort, Markhus et al. ( 200 ) found that the DPA content, DHA content, ω-3 index, ω-3/ω-6 ratio, total PUFA score, and the ω-3 PUFA score were all inversely correlated with the EPDS score. Sallis et al. ( 201 ) found a weak positive correlation between ω-3 fatty acids and PPD. In another study, no association between plasma PUFAs and PPD was found ( 202 ).
For vitamin B12 and folate, a cross-sectional study reported no association between depressive symptoms and blood levels of vitamin B12 and folate ( 203 ). Lewis et al. ( 204 ) did not find strong evidence that folic acid supplementation can reduce the risk of depression during first 8 months of pregnancy. Another study found significantly higher homocysteine levels in women with PPD than in healthy controls, suggesting that the level of serum homocysteine might be a risk biomarker for PPD ( 205 ).
An increased kynurenine level has also been reported to be associated with the induction of depression. Depressive and anxiety symptoms in the early puerperium are associated with increased catabolism of tryptophan into kynurenine ( 206 ), and the increases in plasma kynurenine and the kynurenine/tryptophan (K/T) ratio were positively correlated with the anxiety and depression scores in the puerperium.
There have been some studies on gut microbiome as potential biomarkers recently ( 207 ). The coordination between the gut, the central nervous system, and the neuroendocrine and neuroimmune axes is referred to as the gut-brain axis ( 208 ). There are not many studies and thus not much has been known, regarding what role the gut-brain axis may play in the development of PPD. In a study of nearly 400 pregnant women ( 209 ), it was found that probiotic supplementation with Lactobacillus rhamnosus HN001 in pregnancy and postpartum period reduces the prevalence of PPD.
In summary, biochemical studies seem to suggest that low serum 25(OH)D levels during pregnancy may be a biomarker for PPD. Evidence for other biochemical markers for PPD is either mixed, very weak or negative, including levels of serum cholesterol, PUFA status, vitamin B12 and folate, kynurenine level, and gut microbiome.
Omics-Based Biomarker Studies
Biomarker identification in neuropsychiatric disorders such as PPD and MDD can have important advantages and benefits, in terms of prediction and accurate diagnosis of a disease, and may provide more accurate and reliable information which can guide the selection and development of a cure or treatment. Gadad et al. ( 210 ) conducted a review on various omics approaches for identifying biomarkers of neuropsychiatric disorders. Many of the biomarkers mentioned in the review can be identified using multi-omics, which includes genomics, epigenomics, transcriptomics, proteomics, metabolomics, and lipidomics. These omics technologies have been actively applied in studies on MDD. See, e.g., the review by Sethi and Brietzke ( 211 ). Given the strong similarity between PPD and MDD, these omics technologies can in principle be quickly applied to the identification of biomarkers for PPD.
Metabolomics has recently been applied to unravel the serum metabolomic profile of PPD ( 212 ). Serum metabolomes of a group of women ( n = 10) with PPD and a healthy control group ( n = 10), all from Greece, were analyzed for targeted metabolomics using mass spectrometry. In the PPD group, increased levels of five metabolites were found, such as glutathione-disulfide, adenylosuccinate, and ATP. The data showed that molecular changes related to PPD were indeed detectable in peripheral material, and thus these changes may serve as diagnostic biomarkers.
A metabolomic profiling of morning urine samples of women with PPD, postpartum women without depression (PPWD), and healthy controls (HCs) was recently characterized using gas chromatography-mass spectroscopy ( 213 ). Twenty two (22) differential metabolites (14 up regulated and 8 down regulated) were found to separate PPD subjects from HCs and PPWD. Meanwhile, a panel of five potential biomarkers – formate, succinate, 1-methylhistidine, α-glucose and dimethylamine – was identified, which could be used to effectively distinguish PPD subjects from HCs and PPWD. Recently, using Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry, Zhang et al. ( 214 ) found that the urine metabolomic profiles of patients with PPD were different from those of HCs. Ten differentiating metabolites were found as main contributors to this difference.
So far, there have been far fewer studies on the identification of biomarkers for PPD than for MDD. Nevertheless, we expect that multi-omics technologies will be widely used in identifying biomarkers for PPD in future studies. Indeed, given the enormous advantages of the omics, they should be a major direction of PPD research in the future.
It should be cautioned that, due to possible partial overlap in genetic and biological risk factors and biomarkers among PPD, MDD and BD, a panel of multiple biomarkers may be needed to avoid misdiagnosis.
Conclusions
PPD is a serious health issue for new mothers and has negative consequences on both the mothers and the children. Its high prevalence rate raises strong public health concerns. PPD is likely to be influenced by a multitude of risk factors, including biological, psychosocial, and even environmental factors. There have been various etiological models for PPD. However, no consensus has been reached so far. Typical treatments for PPD include psychotherapy and phamacotherapy, in the form of psychotherapy/counseling and antidepressant medications. While the US FDA has recently approved the first antidepressant medication for PPD, this medicine, Zulresso, has turned out to be extremely expensive, and thus is out of the reach for most PPD patients. This makes preventive measures more important, to protect one from developing PPD.
There has been increasing effort in the diagnosis of PPD using predictors, from both psychosocial and biological aspects. Furthermore, there are a variety of researches on PPD biomarkers so far, using different methods and approaches for characterizing and assessing PPD. While biomarker identification has shown a lot of promise for PPD research, nevertheless no biomarker is ready for clinical use as of today. From etiologic point of view, (epi)genetics and hormones may play a more fundamental role than biochemicals in the development of PPD. Nonetheless, biochemicals may as well be the right signatures or indicators that can be used for diagnosing and predicting PPD. Biomarkers in genetics and epigenetics may have a big potential for personal risk prediction. Several hormones, neurosteroids, and biochemicals have been identified in preliminary studies as potential biomarkers for predicting PPD, but further studies and substantiation are needed before they can be put into clinical use. So far, there are strong inconsistencies in various findings regarding predictors and biomarkers of PPD. These inconsistencies presumably have to do with the limited sample sizes, inconsistent depressive rating scales and timing for sampling, and inconsistent designs across different studies, as well as the high complexity of PPD. Further large-scale, integrative studies are needed to fully understand PPD. Given the objectiveness of biomarkers, we expect that the identification of biomarkers of PPD will be an important subject in future research. Despite that the application of multi-omics to the study of PPD has just begun recently, we strongly believe that modern multi-omics technologies will have a great potential in this arena.
While many studies have found associations or correlations between certain risk factors, predictors, or biomarkers and PPD, these correlations do not necessarily tell whether these risk factors, predictors, and markers are consequences or causes of PPD. A correct etiological model which is subject to comprehensive testing is crucial for uncovering the underlying cause of PPD and for developing the right medication and cure for PPD.
Finally, we end this review by presenting a brief speculative model for the etiology of PPD, based on the findings summarized hereinabove. The hormonal withdrawal theory has largely been proved to be wrong, as the hormonal withdrawal is a normal process every pregnant woman has to undergo upon parturition. This is a desired and necessary biological change that is adapted to pregnancy and parturition, as a result of human evolution. However, for a portion of women the biological system may not perform as perfectly as expected, due to the high complexity of human body and a wide range of genetic and epigenetic variations, as well as environmental, psychosocial and biological factors. In other words, for those the body does not fulfill and cope with the hormonal withdrawal in a perfect manner, depressive disorders to various degrees may develop. Thus, the ultimate goal of studying various predictors and biomarkers for PPD will be to catch such an imperfection at an early stage so that it can be remedied or prevented in time.
Author Contributions
YY completed the literature survey and manuscript writing. J-CL initiated and oversaw the project. H-FL, JC, Z-BL, Y-SH, and J-XC participated in the discussion and the manuscript preparation. All authors contributed to the article and approved the submitted version.
This work was supported in part by the Science Technology Department of Zhejiang Province, China (Project for Applications of Technology, Grant No. 2017C37004), National Natural Science Foundation of China (Grant No. 81772266), and Guangzhou Science and Technology Project (Grant No. 201804010369).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Yim IS, Schetter CD. Biopsychosocial predictors of perinatal depressive symptoms: moving toward an integrative approach. Biol Psychol. (2019) 147:107720. doi: 10.1016/j.biopsycho.2019.107720
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Serati M, Carnevali G. Perinatal depression. In: Clinical Cases in Psychiatry: Integrating Translational Neuroscience Approaches . Cham: Springer International Publishing. (2019) p. 155–70. doi: 10.1007/978-3-319-91557-9_9
CrossRef Full Text | Google Scholar
3. Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Womens. Ment Health. (2003) 6:263–74. doi: 10.1007/s00737-003-0024-6
4. Dennis CLC-L, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. (2009) 123:e736–51. doi: 10.1542/peds.2008-1629
5. Blum LD. Psychodynamics of postpartum depression. Psychoanal Psychol. (2007) 24:45–62. doi: 10.1037/0736-9735.24.1.45
6. Yawn BP, Olson AL, Bertram S, Pace W, Wollan P, Dietrich AJ. Postpartum depression: screening, diagnosis, and management programs 2000 through 2010. Depress Res Treat. (2012) 2012:363964. doi: 10.1155/2012/363964
7. O'Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol. (2013) 9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612
8. Anderson G, Maes M. Postpartum depression: psychoneuroimmunological underpinnings and treatment. Neuropsychiatr Dis Treat. (2013) 9:277–87. doi: 10.2147/NDT.S25320
9. Kim S, Soeken TA, Cromer SJ, Martinez SR, Hardy LR, Strathearn L. Oxytocin and postpartum depression: delivering on what's known and what's not. Brain Res. (2014) 1580:219–32. doi: 10.1016/j.brainres.2013.11.009
10. Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. (2015) 11:99–137. doi: 10.1146/annurev-clinpsy-101414-020426
11. Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. (2003) 44:234–46. doi: 10.1016/S0010-440X(03)00034-8
12. Brummelte S, Galea LAM. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuro-Psychopharmacol Biol Psychiatry. (2010) 34:766–76. doi: 10.1016/j.pnpbp.2009.09.006
13. Corwin EJ, Kohen R, Jarrett M, Stafford B. The heritability of postpartum depression. Biol Res Nurs. (2010) 12:73–83. doi: 10.1177/1099800410362112
14. Skalkidou A, Hellgren C, Comasco E, Sylvén S, Sundström Poromaa I. Biological aspects of postpartum depression. Womens Heal. (2012) 8:659–72. doi: 10.2217/WHE.12.55
15. O'Hara MW, Swain AM. Rates and risk of postpartum depression — a meta-analysis. Int Rev Psychiatry. (1996) 8:37–54. doi: 10.3109/09540269609037816
16. Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. (2004) 26:289–95. doi: 10.1016/j.genhosppsych.2004.02.006
17. O'Hara MW. Postpartum depression: what we know. J Clin Psychol. (2009) 65:1258–69. doi: 10.1002/jclp.20644
18. Hopkins J, Marcus M, Campbell SB. Postpartum depression: a critical review. Psychol Bull. (1984) 95:498–515. doi: 10.1037/0033-2909.95.3.498
19. Meltzer-Brody S, Howard LM, Bergink V, Vigod S, Jones I, Munk-Olsen T, et al. Postpartum psychiatric disorders. Nat Rev Dis Prim. (2018) 4:1–18. doi: 10.1038/nrdp.2018.22
20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders . 5th ed. Washington, DC: American Psychiatric Association (2013). doi: 10.1176/appi.books.9780890425596
21. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) . New York, NY: New York State Psychiatric Institute (2002).
Google Scholar
22. Cox JL, Holden JM, Sagovsky R. Edinburgh postnatal depression scale (EPDS). Br J Psychiatry . (1987). 150:782–6. doi: 10.1192/bjp.150.6.782
23. Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S, Ove Samuelsen S. Review of validation studies of the Edinburgh postnatal depression scale. Acta Psychiatr Scand. (2008) 104:243–9. doi: 10.1111/j.1600-0447.2001.00187.x
24. Arroll B, Goodyear-Smith F, Crengle S, Gunn J, Kerse N, Fishman T, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. (2010) 8:348–53. doi: 10.1370/afm.1139
25. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
26. Venkatesh KK, Zlotnick C, Triche EW, Ware C, Phipps MG. Accuracy of brief screening tools for identifying postpartum depression among adolescent mothers. Pediatrics. (2014) 133:45–53. doi: 10.1542/peds.2013-1628
27. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
28. Sharp R. The Hamilton rating scale for depression. Occup Med. (2015) 65:340. doi: 10.1093/occmed/kqv043
29. Nassir Ghaemi S, Miller CJ, Berv DA, Klugman J, Rosenquist KJ, Pies RW. Sensitivity and specificity of a new bipolar spectrum diagnostic scale. J Affect Disord. (2005) 84:273–7. doi: 10.1016/S0165-0327(03)00196-4
30. Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders. Adv Nurs Sci. (2005) 28:364–75. doi: 10.1097/00012272-200510000-00008
31. Glynn LM, Davis EP, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. (2013) 47:363–70. doi: 10.1016/j.npep.2013.10.007
32. O'Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J Abnorm Psychol. (1991) 100:63–73. doi: 10.1037/0021-843X.100.1.63
33. Workman JL, Barha CK, Galea LAM. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci. (2012) 126:54–72. doi: 10.1037/a0025538
34. Meltzer-Brody S, Stuebe A, Dole N, Savitz D, Rubinow D, Thorp J. Elevated Corticotropin Releasing Hormone (CRH) during pregnancy and risk of Postpartum Depression (PPD). J Clin Endocrinol Metab. (2011) 96:40–7. doi: 10.1210/jc.2010-0978
35. Byrnes EM, Byrnes JJ, Bridges RS. Increased sensitivity of dopamine systems following reproductive experience in rats. Pharmacol Biochem Behav. (2001) 68:481–9. doi: 10.1016/S0091-3057(01)00449-X
36. Licheri V, Talani G, Gorule AA, Mostallino MC, Biggio G, Sanna E. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. (2015) 2015:1–11. doi: 10.1155/2015/170435
37. Pennell KD, Woodin MA, Pennell PB. Quantification of neurosteroids during pregnancy using selective ion monitoring mass spectrometry. Steroids. (2015) 95:24–31. doi: 10.1016/j.steroids.2014.12.007
38. Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, Payne JL. Lower allopregnanolone during pregnancy predicts postpartum depression: an exploratory study. Psychoneuroendocrinology. (2017) 79:116–21. doi: 10.1016/j.psyneuen.2017.02.012
39. Meltzer-Brody S, Kanes SJ. Allopregnanolone in postpartum depression: role in pathophysiology and treatment. Neurobiol Stress. (2020) 12:100212. doi: 10.1016/j.ynstr.2020.100212
40. Beck CT. Theoretical perspectives of postpartum depression and their treatment implications. MCN Am J Matern Nurs. (2002) 27:282–7. doi: 10.1097/00005721-200209000-00008
41. Cutrona CE, Troutman BR. Social support, infant temperament, and parenting self-efficacy: a mediational model of postpartum depression. Child Dev. (1986) 57:1507. doi: 10.2307/1130428
42. Halbreich U. Postpartum disorders: multiple interacting underlying mechanisms and risk factors. J Affect Disord. (2005) 88:1–7. doi: 10.1016/j.jad.2005.05.002
43. Hagen EH. The functions of postpartum depression. Evol Hum Behav. (1999) 20:325–59. doi: 10.1016/S1090-5138(99)00016-1
44. Hahn-Holbrook J, Haselton M. Is postpartum depression a disease of modern civilization Curr Dir Psychol Sci. (2014) 23:395–400. doi: 10.1177/0963721414547736
45. Green SM, Haber E, Frey BN, McCabe RE. Cognitive-behavioral group treatment for perinatal anxiety: a pilot study. Arch Womens Ment Health. (2015) 18:631–8. doi: 10.1007/s00737-015-0498-z
46. Pearlstein TB, Zlotnick C, Battle CL, Stuart S, O'Hara MW, Price AB, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health. (2006) 9:303–8. doi: 10.1007/s00737-006-0145-9
47. Sharp D, Chew-Graham C, Tylee A, Lewis G, Howard L, Anderson I, et al. A pragmatic randomised controlled trial to compare antidepressants with a community-based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial. Health Technol Assess . (2010) 14:1–181. doi: 10.3310/hta14430
48. FDA. FDA Approves First Treatment for Post-Partum Depression . (2019). Available online at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-post-partum-depression (accessed July 12, 2020).
49. Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. (2018) 392:1058–70. doi: 10.1016/S0140-6736(18)31551-4
50. Weissman AM, Levy BT, Hartz AJ, Bentler S, Donohue M, Ellingrod VL, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. (2004) 161:1066–78. doi: 10.1176/appi.ajp.161.6.1066
51. di Scalea TE, Wisner KL. Antidepressant medication use during breastfeeding. Clin Obstet Gynecol. (2009) 52:483–97. doi: 10.1097/GRF.0b013e3181b52bd6
52. Fortinguerra F, Clavenna A, Bonati M. Psychotropic drug use during breastfeeding: a review of the evidence. Pediatrics. (2009) 124:e547–56. doi: 10.1542/peds.2009-0326
53. Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. (2010) 6:89–98. doi: 10.1089/bfm.2010.0019
54. Akiskal HS. Toward a clinical understanding of the relationship of anxiety and depressive-disorders. In: Maser JD, Cloninger CR, editors. Conference on Comorbidity of Mood and Anxiety Disorders . Tuxedo, NY: NIMH Bristol Myers Sandoz Pharm Upjohn (1990). p. 597–607.
55. Chessick CA, Dimidjian S. Screening for bipolar disorder during pregnancy and the postpartum period. Arch Womens Ment Health. (2010) 13:233–48. doi: 10.1007/s00737-010-0151-9
56. Tavormina G. Bipolar disorders and bipolarity: the notion of the “mixity.” Psychiatr Danub . (2019) 31:S434–7. Available online at: http://www.psychiatria-danubina.com/UserDocsImages/pdf/dnb_vol31_noSuppl%203/dnb_vol31_noSuppl%203_434.pdf
PubMed Abstract | Google Scholar
57. Goldberg JF, Ernst CL. Features associated with the delayed initiation of mood stabilizers at illness onset in bipolar disorder. J Clin Psychiatry. (2002) 63:985–91. doi: 10.4088/JCP.v63n1105
58. Schneck CD, Miklowitz DJ, Miyahara S, Araga M, Wisniewski S, Gyulai L, et al. The prospective course of rapid-cycling bipolar disorder: findings from the STEP-BD. Am J Psychiatry. (2008) 165:370–7. doi: 10.1176/appi.ajp.2007.05081484
59. Rihmer Z, Akiskal KK, Rihmer A, Akiskal HS. Current research on affective temperaments. Curr Opin Psychiatry. (2010) 23:12–8. doi: 10.1097/YCO.0b013e32833299d4
60. Tavormina G. An approach to treat bipolar disorders mixed states. Psychiatr Danub. (2016) 28(Suppl. 1):9–12. Available online at: http://www.psychiatria-danubina.com/UserDocsImages/pdf/dnb_vol28_sup1/dnb_vol28_sup1_9.pdf
61. Haga SM, Ulleberg P, Slinning K, Kraft P, Steen TB, Staff A. A longitudinal study of postpartum depressive symptoms: multilevel growth curve analyses of emotion regulation strategies, breastfeeding self-efficacy, and social support. Arch Womens Ment Health. (2012) 15:175–84. doi: 10.1007/s00737-012-0274-2
62. Silverman ME, Reichenberg A, Savitz DA, Cnattingius S, Lichtenstein P, Hultman CM, et al. The risk factors for postpartum depression: a population-based study. Depress Anxiety. (2017) 34:178–87. doi: 10.1002/da.22597
63. Goyal D, Gay C, Lee KA. How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first-time mothers Womens Heal Issues. (2010) 20:96–104. doi: 10.1016/j.whi.2009.11.003
64. Wassif OM, Abdo AS, Elawady MA, Abd Elmaksoud AE, Eldesouky RS. Assessment of postpartum depression and anxiety among females attending primary health care facilities in Qaliubeya Governorate, Egypt. J Environ Public Health. (2019) 2019:1–9. doi: 10.1155/2019/3691752
65. Sockol LE, Epperson CN, Barber JP. The relationship between maternal attitudes and symptoms of depression and anxiety among pregnant and postpartum first-time mothers. Arch Womens Ment Health. (2014) 17:199–212. doi: 10.1007/s00737-014-0424-9
66. Blom E, Jansen P, Verhulst F, Hofman A, Raat H, Jaddoe V, et al. Perinatal complications increase the risk of postpartum depression. The Generation R Study. BJOG. (2010) 117:1390–8. doi: 10.1111/j.1471-0528.2010.02660.x
67. Vigod S, Villegas L, Dennis C-L, Ross L. Prevalence and risk factors for postpartum depression among women with preterm and low-birth-weight infants: a systematic review. BJOG. (2010) 117:540–50. doi: 10.1111/j.1471-0528.2009.02493.x
68. Ihongbe TO, Masho SW. Do successive preterm births increase the risk of postpartum depressive symptoms J Pregnancy. (2017) 2017:1–10. doi: 10.1155/2017/4148136
69. Helle N, Barkmann C, Bartz-Seel J, Diehl T, Ehrhardt S, Hendel A, et al. Very low birth-weight as a risk factor for postpartum depression four to six weeks postbirth in mothers and fathers: cross-sectional results from a controlled multicentre cohort study. J Affect Disord. (2015) 180:154–61. doi: 10.1016/j.jad.2015.04.001
70. Payne JL. Genetic basis for postpartum depression. In: Biomarkers of Postpartum Psychiatric Disorders . London: Elsevier. (2020) p. 15–34. doi: 10.1016/B978-0-12-815508-0.00002-3
71. Viktorin A, Meltzer-Brody S, Kuja-Halkola R, Sullivan PF, Landén M, Lichtenstein P, et al. Heritability of perinatal depression and genetic overlap with non-perinatal depression. Am J Psychiatry. (2016) 173:158–65. doi: 10.1176/appi.ajp.2015.15010085
72. Forty L, Zammit S, Craddock N. Genetic risk and familial transmission of depression. In: Dobson KS, Dozois DJA, editors. Risk Factors in Depression . San Diego: Elsevier (2008). doi: 10.1016/B978-0-08-045078-0.00002-2
73. Murphy-Eberenz K, Zandi PP, March D, Crowe RR, Scheftner WA, Alexander M, et al. Is perinatal depression familial J Affect Disord. (2006) 90:49–55. doi: 10.1016/j.jad.2005.10.006
74. Mahon PB, Payne JL, MacKinnon DF, Mondimore FM, Goes FS, Schweizer B, et al. Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am J Psychiatry. (2009) 166:1229–37. doi: 10.1176/appi.ajp.2009.09030417
75. Alvim-Soares AM, Miranda DM, Campos SB, Figueira P, Correa H, Romano-Silva MA. HMNC1 gene polymorphism associated with postpartum depression. Rev Bras Psiquiatr. (2014) 36:96–7. doi: 10.1590/1516-4446-2013-3507
76. Costas J, Gratacòs M, Escaramís G, Martín-Santos R, de Diego Y, Baca-García E, et al. Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. J Psychiatr Res. (2010) 44:717–24. doi: 10.1016/j.jpsychires.2009.12.012
77. Segman RH, Goltser-Dubner T, Weiner I, Canetti L, Galili-Weisstub E, Milwidsky A, et al. Blood mononuclear cell gene expression signature of postpartum depression. Mol Psychiatry. (2010) 15:93–100. doi: 10.1038/mp.2009.65
78. Licinio J. Potential diagnostic markers for postpartum depression point out to altered immune signaling. Mol Psychiatry. (2010) 15:1. doi: 10.1038/mp.2009.142
79. Lesch K-P, Mössner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders Biol Psychiatry. (1998) 44:179–92. doi: 10.1016/S0006-3223(98)00121-8
80. Comasco E, Sylvén SM, Papadopoulos FC, Oreland L, Sundström-Poromaa I, Skalkidou A. Postpartum depressive symptoms and the BDNF Val66Met functional polymorphism: effect of season of delivery. Arch Womens Ment Health. (2011) 14:453–63. doi: 10.1007/s00737-011-0239-x
81. Comasco E, Sylvén SM, Papadopoulos FC, Sundström-Poromaa I, Oreland L, Skalkidou A. Postpartum depression symptoms: a case–control study on monoaminergic functional polymorphisms and environmental stressors. Psychiatr Genet. (2011) 21:19–28. doi: 10.1097/YPG.0b013e328341a3c1
82. Zhang X, Wang L, Huang F, Li J, Xiong L, Xue H, et al. Gene-environment interaction in postpartum depression: a Chinese clinical study. J Affect Disord. (2014) 165:208–12. doi: 10.1016/j.jad.2014.04.049
83. Pinsonneault JK, Sullivan D, Sadee W, Soares CN, Hampson E, Steiner M. Association study of the estrogen receptor gene ESR1 with postpartum depression—a pilot study. Arch Womens Ment Health. (2013) 16:499–509. doi: 10.1007/s00737-013-0373-8
84. Mehta D, Newport DJ, Frishman G, Kraus L, Rex-Haffner M, Ritchie JC, et al. Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychol Med. (2014) 44:2309–22. doi: 10.1017/S0033291713003231
85. Mandelli L, Serretti A. Gene environment interaction studies in depression and suicidal behavior: an update. Neurosci Biobehav Rev. (2013) 37:2375–97. doi: 10.1016/j.neubiorev.2013.07.011
86. Doornbos B, Dijck-Brouwer DAJ, Kema IP, Tanke MAC, van Goor SA, Muskiet FAJ, et al. The development of peripartum depressive symptoms is associated with gene polymorphisms of MAOA, 5-HTT and COMT. Prog Neuro-Psychopharmacol Biol Psychiatry. (2009) 33:1250–4. doi: 10.1016/j.pnpbp.2009.07.013
87. Sacher J, Rekkas PV, Wilson AA, Houle S, Romano L, Hamidi J, et al. Relationship of monoamine oxidase-A distribution volume to postpartum depression and postpartum crying. Neuropsychopharmacology. (2015) 40:429–35. doi: 10.1038/npp.2014.190
88. Carter CS. Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol. (2014) 65:17–39. doi: 10.1146/annurev-psych-010213-115110
89. Feldman R. Oxytocin and social affiliation in humans. Horm Behav. (2012) 61:380–91. doi: 10.1016/j.yhbeh.2012.01.008
90. Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. (2011) 36:1886–93. doi: 10.1038/npp.2011.74
91. Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J Womens Heal. (2013) 22:352–61. doi: 10.1089/jwh.2012.3768
92. Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, Kennedy JL, et al. Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS ONE. (2013) 8:e61443. doi: 10.1371/journal.pone.0061443
93. Engineer N, Darwin L, Nishigandh D, Ngianga-Bakwin K, Smith SC, Grammatopoulos DK. Association of glucocorticoid and type 1 corticotropin-releasing hormone receptors gene variants and risk for depression during pregnancy and post-partum. J Psychiatr Res. (2013) 47:1166–73. doi: 10.1016/j.jpsychires.2013.05.003
94. Jonas W, Mileva-Seitz V, Girard AW, Bisceglia R, Kennedy JL, Sokolowski M, et al. Genetic variation in oxytocin rs2740210 and early adversity associated with postpartum depression and breastfeeding duration. Genes Brain Behav. (2013) 12:681–94. doi: 10.1111/gbb.12069
95. Bell AF, Carter CS, Steer CD, Golding J, Davis JM, Steffen AD, et al. Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Front Genet. (2015) 6:00243. doi: 10.3389/fgene.2015.00243
96. Kimmel M, Clive M, Gispen F, Guintivano J, Brown T, Cox O, et al. Oxytocin receptor DNA methylation in postpartum depression. Psychoneuroendocrinology. (2016) 69:150–60. doi: 10.1016/j.psyneuen.2016.04.008
97. King L, Robins S, Chen G, Yerko V, Zhou Y, Nagy C, et al. Perinatal depression and DNA methylation of oxytocin-related genes: a study of mothers and their children. Horm Behav. (2017) 96:84–94. doi: 10.1016/j.yhbeh.2017.09.006
98. Christian LM, Mitchell AM, Gillespie SL, Palettas M. Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology. (2016) 74:69–76. doi: 10.1016/j.psyneuen.2016.08.025
99. Lommatzsch M, Hornych K, Zingler C, Schuffwerner P, Hoppner J, Virchow J. Maternal serum concentrations of BDNF and depression in the perinatal period. Psychoneuroendocrinology. (2006) 31:388–94. doi: 10.1016/j.psyneuen.2005.09.003
100. Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, et al. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuro-Psychopharmacol Biol Psychiatry. (2006) 30:1256–60. doi: 10.1016/j.pnpbp.2006.03.025
101. Gazal M, Motta LS, Wiener CD, Fernandes JC, Quevedo LÁ, Jansen K, et al. Brain-derived neurotrophic factor in post-partum depressive mothers. Neurochem Res. (2012) 37:583–7. doi: 10.1007/s11064-011-0647-3
102. Pinheiro RT, Pinheiro KAT, da Cunha Coelho FM, de Ávila Quevedo L, Gazal M, da Silva RA, et al. Brain-derived neurotrophic factor levels in women with postpartum affective disorder and suicidality. Neurochem Res. (2012) 37:2229–34. doi: 10.1007/s11064-012-0851-9
103. Fung J, Gelaye B, Zhong Q-Y, Rondon MB, Sanchez SE, Barrios Y V., et al. Association of decreased serum brain-derived neurotrophic factor (BDNF) concentrations in early pregnancy with antepartum depression. BMC Psychiatry. (2015) 15:43. doi: 10.1186/s12888-015-0428-7
104. Figueira P, Malloy-Diniz L, Campos SB, Miranda DM, Romano-Silva MA, De Marco L, et al. An association study between the Val66Met polymorphism of the BDNF gene and postpartum depression. Arch Womens Ment Health. (2010) 13:285–9. doi: 10.1007/s00737-010-0146-6
105. Katz ER, Stowe ZN, Newport DJ, Kelley ME, Pace TW, Cubells JF, et al. Regulation of mRNA expression encoding chaperone and co-chaperone proteins of the glucocorticoid receptor in peripheral blood: association with depressive symptoms during pregnancy. Psychol Med. (2012) 42:943–56. doi: 10.1017/S0033291711002121
106. Reynolds RM, Pesonen A-K, O'Reilly JR, Tuovinen S, Lahti M, Kajantie E, et al. Maternal depressive symptoms throughout pregnancy are associated with increased placental glucocorticoid sensitivity. Psychol Med. (2015) 45:2023–30. doi: 10.1017/S003329171400316X
107. Pan D, Xu Y, Zhang L, Su Q, Chen M, Li B, et al. Gene expression profile in peripheral blood mononuclear cells of postpartum depression patients. Sci Rep. (2018) 8:1–9. doi: 10.1038/s41598-018-28509-4
108. Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology. (2013) 38:124–37. doi: 10.1038/npp.2012.73
109. Guintivano J, Arad M, Gould TD, Payne JL, Kaminsky ZA. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol Psychiatry. (2014) 19:560–7. doi: 10.1038/mp.2013.62
110. Kaminsky Z, Payne J. Seeing the future: epigenetic biomarkers of postpartum depression. Neuropsychopharmacology. (2014) 39:233–4. doi: 10.1038/npp.2013.238
111. Osborne LM, Clive M, Kimmel M, Gispen F, Guintivano J, Brown T, et al. Replication of epigenetic postpartum depression biomarkers and variation with hormone levels. Neuropsychopharmacology. (2016) 41:1648–58. doi: 10.1038/npp.2015.333
112. Osborne LM, Betz JF, Yenokyan G, Standeven LR, Payne JL. The role of allopregnanolone in pregnancy in predicting postpartum anxiety symptoms. Front Psychol. (2019) 10:1033. doi: 10.3389/fpsyg.2019.01033
113. Matrisciano F, Pinna G. PPAR and functional foods: rationale for natural neurosteroid-based interventions for postpartum depression. Neurobiol Stress. (2020) 12:100222. doi: 10.1016/j.ynstr.2020.100222
114. Pinna G. Allopregnanolone, the neuromodulator turned therapeutic agent: thank you, next Front Endocrinol . (2020) 11:236. doi: 10.3389/fendo.2020.00236
115. Payne JL, Osborne LM, Cox O, Kelly J, Meilman S, Jones I, et al. DNA methylation biomarkers prospectively predict both antenatal and postpartum depression. Psychiatry Res. (2020) 285:112711. doi: 10.1016/j.psychres.2019.112711
116. Mamrut S, Harony H, Sood R, Shahar-Gold H, Gainer H, Shi Y-J, et al. DNA methylation of specific CpG sites in the promoter region regulates the transcription of the mouse oxytocin receptor. PLoS ONE. (2013) 8:e56869. doi: 10.1371/journal.pone.0056869
117. Kaminsky Z, Payne JL. Epigenetic biomarkers of postpartum depression. In: Biomarkers of Postpartum Psychiatric Disorders . London: Elsevier. (2020). p. 35–43. doi: 10.1016/B978-0-12-815508-0.00003-5
118. Quiñones-Jenab V, Jenab S, Ogawa S, Adan RAM, Burbach PH, Pfaff DW. Effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the uterus, pituitary, and forebrain of the female rat. Neuroendocrinology. (1997) 65:9–17. doi: 10.1159/000127160
119. Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. (2011) 36:144–7. doi: 10.1016/j.psyneuen.2010.07.003
120. Kusui C, Kimura T, Ogita K, Nakamura H, Matsumura Y, Koyama M, et al. DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem Biophys Res Commun. (2001) 289:681–6. doi: 10.1006/bbrc.2001.6024
121. Bloch M. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. (2000) 157:924–30. doi: 10.1176/appi.ajp.157.6.924
122. Klier CM, Muzik M, Dervic K, Mossaheb N, Benesch T, Ulm B, et al. The role of estrogen and progesterone in depression after birth. J Psychiatr Res. (2007) 41:273–9. doi: 10.1016/j.jpsychires.2006.09.002
123. Serati M, Redaelli M, Buoli M, Altamura AC. Perinatal major depression biomarkers: a systematic review. J Affect Disord. (2016) 193:391–404. doi: 10.1016/j.jad.2016.01.027
124. Luisi S, Petraglia F, Benedetto C, Nappi RE, Bernardi F, Fadalti M, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. (2000) 85:2429–33. doi: 10.1210/jcem.85.7.6675
125. Paoletti AM, Romagnino S, Contu R, Orrù MM, Marotto MF, Zedda P, et al. Observational study on the stability of the psychological status during normal pregnancy and increased blood levels of neuroactive steroids with GABA-A receptor agonist activity. Psychoneuroendocrinology. (2006) 31:485–92. doi: 10.1016/j.psyneuen.2005.11.006
126. Hellgren C, Åkerud H, Skalkidou A, Bäckström T, Sundström-Poromaa I. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology. (2014) 69:147–53. doi: 10.1159/000358838
127. Nappi R. Serum allopregnanolone in women with postpartum “blues.” Obstet Gynecol . (2001) 97:77–80. doi: 10.1016/S0029-7844(00)01112-1
128. Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology. (2006) 186:425–33. doi: 10.1007/s00213-006-0313-7
129. Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABAA receptors: focus on the α4 and δ subunits. Pharmacol Ther. (2007) 116:58–76. doi: 10.1016/j.pharmthera.2007.03.008
130. Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. (2002) 5:249–57. doi: 10.1080/1025389021000048638
131. Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology. (2007) 32:133–9. doi: 10.1016/j.psyneuen.2006.11.007
132. Groër MW. Differences between exclusive breastfeeders, formula-feeders, and controls: a study of stress, mood, and endocrine variables. Biol Res Nurs. (2005) 7:106–17. doi: 10.1177/1099800405280936
133. Jobst A, Krause D, Maiwald C, Härtl K, Myint A-M, Kästner R, et al. Oxytocin course over pregnancy and postpartum period and the association with postpartum depressive symptoms. Arch Womens Ment Health. (2016) 19:571–9. doi: 10.1007/s00737-016-0644-2
134. Massey SH, Schuette SA, Pournajafi-Nazarloo H, Wisner KL, Carter CS. Interaction of oxytocin level and past depression may predict postpartum depressive symptom severity. Arch Womens Ment Health. (2016) 19:799–808. doi: 10.1007/s00737-016-0616-6
135. Thul TA, Corwin EJ, Carlson NS, Brennan PA, Young LJ. Oxytocin and postpartum depression: a systematic review. Psychoneuroendocrinology. (2020) 120:104793. doi: 10.1016/j.psyneuen.2020.104793
136. Kallak TK, Hellgren C, Skalkidou A, Sandelin-Francke L, Ubhayasekhera K, Bergquist J, et al. Maternal and female fetal testosterone levels are associated with maternal age and gestational weight gain. Eur J Endocrinol. (2017) 177:379–88. doi: 10.1530/EJE-17-0207
137. Aswathi A, Rajendiren S, Nimesh A, Philip RR, Kattimani S, Jayalakshmi D, et al. High serum testosterone levels during postpartum period are associated with postpartum depression. Asian J Psychiatr. (2015) 17:85–8. doi: 10.1016/j.ajp.2015.08.008
138. Chatzicharalampous C, Rizos D, Pliatsika P, Leonardou A, Hasiakos D, Zervas I, et al. Reproductive hormones and postpartum mood disturbances in Greek women. Gynecol Endocrinol. (2011) 27:543–50. doi: 10.3109/09513590.2010.501886
139. Ijuin T, Douchi T, Yamamoto S, Ijuin Y, Nagata Y. The relationship between maternity blues and thyroid dysfunction. J Obstet Gynaecol Res. (1998) 24:49–55. doi: 10.1111/j.1447-0756.1998.tb00052.x
140. Kuijpens JL, Vader HL, Drexhage HA, Wiersinga WM, Van Son MJ, Pop VJ. Thyroid peroxidase antibodies during gestation are a marker for subsequent depression postpartum. Eur J Endocrinol . (2001). 145:579–84. doi: 10.1530/eje.0.1450579
141. Sylvén SM, Elenis E, Michelakos T, Larsson A, Olovsson M, Poromaa IS. Thyroid function tests at delivery and risk for postpartum depressive symptoms. Psychoneuroendocrinology. (2013) 38:1007–13. doi: 10.1016/j.psyneuen.2012.10.004
142. Albacar G, Sans T, Martín-Santos R, García-Esteve L, Guillamat R, Sanjuan J, et al. Thyroid function 48h after delivery as a marker for subsequent postpartum depression. Psychoneuroendocrinology. (2010) 35:738–42. doi: 10.1016/j.psyneuen.2009.10.015
143. Groer MW, Vaughan JH. Positive thyroid peroxidase antibody titer is associated with dysphoric moods during pregnancy and postpartum. J Obstet Gynecol Neonatal Nurs. (2013) 42:E26–32. doi: 10.1111/j.1552-6909.2012.01425.x
144. Dama M, Steiner M, Lieshout R Van. Thyroid peroxidase autoantibodies perinatal depression risk: a systematic review. J Affect Disord. (2016) 198:108–21. doi: 10.1016/j.jad.2016.03.021
145. Wesseloo R, Kamperman AM, Bergink V, Pop VJM. Thyroid peroxidase antibodies during early gestation and the subsequent risk of first-onset postpartum depression: a prospective cohort study. J Affect Disord. (2018) 225:399–403. doi: 10.1016/j.jad.2017.08.058
146. Li D, Li Y, Chen Y, Li H, She Y, Zhang X, et al. Neuroprotection of reduced thyroid hormone with increased estrogen and progestogen in postpartum depression. Biosci Rep. (2019) 39:1–11. doi: 10.1042/BSR20182382
147. Pedersen C, Leserman J, Garcia N, Stansbury M, Meltzer-Brody S, Johnson J. Late pregnancy thyroid-binding globulin predicts perinatal depression. Psychoneuroendocrinology. (2016) 65:84–93. doi: 10.1016/j.psyneuen.2015.12.010
148. Bao A-M, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. (2008) 57:531–53. doi: 10.1016/j.brainresrev.2007.04.005
149. Lightman SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Chapter 8 peripartum plasticity within the hypothalamo-pituitary-adrenal axis. In : Progress in Brain Research. Elsevier . (2001). p. 111–29. doi: 10.1016/S0079-6123(01)33009-1
150. Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. (2006) 27:1457–63. doi: 10.1016/j.peptides.2005.10.002
151. Atsushi S, Anthony SL, Marjorie ML, Andrew NM, Toshihiro S, Dorothy TK. Immunoreactive corticotropin-releasing factor is present in human maternal plasma during the third trimester of pregnancy. J Clin Endocrinol Metab. (1984) 59:812–4. doi: 10.1210/jcem-59-4-812
152. Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. (2003) 997:136–49. doi: 10.1196/annals.1290.016
153. Yim IS, Glynn LM, Schetter CD, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin- releasing hormone in human pregnancy. Arch Gen Psychiatry. (2009) 66:162–9. doi: 10.1001/archgenpsychiatry.2008.533
154. Hahn-Holbrook J, Dunkel Schetter C, Arora C, Hobel CJ. Placental corticotropin-releasing hormone mediates the association between prenatal social support and postpartum depression. Clin Psychol Sci. (2013) 1:253–65. doi: 10.1177/2167702612470646
155. Iliadis SI, Sylvén S, Hellgren C, Olivier JD, Schijven D, Comasco E, et al. Mid-pregnancy corticotropin-releasing hormone levels in association with postpartum depressive symptoms. Depress Anxiety. (2016) 33:1023–30. doi: 10.1002/da.22529
156. Glynn LM, Sandman CA. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med. (2014) 76:355–62. doi: 10.1097/PSY.0000000000000066
157. O'Connor TG, Tang W, Gilchrist MA, Moynihan JA, Pressman EK, Blackmore ER. Diurnal cortisol patterns and psychiatric symptoms in pregnancy: short-term longitudinal study. Biol Psychol. (2014) 96:35–41. doi: 10.1016/j.biopsycho.2013.11.002
158. Labad J, Vilella E, Reynolds RM, Sans T, Cavallé P, Valero J, et al. Increased morning adrenocorticotrophin hormone (ACTH) levels in women with postpartum thoughts of harming the infant. Psychoneuroendocrinology. (2011) 36:924–8. doi: 10.1016/j.psyneuen.2010.11.006
159. Jolley SN, Elmore S, Barnard KE, Carr DB. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol Res Nurs. (2007) 8:210–22. doi: 10.1177/1099800406294598
160. Lara-Cinisomo S, Grewen KM, Girdler SS, Wood J, Meltzer-Brody S. Perinatal depression, adverse life events, and hypothalamic–adrenal–pituitary axis response to cold pressor stress in latinas: an exploratory study. Womens Heal Issues. (2017) 27:673–82. doi: 10.1016/j.whi.2017.06.004
161. Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab. (1996) 81:1912–7. doi: 10.1210/jcem.81.5.8626857
162. Ferguson EH, Di Florio A, Pearson B, Putnam KT, Girdler S, Rubinow DR, et al. HPA axis reactivity to pharmacologic and psychological stressors in euthymic women with histories of postpartum versus major depression. Arch Womens Ment Health. (2017) 20:411–20. doi: 10.1007/s00737-017-0716-y
163. Yim IS, Glynn LM, Schetter CD, Hobel CJ, Chicz-DeMet A, Sandman CA. Prenatal β-endorphin as an early predictor of postpartum depressive symptoms in euthymic women. J Affect Disord. (2010) 125:128–33. doi: 10.1016/j.jad.2009.12.009
164. Parcells DA. Women's mental health nursing: depression, anxiety, and stress during pregnancy. J Psychiatr Ment Health Nurs. (2010) 17:813–20. doi: 10.1111/j.1365-2850.2010.01588.x
165. Iliadis SI, Comasco E, Sylvén S, Hellgren C, Sundström Poromaa I, Skalkidou A. Prenatal and postpartum evening salivary cortisol levels in association with peripartum depressive symptoms. PLoS ONE. (2015) 10:e0135471. doi: 10.1371/journal.pone.0135471
166. Caparros-Gonzalez RA, Romero-Gonzalez B, Strivens-Vilchez H, Gonzalez-Perez R, Martinez-Augustin O, Peralta-Ramirez MI. Hair cortisol levels, psychological stress and psychopathological symptoms as predictors of postpartum depression. PLoS ONE. (2017) 12:e0182817. doi: 10.1371/journal.pone.0182817
167. Harris B, Lovett L, Smith J, Read G, Walker R, Newcombe R. Cardiff puerperal mood and hormone study. III. postnatal depression at 5 to 6 weeks postpartum, and its hormonal correlates across the peripartum period. Br J Psychiatry. (1996) 168:739–44. doi: 10.1192/bjp.168.6.739
168. Corwin EJ, Pajer K, Paul S, Lowe N, Weber M, McCarthy DO. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav Immun. (2015) 49:86–93. doi: 10.1016/j.bbi.2015.04.012
169. Orta OR, Gelaye B, Bain PA, Williams MA. The association between maternal cortisol and depression during pregnancy, a systematic review. Arch Womens Ment Health. (2018) 21:43–53. doi: 10.1007/s00737-017-0777-y
170. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. (2006) 27:24–31. doi: 10.1016/j.it.2005.11.006
171. Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. (2005) 29:201–17. doi: 10.1016/j.pnpbp.2004.11.003
172. Osborne LM, Monk C. Perinatal depression-The fourth inflammatory morbidity of pregnancy. Theory and literature review. Psychoneuroendocrinology. (2013) 38:1929–52. doi: 10.1016/j.psyneuen.2013.03.019
173. Kendall-Tackett K. A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. Int Breastfeed J. (2007) 2:6. doi: 10.1186/1746-4358-2-6
174. Maes M, Lin A, Ombelet W, Stevens K, Kenis G, De Jongh R, et al. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. (2000) 25:121–37. doi: 10.1016/S0306-4530(99)00043-8
175. Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol Res Nurs. (2008) 10:128–33. doi: 10.1177/1099800408323220
176. Boufidou F, Lambrinoudaki I, Argeitis J, Zervas IM, Pliatsika P, Leonardou AA, et al. CSF and plasma cytokines at delivery and postpartum mood disturbances. J Affect Disord. (2009) 115:287–92. doi: 10.1016/j.jad.2008.07.008
177. Krause D, Jobst A, Kirchberg F, Kieper S, Härtl K, Kästner R, et al. Prenatal immunologic predictors of postpartum depressive symptoms: a prospective study for potential diagnostic markers. Eur Arch Psychiatry Clin Neurosci. (2014) 264:615–24. doi: 10.1007/s00406-014-0494-8
178. Liu H, Zhang Y, Gao Y, Zhang Z. Elevated levels of Hs-CRP and IL-6 after delivery are associated with depression during the 6 months post partum. Psychiatry Res. (2016) 243:43–8. doi: 10.1016/j.psychres.2016.02.022
179. Simpson W, Steiner M, Coote M, Frey BN. Relationship between inflammatory biomarkers and depressive symptoms during late pregnancy and the early postpartum period: a longitudinal study. Rev Bras Psiquiatr. (2016) 38:190–6. doi: 10.1590/1516-4446-2015-1899
180. Skalkidou A, Sylvén SM, Papadopoulos FC, Olovsson M, Larsson A, Sundström-Poromaa I. Risk of postpartum depression in association with serum leptin and interleukin-6 levels at delivery: a nested case–control study within the UPPSAT cohort. Psychoneuroendocrinology. (2009) 34:1329–37. doi: 10.1016/j.psyneuen.2009.04.003
181. Chen C, Gao J, Zhang J, Jia L, Yu T, Zheng Y. Serum leptin level measured 48 h after delivery is associated with development of postpartum depressive symptoms: a 3-month follow-up study. Arch Womens Ment Health. (2016) 19:1001–8. doi: 10.1007/s00737-016-0647-z
182. Fransson E, Dubicke A, Byström B, Ekman-Ordeberg G, Hjelmstedt A, Lekander M. Negative emotions and cytokines in maternal and cord serum at preterm birth. Am J Reprod Immunol. (2012) 67:506–14. doi: 10.1111/j.1600-0897.2011.01081.x
183. Maes M, Ombelet W, Libbrecht I, Stevens K, Kenis G, De Jongh R, et al. Effects of pregnancy and delivery on serum concentrations of Clara Cell Protein (CC16), an endogenous anticytokine: lower serum CC16 is related to postpartum depression. Psychiatry Res. (1999) 87:117–27. doi: 10.1016/S0165-1781(99)00073-6
184. Bränn E, Fransson E, White RA, Papadopoulos FC, Edvinsson Å, Kamali-Moghaddam M, et al. Inflammatory markers in women with postpartum depressive symptoms. J Neurosci Res. (2020) 98:1309–21. doi: 10.1002/jnr.24312
185. Lambert M, Gressier F. Inflammatory biomarkers and postpartum depression: a systematic review of literature. Can J Psychiatry. (2019) 64:070674371982897. doi: 10.1177/0706743719828970
186. Bränn E, Papadopoulos F, Fransson E, White R, Edvinsson Å, Hellgren C, et al. Inflammatory markers in late pregnancy in association with postpartum depression—a nested case-control study. Psychoneuroendocrinology. (2017) 79:146–59. doi: 10.1016/j.psyneuen.2017.02.029
187. Wójcik J, Dudek D, Schlegel-Zawadzka M, Grabowska M, Marcinek A, Florek E, et al. Antepartum/postpartum depressive symptoms and serum zinc and magnesium levels. Pharmacol Rep. (2006) 58:571–6. Available online at: http://rabbit.if-pan.krakow.pl/pjp/pdf/2006/4_571.pdf
188. Roomruangwong C, Kanchanatawan B, Sirivichayakul S, Mahieu B, Nowak G, Maes M. Lower serum zinc and higher crp strongly predict prenatal depression and physio-somatic symptoms, which all together predict postnatal depressive symptoms. Mol Neurobiol. (2017) 54:1500–12. doi: 10.1007/s12035-016-9741-5
189. Christesen HT, Falkenberg T, Lamont RF, JØrgensen JS. The impact of vitamin D on pregnancy: a systematic review. Acta Obstet Gynecol Scand. (2012) 91:1357–67. doi: 10.1111/aogs.12000
190. Murphy PK, Mueller M, Hulsey TC, Ebeling MD, Wagner CL. An exploratory study of postpartum depression and vitamin D. J Am Psychiatr Nurses Assoc. (2010) 16:170–7. doi: 10.1177/1078390310370476
191. Brandenbarg J, Vrijkotte TGM, Goedhart G, van Eijsden M. Maternal early-pregnancy vitamin d status is associated with maternal depressive symptoms in the amsterdam born children and their development cohort. Psychosom Med. (2012) 74:751–7. doi: 10.1097/PSY.0b013e3182639fdb
192. Gur EB, Gokduman A, Turan GA, Tatar S, Hepyilmaz I, Zengin EB, et al. Mid-pregnancy vitamin D levels and postpartum depression. Eur J Obstet Gynecol Reprod Biol. (2014) 179:110–6. doi: 10.1016/j.ejogrb.2014.05.017
193. Robinson M, Whitehouse AJO, Newnham JP, Gorman S, Jacoby P, Holt BJ, et al. Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Arch Womens Ment Health. (2014) 17:213–9. doi: 10.1007/s00737-014-0422-y
194. Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Dietary vitamin D intake and prevalence of depressive symptoms during pregnancy in Japan. Nutrition. (2015) 31:160–5. doi: 10.1016/j.nut.2014.06.013
195. Accortt EE, Schetter CD, Peters RM, Cassidy-Bushrow AE. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: preliminary evidence for moderation by inflammatory cytokines. Arch Womens Ment Health. (2016) 19:373–83. doi: 10.1007/s00737-015-0585-1
196. Fu C-W, Liu J-T, Tu W-J, Yang J-Q, Cao Y. Association between serum 25-hydroxyvitamin D levels measured 24 hours after delivery and postpartum depression. BJOG. (2015) 122:1688–94. doi: 10.1111/1471-0528.13111
197. Teofilo MMA, Farias DR, Pinto T, de JP, Vilela AAF, Vaz J, dos S, Nardi AE, et al. HDL-cholesterol concentrations are inversely associated with Edinburgh Postnatal Depression Scale scores during pregnancy: results from a Brazilian cohort study. J Psychiatr Res. (2014) 58:181–8. doi: 10.1016/j.jpsychires.2014.07.030
198. Van Dam RM, Schuit AJ, Schouten EG, Vader HL, Pop VJM. Serum cholesterol decline and depression in the postpartum period. J Psychosom Res. (1999) 46:385–90. doi: 10.1016/S0022-3999(98)00100-7
199. Parker G, Hegarty B, Granville-Smith I, Ho J, Paterson A, Gokiert A, et al. Is essential fatty acid status in late pregnancy predictive of post-natal depression Acta Psychiatr Scand . (2015) 131:148–56. doi: 10.1111/acps.12321
200. Markhus MW, Skotheim S, Graff IE, Frøyland L, Braarud HC, Stormark KM, et al. Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS ONE. (2013) 8:e67617. doi: 10.1371/journal.pone.0067617
201. Sallis H, Steer C, Paternoster L, Davey Smith G, Evans J. Perinatal depression and omega-3 fatty acids: a Mendelian randomisation study. J Affect Disord. (2014) 166:124–31. doi: 10.1016/j.jad.2014.04.077
202. Chong MFF, Ong Y-L, Calder PC, Colega M, Wong JXY, Tan CS, et al. Long-chain polyunsaturated fatty acid status during pregnancy and maternal mental health in pregnancy and the postpartum period. J Clin Psychiatry. (2015) 76:e848–56. doi: 10.4088/JCP.14m09191
203. Lukose A, Ramthal A, Thomas T, Bosch R, Kurpad A V., Duggan C, et al. Nutritional factors associated with antenatal depressive symptoms in the early stage of pregnancy among Urban South Indian women. Matern Child Health J. (2014) 18:161–70. doi: 10.1007/s10995-013-1249-2
204. Lewis SJ, Araya R, Leary S, Smith GD, Ness A. Folic acid supplementation during pregnancy may protect against depression 21 months after pregnancy, an effect modified by MTHFR C677T genotype. Eur J Clin Nutr. (2012) 66:97–103. doi: 10.1038/ejcn.2011.136
205. Huang J, Zhang L, He M, Qiang X, Xiao X, Huang S, et al. Comprehensive evaluation of postpartum depression and correlations between postpartum depression and serum levels of homocysteine in chinese women. J Cent South Univ. (2015) 40:311–6. doi: 10.11817/j.issn.1672-7347.2015.03.013
206. Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpé S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. (2002) 71:1837–48. doi: 10.1016/S0024-3205(02)01853-2
207. Hantsoo L. Potential Hormonal and Neurochemical Biomarkers for Postpartum Depression. In: Biomarkers of Postpartum Psychiatric Disorders . London: Elsevier (2019). doi: 10.1016/B978-0-12-815508-0.00004-7
208. Foster JA, McVey Neufeld K-A. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
209. Slykerman RF, Hood F, Wickens K, Thompson JMD, Barthow C, Murphy R, et al. Effect of lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. (2017) 24:159–65. doi: 10.1016/j.ebiom.2017.09.013
210. Gadad BS, Jha MK, Czysz A, Furman JL, Mayes TL, Emslie MP, et al. Peripheral biomarkers of major depression and antidepressant treatment response: current knowledge and future outlooks. J Affect Disord. (2018) 233:3–14. doi: 10.1016/j.jad.2017.07.001
211. Sethi S, Brietzke E. Omics-based biomarkers: application of metabolomics in neuropsychiatric disorders. Int J Neuropsychopharmacol. (2015) 19:1–13. doi: 10.1093/ijnp/pyv096
212. Papadopoulou Z, Vlaikou AM, Theodoridou D, Komini C, Chalkiadaki G, Vafeiadi M, et al. Unraveling the serum metabolomic profile of post-partum depression. Front Neurosci. (2019) 13:1–9. doi: 10.3389/fnins.2019.00833
213. Lin L, Chen XM, Liu RH. Novel urinary metabolite signature for diagnosing postpartum depression. Neuropsychiatr Dis Treat. (2017) 13:1263–70. doi: 10.2147/NDT.S135190
214. Zhang L, Zou W, Huang Y, Wen X, Huang J, Wang Y, et al. A preliminary study of uric metabolomic alteration for postpartum depression based on liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Dis Markers. (2019) 2019:1–9. doi: 10.1155/2019/4264803
Keywords: postpartum depression, postnatal depression, biological markers, biomarkers, multi-omics
Citation: Yu Y, Liang H-F, Chen J, Li Z-B, Han Y-S, Chen J-X and Li J-C (2021) Postpartum Depression: Current Status and Possible Identification Using Biomarkers. Front. Psychiatry 12:620371. doi: 10.3389/fpsyt.2021.620371
Received: 22 October 2020; Accepted: 19 May 2021; Published: 11 June 2021.
Reviewed by:
Copyright © 2021 Yu, Liang, Chen, Li, Han, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Cheng Li, lijichen@zju.edu.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Breastfeeding Special Circumstances
- Contraindications to Breastfeeding
- Breastfeeding: Diet, Vitamins, and Minerals
- Environmental and Chemical Exposures
- Travel and Breastfeeding
- Illnesses or Conditions and Breastfeeding
- Relactation
Postpartum Depression
At a glance.
Mothers with postpartum depression (following childbirth) can usually continue to breastfeed. Health care providers should work with mothers experiencing postpartum depression to ensure they receive appropriate treatment, support, and safe medications while breastfeeding.

There is not enough evidence to know if breastfeeding is associated with a higher or lower risk of postpartum depression (following childbirth). According to a 2018 systematic review by the Agency for Healthcare Research and Quality (AHRQ) , understanding the relationship is challenging "because women with depression may have difficulty initiating and sustaining breastfeeding, and women who experience breastfeeding difficulties may develop depression" (p. 107).
Antidepressants while breastfeeding
It may be safe to take antidepressant medications while breastfeeding. Although many medications pass into breast milk, most have little or no effect on milk supply or infant well-being.
When discussing depression medications , the health care provider needs to ask a mother if she is breastfeeding. Together, they can decide which medications are right for her and safe to use while breastfeeding.
LactMed® is a database of information on medications to which breastfeeding mothers may be exposed.
Breastfeeding with postpartum depression
Mothers with postpartum depression can usually continue to breastfeed. While some mothers experience positive feelings from breastfeeding, others may not. Health care providers can:
- Address mothers' depression promptly and help them reach their breastfeeding goals.
- Talk to mothers about treatment options, including medications and non-pharmacological options, such as individual or group therapy.
- Help mothers access professional breastfeeding support as needed.
Learn more:
Postpartum Depression —Office on Women's Health
Incorporating Recognition and Management of Perinatal Depression Into Pediatric Practice —American Academy of Pediatrics
ABM Clinical Protocol #18: Use of Antidepressants in Breastfeeding Mothers —Academy of Breastfeeding Medicine
Breastfeeding special circumstances
Health care providers and public health practitioners will find information about breastfeeding in special circumstances.
For Everyone
Health care providers.
The University of Tennessee, Knoxville
The graduate school, spring 2024 gsra award winners – human health and culture.
The Graduate Student Research Awards are used to advance the scholarship of graduate students and faculty working in partnership. Grants up to $5,000 are awarded to the selected student/faculty pairs and are intended to help support student research, scholarship, and creative activity; give students experience writing grants; and foster the mentoring relationship between faculty and graduate students.
Twenty-two outstanding research programs were awarded for spring 2024 across many colleges and departments. We hope that you will read about the work these graduate students and faculty partners are engaged in here at UT.
Beyond resilience: A strength-based intervention for LGBTQ+ communities of color
Kriti Jain —PhD student, psychology
Intersectionality suggests that individuals who hold multiple marginalized identities face unique challenges to their mental health. Queer and trans people of color (QTPOC) are a group that experiences additive minority stress, and it is necessary for research to examine the unique experiences of this understudied and underserved population to design interventions to address their specific needs. Kriti Jain, a doctoral student in psychology, proposes a trial to examine the feasibility and preliminary effectiveness of a strengths-based intervention to improve mental health, well-being, and positive identity in this population. The intervention is based on the psychology of radical healing and aims to empower QTPOC, promote radical hope, and support collective liberation.
The Event: A movie musical
Michael Ray —MMusic student, music
Michael Ray, a master’s student in music, used the GSRA award to write, record, video, and edit an original 45-minute musical. Entitled “The Event,” the musical is an exploration of humanity’s relationship with Earth. Ray aspires to pull the audience into a fictional world that will allow them to feel deeply for the characters in situations not so very different from our own. Ray collaborated with a lyricist to write and create demos for eight songs, recruited recording artists, collaborated with a recording engineer to solidify the soundtrack, cast actors, hired a choreographer and costumer/props designer, and created the final project which was presented recently in the Knoxville area.
A multi-proxy study of human dispersals and paleodiets in the south-central Andes
Rebecca Ann Kraus —MA student, anthropology
The peopling of the Andes Mountains is significant in South America’s population history as it fostered the formation of highly complex pre-Columbian societies. Because European colonization strongly affected the genomic diversity of Indigenous South American groups, a precise characterization of past population interactions and migration patterns can only be achieved by analyzing ancient DNA from pre-colonial populations. Rebecca Kraus, a master’s student in anthropology, proposes to study eight pre-colonial individuals from an Argentine archaeological collection by sequencing complete mitochondrial genomes, analyzing stable isotopes, and radiocarbon dating. These combined data will advance our understanding of genomic diversity, geographic mobility, and dietary trends among pre-colonial South-Central Andeans. The research outcomes will also contribute to locally-desired knowledge of the region’s past among the invested communities of northwest Argentina.
Assessing visual short-term memory in infants and comparing 2D vs. 3D performance
Victoria Jones —PhD student, psychology
Navigating the visual world requires perception, maintenance, and processing of the sensory input in visual short-term memory (VSTM). Because an individual’s VSTM capacity may be predictive of later cognitive performances, it is critical to understand the development of this mechanism and the persistence of individual differences throughout infancy. VSTM capacity is difficult to assess in infants, relying primarily on 2D stimuli, like multi-colored circles, to assess memory. Victoria Jones, a doctoral student in psychology, proposes to study how well these 2D tasks translate to real-world 3D visual processing. This study will test infants using two visual short-term memory tasks; one using physical blocks (3D), and one using scanned images of the blocks (2D). The data collected from these tasks using similar measures could critically inform the generalizability of how well the skills being tested using 2D methods translate to real-world 3D tasks.
Zooarchaeology by Mass Spectrometry (ZooMS) analysis of burned mammal and fish remains
Taylor Bowden-Gray —PhD student, anthropology
Archaeologists and forensic anthropologists depend upon accurate species identification of skeletal remains for their investigations. However, this can be challenging due to high fragmentation rates and poor preservation of bones in some contexts, which have rendered specimens unidentifiable beyond very broad categories. Zooarchaeology by Mass Spectrometry (ZooMS), based on analysis of the amino acid composition of collagen, offers a method for identifying ambiguous or unidentifiable bone fragments. Taylor Bowden-Gray, a doctoral student in anthropology, aims to investigate the feasibility of analyzing burned bones, as high temperatures change the nature of collagen, possibly resulting in a loss of usable samples. Bowden-Gray plans to identify the degree of burning that can reliably yield collagen to identify different mammal and fish bone elements. By developing a system of visual criteria, in conjunction with the bone structures, researchers can make informed decisions on whether ZooMS will be a good option for species identification of burned remains.
Feeling manipulated: analysis of the human cytomegalovirus’ viral protein vCXCL-1 on monocyte function
Morgan Hetzel —PhD student, microbiology
Human cytomegalovirus (HCMV) is a virus that remains dormant within humans until the host becomes immunocompromised due to cancer treatments, late-stage AIDS, organ transplantation or the lack of an immune system in a developing fetus. Approximately 85% of the adult population is infected with HCMV, and when it emerges, it can contribute to complications that can lead to hearing loss, long-term disabilities, and death. Morgan Hetzel, a doctoral student in microbiology, plans to study HCMV and how it has evolved genes that manipulate the immune system to work in the virus’ favor. One of the strategies employed by HCMV is to mimic proteins produced by our own immune system and Hetzel’s study will make it possible to distinguish between responses induced by host proteins versus those induced by HCMV. Data generated from this work will not only allow us to gain a deeper understanding of the mechanisms behind HCMV dissemination, but it could also lead to development of better treatment strategies for mitigating the symptoms of disease resulting from HCMV.
Determining selection or training of constricted migration of melanoma cells
Christopher Playter —PhD student, biochemistry & cellular and molecular biology
The leading cause of death associated with cancer diagnosis is metastasis, where cancer cells spread from where they first formed to another part of the body. Why some tumor cells metastasize while others do not is still unknown, as is why these metastasizing cells are different and more aggressive than the original tumors. Christopher Playter, a doctoral student in biochemistry & cellular and molecular biology, intends to investigate two possible reasons for these differences. One possibility is that these cells are more aggressive due to selection, representing the initially aggressive subset of the tumor. The other possibility is that the cells have undergone training during migration, where force and stress exerted during the process of migration change the properties of the cell over time. Playter will use single-cell RNA-sequencing to compare cells from both migratory and non-migratory cells to determine the relative contributions of selection and training to the final aggressive migratory state. This knowledge will help in understanding how metastatic cells become aggressive in cancer patients and suggest gene profiles that could inform future clinical prognosis of a cancer patient’s likelihood of experiencing metastasis.
Reevaluating plant δ15N as a forensic tool to identify clandestine graves
Sarah Schwing —PhD student, anthropology
When a human body decomposes, the 15N-enriched nitrogen from the body becomes available to plants. Sarah Schwing, a doctoral student in anthropology, proposes a study to test a non-destructive technique for detecting clandestine graves where the ground surface has long since healed the visible scar of disturbance. The goal is to examine whether plant δ15N values can be used to detect hidden human burials. Schwing’s project design utilizes four pre-existing graves and two control graves to compare the original δ15N levels to the plant growth. Results from this study can contribute to efforts to recover buried human remains and associated forensic evidence for use in identifying victims and as potential evidence in a court of law.
Exploring the experiences of veterinary students with disabilities
Claire Burdick —MSSW student, social work
In higher education, apparent and less apparent disabilities are underrepresented due to the history of exclusion for disabled people due to assumptions about their capabilities and lack of legislation protecting disability rights. Many colleges of veterinary medicine (CVMs) in the U.S. are working towards increasing the diversity of their student bodies and the veterinary profession with cultural competency training, outreach to communities that are underrepresented, and examining admission criteria. Claire Burdick, a master’s student in social work, seeks to expand upon emerging research on diversity and inclusion in veterinary education by exploring the experiences of veterinary students with disabilities in academic and experiential learning. Burdick’s project will amplify underrepresented voices in research by listening directly to them and learning what supports they want and need for a successful higher education experience.
Bacterial analysis of expressed breast milk pre- and post-bottle feeding
Emily Wojtowicz —PhD student, nutritional sciences
Breastfeeding is widely recognized as the ideal feeding method for infants due to its numerous health benefits for mother and child and its positive impacts on society, the environment, and the economy. Unfortunately, several barriers to breastfeeding exist, including perceived insufficient milk supply and returning to work. More than 84% of breastfeeding parents express breastmilk within the first four months postpartum, often to provide breastmilk to alternative caregivers during times of separation. Leading health agencies recommend discarding breastmilk remaining in the bottle within two hours of initiating feeding due to safety concerns despite major gaps in the literature to support these recommendations. This burdens mothers trying to compensate for discarded breast milk and exacerbates concerns about perceived milk insufficiency. Emily Wojtowicz, a doctoral candidate in nutritional sciences, will utilize her skills as a registered dietitian (RD) and international board certified lactation consultant (IBCLC) with support from the biomedical nutritional science program to investigate the bacterial composition and potentially pathogenic bacteria within expressed breastmilk pre- and post-bottle feeding. Investigating the safety parameters surrounding re-feeding expressed breastmilk is crucial in establishing evidence-based recommendations, safeguarding infant health, and potentially revising outdated guidelines to ensure the optimal utilization of expressed breastmilk without compromising maternal and infant well-being. .
Impact of social determinants of health and profiles of traumatic, adverse, and positive childhood experiences on cognitive and psychological symptoms in multiple sclerosis
Caterina Obenauf —PhD student, psychology
Multiple Sclerosis (MS) is a chronic autoimmune disease impacting the central nervous system, manifesting in symptoms ranging from depression to fatigue. Understanding risk factors for non-visible symptoms of MS is vital for early detection and monitoring since worsening of cognitive symptoms precede worsening of physical symptoms in MS. Social determinants of health, such as traumatic and adverse childhood experiences, are recognized as contributing significantly to MS-related disability and access to healthcare, but limited research exists on their impact on mental health. Caterina Obenauf, a doctoral student in psychology, intends to study the effects of traumatic and childhood experiences (both positive and negative) on people with MS. The approach taken in this study will ensure that experiences of people with MS are not overshadowed by a deficit-oriented perspective, taking a nuanced approach that explores positive childhood experiences and post-traumatic growth. This not only challenges prevailing assumptions about the capabilities of people with disabilities, but also promotes their dignity by recognizing the significance of resilience.
The flagship campus of the University of Tennessee System and partner in the Tennessee Transfer Pathway .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Postpartum depression
Postpartum depression (PPD) affects up to 15% of mothers. Recent research has identified several psychosocial and biologic risk factors for PPD. The negative short-term and long-term effects on child development are well-established. PPD is under recognized and under treated. The obstetrician and pediatrician can serve important roles in screening for and treating PPD. Treatment options include psychotherapy and antidepressant medication. Obstacles to compliance with treatment recommendations include access to psychotherapists and concerns of breastfeeding mothers about exposure of the infant to antidepressant medication. Further research is needed to examine systematically the short-term and long-term effect of medication exposure through breastmilk on infant and child development.
We reviewed selected studies about the diagnosis and treatment of postpartum depression (PPD). Despite methodologic limitations, the results of several studies can provide treatment options for women with PPD. Women face difficult dilemmas about the negative effects of untreated psychiatric disorder in the postpartum period vs the risks of exposure to the breastfeeding infant from psychotropic medication. We have included a limited discussion about postpartum blues and postpartum psychosis.
Postpartum blues
Postpartum blues have been reported to occur in 15–85% of women within the first 10 days after giving birth, with a peak incidence at the fifth day. 1 Common symptoms include mood swings, mild elation, irritability, tearfulness, fatigue, and confusion. 1 , 2 Antenatal depression, previous depression not related to pregnancy, and previous premenstrual dysphoria have been identified as risk factors. 1 No clear biologic measure has been identified to be causative or predictive of postpartum blues. Although postpartum blues is a common and transient postpartum occurrence and generally does not require intervention, its recognition is important because postpartum blues is a risk factor for subsequent PPD. 3
PPD: diagnosis and epidemiologic factors
PPD is defined strictly in the psychiatric nomenclature as a major depressive disorder (MDD) with a specifier of postpartum onset within 1 month after childbirth. 4 However, depression in women during the postpartum period may start during pregnancy or may have onset beyond the first postpartum month. 5 To meet criteria for MDD, depressed mood or loss of interest or pleasure in activities must be present for at least 2 weeks. In addition, symptoms of sleep disturbance, appetite disturbance, loss of energy, feelings of worthlessness or guilt, diminished concentration, and thoughts of suicide may be present. 4 The diagnosis of PPD is challenging because of changes in sleep patterns, changes in appetite, and excessive fatigue being routine for women after delivery. 6
The optimal time to screen for PPD is between 2 weeks and 6 months after delivery. 6 Several self-report measures that are available to screen for PPD include the Edinburgh Postnatal Depression Scale, 7 which is a validated and widely used 10-item questionnaire. An Edinburgh Postnatal Depression Scale score of ≥12 is indicative of probable PPD. 7 The Postpartum Depression Screening Scale 8 is another self-report screening measure that is popular with clinicians because of its construct validity and emphasis on clinical domains; however, because of high false-positive rates for PPD, it has been reported to be less accurate than the Edinburgh Postnatal Depression Scale. 9
A systematic review of studies that diagnosed depression by clinical structured interview reported that the point prevalence of MDD and minor depression ranged from 6.5–12.9% through the first 6 postpartum months, peaking at 2 and 6 months after delivery. 5 A large cohort study that was conducted in Denmark reported that the first 90 days after delivery represented a time of increased risk of new-onset psychiatric disorder (mostly PPD) in new primiparous mothers, but not in new fathers. 10 Other recent studies document an increased risk of MDD during the postpartum period. 11 , 12 The prevalence of PPD varies in non-Western countries from 0.5–60%; cultural factors can influence the development and reporting of PPD. 13
Psychosocial risk factors for PPD include MDD during pregnancy, anxiety during pregnancy, previous nonpuerperal MDD, previous premenstrual dysphoria, stressful life events during pregnancy or the early puerperium, poor social support, marital conflict, low income, immigrant status, and young maternal age. 14 , 15 A recent study identified previous depression, current depression and anxiety, and low partner support as key risk factors. 16
PPD may be related to a differential sensitivity to hormonal fluctuations. Euthymic women with previous PPD experienced dysphoria after both the addition and withdrawal of supraphysiologic doses of estradiol and progesterone, compared with healthy control subjects. 17 In addition to sensitivity to estrogen and progesterone fluctuations, biologic theories have included fluctuations of other gonadal hormone and neuroactive steroid levels after delivery, altered cytokines and HPA axis hormones, and altered fatty acid, oxytocin, and arginine vasopressin levels. 18 , 19 Involvement of the serotonin system has been suggested by reports of altered platelet serotonin transporter binding 20 and decreased postsynaptic serotonin-1A receptor binding in the anterior cingulate and mesiotemporal cortices. 21 A recent study that used a functional magnetic resonance imaging (fMRI) neuropsychologic activation paradigm suggested altered neural processing in women with PPD. 22
Normal fluctuations in hormonal levels during pregnancy and after delivery result in changes in sleep patterns. Declining levels of progesterone in the early postpartum period promote insomnia. 23 In the first postpartum month, decreased sleep efficiency and increased slow wave sleep have been reported. 23 , 24 The changes in hormones and sleep during the early postpartum period may contribute major vulnerability to the onset of PPD. A recent study identified difficulty falling asleep in the first 3 months after delivery as a possible risk factor for PPD. 25 In addition, infant sleep disturbance may be both a risk factor for and an outcome of PPD in the early postpartum period. 26 , 27 Studies have suggested that persistent infant and child sleep problems are related to maternal depression. 28 , 29 Despite the consistent findings of a relationship between maternal depression and infant and child sleep problems, a causal pathway has not been determined, and few studies have measured infant sleep objectively.
Role of obstetricians and pediatricians
Numerous studies have reported on the low rates of screening, diagnosis, and treatment of perinatal depression in medical settings. Clinician discomfort with psychiatric disorders, time constraints, low belief in maternal mental health having an important effect on child development, and lack of knowledge about resources are some of the barriers to clinician screening for psychiatric disorders in medical settings. 30 – 32 However, the postpartum obstetric visit and pediatric well-baby visits are opportunities for the clinician to assess the mother’s clinical status. 31 , 33 Although women with PPD are often hesitant to divulge their mood and anxiety symptoms to their clinician because of guilt about having symptoms when motherhood is expected to be joyful, there may be indicators that further evaluation is needed. For example, PPD may lead to negative maternal perceptions of infant temperament and behavioral patterns; such complaints should be addressed in the context of the infant’s behavior and how well the mother is coping with these difficulties. 34 PPD has been associated with frequent nonroutine visits to the pediatrician; such visits and telephone contacts may be warranted but could also be an indicator for further assessment of maternal mood and family functioning. 35 Follow-up with the woman who is referred for treatment within the practice or to a mental health clinician reinforces the importance of treatment recommendations.
Risks to children of not treating PPD
There is a well-established relationship between untreated maternal depression and impaired child development. 36 , 37 Infant and child outcomes that are associated with PPD include a higher incidence of excessive infant crying or colic, sleep problems, and temperamental difficulties. 34 , 38 Infant crying and sleeping problems may increase the risk for new onset PPD but may also be reported more frequently by women with PPD. In a study of > 600 infants, objective evidence of infant regulation difficulties were found as early as 1 month after delivery, with infants of mothers with PPD having poorer self-regulation, more stress signs, and heightened arousal compared with infants of mothers without PPD. 39 PPD is associated with negative mother-infant interactions that include maternal withdrawal, disengagement, intrusion, and hostility. 40 , 41 Women with PPD may be less likely to initiate or maintain breastfeeding; depressive symptoms commonly precede the early cessation of breastfeeding. 42 , 43
PPD is linked to poor cognitive functioning, behavioral inhibition, and emotional maladjustment in infants and children. 44 – 46 Persistent untreated maternal depression is associated with violent behavior and externalizing disorders (eg, conduct disorders) 47 – 49 and with psychiatric and medical disorders in adolescence. 50 The complex relationship between maternal depression and child behavioral-emotional development is not yet understood but is likely to be a multidimensional progression that may onset during pregnancy. Women with PPD often have been depressed during pregnancy, 5 which is a potential source of exposure or influence on the fetus. The few published studies on the effects of antenatal depression on fetal outcomes have not always used a diagnosis of MDD but have shown that higher levels of self-reported depressive symptoms during pregnancy were related to heightened fetal behavioral and physiologic reactivity. 51 Alterations in fetal neurobehavioral development are likely to influence infant outcomes. The serious negative effects of PPD on the mother, the infant, and the other family members have made the recognition, prevention, and treatment of PPD a current area of noted public health significance. Recent evidence suggests that successful treatment of PPD may not be sufficient to improve attachment, temperament, and cognitive development in infants and toddlers, 52 , 53 which indicates that efforts toward the prevention and treatment of depression during pregnancy and after delivery are critical. Additional focus on mother-infant attachment and the needs of the family are also indicated.
Suicide during the postpartum period
Completed suicide rates are lower during the postpartum period compared with nonpuerperal time periods, although rates in postpartum adolescents are higher than in older postpartum women. 54 A study of perinatal maternal deaths in the United Kingdom from 1997–1999 reported that suicide was the leading cause of maternal death, was increased in women with psychiatric and substance abuse disorders, and was more likely to be a violent death compared with the suicides of men and nonpuerperal women. 55 Suicide may also be a leading cause of maternal deaths in Australia. 56
A study of a United States population sample reported that there was a 3 times greater risk of a suicide attempt and that inpatient psychiatric admissions were increased after fetal death or infant death in the first postpartum year. 57 In this study, labor and delivery complications, cesarean section, pre-term delivery, low birthweight, and congenital malformations were not associated with increased risk of suicide attempts. A review of studies that confirmed that suicide rates are lower during pregnancy and the postpartum period emphasized that perinatal women complete suicide by more violent and lethal means than do women who are not perinatal. 58 Assessment of suicidality in the perinatal woman should include specific inquiry about depressed mood, substance abuse, previous suicide attempts, current or previous psychiatric illness, previous trauma, current intimate partner violence, and access to firearms. 58 , 59
Postpartum psychosis
Postpartum psychosis occurs in 1 of 500 mothers, with rapid onset in the first 2–4 weeks after delivery. 60 Postpartum psychosis includes confused thinking, mood swings, delusions, paranoia, disorganized behavior, poor judgment, and impaired functioning. 61 Postpartum psychosis is considered a psychiatric emergency and usually results in inpatient psychiatric hospitalization. Risk factors include a previous episode of postpartum psychosis, previous hospitalization for a manic or psychotic episode, recent discontinuation of mood stabilizers, primiparity, obstetric complications, sleep deprivation, and a family history of bipolar disorder or postpartum psychosis. 61 – 63 Longitudinal studies suggest that most cases of postpartum psychosis are related to bipolar disorder, not schizophrenia. 61
Neonaticide and infanticide
Infanticide is 1 of the most serious risks of postpartum psychosis. The rate of homicide of infants up to 1 year of age is 8 per 100,000 in the United States, 64 but it is unknown how many women with postpartum psychosis commit infanticide. Symptom exacerbation, command hallucinations, and the stressor of new infant care can increase the risk of infanticide after delivery in a mother with psychosis. 65 Infanticide may also occur in the context of severe PPD, caused by neglect and abuse, because of the child being unwanted or as revenge against the infant’s father. 65 , 66 Between 16% and 29% of mothers who kill their children also kill themselves. 64 Neonaticide is defined as killing a newborn infant within 24 hours of birth and is associated with denial of pregnancy, lack of prenatal care, dissociation, depersonalization, and intermittent amnesia of delivery. 64 , 67 More study is needed of risk factors for neonaticide and infanticide. 64 Intrusive thoughts of potential accidental harm occurring to a newborn infant are ubiquitous, and intrusive thoughts of intentionally harming an infant are also common. 68 It is important to reassure women that intrusive thoughts of harm to an infant or thoughts of infanticide rarely are acted upon.
Treatment of PPD
Psychotherapy.
Interpersonal psychotherapy (IPT), a short-term efficacious treatment for MDD that addresses interpersonal issues (such as role change, the marital relationship, social support, and life stressors) is highly pertinent to the needs of women during the postpartum period. 69 A randomized controlled trial (RCT) reported that 12 sessions of individual IPT was superior in efficacy to a waitlist control in 120 women with PPD in reducing depression and improving social adjustment. 70 A smaller RCT in women with PPD also reported that individual IPT was superior to a wait-list condition. 71 Additionally, 2 small open studies of group IPT demonstrated significant reduction of depression in women with PPD. 72 , 73
Systematic reviews of treatments for PPD have suggested that individual IPT, cognitive-behavior therapy (CBT), and psychodynamic therapy may be effective psychologic treatments for PPD. 74 Overall, psychologic treatments for PPD demonstrate moderate effect sizes 75 ; antidepressant medications demonstrate larger effect sizes. 76 Methodologic flaws of studies of psychosocial treatments include small sample sizes, short-term treatments, lack of control groups, poorly defined treatment interventions and outcome measures, lack of partner participation, and lack of assessment of infant outcome. 74 Although 1 study included partners as 1 component of psychologic treatments, 77 there has not been systematic study of couples therapy in women with PPD. Initial positive reports that deserve further study include telephone support, lay peer support, individual counseling in the home, nurse-led or health visitor–led support groups, and group therapy led by mental health clinicians. 74 , 78 Women with mild PPD may respond to treatment by nonmental health professionals or to individual or group counseling with a mental health professional, although women with more severe PPD may need IPT or CBT to be administered by trained professionals and/or antidepressant medication. 78 Women who are breastfeeding may prefer psychotherapy over medication for the treatment of PPD. 79 – 81 Barriers to participation in psychotherapy include perceived negative stigma, lack of availability of a trained therapist in IPT or CBT, time commitment, child-care needs, and cost. 82
Mother-baby units
The United States has lagged behind Europe and Australia in the recognition and treatment of perinatal psychiatric disorders. The practice of joint admission of mothers and infants was prompted by concerns about disrupting the mother-infant relationship during intensive psychiatric treatment. The first joint mother-baby admission occurred in the United Kingdom 60 years ago, and joint admission now takes place routinely in the United Kingdom, Australia, France, Belgium, Germany, and the Netherlands. Parent-infant units have been established in Australia. The only known current mother-baby unit in the United States is conducted as a psychiatric partial hospital. 83 Advantages of mother-baby units include support, absence of breastfeeding disruption or cessation, multidisciplinary treatment of PPD, direct observation of mother-infant interaction, and the promotion and modeling of a healthy maternal-child relationship.
Antidepressant treatment
Four RCTs with antidepressant medication have been conducted in women with PPD; 2 were placebo-controlled, and 2 were active comparator studies. One placebo-controlled RCT compared immediate-release flexible-dosed paroxetine with placebo in 70 women with postpartum onset of MDD. 84 After 8 weeks of treatment, both groups improved significantly over time, but paroxetine was superior to placebo in terms of remission of depression (remission rates were 37% and 15%, respectively). Approximately 40% of the subjects in this study were breastfeeding, but the effects in infants were not described in the published study. 84 Another placebo-controlled RCT compared fluoxetine, placebo, and counseling (based loosely on CBT principles) in 87 women with PPD. 85 Women were assigned randomly to 12 weeks of fluoxetine 20 mg daily and 6 counseling sessions, fluoxetine 20 mg daily and 1 counseling session, placebo and 6 counseling sessions, or placebo and 1 counseling session. Fluoxetine was significantly superior to placebo in reducing the severity of depressive symptoms. The combination of fluoxetine and 6 sessions of counseling were not superior to either treatment alone. Women who were breastfeeding were excluded from this study; most of the women who were enrolled had mild-to-moderate severity of depressive symptoms.
A comparator RCT randomly assigned 109 women with PPD to sertraline or nortriptyline, both of which were administered in an escalating dose regimen over 8 weeks. 86 Almost one-half of the subjects remitted by week 8 on either antidepressant. No adverse effects in breastfeeding infants were reported, and infant serum levels were near or below quantifiable levels. 86 Another comparator RCT compared paroxetine with combined paroxetine/CBT in 35 women with PPD and comorbid anxiety disorders. 87 Paroxetine was flexibly dosed over 12 weeks, and CBT was provided in 12 individual sessions. Both treatments led to significant improvements on measures of depression, and there were no significant differences between treatments. Approximately one-half of the subjects were breastfeeding, but antidepressant side-effects and serum levels in infants were not reported. The anxiety comorbidity in the latter study and the lack of a placebo control in both of these comparator RCTs limits conclusions about the efficacy of these treatments for PPD. Notably, the remission rate with paroxetine was lower in the paroxetine study that included a placebo control. 84 Small open trials and case reports have also suggested efficacy of antidepressants for the treatment of PPD. 82 , 88
Additional treatments
Studies have suggested a benefit with infant massage, 89 exercise, 90 sleep deprivation, 91 infant sleep intervention, 92 and electroconvulsive therapy. 93 Studies have reported that postpartum use of estrogen may have a role, 94 , 95 although the postpartum use of progesterone has not been promising. 82 A small study reported that early morning bright light therapy was not more effective than sham dim red light in the reduction of depressive symptoms. 96 Two recent RCTs failed to demonstrate superior efficacy of omega-3 supplementation, compared with placebo. 97 , 98
Antidepressants and breastfeeding
The breastfeeding woman with PPD must weigh the potential efficacy of antidepressant medication for her depression, the potential risks of exposure of her infant to antidepressant medication through the breastfeeding, and the known negative effects of not treating her depression on child development. Breastfeeding has multiple benefits for a developing infant, 42 and a woman with PPD may believe that breastfeeding is an important positive experience that she is able to share with her infant in her depressed state. There is a growing observational database of side-effects in infants who are exposed to antidepressants through breast milk, and the choice of medication should be chosen after review of these data. 99 The Food and Drug Administration has announced that, in the future, medications will be classified by their risk summary, clinical considerations, and data in terms of lactation. 100 Measurement of infant antidepressant serum levels and breast milk analyses are not obtained routinely in clinical care, 101 and milk-to-plasma ratios may not be relevant to adverse effects. 102 When an antidepressant is started in the woman after delivery, it is recommended to start with low doses and to titrate the dose up slowly while monitoring the infant for adverse effects. 82 Possible adverse effects in the breastfeeding infants include irritability, sedation, poor weight gain, or a change in feeding patterns. 103 , 104 Adverse events are most likely to occur in newborn infants up to 8 weeks of age, and infants who are born prematurely or with medical problems may be at increased risk. 103 Infant exposure to antidepressant medication can be minimized by avoiding breastfeeding at the time of peak antidepressant concentration in the breast milk. 105 If adverse effects in the infant are noted, options include decreasing the dose, changing to partial or full bottlefeeding, or changing the medication. Collaboration between the pediatrician and mental health clinician is important.
Several reviews of the safety of selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and newer antidepressants with breast-feeding have been conducted. 99 , 104 , 106 , 107 A pooled analysis of antidepressant levels in mother-infant dyads concluded that sertraline, paroxetine, and nortriptyline usually yield undetectable infant serum levels and that elevated infant levels are more likely with fluoxetine and citalopram. 107 Sertraline has been reported to have minimal or no effect on central serotonin transport in the infant. 108 Case reports of adverse effects in breastfeeding infants have been reported with fluoxetine, citalopram, doxepin, bupropion, and nefazodone. 82 , 88 , 101 If after delivery, a woman is euthymic with antidepressant therapy that is known to be associated potentially with mild adverse effects or high infant serum levels, it may be more advisable to monitor the infant carefully rather than to switch the antidepressant. 82 , 104 Even if there are no adverse effects and unquantifiable levels in infants, the long-term effects of antidepressant exposure through breast milk on child cognitive, motor, neurologic, and behavioral development are unclear. 109
Other psychotropic medications and breastfeeding
Some women with PPD may be administered an adjunctive benzodiazepine for anxiety or insomnia. Sedation and poor feeding have been reported in breast-feeding infants who are exposed to benzodiazepines, and divided low doses has been advised. 101 Other psychotropic medication may be used by breastfeeding women with bipolar or psychotic illness or severe depression. Even though it was reported recently that lithium could be used during breastfeeding with careful infant serum level monitoring, 110 lithium generally has not been recommended during breastfeeding because of reports of hypothermia, hypotonia, cyanosis, T-wave inversion, and lethargy reported in infants. 61 , 101 , 111 There is a paucity of data about the safety of the newer antiepileptic drugs and atypical antipsy-chotics. 105 Valproate and carbamazepine have been used safely during breastfeeding. It was reported recently that infant serum levels of lamotrigine are variable and sometimes high after breastfeeding. 112 Preliminary data have suggested that oxcarbazepine, topiramate, gabapentin, and levetiracetam are not associated with adverse effects. 61 , 105 , 111 Sporadic adverse effects have been reported with olanzapine, clozapine, and traditional antipsychotics. 113 Infant monitoring should match the monitoring of potential adverse events that is used in adults. 105 Studies that evaluate the long-term effect on child development after breastfeeding exposure to anxiolytics, mood stabilizers, and antipsychotics are needed.
Treatment dilemmas for women with PPD
It can be argued that the risks of exposure to PPD outweigh at least the short-term risks of infant exposure to antidepressants through breast milk, because the multiple negative effects of untreated PPD on short-term and long-term child development are well-established. In addition to the multiple known benefits for infants with breastfeeding, 42 a recent large sample study reported that prolonged and exclusive breastfeeding was associated with improved cognitive development in 6-year-old children. 114 Women who are breastfeeding may prefer psychotherapy over medication for the treatment of PPD, but it may be less effective than pharmacotherapy for severely depressed women. For these women and for women whose symptoms are unresponsive to nonpharmacologic treatments, the consideration of antidepressant medication may be necessary. All psychotropic medications pass into breast milk, and the potential for infant exposure exists with each medication. Although observational reports suggest a lack of short-term adverse effects in infants with many psychotropic medications, few studies have examined long-term effects. Discussions of the treatment options with the patient and her partner after delivery must include the patient’s personal psychiatric history and previous response to treatment, the risks of no treatment, available data about the safety of medications with breastfeeding, and her individual expectations and treatment preferences. 103 Time constraints, financial restraints, and perceived cultural dissonance can lead to poor treatment adherence. Even with treatment adherence support in low-income mothers in Chile, the initial benefit of multicomponent care (including psychosocial support and medication) for PPD, compared with usual care, was attenuated after 6 months. 115
Future efforts hopefully will improve the screening and identification of psychiatric disorders in women at their postpartum visit with the obstetrician and at well-baby visits with the pediatrician. Untreated depression and psychotropic medications for the breastfeeding woman each involve exposure of the child to potential short-term and long-term negative effects. Psychotherapy is a treatment option for women with PPD, with IPT being the most validated psychotherapy to be studied to date. Antidepressant medications are also efficacious for PPD. The critical goal of treatment is the resolution of the mother’s psychiatric symptoms. Breastfeeding has multiple known benefits for infant development, and a breastfeeding woman with PPD does not need necessarily to decline pharmacotherapy. Sertraline is the first-line antidepressant used in PPD in breastfeeding women because of the paucity of adverse effects that have been reported in breastfeeding infants. Paroxetine or nortriptyline are second-line agents in women who are unable to tolerate or who do not respond to sertraline. Clinicians and patients can monitor current knowledge about breastfeeding and medications through publications 116 and websites that update and review published information frequently (such as LactMed on http://toxnet.nlm.nih.gov , www.mededppd.org , www.postpartum.net , www.womensmental-health.org , and www.motherrisk.org ). Although antidepressants appear to be effective for PPD, there is a need for large placebo-controlled RCTs of antidepressants in women with PPD of a least moderate severity. Breastfeeding women must be included in pharmacotherapy trials, and potential adverse effects in infants must be assessed systematically. Future studies are needed to confirm the efficacy of psychotherapies for PPD, compare antidepressants to psychotherapy, and compare combined psychotherapy/antidepressant treatment to either treatment alone. Further studies of the factors that govern treatment selection and systematic studies of nonpharmacologic and alternative treatments are needed. Longitudinal follow-up studies that will examine the long-term effects of untreated maternal depression and exposure to psychotropic medication on infant and child cognitive, motor, behavioral, and neurologic development are critically needed to help guide women with depression during the postpartum period.
Authorship and contribution to the manuscript is limited to the 4 authors indicated. There was no outside funding or technical assistance with the production of this article.

IMAGES
COMMENTS
Postpartum depression: Proposal for prevention through an integrated care and support network. September 1997. Applied and Preventive Psychology 6 (4):169-178. DOI: 10.1016/S0962-1849 (97)80006-6 ...
Postpartum depression (PPD) is a serious health issue that can affect about 15% of the female population within after giving birth. It often conveys significant negative consequences to the offsprings. The symptoms and risk factors are somewhat similar to those found in non-postpartum depression. The main difference resides in the fact that PPD ...
Introduction Postpartum depression has costly consequences for the mother, baby, and society. Numerous pharmacological and non-pharmacological interventions are available for the prevention and treatment of postpartum depression. To date, no attempt has been made to synthesize the evidence from comparisons of interventions both within and across these categories. Methods We will perform a ...
Objective: To explore awareness of postpartum depression and its symptoms and available community resources for women with postpartum depression.Design: Cross-sectional surveillance research, using population-based data.Setting: Eight communities in southern and eastern Ontario, Canada.Participants: A random selection of adults 18 years of age and older with telephones.Method: Logistic ...
Recent research suggests that identifying risk for perinatal depression including historical diagnoses of depression, anxiety, trauma, and comorbid substance use and intimate partner violence may move the field to focus on preventive care in peripartum populations. ... Postpartum depression was extremely common before the COVID-19 pandemic and ...
The estimated prevalence of postpartum depression ranges from 6.5 to 12.9% or even higher in lower-income and middle-income countries. 1,4,5 Some studies have shown increased rates of depression ...
Postpartum depression (PPD) is a significant health issue for many new mothers in the weeks and months following a child's birth. Quantitative data suggest that a mother's PPD negatively impacts healthcare decision-making for the child via routine well-baby visits and pediatric care.
Recent research on the biological risk factors includes studies on oxytocin (Skrundz et al., 2011) and inflammatory markers (Osborne & Monk, 2013). ... Postpartum depression is the most common complication of childbirth, with close to 20% of new mothers experiencing an episode. We are only at the beginning stages of understanding the biological ...
Postpartum depression: Proposal for prevention through an integrated care and support network. Author links open overlay panel Alice K. Locicero, Dianne M. Weiss, Deborah Issokson. ... It is useful as both a clinical and research tool, and thus complements other scales that focus just on women's mood or confidence in the postpartum period.
Perinatal mental health conditions are the leading cause of overall and preventable maternal mortality and include a wide array of mental health conditions including anxiety, depression, and substance use disorders. 1,2 Perinatal depression specifically affects 1 in 7 perinatal individuals. 3 While commonly referred to as postpartum depression ...
Current proposals for mandatory depression screening for women in the perinatal and postpartum periods have been supported by many professional organizations, including ACOG, the American Academy of Pediatrics, the U.S. Preventive Services Task Force, and most recently the American Medical Association
Background To provide a comprehensive, systematic evaluation of the literature on experiences of psychological interventions for postpartum depression (PPD) in women. Depression is one of the most common postpartum mental disorders. Studies have identified that psychological interventions reduce depressive symptoms. However, less is known about the experiences of women who have received such ...
Background Postpartum depression explains various groups of depressive symptoms and syndromes that can take place during the first 6 weeks following birth. The postpartum period is a critical time where both mild and severe mood disorders can occur. The familiar forms are baby blues and postpartum depression. Understanding the prevalence and associated factors of postpartum depression is ...
Abstract. Postpartum depression (PPD) is a devastating mental disorder affecting 10-20% of new mothers. Psychiatric disorders always go unnoticed, even with the high prevalence. This review ...
Background Postpartum depression (PPD) affects around 10% of women, or 1 in 7 women, after giving birth. Undiagnosed PPD was observed among 50% of mothers. PPD has an unfavorable relationship with women's functioning, marital and personal relationships, the quality of the mother-infant connection, and the social, behavioral, and cognitive development of children. We aim to determine the ...
Postpartum depression: Proposal for prevention through an integrated care and support network. Author links open overlay panel Alice K. Locicero, Dianne M. Weiss, ... and publishes an extensive bibliography of research on postpartum mood and anxiety disorders (Kruckman, I994). 9Krauss and Jacobs (1990) recommend a risk-assessment model that ...
Limitations: Use of a self-report instrument, potential bias by postpartum mood status, and no inclusion of emerging depression cases after one month postpartum. Conclusions: The shared and separate risk factors for postpartum depression and anxiety may help professionals in identifying mothers at increased risk and provide opportunities for ...
Postpartum depression and pain negatively affect maternal well-being, and postpartum depression has been associated with adverse outcomes in children. However, there is a dearth of information about the effects of postpartum depression and pain on infant care and development. ... Depression Research & Treatment, 2019. 2016; (Article 4518979 ...
Chi-square test and logistic regression analysis were conducted to analyze the associations between social support (and other covariates) and postpartum depression. Among participants, 266 (16.1%) had postpartum depression. Depending on the level of social support, 6.0%, 53.9%, and 40.1% of them had low, moderate, and high social support ...
Postpartum depression can have lasting consequences for the mother, child, and family. After each birth, 1 in 7 women will experience postpartum depression. ... Health System and Henry Ford Health is collaborating on a $6.2 million National Institutes of Health mental health research grant, "The ROSE Scale-Up Study: Informing a decision about ...
At a Glance. Postpartum depression (PPD) is a common mental disorder that many women experience after giving birth. Onset of PPD coincides with a dramatic drop in levels of a brain-derived steroid (neurosteroid) known as allopregnanolone.
Research suggests that it is triggered by changes in hormones and that women with PPD are sensitive to those changes. PPD occurs in approximately 15% of births. You are invited to call the National Institute of Mental Health at 301-496-9576 for information on our research studies of Postpartum Depression (PPD). If you had PPD in the past ...
Postpartum Depression: Current Status and Possible Identification Using Biomarkers. Yi Yu 1,2 Hong-Feng Liang 1 Jing Chen 1,3 Zhi-Bin Li 1,3 Yu-Shuai Han 1,3 Jia-Xi Chen 1,3 Ji-Cheng Li 1,3 *. 1 Central Laboratory, Yangjiang People's Hospital, Yangjiang, China. 2 Center for Analyses and Measurements, College of Chemical Engineering, Zhejiang ...
There is not enough evidence to know if breastfeeding is associated with a higher or lower risk of postpartum depression (following childbirth). According to a 2018 systematic review by the Agency for Healthcare Research and Quality (AHRQ), understanding the relationship is challenging "because women with depression may have difficulty ...
Postpartum depression is a debilitating mental disorder with a high prevalence. The aim of this study was review of the related studies. In this narrative review, we report studies that investigated risk factors of postpartum depression by searching the database, Scopus, PubMed, ScienceDirect, Uptodate, Proquest in the period 2000-2015 published articles about the factors associated with ...
Further research from the International Journal of Public Health Science points out the significant risks that unchecked postpartum can pose to both mothers and their children. This intense anger ...
Twenty-two outstanding proposals were awarded the Graduate Student Research Award for spring 2024 across many colleges and departments. ... More than 84% of breastfeeding parents express breastmilk within the first four months postpartum, often to provide breastmilk to alternative caregivers during times of separation. ...
Postpartum depression (PPD) affects up to 15% of mothers. Recent research has identified several psychosocial and biologic risk factors for PPD. The negative short-term and long-term effects on child development are well-established. PPD is under recognized and under treated. The obstetrician and pediatrician can serve important roles in ...