Cornell Chronicle
- Architecture & Design
- Arts & Humanities
- Business, Economics & Entrepreneurship
- Computing & Information Sciences
- Energy, Environment & Sustainability
- Food & Agriculture
- Global Reach
- Health, Nutrition & Medicine
- Law, Government & Public Policy
- Life Sciences & Veterinary Medicine
- Physical Sciences & Engineering
- Social & Behavioral Sciences
- Coronavirus
- News & Events
- Public Engagement
- New York City
- Photos of the Week
- Big Red Sports
- Freedom of Expression
- Student Life
- University Statements
- Around Cornell
- All Stories
- In the News
- Expert Quotes
- Cornellians

Large-scale study reveals new genetic details of diabetes
By wynne parry weill cornell medicine.
In experiments of unprecedented scale, investigators at Weill Cornell Medicine and the National Institutes of Health have revealed new aspects of the complex genetics behind Type 2 diabetes. Through these discoveries, and by providing a template for future studies, this research furthers efforts to better understand and ultimately treat this common metabolic disease.
Previous studies have generally examined the influence of individual genes. In research described Oct. 18 in Cell Metabolism, senior co-author Shuibing Chen , the Kilts Family Professor of Surgery at Weill Cornell Medicine, working alongside senior co-author Dr. Francis Collins , a senior investigator at the Center for Precision Health Research within the National Human Genome Research Institute of the U.S. National Institutes of Health, took a more comprehensive approach. Together, they looked at the contribution of 20 genes in a single effort.
“It’s very difficult to believe all these diabetes-related genes act independently of each other,” Chen said. By using a combination of technologies, the team examined the effects of shutting each down. By comparing the consequences for cell behavior and genetics, she said, “we found some common themes.”
As with other types of diabetes, Type 2 diabetes occurs when sugar levels in the blood are too high. In Type 2 diabetes, this happens in part because specialized cells in the pancreas, known as β-cells, don’t produce enough insulin, a hormone that tells cells to take sugar out of the blood for use as an energy source. Over time, high levels of blood sugar damage tissues and cause other problems, such as heart and kidney disease. According to the United States Centers for Disease Control and Prevention, nearly 9% of adults in the United States have been diagnosed with Type 2 diabetes.
Both genetic and environmental factors, such as obesity and chronic stress, can increase risk for it. Yet evaluating the role of the genetic contributors alone is a massive project. So far, researchers have identified more than 290 locations within the genome where changes to DNA can raise the likelihood of developing the disease. Some of these locations fall within known genes, but most are found in regions that regulate the expression of nearby genes.
For the new research, the team focused on 20 genes clearly identified as contributors. They began their investigation by using the gene editing system CRISPR-Cas9 to shut down these genes, one at a time, within 20 sets of identical stem cells.
These stem cells had the potential to generate any kind of mature cell, but the researchers coaxed them into becoming insulin-producing β-cells. They then examined the effects of losing each gene on five traits related to insulin production and the health of β-cells. They also documented the accompanying changes in gene expression and the accessibility of DNA for expression.
To make sense of the massive amount of data they collected, the team developed their own computational models to analyze it, leading to several discoveries: By comparing the effects of all 20 mutations on β-cells, they identified four additional genes, each representing a newly discovered pathway that contributes to insulin production. They also found that, of the original 20 genes, only one, called HNF4A, contributed to all five traits, apparently by acting as a master controller that regulates the activity of other genes. In one specific example, they explained how a small variation, located in a space between genes, contributes to the risk of diabetes by interfering with HNF4A’s ability to regulate nearby genes.
Ultimately, this study and others like it hold the promise of benefiting patients, Collins said. “We need to understand all the genetic and environmental factors involved so we can do a better job of preventing diabetes, and to develop new ideas about how to effectively treat it.”
Collins and Chen note that their approach may have relevance beyond diabetes, to other common diseases, such as Alzheimer’s, Parkinson’s and Crohn’s disease, that involve many genetic factors.
The work reported in this newsroom story was supported in part by the United States’ National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and the American Diabetes Association.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, see the profile for Shuibing Chen .
Wynne Parry is a freelance writer for Weill Cornell Medicine.
Media Contact
Krystle lopez.
Get Cornell news delivered right to your inbox.
You might also like

Gallery Heading
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmacological Reviews

New Aspects of Diabetes Research and Therapeutic Development
Both type 1 and type 2 diabetes mellitus are advancing at exponential rates, placing significant burdens on health care networks worldwide. Although traditional pharmacologic therapies such as insulin and oral antidiabetic stalwarts like metformin and the sulfonylureas continue to be used, newer drugs are now on the market targeting novel blood glucose–lowering pathways. Furthermore, exciting new developments in the understanding of beta cell and islet biology are driving the potential for treatments targeting incretin action, islet transplantation with new methods for immunologic protection, and the generation of functional beta cells from stem cells. Here we discuss the mechanistic details underlying past, present, and future diabetes therapies and evaluate their potential to treat and possibly reverse type 1 and 2 diabetes in humans.
Significance Statement
Diabetes mellitus has reached epidemic proportions in the developed and developing world alike. As the last several years have seen many new developments in the field, a new and up to date review of these advances and their careful evaluation will help both clinical and research diabetologists to better understand where the field is currently heading.
I. Introduction
Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020 ). Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing pancreatic beta cells. The much more prevalent T2D arises in conjunction with peripheral tissue insulin resistance and beta cell failure and is estimated to increase to 21%–33% of the US population by the year 2050 (Boyle et al., 2010 ). To combat this growing health threat and its cardiac, renal, and neurologic comorbidities, new and more effective diabetes drugs and treatments are essential. As the last several years have seen many new developments in the field of diabetes pharmacology and therapy, we determined that a new and up to date review of these advances was in order. Our aim is to provide a careful evaluation of both old and new therapies ( Fig. 1 ) in a manner that we hope will be of interest to both clinical and bench diabetologists. Instead of the usual encyclopedic approach to this topic, we provide here a targeted and selective consideration of the underlying issues, promising new treatments, and a re-examination of more traditional approaches. Thus, we do not discuss less frequently used diabetes agents, such as alpha-glucosidase inhibitors; these were discussed in other recent reviews (Hedrington and Davis, 2019 ; Lebovitz, 2019 ).

Pharmacologic targeting of numerous organ systems for the treatment of diabetes. Treatment of diabetes involves targeting of various organ systems, including the kidney by SGLT2 inhibitors; the liver, gut, and adipose tissue by metformin; and direct actions upon the pancreatic beta cell. Beta cell compounds aim to increase secretion or mass and/or to protect from autoimmunity destruction. Ultimately, insulin therapy remains the final line of diabetes treatment with new technologies under development to more tightly regulate blood glucose levels similar to healthy beta cells. hESC, human embryonic stem cell.
II. Diabetes Therapies
A. metformin.
Metformin is a biguanide originally based on the natural product galegine, which was extracted from the French lilac (Bailey, 1992 ; Rojas and Gomes, 2013 ; Witters, 2001 ). A closely related biguanide, phenformin, was also used initially for its hypoglycemic actions. Based on its successful track record as a safe, effective, and inexpensive oral medication, metformin has become the most widely prescribed oral agent in the world in treating T2D (Rojas and Gomes, 2013 ; He and Wondisford, 2015 ; Witters, 2001 ), whereas phenformin has been largely bypassed due to its unacceptably high association with lactic acidosis (Misbin, 2004 ). Unlike sulfonylureas, metformin lowers blood glucose without provoking hypoglycemia and improves insulin sensitivity (Bailey, 1992 ). Despite these well known beneficial metabolic actions, metformin’s mechanism of action and even its main target organ remain controversial. In fact, metformin has multiple mechanisms of action at the organ as well as the cellular level, which has hindered our understanding of its most important molecular effects on glucose metabolism (Witters, 2001 ). Adding to this, a specific receptor for metformin has never been identified. Metformin has actions on several tissues, although the primary foci of most studies have been the liver, skeletal muscle, and the intestine (Foretz et al., 2014 ; Rena et al., 2017 ). Metformin and phenformin clearly suppress hepatic glucose production and gluconeogenesis, and they improve insulin sensitivity in the liver and elsewhere (Bailey, 1992 ). The hepatic actions of metformin have been the most exhaustively studied to date, and there is little doubt that these actions are of some importance. However, several of the studies remain highly controversial, and there are still open questions.
One of the first reported specific molecular targets of metformin was mitochondrial complex I of the electron transport chain. Inhibition of this complex results in reduced oxidative phosphorylation and consequently decreased hepatic ATP production (El-Mir et al., 2008 ; Evans et al., 2005 ; Owen et al., 2000 ). As is the case in many other studies of metformin, however, high concentrations of the drug were found to be necessary to depress metabolism at this site (El-Mir et al., 2000 ; He and Wondisford, 2015 ; Owen et al., 2000 ). Also controversial is whether metformin works by activating 5′ AMP-activated protein kinase (AMPK), a molecular energy sensor that is known to be a major metabolic sensor in cells, or if not AMPK directly, then one of its upstream regulators such as liver kinase B2 (Zhou et al., 2001 ). Although metformin was shown to activate AMPK in several excellent studies, other studies directly contradicted the AMPK hypothesis. Most dramatic were studies showing that metformin’s actions to suppress hepatic gluconeogenesis persisted despite genetic deletion of the AMPK’s catalytic domain (Foretz et al., 2010 ). More recent studies identified additional or alternative targets, such as cAMP signaling in the liver (Miller et al., 2013 ) or glycogen synthase kinase-3 (Link, 2003 ). Other work showed that the phosphorylation of acetyl-CoA carboxylase and acetyl-CoA carboxylase 2 are involved in regulating lipid homeostasis and improving insulin sensitivity after exposure to metformin (Fullerton et al., 2013 ).
Although there are strong data to support each of these pathways, it is not entirely clear which signaling pathway(s) is most essential to the actions of metformin in hepatocytes. Metformin clearly inhibits complex I and concomitantly decreases ATP and increases AMP. The latter results in AMPK activation, reduced fatty acid synthesis, and improved insulin receptor activation, and increased AMP has been shown to inhibit adenylate cyclase to reduce cAMP and thus protein kinase A activation. Downstream, this reduces the expression of phosphoenolpyruvate carboxykinase and glucose 6-phosphatase via decreased cAMP response element-binding protein, the cAMP-sensitive transcription factor. Decreased PKA also promotes ATP-dependent 6-phosphofructokinase, liver type activity via fructose 2,6-bisphosphate and reduces gluconeogenesis, as fructose-bisphosphatase 1 is inhibited by fructose 2,6-bisphosphate, along with other mechanisms (Rena et al., 2017 ; Pernicova and Korbonits, 2014 ).
More recent work has shown that metformin at pharmacological rather than suprapharmacological doses increases mitochondrial respiration and complex 1 activity and also increases mitochondrial fission, now thought to be critical for maintaining proper mitochondrial density in hepatocytes and other cells. This improvement in respiratory activity occurs via AMPK activation (Wang et al., 2019 ).
Although the liver has historically been the major suspected site of metformin action, recent studies have suggested that the gut instead of the liver is a major target, a concept supported by the increased efficacy of extended-release formulations of metformin that reside for a longer duration in the gut after their administration (Buse et al., 2016 ). An older, but in our view an important observation, is that the intravenous administration of metformin has little or no effect on blood glucose, whereas, in contrast, orally administered metformin is much more effective (Bonora et al., 1984 ). Recent imaging studies using labeled glucose have shown directly that metformin stimulates glucose uptake by the gut in patients with T2D to reduce plasma glucose concentrations (Koffert et al., 2017 ; Massollo et al., 2013 ). Additionally, it is possible that metformin may exert its effect in the gut by inducing intestinal glucagon-like peptide-1 (GLP-1) release (Mulherin et al., 2011 ; Preiss et al., 2017) to potentiate beta cell insulin secretion and by stimulating the central nervous system (CNS) to exert control over both blood glucose and liver function. Indeed, CNS effects produced by metformin have been proposed to occur via the local release of GLP-1 to activate intestinal nerve endings of ascending nerve pathways that are involved in CNS glucose regulation (Duca et al., 2015 ). Lastly, several papers have now implicated that metformin may act by altering the gut microbiome, suggesting that changes in gut flora may be critical for metformin’s actions (McCreight et al., 2016 ; Wu et al., 2017 ; Devaraj et al., 2016 ). A new study proposed that activation of the intestinal farnesoid X receptor may be the means by which microbiota alter hyperglycemia (Sun et al., 2018 ). However, these studies will require more mechanistic detail and confirmation before they can be fully accepted by the field. In addition to the action of metformin on gut flora, the production of imidazole propionate by gut microbes in turn has been shown to interfere with metformin action through a p38-dependent mechanism and AMPK inhibition. Levels of imidazole propionate are especially higher in patients with T2D who are treated with metformin (Koh et al., 2020 ).
In summary, the combined contribution of these various effects of metformin on multiple cellular targets residing in many tissues may be key to the benefits of metformin treatment on lowering blood glucose in patients with type 2 diabetes (Foretz et al., 2019 ). In contrast, exciting new work showing metformin leads to weight loss by increasing circulating levels of the peptide hormone growth differentiation factor 15 and activation of brainstem glial cell-derived neurotropic factor family receptor alpha like receptors to reduce food intake and energy expenditure works independently of metformin’s glucose-lowering effect (Coll et al., 2020 ).
B. Sulfonylureas and Beta Cell Burnout
The class of compounds known as sulfonylureas includes one of the oldest oral antidiabetic drugs in the pharmacopoeia: tolbutamide. Tolbutamide is a “first generation” oral sulfonylurea secretagogue whose clinical usefulness is due to its prompt stimulation of insulin release from pancreatic beta cells. “Second generation” sulfonylureas include drugs such as glyburide, gliclazide, and glipizide. Sulfonylureas act by binding to a high affinity sulfonylurea binding site, the sulfonylurea receptor 1 subunit of the K(ATP) channel, which closes the channel. These drugs mimic the physiologic effects of glucose, which closes the K(ATP) channel by raising cytosolic ATP/ADP. This in turn provokes beta cell depolarization, resulting in increased Ca 2+ influx into the beta cell (Ozanne et al., 1995 ; Ashcroft and Rorsman, 1989 ; Nichols, 2006 ). Importantly, sulfonylureas, and all drugs that directly increase insulin secretion, are associated with hypoglycemia, which can be severe, and which limits their widespread use in the clinic (Yu et al., 2018 ). Meglitinides are another class of oral insulin secretagogues that, like the sulfonylureas, bind to sulfonylurea receptor 1 and inhibit K(ATP) channel activity (although at a different site of action). The rapid kinetics of the meglitinides enable them to effectively blunt the postprandial glycemic excursions that are a hallmark (along with elevated fasting glucose) of T2D (Rosenstock et al., 2004). However, the need for their frequent dosing (e.g., administration before each meal) has limited their appeal to patients.
The efficacy of sulfonylureas is known to decrease over time, leading to failure of the class for effective long-term treatment of T2D (Harrower, 1991 ). More broadly, it is now widely accepted that the number of functional beta cells in humans declines during the progression of T2D. Thus, one would expect that due to this decline, all manner of oral agents intended to target the beta cell and increase its cell function (and especially insulin secretion) will fail over time (RISE Consortium, 2019 ), a process referred to as “beta cell failure” (Prentki and Nolan, 2006 ). Currently, treatments that can expand beta cell mass or improve beta cell function or survival over time are not yet available for use in the clinic. As a result, treatments that may be able to help patients cope with beta cell burnout such as islet cell transplantation, insulin pumps, or stem cell therapy are alternatives that will be discussed below.
C. Ca 2+ Channel Blockers and Type 1 Diabetes
Strategies to treat and prevent T1D have historically focused on ameliorating the toxic consequences of immune dysregulation resulting in autoimmune destruction of pancreatic beta cells. More recently, a concerted focus on alleviating the intrinsic beta cell defects (Sims et al., 2020 ; Soleimanpour and Stoffers, 2013 ) that also contribute to T1D pathogenesis have been gaining traction at both the bench and the bedside. Several recent preclinical studies suggest that Ca 2+ -induced metabolic overload induces beta cell failure (Osipovich et al., 2020 ; Stancill et al., 2017 ; Xu et al., 2012 ), with the potential that excitotoxicity contributes to beta cell demise in both T1D and T2D, similar to the well known connection between excitotoxicity and, concomitantly, increased Ca 2+ loading of the cells and neuronal dysfunction. Indeed, the use of the phenylalkylamine Ca 2+ channel blocker verapamil has been successful in ameliorating beta cell dysfunction in preclinical models of both T1D and T2D (Stancill et al., 2017 ; Xu et al., 2012 ). Verapamil is a well known blocker of L-type Ca 2+ channels, and, in normally activated beta cells, it limits Ca 2+ entry into the beta cell (Ohnishi and Endo, 1981 ; Vasseur et al., 1987 ). This would be expected to, in turn, alter the expression of many Ca 2+ influx–dependent beta cell genes (Stancill et al., 2017 ), and the evidence to date suggests it is likely that verapamil preserves beta cell function in diabetes models by repressing thioredoxin-interacting protein (TXNIP) expression and thus protecting the beta cell. This is somewhat surprising given the physiologic role of Ca 2+ is to acutely trigger insulin secretion; this process would be expected to be inhibited by L-type Ca 2+ channel blockers (Ashcroft and Rorsman, 1989 ; Satin et al., 1995 ).
Hyperglycemia is a well known inducer of TXNIP expression, and a lack of TXNIP has been shown to protect against beta cell apoptosis after inflammatory stress (Chen et al., 2008a ; Shalev et al., 2002 ; Chen et al., 2008b ). Excitingly, the use of verapamil in patients with recent-onset T1D improved beta cell function and improved glycemic control for up to 12 months after the initiation of therapy, suggesting there is indeed promise for targeting calcium and TXNIP activation in T1D. Use of verapamil for a repurposed indication in the preservation of beta cell function in T1D is attractive due its well known safety profile as well as its cardiac benefits (Chen et al., 2009 ). Although the long-term efficacy of verapamil to maintain beta cell function in vivo is unclear, a recently described TXNIP inhibitor may also show promise in suppressing the hyperglucagonemia that also contributes to glucose intolerance in T2D (Thielen et al., 2020 ). As there is a clear need for increased Ca 2+ influx into the beta cell to trigger and maintain glucose-dependent insulin secretion (Ashcroft and Rorsman, 1990 ; Satin et al., 1995 ), it remains to be seen how well regulated insulin secretion is preserved in the presence of L-type Ca 2+ channel blockers like verapamil in the system. One might speculate that reducing but not fully eliminating beta cell Ca 2+ influx might reduce TXNIP levels while preserving enough influx to maintain glucose-stimulated insulin release. Alternatively, these two phenomena may operate on entirely different time scales. At present, these issues clearly will require further investigation.
D. GLP-1 and the Incretins
Studies dating back to the 1960s revealed that administering glucose in equal amounts via the peripheral circulation versus the gastrointestinal tract led to dramatically different amounts of glucose-induced insulin secretion (Elrick et al., 1964 ; McIntyre et al., 1964 ; Perley and Kipnis, 1967 ). Gastrointestinal glucose administration greatly increased insulin secretion versus intravenous glucose, and this came to be known as the “incretin effect” (Nauck et al., 1986a ; Nauck et al., 1986b ). Subsequent work showed that release of the gut hormone GLP-1 mediated this effect such that food ingestion induced intestinal cell hormone secretion. GLP-1 so released would then circulate to the pancreas via the blood to prime beta cells to secrete more insulin when glucose became elevated because these hormones stimulated beta cell cAMP formation (Drucker et al., 1987 ). The discovery that a natural peptide corresponding to GLP-1 could be found in the saliva of the Gila monster, a desert lizard, hastened progress in the field, and ample in vitro studies subsequently confirmed that GLP-1 potentiated insulin secretion in a glucose-dependent manner. GLP-1 has little or no significant action on insulin secretion in the absence of elevated glucose (such as might typically correspond to the postprandial case or during fasting), thus minimizing the likelihood of hypoglycemia provoked by GLP-1 in treated patients (Kreymann et al., 1987 ). Although not completely understood, the glucose dependence of GLP-1 likely reflects the requirement for adenine nucleotides to close glucose-inhibited K(ATP) channels and thus subsequently activate Ca 2+ influx–dependent insulin exocytosis. Besides potentiating GSIS at the level of the beta cell, glucagon-like peptide-1 receptor (GLP-1R) agonists also decrease glucagon secretion from pancreatic islet alpha cells, reduce gastric emptying, and may also increase beta cell proliferation, among other cellular actions (reviewed in Drucker, 2018 ; Muller et al., 2019).
Intense interest in the incretins by basic scientists, clinicians, and the pharma community led to the rapid development of new drugs for treating primarily T2D. These drugs include a range of GLP-1R agonists and inhibitors of the incretin hormone degrading enzyme dipeptidyl peptidase 4 (DPP4), whose targeting increases the half-lives of GLP-1 and gastric inhibitory polypeptide (GIP) and thereby increases protein hormone levels in plasma. GLP-1R agonists have been associated with not only a lowering of plasma glucose but also weight loss, decreased appetite, reduced risk of cardiovascular events, and other favorable outcomes (Gerstein et al., 2019; Hernandez et al., 2018; Husain et al., 2019; Marso et al., 2016a; Marso et al., 2016b ; Buse et al., 2004). Regarding their untoward actions, although hypoglycemia is not a major concern, there have been reports of pancreatitis and pancreatic cancer from use of GLP-1R agonists. However, a recent meta-analysis covering four large-scale clinical trials and over 33,000 participants noted no significantly increased risk for pancreatitis/pancreatic cancer in patients using GLP-1R agonists (Bethel et al., 2018).
Ongoing and future developments in the use of proglucagon-derived peptides such as GLP-1 and glucagon include the use of combined GLP-1/GIP, glucagon/GLP-1, and agents targeting all three peptides in combination (reviewed in Alexiadou and Tan, 2020 ). Although short-term infusions of GLP-1 with GIP failed to yield metabolic benefits beyond those seen with GLP-1 alone (Bergmann et al., 2019 ), several GLP-1/GIP dual agonists are currently in development and have shown promising metabolic results in clinical trials (Frias et al., 2017 ; Frias et al., 2020 ; Frias et al., 2018 ). At the level of the pancreatic islet, beneficial effects of dual GLP-1/GIP agonists may be related to imbalanced and biased preferences of these agonists for the gastric inhibitory polypeptide receptor over the GLP-1R (Willard et al., 2020 ) and possibly were not simply to dual hormone agonism in parallel. Dual glucagon/GLP-1 agonist therapy has also been shown to have promising metabolic effects in humans (Ambery et al., 2018 ; Tillner et al., 2019 ). Oxyntomodulin is a natural dual glucagon/GLP-1 receptor agonist and proglucagon cleavage product that is also secreted from intestinal enteroendocrine cells, which has beneficial effects on insulin secretion, appetite regulation, and body weight in both humans and rodents (Cohen et al., 2003 ; Dakin et al., 2001 ; Dakin et al., 2002 ; Shankar et al., 2018 ; Wynne et al., 2005 ). Interestingly, alpha cell crosstalk to beta cells through the combined effects of glucagon and GLP-1 is necessary to obtain optimal glycemic control, suggesting a potential pathway for therapeutic dual glucagon/GLP-1 agonism within the islets of patients with T2D (Capozzi et al., 2019a ; Capozzi et al., 2019b ). Although the early results appear promising, more studies will be necessary to better understand the mechanistic and clinical impacts of these multiagonist agents.
E. DPP4 Inhibitors
Inhibition of DPP4, the incretin hormone degrading enzyme, is one of the most common T2D treatments to increase GLP-1 and GIP plasma hormone levels. These DPP4 inhibitors or “gliptins” are generally used in conjunction with other T2D drugs such as metformin or sulfonylureas to obtain the positive benefits discussed above (Lambeir et al., 2008 ). DPP4 is a primarily membrane-bound peptidase belonging to the serine peptidase/prolyl oligopeptidase gene family, which cleaves a large number of substrates in addition to the incretin hormones (Makrilakis, 2019 ). DPP4 inhibitors provide glucose-lowering benefits while being generally well tolerated, and the variety of available drugs (including sitagliptin, saxagliptin, vildagliptin, alogliptin, and linagliptin) with slightly different dosing frequency, half-life, and mode of excretion/metabolism allows for use in multiple patient populations (Makrilakis, 2019 ). This includes the elderly and individuals with renal or hepatic insufficiency (Makrilakis, 2019 ).
Although hypoglycemia is not a concern for DPP4 inhibitor use, other considerations should be made. DPP4 inhibitors tend to be more expensive than metformin or other second-line oral drugs in addition to having more modest glycemic effects than GLP-1R agonists (Munir and Lamos, 2017 ). Finally, meta-analysis of randomized and observational studies concluded that heart failure in patients with T2D was not associated with use of DPP4 inhibitors; however, this study was limited by the short follow-up and lack of high-quality data (Li et al., 2016 ). Thus, the US Food and Drug Administration (FDA) did recommend assessing risk of heart failure hospitalization in patients with pre-existing cardiovascular disease, prior heart failure, and chronic kidney disease when using saxagliptin and alogliptin (Munir and Lamos, 2017 ).
F. Sodium Glucose Cotransporter 2 Inhibitors
A recent development in the field of T2D drugs are sodium glucose cotransporter 2 (SGLT2) inhibitors, which have an interesting and very different mechanism of action. Within the proximal tubule of the nephron, SGLT2 transports ingested glucose into the lumen of the proximal tubule between the epithelial layers, thereby reclaiming glucose by this reabsorption process (reviewed in Vallon, 2015 ). SGLT2 inhibitors target this transporter and increase glucose in the tubular fluid and ultimately increase it in the urine. In patients with diabetes, SGLT2 inhibition results in a lowering of plasma glucose with urine glucose content rising substantially (Adachi et al., 2000 ; Vallon, 2015 ). These drugs, although they are relatively new, have become an area of great interest for not only patients with T2D (Grempler et al., 2012 ; Imamura et al., 2012 ; Meng et al., 2008 ; Nomura et al., 2010 ) but also for patients with T1D (Luippold et al., 2012 ; Mudaliar et al., 2012 ). Part of their appeal also rests on reports that their use can lead to a statistically significant decline in cardiac events that are known to occur secondarily to diabetes, possibly independently of plasma glucose regulation (reviewed in Kurosaki and Ogasawara, 2013 ). Although the long-term consequences of their clinical use cannot yet be determined, raising the glucose content of the urogenital tract leads to an increased risk of urinary tract infections and other related infections in some patients (Kurosaki and Ogasawara, 2013 ).
Another recent concern about the use of SGLT2 inhibitors has been the development of normoglycemic diabetic ketoacidosis (DKA). Despite the efficacy of SGLT2 inhibitors, observations of hyperglucagonemia in patients with euglycemic DKA has led to a number of recent studies focused on SGLT2 actions on pancreatic islets. Initial studies of isolated human islets treated with small interfering RNA directed against SGLT2 and/or SGLT2 inhibitors demonstrated increased glucagon release. These studies were complemented by the finding of elevations in glucagon release in mice that were administered SGLT2 inhibitors in vivo (Bonner et al., 2015 ). Insights into the possible mechanistic links between SGLT2 inhibition, DKA frequency, and glucagon secretion in humans may relate to the observation of heterogeneity in SGLT2 expression, as SGLT2 expression appears to have a high frequency of interdonor and intradonor variability (Saponaro et al., 2020 ). More recently, both insulin and GLP-1 have been demonstrated to modulate SGLT2-dependent glucagon release through effects on somatostatin release from delta cells (Vergari et al., 2019 ; Saponaro et al., 2019 ), suggesting potentially complex paracrine effects that may affect the efficacy of these compounds.
On the other hand, several recent studies question that the development of euglycemic DKA after SGLT2 inhibitor therapy may be through alpha cell–dependent mechanisms. Three recent studies found no effect of SGLT2 inhibitors to promote glucagon secretion in mouse and/or rat models and could not detect SGLT2 expression in human alpha cells (Chae et al., 2020 ; Kuhre et al., 2019 ; Suga et al., 2019 ). A fourth study demonstrated only a brief transient effect of SGLT2 inhibition to raise circulating glucagon concentrations in immunodeficient mice transplanted with human islets, which returned to baseline levels after longer exposures to SGLT2 inhibitors (Dai et al., 2020 ). Furthermore, SGLT2 protein levels were again undetectable in human islets (Dai et al., 2020 ). These results could suggest alternative islet-independent mechanisms by which patients develop DKA, including alterations in ketone generation and/or clearance, which underscore the additional need for further studies both in molecular models and at the bedside. Nevertheless, SGLT2 inhibitors continue to hold promise as a valuable therapy for T2D, especially in the large segment of patients who also have superimposed cardiovascular risk (McMurray et al., 2019; Wiviott et al., 2019; Zinman et al., 2015).
G. Thiazolidinediones
Once among the most commonly used oral agents in the armamentarium to treat T2D, thiazolidinediones (TZDs) were clinically popular in their utilization to act specifically as insulin sensitizers. TZDs improve peripheral insulin sensitivity through their action as peroxisome proliferator-activated receptor (PPAR) γ agonists, but their clinical use fell sharply after studies suggested a connection between cardiovascular toxicity with rosiglitazone and bladder cancer risk with pioglitazone (Lebovitz, 2019 ). Importantly, an FDA panel eventually removed restrictions related to cardiovascular risk with rosiglitazone in 2013 (Hiatt et al., 2013 ). Similarly, concerns regarding use of bladder cancer risk with pioglitazone were later abated after a series of large clinical studies found that pioglitazone did not increase bladder cancer (Lewis et al., 2015 ; Schwartz et al., 2015 ). However, usage of TZDs had already substantially decreased and has not since recovered.
Although concerns regarding edema, congestive heart failure, and fractures persist with TZD use, there have been several studies suggesting that TZDs protect beta cell function. In the ADOPT study, use of rosiglitazone monotherapy in patients newly diagnosed with T2D led to improved glycemic control compared with metformin or sulfonylureas (Kahn et al., 2006). Later analyses revealed that TZD-treated subjects had a slower deterioration of beta cell function than metformin- or sulfonylurea-treated subjects (Kahn et al., 2011). Furthermore, pioglitazone use improved beta cell function in the prevention of T2D in the ACT NOW study (Defronzo et al., 2013; Kahn et al., 2011). Mechanistically, it is unclear if TZDs lead to beneficial beta cell function through direct effects or through indirect effects of reduced beta cell demand due to enhanced peripheral insulin sensitivity. Indeed, a beta cell–specific knockout of PPAR γ did not impair glucose homeostasis, nor did it impair the antidiabetic effects of TZD use in mice (Rosen et al., 2003 ). However, other reports demonstrated PPAR-responsive elements within the promoters of both glucose transporter 2 and glucokinase that enhance beta cell glucose sensing and function, which could explain beta cell–specific benefits for TZDs (Kim et al., 2002 ; Kim et al., 2000 ). Furthermore, TZDs have been shown to improve beta cell function by upregulating cholesterol transport (Brunham et al., 2007 ; Sturek et al., 2010 ). Additionally, use of TZDs in the nonobese diabetic (NOD) mouse model of T1D augmented the beta cell unfolded protein response and prevented beta cell death, suggesting potential benefits for TZDs in both T1D and T2D (Evans-Molina et al., 2009 ; Maganti et al., 2016 ). With a now refined knowledge of demographics in which to avoid TZD treatment due to adverse effects, together with genetic approaches to identify candidates more likely to respond effectively to TZD therapy (Hu et al., 2019 ; Soccio et al., 2015 ), it remains to be seen if TZD therapy will return to more prominent use in the treatment of diabetes.
H. Insulin and Beyond: The Use of “Smart” Insulin and Closed Loop Systems in Diabetes Treatment
Due to recombinant DNA technology, numerous insulin analogs are now available in various forms ranging from fast acting crystalline insulin to insulin glargine; all of these analogs exhibit equally effective insulin receptor binding. Most are generated by altering amino acids in the B26–B30 region of the molecule (Kurtzhals et al., 2000 ). The American Diabetes Association delineates these insulins by their 1) onset or time before insulin reaches the blood stream, 2) peak time or duration of maximum blood glucose–lowering efficacy, and 3) the duration of blood glucose–lowering time. Insulin administration is independent of the residuum of surviving and/or functioning beta cells in the patient and remains the principal pharmacological treatment of both T1D and T2D. The availability of multiple types of delivery methods, i.e., insulin pens, syringes, pumps, and inhalants, provides clinicians with a solid and varied tool kit with which to treat diabetes. The downsides, however, are that 1) hypoglycemia is a constant threat, 2) proper insulin doses are not trivial to calculate, 3) compliance can vary especially in children and young adults, and 4) there can be side effects of a variety of types. Nonetheless, insulin therapy remains a mainstay treatment of diabetes.
To eliminate the downsides of insulin therapy, research in the past several decades has worked toward generating glucose-sensitive or “smart” insulin molecules. These molecules change insulin bioavailability and become active only upon high blood glucose using glucose-binding proteins such as concanavalin A, glucose oxidase to alter pH sensitivity, and phenylboronic acid (PBA), which forms reversible ester linkages with diol-containing molecules including glucose itself (reviewed in Rege et al., 2017 ). Indeed, promising recent studies included various PBA moieties covalently bonded to an acylated insulin analog (insulin detemir, which contains myristic acid coupled to Lys B29 ). The detemir allows for binding to serum albumin to prolong insulin’s half-life in the circulation, and PBA provided reversible glucose binding (Chou et al., 2015 ). The most promising of the PBA-modified conjugates showed higher potency and responsiveness in lowering blood glucose levels compared with native insulin in diabetic mouse models and decreased hypoglycemia in healthy mice, although the molecular mechanisms have not yet been determined (Chou et al., 2015 ).
An additional active area of research includes structurally defining the interaction between insulin and the insulin receptor ectodomain. Importantly, a major conformational change was discovered that may be exploited to impair insulin receptor binding under hypoglycemic conditions (Menting et al., 2013 ; Rege et al., 2017 ). Challenges in the design, testing, and execution of glucose-responsive insulins may be overcome by the adaptation of novel modeling approaches (Yang et al., 2020 ), which may allow for more rapid screening of candidate compounds.
Technologies have also progressed in the field of artificial pancreas design and development. Currently two “closed loop” systems are now available: Minimed 670G from Medtronic and Control-IQ from Tandem Diabetes Care. Both systems use a continuous glucose monitor, insulin pump, and computer algorithm to predict correct insulin doses and administer them in real time. Such algorithm systems also take into account insulin potency, the rate of blood glucose increase, and the patient’s heart rate and temperature to adjust insulin delivery levels during exercise and after a meal. In addition, so-called “artificial pancreas” systems have also been clinically tested, which use both insulin and glucagon and as such result in fewer reports of hypoglycemic episodes (El-Khatib et al., 2017 ). These types of systems will continue to become more popular as the development of room temperature–stable glucagon analogs continue, such as GVOKE by Xeris Pharmaceuticals (currently available in an injectable syringe) and Baqsimi, a nasally administered glucagon from Eli Lilly.
I. Present and Future Therapies: Beta Cell Transplantation, Replication, and Immune Protection
1. islet transplantation.
The idea to use pancreatic allo/xenografts to treat diabetes remarkably dates back to the late 1800s (Minkowski, 1892 ; Pybus, 1924 ; Williams, 1894 ). Before proceeding to the discovery of insulin (together with Best, MacLeod, and Collip), Frederick Banting also postulated the potential for transplantation of pancreatic tissue emulsions to treat diabetes in dog models in a notebook entry in 1921 (Bliss, 1982 ). Decades later, Paul Lacy, David Scharp, and colleagues successfully isolated intact functional pancreatic islets and transplanted them into rodent models (Kemp et al., 1973 ). These studies led to the initial proof of concept studies for humans, with the first successful islet transplant in a patient with T1D occurring in 1977 (Sutherland et al., 1978 ). A rapid expansion of islet transplantation, inspired by these original studies led to key observations of successfully prolonged islet engraftment by the “Edmonton protocol” whereby corticosteroid-sparing immunosuppression was applied, and islets from at least two allogeneic donors were used to achieve insulin independence (Shapiro et al., 2000 ). More recent work has focused on improving upon the efficiency and long-term engraftment of allogeneic transplants leading to more prolonged graft function (to the 5-year mark) and successful transplantation from a single islet donor (Hering et al., 2016; Hering et al., 2005 ; Rickels et al., 2013 ). Critical to these efforts to improve the success rate was the recognition that the earlier generation of immunosuppressive agents to counter tissue rejection was toxic to islets (Delaunay et al., 1997 ; Paty et al., 2002 ; Soleimanpour et al., 2010 ) and that more appropriate and less toxic agents were needed (Hirshberg et al., 2003 ; Soleimanpour et al., 2012 ).
Certainly, islet transplantation as a therapeutic approach for patients with T1D has been scrutinized due to several challenges, including (but not limited to) the lack of available donor supply to contend with demand, limited long-term functional efficacy of islet allografts, the potential for re-emergence of autoimmune islet destruction and/or metabolic overload-induced islet failure, and significant adverse effects of prolonged immunosuppression (Harlan, 2016 ). Furthermore, although islet transplantation is not currently available for individuals with T2D, simultaneous pancreas-kidney transplantation in T2D had similar favorable outcomes to simultaneous pancreas-kidney transplantation in T1D; therefore, islet-kidney transplantation may eventually be a feasible option to treat T2D, as patients will already be on immunosuppressors (Sampaio et al., 2011 ; Westerman et al., 1983 ). An additional significant obstacle is the tremendous expense associated with islet transplantation therapy. Indeed, the maintenance, operation, and utilization of an FDA-approved and Good Manufacturing Practice–compliant islet laboratory can lead to operating costs at nearly $150,000 per islet transplant, which is not cost effective for the vast majority of patients with T1D (Naftanel and Harlan, 2004 ; Wallner et al., 2016 ). At present, the focus has been to obtain FDA approval for islet allo-transplantation as a therapy for T1D to allow for insurance compensation (Hering et al., 2016; Rickels and Robertson, 2019 ). In the interim, the islet biology, stem cell, immunology, and bioengineering communities have continued the development of cell-based therapies for T1D by other approaches to overcome the challenges identified during the islet transplantation boom of the 1990s and 2000s.
2. Pharmacologic Induction of Beta Cell Replication
Besides transplantation, progress in islet cell biology and especially in developmental biology of beta cells over several decades raised the additional possibility that beta cell mass reduction in diabetes might be countered by increasing beta cell number through mitogenic means. A key method to expand pancreatic beta cell mass is through the enhancement of beta cell replication. Although the study of pancreatic beta cell replication has been an area of intense focus in the beta cell biology field for several decades, only recently has this seemed truly feasible. Seminal studies identified that human beta cells are essentially postmitotic, with a rapid phase of growth occurring in the prenatal period that dramatically tapers off shortly thereafter (Gregg et al., 2012 ; Meier et al., 2008 ). The plasticity of rodent beta cells is considerably higher than that of human beta cells (Dai et al., 2016 ), which has led to a renewed focus on validation of pharmacologic agents to enhance rodent beta cell replication using isolated and/or engrafted human islets (Bernal-Mizrachi et al., 2014 ; Kulkarni et al., 2012 ; Stewart et al., 2015 ). Indeed, a large percentage of agents that were successful when applied to rodent systems were largely unsuccessful at inducing replication in human beta cells (Bernal-Mizrachi et al., 2014 ; Kulkarni et al., 2012 ; Stewart et al., 2015 ). However, several recent studies have begun to make significant progress on successfully pushing human beta cells to replicate.
Several groups have reported successful human beta cell proliferation, both in vitro and in vivo, in response to inhibitors of the dual specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A). These inhibitors include harmine, INDY, GNF4877, 5-iodotubericidin, leucettine-42, TG003, AZ191, CC-401, and more specific, recently developed DYRK1A inhibitors (Ackeifi et al., 2020 ). Although DYRK1A is conclusively established as the important mediator of human beta cell proliferation, comprehensively determining other cellular targets and if additional gene inhibition amplifies the proliferative response is still in process. New evidence from Wang and Stewart shows dual specificity tyrosine phosphorylation-regulated kinase 1B to be an additional mitogenic target and also describes variability in the range of activated kinases within cells and/or levels of inhibition for the many DYRK1A inhibitors listed above (Ackeifi et al., 2020 ). Interestingly, opposite to these human studies, earlier mouse studies from the Scharfmann group demonstrated that Dyrk1a haploinsufficiency leads to decreased proliferation and loss of beta cell mass (Rachdi et al., 2014b ). In addition, overexpression of Dyrk1a in mice led to beta cell mass expansion with increased glucose tolerance (Rachdi et al., 2014a ).
Although important differences in beta cell proliferative capacity have been shown between human and rodent species, there are also significant differences in the mitogenic capacity of beta cells from juvenile, adult, and pregnant individuals. This demonstrates that proliferative stimuli appear to act within the complex islet, pancreas, and whole-body environments unique to each time point. For example, the administration of the hormones platelet-derived growth factor alpha or GLP-1 result in enhanced proliferation in juvenile human beta cells yet are ineffective in adult human beta cells (Chen et al., 2011 ; Dai et al., 2017 ). This has been shown to be due to a loss of platelet-derived growth factor alpha receptor expression as beta cells age but appears to be unrelated to GLP-1 receptor expression levels (Chen et al., 2011 ). Indeed, the GLP-1 receptor is highly expressed in adult beta cells, and GLP-1 secretion increases insulin secretion, as detailed previously; however, the induction of proliferative factors such as nuclear factor of activated T cells, cytoplasmic 1; forkhead box protein 1; and cyclin A1 is only seen in juvenile islets (Dai et al., 2017 ). Human studies using cadaveric pancreata from pregnant donors also showed increased beta cell mass, yet lactogenic hormones from the pituitary or placenta (prolactin, placental lactogen, or growth hormone) are unable to stimulate proliferation in human beta cells despite their ability to produce robust proliferation in mouse beta cells (reviewed in Baeyens et al., 2016 ). Experiments overexpressing mouse versus human signal transducer and activator of transcription 5, the final signaling factor inducing beta cell adaptation, in human beta cells allows for prolactin-mediated proliferation revealing fundamental differences in prolactin pathway competency in human (Chen et al., 2015 ). Overcoming the barrier of recapitulating human pregnancy’s effect on beta cells through isolating placental cells or blood serum during pregnancy may result in the discovery of a factor(s) that facilitates the increase in beta cell mass observed during human pregnancy.
Mechanisms that stimulate beta cell proliferation have also been discovered from studying genetic mutations that result in insulinomas, spontaneous insulin-producing beta cell adenomas. The most common hereditary mutation occurs in the multiple endocrine neoplasia type 1 (MEN1) gene. Indeed, administration of a MEN1 inhibitor in addition to a GLP-1 agonist (which cannot induce proliferation alone) is able to increase beta cell proliferation in isolated human islets through synergistic activation of KRAS proto-oncogene, GTPase downstream signals (Chamberlain et al., 2014 ). Interestingly, MEN1 mutations are uncommon in sporadic insulinomas, yet assaying genomic and epigenetic changes in a large cohort of non-MEN1 insulinomas found alterations in trithorax and polycomb chromatin modifying genes that were functionally related to MEN1 (Wang et al., 2017 ). Stewart and colleagues hypothesized that changes in histone 3 lysine 27 and histone 3 lysine 4 methylation status led to increased enhancer of zeste homolog 2 and lysine demethylase 6A, decreased cyclin-dependent kinase inhibitor 1C, and thereby increased beta cell proliferation, among other phenotypes. They also proposed that these findings help to explain why increased proliferation always occurs despite broad heterogeneity of mutations found between individual insulinomas (Wang et al., 2017 ).
Although factors that induce proliferation are continuing to be discovered, there are drawbacks that still limit their clinical application. Harmine and other DYRK1A inhibitors are not beta cell specific, nor have all their cellular targets been determined (Ackeifi et al., 2020 ). Targeting other pathways to induce human beta cell proliferation such as modulation of prostaglandin E2 receptors (i.e., inhibition of prostaglandin E receptor 3 alone or in combination with prostaglandin E receptor 4 activation) showed promising increases in proliferative rate yet suffers from the same lack of specificity (Carboneau et al., 2017 ). Induction of proliferation may also come at the expense of glucose sensing as in insulinomas, which have an increased expression of “disallowed genes” and alterations in glucose transporter and hexokinase expression (Wang et al., 2017 ). A further untoward consequence that must be avoided is the production of cancerous cells through unchecked proliferation. Finally, increasing beta cell mass through low rates of proliferation may increase the pool of functional insulin-secreting cells in T2D, but without additional measures, these beta cells will still ultimately be targeted for immune cell destruction in T1D.
3. Beta Cell Stress Relieving Therapies
Metabolic, inflammatory, and endoplasmic reticulum (ER) stress contribute to beta cell dysfunction and failure in both T1D and T2D. Although reduction of metabolic overload of beta cells by early exogenous insulin therapy or insulin sensitizers can temporarily reduce loss of beta cell mass/function early in diabetes, a focus on relieving ER and inflammatory stress is also of interest to preserve beta cell health.
ER stress is a well known contributor to beta cell demise both in T1D and T2D (Laybutt et al., 2007 ; Marchetti et al., 2007 ; Marhfour et al., 2012 ; Tersey et al., 2012 ) and a target of interest in the prevention of beta cell loss in both diseases. Preclinical studies suggest that the use of chemical chaperones, including 4-phenylbutyric acid and tauroursodeoxycholic acid (TUDCA), to alleviate ER stress improves beta cell function and insulin sensitivity in mouse models of T2D (Cnop et al., 2017 ; Ozcan et al., 2006 ). Furthermore, TUDCA has been shown to preserve beta cell mass and reduce ER stress in mouse models of T1D (Engin et al., 2013 ). Interestingly, TUDCA has shown promise at improving insulin action in obese nondiabetic human subjects, yet beta cell function and insulin secretion were not assessed (Kars et al., 2010 ). A clinical trial regarding the use of TUDCA for humans with new-onset T1D is also ongoing ( {"type":"clinical-trial","attrs":{"text":"NCT02218619","term_id":"NCT02218619"}} NCT02218619 ). However, a note of caution regarding use of ER chaperones is that they may prevent low level ER stress necessary to potentiate beta cell replication during states of increased insulin demand (Sharma et al., 2015 ), suggesting that the broad use of ER chaperone therapies should be carefully considered.
The blockade of inflammatory stress has long been an area of interest for treatments of both T1D and T2D (Donath et al., 2019 ; Eguchi and Nagai, 2017 ). Indeed, use of nonsteroidal anti-inflammatory drugs (NSAIDs), which block cyclooxygenase, have been observed to improve metabolic control in patients with diabetes since the turn of the 20th century (Williamson, 1901 ). Salicylates have been shown to improve insulin secretion and beta cell function in both obese human subjects and those with T2D (Fernandez-Real et al., 2008; Giugliano et al., 1985 ). However, another NSAID, salsalate, has not been shown to improve beta cell function while improving other metabolic outcomes (Kim et al., 2014 ; Penesova et al., 2015 ), possibly suggesting distinct mechanisms of action for anti-inflammatory compounds. The regular use of NSAIDs to enhance metabolic outcomes is also often limited to the tolerability of long-term use of these agents due to adverse effects. Recently, golilumab, a monoclonal antibody against the proinflammatory cytokine tumor necrosis factor alpha, was demonstrated to improve beta cell function in new-onset T1D, suggesting that targeting the underlying inflammatory milieu may have benefits to preserve beta cell mass and function in T1D (Quattrin et al., 2020). Taken together, both new and old approaches to target beta cell stressors still remain of long-term interest to improve beta cell viability and function in both T1D and T2D.
3. New Players to Induce Islet Immune Protection
Countless researchers have expended intense industry to determine T1D disease etiology and treatments focused on immunotherapy and tolerogenic methods. Multiple, highly comprehensive reviews are available describing these efforts (Goudy and Tisch, 2005 ; Rewers and Gottlieb, 2009 ; Stojanovic et al., 2017 ). Here we will focus on the protection of beta cells through programmed cell death protein-1 ligand (PD-L1) overexpression, major histocompatibility complex class I, A, B, C (HLA-A,B,C) mutated human embryonic stem cell–derived beta cells, and islet encapsulation methods.
Cancer immunotherapies that block immune checkpoints are beneficial for treating advanced stage cancers, yet induction of autoimmune diseases, including T1D, remains a potential side effect (Stamatouli et al., 2018 ; Perdigoto et al., 2019 ). A subset of these drugs target either the programmed cell death-1 protein on the surface of activated T lymphocytes or its receptor PD-L1 (Stamatouli et al., 2018 ; Perdigoto et al., 2019 ). PD-L1 expression was found in insulin-positive beta cells from T1D but not insulin-negative islets or nondiabetic islets, leading to the hypothesis that PD-L1 is upregulated in an attempt to drive immune cell attenuation (Osum et al., 2018 ; Colli et al., 2018 ). Adenoviral overexpression of PD-L1 specifically in beta cells rescued hyperglycemia in the NOD mouse model of T1D, but these animals eventually succumbed to diabetes by the study’s termination (El Khatib et al., 2015 ). A more promising report from Ben Nasr et al. ( 2017 ) demonstrated that pharmacologically or genetically induced overexpression of PD-L1 in hematopoietic stem and progenitor cells inhibited beta cell autoimmunity in the NOD mouse as well as in vitro using human hematopoietic stem and progenitor cells from patients with T1D.
As mentioned above, islet transplantation to treat T1D is limited by islet availability, cost, and the requirement for continuous immunosuppression. Islet cells generated by differentiating embryonic or induced pluripotent stem (iPS) cells could circumvent these limitations. Ideally, iPS-derived beta cells could be manipulated to eliminate the expression of polymorphic HLA-A,B,C molecules, which were found to be upregulated in T1D beta cells (Bottazzo et al., 1985 ; Richardson et al., 2016 ). These molecules allow peptide presentation to CD8+ T cells or cytotoxic T lymphocytes and may lead to beta cell removal. Interestingly, remaining insulin-positive cells in T1D donor pancreas are not HLA-A,B,C positive (Nejentsev et al., 2007; Rodriguez-Calvo et al., 2015 ). However, current differentiation protocols are still limited in their ability to produce fully glucose-responsive beta cells without transplantation into animal models to induce mature characteristics. Additionally, use of iPS-derived beta cells will still lead to concerns regarding DNA mutagenesis resulting from the methods used to obtain pluripotency or teratoma formation from cells that have escaped differentiation.
Encapsulation devices would protect islets or stem cells from immune cell infiltration while allowing for the proper exchange of nutrients and hormones. Macroencapsulation uses removable devices that would help assuage fears surrounding mutation or tumor formation; indeed, the first human trial using encapsulated hESC-derived beta cells will be completed in January 2021 ( {"type":"clinical-trial","attrs":{"text":"NCT02239354","term_id":"NCT02239354"}} NCT02239354 ). Macroencapsulation of islets prior to transplantation using various alginate-based hydrogels has historically been impeded by a strong in vivo foreign body immune response (Desai and Shea, 2017 ; Doloff et al., 2017 ; Pueyo et al., 1993 ). More recently, chemically modified forms of alginate that avoid macrophage recognition and fibrous deposition have been successfully used in rodents and for up to 6 months in nonhuman primates (Vegas et al., 2016 ). Indeed, Bochenek et al. ( 2018 ) successfully transplanted alginate protected islets for 4 months without immunosuppression in the bursa omentalis of nonhuman primates demonstrating the feasibility for this approach to be extended to humans. It remains to be seen if these devices will be successful for long-term use, perhaps decades, in patients with diabetes.
III. Summary
Although existing drug therapies using classic oral antidiabetic drugs like sulfonylureas and metformin or injected insulin remain mainstays of diabetes treatment, newer drugs based on incretin hormone actions or SGLT2 inhibitors have increased the pharmacological armamentarium available to diabetologists ( Fig. 1 ). However, the explosion of progress in beta cell biology has identified potential avenues that can increase beta cell mass in sophisticated ways by employing stem cell differentiation or enhancement of beta cell proliferation. Taken together, there should be optimism that the increased incidence of both T1D and T2D is being matched by the creativity and hard work of the diabetes research community.
Abbreviations
Authorship contributions.
Wrote and contributed to the writing of the manuscript: Satin, Soleimanpour, Walker
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [Grant R01-DK46409] (to L.S.S.), [Grant R01-DK108921] (to S.A.S.), and [Grant P30-DK020572 pilot and feasibility grant] (to S.A.S.), the Juvenile Diabetes Research Foundation (JDRF) [Grant CDA-2016-189] (to L.S.S. and S.A.S.), [Grant SRA-2018-539] (to S.A.S.), and [Grant COE-2019-861] (to S.A.S.), and the US Department of Veterans Affairs [Grant I01 BX004444] (to S.A.S.). The JDRF Career Development Award to S.A.S. is partly supported by the Danish Diabetes Academy and the Novo Nordisk Foundation.
https://doi.org/10.1124/pharmrev.120.000160
- Ackeifi C, Swartz E, Kumar K, Liu H, Chalada S, Karakose E, Scott DK, Garcia-Ocaña A, Sanchez R, DeVita RJ, et al. (2020) Pharmacologic and genetic approaches define human pancreatic β cell mitogenic targets of DYRK1A inhibitors . JCI Insight 5 :e132594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Adachi T, Yasuda K, Okamoto Y, Shihara N, Oku A, Ueta K, Kitamura K, Saito A, Iwakura I, Yamada Y, et al. (2000) T-1095, a renal Na+-glucose transporter inhibitor, improves hyperglycemia in streptozotocin-induced diabetic rats . Metabolism 49 :990–995. [ PubMed ] [ Google Scholar ]
- Alexiadou K, Tan TM (2020) Gastrointestinal peptides as therapeutic targets to mitigate obesity and metabolic syndrome . Curr Diab Rep 20 :26. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, Tsai LF, Robertson D, Jain M, Petrone M, et al. (2018) MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study . Lancet 391 :2607–2618. [ PubMed ] [ Google Scholar ]
- Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell . Prog Biophys Mol Biol 54 :87–143. [ PubMed ] [ Google Scholar ]
- Ashcroft FM, Rorsman P (1990) ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion . Biochem Soc Trans 18 :109–111. [ PubMed ] [ Google Scholar ]
- Baeyens L, Hindi S, Sorenson RL, German MS (2016) β-Cell adaptation in pregnancy . Diabetes Obes Metab 18 ( Suppl 1 ):63–70. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bailey CJ (1992) Biguanides and NIDDM . Diabetes Care 15 :755–772. [ PubMed ] [ Google Scholar ]
- Ben Nasr M, Tezza S, D’Addio F, Mameli C, Usuelli V, Maestroni A, Corradi D, Belletti S, Albarello L, Becchi G, et al. (2017) PD-L1 genetic overexpression or pharmacological restoration in hematopoietic stem and progenitor cells reverses autoimmune diabetes . Sci Transl Med 9 :eaam7543. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bergmann NC, Lund A, Gasbjerg LS, Meessen ECE, Andersen MM, Bergmann S, Hartmann B, Holst JJ, Jessen L, Christensen MB, et al. (2019) Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study . Diabetologia 62 :665–675. [ PubMed ] [ Google Scholar ]
- Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocaña A (2014) Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map . Diabetes 63 :819–831. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, et al.; EXSCEL Study Group (2018) Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis . Lancet Diabetes Endocrinol 6 :105–113. [ PubMed ] [ Google Scholar ]
- Bliss M (1982) Banting’s, Best’s, and Collip’s accounts of the discovery of insulin . Bull Hist Med 56 :554–568. [ PubMed ] [ Google Scholar ]
- Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, et al. (2018) Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques . Nat Biomed Eng 2 :810–821. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, et al. (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion . Nat Med 21 :512–517. [ PubMed ] [ Google Scholar ]
- Bonora E, Cigolini M, Bosello O, Zancanaro C, Capretti L, Zavaroni I, Coscelli C, Butturini U (1984) Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C-peptide, glucagon and growth hormone in non-diabetic subjects . Curr Med Res Opin 9 :47–51. [ PubMed ] [ Google Scholar ]
- Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR (1985) In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis . N Engl J Med 313 :353–360. [ PubMed ] [ Google Scholar ]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence . Popul Health Metr 8 :29. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, et al. (2007) Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment . Nat Med 13 :340–347. [ PubMed ] [ Google Scholar ]
- Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M (2016) The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies . Diabetes Care 39 :198–205. [ PubMed ] [ Google Scholar ]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD; Exenatide-113 Clinical Study Group (2004) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes . Diabetes Care 27 :2628–2635. [ PubMed ] [ Google Scholar ]
- Capozzi ME, Svendsen B, Encisco SE, Lewandowski SL, Martin MD, Lin H, Jaffe JL, Coch RW, Haldeman JM, MacDonald PE, et al. (2019a) β Cell tone is defined by proglucagon peptides through cAMP signaling . JCI Insight 4 :e126742. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Capozzi ME, Wait JB, Koech J, Gordon AN, Coch RW, Svendsen B, Finan B, D’Alessio DA, Campbell JE (2019b) Glucagon lowers glycemia when β-cells are active . JCI Insight 5 :e129954. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carboneau BA, Allan JA, Townsend SE, Kimple ME, Breyer RM, Gannon M (2017) Opposing effects of prostaglandin E 2 receptors EP3 and EP4 on mouse and human β-cell survival and proliferation . Mol Metab 6 :548–559. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chae H, Augustin R, Gatineau E, Mayoux E, Bensellam M, Antoine N, Khattab F, Lai BK, Brusa D, Stierstorfer B, et al. (2020) SGLT2 is not expressed in pancreatic α- and β-cells, and its inhibition does not directly affect glucagon and insulin secretion in rodents and humans . Mol Metab 42 :101071. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chamberlain CE, Scheel DW, McGlynn K, Kim H, Miyatsuka T, Wang J, Nguyen V, Zhao S, Mavropoulos A, Abraham AG, et al. (2014) Menin determines K-RAS proliferative outputs in endocrine cells . J Clin Invest 124 :4093–4101. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK (2011) PDGF signalling controls age-dependent proliferation in pancreatic β-cells . Nature 478 :349–355. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, Parsons R, Jiang J, Zhang Y, Liu H, et al. (2015) Augmented Stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human β-cells . Diabetes 64 :3784–3797. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Cha-Molstad H, Szabo A, Shalev A (2009) Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein . Am J Physiol Endocrinol Metab 296 :E1133–E1139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Hui ST, Couto FM, Mungrue IN, Davis DB, Attie AD, Lusis AJ, Davis RA, Shalev A (2008a) Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes . FASEB J 22 :3581–3594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A (2008b) Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis . Diabetes 57 :938–944. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chou DH, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG (2015) Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates . Proc Natl Acad Sci USA 112 :2401–2406. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P (2017) Endoplasmic reticulum stress and eIF2α phosphorylation: the Achilles heel of pancreatic β cells . Mol Metab 6 :1024–1039. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR (2003) Oxyntomodulin suppresses appetite and reduces food intake in humans . J Clin Endocrinol Metab 88 :4696–4701. [ PubMed ] [ Google Scholar ]
- Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, Cimino I, Yang M, Welsh P, Virtue S, et al. (2020) GDF15 mediates the effects of metformin on body weight and energy balance . Nature 578 :444–448. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Colli ML, Hill JLE, Marroquí L, Chaffey J, Dos Santos RS, Leete P, Coomans de Brachène A, Paula FMM, Op de Beeck A, Castela A, et al. (2018) PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-α and-γ via IRF1 induction . EBioMedicine 36 :367–375. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- RISE Consortium (2019) Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes . Diabetes Care 42 :1742–1751. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, Phillips N, Levy SE, Greiner DL, Shultz LD, et al. (2017) Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling . J Clin Invest 127 :3835–3844. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Kayton NS, Shostak A, Poffenberger G, Cyphert HA, Aramandla R, Thompson C, Papagiannis IG, Emfinger C, Shiota M, et al. (2016) Stress-impaired transcription factor expression and insulin secretion in transplanted human islets . J Clin Invest 126 :1857–1870. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Walker JT, Shostak A, Bouchi Y, Poffenberger G, Hart NJ, Jacobson DA, Calcutt MW, Bottino R, Greiner DL, et al. (2020) Dapagliflozin does not directly affect human α or β cells . Endocrinology 161 :bqaa080. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR (2001) Oxyntomodulin inhibits food intake in the rat . Endocrinology 142 :4244–4250. [ PubMed ] [ Google Scholar ]
- Dakin CL, Small CJ, Park AJ, Seth A, Ghatei MA, Bloom SR (2002) Repeated ICV administration of oxyntomodulin causes a greater reduction in body weight gain than in pair-fed rats . Am J Physiol Endocrinol Metab 283 :E1173–E1177. [ PubMed ] [ Google Scholar ]
- Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Gastaldelli A, Henry RR, Kitabchi AE, et al.; ACT NOW Study (2013) Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates . Diabetes 62 :3920–3926. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S (1997) Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids . J Clin Invest 100 :2094–2098. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Desai T, Shea LD (2017) Advances in islet encapsulation technologies . Nat Rev Drug Discov 16 :338–350. [ PubMed ] [ Google Scholar ]
- Devaraj S, Venkatachalam A, Chen X (2016) Metformin and the gut microbiome in diabetes . Clin Chem 62 :1554–1555. [ PubMed ] [ Google Scholar ]
- Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, et al. (2017) Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates . Nat Mater 16 :671–680. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Donath MY, Dinarello CA, Mandrup-Poulsen T (2019) Targeting innate immune mediators in type 1 and type 2 diabetes . Nat Rev Immunol 19 :734–746. [ PubMed ] [ Google Scholar ]
- Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1 . Cell Metab 27 :740–756. [ PubMed ] [ Google Scholar ]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF (1987) Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line . Proc Natl Acad Sci USA 84 :3434–3438. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK (2015) Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats . Nat Med 21 :506–511. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eguchi K, Nagai R (2017) Islet inflammation in type 2 diabetes and physiology . J Clin Invest 127 :14–23. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El Khatib MM, Sakuma T, Tonne JM, Mohamed MS, Holditch SJ, Lu B, Kudva YC, Ikeda Y (2015) β-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection . Gene Ther 22 :430–438. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, Mondesir D, Esmaeili A, Hartigan C, Thompson MJ, et al. (2017) Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial . Lancet 389 :369–380. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, Fontaine E, Almeida A, Leverve X (2008) Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons . J Mol Neurosci 34 :77–87. [ PubMed ] [ Google Scholar ]
- El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I . J Biol Chem 275 :223–228. [ PubMed ] [ Google Scholar ]
- Elrick H, Stimmler L, Hlad CJ Jr, Arai Y (1964) Plasma Insulin Response to Oral and Intravenous Glucose Administration . J Clin Endocrinol Metab 24 :1076–1082. [ PubMed ] [ Google Scholar ]
- Engin F, Yermalovich A, Nguyen T, Hummasti S, Fu W, Eizirik DL, Mathis D, Hotamisligil GS (2013) Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes [published correction appears in Sci Transl Med (2013) 5 :214er11] . Sci Transl Med 5 :211ra156. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients . BMJ 330 :1304–1305. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, et al. (2009) Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure . Mol Cell Biol 29 :2053–2067. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fernández-Real JM, López-Bermejo A, Ropero AB, Piquer S, Nadal A, Bassols J, Casamitjana R, Gomis R, Arnaiz E, Pérez I, et al. (2008) Salicylates increase insulin secretion in healthy obese subjects . J Clin Endocrinol Metab 93 :2523–2530. [ PubMed ] [ Google Scholar ]
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B (2014) Metformin: from mechanisms of action to therapies . Cell Metab 20 :953–966. [ PubMed ] [ Google Scholar ]
- Foretz M, Guigas B, Viollet B (2019) Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus . Nat Rev Endocrinol 15 :569–589. [ PubMed ] [ Google Scholar ]
- Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state . J Clin Invest 120 :2355–2369. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Frias JPBastyr EJ 3rd, Vignati L, Tschöp MH, Schmitt C, Owen K, Christensen RHDiMarchi RD (2017) The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes . Cell Metab 26 :343–352.e2. [ PubMed ] [ Google Scholar ]
- Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, Milicevic Z, Urva S, Haupt A, Robins DA (2020) Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens . Diabetes Obes Metab 22 :938–946. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, Urva S, Gimeno RE, Milicevic Z, Robins D, et al. (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial . Lancet 392 :2180–2193. [ PubMed ] [ Google Scholar ]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, et al. (2013) Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin . Nat Med 19 :1649–1654. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, et al.; REWIND Investigators (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial . Lancet 394 :121–130. [ PubMed ] [ Google Scholar ]
- Giugliano D, Ceriello A, Saccomanno F, Quatraro A, Paolisso G, D’Onofrio F (1985) Effects of salicylate, tolbutamide, and prostaglandin E2 on insulin responses to glucose in noninsulin-dependent diabetes mellitus . J Clin Endocrinol Metab 61 :160–166. [ PubMed ] [ Google Scholar ]
- Goudy KS, Tisch R (2005) Immunotherapy for the prevention and treatment of type 1 diabetes . Int Rev Immunol 24 :307–326. [ PubMed ] [ Google Scholar ]
- Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ (2012) Formation of a human β-cell population within pancreatic islets is set early in life . J Clin Endocrinol Metab 97 :3197–3206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P (2012) Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors . Diabetes Obes Metab 14 :83–90. [ PubMed ] [ Google Scholar ]
- Harlan DM (2016) Islet transplantation for hypoglycemia unawareness/severe hypoglycemia: caveat emptor . Diabetes Care 39 :1072–1074. [ PubMed ] [ Google Scholar ]
- Harrower AD (1991) Efficacy of gliclazide in comparison with other sulphonylureas in the treatment of NIDDM . Diabetes Res Clin Pract 14 ( Suppl 2 ):S65–S67. [ PubMed ] [ Google Scholar ]
- He L, Wondisford FE (2015) Metformin action: concentrations matter . Cell Metab 21 :159–162. [ PubMed ] [ Google Scholar ]
- Hedrington MS, Davis SN (2019) Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes . Expert Opin Pharmacother 20 :2229–2235. [ PubMed ] [ Google Scholar ]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, et al.; Clinical Islet Transplantation Consortium (2016) Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia . Diabetes Care 39 :1230–1240. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, et al. (2005) Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes . JAMA 293 :830–835. [ PubMed ] [ Google Scholar ]
- Hernandez AFGreen JBJanmohamed SD’Agostino RB Sr , Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MCet al.; Harmony Outcomes committees and investigators (2018) Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial . Lancet 392 :1519–1529. [ PubMed ] [ Google Scholar ]
- Hiatt WR, Kaul S, Smith RJ (2013) The cardiovascular safety of diabetes drugs--insights from the rosiglitazone experience . N Engl J Med 369 :1285–1287. [ PubMed ] [ Google Scholar ]
- Hirshberg B, Preston EH, Xu H, Tal MG, Neeman Z, Bunnell D, Soleimanpour S, Hale DA, Kirk AD, Harlan DM (2003) Rabbit antithymocyte globulin induction and sirolimus monotherapy supports prolonged islet allograft function in a nonhuman primate islet transplantation model . Transplantation 76 :55–60. [ PubMed ] [ Google Scholar ]
- Hu W, Jiang C, Guan D, Dierickx P, Zhang R, Moscati A, Nadkarni GN, Steger DJ, Loos RJF, Hu C, et al. (2019) Patient adipose stem cell-derived adipocytes reveal genetic variation that predicts antidiabetic drug response . Cell Stem Cell 24 :299–308.e6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al.; PIONEER 6 Investigators (2019) Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes . N Engl J Med 381 :841–851. [ PubMed ] [ Google Scholar ]
- Imamura M, Nakanishi K, Suzuki T, Ikegai K, Shiraki R, Ogiyama T, Murakami T, Kurosaki E, Noda A, Kobayashi Y, et al. (2012) Discovery of Ipragliflozin (ASP1941): a novel C-glucoside with benzothiophene structure as a potent and selective sodium glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes mellitus . Bioorg Med Chem 20 :3263–3279. [ PubMed ] [ Google Scholar ]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, et al.; ADOPT Study Group (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy . N Engl J Med 355 :2427–2443. [ PubMed ] [ Google Scholar ]
- Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, Kravitz BG, Herman WH, Viberti G, Holman RR; ADOPT Study Group (2011) Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT . Diabetes 60 :1552–1560. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, et al. (2010) Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women . Diabetes 59 :1899–1905. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kemp CB, Knight MJ, Scharp DW, Lacy PE, Ballinger WF (1973) Transplantation of isolated pancreatic islets into the portal vein of diabetic rats . Nature 244 :447. [ PubMed ] [ Google Scholar ]
- Kim HI, Cha JY, Kim SY, Kim JW, Roh KJ, Seong JK, Lee NT, Choi KY, Kim KS, Ahn YH (2002) Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells . Diabetes 51 :676–685. [ PubMed ] [ Google Scholar ]
- Kim HI, Kim JW, Kim SH, Cha JY, Kim KS, Ahn YH (2000) Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter . Diabetes 49 :1517–1524. [ PubMed ] [ Google Scholar ]
- Kim SH, Liu A, Ariel D, Abbasi F, Lamendola C, Grove K, Tomasso V, Ochoa H, Reaven G (2014) Effect of salsalate on insulin action, secretion, and clearance in nondiabetic, insulin-resistant individuals: a randomized, placebo-controlled study . Diabetes Care 37 :1944–1950. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koffert JP, Mikkola K, Virtanen KA, Andersson AD, Faxius L, Hällsten K, Heglind M, Guiducci L, Pham T, Silvola JMU, et al. (2017) Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial . Diabetes Res Clin Pract 131 :208–216. [ PubMed ] [ Google Scholar ]
- Koh A, Mannerås-Holm L, Yunn NO, Nilsson PM, Ryu SH, Molinaro A, Perkins R, Smith JG, Bäckhed F (2020) Microbial imidazole propionate affects responses to metformin through p38γ-dependent inhibitory AMPK phosphorylation . Cell Metab 32 :643–653.e4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kreymann B, Williams G, Ghatei MA, Bloom SR (1987) Glucagon-like peptide-1 7-36: a physiological incretin in man . Lancet 2 :1300–1304. [ PubMed ] [ Google Scholar ]
- Kuhre RE, Ghiasi SM, Adriaenssens AE, Wewer Albrechtsen NJ, Andersen DB, Aivazidis A, Chen L, Mandrup-Poulsen T, Ørskov C, Gribble FM, et al. (2019) No direct effect of SGLT2 activity on glucagon secretion . Diabetologia 62 :1011–1023. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF (2012) Human β-cell proliferation and intracellular signaling: driving in the dark without a road map . Diabetes 61 :2205–2213. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kurosaki E, Ogasawara H (2013) Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data . Pharmacol Ther 139 :51–59. [ PubMed ] [ Google Scholar ]
- Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C, Trüb T (2000) Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use . Diabetes 49 :999–1005. [ PubMed ] [ Google Scholar ]
- Lambeir AM, Scharpé S, De Meester I (2008) DPP4 inhibitors for diabetes--what next? Biochem Pharmacol 76 :1637–1643. [ PubMed ] [ Google Scholar ]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes . Diabetologia 50 :752–763. [ PubMed ] [ Google Scholar ]
- Lebovitz HE (2019) Thiazolidinediones: the forgotten diabetes medications . Curr Diab Rep 19 :151. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, Ehrlich SF, Mamtani R, Bilker W, Vaughn DJ, et al. (2015) Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes . JAMA 314 :265–277. [ PubMed ] [ Google Scholar ]
- Li L, Li S, Deng K, Liu J, Vandvik PO, Zhao P, Zhang L, Shen J, Bala MM, Sohani ZN, et al. (2016) Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies . BMJ 352 :i610. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Link JT (2003) Pharmacological regulation of hepatic glucose production . Curr Opin Investig Drugs 4 :421–429. [ PubMed ] [ Google Scholar ]
- Luippold G, Klein T, Mark M, Grempler R (2012) Empagliflozin, a novel potent and selective SGLT-2 inhibitor, improves glycaemic control alone and in combination with insulin in streptozotocin-induced diabetic rats, a model of type 1 diabetes mellitus . Diabetes Obes Metab 14 :601–607. [ PubMed ] [ Google Scholar ]
- Maganti AV, Tersey SA, Syed F, Nelson JB, Colvin SC, Maier B, Mirmira RG (2016) Peroxisome proliferator-activated receptor-γ activation augments the β-cell unfolded protein response and rescues early glycemic deterioration and β cell death in non-obese diabetic mice . J Biol Chem 291 :22524–22533. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Makrilakis K (2019) The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: when to select, what to expect . Int J Environ Res Public Health 16 :2720. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M (2007) The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients . Diabetologia 50 :2486–2494. [ PubMed ] [ Google Scholar ]
- Marhfour I, Lopez XM, Lefkaditis D, Salmon I, Allagnat F, Richardson SJ, Morgan NG, Eizirik DL (2012) Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes . Diabetologia 55 :2417–2420. [ PubMed ] [ Google Scholar ]
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al.; SUSTAIN-6 Investigators (2016a) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes . N Engl J Med 375 :1834–1844. [ PubMed ] [ Google Scholar ]
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al.; LEADER Steering Committee; LEADER Trial Investigators (2016b) Liraglutide and cardiovascular outcomes in type 2 diabetes . N Engl J Med 375 :311–322. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Massollo M, Marini C, Brignone M, Emionite L, Salani B, Riondato M, Capitanio S, Fiz F, Democrito A, Amaro A, et al. (2013) Metformin temporal and localized effects on gut glucose metabolism assessed using 18F-FDG PET in mice . J Nucl Med 54 :259–266. [ PubMed ] [ Google Scholar ]
- McCreight LJ, Bailey CJ, Pearson ER (2016) Metformin and the gastrointestinal tract . Diabetologia 59 :426–435. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McIntyre N, Holdsworth CD, Turner DS (1964) New interpretation of oral glucose tolerance . Lancet 2 :20–21. [ PubMed ] [ Google Scholar ]
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al.; DAPA-HF Trial Committees and Investigators (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction . N Engl J Med 381 :1995–2008. [ PubMed ] [ Google Scholar ]
- Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC (2008) Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans . Diabetes 57 :1584–1594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meng W, Ellsworth BA, Nirschl AA, McCann PJ, Patel M, Girotra RN, Wu G, Sher PM, Morrison EP, Biller SA, et al. (2008) Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes . J Med Chem 51 :1145–1149. [ PubMed ] [ Google Scholar ]
- Menting JG, Whittaker J, Margetts MB, Whittaker LJ, Kong GK, Smith BJ, Watson CJ, Záková L, Kletvíková E, Jiráček J, et al. (2013) How insulin engages its primary binding site on the insulin receptor . Nature 493 :241–245. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ (2013) Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP . Nature 494 :256–260. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Minkowski O (1892) Weitere Mitteilungen über den Diabetes mellitus nach Extirpation des Pankreas . Berliner Klinische Wochenschrift 29 :90–93. [ Google Scholar ]
- Misbin RI (2004) The phantom of lactic acidosis due to metformin in patients with diabetes . Diabetes Care 27 :1791–1793. [ PubMed ] [ Google Scholar ]
- Mudaliar S, Armstrong DA, Mavian AA, O’Connor-Semmes R, Mydlow PK, Ye J, Hussey EK, Nunez DJ, Henry RR, Dobbins RL (2012) Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes . Diabetes Care 35 :2198–2200. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, Brubaker PL (2011) Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell . Endocrinology 152 :4610–4619. [ PubMed ] [ Google Scholar ]
- Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, et al. (2019) Glucagon-like peptide 1 (GLP-1) . Mol Metab 30 :72–130. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Munir KM, Lamos EM (2017) Diabetes type 2 management: what are the differences between DPP-4 inhibitors and how do you choose? Expert Opin Pharmacother 18 :839–841. [ PubMed ] [ Google Scholar ]
- Naftanel MA, Harlan DM (2004) Pancreatic islet transplantation . PLoS Med 1 :e58. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W (1986a) Reduced incretin effect in type 2 (non-insulin-dependent) diabetes . Diabetologia 29 :46–52. [ PubMed ] [ Google Scholar ]
- Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W (1986b) Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses . J Clin Endocrinol Metab 63 :492–498. [ PubMed ] [ Google Scholar ]
- Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, et al.; Wellcome Trust Case Control Consortium (2007) Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A . Nature 450 :887–892. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nichols CG (2006) KATP channels as molecular sensors of cellular metabolism . Nature 440 :470–476. [ PubMed ] [ Google Scholar ]
- Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, Yamamoto Y, Ueta K, Kimata H, Nakayama K, et al. (2010) Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus . J Med Chem 53 :6355–6360. [ PubMed ] [ Google Scholar ]
- Ohnishi ST, Endo M, editors. (1981) The Mechanism of Gated Calcium Transport Across Biological Membranes , Academic Press, New York. [ Google Scholar ]
- Osipovich AB, Stancill JS, Cartailler JP, Dudek KD, Magnuson MA (2020) Excitotoxicity and overnutrition additively impair metabolic function and identity of pancreatic β-cells . Diabetes 69 :1476–1491. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Osum KC, Burrack AL, Martinov T, Sahli NL, Mitchell JS, Tucker CG, Pauken KE, Papas K, Appakalai B, Spanier JA, et al. (2018) Interferon-gamma drives programmed death-ligand 1 expression on islet β cells to limit T cell function during autoimmune diabetes . Sci Rep 8 :8295. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain . Biochem J 348 :607–614. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ozanne SE, Guest PC, Hutton JC, Hales CN (1995) Intracellular localization and molecular heterogeneity of the sulphonylurea receptor in insulin-secreting cells . Diabetologia 38 :277–282. [ PubMed ] [ Google Scholar ]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes . Science 313 :1137–1140. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paty BW, Harmon JS, Marsh CL, Robertson RP (2002) Inhibitory effects of immunosuppressive drugs on insulin secretion from HIT-T15 cells and Wistar rat islets . Transplantation 73 :353–357. [ PubMed ] [ Google Scholar ]
- Penesova A, Koska J, Ortega E, Bunt JC, Bogardus C, de Courten B (2015) Salsalate has no effect on insulin secretion but decreases insulin clearance: a randomized, placebo-controlled trial in subjects without diabetes . Diabetes Obes Metab 17 :608–612. [ PubMed ] [ Google Scholar ]
- Perdigoto AL, Quandt Z, Anderson M, Herold KC (2019) Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome . Lancet Diabetes Endocrinol 7 :421–423. [ PubMed ] [ Google Scholar ]
- Perley MJ, Kipnis DM (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects . J Clin Invest 46 :1954–1962. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pernicova I, Korbonits M (2014) Metformin--mode of action and clinical implications for diabetes and cancer . Nat Rev Endocrinol 10 :143–156. [ PubMed ] [ Google Scholar ]
- Preiss D, Dawed A, Welsh P, Heggie A, Jones AG, Dekker J, Koivula R, Hansen TH, Stewart C, Holman RR, et al.; DIRECT consortium group (2017) Sustained influence of metformin therapy on circulating glucagon-like peptide-1 levels in individuals with and without type 2 diabetes . Diabetes Obes Metab 19 :356–363. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes . J Clin Invest 116 :1802–1812. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pueyo ME, Darquy S, Capron F, Reach G (1993) In vitro activation of human macrophages by alginate-polylysine microcapsules . J Biomater Sci Polym Ed 5 :197–203. [ PubMed ] [ Google Scholar ]
- Pybus F (1924) Notes on suprarenal and pancreatic grafting . Lancet 204 :550–551. [ Google Scholar ]
- Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, Leu JH, Zoka R, Hedrick JA, Rigby MR, et al.; T1GER Study Investigators (2020) Golimumab and Beta-Cell Function in Youth with New-Onset Type 1 Diabetes . N Engl J Med 383 :2007–2017. [ PubMed ] [ Google Scholar ]
- Rachdi L, Kariyawasam D, Aïello V, Herault Y, Janel N, Delabar JM, Polak M, Scharfmann R (2014a) Dyrk1A induces pancreatic β cell mass expansion and improves glucose tolerance . Cell Cycle 13 :2221–2229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rachdi L, Kariyawasam D, Guez F, Aïello V, Arbonés ML, Janel N, Delabar JM, Polak M, Scharfmann R (2014b) Dyrk1a haploinsufficiency induces diabetes in mice through decreased pancreatic beta cell mass . Diabetologia 57 :960–969. [ PubMed ] [ Google Scholar ]
- Rege NK, Phillips NFB, Weiss MA (2017) Development of glucose-responsive ‘smart’ insulin systems . Curr Opin Endocrinol Diabetes Obes 24 :267–278. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin . Diabetologia 60 :1577–1585. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rewers M, Gottlieb P (2009) Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future . Diabetes Care 32 :1769–1782. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA, Zeissler M, Leete P, Krogvold L, Dahl-Jørgensen K, et al. (2016) Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes . Diabetologia 59 :2448–2458. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rickels MR, Liu C, Shlansky-Goldberg RD, Soleimanpour SA, Vivek K, Kamoun M, Min Z, Markmann E, Palangian M, Dalton-Bakes C, et al. (2013) Improvement in β-cell secretory capacity after human islet transplantation according to the c7 protocol . Diabetes 62 :2890–2897. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rickels MR, Robertson RP (2019) Pancreatic islet transplantation in humans: recent progress and future directions . Endocr Rev 40 :631–668. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rodriguez-Calvo T, Suwandi JS, Amirian N, Zapardiel-Gonzalo J, Anquetil F, Sabouri S, von Herrath MG (2015) Heterogeneity and lobularity of pancreatic pathology in type 1 diabetes during the prediabetic phase . J Histochem Cytochem 63 :626–636. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rojas LB, Gomes MB (2013) Metformin: an old but still the best treatment for type 2 diabetes . Diabetol Metab Syndr 5 :6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, et al. (2003) Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis . Mol Cell Biol 23 :7222–7229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosenstock J, Hassman DR, Madder RD, Brazinsky SA, Farrell J, Khutoryansky N, Hale PM; Repaglinide Versus Nateglinide Comparison Study Group (2004) Repaglinide versus nateglinide monotherapy: a randomized, multicenter study . Diabetes Care 27 :1265–1270. [ PubMed ] [ Google Scholar ]
- Sampaio MS, Kuo HT, Bunnapradist S (2011) Outcomes of simultaneous pancreas-kidney transplantation in type 2 diabetic recipients . Clin J Am Soc Nephrol 6 :1198–1206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Saponaro C, Gmyr V, Thévenet J, Moerman E, Delalleau N, Pasquetti G, Coddeville A, Quenon A, Daoudi M, Hubert T, et al. (2019) The GLP1R agonist liraglutide reduces hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin via somatostatin release . Cell Rep 28 :1447–1454.e4. [ PubMed ] [ Google Scholar ]
- Saponaro C, Mühlemann M, Acosta-Montalvo A, Piron A, Gmyr V, Delalleau N, Moerman E, Thévenet J, Pasquetti G, Coddeville A, et al. (2020) Interindividual heterogeneity of SGLT2 expression and function in human pancreatic islets . Diabetes 69 :902–914. [ PubMed ] [ Google Scholar ]
- Satin LS, Tavalin SJ, Kinard TA, Teague J (1995) Contribution of L- and non-L-type calcium channels to voltage-gated calcium current and glucose-dependent insulin secretion in HIT-T15 cells . Endocrinology 136 :4589–4601. [ PubMed ] [ Google Scholar ]
- Schwartz AV, Chen H, Ambrosius WT, Sood A, Josse RG, Bonds DE, Schnall AM, Vittinghoff E, Bauer DC, Banerji MA, et al. (2015) Effects of TZD use and discontinuation on fracture rates in ACCORD Bone Study . J Clin Endocrinol Metab 100 :4059–4066. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shalev A, Pise-Masison CA, Radonovich M, Hoffmann SC, Hirshberg B, Brady JN, Harlan DM (2002) Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway . Endocrinology 143 :3695–3698. [ PubMed ] [ Google Scholar ]
- Shankar SS, Shankar RR, Mixson LA, Miller DL, Pramanik B, O’Dowd AK, Williams DM, Frederick CB, Beals CR, Stoch SA, et al. (2018) Native oxyntomodulin has significant glucoregulatory effects independent of weight loss in obese humans with and without type 2 diabetes . Diabetes 67 :1105–1112. [ PubMed ] [ Google Scholar ]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen . N Engl J Med 343 :230–238. [ PubMed ] [ Google Scholar ]
- Sharma RB, O’Donnell AC, Stamateris RE, Ha B, McCloskey KM, Reynolds PR, Arvan P, Alonso LC (2015) Insulin demand regulates β cell number via the unfolded protein response . J Clin Invest 125 :3831–3846. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sims EK, Mirmira RG, Evans-Molina C (2020) The role of beta-cell dysfunction in early type 1 diabetes . Curr Opin Endocrinol Diabetes Obes 27 :215–224. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soccio RE, Chen ER, Rajapurkar SR, Safabakhsh P, Marinis JM, Dispirito JR, Emmett MJ, Briggs ER, Fang B, Everett LJ, et al. (2015) Genetic variation determines PPARγ function and anti-diabetic drug response in vivo . Cell 162 :33–44. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De León DD, Naji A, Kushner JA, et al. (2010) Calcineurin signaling regulates human islet beta-cell survival . J Biol Chem 285 :40050–40059. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Hirshberg B, Bunnell DJ, Sumner AE, Ader M, Remaley AT, Rother KI, Rickels MR, Harlan DM (2012) Metabolic function of a suboptimal transplanted islet mass in nonhuman primates on rapamycin monotherapy . Cell Transplant 21 :1297–1304. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Stoffers DA (2013) The pancreatic β cell and type 1 diabetes: innocent bystander or active participant? Trends Endocrinol Metab 24 :324–331. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, et al. (2018) Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors . Diabetes 67 :1471–1480. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stancill JS, Cartailler JP, Clayton HW, O’Connor JT, Dickerson MT, Dadi PK, Osipovich AB, Jacobson DA, Magnuson MA (2017) Chronic β-cell depolarization impairs β-cell identity by disrupting a network of Ca 2+ -regulated genes . Diabetes 66 :2175–2187. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stewart AF, Hussain MA, García-Ocaña A, Vasavada RC, Bhushan A, Bernal-Mizrachi E, Kulkarni RN (2015) Human β-cell proliferation and intracellular signaling: part 3 . Diabetes 64 :1872–1885. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stojanovic I, Dimitrijevic M, Vives-Pi M, Mansilla MJ, Pujol-Autonell I, Rodríguez-Fernandez S, Palova-Jelínkova L, Funda DP, Gruden-Movsesijan A, Sofronic-Milosavljevic L, et al. (2017) Cell-based tolerogenic therapy, experience from animal models of multiple sclerosis, type 1 diabetes and rheumatoid arthritis . Curr Pharm Des 23 :2623–2643. [ PubMed ] [ Google Scholar ]
- Sturek JM, Castle JD, Trace AP, Page LC, Castle AM, Evans-Molina C, Parks JS, Mirmira RG, Hedrick CC (2010) An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic beta cells . J Clin Invest 120 :2575–2589. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Suga T, Kikuchi O, Kobayashi M, Matsui S, Yokota-Hashimoto H, Wada E, Kohno D, Sasaki T, Takeuchi K, Kakizaki S, et al. (2019) SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels . Mol Metab 19 :1–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, et al. (2018) Gut microbiota and intestinal FXR mediate the clinical benefits of metformin . Nat Med 24 :1919–1929. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sutherland DE, Matas AJ, Najarian JS (1978) Pancreatic islet cell transplantation . Surg Clin North Am 58 :365–382. [ PubMed ] [ Google Scholar ]
- Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, Mirmira RG (2012) Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model . Diabetes 61 :818–827. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thielen LA, Chen J, Jing G, Moukha-Chafiq O, Xu G, Jo S, Grayson TB, Lu B, Li P, Augelli-Szafran CE, et al. (2020) Identification of an anti-diabetic, orally available small molecule that regulates TXNIP expression and glucagon action . Cell Metab 32 :353–365.e8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tillner J, Posch MG, Wagner F, Teichert L, Hijazi Y, Einig C, Keil S, Haack T, Wagner M, Bossart M, et al. (2019) A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials . Diabetes Obes Metab 21 :120–128. [ PubMed ] [ Google Scholar ]
- Vallon V (2015) The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus . Annu Rev Med 66 :255–270. [ PubMed ] [ Google Scholar ]
- Vasseur M, Debuyser A, Joffre M (1987) Sensitivity of pancreatic beta cell to calcium channel blockers. An electrophysiologic study of verapamil and nifedipine . Fundam Clin Pharmacol 1 :95–113. [ PubMed ] [ Google Scholar ]
- Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, et al. (2016) Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates . Nat Biotechnol 34 :345–352. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, Asterholm IW, Benrick A, Briant LJB, Chibalina MV, et al. (2019) Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion . Nat Commun 10 :139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wallner K, Shapiro AM, Senior PA, McCabe C (2016) Cost effectiveness and value of information analyses of islet cell transplantation in the management of ‘unstable’ type 1 diabetes mellitus . BMC Endocr Disord 16 :17. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang H, Bender A, Wang P, Karakose E, Inabnet WB, Libutti SK, Arnold A, Lambertini L, Stang M, Chen H, et al. (2017) Insights into beta cell regeneration for diabetes via integration of molecular landscapes in human insulinomas . Nat Commun 8 :767. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, Radovick S, Hussain M, Maheshwari A, Wondisford FE, O’Rourke B, He L (2019) Metformin improves mitochondrial respiratory activity through activation of AMPK . Cell Rep 29 :1511–1523.e5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Westerman J, Wirtz KW, Berkhout T, van Deenen LL, Radhakrishnan R, Khorana HG (1983) Identification of the lipid-binding site of phosphatidylcholine-transfer protein with phosphatidylcholine analogs containing photoactivable carbene precursors . Eur J Biochem 132 :441–449. [ PubMed ] [ Google Scholar ]
- World Health Organization (2020) World Health Organization Diabetes Fact Sheet . [ Google Scholar ]
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, et al. (2020) Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist . JCI Insight 5 :e140532. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Williams P (1894) Notes on diabetes treated with extract and by grafts of sheep’s pancreas . BMJ 2 :1303–1304. [ Google Scholar ]
- Williamson RT (1901) On the treatment of glycosuria and diabetes mellitus with sodium salicylate . BMJ 1 :760–762. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Witters LA (2001) The blooming of the French lilac . J Clin Invest 108 :1105–1107. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al.; DECLARE–TIMI 58 Investigators (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes . N Engl J Med 380 :347–357. [ PubMed ] [ Google Scholar ]
- Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, et al. (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug . Nat Med 23 :850–858. [ PubMed ] [ Google Scholar ]
- Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, et al. (2005) Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial . Diabetes 54 :2390–2395. [ PubMed ] [ Google Scholar ]
- Xu G, Chen J, Jing G, Shalev A (2012) Preventing β-cell loss and diabetes with calcium channel blockers . Diabetes 61 :848–856. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yang JF, Gong X, Bakh NA, Carr K, Phillips NFB, Ismail-Beigi F, Weiss MA, Strano MS (2020) Connecting rodent and human pharmacokinetic models for the design and translation of glucose-responsive insulin . Diabetes 69 :1815–1826. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yu O, Azoulay L, Yin H, Filion KB, Suissa S (2018) Sulfonylureas as initial treatment for type 2 diabetes and the risk of severe hypoglycemia . Am J Med 131 :317.e11–317.e22. [ PubMed ] [ Google Scholar ]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action . J Clin Invest 108 :1167–1174. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al.; EMPA-REG OUTCOME Investigators (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes . N Engl J Med 373 :2117–2128. [ PubMed ] [ Google Scholar ]
Type 2 Diabetes Research At-a-Glance
The ADA is committed to continuing progress in the fight against type 2 diabetes by funding research, including support for potential new treatments, a better understating of genetic factors, addressing disparities, and more. For specific examples of projects currently funded by the ADA, see below.
Greg J. Morton, PhD
University of Washington
Project: Neurocircuits regulating glucose homeostasis
“The health consequences of diabetes can be devastating, and new treatments and therapies are needed. My research career has focused on understanding how blood sugar levels are regulated and what contributes to the development of diabetes. This research will provide insights into the role of the brain in the control of blood sugar levels and has potential to facilitate the development of novel approaches to diabetes treatment.”
The problem: Type 2 diabetes (T2D) is among the most pressing and costly medical challenges confronting modern society. Even with currently available therapies, the control and management of blood sugar levels remains a challenge in T2D patients and can thereby increase the risk of diabetes-related complications. Continued progress with newer, better therapies is needed to help people with T2D.
The project: Humans have special cells, called brown fat cells, which generate heat to maintain optimal body temperature. Dr. Morton has found that these cells use large amounts of glucose to drive this heat production, thus serving as a potential way to lower blood sugar, a key goal for any diabetes treatment. Dr. Morton is working to understand what role the brain plays in turning these brown fat cells on and off.
The potential outcome: This work has the potential to fundamentally advance our understanding of how the brain regulates blood sugar levels and to identify novel targets for the treatment of T2D.
Tracey Lynn McLaughlin, MD
Stanford University
Project: Role of altered nutrient transit and incretin hormones in glucose lowering after Roux-en-Y gastric bypass surgery
“This award is very important to me personally not only because the enteroinsular axis (gut-insulin-glucose metabolism) is a new kid on the block that requires rigorous physiologic studies in humans to better understand how it contributes to glucose metabolism, but also because the subjects who develop severe hypoglycemia after gastric bypass are largely ignored in society and there is no treatment for this devastating and very dangerous condition.”
The problem: Roux-en-Y gastric bypass (RYGB) surgery is the single-most effective treatment for type 2 diabetes, with persistent remission in 85% of cases. However, the underlying ways by which the surgery improves glucose control is not yet understood, limiting the ability to potentially mimic the surgery in a non-invasive way. Furthermore, a minority of RYGB patients develop severe, disabling, and life-threatening low-blood sugar, for which there is no current treatment.
The project: Utilizing a unique and rigorous human experimental model, the proposed research will attempt to gain a better understanding on how RYGB surgery improves glucose control. Dr. McLaughlin will also test a hypothesis which she believes could play an important role in the persistent low-blood sugar that is observed in some patients post-surgery.
The potential outcome: This research has the potential to identify novel molecules that could represent targets for new antidiabetic therapies. It is also an important step to identifying people at risk for low-blood sugar following RYGB and to develop postsurgical treatment strategies.
Rebekah J. Walker, PhD
Medical College of Wisconsin
Project: Lowering the impact of food insecurity in African Americans with type 2 diabetes
“I became interested in diabetes research during my doctoral training, and since that time have become passionate about addressing social determinants of health and health disparities, specifically in individuals with diabetes. Living in one of the most racially segregated cities in the nation, the burden to address the needs of individuals at particularly high risk of poor outcomes has become important to me both personally and professionally.”
The problem: Food insecurity is defined as the inability to or limitation in accessing nutritionally adequate food and may be one way to address increased diabetes risk in high-risk populations. Food insecure individuals with diabetes have worse diabetes outcomes and have more difficulty following a healthy diet compared to those who are not food insecure.
The project: Dr. Walker’s study will gather information to improve and then will test an intervention to improve blood sugar control, dietary intake, self-care management, and quality of life in food insecure African Americans with diabetes. The intervention will include weekly culturally appropriate food boxes mailed to the participants and telephone-delivered diabetes education and skills training. It will be one of the first studies focused on the unique needs of food insecure African American populations with diabetes using culturally tailored strategies.
The potential outcome: This study has the potential to guide and improve policies impacting low-income minorities with diabetes. In addition, Dr. Walker’s study will help determine if food supplementation is important in improving diabetes outcomes beyond diabetes education alone.
Donate Today and Change Lives!
These New Developments Could Make Living With Type 2 Diabetes More Manageable
E xperts often talk about the “burden” of a disease or illness. The word acts as a tidy container for all the unpleasantness people with that condition may experience—from their symptoms, to the cost of their care, to the restrictions imposed on their lifestyle, to the health complications that may arise. For people with Type 2 diabetes , this burden can be high.
Routine management of Type 2 diabetes often involves major changes to one’s diet and physical activity . And for many, especially those taking insulin to manage their blood sugar, the disease can necessitate daily blood-glucose monitoring, a process that entails pricking a finger to draw blood and then dabbing that blood onto a glucose monitor’s test strip. Doing this several times a week—month after month—can present overlapping challenges. According to a 2013 survey in the journal Diabetes Spectrum, people find finger-prick glucose monitoring to be painful, and the results can be confusing or unhelpful.
“Patients don’t want to prick their fingers, and they come in all the time and say, ‘I’m tired of this,’” says Dr. Francisco Pasquel, a diabetes specialist and associate professor of medicine at Emory University School of Medicine in Atlanta.
But relief is on the way. Continuous glucose monitors, or CGMs, are small devices-—often about the size of a quarter-—that use a small under-the-skin needle to continuously monitor blood-glucose levels. This information can be transmitted—in some cases wirelessly and automatically—to a smartphone app or other device. “You can look at glucose levels for a single point in time, but you can also look at trends in values over time,” says Dr. Roy Beck, medical director of the nonprofit Jaeb Center for Health Research in Tampa. Beck’s work has found that continuous glucose monitoring may provide a number of benefits for people with Type 2 diabetes.
These monitors are just one of several new advancements in Type 2 diabetes care and management. From connected technologies to new drug treatments, medical science is making steady and sometimes life-changing progress in the treatment of this condition. Here, experts describe some of the latest and greatest developments.
Continuous glucose monitors
People with Type 1 diabetes typically have to check their blood-sugar levels on a daily basis, or even multiple times each day. Because testing is such a big part of managing that disease, the research on continuous glucose monitors started with these patients. That work has shown that CGMs provide multiple benefits, including reduced hemoglobin A1C (HbA1c) levels, which is an important measure of healthy blood glucose. Continuous glucose monitors are now being studied in people with Type 2 diabetes, and research points to multiple benefits.
For a study published in 2021 in the Journal of the American Medical Association, Beck and his colleagues compared continuous glucose monitoring to standard finger-prick tests among people with Type 2 diabetes who were using insulin. They found that continuous monitoring was associated with a significantly greater drop in HbA1c. They also found that continuous monitoring helped people avoid risky and severe drops in blood sugar (a.k.a. hypoglycemia). “It’s pretty clear that there’s a benefit for people with Type 2 diabetes who are using insulin,” he says.
More than 90% of people with diabetes have Type 2 diabetes, and Beck says that roughly 30% of these people are using insulin. In other words, there are many people with Type 2 diabetes who stand to benefit from continuous glucose monitoring. However, use of these monitors is still mostly confined to people with Type 1 diabetes. “Use is slowly increasing in Type 2 patients, but I think it’s still too low considering this is a non-pharmacological approach”—something many people prefer because it avoids the side effects of medications—“that can help people,” he says.
Even for people with Type 2 diabetes who are not taking insulin, Beck says that continuous glucose monitoring could be helpful. “There’s a need for more studies to prove it, but it makes sense that it would likely have benefits,” he says. For example, monitoring blood sugar in real time could help people make diet or lifestyle changes that reduce their risks for long-term health complications. “Normally, blood glucose following a meal shouldn’t go above 140 [mg/dL],” he says. But based on factors like diet, meal timing, and exercise habits, someone with Type 2 diabetes may experience post-meal blood-sugar spikes that surpass 200 or even 300 mg/dL. These spikes could cause few symptoms or short-term consequences, Beck says, but over time they can contribute to the development of common diabetes-related complications such as kidney failure, heart disease, or diabetic retinopathy (an eye condition that can cause blurry vision or blindness). “The first time people use these continuous monitors, it can be a real eye-opener,” he adds. “I think they could be most helpful for self-management, and Type 2 diabetes is a disease where self-management through diet and exercise can make a huge difference.”
Other experts second this. “Patients using these devices can receive a graph of their glucose values over time, which helps them understand the effects of nutrition on glucose control, or how they could modify their exercise to make improvements,” says Dr. Ilias Spanakis, an associate professor of medicine in the division of endocrinology, diabetes, and nutrition at the University of Maryland School of Medicine.
For patients who are reliant on insulin to manage their blood glucose, combining continuous glucose monitors with insulin pumps—devices that automatically inject insulin as needed—could also lead to major improvements. “Smart algorithms that connect the two can automatically adjust glucose based on glucose values,” Spanakis says. This is already possible, and it’s likely to become much more commonplace, he adds.
For many people with diabetes, continuous glucose monitoring could provide a safer and simpler path forward.
Read More: The Link Between Type 2 Diabetes and Psychiatric Disorders
Bariatric surgery for Type 2 diabetes
Historically, bariatric (weight-loss) surgery has been used primarily to help people manage severe obesity, which the U.S. Centers for Disease Control and Prevention defines as a BMI of 40 or higher. Many people who are severely obese also have diabetes, and research has found that these surgical procedures can help reduce the burden of Type 2 diabetes or even send it into remission.
A 2018 study from researchers at the University of Oklahoma found that Roux-en-Y gastric bypass surgery, a common bariatric procedure, vastly outperformed typical medical management techniques—such as diet changes, doctor’s visits, and prescription drugs—among people with Type 2 diabetes. Surgery led to diabetes remission in roughly 28% of patients, compared with a remission rate of just 4% among the non-surgery group, according to the study results. More research has found that bariatric surgery may effectively send Type 2 diabetes into remission.
“Surgery does not just lead to weight loss, but also to an improvement in glycemic control, which happens even before the weight loss occurs,” says Emory’s Pasquel, who has published work on the benefits of bariatric surgery for people with Type 2 diabetes. Exactly how the surgery does this isn’t well understood, he says. However, bariatric surgery affects appetite, food intake, caloric absorption, and multiple neuroendocrine pathways—all of which could contribute to its beneficial actions for people with Type 2 diabetes.
In the future, Pasquel says these procedures are likely to become more commonplace even for people with Type 2 diabetes who are not severely obese.
More from TIME
New pharmaceutical drugs.
There are a lot of different diabetes drugs on the market, each with its own risks and benefits. But experts say two types are emerging as potential “game changers” when it comes to Type 2 diabetes treatment.
Glucagon-like peptide 1 (GLP-1) is a hormone released in the gut during digestion—one that plays a role in blood-sugar homeostasis. A class of drugs known as GLP-1 receptor agonists can interact with GLP-1 receptors in ways that lower appetite, slow digestion, and provide other benefits for people with Type 2 diabetes. These GLP-1 drugs aren’t new. But Pasquel says the latest versions are different in that they work on two different receptors, not one. “Recent evidence shows that activating both receptors has a remarkable impact on weight loss and glycemic control,” he says. Especially for people with Type 2 diabetes who are at high risk for heart or arterial disease, he says that these new drugs seem to be a big upgrade over previous medications.
A second category of drug has also emerged as a standout in the treatment of Type 2 diabetes. Known as sodium-glucose cotransporter-2 (SGLT-2) inhibitors, these drugs help the kidneys remove sugar from a person’s blood. Not only does this improve blood-sugar control in people with Type 2 diabetes, but it also helps protect them from heart failure and kidney disease—two common and serious complications. Pasquel says these drugs are so effective that they’re now being used in people with heart failure or kidney disease who do not have Type 2 diabetes.
Read More: The Truth About Fasting and Type 2 Diabetes
Emerging ways to think about weight loss
Experts have long understood that weight loss can help people reduce their Type 2 diabetes symptoms and risks . This recognition has led to research on a number of weight-loss diets . More research is needed, but some of the latest studies suggest that fasting plans—in particular, intermittent fasting—may be particularly beneficial for people with Type 2 diabetes.
Intermittent fasting involves cutting out calorie-containing foods and drinks for an extended period of time—anywhere from 12 hours to two days depending on the approach a person chooses. A 2019 research review in the journal Nutrients found that intermittent fasting promotes weight loss, increases insulin sensitivity, and reduces insulin levels in the blood. All of this is helpful for people with Type 2 diabetes. “Essentially, fasting is doing what we prescribe diabetes medications to do, which is to improve insulin sensitivity,” says Benjamin Horne, director of cardiovascular and genetic epidemiology at Intermountain Healthcare in Utah.
It’s not yet clear which form of intermittent fasting is best. But Horne says that time-restricted eating—a type of fasting that involves squeezing all the day’s calories into single six- or eight-hour feeding windows—is leading the pack, largely because patients are able to stick with it.
There are more new advancements in Type 2 diabetes care. The interventions described here—from continuous glucose monitors to novel drugs—are some of the most promising, but they have company. It’s safe to say that, looking ahead, more people with Type 2 diabetes will be able to effectively manage or mitigate their symptoms.
More Must-Reads From TIME
- The 100 Most Influential People of 2024
- The Revolution of Yulia Navalnaya
- 6 Compliments That Land Every Time
- What's the Deal With the Bitcoin Halving?
- If You're Dating Right Now , You're Brave: Column
- The AI That Could Heal a Divided Internet
- Fallout Is a Brilliant Model for the Future of Video Game Adaptations
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at [email protected]
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 22, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New findings on pancreatic anatomy may affect diabetes research and treatment
by Claes Björnberg, Umea University
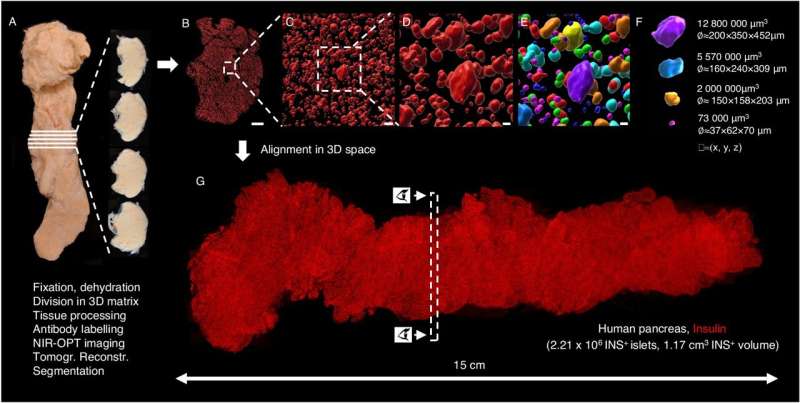
Researchers at Umeå University have succeeded in imaging an entire human organ, a pancreas, in microscopic resolution. By staining different cell-types with antibodies and then using optical 3D imaging techniques to study the entire organ, their data provides a partially new picture of the pancreas.
The results may be of great importance for diabetes research , especially when developing various new forms of treatment. The study is published in Nature Communications .
The pancreas is a key organ for the development of diabetes, a disease that today affects over half a billion people. It contains millions of small cell clusters, the so-called islets of Langerhans, which function to regulate blood sugar levels in the body.
The islets chiefly contain beta- and alpha-cells that produce the hormones insulin and glucagon, respectively. Insulin is secreted into the bloodstream and acts much like a key to unlock the body's cells so that they can take up sugar (glucose) after a meal, the main form of energy used by the body. Glucagon in turn releases glucose stores when we need a supply of energy. These two cell-types also communicate directly with each other to optimize the correct glucose level in the body.
"Both insulin and glucagon cells were discovered over a hundred years ago, and it has long been believed that the islets should contain both cell types to form a fully functioning unit," says Ulf Ahlgren, professor at the Department of Medical and Translational Biology.
Since the islets of Langerhans make up only a small percentage of the pancreas, even though they occur in such large numbers, they have historically been very difficult to study directly within the pancreas. In most cases, researchers have had to study tissue sections that only provide a 2D image of a very small part of the organ. Now, Umeå researchers have used optical 3D techniques in which different cell-types can be marked with fluorescently colored antibodies.
Entire organ at microscopic resolution
"By dividing the entire organ into smaller parts, we enable the antibodies to get where they need to go. Since we know where each piece comes from, we can then, after scanning the different parts individually, 'reassemble' the entire pancreas again using computer software. This allows us to perform a plethora of calculations and study which cell-types are present, as well as where they are located in 3D space, as we know the 3D coordinates, their volume, shape and other parameters for each and every stained object in the entire organ," says Ahlgren.
In addition to new data on how insulin-producing cells are distributed in the pancreas, the researchers now show that glucagon-producing cells are not present in as many as 50% of the islets of Langerhans that do contain insulin cells. This is contrary to what was previously thought, where islets were believed to contain both insulin- and glucagon-expressing cell-types with the same islet.
"This was a surprise to us, and I believe that these results may be of great importance for diabetes research. First, it shows that the islets have a much more uneven composition, or cellularity, than previously thought. This could mean that islets of different composition might be specifically specialized to respond to different signals and/or operate in different metabolic environments. Of course, we really want to find this out," says Ahlgren.
"Second, a great deal of research in the diabetes field is carried out on isolated islets of Langerhans from deceased donors. Since we also show that this uneven composition is largely linked to islet size, it means that results from such experiments may not fully reflect how the islets are structured and function in the living pancreas. This could potentially be important for everything from islet transplants in type 1 diabetes to studies trying to produce islets of Langerhans from stem cells."
Basis for future studies
The research team will now continue to work to see if their methods can be used to determine whether other cell types in the pancreas also contribute to the formation of the islets in a way that has not previously been known. In addition, they will study whether it looks similar in mouse models, which could affect the use of mice for preclinical diabetes research.
"The methods and data we are now publishing will be able to form an important basis for future studies of human material in order to better understand what happens in the pancreas in the development of type 1 and type 2 diabetes, but also for diseases such as pancreatic cancer," says Ahlgren.
Explore further
Feedback to editors

The consumption of certain food additive emulsifiers could be associated with the risk of developing type 2 diabetes
3 hours ago

Perinatal transmission of HIV can lead to cognitive deficits

Study finds suicidal behaviors increased by over 50% in Catalonia, Spain after the COVID-19 pandemic
4 hours ago

Why do we move slower the older we get? New study delivers answers
5 hours ago

Stress activates brain regions linked to alcohol use disorder differently for women than men, finds study

Chemical tool illuminates pathways used by dopamine, opioids and other neuronal signals

Gentle defibrillation for the heart: A milder method developed by researchers for cardiac arrhythmias
6 hours ago

Q&A: Research shows neural connection between learning a second language and learning to code

Gut microbiota acts like an auxiliary liver, study finds

Higher light levels may improve cognitive performance
Related stories.

Pancreas cells in people who have died show significant signs of stress
Apr 2, 2024
Loss of cells in pancreas in the elderly may cause age-related diabetes
Jan 15, 2024
New DNA methylation-based method for precise assessment of pancreas cell composition
Feb 6, 2024
Understanding the differences between healthy and type 2 diabetes-affected pancreatic islets
Nov 28, 2022
Beta cells under fire
Jun 7, 2017

New method enables 3D microscopy of human organs
Sep 13, 2021
Recommended for you

Advancing high-resolution ultrasound imaging with deep learning
Apr 22, 2024

Siblings with unique genetic mutation help scientists progress drug search for type 1 diabetes
Apr 18, 2024

New study focuses on the placenta for clues to the development of gestational diabetes
Apr 16, 2024

GPT-4 matches radiologists in detecting errors in radiology reports

World-first microscopic stiffness probe could advance early cancer diagnosis
Apr 15, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
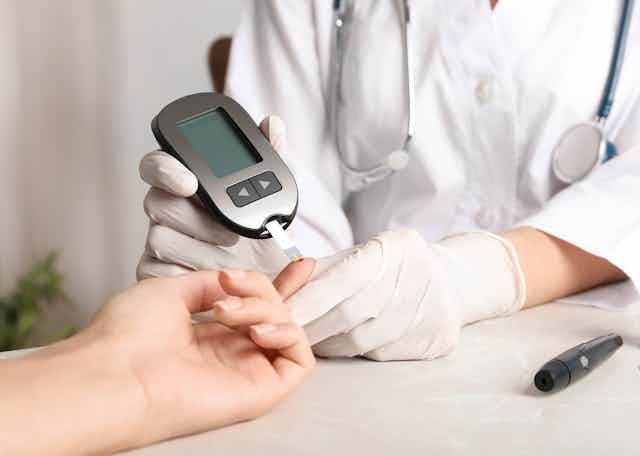
Type 2 diabetes is not one-size - fits-all : Subtypes affect complications and treatment options
PhD Student, Biochemistry, Carleton University
Associate professor, Department of Biology and Institute of Biochemistry, Carleton University
Disclosure statement
Lili Grieco-St-Pierre receives funding from Fonds de recherche du Québec - Santé (FRQS).
Jennifer Bruin receives funding from the Canadian Institutes of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), JDRF, Diabetes Canada.
Carleton University provides funding as a member of The Conversation CA.
Carleton University provides funding as a member of The Conversation CA-FR.
View all partners
You may have heard of Ozempic, the “miracle drug” for weight loss, but did you know that it was actually designed as a new treatment to manage diabetes? In Canada, diabetes affects approximately 10 per cent of the general population . Of those cases, 90 per cent have Type 2 diabetes.
This metabolic disorder is characterized by persistent high blood sugar levels, which can be accompanied by secondary health challenges, including a higher risk of stroke and kidney disease .
Locks and keys
In Type 2 diabetes, the body struggles to maintain blood sugar levels in an acceptable range. Every cell in the body needs sugar as an energy source, but too much sugar can be toxic to cells. This equilibrium needs to be tightly controlled and is regulated by a lock and key system.
In the body’s attempt to manage blood sugar levels and ensure that cells receive the right amount of energy, the pancreatic hormone, insulin, functions like a key . Cells cover themselves with locks that respond perfectly to insulin keys to facilitate the entry of sugar into cells.
Unfortunately, this lock and key system doesn’t always perform as expected. The body can encounter difficulties producing an adequate number of insulin keys, and/or the locks can become stubborn and unresponsive to insulin.
All forms of diabetes share the challenge of high blood sugar levels; however, diabetes is not a singular condition; it exists as a spectrum. Although diabetes is broadly categorized into two main types, Type 1 and Type 2, each presents a diversity of subtypes, especially Type 2 diabetes.
These subtypes carry their own characteristics and risks, and do not respond uniformly to the same treatments .
To better serve people living with Type 2 diabetes, and to move away from a “one size fits all” approach, it is beneficial to understand which subtype of Type 2 diabetes a person lives with. When someone needs a blood transfusion, the medical team needs to know the patient’s blood type. It should be the same for diabetes so a tailored and effective game plan can be implemented.
This article explores four unique subtypes of Type 2 diabetes, shedding light on their causes, complications and some of their specific treatment avenues.
Severe insulin-deficient diabetes: We’re missing keys!
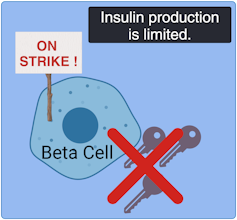
Insulin is produced by beta cells, which are found in the pancreas. In the severe insulin-deficient diabetes (SIDD) subtype, the key factories — the beta cells — are on strike. Ultimately, there are fewer keys in the body to unlock the cells and allow entry of sugar from the blood.
SIDD primarily affects younger, leaner individuals, and unfortunately, increases the risk of eye disease and blindness , among other complications. Why the beta cells go on strike remains largely unknown, but since there is an insulin deficiency, treatment often involves insulin injections.
Severe insulin-resistant diabetes: But it’s always locked!
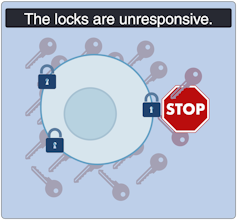
In the severe insulin-resistant diabetes (SIRD) subtype, the locks are overstimulated and start ignoring the keys. As a result, the beta cells produce even more keys to compensate. This can be measured as high levels of insulin in the blood, also known as hyperinsulinemia.
This resistance to insulin is particularly prominent in individuals with higher body weight. Patients with SIRD have an increased risk of complications such as fatty liver disease . There are many treatment avenues for these patients but no consensus about the optimal approach ; patients often require high doses of insulin.
Mild obesity-related diabetes: The locks are sticky!
Mild obesity-related (MOD) diabetes represents a nuanced aspect of Type 2 diabetes, often observed in individuals with higher body weight. Unlike more severe subtypes, MOD is characterized by a more measured response to insulin. The locks are “sticky,” so it is challenging for the key to click in place and open the lock. While MOD is connected to body weight, the comparatively less severe nature of MOD distinguishes it from other diabetes subtypes.
To minimize complications, treatment should include maintaining a healthy diet, managing body weight, and incorporating as much aerobic exercise as possible. This is where drugs like Ozempic can be prescribed to control the evolution of the disease, in part by managing body weight.
Mild age-related diabetes: I’m tired of controlling blood sugar!
Mild age-related diabetes (MARD) happens more often in older people and typically starts later in life. With time, the key factory is not as productive, and the locks become stubborn. People with MARD find it tricky to manage their blood sugar, but it usually doesn’t lead to severe complications.
Among the different subtypes of diabetes, MARD is the most common .
Unique locks, varied keys
While efforts have been made to classify diabetes subtypes, new subtypes are still being identified, making proper clinical assessment and treatment plans challenging.
In Canada, unique cases of Type 2 diabetes were identified in Indigenous children from Northern Manitoba and Northwestern Ontario by Dr. Heather Dean and colleagues in the 1980s and 90s. Despite initial skepticism from the scientific community, which typically associated Type 2 diabetes with adults rather than children, clinical teams persisted in identifying this as a distinct subtype of Type 2 diabetes, called childhood-onset Type 2 diabetes.
Read more: Indigenous community research partnerships can help address health inequities
Childhood-onset Type 2 diabetes is on the rise across Canada, but disproportionately affects Indigenous youth. It is undoubtedly linked to the intergenerational trauma associated with colonization in these communities . While many factors are likely involved, recent studies have discovered that exposure of a fetus to Type 2 diabetes during pregnancy increases the risk that the baby will develop diabetes later in life .
Acknowledging this distinct subtype of Type 2 diabetes in First Nations communities has led to the implementation of a community-based health action plan aimed at addressing the unique challenges faced by Indigenous Peoples. It is hoped that partnered research between communities and researchers will continue to help us understand childhood-onset Type 2 diabetes and how to effectively prevent and treat it.
A mosaic of conditions

Type 2 diabetes is not uniform; it’s a mosaic of conditions, each with its own characteristics. Since diabetes presents so uniquely in every patient, even categorizing into subtypes does not guarantee how the disease will evolve . However, understanding these subtypes is a good starting point to help doctors create personalized plans for people living with the condition.
While Indigenous communities, lower-income households and individuals living with obesity already face a higher risk of developing Type 2 diabetes than the general population, tailored solutions may offer hope for better management. This emphasizes the urgent need for more precise assessments of diabetes subtypes to help customize therapeutic strategies and management strategies. This will improve care for all patients, including those from vulnerable and understudied populations.
- Type 2 diabetes
- Blood sugar

Senior Lecturer - Earth System Science

Strategy Implementation Manager

Sydney Horizon Educators (Identified)


Deputy Social Media Producer

Associate Professor, Occupational Therapy
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Research Programs & Contacts
Clinical Research in Type 2 Diabetes
Studies in humans aimed at the prevention, treatment, and diagnosis of Type 2 Diabetes and the mechanistic aspects of its etiology.
The Clinical Research in Type 2 Diabetes (T2D) program supports human studies across the lifespan aimed at understanding, preventing and treating T2D. This program includes clinical trials that test pharmacologic, behavioral, surgical or practice-level approaches to the treatment and/or prevention of T2D, including promoting the preservation of beta cell function. Studies may also advance the development of new surrogate markers for use in clinical trials. Studies can be designed to understand the pathophysiology of T2D, including the role of gestational diabetes and metabolic imprinting on the development of T2D, as well as factors influencing the response to treatment. The program also encompasses epidemiologic studies that improve our understanding of the natural history and pathogenesis of T2D, and the development of diagnostic criteria to distinguish type 1 and type 2 diabetes, especially in the pediatric population. The program also supports research to understand and test approaches to accelerate the translation of efficacious interventions into real-world practice and adoption; and to address health equity by reducing health disparities in the incidence and/or clinical outcomes of T2D.
NIDDK Program Staff
- Shavon Artis Dickerson, Dr.P.H., M.P.H. Health Equity and Implementation Science
- Henry B. Burch, M.D. Clinical studies utilizing existing digital health technology for the prevention and treatment of type 2 diabetes, clinical and basic science studies involving non-neoplastic disorders of the thyroid, clinical studies involving medical and novel dietary treatment of type 2 diabetes.
- Maureen Monaghan Center, Ph.D., CDCES Health Psychology, Behavioral Science, Clinical Management of Diabetes
- Jean M. Lawrence, Sc.D., M.P.H., M.S.S.A. Type 2 diabetes risk and prevention after gestational diabetes; Studies of adults with diabetes/pre-diabetes using secondary data and observational designs, and natural experiments
- Hanyu Liang, M.D., Ph.D. Hepatic Metabolism; Insulin Resistance; Type 2 Diabetes; Obesity; Bariatric Surgery
- Barbara Linder, M.D., Ph.D. Type 2 diabetes in children and youth; human studies of metabolic imprinting
- Saul Malozowski, M.D., Ph.D., M.B.A. Neuroendocrinology of hypothalamic-pituitary axis, neuropeptide signaling and receptors; hormonal regulation of bone and mineral metabolism; HIV/AIDS-associated metabolic and endocrine dysfunction
- Pamela L. Thornton, Ph.D. Health Equity and Translational Research; Centers for Diabetes Translation Research (P30) Program
- Theresa Teslovich Woo, Ph.D. Human behavior, developmental cognitive neuroscience, and brain-based mechanisms involved in obesity and diabetes
Recent Funding Opportunities
Mentored patient-oriented research career development award (parent k23 independent clinical trial required), mentored patient-oriented research career development award (parent k23 independent clinical trial not allowed), rare diseases clinical research consortia (rdcrc) for the rare diseases clinical research network (rdcrn) (u54 clinical trial optional), adaptation of diabetes control technologies for older adults with t1d (r01 clinical trial optional), diabetes research centers (p30 clinical trial optional), related links.
View related clinical trials from ClinicalTrials.gov.
Study sections conduct initial peer review of applications in a designated scientific area. Visit the NIH’s Center for Scientific Review website to search for study sections.
Research Resources
NIDDK makes publicly supported resources, data sets, and studies available to researchers to accelerate the rate and lower the cost of new discoveries.
- Ancillary Studies to Major Ongoing Clinical Studies to extend our knowledge of the diseases being studied by the parent study investigators under a defined protocol or to study diseases and conditions not within the original scope of the parent study but within the mission of the NIDDK.
- NIDDK Central Repository for access to clinical resources including data and biospecimens from NIDDK-funded studies.
- NIDDK Information Network (dkNET) for simultaneous search of digital resources, including multiple datasets and biomedical resources relevant to the mission of the NIDDK.
Additional Research Programs
Research training.
NIDDK supports the training and career development of medical and graduate students, postdoctoral fellows, and physician scientists through institutional and individual grants.
Diversity Programs
The NIDDK offers and participates in a variety of opportunities for trainees and researchers from communities underrepresented in the biomedical research enterprise. These opportunities include travel and scholarship awards, research supplements, small clinical grants, high school and undergraduate programs, and a network of minority health research investigators.
Small Business
Small business programs.
NIDDK participates in the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs. These programs support innovative research conducted by small businesses that has the potential for commercialization.
Human Subjects Research
NIDDK provides funding for pivotal clinical research, from preliminary clinical feasibility to large multi-center studies.
Translational Research
NIDDK provides funding opportunities and resources to encourage translation of basic discoveries into novel therapeutics.
Meetings & Workshops

Supports researchers with tools to enhance scientific rigor, reproducibility, and transparency, and provides a big data knowledge base for genomic and pathway hypothesis generation.

Providing education and training for the next generation of biomedical and behavioral scientist

Stay informed about the latest events, or connect through social media.

Learn about current projects and view funding opportunities sponsored by the NIH Common Fund .
Registration is required at eRA Commons and grants.gov and can take 4 weeks.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Diabetes articles from across Nature Portfolio
Diabetes describes a group of metabolic diseases characterized by high blood sugar levels. Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes) or by insulin resistance (cells do not respond to insulin; type 2 diabetes).
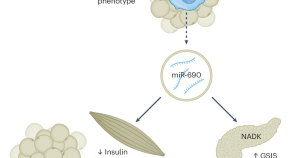
Macrophage vesicles in antidiabetic drug action
Thiazolidinediones (TZDs) are potent insulin-sensitizing drugs, but their use is accompanied by adverse side-effects. Rohm et al. now report that TZD-stimulated macrophages release miR-690-containing vesicles that improve insulin sensitization and bypass unwanted side-effects.
- Rinke Stienstra
- Eric Kalkhoven
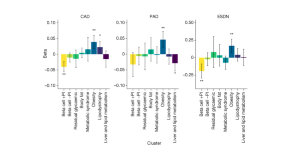
Genetic risk variants lead to type 2 diabetes development through different pathways
The largest genome-wide association study for type 2 diabetes so far, which included several ancestry groups, led to the identification of eight clusters of genetic risk variants. The clusters capture different biological pathways that contribute to the disease, and some clusters are associated with vascular complications.
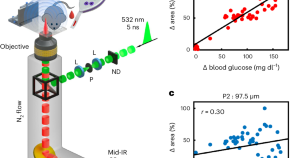
Blood glucose concentration measurement without finger pricking
A new sensor that detects optoacoustic signals generated by mid-infrared light enables measurement of glucose concentration from intracutaneous tissue rich in blood. This technology does not rely on glucose measurements in interstitial fluid or blood sampling and might yield the next generation of non-invasive glucose-sensing devices for improved diabetes management.
Related Subjects
- Diabetes complications
- Diabetes insipidus
- Gestational diabetes
- Type 1 diabetes
- Type 2 diabetes
Latest Research and Reviews
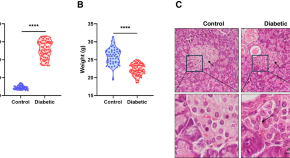
Nicotinamide Mononucleotide improves oocyte maturation of mice with type 1 diabetes
- Fucheng Guo
- Xiaoling Zhang
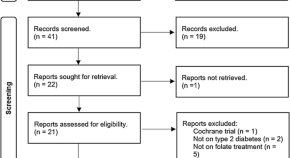
Folic acid supplementation on inflammation and homocysteine in type 2 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials
- Kabelo Mokgalaboni
- Given. R. Mashaba
- Sogolo. L. Lebelo
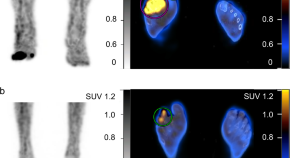
The usefulness of quantitative 99m Tc-HMPAO WBC SPECT/CT for predicting lower extremity amputation in diabetic foot infection
- Soo Bin Park
- Chae Hong Lim
- Jung Mi Park
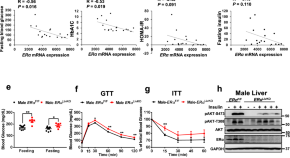
An estrogen receptor α-derived peptide improves glucose homeostasis during obesity
Type 2 diabetes mellitus is an increasing global health issue, which is caused by systemic insulin resistance. Here, the authors show a ligand-independent effect of hepatic ERα in regulating insulin sensitivity and identify an ERα-derived peptide that functions as an insulin sensitizer.
- Wanbao Yang
- Shaodong Guo
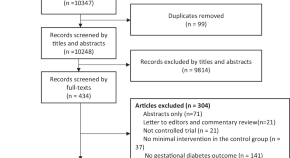
Effective interventions in preventing gestational diabetes mellitus: A systematic review and meta-analysis
Takele et al. conduct a systematic review and meta-analysis of the effects of intervention characteristics on preventing gestational diabetes. Group or healthcare facility-based physical activity interventions are found to be more effective in preventing gestational diabetes than community-based interventions.
- Wubet Worku Takele
- Kimberly K. Vesco
Illuminating the complete ß-cell mass of the human pancreas- signifying a new view on the islets of Langerhans
The pancreatic islets of Langerhans play a pivotal role in regulating blood glucose homeostasis through the regulated secretion of the hormones insulin and glucagon. Here, the authors use deep tissue 3D imaging to re-construct the entire human pancreas at microscopic resolution and display previously unrecognized heterogeneities in the islet’s cellularity with pre-clinical and clinical implications.
- Joakim Lehrstrand
- Wayne I. L. Davies
- Ulf Ahlgren
News and Comment
Repurposing a diabetes drug to treat Parkinson’s disease
In a multicenter clinical trial, patients with early-stage Parkinson’s disease treated with lixisenatide, a drug currently used for the treatment of diabetes, showed improvement in their motor scores compared with those on placebo.
- Sonia Muliyil
A novel system for non-invasive measurement of blood levels of glucose
- Olivia Tysoe
Diabetes drug slows development of Parkinson’s disease
The drug, which is in the same family as blockbuster weight-loss drugs such as Wegovy, slowed development of symptoms by a small but statistically significant amount.
Metformin acts through appetite-suppressing metabolite: Lac-Phe
- Shimona Starling
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Clinical Trials
Type 2 diabetes.
Displaying 96 studies
The purpose of this study is to identify changes to the metabolome (range of chemicals produced in the body) and microbiome (intestine microbe environment) that are unique to Roux-en-Y gastric bypass surgery and assess the associated effect on the metabolism of patients with type 2 diabetes.
The purpose of this study is to evaluate the impact of a digital storytelling intervention derived through a community-based participatory research (CBPR) approach on type 2 diabetes mellitus (T2D) outcomes among Hispanic adults with poorly controlled type 2 diabetes mellitus (T2D) in primary care settings through a randomized clinical trial.
The purpose of this study is to assess the impact of a whole food plant-based diet on blood sugar control in diabetic patients versus a control group on the American Diabetics Association diet before having a total hip, knee, or shoulder replacement surgery.
The purpose of this study is to learn more about if the medication, Entresto, could help the function of the heart and kidneys.
The primary aim of this study is to compare the outcome measures of adult ECH type 2 diabetes patients who were referred to onsite pharmacist services for management of their diabetes to similar patients who were not referred for pharmacy service management of their diabetes. A secondary aim of the study is to assess the Kasson providers’ satisfaction level and estimated pharmacy service referral frequency to their patients. A tertiary aim of the study is to compare the hospitalization rates of type 2 diabetes rates who were referred to onsite pharmacist services for management of their diabetes to similar patients ...
To explore the feasibility of conducting a family centered wellness coaching program for patients at high risk for developing diabetes, in a primary care setting.
To determine engagement patterns.
To describe characteristics of families who are likely to participate.
To identify barriers/limitations to family centered wellness coaching.
To assess whether a family centered 8 week wellness coaching intervention for primary care patients at high risk for diabetes will improve self-care behaviors as measured by self-reported changes in physical activity level and food choices.
This study is being done to understand metformin's mechanisms of action regarding glucose production, protein metabolism, and mitochondrial function.
The purpose of this study is to assess the effectiveness of Revita® DMR for improving HbA1c to ≤ 7% without the need of insulin in subjects with T2D compared to sham and to assess the effectiveness of DMR versus Sham on improvement in Glycemic, Hepatic and Cardiovascular endpoints.
The purpose of this study is to evaluate 6 weeks of home use of the Control-IQ automated insulin delivery system in individuals with type 2 diabetes.
This study will evaluate whether bile acids are able to increase insulin sensitivity and enhance glycemic control in T2DM patients, as well as exploring the mechanisms that enhance glycemic control. These observations will provide the preliminary data for proposing future therapeutic as well as further mechanistic studies of the role of bile acids in the control of glycemia in T2DM.
The purpose of this study is to determine if Inpatient Stress Hyperglycemia is an indicator of future risk of developing type 2 Diabetes Mellitus.
The purpose of this study is to assess the effectiveness of a digital storytelling intervention derived through a community based participatory research (CBPR) approach on self-management of type 2 diabetes (T2D) among Somali adults.
The GRADE Study is a pragmatic, unmasked clinical trial that will compare commonly used diabetes medications, when combined with metformin, on glycemia-lowering effectiveness and patient-centered outcomes.
The overall goal of this proposal is to determine the effects of acute hyperglycemia and its modulation by Glucagon-like Peptide-1 (GLP-1) on myocardial perfusion in type 2 diabetes (DM). This study plan utilizes myocardial contrast echocardiography (MCE) to explore a) the effects of acute hyperglycemia on myocardial perfusion and coronary flow reserve in individuals with and without DM; and b) the effects of GLP-1 on myocardial perfusion and coronary flow reserve during euglycemia and hyperglycemia in DM. The investigators will recruit individuals with and without DM matched for age, gender and degree of obesity. The investigators will measure myocardial perfusion ...
The purpose of this study is to test the hypothesis that patients with T2DM will have greater deterioration in BMSi and in cortical porosity over 3 yrs as compared to sex- and age-matched non-diabetic controls; and identify the circulating hormonal (e.g., estradiol [E2], testosterone [T]) and biochemical (e.g., bone turnover markers, AGEs) determinants of changes in these key parameters of bone quality, and evaluate the possible relationship between existing diabetic complications and skeletal deterioration over time in the T2DM patients.
The purpose of this study is to determine the effect of endogenous GLP-1 secretion on islet function in people with Typr 2 Diabetes Mellitus (T2DM).
GLP-1 is a hormone made by the body that promotes the production of insulin in response to eating. However, there is increasing evidence that this hormone might help support the body’s ability to produce insulin when diabetes develops.
The purpose of this study is to assess whether psyllium is more effective in lowering fasting blood sugar and HbA1c, and to evaluate the effect of psyllium compared to wheat dextrin on the following laboratory markers: LDL-C, inflammatory markers such as ceramides and hsCRP, and branch chain amino acids which predict Diabetes Mellitus (DM).
This trial is a multi-center, adaptive, randomized, double-blind, placebo- and active- controlled, parallel group, phase 2 study in subjects with Type 2 Diabetes Mellitus to evaluate the effect of TTP399 on HbA1c following administration for 6 months.
The purpose of this study is to find the inheritable changes in genetic makeup that are related to the development of type 2 diabetes in Latino families.
The objective of this early feasibility study is to assess the feasibility and preliminary safety of the Endogenex Divice for endoscopic duodenal mucosal regeneration in patients with type 2 diabetes (T2D) inadequately controlled on 2-3 non-insulin glucose-lowering medications.
This mixed methods study aims to answer the question: "What is the work of being a patient with type 2 diabetes mellitus?" .
The purpose of this study is to assess penile length pre- and post-completion of RestoreX® traction therapy compared to control groups (no treatment) among men with type II diabetes.
This observational study is conducted to determine how the duodenal layer thicknesses (mucosa, submucosa, and muscularis) vary with several factors in patients with and without type 2 diabetes.
The purpose of this study is to evaluate if breathing pure oxygen overnight affects insulin sensitivity in participants with diabetes.
The purpose of this study is to determine the impact of patient decision aids compared to usual care on measures of patient involvement in decision-making, diabetes care processes, medication adherence, glycemic and cardiovascular risk factor control, and use of resources in nonurban practices in the Midwestern United States.
The purpose of this study is to estimate the risk of diabetes related complications after total pancreatectomy. We will contact long term survivors after total pancreatectomy to obtain data regarding diabetes related end organ complications.
The purpose of this study is to understand nighttime glucose regulation in humans and find if the pattern is different in people with Type 2 diabetes
The study is being undertaken to understand how a gastric bypass can affect a subject's diabetes even prior to their losing significant amounts of weight. The hypothesis of this study is that increased glucagon-like peptide-1 (GLP-1) secretion explains the amelioration in insulin secretion after Roux-en-Y Gastric Bypass (RYGB) surgery.
The study purpose is to understand patients’ with the diagnosis of Diabetes Mellitus type 1 or 2 perception of the care they receive in the Diabetes clinic or Diabetes technology clinic at Mayo Clinic and to explore and to identify the healthcare system components patients consider important to be part of the comprehensive regenerative care in the clinical setting.
However, before we can implement structural changes or design interventions to promote comprehensive regenerative care in clinical practice, we first need to characterize those regenerative practices occurring today, patients expectations, perceptions and experiences about comprehensive regenerative care and determine the ...
It is unknown how patient preferences and values impact the comparative effectiveness of second-line medications for Type 2 diabetes (T2D). The purpose of this study is to elicit patient preferences toward various treatment outcomes (e.g., hospitalization, kidney disease) using a participatory ranking exercise, use these rankings to generate individually weighted composite outcomes, and estimate patient-centered treatment effects of four different second-line T2D medications that reflect the patient's value for each outcome.
The purpose of this mixed-methods study is to deploy the tenets of Health and Wellness Coaching (HWC) through a program called BeWell360 model , tailored to the needs of Healthcare Workers (HCWs) as patients living with poorly-controlled Type 2 Diabetes (T2D). The objective of this study is to pilot-test this novel, scalable, and sustainable BeWell360 model that is embedded and integrated as part of primary care for Mayo Clinic Employees within Mayo Clinic Florida who are identified as patients li)ving with poorly-controlled T2D.
The investigators will determine whether people with high muscle mitochondrial capacity produce higher amount of reactive oxygen species (ROS) on consuming high fat /high glycemic diet and thus exhibit elevated cellular oxidative damage. The investigators previously found that Asian Indian immigrants have high mitochondrial capacity in spite of severe insulin resistance. Somalians are another new immigrant population with rapidly increasing prevalence of diabetes. Both of these groups traditionally consume low caloric density diets, and the investigators hypothesize that when these groups are exposed to high-calorie Western diets, they exhibit increased oxidative stress, oxidative damage, and insulin resistance. The investigators will ...
The purpose of this research is to find out how genetic variations in GLP1R, alters insulin secretion, in the fasting state and when blood sugars levels are elevated. Results from this study may help us identify therapies to prevent or reverse type 2 diabetes mellitus.
Can QBSAfe be implemented in a clinical practice setting and improve quality of life, reduce treatment burden and hypoglycemia among older, complex patients with type 2 diabetes?
Questionnaire administered to diabetic patients in primary care practice (La Crosse Mayo Family Medicine Residency /Family Health Clinic) to assess patient’s diabetic knowledge. Retrospective chart review will also be done to assess objective diabetic control based on most recent hemoglobin A1c.
To determine if the EndoBarrier safely and effectively improves glycemic control in obese subjects with type 2 diabetes.
The purpose of this study is to assess key characteristics of bone quality, specifically material strength and porosity, in patients who have type 2 diabetes. These patients are at an unexplained increased risk for fractures and there is an urgent need to refine clinical assessment for this risk.
Muscle insulin resistance is a hallmark of upper body obesity (UBO) and Type 2 diabetes (T2DM). It is unknown whether muscle free fatty acid (FFA) availability or intramyocellular fatty acid trafficking is responsible for muscle insulin resistance, although it has been shown that raising FFA with Intralipid can cause muscle insulin resistance within 4 hours. We do not understand to what extent the incorporation of FFA into ceramides or diacylglycerols (DG) affect insulin signaling and muscle glucose uptake. We propose to alter the profile and concentrations of FFA of healthy, non-obese adults using an overnight, intra-duodenal palm oil infusion vs. ...
The objectives of this study are to identify circulating extracellular vesicle (EV)-derived protein and RNA signatures associated with Type 2 Diabetes (T2D), and to identify changes in circulating EV cargo in patients whose T2D resolves after sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB).
This research study is being done to develop educational materials that will help patients and clinicians talk about diabetes treatment and management options.
The purpose of this study evaluates a subset of people with isolated Impaired Fasting Glucose with Normal Glucose Tolerance (i.e., IFG/NGT) believed to have normal β-cell function in response to a glucose challenge, suggesting that – at least in this subset of prediabetes – fasting glucose is regulated independently of glucose in the postprandial period. To some extent this is borne out by genetic association studies which have identified loci that affect fasting glucose but not glucose tolerance and vice-versa.
The purpose of this study is to evaluate whether or not a 6 month supply (1 meal//day) of healthy food choices readily available in the patient's home and self management training including understanding of how foods impact diabetes, improved food choices and how to prepare those foods, improve glucose control. In addition, it will evaluate whether or not there will be lasting behavior change modification after the program.
Assessment of glucose metabolism and liver fat after 12 week dietary intervention in pre diabetes subjects. Subjects will be randomized to either high fat (olive oil supplemented),high carb/high fiber (beans supplemented) and high carb/low fiber diets. Glucose metabolism will be assessed by labeled oral glucose tolerance test and liver fat by magnetic resonance spectroscopy pre randomization and at 8 and 12 week after starting dietary intervention.
To study the effect of an ileocolonic formulation of ox bile extract on insulin sensitivity, postprandial glycemia and incretin levels, gastric emptying, body weight and fasting serum FGF-19 (fibroblast growth factor) levels in overweight or obese type 2 diabetic subjects on therapy with DPP4 (dipeptidyl peptidase-4) inhibitors (e.g. sitagliptin) alone or in combination with metformin.
The purpose of this study is to compare the rate of progression from prediabetes at 4 months to frank diabetes at 12 months (as defined by increase in HbA1C or fasting BS to diabetic range based on the ADA criteria) after transplantation in kidney transplant recipients on Exenatide SR + SOC vs. standard-of-care alone.
The purpose of this study is to learn more about how the body stores dietary fat. Medical research has shown that fat stored in different parts of the body can affect the risk for diabetes, heart disease and other major health conditions.
The purpose of this study is to see why the ability of fat cells to respond to insulin is different depending on body shape and how fat tissue inflammation is involved.
The purpose of this study is to determine the mechanism(s) by which common bariatric surgical procedures alter carbohydrate metabolism. Understanding these mechanisms may ultimately lead to the development of new interventions for the prevention and treatment of type 2 diabetes and obesity.
A research study to enhance clinical discussion between patients and pharmacists using a shared decision making tool for type 2 diabetes or usual care.
While the potential clinical uses of pulsed electromagnetic field therapy (PEMF) are extensive, we are focusing on the potential benefits of PEMF on vascular health. We are targeting, the pre diabetic - metabolic syndrome population, a group with high prevalence in the American population. This population tends to be overweight, low fitness, high blood pressure, high triglycerides and borderline high blood glucose.
The purpose of this study is to evaluate the effects of improving glycemic control, and/or reducing glycemic variability on gastric emptying, intestinal barrier function, autonomic nerve functions, and epigenetic changes in subjects with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) who are switched to intensive insulin therapy as part of clinical practice.
This study is designed to compare an intensive lifestyle and activity coaching program ("Sessions") to usual care for diabetic patients who are sedentary. The question to be answered is whether the Sessions program improves clinical or patient centric outcomes. Recruitment is through invitiation only.
This is a study to evaluate a new Point of Care test for blood glucose monitoring.
The purpose of this study is to determine the metabolic effects of Colesevelam, particularly for the ability to lower blood sugar after a meal in type 2 diabetics, in order to develop a better understanding of it's potential role in the treatment of obesity.
The purpose of this study is to test whether markers of cellular aging and the SASP are elevated in subjects with obesity and further increased in patients with obesity and Type 2 Diabetes Mellitus (T2DM) and to relate markers of cellular aging (senescence) and the SASP to skeletal parameters (DXA, HRpQCT, bone turnover markers) in each of these groups.
Integration of Diabetes Prevention Program (DPP) and Diabetes Self Management Program (DSMP) into WellConnect.
This protocol is being conducted to determine the mechanisms responsible for insulin resistance, obesity and type 2 diabetes.
The purpose of this study is to assess the effects of a nighttime rise in cortisol on the body's glucose production in type 2 diabetes.
The goal of this study is to evaluate a new format for delivery of a culturally tailored digital storytelling intervention by incorporating a facilitated group discussion following the videos, for management of type II diabetes in Latino communities.
Using stem cell derived intestinal epithelial cultures (enteroids) derived from obese (BMI> 30) patients and non-obese and metabolically normal patients (either post-bariatric surgery (BS) or BS-naïve with BMI < 25), dietary glucose absorption was measured. We identified that enteroids from obese patients were characterized by glucose hyper-absorption (~ 5 fold) compared to non-obese patients. Significant upregulation of major intestinal sugar transporters, including SGLT1, GLU2 and GLUT5 was responsible for hyper-absorptive phenotype and their pharmacologic inhibition significantly decreased glucose absorption. Importantly, we observed that enteroids from post-BS non-obese patients exhibited low dietary glucose absorption, indicating that altered glucose absorption ...
Muscle insulin resistance is a hallmark of upper body obesity (UBO) and Type 2 diabetes (T2DM). It is unknown whether muscle free fatty acid (FFA) availability or intramyocellular fatty acid trafficking is responsible for the abnormal response to insulin. Likewise, we do not understand to what extent the incorporation of FFA into ceramides or diacylglycerols (DG) affect insulin signaling and muscle glucose uptake. We will measure muscle FFA storage into intramyocellular triglyceride, intramyocellular fatty acid trafficking, activation of the insulin signaling pathway and glucose disposal rates under both saline control (high overnight FFA) and after an overnight infusion of intravenous ...
The purpose of this study is to improve our understanding of why gastrointestinal symptoms occur in diabetes mellitus patients and identify new treatment(s) in the future.
These symptoms are often distressing and may impair glycemic control. We do not understand how diabetes mellitus affects the GI tracy. In 45 patients undergoing sleeve gastrectomy, we plan to compare the cellular composition of circulating peripheral mononuclear cells, stomach immune cells, and interstitial cells of Cajal in the stomach.
Muscle insulin resistance is a hallmark of upper body obesity (UBO) and Type 2 diabetes (T2DM), whereas lower body obesity (LBO) is characterized by near-normal insulin sensitivity. It is unknown whether muscle free fatty acid (FFA) availability or intramyocellular fatty acid trafficking differs between different obesity phenotypes. Likewise, we do not understand to what extent the incorporation of FFA into ceramides or diacylglycerols (DG) affect insulin signaling and muscle glucose uptake. By measuring muscle FFA storage into intramyocellular triglyceride, intramyocellular fatty acid trafficking, activation of the insulin signaling pathway and glucose disposal rates we will provide the first integrated examination ...
The goal of this study is to evaluate the presence of podocytes (special cells in the kidney that prevent protein loss) in the urine in patients with diabetes or glomerulonephritis (inflammation in the kidneys). Loss of podocyte in the urine may be an earlier sign of kidney injury (before protein loss) and the goal of this study is to evaluate the association between protein in the urine and podocytes in the urine.
The purpose of this study is to evaluate the effects of multiple dose regimens of RM-131 on vomiting episodes, stomach emptying and stomach paralysis symptoms in patients with Type 1 and Type 2 diabetes and gastroparesis.
The purpose of this study is assess the feasibility, effectiveness, and acceptability of Diabetes-REM (Rescue, Engagement, and Management), a comprehensive community paramedic (CP) program to improve diabetes self-management among adults in Southeast Minnesota (SEMN) treated for servere hypoglycemia by the Mayo Clinic Ambulance Services (MCAS).
The purpose of this study is to determine if a blood test called "pancreatic polypeptide" can help distinguish between patients with diabetes mellitus with and without pancreatic cancer.
The purpose of this study is to create a prospective cohort of subjects with increased probability of being diagnosed with pancreatic cancer and then screen this cohort for pancreatic cancer
The purpose of this study is to evaluate the effectiveness and safety of brolucizumab vs. aflibercept in the treatment of patients with visual impairment due to diabetic macular edema (DME).
Women with gestational diabetes mellitus (GDM) are likely to have insulin resistance that persists long after pregnancy, resulting in greater risk of developing type 2 diabetes mellitus (T2DM). The study will compare women with and without a previous diagnosis of GDM to determine if women with a history of GDM have abnormal fatty acid metabolism, specifically impaired adipose tissue lipolysis. The study will aim to determine whether women with a history of GDM have impaired pancreatic β-cell function. The study will determine whether women with a history of GDM have tissue specific defects in insulin action, and also identify the effect of a ...
Although vitreous hemorrhage (VH) from proliferative diabetic retinopathy (PDR) can cause acute and dramatic vision loss for patients with diabetes, there is no current, evidence-based clinical guidance as to what treatment method is most likely to provide the best visual outcomes once intervention is desired. Intravitreous anti-vascular endothelial growth factor (anti-VEGF) therapy alone or vitrectomy combined with intraoperative PRP each provide the opportunity to stabilize or regress retinal neovascularization. However, clinical trials are lacking to elucidate the relative time frame of visual recovery or final visual outcome in prompt vitrectomy compared with initial anti-VEGF treatment. The Diabetic Retinopathy Clinical Research ...
The purpose of this study is to demonstrate feasibility of dynamic 11C-ER176 PET imaging to identify macrophage-driven immune dysregulation in gastric muscle of patients with DG. Non-invasive quantitative assessment with PET can significantly add to our diagnostic armamentarium for patients with diabetic gastroenteropathy.
The purpose of this study is to assess the safety and tolerability of intra-arterially delivered mesenchymal stem/stromal cells (MSC) to a single kidney in one of two fixed doses at two time points in patients with progressive diabetic kidney disease.
Diabetic kidney disease, also known as diabetic nephropathy, is the most common cause of chronic kidney disease and end-stage kidney failure requiring dialysis or kidney transplantation. Regenerative, cell-based therapy applying MSCs holds promise to delay the progression of kidney disease in individuals with diabetes mellitus. Our clinical trial will use MSCs processed from each study participant to test the ...
The purpose of this study is to look at how participants' daily life is affected by their heart failure. The study will also look at the change in participants' body weight. This study will compare the effect of semaglutide (a new medicine) compared to "dummy" medicine on body weight and heart failure symptoms. Participants will either get semaglutide or "dummy" medicine, which treatment participants get is decided by chance. Participants will need to take 1 injection once a week.
This study aims to measure the percentage of time spent in hyperglycemia in patients on insulin therapy and evaluate diabetes related patient reported outcomes in kidney transplant recipients with type 2 diabetes. It also aimes to evaluate immunosuppression related patient reported outcomes in kidney transplant recipients with type 2 diabetes.
The purpose of this study is to evaluate whether or not semaglutide can slow down the growth and worsening of chronic kidney disease in people with type 2 diabetes. Participants will receive semaglutide (active medicine) or placebo ('dummy medicine'). This is known as participants' study medicine - which treatment participants get is decided by chance. Semaglutide is a medicine, doctors can prescribe in some countries for the treatment of type 2 diabetes. Participants will get the study medicine in a pen. Participants will use the pen to inject the medicine in a skin fold once a week. The study will close when ...
The objectives of this study are to evaluate the safety of IW-9179 in patients with diabetic gastroparesis (DGP) and the effect of treatment on the cardinal symptoms of DGP.
The purpose of this study is to understand why patients with indigestion, with or without diabetes, have gastrointestinal symptoms and, in particular, to understand where the symptoms are related to increased sensitivity to nutrients.Subsequently, look at the effects of Ondansetron on these patients' symptoms.
The purpose of this study is to evaluate the safety, tolerability, pharmacokinetics, and exploratory effectiveness of nimacimab in patients with diabetic gastroparesis.
The purpose of this study is to prospectively assemble a cohort of subjects >50 and ≤85 years of age with New-onset Diabetes (NOD):
- Estimate the probability of pancreatic ductal adenocarcinoma (PDAC) in the NOD Cohort;
- Establish a biobank of clinically annotated biospecimens including a reference set of biospecimens from pre-symptomatic PDAC and control new-onset type 2 diabetes mellitus (DM) subjects;
- Facilitate validation of emerging tests for identifying NOD subjects at high risk for having PDAC using the reference set; and
- Provide a platform for development of an interventional protocol for early detection of sporadic PDAC ...
The purpose of this study is to demonstrate the performance of the Guardian™ Sensor (3) with an advanced algorithm in subjects age 2 - 80 years, for the span of 170 hours (7 days).
The purpose of this study is to look at the relationship of patient-centered education, the Electronic Medical Record (patient portal) and the use of digital photography to improve the practice of routine foot care and reduce the number of foot ulcers/wounds in patients with diabetes.
Diabetes mellitus is a common condition which is defined by persistently high blood sugar levels. This is a frequent problem that is most commonly due to type 2 diabetes. However, it is now recognized that a small portion of the population with diabetes have an underlying problem with their pancreas, such as chronic pancreatitis or pancreatic cancer, as the cause of their diabetes. Currently, there is no test to identify the small number of patients who have diabetes caused by a primary problem with their pancreas.
The goal of this study is to develop a test to distinguish these ...
The primary purpose of this study is to evaluate the impact of dapagliflozin, as compared with placebo, on heart failure, disease specific biomarkers, symptoms, health status and quality of life in patients with type 2 diabetes or prediabetes and chronic heart failure with preserved systolic function.
The primary purpose of this study is to prospectively assess symptoms of bloating (severity, prevalence) in patients with diabetic gastroparesis.
The purpose of this study is to track the treatment burden experienced by patients living with Type 2 Diabetes Mellitus (T2DM) experience as they work to manage their illness in the context of social distancing measures.
To promote social distancing during the COVID-19 pandemic, health care institutions around the world have rapidly expanded their use of telemedicine to replace in-office appointments where possible.1 For patients with diabetes, who spend considerable time and energy engaging with various components of the health care system,2,3 this unexpected and abrupt transition to virtual health care may signal significant changes to ...
The purpose of this study is to evaluate the safety and efficacy of oral Pyridorin 300 mg BID in reducing the rate of progression of nephropathy due to type 2 diabetes mellitus.
The purpose of this study is to evaluate the effect of Aramchol as compared to placebo on NASH resolution, fibrosis improvement and clinical outcomes related to progression of liver disease (fibrosis stages 2-3 who are overweight or obese and have prediabetes or type 2 diabetes).
The purpose of this study is to evaluate the ability of appropriately-trained family physicians to screen for and identify Diabetic Retinopathy using retinal camera and, secondarily, to describe patients’ perception of the convenience and cost-effectiveness of retinal imaging.
The primary purpose of this study is to evaluate the impact of dapagliflozin, as compared with placebo, on heart failure disease-specific biomarkers, symptoms, health status, and quality of life in patients who have type 2 diabetes and chronic heart failure with reduced systolic function.
Hypothesis: We hypothesize that patients from the Family Medicine Department at Mayo Clinic Florida who participate in RPM will have significantly reduced emergency room visits, hospitalizations, and hospital contacts.
Aims, purpose, or objectives: In this study, we will compare the RPM group to a control group that does not receive RPM. The primary objective is to determine if there are significant group differences in emergency room visits, hospitalizations, outpatient primary care visits, outpatient specialty care visits, and hospital contacts (inbound patient portal messages and phone calls). The secondary objective is to determine if there are ...
The purpose of this research is to determine if CGM (continuous glucose monitors) used in the hospital in patients with COVID-19 and diabetes treated with insulin will be as accurate as POC (point of care) glucose monitors. Also if found to be accurate, CGM reading data will be used together with POC glucometers to dose insulin therapy.
The purpose of this study is to evaluate the effect of fenofibrate compared with placebo for prevention of diabetic retinopathy (DR) worsening or center-involved diabetic macular edema (CI-DME) with vision loss through 4 years of follow-up in participants with mild to moderately severe non-proliferative DR (NPDR) and no CI-DME at baseline.
The purpose of this study is to assess painful diabetic peripheral neuropathy after high-frequency spinal cord stimulation.
The purpose of this study is to examine the evolution of diabetic kindey injury over an extended period in a group of subjects who previously completed a clinical trial which assessed the ability of losartan to protect the kidney from injury in early diabetic kidney disease. We will also explore the relationship between diabetic kidney disease and other diabetes complications, including neuropathy and retinopathy.
The purpose of this study is to evaluate the effietiveness of remdesivir (RDV) in reducing the rate of of all-cause medically attended visits (MAVs; medical visits attended in person by the participant and a health care professional) or death in non-hospitalized participants with early stage coronavirus disease 2019 (COVID-19) and to evaluate the safety of RDV administered in an outpatient setting.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- Patient Care & Health Information
- Diseases & Conditions
- Type 2 diabetes
Type 2 diabetes is usually diagnosed using the glycated hemoglobin (A1C) test. This blood test indicates your average blood sugar level for the past two to three months. Results are interpreted as follows:
- Below 5.7% is normal.
- 5.7% to 6.4% is diagnosed as prediabetes.
- 6.5% or higher on two separate tests indicates diabetes.
If the A1C test isn't available, or if you have certain conditions that interfere with an A1C test, your health care provider may use the following tests to diagnose diabetes:
Random blood sugar test. Blood sugar values are expressed in milligrams of sugar per deciliter ( mg/dL ) or millimoles of sugar per liter ( mmol/L ) of blood. Regardless of when you last ate, a level of 200 mg/dL (11.1 mmol/L ) or higher suggests diabetes, especially if you also have symptoms of diabetes, such as frequent urination and extreme thirst.
Fasting blood sugar test. A blood sample is taken after you haven't eaten overnight. Results are interpreted as follows:
- Less than 100 mg/dL (5.6 mmol/L ) is considered healthy.
- 100 to 125 mg/dL (5.6 to 6.9 mmol/L ) is diagnosed as prediabetes.
- 126 mg/dL (7 mmol/L ) or higher on two separate tests is diagnosed as diabetes.
Oral glucose tolerance test. This test is less commonly used than the others, except during pregnancy. You'll need to not eat for a certain amount of time and then drink a sugary liquid at your health care provider's office. Blood sugar levels then are tested periodically for two hours. Results are interpreted as follows:
- Less than 140 mg/dL (7.8 mmol/L ) after two hours is considered healthy.
- 140 to 199 mg/dL (7.8 mmol/L and 11.0 mmol/L ) is diagnosed as prediabetes.
- 200 mg/dL (11.1 mmol/L ) or higher after two hours suggests diabetes.
Screening. The American Diabetes Association recommends routine screening with diagnostic tests for type 2 diabetes in all adults age 35 or older and in the following groups:
- People younger than 35 who are overweight or obese and have one or more risk factors associated with diabetes.
- Women who have had gestational diabetes.
- People who have been diagnosed with prediabetes.
- Children who are overweight or obese and who have a family history of type 2 diabetes or other risk factors.
After a diagnosis
If you're diagnosed with diabetes, your health care provider may do other tests to distinguish between type 1 and type 2 diabetes because the two conditions often require different treatments.
Your health care provider will test A1C levels at least two times a year and when there are any changes in treatment. Target A1C goals vary depending on age and other factors. For most people, the American Diabetes Association recommends an A1C level below 7%.
You also receive tests to screen for complications of diabetes and other medical conditions.
More Information
- Glucose tolerance test
Management of type 2 diabetes includes:
- Healthy eating.
- Regular exercise.
- Weight loss.
- Possibly, diabetes medication or insulin therapy.
- Blood sugar monitoring.
These steps make it more likely that blood sugar will stay in a healthy range. And they may help to delay or prevent complications.
Healthy eating
There's no specific diabetes diet. However, it's important to center your diet around:
- A regular schedule for meals and healthy snacks.
- Smaller portion sizes.
- More high-fiber foods, such as fruits, nonstarchy vegetables and whole grains.
- Fewer refined grains, starchy vegetables and sweets.
- Modest servings of low-fat dairy, low-fat meats and fish.
- Healthy cooking oils, such as olive oil or canola oil.
- Fewer calories.
Your health care provider may recommend seeing a registered dietitian, who can help you:
- Identify healthy food choices.
- Plan well-balanced, nutritional meals.
- Develop new habits and address barriers to changing habits.
- Monitor carbohydrate intake to keep your blood sugar levels more stable.
Physical activity
Exercise is important for losing weight or maintaining a healthy weight. It also helps with managing blood sugar. Talk to your health care provider before starting or changing your exercise program to ensure that activities are safe for you.
- Aerobic exercise. Choose an aerobic exercise that you enjoy, such as walking, swimming, biking or running. Adults should aim for 30 minutes or more of moderate aerobic exercise on most days of the week, or at least 150 minutes a week.
- Resistance exercise. Resistance exercise increases your strength, balance and ability to perform activities of daily living more easily. Resistance training includes weightlifting, yoga and calisthenics. Adults living with type 2 diabetes should aim for 2 to 3 sessions of resistance exercise each week.
- Limit inactivity. Breaking up long periods of inactivity, such as sitting at the computer, can help control blood sugar levels. Take a few minutes to stand, walk around or do some light activity every 30 minutes.
Weight loss
Weight loss results in better control of blood sugar levels, cholesterol, triglycerides and blood pressure. If you're overweight, you may begin to see improvements in these factors after losing as little as 5% of your body weight. However, the more weight you lose, the greater the benefit to your health. In some cases, losing up to 15% of body weight may be recommended.
Your health care provider or dietitian can help you set appropriate weight-loss goals and encourage lifestyle changes to help you achieve them.
Monitoring your blood sugar
Your health care provider will advise you on how often to check your blood sugar level to make sure you remain within your target range. You may, for example, need to check it once a day and before or after exercise. If you take insulin, you may need to check your blood sugar multiple times a day.
Monitoring is usually done with a small, at-home device called a blood glucose meter, which measures the amount of sugar in a drop of blood. Keep a record of your measurements to share with your health care team.
Continuous glucose monitoring is an electronic system that records glucose levels every few minutes from a sensor placed under the skin. Information can be transmitted to a mobile device such as a phone, and the system can send alerts when levels are too high or too low.
Diabetes medications
If you can't maintain your target blood sugar level with diet and exercise, your health care provider may prescribe diabetes medications that help lower glucose levels, or your provider may suggest insulin therapy. Medicines for type 2 diabetes include the following.
Metformin (Fortamet, Glumetza, others) is generally the first medicine prescribed for type 2 diabetes. It works mainly by lowering glucose production in the liver and improving the body's sensitivity to insulin so it uses insulin more effectively.
Some people experience B-12 deficiency and may need to take supplements. Other possible side effects, which may improve over time, include:
- Abdominal pain.
Sulfonylureas help the body secrete more insulin. Examples include glyburide (DiaBeta, Glynase), glipizide (Glucotrol XL) and glimepiride (Amaryl). Possible side effects include:
- Low blood sugar.
- Weight gain.
Glinides stimulate the pancreas to secrete more insulin. They're faster acting than sulfonylureas. But their effect in the body is shorter. Examples include repaglinide and nateglinide. Possible side effects include:
Thiazolidinediones make the body's tissues more sensitive to insulin. An example of this medicine is pioglitazone (Actos). Possible side effects include:
- Risk of congestive heart failure.
- Risk of bladder cancer (pioglitazone).
- Risk of bone fractures.
DPP-4 inhibitors help reduce blood sugar levels but tend to have a very modest effect. Examples include sitagliptin (Januvia), saxagliptin (Onglyza) and linagliptin (Tradjenta). Possible side effects include:
- Risk of pancreatitis.
- Joint pain.
GLP-1 receptor agonists are injectable medications that slow digestion and help lower blood sugar levels. Their use is often associated with weight loss, and some may reduce the risk of heart attack and stroke. Examples include exenatide (Byetta, Bydureon Bcise), liraglutide (Saxenda, Victoza) and semaglutide (Rybelsus, Ozempic, Wegovy). Possible side effects include:
SGLT2 inhibitors affect the blood-filtering functions in the kidneys by blocking the return of glucose to the bloodstream. As a result, glucose is removed in the urine. These medicines may reduce the risk of heart attack and stroke in people with a high risk of those conditions. Examples include canagliflozin (Invokana), dapagliflozin (Farxiga) and empagliflozin (Jardiance). Possible side effects include:
- Vaginal yeast infections.
- Urinary tract infections.
- Low blood pressure.
- High cholesterol.
- Risk of gangrene.
- Risk of bone fractures (canagliflozin).
- Risk of amputation (canagliflozin).
Other medicines your health care provider might prescribe in addition to diabetes medications include blood pressure and cholesterol-lowering medicines, as well as low-dose aspirin, to help prevent heart and blood vessel disease.
Insulin therapy
Some people who have type 2 diabetes need insulin therapy. In the past, insulin therapy was used as a last resort, but today it may be prescribed sooner if blood sugar targets aren't met with lifestyle changes and other medicines.
Different types of insulin vary on how quickly they begin to work and how long they have an effect. Long-acting insulin, for example, is designed to work overnight or throughout the day to keep blood sugar levels stable. Short-acting insulin generally is used at mealtime.
Your health care provider will determine what type of insulin is right for you and when you should take it. Your insulin type, dosage and schedule may change depending on how stable your blood sugar levels are. Most types of insulin are taken by injection.
Side effects of insulin include the risk of low blood sugar — a condition called hypoglycemia — diabetic ketoacidosis and high triglycerides.
Weight-loss surgery
Weight-loss surgery changes the shape and function of the digestive system. This surgery may help you lose weight and manage type 2 diabetes and other conditions related to obesity. There are several surgical procedures. All of them help people lose weight by limiting how much food they can eat. Some procedures also limit the amount of nutrients the body can absorb.
Weight-loss surgery is only one part of an overall treatment plan. Treatment also includes diet and nutritional supplement guidelines, exercise and mental health care.
Generally, weight-loss surgery may be an option for adults living with type 2 diabetes who have a body mass index (BMI) of 35 or higher. BMI is a formula that uses weight and height to estimate body fat. Depending on the severity of diabetes or the presence of other medical conditions, surgery may be an option for someone with a BMI lower than 35.
Weight-loss surgery requires a lifelong commitment to lifestyle changes. Long-term side effects may include nutritional deficiencies and osteoporosis.
People living with type 2 diabetes often need to change their treatment plan during pregnancy and follow a diet that controls carbohydrates. Many people need insulin therapy during pregnancy. They also may need to stop other treatments, such as blood pressure medicines.
There is an increased risk during pregnancy of developing a condition that affects the eyes called diabetic retinopathy. In some cases, this condition may get worse during pregnancy. If you are pregnant, visit an ophthalmologist during each trimester of your pregnancy and one year after you give birth. Or as often as your health care provider suggests.
Signs of trouble
Regularly monitoring your blood sugar levels is important to avoid severe complications. Also, be aware of symptoms that may suggest irregular blood sugar levels and the need for immediate care:
High blood sugar. This condition also is called hyperglycemia. Eating certain foods or too much food, being sick, or not taking medications at the right time can cause high blood sugar. Symptoms include:
- Frequent urination.
- Increased thirst.
- Blurred vision.
Hyperglycemic hyperosmolar nonketotic syndrome (HHNS). This life-threatening condition includes a blood sugar reading higher than 600 mg/dL (33.3 mmol/L ). HHNS may be more likely if you have an infection, are not taking medicines as prescribed, or take certain steroids or drugs that cause frequent urination. Symptoms include:
- Extreme thirst.
- Drowsiness.
- Dark urine.
Diabetic ketoacidosis. Diabetic ketoacidosis occurs when a lack of insulin results in the body breaking down fat for fuel rather than sugar. This results in a buildup of acids called ketones in the bloodstream. Triggers of diabetic ketoacidosis include certain illnesses, pregnancy, trauma and medicines — including the diabetes medicines called SGLT2 inhibitors.
The toxicity of the acids made by diabetic ketoacidosis can be life-threatening. In addition to the symptoms of hyperglycemia, such as frequent urination and increased thirst, ketoacidosis may cause:
- Shortness of breath.
- Fruity-smelling breath.
Low blood sugar. If your blood sugar level drops below your target range, it's known as low blood sugar. This condition also is called hypoglycemia. Your blood sugar level can drop for many reasons, including skipping a meal, unintentionally taking more medication than usual or being more physically active than usual. Symptoms include:
- Irritability.
- Heart palpitations.
- Slurred speech.
If you have symptoms of low blood sugar, drink or eat something that will quickly raise your blood sugar level. Examples include fruit juice, glucose tablets, hard candy or another source of sugar. Retest your blood in 15 minutes. If levels are not at your target, eat or drink another source of sugar. Eat a meal after your blood sugar level returns to normal.
If you lose consciousness, you need to be given an emergency injection of glucagon, a hormone that stimulates the release of sugar into the blood.
- Medications for type 2 diabetes
- GLP-1 agonists: Diabetes drugs and weight loss
- Bariatric surgery
- Endoscopic sleeve gastroplasty
- Gastric bypass (Roux-en-Y)
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
Careful management of type 2 diabetes can reduce the risk of serious — even life-threatening — complications. Consider these tips:
- Commit to managing your diabetes. Learn all you can about type 2 diabetes. Make healthy eating and physical activity part of your daily routine.
- Work with your team. Establish a relationship with a certified diabetes education specialist, and ask your diabetes treatment team for help when you need it.
- Identify yourself. Wear a necklace or bracelet that says you are living with diabetes, especially if you take insulin or other blood sugar-lowering medicine.
- Schedule a yearly physical exam and regular eye exams. Your diabetes checkups aren't meant to replace regular physicals or routine eye exams.
- Keep your vaccinations up to date. High blood sugar can weaken your immune system. Get a flu shot every year. Your health care provider also may recommend the pneumonia vaccine. The Centers for Disease Control and Prevention (CDC) also recommends the hepatitis B vaccination if you haven't previously received this vaccine and you're 19 to 59 years old. Talk to your health care provider about other vaccinations you may need.
- Take care of your teeth. Diabetes may leave you prone to more-serious gum infections. Brush and floss your teeth regularly and schedule recommended dental exams. Contact your dentist right away if your gums bleed or look red or swollen.
- Pay attention to your feet. Wash your feet daily in lukewarm water, dry them gently, especially between the toes, and moisturize them with lotion. Check your feet every day for blisters, cuts, sores, redness and swelling. Contact your health care provider if you have a sore or other foot problem that isn't healing.
- Keep your blood pressure and cholesterol under control. Eating healthy foods and exercising regularly can go a long way toward controlling high blood pressure and cholesterol. Take medication as prescribed.
- If you smoke or use other types of tobacco, ask your health care provider to help you quit. Smoking increases your risk of diabetes complications. Talk to your health care provider about ways to stop using tobacco.
- Use alcohol sparingly. Depending on the type of drink, alcohol may lower or raise blood sugar levels. If you choose to drink alcohol, only do so with a meal. The recommendation is no more than one drink daily for women and no more than two drinks daily for men. Check your blood sugar frequently after drinking alcohol.
- Make healthy sleep a priority. Many people with type 2 diabetes have sleep problems. And not getting enough sleep may make it harder to keep blood sugar levels in a healthy range. If you have trouble sleeping, talk to your health care provider about treatment options.
- Caffeine: Does it affect blood sugar?
Alternative medicine
Many alternative medicine treatments claim to help people living with diabetes. According to the National Center for Complementary and Integrative Health, studies haven't provided enough evidence to recommend any alternative therapies for blood sugar management. Research has shown the following results about popular supplements for type 2 diabetes:
- Chromium supplements have been shown to have few or no benefits. Large doses can result in kidney damage, muscle problems and skin reactions.
- Magnesium supplements have shown benefits for blood sugar control in some but not all studies. Side effects include diarrhea and cramping. Very large doses — more than 5,000 mg a day — can be fatal.
- Cinnamon, in some studies, has lowered fasting glucose levels but not A1C levels. Therefore, there's no evidence of overall improved glucose management.
Talk to your health care provider before starting a dietary supplement or natural remedy. Do not replace your prescribed diabetes medicines with alternative medicines.
Coping and support
Type 2 diabetes is a serious disease, and following your diabetes treatment plan takes commitment. To effectively manage diabetes, you may need a good support network.
Anxiety and depression are common in people living with diabetes. Talking to a counselor or therapist may help you cope with the lifestyle changes and stress that come with a type 2 diabetes diagnosis.
Support groups can be good sources of diabetes education, emotional support and helpful information, such as how to find local resources or where to find carbohydrate counts for a favorite restaurant. If you're interested, your health care provider may be able to recommend a group in your area.
You can visit the American Diabetes Association website to check out local activities and support groups for people living with type 2 diabetes. The American Diabetes Association also offers online information and online forums where you can chat with others who are living with diabetes. You also can call the organization at 800-DIABETES ( 800-342-2383 ).
Preparing for your appointment
At your annual wellness visit, your health care provider can screen for diabetes and monitor and treat conditions that increase your risk of diabetes, such as high blood pressure, high cholesterol or a high BMI .
If you are seeing your health care provider because of symptoms that may be related to diabetes, you can prepare for your appointment by being ready to answer the following questions:
- When did your symptoms begin?
- Does anything improve the symptoms or worsen the symptoms?
- What medicines do you take regularly, including dietary supplements and herbal remedies?
- What are your typical daily meals? Do you eat between meals or before bedtime?
- How much alcohol do you drink?
- How much daily exercise do you get?
- Is there a history of diabetes in your family?
If you are diagnosed with diabetes, your health care provider may begin a treatment plan. Or you may be referred to a doctor who specializes in hormonal disorders, called an endocrinologist. Your care team also may include the following specialists:
- Certified diabetes education specialist.
- Foot doctor, also called a podiatrist.
- Doctor who specializes in eye care, called an ophthalmologist.
Talk to your health care provider about referrals to other specialists who may be providing care.
Questions for ongoing appointments
Before any appointment with a member of your treatment team, make sure you know whether there are any restrictions, such as not eating or drinking before taking a test. Questions that you should regularly talk about with your health care provider or other members of the team include:
- How often do I need to monitor my blood sugar, and what is my target range?
- What changes in my diet would help me better manage my blood sugar?
- What is the right dosage for prescribed medications?
- When do I take the medications? Do I take them with food?
- How does management of diabetes affect treatment for other conditions? How can I better coordinate treatments or care?
- When do I need to make a follow-up appointment?
- Under what conditions should I call you or seek emergency care?
- Are there brochures or online sources you recommend?
- Are there resources available if I'm having trouble paying for diabetes supplies?
What to expect from your doctor
Your health care provider is likely to ask you questions at your appointments. Those questions may include:
- Do you understand your treatment plan and feel confident you can follow it?
- How are you coping with diabetes?
- Have you had any low blood sugar?
- Do you know what to do if your blood sugar is too low or too high?
- What's a typical day's diet like?
- Are you exercising? If so, what type of exercise? How often?
- Do you sit for long periods of time?
- What challenges are you experiencing in managing your diabetes?
- Professional Practice Committee: Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020; doi:10.2337/dc20-Sppc.
- Diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed Dec. 7, 2020.
- Melmed S, et al. Williams Textbook of Endocrinology. 14th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 3, 2020.
- Diabetes overview. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/all-content. Accessed Dec. 4, 2020.
- AskMayoExpert. Type 2 diabetes. Mayo Clinic; 2018.
- Feldman M, et al., eds. Surgical and endoscopic treatment of obesity. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 20, 2020.
- Hypersmolar hyperglycemic state (HHS). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hyperosmolar-hyperglycemic-state-hhs. Accessed Dec. 11, 2020.
- Diabetic ketoacidosis (DKA). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetic-ketoacidosis-dka. Accessed Dec. 11, 2020.
- Hypoglycemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hypoglycemia. Accessed Dec. 11, 2020.
- 6 things to know about diabetes and dietary supplements. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/tips/things-to-know-about-type-diabetes-and-dietary-supplements. Accessed Dec. 11, 2020.
- Type 2 diabetes and dietary supplements: What the science says. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/providers/digest/type-2-diabetes-and-dietary-supplements-science. Accessed Dec. 11, 2020.
- Preventing diabetes problems. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/all-content. Accessed Dec. 3, 2020.
- Schillie S, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and Reports. 2018; doi:10.15585/mmwr.rr6701a1.
- Diabetes prevention: 5 tips for taking control
- Hyperinsulinemia: Is it diabetes?
Associated Procedures
News from mayo clinic.
- Mayo study uses electronic health record data to assess metformin failure risk, optimize care Feb. 10, 2023, 02:30 p.m. CDT
- Mayo Clinic Minute: Strategies to break the heart disease and diabetes link Nov. 28, 2022, 05:15 p.m. CDT
- Mayo Clinic Q and A: Diabetes risk in Hispanic people Oct. 20, 2022, 12:15 p.m. CDT
- The importance of diagnosing, treating diabetes in the Hispanic population in the US Sept. 28, 2022, 04:00 p.m. CDT
- Mayo Clinic Minute: Managing Type 2 diabetes Sept. 28, 2022, 02:30 p.m. CDT
Products & Services
- A Book: The Essential Diabetes Book
- A Book: The Mayo Clinic Diabetes Diet
- Assortment of Health Products from Mayo Clinic Store
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
How to Thrive as You Age
A cheap drug may slow down aging. a study will determine if it works.

Allison Aubrey

A drug taken by millions of people to control diabetes may do more than lower blood sugar.
Research suggests metformin has anti-inflammatory effects that could help protect against common age-related diseases including heart disease, cancer, and cognitive decline.
Scientists who study the biology of aging have designed a clinical study, known as The TAME Trial, to test whether metformin can help prevent these diseases and promote a longer healthspan in healthy, older adults.
Michael Cantor, an attorney, and his wife Shari Cantor , the mayor of West Hartford, Connecticut both take metformin. "I tell all my friends about it," Michael Cantor says. "We all want to live a little longer, high-quality life if we can," he says.
Michael Cantor started on metformin about a decade ago when his weight and blood sugar were creeping up. Shari Cantor began taking metformin during the pandemic after she read that it may help protect against serious infections.

Shari and Michael Cantor both take metformin. They are both in their mid-60s and say they feel healthy and full of energy. Theresa Oberst/Michael Cantor hide caption
Shari and Michael Cantor both take metformin. They are both in their mid-60s and say they feel healthy and full of energy.
The Cantors are in their mid-60s and both say they feel healthy and have lots of energy. Both noticed improvements in their digestive systems – feeling more "regular" after they started on the drug,
Metformin costs less than a dollar a day, and depending on insurance, many people pay no out-of-pocket costs for the drug.
"I don't know if metformin increases lifespan in people, but the evidence that exists suggests that it very well might," says Steven Austad , a senior scientific advisor at the American Federation for Aging Research who studies the biology of aging.
An old drug with surprising benefits
Metformin was first used to treat diabetes in the 1950s in France. The drug is a derivative of guanidine , a compound found in Goat's Rue, an herbal medicine long used in Europe.
The FDA approved metformin for the treatment of type 2 diabetes in the U.S. in the 1990s. Since then, researchers have documented several surprises, including a reduced risk of cancer. "That was a bit of a shock," Austad says. A meta-analysis that included data from dozens of studies, found people who took metformin had a lower risk of several types of cancers , including gastrointestinal, urologic and blood cancers.
Austad also points to a British study that found a lower risk of dementia and mild cognitive decline among people with type 2 diabetes taking metformin. In addition, there's research pointing to improved cardiovascular outcomes in people who take metformin including a reduced risk of cardiovascular death .
As promising as this sounds, Austad says most of the evidence is observational, pointing only to an association between metformin and the reduced risk. The evidence stops short of proving cause and effect. Also, it's unknown if the benefits documented in people with diabetes will also reduce the risk of age-related diseases in healthy, older adults.
"That's what we need to figure out," says Steve Kritchevsky , a professor of gerontology at Wake Forest School of Medicine, who is a lead investigator for the Tame Trial.
The goal is to better understand the mechanisms and pathways by which metformin works in the body. For instance, researchers are looking at how the drug may help improve energy in the cells by stimulating autophagy, which is the process of clearing out or recycling damaged bits inside cells.

Shots - Health News
Scientists can tell how fast you're aging. now, the trick is to slow it down.

You can order a test to find out your biological age. Is it worth it?
Researchers also want to know more about how metformin can help reduce inflammation and oxidative stress, which may slow biological aging.
"When there's an excess of oxidative stress, it will damage the cell. And that accumulation of damage is essentially what aging is," Kritchevsky explains.
When the forces that are damaging cells are running faster than the forces that are repairing or replacing cells, that's aging, Kritchevsky says. And it's possible that drugs like metformin could slow this process down.
By targeting the biology of aging, the hope is to prevent or delay multiple diseases, says Dr. Nir Barzilai of Albert Einstein College of Medicine, who leads the effort to get the trial started.
The ultimate in preventative medicine
Back in 2015, Austad and a bunch of aging researchers began pushing for a clinical trial.
"A bunch of us went to the FDA to ask them to approve a trial for metformin,' Austad recalls, and the agency was receptive. "If you could help prevent multiple problems at the same time, like we think metformin may do, then that's almost the ultimate in preventative medicine," Austad says.
The aim is to enroll 3,000 people between the ages of 65 and 79 for a six-year trial. But Dr. Barzilai says it's been slow going to get it funded. "The main obstacle with funding this study is that metformin is a generic drug, so no pharmaceutical company is standing to make money," he says.
Barzilai has turned to philanthropists and foundations, and has some pledges. The National Institute on Aging, part of the National Institutes of Health, set aside about $5 million for the research, but that's not enough to pay for the study which is estimated to cost between $45 and $70 million.
The frustration over the lack of funding is that if the trial points to protective effects, millions of people could benefit. "It's something that everybody will be able to afford," Barzilai says.
Currently the FDA doesn't recognize aging as a disease to treat, but the researchers hope this would usher in a paradigm shift — from treating each age-related medical condition separately, to treating these conditions together, by targeting aging itself.
For now, metformin is only approved to treat type 2 diabetes in the U.S., but doctors can prescribe it off-label for conditions other than its approved use .
Michael and Shari Cantor's doctors were comfortable prescribing it to them, given the drug's long history of safety and the possible benefits in delaying age-related disease.
"I walk a lot, I hike, and at 65 I have a lot of energy," Michael Cantor says. I feel like the metformin helps," he says. He and Shari say they have not experienced any negative side effects.
Research shows a small percentage of people who take metformin experience GI distress that makes the drug intolerable. And, some people develop a b12 vitamin deficiency. One study found people over the age of 65 who take metformin may have a harder time building new muscle.

Millions of women are 'under-muscled.' These foods help build strength
"There's some evidence that people who exercise who are on metformin have less gain in muscle mass, says Dr. Eric Verdin , President of the Buck Institute for Research on Aging. That could be a concern for people who are under-muscled .
But Verdin says it may be possible to repurpose metformin in other ways "There are a number of companies that are exploring metformin in combination with other drugs," he says. He points to research underway to combine metformin with a drug called galantamine for the treatment of sarcopenia , which is the medical term for age-related muscle loss. Sarcopenia affects millions of older people, especially women .
The science of testing drugs to target aging is rapidly advancing, and metformin isn't the only medicine that may treat the underlying biology.
"Nobody thinks this is the be all and end all of drugs that target aging," Austad says. He says data from the clinical trial could stimulate investment by the big pharmaceutical companies in this area. "They may come up with much better drugs," he says.
Michael Cantor knows there's no guarantee with metformin. "Maybe it doesn't do what we think it does in terms of longevity, but it's certainly not going to do me any harm," he says.
Cantor's father had his first heart attack at 51. He says he wants to do all he can to prevent disease and live a healthy life, and he thinks Metformin is one tool that may help.
For now, Dr. Barzilai says the metformin clinical trial can get underway when the money comes in.

7 habits to live a healthier life, inspired by the world's longest-lived communities
This story was edited by Jane Greenhalgh
Proteomic Analyses in Diverse Populations Improved Risk Prediction and Identified New Drug Targets for Type 2 Diabetes
Affiliations.
- 1 Clinical Trial Service Unit & Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, Oxford, U.K.
- 2 Medical Research Council Health Research Unit, Nuffield Department of Population Health, University of Oxford, Oxford, U.K.
- 3 Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China.
- 4 Peking University Center for Public Health and Epidemic Preparedness & Response, Beijing, China.
- 5 Key Laboratory of Epidemiology of Major Diseases (Peking University), Ministry of Education, Beijing, China.
- PMID: 38623619
- DOI: 10.2337/dc23-2145
Objective: Integrated analyses of plasma proteomics and genetic data in prospective studies can help assess the causal relevance of proteins, improve risk prediction, and discover novel protein drug targets for type 2 diabetes (T2D).
Research design and methods: We measured plasma levels of 2,923 proteins using Olink Explore among ∼2,000 randomly selected participants from China Kadoorie Biobank (CKB) without prior diabetes at baseline. Cox regression assessed associations of individual protein with incident T2D (n = 92 cases). Proteomic-based risk models were developed with discrimination, calibration, reclassification assessed using area under the curve (AUC), calibration plots, and net reclassification index (NRI), respectively. Two-sample Mendelian randomization (MR) analyses using cis-protein quantitative trait loci identified in a genome-wide association study of CKB and UK Biobank for specific proteins were conducted to assess their causal relevance for T2D, along with colocalization analyses to examine shared causal variants between proteins and T2D.
Results: Overall, 33 proteins were significantly associated (false discovery rate < 0.05) with risk of incident T2D, including IGFBP1, GHR, and amylase. The addition of these 33 proteins to a conventional risk prediction model improved AUC from 0.77 (0.73-0.82) to 0.88 (0.85-0.91) and NRI by 38%, with predicted risks well calibrated with observed risks. MR analyses provided support for the causal relevance for T2D of ENTR1, LPL, and PON3, with replication of ENTR1 and LPL in Europeans using different genetic instruments. Moreover, colocalization analyses showed strong evidence (pH4 > 0.6) of shared genetic variants of LPL and PON3 with T2D.
Conclusions: Proteomic analyses in Chinese adults identified novel associations of multiple proteins with T2D with strong genetic evidence supporting their causal relevance and potential as novel drug targets for prevention and treatment of T2D.
© 2024 by the American Diabetes Association.
Grants and funding
- C16077/A29186, C500/A16896/CRUK_/Cancer Research UK/United Kingdom
- CH/1996001/9454/BHF_/British Heart Foundation/United Kingdom
Siblings with unique genetic change help scientists progress drug search for type 1 diabetes
Two siblings who have the only known mutations in a key gene anywhere in the world have helped scientists gain new insights that could help progress the search for new treatments in type 1 diabetes.
Type 1 diabetes (also known as autoimmune diabetes) is a devastating and life-long disease, in which the patient's immune cells wrongly destroy the insulin producing beta cells in the pancreas. People living with autoimmune diabetes need to test their blood sugar and inject insulin throughout their lives to control their blood sugars and prevent complications.
Autoimmune diabetes with clinical onset in very early childhood is rare and can result from a variety of genetic variants. However, there are many cases of early onset diabetes without known genetic explanation. In addition, some cancer patients treated with a category of immunotherapy known as immune checkpoint inhibitors -- which target the same pathway that the mutation was found in -- are prone to developing autoimmune diabetes. The reason why only this category of cancer immunotherapy can trigger autoimmune diabetes is not well understood. Like type 1 diabetes, genetic or immunotherapy-associated autoimmune diabetes requires life-long insulin replacement therapy -- there is currently no cure.
The new research, published in the Journal of Experimental Medicine , began when researchers studied two siblings who were diagnosed with a rare genetic form of autoimmune diabetes in the first weeks of life. The University of Exeter offers free genetic testing worldwide for babies diagnosed with diabetes before they are nine months old. For most of these babies, this service provides a genetic diagnosis and in around half of these babies, it allows for a change in treatment.
When researchers tested the two siblings in the study, no mutation in any of the known causes was identified. The Exeter team then performed whole genome sequencing to look for previously unknown causes of autoimmune diabetes. Through this sequencing, they found a mutation in the gene encoding PD-L1 in the siblings and realised it could be responsible for their very-early-onset autoimmune diabetes.
Study authorDr Matthew Johnson, from the University of Exeter, UK, said: "PD-L1 has been particularly well studied in animal models because of its crucial function in sending a stop signal to the immune system and its relevance to cancer immunotherapy. But, to our knowledge, nobody has ever found humans with a disease-causing mutation in the gene encoding PD-L1. We searched the globe, looking at all the large-scale datasets that we know of, and we haven't been able to find another family. These siblings therefore provide us with a unique and incredibly important opportunity to investigate what happens when this gene is disabled in humans."
The PD-L1 protein is expressed on many different cell types. Its receptor, PD-1, is expressed exclusively on immune cells. When the two proteins bind together it provides a stop signal to the immune system, preventing collateral damage to the bodies tissues and organs.
Researchers from the Rockefeller Institute in New York and King's College London joined forces with Exeter to study the siblings, with funding from Wellcome, The Leona M. and Harry B. Helmsley Charitable Trust, Diabetes UK, and the US National Institutes for Health. After contacting the family's clinician in Morocco, the Exeter team visited the siblings where they were living to collect samples and return them to King's College London, within the crucial ten-hour window for analysis while the immune cells were still alive. The London and New York teams then performed extensive analysis on the siblings' cells.
Study co-author Dr Masato Ogishi, from the Rockefeller University in New York, said: "We first showed that the mutation completely disabled the function of PD-L1 protein. We then studied the immune system of the siblings to look for immunological abnormalities that could account for their extremely early-onset diabetes. As we previously described another two siblings with PD-1 deficiency, both of whom had multi-organ autoimmunity including autoimmune diabetes and extensive dysregulation in their immune cells, we expected to find severe dysregulation of the immune system in the PD-L1-deficient siblings. To our great surprise, their immune systems looked pretty much normal in almost all aspects throughout the study. Therefore, PD-L1 is certainly indispensable for preventing autoimmune diabetes but is dispensable for many other aspects of human immune system. We think that PD-L2, another ligand of PD-1, albeit less well-studied than PD-L1, may be serving as a back-up system when PD-L1 is not available. This concept needs to be further investigated in the context of artificial blockade for PD-L1 as cancer immunotherapy."
Study co-author Professor Timothy Tree, from King's College London, said: "Through studying this one set of siblings -- unique in the world to our knowledge -- we have found that the PD-L1 gene is essential for avoiding autoimmune diabetes, but is not essential for 'everyday' immune function. This leads us to the grand question; 'what is the role of PD-L1 in our pancreas making it critical for preventing our immune cells destroying our beta cells?' We know that under certain conditions beta cells express PD-L1. However, certain types of immune cells in the pancreas also express PD-L1. We now need to work out the "communication" between different cell types that is critical for preventing autoimmune diabetes.
"This finding increases our knowledge of how autoimmune forms of diabetes such as type 1 diabetes develop. It opens up a new potential target for treatments that could prevent diabetes in the future. Simultaneously, it gives new knowledge to the cancer immunotherapy field by uniquely providing the results of completely disabling PD-L1 in a person, something you could never manipulate in studies. Reducing PD-L1 is already effective for cancer treatment, and boosting it is now being investigated as a type 1 diabetes treatment -- our findings will help accelerate the search for new and better drugs."
Dr Lucy Chambers, Head of Research Communications at Diabetes UK, said: "Pioneering treatments that alter the behaviour of the immune system to hold off its attack on the pancreas are already advancing type 1 diabetes treatment in the USA, and are awaiting approval here in the UK.
"By zeroing in on the precise role of an important player in the type 1 diabetes immune attack, this exciting discovery could pave the way for treatments that are more effective, more targeted and more transformational for people with or at risk of type 1 diabetes."
Helmsley Program Officer Ben Williams said: "New drugs often fail in development because scientific discoveries made in animal models don't translate into humans. As such, drug developers strongly prefer to pursue new drugs where human genetic evidence supports the drug's target. This study provides such compelling evidence that PD-L1 is a high-priority target to treat T1D, and should be pursued with the ambition of eventually reducing the burden of this difficult to manage disease."
The paper is entitled 'Human inherited PD-L1 deficiency is clinically and immunologically less severe than PD-1 deficiency' and is published in the Journal of Experimental Medicine. The research was supported by the National Institute of Health and Care Research (NIHR) Exeter Biomedical Research Centre and The NIHR Exeter Clinical Research Facility.
- Immune System
- Personalized Medicine
- Diseases and Conditions
- Hormone Disorders
- Medical Topics
- Diabetes mellitus type 1
- Diabetes mellitus type 2
- Erectile dysfunction
- Gene therapy
- Alzheimer's disease
Story Source:
Materials provided by University of Exeter . Original written by Louise Vennells. Note: Content may be edited for style and length.
Journal Reference :
- Matthew B. Johnson et al. Human inherited PD-L1 deficiency is clinically and immunologically less severe than PD-1 deficiency . JEM , 2024 DOI: 10.1084/jem.20231704
Cite This Page :
Explore More
- Fossil Frogs Share Their Skincare Secrets
- Fussy Eater? Most Parents Play Short Order Cook
- Precise Time Measurement: Superradiant Atoms
- Artificial Cells That Act Like Living Cells
- Affordable and Targeted Anticancer Agent
- This Alloy Is Kinky
- Giant Galactic Explosion: Galaxy Pollution
- Flare Erupting Around a Black Hole
- Two Species Interbreeding Created New Butterfly
- Warming Antarctic Deep-Sea and Sea Level Rise
Trending Topics
Strange & offbeat.

IMAGES
VIDEO
COMMENTS
The new research, published in the journal Nature Communications, offers a potential strategy for developing new therapies that could restore dysfunctional pancreatic beta-cells or, perhaps, even prevent Type 2 diabetes from developing. The new study shows that the beta-cells of Type 2 diabetes patients are deficient in a cell trafficking ...
New cause of diabetes discovered, offering potential target for new classes of drugs to treat the disease. ScienceDaily . Retrieved April 22, 2024 from www.sciencedaily.com / releases / 2023 / 12 ...
Type 2 diabetes mellitus, the most frequent subtype of diabetes, is a disease characterized by high levels of blood glucose (hyperglycaemia). ... Latest Research and Reviews. ... including the ...
June 24, 2021 — Across the world, type 2 diabetes is on the rise. A research group has discovered a new gene that may hold the key to preventing and treating lifestyle related diseases such as ...
Type 2 diabetes affects more than 34 million U.S. adults and significantly increases the risk of ... In line with recent research, 11 updated estimates in our study showed that glycemic control ...
Recent Advances. ADA-funded researchers use the money from their awards to conduct critical diabetes research. In time, they publish their findings in order to inform fellow scientists of their results, which ensures that others will build upon their work. Ultimately, this cycle drives advances to prevent diabetes and to help people burdened by it.
Large-scale study reveals new genetic details of diabetes. In experiments of unprecedented scale, investigators at Weill Cornell Medicine and the National Institutes of Health have revealed new aspects of the complex genetics behind Type 2 diabetes. Through these discoveries, and by providing a template for future studies, this research ...
Despite the successful development of new therapies for the treatment of type 2 diabetes, such as glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 inhibitors, the search for novel treatment options that can provide better glycaemic control and at reduce complications is a continuous effort. The present Review aims to present an overview of novel targets and ...
In people with obesity, bariatric surgery can cause remission of type 2 diabetes, and therapeutic alternatives are being sought to achieve levels of weight loss comparable to surgery 2. Insulin ...
Read the latest Research articles in Type 2 diabetes from Nature Reviews Endocrinology ... Evidence indicates an elevated risk of type 2 diabetes mellitus after breast cancer treatment ...
July 22, 2022 — Scientists have discovered a pathway to the regeneration of insulin in pancreatic stem cells, a major breakthrough toward new therapies to treat Type 1 and Type 2 diabetes. Using ...
The chronic complications of type 2 diabetes are a major cause of mortality and disability worldwide [ 1, 2 ]. Clinical research is the main way to gain knowledge about long-term diabetic complications and reduce the burden of diabetes. This allows for designing effective programs for screening and follow-up and fine-targeted therapeutic ...
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Testing and keeping track of your blood glucose levels. Eating a balanced diet with the right ratio of carbs, protein, and fiber for you. Exercising regularly. Taking your prescribed medications ...
The ADA is committed to continuing progress in the fight against type 2 diabetes by funding research, including support for potential new treatments, a better understating of genetic factors, addressing disparities, and more. For specific examples of projects currently funded by the ADA, see below. Greg J. Morton, PhD.
Recent diabetes research includes advances in insulin therapy, cutting-edge diabetes management devices and promising therapies such as immunotherapy and β-cell regeneration. ... Type 2 diabetes ...
Insulin-inhibitory receptor research offers hope for type 2 diabetes therapy. by Verena Schulz, Helmholtz Association of German Research Centres. Central inceptor immunoreactivity is restricted to ...
Surgery led to diabetes remission in roughly 28% of patients, compared with a remission rate of just 4% among the non-surgery group, according to the study results. More research has found that ...
New findings on pancreatic anatomy may affect diabetes research and treatment. Generation of volumetric and 3D-spatial data sets of the complete β-cell distribution across the human pancreas ...
The study recently published in Nature is the largest genome-wide association study of type 2 diabetes to date, and it included genomic data from 2,535,601 individuals, of whom 428,452 had type 2 ...
In Type 2 diabetes, the body struggles to maintain blood sugar levels in an acceptable range. Every cell in the body needs sugar as an energy source, but too much sugar can be toxic to cells. This ...
NIDDK Program Staff. Henry B. Burch, M.D. Clinical studies utilizing existing digital health technology for the prevention and treatment of type 2 diabetes, clinical and basic science studies involving non-neoplastic disorders of the thyroid, clinical studies involving medical and novel dietary treatment of type 2 diabetes.
New guidelines recommend GLP-1 drugs such as Ozempic to help treat type 2 diabetes in adults. A physicians' group says weight loss drugs such as Ozempic can help manage type 2 diabetes. NurPhoto ...
Type 1 diabetes; Type 2 diabetes; Latest Research and Reviews. ... The largest genome-wide association study for type 2 diabetes so far, which included several ancestry groups, led to the ...
A Comparative Effectiveness Study of Major Glycemia-lowering Medications for Treatment of Type 2 Diabetes Rochester, MN. The GRADE Study is a pragmatic, unmasked clinical trial that will compare commonly used diabetes medications, when combined with metformin, on glycemia-lowering effectiveness and patient-centered outcomes.
140 to 199 mg/dL (7.8 mmol/L and 11.0 mmol/L) is diagnosed as prediabetes. 200 mg/dL (11.1 mmol/L) or higher after two hours suggests diabetes. Screening. The American Diabetes Association recommends routine screening with diagnostic tests for type 2 diabetes in all adults age 35 or older and in the following groups:
The FDA approved metformin for the treatment of type 2 diabetes in the U.S. in the 1990s. Since then, researchers have documented several surprises, including a reduced risk of cancer. "That was a ...
Recent estimates reveal that more than 529 million people worldwide are living with diabetes (>90% have type 2 diabetes), with a projection of 1·31 billion cases by 2050. 1 Although the causes of type 2 diabetes are multifaceted, suboptimal dietary intakes play an important role. 2 Among various unhealthy dietary components, ultra-processed ...
Objective: Integrated analyses of plasma proteomics and genetic data in prospective studies can help assess the causal relevance of proteins, improve risk prediction, and discover novel protein drug targets for type 2 diabetes (T2D). Research design and methods: We measured plasma levels of 2,923 proteins using Olink Explore among ∼2,000 randomly selected participants from China Kadoorie ...
June 24, 2021 — Across the world, type 2 diabetes is on the rise. A research group has discovered a new gene that may hold the key to preventing and treating lifestyle related diseases such as ...