- A-Z Publications

Annual Review of Psychology
Volume 70, 2019, review article, how to do a systematic review: a best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses.
- Andy P. Siddaway 1 , Alex M. Wood 2 , and Larry V. Hedges 3
- View Affiliations Hide Affiliations Affiliations: 1 Behavioural Science Centre, Stirling Management School, University of Stirling, Stirling FK9 4LA, United Kingdom; email: [email protected] 2 Department of Psychological and Behavioural Science, London School of Economics and Political Science, London WC2A 2AE, United Kingdom 3 Department of Statistics, Northwestern University, Evanston, Illinois 60208, USA; email: [email protected]
- Vol. 70:747-770 (Volume publication date January 2019) https://doi.org/10.1146/annurev-psych-010418-102803
- First published as a Review in Advance on August 08, 2018
- Copyright © 2019 by Annual Reviews. All rights reserved
Systematic reviews are characterized by a methodical and replicable methodology and presentation. They involve a comprehensive search to locate all relevant published and unpublished work on a subject; a systematic integration of search results; and a critique of the extent, nature, and quality of evidence in relation to a particular research question. The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information. We outline core standards and principles and describe commonly encountered problems. Although this guide targets psychological scientists, its high level of abstraction makes it potentially relevant to any subject area or discipline. We argue that systematic reviews are a key methodology for clarifying whether and how research findings replicate and for explaining possible inconsistencies, and we call for researchers to conduct systematic reviews to help elucidate whether there is a replication crisis.
Article metrics loading...
Full text loading...
Literature Cited
- APA Publ. Commun. Board Work. Group J. Artic. Rep. Stand. 2008 . Reporting standards for research in psychology: Why do we need them? What might they be?. Am. Psychol . 63 : 848– 49 [Google Scholar]
- Baumeister RF 2013 . Writing a literature review. The Portable Mentor: Expert Guide to a Successful Career in Psychology MJ Prinstein, MD Patterson 119– 32 New York: Springer, 2nd ed.. [Google Scholar]
- Baumeister RF , Leary MR 1995 . The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 117 : 497– 529 [Google Scholar]
- Baumeister RF , Leary MR 1997 . Writing narrative literature reviews. Rev. Gen. Psychol. 3 : 311– 20 Presents a thorough and thoughtful guide to conducting narrative reviews. [Google Scholar]
- Bem DJ 1995 . Writing a review article for Psychological Bulletin. Psychol . Bull 118 : 172– 77 [Google Scholar]
- Borenstein M , Hedges LV , Higgins JPT , Rothstein HR 2009 . Introduction to Meta-Analysis New York: Wiley Presents a comprehensive introduction to meta-analysis. [Google Scholar]
- Borenstein M , Higgins JPT , Hedges LV , Rothstein HR 2017 . Basics of meta-analysis: I 2 is not an absolute measure of heterogeneity. Res. Synth. Methods 8 : 5– 18 [Google Scholar]
- Braver SL , Thoemmes FJ , Rosenthal R 2014 . Continuously cumulating meta-analysis and replicability. Perspect. Psychol. Sci. 9 : 333– 42 [Google Scholar]
- Bushman BJ 1994 . Vote-counting procedures. The Handbook of Research Synthesis H Cooper, LV Hedges 193– 214 New York: Russell Sage Found. [Google Scholar]
- Cesario J 2014 . Priming, replication, and the hardest science. Perspect. Psychol. Sci. 9 : 40– 48 [Google Scholar]
- Chalmers I 2007 . The lethal consequences of failing to make use of all relevant evidence about the effects of medical treatments: the importance of systematic reviews. Treating Individuals: From Randomised Trials to Personalised Medicine PM Rothwell 37– 58 London: Lancet [Google Scholar]
- Cochrane Collab. 2003 . Glossary Rep., Cochrane Collab. London: http://community.cochrane.org/glossary Presents a comprehensive glossary of terms relevant to systematic reviews. [Google Scholar]
- Cohn LD , Becker BJ 2003 . How meta-analysis increases statistical power. Psychol. Methods 8 : 243– 53 [Google Scholar]
- Cooper HM 2003 . Editorial. Psychol. Bull. 129 : 3– 9 [Google Scholar]
- Cooper HM 2016 . Research Synthesis and Meta-Analysis: A Step-by-Step Approach Thousand Oaks, CA: Sage, 5th ed.. Presents a comprehensive introduction to research synthesis and meta-analysis. [Google Scholar]
- Cooper HM , Hedges LV , Valentine JC 2009 . The Handbook of Research Synthesis and Meta-Analysis New York: Russell Sage Found, 2nd ed.. [Google Scholar]
- Cumming G 2014 . The new statistics: why and how. Psychol. Sci. 25 : 7– 29 Discusses the limitations of null hypothesis significance testing and viable alternative approaches. [Google Scholar]
- Earp BD , Trafimow D 2015 . Replication, falsification, and the crisis of confidence in social psychology. Front. Psychol. 6 : 621 [Google Scholar]
- Etz A , Vandekerckhove J 2016 . A Bayesian perspective on the reproducibility project: psychology. PLOS ONE 11 : e0149794 [Google Scholar]
- Ferguson CJ , Brannick MT 2012 . Publication bias in psychological science: prevalence, methods for identifying and controlling, and implications for the use of meta-analyses. Psychol. Methods 17 : 120– 28 [Google Scholar]
- Fleiss JL , Berlin JA 2009 . Effect sizes for dichotomous data. The Handbook of Research Synthesis and Meta-Analysis H Cooper, LV Hedges, JC Valentine 237– 53 New York: Russell Sage Found, 2nd ed.. [Google Scholar]
- Garside R 2014 . Should we appraise the quality of qualitative research reports for systematic reviews, and if so, how. Innovation 27 : 67– 79 [Google Scholar]
- Hedges LV , Olkin I 1980 . Vote count methods in research synthesis. Psychol. Bull. 88 : 359– 69 [Google Scholar]
- Hedges LV , Pigott TD 2001 . The power of statistical tests in meta-analysis. Psychol. Methods 6 : 203– 17 [Google Scholar]
- Higgins JPT , Green S 2011 . Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 London: Cochrane Collab. Presents comprehensive and regularly updated guidelines on systematic reviews. [Google Scholar]
- John LK , Loewenstein G , Prelec D 2012 . Measuring the prevalence of questionable research practices with incentives for truth telling. Psychol. Sci. 23 : 524– 32 [Google Scholar]
- Juni P , Witschi A , Bloch R , Egger M 1999 . The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 282 : 1054– 60 [Google Scholar]
- Klein O , Doyen S , Leys C , Magalhães de Saldanha da Gama PA , Miller S et al. 2012 . Low hopes, high expectations: expectancy effects and the replicability of behavioral experiments. Perspect. Psychol. Sci. 7 : 6 572– 84 [Google Scholar]
- Lau J , Antman EM , Jimenez-Silva J , Kupelnick B , Mosteller F , Chalmers TC 1992 . Cumulative meta-analysis of therapeutic trials for myocardial infarction. N. Engl. J. Med. 327 : 248– 54 [Google Scholar]
- Light RJ , Smith PV 1971 . Accumulating evidence: procedures for resolving contradictions among different research studies. Harvard Educ. Rev. 41 : 429– 71 [Google Scholar]
- Lipsey MW , Wilson D 2001 . Practical Meta-Analysis London: Sage Comprehensive and clear explanation of meta-analysis. [Google Scholar]
- Matt GE , Cook TD 1994 . Threats to the validity of research synthesis. The Handbook of Research Synthesis H Cooper, LV Hedges 503– 20 New York: Russell Sage Found. [Google Scholar]
- Maxwell SE , Lau MY , Howard GS 2015 . Is psychology suffering from a replication crisis? What does “failure to replicate” really mean?. Am. Psychol. 70 : 487– 98 [Google Scholar]
- Moher D , Hopewell S , Schulz KF , Montori V , Gøtzsche PC et al. 2010 . CONSORT explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340 : c869 [Google Scholar]
- Moher D , Liberati A , Tetzlaff J , Altman DG PRISMA Group. 2009 . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339 : 332– 36 Comprehensive reporting guidelines for systematic reviews. [Google Scholar]
- Morrison A , Polisena J , Husereau D , Moulton K , Clark M et al. 2012 . The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int. J. Technol. Assess. Health Care 28 : 138– 44 [Google Scholar]
- Nelson LD , Simmons J , Simonsohn U 2018 . Psychology's renaissance. Annu. Rev. Psychol. 69 : 511– 34 [Google Scholar]
- Noblit GW , Hare RD 1988 . Meta-Ethnography: Synthesizing Qualitative Studies Newbury Park, CA: Sage [Google Scholar]
- Olivo SA , Macedo LG , Gadotti IC , Fuentes J , Stanton T , Magee DJ 2008 . Scales to assess the quality of randomized controlled trials: a systematic review. Phys. Ther. 88 : 156– 75 [Google Scholar]
- Open Sci. Collab. 2015 . Estimating the reproducibility of psychological science. Science 349 : 943 [Google Scholar]
- Paterson BL , Thorne SE , Canam C , Jillings C 2001 . Meta-Study of Qualitative Health Research: A Practical Guide to Meta-Analysis and Meta-Synthesis Thousand Oaks, CA: Sage [Google Scholar]
- Patil P , Peng RD , Leek JT 2016 . What should researchers expect when they replicate studies? A statistical view of replicability in psychological science. Perspect. Psychol. Sci. 11 : 539– 44 [Google Scholar]
- Rosenthal R 1979 . The “file drawer problem” and tolerance for null results. Psychol. Bull. 86 : 638– 41 [Google Scholar]
- Rosnow RL , Rosenthal R 1989 . Statistical procedures and the justification of knowledge in psychological science. Am. Psychol. 44 : 1276– 84 [Google Scholar]
- Sanderson S , Tatt ID , Higgins JP 2007 . Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int. J. Epidemiol. 36 : 666– 76 [Google Scholar]
- Schreiber R , Crooks D , Stern PN 1997 . Qualitative meta-analysis. Completing a Qualitative Project: Details and Dialogue JM Morse 311– 26 Thousand Oaks, CA: Sage [Google Scholar]
- Shrout PE , Rodgers JL 2018 . Psychology, science, and knowledge construction: broadening perspectives from the replication crisis. Annu. Rev. Psychol. 69 : 487– 510 [Google Scholar]
- Stroebe W , Strack F 2014 . The alleged crisis and the illusion of exact replication. Perspect. Psychol. Sci. 9 : 59– 71 [Google Scholar]
- Stroup DF , Berlin JA , Morton SC , Olkin I , Williamson GD et al. 2000 . Meta-analysis of observational studies in epidemiology (MOOSE): a proposal for reporting. JAMA 283 : 2008– 12 [Google Scholar]
- Thorne S , Jensen L , Kearney MH , Noblit G , Sandelowski M 2004 . Qualitative meta-synthesis: reflections on methodological orientation and ideological agenda. Qual. Health Res. 14 : 1342– 65 [Google Scholar]
- Tong A , Flemming K , McInnes E , Oliver S , Craig J 2012 . Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med. Res. Methodol. 12 : 181– 88 [Google Scholar]
- Trickey D , Siddaway AP , Meiser-Stedman R , Serpell L , Field AP 2012 . A meta-analysis of risk factors for post-traumatic stress disorder in children and adolescents. Clin. Psychol. Rev. 32 : 122– 38 [Google Scholar]
- Valentine JC , Biglan A , Boruch RF , Castro FG , Collins LM et al. 2011 . Replication in prevention science. Prev. Sci. 12 : 103– 17 [Google Scholar]
- Article Type: Review Article
Most Read This Month
Most cited most cited rss feed, job burnout, executive functions, social cognitive theory: an agentic perspective, on happiness and human potentials: a review of research on hedonic and eudaimonic well-being, sources of method bias in social science research and recommendations on how to control it, mediation analysis, missing data analysis: making it work in the real world, grounded cognition, personality structure: emergence of the five-factor model, motivational beliefs, values, and goals.
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Synthesis without meta...
Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline
Linked opinion.
Grasping the nettle of narrative synthesis
- Related content
- Peer review
- Joanne E McKenzie , associate professor 2 ,
- Amanda Sowden , professor 3 ,
- Srinivasa Vittal Katikireddi , clinical senior research fellow 1 ,
- Sue E Brennan , research fellow 2 ,
- Simon Ellis , associate director 4 ,
- Jamie Hartmann-Boyce , senior researcher 5 ,
- Rebecca Ryan , senior esearch fellow 6 ,
- Sasha Shepperd , professor 7 ,
- James Thomas , professor 8 ,
- Vivian Welch , associate professor 9 ,
- Hilary Thomson , senior research fellow 1
- 1 MRC/CSO Social and Public Health Sciences Unit, University of Glasgow, UK
- 2 School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
- 3 Centre for Reviews and Dissemination, University of York, York, UK
- 4 Centre for Guidelines, National Institute for Health and Care Excellence, London, UK
- 5 Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK
- 6 School of Psychology and Public Health, La Trobe University, Melbourne, Australia
- 7 Nuffield Department of Population Health, University of Oxford, Oxford, UK
- 8 Evidence for Policy and Practice Information and Coordinating Centre, University College London, London, UK
- 9 Bruyere Research Institute, Ottawa, Canada
- Correspondence to: M Campbell Mhairi.Campbell{at}glasgow.ac.uk
- Accepted 8 October 2019
In systematic reviews that lack data amenable to meta-analysis, alternative synthesis methods are commonly used, but these methods are rarely reported. This lack of transparency in the methods can cast doubt on the validity of the review findings. The Synthesis Without Meta-analysis (SWiM) guideline has been developed to guide clear reporting in reviews of interventions in which alternative synthesis methods to meta-analysis of effect estimates are used. This article describes the development of the SWiM guideline for the synthesis of quantitative data of intervention effects and presents the nine SWiM reporting items with accompanying explanations and examples.
Summary points
Systematic reviews of health related interventions often use alternative methods of synthesis to meta-analysis of effect estimates, methods often described as “narrative synthesis”
Serious shortcomings in reviews that use “narrative synthesis” have been identified, including a lack of description of the methods used; unclear links between the included data, the synthesis, and the conclusions; and inadequate reporting of the limitations of the synthesis
The Synthesis Without Meta-analysis (SWiM) guideline is a nine item checklist to promote transparent reporting for reviews of interventions that use alternative synthesis methods
The SWiM items prompt users to report how studies are grouped, the standardised metric used for the synthesis, the synthesis method, how data are presented, a summary of the synthesis findings, and limitations of the synthesis
The SWiM guideline has been developed using a best practice approach, involving extensive consultation and formal consensus
Decision makers consider systematic reviews to be an essential source of evidence. 1 Complete and transparent reporting of the methods and results of reviews allows users to assess the validity of review findings. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; http://www.prisma-statement.org/ ) statement, consisting of a 27 item checklist, was developed to facilitate improved reporting of systematic reviews. 2 Extensions are available for different approaches to conducting reviews (for example, scoping reviews 3 ), reviews with a particular focus (for example, harms 4 ), and reviews that use specific methods (for example, network meta-analysis. 5 ) However, PRISMA provides limited guidance on reporting certain aspects of the review, such as the methods for presentation and synthesis, and no reporting guideline exists for synthesis without meta-analysis of effect estimates. We estimate that 32% of health related systematic reviews of interventions do not do meta-analysis, 6 7 8 instead using alternative approaches to synthesis that typically rely on textual description of effects and are often referred to as narrative synthesis. 9 Recent work highlights serious shortcomings in the reporting of narrative synthesis, including a lack of description of the methods used, lack of transparent links between study level data and the text reporting the synthesis and its conclusions, and inadequate reporting of the limitations of the synthesis. 7 This suggests widespread lack of familiarity and misunderstanding around the requirements for transparent reporting of synthesis when meta-analysis is not used and indicates the need for a reporting guideline.
Scope of SWiM reporting guideline
This paper presents the Synthesis Without Meta-analysis (SWiM) reporting guideline. The SWiM guideline is intended for use in systematic reviews examining the quantitative effects of interventions for which meta-analysis of effect estimates is not possible, or not appropriate, for a least some outcomes. 10 Such situations may arise when effect estimates are incompletely reported or because characteristics of studies (such as study designs, intervention types, or outcomes) are too diverse to yield a meaningful summary estimate of effect. 11 In these reviews, alternative presentation and synthesis methods may be adopted, (for example, calculating summary statistics of intervention effect estimates, vote counting based on direction of effect, and combining P values), and SWiM provides guidance for reporting these methods and results. 11 Specifically, the SWiM guideline expands guidance on “synthesis of results” items currently available, such as PRISMA (items 14 and 21) and RAMESES (items 11, 14, and 15). 2 12 13 SWiM covers reporting of the key features of synthesis including how studies are grouped, synthesis methods used, presentation of data and summary text, and limitations of the synthesis.
SWiM is not intended for use in reviews that synthesise qualitative data, for which reporting guidelines are already available, including ENTREQ for qualitative evidence synthesis and eMERGe for meta-ethnography. 14 15
Development of SWiM reporting guideline
A protocol for the project is available, 10 and the guideline development was registered with the EQUATOR Network, after confirmation that no similar guideline was in development. All of the SWiM project team are experienced systematic reviewers, and one was a co-author on guidance on the conduct of narrative synthesis (AS). 9 A project advisory group was convened to provide greater diversity in expertise. The project advisory group included representatives from collaborating Cochrane review groups, the Campbell Collaboration, and the UK National Institute for Health and Care Excellence (see supplementary file 1).
The project was informed by recommendations for developing guidelines for reporting of health research. 16 We assessed current practice in reporting synthesis of effect estimates without meta-analysis and used the findings to devise an initial checklist of reporting items in consultation with the project advisory group. We invited 91 people, all systematic review methodologists or authors of reviews that synthesised results from studies without using meta-analysis, to participate in a three round Delphi exercise, with a response rate of 48% (n=44/91) in round one, 54% (n=37/68) in round two, and 82% (n=32/39) in round three. The results were discussed at a consensus meeting of an expert panel (the project advisory group plus one additional methodological expert) (see supplementary file 1). After the meeting, we piloted the revised guideline to assess ease of use and face validity. Eight systematic reviewers with varying levels of experience, who had not been involved in the Delphi exercise, were asked to read and apply the guideline. We conducted short interviews with the pilot participants to identify any clarification needed in the items or their explanations. We subsequently revised the items and circulated them for comment among the expert panel, before finalising them. Full methodological details of the SWiM guideline development process are provided in supplementary file 1.
Synthesis without meta-analysis reporting items
We identified nine items to guide the reporting of synthesis without meta-analysis. Table 1 shows these SWiM reporting items. An online version is available at www.equator-network.org/reporting-guidelines . An explanation and elaboration for each of the reporting items is provided below. Examples to illustrate the reporting items and explanations are provided in supplementary file 2.
Synthesis Without Meta-analysis (SWiM) items: SWiM is intended to complement and be used as an extension to PRISMA
- View inline
Item 1: grouping studies for synthesis
1a) description.
Provide a description of, and rationale for, the groups used in the synthesis (for example, groupings of interventions, population, outcomes, study design).
1a) Explanation
Methodological and clinical or conceptual diversity may occur (for example, owing to inclusion of diverse study designs, outcomes, interventions, contexts, populations), and it is necessary to clearly report how these study characteristics are grouped for the synthesis, along with the rationale for the groups (see Cochrane Handbook Chapter 3 17 ). Although reporting the grouping of study characteristics in all reviews is important, it is particularly important in reviews without meta-analysis, as the groupings may be less evident than when meta-analysis is used.
Providing the rationale, or theory of change, for how the intervention is expected to work and affect the outcome(s) will inform authors’ and review users’ decisions about the appropriateness and usefulness of the groupings. A diagram, or logic model, 18 19 can be used to visually articulate the underlying theory of change used in the review. If the theory of change for the intervention is provided in full elsewhere (for example, in the protocol), this should be referenced. In Cochrane reviews, the rationale for the groups can be outlined in the section “How the intervention is expected to work.”
1b) Description
Detail and provide rationale for any changes made subsequent to the protocol in the groups used in the synthesis.
1b) Explanation
Decisions about the planned groups for the syntheses may need to be changed following study selection and data extraction. This may occur as a result of important variations in the population, intervention, comparison, and/or outcomes identified after the data are collected, or where limited data are available for the pre-specified groupings, and the groupings may need to be modified to facilitate synthesis (Cochrane Handbook Chapter 2 20 ). Reporting changes to the planned groups, and the reason(s) for these, is important for transparency, as this allows readers to assess whether the changes may have been influenced by study findings. Furthermore, grouping at a broader level of (any or multiple) intervention, population, or outcome will have implications for the interpretation of the synthesis findings (see item 8).
Item 2: describe the standardised metric and transformation method used
Description.
Describe the standardised metric for each outcome. Explain why the metric(s) was chosen, and describe any methods used to transform the intervention effects, as reported in the study, to the standardised metric, citing any methodological guidance used.
Explanation
The term “standardised metric” refers to the metric that is used to present intervention effects across the studies for the purpose of synthesis or interpretation, or both. Examples of standardised metrics include measures of intervention effect (for example, risk ratios, odds ratios, risk differences, mean differences, standardised mean differences, ratio of means), direction of effect, or P values. An example of a statistical method to convert an odds ratio to a standardised mean difference is that proposed by Chinn (2000). 21 For other methods and metrics, see Cochrane Handbook Chapter 6. 22
Item 3: describe the synthesis methods
Describe and justify the methods used to synthesise the effects for each outcome when it was not possible to undertake a meta-analysis of effect estimates.
For various reasons, it may not be possible to do a meta-analysis of effect estimates. In these circumstances, other synthesis methods need to be considered and specified. Examples include combining P values, calculating summary statistics of intervention effect estimates (for example, median, interquartile range) or vote counting based on direction of effect. See table 2 for a summary of possible synthesis methods (for further details, see McKenzie and Brennan 2019 11 ). Justification should be provided for the chosen synthesis method.
Questions answered according to types of synthesis methods and types of data used
Item 4: criteria used to prioritise results for summary and synthesis
Where applicable, provide the criteria used, with supporting justification, to select particular studies, or a particular study, for the main synthesis or to draw conclusions from the synthesis (for example, based on study design, risk of bias assessments, directness in relation to the review question).
Criteria may be used to prioritise the reporting of some study findings over others or to restrict the synthesis to a subset of studies. Examples of criteria include the type of study design (for example, only randomised trials), risk of bias assessment (for example, only studies at a low risk of bias), sample size, the relevance of the evidence (outcome, population/context, or intervention) pertaining to the review question, or the certainty of the evidence. Pre-specification of these criteria provides transparency as to why certain studies are prioritised and limits the risk of selective reporting of study findings.
Item 5: investigation of heterogeneity in reported effects
State the method(s) used to examine heterogeneity in reported effects when it is not possible to do a meta-analysis of effect estimates and its extensions to investigate heterogeneity.
Informal methods to investigate heterogeneity in the findings may be considered when a formal statistical investigation using methods such as subgroup analysis and meta-regression is not possible. Informal methods could involve ordering tables or structuring figures by hypothesised modifiers such as methodological characteristics (for example, study design), subpopulations (for example, sex, age), intervention components, and/or contextual/setting factors (see Cochrane Handbook Chapter 12 11 ). The methods used and justification for the chosen methods should be reported. Investigations of heterogeneity should be limited, as they are rarely definitive; this is more likely to be the case when informal methods are used. It should also be noted if the investigation of heterogeneity was not pre-specified.
Item 6: certainty of evidence
Describe the methods used to assess the certainty of the synthesis findings.
The assessment of the certainty of the evidence should aim to take into consideration the precision of the synthesis finding (confidence interval if available), the number of studies and participants, the consistency of effects across studies, the risk of bias of the studies, how directly the included studies address the planned question (directness), and the risk of publication bias. GRADE (Grading of Recommendations, Assessment, Development and Evaluations) is the most widely used framework for assessing certainty (Cochrane Handbook Chapter 14 23 ). However, depending on the synthesis method used, assessing some domains (for example, consistency of effects when vote counting is undertaken) may be difficult.
Item 7: data presentation methods
Describe the graphical and tabular methods used to present the effects (for example, tables, forest plots, harvest plots).
Specify key study characteristics (for example, study design, risk of bias) used to order the studies, in the text and any tables or graphs, clearly referencing the studies included
Study findings presented in tables or graphs should be ordered in the same way as the syntheses are reported in the narrative text to facilitate the comparison of findings from each included study. Key characteristics, such as study design, sample size, and risk of bias, which may affect interpretation of the data, should also be presented. Examples of visual displays include forest plots, 24 harvest plots, 25 effect direction plots, 26 albatross plots, 27 bubble plots, 28 and box and whisker plots. 29 McKenzie and Brennan (2019) provide a description of these plots, when they should be used, and their pros and cons. 11
Item 8: reporting results
For each comparison and outcome, provide a description of the synthesised findings and the certainty of the findings. Describe the result in language that is consistent with the question the synthesis addresses and indicate which studies contribute to the synthesis.
For each comparison and outcome, a description of the synthesis findings should be provided, making clear which studies contribute to each synthesis (for example, listing in the text or tabulated). In describing these findings, authors should be clear about the nature of the question(s) addressed (see table 2 , column 1), the metric and synthesis method used, the number of studies and participants, and the key characteristics of the included studies (population/settings, interventions, outcomes). When possible, the synthesis finding should be accompanied by a confidence interval.An assessment of the certainty of the effect should be reported.
Results of any investigation of heterogeneity should be described, noting if it was not pre-planned and avoiding over-interpretation of the findings.
If a pre-specified logic model was used, authors may report any changes made to the logic model during the review or as a result of the review findings. 30
Item 9: limitations of the synthesis
Report the limitations of the synthesis methods used and/or the groupings used in the synthesis and how these affect the conclusions that can be drawn in relation to the original review question.
When reporting limitations of the synthesis, factors to consider are the standardised metric(s) used, the synthesis method used, and any reconfiguration of the groups used to structure the synthesis (comparison, intervention, population, outcome).
The choice of metric and synthesis method will affect the question addressed (see table 2 ). For example, if the standardised metric is direction of effect, and vote counting is used, the question will ask “is there any evidence of an effect?” rather than “what is the average intervention effect?” had a random effects meta-analysis been used.
Limitations of the synthesis might arise from post-protocol changes in how the synthesis was structured and the synthesis method selected. These changes may occur because of limited evidence, or incompletely reported outcome or effect estimates, or if different effect measures are used across the included studies. These limitations may affect the ability of the synthesis to answer the planned review question—for example, when a meta-analysis of effect estimates was planned but was not possible.
The SWiM reporting guideline is intended to facilitate transparent reporting of the synthesis of effect estimates when meta-analysis is not used. The guideline relates specifically to transparently reporting synthesis and presentation methods and results, and it is likely to be of greatest relevance to reviews that incorporate diverse sources of data that are not amenable to meta-analysis. The SWiM guideline should be used in conjunction with other reporting guidelines that cover other aspects of the conduct of reviews, such as PRISMA. 31 We intend SWiM to be a resource for authors of reviews and to support journal editors and readers in assessing the conduct of a review and the validity of its findings.
The SWiM reporting items are intended to cover aspects of presentation and synthesis of study findings that are often left unreported when methods other than meta-analysis have been used. 7 These include reporting of the synthesis structure and comparison groupings (items 1, 4, 5, and 6), the standardised metric used for the synthesis (item 2), the synthesis method (items 3 and 9), presentation of data (item 7), and a summary of the synthesis findings that is clearly linked to supporting data (item 8). Although the SWiM items have been developed specifically for the many reviews that do not include meta-analysis, SWiM promotes the core principles needed for transparent reporting of all synthesis methods including meta-analysis. Therefore, the SWiM items are relevant when reporting synthesis of quantitative effect data regardless of the method used.
Reporting guidelines are sometimes interpreted as providing guidance on conduct or used to assess the quality of a study or review; this is not an appropriate application of a reporting guideline, and SWiM should not be used to guide the conduct of the synthesis. For guidance on how to conduct synthesis using the methods referred to in SWiM, we direct readers to the second edition of the Cochrane Handbook for Systematic Reviews of Interventions, specifically chapter 12. 11 Although an overlap inevitably exists between reporting and conduct, the SWiM reporting guideline is not intended to be prescriptive about choice of methods, and the level of detail for each item should be appropriate. For example, investigation of heterogeneity (item 5) may not always be necessary or useful. In relation to SWiM, we anticipate that the forthcoming update of PRISMA will include new items covering a broader range of synthesis methods, 32 but it will not provide detailed guidance and examples on synthesis without meta-analysis.
The SWiM reporting guideline emerged from a project aiming to improve the transparency and conduct of narrative synthesis (ICONS-Quant: Improving the CONduct and reporting of Narrative Synthesis). 10 Avoidance of the term “narrative synthesis” in SWiM is a deliberate move to promote clarity in the methods used in reviews in which the synthesis does not rely on meta-analysis. The use of narrative is ubiquitous across all research and can serve a valuable purpose in the development of a coherent story from diverse data. 33 34 However, within the field of evidence synthesis, narrative approaches to synthesis of quantitative effect estimates are characterised by a lack of transparency, making assessment of the validity of their findings difficult. 7 Together with the recently published guidance on conduct of alternative methods of synthesis, 11 the SWiM guideline aims to improve the transparency of, and subsequently trust in, the many reviews that synthesise quantitative data without meta-analysis, particularly for reviews of intervention effects.
Acknowledgments
We thank the participants of the Delphi survey and colleagues who informally piloted the guideline.
Contributors: All authors contributed to the development of SWiM. HT had the idea for the study. HT, SVK, AS, JEM, and MC designed the study methods. JT, JHB, RR, SB, SE, SS, and VW contributed to the consensus meeting and finalising the guideline items. MC prepared the first draft of the manuscript, and all authors critically reviewed and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. HT is the guarantor.
Project advisory group members: Simon Ellis, Jamie Hartmann-Boyce, Mark Petticrew, Rebecca Ryan, Sasha Shepperd, James Thomas, Vivian Welch.
Expert panel members: Sue Brennan, Simon Ellis, Jamie Hartmann-Boyce, Rebecca Ryan, Sasha Shepperd, James Thomas, Vivian Welch.
Funding: This project was supported by funds provided by the Cochrane Methods Innovation Fund. MC, HT, and SVK receive funding from the UK Medical Research Council (MC_UU_12017-13 and MC_UU_12017-15) and the Scottish Government Chief Scientist Office (SPHSU13 and SPHSU15). SVK is supported by an NHS Research Scotland senior clinical fellowship (SCAF/15/02). JEM is supported by an NHMRC career development fellowship (1143429). RR’s position is funded by the NHMRC Cochrane Collaboration Funding Program (2017-2010). The views expressed in this article are those of the authors and not necessarily those of their employer/host organisations or of Cochrane or its registered entities, committees, or working groups.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: funding for the project as described above; HT is co-ordinating editor for Cochrane Public Health; SVK, SE, JHB, RR, and SS are Cochrane editors; JEM is co-convenor of the Cochrane Statistical Methods Group; JT is a senior editor of the second edition of the Cochrane Handbook; VW is editor in chief of the Campbell Collaboration and an associate scientific editor of the second edition of the Cochrane Handbook; SB is a research fellow at Cochrane Australia; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Ethical approval was obtained from the University of Glasgow College of Social Sciences Ethics Committee (reference number 400170060).
Transparency: The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Patient and public involvement: This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop outcomes or interpret the results.
Dissemination to participants and related patient and public communities: The authors plan to disseminate the research through peer reviewed publications, national and international conferences, webinars, and an online training module and by establishing an email discussion group.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/ .
- Donnelly CA ,
- Campbell P ,
- Liberati A ,
- Altman DG ,
- Tetzlaff J ,
- Tricco AC ,
- Zorzela L ,
- Ioannidis JP ,
- PRISMAHarms Group
- Salanti G ,
- Caldwell DM ,
- Shamseer L ,
- Campbell M ,
- Katikireddi SV ,
- Katikireddi S ,
- Roberts H ,
- Higgins J ,
- Chandler J ,
- McKenzie J ,
- Greenhalgh T ,
- Westhorp G ,
- Buckingham J ,
- Flemming K ,
- McInnes E ,
- France EF ,
- Cunningham M ,
- Schulz KF ,
- Higgins JPT ,
- McKenzie JE ,
- Brennan SE ,
- Anderson LM ,
- Petticrew M ,
- Rehfuess E ,
- Schünemann HJ ,
- Ogilvie D ,
- Thomson HJ ,
- Harrison S ,
- Martin RM ,
- Higgins JPT
- Schriger DL ,
- Schroter S ,
- Rehfuess EA ,
- Brereton L ,
- PRISMA Group
- ↵ Page M, McKenzie J, Bossuyt P, et al. Updating the PRISMA reporting guideline for systematic reviews and meta-analyses: study protocol. 2018. https://osf.io/xfg5n .
- Melendez-Torres GJ ,
- O’Mara-Eves A ,
- Petticrew M
Jump to navigation

Cochrane Training
“narrative synthesis” of quantitative effect data in cochrane reviews: current issues and ways forward.

In these videos, the presenters give an overview of current use of, and reasons for using narrative approaches to synthesis. They describe use of the term “narrative synthesis” and common issues in narrative synthesis including transparency in reporting and ambiguity about narrative synthesis as a method. They also provide an overview of how transparency can be improved and finish by introducing the Synthesis Without Meta-analysis (SWiM) reporting guideline, which is the focus of the second webinar. The work presented is from the ICONS-Quant (Improving the Conduct and reporting of Narrative Synthesis of Quantitative data) project which is funded by the Cochrane Strategic Methods Fund (May 2017-May 2019).
The webinar was delivered in February 2020 and below you will find the videos from the webinar, together with accompanying slides to download [PDF].
Part 1: Definition and use of ‘narrative synthesis’ Part 2: Reasons for using ‘narrative synthesis’ Part 3: Common issues in ‘narrative synthesis’ Part 4: Improving transparency in synthesis without meta-analysis; moving from ‘narrative synthesis’ to SWiM Part 5: Questions and answers
Presenter bios.
Dr Hilary Thomson , co-ordinating editor of Cochrane Public Health, Senior Research Fellow, University of Glasgow. Hilary Thomson has extensive experience in conducting large complex reviews of questions about the health impacts of social policy interventions such as housing, transport, and welfare. Her work focusses on ways to improve the reliability and utility of systematic reviews that address public health policy relevant questions.
Mhairi Campbell , Systematic Reviewer, University of Glasgow. Mhairi Campbell has broad experience of conducting complex systematic reviews, including: qualitative evidence of policy interventions, review of theories linking income and health, and research investigating the reporting of narrative synthesis methods of quantitative data in public health systematic reviews.
Part 1: Definition and use of ‘narrative synthesis’
Part 2: Reasons for using ‘narrative synthesis’
Part 3: Common issues in ‘narrative synthesis’
Part 4: Improving transparency in synthesis without meta-analysis; moving from ‘narrative synthesis’ to SWiM
Part 5: Questions and answers
Additional materials
Download the slides from the webinar [PDF]

Which review is that? A guide to review types
- Which review is that?
- Review Comparison Chart
- Decision Tool
- Critical Review
- Integrative Review
- Narrative Review
- State of the Art Review
- Narrative Summary
- Systematic Review
- Meta-analysis
- Comparative Effectiveness Review
- Diagnostic Systematic Review
- Network Meta-analysis
- Prognostic Review
- Psychometric Review
- Review of Economic Evaluations
- Systematic Review of Epidemiology Studies
- Living Systematic Reviews
- Umbrella Review
- Review of Reviews
- Rapid Review
- Rapid Evidence Assessment
- Rapid Realist Review
- Qualitative Evidence Synthesis
- Qualitative Interpretive Meta-synthesis
- Qualitative Meta-synthesis
- Qualitative Research Synthesis
- Framework Synthesis - Best-fit Framework Synthesis
- Meta-aggregation
- Meta-ethnography
- Meta-interpretation
- Meta-narrative Review
- Meta-summary
- Thematic Synthesis
- Mixed Methods Synthesis
Narrative Synthesis
- Bayesian Meta-analysis
- EPPI-Centre Review
- Critical Interpretive Synthesis
- Realist Synthesis - Realist Review
- Scoping Review
- Mapping Review
- Systematised Review
- Concept Synthesis
- Expert Opinion - Policy Review
- Technology Assessment Review
- Methodological Review
- Systematic Search and Review
Narrative’ synthesis’ refers to an approach to the systematic review and synthesis of findings from multiple studies that relies primarily on the use of words and text to summarise and explain the findings of the synthesis. Whilst narrative synthesis can involve the manipulation of statistical data, the defining characteristic is that it adopts a textual approach to the process of synthesis to ‘tell the story’ of the findings from the included studies. As used here ‘narrative synthesis’ refers to a process of synthesis that can be used in systematic reviews focusing on a wide range of questions, not only those relating to the effectiveness of a particular intervention. (Popay et al. 2006)
Further Reading/Resources
Guidelines Campbell, M., McKenzie, J. E., Sowden, A., Katikireddi, S. V., Brennan, S. E., Ellis, S., ... & Thomson, H. (2020). Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. bmj , 368 . Full Text Other
Popay, J., Roberts, H., Sowden, A., Petticrew, M., Arai, L., Rodgers, M., ... & Duffy, S. (2006). Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version , 1 (1), b92. Full Text
Thomson H, Campbell M. “Narrative synthesis” of quantitative effect data in Cochrane reviews: Current issues and ways forward [Internet]. Cochrane Learning Live Webinar Series 2020 Feb. Full Text
Morley, G., Ives, J., Bradbury-Jones, C., & Irvine, F. (2019). What is 'moral distress'? A narrative synthesis of the literature. Nursing ethics , 26 (3), 646–662. https://doi.org/10.1177/0969733017724354 Link
References Popay, J., Roberts, H., Sowden, A., Petticrew, M., Arai, L., Rodgers, M., ... & Duffy, S. (2006). Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version , 1 (1), b92. Full Text
- << Previous: Mixed Methods Synthesis
- Next: Meta-narrative Review >>
- Last Updated: Aug 19, 2024 1:08 PM
- URL: https://unimelb.libguides.com/whichreview
- Research article
- Open access
- Published: 12 March 2019
A systematic literature review and narrative synthesis on the risks of medical discharge letters for patients’ safety
- Christine Maria Schwarz 1 ,
- Magdalena Hoffmann ORCID: orcid.org/0000-0003-1668-4294 1 , 2 ,
- Petra Schwarz 3 ,
- Lars-Peter Kamolz 1 ,
- Gernot Brunner 1 &
- Gerald Sendlhofer 1 , 2
BMC Health Services Research volume 19 , Article number: 158 ( 2019 ) Cite this article
28k Accesses
51 Citations
5 Altmetric
Metrics details
The medical discharge letter is an important communication tool between hospitals and other healthcare providers. Despite its high status, it often does not meet the desired requirements in everyday clinical practice. Occurring risks create barriers for patients and doctors. This present review summarizes risks of the medical discharge letter.
The research question was answered with a systematic literature research and results were summarized narratively. A literature search in the databases PubMed and Cochrane Library for Studies between January 2008 and May 2018 was performed. Two authors reviewed the full texts of potentially relevant studies to determine eligibility for inclusion. Literature on possible risks associated with the medical discharge letter was discussed.
In total, 29 studies were included in this review. The major identified risk factors are the delayed sending of the discharge letter to doctors for further treatments, unintelligible (not patient-centered) medical discharge letters, low quality of the discharge letter, and lack of information as well as absence of training in writing medical discharge letters during medical education.
Conclusions
Multiple risks factors are associated with the medical discharge letter. There is a need for further research to improve the quality of the medical discharge letter to minimize risks and increase patients’ safety.
Peer Review reports
The medical discharge letter is an important communication medium between hospitals and general practitioners (GPs) and an important legal document for any queries from insurance carriers, health insurance companies, and lawyers [ 1 ]. Furthermore, the medical discharge letter is an important document for the patient itself.
A timely transmission of the letter, a clear documentation of findings, an adequate assessment of the disease as well as understandable recommendations for follow-up care are essential aspects of the medical discharge letter [ 2 ]. Despite this importance, medical discharge letters are often insufficient in content and form [ 3 ]. It is also remarkable that writing of medical discharge letters is often not a particular subject in the medical education [ 4 ]. Nevertheless, the medical discharge letter is an important medical document as it contains a summary of the patient’s hospital admission, diagnosis and therapy, information on the patient’s medical history, medication, as well as recommendations for continuity of treatment. A rapid transmission of essential findings and recommendations for further treatment is of great interest to the patient (as well as relatives and other persons that are involved in the patients’ caring) and their current and future physicians. In most acute care hospitals, patients receive a preliminary medical discharge letter (short discharge letter) with diagnoses and treatment recommendations on the day of discharge [ 5 ]. Unfortunately, though, the full hospital medical discharge letter, which is often received with great delay, is an area of constant conflict between GPs and hospital doctors [ 1 ]. Thus the medical discharge letter does not only represent a feature of process and outcome quality of a clinic, but also influences confidence building and binding of resident physicians to the hospital [ 6 ].
Beside the transmission of patients’ findings from physician to physician, the delivery of essential information to the patient is an underestimated purpose of the medical discharge letter [ 7 ]. The medical discharge letter is often characterized by a complex medical language that is often not understood by the patients. In recent years, patient-centered/patient-directed medical discharge letters are more in discussion [ 8 ]. Thus, the medical discharge letter points out risks for patients and physicians while simultaneously creating barriers between them.
A systematic review of the literature was undertaken to identify patient safety risks associated with the medical discharge letter.
Search strategy
A systematic literature search was conducted using the electronic databases PubMed and Cochrane Database. Additionally, we scanned the reference lists of selected articles (snowballing). The following search terms were used: “discharge summary AND risks”, “discharge summary AND risks AND patient safety” and “discharge letter AND risks” and “discharge letter AND risks AND patient safety”. We reviewed relevant titles and abstracts on English and German literature published between January 2008 and May 2018 and started the search at the beginning of February 2018 and finished it at the end of May 2018.
Eligibility criteria
In this systematic review, articles were included if the title and/or abstract indicated the report of results of original research studies using quantitative, qualitative, or mixed method approaches. Studies in paediatric settings or studies that do not handle possible risks of the medical discharge letter were excluded, as well as reports, commentaries and letters. Electronic citations, including available abstracts of all articles retrieved from the search, were screened by two authors to select reports for full-text review. Duplicates were removed from the initial search. Nevertheless, during the search of articles the selection, publication as well as language bias must be considered. Thereafter, full-texts of potentially relevant studies were reviewed to determine eligibility for inclusion. In the following Table 1 inclusion and exclusion criteria for the studies are listed. Afterwards, key outcomes and main results were summarized. Differences were resolved by consensus. Finally, a narrative synthesis of studies meeting the inclusion criteria was conducted. Reference management software MENDELEY (Version 1.19.3) was used to organise and store the literature.
Data extraction
The data extraction in form of a table was used to summarize study results. The two authors extracted the data relating to author, country, year, study design, and outcome measure as well as potential risk factors to patient safety directly into a pre-formatted data collection form. After data extraction, the literature was discussed and synthesized into themes. The evaluation of the single studies was done using checklists [STROBE (combined) and the Cochrane Data collection form for intervention reviews (RCTs and non-RCTs)]. Meta-analysis was not considered appropriate for this body of literature because of the wide variability of studies in relation to research design, study population, types of interventions and outcomes.
Then a narrative synthesis was performed to synthesize the findings of the different studies. Because of the range of very different studies that were included in this systematic review, we have decided that a narrative synthesis constitutes the best instrument to synthesise the findings of the studies. First, a preliminary synthesis was undertaken in form of a thematic analysis involving searching of studies, listing and presenting results in tabular form. Then the results were discussed again and structured into themes. Afterwards, summarizing of included studies in a narrative synthesis within a framework was performed by one author.
This framework consisted of the following factors: the individuals and the environment involved in the studies (doctors, hospitals), the tools and technology (such as discharge letter delivery systems), the content of the medical discharge letter (such as missing content, quality of content), the accuracy and timeliness of transfer. These themes were discussed in relation to potential risks for patient’s safety. All articles that were included in this review were published before. The framework of this study was chosen following a previously published systematic review dealing with patient risks associated with telecare [ 9 ].
The initial literature search in the two online databases identified 940 records. From these records, 65 full text articles were screened for eligibility. Then 36 full-text articles were excluded because they pertained to patient transfer within the hospital or to another hospital, or to patient hand-over situations. Finally, 29 studies were included in this review. Included studies are listed in Table 2 . All document types were searched with a focus on primary research studies. The results of the search strategy are shown in Fig. 1 .
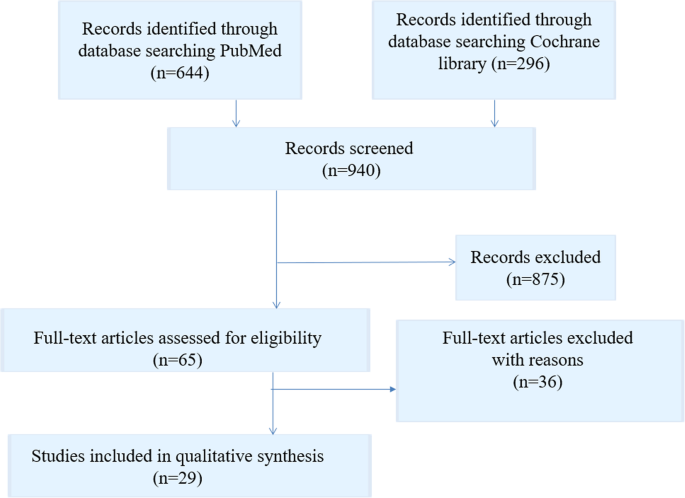
Flow chart literature search strategy
From these 29 studies, 13 studies dealt with the quality analysis of discharge letters, 12 studies with delayed transmission of medical discharge letters and just as many with the lack of information in medical discharge letters. Only few studies dealt with training on writing medical discharge letters and with understanding of patients of their medical discharge letters. The descriptive information of the included articles is presented in Table 2 . Overall quality of the articles was found to be acceptable, with clearly stated research questions and appropriate used methods.
Risk factors
In the following the identified major risk factors concerning the medical discharge letter are presented in a narrative summary.
Delayed delivery
The medical discharge letters should arrive at the GP soon after hospital discharge to ensure the quickest possible further treatment [ 4 ]. If letters are delivered weeks after the hospital stay, a continuous treatment of the patient cannot be ensured. Furthermore, the author of the medical discharge letter will no longer have current data after the discharge of the patient, which may result in a loss of important information [ 10 ]. Interfaces between different treatment areas and organizational units are known to cause a loss of information and a lack of quality in patient handling [ 11 ]. The improvement of information transfer between different healthcare providers during the transition of patients has been recommended to improve patient care [ 12 , 13 ]. Delayed communication of findings may lead to a lack of continuity of care and suboptimal outcomes, as well as decreased satisfaction levels for both patients and GPs [ 14 , 15 , 16 ]. In a review of Kripalani et al., it was shown that 25% of discharge summaries were never received by GPs [ 17 ]. This has several negative consequences for patients. Li et al. [ 18 ] found that a delayed transmission or absence of the medical discharge summary is related to patient readmission, and a study by Gilmore-Bykovskyi [ 19 ] found a strong relationship between patients whose discharge summaries omitted designation of a responsible clinician/clinic for follow-up care and re-hospitalisation and/or death. A Swedish study by Carlsson et al. [ 20 ] points out that a lack of accuracy and continuity in discharge information on eating difficulties may increase risk of undernutrition and related complications. A study of Were et al. [ 18 ] investigated pending lab results in medical discharge summaries and found that only 16% of tests with pending results were mentioned in the discharge summaries, and Walz et al. [ 21 ] found that approximately one third of the sub-acute care patients had pending lab results at discharge, but only 11% of these were documented in the medical discharge summaries.
Quality, lack of information
Medical discharge letters are a key communication tool for patient safety issues [ 17 ]. Incomplete and insufficient medical discharge letters increase the risks of readmission and myriad other complications [ 22 ]. Langelaan et al. (2017) evaluated more than 2000 medical discharge letters and found that in about 60% of the letters essential information was missing, such as a change of the existing medication, laboratory data, and even data on the patients themselves [ 23 ]. Accurate and complete medical discharge summaries are essential for patient safety [ 17 , 24 , 25 ]. Addresses; patient data, including duration of stay; diagnoses; procedures; operations; epicrisis and therapy recommendations; as well as findings in the appendix; are minimum requirements that are supposed to be included in the medical discharge letter [ 4 ]. However, it was found that key components are often lacking in medical discharge letters, including information about follow-up and management plans [ 23 , 26 ], test results [ 27 , 28 , 29 ], and medication adjustments [ 30 , 31 , 32 , 33 , 34 , 35 ]. In a review of Wimsett et al. [ 36 ] key components of a high-quality medical discharge summary were identified in 32 studies. These important components were discharge diagnosis, the received treatment, results of investigations as well as follow-up plans.
Accuracy of patients’ medication information is important to ensure patient safety. Hospital doctors expect GPs to continue with the prescribed (or modified) drug therapy. However, the selection of certain drugs is not always transparent for the GPs. A study by Grimes et al. [ 30 ] found that a discrepancy in medication documentation at discharge occurred in 10.8% of patients. From these patients nearly 65.5% were affected by discrepancies in medication documentation. The most prevalent inconsistency was drug omission (20.9%). Only 2% of patients were contacted, although general patient harm was assessed. A Swedish study of 2009 [ 37 ] investigated the quality improvement of medical discharge summaries. A higher quality of discharge letter led to an average of 45% fewer medication errors per patient.
A recent study by Tong et al. [ 38 ] revealed a reduced rate of medication errors in medical discharge summaries that were completed by a hospital pharmacist. Hospital pharmacists play a key role in preparing the discharge medication information transferred to GPs upon patient discharge and should work closely with hospital doctors to ensure accurate medication information that is quickly communicated to GPs at transitions of care [ 39 ]. Most hospitals have introduced electronic systems to improve the discharge communication, and many studies found a significant overall improvement in electronic transfer systems due to better documentation of information about follow-up care, pending test results, and information provided to patients and relatives [ 40 , 41 , 42 ]. Mehta et al. [ 43 ] found that the changeover to a new electronic system resulted in an increased completeness of discharge summaries from 60.7 to 75.0% and significant improvements in levels of completeness in certain categories.
Writing of medical discharge letter is missing in medical education
Both junior doctors as well as medical students reported that they received inadequate guidance and training on how to write medical discharge summaries [ 44 , 45 ] and recognized that higher priority is often given to pressing clinical tasks [ 46 ]. Research into the causes of prescribing errors by junior doctors at hospitals in the UK has revealed that latent conditions like organizational processes, busy environments, and medical care for complex patients can lead to medication errors in the medical discharge summary [ 47 ].
Fortunately, some study results demonstrate that information and education on writing medical discharge letters would enhance communication to the GPs and prevent errors during the patient discharge process [ 37 ]. Minimal formal teaching about writing medical discharge summaries is common in most medical schools [ 39 , 46 ]; however, a study by Shivji et al. has shown that simple, intensive educational sessions can lead to an improvement in the writing process of medical discharge summaries and communication with primary care [ 48 ].
Since the medical discharge letter should meet specific quality criteria, senior physicians and/or the head physician correct(s) and validate(s) the letter. The medical discharge letter therefore represents an essential learning target [ 8 ]. Training activities and workshops are necessary for junior doctors to improve writing medical discharge letters [ 44 , 49 ]. It might be also useful for young doctors to use checklists or other structured procedures to improve writing [ 4 ]. Maher et al. showed that the use of a checklist enhanced the quality (content, structure, and clarity) of medical discharge letters written by medical students [ 50 ].
In the following Table 3 main risk factors of the medical discharge letter are summarized.
The results of this systematic literature research indicate notable risk factors relating to the medical discharge letter. In a study by Sendlhofer et al., 360 risks were identified in hospital settings [ 51 ]. From these, 176 risks were scored as strategic and clustered into “top risks”. Top risks included medication errors, information errors, and lack of communication, among others. During this review, these potential risk factors were also identified in terms of the medical discharge letter.
Delayed sending and low quality of medical discharge letters to the referring physicians, may adversely affect the further course of treatment. However, a study of Spencer et al. has determined rates of failures in processing actions requested in hospital discharge summaries in general practice. It was found that requested medication changes were not made in 17% and patient harm occurred in 8% in relation to failures [ 52 ].
Despite the existence of reliable standards [ 53 ] many physicians are not adequately trained for writing medical discharge letters during their studies. Regular trainings and workshops and standardized checklists may optimize the quality of the medical discharge letter. Furthermore, electronic discharge letters have the potential to easily and quickly extract important information such as diagnoses, medication, and test results into a structured discharge document, and offer important advantages such as reliability, speed of information transfer, and standardization of content. Comprehensive discharge letters reduce the readmission rate and increase safety and quality by discharging of the patient. A missing structure, as well as a complex language, illegible handwriting, and unknown abbreviations, make reading medical discharge letters more complicated [ 4 ]. At least, poor patient understanding of their diagnosis and treatment plans and incomprehensible recommendations can adversely impact clinical outcome following hospital discharge. Many studies confirm that inadequate communication of findings [ 3 , 39 , 54 ] is an important risk factor in patients’ safety [ 51 ].
Most medical information in the discharge letter is not understood by patients (as well as relatives and other persons that are involved in the patients’ caring) and patients themselves do not receive a comprehensible medical discharge letter. The content of the medical discharge letter is often useless for the patient due to its medical terminology and content that is not matching with the patient’s level of knowledge or health literacy [ 55 , 56 , 57 ]. Poor understanding of diagnoses and related discharge plans are common among patients and family members and often accompanied by unplanned hospital readmissions [ 58 , 59 , 60 , 61 ]. In a study by Lin et al., it was shown that a patient-directed discharge letter enhanced understanding for hospitalization and for recommendations. Furthermore, verbal communication of the letter contents, explanation of every section of the medical discharge letter, and the opportunity for discussion and asking questions improved patient comprehension [ 7 ]. A study by O’Leary et al. showed that roughly 80–95% of patients with breast tumours want to be informed and educated about their illness, treatment, and prognosis [ 62 ].
High quality of care is characterized by a patient-centered communication, where the patient’s personal needs are also in focus [ 63 ]. Translation of medical terms in reports and letters leads to a better understanding of the disease and, interestingly, the avoidance of medical terms did not lead to deterioration in the transmission of information between the treating physicians. Moreover, it was found that the minimisation of medical terminology in medical discharge letters improved understanding and perception of patients’ ability to manage chronic health conditions [ 64 ]. In effect, it is clear that patient-centered communication improves outcome, mental health, patient satisfaction and reduces the use of health services [ 65 ].
Strengths and limitations
We have identified key problems with the medical discharge summaries that negatively impact patients’ safety and wellbeing. However, there is a heterogeneous nature of the included studies in terms of study design, sample size, outcomes, and language. Only two reviewers screened the studies for eligibility and only full-text articles were included in the literature review; furthermore, only the databases Pubmed and Cochrane library were screened for appropriate studies. Due to these constraints, there is a chance that other relevant studies may have been missed.
High-quality medical discharge letters are essential to ensure patient safety. To address this, the current review identified the major risk factors as delayed sending and low quality of medical discharge letters, lack of information and patient understanding, and inadequate training in writing medical discharge letters. In future, research studies should focus on improving the communication of pending test results and findings at discharge, and on evaluating the impact that this improved communication has on patient outcomes. Moreover, a simple patient-centered medical discharge letter may improve the patient’s (as well as family members’ and other caregivers’) understanding of disease, treatment and post-discharge recommendations.
Abbreviations
General practitioner
Randomized Controlled Trial
STrengthening the Reporting of OBservational studies in Epidemiology
United Kingdom
Kreße B, Dinser R. Anforderungen an Arztberichte- ein haftungsrechtlicher Ansatz. Medizinrecht. 2010;28(6):396–400.
Google Scholar
Möller K-H, Makoski K. Der Arztbrief - Rechtliche Rahmen- Bedingungen. 2015;5:186–94.
Van Walraven C, Weinberg AL. Quality assessment of a discharge summary system. CMAJ. 1995;152(9):1437–42.
PubMed Google Scholar
Unnewehr M, Schaaf B, Friederichs H. Die Kommunikation optimieren. Dtsch Arztebl Int. 2013;110(37):831–4.
Roth-Isigkeit A, Harder S. Die Entlassungsmedikation im Arztbrief. Eine explorative Befragung von Hausärzten/−innen. Vol. 100, Medizinische Klinik. 2005. p. 87–93.
Bohnenkamp B. Arbeitsorganisation: Der Arztbrief - Viel mehr als nur lästige Pflicht. Vol. 113, Deutsches Ärzteblatt International. 2016. p. 2–4.
Lin R, Tofler G, Spinaze M, Dennis C, Clifton-Bligh R, Nojoumian H, Gallagher R, et al. Patient-directed discharge letter (PADDLE)-a simple and brief intervention to improve patient knowledge and understanding at time of hospital discharge. Hear Lung Circ. 2012;21:S312.
Hammerer P. Patientenverständliche Arztbriefe und Befunde. Springer Medizin. 2018;33(2):119–23.
Guise V, Anderson J, Wiig S. Patient safety risks associated with telecare: a systematic review and narrative synthesis of the literature. BMC Health Serv Res. 2014;14(1):588.
Raab, A., & Drissner A. Einweiserbeziehungsmanagement: Wie Krankenhäuser erfolgreich Win-Win-Beziehungen zu niedergelassenen Ärzten aufbauen. Kohlhammer Verlag; 2011. 240 p.143.
Hart D. Vertrauen, Kooperation, Organisation. Berlin Heidelberg: Springer Verlag; 2006. p. 845–7.
Cook RI. Gaps in the continuity of care and progress on patient safety. BMJ. 2000;320(7237):791–4.
CAS PubMed Google Scholar
Duggan C, Feldman R, Hough J, Bates I. Reducing adverse prescribing discrepancies following hospital discharge. Int J Pharm Pract. 1998;6(2):77–82.
Polyzotis PA, Suskin N, Unsworth K, Reid RD, Jamnik V, Parsons C, et al. Primary care provider receipt of cardiac rehabilitation discharge summaries are they getting what they want to promote long-term risk reduction. Circ Cardiovasc Qual Outcomes. 2013;6(1):83–9.
Poon EG, Gandhi TK, Sequist TD, Murff HJ, Karson AS, Bates DW. “I wish i had seen this test result earlier!”: Dissatisfaction with test result management systems in primary care. Vol. 164, Archives of Internal Medicine. 2004. p. 2223–8.
Coleman EA, Berenson RA. Lost in transition: Challenges and opportunities for improving the quality of transitional care. Vol. 141, Annals of Internal Medicine. 2004. p. 533–6.
Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–41.
Were MC, Li X, Kesterson J, Cadwallader J, Asirwa C, Khan B, et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow-up providers. J Gen Intern Med. 2009;24(9):1002–6.
Gilmore-Bykovskyi AL, Kennelty KA, Dugoff E, Kind AJH. Hospital discharge documentation of a designated clinician for follow-up care and 30-day outcomes in hip fracture and stroke patients discharged to sub-acute care. BMC Health Serv Res. 2018;18(1).
Carlsson E, Ehnfors M, Eldh AC, Ehrenberg A. Accuracy and continuity in discharge information for patients with eating difficulties after stroke. J Clin Nurs. 2012;21(1–2):21–31.
Walz SE, Smith M, Cox E, Sattin J, Kind AJH. Pending laboratory tests and the hospital discharge summary in patients discharged to sub-acute care. J Gen Intern Med. 2011;26(4):393–8.
Horwitz LI, Jenq GY, Brewster UC, Chen C, Kanade S, Van Ness PH, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436–43.
Alderton M, Callen JL. Are general practitioners satisfied with electronic discharge summaries? Vol. 36, Health Information Management Journal. 2007. p. 7–12.
Harel Z, Wald R, Perl J, Schwartz D, Bell CM. Evaluation of deficiencies in current discharge summaries for dialysis patients in Canada. J Multidiscip Healthc. 2012;5:77–84.
Philibert I, Barach P. The European HANDOVER Project: A multi-nation program to improve transitions at the primary care - Inpatient interface. BMJ Quality and Safety. 2012;21(SUPPL. 1).
Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE. Hospital discharge communications during care transitions for patients with acute kidney injury: a cross-sectional study. BMC Health Serv Res. 2016;16:449.
Belleli E, Naccarella L, Pirotta M. Communication at the interface between hospitals and primary care: a general practice audit of hospital discharge summaries. Aust Fam Physician. 2013;42(12):886–90.
Roy CL, Poon EC, Karson AS, Ladak-Merchant Z, Johnson RE, Maviglia SM, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–8.
Gandara E, Moniz T, Ungar J, Lee J, Chan-Macrae M, O’Malley T, et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–33.
Grimes T, Delaney T, Duggan C, Kelly JG, Graham IM. Survey of medication documentation at hospital discharge: implications for patient safety and continuity of care. Ir J Med Sci. 2008;177(2):93–7.
Uitvlugt EB, Siegert CEH, Janssen MJA, Nijpels G, Karapinar-Çarkit F. Completeness of medication-related information in discharge letters and post-discharge general practitioner overviews. Int J Clin Pharm. 2015;37(6):1206–12.
Ooi CE, Rofe O, Vienet M, Elliott RA. Improving communication of medication changes using a pharmacist-prepared discharge medication management summary. Int J Clin Pharm. 2017;39(2):394–402.
Perren A, Previsdomini M, Cerutti B, Soldini D, Donghi D, Marone C. Omitted and unjustified medications in the discharge summary. Qual Saf Heal Care. 2009;18(3):205–8.
CAS Google Scholar
Garcia BH, Djønne BS, Skjold F, Mellingen EM, Aag TI. Quality of medication information in discharge summaries from hospitals: an audit of electronic patient records. Int J Clin Pharm. 2017;39(6):1331–7.
Monfort AS, Curatolo N, Begue T, Rieutord A, Roy S. Medication at discharge in an orthopaedic surgical ward: quality of information transmission and implementation of a medication reconciliation form. Int J Clin Pharm. 2016;38(4):838–47.
Wimsett J, Harper A, Jones P. Review article: Components of a good quality discharge summary: A systematic review. Vol. 26, EMA - Emergency Medicine Australasia. 2014. p. 430–8.
Bergkvist A, Midlöv P, Höglund P, Larsson L, Bondesson Å, Eriksson T. Improved quality in the hospital discharge summary reduces medication errors-LIMM: Landskrona integrated medicines management. Eur J Clin Pharmacol. 2009;65(10):1037–46.
Tong EY, Roman CP, Mitra B, Yip GS, Gibbs H, Newnham HH, et al. Reducing medication errors in hospital discharge summaries: a randomised controlled trial. Med J Aust. 2017;206(1):36–9.
Yemm R, Bhattacharya D, Wright D, Poland F. What constitutes a high quality discharge summary? A comparison between the views of secondary and primary care doctors. Int J Med Educ. 2014;5:125–31.
O’Leary KJ, Liebovitz DM, Feinglass J, Liss DT, Evans DB, Kulkarni N, et al. Creating a better discharge summary: improvement in quality and timeliness using an electronic discharge summary. J Hosp Med. 2009;4(4):219–25.
Chan S. P Maurice a, W pollard C, Ayre SJ, Walters DL, Ward HE. Improving the efficiency of discharge summary completion by linking to preexisiting patient information databases. BMJ Qual Improv Reports. 2014;3(1):1–5.
Lehnbom EC, Raban MZ, Walter SR, Richardson K, Westbrook JI. Do electronic discharge summaries contain more complete medication information? A retrospective analysis of paper versus electronic discharge summaries. Heal Inf Manag J. 2014;43(3):4–12.
Mehta RL, Baxendale B, Roth K, Caswell V, Le Jeune I, Hawkins J, et al. Assessing the impact of the introduction of an electronic hospital discharge system on the completeness and timeliness of discharge communication: A before and after study. BMC Health Serv Res. 2017;17(1).
Heaton A, Webb DJ, Maxwell SRJ. Undergraduate preparation for prescribing: the views of 2413 UK medical students and recent graduates. Br J Clin Pharmacol. 2008;66(1):128–34.
Maxwell S, Walley T. Teaching safe and effective prescribing in UK medical schools: A core curriculum for tomorrow’s doctors. Vol. 55, British Journal of Clinical Pharmacology. 2003. p. 496–503.
Frain JP, Frain AE, Carr PH. Experience of medical senior house officers in preparing discharge summaries. Br Med J. 1996;312(7027):350.
Dornan T, Investigator P, Ashcroft D, Lewis P, Miles J, Taylor D, et al. An in depth investigation into causes of prescribing errors by foundation trainees in relation to their medical education. EQUIP study. Vol. 44, Methods. 2010.
Shivji FS, Ramoutar DN, Bailey C, Hunter JB. Improving communication with primary care to ensure patient safety post-hospital discharge. Br J Hosp Med. 2015;76(1):46–9.
Cresswell A, Hart M, Suchanek O, Young T, Leaver L, Hibbs S. Mind the gap: Improving discharge communication between secondary and primary care. BMJ Qual Improv Reports. 2015;4(1):u207936.w3197.
Maher B, Drachsler H, Kalz M, Hoare C, Sorensen H, Lezcano L, et al. Use of Mobile applications for hospital discharge letters - improving handover at point of practice. Int J Mob Blended Learn. 2013;5(4):29.
Sendlhofer G, Brunner G, Tax C, Falzberger G, Smolle J, Leitgeb K, et al. Systematic implementation of clinical risk management in a large university hospital: the impact of risk managers. Wien Klin Wochenschr [Internet]. 2015; 127:1–11. Available from: https://doi.org/10.1007/s00508-014-0620-7
Spencer RA, Spencer SEF, Rodgers S, Campbell SM, Avery AJ. Processing of discharge summaries in general practice: a retrospective record review. Br J Gen Pract. 2018;68(673):e576–85.
Carpenter I. A Clinician’s guide to record standards - part 2: standards for the structure and content of medical records and communications when patients are admitted to hospital. Acad Med R Coll R Coll Physicians. 2008;10(5):24.
GMC. Good practice in prescribing and managing medicines and devices. Good Medical Practice. 2013. p. 1–11.
Jolly BT, Scott JL, Sanford SM. Simplification of emergency department discharge instructions improves patient comprehension. Ann Emerg Med. 1995;26(4):443–6.
Williams DM, Counselman FL, Caggiano CD. Emergency department discharge instructions and patient literacy: a problem of disparity. Am J Emerg Med. 1996;14(1):19–22.
Jolly BT, Scott JL, Feied CF, Sanford SM. Functional illiteracy among emergency department patients: a preliminary study. Ann Emerg Med. 1993;22(3):573–8.
Soler RS, Juvinyà Canal D, Noguer CB, Poch CG, Brugada Motge N, Garcia D m, Gil M. Continuity of care and monitoring pain after discharge: patient perspective. J Adv Nurs. 2010;66(1):40–8.
Grover G, Berkowitz CD, Lewis RJ. Parental recall after a visit to the emergency department. Clin Pediatr (Phila). 1994;33(4):194–201.
Witherington EMA, Pirzada OM, Avery AJ. Communication gaps and readmissions to hospital for patients aged 75 years and older: observational study. Qual Saf Heal Care. 2008;17(1):71–5.
Marcantonio ER, McKean S, Goldfinger M, Kleefield S, Yurkofsky M, Brennan T. a. Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan. Am J Med. 1999;107(1):13–7.
O’Leary KA, Estabrooks CA, Olson K, Cumming C. Information acquisition for women facing surgical treatment for breast cancer: Influencing factors and selected outcomes. Vol. 69, Patient Education and Counseling. 2007. p. 5–19.
Vogel BA, Helmes AW, Bengel J. Arzt-Patienten-Kommunikation in der Tumorbehandlung: Erwartungen und Erfahrungen aus Patientensicht. Zeitschrift für Medizinische Psychol. 2006;15(4):149–61.
Wernick M, Hale P, Anticich N, Busch S, Merriman L, King B, et al. A randomised crossover trial of minimising medical terminology in secondary care correspondence in patients with chronic health conditions: impact on understanding and patient reported outcomes. Intern Med J. 2016;46(5):596–601.
Woods SS, Schwartz E, Tuepker A, Press NA, Nazi KM, Turvey CL, et al. Patient experiences with full electronic access to health records and clinical notes through the my healthevet personal health record pilot: Qualitative study. J Med Internet Res. 2013;(15, 3).
Weiskopf NG, Rusanov A, Weng C. Sick patients have more data: the non-random completeness of electronic health records. MIA Symp. 2013;2013:1472–7.
Choudhry AJ, Baghdadi YMK, Wagie AE, Habermann EB, Heller SF, Jenkins DH, et al. Readability of discharge summaries: with what level of information are we dismissing our patients? In: Am J Surg. 2016. p. 631–6.
Li JYZ, Yong TY, Hakendorf P, Ben-Tovim D, Thompson CH. Timeliness in discharge summary dissemination is associated with patients’ clinical outcomes. J Eval Clin Pract. 2013;19(1):76–9.
Download references
Acknowledgements
Not applicable.
This research project was part of a project funded by the Gesundheitsfonds Steiermark. The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Author information
Authors and affiliations.
Research Unit for Safety in Health, c/o Division of Plastic, Aesthetic and Reconstructive Surgery, Department of Surgery, Medical University of Graz, Graz, Austria
Christine Maria Schwarz, Magdalena Hoffmann, Lars-Peter Kamolz, Gernot Brunner & Gerald Sendlhofer
Executive Department for Quality and Risk Management, University Hospital Graz, Auenbruggerplatz 1/3, 8036, Graz, Austria
Magdalena Hoffmann & Gerald Sendlhofer
Carinthia University of Applied Science, Feldkirchen, Austria
Petra Schwarz
You can also search for this author in PubMed Google Scholar
Contributions
CS wrote the manuscript; CS, MH and PS performed the literature search; LK contributed to the conception of this work; GB contributed to the interpretation of data and GS supervised the project. All authors were critically revising the manuscript and all authors have read and approved the final manuscript.
Corresponding author
Correspondence to Magdalena Hoffmann .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Schwarz, C.M., Hoffmann, M., Schwarz, P. et al. A systematic literature review and narrative synthesis on the risks of medical discharge letters for patients’ safety. BMC Health Serv Res 19 , 158 (2019). https://doi.org/10.1186/s12913-019-3989-1
Download citation
Received : 09 August 2018
Accepted : 06 March 2019
Published : 12 March 2019
DOI : https://doi.org/10.1186/s12913-019-3989-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Discharge letter
- Discharge summary
- Patient safety
- Hospital discharge
- Systematic review
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Strengths and limitations of early warning scores: A systematic review and narrative synthesis
Affiliations.
- 1 Leeds Institute of Biomedical & Clinical Sciences, Clinical Sciences Building St. James's University Hospital, University of Leeds, Leeds, LS9 7TF, United Kingdom. Electronic address: [email protected].
- 2 Leeds Institute of Biomedical & Clinical Sciences, Clinical Sciences Building St. James's University Hospital, University of Leeds, Leeds, LS9 7TF, United Kingdom.
- 3 School of Healthcare, Baines Wing, University of Leeds, Leeds LS2 9JT, United Kingdom.
- 4 Leeds Institute of Clinical Trials Research, Worsley Building, University of Leeds, Leeds LS2 9NL, United Kingdom.
- PMID: 28950188
- DOI: 10.1016/j.ijnurstu.2017.09.003
Background: Early warning scores are widely used to identify deteriorating patients. Whilst their ability to predict clinical outcomes has been extensively reviewed, there has been no attempt to summarise the overall strengths and limitations of these scores for patients, staff and systems. This review aims to address this gap in the literature to guide improvements for the optimization of patient safety.
Methods: A systematic review was conducted of MEDLINE ® , PubMed, CINAHL and The Cochrane Library in September 2016. The citations and reference lists of selected studies were reviewed for completeness. Studies were included if they evaluated vital signs monitoring in adult human subjects. Studies regarding the paediatric population were excluded, as were studies describing the development or validation of monitoring models. A narrative synthesis of qualitative, quantitative and mixed- methods studies was undertaken.
Findings: 232 studies met the inclusion criteria. Twelve themes were identified from synthesis of the data: Strengths of early warning scores included their prediction value, influence on clinical outcomes, cross-specialty application, international relevance, interaction with other variables, impact on communication and opportunity for automation. Limitations included their sensitivity, the need for practitioner engagement, the need for reaction to escalation and the need for clinical judgment, and the intermittent nature of recording. Early warning scores are known to have good predictive value for patient deterioration and have been shown to improve patient outcomes across a variety of specialties and international settings. This is partly due to their facilitation of communication between healthcare workers. There is evidence that the prediction value of generic early warning scores suffers in comparison to specialty-specific scores, and that their sensitivity can be improved by the addition of other variables. They are also prone to inaccurate recording and user error, which can be partly overcome by automation.
Conclusions: Early warning scores provide the right language and environment for the timely escalation of patient care. They are limited by their intermittent and user-dependent nature, which can be partially overcome by automation and new continuous monitoring technologies, although clinical judgment remains paramount.
Keywords: Early warning scores; Limitations; Strengths; Systematic review; Vital signs.
Copyright © 2017 Elsevier Ltd. All rights reserved.
PubMed Disclaimer
Similar articles
- Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Smith ME, Chiovaro JC, O'Neil M, Kansagara D, Quiñones AR, Freeman M, Motu'apuaka ML, Slatore CG. Smith ME, et al. Ann Am Thorac Soc. 2014 Nov;11(9):1454-65. doi: 10.1513/AnnalsATS.201403-102OC. Ann Am Thorac Soc. 2014. PMID: 25296111 Review.
- What is the nursing time and workload involved in taking and recording patients' vital signs? A systematic review. Dall'Ora C, Griffiths P, Hope J, Barker H, Smith GB. Dall'Ora C, et al. J Clin Nurs. 2020 Jul;29(13-14):2053-2068. doi: 10.1111/jocn.15202. Epub 2020 Feb 3. J Clin Nurs. 2020. PMID: 32017272
- Promoting and supporting self-management for adults living in the community with physical chronic illness: A systematic review of the effectiveness and meaningfulness of the patient-practitioner encounter. Rees S, Williams A. Rees S, et al. JBI Libr Syst Rev. 2009;7(13):492-582. doi: 10.11124/01938924-200907130-00001. JBI Libr Syst Rev. 2009. PMID: 27819974
- Development and validation of early warning score system: A systematic literature review. Fu LH, Schwartz J, Moy A, Knaplund C, Kang MJ, Schnock KO, Garcia JP, Jia H, Dykes PC, Cato K, Albers D, Rossetti SC. Fu LH, et al. J Biomed Inform. 2020 May;105:103410. doi: 10.1016/j.jbi.2020.103410. Epub 2020 Apr 8. J Biomed Inform. 2020. PMID: 32278089 Free PMC article. Review.
- The impact of continuous versus intermittent vital signs monitoring in hospitals: A systematic review and narrative synthesis. Downey CL, Chapman S, Randell R, Brown JM, Jayne DG. Downey CL, et al. Int J Nurs Stud. 2018 Aug;84:19-27. doi: 10.1016/j.ijnurstu.2018.04.013. Epub 2018 Apr 21. Int J Nurs Stud. 2018. PMID: 29729558 Review.
- Variation in nurses' compliance with an Early Warning Score protocol: A retrospective cohort study. Leenen JP, Mondria CL. Leenen JP, et al. Heliyon. 2024 Aug 13;10(16):e36147. doi: 10.1016/j.heliyon.2024.e36147. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39247370 Free PMC article.
- AI-enabled electrocardiography alert intervention and all-cause mortality: a pragmatic randomized clinical trial. Lin CS, Liu WT, Tsai DJ, Lou YS, Chang CH, Lee CC, Fang WH, Wang CC, Chen YY, Lin WS, Cheng CC, Lee CC, Wang CH, Tsai CS, Lin SH, Lin C. Lin CS, et al. Nat Med. 2024 May;30(5):1461-1470. doi: 10.1038/s41591-024-02961-4. Epub 2024 Apr 29. Nat Med. 2024. PMID: 38684860 Clinical Trial.
- Impact of wearable wireless continuous vital sign monitoring in abdominal surgical patients: before-after study. Leenen JPL, Ardesch V, Kalkman CJ, Schoonhoven L, Patijn GA. Leenen JPL, et al. BJS Open. 2024 Jan 3;8(1):zrad128. doi: 10.1093/bjsopen/zrad128. BJS Open. 2024. PMID: 38235573 Free PMC article.
- Effect of the Ramathibodi Rapid Response System Triggered by the Ramathibodi Early Warning Score and Clinical Warning Signs on in-Hospital Mortality and the Incidence of Cardiopulmonary Resuscitation in Adult Hospitalized Patients. Kwantong C, Sutherasan Y, Junhasavasdikul D, Petnak T, Theerawit P. Kwantong C, et al. Ther Clin Risk Manag. 2023 Dec 5;19:1025-1038. doi: 10.2147/TCRM.S426061. eCollection 2023. Ther Clin Risk Manag. 2023. PMID: 38074484 Free PMC article.
- Predicting Cardiopulmonary Arrest with Digital Biomarkers: A Systematic Review. De Sario Velasquez GD, Forte AJ, McLeod CJ, Bruce CJ, Pacheco-Spann LM, Maita KC, Avila FR, Torres-Guzman RA, Garcia JP, Borna S, Felton CL, Carter RE, Haider CR. De Sario Velasquez GD, et al. J Clin Med. 2023 Nov 30;12(23):7430. doi: 10.3390/jcm12237430. J Clin Med. 2023. PMID: 38068481 Free PMC article. Review.
Publication types
- Search in MeSH
Related information
- Cited in Books
Grants and funding
- DRF-2016-09-037/DH_/Department of Health/United Kingdom
- NIHR-RP-011-029/DH_/Department of Health/United Kingdom
LinkOut - more resources
Full text sources.
- Elsevier Science
- White Rose Research Online
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the Department of Health & Human Services
- Search All AHRQ Sites
- Email Updates

1. Use quotes to search for an exact match of a phrase.
2. Put a minus sign just before words you don't want.
3. Enter any important keywords in any order to find entries where all these terms appear.
- The PSNet Collection
- All Content
- Perspectives
- Current Weekly Issue
- Past Weekly Issues
- Curated Libraries
- Clinical Areas
- Patient Safety 101
- The Fundamentals
- Training and Education
- Continuing Education
- WebM&M: Case Studies
- Training Catalog
- Submit a WebM&M Case
- Submit a Training NEW
- Improvement Resources
- Innovations
- Submit an Innovation
- Submit a Toolkit NEW
- About PSNet
- Editorial Team
- Technical Expert Panel
Components of pharmacist-led medication reviews and their relationship to outcomes: a systematic review and narrative synthesis.
Craske ME, Hardeman W, Steel N, et al. Components of pharmacist-led medication reviews and their relationship to outcomes: a systematic review and narrative synthesis. BMJ Qual Saf. 2024;Epub Jul 16. doi:10.1136/bmjqs-2024-017283.
At several points during a hospital stay, a patient may receive a medication review with a pharmacist to reduce the risk of medication errors. This review characterizes themes and components of pharmacist-led medication reviews associated with positive patient outcomes . Patient involvement in goal setting was identified as a successful component that would benefit from additional research.
Implementation of the I-PASS handoff program in diverse clinical environments: a multicenter prospective effectiveness implementation study. November 16, 2022
A realist synthesis of pharmacist-conducted medication reviews in primary care after leaving hospital: what works for whom and why? December 2, 2020
Families as partners in hospital error and adverse event surveillance. March 8, 2017
Optimizing Pediatric Patient Safety in the Emergency Care Setting. October 19, 2022
Association of adverse effects of medical treatment with mortality in the United States: a secondary analysis of the Global Burden of Diseases, Injuries, and Risk Factors study. January 30, 2019
Effects of a brief team training program on surgical teams' nontechnical skills: an interrupted time-series study. August 11, 2021
Association of diagnostic stewardship for blood cultures in critically ill children with culture rates, antibiotic use, and patient outcomes: results of the Bright STAR Collaborative. May 18, 2022
Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. September 26, 2018
The Lancet Commission on lessons for the future from the COVID-19 pandemic. October 12, 2022
Return on investment for vendor computerized physician order entry in four community hospitals: the importance of decision support. July 10, 2013
Impact of pharmacist-led discharge medication reconciliation on error and patient harm prevention at a large academic medical center. July 17, 2024
Pharmacy-led medication reconciliation is best practice. October 11, 2023
Impact of pharmacist-led admission medication reconciliation on patient outcomes in a large health system. September 20, 2023
Interdisciplinary collaboration across secondary and primary care to improve medication safety in the elderly (The IMMENSE study) - a randomized controlled trial. November 30, 2022
Criteria for the selection of paediatric patients susceptible to reconciliation error. November 16, 2022
Analysis of pharmacist-identified medication-related problems at two United Kingdom hospitals: a prospective observational study. March 18, 2020
Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). February 3, 2016
Results of the Medications At Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. March 17, 2010
Medication safety in acute care in Australia: where are we now? Part 2: a review of strategies and activities for improving medication safety 2002-2008. October 14, 2009
ISMP medication error report analysis. May 7, 2008

- Submit a Training
- Submit a Toolkit
Connect With Us

Sign up for Email Updates
To sign up for updates or to access your subscriber preferences, please enter your email address below.
Agency for Healthcare Research and Quality
5600 Fishers Lane Rockville, MD 20857 Telephone: (301) 427-1364
- Accessibility
- Disclaimers
- Electronic Policies
- HHS Digital Strategy
- HHS Nondiscrimination Notice
- Inspector General
- Plain Writing Act
- Privacy Policy
- Viewers & Players
- U.S. Department of Health & Human Services
- The White House
- Don't have an account? Sign up to PSNet
Submit Your Innovations
Please select your preferred way to submit an innovation.
Continue as a Guest
Track and save your innovation
in My Innovations
Edit your innovation as a draft
Continue Logged In
Please select your preferred way to submit an innovation. Note that even if you have an account, you can still choose to submit an innovation as a guest.
Continue logged in
New users to the psnet site.
Access to quizzes and start earning
CME, CEU, or Trainee Certification.
Get email alerts when new content
matching your topics of interest
in My Innovations.
Submit Your Training
Please select your preferred way to submit a training.
Track and save your training in My
Edit your training as a draft
Please select your preferred way to submit a training. Note that even if you have an account, you can still choose to submit a training as a guest.
Submit Your Toolkit
Please select your preferred way to submit a toolkit.
Track and save your toolkit in My
Edit your toolkit as a draft
Please select your preferred way to submit a toolkit. Note that even if you have an account, you can still choose to submit a toolkit as a guest.
Submit Your WebM&M Case
Please select your preferred way to submit a case.
Track and save your case in My
Edit your case as a draft
Your name will not be publicly
associated with the case
Please select your preferred way to submit a case. Note that even if you have an account, you can still choose to submit a case as a guest. And if you do choose to submit as a logged-in user, your name will not be publicly associated with the case. Learn more information here.
Already have a PSNet
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Study Protocol
Health professionals’ attitudes towards traditional healing for mental illness: A systematic review protocol
Roles Conceptualization, Methodology, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Faculty of Medicine and Health, School of Health, University of New England, Armidale, NSW, Australia, Department of Psychiatry, College of Medicine and Health Sciences, Injibara University, Injibara, Ethiopia
Roles Supervision, Validation, Visualization, Writing – review & editing
Affiliation Faculty of Medicine and Health, School of Health, University of New England, Armidale, NSW, Australia
- Alemayehu Molla Wollie,
- Kim Usher,
- Reshin Maharaj,
- Md Shahidul Islam

- Published: September 9, 2024
- https://doi.org/10.1371/journal.pone.0310255
- Peer Review
- Reader Comments
Mental illness is a global problem that receives less attention, particularly in developing countries. Integrating modern treatment with traditional healing approaches has been proposed as one way to address mental health problems, especially in developing countries. Despite health professionals’ participation in traditional healing being crucial to integrative approaches, their participation is limited to date. This review protocol is designed to explore the attitudes of health professionals towards traditional healing practices in mental health services.
The review will follow the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. Searching databases, including PubMed/Medline, PsychINFO, EMBASE, Scopus, and the Web of sciences will be conducted. Additionally, Google and Google Scholar will be searched for other information, including grey literature. Moreover, a manual search of identified articles’ reference lists will also be conducted to help ensure all potential papers are included in the review. Qualitative, quantitative, and mixed study methods published in English between January 2014 and April 2024 will be included. The qualities of the included studies will be assessed using the Mixed Methods Appraisal Tool (MMAT) Version 2018. A mixed-method synthesis will be used to synthesis the results.
It is crucial for healthcare professionals to provide culturally sensitive care to empower people to manage their health. This systematic review will summarize the attitudes of health professionals towards the adoption and delivery of traditional healing approaches to people experiencing mental illness. Therefore, the findings of this review will support integration between traditional healers and modern mental health practitioners for the treatment of mental illness.
Trial registration
Protocol registration number: CRD42024535136 .
Citation: Wollie AM, Usher K, Maharaj R, Islam MS (2024) Health professionals’ attitudes towards traditional healing for mental illness: A systematic review protocol. PLoS ONE 19(9): e0310255. https://doi.org/10.1371/journal.pone.0310255
Editor: Madhulika Sahoo, Kalahandi University, INDIA
Received: May 8, 2024; Accepted: August 27, 2024; Published: September 9, 2024
Copyright: © 2024 Wollie et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data are in the manuscript.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that co competing interest exists.
Mental health is the overall wellbeing and functioning of an individual, family, and community [ 1 , 2 ]. It is “a state of wellbeing in which the individual realizes his or her own abilities, is able to contribute to his or her environment, copes with the normal stress of life, works fruitfully, and is able to cooperate with others [ 3 ].” In contrast, mental illness has a substantial impact on a person’s feelings, thoughts, behaviour, and social interactions [ 4 , 5 ]. It is influenced by numerous factors, including psychological, physical, social, cultural, and spiritual [ 6 , 7 ].
Currently, mental illness is a major public health burden and accounts for 32.4% of years lived with disability worldwide [ 8 ]. Even though mental health issues are a global health concern, they are highly prevalent in low-income countries [ 9 , 10 ]. Developing nations have fewer health professionals [ 11 ], resulting in a treatment gap for people with mental illnesses in these countries [ 12 ]. Furthermore, negative attitudes, stigma, limited resources, and low priority are reasons for the high magnitude of mental illness, in addition to a shortage of health professionals [ 13 – 16 ].
People with mental health issues often seek care from indigenous or traditional healers [ 17 – 19 ]. Traditional healing practices include but are not limited to a set of beliefs that use culturally accepted spiritual treatments and plant products [ 20 ]. Traditional healers form a major part of the mental health workforce in developing countries [ 21 ]. Nearly 80% of people from Africa seek medical attention from traditional healers [ 21 ], largely because traditional healers provide culturally and socially accepted care for individuals and communities. This is the preferred treatment approach for most people due to its perceived effectiveness, affordability, and accessibility within communities [ 22 – 24 ]. In order to close the significant treatment gap in low-and middle-income countries, “the World Health Organization’s (WHO) 2003–2020 Mental Health Action Plan recommended that government health programs incorporate traditional healers as treatment resources [ 25 ].”
Studies indicate that traditional healers can offer efficacious therapies that may be beneficial for prevalent mental illnesses such as depression and anxiety [ 26 , 27 ]. Healers usually come from extended family branches with experience providing care like counselling, bible interpretation, and using prayer aids such as holy water and oil for treatment [ 27 , 28 ]. They either obtain experience through on-the-job training or apply their practices through ancestors who serve as mediators by providing access to spiritual guidance and power [ 15 , 29 , 30 ].
Despite the fact that traditional healing approaches are practiced by large groups of people, particularly in low-income countries, modern health professionals tend to differ in their opinions about this approach [ 31 , 32 ]. Studies indicate that some health professionals hold a positive view of traditional healing modalities [ 33 ], while many others express doubt about the effectiveness of traditional treatments. Furthermore, there is a negative attitude among health professionals regarding the integration of traditional healing with biomedical therapies [ 34 , 35 ]. Traditional healers are known to consult with modern health professionals when faced with problems beyond their ability to treat, but it is not common for biomedical professionals to refer back to healers [ 27 ]. This is often due to their negative attitudes toward traditional healing practices, including the belief that traditional healers do not incorporate a human right approach and/or compassionate care when dealing with people with mental health problems [ 35 , 36 ]. Their negative attitudes have a detrimental effect on the WHO’s recommended collaboration of biomedical treatment and traditional healing to close the treatment gap in low and middle-income countries because cooperation between the two needs mutual respect and recognition. In addition to providing biological therapies, it is good to emphasize spiritual and cultural approaches for mental illness since mental illness is due to multidimensional factors like spiritual, social, psychological, and physical. Moreover, it is crucial for healthcare providers to understand their patients’ cultural beliefs and practices in order to manage and counsel them appropriately [ 34 ] and empower them towards healthful healing. There is no summarized research regarding the attitudes of health professionals toward traditional healing approaches for mental illness, despite the existence of numerous singe studies. Therefor, this review protocol is aimed to explore the summarized evidences on health care providers’ attitudes toward traditional healing for mental illness, and this will be crucial in developing appropriate guidelines for holistic treatment.
Research question
What are the health professionals’ attitudes towards traditional healing for mental illness?
Methods and materials
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2020 guidelines will be used to conduct this review [ 37 ]. This protocol is also prepared in accordance with the PRISAM-Protocol guidelines (Supporting Information). Primary studies conducted using quantitative, quantitative, and mixed-methods will be included. The review protocol is registered with the International Prospective Register of Systematic Reviews (PROSPERO) with a registration number of CRD42014535136.
Eligibility criteria
The eligibility assessment format will be used to select articles to be included in the systematic review. Globally published original articles focusing on health professionals’ attitude and/or perception towards traditional healing of mental illness will be considered. Qualitative, quantitative, and mixed-method studies will be considered without restrictions in their study design. Studies published in English between January 2014 and April 2024 will be considered to summarize contemporary evidence on the topic. Conference summaries, reviews, dissertations, case studies, governmental and non-government reports, and letters will be excluded from the study.
Searching strategy
A literature search of databases such as PubMed/Medline, PsychINFO, EMBASE, Scopus, and the Web of Sciences will be conducted. The key terms will be searched by connecting Boolean operators OR/AND to specifically address published studies. Searching words will be (attitude) OR (perception) OR (belief) OR (opinion) OR (view) AND (health professionals) OR (health practitioners) OR (health personnel) OR (nurses) OR (medical doctors) OR (psychiatrist) OR (psychologist) AND (traditional healing) OR (traditional medicine) OR (herbal medicine) OR (indigenous treatment) OR (spiritual therapy) OR (religious healing) AND (mental illness) OR (mental disorder) OR (mental health) OR (psychological distress) OR (psychiatric disorder). In addition to database and manual searches, we will use references to articles obtained from the database to filter the remaining studies. Database searching and the initial screening process will be finalized in consultation with a senior health librarian from the University of New England (UNE) Dixon Library and other authors.
An example of a search strategy for Scopus ( (ALL ("health professional") OR TITLE-ABS-KEY ("health personnel") OR TITLE-ABS-KEY ("medical doctor") OR TITLE-ABS-KEY ("medical practitioner") OR TITLE-ABS-KEY (nurse) OR TITLE-ABS-KEY (psychiatrist) OR TITLE-ABS-KEY (psychologist) ) ) AND ( (ALL (perception) OR TITLE-ABS-KEY (attitude) OR TITLE-ABS-KEY (belief) OR TITLE-ABS-KEY (view) OR TITLE-ABS-KEY (opinion) ) ) AND ( (ALL ("traditional healing") OR TITLE-ABS-KEY ("traditional medicine") OR TITLE-ABS-KEY ("indigenous treatment") OR TITLE-ABS-KEY ("spiritual therapy") OR TITLE-ABS-KEY ("religious healing") OR TITLE-ABS-KEY ("herbal medicine") ) ) AND ( (ALL ("mental illness") OR TITLE-ABS-KEY ("mental disorder") OR TITLE-ABS-KEY ("mental health") OR TITLE-ABS-KEY ("psychiatric disorder") OR TITLE-ABS-KEY ("psychological distress") ) ) AND PUBYEAR > 2013 AND PUBYEAR < 2025 AND (LIMIT-TO (DOCTYPE, "ar") ) AND (LIMIT-TO (LANGUAGE, "English") )
Study selection process
PRISMA (preferred reporting items for the systematic review and meta-analysis) guidelines [ 37 ] will be followed to ensure the quality and transparency of the review process. After critically searching articles from databases, we will start the selection process by importing all records to an Endnote library. Duplicates will be detected and removed automatically. At the beginning, the selection process will be carried out by the first author (AMW), and it will be checked by one of the co-authors (KU, RM, and SI). The reviewer will evaluate article titles and abstracts for relevance, keeping those that are relevant to the outcome variable attitudes, and/or perceptions of health professionals towards traditional healing. The full texts of possible relevant papers will then be screened for eligibility. Any disagreements will be handled through a discussion to finalize the selection of the papers.
Data extraction or collection process
With the use of a predetermined, uniform data extraction structure, the first author will extract important data, and then it will be confirmed by another author. Any significant differences will be discussed, and if a consensus cannot be reached, a third author will adjudicate on the decision. The first author’s name, the year of publication, the country where the study is conducted, the methodology used, the health professional type, the data collection and analysis method, the major findings, and the conclusions will be included in the standardized data extraction form.
Quality assessment
Each study’s methodological quality and bias risk will be assessed to determine the validity of the findings. The qualities of the included articles will be assessed using the Mixed Methods Appraisal Tool (MMAT) Version 2018 Checklist [ 38 ]. This tool is designed to assess the methodological qualities of different types of studies, like qualitative, quantitative, and mixed studies. The differences in quality evaluation scores will be resolved by through discussion within a team.
Data synthesis
An integrated mixed-method synthesis technique will be used to summarize all quantitative, qualitative, and mixed-method data in one combined synthesis [ 39 ]. Quantitative data will be described qualitatively to facilitate integration with qualitative data. The overall nature of the articles will be presented in a table. Themes and subthemes will be formulated based on the data generated. All the data will be analysed and reported as a narrative summary. To minimize bias, the research team will thoroughly evaluate and discuss the entire process, and any disagreements will be resolved through discussion.
The review will be conducted to summarize the attitudes of health professionals towards traditional healing approaches for mental illness in a global context. Even though independent studies have been undertaken on health professionals’ attitudes and/or perceptions towards traditional healing, there is no globally summarized general evidence on the topic. Some previous studies present opposing evidence on the attitudes of health professionals towards traditional healing practices. Even if some health professionals encourage traditional methods, there are other studies that show negative attitudes of health professionals towards traditional healing [ 34 , 35 ]. Traditional healing practices are considered appropriate options for mental illness, particularly in low-income countries where there is a scarcity of modern mental health professionals [ 21 ]. Most people with mental health problems seek these treatments because they provide culturally and socially accepted care for individuals and the community [ 22 – 24 ]. But there is limited collaboration between modern treatment and traditional healing. Therefore, this review will summarize all the literature and clearly show the positions of health professionals toward traditional healing approaches. The findings of this review will be important in developing directions for governmental organizations and other concerned bodies to strengthen communication and integration of traditional healing with biomedical treatment options for mental illness.
Expected limitations
This systematic review has the limitation of excluding articles that are not published in English. In addition, the exclusion of conference reports, letter, short communications, and review articles may be a reason to miss important data.
Publication status
This protocol is prepared for the ongoing systematic review of articles entitled “The health professionals’ attitude towards traditional healing approaches to mental illness.” If there is any change during the review, an updated version will be published with a final systematic review.
Supporting information
S1 checklist. prisma-protocol guidelines..
https://doi.org/10.1371/journal.pone.0310255.s001
- View Article
- Google Scholar
- PubMed/NCBI
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals
You are here
- Volume 14, Issue 9
- Guideline concordant screening and monitoring of extrapyramidal symptoms in patients prescribed antipsychotic medication: a protocol for a systematic literature review and narrative synthesis
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0009-0008-8045-1704 Rebekah Aubry 1 ,
- http://orcid.org/0000-0002-7777-0981 Thomas Hastings 2 , 3 ,
- http://orcid.org/0009-0001-5954-4702 Micheal Morgan 4 ,
- Jacqueline Hastings 5 ,
- Marie Bolton 6 ,
- Maura Grummell 7 ,
- Sinead Killeen 1 ,
- Cathal Coyne 8 ,
- Risa Shorr 9 ,
- http://orcid.org/0000-0003-4877-7233 Marco Solmi 10
- 1 Department of Psychiatry , Lucena Clinic Services , Dublin , Ireland
- 2 Department of Psychiatry , McMaster University , Hamilton , Ontario , Canada
- 3 Department of Psychiatry , University of Toronto , Toronto , Ontario , Canada
- 4 Department of Psychiatry , South Louth CAMHS , Drogheda , Ireland
- 5 School of Medicine , UCD , Dublin , Ireland
- 6 Department of Child and Adolescent Psychiatry , St Vincent's Hospital Fairview , Dublin , Ireland
- 7 Department of Psychiatry , Mater Misericordiae University Hospital , Dublin , Ireland
- 8 Department of Child and Adolescent Psychiatry , West Kildare CAMHS Linn Dara , Abbeylands Clane , Ireland
- 9 Learning Services , Ottawa Hospital , Ottawa , Ontario , Canada
- 10 Ottawa Hospital Research Institute , Ottawa , Ontario , Canada
- Correspondence to Dr Rebekah Aubry; rebekah.aubry{at}sjog.ie
Introduction Given the increasing rates of antipsychotic use in multiple psychiatric conditions, greater attention to the assessment, monitoring and documentation of their side effects is warranted. While a significant degree of attention has been provided to metabolic side effect monitoring, comparatively little is known about how clinicians screen for, document and monitor the motor side effects of antipsychotics (ie, parkinsonism, akathisia, dystonia and dyskinesias, collectively ‘extrapyramidal side effects’, EPS). This review aims to systematically assess the literature for insights into current trends in EPS monitoring practices within various mental health settings globally.
Methods and analysis An electronic search will be performed using the OVID Medline, PubMed, Embase, CINAHL and APA PsycINFO databases for studies published in the last quarter century (1998 to present day). Two independent reviewers will conduct the initial title and abstract screenings, using predetermined criteria for inclusion and exclusion. A third reviewer will resolve disagreements if consensus cannot be reached. If selected for inclusion, full-text data extraction will then be conducted using a pilot-tested data extraction form. Quality assessment will be conducted for all included studies using a modified version of the Quality Improvement Minimum Quality Criteria Set. A narrative synthesis and summary of the data will be provided. All stages of the review process will be reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Ethics and dissemination Ethical approval is not required. Findings will be peer reviewed, published and shared verbally, electronically and in print with interested clinicians and will also be presented as posters or talks at relevant medical conferences and meetings.
PROSPERO registration number CRD42023482372.
- Systematic Review
- Schizophrenia & psychotic disorders
- Protocols & guidelines
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2024-087632
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
STRENGTHS AND LIMITATIONS OF THIS STUDY
The search strategy was developed a priori in collaboration with an experienced health sciences librarian and involves a comprehensive search across five large databases and platforms.
The protocol follows the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines enhancing replicability and transparency.
Included studies will be rated based on their methodological quality using a modified version of the Quality Improvement Minimum Quality Criteria Set quality assessment tool developed by Hempel et al , which is suitable for the quality assessment of various types of service evaluation studies.
Due to resource constraints, the literature search will be restricted to English-only, peer-reviewed publications, possibly increasing the risk of selection bias and limiting the generalisability of review findings.
Introduction
Second generation antipsychotics (SGAs) are broadly used in clinical practice, not only for the treatment of psychotic and bipolar disorders but also for a variety of other conditions. 1–3 While SGAs are associated with a lower risk of motor side effects (ie, parkinsonism, akathisia, dystonia and dyskinesias, collectively ‘extrapyramidal side effects’, EPSs) than first-generation antipsychotics the rates of EPS remain significant. 4–8 Furthermore, EPSs are associated with impaired quality of life, medication non-adherence, increased morbidity, mortality, caregiver burden, utilisation of healthcare resources and higher medical costs. 8–16 This has resulted in some advocating for ‘better monitoring … to assess their true effect on patients’ quality of life and functioning and to prevent underascertainment’, 17 something especially important in higher risk populations, for instance, children, adolescents and the elderly. 18–20 The most recent American Psychiatric Association’s guidelines (2020) for the treatment of patients with schizophrenia calls for clinical assessment of EPS at baseline or initial assessment, at each subsequent visit as well as an assessment using a ‘structured instrument’ every 6 months in patients at increased risk of tardive dyskinesia and every 12 months for all other patients. 21 In the UK, the National Institute for Health and Care Excellence guidelines recommend assessment of any movement disorders before starting antipsychotic medication as part of baseline investigations and to monitor and record side effects of treatment and their impact on functioning, and the emergence of movement disorders, regularly and systematically throughout treatment and especially during titration. 22 Unfortunately, evidence demonstrates that actual monitoring rates fall far below these standards. 23–25
Rationale for the review
While a significant degree of attention has been provided to metabolic side effect monitoring, with several systematic reviews conducted on the subject, 26 27 comparatively little is known about EPS monitoring practices.
When it comes to EPS, its incidence and prevalence in research and naturalistic settings have been thoroughly investigated in numerous studies and reviews. 4–6 28 However, there seems to be a paucity of data about current practices relating to how clinicians screen for, monitor and document EPS in patients prescribed antipsychotics. Gaining a better understanding of current practice may allow for the introduction of effective interventions that help address the existing discrepancy between current practice and best practice.
Aim and objectives
The aim of this review is to systematically assess the literature, seeking insights into current EPS monitoring practices within various mental health settings globally.
Our three main objectives are as follows: (1) to identify the extent to which patients prescribed antipsychotic medication receive guideline concordant monitoring, (2) to gather data on interventions that have been proposed to improve this aspect of care and (3) to identify any existing barriers.
Research questions
In accordance with the aim and objectives outlined above, this review will seek to answer the following questions as regards EPS monitoring for patients who are prescribed antipsychotic medication:
Which guidelines if any are being used to guide current practice and arerecommended standards being met? What screening tools are being used?
What is the frequency of monitoring? Has it improved or worsened over the years?
What interventions have been proposed to improve monitoring standards?
What are some of the possible barriers to adequate monitoring?
Methods and design
All stages of the review process including literature searching, screening, applying inclusion and exclusion criteria and data extraction will be reported and documented in accordance with the Preferred Reporting Items for Systematic Review and Met-Analysis Protocol (PRISMA-P) statement. 29 The PRISMA-P was used to guide the development of the review protocol (see online supplemental file 1 for PRISMA-P checklist). 30 In accordance with the guidelines, this systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the reference number CRD42023482372. Any amendments to the protocol will be reported when publishing the results.
Supplemental material
Inclusion and exclusion criteria (eligibility of studies).
These are grouped under the following seven subsections:
Study design
Study designs aimed at gathering data on current practices relating to EPS documentation and monitoring as well as studies describing interventions developed to improve clinical performance in the area of documentation and monitoring of EPS will be included in the review. Examples of study designs that will be included are as follows:
Clinical audits without intervention.
Clinical audits with completed audit cycles after intervention.
Service evaluations without a quality improvement intervention.
Service evaluations following a quality improvement intervention.
However, the following study design types will be excluded:
Case reports.
Any trial design, including randomized controlled trials(RCTs).
Literature reviews.
Discussion and viewpoint studies.
Grey literature.
Abstract-only publications.
Epidemiological studies of incidence/prevalence of EPS.
Survey designs.
Types of intervention
All types of interventions concerned with the assessment, screening and monitoring of EPS will be included. This will involve gathering data on the types of processes currently used to carry out EPS monitoring and documentation as well as on any proposed interventions aimed at improving EPS documentation and monitoring such as educational interventions, adoption of novel screening instruments, etc.
Study language
This systematic review will be restricted to English language studies only.
Publication dates
Studies published from 1998 to the present will be included, spanning the last 25 years of clinical practice. We consider this sufficiently representative of contemporary trends in practice.
Study population/demographics
The first population of interest includes patients of all ages and genders receiving treatment for one or more mental health conditions and prescribed one or more antipsychotic medications. While it is true that EPS can manifest spontaneously in patients who were never exposed to antipsychotic agents 31 32 or can be caused by substances other than antipsychotics, 33–35 a substantial proportion of reported EPS is attributed to antipsychotic medication. 6 36 37 Moreover, even within cohorts of previously neuroleptic naïve patients, research suggests that dopamine D2 receptor antagonist antipsychotics interact with the disease process in such a way that ‘precipitates’ and ‘accentuates’ movement disorders intrinsic to schizophrenia’. 38 This review will, therefore, focus on patients prescribed antipsychotic medication, as they may be at higher risk of developing severe EPS. In addition, most available guidelines on EPS monitoring specifically refer to patients prescribed antipsychotic medications.
The second population of interest includes the healthcare professionals involved in the care of the patients (eg, nurses, residents, clinicians and pharmacists) and tasked with carrying out EPS monitoring.
Study settings
Studies reporting on EPS monitoring practices in any naturalistic, real-world clinical setting, including inpatient hospitals, day hospitals, outpatient clinics, community settings, etc will be included.
Other phenomena of interest
Where available, data on the views, experiences and behaviours of healthcare professionals and patients involved in the assessment, screening and monitoring of EPS will also be collected.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this protocol.
Information sources
Electronic sources.
The literature search was conducted using the following five databases and search platforms: OVID Medline, PubMed, EMBASE, PsycINFO and CINAHL. The initial search covers 25 years and includes studies published between April 1998 and April 2023. These searches will be re-run immediately prior to the final analysis (projected to take place in September 2024) and potential further studies will be retrieved for inclusion, ensuring that the most up-to-date information is presented in the review. The reference lists of all eligible articles will be manually searched to identify any additional relevant citations to ensure a comprehensive search.
Search strategy
Review authors RA and RS (librarian and information specialist with expertise in electronic searching) developed and ran a comprehensive search strategy. A scoping search was undertaken against each database to inform how the search terms were being translated and hence to identify the corresponding text words in each database. Following this, the complete search strategy was tested for its sensitivity to locate the key papers that the researchers are already aware of, along with relevant articles which are consistent with the inclusion criteria just before running the search through all the selected search engines.
The search strategy used variations in text words found in the title, abstract or keyword fields, and relevant focused subject headings to retrieve articles combining the following three search concepts, linked by the Boolean operator ‘AND’:
(1) One or more medication terms: antipsychotic* OR psychotropic* OR haloperidol OR olanzapine OR quetiapine OR risperidone OR cariprazine OR amisulpride OR aripiprazole OR lurasidone etc… (to include full list of antipsychotic medication listed as per the WHO Collaboration Centre for Drug Statistics Methodology ATC classification).
(2) One or more EPS terms: “Extrapyramidal symptom*” OR “Extrapyramidal side effect*” OR “drug-induced movement disorder*” OR ‘Drug-Related Side Effects and Adverse Reactions’ OR ‘movement side effects’ OR Dystonia OR ‘acute dystonia’ OR parkinsonism OR ‘drug-induced parkinsonism’ OR akathisia OR “tardive dyskinesia” OR tremor
(3) One or more terms relating to monitoring, screening, documenting or auditing clinical practice (including screening instruments): ‘Monitoring’ OR ‘Screening’ OR ‘Documenting’ OR ‘Documentation’ OR ‘Assessing’ OR ‘Assessment’ OR ‘Abnormal Involuntary Movement Scale’ OR ‘Extrapyramidal Symptom Rating Scale’ OR ‘Simpson-Angus Scale’ OR ‘Barnes Akathisia Scale’.
The search included all relevant synonyms, truncations and Mesh terms. Full details of search terms used for the OVID Medline search are shown in online supplemental file 2 . A similar search was conducted using the other databases and search platforms. The full search strategy is available on request from the corresponding author.
Study records
Data management.
The search results will be uploaded into web-based, systematic review management software (Covidence). Duplicates will be removed automatically by Covidence software. Authors RA and MM will scan through the results to remove any remaining duplicate records manually. Using Covidence, the initial title and abstract screening, and the full-text review will be logged. All standardised forms will be piloted and revised as needed by the reviewers before starting the review.
Screening and selection process
After identification of articles from searching the electronic databases, titles and abstracts will be screened independently by two review authors according to the predefined eligibility criteria. Disagreements will be resolved by consensus and the opinion of a third reviewer will be sought if necessary. The full-text copies of each potentially relevant study will then be retrieved and screened independently by at least two reviewers including the first author (RA). Consensus will be reached through discussion, and in the event that no consensus can be reached for a study, a third reviewer will arbitrate. All studies not meeting the eligibility criteria will be excluded. The results will be reported using the PRISMA flow diagram.
Data extraction and reporting of results
A standardised data extraction form will be developed to extract all relevant data from included studies. Information to be extracted will be as follows:
Study characteristics: authors, date, settings, country of origin, study design and sample size.
Patient characteristics: demographic data (age, gender, diagnosis, type of antipsychotic prescribed, etc.).
Monitoring characteristics: frequency, use of a structured tool, healthcare professionals involved in monitoring, guidelines followed, etc.
Intervention characteristics: (if study incorporated a preintervention/postintervention design): educational intervention, adoption of a new instrument, etc.
The data extraction form will be piloted on a small random sample (n=3) of the illegible studies to assess its reliability in extracting the targeted study data. Review authors TH, MB and SK will each independently conduct data extraction on the three studies. Review authors RA and MM will then review this extracted data, checking against the full text of the three studies for any discrepancies (eg, errors, omissions or failure to have consensus in any area) and will decide on how to resolve any that may arise. If the above pilot data extraction process is deemed reliable then the review authors TH, MB and SK will each independently conduct data extraction on the remaining studies in the systematic review. Review authors RA and MM will then cross-check the extracted data against the full-text articles in a similar process to that highlighted above.
Additionally, study authors will be contacted if necessary to gain information for clarification purposes and access to raw material when needed.
Critical appraisal of study quality
Authors RA and MM will use the Quality Improvement Minimum Quality Criteria Set (QI-MQCS) developed by Hempel et al to conduct the quality assessment of included studies. 39 Disagreements will be resolved by consensus; the opinion of a third reviewer (MG) will be sought if necessary. The QI-MQCS is a 16-domain, validated, reliable critical appraisal tool that assesses expert-endorsed QI domains for studies that include a QI intervention component. The QI-MQCS will be modified to be suitable for the body of studies included in our review, and in particular, to be able to assess studies with no intervention component, that is, clinical audits and service evaluations with no intervention. This will involve accepting a broader definition of several domains of the appraisal instrument to include studies evaluating existing services or standards in addition to QI intervention. This approach was chosen in the absence of a suitable tool for critical appraisal of service evaluation studies with no intervention component.
The QI-MQCS tool is designed to provide a score for each domain as well as a total score, which is expressed as a percentage of the maximum possible score.
Data synthesis
In this review, the search is expected to reveal heterogeneous studies and meta-analysis of study findings is therefore not a study objective. Therefore, data synthesis will take the form of a structured narrative synthesis of the included studies. The defining characteristic of a narrative synthesis is that it adopts a textual approach to the process of synthesis in order to provide answers to the identified research questions in a structured manner. Study findings pertaining to the following three themes will be examined and synthesised: (1) Data concerning the extent and quality of EPS monitoring being carried out in various mental health settings will be summarised. (2) Following this, details about any potential interventions employed to improve monitoring practices will be synthesised. And finally, (3) Information about any identifiable barriers or facilitators to guideline concordant EPS monitoring will be synthesised and discussed.
Study status
The study is ongoing and is expected to be completed by September 2024.
Proposed value of the systematic review and use of the findings
This systematic review seeks to shed light on the existing patterns of EPS monitoring occurring within various mental health settings. The findings of this systematic review may be of interest to mental health organisations and services as they are expected to provide insights into the potential barriers or facilitators (including possible quality improvement interventions) influencing whether EPS monitoring is carried out in a guideline concordant manner. This may in turn encourage organisations and services to assess their existing EPS monitoring practice and/or lead them to consider the adoption or development of interventions to improve monitoring standards.
Ethics statements
Patient consent for publication.
Not applicable.
- Radojčić MR ,
- Hope H , et al
- McGrath J ,
- Teeling M , et al
- Kelleher JE ,
- Hsieh C-H ,
- Kane JM , et al
- Misdrahi D ,
- Tessier A ,
- Daubigney A , et al
- Martino D ,
- Osland S , et al
- Baba Shettima F ,
- Lateef Sheikh T ,
- Abba Wakil M , et al
- Kadakia A ,
- Dembek C , et al
- Schouten HJ ,
- Egberts TCG , et al
- Gandhi SK ,
- Rizio AA , et al
- Millier A ,
- Boyer L , et al
- V G , et al
- Cutler AJ ,
- Caroff SN ,
- Tanner CM , et al
- Yeomans K ,
- Lenderking WR , et al
- Monteleone P ,
- Cascino G ,
- Monteleone AM , et al
- Vitiello B ,
- Correll C ,
- van Zwieten-Boot B , et al
- Estevez-Fraga C ,
- López-Sendón Moreno JL
- McInerney BE ,
- Alderman CP , et al
- Keepers GA ,
- Fochtmann LJ ,
- Anzia JM , et al
- NICE clinical guideline
- Cortese L ,
- McAuley TJ , et al
- Butler MI ,
- Chandrakanth J
- Mitchell AJ ,
- Delaffon V ,
- Vancampfort D , et al
- Blake JA , et al
- Tariku M , et al
- Liberati A ,
- Shamseer L ,
- Clarke M , et al
- Perju-Dumbrava L ,
- Procyshyn RM ,
- Jones AA , et al
- Seltenreich D ,
- Letmaier M , et al
- Blanchet P ,
- Wang D , et al
- Waddington JL
- Shekelle PG ,
- Liu JL , et al
Contributors RA is the author acting as guarantor. The study was conceived by RA, MS, MM and TH. RA and MM developed the eligibility criteria, search strategy, quality assessment strategy and data extraction plan with guidance from MS and RS. RA, TH and MM wrote the manuscript. MS, MB, MM, MG, JH, SK and CC read all drafts of the manuscript, provided feedback and approved the final manuscript. All contributors meet the ICMJE criteria for authorship.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests MS has received honoraria/has been a consultant for AbbVie, Angelini, Lundbeck, Otsuka.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Rheumatol Adv Pract
- v.8(3); 2024
- PMC11368408

Prevalence, incidence, and mortality of Raynaud’s phenomenon, Sjögren’s syndrome and scleroderma: an umbrella review of systematic reviews
Anthony chen.
Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK
Stephanie J Lax
Matthew j grainge, peter c lanyon.
Department of Rheumatology, Nottingham University Hospitals NHS Trust, Nottingham, UK
Fiona A Pearce
Associated data.
The data and associated materials used in this study will be made available upon request.
To comprehensively review systematic reviews of prevalence, incidence, and mortality of Raynaud’s, Sjögren’s and Scleroderma, and to identify any research gaps.
An umbrella review of English language systematic reviews was undertaken using PubMed and Embase (OVID) covering the period 2000–2023 (PROSPERO CRD42023434865). The estimate and its corresponding 95% confidence interval were reported when available from each systematic review. The quality of systematic reviews was assessed using the Scottish Intercollegiate Guidelines Network (SIGN) tool. A narrative synthesis was undertaken.
Seventeen systematic reviews were identified, of which 1 was for RP, 5 for Sjögren’s and 11 for Scleroderma. There were some high-quality systematic reviews for Sjögren’s and mortality of Scleroderma. However, there were only low-quality systematic reviews of prevalence and incidence of RP and Scleroderma. Furthermore, there were no systematic reviews for the mortality of RP. For RP, the pooled prevalence was 4850 per 100 000; pooled annual incidence was 250 per 100 000. For Sjögren’s, prevalence was 60–70 per 100 000; annual incidence was 6.92 per 100 000 and the pooled standardized mortality ratio ranged from 1.38 to 1.48. For Scleroderma, pooled prevalence ranged from 17.6 to 23 per 100 000; annual incidence was 1.4 per 100 000; and the pooled standardized mortality ratio ranged from 2.72 to 3.53.
The outcomes of RP were less well described compared with Sjögren’s and Scleroderma. There was a lack of high-quality systematic reviews for the prevalence and incidence of RP and Scleroderma. Therefore, further studies and systematic reviews with rigorous case definitions, assessing different ethnic groups are warranted in this area.
Key messages
- There were no systematic reviews for the mortality of Raynaud’s.
- There were only low-quality systematic reviews for the prevalence and incidence of Raynaud’s and Scleroderma.
- There were high-quality systematic reviews for Sjögren’s and mortality of Scleroderma.
Introduction
Raynaud’s Phenomenon, Sjögren’s Syndrome and Scleroderma are diseases of unknown aetiology that share some common features and risk factors [ 1–3 ]. Over the past two decades, a number of studies were conducted to investigate the prevalence, incidence and mortality of these conditions. These studies also examined the severity and prognosis of conditions which in turn would help plan healthcare resources in the populations, improve healthcare and mean economic costing. There have also been several systematic reviews on the conditions. However, there have not been good-quality, recent, systematic reviews of all aspects of the epidemiology of these conditions. Where there have been several previous systematic reviews, these contained variations in findings relating to prevalence, incidence and mortality [ 4 , 5 ]. It is unknown why they varied; whether these differences were due to their methodology and study quality, databases searched or criteria of including primary studies. This has created a confusing landscape. There have been no reviews of reviews that have sought to explain these variations or to identify potential gaps in the literature. Therefore, this umbrella review aims to comprehensively review existing systematic reviews on the prevalence, incidence and mortality of Raynaud’s, Sjögren’s and scleroderma and to identify any gaps in the current literature. This will help to identify priorities for future research.
This umbrella review was prospectively registered in PROSPERO (CRD42023434865) [ 6 ] on 15 June 2023. It was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines and recommendations for umbrella reviews. No revisions were made to the protocol registered in PROSPERO during the study period. Ethical approval was not required because this study retrieved and synthesized data from previously published studies.
Search strategy and selection criteria
A comprehensive systematic literature search was undertaken in PubMed (this includes Medline and PubMed Central databases) and Embase (OVID) from 1 January 2000 to 1 June 2023. We searched using MeSH terms and keywords for ‘RP’, ‘SS’ or ‘Scleroderma’ or ‘SSc’ and terms such as ‘prevalence’, ‘incidence’ or ‘mortality’ (see Supplementary Tables S1–S3 , available at Rheumatology Advances in Practice online, for full details of the search strategy). For mortality, we included systematic reviews investigating all-cause or cause-specific mortality. We limited our search to systematic reviews published in English and excluded conference abstracts. We included systematic reviews of patients of any age, sex and geographical area, based in the general population. Occupational cohorts, indigenous populations, critically ill patients and cohorts identified from specialist centres were excluded. We excluded systematic reviews which focused on a specific sub-condition (such as scleroderma renal crisis, scleroderma overlap syndrome or scleroderma-associated digital-ulcers; see Supplementary Table S6 , available at Rheumatology Advances in Practice online for the list of excluded studies). We searched PROSPERO for any systematic review protocols on topic [ 7–10 ]. We emailed the authors of four protocols [ 7–10 ] for further information and received one reply [ 10 ]. On screening the full text, it was found to be out of scope. Duplicate systematic reviews were removed manually. Two reviewers (AC and SL) independently screened titles and abstracts for relevance using Rayyan [ 11 ], and full-text papers for eligibility using AirTable . Discrepancies were resolved through discussion with a third reviewer (FP or PL) where required.
Data extraction and quality assessment
AC independently extracted the following data from each eligible systematic review: first author; publication year and affiliation; number of primary studies included in the systematic review (databases searched and eligibility criteria); study characteristics; main outcome estimates with assessments of heterogeneity (e.g. I 2 ); whether risk of bias was reported (see Tables 1–3 ) and SIGN quality assessment (SIGN checklist in Supplementary Table S4 , available at Rheumatology Advances in Practice online). This was then checked by SL. For each systematic review, we recorded either a narrative statement summarizing the authors’ main findings or extracted a pooled estimate and its corresponding confidence interval (95% unless stated otherwise). Rates were converted to a per 100 000 denominator where required. The methodological quality of each systematic review was assessed by AC and SL using the Scottish Intercollegiate Guidelines Network (SIGN), a 12-question tool appropriate for systematic reviews of observational studies [ 12 ]. Any discrepancies in quality assessment were resolved through a discussion between AC and SL. Systematic reviews were reported to be of high quality if most of the SIGN criteria (i.e. had their own quality assessment) were met and had little to no risk of bias. Systematic reviews that met most of the SIGN criteria with only minor flaws (i.e. did not consider publication bias or state conflicts of interests) were deemed to be of acceptable quality. Systematic reviews were rated low quality if they had major flaws such as a lack of quality assessment of their included studies and/or lack of relevant characteristics in the tables. Any discrepancies were resolved through a discussion between the two reviewers (AC and SL). If an agreement could not be reached, a third reviewer (FP or PL) was contacted.
Synthesis of RP systematic reviews
| First authors (publication year) and affiliation | Number of primary studies identified, searched database and inclusion/exclusion criteria | Study characteristics | Main findings and I statistics | Risk of bias reported or mitigated | SIGN quality assessment and score |
|---|---|---|---|---|---|
| (2015) [ ] | = 98.2% for pooled prevalence definite PRP | Authors stated they mitigated no numeric value reported | |||
| (2015) [ ] | = not reported | Same as above | |||
Synthesis of SS systematic reviews
| First authors (publication year) | Number of primary studies identified, searched database and inclusion/exclusion criteria | Study characteristics | Main findings and I statistics | Risk of bias reported or mitigated | SIGN quality assessment and score |
|---|---|---|---|---|---|
| (2015) [ ] | = 98.95 | = 0.566 | |||
| (2023) [ ] | All studies have a moderate to high risk of bias | ||||
| (2015) [ ] | and ] | =98.51 | = 0.566 | ||
| (2016) [ ] | = 94% | No publication bias was found. | |||
| (2020) [ ] | = 0.24). = 44% | No publication bias was found | |||
| (2021) [ ] | general population. | = 26.2 = 92.4% | No publication bias was found | ||
Synthesis of Scleroderma systematic reviews
| First authors (publication year) | Number of primary studies identified, searched database and inclusion/exclusion criteria | Study characteristics | Main findings and I statistics | Risk of bias reported or mitigated | SIGN quality assessment and score |
|---|---|---|---|---|---|
| (2008) [ ] | Risk of bias not reported | ||||
| (2017) [ ] | = Not reported | Evaluated the potential for bias, but could not see results of bias | |||
| (2019) [ ] | statistic between-study heterogeneity | Risk of bias not reported | |||
| (2021) [ ] | = 97% = 100% | Some publication bias examined funnel plot visually. Egger’s test otherwise ( = 0.19) | |||
| (2008) [ ] | and | Incidence of Scleroderma ranges from 0.06/thousand/year to 12.2/thousand/year. | Risk of bias not reported | ||
| (2019) [ ] | and | statistic between-study heterogeneity | Risk of bias not reported | ||
| (2021) [ ] | and | = 100% | Probable publication bias—funnel plot. Not confirmed by Egger’s test ( = 0.18) | ||
| (2012) [ ] | = 2691 patients from nine studies. | = 93% | Publication bias unlikely. | ||
| (2012) [ ] | = 12 829 patients | = 68% = 68% = 82% = 75% | Risk of bias not reported | ||
| (2012) [ ] | =‘ . | Only moderate to high quality studies were included. | |||
| (2014) [ ] | = 9239 patients | = Not reported | Risk of bias not reported | ||
| (2017) [ ] | Evaluated the potential for bias, but could not see results of bias | ||||
| (2018) [ ] | = 11 719 patients | = 88.8% | Publication bias unlikely | ||
| ] | = 13 214 patients with Scleroderma with = 4218 deaths | < 0.001) < 0.001. < 0.001. = Not reported | Risk of bias not reported | ||
| (2020) [ ] | = 1884 participants | Risk of bias not reported | |||
Literature search and selection process
PubMed and Embase yielded 63 studies for Raynaud’s ( Fig. 1) , 158 for Sjögren’s ( Fig. 2) and 178 for scleroderma ( Fig. 3 ). After title and abstract screening, 6 studies remained for Raynaud’s, 15 for Sjögren’s and 23 for scleroderma. After full-text screening, 1 systematic review was included for Raynaud’s [ 1 ], 5 for Sjögren’s [ 4 , 5 , 13–15 ] and 11 for scleroderma [ 14–24 ]. The earliest included systematic review was published in 2008, with an increasing frequency over time: one systematic review was published in 2005–2009, four in 2010–2014, seven in 2015–2019 and five in 2020–2023. According to the SIGN quality assessment, six systematic reviews were of high quality, four were of acceptable quality and seven were of low quality.

PRISMA diagram of identification and screening of RP studies

PRISMA diagram of identification and screening of SS studies

PRISMA diagram of identification and screening of Scleroderma studies
Raynaud’s Phenomenon (RP)
One systematic review by Garner et al. [ 1 ] met the inclusion criteria, reporting the prevalence and incidence but not mortality of Raynaud’s. It included studies of the general population from high-income countries. According to the SIGN quality assessment checklist, it was reported to be of low quality due to a lack of quality assessment of their included studies. The authors included 17 studies of prevalence from 4 of the 6 World Health Organisation (WHO) world regions (the Americas, Southeast Asia, Europe, Western Pacific; but not Africa nor the Eastern Mediterranean region). The pooled estimate of prevalence (pooled prevalence from the five studies of definite Raynaud’s in the general adult population) was 4850 per 100 000 (95% CI 2080, 8710), with the pooled prevalence for females being 5740 per 100 000 (2740–9750) and for males 4120 per 100 000 (95% CI 1600, 7740). This review identified only two studies of incidence, which came from Europe and North America. The pooled annual incidence from two studies of incidence was 250 per 100 000 (95% CI 190, 320). There were no systematic reviews of mortality among people with Raynaud’s.
Sjogren’s Syndrome (SS)
Five systematic reviews were included for SS: one systematic review examined both prevalence and incidence [ 16 ], one examined prevalence [ 17 ], and three examined mortality [ 4 , 5 , 15 ]. Of the five systematic reviews, three were of high quality [ 4 , 5 , 13 ] and two was acceptable [ 14 , 15 ]. Two systematic reviews reported the prevalence of Sjögren’s. Qin et al. [ 13 ] conducted a systematic literature search of PubMed and Embase and included 18 studies conducted worldwide, published in English, between 1995 and 2013. The pooled prevalence of primary Sjögren’s was 60.82 (95% CI 43.69, 77.94) per 100 000. The authors found the prevalence rate (per 100 000) of Sjögren’s in women was higher than in men and a higher rate Sjögren’s prevalence in Europe compared with Taiwan. Albrecht et al. [ 14 ] included 20 studies published from 2014 to 2022, conducted in the German population only and including sicca syndrome as well as SS. The authors [ 14 ] found a pooled prevalence of primary Sjögren’s including sicca syndrome of 70 per 100 000 people in Germany.
One systematic review of Sjögren’s incidence, by Qin et al. [ 13 ] who included six studies of incidence reported a pooled annual incidence rate of 6.92 (4.98–8.86) per 100 000. The authors found a higher incidence rate of primary Sjögren’s in females (12.30, 9.07–15.53) than males (1.47, 0.81–2.12), and there tended to be a higher incidence rate in Asia (Taiwan) than in other regions (EU and North America).
There were three systematic reviews of mortality in Sjögren’s including two studies [ 4 , 5 ] of all-cause mortality and one of only cardiovascular mortality [ 15 ]. Singh et al. [ 4 ] reviewed 10 studies of 7888 primary Sjögren’s patients with 682 deaths over a median follow-up of 9 years, which reported a pooled standardized mortality ratio of 1.38 (0.94–2.01), suggesting a possible association of primary Sjögren’s with increased all-cause mortality. Huang et al. [ 5 ] performed a systematic review of 14 studies (14 584 primary Sjögren’s patients and 902 deaths observed) and found a 1.46-fold (95% CI 1.10, 1.93) increased risk of death in primary Sjögren’s. The authors further showed the risk of mortality was highest among Sjögren’s individuals with vasculitis. The results for the one study of cardiovascular mortality were similar to the findings for all-cause mortality. Beltai et al. [ 15 ] reviewed 14 studies of 67 124 patients with primary Sjögren’s found that the patients had a raised risk of mortality compared with the general population cardiovascular mortality (RR = 1.48, 0.77–2.85), which did not reach statistical significance.
Scleroderma
Eleven systematic reviews were included for scleroderma: three systematic reviews [ 16–18 ] reported both prevalence and incidence, one systematic review [ 19 ] reported both prevalence and mortality and seven systematic reviews [ 18–24 ] reported mortality only. Of the 11 systematic reviews, four were of high quality [ 20–23 ], one was acceptable [ 24 ] and six were of low quality [ 16–19 , 25 , 26 ]. The papers reported showed wide ranges of estimates of prevalence with Chifflot et al. [ 16 ] reviewed 32 studies reporting prevalence ranging from 0.7 in Japan in 1974–6 to 48.9 in Italy in 1992. Morrisroe et al. [ 19 ] reviewing 54 studies from Australia reporting that the prevalence of scleroderma increased over time from 0.46 per 100 000 in 1974 to 20.0 per 100 000 in 1993 to 86.0 per 100 000, most recently. The prevalence was found to be similar between the general and the Aboriginal populations in the country. The two meta-analyses by Zhong [ 18 ] and Bairkdar [ 17 ] reported similar pooled estimates. Zhong et al. [ 18 ] pooled the data of 18 studies of 11 574 individuals and estimated a scleroderma prevalence of 23 per 100 000 (16–29 per 100 000). The prevalence of scleroderma varied across countries, ranging from 3.8 per 100 000 in Taiwan to 50 per 100 000 in the USA. Bairkdar et al. [ 17 ] reviewed 46 studies published up to October 2020 and reported a pooled prevalence of 17.6 (15.1–20.5) per 100 000, with a prevalence of 28.0 (23.1–33.9) per 100 000 in women, and 6.0 per 100 000 (4.8–7.5) in men. The highest prevalence of scleroderma was found to be in North America 25.9 per 100 000 (21.5–31.2).
Three systematic reviews [ 16–18 ] also examined the incidence rates of scleroderma, with two reporting narrative results and only one meta-analysis. In the narrative results, Chifflot et al. [ 16 ] reported incidence rates ranging from 0.06 to 12.2 per 100 000 person-years. Zhong et al. [ 18 ] reviewed 12 studies and reported incidence rates of scleroderma ranging from 0.77 per 100 000 person-years in the Netherlands to 5.6 per 100 000 person-years in the USA. Bairkdar et al. [ 17 ] performed a meta-analysis of the data from 28 studies published up to October 2020, finding the pooled incidence rate of scleroderma in the population was 1.4 (1.1–1.9) per 100 000 person-years and there was a higher incidence in women (2.3, 1.8–2.9) than in men (0.5, 0.4–0.7).
Eight systematic reviews [ 19–26 ] reviewed mortality in people with scleroderma, with six presenting results of a meta-analysis and two were systematic reviews [ 19 , 26 ]. Of the six meta-analyses, three were rated high quality but the latest paper included was published in 2012 and three later systematic reviews including papers up to 2019 were rated acceptable or low quality. The results of the six meta-analyses were similar (irrespective of quality rating) reporting a pooled SMR for scleroderma of around 3.5, which is substantially higher than for Sjögren’s. Three high-quality studies including papers published in 2013 or earlier: Elhai et al. [ 21 ] who reviewed nine studies and pooled the data of scleroderma SMR as 3.53 (95% CI 3.03, 4.11). Komosci et al. [ 22 ] reviewed 18 studies and found the risk of death was significantly increased among patients with cardiac involvement HR (3.15, 2.33–4.26), pulmonary interstitial disease (HR 2.58, 1.98–3.37), with pulmonary hypertension (HR 3.50, 1.94–6.30) and with renal manifestations (HR 2.76, 1.91–4.00). Toledano et al. [ 20 ] reviewed seven studies and reported a pooled SMR of 3.51 (2.74–4.50).
There were five low or acceptable quality scleroderma systematic reviews. Morrisroe et al. [ 19 ], a systematic review of 54 studies, found that despite improvements in Australian healthcare, the mortality remained high with an SMR of 3.4 (no confidence interval was given). Reviewing 18 studies of 11 719 patients with scleroderma, Pokeerbux et al. [ 25 ] reported a pooled SMR of 3.45 (3.03–3.94). Reviewing 22 studies of 13 214 patients and 4218 deaths, Lee et al. [ 24 ] found an overall SMR of 2.82 (2.22–3.59). Reviewing 91 studies including 1884 patients, Erzer et al. [ 26 ] reported a scleroderma mortality of 8.49% of all patients (160 out of 1884 total patients from 1945 to 2018). Rubio-Rivas et al. [ 23 ] in their systematic review of 17 studies found that scleroderma patients had an increased risk of mortality (SMR =2.72, 1.93–3.83) compared with the general population.
In our umbrella review, the outcomes of Raynaud’s were less well-described compared with the other conditions. We identified that there were no systematic reviews for the mortality of Raynaud’s and no high-quality systematic reviews covering the prevalence and incidence of Raynaud’s and scleroderma (prevalence and incidence) (see Supplementary Fig. S1 , available at Rheumatology Advances in Practice online). A common theme among the low-quality systematic reviews ( n = 7) was a lack of quality assessment or investigation for publication bias among the included studies. Some of these systematic reviews lacked information on whether two authors selected and extracted data and relevant characteristics in their main finding tables. None of the systematic reviews included a list of excluded studies (see Supplementary Table S5 and Supplementary Data S1 , available at Rheumatology Advances in Practice online).
There was little evidence about the epidemiology of Raynaud’s, identifying only one systematic review, which was rated low quality. The pooled prevalence was reported as 4850 per 100 000, and incidence was reported as 250 per 100 000 per year. However, the heterogeneity in the prevalence estimate was extremely high (I 2 = 98.2%) and the incidence findings were based only on two papers, making it difficult to draw firm conclusions. There were no systematic reviews of mortality in Raynaud’s, probably because of a lack of primary research studies to include.
There was more evidence about Sjögren’s. Our umbrella review identified four high or acceptable quality systematic reviews for prevalence, incidence and mortality of Sjögren’s. Most of these systematic reviews reported their own quality assessment or acknowledged publication bias. Overall, the prevalence was found by two systematic reviews [ 13 , 14 ] to be between 60 and 70 per 100 000; the incidence was reported by one acceptable quality study to be around 7 per 100 000 per year. The incidence of Sjögren’s was eight times higher among females than males; it was higher in studies from Asia (Taiwan) than Europe or North America. The mortality was found in two systematic reviews [ 4 , 5 ] to be 1.4 times higher than the general population. Two Sjögren’s systematic reviews [ 4 , 5 ] reported a variation in the significance of Sjögren’s with all-cause mortality. This variations between these findings could be owing to the Huang et al. [ 5 ] later publication date including four additional studies that were not in Singh et al. [ 4 ] systematic review. Although the largest number of the systematic reviews were about scleroderma, there were no high-quality systematic reviews of prevalence or incidence. Two low-quality systematic reviews [ 17 , 18 ] reported a pooled prevalence around 20 per 100 000, and the most recent study of incidence reported it around 1.4 per 100 000 per year. Heterogeneity in these studies was very high (>90%) again making it difficult to draw firm conclusions. There were several high-quality studies of mortality, which reported similar pooled estimates of mortality around 3.5 times higher than the general population, which is substantially (about 2.5 times) higher than in Sjögren’s. In scleroderma, the main causes of deaths were highlighted as having cardiovascular, renal and pulmonary involvement [ 19 , 21–23 , 25 ].
Our umbrella review reported a higher proportion of women than men suffering from each condition [ 5 , 17 , 18 ]. This is expected as previous studies demonstrated that high levels of oestrogen were associated with the high prevalence of Raynaud’s in pre- and post-menopausal women [ 27 , 28 ]. A systematic review [ 13 ] found that studies from Europe showed a higher prevalence of primary Sjögren’s compared with Asian counterparts, but the incidence was higher in Taiwan (Asia) compared with European and American studies. However, note that this difference should be interpreted cautiously due to difference in the number of studies from each region. There was a higher prevalence and incidence of scleroderma [ 18 ] in America compared with Taiwan and Netherlands, respectively. These geographical differences could be due to differences in genetic make-up, environment, lifestyle and cultural factors, diagnostic criteria and research methods between countries and healthcare resources [ 13 , 22 ]. Furthermore, methodological differences such as the number of studies examined and differences in publication period of the original study can be contributing factors to the difference in findings.
A recent study [ 29 ] published in May 2023 using linked data from the Clinical Practice Research Datalink (CPRD) investigated the prevalence and incidence of autoimmune diseases in a cohort study of 22 million people in the UK. The authors had found that the incidence of Sjögren’s out of 12 292 individuals changed from 6.0 per 100 000 in 2000–02 to 10.7 per 100 000 in 2017–19; SSc 4880 patients 2.6 per 100 000 in 2000–02 to 3.3 per 100 000 in 2017–19. Therefore, the incidence rate ratio (95% CI) for these two auto-immune diseases were 2.09 (1.84–2.37) and 1.23 (1.06–1.43), respectively. However, this study did not include any information regarding on the prevalence, incidence and mortality of Raynaud’s. This study was not included in any of the systematic reviews because their search dates were before November 2022.
Strengths and limitations
To our knowledge, our umbrella review is the first to examine the prevalence, incidence and mortality of Raynaud’s, Sjögren’s and Scleroderma. We captured various forms of measuring outcomes such as trend in time and differences in sex and geography. The umbrella review provides a comprehensive overview of available evidence of the diseases of interests, and the contradictions and consistencies between the systematic reviews. This will help clinicians and researchers to easily understand the depth and breadth of systematic reviews available on the diseases of interest’s outcomes. We assessed the quality of the systematic reviews in our umbrella review using the SIGN guidelines. This will help to identify areas for improvement.
This study has some limitations. First, we limited our search to 2000–2023. Second, only English-language texts were included. Therefore, there remains a potential we have missed studies that are relevant studies to our research question. Third, there were a few systematic reviews available for Raynaud’s. This indicates further research is needed. Finally, due to the number of systematic reviews rated low quality the implications for clinical practise and healthcare planning are limited.
In our umbrella review, gaps in available high-quality systematic reviews of epidemiology were found in Raynaud’s and scleroderma. Furthermore, there were little to no systematic reviews investigating the prevalence, incidence and mortality of Raynaud’s in comparison to Sjogren’s and scleroderma. Therefore, further studies with rigorous case definitions, assessing different ethnic groups and updated systematic reviews are warranted in this area.
Supplementary Material
Rkae086_supplementary_data, contributor information.
Anthony Chen, Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK.
Stephanie J Lax, Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK.
Matthew J Grainge, Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK.
Peter C Lanyon, Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK. Department of Rheumatology, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Fiona A Pearce, Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK. Department of Rheumatology, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Supplementary material
Supplementary material is available at Rheumatology Advances in Practice online.
Data availability
This work was supported by a PhD studentship to Anthony Chen from a benefactor donation to the University of Nottingham. F.P. is funded by an NIHR Advanced Fellowship (NIHR300863). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Disclosure statement: The authors have declared no conflicts of interest.

IMAGES
VIDEO
COMMENTS
in the synthesis. At review stage, authors will make further decisions about how best to organise and present the data based on the actual review findings. ... 2 A copy of the guidelines (Popay et al (2006) Guidance on the conduct of narrative synthesis in systematic reviews) can be obtained from Libby Osborn via email at [email protected] ...
In this study, the steps for a systematic review such as research question design and identification, the search for qualified published studies, the extraction and synthesis of information that pertain to the research question, and interpretation of the results are presented in details. This will be helpful to all interested researchers.
1.2 Narrative synthesis, narrative reviews and evide nce synthesis 'Narrative' synthesis' refers to an approach to the systematic review and synthesi s of findings from
Narrative synthesis of quantitative data (NS) is a method commonly used in systematic reviews where it may not be appropriate, or possible, to meta-analyse estimates of intervention effects. A common criticism of NS is that it is opaque and subject to author interpretation, casting doubt on the trustworthiness of a review's conclusions.
The fourth step of structuring a systematic review can be simpler than that for a narrative review, in that a systematic review is likely to follow the traditional IMRAD (Introduction, Methods, ... Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med, 126 (1996), pp. 376-380. Google Scholar [11]
The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information.
meta-analysis as part of a previous Cochrane review which investigated the effects of interventions for promoting smoke alarm ownership and function.1. The reviewers carrying out the new narrative synthesis were blinded to the findings of the original Cochrane review. We then compared the results and conclusions of the two different approaches.
Introduction. Narrative reviews are a type of knowledge synthesis grounded in a distinct research tradition. They are often framed as non-systematic, which implies that there is a hierarchy of evidence placing narrative reviews below other review forms. 1 However, narrative reviews are highly useful to medical educators and researchers. While a systematic review often focuses on a narrow ...
This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information. We outline core ...
The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information.
In systematic reviews that lack data amenable to meta-analysis, alternative synthesis methods are commonly used, but these methods are rarely reported. This lack of transparency in the methods can cast doubt on the validity of the review findings. The Synthesis Without Meta-analysis (SWiM) guideline has been developed to guide clear reporting in reviews of interventions in which alternative ...
Mhairi Campbell has broad experience of conducting complex systematic reviews, including: qualitative evidence of policy interventions, review of theories linking income and health, and research investigating the reporting of narrative synthesis methods of quantitative data in public health systematic reviews.
Narrative' synthesis' refers to an approach to the systematic review and synthesis of findings from multiple studies that relies primarily on the use of words and text to summarise and explain the findings of the synthesis. ... Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme ...
Synthesis. Then a narrative synthesis was performed to synthesize the findings of the different studies. Because of the range of very different studies that were included in this systematic review, we have decided that a narrative synthesis constitutes the best instrument to synthesise the findings of the studies.
Narrative methods of synthesis can be used to synthesise both quantitative and qualitative studies and have been used when the experimental and quasi-experimental studies included in a systematic review are not sufficiently similar for a meta-analysis to be appropriate (Mays et al. Citation 2005a). Narrative synthesis is used in different ways.
What is Narrative Synthesis ? • Variants on NS widely used in evidence synthesis and recommended in some guidelines on the conduct of systematic reviews of effectiveness but: - no consensus on ...
Narrative synthesis of quantitative data in public health reviews is often inadequate. Reporting of methods is limited, and available guidance is rarely referred to. Links between the data and the narrative summary are often unclear. This lack of transparency prevents assessment of the reliability of review findings, and threatens the credibility of systematic reviews that use narrative synthesis.
Rodgers et al.: Narrative synthesis in systematic Reviews 49 Objectives The aim of this article is to demonstrate the way in which narrative synthesis guidance can be used in the context of a review of effectiveness, and to evaluate what the guidance might add (or otherwise) to the fi ndings of a systematic review.
Our goal with this paper is to conduct a narrative review of the literature about systematic reviews and outline the essential elements of a systematic review along with the limitations of such a review. Keywords: systematic reviews, meta-analysis, narrative literature review, prisma checklist. 1 2.
We revisited a published systematic review on plain packaging of tobacco products. We compared the findings from the narrative synthesis originally used in the review that accounted for study quality with the findings from a new multilevel meta-analysis that included all available effect sizes.
Using a systematic review, we synthesised existing empirical studies on children's PWB from a eudaimonic perspective. We identified 32 quantitative articles, which presented in four different categories, namely validation studies, predictors and correlates of PWB, descriptive studies, and intervention studies. ... a Narrative Synthesis ...
Methods: A systematic review was conducted of MEDLINE ® ... A narrative synthesis of qualitative, quantitative and mixed- methods studies was undertaken. Findings: 232 studies met the inclusion criteria. Twelve themes were identified from synthesis of the data: Strengths of early warning scores included their prediction value, influence on ...
At several points during a hospital stay, a patient may receive a medication review with a pharmacist to reduce the risk of medication errors. This review characterizes themes and components of pharmacist-led medication reviews associated with positive patient outcomes. Patient involvement in goal setting was identified as a successful component that would benefit from additional research.
While our earlier systematic review - narrative synthesis 36 evaluated rural palliative care models from a quantitative perspective, the search process revealed many qualitative studies reporting on patient and family perspectives about their experiences of receiving care through such models. This prompted a meta-synthesis of raw qualitative ...
Systematic review protocols were scanned in Prospero and Cochrane library to ensure novelty of the review question. A scoping search of databases was conducted to inform the development of the search strategy. ... Because the studies were heterogenous, a narrative synthesis was performed. The database search returned 6184 articles, which were ...
This systematic review will summarize the attitudes of health professionals towards the adoption and delivery of traditional healing approaches to people experiencing mental illness. Therefore, the findings of this review will support integration between traditional healers and modern mental health practitioners for the treatment of mental illness.
All stages of the review process including literature searching, screening, applying inclusion and exclusion criteria and data extraction will be reported and documented in accordance with the Preferred Reporting Items for Systematic Review and Met-Analysis Protocol (PRISMA-P) statement.29 The PRISMA-P was used to guide the development of the ...
1. INTRODUCTION. Evidence synthesis is a prerequisite for knowledge translation. 1 A well conducted systematic review (SR), often in conjunction with meta‐analyses (MA) when appropriate, is considered the "gold standard" of methods for synthesizing evidence related to a topic of interest. 2 The central strength of an SR is the transparency of the methods used to systematically search ...
The purpose of this review is to evaluate the impact of MMT on health outcomes, identify predictive factors for mortality, and assess mortality rates among older individuals receiving MMT. A systematic search was performed across databases, including PubMed, Scopus, Web of Science, and Google Scholar, adhering to the Preferred Reporting Items ...
The quality of systematic reviews was assessed using the Scottish Intercollegiate Guidelines Network (SIGN) tool. A narrative synthesis was undertaken. ... A systematic review found that studies from Europe showed a higher prevalence of primary Sjögren's compared with Asian counterparts, but the incidence was higher in Taiwan (Asia) compared ...