Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Cucumber Economic Values and Its Cultivation and Breeding

Special Offer!
Applies to all print copy prices | Offer ends 15 December 2024 Enter the code 20PRINT to unlock the offer

Order this book in hard copy and enjoy a 20% discount! Use the promo code 20PRINT during the ordering process.
And that's not all! You'll also receive a complimentary bag with an inspiring quote.
Code copied to clipboard!
Valid Until: 15 December 2024
Note: All orders include FREE SHIPPING
(DAP Incoterms – Import duties and taxes are not included).
If you require assistance, contact us at [email protected].
Introductory Chapter: Studies on Cucumber
Submitted: 09 March 2021 Published: 08 May 2021
DOI: 10.5772/intechopen.97360
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Cucumber Economic Values and Its Cultivation and Breeding
Edited by Haiping Wang
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
1,858 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Author Information
Huixia jia *.
- Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing, China
Haiping Wang *
*Address all correspondence to: [email protected] and [email protected]
1. Introduction
Cucumber ( Cucumis sativus L.) belongs to Cucumis genus in Cucurbitaceae family and is an economically important fruit vegetable. There are three wild or semi-wild varieties of cucumber: C. sativus L. var. hardwickii, C. sativus L. var. sikkimensis, C. sativus L. var. xishuangbannanesis. Cucumber is indigenous to India and likely originated from the foothills of the Himalayan Mountain [ 1 , 2 ]. Cucumber was cultivated ~3000 years ago in India, and it seems to spread rapidly to Western Asia, and then to Southern Europe [ 2 ]. Cucumber was introduced respectively to North China through the Silk Route and to South China from Burma and India-China border, and subsequently spread to East Asia [ 2 ]. Genome variation analysis showed cucumber core germplasms were divided into four geographic groups including India, Eurasia, East Asia, and Xishuangbanna [ 3 ]. Nowadays, cucumber is widely cultivated in temperate and tropical regions throughout the world [ 4 ]. The total production of cucumber was 87,805,086 tons worldwide, and Asia was the largest producer accounting for 84.9% of the world’s total production in 2019 ( www.fao.org/faostat/en/ ). With abundant water, nutrients and phytochemical composition, cucumber has versatile uses in culinary, therapeutic and cosmetic purposes [ 5 , 6 ]. Cucumber has multiple advantages such as diploid, small genome, short life cycle and self-compatible mating system, so it is suitable for genetic studies. Moreover, cucumber has been identified as a model plant for studying sex determination and plant vascular biology [ 7 ]. Consequently, numerous studies have been conducted to discover the miracle of cucumber. The book will cover the extensive benefits, production and market, cultivation and management, pests and diseases, breeding progress of cucumber.
2. Biological characteristics
Cucumber is an annual climbing herbaceous plant. The root system is shallow and mainly distributes in the cultivated land layer of 30 cm. The stem is vine with different degree of apical dominance. The cross section of the stem is rhombus, and the epidermis of the stem has burrs. The axillae on the stem have the ability of branching, and the number of branching varies greatly among varieties. The cotyledons of cucumber are opposite and long elliptic; euphylla are alternate, simple, pentagonal palmate or cordate in outline, and the blades are 3–7 lobed. The flower is axillary, unisexual and occasionally hermaphrodite. The calyx is green with bristles, and the corolla is yellow. The colour of young fruit changes from white to pale green, while mature fruit is yellow or brown when ripened. The shape of the fruit is diverse, such as clublike, cylindrical and spherical. Each fruit has 100–400 seeds. The weight of 1000 seeds is about 20–40 g.
3. Culinary, therapeutic and cosmetic uses of cucumber
At present, cucumber is the fourth most widely cultivated vegetable after tomato, cabbage and onion [ 8 ]. Cucumber has versatile uses in culinary, therapeutic and cosmetic purposes [ 5 , 6 ]. Nutritional and epidemiological researches have shown various benefits of cucumber. For example, cucumber contains abundant nutrients and has crunchy texture and unique flavor, so it is a quintessential vegetable used for a variety of dishes, and it is also indispensable for salad, soup and smoothie. Cucumber is rich in superior hydration and phytochemicals, which have diverse health benefits including weight loss, anti-inflammation, remedy for multiple diseases of eczema, constipation, hypertension, atherosclerosis, cancer, etc. [ 9 ]. Recent studies found that the presence of kaempferol in cucumber is an important antidiabetic agent [ 10 ]. Furthermore, cucumber is popularly used for natural beautification and for skin treatments [ 11 ].
4. Influence factors and solutions of cucumber fermentation
Cucumber pickles are most commonly fermented vegetable and widely consumed throughout the world [ 12 ]. Good fermentation depends on the proper combinations and interactions of multiple physical, chemical and microbiological factors [ 13 ]. Brine storage and process operations are susceptible to oxidation reactions during the fermentation process, and this has adverse influence on the quality property of cucumber pickles. To control the influence factors of cucumber fermentation, researchers have done many efforts on modern and advanced technologies, such as reducing the concentration of brining sodium chloride, developing the brining properties using lactic acid bacteria cultures, developing an anaerobic tank system, preventing cucumber gaseous deterioration by pouring of CO2 from fermentation brines [ 13 ]. After storing the brine, excess salt and organic wastes need to be leached to complete the product processing, and these wastes are sources of serious environmental concern. Thus, the waste disposal needs to be solved in the cucumber pickle industry.
5. Performance, structure and constraints of cucumber market
Marketing is vital for linking production and consumption and facilitating agricultural productivity and employment [ 14 ]. Market performance is the ultimate result of various market activities, and market structure is the organization characteristics of the market that influence the nature of competition and pricing [ 15 ]. Both male and female participate in cucumber marketing, and the male–female rate has great differences in different regions. The wholesalers are older than the retailers. In Ibadan, most of the retailers were within 31–40 years age, whereas most of the wholesalers were within 41–50 years age. It’s gratifying that cucumber marketing is usually profitable for the retailers and wholesalers at both peak and lean seasons of cucumber production. However, the cucumber market is competitive, and inequality exists in the market. Commodity perishability is an important constraint in cucumber market. Thus, it is indispensable to reduce perishable degree and prolong storage time after post-harvest.
6. Soil moisture and fertilizer management of cucumber
Inappropriate farming systems and poor agronomic management are responsible for low yield of cucumber. The quality/fertility status of soils is essential for growth and development for cucumber [ 16 ]. With good moisture and fertilizer management, optimum yield of cucumber might be attained. The conventional irrigation methods including flooding irrigation, furrow irrigation and drip irrigation have been widely applied for a long time in cucumber cultivation because of their low cost or simple operation [ 17 , 18 ]. However, these irrigation methods are surface irrigation and are driven by positive pressure, which may cause low water use efficiency, water wastage and nutrient loss [ 16 , 19 ]. To solve these problems, new irrigation technique such as negative pressure irrigation that controls automatically water release based on the soil water potential difference should be encouraged [ 16 ]. Inadequate fertilizer use causes low soil fertility that cannot provide sufficient nutrients for the normal growth of cucumber. The integration application of inorganic and organic fertilizer is more beneficial than the sole use of inorganic fertilizer or organic manures in cucumber production [ 20 ]. Moreover, fertilizer sources need to dissolve or decompose to make nutrients available for cucumber plants, so soil fertility also depends on soil water, temperature and density. Consequently, the soil management strategies such as negative pressure irrigation, seasonable fertilization, application of organic mulches and conservation tillage should be appropriately applied for sustainable production of cucumber.
7. Biostimulators promote growth of cucumber under soilless cultivated condition
Soilless cultivation in substrate culture is an important cultivation pattern for cucumber in greenhouses. The substrates should have specific physical properties including pore volume, air and water capacity, and density of substrates. Studies indicate that biostimulators can stabilize the production process to enhance plant growth under stress conditions. For instance, humate can increase vitality and growth of plants, improve seed germination, promote nutrient uptake, enhance transport and availability of micronutrients, and increase ion-exchange capacity. Lactates can produce bioregulatory effects to improve nutrient balance and plant vitality [ 21 ]. Bacillus subtilis , as a microorganism from the rhizosphere, can accelerate plant growth, stimulate the process of formation of plant organs, and enhance the resistance of biotic and abiotic stresses [ 21 ]. Application of biostimulators mixture (humate, lactate, and Bacillus subtilis ) prevent growth reduction of cucumber under pH and temperature stresses through enhancing the root growth, whereas the growth is markedly reduced under stresses if no biostimulator is applied.
8. Pests and diseases during cucumber cultivation and production
During growth process, cucumber might be affected by multiple insect pests and diseases, resulting in decrease of yield and quality. The major insect pests in cucumber including Diabrotica undecimpunctata , Acalymma vitatum , Bactrocera cucurbitae , Raphidopalpa foveicollis , Epilachna implicate , Myzus persicae , Aphis gossypii , Anasa tristis , Trialeurodes vaparariorus , Bemisia tabaci and B. argentifolii [ 22 , 23 ]. Currently, the pest management mainly relies on chemical pesticides that cause environmental pollution, pest resistance, and disturbance of balance between the pests and natural enemies. Moreover, this control strategy is harmful to human health. Therefore, an integrated pest management including pest monitoring, cultural method, host resistance, botanicals, biological control, and judicious use of chemicals is recommended for controlling these pests [ 24 , 25 ] Many diseases caused by viral, bacterial, fungal and nematode pathogens severely affect the cultivation and production of cucumber. Viruses infecting cucumber belong to three genera: Potyvirus , Cucumovirus and Crinivirus [ 26 ]. Especially, the CMV, ZYMV, WMV, MWMV, PRSV and BPYV are major viruses that cause severe symptoms to cucumber. Downy mildew, powdery mildew and anthracnose also cause substantial losses of cucumber production [ 27 ]. Some pathogenic fungi including Alternaria tenuis , Fusarium equisett , Phytophthora capsici , Botrytis cinerea and Cladosporium tenuissimum cause rotting and high post-harvest losses of cucumber [ 28 ]. Furthermore, root-knot nematodes are prevalent destructive pathogens of cucumber [ 29 ]. Though a series of chemicals have been evaluated and screened to control these diseases, the biological control strategy and high-resistant varieties of cucumber need be developed and created to resist diseases in efficient and environmental ways.
9. Polyphenols act as antioxidants in cucumber to defense stresses
Plant secondary metabolites play important roles in adapting to various environments and defensing against biotic and abiotic stresses. Cucumber is a rich source of phenolic compounds that are important secondary metabolites [ 30 , 31 ]. The antioxidant capacity of cucumber seems to be attributing to polyphenols that scavenge singlet oxygen, hydroxyl and lipid peroxyl radicals to prevent lipid oxidation. Better understanding of the molecular regulation of polyphenols biosynthesis is crucial to increase the production of polyphenols. Polyphenols are derivatives of phenylpropanoid pathway which involves an array of enzymes. Among these, phenylalanine ammonia lyase, chalcone synthase, cinnamate 4-hydroxylase and dihydroflavonol reductase play important roles [ 32 ]. In-depth study of these key enzymes in cucumber will facilitate to reveal the molecular mechanism of polyphenol synthesis, which is helpful for advancement in biotechnological and industrial applications.
10. Progress of traditional breeding and molecular breeding in cucumber
In the past decades, traditional breeding has played essential roles in cultivar innovation of cucumber. Some superior varieties with early maturity, high yield and high resistance have been developed through hybridization and mutagenesis [ 33 ]. However, this progress is slow because of the long cycle and difficulty in selection of stable genetic characters or genotypes. To overcome the obstacle of traditional breeding, molecular breeding technologies including molecular marker assisted breeding, genome-wide design breeding and genetic engineering have been applied in cucumber to accelerate the breeding cycle and select desirable traits. Molecular breeding of cucumber has made some progress and achievements on completion of genomics, genetic architecture and molecular mechanism underlying important traits, and creation of high quality and multi-resistant varieties [ 7 , 34 , 35 , 36 ]. With increasing consumption demand of cucumber, more new varieties with excellent comprehensive properties are in need, and we might make some efforts from the following aspects: (i) expanding collection and utilization of cucumber germplasm resources; (ii) establishing highly efficient gene editing and genetic transformation technologies in cucumber; (iii) identifying new loci or genes associated with key agronomic traits of cucumber and combining multiple molecular markers of excellent traits into one variety; (iv) realizing rapid accumulation of omics genotypes and phenomics [ 37 ].
- 1. Sebastian P, Schaefer H, Telford IRH, Renner SS. Cucumber ( Cucumis sativus ) and melon ( C. melo ) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proceedings of the National Academy of Sciences of the United States of America. 2010; 107 (32):14269-14273
- 2. Lv J, Qi JJ, Shi QX, Shen D, Zhang SP, Shao GJ, et al. Genetic diversity and population structure of cucumber ( Cucumis sativus L.). Plos One. 2012; 7 (10):e46919
- 3. Qi JJ, Liu X, Shen D, Miao H, Xie BY, Li XX, et al. A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nature Genetics. 2013; 45 (12):1510-1515
- 4. Vora JD. Biochemical, Anti-microbial and organoleptic studies of cucumber ( Cucumis Sativus ). International Journal of Science & Research. 2014; 3 (3):662-664
- 5. Mukherjee PK, Nema NK, Maity N, Sarkar BK. Phytochemical and therapeutic potential of cucumber. Fitoterapia. 2013; 84 :227-236
- 6. Muruganantham N, Solomon S, Senthamilselvi MM. Anti-cancer activity of Cucumis sativus (cucumber) flowers against human liver cancer. International Journal of Clinical Pharmacology Research. 2016; 8 :39-41
- 7. Huang SW, Li RQ, Zhang ZH, Li L, Gu XF, Fan W, et al. The genome of the cucumber, Cucumis sativus L. Nature Genetics. 2009; 41 (12):1275-1281
- 8. Jamir MSharma A. A Sustainable production and marketing of cucumber crop in the hilly zone of Nagaland. TECHNOFAME-A Journal of Multidisciplinary Advance Research. 2014; 3 (1):61-66
- 9. Oboh G, Ademiluyi AO, Ogunsuyi OB, Oyeleye SI, Dada AF, Boligon AA. Cabbage and cucumber extracts exhibited anticholinesterase, antimonoamine oxidase and antioxidant properties. Journal of Food Biochemistry. 2017; 41 (3):e12358
- 10. Ibitoye OB, Uwazie JN, Ajiboye T. Bioactivity-guided isolation of kaempferol as the antidiabetic principle from Cucumis sativus L. fruits. Journal of Food Biochemistry. 2018; 42 (3):e12479
- 11. Fiume MM, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, et al. Safety assessment of Cucumis Sativus (cucumber)-derived ingredients as used in cosmetics. International Journal of Toxicology. 2014; 33 :47-64
- 12. Moore JF, Duvivier R, Johanningsmeier SD. Formation of γ-aminobutyric acid (GABA) during the natural lactic acid fermentation of cucumber. Journal of Food Composition and Analysis. 2021; 96 :103711
- 13. Fleming HP. Developments in cucumber fermentation. Journal of Chemical Technology & Biotechnology Biotechnology. 2010; 34 (4):241-252
- 14. Girei A, Rabirou K, Ogezi E, Nnodu ON. Analysis of structure and performance of pumpkin ( CucurbitaPepo L.) marketing in Nasarawa State. Direct Research Journal of Agricultural and Food Science. 2018; 6 (7):166-172
- 15. Futrell G, Others A. Introduction to agricultural marketing and Prices. Agricultural Production. 1983; 65 (4):114
- 16. Li YK, Xue XZ, Guo WZ, Wang LC, Duan MJ, Chen H, et al. Soil moisture and nitrate-nitrogen dynamics and economic yield in the greenhouse cultivation of tomato and cucumber under negative pressure irrigation in the North China Plain. Scientific Reports. 2019; 9 :4439
- 17. Zhang LD, Gao LH, Zhang LX, Wang SZ, Sui XL, Zhang ZX. Alternate furrow irrigation and nitrogen level effects on migration of water and nitrate-nitrogen in soil and root growth of cucumber in solar-greenhouse. Scientia Horticulturae. 2012; 138 :43-49
- 18. Fan ZB, Lin S, Zhang XM, Jiang ZM, Yang KC, Jian DD, et al. Conventional flooding irrigation causes an overuse of nitrogen fertilizer and low nitrogen use efficiency in intensively used solar greenhouse vegetable production. Agricultural Water Management. 2014; 144 :11-19
- 19. Qiu RJ, Kang SZ, Li FS, Du TS, Ling T, Wang F, et al. Energy partitioning and evapotranspiration of hot pepper grown in greenhouse with furrow and drip irrigation methods. Scientia Horticulturae. 2011; 129 (4):790-797
- 20. Zhang HM, Jin HJ, Ding XT, Yu JZ, Hao T. Efects of application of organic and inorganic fertilizers on the growth, yield and quality of cucumber in greenhouse. Journal of Plant Nutrition and Fertilizer. 2014; 20 (1):247-253
- 21. Böhme MH, Pinker I, Junge H. Effect of organic biostimulators with Bacillus amyloliquefaciens ssp. plantarum (Former Bacillus subtilis ) as the main agent in vegetable cultivation. Bacilli and Agrobiotechnology. 2016;345-366
- 22. Dhillon MK, Singh R, Naresh JS, Sharma HC. The melon fruit fly, Bactrocera cucurbitae : a review of its biology and management. Journal of Insect Science. 2005; 5 (1):40
- 23. Abrahamian PE, Abou-Jawdah Y. Whitefly-transmitted criniviruses of cucurbits: current status and future prospects. Virusdisease. 2014; 25 (1):26-38
- 24. Gerling D, Alomar O, Arnó J. Biological control of Bemisia tabaci using predators and parasitoids. Crop Protection. 2001; 20 (9):779-799
- 25. Mahmood T, Tariq MS, Khokar KM, Hidayatullah, Hussain SI. Comparative effect of different plant extracts and insecticide application as dust to control the attack of red pumpkin beetle on cucumber. Pakistan Journal of Agricultural Research. 2010; 23 :196-199
- 26. Cradock KR, Graca JVD, Laing MD. Viruses infecting cucurbits in KwaZulu-Natal, South Africa. Revista Mexicana de Fitopatología. 2001; 19 (2):251-252
- 27. Ma JC, Du KM, Zheng FX, Zhang LX, Gong ZH, Sun ZF. A recognition method for cucumber diseases using leaf symptom images based on deep convolutional neural network. Computers and Electronics in Agriculture. 2018; 154 :18-24
- 28. Al-Sadi AM, Al-Said FA, Al-Quraini SM, kaabi SA, Al-Mazroui SS, Al-Mahmooli IHS, et al. Occurrence, characterization and management of fruit rot of immature cucumbers under greenhouse conditions in Oman. Phytopathologia Mediterranea. 2011; 50 (3):421-429
- 29. Mukhtar T, Muhammad ZK, Hussainc MA. Response of selected cucumber cultivars to Meloidogyne incognita . Crop Protection. 2013; 44 :13-17
- 30. Abu-Reidah IM, Arráez-Román D, Quirantes-Piné R, Arroyo SF, Carretero AS, Fernández-Gutiérrez A. HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Research International. 2012; 46 (1):108-117
- 31. Murcia MA, Jiménez AM, Martínez-Tomé M. Vegetables antioxidant losses during industrial processing and refrigerated storage. Food Research International. 2009; 42 (8):1046-1052
- 32. Peukert M, Weise S, Röder MS, Matthies IE. Development of SNP markers for genes of the phenylpropanoid pathway and their association to kernel and malting traits in barley. BMC Genetics. 2013; 14 :97
- 33. Feng SJ, Zhang JP, Mu ZH, Wang YJ, Wen CL, Wu T, et al. Recent progress on the molecular breeding of Cucumis sativus L. in China. Theoretical and Applied Genetics. 2020; 133 :1777-1790
- 34. Wang YH, Bo KL, Gu XF, Pan JS, Li YH, Chen JF, et al. Molecularly tagged genes and quantitative trait loci in cucumber with recommendations for QTL nomenclature. Horticulture Research. 2020; 7 :3
- 35. Wang YH, Jiang B, Dymerski R, Xu XW, Weng YQ. Quantitative trait loci for horticulturally important traits defining the Sikkim cucumber, Cucumis sativus var. sikkimensis . Theoretical and Applied Genetics. 2020; 134 (1):1-19
- 36. Zhang ZH, Map LY, Chen HM, Bu FJ, Li GC, Sun JJ, et al. Genome-wide mapping of structural variations reveals a copy number variant that determines reproductive morphology in cucumber. Plant Cell. 2015; 27 (6):1595-1604
- 37. Feng SJ, Zhang JP, Mu ZH, Wang YJ, Wen CL, Wu T, et al. Recent progress on the molecular breeding of Cucumis sativus L. in China. Theoretical and Applied Genetics. 2020; 133 (7):1777-1790
© 2021 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Published: 06 October 2021
By Ibitoye Oluwayemisi Beatrice, Ajiboye Taofeek Olak...
685 downloads
By Sarmad Ghazi Al-Shawi and Sadiq Jaafir Aziz Alneam...
1128 downloads
By Chidiebere Ugwu and Stephen Suru
1071 downloads
IntechOpen Author/Editor? To get your discount, log in .
Discounts available on purchase of multiple copies. View rates
Local taxes (VAT) are calculated in later steps, if applicable.
Support: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Research Advances in Genetic Mechanisms of Major Cucumber Diseases Resistance
Mingming wei, xiangtao zhu, lingling wei, huasen wang.
- Author information
- Article notes
- Copyright and License information
Edited by: Pei Xu, China Jiliang University, China
Reviewed by: Tianshu Sun, University of Cambridge, United Kingdom; Changlong Wen, Beijing Vegetable Research Center, China
*Correspondence: Xiangtao Zhu, [email protected]
Lingling Wei, [email protected]
Huasen Wang, [email protected]
Li Miao, [email protected]
† These authors have contributed equally to this work
This article was submitted to Crop and Product Physiology, a section of the journal Frontiers in Plant Science
Received 2022 Jan 26; Accepted 2022 Feb 22; Collection date 2022.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Cucumber ( Cucumis sativus L.) is an important economic vegetable crop worldwide that is susceptible to various common pathogens, including powdery mildew (PM), downy mildew (DM), and Fusarium wilt (FM). In cucumber breeding programs, identifying disease resistance and related molecular markers is generally a top priority. PM, DM, and FW are the major diseases of cucumber in China that cause severe yield losses and the genetic-based cucumber resistance against these diseases has been developed over the last decade. Still, the molecular mechanisms of cucumber disease resistance remain unclear. In this review, we summarize recent findings on the inheritance, molecular markers, and quantitative trait locus mapping of cucumber PM, DM, and FM resistance. In addition, several candidate genes, such as PM, DM, and FM resistance genes, with or without functional verification are reviewed. The data help to reveal the molecular mechanisms of cucumber disease resistance and provide exciting new opportunities for further resistance breeding.
Keywords: cucumber, powdery mildew, downy mildew, Fusarium wilt, genetic mechanism
Introduction
Cucumber ( Cucumis sativus L.) is a popular vegetable grown on a large scale worldwide. It has an edible fruit with refreshing tastes and is enriched with vitamin E. In the recent years, with the increasing cultivation area of cucumber, it has gradually moved towards a large-scale planting model. However, because cucumber is susceptible to horticultural diseases, including powdery mildew (PM), downy mildew (DM), Fusarium wilt (FW), Verticillium wilt, Cladosporium cucumerinum , Corynespora leaf spot, green mottle mosaic virus, and bacterial soft rot, it does not help for industrialized production, which results in substantial economic losses to cucumber producers. Among the diseases, PM, DM, and FW are the serious main fungal diseases of cucumber that result in severe production and quality losses ( Block and Reitsma, 2005 ; Zhang et al., 2016 ; Vakalounakis and Lamprou, 2018 ). Several effective approaches have been widely used to control these diseases, such as various fungicides, biofungicides, and grafting. However, the variable adaptability of pathogens, fungicides residues on plants and in the environment, and higher production costs associated with these approaches indicate that better methods are required ( Mahmood et al., 2016 ; Chen et al., 2021 ). Therefore, breeding more resistant cultivars is an efficient approach to control cucumber diseases and understanding the genetic and molecular mechanisms of cucumber disease resistance is a crucial focus of cucumber breeding programs.
There is no conclusive genetic data on cucumber disease resistance at present. Some studies have shown that PM, DM, and FW resistance are quantitative traits controlled by multiple genes, respectively. For instance, the resistance to PM is controlled by a recessive single gene, and susceptibility is controlled by partial dominant genes ( Nie et al., 2015a ). In cucumber, the DM resistance was controlled by multiple recessive genes and has the duplicate recessive epistasis and the additive effects data confirmed the detected 14 quantitative trait loci (QTLs) for DM resistance ( Innark et al., 2020 ). Dong et al. (2019) found that the inheritance of FW resistance in cucumber is a quantitative resistance trait controlled by multiple genes, including two pairs of additive dominance-epistatic major genes and an additive-dominance polygene. However, resistance to PM, DM, and FW is also controlled by a single gene. For example, a single recessive gene, pm , for PM resistance in leaves, has been mapped to an approximately 468 kb region on chromosome 5 in IL52 ( Zhang et al., 2018 ). A recessive resistance gene, dm-1 , has been identified in many DM-resistant plant introduction (PI) lines, including PI 197087, Gy4, Chipper, and the Market more series ( Barnes and Epps, 1954 ; Wehner and Shetty, 1997 ; Call et al., 2012a ). Foc has been incorporated in the Dutch-type cucumber hybrids and has widely controlled FW of cucumber for 40 years ( Vakalounakis and Fragkiadakis, 2003 ). Variety is a major factor in the inheritance of cucumber disease resistance. At present, the mapping population of cucumber PM-resistance genes has been mainly constructed using PI 197088, S06, WI 2757, H136, K8, and IL52. In the constructed segregation population, 19 possible PM resistance QTLs were identified ( Wang et al., 2020 ). In the population generated using the high-resistance variety PI 197088, pm1.1 , pm1.3 , pm4.3 , pm5.1 , pm5.3 , pm5.4 , pm6.2 , pm6.3 , and pm7.1 are the main resistance QTLs and pm4.1 and pm6.3 are two major QTLs in the population constructed using high-resistance variety S06. Additionally, pm5.3 is the most important QTL for PM resistance in the population constructed using the high-resistance cultivar IL52 ( Sakata et al., 2006 ; Liu et al., 2008 ; Fukino et al., 2013 ; Yoshioka et al., 2014 ; Zhang et al., 2018 ). Most PM-related genes are closely linked to DM-related genes; therefore, they may also play equally important roles in DM resistance ( Wang et al., 2018 ; Zhang et al., 2018 ). The materials used for mapping genes associated with PM are also used for selecting genes associated with DM. At present, the DM-resistance gene mapping population has mainly been constructed using PI 197085, PI 197088, WI 7120 (PI 330628), WI 2757, S94, TH118FLM, IL52, and K8. In total, 16, 5, and 2 QTLs have been identified in PI 197085, PI 330628, and WI 2757, respectively ( Wang et al., 2020 ). For example, Wang et al. (2018) developed 55 microsatellite markers and found that dm5.1 , dm5.2 , and dm5.3 are the main resistance QTLs in the highly resistant variety PI 197088 and dm1.1 , dm2.1 , and dm6.2 are the main sensitivity-related QTLs in the highly susceptible variety Coolgreen. Li et al. (2018) used 141 simple sequence repeat (SSR) markers to identify 5 QTLs, namely, dm1.1 , dm3.1 , dm4.1 , and dm5.1/dm5.2 , among which, dm4.1 is a major resistance QTL in the cross-population derived from PI 197088 and Changchunmici. The development of resistance mechanisms against FW occurred more slowly than against PM and DM and few materials resistant to FW have been identified. Dong et al. (2019) detected a major effect QTL, fw2.1 , in a 1.91-Mb region on chromosome 2 using F2 segregating populations derived from Superina (P1) and Rijiecheng (P2). Additionally, different identification and evaluation methods, mapping population, infection site, and pathogen races also influence the inheritance of cucumber disease resistance ( Vakalounakis and Lamprou, 2018 ; Innark et al., 2020 ; Wang et al., 2020 ). The epigenetic variations also play important roles in crop disease resistance and are affected by environmental factors ( Zhi and Chang, 2021 ), but the epigenetic regulation in cucumber disease resistance has not been found yet.
Many candidate genes for PM, DM, and FW disease resistance in cucumber have been identified using genetic mapping as well as transcriptomic and proteomic analyses and several genes have been cloned for functional verification. The candidate genes for these diseases are all involved in plant hormone signal transduction, cell redox homeostasis, and transcriptional regulation. In PM disease, the Mildew Resistance Locus O ( MLO ) -like genes, including CsaMLO1–13 , but especially CsMLO1 , −8 , and −11 , are the most studied PM genes in cucumber ( Schouten et al., 2014 ; Nie et al., 2015b ; Berg et al., 2017 ) and MLO-based PM resistance caused by the formation of cell wall depositions (papillae) by the plant cell directly beneath the site of PM penetration ( Wolter et al., 1993 ), but the function is not yet unraveled. The candidate genes for DM resistance are involved in various metabolic pathways. For example, STAYGREEN ( CsSGR ), which is involved in the chlorophyll degradation pathway, plays important roles in DM disease resistance ( Wang et al., 2019 ) and the transient expression of CsLRK10L2 , which is a Damage-associated Molecular Pattern Molecule (DAMP) oligogalacturonan receptor and is involved in the breakdown of pectin, in Nicotiana benthamiana ( N. benthamiana ) leaves causes necrosis and results in high DM resistance ( Berg et al., 2020 ). Several candidate genes of both DM and PM resistance have also been identified. The gene Csa5M622830.1 , a GATA transcriptional factor gene, may prevent supplement nutrition from reaching DM and PM pathogens ( Zhang et al., 2018 ). Compared with PM and DM, fewer candidate genes resist to FW have been identified. However, resistance to FW is enhanced in transgenic cucumber harboring Ginkbilobin2-1 ( GNK2-1 ) ( Liu et al., 2010 ). Additionally, a great number of candidate microRNAs (miRNAs), long non-coding RNAs (lncRNAs), proteins, and metabolites related to DM, PM, and FW in cucumber have also been identified ( Li et al., 2011 ; Xu et al., 2019 , 2021 ; Nie et al., 2021 ; Sun et al., 2021 ).
Although a number of molecular markers, QTLs, and candidate genes have been identified, the genetic mechanisms of cucumber disease resistance are not well understood. Here, we independently review the genetic mechanisms of cucumber resistance to PM, FW, and DM and also provide new insights into future management strategies.
Inheritance, Quantitative Trait Loci Mapping, and Candidate Genes of Cucumber Resistance to Powdery Mildew
Powdery mildew mainly invades cotyledons, leaves, and stems, resulting in yellow, crisp dry leaves in which photosynthesis is seriously affected, thereby reducing cucumber yield. PM in cucumber is commonly caused by Podosphaera xanthii ( Sphaerotheca fuliginea ) and Golovinomyces cichoracearum ( Erysiphe cichoracearum ) ( Block and Reitsma, 2005 ), which share the characteristics of frequent infection, short incubation period, and strong transmission. They also can occur annually during cucumber production.
Inheritance of Powdery Mildew Resistance in Cucumber
A classical genetic analysis demonstrated that cucumber PM resistance is a quantitative trait controlled by multiple recessive genes in different germplasms ( Smith, 1948 ; Kooistra, 1968 ; Morishita et al., 2003 ; He et al., 2013 ). Early in 1948, Smith (1948) suggested that PM resistance in Puerto Rico 37 was controlled by recessive genes and then, associated recessive genes were identified in the PI 2008151 and Natsufushinari varieties ( Kooistra, 1968 ). The two recessively inherited genes linked to the QTL in chromosome 5 are responsible for PM in WI2757 ( He et al., 2013 ). Additionally, studies have shown that PM resistance in cucumber is controlled by a single recessive gene. A single recessive gene pm for PM resistance in leaves has been mapped to an approximately 468 kb region on chromosome 5 in IL52 ( Zhang et al., 2018 ). The resistance to PM in the stem of NCG-12 is also controlled by a single recessive nuclear gene ( pm-s ) ( Liu et al., 2017 ). The recessive inheritance of PM is not convenient to use in cucumber breeding ( Xu X. et al., 2016 ). The temperature-dependent PM resistance in PI 197088-5 is due to one recessive gene and another incompletely dominant gene ( Morishita et al., 2003 ). Shen et al. (2011) found that cucumber PM traits are determined by the interaction of major genes and polygenes in the JIN 5-508 variety and the inheritance of major genes dominates. Xu X. et al. (2016) first reported the dominantly inherited major-effect QTL ( Pm1.1 ) for PM in the Jin5-508-derived SSSL0.7 line. However, quantitative resistance under polygenic control is generally more durable than that conferred by a single dominant gene ( Kelly and Vallejo, 2006 ). The inheritance of cucumber disease resistance is dependent on the variety and material ( Wang et al., 2020 ) and the genetic laws governing cucumber PM resistance are still not well understood.
Molecular Markers and Quantitative Trait Loci of Powdery Mildew Resistance in Cucumber
Effective molecular markers and QTLs controlling resistance to PM in cucumber have also been reported in recent years (de Ruiter et al., 2008 ; Fukino et al., 2013 ; He et al., 2013 ; Nie et al., 2015a ; Wang et al., 2019 ). Various molecular markers have been used for mapping PM-associated loci in different cucumber species. In total, 140 PM-associated Specific-locus Amplified Fragment Sequencing (SLAFs) and two hot regions ( pm5.3 and pm6.1 ) have been identified on chromosomes 1 and 6 using an F2 segregating population derived from H136 as the susceptible parent and BK2 as the resistance donor ( Zhang et al., 2015 ). In total, 17 SSR markers have been discovered to be linked to the pm-s gene, which maps to chromosome 5 between the pmSSR27 and pmSSR17 markers ( Liu et al., 2017 ). The introgression of the 6.8-Mb segment that contains 3,016 single nucleotide polymorphisms (SNPs) causes the phenotypic variation in PM resistance between SSL508-28 and D8 ( Xu et al., 2017 ) and this region, pm5.1 , is consistent with major loci for PM resistance found in many studies ( Nie et al., 2015a ; Xu Q. et al., 2016 ; Wang et al., 2018 ). In total, 113 SNP and InDel markers significantly associated with PM resistance have been identified on chromosomes 4 and 5 using a genome-wide association analysis (GWAS) ( Tan, 2021 ). Additionally, four QTLs ( pm1.1 , pm2.1 , pm5.1 , and pm6.1 ) have been identified on chromosomes 1, 2, 5, and 6 using the recombinant inbred line (RIL) population derived from a cross between PI 197088 and the susceptible line Coolgreen. Among them, pm5.1 is the major-effect QTL, explaining 32.4% phenotypic variance, whereas the minor-effect QTL, pm6.1 , contributed to disease susceptibility ( Wang et al., 2018 ). Recently, pm5.2 (30% R2 at LOD 11) and pm6.1 (11% R2 at LOD 3.2) conferred PM resistance in an F2 population derived from a cross between PM-R (resistant) and PM-S (susceptible) ( Zhang C. et al., 2021 ). After further studies on the segregation populations constructed from PI 197088, S06, WI 2757, H136, K8, and IL52, 19 possible QTLs for PM resistance were mapped ( Wang et al., 2020 ). Moreover, PM resistance QTLs are also organ-dependent in cucumber. The disease indices of the hypocotyl, cotyledon, and true leaf of WI2757 were analyzed by multiple QTL mapping. pm5.1 was the major QTL for cotyledon resistance, pm5.2 controlled hypocotyl resistance, pm1.1 and pm1.2 controlled leaf resistance and both the minor QTLs, pm3.1 and pm4.1 , caused leaves or hypocotyls to have an increased PM susceptibility ( He et al., 2013 ). Liu et al. (2017) showed that pm-s , located on chromosome 5, controls PM resistance in cucumber stem and the gene Csa5G623470 , encoding an MLO protein, is closely related to the PM resistance of stem. Environmental factors also play important roles in the resistance to PM. Sakata et al. (2006) constructed a cucumber RIL using the PI197088-1 variety, resistant to PM, and the Santou variety, susceptible to PM, under both the high and low temperatures. Only one QTL played a role at high (26°C) and low (20°C) temperatures, which suggested that resistance was related to temperature. This was the first study on the QTL mapping of PM resistance genes at different temperatures. Like PM, DM is also an important disease in cucumber production. Many PM QTLs or genes are closely linked to DM QTLs or genes; consequently, they may also play equally important roles in DM resistance ( Wang et al., 2018 ; Zhang et al., 2018 ). For example, pm2.1 , pm5.1 , and pm6.1 associated with PM QTLs are colocalized with the DM QTLs dm2.1 , dm5.2 , and dm6.1 , respectively ( Wang et al., 2018 ). These studies showed inconsistent results regarding the number and locations of QTLs underlying PM and this may be due to differences in the germplasms, genetic maps, analysis methods, and environmental conditions.
Candidate Genes or Proteins Involved in the Powdery Mildew Resistance of Cucumber
In the recent years, candidate genes or proteins associated with PM resistance have been identified using transcriptomic and proteomic analyses and genetic mapping. Differentially expressed genes (DEGs) have been identified between PM-resistant species and susceptible species, such as SSL508-28 and D8, XY09-118 and Q10, BK2 and H136, and NILs of S1003 and Near Iso-genic Lines (NIL) ( pm5.1 ), using transcriptomes ( Xu et al., 2017 ; Nie et al., 2021 ; Zhang P. et al., 2021 ; Zheng et al., 2021 ). These DEGs function in plant hormone signal transduction, phenylpropanoid biosynthesis, phenylalanine metabolism, ubiquinone and other terpenoid-quinone biosynthesis, endocytosis, plant–pathogen interaction, and Mitogen-activated Protein Kinases (MAPKS). In particular, genes encoding the transcriptome factors (WRKY, NAC, and TCP), peroxidase, nucleotide-binding site (NBS), glucanase, and chitinase have been analyzed ( Zhang P. et al., 2021 ; Zheng et al., 2021 ). The miRNAs Csa-miR172c-3p and Csa-miR395a-3p are upregulated in PM-resistant D8 and Csa-miR395d-3p and Csa-miR398b-3p are downregulated in PM-susceptible SSSL508-28, suggesting that their target genes AP2 , bHLH , Dof , UGT , and LASPO may play important roles in PM-inoculated cucumber leaves ( Xu et al., 2020 ). Nie et al. (2021) showed that 49 differentially expressed lncRNAs may function as target mimics for 106 miRNAs during cucumber_PM interaction, including miR156 , miR159 , miR164 , miR166 , miR169 , miR171 , miR172 , miR6173, miR319 , miR390, miR393 , miR396 , and miR5658 . Moreover, differentially regulated processes, proteins, and accumulated metabolites between different PM-resistant materials have also been detected, including flavonoid, hormones, fatty acid, diterpenoid metabolism, tetrapyrrole biosynthetic process, sulfur metabolic process, and cell redox homeostasis ( Xu et al., 2019 ; Zhang P. et al., 2021 ).
A larger number of potential genes related to PM in cucumber have been identified using genetic mapping. Nie et al. (2015a) delimited the recessive major QTL pm5.1 for PM resistance in an approximately 1.7-kb region between markers UW065021 and UW065094 and they identified an MLO-like gene CsMLO1 , which encodes a cell membrane protein, as a candidate gene for PM resistance ( Nie et al., 2015b ). Schouten et al. (2014) obtained 13 MLO homologs, CsaMLO1-13 , in cucumber. Among them, the ectopic expression of CsMLO1 in the PM-resistant Atmlo2-Atmlo12 double-mutant results in PM sensitivity recovery. The overexpression of CsaMLO1 or CsaMLO8 completely restores PM susceptibility in a tomato mlo mutant, whereas the overexpression of CsaMLO11 only partially restores PM susceptibility ( Nie et al., 2015b ; Berg et al., 2017 ). To date, only MLO genes in cucumber have been functionally verified as being involved in PM resistance. In addition to MLO genes, other candidate PM resistance genes have been identified. Csa1M064780 and Csa1M064790 , encoding a cysteine-rich receptor-like protein kinase, are the most likely candidate PM resistance genes ( Xu Q. et al., 2016 ). The single recessive gene Csa5M622830 , which encodes a GATA transcriptional factor, is likely the gene for the complete PM resistance introgressed from Cucumis hystrix ( Zhang et al., 2018 ). CsGy5G015660 , which encodes a putative leucine-rich repeat receptor-like serine/threonine-protein kinase, is currently considered a strong candidate gene for PM resistance in cucumber ( Liu et al., 2021 ; Zhang C. et al., 2021 ). Moreover, proteins related to PM resistance have also been identified and functionally verified. Two NBS-Leucine-rich Repeat (LRR) proteins (CsRSF1 and CsRSF2), closely correlated with Abscisic Acid (ABA) and Gibberellin (GA) signals in cucumber, are predicted to have a similar domain sequence with the Arabidopsis PM-resistance protein RESISTANCE TO POWDERY MILDEW8 (RPW8) ( Xiao et al., 2001 ). The transient silencing of CsRSF1 and CsRSF2 reduces the resistance of cucumber to PM, whereas the transient overexpression of CsRSF1 and CsRSF2 improves the resistance of cucumber to PM ( Wang et al., 2021 ). Transcription factors, such as GRAS, DNA-binding with One Finger (DoF), Eukaryotic Initiation Factor 2 (eIF2α), Polygalacturonase (PG), UDP-Glycosyltransferase (UGT), and Serine/threonine Protein Kinases (STPKs) and their target genes, are also differentially expressed after PM inoculation ( Zhong, 2020 ). Translationally Controlled Tumor Protein (TCTP) is a highly conserved and multifunctional protein and CsTCTP1 may regulate the defense responses of cucumber or ABA signaling to control PM disease in cucumber. CsTCTP2 may regulate the Target of Rapamycin (TOR) signal in response to PM stress ( Meng et al., 2018 ). These studies provide new insights into cucumber responses to PM and the potential genes related to PM will be highly helpful in breeding cucumber varieties with enhanced PM resistance.
Inheritance, Quantitative Trait Loci Mapping, and Candidate Genes of Cucumber Resistance to Downy Mildew
Downy mildew of cucumber is caused by the obligate biotrophic oomycete Pseudoperonospora cubensis . It mainly infects leaves, but can also harm stems and inflorescences. It can occur from seedling to adult stage, but is particularly prevalent when cucumber enters the harvest stage. During the period of seedling infection, irregular chlorotic and withered yellow spots are produced on the reverse sides of cotyledons. A gray-black mold layer is produced when the plant becomes wet and cotyledons die when the infection is serious. During the adult stage, the disease gradually spreads upward from the lower leaves. At the beginning of the disease, light green water-immersion spots appear on the backs of the leaves. At the middle stage of the disease, the leaf spots fade from green to light yellow and the leaf backs become yellowish-brown. At the later stage, the disease spots converge and shrink upward from the leaf edges and finally, the whole leaf withers. In serious cases, all the leaves on the plant die ( Zhang et al., 2016 ).
Inheritance of Downy Mildew Resistance in Cucumber
Researchers have studied the inheritance of cucumber DM resistance. However, due to different resistance germplasms and inconsistent identification methods, there is no consensus on the genetic laws governing cucumber DM resistance. As early as 1942, DM-resistant lines were screened and DM resistance is controlled by a recessive resistance gene, dm-1 , in many resistant PI lines, including PI 197087, Gy4, Chipper, and the Marketmore series ( Jenkins, 1942 ; Barnes and Epps, 1954 ; Wehner and Shetty, 1997 ; Call et al., 2012a ). Simultaneously, multiple recessive genes are also involved in the regulation of cucumber DM resistance in resistant germplasms, including cucumber varieties WI4783, Wisconsin SMR18, K8 and K18, PI19708, CSL0067, and CSL0139 ( Doruchowski and Lakowska-Ryk, 1992 ; Zhang et al., 2013 ; Szczechura et al., 2015 ; Wang et al., 2016 ). Call et al. (2012b) identified three highly DM-resistant materials, PI 197088, PI 330628, and PI 605996, from 1,300 cucumber collections. Among them, PI 197088 is the most studied for DM resistance, with multiple genes being controlled in breeding programs ( Li et al., 2018 ; Liu et al., 2021 ). PI 197088 also has high resistance to PM. There are different genetic bases of DM-resistant germplasms. Therefore, the identification of DM-associated molecular markers and QTLs in various resistant materials may help to increase the inheritance of DM through breeding programs.
Molecular Markers and Quantitative Trait Loci of Downy Mildew Resistance in Cucumber
A variety of DM-associated QTLs has been identified in different varieties using Sequence Characterized Amplified Regions (SCAR), SSR, and SNP markers in recent years. The genetic linkage map was constructed using 66 polymorphic SSR markers and using this linkage map, 14 QTLs have been detected by evaluating DM in cotyledons as well as first and second true leaves after inoculation. LG5.1, located between the SSR03943 and SSR19172 markers, was detected at all the leaf stages ( Innark et al., 2020 ). Based on the linkage map having 328 SSR and SNP markers, dm4.1 , and dm5.1 , compared with dm2.1 and dm6.1 , were determined to be the major effect of QTL ( R 2 = 15–30%) with additive effects and this has been reproducibly detected in four environments (US2013, US2014, IT2013, and NL2013) ( Wang et al., 2016 ). In total, five QTLs associated with DM resistance have been identified on chromosomes 1, 3, 4, and 5 in seven independent experiments and dm4.1 , explaining 27% of the phenotypic variance, has been reliably detected in all the indoor experiments ( Li et al., 2018 ). The DM candidate QTLs related to DM have been detected using diverse evaluation methods that consist of different plant organs (cotyledons and true leaves), developmental stages (seedlings and adult plants), and evaluation criteria (lesion expansion and sporulation extent) and the dm1.1 QTL has the largest effect on resistance among the nine QTLs detected ( Yoshioka et al., 2014 ). In addition to QTL mapping methods, bulked segregant analyses (BSAs), next-generation sequencing (NGS), and GWASs have been the most rapid and effective ways of studying the genetic inheritance of DM resistant in cucumber. In total, five QTLs ( dm2.2 , dm4.1 , dm5.1 , dm5.2 , and dm6.1 ) have been identified and dm2.2 has the largest effect on DM resistance as assessed by combining BSA and NGS methods based on SNP markers ( Win et al., 2017 ). Additionally, 18 QTLs have been detected through the GWAS of a core database of 97 cucumber lines, but only six QTLs ( dmG1.4 , dmG4.1 , dmG4.3 , dmG5.2 , dmG7.1 , and dmG7.2 ) are associated with stable DM resistance ( Liu et al., 2021 ). To date, PI 197085, PI 197088, WI 7120 (PI 330628), WI 2757, S94, TH118FLM, IL52, and K8 have been used for mapping QTLs associated with DM resistance. Different cucumber germplasm resources may show stable genetic bases and QTLs for DM. For example, dm5.1 and dm5.2 have been detected in five resistance sources ( Wang et al., 2020 ). New QTLs have also been detected in commonly used disease-resistant materials. In PI 197087, Berg et al. (2020) focused on a QTL on chromosome 4-DM4.1 in the NILs produced by PI 197087 and a susceptible cucumber line (HS279) and this contained three sub-QTLs: DM4.1.1 that affects pathogen-induced necrosis, DM4.1.2 that has additive effects on sporulation, and DM4.1.3 that has recessive effects on chlorosis and sporulation. In general, the DM-associated QTLs varied depending on the germplasm and plant tissue as well as the developmental stage used in these analyses.
Candidate Genes or Proteins Involved in the Downy Mildew Resistance of Cucumber
A series of candidate genes or proteins related to DM resistance have been identified in cucumber through transcriptome profiling, proteomic analysis, and fine mapping. A large number of DEGs between DM-resistant and susceptible materials were identified by transcriptome analyses and these DEGs are involved in multiple defense response-related functions, including response: hormone signaling, regulation of nutrient supply, pathogen-associated molecular pattern recognition, signal transduction, reactive oxygen species and lignin accumulation, cell cycle, protein binding and metabolism, and transcriptional regulation ( Li et al., 2011 ; Burkhardt and Day, 2016 ; Gao et al., 2021 ). For example, five genes play important roles in the cucumber DM defense pathway: Csa5G139760 encodes an acidic chitin endonuclease, Csa6G080320 encodes a kinase having an LRR domain and transmembrane domain, Csa5G471600 is a retroviral receptor-like protein, and Csa5G544050 and Csa5G564290 encode the RNA-dependent RNA polymerase gene ( Gao et al., 2021 ). Consistently, differentially expressed proteins between the resistant and susceptible cucumber lines have also been identified and most of these proteins focus on cell rescue, defense, and energy metabolism ( Sun et al., 2021 ). Zinc finger-homeodomain (ZHD) proteins encode a family of plant-specific transcription factors that are responsive to DM in cucumber, such as CsZHD1–3 , CsZHD6 , CsZHD8 , and CsZHD10 ( Lai et al., 2021 ). Many novel QTLs for DM resistance in different cucumber species have been detected, such as dm2.1 , dm4.1 , dm4.1.2 , dm4.1.3 , dmG2.1 , and dmG7.1 ( Win et al., 2017 ; Berg et al., 2020 ; Liu et al., 2021 ), and these precise molecular markers and QTLs for DM resistance are helpful for the consequent fine mapping and positional cloning of QTLs. Liu et al. (2021) identified seven DM-resistance candidate genes using GWAS, including Csa1G575030 for dmG1.4 , Csa2G060360 for dmG2.1 , Csa4G064680 for dmG4.1 , Csa5G606470 for dmG5.2 , and Csa7G004020 for dmG7.1 . Among them, Csa5G606470 is a WRKY transcription factor and it was also identified within the DM-associated QTL dm5.2 using a Bulked Sergeant Analysis with Whole-genome Resequencing (BSA-seq) analysis ( Zhang et al., 2018 ). Cucumber CsSGR encodes a magnesium dechelatase and plays critical regulatory roles in the chlorophyll degradation pathway and a loss-of-susceptibility mutation of CsSGR results in durable broad-spectrum DM disease resistance ( Wang et al., 2019 ). CsLRK10L2 acts as a DAMP oligogalacturonan receptor and is involved in the breakdown of pectin, which is involved in the production of plant cell walls. This gene has been identified as a likely candidate for the sub-QTL DM4.1.2 because the transient expression of its loss-of-function mutation CsLRK10L2 from the DM-susceptible parent HS279 in N. benthamiana leaves causes necrosis ( Berg et al., 2020 ). A series of DM- and PM-associated QTLs were also colocalized in typical Northern Chinese type cucumber K8, PI 197088, and PI 197088-derived line CS-PMR1. For example, dm2.1/pm2.1 , dm5.3/pm5.1 , and dm6.2/pm6.1 have been colocated in PI 197088 ( Wang et al., 2018 ). Several candidate genes for both the DM and PM resistance have also been identified, including Csa5M622800.1 , Csa5M622830.1 , and Csa5M623490.1 . The gene Csa5M622830.1 is a GATA transcriptional factor gene and it may prevent the nutrition from reaching DM and PM pathogens ( Zhang et al., 2018 ). In addition, Cucumis sativus Irregular Vasculature Patterning ( CsIVP )-RNA interference ( RNAi ) plants having higher salicylic acid levels show higher resistance to DM than wild type (WT) and it was proposed that CsIVP may interact with CsNIMIN1 , which is a negative regulator in the salicylic acid-signaling pathway, to improve DM resistance in cucumber ( Yan et al., 2020 ). At present, the candidate genes for DM resistance in cucumber identified by forward genetic analysis methods need to be verified by overexpression or knockout experiments in cucumber.
Inheritance, Quantitative Trait Loci Mapping, and Candidate Genes of Cucumber Resistance to Fusarium Wilt
Cucumber FW, caused by Fusarium oxysporum f. sp. cucumerinum Owen (FOC), is a systemic soil-borne fungal disease and the hyphae of this pathogen penetrate cucumber roots, which causes vascular wilt. The disease causes necrotic lesions on the stem bases, foliar wilting, and eventually whole-plant wilt and even death and it occurs throughout cucumber development ( Vakalounakis and Lamprou, 2018 ). The main factor affecting the incidence of FW is the number of FOC in the soil, which is positively correlated.
Inheritance of Fusarium Wilt Resistance in Cucumber
To understand the genetic inheritance of FW resistance, it is important to develop resistance breeding resources and breed-resistant varieties. The inheritance of FW resistance in cucumber has been studied for a long time, but with different conclusions ( Toshimitsu and Noguchi, 1975 ; Netzer et al., 1977 ; Vakalounakis, 1993 , 1995 ; Zhang et al., 2014 ; Vakalounakis and Lamprou, 2018 ; Dong et al., 2019 ; Jaber et al., 2020 ). Dong et al. (2019) found that the inheritance of FW resistance in cucumber is a quantitative trait controlled by multiple genes using an F2 population derived from a cross between the susceptible line Superina and the resistant line Rijiecheng and several studies agreed with this inheritance of FW resistance in cucumber ( Toshimitsu and Noguchi, 1975 ; Zhang et al., 2014 ). Other researchers have reported that the FW resistance in cucumber is a qualitative trait controlled by a single Foc gene ( Netzer et al., 1977 ; Vakalounakis, 1993 , 1995 ; Vakalounakis and Lamprou, 2018 ; Jaber et al., 2020 ). The Foc gene has been incorporated in the Dutch-type cucumber hybrids and has widely controlled FW in cucumber for 40 years ( Vakalounakis and Fragkiadakis, 2003 ). The different patterns of FW inheritance in cucumber are also influenced by pathogen races, including races 1–3 from America, Israel, and Japan, respectively, and race 4 from China ( Zhang et al., 2014 ). Vakalounakis and Lamprou (2018) found that Foc (syn. Fcu -1), which has been identified as a dominant FW resistance gene in the cultivars SMR-18 and WIS2757, controls FW resistance to races 1, 2, and 3, which indicates that FW resistance is not related to different pathogen races. Additionally, the Foc gene was found to be linked to the Ccu gene, which controls resistance to scab in cucumber inbred line 9110Gt, possible due to the FW and scab resistance in cucumber both being controlled by an NBS-type R gene ( Vakalounakis, 1993 ; Mao et al., 2008 ). In the future, the availability of more natural FW-resistant resources aids in revealing the inheritance pattern of FW resistance in cucumber.
Molecular Markers and Quantitative Trait Loci of Fusarium Wilt Resistance in Cucumber
Compared with PM and DM, there are limited reports on molecular linkage markers and QTL mapping related to the inheritance of FW resistance in cucumber. Wang (2005) identified an Amplified Fragment Length Polymorphisms (AFLP) marker E25M70 and an SSR marker CSWCT06A linked to cucumber Foc2.1 at genetic distances of 8.12 and 5.98 cM, respectively. One major QTL, Foc2.1 , has been screened from the F9 RILs derived from the cross between 9110Gt and 9930 and it is located between SSR03084 and SSR17631 on chromosome 2. The marker SSR17631 has been validated with an 87.88% accuracy among 46 cucumber germplasms ( Zhang et al., 2014 ). Moreover, Zhou et al. (2015) mapped the QTL of Foc4 resistance to FW in the region of SSR17631 and SSR00684 on chromosome 2. Another major QTL, fw2.1 , located on chromosome 2, has also been detected and fine-mapped, with a physical distance of 0.60 Mb (InDel1248093–InDel1817308) and it contains 80 candidate genes ( Dong et al., 2019 ). One AFLP marker of FW resistance in cucumber has been identified at a distance of 6.0 cm from the Foc gene and it was converted into SCE12M50 B and SCE12M50 A codominant markers ( Jaber et al., 2020 ). The SCE12M50 B marker is located 7.0 cm away from SSR03084 and is linked to the Ccu locus that controls resistance to scab in cultivar SMR-18 ( Mao et al., 2008 ; Jaber et al., 2020 ). Owing to the complexity of FW symptoms and the defects of related research techniques, the mechanisms and functions of these loci have not been determined and require further exploration.
Candidate Genes or Proteins Involved in the Fusarium Wilt Resistance of Cucumber
Some FW candidate proteins and genes in cucumber have been identified using proteomic and transcriptomic analyses in different FW-resistant varieties. A comparative proteomic analysis of root proteins isolated from infected highly susceptible 995 and highly resistant F9 revealed that 15 overaccumulated proteins are mainly involved in defense and stress responses, oxidation-reduction, metabolism, transport and other processes, and jasmonic acid and redox signaling components. LRR family- and stress-related proteins may be crucial in the defense responses to FW in cucumber ( Zhang et al., 2016 ). Moreover, defense mechanisms against oxidation and detoxification as well as carbohydrate metabolism may also be necessary for FW resistance in cucumber ( Du et al., 2016 ). Xu et al. (2021) identified 210 and 243 differentially regulated proteins in the FW resistance Rijiecheng and high-susceptibility Superina after Foc infection. Additionally, four genes, TMEM115 ( CsaV3_5G025750 ), which encodes a transmembrane protein, TET8 ( CsaV3_2G007840 ), which functions as a tetraspanin, TPS10 ( CsaV3_2G017980 ), which encodes a terpene synthase, and MGT2 ( CsaV3_7G006660 ), which encodes a glycosyltransferase, are remarkably upregulated in both the cultivars after Foc inoculation, but with higher expression levels in Superina. In total, 14 chitinase defense-related genes have higher expression levels in FW susceptible and resistant lines and CsChi23 may play an important role in activating a rapid immune reaction against FW ( Bartholomew et al., 2019 ). Furthermore, other defense-related genes are activated to regulate the defense responses of cucumber to a Foc inoculation, including several genes related to ABA and ethylene ( Zhou and Wu, 2009 ; Dong et al., 2020 ). miR319a-JRL3 , miR6300-BEE1 , miR6300-DAHP1 , and miR6300-PERK2 also regulate cucumber defenses against FW ( Xu et al., 2021 ). Dong et al. (2019) identified five candidate FW-resistance genes in fw2.1 by combining genetic mapping and a transcriptome analysis, Csa2G007990 , which encodes calmodulin, Csa2G009430 , which encodes a transmembrane protein, Csa2G009440 , which encodes a serine-rich protein, and Csa2G008780 and Csa2G009330 , which are novel genes. This is the only report of mapping FW candidate genes in cucumber, but the functions of the candidate genes have not been verified.
Future Prospects for Enhancing Cucumber Disease Resistance
In summary, the inheritance of PM, DM, and FW resistance in cucumber has been widely investigated and cucumber resistance traits are generally considered as quantitative traits controlled by more one gene. Because of the complicated inheritance of resistance to cucumber diseases, the results are not unified. Simultaneously, several molecular markers and QTLs for PM, DM, and FW resistance in cucumber have been identified ( Figure 1 and Supplementary Table 1 ). Many factors affect cucumber resistance to these three diseases, including pathogen species, plant materials, pathogen invasion site, environment, and genetic linkage, resulting in a variety of effective molecular markers and QTLs for cucumber disease. Large numbers of candidate genes and immune proteins associated with DM, PM, and FW have been identified using mapping, GWAS, RNA sequencing (RNA-seq), and proteomic assay technology, but only a few have been functionally verified ( Figure 1 and Supplementary Table 1 ). For example, only the functions of MLO-like genes that are important in PM resistance have been verified in cucumber. The transient silencing of the two NBS-LRR genes ( CsRSF1 and CsRSF2 ) reduces cucumber resistance to PM. Among the DM-resistant candidate genes, CsSGR , CsLRK10L2 , and CsIVP have been functionally verified through mutation, transient expression, or RNAi. Additionally, the resistance of GNK2-1 transgenic cucumber to FW is enhanced compared with WT ( Liu et al., 2010 ). Because of a lack of cucumber FW-resistant germplasms in China, the susceptibility of most cultivars, and the relatively narrow genetic variation among cucumber FW, the breeding of cucumber FW-resistant cultivars has been restricted to a certain extent and research on the molecular mechanisms of FW has not progressed as far as research on PM and DW.
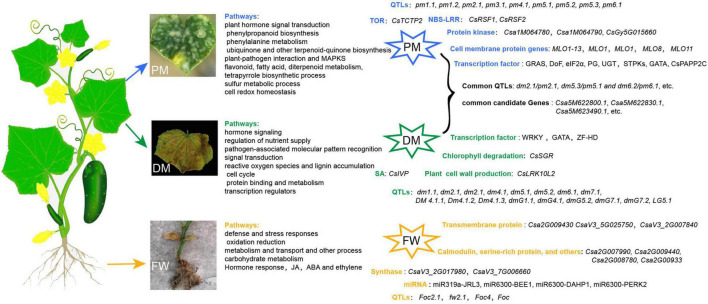
The symptoms, QTL mapping and candidate genes or proteins related to cucumber PM, DM, and FW, respectively.
To better effectively prevent cucumber diseases and explore the genetic and molecular mechanisms of cucumber resistance to PM, DM, and FW, respectively, we propose five aspects of work that need to be performed in the future: (1) collect more disease-resistant cucumber germplasms, especially materials that are resistant to multiple pathogens, including wild germplasm resources, cultivars, and mutants; (2) identify more effective molecular markers and QTLs associated with PM, DM, and FW to be used in selecting germplasms and accelerating resistance breeding; (3) analyze more differently expressed DNA, RNA, miRNAs, lncRNAs, or metabolic related to PM, DM, or FW, respectively, through omics or multiomics and bioinformatics tools would provide considerable experimental information for mechanistic investigations and understand the regulatory network for cucumber diseases, such as transcriptomics, proteomics, metabolomics, epigenomics, and interactomics. Additionally, the data-driven interface through a user-friendly web interface would also be helpful for the mechanism of cucumber diseases, such as plant regulomics ( Ran et al., 2020 ); (4) improve the efficiency and stability of genetic transformation in cucumber. There are now effective methods for gene functional verification that use biotechnology, such as transgenes, RNAi, Transcription Activator-like Effector Nucleases (TALENs), and CRISPR-Cas; (5) develop persistent and safe preventive measures, including chemical, biological, and physical controls. For example, maintaining an optimization of blue light in the growth light before nighttime UV is important for the management of PW in cucumber ( Palma et al., 2021 ). A balance between effective defense and crops yield should be established through these preventive measures. The plant immunity engineering toolbox that integrates genetics, technology, and engineering is required for enhancing disease resistance in crops in the future and the molecular mechanisms of cucumber resistance to PM, DM, and FW need to be further studied.
Author Contributions
YH, MW, and YY drafted the manuscript. CY, SC, and YS modified the manuscript. LM, HW, XZ, and LW designed the project and gave suggestions on the revision of the manuscript. All the authors approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study was supported by “Pioneer” and “Leading Goose” R&D Program of Zhejiang (No. 2022C02051), the Zhejiang Natural Science Foundation of China (Grant No. LY19C150008), Opening Project Fund of Key Laboratory of Biology and Genetic Resources of Rubber Tree, Ministry of Agriculture and Rural Affairs, PR China/State Key Laboratory Breeding Base of Cultivation and Physiology for Tropical Crops/Danzhou Investigation and Experiment Station of Tropical Crops, Ministry of Agriculture and Rural Affairs, PR China (No. RRI-KLOF202102), the Natural Science Foundation of Zhejiang province (Grant Nos. LY21C150002 and LQY19C150001), the National Natural Science Foundation of China (Grant Nos. 31872105, 31972221, 32002048, 31801862, and 32172595), the National College Students Innovation and Entrepreneurship Training Program in 2019 and 2021 (Nos. 202110341043 and 201910341005), and the Student Scientific research training program of Zhejiang Agriculture and Forestry University (Nos. 2021KX0196 and 2021KX019), Ministry of Agriculture, and the National Key Research and Development Program of China (Nos. 2018YFD1000800 and 2019YFD1000300).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.862486/full#supplementary-material
- Barnes W. C., Epps W. M. (1954). An unreported type of resistance to cucumber downy mildew. Plant Dis . Rep. 38:620. [ Google Scholar ]
- Bartholomew E. S., Black K., Feng Z., Wan L., Nan S., Xiao Z., et al. (2019). Comprehensive analysis of the chitinase gene family in cucumber ( Cucumis sativus L.): from gene identification and evolution to expression in response to Fusarium oxysporum. Int. J. Mol. Sci . 20:5309. 10.3390/ijms20215309 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Berg J. A., Appiano M., Bijsterbosch G., Richard G. F. V., Henk J. S., Yuling B. (2017). Functional characterization of cucumber ( Cucumis sativus L.) Clade V MLO genes. BMC Plant Biol . 17:80. 10.1186/s12870-017-1029-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Berg J. A., Hermans F. W., Beenders F., Lou L., Vriezen W. H., Visser R. G., et al. (2020). Analysis of QTL DM4. 1 for downy mildew resistance in cucumber reveals multiple subQTL: a novel RLK as candidate gene for the most important subQTL. Front. Plant Sci . 11:1601. 10.3389/fpls.2020.569876 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Block C. C., Reitsma K. R. (2005). Powdery mildew resistance in the US national plant germplasm system cucumber collection. HortScience 40 416–420. 10.21273/hortsci.40.2.416 [ DOI ] [ Google Scholar ]
- Burkhardt A., Day B. (2016). Transcriptome and small RNAome dynamics during a resistant and susceptible interaction between cucumber and downy mildew. Plant Genome 9:plantgenome2015.08.0069. 10.3835/plantgenome2015.08.0069 [ DOI ] [ PubMed ] [ Google Scholar ]
- Call A. D., Criswell A. D., Wehner T. C., Ando K., Grumet R. (2012a). Resistance of cucumber cultivars to a new strain of cucurbit downy mildew. HortScience 47 171–178. 10.21273/hortsci.47.2.171 [ DOI ] [ Google Scholar ]
- Call A. D., Criswell A. D., Wehner T. C., Klosinska U., Kozik E. U. (2012b). Screening cucumber for resistance to downy mildew caused by Pseudoperonospora cubensis (Berk. and Curt.) Rostov. Crop Sci . 52 577–592. 10.2135/cropsci2011.06.0296 [ DOI ] [ Google Scholar ]
- Chen Q., Yu G., Wang X., Meng X., Lv C. (2021). Genetics and resistance mechanism of the cucumber ( Cucumis sativus L.) against powdery mildew. J. Plant Growth Regul. 40 147–153. 10.1007/s00344-020-10075-7 [ DOI ] [ Google Scholar ]
- Dong J., Wang Y., Xian Q., Chen X., Xu J. (2020). Transcriptome analysis reveals ethylene-mediated defense responses to Fusarium oxysporum f. sp. cucumerinum infection in Cucumis sativus L. BMC Plant Biol . 20:334. 10.1186/s12870-020-02537-7 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dong J., Xu J., Xu X., Xu Q., Chen X. (2019). Inheritance and quantitative trait locus mapping of Fusarium wilt resistance in cucumber. Front. Plant Sci . 10:1425. 10.3389/fpls.2019.01425 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Doruchowski R. W., Lakowska-Ryk E. (1992). “Inheritance of resistance to downy mildew ( Pseudoperonospora cubensis Berk & Curt) in Cucumis sativus ,” in Proceedings of the 5th EUCARPIA Cucurbitaceae Symposiyum , 66–69. 10.1007/BF00035865 [ DOI ] [ Google Scholar ]
- Du N., Shi L., Yuan Y., Li B., Shu S., Sun J., et al. (2016). Proteomic analysis reveals the positive roles of the plant-growth-promoting rhizobacterium NSY50 in the response of cucumber roots to Fusarium oxysporum f. sp. cucumerinum inoculation. Front. Plant Sci . 7:1859. 10.3389/fpls.2016.01859 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fukino N., Yoshioka Y., Sugiyama M., Sakata Y., Matsumoto S. (2013). Identification and validation of powdery mildew ( Podosphaera xanthii )-resistant loci in recombinant inbred lines of cucumber ( Cucumis sativus L.). Mol. Breed . 32 267–277. 10.1007/s11032-013-9867-3 [ DOI ] [ Google Scholar ]
- Gao X., Guo P., Wang Z., Chen C., Ren Z. (2021). Transcriptome profiling reveals response genes for downy mildew resistance in cucumber. Planta 253:112. 10.1007/s00425-021-03603-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- He X., Li Y., Pandey S., Yandell B. S., Pathak M., Weng Y. (2013). QTL mapping of powdery mildew resistance in WI 2757 cucumber ( Cucumis sativus L.). Theor. Appl. Genet . 126 2149–2161. 10.1007/s00122-013-2125-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- Innark P., Panyanitikoon H., Khanobdee C., Samipak S., Jantasuriyarat C. (2020). QTL identification for downy mildew resistance in cucumber using genetic linkage map based on SSR markers. J. Genet. Genomics 99:81. 10.1007/s12041-020-01242-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- Jaber E. H. A., Srour A. Y., Zambounis A. G., Vakalounakis D. J., Doulis A. G. (2020). Identification of SCAR markers linked to the Foc gene governing resistance to Fusarium oxysporum f. sp. cucumerinum in cucumber cv. SMR-18. Eur. J. Plant Pathol . 157 845–855. 10.1007/s10658-020-02045-2 [ DOI ] [ Google Scholar ]
- Jenkins J. M., Jr. (1942). Downy mildew resistance in cucumbers. J. Hered . 33 35–38. 10.1093/oxfordjournals.jhered.a105122 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kelly J. D., Vallejo V. (2006). “QTL analysis of multigenic disease resistance in plant breeding,” in Multigenic and Induced Systemic Resistance in Plants , eds Tuzan S., Bent E. (Boston, MA: Springer; ), 21–48. 10.1007/0-387-23266-4_3 [ DOI ] [ Google Scholar ]
- Kooistra E. (1968). Powdery mildew resistance in cucumber. Euphytica 17 236–244. 10.1007/BF00021216 [ DOI ] [ Google Scholar ]
- Lai W., Zhu C., Hu Z., Liu S., Wu H., Zhou Y. (2021). Identification and transcriptional analysis of Zinc Finger-Homeodomain (ZF-HD) family genes in cucumber. Biochem. Genet . 59 884–901. 10.1007/s10528-021-10036-z [ DOI ] [ PubMed ] [ Google Scholar ]
- Li J. W., Liu J., Zhang H., Xie C. H. (2011). Identification and transcriptional profiling of differentially expressed genes associated with resistance to Pseudoperonospora cubensis in cucumber. Plant Cell Rep . 30 345–357. 10.1007/s00299-010-0959-9 [ DOI ] [ PubMed ] [ Google Scholar ]
- Li L., He H., Zou Z., Li Y. (2018). QTL analysis for downy mildew resistance in cucumber inbred line PI 197088. Plant Dis . 102 1240–1245. 10.1094/PDIS-04-17-0491-RE [ DOI ] [ PubMed ] [ Google Scholar ]
- Liu J., Tian H., Wang Y., Guo A. (2010). Overexpression of a novel antifungal protein gene GNK2-1 results in elevated resistance of transgenic cucumber to Fusarium oxysporum . Chin. Bull. Bot. 45:411. 10.3969/j.issn.1674-3466.2010.04.003 [ DOI ] [ Google Scholar ]
- Liu L., Cai R., Yuan X., He H., Pan J. (2008). QTL molecular marker location of powdery mildew resistance in cucumber ( Cucumis sativus L.). Sci. China Life Sci . 51 1003–1008. 10.1007/s11427-008-0110-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- Liu P., Miao H., Lu H., Cui J., Tian G., Wehner T. C., et al. (2017). Molecular mapping and candidate gene analysis for resistance to powdery mildew in Cucumis sativus stem. Genet. Mol. Res . 16:gmr16039680. 10.4238/gmr16039680 [ DOI ] [ PubMed ] [ Google Scholar ]
- Liu X., Gu X., Lu H., Liu P., Miao H., Bai Y., et al. (2021). Identification of novel loci and candidate genes for resistance to powdery mildew in a resequenced cucumber germplasm. Genes 12:584. 10.3390/genes12040584 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mahmood I., Imadi S. R., Shazadi K., Gul A., Hakeem K. R. (2016). “Effects of pesticides on environment,” in Plant, Soil and Microbes , eds Hakeem K., Akhtar M., Abdullah S. (Cham: Springer; ), 253–269. 10.1007/978-3-319-27455-3_13 [ DOI ] [ Google Scholar ]
- Mao A., Zhang Z., Zhang L. (2008). Analysis on the inheritance of resistance to fusarium wilt race 4 and cucumber scab and their linkage in cucumber WLS2757. Zhongguo Nong Ye Ke Xue 41 3382–3388. 10.3864/j.issn.0578-1752.2008.10.062 [ DOI ] [ Google Scholar ]
- Meng X., Yu Y., Zhao J., Cui N., Song T., Yang Y., et al. (2018). The two translationally controlled tumor protein genes, CsTCTP1 and CsTCTP2 , are negative modulators in the Cucumis sativus defense response to Sphaerotheca fuliginea . Front. Plant Sci . 9:544. 10.3389/fpls.2018.00544 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morishita M., Sugiyama K., Saito T., Sakata Y. (2003). Powdery mildew resistance in cucumber. Jpn. Agric. Res. Q . 37 7–14. 10.6090/jarq.37.7 [ DOI ] [ Google Scholar ]
- Netzer D., Niego S., Galun E. (1977). A dominant gene conferring resistance to Fusarium wilt in cucumber. Phytopathology 67 525–527. 10.1094/Phyto-67-525 [ DOI ] [ Google Scholar ]
- Nie J., He H., Peng J., Yang X., Bie B., Zhao J., et al. (2015a). Identification and fine mapping of pm5. 1 : a recessive gene for powdery mildew resistance in cucumber ( Cucumis sativus L.). Mol. Breed. 35:7. 10.1007/s11032-015-0206-8 [ DOI ] [ Google Scholar ]
- Nie J., Wang Y., He H., Guo C., Zhu W., Pan J., et al. (2015b). Loss-of-function mutations in CsMLO1 confer durable powdery mildew resistance in cucumber ( Cucumis sativus L.). Front. Plant Sci . 6:1155. 10.3389/fpls.2015.01155 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nie J., Wang H., Zhang W., Teng X., Yu C., Cai R., et al. (2021). Characterization of lncRNAs and mRNAs involved in powdery mildew resistance in cucumber. Phytopathology® 111 1613–1624. 10.1094/PHYTO-11-20-0521-R [ DOI ] [ PubMed ] [ Google Scholar ]
- Palma C. F. F., Castro-Alves V., Morales L. O., Rosenqvist E., Ottosen C. O., Strid Å. (2021). Spectral composition of light affects sensitivity to UV-B and photoinhibition in cucumber. Front. Plant Sci . 11:610011. 10.3389/fpls.2020.610011 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ran X., Zhao F., Wang Y., Liu J., Zhuang Y., Ye L., et al. (2020). Plant regulomics: a data-driven interface for retrieving upstream regulators from plant multi-omics data. Plant J . 101 237–248. 10.1111/tpj.14526 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ruiter W. D., Hofstede R., Vries J. D., Heuvel H. (2008). “Combining QTLs for resistance to CYSDV and powdery mildew in a single cucumber line. In Cucurbitaceae 2008,” in Proceedings of the IXth EUCARPIA Meeting on Genetics and Breeding of Cucurbitaceae, Avignon, France, 21-24 May 2008 , (Paris: Institut National de la Recherche Agronomique (INRA)), 181–188. [ Google Scholar ]
- Sakata Y., Kubo N., Morishita M., Kitadani E., Sugiyama M., Hirai M. (2006). QTL analysis of powdery mildew resistance in cucumber ( Cucumis sativus L.). Theor. Appl. Genet . 112 243–250. 10.1007/s00122-005-0121-1 [ DOI ] [ PubMed ] [ Google Scholar ]
- Schouten H. J., Krauskopf J., Visser R. G., Bai Y. (2014). Identification of candidate genes required for susceptibility to powdery or downy mildew in cucumber. Euphytica 200 475–486. 10.1007/s10681-014-1216-z [ DOI ] [ Google Scholar ]
- Shen L., Xu Q., Qi X., Chen X. (2011). Genetic model analysis of cucumber powdery mildew resistance. J. Jiangsu Agric. 27 361–365. [ Google Scholar ]
- Smith P. G. (1948). Powdery mildew resistance in cucumber. Phytopathology 38 1027–1028. [ Google Scholar ]
- Sun C., Song X., Zheng J., Li X., Feng Z., Yan L. (2021). Comparative proteomic profiles of resistant/susceptible cucumber leaves in response to downy mildew infection. Hortic. Plant J . 7 327–340. 10.1016/j.hpj.2021.01.008 [ DOI ] [ Google Scholar ]
- Szczechura W., Staniaszek M., Klosinska U., Kozik E. U. (2015). Molecular analysis of new sources of resistance to Pseudoperonospora cubensis (Berk. et Curt.) Rostovzev in cucumber. Russ. J. Genet . 51 974–979. 10.7868/s0016675815090118 [ DOI ] [ PubMed ] [ Google Scholar ]
- Tan M. (2021). Cucumber Powdery Mildew Resistance Key Genes Identification Based on Genome-Wide Association Analysis. Yangzhou: Yangzhou University. 10.27441/d.cnki.gyzdu.2021.001588 [ DOI ] [ Google Scholar ]
- Toshimitsu Y., Noguchi T. (1975). “Inheritance of resistance to Fusarium wilt in cucumber,” in Proceedings of the Meeting of the Japanese Horticultural Society , 150–151. [ Google Scholar ]
- Vakalounakis D. J. (1993). Inheritance and genetic linkage of fusarium wilt { Fusarium oxysporum f. sp. cucumerinum race 1) and scab { Cladosporium cucumerinum ) resistance genes in cucumber ( Cucumis sativus ). Ann. Appl. Biol . 123 359–365. 10.1111/j.1744-7348.1993.tb04098.x [ DOI ] [ Google Scholar ]
- Vakalounakis D. J. (1995). Inheritance and linkage of resistance in cucumber line SMR-18 to races 1 and 2 of Fusarium oxysporum f. sp. cucumerinum . Plant Pathol . 44 169–172. [ Google Scholar ]
- Vakalounakis D. J., Fragkiadakis G. A. (2003). Plant Pathobreeding with Emphasis in Tomato and Cucurbits , ed. Vakalounakis D. J. (Heraklio: ). [ Google Scholar ]
- Vakalounakis D. J., Lamprou K. (2018). The Foc gene governs resistance to race 3 of Fusarium oxysporum f. sp. cucumerinum in the cucumber cv. SMR-18. Eur. J. Plant Pathol . 152 653–656. 10.1007/s10658-018-1508-6 [ DOI ] [ Google Scholar ]
- Wang X., Chen Q., Huang J., Meng X., Cui N., Yu Y., et al. (2021). Nucleotide-binding leucine-rich repeat genes CsRSF1 and CsRSF2 are positive modulators in the Cucumis sativus defense response to Sphaerotheca fuliginea . Int. J. Mol. Sci . 22:3986. 10.3390/ijms22083986 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang Y. (2005). Molecular Markers of Fusarium Wilt Resistance-Related Genes in Cucumber (Cucumis sativus L.). Yangling: Northwest A & F University. [ Google Scholar ]
- Wang Y., Bo K., Gu X., Pan J., Li Y., Chen J., et al. (2020). Molecularly tagged genes and quantitative trait loci in cucumber with recommendations for QTL nomenclature. Hort. Res. 7:3. 10.1038/s41438-019-0226-3 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang Y., Tan J., Wu Z., VandenLangenberg K., Wehner T. C., Wen C., et al. (2019). STAYGREEN, STAY HEALTHY: a loss-of-susceptibility mutation in the STAYGREEN gene provides durable, broad-spectrum disease resistances for over 50 years of US cucumber production. New Phytol . 221 415–430. 10.1111/nph.15353 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wang Y., VandenLangenberg K., Wehner T. C., Kraan P. A., Suelmann J., Zheng X., et al. (2016). QTL mapping for downy mildew resistance in cucumber inbred line WI7120 (PI 330628). Theor. Appl. Genet . 129 1493–1505. 10.1007/s00122-016-2719-x [ DOI ] [ PubMed ] [ Google Scholar ]
- Wang Y., VandenLangenberg K., Wen C., Wehner T. C., Weng Y. (2018). QTL mapping of downy and powdery mildew resistances in PI 197088 cucumber with genotyping-by-sequencing in RIL population. Theor. Appl. Genet . 131 597–611. 10.1007/s00122-017-3022-1 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wehner T. C., Shetty N. V. (1997). Downy mildew resistance of the cucumber germplasm collection in North Carolina field tests. HortScience 32:450. 10.2135/cropsci1997.0011183X003700040050x [ DOI ] [ Google Scholar ]
- Win K. T., Vegas J., Zhang C., Song K., Lee S. (2017). QTL mapping for downy mildew resistance in cucumber via bulked segregant analysis using next-generation sequencing and conventional methods. Theor. Appl. Genet . 130 199–211. 10.1007/s00122-016-2806-z [ DOI ] [ PubMed ] [ Google Scholar ]
- Wolter M., Hollricher K., Salamini F., Schulze-Lefert P. (1993). The mlo resistance alleles to powdery mildew infection in barley trigger a developmentally controlled defence mimic phenotype. Mol Gen Genet . 239 122–128. 10.1007/BF00281610 [ DOI ] [ PubMed ] [ Google Scholar ]
- Xiao S., Ellwood S., Calis O., Patrick E., Li T., Coleman M., et al. (2001). Broad spectrum mildew resistance in Arabidopsis thaliana mediated by RPW8. Science 291, 118–120. 10.1126/science.291.5501.118 [ DOI ] [ PubMed ] [ Google Scholar ]
- Xu J., Wang K., Xian Q., Zhang N., Dong J., Chen X. (2021). Identification of susceptibility genes for Fusarium oxysporum in cucumber via comparative proteomic analysis. Genes 12:1781. 10.3390/genes12111781 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Xu Q., Shi Y., Yu T., Xu X., Yan Y., Qi X., et al. (2016). Whole-genome resequencing of a cucumber chromosome segment substitution line and its recurrent parent to identify candidate genes governing powdery mildew resistance. PLoS One 11:e0164469. 10.1371/journal.pone.0164469 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Xu Q., Xu X., Shi Y., Qi X., Chen X. (2017). Elucidation of the molecular responses of a cucumber segment substitution line carrying Pm5. 1 and its recurrent parent triggered by powdery mildew by comparative transcriptome profiling. BMC Genom . 18:21. 10.1186/s12864-016-3438-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Xu X., Liu X., Yan Y., Wang W., Gebretsadik K., Qi X., et al. (2019). Comparative proteomic analysis of cucumber powdery mildew resistance between a single-segment substitution line and its recurrent parent. Hort. Res . 6:115. 10.1038/s41438-019-0198-3 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Xu X., Yu T., Xu R., Shi Y., Lin X., Xu Q., et al. (2016). Fine mapping of a dominantly inherited powdery mildew resistance major-effect QTL, Pm1. 1, in cucumber identifies a 41.1 kb region containing two tandemly arrayed cysteine-rich receptor-like protein kinase genes. Theor. Appl. Genet . 129 507–516. 10.1007/s00122-015-2644-4 [ DOI ] [ PubMed ] [ Google Scholar ]
- Xu X., Zhong C., Tan M., Song Y., Qi X., Xu Q., et al. (2020). Identification of MicroRNAs and their targets that respond to powdery mildew infection in cucumber by small RNA and degradome sequencing. Front. Genet . 11:246. 10.3389/fgene.2020.00246 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yan S., Ning K., Wang Z., Liu X., Zhong Y., Ding L., et al. (2020). CsIVP functions in vasculature development and downy mildew resistance in cucumber. PLoS Biol . 18:e3000671. 10.1371/journal.pbio.3000671 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yoshioka Y., Sakata Y., Sugiyama M., Fukino N. (2014). Identification of quantitative trait loci for downy mildew resistance in cucumber ( Cucumis sativus L.). Euphytica 198 265–276. 10.1007/s10681-014-1102-8 [ DOI ] [ Google Scholar ]
- Zhang C., Anarjan M. B., Win K. T., Begum S., Lee S. (2021). QTL-seq analysis of powdery mildew resistance in a Korean cucumber inbred line. Theor. Appl. Genet . 134 435–451. 10.1007/s00122-020-03705-x [ DOI ] [ PubMed ] [ Google Scholar ]
- Zhang D., Meng K., Hao Y., Fan H., Cui N., Wang S., et al. (2016). Comparative proteomic analysis of cucumber roots infected by Fusarium oxysporum f. sp. cucumerium Owen. Physiol. Mol. Plant Pathol . 96 77–84. 10.1016/j.pmpp.2016.09.002 [ DOI ] [ Google Scholar ]
- Zhang K., Wang X., Zhu W., Qin X., Xu J., Cheng C., et al. (2018). Complete resistance to powdery mildew and partial resistance to downy mildew in a Cucumis hystrix introgression line of cucumber were controlled by a co-localized locus. Theor. Appl. Genet . 131 2229–2243. 10.1007/s00122-018-3150-2 [ DOI ] [ PubMed ] [ Google Scholar ]
- Zhang P., Zhu Y., Zhou S. (2021). Comparative analysis of powdery mildew resistant and susceptible cultivated cucumber ( Cucumis sativus L.) varieties to reveal the metabolic responses to Sphaerotheca fuliginea infection. BMC Plant Biol . 21:24. 10.1186/s12870-020-02797-3 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhang S., Liu M., Miao H., Zhang S., Yang Y., Xie B., et al. (2013). Chromosomal mapping and QTL analysis of resistance to downy mildew in Cucumis sativus . Plant Dis . 97 245–251. 10.1094/PDIS-11-11-0941-RE [ DOI ] [ PubMed ] [ Google Scholar ]
- Zhang S., Miao H., Yang Y., Xie B., Wang Y., Gu X. (2014). A major quantitative trait locus conferring resistance to Fusarium wilt was detected in cucumber by using recombinant inbred lines. Mol. Breed . 34 1805–1815. 10.1007/s11032-014-0140-1 [ DOI ] [ Google Scholar ]
- Zhang Z., Mao L., Chen H., Bu F., Li G., Sun J. (2015). Genome-wide mapping of structural variations reveals a copy number variant that determines reproductive morphology in cucumber. Plant Cell 27 1595–1604. 10.1105/tpc.114.135848 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zheng L., Zhang M., Zhuo Z., Wang Y., Gao X., Li Y. (2021). Transcriptome profiling analysis reveals distinct resistance response of cucumber leaves infected with powdery mildew. Plant Biol . 23 327–340. 10.1111/plb.13213 [ DOI ] [ PubMed ] [ Google Scholar ]
- Zhi P., Chang C. (2021). Exploiting epigenetic variations for crop disease resistance improvement. Front. Plant Sci . 12:953. 10.3389/fpls.2021.692328 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhong C. (2020). Identification and Functional Analysis of miRNAs Against Powdery Mildew in Cucumber. Yangzhou: Yangzhou University. 10.27441/d.cnki.gyzdu.2020.001914 [ DOI ] [ Google Scholar ]
- Zhou H., Dong C., Zhang H., Ren Y., Guo S., Yang W. (2015). SSR molecular markers and location of the gene Foc-4 linked to the resistance to Fusarium wilt of cucumber. Mol. Plant Breed . 13 1980–1986. 10.13271/j.mpb.013.001980 [ DOI ] [ Google Scholar ]
- Zhou X., Wu F. (2009). Differentially expressed transcripts from cucumber ( Cucumis sativus L.) root upon inoculation with Fusarium oxysporum f. sp. cucumerinum Owen. Physiol. Mol. Plant Pathol . 74 142–150. 10.1016/j.pmpp.2009.10.005 [ DOI ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (866.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
REVIEW article
The formation of fruit quality in cucumis sativus l..

- 1 Collaborative Innovation Center for Efficient and Green Production of Agriculture in Mountainous Areas, College of Horticulture Science, Zhejiang A&F University, Hangzhou, China
- 2 State Key Laboratory of Subtropical Silviculture, Laboratory of Plant Molecular and Developmental Biology, College of Forestry and Biotechnology, Zhejiang A&F University, Hangzhou, China
- 3 Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing, China
Cucumber is one of the most widely grown vegetables in China and an indispensable fresh fruit in the diet. With the development of society, the demand of people for cucumber quality is higher and higher. Therefore, cultivating high-quality cucumber varieties is one of the main goals of cucumber breeding. With the rapid development of biotechnology such as molecular marker, cucumber quality control network is becoming clear. In this review, we describe the formation mechanism of cucumber fruit quality from three aspects: (1) the commercial quality of cucumber fruit, (2) nutritional quality formation, and (3) flavor quality of cucumber fruit. In addition, the determinants of cucumber fruit quality were summarized from two aspects of genetic regulation and cultivation methods in order to provide ideas for cucumber researchers and cultivators to improve fruit quality.
Introduction
Cucumber ( Cucumis sativus L.) is an annual herbaceous climbing fruit vegetable that belongs to the Cucurbitaceae family and originates from the tropical rainforest in the southern foot of the Himalayas. According to its geographical locations, it can be categorized into four groups such as the Indian group, the Eurasian group, the East Asian group, and the Xishuangbanna group ( Qi et al., 2013 ). In China, cucumber can be subdivided into two geographic groups, such as the northern China group with dark green, dense spines and warts on fruits, and the southern China group with light green, sparse spines and warts on fruits ( Jiang et al., 2015 ). Cucumber is an economically important crop in the world. Its fruits are fragrant and delicious with nutrient enrichment that can be consumed in fresh or processed into pickles. In addition, its fruits are also used in beauty products. However, with the constantly change in the cultivation environment and techniques, and the ever-rising living standards of people, the cucumber fruit quality is becoming much more concerned by the consumers.
With the continuous work on the formation of cucumber fruit quality by various research groups in recent years, some of the influencing factors and regulation mechanism of the formation of cucumber fruit quality can be understood now in a more in-depth and thorough manner. This review attempted to summarize the recent advances in the studies on the formation of cucumber fruit quality through physiological and molecular biological approaches in order to provide insights for further research studies on the formation of fruit quality of cucumber and other melons.
Fruit quality in cucumber can be defined by three aspects, namely, commercial quality, nutrient quality, and flavor quality ( Lv et al., 1992 ). The commercial quality in cucumber contains the fruit size and shape, fruit spine characteristics (color, size, and density), fruit skin characteristics (color, ridges, and speckles), and flesh characteristics (color) ( Wang et al., 2007 ; Lu et al., 2015 ; Zhang et al., 2016 ; Zhang C. et al., 2019 ). Fruit size and shape are the two most obvious appearances of quality traits in cucumber, which had become one of the criterions for breeders to select high fruit quality cultivars. The external fruit qualities, such as the fruit skin and spine color, the presence or absence of the wax on the cucumber surface, and the number, shape, size, distribution, and density of fruit spines (trichomes on cucumber fruit are called spines), are also important fruit quality traits for cucumber production ( Choi et al., 2013 ; Li et al., 2013 ; Chen et al., 2014 ; Liu X. et al., 2016 ). In the late 1980s, the bloomless cucumber fruits are popular in Japan due to their distinct shiny appearance ( Ohara et al., 2006 ). Besides, the character of spines could also have a big influence on consumer preference. For example, people in Europe prefer cucumber without spines, whereas people in Asia like cucumber with spines ( Chen et al., 2014 ). Furthermore, epicuticular wax, which acts as the outermost barrier between the plants and their environment, is also one of the significant commercial quality traits in cucumber that can play significant roles in protecting the tissues against various biotic and abiotic stress ( Samuels et al., 2008 ). Moreover, fruit flesh color that varies from white to green or yellow to orange is also an important fruit quality trait that influences the preference of consumers ( Cuevas et al., 2010 ).
On the other hand, nutrition elements, such as soluble solids, vitamins, and minerals, constitute the nutrient quality in cucumber. In addition, flavor quality in cucumber, for one reason, contains all of the volatile compounds from cucumber, and for another reason, it is related to the non-volatile flavor substances ( Kemp et al., 1974 ; Malundo et al., 1995 ). Ever since the 1960s, there have had research studies on the flavor of cucumber ( Forss et al., 1962 ). Nowadays, there are more and more research studies on fruit flavor, which would help us to improve the fruit quality of cucumber.
Genetic Regulation of Fruit Quality in Cucumber
Genetic regulation of commercial quality in cucumber.
Cucumber commercial quality is determined mainly by its fruit-related traits. The genes related to the commercial quality traits of cucumber fruits are shown in Figure 1 and Table 1 . The ability of cucumber to produce large fruits is believed to have been evolved through long time domestication of wild cucumber, and Cucumis sativus L. var. hardwickii is regarded as the ancestor of cultivated cucumber. Its fruit is small and round and about 3–5 cm long. In addition, it has large amounts of spines on its epidermis. It is so bitter that it is difficult to eat ( Sebastian et al., 2010 ; Yang et al., 2012 ). Nowadays, the fruit length of widely cultivated cucumber is about 10–30 cm, and the fruit shape has also become oblong, which has shown great variation compared with its ancestors. Besides, based on the whole genome sequencing and the construction of the high-resolution genetic map, we have gained a clearer understanding of the genetic mechanism of formation of fruit quality during their domestication processes ( Huang et al., 2009 ; Qi et al., 2013 ).
Figure 1. Genes related to cucumber fruit quality. Genes regulating fruit length, fruit shape, weight, diameter, thickness, carpel number, trichome, wax, spine color, and synthesis of secondary metabolite in cucumber.
Table 1. Genes related to the commercial quality of cucumber fruits.
The Size and Shape of Cucumber Fruits
Fruit size and shape, especially fruit length, are important fruit traits during cucumber domestication and diversifying selection. Wenzel et al. (1995) first identified the overall information of gene regulation on the cucumber fruit length, diameter, and other genetic traits. Yuan et al. (2008) constructed a 257-point genetic linkage map and found 78 quantitative trait locus (QTLs) related to fruit weight, fruit length, fruit diameter, and other four fruit-related traits, which can be used to conduct marker-assisted selection in cucumber breeding. Bo et al., also detected several QTLs of cucumber fruit-related traits, including five fruit length-related QTLs ( fl1.1, fl3.1, fl4.1, fl6.1 , and fl7.1 ), three fruit diameter-related QTLs ( fd1.1, fd4.1 , and fd6.1 ), and three fruit weight-related QTLs ( fw2.1, fw4.1 , and fw6.1 ). At the same time, they also found that the chromosomal rearrangements of cucumber ancestors between wild and cultivated cucumbers were mainly concentrated on chromosomes 4, 5, and 7 ( Bo et al., 2015 ). Besides, Cheng et al. (2010) and Wang et al. (2014) also found 5 fruit length (Fl)-related QTLs that were distributed in chromosome 1, 4, and 6 and 4 stalk length (Fsl)-related QTLs that were located on chromosome 3, 4, and 6, respectively.
The enzyme 1-aminocyclopropane-1-carboxylic acid synthase (ACS) in the process of plant hormone ethylene synthesis plays an important role in cucumber sex determination. The genes involved in this process include CsACS1G ( Kamachi et al., 1997 ), CsACS2 ( Knopf and Trebitsh, 2006 ; Li et al., 2009 ), and CsACS11 ( Boualem et al., 2015 ). Among them, the CsACS2 mutation makes cucumber produce a bisexual flower phenotype. CsACS2 gene was eliminated in addition to the above functions, and it is also related to the length of the cucumber fruit. Boualem et al. (2009) proved that an allele of CsACS2 co-segregated with the M (andromonoecious) locus, resulting in a round fruit phenotype after the gene mutation. Subsequently, Tan et al. (2015) further studied through fine mapping and found that another allele mutation of CsACS2 caused the cucumber to appear a long fruit phenotype. Later, Pan et al. (2017) found that round fruit shape in WI7239 cucumber was controlled by two interacting quantitative trait loci, such as FS1.2 and FS2.1 , and demonstrated that FS2.1 may encode a homolog of tomato fruit shape gene SUN . They also identified a FS5.2 QTL in Xishuangbanna cucumber that has great significance on round fruit determination ( Pan et al., 2017 ). Besides, Zhao et al. (2019) further demonstrated that among the QTLs that have putative functions in regulating cucumber fruit length, a gain-of-function allele CsFUL1 A can prevent the accumulation of auxin by inhibiting the expression of its transporters PIN-FORMED1 ( PIN1 ) and PIN7 . This further analyzes the molecular mechanism of auxin regulating cucumber fruit development.
The Carpel Number and Flesh Thickness of Cucumber Fruits
In addition, factors that affect important fruit characteristics such as cucumber fruit shape, size, and internal quality also include the carpel number and the thickness of fruit flesh. The expression of CsCLV3 in cucumber was negatively correlated with the number of carpels. CsCLV3 and CsWUS act as negative regulators and positive regulators of changes in carpel number, respectively, and CsWUS can be directly combined with the promoter of CsCLV3 to activate its expression. CsFUL1 A overexpression plants showed increased petals and carpels. Through the interaction of CsARF14 and CsWUS , auxin can also participate in the change of cucumber carpel number ( Che et al., 2020 ). The QTL mapping of Xishuangbanna cucumber revealed that ln1.1 and ln1.3 located on chromosome 1 are the main QTLs controlling multi-ventricular traits. At the same time, two genes, namely, Csa1M207820.1 and Csa1M231530.1 , involved in plant hormone signal transduction and two genes, namely, Csa1M071910.1 and Csa1M072490.1 , related to WD40 repeat protein are predicted as candidate genes ( Zhang et al., 2015 ). By combining the separation and segmentation analysis with the sequencing of amplified fragments of specific lengths, the genes that regulate the pulp thickness of cucumber fruit were finely mapped, and the quantitative trait locus that controls the pulp thickness was located in the interval of about 0.19 Mb on chromosome 2. This 0.19-Mb region predicts and recognizes 20 genes, among which there is a 4 bp deletion mutation in the promoter region of the candidate gene Csa2M058670.1 (a protein-lysine methyltransferase, PKMT), which may lead to the loss of its activity in the thin fruit line. This suggests that Csa2M058670.1 may be a candidate gene for controlling cucumber pulp thickness ( Xu et al., 2015 ). Csa2M058670.1 belongs to the same subfamily as At2g18850, and the latter is related to the cell division and growth process of Arabidopsis ( Horvath et al., 2003 ). At present, there are too many factors affecting the thickness of pulp and the number of ventricles in cucumber, and these two traits are easily affected by the environment, so a consistent conclusion has not yet been reached.
The Color of the Flesh and Skin of Cucumber Fruit
The colors of the fruit flesh and skin are also significant commercial quality traits in cucumber that have obvious influences on the choice of the consumers. Fruit color was determined by the regulation of pigment in the plants, and chlorophylls were declared to be the main factor to determine the fruit skin color ( Egea et al., 2010 ). In cucumber, five genes controlled fruit skin color, such as dark green ( DG ), green ( dg ), yellow green ( yg ), light green ( lgp ), and white ( w ) peeling genes ( Pierce and Wehner, 1990 ; Dong et al., 2012 ). Now, the fruit light green peel gene lgp and the white peel gene w have been identified ( Wehner et al., 2001 ; Dong et al., 2012 ). The mutation of CsaARC5 ( ACCUMULATION AND REPLICATION OF CHLOROPLASTS 5 ), the ortholog gene of Arabidopsis ARC5 , led to a light green fruit peel phenotype in cucumber ( Zhou et al., 2015 ). Another Ycf54-like protein-encoding gene Csa6G133820 can also determine the formation of light green fruits ( Lun et al., 2016 ). Besides, a single-nucleotide insertion on APRR2 disturbed the chlorophyll accumulation and chloroplast development so that leading to a white fruit color in cucumber ( Liu H. et al., 2016 ). Identification of cucumber yellow green peel-related genes and research studies on their regulation mechanism have also progressed greatly in recent years. Research studies on a cucumber yellow green peel mutant ( ygp ) identified a Csa2G352940 gene, encoding a MYB36 transcription factor, functioned to regulate a yellow green peel determination in cucumber. This study also revealed that CsMYB36 may interact with the peel color development-related genes, such as Casparian strip ( CsCASP1 ) and pigment synthesis protein ( CsMYC2 ), to regulate yellow green peel determination in cucumber ( Hao et al., 2018 ).
Besides fruit skin color, chlorophylls can also influence the formation of flesh color. Bo et al. (2019) revealed that two QTLs, qgf3.1 and qgf5.1 , can function together in regulating the formation of green fruit flesh in cucumber. When cucumber fruits developed to the mature stage, their flesh color changed from green to yellow or green to orange. High β-carotene content was demonstrated to be the main reason for cucumber to form fruits with orange flesh color, and further genetic analysis demonstrated that the quantity of β-carotene was controlled by the orange endocarp ( ore ) gene ( Bo et al., 2012 ) and CsaBCH1 ( Qi et al., 2013 ). Cucumber fruits with white flesh and yellow flesh were proven to be controlled by two genes, wf and a single recessive gene named yf , respectively ( Kooistra, 1971 ; Whalen, 2005 ; Lu et al., 2015 ). The study on the color of cucumber flesh and skin will help to promote the breeding process of cucumber with a different color.
External Quality of Cucumber Fruits
Several genes and transcription factors are involved in the regulation of the external quality of cucumber fruits. Zhang C. et al. (2019) found that spine color was regulated by the HEUKCHEEM gene, mutations in HEUKCHEEM leading to a white spine in cucumber. Wang et al. (2015) revealed that CsCER1 significantly influenced the biosynthesis of alkane so that further influenced the wax synthesis of cucumber, and CsCER1 overexpression lines showed more wax crystallization phenotypes, whereas its RNA interference (RNAi) lines exhibited fewer wax crystallizations. Liu et al. (2018) analyzed 91 NAC gene homologs in cucumber and identified 13 NAC genes that can control fruit spine development. Yang et al. (2018) found that CsMYB6 and CsTRY can negatively regulate the trichome initiation in cucumber and revealed that CsMYB6 functions the upstream of CsTRY and that they can also form CsMYB6 - CsTRY complex to function together in this progress. These results provide a reference for the cultivation of non-spiny and prickly cucumbers.
Genetic Regulation of Flavor Quality in Cucumber
Research studies on flavor quality have become popular in recent years. Foods with good or special tastes will increase the pleasure in people and influence the digestion in people and absorption of nutrients ( Beaulieu and Lea, 2006 ). The genes contributed to the flavor quality traits of cucumber fruits are shown again in Figure 1 and Table 2 . It is confirmed that the degradation of linoleic acid and linolenic acid occurred rapidly after the disruption of cucumber tissues and gave rise to the flavor of fresh cucumber ( Ligor and Buszewski, 2008 ).
Table 2. Genes related to the nutrient quality and flavor quality of cucumber fruits.
The Scent of Cucumber Fruits
Aldehydes and alcohols are thought to mainly contribute to the fresh cucumber scent ( Kemp et al., 1974 ; Hatanaka et al., 1975 ; Palma-Harris et al., 2001 ; Ligor and Buszewski, 2008 ; Hao et al., 2013 ). Forss et al. (1962) first isolated 2,6-nonadienal from cucumber. Subsequently, Schieberle et al. (1990) found that ( E,Z )-2,6-nonadienal mainly caused the flavor of cucumbers with fresh cucumber odor and identified ( E )-2-nonenal as the second important odor compound in cucumber. Since six-carbon (C6) and nine-carbon (C9) aldehydes play an important role in flavor during fruit development, changes in volatile substances in developing cucumber fruits were investigated in two Cucumis sativus L. lines (No. 26 and No. 14). C6 aldehyde content was higher during the early stages, whereas the C9 aldehyde content was higher during the latter stages in both lines ( Chen et al., 2015 ). Lipoxygenase (LOX) and hydroperoxide lyase (HPL) are the two key pre-regulatory factors in the synthesis of cucumber aldehydes. Thereinto, the expression patterns of 9-CsHPL are similar to the trend of C9, and the expression of CsLOX2 is also significantly correlated with changes in C9 aldehyde aroma content ( Liu, 2018 ; Wei, 2018 ). Subsequently, Wei et al. (2016) analyzed 85 volatile chemicals in 23 different tissues of cucumber and further found that TPS15 (encoded by Csa3G040850 ) mediated the biosynthesis of volatile terpenoid in the fruit tissues, which will promote future research studies on the physiological function of volatiles and improve the cucumber flavor breeding. Except for fresh cucumber odor, Yundaeng et al. (2015) also found a special pandan ( Pandanus amaryllifolius ), like fragrance in PK2011T202 (PKT) cucumber cultivar in Thailand. They further found that 2-acetyl-1-pyrroline (2AP) generated this fragrance, and a betaine aldehyde dehydrogenase 2 ( BADH2 ) mutant caused the biosynthesis of 2AP so that produced the pandan-like fragrance in PKT ( Yundaeng et al., 2015 ). Two cucumber accessions, PKT and 301,176 (301), an inbred line from Clover Seed Company, Hong Kong, possessing no fragrance, were used to determine the mode of inheritance of these recessive fragrance traits and each controlled by a specific gene ( Pramnoi et al., 2013 ).
The Bitterness of Cucumber Fruits
The bitterness of cucumber fruits is also of great popularity in the research study of flavor quality traits. Research studies have demonstrated that the Cucurbitacins (Ct) caused the bitterness in cucumber fruits ( Rice et al., 1981 ; Balkema-Boomstra et al., 2003 ). Since the production of bitterness will lead to fatal losses in the sale of cucumbers, cultivating varieties of cucumbers without bitterness is of great significance for improving the efficiency of cucumber sales. Genetic mechanism of cucumber bitterness showed great complexity. Wehner et al. (2001) found that two dominant genes, Bt (bitter fruit) and Bt-2 (bitter fruit-2), controlled the bitterness of cucumber. In addition, bi (bitter-free cotyledons) gene and the fruit bitterness need both Bi and Bt genes ( Andeweg and Bruyn, 1959 ; Gu et al., 2004 ; Shang et al., 2014 ). Further research studies by Zhang et al. (2013) proved that the candidate gene of bi-1 is considered to be the terpene synthase gene named Csa008595 . Besides, Shang et al. (2014) further demonstrated that Bt can regulate the biosynthesis of cucurbitacin C (CuC) in the cucumber fruits and identified 11 cucumber bitterness biosynthesis, regulation, and domestication-related genes. In addition, the biosynthetic pathway and main regulators of cucurbitacin in cucumber have also been identified. By applying a comparative genomic study, Zhou et al., reported that the independent mutations of the homologous transcription factor genes in the three cucurbits may lead to a significant reduction in fruit bitterness, which may be the reason for the convergence and domestication of bitter wild cucurbits. A syntenic gene cluster that regulates both the tissue-specific biosynthesis of cucurbitacin and the loss of bitter phenotypes associated with the fusion and domestication of wild cucurbits has also been reported in this study. They also found that Csa6G088160 , Csa6G088170 , and Csa6G088180 in cucumber can participate in the biosynthesis of cucurbitacin C ( Zhou et al., 2016 ).
In addition to cucurbitacin, catechins are also one of the key factors that cause cucumber to produce astringency. Xu et al. (2019a) found that tryptophan–aspartate acid (WD40)-repeat protein, avian myeloblastosis viral oncogene homolog (MYB), and basic helix–loop–helix (bHLH) also play an important role in the biosynthesis of catechins. They further found that some genes related to phenylalanine ammonia lyase (PAL)- CsPAL3 and CsPAL5 , to cinnamate 4-hydroxylase (C4H)- CsC4H1 , to 4-coumarate-CoA ligase (4CL)- Cs4CL2 , to chalcone synthase (CHS)- CsCHS2 , to chalcone isomerase (CHI)- CsCHI2 , to flavanone 3-hydroxylase (F3H)- CsF3H3 , to dihydroflavonol 4-reductase (DFR)- CsDFR2 , and to anthocyanidin synthase (ANS)- CsANS are important regulators of catechin biosynthesis in cucumber fruits ( Xu et al., 2019b ). But there is currently no clear evidence on how catechins are regulated.
Genetic Regulation of Nutrient Quality in Cucumber
Qiao et al. (2005) reported that crude protein, Vitamin C (VC), soluble reductive sugar, soluble solids, and moisture were five important nutrient components in cucumber. Meanwhile, soluble solids can directly affect nutritional quality, with the greatest impact, while VC, soluble reducing sugars, and crude protein indirectly affect nutritional quality through soluble solids ( Qiao et al., 2005 ). The genes related to the nutrient quality traits of cucumber fruits are shown in Figure 1 and Table 2 . The heritability of the soluble sugar content of cucumber is higher, and the selection of early generation has a better effect on it ( Xu et al., 2001 ). Zhang et al. (2020) found that the relationship between soluble sugar and water is positively correlated, while ascorbic acid is negatively correlated with water and soluble sugar. Besides, the contents of β-carotene, a provitamin A, were also important nutrient qualities in cucumber, its contents in cucumber were regulated by an ore gene, and seven simple sequence repeat (SSR) markers were identified linking to the locus controlling β-carotene quantity ( Bo et al., 2012 ). On this basis, Wang et al. (2012) used RACE technology to successfully clone the complementary DNA (cDNA) sequence of the ζ-carotene dehydrogenase (ZDS) gene ( CsZds ) and speculated that the gene may be related to the accumulation of β-carotene in cucumber fruits. However, there existed fewer genetic research studies on nutrient qualities in cucumber even until now, and further studies are needed to identify factors related to nutrient qualities formation in cucumber.
Physiological Regulation of Fruit Quality in Cucumber
Physiological regulation of commercial quality in cucumber.
Cucumber plants usually have poorly developed root systems, rending them vulnerable to infection by various pests and diseases; thus, the cucumber growers have to apply various techniques to improve the commercial quality of cucumber fruits ( Figure 2 ). Grafting was wildly used to improve the stress resistance in cucumber, which could also generate positive influences on improvement in their fruit commercial quality ( King et al., 2008 ; Colla et al., 2013 ). Grafting can also influence the transcript expression levels in cucumber. For example, Zhang J. et al. (2019) found that compared to those self-rooted cucumber, the grafted cucumber showed a higher expression level of the Apetala2/ethylene-responsive factor (AP2/ERF)-type transcription factor CsWIN1 , and CsWIN1 further promoted the expression of several key wax biosynthesis and transporter genes so that reflected a glossier appearance. Besides, the usage of plant growth regulators is also an effective measure to alter the fruit qualities in cucumber. Qian et al. (2018) found that N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU) treatment produced a positive effect on cucumber appearance for the increased flesh firmness. However, gibberellin A4 + A7 (GA4 + 7) treatment reduced its commercial quality ( Qian et al., 2018 ). Hypoxia treatment can inhibit the fresh weight of cucumber fruit. Under hypoxia stress, increasing the amount of exogenous calcium can increase the fresh weight of the fruit ( He et al., 2018 ).
Figure 2. Illustration of the cultivation techniques in cucumber fruit quality breeding. Grafting technique (A) , application of exogenous fertilizers and growth regulators (B) , and bagging technology (C) are widely used in cucumber fruit quality breeding.
Physiological Regulation of Flavor Quality in Cucumber
Grafting not only affects the commercial quality of cucumbers but also affects the total amount of aroma substances and characteristic esters in the fruit ( Pérez et al., 2002 ). Dong et al. (2013) found that the increased content of volatile substances such as acetaldehyde in the fruits of cucumbers grafted to pumpkins led to a decrease in the flavor and taste of the fruits. Zhao et al., found that compared with those self-grafted cucumbers, the total content of alcohols, aldehydes, olefins, and acids increased of those cucumber plants that were grafted onto “Weisheng NO.1” ( Cucurbita moschata hybrids) rootstock, so the flavor quality was significantly improved, while the cucumber plants grafted onto Cucurbita ficifolia rootstock showed the opposite trend. Grafting of different rootstocks will also significantly affect the expression of genes related to glycolysis, fructose metabolism, and α-linolenic acid metabolism in the scion, thereby changing the flavor quality of grafted cucumbers ( Zhao et al., 2018 ). Different grafting methods will also affect the flavor of cucumber fruits ( Lee, 1994 ). Peng et al. (2010) comprehensively evaluated the sweetness, bitterness, astringency, moisture, and other aspects of cucumber fruit and found that the taste quality of cucumber after double-cut root grafting was the best, while the taste quality of cucumber after double-root grafting was poor. Wang et al. (2019) found that compared with conventional grafting and self-rooting seedlings, the method of interstock grafting can significantly increase the types and contents of volatile substances in cucumber fruits. Besides, bagging was also an effective measure to improve the fruit quality. Shan et al. (2020) demonstrated that cucumber fruits were found to have enhanced fruit flavor quality after bagging, for the elevated relative proportion of C6 aldehyde, ( E,Z )-2,6-nonadienal/( E )-2-nonenal ratio, and linoleic/α-linolenic acid ratio.
Physiological Regulation of Nutrient Quality in Cucumber
Grafting also has an influence on nutrient qualities in cucumber fruits. For example, in the study of Zhao et al. (2018) when cucumber was grafted onto “Weisheng NO.1,” the soluble solid content in the fruit was significantly higher than that of the self-grafted group. Besides, the contents of the sugar, organic acids, amino acids, and alcohols were greatly increased when grafting “xintaimici” cucumber on the “GNo.45” pumpkin ( Cucurbita moschata ) ( Miao et al., 2019 ). Dong et al. (2018) also found that elevated carbon dioxide (CO 2 ) concentration and high nitrogen (N) application can also increase the content of nutrients such as fructose and glucose in cucumber by promoting the carbon translocation from source leaves to fruits. Qian et al. (2018) also found that gibberellin GA4 + 7 treatment improved the nutrient quality in cucumber fruits but decreased its commercial quality. On the contrary, CPPU treatment had a negative effect on the nutritional quality of cucumber ( Qian et al., 2018 ). Moreover, He et al. (2018) found that exogenous calcium treatment on cucumbers under hypoxic stress results in an increased soluble sugar content in cucumber fruits so that enhances its nutrient quality. Ali et al. (2019) found that cucumber growth can be improved by adding arbuscular mycorrhizal strain (AM: Glomus versiforme L.) inoculant with organic substrates (GS), and GS + AMF (arbuscular mycorrhizal fungi) treatments increase the total soluble solids of cucumber fruits and soluble sugar content, thereby improving the nutrient qualities of cucumber fruits. With the rapid development of facility cultivation technology, cucumber has now become one of the main crops cultivated in protected areas. Di-n-butyl phthalate (DBP) is widely used as a plasticizer in plastic films because it can increase the toughness and elasticity of products. However, one of its main components, dibromophenol, can cause agricultural pollution that leads to food safety problems, and it has been widely concerned ( Heudorf et al., 2007 ). DBP stress has also a detrimental effect on the contents of organic acids, vitamin C, soluble protein, and soluble sugar in cucumber fruits and resulted in the residue of dibromophenol under protected cultivation conditions. Although the residual dibromophenol in cucumber fruits is below the risk threshold, the potential health risks cannot be ignored ( Wang et al., 2016 ). Therefore, in the process of studying cucumbers, attention should also be paid to the safety of the protected cultivation of cucumbers.
Perspective
Compared to the traditional model plants such as Arabidopsis and rice, cucumber has some advantages as a new model plant for studies on gene function during fruit development. However, the fruit development of cucumber is a complex biological process, which is affected both by internal genes and external environmental factors. We still need firstly a comprehensive understanding of cucumber quality traits and problems of cultivation skills such as the production of grafted rootstocks, genetic deterioration in breeding, and soil renovation under protected cultivation. Then, we should analyze the compositions of good-quality fruits, such as the types and content of flavor substances, the nutrients, and pigments in cucumber fruits by using high-throughput, high-resolution, and high-sensitivity modern instrumental analysis methods. Combining with the taste evaluation system of consumers, the breeding goal of cucumber flavor quality can be established and then the genetic mechanism of formation of cucumber fruit quality can be explored. Molecular breeding techniques and methods are used to create new varieties with the best commercial quality (the top flower with thorns and straight strips), nutritional quality (rich in nutrients), and flavor quality (clear fragrance and no bitterness) to meet consumer demand eventually.
In recent years, biotechnologies such as fine mapping, cloning, and transgenesis of genes for important cucumber fruit traits have developed rapidly, and molecular markers combined with traditional breeding methods have been widely used. We will accelerate the completion of the improvement of fruit quality and achieve the breeding goal of high quality, high yield, and stable yield in cucumber fruit production.
Author Contributions
CY, JZ, JY, and HW conceived the review, conducted the literature review, and wrote the manuscript. CW and TL collected the literature. SF provided critical comments on the manuscript. All authors read and approved the manuscript.
This study was supported by the National Key Research and Development Program of China (2018YFD1000800 and 2019YFD1000300), Natural Science Foundation of Zhejiang Province (LY21C150002), and National Natural Science Foundation of China (Grant Nos. 31872105, 31972221, 32002048, and 31801862).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.

Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ali, A., Ghani, M. I., Ding, H., Fan, Y., Cheng, Z., and Iqbal, M. (2019). Co-amended synergistic Interactions between arbuscular mycorrhizal fungi and the organic substrate-induced cucumber yield and fruit quality associated with the regulation of the AM-Fungal community structure under anthropogenic cultivated soil. Int. J. Mol. Sci. 20:1539. doi: 10.3390/ijms20071539
PubMed Abstract | CrossRef Full Text | Google Scholar
Andeweg, J. M., and Bruyn, J. (1959). Breeding of non-bitter cucumbers. Euphytica 8, 13–20. doi: 10.1007/BF00022084
CrossRef Full Text | Google Scholar
Balkema-Boomstra, A. G., Zijlstra, S., Verstappen, F. W. A., Inggamer, H., Mercke, P. E., Jongsma, M. A., et al. (2003). Role of cucurbitacin C in resistance to spider mite ( Tetranychus urticae ) in Cucumber ( Cucumis sativus L.). J. Chem. Ecol. 29, 225–235. doi: 10.1023/A:1021945101308
Beaulieu, J. C., and Lea, J. M. (2006). Quality changes in cantaloupe during growth, maturation, and in stored fresh-cut cubes prepared from fruit harvested at various maturities. J. Am. Soc. Hortic. Sci. 132, 127–139. doi: 10.1007/s10658-007-9180-2
Bo, K., Ma, Z., Chen, J., and Weng, Y. (2015). Molecular mapping reveals structural rearrangements and quantitative trait loci underlying traits with local adaptation in semi-wild Xishuangbanna cucumber ( Cucumis sativus L. var. xishuangbannanesis Qi et Yuan). Theor. Appl. Genet. 128, 25–39. doi: 10.1007/s00122-014-2410-z
Bo, K., Song, H., Shen, J., Qian, C., Staub, J. E., Simon, P. W., et al. (2012). Inheritance and mapping of the ore gene controlling the quantity of β -carotene in cucumber ( Cucumis sativus L.) endocarp. Mol. Breed. 30, 335–344. doi: 10.1007/s11032-011-9624-4
Bo, K., Wei, S., Wang, W., Miao, H., Dong, S., Zhang, S., et al. (2019). QTL mapping and genome-wide association study reveal two novel loci associated with green flesh color in cucumber. BMC Plant Biol. 19:243. doi: 10.1186/s12870-019-1835-6
Boualem, A., Troadec, C., Camps, C., Lemhemdi, A., Morin, H., Sari, M.-A., et al. (2015). A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 350, 688–691. doi: 10.1126/science.aac8370
Boualem, A., Troadec, C., Kovalski, I., Sari, M.-A., Perl-Treves, R., and Bendahmane, A. (2009). A conserved ethylene biosynthesis enzyme leads to andromonoecy in two cucumis species. PLoS One 4:e6144. doi: 10.1371/journal.pone.0006144
Che, G., Gu, R., Zhao, J., Liu, X., Song, X., Zi, H., et al. (2020). Gene regulatory network controlling carpel number variation in cucumber. Development 147:dev184788. doi: 10.1242/dev.184788
Chen, C., Liu, M., Jiang, L., Liu, X., Zhao, J., Yan, S., et al. (2014). Transcriptome profiling reveals roles of meristem regulators and polarity genes during fruit trichome development in cucumber ( Cucumis sativus L.). J. Exp. Botany 65, 4943–4958. doi: 10.1093/jxb/eru258
Chen, S., Zhang, R., Hao, L., Chen, W., and Cheng, S. (2015). Profiling of volatile compounds and associated gene expression and enzyme activity during fruit development in two cucumber cultivars. PLoS One 10:e0119444. doi: 10.1371/journal.pone.0119444
Cheng, Z., Gu, X., Zhang, S., Miao, H., Zhang, R., Liu, M., et al. (2010). QTL analysis for fruit length of cucumber. China Vegetables 12, 20–25.
Google Scholar
Choi, E., Kim, B., Hwang, U., Han, W., and Dong, H. (2013). Characterization of blooming on cucumber fruits. Korean J. Hortic. Sci. Technol. 31, 159–164. doi: 10.7235/hort.2013.12160
Colla, G., Rouphael, Y., Jawad, R., Kumar, P., Rea, E., and Cardarelli, M. (2013). The effectiveness of grafting to improve NaCl and CaCl2 tolerance in cucumber. Sci. Hortic. 164, 380–391. doi: 10.1016/j.scienta.2013.09.023
Cuevas, H. E., Song, H., Staub, J. E., and Simon, P. W. (2010). Inheritance of beta-carotene-associated flesh color in cucumber ( Cucumis sativus L.) fruit. Euphytica 171, 301–311. doi: 10.1007/s10681-009-0017-2
Dong, J., Xu, Q., Gruda, N., Chu, W., Li, X., and Duan, Z. (2018). Elevated and super-elevated CO2 differ in their interactive effects with nitrogen availability on fruit yield and quality of cucumber. J. Sci. Food Agric. 98, 4509–4516. doi: 10.1002/jsfa.8976
Dong, S., Cao, L., Zhang, S., Wang, G., Miao, H., Xie, B., et al. (2013). Effect of grafting on fruit appearance quality and flavor substances in cucumber. China Vegetables 22, 44–51.
Dong, S., Miao, H., Zhang, S., Liu, M., Wang, Y., and Gu, X. (2012). Genetic analysis and gene mapping of white fruit skin in cucumber ( Cucumis sativus L.). Acta Botanica Boreali-Occidentalia Sin. 32, 2177–2181. doi: 10.1007/s11783-011-0280-z
Egea, I., Barsan, C., Bian, W., Purgatto, E., Latché, A., Chervin, C., et al. (2010). Chromoplast differentiation: current status and perspectives. Plant Cell Physiol. 51, 1601–1611. doi: 10.1093/pcp/pcq136
Forss, D., Dunstone, E., Ramshaw, E., and Stark, W. (1962). The flavor of cucumbers. J. Food Sci. 27, 90–93. doi: 10.1111/j.1365-2621.1962.tb00064.x
Gu, X., Zhang, S., Guo, Y., and Xu, C. (2004). Inheritance of bitterness in cucumber. Acta Hortic. Sin. 31, 613–616.
Hao, L., Chen, S., Wang, C., Chen, Q., Wan, X., Shen, X., et al. (2013). Aroma components and their contents in cucumbers from different genotypes. J. Northwest A F Univ. 41, 139–146. doi: 10.13207/j.cnki.jnwafu.2013.06.027
Hao, N., Du, Y., Li, H., Wang, C., Wang, C., Gong, S., et al. (2018). CsMYB36 is involved in the formation of yellow green peel in cucumber ( Cucumis sativus L.). Theor. Appl. Genet. 131, 1659–1669. doi: 10.1007/s00122-018-3105-7
Hatanaka, A., Kajiwara, T., and Harada, T. (1975). Biosynthetic pathway of cucumber alcohol: tr ans -2, cis -6-nonadienol via cis -3, cis -6-nonadienal. Phytochemistry 14, 2589–2592. doi: 10.1016/0031-9422(75)85230-7
He, L., Li, Y., Li, B., Du, N., and Guo, S. (2018). The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. BMC Plant Biol. 18:180. doi: 10.1186/s12870-018-1393-3
Heudorf, U., Mersch-Sundermann, V., and Angerer, J. (2007). Phthalates: toxicology and exposure. Int. J. Hygiene Environ. Health 210, 623–634. doi: 10.1016/j.ijheh.2007.07.011
Horvath, D. P., Schaffer, R., West, M., and Wisman, E. (2003). Arabidopsis microarrays identify conserved and differentially expressed genes involved in shoot growth and development from distantly related plant species. Plant J. 34, 125–134. doi: 10.1046/j.1365-313X.2003.01706.x
Huang, S., Li, R., Zhang, Z., Li, L., Gu, X., Fan, W., et al. (2009). The genome of the cucumber, Cucumis sativus L. Nat. Genet. 41, 1275–1281. doi: 10.1038/ng.475
Jiang, L., Yan, S., Yang, W., Li, Y., Xia, M., Chen, Z., et al. (2015). Transcriptomic analysis reveals the roles of microtubule-related genes and transcription factors in fruit length regulation in cucumber ( Cucumis sativus L.). Sci. Rep. 5, 207–228. doi: 10.1038/srep08031
Kamachi, S., Sekimoto, H., Kondo, N., and Sakai, S. (1997). Cloning of a cDNA for a 1-aminocyclopropane-1-carboxylate synthase that is expressed during development of female flowers at the apices of Cucumis sativus L. Plant Cell Physiol. 38, 1197–1206. doi: 10.1093/oxfordjournals.pcp.a029106
Kemp, T., Knavel, D., and Stoltz, L. (1974). Identification of some volatile compounds from cucumber. J. Agric. Food Chem. 22, 717–718. doi: 10.1021/jf60194a006
King, S., Davis, A., Liu, W., and Levi, A. (2008). Grafting for Disease Resistance. HortScience 43, 1673–1676. doi: 10.21273/HORTSCI.43.6.1673
Knopf, R. R., and Trebitsh, T. (2006). The Female-Specific Cs-ACS1G Gene of Cucumber. A Case of Gene Duplication and Recombination between the Non-Sex-Specific 1-Aminocyclopropane-1-Carboxylate Synthase Gene and a Branched-Chain Amino Acid Transaminase Gene. Plant Cell Physiol. 47, 1217–1228. doi: 10.1093/pcp/pcj09
Kooistra, E. (1971). Inheritance of fruit flesh and skin colours in powdery mildew resistant cucumbers ( Cucumis Sativus L.). Euphytica 20, 521–523. doi: 10.1007/BF00034206
Lee, J. (1994). Cultivation of Grafted Vegetables I. Current Status, Grafting Methods, and Benefits. Hortscience 29, 235–239. doi: 10.21273/HORTSCI.29.4.235
Li, Y., Wen, C., and Weng, Y. (2013). Fine mapping of the pleiotropic locus B for black spine and orange mature fruit color in cucumber identifies a 50kb region containing a R2R3-MYB transcription factor. Theor. Appl. Genet. 126, 2187–2196. doi: 10.1007/s00122-013-2128-3
Li, Z., Huang, S., Liu, S., Pan, J., Zhang, Z., Tao, Q., et al. (2009). Molecular isolation of the M gene suggests that a conserved-residue conversion induces the formation of bisexual flowers in cucumber plants. Genetics 182, 1381–1385. doi: 10.1534/genetics.109.104737
Ligor, T., and Buszewski, B. (2008). Single-drop microextraction and gas chromatography–mass spectrometry for the determination of volatile aldehydes in fresh cucumbers. Anal. Bioanal. Chem. 391, 2283–2289. doi: 10.1007/s00216-008-2098-5
Liu, H., Jiao, J., Liang, X., Liu, J., Meng, H., Chen, S., et al. (2016). Map-based cloning, identification and characterization of the w gene controlling white immature fruit color in cucumber ( Cucumis sativus L .). Theor. Appl. Genet. 129, 1247–1256. doi: 10.1007/s00122-016-2700-8
Liu, X., Ezra, B., Cai, Y., and Ren, H. (2016). Trichome-related mutants provide a new perspective on multicellular trichome initiation and development in cucumber ( Cucumis sativus L). Front. Plant Sci. 7:1187. doi: 10.3389/fpls.2016.01187
Liu, M. (2018). Preliminary Functional Analysis of Cucumber Lipoxygenase CsLOX2 Involved in the Synthesis of C9 Aldehydes. Xianyang: Northwest A&F University.
Liu, X., Wang, T., Ezra, B., Kezia, B., Dong, M., Zhang, Y., et al. (2018). Comprehensive analysis of NAC transcription factors and their expression during fruit spine development in cucumber ( Cucumis sativus L.). Hortic. Res. 5:31. doi: 10.1038/s41438-018-0036-z
Lu, H., Miao, H., Tian, G., Wehner, T., Gu, X., and Zhang, S. (2015). Molecular mapping and candidate gene analysis for yellow fruit flesh in cucumber. Mol. Breed. 35, 1–8. doi: 10.1007/s11032-015-0263-z
Lun, Y., Wang, X., Zhang, C., Yang, L., Gao, D., Chen, H., et al. (2016). A CsYcf54 variant conferring light green coloration in cucumber. Euphytica 208, 509–517. doi: 10.1007/s10681-015-1592-z
Lv, J., Li, M., Qian, W., and Qi, W. (1992). Vegetable quality, criterion and sensory identification. J. Changjiang Vegetables 6, 3–5.
Malundo, T., Shewfelt, R., and Scott, J. (1995). Flavor quality of fresh tomato ( Lycopersicon esculentum Mill.) as affected by sugar and acid levels. Postharvest Biol. Technol. 6, 103–110. doi: 10.1016/0925-5214(94)00052-T
Miao, L., Di, Q., Sun, T., Li, Y., and Yu, X. (2019). Integrated metabolome and transcriptome analysis provide insights into the effects of grafting on fruit flavor of cucumber with different rootstocks. Int. J. Mol. Sci. 20, 289–302. doi: 10.3390/ijms20143592
Ohara, T., Sugiyama, M., and Sakata, Y. (2006). The history of melon and cucumber grafting in Japan. Acta Hortic. 767, 217–228. doi: 10.17660/ActaHortic.2008.767.22
Palma-Harris, C., Mcfeeters, R., and Fleming, H. (2001). Solid-phase microextraction (SPME) technique for measurement of generation of fresh cucumber flavor compounds. J. Agric. Food Chem. 49, 4203–4207. doi: 10.1021/jf010182w
Pan, Y., Qu, S., Bo, K., Gao, M., Haider, K. R., and Weng, Y. (2017). QTL mapping of domestication and diversifying selection related traits in round-fruited semi-wild Xishuangbanna cucumber ( Cucumis sativus L. var. xishuangbannanesis ). Theor. Appl. Genet. 130, 1531–1548. doi: 10.1007/s00122-017-2908-2
Peng, X., Chen, Z., Shi, F., Wu, X., Wang, Y., and Gao, L. (2010). Effects of different grafting methods on growth and fruit quality of cucumber in solar greenhouse. Northern Hortic. 15, 122–124.
Pérez, A. G., Olías, R., Luaces, P., and Sanz, C. (2002). Biosynthesis of strawberry aroma compounds through amino acid metabolism. J. Agric. Food Chem. 50, 4037–4042. doi: 10.1021/jf011465r
Pierce, L., and Wehner, T. (1990). Review of genes and linkage groups in cucumber. Hortic. Sci. 25, 605–615. doi: 10.1016/0304-4238(90)90105-N
Pramnoi, P., Somta, P., Chankaew, S., Juwattansomran, R., and Srinives, P. (2013). A single recessive gene controls fragrance in cucumber ( Cucumis sativus L.). J. Genet. 92, 147–149. doi: 10.1007/s12041-013-0228-0
Qi, J., Liu, X., Shen, D., Miao, H., Xie, B., Li, X., et al. (2013). A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat. Genet. 45, 1510–1515. doi: 10.1038/ng.2801
Qian, C., Ren, N., Wang, J., Xu, Q., Chen, X., and Qi, X. (2018). Effects of exogenous application of CPPU, NAA and GA 4+7 on parthenocarpy and fruit quality in cucumber ( Cucumis sativus L.). Food Chem. 243, 410–413. doi: 10.1016/j.foodchem.2017.09.150
Qiao, H., Zhu, F., Li, C., and Zhang, H. (2005). Inherited analysis on nutrient quality character of cucumber. J. Northeast Agric. Univ. 36, 290–293. doi: 10.19720/j.cnki.issn.1005-9369.2005.03.008
Rice, C., Rymal, K., Chambliss, O., and Johnson, F. (1981). Chromatographic and mass spectral analysis of cucurbitacins of three Cucumis sativus cultivars. J. Agric. Food Chem. 29, 194–196. doi: 10.1021/jf00103a051
Samuels, L., Kunst, L., and Jetter, R. (2008). Sealing plant surfaces: cuticular wax formation by epidermal cells. Ann. Rev. Plant Biol. 59, 683–707. doi: 10.1146/ANNUREV.ARPLANT.59.103006.093219
Schieberle, P., Ofner, S., and Grosch, W. (1990). Evaluation of potent odorants in cucumbers ( Cucumis sativus ) and muskmelons ( Cucumis melo ) by aroma extract dilution analysis. J. Food Sci. 55, 193–195. doi: 10.1111/j.1365-2621.1990.tb06050.x
Sebastian, P., Schaefer, H., Telford, I., and Renner, S. (2010). Cucumber ( Cucumis sativus ) and melon ( C. melo ) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc. Natl. Acad. Sci. U. S. A. 107, 14269–14273. doi: 10.1073/pnas.1005338107
Shan, N., Gan, Z., Nie, J., Liu, H., Wang, Z., and Sui, X. (2020). Comprehensive characterization of fruit volatiles and nutritional quality of three cucumber ( Cucumis sativus L.) genotypes from different geographic groups after bagging treatment. Foods 9:294. doi: 10.3390/foods9030294
Shang, Y., Ma, Y., Zhou, Y., Zhang, H., Duan, L., Chen, H., et al. (2014). Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 346, 1084–1088. doi: 10.1126/science.1259215
Tan, J., Tao, Q., Niu, H., Zhang, Z., Li, D., Gong, Z., et al. (2015). A novel allele of monoecious ( m ) locus is responsible for elongated fruit shape and perfect flowers in cucumber ( Cucumis sativus L.). Theor. Appl. Genet. 128, 2483–2493. doi: 10.1007/s00122-015-2603-0
Wang, G., Qin, Z., Zhou, X., and Zhao, Z. (2007). Genetic analysis and SSR markers of tuberculate trait in Cucumis sativus . Chin. Bull. Botany 24, 168–172.
Wang, J., Li, X., Wang, H., Qiu, Y., Song, J., Zhang, X., et al. (2012). Molecular cloning and expression analysis of ζ-carotene desaturase gene from Cucumis sativus L. Mol. Plant Breed. 10, 520–527. doi: 10.3969/mpb.010.000520
Wang, L., Dong, Y., Sun, X., and Liu, S. (2019). Effects of different grafting methods and different interstock graftings on fruit quality and volatile compounds in cucumber. Plant Physiol. J. 55, 867–874. doi: 10.13592/j.cnki.ppj.2018.0469
Wang, L., Sun, X., Chang, Q., Tao, Y., Wang, L., Dong, J., et al. (2016). Effect of di-n-butyl phthalate (DBP) on the fruit quality of cucumber and the health risk. Environ. Sci. Pollut. Res. 23, 24298–24304. doi: 10.1007/s11356-016-7658-1
Wang, M., Liu, S., Zhang, S., Miao, H., Wang, Y., Tian, G., et al. (2014). Quantitative trait loci associated with fruit length and stalk length in cucumber using RIL population. Acta Botanica Boreali-Occidentalia Sin. 34, 1764–1770.
Wang, W., Zhang, Y., Xu, C., Ren, J., Liu, X., Black, K., et al. (2015). Cucumber ECERIFERUM1 ( CsCER1 ), which influences the cuticle properties and drought tolerance of cucumber, plays a key role in VLC alkanes biosynthesis. Plant Mol. Biol. 87, 219–233. doi: 10.1007/s11103-014-0271-0
Wehner, T., Shetty, N., and Walters, S. (2001). Segregation and linkage of several genes in cucumber. J. Am. Soc. Hortic. Sci. 126, 442–450.
Wei, G., Tian, P., Zhang, F., Qin, H., Miao, H., Chen, Q., et al. (2016). Integrative analyses of nontargeted volatile profiling and transcriptome data provide molecular insight into VOC diversity in cucumber plants ( Cucumis sativus ). Plant Physiol. 172, 603–618. doi: 10.1104/pp.16.01051
Wei, W. (2018). Preliminary Study on the Role of Hydroperoxide lyase CsHPLs in the Function of Aldehydes Aroma Synthesis in Cucumber. Xianyang: Northwest A&F University.
Wenzel, G., Kennard, W., and Havey, M. (1995). Quantitative trait analysis of fruit quality in cucumber: QTL detection, confirmation, and comparison with mating-design variation. Theor. Appl. Genet. 91, 53–61. doi: 10.1007/bf00220858
Whalen, M. (2005). Host defence in a developmental context. Mol. Plant Pathol. 6, 347–360. doi: 10.1111/j.1364-3703.2005.00286.x
Xu, Q., Chen, X., Yu, J., Li, L., and Cao, B. (2001). Preliminary study on heritability and genetic correlation of quality characters in pickling cucumber ( Cucumis sativus L.). Jiangsu Agric. Res. 22, 18–20.
Xu, X., Lu, L., Zhu, B., Xu, Q., Qi, X., and Chen, X. (2015). QTL mapping of cucumber fruit flesh thickness by SLAF-seq. Sci. Rep. 5, 895–906. doi: 10.1038/srep15829
Xu, X., Pan, J., He, M., Tian, H., Qi, X., Xu, Q., et al. (2019a). Transcriptome profiling reveals key genes related to astringency during cucumber fruit development. 3 Biotech 9, 390–398. doi: 10.1007/s13205-019-1922-2
Xu, X., Tian, H., He, M., Gebretsadik, K., Qi, X., Xu, Q., et al. (2019b). Changes in catechin contents and expression of catechin biosynthesis-associated genes during early cucumber fruit development. Acta Physiol. Plant. 41, 130–138. doi: 10.1007/s11738-019-2925-7
Yang, L., Koo, D.-H., Li, Y., Zhang, X., Luan, F., Havey, M. J., et al. (2012). Chromosome rearrangements during domestication of cucumber as revealed by high-density genetic mapping and draft genome assembly. Plant J. 71, 895–906. doi: 10.1111/j.1365-313x.2012.05017.x
Yang, S., Cai, Y., Liu, X., Dong, M., Zhang, Y., Chen, S., et al. (2018). A CsMYB6 - CsTRY module regulates fruit trichome initiation in cucumber. J. Exp. Bot. 69, 1887–1902. doi: 10.1093/jxb/ery047
Yuan, X., Pan, J., Cai, R., Guan, Y., Liu, L., Zhang, W., et al. (2008). Genetic mapping and QTL analysis of fruit and flower related traits in cucumber ( Cucumis sativus L.) using recombinant inbred lines. Euphytica 164, 473–491. doi: 10.1007/s10681-008-9722-5
Yundaeng, C., Somta, P., Tangphatsornruang, S., Chankaew, S., and Srinives, P. (2015). A single base substitution in BADH/AMADH is responsible for fragrance in cucumber ( Cucumis sativus L.), and development of SNAP markers for the fragrance. Theor. Appl. Genet. 128, 1881–1892. doi: 10.1007/s00122-015-2554-5
Zhang, C., Win, K. T., Kim, Y.-C., and Lee, S. (2019). Two types of mutations in the HEUKCHEEM gene functioning in cucumber spine color development can be used as signatures for cucumber domestication. Planta 250, 1491–1504. doi: 10.1007/s00425-019-03244-w
Zhang, J., Yang, J., Luo, J., Zheng, X., Wen, C., and Xu, Y. (2019). Transcription factor CsWIN1 regulates pericarp wax biosynthesis in cucumber grafted on pumpkin. Front. Plant Sci. 10:1564. doi: 10.3389/fpls.2019.01564
Zhang, K., Song, H., Kai-Liang, B., Ji, L., Zheng, M., Lou, Q., et al. (2015). QTL Mapping of Multi-Locule-Number Trait in Xishuangbanna Cucumber. Sci. Agric. Sin. 48, 3211–3220.
Zhang, S., Liu, S., Miao, H., Wang, M., Liu, P., Wehner, T. C., et al. (2016). Molecular mapping and candidate gene analysis for numerous spines on the fruit of cucumber. J. Heredity 107, 471–477. doi: 10.1093/jhered/esw028
Zhang, S., Miao, H., Sun, R., Wang, X., Huang, S., Wehner, T., et al. (2013). Localization of a new gene for bitterness in cucumber. J. Heredity 104, 134–139. doi: 10.1093/jhered/ess075
Zhang, X., Zhang, B., Liu, L., and Liu, Z. (2020). Correlation analysis between main fruit characters and quality characters of South China type cucumber. Hubei Agric. Sci. 59, 389–391. doi: 10.14088/j.cnki.issn0439-8114.2020.S1.106
Zhao, J., Jiang, L., Che, G., Pan, Y., Li, Y., Hou, Y., et al. (2019). A functional allele of CsFUL1 regulates fruit length through repressing CsSUP and inhibiting auxin transport in cucumber. Plant Cell 31, 1289–1307. doi: 10.1105/tpc.18.00905
Zhao, L., Liu, A., Song, T., Jin, Y., Xu, X., Gao, Y., et al. (2018). Transcriptome analysis reveals the effects of grafting on sugar and α-linolenic acid metabolisms in fruits of cucumber with two different rootstocks. Plant Physiol. Biochem. 130, 289–302. doi: 10.1016/j.plaphy.2018.07.008
Zhou, Q., Wang, S., Hu, B., Chen, H., Zhang, Z., and Huang, S. (2015). An ACCUMULATION AND REPLICATION OF CHLOROPLASTS 5 gene mutation confers light green peel in cucumber. J. Integr. Plant Biol. 57, 936–942. doi: 10.1111/jipb.12355
Zhou, Y., Ma, Y., Zeng, J., Duan, L., Xue, X., Wang, H., et al. (2016). Convergence and divergence of bitterness biosynthesis and regulation in Cucurbitaceae. Nat. Plants 2, 1251–1263. doi: 10.1038/nplants.2016.183
Keywords : cucumber, fruit quality, QTLs, spine, bitterness, grafting
Citation: Zhang J, Feng S, Yuan J, Wang C, Lu T, Wang H and Yu C (2021) The Formation of Fruit Quality in Cucumis sativus L.. Front. Plant Sci. 12:729448. doi: 10.3389/fpls.2021.729448
Received: 23 June 2021; Accepted: 18 August 2021; Published: 23 September 2021.
Reviewed by:
Copyright © 2021 Zhang, Feng, Yuan, Wang, Lu, Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huasen Wang, [email protected] ; Chao Yu, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Open access
- Published: 01 October 2018
The USDA cucumber ( Cucumis sativus L.) collection: genetic diversity, population structure, genome-wide association studies, and core collection development
- Xin Wang 1 ,
- Kan Bao 1 ,
- Umesh K. Reddy 2 ,
- Yang Bai 1 ,
- Sue A. Hammar 3 ,
- Chen Jiao 1 ,
- Todd C. Wehner 4 ,
- Axel O. Ramírez-Madera ORCID: orcid.org/0000-0001-6296-1059 5 ,
- Yiqun Weng 5 , 6 ,
- Rebecca Grumet 3 &
- Zhangjun Fei ORCID: orcid.org/0000-0001-9684-1450 1 , 7
Horticulture Research volume 5 , Article number: 64 ( 2018 ) Cite this article
12k Accesses
69 Citations
4 Altmetric
Metrics details
- Genetic variation
- Plant genetics
Germplasm collections are a crucial resource to conserve natural genetic diversity and provide a source of novel traits essential for sustained crop improvement. Optimal collection, preservation and utilization of these materials depends upon knowledge of the genetic variation present within the collection. Here we use the high-throughput genotyping-by-sequencing (GBS) technology to characterize the United States National Plant Germplasm System (NPGS) collection of cucumber ( Cucumis sativus L.). The GBS data, derived from 1234 cucumber accessions, provided more than 23 K high-quality single-nucleotide polymorphisms (SNPs) that are well distributed at high density in the genome (~1 SNP/10.6 kb). The SNP markers were used to characterize genetic diversity, population structure, phylogenetic relationships, linkage disequilibrium, and population differentiation of the NPGS cucumber collection. These results, providing detailed genetic analysis of the U.S. cucumber collection, complement NPGS descriptive information regarding geographic origin and phenotypic characterization. We also identified genome regions significantly associated with 13 horticulturally important traits through genome-wide association studies (GWAS). Finally, we developed a molecularly informed, publicly accessible core collection of 395 accessions that represents at least 96% of the genetic variation present in the NPGS. Collectively, the information obtained from the GBS data enabled deep insight into the diversity present and genetic relationships among accessions within the collection, and will provide a valuable resource for genetic analyses, gene discovery, crop improvement, and germplasm preservation.
Similar content being viewed by others

Genome-wide SNP discovery and core marker sets for assessment of genetic variations in cultivated pumpkin ( Cucurbita spp.)

Almond diversity and homozygosity define structure, kinship, inbreeding, and linkage disequilibrium in cultivated germplasm, and reveal genomic associations with nut and seed weight
Genome-wide diversity analysis to infer population structure and linkage disequilibrium among Colombian coconut germplasm
Introduction.
Improvements in crop yield, ability to withstand abiotic and biotic stresses, and superior product quality all depend on genetic variation for key agronomic and horticultural traits. In search of such variation, breeders frequently turn to germplasm collections to find new sources of valuable characteristics, especially resistances to diseases, insects, and environmental stresses such as heat, drought, salt, or cold. To facilitate these breeding efforts and maintain critical diversity for future generations, many national and international institutions have developed extensive germplasm collections to provide repositories of genetic variation. More than 1750 gene banks have been established worldwide 1 . Collections are typically made from locations throughout the globe, with particular emphasis on centers of crop diversity. The importance of such collections as a critical first step to conserve biological variation, especially in light of genetic erosion resulting from habitat loss, adoption of modern varieties, and climate change, is increasingly recognized as a critical global good, both in scientific and broader public spheres 2 , 3 . While creation and maintenance of these valuable collections is essential, questions arise as to how to catalog, unlock, manage, and preserve the valuable diversity they contain. How do we evaluate the extent and nature of variation that exists within the collection? How can we access that variation for crop improvement? Fortunately, the past decade has ushered in powerful genomic tools that allow for high throughput, high resolution, genetic characterization, while also providing breeders more efficient access to, and use of, the diversity available within collections.
Collections for the Cucurbitaceae family, which includes many high-value crops consumed as vegetables and fruits throughout the world, face the above-mentioned challenges for germplasm preservation and utilization 4 . Cucumber ( Cucumis sativus L.), a member of the Cucurbitaceae family with origins in India, China, Burma, Thailand, is thought to have been domesticated ~3000 years ago 5 , 6 . The primary and secondary centers of diversity for the species are located in India and Southeast Asia, respectively 7 , 8 . Genomic analysis of cultivated cucumber ( C. s . var. sativus) divided it into four geographic groups: India; Eurasia and the West; East Asia and China; and Xishuangbanna from Southwestern China 9 , 10 . The Indian group, which is thought to form the basal group, maintains a large proportion of the genetic diversity and also includes the wild cucumber, C. s . var. hardwickii , a feral form of var. sativus 9 , 10 , 11 . Deep resequencing of a core collection of 115 cucumber lines, sampled from 3342 accessions worldwide, suggests that the domestication process led to a severe genetic bottleneck, resulting in reduction in diversity relative to wild accessions 10 . More than 100 putative selective sweeps appear to be associated with domestication, including extended linkage disequilibrium in regions surrounding loci associated with key fruit traits such as size and bitterness. Results of the genomic analyses, including assignment of a basal role of the Indian group and separation of the orange-endocarp Xishuangbanna group, complement prior genetic and morphological assessments 12 , 13 , 14 , 15 , 16 . These analyses have allowed for evolutionary insight into the relationships and domestication trajectories among cucumber accessions.
The cucumber collection in the United States is maintained at the Ames, Iowa facility of the USDA Agriculture Research Service National Plant Germplasm System (NPGS; https://npgsweb.ars-grin.gov/gringlobal/site.aspx?id=16 ). The NPGS collection comprises 1314 cucumber accessions representing the primary cucumber gene pool ( C. s . var. sativus and C. s . var. hardwickii ). This collection, which is primarily composed of cultivars, land races, and varieties collected from around the world, has been extensively utilized by breeders searching for a variety of traits, including resistance to downy mildew 17 (causal agent: Pseudoperonospora cubensis ), powdery mildew 18 (causal agent: Podosphaera xanthii ), Phytophthora fruit rot 19 , 20 (causal agent: Phytophthora capsici ), belly rot 21 , 22 (causal agent: Rhizoctonia solani ), and root knot nematodes ( Meloidogyne spp.) 23 , as well as variations for fruit yield, fruit quality 24 , and above-ground and below-ground plant architecture 25 , 26 . However, to date, there have been very limited efforts to genetically characterize the US cucumber collection. Meglic et al. 27 examined 757 accessions using seven isozyme loci, and Horejsi et al. 28 characterized 118 accessions with 71 RAPD loci. Lv et al. 9 included 883 accessions from the U.S. collection, which were characterized using a set of 23 SSR markers and 316 alleles. Current genomic technologies allow for much higher throughput and full genome analyses. The dramatically reduced cost of sequencing, high-throughput sample preparation, and efficient bioinformatics now make it feasible to perform genomic analysis on increasingly large numbers of samples for plant germplasm research 29 , 30 . In this study, we have performed genotyping on 1234 cucumber accessions from the NPGS, using genotyping-by-sequencing 30 (GBS). The resultant high-throughput single-nucleotide polymorphism (SNP) markers provided high-definition genetic characterization of the US cucumber germplasm collection, allowing for assessment of genetic diversity and population structure, identification of markers that are highly associated with important agronomic traits through genome-wide association studies (GWAS), and development of a molecularly informed publicly accessible core population to facilitate breeding and preservation efforts.
Materials and methods
Plant materials and dna extraction.
Tissue samples (50–100 mg fresh weight) were collected from young (not fully expanded) leaves, freeze-dried, and ground to a fine powder using 5/32” stainless steel balls (AbbottBall, West Hartford, CT) in a Retsch Mixer Mill (Retsch, Newtown, PA). DNA was isolated using the Omega Mag-Bind Plant DNA DS Kit (M1130, Omega Bio-Tek, Norcross, GA) on a Kingfisher Flex Magnetic Particle Processor (Thermo Scientific, Waltham, MA). The kit protocol was followed except that the initial 56 °C incubation was extended to 60 min instead of 30 min. The DNA was quantified using the Quant-iT PicoGreen dsDNA Kit (Invitrogen, Carlsbad, CA) in a 384-well format on a CFX384 C1000 Real-Time thermal cycler (BioRad, Hercules, CA). Normalization to 30–100 ng/ul was done using a GBFit Arise Pipetting System (Pacgen Inc., Irvine, CA). Quality checks were performed on 10% of the genomic DNA samples from each batch of 96 samples by agarose gel observation of 300 ng of undigested and Hind III digested DNA per sample.
GBS and SNP calling
Genotyping of the cucumber accessions was performed following the GBS protocol 30 , using ApeK I as the restriction enzyme. The resulting 96-plex or 384-plex libraries were sequenced on a HiSeq 2500 system (Illumina Inc., USA) with the single-end mode and read length of 101 bp.
SNP identification was performed using TASSEL 5.0 GBS Discovery Pipeline 31 , using the cucumber Gy14 draft genome (v2; http://cucurbitgenomics.org ) as the reference. Briefly, the raw reads were first processed to retain reads possessing a barcode and a restriction enzyme cut site using GBSSeqToTagDBPlugin with the parameters “-kmerLength 90-minKemrL 30-mnQS 10-c 100-maKmerNum 200000000”. The resulting reads were then concatenated into distinct tags using the FastqToTagCount plug-in in TASSEL, and tags supported by at least ten reads were kept and mapped to the cucumber reference genome sequence using BWA (version 0.7.16a) with default parameters 32 . Based on the alignments, positions of aligned tags were determined using SAMtoGBSdbPlugin, and SNPs were identified from the aligned tags using DiscoverySNPCallerPluginV2 with default parameters. The identified SNPs were scored according to the coverage, depth, and genotypic statistics for a given set of samples using SNPQualityProfilerPlugin. SNPs were filtered based on their missing data rate and minor allele frequencies (MAF) using VCFtools 33 .
Phylogenetic and population genomic analyses
SNPs with MAF ≥ 1% and missing data rate ≤ 50% were used for phylogenetic and population structure analyses. The maximum-likelihood (ML) phylogenetic tree was constructed using SNPhylo 34 with parameters “-r -M 0.5 -m 0.01 -l 0.1 -B 100” and visualized using the ggtree package 35 . PI 618817 ( Cucumis myriocarpus ) and PI 282446 ( C. heptadactylus ) were used as the outgroup. Principal component analysis (PCA) was performed using Plink-1.9 (ref. 36 ). Population structure analysis was performed using the STRUCTURE program 37 . A total of 11,745 SNPs with linkage disequilibrium (LD) decay ( r 2 ) < 0.4 were used for the analysis. To determine the most likely group number, STRUCTURE was run 20 times using 8000 SNPs randomly selected from the 11,745 SNPs, for each K ( K = 2–20). The highest ∆K , which indicates the most likely number of clusters in the population, was obtained. After determining the best K ( K = 3), we then ran STRUCTURE using all 11,745 SNPs with 10,000 iterations for each K ( K = 2–4).
LD decay was measured by correlation coefficients ( r 2 ) for all pairs of SNPs within 500 kb that were calculated using PopLDdecay v3.27 ( https://github.com/BGI-shenzhen/PopLDdecay ) with the following parameters: -MaxDist 500 -MAF 0.05 -Het 0.88 -Miss 0.999. The maximum value of r 2 was calculated using all pairs of SNPs within 500 bp. The nucleotide diversity (π) and population fixation index ( F ST ) were calculated using Bio:PopGen implemented in the BioPerl package 38 . To visualize the pairwise F ST values among different groups, multidimensional scaling (MDS) was conducted using the cmdscale function in R to transform F ST values into two-dimensional values, which were used for plotting.
The USDA-GRIN database archives phenotypic data for Cucumis ( https://npgsweb.ars-grin.gov/gringlobal/cropdetail.aspx?type=descriptor&id=123 ). The phenotypic data of 13 important traits for cucumber, including three related to disease resistance (anthracnose, downy mildew, and gummy stem blight (GSB) resistance), three related to root knot nematode resistance (resistance to Meloidogyne hapla race 1, M. arenaria race 2, or M. incognita race 3), three related to fruit shelf life (weight loss, firmness loss, and shriveling), and four other traits (chilling tolerance, days to flower, root size, and fruit yield), were downloaded from the GRIN database. The phenotypic data were collected over the last 30 years by the Cucurbit Breeding program of North Carolina State University. Data sets for each trait were collected over multiple years and locations ( http://cucurbitbreeding.com ) for 750–950 cultigens per trait. Description of the data collection is available at Supplementary Note . Phenotypic data from accessions genotyped in the present study were used for GWAS.
We used a total of 72,982 biallelic SNPs without any filtering to construct the kinship (K) matrix, which was used to correct for population structure and kinship in the GWAS analyses. For GWAS, the missing genotypes in the raw biallelic SNP dataset were imputed using the k-nearest neighbor (KNN) algorithm implemented in the fillGenotype software 39 . In order to obtain the optimal imputation accuracy and filling rate, three accessions with few missing genotypes (Amex 7735, NSL 32744, and PI 167052) were selected and 10%, 20%, and 30% SNP sites were randomly masked as missing genotypes for imputing. The imputation was performed using the fillGenotype with the following parameters: w (20, 30, 50, 65, 80), p (−3, −5, −7, −9), k (3, 5, 7, 9), and r (0.65, 0.7, 0.75, 0.8). The optimal combination of parameters (w = 30, k = 9, p = −9, r = 0.8) was selected after comparing the filling rate and imputation accuracy of each combination of parameters, to impute the missing genotypes in the raw dataset. Only biallelic imputed SNPs with minor allele frequency ≥ 1% and missing data rate ≤ 20% (a total of 28,650 SNPs) were used for GWAS. GWAS were performed using the linear mixed model (LMM) implemented in Fast-LMM 40 . The genome-wide significance thresholds of the GWAS were determined using the Bonferroni correction at α = 0.05 for significant and α = 0.01 for extremely significant associations as described in Li et al. 41 . In this study, the significance thresholds of α = 0.05 and α = 0.01 corresponded to raw P values of 1.75 × 10 –6 and 3.49 × 10 −7 , or −log10( P ) values of 5.76 and 6.46, respectively.
Core collection selection
GenoCore 42 was used to select a subset of accessions that captured the majority of the allelic diversity of the 1234 cucumber accessions, with the following parameters: -d 0.01%, -cv 100%. Combined with phenotypic analysis, we obtained the final core collection containing 395 cucumber accessions, of which 354 were genotyped in the current study. The percentage of the allelic diversity captured by the 354 accessions in this core collection was determined using GenoCore. The core collection was further evaluated by PCA, using the same methods described above for the entire collection.
Genotyping of cucumber germplasm collection and variation identification
Seed was successfully germinated for 1234 cucumber accessions from the NPGS, which represents 94% of the collection (1314 accessions for which seed was available). Based on their geographic distribution (countries of origins), we classified these accessions mainly into seven groups, 216 from India/South Asia, 293 from East Asia, 113 from Central/West Asia, 161 from Turkey, 314 from Europe, 33 from Africa, 97 from North America, as well as 7 from other regions (Fig. 1 and Supplementary Table S 1 ). The accessions from India (184) along with 32 plant introductions (PIs) from the surrounding regions of Bhutan, Malaysia, Nepal, Myanmar, Pakistan, Sri Lanka, and Thailand were classified separately from accessions from other Asian countries, since India and the surrounding regions are considered as the center of origin of cultivated cucumber 6 , 43 . The Indian/South Asia group also included three accessions of C. s . var. hardwickii . In addition, since Turkey is a country straddling Asia and Europe, we put accessions from Turkey as an independent group. We genotyped these cucumber accessions, as well as two non-cucumber but closely related accessions PI 618817 ( C. myriocarpus ) and PI 282446 ( C. heptadactylus ), using the GBS technology, which generated a total of ~1.35 billion reads of 101 bp in length. The numbers of reads for each sample ranged from ~176 K to 4.8 million, with a median of 677 K reads (Supplementary Table S 1 ). A total of 554 K unique tags with at least 10 read counts, which corresponded to ~1.23 billion reads, were obtained and used for SNP calling. The 1.23 billion GBS reads were aligned to the reference Gy14 genome (version 2; http://cucurbitgenomics.org ), with 55.5% (0.69 billion corresponding to 279 K tags) aligned to unique positions and 17.3% (0.21 billion corresponding to 50 K tags) to multiple locations; the remaining 27.2% (0.33 billion corresponding to 225 K tags) unaligned reads were mainly from mitochondrion and chloroplast, as well as genome regions that were absent in the reference genome. Approximately 3.7% (9.5 Mb out of 258.6 Mb) of the Gy14 genome was covered by the aligned GBS reads, which is typical for reduced complexity GBS data 30 .
Size of the circles indicates the relative sampling size in each country
Based on the alignments, we identified a total of 114,760 variation sites, of which 113,854 were SNPs and 906 were small insertions/deletions (InDels). After retaining SNPs with ≤ 50% missing data (representing at least 617 accessions) and MAF ≥ 0.01 (i.e., SNPs present in at least 7 accessions), we obtained a total of 24,319 SNPs distributed across the cucumber Gy14 genome with an average of one SNP per 10.6 kb (Table 1 ). Only eight regions > 500 kb in the Gy14 genome were not covered by SNPs, and all these eight regions were centromeric or pericentromeric (Fig. 2a ). The distribution of MAF of these SNPs is shown in Fig. 2b . The average MAF was 0.13; nearly half (11,798; 48.5%) of the SNPs had MAF between 0.01 and 0.05. Among the 24,319 SNPs with ≤ 50% missing data and MAF ≥ 0.01, only those that were biallelic were retained as the final SNP dataset (23,552 SNPs) used in the downstream analyses, unless otherwise specified.
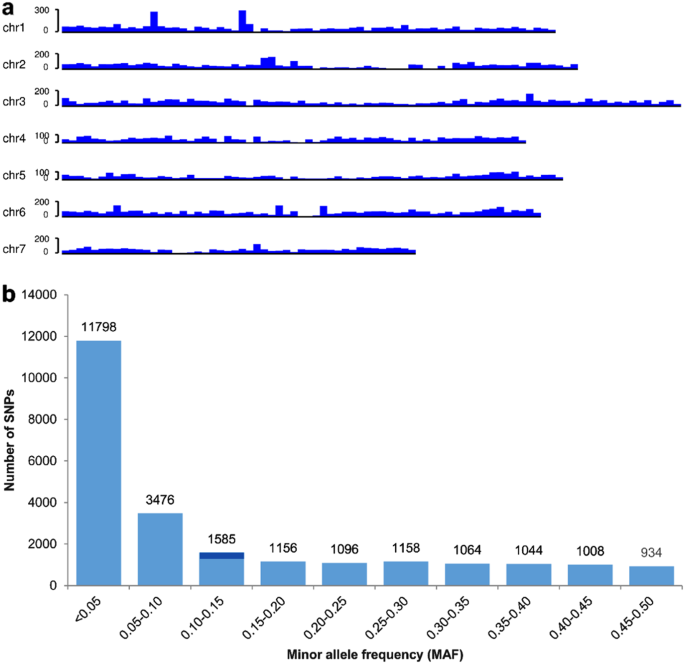
a SNP density across the seven cucumber chromosomes. Number of GBS-SNPs in each 500 kb non-overlapping window are shown. b Distribution of minor allele frequency (MAF) for the filtered SNPs
Phylogenetic relationships and population structure of the cucumber accessions
Using the final SNP dataset, we constructed a rooted ML tree to infer phylogenetic relationships among the cucumber accessions, using PI 618817 ( C. myriocarpus ) and PI 282446 ( C. heptadactylus ) as the outgroup (Fig. 3a and Supplementary File 1 ). Three major clades were identified. Consistent with India as the center of origin for cucumber, the clade with the deepest branches was the India/South Asia group. The remaining accessions were separated into two major clades. One mainly contained accessions from East Asia, while the second encompassed accessions from Central/West Asia, Turkey, Europe, Africa, and North America.
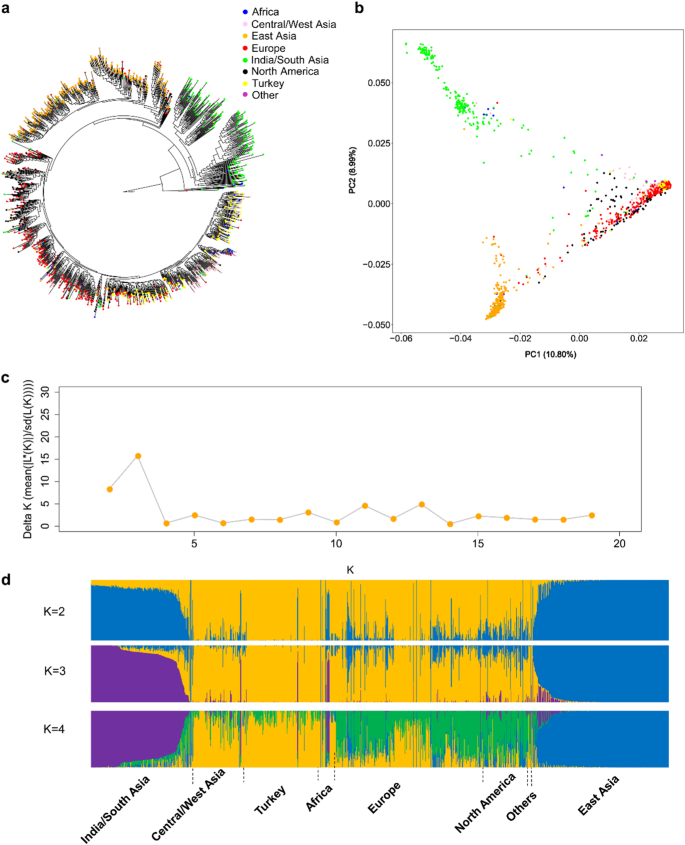
a Rooted maximum-likelihood phylogenetic tree of the 1234 cucumber accessions constructed using GBS-SNPs. PI 618817, C. myriocarpus , and PI 282446, C. heptadactylus were used as the outgroup. An enlarged version of the tree with searchable accession names is provided as Supplementary File 1 in pdf format. b Principal component analysis (PCA) of the 1234 cucumber accessions. The first two PCs explain about 20% of variance, with PC1 and PC2 explaining 10.80% and 8.99%, respectively. c Plot of ΔK values with K from 2 to 19 in the STRUCTURE analysis. d Population structure analysis of cucumber accessions with K from 2 and 4. Each accession is represented by a vertical bar. Each color represents one ancestral population, and the length of each colored segment in each vertical bar represents the proportion contributed by ancestral populations
PCA of these cucumber accessions illustrated a similar pattern of their phylogenetic relationships (Fig. 3b ). Our results are consistent with those reported in Qi et al. 10 , which also classified cucumbers into three primary groups, with the exception of the Xishuangbanna group, for which no accessions were included in our GBS set.
To investigate the population structure of cucumber, the Bayesian clustering algorithm implemented in the STUCTURE program 37 was first used to estimate ancestry proportions for each cucumber accession. ∆ K analysis showed that three populations ( K = 3) represented the best number of clusters for these 1234 cucumber accessions (Fig. 3c ). As shown in Fig. 3d , at K = 2, accessions from East Asia and India were clearly separated from other accessions. At K = 3 (optimal), the India/South Asia group was clearly separated from the East Asia group. The population structure result at this optimal K was consistent with the phylogenetic tree and PCA results; all suggested three primary clusters in the cucumber accessions collected from NPGS. At K = 4, a new subpopulation emerged mainly in accessions from Europe and North America. A large portion of accessions from Europe, North America, Africa, Turkey, and Central/West Asia showed genetic admixture, while most of the East Asia accessions had a homogeneous genetic background.
Within the India/South Asia clade were several subclades (Fig. 3a and Supplementary Fig. S1 ). The Indian accessions within the U.S. NPGS were collected in two time periods: a first set of materials was entered into the system prior to 1972, and a second set collected in 1992. The accessions collected in 1992 were primarily from the states of Rajasthan, Uttar Pradesh, and Madhya Pradesh, representing regions in North and Central India that were largely missed in the prior collection 44 . The Indian accessions were differentially distributed among the different subclades, especially those from Rajasthan that were primarily associated with subclade 2, suggesting that the subclades, in part, reflect geographic distribution within India. Accessions from prior collections from South or Southwest India (Maharashtra, Karnataka, and Kerala) clustered in subclade 3. Subclade 1 primarily contained accessions from Madhya Pradesh in central India. The great majority of the East Asian accessions were collected from China. Those from Japan and South Korea largely clustered with each other; the remaining subclades were almost exclusively composed of accessions from China (Supplementary Fig. S1 ). For accessions from Turkey, two subclades were identified, one clustered with accessions from Central/West Asia group, and the other clustered with accessions from Europe (Supplementary Fig. S1 ). The North American accessions, also showed division into two distinct subclades. One group was largely comprised of pickling (processing) cultigens and the other of slicing (fresh market) cultigens (Supplementary Fig. S1 ), reflecting the two predominant market classes produced in the US.
LD patterns, genetic diversity, and population differentiation in cucumber
The LD decay ( r 2 ) with increasing physical distance between SNPs was calculated for each group (Supplementary Fig. S2 ). When the entire population was analyzed, the average physical distance over which LD decayed to half of its maximum value was around 24 kb ( r 2 = 0.0930; maximum r 2 = 0.1830). Variable LD decays were detected in different groups. The Africa group and the North American group had the longest physical distances over which LD decayed to half of its maximum value, 64 kb and 96 kb, respectively, while the India group had the shortest, 16 kb. The Europe, Turkey, East Asia, and the Central/West Asia groups showed comparable LD decay patterns and physical distances (48 kb, 40 kb, and 32 kb for Europe, East Asia, and Central/West Asia, respectively).
We then evaluated the genetic diversity within different groups. The average values of genome-wide nucleotide diversity (π) for Central/West Asia, Europe, North America, Africa, Turkey, East Asia, and India/South Asia groups were 0.87 × 10 −3 , 0.90 × 10 −3 , 0.93 × 10 −3 , 0.98 × 10 −3 , 0.81 × 10 −3 , 0.74 × 10 −3 , and 1.22 × 10 −3 , respectively. The π value of the India/South Asia group was higher than those of other groups, consistent with India being the center of origin of cultivated cucumber where cucumber accessions are expected to be more genetically diverse.
We further investigated population divergence among different groups by calculating pairwise fixation index ( F ST ) values. Pairwise weighted F ST values among North America, Central/West Asia, Africa, Turkey, and Europe groups ranged from 0.042 to 0.14, while the values between East Asia and other six groups ranged from 0.284 to 0.413, and between India/South Asia and other six groups from 0.176 to 0.284 (Supplementary Table S 2 ). Visualization of pairwise weighted F ST values using MDS showed a clear distinction between the East Asia group and other groups. F ST between East Asia vs. India/South Asia was 0.284 and between East Asia vs. the Western group (North America, Europe, Africa, and Central/West Asia) was 0.269. There was much less divergence among the North America, Europe, Turkey, Africa, and Central/West Asia groups, and between the Western group and India/South Asia (0.15) (Fig. 4 ). Collectively, both π and F ST values suggested that domestication and improvement of cultivated cucumbers from Indian cucumbers occurred independently in East Asia compared to other regions.
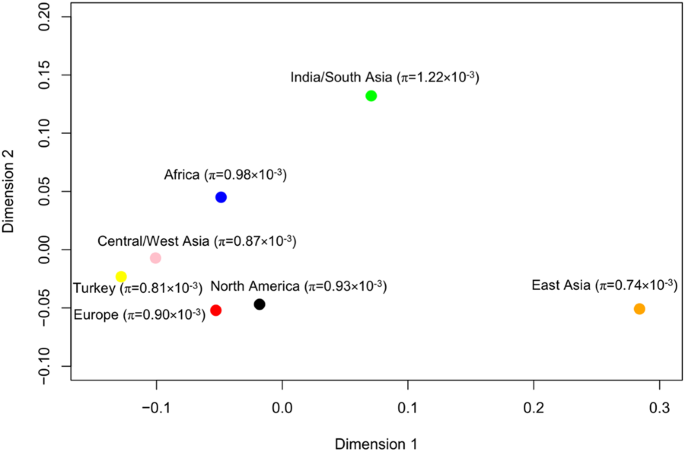
Multidimensional scaling of pairwise F ST values between different cucumber groups
Genome-wide association studies in cucumber
The high-density SNP markers combined with GWAS provide a powerful resource to identify quantitative trait loci (QTL) and possible candidate genes for important horticultural traits. We collected historical phenotypic data of cucumber accessions from the NPGS for 13 agronomic traits, which included three traits related to disease resistance (anthracnose, downy mildew, and GSB resistance), three related to root knot nematode resistance (resistance to Meloidogyne hapla race 1, M. arenaria race 2, or M. incognita race 3), three related to fruit shelf life (weight loss, firmness loss, and shriveling), and four other traits (cold tolerance, days to flower, root size, and fruit yield). For each trait, data were available for around 600–700 accessions that were genotyped using GBS in this study (Supplementary Table S 3 ). The phenotypic data largely followed normal distribution without significant skewness except for resistance to M. hapla race 1 (Supplementary Fig. S3 ). GWAS were performed for these traits with the imputed SNPs, which had an imputation accuracy of > 99% and missing data filling ratio of > 96.5% (Supplementary Table S 4 ), using the LMM accounting for population structure and kinship. Significantly associated SNPs could be identified except for resistance to M. incognita race 3 and root size (Supplementary Table S 5 ).
GWAS for disease and nematode resistance
For anthracnose resistance, two regions on chromosome 7 were identified (Fig. 5a ). A total of 11 SNPs spanning one region (from 1.0 to 1.1 Mb) and a total of five SNPs spanning another region (from 12.52 to 12.55 Mb) were found to be significantly associated with anthracnose resistance (Supplementary Table S 5 ). Other significantly associated SNPs were identified at 33.1 Mb of chromosome 3 and 10.06 Mb of chromosome 5.
a GWAS for disease resistance traits including resistance to downy mildew, anthracnose, or gummy stem blight. b GWAS for root knot nematode resistance traits including resistance to Meloidogyne hapla race 1, M. arenaria race 2, or M. incognita race 3
For downy mildew resistance, a region on chromosome 5 spanning from 29.38 to 32.46 Mb was identified to contain 27 significantly associated SNPs (Fig. 5a and Supplementary Table S 5 ). Eight other SNPs significantly associated with downy mildew resistance were identified, with one on chromosome 3 (40.78 Mb), four on chromosome 5 (4.26, 6.54, 14.64, and 22.49 Mb), and three on chromosome 7 (9.55, 10.96, and 19.73 Mb).
For GSB resistance, three regions, one on chromosome 2, one on chromosome 5 and one on chromosome 7 were identified (Fig. 5a ). The region on chromosome 2 spanned from 30.67 to 31.83 Mb and contained four significantly associated SNPs; the region on chromosome 5 spanned from 28.68 to 31.34 Mb and contained 25 significantly associated SNPs (Supplementary Table S 5 ). Another two SNPs, on chromosome 3 (13.05 Mb) and 5 (23.05 Mb), respectively, were identified to be significantly associated with GSB resistance.
For root knot nematode resistance, no regions were identified to be significantly associated with resistance to M. incognita race 3; while a SNP on chromosome 1 (3.18 Mb) was identified to be significantly associated with resistance to M. arenaria race 2 (Fig. 5b ). Six SNPs, one on chromosome 3 (26.48 Mb), one on chromosome 5 (19.67 Mb), two on chromosome 6 (7.37 and 28.88 Mb), and two on chromosome 7 (1.35 and 16.72 Mb) were significantly associated with resistance to M. hapla race 1 (Supplementary Table S 5 ).
GWAS for fruit yield and physiological traits
Fruit yield trait in the cucumber accessions was investigated at two locations, Iowa and North Carolina. GWAS for fruit yield using data from each of the two locations as well as combined identified a total of nine significantly associated SNPs, one on chromosome 2 (30.07 Mb), two on chromosome 3 in a region at 27.86 Mb, three on chromosome 4 (27.00, 28.27 and 29.83 Mb), and three on chromosome 5 in a region spanning from 2.847 to 2.864 Mb (Supplementary Fig. S4a and Supplementary Table S 5 ).
GWAS were performed for three traits related to fruit shelf life, weight loss, loss of firmness, and shriveling. Five SNPs, three on chromosome 4 (2.22 Mb and two at 28.78 Mb) and two on chromosome 7 (696 and 978 kb) were identified for weight loss, one SNP on chromosome 3 (27.23 Mb) was identified for loss of firmness, and one SNP on chromosome 2 (3.18 Mb) was identified for shriveling (Supplementary Fig. S4b and Supplementary Table S 5 ).
For chilling tolerance, eighteen SNPs were identified, with ten on chromosome 1, one on chromosome 2, two on chromosome 4 and five on chromosome 7. For days to flower, six SNPs, one on chromosome 1, one on chromosome 3, two on chromosome 4, and two on chromosome 6 were identified (Supplementary Fig. S5 and Supplementary Table S 5 ). No significant associations were found for root size.
Development of a publicly accessible core cucumber germplasm collection
Our main objective of developing a core collection from the cucumber accessions in the NPGS is to provide the community with a subset of representative cucumber accessions that can be used for future GWAS, QTL mapping, marker development, and gene cloning studies. The selected core collection would have a reasonable size (~400 accessions) and capture largely the allelic diversity of the entire collection, and also include accessions with some unique and important agronomic traits.
To develop this core collection, we first analyzed the 1234 cucumber accessions using the GenoCore program 42 . The results showed that the 720 top-ranked accessions captured 100% of the allelic diversity of the whole set, and the top 100, 200, 300, and 400 top-ranked accessions captured 93.87%, 97.09%, 98.47%, and 99.20% of the allelic diversity, respectively (Fig. 6a ). According to this analysis, we first selected 354 accessions which captured 95.9% of the allele diversity in the collection of the 1234 cucumber accessions. Of the 354 accessions, 70 (19.8%) were from India/South Asia, 35 (9.9%) from Central/West Asia, 94 (26.6%) from East Asia, 10 (2.8%) from Africa, 20 (6.0%) from North America, 74 (20.9%) from Europe, 48 (13.6%) from Turkey, and 3 from other regions (Supplementary Table S 6 ). PCA analysis of these 354 accessions in the core collection (Fig. 6b ) showed the nearly identical patterns to those of the 1234 accessions in the entire collection.
a Coverage of allelic diversity versus number of selected accessions analyzed by GenoCore. b Principle component analysis (PCA) of cucumber accessions. Red dots: accessions in the core collection; gray dots: accessions not in the core collection
An additional 41 historical varieties with important horticultural and disease resistance traits were added to this core collection, making the final core collection containing a total of 395 accessions. The additional accessions included some cucumber cultivars or germplasm that have played important roles in cucumber breeding in the US for the processing and fresh markets (Supplementary Table S 6 ).
Genetic characterization of the US NPGS cucumber collection
The genetic composition of the U.S. cucumber germplasm collection was characterized using high-throughput GBS analysis. A total of 1234 accessions, predominantly collected from India and South Asia, East Asia, Central and West Asia, Europe, North America, and Africa were genotyped, providing 279 K uniquely aligned sequence tags. From these data ~23.5 K biallelic SNPs representing minor alleles present in at least seven accessions (frequency > 0.01 and missing data rate < 0.5) were identified. With the exception of highly methylated centromeric or pericentromeric regions, the SNPs were well distributed at high density in the genome with an approximate frequency of 1 SNP per 10.6 kb. These data allowed for comprehensive analysis of phylogentic relationships, population structure, and LD patterns of accessions in the collection and provide a resource for genetic analysis and gene discovery.
Consistent with our current understanding about the geographic origin of cucumber 6 and prior phylogenetic analyses 9 , 10 , the U.S. cucumber PI collection comprised three major clades. The basal clade predominantly comprised accessions from India/South Asia, the presumed center of domestication for C. sativus . While this clade had the deepest branches suggesting greater divergence among members of this clade, the overall diversity of accessions from this region was reduced relative to the study of Qi et al. 10 . This is likely due to sampling. The 216 accessions from India/South Asia in the U.S. collection included only three (1.4%) accessions of C. s . var. hardwickii , a highly diverse wild botanical variety believed to be either a progenitor or a feral relative of the cultivated cucumber, C. s . var. sativus 6 , 11 , 44 . In contrast, 13 of 30 accessions (43.3%) from the Indian accessions studied by Qi et al. 10 were var. hardwickii .
From its origins in India and initial domestication ~3000 years ago, it appears that cucumber moved both East (to East Asia) and West (to Central and West Asia, Europe, Africa, and North America), following distinct trajectories in each case 27 , 45 . The strong differentiation between the East and West groups likely reflects a long period of divergent domestication (written Chinese records mentioning cucumber date as early as 164 BCE) as well as geographical isolation due to the Himalayan mountains 10 , 28 , 46 . Patterns of LD decay were consistent with the phylogenetic and population structure analyses. The Indian group had the shortest physical distance to reach half-maximal value (16 kb), vs. 32–48 kb for East Asia, Central/West Asia, and Europe. The reduced rate of LD for North American and Africa accessions (96 and 88 kb) may reflect the greater genetic relatedness of the samples in this collection, or the migration route for cucumber, which is thought to have been introduced into these regions comparatively recently from Europe 47 .
Several studies have indicated that the overall level of genetic diversity within cultivated cucumber is quite narrow, and that most of the genetic differentiation was observed between geographic regions or market classes 9 , 10 , 28 , 44 , 46 , 48 . Our phylogenetic analyses also reflected these sources of divergence. In addition to the separation observed among the three primary clades, we saw examples of differentiation within clades as evidenced by countries of origin, regions of collection within India, subgroups from Turkey, and between processing and fresh market cucumbers in North America. Among the Indian PIs, accessions from Madhya Pradesh, Uttar Pradesh, and Rajasthan were preferentially, but not exclusively, distributed in different subclades, suggesting diversity both within and between regions. Separation of accessions from Rajasthan relative to other regions in India was previously observed based on isozyme analysis performed following initial collection 44 . The current SNP-based analysis allowed for more nuanced assessment of relationships among the accessions. The Turkish germplasm also was associated with several subclades. For the two largest subclades containing Turkish accessions, one was extensively mixed with accessions from Central/West Asia, while the second was extensively mixed with accessions from Europe. Examination of collection locations within Turkey showed predominance of samples from the European-mixed subclade from western Turkey and samples from the Asian-mixed subclade from Eastern Turkey. There were some exceptions, however, possibly reflecting seed exchange across different regions of the country. Separation among the North American accessions reflected market class. As public and commercial breeding efforts have largely catered to either pickling or slicing cucumber, with delineated breeding efforts, it is not surprising to observe genetic divergence. Differentiation between pickling and slicing cucumbers also has been observed with RFLP markers and metabolomic analyses of cucumber fruit peels 28 , 49 .
Development of genomic breeding tools
An important value of genetic characterization of the collection is the development of genomic tools for breeders. QTL analyses of key traits of economic importance can allow for the development of markers for marker assisted selection, focusing phenotypic selection on population subsets containing desired markers and facilitating gene pyramiding for complex traits. The GWAS presented here using the high-density SNP markers and historical phenotyping data for several disease resistance and physiological traits show the identification of significantly associated genomic regions. At this time QTL have been mapped for a limited number of traits in cucumber. Of the traits examined here, recent studies have reported QTL for downy mildew, GSB, and flowering time 50 , 51 , 52 , 53 , 54 , 55 , 56 .
Recent QTL mapping studies for downy mildew resistances in two PI lines (PI 330628 or WI 7120, and PI 197088) identified eight resistance QTL, dm2.1, dm3.1, dm3.2, dm4.1, dm5.1, dm5.2, dm5.3 , and dm6.1 . Among them, dm2.1, dm4.1, dm5.2 and dm6.1 seem to be shared by the two PI lines 50 , 51 . Another cucumber accession, PI 197087, possesses multiple resistances to downy mildew (pre-2004 strain, by the dm1 locus), anthracnose (by the cla locus) and angular leaf spot (by the psl locus). Pan et al. 52 and Wang et al. 54 showed that dm1/cla/psl locus for the triple disease resistances in this PI line was controlled by the same staygreen gene ( CsSGR ), which was located in the short arm of chromosome 5 (~5 Mb in Gy14 V2.0). The several peaks on chromosome 5 detected from GWAS in this study (Supplementary Table S 5 ) seem to correspond well to dm1 , dm5.1 , dm5.2, and dm5.3 detected in Wang et al. 50 , 51 , 54 . In addition, the peak on chromosome 5 detected in GWAS for anthracnose resistance is likely the same as the dm1/cla/psl locus (CsSGR) originated from PI 197087 (ref. 52 , 54 ). However, no downy mildew QTL was detected on chromosome 4 in the natural population, whereas no QTL on chromosome 7 were detected from bi-parental mapping populations, which was identified in GWAS. These differences may reflect the power of QTL detection with different approaches. The different virulence structure of field downy mildew pathogens may also contribute to the observed differences.
QTL for GSB resistance have been recently identified in cucumber and melon. Two QTL mapping studies on GSB resistances from the wild cucumber ( C.s . var. hardwickii ) accession PI 183967 have been reported with some contradictory results 53 , 55 . Liu et al. 55 identified six QTL on chromosomes 3, 4, 5, and 6 ( gsb3.1, gsb3.2, gsb3.3, gsb4.1, gsb5.1, and gsb6.1 ) with gsb5.1 as a major QTL. On the other hand, Zhang et al. 53 identified five QTL ( gsb-s1.1, gsb-s2.1, gsb-s6.1, gsb-s6.2 , and gsb-s6.3 ) for resistance to GSB in PI 183967 with gsb-s6.2 having the largest effect. While not directly overlapping, gsb-s2.1 (ref. 53 ) and gsb5.1 (ref. 55 ) seem to be at nearby regions of the two peaks we identified from GWAS in this study on chromosomes 2 and 5, respectively, which obviously need further investigation to confirm. In addition, a recent report also identified a candidate gene for GSB resistance in melon located on chromosome 4 around 4.0 Mb 57 ; however, it does not appear to reside in syntenic regions 58 with the QTL identified this study.
QTL for flowering time was previously mapped on chromosomes 1, 2, 5, and 6 in recombinant inbred lines derived from a cross between an American pickling type and little leaf (ll) line H-19 (ref. 56 ), and on chromosomes 1, 5, and 6 in a cross between American pickling cucumber and semi-wild var. Xishuangbanna 16 . There appears to be potential overlap among the identified QTL in those studies and the current GWAS. In all three studies significant regions were located on the distal end of chromosome 1 and on the central region of chromosome 6, suggesting potentially robust loci influencing flowering time over a range of genetic backgrounds.
Genetic characterization of accessions within a germplasm collection and knowledge of their genetic relationships also enables definition of a core population, i.e., a subset of the full collection that captures the majority of diversity of the species 29 , 59 . Core collections can greatly facilitate breeding and preservation efforts by providing a common starting point for screening the population for traits of importance for crop improvement. By allowing for reduced numbers in the initial screening stages, they can be especially helpful when phenotyping a trait of interest that is particularly expensive or labor-intensive. A defined core population also can allow for more focused management of seed supplies for distribution. While core populations can be defined using geographic or phenotypic characteristics, establishment of maximally valuable core populations, relies on effective measures of genetic diversity among the accessions 29 .
A prior core of 147 accessions from the NPGS cucumber collection was proposed based on isozyme analysis of 970 PIs, along with data regarding disease resistance (angular leaf spot, anthracnose, downy mildew, rhizoctonia fruit rot, and target leaf spot), water and heat stress tolerance, and morphological characteristics 45 . Current next-generation sequencing technology allows for more robust genotypic assessment. From the analyses performed here, we have designed a core collection of 354 accessions that represent 96% of the genetic variation present in the NPGS. Approximately half (76) of the PIs from the prior core 45 were included in the current core collection. It has also been recommended that germplasm collections include important breeding materials where key traits have been introgressed into cultivated inbred lines 4 , 29 . To this end, the proposed core also includes 41 accessions, including historical cultivars, widely used breeding lines and individuals with identified traits of interest. To make the core maximally valuable for future breeding efforts and genetic studies, we are in the process of deep resequencing of the genomes, and creating seed stocks of the selected accessions in the final core collection, under the current USDA CucCAP project ( https://cuccap.org/ ). Both the genotype data and seeds of the core collection will be accessible to the public.
Conclusions
This work has provided detailed genetic analysis of the cucumber germplasm collection maintained by the US NPGS, which includes more than 1200 accessions collected throughout the world. The information provided by the GBS data has provided deep insight into the diversity present within the collection and genetic relationships among the accessions. These data can be used for genetic analyses such as GWAS to identify potential genomic regions associated with valuable traits, and for informed management of the collection to conserve genetic resources. Development of the genetically informed core collection will enable more efficient genetic analyses that can be coupled with sophisticated genomic tools to facilitate crop improvement. While it is clear that a great deal of valuable diversity is represented among the materials in the NPGS collection, these observations also illustrate the importance of careful and extensive germplasm collection to ensure that our collections reflect the extant diversity available worldwide.
Data availability
Raw GBS reads for all individual cucumber accessions have been deposited in the NCBI sequence read archive (SRA) under accession numbers SRP149275 and SRP149431. Raw and filtered SNPs in VCF format are available at ftp://cucurbitgenomics.org/pub/cucurbit/GBS_SNP/cucumber.
Tyagi, R. K. & Agrawal, A. Revised genebank standards for management of plant genetic resources. Indian. J. Agric. Sci. 85 , 157–165 (2015).
Google Scholar
Davies, L. R. & Allender, C. J. Who is sowing our seeds? A systematic review of the use of plant genetic resources in research. Genet. Resour. Crop Evol. 64 , 1999–2008 (2017).
Article CAS Google Scholar
Fu, Y. B. The vulnerability of plant genetic resources conserved ex situ. Crop Sci. 57 , 2314–2328 (2017).
Article Google Scholar
Grumet, R., Garcia-Mas, J. & Katzir, N. Cucurbit genetics and genomics: a look to the future. In Genetics and Genomics of the Cucurbitaceae (eds Grumet, R., Katzir, N., Garcia-Mas, J.). pp 409–415 (Springer, 2017).
Renner, S. S., Schaefer, H. & Kocyan, A. Phylogenetics of Cucumis (Cucurbitaceae): Cucumber ( C. sativus ) belongs in an Asian/Australian clade far from melon ( C. melo ). BMC Evol. Biol. 7 , 58 (2007).
Article PubMed PubMed Central CAS Google Scholar
Sebastian, P. M., Schaefer, H., Telford, I. R. H. & Renner, S. S. Cucumber and melon have their wild progenitors in India, and the sister species of Cucumis melo is from Australia. Proc. Nat. Acad. Sci. USA 107 , 14269–14273 (2010).
Article PubMed Google Scholar
McCreight, J. D., Staub, J. E., Wehner, T. C. & Dhillon, N. P. S. Gone global: familiar and exotic cucurbits have Asian origins. HortScience 48 , 1078–1089 (2013).
Naegele, R. P. & Wehner, T. C. Genetic resources of cucumber. In Genetics and genomics of the Cucurbitacae (eds Grumet, R., Katzir, N., Garcia-Mas, J.). pp 61–86 (Springer, 2016).
Lv, J. et al. Genetic diversity and population structure of cucumber ( Cucumis sativus L.). PLoS. One. 7 , e46919 (2012).
Qi, J. et al. A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat. Genet. 45 , 1510–1515 (2013).
Article PubMed CAS Google Scholar
Yang, L. M. et al. Chromosome rearrangements during domestication of cucumber as revealed by high-density genetic mapping and draft genome assembly. Plant J. 71 , 895–906 (2012).
Kirkbride, J. H. Biosystematic monograph of the genus Cucumis (Cucurbitaceae) . (Parkway Publishers, Boone, NC, 1993).
Staub, J. E., Serquen, F. C., Horejsi, T. & Chen, J. Genetic diversity in cucumber ( Cucumis sativus L.): IV. An evaluation of Chinese germplasm. Genet. Resour. Crop Evol. 46 , 297–310 (1999).
Simon, P. W. & Navazio, J. P. Early orange mass 400, early orange mass 402, and late orange mass 404: High-carotene cucumber germplasm. HortScience 32 , 144–145 (1997).
CAS Google Scholar
Bo, K. L., Ma, Z., Chen, J. F. & Weng, Y. Molecular mapping reveals structural rearrangements and quantitative trait loci underlying traits with local adaptation in semi-wild Xishuangbanna cucumber ( Cucumis sativus L. var. xishuangbannanesis Qi et Yuan). Theor. Appl. Genet. 128 , 25–39 (2014).
Pan, Y. et al. QTL mapping of domestication and diversifying selection related traits in round-fruited semi-wild Xishuangbanna cucumber ( Cucumis sativus L. var. xishuangbannanesis ). Theor. Appl. Genet. 130 , 1531–1548 (2017).
Call, A. D., Criswell, A. D., Wehner, T. C., Ando, K. & Grumet, R. Resistance of current cucumber cultivars to a new strain of cucurbit downy mildew. HortScience 47 , 171–178 (2012).
Block, C. & Reitsma, K. R. Powdery mildew resistance in the U.S. national plant germplasm system cucumber collection. HortScience 40 , 416–420 (2005).
Colle, M., Straley, E. N., Makela, S. B., Hammar, S. A. & Grumet, R. Screening the cucumber plant introduction collection for young fruit resistance to Phytophthora capsici . HortScience 49 , 244–249 (2014).
Gevens, A. J., Ando, K., Lamour, K. H., Grumet, R. & Hausbeck, M. K. Development of a detached cucumber fruit assay to screen for resistance and effect of fruit age on susceptibility to infection by Phytophthora capsici . Plant Dis. 90 , 1276–1282 (2006).
Uchneat, M. S. & Wehner, T. C. Resistance to belly rot in cucumber identified through field and detached-fruit evaluations. J. Am. Soc. Hort. Sci. 123 , 78–84 (1998).
Wehner, T. C., Shetty, N. V. & Sloane, J. T. Field and detached-fruit screening tests for resistance to belly rot in cucumber. HortScience 38 , 149–152 (2004).
Walters, S. A., Wehner, T. C. & Barker, K. R. NC-42 and NC-43: root-knot nematode-resistant cucumber germplasm. HortScience 31 , 1246–1247 (1996).
Shetty, N. V. & Wehner, T. C. Screening the cucumber germplasm collection for fruit yield and quality. Crop Sci. 42 , 2174–2183 (2001).
Ando, K. & Grumet, R. Evaluation of altered plant architecture as a means to reduce Phytophthora capsici disease incidence on cucumber fruit. J. Am. Soc. Hort. Sci. 131 , 491–498 (2006).
Grumet, R., Barczak, M., Tabaka, C. & Duvall, R. Above-ground screening for genotypic differences in cucumber root growth in the greenhouse and field. J. Am. Soc. Hort. Sci. 117 , 1006–1011 (1992).
Meglic, V., Serquen, F. & Staub, J. E. Genetic diversity in cucumber (Cucumis sativu s L.): I. A reevaluation of the U.S. germplasm collection. Genet. Res. Crop Evol. 43 , 533–546 (1996).
Horejsi, T. & Staub, J. E. Genetic variation in cucumber (Cucumis sativu s L.) as assessed by random amplified polymorphic DNA. Genet. Resour. Crop Evol. 46 , 337–350 (1999).
Jia, J. Z., Li, H. J., Zhang, X. Y., Li, Z. C. & Qiu, L. J. Genomics-based plant germplasm research (GPGR). Crop J. 5 , 166–174 (2017).
Elshire, R. J. et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS. One. 6 , e19379 (2011).
Glaubitz, J. C. et al. TASSEL-GBS: a high capacity genotyping by sequencing analysis pipeline. PLoS. One. 9 , e90346 (2014).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25 , 1754–1760 (2009).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27 , 2156–2158 (2011).
Lee, T. H., Guo, H., Wang, X., Kim, C. & Paterson, A. H. SNPhylo, a pipeline to construct a phylogenetic tree from huge SNP data. BMC Genom. 15 , 162 (2014).
Yu, G. et al. ggtree, anrpackage for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 8 , 28–36 (2017).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81 , 559–575 (2007).
Falush, D., Stephens, M. & Pritchard, J. K. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164 , 1567–1587 (2003).
PubMed PubMed Central CAS Google Scholar
Stajich, J. E. et al. The bioperl toolkit: perl modules for the life sciences. Genome Res. 12 , 1611–1618 (2002).
Huang, X. et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 42 , 961–967 (2010).
Lippert, C. et al. FaST linear mixed models for genome-wide association studies. Nat. Methods 8 , 833–835 (2011).
Li, M. X., Yeung, J. M., Cherny, S. S. & Sham, P. C. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 131 , 747–756 (2012).
Jeong, S. et al. GenoCore: a simple and fast algorithm for core subset selection from large genotype datasets. PLoS. One. 12 , e0181420 (2017).
Whitaker, T. W. & Davis, G. N. Cucurbits: Botany, cultivation, and utilization . (Interscience Publishers, New York, 1962).
Staub, J. E., Serquen, F. C. & McCreight, J. D. Genetic diversity in cucumber ( Cucumis sativus L.): III. An evaluation of Indian germplasm. Genet. Resour. Crop Evol. 44 , 315–326 (1997).
Staub, J. E., Dane, F., Reitsma, K., Fazio, G. & Lopez-Sese, A. The formation of test arrays and a core collection in cucumber using phenotypic and molecular marker data. J. Am. Soc. Hortic. Sci. 127 , 558–567 (2002).
Walters, T. W. Historical overview on domesticated plants in China with special emphasis on the Cucurbitaceae. Econ. Bot. 43 , 297–313 (1989).
Paris, H. S., Daunay, M. C. & Janick, J. Occidental diffusion of cucumber ( Cucumis sativus ) 500-1300 CE: two routes to Europe. Ann. Bot. 109 , 117–126 (2012).
Pandey, S., Ansari, W. A., Mishra, V. K., Singh, A. K. & Singh, M. Genetic diversity in Indian cucumber based on microsatellite and morphological markers. Biochem. Syst. Ecol. 51 , 19–27 (2013).
Mansfeld, B. & Grumet, R. Metabolomic plasticity of cucumber fruit peel - effects of developmental stage and market class. In Proceedings of Cucurbitaceae 2016 (eds Kozik, E. U., Paris, H. S.). pp 174–179 (2016).
Wang, Y. et al. QTL mapping for downy mildew resistance in cucumber inbred line WI7120 (PI 330628). Theor. Appl. Genet. 129 , 1493–1505 (2016).
Wang, Y., VandenLangenberg, K., Wen, C., Wehner, T. C. & Weng, Y. QTL mapping of downy and powdery mildew resistances in PI197088 cucumber with genotyping-by-sequencing in RIL population. Theor. Appl. Genet. 131 , 597–611 (2018).
Pan, J. et al. STAYGREEN (CsSGR) is a candidate for the anthracnose ( Colletotrichum orbiculare ) resistance locus cla in Gy14 cucumber. Theor. Appl. Genet. 131 , 1577–1587 (2018).
Zhang, S. P. et al. Inheritance and QTL mapping of resistance to gummy stem blight in cucumber stem. Mol. Breed. 37 , 49 (2017).
Wang, Y. H. et al. STAYGREEN, STAY HEALTHY: a loss-of-susceptibility mutation in the STAYGREEN gene provides durable, broad-spectrum disease resistances for over 50 years of US cucumber production. New Phytol . (2018). https://doi.org/10.1111/nph.15353 .
Liu, S. L. et al. Genetic analysis and QTL mapping of resistance to gummy stem blight in Cucumis sativus seedling stage. Plant Dis. 101 , 1145–1152 (2017).
Fazio, G., Staub, J. E. & Stevens, M. R. Genetic mapping and QTL analysis of horticultural traits in cucumber ( Cucumis sativus L.) using recombinant inbred lines. Theor. Appl. Genet. 107 , 864–874 (2003).
Hu, Z. et al. A re-sequencing-based ultra-dense genetic map reveals a gummy stem blight resistance-associated gene in Cucumis melo . DNA Res. 25 , 1–10 (2018).
Yang, L. M. et al. Next-generation sequencing, FISH mapping, and synteny-based modeling reveal mechanisms of dysploid chromosome reduction in Cucumis . Plant J. 77 , 16–30 (2004).
Brown, A. H. D. Core collections: a practical approach to genetic resources management. Genome 31 , 818–824 (1989).
Download references
Acknowledgements
We thank Dr. Jim Smith (MSU) for helpful advice regarding phylogenetic analyses and Dr. Marivi Colle (MSU) for assistance in developing the high throughput DNA extraction methods. This research was supported by grants from USDA National Institute of Food and Agriculture Specialty Crop Research Initiative (2015-51181-24285).
Author information
Authors and affiliations.
Boyce Thompson Institute, Cornell University, Ithaca, NY, 14853, USA
Xin Wang, Kan Bao, Yang Bai, Chen Jiao & Zhangjun Fei
Gus R. Douglass Institute and Department of Biology, West Virginia State University, Institute, Virginia, WV, 25112, USA
Umesh K. Reddy
Department of Horticulture, Michigan State University, East Lansing, MI, 48824, USA
Sue A. Hammar & Rebecca Grumet
Horticultural Science Department, North Carolina State University, Raleigh, NC, 27695, USA
Todd C. Wehner
Horticulture Department, University of Wisconsin, Madison, WI, 53706, USA
Axel O. Ramírez-Madera & Yiqun Weng
USDA-ARS Vegetable Crops Research Unit, Madison, WI, 53706, USA
USDA-ARS Robert W. Holley Center for Agriculture and Health, Ithaca, NY, 14853, USA
Zhangjun Fei
You can also search for this author in PubMed Google Scholar
Contributions
Z.F., R.G., and Y.W. designed and managed the project. S.A.H., C.J., A.O.R-M., and Y.W. prepared and handled samples. T.C.W. collected phenotypic data. X.W., K.B., U.K.R., and Y.B. performed data analyses. X.W., Z.F., and R.G. wrote the paper.
Corresponding authors
Correspondence to Rebecca Grumet or Zhangjun Fei .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary note and figures, supplementary file 1, supplementary tables, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Wang, X., Bao, K., Reddy, U.K. et al. The USDA cucumber ( Cucumis sativus L.) collection: genetic diversity, population structure, genome-wide association studies, and core collection development. Hortic Res 5 , 64 (2018). https://doi.org/10.1038/s41438-018-0080-8
Download citation
Received : 05 June 2018
Revised : 07 August 2018
Accepted : 08 August 2018
Published : 01 October 2018
DOI : https://doi.org/10.1038/s41438-018-0080-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A genome-wide association study reveals molecular mechanism underlying powdery mildew resistance in cucumber.
- Xuehao Chen
Genome Biology (2024)
A single nucleotide substitution introducing premature stop codon within CsTFL1 explains the determinate-2 phenotype in cucumber (Cucumis sativus L.)
- Bartosz Biernacik
- Renata Słomnicka
- Grzegorz Bartoszewski
Scientific Reports (2024)
Genetic characterization of cucumber genetic resources in the NARO Genebank indicates their multiple dispersal trajectories to the East
- Gentaro Shigita
- Koichiro Shimomura
Theoretical and Applied Genetics (2024)
ShinyCore: An R/Shiny program for establishing core collection based on single nucleotide polymorphism data
- Dong Sub Kim
Plant Methods (2023)
Complete chloroplast genome of Lilium ledebourii (Baker) Boiss and its comparative analysis: lights into selective pressure and adaptive evolution
- Morteza Sheikh-Assadi
- Roohangiz Naderi
- Vahid Shariati
Scientific Reports (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Advertisement
Molecular research progress and improvement approach of fruit quality traits in cucumber
- Published: 28 June 2021
- Volume 134 , pages 3535–3552, ( 2021 )
Cite this article

- Kiros Gebretsadik 1 , 2 na1 ,
- Xiyan Qiu 1 na1 ,
- Shaoyun Dong 1 ,
- Han Miao 1 &
- Kailiang Bo ORCID: orcid.org/0000-0001-8841-5195 1
2793 Accesses
24 Citations
4 Altmetric
Explore all metrics
Key message
Recent molecular studies revealed new opportunities to improve cucumber fruit quality. However, the fruit color and spine traits molecular basis remain vague despite the vast sources of genetic diversity.
Cucumber is agriculturally, economically and nutritionally important vegetable crop. China produces three-fourths of the world’s total cucumber production. Cucumber fruit quality depends on a number of traits such as the fruit color (peel and flesh color), spine (density, size and color), fruit shape, fruit size, defects, texture, firmness, taste, maturity stage and nutritional composition. Fruit color and spine traits determine critical quality attributes and have been the interest of researchers at the molecular level. Evaluating the molecular mechanisms of fruit quality traits is important to improve production and quality of cucumber varieties. Genes and qualitative trait locus (QTL) that are responsible for cucumber fruit color and fruit spine have been identified. The purpose of this paper is to reveal the molecular research progress of fruit color and spines as key quality traits of cucumber. The markers and genes identified so far could help for marker-assisted selection of the fruit color and spine trait in cucumber breeding and its associated nutritional improvement. Based on the previous studies, peel color and spine density as examples, we proposed a comprehensive approach for cucumber fruit quality traits improvement. Moreover, the markers and genes can be useful to facilitate cloning-mediated genetic breeding in cucumber. However, in the era of climate change, increased human population and high-quality demand of consumers, studies on molecular mechanisms of cucumber fruit quality traits are limited.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Molecular mapping and candidate gene analysis for yellow fruit flesh in cucumber

Mapping for Quality Traits

Identification and fine mapping of molecular markers closely linked to fruit spines size ss gene in cucumber ( Cucumis sativus L.)
Abbreviations.
Arabidopsis Pseudo-Resoibse Regulator
Accumulation and Replication of Chloroplasts
Qualitative Trait Locus
Simple Sequence Repeat
Proanthocyanins
Abbott JA (1999) Quality measurement of fruits and vegetables. Postharvest Biol Technol 15(3):207–225
Article Google Scholar
Abou-Zaid MM, Lombardo DA, Kite GC, Grayer RJ, Veitch NC (2001) Acylated flavones C-glycosides from Cucumis sativus . Phytochemistry 58:167–172
Article CAS PubMed Google Scholar
Abu-Reidah IM, Arráez-Román D, Quirantes-Piné R et al (2012) HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res Int 46:108–117
Article CAS Google Scholar
Ando K, Carr K, Grumet R (2012) Transcriptome analyses of early cucumber fruit growth identifies distinct gene modules associated with phases of development. BMC Genomics 13:518–533
Article CAS PubMed PubMed Central Google Scholar
Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Report 9:208–218
Balkema-Boomstra AG, Zijlstra S, Verstappen FWA et al (2003) Role of cucurbitacin C in resistance to spider mite ( Tetranychus urticae ) in cucumber ( Cucumis sativus L.). J Chem Ecol 29:225–235
Barrett D, Beaulieu JC, Shewfelt R (2010) Color, Flavor, Texture, and nutritional quality of fresh-cut fruits and vegetables: desirable levels, instrumental and sensory measurement, and the effects of processing. Crit Rev Food Sci Nutr 50:369–389
Article PubMed Google Scholar
Bo K, Song H, Shen J et al (2011) Inheritance and mapping of the ore gene controlling the quantity of beta-carotene in cucumber (Cucumis sativus L.) endocarp. Mol Breed 30(1):1–10
Google Scholar
Bo K, Ma Z, Chen J, Weng Y (2015) Molecular mapping reveals structural rearrangements and quantitative trait loci underlying traits with local adaptation in semi-wild Xishuangbanna cucumber ( Cucumis sativus L. var . xishuangbannanesis Qi et Yuan). Theor Appl Genet 128:25–39
Bo K, Wei S, Wang W, Miao H, Dong S, Zhang S, Gu X (2019a) QTL mapping and genome-wide association study reveal two novel loci associated with green flesh color in cucumber. BMC Plant Biol 19:243
Article PubMed PubMed Central CAS Google Scholar
Bo K, Miao H, Wang M et al (2019b) Novel loci fsd6.1 and Csgl3 regulate ultra-high fruit spine density in cucumber. Theor Appl Genet 132:27–40
Bungau S, Abdel-Daim MM, Tit DM et al (2019) Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid Med Cell Longev 2019:1–22
Call AD, Wehner TC (2010) Gene list for cucumber. Cucurbit Genet Coop Rpt 33–34:69–103
Cao C, Zhang S, Guo H (2001) The genetic relationship between glabrous foliage character and warty fruit of cucumber. Acta Horticulturae Sinica 28(6):565–566 ( in Chinese )
Carrara S, Pardossi A, Soldatini GF, Tognoni F, Guidi L (2001) Photosynthetic activity of ripening tomato fruit. Photosynthetica 39:75–78
Cavagnaro PF, Senalik DA, Yang L, Simon PW et al (2010) Genome-wide characterization of simple sequence repeats in cucumber (Cucumis sativus L). BMC Genom 11:569
Chen C, Liu M, Jiang L, Liu X, Zhao J, Yan S, Yang S, Ren H, Liu R, Zhang X (2014) Transcriptome profiling reveals roles of meristem regulators and polarity genes during fruit trichome development in cucumber ( Cucumis sativus L.). J Exp Bot 65:4943–4958
Chen CH, Yin S, Liu XW et al (2016) The WD-repeat protein CsTTG1 regulates fruit wart formation through interaction with the homeodomain-leucine zipper I protein Mict1. Plant Physiol 171:1156–1168
CAS PubMed PubMed Central Google Scholar
Cuevas HE, Song H, Staub JE, Simon PW (2010) Inheritance of beta-carotene-associated flesh color in cucumber ( Cucumis sativus L.) fruit. Euphytica 171:301–311
Cui J, Miao H, Ding L et al (2016) A new glabrous Gene (csgl3) identified in trichome development incucumber (Cucumis sativus L.). PLoSONE 11:e0148422
Dhaliwal MS (2017) Cucurbits. Handbook of Vegetable Crops, 3rd edn. Kalyani Publishers, New Delhi, p 92
Dixit Y, Kar A (2010) Protective role of three vegetable peels in alloxan induced diabetes mellitus in male mice. Plant Foods Hum Nutr 65:284–289
Dou J, Lu X, Ali A et al (2018) Genetic mapping reveals a marker for yellow skin in watermelon (Citrullus lanatus L.). PLoS ONE 13(9):e0200617
Duthie SJ, Duthie GG, Russell WR et al (2018) Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: a randomised trial. Eur J Nutr 57:1855–1872
Egea I, Barsan C, Bian W et al (2010) Chromoplast differentiation: current status and perspectives. Plant Cell Physiol 51:1601–1611
Esteras C, Rambla JL, Sánchez G et al (2018) Fruit flesh volatile and carotenoid profile analysis within the cucumis melo L species reveals unexploited variability for future genetic breeding. J Sci Food Agric 98(10):3915–3925
Fanourakis NE, Simon PW (1987) Inheritance and linkage studies of the fruit epidermis structure in cucumber. J Hered 78:369–371
Feder A, Burger J, Gao S et al (2015) A Kelch domain-containing FBox coding gene negatively regulates flavonoid accumulation in muskmelon. Plant Physiol 169(3):1714–1726
PubMed PubMed Central Google Scholar
Ferro-Luzzi A, Cialfa E, Leclercql C, Toti E (1994) The mediterranean diet revisited. Focus on fruit and vegetables. Int J Food Sci Nutr 45:291–300
Fiedor J, Burda K (2014) Potential role of carotenoids as antioxidants in human health and disease. Nutrients 6:466–488
Gen C, Zhang X (2018) Molecular basis of cucumber fruit domestication. Curr Opin Plant Biol 47:38–46
Gill NS, Sood S, Muthuraman A et al (2010) Antioxidant, anti-inflammatory and analgesic potential of Cucumis sativus seed extract. Lat Am J Pharm 29:927–932
Guan Y (2008) Mapping and cloning of related gene for fruit spines formation in cucumber (in Chinese with English abstract). Dissertation, Shanghai Jiaotong University
Guo P, Chang H, Li Q, Wang L, Ren Z, Ren H, Chen C (2020) Transcriptome profiling reveals genes involved in spine development during CsTTG1-regulated pathway in cucumber (Cucumis sativus L.). Plant Sci 291:110354
Hao N, Hao N, Du Y, Li H et al (2018) CsMYB36 is involved in the formation of yellow green peel in cucumber (Cucumis sativus L.). Theor Appl Genet 131(8):1659–1669
Hao N, Han D, Huang K (2020) Genome-based breeding approaches in major vegetable crops. Theor Appl Genet 133(5):1739–1752
Herbst ST (2001) The new food lover’s companion: comprehensive definitions of nearly 6,000 food, drink, and culinary terms, 3rd edn. Barron’s cooking guide, Hauppauge, New York
Hetherington SE, Smillie RM, Davies WJ (1998) Photosynthetic activities of vegetative and fruiting tissues of tomato. J Exp Bot 49:1173–1181
Hollingshead S, Kopecˇna´ J, Jackson PJ et al (2012) Conserved chloroplast open-reading frame ycf54 is required for activity of the magenisium protoporphyrin monomethylester oxidative cyclase in Synechocystis PCC 6803. J Biol Chem 287:27823–27833
Horie H, Ito H, Ippoushi K, Azuma K, Sakata Y, Igarashi I (2007) Cucurbitacin C - bitter principle in cucumber plants. JARQ-Jpn Agric Res Q 41:65–68
Hu YY, Zhang YL, Luo HH, Li W, Oguchi R, Fan DY, Chow WS, Zhang WF (2012) Important photosynthetic contribution from the non-foliar green organs in cotton at the late growth stage. Planta 235:325–336
Huang HY, Hsieh CH (2017) Genetic research on fruit color traits of the bitter gourd (Momordica charantia L). Horticult J 86(2):238–243
Huang S, Li R, Zhang Z et al (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41:1275–1281
Hui Du, Wang G, Pan J et al (2020) The HD-ZIP IV transcription factor Tril regulates fruit spine density through gene dosage effects in cucumber. J Exp Bot 71(20):6297–6310
Hutchins AE (1940) Inheritance in the cucumber. J Agric Res 60:117–128
Jakoby MJ, Falkenhan D, Mader MT et al (2008) Transcriptional profiling of mature Arabidopsis trichomes reveals that NOECK encodes the MIXTA-like transcriptional regulator MYB106. Plant Physiol 148:1583–1602
John M, John F, Jeffrey H, Chang L (2018) Principles of Food Chemistry, 4th edn. Springer, Switzerland
Kai H, Baba M, Okuyama T (2008) Inhibitory effect of Cucumis sativus on melanin production in melanoma B16 Cells by down regulation of tyrosinase expression. Planta Med 74:1785–1788
Kang S, Hwang I, Goswami G et al (2017) Molecular insights reveal Psy1, SGR, and SlMYB12 genes are associated with diverse fruit color pigments in tomato (Solanum lycopersicum L). Molecules 22(12):2180
Article PubMed Central Google Scholar
Khoo HE, Prasad KN, Kong KW et al (2011) Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules 16:1710–1738
Kohyama K, Nagata A, Tamaki Y, Sakurai N (2009) Comparison of human-bite and instrument puncture tests of cucumber texture. Postharvest Biol Technol 52:243–246
Kong KW, Khoo HE, Prasad KN et al (2010) Revealing the power of the natural red pigment lycopene. Molecules 15:959–987
Lado J, Cronje P, Alquezar B et al (2015) Fruit shading enhances peel color, carotenes accumulation and chromoplast differentiation in red grapefruit. Physiol Plantarum 154(4):469–484
Langi P, Kiokias S, Varzakas T, Proestos C (2018) Carotenoids: From plants to food and feed industries. In: Barreiro C, Barredo JL (eds) Microbial Carotenoids. Humana Press, New York, Methods in Molecular Biology, pp 57–71
Chapter Google Scholar
Lee J, Shin A, Oh JH, Kim J (2017) Colors of vegetables and fruits and the risks of colorectal cancer. World J Gastroenterol 23(14):2527–2538
Article PubMed PubMed Central Google Scholar
Lemaire-Chamley M, Petit J, Garcia V et al (2005) Changes in transcriptional profiles are associated with early fruit tissue specialization in tomato. Plant Physiol 139:750–769
Li L, Yuan H (2013) Chromoplast biogenesis and carotenoid accumulation. Arch Biochem Biophys 539:102–109
Li XJ, Wang HG, Li HB et al (2006) Awns play a dominant role in carbohydrate production during the grain-filling stages in wheat ( Triticum aestivum L). Physiol Plant 127:701–709
Li Y, Wen CL, Weng Y (2013) Fine mapping of the pleiotropic locus B for black spine and orange mature fruit color in cucumber identifies a 50 kb region containing a R2R3-MYB transcription factor. Theor Appl Genet 126:2187–2196
Li Q, Cao C, Zhang C, Zheng S, Wang Z, Wang L, Ren Z (2015) The identification of Cucumis sativus Glabrous 1 ( CsGL1 ) required for the formation of trichomes uncovers a novel function for the homeo domain leucine zipper I gene. J Exp Bot 66:2515–2526
Li J, Luan Q, Han J et al (2020) CsMYB60 directly and indirectly activates structural genes to promote the biosynthesis of flavonols and proanthocyanidins in cucumber. Hortic Res 7:103
Lim SS, Vos T, Flaxman AD et al (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2224–2260
Liu H, Meng H, Pan Y et al (2015) Fine genetic mapping of the white immature fruit color gene w to a 33.0-kb region in cucumber (Cucumis sativus L.). Theor Appl Genet 128(12):2375–2385
Liu H, Jiao JQ, Liang XJ, Liu J, Meng HW, Chen SX, Li YH, Cheng ZH (2016a) Map-based cloning, identification and characterization of the w gene controlling white immature fruit color in cucumber ( Cucumis sativus L.). Theor Appl Genet 129:1247–1256
Liu X, Bartholomew E, Cai Y, Ren H (2016b) Trichome-related mutants provide a new perspective on multicellular trichome initiation and development in cucumber ( Cucumis sativus L). Front Plant Sci 7:1187
Liu M, Zhang C, Duan L (2019) CsMYB60 is a key regulator of flavonols and proanthocyanidans that determine the colour of fruit spines in cucumber. J Exp Bot 70(1):69–84
Lu P (2016) Analysis of β-carotene biosynthesis and expression of genes related to fruit development in cucumber of Xishuangbanna [D]. Chinese Academy of Agricultural Sciences.
Lu HW, Miao H, Tian GL, Wehner TC, Gu XF, Zhang SP (2015) Molecular mapping and candidate gene analysis for yellow fruit flesh in cucumber. Mol Breed 35:64
Lun Y, Wang X, Zhang C, Yang L, Gao D, Chen H, Huang SA (2016) CsYcf54 variant conferring light green coloration in cucumber. Euphytica 208:509–517
Marín A, Ferreres F, Tomas-Barberan FA, Gil MI (2004) Characterization and quantitation of antioxidant constituents of sweet pepper ( Capsicum annuum L.). J Agric Food Chem 52:3861–3869
Article PubMed CAS Google Scholar
Miao H, Zhang S, Wang X et al (2011) A linkage map of cultivated cucumber ( Cucumis sativus L.) with 248 microsatellite marker loci and seven genes for horticulturally important traits. Euphytica 182:167–176
Minguez-Mosquera MI, Gandul-Rojas B, Garrido-Fernandez J, Gallardo-Guerrero L (1990) Pigments present in virgin olive oil. J Am Oil Chem Soc 67:192–196
Mounet F, Moing A, Garcia V et al (2009) Gene and metabolite regulatory network analysis of early developing fruit tissues highlights new candidate genes for the control of tomato fruit composition and development. Plant Physiol 149:1505–1528
Murcia MA, Jiménez AM, Martinez-Tomé M (2009) Vegetables antioxidant losses during industrial processing and refrigerated storage. Food Res Int 42:1046–1052
Oren E, Tzuri G, Vexler L, Dafna A, Meir A, Faigenboim A, Kenigswald M, Portnoy V, Schaffer AA, Levi A (2019) The multi-allelic APRR2 gene is associated with fruit pigment accumulation in melon and watermelon. J Exp Bot 70:3781–3794
Osipowski P, Pawełkowicz M, Wojcieszek M et al (2020) A high-quality cucumber genome assembly enhances computational comparative genomics. Mol Genet Genom 295:177–193
Pan YP, Qu SP, Bo KL, Gao ML, Haider KR, Weng YQ (2017) QTL mapping of domestication and diversifying selection related traits in round-fruited semi-wild Xishuangbanna cucumber ( Cucumis sativus , L. var. xishuangbannanesis ). Theor Appl Genet 130:1531–1548
Pan J, Zhang L, Chen G et al (2021) Study of micro-trichome (mict) reveals novel connections between transcriptional regulation of multicellular trichome development and specific metabolism in cucumber. Hortic Res 8:21
Pawełkowicz ME, Skarzyńska A, Sroka M, Szwacka M, Pniewski T, Pląder W (2020) Effect of transgenesis on mRNA and miRNA profiles in cucumber fruits expressing Thaumatin II. Genes 11:334
Article PubMed Central CAS Google Scholar
Pawełkowicz ME, Skarzyńska A, Mróz T, Bystrzycki E, Pląder W (2021) Molecular insight into somaclonal variation phenomena from transcriptome profiling of cucumber ( Cucumis sativus L.) lines. Plant Cell Tiss Organ Cult 145:239–259
Peterson GC, Pike LM (1992) Inheritance of green mature seed-stage fruit color in Cucumis sativus L. J Am Soc Hortic Sci 117:643–645
Qi J, Liu X, Shen D, Miao H, Xie B, Li X, Zeng P, Wang S, Shang Y, Gu X et al (2013) A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat Genet 45:1510–1515
Rett-Cadman S, Colle M, Mansfeld B et al (2019) QTL and transcriptomic analyses implicate cuticle transcription factor SHINE as a source of natural variation for epidermal traits in cucumber fruit. Front Plant Sci 10:1536
RHS (Royal Horticultural Society) (2005) Mini-color Chart. The Royal Horticultural Society, London
Schlosser J, Olsson N, Weis M, Reid K, Peng F, Lund S, Bowen P (2008) Cellular expansion and gene expression in the developing grape ( Vitis vinifera L.). Protoplasma 232:255–265
Sebastian P, Schaefer H, Telford IR, Renner SS (2010) Cucumber ( Cucumis sativus ) and melon ( C. melo ) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc Natl Acad Sci USA 107:14269–14273
Shan N, Gan Z, Nie J, Liu H, Wang Z, Sui X (2020) Comprehensive characterization of fruit volatiles and nutritional quality of three cucumber (Cucumis sativus L.) genotypes from different geographic groups after bagging treatment. Foods 9(3):294
Article CAS PubMed Central Google Scholar
Shang Y, Ma Y, Zhou Y et al (2014) Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 346:1084–1088
Shen D, Li X, Fang Z et al (2009) Analysis on the nutrition components in the fruits of Cucumis sativus L. var. xishuangbannanesis during maturation. J Plant Genetic Res 10:594–598 (In Chinese)
Shareck M, Rousseau MC, Koushik A, Siemiatycki J, Parent ME (2017) Inverse association between dietary intake of selected carotenoids and vitamin C and risk of lung cancer. Front Oncol 7:23
Shendure J, Mitra RD, Varma C, Church GM (2004) Advanced sequencing technologies: methods and goals. Nat Rev Genet 5:335–344
Shimomura K, Fukino N, Sugiyama M et al (2017) Quantitative trait locus analysis of cucumber fruit morphological traits based on image analysis. Euphytica 213:138
Simon PW, Navazio JP (1997) Early orange mass 400, early orange mass 402, and late orange mass 404: high-carotene cucumber germplasm. Hort Sci 32:144–145
CAS Google Scholar
Slavin JL, Lloyd B (2012) Health benefits of fruits and vegetables. Adv Nutr 3:506–516
Song H, Chen JF, Staub J, Simon P (2010) QTL Analyses of orange color and carotenoid content and mapping of carotenoid biosynthesis genes in cucumber (Cucumis sativus L.). Acta Hortic 871:607–614
Sui X, Shan N, Hu L et al (2017) The complex character of photosynthesis in cucumber fruit. J Exp Bot 7:1625–1637
Tadmor Y, Burger J, Yaakov I et al (2010) Genetics of flavonoid, carotenoid, and chlorophyll pigments in melon fruit rinds. J Agric Food Chem 58:10722–10728
Takyi EK (2001) Bioavailability of Carotenoids from Vegetables versus Supplements. In: Watson RR (ed) Vegetables, Fruits, and Herbs in Health Promotion. CRC Press, Danvers, pp 19–31
Tang HY, Dong X, Wang JK et al (2018) Fine mapping and candidate gene prediction for white immature fruit skin in cucumber (Cucumis sativus L.). Int J Mol Sci 19:1493
Tian N, Liu F, Wang P et al (2018) Overexpression of BraLTP2, a lipid transfer protein of Brassica napus, results in increased trichome density and altered concentration of secondary metabolites. Int J Mol Sci 19:1733
Vicente VJ, Peiró RM, Pérez-de-Castro A, José Díez M (2018) Morphological characterization of the cucumber ( Cucumis sativus L.) collection of the COMAV’s Genebank. Genet Resour Crop Evol 65:1293–1306
Walters SA, Shetty NV, Wehner TC (2001) Segregation and linkage of several genes in cucumber. J Am Soc Hortic Sci 126:442–450
Wang J R (2012) Cloning and expression analysis of β-carotene biosynthesis related genes in cucumber (Cucumis sativus L.) [D].Chinese Academy of Agricultural Sciences.
Wang GL, Qin ZW, Zhou XY, Zhao ZY (2007) Genetic analysis and SSR markers of tuberculate trait in Cucumis sativus. Chin Bull Bot 24:168–172
Wang YL, Nie JT, Chen HM et al (2016) Identification and mapping of Tril , a homeodomainleucine zipper gene involved in multicellular trichome initiation in Cucumis sativus . Theor Appl Genet 129:305–316
Wang Y, Bo K, Gu X, Pan J, Li Y, Chen J, Wen C, Ren Z, Ren H, Chen X (2020a) Molecularly tagged genes and quantitative trait loci in cucumber with recommendations for QTL nomenclature. Hortic Res 7:1–20
Wang M, Chen L, Liang Z et al (2020b) Metabolome and transcriptome analyses reveal chlorophyll and anthocyanin metabolism pathway associated with cucumber fruit skin color. BMC Plant Biol 20:386
Wehner TC (2005) Gene list 2005 for cucumber. Cucurbit Genet Coop Rpt 28–29:105–141
Weng Y, Sun ZY (2012) Major Cucurbits. In: Wang YH, Behera TK, Kole C (eds) Genetics, genomics and breeding of cucurbits. CRC Press, New York, pp 1–16
Weng Y, Wehner TC (2017) Cucumber gene catalog 2017. Cucurbit Genet Coop Rep 40:17–54
Weng YQ, Colle M, Wang YH et al (2015) QTL mapping in multiple populations and development stages reveals dynamic quantitative trait loci for fruit size in cucumbers of different market classes. Theor Appl Genet 128(9):1747–1763
Xie J, Wehner TC (2001) Gene list 2001 for cucumber. Cucurbit Genet Coop Rep 24:110–136
Xie Q, Liu PN, Shi LX, Miao H, Bo KL, Wang Y et al (2018) Combined fine mapping, genetic diversity, and transcriptome profiling reveals that the auxin transporter gene ns plays an important role in cucumber fruit spine development. Theor Appl Genet 131:1239–1252
Xu HL, Gauthier L, Desjardins Y, Gosselin A (1997) Photosynthesis in leaves, fruits, stem and petioles of greenhouse-grown tomato plants. Photosynthetica 33:113–123
Xue S, Dong M, Liu X et al (2019) Classification of fruit trichomes in cucumber and effects of plant hormones on type II fruit trichome development. Planta 249:407–416
Yang SL, Pu H, Liu PY, Walters TW (1991) Preliminary studies on Cucumis sativus var. xishuangbannanesis [sic]. Cucurbit Genet Coop Rep 14:29–31
Yang F, Li L, Li M, Liu L, Ren H (2009) Genetic diversity and phylogenetic relationship of Chinese warty cucumber germplasm based on AFLPs. Acta Agric Boreal Occident Sin 18:205–211 ( in Chinese )
Yang SJ, Miao H, Zhang SP, Cheng ZC (2011) Genetic analysis and mapping of gl-2 gene in cucumber (Cucumis sativus L). Acta Horti Sin 38:1685–92 ( In Chinese )
Yang L, Koo DH, Li Y, Zhang X, Luan F, Havey MJ, Jiang J, Weng Y (2012) Chromosome rearrangements during domestication of cucumber as revealed by high-density genetic mapping and draft genome assembly. Plant J 71:895–906
Yang X, Zhang W, He H et al (2014) High-resolution mapping of the dull fruit skin gene D in cucumber (Cucumis sativus L). Mol Breed 33:15–22
Yang X, Li ZW et al (2014a) Fine mapping of the uniform immature fruit color gene u in cucumber ( Cucumis sativus L.). Euphytica 196:341–348
Yang X, Zhang W, He H et al (2014c) Tuberculate fruit gene Tu encodes a C2H2 zinc finger protein that is required for the warty fruit phenotype in cucumber (Cucumis sativus L.). Plant J 78:1034–1046
Yang S, Cai YL, Liu XW, Dong MM, Zhang YQ, Chen SY et al (2018) Csmyb6 - cstry module regulates fruit trichome initiation in cucumber. J Exp Bot 69:1887–1902
Yang S, Wen C, Liu B et al (2019) A CsTu-TS1 regulatory module promotes fruit tubercule formation in cucumber. Plant Biotechnol J 17(1):289–301
Yeats TH, Rose JC (2013) The formation and function of plant cuticles. Plant Physiol 163:5–20
Yu B, Zheng ZH, Kong LY, Xiao SG (2007) Standardization and quality control in cucumber processing. Chin Agri Sci Bull 23(3):109–112
Yuan XJ, Pan JS, Cai R, Guan Y, Liu LZ et al (2008) Genetic mapping and QTL analysis of fruit and flower related traits in cucumber ( Cucumis sativus L.) using recombinant inbred lines. Euphytica 164:473–491
Yuan H, Zhang J, Nageswaran D, Li L (2015) Carotenoid metabolism and regulation in horticultural crops. Hortic Res 2:15036
Yundaeng C, Somta P, Tangphatsornruang S et al (2015) A single base substitution in BADH/AMADH is responsible for fragrance in cucumber ( Cucumis sativus L.), and development of SNAP markers for the fragrance. Theor Appl Genet 128:1881–1892
Zhang W, He H, Guan Y et al (2010) Identification and mapping of molecular markers linked to the tuberculate fruit gene in the cucumber ( Cucumis sativus L). Theor Appl Genet 120:645–654
Zhang XD, Allan AC et al (2011) Differential gene expression analysis of yunnan red pear, pyrus pyrifolia, during fruit skin coloration. Plant Mol Biol Rep 29(2):305–314
Zhang S, Liu S, Miao H, Wang M, Liu P, Wehner T, Gu X (2016a) Molecular mapping and candidate gene analysis for numerous spines on the fruit of cucumber. J Hered 107(5):471–477
Zhang HY, Wang LN, Zheng SS et al (2016b) A fragment substitution in the promoter of CsHDZIV11/CsGL3 is responsible for fruit spine density in cucumber ( Cucumis sativus L). Theor Appl Genet 129:1289–1301
Zhang S, Miao H, Song Z et al (2017) Molecular mapping and candidate gene analysis for fruit epidermal structure in cucumber. Plant Breed 136:767–774
Zhang W, Chen Y, Zhou P et al (2018) Identification and fine mapping of molecular markers closely linked to fruit spines size ss gene in cucumber ( Cucumis sativus L.). Euphytica 214:213
Zhang C, Win KT, Kim YC, Lee S (2019) Two types of mutations in the HEUKCHEEM gene functioning in cucumber spine color development can be used as signatures for cucumber domestication. Planta 250:1491–1504
Zhang Y, Shen J, Bartholomew E, Don M, Chen S, Yin S, Zhai X, Feng Z, Ren H, Liu X (2021) TINY BRANCHED HAIR functions in multicellular trichome development through an ethylene pathway in Cucumis sativus L. Plant J 106(3):753–765
Zhao JL, Pan JS, Guan Y et al (2015a) Micro-trichome as a class I homeodomainleucine zipper gene regulates multicellular trichome development in Cucumis sativus . J Integr Plant Biol 57:925–935
Zhao JL, Pan JS, Guan Y et al (2015b) Transcriptome analysis in Cucumis sativus identifies genes involved in multicellular trichome development. Genomics 105(5–6):296–303
Zhao L, Zhu H, Zhang K et al (2020) The MIXTA-LIKE transcription factor CsMYB6 regulates fruit spine and tubercule formation in cucumber. Plant Sci 300:110636
Zhou Q, Wang S, Hu B, Chen H, Zhang Z, Huang S (2015) An ACCUMULATION AND REPLICATION OF CHLOROPLASTS 5 gene mutation confers light green peel in cucumber. J Integr Plant Biol 57:936–942
Zhou Y, Ma Y, Zeng J et al (2016) Convergence and divergence of bitterness biosynthesis and regulation in Cucurbitaceae . Nat Plants 2:16183
Download references
Acknowledgements
This work was supported by the Earmarked Fund for Modern Agro–industry Technology Research System [CARS–23], Science and Technology Innovation Program of the Chinese Academy of Agricultural Science (CAAS-ASTIP-IVFCAAS), the National Natural Science Foundation of China (31972413), and the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, Ministry of Agriculture, China.
Author information
Kiros Gebretsadik and Xiyan Qiu have contributed equally to this work.
Authors and Affiliations
Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing, China
Kiros Gebretsadik, Xiyan Qiu, Shaoyun Dong, Han Miao & Kailiang Bo
Department of Plant Science, Aksum University, Shire Campus, Shire, Ethiopia
Kiros Gebretsadik
You can also search for this author in PubMed Google Scholar
Contributions
KB initiated the idea and drew the figures. KG, XQ and KB conducted literature review and wrote the manuscript. SD and HM provided critical comments on the manuscript. All authors reviewed and approved the final submission.
Corresponding author
Correspondence to Kailiang Bo .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Rajeev K. Varshney.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 103 KB)
Rights and permissions.
Reprints and permissions
About this article
Gebretsadik, K., Qiu, X., Dong, S. et al. Molecular research progress and improvement approach of fruit quality traits in cucumber. Theor Appl Genet 134 , 3535–3552 (2021). https://doi.org/10.1007/s00122-021-03895-y
Download citation
Received : 26 January 2021
Accepted : 21 June 2021
Published : 28 June 2021
Issue Date : November 2021
DOI : https://doi.org/10.1007/s00122-021-03895-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fruit color
- Molecular improvement
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Cucumber is a one of healthy fruit which have many health benefits of cucurbitaceae family. It maintains blood pressure, regulates hydration, controls sugar, and soothes the skin, helpful in...
Consequently, numerous studies have been conducted to discover the miracle of cucumber. The book will cover the extensive benefits, production and market, cultivation and management, pests and diseases, breeding progress of cucumber.
Here, we first summarize the achievements of conventional cucumber breeding such as crossing and mutagenesis, and then focus on the current status of molecular breeding of cucumber in China ...
The present study deals with the antimicrobial activity and phytochemical screening of seeds extract of five plants of Cucurbitaceae family-Momordica charantia (Karella), Cucumis sativa...
Different types of cucumbers that vary in these morphological traits are preferred throughout the world. Numerous studies in recent years have added greatly to our understanding of cucumber fruit development and have identified a variety of genetic factors leading to extensive diversity.
In this review, we summarize recent findings on the inheritance, molecular markers, and quantitative trait locus mapping of cucumber PM, DM, and FM resistance. In addition, several candidate genes, such as PM, DM, and FM resistance genes, with or without functional verification are reviewed.
In this review, we describe the formation mechanism of cucumber fruit quality from three aspects: (1) the commercial quality of cucumber fruit, (2) nutritional quality formation, and (3) flavor quality of cucumber fruit.
Genetic analysis of the US cucumber collection provides valuable insights into the plant’s diversity and generates a core resource for future research.
Recent molecular studies revealed new opportunities to improve cucumber fruit quality. However, the fruit color and spine traits molecular basis remain vague despite the vast sources of genetic diversity. Cucumber is agriculturally, economically and nutritionally important vegetable crop.
In this review, various technologies used for the improvement of cucumber crop are discussed. The current eminence of improvement in cucumber crop over a period of time for various traits like sex expression, disease resistance, quality improvement, genetic engineering are described in detail.