
- Book Solutions
- State Boards

Case Study Questions Class 10 Science Acids, Bases and Salts
Case study questions class 10 science chapter 2 acids, bases and salts.

CBSE Class 10 Case Study Questions Science Acids, Bases and Salts. Important Case Study Questions for Class 10 Board Exam Students. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Acids, Bases and Salts.
At Case Study Questions there will given a Paragraph. In where some Important Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks, 4 marks.
CBSE Case Study Questions Class 10 Science Acids, Bases and Salts
Case study : 1.
The reaction between carbon dioxide and calcium hydroxide (lime water), Calcium hydroxide, which is a base, reacts with carbon dioxide to produce a salt and water. Since this is similar to the reaction between a base and an acid, we can conclude that nonmetallic oxides are acidic in nature.
Based on the above paragraph answer the following questions:
i) What is the nature of Carbon dioxide?
Ans: It is a non- metallic oxide as carbon belongs to non- metals group i.e P – Block elements group 6.
ii) Give another reaction of non- metallic oxide and a base?
Ans: CO2(g) + 2NaOH(aq)→ Na2CO3 (aq) + H2O(aq)
iii) Arrange the following bases in increasing order: NaOH, Ca(OH)2 & Mg(OH)2 .
Ans: Mg(OH)2 < Ca(OH)2 < NaOH.
iv) Write the complete reaction between calcium hydroxide and carbon dioxide with physical states?
Ans: Ca(OH)2 (aq) + CO2 (g) → CaCO3 (s) + H2O(l)
v) What is the nature of non- metallic oxide?
Ans: The non- metallic oxide are acidic in nature because when they dissolved in water, they form acidic substance turning blue litmus into red.
CASE STUDY : 2
Take solutions of glucose, alcohol, hydrochloric acid, sulphuric acid, etc. n Fix two nails on a cork, and place the cork in a 100 mL beaker. Connect the nails to the two terminals of a 6 volt battery through a bulb and a switch, as shown in. Now pour some dilute HCl in the beaker and switch on the current. Repeat with dilute sulphuric acid. What do you observe? Repeat the experiment separately with glucose and alcohol solutions. What do you observe now? Does the bulb glow in all cases?
Following the above paragraph, answer the following questions;
i) What was the changes occur in case of acids i.e HCl, H2SO4?
Ans: The bulbs will start glowing as it contains hydrogen ions H+ ions (aq) as cation and Cl- or SO4^2- as anion.
ii) Why do glucose and alcohol do not conduct electricity?
Ans: They do not contains free ions neither cation nor anion. To conduct electricity, free ions are required.
iii) Why do acids do not show acidic behaviour in absence of water?
Ans: Acidic behaviour are shown by releasing of H+ ions from acids. To dissociate into H+ ions, the acids need medium i.e water.
iv) Does rain water or distilled water will conduct electricity?
Ans: Rain water will conduct electricity as it contains both positive and negative ions of different salts in it.
V) Why do aqueous solution of acids conduct electricity?
Ans: The acid contains Hydrogen ions in solutions as well as anions. Due to the presence of free ions they conduct electricity.
CASE STUDY : 3
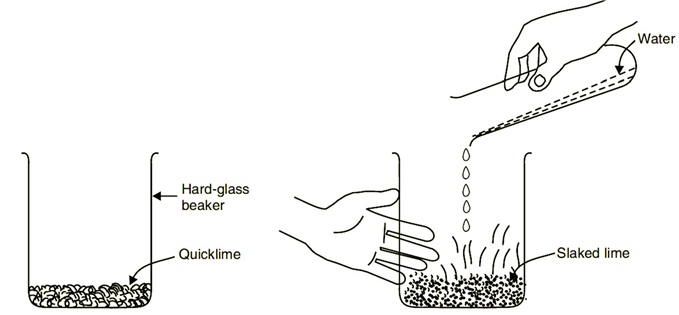
Plaster of Paris
On heating gypsum at 373 K, it loses water molecules and becomes calcium sulphate hemihydrate ( CaSO 4 .½ H 2 O ). This is called Plaster of Paris.Plaster of Paris is a white powder and on mixing with water, it changes to gypsum once again giving a hard solid mass.
Water of crystallisation is the fixed number of water molecules present in one formula unit of a salt. Five water molecules are present in one formula unit of copper sulphate. Chemical formula for hydrated copper sulphate is Cu SO4 . 5H2O. Now you would be able to answer the question whether the molecule of Na2CO3 .10H2O is wet.
Answer the following questions on the basis of the above paragraph:
i) What is the molecular formula of gypsum?
Ans: CaSO4. 2H2O
ii) Write the equation of formation of plaster of paris by heating gypsum?
Ans: CaSO4. 2H2O + heat👉 CaSO4. 1/2 H2O + 1^1/2 H2O
iii) What are the uses of Plaster of Paris?
Ans: It is used by doctor for supporting of fractured bones, to make toys etc.
iv) Give the equation when POP is mixed with water?
Ans: CaSO4. 1/2H2O + 1^1/2 H2O 👉 CaSO4. 2H2O
v) What does this 2 denotes in CaSO4. 2 H2O?
Ans: 2 denotes the two water molecules as water of crystallisation.
CASE STUDY : 4
Sodium hydroxide When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed– chlor for chlorine and alkali for sodium hydroxide.
Based on the above given information, answer the following questions:
i) Write the chemical equation involved in this process?
Ans: 2NaCl (aq)+ 2H2O(l) → 2 NaOH(aq) + Cl2 (g) + H2(g)
ii) What are the substance that are formed at anode and cathode on chlor- alkali process?
Ans: At anode Chlorine gas & at cathode hydrogen gas are formed.
iii) What are the uses of chlorine?
Ans: •Used for water treatment
Disinfectants
pesticides.
iv) Where does the sodium hydroxide solution is formed?
Ans: It is formed near the cathode.
V) What are the uses of Sodium hydroxide?
Ans: • uses in making soaps and detergents
artificial fibres
paper making
CASE STUDY : 5 (Acids Bases and Salts)
Salts of a strong acid and a strong base are neutral with pH value of 7. On the other hand, salts of a strong acid and weak base are acidic with pH value less than 7 and those of a strong base and weak acid are basic in nature, with pH value more than 7.
Answer the following in reference to the above paragraph:
i) Classify the following as strong bases and weak bases: KOH, NaOH, CsOH, NH4OH
Ans: Strong bases: KOH, NaOH, CsOH
Weak base: NH4OH
ii) Write a reaction of a strong acid and a weak base?
Ans- HCl(aq) + NH4OH(aq) → NH4Cl(aq) + H2O(aq)
iii) What happens when strong acids and bases react to each other? Explain by giving example.
Ans: HCl + NaOH → NaCl + H2O
i.e neutral salt is formed.
iv) Identify the following as strong acid: CH3COOH, H2SO4, HNO3, H3PO4, H2CO3, HCl.
Ans: Strong acid: HCl, HNO3, H2SO4.
v) Classify the following acis as strong or weak acid: acetic acid, citric acid, tartaric acid, oxalic acid.
Ans: All are weak acid present in fruits and vegetables.
CASE STUDY : 6
A scale for measuring hydrogen ion concentration in a solution, called pH scale has been developed. The p in pH stands for ‘potenz’ in German, meaning power. On the pH scale we can measure pH generally from 0 (very acidic) to 14 (very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower is the pH value.
Answer the following on the basis of above paragraph:
i) What does the scale represent when pH value increases from 7 to 14?
Ans: It represents an increase in OH- ions concentration in the solution i.e the increment in the strength of alkali.
ii) What is the pH value of milk of magnesia?
Ans: It is 10.
iii) What are the important of pH in everyday life?
Ans: We human beings, plants and animals all are sensitive to pH i.e their body work on normal pH such as plants grow between the pH range of 6 to 8. Our human body work within the pH range of 7 to 7.8.
iv) What happens when the pH of mouth is lower than 5.5?
Ans: Tooth decay starts in which the enamel gets corroded due to the much production of acids in mouth by bacteria.
V) Two solutions X&Y. The pH of X is 4 and the pH of Y is 7. What is the nature of two solution?
Ans: Solution X is acidic in nature and the solution Y is neutral in nature.
For more update follow net explanations page
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
Sikkim scert class 5 english chapter 6a picture reading solution, rd sharma class 9 solution pdf chapter 4 algebraic identities, rd sharma class 9 solution pdf chapter 3 rationalisation, rd sharma class 9 solution pdf chapter 2 exponents of real numbers.
Sign in to your account
Username or Email Address
Remember Me

Case Study Questions Class 10 Science Chapter 2 Acids, Bases, and Salts
- Post author: studyrate
- Post published:
- Post category: class 10th
- Post comments: 0 Comments
CBSE Board Exam is on the way, so you must practice some good Case Study Questions Class 10 Science to boost your preparation to score 95+% on Boards. In this post, you will get Case Study and Passage Based Questions that will come in CBSE Class 10 Science Board Exams.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Re a son . There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Acids, Bases, and Salts Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 2 Acids, Bases, and Salts
Case Study/Passage-Based Questions
Case Study 1:
A compound, X of sodium forms a white powder. It is a constituent of baking powder and is used in some antacids. When heated it gives a compound, Y which is anhydrous and absorbs water to become a hydrated salt. When this salt is kept in the open air, it loses water molecules in a process called efflorescence. When dissolved in water it forms a strong base and a weak acid, Z.
(i) What is the compound, X?
Answer: (c) NaOH.
(ii) The compound, Y is
Answer: (c) Na2CO3.10H2O
(iii) What is the nature of the solution formed by dissolving Y in water?
Answer: (a) Alkaline
(iv) Identify the compound, Z.
Answer: (b) H2CO3
(v) Sodium carbonate is a basic compound because it is a salt of a
Answer: (c) strong acid and weak base
Case Study 2:
pH is quite useful to us in a number of ways in daily life. Some of its applications are:
Control of pH of the soil : Plants need a specific pH range for proper growth. The soil may be acidic, basic, or neutral depending upon the relative concentration of H* and OH-. The pH of any soil can be determined by using pH paper. If the soil is too acidic, it can be corrected by adding lime to it. If the soil is too basic, it can be corrected by adding organic manure which contains acidic materials.
Regaining shine of a tarnished copper vessel by use of acids : A copper vessel gets tarnished due to formation of an oxide layer on its surface. On rubbing lenion on the vessel, the surface is cleaned and the vessel begins to shine again. This is due to the fact that copper oxide is basic in nature, which reacts with the acid (citric acid) present in lemon to form a salt (copper citrate) which is washed away with water. As a result, the layer of copper oxide is removed from the surface of the vessel and the shining surface is exposed.
Self-defence by animals through chemical warfare : Stings of bees and ants contain methanoic acid. When stung, it causes lot of pain and irritation. This can be cured by rubbing the affected area with mild base like baking soda.
(i) When black copper oxide placed in a beaker is treated with dilute HCl, its color changes to ( a) white (b) dark red (c) bluish-green (d) no change.
Answer: (c) bluish green
(ii) P is an aqueous solution of acid and Q is an aqueous solution of base. When these two are diluted separately, then (a) pH of P increases while that of Q decreases till neutralization.
(b) pH of P decreases while that of Q increases till neutralization. (C) pH of both P and Q decrease. (d) pH of both P and Q increase.
Answer: (a) pH of P increases while that of Q decreases till neutralisation.
(iii) Which of the following acids is present in bee sting? (a) Formic acid (b) Acetic acid (c) Citric acid (d) Hydrochloric acid
Answer: (c) Citric acid
(iv) Sting of ant can be cured by rubbing the affected area with soap because (a) it contains oxalic acid which neutralises the effect of formic acid (b) it contains aluminium hydroxide which neutralises the effect of formic acid (c) it contains sodium hydroxide which neutralises the effect of formic acid (d) none of these
Answer: (c) it contains sodium hydroxide which neutralises the effect of formic acid
(v) The pH of soil X is 7.5 while that of soil Y is 4.5. Which of the two soils, should be treated with powdered chalk to adjust its pH? (a) X only (b) Y only (c) Both X and Y (d) none of these
Answer: (b) Y only
Case Study 3: Acids, bases, and salts are essential substances in our daily lives and play crucial roles in various chemical reactions and processes. Acids are sour-tasting substances that can donate hydrogen ions (H+) when dissolved in water. They turn blue litmus paper red and have a pH value less than 7. Bases, on the other hand, are bitter-tasting substances that can accept hydrogen ions or donate hydroxide ions (OH-) when dissolved in water. They turn red litmus paper blue and have a pH value greater than 7. Salts are formed when acids react with bases, resulting in the neutralization process. They are formed by the combination of positive ions (cations) from bases and negative ions (anions) from acids. Understanding the properties and uses of acids, bases, and salts is important in various applications, such as in the preparation of medicines, household cleaning agents, and agricultural practices.
What are acids? a) Substances that can donate hydrogen ions when dissolved in water b) Substances that can accept hydrogen ions when dissolved in water c) Substances that turn blue litmus paper red d) Substances with a pH value less than 7 Answer: a) Substances that can donate hydrogen ions when dissolved in water
How are bases characterized? a) Sour-tasting substances b) Substances that can donate hydrogen ions c) Substances that turn red litmus paper blue d) Substances with a pH value less than 7 Answer: c) Substances that turn red litmus paper blue
What are salts formed from? a) Acids and bases b) Acids and metals c) Bases and metals d) Bases and water Answer: a) Acids and bases
What is the pH value of acids? a) Less than 7 b) Equal to 7 c) Greater than 7 d) Variable Answer: a) Less than 7
In which applications are acids, bases, and salts commonly used? a) Preparation of medicines b) Household cleaning agents c) Agricultural practices d) All of the above Answer: d) All of the above
Hope the information shed above regarding Case Study and Passage Based Questions for Class 10 Science Chapter 2 Acids, Bases, and Salts with Answers Pdf free download has been useful to an extent. If you have any other queries about CBSE Class 10 Science Acids, Bases, and Salts Case Study and Passage Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible. By Team Study Rate
You Might Also Like
Mcq class 10 english fire and ice questions with answers english poem 2.

CBSE Class 10 Science Electricity MCQ Quiz with Answers
Mcq class 10 english footprints without feet questions with answers english chapter 5, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
CBSE NCERT Solutions
NCERT and CBSE Solutions for free
Case Study Chapter 2 Acids Bases Salts
Please refer to Chapter 2 Acids Bases Salts Case Study Questions with answers provided below. We have provided Case Study Questions for Class 10 Science for all chapters as per CBSE, NCERT and KVS examination guidelines. These case based questions are expected to come in your exams this year. Please practise these case study based Class 10 Science Questions and answers to get more marks in examinations.
Case Study Questions Chapter 2 Acids Bases Salts
Case/Passage – 1 Marble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

Question: A student added 10g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition took place?

(a) 15-20 min (b) 10-15 min (c) 5-10 min (d) 0-5 min
Question: Marble statues are corroded or stained when they repeatedly come into contact with polluted rain water.Identify the main reason.

(a) decomposition of calcium carbonate to calcium oxide (b) polluted water is basic in nature hence it reacts with calcium carbonate (c) polluted water is acidic in nature hence it reacts with calciumcarbonate (d) calcium carbonate dissolves in water to give calcium hydroxide.
Question: Gas A, obtained above is a reactant for a very important biochemical process which occurs in the presence of sunlight. Identify the name of the process – (a) Respiration (b) Photosynthesis (c) Transpiration (d) Photolysis and buildings.
Question: The substance not likely to contain CaCO 3 is (a) Dolomite (b) A marble statue (c) Calcined gypsum (d) Sea shells.
Question: Calcium oxide can be reduced to calcium, by heating with sodium metal. Which compound would act as an oxidizing agent in the above process? (a) sodium (b) sodium oxide (c) calcium (d) calcium oxide Frothing in Yamuna:
Case/Passage – 2 The primary reason behind the formation of the toxic foam is high phosphate content in the wastewater because of detergents used in dyeing industries, dhobi ghats and households.Yamuna’s pollution level is so bad that parts of it have been labelled ‘dead’ as there is no oxygen in it for aquatic life to survive.

Question: High content of phosphate ion in river Yamuna may lead to: (a) decreased level of dissolved oxygen and increased growth of algae (b) decreased level of dissolved oxygen and no effect of growth of algae (c) increased level of dissolved oxygen and increased growth of algae (d) decreased level of dissolved oxygen and decreased growth of algae
Question: Which of the following correctly represents the solutions in increasing order of their hydronium ion concentration? (a) P > Q > R > S (b) P > S > Q > R (c) S < Q < R < P (d) S < P < Q < R
Question: Which of the following statements is correct for the water with detergents dissolved in it? (a) low concentration of hydroxide ion (OH– )and high concentration of hydronium ion (H3 O +) (b) high concentration of hydroxide ion (OH–)and low concentration of hydronium ion (H 3 O+) (c) high concentration of hydroxide ion (OH–) as well as hydronium ion (H 3 O+) (d) equal concentration of both hydroxide ion (OH–) and hydronium ion (H 3 O+). The table provides the pH value of four solutions P, Q, R and S

Question: Predict the pH value of the water of river Yamuna if the reason for froth is high content of detergents dissolved in it. (a) 10-11 (b) 5-7 (c) 2-5 (d) 7
Question: If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it? (a) Treating the water with baking soda (b) Treating the water with vinegar (c) Treating the water with caustic soda (d) Treating the water with washing soda

Related Posts
Control and coordination class 10 science notes and questions.

Carbon And Its Compound Class 10 Science Notes And Questions

Sustainable Management of Natural Resources Class 10 Science Notes And Questions
CBSE Expert
Class 10 Science: Case Study Chapter 2 Acids, Bases, and Salts PDF Download
In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason . There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given.

Here we are providing you with Class 10 Science Chapter 2 Acids, Bases, and Salts Case Study Questions, by practicing this Case Study and Passage Based Questions will help you in your Class 10th Board Exam.
Case Study Chapter 2 Acids, Bases, and Salts
Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 2 Acids, Bases, and Salts
Case Study/Passage-Based Questions
Question 1:
A compound, X of sodium forms a white powder. It is a constituent of baking powder and is used in some antacids. When heated it gives a compound, Y which is anhydrous and absorbs water to become a hydrated salt. When this salt is kept in the open air, it loses water molecules in a process called efflorescence. When dissolved in water it forms a strong base and a weak acid, Z.
(i) What is the compound, X?
Answer: (c) NaOH.
(ii) The compound, Y is
Answer: (c) Na2CO3.10H2O
(iii) What is the nature of the solution formed by dissolving Y in water?
Answer: (a) Alkaline
(iv) Identify the compound, Z.
Answer: (b) H2CO3
(v) Sodium carbonate is a basic compound because it is a salt of a
Answer: (c) strong acid and weak base
Question 2:
pH is quite useful to us in a number of ways in daily life. Some of its applications are:
Control of pH of the soil : Plants need a specific pH range for proper growth. The soil may be acidic, basic, or neutral depending upon the relative concentration of H* and OH-. The pH of any soil can be determined by using pH paper. If the soil is too acidic, it can be corrected by adding lime to it. If the soil is too basic, it can be corrected by adding organic manure which contains acidic materials.
Regaining shine of a tarnished copper vessel by use of acids : A copper vessel gets tarnished due to formation of an oxide layer on its surface. On rubbing lenion on the vessel, the surface is cleaned and the vessel begins to shine again. This is due to the fact that copper oxide is basic in nature, which reacts with the acid (citric acid) present in lemon to form a salt (copper citrate) which is washed away with water. As a result, the layer of copper oxide is removed from the surface of the vessel and the shining surface is exposed.
Self-defence by animals through chemical warfare : Stings of bees and ants contain methanoic acid. When stung, it causes lot of pain and irritation. This can be cured by rubbing the affected area with mild base like baking soda.
(i) When black copper oxide placed in a beaker is treated with dilute HCl, its color changes to ( a) white (b) dark red (c) bluish-green (d) no change.
Answer: (c) bluish green
(ii) P is an aqueous solution of acid and Q is an aqueous solution of base. When these two are diluted separately, then (a) pH of P increases while that of Q decreases till neutralization.
(b) pH of P decreases while that of Q increases till neutralization. (C) pH of both P and Q decrease. (d) pH of both P and Q increase.
Answer: (a) pH of P increases while that of Q decreases till neutralisation.
(iii) Which of the following acids is present in bee sting? (a) Formic acid (b) Acetic acid (c) Citric acid (d) Hydrochloric acid
Answer: (c) Citric acid
(iv) Sting of ant can be cured by rubbing the affected area with soap because (a) it contains oxalic acid which neutralises the effect of formic acid (b) it contains aluminium hydroxide which neutralises the effect of formic acid (c) it contains sodium hydroxide which neutralises the effect of formic acid (d) none of these
Answer: (c) it contains sodium hydroxide which neutralises the effect of formic acid
(v) The pH of soil X is 7.5 while that of soil Y is 4.5. Which of the two soils, should be treated with powdered chalk to adjust its pH? (a) X only (b) Y only (c) Both X and Y (d) none of these
Answer: (b) Y only
You can also practice Class 10 Science MCQ Questions for Board Exams.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!
myCBSEguide
- Case Study Questions Class...
Case Study Questions Class 10 Science
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Download Case study questions for CBSE class 10 Science in PDF format from the myCBSEguide App . We have the new pattern case study-based questions for free download. Class 10 Science case study questions
This article will guide you through:
What are case study questions?
- Sample Papers with Case Study questions
- Class 10 Science Case Study question examples
- How to get case-based questions for free?
- How to attempt the case-based questions in Science?
Questions based on case studies are some real-life examples. The questions are asked based on a given paragraph i.e. Case Study. Usually, 4-5 questions are asked on the basis of the given passage. In most cases, these are either MCQs or assertion & reason type questions. Let’s take an example to understand. There is one paragraph on how nitrogen is generated in the atmosphere. On the basis of this paragraph, the board asks a few objective-type questions. In other words, it is very similar to the unseen passages given in language papers. But the real cases may be different. So, read this article till the end to understand it thoroughly.
What is CBE?
CBSE stands for competency-based education. The case study questions are part of this CBE. The purpose of CBE is to demonstrate the learning outcomes and attain proficiency in particular competencies.
Questions on Real-life Situations
As discussed the case study questions are based on real-life situations. Especially for grade 10 science, it is very essential to have the practical knowledge to solve such questions. Here on the myCBSEguide app, we have given many such case study paragraphs that are directly related to real-life implications of the knowledge.
Sample Papers with Case Study Questions
Class 10 Science Sample Papers with case study questions are available in the myCBSEguide App . There are 4 such questions (Q.No.17 to 20) in the CBSE model question paper. If you analyze the format, you will find that the MCQs are very easy to answer. So, we suggest you, read the given paragraph carefully and then start answering the questions. In some cases, you will find that the question is not asked directly from the passage but is based on the concept that is discussed there. That’s why it is very much important to understand the background of the case study paragraph.
CBSE Case Study Sample Papers
You can download CBSE case study sample papers from the myCBSEguide App or Student Dashboard. Here is the direct link to access it.
Case Study Question Bank
As we mentioned that case study questions are coming in your exams for the last few years. You can get them in all previous year question papers issued by CBSE for class 1o Science. Here is the direct link to get them too.
Class 10 Science Case Study Question Examples
As you have already gone through the four questions provided in the CBSE model question paper , we are proving you with other examples of the case-based questions in the CBSE class 10 Science. If you wish to get similar questions, you can download the myCBSEguide App and access the Sample question papers with case study-type questions.
Case-based Question -1
Read the following and answer any four questions: Salt of a strong acid and strong base is neutral with a pH value of 7. NaCl common salt is formed by a combination of hydrochloride and sodium hydroxide solution. This is the salt that is used in food. Some salt is called rock salt bed of rack salt was formed when seas of bygone ages dried up. The common salt thus obtained is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, and bleaching powder.
- Phosphoric acid
- Carbonic acid
- Hydrochloric acid
- Sulphuric acid
- Blue vitriol
- Washing soda
- Baking soda
- Bleaching powder
Case-based Question -2
- V 1 + V 2 + V 3
- V 1 – V 2 +V 2
- None of these
- same at every point of the circuit
- different at every point of the circuit
- can not be determined
- 20 3 Ω 203Ω
- 15 2 Ω 152Ω
Case-based Question -3
- pure strips
- impure copper
- refined copper
- none of these
- insoluble impurities
- soluble impurities
- impure metal
- bottom of cathode
- bottom of anode
How to Attempt the Case-Based Questions in Science?
Before answering this question, let’s read the text given in question number 17 of the CBSE Model Question Paper.
All living cells require energy for various activities. This energy is available by the breakdown of simple carbohydrates either using oxygen or without using oxygen.
See, there are only two sentences and CBSE is asking you 5 questions based on these two sentences. Now let’s check the first questions given there.
Energy in the case of higher plants and animals is obtained by a) Breathing b) Tissue respiration c) Organ respiration d) Digestion of food
Now let us know if you can relate the question to the paragraph directly. The two sentences are about energy and how it is obtained. But neither the question nor the options have any similar text in the paragraph.
So the conclusion is, in most cases, you will not get direct answers from the passage. You will get only an idea about the concept. If you know it, you can answer it but reading the paragraph even 100 times is not going to help you.
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- CBSE Practice Papers 2023
- Class 10 Science Sample Papers 2024
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions

2 thoughts on “Case Study Questions Class 10 Science”
Where is the answer
Class 10 Science MCQ
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.
- New QB365-SLMS
- NEET Materials
- JEE Materials
- Banking first yr Materials
- TNPSC Materials
- DIPLOMA COURSE Materials
- 5th Standard Materials
- 12th Standard Materials
- 11th Standard Materials
- 10th Standard Materials
- 9th Standard Materials
- 8th Standard Materials
- 7th Standard Materials
- 6th Standard Materials
- 12th Standard CBSE Materials
- 11th Standard CBSE Materials
- 10th Standard CBSE Materials
- 9th Standard CBSE Materials
- 8th Standard CBSE Materials
- 7th Standard CBSE Materials
- 6th Standard CBSE Materials
- Tamilnadu Stateboard
- Scholarship Exams
- Scholarships

Class 10th Science - Acids, Bases and Salts Case Study Questions and Answers 2022 - 2023
By QB365 on 09 Sep, 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 10 Science Subject - Acids, Bases and Salts, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Acids, bases and salts case study questions with answer key.
10th Standard CBSE
Final Semester - June 2015
(ii) If the pH of a solution is 8, then its [H + ] ion is
(iii) In terms of acidic strength, which one of the following is in the correct increasing order?
(iv) Which of the following compounds does not give H + ions in aqueous solution?
(v) Four solutions labelled as P, Q, Rand Shave pH values 1, 9, 3 and 13 respectively. Which of the following statements about the given solutions is incorrect?
A compound, X of sodium forms a white powder. It is a constituent of baking powder and is used in some antacids. When heated it gives a compound, Y which is anhydrous and absorbs water to become a hydrated salt. When this salt is kept in open air, it loses water molecules in a process called efflorescence. When dissolved in water it forms a strong base and a weak acid, Z. (i) What is the compound, X?
(ii) The compound, Y is
(iii) What is the nature of the solution formed by dissolving Y in water?
(iv) Identify the compound, Z.
(v) Sodium carbonate is a basic compound because it is a salt of a
Sodium chloride obtained from sea water or from lakes contains many impurities such as sulphates of sodium and magnesium along with chlorides of calcium and magnesium. The chlorides of calcium and magnesium are particularly undesirable on account of their deliquescent nature. For its purification, common salt is dissolved in minimum quantity of water to get a saturated solution from which insoluble impurities are filtered off. Then hydrogen chloride gas is passed through the saturated solution and the crystals of pure NaCl separate out. The soluble impurities remain in the mother liquor. The crystals are filtered, washed and dried. (i) Select the correct statement regarding salt NaCl.
(ii) Nature of aqueous solution of common salt is
(iv) Which of the following compounds is alkaline in aqueous medium?
(v) Some statements regarding salt NaCI are given below (I) It is prepared by chlor-alkali process (II) It is a white crystalline substance (III) It also exists in the form of rocks and is called rock salt (IV) It is a neutral salt, pH value of NaCI is 7
Chemically, Plaster of Paris (POP) is calcium sulphate hemihydrate, i.e., containing half molecule of water of crystallisation. It is represented by the formula, CaSO 4 · 1/2H 2 O. Half molecule of water of crystallisation means that one water molecule is shared by two formula units of CaSO 4. Hence, we also represent its formula as (CaSO 4 ) 2· H 2 O. The name, plaster of Paris, was given to this compound because for the first time, it was made from gypsum which was mainly found in Paris. (i) The difference of water molecules in gypsum and plaster of Paris is
(ii) Plaster of Paris hardens by
(iii) Which of the following statements is incorrect?
(iv) Select the incorrect statement with respect to gypsum
(v) Plaster of Paris is obtained by
pH is quite useful to us in a number of ways in daily life. Some of its applications are: Control of pH of the soil: Plants need a specific pH range for proper growth. The soil may be acidic, basic or neutral depending upon the relative concentration of H + and OH - . The pH of any soil can be determined by using pH paper. If the soil is too acidic, it can be corrected by adding lime to it. If the soil is too basic, it can be corrected by adding organic manure which contains acidic materials Regaining shine of a tarnished copper vessel by use of acids: A copper vessel gets tarnished due to formation of an oxide layer on its surface. On rubbing lemon on the vessel, the surface is cleaned and the vessel begins to shine again. This is due to the fact that copper oxide is basic in nature, which reacts with the acid (citric acid) present in lemon to form a salt (copper citrate) which is washed away with water. As a result, the layer of copper oxide is removed from the surface of the vessel and the shining surface is exposed (i) When black copper oxide placed in a beaker is treated with dilute HCl, its colour changes to
(ii) P is an aqueous solution of acid and Q is an aqueous solution of base. When these two are diluted separately, then
(iii) Which of the following acids is present in bee sting?
(iv) Sting of ant can be cured by rubbing the affected area with soap because
(v) The pH of soil X is 7.5 while that of soil Y is 4.5. Which of the two soils, should be treated with powdered chalk to adjust its pH?
Baking powder produces carbon dioxide on heating, so it is used in cooking to make the batter spongy. Although, baking soda also produces CO 2 on heating, but it is not used in cooking because on heating, baking soda produces sodium carbonate along with carbon dioxide. Sodium carbonate, thus, produced, makes the taste bitter. Baking powder is the mixture of baking soda and a mild edible acid. Generally, tartaric acid is mixed with baking soda to make baking powder. When baking powder is heated, NaHCO 3 decomposes to give CO 2 which makes bread and cake fluffy. Tartaric acid helps to remove bitter taste due to formation of sodium tartrate. \(2 \mathrm{NaHCO}_{3}+ \ \ \mathrm{C}_{4} \mathrm{H}_{6} \mathrm{O}_{6} \quad \longrightarrow \quad 2 \mathrm{CO}_{2}+2 \mathrm{H}_{2} \mathrm{O}+\mathrm{Na}_{2} \mathrm{C}_{4} \mathrm{H}_{4} \mathrm{O}_{6}\) Baking soda Tartaric acid Carbon dioxide Sodium tartrate (i) On passing excess CO 2 gas in aqueous solution of sodium carbonate, the substance obtained is
(ii) When sodium hydrogen carbonate is added to acetic acid, it evolves a gas. Which of the following statements are true about the gas evolved? (I) It turns lime water milky (II) It extinguishes a burning splinter (III) It dissolves in a solution of sodium hydroxide (IV) It has a pungent odour
(iii) Select the correct statement regarding sodium hydrogen carbonate.
(iv) Acetic acid was added to a solid X kept in a test tube. A colourless and odourless gas was evolved. The gas was passed through lime water which turned milky. It was concluded that
(v) Which of the following statements are correct regarding baking soda? (I) Baking soda is sodium hydrogen carbonate (II) On heating, baking soda gives sodium carbonate (III) It is used for manufacture of soap (IV) It is an ingredient of baking powder
Bleaching powder is also known as chloride of lime. It is a solid and yellowish white in colour. Bleaching powder can be easily identified by the strong smell of chlorine. When calcium hydroxide (slaked lime) reacts with chlorine, it gives calcium oxychloride (bleaching powder) and water is formed. Aqueous solution of bleaching powder is basic in nature. The material to be bleached is first passed through solution of NaOH to remove greasy matter. Then it is passed through aqueous solution of bleaching powder and very dil. HCI solution. HCI reacts with bleaching powder to liberate nascent oxygen which bleaches material. (i) Bleaching powder is used as
(ii) Bleaching powder is also known as
(iii) Bleaching powder gives smell of chlorine because it
(iv) Select the correct statement(s) regarding bleaching powder.
(v) Identify the product 'X' in the given reaction \(\mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{Cl}_{2} \longrightarrow X+\mathrm{H}_{2} \mathrm{O}\)
The preparation of washing soda is carried out through following steps: Step-I: Manufacture of sodium hydrogen carbonate: \(\mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O}+\mathrm{NH}_{3}+\mathrm{CO}_{2} \longrightarrow \ \mathrm{NaHCO}_{3}+ \ \mathrm{NH}_{4} \mathrm{Cl}\) Sodium hydrogen carbonate Step-II : Thermal decomposition of sodium hydrogen carbonate: When dry crystals of sodium hydrogen carbonate are heated strongly, they decompose to form anhydrous sodium carbonate (soda ash). \(2 \mathrm{NaHCO}_{3(s)} \longrightarrow \mathrm{Na}_{2} \mathrm{CO}_{3(s)}+\mathrm{CO}_{2(g)}+\mathrm{H}_{2} \mathrm{O}_{(g)}\) Step-III : Recrystallisation of sodium carbonate: Sodium carbonate thus obtained is recrystallised to form crystals of washing soda. \(\mathrm{Na}_{2} \mathrm{CO}_{3(s)}+ 10 \mathrm{H}_{2} \mathrm{O}_{(l)} \longrightarrow \mathrm{Na}_{2} \mathrm{CO}_{3} \cdot 10 \mathrm{H}_{2} \mathrm{O}_{(s)}\) Anhydrous Washing soda sodium carbonate (i) Some of the uses of washing soda are given below: (I) It is used for removing permanent hardness of water (II) It is used in glass industry (III) It is used in paper industry (IV) It is used in the manufacture of sodium compounds such as borax Select the correct option regarding uses of washing soda
(ii) What products will be formed along with water when sodium carbonate reacts with dilute hydrochloric acid?
(iii) Chief raw materials for the manufacture of washing soda are
(iv) What is the action of sodium carbonate on litmus paper?
(v) What products will be obtained when solution of sodium carbonate and slaked lime is heated?
"Indicator is a chemical compound which is added to the solution in very small amount to detect its acidic or basic nature:" As they show colour change in acidic and basic medium, they are also called acid-base indicators. In other words, "an acid-base indicator is that substance which possesses one colour in acidic medium and a different colour in alkaline medium:'' Indicators, basically, are coloured organic substances either extracted from plants (natural indicators) or synthesised in the laboratory (synthetic indicators). A few common acid base indicators are: Litmus, phenolphthalein, methyl orange etc. In addition to these there are some naturally occurring substances which have different smell in acidic and basic medium. These substances are called olfactory indicators. (i) Which one of the following will turn red litmus blue?
(ii) A solution turns blue litmus red. The pH of the solution is probably
(iii) A solution in test tube 'A' turns red litmus blue, evolves hydrogen gas on reaction with zinc and does not react with sodium carbonate. Whereas, solution in test tube 'B' turns blue litmus red, liberates hydrogen gas on reaction with zinc and evolves carbon dioxide gas with sodium carbonate. Identify 'A' and 'B'
(iv) Select the incorrect option
(v) Which one of the following can be used as an acid-base indicator by visually impaired student?
Acids turn blue litmus red but have no effect on red litmus. Bases turn red litmus blue but have no effect on blue litmus. The sample in which phenolphthalein remains colourless while methyl orange changes to pink/ red are acids while the samples in which phenolphthalein colour changes to pink and methyl orange changes to yellow are bases. Some observations of different sample solutions in litmus, phenolphthalein and methyl orange indicator are given in the table.
(i) Which of the following substances does not turn red litmus solution to blue?
(ii) Phenolphthalein's colour in basic medium is ___________but in acid it is ___________ .
(iii) Which of the following acids are edible? (I) Citric acid (II) Tartaric acid (III) Hydrochloric acid (IV) Carbonic acid.
(iv) The colour of methyl orange in neutral solution is
(v) Which of the following cannot act as an indicator?
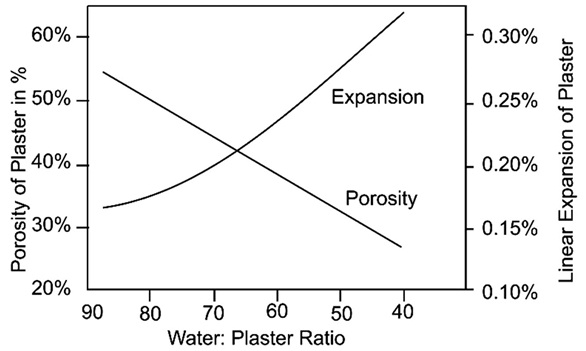
(iv) The reaction involved in the formation of white mass is:
(v) Study the following reaction and choose the correct option: \(\mathrm{CaSO}_{4} \cdot \frac{1}{2} \mathrm{H}_{2} \mathrm{O}+\frac{3}{2} \mathrm{H}_{2} \mathrm{O} \longrightarrow \mathrm{CaSO}_{4} \cdot 2 \mathrm{H}_{2} \mathrm{O}\) (a) Reactant is calcium hemihydrate, product is Gypsum (b) Reactant is Gypsum, product is calcium hemihydrate (c) Reactant is Gypsum, product is calcium sulphate hemihydrate (d) Reactant is calcium sulphate hemihydrate, product is Gypsum
Whenever a solution has a pH of less than 7, it will be an acidic solution. For example, a solution having a pH of 4 will be acidic in nature (or it will be an acid). Please note that more acidic a solution is, the lower will be its pH. For example, a solution of pH 1 is much more acidic than another solution of pH 4. In other words, a solution of pH 1 will be a much more stronger acid than another acid having pH 4 (see the figure). The solutions having pH of 0, 1, 2 and 3 are usually considered to be strong acids. And the solutions having pH of 4, 5 and 6 are considered to be weak acid solutions. It is clear that the acidity of a substance is related to its pH. Strongly acidic substances have a very low pH. In fact, lower the pH, the stronger the acid. (i) A solution turns red litmus blue. Its pH is likely to be : (a) 1 (b) 4 (c) 5 (d) 10 (ii) The pH values of six solutions A to F are given below : A = 0, B = 11, C = 6, D = 3, E = 13, F = 8 Which of the above solutions are acids (a) A, C, D (b) A, B, C (c) A, C, D, F (d) A, C, D, E (iii) Fresh milk has a pH of 6. When milk changes into curd, the pH value will : (a) become 7 (b) become less than 6 (c) become more than 7 (d) remain unchanged (iv) The pH values of three acids A, B and C having equal molar concentrations are 5.0, 2.8 and 3.5 respectively. Arrange these acids in order of the increasing acid strengths. (a) A, C, B (b) B, C, A (c) A, B, C (d) C, B, A "Lesser the pH, stronger the acid.“ Considering the above statement, the order of increasing acidic strength is A < C < B (v) A beaker of concentrated hydrochloric acid has a pH of 1. What colour will full range universal indicator turn if it is added to this beaker? (a) red (b) blue (c) pink (d) no change in colour
A group of students measured the pH of some substances they found in their homes. Their results are given in the following table :
(i) Which solution is the most acidic? (a) Apples (b) Vinegar (c) Lemon Juice (d) Black Coffee (ii) Which solution is the most alkaline? (a) Household ammonia (b) Washing soda (c) Baking soda (d) Toothpaste (iii) Which solutions are neutral? (a) Salt (b) Sugar (c) Milk (d) Both (i) and (ii ) (iv) What will be the litmus test if the solution is basic? (a) Red litmus will turn to blue (b) Blue litmus will turn to red (c) No change in colour (d) It will change into orange pink. (v) Arrange the following in order of the increasing basic strengths. Baking soda, Toothpaste, Household ammonia, Washing soda (a) Baking soda, Toothpaste, Household ammonia, Washing soda (b) Baking soda, Toothpaste, Washing soda, Household ammonia (c) Washing soda, Baking soda, Toothpaste, Household ammonia (d) Baking soda, Household ammonia, Toothpaste, Washing soda Higher the pH, the stronger the base
*****************************************
Acids, bases and salts case study questions with answer key answer keys.
(i) (c): As the pH value increases from 7 to 14, it represents decrease in H+ ion concentration in the solution. (ii) (c) : pH = -log l0 [H + ] = 8 log l0 [H + ] =-8 [H + ] = 10 - 8 mol/L (iii) (a) (iv) (b): C 2 H 5 OH is not an ionic compound, it is a covalent compound and hence does not give H + ions in aqueous solution. (v) (c) : (a) Lower the pH of the solution, more acidic is the solution and higher is the [H + ] ions Thus, solution P (pH = 1) has higher [H + ] ions than solution R (pH = 3). (b) Higher the pH of the solution, more basic is the solution and higher is the [OH - ] ions Thus, solution Q (pH = 9) has lower [OH - ] ions than solution S (pH = l3). (c) Solution P (pH = 1) is acidic which turns blue litmus solution red whereas solution Q (pH = 9) is basic which turns red litmus solution blue. (d) Solution P (pH = 1) is highly acidic while solution S (pH = l3) is highly basic and solution Q (pH = 9) is weakly basic.
(i) (d): Gypsum is CaSO 4 ·2H 2 Oand plaster of Paris is CaSO 4 \(\frac{1}{2}\) H 2 O. Difference in number of water molecules \(=\frac{3}{2}\) . (ii) (c): Plaster of Paris is hardened by combining with water. (iii) (c): Dead burnt plaster is CaSO 4 (anhydrous calcium sulphate). (iv) (d): Gypsum: CaSO 4 ·2H 2 O Plaster of paris: CaSO 4 ·1/2H 2 O (v) (d): Gypsum on heating upto lOO°C gives plaster of Paris. \(\begin{array}{c} \mathrm{CaSO}_{4} \cdot 2 \mathrm{H}_{2} \mathrm{O} \stackrel{100^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{CaSO}_{4} \cdot \frac{1}{2} \mathrm{H}_{2} \mathrm{O}+1 \frac{1}{2} \mathrm{H}_{2} \mathrm{O} { } \end{array}\) Gypsum Plaster of Paris
\(\text { (i) }(\mathrm{c}): \mathrm{CuO}+2 \mathrm{HCl} \longrightarrow \mathrm{CuCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}\) (Bluish green) (ii) (a): On diluting, H + ion concentration reduces per unit volume thus, pH increases. On the other hand, on diluting, OH - concentration also reduces, pOH increases and pH decreases. As, pOH + pH = 14. Thus, pH of Q (basic solution) decreases while that of P (acidic solution) increases on dilution (iii) (c): Formic acid is the common name of methanoic acid, and it is present in bee sting (iv) (c) (v) (b): Soil Y is acidic. Hence, it should be treated with powdered chalk to reduce its acidity
\({ (i) }(\mathrm{b}): \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2} \longrightarrow 2 \mathrm{NaHCO}_{3}\) (ii) (b) : \(\mathrm{NaHCO}_{3}+\mathrm{CH}_{3} \mathrm{COOH} \longrightarrow \mathrm{CH}_{3} \mathrm{COONa}\) \(+\mathrm{CO}_{2} \uparrow+\mathrm{H}_{2} \mathrm{O}\) Carbon dioxide gas is evolved which turns limewater milky. It extinguishes a burning splinter since it is not a supporter of combustion. It dissolves in sodium hydroxide solution and it is an odourless gas. \({ (iii) }(\mathrm{c}): 2 \mathrm{NaHCO}_{3} \stackrel{\text { Heat }}{\longrightarrow} \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}\) NaHCO 3 is soluble in water. \({ (iv) }(\mathbf{b}): \mathrm{NaHCO}_{3}+\mathrm{CH}_{3} \mathrm{COOH} \longrightarrow\) \(\mathrm{CH}_{3} \mathrm{COONa}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}\) (v) (c): It is not used in manufacture of soap .
(i) (d) (ii) (d) (iii) (b): Bleaching powder gives chlorine on exposure to air by reacting with CO 2 . \(\mathrm{CaOCl}_{2}+\mathrm{CO}_{2} \longrightarrow \mathrm{CaCO}_{3}+\mathrm{Cl}_{2}\) (iv) (d) \({ (v) }(\mathrm{a}): \mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{Cl}_{2} \longrightarrow \mathrm{CaOCl}_{2}+\mathrm{H}_{2} \mathrm{O}\)
(i) (d) (ii) (c): Na2CO 3 reacts with dilute acids to give COgas with brisk effervescence. \(\mathrm{Na}_{2} \mathrm{CO}_{3(s)}+2 \mathrm{HCl}_{(a q)} \longrightarrow 2 \mathrm{NaCl}_{(a q)}+\mathrm{H}_{2} \mathrm{O}_{(l)}\) \(+\mathrm{CO}_{2(g)} \uparrow\) Sodium Dil. Hydrochloric Sodium Water Carbon dioxide carbonate acid chloride (iii) (a): Chief raw materials for the manufacture of washing soda are sodium chloride (NaCl), ammonia (NH 3 ) and limestone (CaCO 3 ) (iv) (a): Sodium carbonate turns red litmus blue (v) (b): Sodium hydroxide and calcium carbonate are formed when the solution of sodium carbonate and slaked lime, Ca(OH) 2 is heated \(\mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{Ca}(\mathrm{OH})_{2} \longrightarrow 2 \mathrm{NaOH}+\mathrm{CaCO}_{3}\)
(i) (b) : Baking soda (NaHCO 3 ) is basic in nature. (ii) (d) : The solution turns blue litmus red, hence it is acidic. (iii) (b): Acids turn blue litmus red, liberate hydrogen gas with zinc and evolve carbon dioxide gas with metal carbonates. Bases turn red litmus blue, evolve hydrogen gas with zinc and do not react with metal carbonates (iv) (b):
(v) (c): Vanilla essence is an olfactory indicator. So, its smell is different in acidic and basic medium which can be detected easily by a visually impaired student.
(i) (c) (ii) (a) (iii) (b): Citric and tartaric acid are from organic substances such as lemon and tamarind respectively and they are edible. Hydrochloric acid though formed inside stomach is not edible. Carbonic acid is a mild acid and is edible in the form of soda water. (iv) (b) (v) (b)
(i) (b) The powder would react to moisture and turn into solid mass (ii) (c) Plaster of Paris (iii) (a) 373K (iv) (d) Crystallisation Crystallization is defined as a process by which a chemical is converted from a liquid solution into a solid crystalline state. (v) (d) Reactant is calcium sulphate hemihydrate, product is Gypsum
(i) (c) Calcium hydroxide, Ca(OH) 2 (ii) (b) Calcium hydroxide is basic in nature (iii) (c) change in temperature of mixture (iv) (b) II and III (v) \(\text { (d) } \mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}+6 \mathrm{O}_{2} \rightarrow 6 \mathrm{CO}_{2}+6 \mathrm{H}_{2} \mathrm{O}\)
(i) (d) 10 (ii) (a) A, C, D (iii) (b) become less than 6 (iv) (a) A, C, B (v) (a) red
(i) (c) Lemon Juice (ii) (a) Household ammonia (iii) (d) Both (i) and (ii) (iv) (a) Red litmus will turn to blue (v) (b) Baking soda, Toothpaste, Washing soda, Household ammonia
Related 10th Standard CBSE Science Materials
10th standard cbse syllabus & materials, cbse 10th maths probability chapter case study question with answers, cbse 10th maths statistics chapter case study question with answers, cbse 10th maths surface areas and volumes chapter case study question with answers, cbse 10th maths areas related to circles chapter case study question with answers, cbse 10th maths circles chapter case study question with answers, cbse 10th maths some applications of trigonometry chapter case study question with answers, cbse 10th maths introduction to trigonometry chapter case study question with answers, cbse 10th maths coordinate geometry chapter case study question with answers, cbse 10th maths triangles chapter case study question with answers, cbse 10th maths arithmetic progressions chapter case study questions with answers, cbse 10th maths quadratic equations chapter case study questions with answers, cbse 10th social science the making of a global world chapter case study question with answers, cbse 10th social science nationalism in india chapter case study question with answers, cbse 10th social science the rise of nationalism in europe chapter case study question with answers, cbse 10th maths pair of linear equation in two variables chapter case study question with answers.

Class VI to XII
Tn state board / cbse, 3000+ q&a's per subject, score high marks.

10th Standard CBSE Study Materials

10th Standard CBSE Subjects

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- ML Aggarwal Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- HOTS Question
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Crash Course
- JEE Previous Year Paper
- Important Info
- JEE Mock Test
- JEE Sample Papers
- SRM-JEEE Mock Test
- VITEEE Mock Test
- BITSAT Mock Test
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- COMEDK Previous Year Paper
- GUJCET Previous Year Paper
- KCET Previous Year Paper
- KEAM Previous Year Paper
- Manipal Previous Year Paper
- MHT CET Previous Year Paper
- WBJEE Previous Year Paper
- AMU Previous Year Paper
- TS EAMCET Previous Year Paper
- SRM-JEEE Previous Year Paper
- VITEEE Previous Year Paper
- BITSAT Previous Year Paper
- UPSEE Previous Year Paper
- CGPET Previous Year Paper
- CUSAT Previous Year Paper
- AEEE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam

SHARING IS CARING If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences
- Chemical Reactions and Equations 14 April, 2021, 6:02 pm
- Acids, Bases, and Salts 14 April, 2021, 6:02 pm
- Metals and Non-Metals 14 April, 2021, 6:02 pm
- Carbon and its Compounds 14 April, 2021, 6:02 pm
- Periodic Classification of Elements 14 April, 2021, 6:02 pm
- Life Processes 14 April, 2021, 6:02 pm
- How Do Organisms Reproduce 14 April, 2021, 6:02 pm
- Heredity and Evolution 14 April, 2021, 6:02 pm
- Light Reflection and Refraction 14 April, 2021, 6:02 pm

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.


- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

3.E: Acid–Base (more practice questions with answers)
- Last updated
- Save as PDF
- Page ID 292080
16.1: Acids and Bases: A Brief Review
16.2: brønsted–lowry acids and bases, conceptual problems.
- \(HSO^−_{4}\,(aq)+H_2O\,(l) \rightleftharpoons SO^{2−}_{4}\,(aq)+H_3O^{+}\,(aq)\)
- \(C_{3}H_{7}NO_{2}\,(aq)+H_{3}O^{+}\,(aq) \rightleftharpoons C_{3}H_{8}NO^{+}_{2}\,(aq)+H_{2}O\,(l)\)
- \(CH_{3}O_{2}H\,(aq)+NH_{3}\,(aq) \rightleftharpoons CH_{3}CO^{−}_{2}\,(aq)+NH^{+}_{4}\,(aq)\)
- \(SbF_{5}\,(aq)+2\,HF\,(aq) \rightleftharpoons H_{2}F^{+}\,(aq)+SbF^{−}_{6}\,(aq)\)
- \(HF\,(aq)+H_{2}O\,(l) \rightleftharpoons H_3O^{+}\,(aq)+F^{−}\,(aq)\)
- \(CH_3CH_2NH_{2}\,(aq)+H_{2}O\,(l) \rightleftharpoons CH_3CH_2NH^{+}_{3}\,(aq)+OH^{−}\,(aq)\)
- \(C_3H_7NO_{2}\,(aq)+OH^{−}\,(aq) \rightleftharpoons C_3H_6NO^{−}_{2}\,(aq)+H_{2}O\,(l)\)
- \(CH_3CO_2H\,(aq)+2\,HF\,(aq) \rightleftharpoons CH_3C(OH)_{2}^{+}\,(aq)+HF^{−}_{2}\,(aq)\)
- Salts such as NaH contain the hydride ion (\(H^−\)). When sodium hydride is added to water, it produces hydrogen gas in a highly vigorous reaction. Write a balanced chemical equation for this reaction and identify the conjugate acid–base pairs.
- \(HCO^−_{3}\,(aq)+H_2O\,(l) \rightleftharpoons CO^{2−}_{3}\,(aq)+H_3O^{+}\,(aq)\)
- \(formic\;acid\,(aq)+H_2O\,(l) \rightleftharpoons formate\,(aq)+H_3O^+\,(aq)\)
- \(H_3PO_{4}\,(aq)+H_2O\,(l) \rightleftharpoons H_2PO^−_{4}\,(aq)+H_3O^+\,(aq)\)
- \(OCH^{−}_{3}\,(aq)+H_2O\,(l) \rightleftharpoons HOCH_{3}\,(aq)+OH^{-}\,(aq)\)
- \(NH^−_{2}\,(aq)+H_2O\,(l) \rightleftharpoons NH_{3}\,(aq)+OH^{−}\,(aq)\)
- \(S^{2−}\,(aq)+H_2O\,(l) \rightleftharpoons HS^−\,(aq)+OH^−\,(aq)\)
- \(HBr\,(aq)+H_2O\,(l) \rightleftharpoons H_3O^+\,(aq)+Br^−\,(aq)\)
- \(NaH\,(s)+NH_{3}\,(aq) \rightleftharpoons H_{2}\,(g)+NaNH_{2}\,(s)\)
- \(OCH^{−}_{3}\,(aq)+NH_{3}\,(aq) \rightleftharpoons CH_{3}OH\,(aq)+NH^−_{2}\,(aq)\)
- \(NH_{3}\,(aq)+HCl\,(aq) \rightleftharpoons NH^{+}_{4}\,(aq)+Cl^−\,(aq)\)
- Species that are strong bases in water, such as \(CH_3^−\), \(NH_2^−\), and \(S^{2−}\), are leveled to the strength of \(OH^−\), the conjugate base of \(H_2O\). Because their relative base strengths are indistinguishable in water, suggest a method for identifying which is the strongest base. How would you distinguish between the strength of the acids \(HIO_3\), \(H_2SO_4\), and \(HClO_4\)?
- Is it accurate to say that a 2.0 M solution of \(H_2SO_4\), which contains two acidic protons per molecule, is 4.0 M in \(H^+\)? Explain your answer.
- The alkalinity of soil is defined by the following equation: alkalinity = \([HCO_3^−] + 2[CO_3^{2−}] + [OH^−] − [H^+]\). The source of both \(HCO_3^−\) and \(CO_3^{2−}\) is \(H_2CO_3\). Explain why the basicity of soil is defined in this way.
- Why are aqueous solutions of salts such as \(CaCl_2\) neutral? Why is an aqueous solution of \(NaNH_2\) basic?
- \(C_2H_5NH_3Cl\)
- \((CH_3)_2NH_2^+Br^−\)
- Which complex ion would you expect to be more acidic: \(Pb(H_2O)_4^{2+}\) or \(Sn(H_2O)_4^{2+}\)? Why?
- Would you expect \(Sn(H_2O)_4^{2+}\) or \(Sn(H_2O)_6^{4+}\) to be more acidic in aqueous solutions? Why?
- Is it possible to arrange the hydrides \(LiH\), \(RbH\), \(KH\), \(CsH\), and \(NaH\) in order of increasing base strength in aqueous solution? Why or why not?
Conceptual Answer
a. \(\underset{\text{acid}}{HSO^−_{4}\,(aq)} + \underset{\text{base}}{H_2O\,(l)} \rightleftharpoons \underset{\text{conjugate base}}{SO^{2−}_{4}\,(aq)} + \underset{\text{conjugate acid}}{H_3O^+\,(aq)}\)
b. \(\underset{\text{base}}{C_3H_7NO_{2}\,(aq)} + \underset{\text{acid}}{H_3O^+\,(aq)} \rightleftharpoons \underset{\text{conjugate acid}}{C_3H_8NO^+_{2}\,(aq)} + \underset{\text{conjugate base}}{H_2O\,(l)}\)
c. \(\underset{\text{acid}}{HOAc\,(aq)} + \underset{\text{base}}{NH_{3}\,(aq)} \rightleftharpoons \underset{\text{conjugate base}}{CH_3CO^−_{2}\,(aq)} + \underset{\text{conjugate acid}}{NH^+_{4}\,(aq)}\)
d. \(\underset{\text{acid}}{SbF_{5}\,(aq)} + \underset{\text{base}}{2\,HF\,(aq)} \rightleftharpoons \underset{\text{conjugate acid}}{H_2F^+\,(aq)} + \underset{\text{conjugate base}}{SbF_6^−(aq)}\)
a. \(\underset{\text{acid}}{HF\,(aq)} + \underset{\text{base}}{H_2O\,(l)} \rightleftharpoons \underset{\text{conjugate acid}}{H_{3}O^{+}\,(aq)} + \underset{\text{conjugate base}}{F^{-}\,(aq)}\)
b. \(\underset{\text{base}}{CH_{3}CH_{2}NH_{2}\,(aq)} + \underset{\text{acid}}{H_{2}O\,(l)} \rightleftharpoons \underset{\text{conjugate acid}}{CH_{3}CH_{2}NH_{3}^{+}\,(aq)} + \underset{\text{conjugate base}}{OH^{-}\,(aq)}\)
c. \(\underset{\text{acid}}{C_{3}H_{7}NO_{2}\,(aq)} + \underset{\text{base}}{OH^{-}\,(aq)} \rightleftharpoons \underset{\text{conjugate base}}{C_{3}H_{6}NO_{2}^{-}\,(aq)} + \underset{\text{conjugate acid}}{H_{2}O\,(l)}\)
d. \(\underset{\text{base}}{CH_{3}CO_{2}H\,(aq)} + \underset{\text{acid}}{2\,HF\,(aq)} \rightleftharpoons \underset{\text{conjugate acid}}{CH_{3}C(OH)_{2}^{+}\,(aq)} + \underset{\text{conjugate base}}{HF_{2}^{-}(aq)}\)
3. \(\underset{\text{base}}{NaH\,(s)} + \underset{\text{acid}}{H_{2}O\,(l)} \rightleftharpoons \underset{\text{conjugate acid}}{H_{2}\,(g)} + \underset{\text{conjugate base}}{NaOH\,(aq)}\)
a. \(K_a=\frac{[CO_{3}^{2-}][H_{3}O^{+}]}{[HCO_{3}^{-}]}\)
b. \(K_a=\frac{[formate][H_{3}O^{+}]}{[formic\,acid]}\)
c. \(K_a=\frac{[H_{2}PO_{4}^{-}][H_{3}O^{+}]}{[H_{3}PO_{4}]}\)
a. \(K_b=\frac{[CO_{3}^{2-}][H_{3}O^{+}]}{[HCO_{3}^{-}]}\)
b. \(K_b=\frac{[NH_{3}][OH^{-}]}{[NH_{2}^{-}]}\)
c. \(K_b=\frac{[HS^{-}][OH^{-}]}{[S^{2-}]}\)
6. Strong acids have the smaller \(pK_a\).
a. Equilibrium lies primarily to the right because \(HBr\) (\(pK_a=-8.7\)) is a stronger acid than \(H_{3}O^{+}\) (\(pK_a=-1.7\)) and \(H_{2}O\) (\(pK_a=14\)) is a stronger base than \(Br^-\) ( \(pK_a=-8.7\)).
b. Equilibrium lies primarily to the left because \(H_{2}\) (\(pK_a=36\)) is a stronger acid than \(NH_{3}\) (\(pK_a=38\)) and (\(NaNH_2\)) (\(pK_a=38\)) is a stronger base than \(NaH\) (\(pK_a=35\)).
c. Equilibrium lies primarily to the left because \(CH_{3}OH\) (\(pK_a=17\)) is a stronger acid than \(NH_{3}\) (\(pK_a=38\)) and \(NH_{2}^{-}\) (\(pK_a=38\)) is a stronger base than \(OCH_{3}^{-}\) (\(pK_a=25\)).
d. Equilibrium lies to the right because \(HCl\) (\(pK_a=-7\)) is a stronger acid than \(NH_{4}^{+}\) (\(pK_a=9.3\)) and \(NH_{3}\) is a stronger base than \(Cl^{-}\) (\(pK_a=-7\)).
7. To identify the strongest base we can determine their weakest conjugate acid. The conjugate acids of \(CH_{3}^{-}\), \(NH_{2}^{-}\), and \(S_{2}^{-}\) are \(CH_{4}\), \(NH_{3}\), and \(HS^{-}\), respectively. Next, we consider that acidity increases with positive charge on the molecule, thus ruling out that \(S_{2}^{-}\) is the weakest base. Finally, we consider that acidity increases with electronegativity, therefore \(NH_{3}\) is the second most basic and \(CH_{4}\) is the most basic. To distinguish between the strength of the acids \(HIO_3\), \(H_{2}SO_{4}\), and \(HClO_4\) we can consider that the higher electronegativity and oxidation state of the central nonmetal is the more acidic, therefore the order of acidity is: \(HIO_3\)<\(H_{2}SO_{4}\)<\(HClO_4\) because electronegativity and oxidation state increases as follows: \(I(+5)<S(+6)<Cl(+7)\).
8. It is not accurate to say that a 2.0 M solution of \(H_2SO_4\), which contains two acidic protons per molecule, is 4.0 M in \(H^+\) because a 2.0 M solution of \(H_2SO_4\) is equivalent to 4.0 N in \(H^+\).
\(\frac{2.0\,mol\,H_{2}SO_{4}}{1\,L} \times \frac{2\,eq\,H^{+}}{1\,mol\,H_{2}SO_{4}}=\frac{4\,eq\,H^{+}}{L}=4\,N\,H^{+}\)
9. Alkalinity is a measure of acid neutralizing capability. The basicity of the soil is defined this way because bases such as \(HCO_{3}^{-}\) and \(CO_{3}^{2-}\) can neutralize acids in soil. Because most soil has a pH between 6 and 8, alkalinity can be estimated by its carbonate species alone. At a near neutral pH, most carbonate species are bicarbonate.
10. Aqueous solutions of salts such as \(CaCl_{2}\) are neutral because it is created from hydrochloric acid (a strong acid) and calcium hydroxide (a strong base). An aqueous solution of \(NaNH_2\) is basic because it can deprotonate alkynes, alcohols, and a host of other functional groups with acidic protons such as esters and ketones.
a. \(Li_3N\) is a base because the lone pair on the nitrogen can accept a proton.
b. \(NaH\) is a base because the hydrogen has a negative charge.
c. \(KBr\) is neutral because it is formed from \(HBr\) (a strong acid) and \(KOH\) (a strong base).
d. \(C_2H_5NH_3Cl\) is acidic because it can donate a proton.
a. The pH is expected to increase. \(\underset{\text{acid}}{LiCH_{3}\,(aq)} + \underset{\text{base}}{H_2O\,(l)} \rightleftharpoons \underset{\text{conjugate base}}{LiOH\,(aq)} + \underset{\text{conjugate acid}}{CH_{4}\,(aq)}\)
b. The pH is expected to increase. \(\underset{\text{acid}}{MgCl_{2}\,(aq)} + \underset{\text{base}}{H_2O\,(l)} \rightleftharpoons \underset{\text{conjugate acid}}{2\,HCl\,(aq)} + \underset{\text{conjugate base}}{MgO\,(aq)}\)
c. The pH is expected to remain the same. \(K_{2}O\,(aq)+H_2O\,(l) \rightleftharpoons 2\,KOH\,(aq)\)
d. The pH is expected to increase. \(\underset{\text{acid}}{(CH_3)_2NH_2^+Br^−\,(aq)} + \underset{\text{base}}{H_2O\,(l)} \rightleftharpoons \underset{\text{conjugate acid}}{H_3O^{+}\,(aq)} + \underset{\text{conjugate base}}{(CH_{3})_{2}NH\,(aq)}\)
13. \(Sn(H_2O)_4^{2+}\) is expected to be more acidic than \(Pb(H_2O)_4^{2+}\) because \(Sn\) is more electronegative than \(Pb\).
14. \(Sn(H_2O)_6^{4+}\) is expected to be more acidic than \(Sn(H_2O)_4^{2+}\) because the charge on \(Sn\) is greater (\(4^+>2^+\)).
15. Yes, it is possible the order of increasing base strength is: \(LiH<NaH<RbH<CsH\) because increasing base strength is dependent on decreasing electronegativity.
Numerical Problems
- acid A: \(pK_a = 1.52\)
- acid B: \(pK_a = 6.93\)
- acid C: \(pK_a = 3.86\)
Given solutions with the same initial concentration of each acid, which would have the highest percent ionization?
- base A: \(pK_b = 13.10\)
- base B: \(pK_b = 8.74\)
- base C: \(pK_b = 11.87\)
Given solutions with the same initial concentration of each base, which would have the highest percent ionization?
- −2.50
- Benzoic acid is a food preservative with a \(pK_a\) of 4.20. Determine the \(K_b\) and the \(pK_b\) for the benzoate ion.
- Determine \(K_a\) and \(pK_a\) of boric acid \([B(OH)_3]\), solutions of which are occasionally used as an eyewash; the \(pK_b\) of its conjugate base is 4.80.
Numerical Answers
1. Acids in order of increasing strength: \(acid\,B<acid\,C<acid\,A\). Given the same initial concentration of each acid, the highest percent of ionization is acid A because it is the strongest acid.
2. Bases in order of increasing strength: \(base\,A<base\,C<base\,B\). Given the solutions with the same initial concentration of each base, the higher percent of ionization is base A because it is the weakest base.
\(pK_a+pK_b=14 \rightarrow pK_a=14-pK_b=14-3.80=10.2\)
\(K_a=10^{-pK_a}=10^{-10.2}=6.31 \times 10^{-11}\)
\(pK_a+pK_b=14 \rightarrow pK_a=14-pK_b=14-7.90=6.10\)
\(K_a=10^{-pK_a}=10^{-6.10}=7.94 \times 10^{-7}\)
\(pK_a+pK_b=14 \rightarrow pK_a=14-pK_b=14-7.90=3.000 \times 10^{-1}\)
\(K_a=10^{-pK_a}=10^{-3.000 \times 10^{-1}}=-5.012 \times 10^{-1}\)
\(pK_a+pK_b=14 \rightarrow pK_a=14-pK_b=14-1.40=12.6\)
\(K_a=10^{-pK_a}=10^{-12.6}=2.51 \times 10^{-13}\)
e. \(pK_a+pK_b=14 \rightarrow pK_a=14-pK_b=14-7.90=16.5\)
\(K_a=10^{-pK_a}=10^{-16.5}=3.16 \times 10^{-17}\)
\(pK_a+pK_b=14 \rightarrow pK_b=14-pK_a=14-4.20=9.80\)
\(K_b=10^{-pK_b}=10^{-9.80}=1.58 \times 10^{-10}\)
\(pK_a+pK_b=14 \rightarrow pK_a=14-pK_b=14-4.80=9.20\)
\(K_a=10^{-pK_a}=10^{-9.20}=6.31 \times 10^{-10}\)
16.3: The Autoionization of Water
- What is the relationship between the value of the equilibrium constant for the autoionization of liquid water and the tabulated value of the ion-product constant of liquid water (\(K_w\))?
- The density of liquid water decreases as the temperature increases from 25°C to 50°C. Will this effect cause \(K_w\) to increase or decrease? Why?
- Show that water is amphiprotic by writing balanced chemical equations for the reactions of water with \(HNO_3\) and \(NH_3\). In which reaction does water act as the acid? In which does it act as the base?
- Nitric acid is added to water.
- Potassium hydroxide is added to water.
- Calcium hydroxide is added to water.
- Sulfuric acid is added to water.
- Show that \(K\) for the sum of the following reactions is equal to \(K_w\).
\[HMnO_{4}\,(aq) \rightleftharpoons H^+\,(aq) + MnO^−_{4}\,(aq)\]
\[MnO^−_{4}\,(aq)+H_2O\,(l) \rightarrow HMnO_{4}\,(aq) + OH^−\,(aq)\]
Conceptual Answers
\[K_{auto} = \dfrac{[H_3O^+][OH^−]}{[H_2O]^2}\]
\[K_w = [H_3O^+][OH^−] = K_{auto}[H_2O]^2\]
2. This will affect \(K_w\) as it is dependent on temperature. As the temperature increases, an endothermic process occurs (energy must be absorbed to break the bonds). Consequently, according to Le Chatelier, an increase in temperature favors the forward reaction thus the position of equilibrium shifts toward the right-hand side and \(K_w\) becomes larger.
a. \(HNO_3\,(aq)+H_2O\,(l) \rightleftharpoons H_3O^{+}\,(aq)+ HNO_{2}^{-}\,(aq)\)
b. \(KOH\,(s)+H_2O\,(l) \rightleftharpoons K^{-}\,(aq)+OH^{-}\,(aq)\)
c. \(Ca(OH)_{2}\,(s)+H_2O\,(l) \rightleftharpoons Ca^{2+}\,(aq)+2\,OH^{-}\,(aq)\)
d. \(H_2SO_4\, (aq)+H_2O\,(l) \rightleftharpoons HSO_4^{-}\,(aq)+H^{+}\,(aq)\)
\(H_{2}O\,(l) \rightleftharpoons H^{+}\,(aq)+OH^{-}\,(aq)\)
\(K_w=[H^{+}][OH^{-}]\)
- The autoionization of sulfuric acid can be described by the following chemical equation: \[H_2SO_{4}\,(l)+H_2SO_{4}\,(aq) \rightleftharpoons H_3SO^+_{4}\,(aq)+HSO_{4}^{-}\,(aq)\] At 25°C, \(K = 3 \times 10^{−4}\). Write an equilibrium constant expression for \(K_{H_2SO_4}\) that is analogous to \(K_w\). The density of \(H_2SO_4\) is \(1.8\frac{g}{cm^{3}}\) at 25°C. What is the concentration of \(H_3SO_{4}^{+}\) ? What fraction of \(H_2SO_4\) is ionized?
- An aqueous solution of a substance is found to have \([H_3O]^+ = 2.48 \times 10^{−8}\; M\). Is the solution acidic, neutral, or basic?
- The pH of a solution is 5.63. What is its pOH? What is the \([OH^{−}]\)? Is the solution acidic or basic?
- \([H_3O^+] = 8.6 \times 10^{−3}\; M\)
- \([H_3O^+] = 3.7 \times 10^{−9}\; M\)
- \([H_3O^+] = 2.1 \times 10^{−7}\; M\)
- \([H_3O^+] = 1.4 \times 10^{−6}\; M\)
- 0.15 \(M\,HBr\)
- 0.03 \(M\,KOH\)
- \(2.3 \times 10^{−3}\; M\; HNO_3\)
- \(9.78 \times 10^{−2} \;M\; NaOH\)
- 0.00017 \(M\,HCl\)
- 5.78 \(M\,HI\)
- 25.0 mL of \(2.3 \times 10^{−2}\;M\;HCl\), diluted to 100 mL
- 5.0 mL of \(1.87\,M\,NaOH\), diluted to 125 mL
- 5.0 mL of \(5.98\,M\,HCl\) added to 100 mL of water
- 25.0 mL of \(3.7\,M\,HNO_3\) added to 250 mL of water
- 35.0 mL of \(0.046\,M\,HI\) added to 500 mL of water
- 15.0 mL of \(0.0087\,M\,KOH\) added to 250 mL of water.
- The pH of stomach acid is approximately 1.5. What is the \([H^+]\)?
- household bleach (11.4)
- orange juice (3.5)
- seawater (8.5)
- tomato juice (4.2)
- A reaction requires the addition of 250.0 mL of a solution with a pH of 3.50. What mass of HCl (in milligrams) must be dissolved in 250 mL of water to produce a solution with this pH?
- If you require 333 mL of a pH 12.50 solution, how would you prepare it using a 0.500 M sodium hydroxide stock solution?
\[K_{H_2SO_4}=[H_3SO_4^+][HSO_4^−]=K[H_2SO_4]_2\]
\[[H_3SO_4^+] = 0.3\,M\]
So the fraction ionized is 0.02.
2. The solution is basic because the \(pH=-log([H_3O^{+}])=-log(2.48 \times 10^{−8})=7.61>7\).
\(pH+pOH=14 \rightarrow pOH=14-pH=14-5.63=8.37\)
\([OH^{-}]=10^{-pOH}=-4.27 \times 10^{-9}\)
The \(pH=5.63<7\), therefore the solution is acidic.
a. The solution is acidic. \(pH=-log([H_3O^{+}])=-log(8.6 \times 10^{−3})=2.1<7\)
b. The solution is basic. \(pH=-log([H_3O^{+}])=-log(3.7 \times 10^{−9})=8.4>7\)
c. The solution is acidic. \(pH=-log([H_3O^{+}])=-log(2.1 \times 10^{−7})=6.7<7\)
d. The solution is acidic. \(pH=-log([H_3O^{+}])=-log(1.4 \times 10^{−6})=5.9<7\)
\(pH=-log([H_3O^{+}])=-log(0.15)=0.82\)
\(pH+pOH=14 \rightarrow pOH=14-pH=14-0.82=13\)
\(pOH=-log([OH^{-}])=-log(0.03)=2\)
\(pH+pOH=14 \rightarrow pH=14-pOH=14-2=10\)
\(pH=-log([H_3O^{+}])=-log(2.3 \times 10^{−3})=2.6\)
\(pH+pOH=14 \rightarrow pOH=14-pH=14-2.6=11\)
\(pOH=-log([OH^{-}])=-log(9.78 \times 10^{−2})=1.01\)
\(pH+pOH=14 \rightarrow pH=14-pOH=14-1.01=13.0\)
\(pH=-log([H_3O^{+}])=-log(0.00017)=3.8\)
\(pH+pOH=14 \rightarrow pOH=14-pH=14-3.8=10\)
\(pH=-log([H_3O^{+}])=-log(5.78)=-0.762\)
\(pH+pOH=14 \rightarrow pOH=14-pH=14-(-0.762)=14.8\)
a. \(25.0\,mL \times \frac{1\,L}{1,000\,mL} \times \frac{2.3 \times 10^{-2}\,mol}{1\,L} \times \frac{1}{100\,mL \times \frac{1\,L}{1,000\,mL}}=0.060\,M\,HCl\)
\(pH=-log([H_3O^{+}])=-log(0.060)=1.22\)
\(pH+pOH=14 \rightarrow pOH=14-pH=14-1.22=12.78\)
b. \(5.0\,mL \times \frac{1\,L}{1,000\,mL} \times \frac{1.87\,mol}{1\,L} \times \frac{1}{125\,mL \times \frac{1\,L}{1,000\,mL}}=7.5 \times 10^{-2}\,M\,NaOH\)
\(pOH=-log([OH^{-}])=-log(7.5 \times 10^{-2})=1.1\)
\(pH+pOH=14 \rightarrow pH=14-pOH=14-1.1=12.9\)
c. \(5.0\,mL \times \frac{1\,L}{1,000\,mL} \times \frac{5.98\,mol}{1\,L} \times \frac{1}{100\,mL \times \frac{1\,L}{1,000\,mL}}=0.20\,M\,HCl\)
\(pH=-log([H_3O^{+}])=-log(0.20)=0.70\)
\(pH+pOH=14 \rightarrow pOH=14-pH=14-0.70=13.3\)
d. \(25.0\,mL \times \frac{1\,L}{1,000\,mL} \times \frac{3.7\,mol}{1\,L} \times \frac{1}{250\,mL \times \frac{1\,L}{1,000\,mL}}=0.370\,M\,HNO_3\)
\(pH=-log([H_3O^{+}])=-log(0.370)=0.432\)
\(pH+pOH=14 \rightarrow pOH=14-pH=14-0.432=13.568\)
e. \(35.0\,mL \times \frac{1\,L}{1,000\,mL} \times \frac{0.046\,mol}{1\,L} \times \frac{1}{500\,mL \times \frac{1\,L}{1,000\,mL}}=3 \times 10^{-3}\,M\,HI\)
\(pH=-log([H_3O^{+}])=-log(3 \times 10^{-3})=2.52\)
\(pH+pOH=14 \rightarrow pOH=14-pH=14-2.52=11.48\)
f. \(15.0\,mL \times \frac{1\,L}{1,000\,mL} \times \frac{0.0087\,mol}{1\,L} \times \frac{1}{125\,mL \times \frac{1\,L}{1,000\,mL}}=5.20 \times 10^{-4}\,M\,KOH\)
\(pOH=-log([OH^{-}])=-log(5.20 \times 10^{-4})=3.28\)
\(pH+pOH=14 \rightarrow pH=14-pOH=14-3.28=10.72\)
7. \([H^+]=10^{-pH}=10^{-1.5}=3.2 \times 10^{-2}\,M\)
a. \([H^{+}]=10^{-11.4}=3.98 \times 10^{-12}\,M\)
b. \([H^{+}]=10^{-6.5}=3.2 \times 10^{-7}\,M\)
c. \([H^{+}]=10^{-3.5}=3.2 \times 10^{-4}\,M\)
d. \([H^{+}]=10^{-8.5}=3.2 \times 10^{-9}\,M\)
e. \([H^{+}]=10^{-4.2}=6.3 \times 10^{-5}\,M\)
9. 2.9 mg \(HCl\)
\([H^{+}]=10^{-pH}=10^{-3.50}=3.1622 \times 10^{-4}\,M\)
\(x\,mg\,HCl \times \frac{1\,g\,HCl}{1,000\,mg\,HCl} \times \frac{1\,mol\,HCl}{36.46\,g\,HCl} \times \frac{1}{250\,mL\,H_2O \times \frac{1\,L}{1,000\,mL\,H_2O}}=3.1622 \times 10^{-4}\,M\,HCl \rightarrow \frac{x\,mol\,HCl}{9115\,L\,HCl}=3.1622 \times 10^{-4}\,M\,HCl \rightarrow x\,mol\,HCl=3.1622\times 10^{-4}\,M \times 9115\,L\,HCl \rightarrow x\,mol\,HCl=2.9\,mol\,HCl \rightarrow x=2.9\)
10. To prepare the stock solution, \(2.11 \times 10^{-2}\,L\) of \(0.500\,M\,NaOH\) solution is required.
\(\frac{0.03162\,mol\,NaOH}{1\,L\,NaOH} \times \frac{1\,L\,NaOH}{0.5\,mol\,NaOH} \times 0.333\,L=2.11 \times 10^{-2}\,g\,NaOH\).
\([OH^{-}]=10^{-1.5}=0.03162\,M\)
\(pH+pOH=14 \rightarrow pOH=14-12.50=1.5\)
16.4: The pH Scale
16.5: strong acids and bases, 16.6: weak acids, 16.7: weak bases, 16.8: relationship between ka and kb, 16.9: acid-base properties of salt solutions, 16.10: acid-base behavior and chemical structure.
- \(CH_3CCl_2CH_2CO_2H\) versus \(CH_3CH_2CH_2CO_2H\)
- \(CH_3CO_2H\) versus \(CH_3CH_2OH\)
- \(HClO\) versus \(HBrO\)
- \(\ce{CH_3C(=O)NH_2}\) versus \(CH_3CH_2NH_2\)
- \(H_3AsO_4\) versus \(H_3AsO_3\)
- The stability of the conjugate base is an important factor in determining the strength of an acid. Which would you expect to be the stronger acid in aqueous solution—\(C_6H_5NH_3^+\) or \(NH_4^+\)? Justify your reasoning.
- Explain why \(H_2Se\) is a weaker acid than \(HBr\).
- Arrange the following in order of decreasing acid strength in aqueous solution: \(H_3PO_4\), \(CH_3PO_3H_2\), and \(HClO_3\).
- Arrange the following in order of increasing base strength in aqueous solution: \(\ce{CH_3S−}\), \(OH^−\), and \(CF_3S^−\).
- Arrange the following in order of increasing acid strength in aqueous solution: \(HClO_2\), \(HNO_2\), and \(HNO_3\).
- Do you expect \(H_2SO_3\) or \(H_2SeO_3\) to be the stronger acid? Why?
- Give a plausible explanation for why \(CF_3OH\) is a stronger acid than \(CH_3OH\) in aqueous solution. Do you expect \(CHCl_2CH_2OH\) to be a stronger or a weaker acid than \(CH_3OH\)? Why?
- Do you expect \(Cl_2NH\) or \(NH_3\) to be the stronger base in aqueous solution? Why?
a. The most important factor in determining the stronger acid is considering the inductive effect. Chlorine is an electron-withdrawing group. It pulls electron density away from the compound by means of the inductive effect through the sigma bond. In considering the conjugate base of \(CH_3CCl_2CH_2CO_2H\), Chlorine absorbs some of the electron density or excess negative charge on the oxygen atom. This causes the C bonded to the attached Chlorine atoms to be partially positive. The conjugate base of \(CH_3CCl_2CH_2CO_2H\) is more stable, thus more acidic than the conjugate base of \(CH_3CH_2CH_2CO_2H\).
b. The most important factor in determining the stronger acid is knowing the \(pK_a\) values for functional groups. The \(pK_a\) of alcohol is about 16 while the \(pK_a\) of a carboxylic acid is about 5. Therefore, \(CH_3CO_2H\) is more acidic than \(CH_3CH_2OH\).
c. The most important factor in determining the stronger acid is electronegativity. The chlorine atom is more electronegative than the bromine atom, therefore \(HClO\) is more acidic than \(HBrO\).
d. The most important factor in determining the stronger acid is considering resonance. The \(\ce{CH_3C(=O)NH_2}\) has a resonance which increases the stability of the conjugate base (therefore increasing acidity) because the negative charge can be delocalized. Thus, \(\ce{CH_3C(=O)NH_2}\) is more acidic than \(CH_3CH_2NH_2\).
e. The most important factor in determining the stronger acid is considering oxidation states on the central nonmetal. \(H_3AsO_4\) has an oxidation state of +5 which is larger and thus more acidic than \(H_3AsO_3\) which has an oxidation state of +3.
\(CF_3S^− < CH_3S^− < OH^−\) (strongest base)
\(NH_3\); \(Cl\) atoms withdraw electron density from \(N\) in \(Cl_2NH\).
2. It is expected that the stronger acid is \(C_6H_5NH_3^+\) because in considering the conjugate base the lone pair of electrons on nitrogen is involved in resonance, hence the molecule is stable.
3. \(H_2Se\) is a weaker acid than HBr because \(Br\) is more electronegative than \(Se\) thus more stable.
4. \(HClO_3>CH_3PO_3H_2>H_3PO_4\)
This is because \(H_3PO_4\) is a polyprotic acid which contains more than one ionizable proton, and the protons are lost in a stepwise manner. The fully protonated species is always the strongest acid because it is easier to remove a proton from a neutral molecule than from a negatively charged ion. Thus, acid strength decreases with the loss of subsequent protons, and, correspondingly, the \(pK_a\) increases which indicate it is the most basic. The conjugate base of \(ClO^{3-}\) has a much smaller charge to volume ratio, thus most stable and acidic.
5. \(CF_{3}S^{-}<CH_{3}S^{-}<OH^{-}\)
\(CF_{3}S^{-}\) is the most acidic because of the three electronegative Fluorines. \(OH^{-}\) is the strongest base in water. Thus, \(CH_{3}S^{-}\) is in between these aqueous solutions.
6. \(HNO_2<HClO_2<HNO_3\)
\(HNO_3\) has resonance stabilization, therefore it is the most acidic. Between \(HClO_2\) and \(HNO_2\), \(Cl\) is the most electronegative therefore \(HClO_2\) is more acidic than \(HNO_2\).
7. I expect \(H_2SO_3\) to be the stronger acid because it is more electronegative or has greater attraction which means it’ll be less inclined to share their electrons with a proton.
8. \(CF_3OH\) is a stronger acid than \(CH_3OH\) in aqueous solution because \(F\) is more electronegative than \(H\).
9. It would be expected that \(NH_3\) be a stronger base than \(Cl_2NH\) because it is electronegative due to the two \(Cl\) atoms.
16.11: Lewis Acids and Bases
1. Identify the nature of each of the following as either a Lewis Acid or a Lewis Base:
- \(Ni^{2+}\)
- \(Pt^{4+}\)
2. Explain why \(SiF_4\) can act as a Lewis Acid.
3. Identify the nature of each of the following as either a Lewis Acid or a Lewis Base:
- \([Fe(CN)_6]^{3-}\)
- \([Ni(NH_3)_6]^{2+}\)
- \(CdBr_4^{2-}\)
4. What is the product of the reaction of \(CO_2 + OH^- \rightarrow \) ?
5. In the reactions below, which is the Lewis Acid and/or which is the Lewis Base?
- \(NH_3 + H^+ \rightarrow NH_4^+\)
- \(H_2O + H^+ \rightarrow H_3O^+\)
6. In the complex ion, \([PtCl_6]^{2-}\) w hich is the Lewis Acid and which is the Lewis base?
7. The reaction of \(AgCl\) + \(NH_3\) pr oduces what complex ion?
a. \(NH_3\) is a lewis base because nitrogen has a lone pair of electrons to "donate."
b. \(Ag^{+}\) is a lewis acid because it has an unfilled octet and thus is able to accept a pair of electrons.
c. \(Ni^{+}\) is a lewis acid because it has an unfilled octet and thus is able to accept a pair of electrons.
d. \(Pt^{4+}\) is a lewis acid because it has an unfilled octet and thus is able to accept a pair of electrons.
e. \(H_2O\) is a lewis base because oxygen has two lone pairs of electrons to "donate."
f. \(SO_2\) is a lewis acid because sulfur has an unfilled octet and thus is able to accept a pair of electrons.
2. \( SiF _4\) has a central Silicon Atom which can expand its octet to 12 (compared to the typical 8) so that it forms \([ SiF _6]^{2-}\).
- Lewis Acid: \(Fe^{3+}\), Lewis Base: \(CN^-\)
- Lewis Acid: \(Ni^{2+}\), Lewis Base: \(NH_3\)
- Lewis Acid: \(Cd^{2+}\), Lewis Base: \(Br^-\)
4. This reaction forms a bicarbonate ion. \(CO_2 + OH^- \rightarrow O--COH=O\).
a. Lewis Acid: \(H^+\), Lewis Base: \(NH_3\)
b. Lewis Acid: \(H^+\), Lewis Base: \(H_2O\)
6. \(Pt^{4+}\) is the Lewis acid and \(Cl^-\) is the Lewis base.
7. \[AgCl + 2\,NH_3 \rightarrow [Ag(NH_3)_2]^+ + Cl^-\]
Construct a table comparing how OH − , NH 3 , H 2 O, and BCl 3 are classified according to the Arrhenius, the Brønsted–Lowry, and the Lewis definitions of acids and bases
Describe how the proton \((H^{+})\) can simultaneously behave as an Arrhenius acid, a Brønsted–Lowry acid, and a Lewis acid.
Would you expect aluminum to form compounds with covalent bonds or coordinate covalent bonds? Explain your answer.
Classify each compound as a Lewis acid or a Lewis base and justify your choice.
a. \(AlCl_{3}\)
b. \(CH_{3}N\)
c. \(IO_{3}^{-}\)
Explain how a carboxylate ion \((RCO_{2}^{-})\) can act as both a Brønsted–Lowry base and a Lewis base.
An Arrhenius acid is a molecule that when dissolved in water it will donate an \(H^{+}\) in solution.
An Arrhenius base is a molecule that when dissolved in water it will donate an \(OH^{-}\) in solution.
A Brønsted–Lowry acid is a molecule that when dissolved in a solution it will donate an \(H^{+}\) in solution.
A Brønsted–Lowry base is a molecule that when dissolved in a solution it will donate an atom or ion capable of accepting or bonding to a free proton in solution.
A Lewis acid is an atom or molecule that accepts an electron pair.
A Lewis base is an atom or molecule that donates an electron pair.
The proton \((H^{+})\) can simultaneously behave as an Arrhenius acid because when it is dissolved in water it will donate itself. \(H^{+}+H_{2}O \rightleftharpoons H_{3}O^{+}\)
The proton \((H^{+})\) can simultaneously behave as a Brønsted–Lowry acid because when it is dissolved in solution it will donate itself.
\(H^{+}+B^{-} \rightleftharpoons HB\)
The proton \((H^{+})\) can simultaneously behave as a Lewis acid as it can accept an electron pair.
3. It is expected that Aluminum forms a coordinate covalent bond as it can participate in a Lewis acid and a Lewis base interaction. For example \(Al^{3+}+H_{2}O \rightleftharpoons [Al(OH_2)_{6}]^{3+}\)
a. \(AlCl_{3}\) is a Lewis acid as \(Al\) can accept an electron pair.
b. \(CH_{3}N\) is a Lewis base as \(N\) can donate an electron pair.
c. \(IO_{3}^{-}\) is a Lewis base as \(I\) can donate an electron pair.
5. The carboxylate ion \((RCO_{2}^{-})\) can act as Brønsted–Lowry base because when dissolved in a solution the electron rich \(O\) is capable of accepting a proton. The carboxylate ion \((RCO_{2}^{-})\) can act as a Lewis base because the electron rich \(O\) can donate an electron pair.
1. In each reaction, identify the Lewis acid and the Lewis base and complete the reaction by writing the products(s).
a. (CH 3 ) 2 O + AlCl 3
b. SnCl 4 + 2 Cl −
2. Use Lewis dot symbols to depict the reaction of BCl 3 with dimethyl ether [(CH 3 ) 2 O]. How is this reaction similar to that in which a proton is added to ammonia?
a. The Lewis acid is \(AlCl_{3}\) and the Lewis base is \((CH_{3})_{2}O\).
\((CH_{3})_{2}O+AlCl_{3} \rightleftharpoons AlCl_{3} \cdot O(CH_{3})_{2}\)
b. The Lewis acid is \(SnCl_{4}\) and the Lewis base is \(Cl^{-}\).
\(SnCl_{4}+2\,Cl^{-} \rightleftharpoons SnCl_{6}^{2-}\)
\(BCl_{3}+(CH_{3})_{2}O \rightleftharpoons BCl_{3}\cdot O(CH_{3})_{2}\)
This reaction is similar to that in which a proton is added to ammonia as it also involves a Lewis acid and a Lewis base interaction.
- Important Questions
- Important Questions Class 10 Chemistry
- Important Questions Class 10 Chemistry Chapter 2 Acids Bases and Salts
Class 10 Chemistry Chapter 2 - Acids, Bases and Salts Important Questions with Answers
Class 10 chemistry important questions with answers are provided here for Chapter 2 Acids, Bases and Salts. These important questions are based on the CBSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practicing these Class 10 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Annual examinations.
Download Class 10 Chemistry Chapter 2 Acids, Bases and Salts Important Questions with Answers PDF by clicking on the button below. Download PDF
Recommended Video
Acids, bases and salts class 10 chemistry – (theory+questions+tips).

Class 10 Chapter 2 Acids, Bases and Salts Important Questions with Answers
Matching answer type questions.
Note: Match the items of Column A and Column B in the following questions.
Q1. Match the acids given in Column (A) with their correct source given in Column (B)
Q2. Match the important chemicals given in Column (A) with the chemical formulae given in Column (B).
Short Answer Type Questions
Q1. What will be the action of the following substances on litmus paper?
- Dry HCI gas
- Moistened NH 3 gas
- Lemon juice
- Carbonated soft drinks
- Soap solution
The action of enlisted compounds on litmus paper will be as follows.
- Dry HCI gas: Dry HCl gas would not affect the litmus paper.
- Moistened NH 3 gas: Moistened NH 3 gas is alkaline in nature. Thus it will turn red litmus blue.
- Lemon juice: Lemon juice is acidic in nature. Thus it will turn blue litmus red.
- Carbonated soft drinks: Carbonated soft drinks contains carbonic acid. Thus it will turn blue litmus red.
- Curd: Curd contains lactic acid. Thus it will turn blue litmus red.
- Soap solution: Soap is a salt of a strong base. Thus it will turn red litmus blue.
Q2. Name the acid present in ant sting and give its chemical formula. Also, give the common method to get relief from the discomfort caused by the ant sting.
Ant sting contains formic acid or methanoic acid. The chemical formula of formic acid is HCOOH.
- We can get relief from the discomfort caused by the ant sting by rubbing baking soda (NaHCO 3 ) in the affected area. Baking soda (NaHCO 3 ) is a salt of a strong base, i.e. basic in nature, it neutralises the acid effect of the ant bite when applied to the skin.
- We can also use a calamine solution. Zinc carbonate is present in calamine solution, which neutralises the acid effect of the ant bite when applied to the skin.
Q3. What happens when nitric acid is added to the eggshells?
Eggshells contain calcium carbonate. Calcium carbonate reacts with nitric acid to form calcium nitrate and carbon dioxide gas.
CaCO 3 (s) + HNO 3 (aq) → CaNO 3 (aq) + CO 2 (g) + H 2 O (l)
Q4. A student prepared solutions of (i) an acid and (ii) a base in two separate beakers. She forgot to label the solutions, and litmus paper was not available in the laboratory. Since both the solutions are colourless, how will she distinguish between the two?
We can use phenolphthalein to check which beakers contain acid and which one contains a base. Phenolphthalein turns colourless in acidic solutions and pink in basic solutions.
Apart from that, we can also use other natural indicators, like China rose or turmeric.
Turmeric is a natural indicator. It is yellow coloured. Turmeric paper turns red when it is dipped into a basic solution while it does not change its colour with acid.
China rose is another natural indicator. China rose solution gives dark pink (magenta) colour with acid and green with base.
Q5. How would you distinguish between baking powder and washing soda by heating?
Baking soda (NaHCO 3 ) liberates carbon dioxide gas on heating, confirmed by passing it in lime water. Whereas on heating washing soda Na 2 CO 3 .10H 2 O water of crystallisation is given out, the salt becomes anhydrous.
2 NaHCO 3 → Na 2 CO 3 + H 2 O + CO 2
Na 2 CO 3 .10H 2 O → Na 2 CO 3 + 10 H 2 O
Q6. Salt A is commonly used in bakery products on heating gets converted into another salt B, which is used to remove the hardness of water, and a gas C is evolved. The gas C, when passed through lime water, turns it milky. Identify A, B and C.
Baking powder is the salt used in bakery products that give sodium carbonate, and carbon dioxide gas on heating.
Sodium carbonate is used to remove the hardness of the water.
Carbon dioxide turns lime water milky due to the formation of insoluble calcium carbonate.
CO 2 + Ca(OH) 2 → CaCO 3 + H 2 O.
Thus, A is sodium bicarbonate (Baking powder), B is sodium carbonate, and C is carbon dioxide gas.
Q7. In one of the industrial processes used to manufacture sodium hydroxide, a gas X is formed as a byproduct. The gas X reacts with lime water to give a compound Y used as a bleaching agent in the chemical industry. Identify X and Y giving the chemical equation of the reactions involved.
Sodium chloride is used to manufacture sodium hydroxide, called the chloralkali process. In this process, chlorine and hydrogen gas are formed as byproducts and sodium hydroxide.
2 NaCl (aq) + 2H 2 O (l) → 2 NaOH (aq) + Cl 2 (g) + H 2 (g)
Chlorine gas gives bleaching power when it reacts with lime water and is used as a bleaching agent in chemical industries.
Ca(OH) 2 + Cl 2 ⟶ CaOCl 2 + H 2 O.
Therefore, the gas X is chlorine. Compound Y is calcium oxychloride, commonly known as bleaching powder and is used as a bleaching agent in chemical industries.
Q8. Fill in the missing data in the following table.
Q9. What are strong and weak acids? In the following list of acids, separate strong acids from weak acids. Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Acids that get completely ionised in an aqueous solution are called strong acids, whereas acids that do not get utterly ionised in an aqueous solution are called weak acids.
Hydrochloric acid, nitric acid, and sulphuric acid are examples of strong acids, while citric acid, acetic acid, and formic acid are examples of weak acids.
Q10. When zinc metal is treated with a dilute solution of a strong acid, a gas is evolved, which is utilised in the hydrogenation of oil. Name the gas evolved. Write the chemical equation of the reaction involved and also write a test to detect the gas formed.
Zinc metal gives hydrogen gas when treated with dilute sulphuric acid. Hydrogen gas is utilised in the hydrogenation of oil.
Zn + 2 HCl → ZnCl 2 + H 2 .
Therefore, the gas that evolved is hydrogen.
Test for hydrogen gas: When a burning candle is brought near the hydrogen gas, it burns with a pop sound that confirms hydrogen gas’s presence.
Long Answer Type Questions
Q1. In the following schematic diagram for the preparation of hydrogen gas as shown in Figure 2.3, what would happen if following changes are made?

(b ) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken
(c ) In place of zinc, copper turnings are taken
(d ) Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
(a) If the same amount of zinc dust is taken in the test tube in the place of zinc granules, the reaction rate will increase, and the reaction will occur more quickly. This is because the surface area per unit volume of powder is more than that of the zinc granules, and hence the powder will provide more surface area for the reaction to occur.
Zn + H 2 SO 4 → ZnSO 4 + H 2
(b) If we use dilute hydrochloric acid instead of dilute sulphuric acid, the reaction will occur as usual because we are mixing a strong acid with metal.
Zn + 2 HCl → ZnCl 2 + H 2
(c) With copper turnings, hydrogen gas will not evolve because copper is less reactive, so it does not react with dil.H 2 SO 4 of dil. HCl. Hence, no reaction will take place.
(d) If sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated, the metal will react with the base to form the corresponding salt and evolve hydrogen gas.
Zn + 2 NaOH → Na 2 ZnO 2 + H 2
Q2. For making cake, baking powder is taken. If your mother uses baking soda instead of baking powder in cake at home,
(a ) How will it affect the taste of the cake and why?
(b ) How can baking soda be converted into baking powder?
(c ) What is the role of tartaric acid added to baking soda?
(a ) If we use baking soda instead of baking powder, the taste of the cake will be bitter. On heating baking soda, sodium carbonate will be formed, which will make the cake taste bitter.
2 NaHCO 3 + Heat → Na 2 CO 3 + CO 2 + H 2 O
(b ) Baking soda can be converted to baking powder by adding an edible weak acid like tartaric acid in the baking soda.
(c ) When tartaric acid is dissolved in water, it liberates hydrogen ions. Hydrogen ions react with sodium carbonate to release carbon dioxide gas, which will make the cake fluffy.
Q3. A metal carbonate X reacting with acid gives a gas that gives the carbonate back when passed through a solution Y. On the other hand, a gas G obtained at the anode during electrolysis of brine is passed on dry Y, it gives a compound Z, used for disinfecting drinking water. Identity X, Y, G and Z.
Here, X is calcium carbonate (CaCO 3 ), Y is slaked lime [Ca(OH) 2 ], Z is bleaching powder (CaOCl 2 ), and G is chlorine (Cl 2 ) gas.
When calcium carbonate (CaCO 3 ) reacts with HCl, it liberates carbon dioxide gas.
CaCO 3 + HCl → CaCl 2 + CO 2 + H 2 O
When CO 2 is passed into lime water [Ca(OH) 2 ], it turns milky due to the formation of Calcium carbonate (CaCO 3 ).
CO 2 + Ca(OH) 2 → CaCO 3 + H 2 O
Hence, solution Y is lime water.
When chlorine (Cl 2 ) gas is passed on dry lime water, it gives bleaching powder which is used for disinfecting water.
Cl 2 + Ca(OH) 2 → CaOCl 2 + H 2 O
Q4. A dry pellet of a common base B absorbs moisture and turns sticky when kept open. The compound is also a by-product of the chloralkali process. Identify B. What type of reaction occurs when B is treated with an acidic oxide? Write a balanced chemical equation for one such solution.
Sodium hydroxide (NaOH) is a commonly used base and is hygroscopic; it absorbs moisture from the atmosphere and becomes sticky. A neutralisation reaction occurs when acidic oxides react with the base to give salt and water.
2 NaOH + CO 2 → Na 2 CO 3 + H 2 O
Q5. A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance, which can be moulded into different shapes by making its dough. When this compound is left open for some time, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt and why does it show such behaviour? Give the reaction involved.
Calcium belongs to group 2. Calcium sulphate is a soft white substance. It is known as the Plaster of Paris, which can be moulded into different shapes by making its dough.
When the Plaster of Paris is left open, it turns into a solid mass because of a reaction with moisture present in the atmosphere. The solid mass so formed is known as gypsum and cannot be further used for moulding.
CaSO 4 . ½ H 2 O + 1.5 H 2 O → CaSO 4 . 2 H 2 O
Plaster of Paris shows such behaviour because of half water molecules as water of crystallisation. It absorbs moisture from the atmosphere and forms gypsum, a hard solid mass when left open for some time.
Q6. Identify the compound X based on the reactions given below. Also, write the name and chemical formulae of A, B and C.

Here, X is sodium hydroxide (NaOH), A is sodium zincate (Na 2 ZnO 2 ), B is sodium chloride (NaCl), and C is sodium acetate (CH 3 COONa).
NaOH + Zn → Na 2 ZnO 2 + H 2
NaOH + HCl → NaCl + H 2 O
NaOH + CH 3 COOH → CH 3 COONa + H 2 O
Acids Bases and Salts Class 10 Science – Previous Year Questions

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Case Based Questions Test: Acids, Bases & Salts - 1 - Class 9 MCQ
15 questions mcq test - case based questions test: acids, bases & salts - 1, read the following and answer any questions: the reaction between mno 2 with hcl is depicted in the following diagram. it was observed that a gas with bleaching abilities was released. the chemical reaction between mno 2 and hcl is an example of:.
displacement reaction
combination reaction
redox reaction
decomposition reaction.
MnO 2 gets reduced to MnCl 2 as it loses Oxygen
HCl gets oxidised to H 2 O

Identify the correct statement from the following:
- A. MnO 2 is getting reduced whereas HCl is getting oxidized
- B. MnO 2 is getting oxidized whereas HCl is getting reduced.
- C. MnO 2 and HCl both are getting reduced.
- D. MnO 2 and HCl both are getting oxidized.
Reaction of MnO 2 with aqueous HCI
What will happen if we take dry HCl gas instead of aqueous solution of HCl?
- A. Reaction will occur faster.
- B. Reaction will not occur.
- C. Reaction rate will be slow
- D. Reaction rate will remain the same.
Reaction of MnO 2 with Aqueous and Dry HCI

Read the following and answer any questions:
Frothing in Yamuna:
The primary reason behind the formation of the toxic foam is high phosphate content in the wastewater because of detergents used in dyeing industries, dhobi ghat Yamuna’s pollution level is so bad that parts of it have been labelled ‘dead’ as there is no oxygen in it for aquatic life to survive.

Predict the pH value of the water of river Yamuna if the reason for froth is high content of detergents dissolved in it.
The table provides the pH value of four solutions P, Q, R and S
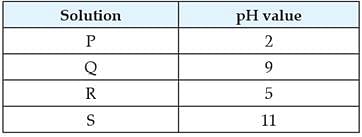
Which of the following correctly represents the solutions in increasing order of their hydronium ion concentration?
P>Q>R>S
P>S>Q> R
S<Q<R<P
S<P<R<Q
If the hydronium concentration increases, the pH decreases , causing the solution to become more acidic
pH → 11 < 9="" />< 5="" />< />
So, hydronium ion concentration will be of the order, S < q="" />< r="" />< />
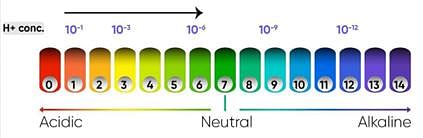
If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?
- A. Treating the water with baking soda
- B. Treating the water with vinegar
- C. Treating the water with caustic soda
- D. Treating the water with washing soda
Acid + Base → Salt + Water
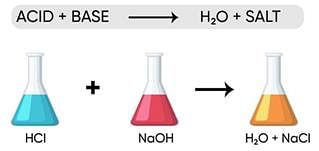
Study the given table and answer the following questions. It shows the pH value of the plaque (which collects around teeth) surrounding the teeth of a child over 3 hrs.
The three constituents of plaque are
All of these
The constituents of plaque are acid, saliva, bacteria and food.
State the time during the day when the condition is most favourable for the process of tooth decay.
Suhana takes three beakers A, B and C filled with aqueous solutions of glucose, alcohol and hydrochloric acid respectively as shown in the following figure:
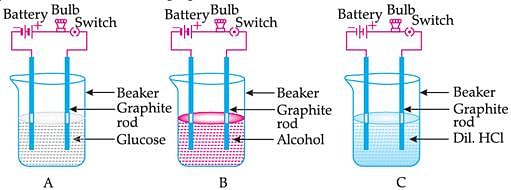
Which of the following statements is correct in terms of glowing of the bulb when the switch is ON?
- A. Bulb A and B do not glow but bulb C glows.
- B. Bulb A and C do not glow but bulb B glows.
- C. Bulb B and C do not glow but bulb A glows.
- D. All the bulbs glow.
Which of the following are present in a dilute aqueous solution of hydrochloric acid?
- A. H 3 O + + Cl –
- B. H 3 O + + OH –
- C. Cl – + OH –
- D. Unionized HCl
Study the given experimental set-up and answer the following questions.
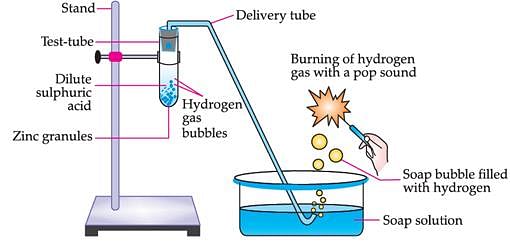
The above experimental set up shows reaction between metal and
- B. Metal carbonate
- C. Metal hydrogen carbonate
- D. Metal oxide
Write the products formed in the above process:
Zn (s) + H 2 SO 4 (g) →
Zinc sulphate only
Only hydrogen gas
Zinc sulphate and hydrogen gas
Zinc sulphide and hydrogen gas
Zn (s) + H 2 SO 4 (g) → ZnSO 4 (s) + H 2 (Zinc)
A metal is treated with dilute sulphuric acid. The gas evolved is collected by the method shown in the figure:
Name the gas evolved:
- A. Hydrogen
- C. Sulphur dioxide gas
- D. Carbon dioxide
What nature of hydrogen is used as a fuel in rocket:
- D. all of the above
P, Q, R are different colourless solids, while S is a colourless solution. They are (in random order) Sodium chloride (NaCl), Calcium Carbonate (CaCO 3 ), Acetic acid (CH 3 COOH) and Phenolphthalein indicator. Small amount of the above substances were added in pairs (e.g. P with Q; Q with R etc.) to a small amount of water in a test tube. They give the following results as shown in the observation table. Observation Table:
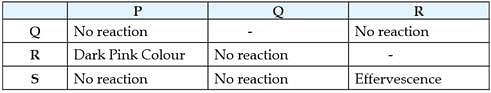
Which of the following reaction is incorrect:
Phenolphthalein + NaCl →Acidic medium (Blue colour)
Phenolphthalein + CaCO 3 (R) → Alkaline medium (Dark Pink Colour)
Phenolphthalein (P) + NaCl (Q) → No reaction
CaCO 3 (R) + 2CH 3 COOH (S) → (CH 3 COO)2Ca + CO 2 (effervescence) + 2H 2 O
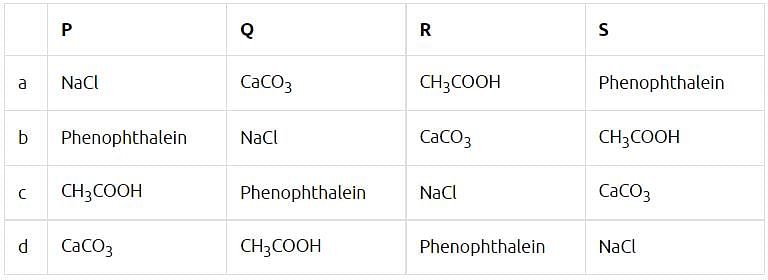
Top Courses for Class 9
Important Questions for Case Based Questions Test: Acids, Bases & Salts - 1
Case based questions test: acids, bases & salts - 1 mcqs with answers, online tests for case based questions test: acids, bases & salts - 1, welcome back, create your account for free.

Forgot Password

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Tag: acids bases and salts case study questions
Join our Telegram Channel for Free PDF Download
Case Study and Passage Based Questions for Class 10 Science Chapter 2 Acids, Bases and Salts
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs

NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts

Chapter 2 Acids, Bases and Salts Class 10 NCERT Solutions
Ncert solutions for class 10 science chapters:, why does tooth decay start when the ph of mouth is lower than 5.5, what would be the colour of litmus in a solution of sodium carbonate, what is the common name of the compound caocl 2 , what is bleaching powder how is it prepared , contact form.

IMAGES
VIDEO
COMMENTS
CASE STUDY : 5 (Acids Bases and Salts) Salts of a strong acid and a strong base are neutral with pH value of 7. On the other hand, salts of a strong acid and weak base are acidic with pH value less than 7 and those of a strong base and weak acid are basic in nature, with pH value more than 7. Answer the following in reference to the above ...
There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. Acids, Bases, and Salts Case Study Questions With Answers. Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 2 Acids, Bases, and Salts
Case Study/Passage Based Questions on Acids, Bases and Salts. Case Study/Passage Based Questions. Question 1: pH is quite useful to us in a number of ways in daily life. Some of its applications are: Control of pH of the soil : Plants need a specific pH range for proper growth. The soil may be acidic, basic or neutral depending upon the ...
Here we are providing case study or passage-based questions for class 7 science chapter 5 Acids, Bases and Salts. Case Study/Passage Based Questions. Passage-1. The reaction between an acid and a base is known as neutralisation reaction. In this reaction salt and water are produced with evolution of heat. 1. Salt is a product formed as a result ...
CBSE 10th Standard Science Subject Acids, Bases and Salts Case Study Questions With Solution 2021. The acidic behaviour of acids is due to the presence of hydrogenil (H + )ions in them. They produce hydrogen ions in the presence of water. Water is a polar solvent and this property of water helps in weakening the bond between the ions and makes ...
The Case Based Questions: Acids, Bases and Salts is an invaluable resource that delves deep into the core of the Class 10 exam. These study notes are curated by experts and cover all the essential topics and concepts, making your preparation more efficient and effective.
CBSE 10th Standard Science Subject Acids, Bases and Salts Case Study Questions 2021. Baking powder produces carbon dioxide on heating, so it is used in cooking to make the batter spongy. Although, baking soda also produces CO 2 on heating, but it is not used in cooking because on heating, baking soda produces sodium carbonate along with carbon ...
Students should follow some basic tips to solve Acids, Bases, and Salts Case Study Based Questions. These tips can help students to score good marks in CBSE Class 10 Science. Generally, the case based questions are in the form of Multiple Choice Questions (MCQs). Students should start solving the case based questions through reading the given ...
Chapter 2 - Acids, Bases and salts Case study-based questions Question 1 Salt is an ionic compound that results from the neutralization reaction of an acid and a base.It is composed of related numbers of cations (positively charged ions) and anions (negative ions) so that the product is electrically neutral (without a net charge).
Select the number of questions for the test: TopperLearning provides a complete collection of case studies for CBSE Class 10 Chemistry Acids, Bases and Salts chapter. Improve your understanding of biological concepts and develop problem-solving skills with expert advice.
Case Study Questions Chapter 2 Acids Bases Salts. Marble's popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings. Question: A student added 10g of calcium carbonate in a rigid container, secured it tightly and ...
Case Study Chapter 2 Acids, Bases, and Salts. Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 2 Acids, Bases, and Salts. Case Study/Passage-Based Questions. Question 1: A compound, X of sodium forms a white powder. It is a constituent of baking powder and is used in some antacids.
Learn Acids, Bases, and Salts Class 10 | Class 10 Science Chapter 2 | Class 10 Chemistry Chapter 2 | case study Questions | case study based question | case ...
We have the new pattern case study-based questions for free download. Class 10 Science case study questions. This article will guide you through: ... Read the following and answer any four questions: Salt of a strong acid and strong base is neutral with a pH value of 7. NaCl common salt is formed by a combination of hydrochloride and sodium ...
Join here for V Quiz - https://vdnt.in/CumQaIn this video, you will watch the CBSE Class 10 2022-23 Session about "Acids Bases Salts Class 10 ". Anubha Ma'am...
5. (a) A compound, X of sodium forms a white powder. It is a constituent of baking powder and is used in some antacids. When heated it gives a compound, Y which is anhydrous and absorbs water to become a hydrated salt. When this salt is kept in open air, it loses water molecules in a process called efflorescence.
Acids, Bases, and Salts Case Study: Here, Students can read Class 10 Acids, Bases, and Salts Case Based Questions with Answers in PDF File format. Apart from this ...
Salt + Water. Report a problem. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.
Aqueous solutions of salts such as \(CaCl_{2}\) are neutral because it is created from hydrochloric acid (a strong acid) and calcium hydroxide (a strong base). An aqueous solution of \(NaNH_2\) is basic because it can deprotonate alkynes, alcohols, and a host of other functional groups with acidic protons such as esters and ketones.
Class 10 chemistry important questions with answers are provided here for Chapter 2 Acids, Bases and Salts. These important questions are based on the CBSE board curriculum and correspond to the most recent Class 10 chemistry syllabus.
Solutions of Case Based Questions Test: Acids, Bases & Salts - 1 questions in English are available as part of our course for Class 9 & Case Based Questions Test: Acids, Bases & Salts - 1 solutions in Hindi for Class 9 course. Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
August 1, 2021 April 11, 2024 Physics Gurukul 3 Comments on Case Study and Passage Based Questions for Class 10 Science Chapter 2 Acids, Bases and Salts. Case Study and Passage Based Questions for Class 10 Science Chapter 2 Acids, Bases and Salts. Join our Telegram Channel for Free PDF Download.
Answer. H ydrogen gas is usually liberated when an acid reacts with metal. Take few pieces of zinc granules and add 5 ml of dilute H2SO4. Shake it and pass the gas produced into a soap solution. The bubbles of the soap solution are formed. These soap bubbles contain hydrogen gas. H 2 SO 4 + Zn → ZnSO 4 + H 2 ↑.