

Clinical Research Certificate
The Clinical Research Certificate provides training in research methods for faculty members, postgraduate residents, international fellows and community-based health care professionals who want to improve their knowledge and skills in research methodology and quality improvement. Completion of the certificate will significantly enhance your ability to understand, effectively use, engage in and collaborate in research. It is not intended as a substitute for a research-based graduate degree, nor is it intended to lead to independent research projects (i.e., to take on a Principal Investigator role on large projects).
Program Objectives
After completing this certificate program, students will be better able to:
- Construct meaningful research questions applicable to primary care research
- Compare and describe qualitative and quantitative research methodologies
- Design and contrast quantitative and qualitative data collection and analysis procedures
- Appraise and critique data reported in the literature from research studies
- Identify, discuss and complete a grant proposal for submission to a research ethics board
Target Audience
Clinicians seeking greater expertise and confidence in the use of research in their practice
Applicants must be health care professionals licensed and in active practice in their country of primary residence.
As of 2021 term admission cycles, we will be considering applications from Internationally-trained Family Physicians or General Practitioners who are citizens or permanent residents of Canada and intending to re-position their careers with a research focus.
Program Length
This 4-module program can be completed within 8-12 months up to a maximum of 24 months.
Certificate
A Departmental Certificate of Completion will be issued by the Department of Family and Community Medicine, University of Toronto, to participants who satisfactorily complete the program.
Learners are evaluated on each component on a pass / fail basis. There is no terminal examination or thesis. All components must be passed.
Or contact us if you have any questions
Program Requirements
Required course.
Choose one(1) of:
- FD05: Research Issues in Family Medicine and Primary Care
- FD01: Appraising and Applying Evidence to Assist Clinical Decision-Making (online course)
Required Practicum
The practicum provides an opportunity for reflective hands-on practice of knowledge and skills in clinical research.
- FD91: Clinical Research Practicum
Two Elective Courses
The elective courses allow students to learn more about a relevant topic of clinical research.
Choose two from the following list:
- FD13: Leading Improvement in the Quality of Health Care for Community Populations
- FD16: Applied Survey Methods for Health Care Professionals
- FD18: Family Medicine and Interprofessional Primary Care in the Global Health Context
- FD23: Practical Management Concepts and Cases in Leading Small Health Organizations
For detailed course information and scheduling, please view our course menu.

Clinical Research
Online full-time programs.
Online full-time programs are offered as either Daytime, or a combination of Evenings and Saturdays. Check your program Dates and Times to see what the program commitment will be.
Find out more about Full-Time Online programs
Humber is proud to have the highest graduate employment and employer satisfaction rate of the GTA colleges based on Colleges Ontario’s key performance indicators for college graduates in 2022-2023.
Program Overview
Humber’s Clinical Research graduate certificate program prepares graduates with the transferable skills needed to build successful careers in a variety of clinical trial management and research sectors. This program focuses on developing the concepts, skills and techniques required to work in the clinical research field. You will gain knowledge and skills in the planning and management of clinical research including practices related to the organization, execution and monitoring of clinical trials. You will learn clinical trial protocol development as part of the principles and processes of clinical trial design.
There is an emphasis on maintaining good clinical practice (GCP) as presented by the International Conference on Harmonization (ICH), along with the importance of data collection, analysis, recording and auditing - all to ensure that clinical trial data are credible and accurate. You will become familiar with the many regulations and guidelines established to ensure trials are conducted ethically and in ways that respect the rights of clinical trial participants, while ensuring the execution of robust scientific research.
Teamwork and communication skills are reinforced throughout the program, and you will acquire the necessary technological skills to assist with data management specific to the field.
You will also benefit from:
- a unique integrated approach
- acquiring a recognized skillset applicable to a wide variety of employment opportunities
- integrative project work that links applied and academic fields
- learning to adapt to the changing field and staying current
- simulation trials mimicking real work experiences
Program Delivery: Courses are scheduled over two 14-week semesters, and are asynchronous, self-directed online modules, with set dates for evaluations. A field experience placement occurs in Semester 3 and is in-person.
This program is not available to international applicants.
At Humber, courses are delivered in a variety of formats:
In-Person - An in-person course is delivered fully on campus.
Online Asynchronous (A) - An online asynchronous course has no fixed class schedule and allows students to engage with the course at different times according to their needs. Faculty provide modules, which are completed independently by the students according to established deadlines.
Online Synchronous (S) - An online synchronous course is delivered fully online and requires faculty and students to participate in real-time according to a fixed schedule. Classes are scheduled for a specific day and time.
Hybrid - A hybrid course is a combination of in-person and online classes and follows a set schedule. Students must be available to attend in-person classes at scheduled times during the semester.
The chart below outlines the delivery options available for each course in this program, by campus. For some academic terms, there may be more than one delivery option available. You’ll be able to select your preferred options when building your course schedule during open enrolment. Preferences for course delivery will be considered on a first come, first served basis. Some Humber programs are also delivered fully online, where all courses are delivered online.
International students: the impact of studying from outside of Canada on Post-Graduation Work Permit (PGWP) eligibility differs significantly based on when you start your program. Please review the PGWP eligibility before choosing your program and course delivery.
Work-Integrated Learning
Work-integrated learning .
Following two online academic semesters, students complete a three-month (450 hours) field experience that provides opportunities to apply and integrate theoretical knowledge and skills into real-world work settings. Field experiences are conducted in a wide range of organizations such as pharmaceutical, natural health products (NHP), medical device and biotechnology companies, as well as contract research organizations, hospitals, government agencies/departments and scientific institutions.
During Semester 3, students complete their learning at field experience sites, and assessments are carried out by assigned supervisors at the site of field experience. While Humber does assist students in finding their field experience by working with industry partners to identify openings, students are responsible for finding their own field experience that is aligned with the learning outcomes of our program.
Work-Integrated Learning (WIL) at Humber
Work-integrated learning.
Work-integrated learning opportunities prepare you for your future career. You will apply what you’ve learned in class and in real-world environments through a wide range of academic, community and industry partnerships. These work-integrated learning opportunities may include field experiences, professional practicums and co-operative education.
Field Experience
A field experience offers students an opportunity to engage in intensive experiences related to their field of study or career goals to build their skills, knowledge and abilities. Field experiences may be paid or unpaid.
Professional Practicum
Programs requiring a professional practicum offer practice-based experience or work hours for a professional license or certification. Students work under the direct supervision of an experienced professional. Placements are unpaid.
Co-operative Education
Students in co-op programs gain experience through paid work terms in their field of study that become progressively more complex as their skill level increases.
Optional Co-operative Education
Students in co-op programs gain experience through paid work terms in their field of study that become progressively more complex as their skill level increases. The co-op portion of this program is optional.
If you would like to learn more about work-integrated learning at Humber, visit WIL AT HUMBER

Watch the video to learn what is work-integrated learning.
An Education in High Demand
The medical, pharmaceutical, and natural products industry is facing constant development. As a society focused on health and wellness much attention is being given to the development of new interventions, supplements and drugs as well as improvement of health care. Comprehensive training combined with work experience and previous degrees make Humber students highly marketable.

Clinical Trials
Clinical research involves human studies where products are tested in multiple phases to assess their safety and efficacy. Clinical research is a necessary step (ethical and regulatory) in the development of new therapeutic/diagnostic products. Industry has the responsibility to follow stringent international, federal, and provincial regulations when planning and implementing preclinical and clinical studies; along with the development and manufacturing of medical products.
Responsibilities
Clinical Research Professionals are responsible for conducting clinical trials, which includes planning, designing. and monitoring clinical experimentation and later, analyzing data to draw conclusions about the treatments. Furthermore, they are responsible for carrying out clinical trials in compliance with Good Clinical Practices, clinical trial protocols, international and local regulations. When working in the industry, interactions with medical personnel (nurses and physicians) and plenty of travelling are a regular part of life for a Clinical Research Professional. When employed by a clinic or hospital the Clinical Researchers are responsible for conducting studies at that facility as well as interacting with sponsoring companies regarding the planning and execution of clinical trials.

The Humber Advantage
Industry recognition.
Every year our program receives excellent feedback from the industry regarding the professional preparation of the graduates and the content of the program; our graduates are an important resource for the health care industry. This is an outcome of our ongoing efforts to ensure that students have solid science, medical, and clinical research knowledge.
Superior Program Design
The Clinical Research Graduate Certificate program at Humber is designed, developed and created so that it can be delivered online with high efficiency, during the first two semesters of the program. Subsequently, theoretical learning is extensively applied through hands-on experiences during the third semester, including a 450-hour internship placement.
The fundamental skills are taught, developed, and reinforced throughout the one-year comprehensive program. Work ready graduates are developed in this program to confidently transition into the workforce.
The Humber Experience
The right fit.
If you are science-oriented, self-regulated, have collaborative interpersonal skills and enjoy managing and working in variety of laboratory and research settings, the Clinical Research program and a career in this field may be for you.
Testimonials
Interested in Clinical Research at Humber College yet want to know more before enrolling?
Your Career
Our graduates typically pursue careers in research settings such as pharmaceutical, medical device and biotechnology industries, hospitals, and research institutes. Their work may help lead to the development of new treatments and therapeutic approaches to enhance the quality of life.
Related Programs

Regulatory Affairs
Credential: Ontario Graduate Certificate Length: 3 semesters

Health Sector Regulatory Compliance
Program Availability
Humber is a publicly-funded institution and does not have a public-private partnership. International students graduating from Humber or Humber’s International Graduate School (IGS) are eligible to apply for a Post-Graduation Work Permit .
International Students in Canada who apply for September 2024 start could be eligible for an automatic scholarship*. Apply now
Please note the new International Admissions Process and Provincial Attestation Letters. Read the update
International Students Out of Canada can Apply through Humber International
Recruitment Events
Open House Book a Tour
Can't make it to campus?
Experience Humber Virtually
Program Delivery Types
Block-based: Students select a pre-set weekly schedule of courses that best meets their needs. Block-Based schedules may include in-person, hybrid and online courses.
Course-based: Students create their own schedule of courses from among in-person, hybrid and online options.
Condensed Week - Courses requiring students to come to campus are scheduled over 2-3 days per week. Online courses are scheduled on other days.
Online - Courses are scheduled only online and may be delivered asynchronously, where students study independently or synchronously, where students attend the online class on a specified time and day.
Twilight - In-person, online synchronous and hybrid courses are generally scheduled after 3:00pm.
Twilight-Online: Online synchronous courses are generally scheduled after 3:00 pm.

IPE Blackboard Site
Wed, December 20, 2023
The Faculty of Health Sciences & Wellness is launching a new tutorial Blackboard site entirely dedicated to IPE!

Navigating Health Care
Thu, September 28, 2023
Humber’s North Campus was proud to host The Central West Navigation Conference on Tues Sep 26.

Humber Launching Student Led Hearing Clinic
Mon, August 21, 2023
A multi-service clinic servicing the Humber community and area.
No news at this time.
Every attempt is made to ensure that information contained on this website is current and accurate. Humber reserves the right to correct any error or omission, modify or cancel any course, program, fee, timetable or campus location at any time without prior notice or liability to users or any other Person.
- Close Menu ×
- How To Apply How To Apply
- Admission Requirements Admission Requirements
- Equipment & Device Requirements Equipment & Device Requirements
- Fees & Financial Aid Fees & Financial Aid
- Contact Us Contact Us
- Apply Now APPLY NOW
Admissions Questions
General enquiries.
Call 416-675-3111 or email [email protected] . If you have already applied, be sure to check your application status on myhumber.ca .
Domestic Applicants Enquiries
Domestic applicants can book a one-on-one advising appointment with an admissions representative.
International Applicants Enquiries
Contact the International Centre for information about full-time programs (including the International Graduate School), how to apply and to follow up on your submitted application.
Program-Specific Questions
Speak to the Program Co-ordinator about the course curriculum, projects and career options.
Aparna Bhan, program manager [email protected]
Campus Information
Book a campus tour to take a closer look at what it's like to be a student at Humber.
Want More Info?
Find out more about the student experience and everything that Humber has to offer Future Students .
Sign-up now for more info on Humber, including programs, special events and more!
How To Become An Apprentice
Becoming an apprentice.
Find an employer willing to sponsor you as an apprentice.
Contact the Ministry of Labour, Immigration, Training and Skills Development to register as an apprentice.
Work with your employer approximately one year before attending Humber.
View Instructions
Ontario Youth Apprenticeship Program (OYAP)
If you’re in high school – grade 11 or 12 – you can earn co-op education credits through work placements in some skilled trades.
Visit OYAP
How to Apply
Domestic students.
Applications to Humber are made through ontariocolleges.ca . Be sure to submit your application by the equal consideration deadline of February 1. You may apply after February 1, however, post-February 1 applications will be considered on a first-come, first-served basis depending on the availability of the space in the program.
To check program availability refer to the Campus/Availability listing on Humber’s program pages, search by availability , or ontariocolleges.ca .
To see where you are in the admissions process, visit the Admissions Road Map .
International Students
If you’re an international student, you can apply directly to Humber via our International Centre .
Need Advice?
Program advising appointments.
Get help narrowing down your program options or book a one-on-one pre-enrolment advising appointment with one of our Recruitment Officers.
Transfer & Pathway Advising
Book a virtual appointment with a Student Mobility Advisor learn more about getting Transfer Credit(s) for previous post-secondary experience, Prior Learning Assessment and Recognition (PLAR), and Pathways options.
Admission Requirements
Admission selection is based on the academic criteria indicated. Meeting minimum eligibility requirements does not guarantee admission.
Admission selection is based on the following three requirements:
To be eligible for admission, you must possess the following:
- A Bachelor of Science degree, majoring in health science, pharmacy, some area in life sciences or a related field.
Mature Applicants
Diplomas and certificates.
An applicant is considered a mature applicant if they have not completed secondary school or other postsecondary school, and will be 19 or older as of the first day of classes. Humber will invite you for testing to demonstrate that you meet all listed course requirements.
An applicant is considered a mature applicant if they have not completed secondary school or attended postsecondary studies, and will be 21 or older as of the first day of classes. Mature applicants for degree programs will be required to meet course requirements at the U/M level or equivalent.
College Transfer Applicants
An applicant is considered a college transfer applicant if they have completed some or all of a college-level credential. Humber may use a combination of secondary school and/or college courses and grades to determine program eligibility.
An applicant is considered a college transfer applicant if they have completed some or all of a college-level credential. Humber may use a combination of secondary school and/or college courses and grades to determine program eligibility. Applicants must have an overall minimum grade point average (GPA) of 65 per cent in the program. Applicants are required to disclose and provide academic transcripts for all course work completed at the postsecondary level.
University Transfer Applicants
An applicant is considered a university transfer applicant if they have completed some or all of a university-level credential. Humber may use a combination of secondary school and/or university courses and grades to determine program eligibility.
An applicant is considered a university transfer applicant if they have completed some or all of a university-level credential. Humber may use a combination of secondary school and/or university courses and grades to determine program eligibility. Applicants are required to disclose and provide academic transcripts for all course work completed at the postsecondary level.
English Language Proficiency
All applicants whose first language is not English must meet Humber’s English Language Proficiency Policy .
International Credit Evaluation
Canadian citizens or permanent residents with international education are required to provide a credential evaluation. Note, for international High school education course by course evaluations, ICAS must be used. For international post-secondary education, a WES evaluation must be provided. In situations where you expect to apply for transfer credit, it is recommended that a course by course WES evaluation is completed.
Please note: A WES course by course evaluation is required for this program.
International Academic Equivalency
Admission equivalencies for Humber depend on your country of study. Please enter your location or choose detect my location to see the requirements for your country below.
Applying with an International Baccalaureate (IB)
Post-Admission Requirements
Once you have been accepted, and have confirmed your offer, you may need to complete a further set of requirements related to your program (Post-Admission Requirements).
Equipment & Device Requirements
Fees & financial aid.
The 2024/2025 fee for three semesters is:
- domestic: $7,501.08
- international: N/A
Fees are subject to change.
Fees by Semester
Domestic Fees by Semester
International fees by semester.
*Plus Mandatory Health Insurance fee once per academic year: Fall start - $420 Winter start - $280 Summer start - $140
Financial Aid, Scholarships and Bursaries
Understand the costs associated with coming to Humber and explore resources available from first year to your final year on Student Fees and Financial Resources .
Scholarships
Humber scholarships.
Find out more about scholarships and bursaries that you may be eligible for, visit Student Scholarships . International students can visit International Student Scholarships .
Humber Bursaries
Bursaries are available for Certificate, Diploma and Degree programs primarily based on financial need, visit Humber Bursaries.
External Awards, Bursaries & Scholarships
Find out more information about external scholarships and bursaries, visit External Awards.
Indigenous Student Awards, Bursaries & Scholarships
Humber offers a variety of bursaries and scholarships for Indigenous students, visit Indigenous Student Awards.
Explore Opportunities through Humber Pathways
Humber Pathways include:
- Opportunities to build on your college education and complete your diploma or degree at Humber.
- Degree and graduate study opportunities at other institutions in Ontario, Canada and abroad.
Additional information will be made available to students from their program before the beginning of the Winter term. Courses with in-person requirements will likely also have online components. The delivery mode of some courses is still being determined. Humber may need to change plans for in-person learning, subject to government and public health directives and/or additional health and safety considerations.
You can find a complete list of programs with downloads including program and course details at Current Student Resources
Students in programs marked as online/in-person will have a combination of those two types of delivery. Additional information will be made available to students from their program in the first week of June. Courses with in-person requirements will likely also have online components. The delivery mode of some courses is still being determined. Humber may need to change plans for in-person learning, subject to government and public health directives and/or additional health and safety considerations.
Learning Outcomes:
Upon successful completion of the program, a graduate will:
- Perform the duties of a clinical research professional, as part of a project team, at all phases of the product/treatment development and post-market processes.
- Consider political, social, and economic factors when making decisions related to clinical research practices in order to plan responses for potentially challenging and complex outcomes.
- Analyze clinical research processes and products from multiple perspectives to identify potential impacts on industry.
- Synthesize scientific, regulatory, and business information from various sources to prepare effective clinical research documents.
- Maintain ethical, legal, regulatory, and professional standards associated with clinical research.
- Create a clinical development plan for a novel therapeutic product.
- Evaluate clinical research practices according to recognized Quality Assurance Process.
- Integrate effective technology and record-keeping practices within all stages of clinical research and post marketing processes to ensure compliance with research approvals and professional and ethical standards of practice.
- Adhere to the principles and practices of specific Standard Operating Procedures to prepare and manage documentation and data in compliance with approved protocols.
- Prepare and critique submissions for clinical trials and marketing approvals that meet regulatory and industry requirements.
- Apply critical analysis, problem solving, and project management skills to recognize and respond to complex clinical research challenges.
- Engage in knowledge translation to contribute to the advancement of the health care industry.
- Collaborate with study participants, research teams, and regulatory and business professionals to contribute to high quality clinical research processes.
Main navigation
- Graduate programs
- How to apply
- Research & supervision
- Student experience
- Connect with us
The majority of graduate programs are NOT impacted by recent government announcements about tuition increases. PhD students from the rest of Canada will continue to pay Quebec fees. International PhD fees will see the same 3% increase as Quebec fees.
Clinical Research (Grad. Dip.)
Program description.
The Graduate Diploma (Gr. Dip.) in Clinical Research offered by the Division of Experimental Medicine in the Faculty of Medicine & Health Sciences is a course-based program that emphasizes practical and hands-on learning opportunities. Our comprehensive and innovative program offers a specialized and practical curriculum designed to equip students with advanced skills in clinical research.
Unique Program Features
- The part-time program comprises practicum, electives, and workshops provided by experts in the academic, industrial, and government sectors, and covering wide-ranging issues pertinent to the conduct of clinical research;
- The program provides students with exposure to both theoretical and practical issues relevant to the conception and conduct of a clinical research study, and to put these principles into practice by participating in an ongoing clinical trial;
- Graduates are well-equipped to manage and design clinical research studies in both academic and industrial settings.
University-Level Admission Requirements
- An eligible Bachelor's degree with a minimum 3.0 GPA out of a possible 4.0 GPA
- English-language proficiency
Each program has specific admission requirements including required application documents. Please visit the program website for more details.
Visit our Educational credentials and grade equivalencies and English language proficiency webpages for additional information.
Program Website
Grad Dip in Clinical Research website
Department Contact
Graduate Program experimental.medicine [at] mcgill.ca (subject: Graduate%20Diploma%20in%20Clinical%20Research) (email)
Available Intakes
Application deadlines.
Note : Application deadlines are subject to change without notice. Please check the application portal for the most up-to-date information.
Application Resources
- Application Steps webpage
- Submit Your Application webpage
Application Workshops
Consult our full list of our virtual application-focused workshops on the Events webpage.
Department and University Information
Graduate and postdoctoral studies.
Continuing Education
Applied clinical research certificate, certificate in applied clinical research, earn your certificate in applied clinical research with mcmaster continuing education.
Explore the requirements below and register today!
Information Box Group

{Link Label}
Certificate in applied clinical research learn more.
Earn the Certificate in Applied Clinical Research by completing all 5 courses listed below.
Certificate in Applied Clinical Research Requirements
Academic Credit – 15 Units
Admission requirement: A completed post-secondary education (diploma or degree) is strongly recommended.
Students are given a three-year period to complete all required components of the certificate program. This requirement is based on the need to remain current with legal, regulatory and ethical considerations in the field of work.
Note: Courses must be taken in the following order:
- Principles of Clinical Research
- Research Ethics
- Clinical Trial Design
- Clinical Trial Management
- Clinical Research Capstone
Courses (complete all 5)
Acr 101 principles of clinical research, acr 102 research ethics, acr 103 clinical trial design, acr 104 clinical trial management, acr 105 clinical research capstone.

Certificate in Clinical Research
Become an essential member of the clinical research process by learning how to protect patient safety, ensure trial integrity, and manage adherence to research ethics, best practices and regulations., canada is a world leader in clinical research.
Canada currently ranks fourth in the world for number of clinical trial sites. The volume and growth of clinical trials taking place nationally signals promising job and career advancement opportunities.
Canada is favourably positioned in clinical research with:
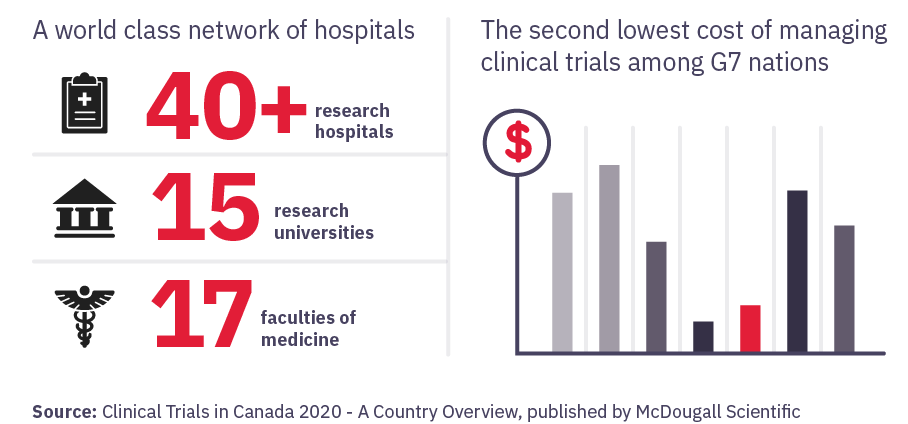
Based on data from Burning Glass, there were:
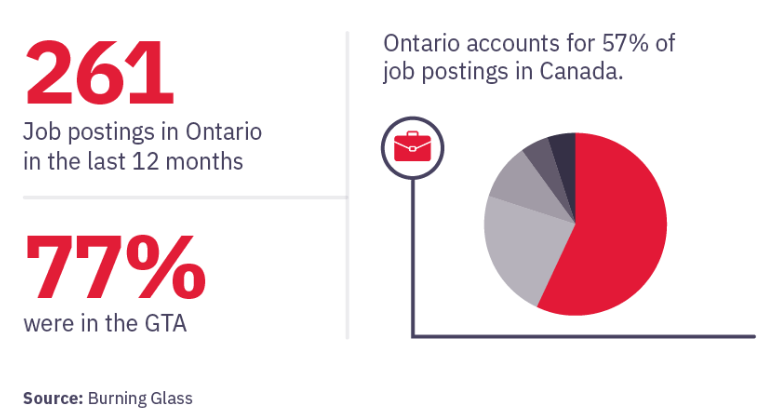
Canada ranks fourth in the world for number of clinical trials sites.

Program Overview
What you will learn.
- Understand the stages in setting up clinical trials
- Plan, manage, and monitor clinical research and trials
- Adhere to good clinical practice including patient consent, privacy, and data integrity protocols
- Abide by regulations and legislation to ensure that trials are conducted ethically, while upholding scientific research principles
- Demonstrate accuracy and reliability in data collection, management, and analysis
Program benefits
- Develop your skills through experiential assignments, projects, and case studies
- Learn from instructors who are leaders in the clinical research field
- Advance through the program with the same cohort of peers and build your professional network
- Participate in an applied clinical research management simulation capstone project
- Complete the program in 9 months through a combination of live classes and online learnings

Who Should Take This Program?
This program is designed for individuals with a health sciences background who are looking to enter this exciting field.
- New Graduates and Early Career Professionals with a related degree
- Internationally Educated Medical Doctors (IMDs) and Internationally Educated Health Practitioners (IEHPs)
- Trained Nurses (RNs, RPNs)
- Laboratory and Medical Technicians & Technologists
- Clinical Research professionals looking to upskill
Get Program Information
Enrolment open.

Program start date
Program delivery information for new students
- Guidance Counsellors
- International Agents

Clinical Research (CRQC)
Starts in January, May, September
Credential Awarded
- Admission requirements
Delivery: At Seneca, courses are delivered in the following formats : online, in-person, hybrid (an online, in-person combination) or flexible (offered in-person and online at the same time).
The chart below outlines the delivery options available for each course in this program. For some academic terms, there may be more than one delivery option available. You’ll be able to select your preferred options when building your course schedule during open enrolment.
Preferences for course delivery will be considered on a first come, first served basis.
Work-Integrated Learning Term
Program Learning Outcomes
JavaScript is disabled. Please enable to view full site properly and for successful submission of the forms.
Updates on study permits and Provincial Attestation Letters (PALs) for international students
From: Wednesday, 24 April 2024
To: Friday, 26 April 2024
Updated: about 21 days ago
From: Monday, 22 April 2024
To: Friday, 30 August 2024
Updated: about 1 months ago
- Sheridan Central
- myStudentCentre
- Applicant Portal
- Faculty & Staff Directory
- Career Portal
- Library Login
- Future Students
- International Students
- Continuing Education Students
- Parents & Counsellors
Ontario College Graduate Certificate
Clinical research.
Co-op | 1 year (2 semesters)
( Remote Learning )
Admission Requirements
- Fees & Financial Aid
Learning Outcomes
Learn how to lead clinical research studies from start to finish., program overview, become a well-rounded clinical research specialist.
Develop both the general and specialized skillsets needed to work in clinical research. In your first semester, you'll learn fundamentals of clinical trials, research methods, project management, regulations, quality and compliance, good practices and more. You'll then build on that knowledge with training in oncology, immunology, cardiology, neurology, medical devices and diagnostics.
Online learning that simulates in-person experiences
Study in the comfort of your own home while still getting hands-on experience. This program is delivered remotely, preparing you for an industry that conducts most of its work online. Video lectures are supported by virtual reality (VR) labs that simulate a real-world consenting room, enabling you to interact with patients and doctors. All costs of your VR equipment will be covered in your fees.
Join a growing field and get paid during your placement
The use of evidence-based medicine is growing globally, prompting governments to invest more money in research and development. The high demand for clinical research associates enables you to be paid during a 360-hour in-person placement at the end of your program. We'll help you secure your co-op at a clinical research organization, hospital, research institution or office in your area.
Upgrade your existing skillset
Government oversight of clinical trials has increased and any violation of research regulations can be extremely damaging to a company's reputation. Even if you're already working in this field, upgrading your knowledge and skills could make you more attractive to employers. You can also apply to receive credit for previous workplace experience or learning , giving you a head start towards your new credential.
This program is delivered fully online and requires faculty and students to participate in real-time according to a fixed provided schedule. Classes are scheduled for a specific day and time.
Book a campus tour
Have questions? Get answers.
Faculty of Applied Health & Community Studies
Designation
Online, Remote Learning
Helpful links
Program Summary
Creative, innovative learning is at the core of all Sheridan’s courses. Here are the courses you’ll take in this program.
Total credits: 19
Total credits: 23
Courses subject to change.
Current students should refer to their Academic Requirements in myStudentCentre to track their academic progress and outstanding course requirements.
Program Eligibility
- Postsecondary bachelor’s degree, advanced diploma or diploma majoring in life science, health science or a related field.*
- Other professional degree designations such as a MA, PhD, MD majoring in life science, health science or a related field* will be considered.
- Minimum overall GPA of 65%
- The results of their Letter of Intent
Applicant Selection
Candidates to the program are selected on the basis of academic achievement and the evaluation of a Letter of Intent that demonstrates their interest in the industry.
Sample Letter of Intent submission instructions (for reference purposes only) are available.
Domestic applicants
- Postsecondary transcripts indicating courses completed to date must be submitted to ontariocolleges.ca at the time of application.
- Domestic applicants with education outside of Canada must have their transcripts assessed for equivalency through ICAS or WES. A comprehensive (course-by-course) postsecondary credential assessment is required.
- Private career college courses and/or credentials are not accepted as admission requirements for Sheridan programs.
English Language Proficiency
All applicants whose first language is not English must meet Sheridan’s English proficiency requirements .
*Related fields
Postsecondary programs related to life or health science include, but are not limited to:
- Biology, biochemistry, biomedical
- Biomedical engineering
- Health policy / health studies
- Health sciences
- Health-care administration or management
- Homeopathic medicine
- Human kinetics, kinesiology
- Life sciences
- Medical laboratory science
- Paramedic, paramedicine
- Pharmacology
- Physical therapy, occupational therapy
- Public health
- BSc psychology
- Veterinary sciences
For applicants with education credentials in a related field that may not be in the life or health science, relevant experience is also very important for consideration into the program.
Career Opportunities
As a graduate of this program, you’ll be prepared to contribute to clinical research projects in a variety of roles.
Potential career opportunities include:
- Clinical Research Associate
- Data Entry Coordinator
- Clinical Research Coordinator
- Clinical Research Manager
- Project Manager
- Research Program Manager
- Clinical Research Assistant
- Clinical Trial Monitor
Following successful completion of two online academic semesters, students in this program complete a mandatory 360-hour in-person co-op semester. We'll help you secure your co-op at a clinical research organization, hospital, research institution or office in your area.
The program-specific academic standard required for participation in co-op can be found here: Academic Standards for Co-op and Internship .
Cooperative Education is a form of work-integrated learning that links classroom learning with paid or unpaid work experience within a professional environment. Co-op work terms are related specifically to the academic studies of each student. The work terms provide an opportunity to learn by doing. You’ll apply theory to practice, develop a meaningful view of the working world, and cultivate an awareness of yourself as a professional.
Students receive in-class and 1-on-1 career education support to help prepare for the work term. The co-op component is delivered by Sheridan’s Cooperative Education Office, which facilitates over 1,800 Co-op/Internship work terms per year Sheridan-wide.
Learn more about Cooperative Education and Internships at Sheridan .
Degree Completion
Advanced entry challenge exams, get credit for what you know.
Sheridan recognizes and appreciates that advanced learning doesn’t always require standard classroom instruction, and that opportunities to advance skills and learn new technologies are everywhere.
If you have significant knowledge and experience in programming, web development, database and/or networking, you may choose to complete Advanced Entry Prior Learning Assessment and Recognition (AEPLAR) Challenge Exams for academic credit towards your Sheridan Computer Systems Technology advanced diploma.
How do Challenge Exams work?
AEPLAR Challenge Exams are administered by and through the Sheridan College Assessment Centre or through an approved Test Centre. There are fees to write each exam (an Assessment Centre fee and a Challenge Exam fee).
Your completed Challenge Exam(s) will be evaluated to determine whether you’re eligible for credit in one or more courses within the identified program streams.
Prior Learning Assessment and Recognition (PLAR) and Advanced Standing credits are subject to Sheridan’s policies and procedures. Learn more about Sheridan’s Credit Transfer policies and procedures .
I have previous postsecondary education. Should I write a Challenge Exam?
No. Challenge Exams are designed for applicants with knowledge and experience gained outside of formal education. If you have previous postsecondary education, we encourage you to apply for Advanced Standing instead — you may be eligible to apply your previously earned credits towards your new program at Sheridan .
What subjects can I complete Challenge Exams for?
Challenge Exams for applicants to the Computer Systems Technology – Software Development and Network Engineering program are designed to test skills in specific knowledge streams within this program:
- Programming – Introductory
- Programming – .NET
- Programming – Advanced Java
- Web Development
- Operating Systems
- Software Design
- Mobile App Development – Android
- Mobile App Development – iOS
- Game Development – UNITY
You can also choose to complete Challenge Exams for specific individual courses within the Computer Systems Technology program.
Complete a free self-assessment test to help you determine whether Challenge Exams are a good option for you.
How do I apply to complete a Challenge Exam?
Step 1: make sure you meet the program admission requirements.
You must meet the program admission requirements to be eligible for admission and PLAR.
Step 2: Complete a free self-assessment
Complete a free self-assessment test for each knowledge stream you wish to challenge. This will help you determine whether your current skills will enable you to succeed in the Challenge Exam(s).
Step 3: Apply to Sheridan's Computer Systems Technology advanced diploma program
Apply to the Computer Systems Technology – Software Development and Network Engineering program .
To be eligible to complete Challenge Exams for this program, you must select "Advanced Entry" as the level of the program on your application.
Step 4: Follow the instructions to complete your Challenge Exam(s)
Once Sheridan receives your application, we’ll send you an email with instructions on how to register and pay for your Challenge Exam(s). Follow those instructions.
Once you’ve completed your Challenge Exam(s), subject matter experts in Sheridan’s Faculty of Applied Science and Technology will evaluate them within 10 business days. The Faculty will forward your results to the Office of the Registrar, and any approved credit transfers will be posted in your Credit Transfer centre. Exam feedback will not be provided.
Step 5: Accept your offer of admission and pay your program fees
If you receive an offer of admission to Sheridan, please follow the instructions to confirm your offer and pay your fees by the due dates indicated in order to reserve your spot in the program.
We wish you success in this process and look forward to seeing you at Sheridan!
Program Fees
Fees shown here are estimates only. Fees are in Canadian dollars and include tuition, health insurance and ancillary charges.
The fees shown here are for the 2024–2025 academic year, and are subject to change. The fees displayed are for the first two (2) academic semesters of study at Sheridan unless otherwise noted; fees for subsequent semesters are not reflected on this website.
Fees for Canadian students
Notes: Co-op students must pay an additional fee of $535 in the term prior to each co-op work term.
Fees for International students
Financial aid & awards.
Your education is a big investment, and we're here to help! Keep an eye on our Financial Aid & Awards page for regular updates, and check out these important links:
- Ontario Student Assistance Program (OSAP)
- Scholarships, awards and bursaries
- Work Study Assistance Program
International Entrance Scholarship
All new international students* beginning their full-time postsecondary studies (Year 1, Semester 1) in Spring 2024, Fall 2024 or Winter 2025 semesters will receive an entrance scholarship ranging from $1,000 to $3,000.
*ESL and programs with tuition reduction are excluded
To achieve the critical performance, students will have demonstrated the ability to:
- Develop, implement and maintain processes and Standard Operating Procedures to execute clinical research projects and to ensure alignment with principles of quality assurance in compliance with applicable regulatory frameworks and best practices.
- Obtain and maintain required ethical and regulatory approvals to ensure the protection and safety of clinical trial participants and the integrity of research data.
- Communicate and collaborate with relevant stakeholders to ensure every aspect of the study can be completed according to requirements.
- Accurately collect and verify, or oversee, the integrity of research data from acquisition to recording.
- Evaluate, recognize and respond to project-related challenges to proactively resolve issues, mitigate risks and improve quality of projects.
- Manage the operations of research projects to ensure compliance and timely and on-budget conduct.
- Participate in professional development activities to maintain up-to-date knowledge and awareness of current developments in order to meet compliance in a changing clinical research industry.
- Identify and apply strategies to support culturally competent research settings involving diverse communities including indigenous peoples in order to recognize and prioritize health equity.
Frequently Asked Questions
Labs & technology, financial planning certification, professional sales certificate, program transition, earn your masters degree, transfer opportunities, clinical placements, articulation agreements, educational philosophy.
Sheridan's Honours Bachelor of Interior Design curriculum and its delivery are designed to address current social issues pertinent to the design industry. This enables students to broaden their understanding of their place within the world.
Fundamental to the vision of the program is the balance of creative and conceptual thinking with the technical and business aspects of the profession. Courses follow a logical sequence with the degree of difficulty building vertically from first year to fourth year.
The various streams provide a range of design problems building in size and scope, from simple to complex in various design sectors. The curriculum builds on fundamental introductions that ensures a strong foundation for students to apply additional more complex learning and skills developed later in the program.
Studio projects are designed to mimic professional practice and require applied theory, creativity and strong technical knowledge. Curriculum is also connected horizontally across each semester to help support the learning in courses happening simultaneously. This demonstrates the inter-connected knowledge and skills required to practice in the industry.
The program provides many opportunities that expose students to practicing professionals and professional practice. Industry leaders and representatives from professional associations like ARIDO and IDC are invited into the classroom as jurors and guest speakers throughout the four years. The internship also provides professional design work experience for students before they enter their final year of study.
The curriculum is student-centric and designed to equip students with the skills that are required for entry-level design positions and advanced study. Manual skills such as drafting, drawing and model making are developed alongside digital skills using current software. Students are also well-versed in the applicable building codes and regulations required to practice in Ontario. Students are exposed to valuable research skills and encouraged to continue their learning past graduation.
Program goals
Sheridan's Honours Bachelor of Interior Design program aspires to:
- Develop confident, creative and critical thinkers that can solve a variety of problems thoughtfully, improving the quality of the built environment and protecting the health, safety and welfare of the public.
- Equip students with the tools to think independently and ethically to ensure technically, environmentally, and socially responsible decision-making.
- Deliver current and relevant curriculum that incorporates equity, diversity and inclusion. This encourages students to create spaces that foster inclusivity and look at design from various perspectives within diverse communities, involving numerous stakeholders.
- Inform students regarding the various facets of the profession, engaging them with designers and industry affiliates.
- Encourage continuous professional and personal growth, instilling a desire to contribute to the profession and society at large. The program encourages students to become active participants in the design community, become members of their local Association, write their NCIDQ exams, give back to the community and become progressive leaders in the profession.
- Prepare graduates for entry-level design positions, equipped with the required knowledge and skills for employment in the diverse design industry, ensuring they possess the body of knowledge necessary to respond to social and environmental issues, while designing interior spaces that are technically proficient, code-compliant, conceptually strong, sustainable and all-inclusive.
- Provide enhanced opportunities for students to pursue post-graduate studies, employment, research and/or further their academic studies and/or credentials.
Pathways from Athletic Therapy and Kinesiology
If you've graduated from a health-science degree other than athletic therapy or kinesiology, your application will be assessed on an individual basis.
Get your Osteopathy degree in less time
If you're a graduate of Sheridan's Athletic Therapy or Kinesiology degree program, you may be eligible to start in the second year of this program after completing three bridging courses.
This bridging program will be available in Spring/Summer 2024.
How to apply: Submit an application using the program code PBHSB .
Bridging courses (Spring/Summer Semester)
- OSTP 17927D: The Science of Osteopathy (3 credits)
- OSTP 14859: Theoretical Pathways to Osteopathy (5 credits)
- OSTP 16333: Practical Pathways to Osteopathy (1 credit)
Course exceptions
After successfully completing the assigned bridging courses, you'll be admitted into Year 2 (Semester 3) of our Osteopathy degree. In order to earn your Osteopathy degree, you'll need to complete all courses in the remaining three years of the program , with the following exceptions.
You will additionally take:
- Year 2: OSTP 11271D: Clinical Methodology (3 credits)
- Year 2: SCIE 22437D: Human Physiology for Allied Health (2 credits)
- Year 3: SCIE 31116D: Pathophysiology for Allied Health (3 credits)
You will not need to take:*
- SCIE 26661D: Pathophysiology 1 (3 credits)
- OSTP 24645D: Structure & Function 2 – Lower Cervicals, Thorax & Upper Extremity (3 credits)
- OSTP 22482D: Applied Clinical Practice 2 (1 credit)
- FLPL 21839D: Internship Prep (1 credit)
- SCIE 39622D: Pathophysiology 2 – Systemic Interactions (3 credits)
- RESE 37626D: Statistical Methods in Health Sciences (3 credits)
- RESE 42279D: Applied Research Methods for Health Sciences (3 credits)
- BUSM 44956D: Business Entrepreneurship for Clinical Practitioners (3 credits)
- 6 Degree Breadth Electives (3 credits each)
*Athletic Therapy graduates are also exempt from the following course:
- OSTP 20782D: Clinical Experience 2 (0.5 credits)
Program Mission
Our mission is to utilize harmonious, comprehensive and specialized training to empower our learners with the knowledge, skills and attitude required to demonstrate the Professional Competencies for Canadian Pharmacy Technicians at Entry to Practice.
Program Vision
Our vision is to graduate highly-skilled life-long learners who uphold professional integrity and promote quality and safety in practice.
Critical Performance Statement
Upon graduation, students in Sheridan’s Pharmacy Technician diploma program will have demonstrated the ability to pursue the career of pharmacy technician and to practice safely in a community or hospital while adhering to the scope of practice for pharmacy technicians.
Field Placements
Writer-in-residence program.
Each year, Sheridan’s Honours Bachelor of Creative Writing & Publishing (CW&P) program hosts a Writer-in-Residence. The 8-month residency is awarded to a writer who embodies the distinctiveness and dynamism of the & in our program name.
In addition to working on at least one specific writing project of their own during their tenure, the Writer-in-Residence is responsible for creative leadership, mentoring and public outreach in the area of creative writing and/or publishing.
How our Writer-in-Residence supports Sheridan students
Students in our CW&P program benefit from the work and mentorship of our Writer-in-Residence, who:
- shares their expertise and experience as a creative professional and working writer;
- performs public readings from their recently published work and/or current work-in-progress;
- organizes public lectures and/or workshops; and
- works with faculty to augment existing curriculum in the area of creative writing, publishing and/or creativity.
2023–24 Writer-in-Residence

Award-winning fiction writer, playwright and poet Kate Cayley is joining Sheridan as the 2023–24 CW&P Writer-in-Residence.
Cayley has published two short story collections and three collections of poetry, and her plays have been performed in Canada, the U.S. and the UK. She has won the Trillium Book Award, an O. Henry Prize and the Mitchell Prize for Poetry, and been a finalist for the Governor General’s Award for Fiction, the Firecracker Award for Fiction, the ReLit Award for both fiction and poetry, and the K.M. Hunter Award for Fiction.
Her writing has appeared in such literary publications as Brick , Electric Literature , Joyland , Best Canadian Poetry and Best Canadian Stories , and she has been a writer-in-residence at McMaster University and the Toronto Public Library.
"Kate’s diverse work plumbs meaning, strangeness and beauty from the spaces we inhabit, even in our domestic lives, while tackling some of the big philosophical questions we all face," says Dr. Genevieve Amaral, Associate Dean, School of Humanities and Creativity. "Our Sheridan community welcomes her capacious intellect, limpid style and wonderful mentorship with full hearts, open ears, and pens, paper and keyboards at the ready!"
From ideas to impact: a masterclass in applied creativity and innovation
Are you a business lead who's looking to level up your company's culture of creativity and entrepreneurship?
Sheridan offers FREE, one-hour masterclasses on topics such as:
- Creative Strategies for an EDI-driven World
- The Science of Creativity and Innovation
- Intra- and Entrepreneurial Thinking, and
- Creative Team Leadership
These masterclasses will catalyze your climate of innovation and give your team an exclusive look into Sheridan’s one-of-a-kind graduate certificate in Applied Creativity and Innovation.*
Register your team for a FREE online one-hour masterclass!
*Advance further with our Applied Creativity and Innovation graduate certificate
Our new graduate certificate in Applied Creativity and Innovation is a great professional development (PD) opportunity for many company teams. The program teaches complex problem-solving, creativity, innovation and negotiation skills — competencies that make companies more resilient and adaptable as they face the unique challenges presented by the future of work.
Why this program is a great choice for PD:
- Flexible hours — your employees can complete their graduate certificate online, at their own pace.
- Relevant coursework – they can choose electives that are most applicable to their position at your company.
- Award-winning faculty – they’ll learn from instructors who are on the cutting-edge of creativity and innovation research.
- Practicum project – they’ll work alongside our team of experts to resolve an issue that your business is facing today — whether it’s how to leverage artificial intelligence technology, advance your sustainability efforts, update your marketing strategies, address supply chain inefficiencies or another challenge.
Apprenticeship Exemption Test
The Apprenticeship Exemption Test (AET) provides a chance for students who are learning a skilled trade to bypass in-class studies.
In most cases, you need to score 70% or higher to pass the AET. If you score lower than 70%, you'll need to wait three months before you can try again (with some exceptions for certain exams).
Apprenticeship Exemption Tests are administered at the Hazel McCallion Campus in Mississauga, through Sheridan's Assessment Centre.
Learn more about the AET, including eligibility criteria, available tests and how to register.
Sheridan is a Ministry-approved Apprenticeship Exemption Test centre, authorized through the Ministry of Labour, Immigration, Training & Skills Development (MLITSD), for both apprentices and non-apprentices.
Program availability
Full-time 1 year Program code: PCRSH


Get a feel for your future
Find your fit and choose with confidence. Choosing the right education is a big decision. At Sheridan we’re committed to providing you with the learning, support and services you need to achieve your goals and reach your full potential. Take a look around, and please connect with us if you have any questions!
Check out our Digital Viewbook 2024–25
Learn about Sheridan’s campuses, programs, support services, alumni and more.
Sign up for a webinar
Join us for a webinar and get answers to those questions on your list.
Book a Campus Tour
In-person tours are offered at all three of Sheridan's campuses. Book your campus tour today!
You might also be interested in:

Honours Bachelor of Applied Health Sciences – Athletic Therapy
Honours baccalaureate degree 4 years.
Gain skills and experience to become a certified athletic therapist.

Clinical Kinesiology
Ontario college graduate certificate 1 year.
Get experience applying kinesiology skills in a clinical environment.
Not sure which program to take?
In 5 easy steps, discover your career preferences — then find programs that could be a great fit!
Have a question? Contact us.
We’re happy to help with any questions you may have. Give us a call at any of these numbers and select Option 4 after the prompt, or simply fill out the contact form and we’ll get back to you as soon as possible.
905-845-9430 (Oakville/Mississauga) 905-459-7533 (Brampton)
Contact Centre hours of operation:
Monday–Thursday, 8:30 a.m.–5 p.m. Friday, 8:30 a.m.–4:30 p.m.
- See Sheridan on Facebook
- See Sheridan on Twitter
- See Sheridan on Youtube
Contact Sheridan

- Office of the Dean
- Strategic Planning
- Awards & Recognition
- Interprofessional Education
- Request IT Support
- DesignED: Course Design Support
- Join our Team: Faculty Position Postings
- Policies and Supports
- School-Based Graduate and Undergraduate Programs
- Advanced Health Care Practice (Master's)
- Applied Health Sciences
- Health & Rehabilitation Sciences
- Health Information Science
- Global Health Systems
- Events for Future Students
- Graduate Student Affordability Calculator
- Communication Sciences and Disorders
- Food and Nutritional Sciences
- Health Studies
- Kinesiology
- Occupational Therapy
- Physical Therapy
- Signature Research Areas & Strategic Planning
- Research Chairs
- Research Centres
- Internal Funding
- Support Team
- Statistical Analysis
- Knowledge Mobilization
- Scholarship Reports
- Musculoskeletal Innovation Factory
- 2024 Research Events
- Undergraduate Summer Research Internships
- Scholarships
- Required Permits
- Anti-Racism Task Force
- Indigenous Committee
- International
- Equity in FHS
- Clinical Epidemiology and Research Management
- Sport and Exercise Medicine
- Upper Extremity Rehabilitation
- Comprehensive Musculoskeletal Physiotherapy
- Wound Healing
- Interdisciplinary Pain Management
- Health Equity and Sustainability
Jointly hosted by

Connect With Us
Email: [email protected] tel: 519-661-2111 x82719, apply online.
Applications are submitted online through Western's School of Graduate and Postdoctoral Studies.

Excellence in Clinical Research
Interested in learning more about this program, complete the form below to get started..
The Clinical Epidemiology and Research Management (CERM) field within the Advanced Health Care Practice master's program gives you the skills needed to build and lead research teams, design fundable studies, build and maintain the infrastructure needed to successfully carry out clinical research, and advance evidence-based thinking in your field. This one-year hybrid program means you will study online through asynchronous and synchronous learning opportunities and take advantage of networking during two short in-person residency periods.
Across the year-long program you’ll develop practical skills related to:
- Clinical research methods
- Biostatistics
- Critical appraisal and evidence-based practice
- Grant writing, research administration and knowledge mobilization
Clinical Research Leadership
Western’s CERM program promotes team-building and evidence-informed thinking. You’ll gain competence as a clinical research leader capable of building and maintaining quality research teams whose outputs contribute to evidence-informed decision making.
Program Highlights
The CERM program’s MBA-style case-based approach gives you the tools to make meaningful contributions to the discussion and advancement of thought in your field. Two on-site residency periods allow for student interaction and for students to:
- Understand research methodology and evidence-based medicine methods
- Learn how to properly initiate, lead, and manage a research study
- Develop quality research questions
- Learn how to build, lead and mentor a research team
- Understand data interpretation and analysis
- Explore successful grant writing
- Learn the importance of writing and justifying a budget for your study
- Develop communication strategies that lead to effective knowledge mobilization
Admission and Applying/Tuition and Fees
Admission requirements.
All applicants must meet the following general requirements, in addition to the specific requirements outlined below for each applicant group.
- A minimum bachelor's degree from a recognized university and at least a (B) standing (or equivalent) over the final two years of the program
- Advanced computer skills
- Academic (if attended university within the last five years) and professional
- See below for more information
- Completion of supplemental questionnaire
In addition to the general requirements outlined above, applicants to the CERM program must meet the following criteria:
Clinicians/Practitioners:
- Evidence of more than 2 years of experience in clinical practice or
- Physician trainees may enter after postgraduate year 3 (PGY3) or beyond
Non-Clinicians Working in a Health Care Setting:
- Evidence of more than two years of related experience, post-undergrad (including participants in the MD+ program)
English Language Proficiency
- Applicants whose first language is not English must provide evidence of English language proficiency (TOEFL or IELTS is recommended)
- A minimum TOEFL score of 620 (paper-based), 105 (internet-based), 260 (computer-based) or an IELTS score of 7.5 is required
- Students who, after admission, show an inadequate command of spoken or written English must improve their proficiency to the satisfaction of the Faculty of Health Sciences
- Students may be asked to withdraw from the program if their command of English interferes with their ability to provide quality professional services
- Students who are required to present evidence of proficiency in English must make their own arrangements to write the TOEFL and to have the official results sent directly to the School of Graduate and Postdoctoral Studies
- The English language proficiency requirements outlined above do not apply to students from Quebec
Applying to the Program
Have questions about applying.
Email [email protected] .
To apply to the Advanced Health Care Practice program, students should:
- Visit the online application website
- Follow the instructions on that page to access the application
- Select "Advanced Health Care Practice" from the program options in the application and select the appropriate field
Application Deadline
- Applications will open November 1 and close June 1
- Applications maybe accepted after that date if space is available
- Offers of admission are sent out beginning in late April and continue until the program is filled
Tuition and Fees
The annual domestic tuition fee for this program is approximately $14,000 CAD including ancillary fees (plus $2,100 CAD mentor fee i.e. $700 CAD/term***) which is payable over three terms (September, January and May).
The annual international tuition fee for this program is approximately $37,000 CAD including ancillary fees (plus $2,100 CAD mentor fee i.e. $700 CAD/term***) which is payable over three terms (September, January and May).
These fees are subject to change and are set by Western University . Instructions for students paying tuition from a Canadian bank are available from the Office of the Registrar .
***Please note all fees including mentorship fees and course fees are currently under review and are subject to change and adjustment*
OSAP Eligible
The calculator was designed for you to get a better estimate of what it will cost to attend one of Western's graduate programs for one year.
Click here to learn more and access the calculator
Capstone Experience
The Capstone Experience consists of a mentorship opportunity in the student's area of concentration. A minimum of 100 hours will be completed under the supervision of one or more approved mentors in a health setting. Students will identify potential mentorship opportunities within their own community. Together, with their academic mentor (appointed by the Program), an appropriate, available opportunity will be secured.
Students are expected to secure mentors in the Fall term. Students will consult with their professional mentors and a faculty supervisor to determine learning objectives and goals for the duration of the capstone experience.
Learning Outcomes
Upon successfully completing the program, students should have the ability to:
- To locate and critically analyze the scientific literature to inform decision making in your field
- To formulate research questions that are unique and advance the science in your field
- To design clinical research using the appropriate methods to test research questions
- To synthesize relevant literature to create evidence informed recommendations in your field
- To build and lead research teams and build and maintain research infrastructure
- To write grant proposals to funding organizations for clinical and methodologic research
- To communicate and apply ethical principles and practices in research activities
- To develop communication and dissemination strategies for knowledge production from clinical research
- To develop iterative self-reflection skills for continued life-long learning
Program Structure and Course Offerings
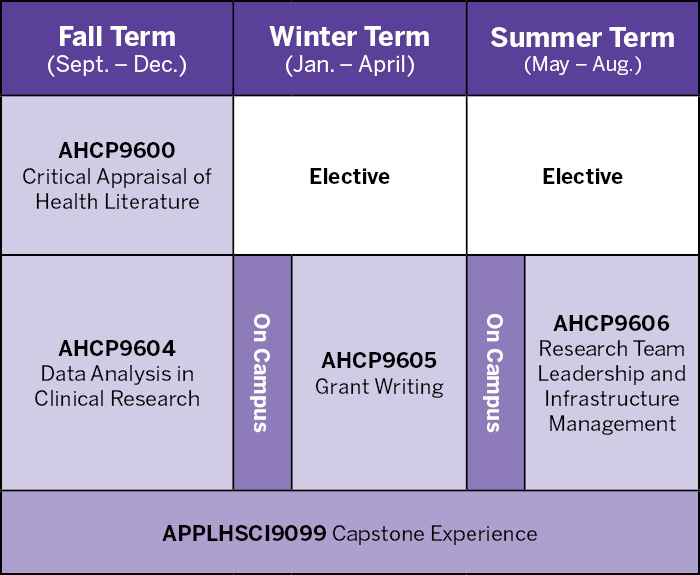
Fall Term (September - December)
- AHCP 9600: Critical Appraisal of Health Literature
- AHCP 9604: Data Analysis in Clinical Research
- APPLHSCI 9099: Capstone Experience
Winter Term (January - April)
- Elective #1 (see below for options)
- AHCP 9605: Grant Writing
- APPLHSCI 9099: Capstone Experience (continued)
- On Campus Residency
Summer Term (May - August)
- Elective #2 (see below for options)
- AHCP9606: Research Team Leadership and Infrastructure Management
- APPLHSCI9099: Capstone Experience (continued)
Elective Course Options
Students in the program are required to complete two of the following elective courses:
- APPLHSCI 9009: Project Management
- APPLHSCI 9010: Health Services, Systems and Policy
- APPLHSCI 9012: Health Program Evaluation
- APPLHSCI 9015: Evidence-Based Leadership in Healthcare
- APPLHSCI 9016: Economic Evaluation for Health Innovations
- APPLHSCI 9017: Implementation Science in Practice
Learn more about our elective course offerings .
Opt In To The Certified Health Executive (CHE) Certificate
As a student in Advanced Health Care Practice master's program, you're eligible for advanced standing toward the Certified Health Executive (CHE) designation.
The CHE is Canada's top professional leadership designation for healthcare leaders looking to expand their institutional leadership career.

If you opt in to the CHE, you must complete the following:
- Submit a detailed project proposal by mid-September
- Complete APPLHSCI 9001 and APPLHSCI 9015
- Complete a LEADS 360 Assessment and 90-minute debriefing
- Meeting regularly with your CHE mentor
- Developing an organizational change, strategic planning or program evaluation project
- Organizing and facilitating a program-wide leadership day in collaboration with the other CHE students
For more information, email: [email protected]
1151 Richmond Street London, Ontario, Canada, N6A 3K7 Privacy | Web Standards | Terms of Use | Accessibility
Popular search terms:
No results found for “ ”.
Search another word or try popular search words.
Clinical Epidemiology and Health Care Research (CEHCR)
Clinical epidemiology prepares future clinician scientists to conduct patient-oriented research and translate this knowledge into clinical practice.

CEHCR is designed to train individuals with a health professional background to become independent investigators.
Clinical epidemiology, also known as ClinEpi, applies epidemiologic principles and methods to the delivery of healthcare, sometimes referred to as “the basic science of clinical medicine.” Clinical epidemiology covers a wide range of disciplines, emphasizing research on improving prevention, diagnosis, prognosis, and treatment in patients.
Students will learn the principles and methodologies of epidemiology to investigate gaps and barriers in patient care, test hypotheses, and translate their research into practice and health policy.
CEHCR has an international reputation for excellence in clinical, health services, knowledge translation, and decision sciences research. Graduates will join an extensive network of leaders in healthcare research, policy, and practice throughout Canada.
MSc – Master of Science
CEHCR Application Deadline: November 15, 2023
Explore MSc Degree
Phd – doctor of philosophy, explore phd degree, program highlights.

Collaborate
Gain access to the program’s highly connected and integrated network, situated near the Faculty of Medicine , research institutes, and leading academic hospitals.

Lead Research
Lead high-calibre research with the opportunity to publish in peer-reviewed scholarly journals. An ICES satellite is on campus, providing the opportunity for access to its extensive holdings of Ontario’s population health data.

Make Connections
Learn from internationally recognized faculty and make lasting connections in the field of clinical epidemiology and health service research.
Clinical Epidemiology Careers
- Since 1992, the program has graduated more than 500 students from varied backgrounds, including medicine, nursing, pharmacy, chiropractory, physiotherapy, and midwifery, among others.
- CEHCR graduates typically continue to practice clinically while pursuing careers as independent clinical investigators.
- Our graduates hail from across Canada, the US, Mexico, South America, Europe, the Middle East, Asia, Africa and Oceania.
Clinical Epidemiology and Health Care Research (CEHCR) Directors Past and Present
CEHCR Directors are internationally recognized leaders that bring their expertise to the program and ensure graduates receive exceptional training in clinical, health services, knowledge translation, and decision sciences research.
Sindhu Johnson : 2023-Present (Associate Director 2020-2023)
Nick Daneman : 2023-Present Associate Director
Jill Tinmouth : 2020-2023 (Associate Director 2017-2020)
Rob Fowler : 2015-2020 (Associate Director 2014-2015)
Sharon Dell : 2012-2015 (Associate Director 2010-2012)
Ahmed Bayoumi: 2006-2012 (Associate Director 2005-2006)
Gillian Hawker : 2000-2006
Claire Bombardier : 1988-2000
People from Clinical Epidemiology and Health Care Research (CEHCR)

Accepting students Find by research interest or program

Graduate Students
Get the latest student theses

Administrative Staff
Get the help you are looking for
Latest Alumni Stories

Duminda Wijeysundera
Duminda Wijeysundera shares how CEHCR was the necessary springboard to an exciting and fulfilling career in academic healthcare.

Michael Hillmer
Michael Hillmer shares three highlights that stood out as a learner: the supervision, training environment, and becoming a leading expert.

Harindra Wijeysundera
Harindra Wijeysundera shares how CEHCR prepares researchers through a collaborative environment.

Fahima Dossa
Fahima Dossa encourages CEHCR PhD students to dive in, and dive in deeply.
Latest CEHCR News

Research and Impact Day 2024: Event Recap
April 30, 2024

IHPME Team Leads Work on World Bank Report
May 30, 2023
Faculty / Research / Students

Research and Impact Day 2023: Event Recap
May 19, 2023

Research and Impact Day 2023
March 22, 2023
Education / Faculty / Research / Students

IHPME Celebrates International Women and Girls in Science Day
February 13, 2023

IHPME Students Named 2022 Vanier Scholars
December 19, 2022

New faculty spotlight: A Q&A with Dr. Zahra Shakeri
June 1, 2022

PhD students awarded Canada Graduate Scholarships from CIHR
May 13, 2022
Research / Students

Celebrating International Women’s Day: A Q&A with surgeon-scientist-in-training Dr. Fahima Dossa
March 2, 2022

‘It reminds me of the responsibility I have to my community in the future’: Opportunity Scholarship recipients committed to addressing health care disparities
October 26, 2021
Upcoming Events

Digital Health Transformation in Europe: the new data strategy
May 24, 2024 from Noon-1pm (EDT)
Online via Zoom
Partner & Affiliate Events
C onnect with Clinical Epidemiology and Health Care Research (CEHCR)
Cehcr program director.
Sindhu Johnson Phone Number: 416-603-6417 Email Address: sindhu.johnson@uhn.ca
CEHCR Associate Director
Nick Daneman Email Address: nick.daneman@sunnybrook.ca
Co-leads the management of the Clinical Epidemiology and Healthcare Research (CEHCR) program.
Graduate Administrator
Zoe Downie-Ross Phone Number: (416) 946-3486 Email Address: ihpme.grad.admin@utoronto.ca
Coordinates student records, graduate funding, and student-related awards.
Graduate Admissions
Christina Lopez Email Address: ihpme.admissions@utoronto.ca
Manages admissions and responds to all related inquiries.
Graduate Assistant
Nadia Ismail Phone Number: (416) 946-4100 Email Address: ihpme.grad.assist@utoronto.ca
Coordinates various graduate initiatives including defences, student events, and graduation.
Program Assistant – CEHCR
Aileen O’Dowd Email Address: clinepi.courses@utoronto.ca
Manages the CEHCR courses including course enrolment, grades, and access to Quercus. For admissions inquiries contact ihpme.admissions@utoronto.ca .
Graduate Placements
Christina Lopez Phone Number: (416) 978-1108 Email Address: ihpme.placements@utoronto.ca
Coordinates details involving student placement and experiential learning
The University of Manitoba campuses are located on original lands of Anishinaabeg, Ininew, Anisininew, Dakota and Dene peoples, and on the National Homeland of the Red River Métis. More
Max Rady College of Medicine
University of manitoba.
University of Manitoba Winnipeg, Manitoba Canada, R3T 2N2
Clinician Investigator Program
To further enhance the Max Rady College of Medicine's strategy to improve the health of society, the Clinician Investigator Program provides the next generation of medical and surgical specialists with the leading-edge graduate research training necessary to become successful clinician-scientists.
Program details
Admission and application requirements.

• Postgraduate Medical Education (PGME)
Accreditation
• Royal College of Physicians and Surgeons of Canada
Expected duration
• 2-4 years
With flexible options, you can take Clinician Investigator Program research training during or immediately after a Royal College of Physician and Surgeons of Canada clinical residency program. The program requires a minimum of 24 months of protected research time and includes enrollment in a graduate degree program (graduate stream), or in a postdoctoral fellowship program, if the trainee already has a research-based graduate degree (postdoctoral stream).
Academic curriculum
The entire CIP curriculum is comprised of one orientation event and one academic week (five full days) plus one to three academic half-days per year.
CIP is an accredited postgraduate medical education program of the Royal College of Physicians and Surgeons of Canada , and all curriculum sessions are designed to address all of the CanMEDs goals and objectives relating to becoming a clinician investigator.
The program is a minimum of 24 months of active research (minimum 80 per cent protected research time) but can often span up to four years, depending on the type of research project and graduate degree.
Areas of research
Eligible areas of research include all four Canadian Institutes of Health Research (CIHR) pillars:
- population health
- health services research
Also captured is research in related areas such as economics, education, e-health, engineering or ethics.
Enhance your training
Many institutions are now including the completion of a graduate degree as an application requirement for all faculty positions. CIP includes the completion of a Masters or PhD, while being fully funded at postgraduate year (PGY) level.
And there are many other benefits to consider:
- Hands-on learning to develop and execute a self-directed research project
- 24+ months of mentored research training with a focus on the integration of clinical and research responsibilities
- Protected research time (during which the trainee must dedicate a minimum of 80 per cent of their time to research)
- Completion of a research-based graduate degree program
- Enhanced academic curriculum to prepare for a career as a clinician investigator
- Progress monitoring and evaluations
- Full salary (at PGY level)
- Team environment working alongside other trainees working toward a similar career/goal
- The opportunity to work with leaders in the research field of interest
- National brand of a RCPSC-accredited residency program
- Support from the faculty CIP committee
- The opportunity to develop contacts and network within a specific clinical research field
- The opportunity to create a more competitive body of work for application to faculty positions
Trainee support and funding
Building a great support committee and designing a research project can take time. It is highly recommended that you start planning your time in CIP as early as possible. The CIP Director is happy to help trainees develop their plans. We encourage you to involve CIP and your clinical department faculty throughout the planning process.
Accepted trainees are guaranteed funding for the full 24 month program at appropriate PGY level through the use of PGME Flex Spots and other funding awards and partnerships. However, CIP trainees are required to apply for external salary awards for their second year. This improves the trainee's resume and, if successful, frees up the Flex Spot funding so that it can be used to sponsor more future CIP trainees.
Some surgery specialties offer one year of funding to complete a research-based Masters degree. This program may be combined with CIP to fund one of the two years provided this is arranged ahead of time and the trainee is enrolled in CIP prior to starting the Master of Surgery year.

Late applications may be considered but must be accompanied by a letter of support from the trainee’s clinical department head requesting an exception for the trainee.
The Residency Program Committee reviews all applications and approves those who are selected for entry.
Conditional acceptance into the program can occur before acceptance into a graduate studies program, however, proof of enrollment will be required prior to final acceptance.
Trainees are also only conditionally accepted until full funding has been confirmed for their first two years in the program.
We thank all applicants for their interest in our program, however only those accepted will be formally contacted. Unsuccessful applicants will be notified by email.
How to apply
In addition to the required documents detailed below, your application to the Clinician Investigator Program must include:
A completed CIP Application Form and Checklist (PDF)
A completed CIP Registration Form (PDF)
Required documents
Required documents include:
- Curriculum vitae
- Statement of your career plans, including those relating to practicing as a clinician scientist (one page)
- Project description including timeline, specifying your roles within the overall research project (three pages)
- Names of at least three research advisory committee members, at least one of whom must be a clinician scientist in your specialty
- Your supervisor’s curriculum vitae
- Letter from your supervisor(s) indicating the role(s) and responsibilities that they will have in your mentorship or training
For PhD applicants only:
- Letter from your clinical department head and research supervisor describing availability of funding for years three and four
- Letter of support from your current clinical program director (if required)
- Your status and year of training in your postgraduate medical education program
- Endorsement of your registration in CIP
- Your research potential, interest, and ability to complete the program
- Your expected completion date of clinical training
- Copies of your publications (for post-doctoral stream applicants only)
- Two letters of reference emailed directly to the CIP office
Please ask referees to personalize their letters to speak to your potential as a researcher, your commitment to your degree program, your proposed project, the proposed learning environment, and your commitment to a career as a physician-scientist.
The letter should also contain a statement that the applicant has not and will not see a copy of the letter.
Required certifications
You must submit certificates for the following courses to the CIP office within six months of your program start date:
TCPS 2: CORE (Course on Research Ethics)
The online tutorial is an introduction to the second edition of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2).
It consists of eight modules focusing on the guidance in TCPS 2 that is applicable to all research regardless of discipline or methodology.
Visit the TCPS 2: CORE (Course on Research Ethics)webpage to register. If you have already completed this tutorial but do not have a copy of your certificate, you should be able to log on and print another copy through the TCPS2 portal .
UM Research Integrity Online Course
This highly interactive course gives participants the opportunity to learn the principles and professional responsibilities of what doing good research implies.
You will be exposed to practical advice on how to deal with the challenging situations in which they may find themselves doing research within their own area of expertise, along with the latest standards, codes and policies in the responsible conduct of research both locally and on a global basis.
For information on how to register for this course, visit the Research Integrity Online Course webpage .
Please email your application and registration forms, along with all requested supporting documents, to [email protected] .
All other inquiries can be directed to:
Shayne Taback Program director Clinician Investigator Program Phone: 204-789-3618 [email protected]
Tashanna Gabbidon Program administrator Clinician Investigator Program Phone: 204-789-3498 [email protected]
Explore the University of Manitoba
We attract people from around the world who share our ideals and vision for positive change. We believe in embracing challenges and taking action. Our students, researchers and alumni bring their unique voices to learning and discovery, shaping new ways of doing things and contributing to important conversations in topics that matter most, from human rights to global health to climate change. We are where imagination and action collide.

For over 125 years, the Max Rady College of Medicine has contributed to education, research and clinical service. Western Canada’s first medical school, the College develops qualified medical graduates who distinguish themselves through excellence in clinical care, health system innovation and leadership, and internationally recognized research.
- Programs of Study
- Student experience
- Research and scholarly activity
- Community and partners
Director, Clinician Investigator Program Dr. Shayne Taback Max Rady College of Medicine University of Manitoba (Bannatyne campus) Room 510 - 715 McDermot Avenue Winnipeg, Manitoba R3E 3P4
You are using an outdated browser. Please upgrade your browser to improve your experience.

Get the Skills to Become a Clinical Researcher

1 Year Clinical Research Program in the Greater Toronto Area
Do you want to make a major impact on healthcare? Could you see yourself maintaining the right testing environment that would allow for the development of new, potentially life-saving drugs? If so, you have the makings of a great Clinical Researcher.
As a Clinical Researcher, you would organize the collection, delivery, and storage of data taken during clinical trials. A key part of this role is overseeing large clinical trials, which is similar to managing big projects. Therefore, you must have strong project management skills. Attention to detail and data analysis is crucial! The approval of the drug hinges on you delivering uncompromised data to regulatory authorities. You must also be able to educate doctors and patients about the trial and present your findings via research papers, presentations, and reports.
Flexible Program Options
If you’re interested in starting a rewarding new career, but you can’t put your entire life on hold to pursue it, you’re in the right place. At Oxford College, we offer flexible class schedules including Morning, Afternoon, Evening and Weekend sessions. We also offer flexible learning options (Online, Hybrid and In-person) for many of our programs.
For more information about Oxford College’s flexible program options, inquire today!
Refer a Friend to Oxford College
Do you know someone who is looking for a change in their life? Refer a friend to Oxford College to help propel their career! If your friend is interested in following in your footsteps, you could be eligible for a $500 reward.
Refer a Friend Now!
The best way to see if Oxford College is the right fit for you is to come in and visit the school. You can tour our facilities, and meet with both students and instructors. Schedule a Tour Now or Get More Info .

Clinical Research
1-year postgraduate diploma | $76,960 median annual salary***.
- Graduates hired by government departments and agencies, research institutes & hospitals
- Practical, hands-on training through Oxford’s fully-equipped Medical Lab
- Advance into Clinical Technology, Laboratory Science, Clinical Management and much more!
*** https://www.jobbank.gc.ca/marketreport/wages-occupation/23070/ON visited on April 5, 2024 . Salary source for clinical research associates in Ontario with lowest 10% earning $37,440 to the highest 10% of workers earning $114,795.
Become Qualified in Clinical Research
The Clinical Research postgraduate program at Oxford College prepares you for a range of exciting professional opportunities. You will develop the concepts, skills, and techniques necessary to succeed in the clinical research field.
As a Clinical Researcher, you will conduct research, produce reports, and administer healthcare policies and programs. Clinical Research professionals contribute to research studies that have major impacts on healthcare. You will learn the latest developments in the clinical research field and gain a competitive advantage in this dynamic industry.
During the Clinical Research postgraduate program, you’ll learn how to:
- Assist in developing government health policy by reviewing relevant literature, conducting interviews, collecting and analyzing statistical data, and providing advice to senior managers and officials on issues such as health promotion, regulation, standards, and financing
- Design and implement health projects or programs
- Maintain, update, and manage health information databases
- Compile and analyze statistical information provided by private and public healthcare institutions and organizations, and produce reports
- Monitor and evaluate healthcare programs operated by government departments and agencies or private organizations
- Assess compliance with health standards and identify remedial action if necessary
- Conduct evaluations and assessments of health projects and programs
- Provide consulting services to clients in private establishments or government departments or agencies
- Respond to internal and external program and policy information requests
- Present the views of an association or organization to politicians, government officials, the media, or the general public
This program requires a minimum B.Sc. or Equivalent or a Diploma in Allied Health or Pharmaceuticals.
Is a Career in Clinical Research Right for You? Take the 'Oxford College Clinical Research Career Training Readiness Quiz'
This fun, online quiz takes 3-minutes to complete and you’ll get a personalized report. Identify your strengths and social style plus the training and positions you’re best suited for. Get your Clinical Research Career Training Readiness score now!
Never a Dull Day
This fascinating career never gets stale, because the circumstances of a trial are always changing. You may work for various departments, with different populations, parameters, and sites. Even within one clinical trial, you’ll get variety. That’s because clinical trials are carried out in phases at various locations and you’ll be involved in each stage of the trail.
Countless Career Opportunities
Graduates are employed by government departments and agencies, consulting establishments, universities, research institutes, hospitals, community agencies, educational institutions, professional associations, non-governmental organizations, and international organizations.
Upon completion of the program, you may find employment as:
- Clinical Research Coordinator
- Clinical Research Associate
- Clinical Research Project Leader
- Medical Information Associate
- Clinical Data Management Associate
Career Services
After your training, we won’t leave you hanging. Career Services is here to help you throughout your entire career training journey and afterwards, too. From organizing placements at prominent companies to helping you with your resume, cover letter, and interview tips, you’ll feel confident that you’re putting your best foot forward when it comes time for you to enter into the profession. What’s more, you can access our services after graduation if you need help with the employment hunt. Because when you become a student at Oxford College, you join the Oxford family for life!
Financial Aid
Many people need extra financial assistance to attend school. At Oxford, we believe that finances should not be a barrier for anyone seeking higher education. That’s why we have many funding programs in place, including OSAP, Second Career, and private student loans, to name a few. We will also work with you to set up manageable monthly payment plans for you. Sit down with a financial aid advisor, and they’ll help to assess your situation and create a funding plan that works for you.
Is a Rewarding Career in Clinical Research Right For You? Get More Info…
If you’re interested in learning more about Clinical Research and exploring if this is the right career path for you, fill out the form on this page to receive more information.
For immediate questions, call 1-866-604-5739
- Program Overview
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning, and advancing global health.
The Society of Clinical Research Associates (SOCRA) established the Certification Program for Clinical Research Professionals in order to create an internationally accepted standard of knowledge, education, and experience by which clinical research professionals will be recognized by the clinical research community. Those individuals so recognized may use the "Certified Clinical Research Professional" or "CCRP ® " designation.
Path to Certification
CCRP certification is awarded upon meeting two criteria: a successful written application and a passing CCRP examination score. The benefits of obtaining certification are numerous. It not only validates knowledge, skills, and abilities but also enhances credibility and peer recognition. Career advancement and increased earning potential become tangible outcomes, reflecting a commitment to standards, compliance, and integrity.
Scope and Standards of Practice
The standards upon which this certification program is based have been set forth by SOCRA to promote recognition and continuing excellence in the ethical conduct of clinical trials. It is the goal of SOCRA to encourage members, and assure the competency of certified members, in their knowledge, understanding, and application of the conduct of clinical investigations involving humans in accordance with the ICH Guidelines, the U.S. Code of Federal Regulations, and the ethical principles that guide clinical research. Members are expected to adhere to national, state, local and provincial regulations and to international guidelines published by the International Conference for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and all applicable federal, state and local laws and policies.
Standards of Practice include an understanding of and application of basic concepts of Good Clinical (Research) Practice, including:
- The Nuremberg Code
- The Belmont Report
- The Declaration of Helsinki
- 21 U.S. Code of Federal Regulations – Parts 11, 50, 56, 312, 812
- 45 U.S. Code of Federal Regulations - Part 46
- ICH Harmonised Guideline for Good Clinical Practice E6(R2), and
- ICH Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (E2A)
- 42 CFR Part 11 (ClinicalTrials.gov)
Certification Exam
The SOCRA Certification Examination is offered in two formats: paper and pencil (at SOCRA sponsored sites), and computer based (at Prometric testing centers or through Home Proctoring).
SOCRA Sponsored Sites: Paper and Pencil
- Hosted exams offered in various location throughout the US and Canada.
- Visit the paper and pencil exam schedule for dates and locations.
- A complete application must be received by the deadline date as stated on the examination schedule.
- Score reports mailed to you in 4-6 weeks after exam.
Computer Based Testing: Testing Centers and Remote Proctoring
- Offered at Prometric testing centers throughout the world or through Home Proctoring
- Click here for a list of test centers.
- Allow 2-4 weeks for application processing.
- Once application is approved, schedule exam at a testing center. Exam sessions are available at least 6 weeks in advance.
- Score reports received immediately upon completion of exam.
Candidate Handbook
For more information, please view the Candidate Handbook.
Certification
- CCRP Certification Quick Facts
- Definition of a Clinical Research Professional
- Certification Program Policies
- Removal of CCRP® Credential
- Verify Certification
- Exam Overview
- Candidate Eligibility
- Application and Fee
- Computer Based Testing Exams
- Paper and Pencil Exams
- Refunds, Rescheduling and Retesting
- SOCRA Sponsored Exam Schedule
- Preparing for the Exam
- Preparation Resources
- Examination Results
- Host an Exam at Your Site
- Apply Online
- Exam Schedule SOCRA Sponsored Sites
- Requirements for Maintaining Certification
- Continuing Education Requirements
- Descriptions of Acceptable CE
- CE Recordkeeping Requirements
- Request for SOCRA CE for Courses / Workshops
- Installment Plan Payment
- Renewal of Certification
- Recertification Audit
- Recertification Learning Module
- Accreditation
Summary of Certification Activities
11,145 CCRPs (as of 12/31/2022)
- 1,391 candidates took CCRP exam
- 73% passed CCRP exam
- 2,649 CCRPs recertified
- 946 candidates took CCRP exam
- 65% passed CCRP exam
- 2,783 CCRPs recertified
- 2,060 candidates took CCRP exam
- 70% passed CCRP exam
- 3,801 CCRPs recertified
- 1,980 candidates took CCRP exam
- 71% passed CCRP exam
- 3,188 CCRPs recertified
- 104 exam sites hosted
- 2,175 candidates took CCRP exam
- 2,491 CCRPs recertified
- 91 exam sites hosted
- 2,141 candidates took CCRP exam
- 2,421CCRPs recertified
- Course Development & Customization
- Forms & Policies
- Certificate Programs
- Bridging Programs
- Registration & Course Formats
- Advanced Life Support
- ACLS UofT Residents
- All CE Courses
- CEPD Accreditation
- Micro-credentials
- Michener Centre for Post-Graduate Nursing Education
Principles of Clinical Research I
- Continuing Education
Home › Continuing Education Courses › Principles of Clinical Research I
Course Information
Course code, instruction method.
Online – login information will be emailed on the course start date
Domestic: $898 International: $1167
12 weeks – 4.8 CEU
Instructor(s)
Debbie Warner, RN, CCRC
Learner Outcomes
Upon successful completion of the course, the learner will be able to:
- Explain the drug development process
- Describe the Canadian drug review process
- Describe the elements of a clinical trial
- Describe the roles and responsibilities of personnel and organizations involved in clinical trials.
- Understand and use the terminology associated with clinical research trials
- List and describe the importance of the regulations relating to the conduct of clinical trials (ICH, Health Canada (Division 5), and FDA).
- Explain the ethical principles governing clinical research
- Describe Good Documentation Practices
- Understand Electronic Data Capture (EDC) Principles
- Describe the primary aspects of privacy and protection of personal information as related to clinical research.
- Discuss recruiting challenges and strategies.
- Describe the requirements for collecting and reporting of Adverse Events in clinical trials
Course Objectives
The learner will begin his/her development as a member of the clinical research team through the study of clinical research Good Clinical Practices and the development of practical skills in clinical trials. During this process, students will acquire the core skills required to become a part of the clinical research team. The course will introduce the learner to the role of clinical trials and research in the health care sector and in industry. The phases and elements of clinical trials will be considered and current issues relating to clinical research will be discussed.
Evaluation Method
Individual assignments, group case study, test, participation
Intended For
Healthcare professionals interested in clinical research
Course registration will close once maximum number of students enroll. Register as soon as you can!
Note: If you are currently working in Clinical Research you may be eligible for a Prior Learning Assessment, for the course Principles of Clinical Research I- CRCR130. Please contact [email protected] for more information.
Certificate Program
Prerequisites, topics covered.
– History of Clinical Research
– Roles and responsibilities of key personnel involved in clinical research
– Role of clinical research in the development and marketing of therapeutic interventions
– Elements of a clinical trial
– Phases and types of clinical trials
– Terminology associated with clinical trials
– Importance of regulations relating to the conduct of clinical trials [ICH, FDA, TPD]
– Good Clinical Practices Guidelines
– Recruiting study subjects
– Requirements for the reporting of Adverse Events
Principles of Clinical Research I (CRCR130) is part of the Clinical Research Program . This course is for healthcare professionals interested in clinical research that need to develop the basic skills for a clinical trial environment.
Registration instructions:
- Select button “View Available Sections and Registration”
- This page contains 3 boxes. Click on the “Sign In” box
- If you have a Self service account, sign in here. If you do not, then create an account.
- Select your course/section by clicking the “Add” button which will send the course to your cart.
- To move on to payment, click the blue “Register” button.
- Once payment has been processed, registration is complete.
COVID Vaccine Requirement:
Proof of full vaccination (2 doses) for all learners is required for all onsite attendance at Michener. Fully online courses do not require proof of vaccination.
Instructions to upload proof of COVID vaccination to Self Service
Privacy Overview

- RECRUITMENT

Clinical Research, Pharmacovigilance and Regulatory Affairs (Online)
Program details.
- Start Date: Open
- Duration: Self-Paced
- Admission Requirement:
- » Post Graduate Diploma – BSc. or higher level of education.
Online Course Outline
- CR09 The Canadian Pharmaceutical Industry: Big Picture
- PRA101 Introduction to Regulatory Affairs
- GMP1001 Introduction to Good Manufacturing Practices – Level I
- CR013 Clinical Pharmacology and Clinical Safety Assessments
- CR014 Good Clinical Practices (GCP), Research Ethics and Clinical Research Regulations

- PRA302 Regulatory Affairs Generic Drugs
- PRA204 Regulatory Affairs for Medical Devices
- CR019 Organization of Clinical Trials and Clinical Monitoring Plan Development
- CR020 Clinical Project Management and Project Chart Development
- CR021 Clinical Data Acquisition and Data Management
- CR023 Drug Lifecycle Safety Management and Pharmacovigilance Compliance
- TWC3001 Technical Writing and Scientific Communication
- CR025 Clinical Study Protocol and ICF Development
- PRA305 Global Regulatory Affairs
- PRA205 Regulatory Affairs for Natural Health Products
- CR028 Global Clinical Research and Pharmacovigilance
- PRA202 Regulatory Affairs for Clinical Research and Preclinical - Drugs
- CR030 Clinical Research, Drug Safety and Pharmacovigilance Project
- PRA203 Regulatory Affairs Biotech/Biologics
Program Overview
The demand has never been greater for professionals who can help companies ensure compliance with applicable laws and regulations in the development and commercialization of new drugs and healthcare products. The Clinical Research, Pharmacovigilance and Regulatory Affairs program will provide you with knowledge and insight into the most recent developments in the clinical research, pharmacovigilance and regulatory affairs field. You will learn current regulatory and pharmacovigilance research current clinical research topics including Good Clinical Practices (GCP), regulatory submission, pharmacovigilance regulations and Good Pharmacovigilance Practices (GVP), and quality assurance concepts in clinical trials and pharmacovigilance. As a student in this program, you will benefit from an applied and practical approach to training and develop a network of industry contacts ahead of graduation.
Career Opportunities
Graduates of the C linical Research, Pharmacovigilance and Regulatory Affairs Post-Graduate Diploma Program can pursue careers in the Pharmaceutical, Biotechnological, Medical devices, Cosmetics, Natural health Products, and allied industries. Trained and qualified RA professionals are in demand for pharmaceutical, biotech, medical device and natural health product companies as they are needed to navigate the intricacies of regulatory submissions for new products.
AAPS graduates have been hired as:
- Clinical Research Coordinator
- Pharmacovigilance Data Management Associate
- Clinical Research Associate
- Pharmacovigilance Associate
- Clinical Data Management Associate
- Clinical Research Project Leader
- Pharmacovigilance Project Leader
- Clinical Research Monitor
- Medical Information Associate
- Pharmacovigilance Officer
- Quality Assurance in Clinical Research
- Quality Assurance in Pharmacovigilance
- Auditor in Clinical Research and Pharmacovigilance
- Quality Assurance Associate/Technician
- Auditor/Inspector
- Technical Writer
- Document Reviewer
- Regulatory Affairs Associate
- Regulatory Compliance
- Regulatory Operations Associate
Organizations who have hired AAPS Graduates

More Companies

Certifications
Graduates will receive an Online Post-Graduate Diploma on Clinical Research, Pharmacovigilance and Regulatory Affairs. This program is approved as a vocational program under the Private Career Colleges Act, 2005 . The Private Career Colleges Branch is part of the Ministry of Colleges and Universities and the Superintendent of Private Career Colleges is an appointed position by the Minister of Colleges and Universities.
AAPS Graduates will also qualify for Certified Clinical Research Professional – CCRP Certification awarded by Society of Clinical Research Associates (SOCRA).
For more information on benefit of CCRP certification please visit https://www.socra.org/certification
To register, enroll into any online course or contact us via telephone at 416-502-2277 or by email at [email protected].
Online Registration Process
Faq – aaps elearning, financial aid.

Interested in AAPS? Contact us directly or provide your information for a call back.
Toronto campus, hours of operation.

How to Become a CRA in Canada: Clinical Research Associate Certification
How to become a cra in canada, how to get a clinical research associate certificate online in canada.
CCRPS offers Canadian CRA certification for students living and working in Canada. A CRA certification in Canada provides students with additional knowledge of resources and guidelines in order to work in clinical research.
Our certification specifically state and prove that you have reviewed Canadian guidelines in clinical research and meet the competencies required for all clinical research associates.While our certificate is accredited by the ACCRE, 4 other organisations, and shows employers you have the working knowledge to act as a CRA; the CRAC credentialing exam will help further establish your professionalism. If you already have experience as a clinical research associate, you can use this course to prepare and sit for the CRAC examination.
In order to become a certified Canadian CRA, you must take the CRAC credentialing examination. To sit for the examination, you must fulfill one of these requirements :
2 years of clinical research expereience within the last 5 years
3,500 hrs of part time experience with at least 1 year of Canadian experience within the last 5 years
A post-graduate certificate in clinical research and at least 1 years’ experience over the past 2 years in clinical research. You must have at least 1 year of Canadian experience.
2. Clinical Research Associate Canada (CRAC) Membership
While CCRPS membership is free, you still benefit from obtaining CRAC membership even if you do not have enough experience to sit for the CRAC credentialing exam. Membership can create networking and job opportunities, as well as provide insight into the field. All interested professionals should become a Member of the Official Canadian CRA Association and list this involvement on their CV and cover letter.
3. CCRPS Canadian CRA Certification (CCRAC) for Resume Building
As we teach nearly 30 Canadian students each quarter, our Canadian Clinical Research Associate Certification will allow students to strengthen their application and resume, specifically towards Canadian guidelines and policies.
4. Landing A Entry Level Clinical Research Associate Job in Canada
Unfortunately, many professionals have trouble securing a job in order to get experience and take the CRAC exam. Our certification, specifically created for Canadian CRAs, provides entry-level science professionals with all the resources they need to be a working CRA in Canada. Our online exam can be taken at any time, if you enroll or demo our course .
5. Clinical Research Associate Programs in Canada
In addition to providing 130 modules, our CRA certification exam is priced affordably and the same as our American students. Students can gain valuable work experience by applying to be one of our CCRPS Clinical Research Education Fellows for a 1 month remote internship to develop and strengthen your resume.
6. Salary of Canadian Clinical Research Associate
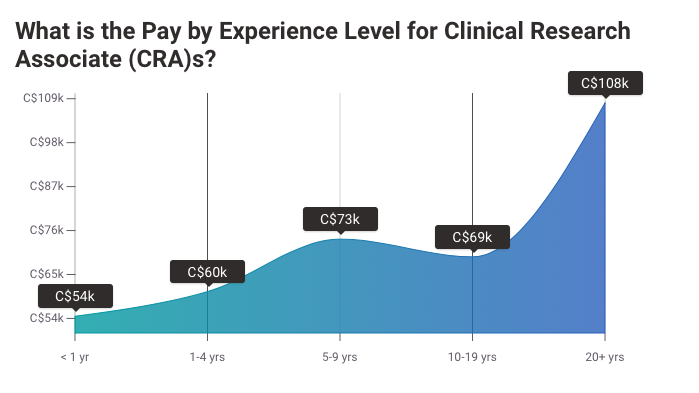
Payscale Canadian CRA Salary

Payscale Canadian Pay Difference CRA
Clinical Research Certification Specifically for Canadian CRAs
CCRPS offers one of the only online certifications specifically designed for entry-level and mid-level professionals in clinical research associate knowledge. We prepare you to understand and apply all skills required to secure a job in clinical research.
Canadian Clinical Research Associate Certificate Course Preview
Want to Learn More About Clinical Research?
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Coordinator
Pharmacovigilance Certification
Advanced Clinical Research Project Manager Certification
Advanced Principal Investigator Physician Certification
Medical Monitor Certification

How much is a Clinical Research Coordinator’s salary?

IMAGES
VIDEO
COMMENTS
Canada is a World Leader in Clinical Research Canada currently ranks fourth in the world for number of clinical trial sites. The volume and growth of clinical trials taking place nationally signals promising job and career advancement opportunities. ... Technology Requirements for Remote/Online Courses Please review the technology and software ...
The Clinical Research Certificate program is an online program that responds to the needs of the rapidly expanding field of Clinical Research, creating exciting opportunities for trained professionals. Please note, NEW program requirements as of September 1, 2022. All applications to the program after September 1, 2022 must meet the new ...
Earn a Certificate with online learning that fits your life. Affiliated with the Faculty of Health Sciences, the Applied Clinical Research program is designed for individuals seeking to enter the field of clinical research. Program content is based on knowledge and skills for clinical research associates and managers as identified by the ...
Required Course. Choose one(1) of: FD05: Research Issues in Family Medicine and Primary Care; FD01: Appraising and Applying Evidence to Assist Clinical Decision-Making (online course) Required Practicum. The practicum provides an opportunity for reflective hands-on practice of knowledge and skills in clinical research. FD91: Clinical Research ...
Program Overview. Humber's Clinical Research graduate certificate program prepares graduates with the transferable skills needed to build successful careers in a variety of clinical trial management and research sectors. This program focuses on developing the concepts, skills and techniques required to work in the clinical research field.
Throughout this program you will develop the following skills: Conduct research design concepts. Analysis and quality assurance of daily operations in clinical trials development process for products such as drugs, medical devices, biopharmaceuticals and natural health products. Identify the roles and responsibilities of the different positions ...
Program Description. The Graduate Diploma (Gr. Dip.) in Clinical Research offered by the Division of Experimental Medicine in the Faculty of Medicine & Health Sciences is a course-based program that emphasizes practical and hands-on learning opportunities. Our comprehensive and innovative program offers a specialized and practical curriculum ...
AAPS' Clinical Research online courses are designed to develop specialized knowledge and skills required to; write study protocols, monitor and manage clinical trials, and to conduct drug safety and pharmacovigilance activities. ... Canada Mississauga Campus 2960 Drew Road, Unit 140 Mississauga, Ontario L4T 0A5, Canada. Hours of Operation Mon ...
ACR 101Principles of Clinical Research. $952.83. Register Now. 3.0 UNITS.
Certificate Program: (consists of four required courses and one elective) Clinical Research Certificate Program Facilitated-Online; Courses Include: ... Toronto, Ontario Canada M5T 1V4 Phone: (416) 596-3101 Toll Free: 1-800-387-9066 (within Canada, outside the Greater Toronto Area) Map. Academic Programs;
Program benefits. Develop your skills through experiential assignments, projects, and case studies. Learn from instructors who are leaders in the clinical research field. Advance through the program with the same cohort of peers and build your professional network. Participate in an applied clinical research management simulation capstone ...
Delivery: At Seneca, courses are delivered in the following formats: online, in-person, hybrid (an online, in-person combination) or flexible (offered in-person and online at the same time). The chart below outlines the delivery options available for each course in this program. For some academic terms, there may be more than one delivery ...
The high demand for clinical research associates enables you to be paid during a 360-hour in-person placement at the end of your program. We'll help you secure your co-op at a clinical research organization, hospital, research institution or office in your area. ... *Athletic Therapy graduates are also exempt from the following course: OSTP ...
The Clinical Epidemiology and Research Management (CERM) field within the Advanced Health Care Practice master's program gives you the skills needed to build and lead research teams, design fundable studies, build and maintain the infrastructure needed to successfully carry out clinical research, and advance evidence-based thinking in your ...
Clinical epidemiology covers a wide range of disciplines, emphasizing research on improving prevention, diagnosis, prognosis, and treatment in patients. Students will learn the principles and methodologies of epidemiology to investigate gaps and barriers in patient care, test hypotheses, and translate their research into practice and health policy.
UM Research Integrity Online Course. ... For over 125 years, the Max Rady College of Medicine has contributed to education, research and clinical service. Western Canada's first medical school, the College develops qualified medical graduates who distinguish themselves through excellence in clinical care, health system innovation and ...
Burlington: 289-778-0305 | Barrie: 705-243-2580 | Peterborough: 705-304-1810Scarborough: 647-931-5399 | Toronto: 647-799-3759 | Mississauga: 289-768-7919. If you're looking for a Clinical Research Program, consider Oxford College. You can choose to attend 6 different locations. Call for more information today!
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning ...
Healthcare professionals interested in clinical research. Note: Course registration will close once maximum number of students enroll. Register as soon as you can! ... Toronto, Ontario Canada M5T 1V4 Phone: (416) 596-3101 Toll Free: 1-800-387-9066 (within Canada, outside the Greater Toronto Area) Map. Academic Programs; Admissions;
The Clinical Research, ... To register, enroll into any online course or contact us via telephone at 416-502-2277 or by email at [email protected]. ... Canada Mississauga Campus 2960 Drew Road, Unit 140 Mississauga, Ontario L4T 0A5, Canada. Hours of Operation Mon-Fri 8:30am - 5:00pm Sat - by appointment only
Clinical research, pharmacovigilance and regulatory affairs are essential for the pharmaceutical industry. ... To register, enroll into any online course or contact us via telephone at 416-502-2277 or by email at [email protected]. ... Canada. Hours of Operation Mon-Fri 8:30am - 5:00pm Sat - by appointment only. Telephone: 416-502-2277 Toll Free: 1 ...
In order to become a certified Canadian CRA, you must take the CRAC credentialing examination. To sit for the examination, you must fulfill one of these requirements: A post-graduate certificate in clinical research and at least 1 years' experience over the past 2 years in clinical research. You must have at least 1 year of Canadian ...
3.4 4. University of London International Programs. 4 Clinical Research Associate Certification Programs in Canada. 5 Eligibility Requirements For Clinical Research Courses in Canada. 6 Top Clinical Research Courses in Canada. 6.1 1. Ontario Tech University. 6.2 2. Wilfrid Laurier University.
During this course, you will explore the essential elements of Good Clinical Practice and gain insights into its significance in the global clinical research arena. By the end of the course, you will have a solid understanding of the principles of GCP and its role in ensuring the integrity and reliability of clinical trial data. The course is ...