EDITORIAL article
Editorial: emerging mosquito-borne diseases and novel biocontrol strategies.

- 1 Department of Pathogen Biology, the Key Laboratory of Microbiology and Parasitology of Anhui Province, the Key Laboratory of Zoonoses of High Institutions in Anhui, School of Basic Medical Sciences, Anhui Medical University, Hefei, China
- 2 Department of Entomology, Faculty of Science, Ain Sham University, Cairo, Egypt
- 3 Public Health Pests Laboratory, Municipality of Jeddah Governorate, Jeddah, Saudi Arabia
- 4 National National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Chinese Center for Tropical Diseases Research), National Health Commission Key Laboratory of Parasite and Vector Biology, WHO Collaborating Center for Tropical Diseases, National Center for International Research on Tropical Diseases, Shanghai, China
Editorial on the Research Topic Emerging mosquito-borne diseases and novel biocontrol strategies
Mosquito-borne diseases threaten more than 40% of the world’s population and are an increasingly serious global health challenge ( Franklinos et al., 2019 ). A report released by the World Health Organization (WHO) showed that malaria caused 247 million cases and 619,000 deaths in 2021, and there is no significant progress in current malaria control ( World Health Organization, 2023a ). The global incidence and number of reported epidemic areas of dengue have also grown dramatically ( World Health Organization, 2023 ). Moreover, Zika, a newly emerged mosquito-borne disease associated with neurological complications, has recently caused several large outbreaks involving 89 countries and territories ( World Health Organization, 2023b ). Furthermore, no efficient vaccines or drugs for diseases such as dengue and Zika are publicly available, and vector control remains largely dependent on traditional insecticide-based strategies ( Namias et al., 2021 ).
Notably, the limitation of the current vector control effect is partly due to the overreliance on chemical control ( Fernandes et al., 2018 ). Chemical insecticides used to be the primary strategy for mosquito control, but insecticide resistance has widely emerged in mosquitoes in recent years ( World Health Organization, 2018 ; Peng et al., 2022 ). Extensive use of insecticides both in mosquito control and agriculture led to environmental pollution and exerted effects on non-targeted organisms ( Deng et al., 2019 ). Thus, there is a growing need for more sustainable, environmentally friendly, and low-cost vector control strategies that can be implemented on a large scale to harness insecticide-resistant mosquitoes and reduce mosquito-borne disease burden.
Biological control agents are important alternatives or complements to chemical insecticides. Combined with genetic approaches (e.g., transgenesis and paratransgenesis) and other biological rear and release theories, novel approaches, including entomopathogenic fungi ( Metarhizium anisopliae and Beauveria bassiana ) ( Deng et al., 2019 ; Peng et al., 2022 ), symbiotic bacteria ( Wolbachia ) ( Turelli et al., 2022 ), lethal bacteria ( Bacillus thuringiensis ) ( Brühl et al., 2020 ), and the release of sterile male mosquitoes ( Wang et al., 2023 ) or disease-refractory mosquitoes (introducing a pathogen effector gene to replace populations) ( Gao et al., 2020 ; Chen et al., 2023 ), shed light on a promising future harnessing insecticide resistance. These strategies are sustainable, inexpensive, and safe for humans and create no pollution to the environment. Gene-drive-based technologies have been encouraged to be combined with biological strategies by the World Health Organization Vector Control Advisory Group due to their broad utility in biological strategies and potential to overcome challenges in current vector control ( Wang et al., 2021 ; World Health Organization, 2022 ). Further epidemiological evidence and field-trial evaluation are needed to support the implementation of these biological measures on a large scale.
This Research Topic, “ Emerging Mosquito-Borne Diseases and Novel Biocontrol Strategies ”, focuses on current and sound research addressing one or more of the abovementioned biocontrol strategies, related genomic surveillance, evolutionary genomics of mosquito species, and insecticide resistance. The Research Topic brings a collection of three original research articles and two reviews. A systematic review and meta-analysis by Wu et al . addressed the impact of COVID-19 non-pharmacological interventions (NPIs) on dengue infection. They searched all qualified articles focusing on NPI efficacy on dengue infection and collected public data on dengue cases to analyze their effects more comprehensively. The study stressed that the changing intensity and scope of internal movement restrictions are more likely to reduce the fundamental level of dengue transmission by reducing the spread of dengue fever among regions in a country, which is conducive to the development of a more comprehensive and sustainable strategy to control dengue fever. Another review by Hou et al. summarized the current development of tetravalent live-attenuated dengue vaccines. CYD-TDV developed by Sanofi Pasteur has been approved, but it is limited to patients who have been infected with dengue fever in the past. The other two candidates for the tetravalent live-attenuated vaccine, TAK-003 of Takeda and TV003 of the National Institute of Allergy and Infectious Diseases, have completed phase III and phase II clinical trials, respectively. They emphasized the specific lessons in the existing research and the challenges that must be overcome in the development of the dengue vaccine, which can effectively protect from all four dengue virus serotypes while causing the fewest side effects. Moreover, Meuren et al. demonstrated that mitochondrial-derived reactive oxygen species (ROS) were a significant inducer of human brain microvascular endothelial cell permeability. In contrast, NADPH oxidase-derived ROS were relevant in producing inflammatory mediators and endothelial activation.
In addition, a study by Qin et al. using the human hepatoma cell model (Huh7), explored the roles of 5’ adenosine monophosphate-activated protein kinase (AMPK), its downstream unc-51-like kinase 1 (ULK1), and mammalian target of rapamycin (mTOR) signaling pathways during the Zika virus infection process. They suggested that Zika virus infection triggers AMPK-mediated lipophagy and that lipid droplet-related lipid metabolism is mainly regulated by the AMPK-ULK1 signaling pathway.
Furthermore, Kimingi et al. used controlled human malaria infection (CHMI) studies in Kenya to further explore the role of anti- Plasmodium falciparum variant surface antigen (VSA) antibodies in malaria immunity. The breadth of IgG antibodies against VSAs is related to the protection in CHMI rather than against individual isolate VSAs.
In conclusion, although this special issue does not include enough articles on biological control strategies, it provides new reference materials for researching malaria, dengue, and Zika. The emergence and re-emergence of mosquito-borne diseases deserve our attention, and new biological control methods deserve our in-depth exploration.

Author contributions
All authors listed have made substantial, direct, and intellectual contributions to the work and approved it for publication.
This work is supported by the National Natural Science Foundation of China (8210082025) and the Anhui Provincial Natural Science Foundation Project (2108085QH347) to S-QD.
Acknowledgments
We thank all researchers contributing to this Research Topic, including the authors and reviewers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Brühl, C. A., Després, L., Frör, O., Patil, C. D., Poulin, B., Tetreau, G., et al. (2020). Environmental and socioeconomic effects of mosquito control in Europe using the biocide bacillus thuringiensis subsp. israelensis (Bti). Sci. Total Environ. 724, 137800. doi: 10.1016/j.scitotenv.2020.137800
CrossRef Full Text | Google Scholar
Chen, J., Deng, S., Peng, H. (2023). Insect-specific viruses used in biocontrol of mosquito-borne diseases. Interdiscip. Med. , e20220001. doi: 10.1002/INMD.20220001
Deng, S., Huang, Q., Wei, H., Zhou, L., Yao, L., Li, D., et al. (2019). Beauveria bassiana infection reduces the vectorial capacity of Aedes albopictus for the zika virus. J. Pest Sci. 92 (2), 781–789. doi: 10.1007/s10340-019-01081-0
Deng, S. Q., Zou, W. H., Li, D. L., Chen, J. T., Huang, Q., Zhou, L. J., et al. (2019). Expression of Bacillus thuringiensis toxin Cyt2Ba in the entomopathogenic fungus Beauveria bassiana increases its virulence towards aedes mosquitoes. PloS Negl. Trop. Dis. 13 (7), e0007590. doi: 10.1371/journal.pntd.0007590
PubMed Abstract | CrossRef Full Text | Google Scholar
Fernandes, J. N., Moise, I. K., Maranto, G. L., Beier, J. C. (2018). Revamping mosquito-borne disease control to tackle future threats. Trends Parasitol. 34 (5), 359–368. doi: 10.1016/j.pt.2018.01.005
Franklinos, L. H. V., Jones, K. E., Redding, D. W., Abubakar, I. (2019). The effect of global change on mosquito-borne disease. Lancet Infect. Dis. 19 (9), e302–ee12. doi: 10.1016/s1473-3099(19)30161-6
Gao, H., Cui, C., Wang, L., Jacobs-Lorena, M., Wang, S. (2020). Mosquito microbiota and implications for disease control. Trends Parasitol. 36 (2), 98–111. doi: 10.1016/j.pt.2019.12.001
Namias, A., Jobe, N. B., Paaijmans, K. P., Huijben, S. (2021). The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs. Elife 10. doi: 10.7554/eLife.65655
Peng, Z. Y., He, M. Z., Zhou, L. Y., Wu, X. Y., Wang, L. M., Li, N., et al. (2022). Mosquito repellents: Efficacy tests of commercial skin-applied products in China. Molecules 27 (17). doi: 10.3390/molecules27175534
Peng, Z.-Y., Huang, S.-T., Chen, J.-T., Li, N., Wei, Y., Nawaz, A., et al. (2022). An update of a green pesticide: Metarhizium anisopliae. All Life 15 (1), 1141–1159. doi: 10.1080/26895293.2022.2147224
Turelli, M., Katznelson, A., Ginsberg, P. S. (2022). Why wolbachia-induced cytoplasmic incompatibility is so common. Proc. Natl. Acad. Sci. U.S.A. 119 (47), e2211637119. doi: 10.1073/pnas.2211637119
Wang, G. H., Gamez, S., Raban, R. R., Marshall, J. M., Alphey, L., Li, M., et al. (2021). Combating mosquito-borne diseases using genetic control technologies. Nat. Commun. 12 (1), 4388. doi: 10.1038/s41467-021-24654-z
Wang, L.-M., Li, N., Ren, C.-P., Peng, Z.-Y., Lu, H.-Z., Li, D., et al. (2023). Sterility of Aedes albopictus by X-ray irradiation as an alternative to gamma-ray irradiation for the sterile insect technique. Pathogens 12 (1), 102. doi: 10.3390/pathogens12010102
World Health Organization (2018) Global report on insecticide resistance in malaria vectors: 2010–2016 . Available at: https://apps.who.int/iris/handle/10665/272533 (Accessed January 10, 2023).
Google Scholar
World Health Organization (2022) Sixteenth meeting of the WHO vector control advisory group . Available at: https://www.who.int/publications/i/item/9789240052673 (Accessed January 10, 2023).
World Health Organization (2023a) World malaria report 2022 . Available at: https://www.who.int/publications/i/item/9789240064898 (Accessed January 10, 2023).
World Health Organization (2023) Dengue and severe dengue . Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (Accessed January 10, 2023).
World Health Organization (2023b) Zika virus . Available at: https://www.who.int/news-room/fact-sheets/detail/zika-virus (Accessed January 10, 2023).
Keywords: mosquito-borne disease, biocontrol, malaria, dengue virus (DENV), Zika virus
Citation: Deng S-Q, Khater EIM, Tambo E and Wang D-Q (2023) Editorial: Emerging mosquito-borne diseases and novel biocontrol strategies. Front. Cell. Infect. Microbiol. 13:1143165. doi: 10.3389/fcimb.2023.1143165
Received: 12 January 2023; Accepted: 26 January 2023; Published: 10 February 2023.
Edited and Reviewed by:
Copyright © 2023 Deng, Khater, Tambo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Duo-Quan Wang, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Mosquito-Borne Diseases
Affiliations.
- 1 Department of Family Medicine, Loma Linda University School of Medicine, 1200 California Street, Suite 240, Redlands, CA 92374, USA. Electronic address: [email protected].
- 2 Department of Family Medicine, Loma Linda University School of Medicine, 1200 California Street, Suite 240, Redlands, CA 92374, USA.
- PMID: 30115330
- DOI: 10.1016/j.pop.2018.05.001
Mosquito-borne diseases have become more common as previously geographically isolated diseases have spread globally. Chikungunya, dengue, Japanese encephalitis, malaria, West Nile, yellow fever, and Zika are a few of the common and emerging viral diseases spread by mosquitoes. A thorough patient history, physical, and knowledge of diagnostic testing based on symptom duration is important to make a quick and accurate diagnosis. Because the treatment for many of these diseases is supportive, the emphasis is on reducing risk and spread of infection.
Keywords: Chikungunya; Dengue; Japanese encephalitis; Malaria; Mosquitoes; West Nile; Yellow fever; Zika.
Copyright © 2018 Elsevier Inc. All rights reserved.
PubMed Disclaimer
Similar articles
- [Mosquito-transmitted infections]. Jelinek T. Jelinek T. Internist (Berl). 2018 Jan;59(1):57-73. doi: 10.1007/s00108-017-0361-6. Internist (Berl). 2018. PMID: 29270717 German.
- Current Problems in Pediatric and Adolescent Health Care. Mosquito-borne diseases. Foreword. Moyer VA. Moyer VA. Curr Probl Pediatr Adolesc Health Care. 2009 Apr;39(4):95-6. doi: 10.1016/j.cppeds.2009.01.002. Curr Probl Pediatr Adolesc Health Care. 2009. PMID: 19327646 No abstract available.
- Mosquito-borne diseases. Tolle MA. Tolle MA. Curr Probl Pediatr Adolesc Health Care. 2009 Apr;39(4):97-140. doi: 10.1016/j.cppeds.2009.01.001. Curr Probl Pediatr Adolesc Health Care. 2009. PMID: 19327647 Review.
- Arboviral diseases and malaria in Australia, 2013-14: Annual report of the National Arbovirus and Malaria Advisory Committee. Knope KE, Muller M, Kurucz N, Doggett SL, Feldman R, Johansen CA, Hobby M, Bennett S, Lynch S, Sly A, Currie BJ; and the National Arbovirus and Malaria Advisory Committee. Knope KE, et al. Commun Dis Intell Q Rep. 2016 Sep 30;40(3):E400-E436. Commun Dis Intell Q Rep. 2016. PMID: 28278416
- Four human diseases with significant public health impact caused by mosquito-borne flaviviruses: West Nile, Zika, dengue and yellow fever. Guarner J, Hale GL. Guarner J, et al. Semin Diagn Pathol. 2019 May;36(3):170-176. doi: 10.1053/j.semdp.2019.04.009. Epub 2019 Apr 17. Semin Diagn Pathol. 2019. PMID: 31006554 Review.
- Mosquito Gut Microbiota: A Review. Liu H, Yin J, Huang X, Zang C, Zhang Y, Cao J, Gong M. Liu H, et al. Pathogens. 2024 Aug 15;13(8):691. doi: 10.3390/pathogens13080691. Pathogens. 2024. PMID: 39204291 Free PMC article. Review.
- Effect of salinity on the oviposition and growth of Ochlerotatus togoi . Choi JW, Choi KS. Choi JW, et al. Ecol Evol. 2024 Apr 23;14(4):e11289. doi: 10.1002/ece3.11289. eCollection 2024 Apr. Ecol Evol. 2024. PMID: 38660469 Free PMC article.
- Aedes albopictus in a recently invaded area in Spain: effects of trap type, locality, and season on mosquito captures. Garrido M, Veiga J, Garrigós M, Morales-Yuste M, Recuero-Gil J, Martínez-de la Puente J. Garrido M, et al. Sci Rep. 2024 Jan 25;14(1):2131. doi: 10.1038/s41598-024-52040-4. Sci Rep. 2024. PMID: 38267495 Free PMC article.
- Climate Stressors and Physiological Dysregulations: Mechanistic Connections to Pathologies. Heidari H, Lawrence DA. Heidari H, et al. Int J Environ Res Public Health. 2023 Dec 23;21(1):28. doi: 10.3390/ijerph21010028. Int J Environ Res Public Health. 2023. PMID: 38248493 Free PMC article. Review.
- Does the Presence or a High Titer of Yellow Fever Virus Antibodies Interfere with Pregnancy Outcomes in Women with Zika Virus Infection? Piauilino ICR, Souza RKDS, Lima MT, Rodrigues YKB, da Silva LFA, Gouveia AS, Neto AVDS, Chaves BA, Alecrim MDGC, de Menezes CHAB, Castilho MDC, Baia-da-Silva DC, Espinosa FEM. Piauilino ICR, et al. Viruses. 2023 Nov 11;15(11):2244. doi: 10.3390/v15112244. Viruses. 2023. PMID: 38005922 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Elsevier Science
- W.B. Saunders
Other Literature Sources
- scite Smart Citations
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 02 November 2022
Mosquito matters
Nature Ecology & Evolution volume 6 , page 1587 ( 2022 ) Cite this article
2758 Accesses
1 Citations
7 Altmetric
Metrics details
Three studies of disease-carrying mosquitoes in this issue illustrate the need for both interdisciplinary approaches and more research into fundamental biology.
Mosquitoes are a large family of dipterans with a cosmopolitan distribution. Although they occupy a range of niches and provide certain ecosystem services, their best-known — and most unfortunate — role is as vectors of infectious disease. Species mainly from three genera — Aedes , Anopheles and Culex — transmit a range of parasitic and viral diseases that are responsible for high burdens of mortality and morbidity, predominantly in warm climates. And, of course, their ranges and potential impacts are shifting with climate change ( Ryan, S. J. et al. PLoS Negl. Trop. Dis . 13 , e0007213; 2019 ).
Despite some major wins, such as the malaria vaccine that was recently approved by the WHO (Ledford, H. Nature , https://doi.org/10.1038/d41586-022-02902-6 ; 2022), widespread management of mosquito-borne diseases with medical intervention is expensive and still needs to be complemented by a range of vector-control strategies. Strategies will differ in rural and urban settings, but with urbanization (and urban mosquitos) on the rise it is especially timely to consider mosquito control in urban areas. Cities have high densities of mosquito hosts (that is, people), fewer predators, often suitably warm microclimates, and abundant still-water containers, and are more interconnected than rural areas. Although these properties are beneficial to mosquitoes, they are also all aspects of urban geography that we can modify and control. Kache et al. argue in a Perspective in this issue that an integrative ecological and urban systems approach is needed to control Aedes -borne disease. Patterns of human movement and employment, water management, and buildings and local-area infrastructure are all parts of this complex urban system that contribute to mosquito-borne disease as an emergent property. These human dimensions can be mapped and modelled using tools from landscape ecology, and the authors make the case for a coordinated effort across disciplines and different types of local actors.
Far from the complexities of urban systems and practicalities of vector control, there is still much we do not understand about the fundamental biology of mosquitoes. Also in this issue, and focusing on Anopheles in a rural region of Africa that is heavily burdened by malaria, Faiman et al. investigate where mosquitoes go during the dry season, when they seem to disappear and are yet able to rebound rapidly when the rains return. One tentative explanation for this unexplained adult persistence is a form of dormancy known as aestivation. The authors developed a deuterium-based isotope tracking approach that allowed them to show that at two village sites in Mali, aestivation by Anopheles coluzzii accounts for at least 20% of the rapid rebound. This insight into how mosquitoes endure the dry season could be important in refining control measures, such as more targeted insecticidal applications to stymie population explosions at the start of rainy seasons. However, more research is needed to identify the actual locations where the mosquitoes aestivate.
Also in this issue, Poda et al. examine another aspect of fundamental mosquito biology with applied implications: how mosquitoes aggregate for mating. Several Anopheles species exist in sympatry yet maintain premating barriers, and hybrids are rare. It is not known how species-specific mating swarms of Anopheles gambiae and Anopheles coluzzii maintain their monospecific nature, but several types of cue might be involved, including acoustic, visual and chemical. Many of these work only over short distances, so long-distance pheromones have been suggested. Poda et al. note that the evidence for these pheromones is not conclusive, so they set out to test for them using a range of different behavioural, physiological and chemical analyses under conditions that they argue maximize the chance of success. Included within their analyses are replications of an earlier study in this journal ( Mozūraitis, R. et al. Nat. Ecol. Evol . 4 , 1395–1401; 2020 ), in which the authors concluded in favour of long-distance pheromones. On the basis of all their new analyses, Poda et al. conclude that there is currently no evidence for such pheromones, although they are careful to note that absence of evidence is not necessarily evidence of absence, and that further work is needed. They identify differences in the timing of observation and the control experiments that may explain the discrepancy with Mozūraitis et al. The study by Poda et al. is not an exact replication of Mozūraitis et al., because it includes a wider range of assays. In doing so, it has shifted the evidence considerably against the existence of long-range pheromones, but it neither invalidates the specific results of Mozūraitis et al. nor provides the final word on the subject. For a start, even if long-range pheromones do not contribute to species segregation in Anopheles , we still need an alternative explanation for what does.
As with the location of aestivating mosquitoes, the mechanism of mating is a potential target for control interventions, so further research understanding the basic biology of these species is essential. Some of these interventions could be low-tech (such as nets and responsibly applied sprays), but another major topic of mosquito research is into high-tech genomic solutions, including transgenic gene drives and the use of reproductive parasites such as Wolbachia ( Wang, G.-H. et al. Nat. Commun . 12 , 4388; 2021 ). Although all of these biological approaches should continue apace, the Perspective by Kache et al. makes the important point that, alongside them, there are huge gains to be made from an interdisciplinary approach that considers mosquito-borne disease as an emergent property of coupled socioecological systems.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Mosquito matters. Nat Ecol Evol 6 , 1587 (2022). https://doi.org/10.1038/s41559-022-01938-1
Download citation
Published : 02 November 2022
Issue Date : November 2022
DOI : https://doi.org/10.1038/s41559-022-01938-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
General Overview of Mosquito Borne Diseases (MBD): A Great Burden on Humanity
- August 2022
- In book: Research Trends in Applied Research (pp.25-50)
- Publisher: Weser books, Germany

- Mohan Lal Sukhadia University
- This person is not on ResearchGate, or hasn't claimed this research yet.

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Glauco Scelza Cavalcanti
- Veronica de Camargo Golfetto

- Gen-Fu Feng

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- Open access
- Published: 28 August 2023
Trends in mosquito species distribution modeling: insights for vector surveillance and disease control
- Catherine A. Lippi 1 , 2 ,
- Stephanie J. Mundis 1 ,
- Rachel Sippy 1 , 3 ,
- J. Matthew Flenniken 1 ,
- Anusha Chaudhary 1 ,
- Gavriella Hecht 1 , 2 ,
- Colin J. Carlson 4 &
- Sadie J. Ryan 1 , 2
Parasites & Vectors volume 16 , Article number: 302 ( 2023 ) Cite this article
4427 Accesses
9 Citations
60 Altmetric
Metrics details
Species distribution modeling (SDM) has become an increasingly common approach to explore questions about ecology, geography, outbreak risk, and global change as they relate to infectious disease vectors. Here, we conducted a systematic review of the scientific literature, screening 563 abstracts and identifying 204 studies that used SDMs to produce distribution estimates for mosquito species. While the number of studies employing SDM methods has increased markedly over the past decade, the overwhelming majority used a single method (maximum entropy modeling; MaxEnt) and focused on human infectious disease vectors or their close relatives. The majority of regional models were developed for areas in Africa and Asia, while more localized modeling efforts were most common for North America and Europe. Findings from this study highlight gaps in taxonomic, geographic, and methodological foci of current SDM literature for mosquitoes that can guide future efforts to study the geography of mosquito-borne disease risk.
Graphical Abstract

Mosquito-borne diseases have long imposed a heavy burden on both human and animal health worldwide [ 1 ]. There is an extensive history of mosquito control efforts to reduce the transmission of mosquito-borne diseases of global public health importance, notably malaria, yellow fever, and dengue fever [ 2 , 3 , 4 ]. However, these efforts are increasingly undermined by the combined effects of climate change, urbanization, and health system erosion, all of which are implicated in the expansion of mosquito-borne diseases to higher latitudes and elevations [ 3 , 5 , 6 ], the re-emergence of diseases like malaria and yellow fever [ 7 , 8 , 9 , 10 ], and the emergence of novel pathogens like chikungunya and Zika virus [ 11 , 12 , 13 , 14 ] . Despite their public health importance, most of these diseases are undersurveilled and underreported, particularly in areas where poverty overlaps with a growing number of syndemic and syndromically hard-to-distinguish mosquito-borne diseases [ 15 , 16 , 17 ]. As a result, mapping the geographic distribution of mosquito vectors is often used as a first step towards describing the shifting landscapes of infectious disease risk.
One of the most commonly applied tools to study mosquito geographic distributions is species distribution modeling (SDM), also commonly known as ecological niche modeling. Species distribution models relate presence-absence or presence-only occurrence data to explanatory landscape factors, producing estimates of suitable habitat [ 18 , 19 ]. Inputs for SDMs typically include geolocated data on the presence of the species of interest as the response variable, often in the form of occurrence records derived from literature reviews, databases, or aggregated abundance sampling [ 18 ]. Explanatory variables are extracted from a wide range of sources, and ideally represent aspects of the species’ ecology that impact whether the organism can persist in a particular environment. Researchers often consider climatological factors, as well as place and organism-specific factors such as land cover, slope, aspect, elevation, soil type, and human effects on the landscape [ 20 , 21 , 22 ].
Given the flexibility of the approach, the motivations and objectives for developing SDMs of mosquito species often vary alongside model inputs, methods, and spatial scope. For example, global, regional, or national-scale SDMs may aim to anticipate broad distributions of present or future disease risk [ 23 , 24 , 25 ], while models developed at a finer spatial scale play an increasingly important role in vector control strategies. Public health vector control and mosquito source reduction are cornerstones of disease management, curbing transmission when clinical treatment and prophylactic options are limited or non-existent, as is currently the case for many arboviruses [ 26 ]. Identifying areas at risk from mosquito-borne disease transmission is integral to the development of effective policies, formation of mitigation strategies, and allocation of resources [ 27 , 28 ]; however, vector surveillance activities can be resource intensive and geographically limited [ 29 , 30 ]. Research-guided mosquito surveillance and abatement efforts are therefore often cited as necessary for practitioners to precisely know not only when, but also where to both monitor and intervene [ 31 , 32 , 33 , 34 , 35 , 36 , 37 ].
Here, we undertake a systematic review of the current body of literature on mosquito SDM research, motivated by the desire to understand these different applications, and to identify trends, challenges, and gaps in the current body of knowledge generated around mosquito ecology and biogeography. We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to identify and compile studies that developed SDMs of mosquito species in the past 20 years, and extracted information on the species, methods, input variables, and location and scale of each relevant published, peer-reviewed study [ 38 ].
We conducted literature searches following PRISMA statement guidelines, a checklist of criteria to ensure transparency in systematic reviews [ 38 , 39 ]. We conducted searches across all Web of Science databases through January 2023 to identify studies using SDMs to estimate mosquito geographic distributions. Combinations of key terms used in searches included “Aedes,” “Culex,” “Anopheles,” and “mosquito” with “species distribution model*” and “ecological niche model*.” We included Aedes , Anopheles and Culex as explicit search terms, as these genera comprise the disease vectors most targeted in public health initiatives, and are intensely studied as disease vectors [ 40 , 41 , 42 ]. The inclusion of “mosquito” as a search term was to ensure that we captured studies on species beyond these three taxonomic groupings, and we did not restrict our searches to species implicated in human disease transmission. While there were no restrictions on the geographic region of study or date of publication, searches were limited to English language results.
Duplicate records were removed from our search results before screening. We screened the remaining abstracts for subject relevance (i.e., studies on mosquitoes), additionally removing publications that were literature reviews, expert commentaries, synthesis papers, phylogenetic studies, or gray literature. The remaining studies were reviewed in full for inclusion, excluding studies with methodologies that were not within the scope of this review, including papers that were purely descriptive, used mosquito-borne disease cases as response variables, or modeled mosquito presence, abundance, or behaviors (e.g., such as oviposition or bite rates) using data with no geospatial component.
We extracted information from the full text of the remaining studies, which included information on publication (e.g., digital object identifier link), the mosquito species of interest as identified in the studies, SDM methods used, geographic location of study, spatial scale of analysis, and data sources for both species occurrence records and explanatory environmental variables. We noted methods used for model fitting, addressing collinearity, and if modeled distributions were projected beyond their initial training scope (e.g., models projected to other geographic locations, or future time horizons) when available. The methods used in the studies were classified into nine categories, which are outlined in Table 1 .
Data extracted from the final collection of screened literature were synthesized to describe trends in mosquito SDMs. Data visualization was conducted in R (v4.1.2.) using code adapted from Lippi et al. [ 43 ], and mapping was performed in ArcMap (v10.8.1). The database of screened literature is available on GitHub ( https://github.com/RyanLab/MOSQ_SDM_Table ).
The initial search returned 1185 records (Fig. 1 ), and 563 records remained after duplicates were removed. In initial abstract screening, 298 records for studies that did not fit the scope of this review were removed. After reviewing the full text of the remaining 265 records, we retained 204 studies that met our criteria for inclusion ( https://github.com/RyanLab/MOSQ_SDM_Table ).
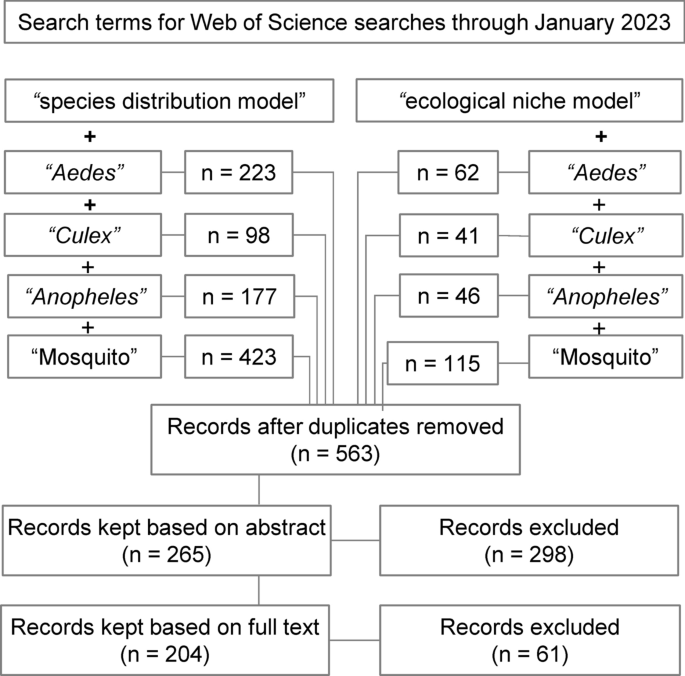
Flow diagram of the systematic review process, indicating combinations of search terms and number of studies screened
Taxonomic focus of SDMs
There were 138 mosquito species featured in SDMs produced in the reviewed literature, which included 78 species in Anopheles , 25 species in Culex , 24 species in Aedes (= Ochlerotatus) , and 11 species in other genera, including Coquillettidia , Culiseta , Haemagogus , and Sabethes . By species, most SDM studies developed models for Aedes aegypti ( n = 55), Aedes albopictus ( n = 50), Culex pipiens ( n = 20), Anopheles gambiae ( n = 17), and Anopheles arabiensis ( n = 15) (Fig. 2 ).
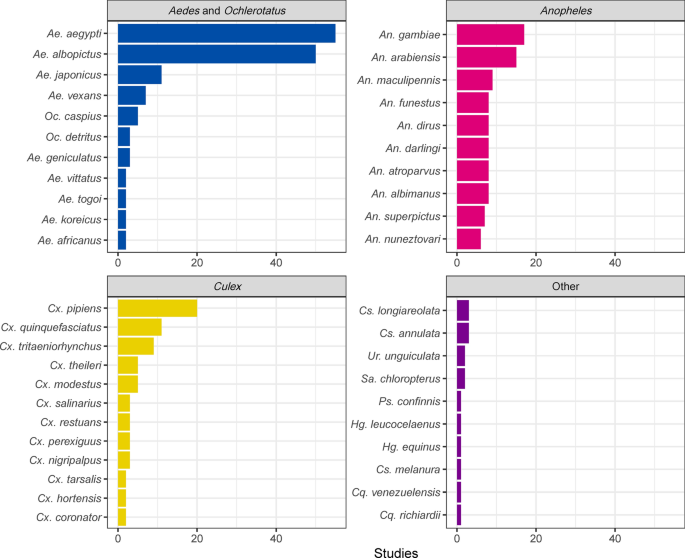
Top 10 mosquito species, within each genus, that have been studied with species distribution models (SDMs). Most efforts focused on Aedes aegypti and Aedes albopictus , followed by Culex pipiens , though collectively species in the genus Anopheles were also extensively modeled. Cs. Culiseta , Cq. Coquillettidia , Hg. Haemagogus , Ps. Psorophora , Sa. Sabethes , Ur. Uranotaenia
Grouping by genera, mosquitoes featured in SDM studies have changed over time (Fig. 3 a).
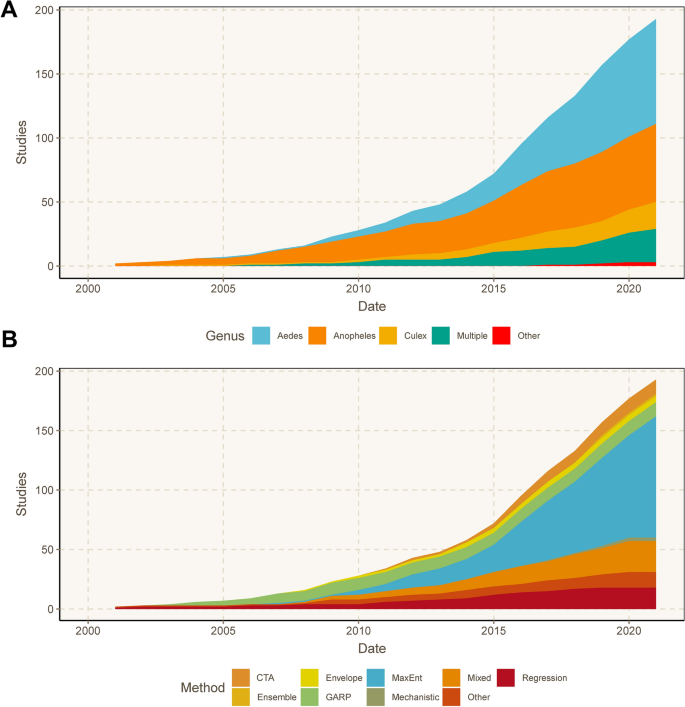
Cumulative number of studies on mosquitoes that modeled a given mosquito genus ( a ), and the SDM methods used in studies over time ( b ). CTA Classification tree analysis, MaxEnt maximum entropy, GARP genetic algorithm for rule-set production
The early mosquito SDM literature predominantly focused on mosquitoes in the genus Anopheles , comprising 64.3% of studies published through 2010. In recent years, studies on Aedes mosquitoes have become more prevalent, and these mosquitoes were the taxonomic focus of nearly half (48.6%) of all mosquito SDMs produced since 2015. In the same period, roughly a quarter of studies were on mosquitoes in the genus Anopheles (24.0%), and 11.6% on mosquitoes in the genus Culex .
Methods used to build SDMs
Overall, 169 of the 204 studies reviewed (82.8%) used a single method to estimate mosquito distributions, while the remaining 35 (17.2%) used more than one method or used model ensemble approaches (Fig. 3 b). More than half ( n = 108, 52.9%) of the studies used MaxEnt exclusively, a trend which also holds across genera (Fig. 4 ). An additional 21 (10.3%) used MaxEnt with one or more additional SDM methods. Non-machine learning regression models (e.g., logistic regression, generalized linear models, etc.) were used exclusively in 18 studies (8.8%), and used in combination with other methods in an additional 16 studies (7.8%). CTA methods, which included classification and regression trees, boosted regression trees, and random forest, were used as the sole SDM method in 14 studies (6.9%), and the genetic algorithm for rule set prediction (GARP) was the sole method in 12 studies (5.9%). CTA methods were combined with other SDMs in 18 additional studies (8.8%), while GARP was used with other methods in five additional studies (2.5%). Mechanistic models were used as the only SDM method in three studies (1.5%), and bioclimatic envelope models were used in five studies (2.5%). Thirteen studies (6.4%), which were characterized as “Other,” featured uncommon methods such as ecological niche factor analysis, and other types of environmental suitability or logic thresholds.
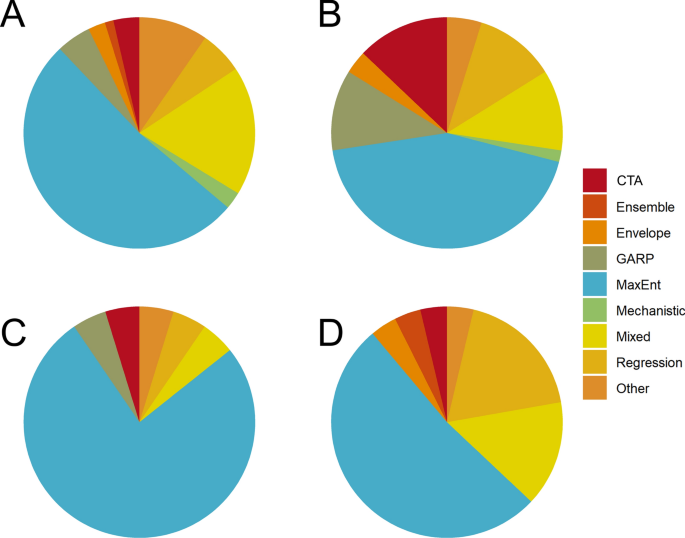
Breakdowns of methods used, shown by taxonomic groups for Aedes ( a ), Anopheles ( b ), Culex ( c ), and studies that estimated distributions for multiple genera ( d ). For abbreviations, see Fig. 3
The frequency of using SDMs to estimate mosquito distributions increased markedly over time, particularly from 2015 onward (Fig. 3 b). Concurrently, the evolution of SDM algorithms led to a more diverse methodological landscape. In the early 2000s, GARP was the most commonly implemented method among these studies, and remained a frequently used method until approximately 2010. MaxEnt software was released in 2006, and the first mosquito SDM study using MaxEnt was published shortly thereafter, in 2007. By 2011, it was the most common SDM approach, with 19 of 43 studies (44.2%) published during those years solely using MaxEnt. More than half (62.3%) of the mosquito SDM studies published since 2015 solely used MaxEnt to estimate mosquito distributions. To a lesser extent, the frequency of using multiple SDM approaches in a single study [“Mixed” (Figs. 3 , 4 )] has also increased over time. The first mixed methods study based on our inclusion criteria was published in 2008, and studies that used multiple SDM approaches have accounted for 13.7% of those published since 2015. CTAs and regression methods have seen modest increases in use over the past decade, and were used in 8.2% and 6.2% of studies, respectively, since 2015. The increased availability of gridded data layers of ecological and climate products, representing a host of environmental factors, has also been a fundamental piece in the rapid expansion of SDM research. A notable example is the WorldClim database, which was first released in 2005 (with version 2 released in 2017) and made long-term averages of historical and projected future climate data accessible for many SDM studies [ 44 ].
Spatial scale of SDMs
The scale of analysis varied considerably throughout the mosquito SDM literature. The majority of studies were conducted at the sub-national (34.8%) or national (31.4%) level. Most national or sub-national studies were conducted in the USA (11.1%), followed by Germany (6.7%), Mexico (6.7%), Australia (5.2%), Brazil (4.4%), China (4.4%), Colombia (4.4%), Italy (4.4%), Argentina (3.7%), Iran (3.7%), and Tanzania (3.7%) (Fig. 5 ). Approximately one-quarter of studies (24.5%) were regional, projecting models over large geographic areas that encompassed multiple countries. Most of the regional models were developed for portions of Africa (30.0%) and Europe (30.0%), followed by SDMs developed for regions in Asia (24.0%), North America (16.0%), and South America (16.0%). Nearly half of all regional models (44.0%) were developed for species of Anopheles . Relatively few SDMs (9.3%) focused on a global extent, but of those, the majority (68.4%) focused on mosquitoes in the genus Aedes (Fig. 6 A). Studies conducted in African countries typically modeled the distributions of Anopheles , while European studies tended to focus on Aedes . In comparison, studies conducted in North America, South America, the Middle East, and countries in Asia more frequently included multiple species, or other taxonomic groups (Fig. 5 ).
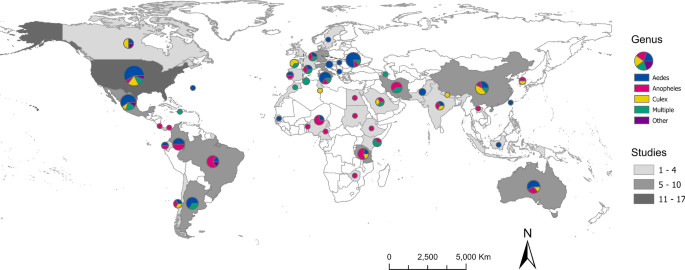
Map representing the number of SDM studies conducted at the national or sub-national level (country, greyscale) and the mosquito genera modeled in the studies (pie chart, color breakdown)
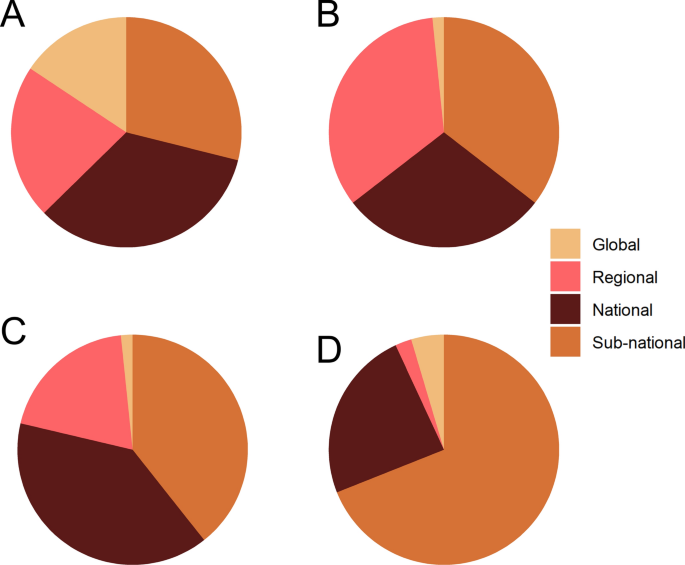
The proportion of spatial scales represented in the reviewed SDM studies, shown by genera for Aedes ( a ), Anopheles ( b ), Culex ( c ), and multiple genera ( d )
The scale of analysis used to build SDMs also varied by taxa. Species distribution models were built for Aedes mosquitoes in roughly equivalent proportions (Fig. 6 a), possibly driven by the global invasions of Ae. aegypti and Ae. albopictus . In contrast, global suitability models for Anopheles and Culex were relatively uncommon (Fig. 6 b, c), regional and sub-national models were most common for Anopheles (Fig. 6 b), and SDMs for Culex and multiple genera primarily consisted of national and sub-national models (Fig. 6 c, d).
Data sources used to build SDMs
Mosquito occurrence records used as data inputs for SDM workflows were obtained from a variety of sources. Over one-third of the reviewed studies (36.8%) included georeferenced locations from online data repositories when building models, the majority of which (49.3%) used the Global Bioinformatics Information Facility to obtain data. Georeferenced data obtained from published sources and literature reviews were also frequently used as data inputs in studies (36.8%), with the database published by Kraemer et al. [ 45 ] explicitly cited as a data source in 5.4% of all studies. Use of existing public health surveillance systems for mosquito records, which included databases from national public health authorities, was indicated in 11.8% of studies.
Collection of novel mosquito records through entomological sampling was indicated in approximately one-third (32.2%) of published studies, of which 16.1% supplemented collection records with data from other sources (e.g., published data, online repositories, etc.). Of the studies that collected entomological data, 59.3% reported larval sampling, of which 18.8% reported sampling with oviposition or gravid traps. Diverse sampling methods were reported in studies with entomological data that targeted the adult life stage, including Centers for Disease Control and Prevention light traps (37.8%), BG-Sentinel traps (22.2%), Mosquito Magnet traps (15.6%), aspiration (13.3%), and human landing catch (11.1%). While studies that included a field component typically described the mosquito life stage targeted in sampling, across all studies the life stage of mosquitoes used for species presence (i.e., adults, immatures, or both) was not specified in most instances (53.9%).
Environmental predictors of mosquito presence
Most studies (89.7%) incorporated climate variables to estimate mosquito distributions. While some studies (20.1%) used climate data exclusively, many (69.6%) used a combination of climate data and other environmental covariates (e.g., land cover class, elevation, soil classifications, etc.) in their model predictions. Most studies (69.2%) that used climate data to produce SDMs employed WorldClim data products. Many studies (27.9%) projected models to estimate mosquito distributions under future climate conditions, using products such as downscaled global climate models as environmental predictors. A variety of methods were used to control for collinearity in environmental predictors before building SDMs, including use of correlation coefficients with a threshold (27.5%), principal component analysis (8.3%), and variance inflation factor (5.9%). Nearly half of the studies (48.5%) did not explicitly address predictor collinearity.
Variables identified as important for predicting mosquito distributions were reported in 80.3% of studies. Identified drivers varied considerably between studies and taxa. Measures of temperature were most often described as important predictors of mosquitoes (54.9% of studies), followed by precipitation (42.6%), land cover and land use (31.4%), and elevation (18.6%). Of the studies that incorporated climate variables, 62.6% identified temperature, and 52.5% identified precipitation as important predictors of mosquito habitat suitability. By taxonomic group, temperature variables were top predictors in 44 (50.0%) studies on Aedes , 37 (58.7%) studies on Anopheles , and 16 (69.6%) studies on Culex . Precipitation variables were top predictors in 32 (36.4%) studies on Aedes , 36 (57.1%) studies on Anopheles , and 12 (52.2%) studies on Culex . Variable importance was not reported in 19.6% of studies.
SDM has become a frequently used methodological approach to estimate the distribution, and implicit risk, of vector-borne diseases [ 35 , 36 ]. In this study, we conducted a systematic review of scientific literature that used SDMs to estimate geographic distributions of mosquitoes. By quantifying data from the screened literature to identify patterns and trends, we were able to summarize the methods, taxonomic foci, geographic scope, and other attributes reported in SDM studies. Importantly, this also enabled us to identify potential gaps in the current literature, and thus provide guidance for future modeling efforts.
Current trends in mosquito SDMs
Although there is diversity in the landscape of available modeling approaches and tools, MaxEnt is the most commonly implemented method for conducting SDM studies on mosquitoes [ 46 ]. After its release in 2006, MaxEnt quickly gained favor over previously common methods, like GARP, and remains the most frequently used approach for the estimation of mosquito distributions [ 47 ]. While MaxEnt may be the most appropriate methodology for some studies, the popularity of the method also results from an interpretable graphic user interface, prolific training guides, and general ease of implementation for users. Further, the release of WorldClim climate model output data in 2005, and the subsequent availability of other gridded environmental data products, allowed users to perform analyses without collecting primary environmental data, facilitating the use of SDMs [ 48 ].
The majority of the SDM studies in this review were on Anopheles or Aedes . This is not surprising, given the emphasis on the global health importance of malaria transmitted by mosquitoes in the genus Anopheles , and arboviral pathogens transmitted by some species in the genus Aedes , including yellow fever virus and dengue virus. Moreover, most global modeling efforts involve mosquitoes in the genus Aedes , likely owing not only to the medical importance of two key species ( Ae. aegypti and Ae. albopictus ), but also to their cosmopolitan success as invasive species. In contrast, SDMs for Anopheles were typically conducted at regional, national, and local scales, and in locations predominantly on the African continent, reflecting the disproportionate research effort focused on malaria caused by Plasmodium falciparum .
Geographic gaps exist for central Asia, Southeast Asia, eastern Europe, and portions of Africa and South America, where few highly localized studies have been conducted. Notably, many of these regions have rich mosquito diversity, including dozens of understudied vectors of current or potential future emerging infections [ 49 ]. Modeling studies in these regions were also underdeveloped in respect to other methodological aspects; for example, temperature and precipitation in these regions were most commonly represented by WorldClim bioclimatic variables rather than regionally developed climate products. Regionally targeted research efforts may benefit from locally created and locally validated climate and land cover products.
Challenges of SDMs
We found considerable variation in which environmental drivers were identified as predictive of mosquito distributions, such that few generalizations could be made even for a given species. Variable importance is influenced by nearly every step of the SDM building process, such as choice of data products, scale of analysis, collinearity reduction techniques, and choice of SDM algorithm. In studies that reported variable importance, actual values of environmental predictors (i.e., numerical thresholds for occurrence) were rarely reported. The prevalence of studies that failed to reduce the number of variables to address collinearity, or lacked justification for choice of environmental predictors, points to a potentially troubling lack of biological grounding and hypothesis testing. These challenges can be readily addressed in future studies through adherence to best practices and standards in building models and reporting results [ 50 , 51 , 52 ]. Assessing the quality of models and adherence to best practices is beyond the scope of this review but has been recently assessed by Barker and MacIsaac [ 51 ]. Given some of these underlying heterogeneities among studies, care must be taken when interpreting the results of SDMs, especially those potentially used for guiding public health decision-making, as basing decisions on the results of poor-quality models can lead to the diversion of resources and miscommunication of the true risk of exposure.
Opportunities for future modeling efforts
Many efforts have been made to delineate the geographic extent of mosquitoes, but the current literature still may not capture the full landscape of risk, especially in the biodiverse areas where new infections are more likely to originate [ 53 , 54 ]. Moreover, older range maps may not reflect the most current understanding of mosquito taxonomy (e.g., grouping members of a species complex together), and may need reassessment. The movement of vectors and pathogens may also serve as the catalyst for new public health challenges, for example, when mosquito vectors aggressively invade new locations [ 6 , 55 ], or when the introduction of pathogens increases the medical significance of local mosquito populations [ 35 ]. These changes also create a problem for the scientific literature itself: the estimation of range boundaries based on baseline climate conditions has diminishing value in a rapidly warming world, where mosquito ranges have already become non-stationary in both invasive ranges [ 25 ] and endemic areas [ 6 ].
Conclusions
Our findings indicate an opportunity not only to expand data collection and distribution modeling efforts for underrepresented mosquito species and in underrepresented areas [ 43 , 56 ] but also to more broadly rethink the SDM workflow as it is currently used in vector surveillance and control. An iterative workflow is technologically feasible and cost-effective, where (1) existing surveillance data and local knowledge are used to generate or update mosquito distribution models; (2) new forecasts are generated that anticipate areas at risk of range expansions, based on existing trends and climate projections; and (3) models are used to guide the collection of new surveillance data, which can also be used for model validation (Fig. 7 ). Field efforts to collect data used for model improvement can be a daunting endeavor, yet we found a surprisingly high number of studies which collected novel entomological survey data for building models. Nevertheless, relatively few studies incorporated data from public health surveillance systems, highlighting a potential avenue to future collaborations between modelers, public health authorities, and vector control agencies. This new workflow presents opportunities on the technological front, where efforts to employ newer approaches with updated methodologies and software, and adherence to best practices, may enable us to refine estimates of spatial risk; moreover, adoption of automated approaches that update range estimates from incoming data could aid in making timely predictions that are more accessible to decision-makers. Partnerships with local experts and agencies will be key both to improving model predictions and maximizing their applied utility [ 57 ]. Ultimately, working more closely with end users may facilitate the uptake of modeling workflows, ensuring that SDMs are appropriately contextualized and regularly updated.
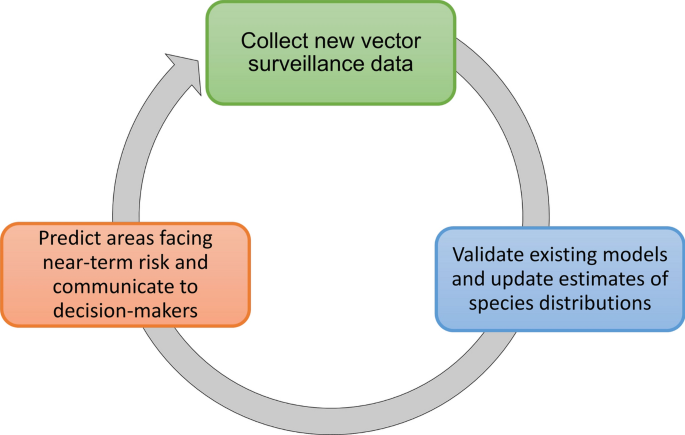
A conceptual workflow for dynamic mosquito species distribution modeling
Availability of data and materials
All of the papers reviewed in this study are included in the References [ 24 , 25 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 ]. Data associated with this analysis are available on GitHub ( https://github.com/RyanLab/MOSQ_SDM_Table ).
Abbreviations
Classification tree analysis
Genetic algorithm for rule-set production
- Maximum entropy modeling
Species distribution model
WHO. A global brief on vector-borne diseases. World Health Organization; 2014. Report no.: WHO/DCO/WHD/2014.1.
Alonso P, Noor AM. The global fight against malaria is at a crossroads. The Lancet. 2017;390:2532–4.
Google Scholar
Gubler D. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health. 2011;39:3–11.
PubMed PubMed Central Google Scholar
Gianchecchi E, Cianchi V, Torelli A, Montomoli E. Yellow fever: origin, epidemiology, preventive strategies and future prospects. Vaccines. 2022;10:372.
Brito AF, Machado LC, Oidtman RJ, Siconelli MJL, Tran QM, Fauver JR, et al. Lying in wait: the resurgence of dengue virus after the Zika epidemic in Brazil. Nat Commun. 2021;12:1–13.
Carlson CJ, Bannon E, Mendenhall E, Newfield T, Bansal S. Rapid range shifts in African Anopheles mosquitoes over the last century. Biol Let. 2023;19:20220365.
Linthicum KJ, Britch SC, Anyamba A. Rift Valley fever: an emerging mosquito-borne disease. Annu Rev Entomol. 2016;61:395–415.
CAS PubMed Google Scholar
Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98-109.
Correa-Salazar C, Amon JJ. Cross-border COVID-19 spread amidst malaria re-emergence in Venezuela: a human rights analysis. Global Health BioMed Central. 2020;16:1–8.
de Oliveira FP, Stoffella-Dutra AG, Barbosa Costa G, Silva de Oliveira J, Dourado Amaral C, Duarte Santos J, et al. Re-emergence of yellow fever in Brazil during 2016–2019: challenges, lessons learned, and perspectives. Viruses. 2020;12:1233.
Roth A, Hoy D, Horwood PF, Ropa B, Hancock T, Guillaumot L, et al. Preparedness for threat of chikungunya in the Pacific. Emerg Infect Dis. 2014;20:e130696.
Lippi CA, Stewart-Ibarra AM, Romero M, Lowe R, Mahon R, Van Meerbeeck CJ, et al. Spatiotemporal tools for emerging and endemic disease hotspots in small areas: an analysis of dengue and chikungunya in Barbados, 2013–2016. Am J Trop Med Hyg. 2020;103:149–56.
Lucey DR, Gostin LO. The emerging Zika pandemic: enhancing preparedness. J Am Med Assoc. 2016;315:865–6.
CAS Google Scholar
Chen B, Sweeny AR, Wu VY, Christofferson R, Ebel G, Fagre AC, et al. Exploring the mosquito-arbovirus network: a survey of vector competence experiments. Am J Trop Med Hyg. 2023;108:987–94.
Vogels CBF, Rückert C, Cavany SM, Alex Perkins T, Ebel GD, Grubaugh ND. Arbovirus coinfection and co-transmission: a neglected public health concern? PLoS Biol. 2019;17:e3000130.
Carlson CJ, Mendenhall E. Preparing for emerging infections means expecting new syndemics. Lancet. 2019;394:297.
PubMed Google Scholar
Glennon EE, Jephcott FL, Oti A, Carlson CJ, Bustos Carillo FA, Hranac CR, et al. Syndromic detectability of haemorrhagic fever outbreaks. medRxiv. 2020. https://doi.org/10.1101/2020.03.28.20019463 .
Article Google Scholar
Elith J, Leathwick JR. Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst. 2009;40:677–97. https://doi.org/10.1146/annurev.ecolsys.110308.120159 .
Elith J, Franklin J. Species distribution modeling. In: Reference module in life sciences. 2017. https://doi.org/10.1016/b978-0-12-809633-8.02390-6
Johnson CJ, Gillingham MP. An evaluation of mapped species distribution models used for conservation planning. Environ Conserv. 2005;32:117–28.
Madzokere ET, Hallgren W, Sahin O, Webster JA, Webb CE, Mackey B, et al. Integrating statistical and mechanistic approaches with biotic and environmental variables improves model predictions of the impact of climate and land-use changes on future mosquito-vector abundance, diversity and distributions in Australia. Parasit Vectors. 2020;13:484. https://doi.org/10.1186/s13071-020-04360-3 .
Article PubMed PubMed Central Google Scholar
Wiese D, Escalante AA, Murphy H, Henry KA, Gutierrez-Velez VH. Integrating environmental and neighborhood factors in MaxEnt modeling to predict species distributions: a case study of Aedes albopictus in southeastern Pennsylvania. PLoS ONE. 2019;14:e0223821.
CAS PubMed PubMed Central Google Scholar
El-Gabbas A, Dormann CF. Wrong, but useful: regional species distribution models may not be improved by range-wide data under biased sampling. Ecol Evol. 2018;8:2196–206.
Lippi CA, Stewart-Ibarra AM, Loor MEFB, Zambrano JED, Lopez NAE, Blackburn JK, et al. Geographic shifts in Aedes aegypti habitat suitability in Ecuador using larval surveillance data and ecological niche modeling: implications of climate change for public health vector control. PLoS Negl Trop Dis. 2019;13:e0007322.
Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus . eLife. 2015;4:e08347.
Rodriguez-Morales AJ, Cardona-Ospina JA, Collins MH. Emerging and re-emerging vector-borne and zoonotic diseases. Front Med. 2021;8:714630. https://doi.org/10.3389/fmed.2021.714630 .
Brookes VJ, Hernández-Jover M, Black PF, Ward MP. Preparedness for emerging infectious diseases: pathways from anticipation to action. Epidemiol Infect. 2015;143:2043–58.
Centers for Disease Control and Prevention. A National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans. Centers for Disease Control and Prevention; 2020;1–16.
Smith Gueye C, Newby G, Gosling RD, Whittaker MA, Chandramohan D, Slutsker L, et al. Strategies and approaches to vector control in nine malaria-eliminating countries: a cross-case study analysis. Malar J. 2016;15:1–14.
Lippi CA, Mao L, Stewart-Ibarra AM, Heydari N, Ayala EB, Burkett-Cadena ND, et al. A network analysis framework to improve the delivery of mosquito abatement services in Machala, Ecuador. Int J Health Geogr. 2020;19:1–14.
Fouet C, Kamdem C. Integrated mosquito management: is precision control a luxury or necessity? Trends Parasitol. 2019;35:85–95.
Dye-Braumuller K, Fredregill C, Debboun M. Mosquito control. In: Mosquitoes, communities, and public health in Texas. Mosquito and Vector Control Division, Harris County Public Health, Houston, TX. 2020; pp. 249–78.
Impoinvil DE, Ahmad S, Troyo A, Keating J, Githeko AK, Mbogo CM, et al. Comparison of mosquito control programs in seven urban sites in Africa, the Middle East, and the Americas. Health Policy. 2007;83:196–212.
Alimi TO, Fuller DO, Quinones ML, Xue R-D, Herrera SV, Arevalo-Herrera M, et al. Prospects and recommendations for risk mapping to improve strategies for effective malaria vector control interventions in Latin America. Malar J. 2015;14:519.
Coatsworth H, Lippi CA, Vasquez C, Ayers JB, Stephenson CJ, Waits C, et al. A molecular surveillance-guided vector control response to concurrent dengue and West Nile virus outbreaks in a COVID-19 hotspot of Florida. Lancet Reg Health Am. 2022;11:100231. https://doi.org/10.1101/2021.10.08.21264776 .
Chanda E, Ameneshewa B, Angula HA, Iitula I, Uusiku P, Trune D, et al. Strengthening tactical planning and operational frameworks for vector control: the roadmap for malaria elimination in Namibia. Malar J. 2015;14:302.
Ryan SJ, Lippi CA, Zermoglio F. Shifting transmission risk for malaria in Africa with climate change: a framework for planning and intervention. Malar J. 2020;19:170.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34.
WHO. Global vector control response 2017–2030. World Health Organization. 2017;1–53.
WHO. Global Strategy for dengue prevention and control, 2012–2020. World Health Organization. 2012; 1–43.
Moonen JP, Schinkel M, van der Most T, Miesen P, van Rij RP. Composition and global distribution of the mosquito virome—a comprehensive database of insect-specific viruses. One Health. 2023;16:100490.
Lippi CA, Ryan SJ, White AL, Gaff HD, Carlson CJ. Trends and opportunities in tick-borne disease geography. J Med Entomol. 2021;58:2021–9.
Fick SE, Hijmans RJ. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol. 2017;37:4302–15.
Kraemer MUG, Sinka ME, Duda KA, Mylne A, Shearer FM, Brady OJ, et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data. 2015;2:150035.
Srivastava V, Lafond V, Griess VC. Species distribution models (SDM): applications, benefits and challenges in invasive species management. CABI Rev. 2019;2:1–13. https://doi.org/10.1079/pavsnnr201914020 .
Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190:231–59. https://doi.org/10.1016/j.ecolmodel.2005.03.026 .
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;15:1965–78. https://doi.org/10.1002/joc.1276 .
Soghigian J, Sither C, Justi S, Morinaga G, Cassel B, Vitek C, et al. An enduring enemy: phylogenomics reveals the history of host use in mosquitoes. 2023; Available from: https://www.researchsquare.com/article/rs-2515328/latest.pdf .
Araújo MB, Anderson RP, Márcia Barbosa A, Beale CM, Dormann CF, Early R, et al. Standards for distribution models in biodiversity assessments. Sci Adv. 2019;5:e4858.
Barker JR, MacIsaac HJ. Species distribution models applied to mosquitoes: use, quality assessment, and recommendations for best practice. Ecol Model. 2022;472:110073. https://doi.org/10.1016/j.ecolmodel.2022.110073 .
Zurell D, Franklin J, König C, Bouchet PJ, Dormann CF, Elith J, et al. A standard protocol for reporting species distribution models. Ecography. 2020;43:1261–77.
Carlson CJ, Albery GF, Merow C, Trisos CH, Zipfel CM, Eskew EA, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607:555–62.
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–3.
World Health Organization. Vector alert: Anopheles stephensi invasion and spread in Africa and Sri Lanka. World Health Organization. 2022;1–4.
Schluth CG, Standley CJ, Bansal S, Carlson CJ. Spatial parasitology and the unmapped human helminthiases. Parasitology. 2023;150:391–9.
PubMed Central Google Scholar
Judson SD, LeBreton M, Fuller T, Hoffman RM, Njabo K, Brewer TF, et al. Translating predictions of zoonotic viruses for policymakers. EcoHealth. 2017;15:52–62.
Foley DH, Weitzman AL, Miller SE, Faran ME, Rueda LM, Wilkerson RC. The value of georeferenced collection records for predicting patterns of mosquito species richness and endemism in the Neotropics. Ecol Entomol. 2007;33:12–23.
Trájer A. The complex investigation of the colonization potential of Aedes albopictus (Diptera: Culicidae) in the South Pannonian Ecoregion. Appl Ecol Env Res. 2017;15:275–98.
Tonnang HE, Kangalawe RY, Yanda PZ. Predicting and mapping malaria under climate change scenarios: the potential redistribution of malaria vectors in Africa. Malar J. 2010;9:111.
Khormi HM, Kumar L. Climate change and the potential global distribution of Aedes aegypti : spatial modelling using geographical information system and CLIMEX. Geospat Health. 2014;8:405.
Tonnang HE, Tchouassi DP, Juarez HS, Igweta LK, Djouaka RF. Zoom in at African country level: potential climate-induced changes in areas of suitability for survival of malaria vectors. Int J Health Geogr. 2014;13:12.
Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89.
Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69.
Lühken R, Czajka C, Steinke S, Jöst H, Schmidt-Chanasit J, Pfitzner W, et al. Distribution of individual members of the mosquito Anopheles maculipennis complex in Germany identified by newly developed real-time PCR assays: the Anopheles maculipennis complex in Germany. Med Vet Entomol. 2016;30:144–54.
Moyes CL, Shearer FM, Huang Z, Wiebe A, Gibson HS, Nijman V, et al. Predicting the geographical distributions of the macaque hosts and mosquito vectors of Plasmodium knowlesi malaria in forested and non-forested areas. Parasit Vectors. 2016;9:242.
Sinka ME, Golding N, Massey NC, Wiebe A, Huang Z, Hay SI, et al. Modelling the relative abundance of the primary African vectors of malaria before and after the implementation of indoor, insecticide-based vector control. Malar J. 2016;15:142.
Wiebe A, Longbottom J, Gleave K, Shearer FM, Sinka ME, Massey NC, et al. Geographical distributions of African malaria vector sibling species and evidence for insecticide resistance. Malar J. 2017;16:85.
Longbottom J, Browne AJ, Pigott DM, Sinka ME, Golding N, Hay SI, et al. Mapping the spatial distribution of the Japanese encephalitis vector, Culex tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) within areas of Japanese encephalitis risk. Parasit Vectors. 2017;10:148.
Ducheyne E, Tran Minh NN, Haddad N, Bryssinckx W, Buliva E, Simard F, et al. Current and future distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in WHO Eastern Mediterranean Region. Int J Health Geogr. 2018;17:4.
Hertig E. Distribution of Anopheles vectors and potential malaria transmission stability in Europe and the Mediterranean area under future climate change. Parasit Vectors. 2019;12:18.
Khan SU, Ogden NH, Fazil AA, Gachon PH, Dueymes GU, Greer AL, et al. Current and projected distributions of Aedes aegypti and Ae. albopictus in Canada and the US. Environ Health Perspect. 2020;128:057007.
Polineni S, Shastri O, Bagchi A, Gnanakumar G, Rasamsetti S, Sundaravadivel P. MOSQUITO EDGE: an edge-intelligent real-time mosquito threat prediction using an IoT-enabled hardware system. Sensors. 2022;22:695.
Ayala D, Costantini C, Ose K, Kamdem GC, Antonio-Nkondjio C, Agbor J-P, et al. Habitat suitability and ecological niche profile of major malaria vectors in Cameroon. Malar J. 2009;8:307.
Monaghan AJ, Eisen RJ, Eisen L, McAllister J, Savage HM, Mutebi J-P, et al. Consensus and uncertainty in the geographic range of Aedes aegypti and Aedes albopictus in the contiguous United States: multi-model assessment and synthesis. PLoS Comput Biol. 2019;15:e1007369.
Simons RRL, Croft S, Rees E, Tearne O, Arnold ME, Johnson N. Using species distribution models to predict potential hot-spots for Rift Valley fever establishment in the United Kingdom. PLoS ONE. 2019;14:e0225250.
Peterson AT, Vieglais DA, Andreasen JK. Migratory birds modeled as critical transport agents for West Nile virus in North America. Vector-Borne Zoonotic Dis. 2003;3:27–37.
Levine RS, Peterson AT, Benedict MQ. Distribution of members of Anopheles quadrimaculatus Say s.l. (Diptera: Culicidae) and implications for their roles in malaria transmission in the United States. J Med Entomol. 2004;41:607–13.
Levine RS, Peterson AT, Benedict MQ. Geographic and ecologic distributions of the Anopheles gambiae complex predicted using a genetic algorithm. Am J Trop Med Hyg. 2004;70:105–9.
Peterson AT, Martínez-Campos C, Nakazawa Y, Martínez-Meyer E. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans R Soc Trop Med Hyg. 2005;99:647–55.
Sweeney AW, Beebe NW, Cooper RD, Bauer JT, Peterson AT. Environmental factors associated with distribution and range limits of malaria vector Anopheles farauti in Australia. J Med Entomol. 2006;43:1068–75.
Sweeney AW, Beebe NW, Cooper RD. Analysis of environmental factors influencing the range of anopheline mosquitoes in northern Australia using a genetic algorithm and data mining methods. Ecol Model. 2007;203:375–86.
De Barros FSM, De Aguiar DB, Rosa-Freitas MG, Luitgards-Moura JF, Da Costa GH, Honório NA, et al. Distribution summaries of malaria vectors in the northern Brazilian Amazon. J Vect Ecol. 2007;32:161.
Rosa-Freitas MG, Tsouris P, Peterson AT, Honório NA, Barros FSMD, Aguiar DBD, et al. An ecoregional classification for the state of Roraima, Brazil: the importance of landscape in malaria biology. Mem Inst Oswaldo Cruz. 2007;102:349–58.
Beebe NW, Cooper RD, Mottram P, Sweeney AW. Australia’s dengue risk driven by human adaptation to climate change. PLoS Negl Trop Dis. 2009;3:e429.
Peterson AT. Shifting suitability for malaria vectors across Africa with warming climates. BMC Infect Dis. 2009;9:59.
Pech-May A, Moo-Llanes DA, Puerto-Avila MB, Casas M, Danis-Lozano R, Ponce G, et al. Population genetics and ecological niche of invasive Aedes albopictus in Mexico. Acta Trop. 2016;157:30–41.
Moffett A, Shackelford N, Sarkar S. Malaria in Africa: vector species’ niche models and relative risk maps. PLoS ONE. 2007;2:e824.
Kulkarni MA, Desrochers RE, Kerr JT. High resolution niche models of malaria vectors in northern Tanzania: a new capacity to predict malaria risk? PLoS ONE. 2010;5:e9396.
Masuoka P, Klein TA, Kim H-C, Claborn DM, Achee N, Andre R, et al. Modeling the distribution of Culex tritaeniorhynchus to predict Japanese encephalitis distribution in the Republic of Korea. Geospat Health. 2010;5:45.
Medley KA. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models: niche shifts and global invasion. Glob Ecol Biogeogr. 2010;19:122–33.
Laporta GZ, Ramos DG, Ribeiro MC, Sallum MAM. Habitat suitability of Anopheles vector species and association with human malaria in the Atlantic Forest in south-eastern Brazil. Mem Inst Oswaldo Cruz. 2011;106:239–45.
Fischer D, Thomas SM, Niemitz F, Reineking B, Beierkuhnlein C. Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Global Planet Change. 2011;78:54–64.
Miller RH, Masuoka P, Klein TA, Kim H-C, Somer T, Grieco J. Ecological niche modeling to estimate the distribution of Japanese encephalitis virus in Asia. PLoS Negl Trop Dis. 2012;6:e1678.
Porretta D, Mastrantonio V, Bellini R, Somboon P, Urbanelli S. Glacial history of a modern invader: phylogeography and species distribution modelling of the Asian tiger mosquito Aedes albopictus . PLoS ONE. 2012;7:e44515.
Fuller DO, Ahumada ML, Quiñones ML, Herrera S, Beier JC. Near-present and future distribution of Anopheles albimanus in Mesoamerica and the Caribbean Basin modeled with climate and topographic data. Int J Health Geogr. 2012;11:13.
Obsomer V, Defourny P, Coosemans M. Predicted distribution of major malaria vectors belonging to the complex in Asia: ecological niche and environmental influences. PLoS ONE. 2012;7:e50475.
Fuller DO, Parenti MS, Hassan AN, Beier JC. Linking land cover and species distribution models to project potential ranges of malaria vectors: an example using Anopheles arabiensis in Sudan and Upper Egypt. Malar J. 2012;11:264.
Rochlin I, Ninivaggi DV, Hutchinson ML, Farajollahi A. Climate change and range expansion of the Asian tiger mosquito ( Aedes albopictus ) in northeastern USA: implications for public health practitioners. PLoS ONE. 2013;8:e60874.
Sallam MF, Al Ahmed AM, Abdel-Dayem MS, Abdullah MAR. Ecological niche modeling and land cover risk areas for Rift Valley fever vector, Culex tritaeniorhynchus Giles in Jazan, Saudi Arabia. PLoS ONE. 2013;8:e65786.
Gardner L, Sarkar S. A global airport-based risk model for the spread of dengue infection via the air transport network. PLoS ONE. 2013;8:e72129.
Mughini-Gras L, Mulatti P, Severini F, Boccolini D, Romi R, Bongiorno G, et al. Ecological niche modelling of potential West Nile virus vector mosquito species and their geographical association with equine epizootics in Italy. EcoHealth. 2014;11:120–32.
Foley DH, Linton Y-M, Ruiz-Lopez JF, Conn JE, Sallum MAM, Póvoa MM, et al. Geographic distribution, evolution, and disease importance of species within the Neotropical Anopheles albitarsis group (Diptera, Culicidae). J Vector Ecol. 2014;39:168–81.
Cardoso-Leite R, Vilarinho AC, Novaes MC, Tonetto AF, Vilardi GC, Guillermo-Ferreira R. Recent and future environmental suitability to dengue fever in Brazil using species distribution model. Trans R Soc Trop Med Hyg. 2014;108:99–104.
Olave MET, Rojas-Villalobos H, Zesati G, Bravo-Pena L, Alatorre-Cejudo L. Modelo biogeográfico de los mosquitos Culex spp. (Diptera: Culicidae) en México. Bol Geogr. 2015;37:43–58.
Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, Danis-Lozano R, Peterson AT. Climate change influences on global distributions of dengue and chikungunya virus vectors. Phil Trans R Soc B. 2015;370:20140135.
Alahmed AM, Naeem M, Kheir SM, Sallam MF. Ecological distribution modeling of two malaria mosquito vectors using geographical information system in Al-Baha Province, Kingdom of Saudi Arabia. Pak J Zool. 2015;47:1797–806.
Melaun C, Werblow A, Cunze S, Zotzmann S, Koch LK, Mehlhorn H, et al. Modeling of the putative distribution of the arbovirus vector Ochlerotatus japonicus japonicus (Diptera: Culicidae) in Germany. Parasitol Res. 2015;114:1051–61.
Samson DM, Archer RS, Alimi TO, Arheart KL, Impoinvil DE, Oscar R, et al. New baseline environmental assessment of mosquito ecology in northern Haiti during increased urbanization. J Vector Ecol. 2015;40:46–58.
Acheson ES, Plowright AA, Kerr JT. Where have all the mosquito nets gone? Spatial modelling reveals mosquito net distributions across Tanzania do not target optimal Anopheles mosquito habitats. Malar J. 2015;14:322.
Kulkarni MA, Desrochers RE, Kajeguka DC, Kaaya RD, Tomayer A, Kweka EJ, et al. Ten years of environmental change on the slopes of Mount Kilimanjaro and its associated shift in malaria vector distributions. Front Public Health. 2016;4:281.
Cunze S, Kochmann J, Koch LK, Klimpel S. Aedes albopictus and its environmental limits in Europe. PLoS ONE. 2016;11:e0162116.
Samy AM, Elaagip AH, Kenawy MA, Ayres CFJ, Peterson AT, Soliman DE. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus , vector of West Nile virus and lymphatic filariasis. PLoS ONE. 2016;11:e0163863.
Mweya CN, Kimera SI, Stanley G, Misinzo G, Mboera LEG. Climate change influences potential distribution of infected Aedes aegypti co-occurrence with dengue epidemics risk areas in Tanzania. PLoS ONE. 2016;11:e0162649.
Sallam MF, Xue R-D, Pereira RM, Koehler PG. Ecological niche modeling of mosquito vectors of West Nile virus in St. John’s County, Florida, USA. Parasit Vectors. 2016;9:371.
Lainhart W, Dutari LC, Rovira JR, Sucupira IMC, Póvoa MM, Conn JE, et al. Epidemic and non-epidemic hot spots of malaria transmission occur in indigenous Comarcas of Panama. PLoS Negl Trop Dis. 2016;10:e0004718.
Ganser C, Gregory AJ, McNew LB, Hunt LA, Sandercock BK, Wisely SM. Fine-scale distribution modeling of avian malaria vectors in north-central Kansas. J Vector Ecol. 2016;41:114–22.
Koch LK, Cunze S, Werblow A, Kochmann J, Dörge DD, Mehlhorn H, et al. Modeling the habitat suitability for the arbovirus vector Aedes albopictus (Diptera: Culicidae) in Germany. Parasitol Res. 2016;115:957–64.
Ren Z, Wang D, Ma A, Hwang J, Bennett A, Sturrock HJW, et al. Predicting malaria vector distribution under climate change scenarios in China: challenges for malaria elimination. Sci Rep. 2016;6:20604.
Fuller DO, Alimi T, Herrera S, Beier JC, Quiñones ML. Spatial association between malaria vector species richness and malaria in Colombia. Acta Trop. 2016;158:197–200.
Naeem M, Alahmed AM, Kheir SM, Sallam MF. Spatial distribution modeling of Stegomyia aegypti and Culex tritaeniorhynchus (Diptera: Culicidae) in Al-bahah Province. Kingdom Saudi Arabia Trop Biomed. 2016;33:295–310.
Espinosa MO, Polop F, Rotela CH, Abril M, Scavuzzo CM. Spatial pattern evolution of Aedes aegypti breeding sites in an Argentinean city without a dengue vector control programme. Geospat Health. 2016;11:471.
Fatima SH, Atif S, Rasheed SB, Zaidi F, Hussain E. Species distribution modelling of Aedes aegypt i in two dengue-endemic regions of Pakistan. Trop Med Int Health. 2016;21:427–36.
Espinosa M, Weinberg D, Rotela CH, Polop F, Abril M, Scavuzzo CM. Temporal dynamics and spatial patterns of Aedes aegypti b reeding sites, in the context of a dengue control program in Tartagal (Salta Province, Argentina). PLoS Negl Trop Dis. 2016;10:e0004621.
Santos J, Meneses BM. An integrated approach for the assessment of the Aedes aegypti and Aedes albopictus global spatial distribution, and determination of the zones susceptible to the development of Zika virus. Acta Trop. 2017;168:80–90.
Kimera SI, Mweya CN, Mboera LEG. Climate influence on emerging risk areas for Rift Valley fever epidemics in Tanzania. Am J Trop Med Hyg. 2017;97:109–14.
Moua Y, Roux E, Girod R, Dusfour I, De Thoisy B, Seyler F, et al. Distribution of the habitat suitability of the main malaria vector in French Guiana using maximum entropy modeling. J Med Entomol. 2016;54:606.
Baak-Baak CM, Moo-Llanes DA, Cigarroa-Toledo N, Puerto FI, Machain-Williams C, Reyes-Solis G, et al. Ecological niche model for predicting distribution of disease-vector mosquitoes in Yucatán State, México. J Med Entomol. 2017;54:854–61.
Filatov S. Little pigeons can carry great messages: potential distribution and ecology of Uranotaenia ( Pseudoficalbia ) unguiculata Edwards, 1913 (Diptera: Culicidae), a lesser-known mosquito species from the Western Palaearctic. Parasit Vectors. 2017;10:464.
Johnson TL, Haque U, Monaghan AJ, Eisen L, Hahn MB, Hayden MH, et al. Modeling the environmental suitability for Aedes ( Stegomyia ) aegypti and Aedes ( Stegomyia ) albopictus (Diptera: Culicidae) in the contiguous United States. J Med Entomol. 2017;54:1605–14.
Altamiranda-Saavedra M, Arboleda S, Parra JL, Peterson AT, Correa MM. Potential distribution of mosquito vector species in a primary malaria endemic region of Colombia. PLoS ONE. 2017;12:e0179093.
Pakdad K, Hanafi-Bojd AA, Vatandoost H, Sedaghat MM, Raeisi A, Moghaddam AS, et al. Predicting the potential distribution of main malaria vectors Anopheles stephensi , An. culicifacies s.l. and An. fluviatilis s.l. in Iran based on maximum entropy model. Acta Trop. 2017;169:93–9.
Kalan K, Ivović V, Glasnović P, Buzan E. Presence and potential distribution of Aedes albopictus and Aedes japonicus japonicus (Diptera: Culicidae) in Slovenia. J Med Entomol. 2017;54:1510–8.
Alaniz AJ, Bacigalupo A, Cattan PE. Spatial quantification of the world population potentially exposed to Zika virus. Int J Epidemiol. 2017;46:966–75.
Sallam M, Michaels S, Riegel C, Pereira R, Zipperer W, Lockaby B, et al. Spatio-temporal distribution of vector–host contact (VHC) ratios and ecological niche modeling of the West Nile virus mosquito vector, Culex quinquefasciatus , in the city of New Orleans, LA, USA. IJERPH. 2017;14:892.
Obenauer JF, Andrew Joyner T, Harris JB. The importance of human population characteristics in modeling Aedes aegypti distributions and assessing risk of mosquito-borne infectious diseases. Trop Med Health. 2017;45:38.
Alaniz AJ, Bacigalupo A, Cattan PE. Zika: probability of establishment of its vector, Aedes aegypti , in Chile. Rev Chilena Infectol. 2017;34:553–6.
Yañez-Arenas C, Rioja-Nieto R, Martín GA, Dzul-Manzanilla F, Chiappa-Carrara X, Buenfil-Ávila A, et al. Characterizing environmental suitability of Aedes albopictus (Diptera: Culicidae) in Mexico based on regional and global niche models. J Med Entomol. 2018;55:69–77.
Mosomtai G, Evander M, Mundia C, Sandström P, Ahlm C, Hassan OA, et al. Datasets for mapping pastoralist movement patterns and risk zones of Rift Valley fever occurrence. Data Brief. 2018;16:762–70.
Akpan GE, Adepoju KA, Oladosu OR, Adelabu SA. Dominant malaria vector species in Nigeria: modelling potential distribution of Anopheles gambiae sensu lato and its siblings with MaxEnt. PLoS ONE. 2018;13:e0204233.
Richman R, Diallo D, Diallo M, Sall AA, Faye O, Diagne CT, et al. Ecological niche modeling of Aedes mosquito vectors of chikungunya virus in southeastern Senegal. Parasit Vectors. 2018;11:255.
Liu B, Gao X, Ma J, Jiao Z, Xiao J, Wang H. Influence of host and environmental factors on the distribution of the Japanese encephalitis vector Culex tritaeniorhynchus in China. IJERPH. 2018;15:1848.
Kamal M, Kenawy MA, Rady MH, Khaled AS, Samy AM. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae albopictus under changing climate. PLoS ONE. 2018;13:e0210122.
Samy AM, Alkishe AA, Thomas SM, Wang L, Zhang W. Mapping the potential distributions of etiological agent, vectors, and reservoirs of Japanese encephalitis in Asia and Australia. Acta Trop. 2018;188:108–17.
Estallo EL, Sangermano F, Grech M, Ludueña-Almeida F, Frías-Cespedes M, Ainete M, et al. Modelling the distribution of the vector Aedes aegypti in a central Argentine city: modelling Aedes aegypti distribution. Med Vet Entomol. 2018;32:451–61.
Hira FS, Asad A, Farrah Z, Basit RS, Mehreen F, Muhammad K. Patterns of occurrence of dengue and chikungunya, and spatial distribution of mosquito vector Aedes albopictus in Swabi district, Pakistan. Trop Med Int Health. 2018;23:1002–13.
Hanafi-Bojd AA, Sedaghat MM, Vatandoost H, Azari-Hamidian S, Pakdad K. Predicting environmentally suitable areas for Anopheles superpictus Grassi (s.l.), Anopheles maculipennis Meigen (s.l.) and Anopheles sacharovi Favre (Diptera: Culicidae) in Iran. Parasit Vectors. 2018;11:382.
Alaniz AJ, Carvajal MA, Bacigalupo A, Cattan PE. Global spatial assessment of Aedes aegypti and Culex quinquefasciatus : a scenario of Zika virus exposure. Epidemiol Infect. 2019;147:e52.
Peach DAH, Almond M, Pol JC. Modeled distributions of Aedes japonicus japonicus and Aedes togoi (Diptera: Culicidae) in the United States, Canada, and northern Latin America. J Vector Ecol. 2019;44:119–29.
Liu B, Gao X, Ma J, Jiao Z, Xiao J, Hayat MA, et al. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in mainland China. Sci Total Environ. 2019;664:203–14.
Liu B, Jiao Z, Ma J, Gao X, Xiao J, Hayat MA, et al. Modelling the potential distribution of arbovirus vector Aedes aegypti under current and future climate scenarios in Taiwan. China Pest Manag Sci. 2019;75:3076–83.
Akpan GE, Adepoju KA, Oladosu OR. Potential distribution of dominant malaria vector species in tropical region under climate change scenarios. PLoS ONE. 2019;14:e0218523.
De Almeida MAB, Dos Santos E, Cardoso JDC, Da Silva LG, Rabelo RM, Bicca-Marques JC. Predicting yellow fever through species distribution modeling of virus, vector, and monkeys. EcoHealth. 2019;16:95–108.
Tiffin HS, Peper ST, Wilson-Fallon AN, Haydett KM, Cao G, Presley SM. The influence of new surveillance data on predictive species distribution modeling of Aedes aegypti and Aedes albopictus in the United States. Insects. 2019;10:400.
Chen X, Dimitrov NB, Meyers LA. Uncertainty analysis of species distribution models. PLoS ONE. 2019;14:e0214190.
Hesami N, Abai MR, Vatandoost H, Alizadeh M, Fatemi M, Ramazanpour J, et al. Using ecological niche modeling to predict the spatial distribution of Anopheles maculipennis s.l. and Culex theileri (Diptera: Culicidae) in Central Iran. J Arthropod Borne Dis. 2019;13:165–76.
Cunze S, Kochmann J, Koch LK, Genthner E, Klimpel S. Vector distribution and transmission risk of the Zika virus in South and Central America. PeerJ. 2019;7:e7920.
Gwitira I, Murwira A, Masocha M, Zengeya FM, Shekede MD, Chirenda J, et al. GIS-based stratification of malaria risk zones for Zimbabwe. Geocarto Int. 2019;34:1163–76.
Abrha H, Hagos H, Brhane E, Hadgu M, Mamo G. Spatio-temporal dynamics of malaria expansion under climate change in semi-arid areas of Ethiopia. Environ Hazards. 2019;18:400–13.
Ordoñez-Sierra R, Mastachi-Loza CA, Díaz-Delgado C, Cuervo-Robayo AP, Fonseca Ortiz CR, Gómez-Albores MA, et al. Spatial risk distribution of dengue based on the ecological niche model of Aedes aegypti (Diptera: Culicidae) in the Central Mexican highlands. J Med Entomol. 2020;57:728–37.
Hanafi-Bojd AA, Vatandoost H, Yaghoobi-Ershadi MR. Climate change and the risk of malaria transmission in Iran. J Med Entomol. 2020;57:50–64.
Portilla Cabrera CV, Selvaraj JJ. Geographic shifts in the bioclimatic suitability for Aedes aegypti under climate change scenarios in Colombia. Heliyon. 2020;6:e03101.
Cunze S, Kochmann J, Klimpel S. Global occurrence data improve potential distribution models for Aedes japonicus japonicus in non-native regions. Pest Manag Sci. 2020;76:1814–22.
Peach DAH, Matthews BJ. Modeling the putative ancient distribution of Aedes togoi (Diptera: Culicidae). J Insect Sci. 2020;20:7.
Espinosa-Vélez Y, Altamiranda-Saavedra M, Correa MM. Potential distribution of main malaria vector species in the endemic Colombian Pacific region. Trop Med Int Health. 2020;25:861–73.
Liu B, Gao X, Zheng K, Ma J, Jiao Z, Xiao J, et al. The potential distribution and dynamics of important vectors Culex pipiens pallens and Culex pipiens quinquefasciatus in China under climate change scenarios: an ecological niche modelling approach. Pest Manag Sci. 2020;76:3096–107.
Liu B, Ma J, Jiao Z, Gao X, Xiao J, Wang H. Risk assessment for the Rift Valley fever occurrence in China: special concern in south-west border areas. Transbound Emerg Dis. 2021;68:445–57.
Figueroa DP, Scott S, González CR, Bizama G, Flores-Mara R, Bustamante R, et al. Estimating the climate change consequences on the potential distribution of Culex pipiens L. 1758, to assess the risk of West Nile virus establishment in Chile. Gayana. 2020;84:46–53.
Marshall DS, Butler CJ. Potential distribution of the biocontrol agent Toxorhynchites rutilus by 2070. J Am Mosq Control Assoc. 2020;36:131–8.
Castaño-Quintero S, Escobar-Luján J, Osorio-Olvera L, Peterson AT, Chiappa-Carrara X, Martínez-Meyer E, et al. Supraspecific units in correlative niche modeling improves the prediction of geographic potential of biological invasions. PeerJ. 2020;8:e10454.
Bond JG, Moo-Llanes DA, Ortega-Morales AI, Marina CF, Casas-Martínez M, Danis-Lozano R. Diversity and potential distribution of culicids of medical importance of the Yucatan Peninsula, Mexico. Salud Publica Mexico. 2020;62:379–87.
Shoraka H, Sofizadeh A, Mehravaran A. Larval habitat characteristics and predicting the distribution of Culex tritaeniorhynchus using maximum entropy (MaxEnt) model in Golestan Province (north of Iran). J Vector Borne Dis. 2020;57:259.
Valderrama L, Ayala S, Reyes C, González CR. Modeling the potential distribution of the malaria vector Anopheles ( Ano .) pseudopunctipennis Theobald (Diptera: Culicidae) in arid regions of northern Chile. Front Public Health. 2021;9:611152.
Gorris ME, Bartlow AW, Temple SD, Romero-Alvarez D, Shutt DP, Fair JM, et al. Updated distribution maps of predominant Culex mosquitoes across the Americas. Parasit Vectors. 2021;14:547.
Tjaden NB, Cheng Y, Beierkuhnlein C, Thomas SM. Chikungunya beyond the Tropics: where and when do we expect disease transmission in Europe? Viruses. 2021;13:1024.
Moo-Llanes DA, López-Ordóñez T, Torres-Monzón JA, Mosso-González C, Casas-Martínez M, Samy AM. Assessing the potential distributions of the invasive mosquito vector Aedes albopictus and its natural Wolbachia infections in México. Insects. 2021;12:143.
Omar K, Thabet HS, TagEldin RA, Asadu CC, Chukwuekezie OC, Ochu JC, et al. Ecological niche modeling for predicting the potential geographical distribution of Aedes species (Diptera: Culicidae): a case study of Enugu State, Nigeria. Parasit Epidemiol Control. 2021;15:e00225.
Echeverry-Cárdenas E, López-Castañeda C, Carvajal-Castro JD, Aguirre-Obando OA. Potential geographic distribution of the tiger mosquito Aedes albopictus (Skuse, 1894) (Diptera: Culicidae) in current and future conditions for Colombia. PLoS Negl Trop Dis. 2021;15:e0008212.
Outammassine A, Zouhair S, Loqman S. Global potential distribution of three underappreciated arboviruses vectors ( Aedes japonicus , Aedes vexans and Aedes vittatus ) under current and future climate conditions. Transbound Emerg Dis. 2022;69:e1160–71.
Campbell LP, Burkett-Cadena ND, Miqueli E, Unlu I, Sloyer KE, Medina J, et al. Potential distribution of Aedes ( Ochlerotatus ) scapularis (Diptera: Culicidae): a vector mosquito new to the Florida peninsula. Insects. 2021;12:213.
Andreo V, Cuervo PF, Porcasi X, Lopez L, Guzman C, Scavuzzo CM. Towards a workflow for operational mapping of Aedes aegypti at urban scale based on remote sensing. Remote Sens Appl Soc Environ. 2021;23:100554.
Hanafi-Bojd AA, Motazakker M, Vatandoost H, Dabiri F, Chavshin AR. Sindbis virus infection of mosquito species in the wetlands of northwestern Iran and modeling the probable ecological niches of SINV vectors in the country. Acta Trop. 2021;220:105952.
Cuervo PF, Artigas P, Mas-Coma S, Bargues MD. West Nile virus in Spain: Forecasting the geographical distribution of risky areas with an ecological niche modelling approach. Transbound Emerg Dis. 2022;69:e1113. https://doi.org/10.1111/tbed.14398 .
Article PubMed Google Scholar
Outammassine A, Zouhair S, Loqman S. Rift Valley fever and West Nile virus vectors in Morocco: current situation and future anticipated scenarios. Transbound Emerg Dis. 2022;69:1466–78.
Olabimi IO, Ileke KD, Adu BW, Arotolu TE. Potential distribution of the primary malaria vector Anopheles gambiae Giles [Diptera: Culicidae] in southwest Nigeria under current and future climatic conditions. JoBAZ. 2021;82:63.
Calzolari M, Desiato R, Albieri A, Bellavia V, Bertola M, Bonilauri P, et al. Mosquitoes of the maculipennis complex in northern Italy. Sci Rep. 2021;11:6421.
Marques R, Krüger RF, Cunha SK, Silveira AS, Alves DMCC, Rodrigues GD, et al. Climate change impacts on Anopheles (K.) cruzii in urban areas of Atlantic Forest of Brazil: challenges for malaria diseases. Acta Trop. 2021;224:106123.
Moua Y, Kotchi SO, Ludwig A, Brazeau S. Mapping the habitat suitability of West Nile virus vectors in southern Quebec and eastern Ontario, Canada, with species distribution modeling and satellite earth observation data. Remote Sens. 2021;13:1637.
Arnoldi I, Negri A, Soresinetti L, Brambilla M, Carraretto D, Montarsi F, et al. Assessing the distribution of invasive Asian mosquitoes in northern Italy and modelling the potential spread of Aedes koreicus in Europe. Acta Trop. 2022;232:106536.
Souris M, Marcombe S, Laforet J, Brey PT, Corbel V, Overgaard HJ. Modeling spatial variation in risk of presence and insecticide resistance for malaria vectors in Laos. PLoS ONE. 2017;12:e0177274.
Wieser A, Reuss F, Niamir A, Müller R, O’Hara RB, Pfenninger M. Modelling seasonal dynamics, population stability, and pest control in Aedes japonicus japonicus (Diptera: Culicidae). Parasit Vectors. 2019;12:142.
Pasquali S, Mariani L, Calvitti M, Moretti R, Ponti L, Chiari M, et al. Development and calibration of a model for the potential establishment and impact of Aedes albopictus in Europe. Acta Trop. 2020;202:105228.
Foley DH, Rueda LM, Peterson AT, Wilkerson RC. Potential distribution of two species in the medically important Anopheles minimus complex (Diptera: Culicidae). J Med Entomol. 2008;45:852–60.
Foley DH, Klein TA, Kim HC, Sames WJ, Wilkerson RC, Rueda LM. Geographic distribution and ecology of potential malaria vectors in the Republic of Korea. J Med Entomol. 2009;46:680–92.
Capinha C, Gomes E, Reis E, Rocha J, Sousa CA, Do Rosário VE, et al. Present habitat suitability for Anopheles atroparvus (Diptera, Culicidae) and its coincidence with former malaria areas in mainland Portugal. Geospat Health. 2009;3:177.
Larson SR, DeGroote JP, Bartholomay LC, Sugumaran R. Ecological niche modeling of potential West Nile virus vector mosquito species in Iowa. J Insect Sci. 2010;10:1–17.
Khatchikian C, Sangermano F, Kendell D, Livdahl T. Evaluation of species distribution model algorithms for fine-scale container-breeding mosquito risk prediction. Med Vet Entomol. 2011;25:268–75.
Arboleda S, Jaramillo-O N, Peterson AT. Spatial and temporal dynamics of Aedes aegypti larval sites in Bello, Colombia. J Vector Ecol. 2012;37:37–48.
Senay SD, Worner SP, Ikeda T. Novel three-step pseudo-absence selection technique for improved species distribution modelling. PLoS ONE. 2013;8:e71218.
Hill MP, Axford JK, Hoffmann AA. Predicting the spread of Aedes albopictus in Australia under current and future climates: multiple approaches and datasets to incorporate potential evolutionary divergence: invasion potential of A. albopictus in Australia. Austral Ecol. 2014;39:469–78.
Conley AK, Fuller DO, Haddad N, Hassan AN, Gad AM, Beier JC. Modeling the distribution of the West Nile and Rift Valley fever vector Culex pipiens in arid and semi-arid regions of the Middle East and North Africa. Parasit Vectors. 2014;7:289.
Golding N, Nunn MA, Purse BV. Identifying biotic interactions which drive the spatial distribution of a mosquito community. Parasit Vectors. 2015;8:367.
Laporta GZ, Linton Y-M, Wilkerson RC, Bergo ES, Nagaki SS, SantAna DC, et al. Malaria vectors in South America: current and future scenarios. Parasit Vectors. 2015;8:426.
Cianci D, Hartemink N, Ibáñez-Justicia A. Modelling the potential spatial distribution of mosquito species using three different techniques. Int J Health Geogr. 2015;14:10.
Cunze S, Koch LK, Kochmann J, Klimpel S. Aedes albopictus and Aedes japonicus— two invasive mosquito species with different temperature niches in Europe. Parasit Vectors. 2016;9:573.
Carlson CJ, Dougherty ER, Getz W. An ecological assessment of the pandemic threat of Zika virus. PLoS Negl Trop Dis. 2016;10:e0004968.
Marcantonio M, Metz M, Baldacchino F, Arnoldi D, Montarsi F, Capelli G, et al. First assessment of potential distribution and dispersal capacity of the emerging invasive mosquito Aedes koreicus in northeast Italy. Parasit Vectors. 2016;9:63.
Padilla O, Rosas P, Moreno W, Toulkeridis T. Modeling of the ecological niches of the Anopheles spp. in Ecuador by the use of geo-informatic tools. Spatial Spatio-temporal Epidemiol. 2017;21:1–11.
Thomas S, Tjaden N, Frank C, Jaeschke A, Zipfel L, Wagner-Wiening C, et al. Areas with high hazard potential for autochthonous transmission of Aedes albopictus -associated arboviruses in Germany. IJERPH. 2018;15:1270.
Dickens BL, Sun H, Jit M, Cook AR, Carrasco LR. Determining environmental and anthropogenic factors which explain the global distribution of Aedes aegypti and Ae. albopictus . BMJ Glob Health. 2018;3:e000801.
Ding F, Fu J, Jiang D, Hao M, Lin G. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus . Acta Trop. 2018;178:155–62.
Früh L, Kampen H, Kerkow A, Schaub GA, Walther D, Wieland R. Modelling the potential distribution of an invasive mosquito species: comparative evaluation of four machine learning methods and their combinations. Ecol Model. 2018;388:136–44.
Sherpa S, Guéguen M, Renaud J, Blum MGB, Gaude T, Laporte F, et al. Predicting the success of an invader: niche shift versus niche conservatism. Ecol Evol. 2019;9:12658–75.
Uusitalo R, Siljander M, Culverwell CL, Mutai NC, Forbes KM, Vapalahti O, et al. Predictive mapping of mosquito distribution based on environmental and anthropogenic factors in Taita Hills, Kenya. Int J Appl Earth Obs Geoinf. 2019;76:84–92.
Ibáñez-Justicia A, Alcaraz-Hernández JD, Van Lammeren R, Koenraadt CJM, Bergsma A, Delucchi L, et al. Habitat suitability modelling to assess the introductions of Aedes albopictus (Diptera: Culicidae) in the Netherlands. Parasit Vectors. 2020;13:217.
Sherpa S, Renaud J, Guéguen M, Besnard G, Mouyon L, Rey D, et al. Landscape does matter: disentangling founder effects from natural and human-aided post-introduction dispersal during an ongoing biological invasion. J Anim Ecol. 2020;89:2027–42.
Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci USA. 2020;117:24900–8.
Sintayehu DW, Tassie N, De Boer WF. Present and future climatic suitability for dengue fever in Africa. Infect Ecol Epidemiol. 2020;10:1782042.
Rhodes CG, Loaiza JR, Romero LM, Gutiérrez Alvarado JM, Delgado G, Rojas Salas O, et al. Anopheles albimanus (Diptera: Culicidae) ensemble distribution modeling: applications for malaria elimination. Insects. 2022;13:221.
Srivastava A, Nagpal B, Saxena R, Subbarao S. Predictive habitat modelling for forest malaria vector species An. dirus in India—a GIS-based approach. Curr Sci. 2001;80:1129–34.
Kearney M, Porter WP, Williams C, Ritchie S, Hoffmann AA. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: the dengue mosquito Aedes aegypti in Australia. Funct Ecol. 2009;23:528–38.
Schäfer ML, Lundström JO. The present distribution and predicted geographic expansion of the floodwater mosquito Aedes sticticus in Sweden. J Vector Ecol. 2009;34:141–7.
Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus : recent trends and future scenarios. J R Soc Interface. 2012;9:2708–17.
Drake JM, Beier JC. Ecological niche and potential distribution of Anopheles arabiensis in Africa in 2050. Malar J. 2014;13:213.
Capinha C, Rocha J, Sousa CA. Macroclimate determines the global range limit of Aedes aegypti . EcoHealth. 2014;11:420–8.
Equihua M, Ibáñez-Bernal S, Benítez G, Estrada-Contreras I, Sandoval-Ruiz CA, Mendoza-Palmero FS. Establishment of Aedes aegypti (L.) in mountainous regions in Mexico: increasing number of populations at risk of mosquito-borne disease and future climate conditions. Acta Trop. 2017;166:316–27.
Zheng X, Zhong D, He Y, Zhou G. Seasonality modeling of the distribution of Aedes albopictus in China based on climatic and environmental suitability. Infect Dis Poverty. 2019;8:98.
Kerkow A, Wieland R, Koban MB, Hölker F, Jeschke JM, Werner D, et al. What makes the Asian bush mosquito Aedes japonicus japonicus feel comfortable in Germany? A fuzzy modelling approach. Parasit Vectors. 2019;12:106.
Gangoso L, Aragonés D, Martínez-de La Puente J, Lucientes J, Delacour-Estrella S, Estrada Peña R, et al. Determinants of the current and future distribution of the West Nile virus mosquito vector Culex pipiens in Spain. Environ Res. 2020;188:109837.
Bayoh MN, Thomas CJ, Lindsay SW. Mapping distributions of chromosomal forms of Anopheles gambiae in West Africa using climate data: climatic mapping of Anopheles gambiae chromosomal forms. Med Vet Entomol. 2001;15:267–74.
Kuhn KG, Campbell-Lendrum DH, Davies CR. A continental risk map for malaria mosquito (Diptera: Culicidae) vectors in Europe. J Med Entomol. 2002;39:621–30.
Diuk-Wasser MA, Brown HE, Andreadis TG, Fish D. Modeling the spatial distribution of mosquito vectors for West Nile virus in Connecticut, USA. Vector-Borne Zoonotic Dis. 2006;6:283–95.
Tran A, Ponçon N, Toty C, Linard C, Guis H, Ferré J-B, et al. Using remote sensing to map larval and adult populations of Anopheles hyrcanus (Diptera: Culicidae) a potential malaria vector in southern France. Int J Health Geogr. 2008;7:9.
Cardo MV, Vezzani D, Carbajo AE. Environmental predictors of the occurrence of ground water mosquito immatures in the Paraná Lower Delta. Argentina J Med Entomol. 2011;48:991–8.
Cailly P, Balenghien T, Ezanno P, Fontenille D, Toty C, Tran A. Role of the repartition of wetland breeding sites on the spatial distribution of Anopheles and Culex , human disease vectors in southern France. Parasit Vectors. 2011;4:65.
Hongoh V, Berrang-Ford L, Scott ME, Lindsay LR. Expanding geographical distribution of the mosquito, Culex pipiens , in Canada under climate change. Appl Geogr. 2012;33:53–62.
Moiroux N, Bio-Bangana AS, Djènontin A, Chandre F, Corbel V, Guis H. Modelling the risk of being bitten by malaria vectors in a vector control area in southern Benin, West Africa. Parasit Vectors. 2013;6:71.
Cardo MV, Vezzani D, Rubio A, Carbajo AE. Integrating demographic and meteorological data in urban ecology: a case study of container-breeding mosquitoes in temperate Argentina: integrating demographic and meteorological data in urban ecology. Area. 2014;46:18–26.
TeneFossog B, Ayala D, Acevedo P, Kengne P, NgomoAbesoMebuy I, Makanga B, et al. Habitat segregation and ecological character displacement in cryptic African malaria mosquitoes. Evol Appl. 2015;8:326–45.
Roiz D, Ruiz S, Soriguer R, Figuerola J. Landscape effects on the presence, abundance and diversity of mosquitoes in Mediterranean wetlands. PLoS ONE. 2015;10:e0128112.
Cianci D, Hartemink N, Zeimes CB, Vanwambeke SO, Ienco A, Caputo B. High resolution spatial analysis of habitat preference of Aedes albopictus (Diptera: Culicidae) in an urban environment. J Med Entomol. 2015;52:329–35.
Adde A, Dusfour I, Roux E, Girod R, Briolant S. Anopheles fauna of coastal Cayenne, French Guiana: modelling and mapping of species presence using remotely sensed land cover data. Mem Inst Oswaldo Cruz. 2016;111:750–6.
Heersink DK, Meyers J, Caley P, Barnett G, Trewin B, Hurst T, et al. Statistical modeling of a larval mosquito population distribution and abundance in residential Brisbane. J Pest Sci. 2016;89:267–79.
Baldacchino F, Marcantonio M, Manica M, Marini G, Zorer R, Delucchi L, et al. Mapping of Aedes albopictus abundance at a local scale in Italy. Remote Sensing. 2017;9:749.
Tisseuil C, Velo E, Bino S, Kadriaj P, Mersini K, Shukullari A, et al. Forecasting the spatial and seasonal dynamic of Aedes albopictus oviposition activity in Albania and Balkan countries. PLoS Negl Trop Dis. 2018;12:e0006236.
Amadi JA, Ong’amo GO, Olago DO, Oriaso SO, Nyamongo IK, Estambale BBA. Mapping potential Anopheles gambiae s.l. larval distribution using remotely sensed climatic and environmental variables in Baringo, Kenya: mapping malaria vectors distribution. Med Vet Entomol. 2018;32:417–26.
Romero D, Olivero J, Real R, Guerrero JC. Applying fuzzy logic to assess the biogeographical risk of dengue in South America. Parasit Vectors. 2019;12:428.
Ibañez-Justicia A, Cianci D. Modelling the spatial distribution of the nuisance mosquito species Anopheles plumbeus (Diptera: Culicidae) in the Netherlands. Parasit Vectors. 2015;8:258.
Wieland R, Kerkow A, Früh L, Kampen H, Walther D. Automated feature selection for a machine learning approach toward modeling a mosquito distribution. Ecol Model. 2017;352:108–12.
Kerkow A, Wieland R, Früh L, Hölker F, Jeschke JM, Werner D, et al. Can data from native mosquitoes support determining invasive species habitats? Modelling the climatic niche of Aedes japonicus japonicus (Diptera, Culicidae) in Germany. Parasitol Res. 2020;119:31–42.
Nurjanah S, et al. Distribution modelling of Aedes aegypti in three dengue-endemic areas in Sumatera, Indonesia. Trop Biomed. 2022;39:373–83.
Hussain SSA, Dhiman RC. Distribution expansion of dengue vectors and climate change in India. GeoHealth. 2022;6:e2021GH000477. https://doi.org/10.1029/2021GH000477 .
Adeleke ED, Shittu RA, Beierkuhnlein C, Thomas SM. High wind speed prevents the establishment of the disease vector mosquito Aedes albopictus in its climatic niche in Europe. Front Environ Sci. 2022;10:846243.
Liu X, Song C, Ren Z, Wang S. Predicting the geographical eistribution of malaria-associated Anopheles dirus in the South-East Asia and Western Pacific regions under climate change scenarios. Front Environ Sci. 2022;10:841966.
Holeva-Eklund WM, Young SJ, Will J, Busser N, Townsend J, Hepp CM. Species distribution modeling of Aedes aegypti in Maricopa County, Arizona from 2014 to 2020. Front Environ Sci. 2022;10:1001190.
Santos JM, Capinha C, Rocha J, Sousa CA. The current and future distribution of the yellow fever mosquito ( Aedes aegypti ) on Madeira Island. PLoS Negl Trop Dis. 2022;16:e0010715.
Furlong M, Adamu A, Hickson R, Horwood P, Golchin M, Hoskins A, et al. Estimating the distribution of Japanese encephalitis vectors in Australia using ecological niche modelling. TropicalMed. 2022;7:393.
Amdouni J, Conte A, Ippoliti C, Candeloro L, Tora S, Sghaier S, et al. Culex pipiens distribution in Tunisia: identification of suitable areas through random forest and MaxEnt approaches. Vet Med Sci. 2022;8:2703–15.
Download references
Acknowledgements
We would like to thank members of the Quantitative Disease Ecology and Conservation (QDEC) Lab for conversations and company while creating and revising this paper.
CAL and SJR were supported by CIBR: VectorByte: a Global Informatics Platform for studying the Ecology of Vector-Borne Diseases [Division of Biological Infrastructure, National Science Foundation (NSF) 2016265]. SJR and CJC were additionally supported by funding to the Viral Emergence Research Initiative (Verena; viralemergence.org), including NSF BII 2021909 and NSF BII 2213854.
Author information
Authors and affiliations.
Quantitative Disease Ecology and Conservation (QDEC) Lab, Department of Geography, University of Florida, Gainesville, FL, 32601, USA
Catherine A. Lippi, Stephanie J. Mundis, Rachel Sippy, J. Matthew Flenniken, Anusha Chaudhary, Gavriella Hecht & Sadie J. Ryan
Emerging Pathogens Institute, University of Florida, Gainesville, FL, 32601, USA
Catherine A. Lippi, Gavriella Hecht & Sadie J. Ryan
School of Mathematics and Statistics, University of St Andrews, St Andrews, KY16 9SS, UK
Rachel Sippy
Center for Global Health Science and Security, Georgetown University Medical Center, Georgetown University, Washington, DC, USA
Colin J. Carlson
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: CAL, SJM, and SJR. Analysis: CAL, SJM, RS, MF, AC, and GH. Visualizations: CAL, CJC, and SJR. First draft: CAL, SJM, SJR, and RS. Final draft and reviewing: all authors.
Corresponding authors
Correspondence to Catherine A. Lippi or Sadie J. Ryan .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lippi, C.A., Mundis, S.J., Sippy, R. et al. Trends in mosquito species distribution modeling: insights for vector surveillance and disease control. Parasites Vectors 16 , 302 (2023). https://doi.org/10.1186/s13071-023-05912-z
Download citation
Received : 17 March 2023
Accepted : 04 August 2023
Published : 28 August 2023
DOI : https://doi.org/10.1186/s13071-023-05912-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Species distribution modeling
Parasites & Vectors
ISSN: 1756-3305
- Submission enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu
- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Mosquito vectors (diptera: culicidae ) and mosquito-borne diseases in north africa.

Simple Summary
Graphical Abstract
1. Introduction
2. methodology, 3. salient data on north africa, 4. morphology, life cycle, taxonomy, genomics and identification methods of culicidae, 5. culicidae fauna of medical and veterinary importance in north africa, 5.1. sub-family culicinae, 5.1.1. aedes (meigen), 5.1.2. culex linnaeus, 5.1.3. culiseta (felt), 5.2. sub-family anophelinae, anopheles (meigen), 6. mosquito-borne diseases in north africa, 6.1. parasitic infections, 6.1.1. plasmodium, 6.1.2. filariasis, wuchereria bancrofti , dirofilaria immitis and dirofilaria repens, 6.2. arboviruses, 6.2.1. west nile virus (wnv), 6.2.2. rift valley fever (rvf), 6.2.3. chikungunya virus (chikv), 6.2.4. dengue, 6.2.5. yellow fever (yf), 6.2.6. zika virus, 6.2.7. sindbis virus (sinv), 6.2.8. usutu virus (usuv), 6.3. bacteria, 7. mosquito control strategies in north africa, insecticide resistance, 8. potential factors contributing to the future spread of mbds within the north africa region, 9. conclusions, supplementary materials, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Lecuit, M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2017 , 69 , 395–408. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Parola, P.; Musso, D.; Raoult, D. Rickettsia felis: The next mosquito-borne outbreak? Lancet Infect. Dis. 2016 , 16 , 1112–1113. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- World Malaria Report. 2020. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020. (accessed on 1 September 2022).
- Tolle, M.A. Mosquito-borne Diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2009 , 39 , 97–140. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ramasamy, R.; Surendran, S.N. Possible impact of rising sea levels on vector-borne infectious diseases. BMC Infect. Dis. 2011 , 11 , 18. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Blagrove, M.S.C.; Caminade, C.; Waldmann, E.; Sutton, E.R.; Wardeh, M.; Baylis, M. Co-occurrence of viruses and mosquitoes at the vectors’ optimal climate range: An underestimated risk to temperate regions? PLoS Negl. Trop. Dis. 2017 , 11 , e0005604. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Semenza, J.C.; Paz, S. Climate change and infectious disease in Europe: Impact, projection and adaptation. Lancet Reg. Health Eur. 2021 , 9 , 100230. [ Google Scholar ] [ CrossRef ]
- Benjelloun, A.; El Harrak, M.; Belkadi, B. West Nile Disease Epidemiology in North-West Africa: Bibliographical Review. Transbound. Emerg. Dis. 2016 , 63 , e153–e159. [ Google Scholar ] [ CrossRef ]
- Tambo, E.; Olalubi, O.A.; Sacko, M. Rift valley fever epidemic in Niger near border with Mali. Lancet Infect. Dis. 2016 , 16 , 1319–1320. [ Google Scholar ] [ CrossRef ]
- Lemine, A.M.M.; Lemrabott, M.A.O.; Ebou, M.H.; Lekweiry, K.M.; Salem, M.S.O.A.; Brahim, K.O.; Moukah, M.O.; Bouraya, I.N.O.; Brengues, C.; Trape, J.-F.; et al. Mosquitoes (Diptera: Culicidae) in Mauritania: A review of their biodiversity, distribution and medical importance. Parasites Vectors 2017 , 10 , 35. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Jourdain, F.; Picard, M.; Sulesco, T.; Haddad, N.; Harrat, Z.; Sawalha, S.S.; Günay, F.; Kanani, K.; Shaibi, T.; Akhramenko, D.; et al. Identification of mosquitoes (Diptera: Culicidae): An external quality assessment of medical entomology laboratories in the MediLabSecure Network. Parasites Vectors 2018 , 11 , 553. [ Google Scholar ] [ CrossRef ]
- Jourdain, F.; Samy, A.M.; Hamidi, A.; Bouattour, A.; Alten, B.; Faraj, C.; Roiz, D.; Petrić, D.; Pérez-Ramírez, E.; Velo, E.; et al. Towards harmonisation of entomological surveillance in the Mediterranean area. PLoS Negl. Trop. Dis. 2019 , 13 , e0007314. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- VectorNet. 2021. Available online: https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/vector-net (accessed on 1 September 2022).
- Dedet, J.-P. Edmond and Etienne Sergent. The epic of the Pasteur Institute of Algeria. Rev. Prat. 2012 , 62 , 1029–1033. [ Google Scholar ]
- Senevet, G.; Andarelli, L. Les Anophèles de l’Afrique du Nord et du Bassin Méditerranéen ; P. Lechevalier: Bois Guillaume, France, 1956. [ Google Scholar ]
- Senevet, G.; Andarelli, L. Les Moustiques de l’Afrique du Nord et du Bassin Méditerranéen: Les Genres Cu-lex-Uranotaenia-Theobaldia-Orthopodomya et Mansonia ; P. Lechevalier: Bois Guillaume, France, 1959. [ Google Scholar ]
- Brunhes, J.; Rhaim, A.; Geoffroy, B.; Angel, G.; Hervy, J.P. The Mosquitoes of the Mediterranean Africa: An Identification and Training Program ; IRD: Montpellier, France, 2000. [ Google Scholar ]
- Robert, V.; Günay, F.; Le Goff, G.; Boussès, P.; Sulesco, T.; Khalin, A.; Medlock, J.M.; Kampen, H.; Petric, D.; Schaffner, F. Distribution Chart for Euro-Mediterranean Mosquitoes (Western Palaearctic Region). J. Eur. Mosq. Control. Assoc. 2019 , 37 , 1–28. [ Google Scholar ]
- Sissani, I.; Boutellis, A.; Ramdan, A.; Hallouane, F.; Chahbar, N.; Bitam, I. Biological effect of the entomopathogenic fungus Metarhi-zium anisopliae variety acridum against the house-mosquito Culex pipiens. Int. J. Bot. Res. 2014 , 4 , 31–38. [ Google Scholar ]
- Kharoubi, R.; Rehimi, N.; Khaldi, R.; Haouari-Abderrahim, J.; Soltani, N. Phytochemical Screening and Insecticidal Activities of Essential oil of Mentha × piperita L. (Lamiales: Lamiaceae) and their Enzymatic Properties against Mosquito Culex pipiens L. (Diptera: Culicidae). J. Essent. Oil Bear. Plants 2021 , 24 , 134–146. [ Google Scholar ] [ CrossRef ]
- Merabti, B.; Boumaza, M.; Ouakid, M.; Carvajal, T.M.; Harbach, R.E. An updated checklist of the mosquitoes (Diptera: Culicidae) present in Algeria, with assessments of doubtful records and problematic species. Zootaxa 2021 , 5027 , 515–545. [ Google Scholar ] [ CrossRef ]
- Tahir, D.; Damene, H.; Davoust, B.; Parola, P. First molecular detection of Dirofilaria immitis (Spirurida: Onchocercidae) infection in dogs from Northern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2017 , 51 , 66–68. [ Google Scholar ] [ CrossRef ]
- Lafri, I.; Prat, C.M.; Bitam, I.; Gravier, P.; Besbaci, M.; Zeroual, F.; Ben-Mahdi, M.H.; Davoust, B.; Leparc-Goffart, I. Seroprevalence of West Nile virus antibodies in equids in the North-East of Algeria and detection of virus circulation in 2014. Comp. Immunol. Microbiol. Infect. Dis. 2017 , 50 , 8–12. [ Google Scholar ] [ CrossRef ]
- Benbetka, S.; Hachid, A.; Benallal, K.; Benbetka, C.; Khaldi, A.; Bitam, I.; Harrat, Z. First field evidence infection of Culex perexiguus by West Nile virus in Sahara Oasis of Algeria. J. Vector Borne Dis. 2018 , 55 , 305–309. [ Google Scholar ] [ CrossRef ]
- Charrel, R.; Berenger, J.-M.; Laroche, M.; Ayhan, N.; Bitam, I.; Delaunay, P.; Parola, P. Neglected vector-borne bacterial diseases and arboviruses in the Mediterranean area. New Microbes New Infect. 2018 , 26 , S31–S36. [ Google Scholar ] [ CrossRef ]
- Lafri, I.; Hachid, A.; Bitam, I. West Nile virus in Algeria: A comprehensive overview. New Microbes New Infect. 2018 , 27 , 9–13. [ Google Scholar ] [ CrossRef ]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007 , 11 , 1633–1644. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ramsar. Country Profiles. 2017. Available online: https://www.ramsar.org/country-profiles. (accessed on 1 September 2022).
- Hagemeijer, W.; Mundkur, T. Migratory Flyways in Europe, Africa and Asia and the Spread of HPAI H5N1. In Proceedings of the International Scientific Conference on Avian Influenza and Wild Birds, Rome, Italy, 30–31 May 2006. [ Google Scholar ]
- Arnal, A.; Gómez-Díaz, E.; Cerdà-Cuéllar, M.; Lecollinet, S.; Pearce-Duvet, J.; Busquets, N.; García-Bocanegra, I.; Pagès, N.; Vittecoq, M.; Hammouda, A.; et al. Circulation of a Meaban-Like Virus in Yellow-Legged Gulls and Seabird Ticks in the Western Mediterranean Basin. PLoS ONE 2014 , 9 , e89601. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bouguedour, R.; Ripani, A. Review of the foot and mouth disease situation in North Africa and the risk of introducing the disease into Europe. Rev. Sci. Tech. 2016 , 35 , 757–768. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Eurostat. Agriculture Statistics—North Africa and Eastern Mediterranean ; Eurostat: Luxembourg, 2017. [ Google Scholar ]
- Puechmaille, S.J.; Allegrini, B.; Benda, P.; Gürün, K.; Srámek, J.; Ibañez, C.; Juste, J.; Bilgin, R. A new species of the Miniopterus schreibersii species complex (Chiroptera: Miniopteridae) from the Maghreb Region, North Africa. Zootaxa 2014 , 3794 , 108–124. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khelfaoui, F.; Kebaci, A.; Benyacoub, S. New Data on Insecta and Acarina Parasitizing Bats (Mammalia: Chiroptera) in Numidia, Eastern Algeria. Bull. Soc. Zool. Fr. 2018 , 143 , 63–73. [ Google Scholar ]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Dahl, C.; Madon, M.; Kaiser, A. Mosquitoes and Their Control ; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [ Google Scholar ]
- EPA. CDC. Joint Statement on Mosquito Control in the United States. 2012. Available online: https://www.epa.gov/mosquitocontrol/joint-statement-mosquito-control-united-states#:~:text=Mosquito%20control%20activities%20are%20important,when%20requested%20by%20the%20states (accessed on 1 September 2022).
- Wilkerson, R.C.; Linton, Y.-M.; Fonseca, D.; Schultz, T.R.; Price, D.C.; Strickman, D.A. Making Mosquito Taxonomy Useful: A Stable Classification of Tribe Aedini that Balances Utility with Current Knowledge of Evolutionary Relationships. PLoS ONE 2015 , 10 , e0133602. [ Google Scholar ] [ CrossRef ]
- Reinert, J.F. List of abbreviations for currently valid generic-level taxa in family Culicidae (Diptera). Eur. Mosq. Bull. 2009 , 27 , 68–76. [ Google Scholar ]
- Motta, D.; Santos, A.B.; Winkler, I.; Machado, B.A.S.; Pereira, D.A.D.I.; Cavalcanti, A.M.; Fonseca, E.O.L.; Kirchner, F.; Badaró, R. Application of convolutional neural networks for classification of adult mosquitoes in the field. PLoS ONE 2019 , 14 , e0210829. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Schaffner, F. Identifying a mosquito: Traditional morphology and new molecular techniques associated for integrated taxonomy. Rev. Francoph. Lab. 2020 , 524 , 24–33. [ Google Scholar ]
- Zamyatin, A.; Avdeyev, P.; Liang, J.; Sharma, A.; Chen, C.; Lukyanchikova, V.; Alexeev, N.; Tu, Z.; Alekseyev, M.A.; Sharakhov, I.V. Chromosome-level genome assemblies of the malaria vectors Anopheles coluzzii and Anopheles arabiensis . GigaScience 2021 , 10 , giab017. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jiang, X.; Peery, A.; Hall, A.B.; Sharma, A.; Chen, X.-G.; Waterhouse, R.M.; Komissarov, A.; Riehle, M.M.; Shouche, Y.; Sharakhova, M.V.; et al. Genome analysis of a major urban malaria vector mosquito, Anopheles stephensi . Genome Biol. 2014 , 15 , 459. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, X.-G.; Jiang, X.; Gu, J.; Xu, M.; Wu, Y.; Deng, Y.; Zhang, C.; Bonizzoni, M.; Dermauw, W.; Vontas, J.; et al. Genome sequence of the Asian Tiger mosquito, Aedes albopictus , reveals insights into its biology, genetics, and evolution. Proc. Natl. Acad. Sci. USA 2015 , 112 , E5907–E5915. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Nene, V.; Wortman, J.R.; Lawson, D.; Haas, B.; Kodira, C.; Tu, Z.; Loftus, B.; Xi, Z.; Megy, K.; Grabherr, M.; et al. Genome Sequence of Aedes aegypti , a Major Arbovirus Vector. Science 2007 , 316 , 1718–1723. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Behura, S.K.; Lobo, N.F.; Haas, B.; Debruyn, B.; Lovin, D.D.; Shumway, M.F.; Puiu, D.; Romero-Severson, J.; Nene, V.; Severson, D.W. Complete sequences of mitochondria genomes of Aedes aegypti and Culex quinquefasciatus and comparative analysis of mitochondrial DNA fragments inserted in the nuclear genomes. Insect Biochem. Mol. Biol. 2011 , 41 , 770–777. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Schenkel, C.D.; Kamber, T.; Schaffner, F.; Mathis, A.; Silaghi, C. Loop-mediated isothermal amplification (LAMP) for the identification of invasive Aedes mosquito species. Med. Vet. Èntomol. 2019 , 33 , 345–351. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nebbak, A.; Koumare, S.; Willcox, A.C.; Berenger, J.-M.; Raoult, D.; Almeras, L.; Parola, P. Field application of MALDI-TOF MS on mosquito larvae identification. Parasitology 2017 , 145 , 677–687. [ Google Scholar ] [ CrossRef ]
- Dieme, C.; Yssouf, A.; Vega-Rúa, A.; Berenger, J.-M.; Failloux, A.-B.; Raoult, D.; Parola, P.; Almeras, L. Accurate identification of Culicidae at aquatic developmental stages by MALDI-TOF MS profiling. Parasites Vectors 2014 , 7 , 544. [ Google Scholar ] [ CrossRef ]
- Yssouf, A.; Socolovschi, C.; Flaudrops, C.; Ndiath, M.O.; Sougoufara, S.; Dehecq, J.-S.; Lacour, G.; Berenger, J.-M.; Sokhna, C.S.; Raoult, D.; et al. Matrix-Assisted Laser Desorption Ionization—Time of Flight Mass Spectrometry: An Emerging Tool for the Rapid Identification of Mosquito Vectors. PLoS ONE 2013 , 8 , e72380. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Yssouf, A.; Parola, P.; Lindström, A.; Lilja, T.; L’Ambert, G.; Bondesson, U.; Berenger, J.-M.; Raoult, D.; Almeras, L. Identification of European mosquito species by MALDI-TOF MS. Parasitol. Res. 2014 , 113 , 2375–2378. [ Google Scholar ] [ CrossRef ]
- Nebbak, A.; Almeras, L. Identification of Aedes mosquitoes by MALDI-TOF MS biotyping using protein signatures from larval and pupal exuviae. Parasites Vectors 2020 , 13 , 161. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Sevestre, J.; Diarra, A.Z.; Laroche, M.; Almeras, L.; Parola, P. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: An emerging tool for studying the vectors of human infectious diseases. Future Microbiol. 2021 , 16 , 323–340. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Abdellahoum, Z.; Nebbak, A.; Lafri, I.; Kaced, A.; Bouhenna, M.M.; Bachari, K.; Boumegoura, A.; Agred, R.; Boudchicha, R.H.; Smadi, M.A.; et al. Identification of Algerian field-caught mosquito vectors by MALDI-TOF MS. Vet. Parasitol. Reg. Stud. Rep. 2022 , 31 , 100735. [ Google Scholar ] [ CrossRef ]
- Izri, A.; Bitam, I.; Charrel, R.N. First entomological documentation of Aedes (Stegomyia) albopictus (Skuse, 1894) in Algeria. Clin. Microbiol. Infect. 2011 , 17 , 1116–1118. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Benallal, K.; Allal-Ikhlef, A.; Benhamouda, K.; Schaffner, F.; Harrat, Z. First report of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Oran, West of Algeria. Acta Trop. 2016 , 164 , 411–413. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shoukry, N.M.; Elwan, M.A.; Morsy, T.A. Aedes Aegypti (Linnaeus) Re-Emerging in Southern Egypt. J. Egypt. Soc. Parasitol. 2012 , 42 , 41–50. [ Google Scholar ] [ CrossRef ]
- Bèji, M.; Rhim, A.; Roiz, D.; Bouattour, A. Ecophysiological characterization and molecular differentiation of Culex pipiens forms (Diptera: Culicidae) in Tunisia. Parasites Vectors 2017 , 10 , 327. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Mikhail, M.W.; Al-Bursheed, K.M.; El-Halim, A.S.A.; Morsy, T.A. Studies on mosquito borne dieases in Egypt and Qatar. J. Egypt. Soc. Parasitol. 2009 , 39 , 745–756. [ Google Scholar ]
- Amara Korba, R.; Boukraa, S.; Alayat, M.S.; Bendjeddou, M.L.; Francis, F.; Boubidi, S.C.; Bouslama, Z. Preliminary report of mosquitoes survey at Tonga Lake (North-East Algeria). Adv. Environ. Biol. 2015 , 9 , 288–294. [ Google Scholar ]
- Trari, B.; Dakki, M.; Harbach, R.E. An updated checklist of the Culicidae (Diptera) of Morocco, with notes on species of historical and current medical importance. J. Vector Ecol. 2017 , 42 , 94–104. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Dyab, A.K.; Galal, L.; Mahmoud, A.E.-S.; Mokhtar, Y. Xenomonitoring of Different Filarial Nematodes Using Single and Multiplex PCR in Mosquitoes from Assiut Governorate, Egypt. Korean J. Parasitol. 2015 , 53 , 77–83. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shaikevich, E.V.; Vinogradova, E.B.; Bouattour, A.; de Almeida, A.P.G. Genetic diversity of Culex pipiens mosquitoes in distinct populations from Europe: Contribution of Cx. quinquefasciatus in Mediterranean populations. Parasites Vectors 2016 , 9 , 47. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Berčič, R.L.; Bányai, K.; Růžek, D.; Fehér, E.; Domán, M.; Danielová, V.; Bakonyi, T.; Nowotny, N. Phylogenetic Analysis of Lednice Orthobunyavirus. Microorganisms 2019 , 7 , 447. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Tabbabi, A.; Simard, F.; Brengues, C.; Rhim, A.; Boussès, P.; Bouratbine, A.; Fontenille, D.; Ben-Alaya-Bouafif, N.; Daaboub, J.; Aoun, K. Larval Habitats Characterization and Species Composition of Anopheles Mosquitoes in Tunisia, with Particular Attention to Anopheles maculipennis Complex. Am. J. Trop. Med. Hyg. 2015 , 92 , 653–659. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Duvallet, G.; Fontenille, D.; Robert, V. Entomologie Médicale et Vétérinaire ; Editions Quae: Versailles, France, 2017. [ Google Scholar ]
- Faraj, C.; Adlaoui, E.; Rhajaoui, M.; Lyagoubi, M. Estimation of malaria transmission in high-risk provinces of Morocco. East. Mediterr. Health J. 2003 , 9 , 542–547. [ Google Scholar ] [ CrossRef ]
- Hamaidia, H.; Berchi, S. Biosystematic study of culicidae (Diptera, Nematocera) nuisance source in Tébessa (Algeria). J. Entomol. Zool. Stud. 2018 , 6 , 1226–1231. [ Google Scholar ]
- Boudemagh, N.; Bendali-Saoudi, F.; Soltani, N. Inventory of Culicidae (Diptera: Nematocera) in the region of Collo (North-East Algeria). Ann. Biol. Res. 2013 , 4 , 94–99. [ Google Scholar ]
- Boubidi, S.C.; Gassen, I.; Khechache, Y.; Lamali, K.; Tchicha, B.; Brengues, C.; Menegon, M.; Severini, C.; Fontenille, D.; Harrat, Z. PlasmodiumfalciparumMalaria, Southern Algeria, 2007. Emerg. Infect. Dis. 2010 , 16 , 301–303. [ Google Scholar ] [ CrossRef ]
- El-Bahnasawy, M.M.; Fadil, E.E.; Morsy, T.A. Mosquito vectors of infectious diseases: Are they neglected health disaster in Egypt? J. Egypt Soc. Parasitol. 2013 , 43 , 373–386. [ Google Scholar ]
- Frank, J.H.; Service, M.W. Medical Entomology for Students. Fla. Èntomol. 2000 , 83 , 384. [ Google Scholar ] [ CrossRef ]
- Bonizzoni, M.; Gasperi, G.; Chen, X.; James, A.A. The invasive mosquito species Aedes albopictus : Current knowledge and future perspectives. Trends Parasitol. 2013 , 29 , 460–468. [ Google Scholar ] [ CrossRef ]
- Lafri, I.; Bitam, I.; Beneldjouzi, A.; Ben Mahdi, M.H. An inventory of mosquitoes (Diptera: Culicidae) in Algeria. Bull. Soc. Zool. Fr. 2014 , 139 , 255–261. [ Google Scholar ]
- Bennouna, A.; Balenghien, T.; EL Rhaffouli, H.; Schaffner, F.; Garros, C.; Gardès, L.; Lhor, Y.; Hammoumi, S.; Chlyeh, G.; Fihri, O.F. First record of Stegomyia albopicta (= Aedes albopictus ) in Morocco: A major threat to public health in North Africa? Med. Vet. Èntomol. 2016 , 31 , 102–106. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Amraoui, F.; Ben Ayed, W.; Madec, Y.; Faraj, C.; Himmi, O.; Btissam, A.; Sarih, M.; Failloux, A.-B. Potential of Aedes albopictus to cause the emergence of arboviruses in Morocco. PLoS Negl. Trop. Dis. 2019 , 13 , e0006997. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Failloux, A.-B.; Bouattour, A.; Faraj, C.; Gunay, F.; Haddad, N.; Harrat, Z.; Jancheska, E.; Kanani, K.; Kenawy, M.A.; Kota, M.; et al. Surveillance of Arthropod-Borne Viruses and Their Vectors in the Mediterranean and Black Sea Regions Within the MediLabSecure Network. Curr. Trop. Med. Rep. 2017 , 4 , 27–39. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Benallal, K.E.; Garni, R.; Bouiba, L.; Harrat, Z. First Detection of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Algiers, the Capital City of Algeria. J. Arthropod-Borne Dis. 2019 , 13 , 420. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Arroussi, D.E.R.; Bouaziz, A.; Boudjelida, H. Mosquito survey reveals the first record of Aedes (Diptera: Culicidae) species in urban area, Annaba district, Northeastern Algeria. Pol. J. Èntomol. 2021 , 90 , 14–26. [ Google Scholar ] [ CrossRef ]
- Bouattour, A.; Khrouf, F.; Rhim, A.; M’Ghirbi, Y. First Detection of the Asian Tiger Mosquito, Aedes (Stegomyia) albopictus (Diptera: Culicidae), in Tunisia. J. Med. Èntomol. 2019 , 56 , 1112–1115. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2018 , 13 , e0210122. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Brustolin, M.; Talavera, S.; Nuñez, A.; Santamaría, C.; Rivas, R.; Pujol, N.; Valle, M.; Verdún, M.; Brun, A.; Pagès, N.; et al. Rift Valley fever virus and European mosquitoes: Vector competence of Culex pipiens and Stegomyia albopicta (= Aedes albopictus ). Med. Vet. Èntomol. 2017 , 31 , 365–372. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus , an arbovirus vector: From the darkness to the light. Microbes Infect. 2009 , 11 , 1177–1185. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cancrini, G.; Scaramozzino, P.; Gabrielli, S.; Di Paolo, M.; Toma, L.; Romi, R. Aedes albopictus and Culex pipiens Implicated as Natural Vectors of Dirofilaria repens in Central Italy. J. Med. Èntomol. 2007 , 44 , 1064–1066. [ Google Scholar ] [ CrossRef ]
- Socolovschi, C.; Pagés, F.; Raoult, D. Rickettsia felis in Aedes albopictus Mosquitoes, Libreville, Gabon. Emerg. Infect. Dis. 2012 , 18 , 1687–1689. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lounibos, L.P.; Kramer, L.D. Invasiveness of Aedes aegypti and Aedes albopictus and Vectorial Capacity for Chikungunya Virus. J. Infect. Dis. 2016 , 214 , S453–S458. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Heikal, O.M.; El-Bahnasawy, M.M.; Morsy, A.T.; Khalil, H.H. Aedes aegypti re-emerging in Egypt: A review and what should be done? J. Egypt. Soc. Parasitol. 2011 , 41 , 801–814. [ Google Scholar ]
- Arsevska, E.; Hellal, J.; Mejri, S.; Hammami, S.; Marianneau, P.; Calavas, D.; Hénaux, V. Identifying Areas Suitable for the Occurrence of Rift Valley Fever in North Africa: Implications for Surveillance. Transbound. Emerg. Dis. 2015 , 63 , 658–674. [ Google Scholar ] [ CrossRef ]
- Mancini, G.; Montarsi, F.; Calzolari, M.; Capelli, G.; Dottori, M.; Ravagnan, S.; Lelli, D.; Chiari, M.; Santilli, A.; Quaglia, M.; et al. Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy. Vet. Ital. 2017 , 53 , 97–110. [ Google Scholar ] [ CrossRef ]
- Ben Ayed, W.; Amraoui, F.; M’Ghirbi, Y.; Schaffner, F.; Rhaim, A.; Failloux, A.-B.; Bouattour, A. A Survey of Aedes (Diptera: Culicidae) Mosquitoes in Tunisia and the Potential Role of Aedes detritus and Aedes caspius in the Transmission of Zika Virus. J. Med. Èntomol. 2019 , 56 , 1377–1383. [ Google Scholar ] [ CrossRef ]
- Mackenzie-Impoinvil, L.; Impoinvil, D.E.; Galbraith, S.E.; Dillon, R.J.; Ranson, H.; Johnson, N.; Fooks, A.R.; Solomon, T.; Baylis, M. Evaluation of a temperate climate mosquito, Ochlerotatus detritus (= Aedes detritus ), as a potential vector of Japanese encephalitis virus. Med. Vet. Èntomol. 2014 , 29 , 1–9. [ Google Scholar ] [ CrossRef ]
- Blagrove, M.S.C.; Sherlock, K.; Chapman, G.E.; Impoinvil, D.E.; McCall, P.J.; Medlock, J.M.; Lycett, G.; Solomon, T.; Baylis, M. Evaluation of the vector competence of a native UK mosquito Ochlerotatus detritus (Aedes detritus) for dengue, chikungunya and West Nile viruses. Parasites Vectors 2016 , 9 , 452. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Moutailler, S.; Krida, G.; Schaffner, F.; Vazeille, M.; Failloux, A.-B. Potential Vectors of Rift Valley Fever Virus in the Mediterranean Region. Vector-Borne Zoonotic Dis. 2008 , 8 , 749–754. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- O’Donnell, K.L.; Bixby, M.A.; Morin, K.J.; Bradley, D.S.; Vaughan, J.A. Potential of a Northern Population of Aedes vexans (Diptera: Culicidae) to Transmit Zika Virus. J. Med. Èntomol. 2017 , 54 , 1354–1359. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Kurucz, K.; Kepner, A.; Krtinic, B.; Zana, B.; Földes, F.; Bányai, K.; Oldal, M.; Jakab, F.; Kemenesi, G. First molecular identification of Dirofilaria spp. (Onchocercidae) in mosquitoes from Serbia. Parasitol. Res. 2016 , 115 , 3257–3260. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Anderson, J.F.; Main, A.J.; Ferrandino, F.J. Horizontal and Vertical Transmission of West Nile Virus by Aedes vexans (Diptera: Culicidae). J. Med. Èntomol. 2020 , 57 , 1614–1618. [ Google Scholar ] [ CrossRef ]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011 , 11 , 1577–1585. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Amraoui, F.; Tijane, M.; Sarih, M.; Failloux, A.-B. Molecular evidence of Culex pipiens form molestus and hybrids pipiens/molestus in Morocco, North Africa. Parasites Vectors 2012 , 5 , 83. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Benallal, K.; Benbetka, S.; Tail, G.; Harrat, Z. Molecular characterization of Culex pipiens (Diptera, Culicidae) in Reghaïa lake, Algeria. Ann. Biol. Sci. 2015 , 3 , 20–24. [ Google Scholar ]
- Korba, R.A.; Alayat, M.S.; Bouiba, L.; Boudrissa, A.; Bouslama, Z.; Boukraa, S.; Francis, F.; Failloux, A.-B.; Boubidi, S.C. Ecological differentiation of members of the Culex pipiens complex, potential vectors of West Nile virus and Rift Valley fever virus in Algeria. Parasites Vectors 2016 , 9 , 455. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Amraoui, F.; Krida, G.; Bouattour, A.; Rhim, A.; Daaboub, J.; Harrat, Z.; Boubidi, S.-C.; Tijane, M.; Sarih, M.; Failloux, A.-B. Culex pipiens , an Experimental Efficient Vector of West Nile and Rift Valley Fever Viruses in the Maghreb Region. PLoS ONE 2012 , 7 , e36757. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Khalifa, R.M.; El-Nadi, N.A.; Ahmed, A.M.; Hassan, F.A. Histological and PCR xenomonitoring of culicine mosquitoes for filarial infestation in Sohag Governorate, upper Egypt. J. Egypt Soc. Parasitol. 2013 , 43 , 591–600. [ Google Scholar ]
- Samy, A.; Elaagip, A.H.; Kenawy, M.; Ayres, C.F.J.; Peterson, A.T.; Soliman, D. Climate Change Influences on the Global Potential Distribution of the Mosquito Culex quinquefasciatus , Vector of West Nile Virus and Lymphatic Filariasis. PLoS ONE 2016 , 11 , e0163863. [ Google Scholar ] [ CrossRef ]
- Guedes, D.R.; Paiva, M.H.; Donato, M.M.; Barbosa, P.P.; Krokovsky, L.; Rocha, S.W.D.S.; LA Saraiva, K.; Crespo, M.M.; Rezende, T.M.; Wallau, G.L.; et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg. Microbes Infect. 2017 , 6 , 1–11. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Hanafi, H.A.; Fryauff, D.J.; Saad, M.D.; Soliman, A.K.; Mohareb, E.W.; Medhat, I.; Zayed, A.B.; Szumlas, D.E.; Earhart, K.C. Virus isolations and high population density implicate Culex antennatus (Becker) (Diptera: Culicidae) as a vector of Rift Valley Fever virus during an outbreak in the Nile Delta of Egypt. Acta Trop. 2011 , 119 , 119–124. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Ratovonjato, J.; Olive, M.-M.; Tantely, L.M.; Andrianaivolambo, L.; Tata, E.; Razainirina, J.; Jeanmaire, E.; Reynes, J.-M.; Elissa, N. Detection, Isolation, and Genetic Characterization of Rift Valley Fever Virus from Anopheles ( Anopheles ) coustani , Anopheles ( Anopheles ) squamosus , and Culex ( Culex ) antennatus of the Haute Matsiatra Region, Madagascar. Vector-Borne Zoonotic Dis. 2011 , 11 , 753–759. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kolodziejek, J.; Pachler, K.; Bin, H.; Mendelson, E.; Shulman, L.M.; Orshan, L.; Nowotny, N. Barkedji virus, a novel mosquito-borne flavivirus identified in Culex perexiguus mosquitoes, Israel, 2011. J. Gen. Virol. 2013 , 94 , 2449–2457. [ Google Scholar ] [ CrossRef ]
- McIntosh, B.M.; Jupp, P.G.; Dos Santos, I.; Meenehan, G.M. Epidemics of West Nile and Sindbis viruses in South Africa with Culex (Culex) univittatus Theobald as vector. S. Afr. J. Sci. 1976 , 72 , 295–300. [ Google Scholar ]
- Miller, B.R.; Nasci, R.S.; Lutwama, J.; Godsey, M.S.; Savage, H.; Lanciotti, R.; Peters, C.J. First field evidence for natural vertical transmission of West Nile virus in Culex univittatus complex mosquitoes from Rift Valley province, Kenya. Am. J. Trop. Med. Hyg. 2000 , 62 , 240–246. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ergünay, K.; Litzba, N.; Brinkmann, A.; Gunay, F.; Kar, S.; Öter, K.; Örsten, S.; Sarıkaya, Y.; Alten, B.; Nitsche, A.; et al. Isolation and genomic characterization of Culex theileri flaviviruses in field-collected mosquitoes from Turkey. Infect. Genet. Evol. 2016 , 46 , 138–147. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Carnevale, P.; Robert, V. Les Anophèles Biologie, Transmission du Plasmodium et Lutte Antivectorielle ; IRD: Montpellier, France, 2009. [ Google Scholar ]
- Larhbali, Y.; Belghyti, D.; El Guamri, Y.; Lahlou, O.; El Kharrim, K.; Kirami, A.; Khamri, Z. Epidemiology of imported malaria and entomological study of breeding sites of potential risk areas in the province of Khemisset (Morocco). Med. Sante Trop. 2014 , 24 , 397–402. [ Google Scholar ] [ CrossRef ]
- Laboudi, M.; Faraj, C.; Sadak, A.; Harrat, Z.; Boubidi, S.C.; Harbach, R.E.; EL Aouad, R.; Linton, Y.-M. DNA barcodes confirm the presence of a single member of the Anopheles maculipennis group in Morocco and Algeria: An. sicaulti is conspecific with An. labranchiae . Acta Trop. 2011 , 118 , 6–13. [ Google Scholar ] [ CrossRef ]
- Mahmoud, D.M.; Hussein, H.M.; El Gozamy, B.M.R.; Thabet, H.S.; Hassan, M.A.; Meselhey, R.A.-A. Screening of Plasmodium parasite in vectors and humans in three villages in Aswan Governorate, Egypt. J. Parasit. Dis. 2018 , 43 , 158–163. [ Google Scholar ] [ CrossRef ]
- Faraj, C.; Adlaoui, E.; Ouahabi, S.; Rhajaoui, M.; Fontenille, D.; Lyagoubi, M. Entomological investigations in the region of the last malaria focus in Morocco. Acta Trop. 2009 , 109 , 70–73. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Smith, D.M. Mosquito records from the Republic of Niger, with reference to the construction of the new ‘Trans-Sahara Highway’. J. Trop. Med. Hyg. 1981 , 84 , 95–100. [ Google Scholar ] [ PubMed ]
- Yamany, A.S.; Adham, F.K.; Mehlhorn, H. Structural changes of the follicular cells during developmental stages of the malaria vector mosquitoes Anopheles pharoensis (Diptera: Culicidae) in Egypt. Parasitol. Res. 2014 , 113 , 4233–4241. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wassim, N.M. Secondray Structure and Sequenceofits2-Rdna of the Egyptian Malaria Vectoranopheles Pharoensis (Theobald). J. Egypt. Soc. Parasitol. 2014 , 44 , 197–204. [ Google Scholar ] [ CrossRef ]
- Epelboin, Y.; Talaga, S.; Epelboin, L.; Dusfour, I. Zika virus: An updated review of competent or naturally infected mosquitoes. PLoS Negl. Trop. Dis. 2017 , 11 , e0005933. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tchicha, B. Historique de la Lutte Antipaludique en Algérie. Service du Paludisme et des Maladies Parasitaires ; INSP: Indian Land, SC, USA, 2011. [ Google Scholar ]
- Hammadi, D.; Boubidi, S.C.; Chaib, S.E.; Saber, A.; Khechache, Y.; Gasmi, M.; Harrat, Z. Malaria in Algerian Sahara. Bull. Soc. Pathol. Exot. 2009 , 102 , 185–192. [ Google Scholar ]
- Gebreel, A.O.; Gilles, H.M.; Prescott, J.E. Studies on the sero-epidemiology of endemic diseases in Libya, IV Malaria. Ann. Trop. Med. Parasitol. 1985 , 79 , 341–347. [ Google Scholar ] [ CrossRef ]
- Trari, B.; Carnevale, P. Malaria in Morocco: From pre-elimination to elimination, what risks for the future? Bull. Soc. Pathol. Exot. 2011 , 104 , 291–295. [ Google Scholar ] [ CrossRef ]
- Mesbah, S. Maladies infectieuses émergentes et réémergentes: Le risque et la riposte en Algérie. Med. Trop. 2009 , 69 , 27–32. [ Google Scholar ]
- Lalami, A.E.O.; Cherigui, M.; Koraichi, S.I.; Maniar, S.; El Maimouni, N.; Rhajaoui, M. Imported malaria in northern central Morocco, 1997–2007. Sante 2009 , 19 , 43–47. [ Google Scholar ] [ CrossRef ]
- Kandeel, A.; Haggag, A.; El Fetouh, M.A.; Refaey, S.; Naiel, M.; Hassan, A.; Ramzy, R. Control of malaria outbreak due to Plasmodium vivax in Aswan Governorate, Egypt. East. Mediterr. Health J. 2016 , 22 , 274–279. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Martelli, G.; Girometti, N.; Vanino, E.; Bottieau, E.; Viale, P. Plasmodium falciparum malaria in migrants who transited Libya—Where did they contract malaria? Travel Med. Infect. Dis. 2015 , 13 , 499–500. [ Google Scholar ] [ CrossRef ]
- WHO. World Health Organization: Algeria and Argentina Certified Malaria-Free by WHO ; WHO: Geneva, Switzerland, 2019. [ Google Scholar ]
- Mendis, K. Eliminating malaria should not be the end of vigilance. Nature 2019 , 573 , 7. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Hotez, P.J.; Savioli, L.; Fenwick, A. Neglected Tropical Diseases of the Middle East and North Africa: Review of Their Prevalence, Distribution, and Opportunities for Control. PLoS Negl. Trop. Dis. 2012 , 6 , e1475. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Abdel-Shafi, I.R.; Shoeib, E.Y.; Attia, S.S.; Rubio, J.M.; Edmardash, Y.; El-Badry, A.A. Mosquito identification and molecular xenomon-itoring of lymphatic filariasis in selected endemic areas in giza and qualioubiya governorates. Egypt. J. Egypt Soc. Parasitol. 2016 , 46 , 93–100. [ Google Scholar ]
- Abdel-Shafi, I.R.; Shoieb, E.Y.; Attia, S.S.; Rubio, J.M.; Ta-Tang, T.-H.; El-Badry, A.A. Molecular identification and phylogenetic analysis of Wuchereria bancrofti from human blood samples in Egypt. Parasitol Res. 2017 , 116 , 963–970. [ Google Scholar ] [ CrossRef ]
- Mehdi, B. Prevalence of canine Dirofilaria immitis infection in the city of Algiers, Algeria. Afr. J. Agric. Res. 2009 , 4 , 1097–1100. [ Google Scholar ]
- Khatat, S.E.; Khallaayoune, K.; Errafyk, N.; van Gool, F.; Duchateau, L.; Daminet, S.; Kachani, M.; El Amri, H.; Azrib, R.; Sahibi, H. Detection of Anaplasma spp. and Ehrlichia spp. anibodies, and Dirofilaria immitis antigens in dogs from seven locations of Morocco. Vet. Parasitol. 2017 , 239 , 86–89. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rjeibi, M.R.; Rouatbi, M.; Mabrouk, M.; Tabib, I.; Rekik, M.; Gharbi, M. Molecular Study of Dirofilaria immitis and Dirofilaria repens in Dogs from Tunisia. Transbound. Emerg. Dis. 2016 , 64 , 1505–1509. [ Google Scholar ] [ CrossRef ]
- Dyab, A.K.; Galal, L.A.; Mahmoud, A.E.; Mokhtar, Y. Finding Wolbachia in Filarial larvae and Culicidae Mosquitoes in Upper Egypt Governorate. Korean J. Parasitol. 2016 , 54 , 265–272. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Soussi, A.; Farah, K.F.; Zermani, R.; Rammeh, S.; Ismail, O.; Zakraoui, A.; Atallah, K.; Ben, J.S. Subcutaneous dirofilariasis due to Diro-filaria repens in Tunisia: A case involving the scrotum. Med. Trop. 2004 , 64 , 375–378. [ Google Scholar ]
- Makni, F.; Hachicha, L.; Abdelkafi, N.; Guiaa, N.; Sellami, H.; Sellami, T.; Ayadi, A. Subcutaneous Dirofilaria repens dirofilariasis in the Sfax region (Tunisia). Ann. Dermatol. Venereol. 2007 , 134 , 53–54. [ Google Scholar ] [ CrossRef ]
- Kaouech, E.; Becheur, M.; Cheikh, M.; Belhadj, S.; Kallel, K.; Chaker, E. Subcutaneous dirofilariasis of the upper lip in Tunisia. Sante 2010 , 20 , 47–48. [ Google Scholar ]
- Ziadi, S.; Trimeche, M.; Mestiri, S.; Mokni, M.; Trabelsi, A.; Ben, A.A.; Ben, S.M.; Ben Hadj, H.F.; Korbi, S. Human subconjunctival diro-filariasis: Two Tunisian case studies. J. Fr. Ophtalmol. 2005 , 28 , 773.e1–773.e4. [ Google Scholar ] [ CrossRef ]
- Sassi, S.H.; Abid, L.; Dhouib, R.; Mrad, K.; Bouguila, H.; Abbes, I.; Driss, M.; Ben, G.R.; Ben, R.K. Conjunctival dirofilariasis due to Diro-filaria Repens. A new Tunisian case. J. Fr. Ophtalmol. 2006 , 29 , e5. [ Google Scholar ]
- Saïed, W.; Amara, K.; Bouchoucha, S.; Khaled, S.; Mrad, K.; Nessib, M.; Smida, M.; Ben Ghachem, M. An unusual cause of hand nodule: Peri-tendon dirofilariasis. Chir. Main. 2011 , 30 , 66–68. [ Google Scholar ] [ CrossRef ]
- Ben Hassouna, J.; Jbir, I.; Mezghani, B.; El Amine, O.; Zemni, I.; Mrad, K.; Ben Dhieb, T.; Gamoudi, A.; Rahal, K. Dirofilariasis of the breast: Two new cases in Tunisia. Med. Sante. Trop. 2015 , 25 , 327–330. [ Google Scholar ] [ CrossRef ]
- Al-Kappany, Y.M.; Lappin, M.R.; Kwok, O.C.H.; Abu-Elwafa, S.A.; Hilali, M.A.; Dubey, J.P. Seroprevalence of Toxoplasma gondii and Concurrent Bartonella spp., Feline Immunodeficiency Virus, Feline Leukemia Virus, and Dirofilaria immitis Infections in Egyptian Cats. J. Parasitol. 2011 , 97 , 256–258. [ Google Scholar ] [ CrossRef ]
- Abdel-Rahman, S.M.; Mahmoud, A.E.; Galal, L.A.A.; Gustinelli, A.; Pampiglione, S. Three new cases of human infection with Dirofilaria repens , one pulmonary and two subcutaneous, in the Egyptian governorate of Assiut. Ann. Trop. Med. Parasitol. 2008 , 102 , 499–507. [ Google Scholar ] [ CrossRef ]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2016 , 166 , 155–163. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Metallaoui, A. Renforcement de la Surveillance et des Systèmes D’alerte Pour la Fièvre Catarrhale Ovine, la Fièvre du Nil Occi-Dentale et la Rage au Maroc, Algérie et Tunisie. 2008. Available online: https://www.ipcinfo.org/fileadmin/user_upload/faoectad/library/WN%20Algerie.pdf (accessed on 1 September 2022).
- El-Harrak, M.; Le Guenno, B.; Gounon, P. Isolation of West Nile Virus in Morocco. Virologie 1997 , 1 , 248–249. [ Google Scholar ]
- Le Guenno, B.; Bougermouh, A.; Azzam, T.; Bouakaz, R. West Nile: A deadly Virus? Lancet 1996 , 348 , 1315. [ Google Scholar ] [ CrossRef ]
- Giese, C.; Ait El Belghiti, F.; Barboza, P. National EpiSouth Focal Points. West Nile Virus Circulation in the Episouth Countries and Neighbouring Areas Seasons 2010 and 2011 Update 1st July 2012. 2012. Available online: http://www.episouthnetwork.org/sites/default/files/bulletin_file/note_west_nile_episouth_2010_2011_july2012.pdf. (accessed on 1 September 2022).
- Institut de Veille Sanitaire. Bulletin Hebdomadaire International N°369: 10 au 16 Octobre 2012 ; Institut de Veille Sanitaire: Paris, France, 2012. [ Google Scholar ]
- Ben Hassine, T.; de Massis, F.; Calistri, P.; Savini, G.; Mohamed, B.B.; Ranen, A.; Di Gennaro, A.; Sghaier, S.; Hammami, S. First Detection of Co-circulation of West Nile and Usutu Viruses in Equids in the South-west of Tunisia. Transbound. Emerg. Dis. 2014 , 61 , 385–389. [ Google Scholar ] [ CrossRef ]
- Wasfi, F.; Dachraoui, K.; Cherni, S.; Bosworth, A.; Barhoumi, W.; Dowall, S.; Chelbi, I.; Derbali, M.; Zoghlami, Z.; Beier, J.; et al. West Nile virus in Tunisia, 2014: First isolation from mosquitoes. Acta Trop. 2016 , 159 , 106–110. [ Google Scholar ] [ CrossRef ]
- Shaibi, T.; Saadawi, W.K.; Aghila, H.; Annajar, B.B. Prevalence of IgG antibodies for the West Nile virus in human population in Tripoli, Libya. J. Vector Borne Dis. 2017 , 54 , 183–186. [ Google Scholar ]
- Hachid, A.; Beloufa, M.; Seghier, M.; Bahoura, N.; Dia, M.; Fall, G.; Sall, A. Evidence of West Nile virus circulation among humans in central northern Algeria. New Microbes New Infect. 2019 , 29 , 100512. [ Google Scholar ] [ CrossRef ]
- Conte, A.; Candeloro, L.; Ippoliti, C.; Monaco, F.; de Massis, F.; Bruno, R.; Di Sabatino, D.; Danzetta, M.L.; Benjelloun, A.; Belkadi, B.; et al. Spatio-Temporal Identification of Areas Suitable for West Nile Disease in the Mediterranean Basin and Central Europe. PLoS ONE 2015 , 10 , e0146024. [ Google Scholar ] [ CrossRef ]
- Saiz, J.-C. Animal and Human Vaccines against West Nile Virus. Pathogens 2020 , 9 , 1073. [ Google Scholar ] [ CrossRef ]
- Kaiser, J.A.; Wang, T.; Barrett, A.D. Virulence determinants of West Nile virus: How can these be used for vaccine design? Futur. Virol. 2017 , 12 , 283–295. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Assaid, N.; Mousson, L.; Moutailler, S.; Arich, S.; Akarid, K.; Monier, M.; Beck, C.; Lecollinet, S.; Failloux, A.-B.; Sarih, M. Evidence of circulation of West Nile virus in Culex pipiens mosquitoes and horses in Morocco. Acta Trop. 2020 , 205 , 105414. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Assaid, N.; Arich, S.; Ezzikouri, S.; Benjelloun, S.; Dia, M.; Faye, O.; Akarid, K.; Beck, C.; Lecollinet, S.; Failloux, A.-B.; et al. Serological evidence of West Nile virus infection in human populations and domestic birds in the Northwest of Morocco. Comp. Immunol. Microbiol. Infect. Dis. 2021 , 76 , 101646. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lumley, S.; Horton, D.L.; Hernandez-Triana, L.L.M.; Johnson, N.; Fooks, A.R.; Hewson, R. Rift Valley fever virus: Strategies for maintenance, survival and vertical transmission in mosquitoes. J. Gen. Virol. 2017 , 98 , 875–887. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Clements, A.C.A.; Pfeiffer, D.U.; Martin, V. Application of knowledge-driven spatial modelling approaches and uncertainty management to a study of Rift Valley fever in Africa. Int. J. Health Geogr. 2006 , 5 , 57. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Mahmoud, A.S.; Di Sabatino, D.; Danzetta, M.L.; Iapaolo, F.; Tolari, F.; Forzan, M.; Mazzei, M.; Dayhum, A.; de Massis, F.; Monaco, F. Rift Valley fever virus: A serological survey in Libyan ruminants. Open Vet. J. 2018 , 8 , 204–207. [ Google Scholar ] [ CrossRef ]
- Kenawy, M.A.; Abdel-Hamid, Y.M.; Beier, J.C. Rift Valley Fever in Egypt and other African countries: Historical review, recent outbreaks and possibility of disease occurrence in Egypt. Acta Trop. 2018 , 181 , 40–49. [ Google Scholar ] [ CrossRef ]
- Humphrey, J.M.; Cleton, N.B.; Reusken, C.B.E.M.; Glesby, M.J.; Koopmans, M.; Abu-Raddad, L.J. Urban Chikungunya in the Middle East and North Africa: A systematic review. PLoS Negl. Trop. Dis. 2017 , 11 , e0005707. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Darwish, M.A.; Feinsod, F.M.; Scott, R.M.; Ksiazek, T.G.; Botros, B.A.; Farrag, I.H.; El Said, S. Arboviral causes of non-specific fever and myalgia in a fever hospital patient population in Cairo, Egypt. Trans. R. Soc. Trop. Med. Hyg. 1987 , 81 , 1001–1003. [ Google Scholar ] [ CrossRef ]
- World Health Organization. Dengue and Severe Dengue ; WHO: Geneva, Switzerland, 2022. [ Google Scholar ]
- Humphrey, J.M.; Cleton, N.B.; Reusken, C.B.E.M.; Glesby, M.J.; Koopmans, M.P.G.; Abu-Raddad, L.J. Dengue in the Middle East and North Africa: A Systematic Review. PLoS Negl. Trop. Dis. 2016 , 10 , e0005194. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Abozeid, S.; Elsayed, A.K.; Schaffner, F.; Samy, A. Re-emergence of Aedes aegypti in Egypt. Lancet Infect. Dis. 2018 , 18 , 142–143. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Torres-Flores, J.M.; Reyes-Sandoval, A.; Salazar, M.I. Dengue Vaccines: An Update. BioDrugs 2022 , 36 , 325–336. [ Google Scholar ] [ CrossRef ]
- World Health Organization. Yellow Fever ; WHO: Geneva, Switzerland, 2019. [ Google Scholar ]
- Gianchecchi, E.; Cianchi, V.; Torelli, A.; Montomoli, E. Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects. Vaccines 2022 , 10 , 372. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Abreu, A.D.J.L.D.; Cavalcante, J.R.; Lagos, L.W.d.A.; Caetano, R.; Braga, J.U. A Systematic Review and a Meta-Analysis of the Yellow Fever Vaccine in the Elderly Population. Vaccines 2022 , 10 , 711. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Escadafal, C.; Gaayeb, L.; Riccardo, F.; Pérez-Ramírez, E.; Picard, M.; Dente, M.G.; Fernández-Pinero, J.; Manuguerra, J.-C.; Jiménez-Clavero, M.; Declich, S.; et al. Risk of Zika virus transmission in the Euro-Mediterranean area and the added value of building preparedness to arboviral threats from a One Health perspective. BMC Public Health 2016 , 16 , 1219. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Nyaruaba, R.; Mwaliko, C.; Mwau, M.; Mousa, S.; Wei, H. Arboviruses in the East African Community partner states: A review of medically important mosquito-borne Arboviruses. Pathog. Glob. Health 2019 , 113 , 209–228. [ Google Scholar ] [ CrossRef ]
- Turell, M.J.; Morrill, J.C.; Rossi, C.A.; Gad, A.M.; Cope, S.E.; Clements, T.L.; Arthur, R.R.; Wasieloski, L.P.; Dohm, D.J.; Nash, D.; et al. Isolation of West Nile and Sindbis Viruses from Mosquitoes Collected in the Nile Valley of Egypt During an Outbreak of Rift Valley Fever. J. Med. Èntomol. 2002 , 39 , 248–250. [ Google Scholar ] [ CrossRef ]
- Ayhan, N.; Hachid, A.; Thirion, L.; Benallal, K.E.; Pezzi, L.; Khardine, F.A.; Benbetka, C.; Benbetka, S.; Harrat, Z.; Charrel, R. Detection and Isolation of Sindbis Virus from Field Collected Mosquitoes in Timimoun, Algeria. Viruses 2022 , 14 , 894. [ Google Scholar ] [ CrossRef ]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol. Infect. 2019 , 147 , e232. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Socolovschi, C.; Pages, F.; Ndiath, M.O.; Ratmanov, P.; Raoult, D. Rickettsia Species in African Anopheles Mosquitoes. PLoS ONE 2012 , 7 , e48254. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Zhang, J.; Lu, G.; Li, J.; Kelly, P.; Li, M.; Wang, J.; Huang, K.; Qiu, H.; You, J.; Zhang, R.; et al. Molecular Detection of Rickettsia felis and Rickettsia bellii in Mosquitoes. Vector-Borne Zoonotic Dis. 2019 , 19 , 802–809. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Dieme, C.; Bechah, Y.; Socolovschi, C.; Audoly, G.; Berenger, J.-M.; Faye, O.; Raoult, D.; Parola, P. Transmission potential of Rickettsia felis infection by Anopheles gambiae mosquitoes. Proc. Natl. Acad. Sci. USA 2015 , 112 , 8088–8093. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Zhang, J.; Lu, G.; Kelly, P.J.; Wang, C. Seasonal and Gender Differences in Presence of Rickettsia felis and Blood meals Provide Additional Evidence of a Vector Role for Mosquitoes. Can. J. Infect. Dis. Med. Microbiol. 2019 , 2019 , 8543460–8543465. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Thelaus, J.; Andersson, A.; Broman, T.; Bäckman, S.; Granberg, M.; Karlsson, L.; Kuoppa, K.; Larsson, E.; Lundmark, E.; Lundström, J.O.; et al. Francisella tularensis Subspecies holarctica Occurs in Swedish Mosquitoes, Persists Through the Developmental Stages of Laboratory-Infected Mosquitoes and Is Transmissible During Blood Feeding. Microb. Ecol. 2013 , 67 , 96–107. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Bäckman, S.; Näslund, J.; Forsman, M.; Thelaus, J. Transmission of tularemia from a water source by transstadial maintenance in a mosquito vector. Sci. Rep. 2015 , 5 , 7793. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kosik-Bogacka, D.I.; Kużna-Grygiel, W.; Jaborowska, M. Ticks and Mosquitoes as Vectors of Borrelia burgdorferi s. l. in the Forested Areas of Szczecin. Folia Biol. 2007 , 55 , 143–146. [ Google Scholar ] [ CrossRef ]
- Melaun, C.; Zotzmann, S.; Santaella, V.G.; Werblow, A.; Zumkowski-Xylander, H.; Kraiczy, P.; Klimpel, S. Occurrence of Borrelia burgdorferi s.l. in different genera of mosquitoes (Culicidae) in Central Europe. Ticks Tick-Borne Dis. 2016 , 7 , 256–263. [ Google Scholar ] [ CrossRef ]
- Rudolf, I.; Blažejová, H.; Mendel, J.; Straková, P.; Šebesta, O.; Rettich, F.; Čabanová, V.; Miterpáková, M.; Betášová, L.; Peško, J.; et al. Bartonella species in medically important mosquitoes, Central Europe. Parasitol Res. 2020 , 119 , 2713–2717. [ Google Scholar ] [ CrossRef ]
- Tourapi, C.; Tsioutis, C. Circular Policy: A New Approach to Vector and Vector-Borne Diseases’ Management in Line with the Global Vector Control Response (2017–2030). Trop. Med. Infect. Dis. 2022 , 7 , 125. [ Google Scholar ] [ CrossRef ]
- Atyame, C.M.; Labbe, P.; Rousset, F.; Beji, M.; Makoundou, P.; Duron, O.; Dumas, E.; Pasteur, N.; Bouattour, A.; Fort, P.; et al. Stable coexistence of incompatibleWolbachiaalong a narrow contact zone in mosquito field populations. Mol. Ecol. 2015 , 24 , 508–521. [ Google Scholar ] [ CrossRef ]
- Tmimi, F.; Bkhache, M.; Mounaji, K.; Failloux, A.; Sarih, M. First report of the endobacteria Wolbachia in natural populations of Culex pipiens in Morocco. J. Vector Ecol. 2017 , 42 , 349–351. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Alout, H.; Labbé, P.; Pasteur, N.; Weill, M. High incidence of ace-1 duplicated haplotypes in resistant Culex pipiens mosquitoes from Algeria. Insect Biochem. Mol. Biol. 2011 , 41 , 29–35. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Lalami, A.E.O.; El-Akhal, F.; El Amri, N.; Maniar, S.; Faraj, C. State resistance of the mosquito Culex pipiens towards temephos central Morocco. Bull. Soc. Pathol. Exot. 2014 , 107 , 194–198. [ Google Scholar ] [ CrossRef ]
- Bkhache, M.; Tmimi, F.-Z.; Charafeddine, O.; Faraj, C.; Failloux, A.-B.; Sarih, M. First report of L1014F-kdr mutation in Culex pipiens complex from Morocco. Parasites Vectors 2016 , 9 , 644. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Tabbabi, A.; Daaboub, J.; Laamari, A.; Ben Cheikh, R.; Feriani, M.; Boubaker, C.; Ben Jha, I.; Ben Cheikh, H. Evaluation of resistance to temephos insecticide in Culex pipiens pipiens larvae collected from three districts of Tunisia. Afr. Health Sci. 2019 , 19 , 1361–1367. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Tabbabi, A.; Daaboub, J. First studies showing high temephos resistance in Anopheles labranchiae (Diptera: Culicidae) from Tunisia. Afr. Health Sci. 2018 , 18 , 41–47. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tabbabi, A.; Daaboub, J.; Ben Cheikh, R.; Laamari, A.; Feriani, M.; Boubaker, C.; Ben Jha, I.; Ben Cheikh, H. The potential role of urbanization in the resistance to organophosphate insecticide in Culex pipiens pipiens from Tunisia. Afr. Health Sci. 2019 , 19 , 1368–1375. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Tabbabi, A.; Daaboub, J.; Ben Cheikh, R.; Laamari, A.; Feriani, M.; Boubaker, C.; Ben Jha, I.; Ben Cheikh, H. Resistance status to deltamethrin pyrethroid of Culex pipiens pipiens (Diptera: Culicidae) collected from three districts of Tunisia. Afr. Health Sci. 2018 , 18 , 1182–1188. [ Google Scholar ] [ CrossRef ]
- Arich, S.; Assaid, N.; Taki, H.; Weill, M.; Labbé, P.; Sarih, M. Distribution of insecticide resistance and molecular mechanisms involved in the West Nile vector Culex pipiens in Morocco. Pest Manag. Sci. 2020 , 77 , 1178–1186. [ Google Scholar ] [ CrossRef ]
- Promed. 2021. Available online: https://promedmail.org/ (accessed on 1 September 2022).
- Bohers, C.; Mousson, L.; Madec, Y.; Vazeille, M.; Rhim, A.; M’Ghirbi, Y.; Bouattour, A.; Failloux, A.-B. The recently introduced Aedes albopictus in Tunisia has the potential to transmit chikungunya, dengue and Zika viruses. PLoS Negl. Trop. Dis. 2020 , 14 , e0008475. [ Google Scholar ] [ CrossRef ]
- Ibáñez-Justicia, A.; Smitz, N.; Hartog, W.D.; van de Vossenberg, B.; de Wolf, K.; Deblauwe, I.; van Bortel, W.; Jacobs, F.; Vaux, A.G.C.; Medlock, J.M.; et al. Detection of Exotic Mosquito Species (Diptera: Culicidae) at International Airports in Europe. Int. J. Environ. Res. Public Health 2020 , 17 , 3450. [ Google Scholar ] [ CrossRef ]
- Ducheyne, E.; Minh, N.N.T.; Haddad, N.; Bryssinckx, W.; Buliva, E.; Simard, F.; Malik, M.R.; Charlier, J.; de Waele, V.; Mahmoud, O.; et al. Current and future distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in WHO Eastern Mediterranean Region. Int. J. Health Geogr. 2018 , 17 , 4. [ Google Scholar ] [ CrossRef ] [ PubMed ]
| Species | Diseases | Genome Size (Mb) | G + C (%) | Protein Coding Genes | GenBank Genome ID | Geographic Distribution | Reference |
|---|---|---|---|---|---|---|---|
| Anopheles arabiensis | Malaria | 256.8 | 44.8 | 25,532 | 11,544 | Egypt. | [ ] |
| Anopheles coluzzi | Malaria | 273.4 | 44.5 | 23,396 | 41,035 | Algeria, Morocco, Tunisia. | [ ] |
| Anopheles stephensi | Malaria | 221 | 44.8 | 11,789 | 2653 | Egypt. | [ ] |
| Aedes albopictus | Arboviruses Filariasis | 1967 | 40.4 | 17,539 | 45 | Algeria, Morocco, Tunisia. | [ ] |
| Aedes aegypti | Arboviruses | 1376 | 38.2 | 15,419 | 44 | Algeria, Morocco, Egypt, Libya, Tunisia. | [ ] |
| Culex quinquefasciatus | Arboviruses Lymphatic filariasis | 579 | 37.4 | 18,883 | 393 | Algeria, Morocco. | [ ] |
| Subfamily | Genus | Subgenus | Species | Diseases Transmitted | Distribution | References | ||
|---|---|---|---|---|---|---|---|---|
| Viruses | Parasites | Bacteria | ||||||
| Stegomyia | Ae. albopictus | Dengue, Yellow fever **, Chikungunya **, Zika | Filariasis | Rickettsia felis | Algeria Morocco | [ , ] | ||
| Ae. aegypti | Dengue, Yellow fever **, Chikungunya **, Zika, | Filariasis | / | Algeria, Egypt, Libya, Morocco, Tunisia. | [ ] | |||
| Ochlerotatus | Ae. caspius | Rift Valley Fever, West Nile Virus, Usutu Virus | Filariasis | / | Algeria, Egypt, Libya, Morocco, Tunisia. | [ , ] | ||
| Ae. detritus | Rift Valley Fever, WNV | / | / | Algeria, Egypt, Tunisia | [ , ] | |||
| Aedimorphus | Ae. vexans | Rift Valley Fever, | Filariasis | / | Algeria, Morocco | [ , ] | ||
| Culex | Cx. pipiens | West Nile Virus, Rift Valley Fever Virus, Usutu Virus, | Dirofilaria immitis, D. repens, Wuchereria bancrofti | / | Algeria, Egypt, Libya, Morocco, Tunisia | [ , , ] | ||
| Cx. antennatus | West Nile Virus, Rift Valley Fever Virus | Dirofilaria repens | / | Algeria, Egypt, Tunisia | [ , ] | |||
| Cx. perexiguus | Rift Valley Fever Virus, West Nile Virus, Sindbis virus | / | / | Algeria, Egypt, Libya, Morocco, Tunisia. | [ , ] | |||
| Cx. theileri | Rift Valley Fever Virus, West Nile Virus, Sindbis virus | / | / | Algeria, Egypt, Libya, Morocco, Tunisia. | [ , ] | |||
| Cx. univittatus | West Nile Virus, Sindbis virus | / | / | Egypt | [ ] | |||
| Cx. quinquefasciatus | West Nile Virus, Saint Louis encephalitis ** | Wuchereria bancrofti | / | Algeria, Morocco. | [ , ] | |||
| Barraudius | Cx. modestus | WNV, Usutu virus, Tahyna virus **, Lednice virus ** | / | / | Algeria, Morocco | [ ] | ||
| Cx. pusillus | / | Dirofilaria immitis | / | Algeria, Egypt, Libya, Tunisia. | [ ] | |||
| Culiseta | Cs. annulata | Tahyna virus ** | / | / | Algeria, Morocco, Tunisia. | [ ] | ||
| Anopheles | An. labranchiae | / | Plasmodium | / | Algeria, Libya Morocco, Tunisia. | [ ] | ||
| An. algeriensis | / | Plasmodium | / | Algeria, Egypt, Libya, Morocco, Tunisia. | [ , ] | |||
| An. coustani | Rift Valley Fever Virus, Zika | Plasmodium | / | Egypt | [ ] | |||
| An. claviger | / | Plasmodium | / | Algeria, Libya, Morocco, Tunisia. | [ , , , ] | |||
| An. sachorovi | / | Plasmodium | / | Algeria | [ ] | |||
| Cellia | An. multicolor | / | Plasmodium | / | Algeria, Egypt, Libya, Morocco, Tunisia. | [ , ] | ||
| An. coluzzi | O’nyong-nyong ** Tataguine virus **, Nyando virus ** | Plasmodium | Rickettsia felis | Algeria *, Egypt # | [ , ] | |||
| An. sergentii | / | Plasmodium | / | Algeria, Egypt, Libya, Morocco, Tunisia. | [ , ] | |||
| An. superpictus | / | Plasmodium | / | Algeria, Egypt | [ , ] | |||
| An. pharoensis | / | Plasmodium Filariasis | / | Egypt | [ ] | |||
| An. stephensi | / | Plasmodium | / | Egypt | [ ] | |||
| An. arabiensis | / | Plasmodium | / | Egypt | [ ] | |||
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Nebbak, A.; Almeras, L.; Parola, P.; Bitam, I. Mosquito Vectors (Diptera: Culicidae ) and Mosquito-Borne Diseases in North Africa. Insects 2022 , 13 , 962. https://doi.org/10.3390/insects13100962
Nebbak A, Almeras L, Parola P, Bitam I. Mosquito Vectors (Diptera: Culicidae ) and Mosquito-Borne Diseases in North Africa. Insects . 2022; 13(10):962. https://doi.org/10.3390/insects13100962
Nebbak, Amira, Lionel Almeras, Philippe Parola, and Idir Bitam. 2022. "Mosquito Vectors (Diptera: Culicidae ) and Mosquito-Borne Diseases in North Africa" Insects 13, no. 10: 962. https://doi.org/10.3390/insects13100962
Article Metrics
Article access statistics, supplementary material.
ZIP-Document (ZIP, 195 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
More From Forbes
Are mosquito-borne viruses becoming more common and severe a clinical virologist explains.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
The Aedes aegypti mosquito, which can transmit several mosquito-borne viral infections (James ... [+] Gathany/CDC vía AP)
Multiple deaths from eastern equine encephalitis have been reported in the northeast over the past several weeks. Dr. Anthony Fauci was hospitalized in late August after being infected with West Nile virus and is now recovering. And this week, a New Hampshire resident was reported to have been hospitalized after testing positive for EEE, West Nile virus and St. Louis encephalitis virus. This has many asking, “Are viruses transmitted by mosquitoes on the rise and becoming more severe?”
What Viruses Are Transmitted By Mosquitoes In The U.S., And What Are The Symptoms?
While viewed as simply a nuisance to many, mosquitoes are in fact the deadliest animal on the planet. The mosquito can transmit a number of fatal infections, including malaria, dengue, yellow fever, chikungunya, Zika, West Nile and EEE. Each year, diseases resulting from a mosquito bite cause at least 1 million deaths worldwide, according to estimates.
Fortunately, not all types of mosquito-borne illnesses are endemic in the U.S., but that may be changing over time. Climate change , human activity (i.e., development of natural habitats) and changes in the geographic distribution of animals has led to an increase in mosquito- and tick-borne infections over the past decade. Currently, the most common mosquito-borne infection in the U.S. is West Nile virus, although cases of EEE, dengue, St. Louis encephalitis virus, Jamestown Canyon virus and malaria have also resulted from local transmission. Typically, states in the northeast and upper Midwest experience higher rates of mosquito-borne infections, but malaria and dengue have been detected in Texas and Florida, and West Nile virus can occur nationwide.
The vast majority of individuals who are infected with a mosquito-borne virus do not develop any symptoms. However, when symptoms occur, they are often flu-like and non-specific, and may include a fever, headache, chills, rash and joint pain. In cases of dengue, the body aches and joint pain can be so severe that the illness is commonly known as breakbone fever . In rare cases of West Nile virus, EEE and St. Louis encephalitis, the illness may progress to neurologic disease such as encephalitis and meningitis or become fatal.
Posted signs warning of West Nile.
Best High-Yield Savings Accounts Of 2024
Best 5% interest savings accounts of 2024, is testing available for these viruses.
While testing for mosquito-borne viruses is available, it’s mainly limited to large reference laboratories and public health facilities. Unlike testing for COVID-19 and the flu, molecular tests (i.e., PCR) aren’t a great option for diagnosing mosquito-borne viruses. This is because the period of time that viruses like West Nile and EEE are present in the bloodstream is relatively short, and PCR tests may be negative by the time a person goes to see their doctor.
For this reason, serology (i.e., antibody-based) tests are the most common means of diagnosing infections caused by mosquito-borne viruses. During the first week or two after an infection, a type of antibody known as IgM is produced, which is an early immune response to a virus. IgM antibodies usually stay in a person’s bloodstream for three to six months. Serology tests that detect IgM can suggest a recent infection; however, these tests often lack specificity, meaning that false-positive and cross-reactive results can occur.
For example, a person infected with West Nile virus may also test positive by IgM assays for St. Louis encephalitis or dengue. This is because West Nile virus, St. Louis encephalitis and dengue all belong to the same family of viruses (i.e., Flaviviruses), and so IgM antibodies produced in response to West Nile virus may cross-react with tests designed to diagnose other members of the flavivirus family.
Other serology tests are designed to detect IgG antibodies, which take longer for the body to produce but function to provide longer term immunity. Tests that look for IgG antibodies are more specific (i.e., they don’t show the level of cross-reactivity observed with IgM tests), but it may take two to three weeks after an infection for IgG antibodies to reach detectable levels. This limits the use of IgG-based serology tests in the diagnosis of an acute mosquito-borne viral infection.
Man while applying insect repellent on his hand.
What Are Steps You Can Take To Prevent Mosquito-Borne Infections?
Unfortunately, vaccines are not broadly available for the prevention of mosquito-borne infections. Currently, vaccines exist for chikungunya virus, yellow fever, Japanese encephalitis virus and dengue. To prevent other viral infections (e.g., West Nile virus, EEE, St. Louis encephalitis) transmitted through mosquitoes, the best steps you can take are to reduce mosquito breeding locations and the chances of being bitten.
Mosquitoes lay their eggs in standing water, so dump out water in old tires, flowerpots and bird baths. Avoid the times of day when mosquitoes are most active, including before sunrise and after dusk. And if you need to be outside when mosquitoes are present, wear a long-sleeved shirt and pants and apply insect repellent. Taking these steps can help protect you and others from mosquito-borne illnesses, which will continue to be a risk until cooler temperatures arrive.

- Editorial Standards
- Reprints & Permissions
Join The Conversation
One Community. Many Voices. Create a free account to share your thoughts.
Forbes Community Guidelines
Our community is about connecting people through open and thoughtful conversations. We want our readers to share their views and exchange ideas and facts in a safe space.
In order to do so, please follow the posting rules in our site's Terms of Service. We've summarized some of those key rules below. Simply put, keep it civil.
Your post will be rejected if we notice that it seems to contain:
- False or intentionally out-of-context or misleading information
- Insults, profanity, incoherent, obscene or inflammatory language or threats of any kind
- Attacks on the identity of other commenters or the article's author
- Content that otherwise violates our site's terms.
User accounts will be blocked if we notice or believe that users are engaged in:
- Continuous attempts to re-post comments that have been previously moderated/rejected
- Racist, sexist, homophobic or other discriminatory comments
- Attempts or tactics that put the site security at risk
- Actions that otherwise violate our site's terms.
So, how can you be a power user?
- Stay on topic and share your insights
- Feel free to be clear and thoughtful to get your point across
- ‘Like’ or ‘Dislike’ to show your point of view.
- Protect your community.
- Use the report tool to alert us when someone breaks the rules.
Thanks for reading our community guidelines. Please read the full list of posting rules found in our site's Terms of Service.
Loading metrics
Open Access
Peer-reviewed
Research Article
Impact of recent climate extremes on mosquito-borne disease transmission in Kenya
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Program in Human Biology, Stanford University, Stanford, California, United States of America
Roles Conceptualization, Methodology, Software, Validation, Writing – review & editing
Affiliation Department of Pediatrics, Stanford University School of Medicine, Stanford, California, United States of America
Roles Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – review & editing
Affiliation Universities Space Research Association & NASA Goddard Space Flight Center, Greenbelt, Maryland, United States of America
Roles Conceptualization, Data curation, Formal analysis, Investigation, Software, Validation, Writing – review & editing
Affiliation Department of Biology, Stanford University, Stanford, California, United States of America
Roles Data curation, Writing – review & editing
Affiliation Morgan State University & NASA Goddard Space Flight Center, Greenbelt, Maryland, United States of America
Roles Project administration, Resources, Supervision, Writing – review & editing
Affiliation Technical University of Mombasa, Mombasa, Kenya
Affiliation Centre for Global Health Research, Kenya Medical Research Institute, Kisumu, Kenya
Roles Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing
- Cameron Nosrat,
- Jonathan Altamirano,
- Assaf Anyamba,
- Jamie M. Caldwell,
- Richard Damoah,
- Francis Mutuku,
- Bryson Ndenga,
- A. Desiree LaBeaud

- Published: March 18, 2021
- https://doi.org/10.1371/journal.pntd.0009182
- Peer Review
- Reader Comments
Climate change and variability influence temperature and rainfall, which impact vector abundance and the dynamics of vector-borne disease transmission. Climate change is projected to increase the frequency and intensity of extreme climate events. Mosquito-borne diseases, such as dengue fever, are primarily transmitted by Aedes aegypti mosquitoes. Freshwater availability and temperature affect dengue vector populations via a variety of biological processes and thus influence the ability of mosquitoes to effectively transmit disease. However, the effect of droughts, floods, heat waves, and cold waves is not well understood. Using vector, climate, and dengue disease data collected between 2013 and 2019 in Kenya, this retrospective cohort study aims to elucidate the impact of extreme rainfall and temperature on mosquito abundance and the risk of arboviral infections. To define extreme periods of rainfall and land surface temperature (LST), we calculated monthly anomalies as deviations from long-term means (1983–2019 for rainfall, 2000–2019 for LST) across four study locations in Kenya. We classified extreme climate events as the upper and lower 10% of these calculated LST or rainfall deviations. Monthly Ae . aegypti abundance was recorded in Kenya using four trapping methods. Blood samples were also collected from children with febrile illness presenting to four field sites and tested for dengue virus using an IgG enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR). We found that mosquito eggs and adults were significantly more abundant one month following an abnormally wet month. The relationship between mosquito abundance and dengue risk follows a non-linear association. Our findings suggest that early warnings and targeted interventions during periods of abnormal rainfall and temperature, especially flooding, can potentially contribute to reductions in risk of viral transmission.
Author summary
Dengue is a rapidly spreading mosquito-borne disease transmitted primarily by Aedes aegypti mosquitoes. As climate change leads to extremes in rainfall and temperature, the abundance and populations of these vectors will be affected, thus influencing transmission of dengue. Using satellite-derived climate data for Kenya, we classified months that experienced highly abnormal rainfall and temperature as extreme climate events (floods, droughts, heat waves, or cold waves). We compared the average monthly Ae . aegypti abundance and confirmed dengue counts following extreme climate months using lag periods of one month and two months, respectively. This study utilized several statistical models to account for differences among study sites and time. Floods resulted in significantly increased egg and adult abundance. Our results contributed to a better understanding of the effect of climate variability and change on dengue. As suggested by our observed increase in vector counts yet a relatively unchanged dengue infection risk, human behavior can help reduce viral transmission. Targeted interventions should be focused on both reducing vector populations and limiting human-vector contact, especially during these climate anomalies.
Citation: Nosrat C, Altamirano J, Anyamba A, Caldwell JM, Damoah R, Mutuku F, et al. (2021) Impact of recent climate extremes on mosquito-borne disease transmission in Kenya. PLoS Negl Trop Dis 15(3): e0009182. https://doi.org/10.1371/journal.pntd.0009182
Editor: Elvina Viennet, Australian Red Cross Lifelood, AUSTRALIA
Received: June 29, 2020; Accepted: January 26, 2021; Published: March 18, 2021
Copyright: © 2021 Nosrat et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data are available from the Stanford Repository at the following URL: https://purl.stanford.edu/rz262rz1347 .
Funding: This research was supported by National Institutes of Health (NIH) grant R01AI102918 (ADL). The Stanford REDCap platform ( http://redcap.stanford.edu ) is operated by Stanford Medicine Research IT team. The REDCap platform services at Stanford are subsidized by a) Stanford School of Medicine Research Office, and b) the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001085⤉. JMC was supported by a Stanford Woods Institute for the Environment - Environmental Ventures Program grant. The funders had no role in the collection, analysis, or reporting of the data.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Climate change’s influence on temperature and rainfall can dramatically impact vector abundance and thus the dynamics of vector-borne disease transmission. Scientific evidence suggests that as global climate change continues to intensify, so will the frequency of extreme climate events, including floods, drought, heat waves, and cold waves [ 1 – 4 ]. Extreme climate events result from both natural internal climate variability and climate warming [ 5 ]. Climate variability arises spontaneously within the climate system even in the absence of climate forcings [ 5 ]. With regards to climate warming, sea-surface temperature increases of 1–2° Celsius can result in greater trapped greenhouse gas molecules and energy flux in the lower atmosphere, resulting in stronger, more unpredictable weather patterns [ 1 ]. Water availability during extreme climate events, including droughts and floods, can have important implications for mosquito-borne disease transmission, as pools of water provide breeding sites for infectious disease vectors. Similarly, local temperatures can alter mosquito dynamics, including the development of immature mosquitoes, and rates of reproduction and biting [ 6 ]. Most notably, transmission of dengue fever, a prevalent arthropod-borne disease in Kenya that is the focus of this study, can be affected by variability in temperature and rainfall [ 6 , 7 ].
Dengue is the most common and the fastest spreading vector-borne disease globally, resulting in close to half of the world’s population living in areas at risk for dengue virus (DENV) transmission [ 8 ]. DENV is a flavivirus with four distinct serotypes (1–4) found mostly in tropical and sub-tropical regions of the world [ 8 ]. It is transmitted by Aedes aegypti and Aedes albopictus mosquito vectors [ 9 ]. In 2019, the largest number of DENV infections were reported globally; annual infection counts have increased 15-fold over the past two decades, resulting in large dengue epidemics [ 8 ]. DENV is transmitted by mosquitoes throughout daylight hours and human populations living in close contact with mosquito vector breeding sites are at risk for DENV infection. The prevalence of vector breeding sites, both natural and artificial, in combination with ambient temperatures can influence vector abundance, vector growth, and infectious disease transmission. However, the effect of extreme climate events, including floods, droughts, heat waves, and cold waves on mosquito-borne disease transmission is not well understood.
Previous studies indicate that accumulated rainfall increases vector habitats, but floods and excessive rainfall flush breeding sites, thus diminishing vector populations of several mosquito species, including Ae . aegypti [ 10 – 13 ]. For example, in Singapore between 2014–2015, researchers observed excessive rainfall flushed Ae . aegypti breeding sites and decreased the risk of dengue outbreaks six weeks following rainfall [ 11 ]. Several epidemiological studies also support strong associations between accumulated rainfall and higher vector abundance 4+ weeks later [ 14 – 16 ]. For example, Aedes albopictus , a closely related species of Ae . aegypti and vector for dengue virus and chikungunya virus, was observed to be positively associated with accumulated rainfall at a lag of four weeks in southern France. This ultimately contributed to an increased risk of chikungunya transmission in France in 2014 [ 17 ]. Such a relationship held true for dengue as well, as researchers in Jakarta and Bali observed the number of dengue cases to increase with higher monthly mean rainfall up to 16.2 mm over the past decade [ 18 ]. As such, the relationship between rainfall and dengue transmission is not clearly delineated.
While a common view is that reductions in water availability removes vector breeding sites and diminish mosquito populations, drought conditions seem favorable for certain mosquito species [ 19 , 20 ]. There are several modes through which drought is believed to promote greater vector abundance, according to a UK literature review; the primary mechanism through which Ae . aegypti abundance is promoted is through increased storage of water, which increases the availability of aquatic habitats for mosquitoes [ 20 ]. Such droughts contributed to several mosquito-borne diseases outbreaks, including a chikungunya virus outbreak in coastal Kenya between 2004–2005 [ 20 , 21 ].
Temperature can affect many mosquito biological processes (e.g., reproduction, biting rate, development rate, etc.), thus influencing the prevalence of mosquitoes and the extent of disease spread [ 22 ]. Researchers have identified that the thermal response curve for Ae . aegypti transmission of dengue virus peaks at 29°C, which is higher than optimal transmission temperatures for other vector species [ 23 ]. However, much remains unknown about whether such laboratory-measured temperature thresholds lead to defined thresholds in reality. A study examining the effect of climatic factors on dengue transmission between Bali and Australia observed the number of dengue cases in Bali to increase with increasing mean temperature [ 18 ]. Heat waves have also been associated with increased dengue transmission; between December 2010-February 2011, seasonal land surface temperatures were 5–20°C above normal, and these above-normal temperatures were associated with the first known large-scale outbreak of dengue fever in East Africa [ 24 ]. We hope to better contextualize previous temperature thresholds and trends associated with DENV transmission using field data in Kenya.
Kenya is an ideal study site to better understand the relationship between climate variability, vector abundance, and mosquito-borne disease transmission due to the endemicity of mosquito-borne diseases, like dengue fever, and the region’s interannual variable climate due to the El Niño Southern Oscillation [ 25 , 26 ]. Due to the generally low levels of endemic DENV transmission in the region, more than a handful of cases are usually associated with small dengue outbreaks. Using existing vector (January 2014 –September 2018), climate (November 2013 –February 2019), and disease (January 2014 –February 2019) data systematically collected over the past six years at four sites in Kenya ( Fig 1 ), this study aims to identify how periods of extreme rainfall and temperature affect mosquito abundance and the risk of dengue infection in a cohort of Kenyan children.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Image reused and altered from public domain ( vidiani.com ).
https://doi.org/10.1371/journal.pntd.0009182.g001

Ethics statement
The study protocol was approved by the Stanford University Institutional Review Board (Protocol ID #31488) and the Kenya Medical Research Institute (KEMRI) National Scientific and Ethical Review Committee (SSC # 2611). Meetings were held at all four sites with local government administrators (village elders, chiefs, and assistant chiefs) and with the local residents in each sub-location to introduce the research study and staff to the public. Written consent was obtained from all participants to collect blood samples and from household heads to sample mosquitoes within their houses and their compounds. Parents and guardians provided written consent on behalf of children and children >7 years of age also provided written assent. Mature minors provided written consent.
Study sites
This study took place at two sites in western Kenya, Chulaimbo (rural) and Kisumu (urban), and two sites in coastal Kenya, Ukunda (urban) and Msambweni (rural) ( Fig 1 ). The study sites vary in DENV burden, climate, geography, population size, and urbanization.
Climate anomalies
Monthly mean land surface temperatures were extracted from the National Aeronautics and Space Administration’s global monthly MOD11C3 version 6 data set derived from MODIS Terra. LST data are available at a spatial resolution 0.05° x 0.05° (≈5.5 km), thus allowing us to compare more recent monthly LSTs to long-term means for a 30 km x 30 km grid centered on each study location ( S1 Appendix ). The monthly long-term means were calculated using the 2003–2018 base period; that is, the long-term mean for each month was calculated by averaging the monthly mean LST for each month between 2003–2018. The study period was included in the base period, as the inclusion of all available observations allows for a more accurate characterization of the study locations’ climate [ 27 ]. Average monthly temperatures between 2013–2019 were similarly gridded at 30km x 30km centered on each study location.
In order to assess rainfall variability for the study sites in Kenya, we used the daily African Rainfall Climatology Version 2 (ARC2) dataset from the archives of the National Oceanic and Atmospheric Administration (NOAA)–Climate Prediction Center (CPC). The rainfall estimates are gridded at 0.1° x 0.1° (≈11 km) spatial resolution operationally produced by a combination of rain gauge measurements and METEOSTAT satellites, thus providing rainfall estimates from 1982 to the present over Africa. Monthly long-term means were calculated for 30km x 30km grids for two counties, Kwale and Kisumu, using the 1982–2019 base period. As referenced in the WMO’s Guidelines on the Calculation of Climate Normals, the inclusion of all available observations allows for a more accurate characterization of the study locations’ climate, especially when fewer than 30 years of data are available [ 27 ]; as a result, the reference periods for LST and rainfall differ for this study. To calculate absolute monthly rainfall between 2013–2019, we aggregated data to a 30 km x 30 km grid centered on each study location. Absolute monthly rainfall for the coastal sites of Msambweni and Ukunda were compared to the long-term monthly mean rainfall for their county, Kwale. Monthly rainfall for the western sites of Kisumu and Chulaimbo were compared to the long-term monthly mean rainfall for their county, Kisumu.
Extreme climate events were defined as anomalies greater than the 90th percentile and lower than the 10th percentile of anomalies ( Fig 2 ). Since this study is concerned with how climate anomalies resulting from climate variability influence dengue transmission, classification of climate events does not consider absolute values of rainfall and LST but rather deviations from what is typically expected. More specifically, a flood is categorized as a positive rainfall deviation (i.e., above the 90% rainfall threshold), and a drought is categorized as a negative rainfall deviation (i.e., below the 10% rainfall threshold). A heat wave is categorized as a positive LST deviation (i.e., above the 90% LST threshold), and a cold wave is categorized as a negative LST deviation (i.e., below the 10% rainfall threshold). Such a method of defining extreme climate periods allows for sufficient observations for analysis.
Extreme climate anomalies were defined as the upper and lower 10% of all anomalies (difference compared to long-term mean). For rainfall, upper 10% is designated as a “flood”; lower 10% is designated as a “drought.” For LST, upper 10% is designated as a “heat wave”; lower 10% is designated as a “cold wave.”
https://doi.org/10.1371/journal.pntd.0009182.g002
Vector abundance
Mosquitoes of different life stages were sampled and classified by trapping method, date of collection, species and sex. This study is concerned with the abundance of Aedes aegypti and Aedes spp. (i.e. Aedes ochraceus , Aedes fulgens Aedes pembaensis ), as they are primarily responsible for the transmission of DENV in Kenya. While traditionally an endophilic species, these mosquitoes have been observed to be primarily exophilic daytime feeders with peak biting periods early in the morning and in the evening before dusk in our study sites [ 28 ]. Vector abundance was recorded using various sampling methods to recover mosquito life stages, including ovitrap (eggs), pupal sampling (pupae), Prokopack aspiration (adults), and Biogents-sentinal (BG) trapping (adults) ( S2 Appendix ). The use of various collection methods allows for a more representative and accurate estimate of vector abundance, as each method allows for the collection of different mosquito life stages.
Disease transmission
Dengue (DENV) incidence was assessed based on blood samples collected from children with acute febrile illness presenting to one of the four study sites (Mbaka Oromo Health Centre in Chulaimbo, Obama Children’s Hospital in Kisumu, Msambweni District Hospital in Msambweni, and Ukunda/Diani Health Center in Ukunda) ( Fig 1 ). The study population consisted of 7,653 children less than 18 years of age (median = 5 years [1 year, 15 years]). Unlike other places around the world, children in Kenya spend a lot of time outside during the day, which is when Ae . aegypti are actively biting. Blood samples were tested on site in Kenya by Ministry of Health collaborators at Msambweni District Hospital for coastal Kenya sites and KEMRI Kisian Field Station for western Kenya sites; samples were also tested at Stanford University. Cases of DENV were defined as a positive by polymerase chain reaction (PCR) and/or IgG-positive enzyme linked immunosorbent assay (ELISA)–children were considered dengue positive at the initial visit if viremia was found in the blood at the initial visit (e.g. by PCR) and if they seroconverted based on the follow-up visit (e.g. by PCR and IgG-ELISA). If a child presented PCR negative and already had antibodies at the initial visit, they were not included in the totals because this indicates that they had dengue previously at some point in their life and we would not be able to distinguish whether an infection occurred in the last few weeks.
Statistical analyses
We conducted bivariate analyses, including Kruskal-Wallis tests by ranks and Wilcoxon rank sum tests, to investigate whether average Ae . aegypti abundance one month following each extreme climate event was significantly different compared to following “normal” climate ( S3 Appendix ). A one-month lag period was used between mosquito abundance and meteorological variables, as has been traditionally been supported in the literature and also expected by the cycle of infection [ 29 ]. An identical analysis was done for DENV infection counts at a lag of two months following the anomaly ( S3 Appendix ). While there have been observed time lags of 1–3 months between dengue incidence and meteorological variables, we made use of a two month lag period; rainfall and temperature have been observed to have the most prominent effects on dengue incidence at a lag period of two months [ 30 , 31 ].
To test the effect of magnitude of rainfall and temperature anomalies on Ae . aegypti abundance after one month, we developed generalized log-linear mixed models for each mosquito life history stage (i.e., trapping method). The predictor variables for the model include rainfall anomalies and LST anomalies while site, month, and year are included as random effects. We also tested for a possible interaction between rainfall anomalies and LST anomalies. Other variables, such as monthly accumulated rainfall, monthly LST, monthly ambient temperature, and humidity were excluded from the model because of their collinear relationships with rainfall anomalies and LST anomalies.
In addition to a generalized log-linear mixed model, we conducted a multinomial logistic regression model using the classification of rainfall anomalies and LST anomalies as our primary independent variables in explaining expected vector abundance classification the following month. Vector abundance was classified as low, intermediate, and high for each trapping method. Due to the non-normal distributions of the outcome, cutoffs for grouping were established non-uniformly across the trapping methods ( S3 Table ). As such, we were interested in observing how our categorization of climate anomalies helps predict vector counts. We calculated adjusted odds ratios including fixed effects for site and month, allowing us to consider site and time-endogenous variation.
Similarly, we tested the effect of rainfall and temperature anomalies on the number of monthly DENV infections using a two-month lag. We developed a binary logistic regression model, with the first outcome defined as <7 confirmed DENV infection in a month and the second outcome defined as 7+ confirmed DENV infections. 7+ confirmed DENV infections represent the upper 10% of monthly cases and can thus be defined as “higher” than normal in a region with low levels of DENV transmission. The model controlled for seasonality and regional differences by considering month, year, and site.
A second binary regression model tested the effect of rainfall and LST anomaly categorization on dengue transmission, again accounting for month, year, and site. Missing data was excluded from all models ( S4 Table ).
Descriptive and inferential analyses were conducted using the statistical software R (version 1.1.383, 2017, Boston, USA).
Establishing defined thresholds for our categorization of extreme climate events allows us to compare related climatic variables of interest between types of extreme climate. Calculations and categorization of extreme climate events were based on previous guidelines [ 32 ]. A comparison between these groups suggests appropriate categorization of anomalies ( S1 and S2 Tables). However, such categorizations of extreme climate events are unevenly distributed among the study sites (Tables 1 and 2 ).
https://doi.org/10.1371/journal.pntd.0009182.t001
https://doi.org/10.1371/journal.pntd.0009182.t002
Ae . aegypti eggs. In our generalized log-linear model, rainfall anomalies were positively associated with egg abundance (p = 0.017); thus, for every ten-millimeter increase in rainfall anomalies (i.e., more severe floods) in any given month, a 2% increase in Ae . aegypti egg abundance would be expected, when site, month, and year, are included as random effects ( Table 3 ). The effect of LST anomalies, and the interaction between rainfall anomalies and LST anomalies were insignificant.
https://doi.org/10.1371/journal.pntd.0009182.t003
Floods significantly increased the odds of egg abundance being classified as “high” when site, month, year, and LST classification were held constant in our multinomial logistic model (OR = 13.8 [6.5, 29.3], p < 0.001); flood classification increased the odds of “high” mosquito egg abundance by 1280% ( Table 4 ). Drought, on the other hand, decreased the odds of egg abundance to be classified as “high” (OR = 0.70 [0.54, 0.90], p = 0.01) ( Table 4 ). Heat waves decreased the odds of both “low” egg abundance (OR = 0.32 [0.23, 0.44], p < 0.001) and “high” egg abundance (OR = 0.22 [0.20, 0.23], p < 0.001), meaning that excessively increased LST anomalies (i.e., heat waves) would be expected to result in intermediate vector counts the following month. Cold waves decreased the odds of “low” egg abundance the following month (OR = 0.25 [0.20, 0.32], p < 0.001) ( Table 4 ).
https://doi.org/10.1371/journal.pntd.0009182.t004
Ae . aegypti pupae.
Our generalized log-linear model did not find any significant effects of rainfall anomalies, LST anomalies, and the interaction between rainfall anomalies and LST anomalies on pupal abundance.
Similarly, for Ae . aegypti pupae, we observed that lower-than-expected levels of rainfall, as are consistent with the definition of a drought, promoted lower Ae . aegypti pupal abundance (OR = 2.41 [1.09, 5.32], p = 0.03) and significantly lowered the odds of high pupal abundance (OR = 0.85 [0.77, 00.95], p = 0.004) ( Table 4 ). Heat waves significantly increased the odds of low Ae . aegypti pupal abundance (OR = 2.22 [1.04, 4.75], p = 0.04) while cold waves significantly increased the odds of high Ae . aegypti pupal abundance (OR = 2.33 [1.48, 3.64], p < 0.001) ( Table 4 ).
Ae . aegypti adults.
For adult Ae . aegypti abundance collected with Prokopack aspirators, the severity of LST anomalies significantly influenced vector abundance: for every 1°C increase in a monthly LST anomaly, an 8.7% decrease in Ae . aegypti abundance would be expected the following month ( Table 3 ). However, for adult Ae . aegypti collected with BG-traps, we did not observe a significant relationship between anomaly severity and vector abundance ( Table 3 ). Rainfall anomalies and the interaction between rainfall anomalies and LST anomalies remained insignificant for both trapping methods. Compared to Chulaimbo, a significantly increased odds of higher adult abundance as collected by Prokopack aspirators was observed in the model for the urban sites of Kisumu (OR = 6.99 [2.87, 17.00], p < 0.001) and Ukunda (OR = 4.21 [1.71, 10.37], p = 0.002) while a decreased odds of higher adult abundance was observed for Msambweni (OR = 0.12 [0.05, 0.26], p < 0.001). A similar relationship was observed for adults collected by BG-traps. Compared to Chulaimbo, a significantly increased odds of higher adult abundance was observed for Kisumu (OR = 18.17 [6.21, 53.44], p < 0.001) while a decreased odds of higher abundance was observed for Msambweni (OR = 0.17 [0.06, 0.43], p < 0.001).
For adult Ae . aegypti collected with Prokopack aspirators, heat waves reduced the odds of “high” mosquito abundance by 67% (OR = 0.33 [0.19, 0.57], p <0.001). Similarly, drought reduced the odds of “high” Ae . aegypti abundance by 80% (OR = 0.20 [0.17, 0.24], p < 0.001) ( Table 4 ). However, unlike the results from our bivariate analyses ( S2 Fig ), once site, month, year, and rainfall anomalies were accounted for, cold waves did not significantly increase the odds of “high” vector abundance for Prokopack (OR = 2.43 [0.80, 7.39], p = 0.12). Moreover, cold waves were expected to decrease the odds of “low” vector abundance (OR = 0.57 [0.45, 0.72], p < 0.001), suggesting that abnormally cold temperatures promoted intermediate vector counts ( Table 4 ). For adult mosquitoes as recorded by BG-traps, we observed that floods significantly increased the odds of “high” vector abundance (OR = 2.41 [1.36, 4.27], p = 0.002) when the analysis was controlled for site, month, year, and LST anomaly ( Table 4 ).
Dengue transmission
Our binomial logistic regression results suggest that both rainfall and LST anomaly severity are not significantly associated with the transmission of dengue in Kenya when using a two-month lag ( Table 5 ). Moreover, site, month, and year did not significantly influence the results, and the interaction between rainfall and LST anomalies was insignificant at p < 0.05. Similarly, when studying the effect that classification of rainfall and LST events has on dengue transmission, we failed to observe any significant effect ( Table 6 ).
https://doi.org/10.1371/journal.pntd.0009182.t005
https://doi.org/10.1371/journal.pntd.0009182.t006
The impact of recent climate extremes on mosquito vector abundance in Kenya is dependent on various factors, and observed results vary depending on the trapping method. Such a finding suggests that the effect of climate extremes differs based on the particular life stage of Ae . aegypti .
We found that one month following floods resulted in a significantly greater abundance of Ae . aegypti eggs. When adjusting for the potential modifying effects of site, month, and year, we observed flooding to result in a significant increase in the odds of higher egg abundance ( Table 4 ). Moreover, more extreme rainfall resulted in increased egg abundance ( Table 3 ). As suggested by significant differences in the effect of flooding between sites, urbanization likely influences the impact that flooding has on the abundance of Ae . aegypti eggs ( S1A and S2 Figs). In our stratified bivariate analyses, we observe only rural sites to experience significantly higher egg abundance following flooding ( S2 Fig ). Rural areas tend to absorb excess water during periods of extreme rainfall better than urban areas and their human-made water catchments [ 33 ], potentially preventing a “flooding out” effect and instead providing more stable pools of water for mosquito breeding. Modifying human behavior during floods, such as the use of artificial and human-made containers, outdoor trash disposal, are necessary in removing potential breeding sites for mosquito vectors.
For Ae . aegypti pupae, we observed significant increases in abundance following cold waves, but both droughts and heat waves were associated with significantly fewer vectors ( Table 4 ). From the unstratified bivariate analysis, pupal abundance was not significantly associated with extreme climate events ( S1B Fig ). However, in the stratified analysis, pupal abundance was significantly lower following a heat wave in Chulaimbo, a rural study site in western Kenya ( S3B and S4 Figs). Even sites that are close in proximity to one another can experience different climate anomalies due to differences in local topography, wind, etc. Of all trapping methods assessed, pupal counts were consistently the lowest across study sites and were zero for more than a third of the observation periods, potentially affecting this study’s ability to assess the effect of extreme climate events on Ae . aegypti pupal abundance.
Drier climatic conditions, including drought and heat waves, seem to promote lower vector abundance. In terms of anomaly severity, we observed that more anomalous cold temperatures promoted greater adult abundance, as recorded by Prokopack ( Table 3 ). Our bivariate analysis supports such a finding, as we observed an increased abundance of adult Ae . aegypti mosquitoes following periods defined as cold waves, for both trapping methods of Prokopack and BG-traps ( S3C and S4C Figs). However, this association was likely driven by the fact that the average observed LST during cold waves was 29.99°C, which is in line with the 29°C optimal ambient air temperature threshold for Ae . aegypti [ 23 ]. Moreover, site and year were significant predictors of abundance classification, as supported by the multinomial regression and bivariate analyses. For example, many of the of the study’s cold wave observations actually took place during the 2015–2016 summer months ( S5 Fig ). This finding suggests that Ae . aegypti find cooler than expected months to be more favorable for growth because of the extremely warm average Kenyan temperatures year-round. However, the most notable finding was that for adult Ae . aegypti mosquitoes, as recorded by BG-trap, we observed significantly greater odds of higher adult abundance following floods, which is consistent with what we observed for Ae . aegypti eggs ( Table 4 ). Such a finding suggests that of all extreme climate events, flooding most likely contributes to higher adult abundance by providing additional breeding sites for Ae . aegypti .
Our results primarily suggest that flood seasons contribute to significantly higher Ae . aegypti egg and adult abundance after one month. While such a finding is unique for Kenya, it is consistent with several previous epidemiological studies [ 14 – 17 ]. Periods defined as “floods” in Kenya considered a monthly accumulation of rainfall that was extreme but that did not always cause physical floodwaters that might have resulted in mosquito habitats being washed away. This was in contrast to previous tightly controlled simulations and experiments, which resulted in a “flooding-out effect” of vector breeding sites [ 10 – 13 ].
When adjusting for site, month, and year, we did not observe statistically significant relationships between extreme climate anomalies (specifically floods and cold waves) and confirmed cases of dengue infection, but this result may still inform our understanding of these relationships. This finding suggests that mosquito abundance and dengue risk do not necessarily share a linear association, as human behaviors can modify the relationship and influence infection risk. For example, previous studies of dengue dynamics in China have observed nonlinear statistical relationships between vectors, human incidence, and climate [ 29 , 34 ]. This nonlinear relationship is in part driven by the extrinsic incubation period (EIP), the time it takes for the virus to disseminate in the mosquito, and mosquito lifespan. EIP duration and the mosquito lifespan are temperature-dependent. EIP becomes faster at higher temperatures, but the mosquito must survive longer than the EIP for transmission to occur [ 35 ]. The 29° C thermal optima derived from the full suite of Ae . aegypti temperature-dependent traits and widespread occurrence of the Ae . aegypti vector throughout Africa, suggests that as temperatures increase, dengue burden is also likely to increase throughout sub-Saharan Africa [ 36 ].
Preventative measures taken by our study population in the four villages may have contributed to reductions in the risk of dengue transmission. A recent study found that the primary control method utilized by participants in our study sites was bed-nets; of 5,833 enrolled patients at our four Kenyan clinic sites, 4,397 (75.4%) reported always using bed-nets [ 37 ]. While bed-nets can be protective against several mosquito species, they are not as useful against a diurnal mosquito species like Ae . aegypti . In addition to the removal of outdoor breeding sites, greater education and promotion of other preventative measures is necessary. The use of outdoor spray and repellant targeting this exophagic and anthropophagic mosquito species can significantly drive down the increased risk of dengue infection following extreme climate events.
An improved understanding of the relationship between extreme climate and dengue transmission can also allow for the development of climate-based early warning systems in Kenya. The El Niño Southern Oscillation (ENSO) and Indian Ocean Dipole (IOD) are responsible for changes in the sea-surface temperature that moderate seasonal rainfall and temperature variability in eastern Africa. As such, there is an increased likelihood of extreme climate events during extreme ENSO and positive IOD. By discerning the spatiotemporal relationship between extreme climate and dengue transmission, we can use ENSO and IOD indices to predict periods of heightened arboviral infection risk.
This study effectively investigates the impact of recent climate extremes on various life stages of Ae . aegypti abundance. With the availability of long-term satellite data, monthly rainfall and LST anomalies were calculated in order to determine associations with vector abundance and disease risk. A strength of the study is the calculation of monthly anomalies to explain both mosquito and disease data trends. The observational nature of the study offers high external validity. The study’s results can be generalized to dengue endemic regions similar in climate and demography to Kenya and the effects related to rainfall and LST can be more widely applicable. However, there are several limitations that are important to consider for future studies.
Due to the lack of long-term climate data available for analysis, this study makes use of satellite-derived data for four study sites in Kenya, which can provide us with a reference for the effect of extreme climate events more generally in the region ( S6 Fig ). As such, satellite-derived climate data offers the ability to compare deviations in recent climate conditions from long-term records. Minimum and maximum temperature values can impact temperature variability to differing extents. We are unable at the moment to reconstruct the data as produced and provided to tease out the effect of minimum and maximum temperatures. so future studies should consider temperature anomalies in the context of these extreme values rather than the mean. Additionally, future studies can make use of lag periods of differing temporal resolutions. Our lag periods were established based on the traditional cycle of dengue infection [ 29 – 31 ]; however, it is not a perfect model and other lag periods, especially with regards to dengue risk, have been observed in the literature. Furthermore, it is necessary to consider “anomalies” in the context of established biologically relevant thresholds for both temperature and rainfall.
Underreporting and misclassification of fever can influence results as well. Since DENV is heavily underreported in the region, all enrolled participants were tested for dengue and malaria. In a study of undifferentiated fever in Kenya between 2014–2017, 150 (51.5%) of 291 participants with dengue viremia were malaria smear positive, suggesting a large overlap in infections [ 38 ]. Additionally, flaviviruses, which include West Nile virus (WNV), ZIKA virus (ZIKAV), and yellow fever virus (YFV), have the potential to cross-react with DENV. However, WNV has a comparatively lower seroprevalence compared to DENV [ 26 ] while ZIKAV and YFV are essentially absent from our study sites.
As climate change accelerates and increases the intensity and severity of extreme climate events, understanding how they impact infectious disease transmission is essential. Climate change continues to blur and transform previously discrete seasons, which will influence vector dynamics and disease burden in Kenya. Ultimately, efforts should be focused on eliminating mosquito-breeding sites through the removal of human-made containers and trash in order to reduce vector populations. At the same time, encouragement of the use of spray, coils, and repellant can reduce the heightened risk of viral transmission during periods of anomalous climate and more specifically, floods.
Supporting information
S1 appendix. land surface temperature data..
https://doi.org/10.1371/journal.pntd.0009182.s001
S2 Appendix. Mosquito Trapping Method Timing and Frequency.
https://doi.org/10.1371/journal.pntd.0009182.s002
S3 Appendix. Crude Bivariate Analysis Methods.
https://doi.org/10.1371/journal.pntd.0009182.s003
S1 Fig. Effect of Rainfall Anomalies on Vector Abundance.
Boxplot of vector abundance by A) ovitrap B) Prokopack C) BG-trap and D) pupal trap one month following anomaly classified as normal, drought, or flood. Wilcoxon test p-values displayed between groups.
https://doi.org/10.1371/journal.pntd.0009182.s004
S2 Fig. Effect of Rainfall Anomalies on Egg Abundance Stratified by Urbanization.
Boxplot of Ae. aegypti egg abundance one month following anomaly classified as normal, drought or flood. Stratified by rural (Chulaimbo and Msambweni) and urban (Kisumu and Ukunda) sites. Wilcoxon test p-values displayed between groups.
https://doi.org/10.1371/journal.pntd.0009182.s005
S3 Fig. Effect of LST Anomalies on Vector Abundance.
Boxplot of vector abundance by A) ovitrap B) Prokopack C) BG-trap and D) pupal trap one month following anomaly classified as normal, heat wave, or cold wave. Wilcoxon test p-values displayed between groups.
https://doi.org/10.1371/journal.pntd.0009182.s006
S4 Fig. Effect of LST Anomalies on Adult Abundance Stratified by Geography.
Boxplot of adult Ae . aegypti abundance (Prokopack) one month following anomaly classified as normal, heat wave or cold wave. Stratified by western (Kisumu and Chulaimbo) and coastal (Ukunda and Msambweni) sites. Wilcoxon test p-values displayed between groups.
https://doi.org/10.1371/journal.pntd.0009182.s007
S5 Fig. Anomaly Severity by Site.
Heat map displaying monthly anomaly severity for A) accumulated rainfall and B) average LST between November 2013—February 2019, stratified by study sites.
https://doi.org/10.1371/journal.pntd.0009182.s008
S6 Fig. Relationship Between Land Surface Temperature and Ambient Air Temperatures.
Across all four study sites, there is a strong correlation (R > 0.50, p < 0.05) between land surface temperature and ambient air temperatures between November 2013 –February 2019; however, there is clear variability between the two measurements.
https://doi.org/10.1371/journal.pntd.0009182.s009
S1 Table. Characteristics of Rainfall Classification.
Mean and standard deviation of monthly LST (°C), LST anomaly (°C), monthly rainfall (mm), rainfall anomaly (mm), monthly ambient air temperature (°C), and monthly humidity (%) between previously defined groups of “flood,” “drought,” and “normal rainfall.” LST and rainfall anomalies refer to difference between observed monthly values and long-term means. p-values indicate significance values from Kruskal-Wallis Rank Sum tests among three groups. *p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 for Wilcoxon-test where “Normal Rainfall” is considered the reference group.
https://doi.org/10.1371/journal.pntd.0009182.s010
S2 Table. Characteristics of LST Classification.
Mean and standard deviation of monthly LST (°C), LST anomaly (°C), monthly rainfall (mm), rainfall anomaly (mm), monthly ambient air temperature (°C), and monthly humidity (%) between previously defined groups of “heat wave,” “cold wave,” and “normal LST.” LST and rainfall anomalies refer to difference between observed monthly values and long-term means. p-values indicate significance values from Kruskal-Wallis Rank Sum tests among three groups. Note: *p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 for Wilcoxon-test where “Normal LST” is considered the reference group.
https://doi.org/10.1371/journal.pntd.0009182.s011
S3 Table. Outcome Classification Cutoffs.
https://doi.org/10.1371/journal.pntd.0009182.s012
S4 Table. Missing Data for Outcomes.
Missing data was excluded from the regression models.
https://doi.org/10.1371/journal.pntd.0009182.s013
Acknowledgments
This retrospective study assesses the effect of extreme climate events on mosquito abundance and DENV transmission in Kenya using data from a study performed by the LaBeaud Lab, Technical University of Mombasa, and the Kenya Medical Research Institute (KEMRI). Vector, climate, and disease data is housed in the Research Electronic Data Capture Database (REDCap) at Stanford University. The Stanford REDCap platform ( http://redcap.stanford.edu ) is operated by Stanford Medicine Research IT team.
- View Article
- PubMed/NCBI
- Google Scholar
- 5. IPCC, 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri R.K. and Meyer L.A. (eds.)]. IPCC, Geneva, Switzerland, 151 pp.
- 8. World Health O. Dengue guidelines for diagnosis, treatment, prevention and control: new edition. Geneva: World Health Organization; 2009.
- 27. World Meteorological Organization–WMO (2017), WMO Guidelines on the Calculation of Climate Normals, WMO, Geneva. Accessed 1 April 2020.
- 32. IPCC, 2012: Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change [Field C.B., Barros V., Stocker T.F., Qin D., Dokken D.J., Ebi K.L., Mastrandrea M.D., Mach K.J., Plattner G.-K., Allen S.K., Tignor M., and Midgley P.M. (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 582 pp.
What you need to know about mosquito-borne diseases
West nile virus, eastern equine encephalitis and california serogroup viruses are most prevalent in canada.

Social Sharing

Belle River, Ont., teacher Melanie Klimkowski never worried much about mosquitoes, since they never seemed interested in biting her.
After learning more about the insects at a special workshop with her eighth grade class, she says she now carries small amounts of mosquito repellant at all times to make sure the arthropods stay away.
"Just like when we carry hand sanitizer, I have a little thing of the oil in my purse, just in case," said Klimkowski.
Experts say that infection rates in Canada for mosquito-borne diseases are extremely low , but evolving environmental factors — including climate change — are changing the way that mosquitoes live and breed, as well as providing extra opportunities for infected mosquitoes to bite people and increase the potential for disease.
A closer look at mosquitoes in Canada
Canada is home to over 80 different species of mosquitoes, according to Brock University entomologist Fiona Hunter. They can be found in every province and territory.
Not every mosquito carries diseases that infect humans. Mosquitoes of the Culex , Culiseta , Anopheles and Ochlerotatus genuses are some of the most common Canadian mosquitoes that can.
The typical mosquito breeding season is April to September, though Hunter says some mosquitoes can survive the winter.
The process is known as diapause — similar to hibernation in mammals — where they find hiding places like sewers or subway systems to slow down their development.
Adult mosquitoes can survive in winter, but Hunter says it's more common for eggs to overwinter — live through the winter — than full-grown insects.
"If you have a wood stove and then suddenly mosquitoes are flying around, they've actually been overwintering in your woodpile," said Hunter.
Mosquitoes typically breed in stagnant water, and experts advise dumping out unused buckets in garages or even emptying out saucers under flower pots in backyards to avoid providing the insects with a place to reproduce.
Of the many mosquito-borne diseases that affect humans around the world, Hunter says West Nile virus, eastern equine encephalitis (EEE) and the California serogroup viruses are the most prevalent in Canada, but remain rare.
West Nile virus is the most common mosquito-borne disease in Canada, spread by mosquitoes of the Culex genus.

Public health authorities report anywhere between 40 to 200 human cases each year, according to infectious diseases specialist Dr. Sumon Chakrabarti. That number is comparable to other parts of the world where the disease circulates more regularly due to tropical climate conditions, Chakrabarti says.
"Notably in 2007, we saw 2,400 cases across Canada," he told The Dose host Dr. Brian Goldman.
Weather conditions, a higher number of infected birds, as well as increased rainfall that year were likely contributors to the higher than normal case counts, Chakrabarti said.
In contrast, 13 human cases of West Nile virus have been confirmed in Canada in 2024, as of Aug. 26.
Provinces and territories also track mosquitoes for infection by testing mosquito traps in pools set up by public health units. This year, more than 260 pools have tested positive for West Nile virus across Canada.

Mosquitos carrying West Nile survive Prairie winters
Eastern equine encephalitis (EEE) is rarer than West Nile virus in Canada, with Hunter estimating one human case in the last two decades.
The U.S. sees around 11 human cases of EEE per year, according to the Centres for Disease Control and Prevention .
Mosquitoes spread diseases by consuming blood from infected animals like horses, sheep and birds. As the virus develops in the insect, the virus is transmitted through the mosquito's saliva when it bites uninfected hosts, including humans.
Chakrabarti says it's not quite clear why some people are mosquito magnets while other people get bitten less often.
- Quebec towns near U.S. border alerted as Vermont faces mosquito-borne illness threat
- Adult dies after eastern equine encephalitis infection, New Hampshire health officials say
"Pheromones are part of it, the composition of the bacteria on your skin, your body heat," he said, adding that body weight also could play a factor as well.
"People who tend to be a bit bigger, they're putting out more [carbon dioxide]," he said, as female mosquitoes have receptors to detect the gas as we exhale .
Low-grade symptoms resemble cold and flu
People infected with West Nile virus typically present flu-like symptoms such as rashes or muscle aches, says Chakrabarti.
"When you see it in the hospital, it's often people who are coming in with high fevers and particularly headaches," he said.
Chakrabarti says many people who get infected with West Nile are mild cases and tend to recover normally.

Climate change could shift what animals we share our cities with, study suggests
"That's not to take away from the fact that this is something we should be aware of," he said.
There are no specific treatments or vaccines for West Nile virus, nor is there an approved vaccine for EEE.
"It's something that generally you watch and give supportive treatment," said Chakrabarti, including treating fever and muscle aches, as well as any seizures that may occur during more serious infections.
Climate change means longer breeding seasons
Despite low infection rates, Hunter says that climate change is driving a shift in Canadian mosquitoes and the introduction of new species.
She pointed to the tiger mosquito as an example of a tropical species that has recently been spotted in Ontario's Windsor-Essex region, adding that it's a strong vector of transmission for more dangerous diseases like Zika virus.
The mosquito was first identified in Windsor-Essex in 2016, according to the Public Health Agency of Canada , and has been consistently reported in the region between 2018 and 2023.
- A virus spread by tiny insects is on the rise in Brazil and Cuba. Here's how to protect yourself
- New Brunswickers trap mosquitoes all summer for scientific study
Zika virus is spread when a mosquito bites an infected human, and then transmits the virus to an uninfected person through another bite.
Hunter says public health units haven't found positive Zika cases among humans or mosquitoes in Windsor-Essex, because "those mosquitoes haven't been biting people who already have Zika."
If a tiger mosquito bit someone who unknowingly brought over Zika virus from abroad, however, that could then lead to greater infection rates.
"But it's not a high risk," Hunter clarified.
How to protect yourself
Chakrabarti recommends wearing long-sleeve shirts and pants, avoiding wearing bright colours that attract mosquitoes, as well using insect repellent to keep the annoying insects away.
Longer spring and fall seasons caused by human-influenced climate change also mean mosquitoes have more time to breed. At the same time, warmer weather leading to more precipitation means mosquitoes are more comfortable in Canada, despite the cold winters.
"Climate change has brought about those massive rain events that we've had, and when that happens, species that have always been here, their numbers just explode," said Hunter.
Hunter adds that floodwater mosquitoes bite during the day, as well as dawn and dusk when other mosquito species are typically more active.
"All the public messaging of course is, protect yourself at dusk and dawn," she said.
"You should also protect yourself from that little floodwater mosquito in a year where it's hot and you've had all that flooding."
ABOUT THE AUTHOR

Associate Producer
Sameer Chhabra is an associate producer with CBC News: The National, currently assigned to White Coat, Black Art and The Dose. He's previously worked with CBC's Day 6, Spark and Cross Country Checkup radio shows, as well as with CBC Toronto local radio, and with CBC Windsor as a web reporter.
Related Stories
- Medical research and development

deliormanli/E+ via Getty Images
Understanding significant mosquito-borne diseases
Common mosquito-borne illnesses, such as zika virus, west nile virus, chikungunya virus, dengue, and malaria, pose public health threats for various communities..

- Veronica Salib, Associate Editor
As several West Nile and dengue cases emerge across the United States, more patients are being impacted by mosquito-borne diseases. Despite being some of the smallest animals, mosquitos and the illnesses they carry can be some of the deadliest.
The World Mosquito Program estimates that mosquito-borne diseases kill one million people and infect up to 700 million people annually. As the environment continues to deteriorate, climate change and altered weather patterns have lengthened mosquito season and expanded the geographical range of these infectious creatures.
According to the CDC , the primary diseases spread to humans by mosquitos are Zika virus, West Nile virus, Chikungunya virus, dengue, and malaria.
Although anyone exposed to these mosquitos may be at risk of contracting the illnesses they carry, the National Institute for Occupational Safety and Health (NIOSH) notes that people in certain jobs are at a greater risk of contracting these viruses. For example, outdoor workers, those who have to travel for work to areas with mosquito-borne diseases, laboratory workers who handle infected samples, cultures, or arthropods, and healthcare workers who are in contact with infected patients.
The CDC reveals that West Nile virus is the most common mosquito-borne disease in the continental U.S. As of September 3, 2024, there have been 377 total cases of West Nile Virus across 38 states in the U.S. Among those cases, 255 were West Nile virus neuroinvasive disease cases.
West Nile virus is a type of flavivirus that infects mosquitoes when they feed on infected birds. Mosquitoes then bite humans, spreading the disease to them, but the spread generally stops at the human level since the viral levels transmit to other species.
There is a small possibility that human-to-human transmission can occur through blood or organ transplantation. However, the CDC maintains that transmission via transplantation, pregnancy, delivery, and breastfeeding is rare.
Although most people infected with West Nile do not have symptoms, roughly 20% of infected individuals will develop a fever or other common symptoms, including headache, body aches, joint pains, vomiting, diarrhea or rash. A smaller subset of individuals, 1 out of 150, will develop a severe and potentially fatal version of the disease, which causes encephalitis or meningitis.
Without any available vaccines, the only way to reduce the risk of contracting West Nile virus and developing severe illness is to prevent mosquito bites.
Zika virus is a mosquito-borne virus caused by infected Aedes mosquitoes. It is typically contracted from mosquitoes in tropical or subtropical areas of Africa, the Americas, Southern Asia and the Western Pacific.
This disease is transmitted from mosquitos to humans through bites. When the virus is circulating, a mosquito can contract the virus by biting an infected human.
In addition to mosquito bites, humans can contract Zika virus from other humans through sexual contact, including vaginal, anal, and oral sex or the sharing of sex toys. However, the CDC notes that the transmission timeframes vary between males and females, as the virus can stay in semen longer.
Zika can also be transmitted from a pregnant individual to a fetus during pregnancy. While the virus has been identified in breast milk, researchers have not yet confirmed any viral transmission through breastfeeding.
Although many individuals infected with Zika will be asymptomatic or exhibit mild symptoms, including fever, rash, headache, joint pain, conjunctivitis, and muscle pain, some patients are at greater risk of complications from Zika. For example, Zika infections during pregnancy have been linked to birth defects and other pregnancy complications, such as miscarriage, stillbirth, and preterm birth.
In rare cases, the Zika virus can be more severe, causing Guillain-Barré syndrome, encephalitis, meningitis, myelitis or a blood disorder.
In addition to mosquito bite prevention, Zika can also be prevented by using safe sex practices and barriers such as condoms to prevent sexually transmitted Zika.
Chikungunya
Chikungunya virus is an alphavirus caused by mosquito bites, commonly occurring in Africa, the Americas, Asia, Europe and Indian or Pacific Oceans islands. Common symptoms of this virus include fever and joint pain; however, patients may also present with headache, muscle pain, joint swelling, or rash.
While death from Chikungunya is rare, some vulnerable populations, including newborns, elderly adults and those with underlying medical conditions might be at a greater risk for severe disease.
Although the virus does not spread from person to person through coughing, sneezing, or touching, an infected individual can infect mosquitos, which can then infect other people. Additionally, high viral loads in the blood mean that the virus can spread via blood transfusion, laboratory handling of infected blood, or drawing blood from an infected individual. Additionally, pregnant individuals can transmit the virus to their unborn fetus, with the greatest risk around the second trimester.
Unlike many other mosquito-borne illnesses, there is a vaccine available for Chikungunya. The IXCHIQ vaccine is approved as a single dose for individuals 18 and older. The CDC recommends vaccination for the following groups:
- Individuals traveling to areas with an outbreak.
- Individuals over 65 going to regions with evidence of transmission in the past five years.
- Individuals spending six months or longer in areas with evidence of transmission in the past five years.
- Laboratory workers who might be exposed to the virus.
Dengue virus includes four closely related subtypes: dengue-1, dengue-2, dengue-3, and dengue-4. This virus is transmitted through mosquito bites but can also be transmitted from an infected pregnant individual to their fetus. Dengue virus during pregnancy has been linked to fetal death, low birth weight, and premature birth.
Approximately 25% of people infected with the dengue virus will experience symptoms, the most common of which include fever, aches, pains, nausea, vomiting, and rash. Generally, symptoms are mild, and most patients recover within a week.
However, approximately 5% of people infected with the virus will develop severe dengue, which can cause shock, internal bleeding and death. Warning symptoms of severe dengue include belly pain or tenderness, vomiting three or more times within 24 hours, bleeding from the nose or gums, blood in the stool or vomit, and extreme fatigue or extreme restlessness.
Malaria is actually caused by a parasite that infects mosquitos; however, most people who get malaria contract it through a bite from infected mosquitos. While the condition is not endemic to the U.S., there are roughly 2,000 cases of malaria reported domestically each year.
Although malaria is not contagious like a cold or flu, in rare cases, the disease can be spread via blood transfusion, organ transplant, sharing needles or syringes, or from a pregnant individual to a fetus.
Disease symptoms can vary dramatically; however, early symptoms include fever, flu-like illness, chills, headache, muscle aches, tiredness, nausea, vomiting, and diarrhea. Severe infection can cause kidney failure, seizures, mental confusion, and even trigger a coma.
Unlike many other mosquito-related illnesses, prescription drugs are available for treating malaria; however, the type of drug and treatment regimen vary depending on the type of malaria, where a patient was infected, disease progression, age and pregnancy status.
In addition to standard mosquito bite prevention, some medications are available to prevent malaria for those traveling to high-risk areas.
Understanding mosquito-borne diseases and their prevention is crucial for public health.
Veronica Salib has covered news related to the pharmaceutical and life sciences industry since 2022.
Dig Deeper on Medical research and development
Recognizing the growing impact of climate change on infectious diseases

CDC issues health advisory for dengue virus infections across the US

10 Ways CRISPR Advancements Are Reshaping Healthcare

UC San Diego to Develop Dengue Fever, Infectious Disease Risk Platform

As artificial intelligence continues to gain traction in healthcare, health systems and other stakeholders must work on building ...
New report reveals that 96% of healthcare technology leaders see AI as providing a potential competitive advantage, but ...
When planning discharge, care teams must use their best judgment and available data to inform decision-making. Can artificial ...
High healthcare costs have motivated employers to think creatively about their benefits and strategies, from rethinking their ...
If the Inflation Reduction Act's enhanced premium tax cuts are renewed in 2025, they could increase enrollment among populations ...
Understanding the quality bonus payment program is critical to appreciating Medicare Advantage and discussing the calls for ...
About half of clinicians using an ambient AI documentation tool in a clinical trial reported positive outcomes, but some found no...
While California's Data Exchange Framework (DxF) aims to enhance statewide data sharing, success hinges on integrating ...
A 'JAMA Internal Medicine' study found that team-based documentation increased visit volume and reduced physician EHR ...
The personally identifiable information of nearly one million Medicare beneficiaries was affected by a third-party data breach at...
RansomHub listed Planned Parenthood of Montana on its leak site following a cyberattack.
CISA and partners released a cybersecurity advisory regarding cyberthreat actors, such as Pioneer Kitten and UNC757, some of ...
- Skip to main content
- Keyboard shortcuts for audio player
How climate change is affecting the spread of mosquito-borne illnesses
Alejandra Borunda
Mosquito-borne diseases and climate
There's an outbreak of the rare mosquito-borne disease Eastern Equine Encephalitis in the Northeast. Could it be connected to climate change? No one knows.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Plant-Based Bioinsecticides for Mosquito Control: Impact on Insecticide Resistance and Disease Transmission
Associated data.
Not applicable.
Simple Summary
Mosquito-borne diseases cause millions of deaths each year. There has been an increase in the use of insecticides to combat disease transmission caused by mosquitoes. Synthetic insecticides have been effectively used to protect humans from mosquito bites through insecticide-treated mosquito nets, fabrics, and indoor sprays. Despite the considerable progress made in reducing mosquito borne diseases, extensive usage of insecticides has caused serious health problems to humans and animals, insecticide resistance or insensitivity in mosquitoes, and environmental damage. A success in the fight with mosquito disease transmission can only be accomplished by adequate and effective implementation of insecticide resistance monitoring and management programs globally. For this purpose, extensive research focuses on exploring insecticide resistance mechanisms in mosquitoes and how they get resistant to chemical applications over time. The search also focuses on novel compounds that are more effective, safer, and eco-friendly for improved management of mosquito vectors. In this review, we provide the current literature on the synthetic insecticides and how mosquitoes develop resistance to them, with further emphasis on bioinsecticides that could replace conventional synthetic insecticides. In this context, plant-based compounds are explained in detail with their potential applications to control mosquitoes.
The use of synthetic insecticides has been a solution to reduce mosquito-borne disease transmission for decades. Currently, no single intervention is sufficient to reduce the global disease burden caused by mosquitoes. Problems associated with extensive usage of synthetic compounds have increased substantially which makes mosquito-borne disease elimination and prevention more difficult over the years. Thus, it is crucial that much safer and effective mosquito control strategies are developed. Natural compounds from plants have been efficiently used to fight insect pests for a long time. Plant-based bioinsecticides are now considered a much safer and less toxic alternative to synthetic compounds. Here, we discuss candidate plant-based compounds that show larvicidal, adulticidal, and repellent properties. Our discussion also includes their mode of action and potential impact in mosquito disease transmission and circumvention of resistance. This review improves our knowledge on plant-based bioinsecticides and the potential for the development of state-of-the-art mosquito control strategies.
1. Introduction
Mosquitoes have been a big burden to human health for a long time. These insects can invade in different geographic locations and new habitats through global trade and travel [ 1 ] which causes millions of people be at risk of the diseases they transmit. In 2019, an estimated 229 million cases and 409 thousand deaths for malaria and 56 million cases for dengue have been reported worldwide [ 2 , 3 ]. While malaria case incidences were reported to decline, the number of malaria endemic countries has increased in the period 2000–2019 [ 2 ]. The global incidence of dengue is thought to be increased about thirty times over the last fifty years with emergencies in new countries [ 4 , 5 , 6 ]. A recent study also indicates that mosquito species will continue to spread globally over the coming decades, which may cause about 50% of the world’s population at the risk of mosquito-borne viral disease transmission by 2050 [ 7 ]. Even a more serious problem is at our doorstep as the climate change is expected to increase the burden of mosquito-borne diseases despite the ongoing disease control interventions [ 8 , 9 ].
The most common way of keeping mosquitoes away from their human hosts is to use synthetic insecticides in mosquito nets, fabrics, and indoor sprays. The usage of chemical strategies has brought hope in controlling disease transmission in endemic regions, but emergence of insecticide resistance has been a major problem in reducing the disease burden. The uncontrolled usage of insecticides has led to reemergence and increase in mosquito populations over the years. Between the years 2010–2019, about 28 malaria endemic countries (out of 82) have detected resistance to all four classes of the most commonly used insecticides, and 73 have detected resistance to at least one insecticide class, an issue that continues to increase globally [ 2 ]. Thus, insecticide resistance is now considered a serious threat to control mosquito invasion and disease transmission. It is essential that the methods for insecticide monitoring in mosquito populations and interpretation of results are performed adequately, effectively and in a timely manner for improving mosquito control [ 10 , 11 ].
Current research on mosquito control is now focused on understanding the mosquito resistance to synthetic insecticides and developing novel strategies to overcome the resistance issues. Natural compounds that are more effective and less toxic than the synthetic ones continue to get more attention in the research community. The use of bioinsecticides, composed of botanical or plant-based compounds, has been a perfect alternative due to their minimal hazardous effects on human health and environment. In this review, we provide current knowledge on synthetic insecticides that are actively used in mosquito control and how they impact prevalence of insecticide resistance in mosquitoes. Major plant-based insecticides, their mode of action and the research about their potential mosquitocidal activity are discussed. A comprehensive understanding of how biochemical compounds can be advantageous to synthetic ones and how we can circumvent insecticide resistance issues in the fight with mosquito-borne disease transmission is provided.
2. Insecticide-Based Mosquito Control Strategies
Insecticide-based mosquito control plays an important role in efforts to reduce the transmission of mosquito-borne diseases worldwide. Two core insecticidal interventions are in use to control mosquitoes: deployment of insecticide-treated mosquito nets (ITNs) and indoor residual spraying (IRS) of insecticides [ 10 ]. These interventions have been effectively used to kill mosquitoes or interfere with their host-seeking behavior to prevent disease transmission worldwide [ 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ]. The global malaria cases and malaria death rates have declined about 18% and 48%, respectively, between the years 2000 and 2015, and 70% reduction in malaria cases in sub-Saharan Africa was attributed to ITNs, and 10% reduction was due to IRS [ 21 ].
Four classes of insecticides are mostly used in mosquito control programs which include pyrethroids (e.g., deltamethrin, permethrin, cypermethrin, lambda-cyhalothrin), organochlorines (e.g., DTT), organophosphates (e.g., malathion, fenitrothion), and carbamates (e.g., propoxur, bendiocarb) [ 10 ] ( Figure 1 ). Most synthetic insecticides have physiological or behavioral impact on mosquitoes ( Figure 1 ), and predominantly target the central nervous system of insects. Among them, pyrethroids are the most widely used insecticides for IRS and the only synthetic insecticide currently used in ITNs and fabrics, with irritant or repellent activity on mosquitoes and less mammalian toxicity [ 2 ]. They disrupt the voltage-gated sodium channels in neuronal membranes [ 22 ]. When pyrethroids bind an open channel, they prevent its closure, thus leading to a prolonged action potential or disruption of electrical signaling in the nervous system [ 23 , 24 , 25 ]. This causes continuous nerve excitation and paralysis (or knockdown) of the insect and eventually its death [ 26 ].

Classification of insecticides based on mode of action and chemical composition.
While pyrethroids have been effectively used in ITNs to control mosquitoes for a long time, prevalence of pyrethroid resistance in mosquito species causes a major problem to combat disease transmission worldwide [ 27 , 28 , 29 ]. Like pyrethroids, some organochlorines are also inhibitors of the insect’s voltage-gated sodium channels. Dichlorodiphenyltrichloroethane (DDT) is an example that targets sodium channels, and it is the first and the most commonly used synthetic insecticide of organochlorine in residual spraying. Its low cost and high effectiveness have made it a favorable chemical for indoor wall spraying. However, resistance developed to DDT in various mosquito species and its toxic effects on humans and non-target organisms have imposed limitations or restrictions in its usage [ 30 , 31 ]. Other organochlorines (such as cyclodienes, dieldrin and fipronil) target γ-amino butyric acid (GABA) receptors, which are hetero-multimeric gated chloride channels in the insect’s central nervous system [ 32 ]. Cyclodiene insecticides act as neurotoxicants and block the GABA receptors causing hyper-excitation of the central nervous system, convulsions, and eventually death of insects [ 33 , 34 , 35 ]. Organophosphates (OP) and carbamates are two other insecticides sharing similar mode of action. They inhibit acetylcholinesterase (AChE) enzyme, preventing breakdown of the neurotransmitter acetylcholine, resulting in neuromuscular overstimulation and death of insects [ 36 , 37 , 38 ]. Due to pyrethroid and DDT resistance issues worldwide, they have been used as alternative insecticides in IRS, but they have a shorter residual effectiveness, high toxicity to mammals, and are more costly compared to the others that limit their persistent long-term usage.
3. Insecticide Resistance in Mosquitoes
Short after its first usage in California in 1945, the resistance of mosquitoes to DDT was reported [ 39 , 40 ]. Since then, insecticidal resistance in mosquitoes has been reported, with a substantial increase between 2010 and 2016 [ 10 ]. In these years, insecticide resistance was found to be widespread in Anopheles vectors in malaria endemic African regions and insecticide resistance frequency has changed over time [ 10 ]. Understanding pyrethroid resistance development in Anopheles mosquitoes is particularly important because its prevalence can disable pyrethroid-treated ITN-based interventions, which are used successfully for malaria control [ 41 , 42 ]. Pyrethroid resistance was determined to be very high in the WHO African Region (78%), Eastern Mediterranean Region (70%), and in the South-East Asia Region (38%), Western Pacific Region (51%), but was lower in the Region of the Americas (20%). The incidence of organochlorine resistance was also similar in all WHO regions (60–70%). Carbamate resistance prevalence was between 22% and 54%, and organophosphate resistance prevalence varied widely across regions, 14% in the WHO African Region and 65% in the WHO Western Pacific Region [ 10 ]. While resistance frequencies are generally high in most of the endemic regions, those with lower resistance frequencies could be an indication of recent gain of resistance or selection for resistant populations to insecticides [ 43 ].
Despite effective use of insecticide-based mosquito control strategies for decades, their prolonged usage is challenged by high cost, toxicity and, more importantly, the development of resistance to the synthetic insecticides. Insecticide resistance is mostly inferred to the ability of insects to survive exposure to a standard dose of insecticide, owing to physiological or behavioral adaptation [ 44 ]. Resistance can be developed due to misusage or overdose usage of insecticides and selection pressure on the insect populations [ 45 ]. The question “when does the resistance emerge?” depends on the mechanism of resistance, known susceptibility, cost effectiveness and availability [ 45 ]. Various resistance mechanisms have been observed in mosquitoes: changes in their metabolism (changes in enzymes leading due rapid detoxification of insecticides), alterations in target-sites (prevention of insecticides to their target sites), penetration resistance (cuticle barrier diminishes insecticide penetration) and behavioral resistance (changes in their response to insecticidal effect) [ 46 , 47 , 48 , 49 ]. These mechanisms can be determined by using bioassays, biochemical assays, and molecular techniques through assessment of resistance alleles, analyzing whether metabolic enzymes are upregulated, or determination of the percent mortality rate upon exposure to a given insecticide.
In mosquitoes, alterations of target site nerve receptors (e.g., mutations in kdr , Rdl and Ace-1R genes) and detoxification due to increased or modified enzyme activities (e.g., monooxygenases (P450s), glutathione-S-transferases and carboxylesterases) are the two major mechanisms responsible for insecticide resistance. According to the insecticide resistance monitoring data for 2010 to 2016, almost 70% of the assays to test resistance mechanisms included detection of the presence or absence of target-site mutations and their frequencies in WHO regions [ 10 ]. Target site alterations in mosquitoes involve knockdown resistance ( kdr ) mutations (L1014F or L1014S) in the voltage-gated sodium channel gene which causes inability of the insecticides to bind their cognate receptors [ 50 , 51 , 52 , 53 , 54 , 55 ]. Occurrence of kdr mutations causes insensitivity to pyrethroids and DDT [ 56 , 57 ]. A kdr -resistant strain of An. gambiae has shown to be less affected by pyrethroids than the susceptible strain [ 58 ]. In the last few decades, kdr resistance mutations in different mosquito populations have expanded significantly which restricts pyrethroid usage in mosquito control [ 59 ]. Another target-site mutation, the AChE gene mutation ( Ace-1R ), causes resistance to organophosphates and carbamates. In mosquitoes, a G119S mutation in the Ace-1R gene encoding AChE causes resistance to organophosphate and carbamate insecticides and the mutation frequency is increasing in natural mosquito populations [ 60 , 61 , 62 , 63 ]. A substitution mutation of alanine-to-serine/glycine (A296S/G) mutation, Rdl , in the second transmembrane domain of the GABA receptor subunit causes resistance to organochlorine insecticides and insensitivity in mosquitoes [ 35 , 64 , 65 , 66 , 67 , 68 , 69 ].
Mosquitoes have metabolic enzymes, mainly “detoxifying enzymes” that are responsible for biodegradation of insecticides and elimination of their insecticidal effects. Upon exposure to synthetic insecticides, detoxifying enzyme activity increases (due to increased gene amplification or upregulation) which result in insecticide-resistant mosquitoes [ 46 ]. Three classes of detoxifying enzymes are involved in insecticide-resistance in mosquitoes: cytochrome P450 monooxygenases (CYP), glutathione-S-transferases (GST) and carboxyl-cholinesterases (CCE) associated with pyrethroid, organochloride, and OP and carbamate resistances, respectively. Cytochrome P450 enzymes are involved in the metabolism of all four classes of insecticides. It is found that elevated levels of P450 activity resulted in pyrethroid resistant mosquito vectors [ 70 , 71 , 72 , 73 , 74 ]. Several CYPs are identified in mosquitoes and CYP overexpression is reported from insecticide resistant mosquito populations [ 45 , 59 , 75 , 76 , 77 ]. Knockdown of the CYP through the RNA-interference technique also showed that mosquitoes become sensitive to pyrethroids [ 78 , 79 , 80 ]. Glutathione S-transferases comprise a diverse family of enzymes involved in detoxification of insecticides (e.g., pyrethroids and DTT) in mosquitoes [ 81 ]. An increase in the gene expression levels of various GSTs has been detected in DDT-resistant and pyrethroid-resistant mosquitoes [ 82 , 83 , 84 , 85 , 86 , 87 , 88 ]. Additionally, a GST gene silencing study indicated an increase in the susceptibility to pyrethroid insecticide which shows that GSTs are involved in insecticide-resistance in mosquitoes [ 86 ]. Increased esterase detoxification in OP resistance has been studied most extensively in Culex mosquitoes [ 72 , 89 ]. These enzymes sequester the insecticide and interfere with its association with the target AChE by rapid binding and slow turning over of the insecticide [ 90 ]. The increase in the activity of esterases was due to overproduction of the enzymes, resulting from co-amplification of two esterase genes, estα2 and estβ2 , in OP-resistant individuals [ 91 , 92 ].
It is evident that cross-resistance causes major issues in the management of insecticide resistance through the approaches discussed above. These mechanisms can cause resistance to more than one class of insecticide (with similar mode of action) due to prolonged and intensive usage of these chemicals. For example, Culex mosquitoes that are resistant to a pyrethroid insecticide also show resistance to OP and other insecticides [ 93 , 94 ]. Pyrethroid-resistant Anopheline mosquitoes also show resistance to OPs due to constitutively elevated P450 levels leading to cross-resistance [ 95 ]. Moreover, insecticide resistance is genetically mediated and can be fixed in mosquito populations in such that individuals with the resistance gene will probably have a selective advantage in the presence of the insecticide [ 96 , 97 ]. Furthermore, mosquitoes that survive insecticide exposures possibly have the chance of passing those traits to their offspring which causes an increase in the percentage of resistant individuals in the next generations in those populations [ 48 ]. If resistance gene frequency increases in the populations, this can cause more resistant individuals to circumvent insecticidal exposures. Taken together, the emergence and spread of insecticide resistance, cross-resistance, and increased resistance gene frequencies in mosquito populations significantly effects mosquito-borne disease control and elimination and highlights the need for alternative strategies. There has been a great interest for safe and healthy biological control strategies and development of novel interventions to overcome problems associated with synthetic insecticides. Hence, extensive research for another class of insecticide for mosquito control, named “bioinsecticide”, is an ongoing process and novel natural compounds are being investigated to replace conventional synthetic insecticides. In this review, we will focus on plant-based bioinsecticides with potential activity in mosquito control.
4. Plant-Based Bioinsecticides
Bioinsecticides are derived from natural products, such as bioactive compounds of plants, pheromones, and from microorganisms, such as bacteria, fungi, virus, or protozoan. There are four major classes of bioinsecticides based on their nature of origin: phytochemicals, microbial pesticides, plant-incorporated protectants (PIPs), and pheromones [ 98 ] ( Figure 1 ). They have been effectively used in pest management and generation of sustainable agricultural products [ 99 , 100 ]. They are less toxic, target-specific, highly effective in small quantities and biodegradable, which makes them excellent alternatives to synthetic compounds. More importantly, mosquitoes are developing resistance to synthetic compounds, a burden that needs to be resolved for successful mosquito disease control. Since biopesticides induce less insect resistance [ 101 , 102 ], most studies now focus on discovery of candidate natural compounds with potential effects on mosquitoes to combat mosquito-borne disease transmission.
Plants have evolved to develop many defensive chemical compounds against pathogenic microorganisms and insects. These biologically active chemical compounds, referred to as “phytochemicals”, function as repellents, toxins, feeding deterrents, and growth regulators against insects [ 103 ]. Various parts of higher plants (leaves, roots, stems, seeds, barks, fruits, peels of fruit and resin), the whole body of little herbs, or mixture of different plants can be used for an effective plant-based insecticide. The activity of a phytochemical can change significantly depending on the plant species, plant part and its age, polarity of solvents used during extraction procedures and mosquito species [ 104 ]. Phytochemicals show their effects through targeting important cell components and affecting insect physiology in different ways; via inhibition of AChE and GABA-gated chloride channel activity, disruption of sodium-potassium ion exchange and nerve cell membrane action, blocking calcium channels, and activation of nicotinic acetylcholine receptors and octopamine receptors [ 105 ]. Moreover, phytochemicals can cause cellular destruction of epithelial cells in the midgut of mosquitoes and affect metamorphosis [ 106 , 107 ].
Several phytochemicals have been reported for their mosquitocidal activities [ 104 , 108 ]. These chemical compounds are mostly secondary metabolites, such as essential oils, alkaloids, phenols, terpenoids, steroids, and phenolics from different plants. Phytochemicals in plant species are diverse and discovery of those with mosquitocidal activities, which are governed by changes in expression levels of detoxifying enzymes, are of great importance to control mosquitoes. In the following sections, we provide the current knowledge on mosquitocidal plant-based compounds and their activities for a better understanding of their efficacy to prevent mosquito-borne diseases.
5. Plant-Based Compounds and Mosquito Control
Plant-based compounds possess larvicidal, ovicidal and repellent activities on early or adult stages of mosquitoes, affecting nervous, respiratory, endocrine, and water balance systems. Ovicidal and larvicidal effects of many plant compounds have been extensively studied since mosquitoes are immobile at these stages and they can be efficiently eliminated before they emerge as adults. Repellent compounds are effective in keeping human hosts from mosquito bites for a blood-meal. Thus, understanding the mosquito olfactory system is vital for determination of repellent compounds. Insect repellents affect the olfactory receptor neurons via modifying or blocking its response, which in turn, elicit avoidance behavior or a change in the host-seeking behavior of mosquitoes [ 109 , 110 ]. There are many plant compounds with repellent activities. Essential oils, alkaloids, and aromatic compounds from various plants are commonly used for plant-based mosquito repellents [ 111 ] and they have shown to interfere with the mosquito host-seeking behavior when applied on human skin or used as indoor spraying [ 112 ]. Insecticidal and repellent activities of four major plant metabolites (essential oils, neem, pyrethrum, alkaloids) and other plant compounds (flavonoids and rotenone) are discussed in detail ( Table 1 ).
An overview of insecticidal activity and mechanism of action of various plant-based compounds against mosquito species.
| Type of Botanical Product | Plant Family | Activity | Mechanism of Action | Mosquito Species | References |
|---|---|---|---|---|---|
| Essential Oils Monoterpenes: linalool, cuminaldehyde, 1,8-cineole, limonene, fenchone, eugenol, γ-terpineol, cinnamic alcohol, geraniol, β-citronellol, -menthane-3,8 diol, α-pinene, β-pinene, -cymene, thymol, terpinolene, camphor, citronellal, sabinene, carvacrol Sesquiterpenes: guaiol, α-bisabolol, α-cadinol, germacrene D, β-caryophyllene, nootkatone Diterpenoids: diterpene alcohol, phytol Aromatic phenol Coumarin | Anacardiaceae Annonaceae Apiaceae Asteraceae Geraniaceae Lamiaceae Lauraceae Poaceae Rutaceae Myrtaceae Verbenaceae | larvicidal, pupaecidal, ovicidal, adulticidal, repellent, antifeedant, growth and reproduction inhibitors | Inhibition of AChE Blockage of GABA-gated chloride channels Agonist of octopamine receptors | | [ , , , , , , , , , , , , , , , , , , , , , , , , , , ] |
| Neem oil azadirachtin, meliantriol, salannin, desacetyl salannin, nimbin, desacetyl nimbin, nimbidin, nimbolide, deacetylgedunin, gedunin, 17-hydroxyazadiradione, deacetylnimbin | Meliaceae | repellent, ovicidal, larvicidal, feeding deterrence, fecundity suppression, toxicity, growth regulation, oviposition deterrence | growth inhibitors, hormonal disruption (ecdysone blocker), molting aberrations, interference with phagostimulants | | [ , , , , , , , , , , , , , , , , , , ] |
| Pyrethrum esters of chrysanthemic acid: pyrethrin I, cinerin I, jasmolin I esters of pyrethric acid: pyrethrin II, cinerin II, jasmolin II | Asteraceae | repellent, knock-down effect, blood-feding inhibition | voltage-gated sodium channel modulator | [ , , , , ] | |
| Alkaloids alpha-solanin ricinine pyridine nicotine diterpene nornicotine anabasine | Berberidaceae Fabaceae Solanaceae Ranunculaceae Euphorbiaceae | repellent, larvicidal | interfering with cellular and physiological functions, inhibition of AChE activity, regulation of hormone activity, toxicity, agonist of acetycholine receptor | | [ , , , , , , , , , , ] |
| Flavonoids | Zingiberaceae | larvicidal | inhibition of AChE, degradation of cell membranes acting as stomach poisons | [ , , ] | |
| Rotenone | Fabaceae | larvicidal | inhibitor of the cellular respiration system | [ ] |
5.1. Essential Oils
Essential oils have been efficiently used against a variety of pests and for crop protection in the world and they are potential alternatives to synthetic insecticides used against mosquitoes. Essential oils are very complex natural mixtures that consist of a variety of volatile molecules, which are hydrocarbons (terpenes and sesquiterpenes), oxygenated hydrocarbons and phenylpropenes ( Table 1 ). Essential oils are synthesized in the cytoplasm and plastids of plant cells through mevalonic acid and 2- C -methyl-erythritol 4-phosphate (MEP) pathways, respectively [ 113 ]. Essential oils target the insect nervous system and cause neurotoxic effects through several mechanisms by inhibiting the activity of AChE, and blocking octopamine receptors and GABA-gated chloride channels [ 114 , 115 ]. About 90% of essential oils are composed of monoterpenes, which are determined to be active ingredients for potential plant-based larvicides and cause inhibition of AChE activity in insects [ 116 ]. Monoterpenes, such as linalool, cuminaldehyde, 1,8-cineole, limonene and fenchone, cause inhibition of AChE and accumulation of acetylcholine in synapses and state of permanent stimulation, which results in ataxia [ 117 , 118 ]. According to Hideyukiu and Mitsuo [ 119 ], a mixture of monoterpenoids is a more potent inhibitor of AChE than single monoterpenoid application and acts synergistically.
The octopaminergic system of insects is another target for essential oils that block octopamine receptors and cause acute and sub-lethal behavioral effects on insects. The increase in cyclic AMP levels, induced upon binding of octopamine to octopamine-receptors, can be inhibited by a mixture of essential oils (eugenol, γ-terpineol and cinnamic alcohol). Moreover, octopamine receptor binding is significantly reduced with low doses of eugenol alone [ 120 , 121 ]. Another possible target for essential oils is ligand-gated chloride channels. Essential oils consist of monoterpenes, such as linalool, methyl eugenol, estragole, citronellal, inhibit GABA-gated chloride channels by binding at the receptor site and increase the chloride anion influx into the neurons, which lead to hyper-excitation of the central nervous system, convulsions, and finally death of insects [ 122 , 123 ].
Many plant oils possess ovicidal, larvicidal, pupaecidal and repellent activities against various mosquito species, some of which will be discussed below. Essential oils of plants from the Lamiaceae, Poaceae, Rutaceae and Myrtaceae families are well-known for repellent activity [ 103 ]. Essential oils obtained from citronella, lemon and eucalyptus are commercially available and recommended by the U.S. Environmental Protection Agency (US EPA) as repellent ingredients for application on the skin because of their low toxicity. For example, P -menthane-3,8 diol (PMD) is an active component of the lemon eucalyptus plant and responsible for the repellency in mosquitoes [ 124 ].
Most of the monoterpenes and sesquiterpenes of essential oils are known with repellent activities [ 125 ]. Among monoterpenes, α-pinene, γ-pinene, p -cymene, eugenol, limonene, thymol, terpinolene, citronellol, camphor and citronellal are responsible for mosquito repellency [ 126 , 127 ]. Representative molecules of sesquiterpenes are guaiol, α-bisabolol, α-cadinol, germacrene D, β-caryophyllene and nootkatone. β-caryophyllene is known to exhibit strong repellent activity against Aedes mosquitoes [ 126 ]. Repellent and larvicidal activities of monoterpenes from the essential oils of Thymus plant against Cx. pipiens pallens , Cx. quinquefasciatus , and Cx. pipiens biotype molestus have been determined [ 128 , 129 , 130 ]. Larvicidal activities of phenolic terpenes, such as thymol and carvacrol, of Satureja species were observed against Cx. pipiens biotype molestus [ 131 ]. Moreover, repellent and larvicidal activities of carvacrol were determined in the field trials against Ae. albopictus mosquitoes in Bologna (Italy) [ 132 ]. Cinnamomum osmophloeum and Carum copticum essential oils had larvicidal activity against Cx. quinquefasciatus and Cx. pipiens , respectively [ 107 , 133 ]. Toxicity of β-citronellol, geraniol and linalool from Pelargonium roseum essential oil was also detected in Cx. pipiens [ 134 ]. High larvicidal and pupaecidal activities of essential oils from Cinnamomum verum , Citrus aurantifolia , Cuminum cyminum , Syzygium aromaticum , Laurus nobilis , Lippia berlandieri and Pimpinella anisum were reported from Cx. quinquefasciatus [ 135 ]. Artemisia absinthium essential oils also showed toxic effects against larval populations of Aedes , Anopheles , and Culex mosquitoes [ 136 ]. Essential oils isolated from Tagetes lucida , Lippia alba , Lippia origanoides , Eucalyptus citriodora , Cymbopogon citratus , Cymbopogon flexuosus , Citrus sinensis , Swinglea glutinosa , and Cananga odorata plants showed larvicidal activities on Ae. aegypti larvae [ 137 ]. Oviposition deterrence and ovicidal activity of some of essential oils, peppermint oil, basil oil, rosemary oil, and citronella oil from Mentha piperita , Ocimum basilicum , Rosmarinus officinalis , Cymbopogon nardus and Apium graveolens were also reported in Ae. aegypti [ 138 ]. Manh et al. [ 139 ] also showed toxicity of essential oils from Eucalyptus and Cymbopogon aromatic plants to the larvae of Ae. aegypti . Essential oils also cause toxicity at different developmental stages and have repellent activities against adult Anopheles mosquitoes [ 140 ]. Essential oils extracted from Cymbopogon proximus , Lippia multiflora and Ocimum canum had larvicidal and ovicidal activities against An. gambiae and Ae. aegypti mosquitoes [ 141 ]. Besides monoterpenes and sesquiterpenes, phytol (a diterpene alcohol) and coumarin (an aromatic phenol) were both determined to be responsible for the biting deterrence effect in Ae. aegypti [ 142 ].
Repellent activity of essential oils is generally attributed to individual chemical compounds, but synergistic effects of plant metabolites have been observed when the effect of an active compound is enhanced by other major compounds or modulated by minor compounds. The efficacy of the major compounds is enhanced by minor compounds through different mechanisms, which may cause higher bioreactivity compared to isolated compounds of essential oils. The synergistic effect is also observed with mixture of oils. The synergistic action of the major compounds in essential oils results in higher repellent and larvicidal activity and toxicity to insects [ 140 , 143 , 144 , 145 ]. A combination of blends assayed on An. gambiae mosquitoes indicated that blends of oils showed higher repellency compared to the individual oil used [ 146 ]. It has been also reported that essential oils composed of a mixture of active components might reduce resistance in mosquito population by acting at different target sites or with a different mode of action [ 139 ].
Neem-based insecticides are extensively used for protection against various pests all over the world. Neem trees, Azadirachta indica , is a member of the Meliaceae family and are originated from India and distributed throughout all South- and Southeast-Asian countries, including Pakistan, Sri Lanka, Thailand, Malaysia, and Indonesia [ 147 ]. The main product of the neem is the oil extracted from the seeds and contains at least 100 active compounds, including azadirachtin, meliantriol, salannin, desacetyl salannin, nimbin, desacetyl nimbin, nimbidin and nimbolides [ 148 ]. Limonoids are the major active compound of the neem oil and act as an insect growth inhibitor. Azadirachtin is a triterpenoid and highly oxidized limonoid, one of the most potent active compounds of the neem extract and found in higher concentrations (0.2–0.6%) in the seeds of the neem compared to other parts of the neem tree [ 149 , 150 ]. Various isomers of azadirachtin (azadirachtin A to G) were identified and azadirachtin A and B isomers are the most abundant isomers in the plant tissues. In addition, azadirachtin A is the most active biological ingredient which shows insecticidal activity compared to the other analogs [ 151 , 152 , 153 ].
Generally, neem-based products are effective in the juvenile stages of insects. Azadirachtin is structurally similar to insect hormones known as ecdysones that are involved in the process of metamorphosis. The main mechanism of action of azadirachtin is to impair the homeostasis of insect hormones by interfering with the endocrine system. Azadirachtin acts as ecdysone blocker and causes severe growth and molting aberrations by affecting ecdysteroid and juvenile hormone titers [ 154 ]. The feeding deterrent activity of azadirachtin is mediated through azadirachtin’s interference with phagostimulants that are important in normal feeding behavior of mosquitos [ 155 ].
Neem-based biopesticides have a wide range of effects against insects, such as re-pellency, feeding deterrence, ovicidal activity, fecundity suppression, toxicity, insect growth regulation, deterrence of egg-laying, disruption of growth and reproduction, and inhibition of metamorphosis [ 156 , 157 , 158 , 159 , 160 ]. Larvicidal activity of the neem oil has been reported in controlling mosquito larvae in different breeding sites under natural field conditions [ 161 ]. Ayinde et al. [ 162 ] reported the repellent and larvicidal potential of the emulsified neem seed oil formulation as a suitable alternative for commercially available insecticides against An. gambiae in Nigeria. Oils of neem and karanj were also found to have larvicidal, ovicidal and oviposition deterrent activities against Ae. aegypti and Ae. albopictus mosquitoes [ 163 ]. The effects of the neem limonoids azadirachtin, salannin, deacetylgedunin, gedunin, 17-hydroxyazadiradione and deacetylnimbin were analyzed, and azadirachtin, salannin and deacetylgedunin showed the highest larvicidal activity against An. stephensi [ 164 ]. Larval mortality and repellent activity were also achieved from neem essential oils against An. gambiae [ 162 ]. A neem extract, neemarin, also showed significant mortality rates at larvae, pupae, and adult stages of Cx. quinquefasciatus and An. stephensi , where the former showed lower mortality rates [ 165 ].
5.3. Pyrethrum
Pyrethrum is a plant-based insecticide obtained from flower heads of Tanacetum cinerariifolium . Pyrethrum extract is composed of six active ingredients derived from esters of chrysanthemic acid: pyrethrin I, cinerin I, and jasmolin I, and esters of pyrethric acid: pyrethrin II, cinerin II, and jasmolin II [ 166 ]. They target the nervous system of insects and cause neurotoxic effects through blocking the voltage-gated sodium channels in nerve axons, thereby cause hyperactivity and convulsions by a rapid knockdown effect [ 167 ]. The mode of action of pyrethrins is similar to that of DDT and many synthetic organochlorine insecticides. Thus, pyrethrins can be alternatively used instead of organophosphates and organochlorides. While it is less toxic to mammals, it has higher toxicity to fish and aquatic invertebrates. When used together with a conventional synergist, such as piperonyl butoxide (PBO), their activity is increased and harmful effects to non-target organisms are reduced [ 168 ]. The usage of natural pyrethrins in mosquito control is supported with the finding that pyrethrum had knock-down effect, repellency, and blood-feeding inhibition in pyrethroid-resistant An. gambiae strains [ 169 ]. Electroantennogram responses of pyrethrum in Ae. aegypti and An. gambiae mosquitoes were detected while no response is observed in maxillary palps, indicating that the repellency effect of pyrethrum is mediated by the olfactory systems of mosquitoes [ 170 ]. Moreover, the molecular mechanism of pyrethrum repellency was investigated and a synergistic mechanism involving dual activation of olfactory repellency pathways and voltage-gated sodium channels has been determined [ 170 ].
5.4. Alkaloids
Alkaloids are nitrogen-containing natural products found in bacteria, fungi, animals, and plants. They are commonly isolated from plants and found in large quantities in many members of the Berberidaceae, Fabaceae, Solanaceae, and Ranunculaceae families. The alkaloids obtained from these plants are used extensively in conventional insect repellents [ 171 , 172 , 173 ]. The mode of action of alkaloids varies depending on the type of alkaloids and interferes with major cellular and physiological functions by affecting AChE receptors in the nervous system, regulating hormonal activity, and causing toxicity [ 174 ]. Alkaloids are not volatile like essential oils. However, they could be used as repellents against mosquitoes by burning plants to generate an insecticidal smoke that repels insects and directly causes toxicity [ 124 ]. In Ae. aegypti , the inhibitory effect of natural alkaloids on AChE activity was determined by using molecular docking studies. Among the 25 different alkaloids tested, alpha-solanine has been found to fit into the AChE1 binding pocket and potentially be the best inhibitor of AChE1 [ 175 ].
Extracts of the castor bean ( Ricinus communis , Euphorbiaceae) contain the alkaloid ricinine and have a strong insecticidal effect. It showed strong larvicidal activity against larvae of An. arabiensis [ 176 ]. Additionally, pyridine alkaloid from R. communis showed bioactivity against An. gambiae larvae and adults [ 177 ]. The larvicidal activity of alkaloids against Ae. albopictus , Cx. pipiens pallens and Ae. aegypti has also been determined [ 178 , 179 ]. Alkaloid from Arachis hypogaea plant also had larvicidal toxicity against An. stephensi and Ae. aegypti mosquitoes [ 180 ].
Nicotine is an alkaloid derived from tobacco plant ( Nicotiana tobacco ) that mostly consists of phenolic compounds, such as nicotine and diterpene. Nicotine, nornicotine and anabasine mimic the neurotransmitter acetylcholine, which causes symptoms similar to organophosphate or carbamate insecticides [ 160 ]. Extracts of tobacco leaves were mixed with bio-oil and high repellent activity was observed against Ae. aegypti [ 181 ]. Furthermore, nicotine has been found to be the most dominant compound among the other active compounds of the repellent mixture, including nicotine, d-limonene, indole, and pyridine. In addition, the repellent compound was harmless to human skin as confirmed by sensitivity tests on volunteers.
5.5. Other Plant Compounds
Besides the most common plant-based bioinsecticides mentioned above, there are other natural plant metabolites that show insecticidal properties. Among them, flavonoids elicit larvicidal activity by inhibiting AChE in mosquito larvae [ 182 ]. They could also act as respiratory inhibitors and result in the disturbance of the larval respiratory system. Alkaloids have multiple effects including inhibition of the AChE enzyme, degradation of cell membranes, and they may act as stomach poisons [ 182 ]. It has been shown that flavonoid and alkaloid components of bangle rhizome extract from Zingiber montanum act differently against Ae. aegypti [ 183 ]. Flavonoids from Derris trifoliata extract also exhibited larvicidal activity against Ae. aegypti [ 184 ]. Rotenone is an isoflavonoid extracted from roots and stems of Derris ( Derris elliptica , Derris involute ), Lonchocarpus ( Lonchocarpus utilis , Lonchocarpus urucu ) and Tephrosia virginiana [ 160 ]. It has long been used as a biopesticide due to less harmful effects to the environment. Rotenone has the potential to be used as a larvicide to control mosquitoes and interferes with the cellular respiration system of insects and prevents energy production [ 185 ].
6. Assessment of Plant-Based Bioinsecticide Efficacy in Mosquito Control
It is important that inherent activity of candidate bioinsecticides should be assessed before they can be effectively used against mosquito populations. The World Health Organization has established methods to screen the efficacy and field application acceptability of new compounds as potential mosquito larvicides and adulticides (for IRS and ITNs); they are laboratory studies, small-scale and large-scale field trials [ 186 , 187 , 188 ]. Laboratory studies focus on determination of biopotency, efficacy, residual activity, irritant or repellent properties, diagnostic concentration, and possible cross-resistance of candidate larvicides or adulticides. In laboratory bioassays, mosquito larvae are exposed to various concentrations of larvicides, and a mortality rate based on lethal concentration (LC) of the larvicide for 50% and 90% mortality (LC50 and LC90) or for 50% and 90% inhibition of adult emergence (IE50 and IE90) is recorded. LC values are determined and can then be compared with the LC50 or LC90 values of other insecticides to assess the activity of the compound as “sufficiently effective”. For adulticides, LC is determined by tarsal contact to treated papers. The “time to first take-off” (FT) for the 50% and 90% of the mosquitoes to take off (FT50 and FT90) after exposure to treated substrates are measured to determine the irritant or repellent activity of an adulticide. Insecticide-treated nets are used for bioassays of adult mosquitoes to determine the efficacy and residual activity of different dosages of the candidate compounds. Moreover, efficacy and wash-resistance of ITNs against susceptible mosquito species should be determined using standard WHO cone bioassays or tunnel tests [ 188 ]. The efficacy criteria for cone bioassays are ≥80% mortality or ≥95% knock-down, and for the tunnel test, it is ≥80% mortality or ≥90% blood-feeding inhibition. Candidate larvicides and adulticides are also tested against multi-resistant mosquito strains and a susceptible reference strain to assess the cross-resistance and, if detected, biochemical, immunological, and molecular methods are used to determine the mechanism of resistance [ 189 ].
Once candidate compounds are selected from laboratory tests, they are subjected to small-scale field testing in natural breeding sites (such as drains sewage water tanks, ponds, rice plots, etc.) or under simulated field conditions (artificial containers filled with water, experimental huts). Larvicidal efficacy is determined by the level of inhibition of emergence of adults and the percentage reduction in larval and pupal densities, while adulticidal efficacy can be assessed in terms of mortality, residual effect, deterrence, blood-feeding inhibition and induced exophily. These trials elucidate efficacy of candidate compounds against different mosquito species in different breeding sites, determine optimum field application dosage of the compound and possible impact on the mosquito behavior. Abiotic parameters that may influence the efficacy of the product and effect on non-target organisms can also be observed. Those larvicides and adulticides that show promise in small-scale field trials should be validated in larger-scale field trials against natural mosquito populations in natural breeding habitats using optimum field dosages. At this stage, the storage, handling, and application of the insecticide formulation should be considered for proper functioning of application and dispersal of the bioinsecticide in natural ecosystems.
There are also potential limitations to the efficacy of bioinsecticides, such as environmental conditions, mosquito fitness, mosquito resistance as well as the parts of the plants used, solvents used in extraction steps, insecticide dose and exposure time [ 190 , 191 ]. These effects should be considered for successful assessment of novel bioinsecticides in mosquito control. While efficacy tests provide promising information on possible mosquitocidal effects, new compounds from plant origin, the identification of actual active ingredient for efficacy and their mode of action are still waiting to be resolved.
7. Effective Use of Plant-Based Bioinsecticides in Resistant Mosquito Populations
Most of the bioinsecticides are now effective alternatives to chemical insecticides and have become an integral part of the integrated mosquito management (IMM) programs because the development of resistance to bioinsecticides is low due to their multiple mode of actions [ 192 , 193 ]. The synergic mixture of the active compounds in plant extracts also minimizes resistance development [ 167 ]. However, resistance already developed to extensively used chemical insecticides is a major problem that limits the success rate of novel bioinsecticides against mosquito populations. Insecticide resistance should be reduced or reverted (which takes time) in order to apply new and effective bioinsecticides in resistant populations. Surveillance of mosquito resistance and effective resistance management strategies should be routinely conducted to determine the levels, mechanisms, and geographic distribution of resistance in field populations of mosquitoes for increasing efficacy of bioinsecticides [ 44 ]. Moreover, proper application technologies should be considered as they greatly influence the bioinsecticide efficacy.
Surveillance of resistance development to many different insecticides are determined by dose-mortality bioassays, the World Health Organization tube testing, and Centers for Disease Control and Prevention (CDC) bottle bioassay for mosquitoes [ 11 , 44 , 194 , 195 ]. In the dose-mortality assay, the resistance ratio (RR) is determined in a susceptible population to monitor changes in resistance over time. The RR is calculated from LC50 values of the field and susceptible populations, in which an RR lower than five indicates susceptibility or low resistance and an RR value higher than ten indicates high resistance. In the WHO tube testing, the insecticide susceptibility status of the selected mosquitoes is evaluated through susceptibility tests measuring the mortality rate twenty-four hour after exposure [ 44 ]. A mortality rate lower than 98% indicates occurrence of resistance and should be confirmed with biochemical and molecular analysis. A mortality rate less than 90% confirms the existence of resistant genes in the tested mosquito populations. The CDC bottle bioassay is a measure of insecticide effectiveness, where diagnostic doses (DDs) and diagnostic times (DTs) are determined for candidate compounds using susceptible mosquitoes prior to testing in field mosquito populations. The DD is a measure of insecticide dose that kills 100% of susceptible mosquitoes within a certain period of time (DT). A mortality rate lower than 97% is an indication of resistance that needs to be confirmed, and below 80% suggests strong resistance at the recommended DT. The DD and DT values for some active ingredients are available for Anopheles and Aedes mosquito populations and these parameters should be defined for a particular insecticide and mosquito population [ 195 ].
It is evident that no single strategy is effective enough to solve insecticide resistance of mosquitoes. According to the WHO [ 44 ], one strategy to prevent the resistance problem is rotational usage of different classes of bioinsecticides with different modes of action. There are several new plant-based larvicides with different modes of action (discussed in Section 5 ) and they could be good alternatives for mosquito control in larval stages. Additionally, multiple interventions that affect different stages of mosquitoes (such as larvae and adults) can be used together to manage insecticide resistance. It is also suggested that different classes of insecticides with different modes of action can be used in neighboring geographic locations. To successfully implement these strategies, knowledge of the mode of action of the novel bioinsecticide is essential. The resistance mechanism developed by the local population of mosquitoes should also be determined to reduce cross-resistance effects.
RNA interference (RNAi) mediated loss-of-function technique has been proposed for pest management programs [ 196 , 197 ] and to study insecticide resistance [ 198 ]. Genes responsible for resistance development in insects (e.g., genes for DDT or pyrethroid resistance) can be identified and used as a target for the development of novel RNAi based insecticides. Several delivery methods including nonmicrobial and microbial are used routinely to induce RNAi in mosquito larvae [ 199 ]. Nonmicrobial delivery methods consist of soaking, injection, nanoparticles and dehydration and rehydration. Although soaking and injection methods are used as excellent tools in RNAi research, they have no application in the field. Soaking, injection methods and nanoparticles have been effectively used to introduce dsRNA into first-instar Ae. aegypti larvae [ 200 ] and fourth instars of Ae. aegypti [ 201 ]. In mosquitoes, a chitosan/dsRNA-based nanoparticle has also been used in feeding the larvae of An. gambiae mosquitoes which led to successful gene silencing of two chitin synthase genes and increased susceptibilities to DTT [ 202 ]. Such an RNAi-based bioinsecticide can be potentially used as an effective strategy to enhance the efficacy of new bioinsecticides for mosquito control.
Another technology used for the manipulation of insect behavior is “Specialized Pheromone and Lure Application Technology (SPLAT)”. SPLAT is a chemical controlled-release emulsion technology, and it has been used as an alternative management strategy to target the aquatic life stages of mosquitoes [ 203 ]. SPLAT emulsions can be formulated by using a variety of compounds, such as sex pheromones, attractants, repellents, phagostimulants and insecticides. SPLAT consists of both aqueous and non-aqueous components. The aqueous component of the SPLAT emulsion is involved in the liquid property of the product and evaporates within 3 h upon application. The non-aqueous component of the emulsion is the controlled-release device that releases active ingredients (e.g., semio-chemical or pesticides) at a controlled rate for 2 weeks to 6 months by protecting the active ingredients from environmental, chemical, and biological degradation. It has been reported that combination of attractant and larvicidal agents in a single formulation and biodegradable matrices causes significant increase in larval mosquito mortality, specifically Cx. quinquefasciatus , compared to formulations consisting of larvicidal agents alone in semi-field trials (e.g., large-screened greenhouse and emulating field conditions) [ 204 ]. The major benefits of this technology are a timely-manner release of both pheromone and insecticide, reduced insecticide resistance, and persistence in the field [ 203 ].
8. How to Improve Plant-Based Bioinsecticide Efficacy in Mosquito Control Strategies?
Synthetic chemicals used to control mosquitoes are now causing serious health problems and, more importantly, resistant mosquitoes that lead to search for more effective, healthier, safer, and eco-friendly natural solutions. Phytochemicals derived from plant resources are excellent targets to search for bioactive compounds because plants synthesize these chemicals naturally in response to their environment (such as against insect predators and microbial attacks), thus, plants are indeed natural insecticide sources. While searching the literature for plant-based compounds, we have encountered a tremendous number of efforts to identify and evaluate compounds that could have potential mosquitocidal activity with negative impact on mosquito physiology at different development stages. Since phytochemicals have multiple modes of action and exert their effects on multiple target sites in insects, their efficacy can be enhanced when used as a blend (e.g., mixture of oils) against mosquitoes. In addition, insects are more likely to develop resistance to a single chemical compound rather than a mixture of compounds. Thus, a combinatorial usage of phytochemicals would limit development of resistance in mosquitoes. Phytochemicals have short residual half-life which could be advantageous when synergistically used together with other biological control agents [ 205 ]. It is encouraging that these features of phytochemicals make them alternative natural solutions for the development of suitable products to interfere with the mosquito–host interaction and reduce disease transmission.
Among the phytochemicals, essential oils are extensively studied and their repellent activities against mosquitoes makes them favorable natural chemicals. However, they are volatile compounds, and this causes issues in their long-term applications in mosquito control. In recent years, new technologies, such as microencapsulation and nanoemulsion, have been used to overcome this problem by enhancing the duration and efficacy of essential oils [ 140 ]. Since ITNs are one of the major intervention methods to control mosquitoes, the incorporation of plant-based insect repellents in fabrics seems a prompt and alternative way to provide safer protection against mosquito bites. Fabrics treated with microencapsulated citronella essential oil have been reported to provide higher repellent activity and longer lasting protection, up to three weeks, against insects compared to the fabrics sprayed with ethanol solution of the essential oil [ 206 ]. Grancaric et al. [ 207 ] also reported that microencapsulated immortelle oil had the highest repellent efficacy against Ae. aegypti compared to immortelle oil alone on cotton samples. In another study, microcapsules composed of two biopesticides, namely citronella essential oil and citriodiol, were prepared and applied to cotton textiles using a variety of techniques. As a result, citriodiol-treated cotton fabrics had a prolonged durability, and 100% repellent activity for more than 30 days after its application [ 208 ]. Additionally, encapsulation of citronella oil into microcapsules of poly ε-caprolactone has been considered as an effective and sustained release system with potential application in protection against mosquitoes [ 209 ]. Encapsulated citronella oil nanoemulsions prepared by high pressure homogenization at varying amounts of surfactant and glycerol were tested for mosquito repellency. It has been shown that increasing concentration of glycerol and surfactant improved the stability of the emulsion causing prolonged mosquito protection [ 210 ]. These results clearly indicate that through microencapsulation and nanoemulsion formulation technologies, effective and longer usage of essential oils on cotton fabrics or ITNs can be achieved.
Neem-based insecticides can also be effectively used for the control of mosquitoes. They are considered more eco-friendly than synthetic insecticides and are less prone to induce resistance because of their multiple modes of action on insects. Another advantage of neem oil formulations is that it causes mortality at relatively low concentrations making them potential alternatives to synthetic insecticides in the control of malaria vectors. Microencapsulation of neem seed oil and karanja oil has been used for the control of larvae of Ae. aegypti [ 211 ]. The major drawback of using neem oil is that its dosage should be considered when applied in the field because neem can cause risks to non-target organisms at higher doses.
Natural pyrethrins are now considered as a potential alternative to synthetic DTT and can overcome hazardous effects of pyrethroids. However, they have major drawbacks which include their high instability and quick degradation in the presence of sunlight. Stability concerns and short duration of their knockdown effect cause inadequate field applications against mosquito populations during the day [ 212 ]. However, the application of pyrethrin-based insecticides after sunset against Culex and Anopheles has shown a decrease in mosquito populations and protection against non-target insects [ 213 ]. Pyrethrins are also more effective when used with a synergist. They can be easily degraded before having an impact on mosquitoes, thus should be applied with a synergist of non-synthetic origin. Since pyrethrin-based chemicals are detected via mosquito olfactory organs and processed through olfactory signal transduction mechanisms, pyrethrin-based repellent molecules should be developed and implemented in order to interfere with the host-seeking behavior of mosquitoes for an effective reduction in disease transmission.
Despite our increasing knowledge on plant-based bioactive compounds and their multiple mode of actions on insects, a few of them, such as essential oil-based and neem-based insecticides, have been commercially available for pest management [ 205 ]. One of the reasons that causes their limited usage in the field is the formulation problem to overcome phytotoxic effects. The chemical composition of each compound should be formulated in such a way that it should be bioactive to target insects and non-toxic to non-target organisms. In addition, formulation of plant-based bioinsecticides should ensure that it can be produced in large quantities through biomass production of plants and administered in recommended dosages to minimize toxic effects, and biological activity can be maintained for longer shelf-life. As discussed above, microencapsulation and nanoemulsion technologies have benefits in solving formulation issues of phytochemicals. A new formulation in the form of tablets containing a lectin preparation showed mosquitocidal activity against different developmental stages of Ae. aegypti mosquitoes, and this formulation method is proposed as a new control strategy for Ae. aegypti populations [ 214 ]. Phytochemicals break down rapidly and this causes a need for continuous and more frequent applications in the field for a satisfactory impact on mosquito control. Further studies are needed with the implementation of new methods for the development of effective bioinsecticides from other plant-based bioactive compounds.
9. Conclusions
Mosquitoes are important vectors of devastating diseases, and their hazardous effects are far beyond eradication. The occurrence/reoccurrence of mosquitoes in endemic, non-endemic, and new regions of the world has led to extensive use of synthetic chemicals to control transmission of mosquito-borne diseases. With the increase of resistant mosquitoes and toxicity issues to target and non-target organisms, safer, biodegradable, target-specific alternatives have been considered to replace conventional mosquito control strategies. Phytochemicals have gained importance to overcome mosquito control problems as being considered natural, environmentally safe, less toxic, inexpensive, and, more importantly, less prone to mosquito resistance. Variety of plant extracts have been reported to have mosquitocidal or repellent activity against mosquito vectors, mostly depending on laboratory assays, but there are limitations for their efficacy and applicability in the field. Problems associated with their formulation and commercialization, non-standardization in evaluation of their bioactivities, and their persistence for longer durations should be resolved for development of effective and sustainable methods for their usage. There is no doubt that there are more bioactive compounds that require exploring, and future research should focus on searching for plant-based products with the ultimate goal of deploying them as a reliable remedy to control mosquito populations and mosquito-borne diseases.
Acknowledgments
The authors thank the editorial board of Insects and the reviewers for comments and suggestions.
Author Contributions
Conceptualization, M.Ş.Ş.D.; original draft preparation, M.Ş.Ş.D. and E.C.; review and editing, M.Ş.Ş.D. and E.C. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Vector-borne diseases account for more than 17% of all infectious diseases, causing more than 700,000 deaths annually . More than 80% of the global population is at risk of vector-borne diseases, with mosquito-borne diseases (MBDs) being the largest contributor . MBDs such as dengue, Zika, chikungunya, West Nile virus (WNV), eastern equine ...
The issue will highlight mosquito control technologies at varying stages of development and includes both opinion pieces and research articles with laboratory and field-based data on control strategy development. This article is part of the theme issue 'Novel control strategies for mosquito-borne diseases'.
1. Introduction. Vector-borne diseases (VBDs) cause significant morbidity and mortality worldwide, accounting for as much as 17% of the global infectious disease burden (Organization, W.H, 2017a).Over one billion people are infected with VBDs annually and more than one million die from those infections each year (Organization, W.H, 2014).Of all the known VBDs, mosquito-borne infectious ...
The issue will highlight mosquito control technologies at varying stages of development and includes both opinion pieces and research articles with laboratory and field-based data on control strategy development. This article is part of the theme issue 'Novel control strategies for mosquito-borne diseases'. Keywords: mosquito; novel approach ...
Combating mosquito-borne diseases using genetic control ...
Mosquitoes act as vectors of pathogens that cause most life-threatening diseases, such as malaria, Dengue, Chikungunya, Yellow fever, Zika, West Nile, Lymphatic filariasis, etc. To reduce the transmission of these mosquito-borne diseases in humans, several chemical, biological, mechanical, and pharmaceutical methods of control are used. However, these different strategies are facing important ...
Knowledge Gaps in Mosquito Biology and the Need for Multiscale, Multidisciplinary Perspectives. Mosquito-borne diseases claim millions of human lives annually, and their prevention and eradication have been a major priority in the global public health agenda over the last two decades [1].In a bid to identify solutions for the health risks posed by mosquitoes, research across scales ranging ...
More than 80% of the global population is at risk of a vector-borne disease, with mosquito-borne diseases being the largest contributor to human vector-borne disease burden. Although many global processes, such as land-use and socioeconomic change, are thought to affect mosquito-borne disease dynamics, research to date has strongly focused on the role of climate change. Here, we show, through ...
This Research Topic, " Emerging Mosquito-Borne Diseases and Novel Biocontrol Strategies ", focuses on current and sound research addressing one or more of the abovementioned biocontrol strategies, related genomic surveillance, evolutionary genomics of mosquito species, and insecticide resistance. The Research Topic brings a collection of ...
Projecting the risk of mosquito-borne diseases in a warmer ...
Mosquito-borne diseases have become more common as previously geographically isolated diseases have spread globally. Chikungunya, dengue, Japanese encephalitis, malaria, West Nile, yellow fever, and Zika are a few of the common and emerging viral diseases spread by mosquitoes. A thorough patient history, physical, and knowledge of diagnostic ...
Author summary Mosquito control interventions are widely used to reduce mosquito-borne diseases, but it is unclear what combination of interventions are most effective in reducing human disease. Given the wide range of mosquito species and the diseases they transmit, different interventions strategies have been implemented across many regions globally, with varying degrees of success. This ...
Patterns of human movement and employment, water management, and buildings and local-area infrastructure are all parts of this complex urban system that contribute to mosquito-borne disease as an ...
Mosquito control interventions are widely used to reduce mosquito-borne diseases, but it is unclear what combination of interventions are most effective in reducing human disease. Given the wide range of mosquito species and the diseases they transmit, different interventions strategies have been implemented across many regions globally, with ...
The expansion of mosquito-borne diseases such as dengue, yellow fever, and chikungunya in the past 15 years has ignited the need for active surveillance of common and neglected mosquito-borne infectious diseases. The surveillance should be designed to detect diseases and to provide relevant field-based data for developing and implementing effective control measures to prevent outbreaks before ...
For more than two decades, extensive research has been conducted into the use of EO data as a tool to inform responses to mosquito-borne diseases (Hay et al., 1998a; Kalluri et al., 2007; Kotchi et al., 2019).Main objectives include identifying risk areas at various spatial scales (Rogers et al., 2002), identifying seasonality in risk in different locations (Hay et al., 1998b), and forecasting ...
Although. Mosquito-borne diseases are distributed by different mosquito types that cause malaria, dengue, chikungunya and Zika virus. Viruses and protozoan parasites mainly cause essential vector ...
Trends in mosquito species distribution modeling: insights for ...
2. Resurgence of Diseases Transmitted by Mosquitoes. The three main mosquito genera, Anopheles, Aedes, and Culex, transmit the causative agents of numerous important diseases to humans as well as animals [11,12,13,14].In this chapter, we briefly describe the resurgence of essential disease agents transmitted by mosquitoes and their impact on humans and animals.
Culicidae) and Mosquito-Borne Diseases in North Africa
Each year, diseases resulting from a mosquito bite cause at least 1 million deaths worldwide, according to estimates. Fortunately, not all types of mosquito-borne illnesses are endemic in the U.S ...
Author summary Dengue is a rapidly spreading mosquito-borne disease transmitted primarily by Aedes aegypti mosquitoes. As climate change leads to extremes in rainfall and temperature, the abundance and populations of these vectors will be affected, thus influencing transmission of dengue. Using satellite-derived climate data for Kenya, we classified months that experienced highly abnormal ...
Experts say that infection rates in Canada for mosquito-borne diseases are extremely low, but evolving environmental factors — including climate change — are changing the way that mosquitoes ...
This Research Topic, " Emerging Mosquito-Borne Diseases and Novel Biocontrol Strategies ", focuses on current and sound research addressing one or more of the abovementioned biocontrol strategies, related genomic surveillance, evolutionary genomics of mosquito species, and insecticide resistance. The Research Topic brings a collection of ...
The World Mosquito Program estimates that mosquito-borne diseases kill one million people and infect up to 700 million people annually. As the environment continues to deteriorate, climate change and altered weather patterns have lengthened mosquito season and expanded the geographical range of these infectious creatures. According to the CDC, the primary diseases spread to humans by mosquitos ...
There's an outbreak of the rare mosquito-borne disease Eastern Equine Encephalitis in the Northeast. ... How climate change is affecting the spread of mosquito-borne illnesses There's an outbreak ...
Plant-Based Bioinsecticides for Mosquito Control