Malaria Vaccines: Recent Advances and New Horizons
Affiliations.
- 1 The Jenner Institute, University of Oxford, Old Road Campus Research Building, Oxford, OX3 7DQ, UK. Electronic address: [email protected].
- 2 Center for Infectious Disease Research, 307 Westlake Ave N., Seattle, WA 98109, USA.
- 3 PATH's Malaria Vaccine Initiative (MVI), 455 Massachusetts Avenue NW, Suite 1000, Washington, DC 20001-2621, USA.
- 4 The Jenner Institute, University of Oxford, Old Road Campus Research Building, Oxford, OX3 7DQ, UK.
- 5 Malaria Programme, Wellcome Sanger Institute, Cambridge, CB10 1SA, UK.
- 6 Department of Biochemistry, University of Oxford, South Parks Road, Oxford, OX1 3QU, UK.
- 7 Laboratory of Malaria and Vector Research, NIAID/NIH, Rockville, MD 20852, USA.
- 8 Vaccine Research Center, NIAID/NIH, Bethesda, MD 20892, USA.
- PMID: 30001524
- PMCID: PMC6054918
- DOI: 10.1016/j.chom.2018.06.008
The development of highly effective and durable vaccines against the human malaria parasites Plasmodium falciparum and P. vivax remains a key priority. Decades of endeavor have taught that achieving this goal will be challenging; however, recent innovation in malaria vaccine research and a diverse pipeline of novel vaccine candidates for clinical assessment provides optimism. With first-generation pre-erythrocytic vaccines aiming for licensure in the coming years, it is important to reflect on how next-generation approaches can improve on their success. Here we review the latest vaccine approaches that seek to prevent malaria infection, disease, and transmission and highlight some of the major underlying immunological and molecular mechanisms of protection. The synthesis of rational antigen selection, immunogen design, and immunization strategies to induce quantitatively and qualitatively improved immune effector mechanisms offers promise for achieving sustained high-level protection.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.

Publication types
- Research Support, N.I.H., Intramural
- Research Support, Non-U.S. Gov't
- Antigens, Protozoan / immunology*
- Disease Models, Animal
- Immunization
- Malaria Vaccines / immunology*
- Malaria, Falciparum / parasitology
- Malaria, Falciparum / prevention & control*
- Malaria, Falciparum / therapy
- Malaria, Falciparum / transmission
- Malaria, Vivax / parasitology
- Malaria, Vivax / prevention & control*
- Malaria, Vivax / therapy
- Malaria, Vivax / transmission
- Plasmodium falciparum / immunology*
- Plasmodium vivax / immunology*
- Sporozoites / immunology
- Vaccines, Subunit / immunology
- Antigens, Protozoan
- Malaria Vaccines
- Vaccines, Subunit
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- 209200/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- Open access
- Published: 23 November 2020
Building momentum for malaria vaccine research and development: key considerations
- Chetan E. Chitnis 1 ,
- David Schellenberg ORCID: orcid.org/0000-0001-8222-0186 2 ,
- Johan Vekemans 2 ,
- Edwin J. Asturias 3 ,
- Philip Bejon 4 ,
- Katharine A. Collins 5 ,
- Brendan S. Crabb 6 ,
- Socrates Herrera 7 ,
- Miriam Laufer 8 ,
- N. Regina Rabinovich 9 , 10 ,
- Meta Roestenberg 11 ,
- Adelaide Shearley 12 ,
- Halidou Tinto 13 ,
- Marian Wentworth 14 ,
- Kate O’Brien 2 &
- Pedro Alonso 2
Malaria Journal volume 19 , Article number: 421 ( 2020 ) Cite this article
4 Citations
4 Altmetric
Metrics details
To maintain momentum towards improved malaria control and elimination, a vaccine would be a key addition to the intervention toolkit. Two approaches are recommended: (1) promote the development and short to medium term deployment of first generation vaccine candidates and (2) support innovation and discovery to identify and develop highly effective, long-lasting and affordable next generation malaria vaccines.
In what is a truly great public health success story, expanded efforts to control and eliminate malaria have effectively halved malaria incidence and mortality since 2000. Several million lives have been saved in that time and a number of previously endemic countries in Asia, South and Central America and Africa have been formally declared malaria free.
This astonishing success has been achieved with a limited toolkit, largely comprising methods to prevent transmission by the mosquito vector through the use of insecticide-treated bed nets and indoor residual spraying, the use of chemoprevention in specific, vulnerable groups, and effective chemotherapy following rapid point-of-care diagnosis. Current vector control and effective anti-malarial treatment strategies represent significant success in both product development and implementation science.
However, progress in areas with high transmission has slowed and further reduction in malaria incidence and deaths has stalled in recent years. The 2018 and 2019, World Health Organization (WHO) World Malaria Reports documented a global increase in the number of malaria cases. Despite some countries achieving elimination, malaria increased in both the 10 most highly burdened countries and 11 of the 21 countries earmarked for elimination by 2020 [ 1 ].
A number of daunting realities impact on the potential for substantial further progress. These include: (1) malaria remains a staggeringly large human health problem with 1,200 malaria deaths every day, (2) longitudinal tracking of the effective implementation of existing tools show imperfect outcomes and suggests that existing tools may be insufficient to control malaria in high-transmission settings, no matter how well they are applied, (3) shifts in climate, population growth and movement, and changes in the location and species of vector, threaten to introduce malaria into new settings (for example, greater urban transmission in Africa by Anopheles stephensi ), (4) problems achieving high coverage of current interventions are exacerbated by the emergence of vectors resistant to insecticides, parasites resistant to first-line treatment and parasite strains that evade diagnosis, (5) lessons from the 1970s and our knowledge of parasite biology and ecology tell us that resurgence can be rapid and devastating if public health measures fail or are not maintained, and (6) the COVID-19 pandemic has exposed the vulnerability of global supply chains and the health systems in many malaria endemic settings. Hard won gains can rapidly be lost.
New interventions are needed to reignite the fight against malaria. As for other infectious diseases, vaccines have the potential to impact burden in a cost-effective way and may, in the long term, contribute to the goal of malaria eradication. The feasibility of vaccine-induced protection against malaria has been demonstrated [ 2 ], but the development of malaria vaccines requires the vigorous and sustained engagement of many stakeholders. Recent advances in the understanding of malaria parasite biology, vaccinology and passive immunization approaches, suggest that the next advance in malaria vaccines is within reach─but only with sustained research and development efforts.
The WHO reconvened the Malaria Vaccine Advisory Committee (MALVAC) in 2019, and organized a stakeholder consultation about the state-of-the-art in malaria vaccine development [Vekemans et al. pers. commun.]. MALVAC’s mandate is to provide guidance on research priorities for the development of new malaria vaccines. Detailed WHO perspectives on the medical need and research priorities in malaria vaccine R&D will emerge over the next 12–24 months, but consultations and MALVAC discussions led to the recognition of the need to advance in parallel two distinct strategies:
To support continued engagement to ensure the availability of 1st and 2nd generation vaccine candidates with moderate efficacy, that show potential for widespread use in the next 3–10 years.
To support innovation and stimulate the discovery of next generation, highly protective and long-lasting malaria vaccines; for this to succeed, identifying efficient and cost-effective clinical development, financing and regulatory pathways will be key. Lessons can no doubt be learnt from the accelerated development pathways and approaches being developed for COVID-19 vaccines.
1st Generation Vaccines with partial protection—an important addition to the intervention toolkit
The most advanced malaria vaccine is RTS,S/AS01, developed by Glaxo Smith Kline with support from the Bill and Melinda Gates Foundation, the Walter Reed Army Institute of Research and PATH, and the collaboration of a large number of African and other international research institutions. RTS,S/AS01 targets Plasmodium falciparum sporozoites and demonstrated an efficacy of 39% over 4 years against malaria incidence in Phase III trials in African children aged 5–17 months at the time of dose 1 [ 3 ]. This moderate efficacy, documented in the context of high mosquito net use and similar to the level of protection afforded by well-implemented vector control, is potentially valuable to complement existing strategies for the reduction of malaria disease and death among young children in endemic areas. RTS,S/AS01 pilot implementation is ongoing in three malaria endemic countries—Ghana, Malawi and Kenya [ 4 ]. In addition to consolidating the vaccine’s safety profile, the pilot implementation will generate data on its survival impact and test the feasibility of delivering the four-dose RTS,S/AS01 regimen under routine conditions. Results of the implementation studies are keenly awaited and will be used to guide policy recommendations on the roll out of RTS,S/AS01 in malaria endemic countries.
RTS,S/AS01 has demonstrated the feasibility of developing a malaria vaccine and has laid down a clinical development path for future vaccines. Its use in programmatic contexts will inform our understanding of the potential value of malaria vaccines in combination with other tools for malaria control and elimination.
In addition to RTS,S/AS01, R21/Matrix-M, an RTS,S-like vaccine, is one of several potential second generation vaccines and is currently being tested for efficacy in the field. Notwithstanding enormous technical and practical challenges, the radiation-attenuated sporozoite vaccine, PfSPZ, has undergone extensive testing including in endemic African countries. Although high efficacy has been demonstrated in adults under experimental challenge conditions, efficacy in naturally exposed children is considerably lower, warranting further improvements. Progress is also being made through the evaluation of Rh5, a promising P. falciparum blood stage vaccine candidate, although it will be necessary to achieve higher rates of growth inhibition for such vaccines to yield clinically relevant efficacy.
The evaluation of sexual-stage candidates continues, and new tools to test vaccines designed to interrupt man-to mosquito transmission are being developed. Subunit vaccines that combine multiple antigens from the pre-erythrocytic and blood stages could synergize immune responses and yield higher efficacy. The addition of sexual-stage antigens to these vaccines could potentially enhance their impact on malaria transmission [ 2 ]. Continued investment in the development of these approaches is warranted given the progress to date and the scale of their potential impact on public health. In addition to subunit vaccines, innovations in the development of whole organism attenuated sporozoite vaccines are needed to develop formulations and delivery strategies that facilitate programmatic implementation in endemic countries.
Future malaria vaccines–towards highly efficacious, long-lasting vaccines and a more streamlined development pathway
Malaria vaccines that confer long-term, robust protection, that are inexpensive and relatively simple to deploy, are not on the short-term horizon. To accelerate progress in the development of such vaccines, a deliberate strategic pivot to fundamental discovery science is needed. Breakthrough science, with possibly unconventional approaches, will be required to meet these ambitious goals [ 5 ].
Decoding of the malaria parasite genome together with functional studies using molecular genetic tools, whole genome approaches as well as classical biochemistry and cell biology, are helping unravel the complex biology of the malaria parasite. Advances in understanding how malaria parasites interact with the human host and its immune system should enable new strategies to target the parasite at different stages with novel vaccine approaches. Advances in our understanding of basic human immunology and powerful new tools that enable dissection of immune responses at a systems level need to be brought to bear on malaria. Other advances such as structural vaccinology can provide unique insights into the molecular basis of protective antibody responses that could lead to therapeutic or prophylactic monoclonal antibodies and inform optimization of vaccine antigens to achieve higher efficacy.
The development of vaccines against parasitic diseases is complex and difficult due to the long history of co-evolution of parasites with their hosts. Malaria vaccines are currently envisioned as complementary tools to be added to the core package of interventions. However, the progress made in understanding malaria parasite biology and pathogenesis, as well as both basic and technological advances in human immunology and vaccinology, means the time is right to attempt the development of malaria vaccines with high efficacy. It is time to deepen and expand our ambitions at all levels, basic and translational, to develop future malaria vaccines that are game changers in efforts to eliminate malaria and create a pathway for other parasitic diseases. A highly efficacious malaria vaccine remains an ambitious target, but with commitment of necessary resources, it is more within reach today than ever before.
Availability of data and materials
Not applicable.
Abbreviations
World Health Organization
Malaria Vaccine Advisory Committee
WHO. World Malaria Report, 2019. Geneva, World Health Organization, 2019. https://www.who.int/publications-detail/world-malaria-report-2019
Laurens MB. The promise of a malaria vaccine-are we closer? Annu Rev Microbiol. 2018;72:273–92.
Article CAS Google Scholar
Vandoolaeghe P, Schuerman L. The RTS, S/AS01 malaria vaccine in children 5 to 17 months of age at first vaccination. Exp Rev Vaccines. 2016;15:1481–93.
WHO. The Malaria Vaccine Implementation Programme. 2020 Geneva, World Health Organization, 2020 March. https://www.who.int/immunization/diseases/malaria/malaria_vaccine_implementation_programme/en/
The malERA Refresh Consultative Panel on Basic Science and Enabling Technologies. malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication. PLoS Med 2017;14:e1002451.
Download references
Acknowledgements
Chetan E. Chitnis, Edwin J. Asturias, Philip Bejon, Katharine A. Collins, Brendan S. Crabb, Socrates Herrera, Miriam Laufer, N. Regina Rabinovich, Meta Roestenberg, Adelaide Shearley, Halidou Tinto and Marian Wentworth Members of the Malaria Vaccine WHO Advisory Committee (MALVAC).
The opinions expressed herein are those of the authors and do not necessarily reflect the views and decisions of the World Health Organization.
WHO is supported financially by the Bill and Melinda Gates foundation for malaria vaccine development-related work. The funder played no role in the present manuscript.
Author information
Authors and affiliations.
Institut Pasteur, Paris, France
Chetan E. Chitnis
World Health Organization, Geneva, Switzerland
David Schellenberg, Johan Vekemans, Kate O’Brien & Pedro Alonso
University of Colorado School of Medicine and Colorado School of Public Health, Denver, USA
Edwin J. Asturias
KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya
Philip Bejon
Department of Medical Microbiology, Radboud University Medical Center, Nijmegen, The Netherlands
Katharine A. Collins
Burnet Institute, Melbourne, Australia
Brendan S. Crabb
Consorcio Para La Investigacion Cientifica, Cali, Colombia
Socrates Herrera
University of Maryland School of Medicine, Baltimore, USA
Miriam Laufer
IS Global, Barcelona, Spain
N. Regina Rabinovich
Harvard TH Chan School of Public Health, Boston, USA
Leiden University Medical Center, Leiden, The Netherlands
Meta Roestenberg
John Snow Inc, Research & Training Institute, Boston, USA
Adelaide Shearley
Institut de Recherche en Sciences de La Santé, Ouagadougou, Burkina Faso
Halidou Tinto
Management Sciences for Health, Arlington, USA
Marian Wentworth
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the writing, read and approved the final manuscript.
Corresponding author
Correspondence to David Schellenberg .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
KAC is a malaria vaccine patent holder. All authors report institutional funding for malaria or vaccine-related research activities.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chitnis, C.E., Schellenberg, D., Vekemans, J. et al. Building momentum for malaria vaccine research and development: key considerations. Malar J 19 , 421 (2020). https://doi.org/10.1186/s12936-020-03491-3
Download citation
Received : 31 August 2020
Accepted : 11 November 2020
Published : 23 November 2020
DOI : https://doi.org/10.1186/s12936-020-03491-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Elimination
- Research and development
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 November 2020
Malaria vaccine research & innovation: the intersection of IA2030 and zero malaria
- David C. Kaslow ORCID: orcid.org/0000-0003-3557-383X 1
npj Vaccines volume 5 , Article number: 109 ( 2020 ) Cite this article
4 Citations
5 Altmetric
Metrics details
- Infectious diseases
This collection of malaria vaccine research and innovation papers highlights the intersection of efforts to: (1) achieve the pan-African 1 and global goal 2 of “Zero Malaria”; and (2) make the compelling case for immunization 3 as a set of new tools for malaria control and elimination.
Problem and opportunity statement
Despite a ~30% reduction in clinical cases and ~60% reduction in lives lost from Plasmodium ssp . infections over the past two decades, malaria continues to relentlessly sap the well-being of an estimated 228 million people worldwide (95% confidence interval [CI]: 206–258 million) and result in the global demise of an estimated 405,000 persons annually. The effort, supported by a US$ 2.7–3.2 billion annual investment, to “bend the curve” towards zero malaria by 2030 has stalled over the past five years 4 . This plateau is reminiscent of the decades-long effort to break through the ~80% ceiling on reaching the goal of fully immunizing every child worldwide. Despite immunization of 116 million children annually, 20 million infants fail to receive a full course of essential vaccines and 13 million infants receive no vaccines whatsoever—these “zero dose” children are highlighted in the Immunization Agenda 2030 3 (IA2030), recently adopted by the World Health Assembly 5 . The shared state of stalled progress towards equity and coverage of effective malaria interventions and essential vaccines has a common solution—new tools.
The early promise of innovative vector control and single-dose radical cure for malaria have yet to deliver the impact needed to achieve “zero malaria”. That said, steadily over the last two decades, as reviewed by Duffy and Gorres, a pipeline of vaccine candidates against the most lethal human malaria, P. falciparum , and the most prevalent, P. vivax , has been assembled 6 . Novel vaccine platforms (e.g., P. berghei sporozoite-based human vaccine candidates 7 ), better defined correlates of protection in non-human primates 8 , and orthogonal learnings from other mosquito-transmitted pathogens (e.g., West Nile Virus) 9 portend an even more robust pipeline of needed new tools, if adequate investments are made.
P. falciparum vaccine development stage-by-stage and step-by-step
Malaria vaccine targets are typically assigned to one of three sequential stages of the parasite’s lifecycle: blood stage—through which the parasite causes all human pathology; sexual and sporogony stage—through which the parasite is transmitted from human host to female mosquito vector; and, pre-erythrocytic stage—through which the parasite infects humans during a subsequent bloodmeal by an infected mosquito (see Fig. 1, ref. 6 ). Despite decades of efforts directed against targets throughout the lifecycle, only one vaccine candidate, the pre-erythrocytic circumsporozoite (CSP)-based RTS,S/AS01 E , has advanced through licensure and pilot implementation 6 . A myriad of biological and technical barriers have been encountered; however, step-by-step, these barriers have been chipped away. Advances include: new insights into the evolution of the immune response to the major pre-erythrocytic target, CSP 10 , and into the role of complement-fixing antibodies in blood stage clinical immunity 11 ; the ability to express and manufacture full length versions of blood stage targets, e.g., the highly promising, highly conserved reticulocyte-binding protein homolog 5 (PfRH5) 12 , as well as the 196-kDa merozoite surface protein 1 (the primary structure first described by Holder, et al. in 1985 13 ), the latter of which demonstrated favorable safety and immunogenicity in a first-in human study 14 ; and, the delivery of target malaria parasite antigens by measles vectors to overcome the barrier of waning immune responses, resulting in durable memory and protection, at least in a murine model 15 .
To be clear, significant challenges remain—for example, immune interference when concomitantly administering other vaccines, such as BCG for tuberculosis 16 , or administering multi-stage targets by multiple vaccine platforms, as observed with an adjuvanted virus-like particle, RTS,S/AS01 B , and viral-vectors expressing the multiple-epitope thrombospondin-related adhesion protein (ME-TRAP) 17 . That said, molecular approaches to identify and direct immune responses to specific promising epitopes 18 and use of novel particle-forming lipid-based adjuvants 19 provide paths forward for poorly immunogenic targets, including those designed to interrupt transmission from human to mosquito.
Malaria vaccine use cases—pregnancy malaria must not be left behind
The longstanding mindset that vaccine evaluation during pregnancy should be delayed to post-licensure studies is obsolete. Even when pregnant women and their offspring do not have a higher risk of disease, there is an ethical rationale to evaluate at least vaccine safety pre-licensure 20 . Pregnancy malaria presents an even clearer case for prioritizing vaccine development as both mother and offspring have a well-described higher risk of disease 6 . More than two decades ago, antibodies to VAR2CSA, a member of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family, were associated with protection, identifying VAR2CSA as a promising vaccine target 21 . Placenta malaria vaccine (PMVs) research and innovation continues to progress 6 , including through the development of new animal models 22 and down-selection of lead PVM candidates 23 .
P. vivax —the other malaria
Despite being the most prevalent human malaria parasite, investments in P. vivax vaccine development have been significantly smaller than that in the more lethal, albeit similarly morbid, falciparum cousin. Many P. falciparum vaccine targets have homologs in P. vivax 6 , so a fast follow-on vivax malaria vaccines based on safe, effective, affordable falciparum malaria vaccines have a reasonable, high likelihood of success. That said, several distinct differences in P. vivax biology, such as liver stage hypnozoites and rapid development of sexual stages directly from liver schizonts, require a P. vivax -specific vaccine research and innovation strategy. A critical differential feature of blood-stage vivax parasites is the use of Duffy antigen receptor for chemokines (DARCs) on human reticulocytes as a major invasion pathway. Recent insights into the structural basis of anti- P. vivax Duffy Binding Domain (PvDBP) immunity 24 , 25 and early clinical results from a PvDBP vaccine candidate 26 are encouraging; however, given the parasite’s gene amplification mechanisms to evade anti-PvDBP immunity 27 , developing a blood stage vivax malaria vaccine won’t be trivial.
Research and Innovation as a driver for creating a compelling value proposition for malaria vaccines
The promising P. falciparum and P. vivax vaccine pipeline faces a significant resource shortfall as candidates head into late-stage development—this increasingly more apparent resource gap, or second “Valley of Death”, is also faced by most, if not all, late stage vaccine candidates for pathogens afflicting primarily those living in low resource settings 28 . Hopefully, the recent global adoption of the Research and Innovation pillar of IA2030 and the growing African continent-led commitment to zero malaria will lead to the investments needed to generate the evidence that support the compelling value proposition required to build bridges for promising malaria vaccine candidates to become affordable, effective, sustainable new tools—part of the solution to regain the prior trajectory towards zero malaria.
RBM. Zero Malaria Starts with Me (RBM & UNOPS, Geneva, 2018); https://zeromalaria.africa/wp-content/uploads/2018/06/Agenda-setting-EN-1.pdf .
RBM. RBM Partnership to End Malaria Annual Report 2019 (RBM & UNOPS, Geneva, 2020); https://endmalaria.org/sites/default/files/RBM%20Annual%20Report%202019.pdf .
WHO. Immunization Agenda 2030: A Global Strategy to Leave No One Behind (WHO, Geneva, 2020); http://www.who.int/immunization/immunization_agenda_2030/en/ .
WHO. World malaria report 2019 (WHO, Geneva, 2019); https://www.who.int/publications-detail-redirect/9789241565721 .
WHO. Seventy-third World Health Assembly WHA73(9) Agenda item 11.3: Immunization Agenda 2030 (WHO, Geneva, 2020); https://apps.who.int/gb/ebwha/pdf_files/WHA73/A73(9)-en.pdf .
Duffy, P. E. & Patrick Gorres, J. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines 5 , 48 (2020).
Article Google Scholar
Mendes, A. M. et al. A Plasmodium berghei sporozoite-based vaccination platform against human malaria. NPJ Vaccines 3 , 33 (2018).
Douglas, A. D. et al. A defined mechanistic correlate of protection against Plasmodium falciparum malaria in non-human primates. Nat. Commun. 10 , 1953 (2019).
Uraki, R., Hastings, A. K., Brackney, D. E., Armstrong, P. M. & Fikrig, E. AgBR1 antibodies delay lethal Aedes aegypti-borne West Nile virus infection in mice. NPJ Vaccines 4 , 23 (2019).
Murugan, R. et al. Evolution of protective human antibodies against Plasmodium falciparum circumsporozoite protein repeat motifs. Nat. Med. 26 , 1135–1145 (2020).
Article CAS Google Scholar
Reiling, L. et al. Targets of complement-fixing antibodies in protective immunity against malaria in children. Nat. Commun. 10 , 610 (2019).
Jin, J. et al. Production, quality control, stability, and potency of cGMP-produced Plasmodium falciparum RH5.1 protein vaccine expressed in Drosophila S2 cells. NPJ Vaccines 3 , 32 (2018).
Holder, A. A. et al. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature 317 , 270–273 (1985).
Blank, A. et al. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. NPJ Vaccines 5 , 10 (2020).
Mura, M. et al. Recombinant measles vaccine expressing malaria antigens induces long-term memory and protection in mice. NPJ Vaccines 4 , 12 (2019).
Walk, J. et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 10 , 874 (2019).
Rampling, T. et al. Safety and efficacy of novel malaria vaccine regimens of RTS,S/AS01B alone, or with concomitant ChAd63-MVA-vectored vaccines expressing ME-TRAP. NPJ Vaccines 3 , 49 (2018).
Canepa, G. E. et al. Antibody targeting of a specific region of Pfs47 blocks Plasmodium falciparum malaria transmission. NPJ Vaccines 3 , 26 (2018).
Huang, W.-C. et al. Antibody response of a particle-inducing, liposome vaccine adjuvant admixed with a Pfs230 fragment. NPJ Vaccines 5 , 23 (2020).
Krubiner, C. B. et al. Pregnant women & vaccines against emerging epidemic threats: ethics guidance for preparedness, research, and response. Vaccine S0264-410X , 30045–3 (2019).
Google Scholar
Fried, M., Nosten, F., Brockman, A., Brabin, B. J. & Duffy, P. E. Maternal antibodies block malaria. Nature 395 , 851–852 (1998).
Doritchamou, J., Teo, A., Fried, M. & Duffy, P. E. Malaria in pregnancy: the relevance of animal models for vaccine development. Lab Anim. 46 , 388–398 (2017).
Chêne, A. et al. Down-selection of the VAR2CSA DBL1-2 expressed in E. coli as a lead antigen for placental malaria vaccine development. NPJ Vaccines 3 , 28 (2018).
Rawlinson, T. A. et al. Structural basis for inhibition of Plasmodium vivax invasion by a broadly neutralizing vaccine-induced human antibody. Nat. Microbiol. 4 , 1497–1507 (2019).
Urusova, D. et al. Structural basis for neutralization of Plasmodium vivax by naturally acquired human antibodies that target DBP. Nat. Microbiol. 4 , 1486–1496 (2019).
Singh, K. et al. Malaria vaccine candidate based on Duffy-binding protein elicits strain transcending functional antibodies in a Phase I trial. NPJ Vaccines 3 , 48 (2018).
Popovici, J. et al. Amplification of Duffy binding protein-encoding gene allows Plasmodium vivax to evade host anti-DBP humoral immunity. Nat. Commun. 11 , 953 (2020).
Kaslow, D. C. et al. Vaccine candidates for poor nations are going to waste. Nature 564 , 337–339 (2018).
Download references
Acknowledgements
This work was supported by the Bill & Melinda Gates Foundation, Seattle, WA [OPP1180199]. The funder had no role in preparation of the manuscript or decision to publish.
Author information
Authors and affiliations.
PATH, 2201 Westlake Avenue, Suite 200, Seattle, WA, 98121, USA
David C. Kaslow
You can also search for this author in PubMed Google Scholar
Contributions
DCK conceived, wrote, reviewed, approved submission of, and is accountable for this paper.
Corresponding author
Correspondence to David C. Kaslow .
Ethics declarations
Competing interests.
DCK is an employee of PATH (a not-for-profit organization), has no financial interest in any for-profit organization, and declares no competing interests. PATH is funded to innovate and partner in developing and implementing malaria vaccines and other interventions to control and eliminate malaria.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kaslow, D.C. Malaria vaccine research & innovation: the intersection of IA2030 and zero malaria. npj Vaccines 5 , 109 (2020). https://doi.org/10.1038/s41541-020-00259-3
Download citation
Received : 24 October 2020
Accepted : 30 October 2020
Published : 20 November 2020
DOI : https://doi.org/10.1038/s41541-020-00259-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
Malaria vaccine efficacy, safety, and community perception in Africa: a scoping review of recent empirical studies
- Open access
- Published: 05 March 2024
Cite this article
You have full access to this open access article

- Muhammad Chutiyami 1 ,
- Priya Saravanakumar 1 ,
- Umar Muhammad Bello 2 ,
- Dauda Salihu 3 ,
- Khadijat Adeleye 4 ,
- Mustapha Adam Kolo 5 ,
- Kabiru Kasamu Dawa 6 ,
- Dathini Hamina 7 ,
- Pratibha Bhandari 1 ,
- Surajo Kamilu Sulaiman 8 &
- Jenny Sim 9 , 10
1 Altmetric
The review summarizes the recent empirical evidence on the efficacy, safety, and community perception of malaria vaccines in Africa.
Academic Search Complete, African Journals Online, CINAHL, Medline, PsychInfo, and two gray literature sources were searched in January 2023, and updated in June 2023. Relevant studies published from 2012 were included. Studies were screened, appraised, and synthesized in line with the review aim. Statistical results are presented as 95% Confidence Intervals and proportions/percentages.
Sixty-six ( N = 66) studies met the inclusion criteria. Of the vaccines identified, overall efficacy at 12 months was highest for the R21 vaccine ( N = 3) at 77.0%, compared to the RTS,S vaccine ( N = 15) at 55%. The efficacy of other vaccines was BK-SE36 (11.0–50.0%, N = 1), ChAd63/MVA ME-TRAP (− 4.7–19.4%, N = 2), FMP2.1/AS02A (7.6–9.9%, N = 1), GMZ2 (0.6–60.0%, N = 5), PfPZ (20.0–100.0%, N = 5), and PfSPZ-CVac (24.8–33.6%, N = 1). Injection site pain and fever were the most common adverse events ( N = 26), while febrile convulsion ( N = 8) was the most reported, vaccine-related Serious Adverse Event. Mixed perceptions of malaria vaccines were found in African communities ( N = 17); awareness was generally low, ranging from 11% in Tanzania to 60% in Nigeria ( N = 9), compared to willingness to accept the vaccines, which varied from 32.3% in Ethiopia to 96% in Sierra Leone ( N = 15). Other issues include availability, logistics, and misconceptions.
Malaria vaccines protect against malaria infection in varying degrees, with severe side effects rarely occurring. Further research is required to improve vaccine efficacy and community involvement is needed to ensure successful widespread use in African communities.
Similar content being viewed by others
RTS,S/AS01 malaria vaccine pilot implementation in western Kenya: a qualitative longitudinal study to understand immunisation barriers and optimise uptake

Are malaria transmission-blocking vaccines acceptable to high burden communities? Results from a mixed methods study in Bo, Sierra Leone
Post introduction evaluation of the malaria vaccine implementation programme in Ghana, 2021
Avoid common mistakes on your manuscript.
Introduction
Malaria is prevalent in Africa and poses a significant public health threat with substantial morbidity and mortality [ 1 ]. Despite concerted efforts to curb the disease, its persistence can be attributed to socioeconomic inequality, inadequate infrastructure, and the emergence and spread of drug-resistant strains [ 2 ]. Control measures such as insecticide-treated nets (ITNs), indoor residual spraying (IRS), and antimalarial drugs are critical, but additional complementary interventions are needed. One of the promising emergent strategies is vaccination, which has been identified as a potentially pivotal measure in the fight against malaria [ 3 ].
Developing a malaria vaccine has been an arduous journey, complicated by the inherent complexity of the Plasmodium parasite's life cycle and its diverse antigenic characteristics [ 4 ]. Despite these challenges, there has been substantial progress. One particular advancement in this field is the RTS,S/AS01 and the R21/Matrix-M vaccines. These vaccines demonstrated protective efficacy in large-scale clinical trials, and have been recommended by the World Health Organization (WHO) for use in regions with moderate to high P. falciparum transmission, particularly Sub-Saharan Africa [ 5 ].
Malaria vaccine clinical trials have provided important knowledge and insights to support the implementation of large-scale vaccination programs. Mokuolo et al. [ 6 ] offered several key learnings from these trials, stressing the significance of robust local regulatory and ethical frameworks, effective community engagement and communication, as well as vigilant monitoring for potential disease enhancement or rebound morbidity following temporary interruptions of clinical infections. A critical factor in the success of vaccine implementation is community acceptance. A recent review of the literature suggests high acceptance of the RTS,S malaria vaccine across low- and middle-income countries (LMICs), with an average acceptance rate of 95.3% [ 7 ]. However, acceptance rates vary and appear to be impacted by socio-demographic factors and community apprehensions about safety, efficacy, and vaccine awareness [ 8 , 9 ].
In light of the success of the RTS,S and R21 vaccines, the need for greater global resources for malaria vaccine research and logistics in vaccine implementation cannot be over-emphasized. This study sought to address a current gap in understanding by using an in-depth scoping review to summarize recent empirical evidence on malaria vaccine efficacy, safety, and community perceptions in Africa.
A scoping review was conducted using the methodological framework outlined by Arksey and O’Malley [ 10 ], incorporated quality recomendations [ 11 ], and reported using the PRISMA extension for scoping reviews (PRISMA-ScR), as outlined in Appendix 1 [ 12 ]. The review protocol was registered at Open Science Framework (OSF) at https://doi.org/ https://doi.org/10.17605/OSF.IO/D54YC .
Eligibility criteria
Studies were included if they evaluated the efficacy, safety, or community perception of a malaria vaccine; were published after 2011; were primary/empirical research; conducted in malaria-endemic African countries; and included the general public as participants (e.g., caregivers, parents, children, or adults). Studies published from 2012 were included as a previous review that have explored malaria vaccine research prior to 2012 [ 13 ]. Studies were excluded if the participants were outside Africa, were not primary research (reviews, opinions, editorial, commentaries), and if they evaluated immunogenicity without safety or efficacy as a construct.
Information sources
Five primary databases were searched to identify relevant studies in any language: African Journals Online (AJOL), Academic Search Complete, Medline, CINAHL and PsychInfo. The initial search was conducted in January 2023 for articles published from 2012 to 2022. An update search was conducted in June 2023 for articles published from 2022 to June 2023. The search was supplemented with two gray literature sources; AfricArxiv (Achieve for African Research) and OPUS (Open Publication of UTS Scholars) to identify relevant preprints and thesis/dissertations respectively. Additionally, the reference list of articles that met the inclusion criteria was searched manually and forward literature search on Google Scholar was conducted to identify potentially missing articles. Peer review identified three additional studies published after June 2023 and those studies have also been included.
A combination of MeSh and index terms were formulated based on the PICO framework to aid the search process: Population (P)—African communities, Intervention (I)—malaria vaccine, Comparator (C)—none, and Outcome (O)—efficacy, safety, community perception. The EBSCOhost interface (including Academic Search Complete, CINAHL, Medline with full-text and PsychInfo) and the AJOL database were searched. The full search terms are reported in supplemental Table S1 . The EBSCOhost interface was expanded to; ‘Apply related words’ and ‘Apply equivalent subjects’.
For gray literature sources, the term 'malaria vaccine' was used to search for preprints papers on AfricArxiv, and any relevant thesis/publication on OPUS.
Selection of studies
Two reviewers (MC and KA) screened potentially eligible studies using the eligibility criteria. First, exact duplicates were removed in EBSCOhost and the search was narrowed to studies published from January 2012. Search results were then exported to Endnote. The duplicate screening was conducted in Endnote. The remaining articles were independently screened by 2 reviewers based on the title and abstract. The full text of all potentially relevant articles was then retrieved and screened independently by MC and UMB in-line with the eligibility criteria.
Data charting process
A data extraction form was developed by three authors (MC, UMB, DS) and included study characteristics such as the citation, year of publication, study design, and study setting. Data related to the study findings varied based on the focus of the study and included the study methods, the type of malaria vaccine assessed, the outcome assessments used, and the major findings. Two reviewers (KA and MAK) independently conducted the data extraction. Differences were resolved through discussion between the two reviewers and a third reviewer (MC).
Critical appraisal of included studies
The quality of the included studies was assessed using Joanna Briggs Institute (JBI) appraisal tools [ 14 ] and the Mixed Methods Appraisal Tool (MMAT) [ 15 ]. The appraisal was conducted independently by 2 reviewers (KKD and PB) and differences were resolved by a third reviewer (UMB). No study was excluded based on quality appraisal, but the quality of the study was considered when reaching key conclusions. JBI and MMAT do not provide a scoring guideline, therefore, studies were considered ‘above-average quality’ when they met at least half (average) of the quality criteria assessed in the specific study design. Therefore, the terms ‘below-average quality’ or ‘above-average quality’ were used to refer to study quality in the results.
Efficacy was operationally defined as the vaccine’s estimated effect on all malaria episodes (clinical, severe, or hospitalization). Efficacy was based on Intention-To-Treat (ITT) or According-To-Protocol/Per Protocol (ATP) analyses. Where ITT and ATP analyses were unavailable, efficacy was based on Hazard Ratio (HR), or any other percentage/proportion estimates reported in the studies. Safety was defined based on the presence or absence of Adverse Event (AE) and/or Serious Adverse Event (SAE). Community perception was defined as the different views of communities (general population) about malaria vaccines.
Synthesis of results
Results were synthesized narratively by summarizing the descriptive numerical data followed by a summary of the textual data. The synthesis considered the nature of the research (e.g., design), the type of malaria vaccine (for efficacy and safety), and the quality of the research studies.
Overall efficacy was classified as positive, none/negative or mixed. A result was considered as having positive efficacy if the Confidence Intervals (CI) were within the positive range; mixed efficacy if the CI ranged from negative to positive; and negative efficacy if the CI was within the negative range to zero. Similarly, safety issues were classified based on the number of subjects presenting with at least one SAE, AE, or none. Where the number of affected subjects were not available, a total number of events/incidents was reported. AEs can be solicited, unsolicited or unexpected, and the cumulative number/range was reported based on available information. For community perception, results were synthesized thematically by reporting the overall quantitative results followed by a summary of qualitative results as applicable. Overall percentages/proportions were reported with a range when available. Community perception was further classified based on 3 components: nature of the vaccine (e.g., risks, effect), systems (e.g., mistrust, logistics), or personal reasons (encompassing anything else). N refers to the number of studies reporting the same finding, while n refers to the number of participants reporting a finding in a study in this review.
We initially found 1299 articles (Fig. 1 ) from the five databases, and 661 underwent title/abstract screening. Two non-English articles, in Danish and French, were evaluated and excluded as they were secondary research. In total, 66 studies ( N ) were included (61 from the main search, 2 from the updated search, and 3 were identified during peer review) [ 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 ].
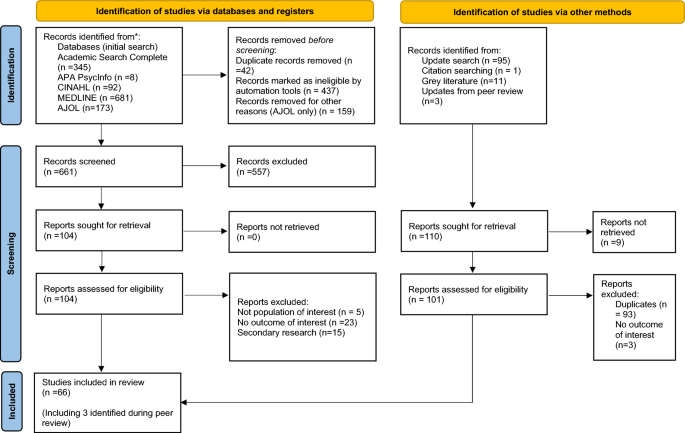
PRISMA flow diagram indicating screening process
Characteristics of included studies
The 66 included studies incorporated 47 Randomized Controlled Trials/clinical trials (71.4%), a case–control study (1.6%), and 17 surveys (27.0%). Sixteen African countries were included, with 64 of the 66 studies (97.0%) being above-average quality (Table S2). Further details are presented in Table S3.
Efficacy of malaria vaccines
Half of the included studies (50%, N = 33) reported vaccine efficacy. At 12 months post-vaccination, the R21 vaccine showed the highest overall efficacy at 77% ( N = 1, n = 146), compared to the RTS,S vaccine at 55% ( N = 1, n = 273). Both of these studies were of above-average quality (Table S2). R21 further demonstrated an efficacy of 79% among younger children (5–17 months compared to 18–36 month-olds) at 12 months [ 86 ] and 80% ( N = 1, n = 137) at 12 months after a booster dose [ 78 ]. Similarly, RTS,S vaccine showed an efficacy of 56% among children aged 5–17 months at 12 months following vaccination [ 62 ]. PfSPZ, though tested on only five individuals, demonstrated an efficacy of 100% at three- or eleven-weeks post-vaccination. This efficacy rose from 20 to 100% at 3 weeks when PfSPZ's dosage regimen was adjusted [ 39 ]. The combined use of RTS,S/AS01 with chemoprevention yielded efficacy between 59.6 to 60.1% against clinical malaria and outperformed the vaccine in isolation against severe malaria and related deaths [ 25 ]. Other vaccines' efficacies varied significantly (Table 1 ).
Two studies [ 55 , 73 ] evaluated the long-term (up to 7 years) efficacy of RTS,S on severe and clinical malaria. While the study by Tinto et al. [ 73 ] demonstrated a decrease in severe malaria cases over time, there was a rebound against clinical malaria among older children (5–7 years). Oluto et al. [ 55 ] identified that vaccine efficacy (clinical malaria) waned over time, including negative efficacy among children with higher exposure to the malaria parasite. Similarly, a negative efficacy of ChAd63/MVA ME-TRAP for an adjusted severe malaria cohort was found [ 74 ]. Vaccine effectiveness was maintained when co-administered with malaria chemoprevention [ 24 , 25 , 27 ] or other childhood vaccinations [ 20 ].
Safety of malaria vaccines
Thirty-six studies (54.5%, N = 36) investigated the safety of the malaria vaccines, all employing Randomized Controlled Trial design with above-average quality (Table S2). Each study reported one or more AEs ( N = 28) or SAEs ( N = 23). The reported AEs and SAEs ranged broadly across various vaccines; RTS,S (AEs: 1.6–87.5%, N = 6; SAEs: 2.8–92.2%, N = 12, vaccine-related SAEs: 0.1–1%, N = 7), BK-SE36 (AEs: 5.6–94.4%, N = 1; SAEs: 4.4–5.6%, N = 2), ChAd63/MVA (AEs: 0–100%, N = 6; SAE: 0.4–8.9%, N = 2), FMP2.1/AS02A (SAE: 4%, N = 1), GMZ2 (AEs: 23–100%, N = 2; SAEs: 49–54.5%, N = 2), PfPZ (AEs: 1.6–83.9%, N = 7; SAEs: 1.6%, N = 1), PfAMA1 (AEs: 5–60%, N = 1), PfSPZ-CVac (AEs: 19.4%, N = 1), Pfs25H-EPA (AEs: 100%, N = 1, SAEs: 1.7%, N = 1) and R21 (AEs 0.7–24.6%, N = 1, SAEs: 2.1%, N = 1).
The local and systemic AEs that were typically reported included injection site pain and fever among other symptoms including redness, warmth, discoloration, bruising, erythema, blistering, pruritis, swelling and induration; headache; allergic rash,; drowsiness; irritability; loss of appetite; fatigue; dizziness; abdominal pain; chills; myalgia; diarrhea; nausea and vomiting [ 18 , 20 , 30 , 31 , 37 , 38 , 39 , 45 , 46 , 52 , 56 , 57 , 59 , 61 , 62 , 63 , 64 , 66 , 67 , 68 , 69 , 70 , 72 , 74 , 75 , 77 , 86 , 87 , 88 ]. Most AEs subsided within 1–7 days [ 18 , 46 , 52 , 74 , 86 ].
Commonly reported SAEs were acute gastritis, anemia, bronchitis, cerebral malaria, severe malaria, dehydration, convulsion, febrile convulsion, gastroenteritis, seizures, meningitis, paralytic ileus, pyrexia, pneumonia, respiratory distress, and death. However, most SAEs were.
deemed unrelated to the vaccination (Table 2 ) and were associated with malaria infection [ 29 , 87 ]. Only 0.1–1% and 4.3% of SAEs were possibly linked to vaccines, mainly febrile convulsion/seizures, associated with RTS,S vaccine [ 25 , 35 , 58 , 61 , 62 , 63 , 66 ] and R21 vaccine [ 86 ] respectively. Malaria vaccine safety when co-administered with other routine childhood immunization was identified [ 20 , 46 ].
Community perception of malaria vaccine
Seventeen studies (27.0%, N = 17) assessed community perception of malaria vaccines, with a mix of below and above-average quality studies (Table S2). The overall perception of participants has been summarized in addition to five key issues that emerged from the studies: acceptance, availability, knowledge/awareness, logistics, and misconceptions about the vaccines (Table 3 ).
Overall perception
Ten of the seventeen studies that assessed community perception (58.8%) reported their overall perception of malaria vaccines (Table 3 ), and were of below and above-average quality (Table S2). Community members agreed that it was essential to have a malaria vaccine [ 44 ] and that the vaccine is necessary for malaria control [ 33 ]. More than three-quarters of participants from each study reported overall positive perceptions [ 26 , 36 , 47 , 48 ], identified malaria as a risk for their children [ 36 ], and identified that the vaccine will keep children healthy [ 23 , 44 ] even though the efficacy of the vaccine may not be 100% [ 47 ]. A significant positive association between positive perception and intent to comply with vaccination was reported [ 26 ]. More than half of respondents recommend the vaccine to others [ 48 ] and were part of the National Program on Immunisation [ 33 , 48 ]. The majority of participants preferred vaccines to malaria drugs/vector control [ 28 , 34 ]. There was a mixed reaction between oral and injectable vaccines in Ghana [ 44 ], while in Tanzania, participants were open to all modes of administration [ 60 ]. The limited side effects experienced by participants in the RTS,S/AS01 vaccine trial reinforced participants’ beliefs about its safety in Nigeria [ 28 ].
Of the studies examined, 88.2%, ( N = 15) reported acceptance of malaria vaccines (Table 3 ), and most studies were above-average quality (Table S2). Acceptance rates varied from 32.3% in Ethiopia [ 21 ] to 96% in Sierra Leone [ 43 ]. Acceptance increased to 98.9% in malaria-endemic areas in Kenya [ 53 ]. Key drivers for acceptance were the high risk of malaria in children [ 17 , 41 ], the desire for self-protection and prevention [ 41 , 43 ], and incentives such as free consultations and medication [ 17 ].
The impact of religion on vaccine acceptance was inconsistent [ 36 , 47 , 71 ]. Some findings showed that Christian mothers were more likely to accept the vaccine than Muslim mothers in Tanzania [ 47 ], while in Ghana [ 36 ] and Nigeria [ 71 ], Christian mothers showed lower odds of accepting the vaccine. Free provision significantly increased vaccine acceptance [ 41 , 43 ], while increased costs decreased acceptance [ 41 , 76 ].
Fear of adverse events and unsuccessful intravenous vaccination attempts were linked to vaccine refusal [ 23 , 43 , 44 , 71 ]. Factors such as marital status, region, knowledge of vaccine, tribe, education level, prior vaccination experience, satisfaction with healthcare services, and parent age influenced willingness to accept vaccination [ 21 , 33 , 41 , 47 , 53 , 76 ].
Availability
Two of the studies (11.8%) reported concerns associated with the availability of malaria vaccines (Table 3 ). The need to provide malaria vaccine to adults in addition to children was reported in Mozambique [ 23 ]. The importance of an adequate supply chain to promote availability was documented from a key informant interview in Sierra Leone [ 43 ].
Knowledge/awareness
Nine of the studies (52.9%) reported knowledge of participants about malaria vaccines (Table 3 ). The percentage of participants having awareness of malaria vaccines ranged from 11% in Tanzania [ 60 ] to 60% in Nigeria [ 33 ]. Additionally, there was a low willingness to learn more about the vaccine in Mozambique [ 23 ]. Confusion and delays related to trial designs were seen to discourage participation in a malaria vaccine trial in Kenya [ 17 ]. The use of mass media, particularly Television, radio, and phones were identified as good sources of information by participants [ 23 , 26 , 44 ]. Information vans, health talks, and information from trusted community members [ 44 ] or health professionals were important but were rated equally with internet sources [ 71 ]. Awareness of vaccines was higher in older people when compared to younger people [ 36 ] and in mothers of Christian children compared to the Islamic faith [ 36 ]. There was evidence of confusion about malaria vaccines and other childhood vaccines in Ghana [ 44 ].
Four of the studies (23.5%) reported findings related to the logistics associated with malaria vaccine enrolments (Table 3 ). The need for community outreach by community health workers, including malaria vaccine campaigns alongside existing vector control programs to encourage participation was reported [ 43 ]. Negative attitudes of health staff were reported and shown to discourage participation in malaria vaccine trials [ 17 ]. Similarly, the system’s capacity to train staff for intravenous administration was noted as important [ 17 ].
Parents’ willingness to pay for the malaria vaccine was reported as a barrier [ 26 , 28 , 43 ]. Although, affordability was noted as a concern in a number of studies [ 26 , 28 , 41 , 76 ], some participants suggested that the provision of malaria vaccines was the sole responsibility of the government [ 28 ].
Misconceptions
Four of the studies (23.5%) reported misconceptions about potential malaria vaccines. Rumors of blood ‘theft and selling’ were linked to early withdrawal from malaria vaccine trials in Kenya [ 17 ]. Similarly, a widespread belief that newborns should have minimum exposure to adults and that the presence of a vaccine scar signifies a nurse had sexual intercourse with the child hindered vaccination programs in Mozambique [ 23 ]. The ideology that vaccines are harmful and can cause sickness was reported as a fear preventing vaccinations [ 23 , 43 ]. Furthermore, rumors of vaccines causing infertility and system mistrust were cited as critical reasons for hesitancy to receive the malaria vaccine [ 43 , 71 ].
This paper summarizes recent evidence on the efficacy, safety, and perception of malaria vaccines in Africa. All vaccines studied showed some degree of protection in terms of reducing the risk of contracting malaria and/or eliciting an antibody response. Overall efficacy varied; the highest overall efficacy (77%) was observed with R21 [ 30 ], which increased to 80% with a booster dose [ 78 ]. Increasing the dosage regimen of PfSPZ may also lead to an increase in efficacy from 20 to 100% [ 39 ]. Vaccination efficacy decreases over time with the highest efficacy expected up to one year after the last dose [ 55 , 73 ]. R21 showed increased efficacy between six months (74%) to one year (77%) after vaccination [ 30 ]. RTS,S, was the most-studied vaccine. RTS,S showed good efficacy (55%) up to one year after vaccination, but this decreased over time [ 24 , 55 ], with efficacy around zero after four years and negative in areas with high malaria exposure at five years of follow-up [ 55 ]. RTS,S was found to prevent clinical malaria cases in infants and children over three to four years and was further enhanced by administering a booster dose [ 63 ]. Emerging evidence suggests that the efficacy of vaccines like RTS,S increases when combined with seasonal malaria chemoprophylaxis [ 63 ]. The concomitant use of malaria vaccines with other control measures is therefore seen to be an important mitigation strategy in areas of high transmission.
Adverse events were reported in all studies. The most common adverse events were injection site pain and fever. Most adverse events were reported to subside within one week of appearance. Serious adverse events were rare (0.1–1%). Serious adverse events can occur following vaccinations, with about 1% of participants developing events such as febrile convulsions following malaria vaccines [ 23 , 25 , 35 , 58 , 61 , 62 , 63 ]. This was particularly observed in children within 2–3 days of receiving the RTS,S vaccine [ 35 ]. It is therefore possible that adverse events may arise following vaccination; however, further research is required.
Fear of unknown side effects associated with vaccines, especially newly developed ones, are often associated with low levels of acceptance [ 79 ]. Willingness to accept the malaria vaccine ranges from 32.3% in Ethiopia to 96% in Sierra Leone [ 21 , 26 ]. However, a number of factors are likely to affect the use of malaria vaccines in many African communities, including inadequate knowledge, misconceptions, availability of vaccines, and logistics.
This review has identified that knowledge about malaria vaccines is not widespread throughout Africa. Vaccine awareness was slightly lower than vaccine acceptance; however, people may have been reluctant to accept the newly developed malaria vaccines because of generalized vaccine hesitancy in some parts of Africa. Vaccine hesitancy has been reported in the literature as a consequence of misinformation about vaccine origin, efficacy, and safety, and psychological factors such as anxiety [ 80 , 81 ]. In addition to these factors, political influences, religious beliefs, and low perception of risk combine to contribute to vaccination rates in sub-Saharan Africa [ 79 , 80 ]. The extent of vaccination hesitancy may vary according to people's commitment to health protection and risk culture and their trust in conventional medicine and public health authorities. Evidence from the literature suggests that the lack of willingness to vaccinate may be due to a lack of knowledge, indifference, and irregular vaccination behavior [ 82 ]. Public education campaigns on vaccination programs are therefore important to support behavior change.
The findings of this review could assist public health experts and policymakers in Africa to develop and implement strategies to address the low acceptance and use of malaria vaccines. Wide-spread adoption of malaria vaccines is possible if awareness campaigns provide adequate factual explanations to counter rumors and mis-information [ 6 , 83 ]. Increasing local vaccine production within the African continent may further promote the use of malaria vaccines. Local production may help reduce mistrust through technology transfer. To raise awareness about vaccination, it is important to take a context-specific approach involving community and religious leaders [ 84 , 85 ]. The provision of credible information to communities by trusted sources is an important strategy to promote vaccination uptake.
There are some limitations to this review. Due to recent advances in malaria vaccines and the recommendations of Schwartz et al. [ 9 ] only studies published since 2012 were included. The scope of this review summarizes the existing evidence and highlights areas for more in-depth analysis in the future.
Different types of malaria vaccines have different efficacy levels, and combining seasonal malaria prophylaxis with a malaria vaccine might increase effectiveness. A variable degree of protection from malaria infection is provided by malaria vaccines with severe adverse events only occurring rarely. Many African communities have a high perception of malaria vaccines, but knowledge of the vaccine is relatively low. Further research and community involvement are needed to respectively improve vaccine efficacy and ensure successful widespread use in African communities.
Data availability
All data used in this review will be made available on request through the corresponding author.
World Health Organization. World malaria report: 20 years of global progress and challenges. https://www.who.int/publications/i/item/9789240015791 . 2020.
Alonso P, Noor AM. The global fight against malaria is at crossroads. The Lancet. 2017;390:2532–4.
Article Google Scholar
Kanoi BN, Maina M, Likhovole C, Kobia FM, Gitaka J. Malaria vaccine approaches leveraging technologies optimized in the COVID-19 era. Front Trop Dis. 2022. https://doi.org/10.3389/fitd.2022.988665 .
Etefia E, Etoh PI. Malaria vaccine development: challenges and prospects. Med Pharm J. 2023;2023:28–42.
World Health Organization malaria vaccine implementation programme.
Mokuolu OA, Bolarinwa OA, Opadiran OR, Ameen HA, Dhorda M, Cheah PY, et al. A framework for stakeholder engagement in the adoption of new anti-malarial treatments in Africa: a case study of Nigeria. Malar J. 2023;22:1–13. https://doi.org/10.1186/s12936-023-04622-2 .
Sulaiman SK, Musa MS, Tsiga-Ahmed FI, Dayyab FM, Sulaiman AK, Bako AT. A systematic review and meta-analysis of the prevalence of caregiver acceptance of malaria vaccine for under-five children in low-income and middle-income countries (LMICs). PLoS One. 2022. https://doi.org/10.1371/journal.pone.0278224 .
Article PubMed PubMed Central Google Scholar
Mumtaz H, Nadeem A, Bilal W, Ansar F, Saleem S, Khan QA, et al. Acceptance, availability, and feasibility of RTS, S/AS01 malaria vaccine: a review. Immun Inflamm Dis. 2023;11: e899. https://doi.org/10.1002/iid3.899 .
Article CAS PubMed PubMed Central Google Scholar
Dimala CA, Kika BT, Kadia BM, Blencowe H. Current challenges and proposed solutions to the effective implementation of the RTS, S/AS01 Malaria vaccine program in sub-Saharan Africa: a systematic review. PLoS ONE. 2018;13: e0209744.
Arksey H, O’Malley L. 2005. Scoping studies: towards a methodological framework. https://doi.org/10.1080/1364557032000119616
Cooper S, Cant R, Kelly M, Levett-Jones T, McKenna L, Seaton P, et al. An evidence-based checklist for improving scoping review quality. Clin Nurs Res. 2019;30:230–40. https://doi.org/10.1177/1054773819846024 .
Article PubMed Google Scholar
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation ann intern med. Am College Phys. 2018. https://doi.org/10.7326/M18-0850 .
Schwartz L, Brown GV, Genton B, Moorthy VS. A review of malaria vaccine clinical projects based on the WHO rainbow table. Malar J. 2012. https://doi.org/10.1186/1475-2875-11-11 .
Joanna Briggs Institute. JBI Critical appraisal tools. https://jbi.global/critical-appraisal-tools .
Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed method appraisal tool (MMAT) Version 2018 - User guide. Montreal. 2018. http://mixedmethodsappraisaltoolpublic.pbworks.com/
Abdulla S, Salim N, Machera F, Kamata R, Juma O, Shomari M, et al. Randomized, controlled trial of the long term safety, immunogenicity and efficacy of RTS, S/AS02D malaria vaccine in infants living in a malaria-endemic region. Malar J. 2013. https://doi.org/10.1186/1475-2875-12-11 .
Achieng F, Rosen JG, Cherop RY, Kariuki S, Hoffman SL, Seder R, et al. Caregiver and community perceptions and experiences participating in an infant malaria prevention trial of PfSPZ Vaccine administered by direct venous inoculation: a qualitative study in Siaya County, western Kenya. Malar J. 2020. https://doi.org/10.1186/s12936-020-03293-7 .
Afolabi MO, Tiono AB, Adetifa UJ, Yaro JB, Drammeh A, Nébié I, et al. Safety and Immunogenicity of ChAd63 and MVA ME-TRAP in West African Children and Infants. Mol Ther. 2016;24:1470–7.
Ajua A, Lell B, Agnandji ST, Asante KP, Owusu-Agyei S, Mwangoka G, et al. The effect of immunization schedule with the malaria vaccine candidate RTS, S/AS01E on protective efficacy and anti-circumsporozoite protein antibody avidity in African infants. Malar J. 2015. https://doi.org/10.1186/s12936-015-0605-7 .
Asante KP, Ansong D, Kaali S, Adjei S, Lievens M, Nana Badu L, et al. Immunogenicity and safety of the RTS, S/AS01 malaria vaccine co-administered with measles, rubella and yellow fever vaccines in Ghanaian children: a phase IIIb, multi-center, non-inferiority, randomized, open, controlled trial. Vaccine. 2020;38:3411–21.
Asmare G. Willingness to accept malaria vaccine among caregivers of under-5 children in Southwest Ethiopia: a community based cross-sectional study. Malar J. 2022. https://doi.org/10.1186/s12936-022-04164-z .
Bejon P, White MT, Olotu A, Bojang K, Lusingu JPA, Salim N, et al. Efficacy of RTS, S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis. 2013;13:319–27.
Bingham A, Gaspar F, Lancaster K, Conjera J, Collymore Y, Ba-Nguz A. Community perceptions of malaria and vaccines in two districts of Mozambique. Malar J. 2012. https://doi.org/10.1186/1475-2875-11-394 .
Cairns M, Barry A, Zongo I, Sagara I, Yerbanga SR, Diarra M, et al. The duration of protection against clinical malaria provided by the combination of seasonal RTS, S/AS01E vaccination and seasonal malaria chemoprevention versus either intervention given alone. BMC Med. 2022. https://doi.org/10.1186/s12916-022-02536-5 .
Chandramohan D, Zongo I, Sagara I, Cairns M, Yerbanga R-S, Diarra M, et al. Seasonal malaria vaccination with or without seasonal malaria chemoprevention. N Engl J Med. 2021;385:1005–17.
Article CAS PubMed Google Scholar
Chukwuocha UM, Okorie PC, Iwuoha GN, Ibe SN, Dozie IN, Nwoke BE. Awareness, perceptions and intent to comply with the prospective malaria vaccine in parts of South Eastern Nigeria. Malar J. 2018. https://doi.org/10.1186/s12936-018-2335-0 .
Coulibaly D, Kone AK, Traore K, Niangaly A, Kouriba B, Arama C, et al. PfSPZ-CVac malaria vaccine demonstrates safety among malaria-experienced adults: a randomized, controlled phase 1 trial. EClinicalMedicine. 2022. 52.
Darkwa S, de Wildt G, Dalaba M, Vidzro E, Ansah EK. “I would have to sell things in order to get the money”: a qualitative exploration of willingness to pay for the RTS, S/AS01 malaria vaccine in the Volta region Ghana. PLoS One. 2022. https://doi.org/10.1371/journal.pone.0268009 .
Dassah S, Adu B, Sirima SB, Mordmüller B, Ngoa UA, Atuguba F, et al. Extended follow-up of children in a phase2b trial of the GMZ2 malaria vaccine. Vaccine. 2021;39:4314–9.
Datoo MS, Natama MH, Somé A, Traoré O, Rouamba T, Bellamy D, et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. The Lancet. 2021;397:1809–18.
Article CAS Google Scholar
Dejon-Agobe JC, Ateba-Ngoa U, Lalremruata A, Homoet A, Engelhorn J, Nouatin OP, et al. Controlled human malaria infection of healthy adults with lifelong malaria exposure to assess safety, immunogenicity, and efficacy of the asexual blood stage malaria vaccine candidate GMZ2. Clin Infect Dis. 2019;69:1377–84.
Dobanõ C, Ubillos I, Jairoce C, Gyan B, Vidal M, Jiménez A, et al. RTS, S/AS01E immunization increases antibody responses to vaccine-unrelated Plasmodium falciparum antigens associated with protection against clinical malaria in African children: a case-control study. BMC Med. 2019. https://doi.org/10.1186/s12916-019-1378-6 .
Etokidem AJ, Ndifon WO, Asibong UE. Perception and acceptability of malaria vaccine among maternal and child health clinic attendees at the University of Calabar teaching Hospital, Calabar, Nigeria. J Commun Med Primary Health Care. 2015;27:51.
Google Scholar
Febir LG, Asante KP, Dzorgbo D-BS, Senah KA, Letsa TS, Owusu-Agyei S. Community perceptions of a malaria vaccine in the Kintampo districts of Ghana. Malaria J. 2013. https://doi.org/10.1186/1475-2875-12-156 .
Guerra Mendoza Y, Garric E, Leach A, Lievens M, Ofori-Anyinam O, Pirçon JY, et al. Safety profile of the RTS, S/AS01 malaria vaccine in infants and children: additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum Vaccin Immunother. 2019;15:2386–98.
Immurana M, Boachie MK, Klu D, Dalaba MA, Manyeh AK, Alhassan RK. Determinants of willingness to accept child vaccination against malaria in Ghana. Int J Health Planning Manage. 2022;37:1439–53.
Jongo SA, Shekalaghe SA, Preston Church LW, Ruben AJ, Schindler T, Zenklusen I, et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg. 2018;99:338–49.
Jongo SA, Church LWP, Mtoro AT, Chakravarty S, Ruben AJ, Swanson PA, et al. Safety and differential antibody and T-cell responses to the plasmodium falciparum sporozoite malaria vaccine, PfSPZ vaccine, by age in tanzanian adults, adolescents, children, and infants. American Journal of Tropical Medicine and Hygiene. American Society of Tropical Medicine and Hygiene. 2019.
Jongo SA, Preston Church LW, Mtoro AT, Schindler T, Chakravarty S, Ruben AJ, et al. Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ vaccine in Tanzanian adults. Clin Infect Dis. 2020;71:2849–57.
Jongo SA, Urbano V, Preston Church LW, Olotu A, Manock SR, Schindler T, et al. Immunogenicity and protective efficacy of radiation-attenuated and chemo-attenuated PfSPZ vaccines in equatoguinean adults. Am J Trop Med Hyg. 2021;104:283–93.
Kpanake L, Sorum PC, Mullet E. The potential acceptability of infant vaccination against malaria: a mapping of parental positions in Togo. Vaccine. 2016;34:408–12.
Laurens MB, Thera MA, Coulibaly D, Ouattara A, Kone AK, Guindo AB, et al. Extended safety, immunogenicity and efficacy of a blood-stage malaria vaccine in Malian children: 24-Month follow-up of a randomized, double-blinded phase 2 trial. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0079323 .
McCoy KD, Weldon CT, Ansumana R, Lamin JM, Stenger DA, Ryan SJ, et al. Are malaria transmission-blocking vaccines acceptable to high burden communities? Results from a mixed methods study in Bo Sierra Leone. Malar J. 2021. https://doi.org/10.1186/s12936-021-03723-0 .
Meñaca A, Tagbor H, Adjei R, Bart-Plange C, Collymore Y, Ba-Nguz A, et al. Factors likely to affect community acceptance of a malaria vaccine in two districts of ghana: a qualitative study. PLoS One. 2014. https://doi.org/10.1371/journal.pone.0109707 .
Mensah VA, Gueye A, Ndiaye M, Edwards NJ, Wright D, Anagnostou NA, et al. Safety, immunogenicity and efficacy of prime-boost vaccination with chad63 and mva encoding me-trap against plasmodium falciparum infection in adults in senegal. PLoS One. 2016. https://doi.org/10.1371/journal.pone.0167951 .
Mensah VA, Roetynck S, Kanteh EK, Bowyer G, Ndaw A, Oko F, et al. Safety and immunogenicity of malaria vectored vaccines given with routine expanded program on immunization vaccines in gambian infants and neonates: a randomized controlled trial. Front Immunol. 2017. https://doi.org/10.3389/fimmu.2017.01551 .
Mtenga S, Kimweri A, Romore I, Ali A, Exavery A, Sicuri E, et al. Stakeholders’ opinions and questions regarding the anticipated malaria vaccine in Tanzania. Malar J. 2016. https://doi.org/10.1186/s12936-016-1209-6 .
Musa S, Olorukooba AA, Muhammad NS, Muhammad B, Makarfi HU. Awareness, perception and acceptance of malaria vaccine among women of the reproductive age group in a rural community in Soba, Kaduna State North-west Nigeria. Kanem J Med Sci. 2022;16:32.
Neafsey DE, Juraska M, Bedford T, Benkeser D, Valim C, Griggs A, et al. Genetic diversity and protective efficacy of the RTS, S/AS01 malaria vaccine. N Engl J Med. 2015;373:2025–37.
Nouatin O, Ateba Ngoa U, Ibáñez J, Dejon-Agobe JC, Mordmüller B, Edoa JR, et al. Effect of immune regulatory pathways after immunization with GMZ2 malaria vaccine candidate in healthy lifelong malaria-exposed adults. Vaccine. 2020;38:4263–72.
Nouatin O, Ibáñez J, Fendel R, Ngoa UA, Lorenz FR, Dejon-Agobé JC, et al. Cellular and antibody response in GMZ2-vaccinated Gabonese volunteers in a controlled human malaria infection trial. Malar J. 2022. https://doi.org/10.1186/s12936-022-04169-8 .
Ogwang C, Afolabi M, Kimani D, Jagne YJ, Sheehy SH, Bliss CM, et al. Safety and immunogenicity of heterologous prime-boost Immunisation with Plasmodium falciparum malaria candidate vaccines, ChAd63 ME-TRAP and MVA ME-TRAP, in healthy Gambian and Kenyan Adults. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0057726 .
Ojakaa DI, Jarvis JD, Matilu MI, Thiam S. Acceptance of a malaria vaccine by caregivers of sick children in Kenya. Malar J. 2014. https://doi.org/10.1186/1475-2875-13-172 .
Olotu A, Fegan G, Wambua J, Nyangweso G, Awuondo KO, Leach A, et al. Four-year efficacy of RTS, S/AS01E and its interaction with malaria exposure. N Engl J Med. 2013;368:1111–20.
Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, et al. Seven-year efficacy of RTS, S/AS01 Malaria vaccine among young African children. N Engl J Med. 2016;374:2519–29.
Olotu A, Urbano V, Hamad A, Eka M, Chemba M, Nyakarungu E, et al. Advancing global health through development and clinical trials partnerships: a randomized, placebo-controlled, double-blind assessment of safety, tolerability, and immunogenicity of pfspz vaccine for malaria in healthy equatoguinean men. Am J Trop Med Hyg. 2018;98:308–18.
Oneko M, Steinhardt LC, Yego R, Wiegand RE, Swanson PA, Kc N, et al. Safety, immunogenicity and efficacy of PfSPZ Vaccine against malaria in infants in western Kenya: a double-blind, randomized, placebo-controlled phase 2 trial. Nat Med. 2021;27:1636–45.
Otieno L, Guerra Mendoza Y, Adjei S, Agbenyega T, Agnandji ST, Aide P, et al. Safety and immunogenicity of the RTS, S/AS01 malaria vaccine in infants and children identified as HIV-infected during a randomized trial in sub-Saharan Africa. Vaccine. 2020;38:897–906.
Palacpac NMQ, Ntege E, Yeka A, Balikagala B, Suzuki N, Shirai H, et al. Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0064073 .
Romore I, Ali AM, Semali I, Mshinda H, Tanner M, Abdulla S. Assessment of parental perception of malaria vaccine in Tanzania. Malar J. 2015. https://doi.org/10.1186/s12936-015-0889-7 .
RTSS Clinical Trial Partnership. a phase 3 trial of RTS, S/AS01 malaria vaccine in african infants. New England J Med. 2012;367:2284–95. https://doi.org/10.1056/NEJMoa1208394 .
RTSS Clinical Trial Partnership. Efficacy and safety of the RTS, S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African Sites. PLoS Med. 2014. https://doi.org/10.1371/journal.pmed.1001685 .
RTSS Clinical Trial Partnership. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. The Lancet. 2015;386:31–45.
Sagara I, Healy SA, Assadou MH, Gabriel EE, Kone M, Sissoko K, et al. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum : a randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect Dis. 2018;18:969–82.
Sagara I, Zongo I, Cairns M, Yerbanga RS, Mahamar A, Nikièma F, et al. The anti-circumsporozoite antibody response of children to seasonal vaccination with the RTS, S/AS01Emalaria vaccine. Clin Infect Dis. 2022;75:613–22.
Samuels AM, Ansong D, Kariuki SK, Adjei S, Bollaerts A, Ockenhouse C, et al. Efficacy of RTS, S/AS01E malaria vaccine administered according to different full, fractional, and delayed third or early fourth dose regimens in children aged 5–17 months in Ghana and Kenya: an open-label, phase 2b, randomised controlled trial. Lancet Infect Dis. 2022;22:1329–42.
Sirima SB, Mordmüller B, Milligan P, Ngoa UA, Kironde F, Atuguba F, et al. A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children. Vaccine. 2016;34:4536–42.
Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17:498–509.
Sissoko MS, Healy SA, Katile A, Zaidi I, Hu Z, Kamate B, et al. Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in mali: a randomised, controlled phase 1 trial. Lancet Infect Dis. 2022;22:377–89.
Steinhardt LC, Richie TL, Yego R, Akach D, Hamel MJ, Gutman JR, et al. Safety, tolerability, and immunogenicity of plasmodium falciparum sporozoite vaccine administered by direct venous inoculation to infants and young children: findings from an age de-escalation, dose-escalation, double-blind, randomized controlled study in Western Kenya. Clin Infect Dis. 2020;71:1063–71.
Sulaiman SK, Tsiga-Ahmed FI, Musa MS, Sulaiman AK, Dayyab FM, Khan MA, et al. Prevalence, determinants, and reasons for malaria vaccine hesitancy among caregivers of under-five children in Nigeria: results from a nationwide cross-sectional survey. Vaccine. 2023;41:1503–12.
Thera MA, Coulibaly D, Kone AK, Guindo AB, Traore K, Sall AH, et al. Phase 1 randomized controlled trial to evaluate the safety and immunogenicity of recombinant Pichia pastoris-expressed Plasmodium falciparum apical membrane antigen 1 (PfAMA1-FVO [25–545]) in healthy Malian adults in Bandiagara. Malar J. 2016;15:1–11.
Tinto H, Otieno W, Gesase S, Sorgho H, Otieno L, Liheluka E, et al. Long-term incidence of severe malaria following RTS, S/AS01 vaccination in children and infants in Africa: an open-label 3-year extension study of a phase 3 randomised controlled trial. Lancet Infect Dis. 2019;19:821–32.
Tiono AB, Nébié I, Anagnostou N, Coulibaly AS, Bowyer G, Lam E, et al. First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5–17 months old infants and children. PLoS One. 2018;13:208328.
Umeh R, Oguche S, Oguonu T, Pitmang S, Shu E, Onyia JT, et al. Immunogenicity and safety of the candidate RTS, S/AS01 vaccine in young Nigerian children: a randomized, double-blind, lot-to-lot consistency trial. Vaccine. 2014;32:6556–62.
Vera Cruz G, Humeau A, Kpanake L, Sorum PC, Mullet E. Infant vaccination against malaria in Mozambique and in Togo: mapping parents’ willingness to get their children vaccinated. Hum Vaccin Immunother. 2020;16:539–47.
Witte D, Cunliffe NA, Turner AM, Ngulube E, Ofori-Anyinam O, Vekemans J, et al. Safety and immunogenicity of seven dosing regimens of the candidate RTS, S/AS01E Malaria vaccine integrated within an expanded program on immunization Regimen: a phase II, single-center, open, controlled trial in infants in Malawi. Pediatric Infect Disease J. 2018;37:483–91.
Datoo MS, Natama HM, Somé A, Bellamy D, Traoré O, Rouamba T, et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. 2022;22:1728–36.
Chutiyami M, Bello UM, Salihu D, Kolo MA, Alsharari AF, Sabo H, et al. Subjective reasons for COVID-19 vaccine hesitancy and sociodemographic predictors of vaccination in Nigeria: an online survey. COVID. 2022;2:1329–40.
Kabakama S, Konje ET, Dinga JN, Kishamawe C, Morhason-Bello I, Hayombe P, et al. Commentary on COVID-19 Vaccine Hesitancy in sub-Saharan Africa. Trop Med Infect Dis MDPI. 2022;77:130.
Chutiyami M, Salihu D, Bello UM, Winser SJ, Gambo AA, Sabo H, et al. Are fear of COVID-19 and vaccine hesitancy associated with COVID-19 vaccine uptake? A population-based online survey in Nigeria. Vaccines. 2022;10:1271.
Peretti-Watel P, Larson HJ, Ward JK, Schulz WS, Verger P. Vaccine hesitancy: clarifying a theoretical framework for an ambiguous notion. PLoS Curr. 2015;25:7.
Grant J, Gyan T, Agbokey F, Webster J, Greenwood B, Asante KP. Challenges and lessons learned during the planning and early implementation of the RTS, S/AS01E malaria vaccine in three regions of Ghana: a qualitative study. Malar J. 2022. https://doi.org/10.1186/s12936-022-04168-9 .
Greenwood BM. Control to elimination: implications for malaria research. Trends Parasitol. 2008;24:449–54.
Moorthy VS, Newman RD, Okwo-Bele JM. Malaria vaccine technology roadmap. Lancet. 2013;382:1700–1.
Datoo MM, Dicko A, Tinto H, Ouédraogo JB, Hamaluba M, Olotu A, Beaumont E, Ramos-Lopez F, Magloire Natama H, Weston S, Chemba M. A Phase III randomised controlled trial evaluating the malaria vaccine candidate R21/Matrix-M™ in African children. 2023. https://doi.org/10.2139/ssrn.4584076
Ouédraogo A, Bougouma EC, Palacpac NM, Houard S, Nebie I, Sawadogo J, Berges GD, Soulama I, Diarra A, Hien D, Ouedraogo AZ. Safety and immunogenicity of BK-SE36/CpG malaria vaccine in healthy Burkinabe adults and children: a phase 1b randomised, controlled, double-blinded, age de-escalation trial. Front Immunol. 2023;16:1267372. https://doi.org/10.3389/fimmu.2023.1267372 .
Silk SE, Kalinga WF, Mtaka IM, Lilolime NS, Mpina M, Milando F, Ahmed S, Diouf A, Mkwepu F, Simon B, Athumani T. Superior antibody immunogenicity of a viral-vectored RH5 blood-stage malaria vaccine in Tanzanian infants as compared to adults. Med. 2023;4:668–86. https://doi.org/10.1016/j.medj.2023.07.003 .
Download references
Open Access funding enabled and organized by CAUL and its Member Institutions. The University of Technology Sydney fully supported the open-access publication of this article as part of its open-access agreement with Springer Nature.
Author information
Authors and affiliations.
School of Nursing and Midwifery, University of Technology Sydney, Sydney, Australia
Muhammad Chutiyami, Priya Saravanakumar & Pratibha Bhandari
Department of Physiotherapy and Paramedicine, School of Health and Life Sciences, Glasgow Caledonian University, Glasgow, UK
Umar Muhammad Bello
College of Nursing, Jouf University, Sakaka, Saudi Arabia
Dauda Salihu
College of Nursing, University of Massachusetts, Amherst, MA, 01003, USA
Khadijat Adeleye
Department of Geography, University of Maiduguri, Maiduguri, Nigeria
Mustapha Adam Kolo
School of Nursing, Midwifery and Health Education, University of Bedfordshire, Luton, UK
Kabiru Kasamu Dawa
Department of Nursing Science, University of Maiduguri, Maiduguri, Nigeria
Dathini Hamina
Department of Physiotherapy, Tishk International University, Erbil, Iraq
Surajo Kamilu Sulaiman
WHO Collaborating Centre for Nursing, Midwifery and Health Development, University of Technology Sydney, Sydney, Australia
School of Nursing, Midwifery and Paramedicine, Australian Catholic University, Sydney, Australia
You can also search for this author in PubMed Google Scholar
Contributions
MC: Conceptualization, protocol and registration, search strategies, article screening, analysis and synthesis, and manuscript drafting. PS: Accessed and verified data, analysis, and manuscript drafting. UMB: Conceptualization, review of quality rating, data analysis, manuscript drafting. DS: Accessed and verified data, conceptualization, data analysis, and manuscript drafting. KA: primary databases search, article screening and data extraction. MAK: data extraction and manuscript drafting. KKD: Quality rating and manuscript drafting. DH: data extraction and manuscript drafting. PB: Quality rating. SKS: Other electronic searches and manuscript drafting. JS: Data verification, qualitative data synthesis, technical and language editing of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Correspondence to Muhammad Chutiyami .
Ethics declarations
Conflict of interests.
All authors declare that there are no conflict of interests.
Additional information
Registration at OSF: https://doi.org/10.17605/OSF.IO/D54YC
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 100 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Chutiyami, M., Saravanakumar, P., Bello, U.M. et al. Malaria vaccine efficacy, safety, and community perception in Africa: a scoping review of recent empirical studies. Infection (2024). https://doi.org/10.1007/s15010-024-02196-y
Download citation
Received : 21 September 2023
Accepted : 22 January 2024
Published : 05 March 2024
DOI : https://doi.org/10.1007/s15010-024-02196-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Malaria vaccine
- Find a journal
- Publish with us
- Track your research
- Skip to main content
- Keyboard shortcuts for audio player
Goats and Soda
- Infectious Disease
- Development
- Women & Girls
- Coronavirus FAQ
New malaria vaccine delivered for the first time

Mothers participate in a pilot program for one of two new malaria vaccines. Yasuyoshi Chiba/AFP via Getty Images hide caption
The Central African Republic is the first country to receive thousands of doses of a new malaria vaccine first recommended by the World Health Organization last October. A total of 43,000 doses arrived by air today from UNICEF, and another 120,000 are scheduled to show up in the coming days.
The vaccine — called R21/Matrix-M — will be used as part of children’s routine immunization.
It comes as welcome news in a country plagued by one of the highest incidences of malaria worldwide; more than 1.7 million cases were reported in 2022. “Previous concerns about supply meeting demand are firmly behind us,” said Leila Pakkala, director of UNICEF Supply Division, in a statement. “Now our priority is for the vaccines to reach every child at risk.”
"As a malaria researcher," says WHO Director-General Tedros Adhanom Ghebreyesus, "I used to dream of the day when we would have a safe and effective vaccine against malaria. Now we have two."
They're the first vaccines designed to work against a human parasite.
The first, called RTS,S, was unveiled more than two-and-a-half years ago. R21/Matrix-M is intended for children between 5 and 36 months, who are among the most vulnerable to the disease.
"A vaccine recruits the human immune system to fight the parasite as soon as it enters the body," says Dyann Wirth , chair of the WHO Malaria Policy Advisory Group. "A vaccinated person is poised to fight off the infection at its earliest stage."
WHO hopes that the combination of these vaccines will make a real difference, especially in Africa where malaria's toll is especially savage.
For example, in Burkina Faso in West Africa, pretty much everyone gets malaria. Last year, out of a population of 20-some million, about half got sick. Halidou Tinto was one of them. He leads the Clinical Research Unit of Nanaro in the country. His six-year-old twins also fell ill with malaria this year.
"As soon as [the children] are febrile or they complain about headache," Tinto says, "you have to think about malaria and treat them immediately. And you can avoid any bad outcome of the disease."
The worst outcome is death. Tinto says 4,000 people died of malaria last year in Burkina Faso alone. In 2021, across Africa, it's estimated that 619,000 died of the mosquito-borne disease, most of them children.
"People are living with the disease," says Tinto. "But of course, we are not happy and we are not proud of this."
This is what made WHO’s approval of the second malaria vaccine such welcome news.
Tinto ran the clinical trials in Burkina Faso that led to its recommendation. Across four African countries, these trials showed a 75% reduction in malaria cases in the year following vaccination of young children.
"I am very, very happy," says Tinto, "and we are pretty sure this vaccine will have a big impact in term[s] of public health."
That impact includes addressing a major shortfall in the supply of the first vaccine. And it comes at an important time in the fight against malaria, since numerous countries are now reporting growing resistance to what had been a powerful anti-malarial drug for decades.
"The estimates are that by adding the vaccine to the current tools that are in place," says Dr. Mary Hamel, senior technical officer with WHO, "tens of thousands of children's lives will be saved every year. So quite substantial."
The idea is that if the number of cases can be lowered, that'll reduce the amount of disease that mosquitoes can transmit. So not only will vaccinated kids receive protection, but even the unvaccinated will have a lower risk of being bitten by a mosquito carrying the parasite.
"It's very important to combine the existing tools," says Tinto, "including vaccine[s], drugs and bednet[s]."
The Serum Institute of India, who will be manufacturing the new vaccine, says a hundred million doses will likely be available to countries by the middle of next year.
"We hope that the objective of the WHO of eliminating malaria by the year 2030," says Tinto, whose twins have now recovered, "will be close if we are able to deploy this vaccine very quickly in Africa."
- Open access
- Published: 17 May 2023
Malaria vaccines: the 60-year journey of hope and final success—lessons learned and future prospects
- Amal A. El-Moamly ORCID: orcid.org/0000-0002-4821-285X 1 &
- Mohamed A. El-Sweify 2
Tropical Medicine and Health volume 51 , Article number: 29 ( 2023 ) Cite this article
14 Citations
153 Altmetric
Metrics details
The world has made great strides towards beating malaria, although about half of the world population is still exposed to the risk of contracting malaria. Developing an effective malaria vaccine was a huge challenge for medical science. In 2021 the World Health Organization (WHO) approved the first malaria vaccine, RTS,S/AS01 vaccine (Mosquirix™), for widespread use.
Main abstract body
This review highlights the history of development, and the different approaches and types of malaria vaccines, and the literature to date. It covers the developmental stages of RTS,S/AS01 and recommends steps for its deployment. The review explores other potential vaccine candidates and their status, and suggests options for their further development. It also recommends future roles for vaccines in eradicating malaria. Questions remain on how RTS,S vaccine will work in widespread use and how it can best be utilized to benefit vulnerable communities.
Malaria vaccines have been in development for almost 60 years. The RTS,S/AS01 vaccine has now been approved, but cannot be a stand-alone solution. Development should continue on promising candidates such as R21, PfSPZ and P. vivax vaccines. Multi-component vaccines may be a useful addition to other malaria control techniques in achieving eradication of malaria.
Developing an effective malaria vaccine has been a huge challenge for medical science and the world has made great strides towards beating malaria. It is one of the oldest of mankind’s deadliest enemies and is still a major health problem in many countries. According to the World Health Organization’s 2020 World Malaria Report, there were 229 million cases reported in 2019 and 409,000 deaths [ 1 ]. Children younger than 5 years old made up 67% of the deaths, and the disease is still killing 1 child every 2 min. In 2019, about half of the world population was exposed to the risk of contracting malaria [ 1 ]. Sub-Saharan Africa suffers the most, accounting for more than 90% of malaria cases and deaths annually.
Recent advances in control efforts have introduced many advances, including highly effective therapies, such as the artemisinin combination therapy, and rapid diagnostic tests. The wider use of insecticide-treated bed nets, various vector control measures, and preventive intermittent chemotherapeutic courses to vulnerable individuals, have all helped reduce the incidence of malaria. However, this reduction has recently slowed and the incidence may be increasing again [ 1 ]. According to the UN’s Sustainable Development Goals, the targets of number 3 (to ensure well-being and promote healthy lives for all individuals at all ages) are a 90% reduction in malaria incidence and mortality, and malaria elimination in at least 35 endemic countries by 2030 [ 2 ]. This increased concern requires extra tools to fight the disease. The approval of the RTS,S vaccine is just in time to maximize the public health benefit of all these efforts [ 3 ].
October 6, 2021 was a historic day, when the WHO approved the first malaria vaccine and parasitic vaccine, RTS,S/AS01 (RTS,S, also known as Mosquirix™) for widespread use. The vaccine significantly reduces total malaria cases, and the deadly form of the disease among young children [ 4 ]. Given that a malaria vaccine has been under development since the 1960s, this is considered to be one of medicine's biggest achievements [ 5 ]. The new vaccine was developed by GlaxoSmithKline (GSK), a British pharmaceutical company, and was first shown to be effective in 2015. We hope its approval will revived the battle against malaria. The WHO has recommended widespread use of the RTS,S vaccine to immunize children in regions with moderate-to-high transmission of P. falciparum malaria, i.e. mainly sub-Saharan Africa [ 5 ]. This decision is justified by good results from a pilot program implemented in three African countries (Kenya, Ghana and Malawi). The pilot program started in 2019 and has vaccinated some 800,000 young children [ 6 ]. The RTS,S vaccine was widely accepted by the communities involved and now it has been on approved, it will be routinely delivered in national childhood healthcare programs.
According to Dr. Tedros Adhanom Ghebreyesus, WHO Director-General, the long-awaited vaccine for children is a breakthrough for science, child health, malaria control, and a gift to the world. This first-ever vaccine for a parasite is a game changer that brings us one step closer to a malaria-free world. Using this vaccine in addition to existing prevention tools could save tens of thousands of young lives each year and change African lives forever [ 6 ].
"It has been a long way of hope for an effective malaria vaccine and now for the first time ever, we have such a vaccine recommended for widespread use…. Today’s recommendation offers a glimmer of hope for Africa, which shoulders the heaviest burden of the disease, and we expect many more African children to be protected from malaria and grow into healthy adults.” Dr. Matshidiso Moeti, WHO Regional Director for Africa [ 6 ].
The search for malaria vaccines was started in 1965 by immunologist Dr. Ruth Nussenzweig [ 7 ], although many scientists and companies also dedicated their lives to ending malaria [ 8 ]. There are now many potential vaccine candidates.
This review highlights the history of malaria vaccines, the different approaches to their development and different types of vaccines. It mainly looks at the stages of RTS,S/AS01 vaccine and recommends steps for deploying RTS,S. It generally explores other potential vaccine candidates, their status and challenges, and suggests prospects for further development. It also makes recommendations on the role of future vaccines in eradicating malaria.
A literature review searched PubMed, Scopus and Clarivate Web of Science up to 30 December 2021 for articles on malaria vaccine development. Terms included were: “malaria”, “WHO", “ Plasmodium falciparum ”, “RTS,S”, “RTS,S/AS01”, “Mosquirix™”, “vaccine”, “vaccination”, " approval", "pilot program", “pre-erythrocytic vaccine”, “erythrocytic vaccine”, "blood stage vaccine", “transmission blocking vaccine”, “circumsporozoite protein”, "whole sporozoite", "sporozoite subunit", "vectored vaccines", "R 21", "PfSPZ", and combinations of these. The initial search and screening of all papers was carried out by a contributor (OME), and the authors (AAE) and (MAE) re-assessed the content of all papers. Subjectively, 131 articles were included based on their relevance to the study objectives and aims. Preference was given to articles that comprehensively and/or appropriately covered the topics of interest. Additional articles were identified by visiting relevant websites, e.g. WHO, PATH global health organization (formerly Program for Appropriate Technology in Health) and major journals. No language restrictions were used.
Why did it take so long to develop a malaria vaccine?
The development of malaria vaccines has taken almost 60 years of hard work. The journey that started in the early 1960s was inspired by the remarkable success of vaccines against polio, measles, diphtheria, tetanus, rabies and other diseases. The complete eradication of smallpox in humans proved the potential of this approach to reduce the global burden of infectious diseases [ 9 ].
Initial attempts to develop a malaria vaccine resulted in great frustration. Researchers realized that vaccines against this disease would be challenging to develop and it became increasingly clear that it is due to a clever parasite. Impediments to successful malaria vaccination are multifactorial. The main difficulties were the malaria parasite’s ( P. falciparum ) extremely complex biology, life cycle and genome in addition to the parasite’s evasion of the human immune system and the absence of sterile immunity to the disease [ 10 ].
It is noteworthy that parasites are difficult to develop vaccines against. The recently approved RTS,S malaria vaccine is the only successful vaccine for a parasitic disease so far. Vaccines against parasites are difficult to develop because the human immune response to parasites is unique, due to their complicated life cycle and the immune escape mechanisms expressed by different parasites. Growing a sufficient number of whole parasites to generate an immune response is also a major challenge in order to develop a vaccine, despite the recent success with malaria [ 11 ]. To overcome this obstacle, efforts were directed at obtaining many types of parasite antigens (mainly proteins) or from vectors trying to induce a protective immune response [ 12 ]. It was also a major challenge to generate an adequate immune response based on small antigens that represented less than 1% of the whole parasite [ 8 ].
Factors like the complex life cycle, genetic diversity, pathophysiologic complexity, and the parasite’s various immune escape mechanisms lead to antigenic variations [ 13 ]. Because of the high number of polymorphisms or allele-specific variations in the proteins, single protein-based vaccines had limited success [ 14 ]. The Plasmodium parasites’ genetic make-up consists of about 5400 coding genes, and with the absence of adequate natural human immunity against the disease, these make malaria unique from other microbial pathogens for which successful vaccines have been developed [ 15 ]. Moreover, malaria has been mutating for 30 million years, and after a person has contracted malaria, they can only acquire partial immunity—unlike a virus which can elicit solid immunity [ 5 ]. The Plasmodium genome is much larger and more complex than bacterial or viral genomes. Its complicated life cycle has an asexual phase (schizogony) in humans and a sexual phase (gametogony) in mosquitoes [ 16 ]. Antigen expression is phase-specific [ 10 ] so different immune system arms are required depending on the parasite’s extracellular or intracellular location and distinct immunogenic properties. The protective antibodies against sporozoites (sexual forms transmitted by the mosquito in man) fail to recognize merozoites (asexual erythrocytic stages that cause clinical malaria). This means that if only one sporozoite evades the antibodies released as response to a vaccine, we can expect approximately 10,000–40,000 merozoites to be active after one week to start clinical disease. This poses a big challenge to developing a highly effective vaccine to malaria [ 8 , 17 , 18 ].
Targeting the erythrocyte stages of the life cycle is also difficult as they are subject to antigenic variation and can easily evade the human immune system [ 10 ]. Another challenge to developing a malaria vaccine is the ability of P. vivax and P. ovale to produce dormant hypnozoite stages in the liver, which are not tackled by the blood-stage vaccine candidates [ 10 ]. Another form of the parasite’s effective immune evasion is its capacity to mimic epidermal cell antigens and induce antigenic variations in blood cells and thereby inhibit apoptosis in liver cells [ 10 ]. Thus, there is no solid natural immune response in the course of malaria; after years of exposure only a weak and partial immunity can develop. Since natural immunity is directed against a wide-range of erythrocytic antigens, immunological studies have found it difficult to identify the best antigens for developing an ideal vaccine [ 19 ]. In addition, the species-specificity of P. falciparum and P. vivax, which do not infect most small animals or old world macaques that are used for models of vaccine evaluation, also posed a challenge. Plasmodium species that infect these animals are different from those that infect humans [ 10 ].
Another problem in developing a malaria vaccine was financial. Malaria mainly affects people in countries with limited resources, where there is little motivation or reward for investing in vaccines; instead manufacturers continued targeting industrialized first-world markets [ 20 , 21 ]. Malaria-endemic countries lack a robust healthcare infrastructure, so they present less attractive investment markets to large corporations, but put their efforts into vaccines for less serious diseases that can make a profit in Western markets [ 5 , 10 ]. In addition, investing in parasitic vaccines carries a higher financial risk because they are significantly more difficult to develop than virus vaccines. [ 5 ]. Malaria vaccine development has therefore suffered from less funding and fewer research initiatives [ 5 ]. Apart from the huge investments made by the Bill and Melinda Gates Foundation, only GSK has invested in a malaria vaccine. However, the evolution of public–private partnerships, such as the Malaria Vaccine Initiative of the Bill and Melinda Gates Foundation, offered hope for enhanced malaria research [ 10 ].
The strict regulations imposed by national vaccine licensing authorities were another barrier to the development of a vaccine. These increase the cost of clinical development pathways heavily. The pharmaceutical industry therefore has to charge more for a new vaccine to recoup its investment if it is not subsidized by non-government organizations and public–private partnerships [ 20 , 21 ].
History of malaria vaccine development
The history of modern malaria vaccine began in the early 1960s with experimental studies on primates, rodents and humans to test irradiated sporozoites [ 7 , 22 ]. The first promising results were documented by Clyde et al. in the 1970s [ 23 ] who found high protective efficacy from using radiation-attenuated sporozoites in persons of a high number of bites by irradiated infectious mosquitoes. Later, complete protection was demonstrated by using attenuated sporozoites using gamma radiation on infected mosquitoes in 2002 [ 24 ].
The promised major component of the sporozoite coat (circumsporozoite protein) was identified and its coded gene cloned and sequenced in the 1980s [ 25 ]. At that time, a range of blood-stage antigens was also identified and expressed, raising hopes for a blood-stage vaccine. However, preliminary trials did not show promising results for the candidate antigens and their efficacy on sporozoite challenge was statistically insignificant [ 26 ]. In 1988, asexual stage vaccine (SPf66 candidate), emerged in Colombia and had an acceptable efficacy in humans and animals (new-world monkeys) [ 27 ]. This peptide-based vaccine was interesting, but disappointing when field studies in Africa and Asia demonstrated insufficient efficacy [ 8 ]. However, the early studies on SPf66 and on sporozoite-based and mosquito-based vaccines led to further field technologies that were used to assess later vaccines (see section “ Types of malaria vaccines ”).
Types of malaria vaccines
Malaria vaccines are categorized according to the parasite’s targeted developmental stage: pre-erythrocytic vaccines (anti-infection), erythrocytic vaccines, and transmission-blocking vaccines (Fig. 1 ). Most malaria vaccines target one of these three phases [ 8 , 17 , 18 , 21 ], although some target two or three phases. A wide range of new vaccine technologies is now used.
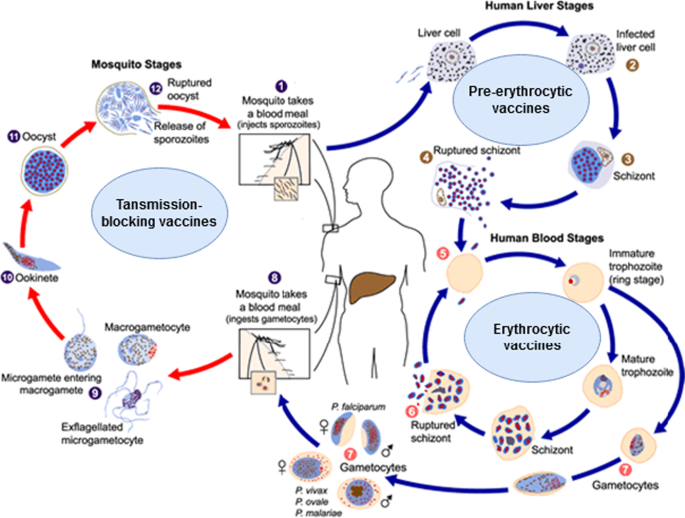
Life cycle of the malaria parasite and the vaccine types targeting various life cycle stages. Image courtesy of DPDx, Centers for Disease Control and Prevention ( https://www.cdc.gov/dpdx ). Image was adapted to show various malaria vaccines' target stages. Detailed information on malaria's life cycle is available on the provided website
Pre-erythrocytic vaccines (PEVs)
Experts believe that the best vaccine is one that attacks the early stages to completely block the development of subsequent stages, infection and transmission [ 3 ]. The pre-erythrocytic (liver stage) vaccines target sporozoites, i.e. the sexual forms transmitted by mosquito to man. PEVs are expected to induce antibodies to sporozoite surface antigens (needed to attack sporozoites in the skin and blood vessels) and prevent the invasion of the liver cells, and also induce a T-cell response needed to clear infected liver cells [ 17 ]. PEVs attack the critical early phase in which the sporozoites infect a few liver cells and need approximately one week of development in the liver phase—which gives enough time for the vaccine to act. However, the infected hepatocytes, unlike the infected erythrocytes, express parasite antigens that can induce T-cells to target and kill these cells, thus preventing merozoites being released into the blood [ 8 , 17 , 21 , 28 , 29 , 30 , 31 , 32 ]. Thus, PEVs with a high efficacy offer the opportunity to completely eradicate the hepatic pre-erythrocytic stages and prevent further infection [ 17 ]. PEVs are thought to be more effective vaccines than those directed against later stages [ 33 ]. They contain whole sporozoites or antigenic subunits of the circumsporozoite proteins [ 8 , 34 ].
Whole sporozoite vaccine (WSV)
Whole sporozoites are managed by radiation or by chemical or genetic attenuation, and are then given to recipients by mosquito bites. After entering the liver, they partially develop in the hepatocytes and induce a broad immune response, including CD4- and CD8-T cells, and antibodies, without causing disease [ 11 , 24 , 35 , 36 , 37 ]. Although whole sporozoite vaccines have induced sterilizing immunity to challenge sporozoites in humans, no further steps have been taken to complete the production of this type of vaccine [ 38 ]. Attenuating sporozoites by irradiation is costly and not easily applicable in a wider setting [ 24 ]. However, there is now renewed interest in the whole-organism vaccine as a result of a highly successful human trial using experimental sporozoite inoculation with chloroquine prophylaxis [ 39 , 40 ].
Genetically attenuated sporozoites were also evaluated as whole-parasite vaccines, in which the favorite candidates were genetically attenuated, late liver-stage parasites [ 41 ]. These parasites are unable to progress beyond the liver stage due to the loss of key genes. This type of vaccine generates a high amount of cross-stage and cross-species protection, and can even offer complete protection when administered by an intradermal or subcutaneous route [ 42 ]. Although genetic attenuation has the advantage of avoiding the irradiation step during the production process, it presents other challenges, like the delivery and manufacturing of a cryo-preserved, viable, whole parasite in a vaccine [ 43 ].
The P. falciparum whole sporozoite vaccine is currently in progress. In 2010, Sanaria Inc. developed a technology to harvest sporozoites of P. falciparum from the salivary gland of cultured, parasite-infected mosquitoes [ 11 ]. The sporozoites were attenuated using various technologies to make the vaccine. Radiation-attenuated vaccine was called PfSPZ, those attenuated in vivo by anti-malarial drugs were called PfSPZ-CVac, and genetically attenuated vaccine, prepared by gene deletion of essential genes [ 35 ], was called PfSPZ-GA1 [ 36 ].
Although there are major challenges to develop irradiated sporozoites, this approach offers a high rate of protection (exceeding 90% in trials). However, this efficacy rate was reported with only a few participants [ 24 ] and the efficacy in humans was dose-dependent [ 44 , 45 , 46 ]. PfSPZ vaccine efficacy showed comparable results with RTS,S vaccine in malaria-endemic settings [ 29 , 47 ]. Three to five doses of PfSPZ vaccine administered intravenously generated almost 100% protection against homologous, controlled human malaria infection (CHMI), when the NF54 strain was used in naive adults [ 44 , 48 , 49 ]. This regimen also showed a durable but partial protection against heterologous CHMI with 7G8-strain parasites in naive patients [ 49 ]. In malaria-endemic areas, a similar dosing in malaria-experienced adults provided more modest immunity against CHMI [ 50 ] and naturally occurring malaria [ 46 ]. There are several ongoing studies of PfSPZ vaccine in both adults and children.
Circumsporozoite protein subunit vaccines
Progress in genetic engineering corresponds with the high efficacy rate reported for whole sporozoite vaccine studies in human. The circumsporozoite protein (CSP) is a protein with a sequence of 412 amino acids; it is a major antigen component on the surface of the malaria sporozoite and is represented early on in the liver phase of infection. Identification of P. falciparum CSP led to the cloning and sequencing of the gene coding for the CSP—the first cloned malaria gene [ 33 , 51 ]. The CSP has continued to be a main focus in protein subunit vaccine development.
RTS,S vaccine The first approved malaria vaccine is RTS,S, a monovalent recombinant protein vaccine that targets a fragment of the CSP. The vaccine contains a truncated CSP of P. falciparum that is then fused with the hepatitis B surface (S) antigen, which acts as a carrier for the CS antigen and an immunogenic adjuvant, AS01 [ 52 ]. In RTS,S, vaccine, the “R” stands for the central repeat region of the P. falciparum CS protein; “T” stands for the T-cell epitope of the CS antigen; the first “S” for “Surface” portion, which when co-expressed on yeast cells display both CS protein and S on their surfaces, while the next “S” stands for the hepatitis B surface antigen. All are assembled in lipoprotein particles (RTS,S) [ 53 ].
RTS,S induces a strong IgG antibody response against the conserved central repeat region of the CS protein and potent T-cell (CD4 +) response [ 22 , 54 ]. Antibody levels reach high concentrations, often of hundreds of micrograms/ml. The levels correlate with the protection from malaria infection or clinical disease in several settings [ 32 , 55 ]. This vaccine has demonstrated 30–50% protection in field trials in humans in Africa [ 56 , 57 ]. Based on the pilot results, RTS,S vaccine has been approved by WHO for widespread use in malaria-endemic African countries. It seems that the RTS,S vaccine generates protective immunity and prevents clinical malaria by reducing the merozoites emerging from the hepatic cells. This low number of merozoites reduces the sexual-stage development in the blood cells to a subclinical level, which in turn induces a natural blood-stage immune response and boosts protection [ 58 ]. Details of the developmental phases of RTS,S vaccine and its efficacy studies are given in section B.
New developments in pre-erythrocytic vaccines
R21 vaccine The R21 vaccine (“next-generation RTS,S-like vaccine”) is an improved version of the RTS,S vaccine developed by the Jenner Institute in Oxford, UK [ 59 ]. The R21 and RTS,S vaccines are both virus-like particle-based vaccines based on CSP. R21, however, is formed solely from CSP-HBsAg fusion particles, with a fused CSP-hepatitis B surface antigen. The removal of the unfused S particles is believed to improve the immune response against the CSP, which comprises a higher proportion in R21 than in RTS,S. In addition, R21 was developed to induce a lower immune response against the HBsAg fraction [ 3 ]. Both RTS,S and R21 are attached to adjuvants that act as carriers that also boost immunity. However, the adjuvant of the R21 can be more easily manufactured than that of RTS,S, which will hopefully make it cheaper to prepare. The R21 with adjuvant Matrix-M (R21/Matrix-M vaccine) has been developed by Oxford University scientists and has shown an enhanced T-cell response and high protection rate in a Phase II clinical trial on children in a high-malaria-transmission setting [ 60 ]. However, questions remain regarding the efficacy of R21 vaccine against CHMI in naive individuals and against naturally occurring malaria in malaria-experienced persons living in endemic areas [ 3 ].
Cell-traversal protein antigen of ookinete and sporozoite (CelTOS) vaccine Another antigenic pre-erythrocytic vaccine candidate has been developed using a novel antigen, the cell-traversal protein antigen found in ookinete and sporozoite (CelTOS). This protein antigen was identified as an essential protein for the traversal of Plasmodium in mammalian and insect hosts [ 61 ]. The evaluation of the CelTOS vaccine candidate in a mouse model revealed a completely sterile immunity against sporozoite challenge [ 62 ].

Viral-vectored vaccines
The viral-vectored vaccine approach has been used to enhance cellular immunity against the pre-erythrocytic stages [ 8 ]. Evaluation of this approach in humans found a strong immune response, mainly from an increased proliferation of CD8-T cells against the viral-vectored-CSP targets. However, the protection rate did not exceed that induced by the RTS,S vaccine [ 63 ]. Many vector vaccine generations have been clinically evaluated in trying to promote comparable efficacy [ 64 , 65 , 66 ].
The vectors used in this approach included chimpanzee adenoviruses [ 64 ], boosted by the modified vaccinia virus Ankara [ 67 ]. This boosted approach, used here for the first time in vaccines, resulted in an improved T-cell immune response compared to using only one viral vector [ 67 , 68 , 69 ]. Other vectors used included the adenoviruses Ad35 and Ad 26 [ 70 ], which, like other chimpanzee viruses, resist the harmful effects of a naturally acquired immune response to human adenoviruses.
The viral-vectored pre-erythrocytic vaccines have encompassed various protein antigens including CSP, and thrombospondin-related adhesion protein (TRAP). Blood-stage antigens such as merozoite surface protein-1 (MSP1) and apical membrane antigen-1 (AMA1) have also been tried. Another approach used plasmid DNA as priming vector, and a human adenovirus, Ad5, to boost the immune response [ 65 ]. Several antigenic inserts from both pre-erythrocytic stage and blood stages showed encouraging efficacy.
Challenges facing the development of circumsporozoite protein vaccines
The targeted CSP antigens of the vaccines, as in many Plasmodium antigens, have shown antigenic variation, including the targeted antigen of the RTS,S C-terminal region. In a Phase III trial on RTS,S, better efficacy was seen against parasites that had a matched sequence with the C-terminal region of the vaccine sporozoites [ 30 ]. This means that the unmatched parasite variants may escape the vaccine’s action and may continue to spread in the community. Another challenge for the RTS,S vaccine is its structure, which does not include an N-terminal region of the CSP that is crucial for the attachment to and invasion of the sporozoites into the liver cells [ 71 ]. The N-terminal region has shown induced natural immunity associated with malaria protection in African children [ 72 ]. Improved CSP-vaccines are being developed to prime vaccine-immune response by selecting various antigenic epitopes that show protective antibodies [ 17 ]. The fact that whole sporozoite vaccines induce better protection than subunit vaccines [ 24 , 73 ] suggests that antigen-combination strategies are necessary. Further research into other potential malaria vaccine antigens and strategies for their delivery is therefore essential [ 52 ].
Erythrocytic vaccines (blood-stage vaccines)
These vaccines act when the merozoites are released from the liver (after completion of the pre-erythrocytic stage) and enter the blood to infect erythrocytes. Hence, these vaccines are also referred to as blood-stage vaccines. Their goal is to block the invasion of red blood cells by the merozoites, prevent the parasite’s asexual reproduction and to elicit anti-invasion and anti-disease responses [ 74 ]. These blood-stage vaccines induce antibodies to the surface antigens of the merozoites and against variant antigens on the red blood cell membranes [ 75 , 76 , 77 ]. Unlike the promising progress in the pre-erythrocytic vaccines, progress in erythrocytic vaccines has been slow [ 78 ]. Development of the blood-stage vaccines faces many challenges, including the very short time that the merozoites are freely available outside the erythrocytes for easy attack by the induced antibodies, the large number of merozoites that need to be targeted compared with the low number of sporozoites in the pre-erythrocytic phase, the antigenic diversity, and the many invasion pathways [ 17 ]. How to address genetic polymorphism is an important issue to explore for this group of vaccines. It has been suggested that efforts should concentrate on antigens or constructs inducing cross-reactive immune responses, which would cover genetic diversity.
Several blood-stage antigens have already been tried: erythrocyte-binding antigen-175 (EBA-175) [ 79 ], apical membrane antigen-1 (AMA-1) [ 80 ], glutamate-rich protein (GLURP) [ 81 , 82 ], serine repeat antigen 5 (SERA5) [ 83 , 84 ] and merozoite surface protein (MSP-1) [ 85 ], MSP-2 [ 86 ], and MSP-3 [ 87 , 88 ]. All these antigens are highly expressed on the surface of the merozoites, but have not shown a significant impact on clinical malaria. After these disappointments, other antigens with strong immunogenicity and great potential as blood-stage vaccine candidates were suggested. For example, the merozoite antigen, P. falciparum reticulocyte-binding protein homologue 5 (PfRH5) has been shown to generate neutralizing antibodies that target its common genetic variants [ 89 , 90 ]. However, PfRH5 has exhibited limited polymorphism and pre-clinical studies showed that the antigen is the first, very conserved blood-stage antigen that generates broadly inhibiting antibodies [ 90 ]. Notably, natural infections induce modest or no antibody against PfRH5 [ 90 , 91 , 92 ]. In addition, rhoptry-associated leucine zipper-like protein-1 (RALP-1), which plays an important role during merozoite invasion into erythrocytes, has been suggested as a target [ 93 ]. Another new blood-stage vaccine, a combination of AMA-1 with the rhoptry-neck protein RON2 (AMA1-RON2) has attracted interest because its binding at the merozoite–erythrocyte junction induces cell invasion. However, AMA1-RON2 showed low efficacy in previous studies. This combined antigen can induce improved immunogenicity of non-combined AMA-1 antigen with more effective anti-invasion inhibitory antibodies [ 94 ].
Other new blood-stage vaccine antigens include those parasite antigens that are expressed on the infected red blood cells; these stay available for hours to be targeted by the induced antibodies. Of these, the PfEMP1 is an immunodominant virulence antigen that facilitates sequestering of the P. falciparum parasites and is targeted by naturally acquired immunity [ 95 ]. No further progress has been made with the PfEMP1 vaccine because the antigen is large and has high genetic polymorphism, with a complicated structure of cysteine-rich content. No evaluations have assessed PfEMP1-vaccine efficacy.
Another erythrocyte surface protein, called PfGARP, has been described as a target for protective antibodies [ 96 ] and P. falciparum Schizont Egress Antigen-1 (PfSEA-1), which emerges from infected blood cells, has also been identified [ 97 ]. After repeated disappointments with various blood-stage vaccine candidates, scientists have tried other erythrocytic-stage antigens which are chemically attenuated by culturing with a DNA-binding agent, tafuramycin-A. These attenuated erythrocytic-phase parasites (CAP) induce homologous as well as heterologous immunity in mice, and their protection depended on CD4 + T cells [ 98 , 99 , 100 ].
Transmission blocking vaccines (TBVs) (mosquito stage vaccines)
TBV vaccines aim to induce antibodies against functionally important proteins that are expressed on developmental stages of the parasite in the mosquito [ 101 ]. They target antigens on parasite gametes, zygotes and ookinetes [ 52 ]. The TBVs block the infection transmission from human to mosquito and so prevent malaria spreading [ 102 ]. These vaccines generate antibodies that prevent the Plasmodium sexual reproduction in the mosquito by blocking either the fertilization of the gametes, the transition of ookinete-to-oocyst, the development of the zygote into sporozoites [ 103 , 104 ], or the sporozoites' invasion of the salivary gland [ 105 ].
The main transmission blocking vaccine candidates that are currently being developed include Pfs-25, Pfs-48/45, and Pfs-230 [ 17 , 106 ]. Both Pfs-48/45 and Pfs-230 are gametocyte-expressed antigens that are present in human and mosquito vectors and continue forming as a protein complex on the P. falciparum gamete surface [ 107 ]. The antibodies formed against the gametocyte and its Pfs-230 and Pfs-48/45 antigens during the naturally acquired immune response have induced transmission blocking activity [ 108 ].
The major limitation of TBVs is that they do not protect the recipient from contracting malaria as they do not impede the infection route. They might be helpful in reducing disease transmission in the long run, after mass immunization has been achieved. So they could benefit the whole community and hence the terms ‘community vaccine’ and ‘altruistic vaccination’ are becoming popular [ 8 ]. However, this approach is unattractive for individuals or for Western travelers, who are the major driver of vaccine development efforts [ 10 ].
Another important limitation of TBVs is their low efficacy, because human immune mechanisms are not naturally exposed to TBV candidate antigens, and thus the boost to immunity is limited [ 109 ]. Some have proposed that malaria might adapt to a new vector, or to an alteration of certain protein compounds required for interaction with the vector [ 10 ]. In addition, because TBVs should target all individuals (including children and infants) who can transmit the disease to accomplish herd immunity, this type of mass vaccination would pose a major logistical challenge [ 8 ]. Furthermore, TBVs must have an exceptional safety profile, since they do not confer a direct benefit to the individual [ 17 ]. Hence, some have recommended their application be combined with efficacious pre-erythrocyte vaccines to prevent both infection in humans and transmission to mosquitoes, and these could also be combined with blood-stage vaccines that would add a synergistic effect by reducing onward transmission [ 101 , 110 ]. Nevertheless, TBVs could still be important tools in malaria elimination and eradication programs, for preventing transmission [ 111 ].
Plasmodium vivax vaccines
Although most research and funding efforts have so far been dedicated to developing P. falciparum vaccines, P. vivax vaccine also deserves attention. P. vivax forms an important public health problem, with a high burden and high rates of morbidity and mortality in many settings [ 112 ]. In addition, P. vivax has been shown to induce sterile heterologous immunity in human studies [ 113 , 114 ]. Some promising attempts have been made to develop a P. vivax vaccine, including a pre-erythrocytic vaccine of circumsporozoite protein (Pv-CSP), a blood-stage vaccine of merozoite Duffy Binding Protein (Pv-DBP), and transmission blocking vaccines (Pv-s25) [ 115 , 116 , 117 ]. These candidates have progressed to pre-clinical and clinical trials with promising results. Viral-vector vaccines [ 116 ] as well as recombinant antigen [ 117 ] approaches have also been used with Pv-DBP. Good transmission blocking was reported with Pv-s25 with a well-tolerated and modest antibody response in mosquito studies [ 115 ] .
Figure 2 shows a summary of the malaria vaccine candidates with their type and developmental phases [ 17 ]. A timeline of the major turning points in the creation of pre-erythrocytic malaria vaccinations is shown in Fig. 3 a. The major turning points in the creation of erythrocytic malaria vaccines are shown in Fig. 3 b. Figure 3 c depicts a timeline of the significant turning points in the creation of transmission-blocking vaccinations.
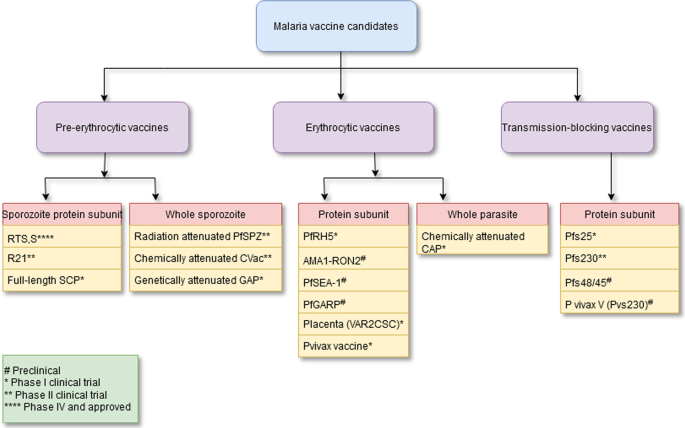
Summary of malaria vaccine candidates with their type and developmental phase. See reference [ 17 ]

a A chronological diagram showing the main milestones in the development of the pre-erythrocytic malaria vaccines. b A chronological diagram showing the main milestones in the development of the erythrocytic malaria vaccines. c A chronological diagram showing the main milestones in the development of the transmission-blocking malaria vaccines
The first approved malaria vaccine: history of RTS,S vaccine development
The first pre-erythrocytic RTS vaccine was created in 1987 and, by 2019, it was tested in a pilot program in seven African countries with endemic malaria. In 2021, RTS vaccine was the first malaria vaccine approved for widespread use. Figure 3a shows the main steps in the development of RTS,S vaccine from creation to approval.
RTS,S—creation and early evaluation
The RTS,S vaccine was created in 1987, as a result of a collaboration that began in 1984 between the multinational pharmaceutical company GSK and the Walter Reed Army Institute of Research (WRAIR, Maryland, U.S.A.) [ 22 ]. At that time both groups were trying to create a vaccine, based on studies that proved good efficacy of attenuated-sporozoites in protecting against malaria [ 23 ]. Other pre-clinical studies revealed that CSP inoculation could induce antibodies that protected against active P. falciparum infection [ 118 ]. This vaccine was effective only against P. falciparum but not against P. vivax or any other types of malaria [ 33 ]. The first attempt to develop RTS vaccine was to identify the CSP antigen as a target of the immune response generated by radiation-attenuated sporozoites. The U.S. National Institutes of Health (NIH) and WRAIR [ 51 , 119 ] then cloned and sequenced this antigen. They found it difficult to produce a whole-length CSP antigen, so they replaced it by using GSK’s Escherichia coli elaboration system and produced a central-repeat region subunit antigen [ 120 ].
The initial structure of the vaccine did not yield good efficacy. It was formed from a CSP-tandem-repeat region, chiefly the NANP 4-amnio acid sequence [ 26 ]. Expressing the central repeat (R), a single polypeptide chain corresponding to a highly conserved, tandem repeat tetrapeptide NANP amino acid sequence, fused to the C-terminal region known to contain T cell epitopes (T). To add a carrier to the central repeat region, the RT particle was fused to the hepatitis-B surface antigen (S), yielding a yeast-expressed protein RTS [ 54 ]. However, to generate immunogenic particles, this protein needed to be co-expressed with large amounts of the unfused S protein. Another unfused hepatitis-B surface antigen portion was added—a second (S)—that spontaneously fuses to the RTS component, hence the name became RTS,S. Then they tried to add many adjuvants to the vaccine. In 1996, a key study assessed several adjuvants and found that the highest efficacy was obtained with the RTS,S vaccine which had an adjuvant that contained monophosphoryl lipid-A, which is an immune stimulant agonist of toll-like receptor 4, and a Quill A derivative [ 54 ]. When using this adjuvant (called AS02) and a related adjuvant (AS01), the RTS,S vaccine produced a protective efficacy of 30–50% in healthy participants challenged by sporozoites in a series of studies. The AS01 adjuvant showed higher protection than AS02, with higher numbers of antibodies against CSP and a higher CD4 + T-cell response in naive participants in a CHMI study [ 121 ]. These findings were confirmed by a study in Kenya [ 47 ]. This RTS,S/AS01 formulation of the vaccine was subsequently tested as part of Phase II and Phase III trials and in the implementation program [ 122 , 123 , 124 , 125 , 126 ]. Their results led to the RTS,S vaccine being approved for widespread use to protect against P. falciparum malaria in African countries.
RTS,S Phase II clinical trials
In 2003 to 2004, and encouraged by earlier results that demonstrated the strong immunogenicity of RTS,S vaccine, a Phase IIb, double-blind randomized controlled clinical trial was implemented with more than 2000 children aged 1 to 4 years in Mozambique [ 56 ]. In this trial, three vaccine doses reduced the incidence of malaria by 37% compared to the control group in the 6 months following the third dose. The efficacy of the vaccine was estimated as 1 minus the ratio of the incidence rates in each group. For all clinical events, efficacy was 27%, while for severe cases it was 58%. The 12-month follow-up raised efficacy to 29% for all malaria cases, whereas it dropped to 39% for severe cases. The 18-month follow-up had an efficacy of 35% for all malaria events and 49% for severe malaria. While the children’s young age in this trial has shown no association with RTS,S efficacy, later Phase IIb clinical trials in African infants has shown an efficacy of 65% in the 6 months following RTS,S vaccination [ 122 , 127 , 128 ].
RTS,S Phase III clinical trials
The encouraging results of the early trials on RTS,S/AS01 vaccine led to a Phase III randomized trial from 2009–2014 (conducted by a collaboration between a private foundation, a vaccine manufacturer, and a public health agency). The Phase III trial enrolled 15,459 children at 11 locations in seven African countries (Burkina Faso, Ghana, Malawi, Gabon, Kenya, Mozambique and Tanzania) [ 129 ]. The vaccine was delivered as three doses of 0.5 mL and administered intramuscularly at monthly intervals, followed by a fourth dose (booster) 18 months after the third dose. The primary end point was clinical malaria events, which were reduced by almost 26% of the pre-trial rate in infants and by almost 36% in young children after four doses. Vaccinated young children, rather than infants, also showed protection against severe malaria. However, the efficacy of the RTS,S vaccine declined with time and clinical malaria events dropped to 68% of the pre-trial rate in the first six months [ 130 ].. RTS,S is about 56% effective over one year and 36% effective over four years. The prevented malaria events were 1774 in 1000 children who were on the 4-dose regime and 1363 in 1000 children who were on the 3-dose regime [ 129 , 130 ]. Most of the prevented events were reported in high transmission settings. However, the vaccine’s efficacy was estimated to be higher in settings of low incidence, but the difference was not statistically significant.
The 2015 milestone: approval by the European Medicines Agency
In 2015, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) documented that the RTS,S/AS01 vaccine had an acceptable safety profile that was to be continually monitored [ 131 , 132 ]. The CHMP gave a supporting scientific decision for use of RTS,S outside the European Union “in areas where malaria is regularly found, for the active immunization of children of six weeks up to seventeen months old against malaria caused by the P. falciparum parasite, and against hepatitis B”[ 131 ].
In late 2015, two main WHO groups, the Strategic Advisory Group of Experts (SAGE) and the Malaria Policy Advisory Group (MPAG), reviewed the findings of the Phase III clinical trial on RTS,S. In January 2016, and based on the recommendations of both advisory groups, the WHO approved a pilot implementation program on RTS,S vaccine in three moderate and high-transmission African countries using the four-dose protocol. The pilot program was started in 2019.
RTS,S 2019 pilot program
In April 2019, the WHO launched the RTS,S pilot program in three African countries (Malawi, Ghana, and Kenya) to assess vaccine effect on childhood mortality, its safety during routine use in the national immunization programs, and the feasibility of delivering four doses to children [ 132 ]. The three-dose intramuscular vaccination schedule for infants was performed at 6, 10, and 14 weeks of age. For older children, the three monthly doses were started at 5–17 months old. The fourth booster dose was given 18 months after the third dose in all age groups [ 132 ]. The vaccine was administered through the routine national immunization program, coordinated by the Ministry of Health in each country.
Up to the end of September 2021, and despite the COVID-19 pandemic, over 800,000 children were included in the pilot program [ 133 ]. Together with the results of earlier clinical trials, the key findings from these three countries have informed the WHO’s decision on RTS,S vaccine. In October 2021, the WHO recommended the widespread use of the vaccine for children in moderate–high transmission settings in Africa and other places [ 133 ].
Summary of the key findings of the pilot program
The pilot program has revealed a high uptake of the RTS,S vaccine and re-confirmed its positive safety profile. RTS,S has significantly reduced severe malaria, life-threatening incidence, and children’s hospitalization due to malaria. The pilot has generated evidence and experience on the feasibility, impact and safety of the vaccine in routine, real-life situations. The pilot has also yielded the following findings [ 133 ]:
RTS delivery was feasible despite the COVID-19 pandemic and equity in the vaccine coverage was achieved everywhere as part of routine child immunization programs.
RTS has been reaching (very nearly) all vulnerable children. The introduction of RTS,S has increased the percentage of children reached by malaria prevention methods to over 90% (insecticide-treated nets or RTS,S vaccine). In the three countries, more than two-thirds of those who were not using bed-nets benefited from the vaccine.
RTS showed a good safety profile: up to October 2021, the number of administered doses exceeded 2.3 million in the three countries with advantageous safety outcomes.
RTS introduction has not negatively affected bed-net use, the child vaccination programs, or people seeking healthcare for other febrile diseases.
RTS has had a major effect on real-life child vaccination settings: the vaccine reduced fatal and severe malaria events by 30%, even in settings that widely used bed-nets for prevention and in the presence of good malaria healthcare.
RTS is highly cost-effective : modeling studies have shown that RTS is cost-effective in endemic settings.
The pilots are planned to continue through 2023 in these three countries, with the aims of evaluating the outcome of the fourth dose and to assess its effect on child mortality in the longer term [ 133 ].
RTS,S supporters
Thirty years of collaboration in research and development between GSK, PATH global health organization, and African research partners have led to the RTS malaria vaccine. The generous funding from the Bill & Melinda Gates Foundation in 2001 through 2015 catalyzed the later stage of the vaccine development [ 133 ]. The pilot program launched in 2019 was financially supported by a significant collaboration between Vaccine Alliance, Gavi, Unitaid, and the Global Fund to Fight AIDS, Tuberculosis and Malaria [ 133 ]. The pilot program was also supported and coordinated by many national and international partners, including the WHO, UNICEF, PATH and GSK (GSK donated ten million RTS,S vaccine doses). National consortiums of evaluation partners collected the data for each pilot program to inform the WHO [ 133 ].
Future prospects for successful malaria vaccines
There are new malaria vaccines on the horizon, features of good malaria vaccine are outlined, and the next steps required for the approved and developing vaccines are discussed in the sections below.
New malaria vaccines on the horizon
There are two main P. falciparum vaccines at an advanced stage of development, R21 and PfSPZ. They are being continually tested in clinical trials in naive and experienced malaria participants for both safety and efficacy. The two vaccines are included in the WHO-Rainbow Tables, along with other candidates [ 134 ] and have recently been reviewed [ 135 , 136 ]. In addition to R21 and PfSPZ vaccines, BioNtech efforts to develop a vaccine based on mRNA technology are ongoing, inspired by their success in COVID-19. This approach may be an answer to the challenges facing malaria vaccine development, which include the evasion of immune mechanisms by the malaria parasite [ 60 ]. It is hoped that an mRNA malaria vaccine will have high efficacy be easily manufactured, and safe for all individuals.
Features of ideal malaria vaccine
Many experts have suggested that a highly effective vaccine is likely to include antigens from multiple stages of the parasite’s life cycle. It is hoped that the multi-component vaccine suggested will induce an effective and sustainable protective response [ 137 ]. The multi-component vaccine should generate protection against sporozoites, sexual and asexual stages, and also against infected liver cells. This vaccine should also elicit different types of immune reaction, i.e. humoral and cell-mediated responses. In addition, to conquer the antigenic and genetic variations, the vaccine should include several epitopes that are represented by various molecules of the major histocompatibility complex (MHC) [ 137 ]. However, there are still some challenges that may impede development of the multi-component vaccine, including increased cost of manufacturing, unless it can be given by a single delivery approach like the pox-viral vector [ 138 , 139 ].
An example of a combination vaccine is to combine a protein/adjuvant vaccine, specifically RTS,S, that induces antibodies to clear sporozoites before they can enter the liver, and vectored vaccines that clear infected liver cells through activation of T cells. When administered as a simple mixture, the two vaccines have shown to provide 90% sterile efficacy [ 140 ]. The RTS,S vaccine can reduce over 95% of the sporozoites before they enter the liver cells, while the vector vaccine can reduce the number by more than 90%. The synergistic effect of both vaccines, based on what has already been reported in clinical trials for each individual vaccine, would speed up the development of the highly effective vaccine [ 8 ]. Besides being highly effective, the ideal malaria vaccine should also be safe, stable under various conditions such as temperature, light and transportation, easy to administer, and must provide long-term immunity. Such vaccines should also be cost-effective and affordable in poor malaria-endemic areas [ 141 ].
Next steps on the road toward successful malaria vaccines
Now the WHO has finally approved the wider utilization of the RTS,S vaccine, the question remains how well the vaccine will work over a wider area and how we can best utilize it to benefit the malaria-endemic communities. The potential impacts of the vaccine on health status, childhood mortality, poverty, and social justice for people living in endemic areas are important issues that need to be monitored. Therefore, evaluations are required to measure these long-term impacts of the vaccine [ 133 ].
Further steps may also include decisions on funding opportunities that will be very important in defining how broadly the vaccine can be used in the most needy communities and in determining national decisions on adopting the vaccine in endemic countries. An operational guide is also needed to lead countries through what is required to integrate the malaria vaccine into the national immunization program and its use alongside other preventive tools like bed-nets [ 60 ].
The RTS vaccine has enabled us to meet the first target of the Malaria Vaccine Technology Roadmap that was published in 2006 [ 142 ]; it is a first-generation vaccine with at least 50% efficacy lasting for one year. Further, we look forward to meeting the second target of the roadmap, which is to have a second-generation vaccine with at least 80% efficacy lasting four years by 2025. There is ongoing work to develop extra types of malaria vaccines and a variety of vaccine candidates are showing promise for the 2025 target [ 8 ]. The recently developed pre-erythrocytic vaccine candidates like PfSPZ, R21 and full-length circumsporozoite protein immunogens are being improved in efficacy [ 17 ]. At the same time, the transmission blocking vaccines have progressed to advanced-phase trials. Combining both transmission blocking vaccines with pre-erythrocytic vaccines like RTS with other tools of malaria control would certainly benefit the malaria eradication programs [ 17 ]. Future advances for the RTS,S vaccine may include improved protection through a schedule of fractionated delayed doses and alternative adjuvants.
Unfortunately, blood-stage vaccines that target merozoite invasion proteins have so far delivered disappointing efficacy. Novel targets of blood-stage vaccines, like infected erythrocytes’ surface proteins, egress antigens that emerge from schizonts and attenuated, intact infected red blood cells, continue to be developed [ 17 ]. In addition, the substantial progress made with P. falciparum vaccine justifies increased efforts and investment in P. vivax vaccines to pursue similar goals and to achieve the ultimate aim of malaria eradication [ 17 ].
Finally, although successful vaccines such as RTS,S/AS01 have proven to prevent clinical malaria in immunized individuals, they might not be sufficient as a stand-alone measure for global malaria eradication. These vaccines should be taken as an addition to current control measures rather than as a replacement for them. A protocol on how to incorporate the vaccine into other control measures, to eradicate malaria successfully is being developed by the WHO [ 60 ]. Figure 4 summarizes the future prospects for successful malaria vaccines, itemizing the conditions for which malaria vaccines are needed.

Features of a needed malaria vaccine and prospects for successful malaria vaccines
After almost 60 years of struggling to achieve the dream of having an efficacious vaccine as a tool to fight malaria and to conquer its enormous burden, the long awaited moment finally arrived in 2021. The complicated life cycle of Plasmodium , its genetic diversity, and the absence of sterile immunity in malaria has long presented a challenge to malaria vaccine development. Modern malaria vaccine development stemmed from studies in the 1960s that immunized mice with irradiated sporozoites. There was continual progress on malaria vaccine candidates. The first-ever malaria vaccine (and also the first parasite vaccine), RTS,S AS01 was approved for widespread use on 6 October 2021. The WHO recommended the broader use of the vaccine among children at risk in African countries and in other areas where P. falciparum has high or moderate transmission. This decision was justified by the favorable efficacy and safety results of the RTS vaccine in Phase II and III clinical trials and in a pilot program conducted in three African countries. The studies showed that RTS was about 56% effective over one year and 36% effective over four years, with an acceptable safety profile. The questions now remain how well RTS,S vaccine will work with widespread use, what are its long-term impacts on child health and on targeted communities, and how can we benefit the most from its use in the worst affected and endemic communities.
Nonetheless, we still need the newer vaccine candidates—including pre-erythrocytic, erythrocytic and transmission-blocking vaccines—to be developed. A multi-component vaccine that increases the probability of a sustainable and effective host response may prove very promising. More efforts and investment in P. vivax vaccines should also be encouraged, in order to attain global malaria eradication.
Finally, although RTS,S vaccine has been approved for wider use in endemic African countries and elsewhere, it might not be sufficient as a stand-alone measure for effective malaria control. In order to achieve malaria elimination, it is wiser to consider the vaccine as an addition to current measures rather than as a replacement for them. A protocol to guide countries on how to incorporate the vaccine into their control measures is being developed.
Availability of data and materials
Not applicable.
Abbreviations
World Health Organization
GlaxoSmithKline
Global health organization (formerly Program for Appropriate Technology in Health)
- Pre-erythrocytic vaccines
Circumsporozoite protein
Whole sporozoite vaccine
Controlled human malaria infection
- Transmission blocking vaccines
Duffy Binding Protein
Walter Reed Army Institute of Research
National Institutes of Health
Committee for Medicinal Products for Human Use
European Medicines Agency
Strategic Advisory Group of Experts
Malaria Policy Advisory Group
Coronavirus disease of 2019
United Nations Children's Fund
Major histocompatibility complex
WHO. World malaria report. 20 years of global progress and challenges. Geneva: World Health Organization; 2020. p. 2020.
Google Scholar
WHO. Towards a global action plan for healthy lives and well-being for all. Geneva: World Health Organization. 2018.
Laurens MB. RTS, S/AS01 vaccine (Mosquirix™): an overview. Hum Vaccin Immunother. 2020;16(3):480–9. https://doi.org/10.1080/21645515.2019.1669415 .
Article CAS PubMed Google Scholar
WHO. Malaria fact sheet 2021. Geneva: World Health Organization; 2021.
WHO. WHO approves first malaria vaccine: why did it take so long? Geneva: World Health Organization. 2021.
WHO. WHO recommends groundbreaking malaria vaccine for children at risk. Geneva: World Health Organization. 2021.
Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–2. https://doi.org/10.1038/216160a0 .
Hill AV. Vaccines against malaria. Philos Trans R Soc Lond B Biol Sci. 2011;366(1579):2806–14. https://doi.org/10.1098/rstb.2011.0091 .
Article PubMed PubMed Central Google Scholar
Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–63.
Article CAS PubMed PubMed Central Google Scholar
Lorenz V, Karanis P. Malaria vaccines: looking back and lessons learnt. Asian Pac J Trop Biomed. 2011;1(1):74–8. https://doi.org/10.1016/S2221-1691(11)60072-5 .
Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccine. 2010;6(1):97–106. https://doi.org/10.4161/hv.6.1.10396 .
Article CAS Google Scholar
Anders RF, Adda CG, Foley M, Norton RS. Recombinant protein vaccines against the asexual blood stages of Plasmodium falciparum . Hum Vaccine. 2010;6:39–53. https://doi.org/10.4161/hv.6.1.10712 .
Rénia L, Goh YS. Malaria parasites: the great escape. Front Immunol. 2016;7(7):463. https://doi.org/10.3389/fimmu.2016.00463 .
Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473:463–9. https://doi.org/10.1038/nature10124 .
Cappadoro M, Giribaldi G, O’Brien E, Turrini F, Manu F, Ulliers D, et al. Early phagocytosis of glucose-6-phosphate dehydrogenase (G6PD)-deficient erythrocytes parasitized by Plasmodium falciparum may explain malaria protection in G6PD deficiency. Blood. 1998;92(7):2527–34.
Pannu AK. Malaria today: advances in management and control. Trop Doct. 2019;49(3):160–4. https://doi.org/10.1177/0049475519846382 .
Article PubMed Google Scholar
Duffy PE, Patrick GJ. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines. 2020;5:48. https://doi.org/10.1038/s41541-020-0196-3 .
Matuschewski K. Vaccines against malaria-still a long way to go. FEBS J. 2017;284(16):2560–8. https://doi.org/10.1111/febs.14107 .
Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. https://doi.org/10.1111/j.1365-3024.2006.00808.x .
Clemens J, Jodar L. Introducing new vaccines into developing countries: obstacles, opportunities and complexities. Nat Med. 2005;11(4 Suppl):S12–5. https://doi.org/10.1038/nm122516 .
Bloom BR, Lambert PH (eds). The vaccine book. 15th edn. Amsterdam: Academic Press; 2003:345–370.
Ballou WR, Cahill CP. Two decades of commitment to malaria vaccine development: Glaxo Smith Kline Biologicals. Am J Trop Med Hyg. 2007;77(6 Suppl):289–95.
Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973;266(3):169–77.
Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–64.
Anonymous. Malaria vaccine is near. New York Times. 1982, 3 August.
Ballou WR, Hoffman SL, Sherwood JA, Hollingdale MR, Neva FA, Hockmeyer WT, et al. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987;1:1277–81. https://doi.org/10.1016/S0140-6736(87)90540-X .
Patarroyo ME, Amador R, Clavijo P, Moreno A, Guzman F, Romero P, et al. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988;332(6160):158–61. https://doi.org/10.1038/332158a0 .
Sadoff JC, Ballou WR, Baron LS, Majarian WR, Brey RN, Hockmeyer WT, et al. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science. 1988;240(4850):336–8. https://doi.org/10.1126/science.3281260 .
RTS,S Malaria Vaccine Trial Team, Bojang KA, Milligan PJ, Pinder M, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358(9297):1927–1934. doi: https://doi.org/10.1016/S0140-6736(01)06957-4 .
Neafsey DE, Juraska M, Bedford T, Benkeser D, Valim C, Griggs A, et al. Genetic diversity and protective efficacy of the RTS, S/AS01 malaria vaccine. N Engl J Med. 2015;373(21):2025–37. https://doi.org/10.1056/NEJMoa1505819 .
Nardin EH, Nussenzweig RS. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 1993;11:687–727. https://doi.org/10.1146/annurev.iy.11.040193.003351 .
Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS, S: a review of the available data. Malar J. 2009;8:312. https://doi.org/10.1186/1475-2875-8-312 .
Arora N, Anbalagan L, Pannu A. Towards Eradication of Malaria: Is the WHO’s RTS, S/AS01 vaccination effective enough? Risk Manag Healthc Policy. 2021;14:1033–9.
Okie S. Betting on a malaria vaccine. N Engl J Med. 2005;353:1877–81.
Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433(7022):164–167. Erratum in: Nature. 2007;446(7131):102. https://doi.org/10.1038/nature03188
Richie TL, Billingsley PF, Sim BK, James ER, Chakravarty S, Epstein JE, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33(52):7452–61. https://doi.org/10.1016/j.vaccine.2015.09.096 .
Jongo SA, Urbano V, Church LWP, Olotu A, Manock SR, Schindler T, et al. Immunogenicity and protective efficacy of radiation-attenuated and chemo-attenuated PfSPZ vaccines in EquatoGuinean adults. Am J Trop Med Hyg. 2021;104(1):283–93. https://doi.org/10.4269/ajtmh.20-0435 .
SmithKlineBeecham Biologicals, Wilde, M. D. Hybrid protein between CS from Plasmodium and HBsAG. (1991).
Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–77.
Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377:1770–6.
Friesen J, Matuschewski K. Comparative efficacy of pre-erythrocytic whole organism vaccine strategies against the malaria parasite. Vaccine. 2011;29:7002–8.
Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011;9:451–62.
Anders RF. The case for a subunit vaccine against malaria. Trends Parasitol. 2011;27:330–4.
Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–65.
Mordmuller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542:445–9.
Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17(5):498–509.
Polhemus ME, Remich SA, Ogutu BR, Waitumbi JN, Otieno L, Apollo S, et al. Evaluation of RTS, S/AS02A and RTS, S/AS01B in adults in a high malaria transmission area. PLoS ONE. 2009;4(7): e6465.
Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med. 2016;22(6):614–23.
Lyke KE, Ishizuka AS, Berry AA, Chakravarty S, DeZure A, Enama ME, et al. Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A. 2017;114(10):2711–6.
Jongo SA, Shekalaghe SA, Church LWP, Ruben AJ, Schindler T, Zenklusen I, et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg. 2018;99(2):338–49.
Dame JB, Williams JL, McCutchan TF, Weber JL, Wirtz RA, Hockmeyer WT, et al. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum . Science. 1984;225(4662):593–9.
Arama C, Troye-Blomberg M. The path of malaria vaccine development: challenges and perspectives. J Intern Med. 2014;275(5):465–6.
Article Google Scholar
Cohen, J. Vaccine composition against malaria. USA patent. 1996. https://patents.google.com/patent/US20060073171A1/en . Accessed 30 December 2021.
RTS,S Malaria Vaccine Evaluation Group, Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336(2): 86–91. https://doi.org/10.1056/NEJM199701093360202 .
White MT, Verity R, Griffin JT, Asante KP, Owusu-Agyei S, Greenwood B, et al. Immunogenicity of the RTS, S/ AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. 2015;15(12):1450–8. https://doi.org/10.1016/S1473-3099(15)00239-X .
Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20.
Rts SCTP, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, et al. A phase 3 trial of RTS, S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–95.
Guinovart C, Aponte JJ, Sacarlal J, Aide P, Leach A, Bassat Q, et al. Insights into long-lasting protection induced by RTS, S/AS02A malaria vaccine: further results from a phase IIb trial in Mozambican children. PLoS ONE. 2009;4: e5165.
Collins KA, Snaith R, Cottingham MG, Gilbert SC, Hill AVS. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci Rep. 2017;7:46621.
WHO. Scientists share data from first WHO-recommended malaria vaccine. Geneva: World Health Organization; 2021.
Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol. 2006;59:1369–79.
Bergmann-Leitner ES, Legler PM, Savranskaya T, Ockenhouse CF, Angov E. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine. 2011;29:5940–9.
Hoffman SL, Vekemans J, Richie TL, Duffy PE. The march toward malaria vaccines. Vaccine. 2015;33(Suppl 4):D13–23.
Hill AV, Reyes-Sandoval A, O’Hara G, Ewer K, Lawrie A, Goodman A, et al. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccine. 2010;6(1):78–83. https://doi.org/10.4161/hv.6.1.10116 .
Bruder JT, Angov E, Limbach KJ, Richie TL. Molecular vaccines for malaria. Hum Vaccine. 2010;6:54–77. https://doi.org/10.4161/hv.6.1.10463 .
Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for antimalarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 2010;6: e1000998. https://doi.org/10.1371/journal.ppat.1000998 .
Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, et al. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8þ T-cell responses. Infect Immun. 2010;78:145–53. https://doi.org/10.1128/IAI.00740-09 .
Capone S, Reyes-Sandoval A, Naddeo M, Siani L, Ammendola V, Rollier CS, et al. Immune responses against a liver-stage malaria antigen induced by simian adenoviral vector AdCh63 and MVA prime-boost immunisation in non-human primates. Vaccine. 2010;29(2):256–65. https://doi.org/10.1016/j.vaccine.2010.10.041 .
Reyes-Sandoval A, Wyllie DH, Bauza K, Milicic A, Forbes EK, Rollier CS, et al. CD8+ T effector memory cells protect against liver-stage malaria. J Immunol. 2011;187(3):1347–57. https://doi.org/10.4049/jimmunol.1100302 .
Radosevic K, Rodriguez A, Lemckert AA, van der Meer M, Gillissen G, Warnar C, et al. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clin Vaccine Immunol. 2010;17:1687–94. https://doi.org/10.1128/CVI.00311-10 .
Rathore D, Sacci JB, de la Vega P, McCutchan TF. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J Biol Chem. 2002;277:7092–8.
Bongfen SE, Ntsama PM, Offner S, Smith T, Felger I, Tanner M, et al. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine. 2009;27(2):328–35. https://doi.org/10.1016/j.vaccine.2008.09.097 .
Richie TL, Saul A. Progress and challenges for malaria vaccines. Nature. 2002;415:694–701.
Moorthy VS, Good MF, Hill AV. Malaria vaccine developments. Lancet. 2004;363:150–6.
Sirima SB, Cousens S, Druilhe P. Protection against malaria by MSP3 candidate vaccine. N Engl J Med. 2011;365(11):1062–4. https://doi.org/10.1056/NEJMc1100670 .
Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365(11):1004–13. https://doi.org/10.1056/NEJMoa1008115 .
Payne RO, Milne KH, Elias SC, Edwards NJ, Douglas AD, Brown RE, et al. Demonstration of the blood-stage plasmodium falciparum controlled human malaria infection model to assess efficacy of the P. falciparum Apical Membrane Antigen 1 Vaccine, FMP2.1/AS01. J Infect Dis. 2016;213 (11):1743–1751. Erratum in: J Infect Dis. 2016;214(6):978. https://doi.org/10.1093/infdis/jiw039.
Goodman AL, Draper SJ. Blood-stage malaria vaccines—recent progress and future challenges. Ann Trop Med Parasitol. 2010;104:189–211. https://doi.org/10.1179/136485910X12647085215534) .
El Sahly HM, Patel SM, Atmar RL, Lanford TA, Dube T, Thompson D, et al. Safety and immunogenicity of a recombinant nonglycosylated erythrocyte binding antigen 175 Region II malaria vaccine in healthy adults living in an area where malaria is not endemic. Clin Vaccine Immunol. 2010;17:1552–9.
Sagara I, Dicko A, Ellis RD, Fay MP, Diawara SI, Assadou MH, et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine. 2009;27:3090–8.
Esen M, Kremsner PG, Schleucher R, Gässler M, Imoukhuede EB, Imbault N, et al. Safety and immunogenicity of GMZ2 - a MSP3-GLURP fusion protein malaria vaccine candidate. Vaccine. 2009;27:6862–8.
Hermsen CC, Verhage DF, Telgt DS, Teelen K, Bousema JT, Roestenberg M, et al. Glutamate-rich protein (GLURP) induces antibodies that inhibit in vitro growth of Plasmodium falciparum in a phase 1 malaria vaccine trial. Vaccine. 2007;25:2930–40.
Horii T, Shirai H, Jie L, Ishii KJ, Palacpac NQ, Tougan T, et al. Evidences of protection against blood-stage infection of Plasmodium falciparum by the novel protein vaccine SE36. Parasitol Int. 2010;59:380–6.
Palacpac NM, Ntege E, Yeka A, Balikagala B, Suzuki N, Shirai H, et al. Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36. PLoS ONE. 2013;8: e64073.
Ogutu BR, Apollo OJ, McKinney D, Okoth W, Siangla J, Dubovsky F, et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE. 2009;4: e4708.
Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, Rare L, Baisor M, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1–2b trial in Papua New Guinea. J Infect Dis. 2002;185:820–7.
Audran R, Cachat M, Lurati F, et al. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect Immun. 2005;73:8017–26.
Jepsen MP, Jogdand PS, Singh SK, et al. The malaria vaccine candidate GMZ2 elicits functional antibodies in individuals from malaria endemic and non-endemic areas. J Infect Dis. 2013;208:479–88.
Bustamante LY, Bartholdson SJ, Crosnier C, Campos MG, Wanaguru M, Nguon C, et al. A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine. 2013;31:373–9.
Douglas AD, Williams AR, Illingworth JJ, et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun. 2011;2:601.
Tran TM, Ongoiba A, Coursen J, Crosnier C, Diouf A, Huang CY, et al. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J Infect Dis. 2014;209(5):789–98. https://doi.org/10.1093/infdis/jit553 .
Villasis E, Lopez-Perez M, Torres K, Gamboa D, Neyra V, Bendezu J, et al. Anti- Plasmodium falciparum invasion ligand antibodies in a low malaria transmission region, Loreto, Peru. Malar J. 2012;11:361. https://doi.org/10.1186/1475-2875-11-361 .
Ito D, Hasegawa T, Miura K, Yamasaki T, Arumugam TU, Thongkukiatkul A, et al. RALP1 is a rhoptry-neck erythrocyte-binding protein of Plasmodium falciparum merozoite and a potential blood-stage vaccine candidate antigen. Infect Immun. 2013;81:4290–8.
Srinivasan P, Baldeviano GC, Miura K, Diouf A, Ventocilla JA, Leiva KP, et al. A malaria vaccine protects Aotus monkeys against virulent Plasmodium falciparum infection. NPJ Vaccines. 2017;2:14. https://doi.org/10.1038/s41541-017-0015-7 .
Tessema SK, Nakajima R, Jasinskas A, Monk SL, Lekieffre L, Lin E, et al. Protective immunity against severe malaria in children is associated with a limited repertoire of antibodies to conserved PfEMP1 variants. Cell Host Microbe. 2019;26(5):579-590.e5. https://doi.org/10.1016/j.chom.2019.10.012 .
Raj DK, Das Mohapatra A, Jnawali A, Zuromski J, Jha A, Cham-Kpu G, et al. Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria. Nature. 2020;582(7810):104–8. https://doi.org/10.1038/s41586-020-2220-1 .
Raj DK, Nixon CP, Nixon CE, Dvorin JD, DiPetrillo CG, Pond-Tor S, et al. Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science. 2014;344(6186):871–7. https://doi.org/10.1126/science.1254417 .
Good MF, Reiman JM, Rodriguez IB, Ito K, Yanow SK, El-Deeb IM, et al. Cross-species malaria immunity induced by chemically attenuated parasites. J Clin Invest. 2013;123(8):3353–62. https://doi.org/10.1172/JCI66634 .
Raja AI, Cai Y, Reiman JM, Groves P, Chakravarty S, McPhun V, et al. Chemically attenuated blood-stage Plasmodium yoelii parasites induce long-lived and strain-transcending protection. Infect Immun. 2016;84(8):2274–88. https://doi.org/10.1128/IAI.00157-16 .
Raja AI, Stanisic DI, Good MF. Chemical attenuation in the development of a whole-organism malaria vaccine. Infect Immun. 2017;85(7):e00062-e117. https://doi.org/10.1128/IAI.00062-17 .
Singh SK, Plieskatt J, Chourasia BK, Singh V, Bengtsson KL, Reimer JM, et al. Preclinical development of a Pfs230-Pfs48/45 chimeric malaria transmission-blocking vaccine. NPJ Vaccines. 2021;6(1):120. https://doi.org/10.1038/s41541-021-00383-8 .
Carter R, Chen DH. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature. 1976;263:57–60.
Duffy PE, Pimenta P, Kaslow DC. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993;177(2):505–10. https://doi.org/10.1084/jem.177.2.505 .
Kubler-Kielb J, Majadly F, Wu Y, Narum DL, Guo C, Miller LH, et al. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc Natl Acad Sci U S A. 2007;104(1):293–8. https://doi.org/10.1073/pnas.0609885104 .
Ghosh AK, Devenport M, Jethwaney D, Kalume DE, Pandey A, Anderson VE, et al. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles salign proteins. PloS Pathog. 2009;5(1): e1000265.
de Jong RM, Tebeje SK, Meerstein-Kessel L, Tadesse FG, Jore MM, Stone W, et al. Immunity against sexual stage Plasmodium falciparum and Plasmodium vivax parasites. Immunol Rev. 2020;293(1):190–215. https://doi.org/10.1111/imr.12828 .
Kumar N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 1987;9:321–35.
Stone WJ, Dantzler KW, Nilsson SK, Drakeley CJ, Marti M, Bousema T, Rijpma SR. Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitology. 2016;143(2):187–98. https://doi.org/10.1017/S0031182015001341 .
Dinglasan RR, Armistead JS, Nyland JF, Jiang X, Mao HQ. Single-dose microparticle delivery of a malaria transmission-blocking vaccine elicits a long-lasting functional antibody response. Curr Mol Med. 2013;13:479–87.
Penny MA, Maire N, Studer A, Schapira A, Smith TA. What should vaccine developers ask? Simulation of the effectiveness of malaria vaccines. PLoS ONE. 2008;3(9): e3193.
Vannice KS, Brown GV, Akanmori BD, Moorthy VS. MALVAC 2012 scientific forum: accelerating development of second-generation malaria vaccines. Malar J. 2012; 11: 372,2875–11–372.
Baird JK. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev. 2013;26:36–57.
Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24:397–401.
Arévalo-Herrera M, Vásquez-Jiménez JM, Lopez-Perez M, Vallejo AF, Amado-Garavito AB, Céspedes N, et al. Protective efficacy of Plasmodium vivax radiation-attenuated sporozoites in colombian volunteers: a randomized controlled trial. PLoS Negl Trop Dis. 2016;10: e0005070.
Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, et al. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine. 2005;23(24):3131–8. https://doi.org/10.1016/j.vaccine.2004.12.019 .
Payne RO, Silk SE, Elias SC, Milne KH, Rawlinson TA, Llewellyn D, et al. Human vaccination against Plasmodium vivax Duffy-binding protein induces strain-transcending antibodies. JCI Insight. 2017;2(12): e93683. https://doi.org/10.1172/jci.insight.93683 .
Singh K, Mukherjee P, Shakri AR, Singh A, Pandey G, Bakshi M, et al. Malaria vaccine candidate based on Duffy-binding protein elicits strain transcending functional antibodies in a Phase I trial. NPJ Vaccines. 2018;3:48.
Hoffman SL, Oster CN, Plowe CV, Woollett GR, Beier JC, Chulay JD, et al. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987;237(4815):639–42. https://doi.org/10.1126/science.3299709 .
Zavala F, Tam JP, Hollingdale MR, Cochrane AH, Quakyi I, Nussenzweig RS, et al. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985;228(4706):1436–40.
Young JF, Ballou WR, Hockmeyer WT. Developing a human malaria sporozoite vaccine. Microb Pathog. 1987;2(4):237–40.
Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS, S/AS01B and RTS, S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200(3):337–46.
Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, et al. Efficacy of RTS, S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359(24):2521–32.
Olotu A, Moris P, Mwacharo J, Vekemans J, Kimani D, Janssens M, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS, S/AS01E and protection against P. falciparum clinical malaria. PLoS ONE. 2011;6(10): e25786.
Owusu-Agyei S, Ansong D, Asante K, Kwarteng Owusu S, Owusu R, WirekoBrobby NA, et al. Randomized controlled trial of RTS, S/AS02D and RTS, S/AS01E malaria candidate vaccines given according to different schedules in Ghanaian children. PLoS ONE. 2009;4(10): e7302.
Lell B, Agnandji S, von Glasenapp I, Haertle S, Oyakhiromen S, Issifou S, et al. A randomized trial assessing the safety and immunogenicity of AS01 and AS02 adjuvanted RTS, S malaria vaccine candidates in children in Gabon. PLoS ONE. 2009;4(10): e7611.
Agnandji ST, Asante KP, Lyimo J, Vekemans J, Soulanoudjingar SS, Owusu R, Shomari M, et al. Evaluation of the safety and immunogenicity of the RTS, S/AS01E malaria candidate vaccine when integrated in the expanded program of immunization. J Infect Dis. 2010;202(7):1076–87.
Aponte JJ, Aide P, Renom M, Bassat Q, Sacarlal J, Manaca MN, et al. Safety of the RTS, S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370(9598):1543–51. https://doi.org/10.1016/S0140-6736(07)61542-6 .
Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, Membi C, et al. Safety and immunogenicity of RTS, S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359(24):2533–44. https://doi.org/10.1056/NEJMoa0807773 .
RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. https://doi.org/10.1016/S0140-6736(15 )60721-8.
Rts SCTP. Efficacy and safety of the RTS, S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11: e1001685.
European Medicines Agency. First malaria vaccine receives positive scientific opinion from EMA EMA/CHMP/488348/2015. Press Office, editor. London: EMA; 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/07/ WC500190447.pdf. Accessed 19 Nov 2021
WHO. Malaria vaccine pilot launched in Malawi 2019. Geneva: World Health Organization; 2019. https://www.who.int/news/item/23-04-2019-malaria-vaccine-pilot-launched-in-malawi . Accessed 3 Nov 2021
WHO. Q &A on RTS,S malaria vaccine. Geneva: World Health Organization; 2021. https://www.who.int/news-room/questions-and-answers/item/q-a-on-rts-s-malaria-vaccine . Accessed 20 Nov 2021.
Tables of malaria vaccine projects globally. Geneva: World Health Organization. https://www.who.int/immunization/research/development/Rainbow_tables/en/ . Accessed 30 Dec 2021.
Laurens MB. The promise of a malaria vaccine-are we closer? Annu Rev Microbiol. 2018;72:273–92. https://doi.org/10.1146/annurev-micro-090817-062427 .
Draper SJ, Sack BK, King CR, Nielsen CM, Rayner JC, Higgins MK, et al. Malaria vaccines: recent advances and new horizons. Cell Host Microbe. 2018;24(1):43–56. https://doi.org/10.1016/j.chom.2018.06.008 .
Shi YP, Hasnain SE, Sacci JB, Holloway BP, Fujioka H, Kumar N, et al. Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine. Proc Natl Acad Sci USA. 1999;96:1615–20.
Ockenhouse CF, Sun PF, Lanar DE, Wellde BT, Hall BT, Kester K, et al. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J Infect Dis. 1998;177(6):1664–73. https://doi.org/10.1086/515331 .
Prieur E, Gilbert SC, Schneider J, Moore AC, Sheu EG, Goonetilleke N, et al. A Plasmodium falciparum candidate vaccine based on a six-antigen polyprotein encoded by recombinant poxviruses. Proc Natl Acad Sci USA. 2004;101:290–5. https://doi.org/10.1073/pnas.0307158101 .
Hutchings CL, Birkett AJ, Moore AC, Hill AV. Combination of protein and viral vaccines induces potent cellular and humoral immune responses and enhanced protection from murine malaria challenge. Infect Immun. 2007;75:5819–26. https://doi.org/10.1128/IAI.00828-07 .
Bonam SR, Rénia L, Tadepalli G, Bayry J, Kumar HMS. Plasmodium falciparum malaria vaccines and vaccine adjuvants. Vaccines (Basel). 2021;9(10):1072. https://doi.org/10.3390/vaccines9101072 .
Malaria Vaccine Technology Roadmap 2006 Executive summary. http://www.malariavaccine.org/files/Malaria_Vaccine_TRM_Final_000.pdf . Accessed 22 Dec 2021.
Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. https://doi.org/10.1038/192733a0 .
Siddiqui WA. An effective immunization of experimental monkeys against a human malaria parasite, Plasmodium falciparum . Science. 1977;197:388–9. https://doi.org/10.1126/science.406671 .
Download references
Acknowledgements
We thank O.M. El-Swify (physician, Medical Services Department, Suez Canal University, Ismailia, Egypt) for his help in performing the literature search, and Jackie Senior (AuthorAID in the Eastern Mediterranean) for improving the use of English in the manuscript.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The work for this manuscript was not funded by an agency in the public, commercial, or not-for-profit sectors, nor any alternative funders.
Author information
Authors and affiliations.
Department of Medical Parasitology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
Amal A. El-Moamly
Department of Medical Microbiology and Immunology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
Mohamed A. El-Sweify
You can also search for this author in PubMed Google Scholar
Contributions
AAE and MAE suggested the research idea designed the study, performed the literature search, wrote the main manuscript, and critically reviewed the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Amal A. El-Moamly .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
El-Moamly, A.A., El-Sweify, M.A. Malaria vaccines: the 60-year journey of hope and final success—lessons learned and future prospects. Trop Med Health 51 , 29 (2023). https://doi.org/10.1186/s41182-023-00516-w
Download citation
Received : 03 October 2022
Accepted : 18 April 2023
Published : 17 May 2023
DOI : https://doi.org/10.1186/s41182-023-00516-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Plasmodium falciparum
- Development
- Implementation program
- Erythrocytic vaccines
- Blood-stage vaccines
- Elimination
Tropical Medicine and Health
ISSN: 1349-4147
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Once a malaria patient, student now has sights set on stopping the deadly disease

May 23, 2024 – Cyrianne Keutcha, PhD ’25, has been around malaria all her life, from growing up in a malaria-endemic country, to being infected herself, and now to studying the parasite in the lab. She is completing her degree in the Harvard Griffin Graduate School of Arts and Sciences (GSAS), studying in the Biological Sciences in Public Health program at Harvard T.H. Chan School of Public Health and doing her dissertation research in the lab of Manoj Duraisingh , John LaPorte Given Professor of Immunology and Infectious Diseases.
I was born and raised in Bafoussam, a city in the west region of Cameroon. Malaria is endemic there, so getting it felt like not a big deal—it was like having a cold.
But when I was 11 years old, I got a serious case of malaria. I was hospitalized for about two weeks, and during that time I was first exposed to how infectious and devastating the disease is. I was in a pediatric facility, and I saw a lot of kids come in and out with different infections, but it was mostly malaria. That put a thought in my head—I’m good at science [at school], I don’t want kids to be sick, so I want to be a doctor.
When I was 12, my grandmother passed away from lung cancer. Then two years after, I came to the U.S. Those were transforming stages in my life. It was like I lost my sense of security, a sense of community. Being put into an environment for better opportunity, you have a pressure to succeed. I became very focused, very driven. I graduated as valedictorian of my high school class, was awarded a few scholarships to attend college, and decided to go to Georgia State University.
I was a chemistry major. I always had a love for chemistry . I went in with a pre-med mindset, so I was shadowing doctors, but chemistry has a requirement that you have to do a research class that includes working in the lab, reading scientific papers, and giving presentations. The person who led that class was Prof. Dabney Dixon, who became my undergraduate research advisor. She was working on the infectious disease diphtheria, which impacts tropical areas, including some countries in Africa. That semester was life-changing. There was just something exhilarating about doing research. You might have a 4.0 GPA, and then learn that you do not know much, because textbook knowledge and research knowledge are different. Science will humble you every day of the week. I love to be in a space of not knowing and enjoy the process of learning, thus I took to research.
After my course ended, I kept working with Prof. Dixon, helping to investigate diphtheria-causing bacteria. During my junior and senior years, in between classes, I worked in her lab and contributed to some great published findings in diptheria. Being in a lab was my happy place. The summer before my senior year, I participated in the Amgen Scholars undergraduate research program at Washington University in St. Louis, which was an amazing experience. In my senior year, I was ready to study for the MCAT [the entrance exam for medical school]. I bought the review books, opened them, and just couldn’t focus—I seemed to have lost interest in studying for that path.
Prof. Dixon asked, “Have you ever thought of getting a PhD?” I needed some time to think about it, thus I applied to post-baccalaureate research programs, and I got into the Johns Hopkins Malaria Research Institute. I thought, if I’m going to go into research, I’m going to do malaria—that was my only condition. I’d had it as a kid, and my family back home in Cameroon still gets it every now and then. There’s always this common link between us.
I conducted research for a year at Johns Hopkins under the supervision of Prof. Sean Prigge, and we published our findings on the metabolism of the malaria parasite. Being in the lab was like a playground. I was exposed not only to malaria research, but to public health. I was challenged to think, what does this science mean in the big scheme of the world? What responsibility do you have in making sure that research gets to the people?
In addition to malaria, I wanted to explore other infectious diseases, and the Biological Sciences in Public Health program at Harvard Chan had experts looking at all the diseases that piqued my interest, such as tuberculosis and HIV . So I decided to pursue a doctoral degree here.
After rotating through three labs, I decided to join Manoj’s lab. I wanted to study the malaria parasite Plasmodium falciparum , because that’s the prevalent species in Africa. The parasite has two hosts, Anopheles mosquitoes and humans. When an infected mosquito bites a human, it’s injecting saliva in you, and in that saliva there are parasites. Our lab focuses on the stage at which the parasites invade red blood cells in humans. This stage is essential for parasite replication and responsible for clinical symptoms. Given that I love protein chemistry, I am interested in knowing what kind of protein from the parasite interacts with proteins from the red blood cell to facilitate invasion of the cell.
I’m working on two projects. The first is to develop a human blood cell line that can be genetically modified, so we can test if certain genes affect parasite invasion. To help do this, our lab generated an immortalized cell line—which replicates endlessly—so that we don’t need to keep getting blood from human donors. We use the immortalized cells to make red blood cells, and then perform experiments to see if parasites invade those red blood cells.
The second project is more parasite-driven. My collaborators and I screened multiple parasite lines and identified two that invaded red blood cells using different invasion strategies. We bred those two parasites together and acquired the resulting parasite’s whole genome sequence to identify the specific genomic regions of the parasite responsible for the invasion difference. We identified two genes of interest—one known, so it’s a beautiful control [to show that our experimental system works]. The other is novel, which is the focus of my project. The aim of my research is to clarify the invasion mechanism, with the goal of contributing to the development of a vaccine targeting the stage of infection when parasites are in the blood of the host.
After graduation, I will take a position as a postdoctoral researcher in a lab at the Yale School of Public Health, focusing on vaccine candidates against malaria.
In the future, I hope to work in the global health arena. I have acquired a world of knowledge just by knowing people [who are affected by malaria] and growing up where I did. Often, those bringing public health interventions to a community may make assumptions about the community’s culture and lifestyles.
When I was hospitalized with malaria as a kid, I was privy to something that made me quite sad. Sick kids had to leave the hospital not because they got better, but because they couldn’t financially be there anymore. The second you can’t pay, they ask you to leave the bed. Severe malaria only gets severe because the average person cannot afford to buy the medication. Malaria kills; poverty kills better. It is my opinion that if you can’t eradicate poverty, you will not eradicate malaria.
I have been blessed in my life to be surrounded by amazing mentors —from my parents to my grandparents to my professors. One of my philosophies in life is that the best way to thank people is to do what they have done for you to someone else, to pass it on. That’s why I’m a mentor in the Health Professions Recruitment and Exposure Program [a Harvard Medical School program that provides science lessons and mentorship to Boston-area high school students, particularly those from underserved and underrepresented backgrounds.]
I’m also the vice president and director of diversity and inclusion of the GSAS Harvard Biotech Club [which provides educational and networking opportunities for students interested in working in the biotech industry]. My proudest moment was that, with the MIT Biotech Group, we established a scholarship program to sponsor incoming college freshmen who are interested in life science and hope to someday join biotech. People don’t know how far a scholarship goes. When I started college, I had several, and it’s like somebody said, “I see you. I think you have potential.” Walking into a room, you feel more confident.
Photo: Kent Dayton
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Microorganisms

Malaria: The Past and the Present
Jasminka talapko.
1 Faculty of Dental Medicine and Health, Josip Juraj Strossmayer University of Osijek, Crkvena 21, HR-31000 Osijek, Croatia; rh.zmdf@okpalatj (J.T.); rh.zmdf@vecva (A.V.)
Ivana Škrlec
Tamara alebić.
2 Faculty of Medicine, Josip Juraj Strossmayer University of Osijek, Josipa Huttlera 4, HR-31000 Osijek, Croatia; [email protected] (T.A.); moc.liamg@71ikujm (M.J.)
Melita Jukić
3 General Hospital Vukovar, Županijska 35, HR-32000 Vukovar, Croatia
Aleksandar Včev
Malaria is a severe disease caused by parasites of the genus Plasmodium , which is transmitted to humans by a bite of an infected female mosquito of the species Anopheles . Malaria remains the leading cause of mortality around the world, and early diagnosis and fast-acting treatment prevent unwanted outcomes. It is the most common disease in Africa and some countries of Asia, while in the developed world malaria occurs as imported from endemic areas. The sweet sagewort plant was used as early as the second century BC to treat malaria fever in China. Much later, quinine started being used as an antimalaria drug. A global battle against malaria started in 1955, and Croatia declared 1964 to be the year of eradication of malaria. The World Health Organization carries out a malaria control program on a global scale, focusing on local strengthening of primary health care, early diagnosis of the disease, timely treatment, and disease prevention. Globally, the burden of malaria is lower than ten years ago. However, in the last few years, there has been an increase in the number of malaria cases around the world. It is moving towards targets established by the WHO, but that progress has slowed down.
1. Introduction
Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [ 1 ]. Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous cases. The malaria mortality rate globally ranges from 0.3–2.2%, and in cases of severe forms of malaria in regions with tropical climate from 11–30% [ 2 ]. Different studies showed that the prevalence of malaria parasite infection has increased since 2015 [ 3 , 4 ].
The causative agent of malaria is a small protozoon belonging to the group of Plasmodium species, and it consists of several subspecies. Some of the Plasmodium species cause disease in human [ 2 , 5 ]. The genus Plasmodium is an amoeboid intracellular parasite which accumulates malaria pigment (an insoluble metabolite of hemoglobin). Parasites on different vertebrates; some in red blood cells, and some in tissue. Of the 172 of Plasmodium species, five species can infect humans. These are P. malariae , P.falciparum , P.vivax , P.ovale , and P.knowlesi . In South-East Asia, the zoonotic malaria P.knowlesi is recorded. Other species rarely infect humans [ 5 , 6 , 7 , 8 ]. All the mentioned Plasmodium species cause the disease commonly known as malaria (Latin for Malus aer —bad air). Likewise, all species have similar morphology and biology [ 9 ].
The Plasmodium life cycle is very complex and takes place in two phases; sexual and asexual, the vector mosquitoes and the vertebrate hosts. In the vectors, mosquitoes, the sexual phase of the parasite’s life cycle occurs. The asexual phase of the life cycle occurs in humans, the intermediate host for malaria [ 9 , 10 ]. Human malaria is transmitted only by female mosquitoes of the genus Anopheles . The parasite, in the form of sporozoite, after a bite by an infected female mosquito, enters the human blood and after half an hour of blood circulation, enters the hepatocytes [ 11 ]. The first phase of Plasmodium asexual development occurs in the hepatocytes, and then in the erythrocytes. All Plasmodium species lead to the rupture of erythrocytes [ 7 , 9 , 12 , 13 ].
The most common species in the Americas and Europe are P.vivax and P.malariae , while in Africa it is P.falciparum [ 14 ].
2. Discovery of Malaria
It is believed that the history of malaria outbreaks goes back to the beginnings of civilization. It is the most widespread disease due to which many people have lost lives and is even thought to have been the cause of major military defeats, as well as the disappearance of some nations [ 15 ]. The first descriptions of malaria are found in ancient Chinese medical records of 2700 BC, and 1200 years later in the Ebers Papyrus [ 2 ]. The military leader Alexander the Great died from malaria [ 15 ]. The evidence that this disease was present within all layers of society is in the fact that Christopher Columbus, Albrecht Dürer, Cesare Borgia, and George Washington all suffered from it [ 16 , 17 ].
Although the ancient people frequently faced malaria and its symptoms, the fever that would occur in patients was attributed to various supernatural forces and angry divinities. It is, thus, stated that the Assyrian-Babylonian deity Nergal was portrayed as a stylized two-winged insect, as was the Canaan Zebub (‘Beelzebub, in translation: the master of the fly’) [ 17 ]. In the 4th century BC, Hippocrates described this disease in a way that completely rejected its demonic origins and linked it with evaporation from swamps which, when inhaled, caused the disease. That interpretation was maintained until 1880 and Laveran’s discovery of the cause of the disease [ 18 ]. Laveran, a French military surgeon, first observed parasites in the blood of malaria patients, and for that discovery he received the Nobel Prize in 1907 [ 19 ].
Cartwright and Biddis state that malaria is considered to be the most widespread African disease [ 14 ]. The causative agent of malaria is a small protozoon belonging to the group of Plasmodium species, and it consists of several subspecies [ 14 ].
3. The Development of Diagnostic Tests for Proving Malaria through History
Malaria can last for three and up to five years, if left untreated, and depending on the cause, may recrudesce. In P. vivax and ovale infections, the persistence of the merozoites in the blood or hypnozoites in hepatocytes can cause relapse months or years after the initial infection. Additionally, relapse of vivax malaria is common after P. falciparum infection in Southeast Asia. Relapse cases were observed in P. falciparum infections, which can lead to a rapid high parasitemia with subsequent destruction of erythrocytes [ 20 , 21 ]. Children, pregnant women, immunocompromised and splenectomized patients are especially vulnerable to malaria infection, as well as healthy people without prior contact with Plasmodium . A laboratory test for malaria should always confirm clinical findings. The proving of malaria is carried out by direct methods such as evidence of parasites or parts of parasites, and indirect methods that prove the antibodies to the causative agents ( Table 1 ) [ 2 , 5 , 22 ].
Diagnostic tests for proving malaria.
The gold standard method for malaria diagnosis is light microscopy of stained blood films by Giemsa. Due to a lack of proper staining material and trained technicians, this method is not available in many parts of sub-Saharan Africa. The sensitivity of the method depends on the professional expertise, and it is possible to detect an infection with 10–100 parasites/μL of blood. A negative finding in patients with symptoms does not exclude malaria, but smears should be repeated three times in intervals of 12–24 h if the disease is still suspected [ 23 , 24 ]. Diagnosis of malaria using serologic testing has traditionally been done by immunofluorescence antibody testing (IFA). IFA is time-consuming and subjective. It is valuable in epidemiological studies, for screening possible blood donors. It also demands fluorescence microscopy and qualified technicians [ 23 , 25 , 26 ].
Rapid Diagnostic Tests (RDT) for the detection of antigens in the blood are immunochromatographic tests to prove the presence of parasite antigens. No electrical equipment, and no special experience or skills are required to perform these tests. The RDTs are now recommended by WHO as the first choice of test all across the world in all malaria-endemic areas. The sensitivity of the antigen test varies depending on the selected antigens represented in the test. For some RDTs is 50–100 parasites/μL (PfHRP2) to <100 parasites/μL [ 27 , 28 ]. The FDA approved the first RDT test in 2007. It is recommended that the results of all RDT tests should be confirmed by microscopic blood analysis [ 29 ]. It is known that antigens detected with RDT test remain in the blood after antimalarial treatment, but the existence of these antigens varies after treatment. The false-positive rates should be less than 10% [ 30 ]. Several RDT tests in the eight rounds of testing revealed malaria at a low-density parasite (200 parasites/μL), had low false-positive rates and could detect P. falciparum or P. vivax infections or both [ 30 ]. False-positive rates of P. vivax were typically small, between 5% and 15%. On the other hand, the false-positive rates of P. falciparum range from 3–32% [ 30 , 31 ]. Good RDTs might occasionally give false-negative results if the parasite density is low, or if variations in the production of parasite antigen reduce the ability of the RDT to detect the parasite. False negative results of the RDT test for P. falciparum ranged between 1% and 11% [ 31 , 32 , 33 , 34 ]. The overall sensitivity of RDTs is 82% (range 81–99%), and specificity is 89% (range 88–99%) [ 35 ].
Polymerase chain reaction (PCR) is another method in the detection of malaria. This method is more sensitive and more specific than all conventional methods in the detection of malaria. It can detect below one parasite/μL. PCR test confirms the presence of parasitic nucleic acid [ 23 , 36 ]. PCR results are often not available fast enough to be useful in malaria diagnosis in endemic areas. However, this method is most helpful in identifying Plasmodium species after diagnosis by microscopy or RDT test in laboratories that might not have microscopic experts. Additionally, PCR is useful for the monitoring of patients receiving antimalaria treatment [ 36 , 37 ].
Indirect methods are used to demonstrate antibodies to malaria-causing agents. Such methods are used in testing people who have been or might be at risk of malaria, such as blood donors and pregnant women. The method is based on an indirect immunofluorescence assay (IFA) or an ELISA test. The IFA is specific and sensitive but not suitable for a large number of samples, and the results are subjective evaluations. For serological testing, ELISA tests are more commonly used [ 26 ].
Rapid and accurate diagnosis of malaria is an integral part of appropriate treatment for affected person and the prevention of the further spread of the infection in the community.
4. Malaria Treatment through History
Already in the 2nd century BC, a sweet sagewort plant named Qinghai (Latin Artemisia annua ) was used for the treatment of malaria in China [ 38 ]. Much later, in the 16th century, the Spanish invaders in Peru took over the cinchona medication against malaria obtained from the bark of the Cinchona tree (Latin Cinchona succirubra ). From this plant in 1820 the French chemists, Pierre Joseph Pelletie, and Joseph Bienaimé Caventou isolated the active ingredient quinine, which had been used for many years in the chemoprophylaxis and treatment of malaria. In 1970, a group of Chinese scientists led by Dr. Youyou Tu isolated the active substance artemisinin from the plant Artemisia annua , an antimalarial that has proved to be very useful in treating malaria. For that discovery, Youyou Tu received the Nobel Prize for Physiology and Medicine in 2015 [ 39 , 40 , 41 ]. Most of the artemisinin-related drugs used today are prodrugs, which are activated by hydrolysis to the metabolite dihydroartemisinin. Artemisinin drugs exhibit its antimalarial activity by forming the radical via a peroxide linkage [ 42 ]. WHO recommends the use of artemisinin-based combination therapies (ACT) to ensure a high cure rate of P. falciparum malaria and reduce the spread of drug resistance. ACT therapies are used due to high resistance to chloroquine, sulfadoxine-pyrimethamine, and amodiaquine [ 1 ]. Due to the unique structure of artemisinins, there is much space for further research. Extensive efforts are devoted to clarification of drug targets and mechanisms of action, the improvement of pharmacokinetic properties, and identifying a new generation of artemisinins against resistant Plasmodium strains [ 42 ].
The German chemist Othmer Zeidler synthesized dichlorodiphenyltrichloroethane (DDT) in 1874 during his Ph.D. At that time, no uses of DDT was found, and it just became a useless chemical [ 43 ]. The insecticide property of DDT was discovered in 1939 by Paul Müller in Switzerland. DDT began to be used to control malaria at the end of the Second World War [ 40 ]. During the Second World War, the success of DDT quickly led to the introduction of other chlorinated hydrocarbons which were used in large amounts for the control of diseases transmitted by mosquito [ 43 ]. From the late Middle Ages until 1940, when DDT began to be applied, two-thirds of the world’s population had been exposed to malaria, a fact that represented a severe health, demographic, and economic problem [ 29 , 40 , 41 , 44 , 45 ]. DDT is an organochlorine pesticide which was applied in liquid and powder form against the insects. During the Second World War people were sprayed with DDT. After the war, DDT became a powerful way of fighting malaria by attacking the vector [ 43 ].
Five Nobel Prizes associated with malaria were awarded: Youyou Tu in 2015. Ronald Ross received the Nobel Prize in 1902 for the discovery and significance of mosquitoes in the biology of the causative agents in malaria. In 1907, the Nobel was awarded to the already-mentioned Charles Louis Alphonse Laveran for the discovery of the causative agent. Julius Wagner-Jauregg received it in 1927 for the induction of malaria as a pyrotherapy procedure in the treatment of paralytic dementia. In 1947 Paul Müller received it for the synthetic pesticide formula dichlorodiphenyltrichloroethane.
Attempts to produce an effective antimalarial vaccine and its clinical trials are underway. Over the past several decades’ numerous efforts have been made to develop effective and affordable preventive antimalaria vaccines. Numerous clinical trials are completed in the past few years. Nowadays are ongoing clinical trials for the development of next-generation malaria vaccines. The main issue is P. vivax vaccine, whose research requires further investigations to identify novel vaccine candidates [ 46 , 47 , 48 ]. Despite decades of research in vaccine development, an effective antimalaria vaccine has not yet been developed (i.e., with efficacy higher than 50%) [ 49 , 50 , 51 ]. The European Union Clinical Trials Register currently displays 48 clinical trials with a EudraCT protocol for malaria, of which 13 are still ongoing clinical trials [ 52 ]. The malaria parasite is a complex organism with a complex life cycle which can avoid the immune system, making it very difficult to create a vaccine. During the different stages of the Plasmodium life cycle, it undergoes morphological changes and exhibits antigenic variations. Plasmodium proteins are highly polymorphic, and its functions are redundant. Also, the development of malaria disease depends on the Plasmodium species. That way, a combination of different adjuvants type into antigen-specific formulations would achieve a higher efficacy [ 53 , 54 ]. Drugs that underwent clinical trials proved to be mostly ineffective [ 5 , 7 , 55 ]. However, many scientists around the world are working on the development of an effective vaccine [ 56 , 57 , 58 ]. Since other methods of suppressing malaria, including medication, insecticides, and bed nets treated with pesticides, have failed to eradicate the disease, and the search for a vaccine is considered to be one of the most important research projects in public health by World Health Organization (WHO).
The best way to fight malaria is to prevent insect bites. Malaria therapy is administered using antimalarial drugs that have evolved from quinine. According to its primary effect, malarial vaccines are divided into pre-erythrocytic (sporozoite and liver-stage), blood-stage, and transmission-blocking vaccines [ 9 , 54 ]. Most medications used in the treatment are active against parasitic forms in the blood (the type that causes disease) ( Table 2 ) [ 59 ]. The two crucial antimalarial medications currently used are derived from plants whose medical importance has been known for centuries: artemisinin from the plant Qinghao ( Artemisia annua L, China, 4th century) and quinine from Cinchona (South America, 17th century). Side-by-side with artemisinin, quinine is one of the most effective antimalarial drugs available today [ 13 , 39 , 40 ]. Doxycycline is indicated for malaria chemoprophylaxis for travel in endemic areas. It is also used in combination with the quinine or artesunate for malaria treatment when ACT is unavailable or when the treatment of severe malaria with artesunate fails. The disadvantage of doxycycline is that children and pregnant women cannot use it [ 29 ]. Due to the global resistance of P. falciparum to chloroquine, ACTs are recommended for the treatment of malaria, except in the first trimester of pregnancy. ACTs consist of a combination of an artemisinin derivative that fast decreases parasitemia and a partner drug that eliminates remaining parasites over a more extended period. The most common ACTs in use are artemether-lumefantrine, artesunate-amodiaquine, dihydroartemisinin-piperaquine, artesunate-mefloquine, and artesunate with sulfadoxine-pyrimethamine. The ACTs were very efficient against all P. falciparum until recently when numbers of treatment failures raised in parts of Southeast Asia. Atovaquone-proguanil is an option non-artemisinin-based treatment that is helpful for individual cases which have failed therapy with usual ACTs. Although, it is not approved for comprehensive implementation in endemic countries because of the ability for the rapid development of atovaquone resistance. Quinine remains efficient, although it needs a long course of treatment, is poorly tolerated, especially by children, and must be combined with another drug, such as doxycycline or clindamycin. Uncomplicated vivax, malariae, and ovale malaria are handled with chloroquine except in case of chloroquine-resistant P. vivax when an ACT is used [ 7 , 29 , 60 , 61 , 62 ].
Overview of the most commonly used antimalarials.
CNS—central nervous system.
4.1. Malaria in Europe
In Europe, malaria outbreaks occurred in the Roman Empire [ 63 , 64 ] and the 17th century. Up until the 17th century it was treated as any fever that people of the time encountered. The methods applied were not sufficient and included the release of blood, starvation, and body cleansing. As the first effective antimalarial drug, the medicinal bark of the Cinchona tree containing quinine was mentioned and was initially used by the Peruvian population [ 14 ]. It is believed that in the fourth decade of the 17th century it was transferred to Europe through the Spanish Jesuit missionaries who spread the treatment to Europe [ 65 ].
Contemporary knowledge of malaria treatment is the result of the work of a few researchers. Some of researchers are Alphonse Laveran, Ronald Ross, and Giovanni Battista Grassi. In November 1880, Laveran, who worked as a military doctor in Algeria, discovered the causative agents of malaria in the blood of mosquitoes and found that it was a kind of protozoa [ 66 ]. Laveran noticed that protozoa could, just like bacteria, live a parasitic way of life within humans and thus cause disease [ 66 ]. Nearly two decades later, more precisely in 1898, Ronald Ross, a military doctor in India, discovered the transmission of bird malaria in the saliva of infected mosquitos, while the Italian physician Giovanni Battista Grassi proved that malaria was transmitted from mosquitoes to humans, in the same year. He also proved that not all mosquitoes transmit malaria, but only a specific species ( Anopheles ) [ 17 ]. This discovery paved the way for further research.
The global battle against malaria started in 1955, and the program was based on the elimination of mosquitoes using DDT and included malarial areas of the United States, Southern Europe, the Caribbean, South Asia, but only three African countries (South Africa, Zimbabwe, and Swaziland). In 1975, the WHO announced that malaria had been eradicated in Europe and all recorded cases were introduced through migration [ 67 , 68 ].
4.2. Malaria in Croatia
In Croatia, the first written document that testifies to the prevention of malaria is the Statute of the town of Korčula from 1265. In 1874, the Law on Health Care of Croatia and Slavonia established the public health service that was directed towards treating malaria. There was no awareness nor proper medical knowledge about malaria, but the drainage was carried out to bring the ‘healthy air’ in the cities [ 69 , 70 ]. In 1798 physician Giuseppe Arduino notified the Austrian government about malaria in Istria. A government representative Vincenzo Benini accepted a proposed sanitary measure of the drainage of wetlands [ 71 ]. In 1864, the drainage of wetlands around Pula and on the coastal islands began, and since 1902 a program for the suppression of malaria by treatment of patients using quinine has been applied [ 72 ]. In 1922, the Institute for Malaria was founded in Trogir. In 1923, on the island of Krk, a project was started to eradicate malaria by the sanitation of water surfaces and the treatment of the patients with quinine, led by Dr. Otmar Trausmiller [ 66 ]. Since 1924, besides chemical treatment, biological control of mosquitoes has been established by introducing the fish Gambusia holbrooki to Istria and the coast [ 73 ]. In 1930 legislation was passed to enforce village sanitation, which included the construction of water infrastructure and safe wells, contributing to the prevention of malaria. Regular mosquito fogging with arsenic green (copper acetoarsenite) was introduced, and larvicidal disinfection of stagnant water was carried out.
Since malaria occurs near swamps, streams, ravines, and places where mosquitoes live near water, this disease has been present throughout history in Croatia, and it has often become an epidemic [ 74 ]. It was widespread in the area of Dalmatia, the Croatian Littoral region, Istria, and river flows. In the area of the Croatian Littoral, it was widespread on some islands, such as Krk, Rab, and Pag, while the mainland was left mainly clear of it [ 75 ]. The ethnographer Alberto Fortis (1741–1803) who traveled to Dalmatia, noted impressions recording details of malaria that was a problem in the Neretva River valley. Fortis wanted to visit that area, but the sailors on ship were afraid, probably because the were afraid to go to a place where there had been a disease outbreak known as the Neretva plague [ 76 ]. This Neretva plague was, in fact, malaria, and it is believed that due to it, the Neretva was nicknamed “Neretva—damned by God” [ 77 , 78 ]. Speaking of the Neretva region, Fortis states that the number of mosquitoes in that wetland area was so high that people had to sleep in stuffy canopy tents to defend themselves. Fortis also states that there were so many mosquitoes that he was affected by it. During the stay, Fortis met a priest who had a bump on the head claiming it had occurred after a mosquito bite and believed that the fever that infected the people of the Neretva Valley was also a consequence of the insect bites there [ 76 ]. In a manuscript, Dugački described some of the epidemics in Croatia. Thus, noted the small population of Nin in 1348, which was the result of the unhealthy air and high mortality of the population. Three centuries later, in 1646, the fever was mentioned in Novigrad, while the year 1717 was crucial for to the Istrian town of Dvigrad, which was utterly deserted due to malaria. At the beginning of the 20th century, more precisely in 1902, the daily press reported that the Provincial Hospital in Zadar was full of people affected by malaria. The extent to which this illness was widespread is proved by the fact that at the beginning of the 20th century about 180,000 people suffered from it in Dalmatia [ 18 ]. The volume and frequency of epidemics in Dalmatia resulted in the arrival of the Italian malariologist Grassi and the German parasitologist Schaudin. The procedures of quininization began to be applied, and in 1908 25 physicians and 423 pill distributors were to visit the villages and divide pills that had to be taken regularly to the people to eradicate malaria [ 75 ].
Likewise, in Slavonia, malaria had also a noticeable effect, and it was widespread in the 18th century due to a large number of swamps that covered the region. Such areas were extremely devastating for settlers who were more vulnerable to the disease than its domestic population [ 79 ]. Friedrich Wilhelm von Taube (1728–1778) recorded the disease and stated that the immigrant Germans were primarily affected by malaria and that the cities of Osijek and Petrovaradin can be nicknamed "German Cemeteries" [ 80 ]. According to Skenderović, the high mortality of German settlers from malaria was not limited only to the Slavonia region but also to the Danubian regions in which the Germans had settled in the 18th century, with Banat and Bačka [ 79 ] having the most significant number of malaria cases. The perception of Slavonia in the 18th century was not a positive one. Even Taube stated that Slavonia was not in good standing in the Habsburg Monarchy and that the nobility avoided living there. As some of the reasons for this avoidance, Taube mentioned the unhealthy air and the many swamps in the area around in which there was a multitude of insects. Taube noted that mosquitoes appear to be larger than in Germany and that its bite was much more painful. A change in the situation could only be brought about by drying the swamp, in his opinion [ 80 ]. Since malaria had led to the death of a large number of people, the solution had to be found to stop its further spread. Swamp drying was finally accepted by the Habsburg Monarchy and some European countries as a practical solution and, thus, its drainage began during the 18th century, resulting in cultivated fields [ 79 ].
Since epidemics of malaria continued to occur, there is one more significant record of the disease in the Medical Journal of 1877. In it, the physician A. Holzer cites his experiences from Lipik and Daruvar where he had been a spa physician for a long time. Holzer warns of the painful illness noticed at spa visitors suffering from the most in July and August. As a physician, Holzer could not remain indifferent to the fact that he did not see anyone looking healthy. It also pointed out that other parts of Croatia were not an exception. As an example, Holzer noted Virovitica County, where malaria was also widespread. He wanted to prevent the development and spread of the illness. Believing that preventing the toxic substances from rising into the air would stop the disease, the solution was to use charcoal that has the properties of absorbing various gases and, thus, prevents vapor rising from the ground [ 81 ].
Dr. Andrija Štampar (1888–1958) holds a prominent place in preventing the spread of malaria. Štampar founded the Department of Malaria, and numerous antimalaria stations, hygiene institutes, and homes of national health. Dr. Štampar devoted his life to educating the broader population about healthy habits and, thus, prevents the spread of infectious diseases. Many films were shown, including a film entitled ‘Malaria of Trogir’ in Osijek in 1927, with numerous health lectures on malaria [ 82 ]. After the end of the Second World War, a proposal for malaria eradication measures was drafted by Dr. Branko Richter. These measures, thanks to Dr. Andrija Štampar, are being used in many malaria-burdened countries. For the eradication of malaria in Croatia and throughout Yugoslavia, DDT has been used since 1947 [ 83 ].
Malaria is still one of the most infectious diseases that cause far more deaths than all parasitic diseases together. Malaria was eradicated in Europe in 1975. After that year, malaria cases in Europe are linked to travel and immigrants coming from endemic areas. Although the potential for malaria spreading in Europe is very low, especially in its western and northern parts, it is still necessary to raise awareness of this disease and keep public health at a high level in order to prevent the possibility of transmitting the disease to the most vulnerable parts of Europe [ 84 ].
Unofficial data show that malaria disappeared from Croatia in 1958, while the World Health Organization cites 1964 as the year when malaria was officially eradicated in Croatia [ 45 , 75 ]. Nonetheless, some cases of imported malaria have been reported in Croatia since 1964. The imported malaria is evident concerning Croatia’s orientation to maritime affairs, tourism, and business trips. Namely, malaria is introduced to Croatia by foreign and domestic sailors, and in rare cases by tourists, mainly from the countries of Africa and Asia [ 75 , 85 ]. According to the reports of the Croatian Institute of Public Health, since the eradication of this disease 423 malaria cases have been reported, all imported [ 86 ]. Figure 1 shows the number of imported malaria cases in Croatia from 1987–2017, and Figure 2 the causative Plasmodium species of those cases ( Figure 1 and Figure 2 ) [ 86 , 87 ].

Imported malaria cases in Croatia from 1987–2017.

The causative agents of imported malaria in Croatia.
There is also massive and uncontrollable migration from Africa and Asia (mostly due to climate change) of both humans and birds, from countries with confirmed epidemics. This issue is an insurmountable problem if measured by the traditional approach. Insecticides (DDT, malathion, etc.) synthetic pyrethroids, in addition to inefficiency, impact the environment (harm bees, fruits, vines, etc.). Consequently, scientists have patiently established a mosquito control strategy (University of Grenoble, Montpellier) which includes a meticulous solution to the mosquito vector effect (malaria, arbovirus infection, West Nile virus) by changes in agriculture, urbanism, public services hygiene [ 88 ].
Northeastern Slavonia is committed to applying methods that are consistent with such achievements, with varying success, as certain limitations apply to protected natural habitats (Kopački rit) [ 89 ].
There is a historical link between population movement and global public health. Due to its unique geostrategic position, in the past, Croatia has been the first to experience epidemics that came to Europe through land and sea routes from the east. Adriatic ports and international airports are still a potential entry for the import of individual cases of communicable diseases. Over the past few years, sailors, as well as soldiers who worked in countries with endemic malaria, played a significant role in importing malaria into Croatia. Successful malaria eradication has been carried out in Croatia. Despite that in Croatia are still many types of Anopheles , which means that the conditions of transmission of the imported malaria from the endemic areas still exist. The risk of malaria recrudesce is determined by the presence of the vector, but also by the number of infected people in the area. Due to climate change, it is necessary to monitor the vectors and people at risk of malaria. Naturally- and artificially-created catastrophes, such as wars and mass people migration from endemic areas, could favor recrudescing of malaria. Once achieved, eradication would be maintained if the vector capacities are low and prevention measures are implemented. The increased number of malaria cases worldwide, the recrudesce of indigenous malaria cases in the countries where the disease has been eradicated, the existence of mosquitoes that transmit malaria and the number of imported malaria cases in Croatia are alarming facts. Health surveillance, including obligatory and appropriate prophylaxis for travelers to endemic areas, remains a necessary public health care measure pointed at managing malaria in Croatia.
5. Malaria Trends in the World
The WHO report on malaria in 2017 shows that it is difficult to achieve two crucial goals of a Global Technical Strategy for Malaria. These are a reduction in mortality and morbidity by at least 40% by 2020. Since 2010, there has been a significant reduction in the burden of malaria, but analysis suggests a slowdown, and even an increase in the number of cases between 2015 and 2017. Thus, the number of malaria cases in 2017 has risen to 219 million, compared to 214 million cases in 2015 and 239 million cases in 2010. Figure 3 presents the reported number of malaria cases per WHO region from 1990–2017 [ 1 , 90 ]. The most critical step in the global eradication of malaria is to reduce the number of cases in countries with the highest burden (many in Africa). The number of deaths from disease is declining, thus, in 2017 there were 435,000 deaths from malaria globally, compared with 451,000 in 2016, and 607,000 deaths in 2010. Figure 4 presents the number of malaria deaths from 1990-2017 [ 1 , 90 ]. Despite the delay in global progress, there are countries with decreasing malaria cases during 2017. Thus, India in 2017, compared with 2016, recorded a 24% decline of malaria cases. The number of countries reporting less than 10,000 malaria cases is growing, from 37 countries in 2010, to 44 in 2016, and to 46 in 2017. Furthermore, the number of countries with fewer than 100 indigenous malaria cases growing from 15 in 2010, to 26 countries in 2017 [ 1 ].

Reported malaria cases per WHO region from 1990–2017.

Reported malaria deaths per WHO region from 1990–2017.
Funding in malaria has not changed much. During 2017, US$3.1 billion was invested in malaria control and elimination globally. That was 47% of the expected amount by 2020. The USA was the largest single international donor for malaria in 2017 [ 1 , 91 ].
The most common global method of preventing malaria is insecticide-treated bed nets (ITNs). The WHO report on insecticide resistance showed that mosquitoes became resistant to the four most frequently used classes of insecticides (pyrethroids, organochlorines, carbamates, and organophosphates), which are widespread in all malaria-endemic countries [ 1 , 7 , 92 ].
Drug resistance is a severe global problem, but the immediate threat is low, and ACT remains an effective therapy in most malaria-endemic countries [ 1 , 93 ].
According to the WHO, Africa still has the highest burden of malaria cases, with 200 million cases (92%) in 2017, then Southeast Asia (5%), and the Eastern Mediterranean region (2%). The WHO Global Technical Strategy for Malaria by 2020 is the eradication of malaria from at least ten countries that were malaria-endemic in 2015 [ 1 ].
The march towards malaria eradication is uneven. Indigenous cases in Europe, Central Asia, and some countries in Latin America are now sporadic. However, in many sub-Saharan African countries, elimination of malaria is more complicated, and there are indications that progress in this direction has delayed. Elimination of vivax and human knowlesi malaria infections are another challenge [ 7 ].
6. Conclusions
The campaign to eradicate malaria began in the 1950s but failed globally due to problems involving the resistance of mosquitoes to the insecticides used, the resistance of malaria parasites to medication used in the treatment, and administrative issues. Additionally, the first eradication campaigns never included most of Africa, where malaria is the most common. Although the majority of forms of malaria are successfully treated with the existing antimalarials, morbidity and mortality caused by malaria are continually increasing. This issue is the consequence of the ever-increasing development of parasite resistance to drugs, but also the increased mosquito resistance to insecticides, and has become one of the most critical problems in controlling malaria over recent years. Resistance has been reported to all antimalarial drugs. Therefore, research into finding and testing new antimalarials, as well as a potential vaccine, is still ongoing, mainly due to the sudden mass migration of humans (birds, parasite disease vector insects) from areas with a large and diverse infestation.
The process towards eradication in some countries confirms that current tools could be sufficient to eradicate malaria. The spread of insecticide resistance among the vectors and the rising ACT failures indicate that eradication of malaria by existing means might not be enough.
Thus, given the already complicated problem of overseeing and preventing the spread of the disease, it will be necessary to supplement and change the principles, strategic control, and treatment of malaria.
Abbreviations
Author contributions.
Writing the manuscript: J.T., I.Š., and T.A.; updating the text: J.T., I.Š., T.A., and A.V.; literature searches: J.T., I.Š., T.A., and M.J.; tables and figures drawing: I.Š. and M.J.; critical reviewing of the manuscript: A.V.; organization and editing of the manuscript: I.Š. and A.V.
This research received no external funding. The article processing charges (APC) was funded by Faculty of Dental Medicine and Health, Osijek, Croatia.
Conflicts of Interest
The authors declare no conflict of interest.
- Nebraska Medicine
- Give to GCHS
Over 750,000 antimicrobial resistance deaths preventable yearly via vaccines, water, sanitation and infection control
- Published May 28, 2024
Medical Express Speaking at the World Health Assembly , authors of a new Lancet Series call for urgent global action on antimicrobial resistance (AMR). Authors say if the world does not prioritize action on AMR now, we will see a steady increase in the global death toll—currently 4.95 million per year from infections linked to AMR—with young infants, elderly people , and people with chronic illnesses or requiring surgical procedures at the highest risk.
Improving and expanding existing methods to prevent infections, such as hand hygiene, regular cleaning and sterilization of equipment in health care facilities, availability of safe drinking water, effective sanitation and use of pediatric vaccines, could prevent over 750,000 deaths associated with AMR every year in low- or middle-income countries (LMICs), estimates a new modeling analysis as part of a new four paper Series published in The Lancet .
Each year, an estimated 7.7 million deaths globally are caused by bacterial infections—1 in 8 of all global deaths, making bacterial infections the second largest cause of death globally. Out of these bacterial infection deaths, almost 5 million are associated with bacteria that have developed resistance to antibiotics. Authors of the new Lancet Series on antimicrobial resistance call for support for sustainable access to antibiotics to be central to ambitious and actionable targets on tackling AMR introduced at the High-Level Meeting of the United Nations General Assembly in September 2024.
Leave a comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 21, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
A boost for HIV vaccine research: Studies present comprehensive platform for validating next steps
by Ragon Institute of MGH, MIT and Harvard
HIV has proven a hard target for vaccine design. The most promising approach, germline-targeting (GT), proposes a series of immunizations: a first shot to activate inexperienced B cells—antibody-producing white blood cells—followed by a sequence of immunogens that are more and more like the HIV Envelope (Env) protein.
The ultimate goal of GT immunization is to coach B cells into producing broadly neutralizing antibodies (bnAbs) capable of binding to conserved sites on HIV Env, which seldom change despite HIV's penchant for rapid diversification.
A substantial amount of research has demonstrated the feasibility of the initial activation step; now, in two papers simultaneously out in Science and Science Immunology , researchers at the Ragon Institute of Mass General, MIT, and Harvard have developed a comprehensive platform for HIV vaccine research capable of both preclinically validating next-step boost immunogens and providing new insights into the basic biology of the antibody response.
These articles have been published in tandem with manuscripts from the Scripps Research Institute group in Science and Science Translational Medicine . This suite of four papers is the outcome of years of intense collaboration between the institutes and represents a major step towards an HIV vaccine.
"The key tool for our HIV program is the humanized mouse model," explains Professor Facundo D. Batista, Ph.D., Associate and Scientific Director of the Ragon Institute and PI of the lab in which the mouse studies were conducted.
"With knock-in human B cell receptors identified as potential bnAb precursors, we can then observe how they respond to immunogens as part of a complete mammalian immune system. We've used a CRISPR-based approach to develop mouse lines to interrogate several known conserved sites on the HIV Envelope for which our collaborators at Scripps have developed immunogens."
While first-author research scientists Zhenfei Xie, Ph.D., and Xuesong Wang, Ph.D., both carried out the work underpinning their respective Science and Science Immunology articles in the Batista lab at the Ragon Institute, each focused on a different conserved site on the HIV Env and a different aspect of the fundamental biology of immunization. Both scientists, however, came to a similar conclusion about delivery format: the mRNA-LNP system made famous by the Pfizer and Moderna COVID-19 vaccines was highly effective for HIV boost immunogens.
For Dr. Xie, the consilience between the work performed by the immunogen design team at Scripps, led by Prof. William Schief, and the immunobiological work in the Batista lab was the key factor.
"bnAbs against HIV-1 are the uncommon outcome of a long journey in HIV-infected patients. Jon Steichen worked backwards from the co-evolutionary trajectory of the bnAb and the virus, understanding how their structures interact, and then we moved everything back into an in vivo model where we could start observing messier phenomena like antibody competition. That collaboration was central to demonstrating that these types of very site-specific boosts can work."
The preclinical platform can address more fundamental biological problems. B cell lines that might develop bnAbs are often rare in humans, and one way the Scripps team has sought to overcome that difficulty is by designing immunogens capable of engaging a wide array of B cell lineages with that potential—but would those B cells compete, limiting immunogen effectiveness?
Dr. Wang produced a model with multiple potential precursors to CD4 binding site (CD4bs) bnAbs.
"The mRNA-LNP prime-boosts Christ Cottrell developed not only induced multi-lineage precursor B cell responses, but also triggered bnAb-like affinity maturation," she says. "Multiple lines could be matured in the same mouse at the same time."
Now, the challenge will be found in combining their work. "We're at the stage where there are an increasing number of immunogens in the pipeline targeting different Env sites," says Prof. Batista.
"Ultimately, our hope is to bring these projects together and start to understand the best way to generate a response to multiple sites: that's when you start really clamping down on the potential for viral escape. We already have this work underway."
Xuesong Wang et al, mRNA-LNP prime boost evolves precursors toward VRC01-like broadly neutralizing antibodies in preclinical humanized mouse models, Science Immunology (2024). DOI: 10.1126/sciimmunol.adn0622
Jon M. Steichen et al, Vaccine priming of rare HIV broadly neutralizing antibody precursors in nonhuman primates, Science (2024). DOI: 10.1126/science.adj8321
Christopher A. Cottrell et al, Heterologous prime-boost vaccination drives early maturation of HIV broadly neutralizing antibody precursors in humanized mice, Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.adn0223
Rogier W. Sanders et al, Progress on priming HIV-1 immunity, Science (2024). DOI: 10.1126/science.adp3459
Explore further
Feedback to editors
Researchers develop minimally invasive scaffold delivery system using dynamic thermoset polyurethane

Study finds text reminders help improve health care workers' mental health
Study of deadly Australian Japanese encephalitis virus strain prompts push for new vaccine

New sequencing method analyzes lung microbiomes to predict mortality in children following bone marrow transplant

Prenatal exposure to air pollution associated with increased mental health risks
2 hours ago

Sleep study finds post-karaoke stress is strengthened by REM sleep

Promising results for hyperlipidemia treatment reduce risk of cardiovascular events
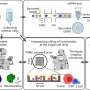
Cancer researchers discover optimal cancer-killing T cells

Genetic mosaicism more common than thought, study shows
Potential future target for treating primary headache disorders identified
3 hours ago
Related Stories
Scientists design and validate promising HIV vaccine strategy
Oct 3, 2022
Groundbreaking HIV vaccine design strategy shows promise in proof-of-principle tests
Nov 7, 2019

Tests of HIV vaccine using mRNA technology have begun
Jan 27, 2022
Immune system can be coaxed into selecting key antibodies to fight HIV
Dec 5, 2019
GPS-like system shows promise as HIV vaccine strategy to elicit critical antibodies
May 9, 2024
One-step method to generate mice for vaccine research
Dec 14, 2020
Recommended for you

Study demonstrates how cytokines produce long lasting humoral immunity following vaccination
4 hours ago
How COVID-19 'breakthrough' infections alter your immune cells
May 24, 2024
HPV vaccines prevent cancer in men as well as women, new research suggests
Researchers develop experimental mRNA avian flu vaccine
May 23, 2024

Cracking the genetic code for COVID-19 vaccine effectiveness
May 22, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Share full article
Advertisement
Supported by
Guest Essay
The End of Polio Is in Sight. What Have We Learned?

By Richard Conniff
Mr. Conniff is the author of “ Ending Epidemics: A History of Escape From Contagion .”
The fight to eradicate polio has been long and difficult. It’s been nearly 50 years since vaccines eliminated the disease in the United States. But polio continues to this day disabling or killing children in some harder to reach parts of the world. The good news is that we are now on the cusp of eradicating this terrible disease everywhere and forever.
The Global Polio Eradication Initiative is a consortium of major players in the fight — the Gates Foundation, Rotary International, the World Health Organization, the Centers for Disease Control and Prevention and Gavi, the Vaccine Alliance. The group has the ambitious aim to end transmission of the virus that causes the disease, wild poliovirus, by the end of the year in Afghanistan and Pakistan, the two countries where it is still actively infecting humans. If the initiative succeeds, it will be the culmination of a campaign that has reduced the incidence of paralytic wild poliovirus from an estimated 350,000 cases in 1988 to just 12 known cases last year.
It will also be a result of what may seem like a counterintuitive strategy: Knowledge about the disease flows not just from medical experts in great research centers to people in developing nations, but the other way as well, with workers on the front lines providing crucial information to stop the disease in their own areas and beyond. The lesson here: The medical tools needed to detect and contain any disease work best in the hands of the people most directly affected by it. Having used this strategy to stop polio, people in developing nations are already looking to apply those same tools against other diseases, both familiar and emerging.
Along the remote, mountainous Afghanistan-Pakistan border, the people on the front lines of the polio eradication effort are mostly women, and mostly members of the communities they serve. Each team is responsible for up to 75 houses, going door to door (or sometimes mosque to mosque), providing a dose of oral polio vaccine to every child in every five-day campaign. Because the communities are poor, and because families can lose patience with repeated visits focused only on polio, the workers also bring nutritional supplements, health information and other resources. Their job is to build trust in villages where people are prone to distrust, and to keep parents engaged in the fight. (In 2011, the fake vaccination campaign reportedly staged by the Central Intelligence Agency in its hunt for Osama bin Laden served only to deepen that distrust.)
The intensity of the national programs — with about 400,000 workers in Pakistan and 86,000 in Afghanistan — has recently reduced 12 genetic clusters of the wild poliovirus in the region to just two, and one of the two hasn’t been seen since November. “From a medical perspective, the virus is gasping in these last corridors,” says Dr. Ananda Bandyopadhyay of the Gates Foundation.
The virus could, of course, spread outside these regions, as it did in 2022, when international air travel carried polio to a handful of other countries, including the United States. But frontline workers in Pakistan and Afghanistan serve as a network for tracking its possible escape routes, as families move back and forth across the border.
Sheeba Afghani, a communication specialist for UNICEF’s polio program, said that when local health workers make a home visit, for instance, and find a family member absent, they ask questions, such as: “If the child is not at home, where are they? Are they out of the district? If out of the district, is it in the same city or another city?” These are questions outsiders could never ask. If the family member has crossed the border, the information gets relayed to polio workers at the reported destination, to locate newcomers in their own 75-house networks.
New tools also help track the virus as it moves in these areas. When India was struggling to eliminate polio in 2010, it had fewer than 10 sites routinely monitoring for the virus in sewage and surface water, said Dr. Hamid Jafari, the World Health Organization’s director of polio eradication in the Eastern Mediterranean region. Back then, to spot an outbreak, health officials had to wait for children to turn up with paralysis. Now, Pakistan has monitoring sites in 84 districts.
Over nine months last year, that monitoring alerted the city of Peshawar to 30 separate introductions of the virus. But the Peshawar district’s 4.7 million people did not suffer a single case of polio, said Dr. Jafari. Knowing where to look for the virus and maintaining a high level of vaccination among permanent residents kept them safe.
A big part of this success is due to the use of the Sabin oral vaccine rather than the Salk injectable vaccine. The oral vaccine, containing a weakened live virus, is easier to deliver and has the critical advantage of inducing immunity not just in recipients’ bloodstream, as the Salk vaccine does, but also in their intestines. That means it stops transmission of the virus in the unsanitary conditions that are common in affected areas (and universal in children). Instead, the live vaccine itself spreads and protects children who might otherwise go unvaccinated.
According to the Global Polio Eradication Initiative, the Sabin vaccine has protected more than three billion children in the past 10 years. But using it involves a trade off: In places with very low levels of polio immunity the vaccine-derived virus can evolve as it spreads, and in rare instances it can revert to a paralytic form. Over the five years through 2023, about 3,600 people, mostly unvaccinated children, have suffered vaccine-derived poliovirus. But the number of cases has already begun to decline thanks to a novel version of the oral vaccine, genetically modified to sharply reduce the risk of reverting.
In Pakistan and Afghanistan, the women on the front lines see the end of polio in sight. This fight has given them the opportunity to work outside the home, earn money and make a lifesaving difference to their villages. When the government of Pakistan recently surveyed them about their experience, one big question they asked was: What can we work on next?
Public health workers everywhere already have the answer. Give them the tools, and developing nations will apply the lessons learned in this fight against infectious diseases like tuberculosis, malaria, measles, typhoid fever and others yet unknown. The end result will be a world that’s safer for all of us.
Richard Conniff is the author of “ Ending Epidemics: A History of Escape From Contagion .”
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .

IMAGES
COMMENTS
The development of highly effective and durable vaccines against the human malaria parasites Plasmodium falciparum and P. vivax remains a key priority. Decades of endeavor have taught that achieving this goal will be challenging; however, recent innovation in malaria vaccine research and a diverse pipeline of novel vaccine candidates for clinical assessment provides optimism.
Introduction. The malaria vaccine RTS,S/AS01E (brand name Mosquirix TM) received a favorable opinion from the European Medicines Agency (EMA) in 2015 after review of its safety and efficacy to ...
Here, we discuss recent clinical advances in vaccine development and highlight ongoing challenges for the future. Malaria is one of the most devastating infectious diseases in humans, responsible ...
RTS,S vaccine The first approved malaria vaccine is RTS,S, a monovalent recombinant protein vaccine that targets a fragment of the CSP. The vaccine contains a truncated CSP of P. falciparum that is then fused with the hepatitis B surface (S) antigen, which acts as a carrier for the CS antigen and an immunogenic adjuvant, AS01 [ 52 ].
This position paper supersedes the 2022 WHO position paper on malaria vaccines.6 It includes the updated WHO recommendations on the use of the RTS,S/AS01 and R21/Matrix-M vaccines for the reduction of malaria morbidity and mortality in children living in endemic areas, prioritizing areas of moderate and high malaria transmission.
A recent trial showed that seasonal malaria chemoprevention plus an annual booster of RTS,S/AS01 through 5 years of age (after the initial three-dose vaccine series is started at 5 to 17 months of ...
There is no doubt that decades of malaria vaccine research have yielded some major outcomes over recent years. The decision to recommend and then implement RTS,S/AS01 vaccination in young children at high risk of P. falciparum infection is a significant moment in the history of malaria vaccine research and control. RTS,S/AS01 is also the first ...
World Health Organization. This position paper supersedes the 2016 publication, "Malaria vaccine: WHO position paper-2016."1 It includes the updated WHO recommendations on the wider use of the RTS,S/AS01 vaccine for the reduction of malaria morbidity and mortality in children living in areas of moderate to high malaria transmission.
Abstract. The development of highly effective and durable vaccines against the human malaria parasites Plasmodium falciparum and P. vivax remains a key priority. Decades of endeavor have taught that achieving this goal will be challenging; however, recent innovation in malaria vaccine research and a diverse pipeline of novel vaccine candidates ...
In 2021, nearly half of the world's population lived at risk from malaria, with over 600 000 deaths annually, of which over 95% occur in the WHO African region and 80% of these in children younger than 5 years.1 WHO recommends several preventive and curative interventions that, when used together, can greatly reduce malaria illness and death, including effective vector control, chemoprevention ...
Malaria vaccine introduction in endemic countries is a game-changing milestone in the fight against the disease. This article examines the inequity in the global pharmaceutical research, development, manufacturing, and trade landscape. The role of inequity in hindering progress towards malaria elimination is explored. The analysis finds that transformational changes are required to create an ...
To maintain momentum towards improved malaria control and elimination, a vaccine would be a key addition to the intervention toolkit. Two approaches are recommended: (1) promote the development and short to medium term deployment of first generation vaccine candidates and (2) support innovation and discovery to identify and develop highly effective, long-lasting and affordable next generation ...
In addition, the paper identifies research priorities for the vaccine and considerations for immunization and health systems. It briefly describes the development of a framework to guide the allocation of the initial limited doses of malaria vaccine; supplies of RTS,S are expected to be limited in the short to medium term.
A booster dose of R21/Matrix-M at 1 year following the primary three-dose regimen maintained high efficacy against first and multiple episodes of clinical malaria. Furthermore, the booster vaccine induced antibody concentrations that correlated with vaccine efficacy. The trial is ongoing to assess long-term follow-up of these participants and the value of further booster vaccinations.
This collection of malaria vaccine research and innovation papers highlights the intersection of efforts to: (1) achieve the pan-African 1 and global goal 2 of "Zero Malaria"; and (2) make the ...
Malaria control remains a challenge in many parts of the Sahel and sub-Sahel regions of Africa. We conducted an individually randomized, controlled trial to assess whether seasonal vaccination with...
While two Plasmodium falciparum circumsporozoite protein-based pre-erythrocytic vaccines (PEV), RTS,S and R21, have been approved by the WHO, no blood-stage vaccine (BSV) or transmission-blocking vaccine (TBV) has reached a phase 3 trial. One of the major obstacles that slows down malaria vaccine development is the shortage (or lack) of in vitro assays or animal models by which investigators ...
Two studies [55, 73] evaluated the long-term (up to 7 years) efficacy of RTS,S on severe and clinical malaria.While the study by Tinto et al. [] demonstrated a decrease in severe malaria cases over time, there was a rebound against clinical malaria among older children (5-7 years).Oluto et al. [] identified that vaccine efficacy (clinical malaria) waned over time, including negative efficacy ...
The 77% efficacy against malaria dipped to 71% in children who got a vaccine with a lower dose of adjuvant. The children's levels of specific antibodies to malaria fell by two-thirds by 9 months, but the booster dose at 12 months restored them, according to the paper, which is in press at The Lancet and was posted 20 April on its preprint server.
The worst outcome is death. Tinto says 4,000 people died of malaria last year in Burkina Faso alone. In 2021, across Africa, it's estimated that 619,000 died of the mosquito-borne disease, most of ...
Background The world has made great strides towards beating malaria, although about half of the world population is still exposed to the risk of contracting malaria. Developing an effective malaria vaccine was a huge challenge for medical science. In 2021 the World Health Organization (WHO) approved the first malaria vaccine, RTS,S/AS01 vaccine (Mosquirix™), for widespread use. Main abstract ...
To explore the data further. Select the vaccine status (active, inactive) and phase of development (phase I-IV) and further stratify the data according to target malaria species, target life cycle stage, vaccine platform, target antigen or adjuvant - or a combination of elements (e.g., by clicking on a bar in a chart or cell in a table) - to display the corresponding data in the other charts.
May 23, 2024 - Cyrianne Keutcha, PhD '25, has been around malaria all her life, from growing up in a malaria-endemic country, to being infected herself, and now to studying the parasite in the lab. She is completing her degree in the Harvard Griffin Graduate School of Arts and Sciences (GSAS), studying in the Biological Sciences in Public Health program at Harvard T.H. Chan School of ...
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
The Global Center is led by experts in biopreparedness and high-consequence infections research, education and clinical care. ... sanitation and use of pediatric vaccines, could prevent over 750,000 deaths associated with AMR every year in low- or middle-income countries (LMICs), estimates a new modeling analysis as part of a new four paper ...
WHO updated its recommendation for malaria vaccines in October 2023. This applies to both RTS,S/AS01 and R21/Matrix-M vaccines. WHO recommends the programmatic use of malaria vaccines for the prevention of P. falciparum malaria in children living in malaria endemic areas, prioritizing areas of moderate and high transmission.. The malaria vaccine should be provided in a schedule of 4 doses in ...
HIV has proven a hard target for vaccine design. The most promising approach, germline-targeting (GT), proposes a series of immunizations: a first shot to activate inexperienced B cells—antibody ...
Mr. Conniff is the author of "Ending Epidemics: A History of Escape From Contagion." The fight to eradicate polio has been long and difficult. It's been nearly 50 years since vaccines ...
As of October 2023, WHO recommends the programmatic use of malaria vaccines for the prevention of P. falciparum malaria in children living in malaria endemic areas, prioritizing areas of moderate and high transmission.This applies to both RTS,S/AS01 and R21/Matrix-M vaccines. The first malaria vaccine, RTS,S, was recommended by WHO to prevent malaria in children in October 2021.
UNICEF delivered over 43 000 doses of the R21/Matrix-M malaria vaccine by air to Bangui, Central African Republic, today, with more than 120 000 doses to follow in the next days. It is the first country to receive the R21 malaria vaccine for use in routine childhood immunization, marking another step forward in preventing the disease and saving children's lives.