An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts


The global epidemiology of hypertension
Hypertension is the leading cause of cardiovascular disease and premature death worldwide. Owing to widespread use of antihypertensive medications, global mean blood pressure (BP) has remained constant or decreased slightly over the past four decades. By contrast, the prevalence of hypertension has increased, especially in low and middle-income countries (LMICs). Estimates suggest that in 2010, 31.1% of adults (1.39 billion) worldwide had hypertension. The prevalence of hypertension among adults was higher in LMICs (31.5%, 1.04 billion people) than in high-income countries (HICs; 28.5%, 349 million people). Variations in the levels of risk factors for hypertension, such as high sodium intake, low potassium intake, obesity, alcohol consumption, physical inactivity and unhealthy diet, may explain some of the regional heterogeneity in hypertension prevalence. Despite the increasing prevalence, the proportions of hypertension awareness, treatment and BP control are low, particularly in LMICs, and few comprehensive assessments of the economic impact of hypertension exist. Future studies are warranted to test implementation strategies for hypertension prevention and control, especially in low-income populations, and to accurately assess the prevalence and financial burden of hypertension worldwide.
INTRODUCTION
Hypertension is the leading preventable risk factor for cardiovascular disease (CVD) and all-cause mortality worldwide. 1 , 2 In 2010, 31.1% of the global adult population (1.39 billion people) had hypertension, defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg. 3 The prevalence of hypertension is rising globally owing to ageing of the population and increases in exposure to lifestyle risk factors including unhealthy diets (i.e. high sodium and low potassium intake and lack of physical activity. 3 However, changes in hypertension prevalence are not uniform worldwide. In the past two decades, high-income countries (HICs) experienced a modest decrease in hypertension prevalence, while low and middle-income countries (LMICs) experienced significant increases. 3 These disparities in hypertension prevalence trends suggest that health care systems in LMICs could be facing a rapidly increasing burden of hypertension and BP-related cardiovascular diseases, in some cases in addition to a substantial burden of infectious diseases.
In this Review, we discuss estimates and trends in mean BP levels and hypertension prevalence, awareness, treatment, and control worldwide. We also examine risk factors for hypertension, strategies for hypertension control, and evidence of the financial burden of hypertension. We conclude by discussing the consequences of current trends in hypertension and areas where more research is needed.
GLOBAL MEAN BLOOD PRESSURE
A study that analyzed data from 844 studies performed in 154 countries with 8.69 million participants estimated that in 2015, the global mean age-standardized systolic BP was 127.0 mmHg in men and 122.3 mmHg in women, whereas the mean age-standardized diastolic BP was 78.7 mmHg in men and 76.7 mmHg in women. 4 Higher mean systolic and diastolic BPs in both men and women were found in South Asia, Sub-Saharan Africa, and Central and Eastern Europe, while lower mean BPs were found in high-income Western and high-income Asia-Pacific regions. 4 Social and environmental factors, including healthcare access, availability of antihypertensive medications, and regional variations in hypertension risk factors, such as obesity, alcohol consumption, unhealthy diet and lack of physical activity, likely contribute to these regional differences 3 , 4
This study also reported that over the past 40 years, estimated mean BP has remained constant or decreased slightly worldwide. 4 Estimated global mean age-standardized systolic BP remained fairly constant in men between 1975 (126.6 mmHg) and 2015 (127.0 mmHg) but decreased slightly in women during this period (from 123.9 mmHg to 122.3 mmHg). Trends for men and women were similar for estimated global mean age-standardized diastolic BP, with very little change in men and a slight decrease in women. However, regional changes in estimated mean BP between 1975 and 2015 were more heterogeneous ( Figure 1 ). In general, HICs experienced a significant BP decrease, while BP in LMIC regions increased. 4 Substantial decreases in estimated age-standardized mean systolic and diastolic BP occurred in both men and women in high-income Western and Asia-Pacific regions between 1975 and 2015. 4 Estimates suggest that the largest decrease in systolic BP was in the high-income Asia-Pacific region in which systolic BP decreased by 2.4 mmHg per decade in men and 3.2 mmHg per decade in women, whereas the largest decrease in diastolic BP was in the high-income Western region in which diastolic BP decreased by 1.5 mmHg per decade in men and 1.8 mmHg per decade in women. Decreases in mean systolic and diastolic BP were also observed in women in Central and Eastern Europe, Latin America and the Caribbean, the Middle East and North Africa, and Central Asia, while no change in BP was seen in men in these regions. 4 In both men and women, systolic and diastolic BP increased in East and Southeast Asia, South Asia, Oceania, and sub-Saharan Africa.

A. Change in systolic blood pressure (BP) in men. B. Change in systolic BP in women. C. Change in diastolic BP in men. D. Change in diastolic BP in Women. Data obtained from reference 4 .
These regional changes in mean systolic and diastolic BP have led to disparities in the burden of hypertension. 3 Improvements in prevention, detection, and treatment of BP in HICs have likely contributed to lower BP levels in these regions. 3 – 7 By contrast, limited healthcare resources together with population ageing and urbanization, which is associated with reductions in physical activity and increases in unhealthy diets, are potential drivers of BP increases in LMICs. 8 – 10 In both HICs and LMICs, women have higher proportions of hypertension awareness, treatment, and control than men. 3 These differences could contribute to the greater BP reductions observed in women than men in some regions
GLOBAL BURDEN OF HYPERTENSION
Based on an analysis of data from 135 population-based studies that included 968,419 adults from 90 countries, we estimated that in 2010 the global age-standardized prevalence of hypertension (defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, and/or current use of antihypertensive medication) was 31.1% (95% CI 30.0–32.2%). 3 The age-standardized prevalence of hypertension was slightly higher in men (31.9%) than in women (30.1%) ( Table 1 ) 3 and was lower in HICs (28.5%) than in LMICs (31.5%) ( Figure 2 ). 3 The lowest prevalence of hypertension in men was found in South Asia (26.4%), whereas the highest prevalence was in Eastern Europe and Central Asia (39.0%). In women, the prevalence of hypertension was lowest in HICs (25.3%) and highest in Sub-Saharan Africa (36.3%). The reasons for these disparities in hypertension prevalence across regions are not fully understood but are likely influenced by differences in the prevalence of risk factors for hypertension, including unhealthy diet, lack of physical activity and obesity. 3 , 8

Prevalence of hypertension defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg or use of antihypertensive medication in A. Men and B. Women. Data obtained from reference 3 .
Estimated prevalence of hypertension in high-income and low and middle-income countries in 2000 and 2010
| Population | 2000 | 2010 | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Age-standardized prevalence % (95% CIs) | ||||
| Global | 26.4 (24.6–28.2) | 25.1 (23.4–26.9) | 31.9 (30.3–33.5) | 30.1 (28.5–31.6) |
| High-income countries | 35.1 (29.8–40.3) | 26.9 (22.6–31.3) | 31.6 (29.6–33.6) | 25.3 (23.9–26.7) |
| Low and middle-income countries | 23.4 (21.6–25.2) | 24.1 (22.4–25.9) | 31.7 (29.7–33.6) | 31.2 (29.3–33.1) |
| Absolute numbers in millions (95% CIs) | ||||
| Global | 457.0 (422.9, 491.2) | 464.1 (432.0, 496.2) | 694.4 (658.7–730.1) | 693.5 (659.5–727.5) |
| High-income countries | 162.8 (140.7–184.9) | 159.5 (138.4–180.6) | 174.2 (165.3–183.2) | 174.7 (167.2–182.1) |
| Low- and middle-income countries | 294.3 (268.2–320.3) | 304.6 (280.5–328.8) | 520.1 (485.6–554.7) | 518.8 (485.7–552.0) |
Data obtained from reference 3
The Prospective Urban Rural Epidemiology (PURE) study included 153,996 adults aged 35–70 years from 628 rural and urban communities in 17 geographically and economically diverse countries who were recruited between 2003 and 2009. 11 This study included 142,042 participants with BP data at baseline, providing a unique opportunity to compare hypertension prevalence between rural and urban populations in different world regions. The PURE study found that 40.8% (95% CI 40.5–41.0%) of participants had hypertension with a higher prevalence in men (41.4%) than in women (37.7%). 11 Residents of rural areas had a higher prevalence of hypertension than urban residents in HICs and middle-income countries (MICs), but the opposite was true in low-income countries (LICs). 11
We estimated that between 2000 and 2010, the global age-standardized prevalence of hypertension in adults aged ≥20 years increased by 5.2%. 3 This estimate is consistent with a 2015 Global Burden of Disease analysis that found that the prevalence of elevated systolic BP (≥140 mmHg) increased 3.2% from 17.3% in 1990 to 20.5% in 2015. 12 The global increase in prevalence of hypertension was consistent by sex (5.5% in men and 5.0% in women) but varied by economic development. 3 From 2000 to 2010, the prevalence of hypertension increased in LMICs and decreased in HICs ( Table 1 ). 3 LMICs experienced a sharp increase in hypertension prevalence from 23.8% in 2000 to 31.5% in 2010. By contrast, HICs experienced a decrease in hypertension prevalence from 31.1% to 28.5% in the same ten-year period. 3 Hypertension prevalence was therefore higher in LMICs (31.5%) than in HICs (28.5%) in 2010. Similar to the trends observed for BP change, population ageing, urbanization and related lifestyle changes (such as unhealthy diet and lack of physical activity) might explain the increased prevalence of hypertension in LMICs. 8 , 10
As mentioned above, an estimated 1.39 billion adults worldwide had hypertension in 2010 with an approximately even distribution between men and women ( Table 1 ). 3 Among those with hypertension, approximately 75% (1.04 billion) lived in LMICs and 25% (349 million) lived in HICs. By contrast, in 2000 an estimated 921 million people had hypertension comprising 322 million (34%) in HICs and 599 million (66%) in LMICs. 3 , 13 Between 2000 and 2010, the absolute burden of hypertension (total number of individuals with hypertension) increased by 440 million in LMICs and only 27 million in HICs. 3 In 2010, the highest absolute burden of hypertension was in East Asia and the Pacific, which had more individuals with hypertension than all HICs combined. 3 The absolute burden of hypertension in men and women increased in all world regions between 2000 and 2010, except for in the Middle East and North Africa where the absolute burden in women decreased slightly. 3 The Global Burden of Disease study estimated that in 2015 around 3.5 billion adults worldwide had systolic BP of at least 110–115 mmHg, a level that is associated with increased risk of ischaemic heart disease (IHD), stroke, and kidney disease. 4 This prevalence represents a marked increase from 1990 when 1.87 billion people had a systolic BP of at least 110–115 mmHg. 4
In 2017, the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines redefined hypertension in adults as systolic BP ≥130 mmHg and/or diastolic BP ≥80 mmHg. 14 This change was based on findings from a number of large-scale, prospective observational studies that reported significant increases in risk of CVD with increasing BP even from levels as low as systolic BP 115 mmHg, as well as the results of randomized clinical trials including the SPRINT trial (discussed further below) that showed that intensive BP lowering (target systolic BP <120 mmHg) reduces CVD and all-cause mortality to an even greater extent than does standard BP lowering (target systolic BP ≤140 mmHg). 15 – 18 When the new definition was applied to the US general population, hypertension prevalence increased from 32.0% (based on the traditional criteria) to 45.4% ( Table 2 ). 19 , 20 In the Chinese general population, the increase was even greater from 23.2% to 46.4%. 19 , 20 These findings suggest that if the new criteria were applied worldwide, the difference in hypertension prevalence between LMICs and HICs would be much greater than previously reported. 3 Full implementation of the new guidelines would require a greater proportion of adults to be treated with antihypertensive medications but could prevent an estimated 610,000 CVD events and 334,000 deaths per year in the US alone. 19
Estimated prevalence of hypertension in the general populations of the US in 2013–2016 and China in 2012–2015
| Population | Age-standardized prevalence of hypertension (% (95% CI) | ||
|---|---|---|---|
| Blood pressure ≥140/90 mmHg | Blood pressure ≥130/80 mmHg | ||
| US 2013–2016 | Men | 32.1 (30.2–34.0) | 48.0 (45.7–50.3) |
| Women | 31.8 (29.8–33.8) | 43.1 (41.3–44.9) | |
| Total | 32.0 (30.3–33.6) | 45.4 (43.9–46.9) | |
| China 2012–2015 | Men | 24.5 (23.0–26.0) | 52.3 (50.0–54.5) |
| Women | 21.9 (20.7–23.1) | 40.4 (38.0–42.9) | |
| Total | 23.2 (21.9–24.5) | 46.4 (44.2–48.7) | |
Data obtained from references 19 and 20
Valid estimation of global BP levels and hypertension prevalence is highly dependent on the availability and quality of BP data from population-based studies around the world. Many factors, such as the representativeness of study populations (e.g. sampling methods and response rates), BP measurement methods (e.g. calibration of BP measuring devices, appropriate BP cuff sizes, and preparation of participants), and number of BP measurements can affect the quality of prevalence data. 21 In many countries, population-based BP studies have not been conducted or BP data are not publicly available. In addition, the number of studies and the quality of the available data varies substantially between regions. As a result, BP estimates for some countries are based entirely on modelling in several pooling projects. 4 , 12 , 22 This problem is particularly apparent in Sub-Saharan Africa for which BP data are sparse. 23 High-quality, population-based studies that accurately measure BP in all countries worldwide, particularly in LMICs, are required to enable accurate assessment of the global burden of hypertension.
BLOOD PRESSURE, CVD, CKD AND MORTALITY
Elevated BP is associated with a large global burden of CVD and premature death. In 2015, the estimated number of all-cause deaths that were associated with systolic BP ≥110–115 mmHg was 10.7 million (19.2% of all deaths) and with systolic BP ≥140 mm Hg was 7.8 million (14.0% of all deaths). 12 The largest numbers of deaths that were related to systolic BP ≥110–115 mmHg were attributed to IHD (4.9 million or 54.5% of IHD deaths), ischaemic stroke (1.5 million or 50.0% of ischaemic stroke deaths), and haemorrhagic stroke (2.0 million or 58.3% of haemorrhagic stroke deaths) ( Figure 3 ). The corresponding numbers of deaths related to systolic BP ≥140 mmHg were 3.6 million (40.1% of IHD deaths), 1.1 million (38.1% of ischemic stroke deaths) and 1.4 million (42.5% of hemorrhagic stroke deaths). 12 Consistent with trends in hypertension prevalence, the estimated numbers of BP-related all-cause and CVD deaths increased substantially from 1990 to 2015, especially in LMICs. 12 Scaling up effective antihypertensive interventions to reduce BP-related morbidity and mortality should be a global public health priority.

A. Estimated numbers of deaths attributed to systolic blood pressure (SBP) ≥110–115 mmHg and B. SBP ≥140 mmHg by cause of death. Data obtained from reference 12 .
Observational epidemiological studies have shown a strong, independent and linear association between BP and the risk of CVD without any evidence of a BP threshold. 15 , 24 – 26 For example, the Prospective Studies Collaboration examined the association between BP and cause-specific mortality in approximately 1 million adults aged 40–89 years with no previous history of CVD at baseline using data from 61 prospective observational studies. 15 During 12.7 million person-years of follow-up, around 56,000 CVD deaths (12,000 stroke, 34,000 IHD, and 10,000 other CVD) and 66,000 deaths owing to other causes were reported. Meta-analyses that corrected for regression dilution showed that the proportional difference in risk of CVD death that was associated with a given absolute difference in usual BP for each decade of age was similar for increases in BP from levels as low as 115 mmHg systolic and 75 mmHg diastolic. 15 In those aged 40–69 years, a difference in usual systolic BP of 20 mmHg or usual diastolic BP of 10 mmHg was associated with a more than twofold difference in the rate of stroke deaths and a twofold difference in the rate of death owing to IHD and other CVD causes. The proportional difference in CVD mortality that was associated with a given absolute difference in usual blood pressure in adults aged 80–89 years was about half that of adults aged 40–49 years, but the annual absolute differences in risk were greater in old age. 15
In adults who are middle aged or older (≥35 years), systolic BP is a more important determinant of CVD risk than diastolic BP. 24 – 26 Among 347,978 men aged 35 to 57 years who were screened in the US for entry into the Multiple Risk Factor Intervention Trial (MRFIT) and had not previously been hospitalized for CVD, 7,150 deaths from IHD and 733 deaths from stroke were identified during an average of 11.6 years of follow-up. 25 In every decile of BP, systolic BP was more strongly associated with risk of IHD and stroke than was diastolic BP. When the highest deciles were compared with the lowest deciles, the relative risks associated with increased systolic and diastolic BP were 3.7 and 2.8, respectively, for IHD, and 8.2 and 4.4, respectively, for stroke.
Several large prospective cohort studies have reported that elevated BP is also a strong independent risk factor for chronic kidney disease (CKD) and end-stage renal disease (ESRD). 27 – 29 The increase in risk that was associated with higher BP was dose-response and continuous throughout the distribution of BP levels above 120 mmHg. 27 – 29 Among 332,544 men aged 35 to 57 years who were screened for entry into the MRFIT trial and did not have ESRD at baseline, a strong independent linear relationship between both systolic and diastolic BP and incidence of ESRD was identified during an average of 16 years of follow-up. 28 Compared with normotensive men who had a systolic BP <120 mmHg and diastolic BP <80 mmHg, the relative risk of ESRD for men with hypertension who had a systolic BP >210 mmHg or diastolic BP >120 mmHg was 22.1 (P<0.001). A similar association between BP and risk of ESRD was reported in a cohort of 158,365 Chinese men and women aged ≥40 years. 29 In addition, BP was significantly and independently associated with progression to ESRD among patients with CKD. 27 Among 3,708 patients with CKD in the Chronic Renal Insufficiency Cohort Study in the US, multivariable-adjusted relative risk (95% CI) for ESRD was 2.37 (CI, 1.48 to 3.80) and 3.37 (CI, 2.26 to 5.03) among those with systolic BP of 130–139 and ≥140 mmHg, respectively, compared with systolic BP <120 mmHg. 27
Randomized clinical trials have demonstrated that BP lowering with commonly used regimens, such as diuretics, angiotensin-converting-enzyme inhibitors, angiotensin-receptor blockers, and calcium channel blockers reduces the risk of CVD and all-cause mortality. 30 , 31 A large meta-analysis of 123 clinical trials with 613,815 participants showed that relative risk reductions of CVD and all-cause mortality were proportional to the magnitude of achieved BP reductions. 31 For example, every 10 mmHg reduction in systolic BP significantly reduced the risk of major CVD events by 20% (relative risk 0.80, 95% CI 0.77–0.83), IHD by 17% (relative risk 0.83, 0.78–0.88), stroke by 27% (relative risk 0.73, 0.68–0.77), heart failure by 28% (relative risk 0.72, 0.67–0.78), and all-cause mortality by 13% (0.87, 0.84–0.91). 31 This study provided no clear evidence that proportional risk reductions in major CVD events differed by baseline BP levels or comorbidities, except for patients with diabetes and CKD, for whom the risk reductions were smaller but still statistically significant.
The Systolic Blood Pressure Intervention Trial (SPRINT) randomly assigned 9,361 patients with systolic BP ≥130 mmHg and increased CVD risk to a systolic BP target of <120 mmHg (intensive treatment) or <140 mmHg (standard treatment). 17 Increased CVD risk was defined as clinical or subclinical CVD, CKD, a 10-year risk of CVD of ≥15% according to the Framingham risk score, or an age of ≥75 years. At 1 year, mean systolic BP was 121.4 mmHg in the intensive treatment group and 136.2 mmHg in the standard-treatment group. Moreover, intensive treatment was associated with a 25% reduction in CVD events (hazard ratio 0.75, 95% CI 0.64–0.89, P<0.001) and a 27% reduction in all-cause mortality (hazard ratio 0.73, 95% CI 0.60–0.90, P=0.003) compared to standard treatment over a median follow-up of 3.26 years. The number needed to treat (NNT) to prevent one CVD event during the follow-up period was 61, and the NNT to prevent one death from any cause was 90. 17 The SPRINT trial demonstrates that intensive BP lowering treatment with a systolic BP target of <120 mmHg is beneficial for patients with hypertension who are at high risk of CVD. The SPRINT trial prompted the revision of the definition of hypertension and BP antihypertensive treatment goals. 14
The results of meta-analyses of randomized controlled trials that compared the efficacy of different BP targets or treatment intensities to reduce the risk of CVD and mortality in patients with hypertension are consistent with the SPRINT findings. 18 , 31 , 32 For example, a 2015 network meta-analysis of 42 trials that included 144,220 patients with hypertension, reported linear associations between mean achieved systolic BP and risk of CVD and mortality, with the lowest risk among those who achieved a systolic BP of 120–124 mmHg. 18 In these trials, study groups that achieved mean systolic BPs of 120–124 mm Hg had hazard ratios for major CVD of 0.71 (95% CI, 0.60–0.83), 0.58 (95% CI, 0.48–0.72), 0.46 (95% CI, 0.34–0.63), and 0.36 (95% CI, 0.26–0.51), compared with study groups that achieved systolic BPs of 130–134 mmHg, 140–144 mmHg, 150–154 mmHg and ≥160 mmHg, respectively. 18 Network meta-analyses offer a unique advantage over traditional meta-regression methods by enabling the simultaneous comparison of the effects of multiple interventions or treatment targets on clinical outcomes while preserving trial-level treatment randomization and its associated protection against bias.
Despite the findings from SPRINT and related meta-analyses, some concerns remain about the appropriateness and effectiveness of intensive BP reduction in certain subgroups, including patients with CKD, diabetes or stroke and older adults (≥65 years). A pre-specified subgroup analysis of outcomes in SPRINT trial participants with CKD found that intensive BP treatment resulted in significant reductions in the risk of CVD and death but no significant increase in the incidence of serious adverse events compared to standard BP treatment. 33 The rate of continuous eGFR decline was higher in the intensive BP treatment group but there was no significant difference in risk of CKD progression (defined as incident ESRD or halving of eGFR) between the two groups. 33 In addition, a subgroup analysis of 978 SPRINT participants (519 in the intensive and 459 in the standard treatment group) showed that while eGFR was lower in the intensive treatment compared to standard treatment group, urine biomarkers of tubule function, injury, inflammation, and repair were not different over 4 years of follow-up. These findings suggest that observed eGFR declines in the intensive treatment group predominantly reflect hemodynamic changes rather than intrinsic damage to kidney tubule cells. 34 An additional SPRINT subgroup analysis in patients aged ≥75 years reported that intensive BP treatment significantly reduced the incidence of CVD and mortality with no increase in serious adverse events compared with standard BP-lowering treatment. 35
As patients with diabetes or stroke were not included in the SPRINT trial, questions regarding the risks and benefits of intensive BP lowering remain in these subgroups. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study reported that intensive BP treatment aimed at achieving a systolic BP target of <120 mmHg did not significantly reduce the risk of CVD events compared with standard BP treatment (systolic BP target of <140 mmHg) in patients with type 2 diabetes (hazard ratio 0.88; 95% CI 0.73–1.06). 36 However, a subgroup analysis suggested a significant reduction in CVD events in the intensive BP treatment group compared to standard treatment group (hazard ratio 0.74, 95% CI 0.55 to 1.00, p=0.049) among participants who received standard glycaemic treatment. 37 In addition, a meta-analysis of four randomized clinical trials with 4,895 participants reported that intensive BP control reduced the risk of recurrent stroke compared with standard BP control (relative risk 0.78; 95% CI 0.64–0.96; P = 0.02). 38 On-going clinical trials (such as the OPTIMAL-DIABETES trial [ {"type":"clinical-trial","attrs":{"text":"NCT04040634","term_id":"NCT04040634"}} NCT04040634 ], the OPTIMAL Stroke trial [ {"type":"clinical-trial","attrs":{"text":"NCT04036409","term_id":"NCT04036409"}} NCT04036409 ], the BPROAD trial [ {"type":"clinical-trial","attrs":{"text":"NCT03808311","term_id":"NCT03808311"}} NCT03808311 ], and the IBIS Trial [ {"type":"clinical-trial","attrs":{"text":"NCT03585595","term_id":"NCT03585595"}} NCT03585595 ]) will likely provide definite answers regarding optimum BP treatment goals for patients with diabetes or stroke. However, the currently available evidence supports a more intensive BP treatment target. 14
RISK FACTORS FOR HYPERTENSION
BP levels and hypertension prevalence increase with age in both sexes. 39 Men have higher BP at younger ages than women, but BP increase per decade is higher in women than in men. By the age of 60 years, women have a higher mean BP and hypertension prevalence than men. Race and ethnicity is also a significant risk factor for hypertension. For example, a study that analyzed National Health and Nutrition Examination Survey (NHANES) 2015–2016 data for 4,821 US adults aged ≥20 years, reported a significantly higher age-standardized prevalence of hypertension in non-Hispanic Black individuals (57.3%) than in non-Hispanic White individuals (43.8%) and Hispanic-Americans (44.7%). 40 There is no evidence that racial and ethnic disparities in risk of hypertension are explained by genetic factors. 41 , 42 Sociodemographic, environmental and behavioral factors are therefore likely to be the main contributors to racial and ethnic differences in mean BP and prevalence of hypertension. 42 In addition, several modifiable risk factors, including high sodium intake, low potassium intake, alcohol consumption, obesity, lack of physical activity, and unhealthy diet are associated with increased risk of hypertension
High sodium intake
The PURE study estimated that in 2010, global sodium intake was 3,950 mg per day, which is considerably higher than the recommended amounts of 2,300 mg or less per day in all published guidelines. 44 , 45 In addition, sodium consumption varied considerably by world region from >4,200 mg per day in East Asia, Central Asia, and Eastern Europe to <3,300 mg per day in Latin America and Sub-Saharan Africa. 44
Animal experiments, observational epidemiologic studies, and randomized clinical trials have demonstrated a causal relationship between sodium intake and elevated BP. 46 The majority of the observational studies were cross-sectional and reported a positive, linear, and significant association of either dietary intake or 24-hour urinary excretion of sodium with BP or hypertension. 47 , 48 For example, the Intersalt study investigated the association between 24-hour urinary sodium excretion and BP among 10,074 men and women aged 20–59 from 52 population samples in 32 countries. 47 In within population analyses, a 100 mmol higher individual 24-hour urinary sodium excretion was associated with a 6.0 mmHg higher average systolic BP and a 2.5 mmHg higher average diastolic BP. In across population analyses, a 100 mmol higher median 24-hour sodium excretion was associated with a median 4.5 mmHg higher systolic and 2.3 mmHg higher diastolic BP. 47 When four remote populations with extremely low urinary sodium excretion were excluded from the across population analyses, a 100 mmol increase in median 24-hour sodium excretion was associated with a 2.5 mmHg increase in systolic BP. 47 The Intersalt study showed consistent patterns of regional differences in salt intake and BP levels. A positive and significant association between spot urinary sodium excretion and BP was also observed in the PURE study. 49
In the DASH-Sodium trial, 412 people with an average systolic BP of 120–159 mmHg and diastolic BP of 80–95 mmHg were randomly assigned to high sodium (mean urinary excretion of 142 mmol per day), intermediate sodium (mean urinary excretion of 107 mmol per day) and lower sodium diets (mean urinary excretion of 65 mmol per day) for 30 days in a cross-over design. 50 The results showed that reducing sodium intake from a high to an intermediate level lowered systolic BP by 2.1 mmHg (P<.001) during consumption of a usual American control diet and by 1.3 mm Hg (P = 0.03) during consumption of a Dietary Approaches to Stop Hypertension (DASH) diet. Further reducing sodium intake from an intermediate to a lower level resulted in an additional reduction in systolic BP of 4.6 mmHg during consumption of the control diet (P<0.001) and a 1.7 mmHg reduction during consumption of the DASH diet (P<0.01). 50
Several meta-analyses of clinical trials have shown that sodium reduction significantly lowers BP in hypertensive and normotensive individuals. 51 – 55 An Agency for Healthcare Research and Quality (AHRQ) meta-analysis that included 48 randomized control trials found a significant BP-lowering effect of dietary sodium reduction in adults both with and without hypertension. 55 In this study, a 42 mmol decrease in the weighted mean sodium intake was associated with a 3.23 mmHg (95% CI 2.41–4.06) reduction in systolic BP and a 2.24 mmHg (95% CI 1.61–2.96) reduction in diastolic BP. 55
Despite the strong positive association between dietary sodium intake, BP and risk of hypertension, the associations of sodium intake with risk of CVD, CKD, and mortality are inconsistent. 55 Some studies have found a positive association between dietary sodium intake and these clinical outcomes, whereas others have found inverse, J-shaped or U-shaped associations. 56 – 65 These conflicting findings can likely be partly explained by methodological limitations, such as systematic and random error in sodium measurements, reverse causality, insufficient statistical power, residual confounding, and inadequate follow-up duration. 55 , 64 , 66 In summary, dietary sodium reduction should be recommended to lower population BP levels and risk of hypertension. More research is needed, however, to determine optimal dietary sodium intake for the prevention of CVD, CKD and mortality.
Low potassium intake
Similar to sodium, considerable regional variation exists in 24-hour urinary potassium excretion (the best available measure of potassium intake) with the highest levels in Europe (e.g., Finland 2,995 mg, Netherlands 2,835 mg, Germany 2,825 mg, Belgium 2,618 mg, and Spain 2,629 mg) and South America (e.g., Brazil 2,940 mg, and Colombia 2,803 mg), and the lowest levels in Asia (e.g., China 1,249 mg and Japan 1,792 mg) and Africa (e.g., Kenya 1,306 mg and Zimbabwe 1,466 mg). 67 , 68 Observational epidemiological studies have reported an inverse association of dietary potassium intake with BP levels and hypertension. In the Intersalt study, a 50 mmol per day higher level of urinary potassium excretion was associated with a 3.4 mmHg (95% CI 1.5–5.2) lower level of systolic BP and 1.9 mmHg (95% CI 0.7–3.0) lower level of diastolic BP after adjustment for important confounding factors, including age, sex, BMI, alcohol consumption, and urinary sodium excretion, and correction for regression dilution bias. 69
Randomized clinical trials have shown that potassium supplementation lowers BP in hypertensive and normotensive individuals. 70 – 73 In a meta-analysis of 33 randomized controlled trials with 2,609 participants, potassium supplementation was associated with significant reductions in mean systolic and diastolic BP of 3.11 mmHg (95% CI 1.91–4.31) and 1.97 mmHg (95% CI 0.52–3.42), respectively. 71 In 31 trials with urinary potassium measurements, the median net increase in potassium excretion in the potassium supplementation group versus the control group was 50 mmol per day; in 21 (68%) of these trials, the net difference in potassium excretion was ≥40 mmol per day. 71 The effects of potassium supplementation seemed to be greater in black individuals and in those eating a high sodium diet. An AHRQ meta-analysis that included 18 randomized controlled trials also reported that potassium supplementation was associated with a significant reduction in systolic (6.43 mmHg; 95% CI 1.80–11.1) and diastolic BP (3.50 mmHg; 95% CI 0.89–6.10). 55 Increasing potassium intake, especially from fruits and vegetables, is therefore recommended for the prevention and treatment of hypertension. 14 , 43
Alcohol consumption
Globally, alcohol consumption varies considerably, with the lowest consumption in North African and the Middle East (most countries <1 L per-capita annually), and the highest consumption in Central and Eastern Europe (many countries >12 L per-capita annually). Global annual alcohol consumption increased from 5.9 L per-capita in 1990 to 6.5 L in 2017. 74 Numerous observational epidemiologic studies have reported that high alcohol consumption is a risk factor for elevated BP. 75 , 76 The Atherosclerosis Risk in Communities Study reported a J-shaped association between alcohol consumption and risk of hypertension. 76 Light to moderate alcohol drinkers were at lower risk of incident hypertension than nondrinkers, whereas heavy drinkers were at higher risk than both light to moderate drinkers and nondrinkers. This pattern was observed in all race and gender groups except for black men, for whom risk was similarly elevated in light to moderate and heavy drinkers compared to nondrinkers. 76 In a large prospective cohort study of over 500,000 Chinese adults, however, self-reported usual alcohol intake and genotype-predicted alcohol intake were both positively and linearly associated with BP. Genetic epidemiological data showed that the observed protective effects of moderate alcohol intake against hypertension and CVD were largely non-causal. 77
A meta-analysis of 15 randomized control trials with a total of 2,234 participants reported that a reduction in alcohol intake (median: 76%, range: 16%–100%) was associated with significant reductions in mean systolic BP (3.31 mmHg, 95% CI 2.52–4.10) and diastolic BP (2.04 mmHg, 95% CI 1.49–2.58). 78 A dose-response relationship was observed between mean percentage reduction in alcohol intake and mean reduction in BP. Moreover, the effects of reducing alcohol intake on BP were enhanced in those with higher BP at baseline. A recent meta-analysis of 36 trials with 2,865 participants showed that a reduction in alcohol intake was not associated with a significant reduction in BP in individuals who drank two or fewer drinks per day. However, a 50% reduction in alcohol intake was significantly associated with a 5.50 (95% CI 4.30 to 6.70) mmHg lower systolic BP and a 3.97 (3.25 to 4·70) mmHg lower diastolic BP in participants who drank six or more drinks per day. 79 These findings suggest that reducing alcohol intake should be recommended as an important component of lifestyle modification for the prevention and treatment of hypertension in people who are heavy drinkers.
Lack of physical activity
Global age-adjusted prevalence of insufficient physical activity (less than 150 min of moderate-intensity, or 75 min of vigorous-intensity physical activity per week, or any equivalent combination of the two) was 27.5% with a higher prevalence in women (31.7%) than in men (23.4%). The highest levels were in women in Latin America and the Caribbean (43.7%), south Asia (43.0%), and high-income Western countries (42.3%), whereas the lowest levels were in men from Oceania (12.3%), east and southeast Asia (17.6%), and sub-Saharan Africa (17.9%). Prevalence of insufficient physical activity was much higher in urbanized populations in high-income countries. 80
Epidemiological studies have reported an inverse relationship between physical activity, BP and hypertension. 81 Even modest levels of physical activity (such as walking to work) are associated with a decrease in the risk of incident hypertension. 82 Randomized controlled trials and meta-analyses have demonstrated that physical activity lowers BP in hypertensive and normotensive individuals. 83 – 85 For example, a meta-analysis of 54 randomized controlled trials with 2,419 participants reported that aerobic exercise was associated with a significant reduction in mean systolic BP of 3.84 mmHg (95% CI 2.72–4.97) and diastolic BP of 2.58 mmHg (95% CI 1.81–3.35). 83 Aerobic exercise-induced BP reductions were consistent in hypertensive and normotensive participants and in overweight and normal-weight participants. These findings indicate that physical activity is an effective lifestyle intervention for the prevention and treatment of hypertension.
Overweight and obesity
The prevalence of obesity has increased rapidly worldwide over the past several decades. From 1975 to 2014, global age-standardized prevalence of obesity increased from 3.2% to 10.8% in men, and from 6.4% to 14.9% in women. During the same period, global age-standardized mean body mass index (BMI) increased from 21.7 kg/m 2 to 24.2 kg/m 2 in men and from 22.1 kg/m 2 in 1975 to 24.4 kg/m 2 in women. BMI varied significantly by world region, from 21.4 kg/m 2 in central Africa and south Asia to 29.2 kg/m 2 in the Pacific Islands for men and from 21.8 kg/m 2 in south Asia to 32.2 kg/m 2 in the Pacific Islands for women in 2014. 86
Epidemiological studies have consistently identified a direct relationship between BMI and BP that is continuous and almost linear, with no evidence of a threshold. 87 , 88 The relationship between BP and waist-to-hip ratio or computed tomographic measures of central fat distribution is even stronger than the relationship between BP and BMI. 89 Attributable risk estimates from the Nurses’ Health Study suggest that obesity is responsible for about 40% of hypertension 80 , whereas the Framingham Offspring Study suggested that obesity is responsible for 78% of hypertension in men and 65% of hypertension in women. 90 , 91 In a meta-analysis of 25 randomized controlled trials with 4,874 participants, a net reduction in body weight of 5.1 kg (95% CI 4.25–6.03) owing to calorie restriction, increased physical activity, or both, reduced systolic BP by 4.44 mmHg (95% CI 2.95–5.93) and diastolic BP by 3.57 mmHg (95% CI 2.25–4.88). BP reductions were 1.05 mmHg (95% CI 0.66–1.43) systolic and 0.92 mmHg (95% CI 0.55–1.28) diastolic when expressed per kilogram of weight loss. 92
Unhealthy diet
In addition to sodium and potassium, several macronutrients are associated with BP, including dietary fiber, 93 , 94 protein, 95 , 96 and fat. 97 , 98 The Global Burden of Diseases Nutrition and Chronic Diseases Expert Group examined two different dietary patterns worldwide: one based on relatively high consumption of ten healthy items (fruits, vegetables, beans and legumes, nuts and seeds, whole grains, milk, total polyunsaturated fatty acids, fish, plant omega-3s, and dietary fiber); and another based on relatively low consumption of seven unhealthy items (unprocessed red meats, processed meats, sugar sweetened beverages, saturated fat, trans fat, dietary cholesterol, and sodium). Diets and their trends were very heterogeneous across world regions. For example, both types of dietary patterns improved in high-income countries but worsened in some low-income countries in Africa and Asia. Middle-income countries showed the largest improvement in dietary patterns based on healthy items but the largest deterioration in dietary patterns based on unhealthy items. 99
The efficacy of dietary interventions for blood pressure lowering has been investigated in randomized controlled trials. For example, in the DASH trial, 459 adults with systolic BP <160 mmHg and diastolic BP of 80–95 mm Hg were randomly assigned to 8 weeks of a control diet low in fruits, vegetables, and dairy products with a fat content typical of the average diet in the US, a diet rich in fruits and vegetables or a DASH diet rich in fruit, vegetables and low-fat dairy products with a total fat and saturated fat content lower than the typical US diet. 100 Sodium intake and body weight were maintained at constant levels. Compared to the control diet, the DASH diet significantly reduced systolic and diastolic BP by 5.5 mmHg and 3.0 mmHg (both P<0.001), respectively, whereas the fruit and vegetable diet reduced systolic and diastolic BP by 2.8 mmHg (P<0.001) and 1.1 mm Hg (P=0.07), respectively. In participants with hypertension, the DASH diet reduced systolic and diastolic BP by 11.4 mmHg and 5.5 mmHg compared with the control diet (both P<0.001). The DASH diet was also effective in reducing BP in normotensive participants, but the BP reductions were smaller than those seen in hypertensive participants [(3.5 mmHg (P<0.001) and 2.1 mmHg (P=0.003) compared with the control diet]. 100 These data indicate that the DASH diet provides an effective nutritional approach to prevent and treat hypertension.
Vegetarian and Mediterranean dietary patterns are also associated with BP reduction. 101 , 102 A meta-analysis of 7 randomized controlled trials with a total of 311 participants reported that vegetarian diets (defined as diets that never or rarely included meat) were associated with a mean reduction in systolic BP of 4.8 mmHg (95% CI 3.1–6.6) and diastolic BP of 2.2 mmHg (95% CI 1.1–3.5). 101 Mediterranean diets are characterized by moderate fat intake (primarily from olive oil and nuts), low consumption of red meat, and high consumption of vegetables. 102 A meta-analysis of 6 trials with a total of 2,650 participants reported a modest but significant reduction in systolic BP of 1.7 mmHg (95% CI 0.1–3.4) and diastolic BP of 1.5 mmHg (95% CI 0.8–2.1) in Mediterranean diets compared to low-fat diets. 102
In summary, numerous lifestyle risk factors are associated with BP levels and hypertension. These risk factors vary by world region and this heterogeneity likely underlies some of the disparities in hypertension trends and prevalence worldwide. Lifestyle interventions that target these risk factors could therefore play an important role in reducing global disparities in hypertension. 22 Implementing lifestyle interventions in HICs has contributed to reductions in hypertension prevalence and BP levels. 43 However, there is limited data on implementing lifestyle intervention programs in LMIC communities.
Other potential risk factors
Several other potential risk factors for hypertension have been proposed, including cigarette smoking, air pollution, psychological stress, sleep disorders and noise exposure. 103 – 114 Cigarette smoking has been shown to be associated with an immediate acute increase in BP, mainly through stimulation of the sympathetic nervous system. 103 , 104 , 107 However, its long-term effects on BP and incidence of hypertension are inconclusive. 107 , 108 , 115 Several prospective cohort studies have reported a weak positive association between cigarette smoking and risk of hypertension. For example, in 13,529 men from the Physicians’ Health Study, former and current smoking were significantly associated with an 8% (RR 1.08, 95% CI 1.01 to 1.15) and 15% (RR 1.15, 95% CI 1.03 to 1.27) increase in the risk of incident hypertension, respectively, compared with never smoking over a median follow-up of 14.5 years. 107 In a prospective cohort study of 28,236 women from the Women’s Health Study, multiple-adjusted hazard ratios of developing hypertension among former smokers and current smokers of 1–14 and ≥15 cigarettes per day were 1.03 (95% CI 0.98 to 1.08), 1.02 (95% CI 0.92 to 1.13), and 1.11 (95% CI 1.03 to 1.21), respectively, compared to never smokers. 108 However, direct causality between cigarette smoking and hypertension cannot be established because cessation of chronic smoking does not lower BP. 116
The association between exposure to ambient air pollutants and risk of hypertension has been investigated in epidemiological studies. 109 , 110 A meta-analysis that examined the effects of a 10 μg/m 3 increase in exposure to air pollutants on hypertension, reported that short-term exposure over several days to sulfur dioxide (OR=1.046, 95% CI 1.012–1.081), particulate matter with a diameter of ≤2.5 μm (PM 2.5 ) (OR=1.069, 95% CI 1.003–1.141), and particulate matter with a diameter of ≤10 μm (PM 10 ) (OR=1.024, 95% CI 1.016–1.032) were all significantly associated with hypertension (based on data from 7 studies). 109 Long-term exposure over years to nitrogen oxide (OR=1.034, 95% CI 1.005–1.063) and PM 10 (OR=1.054, 95% CI 1.036–1.072) were also significantly associated with hypertension (based on data from 11 studies). These results suggest that short-term and long-term exposure to air pollutants might increase the risk of hypertension. Future studies are needed to assess the impacts of chronic exposure to air pollution on global disparities of hypertension. 117 , 118
Psychosocial stress and rotating shift work have also been reported to be associated with risk of hypertension. 111 , 112 In a meta-analysis of two observational studies with 622 participants, mental stress was associated with an increased risk of hypertension (OR 2.40, 95% CI 1.65–3.49, p < 0.001). 111 However, a meta-analyses of 15 trials with 902 participants testing the effects of different stress-reduction techniques, such as biofeedback, relaxation or combined interventions, on BP concluded that the benefit of stress reduction on BP remains unproven. 119 The meta-analysis identified methodological shortcomings in these trials and significant heterogeneity of BP changes: from −12 to 10 mmHg for systolic and from −10 to 1 mmHg for diastolic. In addition, a meta-analysis of 9 cohort studies with a total of 172,824 individuals reported a pooled OR of hypertension in shift workers of 1.31 (95% CI, 1.07–1.60). 112 In addition, ambient noise from road, rail, and air traffic has a modest association with hypertension prevalence but not incidence in cohort studies. 113 , 114 An inconsistent relationship between sleep duration and risk of hypertension has also been reported. 105 Obstructive sleep apnea is a very common risk factor for hypertension, and continuous positive airway pressure was shown to reduce systolic BP by 2.46 mm Hg (95% CI 0.62–4.31) and diastolic BP by 1.83 mm Hg (95% CI 0.61–3.05) in a meta-analysis of 16 randomized clinical trials with 818 participants. 106
In summary, observational studies have reported weak or moderate associations between these potential risk factors (i.e., cigarette smoking, air pollution, psychological stress, sleep disorders and noise exposure) and risk of hypertension. However, there is insufficient evidence from randomized clinical trials to support causal relationships between these potential risk factors and risk of hypertension. Overall, the currently available data suggest that these potential risk factors have limited effect on BP in the general population.
HYPERTENSION CONTROL IN THE COMMUNITY
As discussed above, antihypertensive treatment and lifestyle modifications have been shown to lower BP and CVD risk in randomized clinical trials. 30 , 31 , 120 , 121 Despite these effective interventions, hypertension control remains unacceptably low, particularly in LMICs. 3 The most recent global estimates suggest that in 2010, only 45.6% of people with hypertension were aware of their condition, only 36.9% were receiving treatment, and only 13.8% had achieved BP control (defined as systolic BP <140 mmHg and diastolic BP <90 mmHg. 3 Moreover, HICs had approximately twice the proportion of hypertension awareness and treatment and four times the proportion of hypertension control than that of LMICs, in which the proportion of BP control was only 7.7% in 2010. 3 Between 2000 and 2010, HICs experienced substantial improvements in the proportions of hypertension awareness, treatment, and control. In the same period, awareness and treatment increased much more modestly in LMICs and the proportion of patients with hypertension and controlled BP decreased slightly ( Table 3 ). 3 Similar patterns were observed in the baseline data from the PURE study in which hypertension awareness, treatment, and control were lower in communities in low-income countries (LICs) than in those in HICs. 11
Hypertension control in high income and low and middle income countries in 2000 and 2010
| Population | 2000 | 2010 | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Age-standardized proportion of hypertension control in all patients with hypertension (% (95% CI)) | ||||
| Global | 10.0 (4.0–15.9) | 13.4 (6.5–20.2) | 10.9 (7.7–14.2) | 16.8 (13.1–20.5) |
| High-income countries | 15.5 (2.3–28.7) | 20.3 (4.8–35.8) | 24.6 (16.0–33.2) | 32.2 (23.6–40.8) |
| Low and middle-income countries | 6.9 (1.3–12.6) | 9.7 (3.2–16.3) | 5.2 (2.3–8.1) | 10.2 (6.4–14.0) |
| Age-standardized proportion of hypertension control in patients with treated hypertension (% (95% CI)) | ||||
| Global | 34.2 (24.1–44.2) | 33.7 (23.2–44.2) | 35.8 (30.8–40.7) | 38.0 (33.2–42.8) |
| High-income countries | 38.6 (21.1–56.0) | 38.6 (19.8–57.4) | 49.1 (40.7–57.4) | 51.5 (43.1–59.9) |
| Low and middle-income countries | 29.5 (19.9–39.2) | 29.2 (18.9–39.6) | 23.4 (17.9–28.9) | 28.1 (22.6–33.7) |
Large-scale screening programs in communities or health facilities could be an effective way to address the lack of BP awareness in LMICs. For example, May Measurement Month (MMM) 2017 was a BP screening program aimed at raising global awareness of hypertension. 122 The MMM 2017 program screened over 1.2 million individuals in 80 countries who had not had their BP measured in the past year and found that 34.9% had hypertension, 17.3% of those with hypertension were not receiving treatment, and 46.3% of those with hypertension who were receiving treatment did not have controlled BP (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg). This program demonstrated that BP screening in convenience samples can be cost-effective and can identify large numbers of individuals who could benefit from initiation or enhancement of antihypertensive treatment. 122
Barriers to hypertension control exist at multiple levels: patients, healthcare providers, health systems, and communities. 123 – 125 A meta-analysis of randomized controlled trials that assessed the comparative effectiveness of 8 implementation strategies for BP control in patients with hypertension found that multilevel, multicomponent strategies were most effective for systolic BP reduction. 125 These multicomponent strategies included team-based care with medication titration by a nonphysician mean change −7.1 mmHg, 95% CI −8.9 to −5.2 mmHg]), team-based care with medication titration by a physician (−6.2 mmHg 95% CI, −8.1 to −4.2 mmHg), and multilevel strategies without team-based care (−5.0 mmHg, 95% CI, −8.0 to −2.0 mmHg). Patient-level strategies resulted in systolic BP reductions of −3.9 mmHg (95% CI, −5.4 to −2.3 mm Hg) for health coaching and −2.7 mmHg (95% CI, −3.6 to −1.7 mm Hg) for home BP monitoring. Similar trends were reported for diastolic BP reductions. Strategies that targeted provider-level barriers only, including provider training, audit and feedback, and electronic decision support systems, did not result in significant BP reductions.
Most trials of implementation strategies to overcome barriers to BP control have been conducted in HICs. Whether the same strategies will be effective in LMICs is unclear. A cluster-randomized trial published in 2017 showed that a multifaceted intervention, which included a community health worker-led home intervention (health coaching, home BP monitoring, and BP audit and feedback), a physician intervention, and a text-messaging intervention over 18 months, significantly lowered systolic BP by 6.6 mmHg (95% CI, 4.6–8.6; P <0.001) and diastolic BP by 5.4 mmHg (95% CI, 4.0–6.8 mmHg; P <0.001) in low-income patients in Argentina. 126 This trial indicated that a community health worker-led multifaceted intervention was effective for improving patients’ adherence to antihypertensive medication and physicians’ adherence to clinical guidelines resulting in a significant reduction in BP and an increase in hypertension control among low-income patients with hypertension. Widespread scale-up of this proven effective intervention in LMICs should result in a substantial reduction in uncontrolled hypertension and related CVD and premature death.
Increased use of out-of-office BP measurements for confirmation of hypertension diagnosis and titration of antihypertensive medications could also play an important role in improving BP control. A meta-analysis of 11 prospective cohort studies showed that ambulatory BP measurements (ABPM) predicted long-term CVD outcomes independently of office BP (hazard ratio range, 1.28 to 1.40). 127 An international pooling project with 11,135 participants reported that higher 24-hour and night-time BP measurements using ABPM were significantly associated with greater risks of death and CVD outcomes after adjustment for office BP. 128 However, the incremental improvement in predictive values of clinical outcomes with these measurements compared with office BP was small. Adding 24-hour or nighttime systolic BP to base models that included other BP measures resulted in incremental improvements in the area under the curve (AUC) of 0.0013 to 0.0027 for mortality and 0.0031 to 0.0075 for CVD. 128 However, base models that included single systolic BP measure already had an AUC of 0.83 for mortality and 0.84 for CVD. Home BP measurements (HBPM) are a more economical option than ABPM and are a useful strategy to overcome patient-level barriers to BP control by empowering patients. 129 Use of self HBPM resulted in a significant reduction in systolic and diastolic BP among patients with hypertension in randomized clinical trials. 125 , 130 Out-of-office BP measurements are recommended for diagnostic confirmation to avoid white coat hypertension and masked hypertension and for optimal titration of medications to overcome therapeutic inertia. 130
The prevention and control of hypertension in communities requires a life-course approach. 131 Epidemiological studies have shown that elevated BP in childhood promotes subclinical organ damage (i.e. early vascular ageing) and leads to increased risk of hypertension, CVD, and CKD later in life. 132 – 134 To reduce BP-related risks of CVD, CKD, and premature death over the lifespan, preventive efforts should start in early childhood. Innovative strategies for early detection and optimal treatment of hypertension, such as a multifaceted intervention approach, should be implemented in communities to reduce the burden of this condition.
THE FINANCIAL BURDEN OF HYPERTENSION
Hypertension is associated with a substantial financial burden. Costs include direct healthcare expenditures associated with BP management, such as medications, laboratory tests, and clinic visits, as well as costs associated with hospitalizations for BP-related complications and indirect costs associated with lost productivity resulting from premature mortality and disability owing to hypertension-related cardiovascular and kidney diseases. 135 The global financial burden of high BP in 2001 was estimated to be around 370 billion US dollars or about 10% of the world’s overall healthcare expenditure. 135 However, large regional variations in healthcare costs were observed. For example, high BP accounted for 22.6% of all healthcare expenditures in Eastern Europe and Central Asia but only 7.2% in East Asia and Pacific. 135 To the best of our knowledge, no more recent estimates of the global financial burden of hypertension are available.
More recent national and regional estimates of the financial burden of hypertension are available for some HICs, although they are not always consistent owing to methodologic differences. 136 – 139 A study that used a nationally representative database, the Medical Expenditure Panel Survey, estimated that the average annual adjusted incremental expenditure for patients with hypertension was $1,920 higher compared to individuals without hypertension in the US between 2003 and 2014. Based on an average annual number of individuals with hypertension in the US (n= 68,420,747) from NHANES during this period, the estimated adjusted annual incremental cost is $131 billion higher for the hypertensive adult population compared with the non-hypertensive population.. 136 Using the same database, another study analyzed changes in medical expenditures associated with hypertension between 2000 and 2013. Estimated annual medical expenditures per person associated with hypertension in 2000–2001 ($1,399) were not significantly different from those in 2012–2013 ($1,494), but annual national spending for patients with hypertension increased significantly from US$58.7 billion in 2000–2001 to US$109.1 billion in 2012–2013, mainly owing to an increase in the number of treated patients. 137 In addition to lower estimates of medical costs per patient, the underestimated prevalence of hypertension in the Medical Expenditure Panel Survey which used self-reported history of hypertension to define hypertension might also contribute to the conservative estimates of medical expenditure for hypertension in this study. 121
Payments for prescription medications account for a large proportion of the medical expenditures associated with hypertension. The cost of hypertension medication expenditures in the US in 2007 was estimated to be $68 billion based on an analysis of data for 21,782 adults (aged ≥18 years) who participated in the Medical Expenditure Panel Survey. 139 A study that used data from the National Hospital Discharge Survey reported that annual costs for hypertension-related hospitalizations in the US increased from $40 billion (5.1% of total hospital costs) during 1979–1982 to $113 billion (15.1% of total hospital costs) during 2003–2006. 138 The American Heart Association projected that by 2030, the direct costs of hypertension in the US population would increase to $200 billion and the indirect costs to $40 billion. 140
In a study of 314, 622 beneficiaries of the medical insurance system in Japan, inpatient medical expenditure attributable to hypertension represented 7.2% and 6.9% of the total medical expenditure for men and 2.8% and 3.8% of the total medical expenditure for women aged 40–54 years and 55–69 years, respectively. 141 Estimates from Mexico suggest that combined direct and indirect costs attributable to hypertension amounted to nearly $2.5 billion in 2007, which was between 6% and 8% of the healthcare budget. 122 The economic burden of hypertension in Mexico grew by 24% between 2010 and 2012, and the total direct and indirect costs of hypertension in 2011 were estimated to exceed $5.7 billion. 123
The financial implications of hypertension in LICs are of particular concern because of the rapid increase in the prevalence and absolute burden of hypertension and the low current and projected healthcare spending in these regions. 4 , 124 In 2016, only 0.4% of global health spending was in LICs even though these countries comprise 10% of the global population. 124 Projections suggest that by 2050, only 0.6% of health spending will occur in countries that are currently classed as LICs although these countries will comprise 15.7% of the global population. 124 Many LICs currently rely on development assistance for health spending from international agencies and private philanthropy, which is primarily focused on infectious diseases and could be less sustainable for chronic disease control. 124 Large-scale studies using standardized methods are needed to improve understanding of the economic costs of hypertension, particularly in LMICs.
In general, interventions are considered to be reasonably cost-effective if they cost less than US$50,000–75,000 per quality-adjusted life year (QALY); however, payers routinely cover treatments that cost >$100,000 per QALY. 145 Several analyses have concluded that all antihypertensive medications are cost effective compared with no treatment (placebo). 146 In a meta-analysis of 14 studies, the incremental cost-effectiveness ratio (ICER) of all antihypertensive medications adjusted to 2015 US dollars was $19,945 per QALY gained. 146 The SPRINT trial reported an ICER of $28,000 per QALY gained for the intensive BP intervention compared to the standard intervention if the treatment effects persisted for the remaining lifetime of the patient. 147 Antihypertensive treatment might be even more cost effective in LMICs than in HICs. For example, treating all adults with hypertension and prior CVD for secondary prevention was projected to be cost saving in China with an ICER in 2015 of Int$9,000 per QALY gained in those with BP ≥160/100 mmHg and Int$13,000 per QALY gained in those with BP ≥140/90 mm Hg. 148 Moreover, patient-centered values, such as health-related quality of life, productivity, severity of disease, and equity, are important to factor into analyses when determining the financial burden associated with hypertension and weighing the costs of prevention and treatment versus the costs associated with BP-related CVD. 149 , 150
FUTURE PERSPECTIVES
Without intervention, the prevalence and absolute burden of hypertension is expected to continue to increase, particularly in LMICs. 3 , 4 , 12 , 13 Consequentially, BP-related cardiovascular and kidney disease will become even greater health and financial burdens worldwide. 2 Economic development and urbanization have resulted in rapid epidemiological and nutrition transitions, i.e., decreasing infant mortality and infectious diseases and increasing life expectancy and chronic diseases, in many LMICs, including China and India. 151 – 153 The prevalence of hypertension is high and increasing in these countries, whereas the rates of awareness, treatment and control are low. 3 , 20 Lifestyle risk factors for hypertension, such as obesity, high dietary sodium intake, low dietary potassium intake, alcohol consumption, lack of physical activity and unhealthy diet have reached epidemic proportions in LMICs. 154 , 155 National programs for the prevention, detection, and treatment of hypertension in LMICs are needed to slow and reverse the current trends in the hypertension epidemic. In addition, community-based intervention programs are needed to reduce health disparities and improve hypertension prevention and control in low-income and ethnic minority populations in HICs. 42 To accurately estimate the global burden of hypertension, worldwide prevalence surveys using standardized methodology for sampling of participants, BP measurement, and data quality control and analysis are needed, especially in under-represented regions. 3 Research to develop and test the effective, equitable, and sustainable interventions for implementing evidence-based clinical guidelines and public health policies worldwide are critically important for reducing the global burden of hypertension. 3
CONCLUSIONS
Although the estimated global mean BP appears to be fairly stable, the prevalence and absolute burden of hypertension is increasing globally, especially in LMICs. In 2010, an estimated 1.39 billion adults worldwide had hypertension of whom 1.04 billion were in LMICs and 349 million in HICs. 3 Elevated BP is strongly, independently, and linearly associated with the risk of CVD, CKD, and all-cause mortality. Although effective lifestyle modifications and pharmaceutical treatments are available, proportion of hypertension awareness, treatment, and control are low. Hypertension, therefore, remains a global public health challenge. Few comprehensive assessments of the economic impact of hypertension in global populations exist. It was estimated that the global financial burden of hypertension was about 10% of the world’s overall healthcare expenditures, varying from about 22.6% of all healthcare expenditures in Eastern Europe and Central Asia to 7.2% in East Asia and Pacific. Future studies are warranted to develop multifaceted implementation strategies for hypertension prevention and control, especially in LICs and low-income populations, and to accurately assess the financial burden of hypertension worldwide.
- Hypertension is the leading modifiable risk factor for cardiovascular disease and premature death worldwide.
- The prevalence and absolute burden of hypertension is rising globally, especially in low and middle-income countries (LMICs).
- Awareness, treatment, and control of hypertension are unacceptably low worldwide, particularly in LMICs.
- Reductions in risk factors, including high sodium intake, low potassium intake, obesity, alcohol consumption, physical inactivity and unhealthy diet, are recommended for the prevention and control of hypertension.
- Multifaceted implementation strategies for hypertension prevention and control are needed to address barriers at the patient, provider, system, and community levels.
- Comprehensive assessments are needed to evaluate the economic impact of hypertension worldwide.
Acknowledgements
The authors’ work is supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award Number P20GM109036 and by the National Heart, Lung, and Blood Institute of NIH under Award Number R01HL133790. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Glossary of Terms
| Pooling analysis | A type of meta-analysis in which investigators have access to and analyze the original individual level data from participating study |
| Regression dilution (bias) | In an epidemiological study the regression slope between a response and predictor variable is underestimated when the predictor variable is measured imprecisely |
| Network meta-analysis | A type of meta-analysis in which multiple treatments are being compared using both direct comparisons of interventions within randomized controlled trials and indirect comparisons across trials based on a common comparator |
| Cross-over design | A type of clinical trial in which each participant is randomized to a sequence of two or more treatments; therefore, the participant is used as his or her own control |
| Dietary Approaches to Stop Hypertension (DASH) diet | A diet emphasizing fruits, vegetables, and low-fat dairy foods; including whole grains, poultry, fish, and nuts; and limiting red meat, sweets, and sugar-containing beverages |
| Weighted mean | A type of average that instead of each of the data points contributing equally to the final average, some data points contribute more than others |
| Dose-response relationship | A relationship in which a change in amount, intensity, or duration of exposure is associated with either increasing or decreasing risk of the outcome |
| Convenience samples | A type of non-probability sample in which the study participants are taken from a group of people easy to contact or to reach |
| Quality-adjusted life year (QALY) | A measure of the burden of disease on a defined population which equals the sum of years of life lost (YLLs) and years lived with disability (YLDs). One DALY equals one lost year of healthy life |
| Incremental cost-effectiveness ratio (ICER) | A measure of the cost-effectiveness of new health care interventions defined as the ratio of the difference in cost between two possible interventions divided by the difference in their effect |
Competing interest statement
The authors declare no competing interests.
- Open access
- Published: 07 August 2021
Prevalence of hypertension in Ghanaian society: a systematic review, meta-analysis, and GRADE assessment
- Fidelis Atibila 1 ,
- Gill ten Hoor 2 ,
- Emmanuel Timmy Donkoh 3 ,
- Abdul Iddrisu Wahab 4 &
- Gerjo Kok 5
Systematic Reviews volume 10 , Article number: 220 ( 2021 ) Cite this article
15k Accesses
18 Citations
29 Altmetric
Metrics details
Hypertension has become an important public health concern in the developing world owing to rising prevalence and its adverse impact on ailing health systems. Despite being a modifiable risk factor for cardiovascular disease, hypertension has not received the needed attention in Ghana as a result of various competing interests for scarce health resources. This systematic review and meta-analysis provides a comprehensive and updated summary of the literature on the prevalence of hypertension in Ghana.
Major databases such as MEDLINE, EMBASE, and Google Scholar and local thesis repositories were accessed to identify population-based studies on hypertension among Ghanaians. Data extracted from retrieved reports were screened independently by two reviewers. The quality of eligible studies was evaluated and reported. A reliable pooled estimate of hypertension prevalence was calculated utilizing a random-effects model and reported according to the GRADE framework. Additionally, a meta-regression analysis was performed to analyze the contribution of study-level variables to variance in hypertension prevalence.
In general, a total of 45,470 subjects ( n = 22,866 males and 22,604 females) were enrolled from urban ( n = 12), rural ( n = 8), and mixed populations ( n = 7). Blood pressure (BP) was measured across studies according to a validated and clinically approved protocol by trained field workers or healthcare workers including nurses and physicians. A combined total of 30,033 participants across twenty studies reporting on the population prevalence of hypertension were pooled with 10,625 (35.4%) identified to satisfy study criteria for elevated BP. The pooled prevalence across 24 studies was 30.3% (95% CI 26.1–34.8%) after fitting a random effects model. Prevalence of hypertension was 30.1% (95% CI 25.6–36.0%) among females and 34.0% (95% CI 28.5–40.0%) among males. Significant differences in pooled estimates across regions emerged from subgroup comparisons of regional estimates with an increasing trend in the north-to-south direction and with increasing age. Compared to rural settings, the burden of hypertension in urban populations was significantly higher. Age structure and population type accounted for 65.0% of the observed heterogeneity in hypertension estimates.
Conclusions
The prevalence of hypertension in Ghana is still high. The gap in hypertension prevalence between rural and urban populations is closing especially in elderly populations. These findings must claim the attention of public health authorities in Ghana to explore opportunities to reduce rural hypertension.
Systematic review registration
The protocol for this review has been published previously with PROSPERO ( CRD42020215829 ).
Peer Review reports
Introduction
Hypertension or elevated blood pressure (BP) represents a significant cause of avoidable cardiovascular debility and early death in less-developed countries with inadequately resourced healthcare systems [ 1 , 2 ]. Suboptimal control of BP in the growing hypertensive population is a major contributory factor to the rising burden of non-communicable diseases (NCDs) in low- and middle-income countries [ 3 , 4 ]. Whereas hypertension is a well-known cause of cardiovascular disease and related deaths in the advanced nations, the importance of hypertension in low-resource health settings is less emphasized but believed to be on the ascendancy [ 1 , 5 , 6 , 7 ]. In developing nations, healthcare resources are stretched by a double burden of communicable diseases such as malaria, HIV AIDS, and tuberculosis and non-communicable diseases such as hypertension and diabetes [ 8 ]. In spite of this double burden of diseases, a disproportionate fraction of health resources is allotted to combat and prevent infectious diseases, leaving little to invest in interventions to prevent non-communicable diseases [ 9 ].
In Ghana, available records indicate that hypertension prevalence has been rising with the spate of rural–urban migration and associated changes to lifestyle and dietary choices [ 10 , 11 ]. A number of factors such as positive perception of obesity, more sedentary lifestyles, excessive consumption of high-calorie diets, genetic predisposition, high intake of salt, and increasing life-expectancy have been cited for this disturbing trend [ 12 , 13 ]. Without urgent attention, the current epidemic of hypertension in the country is expected to worsen [ 14 ].
From an adult hypertension prevalence of less than 5% a generation ago, currently, approximately 50% of all adults have hypertension [ 15 ]. The incidence of outpatient hypertension in health facilities increased 11-fold from an estimated 60,000 reported cases in 1990 to approximately 700,000 reported cases in 2010 [ 15 ]. Prevalence estimates of hypertension based on population studies range between 19 and 48% depending on the study protocol and diagnostic criteria used to detect hypertension [ 16 , 17 , 18 , 19 ]. Furthermore, close to half of diagnosed hypertension manifest clinical signs of organ damage, as a consequence of late presentation/detection by the existing health system and suboptimal BP control [ 15 , 20 ].
Several attempts have been made in the past decade to better understand the burden of hypertension in Ghana [ 4 , 19 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ]. These studies extended the scope of preliminary work undertaken in the previous decade and began to dispel popular myths and misconceptions still held from the earliest studies [ 30 , 31 , 32 ]. As a result of their nature, observational studies have several inherent flaws that limit their impact. Systematic reviews have become one way to circumvent these limitations and provide concrete epidemiological data [ 33 ]. The last systematic review focussing exclusively on the prevalence of hypertension in Ghanaians dates back to 2012 [ 34 ]. Since then, a few large continental studies have reported aggregated evidence on a handful of datasets from Ghana [ 8 , 35 , 36 , 37 , 38 ]. Otherwise, population data on hypertension prevalence is sparse. High-quality nationally representative, population-based data on hypertension in the country are needed to monitor trends in disease epidemiology across socioeconomic strata, population demographics, time, and space [ 13 ]. The aim of this review was to identify new observational studies reporting on the prevalence of elevated BP in Ghanaian populations and to consolidate their findings with previous studies to generate high-impact evidence to inform health planning in this setting.
This review was guided by internationally Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for undertaking systematic reviews and the Meta-analyses of Observational Studies in Epidemiology (MOOSE) approach [ 39 , 40 ]. In addition, the protocol for this review has been published previously with PROSPERO (CRD42020215829).
Literature search strategy and terms
The major electronic archives for published research, Medline/PubMed, Web of Science, Embase (via Ovid), CINAHL, and African journals online (AJOL) were queried for publications reporting population estimates of hypertension in Ghana. For grey literature, the authors perused the first 200 results in an advanced Google Scholar search as well as local thesis repositories. In addition, a snowballing technique of scrutinizing bibliographies of all eligible studies was employed to identify additional potential reports. A modified approach with search terms specifying and targeting the defined population, intervention (defined appropriately for non-experimental scenarios), defined outcome of interest, and required setting for studies was used to screen all study titles and abstracts [ 39 ]. Terms specifying the intervention concept were “prevalence”, “proportion”, “survey”, “descriptive”, “cross-sectional”, “cohort”, “longitudinal”, “attributable fraction”, and “incidence”. Outcome of interest was coded in the search strategy as “hypertension”, “blood pressure”, “cardiovascular”, and “cardiometabolic”. Those for the settings were “Ghana” and “Ghanaians”. Search terms were framed with the “OR” and “AND” operators as previously described [ 35 ].
Exclusion and inclusion criteria
The main outcome was the prevalence of elevated BP in the general and various strata of the population. Published articles on studies and follow-up studies published before October 2019 with hypertension, risk factors, management practices, and control in the Ghanaian population as an outcome were eligible for inclusion in this review. An additional search for the period spanning October 2019 to November 2020 yielded an additional 2 studies. Conference abstracts reporting adequately detailed information regarding the population, definition of hypertension, sample size, blood pressure data collection, and a point estimate of hypertension were also included for screening. Population-based studies involving individuals living in Ghana or with well-defined data analysis of Ghanaian cohorts were considered for inclusion. Multicenter studies that include Ghanaian participants were included if there was adequate statistical detail on data pertaining to Ghana. Reports fulfilling inclusion criteria were eligible for the initial screening (Fig. 1 ). Studies that failed to satisfy conditions for inclusion were excluded in this review to prevent impact of related confounding variables. These included case reports, reviews, expert commentaries, and data on Ghanaians living in foreign territories. Although reviews were not included in this work, a manual search of references found in review articles was performed. Additionally, the findings of this work were compared to previous work and in order to build upon existing knowledge. Clinical studies reporting on organ-specific elevated blood pressure such as pulmonary hypertension and studies that were based on hospitalized patients, pregnant women, or utilized hospital-based sampling protocols were excluded on grounds that they do not adequately represent the national population. Studies that did not report absolute population or sub-population estimates on hypertension were further excluded from the quantitative synthesis or meta-analysis.
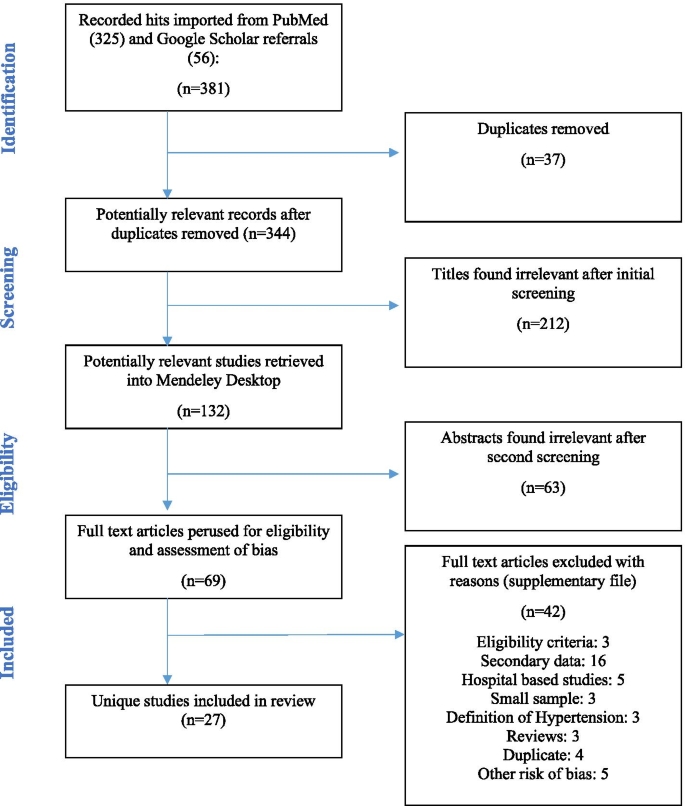
Flow diagram illustrating sequence of important actions
Selection of studies
Citations returned in the database query were imported to Mendeley Desktop 1.19.4, and duplicate reports from multiple sources were identified and removed. The titles and abstracts of articles returned in database queries using the above search strategy were accessed and pre-screened by two co-authors who worked independently to flag non-suitable or extraneous reports for exclusion. Subsequently, full-text reports of the pre-screened and promising studies were accessed and perused by two co-authors who worked independently to ascertain conformity to stated inclusion criteria. A checklist was used for this purpose. Only articles published in the English language were considered. Detected discrepancies in the outcome of independent screening was resolved by consensus of reviewers. Relevant information from selected studies was extracted into a standardized Microsoft Excel template. In accordance with the PRISMA guidelines, the basis for excluding any article during the extensive screening stage was documented and reported (see Additional file 1 ) [ 40 ].
Data extraction
Relevant data was retrieved from selected full-text manuscripts using a standardized extraction form [ 35 ]. Details of publication such as manuscript title, author names, date of publication, digital object identifiers (DOIs), and other vital variables were extracted. In addition, informative variables such as study size, target population, time period of data collection, geographical location, study objective, eligible participants, and other elements of design were captured into format. Furthermore, explanatory and outcome variables, details of data analysis, and the major findings were retrieved as well. Additional data on participant sociodemographic characteristics, anthropometrics, and blood pressure measurement protocols were also extracted. The overall prevalence as well as age-specific prevalence based on the classification of hypertension were of interest. In instances of replicate data reporting affecting the same study population and setting, relevant information was retrieved from the most informative manuscript(s). All such reports were regarded as one unique study report. Also, in such instances, the earliest year of publication was reported.
Definition of hypertension and classification
Studies were scrutinized for and organized according to compliance to the JNC VII criteria which defines elevated BP or hypertension as having either a systolic BP greater or equal to 140 mmHg or concurrent with a diastolic BP greater or equal to 90 mmHg or prescription use of antihypertensive medication [ 41 ]. The three grades of hypertension were all reported as one category: hypertension.
Appraisal of selected studies for risk
All studies were appraised for potential risk of bias according to a suitably validated tool [ 42 ]. The tool concerns itself with assessments of both the internal and external validity of reports from cross-sectional studies. Two independent assessors conducted this appraisal and scored the manuscripts as high, low, or very low for bias based on the presence or absence of each construct being assessed. Specific issues probed included whether participants were adequately representative of the defined population, randomly sampled, and were drawn from an adequate sampling frame with low non-response bias (external validity). Internal validity was assessed from impressions about instrument reliability and validity, uniform administration of instrument, definition of hypertension, primary data collection, and exposure bias. All disagreements were resolved by discussion and regular consultations with more experienced team members. Reports with scanty detail were classified as “limited,” and the primary investigators were contacted for particular information.
Meta-analysis procedures: data analysis
Meta-analysis was conducted in the R computing software using the “meta” package [ 43 , 44 ] (Additional file 2 ). A random intercept logistic regression model, which is a form of generalized linear mixed model (GLMM), was utilized to estimate pooled estimates of hypertension prevalence in Ghana based on available studies [ 45 ]. The proportion of hypertensive individuals in each study was transformed using the logit transformation in order to stabilize the variances of the prevalence estimates. The choice of the logit transformation was made after a thorough review and consideration of the merits and demerits of the various transformation methods of proportions, including the arcsine and Freeman-Tukey double arcsine transformations. Schwarzer et al. [ 46 ] recommended “the use of inverse variance method with the arcsine or logit transformations for the meta-analysis of single proportions that require individual study weights, after reporting seriously misleading results in a meta-analysis with very different sample sizes due to problems with the back-transformation of the Freeman-Tukey transformation.” Gender-, age-, and region-specific estimates were presented.
The I 2 statistic is a measure of variability in pooled estimate attributed to heterogeneity of studies while the Q statistic is a check for homogeneity in effect estimates. Higgins and Thompson’s I 2 statistic [ 47 ] and Cochran’s Q statistic [ 48 ] were used to assess heterogeneity between the included studies. Subgroup analysis was performed by BP measurement device, year of publication, nature of study population, region, and geographical location of study site in order to determine whether prevalence of hypertension was modified by subgroup membership.
Meta-regression was performed in R to determine the extent to which study characteristics could explain the heterogeneity among prevalence estimates of hypertension between studies. Codes for the procedure are shown in Additional file 3 . Whereas σ 2 is typically used to represent within study variance, τ 2 is used to represent the between study variance, also called study heterogeneity [ 49 ]. The estimate of τ 2 in meta-regression analysis with covariate in comparison to τ 2 when the covariate is omitted allows for the calculation of the proportion of study heterogeneity explained by the covariate [ 50 ]. Attention was also given to the R 2 value which indicates the percentage of the variance in the dependent variable that the independent variables explain collectively.
Description of selected studies
The database queries returned 381 hits which were screened down to 344 studies after exclusion of duplicates ( n = 37). An initial screening of titles for relevance narrowed this number down to 132 reports. A further 105 were removed following abstract ( n = 63) and full manuscript review ( n = 42) for a number of reasons stated in Fig. 1 . These reasons include false hits, failure to meet eligibility criteria, reporting on secondary data, hospital-based studies involving convenient samples, small sample size, unexpected definition of hypertension or self-reports, systematic reviews or letters to the editor, duplicate analyses on previous samples, and other risks of bias. The remaining 27 eligible reports retained for analysis represent unique population-based studies on Ghanaian subjects conducted from 1977 to 2020.
At the time of compiling this report, new administrative regions of the country were created out of existing ones. To facilitate comprehension among international audiences and avoid confusion in future, the administrative map used here has been given in Fig. 2 . Most of these studies ( n = 15) were conducted in the Greater Accra ( n = 8 studies) and the Greater Kumasi ( n = 8 studies) areas. There were 3 studies from the Upper East, 2 nationwide reports and one international study. In addition, the Bono, Bono East, Eastern, and Volta regions were represented by a study each. Details of the specific locations and identities of these studies are given in Fig. 2 and Additional file 4 . Data collection ranged from 2 to 36 months. The earliest sampling was conducted in 1972 by Pobee et al. [ 30 ], and the latest was from November 2017 by Acheampong et al. [ 51 ]. There were more published studies available in the past decade (2010–2020) than the two decades (1990–2010) prior. A total of sixteen (16) studies were published after year 2010, and eleven (11) studies were published before this year. The year with the most included studies was 2017 with 5 studies.
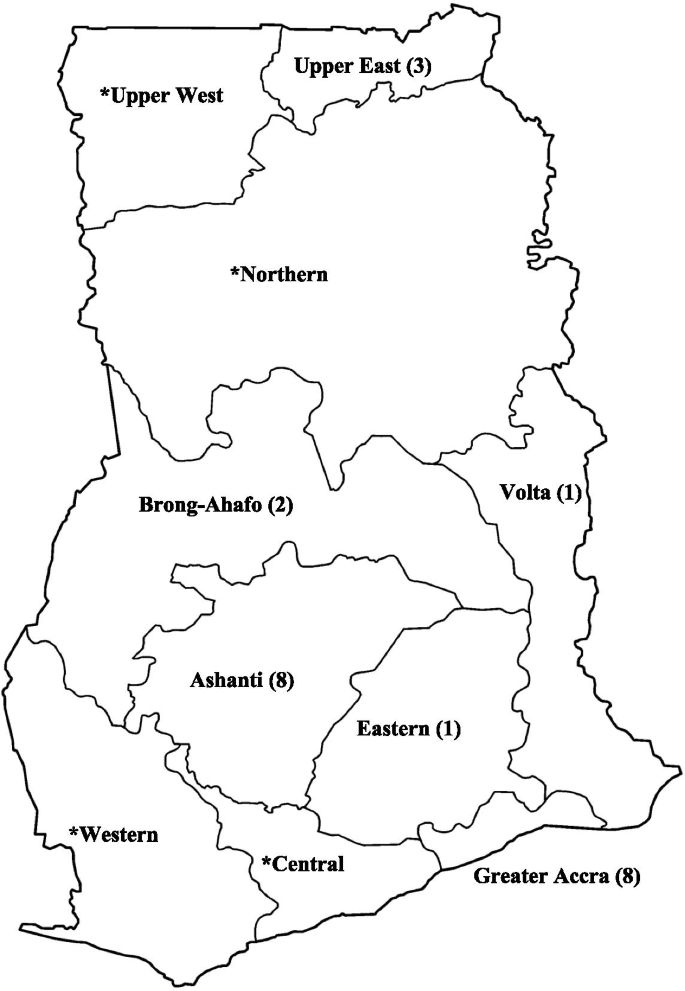
Regional map of Ghana showing distribution of included studies. *Included in one of two (2) nationwide surveys, one (1) multi-city study, and one (1) international study
All studies included in the review were original articles capturing cross-sectional studies and baseline surveys of prospective studies [ 52 , 53 ]. Hypertension prevalence was reported as a primary outcome in all 24 studies included in the meta-analysis. A number of studies also reported on risk factors ( n = 15), awareness ( n = 9), management ( n = 9), and control ( n = 9).
Although a number of reports published on the same dataset were identified, all selected studies reported on unique datasets. These clusters of publications emerged from datasets generated by large-scale multinational population-based cardiovascular risk studies such as the modeling epidemiologic transition study (METS) [ 27 , 54 ] and the WHO SAGE project [ 22 , 23 , 55 ]. One study also [ 21 ] reported results from the University of Witwatersrand’s INDEPTH collaboration with the US National Institutes for Health (NIH) [ 56 ] and H3Africa Consortium [ 57 ]. Six study sites in four sub-Saharan African countries, namely, South Africa, Kenya, Ghana, and Burkina Faso, are involved in the African genomics partnership (AWI-Gen) [ 21 ].
Other smaller, localized datasets informing selected studies included the Accra urban poverty project (UPHS) which examined associations between health and developmental indices from urban poor populations in the capital of Ghana [ 53 , 58 ] and the second phase of the Women’s Health Survey (WHSA-2) conducted in Accra [ 52 , 59 , 60 ]. The WHSA was designed to measure the burden of tropical diseases among adult women. Notably, the Time Use and Health Study of Accra (TUHS) [ 61 ] was not selected on account of using 87% of data reported in the WHSA [ 52 ].
In general, a total of 45,470 subjects ( n = 22,866 males and 22,604 females) enrolled from urban ( n = 12) and rural populations ( n = 8), as well as mixed populations ( n = 7), were captured in this review. Studies from populations designated as mixed covered both urban and rural populations. The definition of “urban” and “rural” given by the original authors were applied. In cases where this was not stated, the entry category used for the location in question in the Ghana Demographic Heath Survey 2010 by the Ghana Statistical Service was used to classify the study. Two studies sampled female subjects exclusively [ 51 , 52 ], and one study reported on males exclusively [ 62 ].
Measurement of blood pressure
In general, BP was measured across studies according to a validated and clinically approved protocol by trained field workers or healthcare workers including nurses and physicians (Additional file 5 ). Mostly, all subjects had their BP readings taken by protocols that were standardized across study sites. Only a handful of studies reported partial fractions of study subjects evaluated for blood pressure. Some studies, per the definition of hypertension given, admitted patients who were on medication into this category [ 63 , 64 ]. Most studies reportedly used the JNC VII criteria which defines elevated BP or hypertension as having either a systolic BP greater or equal to 140 mmHg or concurrent with a diastolic BP greater or equal to 90 mmHg or prescription use of antihypertensives [ 41 ], irrespective of BP [ 28 ]. However, a few studies conducted before this definition was published used the old WHO definition (BP greater or equal 160/95) [ 30 , 65 ]. One study refrained from explicitly providing a prevalence of hypertension but reported the mean systolic and diastolic BP values [ 66 ].
In most studies ( n = 21), participants were seated during the BP measurement, usually in a quiet place. Two (2) reports had patients in the supine position [ 52 , 67 ], and five (5) did not report on posture. In general, study subjects were required to take at least 5 min rest prior to BP measurement, and in one instance, investigators insisted on avoidance of smoking [ 68 ]. One study reported an initial resting period of 3–5 min [ 21 ].
Half of the selected studies ( n = 13, 52%), usually the more recent, reportedly used electronic BP monitors. Commonly, models of the Omron digital brand were employed. In addition, the Boso Medistar Wrist BP Monitor Model S and the semi-automated Microlife Watch BP home were also used. Two studies did not specify the BP measurement apparatus used, and up to 10 (40%) included studies also used manual sphygmomanometers. In such cases, the systolic and diastolic BPs were noted to coincide with the first and the fourth or fifth [ 30 , 68 ] Korotkoff phase sounds heard during auscultation, respectively.
The preferred anatomical site for BP measurement was the upper arm. A dozen studies ( n = 12) indicated using either large or small cuffs depending on which was appropriate for the subject. The Danfa study used a 14-cm large cuff size for all subjects [ 30 ] while the SAGE WAVE 1 used a wrist BP monitor [ 23 ]. BP measurements were performed by teams of trained study staff with varying degrees of experience. Teams were variously constituted with clinical and community health nurses, general field staff/research assistants, allied health workers, and students. Teams were usually supervised by medical officers or research officers. In line with national and international guidelines, most studies measured participant’s blood pressure from the arm, mostly the right upper arm. Minicuci et al. used a device that had to be worn around the wrist to measure the BP. In most cases, participants were seated in a quiet area. Koopman et al. reported blood pressure readings from supine individuals, while Hill et al. had some participants seated and some supine [ 52 ].
Except for four studies [ 20 , 65 , 69 , 70 ], all studies performed one-time BP readings. Studies using BP protocols based on multiple visits performed secondary readings within 24 h [ 65 ], 3 weeks [ 20 ], or 1 month [ 60 ] to confirm an initial BP reading exceeding 140/90 mmHg [ 20 , 60 ]. In Kunutsor et al., BP readings were replicated after 2 weeks for participants ( n = 89 (16%)) in order to adjust for the phenomenon of regression dilution in BP studies where baseline/initial BP measurements are noted to underestimate the actual BP leading to underestimation of cardiovascular risk [ 69 ]. A regression dilution ratio may be calculated from repeat readings for the purpose of correcting for this error and to estimate the actual BP [ 71 ].
On sampling days, with the exception of one study in which BP was measured a minimum of three times [ 19 ], most studies conducted BP readings up to three times to allow confirmation of elevated BP readings [ 65 , 69 , 72 ]. In general, the intervening time between repeat BP measurements was not less than 1 min but less than 1 h in most studies and up to a full day in one study [ 72 ]. Majority of studies ( n = 23) reported the nature of BP statistic used in classifying participants. Most studies computed an average BP from two readings [ 20 , 21 , 23 , 26 , 27 , 28 , 51 , 66 , 68 , 69 , 72 , 73 , 74 , 75 ] or three [ 19 , 30 , 53 , 70 , 76 ]. Frequently, studies would discard the initial reading and rely on the mean of the latter readings [ 11 , 20 , 21 , 23 , 73 ] or the fifth and sixth [ 27 ]. One study, Pobee et al. [ 65 ], used only one BP reading with confirmation for high-for-age readings.
Prevalence of hypertension
Reported prevalence estimates on hypertension ranged from a low of 4.5% in a rural population from the Ashanti Region [ 27 ] to a high of 54.3% in adults above 65 years in the same Region [ 70 ]. A combined total of 30,033 participants across twenty studies reporting on the population prevalence of hypertension were pooled with 10,625 (35.4%) identified to satisfy study criteria for elevated BP. A few studies reporting only mean systolic and diastolic BP were excluded from pooled analysis. After fitting a random effects model to 24 representative studies, the composite prevalence of elevated BP in the general population of Ghanaians stood at 30.3% (95% CI 26.1–34.8%) (Fig. 3 ). Two nationwide studies (9195 participants) in adults above 50 years gave rise to a higher pooled estimate of hypertension of 49.5% (46.2–52.7%) [ 23 , 25 ]. The pooled estimate did not change significantly between studies using manual [32.2% (95% CI 26.1–38.9%)] and electronic measuring devices [29.5% (24.5–35.1%)] ( p = 0.527).

Pooled prevalence of hypertension in Ghana
After fitting a random effects model to 17 exclusive studies with sex-stratified rates, the prevalence of elevated BP was 30.6% (95% CI 25.6–36.0%) among females ( n = 15 data points) and 34.0% (95% CI 28.5–40.0%) among males ( n = 16 data points) (Fig. 4 ). In general, hypertension prevalence was higher in males (see Additional file 6 ) [ 19 , 20 , 23 , 26 , 53 , 67 , 70 , 72 , 74 , 76 , 77 , 78 ]. This trend was reversed in two studies which reported narrowly higher hypertension prevalence in females [ 21 , 68 ].
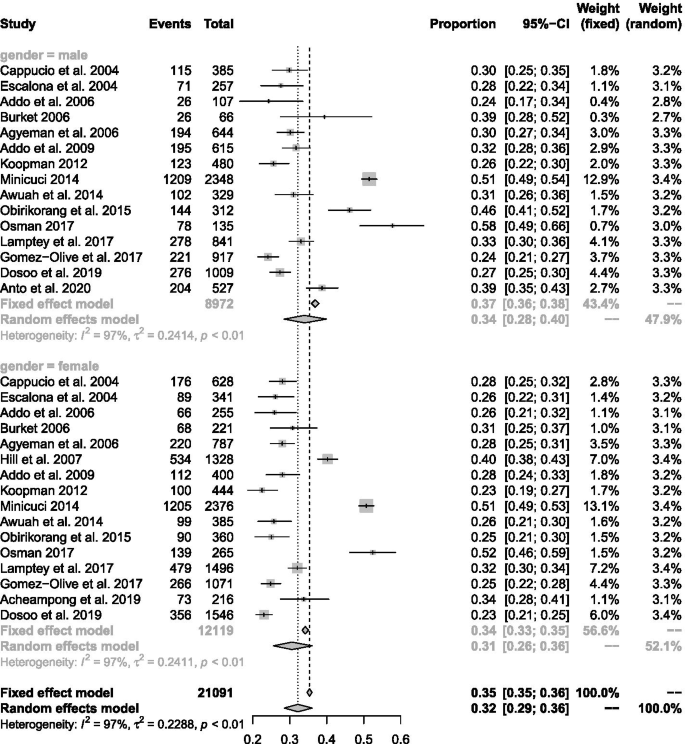
Prevalence of hypertension in males and females
Regionally, hypertension prevalence from studies in the Greater Accra region (30.7%; 95% CI 24.7–37.5%) did not vary significantly from the estimate from the Ashanti Region (29.2%; 95% CI 21.6–37.5%). However, significant differences in pooled estimates across regions emerged from subgroup comparisons of regional estimates in the general population ( p < 0.001).
Temporal variations in the prevalence estimates were not statistically significant ( p = 0.624). Two identical studies were published in 2000–2005 with a combined estimate of 28.0% (95% CI 25.9–30.3%) [ 74 , 78 ]. A further six studies were included from the next 5-year period (2006–2010), giving a pooled estimate of 29.3% (95% CI 23.9–35.3%). In the next 5-year window (2011–2015), another 6 published studies gave a pooled estimate of 35.5% (95% CI 24.5–46.1%). Similarly, there was no significant time-trend when the earliest year of sampling was considered instead of the publication year in the random effects model ( p = 0.829). The pooled estimate for eleven studies conducted in the past decade (2010–2019) was 30.4% (95% CI 24.4–37.2%). For comparison, another eleven studies were conducted before the 2010 population and housing census giving a pooled prevalence of 31.5% (95% CI 25.0–38.9%).
In terms of the developmental indices of populations studied, the highest pooled prevalence of hypertension was seen in studies comprised of a mix of urban and rural dwellers (38.7%; 95% CI 31.4–46.7%). The prevalence of elevated BP in urban populations (31.7%; 95% CI 28.1–35.5%) was significantly higher than in rural populations (23.4%; 95% 18.6–28.9%) (Fig. 5 ). Most of the studies in rural areas were among the general population: just one study in eight recruited participants who are above 50 years [ 67 ] with the potential of skewing the result.
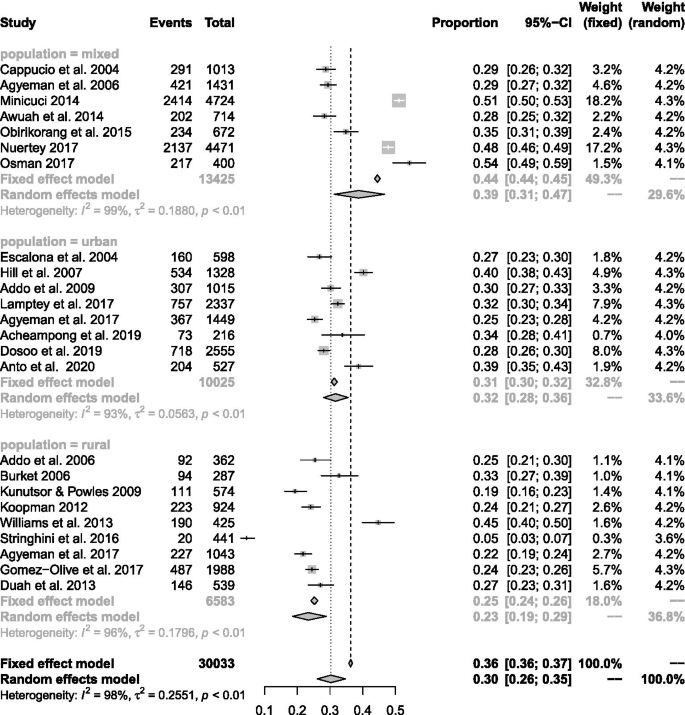
Prevalence of hypertension in rural and urban areas
Prevalence of hypertension increased in the north-to-south direction. The highest prevalence of hypertension was (30.7%; 95% CI 25.8–36.2%) observed for southern Ghana. This was significantly higher than that in the other northern belt 22.9% (95% CI 20.3–25.9%) of Ghana and only marginally higher than that for the middle-belt 30.1% (95% CI 25.4–35.4%).
There was a distinguishable age pattern of hypertension in which prevalence of elevated bp increased with age, peaking in middle-aged individuals [ 51 , 53 , 76 ]. Statistics on the age distribution of participants were extracted to classify studies based on the following criteria: (1) retirees where selection criteria specifying advanced age > 50 or > 65 was used, and (2) senior citizens where the mean ages range from 67.2 to 74.4 years, and (3) studies in the general population with mean ages ranging from 31 to 54.7 years. The pooled prevalence of hypertension from studies focussing on adults was 43.9% (95% CI 36.2–51.9%) while general population studies that recruited younger participants on average gave an estimated prevalence of 27.4% (95% CI 24.5–30.6%). In addition to overall prevalence estimates, some studies also provided age-specific prevalence rates for hypertension. In general, the prevalence of hypertension was always lower in the youngest age group than in the oldest age group [ 51 , 53 , 76 ].
A weighted regression technique was performed to understand systematic differences among studies that potentially explained the heterogeneity between study results (see Additional file 7 ). In univariate analysis, type of study population (rural vs. urban) ( R 2 = 31.4%, p < 0.05) and age structure of the population ( R 2 = 47.2%, p < 0.05) accounted for significant variability in the estimated prevalence of hypertension. All other study characteristics were not responsible for significant variation in hypertension prevalence. A multivariate model based on significant determinants of hypertension in preliminary analysis across studies accounted for 65% of the variability in hypertension prevalence across studies ( p < 0.05).
Risk appraisal of selected studies
A total of 69 full-text articles were perused for eligibility and assessment of bias according to a suitably validated tool [ 42 ]. Available articles were rated as having “low,” “moderate,” “high,” and “very high” risk of bias by consensus of two investigators. The results of this appraisal are presented in supplementary file (Additional file 1 ). Forty-two (42) full-text articles were adjudged to have a very high risk of bias and were discarded, leaving 27 for the meta-analysis. Out of this number, 7 studies [ 23 , 51 , 69 , 70 , 73 , 74 , 76 ] were rated low risk of bias, 18 studies [ 19 , 20 , 21 , 25 , 26 , 27 , 28 , 30 , 52 , 53 , 65 , 67 , 72 , 75 , 77 , 78 , 79 ] were rated moderate risk, and two studies [ 62 , 68 ] were rated high risk of bias.
GRADE assessment of quality of pooled estimate
In terms of quality, the pooled estimate presented here can be adjudged to have a bias rating of 3 + on a scale ranging from 1 + representing an estimate with a low quality and 4 + representing the highest quality. This rating reflects that all the included studies were observational studies. Although some level of bias may arise from the lack of blinding, concealment, and randomization to treatment, selected studies presented adequate sample sizes and simple/systematic random sampling to mitigate bias to moderate levels. In addition, most of the samples were sufficiently representative of the study populations from which they were obtained. In terms of consistency, we assign a low rating based on the elevated I 2 statistic. An elevated I 2 statistic and low Q statistic were indicative of a high proportion of variability in the pooled estimate attributable to heterogeneity of reported estimates. A relatively small confidence interval indicated a moderate level of precision in the effect estimate. In terms of the risk of the influence of publication bias from included studies, after examination of funnel plots and based on the results of Egger’s tests, a moderate level of confidence may be exercised when interpreting the pooled prevalence. The authors have provided subgroup analyses to supplement the interpretation of the pooled prevalence by circumventing some degree of heterogeneity in the composite value.
There is a surge in non-communicable diseases in the developing world, driven by a high prevalence of cardiometabolic risk factors such as hypertension. We have systematically reviewed the available literature on population-based studies in order to present an accurate and updated synthesis on the state of the epidemic in Ghana. The inclusion of a meta-analysis unifies the available literature on hypertension over the longest time span: it includes studies from the 1970s to date conducted in rural and urban settings in both youthful and advanced age groups. A key finding is the high prevalence of hypertension in vulnerable groups such as rural women, urban poor folk, and aged individuals with limited access to healthcare services which has persisted over the last decade in contrast to most regions of the world [ 80 ].
The pooled prevalence of hypertension of 30.3% (95% CI 26.1–34.8%) established in this study coincides with the findings of most studies conducted in the general population of Ghanaians in the past decade [ 51 , 72 , 81 ]. This estimate is higher than estimates reported on the general population of Ghanaians at the beginning of the last decade [ 32 ] and may indicate the worsening in the epidemic of hypertension first recognized and reported by Pobee et al. [ 65 ] and several others much later [ 32 ]. However, it coincides with prevalence of hypertension established for younger adults in Africa or sub-Saharan Africa [ 82 ]. This may be explained by the age dynamics of studies included in the meta-analysis and may reflect general population studies in the region [ 82 ]. An analysis of secondary data from the Ghana demographic and health survey gave the prevalence of hypertension as 13.0% (12.1% for males and 13.4% for females) [ 4 ]. In addition, there was a 22% prevalence of high-risk pre-hypertensives [ 4 ]. The GDHS examined the prevalence of hypertension among Ghanaian’s aged 15–49 years with approximately 90% of participants between 15 and 44 years and mostly women (71%). Exclusion of aged individuals may account for the much lower prevalence.
There have been a few systematic reviews of prevalence studies on hypertension in Ghana [ 32 , 34 , 83 ] and a few nationally representative surveys [ 10 , 23 , 25 ]. According to Bosu, after reviewing 15 unique population-based reports and two academic dissertations, the prevalence estimates ranged from 19 to 48% between studies [ 32 ]. Addo et al. later published a similar review of 11 population-based surveys with an estimated hypertension prevalence of 19.3% in rural areas and 54.6% in urban areas [ 34 ]. Neither of these studies provided a pooled estimate of hypertension making the present study the first to report a pooled prevalence of hypertension in Ghana.
According to study area, the prevalence of hypertension in urban versus rural areas follows the familiar pattern of higher rates of blood pressure and hypertension in urban areas [ 77 ]. However, up to a quarter of rural Ghanaians were estimated to have high blood pressure. There is evidence that the problem of hypertension in rural settings deserves as much attention as in urban settings [ 32 , 77 ]. In a few decades, hypertension has transitioned from being almost unheard of among the rural poor into a genuine public health concern. The principal factors driving this trend need to be investigated. Systemic disparities in healthcare between rural and urban areas are typical in Ghana and need to be addressed. Factors such as shortage of qualified health workforce, low access to care, insurance coverage, and inconsistent supply of medication have been identified [ 84 , 85 ].
An ambiguity in the gender variation in the distribution of blood pressure and prevalence of hypertension has been recognized in most studies in the country and in the sub-region [ 38 ]. This review confirms that in the general population, the prevalence of hypertension may be higher for males compared to females. However, a reversal of this order can be seen in older populations, where females show higher blood pressure on the average [ 35 ]. These variations may reflect differences in obesity between older males and females [ 17 , 24 , 25 ] and may result in a reversal of the trend seen for even younger populations with a high prevalence of female obesity [ 86 ].
During this review, Bosu et al. published a meta-analysis of hypertension in older adults in Africa. The high prevalence of hypertension reported for older adults was confirmed by this study. Furthermore, the detection of higher blood pressure with age is consistent with previous reports [ 4 , 35 , 38 ]. This trend is evident in both the urban and rural settings [ 77 ] and has been attributed to changes in renal sodium metabolism and the renin-aldosterone pathway, oxidative stress resulting in microvascular injury and chronic inflammation, loss of arterial and arteriolar elasticity, suppressed baroreceptor sensitivity, and increased sensitivity to sympathetic nervous system stimuli [ 87 , 88 , 89 ]. Acheampong et al. found a significant age-related trend among a small sample of women in the capital, suggesting that elderly women are not spared. The accumulated data on age-related hypertension supports the assertion that as the life expectancy in Ghana rises, there is the need for practical and effective hypertension management strategies that target the aging population [ 51 ]. However, the large fraction of younger individuals exhibiting high blood pressure warrants equal attention since they are likely to live with the condition long enough to develop complications unchecked.
The spatiotemporal aspects of disease epidemiology have become a topic of interest in recent discourse. We compared studies conducted in the past decade to previous work in an attempt to appreciate trends across time. In consonance with most studies across Africa, the epidemic of hypertension does not seem to have eased up over the past decade [ 80 ]. This has implications for the healthcare system and calls for more innovative and impact-driven public health strategies to curb the trend [ 90 ]. Home-grown strategies that have shown promising results will need to be identified and aggressively scaled up in the general population [ 8 , 14 , 19 , 24 ]. Commonly held myths about the distribution of hypertension in the population need to be dispelled [ 32 ]. The socioeconomic patterning and regional disparities in access to healthcare services will need to be addressed as well.
Strengths and limitations
We have successfully provided a comprehensive update on the prevalence of hypertension from the best available studies providing estimates of hypertension in the general population. This review featured a large number of studies from the population with a good data distribution across all 10 regions of the country, across the longest time span, and across both young and elderly age groups as shown in Fig. 2 . The consolidation of studies with large sample sizes improved the generalizability of findings by making the sample representative of the larger population. In addition, all included studies were assessed for risk of bias. Most studies adopted measures for ensuring the quality of blood pressure measurements such as training of field staff prior to deployment and the use of unified protocols. Adherence to the MOOSE validates the methodology of the meta-analysis against international benchmarks, and the use of the PRISMA approach for reporting standardizes the review process. Furthermore, we have included a GRADE assessment of the quality of evidence provided by the meta-analysis (see Additional file 8 ) [ 91 ].
In spite of these, there are a number of important caveats to bear in mind when interpreting findings enumerated here. By design, the review attracted a number of observational studies reporting on hypertension prevalence in the general population with a few instances of confirmatory screening: the classification of subjects based on a single visit falls short of the recommended protocol for diagnosing hypertension [ 41 ]. It is therefore likely to overestimate the true prevalence of hypertension in the general population by an inclusion of false-positive diagnoses. As has been previously indicated in similar pooled studies [ 12 , 32 , 34 ], there was also evidence of heterogeneity in the pooled estimate, possibly as a result of systematic variations in populations and study protocols [ 82 , 92 ]. This point has been conceded in rating the quality of evidence presented here. Additionally, unlike prospective studies, the absence of participant follow-up reduces the strength of the review for monitoring time-related trends and other changes over time.
Conclusion and recommendations
The results presented in this systematic review indicate that hypertension is an important problem in the country, requiring urgent public health attention. The pooled prevalence of hypertension among the Ghanaian populace was 30.3% or approximately one in every three individuals. Most authors of articles perused were supportive of this conclusion and recommended large-scale intervention to curtail the rising trend of hypertension especially in rural populations. The national health insurance scheme may be relevant in this regard to deal with socioeconomic disparities and providing affordable access to healthcare providers. Also, since hypertension is a known risk factor for cardiovascular complications, measures taken to reduce the level of hypertension will improve quality-of-life for affected individuals and reduce the burden on an already constrained healthcare system. This is especially significant for populations where higher than average prevalence of hypertension was observed such as elderly/senior citizens and urban centers. There was also indication of rising rates in rural areas previously considered to be hypertension safe havens. In general, our findings are corroborated by other reviews and large-scale studies from sub-Saharan Africa.
Availability of data and materials
The data that support the findings of this study are shown in the manuscript or attached as supplementary material. In addition, all data has been lodged with the institutional ethical review board and is fully accessible to the public on reasonable request without the permission of the authors.
Abbreviations
Acquired immune deficiency syndrome
African Journals Online
- Blood pressure
Committee on Human Research, Publication and Ethics, Kwame Nkrumah University of Science and Technology, School of Medical Sciences
Cumulative Index to Nursing & Allied Health
Diastolic blood pressure
Digital object identifier
Generalized linear mixed model
Grading of Recommendations Assessment, Development, and Evaluations
Human Heredity and Health
Human immunodeficiency virus
International Network for the Demographic Evaluation of Populations and Their Health
Joint National Committee
Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure
Millennium Development Goals
Modelling the Epidemiological Transition Study
Ministry of Health
Meta-analyses Of Observational Studies in Epidemiology
Non-communicable diseases
Participants, interventions, comparators, and outcomes
Preferred Reporting Items for Systematic review and Meta-Analysis Protocols
International prospective register of systematic reviews
Study on Global Ageing and Adult Health
Systolic blood pressure
Time Use and Health Study
Urban Poverty and Health Study
World Health Organization
Women’s Health Study of Accra
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23.
Article PubMed Google Scholar
Mazloumi E, Poorolajal J, Sarrafzadegan N, Roohafza HR, Faradmal J, Karami M, et al. Avoidable burden of cardiovascular diseases in the eastern Mediterranean region: contribution of selected risk factors for cardiovascular-related deaths. High Blood Press Cardiovasc Prev. 2019;26(3):227–37.
Beaney T, Schutte AE, Tomaszewski M, Ariti C, Burrell LM, Castillo RR, et al. May measurement month 2017: an analysis of blood pressure screening results worldwide. Lancet Glob Health. 2018;6(7):e736–43.
Sanuade OA, Boatemaa S, Kushitor MK. Hypertension prevalence, awareness, treatment and control in Ghanaian population: evidence from the Ghana demographic and health survey. PLoS One. 2018;13(11):e0205985.
Article PubMed PubMed Central CAS Google Scholar
Appiah LT, Sarfo FS, Agyemang C, Tweneboah HO, Appiah N, Bedu-Addo G, et al. Current trends in admissions and outcomes of cardiac diseases in Ghana. Clin Cardiol. 2017;40(10):783–8.
Article PubMed PubMed Central Google Scholar
Fuentes R, Ilmaniemi N, Laurikainen E, Tuomilehto J, Nissinen A. Hypertension in developing economies: a review of population-based studies carried out from 1980 to 1998. J Hypertens. 2000;18(5):521–9.
Article CAS PubMed Google Scholar
Mensah GA. Descriptive epidemiology of cardiovascular risk factors and diabetes in sub-Saharan Africa. Prog Cardiovasc Dis. 2013;56(3):240–50.
Nyirenda MJ. Non-communicable diseases in sub-Saharan Africa: understanding the drivers of the epidemic to inform intervention strategies. Int Health. 2016;8(3):157–8.
Kushitor MK, Boatemaa S. The double burden of disease and the challenge of health access: evidence from access, bottlenecks, cost and equity facility survey in Ghana. PLoS One. 2018;13(3):e0194677.
GSS. Ghana demographic health survey 2014. Rockville: Ghana Statistical Service, Ghana Health Service, ICF International; 2015.
Google Scholar
Cappuccio FP, Kerry SM, Micah FB, Plange-Rhule J, Eastwood JB. A community programme to reduce salt intake and blood pressure in Ghana [ISRCTN88789643]. BMC Public Health. 2006;6:13.
Bosu WK, Aheto JMK, Zucchelli E, Reilly ST. Determinants of systemic hypertension in older adults in Africa: a systematic review. BMC Cardiovasc Disord. 2019;19(1):173.
Ibrahim MM. Hypertension in developing countries: a major challenge for the future. Curr Hypertens Rep. 2018;20(5):38.
Article Google Scholar
Laar AK, Adler AJ, Kotoh AM, Legido-Quigley H, Lange IL, Perel P, et al. Health system challenges to hypertension and related non-communicable diseases prevention and treatment: perspectives from Ghanaian stakeholders. BMC Health Serv Res. 2019;19(1):693.
MOH. Strategy for the management, prevention and control of chronic non-communicable diseases in Ghana 2012–2016. In: Health Mo, editor. Accra: Ministry of Health; 2012.
Acheampong K, Nyamari JM, Ganu D, Appiah S, Pan X, Kaminga A, et al. Predictors of hypertension among adult female population in Kpone-Katamanso District, Ghana. 2019.
Book Google Scholar
Commodore-Mensah Y, Samuel LJ, Dennison-Himmelfarb CR, Agyemang C. Hypertension and overweight/obesity in Ghanaians and Nigerians living in West Africa and industrialized countries: a systematic review. J Hypertens. 2014;32:464–72.
Article CAS PubMed PubMed Central Google Scholar
Gebreselassie KZ, Padyab M. Epidemiology of hypertension stages in two countries in Sub-Sahara Africa: factors associated with hypertension stages. Int J Hypertens. 2015;2015:959256.
Lamptey P, Laar A, Adler AJ, Dirks R, Caldwell A, Prieto-Merino D, et al. Evaluation of a community-based hypertension improvement program (ComHIP) in Ghana: data from a baseline survey. BMC Public Health. 2017;17:368.
Addo J, Smeeth L, Leon DA. Socioeconomic position and hypertension: a study of urban civil servants in Ghana. J Epidemiol Community Health. 2009;63(8):646–50.
Gomez-Olive FX, Ali SA, Made F, Kyobutungi C, Nonterah E, Micklesfield L, et al. Regional and sex differences in the prevalence and awareness of hypertension: an H3Africa AWI-Gen study across 6 sites in Sub-Saharan Africa. Glob Heart. 2017;12(2):81–90.
Lloyd-Sherlock P, Beard J, Minicuci N, Ebrahim S, Chatterji S. Hypertension among older adults in low- and middle-income countries: prevalence, awareness and control. Int J Epidemiol. 2014;43(1):116–28.
Minicuci N, Biritwum RB, Mensah G, Yawson AE, Naidoo N, Chatterji S, et al. Sociodemographic and socioeconomic patterns of chronic non-communicable disease among the older adult population in Ghana. Glob Health Action. 2014;7:21292.
Mohammed H, Ghosh S, Vuvor F, Mensah-Armah S, Steiner-Asiedu M. Dietary intake and the dynamics of stress, hypertension and obesity in a peri-urban community in Accra. Ghana Med J. 2016;50(1):16–21.
Nuertey BD, Alhassan AI, Nuertey AD, Mensah IA, Adongo V, Kabutey C, et al. Prevalence of obesity and overweight and its associated factors among registered pensioners in Ghana; a cross sectional studies. BMC Obes. 2017;4(1):26.
Obirikorang C, Osakunor DN, Anto EO, Amponsah SO, Adarkwa OK. Obesity and cardio-metabolic risk factors in an urban and rural population in the Ashanti Region-Ghana: a comparative cross-sectional study. PLoS One. 2015;10(6):e0129494.
Stringhini S, Forrester TE, Plange-Rhule J, Lambert EV, Viswanathan B, Riesen W, et al. The social patterning of risk factors for noncommunicable diseases in five countries: evidence from the modeling the epidemiologic transition study (METS). BMC Public Health. 2016;16:956.
Williams EA, Keenan KE, Ansong D, Simpson LM, Boakye I, Boaheng JM, et al. The burden and correlates of hypertension in rural Ghana: a cross-sectional study. Diabetes Metab Syndr. 2013;7(3):123–8.
Duah AF, Werts N, Hutton-Rogers L, Amankwa D, Otupiri EJSO. Prevalence and risk factors for hypertension in Adansi South, Ghana: a case for health promotion. SAGE Open. 2013;3(4):2158244013515689.
Pobee JO, Larbi EB, Belcher DW, Wurapa FK, Dodu SR. Blood pressure distribution in a rural Ghanaian population. Trans R Soc Trop Med Hyg. 1977;71(1):66–72.
Ikeme AC, Pole DJ, Pobee JO, Larbi E, Blankson J, Williams H. Cardiovascular status and blood pressure in a population sample in Ghana–the Mamprobi survey. Trop Geogr Med. 1978;30(3):313–29.
CAS PubMed Google Scholar
Bosu WK. Epidemic of hypertension in Ghana: a systematic review. BMC Public Health. 2010;10(1):418.
Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16(2):e1002742.
Addo J, Agyemang C, Smeeth L, de-Graft Aikins A, Edusei AK, Ogedegbe O. A review of population-based studies on hypertension in Ghana. Ghana Med J. 2012;46(2):4–11.
CAS PubMed PubMed Central Google Scholar
Bosu WK, Reilly ST, Aheto JMK, Zucchelli E. Hypertension in older adults in Africa: a systematic review and meta-analysis. PLoS One. 2019;14(4):e0214934.
Twagirumukiza M, De Bacquer D, Kips JG, de Backer G, Stichele RV, Van Bortel LM. Current and projected prevalence of arterial hypertension in sub-Saharan Africa by sex, age and habitat: an estimate from population studies. J Hypertens. 2011;29(7):1243–52.
Nkyi CA. Review of Hypertension in sub-Saharan Africa. Afri J Cur Med Res. 2017;1(1). Available from: https://myjournal.afrijcmr.org/index.php/ajcmr/article/view/8 . [cited 2020 Jan. 31]
Addo J, Smeeth L, Leon DA. Hypertension in sub-Saharan Africa: a systematic review. Hypertension. 2007;50(6):1012–8.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72.
Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–9.
R Core Team. R: a language and environment for statistical computing computer program, version 3.5.0. Vienna: R Core Team; 2019.
Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–5.
Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29(29):3046–67.
Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10(3):476–83.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Cochran WG. Problems arising in the analysis of a series of similar experiments. Suppl J R Stat Soc. 1937;4(1):102–18.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708.
Ellis SE, Speroff T, Dittus RS, Brown A, Pichert JW, Elasy TA. Diabetes patient education: a meta-analysis and meta-regression. Patient Educ Couns. 2004;52(1):97–105.
Acheampong K, Nyamari JM, Ganu D, Appiah S, Pan X, Kaminga A, et al. Predictors of hypertension among adult female population in Kpone-Katamanso District, Ghana. Int J Hypertens. 2019;2019:1876060.
Hill AG, Darko R, Seffah J, Adanu RM, Anarfi JK, Duda RB. Health of urban Ghanaian women as identified by the Women’s Health Study of Accra. Int J Gynecol Obstet. 2007;99(2):150–6.
Article CAS Google Scholar
Awuah RB, Anarfi JK, Agyemang C, Ogedegbe G, Aikins A. Prevalence, awareness, treatment and control of hypertension in urban poor communities in Accra, Ghana. J Hypertens. 2014;32(6):1203–10.
Luke A, Bovet P, Forrester TE, Lambert EV, Plange-Rhule J, Schoeller DA, et al. Protocol for the modeling the epidemiologic transition study: a longitudinal observational study of energy balance and change in body weight, diabetes and cardiovascular disease risk. BMC Public Health. 2011;11(1):927.
Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R, et al. Data resource profile: the World Health Organization study on global AGEing and adult health (SAGE). Int J Epidemiol. 2012;41(6):1639–49.
Sankoh O, Byass P. The INDEPTH Network: filling vital gaps in global epidemiology. Oxford University Press; 2012.
Rotimi C, Abayomi A, Abimiku A, Adabayeri VM, Adebamowo C, Adebiyi E, et al. Research capacity. Enabling the genomic revolution in Africa. Science. 2014;344(6190):1346–8.
Afrifa-Anane E, Agyemang C, Codjoe SN, Ogedegbe G, de-Graft Aikins A. The association of physical activity, body mass index and the blood pressure levels among urban poor youth in Accra, Ghana. BMC Public Health. 2015;15(1):269.
Duda RB, Anarfi JK, Adanu RM, Seffah J, Darko R, Hill AG. The health of the “older women” in Accra, Ghana: results of the Women’s Health Study of Accra. J Cross Cult Gerontol. 2011;26(3):299–314.
Duda RB, Kim MP, Darko R, Adanu RM, Seffah J, Anarfi JK, et al. Results of the Women’s Health Study of Accra: assessment of blood pressure in urban women. Int J Cardiol. 2007;117(1):115–22.
Fink G, Weeks JR, Hill AG. Income and health in Accra, Ghana: results from a time use and health study. Am J Trop Med Hyg. 2012;87(4):608–15.
Anto EO, Owiredu W, Adua E, Obirikorang C, Fondjo LA, Annani-Akollor ME, et al. Prevalence and lifestyle-related risk factors of obesity and unrecognized hypertension among bus drivers in Ghana. Heliyon. 2020;6(1):e03147.
Mancia G, Fagard R, Narkiewicz K, Redán J, Zanchetti A, Böhm M, et al. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31(10):1925–38.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Pobee JO, Larbi EB, Dodu SR, Pisa Z, Strasser T. Is systemic hypertension a problem in Ghana? Trop Doct. 1979;9(2):89–92.
Kodaman N, Aldrich MC, Sobota R, Asselbergs FW, Poku KA, Brown NJ, et al. Cardiovascular disease risk factors in Ghana during the rural-to-urban transition: a cross-sectional study. PLoS One. 2016;11(10):e0162753.
Koopman JJ, van Bodegom D, Jukema JW, Westendorp RG. Risk of cardiovascular disease in a traditional African population with a high infectious load: a population-based study. PLoS One. 2012;7(10):e46855.
Addo J, Amoah AG, Koram KA. The changing patterns of hypertension in Ghana: a study of four rural communities in the Ga District. Ethn Dis. 2006;16(4):894–9.
PubMed Google Scholar
Kunutsor S, Powles J. Descriptive epidemiology of blood pressure in a rural adult population in Northern Ghana. Rural Remote Health. 2009;9(2):1095.
Osman A. Nutrition and health status, quality of life, and associated factors among non-institutionalized older ghanaians. Kumasi: Kwame Nkrumah University of Science and Technology; 2017.
Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150(4):341–53.
Burket BA. Blood pressure survey in two communities in the Volta region, Ghana, West Africa. Ethn Dis. 2006;16(1):292–4.
Agyemang C, Nyaaba G, Beune E, Meeks K, Owusu-Dabo E, Addo J, et al. Variations in hypertension awareness, treatment, and control among Ghanaian migrants living in Amsterdam, Berlin, London, and nonmigrant Ghanaians living in rural and urban Ghana–the RODAM study. J Hypertens. 2017;36(1):169–77.
Cappuccio FP, Micah FB, Emmett L, Kerry SM, Antwi S, Martin-Peprah R, et al. Prevalence, detection, management, and control of hypertension in Ashanti, West Africa. Hypertension. 2004;43(5):1017–22.
Owiredu W, Adamu M, Amidu N, Woode E, Bam V, Plange-Rhule J, et al. Obesity and cardiovascular risk factors in a Pentecostal population in Kumasi-Ghana. J Med Sci. 2008;8(8):682–90.
Dosoo DK, Nyame S, Enuameh Y, Ayetey H, Danwonno H, Twumasi M, et al. Prevalence of hypertension in the middle belt of Ghana: a community-based screening study. Int J Hypertens. 2019;2019:1089578.
Agyemang C. Rural and urban differences in blood pressure and hypertension in Ghana, West Africa. Public Health. 2006;120(6):525–33.
Escalona AL, Sarfo M, Kudua L. Obesity and systemic hypertension in Accra communities. 2004.
Duah AF, Werts N, Hutton-Rogers L, Amankwa D, Otupiri E. Prevalence and risk factors for hypertension in Adansi South, Ghana. SAGE Open. 2013;3:215824401351568.
Zhou B, Bentham J, Di Cesare M, Bixby H, Danaei G, Cowan MJ, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19· 1 million participants. Lancet. 2017;389(10064):37–55.
Addo J, Smeeth L, Leon DA. Prevalence, detection, management, and control of hypertension in Ghanaian civil servants. Ethn Dis. 2008;18(4):505–11.
Sarki AM, Nduka CU, Stranges S, Kandala NB, Uthman OA. Prevalence of hypertension in low- and middle-income countries: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(50):e1959.
Awuah RB, Afrifa-Anane E, Agyemang CH. Cardiovascular diseases and established risk factors in low- and middle-income countries. 2016. p. 1–13.
Aikins AG, Kushitor M, Koram K, Gyamfi S, Ogedegbe G. Chronic non-communicable diseases and the challenge of universal health coverage: insights from community-based cardiovascular disease research in urban poor communities in Accra, Ghana. BMC Public Health. 2014;14(S2):S3.
Ogedegbe G, Plange-Rhule J, Gyamfi J, Chaplin W, Ntim M, Apusiga K, et al. A cluster-randomized trial of task shifting and blood pressure control in Ghana: study protocol. Implement Sci. 2014;9(1):73.
Oti S, van de Vijver S, Kyobutungi C, Gomez G, Agyemang C, Van Charante EM, Brewster LM, Hendriks ME, Schultsz C, Ettarh R. A community-based intervention for primary prevention of cardiovascular diseases in the slums of Nairobi: the SCALE UP study protocol for a prospective quasi-experimental community-based trial. Trials. 2013;14:409.
Kapoor P, Kapoor A. Hypertension in the elderly: a reappraisal. Clin Queries Nephrol. 2013;2(2):71–7.
Pinto E. Blood pressure and ageing. Postgrad Med J. 2007;83(976):109–14.
Buford TW. Hypertension and aging. Ageing Res Rev. 2016;26:96–111.
Gad M, Lord J, Chalkidou K, Asare B, Lutterodt MG, Ruiz F. Supporting the development of evidence-informed policy options: an economic evaluation of hypertension management in Ghana. Value Health. 2020;23(2):171–9.
Piggott T, Morgan RL, Cuello-Garcia CA, Santesso N, Mustafa RA, Meerpohl JJ, et al. Grading of Recommendations Assessment, Development, and Evaluations (GRADE) notes: extremely serious, GRADE’s terminology for rating down by three levels. J Clin Epidemiol. 2020;120:116–20.
Ezejimofor M, Uthman O, Chen YF, Ezejimofor B, Ezeabasili A, Stranges S, et al. Magnitude and pattern of hypertension in the Niger Delta: a systematic review and meta-analysis of community-based studies. Glob Health. 2018;8(1):010420.
Download references
Acknowledgements
The authors are particularly grateful to authors of included studies for making full text articles available for review and providing additional data when called upon. Special thanks go to Prof. William Kofi Bosu and Prof. Juliet Addo for their contribution to the subject.
The authors received no specific funding for this work.
Author information
Authors and affiliations.
Valley View University, Box 183, Techiman, Ghana
Fidelis Atibila
Department of Works and Social Psychology, Maastricht University, UNS40, 4755, Box 616, Maastricht, 6200 MD, The Netherlands
Gill ten Hoor
Department of Basic and Applied Biology, University of Energy and Natural Resources, UENR, Box 214, Sunyani, Ghana
Emmanuel Timmy Donkoh
Department of Mathematics and Statistics, University of Energy and Natural Resources, UENR, Box 214, Sunyani, Ghana
Abdul Iddrisu Wahab
Maastricht University, UNS40A4.732, Box 616, Maastricht, 6200 MD, The Netherlands
You can also search for this author in PubMed Google Scholar
Contributions
FA, ETD, GH, and GK are credited with conceptualizing the idea for an updated and comprehensive review of the literature on the prevalence of hypertension in the Ghanaian context. FA contributed the foundational draft of the paper, reviewed abstracts and full articles, and retrieved data on studies for data analysis. GH contributed conceptually to the paper and reviewed, effected changes, and provided feedback on all drafts, and also gave approval for submission. ETD contributed conceptually to the paper, reviewed abstracts and full articles, planned and performed statistical analysis with AIW, and approved the final manuscript for submission. GK contributed conceptually to the paper and reviewed and provided feedback on all drafts as well as final approval for submission. AIW contributed conceptually to the paper, carried out statistical analysis, and approved the final version. The author(s) read and approved the final manuscript.
Corresponding authors
Correspondence to Fidelis Atibila or Emmanuel Timmy Donkoh .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was not required for the present study. Details on the protocol used for this work has been published previously with PROSPERO (CRD42020215829).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Summary of findings table for eligibility assessment of full-text articles retrieved indicating reasons for exclusion.
Additional file 2.
R codes for meta-analysis.
Additional file 3.
R codes for meta-regression.
Additional file 4.
Characteristics of selected studies.
Additional file 5.
Blood pressure measurement protocols from selected studies.
Additional file 6.
Full results of meta-analysis
Additional file 7.
Meta regression output.
Additional file 8.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Atibila, F., Hoor, G.t., Donkoh, E.T. et al. Prevalence of hypertension in Ghanaian society: a systematic review, meta-analysis, and GRADE assessment. Syst Rev 10 , 220 (2021). https://doi.org/10.1186/s13643-021-01770-x
Download citation
Received : 26 December 2020
Accepted : 21 July 2021
Published : 07 August 2021
DOI : https://doi.org/10.1186/s13643-021-01770-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hypertension
- Cardiovascular risk
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 01 May 2022
Interventions in hypertension: systematic review and meta-analysis of natural and quasi-experiments
- Tong Xia ORCID: orcid.org/0000-0001-7136-8361 1 ,
- Fan Zhao ORCID: orcid.org/0000-0002-1261-5841 1 &
- Roch A. Nianogo ORCID: orcid.org/0000-0001-5932-6169 1 , 2
Clinical Hypertension volume 28 , Article number: 13 ( 2022 ) Cite this article
8611 Accesses
8 Citations
3 Altmetric
Metrics details
Hypertension is an urgent public health problem. Consistent summary from natural and quasi-experiments employed to evaluate interventions that aim at preventing or controlling hypertension is lacking in the current literature. This study aims to summarize the evidence from natural and quasi-experiments that evaluated interventions used to prevent or control hypertension.
We searched PubMed, Embase and Web of Science for natural and quasi-experiments evaluating interventions used to prevent hypertension, improve blood pressure control or reduce blood pressure levels from January 2008 to November 2018. Descriptions of studies and interventions were systematically summarized, and a meta-analysis was conducted.
Thirty studies were identified, and all used quasi-experimental designs including a difference-in-difference, a pre-post with a control group or a propensity score matching design. Education and counseling on lifestyle modifications such as promoting physical activity (PA), promoting a healthy diet and smoking cessation consultations could help prevent hypertension in healthy people. The use of computerized clinical practice guidelines by general practitioners, education and management of hypertension, the screening for cardiovascular disease (CVD) goals and referral could help improve hypertension control in patients with hypertension. The educating and counseling on PA and diet, the monitoring of patients’ metabolic factors and chronic diseases, the combination of education on lifestyles with management of hypertension, the screening for economic risk factors, medical needs, and CVD risk factors and referral all could help reduce blood pressure. In the meta-analysis, the largest reduction in blood pressure was seen for interventions which combined education, counseling and management strategies: weighted mean difference in systolic blood pressure was − 5.34 mmHg (95% confidence interval [CI], − 7.35 to − 3.33) and in diastolic blood pressure was − 3.23 mmHg (95% CI, − 5.51 to − 0.96).
Conclusions
Interventions that used education and counseling strategies; those that used management strategies; those that used combined education, counseling and management strategies and those that used screening and referral strategies were beneficial in preventing, controlling hypertension and reducing blood pressure levels. The combination of education, counseling and management strategies appeared to be the most beneficial intervention to reduce blood pressure levels.
Cardiovascular diseases (CVD) represent the leading cause of death, accounting for one in three deaths in the United States (US) and worldwide [ 1 , 2 , 3 ]. One of their most potent risk factors, hypertension (also known as high blood pressure), is a common risk factor for CVD [ 3 , 4 ]. Approximately 40% of adults aged 25 and over had elevated blood pressure in 2008 [ 3 ]. What is more, hypertension is responsible for at least 45% of deaths due to heart diseases and 51% of deaths due to stroke worldwide [ 3 , 4 ]. In the US alone, the direct medical and indirect expenses from CVDs were estimated at approximately $329 billion in 2013 to 2014 [ 5 ]. Effective large-scale interventions to prevent or treat hypertension are therefore urgently needed to reverse this trend. Yet, as new and promising interventions are surfacing every day, the need for rigorous evaluation of these interventions to inform evidence-based policies and clinical practice is ever growing.
To this effect, several randomized clinical trials (RCT) have been conducted to evaluate interventions used to prevent hypertension or improve its control [ 6 , 7 , 8 ]. However, although RCTs represent the gold standard for evaluating the efficacy (i.e., impact under ideal conditions) of most health interventions because of their high internal validity [ 9 , 10 ], they are not always feasible, appropriate or ethical for the evaluation of certain types of interventions. Furthermore, results from RCTs are not always generalizable to populations or settings of interest due to the highly selected sample and because the intervention is generally conducted under more stringent conditions ( low external validity ) [ 11 ]. To evaluate the effectiveness of an intervention (i.e., impact under real conditions) and to increase the uptake and implementation of evidence-based health interventions in the communities of interests, other types of experimental designs have been proposed. One such example is natural and quasi-experiments. The terms “natural experiments” and “quasi-experiments” are sometimes used interchangeably. In this study, and as described by others [ 12 ], we will distinguish these two concepts. Natural and quasi-experiments are similar in that, in both cases, there is no randomization of treatments or exposures (i.e., no random assignment). They differ, however, in that, natural experiments are those that involve naturally occurring or unplanned events (e.g., a national policy, new law), while quasi-experiments involve intentional or planned interventions implemented (typically for the purpose of research/evaluation) to change a specific outcome of interest (e.g., a community intervention program). Furthermore, in natural experiments, the investigator does not have control over the treatment assignment whereas in quasi-experiments, the investigator has control over the treatment assignment [ 12 ]. These experiments include difference-in-difference (DID) designs, synthetic controls and regression discontinuity designs to name a few [ 13 , 14 , 15 ].
As utilization of natural and quasi-experiments is increasing in public health and in the biomedical field [ 13 , 14 , 15 ], more natural and quasi-experiments are being conducted to evaluate interventions targeted to prevent or control hypertension [ 16 , 17 , 18 , 19 ]. This could be due to recent development or the reframing of classical approaches for determining causality in natural and quasi- experiments [ 13 , 14 , 15 , 20 ]. However, unlike RCTs of interventions aiming to prevent hypertension or improve its control [ 6 , 7 , 8 ], consistent summary and synthesis of evidence from natural and quasi- experiments is lacking in the current literature. The primary aim of the current systematic review is to summarize the evidence from natural and quasi-experiments that have evaluated interventions used to prevent, control hypertension or reduce blood pressure levels. A secondary aim of this study is to conduct a meta-analysis to summarize intervention effectiveness.
Data sources and strategy
We searched PubMed, Embase and Web of Science from January 2008 to November 2018. This time frame was selected to encompass studies that would have likely benefited from recent development and improvement in natural and quasi- experiments [ 13 , 20 ]. Briefly, the search strategy consisted in intersecting keywords related to the study methods (e.g., natural experiments, quasi-experiments, DID, synthetic control, interrupted time series, etc.) with the environment or settings (e.g., community, nation, organization, etc.) and the outcome (e.g., hypertension, elevated blood pressure, etc.). The full search strategy is described in Table S 1 . This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [ 21 ] (Fig. 1 ).
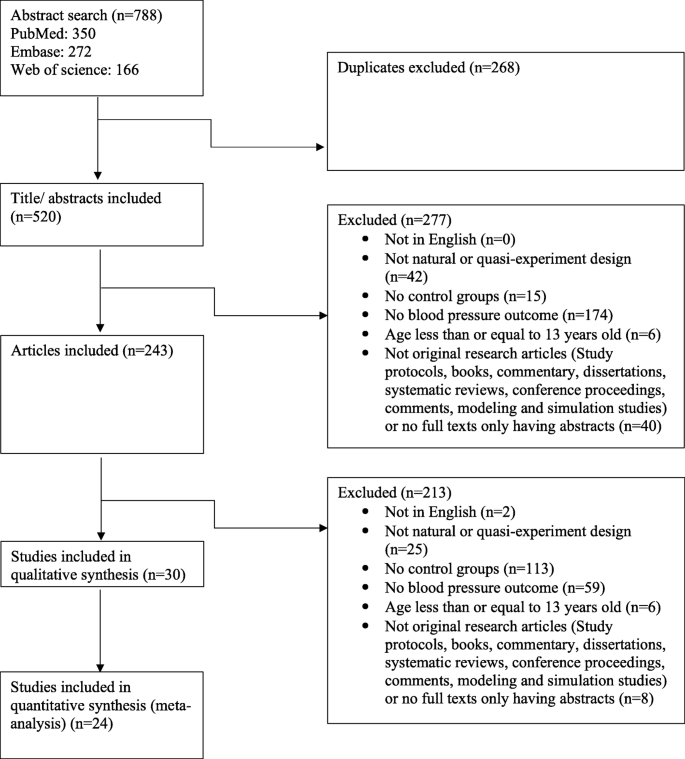
Study search and selection flow
Study selection
Two trained members (TX, FZ) screened abstracts and full-text articles. Disagreements were decided by a third member (RN). We included studies that used natural and quasi-experiments to evaluate interventions aimed at preventing hypertension, controlling hypertension or reducing blood pressure levels. The outcome measures were prevalence of hypertension and changes in mean blood pressure. Studies were excluded if they were not in English, were not a natural experiment or a quasi-experimental design, did not include a control group (as it has higher risk to internal validity due to the absence of comparison to adjust for time trends and confounding) [ 22 ], did not include blood pressure or hypertension as their outcome or included participants that were 13 years old or younger. In addition, we excluded studies that were not original research articles (e.g., study protocol, books, commentary, dissertations, conference proceedings, comments, systematic reviews, modeling and simulation studies), or had no full text available.
Data extraction and quality assessment
The following information was extracted: study design, sample size, study duration, data source, geographic location, participants’ socio-demographic characteristics, intervention types, intervention levels (e.g., individuals, community, school, clinic and national levels as suggested by the socio-ecological model [ 23 ]), behavior targeted and outcome measures (prevalence of hypertension or mean blood pressure change) (Table 1 , Table S 2 ).
The interventions were classified by strategies into four types:
Education and counseling: This subcategory includes strategies that aim at educating and providing knowledge and counseling to participants on lifestyle modifications (e.g., increasing physical activity (PA), eating better, avoiding or stopping smoking, etc.).
Management: This subcategory includes strategies that aim at monitoring patients’ metabolic factors and chronic diseases (e.g., blood pressure, cholesterol level, etc.) as well as patients’ adherence to medication. These strategies are generally done or facilitated by physicians, general practitioners (e.g., by assessing computerized clinical guidelines in the electronic health record management system), nurses, other staffs, or patients themselves.
Education, counseling and management: This subcategory combines education and counseling strategies with management strategies as described above.
Screening and referral for management: This subcategory includes strategies that aim at screening for (i.e., checking for the presence of) economic risk factors, medical needs, and CVD risk factors, followed by the referral of participants who screened positive to professionals who specialize in the management of those needs.
We also classified the interventions by settings into (1) community level; (2) health center level (i.e., primary care center or general practices), (3) organization level and (4) nationwide. In addition, we have classified the intervention by duration of the study into short-term (i.e., participants were followed for less than 12 months) and long-term (i.e., participants were followed for longer than or equal to 12 months).
We implemented the Cochrane Risk of Bias Tool for risk of bias and used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to assess the quality of the evidence for mean blood pressure change outcome [ 50 ], since the meta-analysis focused on this outcome. The risk of bias for studies included in this review could be found in Table S 3 and the quality of studies has also been summarized in Table S 4 .
Meta-analysis
To summarize the effectiveness of interventions on mean blood pressure changes, we also conducted a meta-analysis. Due to the high heterogeneity in the studies and interventions, we undertook a random-effects model and only summarized the effectiveness of intervention strategies by subgroup defined by intervention types, settings and duration. We estimated the weighted mean difference (WMD) of blood pressure and 95% confidence intervals (CIs). The studies included in the meta-analysis were only those whose outcomes were mean differences (MDs) in blood pressure ( n = 27) [ 16 , 19 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ] as these studies provided the data needed for performing the meta-analysis. Three studies [ 38 , 39 , 43 ] were excluded as they did not provide enough information to compute the standard errors (SEs). To estimate the average effect of the intervention when not directly provided, we subtracted the before-and-after change in the intervention group from that in the control group or subtracted the intervention-to-control difference at follow-up to that at baseline (pre-post design with a control group). Methods to calculate intervention impact and SEs were outlined in the appendix (Figs. S 1 , S 2 , Table S 5 ).
We presented the meta-analysis results using forest plots (Table 2 , Fig. 2 , Figs. S 3 , S 4 ). We assessed the heterogeneity by using the I 2 (Table 2 , Fig. 2 , Figs. S 3 , S 4 ). We did not perform meta-regression as it is not recommended when the number of studies is small (< 10 studies per covariate) [ 51 ]. We assessed publication bias by using funnel plots of SEs (Figs. S 5 , S 6 , S 7 ). To test the robustness of our results, we performed sensitivity analyses by removing one study at a time from the pool of studies to assess its impact on the findings (Tables S 6 , S 7 , S 8 , Figs. S 8 , S 9 , S 10 ). Data were analyzed with Stata 15.1 (StataCorp LLC, College Station, TX, USA).
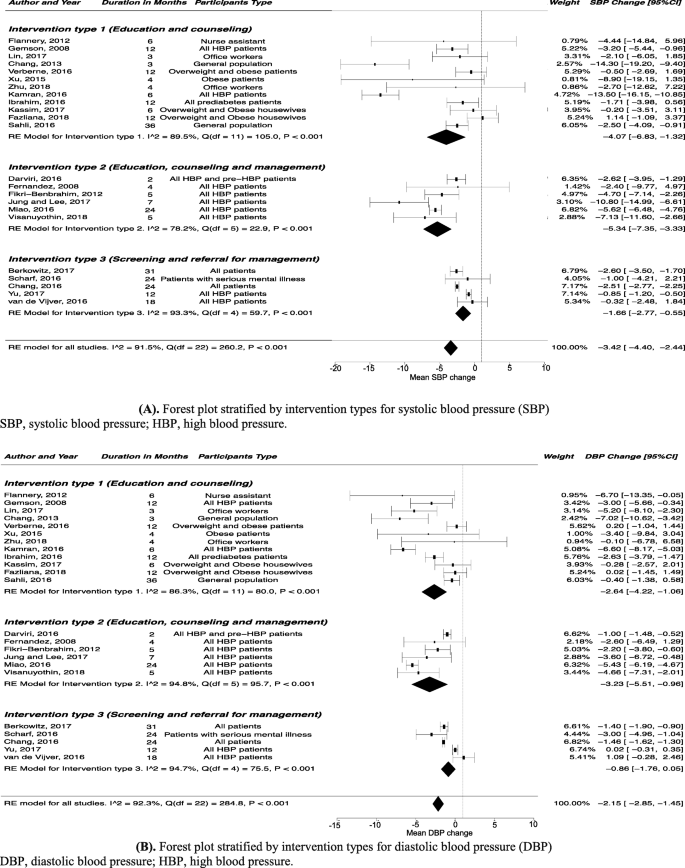
Forest plot stratified by intervention types for blood pressure. A Forest plot stratified by intervention types for systolic blood pressure (SBP). B Forest plot stratified by intervention types for diastolic blood pressure (DBP)
Overall, 788 titles of potentially relevant studies were identified and screened. In total, 545 were excluded and 243 full papers were retrieved, then 30 studies were included in the final sample ( Fig. 1 ) .
Study characteristics
Of the 30 studies included in this review [ 16 , 17 , 18 , 19 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ], three studies reported changes in hypertension prevalence, among which one study reported preventing hypertension in the general population [ 24 ] and two studies reported blood pressure control in patients with hypertension [ 17 , 18 ]; 25 studies reported mean blood pressure changes [ 16 , 19 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ]; two studies reported both outcome measures (changes in hypertension prevalence and mean blood pressure changes) [ 25 , 26 ]. Thirteen studies used education and counseling intervention strategies [ 24 , 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ]; four studies used management intervention strategies [ 18 , 19 , 38 , 39 ]; seven studies combined education, counseling and management intervention strategies [ 26 , 40 , 41 , 42 , 43 , 44 , 45 ]; and six studies used screening and referral for management intervention strategies [ 16 , 17 , 46 , 47 , 48 , 49 ]. Fourteen studies followed participants for less than 12 months (i.e., short-term interventions) [ 17 , 26 , 27 , 29 , 30 , 32 , 33 , 34 , 36 , 40 , 41 , 42 , 43 , 45 ]. Twelve studies were conducted in the US [ 16 , 17 , 19 , 24 , 27 , 28 , 32 , 33 , 39 , 41 , 43 , 46 ] and most studies included both genders [ 16 , 17 , 18 , 19 , 24 , 25 , 26 , 28 , 29 , 30 , 31 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ] and all racial/ethnic groups [ 16 , 17 , 18 , 19 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ]. We found no natural experiments according to the definition used in this study (Table 1 , Table S 2 ).
Quality ratings
According to the Cochrane Risk of Bias Tool, most studies included in this review were found to have a high risk of bias ( Table S 3 ). This was so because the Cochrane Risk of Bias Tool was mostly designed for RCTs. Studies included in this review only used quasi-experiment designs and as such did not use randomization, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. Using the GRADE approach, the quality of evidence was deemed of low quality for the mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) change outcome (Table S 4 ).
Studies that reported prevalence of hypertension in the general population or changes in the prevalence of controlled blood pressure in hypertension patients after intervention
Outcome of interest: prevention of hypertension in healthy people, education and counseling intervention strategies.
Two studies evaluated the education and counseling intervention strategies, and both found that those strategies could help prevent hypertension in healthy people [ 24 , 25 ]. One study in the US found that nutritional education and giving access to fruits and vegetables through community gardens helped reduce hypertension prevalence (61.0% vs. 45.0%; P < 0.01), whereas the prevalence of hypertension in the control group did not change (46.7% vs. 49.8%; P = 0.39) [ 24 ]. The other study in Africa showed that an education strategy which promoted PA and healthy diet and combined with free smoking cessation consultations could help reduce the prevalence of hypertension (22.8% vs. 16.2%; P = 0.01), compared to that in control group (14.0% vs. 15.1%; P = 0.52) [ 25 ].
Outcome of interest: improvement of hypertension control in patients with hypertension
Management intervention strategies.
A study in the US showed that patients whose general practitioners accessed the computerized clinical practice guideline at least twice a day improved their hypertension control compared to the patients whose general practitioners never accessed the computerized clinical practice guideline ( P < 0.001) [ 18 ].
Education, counseling and management intervention strategies
A study in the US found that patients who received education about hypertension and did home blood pressure monitoring had a better control of their hypertension compared to the control group ( P = 0.03) [ 26 ].
Screening and referral for management intervention strategies
A study in the US showed that for White patients, interventions which involved a coordinator who identified and reached out to patients not meeting CVD goals and linked them to management programs could improve the odds of blood pressure control (odds ratio, 1.13; 95% CI, 1.05 to 1.22) compared to no intervention [ 17 ].
Studies that reported mean blood pressure changes after intervention
Outcome of interest: reduction in mean blood pressure.
Seven [ 25 , 27 , 28 , 29 , 30 , 34 , 35 ] of twelve [ 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ] (58.3%) studies showed that the education and counseling intervention strategies could help reduce mean blood pressure compared to the control group. Education and counseling interventions targeting lifestyle modifications (e.g., diet and PA) have been found effective in reducing blood pressure in the workplace. A study in US female nursing assistants found that combining education and continuing motivation (e.g., counseling on questions of interventions and receiving feedback) on diet and PA led to more reduction in DBP compared to the control group who only received the education (MD, − 6.70 mmHg; 95% CI, − 13.35 to − 0.05) [ 27 ]. Two other studies also found that multi-component lifestyle interventions in the workplace including sharing health information by messages, putting up posters, using pedometers, and giving education on PA could help healthy employees or employees with hypertension lower blood pressure [ 28 , 29 ]. Besides the workplace, interventions implemented in a community setting also appeared to work in reducing blood pressure. A study that included participants age 55 years or more in Asia found that people who attended 60-min Tai Chi three times per week for 12 weeks had a larger reduction in SBP (MD, − 14.30 mmHg; 95% CI, − 19.20 to − 9.40) and in DBP (MD, − 7.02 mmHg; 95% CI, − 10.62 to − 3.42) compared to people maintaining usual daily activities [ 30 ]. Another study among patients with hypertension in Asia found that education about the nutritional behavior and guidelines from dietary approaches to stop hypertension (DASH) approach could help reduce blood pressure more in the intervention group compared to the control group who only received the instruction booklets used in intervention group (SBP: MD, − 13.50 mmHg; 95% CI, − 16.15 to − 10.85; DBP: MD, − 6.60 mmHg; 95% CI, − 8.17 to − 5.03) [ 34 ]. One study in Africa also showed that education on promoting PA and healthy diet, combined with free smoking cessation consultations could help reduce SBP in the intervention group [ 25 ].
Two [ 19 , 39 ] of three [ 19 , 38 , 39 ] (66.7%) studies showed that the management intervention strategies could help reduce mean blood pressure compared to the control group. A study in the US showed that supporting diabetes patients’ self-management of hypertension by team-based chronic models (e.g., proactive patient outreach, depression screening, and health coaching) could decrease more DBP over a 6-month period compared to the usual care (MD, − 1.13 mmHg; 95% CI, − 2.23 to − 0.04) [ 19 ]. A study among hypertension patients in Asia showed that improving the social health insurance system by increasing outpatient expenditure reimbursement ratio could help reduce more SBP (MD, − 2.9 mmHg; P = 0.01) compared to outpatient expense not covered [ 38 ]. The other study among diabetes patients in the US also showed that team-based treatment with trained staff on medical management and self-management helped lower SBP (MD, − 0.88 mmHg; P = 0.01), but it did not compare the MD between treatment and control group [ 39 ].
Six [ 26 , 40 , 42 , 43 , 44 , 45 ] of seven [ 26 , 40 , 41 , 42 , 43 , 44 , 45 ] (85.7%) studies showed that the combination of education, counseling and management intervention strategies led to more blood pressure reduction compared to the control group. One study among hypertension patients in Europe found that management of stress by biofeedback-assisted relaxation and lifestyle counseling on diet and PA reduced more SBP (MD, − 2.62 mmHg; 95% CI, − 3.96 to − 1.29) and DBP (MD, − 1.00 mmHg; 95% CI, − 1.90 to − 0.93) compared to the control group [ 40 ]. One study among hypertension patients in the US also found that education about hypertension and home blood pressure monitoring could help reduce more SBP (MD, − 4.70 mmHg; 95% CI, − 7.14 to − 2.26) and DBP (MD, − 2.20 mmHg; 95% CI, − 3.80 to − 0.60) compared to controls [ 26 ]. A study among 65-year-and-older hypertension patients in Asia found that the intervention group who received education on hypertension management, community-based eHealth monitoring, and monthly telephone counseling had more reduction in SBP (MD, − 10.80 mmHg; 95% CI, − 14.99 to − 6.61) compared to the control group who only received a poster about hypertension management [ 42 ]. A study among hypertension patients in the US also showed that interventions on lifestyle modifications, and nutritional, pharmacological therapies as well as medication adherence lowered SBP and DBP compared to the control group [ 43 ]. A study among hypertension patients in Asia found that integration of preventive-curative services delivery and cooperation among village-town-county physicians for education on lifestyle modifications, taking blood pressure drugs regularly and monitoring the blood pressure could help reduce blood pressure more in the intervention group [ 44 ]. The other study in Asia also found that integrated program with health education on home blood pressure monitoring and hypertension measurement skills could help reduce blood pressure more in the intervention group [ 45 ].
Four [ 16 , 46 , 47 , 48 ] of five [ 16 , 46 , 47 , 48 , 49 ] (80.0%) studies showed that the screening and referral for management intervention strategies could help reduce more blood pressure compared to the control group. Screening for medical or economic needs followed by offering treatment and resources has been found helpful. One study in the US found that screening for unmet needs in primary care and offering those who screened positive some resources could reduce SBP (MD, − 2.6 mmHg; 95% CI, − 3.5 to − 1.7]) and DBP (MD, − 1.4 mmHg; 95% CI, − 1.9 to − 0.9) in patients [ 16 ]. The other study among patients with serious mental illness in the US also found that using registry for general medical needs and outcomes, screening and referral for general medical illness prevention and treatment could help reduce more DBP compared to controls (MD, − 3.00 mmHg; 95% CI, − 4.96 to − 1.04) [ 46 ]. Assessing and screening CVD risk followed by a management program has also been found beneficial to reduce blood pressure. A study in Europe showed that participating in CVD risk assessment and management program, including screening and tailored strategies for lifestyle advice on CVD risk factors could reduce more SBP (MD, − 2.51 mmHg; 95% CI, − 2.77 to − 2.25) and DBP (MD, − 1.46 mmHg; 95% CI, − 1.62 to − 1.29) compared to controls [ 47 ]. A study among hypertension patients in Asia also found that a standardized CVD-risk assessment, a hypertension complication screening and adherence to medications could help reduce more blood pressure compared to the usual care [ 48 ].
Meta-analysis of the effectiveness of interventions on mean blood pressure change
Intervention type sub-group analysis.
The largest blood pressure reduction (SBP: WMD, − 5.34 mmHg; 95% CI, − 7.35 to − 3.33; DBP: WMD, − 3.23 mmHg; 95% CI, − 5.51 to − 0.96) was seen for interventions that combined education, counseling and management intervention strategies (Table 2 , Fig. 2 ).
Intervention setting sub-group analysis
Participants who experienced interventions implemented in community settings (WMD, − 3.77 mmHg; 95% CI, − 6.17 to − 1.37) and in health center settings (WMD, − 3.77 mmHg; 95% CI, − 5.78 to − 1.76) had large SBP reduction. Participants experienced interventions implemented in organization settings had large DBP reduction (WMD, − 3.92 mmHg; 95% CI, − 5.80 to − 2.04) (Table 2 , Fig. S 3 ).
Intervention duration sub-group analysis
Participants who were followed for less than 12 months (i.e., short-term interventions) had a large reduction in blood pressure (SBP: WMD, − 6.25 mmHg; 95% CI, − 9.28 to − 3.21; DBP: WMD, − 3.54 mmHg; 95% CI, − 5.21 to − 1.87) and participants who were followed for longer than or equal to 12 months (i.e., long-term interventions) had a moderate reduction in blood pressure (SBP: WMD, − 1.89 mmHg; 95% CI, − 2.80 to − 0.97; DBP: WMD, − 1.33 mmHg; 95% CI, − 2.11 to − 0.55) (Table 2 , Fig. S 4 ).
We summarized the evidence from quasi-experiments that have evaluated interventions used to (1) prevent hypertension in the general population, (2) improve hypertension control in patients with hypertension or (3) reduce blood pressure levels in both the general population and patients.
In this systematic review, we found that the intervention strategies such as (1) education and counseling, (2) management, (3) education, counseling and management and (4) screening and referral for management were beneficial in preventing, controlling hypertension or reducing blood pressure levels. In particular, we found that education and counseling on lifestyle modifications (i.e., promoting PA, healthy diet, smoking cessation consultations) could help prevent hypertension in healthy people. The use of computerized clinical practice guidelines by general practitioners, education and management of hypertension, screening for CVD goals and referral to management could help improve hypertension control in patients with hypertension. The education and counseling on lifestyle modifications, the monitoring of patients’ metabolic factors and chronic diseases (e.g., blood pressure, cholesterol level, etc.) as well as patients’ adherence to medication, the combined education and management of hypertension, the screening for economic risk factors, medical needs, and CVD risk factors, followed by the referral to management all could help reduce blood pressure levels. Our study is one of the few systematic reviews that have summarized the evidence from quasi-experiments on hypertension prevention and control. A previous systematic review [ 52 ] which summarized evidence from cluster-randomized trials and quasi-experimental studies had been conducted and found that education, counseling and management strategies were also beneficial in controlling hypertension and reducing blood pressure. It showed that educating healthcare providers and patients, facilitating relay of clinical data to providers, promoting patients’ accesses to resources were associated with improved hypertension control and decreased blood pressure [ 52 ]. Another systematic review which summarized evidence from RCTs found that several interventions including blood pressure self-monitoring, educational strategies, improving the delivery of care, and appointment reminder systems could help control hypertension and reduce blood pressure [ 6 ]. Another study also found that community-based health workers interventions including health education and counseling, navigating the health care system, managing care, as well as giving social services and support had a significant effect on improving hypertension control and decreasing blood pressure [ 53 ]. A review from observational studies and RCT evidence from the US Preventive Services Task Force found that office measurement of blood pressure could effectively screen adults for hypertension [ 7 ].
Our review did not find natural experiments studies according to the definition used in this study. Quasi-experimental designs included DID, propensity score matching and pre-post designs with a control group (PPCG). While PPCG designs generally involve two groups (intervention and control) and two different time points (before and after the intervention), DID designs generally involve two or more intervention and control groups and multiple time points [ 13 ]. In this review, we did not include pre-post without a control group design because of its higher risk to internal validity due to the absence of comparison to adjust for time trends and confounding [ 22 ]. The findings in this review, highlight that, quasi-experiments are increasingly used to evaluate the effectiveness of health interventions for hypertension management when RCTs are not feasible or appropriate. For instance, several studies included in our systematic review often indicated that RCTs would have been difficult to be implemented given that the intervention was conducted in a particular setting such as a pragmatic clinical setting [ 16 , 43 , 45 , 48 ], a community setting [ 24 , 35 , 36 , 42 ], or a real-world organizational setting [ 33 ] because of ethical concerns and human resources issues. Another reason why quasi-experiments were chosen had to do with the need for translation and generalizability of the evidence in a specific community setting [ 32 ]. In fact, RCTs are not always generalizable to the communities or settings of interests [ 11 ]. The growing interest in and hence the increase in the use of natural and quasi-experiments in public health may be due to the recognition and realization of its usefulness in evaluating health interventions [ 14 , 54 ].
Given that there was high heterogeneity in the studies included in this systematic review, we have performed a random effects model and have only presented the subgroup analysis by intervention types, settings and duration of the study. Overall, our study suggested that interventions that combined education, counseling and management strategies appeared to show a relatively large beneficial effect for reducing blood pressure. However, our finding should be interpreted with caution due to the high-risk of bias and lower quality of evidence given the quasi-experimental nature of the designs (as opposed to evidence from randomized experiments). Nevertheless, the findings here can give us some insights on the benefit of interventions such as education, counseling and management, especially given that our findings are in line with previous studies [ 6 , 8 , 52 , 55 ]. Given that RCTs are not always feasible or appropriate, scientists should develop more rigorous methods to increase the internal validity of non-randomized studies. Compared to previous studies, one systematic review with meta-analysis including cluster-randomized trials and quasi-experiment studies showed that multi-component interventions which incorporated education of health care providers and patients, facilitating relay of clinical data to providers, and promoting patients’ accesses to resources could reduce more blood pressure compared to controls [ 52 ]. A recent systematic review with meta-analysis of RCTs also reported that interventions which included blood pressure self-monitoring, appointment reminder systems, educational strategies, and improving the delivery of care showed beneficial effects on lowering blood pressure [ 6 ]. Another systematic review and meta-analysis of RCTs also showed that self-measured blood pressure monitoring lowered SBP by 3.9 mmHg and DBP by 2.4 mmHg at 6 months compared to the usual care group [ 8 ]. One systematic review and meta-analysis of RCTs found that diet improvement, aerobic exercise, alcohol and sodium restriction, and fish oil supplements reduced blood pressure as well [ 55 ].
Limitations
This review has some limitations. First, the definition of natural and quasi-experiments is not consistent across fields. Second, the main limitation in most if not all the quasi-experimental study designs noted in this review was the potential for unobserved and uncontrolled confounding, which is a threat to internal validity and could lead to biased findings. Third, our findings may not be generalizable to all countries and settings as we only included studies published in the English language in this review. Fourth, as is the case in most other reviews, we could have missed relevant studies despite our best attempt to conduct a thorough search of the literature. Fifth, we found that most studies included in this study had a high risk of bias. It might be because we used the Cochrane Risk of Bias Tool to assess bias which was designed for examining RCTs. Studies in this review only used quasi-experiment designs and did not have randomization, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. Sixth, studies generally reported the measure of intervention impact differently across studies, making it difficult to combine the findings. In addition, studies were highly heterogeneous in terms of the types of individuals included in the study (e.g., healthy individuals and patients). We conducted the subgroup meta-analysis to reduce the heterogeneity, but the high heterogeneity still existed. Therefore, the results from meta-analysis need to be interpreted with caution. The individual impact reported for each individual study and the results from systematic review should be given more consideration.
In this systematic review, interventions that used education and counseling strategies; those that used management strategies; those that combined education, counseling and management strategies and those that used screening and referral for management strategies were beneficial in preventing, controlling hypertension and reducing blood pressure levels. The combination of education, counseling and management strategies appeared to be the most beneficial intervention to reduce blood pressure levels. The findings in this review, highlight that, a number of interventions that aim at preventing, controlling hypertension or reducing blood pressure levels are being evaluated through the use of quasi-experimental studies. Given that RCTs are not always feasible or appropriate, scientists should develop more rigorous methods to increase the internal validity of such quasi-experimental studies.
Availability of data and materials
The data supporting the conclusions of this article is included within the article and the additional file.
Abbreviations
Confidence interval
Cardiovascular disease
Dietary approaches to stop hypertension
Diastolic blood pressure
Difference-in-difference
Grading of Recommendations, Assessment, Development, and Evaluation
Mean difference
Physical activity
Pre-post designs with a control group
Randomized clinical trial
Systolic blood pressure
Standard error
United States
Weighted mean difference
National Center for Health Statistics & Heron, M. Deaths: Leading Causes for 2018. Atlanta: CDC; 2021.
Lewington S, Lacey B, Clarke R, Guo Y, Kong XL, Yang L, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176:524–32.
Article PubMed Google Scholar
World Health Organization (WHO). A global brief on hypertension: silent killer, global public health crisis: World Health Day 2013. Geneva: WHO; 2013.
Sun D, Liu J, Xiao L, Liu Y, Wang Z, Li C, et al. Recent development of risk-prediction models for incident hypertension: an updated systematic review. PLoS One. 2017;12:e0187240.
Article PubMed PubMed Central Google Scholar
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–492.
Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;3:CD005182.
Google Scholar
Sheridan S, Pignone M, Donahue K. Screening for high blood pressure: a review of the evidence for the U.S. preventive services task force. Am J Prev Med. 2003;25:151–8.
Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159:185–94.
de Simone G, Izzo R, Verdecchia P. Are observational studies more informative than randomized controlled trials in hypertension? Pro side of the argument. Hypertension. 2013;62:463–9.
Li DZ, Zhou Y, Yang YN, Ma YT, Li XM, Yu J, et al. Acupuncture for essential hypertension: a meta-analysis of randomized sham-controlled clinical trials. Evid Based Complement Alternat Med. 2014;2014:279478.
Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–6.
Article CAS PubMed Google Scholar
Remler DK, Van Ryzin GG. Research methods in practice: strategies for description and causation. 2nd ed. SAGE: Los Angeles; 2014.
Handley MA, Lyles CR, McCulloch C, Cattamanchi A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu Rev Public Health. 2018;39:5–25.
Basu S, Meghani A, Siddiqi A. Evaluating the health impact of large-scale public policy changes: classical and novel approaches. Annu Rev Public Health. 2017;38:351–70.
Craig P, Katikireddi SV, Leyland A, Popham F. Natural experiments: an overview of methods, approaches, and contributions to public health intervention research. Annu Rev Public Health. 2017;38:39–56.
Berkowitz SA, Hulberg AC, Standish S, Reznor G, Atlas SJ. Addressing unmet basic resource needs as part of chronic cardiometabolic disease management. JAMA Intern Med. 2017;177:244–52.
James A, Berkowitz SA, Ashburner JM, Chang Y, Horn DM, O’Keefe SM, et al. Impact of a population health management intervention on disparities in cardiovascular disease control. J Gen Intern Med. 2018;33:463–70.
Comin E, Catalan-Ramos A, Iglesias-Rodal M, Grau M, Del Val JL, Consola A, et al. Impact of implementing electronic clinical practice guidelines for the diagnosis, control and treatment of cardiovascular risk factors: a pre-post controlled study. Aten Primaria. 2017;49:389–98.
Panattoni L, Hurlimann L, Wilson C, Durbin M, Tai-Seale M. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
Bor J. Capitalizing on natural experiments to improve our understanding of population health. Am J Public Health. 2016;106:1388–9.
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Ho AM, Phelan R, Mizubuti GB, Murdoch JAC, Wickett S, Ho AK, et al. Bias in before-after studies: narrative overview for anesthesiologists. Anesth Analg. 2018;126:1755–62.
Bronfenbrenner U. The ecology of human development: experiments by nature and design. Cambridge: Harvard University Press; 1979.
Barnidge EK, Baker EA, Schootman M, Motton F, Sawicki M, Rose F. The effect of education plus access on perceived fruit and vegetable consumption in a rural African American community intervention. Health Educ Res. 2015;30:773–85.
Article CAS PubMed PubMed Central Google Scholar
Sahli J, Maatoug J, Harrabi I, Ben Fredj S, Dendana E, Ghannem H. Effectiveness of a community-based intervention program to reduce hypertension prevalence among adults: results of a quasiexperimental study with control group in the region of Sousse. Tunisia Glob Heart. 2016;11:131–7.
Fikri-Benbrahim N, Faus MJ, Martínez-Martínez F, Alsina DG, Sabater-Hernandez D. Effect of a pharmacist intervention in Spanish community pharmacies on blood pressure control in hypertensive patients. Am J Health Syst Pharm. 2012;69:1311–8.
Flannery K, Resnick B, Galik E, Lipscomb J, McPhaul K, Shaughnessy M. The worksite heart health improvement project (WHHIP): feasibility and efficacy. Public Health Nurs. 2012;29:455–66.
Gemson DH, Commisso R, Fuente J, Newman J, Benson S. Promoting weight loss and blood pressure control at work: impact of an education and intervention program. J Occup Environ Med. 2008;50:272–81.
Lin YP, Lin CC, Chen MM, Lee KC. Short-term efficacy of a “sit less, walk more” workplace intervention on improving cardiometabolic health and work productivity in office workers. J Occup Environ Med. 2017;59:327–34.
Chang MY, Yeh SC, Chu MC, Wu TM, Huang TH. Associations between Tai chi Chung program, anxiety, and cardiovascular risk factors. Am J Health Promot. 2013;28:16–22.
Verberne LD, Hendriks MR, Rutten GM, Spronk I, Savelberg HH, Veenhof C, et al. Evaluation of a combined lifestyle intervention for overweight and obese patients in primary health care: a quasi-experimental design. Fam Pract. 2016;33:671–7.
Xu F, Letendre J, Bekke J, Beebe N, Mahler L, Lofgren IE, et al. Impact of a program of Tai chi plus behaviorally based dietary weight loss on physical functioning and coronary heart disease risk factors: a community-based study in obese older women. J Nutr Gerontol Geriatr. 2015;34:50–65.
Zhu W, Gutierrez M, Toledo MJ, Mullane S, Stella AP, Diemar R, et al. Long-term effects of sit-stand workstations on workplace sitting: a natural experiment. J Sci Med Sport. 2018;21:811–6.
Kamran A, Sharifirad G, Heydari H, Sharifian E. The effect of theory based nutritional education on fat intake, weight and blood lipids. Electron Physician. 2016;8:3333–42.
Ibrahim N, Ming Moy F, Awalludin IA, Mohd Ali Z, Ismail IS. Effects of a community-based healthy lifestyle intervention program (co-HELP) among adults with prediabetes in a developing country: a quasi-experimental study. PLoS One. 2016;11:e0167123.
Kassim MSA, Manaf MRA, Nor NSM, Ambak R. Effects of lifestyle intervention towards obesity and blood pressure among housewives in Klang Valley: a quasi-experimental study. Malays J Med Sci. 2017;24:83–91.
PubMed PubMed Central Google Scholar
Fazliana M, Liyana AZ, Omar A, Ambak R, Mohamad Nor NS, Shamsudin UK, et al. Effects of weight loss intervention on body composition and blood pressure among overweight and obese women: findings from the MyBFF@home study. BMC Womens Health. 2018;18(Suppl 1):93.
Miao Y, Gu J, Zhang L, He R, Sandeep S, Wu J. Improving the performance of social health insurance system through increasing outpatient expenditure reimbursement ratio: a quasi-experimental evaluation study from rural China. Int J Equity Health. 2018;17:89.
Scanlon DP, Hollenbeak CS, Beich J, Dyer AM, Gabbay RA, Milstein A. Financial and clinical impact of team-based treatment for medicaid enrollees with diabetes in a federally qualified health center. Diabetes Care. 2008;31:2160–5.
Darviri C, Artemiadis AK, Protogerou A, Soldatos P, Kranioutou C, Vasdekis S, et al. A HEALth promotion and STRESS management program (HEAL-STRESS study) for prehypertensive and hypertensive patients: a quasi-experimental study in Greece. J Hum Hypertens. 2016;30:397–403.
Fernandez S, Scales KL, Pineiro JM, Schoenthaler AM, Ogedegbe G. A senior center-based pilot trial of the effect of lifestyle intervention on blood pressure in minority elderly people with hypertension. J Am Geriatr Soc. 2008;56:1860–6.
Jung H, Lee JE. The impact of community-based eHealth self-management intervention among elderly living alone with hypertension. J Telemed Telecare. 2017;23:167–73.
Hussain T, Franz W, Brown E, Kan A, Okoye M, Dietz K, et al. The role of care management as a population health intervention to address disparities and control hypertension: a quasi-experimental observational study. Ethn Dis. 2016;26:285–94.
Miao Y, Zhang L, Sparring V, Sandeep S, Tang W, Sun X, et al. Improving health related quality of life among rural hypertensive patients through the integrative strategy of health services delivery: a quasi-experimental trial from Chongqing. China Int J Equity Health. 2016;15:132.
Visanuyothin S, Plianbangchang S, Somrongthong R. An integrated program with home blood-pressure monitoring and village health volunteers for treating poorly controlled hypertension at the primary care level in an urban community of Thailand. Integr Blood Press Control. 2018;11:25–35.
Scharf DM, Schmidt Hackbarth N, Eberhart NK, Horvitz-Lennon M, Beckman R, Han B, et al. General medical outcomes from the primary and behavioral health care integration grant program. Psychiatr Serv. 2016;67:1226–32.
Chang KC, Lee JT, Vamos EP, Soljak M, Johnston D, Khunti K, et al. Impact of the National Health Service Health Check on cardiovascular disease risk: a difference-in-differences matching analysis. CMAJ. 2016;188:E228–38.
Yu EY, Wan EY, Wong CK, Chan AK, Chan KH, Ho SY, et al. Effects of risk assessment and management programme for hypertension on clinical outcomes and cardiovascular disease risks after 12 months: a population-based matched cohort study. J Hypertens. 2017;35:627–36.
van de Vijver S, Oti SO, Gomez GB, Agyemang C, Egondi T, Moll van Charante E, et al. Impact evaluation of a community-based intervention for prevention of cardiovascular diseases in the slums of Nairobi. the SCALE-UP study Glob Health Action. 2016;9:30922.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Wiley-Blackwell: Hoboken; 2020.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: John Wiley & Sons; 2009.
Book Google Scholar
Walsh JM, McDonald KM, Shojania KG, Sundaram V, Nayak S, Lewis R, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646–57.
Kim K, Choi JS, Choi E, Nieman CL, Joo JH, Lin FR, et al. Effects of community-based health worker interventions to improve chronic disease management and care among vulnerable populations: a systematic review. Am J Public Health. 2016;106:e3–28.
Rehkopf DH, Basu S. A new tool for case studies in epidemiology-the synthetic control method. Epidemiology. 2018;29:503–5.
Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–33.
Download references
Acknowledgments
Not applicable.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles (UCLA), 650 Charles E. Young Drive South, Los Angeles, CA, 90095, USA
Tong Xia, Fan Zhao & Roch A. Nianogo
California Center for Population Research (CCPR), 337 Charles E. Young Drive East, Los Angeles, CA, 90095, USA
Roch A. Nianogo
You can also search for this author in PubMed Google Scholar
Contributions
TX participated in the study conception, design and analysis and wrote the initial first draft of the article. FZ participated in the study conception, design and reviewed the first draft of the article. RN conceived and supervised the design and analysis, finalized the first draft and critically reviewed and revised the manuscript. All authors provided critical input and insights into the development and writing of the article and approved the final manuscript as submitted.
Corresponding author
Correspondence to Roch A. Nianogo .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
Search words. Table S2. Summary of the characteristics of the studies included in this review ( n = 30). Table S3. Risk of Bias Tool Assessments Across Studies (n = 30). Table S4. GRADE Evidence Profiles Across Studies in Meta-analysis ( n = 24). Table S5. Estimates and parameters in studies that reported on the mean difference in blood pressure ( n = 27). Table S6. Sensitivity analysis for systolic blood pressure (SBP) and diastolic blood pressure (DBP) in meta-analysis stratified by intervention type. Table S7. Sensitivity analysis for systolic blood pressure (SBP) and diastolic blood pressure (DBP) in meta-analysis stratified by intervention setting. Table S8. Sensitivity analysis for systolic blood pressure (SBP) and diastolic blood pressure (DBP) in meta-analysis stratified by intervention duration. Fig. S1. Methods to calculate mean differences (MD). Fig. S2. Methods to calculate standard errors (SE). Fig. S3. Forest plot stratified by intervention settings for blood pressure. (A) Forest plot stratified by intervention settings for systolic blood pressure (SBP). (B) Forest plot stratified by intervention settings for diastolic blood pressure (DBP). Fig. S4. Forest plot stratified by intervention duration for blood pressure. ( A) Forest plot stratified by intervention duration for systolic blood pressure (SBP). ( B) Forest plot stratified by intervention duration for diastolic blood pressure (DBP). Fig. S5. Funnel plot of systolic blood pressure (SBP), diastolic blood pressure (DBP) stratified by intervention types. Fig. S6. Funnel plot of systolic blood pressure (SBP), diastolic blood pressure (DBP) stratified by intervention settings. Fig. S7. Funnel plot of systolic blood pressure (SBP), diastolic blood pressure (DBP) stratified by intervention duration. Fig. S8. Sensitivity analysis of systolic blood pressure (SBP), diastolic blood pressure (DBP) stratified by intervention types. Fig. S9. Sensitivity analysis of systolic blood pressure (SBP), diastolic blood pressure (DBP) stratified by intervention settings. Fig. S10. Sensitivity analysis of systolic blood pressure (SBP), diastolic blood pressure (DBP) stratified by intervention duration
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Xia, T., Zhao, F. & Nianogo, R.A. Interventions in hypertension: systematic review and meta-analysis of natural and quasi-experiments. Clin Hypertens 28 , 13 (2022). https://doi.org/10.1186/s40885-022-00198-2
Download citation
Received : 19 February 2021
Accepted : 21 January 2022
Published : 01 May 2022
DOI : https://doi.org/10.1186/s40885-022-00198-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hypertension
- Non-randomized controlled trials as topic
- Comparative effectiveness research
Clinical Hypertension
ISSN: 2056-5909
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- DOI: 10.21275/sr24407123357
- Corpus ID: 269807598
Diagnostic and Therapeutic Advances in Pulmonary Arterial Hypertension Management - A Literature Review
- Upasi Venkatasubba Reddy , Mukka Rajender , +1 author Shaik Siddique
- Published in International Journal of… 5 May 2024
18 References
Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension., unraveling the epigenetic landscape of pulmonary arterial hypertension: implications for personalized medicine development, phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension., pulmonary hypertension inhaled therapies: an updated review., inhaled treprostinil vs iloprost: comparison of adherence, persistence, and health care resource utilization in patients with pulmonary arterial hypertension, pulmonary arterial hypertension: emerging principles of precision medicine across basic science to clinical practice, novel approaches to imaging the pulmonary vasculature and right heart., a systematic review with meta-analysis of biomarkers for detection of pulmonary arterial hypertension, breeze: open‐label clinical study to evaluate the safety and tolerability of treprostinil inhalation powder as tyvaso dpi™ in patients with pulmonary arterial hypertension, interventional and surgical treatments for pulmonary arterial hypertension, related papers.
Showing 1 through 3 of 0 Related Papers
- Open access
- Published: 23 April 2024
Prevalence of metabolic syndrome and associated factors among patient with type 2 diabetes mellitus in Ethiopia, 2023: asystematic review and meta analysis
- Betelhem Mesfin Demissie 1 ,
- Fentaw Girmaw 2 ,
- Nimona Amena 3 &
- Getachew Ashagrie 2
BMC Public Health volume 24 , Article number: 1128 ( 2024 ) Cite this article
638 Accesses
Metrics details
Metabolic syndrome is a complex pathophysiologic state which characterized by abdominal obesity, insulin resistance, hypertension, and hyperlipidaemia. The Adult Treatment Panel III report (ATP III) of the National Cholesterol Education Programme identified the metabolic syndrome as a serious public health issue in the modern era. In Western and Asian nations, the frequency of metabolic syndrome is rising, especially in developing regions experiencing rapid socio-environmental changes, in Sub-Saharan Africa; metabolic syndrome may be present in more than 70% of people with type 2 diabetes mellitus. Therefore the objective of our study was to estimate the pooled prevalence of metabolic syndrome and associated factors among type II diabetes mellitus patient.
This systematic review and meta-analysis included original articles of cross sectional studies published in the English language. Searches were carried out in PubMed, Web of Science, Google Scholar, and grey literature Journals from 2013 to June 2023. A random-effects model was used to estimate the pooled prevalence of metabolic syndrome among type II Diabetes mellitus patient in Ethiopia. Heterogeneity was assessed using the I 2 statistic. Subgroup analysis was also conducted based on study area. Egger’s test was used to assess publication bias. Sensitivity analysis was also conducted.
Out of 300 potential articles, 8 cross sectional studies were included in this systematic review and meta-analysis study. The pooled prevalence of metabolic syndrome among patient with type II diabetes mellitus in Ethiopia was found to be 64.49% (95% CI: 62.39, 66.59) and 52.38% (95% CI: 50.05, 54.73) by using NCEP/ATP III and IDF criteria, respectively. The weighted pooled prevalence of metabolic syndrome among type II diabetes mellitus patients by sub group analysis based on the study region was 63.79% (95% CI: 56.48, 71.11) and 52.23% (95%CI: 47.37, 57.22) by using NCEP/ATP III and IDF criteria, respectively. Being female and increased body mass index were factors associated with metabolic syndrome among type II diabetes mellitus patients.
The prevalence of metabolic syndrome among type II patient is high. Therefore, policymakers, clinicians, and concerned stakeholders shall urge effective strategies in the control, prevention, and management of metabolic syndrome among type II diabetes mellitus.
Peer Review reports
Introduction
The complicated pathophysiologic condition known as the metabolic syndrome is characterised by insulin resistance, hypertension, hyperlipidaemia, and abdominal obesity and which originate primarily from an imbalance between energy expenditure and calorie intake [ 1 ]. Even though the NCEP-ATPIII, IDF, and WHO criteria are the most often utilised clinical criteria for the diagnosis of metabolic syndrome, there are numerous similarities between them, there are also notable differences in the perspectives on the underlying causes of the metabolic syndrome [ 2 ]. The prevalence of metabolic syndrome is increasing in Western and Asian countries, particularly in emerging areas that are undergoing fast socio-environmental change. Numerous studies have demonstrated that metabolic syndrome is a major risk factor for type 2 diabetes mellitus, cardiovascular disease (CVD), and overall mortality [ 3 ].
Global estimates suggest that about one-third of the world’s population, primarily in developing countries, may have metabolic syndrome [ 4 ]. The National Cholesterol Education Programme’s Adult Treatment Panel III report (ATP III) recognised metabolic syndrome as a significant contemporary public health concern. It is a multiplex risk factor for cardiovascular disease (CVD) that requires more therapeutic care, and it also raises the risk of cancer, mental problems, renal illness, and early mortality [ 2 , 5 ].
Compared to those in Western countries, the urban population in several developing nations has a greater prevalence of metabolic syndrome. The two main causes of this disease’s growth are the increase in fast food consumption—high-calorie, low-fiber foods—and the decline in physical activity brought on by sedentary leisure activities and the use of automated transportation [ 1 ].
In Sub-Saharan Africa, metabolic syndrome may be present in more than 70% of people with type 2 diabetes mellitus. According to research which is done in two rural clinics of Ghana, the prevalence of metabolic syndrome among type II diabetes mellitus patients was 68.6% (95% CI: 64.0-72.8), and having diabetes for more than five years, being female, and being overweight are significantly associated with metabolic syndrome [ 4 ]. A study done by Lira Neto JCG et al., Stated that among 201 study participants, 50.7% were diagnosed with metabolic syndrome [ 5 ].
A cross sectional study conducted in Ethiopia reported that the prevalence of metabolic syndrome was 20.3% among 325 study participants [ 6 ]. A Systematic Review and Meta-analysis study conducted in Ethiopia stated that the pooled prevalence of metabolic syndrome in Ethiopia was found to be 34.89% (95% CI: 26.77, 43.01) and 27.92% (95% CI: 21.32, 34.51) by using NCEP/ATP III and IDF criteria, respectively. Subgroup analysis based on the study subjects using NCEP/ATP III showed that the weighted pooled prevalence was 63.78%(95% CI: 56.17, 71.40) among type 2 Diabetes Mellitus patients [ 7 ].
Even though this topic has been studied before, our work is unique since we look at aspects outside prevalence. Thus, our study’s goal was to analyse the pooled prevalence of metabolic syndrome and associated factors among patients with type II diabetes mellitus. How much is the prevalence of metabolic syndrome among Ethiopian patients with type II diabetes mellitus? And what variables are associated with metabolic syndrome patients with type II diabetes in Ethiopian?
Protocol and search strategy
The systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guideline (Fig. 1 ). The study protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews (CRD42023442704). An inclusive literature search was conducted to identify studies about the prevalence of metabolic syndrome among patients with type II diabetes mellitus reported among the Ethiopian population of various study subjects. Both electronic and gray literature searches were carried out systematically. PubMed, Web of Science, Google Scholar, and grey literature, were searched for material between 2013 and 2023. The search terms were used separately and in combination using Boolean operators like “OR” or “AND.” An example of keywords used in PubMed to select relevant studies was as follows: (((“Metabolic Syndrome“[Mesh] OR Metabolic syndrome*[tiab]) AND (“Diabetes Mellitus, Type 2“[Mesh] OR “Diabetes Mellitus, Type 2*“[tiab])) AND (“Prevalence“[Mesh] OR “Magnitude*“[tiab])) AND (“Ethiopia“[Mesh] OR “Ethiopia*“[tiab]). Moreover, each database’s specific search parameters were customized accordingly.
Study selection (inclusion and exclusion criteria)
Inclusion criteria.
Studies were selected according to the following criteria: study design, participants, exposures and condition or outcome(s) of interest. Eligible studies were only quantitative full- text, and observational studies (cross-sectional) reporting prevalence and associated factors in terms of the odds ratio. Only articles written in English were retrieved for review. We included studies involving only type II diabetes mellitus patients aged greater than 30.
The primary outcome was the prevalence of metabolic syndrome. We used author reported definitions (according ATP III &IDF) (Table 1 ). Secondary outcome was factors associated with metabolic syndrome among type 2 diabetes mellitus patients. We were used unpublished articles to identify any potential studies that might have been missed from our search.
Exclusion criteria
We excluded reviews, case reports, case series, qualitative studies, and opinion articles. We were exclude abstract-only papers.

Data extraction and quality assessment
Data extraction was done independently by the two reviewers in a pre-piloted data extraction form created in MS Excel. Any discrepancies in the extracted data were resolved by consensus or discussion with a third reviewer. The following details will be extracted from each study:- details of the study (first author’s last name, year of publication), study region, study design, sample size, Prevalence of metabolic syndrome, associated factors.
The Joanna Brigg Institute’s quality evaluation criteria’s (JBI) were used to evaluate the studies’ quality. We assessed each of the chosen publications using the JBI assessment checklist. Research with a quality score of at least 50% was deemed to be of high quality.
Data analysis
Version 17 of STATA was used to analyse the retrieved data after they were imported into Microsoft Excel. To get a general summary estimate of the prevalence across trials, a random-effects model was employed. We employed point estimate with a 95% confidence interval. Sensitivity analysis was used to evaluate each study’s contribution to the outcome by eliminating each one individually. Using Egger’s test, the existence of publication bias was evaluated. The Cochran’s Q statistic and I2 statistics were used to assess the heterogeneity of the studies. Moreover, meta-regression has been conducted that represents linear predictions for the metabolic syndrome among type 2 diabetes mellitus patients prevalence as a function of published year. Subgroup analysis was performed based on study region and study subjects since there was unexplained significant heterogeneity.
Publication bias
Funnel plot and Egger’s test was used to assess publication bias and a P-value of less than 0.05 was used to declare the publication bias. The included studies were assessed for potential publication bias and separate analyses were done based on IDF and NCEP/ATPIII criteria (p values were 0.58 and 0.88, respectively) which indicated the absence of publication bias (Figs. 2 and 3 ).
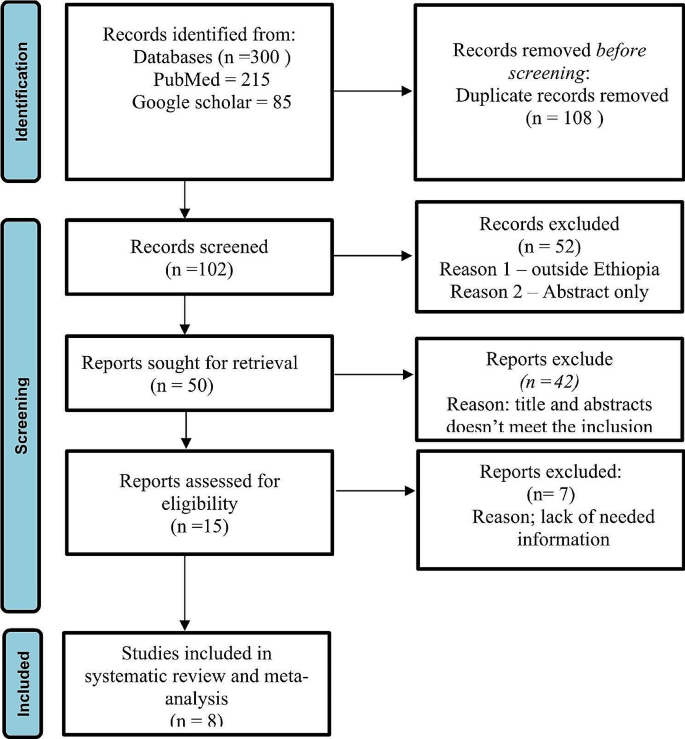
PRISMA flow diagram of study selection for systematic review and meta-analysis of prevalence of metabolic syndrome among type II Diabetes mellitus patients in Ethiopia [ 11 ]
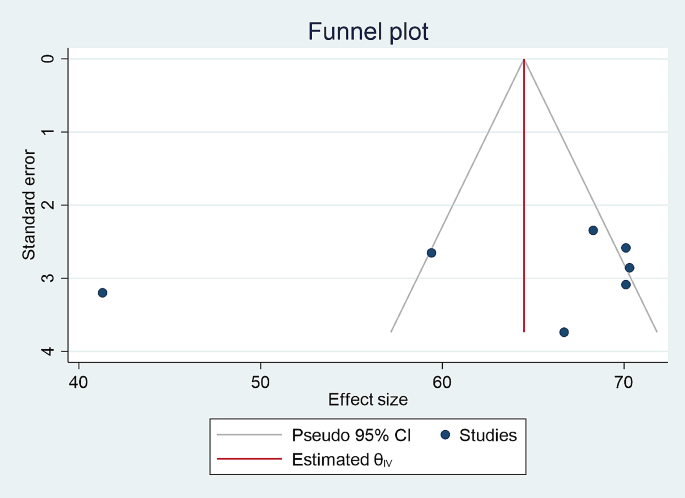
Forest plot showing the pooled prevalence of metabolic syndrome among type II Diabetes mellitus patients in Ethiopia (according to NCEP ATP III Criteria)
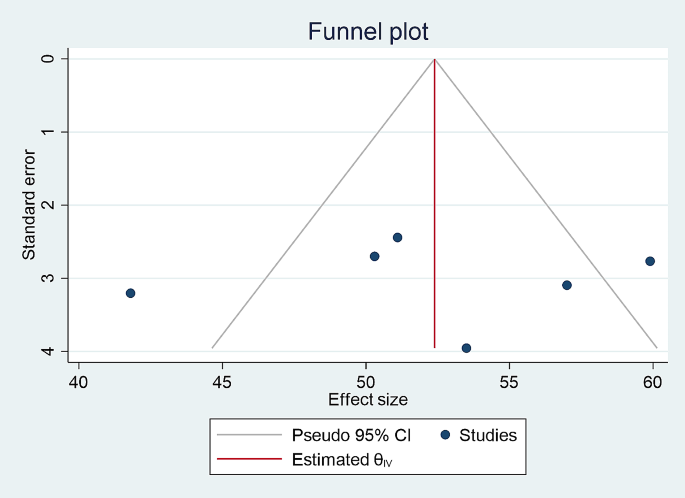
Forest plot showing the pooled prevalence of metabolic syndrome among type II Diabetes mellitus patients in Ethiopia (according to IDF Criteria)
Characteristics of included studies
The title and abstract screening of 300 potential articles yielded 102 that were included relevant to the topic of interest; the full-text screening of 50 of these articles indicated their eligibility for full-text assessment; 8 of these articles, involving 2375 study participants, were found to be eligible for systematic review and meta-analysis. Based on the NCEP/ATPIII and IDF criteria, the prevalence of metabolic syndrome in patients with type 2 diabetes mellitus was assessed among the Ethiopian population of different study participants. Five studies reported the prevalence of metabolic syndrome among type 2 diabetes mellitus based on both IDF and NCEP/ATPIII criteria, whereas seven studies based on NCEP/ATPIII criteria only and six studies by IDF criteria only (Table 2 ).
Prevalence of metabolic syndrome among type 2 diabetes mellitus patients using IDF and NCEP ATP III Criteria
The random-effects model was applied since the heterogeneity index of the studies were significant. The pooled prevalence of metabolic syndrome was found to be 64.49% (95% CI: 62.39, 66.59) by using NCEP/ATP III (Figs. 4 ) and 52.38% (95% CI: 50.05, 54.73) by using IDF criteria (Fig. 5 ). Subgroup analysis based on the study region using NCEP/ ATP III showed that the weighted pooled prevalence was 63.79% (95% CI: 56.48, 71.11) among type 2 diabetes patients (Fig. 6 ). Using IDF criteria, subgroup analysis based on the study region showed that the weighted pooled prevalence was 52.23% (95%CI: 47.37, 57.22).
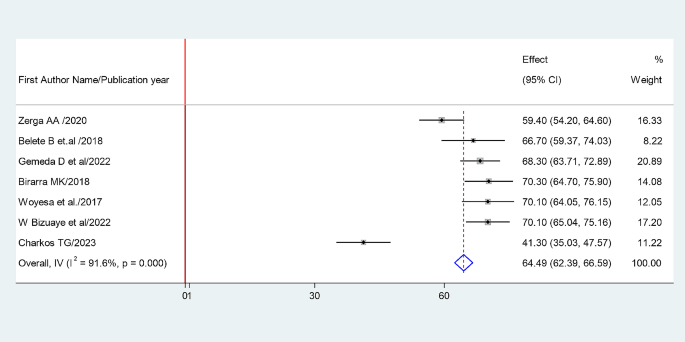
Sub group analysis based on study region using NCEP ATP III Criteria
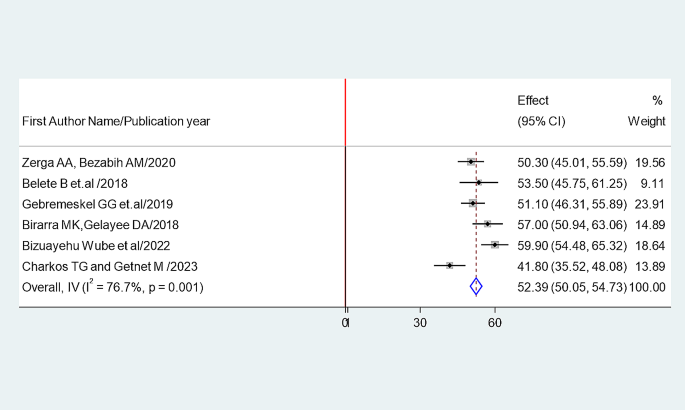
the pooled odds ratio of the association between sex and prevalence of metabolic syndrome among type II diabetes mellitus patients
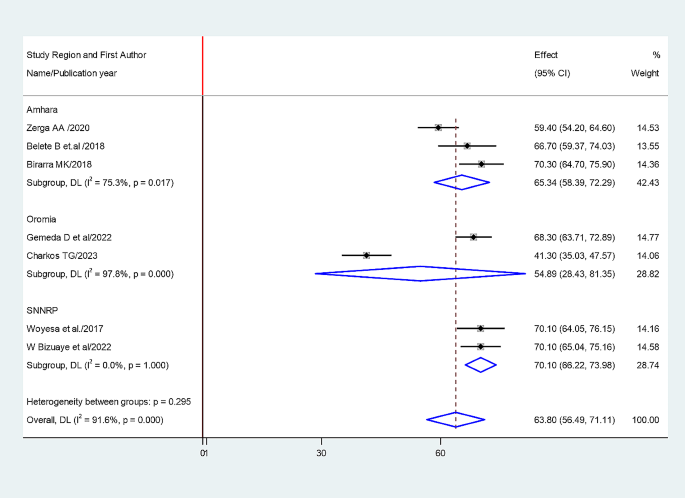
The pooled odds ratio of the association between BMI and prevalence of metabolic
Factors associated with metabolic syndrome among type II DM
Association between sex and prevalence of metabolic syndrome.
The association between being female and prevalence of metabolic syndrome was examined based on the finding from five studies ( 1 , 2 , 4 , 5 and 7 ). The pooled odds ratio (AOR: 0.5, 95% CI: -0.32-1.31) showed that prevalence of metabolic syndrome associated with being female. The studies showed very high heterogeneity (I²=86.3% and P = 0.00) (Fig. 7 ). Hence, a random effects model was employed to do the final analysis.
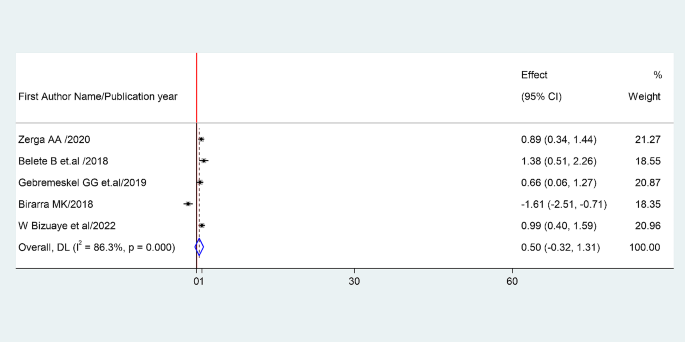
Funnel plot for prevalence of metabolic syndrome according to NCE ATPIII
Association between BMI and prevalence of metabolic syndrome
The association between BMI and prevalence of metabolic syndrome was examined based on the finding from four studies ( 1 , 2 , 4 and 8 ). The pooled odds ratio (AOR: 3.86, 95% CI: 2.57–5.15) showed that prevalence of metabolic syndrome associated with BMI. The studies showed high heterogeneity (I²= 68.2% and P = 0.034) (Fig. 8 ). Hence, a random effects model was employed to do the final analysis.
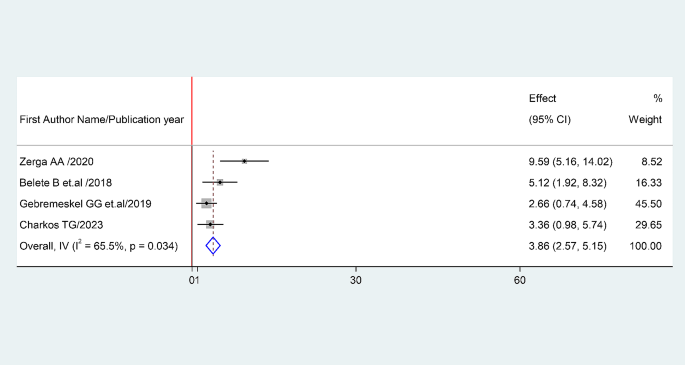
Funnel plot for prevalence of metabolic syndrome according to IDF
Sensitivity analysis
Sensitivity analysis was carried out by gradually removing each research from the analytic process according to the two provided diagnostic criteria (NCEP/ATP III and IDF) in order to evaluate the impact of each study on the pooled estimated prevalence of metabolic syndrome among type II diabetes mellitus patients. The result showed that excluded studies led to significant changes in the shared estimation of the prevalence of metabolic syndrome (Figs. 9 and 10 ).
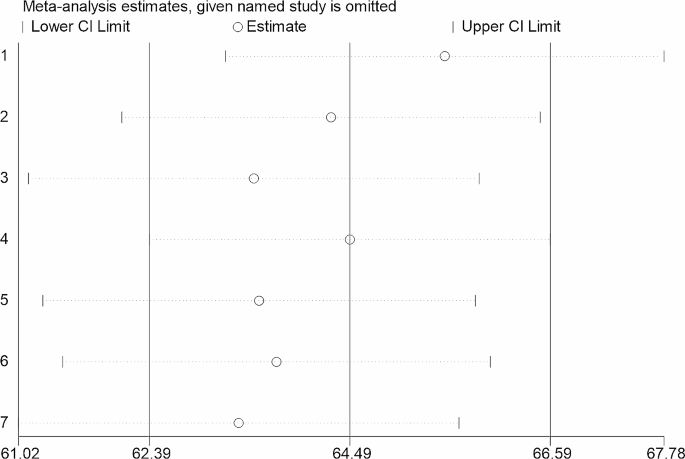
Sensitivity analysis based on NCEP/ATP III diagnostic criteria
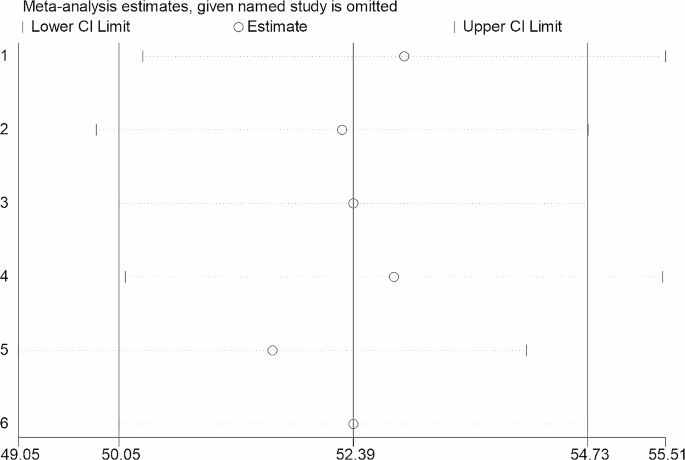
Sensitivity analysis based on IDF diagnostic criteria
This systematic review and meta- analysis study provides evidence of an estimated pooled prevalence of metabolic syndrome among type II diabetes mellitus patients of the Ethiopian population. According to this review, the combined pooled prevalence of Metabolic syndrome among type II diabetes mellitus patients were 64.49% (95% CI: 62.39, 66.59) and 52.38% (95% CI: 50.05, 54.73) by using NCEP/ATP III and IDF criteria, respectively.
The finding of this study similar with the study conducted in African populations, which reported that the prevalence of metabolic syndrome among type 2 diabetes mellitus was 66.9%(95%CI: 60.3–73.1) [ 9 ]. In addition, the study which was done in sub-Saharan African countries in line with this finding which reports that prevalence of metabolic syndrome among type II diabetes melituse patients were 64.8% (95% CI: 54.74, 74.86) and 57.15% by using NCEP/ATP III and IDF criteria, respectively.
Furture more, the study which was done in Sub-Saharan Africa reported that, among others sub-Saharan Africa countries the prevalence of metabolic syndrome was highest in Ethiopia, (61.14%, 95% CI: 51.74, 70.53), which is almost similar with our study findings [ 8 ].
Similarly the study conducted in Ethiopian population showed that the weighted pooled prevalence of metabolic syndrome among type II diabetes mellitus patient was 63.78% (95% CI: 56.17, 71.40) [ 7 ].
This study result supported by the fact that metabolic syndrome has been associated with type 2 diabetes due to its high prevalence worldwide since it is both related to the increase in obesity and a sedentary lifestyle. Several studies suggest that individuals with Metabolic syndrome are 5 times more likely to develop type 2 diabetes [ 10 ].
This meta-analysis assessed factors determining metabolic syndrome among type II diabetes mellitus patients. The current meta-analysis demonstrated that the prevalence rate of metabolic syndrome was higher in females compared to that in males. This has been shown in all the Middle Eastern countries, and the prevalence was much higher among women than men [ 6 ]. The prevalence rate of metabolic syndrome associated with the individual’s body mass index.
This study has implications for clinical practice. Determining the prevalence of metabolic syndrome among type 2 diabetic patients is critical to guide healthcare professionals to minimize the risk of metabolic syndrome by providing guidance to the patient who has undergone diabetic care follow up. Moreover, it gives information about the burden and public health impact of metabolic syndrome for possible consideration during routine diabetic patient care.
This meta-analysis study has its own limitations that should be considered in the future research. Few studies are included due to limited research in Ethiopia which makes it difficult to generalize the findings to all type 2 diabetic patients in the countery and which makes the discussion part more shallow.
In conclusion, according to this systematic review the prevalence of metabolic syndrome among type II patient is high in Ethiopia and recommends an urgent attention from both the clinical and public health viewpoint Therefore, policymakers, clinicians, and concerned stakeholders shall urge effective strategies in the control, prevention, and management of metabolic syndrome among type II diabetes mellitus. In addition country context-specific preventive strategies should be developed to reduce the burden of metabolic syndrome.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
Cardiovascular Disease
National Cholesterol Education Program–Adult Treatment Panel III
International diabetes federation
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
World Health Organization
- Metabolic syndrome
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. https://doi.org/10.1007/s11906-018-0812-z . PMID: 29480368; PMCID: PMC5866840.
Article PubMed PubMed Central Google Scholar
Grundy et al. Definition of metabolic syndrome, January 27, 2004, https://doi.org/10.1161/01.CIR.0000111245.75752.C6 .
Shin JA, Lee JH, Lim SY, Ha HS, Kwon HS, Park YM, Lee WC, Kang MI, Yim HW, Yoon KH, Son HY. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. 2013;4(4):334–43. https://doi.org/10.1111/jdi.12075 . Epub 2013 May 28. PMID: 24843675; PMCID: PMC4020225.
Article CAS PubMed PubMed Central Google Scholar
Abagre, et al. Determinants of metabolic syndrome among patients attending diabetes clinics in two sub-urban hospitals: Bono Region, Ghana. BMC Cardiovasc Disord. 2022;22:366. https://doi.org/10.1186/s12872-022-02805-4 .
Lira Neto JCG, Xavier MA, Borges JWP, Araújo MFM, Damasceno MMC, Freitas RWJF. Prevalence of metabolic syndrome in individuals with type 2 diabetes Mellitus. Rev Bras Enferm [Internet]. 2017;70(2):265–70. https://doi.org/10.1590/0034-7167-2016-0145 .
Article PubMed Google Scholar
Samrawit Solomon and Wudeneh Mulugeta. Disease burden and associated risk factors for metabolic syndrome among adults in Ethiopia. BMC Cardiovasc Disord. 2019;19:236. https://doi.org/10.1186/s12872-019-1201-5 .
Article CAS PubMed Google Scholar
Sintayehu Ambachew A, Endalamaw A, Worede Y, Tegegne M, Melku B, Biadgo. The Prevalence of Metabolic Syndrome in Ethiopian Population: A Systematic Review and Meta-analysis, Journal of Obesity , vol. 2020, Article ID 2701309, 14 pages, 2020. https://doi.org/10.1155/2020/2701309 .
Shiferaw WS, Akalu TY, Gedefaw M, Anthony D, Kassie AM. Worku Misganaw Kebede, Henok Mulugeta, Getenet Dessie, Yared Asmare Aynalem, Metabolic syndrome among type 2 diabetic patients in Sub-Saharan African countries: A systematic review and meta-analysis,Diabetes & Metabolic Syndrome: Clinical Research & Reviews, Volume 14, Issue 5, 2020, Pages 1403–1411, ISSN 1871–4021, https://doi.org/10.1016/j.dsx.2020.07.013 .
Bowo-Ngandji A. Prevalence of the metabolic syndrome in African populations: a systematic review and meta-analysis. PLoS ONE. 2023;18(7):e0289155. https://doi.org/10.1371/journal.pone.0289155 . PMID: 37498832; PMCID: PMC10374159.
Regufe VMG, Pinto CMCB, Perez PMVHC. Metabolic syndrome in type 2 diabetic patients: a review of current evidence. Porto Biomed J. 2020;5(6):e101. https://doi.org/10.1097/j.pbj.0000000000000101 . PMID: 33299950; PMCID: PMC7721212.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71 .
Download references
Not applicable.
Author information
Authors and affiliations.
Department of Nursing, School of Nursing, College of Health Sciences, Woldia University, North Wollo, Amhara, Ethiopia
Betelhem Mesfin Demissie
Department of Pharmacy, College of Health Sciences, Woldia University, North Wollo, Amhara, Ethiopia
Fentaw Girmaw & Getachew Ashagrie
Department of Nursing, College of Health Sciences, Ambo University, Oromia, Ethiopia
Nimona Amena
You can also search for this author in PubMed Google Scholar
Contributions
“B.M involved in the entire work of the manuscript which is a design of the study, review of literature, and data analysis, “F.G.” was involved in, design of the study, review of the literature, data extraction, and statistical analysis. “N.A”. and “G.A” were involved in data analysis and interpretation and drafting of the manuscript. All authors critically revised the paper, and they agreed to be accountable for all aspects of the work.
Corresponding author
Correspondence to Betelhem Mesfin Demissie .
Ethics declarations
Ethical approval and consent to participate, consent for publication, conflict of interest.
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Demissie, B.M., Girmaw, F., Amena, N. et al. Prevalence of metabolic syndrome and associated factors among patient with type 2 diabetes mellitus in Ethiopia, 2023: asystematic review and meta analysis. BMC Public Health 24 , 1128 (2024). https://doi.org/10.1186/s12889-024-18580-0
Download citation
Received : 10 November 2023
Accepted : 12 April 2024
Published : 23 April 2024
DOI : https://doi.org/10.1186/s12889-024-18580-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type II diabetes mellitus
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Self-Monitoring and Managing Medications Improved High Blood Pressure
- Original Investigation Home BP Self-Monitoring Plus Self-Titration for Patients With Hypertension Patricia Martínez-Ibáñez, PhD; Irene Marco-Moreno, PhD; Aníbal García-Sempere, PhD; Salvador Peiró, PhD; Lucia Martínez-Ibáñez, MD; Ignacio Barreira-Franch, MD; Laura Bellot-Pujalte, MD; Eugenia Avelino-Hidalgo, MD; Marina Escrig-Veses, MD; María Bóveda-García, MD; Mercedes Calleja-del-Ser, MD; Celia Robles-Cabaniñas, BBioTech; Isabel Hurtado, PhD; Clara L. Rodríguez-Bernal, PhD; Margarita Giménez-Loreiro, MD; Gabriel Sanfélix-Gimeno, PhD; José Sanfélix-Genovés, PhD; ADAMPA Research Group; Joaquín Abad Carrasco; Maria Virginia Agudo Escagüés; Jorge Navarro-Perez; Rosa Maria Bartual Penella; Rosa Carrión Villanueva; Ana Costa Alcaraz; Isabel Cristófol López; Rosario González Candelas; Ricardo González Espadas; Luis González Luján; Victoria Gosalbes; Enrique Guinot Martínez; Emilio Luis López Torres; Silvia Molla LLosa; Víctor Moreno Comins; Miriam Moreno Prat; Mª José Puchades Company; Ángela Ramos García; Paloma Ramos Ruiz; Pilar Roca Navarro; Rosa Saiz Rodriguez; Julia Lorena Salanova Chilet; Ana Tchang Sanchez; Francisca Torres Asensi; Ruth Uribes Fillol; Cristina Valle García; Macarena Villar Ruiz; Marta Alcocer Escribano; Laura Almudever Campo; Lorena Cruz Bautista; Mª Begoña Fuertes Fernandez; Victor García Olivencia; Carmen Molla Orts; María José Muñoz Sanchíz; Francisca Osuna Sabariego; Emilia Ramón Carretero; Pilar Roca Roda; Esther Rodriguez García; Maria Rosa Serrada Iranzo; Eva Sierra García; Adina A Iftimi; Andreu Ferrero-Gregori JAMA Network Open
People who monitored their blood pressure at home and modified their medications based on the readings lowered their blood pressure more than those in a control group who received routine blood pressure management from a physician, according to results from a randomized clinical trial involving 219 patients in Spain. The participants were aged 40 years or older and had a systolic blood pressure of more than 145 mm Hg, a diastolic blood pressure of more than 90 mm Hg, or both. Both groups also received an educational booklet on reducing blood pressure.
Without consulting a clinician, participants titrated their medications if their daily readings were over the target range for 4 or more consecutive days. At the 2-year follow-up, they’d experienced an average 3.4-mm Hg decrease in systolic blood pressure and an average 2.5-mm Hg decrease in diastolic blood pressure relative to those in the control group. About 39% increased their medication dose during the trial and 55% added a new drug at least once. There was no difference in adverse events between groups.
The findings suggest that “simple, inexpensive, and easy-to-implement self-management interventions have the potential to improve the long-term control of hypertension in routine clinical practice,” the researchers wrote in JAMA Network Open .
Published Online: June 7, 2024. doi:10.1001/jama.2024.10331
See More About
Harris E. Self-Monitoring and Managing Medications Improved High Blood Pressure. JAMA. Published online June 07, 2024. doi:10.1001/jama.2024.10331
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 11 October 2023
Endocrine causes of hypertension: literature review and practical approach
- Jean-Baptiste de Freminville 1 , 2 ,
- Laurence Amar 1 , 2 ,
- Michel Azizi 1 , 2 &
- Julien Mallart-Riancho 1 , 2
Hypertension Research volume 46 , pages 2679–2692 ( 2023 ) Cite this article
602 Accesses
2 Citations
38 Altmetric
Metrics details
Hypertension (HTN) affects more than 30% of adults worldwide. It is the most frequent modifiable cardiovascular (CV) risk factor, and is responsible for more than 10 million death every year. Among patients with HTN, we usually distinguish secondary HTN, that is HTN due to an identified cause, and primary HTN, in which no underlying cause has been found. It is estimated that secondary hypertension represents between 5 and 15% of hypertensive patients [ 1 ]. Therefore, routine screening of patients for secondary HTN would be too costly and is not recommended. In addition to the presence of signs suggesting a specific secondary cause, screening is based on specific criteria. Identifying secondary HTN can be beneficial for patients in certain situations, because it may lead to specific treatments, and allow better control of blood pressure and sometimes even a cure. Besides, it is now known that secondary HTN are more associated with morbidity and mortality than primary HTN. The main causes of secondary HTN are endocrine and renovascular (mainly due to renal arteries abnormalities). The most frequent endocrine cause is primary aldosteronism, which diagnosis can lead to specific therapies. Pheochromocytoma and Cushing syndrome also are important causes, and can have serious complications. Other causes are less frequent and can be suspected on specific situations. In this article, we will describe the endocrine causes of HTN and discuss their treatments.
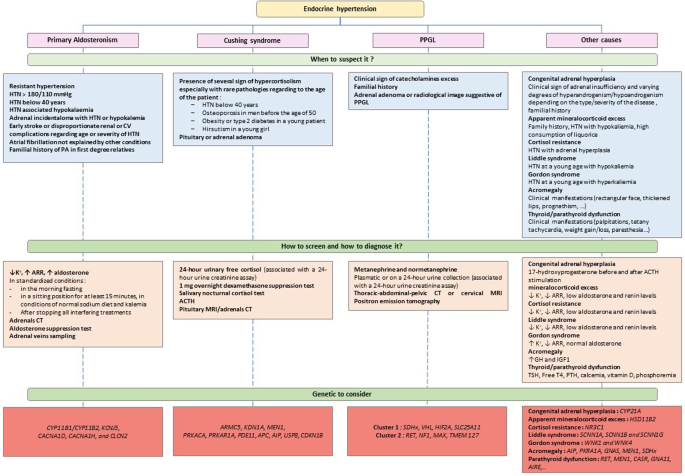
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Prevalence of hypertension and pre-hypertension in the Middle East region: a systematic review & meta-analysis
The burden of hypertension in Ecuador: a systematic review and meta-analysis
Hypertension trials update
Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245–54.
Google Scholar
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37.
CAS PubMed PubMed Central Google Scholar
Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global Burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317:165–82.
PubMed Google Scholar
de Freminville J-B, Amar L. How to explore an endocrine cause of hypertension. JCM. 2022;11:420.
PubMed PubMed Central Google Scholar
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–57.
CAS PubMed Google Scholar
Mancia Chairperson G, Kreutz Co-Chair R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. Published Online First: 21 June 2023. https://doi.org/10.1097/HJH.0000000000003480 .
Braun LT, Vogel F, Reincke M. Long-term morbidity and mortality in patients with Cushing’s syndrome. J Neuroendocrinol. 2022;34:e13113.
Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50.
Januszewicz A, Mulatero P, Dobrowolski P, Monticone S, Van der Niepen P, Sarafidis P, et al. Cardiac phenotypes in secondary hypertension. J Am Coll Cardiol. 2022;80:1480–97.
Conn JW. Plasma renin activity in primary aldosteronism: importance in differential diagnosis and in research of essential hypertension. JAMA. 1964;190:222–5.
Conn JW, Cohen EL, Rovner DR, Nesbit RM. Normokalemic primary aldosteronism: a detectable cause of curable “Essential” hypertension. JAMA. 1965;193:200–6.
Gordon RD, Ziesak MD, Tunny TJ, Stowasser M, Klemm SA. Evidence That Primary Aldosteronism May Not Be Uncommon: 12% Incidence Among Antihypertensive Drug Trial Volunteers. Clin Exp Pharmacol Physiol. 1993;20:296–8.
Anderson GH, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609–15.
Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Rutherford JC. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharm Physiol. 1994;21:315–8.
CAS Google Scholar
Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300.
Plouin P-F, Amar L, Chatellier G, on behalf of the COMETE-Conn Study Group. Trends in the prevalence of primary aldosteronism, aldosterone-producing adenomas, and surgically correctable aldosterone-dependent hypertension. Nephrol Dialysis Transplant. 2004;19:774–7.
Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies–a review of the current literature. Horm Metab Res. 2012;44:157–62.
Buffolo F, Monticone S, Burrello J, Tetti M, Veglio F, Williams TA, et al. Is primary Aldosteronism still largely unrecognized? Horm Metab Res. 2017;49:908–14.
Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–20.
Parasiliti-Caprino M, Lopez C, Prencipe N, Lucatello B, Settanni F, Giraudo G, et al. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J Hypertens. 2020;38:1841–8.
Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173:10–20.
Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021;9:876–92.
Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371:1921–6.
Burrello J, Monticone S, Losano I, Cavaglià G, Buffolo F, Tetti M, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension. 2020;75:1025–33.
Mulatero P, Stowasser M, Loh K-C, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–50.
Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond). 2007;113:267–78.
Stowasser M, Sharman J, Leano R, Gordon RD, Ward G, Cowley D, et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J Clin Endocrinol Metab. 2005;90:5070–6.
Chang Y-Y, Liao C-W, Tsai C-H, Chen C-W, Pan C-T, Chen Z-W, et al. Left ventricular dysfunction in patients with primary aldosteronism: a propensity score-matching follow-up study with tissue doppler imaging. J Am Heart Assoc. 2019;8:e013263.
Hung C-S, Chou C-H, Liao C-W, Lin Y-T, Wu X-M, Chang Y-Y, et al. Aldosterone induces tissue inhibitor of Metalloproteinases-1 expression and further contributes to collagen accumulation: from clinical to bench studies. Hypertension. 2016;67:1309–20.
Parksook WW, Williams GH. Aldosterone and cardiovascular diseases. Cardiovasc Res. 2022; cvac027.
van der Heijden CDCC, Smeets EMM, Aarntzen EHJG, Noz MP, Monajemi H, Kersten S, et al. Arterial wall inflammation and increased hematopoietic activity in patients with primary aldosteronism. J Clin Endocrinol Metab. 2020;105:e1967–80.
Demirkiran A, Everaars H, Elitok A, van de Ven PM, Smulders YM, Dreijerink KM, et al. Hypertension with primary aldosteronism is associated with increased carotid intima-media thickness and endothelial dysfunction. J Clin Hypertens (Greenwich). 2019;21:932–41.
Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, et al. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232–8.
Monticone S, Sconfienza E, D’Ascenzo F, Buffolo F, Satoh F, Sechi LA, et al. Renal damage in primary aldosteronism: a systematic review and meta-analysis. J Hypertens. 2020;38:3–12.
Huby A-C, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation. 2015;132:2134–45.
Spyroglou A, Handgriff L, Müller L, Schwarzlmüller P, Parasiliti-Caprino M, Fuss CT, et al. The metabolic phenotype of patients with primary aldosteronism: impact of subtype and sex - a multicenter-study of 3566 Caucasian and Asian subjects. Eur J Endocrinol. 2022;187:361–72.
Wolley MJ, Pimenta E, Calhoun D, Gordon RD, Cowley D, Stowasser M. Treatment of primary aldosteronism is associated with a reduction in the severity of obstructive sleep apnoea. J Hum Hypertens. 2017;31:561–7.
Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. 2014;63:20–31.
Savard S, Amar L, Plouin P-F, Steichen O. Cardiovascular complications associated with primary aldosteronism. Hypertension. 2013;62:331–6.
Milliez P, Girerd X, Plouin P-F, Blacher J, Safar ME, Mourad J-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–8.
Buffolo F, Tetti M, Mulatero P, Monticone S. Aldosterone as a mediator of cardiovascular damage. Hypertension. 2022;79:1899–911.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–9.
Inoue K, Goldwater D, Allison M, Seeman T, Kestenbaum BR, Watson KE. Serum aldosterone concentration, blood pressure, and coronary artery calcium: The multi-ethnic study of atherosclerosis. Hypertension. 2020;76:113–20.
Amar L, Baguet JP, Bardet S, Chaffanjon P, Chamontin B, Douillard C, et al. SFE/SFHTA/AFCE primary aldosteronism consensus: Introduction and handbook. Ann d’Endocrinologie. 2016;77:179–86.
Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro M-C, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the working group on endocrine hypertension of the european society of hypertension ∗ . J Hypertens. 2020;38:1919–28.
Seccia TM, Letizia C, Muiesan ML, Lerco S, Cesari M, Bisogni V, et al. Atrial fibrillation as presenting sign of primary aldosteronism: results of the Prospective Appraisal on the Prevalence of Primary Aldosteronism in Hypertensive (PAPPHY) Study. J Hypertens. 2020;38:332–9.
Mosso L, Carvajal C, González A, Barraza A, Avila F, Montero J, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42:161–5.
Maiolino G, Rossitto G, Bisogni V, Cesari M, Seccia TM, Plebani M, et al. Quantitative value of aldosterone‐renin ratio for detection of aldosterone‐producing adenoma: the Aldosterone‐Renin Ratio for Primary Aldosteronism (AQUARR) Study. J Am Heart Assoc. 2017;6:e005574.
Reznik Y, Amar L, Tabarin A. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 3: Confirmatory testing. Ann Endocrinol (Paris). 2016;77:202–7.
Baron S, Amar L, Faucon A-L, Blanchard A, Baffalie L, Faucard C, et al. Criteria for diagnosing primary aldosteronism on the basis of liquid chromatography-tandem mass spectrometry determinations of plasma aldosterone concentration. J Hypertens. 2018; 36. https://doi.org/10.1097/HJH.0000000000001735 .
Funder JW. Primary aldosteronism. Hypertension. 2019;74:458–66.
Douillard C, Houillier P, Nussberger J, Girerd X. SFE/SFHTA/AFCE Consensus on Primary Aldosteronism, part 2: First diagnostic steps. Ann Endocrinol (Paris). 2016;77:192–201.
Aldea ML, Barallat J, Martín MA, Rosas I, Pastor MC, Granada ML. Sodium interference in the determination of urinary aldosterone. Clin Biochem. 2016;49:295–7.
Lin DC, Raizman JE, Holmes DT, Don-Wauchope AC, Yip PM. Evaluation of a chemiluminescent immunoassay for urinary aldosterone on the DiaSorin LIAISON automated platform against RIA and LC-MS/MS. Clin Chem Lab Med. 2017;55:e181–3.
Alnazer RM, Veldhuizen GP, Kroon AA, de Leeuw PW. The effect of medication on the aldosterone-to-renin ratio. A critical review of the literature. J Clin Hypertens (Greenwich). 2021;23:208–14.
Veldhuizen GP, Alnazer RM, de Leeuw PW, Kroon AA. The Effects of Verapamil, Hydralazine, and Doxazosin on Renin, Aldosterone, and the Ratio Thereof. Cardiovasc Drugs Ther. Published Online First: 13 September 2021. https://doi.org/10.1007/s10557-021-07262-3 .
Beeftink MMA, van der Sande NGC, Bots ML, Doevendans PA, Blankestijn PJ, Visseren FLJ, et al. Safety of Temporary Discontinuation of Antihypertensive Medication in Patients With Difficult-to-Control Hypertension. Hypertension. 2017;69:927–32.
Zennaro M-C, Boulkroun S, Fernandes-Rosa FL. Pathogenesis and treatment of primary aldosteronism. Nat Rev Endocrinol. 2020;16:578–89.
Boulkroun S, Fernandes-Rosa FL, Zennaro M-C. Old and new genes in primary aldosteronism. Best Pr Res Clin Endocrinol Metab. 2020;34:101375.
Mulatero P, Sechi LA, Williams TA, Lenders JWM, Reincke M, Satoh F, et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension ∗ . J Hypertens. 2020;38:1929–36.
Bardet S, Chamontin B, Douillard C, Pagny J-Y, Hernigou A, Joffre F, et al. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 4: Subtype diagnosis. Ann Endocrinol (Paris). 2016;77:208–13.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916.
Kempers MJE, Lenders JWM, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus ARMM, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–37.
Rossi GP, Crimì F, Rossitto G, Amar L, Azizi M, Riester A, et al. Feasibility of imaging-guided adrenalectomy in young patients with primary aldosteronism. Hypertension. 2022;79:187–95.
Schloetelburg W, Ebert I, Petritsch B, Weng AM, Dischinger U, Kircher S, et al. Adrenal wash-out CT: moderate diagnostic value in distinguishing benign from malignant adrenal masses. Eur J Endocrinol. 2021;186:183–93.
Gao Y, Ding J, Cui Y, Li T, Sun H, Zhao D, et al. Functional nodules in primary aldosteronism: identification of CXCR4 expression with 68Ga-pentixafor PET/CT. Eur Radio. 2023;33:996–1003.
Heinze B, Fuss CT, Mulatero P, Beuschlein F, Reincke M, Mustafa M, et al. Targeting CXCR4 (CXC Chemokine Receptor Type 4) for Molecular Imaging of Aldosterone-Producing Adenoma. Hypertension. 2018;71:317–25.
Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, et al. Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension. 2016;67:139–45.
De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75:1034–44.
Le Floch E, Cosentino T, Larsen CK, Beuschlein F, Reincke M, Amar L, et al. Identification of risk loci for primary aldosteronism in genome-wide association studies. Nat Commun. 2022;13:5198.
Steichen O, Zinzindohoué F, Plouin P-F, Amar L. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review. Horm Metab Res. 2012;44:221–7.
Williams TA, Gong S, Tsurutani Y, Tezuka Y, Thuzar M, Burrello J, et al. Adrenal surgery for bilateral primary aldosteronism: an international retrospective cohort study. Lancet Diabetes Endocrinol. 2022;10:769–71.
Lim PO, Jung RT, MacDonald TM. Raised aldosterone to renin ratio predicts antihypertensive efficacy of spironolactone: a prospective cohort follow-up study. Br J Clin Pharm. 1999;48:756–60.
Parthasarathy HK, Ménard J, White WB, Young WF, Williams GH, Williams B, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29:980–90.
Fagart J, Hillisch A, Huyet J, Bärfacker L, Fay M, Pleiss U, et al. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem. 2010;285:29932–40.
PubMed Central Google Scholar
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in Type 2 diabetes. N. Engl J Med. 2020;383:2219–29.
Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and Type 2 diabetes. N. Engl J Med. 2021;385:2252–63.
Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43:474–84.
Bogman K, Schwab D, Delporte M-L, Palermo G, Amrein K, Mohr S, et al. Preclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone Synthase (CYP11B2). Hypertension. 2017;69:189–96.
Azizi M, L Amar L, Menard J. Aldosterone synthase inhibition in humans. Nephrol, dialysis, Transpl.: official publication of the European Dialysis and Transplant Association - European Renal Association 2013; 28. https://doi.org/10.1093/ndt/gfs388 .
Freeman MW, Halvorsen Y-D, Marshall W, Pater M, Isaacsohn J, Pearce C, et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388:395–405.
Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. <B>Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan</B>. Hypertens Res. 2004;27:193–202.
Lenders JWM, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–75.
Amar L, Servais A, Gimenez-Roqueplo A-P, Zinzindohoue F, Chatellier G, Plouin P-F. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90:2110–6.
Giavarini A, Chedid A, Bobrie G, Plouin P-F, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99:1438–44.
Zelinka T, Petrák O, Turková H, Holaj R, Štrauch B, Kršek M, et al. High Incidence of Cardiovascular Complications in Pheochromocytoma. Horm Metab Res. 2012;44:379–84.
Dobrowolski P, Januszewicz A, Klisiewicz A, Gosk-Przybyłek M, Pęczkowska M, Kabat M, et al. Left ventricular structural and functional alterations in patients with pheochromocytoma/paraganglioma before and after surgery. JACC Cardiovasc Imaging. 2020;13:2498–509.
Robertson V, Poli F, Hobson B, Saratzis A, Ross Naylor A. A systematic review and meta-analysis of the presentation and surgical management of patients with carotid body tumours. Eur J Vasc Endovasc Surg. 2019;57:477–86.
Lenders JWM, Duh Q-Y, Eisenhofer G, Gimenez-Roqueplo A-P, Grebe SKG, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42.
Eisenhofer G, Peitzsch M, Kaden D, Langton K, Mangelis A, Pamporaki C, et al. Reference intervals for LC-MS/MS measurements of plasma free, urinary free and urinary acid-hydrolyzed deconjugated normetanephrine, metanephrine and methoxytyramine. Clin Chim Acta. 2019;490:46–54.
Därr R, Kuhn M, Bode C, Bornstein SR, Pacak K, Lenders JWM, et al. Accuracy of recommended sampling and assay methods for the determination of plasma-free and urinary fractionated metanephrines in the diagnosis of pheochromocytoma and paraganglioma: a systematic review. Endocrine. 2017;56:495–503.
Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo A-P, Grossman AB, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pr Endocrinol Metab. 2007;3:92–102.
Boyle JG, Davidson DF, Perry CG, Connell JMC. Comparison of diagnostic accuracy of urinary free metanephrines, vanillyl mandelic Acid, and catecholamines and plasma catecholamines for diagnosis of pheochromocytoma. J Clin Endocrinol Metab. 2007;92:4602–8.
Pamporaki C, Prejbisz A, Małecki R, Pistrosch F, Peitzsch M, Bishoff S, et al. Optimized procedures for testing plasma metanephrines in patients on hemodialysis. Sci Rep. 2021;11:14706.
Därr R, Pamporaki C, Peitzsch M, Miehle K, Prejbisz A, Peczkowska M, et al. Biochemical diagnosis of phaeochromocytoma using plasma-free normetanephrine, metanephrine and methoxytyramine: importance of supine sampling under fasting conditions. Clin Endocrinol (Oxf). 2014;80:478–86.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041.
Lenders JWM, Kerstens MN, Amar L, Prejbisz A, Robledo M, Taieb D, et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020;38:1443–56.
Plouin P-F, Gimenez-Roqueplo A-P. Pheochromocytomas and secreting paragangliomas. Orphanet J Rare Dis. 2006;1:49.
Timmers HJLM, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–67.
Taïeb D, Hicks RJ, Hindié E, Guillet BA, Avram A, Ghedini P, et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2019;46:2112–37.
Timmers HJLM, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012;104:700–8.
Amar L, Lussey-Lepoutre C, Lenders JWM, Djadi-Prat J, Plouin P-F, Steichen O. Management of endocrine disease: Recurrence or new tumors after complete resection of pheochromocytomas and paragangliomas: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175:R135–45.
Favier J, Amar L, Gimenez-Roqueplo A-P. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11:101–11.
Burnichon N, Buffet A, Gimenez-Roqueplo A-P. Pheochromocytoma and paraganglioma: molecular testing and personalized medicine. Curr Opin Oncol. 2016;28:5–10.
Tian J, Bao Z, Yuan Y, Fang D, Zhan Y, Wang T, et al. The duration of preoperative administration of single α-Receptor Blocker Phenoxybenzamine before Adrenalectomy for Pheochromocytoma: 18 Years of Clinical Experience from Nationwide High-Volume Center. Biomed Res Int. 2019;2019:2613137.
Castinetti F, De Freminville JB, Guerin C, Cornu E, Sarlon G, Amar L. Controversies about the systematic preoperative pharmacological treatment before pheochromocytoma or paraganglioma surgery. Eur J Endocrinol. 2022;186:D17–24.
Gruber LM, Jasim S, Ducharme-Smith A, Weingarten T, Young WF, Bancos I. The Role for Metyrosine in the Treatment of Patients With Pheochromocytoma and Paraganglioma. J Clin Endocrinol Metab. 2021;106:e2393–2401.
Wengander S, Trimpou P, Papakokkinou E, Ragnarsson O. The incidence of endogenous Cushing’s syndrome in the modern era. Clin Endocrinol. 2019;91:263–70.
Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BMK, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4:611–29.
Graversen D, Vestergaard P, Stochholm K, Gravholt CH, Jørgensen JOL. Mortality in Cushing’s syndrome: a systematic review and meta-analysis. Eur J Intern Med. 2012;23:278–82.
Deutschbein T, Reimondo G, Dalmazi GD, Bancos I, Patrova J, Vassiliadi DA, et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: an international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022;10:499–508.
Cicala MV, Mantero F. Hypertension in Cushing’s syndrome: from pathogenesis to treatment. Neuroendocrinology. 2010;92:44–9.
Frey FJ, Odermatt A, Frey BM. Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens. 2004;13:451–8.
Lugat A, Lasolle H, François M, Benhenda N, Bricaire L, Cornu E, et al. Pneumocystis pneumonia in patients with Cushing’s syndrome: a French multicenter retrospective study. Ann Endocrinol (Paris). 2022;S0003-4266:00836–8.
Van Zaane B, Nur E, Squizzato A, Dekkers OM, Twickler MTB, Fliers E, et al. Hypercoagulable state in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. 2009;94:2743–50.
Tabarin A, Assié G, Barat P, Bonnet F, Bonneville JF, Borson-Chazot F, et al. Consensus statement by the French Society of Endocrinology (SFE) and French Society of Pediatric Endocrinology & Diabetology (SFEDP) on diagnosis of Cushing’s syndrome. Ann d’Endocrinologie. 2022;83:119–41.
Nieman, Biller BMK LK, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526–40.
CAS PubMed Central Google Scholar
Braun LT, Vogel F, Zopp S, Marchant Seiter T, Rubinstein G, Berr CM, et al. Whom should we screen for Cushing syndrome? The Endocrine Society Practice Guideline Recommendations 2008 revisited. J Clin Endocrinol Metab. 2022;107:e3723–30.
Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9:847–75.
Galm BP, Qiao N, Klibanski A, Biller BMK, Tritos NA. Accuracy of laboratory tests for the diagnosis of Cushing syndrome. J Clin Endocrinol Metab. 2020;105:2081–94.
Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, et al. The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165:383–92.
Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367:1605–17.
Steffensen C, Bak AM, Rubeck KZ, Jørgensen JOL. Epidemiology of Cushing’s syndrome. NEN. 2010;92:1–5.
Aresta C, Favero V, Morelli V, Giovanelli L, Parazzoli C, Falchetti A, et al. Cardiovascular complications of mild autonomous cortisol secretion. Best Pract Res Clin Endocrinol Metab. 2021;35:101494.
Hakami OA, Ahmed S, Karavitaki N. Epidemiology and mortality of Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab. 2021;35:101521.
Rubinstein G, Osswald A, Braun LT, Vogel F, Kroiss M, Pilz S, et al. The role of adrenal venous sampling (AVS) in primary bilateral macronodular adrenocortical hyperplasia (PBMAH): a study of 16 patients. Endocrine. 2022;76:434–45.
Bertherat J, Bourdeau I, Bouys L, Chasseloup F, Kamenický P, Lacroix A. Clinical, pathophysiologic, genetic, and therapeutic progress in primary bilateral macronodular adrenal hyperplasia. Endocr Rev. 2023;44:567–628.
Theodoropoulou M, Reincke M. Genetics of Cushing’s disease: from the lab to clinical practice. Pituitary. 2022;25:689–92.
Fleseriu M, Biller BMK. Treatment of Cushing’s syndrome with osilodrostat: practical applications of recent studies with case examples. Pituitary. 2022;25:795–809.
Brue T, Rahabi H, Barry A, Barlier A, Bertherat J, Borson-Chazot F, et al. Position statement on the diagnosis and management of acromegaly: the French National Diagnosis and Treatment Protocol (NDTP). Annales d’Endocrinologie 2023;S0003426623006868.
Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM, et al. Acromegaly. Nat Rev Dis Prim. 2019;5:20.
Maione L, Chanson P. National acromegaly registries. Best Pract Res Clin Endocrinol Metab. 2019;33:101264.
Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, et al. A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab. 2020;105:e937–46.
González B, Vargas G, De Los Monteros ALE, Mendoza V, Mercado M. Persistence of diabetes and hypertension after multimodal treatment of acromegaly. J Clin Endocrinol Metab. 2018;103:2369–75.
Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89:667–74.
Garby L, Caron P, Claustrat F, Chanson P, Tabarin A, Rohmer V, et al. Clinical Characteristics and Outcome of Acromegaly Induced by Ectopic Secretion of Growth Hormone-Releasing Hormone (GHRH): A French Nationwide Series of 21 Cases. J Clin Endocrinol Metab. 2012;97:2093–104.
Gadelha MR, Kasuki L, Korbonits M. The genetic background of acromegaly. Pituitary. 2017;20:10–21.
Giustina A, Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B, et al. Multidisciplinary management of acromegaly: A consensus. Rev Endocr Metab Disord. 2020;21:667–78.
El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390:2194–210.
Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. A summary of the endocrine society clinical practice guidelines on congenital adrenal hyperplasia due to Steroid 21-Hydroxylase deficiency. Int J Pediatr Endocrinol. 2010;2010:494173.
Nicolaides NC, Roberts ML, Kino T, Braatvedt G, Hurt DE, Katsantoni E, et al. A Novel point mutation of the human glucocorticoid receptor gene causes primary generalized glucocorticoid resistance through impaired interaction with the LXXLL Motif of the p160 coactivators: dissociation of the transactivating and transreppressive activities. J Clin Endocrinol Metab. 2014;99:E902–7.
Vitellius G, Lombes M. Genetics in endocrinology: glucocorticoid resistance syndrome. Eur J Endocrinol. 2020;182:R15–27.
Jeunemaitre X, Bassilana F, Persu A, Dumont C, Champigny G, Lazdunski M, et al. Genotype-phenotype analysis of a newly discovered family with Liddle’s syndrome. J Hypertens. 1997;15:1091–1100.
Garovic VD, Hilliard AA, Turner ST. Monogenic forms of low-renin hypertension. Nat Clin Pr Nephrol. 2006;2:624–30.
Young WF Jr, Calhoun DA, Lenders JWM, Stowasser M, Textor SC. Screening for endocrine hypertension: an endocrine society scientific statement. Endocr Rev. 2017;38:103–22.
Download references
Author information
Authors and affiliations.
Hypertension Unit, AP-HP, Hôpital Européen Georges Pompidou, F-75015, Paris, France
Jean-Baptiste de Freminville, Laurence Amar, Michel Azizi & Julien Mallart-Riancho
Université Paris Cité,, F-75015, Paris, France
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jean-Baptiste de Freminville .
Ethics declarations
Conflict of interest.
The author declares no competing interests
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
de Freminville, JB., Amar, L., Azizi, M. et al. Endocrine causes of hypertension: literature review and practical approach. Hypertens Res 46 , 2679–2692 (2023). https://doi.org/10.1038/s41440-023-01461-1
Download citation
Received : 21 July 2023
Revised : 05 September 2023
Accepted : 09 September 2023
Published : 11 October 2023
Issue Date : December 2023
DOI : https://doi.org/10.1038/s41440-023-01461-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hypertension
- Aldosteronism
- Pheochromocytoma
- Secondary hypertension.
This article is cited by
Effects of sodium intake, age, gender, blood sampling time on distribution of plasma aldosterone, renin activity, deoxycorticosterone, cortisol, cortisone, and 24 h urinary aldosterone levels in normotensive individuals based on lc-ms/ms.
- Kaijuan Wang
Endocrine (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 01 December 2022
The psychological impact, risk factors and coping strategies to COVID-19 pandemic on healthcare workers in the sub-Saharan Africa: a narrative review of existing literature
- Freddy Wathum Drinkwater Oyat 1 ,
- Johnson Nyeko Oloya 1 , 2 ,
- Pamela Atim 1 , 3 ,
- Eric Nzirakaindi Ikoona 4 ,
- Judith Aloyo 1 , 5 &
- David Lagoro Kitara ORCID: orcid.org/0000-0001-7282-5026 1 , 6 , 7
BMC Psychology volume 10 , Article number: 284 ( 2022 ) Cite this article
6025 Accesses
9 Citations
1 Altmetric
Metrics details
The ongoing COVID-19 pandemic has significantly impacted the physical and mental health of the general population worldwide, with healthcare workers at particular risk. The pandemic's effect on healthcare workers' mental well-being has been characterized by depression, anxiety, work-related stress, sleep disturbances, and post-traumatic stress disorder. Hence, protecting the mental well-being of healthcare workers (HCWs) is a considerable priority. This review aimed to determine risk factors for adverse mental health outcomes and protective or coping measures to mitigate the harmful effects of the COVID-19 crisis among HCWs in sub-Saharan Africa.
We performed a literature search using PubMed, Google Scholar, Cochrane Library, and Embase for relevant materials. We obtained all articles published between March 2020 and April 2022 relevant to the subject of review and met pre-defined eligibility criteria. We selected 23 articles for initial screening and included 12 in the final review.
A total of 5,323 participants in twelve studies, predominantly from Ethiopia (eight studies), one from Uganda, Cameroon, Mali, and Togo, fulfilled the eligibility criteria. Investigators found 16.3–71.9% of HCWs with depressive symptoms, 21.9–73.5% with anxiety symptoms, 15.5–63.7% experienced work-related stress symptoms, 12.4–77% experienced sleep disturbances, and 51.6–56.8% reported PTSD symptoms. Healthcare workers, working in emergency, intensive care units, pharmacies, and laboratories were at higher risk of adverse mental health impacts. HCWs had deep fear, anxious and stressed with the high transmission rate of the virus, high death rates, and lived in fear of infecting themselves and families. Other sources of fear and work-related stress were the lack of PPEs, availability of treatment and vaccines to protect themselves against the virus. HCWs faced stigma, abuse, financial problems, and lack of support from employers and communities.
The prevalence of depression, anxiety, insomnia, and PTSD in HCWs in sub-Saharan Africa during the COVID-19 pandemic has been high. Several organizational, community, and work-related challenges and interventions were identified, including improvement of workplace infrastructures, adoption of correct and shared infection control measures, provision of PPEs, social support, and implementation of resilience training programs. Setting up permanent multidisciplinary mental health teams at regional and national levels to deal with mental health and providing psychological support to HCWs, supported with long-term surveillance, are recommended.
Peer Review reports
Introduction
When coronavirus disease 2019 (COVID-19) was declared a pandemic in March 2020, healthcare workers (HCWs) globally and in sub-Saharan Africa (SSA) were unprepared for the scale of the physical and mental health devastation that was to follow [ 1 ]. The impact of the COVID-19 pandemic on healthcare workers has been profound, characterized by death, disability, and untenable burden on mental health and well-being [ 2 ]. Factors impacting their mental health include high risks of exposure and infection, financial insecurity, separation from loved ones, stigma, difficult triage decisions, stressful work environment, scarcity of supplies including personal protective equipment (PPEs), exhaustion, traumatic experiences due to regular witnessing of deaths among patients and colleagues [ 2 , 3 ]. Greenberg et al. [ 4 ] observed that the COVID-19 pandemic put healthcare professionals worldwide in an unprecedented situation, making difficult decisions to provide care for many severely ill patients with constrained or inadequate resources.
In almost all WHO regions, data indicates that infection rates among healthcare workers are higher than in the general population [ 5 ]. Scholars suggest that the end of the COVID-19 pandemic is not yet in sight. Neither are they sure about the virulence of the following variant when it appears as caseloads are still rising, with more than 621 million infections and 6.5 million deaths reported worldwide by 19th October 2022 [ 6 ]; mainly driven by the newer omicron variants. However, recently in October 2022, we received with gratitude a reassuring message from US President Biden declaring the end of the COVID-19 pandemic in the United States of America.
Meanwhile, previous studies found high levels of depression, anxiety, and PTSD in survivors among the general population and healthcare workers (HCWs) one-to-three years after the control of the SARS epidemic [ 7 ] and the 2014–2016 Ebola epidemic in West Africa [ 8 ]. In addition, recent surveys [ 9 , 10 , 11 , 12 , 13 , 14 ], reviews, and meta-analyses [ 15 , 16 , 17 , 18 ] are pointing to early evidence that a considerable proportion of healthcare workers have experienced stress, anxiety, depression, and sleep disturbances during the COVID-19 pandemic, raising concerns about risks to their long-term mental health.
Studies from the global north countries [ 19 , 20 ], UK [ 21 ], USA [ 22 ], and in India [ 23 ], and China [ 24 , 25 ] have shed light on the vulnerability that characterizes frontline healthcare workers during this pandemic, especially regarding their mental health and well-being. However, evidence in sub-Saharan Africa is scanty, and the pattern and prevalence of psychological disorders are not well understood.
Evidence from a systematic review by Pappa S et al. on 33,062 Chinese HCWs in April 2020 found a pooled prevalence rate of mental health problems among respondents; anxiety 23.2%, depression 22.8%, and insomnia 38.9% [ 26 ]. Similarly, Singapore study, Tan et al . [ 27 ], Li et al . [ 28 ], BMA [ 29 ] and in China [ 31 ] found high levels of psychological disorders among health workers.
Since the beginning of the pandemic, we found one systematic review involving 919 frontline HCWs, 3928 general HCWs, and 2979 medical students conducted in Africa from December 2019 to April 2020 [ 31 ]. The study by Chen J et al . reported a high prevalence of depression, anxiety, and insomnia among frontline HCWs in sub-Saharan Africa (SSA) at 45%, 51%, and 28%, respectively. In comparison, the prevalence of depression, anxiety, and insomnia among the general population was much lower at 30%, 31%, and 24%, respectively [ 31 ]. Furthermore, we found that only a few studies investigated protective and coping measures, given the many uncertainties surrounding the evolution of the COVID-19 pandemic [ 32 ]. Adequate data are needed to equip frontline HCWs and healthcare managers in sub-Saharan Africa to mitigate the medium and long-term adverse effects of the COVID-19 pandemic [ 33 ].
This review aimed to answer three questions (1) What is the psychological impact of the COVID-19 pandemic on HCWs in Sub-Saharan Africa?
(2) What are the associated risk factors during the COVID-19 pandemic?
(3) What interventions (mitigating and coping strategies) protect and support the mental health and well-being of HCWs during the ongoing crises and after the pandemic?
Methodology
Search methodology and article selection.
This current article is a mixed-method narrative review of existing literature on mental health disorders, risk factors, and interventions relevant to the COVID-19 pandemic on HCWs in sub-Saharan. A search on the PubMed electronic database was undertaken using the search terms "novel coronavirus", "COVID-19", "nCoV", "mental health", "psychiatry", "psychology", "anxiety", "depression" and "stress" in various permutations and combinations.
Search processes
We conducted a comprehensive literature search on original articles published from March 2020 to 30 April 2022 in electronic databases of Embase, PubMed, Google Scholar, and the daily updated WHO COVID-19 database. Our search terms included but were not limited to ('COVID-19'/exp OR COVID-19 OR 'coronavirus'/exp OR coronavirus) AND ('psychological'/exp OR psychological OR 'mental'/exp OR mental OR 'stress'/exp OR stress OR 'anxiety' OR anxiety OR 'depression' OR depression OR 'post-traumatic' OR 'post-traumatic'/exp OR 'trauma' OR 'trauma'/exp) OR Health care workers, medical workers of health care professionals, sub-Saharan Africa, for Embase. ("COVID-19" [All Fields] OR "coronavirus" [All Fields]) AND ("Stress, Psychological" [Mesh] OR "mental" OR "anxiety" OR "depression" OR "stress" OR "post-traumatic" OR "trauma") for PubMed, for the WHO COVID-19 database, and ("COVID-19" OR "coronavirus") AND ("Psychological" OR "mental" OR "anxiety" OR "depression" OR "stress" OR "post-traumatic" OR "trauma") for Google Scholar. On reviewing the above citations, twelve articles met the inclusion criteria relevant for this review and are in Table 1 . All twelve articles were cross-sectional, with one qualitative and the others quantitative observational studies.
Eligibility criteria
We included original qualitative and quantitative studies examining the risk factors, psychological impact of COVID-19 and coping strategies of healthcare workers (HCWs) in sub-Saharan Africa during the COVID-19 pandemic. We excluded studies if they were.
1. Not reported in the English language 2. Studies which were not primary research 3. Studies that had not been published in a peer-reviewed journal 4. Studies that did not include data on HCWs’ mental health or psychological well-being 5. Duplicate studies 6. not using validated instruments to measure the risks and psychological impact.
FWDO performed the search of articles. DLK reviewed the articles involving screening of titles, followed by examination of abstracts. The potential articles identified were further reviewed in full text to examine their eligibility. In addition, four of the authors independently reviewed the full articles to abstract the relevant data required for the review. Thereafter, a meeting to harmonise findings were done and presented in a report.
Data extraction and appraisal of the study
We extracted information from each study, including author, study population, year of publication, country, socio-demographic characteristics, sample size, response rate, gender proportion, age, and study time, areas assessed, the validated instrument used and the prevalence. The appraisal involved assessing the research design, recruitment of respondents, inclusion and exclusion criteria, reliability of outcome determination, statistical analyses, ethical compliance, strengths, limitations, and clinical implications of the articles.
Our review protocol was not registered on PROSPERO because of the significant variation in the methodologies of the articles used in the review. The results precluded using a meta-analytic approach and made a narrative review the most suitable for this work. In addition, we did not use the Cochrane Collaboration GRADE method to assess the quality of evidence of outcomes included in this narrative review. Instead, we used the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) 22 items checklist to gauge the quality of the twelve articles included in this review. We qualitatively validated the articles based on additional considerations namely study design, sample sizes, sampling procedures, response rates, statistical methods used, measures taken by the authors to deal with bias and confounding factors and ethical consideration.
Definition of healthcare worker (HCW)
For this narrative review, we adhered to the Centres for Disease Control and Prevention (CDC) definition of HCWs, which includes physicians, nurses, emergency medical personnel, dental professionals and students, medical and nursing students, laboratory technicians, pharmacists, hospital volunteers, and administrative staff [ 34 ].
Search results
The search found twenty-three studies of interest. Full texts of potentially relevant studies underwent eligibility assessment, and twelve articles met the inclusion criteria for this narrative review.
Study characteristics
The twelve articles comprised eleven quantitative and one qualitative study. The common mental health conditions assessed were depression, anxiety, perceived stress, and post-traumatic stress disorder (PTSD). The coping strategy, perceived health status, health distress (including burnout), insomnia, and perceived stigma were also assessed [ 35 , 36 ]. The total number of respondents in these studies was 5,323. The qualitative study had fifty respondents [ 35 ], while the most significant number of participants, 420 was recorded in one of the quantitative studies from Ethiopia [ 37 ]. The questionnaire response rates varied between 90%-100%, with most studies dominated by male respondents at 51.9%-69.2% [ 38 ]. Nurses were the commonest study population, followed by doctors, pharmacists, and laboratory technicians, and no study involved non-HCWs of facilities. Most papers utilized probability sampling procedures, and four quantitative studies used non-random sampling procedures limiting generalizability of their findings and increasing the risk of selection bias. Eight studies were from Ethiopia, and one was from Cameroon, Uganda, Mali, and Togo, respectively (Table 1 ). Most studies were conducted in urban tertiary public hospitals, university teaching hospitals, and rural and urban general hospitals, including primary care facilities operated by Non-Governmental Organizations (NGOs) for example in Mali [ 39 ]. Several validated tools assessed depression, anxiety, insomnia, stress, and PTSD (Table 1 ).
Table 1 provides an overview of the studies selected and validated instruments used to measure psychological disorders.
Table 2 provides comparisons with studies conducted outside of sub-Saharan Africa.
Table 3 provides information on studies showing the classification of psychological outcomes.
Table 4 are studies showing risk factors associated with psychological disorders.
Table 5 are studies that identified protective factors for psychological disorders.
Risks of bias and confounding factors
Most articles selected were cross-sectional studies that employed probability sampling procedures (Table 1 ). Cross-sectional study design minimized selection biases, but many used structured questionnaires, including online self-administered questionnaires, which increased bias due to social desirability. It was not clear how confounding variables were controlled in five papers reviewed [ 38 , 39 , 40 , 43 , 45 ] leading to excessive and perhaps inappropriate determination of associations.
Socio-demographic factors
In this review, the mean age of the respondents ranged between 23 and 35 years, and predominantly males. Age was associated with anxiety, and stress symptoms in 6(50%) of all the studies reviewed [ 35 , 37 , 40 , 41 , 42 , 44 ]. An age of over 40 years was associated with moderate to severe symptoms of PTSD. Two studies concluded that respondents aged over 40 years were more likely to develop PTSD symptoms than their younger counterparts [ 37 , 41 ].
Female gender was significantly associated with depression, anxiety, and stress symptoms among HCWs in seven studies reviewed [ 36 , 37 , 38 , 41 , 42 , 43 ]. Many studies found that being female, married, and a nurse were independent predictors of stress symptoms. Moreover, sex, age, marital status, type of profession, and working environment were significant factors for PTSD symptoms [ 37 , 41 ]. However, one study in Ethiopia found that the odds of depression were twice higher among male healthcare providers than among female healthcare providers [ 35 ].
Psychological impact on healthcare workers
Most studies reviewed directly assessed the prevalence of depression, anxiety, stress, insomnia, and PTSD in HCWs. Common causes of anxiety, fear, or psychological distress that health professionals reported were: lack of access to PPEs and other equipment, being exposed to COVID-19 at work and taking the infection home to their families, uncertainties that their organization will support/take care of their personal and family needs if they got infection, long working hours, death of colleagues, lack of social support, stigmatization, high rates of transmission and poor income [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. However, the prevalence of mental health symptoms exhibited great variations for example depressive symptoms were examined in nine studies [ 35 , 36 , 37 , 39 , 43 , 44 , 45 , 46 ], and varied between 16.3% and 71.9% among HCWs [ 38 , 39 ].
In addition, nine other studies reported high prevalence of anxiety symptoms among HCWs [ 35 , 36 , 37 , 40 , 43 , 44 , 45 , 46 , 47 ] which varied between 21.9% and 73.5% [ 36 , 39 ]. Five studies investigated HCWs' perceived stress during the pandemic; 15.5%-63.7% of HCWs reported high levels of work-related stress [ 35 , 36 , 37 , 43 , 45 ]. Three studies reported 12.4–77% of HCWs experienced sleep disturbances during the COVID-19 pandemic [ 37 , 39 , 40 ].
Post-traumatic stress disorder (PTSD) was in three studies [ 38 , 41 , 42 ], and the prevalence of PTSD-like symptoms varied between 51.6 and 56.8% in HCWs [ 38 , 41 ]. A qualitative study from Uganda reported high symptoms of depression, anxiety, and PTSD among HCWs [ 35 ]. Additionally, factors that increased the risk of PTSD symptoms were for example, working in emergency units and being frontline workers. Furthermore, many studies found that frontline HCWs had increased symptoms of mental disorders and being a frontline worker was an independent risk factor for depression, anxiety, and PTSD [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 ].
Risk factors associated with adverse mental health outcomes
The qualitative study from Uganda reported the factors associated with mental disorder symptoms among HCWs. These were long working hours, lack of equipment (PPEs, testing kits), lack of sleep, exhaustion, high death rates, death of colleagues, and a high COVID-19 transmission rate among HCWs [ 35 ]. Lack of equipment (PPEs, ventilators, and testing kits), overworking, and lack of logistic support were in Ethiopian studies [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 45 ]. Most studies identified several risk factors for adverse mental health outcomes among respondents for example those with medical and mental illnesses, contacts with confirmed COVID-19 patients, and poor social support which were significantly associated with depression [ 42 , 43 ]. Other factors were females, nurses, married, frontline workers, ICU, emergency units, living alone, and lack of social support [ 35 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. Too, participants’ families with chronic illnesses, had contacts with confirmed COVID-19 cases, and poor social support were significantly associated with anxiety. Other risk factors associated with anxiety include exhaustion, long working hours, frontline workers, emergencies, nurses, pharmacists, laboratory technicians, married, older, younger, living alone, being female, working at general and referral hospitals, and perceived stigma. In addition, participants’ families with chronic illnesses, those who had contacts with confirmed COVID-19 cases, and those with poor social support were predictors of stress during the COVID-19 pandemic [ 37 , 38 , 40 , 41 , 42 , 43 , 45 ]. Other stress symptoms include having a medical illness, a mental illness, being a frontline worker, married, nurse, female, pharmacist, laboratory technician, physician, older age, lack of standardized PPE supply, low incomes, and living with a family [ 36 , 37 , 40 , 41 , 42 , 43 , 44 , 45 ]. Healthcare providers with low monthly incomes were significantly more likely to develop stress than those with high monthly incomes [ 38 ]. In addition, participants living alone, living with a family, and being married were associated with symptoms of psychological disorders among HCWs [ 36 , 37 , 38 , 45 ]. Overall, the risk factors for adverse psychological impacts are categorized in three thematic areas (i) occupational, (ii) psychosocial, and (iii) environmental aspects.
Occupational factors
Most studies showed that frontline HCWs, nurses, doctors, pharmacists, and laboratory technicians had significantly higher levels of mental health risks compared to non-frontline HCWs [ 35 , 36 , 37 , 38 , 40 , 42 , 43 , 45 ]. They experienced higher frequency of insomnia, anxiety, depression, and somatization than non-frontline medical HCWs. In contrast, Mali [ 39 ] and Cameroon [ 46 ] studies found a higher prevalence of depression, anxiety, and PTSD in non-frontline HCWs [ 39 , 46 ]. However, among HCWs, physicians were 20% less likely to develop mental health disorders than nurses, pharmacists, and laboratory technicians [ 39 ]. In addition, healthcare workers with low monthly incomes had higher symptoms of depression, anxiety, stress, and insomnia [ 37 ].
Healthcare groups
Five studies found that being a nurse was associated with worse mental disorders than doctors [ 36 , 37 , 40 , 44 , 45 ].
Frontline staff with direct contact with COVID-19
Most papers in the review found that being in a “frontline” position or having direct contact with COVID-19 patients was associated with higher level of psychological distress [ 35 , 36 , 37 , 38 , 40 , 42 , 43 , 45 ]. In addition, studies found that contact with COVID-19 patients was independently associated with an increased risk of sleep disturbances [ 40 , 46 ]. Moreover, HCWs who had contact with confirmed COVID-19 cases were more likely to develop depression, anxiety, and stress symptoms than those who had no contact with COVID-19 patients [ 36 , 37 , 38 , 43 , 45 ].
Lack of personal protective equipment (PPEs)
Most studies reported that the lack of PPEs was associated with higher symptoms of depression, anxiety, stress, and insomnia, while its availability was associated with fewer mental disorder symptoms [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 ]. In Mali, workers from centres that provided facemasks were 51% less likely to suffer from depression, 62% less likely to develop anxiety, and 45% less likely to develop insomnia [ 39 ]. In Ethiopia, the odds of developing post-traumatic stress disorder were much higher among HCWs who did not receive standardized PPEs supplies than those who had [ 38 , 41 , 42 ]. In Uganda, the lack of PPEs was associated with depression, anxiety, and PTSD [ 35 ].
Heavy workload
Longer working hours, increased work intensity, increased patient load, and exhaustion were risk factors in Ugandan [ 35 ] and Ethiopian studies [ 36 ].
Psychosocial factors: perceived stigma and fear of infection
The fear of infection was in the qualitative study from Uganda [ 35 ], one quantitative study from Cameroon [ 47 ] and seven cross-sectional studies from Ethiopia [ 36 , 37 , 38 , 41 , 42 , 43 , 44 ]. Poor social support was associated with PTSD symptoms, depression, anxiety, and stress [ 35 , 36 , 37 , 38 , 42 , 43 ]. Two studies reported that HCWs with perceived stigmatization were more likely to suffer from depression, anxiety, stress, and PTSD [ 37 , 42 ].
family concerns
This came up as one of the main risk factors of stress in almost all studies, especially among those HCWs in direct contact with confirmed COVID-19 cases [ 35 , 36 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 ]. A family member suffering from COVID-19 was associated with poor mental health outcomes in HCWs [ 36 , 37 ].
Protective psychosocial factors
Two studies suggest a reduction of perceived stigma can be achieved by sensitization of communities about COVID-19 [ 37 , 42 ], and four studies recommend solid social support [ 36 , 37 , 42 , 43 ].
Safety of family
Family safety had the most significant impact in reducing stress. Safety from COVID-19 infection and financial protection of families were essential coping strategies for HCWs [ 35 , 36 ].
Underlying illnesses
We found three studies that reported an underlying medical and mental illness as an independent risk factor for poor psychological outcomes [ 42 , 43 , 45 ].
Protective factors against adverse mental health outcomes
The review identified protective factors to adverse mental health outcomes during COVID-19. The qualitative study from Uganda and four quantitative cross-sectional studies from Ethiopia identified some protective factors [ 35 , 38 , 41 , 42 , 45 ]. The protective factors are grouped under three thematic areas (i) occupational, (ii) psychosocial, and (iii) environmental aspects.
The qualitative study identified many social coping strategies among respondents, including family networks, community networks, help from family, responsibility to society, assistance from community members, availability of assistance from strangers, and the symbiotic nature of assistance in the community [ 35 ].
Protective occupational factors
Studies suggest that physicians suffered fewer mental health disorders partly because of their experience with previous epidemics [ 37 , 42 , 45 ].
Some necessary coping measures include good hospital guidance and ongoing training of frontline HCWs [ 37 , 42 , 45 ].
Adequate supply of PPEs
As mentioned above, PPE was a protective factor when adequate and a risk factor for poor mental health outcomes when deemed inadequate [ 35 , 36 , 37 , 42 , 43 ].
The COVID-19 pandemic has been an ongoing global public health emergency that has burdened healthcare workers' physical and mental well-being (HCWs) [ 1 , 5 ]. Our review confirms the enormous magnitude of mental health impact of COVID-19 on healthcare workers in sub-Saharan Africa, and it is widespread, with significant levels of depression, anxiety, distress, and insomnia; especially those working directly with COVID-19 patients at particular risk [ 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. Out of the twelve articles reviewed, eight studies (66%) came from Ethiopia, and this has implications on the results (Table 1 ). This finding indicates few research published to date on the psychological impact of the pandemic on the mental health of HCWs in sub-Saharan Africa; a subregion that the COVID-19 pandemic has severely impacted.
Overview of the study sites
Studies in this review were conducted predominantly in hospital settings. We found only one study relating to primary healthcare workers or facilities [ 38 ]. This finding is of concern, as there is increasing evidence that many non-frontline HCWs continue to suffer psychological symptoms long after the conclusion of infectious disease epidemics [ 7 , 8 ]. In addition, a significant mortality due to COVID-19 was due to excess morbidity, some of which were from primary care facilities. Given that this study is the first narrative review in sub-Saharan Africa, it would be helpful to briefly compare our findings with some published reviews and surveys from other regions (Table 2 ).
High prevalence of psychological disorders among participants
Investigators in this review found 16.3–71.9% HCWs with depressive symptoms, 21.9–73.5% had anxiety symptoms, 15.5–63.7% experienced work-related stress symptoms, 12.4–77% experienced sleep disturbances, and 51.6–56.8% PTSD symptoms [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. This high prevalence of mental health symptoms among HCWs in our review is consistent with previous reviews conducted early in the pandemic in sub-Saharan Africa [ 31 ], Asia [ 17 , 18 , 26 , 28 ], USA & Europe [ 15 , 16 ], and supported by a batch of cross-sectional studies globally [ 11 , 12 , 13 , 14 , 19 , 27 , 30 ]. We found mixed results with significant variations within and among regions and countries, as depicted in Tables 1 and 2 .
Risk factors of psychological disorders among participants
Studies established that HCWs responding to the COVID-19 pandemic in sub-Saharan Africa were exposed to long working hours, overworking, exhaustion, high risk of infection, and shortage of personal protective equipment (Tables 3 and 4 ). In addition, HCWs had deep fear, were anxious and stressed with the high transmission rate of the virus among themselves, high death rates among themselves and their patients, and lived under constant fear of infecting themselves and their families with obvious consequences [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. Some HCWs were deeply worried about the lack of standardized PPEs, known treatments and vaccines to protect against the virus. Many health workers had financial problems, lacked support from families and employers if they contracted the virus [ 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 , 44 ]. An additional source of fear and anxiety was the perceived stigma attached to being infected with COVID-19 by the public [ 36 , 41 ]. Studies found that HCWs, especially those working in emergency, intensive care units, infectious disease wards, pharmacies, and laboratories, were at higher risk of developing adverse mental health impacts compared to others [ 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 ]. This is supported by previous reviews [ 15 , 16 , 17 , 18 , 26 , 28 ] and cross-sectional studies [ 10 , 11 , 12 , 13 , 14 , 20 , 21 , 23 , 25 , 30 ]. However, findings were inconsistent on the impact of COVID-19 on frontline health workers, with ten studies [ 35 , 36 , 37 , 39 , 40 , 41 , 42 , 44 , 45 ] suggesting they are at higher risk than peers and two studies showing no significant difference in psychological disorders relating to the departments [ 38 , 43 ].
The Mali’s study was conducted exclusively in primary care facilities among HCWs not involved in treating COVID-19 cases but still registered a very high prevalence of depression 71.9%, anxiety 73.6%, and insomnia 77.0% [ 39 ]. In contrast, two studies conducted among HCWs at COVID-19 treatment facilities in Ethiopia [ 36 , 38 ] registered much lower prevalence of depression 20.2%, anxiety 21.0%, and insomnia12.4% [ 36 ], and 16.3%, 30.7% and 15.9% respectively, in the second study [ 38 ]. These findings show that not only frontline HCWs experienced mental health disorders during this pandemic but highlight the need for direct interventions for all HCWs regardless of occupation or workstation during this and future pandemics. The significant disparity in the studies could be due to structural, occupational, and environmental issues for example challenges faced by Mali's healthcare systems, characterized by acute equipment shortages, lack of PPEs, human resources, lack of trained and experienced HCWs, ongoing nationwide insecurity, and terrorism compared to Ethiopia. Therefore, local context needs to be considered as contributing factor to mental health disorders among HCWs.
Regional variations of psychological disorders
Tan et al . found a higher prevalence of anxiety among non-medical HCWs in Singapore [ 27 ]. As previously noted, the prevalence of poor psychological outcomes varied between countries. Compared to sub-Saharan Africa and China, data from India [ 23 ] and Singapore [ 27 ] revealed an overall lower prevalence of anxiety and depression than similar cross-sectional data from sub-Saharan Africa [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ] and China [ 9 , 25 , 30 ]. This finding suggests that different contexts and cultures may reveal different psychological findings and that, it is possible that being at different countries’ outbreak curve may play a part, as there is evidence that it is influential.
Tan et al . suggests that medical HCWs in Singapore had experienced a SARS outbreak and thus were well prepared for COVID-19 psychologically and infection control measures [ 27 ]. What can be deduced is that context and cultural factors play a role, not just the cadre or role of healthcare workers [ 16 ]. It also highlights the importance of reviewing evidence regularly as more data emerge from other countries.
One hospital in Ethiopia found that the thought of resignation was associated with higher chances of mental health disorders and that pharmacists and laboratory technicians who did not receive prior training exhibited higher symptoms of mental health disorders compared to others [ 36 ]. Work shift arrangement, considering a dangerous atmosphere presented by working in COVID-19 wards, was one which exacerbated or relieved mental health symptoms among HCWs, with shorter exposure periods being most beneficial [ 36 ]. Meanwhile, studies found that financial worries caused by severe lockdowns and erratic payment of salaries and allowances were also major stressors [ 35 ]. This finding is like studies in Pakistan [ 13 ] and China [ 30 , 32 ].
In this review, HCWs who had contact with confirmed COVID-19 patients were more affected by depression, anxiety, and stress than their counterparts who had not [ 35 , 36 , 37 , 40 , 41 , 43 , 45 ]. This finding is like previous reviews [ 15 , 16 , 17 , 18 , 26 , 28 , 31 ] and cross-sectional studies [ 9 , 10 , 11 , 12 , 13 , 14 , 21 , 23 , 24 , 25 , 27 , 30 ], which reported higher depression, anxiety, and psychological symptoms of distress in HCWs who were in direct contact with confirmed or suspected COVID-19 patients.
A study in Pakistan showed that 80% of participants expected the provision of PPE from authority [ 13 ], and 86% were anxious. Some respondents alluded to forced deployment, while in Mali, 73.3% were anxious, with the majority worrying about the shortage of nurses [ 39 ]. Therefore, prospects of being deployed at a workstation where one had not been trained or oriented contributed to fear among health workers. In the sub-Saharan African context, this scenario can best be represented in HCWs involved in internship who must endure hard work during their training. Tan et al . found that junior doctors were more stressed than nurses in Singapore [ 27 ].
Socio-demographic characteristics
Nearly all studies in our review suggest that socio-demographic variables for example age, gender, marital status, and living alone or with families contribute to the high mental disorder symptoms [ 35 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 ]. We, the authors suggest that these observations are handled cautiously as several investigators of these reviewed articles did not entirely control the influence of confounding variables. An alternative explanation for this study's findings may be the more significant risks of frontline exposure amongst women and junior HCWs, predominantly employed in lower-status roles, many of whom lacked experience and appropriate training within healthcare system globally. It is also important to note that respondents to all studies, when disaggregated by gender, and age, were predominantly younger or female, which may have impacted the outcomes of these findings [ 16 ]. In addition, the consistently higher mortality rates, and risk of severe COVID-19 disease amongst men would suggest that the complete picture regarding gender and mental health during this pandemic is still incomplete [ 16 ]. Moreover, in several studies, both younger and older age groups were equally affected by mental health symptoms but for different reasons. Cai et al . [ 32 ] in a Chinese study on HCWs for example observed that irrespective of age, colleagues' safety, self and families' safety, the lack of treatment for COVID-19 was a factor that induced stress in HCWs. Similarly, in our review, the lack of PPEs, high infection transmission rates, high death rates among HCWs, and the fear of infecting their families were the factors that induced stress in all HCWs [ 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ].
We, the authors propose that paying close attention to concerns of HCWs by employers would greatly relieve some stressors and contribute to increased mental well-being of participants. Compared with physicians, our review showed that nurses were more likely to suffer from depression, anxiety, insomnia, PTSD, and stress [ 35 , 37 , 39 , 40 , 41 , 44 , 45 ]. Workloads and night shifts in healthcare facilities, as well as contacts with risky patients, enhanced nurses' mental distress risks [ 15 , 16 , 17 , 18 , 26 , 27 , 28 ]. In addition, nursing staff have more extended physical contacts and closer interactions with patients than other professionals, providing round-the-clock care required by patients with COVID-19 and thus the increased risk [ 15 ]. On the one hand, we posit that most senior physicians are experienced and always keep well-informed with emerging medical emergencies. The majority become aware of emerging epidemic early and actively protect themselves from infections through regular scientific literature updates compared to their junior counterparts. Senior physicians also spend less time in emergency wards unless there is a need to conduct specific procedures which cannot be undertaken by senior housemen or general medical officers. Cai et al . [ 32 ] concluded that it is essential to have a high level of training and professional experience for healthcare workers engaging in public health emergencies, especially for the new staff. As a result, these findings highlight the importance of focusing on all the frontline HCWs sacrificing to contain the COVID-19 pandemic.
Regular monitoring of high-risk groups
There is a need to continue monitoring the high-at-risk groups, including nursing staff, interns, support staff, and all deployed in emergency wards. These high-at-risk groups should be encouraged to undertake screening, treatment, and vaccination to avoid the medium and long-term consequences of such epidemics [ 15 , 16 , 35 , 37 , 40 , 44 ].
Social support and coping mechanisms
The effect of social support and coping measures is in the qualitative study [ 34 ] and three other quantitative studies [ 36 , 41 , 42 ] which concluded that respondents with good social support were less likely to suffer from severe depression, anxiety, work-related stress, and PTSD. The qualitative study identified several coping measures, including community and organizational support, family, and community networks, help from family, responsibility to society, and assistance from community members and strangers, including the symbiotic nature of assistance in the community [ 35 ]. Other measures include providing accommodation and food to employees [ 35 ].
Interestingly, no study examined the association of resilience and self-efficacy with sleep quality, degrees of anxiety, depression, PTSD, and stress. However, a Chinese study by Cai et al. [ 32 ] suggests that the social support given to HCWs causes a reduction in anxiety and stress levels and increases their self-efficacy. In divergence, Xiao et al . [ 46 ] found no relationship between social support and sleep quality.
Only two studies in our review examined the effects of stigma on the mental health of HCWs [ 36 , 41 ] and found that HCWs with perceived stigma were more likely to be depressed, anxious, stressed, and prone to poor sleep quality [ 36 , 41 ]. We, the authors suggest that better community sensitization by creating public awareness involving appropriate local community structures and networks are essential. The broader community in sub-Saharan Africa may have suffered severely from infodemics with severe consequences on their mental health, especially during the difficult lockdowns. In addition, removing discrimination/inequalities at the workplace based on race and other social standings have a powerful influence on the mental health outcomes of HCWs. Also, because emotional exhaustion is long associated with depression, anxiety, and sleep disturbances, none of the studies in our review examined burnout as an essential component of mental health disorders in HCWs in sub-Saharan Africa.
Protective and coping measures
In this review we have provided evidence about personal, occupational, and environmental factors that were important protective and coping measures against psychological disorders. Based on these factors we suggest some protective and coping measures which can help to reduce the negative effects of the pandemic on mental health of HCWs in sub-Saharan Africa. Organizations and healthcare managers need to be aware that primary prevention is key to any successful interventions to contain and control any epidemic. This should take the form of planned regular training, orientation and continuing medical education grounded on proven infection control measures. These measures need to be backed up by timely provision of protective equipment, drugs, testing facilities, vaccines, isolation facilities, clinical and mental health support, and personal welfare of HCWs [ 35 , 36 , 37 , 42 , 45 ]. The effect of community and organizational support and coping measures was shown by the qualitative study [ 35 ] and five other quantitative studies [ 36 , 37 , 41 , 42 , 43 ] indicating that respondents who had good social and organizational support were less likely to suffer from severe depression, anxiety, work related stress and PTSD. Prior experience with comparable pandemics and training are suggested as beneficial coping strategies for healthcare workers during this pandemic but also local social structural and geopolitical conditions appear to determine the pattern and evolution of mental health symptoms among HCWs [ 14 , 15 , 31 , 32 , 47 ]. In our case the high prevalence of all mental health symptoms in non-frontline primary health care facilities in Mali [ 39 ] which was already plagued with instability and weak healthcare systems prior to the pandemic is a case in point. Results are particularly consistent in showing that provision of PPEs, testing kits, orientation training of workers, work shift arrangements, provision of online counselling, provision of food and accommodation and prompt payment of allowances by employers were important protective measures [ 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 ]. The feeling of being protected is associated with higher work motivation with implication for staff turnover [ 35 , 38 , 43 , 45 ]. Hence, physical protective materials [ 14 ], together with frequent provision of information, should be the cornerstone of any interventions to prevent deterioration in mental health of HCWs (Table 5 ). Finally, provision of rest rooms, online consultation with psychologists/psychiatrists, protection from financial hardships, access to social amenities and religious activities are some important coping measures [ 35 , 36 , 38 , 42 , 45 ]. In this era of digital health care with plentiful internet and smartphones, organization can conduct online trainings, online mental health education, online psychological counselling services, and online psychological self-help intervention tailored to the needs of their HCWs [ 35 , 37 , 42 ]. In addition, it is essential to understand and address the sources of anxiety among healthcare professionals during this COVID-19 pandemic, as this has been one of the most experienced mental health symptoms [ 48 ]. Adequate protective equipment provided by health facilities is one of the most important motivational factors for encouraging continuation of work in future outbreaks. Furthermore, availability of strict infection control guidelines, specialized equipment, recognition of their efforts by facility management, government, and reduction in reported cases of COVID-19 provide psychological benefits [ 15 , 32 ]. Finally, we call upon Governments (the largest employers of HCWs) in sub-Saharan Africa to do what it takes to improve investments in the mental health of HCWs and plan proactively in anticipation of managing infectious disease epidemics, including other expected and unexpected disasters.
Future research direction
There was no study that examined the association of resilience and self-efficacy with sleep quality, degrees of anxiety, depression, PTSD, and stress. Although emotional exhaustion has long been associated with depression, anxiety, and sleep disturbances, no study in our review examined burnout as an important component of mental health disorders in HCWs in sub-Saharan Africa. The impacts of infodemics, stringent lockdown measures, discrimination/inequalities at workplaces based on race, and other social standings on mental health outcomes of HCWs need to be investigated.
Future studies are needed on the above including other critical areas like suicidality, suicidal ideations, and substance abuse during the COVID-19 pandemic. In addition, there is a significant variation of related literature calling for more rigorous research in future. More systematic studies will be required to clarify the full impact of the pandemic so that meaningful interventions can be planned and executed at institutional and national levels in the Sub-Saharan Africa.
Limitations of this study
There are some limitations to this study. First, most of the studies are from one country, limiting the generalizability of the results to the whole African continent. Second, all the studies were cross-sectional and only looked at associations and correlations. There is a need for prospective or retrospective cohort or case–control studies on this subject matter. Longitudinal research studies on the prevalence of mental disorders in the COVID-19 pandemic in the sub-Saharan Africa are urgently required. Third, most studies reviewed did not adequately examine protective factors or coping measures of the health workers in their settings. In addition, most studies did not pay strict attention to confounding variables which could have led to inappropriate results and conclusions. Fourth, most sample sizes were small and unlikely representative of the population and yet larger sample sizes would better identify the extent of mental health problems among health workers in the region. Fifth, depression, anxiety, and stress were assessed solely through self-administered questionnaires rather than face-to-face psychiatric interviews. Sixth, these studies employed various instruments and different cut-off thresholds to assess severity. Notably, the magnitude and severity of reported mental health outcomes may vary based on the validity and sensitivity of the measurement tools. Seventh, there was no mention of mental baseline information among the studied population and therefore it was unknown if the studied population had pre-existing mental health illnesses that decompensated during the pandemic crisis. Eight, investigators did not give much attention to stigma, burnout, resilience, and self-efficacy among study participants.
Furthermore, our review did not employ systematic reviews or meta-analyses methods for the information generated. This narrative review paper precluded deeper insight into the quality of reviewed articles for this paper. Still, our observation was that investigators did not consider the strict lockdown measures, quarantine, and isolation imposed by many countries in sub-Saharan Africa as possible risk factors for mental health disorders among HCWs.
Based on the articles reviewed, the prevalence of depression, anxiety, insomnia, and PTSD in HCWs in the sub-Saharan Africa during the COVID-19 pandemic is high. We implore health authorities to consider setting up permanent multidisciplinary mental health teams at regional and national levels to deal with mental health issues and provide psychological support to patients and HCWs, always supported with sufficient budgetary allocations.
Long-term surveillance is essential to keep track of insidiously rising mental health crises among community members. There is a significant variation of related literature thus calling for more rigorous research in the future. More systematic studies will be needed to clarify the full impact of the pandemic so that meaningful interventions can be planned better and executed at institutional and national levels in sub-Saharan Africa.
Availability of data and materials
Datasets analysed in the current study are available from the corresponding author at a reasonable request.
Abbreviations
Coronavirus disease 2019
Healthcare workers.
Mental health
Public health emergency
Personal protective equipment
World Health Organisation
World Health Organization. Coronavirus disease (COVID-19) outbreak. 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed 1 March 2020).
Veenema TG, Closser S, Thrul J. Mental Health, and Social Support for Healthcare and Hospital Workers During the COVID-19 Pandemic. Baltimore, MD: Johns Hopkins Center for Health Security; 2021.
Chersich MF, Gray G, Fairlie L. COVID-19 in Africa: care and protection for frontline healthcare workers. Global Health. 2020;16:46. https://doi.org/10.1186/s12992-020-00574-3Interventions .
Article PubMed PubMed Central Google Scholar
Greenberg N, Docherty M, Gnanapragasam S, Wessely S. Managing mental health challenges faced by healthcare workers during a COVID-19 pandemic. BMJ. 2020;368: m1211. https://doi.org/10.1136/bmj.m1211 .
Article PubMed Google Scholar
WHO. Keep health workers safe to keep patients safe: WHO. 2020. https:// www.who.int/news/item/17-09-2020-keep-health-workers-safe-to-keep - patients-safe-who. Accessed 21 October 2020.
WHO. Weekly epidemiological update on COVID-19- 19 th October 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---19-october-2022 .
Maunder R, Lancee W, Balderson K, Bennett J, Borgundvaag B, Evans S, et al. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerging Infect Dis. 2006;12(12):1924–32. https://doi.org/10.3201/eid1212.060584 .
Article Google Scholar
Jalloh MF, Li W, Bunnell RE. Impact of Ebola experiences and risk perceptions on mental health in Sierra Leone, July 2015. BMJ Glob Health. 2018;3: e000471. https://doi.org/10.1136/bmjgh-2017-000471 .
Kang L, Li Y, Hu S, Chen M, Yang C, Yang BX, et al. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry. 2020;7(3): e14.
Huang JZ, Han MF, Luo TD, Ren AK, Zhou XP. Mental health survey of 230 medical staff in a tertiary infectious disease hospital for COVID-19. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi. Chinese J Indus Hygiene Occup Dis. 2020;38:E001.
Google Scholar
Cai W, Lian B, Song X, Hou T, Deng G, Li H. A cross-sectional study on mental health among health care workers during the outbreak of coronavirus disease. Asian J Psychiatr. 2020. https://doi.org/10.1016/j.ajp.2020.102111 .
Elhadi M, Msherghi A, Elgzairi M, Alhashimi A, Bouhuwaish A, Biala M, Abuelmeda S, et al. Psychological status of healthcare workers during the civil war and COVID-19 pandemic: a cross-sectional study. J Psychosom Res. 2020;137: 110221.
Urooj U, Ansari A, Siraj A, Khan S, Tariq H. Expectations, fears, and perceptions of doctors during COVID-19 pandemic. Pak J Med Sci. 2020;36:S37–42. https://doi.org/10.12669/pjms.36.COVID19-S4.2643 .
Khanal P, Devkota N, Dahal M, Paudel K, Joshi D. Mental health impacts among health workers during COVID-19 in a low resource setting: a cross-sectional survey from Nepal. Global Health. 2020;16(1):89. https://doi.org/10.1186/s12992-020-00621-z .
Preti E, Di Mattei V, Perego G. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43. https://doi.org/10.1007/s11920-020-01166-z .
De Kock JH, Latham HA, Leslie SJ, Grindle M, Munoz SA, Ellis L, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. https://doi.org/10.1186/s12889-020-10070-3 .
Luo M, Guo L, Yu M, Jiang W, Wang H. The psychological and mental impact of coronavirus disease 2019 (COVID-19) on medical staff and the general public. A systematic review and meta-analysis. Psychiatry Res. 2020;291:113190. https://doi.org/10.1016/j.psychres.2020.113190 .
Bareeqa SB, Ahmed SI, Samar SS, Yasin W, Zehra S, Monese GM, et al. Prevalence of depression, anxiety, and stress in china during COVID-19 pandemic: a systematic review with meta-analysis. Int J Psychiatry Med. 2021;56:210–27.
Søvold LE, Naslund JA, Kousoulis AA. Prioritizing the mental health and well-being of healthcare workers: an urgent global public health priority. Front Public Health. 2021;9: 679397. https://doi.org/10.3389/fpubh.2021.679397 .
Consolo U, Bellini P, Bencivenni D, Iani C, Checchi V. Epidemiological aspects, and psychological reactions to COVID-19 of dental practitioners in the Northern Italy districts of Modena and Reggio Emilia. Int J Environ Res Public Health. 2020;17(10):3459. https://doi.org/10.3390/ijerph17103459 .
Gilleen J, Santaolalla A, Valdearenas L, Salice C, Fusté M. Impact of the COVID-19 pandemic on the mental health and well-being of UK healthcare workers. BJPsych Open. 2021;7(3): e88. https://doi.org/10.1192/bjo.2021.42 .
Shechter A, Diaz F, Moise N, Anstey DE, Ye S, Agarwal S, et al. psychological distress, coping behaviours, and preferences for support among New York healthcare workers during the COVID-19 pandemic. Gen Hosp Psychiatry. 2020;66:1–8. https://doi.org/10.1016/j.genhosppsych.2020.06.007 .
Wilson W, Raj JP, Rao S, Ghiya M, Nedungalaparambil NM, Mundra H, et al. Prevalence and predictors of stress, anxiety, and depression among healthcare workers managing COVID-19 pandemic in India: a nationwide observational study. Indian J Psychol Med. 2020;42(4):353–8. https://doi.org/10.1177/0253717620933992 .
Wang S, Xie L, Xu Y, Yu S, Yao B, Xiang D. Sleep disturbances among medical workers during the outbreak of COVID-2019. Occup Med. 2020. https://doi.org/10.1093/occmed/kqaa074 .
Kang L, Ma S, Chen M, Yang J, Wang Y, Li R. Impact on mental health and perceptions of psychological care among medical and nursing staff in Wuhan during the 2019 novel coronavirus disease outbreak: a cross-sectional study. Brain Behav Immun. 2020. https://doi.org/10.1016/j.bbi.2020.03.028 .
Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behavi Immun. 2020. https://doi.org/10.1016/j.bbi.2020.05.026 .
Tan BYQ, Chew NWS, Lee GKH, Jing M, Goh Y, Yeo LLL. Psychological impact of the COVID-19 pandemic on health care workers in Singapore. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1083 .
Li Y, Scherer N, Felix L, Kuper H. Prevalence of depression, anxiety, and post-traumatic stress disorder in health care workers during the COVID-19 pandemic: a systematic review and meta-analysis. PLoS One. 2021;16(3): e0246454. https://doi.org/10.1371/journal.pone.0246454 .
British Medical Association. The mental health and well-being of the medical workforce – now and beyond COVID-19. 2020. Available from URL: https://www.bma.org.uk/media/2475/bma-covid-19-and-nhs-staff-mental-healthwellbeing-report-may-2020.pdf .
Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3): e203976. https://doi.org/10.1001/jamanetworkopen.2020.3976 .
Chen J, Farah N, Dong RK, Chen RZ, Xu W, Yin J, et al. Mental health during the COVID-19 crisis in Africa: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:10604. https://doi.org/10.3390/ijerph182010604 .
Gupta S, Sahoo S. Pandemic, and mental health of the frontline healthcare workers: a review and implications in the Indian context amidst COVID-19. Gen Psychiatr. 2020;33(5): e100284. https://doi.org/10.1136/gpsych-2020-100284 .
Cai H, Tu B, Ma J, Chen L, Fu L, Jiang Y, et al. psychological impact, and coping strategies of frontline medical staff in Hunan between January and March 2020 during the outbreak of coronavirus disease 2019 (COVID-19) in Hubei, China. Med Sci Monit. 2020;26:924171. https://doi.org/10.12659/MSM.924171 .
CDC. National Center for Immunization and Respiratory Diseases. CDC (2020) Available online at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html .
Muzyamba C, Makova O, Mushibi GS. Exploring health workers’ experiences of mental health challenges during care of patients with COVID-19 in Uganda: a qualitative study. BMC Res Notes. 2021;14(1):286. https://doi.org/10.1186/s13104-021-05707-4 .
GebreEyesus FA, Tarekegn TT, Amlak BT, Shiferaw BZ, Emeria MS, Geleta OT, et al. Levels and predictors of anxiety, depression, and stress during COVID-19 pandemic among frontline healthcare providers in Gurage zonal public hospitals, Southwest Ethiopia, 2020: a multicentre cross-sectional study. PLoS One. 2021;16(11): e0259906. https://doi.org/10.1371/journal.pone.0259906 .
Mulatu HA, Tesfaye M, Woldeyes E. The prevalence of common mental disorders among health care professionals during the COVID-19 pandemic at a tertiary Hospital in East Africa. Med Rxiv. 2020. https://doi.org/10.1101/2020.10.29.20222430 .
Ayalew M, Deribe B, Abraham Y, Reta Y, Tadesse F, Defar S. Post-traumatic stress disorder symptoms and its predictors among healthcare workers following COVID-19 pandemic in Southern Ethiopia: a cross-sectional study. Front Psychiatry. 2021. https://doi.org/10.3389/fpsyt.2021.818910 .
Sagaon-Teyssier L, Kamissoko A, Yattassaye A, Diallo F, Castro DR, Delabre R, et al. Assessment of mental health outcomes and associated factors among workers in community-based HIV care centers in the early stage of the COVID-19 outbreak in Mali. Health Policy Open. 2020. https://doi.org/10.1016/j.hpopen.2020.100017 .
Kemal J. Psychological distress, early behavioral response, and perception toward the COVID-19 pandemic among health care workers in North Shoa Zone, Oromiya region. Front Psychiatry. 2020. https://doi.org/10.3389/fpsyt.2021.628898 .
Chekole YA, Minaye SY, Abate SM, Mekuriaw B. Perceived stress, and its associated factors during COVID-19 among healthcare providers in Ethiopia: a cross-sectional study. Adv Public Heal. 2020. https://doi.org/10.1155/2020/5036861 .
Asnakew S. Prevalence of post-traumatic stress disorder on health professionals in the era of COVID-19 pandemic, Northwest Ethiopia 2020: a multi-centred cross-sectional study. PLoS One. 2021;16(9):e0255340.
Asnakew S, Amha H, Kassew T. Mental health adverse effects of COVID-19 pandemic on health care workers in Northwest Ethiopia: a multicenter cross-sectional study. Neuropsychiatr Dis Treat. 2021;17:1375–84. https://doi.org/10.2147/NDT.S306300 .
Kounou KB, Guédénon KM, Foli AAD, Gnassounou-Akpa E. Mental health of medical professionals during the COVID-19 pandemic in Togo. Psychiatry Clin Neurosci. 2020;74:559–60.
Ayalew MB. Prevalence and determinant factors of mental health problems among healthcare professionals during COVID-19 pandemic in southern Ethiopia: a multicentre cross-sectional study. BMJ Open. 2021. https://doi.org/10.1136/bmjopen-2021-057708 .
Keubo FRN, Mboua PC, Tadongfack TD, Tchoffo EF, Tatang CT, Zeuna JI, et al. psychological distress among health care professionals of the three COVID-19 most affected Regions in Cameroon: Prevalence and associated factors. In: Annales Médico-Psychologiques, Revue Psychiatrique; Elsevier Masson: Paris, France. 2021;179:141–146.
Xiao H, Zhang Y, Kong D, Li S, Yang N. The effects of social support on sleep quality of medical staff treating patients with coronavirus disease 2019 (COVID-19) in January and February 2020 in China. Med Sci Monit. 2020;26:e923549. https://doi.org/10.12659/MSM.923549 .
Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among healthcare professionals during the COVID-19 pandemic. JAMA. 2020. https://doi.org/10.1001/jama.2020.5893 .
Download references
Acknowledgements
We thank Uganda Medical Association Acholi-branch members for the financial assistance which enabled the team to conduct this study successfully.
Author information
Authors and affiliations.
Uganda Medical Association (UMA), UMA-Acholi Branch, Gulu City, Uganda
Freddy Wathum Drinkwater Oyat, Johnson Nyeko Oloya, Pamela Atim, Judith Aloyo & David Lagoro Kitara
Moroto Regional Referral Hospital, Moroto, Uganda
Johnson Nyeko Oloya
St. Joseph’s Hospital, Kitgum District, Uganda
Pamela Atim
ICAP at Columbia University, Freetown, Sierra Leone
Eric Nzirakaindi Ikoona
Rhites-N, Acholi, Gulu City, Uganda
Judith Aloyo
Faculty of Medicine, Department of Surgery, Gulu University, P.O. Box 166, Gulu City, Uganda
David Lagoro Kitara
Harvard University, Cambridge, USA
You can also search for this author in PubMed Google Scholar
Contributions
FWDO, JA, JNO, ENI and DLK searched and screened studies and extracted data from selected articles. FWDO wrote the first draft of the manuscript, and all authors reviewed and edited the final draft. All authors approved the final version of the manuscript.
Corresponding author
Correspondence to David Lagoro Kitara .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
All authors declare no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Oyat, F.W.D., Oloya, J.N., Atim, P. et al. The psychological impact, risk factors and coping strategies to COVID-19 pandemic on healthcare workers in the sub-Saharan Africa: a narrative review of existing literature. BMC Psychol 10 , 284 (2022). https://doi.org/10.1186/s40359-022-00998-z
Download citation
Received : 03 September 2022
Accepted : 22 November 2022
Published : 01 December 2022
DOI : https://doi.org/10.1186/s40359-022-00998-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- COVID-19 pandemic
- Social support
- Occupational health and safety
- Mental health surveillance
- Workplace organization
BMC Psychology
ISSN: 2050-7283
- General enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Obstructive sleep apnea and acute lower respiratory tract infections: a narrative literature review.

1. Introduction
2. literature search strategy, 3. obstructive sleep apnea and community-acquired pneumonia, 4. obstructive sleep apnea and influenza pneumonia, 5. obstructive sleep apnea and covid-19 pneumonia, 6. obstructive sleep apnea and lower respiratory tract infections: pathophysiology, 6.1. altered immunity, 6.2. risk of aspiration, 6.3. the role of obesity and other comorbidities, 7. obstructive sleep apnea and lower respiratory tract infections: treatment, 7.1. settings of care and empiric antibiotics, 7.2. specific risks guiding empiric antibiotic therapy, 7.3. antibiotic pharmacokinetics, side effects, and resistance, 8. discussion, 9. conclusions, supplementary materials, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019 , 7 , 687–698. [ Google Scholar ] [ CrossRef ]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015 , 3 , 310–318. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010 , 90 , 47–112. [ Google Scholar ] [ CrossRef ]
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008 , 5 , 144–153. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Randerath, W.; Bassetti, C.L.; Bonsignore, M.R.; Farre, R.; Ferini-Strambi, L.; Grote, L.; Hedner, J.; Kohler, M.; Martinez-Garcia, M.A.; Mihaicuta, S.; et al. Challenges and perspectives in obstructive sleep apnoea: Report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. Eur. Respir. J. 2018 , 52 , 1702616. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019 , 14 , 8. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, J.Y.; Ko, I.; Kim, D.K. Association of Obstructive Sleep Apnea with the Risk of Affective Disorders. JAMA Otolaryngol. Head. Neck Surg. 2019 , 145 , 1020–1026. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Feldman, C.; Shaddock, E. Epidemiology of lower respiratory tract infections in adults. Expert. Rev. Respir. Med. 2019 , 13 , 63–77. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- International Respiratory Coalition (IRC). Lower Respiratory Tract Infections. Available online: https://international-respiratory-coalition.org/diseases/lower-respiratory-tract-infections/ (accessed on 10 April 2024).
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 April 2024).
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020 , 369 , m1985. [ Google Scholar ] [ CrossRef ]
- Mizgerd, J.P. Acute lower respiratory tract infection. N. Engl. J. Med. 2008 , 358 , 716–727. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ludwig, K.; Huppertz, T.; Radsak, M.; Gouveris, H. Cellular Immune Dysfunction in Obstructive Sleep Apnea. Front. Surg. 2022 , 9 , 890377. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Faverio, P.; Zanini, U.; Monzani, A.; Parati, G.; Luppi, F.; Lombardi, C.; Perger, E. Sleep-Disordered Breathing and Chronic Respiratory Infections: A Narrative Review in Adult and Pediatric Population. Int. J. Mol. Sci. 2023 , 24 , 5504. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Keto, J.; Feuth, T.; Linna, M.; Saaresranta, T. Lower respiratory tract infections among newly diagnosed sleep apnea patients. BMC Pulm. Med. 2023 , 23 , 332. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Grant, L.R.; Meche, A.; McGrath, L.; Miles, A.; Alfred, T.; Yan, Q.; Chilson, E. Risk of Pneumococcal Disease in US Adults by Age and Risk Profile. Open Forum Infect. Dis. 2023 , 10 , ofad192. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lutsey, P.L.; Zineldin, I.; Misialek, J.R.; Full, K.M.; Lakshminarayan, K.; Ishigami, J.; Cowan, L.T.; Matsushita, K.; Demmer, R.T. OSA and Subsequent Risk of Hospitalization with Pneumonia, Respiratory Infection, and Total Infection: The Atherosclerosis Risk in Communities Study. Chest 2023 , 163 , 942–952. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chiner, E.; Llombart, M.; Valls, J.; Pastor, E.; Sancho-Chust, J.N.; Andreu, A.L.; Sánchez-de-la-Torre, M.; Barbé, F. Association between Obstructive Sleep Apnea and Community-Acquired Pneumonia. PLoS ONE 2016 , 11 , e0152749. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Su, V.Y.; Liu, C.J.; Wang, H.K.; Wu, L.A.; Chang, S.C.; Perng, D.W.; Su, W.J.; Chen, Y.M.; Lin, E.Y.; Chen, T.J.; et al. Sleep apnea and risk of pneumonia: A nationwide population-based study. CMAJ 2014 , 186 , 415–421. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lindenauer, P.K.; Stefan, M.S.; Johnson, K.G.; Priya, A.; Pekow, P.S.; Rothberg, M.B. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest 2014 , 145 , 1032–1038. [ Google Scholar ] [ CrossRef ]
- Beumer, M.C.; Koch, R.M.; van Beuningen, D.; OudeLashof, A.M.; van de Veerdonk, F.L.; Kolwijck, E.; van der Hoeven, J.G.; Bergmans, D.C.; Hoedemaekers, C.W.E. Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J. Crit. Care 2019 , 50 , 59–65. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Boattini, M.; Charrier, L.; Almeida, A.; Christaki, E.; Moreira Marques, T.; Tosatto, V.; Bianco, G.; Iannaccone, M.; Tsiolakkis, G.; Karagiannis, C.; et al. Burden of primary influenza and respiratory syncytial virus pneumonia in hospitalised adults: Insights from a 2-year multi-centre cohort study (2017–2018). Intern. Med. J. 2023 , 53 , 404–408. [ Google Scholar ] [ CrossRef ]
- Mok, E.M.; Greenough, G.; Pollack, C.C. Untreated obstructive sleep apnea is associated with increased hospitalization from influenza infection. J. Clin. Sleep Med. 2020 , 16 , 2003–2007. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tsai, M.S.; Chen, H.C.; Li, H.Y.; Tsai, Y.T.; Yang, Y.H.; Liu, C.Y.; Lee, Y.C.; Hsu, C.M.; Lee, L.A. Sleep Apnea and Risk of Influenza-Associated Severe Acute Respiratory Infection: Real-World Evidence. Nat. Sci. Sleep 2022 , 14 , 901–909. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, T.Y.; Chang, R.; Chiu, L.T.; Hung, Y.M.; Wei, J.C. Obstructive sleep apnea and influenza infection: A nationwide population-based cohort study. Sleep Med. 2021 , 81 , 202–209. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mashaqi, S.; Lee-Iannotti, J.; Rangan, P.; Celaya, M.P.; Gozal, D.; Quan, S.F.; Parthasarathy, S. Obstructive sleep apnea and COVID-19 clinical outcomes during hospitalization: A cohort study. J. Clin. Sleep Med. 2021 , 17 , 2197–2204. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maas, M.B.; Kim, M.; Malkani, R.G.; Abbott, S.M.; Zee, P.C. Obstructive Sleep Apnea and Risk of COVID-19 Infection, Hospitalization and Respiratory Failure. Sleep Breath 2021 , 25 , 1155–1157. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Strausz, S.; Kiiskinen, T.; Broberg, M.; Ruotsalainen, S.; Koskela, J.; Bachour, A.; Palotie, A.; Palotie, T.; Ripatti, S.; Ollila, H.M. Sleep apnoea is a risk factor for severe COVID-19. BMJ Open Respir. Res. 2021 , 8 , e000845. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rögnvaldsson, K.G.; Eyþórsson, E.S.; Emilsson, Ö.I.; Eysteinsdóttir, B.; Pálsson, R.; Gottfreðsson, M.; Guðmundsson, G.; Steingrímsson, V. Obstructive sleep apnea is an independent risk factor for severe COVID-19: A population-based study. Sleep 2022 , 45 , zsab272. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cade, B.E.; Dashti, H.S.; Hassan, S.M.; Redline, S.; Karlson, E.W. Sleep Apnea and COVID-19 Mortality and Hospitalization. Am. J. Respir. Crit. Care Med. 2020 , 202 , 1462–1464. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pena Orbea, C.; Wang, L.; Shah, V.; Jehi, L.; Milinovich, A.; Foldvary-Schaefer, N.; Chung, M.K.; Mashaqi, S.; Aboussouan, L.; Seidel, K.; et al. Association of Sleep-Related Hypoxia with Risk of COVID-19 Hospitalizations and Mortality in a Large Integrated Health System. JAMA Netw. Open 2021 , 4 , e2134241. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Oh, T.K.; Song, I.A. Impact of coronavirus disease-2019 on chronic respiratory disease in South Korea: An NHIS COVID-19 database cohort study. BMC Pulm. Med. 2021 , 21 , 12. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gottlieb, M.; Sansom, S.; Frankenberger, C.; Ward, E.; Hota, B. Clinical Course and Factors Associated with Hospitalization and Critical Illness Among COVID-19 Patients in Chicago, Illinois. Acad. Emerg. Med. 2020 , 27 , 963–973. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kendzerska, T.; Povitz, M.; Gershon, A.S.; Ryan, C.M.; Talarico, R.; Franco Avecilla, D.A.; Robillard, R.; Ayas, N.T.; Pendharkar, S.R. Association of clinically significant obstructive sleep apnoea with risks of contracting COVID-19 and serious COVID-19 complications: A retrospective population-based study of health administrative data. Thorax 2023 , 78 , 933–941. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Peker, Y.; Celik, Y.; Arbatli, S.; Isik, S.R.; Balcan, B.; Karataş, F.; Uzel, F.I.; Tabak, L.; Çetin, B.; Baygül, A.; et al. Effect of High-Risk Obstructive Sleep Apnea on Clinical Outcomes in Adults with Coronavirus Disease 2019: A Multicenter, Prospective, Observational Clinical Trial. Ann. Am. Thorac. Soc. 2021 , 18 , 1548–1559. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Girardin, J.L.; Seixas, A.; Ramos Cejudo, J.; Osorio, R.S.; Avirappattu, G.; Reid, M.; Parthasarathy, S. Contribution of pulmonary diseases to COVID-19 mortality in a diverse urban community of New York. Chron. Respir. Dis. 2021 , 18 , 1479973120986806. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gimeno-Miguel, A.; Bliek-Bueno, K.; Poblador-Plou, B.; Carmona-Pírez, J.; Poncel-Falcó, A.; González-Rubio, F.; Ioakeim-Skoufa, I.; Pico-Soler, V.; Aza-Pascual-Salcedo, M.; Prados-Torres, A.; et al. Chronic diseases associated with increased likelihood of hospitalization and mortality in 68,913 COVID-19 confirmed cases in Spain: A population-based cohort study. PLoS ONE 2021 , 16 , e0259822. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020 , 63 , 1500–1515. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ioannou, G.N.; Locke, E.; Green, P.; Berry, K.; O’Hare, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Dominitz, J.A.; Fan, V.S. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans With SARS-CoV-2 Infection. JAMA Netw. Open 2020 , 3 , e2022310. [ Google Scholar ] [ CrossRef ]
- Izquierdo, J.L.; Ancochea, J.; Soriano, J.B. Clinical Characteristics and Prognostic Factors for Intensive Care Unit Admission of Patients with COVID-19: Retrospective Study Using Machine Learning and Natural Language Processing. J. Med. Internet Res. 2020 , 22 , e21801. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lohia, P.; Sreeram, K.; Nguyen, P.; Choudhary, A.; Khicher, S.; Yarandi, H.; Kapur, S.; Badr, M.S. Preexisting respiratory diseases and clinical outcomes in COVID-19: A multihospital cohort study on predominantly African American population. Respir. Res. 2021 , 22 , 37. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Prasad, B.; Mechineni, A.; Talugula, S.; Gardner, J.; Rubinstein, I.; Gordon, H.S. Impact of Obstructive Sleep Apnea on Health Outcomes in Veterans Hospitalized with COVID-19 Infection. Ann. Am. Thorac. Soc. 2024; online ahead of print . [ Google Scholar ] [ CrossRef ]
- Bailly, S.; Galerneau, L.M.; Ruckly, S.; Seiller, A.; Terzi, N.; Schwebel, C.; Dupuis, C.; Tamisier, R.; Mourvillier, B.; Pepin, J.L.; et al. Impact of obstructive sleep apnea on the obesity paradox in critically ill patients. J. Crit. Care 2020 , 56 , 120–124. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bolona, E.; Hahn, P.Y.; Afessa, B. Intensive care unit and hospital mortality in patients with obstructive sleep apnea. J. Crit. Care 2015 , 30 , 178–180. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018 , 31 , 174–183. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tyrrell, C.S.; Allen, J.L.Y.; Gkrania-Klotsas, E. Influenza: Epidemiology and hospital management. Medicine 2021 , 49 , 797–804. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Veerapandian, R.; Snyder, J.D.; Samarasinghe, A.E. Influenza in Asthmatics: For Better or for Worse? Front. Immunol. 2018 , 9 , 1843. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rennard, S.; Decramer, M.; Calverley, P.M.; Pride, N.B.; Soriano, J.B.; Vermeire, P.A.; Vestbo, J. Impact of COPD in North America and Europe in 2000: Subjects’ perspective of Confronting COPD International Survey. Eur. Respir. J. 2002 , 20 , 799–805. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020 , 584 , 430–436. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Denson, J.L.; Gillet, A.S.; Zu, Y.; Brown, M.; Pham, T.; Yoshida, Y.; Mauvais-Jarvis, F.; Douglas, I.S.; Moore, M.; Tea, K.; et al. Metabolic Syndrome and Acute Respiratory Distress Syndrome in Hospitalized Patients With COVID-19. JAMA Netw. Open 2021 , 4 , e2140568. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Miller, M.A.; Cappuccio, F.P. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med. Rev. 2021 , 55 , 101382. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bellou, V.; Tzoulaki, I.; van Smeden, M.; Moons, K.G.M.; Evangelou, E.; Belbasis, L. Prognostic factors for adverse outcomes in patients with COVID-19: A field-wide systematic review and meta-analysis. Eur. Respir. J. 2022 , 59 , 2002964. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vardavas, C.I.; Mathioudakis, A.G.; Nikitara, K.; Stamatelopoulos, K.; Georgiopoulos, G.; Phalkey, R.; Leonardi-Bee, J.; Fernandez, E.; Carnicer-Pont, D.; Vestbo, J.; et al. Prognostic factors for mortality, intensive care unit and hospital admission due to SARS-CoV-2: A systematic review and meta-analysis of cohort studies in Europe. Eur. Respir. Rev. 2022 , 31 , 220098. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hariyanto, T.I.; Kurniawan, A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: A systematic review and meta-analysis. Sleep Med. 2021 , 82 , 47–53. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hu, M.; Han, X.; Ren, J.; Wang, Y.; Yang, H. Significant association of obstructive sleep apnoea with increased risk for fatal COVID-19: A quantitative meta-analysis based on adjusted effect estimates. Sleep Med. Rev. 2022 , 63 , 101624. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mandel, L.H.; Colleen, G.; Abedian, S.; Ammar, N.; Charles Bailey, L.; Bennett, T.D.; Daniel Brannock, M.; Brosnahan, S.B.; Chen, Y.; Chute, C.G.; et al. Risk of post-acute sequelae of SARS-CoV-2 infection associated with pre-coronavirus disease obstructive sleep apnea diagnoses: An electronic health record-based analysis from the RECOVER initiative. Sleep 2023 , 46 , zsad126. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Labarca, G.; Henríquez-Beltrán, M.; Lamperti, L.; Nova-Lamperti, E.; Sanhueza, S.; Cabrera, C.; Quiroga, R.; Antilef, B.; Ormazábal, V.; Zúñiga, F.; et al. Impact of Obstructive Sleep Apnea (OSA) in COVID-19 Survivors, Symptoms Changes Between 4-Months and 1 Year After the COVID-19 Infection. Front. Med. 2022 , 9 , 884218. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chervin, R.D. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest 2000 , 118 , 372–379. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine 2023 , 55 , 101762. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022 , 328 , 1604–1615. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011 , 306 , 613–619. [ Google Scholar ] [ CrossRef ]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021 , 398 , 747–758. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wheaton, A.G.; Perry, G.S.; Chapman, D.P.; Croft, J.B. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005–2008. Sleep 2012 , 35 , 461–467. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rezaeitalab, F.; Moharrari, F.; Saberi, S.; Asadpour, H.; Rezaeetalab, F. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J. Res. Med. Sci. 2014 , 19 , 205–210. [ Google Scholar ] [ PubMed ]
- Menzler, K.; Mayr, P.; Knake, S.; Cassel, W.; Viniol, C.; Reitz, L.; Tsalouchidou, P.E.; Janzen, A.; Anschuetz, K.; Mross, P.; et al. Undiagnosed obstructive sleep apnea syndrome as a treatable cause of new-onset sleepiness in some post-COVID patients. Eur. J. Neurol. 2024 , 31 , e16159. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009 , 6 , e1000132. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019 , 20 , 459. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015 , 147 , 266–274. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kimoff, R.J. Sleep fragmentation in obstructive sleep apnea. Sleep 1996 , 19 , S61–S66. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jun, J.; Savransky, V.; Nanayakkara, A.; Bevans, S.; Li, J.; Smith, P.L.; Polotsky, V.Y. Intermittent hypoxia has organ-specific effects on oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008 , 295 , R1274–R1281. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Sadeghi Bahmani, D.; Brand, S. Evaluation of Serum and Plasma Interleukin-6 Levels in Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Immunol. 2020 , 11 , 1343. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Van der Touw, T.; Andronicos, N.M.; Smart, N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019 , 24 , 429–435. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010 , 16 , 238–246. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021 , 71 , 101422. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shukla, S.D.; Walters, E.H.; Simpson, J.L.; Keely, S.; Wark, P.A.B.; O’Toole, R.F.; Hansbro, P.M. Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology 2020 , 25 , 53–63. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020 , 130 , 5042–5051. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012 , 463 , 121–137. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ibarra-Coronado, E.G.; Pantaleón-Martínez, A.M.; Velazquéz-Moctezuma, J.; Prospéro-García, O.; Méndez-Díaz, M.; Pérez-Tapia, M.; Pavón, L.; Morales-Montor, J. The Bidirectional Relationship between Sleep and Immunity against Infections. J. Immunol. Res. 2015 , 2015 , 678164. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dopp, J.M.; Wiegert, N.A.; Moran, J.J.; Muller, D.; Weber, S.; Hayney, M.S. Humoral immune responses to influenza vaccination in patients with obstructive sleep apnea. Pharmacotherapy 2007 , 27 , 1483–1489. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tufik, S.; Andersen, M.L.; Rosa, D.S.; Tufik, S.B.; Pires, G.N. Effects of Obstructive Sleep Apnea on SARS-CoV-2 Antibody Response After Vaccination Against COVID-19 in Older Adults. Nat. Sci. Sleep 2022 , 14 , 1203–1211. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Quach, H.Q.; Warner, N.D.; Ovsyannikova, I.G.; Covassin, N.; Poland, G.A.; Somers, V.K.; Kennedy, R.B. Excessive daytime sleepiness is associated with impaired antibody response to influenza vaccination in older male adults. Front. Cell Infect. Microbiol. 2023 , 13 , 1229035. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dimitrov, S.; Lange, T.; Tieken, S.; Fehm, H.L.; Born, J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav. Immun. 2004 , 18 , 341–348. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Patel, S.R.; Malhotra, A.; Gao, X.; Hu, F.B.; Neuman, M.I.; Fawzi, W.W. A prospective study of sleep duration and pneumonia risk in women. Sleep 2012 , 35 , 97–101. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Resta, O.; Foschino Barbaro, M.P.; Bonfitto, P.; Talamo, S.; Mastrosimone, V.; Stefano, A.; Giliberti, T. Hypercapnia in obstructive sleep apnoea syndrome. Neth. J. Med. 2000 , 56 , 215–222. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Budhiraja, R.; Siddiqi, T.A.; Quan, S.F. Sleep disorders in chronic obstructive pulmonary disease: Etiology, impact, and management. J. Clin. Sleep Med. 2015 , 11 , 259–270. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Macavei, V.M.; Spurling, K.J.; Loft, J.; Makker, H.K. Diagnostic predictors of obesity-hypoventilation syndrome in patients suspected of having sleep disordered breathing. J. Clin. Sleep Med. 2013 , 9 , 879–884. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gates, K.L.; Howell, H.A.; Nair, A.; Vohwinkel, C.U.; Welch, L.C.; Beitel, G.J.; Hauser, A.R.; Sznajder, J.I.; Sporn, P.H. Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Am. J. Respir. Cell Mol. Biol. 2013 , 49 , 821–828. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhu, Q.; Hua, L.; Chen, L.; Mu, T.; Dong, D.; Xu, J.; Shen, C. Causal association between obstructive sleep apnea and gastroesophageal reflux disease: A bidirectional two-sample Mendelian randomization study. Front. Genet. 2023 , 14 , 1111144. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wu, Z.H.; Yang, X.P.; Niu, X.; Xiao, X.Y.; Chen, X. The relationship between obstructive sleep apnea hypopnea syndrome and gastroesophageal reflux disease: A meta-analysis. Sleep Breath 2019 , 23 , 389–397. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Emilsson, Ö.I.; Bengtsson, A.; Franklin, K.A.; Torén, K.; Benediktsdóttir, B.; Farkhooy, A.; Weyler, J.; Dom, S.; De Backer, W.; Gislason, T.; et al. Nocturnal gastro-oesophageal reflux, asthma and symptoms of OSA: A longitudinal, general population study. Eur. Respir. J. 2013 , 41 , 1347–1354. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- You, C.R.; Oh, J.H.; Seo, M.; Lee, H.Y.; Joo, H.; Jung, S.H.; Lee, S.H.; Choi, M.G. Association Between Non-erosive Reflux Disease and High Risk of Obstructive Sleep Apnea in Korean Population. J. Neurogastroenterol. Motil. 2014 , 20 , 197–204. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hsu, W.T.; Lai, C.C.; Wang, Y.H.; Tseng, P.H.; Wang, K.; Wang, C.Y.; Chen, L. Risk of pneumonia in patients with gastroesophageal reflux disease: A population-based cohort study. PLoS ONE 2017 , 12 , e0183808. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fohl, A.L.; Regal, R.E. Proton pump inhibitor-associated pneumonia: Not a breath of fresh air after all? World J. Gastrointest. Pharmacol. Ther. 2011 , 2 , 17–26. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nguyen, A.T.; Jobin, V.; Payne, R.; Beauregard, J.; Naor, N.; Kimoff, R.J. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep 2005 , 28 , 585–593. [ Google Scholar ] [ CrossRef ]
- Ghannouchi, I.; Speyer, R.; Doma, K.; Cordier, R.; Verin, E. Swallowing function and chronic respiratory diseases: Systematic review. Respir. Med. 2016 , 117 , 54–64. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pizzorni, N.; Radovanovic, D.; Pecis, M.; Lorusso, R.; Annoni, F.; Bartorelli, A.; Rizzi, M.; Schindler, A.; Santus, P. Dysphagia symptoms in obstructive sleep apnea: Prevalence and clinical correlates. Respir. Res. 2021 , 22 , 117. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Teramoto, S.; Sudo, E.; Matsuse, T.; Ohga, E.; Ishii, T.; Ouchi, Y.; Fukuchi, Y. Impaired swallowing reflex in patients with obstructive sleep apnea syndrome. Chest 1999 , 116 , 17–21. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017 , 69 , 841–858. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gleeson, K.; Eggli, D.F.; Maxwell, S.L. Quantitative aspiration during sleep in normal subjects. Chest 1997 , 111 , 1266–1272. [ Google Scholar ] [ CrossRef ]
- Beal, M.; Chesson, A.; Garcia, T.; Caldito, G.; Stucker, F.; Nathan, C.O. A pilot study of quantitative aspiration in patients with symptoms of obstructive sleep apnea: Comparison to a historic control group. Laryngoscope 2004 , 114 , 965–968. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sato, K.; Chitose, S.I.; Sato, K.; Sato, F.; Ono, T.; Umeno, H. Recurrent aspiration pneumonia precipitated by obstructive sleep apnea. Auris Nasus Larynx 2021 , 48 , 659–665. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013 , 177 , 1006–1014. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. 2017 , 1 , 00019. [ Google Scholar ] [ PubMed ]
- Anderson, M.R.; Shashaty, M.G.S. Impact of Obesity in Critical Illness. Chest 2021 , 160 , 2135–2145. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhao, Y.; Li, Z.; Yang, T.; Wang, M.; Xi, X. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS ONE 2018 , 13 , e0198669. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kahlon, S.; Eurich, D.T.; Padwal, R.S.; Malhotra, A.; Minhas-Sandhu, J.K.; Marrie, T.J.; Majumdar, S.R. Obesity and outcomes in patients hospitalized with pneumonia. Clin. Microbiol. Infect. 2013 , 19 , 709–716. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Botros, N.; Concato, J.; Mohsenin, V.; Selim, B.; Doctor, K.; Yaggi, H.K. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am. J. Med. 2009 , 122 , 1122–1127. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000 , 342 , 1378–1384. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005 , 365 , 1046–1053. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010 , 122 , 352–360. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Teodorescu, M.; Broytman, O.; Curran-Everett, D.; Sorkness, R.L.; Crisafi, G.; Bleecker, E.R.; Erzurum, S.; Gaston, B.M.; Wenzel, S.E.; Jarjour, N.N. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J. Allergy Clin. Immunol. Pract. 2015 , 3 , 566–575.e561. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kimmel, P.L.; Miller, G.; Mendelson, W.B. Sleep apnea syndrome in chronic renal disease. Am. J. Med. 1989 , 86 , 308–314. [ Google Scholar ] [ CrossRef ]
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013 , 68 , 1057–1065. [ Google Scholar ] [ CrossRef ]
- Naqvi, S.B.; Collins, A.J. Infectious complications in chronic kidney disease. Adv. Chronic Kidney Dis. 2006 , 13 , 199–204. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Viasus, D.; Garcia-Vidal, C.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J. Infect. 2013 , 66 , 27–33. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Johnsen, R.H.; Heerfordt, C.K.; Boel, J.B.; Dessau, R.B.; Ostergaard, C.; Sivapalan, P.; Eklöf, J.; Jensen, J.S. Inhaled corticosteroids and risk of lower respiratory tract infection with Moraxella catarrhalis in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2023 , 10 , e001726. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019 , 200 , e45–e67. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, M.A.; Park, J.S.; Lee, C.W.; Choi, W.I. Pneumonia severity index in viral community acquired pneumonia in adults. PLoS ONE 2019 , 14 , e0210102. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Restrepo, M.I.; Babu, B.L.; Reyes, L.F.; Chalmers, J.D.; Soni, N.J.; Sibila, O.; Faverio, P.; Cilloniz, C.; Rodriguez-Cintron, W.; Aliberti, S. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: A multinational point prevalence study of hospitalised patients. Eur. Respir. J. 2018 , 52 , 1701190. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Srivali, N.; Chongnarungsin, D.; Ungprasert, P.; Edmonds, L.C. Two cases of Legionnaires’ disease associated with continuous positive airway pressure therapy. Sleep Med. 2013 , 14 , 1038. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schnirman, R.; Nur, N.; Bonitati, A.; Carino, G. A case of legionella pneumonia caused by home use of continuous positive airway pressure. SAGE Open Med. Case Rep. 2017 , 5 , 2050313x17744981. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brady, M.F.; Awosika, A.O.; Sundareshan, V. Legionnaires’ Disease. In StatPearls ; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [ Google Scholar ]
- Kato, H.; Hagihara, M.; Asai, N.; Shibata, Y.; Koizumi, Y.; Yamagishi, Y.; Mikamo, H. Meta-analysis of fluoroquinolones versus macrolides for treatment of legionella pneumonia. J. Infect. Chemother. 2021 , 27 , 424–433. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cesar, L.; Gonzalez, C.; Calia, F.M. Bacteriologic flora of aspiration-induced pulmonary infections. Arch. Intern. Med. 1975 , 135 , 711–714. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Influenza Antiviral Medications: Summary for Clinicians|CDC. Available online: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm#highrisk (accessed on 4 May 2024).
- People with Certain Medical Conditions|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 4 May 2024).
- Zhang, X.B.; Chen, X.Y.; Chiu, K.Y.; He, X.Z.; Wang, J.M.; Zeng, H.Q.; Zeng, Y. Intermittent Hypoxia Inhibits Hepatic CYP1a2 Expression and Delays Aminophylline Metabolism. Evid. Based Complement. Alternat Med. 2022 , 2022 , 2782702. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fradette, C.; Du Souich, P. Effect of hypoxia on cytochrome P450 activity and expression. Curr. Drug Metab. 2004 , 5 , 257–271. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fohner, A.E.; Sparreboom, A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet. Genom. 2017 , 27 , 164–167. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am. J. Respir. Crit. Care Med. 2019 , 199 , 830–841. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Elbarbry, F. Vancomycin Dosing and Monitoring: Critical Evaluation of the Current Practice. Eur. J. Drug Metab. Pharmacokinet. 2018 , 43 , 259–268. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Munar, M.Y.; Singh, H. Drug dosing adjustments in patients with chronic kidney disease. Am. Fam. Physician 2007 , 75 , 1487–1496. [ Google Scholar ] [ PubMed ]
- Gorelik, E.; Masarwa, R.; Perlman, A.; Rotshild, V.; Abbasi, M.; Muszkat, M.; Matok, I. Fluoroquinolones and Cardiovascular Risk: A Systematic Review, Meta-analysis and Network Meta-analysis. Drug Saf. 2019 , 42 , 529–538. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Guo, D.; Cai, Y.; Chai, D.; Liang, B.; Bai, N.; Wang, R. The cardiotoxicity of macrolides: A systematic review. Pharmazie 2010 , 65 , 631–640. [ Google Scholar ] [ PubMed ]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017 , 102 , 459–469. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017 , 10 , 369–378. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McIsaac, D.I.; Gershon, A.; Wijeysundera, D.; Bryson, G.L.; Badner, N.; van Walraven, C. Identifying Obstructive Sleep Apnea in Administrative Data: A Study of Diagnostic Accuracy. Anesthesiology 2015 , 123 , 253–263. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Steinhauer, K.; Goroncy-Bermes, P. Investigation of the hygienic safety of continuous positive airways pressure devices after reprocessing. J. Hosp. Infect. 2005 , 61 , 168–175. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ortolano, G.A.; Schaffer, J.; McAlister, M.B.; Stanchfield, I.; Hill, E.; Vandenburgh, L.; Lewis, M.; John, S.; Canonica, F.P.; Cervia, J.S. Filters reduce the risk of bacterial transmission from contaminated heated humidifiers used with CPAP for obstructive sleep apnea. J. Clin. Sleep Med. 2007 , 3 , 700–705. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sanner, B.M.; Fluerenbrock, N.; Kleiber-Imbeck, A.; Mueller, J.B.; Zidek, W. Effect of continuous positive airway pressure therapy on infectious complications in patients with obstructive sleep apnea syndrome. Respiration 2001 , 68 , 483–487. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jao, L.Y.; Su, W.L.; Chang, H.C.; Lan, C.C.; Wu, Y.K.; Yang, M.C. Pneumocystis jirovecii pneumonia presenting as a solitary pulmonary granuloma due to unclean continuous positive airway pressure equipment: A case report. J. Clin. Sleep Med. 2022 , 18 , 1717–1721. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Caiano Gil, J.; Calisto, R.; Amado, J.; Barreto, V. Eikenella corrodens and Porphyromonas asaccharolytica pleural empyema in a diabetic patient with obstructive sleep apnea syndrome on noninvasive ventilation. Rev. Port. Pneumol. 2013 , 19 , 76–79. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Patel, S.R. Providing Cleaning Recommendations for Positive Airway Pressure Devices. Ann. Am. Thorac. Soc. 2024 , 21 , 27–29. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mercieca, L.; Pullicino, R.; Camilleri, K.; Abela, R.; Mangion, S.A.; Cassar, J.; Zammit, M.; Gatt, C.; Deguara, C.; Barbara, C.; et al. Continuous Positive Airway Pressure: Is it a route for infection in those with Obstructive Sleep Apnoea? Sleep Sci. 2017 , 10 , 28–34. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gavidia, R.; Shieu, M.M.; Dunietz, G.L.; Braley, T.J. Respiratory infection risk in positive airway pressure therapy users: A retrospective cohort study. J. Clin. Sleep Med. 2023 , 19 , 1769–1773. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mutti, C.; Azzi, N.; Soglia, M.; Pollara, I.; Alessandrini, F.; Parrino, L. Obstructive sleep apnea, cpap and COVID-19: A brief review. Acta Biomed. 2020 , 91 , e2020196. [ Google Scholar ] [ CrossRef ]
- Feng, Z.; Glasser, J.W.; Hill, A.N. On the benefits of flattening the curve: A perspective. Math. Biosci. 2020 , 326 , 108389. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Klompas, M.; Baker, M.A.; Rhee, C. Airborne Transmission of SARS-CoV-2: Theoretical Considerations and Available Evidence. JAMA 2020 , 324 , 441–442. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Drummond, M. Sleep labs, lung function tests and COVID-19 pandemic—Only emergencies allowed! Pulmonology 2020 , 26 , 244–245. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Barker, J.; Oyefeso, O.; Koeckerling, D.; Mudalige, N.L.; Pan, D. COVID-19: Community CPAP and NIV should be stopped unless medically necessary to support life. Thorax 2020 , 75 , 367. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sampol, J.; Sáez, M.; Martí, S.; Pallero, M.; Barrecheguren, M.; Ferrer, J.; Sampol, G. Impact of home CPAP-treated obstructive sleep apnea on COVID-19 outcomes in hospitalized patients. J. Clin. Sleep Med. 2022 , 18 , 1857–1864. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pépin, J.L.; Bailly, S.; Borel, J.C.; Logerot, S.; Sapène, M.; Martinot, J.B.; Lévy, P.; Tamisier, R. Detecting COVID-19 and other respiratory infections in obstructive sleep apnoea patients through CPAP device telemonitoring. Digit. Health 2021 , 7 , 20552076211002957. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pneumococcal Vaccination: Who and When to Vaccinate|CDC. Available online: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html#adults-19-64 (accessed on 10 April 2024).
- Influenza Vaccination: A Summary for Clinicians|CDC. Available online: https://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm#vaccinated (accessed on 10 April 2024).
- People at Higher Risk of Flu Complications|CDC. Available online: https://www.cdc.gov/flu/highrisk/index.htm (accessed on 10 April 2024).
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; Hinay, A.A., Jr.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J.; et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024 , 24 , e82. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Song, X.D.; Yang, G.J.; Jiang, X.L.; Wang, X.J.; Zhang, Y.W.; Wu, J.; Wang, M.M.; Chen, R.R.; He, X.J.; Dong, G.; et al. Seroprevalence of SARS-CoV-2 neutralising antibodies and cross-reactivity to JN.1 one year after the BA.5/BF.7 wave in China. Lancet Reg. Health West. Pac. 2024 , 44 , 101040. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jeworowski, L.M.; Mühlemann, B.; Walper, F.; Schmidt, M.L.; Jansen, J.; Krumbholz, A.; Simon-Lorière, E.; Jones, T.C.; Corman, V.M.; Drosten, C. Humoral immune escape by current SARS-CoV-2 variants BA.2.86 and JN.1, December 2023. Euro Surveill. 2024 , 29 , 2300740. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.M.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018 , 18 , 1191–1210. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ford, N.D.; Patel, S.A.; Narayan, K.M. Obesity in Low- and Middle-Income Countries: Burden, Drivers, and Emerging Challenges. Annu. Rev. Public Health 2017 , 38 , 145–164. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Roche, J.; Rae, D.E.; Redman, K.N.; Knutson, K.L.; von Schantz, M.; Gómez-Olivé, F.X.; Scheuermaier, K. Sleep disorders in low- and middle-income countries: A call for action. J. Clin. Sleep Med. 2021 , 17 , 2341–2342. [ Google Scholar ] [ CrossRef ] [ PubMed ]
| (“Obstructive Sleep Apnea” OR “Sleep Apnea Syndromes” OR “Sleep-related breathing disorder” OR OSA) AND (pneumonia OR “acute pneumonia” OR “bacterial pneumonia” OR “community acquired pneumonia” OR CAP OR “lung infection” OR “respiratory infection” OR “bronchopneumonia”) |
| (“Obstructive Sleep Apnea” OR “Sleep Apnea Syndromes” OR “Sleep-related breathing disorder” OR OSA) AND (influenza OR “Influenza A” OR “Influenza B” OR “H1N1” OR “swine flu” OR “avian influenza” OR “H5N1” OR “seasonal influenza” OR “viral pneumonia” OR flu) |
| (“Obstructive Sleep Apnea” OR “Sleep Apnea Syndromes” OR “Sleep-related breathing disorder” OR OSA) AND (COVID-19 OR “SARS-CoV-2” OR “2019-nCoV” OR “coronavirus disease 2019” OR “novel coronavirus” OR “viral pneumonia”) |
| Author and Date | Design | Total N (OSA N) | Inclusion and Exclusion Criteria | Outcomes | Key Findings | Limitations |
|---|---|---|---|---|---|---|
| Keto et al., 2023 [ ] | Case-control from Finland | 50,648 (25,324) | I: ICD code for OSA. E: OSA in the two years preceding the index date. | LRTI, recurring LRTI. | ↑ LRTI in the year preceding OSA RR 1.35, and during the year after OSA RR 1.39. | No PSG data, no data on OSA treatment, no BMI data. |
| Grant et al., 2023 [ ] | Retrospective cohort from healthcare plans database | 38.62M PY (1.29M PY) | I: Minimum 1 year of enrollment in health plan. E: Death date before January 1st of the index year; Overlapping pneumonia inpatient admissions. | All-cause pneumonia, invasive pneumococcal disease, pneumococcal pneumonia. | OSA: ↑ pneumonia (18–49 y RR 3.6, 50–64 y RR 3.6, ≥65 y RR 3.4), ↑ invasive pneumococcal disease (18–49 y RR 5.7, 50–64 y RR 4.2, ≥65 y RR 4.2). | No PSG data, no data on OSA treatment, no BMI data. |
| Lutsey et al., 2023 [ ] | Post-hoc analysis of the multicentric prospective cohort | 1586 (772) | I: Valid PSG data; Self-identify as White. E: CSA; Already had the outcome of interest at the time of visit. | Hospitalization: with pneumonia; with respiratory infection; with any infection. | OSA not linked to outcomes; T90 > 5% ↑ hospitalized pneumonia HR 1.59, ↑ hospitalized respiratory infection HR 1.53, ↑ hospitalized any infection HR 1.25. | No data on OSA treatment, mostly White population. |
| Chiner et al., 2016 [ ] | Single center case-control | 123 (85) | I: Cases: Hospitalized for CAP; Controls: Hospitalized for non-respiratory/non-ENT infection. E: Previous OSA diagnosis and CPAP. | Pneumonia, PSI. | AHI ≥ 10: ↑ pneumonia OR 2.86; AHI ≥ 30: ↑ pneumonia OR 3.184; AHI positively correlated with PSI. | Small sample size, no data on OSA treatment. |
| Su et al., 2014 [ ] | Retrospective cohort from Taiwan | 34,100 (6816) | I: ICD codes for OSA; E: ICD codes for pneumonia, lung abscess, empyema. | Pneumonia. | OSA: ↑ pneumonia HR 1.19; OSA requiring CPAP: ↑ pneumonia HR 1.32. | No PSG data, no BMI data. |
| Lindenauer et al., 2014 [ ] | Multicenter, retrospective cohort | 250,907 (15,569) | I: ICD code for pneumonia; Chest radiography; Antibiotics within 48 h of admission. E: Transfers; Hospital LOS under 2 days; Cystic fibrosis; Pneumonia not present at admission. | ICU, MV, hospital mortality, hospital LOS, costs. | OSA: ↑ ICU OR 1.54, ↑ MV OR 1.68, ↑ hospital LOS RR 1.14, ↑ cost RR 1.22, ↓ mortality OR 0.90. | No PSG data, no data on OSA treatment, no BMI data. |
| Beumer et al., 2019 [ ] | Two center, retrospective cohort | 199 (9) | I: Symptoms and positive influenza PCR; Transfers if not received antibiotics or antivirals. | ICU, ICU mortality. | OSA/CSA: ↑ ICU admission OR 9.73., not linked to mortality. | Small sample size, no PSG data, no data on OSA treatment. |
| Boattini et al., 2023 [ ] | Post-hoc analysis of a multicentric, retrospective cohort | 356 (23) | I: Positive influenza or RSV PCR; Symptoms; Pulmonary infiltrate on imaging. E: Viral co-infections. | NIV failure, hospital mortality. | OSA/OHS: ↑ NIV failure OR 4.66, not linked to mortality. | No PSG data, no data on OSA treatment, no BMI data, no adjustments for obesity. |
| Mok et al., 2020 [ ] | Single center, retrospective cohort | 53 (53) | I: ICD codes for OSA, influenza. E: No PSG data; No OSA treatment data; CSA on PSG. | Hospitalization, complications, hospital LOS. | OSA non-CPAP vs. CPAP: ↑ hospitalization OR 4.7. Severity of OSA not linked to hospitalization in CPAP-non adherent. | Small sample size, no adjustments for obesity and comorbidities. |
| Tsai et al., 2022 [ ] | Retrospective cohort from Taiwan | 32,540 (6508) | I: Cases: ICD codes for OSA; Controls: No OSA; Randomly selected, matched by income, gender, urbanization, and age. E: influenza pneumonia before OSA. | Influenza-associated SARI. | OSA: ↑ influenza-SARI HR 1.98, ↑ cumulative incidence of influenza-SARI. | No PSG data, no data on OSA treatment, no BMI data. |
| Chen et al., 2021 [ ] | Retrospective cohort from Taiwan | 27,501 (5483) | I: Cases: ICD codes for OSA; Controls: No OSA; Randomly selected, matched by age, sex, index years, and comorbidities. E: UPPP; influenza before OSA. | Influenza, composite (pneumonia, hospitalization). | OSA: ↑ influenza HR 1.18, ↑ pneumonia or hospitalization 1.79. | No PSG data, no data on OSA treatment, no BMI data. |
| Mashaqi et al., 2021 [ ] | Multicentric, retrospective cohort | 1738 (139) | I: Hospitalized; ICD codes, PSG report, self-report, STOP-BANG for OSA; ICD codes COVID-19. E: ICD for CSA and unspecified sleep apnea. | MV, ICU, hospital mortality, hospital LOS. | OSA not linked to ICU admission, hospital LOS, MV, or mortality. | No PSG data, no data on OSA treatment. |
| Maas et al., 2021 [ ] | Multicentric, retrospective cohort | 5544,884 (~44,877) | I: All patient encounters; January to June 2020. | COVID-19, hospitalization, respiratory failure. | OSA: ↑ COVID-19, OR 8.6, ↑ hospitalization, OR 1.65, ↑ respiratory failure, OR 1.98. | No PSG data, no data on OSA treatment. |
| Strausz et al., 2021 [ ] | Retrospective cohort from FinnGen biobank | 445 (38) | I: All positive COVID-19 PCR from FinnGen biobank. | Hospitalization, COVID-19. | OSA not linked with COVID-19, ↑ hospitalization, OR 2.93. Link attenuated after adjustment for BMI in meta-analysis. | Small sample size, no PSG data, no data on OSA treatment. |
| Rögnvaldsson et al., 2022 [ ] | Retrospective cohort from Iceland | 4756 (185) | I: Positive COVID-19 PCR. E: Nursing home; COVID-19 during hospitalization or rehabilitation. | Composite (hospitalization, mortality). | OSA: ↑ composite outcome (hospitalization and mortality) OR 2.0. OSA and CPAP: ↑ composite outcome (hospitalization and mortality) OR 2.4. | No PSG data for the control group, no BMI data for 30% of controls and 2% of the OSA group. |
| Cade et al., 2020 [ ] | Multicentric, retrospective cohort | 4668 (443) | I: Positive COVID-19 PCR; A minimum of two clinical notes, two encounters, and three ICD diagnoses. | Mortality, composite (mortality, MV, ICU), hospitalization. | OSA or CPAP not linked with mortality, MV, ICU, and hospitalization. | No PSG data, no data on OSA treatment. |
| PenaOrbea et al., 2021 [ ] | Multicentric, retrospective control and case-control | 5402 (2664) | I: Positive COVID-19 PCR; PSG record available. | COVID-19, WHO-designated COVID-19 clinical outcomes, composite (hospitalization, mortality). | AHI, T90, SaO , ETCO and CPAP not linked with COVID-19. T90 and SaO : ↑ WHO-designated COVID-19 outcomes ↑ hospitalization, ↑ mortality. | Included only patients who had indications for PSG. |
| Oh et al., 2021 [ ] | Retrospective cohort from South Korea | 124,330 (550) | I: ICD codes for COVID-19, chronic respiratory diseases. E: COVID-19 still hospitalized as of June 26, 2020. | COVID-19; hospital mortality. | OSA: ↑ COVID-19, OR 1.65, not linked to mortality. | No PSG data, no data on OSA treatment, no BMI data. |
| Gottlieb et al., 2020 [ ] | Retrospective cohort from Chicago, IL. | 8673 (288) | I: Positive COVID-19 PCR. E: Interhospital transfers. | Hospitalization, ICU. | OSA not linked to hospitalization, ↑ ICU, OR 1.58. | No PSG data, no data on OSA treatment. |
| Kendzerska et al., 2023 [ ] | Retrospective cohort from Ontario, CA. | 4,912,229 (324,029) | I: Alive at the start of the pandemic; Followed until March 31, 2021, or death. | COVID-19, ED, hospitalization, ICU, 30-day mortality. | OSA: ↑ COVID-19, csHR 1.17, ↑ ED, csHR 1.62, ↑ hospitalizations csHR 1.50, ↑ ICU csHR 1.53, not linked to mortality. | No PSG data, no data on OSA treatment, no BMI data. |
| Peker et al., 2021 [ ] | Multicenter, prospective, observational clinical trial | 320 (121) | I: Positive COVID-19 PCR and/or clinical/radiologic. | Clinical improvement, clinical worsening, hospitalization, oxygen, ICU. | OSA: ↑ delayed clinical improvement, OR 0.42, ↑ oxygen OR 1.95, ↑ clinical worsening. | No PSG data, no data on OSA treatment. |
| Girardin et al., 2021 [ ] | Retrospective cohort from NYC and LI | 4446 (290) | I: Positive COVID-19 PCR. | Hospital mortality. | OSA not linked to mortality. | No PSG data, no data on OSA treatment, no BMI data. |
| Gimeno-Miguel et al., 2021 [ ] | Retrospective cohort from Aragon, ES. | 68,913 (1231) | I: Positive COVID-19 PCR/antigen; E: Patients diagnosed from March to May 2020. | Composite (hospitalization, 30-day mortality) | OSA: ↑ composite outcome (hospitalization and 30-day mortality) in women OR 1.43, but not in men. | No PSG data, no data on OSA treatment, no BMI data. |
| Cariou et al., 2020 [ ] | Multicentric, retrospective cohort | 1317 (114) | I: Positive COVID-19 PCR or clinical/radiological diagnosis, hospitalized, diabetics. | Composite (MV, 7-day mortality), mortality on day 7, MV on day 7, ICU, discharge on day 7. | OSA: ↑ mortality by day 7 OR 2.80, not linked to composite outcome (intubation and death within 7 days of admission). | No PSG data, no data on OSA treatment, diabetic population. |
| Ioannou et al., 2020 [ ] | Longitudinal cohort from VA registry. | 10,131 (2720) | I: VA enrollees who had COVID-19 PCR test; E: VA employees. | Hospitalization, MV, mortality. | OSA: ↑ MV HR, 1.22, not linked to hospitalization, mortality. | No PSG data, no data on OSA treatment, male veterans. |
| Izquierdo et al., 2020 [ ] | Multicentric, retrospective cohort | 10,504 (212) | I: Positive COVID-19 PCR or clinical/radiological diagnosis. | ICU. | OSA not linked to ICU admission. | No PSG data, no data on OSA treatment, no BMI data, no adjustments for obesity and comorbidities. |
| Lohia et al., 2021 [ ] | Multicentric, retrospective cohort | 1871 (63) | I: Adults; Positive COVID-19 PCR; E: Readmission; Ambulatory surgery, pregnant, transferred-for-ECMO patients. | Mortality, MV, ICU. | OSA ↑ mortality OR 2.59, ↑ ICU OR 1.95, ↑ MV OR 2.20. | Small OSA sample size, no data on OSA treatment, mostly African Americans. |
| Prasad et al., 2024 [ ] | Retrospective cohort from VA registry | 20,357 (6112) | I: Tested for COVID-19 by PCR; Until 16 December 2023. | COVID-19, LFNC, HFNC, NIV, MV, 30-day readmission; hospital LOS, ICU LOS, adapted WHO severity scale. | OSA ↑ COVID-19 OR 1.37, ↑ NIV OR 1.83, not linked to LFNC, HFNC, MV, 30-day readmission. CPAP adherence not linked to outcomes. | No PSG data. |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Nemet, M.; Vukoja, M. Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review. Antibiotics 2024 , 13 , 532. https://doi.org/10.3390/antibiotics13060532
Nemet M, Vukoja M. Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review. Antibiotics . 2024; 13(6):532. https://doi.org/10.3390/antibiotics13060532
Nemet, Marko, and Marija Vukoja. 2024. "Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review" Antibiotics 13, no. 6: 532. https://doi.org/10.3390/antibiotics13060532
Article Metrics
Article access statistics, supplementary material.
ZIP-Document (ZIP, 196 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

IMAGES
VIDEO
COMMENTS
INTRODUCTION. Hypertension is the leading preventable risk factor for cardiovascular disease (CVD) and all-cause mortality worldwide. 1,2 In 2010, 31.1% of the global adult population (1.39 billion people) had hypertension, defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg. 3 The prevalence of hypertension is rising globally owing to ageing of the population and increases in ...
The global increase in prevalence of hypertension was consistent by sex (5.5% in men and 5.0% in women), but varied by economic development 3. From 2000 to 2010, the prevalence of hypertension ...
patients with hypertension and reducing the risk of its sequelae.1,3-5 Improving the effective coverage of treatment for patients with hypertension is an objective of many global, regional, and national initiatives, and programmes. Comparable data on hypertension detection, treat-ment, and control are needed to learn from good
Background Hypertension has become an important public health concern in the developing world owing to rising prevalence and its adverse impact on ailing health systems. Despite being a modifiable risk factor for cardiovascular disease, hypertension has not received the needed attention in Ghana as a result of various competing interests for scarce health resources. This systematic review and ...
The prevalence was similar by sex, urban-rural residence or peer-review status of the included studies. It did not appear to vary over the study year period 1976-2019.
A study indicated that adoption of the 2017 ACC/AHA hypertension guidelines would make the prevalence of hypertension among adults aged 45-75 years increased from 36% to 63% in the United States ...
Employing a semi-systematic literature review research design, this paper reviews hypertension epidemiology in Ghana from an extensively large pool of research studies that investigate ...
The prevalence of hypertension was 55.0% (3003) and 27.5% (1473) based on the ACC/AHA 2017 guideline and the JNC7 2003 guidelines respectively. Body mass index was positively correlated with systolic and diastolic BP (p=0.000). Conclusions: Over half of the adult population in this major Nigerian city are classified to have hypertension by the
expected rise in both prevalent hypertension from 26.4% to 29.2% and the worldwide population. By 2010, these projec-tions appeared conservative as the worldwide prevalence of hypertension was estimated at 31.1%, affecting 1.39 billion people.2 The large increase in prevalent hypertension globally
A literature review of articles published between January 2000 and June 2021 was performed via the MEDLINE database to assess the influence of SES on the prevalence/
Few studies have characterized the epidemiology and management of hypertension across several communities with comparable methodologies in sub-Saharan Africa. We assessed prevalence, awareness, treatment, and control of hypertension and predicted 10-year cardiovascular disease risk across seven sites in East and West Africa. Between June and August 2018, we conducted household surveys among ...
Background Hypertension is an urgent public health problem. Consistent summary from natural and quasi-experiments employed to evaluate interventions that aim at preventing or controlling hypertension is lacking in the current literature. This study aims to summarize the evidence from natural and quasi-experiments that evaluated interventions used to prevent or control hypertension. Methods We ...
A combined total of 30,033 participants across twenty studies reporting on the population prevalence of hypertension were pooled with 10,625 (35.4%) identified to satisfy study criteria for ...
Awareness and control of HTN (11 studies) ranged from 20 to 54% and 7.5 to 25%, respectively. Increasing age, body mass index, smoking, diabetes and extra salt intake were common risk factors. In ...
The prevalence of arterial hypertension in the reviewed studies ranged from. 23.6% to 54.8%. Discussion: It is more prevalent in female gender, with the highest incidence in adults and. the ...
Developing technologies hold promise for earlier diagnosis and noninvasive monitoring of right heart failure and pulmonary hypertension that will aid in preclinical studies, enhance patient selection and provide surrogate end points in clinical trials, and ultimately improve bedside care.
Metabolic syndrome is a complex pathophysiologic state which characterized by abdominal obesity, insulin resistance, hypertension, and hyperlipidaemia. The Adult Treatment Panel III report (ATP III) of the National Cholesterol Education Programme identified the metabolic syndrome as a serious public health issue in the modern era. In Western and Asian nations, the frequency of metabolic ...
People who monitored their blood pressure at home and modified their medications based on the readings lowered their blood pressure more than those in a control group who received routine blood pressure management from a physician, according to results from a randomized clinical trial involving 219 patients in Spain. The participants were aged 40 years or older and had a systolic blood ...
Hypertension (HTN) affects more than 30% of adults worldwide. It is the most frequent modifiable cardiovascular (CV) risk factor, and is responsible for more than 10 million death every year.
This review aimed to determine risk factors for adverse mental health outcomes and protective or coping measures to mitigate the harmful effects of the COVID-19 crisis among HCWs in sub-Saharan Africa. We performed a literature search using PubMed, Google Scholar, Cochrane Library, and Embase for relevant materials.
The prevalence of hypertension varied broadly in researches, ranging from a minimum of 3.2% to a maximum of 10.1% for children and adolescents, and a minimum of 21.5% to a maximum of 78.5% for adults.
Both obstructive sleep apnea (OSA) and acute lower respiratory tract infections (LRTIs) are important global health issues. The pathophysiological links between OSA and LRTIs include altered immune responses due to chronic intermittent hypoxia and sleep fragmentation, increased aspiration risk, and a high burden of comorbidities. In this narrative review, we evaluated the current evidence on ...
The review also extensively delves into the impact of ethnicity on the prevalence and outcomes of hypertension-induced CKD, highlighting disparities and distinctive trends among diverse ethnic ...