The University of Chicago neuroscience community is host to a diverse group of researchers investigating epilepsy and seizure disorders. The faculty of Department of Neurology play a key role in this research, conducting critical clinical and neurophysiological studies, and providing valuable collaborations with the neuroscience researchers in other departments.
John Ebersole MD directs a research program aimed at:
- Clarifying the relationships between cerebral electrical activity and the resultant scalp EEG; and
- Developing and validating computational techniques of functional imaging and seizure localization using scalp, intracranial EEG, and magnetoencephalography (MEG).
Over the past fifteen years research from his laboratory has established the usefulness of spike and seizure dipole modeling with both EEG and MEG in order to localize non-invasively epileptogenic foci in epilepsy surgery candidates. He is a foremost proponent of and authority on the use of source models in the evaluation of epilepsy. Ongoing projects include studies of the accuracy of dipole and other extended source models of epileptic foci using simultaneously recorded scalp and intracranial EEG, comparisons of real-time EEG imaging with other functional imaging techniques using three-dimensional co-registrations, and the development of a new spatio-temporal analysis technique for intracranial EEG utilizing field and source display on the patient’s reconstructed cortex. These direct applications of neurocomputational, neuroimaging, and electrical engineering developments to the evaluation of epileptic foci in the human brain are an example of translational research at its best.
James Tao MD PhD is interested in determining the cerebral substrates of scalp EEG epileptiform patterns, in order to improve the accuracy of non-invasive seizure localization during epilepsy surgery. His long-term interests include investigations of the mechanisms of epileptogenesis and of new modalities of epilepsy therapy. Current projects include:
- The impact of cerebral source area and synchrony on recording scalp EEG ictal patterns; and
- The pathophysiology of interictal temporal delta activity (ITDA) and its value in localizing epileptogenic zone.
His long term goals are to investigate the electrophysiological behavior of cerebral epileptogenic networks and the mechanism of epileptogenesis at the neural network level in order to identify new preventive and therapeutic modalities.
Wim Van Drongelen PhD (Department of Pediatrics) has focussed his research on the long-range goal of optimizing therapeutic intervention in pediatric epilepsy by improved spatial and temporal localization of seizure activity and examining the underlying mechanisms in the pathogenesis of seizures. His research focuses on:
- Underlying neuronal mechanisms in epilepsy (synchrony, recruitment, oscillation, weak coupling);
- Relationships between neuronal activity at different scales (neuron, network, brain);
- Detection and prediction of brain electrical activity during seizures using various signal processing techniques (correlation dimension, Kolmogorov entropy, wavelet analysis);
- Localization of sources from surface recordings (dipole analysis, MUSIC, LORETA, spatial filtering); and
- Monitoring of the nervous system in the intensive care environment (EEG, evoked potential
James Tao, MD, PhD.
Sandra Rose, MD
Shasha Wu, MD, PhD.
Naoum P. Issa, MD, PhD.
Peter Warnke, MD, FRCS
David Frim, MD, PhD.
Maureen Lacy, PhD.
Vernon L. Towle, PhD.
Shadrach Castillo, RN
Diane Suarez, REEGT
The Comprehensive Epilepsy Center at the University of Chicago Medicine is at the forefront of epilepsy care. Many of our patients come to us after trying a number of different medications and therapies with little success.

Leading Advancements in Epilepsy Care
Backed by the development of groundbreaking diagnostic techniques and a long history of research, our expert team has the experience to manage nearly all epilepsy-related problems from new onset seizures to rare and difficult-to-treat epileptic syndromes. With non-invasive technology and contemporary diagnostic techniques, we can pinpoint the origin of most seizures with precision. Having this advantage can significantly increase diagnostic accuracy and improve patient outcomes.
Epilepsy is a chronic, non-communicable neurologic disorder defined by two or more unprovoked seizures. Provoked seizures result from some immediately recognizable stimulus (for example, low blood sugar in people with diabetes), while unprovoked seizures have no immediately recognizable cause. Provoked seizures are unlikely to recur if the provocations can be avoided; however, unprovoked seizures have the tendency to recur and are the hallmark of epilepsy. When someone has two or more seizures, doctors diagnose epilepsy.
Types of Epilepsy
There are many types of epilepsy. Some types of epilepsy and epilepsy syndromes include:
- Benign rolandic epilepsy
- Childhood absence epilepsy
- Doose syndrome (myoclonic astatic epilepsy of childhood)
- Frontal lobe epilepsy
- Infantile spasms
- Juvenile myoclonic epilepsy
- Landau-Kleffner syndrome
- Lennox-Gastaut syndrome
- Occipital lobe epilepsy
- Parietal lobe epilepsy
- Rasmussen's syndrome
- Sturge-Weber syndrome
- Temporal lobe epilepsy
- West syndrome
A seizure is a sudden surge of abnormal electrical activity in the brain. Electrical activity in the brain is normal. It’s how our brain works. Seizures occur when there is significant disruption of that normal electrical activity in the brain. The general symptoms of seizures may include changes in consciousness, sensation, movement or behavior.
Is a Seizure the Same Thing as Epilepsy?
No. Not all seizures are caused by epilepsy. Approximately 10 percent of the general population may experience a seizure during their lifetime, but only 1 percent of the population has epilepsy. A single seizure may or may not progress to a chronic and recurrent condition or epilepsy.
Although there are many types of seizures, those that people with epilepsy experience commonly fall into generalized and partial (or focal) seizures.
Generalized Seizures
Generalized seizures are characterized by widespread electrical discharges in both sides of the brain. You might think of it as a lightning storm in which the lightning seems to be coming from all areas of the sky at the same time.
There are six types of generalized seizures:
- Absence seizures are also known as petit mal seizures. When people experience an absence seizure, they may seem to disconnect from the world, blank out or stare into space for at least a few seconds. Their eyes may roll as well. People who have absence seizures usually lose awareness for a short time and have no memory of the seizure afterward. This type of seizure usually begins between the ages of 4 and 14, and it can resemble daydreaming. Subtle body movement may accompany the seizure, but it’s not the jerking movements that occur with tonic-clonic or clonic seizures.
- Atonic seizures , also known as drop attacks, drop seizures or akinetic epileptic drop attacks, may involve a sudden loss of muscle tone, a head drop or leg weakening. People suffering an atonic seizure may suddenly collapse. This type of seizure may also cause the person suffering it to drop objects.
- Clonic seizures include jerking muscle movements that are more rhythmic than chaotic. The muscle spasms typically affect the face, neck and arms. They may last for several minutes.
- Myoclonic seizures are typically short and involve uncontrollable jerking, usually of the arms and/or legs, and last for only a second or two
- Tonic-clonic seizures , also known as grand mal seizures, are what most people think of when they imagine a seizure. They involve a loss of consciousness, stiffening of the body and shaking or jerking, sometimes followed by loss of bladder or bowel control
- Tonic seizures include body stiffening, but do not include the clonic phase of uncontrolled jerking or spasms. Back, arm and leg muscles are affected most often. The seizure may cause a patient to fall or collapse.
Partial Seizures
Partial seizures, also known as focal seizures, begin in one side of the brain. They fall into one of two groups:
- Simple partial seizures (also known as simple focal seizures) may only include the aura stage (see below). During this type of seizure, awareness, memory and consciousness remain intact. This type of seizure may alter emotions or change the way things look, smell, feel, taste or sound. It may also result in involuntary jerking of a body part, such as an arm or leg, or spontaneous sensory symptoms, such as tingling, dizziness and flashing lights.
- Complex partial seizures (also known as psychomotor seizures) alter consciousness or responsiveness. The person having the seizure may appear to be staring into space or moving without purpose. Some common movements include hand rubbing, chewing, swallowing, and repetitive motion, such as bicycling leg movements or walking in a circle.
For some types of seizures, an aura happens before a seizure and may alert a person that a seizure may occur. Auras typically begin seconds before the seizure.
The symptoms that accompany an aura can vary depending on the type of seizure and the area of the brain affected. Some symptoms of aura include:
- Abnormal sensations
- Deja vu (familiar feelings) or jamais vu (unfamiliar feelings)
Distorted emotions, such as panic or fear
Forced thinking
Perceived sounds, tastes, or smells (some people report smelling burning rubber, for example)
Physical sensations, like dizziness, headache, numbness, and lightheadedness
- Unusual feelings
At UChicago Medicine, we offer an advanced treatment designed to prevent seizures before they start and often before a patient feels the aura. The NeuroPace Responsive Neurostimulation System (RNS) is a tiny device that detects abnormal brain activity and responds in real time to deliver short bursts of electrical stimulation designed to reduce how often seizures happen.
Similarly, deep brain stimulation (DBS) prevents seizures to spread throughout the brain and stops them from becoming clinically relevant. Neuromodulation is just one of the several treatment options we provide, from the latest anti-epileptic drugs to Visualase MRI-guided laser thermal ablation.
Ictus Stage
Ictus is another word for the seizure itself — the part of the seizure that outsiders can witness. It can be convulsive, commonly called “grand mal,” or non-convulsive, such as staring and inability to respond normally.
Postictal Stage
The postictal stage occurs after the ictus or active stage of the seizure. During the postictal stage, the body begins to relax and aftereffects may set in. The type and length of aftereffects will vary from person to person and may include:
- Confusion and agitation
- Fatigue and drowsiness
- Loss of bowel or bladder control
- Loss of consciousness or unresponsiveness
- Partial paralysis
Duke Comprehensive Epilepsy Center

Basic Science Researchers
Our basic science research focuses on understanding the origins and mechanisms of epilepsy in the brain. This includes work on the genetic causes of epilepsy as well as understanding the biochemical pathways responsible for epilepsy. We are also using data from patients with epilepsy to improve our basic understanding of how the brain works.

Gregory Cogan

James O'Connell McNamara

Translational Science Researchers
Our translational work focuses on developing advanced tools to diagnose, treat, and cure epilepsy. This includes work on biomarkers in the blood to help with the diagnosis and classification of epilepsy and advanced imaging techniques to help identify parts of the brain responsible for seizures We are also currently developing new electrode technologies to better identify seizure onset zones for surgery and tools to modulate brain activity to prevent seizures
Derek Southwell

Jonathan Viventi
Clinical researchers.
Run by Professor of Neurology Birgit Frauscher, MD, PhD, the Lab for Analytical Neurophysiology (ANPHY Lab ) seeks to employ a variety of quantifiable tools in order to shed light on neurophysiological and pathological processes
Our clinical work investigates new techniques for the analysis of EEG and other data from patients with epilepsy, new drugs and devices for treatment of epilepsy, as well as quality and process improvements for a better quality of care for patients. The overall goal of the research program of the DCEC is to provide more extensive and better treatment opportunities for patients suffering from epilepsy.
To find information about clinical trials being done at Duke, please go to Clinicaltrials.gov
Labs Associated with the DCEC
The anphy lab.
Birgit Frauscher's, MD, PhD Lab for Analytical Neurophysiology seeks to employ a variety of quantifiable tools in order to shed light on neurophysiological and pathological processes
The Cogan Lab
The Cogan Lab studies speech, language, and cognition using invasive electrophysiological recordings (ECoG, SEEG, µECoG).
We use engineering approaches to understand and control neural function. Our research and development efforts are focused on devices that use electrical activation of the nervous system to restore function to individuals with neurological impairment.
Southwell Lab
Our research group seeks to improve the treatment of neurologic diseases and injuries. We study the development and function of neurons, one of the cellular components of brain circuits, as well as the neural signals that brain circuits produce.
Viventi Lab
Our research applies innovations in flexible electronics to create new technology for interfacing with the brain at a much finer scale and with broader coverage than previously possible. We create new tools for neuroscience research and technology to diagnose and treat neurological disorders, such as epilepsy.
McNamara Lab
The goal of this laboratory is to elucidate the cellular and molecular mechanisms underlying epileptogenesis, the process by which a normal brain becomes epileptic. The epilepsies constitute a group of common, serious neurological disorders, among which temporal lobe epilepsy (TLE) is the most prevalent and devastating.

DCEC Quarterly Research Symposium Summer 2022
Is Best Always Best? Patients' Preferences for Treatment Alternatives for Drug-resistant focal epilepsy by Shelby Reed, PhD, RPh, Professor of Population Health Sciences, Duke University
Brain Stimulation and Cognition in Epilepsy by Barbara C. Jobst, MD, Chair and Professor of Neurology, Dartmouth Geisel School of Medicine
DCEC Quarterly Research Symposium Spring 2022
EEG Source Imaging (ESI): Source modeling basics and more by Prachi T. Parikh, MD Assistant Professor of Neurology Duke University
Epileptiform activity at the microscale by Sydney S. Cash, M.D., Ph.D. Associate Professor of Neurology Massachusetts General Hospital and Harvard Medical School
DCEC Quarterly Research Symposium Winter 2021
Using Dynamical Network Models to Derive Interictal and Ictal Biomarkers of the Epileptogenic Zone from Intracranial EEG Data by Sridevi V. Sarma, PhD Associate Professor Biomedical Engineering, Johns Hopkins University
Non-invasive optical detection of brain activity using parallelized diffuse correlation spectroscopy by Roarke W. Horstmeyer, PhD Assistant Professor Biomedical Engineering, Duke University
DCEC Quarterly Research Symposium Fall 2021
- How refractory epilepsy patients with SEEG implants help in advancing the science of dose individualization in neuromodulation by Pratik Chhatbar, MD, PhD Assistant Professor of Neurology Department of Neurology Duke University
Adding some science to the art of EEG: classifying seizure Dynamotypes by William Stacey, MD, PhD Associate Professor, Department of Neurology and Biomedical Engineering University of Michigan
DCEC Quarterly Research Symposium Summer 2021
- Research and New Therapies in Epilepsy by Jacqueline French, MD Professor of Neurology NYU Comprehensive Epilepsy Center
The Measure by which you Measure: Quality of life and Quality of Care in Epilepsy by Matthew Luedke, MD Division Chief, Hospital Neurology Assistant Professor of Neurology Duke University
DCEC Quarterly Research Symposium Spring 2021
Beyond the focus – Why networks matter in temporal lobe epilepsy by Vicky Morgan, Ph.D. Professor of Radiology and Radiological Sciences Vanderbilt Universit
DCEC Quarterly Research Symposium Winter 2020
- Localizing epilepsy "hotspots" with quantitative neuroimaging by Kathryn Adamiak Davis, M.D., M.S.T.R. Assistant Professor of Neurology University of Pennsylvania
Using Ultrahigh-Resolution Diffusion MRI to Localize Seizure Foci in Patients with Intractable Epilepsy by Iain Bruce, PhD Medical Instructor Duke Department of Neurology
DCEC Quarterly Research Symposium Fall 2020
- Automating Clinical Neurophysiology by M. Brandon Westover, MD, PhD Associate Professor of Neurology Massachusetts General Hospital and Harvard Medical School
Augmented reality in epilepsy surgery and clinical application of source localization by Muhammad Zafar, MBBS Assistant Professor of Pediatrics Duke University

Biomedical Graduate Studies
Neuroscience graduate group.
- Focused Areas
By Traditional Areas of Neuroscience Research
- Behavioral and Cognitive
- Cellular and Molecular
- Computational Neuroscience
- Developmental
- Neurobiology of Disease
By Focused Areas of Neuroscience Research
- Affective Disorders
- Autism and Other Neurodevelopmental Disorders
- Axon Guidance
- Behavioral Neuroscience
- Cognitive Neuroscience
- Decision Neuroscience
- Genetics and Epigenetics
- Imaging Approaches
- Ion Channels and Receptors
- Learning and Memory
- Motor Systems
- Neural Injury, Regeneration, Repair, and Stem Cells
- Neurobiology of Obesity
- Neurobiology of Sleep and Circadian Rhythms
- Neurobiology of Stress
- Neurodegeneration
- Neuroendocrinology
- Neuroengineering
- Neuroinflammation and Neurovirology
- Neuron-Glia Interactions
- Schizophrenia
- Sensory Systems and Perception
- Synaptic Plasticity
- Synaptogenesis and Circuit Formation
Current Members
Click the faculty member's name to see more detailed information.
© The Trustees of the University of Pennsylvania | Site best viewed in a supported browser . | Report Accessibility Issues and Get Help | Privacy Policy | Site Design: PMACS Web Team.
Follow us on social media
- See us on facebook
- See us on twitter
- See us on youtube
- See us on instagram
- See us on linkedin
Stanford Comprehensive Epilepsy Program Team

Neuroradiology
Neuropsychology, neuropsychiatry, adult epilepsy.

Robert Fisher, MD, PhD Maslah Saul MD Professor of Neurology & Neurological Sciences Director of Stanford Comprehensive Epilepsy Program
Robert S. Fisher, MD, PhD is Maslah Saul MD Professor and Director of the Stanford Epilepsy Center and EEG lab. He had research funding or awards from the Klingenstein Foundation, Epilepsy Foundation, CURE, American Society of Clinical Neurophysiology, NIH and NSF. He has published about 230 peer-reviewed articles and 3 books. He has been named every year from 1996 to 2019 in Best Doctors in America. He received the Ambassador Award from the International League Against Epilepsy, the 2005 American Epilepsy Society Service Award and the 2006 Annual Clinical Research Award. Dr. Fisher is Past-President of the American Epilepsy Society, and has served on the Board of the International League Against Epilepsy and as Editor-in-Chief of the Journal, Epilepsia. He is past Editor-in-Chief of the world’s most visited website about epilepsy, epilepsy.com. Dr. Fisher led the projects to develop a formal definition of who has epilepsy and an update of seizure type classification. His recent research is on new devices to detect and treat seizures. He led the clinical trials on deep brain stimulation for epilepsy and on the next-generation (heart-rate-sensing) vagus nerve stimulation device. Dr. Fisher has additionally won several teaching awards and cares for epilepsy patients in the Stanford Epilepsy Clinics and inpatient epilepsy unit.

Kimford J. Meador, MD Professor of Neurology & Neurological Sciences Director of the Epilepsy Monitoring Unit
Dr. Meador received his MD from the Medical College of Georgia. After an internship at the University of Virginia and service as an officer in the Public Health Corps, he completed a residency in Neurology at the Medical College of Georgia and a fellowship in Behavioral Neurology at the University of Florida. Dr. Meador is currently the Multi-PI on a multicenter NIH investigation on the pregnancy outcomes in women with epilepsy including neurodevelopmental effect of fetal antiepileptic drug exposure. Dr. Meador has authored over 400 peer-reviewed publications and has served on the editorial boards of numerous journals.

Jessica Falco Walter, MD Clinical Associate Professor of Neurology & Neurological Sciences
Dr. Walter received her MD from Georgetown University in Washington, DC. She stayed at Georgetown for her internship in Internal Medicine and then moved to New York City to complete her residency in Neurology at the Icahn School of Medicine at Mount Sinai. She went on to pursue a Clinical Neurophysiology Fellowship at Rush University in Chicago, IL, training in both EEG and EMG. Due to her particular interest in Epilepsy she went on to become the first Epilepsy Fellow at Rush University. Dr. Walter provides clinical care to general neurology patients as well as patients with epilepsy and enjoys teaching residents and medical students. She also has a particular interest in dietary treatments for epilepsy and clinical research.

Kevin Graber, MD Clinical Professor of Neurology & Neurological Sciences Director of Outpatient Epilepsy Clinic
Dr. Kevin Graber earned his MD from Indiana University in 1992 and completed his training in Neurology & Neurological Sciences at Stanford Medical Center. Dr Graber has earned prestigious research awards and has served on several national committees including the American Epilepsy Society, CURE, and Epilepsy Foundation. As a Clinician Educator, Dr. Graber provides clinical care to patients with epilepsy, and teaches fellows, residents, and medical students. Dr. Graber's research is focused on discovering how brain injuries, such as trauma, lead to epilepsy.

Scheherazade Le, MD Clinical Professor of Neurology & Neurological Sciences
Dr. Scheherazade Le is a Clinical Professor within the Stanford Comprehensive Epilepsy Center. Within the AEC, she cares for patients who suffer from immune-mediated seizures/epilepsy. Treatments may include immunotherapy, anti-seizure medications, epilepsy surgery, laser ablation and/or neuromodulation. She is accepting new patient referrals. Her research is focused on identifying and treating undiagnosed immune-mediated seizures and characterizing immune-mediated seizures/epilepsy in patients with autoimmune encephalitis.

Yi Li, MD, PhD Clinical Assistant Professor of Neurology & Neurological Sciences
Dr. Yi Li received her M.D. and Ph.D. from Central South University in China, conducting her Ph.D. research as a visiting graduate student at UCLA. She received two national grants in China to research the role of neurogenesis in animal models of refractory temporal lobe epilepsy. Dr. Li received her residency training from University of Massachusetts Medical School, during which time she received a Safety and Quality Award from the American Academy of Neurology. She then continued her training as an epilepsy clinical and research fellow at Stanford. She is interested in medically refractory epilepsy management, epilepsy clinical research, precision medicine and genetics in epilepsy, and improving quality of patient care.

Martha Morrell, MD Professor of Neurology & Neurological Sciences
Dr. Morrell has focused her career on the treatment of patients with epilepsy, including health issues for women with epilepsy. She attended Stanford Medical School, then completed her residency in Neurology and her fellowship in EEG and epilepsy at University of Pennsylvania. After founding the Stanford Comprehensive Epilepsy Center, she moved to Columbia University where she was the Caitlin Tynan Doyle Professor of Epilepsy and Director of the Columbia Comprehensive Epilepsy Center. She returned in 2004 and sees patients in the Epilepsy Clinic. She is currently the Chief Medical Officer for NeuroPace, a company focused on brain stimulation for epilepsy.

Josef Parvizi, MD, PhD Professor of Neurology & Neurological Sciences Director of Stanford Program for Intractable Epilepsy
Dr. Parvizi’s clinical training is from Mayo Clinic- Rochester, BIDMC-Harvard University, and UCLA. His major interest is in the study of seizure propagation and treating patients with intractable epilepsy. His special expertise is in detecting the epileptic source in patients with uncontrolled seizures and mapping the brain circuitries that underlie development and spread of seizures. He performs functional brain mapping of the brain during epilepsy surgery evaluations. Dr. Parvizi is also the Director of the Stanford Human Intracranial Cognitive Electrophysiology Program (SHICEP), and is involved in multidisciplinary collaborative research projects with several Stanford principal investigators to understand how different parts of the human brain work and how their function may be broken during seizures.

Babak Razavi, MD, PhD Clinical Associate Professor of Neurology & Neurological Sciences
Dr. Razavi's clinical interests are in medically refractory epilepsies and using high density EEG (electroencephalogram) for better localization of seizure foci. His research areas include using engineering techniques for analyzing EEGs, medical devices for evaluation and treatment of epilepsy, and using seizures as a model for understanding consciousness.

Zahra Sadat-Hossieny, MD Clinical Assistant Professor Neurology & Neurological Sciences
Dr. Sadat received her MD from The George Washington University School of Medicine. She spent two months during medical school in South America and obtained a certificate in global health. She completed her residency in Neurology at The Ohio State University and Fellowship in Epilepsy at Stanford University. She is passionate about providing cutting-edge, personalized and evidence-based care that is delivered with compassion. Her research focuses on developing therapeutics that optimize cognition and improve outcomes for patients with epilepsy and neurologic diseases.

Niyatee Samudra, MD Clinical Assistant Professor, Neurology & Neurological Sciences
Dr. Samudra is a clinical assistant professor in the Department of Neurology and Neurological Sciences at Stanford University School of Medicine. She specializes in the care of patients with memory disorders and epilepsy. She has completed fellowship training in behavioral neurology at the University of California, San Francisco, as well as in epilepsy and clinical neurophysiology at Vanderbilt University Medical Center. Dr. Samudra is board-certified in neurology and in epilepsy. Her research interests include clinical trials in memory disorders and epilepsy; early neurophysiological markers of Alzheimer’s disease and related disorders; neuropsychiatric symptoms in neurodegenerative disorders; and the cognitive and neuropsychiatric consequences of epilepsy. She is interested in improving neurologic care for underserved populations. Dr. Samudra has published in the Journal of Alzheimer’s Disease; Current Neurology and Neuroscience Reports; Journal of the Neurological Sciences; Seizure; and Epilepsy and Behavior, among others. She is a member of the American Academy of Neurology.
Instructors

Erica Von Stein, MD Clinical Instructor
Erica graduated summa cum laude with a B.E. in Biomedical Engineering from Vanderbilt University, where she attended on a 4-year full tuition merit scholarship. She earned her M.D. from the University of Pennsylvania Perelman School of Medicine. At Penn she was awarded a Guggenheim Neurosurgery Research Fellowship. She completed her internship at Cedars-Sinai Medical Center followed by Neurology residency and Epilepsy fellowship at Stanford. Her research interests broadly include brain-computer interface, surgical epilepsy, network neuroscience, comparative epilepsy.

Manveer Dilts-Garcha, MD, MA Clinical Instructor/Chief Epilepsy Educational Fellow
Manny graduated from the USF Morsani School of Medicine SELECT MD leadership program and before that completed an undergraduate degree in Biology and a Professional Science Master’s in Stem Cell Research at California State University Sacramento. Manny is passionate about advancing the future of science and medicine, both through academic endeavors as well as through policy. Their accomplishments include research in the field of regenerative medicine, publications in biophotonics, awards for healthcare quality improvement, ongoing engagement at the legislative level to reform health policy and improve resident wellbeing, and serving as a founding member and delegate of the UC Davis Residents Union, CIR. Originally from Vancouver, Canada and the child of immigrant parents who fled religious persecution in their native India, Manny is acutely sensitive to the struggles of underserved communities, and disadvantaged groups and is a strong proponent of universal healthcare for all Americans and for health care reform. In their spare time they enjoy wood working, powerlifting, cooking, photography and spending time with their family. Manny is excited for the next chapter in their academic career as an Epilepsy Fellow at Stanford.

Mehraneh Khadjevand, MD, MPH Clinical Instructor/Second Year Epilepsy Fellow
Mehraneh completed her posdoctral fellowship at Mayo Clinic, MN, where she investigated human memory, and worked on interictal biomarkers of epileptic brain. Afterwards she completed her residency at Tufts medical center, and served as the academic chief resident, and received the Award for Excellence in Medical Student Teaching and the Resident Teaching Award. Her goal is finding a reliable interictal biomarker for guiding epilepsy surgery.
Adult Epilepsy Fellows

Roger Chang, MD, PhD Adult Epilepsy Fellow
Roger earned his undergraduate degree in Biomedical Engineering at Washington University in St. Louis. He completed his doctorate work in the lab of Dr. Robert Edwards at UCSF, determining the unique, dual role of chloride in synaptic vesicular glutamate transport. He subsequently received his MD at UCSF. He completed his internship and Neurology residency at University of Washington. At University of Washington, he worked with Dr. Garret Stuber’s lab investigating neuronal circuits involved in nociception and resultant maladaptive behaviors in Zebrafish. In addition to his clinical interest in providing care for patients with medically refractory epilepsy, one of his main research interests is determining the effects of epilepsy on cognition and behavior.

Christopher Primiani Adult Epilepsy Fellow
Chris Primiani is a Neurology Epilepsy Fellow at Stanford. He graduated with a Bachelor of Science in neuroscience from the University of Florida. After college, he completed a two-year Predoctoral Fellowship in cell biology and gene expression at the National Institute of Aging. Chris completed his medical degree at University of South Florida before completing his adult neurology residency at Johns Hopkins Hospital in Baltimore, MD. His research interest includes the intersection of neuroscience and technology, particularly brain-machine interface, and medical devices in the treatment of epilepsy.

Trevor Rafferty, MD Adult Epilepsy Fellow

Jackie (Jacqueline) Summers Stromberg, MD Adult Epilepsy Fellow

Spencer Nam, MD CNP/Chief Epilepsy Fellow
Capt. Nam received an undergraduate degree in chemistry from Stanford University, and his MD from Uniformed Services University of the Health Sciences. As an officer in the US Air Force, he completed residency at Walter Reed National Military Medical Center. Dedicated to clinical care and teaching, he hopes to eventually open an epilepsy monitoring unit at San Antionio Military Medica Center and influence training of future Air Force neurologists. He also performed as a violin soloist at Carnegie Hall and performed with the US Army Orchestra and with members of the Philadelphia orchestra
Advanced Practice Providers

Mimi Callanan, RN, MSN, ACNS-BC Epilepsy Clinical Nurse Specialist
Ms. Callanan has many years experience as a Clinical Nurse Specialist in Epilepsy. She has been in this role at Stanford since the Center opened in 1990. She received her undergraduate degree at St Louis University and her graduate degree at the University of Pennsylvania. She is a past member of the Professional Advisory Board of the Epilepsy Foundation of America. She is a past President of the Epilepsy Society of San Francisco and was on the Board of Directors of the Epilepsy Foundation of Northern California. She is author of several publications pertaining to education of patients and families about epilepsy, and to the impact of epilepsy on life.

Tenzin D. Lama, DNP, FNP, CNL, RN Nurse Practitioner
Tenzin Lama received her DNP (Doctor of Nursing Practice) with Family Nurse Practitioner degree from University of San Francisco. She has also received her MSN- CNL (Clinic Nurse Leader) from the same university. Tenzin joined the Stanford Comprehensive Epilepsy Center in 2016 and has been working as a Nurse Practitioner in providing care and coordination of services for patients with Epilepsy.
The Pediatric Epilepsy service, led by Dr. Brenda Porter, includes 8 pediatric epileptologists, making it one of the largest services in the country.
Pediatric Epilepsy

Brenda Porter, MD Professor of Neurology & Neurological Sciences and Pediatrics
Dr. Porter has 15 years of experience in taking care of children with epilepsy. Taking care of children with epilepsy and trying to provide them with the best opportunity for seizure freedom, social and emotional well-being is the goal of her practice. She has expertise in medical, surgical and dietary therapy for intractable epilepsy. She directs the Stanford Tuberous Sclerosis Clinic. Her research has focused on improving outcomes in epilepsy surgery and designing therapies for the prevention of epilepsy in at risk patients. Dr. Porter sees patients at Lucille Packard Children’s Hospital.

Fiona Baumer, MD Assistant Professor of Neurology & Neurological Sciences
Dr. Fiona Baumer is an Assistant Professor in Neurology at Stanford University with a focus on pediatric epilepsy. Dr. Baumer received her B.A. at Stanford University and her M.D. from Harvard Medical School. She remained on the east coast to train in pediatrics and child neurology at Boston Children’s Hospital before returning to Stanford in 2015 for epilepsy training as the first Maggie Otto Fellow in Pediatric Epilepsy. She joined the neurology faculty in 2016.
Dr. Baumer’s clinical and research interests include difficult to treat pediatric epilepsies and the interaction between epilepsy and cognition. She is gaining expertise in noninvasive brain stimulation as a clinical and research tool in epilepsy. Dr. Baumer is currently participating in the KL2 Mentored Research Fellowship through which she will conduct a study using transcranial magnetic stimulation to assess cortical excitability and synaptic plasticity in benign epilepsy with centrotemporal spikes (Rolandic Epilepsy).

William Gallentine, DO Clinical Professor of Neurology & Neurological Sciences and Pediatrics
Dr. William Gallentine is a Clinical Professor of Neurology at Stanford University. A product of Waynesburg University, he subsequently received his D.O. from the Philadelphia College of Osteopathic Medicine and then completed a pediatrics residency at Geisinger Medical Center. Dr. Gallentine next undertook his child neurology residency and clinical neurophysiology fellowship at Duke University, where he remained on the faculty as a pediatric epileptogist for 11 years, prior to being recruited to Stanford in 2018.
Dr. Gallentine’s research interests focus on the role of inflammation and genetics in the development of epilepsy, and the ovelap between the two. He is part of a NIH funded multi-center study aimed at discovering novel biomarkers of epileptogenesis and identifying potential therapeutic targets within the inflammatory cascade as a new approach to treating and preventing epilepsy. He is also interested in the exploration of novel mutation-specific therapeutics for children with genetic epilepsies.
Dr. Gallentine’s clinical interests focus on children with refractory epilepsy. He offers clinical expertise in all aspects of epilepsy management, including medications, dietary therapy, surgical planning, and neurostimulation. He has developed specific interests in autoimmune and genetic epilepsies. His epilepsy and inflammation clinic focuses on children with antibody-mediated autoimmune epilepsy (including NMDA receptor encephalitis), Rasmussen’s encephalitis, recurrent febrile seizures, febrile status epilepticus, genetic epilepsies with seizures triggered by fever (Dravet syndrome , GEFS+), fever-induced refractory epileptic encephalopathy (FIRES), mesial temporal temporal lobe epilepsy, and infantile spasms. His epilepsy and genetics clinic provides multi-disciplinary care focused on the evaluation and management of children with known or suspected genetic epilepsy syndromes with an emphasis on mutation-specific therapy.
At Stanford he serves as the Outpatient Medical Director of Pediatric Epilepsy. He is an active member of the American Clinical Neurophysiology Society, American Epilepsy Society, and American Academy of Neurology. Dr. Gallentine has a passion for teaching, too. During his tenure at Duke, he played a prominent role in medical education serving as the Child Neurology Residency Program Director.
When not working, Dr. Gallentine is always up for seeking a new travel adventure with his wife Darla and kids, Caleb, Anya, and Gabe. They definitely consider themselves “foodies,” always looking to try a new dish. He enjoys watching anything sports related, with San Jose Sharks hockey being his most recent obsession.

Jin Hahn, MD Professor of Neurology & Neurological Sciences and Pediatrics, Emeritus
Dr. Hahn is a Child Neurologist who studies brain development, epilepsy and conditions such as dysplasia and holoprosencephaly, in which the two overlap. Dr. Hahn received his MD from Harvard in 1982. After residency training in pediatrics and child neurology at Harvard in 1987, he came to Stanford to do a neurophysiology fellowship. After a brief faculty position at UCSD, he returned to Stanford In 1991 to become the Chief of Neurology Service at Lucile Salter Packard Children's Hospital at Stanford. His research interests are in the areas of neonatal seizures and electoencephalography, neonatal neurology, hypoxic ischemic brain injury and seizure disorders in the first year of life.

Ann Hyslop, MD Clinical Associate Professor of Neurology & Neurological Sciences
Dr. Ann Hyslop is a Clinical Associate Professor Neurology at Stanford University. She received her B.A. at Macalester College and M.D. at the University of Texas, Houston. She then completed her pediatrics residency at the Mount Sinai Hospital in New York City and child neurology residency at the University of Washington. She next completed a clinical neurophysiology fellowship at Miami Children's Hospital after which she had further training in the pre-surgical evaluation of medically intractable epilepsy. She remained as a pediatric epileptologist at Miami Children's Hospital with a concentration in surgical epilepsy for 10 years before joining the Stanford faculty in 2022.
Dr. Hyslop is an avid diver, seeking out shark dives worldwide, and loves yoga, sailing, and traveling with her husband.

Hyunmi Kim, MD, PhD, MPH Clinical Professor of Neurology & Neurological Sciences
Dr. Hyunmi Kim is Clinical Professor of Neurology at Stanford University. She received her M.D. and Ph.D. from Ewha Womans University in Seoul, Korea. She completed her residency in Pediatrics at Asan Medical Center and fellowship in Pediatric Neurology at Ewha Womans University Hospital and Seoul National University Hospital in Seoul, Korea. In the United States, she completed her child neurology residency at Medical College of Georgia and epilepsy fellowship at the Cleveland Clinic. She was on the faculty at University of Alabama at Birmingham and then Emory University, before joining at Stanford in 2018.
Dr. Kim’s clinical and research interests are treatment-resistant epilepsy, epilepsy surgery including stereo EEG monitoring and less-invasive treatment, multimodal brain imaging analysis, and population health research of neurological disorders. She has established a line of research exploring how epidemiological factors relate to childhood epilepsy risk and outcomes.
Dr. Kim’s personal interests are exploring the National Parks, golfing, sailing, and scuba diving.

Juliet K. Knowles, MD, PhD Assistant Professor of Neurology & Neurological Sciences
Dr. Juliet Knowles is Assistant Professor in Neurology, and specializes in epilepsy. She received her B.A. with a double major in Microbiology and Philosophy at the University of Texas-Austin. She then completed her M.D. and Ph.D. in Neurosciences at Stanford University. In graduate school, Dr. Knowles conducted research on novel neurotrophin-based small molecules for the treatment of Alzheimer’s disease under the mentorship of chair Dr. Frank Longo. After deciding to become a pediatric neurologist, Dr. Knowles completed her residencies in Pediatrics and Child Neurology at Stanford, where she also served as Chief Resident in Neurology. Following this, she completed a Pediatric Epilepsy fellowship, also at Stanford.
Currently Dr. Knowles cares for patients with pediatric epilepsy, and is particularly interested in genetic epilepsy and difficult to treat, or refractory, epilepsy. Dr. Knowles has developed a basic and translational research program under the mentorship of Drs Michelle Monje, John Huguenard and Courtney Wusthoff, and she is focused on how recurrent seizures influence brain development in pediatric epilepsy, as well as the mechanisms underlying epileptogenesis and associated cognitive dysfunction. Her current projects are particularly focused on interactions between neurons (gray matter) and myelin (white matter). Dr. Knowles’s research is supported by the American Epilepsy Society, a NIH/NINDS K12 award, the CURE Epilepsy Foundation, the Child Neurology Foundation/Pediatric Epilepsy Research Foundation and the Stanford Child Health Research Institute.
When she is not in the clinic or the lab, Dr. Knowles loves to spend time with her husband, Joshua, and their two children. She also enjoys reading, training and running in marathons, and spending time in the great outdoors of California.

Christopher Lee-Messer, MD, PhD Clinical Associate Professor of Neurology & Neurological Sciences and Pediatrics
Dr. Lee-Messer’s chief clinical focus is in pediatric epilepsy, especially the relationship between stroke and epilepsy. His translational and basic science interests lie in neuronal development and physiology, and in using that knowledge to create treatments for disease, especially in the injured developing brain. To investigate these subjects, he is currently participating as a fellow in the Deisseroth lab, combining techniques of in vivo and in vitro electrophysiology with optogenetics.

Emily Spelbrink, MD, PhD Clinical Associate Professor of Neurology & Neurological Sciences and Pediatrics
Dr. Emily Spelbrink is Clinical Assistant Professor of Neurology at Stanford University. Dr. Spelbrink received her B.S. at Emory University in Atlanta, and then her M.D. and Ph.D. degrees through the Medical Scientist Training Program at the University of California San Diego. She completed her pediatrics residency at Harbor UCLA, followed by child neurology residency at Stanford, and pediatric epilepsy fellowship at the University of California, San Francisco. Her interests in learning and memory were extended by Ph.D. research in these areas, and continue to fuel her interests in brain development and epileptic encephalopathies as she treats epilepsy patients.
Dr. Spelbrink joined the faculty at Stanford in 2016 as a child neurologist and epileptologist. She sees outpatients primarily at California Pacific Medical Center, in addition to inpatient and EEG service and teaching at Stanford.
When not at work, Dr. Spelbrink enjoys travel, time and space in nature (hiking, running, backpacking, camping), reading, knitting, yoga, and being with close friends and family.

Courtney Wusthoff, MD Professor of Neurology & Neurological Sciences and Pediatrics
Dr. Courtney Wusthoff is the Neurology Director for the Neuro Neonatal Intensive Care Unit at Lucile Packard Children's Hospital. She received her BA in neuroscience and behavior at Columbia University in New York, and her MD at the University of California, San Francisco. Dr. Wusthoff completed her pediatrics residency at Children's Hospital Oakland, and her neurology and neurophysiology training at the Children's Hospital of Philadelphia. After her fellowship, Dr. Wusthoff served as Consultant in Perinatal Neurology at the Hammersmith Hospital and Imperial College in London. Her special expertise is in neonatal EEG, neonatal seizures, and complications of newborn brain injury. She also has a major interest in brain monitoring in critically ill infants and children.
Pediatric Epilepsy Fellows

Michaela Costello, MD, PhD Pediatric Epilepsy Fellow
Originally from Baltimore, MD, Michaela Castello comes to Stanford via Child Neurology residency at UC San Diego/Rady Children's Hospital; It was there she developed her fascination with epilepsy. Her research interest in EEG has its origins in the laboratory of Dr. Jessica Mong, where she used it in the characterization of sleep in animal models. She completed her doctoral thesis on lipid regulation and neurodegeneration with Dr. Salvador Soriano. At Stanford she hopes to focus on applying machine learning techniques to seizure localization and epilepsy surgery planning.
While in San Diego, Dr. Castello tried really hard to get better at surfing with dubious success. Now having moved north, she can be found chipping her extra sparkly nail polish on rock climbing walls, exploring new restaurants, and steadfastly insisting that adults can still build with Lego. Occasionally she writes about her misadventures!

Katherine Xiong, MD Pediatric Epilepsy Fellow
Dr. Katherine Xiong is a Pediatric Epilepsy Fellow at Stanford. She earned her undergraduate degree in Neuroscience at Brown University, and her medical degree at University of Texas Southwestern. She went on to complete her child neurology residency at Stanford University, where she served as a chief resident. Her academic interests include neonatal epilepsy, epilepsy genetics, and improving care through quality improvement and medical education.
Epilepsy Surgery

Lawrence Shuer, MD Professor of Neurosurgery Associate Chair of the Department of Neurosurgery
Dr. Shuer performs surgical procedures on patients with uncontrolled seizures. Hetrained in surgery, neurosurgery and neuropathology at Stanford and joined the faculty in 1984. He has been part of the Comprehensive Epilepsy Team at Stanford since 1992. His interests in neurosurgery include the management of craniofacial anomalies, degenerative spine disorders, syringomyelia, surgical treatment of epilepsy and hypothermic brain protection. He was a consultant for Neuropace as it developed the responsive brain stimulator for medically refractory epilepsy. He is past president of the California Association of Neurological Surgeons, past president of the Western Neurosurgical Society and is chair of the California Medical Association Scientific Advisory Panel on Neurosurgery.

Vivek P. Buch, MD Clinical Assistant Professor of Neurosurgery
Dr. Buch is a neurosurgeon with fellowship training in epilepsy, functional, and minimally invasive neurosurgery. He is a clinical assistant professor in the Department of Neurosurgery of Stanford University School of Medicine. Dr. Buch focuses his expertise on the open and minimally invasive treatment of epilepsy, brain disorders, spinal injury and disease, and other conditions. For each patient, he develops a personalized care plan that is designed to be both comprehensive and compassionate.

Jaimie Henderson, MD John and Jene Blume - Robert and Ruth Halperin Professor Director, Stereotactic and Functional Neurosurgery
Dr. Henderson is the director of the program in Stereotactic and Functional Neurosurgery, and has a special interest in movement disorders and epilepsy. Dr. Henderson received his M.D. from Chicago's Rush Medical College in 1988. After completing his residency in Neurosurgery at Saint Louis University and fellowship training in Stereotactic and Functional Neurosurgery, he started the movement disorders surgery program at St. Louis University where he remained on the faculty for 6 years. He joined the Neurosurgery staff of the Cleveland Clinic in 2001, and Stanford's Neurosurgery Department in 2004. Dr. Henderson is an expert in invasive monitoring for surgical evaluation of patients with drug resistant epilepsy.

Candice Osuga Lin, MSN, APRN, BC, ACNP Nurse Practitioner
Candice earned her undegraduate degree in Biological Sciences from the University of California, Davis. She received her Master of Science in Nursing from Vanderbilt University. She is certified as an Acute Care Nurse Practitioner through the American Academy of Nurse Practitioners, and is a member of the American Association of Neurosciences Nursing, American Association of Nurse Practitioners and Sigma Theta Tau. She has been active in the care of adult neurosurgery patients. Her interests include neuro-oncology and degenerative spine disease.

Michael Zeineh, MD, PhD Associate Professor of Neuroradiology
Dr. Michael Zeineh’s background is in Neurosciences and Medicine from California Technological Institute and UCLA with residency training in Diagnostic Radiology and subspeciality in Neuroradiology from Stanford Medical Center. He has extensive experience studying the hippocampus with MRI and is an expert of advanced quantitative neuroimaging in epilepsy and leads the state-of-the art brain imaging of epilepsy patients with 7 Tesla MR scanner.

Gayle K Deutch, PhD Staff Neuropsychologist and Associate Professor Clinical and Research Neuropsychologist
Dr. Deutsch has special expertise in evaluation of brain function in people with epilepsy. She received her doctoral degree in clinical psychology at Drexel University and completed a pre-doctoral internship the University of Pennsylvania and a post-doctoral fellowship in clinical neuropsychology at the Graduate Hospital in Philadelphia, Pennsylvania. She was the Staff Neuropsychologist at the New Jersey Neuroscience Institute and Assistant Professor of Neuroscience at Seton Hall University, Graduate School of Medical Education. Her research interests are the neural basis of dyslexia and learning disorders and cognitive disorders in epilepsy.

Sepideh Bajestan, MD, PhD Clinical Associate Professor of Psychiatry
Dr. Bajestan has received her PhD in Molecular and Cellular Neuroscience. She spent her training during her PhD and post-doctoral course in learning the molecular genetics and biology of neurological and psychiatric disorders. She later trained in Psychiatry at Stanford University and became a board certified General Psychiatrist by the American Board of Psychiatry and Neurology. Dr. Bajestan was the first neuropsychiatry fellow at Stanford University and established this fellowship with Dr. John Barry, her fellowship director and is currently the Associate Neuropsychiatry Fellowship Director. She is also board certified in Neuropsychiatry and Behavioral Neurology by the United Council of Neurological Subspecialties. She has undergone training in different psychotherapy Orientations including Psychodynamic Psychotherapy, Cognitive and Behavioral Therapy (CBT), Psychodynamic Psychotherapy, Acceptance and Commitment Therapy (ACT), Hypnosis and Prolonged Exposure (PE).

John Barry, MD Professor of Psychiatry
Dr. Barry is the Director of the Neuropsychiatry and Psychotherapy Clinics has a special interest in psychiatric problems of people with epilepsy. He has done studies of depression and psychosis in association with epilepsy, and of the psychiatric mimics of seizures, called psychogenic non-epileptic seizure-like events, also known as psychogenic seizures or pseudoseizures. In patients admitted for video-EEG evaluation, he leads the efforts in making the diagnosis of psychogenic disorders and treating the patients disabled by this condition.

Kim Bullock, MD Clinical Professor of Psychiatry
Dr. Bullock is certified in the subspecialty of Behavioral Neurology & Neuropsychiatry. She runs an outpatient Neuro-Behavior Clinic and Laboratory with special emphasis on non-pharmacological interventions and evidenced-based psychotherapies for problems such as psychogenic seizures. Her focus is cognitive behavior group therapy and she trains residents, psychology students and therapists in these methods. She currently is investigating the use of group dialectical behavior for non-epileptic seizures.

Juliana Lockman, MD Clinical Assistant Professor of Psychiatry
Dr. Juliana Lockman is Clinical Assistant Professor in the Neuropsychiatry Division in Department of Psychiatry & Behavioral Sciences at Stanford. She is also appointed to La Selva Group, where she directs the Functional Neurologic Symptom Disorder (FND) Track within their state-of-the-art residential, partial hospitalization and intensive outpatient programs. She completed residencies in both Neurology at the University of Virginia and Psychiatry at Stanford Hospital & Clinics. Her clinical activities include providing pharmacologic and behavioral care for clients with psychiatric and behavioral conditions in the context of neurological illness, including epilepsy, stroke, movement disorders and others. She also teaches and supervises Stanford residents and fellows in Neuropsychiatry. Professional goals include advancement of research and clinical care and improving access for clients suffering from neuropsychiatric conditions, including FND and related disorders.
Basic Science Researchers

Paul Buckmaster, PhD Professor of Comparative Medicine and Neurology & Neurological Sciences
Dr. Buckmaster is one of the world’s leading scientists working on the anatomy and electrophysiology of temporal lobe epilepsy in animals to discover how brains become epileptic and how spontaneous seizures begin. Dr. Buckmaster's laboratory specializes in the studies of the pathophysiological mechanisms of temporal lobe epilepsy and the neuronal circuitry of temporal lobe structures in normal and epileptic brains.

John Huguenard, PhD Professor of Neurology & Neurological Sciences
Dr. Huguenard is the director of the Stanford Neuroscience Graduate Program training PhD students involved in the study of the brain. He is also one of the world's leading scientists working on the relationship between the pacemaker area of the brain (thalamus) and the cerebral cortex, and how dysfunction of this interaction can lead to seizures. Dr. Huguenard's laboratory specializes in the study of neuronal mechanisms underlying synchronous oscillatory activity in the thalamus, cortex and their interconnection and genes whose products, mainly ion channels, play key roles in the regulation of thalamocortical network responses.

Paul Nuyujukian, MD, PhD Assistant Professor of Bioengineering and of Neurosurgery
Paul Nuyujukian is a physician-engineer who directs the Brain Interfacing Laboratory . He received his MD and PhD in Bioengineering from Stanford and joined the Departments of Bioengineering and Neurosurgery as faculty in 2017. His group focuses on the development of brain-machine interfaces towards applications for neurological conditions such as stroke and epilepsy, spanning both translational preclinical models and human clinical studies.


David A. Prince, MD Professor of Neurology & Neurological Sciences
Dr. Prince is one of the world’s leading scientists working on the cellular mechanisms of epilepsy. Dr. Prince has trained dozens of the next generation leading epilepsy researchers in the world, and he and his research associates have made major advances in the understanding of the physiology of epilepsy. He is the holder of numerous research awards, including an NIH Javitz Award, the American Epilepsy Society award for excellence in research and epilepsy leadership, and he is a past president of the American Epilepsy Society. Dr. Prince's laboratory specializes in the studies of neuronal excitability and its regulation in the mammalian brains and mechanisms underlying development and prophylaxis of epilepsy in animal models.

Ivan Soltesz, PhD Professor of Neurosurgery and Neurosciences
Dr. Soltesz's laboratory employs a combination of closely integrated experimental and theoretical techniques, including closed-loop in vivo optogenetics, paired patch clamp recordings, in vivo electrophysiological recordings from identified interneurons in awake mice, 2-photon imaging, machine learning-aided 3D video analysis of behavior, video-EEG recordings, behavioral approaches, and large-scale computational modeling methods using supercomputers. He is the author of a book on GABAergic microcircuits (Diversity in the Neuronal Machine, Oxford University Press), and editor of a book on Computational Neuroscience in Epilepsy (Academic Press/Elsevier). He co-founded the first Gordon Research Conference on the Mechanisms of neuronal synchronization and epilepsy, and taught for five years in the Ion Channels Course at Cold Springs Harbor. He has over 30 years of research experience, with over 20 years as a faculty involved in the training of graduate students (total of 16, 6 of them MD/PhDs) and postdoctoral fellows (20), many of whom received fellowship awards, K99 grants, joined prestigious residency programs and became independent faculty.

The official website for the neurology fellowship programs at University of Pennsylvania in Philadelphia.
Epilepsy Fellowship Program

- Epilepsy Surgery (>80 procedures last year, including phase II monitoring with stereo EEG and subdural electrodes, implantable devices, resections and laser ablations )
- Outpatient epilepsy, including specialty clinics for epilepsy genetics, neuromodulation, transitioning care from pediatrics to adult, tuberous sclerosis and others
- Clinical trials - medications and devices
- Implantable devices, including RNS, DBS and VNS
- International medicine
- Quality of care in collaboration with the Wharton School of Business
Fellowship Curriculum
Clinical fellows in our program evaluate and care for epilepsy patients in both inpatient and outpatient settings. Our Epilepsy Monitoring Unit (currently 8 beds) and our active epilepsy surgical program provide a means for fellows to learn how to evaluate and refer patients for epilepsy surgery and device implantation. This includes very active intracranial EEG program, including functional brain mapping. In addition, there is an active long-term monitoring/ICU EEG program which covers 4 hospitals in downtown Philadelphia, including advanced techniques such as quantitative EEG and intracranial depth electrodes for traumatic brain injury. Fellows also participate in the outpatient management of patients with epilepsy (or concern for epilepsy) by co-managing patients in attending clinics as well as their own longitudinal clinic. The outpatient EEG laboratory is very active and offers routine, prolonged and ambulatory EEG studies at multiple locations.
In addition to the above, fellows rotate at the Children’s Hospital of Philadelphia for pediatric epilepsy and at the Philadelphia Veterans Affairs Medical Center.
A critical aspect of our program is the active integration of clinical, translational and basic research with the clinical programs. This includes active research projects related to intracranial EEG, neuromodulation, neuroimaging, genetics and others. Fellows have the opportunity to participate in these projects based on their interests. Dedicated didactic sessions include core lectures on epilepsy and EEG followed by weekly conferences on EEG/Neurophysiology and Clinical Epilepsy. Active participation by fellows is encouraged including case presentations, journal clubs and presentation of projects. There is also a weekly Epilepsy Surgery conference where the fellows present the vast majority of patients for discussion, with active participation by neuroradiology and neuropsychology. Joint conferences include the NeuroICU and pediatric epilepsy programs.

“Penn provided an ideal environment for diverse training in clinical epileptology, leading the cutting edge on many aspects of EEG and patient care. In addition, there were tremendous opportunities for funded translational research, which were instrumental in establishing my own academic career.”
Application Process
- November 15, 2023 Applicants may begin submitting applications on ERAS.
- December 6, 2023 Programs may begin reviewing applications on ERAS.
- January - April 2024 Penn Epilepsy Fellowship Interviews.
- May 1, 2024 Deadline for submitting Rank Order List.
- May 15, 2024 Match Day.
- July 1, 2025 First Day of Fellowship.
- Personal Statement explaining your interests in Epilepsy
- USMLE Transcript
- Professional photo
- Three Letters of Recommendation.
Thank you for your interest in our program!
Epilepsy faculty.

Kathryn Davis, MD, MTR
Epilepsy division chief medical director, epilepsy monitoring unit medical director, epilepsy surgical program interests: epilepsy anti-epileptic medications, electroencephalogram, seizures faculty profile.

Erin Conrad, MD
Assistant professor of neurology faculty profile.

Ramon Diaz-Arrastia, MD, PhD

Colin Ellis, MD
Taneeta ganguly, md, faculty profile , michael gelfand, md, phd.

Ramya Raghupathi, MD
- Age Span Fellowship in Multiple Sclerosis and related Neuroinflammatory Disorders
- Headache and Facial Pain
- Movement Disorders
- Neurocritical Care
- Neurohospitalist Medicine
- Neuro-Ophthalmology
- Neuromuscular Medicine
- Neuro-Oncology
- Sleep Medicine
- Vascular Neurology
Contact Information
Popular posts.

Copyright 2015 Penn Neurology Fellowship Programs
Sora Templates
See our updated masking policy »
- Doctors, Clinics & Locations, Conditions & Treatments
- Patients & Visitors
- Medical Records
- Support Groups
- Help Paying Your Bill
- COVID-19 Resource Center
- Locations and Parking
- Visitor Policy
- Hospital Check-in
- Video Visits
- International Patients
View the changes to our visitor policy »
View information for Guest Services »
New to MyHealth?
Manage Your Care From Anywhere.
Access your health information from any device with MyHealth. You can message your clinic, view lab results, schedule an appointment, and pay your bill.
ALREADY HAVE AN ACCESS CODE?
Don't have an access code, need more details.
Learn More about MyHealth » Learn More about Video Visits »
MyHealth for Mobile
Get the iPhone MyHealth app » Get the Android MyHealth app »
WELCOME BACK

Yi Li, MD, PhD
Epilepsy specialist.
Clinical Assistant Professor, Neurology & Neurological Sciences
View Professional Summary »
Get In Touch
Professional summary, education & certifications.
- Board Certification: American Board of Psychiatry and Neurology, Epilepsy (2020)
- Fellowship: Stanford University Epilepsy Fellowship (2020) CA
- Board Certification: American Board of Psychiatry and Neurology, Neurology (2018)
- Residency: University of Massachusetts GME Office (2018) MA
- Internship: University of Massachusetts Internal Medicine Residency (2015) MA
- Medical Education: Xiangya School of Medicine South University (2007) China
Publications
- Activation of ERK by spontaneous seizures in neural progenitors of the dentate gyrus in a mouse model of epilepsy Li, Y., Peng, Z., Xiao, B., & Houser, C. R. (2010). Activation of ERK by spontaneous seizures in neural progenitors of the dentate gyrus in a mouse model of epilepsy. EXPERIMENTAL NEUROLOGY , 224 (1), 133–45.
- Electroencephalography of Seizure-Like Movements During General Anesthesia with Propofol: Seizures or Nonepileptic Events? Li, Y., Flood, P., & Cornes, S. (2015). Electroencephalography of Seizure-Like Movements During General Anesthesia with Propofol: Seizures or Nonepileptic Events? A & A Case Reports , 5 (11), 195–98.
- Left Ventricular Ejection Fraction and Clinically Defined Heart Failure to Predict 90-Day Functional Outcome After Ischemic Stroke. Li, Y., Fitzgibbons, T. P., McManus, D. D., Goddeau, R. P., Silver, B., & Henninger, N. (2018). Left Ventricular Ejection Fraction and Clinically Defined Heart Failure to Predict 90-Day Functional Outcome After Ischemic Stroke. Journal of Stroke and Cerebrovascular Diseases : the Official Journal of National Stroke Association .
- Potential use of leukocytosis and anion gap elevation in differentiating psychogenic nonepileptic seizures from epileptic seizures. Li, Y., Matzka, L., Flahive, J., & Weber, D. (2019). Potential use of leukocytosis and anion gap elevation in differentiating psychogenic nonepileptic seizures from epileptic seizures. Epilepsia Open , 4 (1), 210–15.
- Ephrin-b3 modulates hippocampal neurogenesis and the reelin signaling pathway in a pilocarpine-induced model of epilepsy. Liu, T.-T. T., Li, Y., Shu, Y., Xiao, B., & Feng, L. (2018). Ephrin-b3 modulates hippocampal neurogenesis and the reelin signaling pathway in a pilocarpine-induced model of epilepsy. International Journal of Molecular Medicine , 41 (6), 3457–3467.
- Epilepsy with temporal encephalocele: Characteristics of electrocorticography and surgical outcome. Panov, F., Li, Y., Chang, E. F., Knowlton, R., & Cornes, S. B. (2016). Epilepsy with temporal encephalocele: Characteristics of electrocorticography and surgical outcome. Epilepsia , 57 (2), e33–8.
- Anion gap can differentiate between psychogenic and epileptic seizures in the emergency setting. Li, Y., Matzka, L., Maranda, L., & Weber, D. (2017). Anion gap can differentiate between psychogenic and epileptic seizures in the emergency setting. Epilepsia , 58 (9), e132–e135.
- The Ephrin-A5/EphA4 Interaction Modulates Neurogenesis and Angiogenesis by the p-Akt and p-ERK Pathways in a Mouse Model of TLE. Shu, Y., Xiao, B., Wu, Q., Liu, T., Du, Y., Tang, H., … Li, Y. (2016). The Ephrin-A5/EphA4 Interaction Modulates Neurogenesis and Angiogenesis by the p-Akt and p-ERK Pathways in a Mouse Model of TLE. Molecular Neurobiology , 53 (1), 561–576.
- MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. Hu, K., Xie, Y.-Y. Y., Zhang, C., Ouyang, D.-S. S., Long, H.-Y. Y., Sun, D.-N. N., … Xiao, B. (2012). MicroRNA expression profile of the hippocampus in a rat model of temporal lobe epilepsy and miR-34a-targeted neuroprotection against hippocampal neurone cell apoptosis post-status epilepticus. BMC Neuroscience , 13 , 115.
- EphA4 may contribute to microvessel remodeling in the hippocampal CA1 and CA3 areas in a mouse model of temporal lobe epilepsy. Feng, L., Shu, Y., Wu, Q., Liu, T., Long, H., Yang, H., … Xiao, B. (2017). EphA4 may contribute to microvessel remodeling in the hippocampal CA1 and CA3 areas in a mouse model of temporal lobe epilepsy. Molecular Medicine Reports , 15 (1), 37–46.
- [Neuronal synaptic reconstruction in hippocampus in chronic phase of pilocarpine-treated rats]. Yi, F., Abuhamed, M. M., Long, L.-li L., Li, Y., Li, S.-yu Y., Wu, Z.-guo G., & Xiao, B. (2011). [Neuronal synaptic reconstruction in hippocampus in chronic phase of pilocarpine-treated rats]. Zhonghua Yi Xue Za Zhi , 91 (19), 1335–9.
- Altered expression of pannexin proteins in patients with temporal lobe epilepsy. Jiang, T., Long, H., Ma, Y., Long, L., Li, Y., Li, F., … Xiao, B. (2013). Altered expression of pannexin proteins in patients with temporal lobe epilepsy. Molecular Medicine Reports , 8 (6), 1801–6.
- Expression profile of microRNAs in rat hippocampus following lithium-pilocarpine-induced status epilepticus. Hu, K., Zhang, C., Long, L., Long, X., Feng, L., Li, Y., & Xiao, B. (2011). Expression profile of microRNAs in rat hippocampus following lithium-pilocarpine-induced status epilepticus. Neuroscience Letters , 488 (3), 252–7.
- Selective loss and axonal sprouting of GABAergic interneurons in the sclerotic hippocampus induced by LiCl-pilocarpine. Long, L., Xiao, B., Feng, L., Yi, F., Li, G., Li, S., … Li, Y. (2011). Selective loss and axonal sprouting of GABAergic interneurons in the sclerotic hippocampus induced by LiCl-pilocarpine. The International Journal of Neuroscience , 121 (2), 69–85.
- Rapamycin suppresses the recurrent excitatory circuits of dentate gyrus in a mouse model of temporal lobe epilepsy. Tang, H., Long, H., Zeng, C., Li, Y., Bi, F., Wang, J., … Xiao, B. (2012). Rapamycin suppresses the recurrent excitatory circuits of dentate gyrus in a mouse model of temporal lobe epilepsy. Biochemical and Biophysical Research Communications , 420 (1), 199–204.
- DISC1 Regulates the Proliferation and Migration of Mouse Neural Stem/Progenitor Cells through Pax5, Sox2, Dll1 and Neurog2 Wu, Q., Tang, W., Luo, Z., Li, Y., Shu, Y., Yue, Z., … Feng, L. (2017). DISC1 Regulates the Proliferation and Migration of Mouse Neural Stem/Progenitor Cells through Pax5, Sox2, Dll1 and Neurog2. FRONTIERS IN CELLULAR NEUROSCIENCE , 11 , 261.
- NDEL1 was decreased in the CA3 region but increased in the hippocampal blood vessel network during the spontaneous seizure period after pilocarpine-induced status epilepticus. Wu, Q., Li, Y., Shu, Y., Feng, L., Zhou, L., Yue, Z. W., … Xiao, B. (2014). NDEL1 was decreased in the CA3 region but increased in the hippocampal blood vessel network during the spontaneous seizure period after pilocarpine-induced status epilepticus. Neuroscience , 268 , 276–83.
- DISC1-related signaling pathways in adult neurogenesis of the hippocampus. Wu, Q., Li, Y., & Xiao, B. (2013). DISC1-related signaling pathways in adult neurogenesis of the hippocampus. Gene , 518 (2), 223–30.
- The increased expression of CD21 on AchR specified B cells in patients with myasthenia gravis Yin, W., Allman, W., Ouyang, S., Li, Y., Li, J., Christadoss, P., & Yang, H. (2013). The increased expression of CD21 on AchR specified B cells in patients with myasthenia gravis. JOURNAL OF NEUROIMMUNOLOGY , 256 (1-2), 49–54.
- Immature Dendritic Cell-Derived Exosomes: a Promise Subcellular Vaccine for Autoimmunity Yin, W., Ouyang, S., Li, Y., Xiao, B., & Yang, H. (2013). Immature Dendritic Cell-Derived Exosomes: a Promise Subcellular Vaccine for Autoimmunity. INFLAMMATION , 36 (1), 232–40.
- Ultrasound-guided lumbar puncture improves success rate and efficiency in overweight patients. Li, Y., Carandang, R. A., Ade, S., Flahive, J., & Daniello, K. (2020). Ultrasound-guided lumbar puncture improves success rate and efficiency in overweight patients. Neurology. Clinical Practice , 10 (4), 307–13.
- Pregnancy outcomes of refractory epilepsy patients treated with Brain-responsive neurostimulation. Li, Y., Eliashiv, D., LaHue, S. C., Rao, V. R., Martini, M. L., Panov, F., … Meador, K. J. (2020). Pregnancy outcomes of refractory epilepsy patients treated with Brain-responsive neurostimulation. Epilepsy Research , 169 , 106532.
- Antibody Prevalence in Epilepsy before Surgery (APES) in drug-resistant focal epilepsy. Li, Y., Tymchuk, S., Barry, J., Muppidi, S., & Le, S. (2021). Antibody Prevalence in Epilepsy before Surgery (APES) in drug-resistant focal epilepsy. Epilepsia .
- Precision medicine in women with epilepsy: The challenge, systematic review, and future direction. Li, Y., Zhang, S., Snyder, M. P., & Meador, K. J. (2021). Precision medicine in women with epilepsy: The challenge, systematic review, and future direction. Epilepsy & Behavior : E&B , 118 , 107928.
- Impact of high-density EEG in presurgical evaluation for refractory epilepsy patients. Li, Y., Fogarty, A., Razavi, B., Ardestani, P. M., Falco-Walter, J., Werbaneth, K., … Fisher, R. S. (2022). Impact of high-density EEG in presurgical evaluation for refractory epilepsy patients. Clinical Neurology and Neurosurgery , 219 , 107336.
- Precision medicine in epilepsy. McGinn, R. J., Von Stein, E. L., Summers Stromberg, J. E., & Li, Y. (2022). Precision medicine in epilepsy. Progress in Molecular Biology and Translational Science , 190 (1), 147–188.
- How does foetal exposure to valproate produce adverse neurodevelopmental outcomes? Meador, K. J., & Li, Y. (2022). How does foetal exposure to valproate produce adverse neurodevelopmental outcomes? Brain : a Journal of Neurology .
- Epilepsy and Pregnancy. Li, Y., & Meador, K. J. (2022). Epilepsy and Pregnancy. Continuum (Minneapolis, Minn.) , 28 (1), 34–54.
Specializing In
- Epilepsy Clinical and Translational Research
- Epilepsy Genetics
Practice Locations
Comprehensive epilepsy program in palo alto palo alto, ca.
Comprehensive Epilepsy Program in Palo Alto
213 Quarry Road
Palo Alto , CA 94304
(650) 723-6469
Important Information about Our Organizations and Physician Affiliation
Stanford Health Care, Stanford Health Care Tri-Valley, and Stanford Medicine Partners are each independent nonprofit organizations that are affiliated with but separate from each other and from Stanford University. The physicians who provide care at facilities operated by Stanford Health Care, Stanford Health Care Tri-Valley, and Stanford Medicine Partners are faculty, foundation, or community physicians who are not employees, representatives, or agents of Stanford Health Care, Stanford Health Care Tri- Valley, or Stanford Medicine Partners. Stanford Health Care, Stanford Health Care Tri-Valley, and Stanford Medicine Partners do not exercise control over the care provided by such faculty, foundation, and community physicians and are not responsible for their actions.
Patient Ratings
(49 Ratings)
View Ratings by Category
How Our Ratings Work
The Patients Ratings and comments are gathered from our Patient Satisfaction Survey and displayed in their entirety.
Patient Satisfaction Survey Disclaimer
Patient Reviews
(50 reviews), referring physicians, physician helpline.
Stanford Health Care provides comprehensive services to refer and track patients, as well as the latest information and news for physicians and office staff. For help with all referral needs and questions, visit Referral Information .
You may also submit a web referral or complete a referral form and fax it to 650-320-9443 or email the Referral Center at [email protected] .
- Send referrals online
- Place radiology and lab orders
- View referral status
- Access medical records
Learn More About MedLink »
Emory Epilepsy Center section navigation
Emory epilepsy center.
The Emory Epilepsy Center is a multidisciplinary specialized team from the Departments of Neurology and Neurosurgery. The Emory Epilepsy Center is part of the Emory Brain Health Center.

The Emory University Epilepsy Program is a multi-specialty group of physicians, neuropsychologists, and nurses from the departments of Neurology, Neurosurgery, Pediatrics, Physical Medicine and Rehabilitation and Radiology. The Epilepsy Program provides specialized clinical care in the diagnosis and treatment of seizures, epilepsy and conditions that may mimic epilepsy.
Learn more about the Emory Epilepsy Center.
Research Highlights
Emory University is a major research center. The faculty of the Emory Epilepsy Program are national leaders in research and in the forefront of medical discoveries related to epilepsy. Our group is involved in several NIH and foundation supported research projects, as well as innovative partnerships with industry.
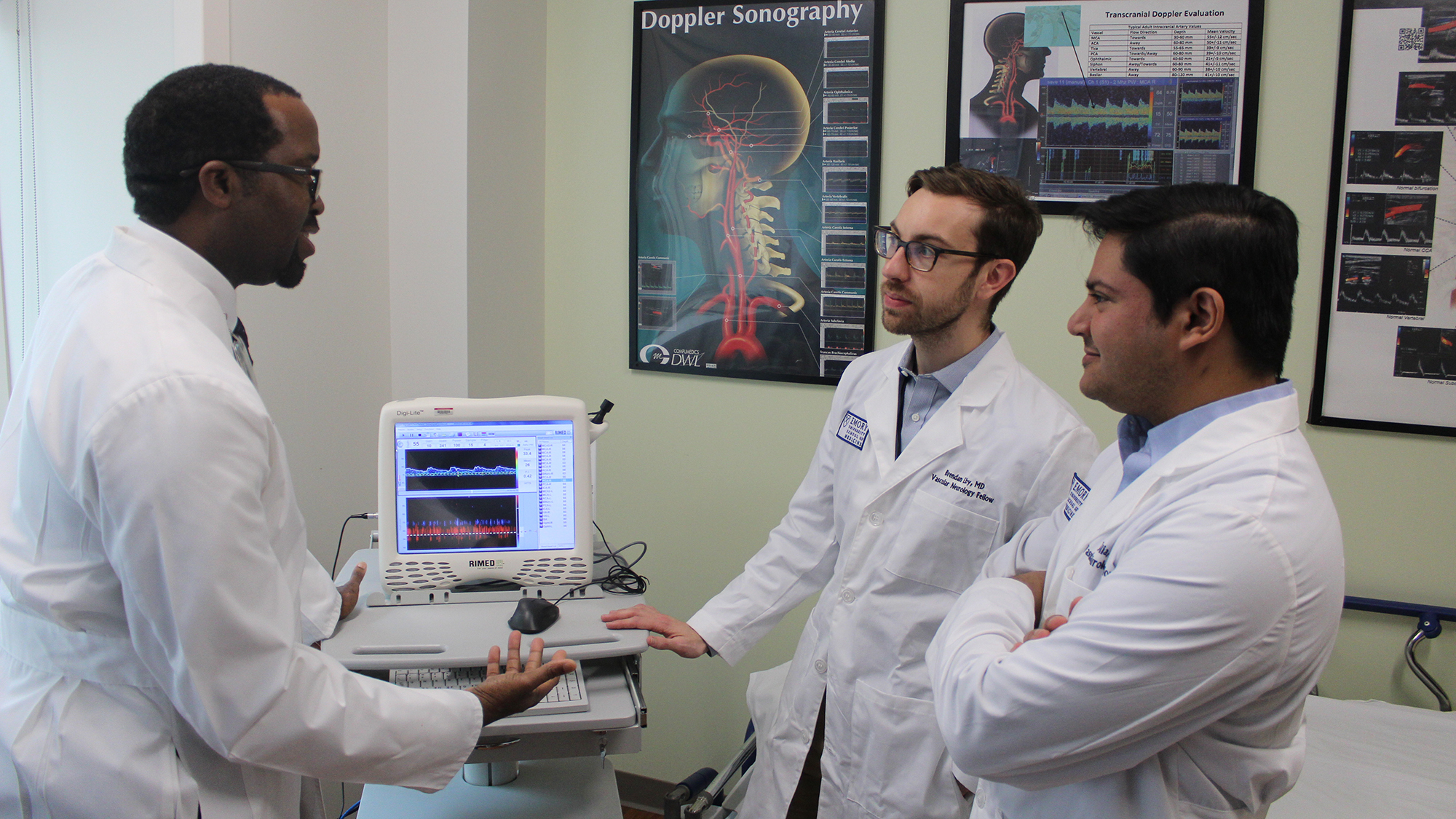
Advanced fellowship training opportunities are available in the Epilepsy Program, and include clinical and research fellowships .
Patient Care
Emory Epilepsy Center is one of the only Level-4 epilepsy centers in the southeastern United States, which means patients can count on us for providing them with the most comprehensive and innovative care available.

Benchmarks Overview
2021 aes/ninds epilepsy research benchmarks .
On January 4-6, 2021 NINDS hosted Curing the Epilepsies 2021: Setting Research Priorities conference virtually via Zoom. This conference was the fourth in a series of Curing the Epilepsies conferences held approximately every seven years since 2000. The goal of the conference is to bring together all stakeholders—including researchers, clinicians, patients, families, and advocates—to evaluate the current state of epilepsy research and consider priorities for future efforts.
As an important outcome, the Curing the Epilepsies conferences have led to the development of Benchmarks for Epilepsy Research, which reflect priorities shared across the epilepsy community for research toward clinically meaningful advances in understanding and treating the epilepsies.
In late 2019, the Epilepsy Benchmark Stewards Committee, coordinated by the American Epilepsy Society, started the process of revising the Epilepsy Research Benchmarks by publishing progress reports for each of the four benchmark areas. These articles were published in Epilepsy Currents in early 2020.
Epilepsy Benchmarks Area I: Understanding the Causes of the Epilepsies and Epilepsy-Related Neurologic, Psychiatric, and Somatic Conditions
Bernard S. Chang, MD; Vaishnav Krishnan, MD, PhD, FAES; Chris G. Dulla, PhD; Nathalie Jette, MD, MScFAES; Eric D. Marsh, MD, PhD; Penny A. Dacks, PhD; Vicky Whittemore, PhD; Annapurna Poduri, MD, MPH, FAES; and for the NINDS/AES Epilepsy Research Benchmark Stewards Committee
Epilepsy Benchmarks Area II: Prevent Epilepsy and Its Progression
Devin K. Binder, MD, PhD; Detlev Boison, PhD; Tore Eid, MD, PhD; Wayne N. Frankel, PhD; Ana Mingorance, PhD; Bret N. Smith, PhD; Penny A. Dacks, PhD; Vicky Whittemore, PhD; Annapurna Poduri, MD, MPH, FAES; and for the AES/NINDS Epilepsy Benchmarks Stewards
Epilepsy Benchmarks Area III: Improved Treatment Options for Controlling Seizures and Epilepsy-Related Conditions Without Side Effects
Stephen F. Traynelis, PhD; Dennis Dlugos, MD, FAES; David Henshall, PhD; Heather C. Mefford, MD, PhD; Michael A. Rogawski, MD, PhD, FAES; Kevin J. Staley, MD, FAES; Penny A. Dacks, PhD; Vicky Whittemore, PhD; Annapurna Poduri, MD, MPH, FAES; and for the National Institutes of Neurological Disorders and Stroke (NINDS)/American Epilepsy Society (AES) Epilepsy Research Benchmark Stewards
Epilepsy Benchmarks Area IV: Limit or Prevent Adverse Consequence of Seizures and Their Treatment Across the Life Span
Jana E. Jones, PhD, FAES; Miya R. Asato, MD; Mesha-Gay Brown, MD; Julia L. Doss, PsyD; Elizabeth A. Felton, MD, PhD, FAES; Jennifer A. Kearney, PhD, FAES; Delia Talos, MD; Penny A. Dacks, PhD; Vicky Whittemore, PhD; Annapurna Poduri, MD, MPH, FAES; and for the NINDS/AES Benchmarks Stewards Committee
Revisions were drafted based on these reports and public comments received through a 2019 NINDS Request for Information (RFI) and were posted as draft 2020 epilepsy research benchmarks as part of a crowdsourcing campaign conducted in September and October 2020. The final 2021 AES/NINDS Epilepsy Research Benchmarks incorporate revisions in response to public comments from the crowd-sourcing campaign and the priorities identified during the conference. These Benchmarks are intended to anchor research over the next 5-7 years in the issues that are key to understanding the epilepsies and improving meaningful outcomes for people with epilepsy through research. A plain language version is also available.
Area I: Understand the causes of the epilepsies and their relationship to epilepsy-associated neurologic, psychiatric, and somatic conditions.
A. Identify the many genes and molecular pathways associated with the epilepsies and epilepsy-related conditions. Define the many trajectories to hypersynchrony and demonstrate how these trajectories are modified by genetic background.
B. Identify and understand the mechanisms by which infections, inflammation, environment, vascular changes, perinatal exposures or insults, trauma, and other causes and risk factors, alone and in combination, contribute to the development of the epilepsies and epilepsy-related conditions.
C. Determine how alterations in molecular and cellular function interact with alterations in circuit and network function in the pathogenesis of cortical hyperexcitability and the clinical epilepsies.
D. Identify and understand the mechanisms by which factors related to age, sex, race/ethnicity, socioeconomic status, and other demographic features modulate epilepsy risk, drawing from discoveries in basic, translational, clinical, and population-level investigation.
E. Define the various mechanisms that explain why seizures commonly present with neuropsychiatric and neurodevelopmental comorbidities, drawing from discoveries in basic, translational, clinical, and population-level investigation.
Area II: Prevent epilepsy and its progression.
A. Understand epileptogenic processes involved in epilepsies with neurodevelopmental origins, including those due to genetic or epigenetic causes.
B. Understand epileptogenic processes involved in the development of epilepsy following traumatic brain injury, stroke, brain tumor, infections, neurodegeneration, or other insults to the brain.
C. Identify biomarkers that will aid in identifying, predicting, and monitoring epileptogenesis and disease progression, including markers early after injury/insult that identify those people at risk for epilepsy.
D. Develop or refine models aligned with the etiologies of human epilepsies to enable improved understanding of epileptogenesis and rigorous preclinical therapy development for epilepsy prevention or disease modification.
E. Identify new targets and develop interventions to prevent or modify epileptogenesis and the progression of epilepsy and epilepsy-related conditions.
F. Combine complex systems and/or machine learning approaches with laboratory studies in order to identify convergent phenotypes or pathways, examine background genetic or epigenetic effects, or consider novel molecular reclassifications of disease and the epileptogenic process.
Area III: Improve treatment options for controlling seizures and epilepsy-related conditions while limiting side effects.
A. In order to identify new antiseizure or disease-modifying therapeutic targets and mechanism-based therapies, we need to (1) understand the mechanisms of initiation, propagation, and termination of seizures at the cellular and network level for different seizure types, including status epilepticus, and in different forms of epilepsy, (2) understand the neural circuits, cell types, cellular interactions, and genetic factors that participate in interictal activity, different seizure types in different forms of epilepsy, and (3) understand the cellular, molecular, and network and systems basis for treatment side effects.
B. Identify genetic, molecular, imaging, immunological, and electrophysiological biomarkers, determine mechanisms of pharmacoresistance, and develop clinical informatics tools so that the most appropriate pharmacological, biological, surgical, or device therapy can be selected for an individual with a common or rare epilepsy. These efforts should take into consideration time, an individual’s unique set of personal characteristics, including sex and life stage (e.g., childhood, pregnancy, elderly), and consider inclusion of non-seizure outcome measures reflecting other epilepsy-related risks.
C. Develop, refine, fully characterize, and deploy epilepsy and seizure models (including in non-rodents) that align with the etiologies, clinical features, rhythmicities, treatment responses, and development of resistance of human epilepsies to improve understanding of epileptogenesis, ictogenesis, seizure initiation, seizure termination, disease progression, and therapeutic targets. Explore the utility of new technologies to model human epilepsies and screen for therapies in a high throughput fashion, including iPSCs and organoids.
D. Identify, develop, and improve pharmacological, surgical, genetic, epigenetic, neuromodulatory, dietary interventions, and devices to detect, predict, prevent, or terminate seizures and mitigate other epilepsy-related health risks while minimizing adverse effects.
E. Develop, improve, implement, and validate strategies, protocols, and interventions for epilepsy self-management in the home or other non-medical settings that allow ongoing assessment of treatment response, therapy adherence, and adverse effects of therapies.
Area IV: Limit, treat, or prevent co-occurring conditions associated with epilepsy across the lifespan in general and special epilepsy populations.
A. Understand and limit the impact of epilepsy on non-seizure outcomes such as neurodevelopment, mental health, cognition, health-related quality of life, and other functions.
B. Understand and limit the impact of anti-seizure treatments (medical, surgical, and other interventions) on non-seizure outcomes, such as neurodevelopment, mental health, cognition, health-related quality of life, and other functions.
C. Understand mechanisms (psychiatric and neurological) involved in non-epileptic seizures (NES). Develop effective pediatric and adult treatments and assess outcomes in NES including psychopathology and quality of life.
D. Identify causes, risk factors, and potential preventative strategies for sudden unexpected death in epilepsy (SUDEP) and other epilepsy-related mortality due to co-occurring conditions including depression, anxiety, and suicide in people with epilepsy.
E. Identify the impact of epilepsy on women’s health outcomes (fertility, pregnancy, bone health, hormones, mental health, QOL) and the health of their offspring (fetal and neonatal development).
F. Understand the role of sleep and circadian rhythms in cognitive and psychiatric and other health-related outcomes. Identify and treat sleep as a target to improve non-seizure outcomes, such as neurodevelopment, mental health, cognition, health-related quality of life, and other functions.
2021 Commentaries
The 2021 Epilepsy Research Benchmarks—Respecting Core Principles, Reflecting Evolving Community Priorities
Eric D. Marsh, MD, PhD; Vicky Whittemore, PhD; Miriam Leenders, PhD; Annapurna Poduri, MD, FAES; and on behalf of the American Epilepsy Society Epilepsy Research Benchmark Stewards Committee
Epilepsy Community at an Inflection Point: Translating Research Toward Curing the Epilepsies and Improving Patient Outcomes
Ilene Penn Miller, JD, LLM; JayEtta Hecker, MS; Brandy Fureman, PhD; Mary Anne Meskis; Steve Roberds, PhD; Monika Jones, JD; Heidi Grabenstatter, PhD, FAES; Vanessa Vogel-Farley, PhD; and Laura Lubbers, PhD
Tools & Resources
- Find Your Local Chapter
- Use my current location

- Our Editors
Robert Wechsler, MD, PhD

Former Medications Co-Editor
Dr. Robert Wechsler is a fellowship-trained and Board certified epileptologist in private practice in Boise, Idaho. He is the owner of Consultants in Epilepsy & Neurology, PLLC, and medical director of the Idaho Comprehensive Epilepsy Center, which he opened in 2005.
Dr. Wechsler received his medical degree from Finch University of Health Sciences/The Chicago Medical School in Illinois, where he also received a doctorate in neuroscience. He completed his residency in neurology at the Barrow Neurological Institute/St. Joseph’s Hospital in Phoenix, Arizona, and his fellowship in epilepsy at the Stanford University Hospital Comprehensive Epilepsy Center in California.
Dr. Wechsler is president of the Board of the Epilepsy Foundation of Idaho and has served as chair of their Professional Advisory Board. He is active in several committees of the American Epilepsy Society and currently serves as chair of its Corporate Advisory Committee. He also is active in a number of epilepsy clinical trials.
Dr. Wechsler recognizes the importance of support and education for people with epilepsy and of educating the public about epilepsy, having lead numerous local and regional education initiatives.
Reviewed By: Epilepsy Foundation Marketing & Communications
Robert Fisher, MD, PhD
Sandra Cushner Weinstein, PT, LICSW, LCSW-C
Epilepsy Centers
Epilepsy centers provide you with a team of specialists to help you diagnose your epilepsy and explore treatment options.
Epilepsy Medication
Find in-depth information on anti-seizure medications so you know what to ask your doctor.
Epilepsy and Seizures 24/7 Helpline
Call our Epilepsy and Seizures 24/7 Helpline and talk with an epilepsy information specialist or submit a question online.
Get information, tips, and more to help you manage your epilepsy.
Related Stories

Press Releases
Patient Organizations Applaud Release of Affordable Care Act Rule to Protect All Patients from Discrimination When Accessing Health Care

What is the Epilepsy Foundation’s Multicultural Outreach Program?
Diagnosis & Treatment
How to Best Prepare for an EMU Stay - Webinar
Sign Up for Emails
Stay up to date with the latest epilepsy news, stories from the community, and more.

UCL Queen Square Institute of Neurology

The following are PhD theses from the UCL Epilepsy Imaging Group with links to the full text in UCL Discovery where available:
- Vakharia, Vejay Niranjan; (2020) Computer-Assisted Planning and Robotics in Epilepsy Surgery .
- De Blasi, Bianca; (2020) Multi-parametric Imaging Using Hybrid PET/MR to Investigate the Epileptogenic Brain
- Lorenzo Caciagli - Neuroimaging of epilepsy: disease severity, cognitive comorbidities and endophenotypes
- Mark Nowell - Novel multimodality imaging in the planning and surgical treatment of epilepsy
- Christian Vollmar - Neuroimaging of functional and structural alterations in juvenile myoclonic epilepsy and frontal lobe epilepsy
- Britta Wandschneider - The effects of genes, antiepileptic drugs and risk of death on functional anatomy and cognitive networks in epilepsy
- Maria Feldmann - Imaging p-glycoprotein function: prediction of treatment response in mesial temporal lobe epilepsy
- Meneka Sidhu - Episodic memory in temporal lobe epilepsy
- Silvia Bonelli-Nauer - Cognitive functional MRI in temporal lobe epilepsy
- Helmut Laufs - EEG-fMRI signatures of spontaneous brain activity in healthy volunteers and epilepsy patients
- Rachel Thornton - Imaging brain networks in focal epilepsy : a prospective study of the clinical application of simultaneous EEG-fMRI in pre-surgical evaluation
- Gavin Winston - Translation of novel imaging techniques into clinical use for patients with epilepsy
- Mahinda Yogarajah - Imaging structural connections of the brain in epilepsy
- Umair Chaudhary - Haemodynamic correlates of interictal and ictal epileptic discharges and ictal semiology using simultaneous scalp video-EEG-fMRI and intracranial EEG-fMRI
- Robert Simister - Magnetic Resonance Spectroscopy as applied to epilepsy
- Serge Vulliemoz - Imaging functional and structural networks in the human epileptic brain
- Beate Diehl - Imaging correlates of the epileptogenic zone and functional deficit zone using diffusion tensor imaging (DTI)
- Khalid Hamandi - Functional MRI of focal and generalised interictal epileptiform discharges
- Afraim Salek-Haddadi - EEG-correlated functional MRI in epilepsy
- Rebecca Samson - Optimisation of quantitative magnetisation transfer (qMT) MRI to study restricted protons in the living human brain
- Robert Powell - Investigating brain structure and function in temporal lobe epilepsy
- Karsten Krakow - Imaging of epileptic activity using EEG-correlated functional MRI
- Rebecca Liu - Investigation of secondary cerebral damage in epilepsy
- Tejal Mitchell - Quantitative MRI in cerebral development disorders and epilepsy
- Fergus Rugg-Gunn - Imaging the neocortex in epilepsy with advanced MRI techniques
- Alex Everitt - The structural basis of the epilepsies: MRI and epidemiological studies
- Alexander Hammers - PET investigations in focal epilepsy
- Udo Wieshmann - New MR imaging techniques in epilepsy
- Matthias Koepp - Central benzodiazepine receptors in hippocampal sclerosis and idiopathic generalised epilepsies and opioid receptors in reading epilepsy
- Mark Richardson - Positron emission tomography investigation of cortical malformations causing epilepsy
- Wim van Paesschen - Quantitative MRI and hippocampal neuropathology of temporal lobe epilepsy
- Sanjay Sisodiya - Qualitative and quantitative analysis of MRI data from patients with epilepsy
Epilepsy Society

Research papers
Please find below a lay summary of research recently published in open access journal by staff based at the Chalfont Centre for Epilepsy.
breadcrumb navigation:
- What we do /
- current page Research papers
Published on 23 February 2020
Updated: 12 July 2022
Authored by Anonymous
Perampanel in routine clinical use across Europe: Pooled, multicenter, observational data.
Authors Rohracher A, Zimmermann G, Villanueva V, Garamendi I, Sander JW, et al.
Journal details Epilepsia. 2018; 59:1727-1739.
Lay summary One of the limitations of clinical trials is related to the difficulty to interpret or generalize the results because the studied population is very different from the population treated in normal life. In this report the researchers aimed to generate real-world evidence of health information from data reflecting routine clinical use over the past 4 years; from a wide variety of people with epilepsy, including those typically underrepresented such as the elderly members of the population who are being treated with the antiepileptic drug Perampanel (sold under the trade name Fycompa). Information was collected from over 2300 patients who had been prescribed this medicine from over 45 centres across Europe. The Perampanel related adverse side effects found in this study were similar to previous reports documenting routine clinical use, no new Perampanel related side effects were found in this study, adverse side effects such as dizziness were less frequent in people, than those events reported in the clinical trials. It was concluded that Perampanel was effective in routine clinical use in a wide variety of people whose epilepsy was not controlled.
The landscape of epilepsy-related GATOR1 variants
Authors Baldassari S, Picard F, Verbeek NE, van Kempen M, Brilstra EH, Lesca G, Conti V, Guerrini R, Bisulli F, Licchetta L, Pippucci T, Tinuper P, Hirsch E, de Saint Martin A, Chelly J, Rudolf G, Chipaux M, Ferrand-Sorbets S, Dorfmüller G, Sisodiya S, Balestrini S, Schoeler N, Hernandez- Hernandez L, Krithika S, et al
Journal details Genet Med. 2019; 21:398-408
Lay summary The objective of this study was to gain further understanding of the three genes that are involved in forming the GATOR1 protein complex (GAP activity towards rags complex 1); mutations in these genes are known to play a role in causing focal epilepsies. The researchers extracted DNA of 73 people with focal epilepsy and looked for changes in these three genes. The results of this study enabled detailed characterisation of epilepsy features related to GATOR1 protein complex. Additionally, it provided updated information to researchers and clinicians on all previously reported and novel GATOR1 epilepsy-related gene changes complete with new guidance for their clinical interpretation.
Genome-wide association study: Exploring the genetic basis for responsiveness to ketogenic dietary therapies for drug- resistant epilepsy.
Authors Schoeler NE, Leu C, Balestrini S, Mudge JM, Steward CA, Frankish A, Leung MA, Mackay M, Scheffer I, Williams R, Sander JW, Cross JH, Sisodiya SM.
Journal details Epilepsia. 2018; 59:1557-1566.
Lay summary Ketogenic dietary therapies (KDTs) are a group of high‐fat, low‐carbohydrate diets that have been used effectively as treatment options for people with drug resistant epilepsy since the early 1900s. We know that certain epilepsies, such as epilepsy with myoclonic‐atonic seizures, tuberous sclerosis complex, and Dravet syndrome, generally respond well to KDTs. However, KDTs are resource‐intensive, require dietary restriction, and can cause adverse side effects. Therefore the aim of this study was to identify a way to predict those who are likely to respond well and those who will not respond to KDTs in order to enable this dietary treatment earlier in the course of epilepsy.
In this study the researchers used a technique called genotyping in 272 patients to look for any specific signals such as changes in the DNA potentially associated with their responses to KDTs. A specific change in a gene called chromodomain Y like (CDYL) was found to be associated in individuals with poor response to KDT, where seizures were not under control. The CDYL gene is known to play a role in neural activities such as migration and therefore is a good candidate to study in more detail.
Effects of carbamazepine and lamotrigine on functional magnetic resonance imaging cognitive networks.
Authors Xiao F, Caciagli L, Wandschneider B, Sander JW, Sidhu M, Winston G, Burdett J, Trimmel K, Hill A, Vollmar C, Vos SB, Ourselin S, Thompson PJ, Zhou D, Duncan JS, Koepp MJ.
Journal details Epilepsia. 2018; 59:1362-1371
Lay summary This investigation took an imaging approach to study the effects of three antiepileptic drugs, Carbamazepine, Lamotrigine and Levetiracetam, on cognitive abilities of people with epilepsy. The 7 year records of functional magnetic resonance imaging (fMRI) brain scans of adult patients, who were fluent in English, were evaluated as part of their pre-surgical evaluation at the National Hospital for Neurology and Neurosurgery and Chalfont Centre for Epilepsy (UK). Activities in brain regions of patients and healthy volunteers were measured using their fMRI scans. Researchers found changes in patterns of activities involved in language tasks in patients on Lamotrigine and those on Carbamazepine showed more pronounced impairment of performance than those on lamotrigine. This study indicated that patients on Carbamazepine perform less well on a verbal fluency tests than those taking Lamotrigine and Levetiracetam. These important findings enhanced understanding of the effects of these medicines on the cognition of people with epilepsy.
Genome-wide mega-analysis identifies 16 loci and highlights diverse biological mechanisms in the common epilepsies
Authors Sander JW, Sisodiya SM.
Journal details International League Against Epilepsy Consortium on Complex Epilepsies. Nat Communications. 2018; 9:5269.
Lay summary This large study was conducted by the International League Against Epilepsy Consortium to help further the understanding of the genetics of the both focal and generalised epilepsies. The researchers compared the genomes of over 15,000 people with epilepsy with the genomes from over 29,000 healthy controls using a technique called genotyping.
Results of this study confirmed association of 16 regions of the genome with common epilepsies. One of these regions was a new association with juvenile myoclonic epilepsy and two novel regions were shown to be associated with focal epilepsy with hippocampal sclerosis. A total of 21 candidate epilepsy genes detected in these regions were studied further, to understand specific variants in these genes and the underlying biological mechanisms, and found a wide range of functions carried out by the products of these genes in the brain. Collectively, their findings provide new therapeutic leads that could potentially develop into drugs for treating common types of epilepsy.
MRI PhD theses
The following are PhD theses from the ES MRI Epilepsy Imaging Group:
- Vejay Vakharia. Computer assisted planning and robotics in epilepsy surgery.
- Bianca DeBlasi. Multi-parametric Imaging Using Hybrid PET/MR to Investigate the Epileptogenic Brain
- Lorenzo Caciagli - Neuroimaging of epilepsy: disease severity, cognitive comorbidities and endophenotypes
- Mark Nowell - Novel multimodality imaging in the planning and surgical treatment of epilepsy
- Christian Vollmar - Neuroimaging of functional and structural alterations in juvenile myoclonic epilepsy and frontal lobe epilepsy
- Britta Wandschneider - The effects of genes, antiepileptic drugs and risk of death on functional anatomy and cognitive networks in epilepsy
- Maria Feldmann - Imaging p-glycoprotein function: prediction of treatment response in mesial temporal lobe epilepsy
- Meneka Sidhu - Episodic memory in temporal lobe epilepsy
- Silvia Bonelli-Nauer - Cognitive functional MRI in temporal lobe epilepsy
- Helmut Laufs - EEG-fMRI signatures of spontaneous brain activity in healthy volunteers and epilepsy patients
- Rachel Thornton - Imaging brain networks in focal epilepsy : a prospective study of the clinical application of simultaneous EEG-fMRI in pre-surgical evaluation
- Gavin Winston - Translation of novel imaging techniques into clinical use for patients with epilepsy
- Mahinda Yogarajah - Imaging structural connections of the brain in epilepsy
- Umair Chaudhary - Haemodynamic correlates of interictal and ictal epileptic discharges and ictal semiology using simultaneous scalp video-EEG-fMRI and intracranial EEG-fMRI
- Robert Simister - Magnetic Resonance Spectroscopy as applied to epilepsy
- Serge Vulliemoz - Imaging functional and structural networks in the human epileptic brain
- Beate Diehl - Imaging correlates of the epileptogenic zone and functional deficit zone using diffusion tensor imaging (DTI)
- Khalid Hamandi - Functional MRI of focal and generalised interictal epileptiform discharges
- Afraim Salek-Haddadi - EEG-correlated functional MRI in epilepsy
- Rebecca Samson - Optimisation of quantitative magnetisation transfer (qMT) MRI to study restricted protons in the living human brain
- Robert Powell - Investigating brain structure and function in temporal lobe epilepsy
- Karsten Krakow - Imaging of epileptic activity using EEG-correlated functional MRI
- Rebecca Liu - Investigation of secondary cerebral damage in epilepsy
- Tejal Mitchell - Quantitative MRI in cerebral development disorders and epilepsy
- Fergus Rugg-Gunn - Imaging the neocortex in epilepsy with advanced MRI techniques
- Alex Everitt - The structural basis of the epilepsies: MRI and epidemiological studies
- Alexander Hammers - PET investigations in focal epilepsy
- Udo Wieshmann - New MR imaging techniques in epilepsy
- Matthias Koepp - Central benzodiazepine receptors in hippocampal sclerosis and idiopathic generalised epilepsies and opioid receptors in reading epilepsy
- Mark Richardson - Positron emission tomography investigation of cortical malformations causing epilepsy
- Wim van Paesschen - Quantitative MRI and hippocampal neuropathology of temporal lobe epilepsy
- Sanjay Sisodiya - Qualitative and quantitative analysis of MRI data from patients with epilepsy

Read how we are working to understand the genetic architecture of each individual person's epilepsy through our world leading genomics research programme.

Neuroimaging
Neuroimaging enables us to look deep inside the brain to learn more about the impact of seizures on its structure and function.

Neuropathology
The Epilepsy Society Brain and Tissue Bank is the first of its kind in the UK. It is dedicated to the study of epilepsy through brain and other tissue samples.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 11 May 2024
Performance investigation of epilepsy detection from noisy EEG signals using base-2-meta stacking classifier
- Torikul Islam 1 , 2 ,
- Redwanul Islam 2 ,
- Monisha Basak 2 ,
- Amit Dutta Roy 2 ,
- Md. Adil Arman 3 ,
- Samanta Paul 4 ,
- Oleksii Shandra 3 &
- Sk. Rahat Ali 2
Scientific Reports volume 14 , Article number: 10792 ( 2024 ) Cite this article
19 Accesses
Metrics details
- Biomedical engineering
Epilepsy is a chronic neurological disease, characterized by spontaneous, unprovoked, recurrent seizures that may lead to long-term disability and premature death. Despite significant efforts made to improve epilepsy detection clinically and pre-clinically, the pervasive presence of noise in EEG signals continues to pose substantial challenges to their effective application. In addition, discriminant features for epilepsy detection have not been investigated yet. The objective of this study is to develop a hybrid model for epilepsy detection from noisy and fragmented EEG signals. We hypothesized that a hybrid model could surpass existing single models in epilepsy detection. Our approach involves manual noise rejection and a novel statistical channel selection technique to detect epilepsy even from noisy EEG signals. Our proposed Base-2-Meta stacking classifier achieved notable accuracy (0.98 ± 0.05), precision (0.98 ± 0.07), recall (0.98 ± 0.05), and F1 score (0.98 ± 0.04) even with noisy 5-s segmented EEG signals. Application of our approach to the specific problem like detection of epilepsy from noisy and fragmented EEG data reveals a performance that is not only superior to others, but also is translationally relevant, highlighting its potential application in a clinic setting, where EEG signals are often noisy or scanty. Our proposed metric DF-A (Discriminant feature-accuracy), for the first time, identified the most discriminant feature with models that give A accuracy or above (A = 95 used in this study). This groundbreaking approach allows for detecting discriminant features and can be used as potential electrographic biomarkers in epilepsy detection research. Moreover, our study introduces innovative insights into the understanding of these features, epilepsy detection, and cross-validation, markedly improving epilepsy detection in ways previously unavailable.
Similar content being viewed by others
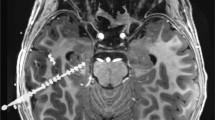
Multicenter intracranial EEG dataset for classification of graphoelements and artifactual signals
An improved GBSO-TAENN-based EEG signal classification model for epileptic seizure detection
A framework for comparative study of databases and computational methods for arrhythmia detection from single-lead ECG
Introduction.
Epilepsy is a prominent neurological disease that is denoted by repetitive and unprovoked seizures 1 , 2 . Seizures result from abnormal sudden electrical discharges in the brain, often typically presenting with loss of consciousness, convulsion, atypical movements, behavioral alterations or emotions 3 , 4 . Additionally, epilepsy is one of the most common neurological diseases since around 50 million people worldwide have it 5 .
The detection of epilepsy remains a significant challenge nowadays. It is a chronic neurological disease that can affect individuals of all ages. Moreover, aging can affect epilepsy detection significantly which makes epilepsy detection more challenging 6 , 7 . The etiology of epilepsy is heterogeneous, with variations observed among individuals. In certain instances, it is associated with underlying conditions such as traumatic brain injuries or developmental disorders 8 . Yet, the exact causes of epilepsy are still unknown and epilepsy detection presents a substantial challenge.
Recent advancements in diagnostic approaches allowed researchers and clinicians to shift from subjective assessments to more objective and quantifiable tests, recognized by improved accuracy since they provide more accurate results. Establishing a diagnosis of epilepsy most of the time involves a review of the exhaustive medical history and a physical examination, along with a series of tests. These tests include an electroencephalogram (EEG) to measure brain activity and imaging scans such as functional magnetic resonance imaging (fMRI) to identify any structural abnormalities in the brain. Epilepsy brings substantial changes to certain parts of the brain regions such as the hippocampus, amygdala, frontal cortex, temporal cortex, and olfactory cortex 9 , 10 , 11 . However, these structural changes occur in a rather slow progression.
EEG is a non-invasive test that measures and records the electrical activity of the brain using electrodes placed on the scalp 12 , 13 . During an EEG, the electrical signals generated by the brain are amplified and displayed as waveforms on a computer screen. The patterns observed in the EEG can provide valuable information including the presence of epilepsy, seizure classification, and seizure prediction 14 . Therefore, EEG plays a crucial role in the detection of epilepsy. Correspondingly, it will also facilitate the treatment of epilepsy which will be immensely helpful for human beings.
Literature review
In a study, a wavelet scalogram was utilized in the dataset described by Andrzejak et al. 15 with Alex net and achieved 100% accuracy 16 . Fourier-based synchro squeezing transform (SST) and convolutional neural network were applied in the publicly available CHB-MIT dataset and achieved 99.63% accuracy 17 . Multi-view feature learning was utilized with convolutional deep learning applied in the CHB-MIT dataset achieving 94.37% accuracy 18 . Till now different studies utilizing neural networks for epilepsy detection are discussed. Although, in various studies, researchers utilized neural networks but, it is having some dilemma too. Here, EEG signals are converted into images by different transform analysis which is an arduous and time-consuming task. Additionally, the convolutional neural network automatically extracts features from the image. Hence, researches are not aware of the features that play significant role in epilepsy detection. In a study, Discrete wavelet transform was used to decompose the data into sub-bands and then the wavelet energy distribution of each band was used as a feature set from the dataset described by Andrzejak et al. 15 utilized in artificial neural network (ANN) for classification achieved 95.0% accuracy 19 . However, ANN is very complex and it requires copious data for training. Thus, it is very time-consuming. Several machine learning models were used for epilepsy detection and they exhibited promising results. One study utilized wavelet transform on a dataset described by Andrzejak et al. 15 with K means clustering has achieved 96.67% accuracy 20 . Another study used the optimum allocation technique on the dataset described by Andrzejak et al. 15 with the logistic model tree achieved 96.67% accuracy 21 . Fuzzy distribution entropy (fDisEn) and wavelet packet decomposition were extracted from the dataset described by Andrzejak et al. 15 and were utilized in K-nearest neighbor. This method achieved 98.33% accuracy as per reference 22 . Symlet wavelet processing and grid search optimizer featured extracted from dataset described by Andrzejak et al. 15 and were used in gradient boosting machine. This method achieved 96.10% accuracy 1 . In some studies, researchers segmented the EEG signals. Thus, prolonged EEG signals were not required for classification. In a study, the 5 s epileptic segment was utilized with sample entropy and distributed entropy, using GA-SVM (genetic algorithm for feature selection and parameter optimization of support vector machine) on the dataset described by Andrzejak et al. 15 and achieved a maximum AUC of 96.67% 23 . In practical while data is recorded from humans there are so many noises and artifacts which makes epilepsy detection more challenging. Spectrogram images of EEG signals were utilized in a pre-trained convolutional neural network applied in the TUH dataset, which achieved the highest 88.30% accuracy as per as the reference 24 . A one-dimensional deep convolutional neural network was used for the automated identification of abnormal EEG signals without any feature extraction and applied in the TUH dataset, which achieved 79.34% accuracy 25 .
Research gap
A common practice in epilepsy research is often based on the use of smooth, noise-free datasets. As the data is mostly noise-free and smooth, researchers only utilized filters such as band pass, high pass, and low pass to put an end to noise from the signal. Therefore, researchers achieved significantly higher accuracy for epilepsy detection. In contrast, the real-world EEG signals are often contaminated by noise and various kinds of artifacts, which raises big questions regarding the practicality of the aforementioned methods. Therefore, the methodology proposed by the researchers will not work flawlessly.
Epilepsy detection from segmented EEG has supplementary utility. However, practical use of segmented EEG in detection still remains a limiting challenge for epilepsy diagnosis, since the accuracy rates drop much below certain conditions that remain to be fully understood. Therefore, although segmented has added value for epilepsy detection, researchers cannot take advantage of it.
Different researchers utilized non-identical features for their study 7 , 26 , 27 , 28 . Additionally, researchers claimed that the feature utilized in their study is the most prominent feature for epilepsy detection 26 . Thus, it is not transparent that actually which features play consequential role in epilepsy detection. Moreover, there appears to be no widely accepted significance order for epilepsy detection. Furthermore, researchers only have a few feature for their study 26 , 27 .
Researchers have not created any metric that can be utilized to exhibit the significance of the features for all the machine learning models. It is a notable issue since different features work differently with disparate machine learning models. Hence, it was not feasible for the researchers to construct a feature significance order for epilepsy detection that would remain accurate for all the models.
The field has thus far mostly focused on the use of either single machine learning or neural network-based models for epilepsy detection, leaving quite unexplored area of more promising hybrid models or the synergistic use of a few models. This narrow vision is missing the opportunity in which the fusion of different methodologies could make the system of epilepsy detection more accurate and robust. It points toward a very significant area for future research and development in this domain.
The main problems that we are striving to address in this paper: are (1) achieving the utmost accuracy in epilepsy detection utilizing noisy data (TUH dataset) which are more likely to practical EEG signals. (2) Finding out the reason behind accuracy mitigation while utilizing segmented EEG signals. (3) Finding out the discriminant features and evolving the feature order for epilepsy detection. (4) proposing a new metric that can be utilized to exhibit the significance of the features for all the machine learning models. (5) proposing a new hybrid model that will detect epilepsy more precisely than a conventional single model. To the best of our knowledge, existing research has not specifically addressed these challenges and hence, our work represents a significant advance, offering innovative solutions rather than mere incremental progress. This highlights the potential for translational application and highlights the innovation and impact of our research. Therefore, this research outcome will be substantial enough for epilepsy detection.
Methodology
Data preparation and processing.
In this study, we utilized the world’s largest publicly available EEG database which is called the TUH EEG corpus database 8 . The number of channels used for the recording of EEG signals is 32. Here, some channels are non EEG, such as (EEG-KKG, and EEG-RESP). We did not use these channels 29 . 10–20 electrode placement systems used in this dataset. This dataset carries annotation files by dint of it we can acquire knowledge about the event of each channel. Here, we used data from 10 disparate patients (5 epileptic, 5 normal). Delineate information regarding patients is provided in the supplementary information.
EEG may contain muscle activity, eye movement, power line interference, and interference from other devices. These are called noise of EEG signals. The primary purpose of EEG signals is to record the cerebral electrical activity of the brain. However, it may also record the electrical activity that is not generated from the cerebral region of the brain which is known as artifact of EEG signals. These noise and artifacts do not contain any significant information that may assist the analyze of EEG signals.
Noise and artifact evolve dilemma while analyzing EEG signals. Noise and artifact removal may increase the output of EEG signal analyze in a positive direction significantly. In the previous study, researchers utilized only filtering owing to remove the noise artifact. In this study, we have also utilized manual noise remove for better results.
Signal analysis and feature extraction
A bandpass filter is the most effective way to remove noise from the EEG signal. In our study, EEG signals were passed across a bandpass filter with a cut-off frequency between 0.1 to 44 Hz, resulting in high-frequency noise along with low-frequency superfluous signal being detached. MATLAB EEGLAB was utilized for the visualization as well as filtering of the signal. EEG signals before as well as after filtering are depicted in supplementary information.
After filtering the signal, noise, and artifacts were obviated from the signal manually by taking advantage of the annotation files. To discern the noise, we employed the image exhibited in the annotation files. EEG signals before as well as after removing the noise along with the artifact are depicted in supplementary information.
As the TUH EEG corpus dataset comprises a multi-channel EEG signal, it is necessary to select the appropriate channels. We used statistical features such as mean, median, and standard deviation for channel selection. Initially, we calculated statistical features for each channel of the EEG signal. Subsequently, we constructed co-relation as well as reckoned the p-value of the mean, median, and standard deviation for each channel of the EEG signal. Afterward, we selected two channels that have the upmost co-relation and p-value.
In this study, we segmented the Normal EEG signal and Epileptic EEG signal into 5-s fragments. Then, this 5-s EEG signal was used for feature extraction. Different signal fragments have been used in other research studies such as 0.1 s 30 , 2 s 31 , 32 , 4 s 33 , 5 s 23 , 34 , and 60 s 25 .
In the context of signal processing, features refer to specific characteristics of a signal. Additionally, features provide more relevant information about the signal context. To extract features various mathematical computations as well as algorithms are applied to the raw signals. For instance, in the case of EEG signals, there are copious features. Different techniques are utilized to extract these features.
Based on the idea of signal processing, a signal can have many features. However, the significance of the features is not alike. Disparate features have different noteworthiness. Feature ranking depicts the importance of the features of that particular signal.
Machine learning framework
A machine learning model is an algorithm that learns patterns from the given data to make prediction, and decisions without being explicitly programmed. These models initially process data, learn from data, and make decisions and predictions for unknown data.
Classification indices imply the performance of the machine learning models for classification tasks. These metrices indicate how well machine learning works, in making classification. Therefore, we can perceive that which model can be utilized for our stipulate classification task. In this study, we used four classification indices such as accuracy, precision, recall, f1 score.
Furthermore, to enhance the robustness of our findings, we used cross-validation. The basic idea of cross-validation is to divide the dataset into multiple subsets, train and test the model on different subsets, and then aggregate the results to get a more robust evaluation of the model's performance.
In this method, multiple base models are trained independently on the training data, each producing its predictions or decisions. The prime reason behind it is different models can capture different aspects of the data. All the models take the same feature value and make their predictions individually. Afterward, this prediction can be used for the classification or prediction that may outperform the single model.
A meta-classifier, is a higher-level model that combines the predictions or decisions of multiple base models to make a final prediction or classification. The purpose of the meta-classifier is to learn how to best combine the predictions of the base models to make a final prediction or classification. It takes into account the strengths and weaknesses of the base models and attempts to leverage the complementary information provided by them.
A key innovation in our methodology is the use of a stacking classifier which is the combination of base model and meta classifier. The idea behind stacking is to escalate predictive performance. In this study, we utilized two base model, and then the output of the base models were given to meta classifier.
Proposed metrics
In this study, we proposed a metric for depicting the significance of features. Initially, all the feature ranks are calculated for different models using permutation feature importance 35 , 36 , 37 , 38 . Different models gave disparate feature ranks. To solve this dilemma, we proposed new metric called DF-A (discriminant feature–accuracy). In this metric, accuracy implies the cut-off accuracy for the selected model. For example, DF-90 implies the models that gave 90 percent or above accuracy are taken into account. Afterward, the cumulative order for selected models is calculated for all the features. Cumulative feature order means the summation of feature order for any particular feature for the selected models. The most discriminant feature has the least feature order value. Then, features are arranged in ascending order of the cumulative feature order. In this way, we calculated the value of DF-A that exhibits the importance of features of a particular signal for any particular task.
Proposed model
In this study, we utilized two models for ensemble to mitigate the computational complexity. The output prediction of the model is utilized to evolve a new dataset. Afterwards, this meta-classifier is used with the new dataset. In this way, our proposed model exhibited in Fig. 1 outperformed all other models.
Stacking classifier (combining base model and meta classifier).
Proposed methodology
Here, Fig. 2 depicts the novel methodology used in this study for epilepsy detection along with feature ranking.
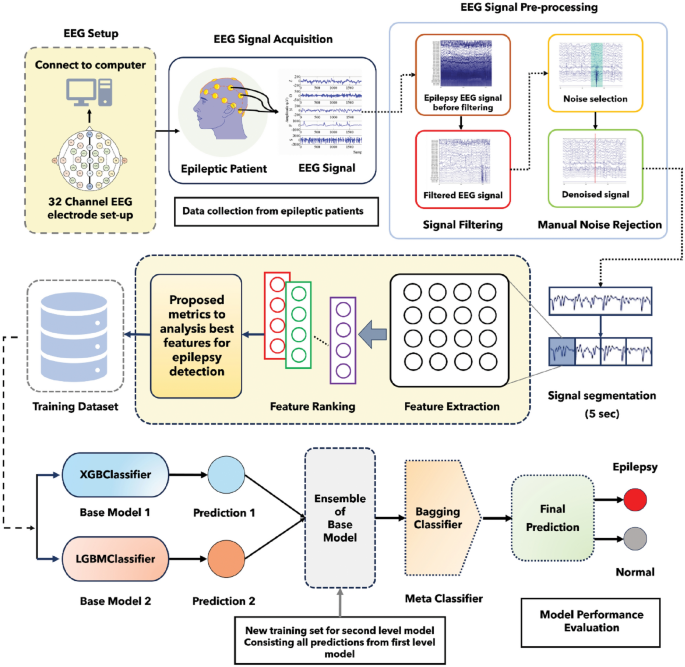
Graphical representation of the proposed methodology of this study.
Single base model
The results for the single model with 32 feature datasets is depicted in supplementary information. We divided the classifier model into two parts such as right and left. In the left side, classifier models that exhibited accuracy 95% or above. On the other hand, Classifier models that exhibited lower than 95% accuracy are on the right side.
DF-95 metrics
In order to exhibit the most significant feature DF-A metric was utilized. In this study, we selected 95% accuracy for DF-A metric as the accuracy parameter is variable and it can be altered. We used all the classifier models that exhibited 95% accuracy or above in single-model epilepsy detection task. Feature orders were calculated for all 32 features and seven classifier models that exhibited selected 95 accuracy or above. In Table 1 , first column shows the 32 features used in this study. From column 2 to column 8 shows the feature order of 32 features used in this study for selected seven classifiers. Here, feature order was calculated by permutation feature importance. Afterward, in column 9 shows cumulative ranking order which is calculated by simple arithmetic addition. Subsequently, DF-95 (discriminant features–accuracy) is calculated by observing the cumulative ranking order. The feature achieving the least cumulative ranking order is entitled as the most discriminant feature. In this way, all 32 features are ranked for DF-95. The results are depicted in Table 1 .
Base 2 meta stacking classifier
To reduce the computational complexity only 2 models were used as a Base model. XGB Classifier along with LGBM Classifier as a base classifier performed better than any other model. It may happened since they also performed significantly better as a single model for epilepsy detection with a 32-feature dataset. Therefore, results are depicted while they were utilized as base classifiers and others models as meta classifiers. We also investigated the impact of cross-validation in this study and utilized disparate CV values such as 10, 25, 50, 75, and 100.
Supplemental information depicts the results of models for cross-validation value 10. We can perceived that the highest accuracy, precision, recall, and F1 score were respectively 0.95 (+ /− 0.04), 0.96 (+ /− 0.06), 1.00 (+ /− 0.00), and 0.95 (+ /− 0.04). However, some models exhibited 1.00 (+ /− 0.00) recall, but accuracy, precision, and F1 score were low for them.
Supplemental information depicts the results of models for cross-validation value 25. We can perceived that the highest accuracy, precision, recall, and F1 score were respectively 0.95 (+ /− 0.04), 0.96 (+ /− 0.06), 1.00 (+ / − 0.00), and 0.96 (+ /− 0.06). However, some models exhibited 1.00 (+ /− 0.00) recall, but accuracy, precision, and F1 score were low for them.
Supplemental information depicts the results of models for cross-validation value 50. we can perceived that the highest accuracy, precision, recall, and F1 score were respectively 0.97 (+ / − 0.06) , 0.97 (+ / − 0.07) , 0.98 (+ / − 0.05), and 0.97 (+ / − 0.05) . Although some models exhibited 1.00 (+ /− 0.00) recall, but accuracy, precision, and F1 score were low for them.
Table 2 depicts the results of models for cross-validation value 75. We can perceived that the highest accuracy, precision, recall, and F1 score were respectively 0.98 (+ / − 0.05) , 0.98 (+ / − 0.07) , 0.98 (+ / − 0.05) and 0.98 (+ / − 0.04) . Although some models exhibited 1.00 (+ /− 0.00) recall, but accuracy, precision, and F1 score were low for them. Meta–Bagging Classifier provided the upmost results when it was used as a meta-classifier.
Supplemental information depicts results of models for cross-validation value 100. We can perceived that the highest accuracy, precision, recall, and F1 score were respectively 0.97 (+ /− 0.06), 0.97 (+ /− 0.07), 0.98 (+ /− 0.05), and 0.97 (+ /− 0.05). Although some models exhibited 1.00 (+ /− 0.00) recall, but accuracy, precision and F1 score were low for them.
Findings of the study
The most significant finding of this study are proposed combination of the Base-2-Meta model that exhibits higher accuracy than the usually used single model. Additionally, finding out the most discriminant feature for epilepsy detection. Moreover, we have proposed a metric DF-A for depicting the most discriminant feature that is persistent with most of the models that give A accuracy or above. Furthermore, we have also investigated the influence of cross-validation in this study. In addition, one of the most significant findings of this study is to work with a challenging dataset containing copious noise and artifacts and achieving higher accuracy by manual noise rejection and filtering. Besides, achieving upmost accuracy with 5-s fragments of EEG signals.
In our proposed model, we utilized XGB and LGBM classifiers as base classifiers. We used the Bagging Classifier as a meta-classifier. We observed from the results that our proposed model gave better accuracy the single base model. Accuracy, precision, recall and F1 score for this Base-2-Meta model are respectively 0.98 (+ /− 0.05), 0.98 (+ /− 0.07), 0.98 (+ /− 0.05) and 0.98 (+ /− 0.04). Additionally, it also renders better results than other Base-2-Meta models. Initially, base model used the dataset and made the initial prediction. The probability of prediction is used as a dataset for the meta-classifier.
We observed from the study that some models gave better accuracy when they were used as meta classifiers than utilized as a single classifier model. We realized from this escalation of results that the output result substantially depends on the Base classifier. While the classifier model works as a single classifier model they used the main feature dataset. Conversely, when they worked as a meta classifier they used the dataset evolved from the probability of prediction predicted by the Base classifier.
Our proposed metric, DF-95 rendered the rankings of the features for epilepsy detection. Previously, there was confusion about the most discriminant feature since different model implies different features as the most discriminant. Additionally, different researchers also claim different features as the most discriminant. However, our proposed metric resolves this dilemma.
We investigated the impact of cross-validation in this study. We observed that when we increased the cross-validation value model training time increased significantly. However, we also observed the escalation of results with the cross-validation. Thus, we have found a tradeoff between execution time and accuracy for cross-validation. Although a higher cross-validation value increases the complexity, it gives better results.
EEG signals contain various noise and artifacts and they lower the results of detection. However, researchers in their research work mostly utilized noise-free and smooth datasets. Thus, they were able to achieve the upmost accuracy with their proposed methodology. However, with the practical data their proposed models do not work up to the mark.
In this study, we utilized a manual noise rejection technique along with filtering the data. In addition, we utilized a novel statistical channel selection procedure to find out the most significant EEG channel. Afterward, features were extracted from these channels. Therefore, our proposed methodology achieved significant accuracy even with the dataset containing noise and artifacts.
We utilized fragments of EEG signals. Thus, a prolonged EEG signal is not required for epilepsy detection. In cases where only a small fragment of EEG signals is present, our proposed methodology can be utilized to accurately detect epilepsy with those small EEG signals.
We have found a tradeoff between signal-to-noise ratio (SNR) with the length of the EEG signals. When the length of the EEG signal is reduced, detection accuracy is also mitigated. Researchers utilized only filtering for noise eradication. However, filtering alone cannot eradicate the noise and artifact accurately. Therefore, when the length of the signals is reduced the signal part of the signal is reduced considerably but noise and artifacts do not reduce at that high level. Thus, a significant reduction of results is observed while the length of the signal is reduced.
Conversely, our methodology worked significantly even with the 5 s fragment of the EEG signals. In our proposed method, we utilized novel manual noise and artifact rejection techniques along with filtering. In this way, noise and artifacts can be removed significantly better than only filtering. Therefore, while the error rate with signal fragments for other studies is significantly high, in our study error rate with 5 s fragments of data is only 2%.
We have achieved up-to-the-mark results with our proposed methodology even though we utilized a dataset that contains copious noise and artifact-like practical data. We achieved this sublime result owing to our novel approaches. Therefore, our proposed methodology will be very useful for the practical application. Tables 3 and 4 exhibit the comparison of our study with previous studies conducted on the same dataset. From the comparison, it is evident that our study surpasses previous studies. This superiority is attributed to our innovative approach.
Limitations
In our study, although we achieved 98% accuracy, it can be further improved with additional novel techniques in the future. Although, we utilized 32 features, the number of features can be further improved which might raise the result. we rendered a ranking of features for epilepsy detection. However, this order of features was only calculated between the 32 features utilized in this study. In the future, if further features are augmented then the orders of the features can be altered. The result of our proposed metric also varied with the accuracy parameter added with it. We utilized 5 s fragments of EEG data. Therefore, while EEG data is less than 5 s, our proposed methodology cannot detect epilepsy as accurately as the result exhibited in Table 3 and Table 4 . Although our proposed methodology has a few drawbacks, it provides intriguing insights for epilepsy detection. However, in the future we are looking forward to providing more significant insights for epilepsy detection and resolving these drawbacks.
Although epilepsy detection is a challenging task, our proposed methodology can do it merely accurately. We utilized a novel manual noise rejection technique with filtering and extracted copious features for epilepsy detection. Afterward, a novel Base-2-Meta stacking model is utilized for the detection of epilepsy. Even though with the noisy data our proposed method can work up to the mark. We have also discovered the most discriminant features order in this study. In addition, our proposed metric DF-A used to exhibit the most discriminant feature unprecedentedly. We have also achieved significantly higher accuracy with 5 s fragments of EEG signals. The influence of cross-validation for the detection of epilepsy is investigated in this study. Our investigation provided significant insights for epilepsy detection. These insights can be considerably useful for the detection and treatment of epilepsy.
Data availability
The TUH EEG Epilepsy Corpus dataset was collected from the web link- https://isip.piconepress.com/projects/tuh_eeg/html/downloads.shtml .
Wang, X., Gong, G. & Li, N. Automated recognition of epileptic EEG states using a combination of symlet wavelet processing, gradient boosting machine, and grid search optimizer. Sensors 19 (2), 219. https://doi.org/10.3390/s19020219 (2019).
Article ADS MathSciNet PubMed PubMed Central Google Scholar
Islam, R. et al. Epileptic Seizure Detection from EEG Signal Using ANN-LSTM Model. In Proceedings of Trends in Electronics and Health Informatics: TEHI 2022 (eds Mahmud, M. et al. ) 129–141 (Springer, 2023). https://doi.org/10.1007/978-981-99-1916-1_10 .
Chapter Google Scholar
Lüders, H. O. et al. Proposal: Different types of alteration and loss of consciousness in epilepsy. Epilepsia https://doi.org/10.1111/epi.12595 (2014).
Article PubMed Google Scholar
Sharma, R., Sircar, P. & Pachori, R. B. Automated seizure classification using deep neural network based on autoencoder. in Handbook of Research on Advancements of Artificial Intelligence in Healthcare Engineering https://doi.org/10.4018/978-1-7998-2120-5.ch001 (2020).
Kumar, H., Debnath, S. & Sharma, A. Can epilepsy be cured? A review. Heal. Sci. Rev. https://doi.org/10.1016/j.hsr.2022.100062 (2022).
Article Google Scholar
Islam, T., Basak, M., Islam, R. & Roy, A. D. Investigating population-specific epilepsy detection from noisy EEG signals using deep-learning models. Heliyon https://doi.org/10.1016/j.heliyon.2023.e22208 (2023).
Article PubMed PubMed Central Google Scholar
Sharma, R., Pachori, R. B. & Sircar, P. Seizures classification based on higher order statistics and deep neural network. Biomed. Signal Process. Control https://doi.org/10.1016/j.bspc.2020.101921 (2020).
Walsh, S. et al. A systematic review of the risks factors associated with the onset and natural progression of epilepsy. Neurotoxicology https://doi.org/10.1016/j.neuro.2016.03.011 (2017).
Zhao, F. et al. Neuropsychological deficits in temporal lobe epilepsy: A comprehensive review. Ann. Indian Acad. Neurol. https://doi.org/10.4103/0972-2327.144003 (2014).
Chatzikonstantinou, A. Epilepsy and the hippocampus. In The Hippocampus in Clinical Neuroscience (eds Szabo, K. & Hennerici, M. G.) 121–142 (S. Karger AG, 2014). https://doi.org/10.1159/000356435 .
Sharma, R. Localization of epileptic surgical area using automated hybrid approach based on higher-order statistics with sensitivity analysis and residual wavelet transform. Biomed. Signal Process. Control https://doi.org/10.1016/j.bspc.2023.105192 (2023).
Kumar, Y., Dewal, M. L. & Anand, R. S. Epileptic seizure detection using DWT based fuzzy approximate entropy and support vector machine. Neurocomputing https://doi.org/10.1016/j.neucom.2013.11.009 (2014).
Sharma, R., Sircar, P. & Pachori, R. B. Automated focal EEG signal detection based on third order cumulant function. Biomed. Signal Process. Control https://doi.org/10.1016/j.bspc.2020.101856 (2020).
Sharma, R., Sircar, P. & Pachori, R. B. Computer-aided diagnosis of epilepsy using bispectrum of EEG signals. Appl. Biomed. Eng. Neurosci. https://doi.org/10.1007/978-981-13-7142-4_10 (2019).
Andrzejak, R. G. et al. Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: Dependence on recording region and brain state. Phys. Rev. E https://doi.org/10.1103/PhysRevE.64.061907 (2001).
Roy, A. D. & Islam, M. M. Detection of Epileptic Seizures from Wavelet Scalogram of EEG Signal Using Transfer Learning with AlexNet Convolutional Neural Network. https://doi.org/10.1109/ICCIT51783.2020.9392720 (2020).
Ozdemir, M. A., Cura, O. K. & Akan, A. Epileptic EEG classification by using time-frequency images for deep Learning. Int. J. Neural Syst. https://doi.org/10.1142/S012906572150026X (2021).
Yuan, Y., Xun, G., Jia, K. & Zhang, A. A multi-view deep learning framework for EEG seizure detection. IEEE J. Biomed. Heal. Informatics https://doi.org/10.1109/JBHI.2018.2871678 (2019).
Wani, S. M., Sabut, S. & Nalbalwar, S. L. Detection of epileptic seizure using wavelet transform and neural network classifier. In Computing, Communication and Signal Processing: Proceedings of ICCASP 2018 (eds Brijesh Iyer, S. L. et al. ) 739–747 (Springer, 2019). https://doi.org/10.1007/978-981-13-1513-8_75 .
Orhan, U., Hekim, M. & Ozer, M. EEG signals classification using the K-means clustering and a multilayer perceptron neural network model. Expert Syst. Appl. https://doi.org/10.1016/j.eswa.2011.04.149 (2011).
Kabir, E. & Siuly, Y. Z. Epileptic seizure detection from EEG signals using logistic model trees. Brain Inf. 3 (2), 93–100. https://doi.org/10.1007/s40708-015-0030-2 (2016).
Zhang, T., Chen, W. & Li, M. Fuzzy distribution entropy and its application in automated seizure detection technique. Biomed. Signal Process. Control 39 , 360–377. https://doi.org/10.1016/j.bspc.2017.08.013 (2018).
Li, P., Karmakar, C., Yan, C., Palaniswami, M. & Liu, C. Classification of 5-S epileptic EEG recordings using distribution entropy and sample entropy. Front. Physiol. https://doi.org/10.3389/fphys.2016.00136 (2016).
Thomas, J., Comoretto, L., Jin, J., Dauwels, J., Cash, S. S. & Westover, M. B. EEG CLassification Via Convolutional Neural Network-Based Interictal Epileptiform Event Detection. in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS , 2018, vol. 2018-July. doi: https://doi.org/10.1109/EMBC.2018.8512930 .
Yıldırım, Ö., Baloglu, U. B. & Rajendra Acharya, U. A deep convolutional neural network model for automated identification of abnormal EEG signals. Neural Comput. Appl. 32 (20), 15857–15868. https://doi.org/10.1007/s00521-018-3889-z (2020).
Boonyakitanont, P., Lek-uthai, A., Chomtho, K. & Songsiri, J. A review of feature extraction and performance evaluation in epileptic seizure detection using EEG. Biomed. Signal Process. Control 57 , 101702. https://doi.org/10.1016/j.bspc.2019.101702 (2020).
Rajendra Acharya, U., Vinitha Sree, S., Swapna, G., Martis, R. J. & Suri, J. S. Automated EEG analysis of epilepsy: A review. Knowl.-Based Syst. 45 , 147–165. https://doi.org/10.1016/j.knosys.2013.02.014 (2013).
Sharma, R., Sircar, P. & Pachori, R. B. A new technique for classification of focal and nonfocal EEG signals using higher-order spectra. J. Mech. Med. Biol. 19 (01), 1940010. https://doi.org/10.1142/S0219519419400104 (2019).
Teplan, M. Fundamentals of EEG measurement. Measurement science review. Meas. Sci. Rev. 2(2), (2002).
Lopez, S., Suarez, G., Jungreis, D., Obeid, I. & Picone, J. Automated identification of abnormal adult EEGs. https://doi.org/10.1109/SPMB.2015.7405423 (2016).
Sharan, R. V. & Berkovsky, S. Epileptic Seizure Detection Using Multi-Channel EEG Wavelet Power Spectra and 1-D Convolutional Neural Networks. in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS , 2020, vol. 2020-July. https://doi.org/10.1109/EMBC44109.2020.9176243 .
Li, M., Sun, X. & Chen, W. Patient-specific seizure detection method using nonlinear mode decomposition for long-term EEG signals. Med. Biol. Eng. Comput. https://doi.org/10.1007/s11517-020-02279-6 (2020).
Hu, X. et al. Scalp EEG classification using deep Bi-LSTM network for seizure detection. Comput. Biol. Med. https://doi.org/10.1016/j.compbiomed.2020.103919 (2020).
Ryu, S. et al. Pilot study of a single-channel EEG seizure detection algorithm using machine learning. Child’s Nerv. Syst. https://doi.org/10.1007/s00381-020-05011-9 (2021).
Altmann, A., Toloşi, L., Sander, O. & Lengauer, T. Permutation importance: A corrected feature importance measure. Bioinformatics https://doi.org/10.1093/bioinformatics/btq134 (2010).
Li, J. et al. Feature selection: A data perspective. ACM Comput. Surv. https://doi.org/10.1145/3136625 (2017).
Peng, G., Nourani, M., Harvey, J. & Dave, H. Feature Selection Using F-statistic Values for EEG Signal Analysis. in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS , 2020, vol. 2020-July. https://doi.org/10.1109/EMBC44109.2020.9176434 .
Tzimourta, K. D. et al. A robust methodology for classification of epileptic seizures in EEG signals. Health Technol. https://doi.org/10.1007/s12553-018-0265-z (2019).
Raghu, S., Sriraam, N., Temel, Y., Rao, S. V. & Kubben, P. L. EEG based multi-class seizure type classification using convolutional neural network and transfer learning. Neural Netw. https://doi.org/10.1016/j.neunet.2020.01.017 (2020).
Download references
Acknowledgements
This study was supported by the facilities the provided by New Jersey Institute of Technology, NJ, USA; Florida International University, FL, USA; University of Cincinnati, OH, USA, and Khulna University of Engineering & Technology, Bangladesh.
Author information
Authors and affiliations.
Department of Biomedical Engineering (BME), New Jersey Institute of Technology, Newark, NJ, USA
Torikul Islam
Department of Biomedical Engineering (BME), Khulna University of Engineering & Technology, Khulna, 9230, Bangladesh
Torikul Islam, Redwanul Islam, Monisha Basak, Amit Dutta Roy & Sk. Rahat Ali
Department of Biomedical Engineering (BME), Florida International University, Miami, FL, USA
Md. Adil Arman & Oleksii Shandra
Department of Biomedical Engineering (BME), University of Cincinnati, Cincinnati, OH, USA
Samanta Paul
You can also search for this author in PubMed Google Scholar
Contributions
T.I.: Conceptualization, Investigation, Formal analysis, Methodology, Software, Validation, Resources, Visualization, Project administration, Writing—original draft, Writing—review & editing. R.I.: Investigation, Methodology, Software, Validation, Visualization. M.B.: Investigation, Methodology, Software, Validation, Visualization. A.D.R.: Conceptualization, Data curation, Supervision, Writing—review & editing. M.A.A.: Investigation. S.P.: Investigation. O.S.: Writing—review & editing. S.R.A.: Writing—review & editing.
Corresponding author
Correspondence to Torikul Islam .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Islam, T., Islam, R., Basak, M. et al. Performance investigation of epilepsy detection from noisy EEG signals using base-2-meta stacking classifier. Sci Rep 14 , 10792 (2024). https://doi.org/10.1038/s41598-024-61338-2
Download citation
Received : 17 January 2024
Accepted : 04 May 2024
Published : 11 May 2024
DOI : https://doi.org/10.1038/s41598-024-61338-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Base-2-Meta stacking classifier
- Machine learning
- Feature extraction
- Feature ranking
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

California Mom Earns PhD To Find Out Why Daughter Had 300 Seizures A Month
T racy Dixon-Salazar is an extraordinary woman. She could have let her daughter Savannah's epilepsy diagnosis defeat her, but instead she got curious. This curiosity led her to get her PhD. In the process, she was also able to help her daughter’s quality of life drastically improve. She told her remarkable story to Today in the hopes of helping others.
Savannah was not born with epilepsy. She had her first seizure at age 2. By age 3 she was experiencing hundreds of them a day.
Tracy thought Savannah’s first seizure was a choking incident. She called 911 for emergency medical help. “Both my husband and I went at the same time, ‘What’s a seizure,’” she recalled. “We actually went six months and she didn’t have any and then at the age of 3 they came back hard and fast. She started having hundreds of seizures a day.”
At first, doctors were hesitant to label Savannah’s seizures. They didn’t want to stigmatize her and called them “spells” or “episodes.” Tracy learned of her daughter’s epilepsy diagnosis by reading her chart for herself. No doctor ever told her.
More from LittleThings: When Antiseizure Medications Fail, Parents Of Epileptic 5-Year-Old Turn To Medical Marijuana
Tracy didn’t understand how her daughter developed epilepsy. There was no family history or traumatic injury to explain it. At age 5, Savannah developed Lennox-Gastaut syndrome because of her frequent seizures. This condition occurs in severe cases of epilepsy and causes developmental delays.
“No one’s born with LGS. LGS is something that develops over time when the brain is barraged by seizures,” Tracy explained. “Your brain is also trying to develop. She was 3 years old, and her brain was trying to put itself together … she’s got all this abnormal seizure electricity going on, so the brain wires itself wrong.”
This was not easy to navigate at home. “The messaging [in the '90s] was ‘You can live a full life with epilepsy,’” she explained. “None of this was what we were experiencing at home. Savannah stopped developing. We’ve had to resuscitate her. She’s in the hospital all the time.” This is when Tracy made the decision to learn everything she could.
Before Savannah was born, Tracy had graduated high school and served in the military. At first, she enrolled in English college classes to better understand the medical literature she was reading. She took a science course and really enjoyed it.
“I was just trying to understand how this kid, this healthy 2-year-old could go from being totally normal to having seizures and her life derailed. And reading those [medical studies] drove me to go to college,” she explained. “I needed an outlet for the pain.”
Over 12 years, Tracy eventually earned her PhD in neurobiology and finished a three-year post-doctoral program. She didn’t think her studies would help her daughter but she needed to do something. Little did she know.
Tracy started working in a lab that was examining the genetics of epilepsy. Savannah’s case was genetic but she did discover that she had “calcium channel mutations." This meant that too much calcium was causing her seizures. Tracy took this new information to Savannah’s doctors and they put her on a calcium blocker.
“There’s actually a drug that blocks these exact calcium channels,” Tracy explained. The results were amazing. “It worked within two weeks. She went from having 300 seizures a month and going into these nonstop seizures a couple of times a week … to a 95% reduction in her seizures and it’s been 11 years, which is pretty incredible.”
This medication has also helped Savannah developmentally. For many years she was essentially cognitively 3 years old because of all of the brain damage from the seizures. She has made big strides since starting the medication.
“She just exploded in her personality and her talking and her walking and her potty training and oh my gosh she is just so sassy,” Tracy explained. She now loves boys, puzzles, and singer-songwriters' music.
Together Tracy and Savannah work as advocates for the disease. They are working to find their place in the world that isn't always kind to them. She tells her story so others can learn from it.

Beacon Biosignals Selected by Longboard Pharmaceuticals to Advance Understanding of Epilepsy and Related Conditions
BOSTON, May 13, 2024 (GLOBE NEWSWIRE) -- Beacon Biosignals announced a strategic partnership with Longboard Pharmaceuticals, Inc. that combines Longboard’s drug development expertise with Beacon’s neurophysiological platform to develop a better understanding of the relationship between sleep and neurological conditions with an initial focus in rare epileptic syndromes.
“We spend a third of our lives sleeping, yet sleep has received little attention when assessing the patient profile in many neurological conditions. As a result, clinical development of therapies that impact the brain have historically ignored the critical physiology of sleep when assessing patient outcomes and treatment effectiveness,” said Jacob Donoghue, MD, PhD, CEO of Beacon Biosignals. “Our partnership with Longboard changes all of that. We’re excited to collaborate in studying the utility of sleep and factors that may impact the management of neurological conditions, particularly in rare epilepsies.”
The Beacon Platform offers unparalleled tools to derive new insights for drug development and research. Initially, this collaboration will deploy Beacon’s FDA-cleared Dreem 3S™ EEG headband device and AI algorithm-derived neurobiomarkers to interrogate the neurophysiology of sleep longitudinally, at-scale, and from the comfort of a person’s home. This collaboration thereby enables Longboard to collect laboratory-quality sleep data while reducing the burden on patients and caregivers.
“Longboard is excited to partner with Beacon with the goal of pioneering a new era of research by leveraging innovative biomarker insights, approaches, and technology. Our hope is to apply data and key learnings obtained from our combined experience in clinical studies and EEG analyses to ultimately improve outcomes for patients and their families,” stated Dr. Randall Kaye, Longboard’s Chief Medical Officer. Dr. Kaye added, “our partnership with Beacon will help us in our mission to transform the lives of patients with neurological and rare diseases.”
About Beacon Biosignals
Beacon Biosignals is the leading at-home EEG platform for precision drug development. Its FDA 510(k)-cleared Dreem 3 EEG headband and FDA-cleared algorithms enable and accelerate research and development of treatments that transform the lives of patients with neurological, psychiatric, and sleep disorders. Beacon’s Clinico-EEG database contains over 100,000 patients’ brain activity data, and its cloud-native analytics platform empowers rapid interrogation of RWD/RWE for retrospective and predictive studies. For more information, visit https://beacon.bio. Follow us on Twitter or LinkedIn.
Beacon Biosignals media contact: [email protected]

IMAGES
VIDEO
COMMENTS
The breakdown of neurovascular coupling in the diseased state specifically Epilepsy and Alzheimer's disease. University of Sheffield Department of Psychology. Epilepsy is the most common neurological condition in the UK, affecting 1 - 2 % of the population. Epilepsies often involve only a small area of the brain - the epileptic focus ...
The University of Manchester Faculty of Biology, Medicine and Health. Epilepsy is a chronic neurological disease characterised by recurrent spontaneous seizures and cognitive co-morbidities. Current small molecule approaches to treating epilepsy are ineffective in a significant portion of cases, and are associated with substantial adverse effects.
Nuyujukian, Paul, MD, PhD (Brain Interfacing Laboratory) The Brain Interfacing Laboratory is interested in the applicability of brain-machine interfaces as a platform technology for a variety of brain-related medical conditions, particularly stroke and epilepsy. This research spans both preclinical models and human clinical studies.
James Tao MD PhD is interested in determining the cerebral substrates of scalp EEG epileptiform patterns, in order to improve the accuracy of non-invasive seizure localization during epilepsy surgery. His long-term interests include investigations of the mechanisms of epileptogenesis and of new modalities of epilepsy therapy.
Run by Professor of Neurology Birgit Frauscher, MD, PhD, the Lab for Analytical Neurophysiology (ANPHY Lab) seeks to employ a variety of quantifiable tools in order to shed light on neurophysiological and pathological processes. Our clinical work investigates new techniques for the analysis of EEG and other data from patients with epilepsy, new drugs and devices for treatment of epilepsy, as ...
Focus On Epilepsy Research. The epilepsies are a set of disorders characterized by recurring seizures, or disturbances in the electrical activity of the brain. Epilepsy affects people of all ages, from infants to the aged, and can result from many causes, including genetic variations, illness, head injury, or abnormal brain development.
Epilepsy. Jerome Engel Jr., MD, PhD. The UCLA Adult Epilepsy Program has been engaged in both clinical and basic research for over 54 years, funded by the National Institutes of Health, as well as by other sources. Clinical investigations involve the development of new diagnostic and therapeutic procedures and the evaluation of the beneficial ...
The NGG is a collaborative and interdisciplinary PhD program that provides training for careers in neuroscience research, teaching and more. Our training program is designed to provide a strong foundation of neuroscience knowledge while at the same time taking into account each student's strengths, needs and career goals.
Our Research Mission. Since 2003, we've funded nearly half of the therapies in the epilepsy clinical pipeline. We are developing an epilepsy research ecosystem that covers all parts of therapy, from idea to market. Our research initiatives include: Innovation programs that test new ideas and follow new research leads.
Roger Chang, MD, PhD Adult Epilepsy Fellow. Roger earned his undergraduate degree in Biomedical Engineering at Washington University in St. Louis. He completed his doctorate work in the lab of Dr. Robert Edwards at UCSF, determining the unique, dual role of chloride in synaptic vesicular glutamate transport. He subsequently received his MD at UCSF.
For help with all referral needs and questions, visit Referral Information. You may also submit a web referral or complete a referral form and fax it to 650-320-9443 or email the Referral Center at [email protected]. Dr. Robert Fisher, an epilepsy specialist at Stanford Health Care, treats epilepsy.
The Penn Epilepsy fellowship is now recruiting fellows starting training on July 1, 2025. The fellowship is participating in the Epilepsy and Clinical Neurophysiology Fellowship Match The New Epilepsy and Clinical Neurophysiology Fellowship Match with applications accepted through ERAS . November 15, 2023 Applicants may begin submitting ...
Epilepsy Clinical Research. The clinical research program led by NYU Langone's Division of Epilepsy studies biomarkers for epilepsy and moves novel therapies from the clinical trial pipeline to the physician's arsenal. After diagnosis, most patients are started on antiseizure medication, but doctors cannot predict who will be treatment ...
Epilepsy, caused by abnormal firing of neurons in the brain, affects 50 million people globally [1]. Almost one-third of epileptic patients do not respond well to antiepileptic drugs, and their side effects are associated with cognitive impairment, psychiatric problems, and recurrent epileptic adverse reactions [2, 3]. Read more.
Phone: 1-866-742-4811. Fax: 650-320-9443. Monday-Friday, 8 a.m.-5 p.m. Stanford Health Care provides comprehensive services to refer and track patients, as well as the latest information and news for physicians and office staff. For help with all referral needs and questions, visit Referral Information.
Building New Technologies to Aid Epilepsy Patients. Brian Litt, MD, a professor of Neurology and Bioengineering. The new epilepsy unit will be a place where tons of systems neuroscience research converges — everything from basic science research to the stuff I do to develop and build devices for people with neurologic disease and epilepsy.
The Emory Epilepsy Center is part of the Emory Brain Health Center. The Emory University Epilepsy Program is a multi-specialty group of physicians, neuropsychologists, and nurses from the departments of Neurology, Neurosurgery, Pediatrics, Physical Medicine and Rehabilitation and Radiology. The Epilepsy Program provides specialized clinical ...
2021 AES/NINDS Epilepsy Research Benchmarks. Area I: Understand the causes of the epilepsies and their relationship to epilepsy-associated neurologic, psychiatric, and somatic conditions. A. Identify the many genes and molecular pathways associated with the epilepsies and epilepsy-related conditions.
Dr. Robert Wechsler is a fellowship-trained and Board certified epileptologist in private practice in Boise, Idaho. He is the owner of Consultants in Epilepsy & Neurology, PLLC, and medical director of the Idaho Comprehensive Epilepsy Center, which he opened in 2005. Dr. Wechsler received his medical degree from Finch University of Health ...
2016. Mark Nowell - Novel multimodality imaging in the planning and surgical treatment of epilepsy. Christian Vollmar - Neuroimaging of functional and structural alterations in juvenile myoclonic epilepsy and frontal lobe epilepsy. Britta Wandschneider - The effects of genes, antiepileptic drugs and risk of death on functional anatomy and ...
Epilepsia. 2018; 59:1557-1566. Lay summary. Ketogenic dietary therapies (KDTs) are a group of high‐fat, low‐carbohydrate diets that have been used effectively as treatment options for people with drug resistant epilepsy since the early 1900s. We know that certain epilepsies, such as epilepsy with myoclonic‐atonic seizures, tuberous ...
Epilepsy is a chronic brain disorder in which groups of nerve cells, or neurons, in the brain sometimes send the wrong signals and cause seizures. Epilepsy (sometimes referred to as a seizure disorder) can have many different causes and seizure types. Epilepsy varies in severity and impact from person to person and can be accompanied by a range of co-existing conditions.
Epilepsy is a chronic neurological disease, characterized by spontaneous, unprovoked, recurrent seizures that may lead to long-term disability and premature death. Despite significant efforts made ...
California Mom Earns PhD To Find Out Why Daughter Had 300 Seizures A Month. Tracy Dixon-Salazar is an extraordinary woman. She could have let her daughter Savannah's epilepsy diagnosis defeat her ...
The University of Manchester Faculty of Biology, Medicine and Health. Epilepsy is a chronic neurological disease characterised by recurrent spontaneous seizures and cognitive co-morbidities. Current small molecule approaches to treating epilepsy are ineffective in a significant portion of cases, and are associated with substantial adverse effects.
Beacon Biosignals is the leading at-home EEG platform for precision drug development. Its FDA 510(k)-cleared Dreem 3 EEG headband and FDA-cleared algorithms enable and accelerate research and ...
Some 30% to 40% of patients do not respond to medications for depression and obsessive-compulsive disorder (OCD), but half of them could be helped by a noninvasive in-office procedure. To mark National Mental Health Awareness Month in May, we talk to psychiatrist Katherine Scangos, MD, PhD, co-director of the Transcranial Magnetic Stimulation ...