- Advanced search

Advanced Search

Speech and language delay in children: a case to learn from
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- Figures & Data
- INTRODUCTION
Speech and language delay in children is a common presentation to primary care either directly to the GP or through the health visitor, affecting approximately 6% of pre-school children. 1 Young children, particularly those with speech delay, can be difficult to examine. Differentiation between an isolated pathology and those with concurrent global developmental delay is crucial. This article presents an example of a common case, considers the learning points, and highlights management principles.
- CASE HISTORY
A 2-year-old boy presented to primary care with fewer words than his peers, and with difficulty in non-family members understanding him. On closer questioning he had <10 words of speech. He was born at 39 weeks by normal delivery, not requiring special care baby unit, and passed his newborn hearing screening. Review of his Personal Child Health Record (red book) showed consistent growth along centile lines, and other developmental milestones attained. In the consultation room he played appropriately, made good eye contact, and followed instructions: identifying his nose and ears when asked. On examination, he had normal facies, and otoscopy revealed bilateral dull tympanic membranes.
Referral to audiology was made and age- appropriate free-field hearing testing with tympanometry performed. He had hearing thresholds of >40 dB (mild-to-moderate hearing loss) with flat tympanograms indicating a conductive loss in keeping with otitis media with effusion (OME).
For 3 months the child was actively observed and then referred to the ear, nose, and throat consultant. With evidence of persistent conductive hearing loss, he was offered hearing aids or grommets, in keeping with National Institute for Health and Care Excellence guidelines. 2 His parents elected for grommet insertion. On follow-up at 2 years, 6 months, his vocabulary had expanded to >100 words, and audiogram showed thresholds <20 dB in the normal range.
- ASSESSMENT AND DIFFERENTIAL DIAGNOSIS
Speech and language delay must be separated from variation in speech development, and is defined by children falling behind recognised milestones. Regression or loss of speech and language are particularly concerning.
Initially, a history with a focus on identifying a cause for the speech delay should be taken, including pregnancy and birth history, developmental milestones, and family history.
Aspects of the antenatal history that may impact on newborn hearing must be explored. These include TORCH interuterine infections (toxoplasmosis, rubella, cytomegalovirus, and herpes simplex) and maternal drug exposure. Important aspects of the perinatal history include prematurity, hypoxia, birth trauma, and neonatal jaundice. Newborn hearing screening does not occur worldwide and should not be assumed in births outwith the UK. General maternal health is useful, particularly for the exclusion of conditions such as hypothyroidism.
The child’s medical history should be covered, including conditions such as meningitis, head trauma, and seizures, and exposure to ototoxic drugs. Developmental milestones should be noted, including social interactions with peers and family. This is not only to explore the possibility of a global developmental delay/disorder and the possibility of an underlying psychological diagnosis, but may also highlight deprivation and neglect.
It is important to enquire about any family history of hearing loss and speech delay including the possibility of consanguinuity, which may point to metabolic or recessive conditions.
In multilingual children total words across all languages should be counted, and will often compensate for the perceived delay. 5
Examination should be global, observing behaviour but with a focus on otoscopy, which may provide instant diagnosis of common conditions such as OME. Observed or formal neurological assessment of fine and gross motor skills may highlight a global development delay, with head circumference a useful adjunct.
There are multiple causes of speech delay, which can be split into psychological, neurological, and otological ( Figure 1 ). There is a known association between confirmed speech and language delay and psychiatric disorders such as autism spectrum disorder, with up to 50% occurring concurrently. 6
- Download figure
- Open in new tab
- Download powerpoint
Venn diagram demonstrating the different causes of speech and language delay (adapted from the Oxford Handbook of Paediatrics 4 ). OME = otitis media with effusion. TORCH = toxoplasmosis, rubella, cytomegalovirus, and herpes simplex.
In syndromic children, especially those with craniofacial abnormalities the speech delay may be multifactorial and a multidisciplinary approach with multiple referrals required.
One of the challenges in assessing a child with speech and language delay is that the order of learning and speech and language acquisition is fixed, but there is significant variation in timings described. 7 Up to 60% of children with speech delay do not require intervention and the problem resolves spontaneously by 3 years of age. 1 It is therefore important to undertake an individualised approach to each child.
Diagnosis of the underlying causation of speech delay is the priority and guides management. All children with suspected speech delay should be referred for audiometry to exclude hearing loss as this is a potentially reversible cause in the setting of OME with appropriate intervention.
Other causes that should not be missed include global developmental delay and psychiatric disorders such as autism spectrum disorder, both of which will require a multidisciplinary approach with enhanced potential outcomes for the child if support and treatment are offered earlier. Ultimately these children will require input from a child development centre.
Children with craniofacial abnormalities, for example, Down’s syndrome, may suffer from both conductive deafness and development delay, which will be confounded if not treated.
In the case described the child was suffering from speech delay secondary to OME. This is the commonest cause of hearing impairment in the developed world 8 and is reversible. OME has two peaks of incidence at 2 and 5 years. 9 The current treatment strategy for OME is grommet insertion after a recommended 3-month period of watchful waiting 2 to allow for spontaneous effusion resolution. Hearing aids are a non-surgical alternative but are generally seen as socially unacceptable. Twenty-five per cent of children will require further grommet insertion within 2 years of the first, 10 with a mean number of grommet insertions per child of 2.1. 11 This emphasises the recurrent nature of OME and the importance of close follow-up for these children.
Speech and language delay may be an early presenting feature in children with global developmental delay, and provides a crucial early opportunity to intervene and provide multidisciplinary support. Prompt audiological assessment is essential in all children with speech and language delay to exclude reversible causes.
Patient consent
The case presented here is fictional and therefore consent was not required.
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
- Received March 12, 2017.
- Revision requested April 6, 2017.
- Accepted June 9, 2017.
- © British Journal of General Practice 2018
- National Institute for Health and Care Excellence.
- McClure R ,
- Bellman M ,
- Beitchman JH
- Reznick JS ,
- Mandel EM ,
- Winther B ,
- Zielhuis GA ,
- Straatman H ,
- van den Broek P
- Prihoda T ,
- Cooper JC Jr .
- Vaghela H ,
- Philpott C ,
In this issue

- Table of Contents
- Index by author
Thank you for recommending British Journal of General Practice.
NOTE: We only request your email address so that the person to whom you are recommending the page knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
More in this toc section.
- How to manage low testosterone level in men: a guide for primary care
- Hyperparathyroidism (primary) NICE guideline: diagnosis, assessment, and initial management
- The atypical presentation of COVID-19 as gastrointestinal disease: key points for primary care
Related Articles
Cited by....

British Journal of General Practice
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
A novel case of global developmental delay syndrome with microdeletion at 10p14–p15.3 and microduplication at 18p11.31–p11.32
Editor(s): Thachangattuthodi., Anish
a Department of Medical Genetics, College of Basic Medical Science, Army Medical University (Third Military Medical University)
b Population and Family Planning Science and Technology Research Institute/Key Laboratory of Birth Defects and Reproductive Health of The National Health and Family Planning Commission
c Department of Pediatrics, Xinqiao Hospital, Army Medical University (Third Military Medical University), Chongqing, PR China.
∗Correspondence: Yun Bai, Department of Medical Genetics, College of Basic Medical Science, Army Medical University (Third Military Medical University), Chongqing, PR China (e-mail: [email protected] ); Yuping Zhang, Department of Pediatrics, Xinqiao Hospital, Army Medical University (Third Military Medical University), Chongqing, PR China (e-mail: [email protected] ).
Abbreviations: aCGH = comparative genomic hybridization, CMA = chromosomal microarray analysis, CNVs = copy number variations, DD = developmental delay, FISH = fluorescent in situ hybridization, GDDS = global developmental delay syndrome, MLPA = multiplex ligation-dependent probe amplification, MR = mental retardation, OMIM = Online Mendelian Inheritance in Man.
DZ, LD, YZ, and XF contributed equally to this work.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This work was supported by the National Natural Science Foundation of China (No. 81172723), and the Fundamental Research Funds for Non-profit Public Scientific Research Institutions of Chongqing (No. 2015cstc-jbky-01703 and 2016cstc-jbky-01703), Foundation of Chongqing Health Commission (No. 2017MSXM069), Natural Science Foundation Project of CQ CSTC (No. 2009CA5001 and 2017jcyjAX0478).
The authors declare that they have no conflict of interest.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc-nd/4.0
To characterize the etiology underlying a novel case of global developmental delay syndrome (GDDS) identified in a female child, aged 3 years old. This syndrome is a common pediatric presentation estimated to affect 3.65% of children aged 3 to 17 years.
The proband's detailed family history was used to infer a likely mode of inheritance for the GDDS. Genomic DNA samples collected from the proband and her parents were evaluated using conventional karyotyping, multiplex ligation-dependent probe amplification (MLPA), comparative genomic hybridization microarray (aCGH), and fluorescent in situ hybridization (FISH) analysis techniques.
An analysis of the proband's family history suggested that she inherited the GDDS from her father. The conducted conventional karyotyping and MLPA methods failed to identify a causative defect for the GDDS; however, the aCGH analysis revealed both a 6.6-Mb deletion at p14–p15.3 of chromosome 10 (arr[hg19]; 100,026–6,710,183), and a 6.3-Mb duplication at p11.31–p11.32 of chromosome 18 (arr[hg19]; 136,226–6,406,733) in the proband. The conducted FISH analysis subsequently determined that these mutations resulted from a balanced translocation t(10;18)(p15.3; p11.32) carried by the proband's father. Finally, a bioinformatic analysis of the proband's mutations revealed ZMYND11 as a promising candidate causative gene for this case of GDDS.
The present study demonstrates that the aCGH method can be used to effectively identify the location and approximate size of microdeletions and/or microduplications, but not balanced reciprocal translocations. The nonconventional analysis methods used in the present study may be applicable to other GDDS cases with elusive etiology, and likewise, ZMYND11 should be considered as a potential causative gene during the investigation of future GDDS cases.
1 Introduction
Global developmental delay syndrome (GDDS) is a common pediatric presentation estimated to affect approximately 3.65% of children aged 3 to 17 years. [1] For children aged less than 5 years, it is characterized as the exhibition of a significant delay in 2 or more developmental domains (ie, intelligence, language, social communication, cognition, and/or daily motor activities). [2] Currently, there is no consensus neuroimaging method used to study and/or diagnose this condition, and furthermore, causes of developmental delay (DD) are difficult to elucidate using only routine diagnostic techniques and detailed clinical information. The chromosomal microarray analysis (CMA) technique facilitates the detection of small chromosome imbalances that are unable to be unidentified via microscope-guided karyotyping. In fact, CMA is already established as a major platform for the identification of copy number variations (CNVs) in patients with autism spectrum disorder and/or mental retardation (MR). [3,4]
In the present study, comparative genomic hybridization array (aCGH) and fluorescence in situ hybridization (FISH) techniques were used to investigate the etiology and pathogenesis of GDDS in a female child aged 3 years old.
2 Materials and methods
2.1 proband family history.
The study participants comprised members of a Chinese-Han family, who were identified and enrolled at the Department of Pediatrics at the Xinqiao Hospital (Third Military Medical University). The proband was a female child aged 8 months, who was diagnosed with GDDS. She was unable to either sit or crawl without assistance. The conducted physical examination of the proband identified no cortex thumb syndrome; however, she was found to exhibit bilateral ankle clonus, a poor active-conscious grip in both hands, grade-IV lower-limb muscle tension and strength, and the ability to support a prone position. Her bilateral knee-jerk and Achilles tendon reflex were found to be normal, and she was also assessed for Babinski (+), Kernig (−), Brudzinski (−), and Auspitz (−) signs. The proband was calculated to have a mental developmental index of 70, and a psychomotor developmental index of 63. The results of the generated electroencephalogram report were abnormal, comprising a small number of sharp waves, and slow spike waves in the central region.
The proband's mother reported a history of 3 spontaneous miscarriages. A chromosomal karyotype analysis did not reveal any positive findings for either the proband or her parents.
2.2 Ethics statement
A written statement of informed consent was obtained from the proband's guardians for her and their participation in the study, which was approved by the Ethics Committee of the Third Military Medical University (Chongqing, China), and by the Population and Family Planning Science and Technology Research Institute.
2.3 DNA extraction
Venous blood samples were collected in vacutainer tubes containing EDTA, and genomic DNA was extracted using the Wizard Genomic DNA Purification Kit (Promega, WI), according to the manufacturer's instructions. The quantity and quality of the extracted DNA were determined using a NanoDrop 1000 spectrophotometer (Thermo, MA).
2.4 Multiplex ligation-dependent probe amplification
Multiplex ligation-dependent probe amplification (MLPA) was performed at numerous sites of the proband's genome using the SALSA MS-MLPA kit P245-B1 (MRC-Holland), according to the manufacturer's instructions. This kit includes 40 probes that target chromosomal regions known to be altered in 23 multiple-microdeletion syndromes (including Prader-Willi/Angelman, Cri-du-chat, DiGeorge, Langer-Giedion, and Miller-Dieker syndrome, among others).
2.5 Array-CGH
The proband's extracted DNA was screened via an aCGH analysis conducted by the KingMed Diagnostics Corporation (Guangzhou, China), using the Affymetrix Genome-Wide CGH CytoScan HD array (ThermoFisher Scientific), according to the manufacturer's instructions. This array includes more than 2,000,000 copy-number and 750,000 SNP probes. Genotype and CNV identification, and an assessment of genotyping integrity were conducted using Affymetrix Chromosome Analysis Suite software (ThermoFisher Scientific).
A blood sample was accordingly collected from the proband's father, and the extracted DNA was used to conduct a FISH analysis of chromosomes 10 and 18. This analysis used 2 probe pairs, one of which comprised an Agilent SureFISH 18p11.32 red fluorescent (R) and a Chr18 CEP green fluorescent (G) label, and the second of which comprised an Agilent SureFISH 10p15.3 red fluorescent (R) and a Chr10 CEP green fluorescent (G) label.
The conducted MLPA analysis of the proband's genomic DNA did not identify any genetic abnormalities. Similarly, while the conducted aCGH analysis detected the proband to harbor a 6.6-Mb deletion between p14–p15.3 of chromosome 10 (arr[hg19]; 100,026–6,710,183), and a 6.3-Mb duplication at p11.31–p11.32 of chromosome 18 (arr[hg19]; 136,226–6,406,733), both mutations were not identified in her healthy parents ( Fig. 1 ).

By analysis of the provided family history, we determined that proband's paternal aunt had a child who exhibited similar symptoms to those displayed by the proband, and that the proband's paternal grandmother reported several spontaneous miscarriages. Taken together, these observations suggest that the proband's genetic disorder was likely paternally inherited. The proband's father was conducted a FISH analysis of chromosomes 10 and 18 ( Fig. 2 ). The results in 10 middle split like cells indicated that a translocation event happened. Furthermore, this translocation was shown to be balanced, as evidenced by the normal aCGH analysis result and phenotype exhibited by the proband's father. Thus, the proband's father was determined to harbor a t(10;18)(p15.3; p11.32) balanced translocation, from which the proband inherited her chromosome 10 p14–p15.3 deletion and chromosome 18 p11.31–p11.31 duplication.

4 Discussion
Balanced reciprocal translocations are the most common chromosomal rearrangements affecting humans, and are estimated to occur in 0.16% to 0.20% (1/625–1/500) of live births. [5] The great majority of cases with apparently balanced structural rearrangements exhibit a normal phenotype; however, 0.6% of patients with MR harbor these balanced structural rearrangements, which likely cause a deleterious phenotype by inducing gene disruption/dysregulation, microdeletion/duplication, and/or position effects at the chromosome breakpoint. Theoretically, balanced reciprocal translocation-carriers can produce 18 types of gametes, including only 1 normal and 1 balanced reciprocal chromosomal translocation, but 16 cytogenetically abnormal gamete types. As a result, the probability of such carriers producing healthy offspring is relatively low, and many carriers are clinically infertile, experience a high rate of miscarriage, and/or produce offspring affected by chromosomal disease. In the present study, the proband's father was identified to carry the balanced translocation t(10;18)(p15.3; p11.32), which was likely the cause of the multiple spontaneous miscarriages reported by his wife.
The proband was identified to harbor a 6.6-Mb deletion at p14–p15.3 of chromosome 10 (arr[hg19]; 100,026–6,710,183) and a 6.3-Mb duplication at p11.31–p11.32 of chromosome 18 (arr[hg19]; 136,2266,406,733), via the conducted aCGH analysis. A literature search using the University of Santa Cruz genome browser ( http://genome.ucsc.edu/ ) and Online Mendelian Inheritance in Man (OMIM) database ( http://www.omim.org/ ) identified ZMYND11 and TGIF1 (located at 10p15.3 and 18p11.3, respectively) as potential causative genes for the proband's observed GDDS phenotype. Coe et al recently reported loss-of-function mutations in ZMYND11 in 7 individuals from 6 families. [6] One of these familial cases comprised a male individual observed to exhibit GDDS, as well as delayed speech, social and behavioral difficulties, and dysmorphic facial features. Moreover, his father exhibited a milder version of this phenotype, comprising GDDS, and behavioral difficulties including aggressive childhood behavior and mood swings. In general, patients with mutations in ZMYND11 exhibit a mild intellectual disability, and subtle facial malformations that may include hypertelorism, ptosis, and/or a wide mouth. Both of the females studied by Coe et al were described as having autistic tendencies, and 3 of the 4 studied males exhibited increased aggression. Taken together, these results support those of the present study, and suggest that ZMYND11 is a promising candidate causative gene for GDDS. In contrast, TGIF1 is a dosage-sensitive gene, and TGIF1 haploinsufficiency is established to induce various human disorders (OMIM: 142946). [7] However, the proband in the present study harbors a chromosome 18 duplication that includes TGIF1 , while she was not observed to exhibit any related clinical phenotypes.
The aCGH technique is routinely used to detect chromosomal imbalances since it enables researchers to achieve a very high level of resolution without requiring specific probes for target sub-regions. It is well established to be effective in detecting CNVs, long-term continuous homozygosity, and chimeras (at a rate of greater than 20%), but it is unable to detect balanced chromosomal translocations such as reciprocal and/or Robertson translocations, inversions, and balanced insertions. [8] It is also unable to detect point mutations, and/or pathogenic tandem repeats (as observed in Fragile-X Syndrome). In the present study, the proband's father was identified as a chr18p–10p-balanced translocation carrier; however, the results of his karyotype and aCGH analyses showed no cytogenetic abnormalities. This is because while large balanced translocations can be identified via a conventional karyotype analysis, small balanced translocations must be detected via more sensitive methods than aCGH. Importantly, this emphasizes the fact that failure of these techniques to detect chromosomal lesion sites in the clinical setting should not be considered sufficient to exclude the possibility of their contribution to disease pathogenesis.
The American College of Medical Genetics (ACMG) has made a guideline on the cytogenetic evaluation of the individual with DD or MR in 2005. And it also made guidance for constitutional cytogenomic microarray analysis to explain CNV. For any child with unexplained MR/DD, even in the absence of dysmorphic facial features, other clinical features or positive family history, routine chromosome analysis is indicated according to these advices of ACMG. FISH or other molecular techniques should be performed before or at the same time as with chromosome analysis for children with clinical features suggestive of a particular microdeletion/microduplication syndrome. [9] In general, unaffected parent carried the detected CNV in patient with MR/DD, which it may be taken as evidence that supports the CNV as unrelated to the clinical features and likely benign in the patient. [10–12] In our study, no abnormal findings were present in karyotype analysis for all individuals, but a microdeletion of chromosome 10 with a microduplication of chromosome 18 was found in patient. For this situation, some doctors may regard the parents as normal individuals, while the patient carried a de novo variation of CNV. Notably, minor balanced translocation between chromosome 10 and 18 may be present in proband's parents, and the results of FISH confirmed our speculation in proband's father. Although our study only involved a rare case, it was important supplementary information to the current guidelines, especially in some special families similar to our case, where the results of FISH in proband's parents will help us identify the genetic pathogenesis.
The present study also demonstrates that the efficacy of genetic counseling in advising patients and their relatives of the risks and consequences associated with an inherited disorder, (particularly with regards to fertility management and family planning), is highly dependent upon the provision of an accurate patient medical history. The present study was initially hampered because the proband's parents did not disclose their full family medical history until a potential genetic basis of the observed GDDS was identified.
Ensuring that accurate genetic counseling is available to the families of patients with GDDS is essential, since the identification of the underlying disease pathogenesis in each GDDS case may facilitate the provision of tailored symptomatic treatment and/or rehabilitation services, thus ensuring that affected individuals are adequately supported. [13] In fact, children with GDDS are usually able to learn in a similar way to most children unaffected by the disorder, but take longer, and require additional support to acquire and develop new skills. Effective genetic counseling may allow the families of patients with GDDS to anticipate their current and future needs, and to thus to psychologically and financially prepare for the provision of future treatments and rehabilitation. This may, in turn, reduce the familial stress caused by the high level of care required to support patients in daily activities (such as eating, dressing, communicating, etc), and by parental anxiety for the future wellbeing of patients with this disorder.
Author contributions
Conceptualization: Danyan Zhang.
Data curation: Yijian Zhu, Xuefei Feng, Letian Zhao, Yuping Zhang.
Formal analysis: Limeng Dai.
Investigation: Xuefei Feng.
Methodology: Danyan Zhang, Mingfu Ma, Lianbing Li.
Project administration: Yijian Zhu, Hong Guo.
Resources: Limeng Dai, Hong Guo, Yuping Zhang.
Supervision: Danyan Zhang, Xuefei Feng, Limeng Dai, Mingfu Ma, Yun Bai.
Validation: Yijian Zhu, Xuefei Feng, Letian Zhao.
Visualization: Lianbing Li.
Writing – original draft: Danyan Zhang, Yun Bai.
Writing – review and editing: Danyan Zhang.
- Cited Here |
- Google Scholar
comparative genomic hybridization; developmental delay; microdeletion; microduplication
- + Favorites
- View in Gallery
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 18 May 2020
Clinical Characteristics of Developmentally Delayed Children based on Interdisciplinary Evaluation
- S. W. Kim 1 ,
- H. R. Jeon 1 ,
- H. J. Jung 2 ,
- J. A. Kim 2 ,
- J.-E. Song 3 &
- J. Kim ORCID: orcid.org/0000-0003-4693-8400 4
Scientific Reports volume 10 , Article number: 8148 ( 2020 ) Cite this article
13k Accesses
11 Citations
8 Altmetric
Metrics details
- Autism spectrum disorders
- Risk factors
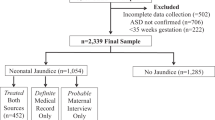
The aim of this study is to examine the clinical characteristics of children suspected to have neurodevelopmental disorders and to present features that could be helpful diagnostic clues at the clinical assessment stage. All children who visited the interdisciplinary clinic for developmental problems from May 2001 to December 2014 were eligible for this study. Medical records of the children were reviewed. A total of 1,877 children were enrolled in this study. Most children were classified into four major diagnostic groups: global developmental delay (GDD), autism spectrum disorder (ASD), developmental language disorder (DLD) and motor delay (MD). GDD was the most common (43.9%), and boys were significantly more predominant than girls in all groups. When evaluating the predictive power of numerous risk factors, the probability of GDD was lower than the probability of ASD among boys, while the probability of GDD increased as independent walking age increased. Compared with GDD and DLD, the probability of GDD was increased when there was neonatal history or when the independent walking age was late. Comparison of ASD and DLD showed that the probability of ASD decreased when a maternal history was present, whereas the probability of ASD increased with male gender. To conclude, the present study revealed the clinical features of children with various neurodevelopmental disorders. These results are expected to be helpful for more effectively flagging children with potential neurodevelopmental disorders in the clinical setting.
Similar content being viewed by others

Association between craniofacial anomalies, intellectual disability and autism spectrum disorder: Western Australian population-based study
Neonatal jaundice in association with autism spectrum disorder and developmental disorder

Divergent neurodevelopmental profiles of very-low-birth-weight infants
Introduction.
Developmental disabilities caused by dysfunction of the central nervous system, including the brain, are called neurodevelopmental disorders, and children with neurodevelopmental disorders have difficulties in various fields including physical, linguistic, behavior and learning 1 . According to a previous study conducted in the United States, 5–17% of children suffer from developmental disabilities, and recent trends have shown a gradual increase 2 . Limitations due to neurodevelopmental disorders might continue throughout life, and individuals with these disorders may require special services, health care and support 3 . These factors cause enormous social costs to a country as well as economic and psychological burdens for the families of children with developmental disabilities 4 .
The cause of neurodevelopmental disorders varies, and it is difficult to distinguish between children with neurodevelopmental disorders and typically developing children in early infancy. Even if the neurodevelopmental disorder is caused by nonprogressive factors, the clinical phenotype may change over time as the central nervous system matures 5 . Therefore, children’s symptoms are different according to their age and severity, and the necessary interventions will vary accordingly. As a result, the diagnosis of a neurodevelopmental disorder can vary greatly depending on the clinician’s perspective, and the treatment or intervention or social support offered may differ according to diagnosis. The time at which an expert is consulted varies widely from newborn to school-aged 6 . As shown in previous studies 7 , 8 , intervention during the period when the brain is developing rapidly can minimize disabilities and reduce the gap in developmental delay; as such, it is important to start precise intervention early. Neurodevelopmental disorders express various features, and the degree of influence by developmental domain varies from case to case. Because of the multi-morbidity feature, attempting to intervene by focusing on only one problem can lead to not only overlooking other accompanying problems but also a problem of inefficient use of limited intervention resources.
To compensate for difficulties in dealing with the complexity of neurodevelopmental disorders, an interdisciplinary clinic named the Developmental Delay Clinic (DDC) has been operating in our hospital. In this clinic, three specialists (a pediatric neurologist, pediatric physiatrist and pediatric psychologist) work together to provide comprehensive diagnoses and intervention plans. The three specialists, depending on area of expertise, each examine children, prescribe necessary tests, share and discuss the results of physical and neurological examinations and various tests and produce a precise diagnosis with a balanced intervention plan for each child. In this study, the authors aimed to identify meaningful factors for diagnosis and to determine if it is possible to distinguish major neurodevelopmental disorders at the clinical assessment stage.
Children who visited the DDC in our hospital with complaints of any developmental problems from May 2001 to December 2014 were included in this study. The total number of subjects was 1,877. Approval to perform this retrospective study was obtained from our Institutional Review Board (IRB) and research ethics committee (National Health Insurance Medical Center, NHIMC 2015-09-016). The need for informed consent was formally waived by the IRB and research ethics committee. All methods were performed in accordance with relevant guidelines and regulations.
All patients who visited the DDC for the first time had a history taken, and data were gathered according to the prescribed protocol. Data such as birth history, prenatal history, family history and other medical history were collected from a paper questionnaire. Birth history included intrauterine period and birth weight. Prenatal history included fetal distress, problems related to amniotic fluid or placenta, intrauterine growth retardation (IUGR), and fetal movement abnormality. Events such as fetal apnea, meconium aspiration and neonatal seizures were considered in the neonatal history. Postnatal history included infections such as sepsis, infantile spasm, and febrile convulsion. The presence of family history, such as language delay, autism spectrum disorder, and intellectual disability, and maternal history during the pregnancy period, such as anxiety or insomnia, depression, smoking and drinking, were also assessed in the survey.
After assessing histories through the questionnaire, the three specialists examined the child and prescribed necessary tests according to protocol. The diagnostic protocol was composed of two categories: required tests applied to all children and selective tests applied to some patients who needed those tests, based on each specialist’s judgment 9 (Fig. 1 , Supplementary 1).
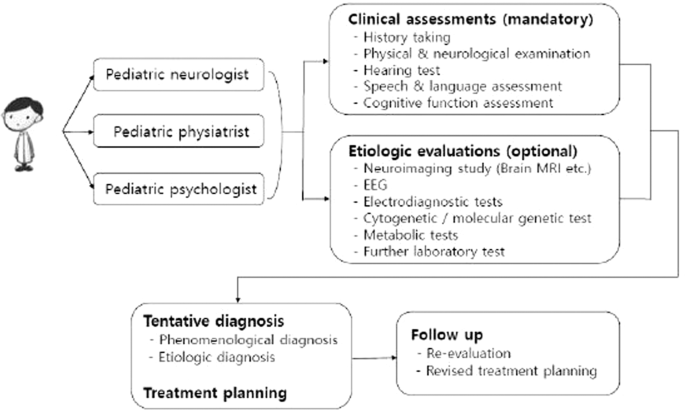
Diagnostic protocol for children visited developmental delay clinic.
The diagnosis was determined by discussion among the three specialists in reference to each child’s clinical findings and standardized developmental assessment results. The diagnoses were divided into two categories: either a phenomenological diagnosis based on the child’s current condition or an etiological diagnosis based on the pathophysiology of the condition. All these phenomenological diagnoses were classified into four major groups according to the child’s main features: global developmental delay (GDD), autism spectrum disorder (ASD), developmental language disorder (DLD) and motor delay (MD). The GDD group included diagnoses such as GDD and intellectual disability. GDD refers to children with significant delays in more than two of the following developmental domains: gross motor/fine motor, speech/language, intelligence, social interaction and self-care. In general, children under five years of age who met the requirements were diagnosed with GDD, while older children who could be examined using a reliable and formal intelligence test were diagnosed with intellectual disability 10 . Diagnoses such as reactive attachment disorder and social communication disorder were included in the ASD group. Those in the ASD group were diagnosed based on diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4 th edition (DSM-IV) 11 . However, since it has been updated from DSM-IV to DSM-V, the term ASD is used in this paper to prevent confusion. MD was defined as significant impairment of gross and/or fine-motor function compared with other developmental domains. Cerebral palsy and developmental coordination disorder were included in this group. DLD was defined as significant impairment of speech and language ability compared with other developmental domains. In this context, “significant” meant more than two standard deviations below the average value for the same age 10 . Etiological diagnoses included chromosomal and genetic anomalies, myopathy, and metabolic disease, among others.
Statistical analysis
SAS ver. 9.2 (SAS Institute, Cary, NC, USA) was used for statistical analysis. The results of the survey were obtained using the Kruskal-Wallis test with Bonferroni correction and logistic regression analysis. The level of significance was set at p < 0.05.
A total of 1,877 children were enrolled in this study. When divided into classes according to major phenomenological diagnosis, GDD accounted for the largest number, with 824 children (43.9%), followed by ASD with 430 (22.9%), DLD with 389 (20.7%) and MD with 72 (3.8%). Only 16 children (0.9%) were finally diagnosed as developing normally after all tests and examinations were given. Boys were more predominant than girls, with 1,316 (70.1%) and 561 (29.9%), respectively (p < 0.05). The age at which children visited the DDC ranged from 2 months to 192 months, and the average age was 50.9 ± 30.0 months. The corrected age was used for preterm children until they reached two years old. Two hundred thirty-four children (12.5%) out of the total could be diagnosed with an etiological diagnosis. Among these, hypoxic ischemic encephalopathy accounted for the largest number, with 58 children (24.8%), followed by chromosomal and/or genetic abnormalities with 53 children (22.6%) and congenital anomalies of the brain with 33 children (14.0%). Among the children who underwent a brain MRI, abnormal findings were mostly found in MD with 27.8%, which was significantly higher than ASD and DLD (p < 0.05) (Table 1 ).
With respect to preterm birth (gestational age less than 37 weeks), the history of preterm birth was the most prevalent in MD (29.2%), which was significantly higher than that in GDD (12.5%), ASD (10.9%) and DLD (8.7%) (p < 0.05). A history of low birth weight (LBW, birth weight less than 2,500 grams) was most common in MD (44.4%), which was significantly higher than that in ASD (20.9%) and DLD (25.4%) (p < 0.05) but not GDD (32.5%) (p = 0.426). Prenatal histories were most prevalent in MD (5.6%), which was significantly higher than in ASD and DLD (p < 0.05). Neonatal histories were also most prevalent in MD (29.2%), which was significantly higher than in the other three groups (p < 0.05). GDD and MD had a significantly higher prevalence of postnatal history compared with ASD and DLD (p < 0.05), but the difference between GDD and MD was not significant. Among family histories, language delay was the most common across all diagnosis groups, but the prevalence of having a family history did not differ significantly among the groups (p = 0.445). With regard to maternal histories, a maternal history of having anxiety or insomnia was the most common type in GDD, ASD and DLD, but drugs or drinking alcohol were the most common in MD. The percentage of cases with a maternal history did not differ significantly across the groups (p = 0.294) (Table 2 ).
Among the various risk factors mentioned above, logistic regression analysis performed to compare the groups and to determine if certain risk factors contributed to being diagnosed with GDD, ASD and DLD. When comparing GDD with ASD, the risk of having GDD decreased with boys and the presence of family history, while the risk increased with the presence of neonatal, postnatal and maternal history, later independent walking age (a representation of delayed motor milestone) and abnormal findings in the brain MRI. After controlling for confounders, gender and independent walking age showed significant between-group differences. When comparing GDD with DLD, the risk of having GDD was lower in boys and with the presence of a family history, while the risk increased with presence of the prenatal, neonatal and postnatal history, later independent walking age and abnormal findings in the brain MRI. After controlling for confounders, neonatal history and independent walking age showed significant between-group differences. When comparing ASD with DLD, the risk of having ASD was higher in boys, while the risk decreased with the presence of maternal history. The results were the same after controlling for confounders (Table 3 , Fig. 2 ).
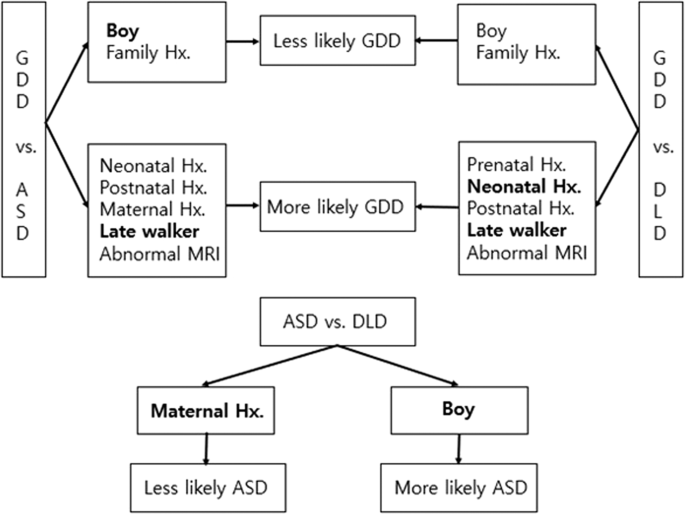
Distinctive clinical features among different diagnosis.
When receiver operating characteristic (ROC) curve analysis was performed to confirm the predictive power of these models, the model comparison of GDD vs. ASD and the model comparison of GDD vs. DLD showed good predictive power, while the model comparison of ASD vs. DLD had poor predictive power. Hosmer and Lemeshow’s Goodness-of-Fit Test revealed that all three logistic regression models were fit to predict the risk factors (Table 4 ).
The prevalence of developmental disabilities has risen in recent years with increases in high-risk pregnancies such as aged pregnancy, improved survival of high-risk infants due to medical technology advancement, and improved awareness and diagnosis of developmental disabilities 2 . The goal of early intervention for children with developmental disabilities is to prevent or minimize delays in all developmental domains, and early intervention allows children to achieve developmental milestones through the provision of enriched environments. Additionally, such interventions help caregivers cope efficiently with their children in daily life 12 . As seen in this study, the symptoms of children with neurodevelopmental disorders are very diverse, and the timing and symptoms of caregivers’ perception of something wrong in their children also vary. In addition, during the brain development period, one developmental domain affects the development of other domains, thus indicating multi-morbidity features. Proper intervention is important, but intervention is not always necessary. In some cases, it is more important to educate parents and modify the home environment than to use special resources. To effectively use limited resources, it is important to accurately diagnose neurodevelopmental disorders, which represent a multi-morbidity feature.
Among the patients who visited the DDC during the past 14 years, boys outnumbered girls in all diagnostic groups, which is consistent with previous studies 2 , 13 . Regarding etiological diagnosis, hypoxic ischemic encephalopathy was the most prevalent, followed by chromosomal and genetic abnormalities and congenital anomalies of the brain. These three factors accounted for 61.5% of the total etiologic causes. This outcome is similar to that of a study conducted by Shevell et al . 14 indicating that four causes, i.e., the three causes mentioned above plus poisoning, accounted for 68.9% of total cases with a known etiological basis. There were no children with poisoning in the present study, which could be due to differences in socio-cultural backgrounds. However, more attention to antenatal poisoning might be needed, based on the recent increase in poisoning cases in Korea 15 .
In cases of preterm birth and LBW, which are known as the strongest risk factors for developmental disabilities 16 , a history of preterm birth was significantly more common in MD than in GDD, ASD and DLD. In contrast, a history of LBW was not significantly different between MD and GDD. It could be posited that the risk of GDD increased in cases of small for gestational age even in full-term births. Arcangeli et al . 17 reported that compared with children of appropriate size for their gestational age, children who had a history of being small for their gestational age or who had fetal growth retardation, even in full-term births, showed lower neurodevelopmental scores. Takeuchi et al . 18 reported that being small for gestational age is a risk factor for developmental disabilities, even in full-term babies. These results were consistent with the present study, and more attentive follow-up regarding developmental course is needed for children with a history of being small for gestational age.
Kumar et al . 19 reported that the prevalence of neurodevelopmental disorders was higher in groups having family histories of neurodevelopmental disorders, such as epilepsy, GDD, MD, vision or hearing defects, compared with groups without such histories. Among the types of family histories, a history of language delay was seen the most in all diagnostic groups in this study. This finding could be explained by several factors: language delay is often present in various neurodevelopmental disorders, and the recognition and diagnosis of various neurodevelopmental disorders has improved in recent years, but this was not the case before. It may have been diagnosed as language delay 13 . In addition, it is possible that ASD has been diagnosed as other diseases, such as GDD or language delay, due to negative social perception of the diagnosis in Korea. Several studies have previously revealed that delay in one developmental domain often correlates with delay in other domains. Rechetnikov et al . 20 stated that there was a correlation between motor impairment and speech and language disorder. Wang et al . 21 reported that motor skill and communication skill were correlated with each other and that the motor skill of a one-and-a-half-year-old could predict the communication skill of a three-year-old. Language delay was predominant among the chief complaints of children who visited the DDC, but their final diagnosis was not limited to DLD. Shevell et al . 22 reported that approximately three-quarters of children who were diagnosed with DLD before their fifth birthday showed some limitation of not only language but also communication, motor skill and social function at an early school age. Overall, the physicians would carefully assess all of the developmental domains, even if the chief complaints of parents were language delay, and would also give them a proper intervention plan focusing on the other domains.
This study has a few limitations. First, it is a single-center study, and most of the included children were from a metropolitan area in the Northern Gyeonggi territory. Second, children suspected to have cerebral palsy often visited the outpatient clinic of the rehabilitation department instead of the DDC for their initial evaluation, so the proportion of children with cerebral palsy was low in this study. Third, although the diagnosis may change over time, the study was conducted based on the initial diagnosis. Nevertheless, this study is meaningful in that it is the first study to present a probabilistic model in the clinical evaluation of children with suspected neurodevelopmental disorders. Several papers on the diagnosis of neurodevelopmental disorders that suggest diagnostic steps for GDD and ASD have been published thus far 23 , 24 , 25 , 26 , 27 . However, in contrast to the present study, there were no articles suggesting probabilistic models that included comprehensive history taking and clinical diagnosis. Additionally, most previous studies were confined to one diagnosis, such as cerebral palsy or intellectual disabilities, whereas this study represents the many children who visited interdisciplinary clinics for 14 years with various chief complaints about development.
In conclusion, the present study revealed the clinical characteristics of children who have developmental problems. In this study, we present a feature that can aid diagnosis in the stage of clinical evaluation for children with suspected neurodevelopmental disorders. These results are expected to be helpful for more effectively identifying children with potential neurodevelopmental disorders in the clinical setting.
Kaufmann, W. E., Capone, G. T., Carter, J. C., Lieberman, D. N. & Disability, G. I. Capute and Accardo’s Neurodevelopmental Disabilities in Infancy and Childhood . (Paul H. Brookes Publishing Company Baltimore, 2008).
Boyle, C. A. et al . Trends in the Prevalence of Developmental Disabilities in US Children, 1997–2008. Pediatrics 127 , 1034–1042 (2011).
Article Google Scholar
Boulet, S. L., Boyle, C. A. & Schieve, L. A. Health care use and health and functional impact of developmental disabilities among US children, 1997-2005. Arch. Pediatr. Adolesc. Med. 163 , 19–26 (2009).
Anderson, D., Dumont, S., Jacobs, P. & Azzaria, L. The Personal Costs of Caring for a Child with a Disability: A Review of the Literature. Public Health Rep. 122 , 3–16 (2007).
Kaplan, B. J., Dewey, D. M., Crawford, S. G. & Wilson, B. N. The Term Comorbidity Is of Questionable Value in Reference to Developmental Disorders: Data and Theory. J. Learn. Disabil. 34 , 555–565 (2001).
Article CAS Google Scholar
Yeargin-Allsopp, M., Oakley, G. P., Murphy, C. C. & Sikes, R. K. A multiple-source method for studying the prevalence of developmental disabilities in children: the Metropolitan Atlanta Developmental Disabilities Study. Pediatrics 89 , 624–630 (1992).
CAS PubMed Google Scholar
Morgan, C. et al . Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev. Med. Child Neurol. 58 , 900–909 (2016).
Zwaigenbaum, L. et al . Early Intervention for Children With Autism Spectrum Disorder Under 3 Years of Age: Recommendations for Practice and Research. Pediatrics 136 , S60–S81 (2015).
Kim, S. W. et al . Diagnosis and Clinical Features in Children Referred to Developmental Delay Clinic. J. Korean Acad. Rehabil. Med . 28 , 132–139 (2004).
Shevell, M. I. et al . Practice parameter: evaluation of the child with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society. Neurology 60 , 367–380 (2003).
Segal, D. L. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). In The Corsini Encyclopedia of Psychology 1–3, https://doi.org/10.1002/9780470479216.corpsy0271 (American Cancer Society, 2010).
Majnemer, A. Benefits of early intervention for children with developmental disabilities. Semin. Pediatr. Neurol. 5 , 62–69 (1998).
Baio, J. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 63 , 1–21 (2014).
ADS Google Scholar
Shevell, M. I., Majnemer, A., Rosenbaum, P. & Abrahamowicz, M. Etiologic yield of subspecialists’ evaluation of young children with global developmental delay. J. Pediatr. 136 , 593–598 (2000).
Yoon, M. S. The current situation and developmental direction of Korean addiction service delivery system. Ment Health Soc Work 35 , 234–266 (2010).
Google Scholar
Salas, A. A. et al . Gestational age and birthweight for risk assessment of neurodevelopmental impairment or death in extremely preterm infants. Arch. Dis. Child.-Fetal Neonatal Ed. 101 , F494–F501 (2016).
Arcangeli, T., Thilaganathan, B., Hooper, R., Khan, K. S. & Bhide, A. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet. Gynecol. 40 , 267–275 (2012).
Takeuchi, A. et al . Neurodevelopment in full-term small for gestational age infants: A nationwide Japanese population-based study. Brain Dev. 38 , 529–537 (2016).
Kumar, R., Bhave, A., Bhargava, R. & Agarwal, G. G. Prevalence and risk factors for neurological disorders in children aged 6 months to 2 years in northern India. Dev. Med. Child Neurol. 55 , 348–356 (2013).
Rechetnikov, R. P. & Maitra, K. Motor impairments in children associated with impairments of speech or language: A meta-analytic review of research literature. Am. J. Occup. Ther. 63 , 255–263 (2009).
Wang, M. V., Lekhal, R., Aarø, L. E. & Schjølberg, S. Co-occurring development of early childhood communication and motor skills: results from a population-based longitudinal study. Child Care Health Dev. 40 , 77–84 (2014).
Shevell, M. I., Majnemer, A., Webster, R. I., Platt, R. W. & Birnbaum, R. Outcomes at school age of preschool children with developmental language impairment. Pediatr. Neurol. 32 , 264–269 (2005).
Moeschler, J. B. & Shevell, M. Clinical genetic evaluation of the child with mental retardation or developmental delays. Pediatrics 117 , 2304–2316 (2006).
Moeschler, J. B. & Shevell, M. Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics 134 , e903–e918 (2014).
Bélanger, S. A. & Caron, J. Evaluation of the child with global developmental delay and intellectual disability. Paediatr. Child Health 23 , 403–410 (2018).
Charman, T. & Gotham, K. Measurement Issues: Screening and diagnostic instruments for autism spectrum disorders–lessons from research and practise. Child Adolesc. Ment. Health 18 , 52–63 (2013).
Charman, T. & Baird, G. Practitioner review: Diagnosis of autism spectrum disorder in 2-and 3-year-old children. J. Child Psychol. Psychiatry 43 , 289–305 (2002).
Download references
Author information
Authors and affiliations.
Department of Physical Medicine and Rehabilitation, National Health Insurance Service Ilsan Hospital, Goyang, Korea
S. W. Kim & H. R. Jeon
Department of Pediatrics, National Health Insurance Service Ilsan Hospital, Goyang, Korea
H. J. Jung & J. A. Kim
Department of Psychiatry, National Health Insurance Service Ilsan Hospital, Goyang, Korea
Department of Rehabilitation Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea
You can also search for this author in PubMed Google Scholar
Contributions
S.W., H.J. and J.-E. conceived of the presented concept and revised the article. J.K. and H.R. developed the theory, interpreted of data and drafted the article. J.A. collected and analyzed the data and drafted the article. All authors discussed the results and contributed to the final manuscript and had complete access to the study data that support the publication. All authors read and approved the final manuscript.
Corresponding author
Correspondence to J. Kim .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kim, S.W., Jeon, H.R., Jung, H.J. et al. Clinical Characteristics of Developmentally Delayed Children based on Interdisciplinary Evaluation. Sci Rep 10 , 8148 (2020). https://doi.org/10.1038/s41598-020-64875-8
Download citation
Received : 28 October 2019
Accepted : 22 April 2020
Published : 18 May 2020
DOI : https://doi.org/10.1038/s41598-020-64875-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Application of symbolic play test in identification of autism spectrum disorder without global developmental delay and developmental language disorder.
- Xuening Chang
BMC Psychiatry (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Sign into My Research
- Create My Research Account
- Company Website
- Our Products
- About Dissertations
- Español (España)
- Support Center
Select language
- Bahasa Indonesia
- Português (Brasil)
- Português (Portugal)
Welcome to My Research!
You may have access to the free features available through My Research. You can save searches, save documents, create alerts and more. Please log in through your library or institution to check if you have access.

Translate this article into 20 different languages!
If you log in through your library or institution you might have access to this article in multiple languages.

Get access to 20+ different citations styles
Styles include MLA, APA, Chicago and many more. This feature may be available for free if you log in through your library or institution.

Looking for a PDF of this document?
You may have access to it for free by logging in through your library or institution.

Want to save this document?
You may have access to different export options including Google Drive and Microsoft OneDrive and citation management tools like RefWorks and EasyBib. Try logging in through your library or institution to get access to these tools.

- More like this
- Preview Available
- Scholarly Journal

Case Study: Child With Global Developmental Delay
No items selected.
Please select one or more items.
You might have access to the full article...
Try and log in through your institution to see if they have access to the full text.
Content area
PURPOSE. This case study focused on the care of a child with global developmental delay.
DATA SOURCES. Data were obtained through the author's clinical practice in long-term care pediatric rehabilitation and literature sources.
DATA SYNTHESIS. NANDA-International Classifications, the Nursing Interventions Classification (NIC), and Nursing Outcomes Classification (NOC) were used to identify the appropriate nursing diagnosis, nursing interventions, and patient outcomes.
CONCLUSIONS. This case study provides the pertinent nursing diagnoses, interventions, and outcomes for a child with global developmental delay. The interdisciplinary team approach and family involvement is addressed.
IMPLICATIONS FOR NURSING. Use of NANDA, NIC, and NOC outcomes constructs for enhancing the care of a child with global developmental delay.
Search terms: Global developmental delay, nursing diagnosis, nursing interventions, health outcomes
© (2010) The Authors, Journal compilation © (2010) NANDA International
doi: 10.1111/j.1744-618X.2010.01159.x
JT, a 5-month-old male infant, was admitted from an acute care hospital setting to the long-term care pediatric rehabilitation unit with a diagnosis of global developmental delay secondary to prematurity. Global developmental delay is a genetic disability that affects all areas of development, including motor, speech, language, cognitive, and social skills (Tervo, 2006).
JT had been institutionalized since birth. He was born at home at 28 weeks' gestation by spontaneous vaginal delivery precipitated by an altercation between his parents. His mother stated that she did not know she was pregnant He was born in the sack and reportedly experienced respiratory arrest prior to transport to the hospital neonatal intensive care unit. He weighed less than 2 lbs at birth. While hospitalized, he developed and was treated for pneumonia with periods of apnea.
The nurses and interdisciplinary team members evaluated JT's delayed development. The areas addressed were speech and language delay, motor delay, fine motor adaptive delay, and personal and social delay (Tervo, 2006). On admission to the longterm care facility,...
You have requested "on-the-fly" machine translation of selected content from our databases. This functionality is provided solely for your convenience and is in no way intended to replace human translation. Show full disclaimer
Neither ProQuest nor its licensors make any representations or warranties with respect to the translations. The translations are automatically generated "AS IS" and "AS AVAILABLE" and are not retained in our systems. PROQUEST AND ITS LICENSORS SPECIFICALLY DISCLAIM ANY AND ALL EXPRESS OR IMPLIED WARRANTIES, INCLUDING WITHOUT LIMITATION, ANY WARRANTIES FOR AVAILABILITY, ACCURACY, TIMELINESS, COMPLETENESS, NON-INFRINGMENT, MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. Your use of the translations is subject to all use restrictions contained in your Electronic Products License Agreement and by using the translation functionality you agree to forgo any and all claims against ProQuest or its licensors for your use of the translation functionality and any output derived there from. Hide full disclaimer
Suggested sources
- About ProQuest
- Terms of Use
- Privacy Policy
- Cookie Policy
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- BMJ Journals
You are here
- Volume 102, Issue 11
- Current evidence-based recommendations on investigating children with global developmental delay
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Renuka Mithyantha 1 ,
- Rachel Kneen 2 , 3 ,
- Emma McCann 4 ,
- http://orcid.org/0000-0002-2579-9301 Melissa Gladstone 1 , 5
- 1 Department of Developmental Paediatrics , Alder Hey Children’s NHS Foundation Trust , Liverpool , UK
- 2 Department of Paediatric Neurology , Alder Hey Children’s NHS Foundation Trust , Liverpool , UK
- 3 Institute of Infection and Global Health, University of Liverpool , Liverpool , UK
- 4 Department of Clinical Genetics , Liverpool Women’s Hospital , Liverpool , UK
- 5 Department of Women and Children’s Health , Institute of Translational Medicine, University of Liverpool, Alder Hey Children’s NHS Foundation Trust , Liverpool , UK
- Correspondence to Dr Melissa Gladstone, Department of Women and Children’s Health, Institute of Translational Medicine, University of Liverpool, Alder Hey Children’s NHS Foundation Trust, Liverpool, L14 5AB, UK; M.J.Gladstone{at}liverpool.ac.uk
Introduction Global developmental delay (GDD) affects 1%–3% of the population of children under 5 years of age, making it one of the most common conditions presenting in paediatric clinics; causes are exogenous, genetic (non-metabolic) or genetic (metabolic). Recent advances in biotechnology and genetic testing mean that the investigations available to perform for children under 5 years are increasing and are more sensitive than previously. This change in availability and type of testing necessitates an update in the recommendations for investigating GDD.
Methods We conducted a review of the literature from 2006 to 2016 to identify articles with evidence relating to the investigation of developmental delay in children under the age of 5 years. We collated the evidence into first-line and second-line investigations and, where available, on their yield and cost implications.
Results We have provided up-to-date guidance for first-line and second-line investigations for children with GDD under the age of 5 years. Recent evidence demonstrates that genetic testing for all children with unexplained GDD should be first line, if an exogenous cause is not already established. Our review of the literature demonstrates that all patients, irrespective of severity of GDD, should have investigations for treatable conditions. Evidence demonstrates that the yield for treatable conditions is higher than previously thought and that investigations for these metabolic conditions should be considered as first line. Additional second-line investigations can be led by history, examination and developmental trajectories.
Discussion We may need to update present recommendations in the UK for investigation of developmental delay. This would include microarray testing as first line and a more thorough approach to investigations for metabolic disorders that can be treated. Clinical assessment remains vital for guiding investigations.
- neurodevelopment
- neurodisability
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
https://doi.org/10.1136/archdischild-2016-311271
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Global developmental delay (GDD) is defined as a delay in two or more developmental domains of gross/fine motor, speech/language, cognition, social/personal and activities of daily living, affecting children under the age of 5 years. 1 2 The degree of developmental delay is further subclassified as: mild (functional age <33% below chronological age), moderate (functional age 34%–66% of chronological age) and severe (functional age <66% of chronological age). 1 GDD is considered significant when there is a deficit in performance of at least 2 SD below the age appropriate mean on accepted standardised assessment tests. 3 With a prevalence of 1%–3%, GDD is one of the the most common conditions encountered in paediatrics with genetic and structural brain abnormalities being the most frequent causes. 1 Establishing a diagnosis enables clinicians to define treatment options and conduct surveillance for known complications as well as provide prognosis and condition-specific family support (including family planning choices). This ensures the best overall outcomes for the child and their families/carers. 4 A diagnosis may also provide an explanation, a source of closure or acceptance to parents and stops clinicians advancing to potentially more expensive and invasive tests 5–7
Previous estimates for the yield of investigations for GDD are broad (10%–81%). 2 The variability may be due to differences in patient populations, clinical settings where tests are performed and the range of tests undertaken. 2 The last evidence-based UK guideline for investigation of developmental delay was published 10 years ago. 8 With the advent of more recent techniques in genetics and a recent burgeoning of guidelines in other countries, 4 9 10 there is a need to review our practice in the UK.
The primary objective of this paper is to provide (1) an update of the latest evidence for investigation of GDD, (2) recommendations for investigations and (3) evidence relating to yield and cost from literature presently available.
We conducted a systematic review of the literature relating to the investigation of GDD published in the last 10 years (since the McDonald review in 2006). We searched Pubmed, Google Scholar and Embase using the MESH terms: ‘developmental delay’, ‘developmental disorders’, ‘mental retardation’, ‘intellectual disability’, ‘learning disorders’ AND ‘guidelines’ AND ‘investigations’. ‘Cost’ and ‘yield’ were included along with the MESH terms. Papers included were reviews, consensus recommendations, retrospective or prospective studies. Relevant articles from reference lists were also included. We included papers published in English that were relevant to children that included investigations for GDD. We excluded papers that targeted specific metabolic, genetic or neurological conditions. We used the term GDD as meaning: delayed developmental domains in children under the age of 5 years and intellectual disability (ID) as the term used after this age when IQ can be reliably tested. 11
For this review, we discuss and categorise investigations into first-line and second-line tests and subcategorised them to genetics, metabolic and imaging. See table 1 for recommended first-line investigations to be considered prior to referral to specialist services. We show a flowchart and decision-making tree for investigations in figure 1 .
- Download figure
- Open in new tab
- Download powerpoint
Flow chart for decision making for investigations for global developmental delay in young children.
- View inline
Table demonstrating recommendations for first-line investigations for global developmental delay from four guidelines and our proposed recommendations
First-line assessment and investigations
History and examination.
Comprehensive clinical assessment remains the core to planning investigations in young children presenting with GDD. 4 8–10 Aetiology can be categorised into exogenous, genetic (non-metabolic) and genetic (metabolic). 11 The diagnosis of exogenous causes includes teratogenic agents (alcohol and drugs); prenatal, perinatal causes (prematurity, infections); and social causes often best assessed by history but must not be assumed.
Investigations following a thorough clinical history (including a family pedigree, pregnancy and birth history) and a detailed physical examination by a trained specialist lead to a higher diagnostic yield. 3 12 Identification and correction of sensory deficits are essential, while evaluating these children and may provide pointers to the underlying aetiology. 2 6
An examination of the child’s developmental status in all domains (gross motor, fine motor, language, socioemotional and cognitive skills) using a recognised tool to provide a normative comparison should also be conducted. Repeated clinical/dysmorphology and developmental assessments over time are more informative than one-off assessments in planning investigations and management.
It is important that the clinician consider investigations in all levels of developmental delay including those with persistent mild GDD, given the variable phenotypic presentations of genetic and metabolic conditions. Some studies, although from tertiary centres, have found that severity did not impact on the diagnostic rate of investigations, 12 while others report higher yield in patients with moderate-to-severe GDD. 13 Serial assessment enables clinicians to identify changing phenotypes over time. When metabolic conditions are clinically suspected, annual evaluation after the first year of life until school age is recommended. 14
Some studies have demonstrated that we can identify the cause of developmental or cognitive delay in a one-third of cases by history and examination alone. With clinical evaluation prompting investigations, we can identify another one-third. It is only the latter one-third that are identified by investigations only. 12 The presence of abnormal neurology, microcephaly, female gender, dysmorphism, abnormal prenatal or perinatal history and absence of autistic features are linked with higher aetiological yield of investigations. 15 Investigations following comprehensive clinical evaluation are also cost effective. 16
Genetic testing
First-line tests .
Genetic investigation by means of standard karyotyping was recommended as a first-line investigation in the UK guidance from 2006. 8 The implementation of ‘molecular karyotyping’ or chromosome microarray (array-based comparative genomic hybridisation (aCGH)) has changed the state of play. Recent evidence-based international guidelines promote the use of aCGH as a first-tier investigation for GDD if no aetiological indicators from history and examination are found. 4 9 10 The higher sensitivity that it has for identifying submicroscopic deletions and duplications (than standard karyotyping methods) and better definition of the breakpoints and size of imbalances all make microarray a suitable first-line test. 4 17 18
Chromosome microarray has been described to be the ‘single most efficient diagnostic test’ for GDD after history and examination. 4 A literature search of 33 studies that used this technique in nearly 22 000 patients has demonstrated that the diagnostic yield of aCGH is between 15% and 20%, while karyotyping is 3%. 18 The diagnostic yield of microarray is supported by a health economics report, which showed cost saving when comparing a National Health Service (NHS) clinical genetics service use of aCGH as a first-tier test while evaluating learning disability, compared with CGH as second line after negative karyotyping. 19
Molecular karyotyping will not detect conditions where structural changes in the chromosomes result in no loss or gain of genetic material such as balanced translocations or inversions, ring chromosomes and low-level mosaicism. 18 20 A standard karyotype is still required if such a disorder is suspected (eg, refractory epilepsy, if a family is known to have a balanced translocation associated with a phenotype, a history of multiple miscarriages or clinical features to suggest mosaicism). Syndromes caused by methylation defects (eg, Beckwith-Wiedemann, Angelman syndrome) or mutations in single genes will also go undetected unless specifically tested.
Fragile X syndrome affects approximately 1/5000 births, typically causing moderate ID in boys and a variable phenotype in girls (unaffected to significant). Phenotypic features evolve and are not as apparent in younger children. 9 The UK genetic testing network and international guidelines therefore do promote testing for fragile X for children with moderate-to-severe GDD, without profound physical disability, as an additional first-tier genetic investigation. 4 9 10 21 Testing criteria are available to help aid clinical decisions in older children. 21
Second-line tests
Clinical syndromes can present with variable phenotypes, and children who have a normal aCGH and FMR1 may be best assessed by a clinical geneticist to ensure that the most appropriate and cost-effective additional tests are undertaken. 22 Use of specific gene tests such as those for Rett syndrome (or its variants) or gene panels for ID has been proposed as second-line tests. 4 There is an increasing number of panels and exome sequencing tests available for ID (UK Genetic Testing Network; http://www.ukgtn.nhs.uk ) or private providers, but specialist services (clinical genetics or paediatric neurology) do most requests for these tests, although this is likely to change as mainstreaming of these investigations advances.
Metabolic and biochemical investigations
There is limited good quality evidence for first-line metabolic investigations. Recommendations from Ireland are based on evidence review by expert committee, 10 while those from Australia are based on a literature review, quoting grade III–IV evidence. 9
Inborn errors of metabolism (IEMs) are rare, their prevalence likely to vary in different populations. There is limited UK data on detecting metabolic disorders in patients with GDD. 14 IEMs are usually associated with systemic features, and previous guidelines recommend selective metabolic investigations. 2 8 Some IEMs are now (partially) treatable, and for others, treatment is in the research stages. Treatment includes dietary supplements (folinic acid for cerebral folate deficiency, pyridoxine or pyridoxal phosphate for B6-responsive epilepsy, creatine in creatine transporter deficiency, uridine in pyrimidine 5-nucleotidase super activity), dietary restriction (homocystinuria, glutaricacidaemia) and ketogenic diet (pyruvate dehydrogenase deficiency, Glut1 transporter deficiency). Other treatments include: haematopoietic stem cell transplantation (mucopolysaccharidoses, metachromatic leucodystrophy), enzyme replacement (Fabry’s disease, Gaucher’s disease, neuronal ceroid lipofuscinosis) or gene therapy (adrenoleucodystophy, lysosomal storage disorders). 23–25
A systematic review of literature by van Karnebeek et al identified 89 conditions presenting with ID as a major feature, which are susceptible to treatment. Of these, 60% could be identified by non-targeted urine and blood tests. Some of these conditions (eg, creatine transporter defects, mild homocystinuria, female ornithine transcarbamylase deficiency) can initially present as GDD alone. 25 26 While individual treatable IEMs are extremely rare in the general population, the prevalence will be higher in the at-risk population. Hence, though small in number, these treatable causes of GDD have been the focus of the more recent US guidance, with recommendations that screening for IEM should be used in all patients with GDD of unknown aetiology. 4 24 A list of tests with treatable conditions they identify is shown in table 2 .
Table demonstrating IEM tested for by first-line metabolic investigations 25
The neonatal screening programme in the UK (Guthrie test) currently includes six IEMs (phenyketonuria, medium-chain acyl-CoA dehydrogenase deficiency, maple syrup urine disease, isovaleric acidaemia, glutaricaciduria type 1, homocystinuria (pyridoxine unresponsive)) and congenital hypothyroidism. It is restricted when compared with other countries (eg, Canada, the USA, The Netherlands), which offer a wider range including urea cycle disorders, organic and some amino acid disorders. Testing for these is, therefore, more relevant in UK patients with GDD, and IEMs should be considered in symptomatic children. 14
There are also some conditions where early diagnosis can be made from simple and cheap biochemical screening tests. This includes creatine kinase and thyroid function tests as well as ferritin, vitamin B12 and lead on a selective basis when Pica, dietary restrictions (vegan diet in child/mother) or environmental exposure risk is possible. 9 While these tests seldom lead to a diagnosis, they also may add to a diagnosis (eg, macrocytic anaemia in organic acidaemias, abnormal triiodothyronine in Allan-Herdon-Dudley syndrome). 10 27
There is limited research on comprehensive metabolic evaluation in larger groups of individuals with GDD. It is, therefore, difficult to estimate the yield of many of the proposed first-line metabolic tests. A recent systematic review conducted for the American Academy of Neurology found that yield of metabolic investigations varied between 0.2% and 4.6%, based on clinical signs and range of tests undertaken in the studies (grade III evidence). 28 Second-line individually tailored testing in a tertiary setting in the Netherlands produced an overall yield of 2.8% for metabolic investigations. 11
Individually tailored second-line testing 4 14 26 and referral to a specialist service is recommended, 4 9 when clinical suspicion remains. An evidence-based, free web-based application ( http://www.treatable-id.org ) may be useful to tailor investigations for treatable IEMs not covered by first-line tests. 29
Neuroimaging
MRI of the brain has been used selectively and non-selectively in evaluating patients with GDD. The diagnostic yield of MRI is higher when used in patients where GDD is associated with clinical signs such as abnormal head circumference (microcephaly, non-familial macrocephaly, rapid change in head circumference), focal neurological signs or epilepsy. Targeted imaging was hence advocated by previous guidelines. 2 8 Previous studies have demonstrated abnormal results in targeted imaging in about 41% compared with 14% with non-selective screening. 3 Recent studies continue to demonstrate higher abnormality detection rates when MRI is performed in patients with GDD with additional clinical/neurological signs. 30 31 More complex MRI protocols (eg, proton magnetic resonance spectroscopy) are promising tools to investigate GDD and enable a non-invasive measure of brain metabolites such as lactate or white matter choline, 32 but studies have so far failed to show an increased diagnostic yield, 31 33 and hence these are best used as second line in selected patients.
MRI is a more sensitive test and has no radiation exposure, making it a preferred choice over CT. However, all children under 5 years will need sedation or a general anaesthetic, which has a slim risk attached, and some children will need further investigations including a lumbar puncture. There is an argument, therefore, that children requiring brain imaging should see a specialist prior to imaging, if an anaesthetic is required.
Special considerations
Ten most common causes of progressive intellectual and neurological deterioration.
10 most common causes of PIND reported in the PIND study in the UK ( www.rcpch.ac.uk/pind ) 34
NCL late infantile
Mucopolysaccharidosis IIIA (San Filippo)
Rett syndrome
Metachromatic leucodystrophy
Adrenoleucodystrophy
NCL juvenile
GM2 gangliosidosis type 1 (Tay-Sachs)
Niemann-Pick type C
GM2 gangliosidosis type 2 (Sandhoff)
NCL, neuronal ceroid lipofuscinosis; PIND, progressive intellectual and neurological deterioration.
Children that should be referred to a specialist in neurodisability or neurology are shown on table 3 . Investigations should be individualised and targeted as they can be invasive (eg, LP, muscle/skin biopsy) or painful (eg, nerve conduction studies and electromyography) and are expensive and time consuming for medical staff and families. Children with regression may also be referred to the clinical genetics team where specific next-generation sequencing panels can be undertaken and, at present, considered for the 100 000 Genome Project ( www.genomicsengland.co.uk/the-100000-genomes-project ).
Clinical pointers to consider referral to a specialist in neurodisability or neurology
Immigrant children
Immigrant children are exposed to a combination of biological, socioeconomical, emotional and environmental adverse events placing them at higher risk of developmental problems. This includes malnutrition and disability from trauma, overcrowding and toxin exposure and loss of parents or trauma from lack of stability. 35 Furthermore, children may have missed new-born screens and vaccinations and been exposed to infectious diseases. In these children, comprehensive clinical assessments should consider all these factors while planning individual investigations.
Despite new advances in technology, particularly in the realm of genetic investigation, clinical assessment continues to be vital in guiding investigation. Clues to investigation may lie in the history and examination with clinical judgement being essential to enabling the right pathways to be taken in making a diagnosis. A good history can help direct which route to take in terms of investigation, particularly when exogenous causes are identified. Assessment over a period will provide clarity as to whether a condition is resolving, static or deteriorating. Assessment over time enables the phenotype to evolve and more appropriate targeting of investigations.
It is clear that establishing a diagnosis enables us to answer questions on: why it has happened (aetiology), what does it mean for our child (prognosis), what treatments might be available (precision medicine) and whether it can be prevented in the future (prenatal testing and preimplantation genetic diagnosis).
In these recommendations, we have also highlighted the recent evidence that promotes metabolic screening tests to detect treatable conditions. This is a move away from older guidance where metabolic investigations were not recommended for children with no features/risk factors other than GDD. 2 Though rare, the possibility of presentation as stable developmental delay and potential for treatment merits their inclusion as first-line tests. Treatment outcomes vary but can potentially improve cognitive development, slow deterioration, prevent metabolic decompensation and improve seizure control and systemic manifestations. 25 26
GDD and ID affect 2%–3% of the worldwide population with a lifetime cost of up to US$1 million. 36 First-line metabolic investigations to identify treatable IEMs cost approximately $C568, 26 with costs in Ireland for all first-line tests at €1335. 10 Costs in the UK NHS laboratory for aCGH are not astronomical (£338–£350), 37 38 with the majority of combined metabolic tests costing under £1000. 38 Not all children will get a diagnosis and cost per diagnosis may be high, but there are obvious long-term cost savings if early diagnosis and treatment are possible. The options of genetic counselling and support for young families also make diagnosis invaluable.
Recent advances in genomic medicine are transforming the investigation of children with significant developmental delay and are likely to transform the way we assess and investigate children. Traditional models of care have relied on history and examination with broad and then specific investigations to funnel down to specific diagnoses. The advent of rapid genetic testing and ‘omic’ medicine is likely to turn this paradigm on its head with whole genome/exome sequencing identifying genes, which may be causing the phenotype in an individual. The clinician will then use knowledge of their patient to make a judgement about whether this is the cause for their patient—‘reverse dysmorphology’.
These advances in genomic medicine will lead to an increase in diagnoses that will modify how the individual is clinically cared for (precision medicine). The Deciphering Developmental Disorders study and the 100 000 Genome Project will both aid our understanding of disorders. We predict that, with time, whole genome sequencing/exome sequencing may become the first-line investigation of choice for all children with unexplained GDD and that other investigations will be secondary to this and used primarily for phenotyping. These will provide answers for families about the underlying cause of their child’s condition and will prevent further costly and potentially distressing investigations taking place.
Conclusions
In this paper, we have outlined the present evidence and recommendations for both first-line and second-line investigations for GDD in children in the UK. We have provided new evidence relating to the use of genetic testing techniques and have demonstrated that this should be a first-line investigation for all children with GDD. Second to this, any treatable metabolic conditions should be always considered. With time, it is likely that the investigation of children with developmental delay will be turned on its head and we will be going from genetic diagnosis to phenotypic diagnosis. Despite this, history and examination will always be crucial for defining the condition and the change over time.
- Majnemer A ,
- Shevell M ,
- Donley D , et al
- Shevell MI ,
- Rosenbaum P , et al
- Moeschler JB ,
- Shonkoff JP ,
- Hauser-Cram P
- McConachie H ,
- Hayeems RZ ,
- Babul-Hirji R ,
- Hoang N , et al
- McDonald L ,
- Tolmie J , et al
- Collins F ,
- O’Byrne JJ ,
- Treacy EP , et al
- Engbers HM ,
- van Hasselt P , et al
- van Karnebeek CD ,
- Scheper FY ,
- Abeling NG , et al
- Cleary MA ,
- Thomas RL , et al
- Miller DT ,
- Aradhya S , et al
- Kharbanda M ,
- Milunsky JM
- Corbella M ,
- Fons C , et al
- Stockler IS
- Zschocke J , et al
- Michelson DJ ,
- Sherr EH , et al
- Popurs MA ,
- Lafek M , et al
- Griffiths PD ,
- Warren D , et al
- Verbruggen KT ,
- Meiners LC ,
- Sijens PE , et al
- Maurits NM ,
- Meiners LC , et al
- Ritter S , et al
- Winstone AM ,
- Stellitano L , et al
- Davidson N ,
- Chaney G , et al
- ↵ Centers for Disease Control and Prevention (CDC) . Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment--United States, 2003 . MMWR Morb Mortal Wkly Rep 2004 ; 53 : 57 – 9 . OpenUrl PubMed
- ↵ Great Ormond Street Hospial for Children NHS Foundation Trust,North East Thames Regional Genetics Service . Pricing , 2014 .
- ↵ Community Children’s Health Partnership . Investigations for developmental delay: blood tests costs (2014) and what results could indicate? 2014 .
Contributors RM, RK, EM and MG contributed to the initial idea for the paper, wrote and reviewed sections of the paper and approved the final version. RM conducted the literature review with the support of MG and wrote the first draft of the paper.
Funding None declared.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Linked Articles
- Original article Aetiological investigations in early developmental impairment: are they worth it? Anthony Richard Hart Ruchi Sharma Mark Atherton Samer Alabed Sally Simpson Stuart Barfield Judith Cohen Nicholas McGlashan Asha Ravi Michael James Parker Daniel JA Connolly Archives of Disease in Childhood 2017; 102 1004-1013 Published Online First: 22 Jul 2017. doi: 10.1136/archdischild-2017-312843
Read the full text or download the PDF:
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplements
- Open Access Articles
- Author Guidelines
- Submission Site
- Open Access
- For Reviewers
- About International Health
- About the Royal Society of Tropical Medicine and Hygiene
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, child development, developmental disability, early intervention for children with developmental disabilities, case studies of eci for children with developmental disabilities, the case for action, author's contributions, acknowledgements, competing interests, ethical approval.
- < Previous
Early intervention for children with developmental disabilities in low and middle-income countries – the case for action
- Article contents
- Figures & tables
- Supplementary Data
Tracey Smythe, Maria Zuurmond, Cally J Tann, Melissa Gladstone, Hannah Kuper, Early intervention for children with developmental disabilities in low and middle-income countries – the case for action, International Health , Volume 13, Issue 3, May 2021, Pages 222–231, https://doi.org/10.1093/inthealth/ihaa044
- Permissions Icon Permissions
In the last two decades, the global community has made significant progress in saving the lives of children <5 y of age. However, these advances are failing to help all children to thrive, especially children with disabilities. Most early child development research has focussed on the impact of biological and psychosocial factors on the developing brain and the effect of early intervention on child development. Yet studies typically exclude children with disabilities, so relatively little is known about which interventions are effective for this high-risk group. In this article we provide an overview of child development and developmental disabilities. We describe family-centred care interventions that aim to provide optimal stimulation for development in a safe, stable and nurturing environment. We make the case for improving opportunities for children with developmental disabilities to achieve their full potential and thrive, including through inclusive early childhood development intervention. Finally, we call for the global research community to adopt a systematic approach for better evidence for and implementation of early interventions for children with developmental disabilities in low-resource settings.
Substantial global progress has been made in reducing child deaths since 1990 and the mortality rate of children <5 y of age has decreased in all world regions. However, non-communicable morbidities and disabilities have not been addressed to the same extent. This review discusses the urgency of taking actions to narrow the inequality gap in early childhood developmental care, especially for the 53 million children <5 y of age living with disabilities and developmental disorders such as epilepsy, intellectual disability, sensory impairments, autism spectrum disorder and attention deficit hyperactivity disorder. 1 A focus on supporting children with disabilities to thrive during their early years is important, as this period is critical for maximising their development. Furthermore, under the United Nations Convention of Rights for a Child and the United Nations Convention of the Rights of Persons with Disabilities, governments are duty-bound to provide early years services that are inclusive of and available to all children. 2 , 3 This article will describe child development and developmental disabilities and make the case for which equitable early childhood development (ECD) interventions may be optimal for helping children with developmental disabilities to achieve their potential.
Early childhood is a period of great opportunity for optimum brain growth, but it is also a period of vulnerability. Development in language, cognition, motor and socio-emotional domains occurs rapidly in these first years. These areas of development do not operate or develop in isolation, but enable each other and mutually interact as the child learns to become more independent. For instance, as a child learns to see, she will increasingly reach for and play with objects and thereby develop motor skills and coordination. Biological, psychosocial 4 , 5 and environmental factors also crucially affect the structure and functioning of the brain as it is developing. 6 For example, if a child experiences adequate nutrition and is provided with opportunities to play, she may progressively explore her environment and interact with her caregiver and by doing so, reinforce her psychosocial development. Furthermore, the time period when these factors influence brain growth are critically important, as there are particular early windows of opportunity that if not harnessed, may prevent optimal brain development and lifelong well-being. 7
It is increasingly apparent that optimal early child development has lifetime beneficial consequences for educational achievement, adult productivity and population health. 8–10 Conversely, exposure to biological and psychosocial risks negatively affects the developing brain and compromises the development of children. 5 Many structural factors determine these early child circumstances. These factors include a lack of nurturing care (nutrition, stimulation, good health) in the early years, as well as inadequate cognitive and psychosocial stimulation. 5 , 11 Children <5 y of age in low- and middle-income countries (LMICs) may be particularly at risk of poor development due to poor health and nutrition. 7
Child development can be encouraged through intervention in early childhood. 11 A number of mutually important elements are needed for maximising children's development. These include supporting responsive relationships, reducing sources of stress in the lives of children and families, building executive function and self-regulation skills and reinforcing contexts in which learning is most achievable across all developmental domains. 12 , 13 ECD interventions work to improve development through integrating family support, health, nutrition and educational services and providing direct learning experiences to young children and families. 14
The strategic focus of the World health Organization (WHO), United Nations Children's Fund (UNICEF) and World Bank ‘Nurturing Care Framework’ is therefore timely. 15–17 This action plan provides a framework for helping children survive and thrive through five strategic actions—lead and invest, focus on families and their communities, strengthen services, monitor progress and use data and innovate—and thereby aims to transform health and human potential. We know that urgent action is necessary to improve early childhood outcomes and ensure that all children reach their full potential as adults. Children with developmental disabilities must be included in this agenda, as they are a marginalised group with additional and specific needs and will otherwise be left behind.
Developmental delay and developmental disability are two distinct concepts. Developmental delay is often defined as a deviation from normative milestones; this may be in terms of delayed cognitive, language, motor and/or socio-emotional development. 18 The term developmental disabilities covers a range of childhood conditions and is used differently across different settings and cultures. 19 In this article we define developmental disability as a heterogeneous group of conditions that can impact on the development of children's function (e.g. sensory, cognitive, physical), with a very wide range of effects. 20 Developmental disability is the most common cause of childhood disability, with an estimated 53 million children <5 y of age living with developmental disabilities globally. 21 This estimate is based on only six conditions (epilepsy, intellectual disability, vision loss, hearing loss, autism and attention deficit hyperactivity disorder) and on present reporting of these conditions. It is likely therefore that the true number of children with developmental disability is much higher than this estimate, particularly if a broader age range is considered.
The majority of children with developmental disabilities live in LMICs, 21 and the prevalence is higher among families with high levels of poverty and low education. 27 However, there remain data gaps for the prevalence, epidemiology and causes of developmental disabilities in LMICs. 28 One reason for the uncertainty in the estimates is that identification of children with or at risk of developmental delay requires assessment using valid developmental evaluation tools to measure ECD 29 (Box 1 ), and these facilities are often not available in LMICs.
Identification of children with developmental disabilities
| In order to meaningfully measure thriving and well-being of children globally, developmental assessment tools need to be culturally relevant and age appropriate and cover the spectrum of developmental domains, including sensory impairments and educational outcomes. Examples of tools with high validity and reliability to measure several developmental domains include the Bayley Scales of Infant and Toddler Development (BSID II or Bayley-III). Regionally developed instruments include the Malawi Development Assessment Tool and the Kilifi Developmental Inventory. However, a recent review found variability in translation, adaptation, piloting and standardisation of tools, with important domains such as vision, hearing, functioning and disability often omitted, which limits holistic understanding of a child's progress. In addition, no tool covers all domains of development and is accurate and feasible in all contexts. |
| In order to meaningfully measure thriving and well-being of children globally, developmental assessment tools need to be culturally relevant and age appropriate and cover the spectrum of developmental domains, including sensory impairments and educational outcomes. Examples of tools with high validity and reliability to measure several developmental domains include the Bayley Scales of Infant and Toddler Development (BSID II or Bayley-III). Regionally developed instruments include the Malawi Development Assessment Tool and the Kilifi Developmental Inventory. However, a recent review found variability in translation, adaptation, piloting and standardisation of tools, with important domains such as vision, hearing, functioning and disability often omitted, which limits holistic understanding of a child's progress. In addition, no tool covers all domains of development and is accurate and feasible in all contexts. |
The impacts of developmental disabilities extend far beyond functional abilities. Children with developmental disabilities and their families are at high risk of social exclusion, exclusion from education and even stigma and violence. 30 Furthermore, looking after a child with developmental disabilities potentially places an enormous strain on families, and caregivers experience high levels of stress, anxiety, depression, physical exhaustion, stigma and discrimination. 31 This further increases the risk of mental ill health and social isolation in caregivers. A recent systematic review found caregivers of children with intellectual and developmental disabilities, when compared with caregivers of children without intellectual and developmental disabilities, experienced elevated levels of depressive symptoms (31% vs 7%, respectively) and anxiety symptoms (31% vs 14%, respectively). 32 There are also substantial costs to childhood disability, both the cost of additional services and resources required by the child and the lost income from parents who are caring for their child. Consequently, childhood disability may exacerbate poverty. 33 , 34 However, there is generally a lack of available services and support for children with disabilities and their families, especially in LMICs, which further compound these risks.
Evidence is limited, but growing, on the effectiveness of ECD interventions for children at risk of and with developmental delays, particularly in LMICs. 35 Indeed, many programmes and studies actively exclude children with developmental disabilities, as additional considerations may be required, and children with developmental disabilities may be unable to show progress when using developmental progress as the primary outcome 9 , 36–38 (Box 2 ).
Inclusion of children with developmental disabilities in clinical trials
| Our review of the first 100 titles of registered clinical trials of ECD interventions (Appendix ), and inclusion of children with disabilities, demonstrated that 50% of the trials exclude children with disabilities, 22% of trials target children with disabilities, 3% of trials target children in general and include children with disabilities and 25% of trials do not specify whether children with disabilities are included or excluded. |
| Our review of the first 100 titles of registered clinical trials of ECD interventions (Appendix ), and inclusion of children with disabilities, demonstrated that 50% of the trials exclude children with disabilities, 22% of trials target children with disabilities, 3% of trials target children in general and include children with disabilities and 25% of trials do not specify whether children with disabilities are included or excluded. |
Consequently, risks to delayed development are compounded for children with developmental disabilities, as they potentially receive less stimulation and fewer learning opportunities through other health service or care routes. 39 Exclusion of children with developmental disabilities from ECD thus perpetuates an already fragile cycle of development. We know that early childhood developmental intervention for these children is imperative, but we cannot inform planning and delivery of inclusive services for all children without better research in this area. For example, there are gaps in evidence-based approaches to monitoring and evaluation of ECD projects in LMICs, such as challenges in measurement of outcomes in routine programmes, which limit comparative understanding of impact, and in defining and monitoring quality and coverage. 25
Early identification of children with developmental disabilities, as well as early childhood intervention (ECI), improves children's opportunities to maximise their developmental potential and functioning as well as their quality of life and social participation. 40 , 41 Early identification and intervention are two distinct complementary strands; timely identification of children with developmental disabilities is required for early intervention, which strengthens the cumulative process of development, helping children acquire new skills and behaviours to reinforce and strengthen learning. In addition, some ECIs may have wider benefits for caregivers, such as through establishing support, thus helping build their knowledge, confidence and coping strategies, 32 with positive impacts for their mental health. However, data are lacking from LMICs and there is a paucity of implementation evidence to guide policymakers and donors. 33
ECI for children with disabilities can comprise a range of coordinated multidisciplinary services and can take many forms, including hospital- or clinic-based care, school-based programmes, parenting and community support and home-based childhood therapies. In high-resource settings, we know that family-centred interventions are more likely to result in the greatest satisfaction with services and improve psychosocial well-being for the child and caregiver. 42 With regards to impact, a systematic review of ECIs for children at risk of cerebral palsy demonstrated improved cognitive outcomes up to preschool age and improved motor outcomes during infancy, although variability in interventions limited the identification of which interventions are most effective. 43 Nevertheless, without such ECIs in LMICs, years lived with disability will be more than 3.3 million. 1
There are broadly two approaches to providing ECI for children with developmental disabilities, including children with disabilities in mainstream ECD interventions and targeted intervention programmes for children with disabilities. These approaches take many different forms, as they are used to support children and families with different needs. For example, universal programmes in the UK, such as the five mandated health visits for young children, are offered to all families. In contrast, targeted programmes, such as the Disabled Children's Outreach Service (DCOS), are aimed specifically at vulnerable families of children with a disability where the children are at higher risk of poor outcomes in later life. 44
While both inclusive and targeted efforts for children with disabilities at the level of early childhood centres have increased, 45 weak country health systems and conflict settings are major impediments to delivering high-quality services. 46 There remains a need for inclusive approaches for children with developmental disabilities in mainstream services, as well as within specialist ECIs. This means that the role of families can be particularly crucial to fill existing gaps in service availability.
A number of case studies have been identified for ECI for children with developmental disabilities. The following have been selected for description, as they illustrate different approaches for children with different developmental disabilities in several LMIC settings.
The WHO has developed Caregiver Skills Training (CST) for caregivers of children with intellectual disabilities. 47 , 48 The CST consists of nine group sessions and three home visits. The programme teaches strategies to promote communication and learning and address challenging behaviours. However, sustainable and scalable quality delivery of the group format by a lay facilitator remains a challenge due to limited integration in health systems. 49 Evidence of effectiveness is currently lacking, but randomised controlled trials are under way in Pakistan (Family Networks [FaNs] for Children with Developmental Disorders and Delays 50 ) and Italy, with future trials planned in China, Ethiopia and Kenya. 51
Interventions that aim to provide contextualised psychological support to caregivers of children with intellectual disabilities include ‘Titukulane’, a community group intervention that aims to reduce mental health problems among the parents of affected children. 52 This community-based intervention consists of eight modules that have been developed and piloted to help parents cope with the challenging role of caring for a child with intellectual disabilities.
Learning through Everyday Activities with Parents (LEAP-CP) is a family-centred intervention delivered peer to peer at home during 30 weekly 2h visits that aims to improve the mobility of children with cerebral palsy. 53 Visits include therapeutic modules (goal-directed active motor and cognitive strategies and LEAP-CP games) and parent education. Randomised controlled trials are currently under way in India. 54 The trial also provides nutrition and health support to all families in the study, which may influence the findings.
The London School of Hygiene & Tropical Medicine (UK) has developed three caregiver group interventions under the ‘Ubuntu’ umbrella (resources available from www.ubuntu-hub.org ). The interventions consist of 10 sessions, the content of which includes information about essential care practices, such as feeding, positioning, communication and play, offered through a local support group format. ‘Getting to know cerebral palsy’ was developed as a resource to empower families using a participatory approach at the community level. 31 , 55 The ABAaNA Early Intervention Programme (EIP) was developed in response to a recognised need to support families of very young children (<2 y) with an evolving developmental disability. 56 ‘Juntos’ was developed for children with congenital Zika syndrome and their families in Latin America and integrates a strengthened component on caregiver emotional well-being, arguably fundamental to a child's early development. 57–60
Interventions for children with autism spectrum disorder include PASS, a parent-mediated intervention for autism spectrum disorder in India and Pakistan. 61 The intervention uses video feedback methods to address parent–child interaction and was adapted for delivery by non-specialist workers. As PASS is focused on improving a child's social communication, common mental health comorbidities such as sleep difficulties will be important to integrate into wider intervention programmes.
These examples provide good case studies of diverse interventions for different children with developmental disabilities in different low-resource settings. These case studies indicate that in LMICs, the gap in meeting the holistic needs of children with developmental disabilities may be addressed through the use of community-based group interventions facilitated by trained and supervised health or peer support workers. Commonality is the focus on caregiver involvement, which is critical, particularly where there are few health services. Yet formal evaluation of their effectiveness and cost-effectiveness is lacking, in addition to limited implementation with education and social welfare, which hampers scaling of these services.
The number of children with developmental disabilities is large and the impacts on the child and family are extensive. There are valuable lessons learned from case studies, yet there remains insufficient progress in ECI for children with developmental disabilities and unmet needs are widespread. The causes of this gap are complex and diverse. An important reason is that in many settings health services are often fragile, poorly coordinated and overstrained, with concerns about the availability and quality of healthcare workers capable of delivering the intervention. Health systems gaps are particularly important in fragile states, including those affected by war and famine, as they experience many competing pressing needs. Furthermore, the policy agenda supporting a focus on children with developmental disabilities is weak internationally and nationally in many cases, limiting the priority given to this issue and the availability of funding for developing services. Ensuring inclusive education is a clear responsibility for United Nations member states under international treaties and Sustainable Development Goal 4, to ‘ensure inclusive, equitable quality education for all’. However, investing in inclusion prior to schooling is not mandated and consequently becomes optional. Cultural challenges also exist, such as widespread stigma and discrimination around children with disabilities and their families. 62 Finally, the evidence base on needs for and effectiveness of services is currently weak and needs to be strengthened. Enhancing environments that provide equal opportunities for children with developmental disabilities for ECI therefore requires a systems approach with global collaboration.
Accordingly, priorities for future research to ensure that all young children reach their development potential include assessment of the effect of interventions for children with developmental disability and their families in different low-resource settings. Further identification of barriers to accessing general services (e.g. primary healthcare) as well as specialist services is also required, as poverty remains a major issue for affected families in LMICs. Furthermore, studies that identify how to maximise the reach and cost-effectiveness of ECD interventions for children with developmental disabilities are warranted. Evaluation of how these interventions can be embedded within health systems are needed to strengthen the service delivery strategies. Global collaboration in these efforts are required in research, and critical steps include providing best evidence on practices to improve knowledge and skills at local levels to avoid children with developmental disabilities being turned away from existing services and evidence of ‘what works’ to provide sustainable, inclusive ECD interventions with impact in resource-constrained settings. We call for international research communities, including funders, to adopt a systematic approach for better evidence.
ECD interventions are aimed at improving the development of children. However, children with developmental disabilities are often excluded from these programmes, even though they have the greatest need for support. There is still a dearth of research about what interventions are effective in improving outcomes for this marginalised group and an even greater lack of evidence on cost-effectiveness and what can be successfully implemented at scale. A two-pronged approach is likely to be optimal, encouraging the inclusion of children with disabilities in mainstream ECD programmes, while also offering targeted approaches, most likely through caregivers. We call for global collaboration among international research communities, including funders, to adopt a systematic approach to strengthening the available evidence base of interventions for children with developmental disabilities and their families. We call for greater attention for this marginalised group, to prioritise public policies and hold governments accountable to ensure that multisectoral services centred around the child and his/her family are provided during this crucial time. This will contribute to ensuring that all children have an early foundation for optimal development, a key factor in equitable long-term health.
HK conceived the study. TS carried out the analysis and interpretation of case study data. TS and HK drafted the manuscript. MZ, CJT, MG and HK critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. TS and HK are guarantors of the paper. The data underlying this article are available in the article and in its online supplementary material.
This work was supported by the Wellcome Trust and Department for International Development (grant 206719/Z/17/Z to HK). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
MG is a member of expert panels for the WHO and UNICEF on measurement of childhood development and disability. This research paper was undertaken outside and separate from these duties.
Not required.
Olusanya BO , de Vries PJ , Olusanya BO et al. . Nurturing care for children with developmental disabilities: a moral imperative for sub-Saharan Africa . Lancet Child Adolesc Health . 2018 ; 2 ( 11 ): 772 – 4 .
Google Scholar
United Nations . Convention on the Rights of the Child 1989 . Available from: http://www2.ohchr.org/english/law/crc.htm .
United Nations Committee on the Rights of Persons with Disabilities . Report of the Committee on the Rights of Persons with Disabilities: First session (23–27 February 2009), Second session (19–23 October 2009), Third session (22–26 February 2010), Fourth session (4–8 October 2010). Available from: https://www.refworld.org/docid/4eef033a2.html [accessed 27 February 2020] .
MAL-ED Network Investigators . Early childhood cognitive development is affected by interactions among illness, diet, enteropathogens and the home environment: findings from the MAL-ED birth cohort study . BMJ Glob Health . 2018 ; 3 ( 4 ): e000752 .
Walker SP , Wachs TD , Grantham-McGregor S et al. . Inequality in early childhood: risk and protective factors for early child development . Lancet . 2011 ; 378 ( 9799 ): 1325 – 38 .
Britto PR , Lye SJ , Proulx K et al. . Nurturing care: promoting early childhood development . Lancet . 2017 ; 389 ( 10064 ): 91 – 102 .
Black MM , Walker SP , Fernald LCH et al. . Early childhood development coming of age: science through the life course . Lancet . 2017 ; 389 ( 10064 ): 77 – 90 .
Attanasio O , Meghir C , Nix E et al. . Human capital growth and poverty: evidence from Ethiopia and Peru . Rev Econ Dyn . 2017 ; 25 : 234 – 59 .
Richter L , Black M , Britto P et al. . Early childhood development: an imperative for action and measurement at scale . BMJ Glob Health . 2019 ; 4 ( Suppl 4 ): e001302 .
Aguilera Vasquez N , Daher J . Do nutrition and cash-based interventions and policies aimed at reducing stunting have an impact on economic development of low-and-middle-income countries? A systematic review . BMC Public Health . 2019 ; 19 ( 1 ): 1419 .
Engle PL , Fernald LC , Alderman H et al. . Strategies for reducing inequalities and improving developmental outcomes for young children in low-income and middle-income countries . Lancet . 2011 ; 378 ( 9799 ): 1339 – 53 .
Aboud FE , Yousafzai AK. Global health and development in early childhood . Annu Rev Psychol . 2015 ; 66 : 433 – 57 .
Institute of Medicine, National Research Council . Transforming the workforce for children birth through age 8: a unifying foundation . Washington, DC : National Academies Press ; 2015 .
Google Preview
Engle PL , Black MM , Behrman JR et al. . Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world . Lancet . 2007 ; 369 ( 9557 ): 229 – 42 .
World Health Organization . Nurturing care framework . Geneva : World Health Organization ; 2018 .
Gove A , Black MM. Measurement of early childhood development and learning under the Sustainable Development Goals . J Hum Dev Capabil . 2016 ; 17 ( 4 ): 599 – 605 .
Daelmans B , Darmstadt GL , Lombardi J et al. . Early childhood development: the foundation of sustainable development . Lancet . 2017 ; 389 ( 10064 ): 9 – 11 .
Boggs D , Milner KM , Chandna J et al. . Rating early child development outcome measurement tools for routine health programme use . Arch Dis Child . 2019 ; 104 ( Suppl 1 ): S22 – 33 .
Wong VCN. Global developmental delay – a delay in development of terminology . Dev Med Child Neurol . 2011 ; 53 ( 7 ): 585 .
Rosenbaum P , Gorter JW. The ‘F-words’ in childhood disability: I swear this is how we should think! Child Care Health Dev . 2012 ; 38 ( 4 ): 457 – 63 .
Global Research on Developmental Disabilities Collaborators . Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016 . Lancet Glob Health . 2018 ; 6 ( 10 ): e1100 – 21 .
Bayley N . Bayley scales of infant and toddler development . 3rd ed. London : Pearson ; 2006 .
Gladstone M , Lancaster GA , Umar E et al. . The Malawi Developmental Assessment Tool (MDAT): the creation, validation, and reliability of a tool to assess child development in rural African settings . PLoS Med . 2010 ; 7 ( 5 ): e1000273 .
Abubakar A , Holding P , van Baar A et al. . Monitoring psychomotor development in a resource-limited setting: an evaluation of the Kilifi Developmental Inventory . Ann Trop Paediatr . 2008 ; 28 ( 3 ): 217 – 26 .
Milner KM , Bhopal S , Black M et al. . Counting outcomes, coverage and quality for early child development programmes . Arch Dis Child . 2019 ; 104 ( Suppl 1 ): S13 – 21 .
Sabanathan S , Wills B , Gladstone M . Child development assessment tools in low-income and middle-income countries: how can we use them more appropriately? Arch Dis Child . 2015 ; 100 ( 5 ): 482 – 8 .
Banks LM , Kuper H , Polack S . Poverty and disability in low- and middle-income countries: a systematic review . PLoS One . 2017 ; 12 ( 12 ): e0189996 .
Black MM , Lawn JE . Early childhood developmental disabilities-data still needed . Lancet Glob Health . 2018 ; 6 ( 10 ): e1050 – 1 .
World Health Organization . Developmental difficulties in early childhood: prevention, early identification, assessment and intervention in low- and middle-income countries . Geneva : World Health Organization ; 2012 .
Jones L , Bellis MA , Wood S et al. . Prevalence and risk of violence against children with disabilities: a systematic review and meta-analysis of observational studies . Lancet . 2012 ; 380 ( 9845 ): 899 – 907 .
Zuurmond M , Nyante G , Baltussen M et al. . A support programme for caregivers of children with disabilities in Ghana: understanding the impact on the wellbeing of caregivers . Child Care Health Dev . 2019 ; 45 ( 1 ): 45 – 53 .
Scherer N , Verhey I , Kuper H . Depression and anxiety in parents of children with intellectual and developmental disabilities: a systematic review and meta-analysis . PLoS One . 2019 ; 14 ( 7 ): e0219888 .
Tomlinson M , Darmstadt GL , Yousafzai AK et al. . Global research priorities to accelerate programming to improve early childhood development in the sustainable development era: a CHNRI exercise . J Glob Health 2019 ; 9 ( 3 ): 020703 .
McGovern ME , Krishna A , Aguayo VM et al. . A review of the evidence linking child stunting to economic outcomes . Int J Epidemiol . 2017 ; 46 ( 4 ): 1171 – 91 .
Luby JL. Poverty's most insidious damage: the developing brain . JAMA Pediatr . 2015 ; 169 ( 9 ): 810 – 1 .
Murphy R , Jolley E , Lynch P et al. . Estimated prevalence of disability and developmental delay among preschool children in rural Malawi: findings from ‘Tikule Limodzi’, a cross-sectional survey . Child Care Health Dev . 2020 ; 46 ( 2 ): 187 – 94 .
Black MM , Perez-Escamilla R , Rao SF . Integrating nutrition and child development interventions: scientific basis, evidence of impact, and implementation considerations . Adv Nutr . 2015 ; 6 ( 6 ): 852 – 9 .
Lu C , Black MM , Richter LM . Risk of poor development in young children in low-income and middle-income countries: an estimation and analysis at the global, regional, and country level . Lancet Glob Health . 2016 ; 4 ( 12 ): e916 – 22 .
Canavera K , Johnson L-M , Harman J . Beyond parenting: the responsibility of multidisciplinary health care providers in early intervention policy guidance . Am J Bioeth . 2018 ; 18 ( 11 ): 58 – 60 .
Collins PY , Pringle B , Alexander C et al. . Global services and support for children with developmental delays and disabilities: bridging research and policy gaps . PLoS Med . 2017 ; 14 ( 9 ): e1002393 .
Scherzer AL , Chhagan M , Kauchali S et al. . Global perspective on early diagnosis and intervention for children with developmental delays and disabilities . Dev Med Child Neurol . 2012 ; 54 ( 12 ): 1079 – 84 .
King S , Teplicky R , King G et al. . Family-centered service for children with cerebral palsy and their families: a review of the literature . Semin Pediatr Neurol . 2004 ; 11 ( 1 ): 78 – 86 .
Spittle A , Orton J , Anderson PJ et al. . Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants . Cochrane Database Syst Rev . 2015 ; 11 : CD005495 .
Powell T . Early intervention. Briefing paper 7647 . London : House of Commons Library ; 2019 .
McLinden M , Lynch P , Soni A et al. . Supporting children with disabilities in low- and middle- income countries: promoting inclusive practice within community-based childcare centres in Malawi through a bioecological systems perspective . Int J Early Child . 2018 ; 50 ( 2 ): 159 – 74 .
Countdown to 2030 Collaboration . Countdown to 2030: tracking progress towards universal coverage for reproductive, maternal, newborn, and child health . Lancet . 2018 ; 391 ( 10129 ): 1538 – 48 .
World Health Organization . The WHO Caregiver Skills Training programme . Available from: https://www.who.int/mental_health/maternal-child/PST/en/ .
Salomone E , Pacione L , Shire S et al. . Development of the WHO Caregiver Skills Training Program for developmental disorders or delays . Front Psychiatry . 2019 ; 10 : 769 .
Philip S , Chaturvedi SK. Musings on task shifting in mental health . J Psychosoc Rehabil Ment Health . 2018 ; 5 ( 2 ): 103 – 7 .
Hamdani SU , Akhtar P , Zill EH et al. . WHO Parents Skills Training (PST) programme for children with developmental disorders and delays delivered by family volunteers in rural Pakistan: study protocol for effectiveness implementation hybrid cluster randomized controlled trial . Glob Ment Health (Camb) . 2017 ; 4 : e11 .
World Health Organization. Training parents to transform children's lives. Available from: https://www.who.int/mental_health/maternal-child/PST/en/ .
Masulani-Mwale C , Kauye F , Gladstone M et al. . Development of a psycho-social intervention for reducing psychological distress among parents of children with intellectual disabilities in Malawi . PLoS One . 2019 ; 14 ( 2 ): e0210855 .
Benfer KA , Novak I , Morgan C et al. . Community-based parent-delivered early detection and intervention programme for infants at high risk of cerebral palsy in a low-resource country (Learning through Everyday Activities with Parents (LEAP-CP): protocol for a randomised controlled trial . BMJ Open . 2018 ; 8 ( 6 ): e021186 .
LEAP-CP Symposium. Early detection and early intervention for children at risk of CP in low-middle income countries. Available from: https://cparf.org/sstposts/StoryId1579066413321 .
Zuurmond M , O'Banion D , Gladstone M et al. . Evaluating the impact of a community-based parent training programme for children with cerebral palsy in Ghana . PLoS One . 2018 ; 13 ( 9 ): e0202096 .
Nampijja M , Webb E , Nanyunja C et al. . Randomised controlled pilot feasibility trial of an early intervention programme for young infants with neurodevelopmental impairment in Uganda: a study protocol . BMJ Open . 2019 ; 9 ( 10 ): e032705 .
Duttine A , Smythe T , Calheiro de Sá MR et al. . Development and assessment of the feasibility of a Zika family support programme: a study protocol . Wellcome Open Res . 2019 ; 4 : 80 .
Kuper H , Smythe T , Duttine A . Reflections on health promotion and disability in low and middle-income countries: case study of parent-support programmes for children with congenital Zika syndrome . Int J Environ Res Public Health . 2018 ; 15 ( 3 ): 514 .
Sa MRC , Vieira ACD , Castro BSM et al. . [The need to act together in every way possible: inter-sector action in health and education for children living with the congenital Zika syndrome] . Cad Saude Publica . 2019 ; 35 ( 12 ): e00233718 .
Smythe T , Duttine A , Vieira ACD et al. . Engagement of fathers in parent group interventions for children with congenital Zika syndrome: a qualitative study . Int J Environ Res Public Health . 2019 ; 16 ( 20 ): 3862 .
Rahman A , Divan G , Hamdani SU et al. . Effectiveness of the parent-mediated intervention for children with autism spectrum disorder in south Asia in India and Pakistan (PASS): a randomised controlled trial . Lancet Psychiatry . 2016 ; 3 ( 2 ): 128 – 36 .
Smythe T , Adelson JD , Polack S . Systematic review of interventions for reducing stigma experienced by children with disabilities and their families in low- and middle-income countries: state of the evidence . Trop Med Int Health . 2020 ; 25 : 508 – 24 .
Completed clinical trials with a focus on developmental outcomes
| . | Title . | Start date . | Country . | Target children with disabilities . | Includes children with disabilities . | Does not include or exclude . | Excludes children with disabilities . |
|---|---|---|---|---|---|---|---|
| 1 | The Pakistan Early Childhood Development Scale Up Trial | 2009 | Pakistan | 1 | |||
| 2 | Iron Treatment for Young Children With Non-anemic Iron Deficiency | 2012 | Canada | 1 | |||
| 3 | Project Grow Smart: Intervention Trial of Multiple Micronutrients and Early Learning Among Infants in India | 2012 | India | 1 | |||
| 4 | Early Child Development and Nutrition in Guatemala | 2015 | Guatemala | 1 | |||
| 5 | Strong Families, Thriving Children “Sugira Muryango”_Activity C | 2018 | Rwanda | 1 | |||
| 6 | Implementation and Adoption of Care for Child Development in Day Care Centers | 2015 | Lebanon | 1 | |||
| 7 | CASITA Intervention for Children at Risk of Delay in Carabayllo, Peru | 2013 | Peru | 1 | |||
| 8 | Family Inclusive Early Brain Stimulation | 2014 | Nigeria | 1 | |||
| 9 | Applying Mindfulness for Economically Disadvantaged Families | 2016 | Hong Kong | 1 | |||
| 10 | Promoting Child Development Practices in the First Year of Life Through a Video Administered at Two Different Times | 2008 | Italy | 1 | |||
| 11 | Promoting Early School Readiness in Primary Health Care | 2005 | USA | 1 | |||
| 12 | Improving Early Childhood Development in Zambia | 2014 | Zambia | 1 | |||
| 13 | Early Intervention for Developmental Delay | 2014 | Taiwan | 1 | |||
| 14 | A Family Centered Intervention to Promote Optimal Child Development | 2013 | USA | 1 | |||
| 15 | Family Strengthening Intervention for Early Childhood Development (ECD) | 2014 | Rwanda | 1 | |||
| 16 | Screening for Therapy and Empowering Parents: A Pilot Study | 2015 | USA | 1 | |||
| 17 | Alliance for Family Strengthening: Improved Early Childhood Development in Rwanda | 2017 | Rwanda | 1 | |||
| 18 | Efficacy of Tools of the Mind for Enhancing Self-Control in Preschoolers | 2012 | Canada | 1 | |||
| 19 | Early Literacy Promotion Intervention | 2016 | USA | 1 | |||
| 20 | Zinc and Biobehavioral Development in Early Childhood | 2004 | Peru | 1 | |||
| 21 | The Effect of a Cash Transfer Program and Preventive Nutrition Packages on Household Welfare and Child Nutritional Status in Mali | 2014 | Mali | 1 | |||
| 22 | Enhancing Ugandan HIV-Affected Child Development With Caregiver Training | 2012 | Uganda | 1 | |||
| 23 | Early Intervention for Preterm Infants | 2006 | Taiwan | 1 | |||
| 24 | Effect of Improving Caregiving on Early Mental Health | 2000 | Russia | 1 | |||
| 25 | Testing the Effectiveness of Telephone-based Early Childhood Developmental Screening | 2015 | USA | 1 | |||
| 26 | Early Family-Centered Prevention of Drug Use Risk (Aka Early Steps) | 2003 | USA | 1 | |||
| 27 | Effect of Power Wheelchairs on the Development and Function of Young Children With Severe Physical Disabilities | 2002 | USA | 1 | |||
| 28 | Effect of Community Based Depression Management and Child Development | 2014 | Bangladesh | 1 | |||
| 29 | Play and Pre-Literacy Among Young Children | 2015 | Canada | 1 | |||
| 30 | Social and Communication Outcomes for Young Children With Autism | 2009 | USA | 1 | |||
| 31 | The Anemia Control Program: Early Intervention | 1992 | Chile | 1 | |||
| 32 | Early Psychosocial Stimulation Program for Children of Depressed Mothers | 2009 | Pakistan | 1 | |||
| 33 | Promoting Infant Mental Health in Foster Care | 2007 | USA | 1 | |||
| 34 | Addressing Systemic Health Disparities in Early Identification and Treatment of Autism Spectrum Disorder (ASD): ABCD Project | 2014 | USA | 1 | |||
| 35 | The MOM Program at the Children's Hospital of Philadelphia | 2001 | USA | 1 | |||
| 36 | Translating Evidence Based Developmental Screening Into Pediatric Primary Care | 2008 | USA | 1 | |||
| 37 | Improving Parental Psychosocial Functioning and Early Developmental Outcomes in Children With Sickle Cell Disease | 2014 | West Indies | 1 | |||
| 38 | Promoting Healthy Development With the Recipe 4 Success Intervention | 2013 | USA | 1 | |||
| 39 | Long Term Effect of Early Iron Supplementation and Psychosocial Stimulation on Growth and Development of Iron-deficient Anaemic Infants | 2015 | Bangladesh | 1 | |||
| 40 | Reduce Childhood Maltreatment and Promote Development | 2015 | Bangladesh | 1 | |||
| 41 | The Impact of Cash and Food Transfers Linked to Preschool Enrollment on Child Nutrition and Cognitive Outcomes | 2010 | Uganda | 1 | |||
| 42 | Effects of Family-Centered Intervention for Preterm Infants at Preschool Age | 2015 | Taiwan | 1 | |||
| 43 | Effectiveness of Parent-Child Interaction and Emotion Development Therapy in Treating Preschool Children With Depression | 2007 | USA | 1 | |||
| 44 | The Effects of Iodized Salt on Cognitive Development in Ethiopia | 2011 | Ethiopia | 1 | |||
| 45 | An Intervention for Enhancing Early Attachment in Primary Health Care | 2013 | Chile | 1 | |||
| 46 | The MOM Program: 5 Year Follow-up Study of a Home Visiting Program at the Children's Hospital of Philadelphia | 2004 | USA | 1 | |||
| 47 | Small Step Intervention for Infants With Cerebral Palsy and Other Neurodevelopmental Disorders | 2014 | Sweden | 1 | |||
| 48 | Intervention Effects of Intensity and Delivery Style for Toddlers With Autism | 2008 | USA | 1 | |||
| 49 | Motivational Interviewing to Increase Parent Engagement in Preventive Parenting Programming | 2013 | USA | 1 | |||
| 50 | Intensive Intervention for Toddlers With Autism (EARLY STEPS) | 2013 | USA | 1 | |||
| 51 | Optimizing Social and Communication Outcomes for Toddlers With Autism | 2008 | USA | 1 | |||
| 52 | Primary Prevention of Allergic Disease in Early Child by | 2001 | Sweden | 1 | |||
| 53 | Intervention for Toddlers at Risk for Autism Spectrum Disorders (ASD) | 2008 | USA | 1 | |||
| 54 | Promoting Development in Toddlers With Communication Delays | 2007 | USA | 1 | |||
| 55 | Early Intervention, Supervision, Quality and Outcome in ASD | 2013 | Sweden | 1 | |||
| 56 | Differential DNA Methylation as a Function of a Parenting Intervention | 2013 | USA | 1 | |||
| 57 | Early Connections, Early Detection and Intervention in Infants at Risk for Autism | 2008 | USA | 1 | |||
| 58 | Early Characteristics of Autism | 2003 | USA | 1 | |||
| 59 | School- and Home-Based Early Intervention for Toddlers With Autism | 2003 | USA | 1 | |||
| 60 | Follow-up of Families in Early Preventive Intervention | 2000 | USA | 1 | |||
| 61 | Parent Training Program for Preschool Children With Autism Spectrum Disorders | 2015 | Taiwan | 1 | |||
| 62 | RESPECT-PLUS: Services for Infants With Prenatal Opiate Exposure | 2013 | USA | 1 | |||
| 63 | Early Nutritional Intervention in Patients With Autism Spectrum Disorders | 2010 | Qatar | 1 | |||
| 64 | Maximizing Language Development in Children With Hearing Loss | 2013 | USA | 1 | |||
| 65 | Mother and Child Education Program in Palestinian Refugee Camps | 2014 | Lebanon | 1 | |||
| 66 | Early Intervention and Autism: Transformation From Research to Practice Through a Competency Based Model | 2017 | Sweden | 1 | |||
| 67 | Mindfulness Training and Parent-coaching Interventions for Autism Spectrum Disorder | 2015 | USA | 1 | |||
| 68 | Impact of an Intervention Program on Parenting Stress After Preterm Birth | 2006 | France | 1 | |||
| 69 | Efficacy Trial of the Kids in Transition to School (KITS) Program for Children With Developmental Disabilities and Behavioral Problems | 2008 | USA | 1 | |||
| 70 | Social-emotional Under 4’s Screening & Intervention S.U.S.I. | 2016 | USA | 1 | |||
| 71 | H3: Healthy Minds, Healthy Children, Healthy Chicago Project Evaluation | 2014 | UK | 1 | |||
| 72 | Reproducibility Inter-session of the Measurement Elastography of the Passive Stiffness of Medial Beams of Gastrocnemius Muscle of the Hemiplegic Cerebral Child | 2017 | France | 1 | |||
| 73 | Transition to Scale of Nutrition and Psychosocial Stimulation Program for Malnourished Children | 2014 | Bangladesh | 1 | |||
| 74 | Electronic Patient-reported Outcomes (e-PROs) in Early Intervention | 2016 | USA | 1 | |||
| 75 | Iron Deficiency Anemia and Psychosocial Stimulation | 2007 | Bangladesh | 1 | |||
| 76 | Omega Tots: A Randomized, Controlled Trial of Long-chain Polyunsaturated Fatty Acid Supplementation of Toddler Diets and Developmental Outcomes | 2012 | USA | 1 | |||
| 77 | Zinc, Iron, Vitamin A and Psychosocial Care for Child Growth and Development | 1998 | Indonesia | 1 | |||
| 78 | Middle Ear Disease Before Age 3, Treatment With Ear Tubes, and Literacy and Attentional Abilities at Ages 9 to 11 | 2002 | USA | 1 | |||
| 79 | The Effect of a Deworming Intervention to Improve Early Childhood Growth and Development in Resource-poor Areas | 2014 | USA | 1 | |||
| 80 | Comparing Parent-Implemented Interventions for Toddlers With Autism Spectrum Disorders | 2007 | USA | 1 | |||
| 81 | Strengthening Families and Reducing Risk Thru Developmental and Legal Collaboration | 2011 | USA | 1 | |||
| 82 | Social Cognitive Development in Young Children With Autism | 2012 | USA | 1 | |||
| 83 | Evaluation of the Healthy Families Alaska Program | 1999 | USA | 1 | |||
| 84 | Initial Efficacy Study of Supporting Play, Exploration, & Early Development Intervention | 2011 | USA | 1 | |||
| 85 | Healthy Habits, Happy Homes: An Intervention to Improve Household Routines for Obesity Prevention | 2011 | USA | 1 | |||
| 86 | Age 12 Follow-up of Early Preventive Intervention (Memphis) | 2003 | USA | 1 | |||
| 87 | Project ASPIRE Efficacy Pilot: Achieving Superior Parental Involvement for Rehabilitative Excellence | 2009 | USA | 1 | |||
| 88 | Interventions for Communication in Autism Network | 2012 | USA | 1 | |||
| 89 | The Effects of a Parental Intervention on Electronic Media Exposure and Sleep Patterns in Adolescents | 2011 | Israel | 1 | |||
| 90 | A Trial of Sertraline in Young Children With Autism Spectrum Disorder | 2015 | USA | 1 | |||
| 91 | A Randomized Controlled Trial of PCIT-ED for Preschool Depression | 2014 | USA | 1 | |||
| 92 | Psychomotor Therapy for Very Premature Infants | 2007 | France | 1 | |||
| 93 | A Website to Teach Children Safety With Dogs | 2015 | USA | 1 | |||
| 94 | Early Physical Therapy Intervention in Preterm Infants | 2017 | Spain | 1 | |||
| 95 | The Children in Action Feasibility Study | 2007 | USA | 1 | |||
| 96 | Development of Appetite Measuring Tool and Appetite Status of Stunted Children | 2016 | Bangladesh | 1 | |||
| 97 | Early Pharmacotherapy Aimed at Neuroplasticity in Autism: Safety and Efficacy | 2004 | USA | 1 | |||
| 98 | Study and Development of Application Models of “Therapeutic Education to the Patient” (TEP) in Asthmatic Children | 2007 | Italy | 1 | |||
| 99 | Development and Effectiveness of Home-based Programs for Preschool Children With Developmental Delay | 2017 | USA | 1 | |||
| 100 | Digital Literacy Promotion | 2016 | Bangladesh | 1 | |||
| TOTAL | 4 | 21 | 25 | 50 |
| . | Title . | Start date . | Country . | Target children with disabilities . | Includes children with disabilities . | Does not include or exclude . | Excludes children with disabilities . |
|---|---|---|---|---|---|---|---|
| 1 | The Pakistan Early Childhood Development Scale Up Trial | 2009 | Pakistan | 1 | |||
| 2 | Iron Treatment for Young Children With Non-anemic Iron Deficiency | 2012 | Canada | 1 | |||
| 3 | Project Grow Smart: Intervention Trial of Multiple Micronutrients and Early Learning Among Infants in India | 2012 | India | 1 | |||
| 4 | Early Child Development and Nutrition in Guatemala | 2015 | Guatemala | 1 | |||
| 5 | Strong Families, Thriving Children “Sugira Muryango”_Activity C | 2018 | Rwanda | 1 | |||
| 6 | Implementation and Adoption of Care for Child Development in Day Care Centers | 2015 | Lebanon | 1 | |||
| 7 | CASITA Intervention for Children at Risk of Delay in Carabayllo, Peru | 2013 | Peru | 1 | |||
| 8 | Family Inclusive Early Brain Stimulation | 2014 | Nigeria | 1 | |||
| 9 | Applying Mindfulness for Economically Disadvantaged Families | 2016 | Hong Kong | 1 | |||
| 10 | Promoting Child Development Practices in the First Year of Life Through a Video Administered at Two Different Times | 2008 | Italy | 1 | |||
| 11 | Promoting Early School Readiness in Primary Health Care | 2005 | USA | 1 | |||
| 12 | Improving Early Childhood Development in Zambia | 2014 | Zambia | 1 | |||
| 13 | Early Intervention for Developmental Delay | 2014 | Taiwan | 1 | |||
| 14 | A Family Centered Intervention to Promote Optimal Child Development | 2013 | USA | 1 | |||
| 15 | Family Strengthening Intervention for Early Childhood Development (ECD) | 2014 | Rwanda | 1 | |||
| 16 | Screening for Therapy and Empowering Parents: A Pilot Study | 2015 | USA | 1 | |||
| 17 | Alliance for Family Strengthening: Improved Early Childhood Development in Rwanda | 2017 | Rwanda | 1 | |||
| 18 | Efficacy of Tools of the Mind for Enhancing Self-Control in Preschoolers | 2012 | Canada | 1 | |||
| 19 | Early Literacy Promotion Intervention | 2016 | USA | 1 | |||
| 20 | Zinc and Biobehavioral Development in Early Childhood | 2004 | Peru | 1 | |||
| 21 | The Effect of a Cash Transfer Program and Preventive Nutrition Packages on Household Welfare and Child Nutritional Status in Mali | 2014 | Mali | 1 | |||
| 22 | Enhancing Ugandan HIV-Affected Child Development With Caregiver Training | 2012 | Uganda | 1 | |||
| 23 | Early Intervention for Preterm Infants | 2006 | Taiwan | 1 | |||
| 24 | Effect of Improving Caregiving on Early Mental Health | 2000 | Russia | 1 | |||
| 25 | Testing the Effectiveness of Telephone-based Early Childhood Developmental Screening | 2015 | USA | 1 | |||
| 26 | Early Family-Centered Prevention of Drug Use Risk (Aka Early Steps) | 2003 | USA | 1 | |||
| 27 | Effect of Power Wheelchairs on the Development and Function of Young Children With Severe Physical Disabilities | 2002 | USA | 1 | |||
| 28 | Effect of Community Based Depression Management and Child Development | 2014 | Bangladesh | 1 | |||
| 29 | Play and Pre-Literacy Among Young Children | 2015 | Canada | 1 | |||
| 30 | Social and Communication Outcomes for Young Children With Autism | 2009 | USA | 1 | |||
| 31 | The Anemia Control Program: Early Intervention | 1992 | Chile | 1 | |||
| 32 | Early Psychosocial Stimulation Program for Children of Depressed Mothers | 2009 | Pakistan | 1 | |||
| 33 | Promoting Infant Mental Health in Foster Care | 2007 | USA | 1 | |||
| 34 | Addressing Systemic Health Disparities in Early Identification and Treatment of Autism Spectrum Disorder (ASD): ABCD Project | 2014 | USA | 1 | |||
| 35 | The MOM Program at the Children's Hospital of Philadelphia | 2001 | USA | 1 | |||
| 36 | Translating Evidence Based Developmental Screening Into Pediatric Primary Care | 2008 | USA | 1 | |||
| 37 | Improving Parental Psychosocial Functioning and Early Developmental Outcomes in Children With Sickle Cell Disease | 2014 | West Indies | 1 | |||
| 38 | Promoting Healthy Development With the Recipe 4 Success Intervention | 2013 | USA | 1 | |||
| 39 | Long Term Effect of Early Iron Supplementation and Psychosocial Stimulation on Growth and Development of Iron-deficient Anaemic Infants | 2015 | Bangladesh | 1 | |||
| 40 | Reduce Childhood Maltreatment and Promote Development | 2015 | Bangladesh | 1 | |||
| 41 | The Impact of Cash and Food Transfers Linked to Preschool Enrollment on Child Nutrition and Cognitive Outcomes | 2010 | Uganda | 1 | |||
| 42 | Effects of Family-Centered Intervention for Preterm Infants at Preschool Age | 2015 | Taiwan | 1 | |||
| 43 | Effectiveness of Parent-Child Interaction and Emotion Development Therapy in Treating Preschool Children With Depression | 2007 | USA | 1 | |||
| 44 | The Effects of Iodized Salt on Cognitive Development in Ethiopia | 2011 | Ethiopia | 1 | |||
| 45 | An Intervention for Enhancing Early Attachment in Primary Health Care | 2013 | Chile | 1 | |||
| 46 | The MOM Program: 5 Year Follow-up Study of a Home Visiting Program at the Children's Hospital of Philadelphia | 2004 | USA | 1 | |||
| 47 | Small Step Intervention for Infants With Cerebral Palsy and Other Neurodevelopmental Disorders | 2014 | Sweden | 1 | |||
| 48 | Intervention Effects of Intensity and Delivery Style for Toddlers With Autism | 2008 | USA | 1 | |||
| 49 | Motivational Interviewing to Increase Parent Engagement in Preventive Parenting Programming | 2013 | USA | 1 | |||
| 50 | Intensive Intervention for Toddlers With Autism (EARLY STEPS) | 2013 | USA | 1 | |||
| 51 | Optimizing Social and Communication Outcomes for Toddlers With Autism | 2008 | USA | 1 | |||
| 52 | Primary Prevention of Allergic Disease in Early Child by | 2001 | Sweden | 1 | |||
| 53 | Intervention for Toddlers at Risk for Autism Spectrum Disorders (ASD) | 2008 | USA | 1 | |||
| 54 | Promoting Development in Toddlers With Communication Delays | 2007 | USA | 1 | |||
| 55 | Early Intervention, Supervision, Quality and Outcome in ASD | 2013 | Sweden | 1 | |||
| 56 | Differential DNA Methylation as a Function of a Parenting Intervention | 2013 | USA | 1 | |||
| 57 | Early Connections, Early Detection and Intervention in Infants at Risk for Autism | 2008 | USA | 1 | |||
| 58 | Early Characteristics of Autism | 2003 | USA | 1 | |||
| 59 | School- and Home-Based Early Intervention for Toddlers With Autism | 2003 | USA | 1 | |||
| 60 | Follow-up of Families in Early Preventive Intervention | 2000 | USA | 1 | |||
| 61 | Parent Training Program for Preschool Children With Autism Spectrum Disorders | 2015 | Taiwan | 1 | |||
| 62 | RESPECT-PLUS: Services for Infants With Prenatal Opiate Exposure | 2013 | USA | 1 | |||
| 63 | Early Nutritional Intervention in Patients With Autism Spectrum Disorders | 2010 | Qatar | 1 | |||
| 64 | Maximizing Language Development in Children With Hearing Loss | 2013 | USA | 1 | |||
| 65 | Mother and Child Education Program in Palestinian Refugee Camps | 2014 | Lebanon | 1 | |||
| 66 | Early Intervention and Autism: Transformation From Research to Practice Through a Competency Based Model | 2017 | Sweden | 1 | |||
| 67 | Mindfulness Training and Parent-coaching Interventions for Autism Spectrum Disorder | 2015 | USA | 1 | |||
| 68 | Impact of an Intervention Program on Parenting Stress After Preterm Birth | 2006 | France | 1 | |||
| 69 | Efficacy Trial of the Kids in Transition to School (KITS) Program for Children With Developmental Disabilities and Behavioral Problems | 2008 | USA | 1 | |||
| 70 | Social-emotional Under 4’s Screening & Intervention S.U.S.I. | 2016 | USA | 1 | |||
| 71 | H3: Healthy Minds, Healthy Children, Healthy Chicago Project Evaluation | 2014 | UK | 1 | |||
| 72 | Reproducibility Inter-session of the Measurement Elastography of the Passive Stiffness of Medial Beams of Gastrocnemius Muscle of the Hemiplegic Cerebral Child | 2017 | France | 1 | |||
| 73 | Transition to Scale of Nutrition and Psychosocial Stimulation Program for Malnourished Children | 2014 | Bangladesh | 1 | |||
| 74 | Electronic Patient-reported Outcomes (e-PROs) in Early Intervention | 2016 | USA | 1 | |||
| 75 | Iron Deficiency Anemia and Psychosocial Stimulation | 2007 | Bangladesh | 1 | |||
| 76 | Omega Tots: A Randomized, Controlled Trial of Long-chain Polyunsaturated Fatty Acid Supplementation of Toddler Diets and Developmental Outcomes | 2012 | USA | 1 | |||
| 77 | Zinc, Iron, Vitamin A and Psychosocial Care for Child Growth and Development | 1998 | Indonesia | 1 | |||
| 78 | Middle Ear Disease Before Age 3, Treatment With Ear Tubes, and Literacy and Attentional Abilities at Ages 9 to 11 | 2002 | USA | 1 | |||
| 79 | The Effect of a Deworming Intervention to Improve Early Childhood Growth and Development in Resource-poor Areas | 2014 | USA | 1 | |||
| 80 | Comparing Parent-Implemented Interventions for Toddlers With Autism Spectrum Disorders | 2007 | USA | 1 | |||
| 81 | Strengthening Families and Reducing Risk Thru Developmental and Legal Collaboration | 2011 | USA | 1 | |||
| 82 | Social Cognitive Development in Young Children With Autism | 2012 | USA | 1 | |||
| 83 | Evaluation of the Healthy Families Alaska Program | 1999 | USA | 1 | |||
| 84 | Initial Efficacy Study of Supporting Play, Exploration, & Early Development Intervention | 2011 | USA | 1 | |||
| 85 | Healthy Habits, Happy Homes: An Intervention to Improve Household Routines for Obesity Prevention | 2011 | USA | 1 | |||
| 86 | Age 12 Follow-up of Early Preventive Intervention (Memphis) | 2003 | USA | 1 | |||
| 87 | Project ASPIRE Efficacy Pilot: Achieving Superior Parental Involvement for Rehabilitative Excellence | 2009 | USA | 1 | |||
| 88 | Interventions for Communication in Autism Network | 2012 | USA | 1 | |||
| 89 | The Effects of a Parental Intervention on Electronic Media Exposure and Sleep Patterns in Adolescents | 2011 | Israel | 1 | |||
| 90 | A Trial of Sertraline in Young Children With Autism Spectrum Disorder | 2015 | USA | 1 | |||
| 91 | A Randomized Controlled Trial of PCIT-ED for Preschool Depression | 2014 | USA | 1 | |||
| 92 | Psychomotor Therapy for Very Premature Infants | 2007 | France | 1 | |||
| 93 | A Website to Teach Children Safety With Dogs | 2015 | USA | 1 | |||
| 94 | Early Physical Therapy Intervention in Preterm Infants | 2017 | Spain | 1 | |||
| 95 | The Children in Action Feasibility Study | 2007 | USA | 1 | |||
| 96 | Development of Appetite Measuring Tool and Appetite Status of Stunted Children | 2016 | Bangladesh | 1 | |||
| 97 | Early Pharmacotherapy Aimed at Neuroplasticity in Autism: Safety and Efficacy | 2004 | USA | 1 | |||
| 98 | Study and Development of Application Models of “Therapeutic Education to the Patient” (TEP) in Asthmatic Children | 2007 | Italy | 1 | |||
| 99 | Development and Effectiveness of Home-based Programs for Preschool Children With Developmental Delay | 2017 | USA | 1 | |||
| 100 | Digital Literacy Promotion | 2016 | Bangladesh | 1 | |||
| TOTAL | 4 | 21 | 25 | 50 |
- child development
- developmental disabilities
- disabled children
- early intervention (education)
| Month: | Total Views: |
|---|---|
| August 2020 | 498 |
| September 2020 | 214 |
| October 2020 | 478 |
| November 2020 | 923 |
| December 2020 | 603 |
| January 2021 | 598 |
| February 2021 | 804 |
| March 2021 | 1,039 |
| April 2021 | 934 |
| May 2021 | 1,112 |
| June 2021 | 858 |
| July 2021 | 830 |
| August 2021 | 751 |
| September 2021 | 871 |
| October 2021 | 816 |
| November 2021 | 910 |
| December 2021 | 669 |
| January 2022 | 628 |
| February 2022 | 937 |
| March 2022 | 1,251 |
| April 2022 | 968 |
| May 2022 | 905 |
| June 2022 | 627 |
| July 2022 | 563 |
| August 2022 | 526 |
| September 2022 | 742 |
| October 2022 | 833 |
| November 2022 | 820 |
| December 2022 | 615 |
| January 2023 | 666 |
| February 2023 | 751 |
| March 2023 | 832 |
| April 2023 | 813 |
| May 2023 | 862 |
| June 2023 | 617 |
| July 2023 | 728 |
| August 2023 | 743 |
| September 2023 | 842 |
| October 2023 | 916 |
| November 2023 | 1,019 |
| December 2023 | 810 |
| January 2024 | 993 |
| February 2024 | 1,086 |
| March 2024 | 1,405 |
| April 2024 | 1,521 |
| May 2024 | 1,363 |
| June 2024 | 808 |
Email alerts
Citing articles via.
- Contact RSTMH
- Recommend to your Library
Affiliations
- Online ISSN 1876-3405
- Copyright © 2024 Royal Society of Tropical Medicine and Hygiene
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- DOI: 10.1111/j.1744-618X.2010.01159.x
- Corpus ID: 22024437
Case study: child with global developmental delay.
- Pearline Okumakpeyi , M. Lunney
- Published in International Journal of… 1 July 2010
One Citation
Nursing diagnoses and theoretical frameworks in neonatal units: a literature review., 2 references, the redefinition of failure to thrive from a case study perspective., identifying patterns of developmental delays can help diagnose neurodevelopmental disorders, related papers.
Showing 1 through 3 of 0 Related Papers
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Exposure of children with developmental delay to social determinants of poor health: cross-sectional case record review study
Affiliation.
- 1 Centre for Disability Research and Policy, University of Sydney, Sydney, NSW, Australia.
- PMID: 24797435
- DOI: 10.1111/cch.12144
Background: Research on child development in general has highlighted the importance that the family environment plays in mediating the pathway between exposure to low socio-economic position (SEP) and child well-being. While child developmental models in intellectual disability have highlighted the interplay between social context, family environment and child development, little empirical work has attempted to formally evaluate the evidence in support of specific mediating pathways between low SEP and child outcomes.
Methods: Secondary analysis of cross-sectional confidentialized needs analysis data collected in three Primary Care Trusts in England covering a total population of 1.25 million people. Case record reviews were undertaken for 46 023 households, 2236 (4.9%) of which contained a child in the target age range with developmental delay.
Results: Children with developmental delay, when compared with their non-disabled peers, were at significantly increased risk of poorer health outcomes and of being exposed to a wide range of social determinants of poor health. Controlling for between-group differences in exposure to social determinants of poor health reduced the risk of developmental delay being associated with poorer health outcomes by 45% for behaviour problems and 89% for risk of significant harm. For children with developmental delay, parenting difficulties appears to play a particularly significant role in partially mediating the effects of low SEP.
Conclusions: The findings of the present study point to the potential effectiveness of family-focused early intervention to prevent the emergence and escalation of behavioural difficulties and health problems in children with developmental delay.
Keywords: developmental delay; developmental disability; health inequalities; parenting; poverty; social determinants of health.
© 2014 John Wiley & Sons Ltd.
PubMed Disclaimer
Similar articles
- Maltreatment in Infancy: A Developmental Perspective on Prevention and Intervention. Harden BJ, Buhler A, Parra LJ. Harden BJ, et al. Trauma Violence Abuse. 2016 Oct;17(4):366-86. doi: 10.1177/1524838016658878. Trauma Violence Abuse. 2016. PMID: 27580663 Review.
- The developmental health of children of parents with intellectual disabilities: cross sectional study. Emerson E, Brigham P. Emerson E, et al. Res Dev Disabil. 2014 Apr;35(4):917-21. doi: 10.1016/j.ridd.2014.01.006. Epub 2014 Jan 29. Res Dev Disabil. 2014. PMID: 24485469
- The social context of parenting 3-year-old children with developmental delay in the UK. Emerson E, Graham H, McCulloch A, Blacher J, Hatton C, Llewellyn G. Emerson E, et al. Child Care Health Dev. 2009 Jan;35(1):63-70. doi: 10.1111/j.1365-2214.2008.00909.x. Epub 2008 Nov 19. Child Care Health Dev. 2009. PMID: 19054011
- Behaviour and emotional problems in toddlers with pervasive developmental disorders and developmental delay: associations with parental mental health and family functioning. Herring S, Gray K, Taffe J, Tonge B, Sweeney D, Einfeld S. Herring S, et al. J Intellect Disabil Res. 2006 Dec;50(Pt 12):874-82. doi: 10.1111/j.1365-2788.2006.00904.x. J Intellect Disabil Res. 2006. PMID: 17100948
- Children with disabilities: a longitudinal study of child development and parent well-being. Hauser-Cram P, Warfield ME, Shonkoff JP, Krauss MW, Sayer A, Upshur CC. Hauser-Cram P, et al. Monogr Soc Res Child Dev. 2001;66(3):i-viii, 1-114; discussion 115-26. Monogr Soc Res Child Dev. 2001. PMID: 11677873 Review.
- Surveying Early Intervention Providers to Identify Opportunities for Workforce Support to Strengthen Family-Centered Care. Griffith SF, Magariño LS, Pedraza FDM, Frazier SL, Berkovits MD, Bagner DM. Griffith SF, et al. Infants Young Child. 2023 Oct-Dec;36(4):314-332. doi: 10.1097/iyc.0000000000000247. Infants Young Child. 2023. PMID: 38107032
- Desirable but not feasible: Measures and interventions to promote early childhood health and development in marginalized Roma communities in Slovakia. Filakovska Bobakova D, Chovan S, Bosakova L, Koky R, de Kroon MLA, Dankulincova Veselska Z. Filakovska Bobakova D, et al. Front Public Health. 2022 Oct 5;10:942550. doi: 10.3389/fpubh.2022.942550. eCollection 2022. Front Public Health. 2022. PMID: 36276342 Free PMC article.
- Risk of Mental Health Problems in Children and Youths Following Concussion. Ledoux AA, Webster RJ, Clarke AE, Fell DB, Knight BD, Gardner W, Cloutier P, Gray C, Tuna M, Zemek R. Ledoux AA, et al. JAMA Netw Open. 2022 Mar 1;5(3):e221235. doi: 10.1001/jamanetworkopen.2022.1235. JAMA Netw Open. 2022. PMID: 35254429 Free PMC article.
- Working memory training in children with borderline intellectual functioning and neuropsychiatric disorders: a triple-blind randomised controlled trial. Roording-Ragetlie S, Spaltman M, de Groot E, Klip H, Buitelaar J, Slaats-Willemse D. Roording-Ragetlie S, et al. J Intellect Disabil Res. 2022 Jan;66(1-2):178-194. doi: 10.1111/jir.12895. Epub 2021 Nov 10. J Intellect Disabil Res. 2022. PMID: 34755919 Free PMC article. Clinical Trial.
- Supporting Child Development Through Parenting Interventions in Low- to Middle-Income Countries: An Updated Systematic Review. Zhang L, Ssewanyana D, Martin MC, Lye S, Moran G, Abubakar A, Marfo K, Marangu J, Proulx K, Malti T. Zhang L, et al. Front Public Health. 2021 Jul 16;9:671988. doi: 10.3389/fpubh.2021.671988. eCollection 2021. Front Public Health. 2021. PMID: 34336768 Free PMC article.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Ovid Technologies, Inc.
Other Literature Sources
- scite Smart Citations
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Skip to content
- Skip to navigation
About developmental delay
When young children are slower to develop physical, emotional, social, communication and thinking skills than expected, it’s called developmental delay.
Developmental delay can show up in the way children move, communicate, think and learn, or behave with others. When more than one of these areas is affected, it might be called global developmental delay.
Developmental delay might be short term or long term.
Long-term developmental delays are also called developmental disabilities. Examples include learning disabilities , cerebral palsy and intellectual disability .
Usually health professionals use the term ‘developmental delay’ only until they can work out what’s causing the delay. If and when they find the cause, they’ll use a term that better explains the child’s condition.
Signs of developmental delay
Children grow and develop at different speeds. If you’re worried about whether your child’s development is ‘normal’, it might help to know that ‘normal’ varies a lot.
But as a general guide, you might be concerned about developmental delay if you notice that, over several months, your child isn’t developing motor, social or language skills at the same rate as other children the same age.
Worried about developmental delay: what to do
As a parent, you know your child better than anyone else.
If you’re concerned about your child’s development, trust your instincts and talk to your GP , child and family health nurse or paediatrician .
These health professionals can diagnose developmental delay after assessing your child. Or they can refer you to other professionals who can help.
Support for children with developmental delay
If your child has a suspected or confirmed developmental delay diagnosis, early intervention can make a difference.
Early intervention includes therapies, supports, education and so on to help children develop the skills they need to take part in everyday activities. Sometimes children who get early intervention need less or no support as they get older.
If your child has developmental delay, you and your child might work with some or all of the following professionals depending on your child’s needs:
- audiologist
- occupational therapist
- physiotherapist
- psychologist
- social worker
- special education teacher
- speech pathologist .
The National Disability Insurance Scheme (NDIS) might support your child with developmental delay, as well as you and your family. Our guide to the NDIS and early intervention explains how.
Living with developmental delay
Like other children, children with developmental delay keep learning. But they take longer to develop new skills, and they might learn in slightly different ways from other children.
For example, most children can learn skills quickly and by example. But children with developmental delay might need to be shown skills in smaller, simpler steps. They might also need more time and opportunities to practise skills.
At preschool or school, your child might need extra support to do well. It’s always a good idea to talk with preschools and schools about your child’s needs. And if your child has a disability diagnosis, you might be able to get funding and other school support .
If your child has developmental delay, it’s easy to get caught up in supporting their needs. But it’s important to look after your own wellbeing and get support for yourself too. If you’re physically and mentally well, you’ll be better able to care for your child.
Causes of developmental delay
Many things can cause children to develop more slowly than others.
Developmental delay might happen because of genetic conditions like Down syndrome or because of complications during pregnancy and birth, like premature birth or fetal alcohol spectrum disorder .
Other causes of short-term delays include physical illness, long periods in hospital, and family stress.
In many cases, the cause of developmental delay isn’t known.
ONLY TWO WEEKS LEFT! The To Be Loved offer features 50% off SSP Training and resources from Dr. Frank Anderson! Learn more →

Case Study: Autism, developmental delays, attachment challenges
Associate Name : Doreen Hunt
Name of Organization : Children’s Therapy of Woodinville, P.L.L.C.
Age/Gender of Client: 5-year-old male with autism, developmental delays, attachment challenges
Background: Thomas is a five-year old who was adopted at the age of 9 months, from a developing country where he spent time in an orphanage and foster care. He suffers from attachment challenges, Autism, and global developmental delays, and was a very angry, inflexible, detached and neurologically disorganized boy when we met a year ago. He has done several iLs Focus programs intensely in clinic-combined with home program and has made beautiful changes from them.
Presenting Problem: Thomas displays behavioral dysregulation, auditory processing difficulties, anxiety with change or unexpected transitions. He has poor social engagement with his brother or peers, obtains brief eye contact, and no two-way communication.
Therapeutic Goals :
Improve self-regulation and ability to engage in purposeful activity (play, learning/pre-academic); Increase happiness and positive interaction with others.
Improve Social engagement and Communication
Improve Fine Motor and Visual Motor development
Improve Visual Perceptual skills
Improve Visual tracking, eye-hand coordination
iLs Program Used: Safe and Sound Protocol (SSP).
Thomas has completed the Sensory Motor, Concentration & Attention, Reading & Auditory Processing in the past.
He seemed an ideal candidate for the SSP, so he completed the program during a break between iLs programs.
Summary of Changes:
BBC Sensory Scales: Auditory Processing – Primary Caregiver Version
This tool assesses behaviors related to auditory processing. Responses are on a 4-point Likert scale (4=Almost Always; 1= Almost Never)
Q: How often does your child hold his/her hands over or plug his/her ears?
A: Pre: 2; Post:1.
Q: How often does your child seem overly aware, distracted, or disturbed by continuous noise in the environment (for example, TV, stereo)?
A: Pre: 4; Post: 1
Q: How often does your child take a long time to respond when spoken to, even to familiar voices?
A: Pre: 4; Post: 2
Q: How concerned are you about the above behaviors?
A: Pre: Very concerned: Post: Moderately concerned
Parents’ Comments:
Thomas’ mother wrote a grateful letter describing her observations since he completed the SSP.
A few highlights are:
- Thomas is now communicating on a deeper and more emotional level
- His language, intonation and eye contact have all improved
- He can think more clearly and connections are made more quickly
Below are segments from the three-page letter:
“ At times, over the last month and a half, I have asked myself if I am making this up. If somehow the changes I’ve observed are the result of wishful thinking. They are not. Something is changing in the connections he is making. The awareness of the world around him. The stress level of interactions with people and his environment….
The biggest change I see is that he wants to communicate. Not just ask for things, but communicate. He has always been an affectionate child. But now he seems aware that we want love from him as much as he wants it from us. It’s like bits of his brain can make connections now where before there was only static…
I believe this program allowed him to rapidly make connections that were laying dormant in his brain. That information that was stored can now be accessed easier, or simply accessed at all. Something changed. Things we had been working and working on are suddenly there at a greater rate. That I am sure is real. “
Therapist’s Comments :
Thomas is more ‘connected to others and teachable’. He greets familiar people unprompted; he is quiet instead of constantly talking about the information racing through his “noisy brain”. He will now sing along, count along to 20 (mostly accurate) and can name most colors correctly. His attention span has increased and he’ll engage positively in activities requiring visual motor, fine motor skills and puzzles (instead of screaming “NO!”). He is happy, smiles frequently and is able to transition easily between activities.
Conclusions and Recommendations:
Thomas’ response to the SSP was very positive. And the progress he has shown in the areas of physiological state, behavioral regulation and social communication have affected his engagement in the world around him, his family and his classroom community.
Thomas will continue with Occupational Therapy and iLs Focus Programs. He is currently doing the Reading and Auditory Processing Program for the 2 nd time. He has very good “body organization” skills (balance, motor planning). He loves to skip, throw and catch balls (except for delayed visual fixation and tracking skills); He rides a 2 wheel bike with training wheels.

Professionals
Events Calendar
Safe and Sound Protocol
Focus System
Rest and Restore Protocol
Return/Cancel
Unyte Provider Criteria
We’re Hiring
We’ll send you the free e-book “Why Effective Processing and Regulation are the Essential Foundation for Health.”

Statements on Unyte’ site have not been evaluated by the FDA, and the products and services are not intended to diagnose, treat, cure, or prevent any disease. Unyte products are not medical devices or medical instruments.
It is solely the responsibility of each user (whether a professional user or personal user) to determine whether the products and/or services may be beneficial for their patients/clients or themselves.
We're not around right now. But you can send us an email and we'll get back to you, asap.
Start typing and press Enter to search
Occipital encephalocele: a retrospective analysis and assessment of post-surgical neurodevelopmental outcome
- Published: 24 June 2024
Cite this article

- Soumen Kanjilal 1 ,
- Pawan Kumar Verma 1 ,
- Sreyash Rai 2 ,
- Ashutosh Kumar 1 ,
- Kamlesh Singh Bhaisora 1 ,
- Ved Prakash Maurya 1 ,
- Kuntal Kanti Das 1 ,
- Anant Mehrotra 1 ,
- Arun Kumar Srivastava 1 &
- Awadhesh Kumar Jaiswal 1
Encephalocele represent a group of disorders which is characterised by extracranial herniation of the leptomeninges, brain, and CSF through a structural defect in the cranium. They are usually associated with other intracranial anomalies which may impact the neurological development.
This study aimed to assess the predictors of neurological development of patients undergone surgical excision of occipital encephalocele.
All patients with occipital encephaloceles operated over the last decade (2012–2022). The sac size, presence of hydrocephalous, and associated anomalies were noted. The biopsy of these patients were reviewed and categorised as those which contains mature neural tissue and those without. The neurological outcomes were assessed by social, language, cognitive, and motor milestone and has been stratified into no delay, mild (1 of 4), moderate (2 or 3 of 4), and severe development delay (4 of 4).
Total of 35 patients were included with median age of 10 months (IQR = 5–20 months). Fifteen (42.9%) patients had sac size of ≥ 5 cm, and 23 (65.7%) patients had mature neural tissues on biopsy. The median follow-up period was 6.4 years (IQR = 4.38–10.65) years. Seventeen (49.6%) patients had moderate to severe developmental delay. The sac size of ≥ 5 cm (AOR = 33.5; 95%CI = 3.35–334.8) ( p = 0.003) and presence of mature neural content in the sac (AOR = 13.32; 95%CI = 1.1–160.36) ( p = 0.041) were associated with significant neurodevelopmental delay.
The presence of a large sac of ≥ 5 cm and the presence of mature neural tissues on histopathological specimen of patients with encephalocele point towards the possibility of poor neurological development.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow.
Mahajan C, Rath GP, Dash HH, Bithal PK (2011) Perioperative management of children with encephalocele: an institutional experience. J Neurosurg Anesthesiol 23(4):352–356. https://doi.org/10.1097/ANA.0b013e31821f93dc
Article PubMed Google Scholar
Abdel-Aziz M, El-Bosraty H, Qotb M et al (2010) Nasal encephalocele: endoscopic excision with anesthetic consideration. Int J Pediatr Otorhinolaryngol 74(8):869–873. https://doi.org/10.1016/j.ijporl.2010.04.015
Thu A, Kyu H (1984) Epidemiology of frontoethmoidal encephalomeningocoele in Burma. J Epidemiol Community Health 38(2):89–98. https://doi.org/10.1136/jech.38.2.89
Article Google Scholar
Suwanwela C (1972) Geographical distribution of fronto-ethmoidal encephalomeningocele. Br J Prev Soc Med 26(3):193–198. https://doi.org/10.1136/jech.26.3.193
Article CAS PubMed PubMed Central Google Scholar
Tirumandas M, Sharma A, Gbenimacho I et al (2013) Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst 29(5):739–744. https://doi.org/10.1007/s00381-012-1998-z
Barnes L (2000) Surgical pathology of the head and neck. CRC Press
Google Scholar
Yindeedej V, Sungpapan R, Duangprasert G, Noiphithak R (2023) The outcome of surgical management for the patient with giant occipital encephalocele: a case illustration and systematic review. Childs Nerv Syst 39(8):2161–2167. https://doi.org/10.1007/s00381-023-05934-z
CDC’s Developmental milestones. Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/actearly/milestones/index.html . Accessed October 27, 2022
Baradaran N, Nejat F, Baradaran N, El Khashab M (2009) Cephalocele: report of 55 cases over 8 years. Pediatr Neurosurg 45(6):461–466. https://doi.org/10.1159/000277622
Protzenko T, dos Santos Gomes Junior SC, Bellas A, Salomão JFM (2021) Hydrocephalus and occipital encephaloceles: presentation of a series and review of the literature. Childs Nerv Syst 37(11):3437-3445. https://doi.org/10.1007/s00381-021-05312-7
Warf BC (2011) Hydrocephalus associated with neural tube defects: characteristics, management, and outcome in sub-Saharan Africa. Childs Nerv Syst 27(10):1589–1594. https://doi.org/10.1007/s00381-011-1484-z
Da Silva SL, Jeelani Y, Dang H, Krieger MD, McComb JG (2015) Risk factors for hydrocephalus and neurological deficit in children born with an encephalocele. J Neurosurg Pediatr 15(4):392–398. https://doi.org/10.3171/2014.10.PEDS14192
Article PubMed PubMed Central Google Scholar
Mahapatra AK (2011) Giant encephalocele: a study of 14 patients. Pediatr Neurosurg 47(6):406–411. https://doi.org/10.1159/000338895
Article CAS PubMed Google Scholar
Refaee EAE, Refaat MI, Reda M (2018) Incidence of secondary hydrocephalus after excision of huge encephaloceles in neonates: case study. J Neurol Surg A Cent Eur Neurosurg 79(1):15–18. https://doi.org/10.1055/s-0036-1597548
Rehman L, Farooq G, Bukhari I (2018) Neurosurgical interventions for occipital encephalocele. Asian J Neurosurg 13(2):233–237. https://doi.org/10.4103/1793-5482.228549
Kankam SB, Tavallaii A, Mohammadi E, Nejat A, Habibi Z, Nejat F (2022) The neurodevelopmental outcomes of children with encephalocele: a series of 102 patients. J Neurosurg Pediatr 31(2):151–158. https://doi.org/10.3171/2022.10.PEDS22304
Ganjoo P, Kaushik S (1993) An unexpected complication of encephalocele repair [published correction appears in J Neurosurg Anesthesiol 1994 Jul; 6(3):228]. J Neurosurg Anesthesiol 5(2):137–138. https://doi.org/10.1097/00008506-199304000-00023
Alexiou GA, Sfakianos G, Prodromou N (2010) Diagnosis and management of cephaloceles. J Craniofac Surg 21(5):1581–1582. https://doi.org/10.1097/SCS.0b013e3181edc3f6
Lo BW, Kulkarni AV, Rutka JT et al (2008) Clinical predictors of developmental outcome in patients with cephaloceles. J Neurosurg Pediatr 2(4):254–257. https://doi.org/10.3171/PED.2008.2.10.254
Aslan A, Eser O, Doğru O, Aktepe F, Yurumez Y, Fidan H (2007) Occipital mega encephalocele associated with acute inflammation. Pediatr Neurosurg 43(1):65–66. https://doi.org/10.1159/000097530
Martínez-Lage JF, Poza M, Sola J et al (1996) The child with a cephalocele: etiology, neuroimaging, and outcome. Childs Nerv Syst 12(9):540–550. https://doi.org/10.1007/BF00261608
Junior JHMF, Junior SP, Pustilnik HN et al (2024) Neurosurgical intervention for the Meckel-Gruber syndrome: a systematic review. Childs Nerv Syst:. https://doi.org/10.1007/s00381-024-06346-3
Gunasekaran PK, Jindal P, Laxmi V et al (2024) Knobloch syndrome — triad of occipital encephalocele, retino-choroidal detachment and epilepsy. Indian J Pediatr 91(3):306. https://doi.org/10.1007/s12098-023-04861-w
Download references
Author information
Authors and affiliations.
Department of Neurosurgery, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, UP, India
Soumen Kanjilal, Pawan Kumar Verma, Ashutosh Kumar, Kamlesh Singh Bhaisora, Ved Prakash Maurya, Kuntal Kanti Das, Anant Mehrotra, Arun Kumar Srivastava & Awadhesh Kumar Jaiswal
Department of Neurosurgery, Ram Manohar Lohia Institute of Medical Sciences, Lucknow, UP, India
Sreyash Rai
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: [SK, PKV]Methodology: [SK, PKV, SR] Formal analysis and investigation: [SK, PKV, SR] Writing—original draft preparation: [SK, PKV, SR] Writing—review and editing: [SK, PKV, AK, VPM] Funding acquisition: [none] Resources: [SK, PKV, AK, VPM, KSB] Supervision: [PKV, AM, AKS, AKJ].
Corresponding author
Correspondence to Pawan Kumar Verma .
Ethics declarations
Declarations.
I, Dr Pawan Kumar Verma, certify that this manuscript is a unique submission and is not being considered for publication, in part or in full, with any other source in any medium.
Ethics approval
This is an observational study. The Institutional Ethics Committee has confirmed that no ethical approval is required.
Consent to participate
As this a retrospective study, the patients’ consent to participate were waived off by the Institutional Ethics Committee.
Consent for publication
The manuscript does not contain any information from which a patient’s identity could be disclosed.
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Kanjilal, S., Verma, P.K., Rai, S. et al. Occipital encephalocele: a retrospective analysis and assessment of post-surgical neurodevelopmental outcome. Childs Nerv Syst (2024). https://doi.org/10.1007/s00381-024-06506-5
Download citation
Received : 03 April 2024
Accepted : 16 June 2024
Published : 24 June 2024
DOI : https://doi.org/10.1007/s00381-024-06506-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Encephalocele
- Hydrocephalous
- Neurodevelopment
- Cognitive delay
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.176(1); 2002 Jan
Evidence-Based Case Reviews
Investigation of children with “developmental delay”, louise hartley.
1 Dubowitz Neuromuscular Centre Hammersmith Hospital Campus Du Cane Rd London W12 0NN UK
Alison Salt
2 Neurodisability Service Institute of Child Health Mecklenburgh Square London WC1N 2AP
Jon Dorling
3 Jenny Lind Children's Department Norfolk and Norwich Hospital Brunswick Rd Norwich NR1 3SR UK
Paul Gringras
4 Imperial College of Science, Technology and Medicine London, UK
A 7-year-old boy is referred to you with concerns about developmental delay. On assessment, he is found to have moderate mental retardation (IQ of 50) but no remarkable physical findings. His parents are considering having another child, and they wonder what caused the retardation in their first child and whether it is likely to recur in future offspring.
Developmental delay is a common problem in pediatrics, with an estimated population prevalence as high as 10%. 1 , 2 , 3 , 4 The etiology includes various genetic and environmental processes, with the most common causes being Down syndrome and the fragile X chromosome. The proportion of children with severe mental retardation found to have an organic cause is reported as 55% to 57%. 5 , 6 , 7 No consensus exists on the choice of investigations for developmental delay, with clinicians using a wide variety of investigations. 8 The example we give in this article is intended to illustrate the process used to evaluate developmental delay in a variety of circumstances, recognizing that the specifics will vary according to the clinical situation.
This scenario raises many clinical questions. You wish to use an evidence-based approach, so you frame your questions to maximize the yield from searching and look first for high-quality systematic reviews and evidence-based practice guidelines to answer your questions. However, because most systematic reviews address issues of therapy, no reviews or guidelines are found that address your questions, which are mostly related to the probability of particular causes of developmental delay. You go to MEDLINE and EMBASE searches to try to answer these questions ( box 1 ).
Focusing a literature search
| In a 7-year-old boy (population) with mental retardation who does not have a diagnosis responsive to specific interventions (exposure), what is the risk of having the fragile X chromosome (outcome)? Search: MEDLINE 1966 to present and EMBASE (Winspirs); search terms (text or MeSH heading) In a boy with mental retardation (population) and no dysmorphic features (negative test result), what is the risk of having the fragile X chromosome (outcome)? In a boy with mental retardation (population) with dysmorphic features (positive test result), what is the risk of having the fragile X chromosome (outcome)?Search: MEDLINE (PubMed Clinical Queries); search term —click on “diagnosis” and “specificity” In a boy with mental retardation (population), does knowing the diagnosis of fragile X chromosome (exposure) improve the parents' ability to plan and cope (outcome)?Search: MEDLINE (PubMed); search terms |
You want to know the best estimate for the prevalence of fragile X chromosome in the general population and the best estimate for the prevalence of fragile X chromosome among children with learning disabilities. From the 133 articles found in your search, 10 described population-based studies of the prevalence of fragile X chromosome that were performed since the cloning of the fragile X mental retardation gene ( FMR1 ) in 1991. Of the 10 studies, only 2 meet most of the criteria for high-quality prevalence studies ( box 2 ).
Criteria for appraising the quality of prevalence studies
You decide to start with the study of Murray et al because it was limited to boys, used a population-based sample, and tested only those aged 18 years or younger. 9 Neither the case definition for mental retardation nor the distribution of IQs in the population is stated in the study, and the low prevalence of fragile X chromosome suggests that this is a relatively lower risk group (higher IQ) than that used in other studies. Only 70% of children with specia educational needs were tested, and no information is available about nonresponders. Because the prevalence estimate of fragile X chromosome from this study would be affected if children with the chromosome were less or more likely to participate, you try varying the prevalence in the nonparticipating group to half or double that of the participating group. This gives a prevalence range for the overall population of between 1 in 3,990 and 1 in 6,171, which is reassuring because these numbers overlap with estimates from other studies identified by your search (de Vries et al, 10 1/6,045; and Turner et al, 11 1/5,000). Applying the same assumptions to the population of learning disabled boys in this study gives a range of 1 in 162 to 1 in 250 (0.6%-0.4%).
In the study by de Vries et al, the learning-disabled group was stratified into mild and moderate-to-severe learning difficulty. 10 Unfortunately, the authors excluded those who already had a diagnosis of fragile X; these cases must be included for accurate prevalence figures. If the prevalence of fragile X chromosome among the nonresponders is similar to that among the responders, then adding those known to have fragile X and new diagnoses to the numerator gives an estimated prevalence of fragile X chromosome for mild mental retardation of 1 in 50, with 1 in 40 for moderate to severe mental retardation. In your 7-year-old child who has moderate intellectual impairment, you estimate the probability of his having the fragile X syndrome as somewhere between 1 in 40 and 1 in 250. You would, therefore, need to test between 40 and 250 children to find 1 child with the fragile X chromosome.
You next consider the usefulness of dysmorphic features in ruling in or ruling out the diagnosis of fragile X syndrome. A search nets 33 articles regarding dysmorphic features in those with the fragile X chromosome. From the abstracts, two articles were found that used a combination of physical and behavioral features to select who, among a group of mentally retarded children, has the highest probability of testing positive for the fragile X chromosome, using molecular testing for the FMR1 gene. 10 , 12
The article by Giangreco et al refines previously defined checklists of phenotypic characteristics associated with the fragile X chromosome into a six-item checklist with a scoring system, shown in table 1 . 12
Phenotypic characteristics associated with fragile X: checklist and scoring system *
| Mental retardation | IQ > 85 | IQ 70-85 | IQ < 70 |
| Family history | None | Maternal female with psychiatric disorder | Maternal history of X-linked mental retardation |
| Elongated face | Not present | Somewhat | Present |
| Large or prominent ears | Not present | Somewhat | Present |
| Attention deficit hyperactivity disorder | Not present | Hyperactivity | Present |
| Autistic-like behavior | Not present | 1 behavior | >1 behavior |
In this study, a score of 5 or more of a maximum of 12 was found to identify all children who had the fragile X chromosome. Using the identification of these features as a “diagnostic test” for fragile X chromosome, with molecular testing as the gold standard, you use the guidelines on assessing diagnostic and screening tests summarized in box 3 . 13
Criteria for appraising studies of diagnostic tests
In this retrospective study, the molecular polymerase chain reaction (PCR) technique was used in all patients, but the authors do not state whether those applying the diagnostic test were blind to the (PCR-defined) fragile X status of the patients. If the assessors already knew the “answer,” the potential for biased assessment is high. The scoring system is clear; however, some of the physical features, such as long face and large or prominent ears, are subjective, and no objective measurements are given. Some of the behavioral characteristics may also be open to interpretation. The provision of genetic testing at the time of the study may have been unique to this study or this location; if so, this could have attracted a highly selected group of children, and the results may not be generalizable. Because the clinical features of fragile X syndrome are well known to clinicians, and all children were referred for testing, the children referred are likely to have had a high prevalence of the chromosome. The data necessary for calculating likelihood ratios (LRs) presented in the article are shown in table 2 .
Calculation of likelihood ratios
| 5 | 12 | 129 | for positive test: (12/12)/(129/323) = 2.5 |
| <5 | 0 | 194 | For negative test: (0/12)/(194/323) = 0 |
| Total | 12 | 323 | |
| PCR = polymerase chain reaction | |||
In this study, a negative result (those with a score <5) will effectively rule out a diagnosis of the fragile X chromosome because it is a highly sensitive test. That is, children with a low score on the clinical assessment are unlikely to have the chromosome. However, the calculated LR of a positive test in this study is 2.5. In general, LRs between 2 and 5 generate only small changes in probability. Indeed, if the pretest probability is 3.5%, then a positive test increases the probability of having the fragile X chromosome to only 8.2%.
de Vries et al studied a prospectively collected sample with examiners blind to the fragile X result 10 using a similar scoring system: the phenotypic criteria described by Laing et al. 14 Scores were divided into three groups: low risk, when dysmorphic features suggested another diagnosis; medium risk, in the absence of dysmorphic features; and high risk, in the presence of fragile X chromosome characteristics. This sample contained many adults in whom the phenotype is more characteristic than in children. Despite this, the outcome was impressive. None of the low- or medium-scoring males had the fragile X chromosome, with all those who had the chromosome scoring in the high range. Of course, this did not mean that all of the high scorers had the syndrome. The LR for a high score was 10, and the LR for a low or medium score was 0. The high LR for a positive test confirms your suspicion that the patients in their group showed more distinct features. This indicates how test performance, including LRs, can vary when a test is applied to different groups.
Although neither of these studies is ideal, both show that children who do not have the fragile X chromosome can be correctly identified clinically (decreasing the number of molecular tests that are done) and that having clinically identified features increases the likelihood of a positive genetic test but does not confirm the diagnosis. If our group were similar to that described by Murray et al, with a prevalence of 0.4% (the lowest possible estimate of prevalence), and the diagnostic test performed in the same way as described by Giangreco et al, with an LR of 2.5, the posttest probability of having the fragile X chromosome would have increased from 0.5% to 1.0%. 9 , 12 However, the high sensitivity of the test suggests that instead of testing 250 children before finding 1 child with the fragile X chromosome, you could exclude 150 of those children (60%) from testing with minimal risk of missing a case. de Vries et al suggest that in a group with moderate or severe mental retardation, a higher prevalence may be expected (the highest estimate being 3.3%). 10 Excluding those with a known diagnosis from the denominator gives an estimated prevalence of about 4%, so the posttest probability is, therefore, increased to 10%. Under these circumstances, for every 24 children at risk, 14 could be excluded from testing, and 1 of the remaining 10 would have the fragile X chromosome.
You wonder about the benefit to the parents of knowing their son's diagnosis but are not able to find randomized trials or cohort studies directly relating to the diagnosis of fragile X chromosome. A table is found that provides a framework with the different values associated with making a diagnosis in children with developmental delay ( table 3 ).
Considerations for genetic diagnostic testing in developmentally delayed children *
| Treatment—such as thyroid replacement therapy if hypothyroid | Harm from testing—the pain of venepuncture or the risk of general anesthetic for some investigations |
| Genetic counseling—such as discussion of chromosome abnormality with extended family | False-positive and false-negative results |
| Explanation—for parents and family, even if no treatment identified | Financial costs |
| Prognostic information | |
| Research—if an investigation may increase understanding of the mechanisms and/or genetics of the developmental delay |
Resolution of the scenario You are now able to estimate the probability of the patient's having the fragile X chromosome as somewhere between 1 in 40 and 1 in 250 and would, therefore, need to test between 40 and 250 children to find 1 child with the chromosome abnormality. If this child has a score of less than 5 for the features described by Giancreco et al, you feel confident in ruling out the chromosome abnormality and not proceeding to molecular testing. 12 Because there is no well-established treatment option, the direct benefit to the patient of making a diagnosis is marginal. However, the use of this clinical diagnostic test avoids subjecting some children to an unnecessary blood test and spares some parents the anxiety of awaiting the results, as well as the unnecessary expenditure. In addition, the resolution of diagnostic uncertainty can provide much relief and stop further investigations for a cause of developmental delay. As more information on the prognosis of this condition becomes available, parents and patients may benefit from this knowledge. Also, for the parents and relatives, the identification of female carriers may allow an informed choice regarding at-risk pregnancies.
Within the limitations of current evidence, some information is now available on the range of the possible prevalence of the fragile X chromosome in different groups, and some understanding of how specific features of the fragile X syndrome may influence your decision making. The decisions that are made depend on the group from which the child comes and the values that the tester and the parents put on having a diagnosis versus the disadvantages of unnecessary testing. In this article, we provide a model for thinking through the issues involved in the investigation of developmental delay and a way of incorporating evidence into this process. We have chosen a common example to illustrate the process, that of fragile X syndrome, the second most common cause of mental retardation after Down syndrome. The prevalence of a particular disorder in different patient groups will influence the outcome of any diagnostic investigations. This method is generalizable to other causes of developmental delay.

Down syndrome is one of the more common causes of developmental delay
Hattie Young/Science Photo Library

Fragile X syndrome: the benefits of testing include diagnostic certainty. (Courtesy of the Fragile X Society, www.fraxa.org )
Competing interests: None declared
This article was edited by Virginia A Moyer of the Department of Pediatrics, University of Texas Medical Center at Houston. Articles in this series are based on chapters from Moyer VA, Elliott EJ, Davis RL, et al, eds. Evidence-Based Pediatrics and Child Health . London: BMJ Books; 2000.
Summary points In a 7-year-old child who has moderate intellectual impairment:
- The likely prior probability of having fragile X syndrome is between 1 in 40 and 1 in 250 (ie, to find 1 child with the fragile X chromosome, between 40 and 250 children would need to be tested)
- Currently available evidence shows that when a scoring system based on physical and behavioral features is used, a diagnosis of fragile X syndrome can be confidently ruled out in those with low scores
- Decisions that are made about testing will depend on the population from which the child comes and the values that the tester and the parents put on having a diagnosis versus the disadvantages of unnecessary testing
- The benefits of using this “clinical diagnostic test” include preventing children from being subjected to an unnecessary blood test, sparing parents the anxiety of awaiting the results, and reducing the cost of investigation
- The benefits of testing for the fragile X chromosome include the resolution of diagnostic uncertainty, the prevention of further investigations, and the identification of female carriers
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Relationship between maternal consumption of fermented foods and the development of the offspring at the age of 3 years: The Japan Environment and Children’s Study
Roles Conceptualization, Investigation, Writing – original draft
Affiliation Faculty of Medicine, Department of Pediatrics, University of Toyama, Toyama, Japan
Roles Writing – review & editing
* E-mail: [email protected]
Affiliations Faculty of Medicine, Department of Pediatrics, University of Toyama, Toyama, Japan, Toyama Regional Center for JECS, University of Toyama, Toyama, Japan
Roles Data curation, Formal analysis, Writing – review & editing
Affiliation Toyama Regional Center for JECS, University of Toyama, Toyama, Japan
Affiliations Toyama Regional Center for JECS, University of Toyama, Toyama, Japan, Faculty of Medicine, Department of Public Health, University of Toyama, Toyama, Japan
Current address: Department of Public Health, Graduate School of Medicine, Gunma University, Maebashi, Gunma, Japan
Affiliation Pediatric Allergy Center, Toyama Red Cross Hospital, Toyama, Japan
¶ The names of the study group members are provided at the end of the manuscript
- Hiroko Hirai,
- Tomomi Tanaka,
- Kenta Matsumura,
- Akiko Tsuchida,
- Kei Hamazaki,
- Yuichi Adachi,
- Hidekuni Inadera,
- the Japan Environment and Children’s Study Group

- Published: June 21, 2024
- https://doi.org/10.1371/journal.pone.0305535
- Reader Comments
It is well known that maternal diet affects the development of offspring. Herein, the relationship between maternal intake of fermented foods during pregnancy and offspring development was investigated.
The diet of 103,060 pregnant women at >4 months of gestation who were enrolled in the Japan Environment and Children’s Study was analyzed. Their intake levels of fermented soybeans ( miso and natto ), yogurt, and cheese were investigated. The developmental status of the offspring at 3 years of age was assessed using the Ages and Stages Questionnaires (ASQ-3). Multivariable logistic regression analysis was performed to determine the risk of maternal intake levels of the fermented foods associated with subsequent developmental delay in the offspring.
Intake of cheese was associated with a reduced risk of child developmental delay in all intake level groups from the second quartile onward. Intakes of miso and yogurt were associated with a reduced risk of developmental delay in communication skills in the fourth quartile. There was no association between intake of natto and developmental delay.
Maternal consumption of fermented foods during pregnancy may reduce the risk of later developmental delay in offspring. It is therefore important to review the mother’s diet for fermented foods during pregnancy. However, further studies are warranted to evaluate the factors influencing the association between diet and offspring development.
Citation: Hirai H, Tanaka T, Matsumura K, Tsuchida A, Hamazaki K, Adachi Y, et al. (2024) Relationship between maternal consumption of fermented foods and the development of the offspring at the age of 3 years: The Japan Environment and Children’s Study. PLoS ONE 19(6): e0305535. https://doi.org/10.1371/journal.pone.0305535
Editor: António Machado, Universidad San Francisco de Quito, ECUADOR
Received: January 19, 2024; Accepted: June 1, 2024; Published: June 21, 2024
Copyright: © 2024 Hirai et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No.57 of May 30, 2003, amendment on September 9, 2015) to publicly deposit data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labor, and Welfare also restrict the open sharing of epidemiologic data. All inquiries about access to data should be sent to [email protected] . This is the e-mail address of Dr. Shoji F. Nakayama, JECS Program Office, National Institute for Environmental Studies, in charge of handling inquiries.
Funding: The Japan Environment and Children’s Study is funded by the Ministry of the Environment, Japan. This funding source played no role in the study’s design, collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit this paper for publication.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Intake of fermented foods affects the regulation of intestinal microbiota and is effective in preventing the progression of various diseases, including diabetes, allergies, depression, obesity, and constipation [ 1 – 5 ]. In addition, previous studies have shown a relationship between autism spectrum disorder or depressive symptoms and gut–brain interaction, as well as between psychiatric symptoms or neurodevelopment and intake of fermented foods [ 6 , 7 ].
Although the intestinal microbiota changes with diet, it is thought that the fetal intestinal microbiota begins to develop in utero and is inherited from the mother [ 8 , 9 ]. This suggests that intake of fermented foods during pregnancy may affect fetal development by improving the intestinal environment. Some fermented foods also contain nutrients that are beneficial to the child’s development and are therefore considered beneficial to child health [ 10 ]. Our research group previously investigated the relationship between maternal intake of four fermented foods commonly consumed in Japan— miso , natto , yogurt, and cheese—during pregnancy and the development of offspring at 1 year of age, and reported a beneficial association [ 11 ]. In the present study, we investigated whether the association persisted in the offspring at 3 years of age.
The JECS protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies (Ethical Number: No. 100910001) and the ethics committees of all participating institutions.
Participants
The present study was based on data from the Japan Environment and Children’s Study (JECS), a nationwide birth cohort study that focuses on the relationship of environmental factors with child health and development. The JECS enrolled pregnant women from study areas throughout Japan between January 2011 and March 2014. Details of the JECS design have been reported previously [ 12 – 14 ]. We used data from the jecs-an-20190930 dataset released in October 2019 in the present study. The dataset includes 103,060 pregnancies. We excluded 5,647 due to multiple enrollment, 948 due to multiple pregnancies, 3,520 due to miscarriage or stillbirth, and 32,305 due to lack of information on maternal diet or developmental delay or due to inability to respond to the survey. Thus, a total of 60,910 mother–infant pairs were analyzed in this study ( Fig 1 ). All maternal dietary data were obtained via a self-administered questionnaire on the consumption of fermented foods during pregnancy. Written informed consent for the study was obtained from all participants.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
This study included 103,060 pregnant women, 5,647 of whom were excluded due to multiple enrollment, 948 due to multiple pregnancies, 3,520 due to miscarriage or stillbirth, and 32,305 due to lack of information on diet or developmental delay or due to inability to respond to the survey. A total of 60,910 mother–infant pairs were analyzed in the study.
https://doi.org/10.1371/journal.pone.0305535.g001
Fermented foods
The intake of four types of fermented foods ( miso , natto , yogurt, and cheese) by the mothers at >4 months of gestation using the Food Frequency Questionnaire [ 15 ]. This semi-quantitative questionnaire contains the following items: name of food, frequency of consumption, and estimated amount consumed in a single meal (weight, nutritional values, and portion size) [ 16 ]. These four foods are commonly consumed in Japan.
Neurodevelopment
The primary outcome was neurodevelopmental status of the offspring at 3 years of age. Neurodevelopment was assessed using the Ages and Stages Questionnaires (ASQ-3). The ASQ-3 is a modified version of the parent-completed child monitoring system, which is a UNICEF-recommended and validated neurodevelopment assessment tool [ 17 , 18 ]. The ASQ consists of 21 age-specific questionnaires administered over time and assesses development in five domains: communication skills, gross motor skills, fine motor skills, problem-solving skills, and social skills. The questionnaire consists of 30 questions: six for each of the five major domains. Each can be answered with “yes,” “no,” “sometimes,” or “not yet.” A score of 10 is given for “yes,” indicating that the child has achieved the item; a score of 5 is given for “sometimes,” indicating that the child is still developing; and a score of 0 is given for “not yet” and “no,” indicating that the child has not yet acquired the ability to achieve the item. If one or two of the six questions were unanswered, a correlation coefficient of 1.2–1.5 was multiplied by the actual number of points earned, and the scores were adjusted to be in the range of 0–60 points; if three or more of the six questions were unanswered, the scores were excluded from the analysis. Participants with scores equal to or below the cut-off values were considered positive cases [ 19 ]. The ASQ-3 was completed by mothers for offspring between 35 and 37 months of age.
Statistical analysis
Data are presented as the mean ± standard deviation or median, unless otherwise stated. To estimate the risk of neurodevelopmental delay with intake of each fermented food, the participants were divided into four groups according to intake level. Odds ratios and 95% confidence intervals were obtained from a multivariate logistic regression analysis. Potential confounders from previous reports included maternal age (<25, 25–<30, 30–<35, ≥35 years), body mass index (<18.5 kg/m 2 , 18.5–25 kg/m 2 , ≥25 kg/m 2 ), parity (primipara, multipara), smoking status (never, previously smoked, current smoker), passive smoking (almost never or never, >1 per week), alcohol intake (never, previously drank, current drinker), quartile of physical activity (MET min/week) [ 20 ], quartile of folic acid intake (μg), quartile of energy intake (cal), marital status (married [including common-law marriage], single [never married], divorced or widowed), highest education level (≤12, 12–<16, ≥16 years), highest education level of partner (≤12, 12–<16, ≥16 years), employed (yes, no), annual household income (<4 million yen, 4–6 million yen, ˃6 million yen), administration of anti-bacteria medicine (yes, no). Potential mediators were not used as covariates.
The intake level of each of the four fermented foods consumed during pregnancy was categorized into quartiles: miso , 0–24 g, 25–74 g, 75–145 g, and 147–2,063 g; natto , 0–1.7 g, 3.3–5.4 g, 10.7–12.5 g, and 16.1−600.0 g; yogurt, 0–8 g, 12–26 g, 30–90 g, and 94–1,440 g; and cheese, 0–0.7 g, 1.3–2.0 g, 2.1–4.3 g, and 5.0–240.0 g. The characteristics of each quartile for cheese intake during pregnancy are shown in Table 1 . The characteristics of the other fermented foods are shown in S1–S3 Tables in S1 File . Compared with mothers who consumed less yogurt, those who consumed more yogurt had a higher level of education and annual income, and a higher percentage were nulliparas. Further, their partners had a higher level of education and a lower percentage were smokers or passive smokers. For all four fermented foods, higher intake groups also had higher energy and folic acid intakes compared with the lowest intake group.
https://doi.org/10.1371/journal.pone.0305535.t001
Table 2 shows the ORs of neurodevelopmental delay in each domain, as assessed by the ASQ-3, according to the quartiles of fermented food intake relative to the first quartile. Multivariable logistic regression analysis revealed that cheese intake during pregnancy significantly reduced the risk of neurodevelopmental delay in offspring at 3 years of age in all five domains.
https://doi.org/10.1371/journal.pone.0305535.t002
When mothers consumed ≥1.3 g of cheese daily during pregnancy, the risk of motor and neurodevelopmental delays in their offspring at the age of 3 years was significantly reduced. Fermented foods are expected to have health-promoting effects due to fermentation by various microorganisms, which enhance the nutritional value of the foods compared with their original non-fermented state. The health-promoting effects of fermented foods may be caused by changes that occur when they are consumed as a meal [ 21 ]. There are many reports on the relationship between consumption of fermented foods and neurodevelopment, and gut–brain interactions have attracted increasing research interest recently [ 6 ]. In addition to fermented foods, Hamazaki et al. [ 22 ], Julvez et al. [ 23 ], and Bolduc et al. [ 24 ] reported positive correlations between maternal intake of fish and fruits during pregnancy and offspring development. There are also numerous reports on the relationship between intake of vitamins (including folic acid) and trace elements, such as iron, and offspring development [ 25 – 29 ]. Our research group previously found a beneficial association between maternal intake during pregnancy of the same four fermented foods that we assessed in the present study–( miso , natto , yogurt, and cheese) and the development of the offspring at 1 year of age [ 11 ]. The present study is the first to evaluate this association in offspring at the age of 3 years. The mechanisms of this association might be explained by gut–brain interactions mediated by the intestinal microbiota, as described above. Development of the intestinal microbiota begins in utero with the microorganisms of the maternal microbiota [ 8 , 9 ]. Although the fetal gut is sterile, the prototype of subsequent intestinal microbiota is formed within the first week after birth, and the predominant bacterial groups change with growth [ 30 ]. Accordingly, maternal intestinal microbiota at the time of birth has a great influence on the composition of the postnatal intestinal microbiota of infants. Thus, improving the intestinal environment of pregnant women through intake of fermented foods could be beneficial to the health of the fetus.
The intestinal microflora affect the nervous system through the action of neurotransmitters produced by various microorganisms and also has anti-inflammatory effects [ 31 – 33 ]. Bifidobacterium spp., which are found in high proportions in the gut throughout life, produce the inhibitory neurotransmitter GABA and play an important role in early infant development [ 6 , 34 ]. Because GABA suppresses nerve excitation, the presence of Bifidobacterium spp. in the gut may affect the mental activity and behavior of children. Disruption of the intestinal environment, so-called dysbiosis, also promotes intestinal inflammation, which is thought to increase intestinal permeability and facilitate the transfer of toxic neurotransmitters to the central nervous system. It has been suggested that the underlying mechanism of autism spectrum disorders might be brain inflammation associated with increased inflammatory cytokines such as IL-6 and TNF-α [ 31 – 33 ]. Although there are presently no established therapies, some animal studies have reported that administration of probiotics improved symptoms in autistic spectrum disorders, and interventions to improve the gut environment may also be effective in improving neurological symptoms [ 35 , 36 ].
Many studies have reported the effects of the gut microbiota on the behavior and mental states of the host, but it is difficult to consider the gut microbiota as the underlying cause of developmental disorders. It is speculated that improving the gut microbiota may be effective in alleviating the symptoms of developmental disorders in predisposed children.
In this study, there were differences in the results of the four fermented foods investigated. Only cheese was associated with a reduced risk of developmental delay in all five domains studied. Previous studies have suggested that intake of fermented dairy products may be effective in preventing cognitive decline in Alzheimer’s disease, and some fermented dairy products are thought to influence cognitive function [ 37 ]. These findings may be explained by differences in the function of the probiotics and nutrients in each fermented food. Various microorganisms are involved in the production of fermented foods: Aspergillus mold in miso , Bacillus in natto , and Lactobacillus spp. in yogurt and cheese. Although the effects of individual microorganisms on development are unclear, each fermented food has a variety of different health-promoting effects. Miso is reported to be effective in preventing hypertension and elevating blood sugar [ 38 ]. Natto is effective in inhibiting the growth of Clostridium spp. and regulating intestinal microbiota [ 39 – 41 ]. Some Lactobacillus spp. are known to improve the overall intestinal microbiota composition. Some Bifidobacterium spp. increase the counts of Bifidobacterium and Lactobacillus spp. [ 34 ]. In addition, the nutritional components differ in each fermented food [ 10 ]. Nutrients such as iron, zinc, long-chain fatty acids, and tryptophan are associated with development [ 42 , 43 ] and are essential in normal neurodevelopment and the differentiation of some brain cells. These nutrients may be transferred from mother to child and affect postnatal development. Regarding the nutritional composition of the four fermented foods investigated in this study, cheese has lower amounts of iron and long-chain fatty acids but higher amounts of protein, zinc, and tryptophan, according to the Japanese standard tables of food composition [ 44 ]. However, even small amounts of cheese (≥1.3 g daily) had observable effects. Because a piece of processed cheese sold in Japan weighs about 15–20 g, the effect was observable with a relatively small intake. It is unclear whether the effects of cheese intake on development may be direct, as seen with individual nutrients.
In the group with high cheese intake, the education level of the mother and her partner was higher, and the rate of passive smoking was lower than in the group with the lowest cheese intake, suggesting that there may be factors such as high health consciousness that could not be adjusted for. In the future, it may be possible to clarify the relationship between diet and development by directly examining the intestinal microbiota of mother–infant pairs.
This study has some limitations. First, the manufacturer of each food was not investigated and detailed ingredients were not available. Second, the microorganisms and nutritional components in the same food were not standardized. Third, the intake levels during pregnancy were not strictly measured because the survey was conducted using a questionnaire. Fourth, the data analyzed was obtained only from mothers who had been recruited and consented to participate in the JECS while pregnant at the participating maternity units. Therefore, there may be a bias in which only women who were amenable to participating in the JECS were analyzed in the present study. Finally, in addition to the influence of the maternal diet during pregnancy, the offspring’s diet after birth may also influence neurodevelopment. Furthermore, although the results of this study suggest that the intestinal microbiota is involved, the actual composition of the intestinal microbiota of the mothers and their children was not investigated. Further studies are warranted to address these limitations.
This study on the relationship between maternal intake of fermented foods during pregnancy and offspring development found the risk of neurodevelopmental delay at the age of 3 years was reduced in the offspring of mothers who consumed more cheese. However, it is possible that the child’s development might have been influenced by the child’s own diet and lifestyle, and thus further studies are needed.
Supporting information
S1 file. s1-s3 tables shows the demographic and obstetric characteristics of participants for miso, yogurt, and natto, respectively..
https://doi.org/10.1371/journal.pone.0305535.s001
Acknowledgments
We are grateful to all the participants of the JECS and to all the individuals involved in data collection. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government agency.
Members of the JECS Groups as of 2023: Michihiro Kamijima (Principal Investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Seiji Kageyama (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Shoichi Ohga (Kyushu University, Fukuoka, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
- View Article
- PubMed/NCBI
- Google Scholar
- 11. Tanaka T,Kenta Matsumura,Akiko Tsuchida,Kei Hamazaki,Haruka Kasamatsu et al.Maternal fermented food intake and infant neurodevelopment:The Japan Environment and children’s Study.Asia Pacific Journal of Clinical Nutrition (in Press)
- 44. Ministry of Education, Culture, Sports, Science and Technology, Science and Technology Council, Resources Research Subcommittee. Japanese Food Standard Ingredient List 2023 Edition (8th ed): 2023. https://fooddb.mext.go.jp/ .
- Open access
- Published: 17 June 2024
Impact of maternal depression and anxiety on immunization status of children: a prospective cohort study
- Shannon E. MacDonald 1 , 2 ,
- Manisha Dhungana 1 ,
- Victoria Stagg 3 ,
- Sheila McDonald 3 ,
- Deborah McNeil 4 ,
- James D. Kellner 2 ,
- Suzanne Tough 2 &
- Vineet Saini 3 , 4
Archives of Public Health volume 82 , Article number: 89 ( 2024 ) Cite this article
205 Accesses
1 Altmetric
Metrics details
Maternal depression and anxiety can have a detrimental impact on birth outcomes and healthy child development; there is limited knowledge on its influence on immunization schedule adherence. Therefore, the objectives of this study were to determine the impact of maternal depression and anxiety in the perinatal period on prolonged vaccine delay of childhood vaccines.
In this prospective cohort study, we analyzed linked survey and administrative data of 2,762 pregnant women in Calgary, Alberta, Canada. Data were collected at two time-points: prenatal (< 25 weeks of gestation) and postpartum (4 months postpartum). We used multivariable logistic regression to examine the association between depression and anxiety with prolonged immunization delay, adjusting for covariates.
In multivariable analysis, maternal depression at either time point was not associated with prolonged delay for DTaP-IPV-Hib (OR 1.16, 95% CI 0.74–1.82), MMR/MMRV (OR 1.03, 95% CI 0.72–1.48), or all routine childhood vaccines combined (OR 1.32, 95% CI 0.86–2.04). Maternal anxiety at either time point was also not associated with prolonged delayed for DTaP-IPV-Hib (OR 1.08, 95% CI 0.77–1.53), MMR/MMRV (OR 1.07, 95% CI 0.82–1.40), or all vaccines combined (OR 1.00, 95% CI 0.80–1.26). In both the depression and anxiety models, children of Canadian-born mothers had higher odds of prolonged delay, as did those with low-income mothers.
Health care providers can be reassured that maternal depression and anxiety do not appear to influence maternal commitment to routine immunization. Findings suggested that low income and household moves may influence adherence to vaccine schedules and health care providers may want to provide anticipatory guidance to these families.
Peer Review reports
|
|---|
• Maternal perinatal depression and anxiety can have a detrimental impact on birth outcomes and healthy child development, but there is limited knowledge on its influence on uptake of childhood vaccines |
• Perinatal depression and/or anxiety was not found to be associated with prolonged delay for most early childhood vaccines |
• Reassuring/negative findings can be useful to guide clinicians and policy-makers in their decision of where to focus scarce resources for targeted immunization efforts. |
Introduction
Depression and anxiety during the prenatal and postpartum period can have a detrimental impact on birth outcomes and healthy child development [ 1 , 2 ]. In particular, maternal mental health challenges in the perinatal period may result in reduced healthcare-seeking behaviours for children, including uptake of preventive health care [ 3 , 4 , 5 ].
Childhood immunization is an essential and cost-effective preventive health intervention to protect children from vaccine-preventable diseases [ 6 , 7 ]. Complete and timely immunization with routinely scheduled childhood vaccines is important to ensure optimal protection from vaccine-preventable diseases [ 8 ]. Thus, missed or delayed immunization increases risk for severe infections, such as pertussis and measles, in young children [ 9 ].
There is limited knowledge on the impact of maternal depression or anxiety on immunization status of children [ 3 , 4 , 10 , 11 , 12 , 13 ] and very few studies have assessed both prenatal and postpartum depression and anxiety [ 3 , 14 ]. The existing literature shows mixed evidence of the relationship between maternal depression or anxiety and complete immunization of the child, and most results come from studies with very small sample sizes [ 4 , 11 , 12 , 13 ]. In addition, no previously published studies have assessed the timeliness of immunization, defined as lack of delays in administration of vaccine(s) according to the recommended vaccine schedule, in this population. This study addresses these gaps in knowledge. Specifically, we aimed to examine the association between prenatal and postpartum depression , and timeliness of immunization for (a) diphtheria, tetanus, acellular pertussis, polio and Hemophilus influenzae type b (DTaP-IPV-Hib) vaccine, (b) measles, mumps and rubella (MMR)/MMR-Varicella (MMRV) vaccines, and (c) for all vaccines in the recommended immunization schedule combined. We also aimed to examine the association between prenatal and postpartum anxiety , and timeliness of immunization for DTaP-IPV-Hib, MMR/MMRV, and all vaccines in the recommended immunization schedule combined.
Study design, population, and setting
The data for this study was obtained from an existing prospective pregnancy cohort study, the “All Our Families” (AOF) study. AOF recruited 3387 pregnant women in Calgary, Alberta, Canada, a city of 1.27 million people, between May 2008 and December 2010 [ 15 ]. The detailed recruitment process for AOF has been described previously [ 16 ]. Two surveys were completed by the cohort in the prenatal period (before 25 weeks and at 34–36 weeks), with six surveys after birth (at 4 months, 1,2,3,5, and 8 years). Women who consented to linkage of their survey data with administrative health records through their personal health number (PHN) provided at the time of recruitment ( N = 2855, 84%) were included in this analysis.
For the purpose of this analysis, we drew data from surveys at two time points: prenatal , defined as < 25 weeks of gestation ( N = 2,849) and postpartum , defined as 4 months postpartum ( N = 2,699). The number of participants who were sent surveys varied slightly by time point. Self-reported demographic and other characteristics from the surveys (including data from self-administered depression and anxiety scales) were linked using unique identifiers to public health immunization data [ 17 ].
Depression symptoms were measured using the Edinburgh Postpartum Depression Scale (EPDS), a 10- item self-report validated instrument used to screen women for depression in their postpartum period [ 18 ]. This tool is also used to detect early prenatal depression [ 19 ]. The maximum score for both prenatal and postpartum periods is 30 and we used the standard cut off score of ≥ 13 that indicates greater risk of depression [ 16 ]. The sensitivity of the EPDS for identifying women with major or minor depression, as diagnosed according to the Research Diagnostic Criteria (RDC), was found to be 86%, while the specificity was 78% [ 18 ]. We created a categorical variable for depression with two levels: (a) prenatal and/or postpartum depression and (b) no depression at either time point.
Anxiety was measured using the Spielberger State Anxiety Scale, a self-report validated questionnaire of twenty questions that was used to measure the mothers’ state of anxiety [ 20 ]. The scale ranges from 20 to 80, and the standard cut off score of ≥ 40 indicates an anxious state in pregnant women, with 81% sensitivity and 79.8% specificity [ 21 ]. We created a categorical variable for anxiety with two levels: (a) prenatal and/or postpartum anxiety and (b) no anxiety at either time point.
Vaccine delay
The AOF survey data were linked to public health administrative databases (Phantim and Medipatient) using a combination of maternal PHN, and maternal and child date of birth [ 22 ]. These public health databases contain records of all vaccines administered to children as part of Alberta’s publicly funded immunization program. Through this program, all childhood vaccines are administered by public health nurses and entered into these immunization registries. Data are routinely audited to ensure accuracy of data entry. For the purpose of this study, we linked AOF survey data to vaccine events (vaccine type, dose, and date of administration) for children up to 24 months of age ( N = 2763 children) (Supplementary Fig. 1 ) [ 22 ]. Later, one child was dropped from the linked database due to invalid age information thereby leaving 2762 observations for the analysis.
Using the method developed by Luman et al. [ 23 ], we calculated delay in administration of all vaccine doses (combined) in the recommended routine childhood immunization schedule by 2 years of age (see Table 1 ), as well as for two individual vaccines: a multi-dose vaccine, DTaP-IPV-Hib, and a single dose vaccine, MMR/MMRV. ‘Delayed immunization’ was defined as doses administered after the end of a grace period, which was the maximum number of days before the child increased in age to be the next month old. It was measured as ‘Days delayed’ and calculated as the cumulative number of days delayed after the grace period for all recommended vaccines of one or more doses, assessed at 2 years of age. For instance, the recommended age for administering the first dose of DTaP-IPV-Hib is at 2 months and the grace period ends at 3 months of age (90–92 days, depending on month). If a child received their first dose of DTaP-IPV-Hib 1 day after the grace period i.e., at 93 days, then it is counted as 1 day of delay for the first dose of DTaP-IPV-Hib vaccine. For multi-dose vaccines, days delayed was calculated by adding up days delayed for each dose. For example, suppose a child received the first dose of DTaP-IPV-Hib vaccine at 110 days (3 ½ months), the 2nd dose at 180 days (6 months) and the 3rd dose at 330 days (11 months). Then, the days delayed would be considered to be from days 93 to 109 for first dose, from days 154 to 179 for 2nd dose, and from days 216 to 329 for the 3rd dose. Thus, the days delayed for the DTaP-IPV-Hib vaccine would be 157 days, i.e., the cumulative delay for the 3 doses = (110 − 93) + (180 − 154) + (330 − 216).
We then calculated our primary outcome, ‘prolonged delay’, which we defined as delayed for more than 6 months (≥ 7 months) for all vaccines combined in the schedule, or the multi-dose vaccine, DTaP-IPV-Hib, or the single dose vaccine, MMR/MMRV. Thereafter, we assessed the association of the primary exposures of maternal depression and anxiety with prolonged delay in immunization for all routine childhood immunizations combined and separately for DTaP-IPV-Hib and MMR/MMRV immunizations.
Various covariates such as parity, maternal age in years at the time of delivery, maternal marital status, born in Canada, maternal education, income, social support, number of household moves, and gestational term of baby were examined for potential inclusion in the models based on association with delayed or incomplete vaccination in the existing literature [ 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 ]. All covariates were obtained during the survey at the 1st trimester of the pregnancy, except maternal age, which was obtained at the time of delivery.
Statistical analysis
To describe the characteristics of the sample, we calculated means and standard deviations (SD) for the normally distributed continuous variables, and medians and interquartile range (IQR) for continuous variables that were not normally distributed. For categorical variables, we calculated frequencies and percentages.
To examine the association between the exposures/covariates and prolonged delay of immunization, we conducted bivariate and multivariable analyses. For the binary outcome (0 = no prolonged delay; 1 = prolonged delay), we used logistic regression. No prolonged delay was defined as delay between 0 and 215 days and prolonged delay was defined as delay ≥ 216 days; 215 days is the maximum number of days that reflects 6 months + 30 days (just prior to turning 7 months old). We ran two logistic regression models, one with depression as the primary exposure and second with anxiety as the primary exposure.
Covariates found to be significantly related to the relevant prolonged delay outcome (DTaP, MMR, or All Vaccines) in bivariate regression (defined as p < = 0.25) were included in the full models. Backward elimination was used to determine the most parsimonious model, with the primary exposure variables (prenatal and/or postpartum depression, prenatal and/or postpartum anxiety) retained in the relevant models throughout the process of backward elimination. Two-way interaction terms were tested for each combination of explanatory variables remaining in the full model, and tested for significance using the likelihood ratio test. Following the process of backward elimination and the selection of a “final” model of covariates, all explanatory (independent) variables that were dropped from the model during the steps of backward elimination were checked for their qualification as a confounder. Any that qualified (changed the coefficient of the exposure variable by ≥ 10%) were retained in the final model.
Sensitivity analysis
As part of a sensitivity analysis, we assessed depression and anxiety separately at two different time points, i.e. prenatal (< 25 weeks gestation) and postpartum (4 months post-partum). The intent was to understand if the timing of depression and anxiety symptoms assessment was associated with immunization delay.
Ethical approval for this study was received from the Conjoint Health Research Ethics Board at the University of Calgary (REB 14–0925).
Of 2,762 participants, 266 (9.63%) and 581 (21.04%) mothers had depression and anxiety symptoms, respectively (Table 2 ). Most mothers were married or in a common-law relationship (94.61%), born in Canada (77.73%), had some or completed post-secondary education (89.10%), had income greater than $60,000 (80.30%), a history of no household moves (75.74%), and had high social support (72.77%).
In bivariate analysis (Table 3 ), neither maternal depression nor maternal anxiety (at either the prenatal and /or postpartum periods) increased the likelihood of a child having a prolonged delay for DTaP-IPV-Hib, MMR/MMRV, or all routine childhood vaccines combined. Some specific covariates, such as parity, country of birth, income, and number of household moves, were associated with the outcome of prolonged delay.
Multivariable association between depression and prolonged delay of DTaP-IPV-Hib, MMR/MMRV, and all vaccines combined
After controlling for covariates, maternal depression at either time point (prenatal and /or postpartum) did not increase the likelihood of a child having a prolonged delay for DTaP-IPV-Hib (OR 1.16, 95% CI 0.74–1.82), MMR/MMRV (OR 1.03, 95% CI 0.72–1.48), or all routine childhood vaccines combined (OR 1.32, 95% CI 0.86–2.04) (Table 4 ).
In the multivariable depression model, some covariates were associated with prolonged delay. Children of multiparous mothers had higher odds of prolonged delay for DTaP-IPV-Hib (OR 1.64, 95% CI 1.22–2.20), and all vaccines combined (OR 1.48, 95% CI 1.21–1.81). Children of mothers born in Canada had higher odds of prolonged delay for MMR/MMRV (OR 1.58, 95% CI 1.17–2.13) and all routine childhood vaccines combined (OR 1.36, 95% CI 1.06–1.73).
Notably for DTaP-IPV-Hib immunization, there were interaction effects for some covariates on prolonged delay (Table 4 , Supplementary Figs. 2 – 5 ), especially among mothers who were born in Canada and moving households. Preterm babies of such mothers had higher odds of prolonged delay of DTaP-IPV-Hib immunization compared to mothers of full term babies (OR = 3.87, 95% CI: 1.69–8.85, Table 4 , Supplementary Fig. 5 ).
Multivariable association between anxiety and prolonged delay of DTaP-IPV-Hib, MMR, and all vaccines combined
Similar to depression, prenatal and/or postpartum anxiety did not increase the likelihood of a child having a prolonged delay for DTaP-IPV-Hib (OR 1.08, 95% CI 0.77–1.53), MMR/MMRV (OR 1.07, 95% CI 0.82–1.40), and all vaccines combined (OR 1.00, 95% CI 0.80–1.26) after controlling for covariates (Table 5 ).
As with the depression model, certain covariates were associated with prolonged delay in the anxiety model. Children of mothers born in Canada had higher odds of prolonged delay for DTaP-IPV-Hib (OR 1.57, 95%CI 1.03–2.39) MMR/MMRV (OR 1.74, 95% CI 1.25–2.42), and all vaccine combined (OR 1.48, 95% CI 1.14–1.93, Table 5 ) than those with mothers born elsewhere.
Notably for DTaP-IPV-Hib vaccine, interaction between some covariates was observed (Table 5 , Supplementary Figs. 6 – 8 ). In the anxiety model, among mothers not moving households, children of high-income mothers had lower odds of prolonged delay of DTaP-IPV-HiB immunization compared to those of low-income mothers (OR: 0.43, 95% CI 0.28–0.67, Table 5 , Supplementary Fig. 6 ). In the anxiety model, preterm babies had higher odds of prolonged DTaP delay than full term babies among mothers experiencing one or more moves (OR: 2.96, 95% CI 1.33–6.57, Table 5 , Supplementary Figure Fig. 7 ).
Interaction between parity and household moves was observed for MMR/MMRV immunization as well. In the anxiety model, among mothers experiencing multiple moves, children of multiparous mothers had higher odds of prolonged delay compared to those of primiparous mothers (OR: 2.0, 95% CI 1.22–3.29, Table 5 , Supplementary Fig. 8 ).
We conducted a sensitivity analysis to assess depression and anxiety only prenatally and only postpartum with no change in the relationships i.e. it did not impact the likelihood of a child having prolonged delayed for DTaP-IPV-Hib and MMR/MMRV, and all vaccines combined (Supplementary Tables 4 – 6 ).
This study assessed the role of both prenatal and/or postpartum depression and anxiety on the relationship with timeliness of routine childhood immunization. Interestingly, prenatal and/or postpartum depression was not associated with prolonged vaccine delay for a multi-dose vaccine (i.e., DTaP-IPV-Hib), a single dose vaccine (i.e., MMR/MMRV), nor for all five routine childhood vaccines combined. Previous studies have reported contradictory findings on the effect of maternal mental depression and childhood immunization. For instance, a recent study in the UK reported that children with mothers having depression between one year prior to child’s birth up to the age of two years and five years had reduced likelihood of receiving DTaP/IPV/Hib and MMR vaccines [ 14 ]. Likewise, a study done in the USA reported that children whose mothers had depressive symptoms in the 2–4 months’ post-partum period were less likely to receive up-to-date immunization at 24 months for MMR, DTP, and polio vaccines [ 10 ]. Similarly, Australian children of mothers experiencing depression were more likely to be immunized late or not at all [ 12 ]. In contrast, consistent with our study findings, some studies in the USA and Zambia reported no association between maternal depression and childhood immunization status [ 3 , 11 , 13 ].
Likewise, prenatal and/or postpartum anxiety had no association with prolonged delay, for all five routine childhood vaccines combined, or for single-dose (i.e., MMR/MMRV) and multi-dose (i.e., DTaP-IPV-Hib) vaccines. Previous studies revealed contradictory findings on the association of maternal anxiety with childhood immunization. In one study, children whose mothers had anxiety symptoms were less likely to receive complete immunization at two years and five years for DTaP/IPV/Hib and MMR vaccines [ 14 ]. In another study, maternal anxiety was associated with incomplete immunization for all vaccines among children younger than three years old [ 4 ]. Likewise, another study found that children of mothers experiencing postpartum anxiety were more likely to be immunized late or not at all [ 12 ]. Consistent with our study findings, a US study found no association between mothers with perinatal anxiety and childhood immunization at 8 months of age [ 3 ].
Comparability with previous studies examining maternal depression and anxiety and routine childhood immunization is limited due to differences in study settings, health system, depression and anxiety measurement or definitions of outcome variables. One possible reason behind lack of association between vaccine delay and either depression or anxiety in our setting could be the increased use of primary health care services (family physicians/public health nurses) by mothers for prenatal and/or postpartum depression and anxiety symptoms, as has been recognized elsewhere [ 32 ]. Mothers receiving mental care support and treatment during prenatal and/or postpartum period could resolve mental health challenges and might have a positive impact on the wellbeing of mother and babies, or the vaccine delivery system is adequate to reach these children despite maternal challenges; however, these assumptions should be investigated in future studies.
Other than depression and anxiety, other factors were associated with delayed childhood immunization status. Children from mothers born in Canada were more likely to have prolonged delay for all vaccines compared to mothers born elsewhere. This finding was consistent with a previous study that reported children from mothers born in Canada, versus immigrant mothers, were less likely to be completely immunized [ 33 ]. In line with previous studies [ 25 , 34 ], we found that a greater number of household moves and low income increased the likelihood of prolonged delay for all vaccines. For example, low income and one or more household moves could influence vaccine compliance, as parents have to orient themselves to the new neighborhood, clinics, healthcare provider, as well as resources.
Although an independent effect of maternal depression and/or anxiety on the timeliness of childhood immunization was not evident, a number of other factors interacted to increase the likelihood of a child having prolonged delay for immunization. For example, joint presence of two or more factors such as one or more household moves, born in Canada, preterm child, multiparous mother, and income was associated with the timeliness of childhood immunization. There is growing recognition that constellations of factors (i.e. multiple overlapping factors such as those stated above) can amplify barriers to immunization, and other health services, beyond the additive impact of the individual factors [ 29 , 34 ]. This concept, referred to as ‘intersectionality’ should be considered further in future immunization coverage research [ 35 , 36 ].
Strengths and limitations
This study was able to link administrative health and survey data; the former aided in obtaining accurate and complete outcome data. The use of validated tools for measuring depression and anxiety at multiple time points during the study was another strength. However, there are some noteworthy limitations of this study. Firstly, this study cohort was representative of urban families in Canada, which may over-represent those with higher socioeconomic status (SES). Thus, the findings may not be generalizable to lower SES families. Secondly, maternal depression and anxiety assessment was based on maternal self-reporting, which could be prone to measurement error and self-report bias. In addition, these scales represent depression and anxiety symptomatology, and not clinical diagnoses. Further, we did not have accurate information on treatment or support that may have been received by mothers during or subsequent to the perinatal period. Such information might help to understand the lack of associations found between maternal mental health symptoms and child vaccine outcomes in the present study.
This study suggests that prenatal and/ or postpartum maternal depression and anxiety was not associated with timeliness of infants’ immunization. Health care providers can be reassured that maternal depression and anxiety do not influence vaccine adherence. Adherence to vaccine schedules was more difficult among Canadian born mothers and those who experienced one or more household moves and of low income. Provision of information about location of immunization services may assist mothers who are relocating in vaccine adherence, particularly those with income insecurity.
Data availability
The data set from this study is held securely in coded and de-identified form at Alberta Health Services (AHS). Although data sharing agreements prohibit AHS from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access. Please contact [email protected] for more information.
Coburn SS, Luecken LJ, Rystad IA, Lin B, Crnic KA, Gonzales NA. Prenatal maternal depressive symptoms predict early infant health concerns. Matern Child Health J. 2018;22(6):786–93. https://doi.org/10.1007/s10995-018-2448-7 .
Article CAS PubMed PubMed Central Google Scholar
Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev. 2010;33(1):1–6. https://doi.org/10.1016/j.infbeh.2009.10.005 .
Article PubMed Google Scholar
Farr SL, Dietz PM, Rizzo JH, Vesco KK, Callaghan WM, Bruce FC, Bulkley JE, Hornbrook MC, Berg CJ. Health care utilisation in the first year of life among infants of mothers with perinatal depression or anxiety. Paediatr Perinat Epidemiol. 2013;27(1):81–8. https://doi.org/10.1111/ppe.12012 .
Article PubMed PubMed Central Google Scholar
Ozkaya E, Eker HH, Aycan N, Samanci N. Impact of maternal anxiety level on the childhood vaccination coverage. Eur J Pediatrics. 2010;169(11):1397–401. https://doi.org/10.1007/s00431-010-1247-y .
Article Google Scholar
Zuckerman BS, Beardslee WR. Maternal depression: a concern for pediatricians. Pediatrics. 1987;79(1):110–7. https://doi.org/10.1542/peds.79.1.110 .
Article CAS PubMed Google Scholar
Public Health Agency of Canada. Canadian Immunization Guide. 2006. http://www.phac-aspc.gc.ca/publicat/cig-gci/index-eng.php .
World Health Organization. Immunization. 2019. https://www.who.int/news-room/facts-in-pictures/detail/immunization .
National Advisory Committee on Immunization. Canadian immunization guide. 6th ed. Canadian Medical Association; 2002.
Hull BP, McIntyre PB. Timeliness of childhood immunisation in Australia. Vaccine. 2006;24(20):4403–8. https://doi.org/10.1016/j.vaccine.2006.02.049 .
Minkovitz CS, Strobino D, Scharfstein D, Hou W, Miller T, Mistry KB, Swartz K. Maternal depressive symptoms and children’s receipt of health care in the first 3 years of life. Pediatrics. 2005;115(2):306–14. https://doi.org/10.1542/peds.2004-0341 .
Ndokera R, MacArthur C. The relationship between maternal depression and adverse infant health outcomes in Zambia: a cross-sectional feasibility study. Child Care Health Dev. 2011;37(1):74–81. https://doi.org/10.1111/j.1365-2214.2010.01129.x .
Turner C, Boyle F, O’Rourke P. Mothers’ health post-partum and their patterns of seeking vaccination for their infants. Int J Nurs Pract. 2003;9(2):120–6. https://doi.org/10.1046/j.1322-7114.2003.00410.x .
Watson J. Maternal factors and child’s health care use. Soc Sci Med. 1995;40(5):623–8. https://doi.org/10.1016/0277-9536(94)E0112-6 .
Osam CS, Pierce M, Hope H, Ashcroft DM, Abel KM. The influence of maternal mental illness on vaccination uptake in children: a UK population-based cohort study. Eur J Epidemiol. 2020;35(9):879–89. https://doi.org/10.1007/s10654-020-00632-5 .
Government of Alberta. Municipal affairs population list. 2018.
McDonald SW, Lyon AW, Benzies KM, McNeil DA, Lye SJ, Dolan SM, Pennell CE, Bocking AD, Tough SC. The all our babies pregnancy cohort: design, methods, and participant characteristics. BMC Pregnancy Childbirth. 2013. https://doi.org/10.1186/1471-2393-13-S1-S2 . 13 Suppl 1.
Premji S, McDonald SW, Metcalfe A, Faris P, Quan H, Tough S, McNeil DA. Examining postpartum depression screening effectiveness in well child clinics in Alberta, Canada: a study using the all our families cohort and administrative data. Prev Med Rep. 2019;14. https://doi.org/10.1016/j.pmedr.2019.100888 .
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Br J Psychiatry. 1987;150(6):782–6. https://doi.org/10.1192/bjp.150.6.782 .
Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EPDS). J Reproductive Infant Psychol. 1990;8(2):99–107. https://doi.org/10.1080/02646839008403615 .
Spielberger C, Gorsuch R, Lushene R, Vagg P. In: Gorsuch RL, Lushene RE, Vagg P, Jacobs GA, editors. Manual for the state-trait anxiety inventory (C spielberge. Consulting Psychologists; 1983.
Grant KA, McMahon C, Austin MA. Maternal anxiety during the transition to parenthood: a prospective study. J Affect Disorder. 2008;108(1–2):101–11.
Saini V, MacDonald SE, McNeil DA, McDonald SW, Kellner JD, Edwards SA, Stagg V, Tough S. Timeliness and completeness of routine childhood vaccinations in children by two years of age in Alberta, Canada. Can J Public Health. 2017;108(2):e124–8. https://doi.org/10.17269/CJPH.108.5885 .
Luman ET, Barker LE, McCauley MM, Drews-Botsch C. Timeliness of Childhood immunizations: a state-specific analysis. Am J Public Health. 2005;95(8):1367–74. https://doi.org/10.2105/AJPH.2004.046284 .
Awasthi A, Pandey CM, Singh U, Kumar S, Singh TB. Maternal determinants of immunization status of children aged 12–23 months in urban slums of Varanasi, India. Clin Epidemiol Global Health. 2015;3(3):110–6. https://doi.org/10.1016/j.cegh.2014.07.004 .
Bell CA, Simmonds KA, MacDonald SE. Exploring the heterogeneity among partially vaccinated children in a population-based cohort. Vaccine. 2015;33(36):4572–8. https://doi.org/10.1016/j.vaccine.2015.07.004 .
Busby C, Chesterley N. A shot in the arm: how to improve vaccination policy in Canada. 2015.
Luman ET, McCauley MM, Shefer A, Chu SY. Maternal characteristics associated with vaccination of young children. Pediatrics, 2003;111(5):1215–1218. http://www.scopus.com/inward/record.url?eid=2-s2.0-0037964380&partnerID=40&md5=c32757bc06fea2b923cdf5e045191195
MacDonald SE, Schopflocher DP, Vaudry W. Parental concern about vaccine safety in Canadian children partially immunized at age 2: a multivariable model including system level factors. Hum Vaccines Immunotherapeutics. 2014;10(9):2603–11. https://doi.org/10.4161/21645515.2014.970075 .
Negussie A, Kassahun W, Assegid S, Hagan AK. Factors associated with incomplete childhood immunization in Arbegona district, southern Ethiopia: a case - control study. BMC Public Health. 2016;16(1). https://doi.org/10.1186/s12889-015-2678-1 .
Nestander M, Dintaman J, Susi A, Gorman G, Hisle-Gorman E. Immunization completion in infants born at low birth weight. J Pediatr Infect Dis Soc. 2018;7(3):E58–64. https://doi.org/10.1093/jpids/pix079 .
Robitaille A, Orpana H, Mcintosh CN. Psychometric properties, factorial structure, and measurement invariance of the English and French versions of the Medical Outcomes Study social support scale. In Statistics Canada (Vol. 22, Issue 2); 2011.
Dennis CL. Influence of depressive symptomatology on maternal health service utilization and general health. Archives Women’s Mental Health. 2004;7(3):183–91. https://doi.org/10.1007/s00737-004-0053-9 .
Guttmann A, Manuel D, Stukel TA, Desmeules M, Cernat G, Glazier RH. Immunization coverage among young children of urban immigrant mothers: findings from a universal health care system. Ambul Pediatr. 2008;8(3):205–9. https://doi.org/10.1016/j.ambp.2008.01.010 .
Gilbert NL, Gilmour H, Wilson SE, Cantin L. Determinants of non-vaccination and incomplete vaccination in Canadian toddlers. Hum Vaccines Immunotherapeutics. 2017;13(6):1–7. https://doi.org/10.1080/21645515.2016.1277847 .
Crenshaw K. Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanford Law Rev. 1991;43(6):1241. https://doi.org/10.2307/1229039 .
Hankivsky O, Grace D, Hunting G, Giesbrecht M, Fridkin A, Rudrum S, Ferlatte O, Clark N. An intersectionality-based policy analysis framework: critical reflections on a methodology for advancing equity. Int J Equity Health. 2014;13(1):119. https://doi.org/10.1186/s12939-014-0119-x .
Download references
Acknowledgements
Not applicable.
No funding was received for conducting this study.
Author information
Authors and affiliations.
Faculty of Nursing, University of Alberta, Edmonton, Canada
Shannon E. MacDonald & Manisha Dhungana
Department of Pediatrics, Cumming School of Medicine, University of Calgary, Calgary, Canada
Shannon E. MacDonald, James D. Kellner & Suzanne Tough
Research and Innovation, Public Health Evidence and Innovation, Alberta Health Services, Calgary, Canada
Victoria Stagg, Sheila McDonald & Vineet Saini
Department of Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Canada
Deborah McNeil & Vineet Saini
You can also search for this author in PubMed Google Scholar
Contributions
SEM was involved in conceptualization, investigation, formal analysis and writing (review and editing). MD was involved in interpretation of data and writing (original draft and editing). VSG (Victoria Stagg) was involved in statistical analysis and writing (original draft, review and editing). VS (Vineet Saini) was involved in conceptualization, investigation, analytical designing, formal analysis, writing (review and editing) and supervision. SMcDonald, DM, JDK, and ST were involved in conceptualization, methodology and writing (review and editing).
Corresponding author
Correspondence to Shannon E. MacDonald .
Ethics declarations
Ethical approval.
The University of Calgary Conjoint Health Research Ethics Board granted ethical approval for this study (REB 14–0925).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
MacDonald, S.E., Dhungana, M., Stagg, V. et al. Impact of maternal depression and anxiety on immunization status of children: a prospective cohort study. Arch Public Health 82 , 89 (2024). https://doi.org/10.1186/s13690-024-01323-3
Download citation
Received : 04 July 2023
Accepted : 09 June 2024
Published : 17 June 2024
DOI : https://doi.org/10.1186/s13690-024-01323-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunization
Archives of Public Health
ISSN: 2049-3258
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
An 18-month-old girl was first referred at eight months of age to a developmental centre because of general developmental delay. She was born after a normal pregnancy and delivery. Her birth weight was 3.7 kg, with Apgar scores of 9 and 10 at 1 min and 5 min, respectively. The neonatal period was uneventful. Her parents are non-consanguineous ...
Shevell, M, Ashwal, S, Donley, D, et al. Practice parameter: evaluation of the child with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of ...
Case studies of ECI for children with developmental disabilities. ... Estimated prevalence of disability and developmental delay among preschool children in rural Malawi: findings from 'Tikule Limodzi', a cross-sectional survey. Child Care Health Dev. 2020; 46 (2):187-94.
Introduction. Global developmental delay (GDD) is term used for children under 5 years of age. It is defined as a significant delay in two or more domains of development, including activities of daily living as well as motor, cognitive, speech/language, and personal/social skills. 1,2 The clear diagnosis of GDD is problematic because of its heterogeneous etiology; thus, the causes of GDD are ...
Speech and language delay in children is a common presentation to primary care either directly to the GP or through the health visitor, affecting approximately 6% of pre-school children.1 Young children, particularly those with speech delay, can be difficult to examine. Differentiation between an isolated pathology and those with concurrent global developmental delay is crucial. This article ...
Abstract. To characterize the etiology underlying a novel case of global developmental delay syndrome (GDDS) identified in a female child, aged 3 years old. This syndrome is a common pediatric presentation estimated to affect 3.65% of children aged 3 to 17 years. The proband's detailed family history was used to infer a likely mode of ...
A total of 1,877 children were enrolled in this study. Most children were classified into four major diagnostic groups: global developmental delay (GDD), autism spectrum disorder (ASD ...
children with developmental. delay - A case-control study. Amulya R Rao, Deepthi Ramamurthy, Uday Kumar. Abstract: BACKGROUND: Caregivers role is crucial in lives of children with ...
This case study provides the pertinent nursing diagnoses, interventions, and outcomes for a child with global developmental delay. The interdisciplinary team approach and family involvement is addressed. IMPLICATIONS FOR NURSING. Use of NANDA, NIC, and NOC outcomes constructs for enhancing the care of a child with global developmental delay.
This case study provides the pertinent nursing diagnoses, interventions, and outcomes for a child with global developmental delay. The interdisciplinary team approach and family involvement is addressed. IMPLICATIONS FOR NURSING. Use of NANDA, NIC, and NOC outcomes constructs for enhancing the care of a child with global developmental delay.
Purpose: This case study focused on the care of a child with global developmental delay. Data sources: Data were obtained through the author's clinical practice in long-term care pediatric rehabilitation and literature sources. Data synthesis: NANDA-International Classifications, the Nursing Interventions Classification (NIC), and Nursing Outcomes Classification (NOC) were used to identify the ...
Evidence based case repor t. Assessing dev elopmental delay. Jon Dorling, Alison Salt. An 18 month old boy was referred to the local child. development centre because he was not walking inde ...
Case record reviews were undertaken for 46 023 households, 2236 (4.9%) of which contained a child in the target age range with developmental delay. Results. Children with developmental delay, when compared with their non-disabled peers, were at significantly increased risk of poorer health outcomes and of being exposed to a wide range of social ...
Introduction Global developmental delay (GDD) affects 1%-3% of the population of children under 5 years of age, making it one of the most common conditions presenting in paediatric clinics; causes are exogenous, genetic (non-metabolic) or genetic (metabolic). Recent advances in biotechnology and genetic testing mean that the investigations available to perform for children under 5 years are ...
Case studies of ECI for children with developmental disabilities. ... Development and Effectiveness of Home-based Programs for Preschool Children With Developmental Delay: 2017: USA: 1: 100: Digital Literacy Promotion: 2016: Bangladesh: 1: TOTAL: 4: 21: 25: 50: Table A1. Open in new tab
This case study provides the pertinent nursing diagnoses, interventions, and outcomes for a child with global developmental delay and the interdisciplinary team approach and family involvement is addressed. PURPOSE This case study focused on the care of a child with global developmental delay. DATA SOURCES Data were obtained through the author's clinical practice in long-term care pediatric ...
Case record reviews were undertaken for 46 023 households, 2236 (4.9%) of which contained a child in the target age range with developmental delay. Results: Children with developmental delay, when compared with their non-disabled peers, were at significantly increased risk of poorer health outcomes and of being exposed to a wide range of social ...
About developmental delay. When young children are slower to develop physical, emotional, social, communication and thinking skills than expected, it's called developmental delay. Developmental delay can show up in the way children move, communicate, think and learn, or behave with others. When more than one of these areas is affected, it ...
This case study focused on the care of a child with global developmental delay. Data were obtained through the author's clinical practice in long-term care pediatric rehabilitation and literature ...
Associate Name: Doreen Hunt. Name of Organization: Children's Therapy of Woodinville, P.L.L.C.. Age/Gender of Client: 5-year-old male with autism, developmental delays, attachment challenges Background: Thomas is a five-year old who was adopted at the age of 9 months, from a developing country where he spent time in an orphanage and foster care.He suffers from attachment challenges, Autism ...
View Case Study #1 - Ben, Developmental Delay from COMD 3600 at Utah State University. Running head: CASE STUDY #1 1 Case Study #1 - Developmental Delay Pamela Boyer A02318168 Utah State ... 2015) believed that the development of language is dynamic and arises in the ZPD, or Zone of Proximal Development, as a child interacts with other kids and ...
The presence of neural tissue in the encephalocele has correlated with the neurological development delay [12, 16]. In our study, the presence of mature neural tissue was also associated with moderate or severe developmental delay (OR = 9.37; 95%CI = 1.64-53.62) (p = 0.012). This study is unique in respect of analysing the histology of sac ...
Evidence-Based Case Reviews: Investigation of children with "developmental delay" - PMC. Journal List. West J Med. v.176 (1); 2002 Jan. PMC1071650. As a library, NLM provides access to scientific literature. Inclusion in an NLM database does not imply endorsement of, or agreement with, the contents by NLM or the National Institutes of Health.
Background It is well known that maternal diet affects the development of offspring. Herein, the relationship between maternal intake of fermented foods during pregnancy and offspring development was investigated. Methods The diet of 103,060 pregnant women at >4 months of gestation who were enrolled in the Japan Environment and Children's Study was analyzed. Their intake levels of fermented ...
Background Maternal depression and anxiety can have a detrimental impact on birth outcomes and healthy child development; there is limited knowledge on its influence on immunization schedule adherence. Therefore, the objectives of this study were to determine the impact of maternal depression and anxiety in the perinatal period on prolonged vaccine delay of childhood vaccines. Methods In this ...