Diathesis–Stress Model
Oliver Sussman
Neuroscience Researcher
Harvard University Undergraduate
Oliver Sussman is an undergraduate at Harvard University studying neuroscience within the interdisciplinary Mind, Brain, and Behavior program.
Learn about our Editorial Process
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
On This Page:
The Diathesis-Stress Model suggests that psychological disorders arise from the interaction of an underlying vulnerability (diathesis) and external stressors. An individual may have a predisposition to a disorder, but it’s the combination of this vulnerability and adverse life events that triggers its manifestation.

What is Diathesis?
The term “diathesis” comes from the Greek word for disposition (“diathesis”). In the context of the diathesis-stress model, this disposition is a factor that makes it more likely that an individual will develop a disorder following a stressful life event.
A diathesis can be a biological factor, like abnormal variations in one or more genes. But other sorts of factors, even if not genetically hard-wired, can also be considered diatheses so long as they form early on and are stable across a person’s life.
For example, traumatic early life experiences, such as the loss of a parent, can act as longstanding predispositions to a psychological disorder. In addition, personality traits like high neuroticism are sometimes also referred to as diatheses.
Finally, diatheses can be situational factors — like living in a low-income household or having a parent with mental illness (Theodore, 2020).
Some of these factors might matter more for some psychological disorders compared to others (for example, a particular genetic variation might increase one’s risk of developing depression but not schizophrenia).
It’s important to note that not all diatheses are created equal. For example, some genetic variations only slightly increase an individual’s risk of a mental disorder, while others increase one’s risk substantially.
As a result, in the diathesis-stress model, different diatheses give rise to different responses to stress.
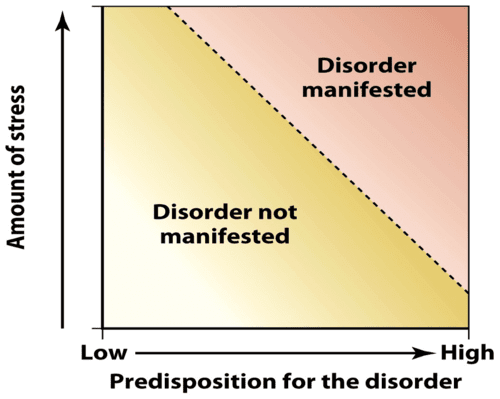
To conceptualize this, consider the “cup analogy.” Imagine several cups filled with different amounts of marbles; when water is poured into those cups, the cups with more marbles will overflow more easily.
Diatheses are like marbles, and stress is like water: the greater the diathesis, the less stress is needed to cause “overflow” (i.e., give rise to mental illness) (Theodore, 2020).
Diathesis-Stress Model
The diathesis-stress model is a concept in psychiatry and psychopathology that offers a theory of how psychological disorders emerge.
It intervenes in the debate about “ nature vs. nurture ” in psychopathology — whether disorders are predominantly caused by innate biological factors (“nature”) or by social and situational factors (“nurture”) — by providing an account of how both might coincide in giving rise to a disorder.
According to the diathesis-stress model, the emergence of a psychological disorder requires first the existence of a diathesis, or an innate predisposition to that disorder in an individual, and second, stress, or a set of challenging life circumstances that trigger the disorder’s development.
Furthermore, individuals with greater innate predispositions to a disorder may require less stress to trigger that disorder, and vice versa.
In this way, the diathesis-stress model explains how psychological disorders might be related to both nature and nurture and how those two components might interact with one another (Broerman, 2017).
The diathesis-stress model is a modern development of a longstanding debate about the causes of mental illness. This debate began as early as ancient Greece and Rome when theories included imbalances in bodily fluids and interactions with the devil.
Later, this evolved into the “nature vs. nurture” debate. By the late 20th century, it became clear that nature interacted with nurture to produce disorder, and the diathesis-stress model came to the forefront (Theodore, 2020).
The model has been useful in explaining why some individuals with biological dispositions to mental illness do not develop a disorder and why some individuals living through stressful life circumstances nonetheless remain psychologically healthy.
It has also opened the door to research into protective factors: positive elements that counteract the effects of diathesis and stress to prevent the onset of a disorder.
Finally, it has proven particularly useful in the context of specific disorders, such as schizophrenia and depression.
Diathesis and Stress Interactions
According to the diathesis-stress model, diatheses interact with stress to bring about mental illness. In this context, “stress” is an umbrella term encompassing any life event that disrupts an individual’s psychological equilibrium — their normal, healthy regulation of thoughts and emotions.
In the diathesis-stress model, these challenging life events are thought to interact with individuals’ innate dispositions to bring psychological disorders to the surface.
Stress comes in many different forms. It may be a single traumatic event, like the death of a close relative or friend. But stress can also be an ongoing, sustained challenge in one’s life, like a chronic illness or an abusive relationship.
It can even be more mundane, the sorts of things we usually mean when we talk about “stress” — like anxiety from work or school (Theodore, 2020).
These events or situations can profoundly impact individual psychology and interact with diatheses to foment mental illness.
The role of stress in the diathesis-stress model is nuanced. For one, some life circumstances may constitute both a diathesis and stress. For instance, a child with a parent who suffers from mental illness may be genetically predisposed to that illness and may also undergo stress as a result of her parent’s condition (Theodore, 2020).
Second, the timing of stress within an individual’s lifespan may be important; certain disorders are thought to have “windows of vulnerability” during which they are more likely to be brought about by stressful life events (Lokuge, 2011)
Moreover, positive life circumstances, called protective factors, may counteract stress, which decrease the likelihood that a disorder will emerge in response to stress.
Finally, different stresses are thought to play different roles across mental disorders — in other words, a particular form of stressful life event may play an especially pronounced role in depression, or schizophrenia, etc. These last two points will be explored in the sections below.
Protective Factors
Just as negative elements in one’s life make the onset of a psychological disorder more likely, there can also be positive elements that make the onset of a disorder less likely. These positive elements are called protective factors.
Protective factors help explain why some people who have both significant diatheses and stresses nonetheless remain psychologically healthy — in these cases, protective factors prevent a disorder from coming to the surface (Theodore, 2020).
Protective factors can be conditions, meaning beneficial life circumstances that protect against mental illness. They can also be attributes: traits or behaviors of an individual that make them more resilient against psychological disorders (“Protective Factors”).
Conditions that act as protective factors include strong parental and social support and assistance from psychotherapists or counselors. Attributes that act as protective factors include social and emotional competence and the use of healthy coping strategies and stress management techniques (Theodore, 2020).
By itself, the diathesis-stress model does not necessarily include protective factors in its assessment of the causes of psychological disorders.
As a result, the model has been updated in recent years to accommodate protective factors. This updated model is sometimes called the stress-vulnerability-protective factors model (Theodore, 2020).
The diathesis-stress model has proven useful in illuminating the causes of specific psychological disorders. One area where the model has had considerable success is schizophrenia, a disease with both genetic and environmental causes.
Schizophrenia
While schizophrenia has a strong genetic component, some individuals with genetic susceptibilities to the disorder nonetheless remain healthy.
As a result, the view currently held by many psychiatrists is that schizophrenia requires a genetic predisposition in combination with stress later on in life, which then triggers the emergence of the disorder.
Some researchers have also put forth a neural diathesis-stress model of schizophrenia, in which they attempt to explain how brain changes resulting from diatheses and stresses give rise to the disorder (Jones and Fernyhough, 2007).
Thus, the diathesis-stress model does well to explain the origins of schizophrenia and has even been supported by evidence from neuroscience.
The diathesis-stress model has also been used to explain the origins of depression. Similarly to schizophrenia, genetic risk factors for depression have been identified, but not all people with those risk factors go on to develop the disorder.
According to the diathesis-stress model of depression, stressful life events interact with genetic predispositions to bring about depressive symptoms.
This model of depression has been validated by research — a study found there to be an interaction effect between genetic risk factors for depression and scores on an inventory of stressful life events in predicting depressive symptoms (Colodro-Conde et al., 2018).
The model has also proven useful in explaining suicidal behavior. Early models of suicidal behavior tended to focus exclusively on stress, which failed to account for why some individuals exposed to extreme stress nonetheless refrain from engaging in suicidal behavior.
Since suicidal behavior likely also relies on an interaction between genetic and early childhood dispositions with stress later in life, researchers have suggested that efforts to treat and prevent suicidal behavior should utilize a diathesis-stress model (van Heeringen, 2012).
Different psychological disorders have different causes. Some may rely more strongly on hard-wired predispositions, while others may respond more to stressful events later in life.
Nevertheless, the diathesis-stress model has been shown to have wide applicability across many areas of psychiatry.
It offers a powerful explanation of how nature and nurture might come together to give rise to mental illness, a much-needed advancement over earlier theories that took one or the other to be completely determinative.
Broerman, R. (2017). Diathesis-Stress Model. In V. Zeigler-Hill & T. K. Shackelford (Eds.), Encyclopedia of Personality and Individual Differences (pp. 1–3). Springer International Publishing. https://doi.org/10.1007/978-3-319-28099-8_891-1
Colodro-Conde, L., Couvy-Duchesne, B., Zhu, G., Coventry, W. L., Byrne, E. M., Gordon, S., Wright, M. J., Montgomery, G. W., Madden, P. a. F., Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, Ripke, S., Eaves, L. J., Heath, A. C., Wray, N. R., Medland, S. E., & Martin, N. G. (2018). A direct test of the diathesis-stress model for depression. Molecular Psychiatry, 23(7), 1590–1596. https://doi.org/10.1038/mp.2017.130
DIATHESIS | Meaning & Definition for UK English | Lexico.com. (n.d.). Lexico Dictionaries | English. Retrieved February 23, 2022, from https://www.lexico.com/definition/diathesis
Jones, S. R., & Fernyhough, C. (2007). A new look at the neural diathesis–stress model of schizophrenia: The primacy of social-evaluative and uncontrollable situations. Schizophrenia Bulletin, 33(5), 1171–1177. https://doi.org/10.1093/schbul/sbl058
Lokuge, S., Frey, B. N., Foster, J. A., Soares, C. N., & Steiner, M. (2011). Depression in women: Windows of vulnerability and new insights into the link between estrogen and serotonin. The Journal of Clinical Psychiatry, 72(11), e1563-1569. https://doi.org/10.4088/JCP.11com07089
Protective Factors to Promote Well-Being and Prevent Child Abuse & Neglect—Child Welfare Information Gateway. (n.d.). Retrieved February 23, 2022, from https://www.childwelfare.gov/topics/preventing/promoting/protectfactors/
Theodore. (2020, April). Diathesis-Stress Model (Definition + Examples). Retrieved from https://practicalpie.com/diathesis-stress-model/.
van Heeringen, K. (2012). Stress–Diathesis Model of Suicidal Behavior. In Y. Dwivedi (Ed.), The Neurobiological Basis of Suicide. CRC Press/Taylor & Francis. http://www.ncbi.nlm.nih.gov/books/NBK107203/
Walker, E. F., & Diforio, D. (1997). Schizophrenia: a neural diathesis-stress model . Psychological review, 104(4), 667.
Related Articles

Clinical Psychology
A Study Of Social Anxiety And Perceived Social Support

Psychological Impact Of Health Misinformation: A Systematic Review

Sleep Loss and Emotion: A Systematic Review and Meta-Analysis

Family History Of Autism And ADHD Vary With Recruitment Approach And SES

Measuring And Validating Autistic Burnout
A Systematic Review of Grief and Depression in Adults
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
What Is the Diathesis-Stress Model?
Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
:max_bytes(150000):strip_icc():format(webp)/IMG_9791-89504ab694d54b66bbd72cb84ffb860e.jpg)
Steven Gans, MD is board-certified in psychiatry and is an active supervisor, teacher, and mentor at Massachusetts General Hospital.
:max_bytes(150000):strip_icc():format(webp)/steven-gans-1000-51582b7f23b6462f8713961deb74959f.jpg)
Fizkes / Getty Images
- How It Works
How to Manage Your Stress
The diathesis-stress model is a theory suggesting that mental disorders and medical conditions are caused by a combination of an inherent predisposition and and the person's experience of stress . Also known as the vulnerability-stress model , it is a framework for understanding how existing vulnerabilities and environmental stresses interact to influence mental health conditions.
While the term sounds unwieldy and complex, the phenomenon it explains is relatively easy to understand.
Diathesis and Stress
- Diathesis refers to a predisposition or vulnerability to developing a mental disorder. This can be due to genetic factors , early life experiences, or other biological susceptibilities.
- Stress refers to the environmental factors that trigger the onset of mental illness or exacerbate existing conditions. These can include significant life events, trauma , and daily stressors.
This article discusses the diathesis-stress model, how it explains the onset of different mental health conditions, and how you can use this information to better moderate the stress in your life.
History of the Diathesis Stress Model
The model can trace its origins to the 1950s, although roots of this theory date to much earlier. Paul Meehl, in the 1960s, applied this approach to explain the origins of schizophrenia . Since then, it has also been utilized to understand the development of depression. According to researchers who use this viewpoint, people with a predisposition for depression are more likely to develop the condition when exposed to stress.
This theory was later expanded to include other mental health conditions, including anxiety and eating disorders.
The diathesis-stress model is one of the most widely accepted theories for explaining the etiology of mental disorders.
How the Diathesis Stress Model Works
Everyone has vulnerabilities due to genes, genetic abnormalities, or the complex interaction of various genes. But just because these predispositions exist does not mean that an individual will develop a particular condition.
In many cases, a disorder will only emerge when stress-related pressures trigger the underlying diathesis. This exposure to stress can trigger the mental disorder's onset or worsen existing symptoms.
However, it is essential to note that not everyone with a predisposition will develop a mental disorder, just as not everyone who experiences stress is destined to experience mental illness. The diathesis-stress model is one way to explain why some people are more vulnerable to mental illness than others. It also explains why some people may develop a mental disorder after exposure to stressful life events while others do not.
The heritability of mental illness ranges from around 40% for depression to about 80% for schizophrenia.
However, it is essential to remember that in most cases, no single gene is responsible for causing a mental disorder. Instead, it is often the result of many genes or other biological factors interacting with environmental variables that determine overall risk.
Types of Conditions Linked to Diathesis and Stress
The diathesis-stress model has been utilized to explain the etiology of many different types of mental health conditions, including:
Anxiety Disorders
While the exact causes of anxiety disorders are unknown, genetic vulnerabilities and exposure to stressful life events can play a role. Anxiety disorders tend to run in families, and having an inherited predisposition means that traumatic experiences are more likely to trigger anxiety. Genetics can also impact how people manage stress.
A combination of factors is believed to contribute to the onset of depression , including genetics, a family history of depression, and exposure to stressful life events.
Schizophrenia
Experts suggest that schizophrenia is caused by a combination of genetics and stressful events. For example, around 25% of individuals with 22q11.2 deletion syndrome (aka DiGeorge Syndrome) develop schizophrenia, indicating a significant genetic component. Not everyone with this genetic variation experiences schizophrenia, suggesting that environmental factors also play a part.
Eating Disorders
Genetic, environmental, and psychosocial factors are believed to contribute to the development of eating disorders. For many people, stressful life events that leave them feeling a loss of control can lead people with inherent vulnerability to extreme behaviors to control weight and food intake.
Uses for the Diathesis Stress Model
The diathesis-stress model has been used to help improve research, diagnosis, and treatment of mental disorders. Some uses for this model include:
- Understanding the causes of mental illness : Where other models of mental illness focus primarily on nature or nurture , the diathesis-stress model suggests that mental disorders are best understood as resulting from biological and environmental influences.
- Reducing the stress that contributes to mental disorders : Because of the emphasis on the role stress plays in causing mental illness, this model encourages the use of stress reduction strategies to help limit disease risk. While genetic and other biological factors cannot be controlled, stress is a modifiable risk factor that people can address through lifestyle changes. This may include stress-relief techniques, such as relaxation and mindfulness .
- Exploring the interactions of biological and environmental influences : The diathesis-stress model is also helpful in understanding the complex interaction of biological and environmental factors in developing mental disorders. It can help guide research to identify the causes of mental illness, ultimately leading to the development of more effective treatments.
- Understanding the impact of genetics and environment : This model helps explain why some people are more likely to develop mental health conditions following stressful events and why others are not.
Impact of the Diathesis-Stress Model
The diathesis-stress model has influenced how researchers investigate mental health conditions. It has helped shift the focus of research from nature vs. nurture debates to a more nuanced understanding of how biological and environmental factors contribute to mental illness.
The diathesis-stress model has also helped change how mental disorders are treated. The model suggests that treatment should reduce stress and address the underlying diathesis to the degree possible. This has led to the use of new therapies, such as mindfulness-based stress reduction , that might mitigate these risks.
The diathesis-stress model is a widely accepted theory with important implications for research and treatment. The model has helped to improve our understanding of mental disorders and develop more effective treatments.
While avoiding all sources of stress is impossible, limiting your stress and developing strong stress management skills may help limit your health risks. Some things you can do include:
- Identify your sources of stress
- Explore ways to avoid or minimize your stress
- Develop healthy coping mechanisms
- Try stress relief techniques such as deep breathing , progressive muscle relaxation , and guided meditation
- Exercise regularly
- Write in a gratitude journal
Making lifestyle changes and developing healthy coping mechanisms can help reduce stress and protect your mental health.
Protective Factors
Stress and genetic factors contribute to increased vulnerability, but protective factors can counteract some of the effects of stress. Some protective factors that can affect the interaction of diathesis and stress include secure attachments , positive relationships, stress management skills, and emotional competence.
Certain traits and characteristics can also make people more resilient to stress. For example, the big five personality traits of extroversion and conscientiousness have been linked to increased resilience.
The diathesis-stress model is a widely accepted theory with important implications for research and treatment. The model suggests that a mental disorder develops when an individual has a vulnerability or predisposition combined with exposure to stressful life events. This exposure can trigger the mental disorder's onset or worsen existing symptoms. The diathesis-stress model has helped to improve our understanding of mental disorders and led to the development of more effective treatments.
Broerman R. Diathesis-stress model . In: Zeigler-Hill V, Shackelford TK, eds. Encyclopedia of Personality and Individual Differences . Springer International Publishing; 2018:1-3. doi:10.1007/978-3-319-28099-8_891-1
Kendler KS. A prehistory of the diathesis-stress model: predisposing and exciting causes of insanity in the 19th century . AJP . 2020;177(7):576-588. doi:10.1176/appi.ajp.2020.19111213
Pettersson E, Lichtenstein P, Larsson H, et al. Genetic influences on eight psychiatric disorders based on family data of 4 408 646 full and half-siblings, and genetic data of 333 748 cases and controls . Psychol Med . 2019;49(07):1166-1173. doi:10.1017/S0033291718002039
Daviu N, Bruchas MR, Moghaddam B, Sandi C, Beyeler A. Neurobiological links between stress and anxiety . Neurobiol Stress . 2019 Aug 13;11:100191. doi:10.1016/j.ynstr.2019.100191
Generation Scotland, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, Arnau-Soler A, et al. A validation of the diathesis-stress model for depression in Generation Scotland . Transl Psychiatry . 2019;9(1):25. doi:10.1038/s41398-018-0356-7
Cleynen I, Engchuan W, Hestand MS, et al. Genetic contributors to risk of schizophrenia in the presence of a 22q11.2 deletion . Mol Psychiatry . 2021;26(8):4496-4510. doi:10.1038/s41380-020-0654-3
Stice E. Interactive and mediational etiologic models of eating disorder onset: evidence from prospective studies . Annu Rev Clin Psychol . 2016;12(1):359-381. doi:10.1146/annurev-clinpsy-021815-093317)
Childs E, de Wit H. Regular exercise is associated with emotional resilience to acute stress in healthy adults . Front Physiol . 2014;5:161. doi:10.3389/fphys.2014.00161
Sharma S, Mustanski B, Dick D, Bolland J, Kertes DA. Protective factors buffer life stress and behavioral health outcomes among high-risk youth . J Abnorm Child Psychol . 2019 Aug;47(8):1289-1301. doi:10.1007/s10802-019-00515-8
de la Fuente J, González-Torres MC, Artuch-Garde R, Vera-Martínez MM, Martínez-Vicente JM, Peralta-S’anchez FJ. Resilience as a buffering variable between the big five components and factors and symptoms of academic stress at university . Front Psychiatry . 2021;12:600240. doi:10.3389/fpsyt.2021.600240
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
Diathesis-Stress Model (Definition + Examples)

At the center of many theories in social psychology is the nature/nurture debate. Do we develop a certain personality and make certain choices because it’s in our nature? Or do our experiences alone shape who we become and how we look at the world? I want to look at one particular model that considers both factors. It’s called the Diathesis Stress Model (the Stress-Vulnerability Model.) This takes genetics and personal experiences into account when assessing a person’s likelihood of developing mental disorders.
What Is the Diathesis-Stress Model?
The diathesis-stress model is a theory about how stress and genetics play into the manifestation of different mental disorders and conditions. High stress and high predisposition significantly increase your risk of developing said disorders. But even high stress and low predisposition can increase your risk.
History of the Diathesis Stress Model
Mental disorders are not always easy to identify. It’s not easy to pinpoint a moment when a person becomes depressed. And the causes attributed to mental illnesses have caused some pretty dramatic treatments over time.
In Ancient Rome, for example, Hippocrates connected depression to an excess of “black bile” in the kidney or spleen. A physician named Asclepiades argued against these terms, saying that emotions of grief or hurt caused depression. Asclepiades was pretty close, but his theories soon were thrown out. For centuries after the Ancient Romans, mental disorders were connected to interactions with the devil. Some patients with mental disorders were burned at the stake.

Fast-forward to 1977. At this point, psychologists and doctors believed that more natural factors played into the development of mental disorders. Could it be genes? Could it be emotions? Maybe trauma from childhood, as Freud theorized? What if a combination could explain a disorder like schizophrenia?
How Does the Diathesis-Stress Model Explain Schizophrenia?
Joseph Zubin, a Lithuanian-American psychologist who specialized in the study of schizophrenia, believed that relapses could be predicted using a model. On one axis was diathesis, or vulnerability. On the other axis was stress. The combination of low vulnerability and stress could keep a person from relapsing or showing symptoms.
Zubin’s model, the Diathesis-Stress model, has since been adapted and applied to a range of mental disorders and conditions, including addiction and depression . Let’s go over what these factors mean and how they can help anyone to keep themselves in check.
The Diathesis-Stress Model Cup Analogy
The first part of this model is diathesis. Regarding the diathesis-stress model, “diathesis” is often used interchangeably with “vulnerability.” If someone is more vulnerable to a mental disorder, it might not take a lot of stress for symptoms to start appearing.
Think of this like a cup. In this analogy, diathesis is the marbles in the cup. Stress is water. A cup that is ¼ full of marbles will need quite a bit of water before it starts to spill over. If the cup is ¾ full of marbles, it won’t take that much water. This is how diathesis and stress work.
So what causes diathesis? A few factors may come into play here:
- Genes or biology
- Cognitive factors, such as perception, memory, problem-solving skills, and decision-making abilities
- Trauma or environmental stressors early in life
- Situational factors (living with a parent with mental illness, living in a low-income household, etc.)
Not all of these things are “ natural ” because they are genetic. But these factors tend to stay very present in a person’s life. If a person could move from a low-income household into a more affluent situation, they may be less vulnerable to certain conditions. Moving these “marbles” out of a person’s cup takes a lot. It might be impossible.
Now let’s talk about stress or the “water” in the analogy I used earlier. When stress is piled onto a person’s life, they are more likely to be “triggered” and display symptoms of a mental disorder. Stressors may be things that are generally considered stressful:
- Death in the family
- A global pandemic
Chronic illnesses or ongoing stressors may not be a one-time event but may continue to “fill your cup.” Stressors don’t have to be traumatic or life-altering, either. The stress of graduating high school, studying for exams, or buying a home may also “fill your cup.”
At some point, too much stress can fill anyone’s cup. You do not have to be predisposed to a specific mental disorder to develop one. You do not have to have a family of addicts to become addicted to alcohol or drugs.
It’s important to note that diathesis can cause stress or vice versa. Growing up with the knowledge that a parent has bipolar disorder can be very stressful. That early stress may remain in the body, making a person more vulnerable. Without addressing the issue or seeking treatment, this process can continue to cause more and more stress and vulnerability.
Protective Factors
But what about someone who is predisposed to a specific mental disorder, has a lot of stress, but still manages to go about life without showing any major symptoms?
These people are probably shielded from certain “protective factors.”
In the past 20 years, the diathesis-stress model has been adapted to include these protective factors. You may hear this version of the “stress-vulnerability-protective factors model.” Stressors and diathesis are still present in this model.
Protective factors make stressors easier to handle. These factors may include:
- A positive relationship with parents or children
- A support group
- Help from a counselor or therapist
- Self-awareness or emotional intelligence
- Understanding of stress management techniques
- A good grasp of healthy coping mechanisms or emotional regulation
Example of the Diathesis-Stress Model
An addict, for example, may be predisposed to an addiction and face stress in their marriage and career. With the help of an AA or NA group, they may be able to face those stressors and overcome them without relapsing.
What Does This Mean?
The Diathesis-Stress Model can be used to assess anyone’s risk. But if you take away one lesson about this model, it’s that stress is dangerous. Stress can be hard to avoid, like during a global pandemic or a tragedy out of your control. But if you notice that more stress is coming into your life, assessing what protective factors are present is especially important. Do you have positive relationships that can help you through a stressful time? Do your diet and daily routine contribute to stress or protect you from it? The more protective factors in your life, the more confident you can be in your health and minimize risks.
How to Reduce Stress and Protect Your Mental Health
Stress can feel inevitable. In some ways, it is. Everyone goes through tough times or worries about the future. But you do not have to let that stress control your life. Take action to reduce your stress and create a healthier environment for you and the people around you.
Where Does Stress Come From?
The feeling of being stressed - sweaty palms, a tight chest, etc. - is often the result of hormones entering your bloodstream. When the brain encounters something stressful, it tells the body to release cortisol, adrenaline, and other "stress" hormones. Hormones are chemical messengers. Hormones like cortisol or adrenaline send a specific message throughout the body: we are in danger.
Your brain doesn't have to be in danger to release these hormones. You might be in front of a grizzly bear or think about your crush rejecting you. The response from the brain is the same. Knowing this, you can manage stress in one of two ways: prevent situations from feeling as dire as being in front of a grizzly bear or calm your body down when the hormones start to flow through the bloodstream.
Preventing Stress
Consider your diet and exercise.
Diet and exercise significantly influence the production and release of stress hormones. Consider this: 95% of serotonin, a key neurotransmitter, is produced in the gut. Serotonin plays a crucial role in regulating mood, anxiety, and happiness. It also impacts other functions like sleep, appetite, and digestion.
This fact underscores how closely our physical and mental health are connected. By taking care of your body through proper nutrition and physical activity, you also support your mental well-being, creating a beneficial cycle where treating your body rightfully positively affects your mind and vice versa.
Getting eight hours of sleep won't just help you grow big and strong. Sleep is part of the body's circadian rhythm. This 24-hour cycle helps the body wake up and fall asleep through the release of - you guessed it - hormones. The body releases cortisol in the morning as you get up. Improper sleep schedules mess with the body's intended cortisol production. This can lead to a myriad of health issues , including increased appetite and high blood pressure. Getting proper sleep is one of the best things you can do for your physical and mental health.
Setting Boundaries
Stress often comes from feeling overwhelmed . There are only 24 hours in a day. (And hopefully, you are spending around eight hours sleeping.) It is okay to say "no" to responsibilities, obligations, projects, or even vacations that feel like "too much." The easiest way to handle a stressful schedule is to lighten it!
Handling Stress
Take some time to write down how you are feeling. Journaling allows you to process your emotions using different parts of your brain. You might gain a new perspective on your situation and what solutions you can implement as you write. You do not have to spend a lot of time journaling each day. Sit down for five minutes, write about your day, and see where your words take you.
Practice Mindful Meditation
Stress can become a nasty cycle. You experience stress, so you indulge in unhealthy habits to reduce stress, and then you stress about your unhealthy habits, and you become stressed again. Break that cycle by sitting down, closing your eyes, and meditating. Mindfulness meditation doesn't require you to take a vow of silence or chant mantras for hours a day. You can be mindful by pausing and being aware of your thoughts. As you become aware of your thoughts, you may find that you are working yourself up over something silly - certainly nothing to put you in danger. Sit, breathe deeply, and remind your body that you are not in danger. You may find that your jaw unclenches, your palms start sweating, and your tense muscles loosen!
Reach Out To Your Support Groups
Belonging is listed as one of Maslow's Hierarchy of Needs. Humans feel the desire to be part of a group that supports them. Humans also want to support other humans! Contact friends if you feel stressed, overwhelmed, or out of control. Reach out to your family. Reach out to a support group of people going through similar things. Meetups for people going through grief, who have family members who are struggling with addiction, or who are addicts themselves can help you. Don't let your stress turn into something more severe. Get help and take care of yourself.
Related posts:
- Hypophyseal Portal System
- Sleep Stages (Light, Deep, REM)
- The 10 Personality Disorders (Clusters A, B, C)
- Hypothalamic Pituitary Adrenal Axis (HPA)
- What Endocrine Functions take place in the Brain?
Reference this article:
About The Author
Free Personality Test
Free Memory Test
Free IQ Test
PracticalPie.com is a participant in the Amazon Associates Program. As an Amazon Associate we earn from qualifying purchases.
Follow Us On:
Youtube Facebook Instagram X/Twitter
Psychology Resources
Developmental
Personality
Relationships
Psychologists
Serial Killers
Psychology Tests
Personality Quiz
Memory Test
Depression test
Type A/B Personality Test
© PracticalPsychology. All rights reserved
Privacy Policy | Terms of Use
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 18 January 2019
A validation of the diathesis-stress model for depression in Generation Scotland
- Aleix Arnau-Soler ORCID: orcid.org/0000-0001-9768-0513 1 ,
- Mark J. Adams ORCID: orcid.org/0000-0002-3599-6018 2 ,
- Toni-Kim Clarke 2 ,
- Donald J. MacIntyre ORCID: orcid.org/0000-0001-6963-1335 2 ,
- Keith Milburn 3 ,
- Lauren Navrady ORCID: orcid.org/0000-0001-5625-7962 2 ,
- Generation Scotland, ,
- Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium ,
- Caroline Hayward ORCID: orcid.org/0000-0002-9405-9550 4 ,
- Andrew McIntosh ORCID: orcid.org/0000-0002-0198-4588 2 , 5 &
- Pippa A. Thomson ORCID: orcid.org/0000-0002-4208-5271 1 , 5
Translational Psychiatry volume 9 , Article number: 25 ( 2019 ) Cite this article
11k Accesses
33 Citations
7 Altmetric
Metrics details
Depression has well-established influences from genetic and environmental risk factors. This has led to the diathesis-stress theory, which assumes a multiplicative gene-by-environment interaction (GxE) effect on risk. Recently, Colodro-Conde et al . empirically tested this theory, using the polygenic risk score for major depressive disorder (PRS, genes) and stressful life events (SLE, environment) effects on depressive symptoms, identifying significant GxE effects with an additive contribution to liability. We have tested the diathesis-stress theory on an independent sample of 4919 individuals. We identified nominally significant positive GxE effects in the full cohort ( R 2 = 0.08%, p = 0.049) and in women ( R 2 = 0.19%, p = 0.017), but not in men ( R 2 = 0.15%, p = 0.07). GxE effects were nominally significant, but only in women, when SLE were split into those in which the respondent plays an active or passive role ( R 2 = 0.15%, p = 0.038; R 2 = 0.16%, p = 0.033, respectively). High PRS increased the risk of depression in participants reporting high numbers of SLE ( p = 2.86 × 10 −4 ). However, in those participants who reported no recent SLE, a higher PRS appeared to increase the risk of depressive symptoms in men ( β = 0.082, p = 0.016) but had a protective effect in women ( β = −0.061, p = 0.037). This difference was nominally significant ( p = 0.017). Our study reinforces the evidence of additional risk in the aetiology of depression due to GxE effects. However, larger sample sizes are required to robustly validate these findings.
Similar content being viewed by others
Stress-related exposures amplify the effects of genetic susceptibility on depression and anxiety

Patterns of stressful life events and polygenic scores for five mental disorders and neuroticism among adults with depression
Multiple dimensions of stress vs. genetic effects on depression
Introduction.
Stressful life events (SLE) have been consistently recognized as a determinant of depressive symptoms, with many studies reporting significant associations between SLE and major depressive disorder (MDD) 1 , 2 , 3 , 4 , 5 , 6 , 7 . Some studies suggest that severe adversity is present before the onset of illness in over 50% of individuals with depression 8 and may characterize a subtype of cases 9 . However, some individuals facing severe stress never present symptoms of depression 10 . This has led to a suggestion that the interaction between stress and an individual’s vulnerability, or diathesis , is a key element in the development of depressive symptoms. Such vulnerability can be conceived as a set of biological factors that predispose to illness. Several diathesis-stress models have been successfully applied across many psychopathologies 11 , 12 , 13 , 14 , 15 .
The diathesis-stress model proposes that a latent diathesis may be activated by stress before psychopathological symptoms manifest. Some levels of diathesis to illness are present in everybody, with a threshold over which symptoms will appear. Exceeding such a threshold depends on the interaction between diathesis and the degree of adversity faced in SLE, which increases the liability to depression beyond the combined additive effects of the diathesis and stress alone 11 . Genetic risk factors can, therefore, be conceived as a genetic diathesis . Thus, this genetically driven effect produced by the diathesis-stress interaction can be seen as a gene-by-environment interaction (GxE).
MDD is characterized by a highly polygenic architecture, composed of common variants with small effect and/or rare variants 16 . Therefore, interactions in depression are also expected to be highly polygenic. In recent years, with the increasing success of genome-wide association studies, GxE studies in depression have shifted towards hypothesis-free genome-wide and polygenic approaches that capture liability to depression using genetic data 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 . Recent advances in genomics and the massive effort from national institutions to collect genetic, clinical and environmental data on large population-based samples now provide an opportunity to empirically test the diathesis-stress model for depression. The construction of polygenic risk scores (PRS) offers a novel paradigm to quantify genetic diathesis into a single genetic measure, allowing us to study GxE effects with more predictive power than any single variant 25 , 26 , 27 , 28 . PRS are genetic indicators of the aggregated number of risk alleles carried by an individual weighted by their allelic effect estimated from genome-wide association studies. This polygenic approach to assessing the diathesis-stress model for depression has been tested using either childhood trauma 17 , 19 , 24 or adult SLE 18 , 23 , 24 as measures of environmental adversity.
Recently, Colodro-Conde et al. 23 provided a direct test of the diathesis-stress model for recent SLE and depressive symptoms. In this study, Colodro-Conde et al. used PRS weighted by the most recent genome-wide meta-analysis conducted by the Psychiatric Genetics Consortium (PGC; N = 159,601), and measures of three environmental exposures: lack of social support, “personal” SLE, and “network” SLE. Colodro-Conde et al. reported a significant additive risk on liability to depression due to a GxE effect in individuals who combine a high genetic predisposition to MDD and a high number of reported “personal” SLE, mainly driven by effects in women. A significant effect of interaction was not detected in males. They found no significant interaction between the genetic diathesis and “network” SLE or social support. They concluded that the effect of stress on risk of depression was dependent on an individual’s diathesis , thus supporting the diathesis-stress theory. In addition, they suggested possible sex-specific differences in the aetiology of depression. However, Colodro-Conde et al. findings have not, to our knowledge, been independently validated.
In the present study, we aim to test the diathesis-stress model in an independent sample of 4919 unrelated white British participants from a further longitudinal follow-up from Generation Scotland, and assess the differences between women and men, using self-reported depressive symptoms and recent SLE.
Materials and methods
Sample description.
Generation Scotland is a family-based population cohort recruited throughout Scotland by a cross-disciplinary collaboration of Scottish medical schools and the National Health Service (NHS) between 2006 and 2011 29 . At baseline, blood and salivary DNA samples from Generation Scotland participants were collected, stored and genotyped at the Wellcome Trust Clinical Research Facility, Edinburgh. Genome-wide genotype data were generated using the Illumina HumanOmniExpressExome-8 v1.0 DNA Analysis BeadChip (San Diego, CA, USA) and Infinium chemistry 30 . The procedures and further details for DNA extraction and genotyping have been extensively described elsewhere 31 , 32 . In 2014, 21,525 participants from Generation Scotland eligible for re-contact were sent self-reported questionnaires as part of a further longitudinal assessment funded by a Wellcome Trust Strategic Award “STratifying Resilience and Depression Longitudinally” (STRADL) 33 to collect new and updated mental health questionnaires including psychiatric symptoms and SLE measures. 9618 re-contacted participants from Generation Scotland agreed to provide new measures to the mental health follow-up 33 (44.7% response rate). Duplicate samples, those showing sex discrepancies with phenotypic data, or that had more than 2% missing genotype data, were removed from the sample, as were samples identified as population outliers in principal component analysis (mainly non-Caucasians and Italian ancestry subgroups). In addition, individuals with diagnoses of bipolar disorder, or with missing SLE data, were excluded from the analyses. SNPs with more than 2% of genotypes missing, Hardy-Weinberg Equilibrium test p < 1 × 10 −6 , or a minor allele frequency lower than 1%, were excluded. Individuals were then filtered by degree of relatedness (pi-hat < 0.05) using PLINK v1.9 34 , maximizing retention of those participants reporting higher numbers of SLE (see phenotype assessment below). After quality control, the final dataset comprised 4919 unrelated individuals of European ancestry and 560 351 SNPs (mean age at questionnaire: 57.2, s.d. = 12.2, range 22–95; women : n = 2990–60.8%, mean age 56.1, s.d. = 12.4; men : n = 1 929–39.2%, mean age 58.7, s.d. = 11.8). Further details on the recruitment procedure and Generation Scotland profile are described in detail elsewhere 29 , 31 , 35 , 36 , 37 . All participants provided written consent. All components of Generation Scotland and STRADL obtained ethical approval from the Tayside Committee on Medical Research Ethics on behalf of the National Health Service (reference 05/s1401/89). Generation Scotland data is available to researchers on application to the Generation Scotland Access Committee ([email protected]).
Phenotype assessment
Participant self-reported current depressive symptoms through the 28-item scaled version of The General Health Questionnaire 38 , 39 . The General Health Questionnaire is a reliable and validated psychometric screening tool to detect common psychiatric and non-psychotic conditions (General Health Questionnaire Cronbach alpha coefficient: 0.82–0.86) 40 . This consists of 28 items designed to identify whether an individual’s current mental state has changed over the last 2 weeks from their typical state. The questionnaire captures core symptoms of depression through subscales for severe depression, emotional (e.g., anxiety and social dysfunction) and somatic symptoms linked to depression. These subscales are highly correlated 41 and suggest an overall general factor of depression 42 . Participants rated the 28 items on a four-point Likert scale from 0 to 3 to assess its degree or severity 40 (e.g., Have you recently felt that life is entirely hopeless? “Not at all”, “No more than usual”, “Rather more than usual”, “Much more than usual”), resulting on an 84-point scale depression score. The Likert scale, which provides a wider and smoother distribution 40 , may be more sensitive to detect changes in mental status in those participants with chronic conditions or chronic stress who may feel their current symptoms as “usual” 43 , and to detect psychopathology changes as response to stress. The final depression score was log transformed to reduce the effect of positive skew and provide a better approximation to a normal distribution. In addition, participants completed the Composite International Diagnostic Interview–Short Form, which diagnoses lifetime history of MDD according to DSM-IV criteria 44 . The depression score predicted lifetime history of MDD (odd ratio = 1.91, 95% confidence intervals 1.80–2.02, p = 1.55 × 10 −102 , N = 8994), with a 3.8-fold increased odds of having a lifetime history of MDD between participants in the top and bottom deciles, thus supporting the usefulness of the depression score in understanding MDD. To improve interpretation, we scaled the depression score to a mean of 0 when required (Fig. 3 ).
Data from a self-reported questionnaire based on the List of Threating Experiences 45 was used to construct a measure of common SLE over the previous 6 months. The List of Threatening Experiences is a reliable psychometric device to measure psychological “stress” 46 , 47 . It consists of a 12-item questionnaire to assess SLE with considerable long-term contextual effects (e.g., Over last 6 months, did you have a serious problem with a close friend, neighbor or relatives? ). A final score reflecting the total number of SLE (TSLE) ranging from 0 to 12 was constructed by summing the “yes” responses. Additionally, TSLE was split into two categories based on those items measuring SLE in which the individual may play and active role exposure to SLE, and therefore in which the SLE is influenced by genetic factors and thus subject to be “dependent” on an individual’s own behavior or symptoms (DSLE; 6 items, e.g., a serious problem with a close friend, neighbor or relatives may be subject to a respondent’s own behavior), or SLE that are not influenced by genetic factors, likely to be “independent” on a participant’s own behavior (ISLE; 5 items, e.g., a serious illness, injury or assault happening to a close relative is potentially independent of a respondent’s own behavior) 45 , 48 . The item “ Did you/your wife or partner give birth?” was excluded from this categorization. In addition, SLE reported were categorized to investigate the diathesis effect at different levels of exposure, including a group to test the diathesis effect when SLE is not reported. Three levels of SLE reported were defined (0 SLE = “none”, 1 or 2 SLE = “low”, and 3 or more SLE = “high”) to retain a large enough sample size for each group to allow meaningful statistical comparison.
Polygenic profiling and statistical analysis
Polygenic risk scores (PRS) were generated by PRSice 49 , whose functionality relies mostly on PLINK v1.9 34 , and were calculated using the genotype data of Generation Scotland participants (i.e., target sample) and summary statistics for MDD from the PGC-MDD2 GWAS release (July 2016, discovery sample) used by Colodro-Conde et al. 23 , with the added contribution from QIMR cohort and the exclusion of Generation Scotland participants, resulting in summary statistics for MDD derived from a sample of 50,455 cases and 105,411 controls.
Briefly, PRSice removed strand-ambiguous SNPs and clump-based pruned ( r 2 = 0.1, within a 10 Mb window) our target sample to obtain the most significant independent SNPs in approximate linkage equilibrium. Independent risk alleles were then weighted by the allelic effect sizes estimated in the independent discovery sample and aggregated into PRS. PRS were generated for eight p thresholds ( p thresholds: < 5 × 10 −8 , < 1 × 10 −5 , < 0.001, < 0.01, < 0.05, < 0.1, < 0.5, < = 1) determined by the discovery sample and standardized (See Supplementary Table 1 for summary of PRS).
A genetic relationship matrix (GRM) was calculated for each dataset (i.e., full cohort , women , and men ) using GCTA 1.26.0 50 . Mixed linear models using the GRM were used to estimate the variance in depression score explained by PRS, SLEs and their interaction; and stratified by sex. Twenty principal components were calculated for the datasets.
The mixed linear model used to assess the effects of PRS is as follows:
Mixed linear models used to assess the effect of the stressors are as follows:
Following Colodro-Conde et al. 23 , covariates (i.e., age, age 2 , sex, age-by-sex and age 2 -by-sex interactions, and 20 principal components) were regressed from PRS (PRS’) and SLE scores (i.e., TSLE’, DSLE’ and ISLE’; SLEs’) before fitting models in GCTA to guard against confounding influences on the PRS-by-SLEs interactions 51 . PRS’ and SLEs’ were standardized to a mean of 0 and a standard deviation of 1. The Mixed linear models (i.e., the diathesis-stress model) used to assess GxE effects are as follows:
\(\begin{array}{lcc}{\mathrm{Depression}} = \beta _0 + \beta _1{\mathrm{PRS}}^\prime + \beta _2{\mathrm{TSLE}}^\prime \\+\, \beta _3{\mathrm{PRS}}^\prime x{\mathrm{TSLE}}^\prime + {\mathrm{GRM}} + {\mathrm{Covariates}}\end{array}\)
Covariates fitted in the models above were age, age 2 , sex, age-by-sex, age 2 -by-sex, and 20 principal components. Sex and its interactions (age-by-sex and age 2 -by-sex) were omitted from the covariates when stratifying by sex. All parameters from the models were estimated using GCTA and the significance of the effect ( β ) from fixed effects assessed using a Wald test. The significance of main effects (PRS and SLEs) allowed for nominally testing the significance of interactions at p -threshold = 0.05. To account for multiple testing correction, a Bonferroni’s adjustment correcting for 8 PRS and 3 measures of SLE tested (24 tests) was used to establish a robust threshold for significance at p = 2.08 × 10 −3 .
The PRS effect on depression score at different levels of exposure was further examined for the detected nominally significant interactions by categorizing participants on three groups based on the number of SLE reported (i.e., “none”, “low” or “high”). Using linear regression, we applied a least squares approach to assess PRS’ effects on the depression score in each SLE category. Further conservative Bonferroni correction to adjust for the 3 SLE categories tested established a threshold for significance of p = 6.94 × 10 −4 .
Differences on the estimated size of GxE effect between women and men were assessed by comparing a z -score to the standard normal distribution (α = 0.05, one-tailed). Z -scores were derived from GxE estimates ( β ) and standard errors (SE) detected in women and men as follows:
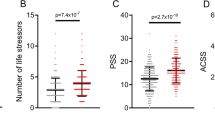
PRS for MDD significantly predicted the depression score across the whole sample ( β = 0.080, s.e. = 0.014, p = 7.53 × 10 −9 ) explaining 0.64% of the variance at its best p- threshold ( p- threshold = 0.1; Fig. 1a ). Stratifying by sex, PRS significantly predicted the depression score in both sexes, explaining 0.59% in men and 0.67% in women ( men : p- threshold = 0.1, β = 0.077, s.e. = 0.022, p = 2.09 × 10 −4 ; women : p- threshold = 0.1, β = 0.082, s.e. = 0.018, p = 4.93 × 10 −6 ; Fig. 1a ). Self-reported SLE over the last 6 months (TSLE, mean = 1.3 SLE, s.d. = 1.5) also significantly predicted depression score for the whole sample and stratified by sex ( full cohort : variance explained = 4.91%, β = 0.222, s.e. = 0.014, p = 9.98 × 10 −59 ; men : 4.19%, β = 0.205, s.e. = 0.021, p = 2.23 × 10 −22 ; women : 5.33%, β = 0.231, s.e. = 0.018, p = 7.48 × 10 −38 ; Fig. 1b ). Overall, significant additive contributions from genetics and SLE to depression score were detected in all participants and across sexes. There was no significant difference in the direct effect of TSLE between women and men ( p = 0.17). However, the variance in depression score explained by the TSLE appeared to be lower than the variance explained by the measure of personal SLE (PSLE) used in Colodro-Conde et al. 23 (12.9%). This may, in part, be explained by different contributions of dependent and independent SLE items screened in Colodro-Conde et al. compared to our study. Although questions about dependent SLE (DSLE, mean = 0.4 SLE) represented over 28% of the TSLE-items reported in our study, the main effect of DSLE explained approximately 93% of the amount of variance explained by TSLE ( full cohort : variance explained = 4.56%, β = 0.212, s.e. = 0.014, p = 1.73 × 10 −54 ; men : 3.74%, β = 0.193, s.e. = 0.021, p = 9.66 × 10 −21 ; women : 5.07%, β = 0.225, s.e. = 0.018, p = 8.09 × 10 −35 ; Fig. 1b ). Independent SLE (ISLE, mean = 0.85 SLE), which represented over 69% of TSLE-items, explained approximately 57% of the amount of variance explained by TSLE ( full cohort : variance explained = 2.80%, β = 0.167, s.e. = 0.014, p = 1.32 × 10 −33 ; men : 2.44%, β = 0.156, s.e. = 0.022, p = 2.88 × 10 −13 ; women : 3.02%, β = 0.174, s.e. = 0.018, p = 5.20 × 10 −22 ; Fig. 1b ). To explore the contribution from each measure, we combined DSLE and ISLE together in a single model. DSLE explained 3.34% of the variance in depression score compared to 1.45% of the variance being explained by ISLE, suggesting that DSLE have a greater effect on liability to depressive symptoms than ISLE.
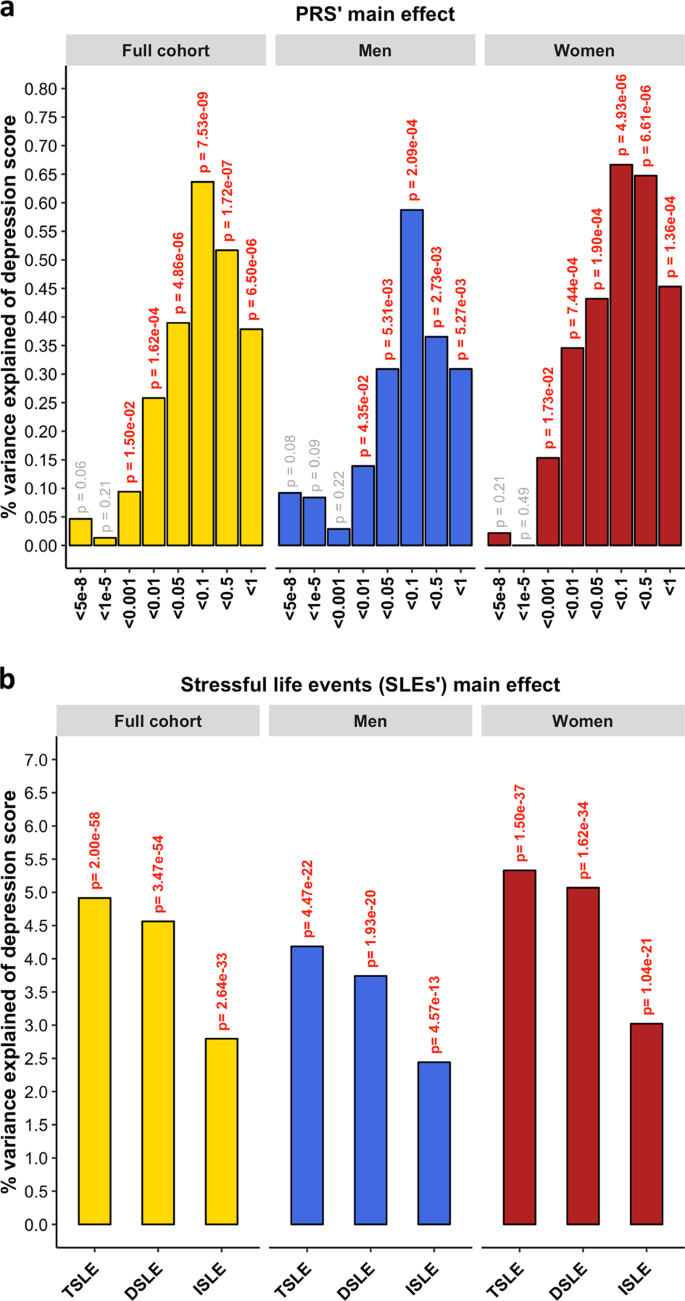
a Association between polygenic risk scores (PRS) and depression score (main effects, one-sided tests). PRS were generated at 8 p -threshold levels using summary statistics from the Psychiatric Genetic Consortium MDD GWAS (released July 2016) with the exclusion of Generation Scotland participants. The depression score was derived from The General Health Questionnaire. The Y -axis represents the % of variance of depression score explained by PRS main effects. The full cohort (yellow) was split into men (blue) and women (red). In Colodro-Conde et al. PRS for MDD significantly explained up to 0.46% of depression score in their sample (~0.39% in women and ~0.70% in men). b Association between reported number of SLE and depression score (main effect, one-sided tests, results expressed in % of variance in depression score explained). SLE were self-reported through a brief life-events questionnaire based on the List of Threatening Experiences and categorized into: total number of SLE reported (TSLE), “dependent” SLE (DSLE) or “independent” SLE (ISLE). The full cohort (yellow) was split into men (blue) and women (red). In Colodro-Conde et al. “personal” SLE significantly explained up to 12.9% of depression score variance in their sample (~11.5% in women and ~16% in men) 23
A diathesis-stress model for depression was tested to assess GxE effects. We detected significant, albeit weak, GxE effects on depression score (Fig. 2 ). The PRS interaction with TSLE was nominally significant in the full cohort ( β = 0.028, s.e. = 0.014, R 2 = 0.08%, p = 0.049) and slightly stronger in women ( β = 0.044, s.e. = 0.018, R 2 = 0.19%, p = 0.017; Fig. 2a ), compared to men in which the effect was not significant ( β = 0.039, s.e. = 0.022, R 2 = 0.15%, p = 0.07). However, these results did not survive correction for multiple testing ( p > 2.08 × 10 -3 ).
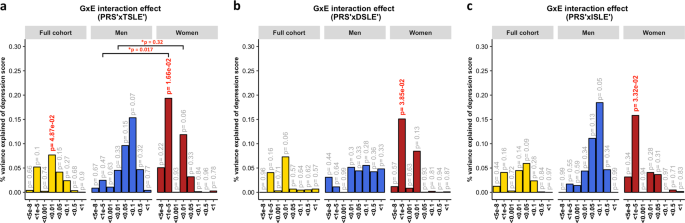
The plots show the percentage of depression score explained by the interaction term (two-sided tests) fitted in linear mixed models to empirically test the diathesis-stress model. Red numbers show significant interactions p -values. *Shows the significance of the difference in variance explained between sexes. The full cohort (yellow) was split into men (blue) and women (red). PRS were generated at 8 p -threshold levels using summary statistics from the Psychiatric Genetic Consortium MDD GWAS (released July 2016) with the exclusion of Generation Scotland participants. The interaction effect was tested with the number of: ( a ) SLE (TSLE), ( b ) “dependent” SLE (DSLE) and c ) “independent” SLE (ISLE). In Colodro-Conde et al., the variance of depression score explained in their sample by GxE was 0.12% ( p = 7 × 10 −3 ). GxE were also significant in women ( p = 2 × 10 -3 ) explaining up to 0.25% of depression score variation, but not in men ( p = 0.059; R 2 = 0.17%; negative/protective effect on depression score)
The best-fit threshold was much lower in women ( p- threshold = 1 × 10 −5 ) compared to the full sample ( p -threshold = 0.01). The size of the GxE effects across sexes at p- threshold = 1 × 10 −5 were significantly different (GxE*sex p = 0.017), but not at the best p-threshold in the full cohort ( p -threshold = 0.01, GxE*sex p = 0.32; Fig. 2a ). In women, GxE effect with DSLE predicted depression score ( p- threshold = 1 × 10 −5 ; β = 0.039, s.e. = 0.019, R 2 = 0.15%, p = 0.038; Fig. 2b and Supplementary Fig. 2a ), as did the GxE effect with ISLE ( p- threshold = 1 × 10 −5 ; β = 0.040, s.e. = 0.019, R 2 = 0.16%, p = 0.033; Fig. 2c and Supplementary Fig. 2b ). No significant interaction was detected in men (best-fit p- threshold = 0.1) with either TSLE ( β = 0.039, s.e. = 0.022, R 2 = 0.15%, p = 0.072; Fig. 2a ), DSLE ( β = 0.024, s.e. = 0.022, R 2 = 0.06%, p = 0.28; Fig. 2b ) or ISLE ( β = 0.043, s.e. = 0.022, R 2 = 0.18%, p = 0.055; Fig. 2c ).
To examine these results further and investigate the diathesis effect at different levels of stress, nominally significant GxE were plotted between PRS and categories of SLE (i.e, “none”, “low”, and “high” SLE reported; Fig. 3 ). Examining the interaction found in the full cohort (PRS at PGC-MDD GWAS p- threshold = 0.01), we detected a significant direct diathesis effect on the risk of depressive symptoms in those participants reporting SLE, with a higher risk when greater numbers of SLE were reported (“low” number of SLE reported: PRS’ β = 0.043, s.e. = 0.021, p = 0.039; “high” number of SLE reported: PRS’ β = 0.142, s.e. = 0.039, p = 2.86 × 10 −4 ; see Table 1 and Fig. 3a ). Whereas, in participants who reported no SLE over the preceding 6 months, the risk of depressive symptoms was the same regardless of their diathesis risk (“none” SLE reported: PRS’ β = 0.021, s.e. = 0.022, p = 0.339). Stratifying these results by sex, we found the same pattern as in the full cohort in women (“none”: p = 0.687; “low”: p = 0.023; “high”: p = 2 × 10 -3 ), but not in men (“none”: p = 0.307; “low”: p = 728; “high”: p = 0.053; see Table 1 and Fig. 3a ). However, the lack of a significant diathesis effect in men may be due to their lower sample size and its corresponding reduced power.
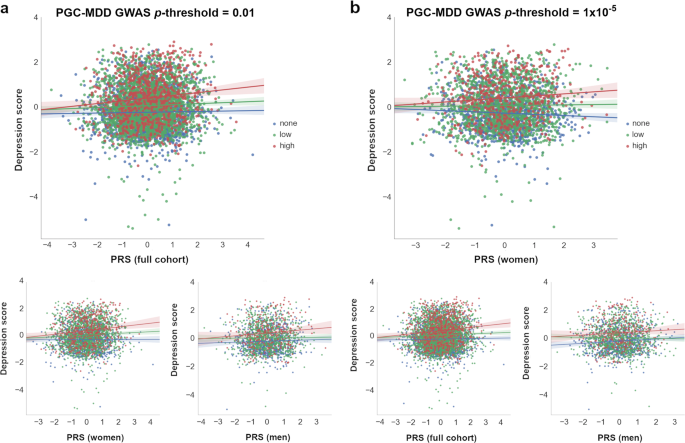
Interactions with PRS at which nominally significant GxE effects were detected in ( a ) full cohort ( p- threshold = 0.01) and ( b ) in women ( p- threshold = 1 × 10 –5 ) are shown. At bottom, the remaining samples (i.e., full cohort , women or men ) at same p- threshold are shown for comparison. The X-axis represents the direct effect of PRS (standard deviation from the mean) based on ( a ) p -threshold = 0.01 and ( b ) p -threshold = 1 × 10 –5 , using the total number of SLE reported by each participant (dot) as environmental exposures at three SLE levels represented by colors. Blue: 0 SLE, “no stress”, n = 1 833/1 041/792; green: 1 or 2 SLE, “low stress”, n = 2 311/1 459/852; red: 3 or more SLE, “high stress”, n = 775/490/285; in the full cohort, women and men, respectively. Y -axis reflects the depression score standardized to mean of 0 and standard deviation of 1. Lines represent the increment in risk of depression at a certain degree of “stress” dependent on a genetic predisposition ( = diathesis )
Examining the interaction with PRS at PGC-MDD GWAS p- threshold = 1 × 10 −5 , with which a significant interaction was detected in women, we detected a significant diathesis effect on depression score only when stratifying by sex in those participants who did not reported SLE over the last 6 months (see Table 1 ). The diathesis effect was positive in men (PRS’ β = 0.082, s.e. = 0.034, p = 0.016, R 2 = 0.7%; Fig. 3b ), consistent with the contribution of risk alleles. Conversely, the diathesis effect was negative in women (PRS’ β = -0.061, s.e. = 0.029, p = 0.037, R 2 = 0.4%; Fig. 3b ), suggesting a protective effect of increasing PRS in those women reporting no SLE, and consistent with the contribution of alleles to individual sensitivity to both positive and negative environmental effects (i.e., “plasticity alleles” rather than “risk alleles”) 52 , 53 . This PRS accounted for the effect of just 34 SNPs, and the size of its GxE across sexes were significantly different (GxE*sex p = 0.017; Fig. 2a ), supporting possible differences in the underlying stress-response mechanisms between women and men.
The findings reported in this study support those from Colodro-Conde et al. 23 , in an independent sample of similar sample size and study design, and also supports possible sex-specific differences in the effect of genetic risk of MDD in response to SLE.
Both Colodro-Conde et al. and our study suggest that individuals with an inherent genetic predisposition to MDD, reporting high number of recent SLE, are at additional risk of depressive symptoms due to GxE effects, thus validating the diathesis-stress theory. We identified nominally significant GxE effects in liability to depression at the population level ( p = 0.049) and in women ( p = 0.017), but not in men ( p = 0.072). However, these interactions did not survive multiple testing correction ( p > 2.08 × 10 −3 ) and the power of both studies to draw robust conclusions remains limited 54 . With increased power these studies could determine more accurately both the presence and magnitude of a GxE effect in depression. To better understand the effect of PRS at different levels of exposure to stress, we examined the nominally significant interactions detected in the full sample by categorizing participants on three groups based on the number of SLE reported (i.e., “none”, “low” or “high”). We detected a significant diathesis effect on risk of depression only in those participants reporting SLE, but not in those participants that reported no SLE over the preceding 6 months. Furthermore, the diathesis effect was stronger on those participants reporting a “high” number of SLE ( β = 0.142, p = 2.86 × 10 −4 ) compared to those participants reporting a “low” number of SLE ( β = 0.043, p = 0.039). The former effect was significant and survived a conservative Bonferroni correction to adjust for multiple testing ( p < 6.94 × 10 −4 ). This finding corroborates the diathesis-stress model for depression and supports the results of Colodro-Conde et al. in an independent sample.
To investigate the relative contribution of the GxE to the variance of depression, we examined in the full cohort the total variance of depression score explained by the PRS main effect and the significant GxE effect jointly. Together, they explained 0.34% of the variance, of which 0.07% of the variance of the depression score was attributed to the GxE effect ( p- threshold = 0.01; PRS p = 1.19 × 10 −4 , GxE p = 0.049; both derived from the full diathesis-model with TSLE). This is lower than the proportion of variance attributed to common SNPs (8.9%) in the full PGC-MDD analysis 16 . As Colodro-Conde et al . noted, this result aligns with estimates from experimental organisms suggesting that around 20% of the heritability may be typically attributed to the effects of GxE 55 , although it is inconsistent with twin studies of the majority of human traits with the potential exception of depression 56 .
Consistent with PRS predicting “personal” SLE in Colodro-Conde et al., PRS for MDD predicted SLE in our study (see Supplementary Fig. 1 ), although not at the p- threshold at which significant GxE effects were detected. Genetic factors predisposing to MDD may contribute to individuals exposing themselves to, or showing an increased reporting of, SLE via behavioral or personality traits 57 , 58 . Such genetic mediation of the association between depression and SLE would disclose a gene-environment correlation (i.e., genetic effects on the probability of undergoing a SLE) that hinders interpretion of our findings as pure GxE effects 59 , 60 . To address this limitation and assess this aspect, following Colodro-Conde et al., we split the 12-items TSLE measure into SLE that are either potentially “dependent” of a participant’s own behavior (DSLE; therefore, potentially driven by genetic factors) or not (“independent” SLE; ISLE) 45 , 48 . DSLE are reported to be more heritable and have stronger associations with MDD than ISLE 48 , 61 57 . In our sample, DSLE is significantly heritable ( h 2 SNP = 0.131, s.e. = 0.071, p = 0.029), supporting a genetic mediation of the association, whereas ISLE is not significantly heritable ( h 2 SNP = 0.000, s.e. = 0.072, p = 0.5) 62 . Nominally significant GxE effects were seen in women for both DSLE and ISLE, suggesting that both GxE and gene-environment correlation co-occur. Colodro-Conde et al. did not identify significant GxE using independent SLE as the exposure.
Between-sex differences in stress response could help to explain previous differences seen between sexes in depression such as those in associated risk (i.e., approximately 1.5–2-fold higher in women), symptoms reported and/or coping strategies (e.g., whereas women tend to cope through verbal and emotional strategies, men tend to cope by doing sport and consuming alcohol) 63 , 64 , 65 , 66 , 67 . This also aligns with an increased risk associated with a lack of social support seen in women compared to men 23 . Furthermore, although we do not know whether participants experienced recent events with positive effects, we saw a protective effect in those women who did not experienced recent SLE ( p = 0.037), suggesting that some genetic variants associated with MDD may operate as “plasticity alleles” and not just as “risk alleles” 52 , 53 . This effect was neutralized in the full cohort due to an opposite effect in men ( p = 0.016), but it is supported by previous protective effects reported when using a serotoninergic multilocus profile score and absence of SLE in young women 68 . These findings would be consistent with a differential-susceptibility model of depression 69 , 70 , also suggested by the interaction effects seen between the serotonin transporter linked promoter region gene (5-HTTLPR) locus and family support and liability to adolescent depression in boys 71 . However, our results and the examples given are only nominally significant and will require replication in larger samples. Robust identification of sex-specific differences in genetic stress-response could improve personalized treatments and therapies such as better coping strategies.
There are notable differences between our study and Colodro-Conde et al. to consider before accepting our findings as a replication of their results. First, differences in PRS profiling may have affected replication power. We used the same equivalent PGC-MDD2 GWAS as discovery sample. However, whereas Colodro-Conde et al. generated PRS in their target sample containing over 9.5 M imputed SNP, in this study we generated PRS in a target sample of over 560 K genotyped SNPs (see Supplementary table 1 for comparison). This potentially results in a less informative PRS in our study, with less predictive power, although the variance explained by our PRS was slightly larger (0.64% vs. 0.46%). The size of the discovery sample is key to constructing an accurate predictive PRS, but to exploit the greatest number of the available variants may be an asset 54 . Secondly, different screening tools were used to measure both current depressive symptoms and recent environmental stressors across the two studies. Both studies transformed their data, using item response theory or by log-transformation, to improve the data distribution. However, neither study used depression scores that were normally distributed. The scale of the instruments used and their corresponding parameterization when testing for an interaction could have a direct effect on the size and significance of their interaction; 55 , 72 so findings from GxE must be taken with caution. Furthermore, although both screening methods have been validated and applied to detect depressive symptoms, different questions may cover and emphasize different features of the illness, which may result in different results. The same applies to the measurement of environmental stressors in the two studies. Both covering of a longer time-period and upweighting by “dependent” SLE items may explain the increased explanatory power of “personal” SLE (12.9%) in Colodro-Conde et al. to predict depression score compared to our “total” SLE measure (4.91%). Finally, the unmeasured aspects of the exposure to SLE or its impact may also contribute to the lack of a stronger replication and positive findings.
In conclusion, despite differences in the measures used across studies, we saw concordance and similar patterns between our results and those of Colodro-Conde et al. 23 Our findings, therefore, add validity to the diathesis-stress theory for depression. Empirically demonstrating the diathesis-stress theory for depression would validate recent 20 , 21 , 22 and future studies using a genome-wide approach to identify genetic mechanisms and interactive pathways involved in GxE underpinning the causative effect of “stress” in the development of depressive symptoms and in mental illness in general. This study adds to our understanding of gene-by-environment interactions, although larger samples will be required to confirm differences in diathesis-stress effects between women and men.
Hammen, C. Stress and depression. Annu Rev. Clin. Psychol. 1 , 293–319 (2005).
Article Google Scholar
Kessler, R. C. The effects of stressful life events on depression. Annu Rev. Psychol. 48 , 191–214 (1997).
Article CAS Google Scholar
Kendler, K. S., Karkowski, L. M. & Prescott, C. A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156 , 837–841 (1999).
Paykel, E. S. Life events and affective disorders. Acta Psychiatr. Scand 108 , 61–66 (2003).
Stroud, C. B., Davila, J. & Moyer, A. The relationship between stress and depression in first onsets versus recurrences: a meta-analytic review. J. Abnorm. Psychol. 117 , 206–213 (2008).
Ensel, W. M., Peek, M. K., Lin, N. & Lai, G. Stress in the life course: a life history approach. J. Aging Health 8 , 389–416 (1996).
Kendler, K. S., Karkowski, L. M. & Prescott, C. A. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J. Nerv. Ment. Dis. 186 , 661–669 (1998).
Mazure, C. M. Life stressors as risk factors in depression. Clin. Psychol. 5 , 291–313 (1998).
Google Scholar
Lichtenberg, P. & Belmaker, R. H. Subtyping major depressive disorder. Psychother. Psychosom. 79 , 131–135 (2010).
Elisei, S., Sciarma, T., Verdolini, N. & Anastasi, S. Resilience and depressive disorders. Psychiatr. Danub. 25 (Suppl 2), S263–S267 (2013).
PubMed Google Scholar
Monroe, S. M. & Simons, A. D. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol. Bull. 110 , 406–425 (1991).
Vogel, F. Schizophrenia genesis: the origins of madness. Am. J. Human. Genet. 48 , 1218–1218 (1991).
Mann, J. J., Waternaux, C., Haas, G. L. & Malone, K. M. Toward a clinical model of suicidal behavior in psychiatric patients. Am. J. Psychiatry 156 , 181–189 (1999).
CAS PubMed Google Scholar
Riemann, D. et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep. Med Rev. 14 , 19–31 (2010).
Bolt, M. A., Helming, L. M. & Tintle, N. L. The associations between self-reported exposure to the chernobyl nuclear disaster zone and mental health disorders in Ukraine. Front. Psychiatry 9 , 32 (2018).
Wray, N. R. & Sullivan, P. F. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature Genetics 50 , 668–681 (2018).
Peyrot, W. J. et al. Effect of polygenic risk scores on depression in childhood trauma. Br. J. Psychiatry 205 , 113–119 (2014).
Musliner, K. L. et al. Polygenic risk, stressful life events and depressive symptoms in older adults: a polygenic score analysis. Psychol. Med. 45 , 1709–1720 (2015).
Peyrot, W. J. et al. Does childhood trauma moderate polygenic risk for depression? A Meta-analysis of 5765 Subjects From the Psychiatric Genomics Consortium. Biol. Psychiatry 84 , 138–147 (2018).
Dunn, E. C. et al. Genome-Wide Association Study (GWAS) and Genome-Wide by Environment Interaction Study (GWEIS) of Depressive Symptoms in African American and Hispanic/Latina Women. Depress. Anxiety 33 , 265–280 (2016).
Otowa, T. et al. The first pilot genome-wide gene-environment study of depression in the Japanese Population. PLoS One 11 , e0160823 (2016).
Ikeda, M. et al. Genome-wide environment interaction between depressive state and stressful life events. J. Clin. Psychiatry 77 , e29–e30 (2016).
Colodro-Conde, L. et al. A direct test of the diathesis-stress model for depression. Mol. Psychiatry 23 , 1590–1596 (2018).
Mullins, N. et al. Polygenic interactions with environmental adversity in the aetiology of major depressive disorder. Psychol. Med 46 , 759–770 (2016).
Iyegbe, C., Campbell, D., Butler, A., Ajnakina, O. & Sham, P. The emerging molecular architecture of schizophrenia, polygenic risk scores and the clinical implications for GxE research. Soc. Psychiatry Psychiatr. Epidemiol. 49 , 169–182 (2014).
McGrath, J. J., Mortensen, P. B., Visscher, P. M. & Wray, N. R. Where GWAS and epidemiology meet: opportunities for the simultaneous study of genetic and environmental risk factors in schizophrenia. Schizophr. Bull. 39 , 955–959 (2013).
Plomin, R. Commentary: missing heritability, polygenic scores, and gene-environment correlation. J. Child Psychol. Psychiatry 54 , 1147–1149 (2013).
Wray, N. R. et al. Research review: polygenic methods and their application to psychiatric traits. J. Child Psychol. Psychiatry 55 , 1068–1087 (2014).
Smith, B. H. et al. Cohort Profile: Generation Scotland: Scottish Family Health Study (GS:SFHS). The study, its participants and their potential for genetic research on health and illness. Int J. Epidemiol. 42 , 689–700 (2013).
Gunderson, K. L. Whole-genome genotyping on bead arrays. In DNA Microarrays for Biomedical Research: Methods and Protocols (ed. Dufva, M.) 197–213 (Humana Press, Totowa, 2009).
Kerr, S. M. et al. Pedigree and genotyping quality analyses of over 10,000 DNA samples from the Generation Scotland: Scottish Family Health Study. BMC Med. Genet . 14 , 38 (2013).
Nagy, R. et al. Exploration of haplotype research consortium imputation for genome-wide association studies in 20,032 Generation Scotland participants. Genome Med . 9 , 23 (2017).
Navrady, L. B. et al. Cohort Profile: Stratifying Resilience and Depression Longitudinally (STRADL): a questionnaire follow-up of Generation Scotland: Scottish Family Health Study (GS:SFHS). Int. J. Epidemiol 47 , 13–14g (2018).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet 81 , 559–575 (2007).
Smith, B. H. et al. Generation Scotland: the Scottish Family Health Study; a new resource for researching genes and heritability. BMC Med. Genet. 7 , 74 (2006).
Fernandez-Pujals, A. M. et al. Epidemiology and Heritability of Major Depressive Disorder, Stratified by Age of Onset, Sex, and Illness Course in Generation Scotland: Scottish Family Health Study (GS:SFHS). PLoS One 10 , e0142197 (2015).
Amador, C. et al. Recent genomic heritage in Scotland. BMC Genom. 16 , 437 (2015).
Goldberg, D. P. & Hillier, V. F. A scaled version of the General Health Questionnaire. Psychol. Med. 9 , 139–145 (1979).
Sterling, M. General Health Questionnaire - 28 (GHQ-28). J. Physiother. 57 , 259 (2011).
Goldberg, D. P. et al. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol. Med. 27 , 191–197 (1997).
Banks, M. H. Validation of the General Health Questionnaire in a young community sample. Psychol. Med. 13 , 349–353 (1983).
Marks, A. D. G., Horrocks, K. A. & Schutte, N. S. Emotional intelligence mediates the relationship between insecure attachment and subjective health outcomes. Personal. Individ. Differ. 98 , 188–192 (2016).
O’Rourke, S., MacHale, S., Signorini, D. & Dennis, M. Detecting psychiatric morbidity after stroke: comparison of the GHQ and the HAD Scale. Stroke 29 , 980–985 (1998).
Kessler, R. C., Andrews, G., Mroczek, D., Ustun, B. & Wittchen, H.-U. The World Health Organization Composite International Diagnostic Interview short-form (CIDI-SF). Int. J. Methods Psychiatr. Res. 7 , 171–185 (1998).
Brugha, T., Bebbington, P., Tennant, C. & Hurry, J. The List of Threatening Experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol. Med. 15 , 189–194 (1985).
Brugha, T. S. & Cragg, D. The list of threatening experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr. Scand. 82 , 77–81 (1990).
Motrico, E. et al. Psychometric properties of the List of Threatening Experiences--LTE and its association with psychosocial factors and mental disorders according to different scoring methods. J. Affect Disord. 150 , 931–940 (2013).
Kendler, K. S., Karkowski, L. M. & Prescott, C. A. The assessment of dependence in the study of stressful life events: validation using a twin design. Psychol. Med . 29 , 1455–1460 (1999).
Euesden, J., Lewis, C. M. & O’Reilly, P. F. PRSice: polygenic risk score software. Bioinformatics 31 , 1466–1468 (2015).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet 88 , 76–82 (2011).
Keller, M. C. Gene x environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biol. Psychiatry 75 , 18–24 (2014).
Belsky, J. & Beaver, K. M. Cumulative-genetic plasticity, parenting and adolescent self-regulation. J. Child Psychol. Psychiatry 52 , 619–626 (2011).
Belsky, J. et al. Vulnerability genes or plasticity genes? Mol. Psychiatry 14 , 746–754 (2009).
Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet 9 , e1003348 (2013).
Eaves, L. J., Last, K., Martin, N. G. & Jinks, J. L. A progressive approach to non-additivity and genotype-environmental covariance in the analysis of human differences. Br. J. Math. Stat. Psychol. 30 , 1–42 (1977).
Polderman, T. J. et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 47 , 702–709 (2015).
Clarke, T. et al. Genetic and environmental determinants of stressful life events and their overlap with depression and neuroticism [version 2; referees: 3 approved with reservations]. Wellcome Open Res 3 , 11 (2019).
Kendler, K. S., Kuhn, J. & Prescott, C. A. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry 161 , 631–636 (2004).
Kendler, K. S. & Eaves, L. J. Models for the joint effect of genotype and environment on liability to psychiatric illness. Am. J. Psychiatry 143 , 279–289 (1986).
Plomin, R., DeFries, J. C. & Loehlin, J. C. Genotype-environment interaction and correlation in the analysis of human behavior. Psychol. Bull. 84 , 309–322 (1977).
Plomin, R., Lichtenstein, P., Pedersen, N. L., McClearn, G. E. & Nesselroade, J. R. Genetic influence on life events during the last half of the life span. Psychol. Aging 5 , 25–30 (1990).
Arnau Soler, A. et al. Genome-wide by environment interaction studies (GWEIS) of depressive symptoms and psychosocial stress in UK Biobank and Generation Scotland. Transl. Psychiatry (in press).
Weissman, M. M. et al. Sex differences in rates of depression: cross-national perspectives. J. Affect Disord. 29 , 77–84 (1993).
Van de Velde, S., Bracke, P. & Levecque, K. Gender differences in depression in 23 European countries. Cross-national variation in the gender gap in depression. Soc. Sci. Med . 71 , 305–313 (2010).
Labonte, B. et al. Sex-specific transcriptional signatures in human depression. Nat. Med 24 , 525 (2018).
Angst, J. et al. Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. Eur. Arch. Psychiatry Clin. Neurosci. 252 , 201–209 (2002).
Piccinelli, M. & Wilkinson, G. Gender differences in depression. Crit. Rev. Br. J. Psychiatry 177 , 486–492 (2000).
Vrshek-Schallhorn, S. et al. Additive genetic risk from five serotonin system polymorphisms interacts with interpersonal stress to predict depression. J. Abnorm. Psychol. 124 , 776–790 (2015).
Belsky, J. & Pluess, M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull. 135 , 885–908 (2009).
Belsky, J., Bakermans-Kranenburg, M. J. & van Ijzendoorn, M. H. For better and for worse: differential susceptibility to environmental influences. Curr. Dir. Psychol. Sci. 16 , 300–304 (2007).
Li, J. J., Berk, M. S. & Lee, S. S. Differential susceptibility in longitudinal models of gene-environment interaction for adolescent depression. Dev. Psychopathol. 25 , 991–1003 (2013).
Kang, S.-M. & Waller, N. G. Moderated multiple regression, spurious interaction effects, and IRT. Applied Psychological Measurement 29 , 87–105 (2005).
Download references
Acknowledgements
A.A.S. is funded by the University of Edinburgh ( www.ed.ac.uk ) and MRC for his PhD study at the University of Edinburgh Institute of Genetics and Molecular Medicine ( www.ed.ac.uk/igmm ). D.J.M. acknowledges the financial support of NHS Research Scotland (NRS) through NHS Lothian. MA is supported by STRADL through a Wellcome Trust Strategic Award (reference 104036/Z/14/Z). Generation Scotland received core support from the Chief Scientist Office of the Scottish Government Health Directorates [CZD/16/6] and the Scottish Funding Council [HR03006]. The genotyping of the GS:SFHS samples was carried out by the Genetics Core Laboratory at the Wellcome Trust Clinical Research Facility, Edinburgh, Scotland and was funded by the Medical Research Council UK and the Wellcome Trust (Wellcome Trust Strategic Award “STratifying Resilience and Depression Longitudinally” (STRADL) Reference 104036/Z/14/Z). The Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium depends on the contributions of many parties.
Author information
Authors and affiliations.
Medical Genetics Section, Centre for Genomic and Experimental Medicine and MRC Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK
Aleix Arnau-Soler & Pippa A. Thomson
Division of Psychiatry, Deanery of Clinical Sciences, Royal Edinburgh Hospital, University of Edinburgh, Morningside Park, Edinburgh, EH10 5HF, UK
Mark J. Adams, Toni-Kim Clarke, Donald J. MacIntyre, Lauren Navrady & Andrew McIntosh
Health Informatics Centre, University of Dundee, Dundee, UK
Keith Milburn
Medical Research Council Human Genetics Unit, Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK
Caroline Hayward
Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, UK
Andrew McIntosh & Pippa A. Thomson
You can also search for this author in PubMed Google Scholar
Generation Scotland,
Major depressive disorder working group of the psychiatric genomics consortium, corresponding authors.
Correspondence to Aleix Arnau-Soler or Pippa A. Thomson .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A collaboration between the University Medical School and NHS in Aberdeen, Dundee, Edinburgh and Glasgow, Scotland, UK
For a full list of MDD working group of the PGC investigators, see the Supplementary Material.
Supplementary information
Supplementary material, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Arnau-Soler, A., Adams, M.J., Clarke, TK. et al. A validation of the diathesis-stress model for depression in Generation Scotland. Transl Psychiatry 9 , 25 (2019). https://doi.org/10.1038/s41398-018-0356-7
Download citation
Received : 05 April 2018
Revised : 28 November 2018
Accepted : 10 December 2018
Published : 18 January 2019
DOI : https://doi.org/10.1038/s41398-018-0356-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Effects of lockdowns on neurobiological and psychometric parameters in unipolar depression during the covid-19 pandemic.
- Jakob Unterholzner
- Alexander Kautzky
- Thomas Vanicek
Translational Psychiatry (2024)
- Jacob J. Crouse
- Shin Ho Park
- Ian B. Hickie
Molecular Psychiatry (2024)
The relationship between disease-specific psychosocial stressors and depressive symptoms in Huntington’s disease
- Ian H. Harding
- Julie C. Stout
Journal of Neurology (2024)
Anxiety, depression, and their comorbidity among Chinese college students during the COVID-19 lockdown in the post-epidemic era: an online cross-sectional survey
- Jinghong Huang
- Xiaojun Liu
BMC Psychiatry (2023)
- Catharina A. Hartman
- Harold Snieder
Translational Psychiatry (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Live revision! Join us for our free exam revision livestreams Watch now →
- Health & Social Care
Reference Library
Collections
- See what's new
- All Resources
- Student Resources
- Assessment Resources
- Teaching Resources
- CPD Courses
- Livestreams
Study notes, videos, interactive activities and more!
Health & Social Care news, insights and enrichment
Currated collections of free resources
Browse resources by topic
- All Health & Social Care Resources
Resource Selections
Currated lists of resources
Study Notes
Stress-Diathesis Model
Last updated 29 Sept 2019
- Share on Facebook
- Share on Twitter
- Share by Email
The stress-diathesis model is the explanation that a disorder or behaviour trait is the result of an interaction between genetic predisposition vulnerability and stress, usually caused by life events and factors.
Schizophrenia can be explained by the stress-diathesis model. A child may have inherited the gene from their parents ( nature ). This means that they are predisposed to the gene.
However, environmental factors, ( nurture ) such as a stressful life event, may trigger the activation of the gene, which then results in a schizophrenic episode.
There is growing evidence that the stress-diathesis model offers a more credible explanation for behaviour than looking at genetics and the environment as separate factors.
- Stress-diathesis model

You might also like
The nature / nurture debate.

Are lockdown babies behind on language development?
15th November 2022
Genetic Factors that Affect Development
Can drumming enhance your wellbeing.
8th August 2022
Babies can learn language sounds in the first few hours of being born
19th August 2022
Do optimists live longer?
24th August 2022
Black people more likely to develop dementia
21st October 2022
The festive season, stress and the sympathetic nervous system
10th January 2023
Our subjects
- › Criminology
- › Economics
- › Geography
- › Health & Social Care
- › Psychology
- › Sociology
- › Teaching & learning resources
- › Student revision workshops
- › Online student courses
- › CPD for teachers
- › Livestreams
- › Teaching jobs
Boston House, 214 High Street, Boston Spa, West Yorkshire, LS23 6AD Tel: 01937 848885
- › Contact us
- › Terms of use
- › Privacy & cookies
© 2002-2024 Tutor2u Limited. Company Reg no: 04489574. VAT reg no 816865400.
- Memberships
Diathesis Stress Model: a psychology theory

Diathesis stress model: This article explains the diathesis stress model in a practical way. It covers a definition of the model, as well as an explanation of its use in psychology, along with examples. It also provides tips on dealing with stress. After reading this article, you will understand the basics of this powerful psychology method. Enjoy reading!
What is the Diathesis stress model?
Diathesis stress model definition.
The Diathesis stress model or stress vulnerability model is a theory from psychology that explains disorders as a result of an interaction between vulnerability, diathesis and stress as a result of life experiences.
The term diathesis comes from Greek and means something like predisposition or sensitivity. A diathesis. There are various forms of this diathesis. These depend on psychological, genetic, biological or situational factors.

Stress arises from life events or other events that have disrupted a person’s psychological balance. Stress has the ability to catalyze the development of a disorder. That is, stress can be a stimulator for the development of a psychological disorder.
The diathesis stress model is used to investigate how biological or genetic factors interact with stressors causing disorders such as anxiety, depression and schizophrenia. The model states that if the combination of predisposition and stress exceeds a certain limit, the person will develop a disorder in the short term.
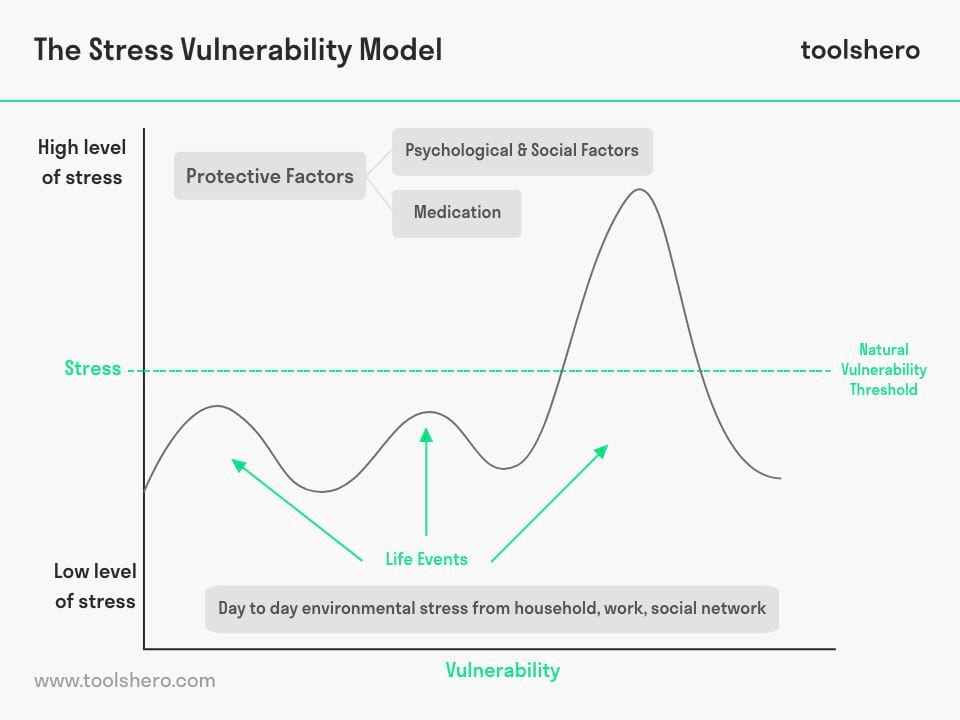
Figure 1 – Diathesis Stress Model / Stress Vulnerability Model
Origin and use in Psychology
The term diathesis was coined in medicine and psychiatry and comes from the 19th century. The diathesis stress model only became popular when it was used in the 1960s to explain schizophrenia. Joseph Zubin , a Lithuanian-American psychologist, used the concept to explain schizophrenia.
How is the diathesis stress model used?
The model is used in many areas of psychology. An example of this is the development of psychopathology.
It is also helpful to understand the relationship and interplay between nature vs. nurture in the search for susceptibility to disorders throughout a human’s lifespan. The model is furthermore useful for explaining the context of a depression. For example, it helps explain why Person A becomes depressed and Person B does not, even though they are exposed to the same factors and stressors.
More recently, the model helped explain why some people are more at risk of developing a disorder than others. A child with a family in which depression is present is more likely to develop a disorder than children who do not. Children who have experienced exclusion from friends or family are also more likely to develop a psychological disorder.
Protective factors for developing a disorder
There are so-called protective factors that counteract the effects of stressors and thus can prevent the consequences of a developed disorder. In many of these disorders, there is a limited amount of time in which the person is more likely to develop a disorder.
Examples of these protective factors are high-quality and positive social networks and a high self-esteem.
The protective factors help explain why some people don’t develop a psychological disorder when they do have severe diathesis and stress. In these cases, therefore, certain factors prevent the condition from coming to the surface.
Psychological disorders during a lifetime
Many models that show how psychopathology works suggest that all people have some degree of vulnerability to developing a psychological disorder. Yet there are major differences in the explanation of exactly when in a human life this happens.
A person with personality traits such as extroversion and friendliness is good at entering into new relationships.
He or she therefore more often has strong social support than people who do not. This social support can later develop into a protective factor when a lot of stress is experienced. They can also delay or even prevent the development of depression.
Conversely, a person who finds it difficult to form new relationships may be more vulnerable to developing a psychological disorder. That’s because this person doesn’t have a strong protective factor. The threshold whether someone develops an abnormality is determined by the interaction of stress and diathesis.
Windows of vulnerability
It is believed that at various times in life a person is especially vulnerable to developing a psychological disorder.
A breakup or other traumatic stressor can be involved in the development of depression. Stressful events can also trigger a manic phase of bipolar disorder. A new stressful event can then hinder recovery and cause another relapse.
Having a genetic predisposition to drink a lot during college with drinking games can be a factor in developing alcoholism later in life.
A family history in which schizophrenia is not unknown, coupled with stress factors such as growing up in an unorganized family, increases the risk of developing a psychological disorder such as schizophrenia .
Genetic predisposition to developing a psychological disorder
Also, receiving a particular temperament trait, such as sensory processing sensitivity (SPS), increases the vulnerability to developing psychological disorders. SPS is associated with increased sensitivity in the central nervous system and deeper cognitive processing of social, physical, and emotional stimuli.
The trait is characterized by the tendency to pause in new situations and to check the situation. These people have a greater sensitivity to stimuli and use deeper cognitive processing strategies for applying coping styles. These are driven by heightened emotional reactivity.
SPS is an inherited and conserved trait. It is associated with an increased level of information processing in the brain, which moderates sensitivity to environments in positive and negative ways.
Interaction with negative experiences increases the risk of a psychopathology, such as a depression or disorder. Interacting with positive experiences actually increases positive results.
Stress Management: 40+ easy ways to deal with stress Stress relief and burnout prevention. Don’t let stress control your life. Beat anxiety and worries. Live, Laugh, Love. >> More information
Diathesis Stress Model and how to deal with stress?
Stress is the culprit or trigger of many different negative influences. It is therefore important to avoid bad stress as much as possible.
Diet and exercise
The extent to which a person moves and eats healthy has a lot of influence on the production and release of stress hormones such as cortisol. Serotonin, one of the neurotransmitters that affects a person’s happiness, is produced in the gut.
It is therefore important to keep the intestines optimally healthy. Physical and mental health are more closely linked than many people think.
A regular sleep pattern with about 8 hours of sleep helps to rest and grow, both physically and mentally. Sleep is important for maintaining the 24-hour clock in the body. That rhythm helps a body fall asleep and wake up. That is regulated by hormones. When you wake up, the body mainly releases cortisol.
A disturbed sleep rhythm therefore stands in the way of a properly functioning hormone release. This can lead to various complaints, such as an increased appetite and increased blood pressure.
Set boundaries
Stress often arises because a person becomes overwhelmed. There are 24 hours in a day, 8 of which you sleep. An excess of tasks and responsibilities can cause a person to reach the limit. It is difficult to learn to say no for many people. Still, it’s a good idea to say no when you’re suffering physically and mentally. The easiest way to deal with a stressful schedule is to relieve it.
Diathesis Stress Model: deal with stress
Do you experience a lot of stress despite taking measures? Then take a moment every now and then to pause, breathe and reflect on how you feel. You might consider keeping an emotion diary . Emotions are processed by different parts of the brain. As you write about your feelings, you may just start to see things from a new perspective.

Now it’s your turn
What do you think? Do you recognize the explanation about the Diathesis stress model/ stress vulnerability model? Do you think you are vulnerable to developing a psychological disorder? Do you have protective factors such as close family and a close circle of friends? Do you have any tips or comments?
Share your experience and knowledge in the comments box below.
More information
- Walker, E. F., & Diforio, D. (1997). Schizophrenia: a neural diathesis-stress model . Psychological review, 104(4), 667.
- Swearer, S. M., & Hymel, S. (2015). Understanding the psychology of bullying: Moving toward a social-ecological diathesis–stress model . American Psychologist, 70(4), 344.
- Broerman, R. (2020). Diathesis-stress model . Encyclopedia of personality and individual differences, 1107-1109.
How to cite this article: Janse, B. (2022). Diathesis Stress Model . Retrieved [insert date] from Toolshero: https://www.toolshero.com/psychology/diathesis-stress-model/
Original publication date: 08/15/2022 | Last update: 12/25/2023
Add a link to this page on your website: <a href=”https://www.toolshero.com/psychology/diathesis-stress-model/”>Toolshero: Diathesis Stress Model</a>
Did you find this article interesting?
Your rating is more than welcome or share this article via Social media!
Average rating 4.2 / 5. Vote count: 5
No votes so far! Be the first to rate this post.
We are sorry that this post was not useful for you!
Let us improve this post!
Tell us how we can improve this post?

Ben Janse is a young professional working at ToolsHero as Content Manager. He is also an International Business student at Rotterdam Business School where he focusses on analyzing and developing management models. Thanks to his theoretical and practical knowledge, he knows how to distinguish main- and side issues and to make the essence of each article clearly visible.
Related ARTICLES

Mandela effect: the meaning, basics and some examples

McGurk Effect in Psychology explained

Stress and Happiness at Work

Fowler’s Stages of Faith Development

Psychotherapy: the Definition and Theory explained

Ulric Neisser biography, quotes and books
Also interesting.

Social Learning Theory by Albert Bandura

Psychological Safety by Amy Edmondson

Barrett Model, a great motivation theory
Leave a reply cancel reply.
You must be logged in to post a comment.
BOOST YOUR SKILLS
Toolshero supports people worldwide ( 10+ million visitors from 100+ countries ) to empower themselves through an easily accessible and high-quality learning platform for personal and professional development.
By making access to scientific knowledge simple and affordable, self-development becomes attainable for everyone, including you! Join our learning platform and boost your skills with Toolshero.

POPULAR TOPICS
- Change Management
- Marketing Theories
- Problem Solving Theories
- Psychology Theories
ABOUT TOOLSHERO
- Free Toolshero e-book
- Memberships & Pricing
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

A direct test of the diathesis–stress model for depression
Lucía colodro-conde.
1 Genetics and Computational Biology, QIMR Berghofer Medical Research Institute, Brisbane, Australia
2 Human Anatomy and Psychobiology, University of Murcia, Murcia, Spain
Baptiste Couvey-Duchesne
3 Queensland Brain Institute, The University of Queensland, Brisbane, Australia
William L Coventry
4 School of Behavioural and Social Sciences, University of New England, Armidale, Australia
Enda M Byrne
5 Institute for Molecular Bioscience, The University of Queensland, Brisbane, Australia
Scott Gordon
Margaret j wright.
6 Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia
Grant W Montgomery
7 Institute for Molecular Bioscience, The University of Queensland, Brisbane, Australia
Pamela AF Madden
8 Department of Psychiatry, Washington University School of Medicine, St Louis, US
Stephan Ripke
9 Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, US
10 Department of Psychiatry and Psychotherapy, Universitätsmedizin Berlin Campus Charité Mitte, Berlin, DE
11 Medical and Population Genetics, Broad Institute, Cambridge, US
Lindon J Eaves
12 Department of Human and Molecular Genetics, Virginia Commonwealth University, Richmond, US
Andrew C Heath
Naomi r wray, sarah e medland, nicholas g martin, associated data.
The diathesis-stress theory for depression states that the effects of stress on the depression risk are dependent on the diathesis or vulnerability, implying multiplicative interactive effects on the liability scale. We used polygenic risk scores for major depressive disorder (MDD) calculated from the results of the most recent analysis from the Psychiatric Genomics Consortium as a direct measure of the vulnerability for depression in a sample of 5 221 individuals from 3 083 families. In the same we also had measures of stressful life events and social support and a depression symptom score, as well as DSM-IV MDD diagnoses for most individuals. In order to estimate the variance in depression explained by the genetic vulnerability, the stressors and their interactions, we fitted linear mixed models controlling for relatedness for the whole sample as well as stratified by sex. We show a significant interaction of the polygenic risk scores with personal life events (0.12% of variance explained, p-value=0.0076) contributing positively to the risk of depression. Additionally, our results suggest possible differences in the aetiology of depression between women and men. In conclusion, our findings point to an extra risk for individuals with combined vulnerability and high number of reported personal life events beyond what would be expected from the additive contributions of these factors to the liability for depression, supporting the multiplicative diathesis-stress model for this disease.
Introduction
A popular explanation for the aetiology of depression is the diathesis-stress model 1 – 6 . Initially developed to explain the origins of schizophrenia in the 1960s 5 , 6 and adapted for the study of depression in the 1980s 1 – 4 , this model states that stress may activate a diathesis or vulnerability, transforming the potential of predisposition into the actuality of psychopathology 7 . The model proposes that there is a synergism between the diathesis and stress that yields an effect beyond their combined separate effects into depressive symptomatology and thus, the effects of stress on the depression risk are dependent on the diathesis. Implicit in this theory is that there will be not only additive but multiplicative interactive effects on the liability scale 7 .
Over fifty years ago David Rosenthal 6 described the diathesis-stress theories as “the ones in which genuine meaning attaches to the commonly repeated statement that heredity and environment interact”. However, he criticised the vague formulations for the predispositions and stressors that these theories propose. This criticism has been highlighted by others like Monroe 7 , who call for more research and more precise measures on the “conceptual essence” of the diathesis-stress premise, i.e. “the nature of the interaction between elements in the etiologic process over time”. The diathesis-stress theory and research have been criticised for being “unproductive, either theoretically or empirically” 8 .
The genetically driven sensitivity to environments proposed by the diathesis-stress model can be operationalised as a gene by environment interaction (GxE). GxE studies have commonly focused on single loci in candidate genes, such as the length polymorphism (5HTTLPR) in the serotonin transporter gene (SLC6A4), with mostly inconsistent or negative results 9 – 13 . This approach has limitations related to poor quality genotyping, inconsistent types of interactions, inconsistent grouping of genotypes, selective presentation of results, interactions arising from the scale of measurement, and publication bias 9 . Moreover, MDD is a polygenic trait, arising from the effect of multiple risk variants, each with small effect sizes 14 , 15 . Therefore, MDD is influenced by many genetic variants of small effect, and it is more likely that affected individuals carry a polygenic burden of risk alleles rather than any single genotype of large effect. However, the progress from a candidate gene to an hypothesis-free genome-wide approach is hampered by the need for extremely large samples due to expected small effect sizes as well as necessarily imperfect assessment of environmental stressors across large cohorts 16 , 17 .
Polygenic risk scores (PRS) provide a novel opportunity to test the diathesis-stress model, since PRS can be conceptualised as an indicator of the diathesis and will likely prove a much stronger instrument than any single risk gene. PRS estimation uses Genome-Wide Association Study (GWAS) results to predict the genetic risk of each individual in an independent genotyped sample; PRS are estimated as the sum of risk alleles weighted by their respective independently estimated effect sizes 18 . Note that, since GWAS are currently underpowered to detect all common genetic risk variants in complex traits, the variance explained by the PRS is usually lower than the twin heritability 18 .
The first ones to use PRS for MDD to test for GxE interaction in MDD were Peyrot et al. 17 . Using a sample of 1 645 participants with a DSM-IV diagnosis for MDD and 340 screened controls from the Netherlands Study of Depression and Anxiety, they showed increased effects of PRS on MDD in the presence of childhood trauma, with evidence for interaction. Musliner et al 19 studied the association between PRS-MDD, SLEs and depressive symptoms in a sample of 8 761 participants from the Health and Retirement Study in the United States. SLEs were operationalised as a dichotomous variable indicating whether participants had experienced at least one stressful event in the previous two years. Depressive symptoms were measured using an 8-item Center for Epidemiological Studies Depression subscale and operationalised as both a dichotomous and a continuous variable. They found that both SLEs and PRS were significantly and independently associated with depressive symptoms, but found no evidence that SLEs moderated the association between PRS-MDD and depressive symptoms. Instead, their results were compatible with an additive model. Most recently, Mullins et al. 20 examined the idea using 1 605 cases with recurrent MDD and 1 064 controls all with SLE data, and a subset of 240 cases and 272 controls with childhood trauma data from in the RADIANT UK study. Both PRS and SLEs were significant predictors of case/control status but no interactions were found between PRS for MDD and SLEs, in agreement with previous findings by Musliner et al. 19 . Significant interactions were found between PRS and childhood trauma but, contrary to Peyrot et al. 17 , there was an inverse association with depression status. In summary, these studies do not present consistent results. Studies to date have used the first wave of GWAS data (MDD1) from the Psychiatric Genomics Consortium (PGC) MDD working group (PGC-MDD), based on 9 240 cases and 9 519 controls 14 , and so are likely underpowered.
We report here a direct test of the diathesis-stress model for depression using PRS for MDD and measures of Stressful Life Events (SLEs) and Social Support (SS; lack of SS being considered a stressor); we predict diathesis using an updated version of PGC-MDD GWAS results (N total=159 601, after excluding QIMR data). Given the higher lifetime risk of MDD in women 21 , we also tested the hypothesis in sexes separately.
Materials and Methods
Phenotypic data were collected as part of a general Health and Lifestyle questionnaire (HLQ) mailed to adult twins enrolled in the Australian Twin Registry between 1988 and 1992 22 – 24 . It included self-report questions about depression, recent personal or network stressful life events (PSLE, NSLE) and SS. The content and details of data collection have been previously described 22 – 24 . Data used in this analysis were collected in 3 waves. The first wave ran between 1988 and 1992 (N=5 843) and targeted adult twins (mean age 41.2, SD=12.8, range 24–86, 61.0% females) 23 . The second wave (N=3 646, collected between 1990 and 1992) focused on younger twins (mean age 23.2, SD=2.2, range 16–31, 65.6% females) and the questionnaire was slightly adapted to cover some of the more common issues of that age group 22 , 23 . Finally, the last wave (N=236) targeted twin pairs whose information was partially missing from the original 1980 survey, using the same questionnaire (collection between 1990–1992, mean age 42.0, SD=9.9, range 27–73, 58.5% females). This study was approved by the Queensland Institute of Medical Research Human Research Ethics Committee and the storage of the data follows national regulations regarding personal data protection. All of the participants provided informed consent.
Depression scores were calculated by combining the 7 depression items from the Delusions-Symptoms-States Inventory (DSSI) 25 , 26 with 5 depression items from the Symptom CheckList (SCL-90) 27 . The factor structure of the scale has been reported previously in the younger dataset 22 and the score has been used in several publications 9 , 22 , 23 , 28 , 29 .
The HLQ also assessed Personal Stressful Life Events (PSLE) and Network Stressful Life Events (NSLE), adapted from the List of Threatening Experiences 30 . For PSLE, participants were asked to report adverse events (divorce, marital separation, broken engagement or steady relationship, separation from other loved one or close friend, serious illness or injury, serious accident, burgled or robbed, laid off or sacked from job, other serious difficulties at work, major financial problems, legal troubles or involvement with police, living in unpleasant surroundings) that happened in the last 12 months. In addition, they were asked if they had had serious problems getting along with their close network (spouse, someone living with you e.g. child/elderly parent, other family member, co-twin, a close friend, neighbour or workmate) in the past 12 months. These 19 yes/no items were summed to calculate the PSLE score.
NSLE was calculated from 21 yes/no questions, in which the participants could report death, injury or crisis that their close network (spouse, child, mother/father, co-twin, other brother/sister, other relative, someone else close to them) experienced in the last 12 months.
Perceived Social Support (PSS) was measured using the Kessler Perceived Social Support (KPSS) Measure 31 . Several publications from our group have made use of these data 9 , 23 , 28 , 32 – 34 .
We used Item Response Theory 35 , 36 , which weights the item responses by their difficulty and discrimination, to calculate individuals’ scores of depression, PSLE, NSLE and SS. First, we performed an exploratory non-parametric IRT analysis the KernSmoothIRT package in R 37 . It allows estimation of the probability of endorsing each option of each item as a function of the latent underlying trait (known as Item Response Step Functions: IRSF), without any constraint on the shape of the fitted curve 36 . We used it to confirm the monotonicity of IRSF necessary to ensure the property of stochastic ordering on the sum score 38 . It further allows choosing the most appropriate parametric IRT models based on the shapes of the non-parametric IRSF.
IRSF plotted in Supplementary Figures 1 – 4 show, for all scales and items, monotonic IRSF in the normal range of the latent trait continuum (−2 2). Small breaches of monotonicity were observed for extreme values of the latent trait and can be attributed to small numbers of participants that lead to unstable non-parametric kernel estimation (as indicated by widening 95% CIs). Consequently, we estimated the IRT scores using a 2-parameter logistic model in WinBUGS v. 1.4.3 39 that constrains all left asymptotes to be 0 and all right asymptotes to be 1. In such a model, the IRSF only differ in term of difficulty and discrimination 35 , 40 . Such IRT scores are maximum likelihood estimates of the latent trait and carry the same information as a sum score while presenting more normal distributions, thus reducing the influence of extreme values in later analyses.
Missingness in the depression items was limited to less than 2% of the respondents and most of the missing answers (88 or 60%) were found in the item “recently, I have lost interest in sex or have found not found sex pleasurable”. The 1.6% of the respondents who omitted this item tended to be females (p-value=4.2e-04), 4 months older on average (p-value=9.7e-06), and with a slightly higher DSSI score (+0.1 pts, p-value=3.6e-05). Missingness not at random (i.e. potentially dependent on depression level) implies that excluding participants may create a sampling bias. Thus, we chose to impute the missing observations using WinBUGS (described in 34 , 41 ) using age, sex and the depression items as predictors. Overall, due the low missingness rate imputation should have little influence on the results.
Lifetime DSM-IV depression diagnoses were obtained in most of the cohort in two telephone interview follow-up studies using the clinical Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA 42 , 43 in 1992–1993 and 1996–2000 (see Supplementary Figure 5 for a summary of the phenotypic data collection). Details of data collection are described elsewhere 29 , 44 – 47 . The depression score significantly predicted lifetime DSM-IV MDD status assessed 4 to 7 years later 44 , 45 (OR=1.96, 95%CI 1.85–2.08, p-value=3.0e-108, N=8 607), which translates to a 6.1 fold increased odds of MDD between participants in the top and bottom deciles of depression IRT scores ( Figure 1a ), so demonstrating the utility of the score. For our analysis we used the continuous IRT score rather than the binary diagnosis as continuous models provide greater statistical power than logistic regressions (>99.9% vs. 88.0%, N= 5 221, O.R.= 1.1, beta = 0.095, with α= 0.05, proportion of cases and SD of outcome and predictor measured in our sample) and is available for larger sample size (5 179 vs. 5 221 with IRT score).

(a) Increased odds of DSM IV MDD diagnosis per decile of depression IRT score assessed 4–7 years previously. (b) Association between MDD-PRS and depression scores (main effects, one-sided tests, results expressed in % of variance explained). Full sample analyses using the two versions of the PRS were run in the same target dataset with the exact same covariates. Red bars indicate positive correlation with the depression score. PRS were calculated using different p-value thresholds from the GWAS summary statistics. The most conservative only includes independent loci with genome wide significant SNPs (p-value<5e-8), while the least conservative include the most significant SNP of each haplotype (p-value<1). (c) Association between self-reported stress (personal stressful life events (PSLE) or network stressful life events (NSLE), lack of SS) and depression IRT score (main effect, one-sided tests, results expressed in % of variance explained). Blue bars indicate negative correlations and red bars indicate positive correlations with the depression score. Dashed bars indicate sex specific effects. (d) Variance of the depression score explained by the interaction between PRS and PSLE (2-sided tests). Dashed bars indicate sex specific effects. We focused on the association with the PRS comprising all haplotypes but the other associations are also reported for completeness. Blue bars indicate negative correlations and red bars indicate positive correlations with the depression score. (e) Increase in depression score (fitted values, vertical axis) as a function of PSLE and MDD-PRS. For example, the effect of the PSLE-diathesis interaction is visible when comparing the bottom (minimal PSLE) and top (maximal PSLE) edges of the surface. The difference in slopes indicates that PSLE mediates the effect of the genetic predisposition on the depression score. From right to left, results for the whole sample, females and males. In all analyses, we accounted for familial relatedness using a kinship matrix (a) or a genetic relatedness matrix calculated from SNPs (b–d) . For (a) we used R package “hglm” 77 to estimate the odds ratios (student test to test the significance of the fixed effects). For panels (b–d) the parameters of the model were estimated using GCTA 1.26.0 (student test to test the significance of the fixed effects) 62 . All analyses controlled for age, age 2 , sex, age*sex and age 2 *sex interactions, study, array, and the first four genetic principal components in the outcome variable and predictors 60 .
DNA collected from blood was genotyped using commercial arrays (Illumina 317K, 370K, 610K, ‘1st generation’, or Core Exome plus Omni-family, ‘2nd generation’ 48 – 50 .) and imputed from a common SNP set to the 1000 Genomes (Phase 3 Release 5) reference panel 51 – 52 , a strategy that allows genotype data from different arrays to be combined. Observed genotypes were cleaned (by batch) for call rate (≥95%); minor allele frequency, MAF (>=1%); Hardy-Weinberg equilibrium (p≥10 −3 ; PLINK1. 9 53 ), GenCall score (≥0.15 per genotype; mean ≥0.7) and standard Illumina filters, before integrating batches and re-running the quality control and Mendelian checks. We imputed the genotype data via the University of Michigan Imputation Server 54 or in-house (chr. X only) using the 1000 genomes Phase 3 Release 5 ‘mixed population’ reference panel) 51 – 52 , with phasing by SHAPEIT 55 , 56 followed by imputation using minimac3 57 . ‘1st generation’ and ‘2nd generation’ were imputed separately due to poor overlap between observed markers. Imputation was based on 277 690 (‘1 st generation’) and 240 297 (‘2 nd generation’) observed markers; and the two combined after imputation to maximise sample size. This resulted in 9 411 304 SNPs available for analysis, after quality control.
PRS 58 , 59 were calculated from the imputed genotype dosages, using GWAS summary statistics from the most recent PGC MDD release [July 9 th 2016], with the exclusion of the contribution of QIMR, for a final sample of 49 524 cases and 110 074 controls (see Supplementary Table 1 for cohort contributions). For comparison, we also calculated the PRS using the first wave GWAS summary statistics published by the PGC-MDD 14 . From our data, we excluded SNPs with low imputation quality (r 2 <0.6) and MAF below 1%. We selected the most significant independent SNPs using PLINK1.9 53 in order to correct for signal inflation due to Linkage Disequilibrium (LD) (criteria LD r 2 <0.1 within windows of 10MBp). We calculated 8 different PRS using different p-value thresholding of the GWAS summary statistics (see Supplementary Table 2 for number of SNPs included in each threshold). Histograms of PRS for MDD (1000G imputation, GWAS results from July 2016), together with the histograms of the IRT scores for depression, PSLE, NSLE and SS scores are reported in Supplementary Figure 6 .
Our final sample comprised 5 221 individuals (from 3 083 twin families) of European ancestry with available phenotypic and genetic data (mean age at questionnaire 35.7, SD=12.2, range 17–85, 65.6% females). Covariates (age, age 2 , sex, age*sex and age 2 *sex interactions, and the first four genetic principal components) were regressed from the PRS and the stress scores before inclusion in the models to guard against confounding influences on the PRS-stress interactions 60 .
In order to estimate the variance explained by the PRS, the stressors and their interactions in the depression score, we then fitted linear mixed models which controlled for relatedness for the whole sample as well as stratified by sex. ). The parameters of the model were estimated using GCTA 1.26.0 (student test to test the significance of the fixed effects) that accounts for twin relatedness using a Genetic Relatedness Matrix (GRM). The linear model used is as follows:
Depression = intercept + b*Covariates + c*PRS_z + d*PSLE_z + e*NSLE_z + f*SS_z + g*PRS_z*PSLE_z + h*PRS_z*NSLE_z + i*PRS_z*SS_z + j*G
With b,c,d,e,f,g,h,i the vectors of fixed effects
Covariates used in this analyses were age, sex, age 2 , sex*age, sex*age 2 , GWAS array, wave, and first 4 genetic principal components. Note that sex and its interaction were not included when stratifying the analyses by sex.
PSLE_z, SS_z, NSLE_z and PRS_z are the residuals of the scores after regressing out the covariates listed above
G is the random effect that models the sample relatedness G~ N(0, GRM), with GRM the NxN matrix of relatedness estimated from SNPs
We used OpenMx 61 to calculate the heritability and correlations (likelihood-ratio test, using a kinship matrix to account for familial relatedness) of the depression score and the stressors, correcting for age, sex, age 2 , sex*age, sex* age 2 , and wave. Following the significant genetic correlations estimated from twin models, we investigated how much of the variance in stress scores could be accounted for by the MDD-PRS. We controlled for age, age 2 , sex (and their interactions), study, imputation batch and 4 genetic principal components. Model parameters were estimated using GCTA 1.26.0 62 that accounts from twin relatedness.
PRS for MDD significantly predicted the depression score (maximum variance explained =0.46%. p-value= 5.01e-08, Figure 1b , right panel), which represents a substantial improvement compared to PRS predictions based on earlier GWAS 14 ( Figure 1b , left panel, variance explained = 0.08%, p-value=0.018), reflecting the increased sample size of the GWAS discovery samples 63 , 64 . The main effects of PSLE, NSLE, and lack of SS were also significant, explaining respectively 12.9%, 0.3% and 3% of the depression score variance ( Figure 1c ), with effects in the expected directions. Lack of SS predicted more of the depression score in women than it did in men (between sex differences, p-value= 4.7e-03) but there were no other differences between sexes that reached significance.
The significance of main effects allowed testing the significance of the interaction between each stress type (PSLE, NSLE, lack of SS) and the most predictive PRS (using all SNPs: p<1). The interaction with PSLE was significant (0.12% of variance explained, p-value=0.0076) and contributed positively to the risk of depression, predominantly in women ( Figure 1d ). Overall, the variance explained by PRS main effect plus the interaction was comparable in men (0.73%) and women (0.60%). The interaction was not significant in men while explaining almost as much variance as the main effect in women. However, there was no significant difference when comparing the size of the interaction across sexes (p-value=0.21). For completeness, interactions between each stressor and all PRS are also reported in Supplementary Figure 7 .
Our finding of a significant diathesis-PSLE interaction points to an extra risk for individuals with combined vulnerability and high number of reported PSLE beyond what would be expected from their additive contributions to liability ( Figure 1d, 1e ). In the full sample 0.58% of the depression score variance was explained by the PRS and interaction, of which ~80% corresponds to the main effects and ~20% to the interaction. As the power of the PRS increases with larger GWAS 63 , and if these proportions are maintained, the interaction explaining about 20% of the heritability would be typical of the size of GxE estimates for other traits in other species 65 . We cannot dismiss the possibility of diathetic interactions with NSLE or SS, as the power of our study is still limited by the PRS instrument 63 and our sample size. This is evidenced by our measure of genetic predisposition still only explaining a small fraction of the depression score variance ( Figure 1b ), in comparison with the twin based heritability ( Supplementary Figure 8 and 66 ), or even the SNP heritability for MDD (h 2 SNP =0.21 18 ). Note that for all psychiatric and complex traits, it is common that the variance explained by PRS corresponds to a fraction of the heritability, especially when only a few variants are known 67 . Much larger GWAS samples are required to better differentiate the true SNP signals from the noise and to provide a greater level of prediction 63 .
We confirmed using a twin analysis of the dataset (1 110 MZ pairs, 1 032 DZ pairs, 961 singletons) that our measure of depression and all 3 stress measures are moderately heritable (30–40%, p-value<2.1e-25; Supplementary Figure 8 ), as reported previously 68 , 69 . Additive genetic and unique environment (AE) models showed the best fit to the data and shared environment could not explain the association (p-value<1.7e-03). We also replicated that self-reported measures of stress are genetically correlated with the depression score ( Supplementary Figure 9 ) 70 , 71 .
PRS for MDD predicted PSLE and SS (p-value<0.001), no significant association was observed with NSLE ( Supplementary Figure 10 ). This is consistent with heritability and genetic correlation results reported from twin models.
On the scale of measurement for depression that we have used, our results support the multiplicative diathesis-stress model for depression proposed in the 1980s. In addition, our results suggest possible differences in the aetiology of depression between women and men, which may have implications for the tailoring of treatments. However, we must caution that the presence and size of interaction are completely dependent on the scale of measurement and more perfectly normal scales for depression and stressors may have produced a different result 65 , 72 . For example, using a simple sum score for depression, with an extreme reverse-J distribution yields an even larger and more significant estimate of interaction (0.39% variance explained; p-value = 6.8e-07) whereas using logistic regression to analyse the binary DSM IV diagnosis, predicated on an underlying normal liability, produces a smaller and only marginally significant estimate (0.06% variance explained; p-value = 0.059), although this analysis has much lower power than using a continuous variable.
In addition, the substantial genetic correlation between PSLE and depression hinders attributing the interaction solely to a GxE effect. To investigate this point, we broke down PSLE into events in which the individual may have played an active role (PSLE-active: divorce, separation, having difficulty at work, financial or legal troubles, not getting along with people) as opposed to passive role 73 , 74 (PSLE-passive: illness, accident, being burgled, sacked or living in unpleasant surroundings) and calculated the IRT score for them. This follows previous publications reporting that PSLE-active was more heritable than PSLE-passive 73 , 74 , which we confirmed in our sample (twin h 2 PSLE-Active =0.29, 95%CI 0.24–0.34; h 2 PSLE- Passive =0.11, 0.05–0.17). We tested whether the less heritable PSLE-passive may drive the observed interaction, which may point towards a more likely GxE interaction. However, in the models, active PSLE explained most of the variance explained by the PSLE score (r 2 PSLE- Active =10.5%, p-value=3.2e-123 vs. r 2 PSLE-Passive =0.77%, p-value=4.1e-12) and the interaction (r 2 PSLE- Active * PRS =0.085%, p-value=0.030 vs. r 2 PSLE-Passive * PRS =0.0084%, p-value=0.46) (results given for the PRS “P<1”, consistent with our other analyses, see Supplementary Figure 12 for all details). This approach was unable to confirm the nature of the interaction, since both PSLE-active and -passive are significantly heritable.
To tackle this question more directly, we calculated environmental and genetic factor scores for PSLE (PSLE-E and PSLE-A) via an independent pathway model fitted to the 19 items of the PSLE questionnaire 75 ( Supplementary Figure 13 ). Conceptually, this divides the IRT PSLE score, which estimates the (phenotypic) latent trait underlying the participants’ responses in the questionnaire, into its additive genetic and environmental dimensions ( Supplementary Figures 13 and 14 ). We then replaced the PSLE IRT score by each of its components in a mixed model to investigate the source of the interaction: GxG if interaction between PRS and PSLE-A or GxE if interaction between PRS and PSLE-E. As in our previous analyses, we regressed covariates out of the factor scores and also included them in the model. The model including PSLE-E was the best fitting as indicated by lower Akaike information criterion, AIC (AIC PSLE-E =2645, AIC PSLE-A =2798). Further, the interaction term points towards a greater GxE effect, as the interaction between PRS and PSLE-E explains 0.11% of the variance (p-value=0.0076), although we cannot rule out the presence of an interaction with PSLE-A (r 2 =0.076%, p-value=0.037) (results given for the PRS “P<1”, see Supplementary Figure 15 for more details). Results were showed a similar pattern when we modelled the A and E factors of PLSE-active.
Notwithstanding the above caveats, more work is needed to evaluate different mechanisms of interaction, including a bi-causal relationship between PSLE and depression or molecular interaction (e.g. through methylation changes). We are aware of potential confounds of interaction analyses: in addition to sensitivity of the analyses to the properties of the scale 76 and unavoidable departures from normality in the outcome and predictors as discussed above ( Supplementary Figures 6 and 11 ), problems may also arise from the stress and depression measures being self-reported in the same questionnaire, and the fact that the stress measures are genetically correlated with the outcome variable. Replication of our findings in independent cohorts, consideration of other variables such as the perceived impact of stressors and improvement of PRS via larger GWAS and larger samples with both depression and risk factors evaluated will allow us further to refine our understanding of the aetiology of depression.
Supplementary Material
Item Response Step Function for each option of each item included in the depression score. All items were 4 points Likert scales (coding 0: not at all, 1: a little, 2: a lot, 3: unbearably).
IRSF of the PSLE scale. All items were yes (1), no (0) questions.
IRSF of the NSLE scale. All items were yes (1), no (0) questions.
IRSF of the SS scale. All items were 4 points Likert scales (coding 0: not at all, 1: a little, 2: quite a bit, 3: a great deal)
Timeline of the phenotypic data collection. Health and Lifestyle Questionnaire, waves 1, 2 and 3 (HLQ1, HLQ2, HLQ3), and follow-up clinical assessment of lifetime major depressive disorder as per DSM-IV criteria (DSM).
Histograms of the IRT scores for depression, personal stressful life events (PSLE), network stressful life events (NSLE), social support (SS) and polygenic risk scores for major depressive disorder (PRS MDD, July 2016 GWAS). There were no sex differences in the distributions of the PRS.
Variance of the depression score explained by the interactions between personal stressful life events (PSLE) network stressful life events (NSLE), social support (SS) and polygenic risk scores for major depressive disorder (PRS MDD, July 2016 GWAS). We focused on the association with the PRS comprising all haplotypes but the other associations are also reported for completeness. Blue bars indicate negative correlations and red bars indicate positive correlation with the depression score.
Twin heritability of depression and self-reported stress levels (diagonal) and phenotypic correlations between measurements (above diagonal). Coloured squares indicate significant association (p-value<0.001).
Genetic (above diagonal) and environmental correlations (below diagonal). Coloured squares indicate significant correlations (p-value<0.001).
Association between MDD-PRS (July 2016 release) and self-reported measures of stress (PSLE, NSLE, SS). Red bars indicate a positive correlation; blue bars a negative correlation between PRS and stress scores.
Jinks-Fulker plot ( 78 , p. 313) of absolute MZ pair differences (1 110 MZ pairs) on corresponding pair sums for IRT depression scores. The significant linear (p-value = 3.24e-12) plus quadratic regression (p-value = 0.0495) indicates heteroscedasticity which may generate scale dependent GxE interaction. However, while significant, the effect is not numerically large and the interaction is almost significant (p-value = 0.059) in the logistic regression analysis of the less powerful binary DSM-IV MDD diagnosis
From left to right: variance of the depression score explained by PSLE- active and -passive, variance explained by the PRS, variance explained by the interaction between PSLE- passive and PRS, variance explained by the interaction between active PSLE and PRS. PSLE-active explained most of the reported association between PSLE and depression score, as well as most of the interaction. Note that the scale of the y-axis is different.
A) Phenotypic model that corresponds to the IRT score calculation, in which items are weighted and combined to provide an accurate ML estimate of the latent trait. B) Twin model that allows estimating the A and E factor scores (we used the regression method 75 ) by explicitly modelling the environmental and additive genetic latent variables.
The high correlation between the PSLE IRT score and the sum of factor scores (PSLE-E + PSLE-A) confirms that the twin model approach appropriately breaks up the IRT score into its additive genetic and environmental components.
From left to right: Variance of the depression score explained by the PSLE-A and PSLE-E factor scores; Variance of the depression score explained by the PRS; Variance of the depression score explained by the PSLE-E*PRS interactions; Variance of the depression score explained by the PSLE-A*PRS interactions. P-values as well as direction of effect are reported as previously. Models including PSLE-E showed lower AIC, hence better fit to the data. For all PRS, the GxE interactions explained a greater proportion of the depression score variance than GxG interactions. Note that the scale of the y-axis is different.
Acknowledgments
Phenotype collection, DNA collection, and genotyping were funded by NHMRC (981351) and NIH (R01 AA013326, R01 AA007535, R01 AA010249, R01 AA013321, R37 AA007728) grants to NGM, ACH and PAFM over the past three decades. The Psychiatric Genomics Consortium MDD Working Group depends on the contributions of many parties. LCC was supported by a post-doctoral fellowship from the Fundación Séneca (Seneca Foundation, Regional Agency for Science and Technology, Murcia, Spain, 19151/PD/13) and is supported by a QIMR Berghofer Fellowship. BCD is supported by a UQI scholarship. SEM is supported by a NHMRC research fellowship (1103623). We are grateful to the QIMR participants, data collectors and data managers.
Conflict of interest
The authors declare no conflict of interest.
Diathesis-Stress Model
- Reference work entry
- Cite this reference work entry

- Kristen Salomon 3 &
- Alvin Jin 3
2284 Accesses
1 Citations
1 Altmetric
Risk factors
Diathesis refers to a predisposition or vulnerability for the development of a pathological state. Diathesis-stress models argue that certain pathological states or diseases emerge from the combination of a predisposition with stressful events (Zuckerman, 1999 ). Most models specify that neither the diathesis nor stress alone is sufficient to produce the disorder. Instead, stress activates the diathesis, which then leads to the disorder. More broadly, diathesis-stress models are similar to the idea of risk-factors for stress-related diseases.
Description
Early diathesis-stress models primarily focused on psychiatric disorders such as schizophrenia, depression, and anxiety disorders, born out of the observation that these disorders tend to be inherited and yet also show a significant relationship to life stress (Zuckerman, 1999 ). These early diathesis-stress models identified fixed biological and/or hereditary factors as predispositions, and...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
References and Readings
Belsky, J., & Pluess, M. (2009). Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin, 135 (6), 885–908.
Article PubMed Google Scholar
Fowles, D. C. (1992). Schizophrenia – diathesis stress revisited. Annual Review of Psychology, 43 , 303–336.
Article PubMed CAS Google Scholar
Lesch, K. P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., et al. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science, 274 , 1527–1531.
Monroe, S. M., & Simons, A. D. (1991). Diathesis-stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin, 110 , 406–425.
Zuckerman, M. (1999). Vulnerability to psychopathology: A biosocial model . Washington, DC: American Psychological Association.
Book Google Scholar
Download references
Author information
Authors and affiliations.
Department of Psychology, University of South Florida College of Arts & Sciences, 4202 East Fowler Ave, PCD 4118Gm, Tampa, FL, 33620-7200, USA
Dr. Kristen Salomon & Alvin Jin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kristen Salomon .
Editor information
Editors and affiliations.
Behavioral Medicine Research Center, Department of Psychology, University of Miami, Miami, FL, USA
Marc D. Gellman
Cardiovascular Safety, Quintiles, Durham, NC, USA
J. Rick Turner
Rights and permissions
Reprints and permissions
Copyright information
© 2013 Springer Science+Business Media, New York
About this entry
Cite this entry.
Salomon, K., Jin, A. (2013). Diathesis-Stress Model. In: Gellman, M.D., Turner, J.R. (eds) Encyclopedia of Behavioral Medicine. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-1005-9_797
Download citation
DOI : https://doi.org/10.1007/978-1-4419-1005-9_797
Publisher Name : Springer, New York, NY
Print ISBN : 978-1-4419-1004-2
Online ISBN : 978-1-4419-1005-9
eBook Packages : Medicine Reference Module Medicine
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
The diathesis-stress model is a modern development of a longstanding debate about the causes of mental illness. This debate began as early as ancient Greece and Rome when theories included imbalances in bodily fluids and interactions with the devil. Later, this evolved into the "nature vs. nurture" debate. By the late 20th century, it ...
The diathesis-stress model is a theory suggesting that mental disorders and medical conditions are caused by a combination of an inherent predisposition and and the person's experience of stress. Also known as the vulnerability-stress model, it is a framework for understanding how existing vulnerabilities and environmental stresses interact to ...
The diathesis-stress model, also known as the vulnerability-stress model, is a psychological theory that attempts to explain a disorder, or its trajectory, as the result of an interaction between a predispositional vulnerability, the diathesis, and stress caused by life experiences. The term diathesis derives from the Greek term ...
The diathesis-stress model is a theory about how stress and genetics play into the manifestation of different mental disorders and conditions. High stress and high predisposition significantly increase your risk of developing said disorders. But even high stress and low predisposition can increase your risk.
The diathesis-stress model, or the vulnerability-stress model, is a psychological theory suggesting that mental disorders result from a combination of genetic risk factors interacting with environmental factors. This model explains that individuals with a genetic or biological predisposition for a certain disorder are more likely to develop it if significant life stressors are also present.
The diathesis-stress model, an influential conceptualization of psychiatric illness over recent generations (3-5), postulates that etiologic factors underlying psychiatric illness can be divided into those that are present from an early age and are temporally stable in their effect (diathesis) and those that are temporally discrete, occurring close in time to disorder onset (stress).
Recently, the basic diathesis-stress model has been expanded to include predispositions that protect individuals from developing stress-related disorders, or resilience (Belsky and Pluess 2009). Instead of focusing on why some people fall victim to disorders in the face of stress, resilience research focuses on why some people seem resistant to ...
The diathesis-stress model of psychopathology is a framework for understanding the development of psychological disorders. According to the general model, each individual possesses some degree of inherent vulnerability (i.e., diathesis) for developing a given disorder. Onset of a disorder can then be triggered by environmental stress; however ...
The stress diathesis model for suicidal behavior addresses clinical and cognitive factors and social context. Its structure reflects what is known about the interrelationship of the many reported causal factors. Mood regulation, decision making, and aggressive/impulsive traits can be linked to biological intermediate phenotypes and in turn to ...
This chapter discusses diathesis-stress models and provides general definitions of diathesis, personality and stress. Diathesis-stress models may take different forms depending on assumptions made about the roles of the diathesis and stress, such as whether they are necessary, sufficient, or contributing but not necessary causes. Human models, and the usefulness of animal models, in the ...
The diathesis-stress model proposes that a latent diathesis may be activated by stress before psychopathological symptoms manifest. Some levels of diathesis to illness are present in everybody, ...
Figure 1 displays the stress-diathesis model and its three main components: stress, which can be physical or emotional or another factor which causes negative effects on a person; diathesis, a genetic or biological disposition/threshold for development of mental ill-health; and vulnerability or protective factors, factors which help keep a ...
Mann et al. (1999) proposed a stress-diathesis model based on the findings from a clinical study of a large sample of patients admitted to a university psychiatric hospital. When compared to patients without a history of suicide attempts, patients who had attempted suicide show higher scores on subjective depression and suicidal ideation, and ...
Scatterplot of diathesis-stress interactions on depression score. Interactions with PRS at which nominally significant GxE effects were detected in ( a) full cohort ( p- threshold = 0.01) and ( b) in women ( p- threshold = 1 × 10 -5) are shown. At bottom, the remaining samples (i.e., full cohort, women or men) at same p- threshold are shown ...
The stress-diathesis model is the explanation that a disorder or behaviour trait is the result of an interaction between genetic predisposition vulnerability and stress, usually caused by life events and factors. Schizophrenia can be explained by the stress-diathesis model. A child may have inherited the gene from their parents (nature). This ...
The diathesis-stress model postulates that major transitions or negative events interact with prior vulnerabilities to psychopathology and result in increased problems or poor outcomes in the face of stressors (Graber and Sontag 2009). According to this model, ...
The Diathesis stress model or stress vulnerability model is a theory from psychology that explains disorders as a result of an interaction between vulnerability, diathesis and stress as a result of life experiences. The term diathesis comes from Greek and means something like predisposition or sensitivity.
The diathesis-stress model is a psychological theory that explains behavior as a result of both biological and genetic vulnerability (the meaning of the greek word diathesis), and stress from life experiences. This model thus assumes that the onset of a certain disorder may result from a combination of one's biological disposition towards ...
A popular explanation for the aetiology of depression is the diathesis-stress model 1 - 6. Initially developed to explain the origins of schizophrenia in the 1960s 5, 6 and adapted for the study of depression in the 1980s 1 - 4, this model states that stress may activate a diathesis or vulnerability, transforming the potential of ...
The diathesis stress is a psychological theory. which relates diathesis (susceptibility) and stress. in order to develop psychopathological disorder. Stress. Stress is a psychological state marked ...
The diathesis-stress model of abnormality says that people develop psychological disorders as a result of a combination of nature and nurture. There are many strengths of that model, including the ...
Diathesis refers to a predisposition or vulnerability for the development of a pathological state. Diathesis-stress models argue that certain pathological states or diseases emerge from the combination of a predisposition with stressful events (Zuckerman, 1999 ). Most models specify that neither the diathesis nor stress alone is sufficient to ...
Updated on 04/19/2018. the theory that mental and physical disorders develop from a genetic or biological predisposition for that illness (diathesis) combined with stressful conditions that play a precipitating or facilitating role. Also called diathesis-stress hypothesis (or paradigm or theory ). See also stress-vulnerability model.