Quality of tuberculosis care in India: assessing diagnostic and treatment practices of health care providers
Downloadable content.
- Satyanarayana, Srinath
- Madhukar Pai (Supervisor)
- MOTIVATION La tuberculose continue d'être un problème de santé publique majeur dans les pays à revenu faible ou intermédiaire. L'Inde, qui a enregistré 2,8 millions de cas d'incidents et 0,48 million de décès en 2015, est le pays le plus lourd de la tuberculose au monde. Un diagnostic précoce et un traitement approprié sont essentiels pour le contrôle de la tuberculose et les prestataires de soins jouent un rôle majeur dans ce processus. Le système de santé de l'Inde est complexe, mal réglementé, avec différents types de fournisseurs de soins de santé (publics / privés, qualifiés / non qualifiés). La façon dont les fournisseurs de soins de santé (en particulier ceux du secteur privé) gèrent les personnes atteintes de la tuberculose et les maladies est largement inconnue. En Inde, il existe une grande incertitude quant à la méthodologie à utiliser pour évaluer la qualité des soins en raison des variations énormes des caractéristiques des prestataires de soins et des patients. En outre, en raison des grandes lacunes dans les connaissances et les pratiques des fournisseurs de soins de santé, l'évaluation des pratiques des fournisseurs de soins de santé dans les environnements cliniques de routine est considérée vitale pour déterminer la qualité des soins de la tuberculose. OBJECTIF L'objectif global de ma thèse est de décrire en utilisant des méthodes appropriées ce que les fournisseurs de soins de santé «faire» dans la pratique courante pour les patients atteints de symptômes de la tuberculose pulmonaire et la maladie. La «qualité» dans le cadre de ma thèse de doctorat est définie comme l'adhésion aux normes internationales et nationales de soins de la tuberculose par les fournisseurs de soins de santé. Ma thèse est basée sur des manuscrits et a les trois études suivantes. La première est une revue systématique de diverses études qui ont évalué les connaissances et les pratiques relatives aux TB en Inde. Dans les deuxième et troisième études, j'ai utilisé la méthodologie standardisée des patients pour évaluer les pratiques des fournisseurs de soins de santé. La deuxième étude décrit la façon dont les pharmacies gèrent les personnes souffrant de symptômes et de maladies de la tuberculose pulmonaire et la troisième étude évalue s'il existe des différences entre les sexes dans la prise en charge des patients et des femmes atteints de tuberculose pulmonaire.
- MOTIVATION Tuberculosis (TB) continues to be a major public health problem in low and middle-income countries. India, with 2.8 million incident cases and 0.48 million deaths in 2015, is the highest TB burden country in the world. Early diagnosis and appropriate treatment is essential for TB control and health care providers play a major role in this process. India's health care system is complex, poorly regulated, with different types of health care providers (public/private, qualified/unqualified). How health care providers (especially those in the private sector) manage persons with TB symptoms and disease is largely unknown. In India, there is considerable uncertainty about what methodology to use for assessing the quality of care due to huge variations in health care provider and patient characteristics. Moreover, due to large gaps in health care providers' knowledge and practices, assessing health care providers 'practices' in routine clinical settings is considered vital for determining the quality of TB care. OBJECTIVE The overall goal of my PhD thesis is to describe using suitable methods what health care providers 'do' in routine practice for patients with pulmonary TB symptoms and disease. 'Quality' in the context of my PhD thesis is defined as adherence to international and national standards of tuberculosis care by health care providers. My thesis is manuscript based and has the following three studies. The first is a systematic review of various studies that have assessed health care providers' TB-related knowledge and practices in India. In the second and third studies, I have used the standardized patient methodology to assess health care providers' practices. The second study describes how pharmacies manage persons with pulmonary TB symptoms & disease and the third study assesses whether there are gender differences in the health care providers' management of male and female patients with pulmonary TB symptoms & disease.
- Epidemiology and Biostatistics
- McGill University
- https://escholarship.mcgill.ca/concern/theses/pn89d915m
- All items in eScholarship@McGill are protected by copyright with all rights reserved unless otherwise indicated.
- Department of Epidemiology, Biostatistics and Occupational Health
- Doctor of Philosophy
- Theses & Dissertations
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Recurrence of pulmonary tuberculosis in India: Findings from the 2019–2021 nationwide community-based TB prevalence survey
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation ICMR- National Institute for Research in Tuberculosis, Chetpet, Chennai, India
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected] (CP); [email protected] (SS)
Affiliation Central TB Division, Ministry of Health and Family Welfare, New Delhi, India
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation National Professional Officer, WHO Country Office, New Delhi, India
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Contributed equally to this work with: Smita Asthana, Rakesh Balachandar, Sampada Dipak Bangar, Avi Kumar Bansal, Jyothi Bhat, Debjit Chakraborty, Vishal Chopra, Dasarathi Das, Shanta Dutta, Kangjam Rekha Devi, Sunil Kumar, Avula Laxmaiah, Major Madhukar, Amarendra Mahapatra, Suman Sundar Mohanty, Chethana Rangaraju, Jyotirmayee Turuk, Kamran Zaman
Roles Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation ICMR- National Institute for Cancer Prevention and Research, Noida, Uttar Pradesh, India
Roles Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation ICMR- National Institute for Occupational Health, Ahmedabad, Gujarat, India
Affiliation ICMR- National AIDS Research Institute, Pune, Maharashtra, India
Affiliation ICMR- National JALMA Institute of Leprosy and other Mycobacterial diseases, Agra, Uttar Pradesh, India
Roles Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation ICMR- National Institute for research in Tribal Health, Jabalpur, Madhya Pradesh, India
Affiliation ICMR- National Institute of Cholera and Enteric Diseases, Kolkata, India
Affiliation State TB Training and Demonstration Centre (STDC), TB Hospital, Lahori, Punjab, India
Affiliation ICMR- Regional Medical Research Centre, Bhubaneshwar, Odisha, India
Affiliation ICMR- Regional Medical Research Centre, Dibrugarh, Assam, India
Affiliation State TB Cell, Trivandrum, Kerala, India
Affiliation ICMR- National Institute for Research in Nutrition, Hyderabad, Telangana, India
Affiliation ICMR- Rajendra Memorial Research Institute of Medical Sciences Agamkuan, Patna, India
Affiliation ICMR- ICMR-National Institute for Implementation Research on Non-Communicable Diseases, Jodhpur, India
Affiliation National Tuberculosis Institute, Bengaluru, Karnataka, India
Roles Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation ICMR- Regional Medical Research Centre, Gorakhpur
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation Indian Council of Medical Research, New Delhi, India
- [ ... ],
Roles Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
- [ view all ]
- [ view less ]
- Prathiksha Giridharan,
- Sriram Selvaraju,
- Raghuram Rao,
- Kiran Rade,
- Kannan Thiruvengadam,
- Smita Asthana,
- Rakesh Balachandar,
- Sampada Dipak Bangar,
- Avi Kumar Bansal,

- Published: December 21, 2023
- https://doi.org/10.1371/journal.pone.0294254
- Reader Comments
Recurrent Tuberculosis patients contribute to a significant proportion of TB burden in India. A nationwide survey was conducted during 2019–2021 across India among adults to estimate the prevalence of TB. A total of 322480 individuals were screened and 1402 were having TB. Of this, 381 (27.1%) had recurrent TB. The crude prevalence (95% CI) of recurrent TB was 118 (107–131) per 100,000 population. The median duration between episodes of TB was 24 months. The proportion of drug resistant TB was 11.3% and 3.6% in the recurrent group and new TB patients respectively. Higher prevalence of recurrent TB was observed in elderly, males, malnourished, known diabetics, smokers, and alcohol users. (p<0.001). To prevent TB recurrence, all treated tuberculosis patients must be followed at least for 24 months, with screening for Chest X-ray, liquid culture every 6 months, smoking cessation, alcohol cessation, nutritional interventions and good diabetic management.
Citation: Giridharan P, Selvaraju S, Rao R, Rade K, Thiruvengadam K, Asthana S, et al. (2023) Recurrence of pulmonary tuberculosis in India: Findings from the 2019–2021 nationwide community-based TB prevalence survey. PLoS ONE 18(12): e0294254. https://doi.org/10.1371/journal.pone.0294254
Editor: Yatin N. Dholakia, The Foundation for Medical Research, INDIA
Received: May 27, 2023; Accepted: October 30, 2023; Published: December 21, 2023
Copyright: © 2023 Giridharan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data contains potentially sensitive information, and data access is governed by the Indian Council of Medical Research (ICMR). Data can be made available through a data-sharing agreement according to Indian government norms. Data are therefore accessible to interested researchers upon request. The ICMR data access request form must be completed and reviewed. The form can be obtained from National Institute for Research in Tuberculosis (NIRT), ICMR, India at the following email address: [email protected] .
Funding: The survey is funded by the Ministry of Health and Family Welfare, Government of India(F.No.5/8/5NTBP/Main File/ECD 1/2018, dt 18.12.2018, awarded to SS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have no conflict of interest to declare.
Introduction
Recurrent episodes of tuberculosis (RTB) is a public health challenge and has immense implications for TB control worldwide [ 1 ]. In areas of high TB and HIV burden, the recurrence is mainly due to exogenous reinfection while in low burden settings, the recurrence is primarily due to endogenous reactivation [ 2 , 3 ]. In India, the proportion of retreatment patients of smear positive pulmonary TB is 24% under the National TB Elimination Programme (NTEP) [ 4 ].
Risk of recurrent TB is high among patients who have recently completed treatment for TB. Individuals with recurrent TB are less likely to complete their treatment and also have a higher risk of mortality when compared with those with first episode of TB [ 5 ]. Hence recurrent TB patients needs to be identified not only for effective treatment but also to curtail the transmission of disease and prevent mortality. Rate of recurrent TB can be used as a proxy measure to assess the effectiveness of TB control programme in the country and emphasizes the emergence of drug resistance in the community [ 6 ]. In addition it increases the ongoing transmission and burden to the health system. Consequently recurrent TB is of high public health importance due to emergence of drug resistance, increased mortality. In this context it is important to understand the burden and epidemiological factors associated with TB recurrence in India, a country with the highest TB burden in the world. This knowledge can be incorporated by the NTEP in its implementation of TB control for achieving END TB goals. We present here the burden of TB recurrence in India based on the world’s largest ever National TB prevalence survey conducted in India, during 2019–2021 [ 7 ].
Survey setting, design and procedures
The NTEP in India recommends sputum testing and chest X-ray (CXR) for presumptive TB patients (those with symptoms of cough> 2weeks, fever> 2weeks, significant weight loss and haemoptysis). Those who are sputum smear positive are classified as microbiologically confirmed pulmonary TB (MCPTB). Those who are smear negative but CXR suggestive of TB and those with high clinical suspicion undergo cartridge based nucleic acid amplification test (CBNAAT) and subjected to universal Drug Susceptibility Testing (UDST) in order to start appropriate regimen for the TB patients. The programme also recommends follow up for 24 months after treatment completion.
A nationwide survey to estimate the prevalence of MCPTB among adults>15 years of age was conducted by ICMR-National Institute for Research in Tuberculosis (ICMR-NIRT) along with other ICMR institutes, NTEP, Central TB Division (CTD), National Institute of Tuberculosis and Respiratory Diseases, National Tuberculosis Institute, Intermediate Reference laboratories (IRLs), State TB Cells and WHO- India between June 2019 to September 2021. This was a cross sectional survey using multistage cluster sampling conducted in 443 clusters with a sample size of 800 in each cluster. Eligible participants underwent symptom screening and CXR and those with symptoms suggestive of TB or with history of previous/ current TB treatment and individuals having abnormal CXR underwent testing for TB by sputum CBNAAT, smear microscopy and liquid culture. All the participants were interviewed about their past episode of TB including time and place of treatment and those with reported history of TB treatment in the past were categorized as “Past TB” cases. For the survey “Recurrent TB (RTB)” was defined as participants identified as MCPTB during survey and/or on current TB treatment among those with a reported history of TB treatment in the past and “Non-Recurrent TB (NRTB)” were defined participants with a history of past TB treatment but were not identified as MCPTB and were not on TB treatment during the survey period. All the data were captured electronically. The investigators had access to information that could identify individual participants during and after data collection.
Data analysis
Descriptive analysis for summarizing the characteristics of survey participants was performed based on "past TB status" and/or "TB history" and RTB. All the statistical analysis were done using Stata16 (Stata Corporation, College Station, TX, USA). The crude prevalence per 100,000 of recurrent TB was estimated along with the confidence interval using the exact binomial formula. Univariate and multiple logistic regression analyses were performed for associations between RTB and risk factors such as age, gender, BMI, smoking, alcohol consumption, TB symptoms, and CXR abnormality. The multivariable analysis included variables known to be associated with PTB identified a-priori by literature review and post-hoc by exploratory data analysis. The statistical difference in duration between the past and current TB diagnosis was compared using an independent t-test. We calculated all estimates and 95% confidence intervals using the Stata svy commands to correct for design-effect. All the tests were two-sided, with a type I error set at a = 0.05.
Ethical statement
The survey was approved by the Institutional Ethics Committee of ICMR- National Institute for Research in Tuberculosis and all other participating institutes. Approval number: 334/ NIRT- IEC/2018 dated 26th November 2018. All Participants above 18 years provided a written informed consent and for participants from 15 years to 18 years written informed assent and Parent’s/ legally authorized representatives’ consent was obtained.
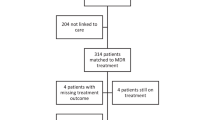
A total of 354,541 individuals aged more than 15 years participated in the survey and of these 322,480 (90.9%) underwent symptom screening and/or CXR ( Fig 1 ) We identified 7056 (2.2%) participants with a past history of TB, of whom 381 (5.3%) participants were identified as recurrent TB as defined in the survey. Of these 381 patients, 160 (42%) were diagnosed by at least two bacteriological evidence during survey, 70 (18.4%) were diagnosed with one bacteriological and one radiological evidence during survey and 151 (39.6%) were on current TB treatment at the time of survey. Among the 381 recurrent TB cases, 191 (50.1%) were currently not on any treatment and were diagnosed as part of the survey activities. ( Fig 2 ) The crude prevalence (95% CI) of RTB was 118 (107–131) per 100,000 population. The RTB patients (n = 381) contributed to 27.1% of the total (n = 1402) TB patients identified in the survey.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0294254.g001
https://doi.org/10.1371/journal.pone.0294254.g002
The characteristics of the participants with RTB and its crude prevalence is given in Table 1 . The prevalence of RTB was more among the 45 to 64 years age group, males, urban residents, extremely underweight, those with past history of alcohol and smoking and diabetics.
https://doi.org/10.1371/journal.pone.0294254.t001
A total of 7056 participants were classified as “Past TB”. Among them, 381 participants were identified as “RTB” and 6675 as “non- recurrent TB” patients. The median duration of recurrence of TB among the past TB patients was 24 months (IQR = 7,104) whereas the median duration of time from the past episode till the survey time point among the non-recurrent group was 108 months (IQR = 36,240) which was statistically significant. The proportion of past TB patients who sought care from public and private health sector were 78.2% (n = 298) and 21.8% (n = 83) respectively. The proportion of males with a habit of smoking was 9.4%, alcohol consumption was 10.8% and both was 16.5% in the recurrent TB group compared to 7.1%, 7.4% and 13.7% respectively in the non-recurrent TB group. This difference was statistically significant both by univariate and multivariate logistic regression. The proportion of RTB patients with diabetes mellitus was 8.4% compared to 7.3% in the non-recurrent group [ Table 2 ]. Out of the 381 RTB patients, 18 (4.7%) had a household contact (HHC) who were diagnosed with TB during the survey.
https://doi.org/10.1371/journal.pone.0294254.t002
The survey identified 1021 new patients with no reported past episode of TB. The difference in characteristics between new TB patients and RTB patients is shown in Table 2 . The Odds Ratio (95% CI) for RTB among males who smoke was 1.269 (0.812–1.984), males with alcohol consumption was 2.157 (1.37–3.396) and males with both smoking and alcohol usage was 1.399 (0.963–2.032). The proportion of RTB patients with symptoms and BMI<18.5 was 33.9%, symptoms and BMI >18.5 was 24.9%, no symptoms and BMI<18.5 was 15.2% and no symptoms and BMI>18.5 was 26% compared to 23.7%, 13.8%, 18.3% and 23.5% respectively among the newly diagnosed patients. There was a statistically significant (p<0.001) difference in age, gender, self-reported alcohol status, symptoms and BMI between the new and recurrent patients ( Table 3 ). The proportion of drug resistance, identified by Gene-Xpert was more among recurrent MCPTB patients (11.3%) when compared with new MCPTB (4.3%) patients identified in the survey. This difference was statistically significant (p<0.001) with an OR (95% CI) of 3.058 (1.666–5.611).
https://doi.org/10.1371/journal.pone.0294254.t003
The RTB observed in the current survey is high, in terms of absolute numbers, for a country like India. The proportion of RTB patients identified in the survey is slightly higher than proportion of sputum smear positive retreatment patients (24%) as reported in the NTEP [ 8 ] This is higher than the reported proportion of relapse in Zambia and other African counties [ 9 ]. of TB indicate that patients were cured but the underlying medical or social conditions have not been addressed completely. Factors like malnutrition, diabetic control, alcohol consumption and smoking that probably led to the previous episode of TB have not been addressed completely which is very essential to be tackled as we march towards TB elimination [ 10 ] RTB cases contributes to more than one fourth of the burden of TB prevalence in India. This emphasis the need for devising further strategies to closely monitor TB patients who have completed treatment. This will help early diagnosis of TB recurrence to prevent emergence of drug resistance and mortality. The prevalence of RTB is more in males (approximately 4 times) than in females. Similar findings were observed in the overall prevalence in the survey as well as in other studies [ 11 , 12 ]. This was in contrary to a systematic review done earlier in India which showed that there was no gender difference in recurrence [ 5 ].
Residing in an urban area, those with a reported past or current smoking status, past or current alcohol user, presence of DM and BMI<18 were all risk factors for RTB. All these are known risk factors for PTB and it would be reasonable to say that the trend applies here too. Similar findings have been reported in various other studies too [ 11 – 14 ]. The median duration of past TB episode from the survey time point in those with recurrent TB was 24 months which aligns with NTEP’s policy to follow TB patients for 2-years after treatment completion. A seven-years prospective study done in China showed that 76% of recurrence occurs in the first 3 years [ 12 ]. Similar results were noted in few other studies [ 15 , 16 ]. We also found that around 11% of recurrent patients had only one symptom. This emphasizes that people who had an episode of TB should be carefully followed up for presumptive TB symptoms and even a single symptom should not be ignored to identify recurrence at the earliest. It should be taken into consideration that past TB patients might have old lesions which would have been reported as X-ray abnormality in the survey. Inclusion of X-ray should be considered in the post treatment follow up strategy since nearly 41.2% of recurrent TB patients did not report any symptoms and identification of any new lesion can pick up the recurrence at the early stage of the disease. Though the participants had a past history of TB, we found that 17% of those with symptoms did not seek health care. The various delays in health seeking behaviour of TB patients may lead to disastrous outcomes like, drug resistance TB and this is even more likely in patients with recurrent TB [ 17 ]. There is a high proportion of MDR TB patients among the recurrent TB patients stressing the need for focused interventions to prevent TB recurrence and circumvent drug resistant TB. The prevalence of MDR-TB among previously treated TB cases in the survey (11.3%) is similar to the findings from another Indian study where the estimated prevalence is 12 to 14% [ 18 ]. From a public health perspective, recurrent TB patients contributes to the ongoing TB transmission to their contacts at home and community. Moreover, the higher risk of drug resistant TB makes it even more important to identify them at the earliest to combat the growing burden of drug resistant TB in the country [ 19 ].
The study has identified many risk factors for recurrence of TB, which can help in targeted post tuberculosis care and monitoring. Various studies conclude that active smoking increase the risk of TB relapse and also states that smokers were less likely to adhere to the tuberculosis treatment [ 20 ]. Around half of the patients with past history of TB (191/381) would have missed TB diagnosis at the National Level in the absence of this survey, indicates gaps in case detection and follow-up among the TB treated patients. When translated to absolute numbers, this may contribute to significant amount of TB transmission, drug resistance and mortality. Similar situation may be found in other high TB burden countries. In the context of moving towards END TB Goals, NTPs in India and other high burden countries, should strengthen the implementation of Post treatment follow-up among TB treated patients, for early diagnosis of recurrent TB, prevention of TB transmission and TB mortality.
Limitations
The recurrent TB was based on self-reporting is a huge limitation, there would be others who had recurrent TB but either did not report or died before diagnosis. The actual proportion of TB patients who experience recurrence may be different in fact higher than 5.3%, the observed figure in this study. The study could not distinguish as to whether these recurrences were due to relapse or reinfection. We did not capture details about the past TB illness in terms of type of TB disease, treatment details, smear status, outcome of the treatment which would have provided much more insights about the factors for TB recurrence. On the other hand, the operational definition used in the survey for MCPTB cases definition may overestimate the recurrent patients to a small extent because CBNAAT and smear may report positive for dead bacilli too. We expect that due to the composite nature of the case definition of MCPTB this would have been minimized.
Given the burden of increasing drug resistance in India, there is need for specific strategies to address risk factors for TB recurrence. The implementation gaps in post treatment follow up among TB treated patients for at least 24 months should be addressed. Health education awareness about benefits of smoking and alcohol cessation to TB patient must be offered to all TB patients. Emphasis on nutritional interventions and good diabetic control in the post treatment follow-up to prevent TB recurrence should be considered.
X-ray should be included in the follow up protocols to identify any new lesions. There is a growing need for effective vaccines or immunomodulators for post treatment prophylaxis to prevent recurrence by boosting immunity. Given the high cost of treatment that programme incurs for each patient in India, it is crucial to control recurrence of TB in treated patients to prevent drug resistance and TB transmission from these patients.
Acknowledgments
We acknowledge all the participants of survey, local community leaders and all staff who conducted the National TB Prevalence survey. We thank the support from the Central TB Division (CTD), National Health Mission (NHM), Ministry of Health and Family Welfare (MOHFW), Government of India, Department of Health Research (DHR), Indian Council of Medical Research (ICMR), Government of India, ICMR-National Institute for Research in Tuberculosis (ICM-NIRT), nodal ICMR Institutes and World Health Organisation, Country Office for India. We acknowledge the Experts who guided us in several Committees for the survey. We acknowledge the support provided by National TB Institute (NTI), National Institute of Tuberculosis and Respiratory Diseases (NITRD), WHO NTEP Consultant Network, State TB Cells and Reference Laboratories. We acknowledge all the NTEP staff at National, State and District level who helped in implementation of the survey. We also acknowledge the support given by various general health care staff, volunteers and the communities which supported the survey.
- View Article
- PubMed/NCBI
- Google Scholar
- 7. National TB prevalence survey in India (2019–2021). Available at https://tbcindia.gov.in/showfile.php?lid=3659 . Accessed on 10th April 2022.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 16 October 2020
Prevalence and factors associated with multidrug-resistant tuberculosis in South India
- Smita S. Shivekar 1 ,
- Venkatesh Kaliaperumal 2 ,
- Usharani Brammacharry 3 ,
- Anbazhagi Sakkaravarthy 4 ,
- C. K. Vidya Raj 1 ,
- Chitra Alagappan 1 &
- Muthuraj Muthaiah 1
Scientific Reports volume 10 , Article number: 17552 ( 2020 ) Cite this article
11k Accesses
35 Citations
23 Altmetric
Metrics details
- Microbiology
- Molecular biology
India accounts for about one-fourth of the global burden of MDR-TB. This study aims to assess the prevalence and factors associated with tuberculosis drug resistance among patients from South India. MTBDRplus assay and MGIT liquid culture performed on 20,245 sputum specimens obtained from presumptive MDR-TB cases during a six-year period from 2013 to 2018 were analyzed retrospectively. Univariate and multivariate logistic regression analysis was carried out to evaluate factors associated with MDR, Rifampicin mono-resistance, and Isoniazid mono-resistance. MDR, Rifampicin mono- resistant and Isoniazid mono-resistant TB were found in 5.4%, 2.5%, and 11.4% cases of presumptive MDR-TB, respectively. Based on the rpoB gene, true resistance, hetero-resistance, and inferred resistance to Rifampicin was found in 38%, 29.3%, and 32.7% of the 1582 MDR cases, respectively. S450L (MUT3) was the most common rpoB mutation present in 59.4% of the Rifampicin resistant cases. Of the 3390 Isoniazid resistant cases, 72.5% had mutations in the katG gene, and 27.5% had mutations in the inhA gene. True resistance, heteroresistance, and inferred resistance accounted for 42.9%, 22.2%, and 17.3% of the 2459 katG resistant cases, respectively. True resistance, heteroresistance, and inferred resistance for the inhA gene were found in 54.5%, 40.7%, and 4.7% cases, respectively. MDR-contact (AOR 3.171 95% CI: 1.747–5.754, p-0.000) treatment failure (AOR 2.17595% CI: 1.703–2.777, p-0.000) and female gender (AOR 1.315 95% CI: 1.117–1.548, p-0.001), were positively associated with MDR-TB. Previous TB treatment did not show a significant positive association with MDR (AOR 1.113 95% CI: 0.801–1.546, p-0.523). Old age (AOR 0.994 95% CI: 0.990–0.999, p-0.023) and HIV seropositivity (AOR 0.580 95% CI: 0.369–0.911, p-0.018) were negatively associated with MDR-TB. Although Rifampicin mono-resistance had a positive association with treatment failure (AOR 2.509 95% CI: 1.804–3.490, p < .001), it did not show any association with previous TB treatment (AOR 1.286 95% CI: 0.765–2.164, p-0.342) or with history of contact with MDR-TB (AOR 1.813 95% CI: 0.591–5.560, p-0.298). However, INH mono-resistance showed a small positive association with the previous history of treatment for TB (AOR 1.303 95% CI: 1.021–1.662, p-0.033). It was also positively associated (AOR 2.094 95% CI: 1.236–3.548, p-0.006) with MDR-TB contacts. Thus INH resistance may develop during treatment if compliance has not adhered too and may be easily passed on to the contacts while Rifampicin resistance is probably due to factors other than treatment compliance. MDR-TB, i.e. resistance to both Rifampicin and Isoniazid, is strongly correlated with treatment failure, spread through contact, and not to treatment compliance. The temporal trend in this region shows a decrease in MDR prevalence from 8.4% in 2015 to 1.3% in 2018. A similar trend is observed for Rifampicin mono-resistance and Isoniazid mono-resistance, pointing to the effectiveness of the TB control program. The higher proportion of inferred resistance observed for Rifampicin compared with INH may indicate a surfeit of mechanisms that enable rifampicin resistance. Association of MDR-TB with age, gender, and HIV status suggest the role of the immune system in the emergence of the MDR phenotype.
Similar content being viewed by others
Rifampicin Resistant Tuberculosis in Lesotho: Diagnosis, Treatment Initiation and Outcomes
Multidrug-resistant tuberculosis
Targeted next-generation sequencing of sputum for diagnosis of drug-resistant TB: results of a national survey in Democratic Republic of the Congo
Introduction.
Tuberculosis is the foremost cause of death from single infectious agent Mycobacterium tuberculosis . About 10 million people worldwide were infected in 2018 1 . According to the World Health Organization, 27% of the global TB cases are from India. Besides, India also accounts for 27% of the worldwide burden of rifampicin-resistant TB 2 . The incidence of TB is highest in the 15–24 year age group in India. The incidence rates in men, women, and children were 60%, 34%, and 6%, respectively 2 . A decreasing trend is observed in the TB incidence and mortality in India and other South-East Asian countries like Vietnam and Myanmar. But, Multi-drug resistant tuberculosis (MDR-TB) with resistance to first-line anti-TB drugs viz. Rifampicin and Isoniazid drugs pose a serious threat to the End TB initiative 3 . The global incidence of MDR-TB is 3.4% in new cases and 18% in previously treated cases. Globally, 78% of the rifampicin-resistant TB (RR-TB) cases were multidrug-resistant. Indian government survey from 2014 to 2016 estimated the incidence of MDR-TB as 2.84% in new cases and 11.6% among previously treated patients 4 . Further, rifampicin mono-resistance was negligible, and INH resistance was invariably associated with rifampicin resistance. Worldwide, INH mono-resistance in new cases is 7.2% and 11.6% in previously treated TB cases. In India, INH mono-resistance was observed in 3.8% and 7.8% of new and previously treated cases, respectively.
Many studies on TB drug resistance are conducted using small sample size, and hence the results may not be extended to a larger population. This study analyzed more than twenty thousand sputum positive and positive culture specimens and described the temporal profile of TB drug resistance from 2013 to 2018 in the state of Tamil Nadu and Puducherry, located in the southern part of India. The association between age, gender, previous treatment/failure, HIV status, and drug resistance were also examined. Treatment of MDR-TB involves toxic and expensive drugs and has a lower success rate of about 56% only. Therefore, it is imperative to assess the burden and pattern of MDR-TB and other factors associated with drug resistance, to plan intervention, prevention, treatment, and to evaluate the outcome.
Materials and methods
Clinical specimens.
The Intermediate Reference Laboratory in Government Hospital for Chest Diseases, Puducherry, India routinely receives specimens for diagnostic workup from Tamil Nadu and Puducherry state since 2013. This retrospective cross-sectional study analyzed clinical and laboratory data collected between January 2013 and December 2018 from 20,245 patients diagnosed as presumptive MDR-TB attending primary healthcare clinics. Specimens were received as per the guidelines described in the Revised National TB Control Programme. The smear-positive sputum samples by fluorescence microscopy were directly processed by GenoType MTBDRplus assay version 2.0 (Hain Life-science, Nehren, German). All the smear-negative sputum samples were processed in the BACTEC MGIT 960 system, and the culture-positive tubes were then processed by GenoType MTBDRplus assay version 2.0. The laboratory requisition form had details regarding the patient’s age, gender, address, treatment history, and HIV status. The study protocol was approved by the Ethical Committee for Intermediate Reference Laboratory of Government Hospital for Chest Diseases, and written informed consent was obtained from each study subject. All methods were applied in accordance with relevant guidelines and regulations.
Sputum samples were refrigerated immediately after collection in primary healthcare clinics and transported at 4 °C to the Intermediate Reference Laboratory at Government Hospital for Chest Disease within 24 h. Sputum decontamination using 4% NALC-NaOH was performed to minimize commensal bacterial flora. The decontaminated sputum specimens were was subjected to microscopy using Auramine O Fluorescent staining.
Genotype MTBDRplus assay
The GenoType MTBDRplus VER 2.0 is a DNA-strip- based in-vitro assay for identifying the M.tuberculosis complex and its resistance to rifampicin (RIF) isoniazid (INH) from smear-positive pulmonary sputum samples and positive culture samples. There are three steps, namely DNA extraction, multiplex PCR amplification with biotinylated primers, and reverse hybridization. Each DNA-strip has five control zones, conjugate control to check the binding of conjugates on strips while doing conjugation process, amplification control to monitor the success of amplification process, and three locus control to check the sensitivity of the reaction for each of the tested gene ( rpoB , katG , and inhA ) loci.
The sputum smear-positive samples and bacterial grown on MGIT tubes were decontaminated using the NALC/NaOH method 5 and centrifuged at 4000 rpm for 20 min in a refrigerated centrifuge. After decontamination, the cell pellet was suspended in 1 to 1.5 ml of sterile phosphate buffer solution. 500 µl suspension of the pellet in phosphate buffer was transferred to a sterile pre-labelled 1.5 ml screw cap micro centrifuge tube and centrifuged at 10,000 rpm for 15 min. The supernatant was discarded and the pellet was suspended in 100 µl Lysis buffer (A-LYS) using a vortex mixer. The tube was incubated at 95 °C in a hot air oven for 5 min, and 100 µl of Neutralisation Buffer (A-NB) was added to each tube. After vortex, the sample was centrifuged at 10000 rpm for 5 min. Approximately 40–80 µl of DNA supernatant was transferred to a fresh sterile screw cap 1.5 ml tube and stored for further amplification process. 5–10 µl of DNA supernatant was transferred to pre-labelled sterile PCR tube containing 40–45 µl of amplification mixes (AM-A and AM-B). 5 µl of sterile Milli-Q water and 5 µl of DNA supernatant from H37Rv were transferred to PCR tubes containing 45 µl of amplification mixes for negative and positive control, respectively. The multiplex PCR reaction for rpoB , katG , and inhA gene loci was performed in a 2720 thermal cycler (Applied Biosystems Inc) under the following conditions; initial denaturation at 95 °C for 15 min; 20 cycles of 95 °C for 30 s, 65 °C for 2 min; 30 cycles of 95 °C for 25 s, 50 °C for 40 s, 70 °C for 40 s; and final elongation at 70 °C for 8 min. The amplicons were stored at 4 °C until further use.
20 µl DEN (denaturing solution) was pipetted out into each well of GT Blot tray and 20 µl of the amplicon was added to each well and mixed well and incubated for 5 min at room temperature. 1 ml of HYB (hybridization solution) was added to each well and gently shaken to homogenize the solution. The pre-labelled DNA-strip was placed into each well with coloured marker facing up. The tray was placed on GT-Blot and incubated for 30 min at 45 °C. After incubation, HYP solution was carefully pipetted with with individual sterile pasture pipette into a beaker containing diluted bleach solution.1 ml STR solution (stringent buffer) was added into each well and incubated for 15 min at 45 °C in GT-Blot. After incubation, STR was carefully pipetted out with individual sterile pasture pipette into a beaker containing a diluted bleach solution. 1 ml RIN (Rinse solution) was added to each well and incubated for 1 min at 25 °C on GT-Blot. After completion of incubation period, the solution was carefully pipetted out with individual sterile pasteur pipette. 1 ml diluted conjugate (Con D-1: 100 ratios) was added into each well and incubated for 30 min at 25 °C on GT-Blot. The solution was aspirated with individual sterile pasture pipette and washed for 1 min at 25 °C with 1 ml RIN per well on GT-Blot. The RIN solution was removed with individual sterile pasture pipette and washed with 1 ml sterile distilled Milli-Q water. 1 ml diluted substrate (Sub D-1: 100 ratios) was added into each well after complete removal of water and incubated for 3 min at 25 °C on GT-Blot. The reaction was stopped as soon as bands are visible by briefly rinsing twice with distilled water. The DNA-strips were removed from the tray using tweezers and dried between two layers of absorbent paper 6 .
MGIT culture and identification
The MGIT PANTA was reconstituted with 15.0 ml of MGIT growth supplement and mixed well to dissolved completely. 0.8 ml of this enrichment was added to each pre-labelled (specimen) MGIT medium tube and Quality control tube before the specimen's inoculation. All sputum specimens were digested and decontaminated by the standard N-acetyl-l-cysteine-NaOH method. The deposits were suspended in 1 ml sterile phosphate-buffered saline (pH 6.8), and 0.5 ml of the processed specimen was inoculated into MGIT 960 tube and supplemented as recommended by the manufacturer 7 . 0.5 ml of diluted (1:100 ratio) reference strains suspension into quality control tubes. Immediately recapped the tube tightly and mixed carefully by inverting the tube several times. All inoculated MGIT tubes were incubated within the MGIT 960 instrument either until they were flagged positive or for a maximum of 6 weeks. All positive MGIT vials were confirmed for acid-fast bacilli by Ziehl–Neelsen staining and subjected to identification of M. tuberculosis complex using rapid immuno-chromatographic test 8 .
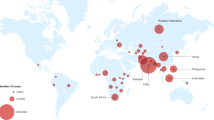
Twenty thousand two hundred forty-five specimens were included in this study from the Southern Indian states of Tamil Nadu and Puducherry (Fig. 1 ). The majority of the specimens analyzed were from Puducherry and its neighbouring districts. All positive sputum specimens were subjected to direct genotyping assay. The negative sputum specimens were cultured, and all the 629 culture-positive isolates were genotyped (Fig. 2 ). The temporal profile of tuberculosis drug resistance from 2013 to 2018 is depicted in Fig. 3 . From the year 2015 onwards, a decreasing trend is observed in the prevalence of tuberculosis drug resistance. Frequency distribution of the prevalence and pattern of molecular drug resistance based on rpoB , katG , and inhA genes is shown in Table 1 . The rpoB and katG and inhA gene mutations were observed in 7.8% (95% CI: 7.4–8.2), 12.1% (95% CI: 11.7–12.6) and 4.6% (95% CI: 4.3–4.9) of the cases respectively. Of the 1582 rifampicin resistance cases, 518 (32.7%) were subcategorized as inferred resistance (absence of WT probes without the expression of the mutant probe), 463(29.3%) as hetero-resistance (presence of WT probe with the expression of the mutant probe), and 601 as true resistance (absence of WT probe with the expression of corresponding mutant probe). The codon S450L (MUT3) in the rpoB gene is the most common mutation associated with rifampicin resistance. Of the 3390 isoniazid drug resistance cases, 2459 (72.5%) had mutations in the katG gene, and 27.5% (931/3390) had mutations in the inhA gene. Of the 2459 katG mutants, 30.5%, 59.2%, and 32.7% were subcategorized as hetero-resistance, true resistance, and inferred resistance, respectively. The katG S351T mutation is the most common mutation associated with isoniazid drug resistance. Of the 931 inhA gene mutants, 40.7% and 54.6% were hetero-resistant and true resistant, respectively. Resistance was inferred in only 4.7% of cases. The promoter region C15T mutation is found in a majority of the inhA resistant cases. Isoniazid inferred resistance for (8.7%) was lower than the inferred resistance (32.7%) for rifampicin.

Geographic distribution of samples across different districts of Tamil Nadu and Puducherry (PD) states. (Courtesy: www.d-maps.com , [email protected]).

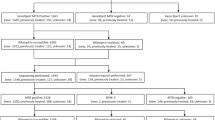
Flow Chart describing the workflow for the study.

Temporal profile of TB drug resistance in presumptive MDR-TB patients during 2013 to 2018.
The distribution of MDR-TB, Rifampicin mono-resistant TB, and Isoniazid mono-resistant TB with respect to age, gender, HIV status, and previous treatment are tabulated in Table 2 . Our study sample involved a high proportion of males belonging to the age group 18–6045 years. The number of female patients included in the study was 3029 (15%). The majority of cases had a history of previous treatment (95.3%) and were HIV negative (97%). A considerable number of cases had a prior history of contact with MDR-TB (n = 124) or were seropositive for HIV (n = 613).
MDR-TB based on line probe assay was detected in 1084 cases (5.35%; 95% CI; 5–5.7).Contact with MDR-TB (AOR 3.171 95% CI: 1.747–5.754, p-0.000), Treatment failure (AOR 2.17595% CI: 1.703–2.777, p-0.000) and female gender (AOR 1.315 95% CI: 1.117–1.548, p-0.001) were positively associated with MDR-TB (Table 3 ). The previous history of TB treatment (p = 0.523) did not show a statistically significant association with MDR-TB. Old age (AOR 0.994 95% CI: 0.990–0.999, p-0.023) and HIV seropositivity (AOR 0.580 95% CI: 0.369–0.91, p-0.018) had a negative association with MDR-TB. The number of cases showing mono-resistance to Rifampicin was 498 (2.45%; 95% CI: 2.2–2.7%). Treatment failure is positively associated with Rifampicin mono-resistance (AOR 2.509 95% CI: 1.804–3.490,p-0.000) (Table 4 ). Isoniazid mono-resistance is positively associated with MDR contact (AOR 2.094 95% CI: 1.236–3.548, p-0.006) and previous treatment (AOR 1.303 95% CI: 1.021–1.662, p-0.033) (Table 5 ).
From the matrix analysis of Rifampicin and Isoniazid resistance genes, it is observed that MUT3 (S450L) of rpoB and katG MUT1 (S315T1) pair contributed to 397 (36.6%; 95% CI: 33.8–39.5) MDR-TB cases (Table 6 ). Independently, rpoB MUT3 and katG MUT1 are associated with 52%, and 71.6% of MDR-TB cases. Of the 1084 MDR-TB cases, inferred resistance due to loss of wild type rpoB , katG , and inhA gene was found in 310 (28.6%), 98 (9%), and 17 (1.6%) cases, respectively. The Line probe assay was performed on direct positive sputum specimens in 19,614 (97%) of cases and from the culture in 604 (3%) cases. The relative proportions of resistant cases were compared across direct and indirect line probe assays (Fig. 4 ). The Indirect line probe assay nearly doubled the detection of drug resistance.

Comparison of Resistance detection by direct and indirect LPA.
India has the highest global burden of MTB and MDR-TB. Nearly half of the world's MDR-TB patients are from three countries, namely, India (27%), China (14%), and Russia (9%) 9 . Indian survey of TB drug resistance in 2016 reports a lower incidence of MDR in treated (11.6% vs. 18%) and new cases (2.84% vs. 3.4%) in comparison with the global WHO 2019 report 2 . This study observed a gradual decline in MDR-TB from 5.06% in 2015 to 1.34% in 2018, although this data pertains to presumptive MDR-TB cases. Mono-resistance to Rifampicin and Isoniazid also shows a decline from 2015 onwards, pointing to the effectiveness of the Revised National TB Control Program (RNTCP) in the state. About 60 to 70% of our rifampicin-resistant TB is multidrug-resistant, in close concordance with the global value of 78% 10 , 11 . The MDR-TB prevalence for untreated cases in our study is 6%, which is higher than the global (3.4%) and the national average (2.84%). The higher prevalence of MDR-TB may be explained by the patient selection bias involved, as only samples from suspected MDR-TB cases were sent to the reference laboratory. In this study, the majority of the suspected (52.5%) and lab-confirmed (56%) MDR-TB patients were from the age group between 15 and 45 years in concordance with the national data. The high frequencies of MDR-TB among young age groups may indicate the possibility of propagation of MDR-TB in the community because of the higher mobility of youth 12 , 13 .
Traditionally, the etiopathogenesis of MDR-TB is attributed to poor compliance and programmatic failure. We do not find any significant difference in the MDR-TB prevalence between previously treated and new cases. Although isoniazid mono-resistance had a small positive association with previous treatment, rifampicin mono-resistance was not associated with earlier treatment. Our results support the observation by Dheda et al. claiming that factors other than poor compliance and program failure are strongly implicated in the prevalence of MDR-TB, and they need to be identified 14 . There is an inherent male bias (1.9:1) in the incidence of tuberculosis, which may be attributed to a biological difference in response to mycobacterium. Post-pubertal females tend to mount a more robust immune response leading to the tuberculoid form of cured or contained disease. This response may likely play a role in the pathogenesis of multidrug-resistance / rifampicin-resistance 15 , 16 . In our study, the proportion of female presenting with MDR-TB and non-MDR-TB is 19.3% (95% CI: 16.3–21.6) and 14.7% (95% CI: 14.2–15.2) respectively. A Chinese study by Liu et al. also attributes the positive association of MDR-TB with the female gender 17 . We also infer that old age and HIV positive individuals are less susceptible to MDR-TB from the multivariate logistic regression analysis. The waning immunity with age and compromised immune response in HIV may be attributed to the lower prevalence of MDR-TB in these groups. Although many studies have suggested the role of HIV in augmenting the prevalence of MDR-TB, our study using a reasonably large sample size did not find evidence for this observation 18 , 19 , 20 , 21 , 22 . This may be attributed to behavioural differences rather than biological differences in studies conducted in a small population. It may also be attributed to regions with a higher prevalence of both HIV and MDR-TB. Our observation that HIV is not a MDR-TB risk factor is in concordance with the study by Baya et al. from Mali in 2019 23 . Our study also revealed a significant positive association between MDR-TB and treatment failure concordance with several other similar reports.
TB drug resistance may be sub-classified as true resistance, heteroresistance and Inferred resistance. In true resistance, only the mutant strain is present. Detection of both the mutant and the wild type strains is known as heteroresistance, while the absence of both mutant and wild type strain is considered as Inferred resistance. Heteroresistance is considered to be the early stage in the development of drug-resistant TB. Heteroresistance to rpoB , katG , and inhA by line probe assay in our study was 29.3%, 22.2%, and 40.7%, respectively. Heteroresistance, reflective of the slow evolution of bacteria from a sensitive to resistant profile, is a well-documented phenomenon in M. tuberculosis 24 . However, to our knowledge, only a few studies using a small number of cases have analysed this property. The clinical implications of heteroresistance are not fully ascertained. The sensitivity of GenoType MTBDRplus for detection of heteroresistance is reported to be about 5% from a mixed liquid culture 25 . Countries of the former Soviet Union have a higher proportion of MDR-TB (> 50%), as documented by WHO. A small study from Uzbekistan conducted in 2009 reports a 20% heteroresistance to rifampicin and/or isoniazid by direct LPA method. Another study from Mumbai, India (2012) based on Indirect LPA done on solid culture medium reports a rifampicin, isoniazid, and inhA heteroresistance of 34%, 39%, and 74%, respectively 26 , 27 . Although Rinder et al. claims that heteroresistance may be obscured by culture, we find that liquid culture enhances heteroresistance detection using GenoType MTBDRplus. Besides, the detection of total resistance and inferred resistance also were doubled by Indirect LPA assay in comparison with direct LPA on sputum specimens 28 . The rate of 72.5% of katG S315T mutation in non-MDR INH resistant isolates is an agreement to the finding by Manson et al., who reported a rate of 79%, and it is the harbinger mutations that often precede MDR 29 . In matrix analysis, 36.6% of strains carrying S315T1 mutations are associated with S450L mutations leading to MDR. We also observe that the single mutation in katG , S315T, accounted for the majority of isoniazid-resistance.
One of the limitations of our study is a bias associated with the selective analysis of presumptive MDR-TB. Therefore the prevalence data from this study may exaggerate the proportion of MDR-TB in the community. Our multivariate analysis did not include other behavioural factors that may be associated with MDR-TB.GenoType MTBDRplus assay is not sensitive to detect less than 5% heteroresistance in the population.
This study shows a temporal decline in the MDR-TB prevalence from 2015 to 2018 in South Indian states of Tamil Nadu and Puducherry. Besides, the factors positively or negatively associated with drug resistance were arrived by analyzing a large number of samples. They hence may represent a less biased estimate of the actual underlying elements related to drug resistance. Our data do not support traditional views on treatment compliance and HIV in the etiopathogenesis of MDR-TB. A positive association of MDR-TB with female gender and negative association with HIV seropositivity and old age suggests mechanisms by which the immune system and sex hormones may be involved in the etiopathogenesis of MDR-TB. Studies on these aspects may pave the way for innovative approaches to target MDR-TB.
World Health Organization (2018) Geneva, Switzerland: WHO; 2018. Global tuberculosis report.
World Health Organization (2019) Geneva, Switzerland: WHO; 2019. Global tuberculosis report.
Ullah, I. et al. Pattern of drug resistance and risk factors associated with development of drug resistant Mycobacterium tuberculosis in Pakistan. PLoS ONE 11 , e0147529. https://doi.org/10.1371/journal.pone.0147529 (2016).
Article CAS PubMed PubMed Central Google Scholar
Yang, Y., Zhou, C., Shi, L., Meng, H. & Yan, H. Prevalence and characterization of drug-resistant tuberculosis in a local hospital of Northeast China. Int. J. Infect. Dis. 22 , 83–86. https://doi.org/10.1016/j.ijid.2013.12.015PMID:24556164 (2014).
Article PubMed Google Scholar
World Health Organization. Molecular line probe assay for rapid screening ofpatients at risk of multidrug-resistant tuberculosis (MDRTB) (WorldHealth Organization, Geneva, 2008).
Google Scholar
Muthuraj, M. et al. Prevalence of mutations in genes associated with rifampicin andisoniazid resistance in Mycobacterium tuberculosis clinical isolates. J. Clin. Tuberc. Other Mycobact. Dis. 8 , 19–25 (2017).
Article Google Scholar
Vidyaraj, C. K. et al. Prevalence of rifampicin-resistant Mycobacterium tuberculosis among human-immunodeficiency-virus-seropositive patients and their treatment outcomes. J. Epidemiol. Glob. Health https://doi.org/10.1016/j.jegh.2017.09.002 (2017).
Article PubMed PubMed Central Google Scholar
Singh, K., Kumari, R., Tripathi, R., Gupta, A. & Anupurba, S. Mutation in MPT64 gene influencing diagnostic accuracy of SD Bioline assay (capilia). BMC Infect. Diseases 19 , 1048–1054 (2019).
Article CAS Google Scholar
Mishra, G. P. & Mulani, J. D. First National Anti-Tuberculosis Drug Resistance Survey (NDRS) from India - An Eye Opener. J. Infectiol. 1 (2), 26–29 (2018).
Zhao, Y. et al. National Survey of Drug-Resistant Tuberculosis in China. N. Engl. J. Med 366 , 2161–2170 (2012).
Wright, A., Zignol, M. & Van Deun, A. Epidemiology of anti-tuberculosis drug resistance 2002–2007: an updated analysis of the global project on anti-tuberculosis drug resistance surveillance. Lancet 373 , 1861–1873 (2009).
Abdella, K., Abdissa, K., Kebede, W. & Abebe, G. Drug resistance patterns of Mycobacterium tuberculosis complex and associated factorsamong retreatment cases around Jimma, Southwest Ethiopia. BMC Public Health 15 (599), 1–7 (2015).
Adane, K., Ameni, G., Bekele, S., Abebe, M. & Aseffa, A. Prevalence and drug resistance profile of Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients attending two public hospitals in EastGojjam zone, northwest Ethiopia. BMC Public Health 15 , 572–577 (2015).
Dheda, K. et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. 5 , 291–360 (2017).
Nhamoyebonde, S. & Leslie, A. Biological differences between the sexes and susceptibility to tuberculosis. J. Infect. 209 (S3), S100–S106 (2014).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nature 16 , 626–638 (2016).
CAS Google Scholar
Liu, Q. et al. Rates and risk factors for drug resistance tuberculosis in Northeastern China. BMC Public Health 13 , 1171–1178 (2013).
Barroso, E. C. et al. Risk factors for acquired multidrug-resistant tuberculosis. Jornal de Pneumologia 29 , 89–97 (2003).
Marahatta, S. B., Kaewkungwal, J., Ramasoota, P. & Singhasivanon, P. Risk factors of multidrug resistant tuberculosis in central Nepal: A pilot study. Kathmandu Univ. Med. J. 8 , 392–397 (2010).
Andrews, J. R. et al. Predictors of multidrug- and extensively drug-resistant tuberculosis in a high HIV prevalence community. PLoS ONE 5 , e15735 (2010).
Article ADS CAS Google Scholar
Liang, L. et al. Factors contributing to the high prevalence of multidrug-resistant tuberculosis: a study from China. Thorax 67 , 632–638. https://doi.org/10.1136/thoraxjnl-2011-200018 (2012).
El Mahalli, A. & Al-Qahtani, M. F. (2015) Predictors of drug resistance in tuberculosis patients in the Eastern Province, Saudi Arabia. J. Egypt Public Health Assoc. 90 , 24–28 (2015).
Baya, B. et al. Clinical risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in Mali. Int. J. Infect. Dis. 81 , 149–155 (2019).
Hofmann-Thiel, S. et al. Mechanisms of hetero-resistance to isoniazid and rifampicin of Mycobacterium tuberculosis in Tashkent, Uzbekistan. Eur. Respir. J. 33 , 368–374 (2009).
Folkvardsen, D. B. et al. Rifampinheteroresistance in Mycobacterium tuberculosis cultures as detected by phenotypic and genotypic drug susceptibility test methods. J. Clin. Microbiol. 51 (12), 4220–4222 (2013).
Tolani, M. P., Dsouza, D. T. B. & Mistry, N. F. Drug resistance mutations and hetero-resistance detected using the GenoTypeMTBDRplus assay and their implication for treatment outcomes in patients from Mumbai, India. BMC Infect. Diseases 12 , 9 (2012).
Audu, E. S., Gambo, M. S. & Yakubu, A. A. Rifampicin resistant mycobacterium tuberculosis in Nasarawa State, Nigeria. Niger. J. Basic Clin. Sci. 14 , 21–25 (2017).
Rinder, H., Mieskes, K. T. & Löscher, T. Hetero-resistance in Mycobacterium tuberculosis . Int. J. Tuberc. Lung Dis. 5 , 339–345 (2001).
CAS PubMed Google Scholar
Manson, A. L. et al. Genomic analysis of globally diverse mycobacterium tuberculosis strains provides insights into the emergence and spread of multidrug resistance. Nat. Genet. 49 (3), 395–402 (2017).
Download references
Acknowledgments
The authors thank all the patients for their participation in this study, and the staff members who cared for them.
All methods were carried out in accordance with relevant guidelines and regulations. This work was supported by the Revised National TB Control Programme, Central TB Divisions, Government of India through state National Rural Health Mission.
Author information
Authors and affiliations.
Department of Microbiology, State TB Training and Demonstration Centre, Government Hospital for Chest Diseases, Puducherry, India
Smita S. Shivekar, C. K. Vidya Raj, Chitra Alagappan & Muthuraj Muthaiah
Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
Venkatesh Kaliaperumal
Department of Genetics, Dr.A.L.M. Postgraduate Institute of Basic Medical Sciences, University of Madras, Tamil Nadu, Chennai, India
Usharani Brammacharry
Department of Environmental Science, Central University, Kasargod, Kerala, India
Anbazhagi Sakkaravarthy
You can also search for this author in PubMed Google Scholar
Contributions
M.M. and U.B.—prepared manuscript C.K.V.R and S.S.S—prepared figures C.A. and A.S.—prepared tables V.K.—prepared manuscript, statistical analysis. All authors reviewed the manuscript.
Corresponding authors
Correspondence to Venkatesh Kaliaperumal , Usharani Brammacharry or Muthuraj Muthaiah .
Ethics declarations
Competing interests.
The authors declare no competing interests .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Shivekar, S.S., Kaliaperumal, V., Brammacharry, U. et al. Prevalence and factors associated with multidrug-resistant tuberculosis in South India. Sci Rep 10 , 17552 (2020). https://doi.org/10.1038/s41598-020-74432-y
Download citation
Received : 31 January 2020
Accepted : 18 September 2020
Published : 16 October 2020
DOI : https://doi.org/10.1038/s41598-020-74432-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Extra-long treatment of mdr-tb osteomyelitis of humerus due to neurotoxicity from the 2nd-line drugs: a case report.
- Naser Naser
- Habib Abdulla
- Husain Kadhem
The Egyptian Journal of Internal Medicine (2023)
Role of Antimicrobial Peptides in Treatment and Prevention of Mycobacterium Tuberculosis: A Review
- Kanchan Mehta
- Prince Sharma
- Ashish Vyas
International Journal of Peptide Research and Therapeutics (2022)
The dynamic impacts of environmental-health and MDR-TB diseases and their influence on environmental sustainability at Chinese hospitals
- Misbah Sadiq
- Mosab I. Tabash
Environmental Science and Pollution Research (2022)
Antibiotic heteroresistance in Mycobacterium tuberculosis isolates: a systematic review and meta-analysis
- Ebrahim Kouhsari
Annals of Clinical Microbiology and Antimicrobials (2021)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
Out of pocket expenditure on tuberculosis in India: Do households face hardship financing?
Affiliations.
- 1 ICMR-National Institute of Medical Statistics (NIMS), Department of Health Research, Ministry of Health & Family Welfare, Govt of India, Medical Enclave Ansari Nagar, New Delhi, 110029, India. Electronic address: [email protected].
- 2 ICMR-National Institute of Medical Statistics (NIMS), Department of Health Research, Ministry of Health & Family Welfare, Govt of India, Medical Enclave Ansari Nagar, New Delhi, 110029, India; Campbell Collaboration, New Delhi, India. Electronic address: [email protected].
- 3 ICMR-National Institute of Medical Statistics (NIMS), Department of Health Research, Ministry of Health & Family Welfare, Govt of India, Medical Enclave Ansari Nagar, New Delhi, 110029, India. Electronic address: [email protected].
- PMID: 31813431
- DOI: 10.1016/j.ijtb.2019.02.016
Background: In 2017, India accounted for 27 percent of the global burden on tuberculosis, and the highest among the top 30 countries with high TB burden. Despite the expansion of DOTS programme many households in India incur high expenditure towards TB treatment. Most of the studies in India have focused on measuring catastrophic health expenditure on TB. Catastrophic health expenditure and its impoverishment effects are difficult to calculate and may misrepresent economic hardship.
Methods: This paper uses hardship financing, i.e. when a household sells assets or borrows money on interest to pay for healthcare expenditure, as an indicator of the hardship of the family when it spends on TB treatment using NSSO 71st Round 2014 data.
Results: Using the NSSO national representative sample, the paper estimated that 26.7% of hospitalized cases and 3.5% percent of patients utilising outpatient care experience hardship financing due to TB in the country. 25.9% of the general population had to sell assets or used borrowings for financing TB hospitalization expenses. Education of head of household, income, type of health facility used, and number of hospitalized days were found to be significant factors influencing hardship financing.
Conclusion: Our study highlights that even with free care for tuberculosis, 21.3% were exposed to hardship financing, suggesting the need to re-look at the subsidy coverage of tuberculosis treatment in the country. The study also suggests the use of hardship financing as an alternative to catastrophic spending method as a index of effectiveness of tuberculosis control programme in the country.
Keywords: Hardship financing; India; NSSO; Out of pocket expenditure; Tuberculosis.
Copyright © 2019 Tuberculosis Association of India. Published by Elsevier B.V. All rights reserved.
- Child, Preschool
- Cost of Illness*
- Family Characteristics*
- Healthcare Disparities*
- Infant, Newborn
- Middle Aged
- Tuberculosis, Pulmonary / economics*
- Young Adult
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMJ Glob Health
- v.5(1); 2020

Tuberculosis case fatality in India: a systematic review and meta-analysis
Sophie huddart.
1 Epidemiology & Biostatistics, McGill University, Montreal, Quebec, Canada
2 McGill International TB Centre, Montreal, Quebec, Canada
Anita Svadzian
Vaidehi nafade, srinath satyanarayana.
3 International Union Against Tuberculosis and Lung Disease, Delhi, India

Madhukar Pai
Associated data.
bmjgh-2019-002080supp001.pdf
bmjgh-2019-002080supp002.csv
Introduction
The WHO End TB Strategy calls for a global reduction in the case fatality ratio (CFR) below 5%. India accounts for a third of global tuberculosis (TB) deaths. This systematic review estimated CFRs among Indian patients with TB both during and after treatment.
We systematically searched Medline, Embase and Global Health for eligible studies published between 1 January 2006 and 8 January 2019, including both cohort studies and intervention study control arms that followed Indian patients with TB for fatality either during treatment or post-treatment. From relevant studies we extracted CFRs in addition to study demographics. Study quality was assessed using modified Scottish Intercollegiate Guidelines Network cohort criteria. Sufficiently homogenous studies were pooled using a random effect generalised linear mixed model. A meta-regression was performed to associate study characteristics with resulting CFRs.
218 relevant studies were identified, of which 211 provided treatment phase CFRs. Most patients (92.4%) were treated in the public sector. Quality concerns were identified in 74% of papers. We estimated a pooled treatment phase CFR of 5.16% (95% CI 4.20% to 6.34%) which fell to 3.78% (2.77% to 5.16%) when restricted to 52 high-quality studies. Treatment phase CFRs were higher for paediatric (n=27, 6.50% (2.65% to 10.36%)), drug-resistant (n=43, 14.06% (10.15% to 19.49%)) and HIV-infected (n=35, 10.91% (7.68% to 15.50%)) patients. Nineteen post-treatment CFR studies were too heterogeneous to pool except when restricting to three high-quality studies (2.69% (–0.79% to 6.18%)). Poor study quality (OR=2.27 (2.01 to 2.57)) and tertiary centres patients (OR=1.15 (1.03 to 1.28)) were significantly associated with increased treatment phase case fatality.
Conclusions
Case fatality is a critical measure of the quality of TB care. While India’s treatment CFRs are in line with WHO targets, several key patient groups remain understudied and most studies suffer from methodological issues. Increased high-quality reporting on patient outcomes will help improve the evidence base on this topic.
Key questions
What is already known.
- India accounts for more than 25% of global tuberculosis (TB) incidence but there are concerns about the quality of care that patients receive.
- One of the WHO’s most important quality of care indicators is the case fatality ratio, with an ideal case fatality ratio below 5%.
What are the new findings?
- A systematic review of the literature yielded an overall case fatality ratio among Indian patients with TB during treatment of 5.16% (95% CI 4.20% to 6.34%).
- Case fatality was higher among key patient subgroups like those living with HIV or fighting drug-resistant TB.
- However, the quality of available studies was generally poor meaning that the literature estimates of case fatality may be biased.
What do the new findings imply?
- The TB field must better estimate case fatality with improved study design and statistical corrections for common biases.
- Special efforts must be made to monitor case fatality in the private sector and among patients who have completed treatment as current evidence for these groups is limited.
Tuberculosis (TB) affected 10.0 million new people in 2017 resulting in 1.6 million deaths globally. 1 A key component to the WHO End TB Strategy 2 is improving the quality of TB care.
The End TB Strategy calls for a 95% reduction in TB deaths by 2035 relative to 2015. One of the most important measures for quality of TB care is the case fatality ratio (CFR). At the country level, the CFR is estimated as the number of TB deaths divided by the number of incident cases in the same years, expressed as a percentage. 1 In order to achieve the 2025 milestone of a 75% reduction in deaths, the End TB Strategy calls for the global CFR to fall from 15% to 6.5%. 2 The WHO’s ideal global TB CFR is under 5%. 1
India accounts for more than 25% of the global TB incidence. 1 India has a complex healthcare system with a large private sector. Many patients with TB seek care from multiple providers before being diagnosed with TB and receiving appropriate treatment. 3 Although India’s Revised National TB Control Programme (RNTCP) offers free TB therapy, over half of Indian patients with TB pay out-of-pocket to receive treatment in the unregulated private sector, where treatment quality often deviates from international standards. 4 5 Publicly treated patients with TB are registered with the RNTCP and their treatment outcomes are recorded; however, no such routine treatment follow-up occurs in the private sector. In both the public and private sectors, no systematic post-treatment follow-up is conducted.
Globally, moderate quality data exist on patient fatality during TB treatment, mostly for publicly treated patients. A recent systematic review found a global CFR of 3.5% among patients who were HIV– and 18.8% among patients who were HIV+ of all ages. 6 Of the Indian studies in this review, treatment phase case fatality ranged from 2.2% to 5.7%; these studies reflected only publicly treated patients. 6 Globally, few studies estimate patient mortality after completing treatment. The available evidence suggests that patients with TB continue to experience significantly higher mortality after treatment when compared with the general population. 7
In this systematic review, we summarise the available literature estimating treatment phase and post-treatment phase CFRs of Indian patients with TB and provide pooled CFRs among key subpopulations including HIV+, privately treated, drug-resistant and paediatric patients.
This systematic review sought to estimate the treatment and post-treatment phase CFRs among Indian patients with TB after directly observed therapy (DOTS) scale-up in India (2006). A protocol with prespecified analyses was developed before conducting this review.
Search strategy
Our search strategy focused on the intersection of concepts related to TB, death and India. The full search strategy can be found in online supplementary data S1.1 .
Supplementary data
On 8 January 2019, the Medline (1946–Present), Embase (1947–Present) and Global Health (1973–Present) databases were searched. We restricted to papers published in 2006 or afterwards to limit our data to the period where modern DOTS treatment was widely available across India.
Supplemental searches were conducted manually in the Indian Journal of Tuberculosis, Lung India and Indian Journal of Chest and Allied Diseases. A supplemental search was also conducted in the IndMed database. Additionally, we included programmatic data from RNTCP progress reports from 2007 to 2018.
Outcome measure
A CFR is defined as the number of patients who die from any cause during the observed period divided by the number of patients forming the cohort at the beginning of the observed period. This differs slightly from the definition used at the country level and seen in the RNTCP reports as the number of incident cases does not need to be estimated; it is fixed by the design of the cohort.
Our primary outcomes were the CFR during the treatment phase and/or the post-treatment phase. The treatment phase was defined as the time period from treatment initiation to treatment completion or treatment cessation. The post-treatment phase was defined as the time period from treatment completion or cessation to the end of follow-up. If fatality data were not delineated between the treatment and post-treatment phase, an overall CFR was extracted.
Eligibility criteria and study selection
We targeted prospective or retrospective cohort studies or control arms of intervention studies which described case fatality of any Indian patients with TB.
The specific inclusion criteria are as follows:
- Published on or after 1 January 2006.
- Covers, prospectively or retrospectively, Indian patients with TB after treatment initiation during either the treatment phase, post-treatment phase or both.
- Records case fatality during these phases.
- Cohort study or intervention study that allows a CFR to be estimated.
We excluded conference abstracts, study designs that did not allow for estimation of CFRs, study designs where patients were not randomly sampled (eg, case series), duplicate study populations and study populations where all patients with TB had the same comorbidity unless that comorbidity was HIV. We also excluded studies where the treatment phase follow-up did not begin at treatment initiation.
A title and abstract screen was performed independently by two reviewers (SH and VN). The full text screen was performed by SH and AS with disagreements resolved by consensus.
Data extraction
Studies were extracted independently by SH and AS and then adjudicated. Extracted data included sample size, number of deaths and length of follow-up for the entire cohort and within any available patient strata in addition to cohort demographics and study quality data. The full list of extracted variables is available in online supplementary data S1.2 and the extracted data are available in online supplementary data S2 .
Quality assessment
Study quality was assessed using a modification of the Scottish Intercollegiate Guidelines Network cohort criteria. 8 Because the included studies were descriptive cohorts and not intervention assessments, existing cohort evaluation tools were not completely suitable. Additional questions were adopted from ROBINS-I 9 and Newcastle Ottawa Scale 10 as appropriate. Major methodological concerns included cohort generalisability, selection bias due to loss to follow-up and appropriateness of length of follow-up. Studies were deemed to have poor generalisability if all patients were hospitalised or all patients had a rare form of TB (eg, TB of the ankle). Studies where more than 15% of patients were lost to follow-up were categorised as having a high risk of selection bias. Studies that followed patients for less than a month were categorised as having an inappropriately short follow-up. A study with one or more of the previous issues was classified as low quality. As a sensitivity analysis, low-quality studies were excluded from the meta-analyses.
Meta-analysis methods
Case fatality estimates were pooled using a random-effects generalised linear mixed model (GLMM), which has been shown to outperform Der-Simonian and Laird models for meta-analysis of proportions because it exactly models the variance structure of binomial data. 11 For each pooling, if the crude CFR (the sum of all deaths in all studies divided by the sum of the study sample sizes) was below 5% a Beta-Binomial GLMM was fit. If the crude CFR was above 5% a Normal-Binomial GLMM was fit. Beta-Binomial GLMMs have been shown via simulation to minimise error compared with Normal-Binomial GLMMs for rare events under 5%. 12
While a forest plot was generated for each strata of interest, results were not pooled if there was substantial methodological or clinical heterogeneity in either the design or populations of the studies or if there is substantial statistical heterogeneity. As the more common I 2 statistic is not available for GLMMs, 13 statistical heterogeneity was assessed using τ 2 , a measure of interstudy heterogeneity. 14 The decision to pool was made based on an assessment of clinic heterogeneity and a τ 2 <4. Values of τ 2 are unique to each dataset and as such a universally applicable cut-off does not exist. For this work, a cut-off of four for τ 2 was based on a holistic assessment of the range of τ 2 across the strata and the precision of pooled CIs.
Treatment phase CFRs were pooled separately from post-treatment phase CFRs. In addition to the overall results, results within the following strata were examined: adult and paediatric patients, primary/secondary health centre and tertiary health centre patients, public and private sector patients, patients who were HIV+ and HIV– and drug-sensitive (DS) and drug-resistant (DR) patients. Studies with <2 patients in a given strata were excluded from the relevant strata pooling.
Routinely collected data on patient outcomes, including CFRs, are provided in annual RNTCP reports. The CFRs stratified by patient type are presented here from 2006 onwards; however, they are presented separately from the peer-reviewed literature and are not included in the pooled analyses. RNTCP reports were not included in pooling as they contain the data of many of the patients described in the included studies and thus would not be independent datapoints, a methodological requirement of meta-analysis. Additionally, they use the country level definition of CFR rather than the exact cohort definition used in the peer-reviewed studies.
Meta-regression
A logistic meta-regression was fit for both the treatment phase and post-treatment phase studies with the relevant CFRs as the dependent variable. In order to not overfit the model, a limited number of study-level covariates were included in each model. Covariates were selected based on degree of missingness in order to maximise the number of studies which could be included in each meta-regression. For the treatment CFR meta-regression, those covariates were the proportion of patients with extrapulmonary TB (EPTB), treated in the private sector, living with HIV and with DR TB, as well as study quality (high or low) and study setting (primary/secondary centre or tertiary centre). The post-treatment CFR meta-regression included the proportion of patients with EPTB and study quality (High or Low). Model coefficients are presented as ORs. An example interpretation of an OR of 2 for study setting would be that the odds of case fatality are double for patient populations in tertiary centres compared with patient populations in primary and secondary centres, after adjustment for all other included variables.
Data analyses were performed in R (V.3.6.1) using the metafor (V.2.1) package and SAS (V.9.4M6).
Patient and public involvement
This research was done without patient or public involvement.
Our search identified 4399 unique papers of which 733 full texts were screened. After screening, 218 relevant papers were identified ( figure 1 ). Two hundred and eleven papers with treatment CFR information were included as well as 19 papers with post-treatment CFR information (one of which did not cover the treatment phase and thus is not included in the count of 211 treatment phase studies), for a total of 212 unique studies included in the quantitative analysis (full citations in online supplementary data S1.3 ). Six papers had the necessary information to calculate a CFR but did not delineate between treatment and post-treatment phases. These studies were not included in the quantitative analyses but can be viewed in online supplementary data S1.4 .

PRISMA flowchart of study selection.
The included studies provide good representation of the highly diverse 15 Indian states ( figure 2 ). About half the studies included patients from tertiary centres. With the exception of patient sex, level of the health centre and study location (which allowed for a public/private sector determination), critical patient demographics were often missing from studies ( table 1 ). More than three quarters of studies failed to report the proportion of patients who received drug sensitivity testing and almost two thirds did not report the proportion of patients who were smear positive or the proportion of patients microbiologically versus clinically diagnosed.
Summary of available study characteristics, n=218
*Some studies reported median ages which are included in this average.
DR-TB, drug-resistant TB; DST, drug sensitivity testing; EPTB, extrapulmonary TB; TB, tuberculosis.

Heat map of included studies across Indian states. X-axis indicates the number of studies from each state.
Study quality
A high risk of poor generalisability was found in 61.0% of papers and a high risk of selection bias was identified in 27.5% of papers. Finally, 5.0% of papers had follow-up periods too short to adequately capture fatality. Overall, 73.9% of papers were of poor quality for the reliable estimation of CFRs ( figure 3 ).

Summary of study quality assessment. If duration of follow-up was less than 1 month, studies were classified as having a high risk of bias. If more than 15% of patients were lost to follow-up, studies were classified as having a high risk of bias. If all patients were hospitalised or had a rare form of TB, studies were classified as having a high risk of bias. Unclear classifications indicate that insufficient information was available to assess these areas. If studies had a high risk of bias in any of the aforementioned areas, the study was classified as low quality. TB, tuberculosis.
RNTCP reports
The RNTCP prepares annual reports of the previous year’s TB programme activity including treatment outcomes stratified by patient categories. The reports from 2007 (covering 2006 patient data) to 2018 (covering 2017 patient data) were included in this systematic review. Beginning in 2017, treatment outcome data was stratified by clinical and microbiological diagnosis status versus sputum smear status. Additionally, in 2011 the reports began to include multidrug-resistant (MDR) TB treatment outcomes and in 2013 HIV-TB specific treatment outcomes. The 2007–2016 report CFRs are available in table 2 and the 2017–2018 report CFRs are available in table 3 . MDR TB and HIV-TB data from the 2011–2018 reports are available in table 4 . The average CFRs for new smear positive (NSP), new smear negative (NSN) and new extrapulmonary TB cases were 4.1%, 3.7% and 2.6%, respectively ( table 2 ). The average CFRs for new microbiologically diagnosed and new clinically diagnosed cases were 4.0% and 3.0%, respectively ( table 3 ). Average CFRs for new HIV-TB, re-treatment HIV-TB and MDR TB cases were 13.0%, 14.4% and 21.0%, respectively ( table 4 ).
RNTCP report treatment CFRs during old classification system
The significant digits appear here as they were reported by the RNTCP.
CFR, case fatality ratio; EPTB, extrapulmonary TB; MDR TB, multidrug-resistant TB; NSN, new smear negative; NSP, new smear positive; RNTCP, Revised National TB Control Programme; TB, tuberculosis.
RNTCP report treatment CFRs with new classification system
CFR, case fatality ratio; MDR TB, multidrug-resistant TB; RNTCP, Revised National TB Control Programme.
RNTCP report treatment CFRs for HIV-TB and MDR TB across classification systems
CFR, case fatality ratio; MDR TB, multidrug-resistant TB; RBTCP, Revised National TB Control Programme; TB, tuberculosis.
Peer-reviewed literature
Treatment phase case fatality ratios.
The 211 studies which described treatment phase CFRs had an overall pooled CFR of 5.16% (4.20% to 6.34%) ( table 5 ). The paediatric pooled CFR (n=27) was 6.50% (2.65% to 10.36%) while higher CFRs were observed for patients with HIV infection (n=35, 10.91% (7.68% to 15.50%)) and DR-TB (n=43, 14.06% (10.15% to 19.49%)). The pooled treatment phase CFRs for primary/secondary centres (n=91, 5.18% (4.07% to 6.60%)) and tertiary centres (n=116, 4.87% (3.42% to 6.94%)) were similar. Fourteen papers with private sector CFRs were identified but their results were too heterogenous to reliably pool ( figure 4 ).

Forest plot of private sector treatment phase CFRs. CFR, case fatality ratio.
Treatment CFRs for all studies
Private sector was not pooled due to high heterogeneity.
CFR, case fatality ratio.
Post-treatment phase case fatality ratios
The 19 studies which described post-treatment phase CFRs were more heterogenous than the treatment CFRs ( table 6 ). Only the HIV-infected patient stratum was sufficiently homogenous giving a pooled post-treatment phase CFR of 4.15% (1.06% to 16.24%). There were substantially fewer studies that examined post-treatment fatality with only one study each providing post-treatment follow-up in the key populations of paediatric, DR and privately treated patients with TB.
Post-treatment CFRs for all studies
Overall, adult, drug sensitive, public sector, primary/secondary centre and tertiary centre strata were not pooled due to high heterogeneity. The HIV− strata was not pooled as the model failed to converge.
Restricting to high-quality studies
Treatment case fatality ratios.
Restricting to high-quality studies left 52 (52/211, 24.6%) studies concerning treatment phase CFRs ( table 7 ). The overall pooled CFR reduced slightly to 3.78% (2.77% to 5.16%). The paediatric (n=11) CFR was substantially reduced to 1.08% (1.06% to 1.10%) while the HIV+ (n=7, 12.17% (5.68% to 26.11%)) and DR-TB (n=5, 11.78% (2.96% to 46.78%)) remained high. No high-quality private sector studies were identified. The high-quality study treatment phase CFRs were similar or slightly lower than the overall results with the exception of the HIV-infected strata, which increased slightly. All tertiary centre studies were excluded as low quality due to poor generalisability; thus, this stratum is not presented for the high-quality studies.
Treatment CFRs for high-quality studies
Post-treatment case fatality ratios
Only three (3/19, 15.8%) high-quality post-treatment CFR studies remained after quality restriction though they were now sufficiently homogenous to pool for an overall post-treatment phase CFR of 2.69% (-0.79%, 6.18%). The three studies were all from the public sector. No high-quality post-treatment phase studies were available for paediatric patients, patients with DR-TB or patients with HIV-TB.
CFRs were regressed on study covariates for both treatment and post-treatment phase CFRs. There were 71 studies which had non-missing values for the required coefficients and a treatment phase CFR ( table 8 ) and 19 studies which had non-missing values for the required coefficients and a post-treatment phase CFR ( table 9 ).
ORs from meta-regression of treatment CFRs, n=71
CFR, case fatality ratio; DR TB, drug-resistant TB; EPTB, extrapulmonary TB; TB, tuberculosis.
ORs from meta-regression for post-treatment CFRs, n=19
CFR, case fatality ratio; EPTB, extrapulmonary TB; TB, tuberculosis.
For treatment phase CFRs, increasing proportions of EPTB (OR=0.95 (0.94 to 0.97)) and privately treated (OR=0.86 (0.84 to 0.89)) patients were significantly associated with lower odds of case fatality. Increasing proportions of patients with HIV infection (OR=1.15 (1.13 to 1.17)) and DR-TB (OR=1.09 (1.08 to 1.10)) were significantly associated with higher odds of case fatality. Studies set in tertiary settings were significantly associated with higher case fatality (OR=1.15 (1.03 to 1.28)) as was poor study quality (OR=2.27 (2.01 to 2.57)).
Post-treatment CFRs were not significantly associated with either proportion of patients with EPTB or study quality.
Treatment phase
Our systematic review of the literature found an overall treatment phase CFR for Indian patients with TB of 5.16% (4.20% to 6.34%) among 211 papers. The pooled treatment phase CFR dropped slightly when restricted to high-quality studies to 3.78% (2.77% to 5.16%). Elevated treatment phase CFRs were identified for key patient subpopulations like those with HIV (10.91% (7.68% to 15.50%)) and those with DR-TB (14.06% (10.15% to 19.49%)). The paediatric TB CFR was found to be 6.50% (2.65% to 10.36%) in the full data but when restricting to high-quality studies it dropped to 1.08% (1.06% to 1.10%). In general, when restricting to high-quality studies, which were defined in part by having good generalisability to the entire TB population, pooled treatment phase CFRs were lower than the mixed quality pooled treatment phase CFRs. Generalisability concerns were the leading cause of declaring a study low quality suggesting that much of the available TB literature focuses on the sickest patients with TB like those who are treated in hospitals. This skewing towards sicker patients may be artificially elevating reporting of TB CFRs in the literature. Interestingly, the pooled treatment phase CFRs for primary/secondary health centres (5.18% (4.07% to 6.60%)) and tertiary health centres (4.87% (3.42% to 6.94%)) were similar but when adjusted for other study characteristics, tertiary centre studies were significantly associated (OR=1.15 (1.03 to 1.28)) with increased case fatality.
The overall pooled treatment phase CFR (5.16% (4.20% to 6.34%)) from the peer-reviewed studies since 2006 was higher than the average annual RNTCP reported CFR for NSP (4.1%), NSN (3.6%) and new EPTB (2.6%) cases over the same period. The pooled treatment CFRs for patients with HIV (10.91% (7.68% to 15.50%)) and DR-TB (14.06% (10.15% to 19.49%)) were lower than the RNTCP reported CFRs for these groups (new HIV-TB: 13.0%, re-treatment HIV-TB: 14.4%, MDR TB: 21.0%).
Our meta-regression associated pulmonary TB, public sector treatment, HIV positivity, drug resistance and tertiary health centre settings with increasing CFRs during treatment. Additionally, poor-quality studies were associated with finding higher CFRs.
Post-treatment phase
The 19 papers that described post-treatment CFRs were highly heterogeneous and could only be reliably pooled when restricted to the three high-quality studies. The high-quality study post-treatment CFR was estimated to be 2.69% (–0.79% to 6.18%). Patient deaths after treatment may indicate either ineffective anti-TB treatment or a failure to address the socioeconomic determinants and physical environment that led to developing a disease like TB in the first place. TB treatment may also leave patients more susceptible to other diseases, both infectious and non-communicable. The goal of anti-TB treatment must extend beyond simply curing the current bout of TB to promoting long-lasting health for the patient.
Extensive quality concerns
As discussed above, 61.0% of studies had poor generalisability due to hospitalised or other specialised patient populations. It is likely that these patients were sicker than the average Indian patients with TB and thus those studies had higher than representative CFRs. Many studies (27.5%) also had selection bias concerns. No study, including the RNTCP reports, corrected for patients lost to follow-up or those who transferred to other TB centres meaning that these patient outcomes are not reflected in the reported CFR. Patients lost to follow-up may have been lost because they had died which could bias reported CFRs downward. Overall, after adjusting for other study variables, poor study quality was associated with higher CFRs (OR=2.27 (2.01 to 2.57)) in our meta-regression.
Strengths and limitations
The pooled overall treatment phase CFR estimated in this work is in line with the WHO End TB Strategy goal which is an important and positive step for India. However, key patient subpopulations are understudied or described in studies with potential biases. In more than a decade, only 14 studies have addressed case fatality during private TB treatment in India, a country where half of patients with TB are treated in the private sector. 4 5 None of these 14 studies was of high quality. For all patient subgroups, post-treatment CFRs are understudied with only 19 studies identified. Support for patients with TB cannot stop when treatment is completed as patients are often in the same or worse social and environmental condition than when they first contracted TB. 16 Study quality is also a major concern as almost three quarters of studies had potential biases. Our meta-regression suggested that studies with methodological issues were likely to find higher CFRs, potentially overestimating patient fatality.
Critically, this study focused on a WHO-identified key indicator of treatment quality: the CFR. This is a value monitored by TB programmes around the world and is relevant for programmatic planning. We were also able to include more than 200 studies thanks to flexible inclusion criteria that allowed for CFRs to be calculated from multiple study designs. Finally, our pooling methodology improved on the more common Der-Simonian and Laird models by exactly modelling the binomial variance of the CFRs and adapting as needed to rare events by using a Beta-Binomial GLMM.
However, it is important to keep in mind that the patients in the published literature are unlikely to perfectly represent the complete patient distribution of India. The literature likely over-represents rare forms of TB and hospitalised patients. We attempted to correct for this by restricting to high-quality studies that were evaluated for generalisability of the studied patient population. While we have reported on CFRs from the RNTCP government reports, we have excluded other grey literature that may exist. Additional data from the grey literature may have added power to this work but it is often difficult to assess the quality of non-peer-reviewed literature. Additionally, this work only reflects fatality during and after treatment. Studies that estimate case fatality before treatment initiation were not included; thus, we cannot speak to pretreatment CFRs. Similarly, the CFR is a measure of all-cause mortality and does not distinguish between TB or non-TB causes of death. In our meta-regression, which associated patient demographics and study characteristics with case fatality, we had to exclude many studies due to incomplete reporting of patient and study variables. Future studies on CFR and patient outcomes must fully report patient characteristics and patient selection. Moving forward, researchers and programmes must apply correction methods for patient loss to follow-up in order to minimise selection bias. 17 Researchers should also recognise that hospitalised patients may systematically differ from most patients with TB and that only limited conclusions can be drawn from these populations.
Case fatality is a critical measure of the quality of TB care. While India’s overall treatment CFR is in line with WHO targets, several key patients groups remain understudied. Increased monitoring of patients treated in the private sector as well as follow-up of patients post-treatment will help ensure that all patients are able to achieve and maintain health after TB.
Acknowledgments
The authors thank Genevieve Gore for her assistance in the development of the search strategy. SH and AS were supported by FRQS Doctoral Awards.
Handling editor: Seye Abimbola
Twitter: @paimadhu
Contributors: SH and MP conceived the study. SH, AS and VN collected data. SH analysed the data. All coauthors reviewed and provided feedback on the manuscript.
Funding: This study was funded by Bill and Melinda Gates Foundation.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. Data is provided as a supplementary CSV file.
CDC and Partners Tackle Drug-resistant TB in India
Posted On: 2016
- Tuberculosis (TB) is the number one infectious disease killer worldwide. Unlike
- drug-resistant TB, TB is preventable, treatable, and curable. We must address TB both at home and abroad to ensure a safer, healthier America.
- Drug-resistant TB threatens our ability to treat and control TB. Drug-resistant forms of TB have spread to every country around the world.
- To protect Americans from resistance threats, CDC is reinforcing international collaborations and capacities for antimicrobial resistance prevention, surveillance, and infection control with countries worldwide.
- Mumbai, India is one of the highest burden municipalities for drug-resistant TB in the world. CDC is working to address key gaps in this serious and growing epidemic, including improving TB infection control, diagnostic and treatment services, and treatment adherence.
- With local experts, CDC helped establish an Airborne Infection Control Unit (AICU) in Mumbai in September 2016. The AICU helps healthcare facilities implement TB infection control best practices to reduce healthcare-associated transmission of drug-resistant TB. CDC provides ongoing training, but the unit is run by local partners.
- In addition, CDC is working with partners in Mumbai to improve TB laboratory quality, TB treatment and adherence, and proficiency testing to improve treatment outcomes.
- By sharing its world-class scientific expertise, CDC can strengthen local capacity to quickly detect and treat the threat of drug-resistant TB and contain its spread.
Tuberculosis (TB) is the number one infectious disease killer worldwide but, unlike drug-resistant TB, it is preventable, treatable, and curable. Drug-resistant TB —when the bacteria that causes TB disease is able to avoid the effects of antibiotics—threatens our ability to treat and control the disease. In October 2016, the World Health Organization estimated [PDF -6.35 MB] that drug-resistant TB had risen to more than half a million cases worldwide. Stronger TB infection control and improved detection and treatment practices can help reduce rates of drug-resistant TB worldwide.

Because TB is an airborne disease that is transmitted from person to person, it knows no borders. We must address TB both at home and abroad to ensure a safer, healthier America. While TB can have a severe economic toll, efforts to end TB are one of the best investments for improving U.S. and global health, security, and economic development. Patients often face staggering costs when seeking TB diagnosis and treatment, which can put families in poverty and undermines economic gains and stability. Efforts to end TB are a best buy—reaping $43 return for every $1 invested.
Transformative Investments
CDC’s AR Solutions Initiative is implementing several TB projects to reduce rates of drug-resistant TB worldwide:
- Conducting a one-year study to optimize treatment protocol using early bactericidal activity study methodology in India.
- Evaluating the use of smartphones and video to monitor patient treatment and ensure therapy is completed.
- Increasing global technology for testing and treating TB to stop it at its source.
- Working closely with more than 25 ministries of health to strengthen TB control efforts
CDC is Combating Drug-resistant TB
To protect Americans from antimicrobial resistance threats, like drug-resistant TB, CDC is reinforcing international collaborations and capacities for antimicrobial resistance prevention, surveillance, and TB infection control with countries worldwide.
CDC is working on the ground in 25 countries to find, cure, and prevent TB and protect Americans both here and abroad by:
- Improving access to better screening, contact tracing, and diagnostic tools to find missing TB cases.
- Evaluating better TB treatment regimens and improving access to care and treatment.
- Breaking the cycle of transmission by strengthening TB infection control, identifying hotspots to target screening efforts, and scaling-up treatment to prevent TB among vulnerable populations.
One example of these efforts can be seen in Mumbai, India, one of the highest burden municipalities for drug-resistant TB in the world. By working together in Mumbai to address key gaps in this serious and growing epidemic, like TB infection control and treatment compliance, CDC and its partners can help reduce drug-resistant TB rates and contain its spread.
Improving TB Infection Control

In September 2016, the Airborne Infection Control Unit (AICU) in Mumbai was officially stood up by CDC with the Mumbai Municipal Corporation and India’s national TB program. The AICU addresses key gaps in seven high burden districts in Mumbai by evaluating key TB infection control gaps and implementing simple, rigorous interventions to decrease TB transmission in healthcare facilities. Simple steps—like ensuring appropriate triage for coughing patients and patients with confirmed TB, increasing airflow in waiting areas or moving them to sheltered spaces outdoors, and improving use of protective gear among healthcare workers—can reduce TB infections among patients and healthcare workers and improve working conditions. As of March 2017, the AICU has assessed and recommended improvements in 12 facilities.
Improving Treatment Adherence
Treating and curing drug-resistant TB is complicated and up to 30% of drug-resistant TB patients are lost to follow-up (do not complete treatment for a variety of reasons).
CDC and Science Health Allied Research Education (SHARE) are collaborating on the Strengthening TB Action and Response (STAR) project to build institutional capacity to improve drug-resistant TB treatment and adherence in Mumbai, as well as improve TB laboratory quality, airborne infection control, and healthcare worker surveillance.
To ensure that patients receive and complete the treatment they need, the STAR project has successfully registered 295 multi-drug-resistant TB patients, 21 extensively drug-resistant TB patients, and 305 caregivers in treatment adherence counseling services. The STAR project teams have made 270 home visits for follow-up counseling and retrieved seven patients whose treatment was previously interrupted.
Improving Lab Diagnostics & Treatment Services
Consistent access to sophisticated diagnostics and high-quality treatment varies in Mumbai and can come at a direct cost to the patient. These inconsistencies can lead to uneven patient care, under-diagnosis, and missed cases—all contributing to potential TB spread.
Each year, the private sector diagnoses more than half of Mumbai’s estimated 50,000 TB cases. Engaging the private sector in Mumbai is essential to avoid these inconsistencies and ensure patients can access quality diagnosis and treatment.
CDC is working with both the public sector (through the Mumbai Municipal Corporation) and the private sector (through Private Provider Interface Agency at PATH) to improve access to cultures, drug susceptibility testing (DST), and DST-based treatment to improve diagnosis and treatment of drug-resistant TB.
Finally, CDC is working to improve lab quality by training India’s National TB Institute microbiologists on proficiency testing. Using proficiency testing panels to measure drug susceptibility (if a bacteria will or won’t be killed or slowed down by a drug) helps facilities rapidly implement new national guidelines and can improve individualized drug-resistant TB treatment and outcomes. By sharing its world-class scientific expertise, CDC can strengthen local capacity to quickly detect and treat the threat of drug-resistant TB and contain its spread.
- What is drug-resistant tuberculosis?
- CDC is transforming how we identify and respond to antimicrobial resistance
- CDC’s international activities to combat antimicrobial resistance
To receive email updates about this page, enter your email address:

IMAGES
VIDEO
COMMENTS
The National Prevalence Survey of India (2019-2021) estimated 31 per cent tuberculosis infection (TBI) burden among individuals above 15 years of age. However, so far little is known about the TBI burden among the different risk groups in India. Thus, this systematic review and meta-analysis, aimed to estimate the prevalence of TBI in India ...
Introduction. Among other global burdens of diseases, the estimated tuberculosis (TB) cases are 130 cases/100,000 population globally, 1 and 131/100,000 population in India. 2 The Global TB Report 2020 indicates that India has a dual burden of tuberculosis (26%) and multidrug-resistant (MDR)/rifampicin-resistant (27%). 3 About 26% of the worldwide incident cases and 31% of the global TB deaths ...
Tuberculosis is one of the ten major causes of mortality worldwide. Many government plans have failed in bringing down the high incidence and prevalence of TB in India. Major challenges: Lack of awareness, poor infrastructure, drug resistance, poor notification and overall negligence. Eradication of poverty and undernourishment, education of ...
According to the Global Tuberculosis Report 2018 (World Health Organization [WHO], 2020a), there are an estimated 10 million incident TB cases, which. Promita Majumdar is a Research Scholar, Department of Social Work, Visva-Bharati University, Santiniketan, India. She can be contacted at [email protected].
PDF | Background & objectives: The National Prevalence Survey of India (2019-2021) estimated 31 per cent tuberculosis infection (TBI) burden among... | Find, read and cite all the research you ...
The National Prevalence Survey of India (2019-2021) estimated 31 per cent tuberculosis infection (TBI) burden among individuals above 15 years of age. However, so far little is known about the TBI burden among the different risk groups in India. Thus, this systematic review and meta-analysis, aimed to estimate the prevalence of TBI in India ...
Tuberculosis (TB) is a highly infectious disease, and it has the highest global burden on India with 21% prevalence rate and 27% of patients who do not receive pertinent medical treatment. Although India spends 23 billion dollars annually towards medical expenses for TB, India still ranks among the top 2 countries with the highest incidence and ...
Conclusion. India's ambitious goal to end TB by the year 2025 is daunting. The World Health Organization estimates that India contributed 2.7 million incident TB cases in 2018, representing a 9% reduction compared to 2015 [ 4 ]. Currently, India contributes 27% of the global burden of TB. We need to do more.
Tuberculosis (TB) remains one of India's most pressing and challenging problems. Globally, India remains the highest TB burden country, inf ecting people of all a ge. groups, mainly affecting ...
India's national TB control program, the Revised National TB Control Program (RNTCP), has taken noteworthy steps for the control of DR-TB. It launched the Programmatic Management of Drug Resistant Tuberculosis (PMDT) in 2007 and made it pan-national in 2013 [5], [6]. However, with a treatment success rate of 46% and a mortality rate of 20% ...
OBJECTIVE The overall goal of my PhD thesis is to describe using suitable methods what health care providers 'do' in routine practice for patients with pulmonary TB symptoms and disease. 'Quality' in the context of my PhD thesis is defined as adherence to international and national standards of tuberculosis care by health care providers.
Recurrent Tuberculosis patients contribute to a significant proportion of TB burden in India. A nationwide survey was conducted during 2019-2021 across India among adults to estimate the prevalence of TB. A total of 322480 individuals were screened and 1402 were having TB. Of this, 381 (27.1%) had recurrent TB. The crude prevalence (95% CI) of recurrent TB was 118 (107-131) per 100,000 ...
about 1.3 million deaths during the year 2020. 2 In India, there is about 38% TB infected deaths due to HIV -negativ e. and about 34% of deaths due TB, HIV -negativ e and HIV -. positive infected ...
India accounts for about one-fourth of the global burden of MDR-TB. This study aims to assess the prevalence and factors associated with tuberculosis drug resistance among patients from South India.
INTRODUCTION. Tuberculosis (TB) is one of the most ancient diseases of mankind and has co-evolved with humans for many thousands of years or perhaps for several million years.[] The oldest known molecular evidence of TB was detected in a fossil of an extinct bison (Pleistocene bison), which was radiocarbon dated at 17,870±230 years[] ; and in 9000, year old human remains which were recovered ...
This thesis draws on innovation studies and Science and Technology Studies to examine innovation dynamics in organizational, strategic, technological and service delivery aspects of ... The results reveal that the dynamics of innovation and control in coping with Tuberculosis in India are a complex interplay of mutual influence and requirement ...
By September 2001, about 3.4 million symptomatic patients had been assessed for tuberculosis, and in the case of nearly 800,000, treatment had been started in the past 12 months. More than 200,000 ...
Background: In 2017, India accounted for 27 percent of the global burden on tuberculosis, and the highest among the top 30 countries with high TB burden. Despite the expansion of DOTS programme many households in India incur high expenditure towards TB treatment. Most of the studies in India have focused on measuring catastrophic health expenditure on TB.
In order to improve this situation, the draft Strategic Plan To End Tuberculosis in India 2020-2025 now proposes an ambitious target to universally roll out of TB preventive treatment for PLHIV and child contacts of TB patients by 2022. A nation-wise catch-up campaign is accordingly being proposed to cover these two eligible groups. Further, it ...
With an estimated 480 000 new cases developing every year,1 multidrug-resistant tuberculosis is one of the greatest public health challenges worldwide. Multidrug-resistant tuberculosis is much more common in patients who have previously been treated for tuberculosis,1 and public health efforts have typically focused on high-quality treatment of drug-susceptible tuberculosis to prevent ...
Introduction. Among other global burdens of diseases, the estimated tuberculosis (TB) cases are 130 cases/100,000 population globally, Citation 1 and 131/100,000 population in India. Citation 2 The Global TB Report 2020 indicates that India has a dual burden of tuberculosis (26%) and multidrug-resistant (MDR)/rifampicin-resistant (27%). Citation 3 About 26% of the worldwide incident cases and ...
Tuberculosis thesis sanaag .pdf. Content uploaded ... records of patients with sputum smear-positive TB registered under the RNTCP in 43 districts across three states of India during a three month ...
Tuberculosis (TB) affected 10.0 million new people in 2017 resulting in 1.6 million deaths globally. 1 A key component to the WHO End TB Strategy 2 is improving the quality of TB care. The End TB Strategy calls for a 95% reduction in TB deaths by 2035 relative to 2015.
The incidence of tuberculosis (TB) among Tibetan refugees in India is 431 cases/100,000 persons, compared with 181 cases/100,000 persons overall in India in 2010.
Tuberculosis (TB) is the number one infectious disease killer worldwide but, unlike drug-resistant TB, it is preventable, treatable, and curable. Drug-resistant TB—when the bacteria that causes TB disease is able to avoid the effects of antibiotics—threatens our ability to treat and control the disease.