Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 28 May 2021

A deep learning system for detecting diabetic retinopathy across the disease spectrum
- Ling Dai 1 , 2 , 3 na1 ,
- Liang Wu ORCID: orcid.org/0000-0002-3763-1274 2 na1 ,
- Huating Li ORCID: orcid.org/0000-0003-0526-5545 2 na1 ,
- Chun Cai ORCID: orcid.org/0000-0001-8725-5433 2 na1 ,
- Qiang Wu 4 na1 ,
- Hongyu Kong ORCID: orcid.org/0000-0002-8133-5990 4 ,
- Ruhan Liu ORCID: orcid.org/0000-0002-0281-8039 1 , 3 ,
- Xiangning Wang 4 ,
- Xuhong Hou 2 ,
- Yuexing Liu 2 ,
- Xiaoxue Long ORCID: orcid.org/0000-0002-7080-8422 2 ,
- Yang Wen ORCID: orcid.org/0000-0001-6303-8178 1 , 3 ,
- Lina Lu 5 ,
- Yaxin Shen ORCID: orcid.org/0000-0003-2169-338X 1 , 3 ,
- Yan Chen 4 ,
- Dinggang Shen ORCID: orcid.org/0000-0002-7934-5698 6 , 7 ,
- Xiaokang Yang 8 ,
- Haidong Zou ORCID: orcid.org/0000-0002-6831-7560 5 ,
- Bin Sheng ORCID: orcid.org/0000-0001-8678-2784 1 , 3 &
- Weiping Jia ORCID: orcid.org/0000-0002-6244-2168 2
Nature Communications volume 12 , Article number: 3242 ( 2021 ) Cite this article
55k Accesses
241 Citations
36 Altmetric
Metrics details
- Diabetes complications
- Retinal diseases
Retinal screening contributes to early detection of diabetic retinopathy and timely treatment. To facilitate the screening process, we develop a deep learning system, named DeepDR, that can detect early-to-late stages of diabetic retinopathy. DeepDR is trained for real-time image quality assessment, lesion detection and grading using 466,247 fundus images from 121,342 patients with diabetes. Evaluation is performed on a local dataset with 200,136 fundus images from 52,004 patients and three external datasets with a total of 209,322 images. The area under the receiver operating characteristic curves for detecting microaneurysms, cotton-wool spots, hard exudates and hemorrhages are 0.901, 0.941, 0.954 and 0.967, respectively. The grading of diabetic retinopathy as mild, moderate, severe and proliferative achieves area under the curves of 0.943, 0.955, 0.960 and 0.972, respectively. In external validations, the area under the curves for grading range from 0.916 to 0.970, which further supports the system is efficient for diabetic retinopathy grading.
Similar content being viewed by others

A deep learning system for predicting time to progression of diabetic retinopathy

Detection of signs of disease in external photographs of the eyes via deep learning
Deep-learning models for the detection and incidence prediction of chronic kidney disease and type 2 diabetes from retinal fundus images
Introduction.
It is estimated that approximately 600 million people will have diabetes by 2040, with one-third expected to have diabetic retinopathy (DR)—the leading cause of vision loss in working-age adults worldwide 1 . Mild non-proliferative DR (NPDR) is the early stage of DR, which is characterized by the presence of microaneurysms. Proliferative DR (PDR) is the more advanced stage of DR and can result in severe vision loss. Regular DR screening is important so that timely treatment can be implemented to prevent vision loss 2 . Early-stage intervention via glycemia and blood pressure control can slow down the progression of DR and late-stage interventions through photocoagulation or intravitreal injection can reduce vision loss 3 . In the United Kingdom and Iceland, where systematic national DR screening has been carried out, DR is no longer the leading cause of blindness among working-age adults 4 , 5 . Although routine DR screening is recommended by all professional societies, comprehensive DR screening is not widely performed 6 , 7 , 8 , 9 , 10 , facing the challenges related to the availability of human assessors 3 , 11 .
China currently has the largest number of patients with diabetes worldwide 12 . In 2016, the State Council issued the “Healthy China 2030” planning outline, which provided further guidance on the future direction of Chinese health reform 13 . The “Healthy China 2030” outlined the goal that all patients with diabetes will receive disease management and intervention by 2030. In China, there are about 40,000 ophthalmologists, with a 1:3000 ratio to patients with diabetes. As a cost-effective preventive measure, regular retinal screening is encouraged at the community level. Task shifting is one way the public health community can address this issue head-on so that ophthalmologists can do the treatment but not the screening. Task shifting is the name given by WHO to a process of delegation whereby tasks are moved, where appropriate, to less specialized health workers 14 . Recent evidence has established a role for screening by healthcare workers, given prior training in grading DR 3 . However, we still face the issues of insufficiency of their training and where they are placed in the system. Thus, diagnostic system using deep learning algorithms is required to help DR screening.
Recently, deep learning algorithms have enabled computers to learn from large datasets in a way that exceeds human capabilities in many areas 15 , 16 , 17 , 18 . Several deep learning algorithms with high specificity and sensitivity have been developed for the classification or detection of certain disease conditions based on medical images, including retinal images 19 , 20 , 21 , 22 , 23 . Current deep learning systems for DR screening have been predominantly focused on the identification of patients with referable DR (moderate NPDR or worse) or vision-threatening DR, which means the patients should be referred to ophthalmologists for treatment or closer follow-up 21 , 22 , 24 . However, the importance of identifying early-stage DR should not be neglected. Evidence suggests that proper intervention at an early stage to achieve optimal control of glucose, blood pressure, and lipid profiles could significantly delay the progression of DR and even reverse mild NPDR to DR-free stage 25 .
In addition, the integration of these deep learning advances into DR screening is not straightforward because of some challenges. First, there are a few end-to-end and multi-task learning methods that can share the multi-scale features extracted from convolutional layers for correlated tasks, and further improve the performance of DR grading based on the lesion detection and segmentation, due to the fact that DR grading inherently relies on the global presence and distribution of the DR lesions 21 , 22 , 26 , 27 , 28 . Second, despite being helpful in DR screening, there are a few deep learning methods providing on-site image quality assessment with latency compatible with real-time use, which is one of the most needed additions at primary DR screening level and will have the impact on screening delivery at the community level.
Here we describe the development and validation of a deep learning-based DR screening system called DeepDR (Deep-learning Diabetic Retinopathy), which was a transfer learning assisted multi-task network to evaluate retinal image quality, retinal lesions, and DR grades. The system was developed using a real-world DR screening dataset consisting of 666,383 fundus images from 173,346 patients. In addition, we annotated retinal lesions, including microaneurysms, cotton-wool spots (CWS), hard exudates, and hemorrhages on 14,901 images, and used transfer learning 29 to enhance the lesion-aware DR grading performance. The system achieved high sensitivity and accuracy in the whole-process detection of DR from early to late stages.
Data sources and network design
DeepDR was developed using the fundus images of patients with diabetes who participated in the Shanghai Integrated Diabetes Prevention and Care System (Shanghai Integration Model, SIM) between 2014 and 2017 (Supplementary Table 1 ). A total of 666,383 fundus images from 173,346 patients with diabetes with integrity fundus examination records were enrolled in this study. Two retinal photographs (macular and optic disc centered) 30 were taken for each eye according to the DR screening guidelines of the World Health Organization 31 . Image quality (overall gradability, artifacts, clarity, and field), DR grades (non-DR, mild NPDR, moderate NPDR, severe NPDR, or PDR), and diabetic macular edema (DME) were labeled for each image. In addition, 14,901 images were labeled with retinal lesions, including microaneurysms, CWS, hard exudates, and hemorrhages.
Among the 173,346 subjects in the SIM cohort (referred as the local dataset in this study), 121,342 subjects (70%) were randomly selected as the training set, and the remaining 52,004 subjects (30%) served as the local validation set (Fig. 1 ). In the SIM cohort, each subject was enrolled only once and was recorded with the unique resident ID. So, the data separation was guaranteed between the training and local validation datasets. The prevalence of DR in the study cohorts is shown in Table 1 . In the training dataset, 12.85% of images had DR, among which 27.94% were mild NPDR. In the local validation dataset of 200,136 images, 12.99% of images had DR, among which 27.30% were mild NPDR.
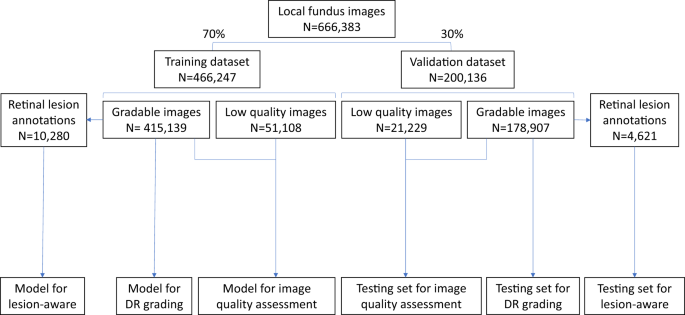
The local dataset was randomly divided into training or validation datasets. All 466,247 images in the training dataset were used for training the image quality assessment sub-network. The lesion detection sub-network was trained using 10,280 gradable images with retinal lesion annotations. Then, 415,139 gradable images in the training set were used for the training of the DR grading sub-network. All 200,136 images in the local validation dataset were used to test the image quality sub-network, and 178,907 gradable images were used to test the DR grading sub-network. Finally, 4621 gradable images labeled with retinal lesions were used to test the lesion detection sub-network. DR, diabetic retinopathy.
The DeepDR system consisted of three deep-learning sub-networks: image quality assessment sub-network, lesion-aware sub-network, and DR grading sub-network (Fig. 2 ). All the 466,247 images in the training dataset were used to train the image quality assessment sub-network to make binary classification of whether the image was gradable and recognize certain quality issues in terms of artifacts, clarity, and field problems of the retinal images; 415,139 images without quality issues were used to train the DR grading sub-network to classify the images into non-DR, mild NPDR, moderate NPDR, severe NPDR, or PDR, and binary classification of whether there was DME. The lesion-aware sub-network was trained using 10,280 images labeled with retinal lesions to achieve detection and segmentation of microaneurysms, CWS, hard exudates, and hemorrhages.
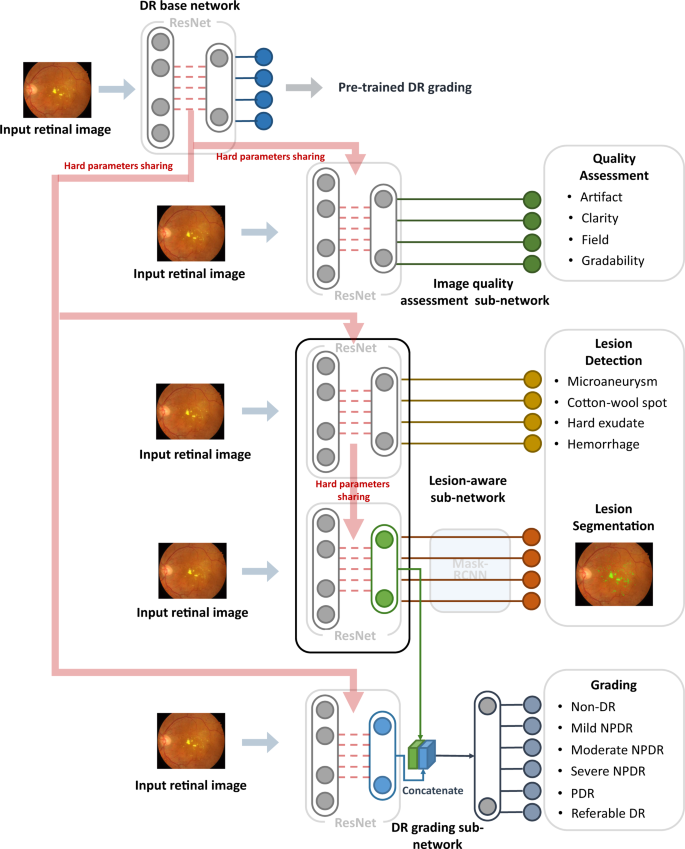
DeepDR system consisted of three sub-networks: image quality assessment sub-network, lesion-aware sub-network, and DR grading sub-network. We first pre-trained the ResNet to form the DR base network (top row). The trained weights of the pre-trained DR base network were then shared in the three different sub-networks of the system, indicated by the red arrow. These three sub-networks took retinal images as input and performed different tasks one-by-one. Furthermore, the lesion features extracted by the segmentation module of the lesion-aware sub-network (indicated by the green arrow) were concatenated with the features extracted by the DR grading sub-network (indicated by the blue arrow). DR, diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
As shown in Fig. 2 , our DeepDR system was designed as the transfer learning assisted multi-task network. Specifically, a DR base network was first pre-trained on ImageNet classification and then fine-tuned on our DR grading task using 415,139 retinal images. Next, we utilized transfer learning 32 to transfer the DR base network to the three sub-networks of the DeepDR system, rather than directly training randomly initialized sub-networks. During the process of transfer learning, we fixed the pre-trained weights in the lower layers of the DR base network and retrained the weights of its upper layers using backpropagation. This process worked well since the features were suitable to all the DR-related learning tasks (evaluating image quality, lesion analysis, and DR grading). Furthermore, we concatenated the lesion features extracted by the segmentation module of the lesion-aware sub-network with the features extracted by the DR grading sub-network to enhance grading performance. To prevent the network from overfitting, an early stopping criterion 33 was used to determine the optimized number of iterations. For every task, we randomly split the training dataset into two parts, 80% of the data were used to train the network and the rest were used for early stopping. The network was tested on early stopping dataset every epoch during training and the performance of the network was recorded. If the area under the receiver operating characteristic curve (AUC) or intersect over union (IoU) increment was less than 0.001 for 5 epochs continuously, we stopped training and selected the best model as the final model.
Performance of the DeepDR system
The image quality assessment sub-network for assessing overall image quality and identifying artifacts, clarity, and field definition problems was tested using 200,136 images in the local validation dataset. DeepDR achieved an AUC of 0.934 (0.929–0.938) for overall image quality. For the identification of artifacts, clarity, and field definition issues, the system achieved AUCs of 0.938 (0.932–0.943), 0.920 (0.914–0.926), and 0.968 (0.962–0.973), respectively.
The lesion-aware sub-network was evaluated using 4621 gradable images with retinal lesion annotations from the local validation dataset. The results are shown in Fig. 3 and Supplementary Table 2 . For microaneurysm, the AUC, sensitivity, specificity, and F -score were 0.901 (0.894–0.906), 88.0% (87.2–88.9%), 73.3% (72.0–74.3%), and 0.815, respectively. For CWS, the AUC, sensitivity, specificity, and IoU were 0.941 (0.935-0.946), 90.0% (87.9-91.9%), 83.1% (82.2–83.9%), and 0.711, respectively. For hard exudate, the AUC, sensitivity, specificity, and IoU were 0.954 (0.949–0.957), 90.5% (88.9–91.5%), 85.8% (85.2–86.6%), and 0.971, respectively. For hemorrhage, the AUC, sensitivity, specificity, and IoU were 0.967 (0.965–0.969), 93.2% (92.6–94.1%), 88.0% (87.6–88.7%), and 0.738, respectively. The lesion-aware sub-network highlighted the lesion areas by masking the fundus images (Fig. 3B ). To facilitate usability in clinical settings, a clinical report could be automatically generated for each patient (example report shown in Supplementary Fig. 1 ). This report showed the original fundus images with highlighted lesions, described the type and location of the retinal lesions along with DR gradings. In addition, we conducted an experiment to evaluate the utility of lesion-aware sub-network by measuring its effect on the grading accuracy of trained primary healthcare workers from community health service centers. Detailed study design is described in the Supplementary Information (Section “Supplementary Methods”). The results were tested using one-sided, two-sample Wilcoxon signed rank test and are shown in Table 2 . The sensitivities of all DR grades and the specificity of severe DR were significantly improved with the aid of the DeepDR system. This suggested that visual hints of retinal lesions significantly improved the diagnostic accuracy of the primary healthcare workers, which can facilitate the task shifting of DR screening.
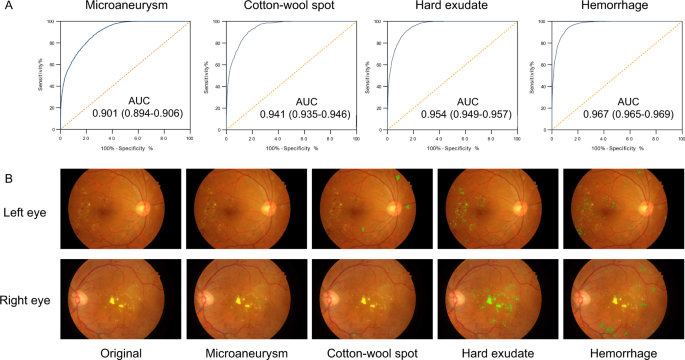
A Receiver operating characteristic curve demonstrating the performance of the lesion-aware sub-network for retinal lesion detection ( n = 4621). B Example images of retinal lesion segmentation: microaneurysms, cotton-wool spots, hard exudates, and hemorrhages are highlighted using green regions.
The DeepDR system achieved the whole-process diagnosis of DR from early to late stages based on the accurate detection of retinal lesions that was especially accurate for microaneurysms. In the local validation dataset, 178,907 gradable images were used to test the DR grading sub-network and the results are shown in Table 3 . For the two images per eye, our DR grading sub-network made separate prediction per image, and then we accepted the more severe DR grade obtained from those images as the grading result for that eye, which was used to calculate the AUC of DR grades. The average AUC was 0.955 for DR grading. In particular, for mild NPDR, the AUC, sensitivity, and specificity were 0.943 (0.940–0.946), 88.8% (87.7–89.7%), and 83.9% (83.7–84.1%), respectively. For DME, the AUC was 0.946 (0.945–0.947), sensitivity was 92.8% (92.4–93.1%), and specificity was 81.3% (81.0–81.6%).
External validation
To test the generalization of the system, we further evaluated the performance of DeepDR using two independent real-world cohorts and the publicly accessible dataset EyePACS for external validation. The first cohort was the China National Diabetic Complications Study (CNDCS) cohort, comprising 92,672 fundus images from 23,186 patients with diabetes and was acquired in 2018. The second cohort was the Nicheng Diabetes Screening Project (NDSP) cohort, comprising 27,948 fundus images from 6987 elderly subjects over 65 years of age and was acquired in 2018. The prevalence of diabetes was 31.7% in the NDSP cohort. The EyePACS dataset is a publicly available dataset from the United States, and consists of 88,702 fundus images.
The results for DR grading are shown in Table 3 . In the CNDCS, the DeepDR system achieved AUCs of 0.916 (0.912–0.920) for mild NPDR, 0.927 (0.925–0.929) for moderate NPDR, 0.962 (0.959–0.965) for severe NPDR, and 0.955 (0.949–0.961) for PDR. In the NDSP and EyePACS dataset, the average AUCs for DR grading were 0.944 and 0.943, respectively. The system had high AUCs for mild NPDR, achieving 0.929 (0.916–0.942) and 0.937 (0.935–0.939) for the NDSP and EyePACS datasets, respectively.
Real-time image quality feedback
We employed DeepDR to provide real-time image quality feedback during the non-mydriatic retinal photography of 1294 elderly subjects from the NDSP cohorts (age over 65 years). Two retinal photographs (macular and optic disc centered) were taken of each eye. If DeepDR determined the quality of the first image of a field to be ungradable, a second image of the same field was recaptured. Only one more photograph was taken of each field to avoid contracted pupils due to the camera flash.
The results are shown in Table 4 . During this process, 5176 retinal images were initially taken from 1294 patients. Of these, 1487 images (28.7%) were recognized as low-quality with artifacts, clarity, and/or field definition issues. Based on the feedback information, a second photograph was taken of these patients. For the 1487 initial low-quality images, 1065 (71.6%) recaptured images were of adequate quality. After replacing the low-quality images with recaptured images, the diagnostic accuracy of each grade of DR was improved. Especially for mild NPDR, the AUC increased from 0.880 (0.859–0.895) to 0.933 (0.918–0.950) ( P < 0.001) and sensitivity increased from 78.5% (72.7–83.4%) to 87.6% (83.2–92.3%).
The DeepDR system achieved high sensitivity and specificity in DR grading. Rather than just generating a DR grading, it offers visual hints that help users to identify the presence and location of different lesion types. Introducing the image quality sub-network and lesion-aware sub-network into DeepDR improved the diagnostic performance and more closely followed the thought process of ophthalmologists. DeepDR can run on a standard personal computer with average-performance processors. Thus, it has great potential to improve the accessibility and efficiency of DR screening.
Several previous studies using deep learning approaches have been conducted on the detection of referable or vision-threatening DR detection. Gulshan et al. tested their deep learning system using 9963 fundus images and achieved a high level of performance for referable DR (AUC = 0.99) 21 . Ting et al. evaluated their deep learning system using 71,896 images and reported excellent results for referable and vision-threatening DR (AUCs of 0.936 and 0.958, respectively) 22 . Li et al. validated their deep learning system in a real-world multiethnic dataset of 35,201 images and achieved an AUC of 0.955 for vision-threatening DR 24 . Although these studies achieved excellent accuracy, they focused only on patients with referable DR who are then referred for specialist eye care. However, mild DR was classified into non-referable DR and was not distinguished from DR-free subjects 21 , 22 , 24 .
The value of detecting early DR is underestimated, as there is little evidence that ophthalmic treatments, such as photocoagulation or anti-VEGF medications, are indicated at this stage 2 . Furthermore, if all the cases of DR are referred to ophthalmologists, it would likely overwhelm our medical systems. However, from the perspective of diabetes management, the screening for mild DR is of great clinical importance and may improve patients’ outcomes. First, the identification of patients with mild DR facilitates health providers, such as family physicians, general practitioners, and endocrinologists, to participate in the patient education and management of blood glucose, lipid profiles, blood pressure, and other risk factors 2 . Secondly, there is no known cure for advanced DR, and some of the damage caused by leakage, oxygen deprivation, and blood vessel growth is permanent 34 . But there is evidence showing that optimal glycemic and blood pressure controls are strongly correlated with the regression from mild DR to DR-free state 25 , and intensive glycemic and lipid control reduces the rate of progression to vision-threatening DR 35 . Thirdly, screening for mild DR provides valuable information for clinical decision making. Although intensive glycemic control reduces the rate of photocoagulation, it increases the risk of severe hypoglycemia and incurs additional burden by way of polypharmacy, side effects, and cost 36 . The optimal glycemic target is controversial. The American College of Physicians guideline 37 set HbA1c levels 7–8% as the optimal target for most patients with diabetes, while the American Diabetes Association guideline 38 set the HbA1c target at 6.5–7.0%. Patients with mild DR could benefit from strict glycemic control 39 . Thus, the detection of mild DR can promote personalized diabetes management.
Accurate detection of microaneurysms is still a problem for deep learning systems 40 . In this study, to improve the performance of detecting specific retinal lesions and DR grading, we introduced an efficient retinal lesion-aware sub-network based on ResNet that avoided the problem of vanishing gradients, which made it a more sensitive feature extractor for small lesions compared to other existing network architectures (e.g., VGG and Inception) 41 . The lesion-aware sub-network contained feature pyramid structure that was designed to capture multi-scale features and mine the relationship of lesion types and position 42 . Meanwhile, transfer learning was used in our study and the lesion-aware sub-network contained the repurposed DR base network layers that were pre-trained by a base DR grading dataset of 415,139 retinal images. This boosted the performance of learning lesion detection and segmentation through the transfer of knowledge from DR grading task that has already been learned. As a result, the DeepDR system achieved AUCs of 0.901–0.967 for lesion detection, including microaneurysms, CWS, hard exudates, and hemorrhages. Retinal lesion detection and segmentation is of great clinical impact. Detecting different types of retinal lesions can provide guidance for clinical decision making. For example, fenofibrate may benefit patient with hard excaudate 43 and antiplatelet drugs should be used carefully in patient with retinal bleeding 44 . More importantly, one of the major problems in DR screening is detecting change or progression, as progression of retinal lesions is indicative of developing sight-threatening DR/DME 45 , 46 , 47 . Due to the fact that DR progression could be detected not only between different DR grades, but even within the same grade, our lesion-aware sub-network has the potential to capture tiny progression of certain kind of retinal lesions through follow-up of DR patients. Further studies are needed to evaluate this application in real-world clinical settings.
In previous studies, the deep learning systems were usually trained directly end-to-end from original fundus images to the labels of DR grades 21 , 22 , 24 , these end-to-end systems might fail to encode the lesion features due to the black-box nature of deep learning 48 . In our study, instead of direct end-to-end training from fundus images to DR grades, an efficient lesion-aware sub-network was introduced to increase the ability of capturing lesion features. Due to the fact that embedding prior knowledge into the end-to-end machine learning algorithms can regulate machine learning models and shrink the search space 49 , and the ophthalmologists read fundus images based on the presence of lesions, our DR grading network can leverage lesion features as prior knowledge to enhance the performance of DR grading. Previous studies, such as Michael D. Abràmoff et al.’s work 50 , used multiple CNNs to detect hemorrhages, exudates, and other lesions, and those detected lesion results were used to classify referable DR by a classic feature fusion model. Differently, our DeepDR network was trained end-to-end with the features extracted from both the lesion-aware sub-network and the original image. In this way, our DR grading sub-network can further exploit the features to minimize the training error, thus improving grading results. As a result, DeepDR achieved a sensitivity of 88.8% and specificity of 83.9% for mild NPDR detection on the local validation dataset. Notably, DeepDR achieved the diagnosis of all stages of DR with sufficient accuracy in real-word datasets.
Despite the continuous optimization in digital fundus cameras, aging, experience, lighting, and other non-biological factors resulting from improper operation still results in high percentage of low-quality fundus images, and reacquisition is time-consuming and sometimes impossible 51 , 52 . Previous studies on image quality assessment have focused on post hoc image data processing 21 , 22 . In this study, a real-time image quality feedback sub-network was implemented to facilitate the DR screening. Based on the feedback information, the artificial intelligence-assisted image quality assessment can reduce the proportion of poor-quality images from 28.7% to 8.2%. Furthermore, with the improvement of image quality, the diagnostic accuracy was significantly improved, especially for mild DR. This real-time image quality feedback function allows the operators to identify image quality issue immediately and the patient does not need to be called back. It is a promising tool to reduce ungradable rate of the fundus images, thus increasing the efficiency of DR screening.
The limitation of this study is, firstly, the single-ethnic cohort used to develop the system. However, we used the publicly available EyePACS dataset from the United States for external validation and achieved satisfactory sensitivity and specificity. Secondly, the lesion-aware sub-network was tested only on the local validation dataset, because of the lack of lesion annotations in external cohorts. Further external validation in multiethnic and multicenter cohorts is needed to confirm the robustness of lesion detection and DR grading of the DeepDR system.
In conclusion, we developed an automated, interpretable, and validated system that performs real-time image quality feedback, retinal lesion detection, and early- to late-stage DR grading. With those functions, DeepDR system is able to improve image collection quality, provide clinical reference, and facilitate DR screening. Further studies are needed to evaluate deep learning system in detecting and predicting DR progression.
Ethical approval
The study was approved by the Ethics Committee of Shanghai Sixth People’s Hospital and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from participants. The study was registered on the Chinese Clinical Trials Registry (ChiCTR.org.cn) under the identifier ChiCTR2000031184.
Image acquisition and reading process
In the SIM project, retinal photographs were captured using desktop retinal cameras from Canon, Topcon, and ZEISS (Supplementary Table 1 ). All the fundus cameras were qualified by the organizer to ensure enough quality for DR grading. The operators of the cameras had all received standard training and the images were read by a centered reading group consisting of 133 certified ophthalmologists. The members in the reading group underwent training by fundus specialists and passed the tests. Original retinal images were uploaded to the online platform, and the images of each eye were assigned separately to two authorized ophthalmologists. They labeled the images using an online reading platform and gave the graded diagnosis of DR (Supplementary Fig. 2 ). The third ophthalmologist who served as the senior supervisor confirmed or corrected when the diagnostic results were contradictory. The final grading result was dependent on the consistency within these three ophthalmologists. At least 20% of the grading results would be randomly re-read to check the consistency. The total eligibility rate of spot-check was equal to or greater than 90%. If the reading group encountered difficult cases, they could apply for consultation from superior medical institutions. The overall disagreement rate in the SIM dataset was 18.9%. The primary cause of the diagnostic divergence was the decision between mild NPDR and non-DR.
For retinal lesion annotation, each fundus image was annotated by two ophthalmologists. For each type of lesion, two ophthalmologists generated two lesion annotations, respectively. We considered the two annotations to be valid if the IoU between them was greater than 0.85. Otherwise, a senior supervisor would check the annotations and give feedback to provide guidance. The image would be re-annotated by the two ophthalmologists until the IoU was larger than 0.85. Finally, we took the union of valid annotations as final ground truth segmentation annotation.
Diagnostic criteria
DR severity was graded into five levels (non-DR, mild NPDR, moderate NPDR, severe NPDR, or PDR, respectively), according to the International Clinical Diabetic Retinopathy Disease Severity Scale (AAO, October 2002) 53 . Mild NPDR was defined as the presence of microaneurysms only. Moderate NPDR was defined as more than just microaneurysms but less than severe NPDR, presenting CWS, hard exudates, and/or retinal hemorrhages. Severe NPDR was defined as any of the following: more than 20 intraretinal hemorrhages in each of the 4 quadrants; definite venous beading in 2+ quadrants; prominent intraretinal microvascular abnormalities (IRMA) in 1+ quadrant, and no signs of PDR. PDR was defined as one or more of the following: neovascularization, vitreous/preretinal hemorrhage 53 . DME was diagnosed if hard exudates were detected within 500 μm of the macular center according to the standard of the Early Treatment for Diabetic Retinopathy study 54 . Referable DR was defined as moderate NPDR or worse, DME, or both. Based on the guidelines for image acquisition and interpretation of diabetic retinopathy screening in China 55 , the image quality was graded according to standards defined in terms of three quality factors, artifacts, clarity, and field definition 56 , as listed in Table 5 . The total score was equal to the score for clarity plus the score for field definition and minus the score for artifacts. A total score less than 12 was considered as ungradable.
Architecture of the DeepDR system
The DeepDR system had three sub-networks: image quality assessment sub-network, lesion-aware sub-network, and DR grading sub-network. Those sub-networks were developed based on ResNet 41 and Mask-RCNN 57 . Both ResNet and Mask-RCNN could be divided into two parts: (1) feature extractor, which took images as input and output features, (2) task-specific header, which took the features as input and generated task-specific outputs (i.e., classification or segmentation). Specifically, we chose to use the Mask-RCNN and ResNet with the same feature extractor architecture, so the feature extractor of one sub-network can be easily transferred to another.
The quality assessment sub-network can identify overall quality including gradability, artifacts, clarity, and field issues for the input images. To train the image quality assessment sub-network effectively, we initialized a ResNet with weights pre-trained on ImageNet and pre-trained the ResNet to form the DR base network. We utilized the weights of the convolution layers in the pre-trained DR base network to initialize the feature extractor of the image quality assessment sub-network. We assessed image quality in terms of multiple factors to determine if: (a) the artifact covered the macular area or the area of artifact was larger than a quadrant of the retinal image; (b) only Level II or wider vascular arch and obvious lesions could be identified (Level II vascular arch was defined as the veins deriving from the first bifurcation); (c) no optic disc or macula was contained in the image; and (d) the image was not gradable.
The lesion-aware sub-network can generate lesion presence and lesion segmentation masks of the input images. There were two modules in our lesion-aware sub-network: one was the lesion detection module and the other was the lesion segmentation module. The lesion detection module was a binary classifier that predicted whether any kind of lesions exist in a quadrant of the retinal image, as shown in Supplementary Fig. 3 . The lesion segmentation module generated mask images to identify different lesions existing in the retinal images, as shown in Fig. 3B . We used ResNet and Mask-RCNN to form the lesion detection module and lesion segmentation module, respectively. Then we transferred the pre-trained DR base network to the lesion detection module by initializing the feature extractor of lesion detection module using the feature extractor of pre-trained DR base network, followed by fine-tuning the lesion detection module. Then we initialized the feature extractor of lesion segmentation module by reusing the feature extractor of the lesion detection module. The feature extractor layers of the lesion segmentation module were then fixed, and the rest of the layers of the module were updated during training. Non-maximum suppression was used in our lesion segmentation sub-module to select the bounding box with the highest objectiveness score from multiple predicted bounding boxes. Specifically, we first selected the bounding box with the highest objectiveness score, and then compared the IoU of this bounding box with other bounding boxes and removed the bounding boxes with IoU > 0.5. Finally, we moved to the next box with the highest objectiveness score and repeated until all boxes were either removed or selected.
The DR grading sub-network can fuse features from lesion-aware network and generate final DR grading results. To retain as much lesion information from the original retinal image as possible, we combined the pre-trained DR base network with the feature extractor of the lesion segmentation module in order to capture more detailed lesion features for DR grading. Then the weights in the extractors of DR grading sub-network were fixed, and the classification header of sub-network was updated during training.
The transfer learning assisted multi-task network was developed in our DeepDR architecture to improve the performance of DR grading based on lesion detection and segmentation. Due to the fact that DR grading inherently relies on the global presences of retinal lesions that contain multi-scale local texture and structures, the central feature of our multi-task learning method was designed to extract multi-scale features encoding local textures and structures of retinal lesions, where the transfer learning was used to improve the performance of DR grading task. Meanwhile, we used hard-parameter sharing in lesion-aware sub-network, and all the layers in the feature extractors of ResNet and Mask-RCNN are shared. Using hard-parameter sharing was important to reduce the risk of overfitting 58 due to the limited number of lesion segmentation labels. Besides, sharing the pre-trained weights can facilitate the training of both lesion detection task and lesion segmentation task. Additional experimental results demonstrated that hard-parameter sharing outperformed soft-parameter sharing for lesion segmentation is shown in Supplementary Table 3 .
Recommended computer configuration
Any desktop or laptop computer with x86 compatible CPU, 10 GB or more of free disk space, and at least 8 GB main memory is capable to run the DeepDR system. There is no specialized hardware requirement, including GPU or any speed up card, to run the software. A powerful computer with more CPU cores and a GPU will speed up the diagnosis procedure significantly, while the diagnosis time on a typical laptop (i.e., with Intel I3 processor, no GPU, more than 8 GB memory) is also acceptable (less than 20 s per image).
Statistical analyses
The performances of DeepDR in assessing image quality, retinal lesion detection, and grading DR were measured by the AUC of the receiver operating characteristic curve generated by plotting sensitivity (the true-positive rate) versus 1-specificity (the false-negative rate). The operating thresholds for sensitivity and specificity were selected using the Youden index. The AUCs were compared using binormal model methods 59 , where a two-sided P value of less than 0.05 was considered statistically significant. For lesion detection, AUC was calculated as a binary classification to determine if a quadrant contained a certain kind of lesion. The performance of lesion segmentation was measured by IoU and F -score.
For CWS, hard exudates, and hemorrhages, we used IoU to measure the performance of segmentation network. The IoU was calculated as:
where \({\rm{A}}\) and \({\rm{B}}\) were set of pixels in the retinal images (e.g., A was the segmented lesion and B was the ground truth).
For microaneurysms, the F -score was used instead of the IoU score, because the average diameter of microaneurysms in the retinal image was usually less than 30 pixels, minor change in the predicted map would result in a large change in IoU score. F -score was calculated as:
\({\rm{P}}\) was the set of all predicted microaneurysms produced by the network, \({\rm{G}}\) was the set of all microaneurysms annotated by ophthalmologists. \({\rm{tp}}=\left({\bf{p}}\in {\bf{P}},|,\exists {\bf{g}}\in {\bf{G}},{\rm{IoU}}({\bf{p}},{\bf{g}})\ge 0.5\right)\) represented the set true-positive predicts of microaneurysms, \({\rm{fp}}=\left({\bf{p}}\in {\bf{P}},|,\forall {\bf{g}}\in {\bf{G}},{\rm{IoU}}({\bf{p}},{\bf{g}}) < 0.5\right)\) represented the set false-positive predicts of microaneurysms, \({\rm{fn}}=\left({\bf{g}}\in {\bf{G}},|,\forall {\bf{p}}\in {\bf{P}},{\rm{IoU}}({\bf{p}},{\bf{g}}) < 0.5\right)\) represented the set of false-negative predictions of microaneurysms. \(\left|\bullet \right|\) represented the cardinality (size) of a set.
Python version 3.7.1 (Python Software Foundation, Delaware, USA) was used for all statistical analyses in this study. The following third-party python packages were used: OpenCV version 2.4.3 (Intel Corporation, California, USA) was used for image loading and decoding image. Pytorch version 1.0.1 (Facebook, Massachusetts, USA) was used for convolutional neural network computing. Scikit-learn version 0.20.0 (David Cournapeau, California, USA) was used for calculating AUC. Pandas version 0.23.4 (Wes McKinney, Connecticut, USA) was used for loading ground truth and metadata. NumPy version 1.15.4 (Travis Oliphant, Texas, USA) was used for calculating IoU and F -score.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The export of human-related data is governed by the Ministry of Science and Technology of China (MOST) in accordance with the Regulations of the People’s Republic of China on Administration of Human Genetic Resources (State Council No.717). Request for the non-profit use of the fundus images and related clinical information in the SIM, NDSP, and CNDCS cohorts should be sent to corresponding author Weiping Jia. The joint application of the corresponding author together with the requester for the data sharing will be generated and submitted to MOST. The data will be provided to the requester after the approval from MOST. The EyePACS dataset is publicly available at https://www.kaggle.com/c/diabetic-retinopathy-detection/data . The rest of the data are available from the corresponding author upon reasonable request.
Code availability
The code being used in this study for developing the algorithm is provided at https://zenodo.org/badge/latestdoi/334570111 .
International Diabetes Federation. IDF Diabetes Atlas 7th edn (International Diabetes Federation, Brussels, Belgium, 2015).
American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care 43 , S135–S151 (2020).
Article Google Scholar
Wang, L. Z. et al. Availability and variability in guidelines on diabetic retinopathy screening in Asian countries. Br. J. Ophthalmol. 101 , 1352–1360 (2017).
Article PubMed Google Scholar
Liew, G., Michaelides, M. & Bunce, C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 4 , e004015 (2014).
Article PubMed PubMed Central Google Scholar
Kristinsson, J. K., Hauksdottir, H., Stefansson, E., Jónasson, F. & Gíslason, I. Active prevention in diabetic eye disease: a 4-year follow-up. Acta Ophthalmol. Scand. 75 , 249–254 (1997).
Article CAS PubMed Google Scholar
Ruta, L. et al. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet. Med. 30 , 387–398 (2013).
Kung, K. et al. Prevalence of complications among Chinese diabetic patients in urban primary care clinics: a cross-sectional study. BMC Fam. Pract. 15 , 8 (2014).
Hu, Y. et al. Prevalence and risk factors of diabetes and diabetic retinopathy in Liaoning province, China: a population-based cross-sectional study. PLoS ONE 10 , e0121477 (2015).
Article PubMed PubMed Central CAS Google Scholar
Pang, C. et al. Determination of diabetic retinopathy prevalence and associated risk factors in Chinese diabetic and pre-diabetic subjects: Shanghai diabetic complications study. Diabetes Metab. Res. Rev. 28 , 276–283 (2012).
Lian, J. X. et al. Systematic screening for diabetic retinopathy (DR) in Hong Kong: prevalence of DR and visual impairment among diabetic population. Br. J. Ophthalmol. 100 , 151–155 (2015).
Ting, D. S. W., Cheung, G. C. M. & Wong, T. Y. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin. Exp. Ophthalmol. 44 , 260–277 (2016).
Hu, C. & Jia, W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes 67 , 3–11 (2018).
Li, L. & Fu, H. China’s health care system reform: progress and prospects. Int. J. Health Plann. Manage. 32 , 240–253 (2017).
World Health Organization. Treat Train Retain. Task Shifting: Global Recommendations and Guidelines (WHO, Geneva, 2008).
Silver, D. et al. Mastering the game of Go without human knowledge. Nature 550 , 354–359 (2017).
Article ADS CAS PubMed Google Scholar
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521 , 436–444 (2015).
Shen, D., Wu, G. & Suk, H.-I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 19 , 221–248 (2017).
Article CAS PubMed PubMed Central Google Scholar
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542 , 115–118 (2017).
Article ADS CAS PubMed PubMed Central Google Scholar
Abràmoff, M. et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest. Ophthalmol. Vis. Sci. 57 , 5200–5206 (2016).
Gargeya, R. & Leng, T. Automated identification of diabetic retinopathy using deep learning. Ophthalmology 124 , 962–969 (2017).
Gulshan, V. et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 316 , 2402–2410 (2016).
Ting, D. S. W. et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 318 , 2211–2223 (2017).
van der Heijden, A. A. et al. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System. Acta Ophthalmol. 96 , 63–68 (2018).
Li, Z. et al. An automated grading system for detection of vision-threatening referable diabetic retinopathy on the basis of color fundus photographs. Diabetes Care 41 , 2509–2516 (2018).
Article ADS PubMed Google Scholar
Liu, Y. et al. Glycemic exposure and blood pressure influencing progression and remission of diabetic retinopathy: a longitudinal cohort study in GoDARTS. Diabetes Care 36 , 3979–3984 (2013).
Kendall, A., Gal, Y. & Cipolla, R. Multi-task learning using uncertainty to weigh losses for scene geometry and semantics. In Proc. IEEE Conference on Computer Vision and Pattern Recognition (IEEE, 2018).
Wang, X., Ju, L., Zhao, X. & Ge, Z. Retinal abnormalities recognition using regional multitask learning. In International Conference on Medical Image Computing and Computer-Assisted Intervention (eds Shen, D. et al.) 30–38 (Springer, 2019).
Playout, C., Duval, R. & Cheriet, F. A novel weakly supervised multitask architecture for retinal lesions segmentation on fundus images. IEEE Trans. Med. Imaging 38 , 2434–2444 (2019).
Yosinski, J., Clune, J., Bengio, Y. & Lipson, H. How transferable are features in deep neural networks? In International Conference on Neural Information Processing Systems 3320–3328 (NIPS, 2014).
Scanlon, P. H. et al. Comparison of two reference standards in validating two field mydriatic digital photography as a method of screening for diabetic retinopathy. Br. J. Ophthalmol. 87 , 1258–1263 (2003).
World Health Organization. Prevention of Blindness from Diabetes Mellitus. Report of a WHO consultation in Geneva, Switzerland, 9–11 November 2005 (World Health Organization, 2006).
Kermany, D. S. et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 172 , 1122–1131 e1129 (2018).
Caruana, R., Lawrence, S. & Giles, C. L. Overfitting in neural nets: backpropagation, conjugate gradient, and early stopping. In Advances in Neural Information Processing Systems 402–408 (NIPS, 2001).
Bakri, S. J. & Kaiser, P. K. Posterior subtenon triamcinolone acetonide for refractory diabetic macular edema. Am. J. Ophthalmol. 139 , 290–294 (2005).
Group, A. S. & AES., Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 363 , 233–244 (2010).
Article CAS Google Scholar
Rodriguez-Gutierrez, R., Gonzalez-Gonzalez, J. G., Zuñiga-Hernandez, J. A. & McCoy, R. G. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ 367 , l5887 (2019).
Qaseem, A. et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann. Intern. Med. 168 , 569–576 (2018).
American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes—2020. Diabetes Care 43 , S66–S76 (2020).
Flaxel, C. J. et al. Diabetic retinopathy preferred practice pattern ® . Ophthalmology 127 , P66–P145 (2020).
Eftekhari, N., Pourreza, H.-R., Masoudi, M., Ghiasi-Shirazi, K. & Saeedi, E. Microaneurysm detection in fundus images using a two-step convolutional neural network. Biomed. Eng. Online 18 , 67 (2019).
He, K., Zhang, X., Ren, S. & Sun, J. Deep residual learning for image recognition. In Proc. IEEE Conference on Computer Vision and Pattern Recognition 770–778 (IEEE, 2016).
Lin T.-Y. et al. Feature pyramid networks for object detection. In Proc. IEEE Conference on Computer Vision and Pattern Recognition 2117–2125 (IEEE, 2017).
Keech, A. C. et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370 , 1687–1697 (2007).
Ying, G.-s. et al. Association between antiplatelet or anticoagulant drugs and retinal or subretinal hemorrhage in the comparison of age-related macular degeneration treatments trials. Ophthalmology 123 , 352–360 (2016).
Ribeiro, M. L., Nunes, S. G. & Cunha-Vaz, J. G. Microaneurysm turnover at the macula predicts risk of development of clinically significant macular edema in persons with mild nonproliferative diabetic retinopathy. Diabetes Care 36 , 1254–1259 (2013).
Hove, M. N., Kristensen, J. K., Lauritzen, T. & Bek, T. Quantitative analysis of retinopathy in type 2 diabetes: identification of prognostic parameters for developing visual loss secondary to diabetic maculopathy. Acta Ophthalmol. Scand. 82 , 679–685 (2004).
Klein, R., Klein, B. E. & Moss, S. E. How many steps of progression of diabetic retinopathy are meaningful? The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch. Ophthalmol. 119 , 547–553 (2001).
Papernot, N. et al. Practical black-box attacks against machine learning. In Proc. 2017 ACM on Asia Conference on Computer and Communications Security 506–519 (ACM, 2017).
Gülçehre, Ç. & Bengio, Y. Knowledge matters: importance of prior information for optimization. J. Mach. Learn. Res. 17 , 226–257 (2016).
MathSciNet MATH Google Scholar
Abràmoff, M. D., Lavin, P. T., Birch, M., Shah, N. & Folk, J. C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. npj Digital Med. 1 , 1–8 (2018).
Fleming, A. D., Philip, S., Goatman, K. A., Olson, J. A. & Sharp, P. F. Automated assessment of diabetic retinal image quality based on clarity and field definition. Invest. Ophthalmol. Vis. Sci. 47 , 1120–1125 (2006).
Boucher, M. C., Gresset, J. A., Angioi, K. & Olivier, S. Effectiveness and safety of screening for diabetic retinopathy with two nonmydriatic digital images compared with the seven standard stereoscopic photographic fields. Can. J. Ophthalmol. 38 , 557–568 (2003).
Wilkinson, C. et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110 , 1677–1682 (2003).
Group ETDRSR. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 2. Ophthalmology 94 , 761–774 (1987).
Fundus disease Group in Ophthalmology Branch of Chinese Medical Association. Guidelines of retinal image acquisition and reading for diabetic retinopathy screening in China. Chin. J. Ophthalmol. 53 , 890–896 (2017).
Google Scholar
Shen, Y. et al. Domain-invariant interpretable fundus image quality assessment. Med. Image Anal. 61 , 101654 (2020).
He, K., Gkioxari, G., Dollár, P. & Girshick, R. Mask R-CNN . In Proc. IEEE International Conference on Computer Vision 2980–2988 (IEEE, 2017).
Ruder S. An overview of multi-task learning in deep neural networks. Preprint at https://arxiv.org/abs/1706.05098 (2017).
McClish, D. K. Analyzing a portion of the ROC curve. Med. Decis. Making 9 , 190–195 (1989).
Download references
Acknowledgements
We would like to thank all the medical staffs and study subjects who participated in the Shanghai Integrated Diabetes Prevention and Care System Study. This work was supported by Shanghai Municipal Grants Award (GWIV-3), National Natural Science Foundation of China (NSFC) - National Health and Medical Research Council of Australia (NHMRC) joint research grant (81561128016), Shanghai Belt and Road Joint Laboratory of Intelligent Diagnosis and Treatment of Metabolic Diseases (18410750700), Science and Technology Innovation Action Plan from Shanghai Science and Technology Commission (17411952600) and Shanghai Municipal Key Clinical Specialty to W.J., and General Project from NSFC (61872241) to B.S., and Science and Technology Innovation Action Plan from Shanghai Science and Technology Commission (16DZ0501100) to Q.W.
Author information
These authors contributed equally: Ling Dai, Liang Wu, Huating Li, Chun Cai, Qiang Wu.
Authors and Affiliations
Department of Computer Science and Engineering, Shanghai Jiao Tong University, Shanghai, 200240, China
Ling Dai, Ruhan Liu, Yang Wen, Yaxin Shen & Bin Sheng
Department of Endocrinology and Metabolism, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai Diabetes Institute, Shanghai Clinical Center for Diabetes, Shanghai, 200233, China
Ling Dai, Liang Wu, Huating Li, Chun Cai, Xuhong Hou, Yuexing Liu, Xiaoxue Long & Weiping Jia
MoE Key Lab of Artificial Intelligence, Artificial Intelligence Institute, Shanghai Jiao Tong University, Shanghai, 200240, China
Department of Ophthalmology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, 200233, China
Qiang Wu, Hongyu Kong, Xiangning Wang & Yan Chen
Department of Ophthalmology, Shanghai General Hospital, Shanghai Jiao Tong University, Shanghai Eye Diseases Prevention and Treatment Center, Shanghai Eye Hospital, Shanghai Engineering Center for Precise Diagnosis and Treatment of Eye Diseases, Shanghai, 200040, China
Lina Lu & Haidong Zou
School of Biomedical Engineering, Shanghai Tech University, Shanghai, China
Dinggang Shen
Shanghai United Imaging Intelligence Co., Ltd., Shanghai, China
Shanghai Institute for Advanced Communication and Data Science, Shanghai Key Laboratory of Digital Media Processing and Transmission, Shanghai Jiao Tong University, Shanghai, 200240, China
Xiaokang Yang
You can also search for this author in PubMed Google Scholar
Contributions
W.J., B.S., and H.Z. conceived and oversaw overall direction. L.D. designed the deep learning algorithm and the computational framework. L.W., H.L., and L.D. designed the study, interpreted the results and drafted the manuscript. C.C., Q.W., H.K., X.W., X.H., Y.L., L.L., and X.L. collected and organized data. Y.S., Y.W., and R.L contributed to data analysis. Q.W., Y.C., D.S., and X.Y. provided critical comments and reviewed the manuscript.
Corresponding authors
Correspondence to Haidong Zou , Bin Sheng or Weiping Jia .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Tunde Peto and Dale Webster for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Dai, L., Wu, L., Li, H. et al. A deep learning system for detecting diabetic retinopathy across the disease spectrum. Nat Commun 12 , 3242 (2021). https://doi.org/10.1038/s41467-021-23458-5
Download citation
Received : 18 December 2019
Accepted : 29 April 2021
Published : 28 May 2021
DOI : https://doi.org/10.1038/s41467-021-23458-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Development and evaluation of a deep learning model for automatic segmentation of non-perfusion area in fundus fluorescein angiography.
- Bingjie Wang
Journal of Big Data (2024)
Novel artificial intelligence algorithms for diabetic retinopathy and diabetic macular edema
- Daniel Shu Wei Ting
Eye and Vision (2024)
Deep learning-based algorithm for the detection of idiopathic full thickness macular holes in spectral domain optical coherence tomography
- Carolina C. S. Valentim
- Katherine E. Talcott
International Journal of Retina and Vitreous (2024)
Fairer AI in ophthalmology via implicit fairness learning for mitigating sexism and ageism
- Qiaoling Wei
Nature Communications (2024)
Deep learning predicts prevalent and incident Parkinson’s disease from UK Biobank fundus imaging
- Charlie Tran
Scientific Reports (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed, diabetic retinopathy, affiliations.
- 1 Centre for Sight new delhi
- 2 ASG Eye Hospital, BT Road, Kolkata, India
- PMID: 32809640
- Bookshelf ID: NBK560805
Diabetic retinopathy (DR) is a microvascular disorder occurring due to the long-term effects of diabetes mellitus. Diabetic retinopathy may lead to vision-threatening damage to the retina, eventually leading to blindness. It is the most common cause of severe vision loss in adults of working age groups in the western world. Early detection and timely intervention are the keys to avoiding blindness due to diabetic retinopathy. The number of patients with diabetic retinopathy in America is estimated to reach 16.0 million by 2050, with vision-threatening complications affecting around 3.4 million of them. The usefulness of strict glycemic control was clearly seen in clinical trials like the UK Prospective Diabetes Study (UKPDS) and Diabetes Control and Complication Trial (DCCT).
Uncontrolled diabetes can lead to many ocular disorders like cataracts, glaucoma, ocular surface disorders, recurrent stye, non-arteritic anterior ischemic optic neuropathy, diabetic papillopathy, and diabetic retinopathy. Diabetic retinopathy may lead to vision-threatening damage to the retina, eventually leading to blindness; it is the most common and severe ocular complication. Poor glycemic control, uncontrolled hypertension, dyslipidemia, nephropathy, male sex, and obesity are associated with worsening diabetic retinopathy. Typical fundus features of diabetic retinopathy include microaneurysms, hard exudates, macular edema (diabetic macular edema or DME), and new vessels (in proliferative DR or PDR). The management options include strict control of the systemic conditions, intravitreal pharmacotherapy, and laser photocoagulation. With early diagnosis and prompt management, good final visual acuity may be achieved in most patients with DR.
Copyright © 2024, StatPearls Publishing LLC.
PubMed Disclaimer
Conflict of interest statement
Disclosure: Unnati Shukla declares no relevant financial relationships with ineligible companies.
Disclosure: Koushik Tripathy declares no relevant financial relationships with ineligible companies.
- Continuing Education Activity
- Introduction
- Epidemiology
- Pathophysiology
- Histopathology
- History and Physical
- Treatment / Management
- Differential Diagnosis
- Complications
- Postoperative and Rehabilitation Care
- Deterrence and Patient Education
- Enhancing Healthcare Team Outcomes
- Review Questions
Similar articles
- Epidemiology of Ocular Functions and Diseases in Persons With Diabetes. Klein R, Klein BEK. Klein R, et al. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, editors. Diabetes in America. 3rd edition. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018 Aug. CHAPTER 21. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, editors. Diabetes in America. 3rd edition. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018 Aug. CHAPTER 21. PMID: 33651534 Free Books & Documents. Review.
- Laser Treatment Modalities for Diabetic Retinopathy. Pande GS, Tidake P. Pande GS, et al. Cureus. 2022 Oct 7;14(10):e30024. doi: 10.7759/cureus.30024. eCollection 2022 Oct. Cureus. 2022. PMID: 36348830 Free PMC article. Review.
- [Diabetic retinopathy]. Barth T, Helbig H. Barth T, et al. Klin Monbl Augenheilkd. 2021 Oct;238(10):1143-1159. doi: 10.1055/a-1545-9927. Epub 2021 Aug 11. Klin Monbl Augenheilkd. 2021. PMID: 34380155 German.
- The Evolving Treatment of Diabetic Retinopathy. Mansour SE, Browning DJ, Wong K, Flynn HW Jr, Bhavsar AR. Mansour SE, et al. Clin Ophthalmol. 2020 Mar 4;14:653-678. doi: 10.2147/OPTH.S236637. eCollection 2020. Clin Ophthalmol. 2020. PMID: 32184554 Free PMC article. Review.
- Epidemiology of Diabetic Retinopathy at Eye Clinic Svjetlost Sarajevo: Two Years Retrospective Single Center Study. Pidro A, Ahmedbegovic-Pjano M, Grisevic S, Sofic-Drino V, Gabric K, Biscevic A. Pidro A, et al. Mater Sociomed. 2019 Dec;31(4):290-293. doi: 10.5455/msm.2019.31.290-293. Mater Sociomed. 2019. PMID: 32082096 Free PMC article.
- Eisma JH, Dulle JE, Fort PE. Current knowledge on diabetic retinopathy from human donor tissues. World J Diabetes. 2015 Mar 15;6(2):312-20. - PMC - PubMed
- Hendrick AM, Gibson MV, Kulshreshtha A. Diabetic Retinopathy. Prim Care. 2015 Sep;42(3):451-64. - PubMed
- Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993 Sep 30;329(14):977-86. - PubMed
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837-53. - PubMed
- Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes. 2015 Feb 15;6(1):92-108. - PMC - PubMed
Publication types
- Search in PubMed
- Search in MeSH
- Add to Search
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- NCBI Bookshelf

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Advertisement
Diabetic retinopathy: emerging concepts of current and potential therapy
- Published: 04 July 2023
- Volume 396 , pages 3395–3406, ( 2023 )
Cite this article

- Muhammad Zulfiqah Sadikan 1 &
- Nurul Alimah Abdul Nasir 2 , 3
911 Accesses
5 Citations
Explore all metrics
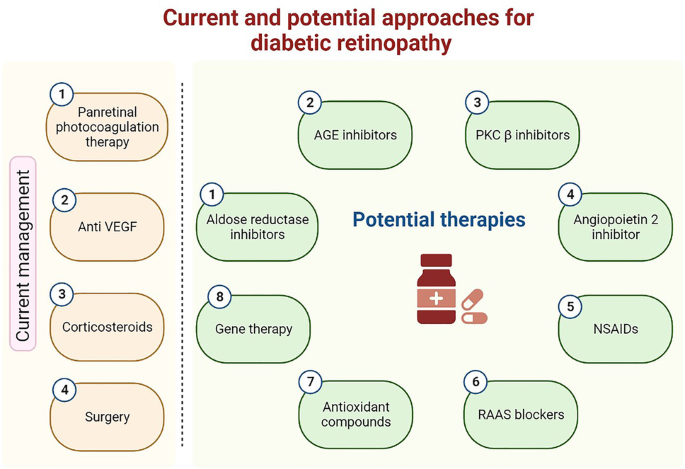
Diabetic retinopathy (DR) is one of the leading causes of permanent central blindness worldwide. Despite the complexity and inadequate understanding of DR pathogenesis, many of the underlying pathways are currently partially understood and may offer potential targets for future treatments. Anti-VEGF medications are currently the main medication for this problem. This article provides an overview of the established pharmacological treatments and those that are being developed to cure DR. We firstly reviewed the widely utilized approaches including pan-retinal photocoagulation therapy, anti-VEGF therapy, corticosteroid therapy, and surgical management of DR. Next, we discussed the mechanisms of action and prospective benefits of novel candidate medications. Current management are far from being a perfect treatment for DR, despite mild-term favorable efficiency and safety profiles. Pharmacological research should work toward developing longer-lasting treatments or new drug delivery systems, as well as on identifying new molecular targets in the pathogenetical mechanism for DR. In order to find a treatment that is specifically designed for each patient, it is also necessary to properly characterize patients, taking into account elements like hereditary factors and intraretinal neovascularization stages for effective utilization of drugs.
Graphical abstract
The current and potential approaches for diabetic retinopathy. Image was constructed using Biorender.com
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Emerging Concepts in the Treatment of Diabetic Retinopathy

Perspectives of diabetic retinopathy—challenges and opportunities

Proliferative Diabetic Retinopathy: Treatment Update
Data availability.
This is not applicable.
Abdou DM, Mohammed NS, El Fouli M, Medaney HA, El Kateb SM, El-Gabrty SA (2021) The association of manganese superoxide dismutase gene polymorphism (Rs4880) with diabetic macular edema in a cohort of type 2 diabetic Egyptian patients. Egypt J Med Hum Genet 22:1–7
Article Google Scholar
Abdul Nasir NA, Sadikan MZ, Agarwal R (2021) Modulation of NFκB signalling pathway by tocotrienol: A systematic review. Asia Pac J Clin Nutr 30(3):537–555
CAS Google Scholar
Abdul Ghani NA, Abdul Nasir NA, Lambuk L, Sadikan MZ, Agarwal R, Ramli N (2023) The effect of palm oil-derived tocotrienol-rich fraction in preserving normal retinal vascular diameter in streptozotocin-induced diabetic rats. Graefe’s Arch Clin Exp Ophthalmol 2023:1–10
Google Scholar
Adhi M, Cashman SM, Kumar-Singh R (2013) Adeno-associated virus mediated delivery of a non-membrane targeted human soluble CD59 attenuates some aspects of diabetic retinopathy in mice. PLoS ONE 8:e79661
Article CAS PubMed PubMed Central Google Scholar
Ahiskali I, Pinar CL, Kiki M, Mammadov R, Ozbek Bilgin A, Hacimuftuoglu A, Cankaya M, Keskin Cimen F, Altuner D (2019) Effect of taxifolin on development of retinopathy in alloxan-induced diabetic rats. Cutan Ocul Toxicol 38:227–232
Article CAS PubMed Google Scholar
Ahmad S, ElSherbiny NM, Jamal MS, Alzahrani FA, Haque R, Khan R, Zaidi SK, AlQahtani MH, Liou GI, Bhatia K (2016) Anti-inflammatory role of sesamin in STZ induced mice model of diabetic retinopathy. J Neuroimmunol 295:47–53
Article PubMed Google Scholar
Aiello LP, Bursell S-E, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M (1997) Vascular endothelial growth factor–induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective β-isoform–selective inhibitor. Diabetes 46:1473–1480
Aiello LP, Beck RW, Bressler NM, Browning DJ, Chalam K, Davis M, Ferris FL III, Glassman AR, Maturi RK, Stockdale CR (2011) Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema. Ophthalmol 118:e5–e14
Al-Dosari DI, Ahmed MM, Al-Rejaie SS, Alhomida AS, Ola MS (2017) Flavonoid naringenin attenuates oxidative stress, apoptosis and improves neurotrophic effects in the diabetic rat retina. Nutrients 9:1161
Article PubMed PubMed Central Google Scholar
Al-Mesallamy HO, Hammad LN, El-Mamoun TA, Khalil BM (2011) Role of advanced glycation end product receptors in the pathogenesis of diabetic retinopathy. J Diabetes Complications 25:168–174
Arevalo JF, Garcia-Amaris RA (2009) Intravitreal bevacizumab for diabetic retinopathy. Curr Diabetes Rev 5:39–46
Arevalo JF, Lasave AF, Wu L, Maia M, Diaz-Llopis M, Alezzandrini AA, Brito M (2017) Intravitreal bevacizumab for proliferative diabetic retinopathy. Retina 37:334–343
Arevalo JF, Liu TA, Group P-ACRS (2018) Intravitreal bevacizumab in diabetic retinopathy. recommendations from the Pan-American Collaborative Retina Study Group (PACORES): The 2016 Knobloch Lecture. Asia-Pac J Ophthalmol 7:36-39
Ayalasomayajula SP, Ashton P, Kompella UB (2009) Fluocinolone inhibits VEGF expression via glucocorticoid receptor in human retinal pigment epithelial (ARPE-19) cells and TNF-α–induced angiogenesis in chick chorioallantoic membrane (CAM). J Ocul Pharmacol Ther 25:97–104
Baptista PM, Marta AA, Heitor J, José D, Almeida D, Ribeiro A, Barbosa I (2021) Long-Term visual function effects of pan-retinal photocoagulation in diabetic retinopathy and its impact in real life. Diabetes Metab Syndr Obes Targets Ther 2021:1281
Barber AJ (2003) A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuro-Psychopharmacol Biol Psychiatry 27(2):283–290. https://doi.org/10.1016/S0278-5846(03)00023-X
Article CAS Google Scholar
Barber AJ, Gardner TW, Abcouwer SF (2011) The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol vis Sci 52:1156–1163
Behl T, Kotwani A (2015a) Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res 99:137–148
Behl T, Kotwani A (2015b) Possible role of endostatin in the antiangiogenic therapy of diabetic retinopathy. Life Sci 135:131–137
Behl T, Kotwani A (2017) Downregulated brain-derived neurotrophic factor-induced oxidative stress in the pathophysiology of diabetic retinopathy. Can J Diabetes 41:241–246
Bolinger MT, Antonetti DA (2016) Moving past anti-VEGF: novel therapies for treating diabetic retinopathy. Int J Mol Sci 17:1498
Bonnin S, Sandali O, Bonnel S, Monin C, El Sanharawi M (2015) Vitrectomy with internal limiting membrane peeling for tractional and nontractional diabetic macular edema: long-term results of a comparative study. Retina 35:921–928
Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ, Li X-Y, Cui H, Hashad Y, Whitcup SM (2014) Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 121:1904–1914
Bressler NM, Beaulieu WT, Bressler SB, Glassman AR, Melia BM, Jampol LM, Jhaveri CD, Salehi-Had H, Velez G, Sun JK (2020) Anti-vascular endothelial growth factor therapy and risk of traction retinal detachment in eyes with proliferative diabetic retinopathy: Pooled Analysis of Five DRCR Retina Network Randomized Clinical Trials. Retina (Philadelphia, Pa) 40:1021
Brown DM, Ou WC, Wong TP, Kim RY, Croft DE, Wykoff CC, Group DS (2018) Targeted retinal photocoagulation for diabetic macular edema with peripheral retinal nonperfusion: three-year randomized DAVE trial. Ophthalmol 125:683-690
Browning DJ, Lee C, Stewart MW, Landers MB III (2016) Vitrectomy for center-involved diabetic macular edema. Clin Ophthalmol (Auckland, NZ) 10:735
Cáceres-del-Carpio J, Costa RD, Haider A, Narayanan R, Kuppermann BD (2016) Corticosteroids: triamcinolone, dexamethasone and fluocinolone. Retinal Pharmacotherapeutics. Karger Publishers, pp 221–231
Cahoon JM, Rai RR, Carroll LS, Uehara H, Zhang X, O’Neil CL, Medina RJ, Das SK, Muddana SK, Olson PR (2015) Intravitreal AAV2. COMP-Ang1 prevents neurovascular degeneration in a murine model of diabetic retinopathy. Diabetes 64:4247–4259
Campochiaro PA, Khanani A, Singer M, Patel S, Boyer D, Dugel P, Kherani S, Withers B, Gambino L, Peters K (2016) Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology 123:1722–1730
Castillo J, Aleman I, Rush SW, Rush RB (2017) Preoperative bevacizumab administration in proliferative diabetic retinopathy patients undergoing vitrectomy: a randomized and controlled trial comparing interval variation. Am J Ophthalmol 183:1–10
Chatziralli IP, Theodossiadis G, Dimitriadis P, Charalambidis M, Agorastos A, Migkos Z, Platogiannis N, Moschos MM, Theodossiadis P, Keryttopoulos P (2017) The effect of vitamin E on oxidative stress indicated by serum malondialdehyde in insulin-dependent type 2 diabetes mellitus patients with retinopathy. Open Ophthalmol J 11:51
Chen J, Lin F-L, Leung JY, Tu L, Wang J-H, Chuang Y-F, Li F, Shen H-H, Dusting GJ, Wong VH (2021) A drug-tunable Flt23k gene therapy for controlled intervention in retinal neovascularization. Angiogenesis 24:97–110
Chiew Y, Tan SMQ, Ahmad B, Khor SE, Kadir KA (2021) Tocotrienol-rich vitamin E from palm oil (Tocovid) and its effects in diabetes and diabetic retinopathy: a pilot phase II clinical trial. Asian J Ophthalmol 17:375–399
Chous AP, Richer SP, Gerson JD, Kowluru RA (2016) The diabetes visual function supplement study (DiVFuSS). Br J Ophthalmol 100:227–234
Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS (2011) Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc Res 89:671–679
Ciulla TA, Pollack JS, Williams DF (2021) Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: a real-world analysis of 28 658 patient eyes. Br J Ophthalmol 105:216–221
Collaboration AT (2011) Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138 769 individuals. J Hypertens 29:623–635
Crawford TN, Alfaro DV III, Kerrison JB, Jablon EP (2009) Diabetic retinopathy and angiogenesis. Curr Diabetes Rev 5:8–13
Domanico D, Fragiotta S, Cutini A, Carnevale C, Zompatori L, Vingolo EM (2015) Circulating levels of reactive oxygen species in patients with nonproliferative diabetic retinopathy and the influence of antioxidant supplementation: 6-month follow-up. Indian J Ophthalmol 63:9
Duan Y, Beli E, Li Calzi S, Quigley JL, Miller RC, Moldovan L, Feng D, Salazar TE, Hazra S, Al-Sabah J (2018) Loss of angiotensin-converting enzyme 2 exacerbates diabetic retinopathy by promoting bone marrow dysfunction. Stem Cells 36:1430–1440
Evliyaoğlu F, Akpolat Ç, Kurt MM, Çekiç O, Nuri Elçioğlu M (2018) Retinal vascular caliber changes after topical nepafenac treatment for diabetic macular edema. Curr Eye Res 43(3):357–361
Fallico M, Maugeri A, Lotery A, Longo A, Bonfiglio V, Russo A, Avitabile T, Pulvirenti A, Furino C, Cennamo G (2020) Intravitreal anti-vascular endothelial growth factors, panretinal photocoagulation and combined treatment for proliferative diabetic retinopathy: a systematic review and network meta-analysis. Acta Ophthalmol (Copenh) 2020:14681
Faure P, Benhamou P, Perard A, Halimi S, Roussel A (1995) Lipid peroxidation in insulin-dependent diabetic patients with early retina degenerative lesions: effects of an oral zinc supplementation. Eur J Clin Nutr 49:282–288
CAS PubMed Google Scholar
Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH (2017) Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health 5:e1221–e1234
Gao S, Lin Z, Shen X (2020) Anti-vascular endothelial growth factor therapy as an alternative or adjunct to pan-retinal photocoagulation in treating proliferative diabetic retinopathy: meta-analysis of randomized trials. Front Pharmacol 11:849
Garcia-Medina JJ, Pinazo-Duran MD, Garcia-Medina M, Zanon-Moreno V, Pons-Vazquez S (2011) A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur J Ophthalmol 21:637–643
Ghadiri Soufi F, Vardyani M, Sheervalilou R, Mohammadi M, Somi MH (2012) Long-term treatment with resveratrol attenuates oxidative stress pro-inflammatory mediators and apoptosis in streptozotocin-nicotinamide-induced diabetic rats. Gen Physiol Biophys 31:431–438
Gholamhossein Y, Behrouz H, Asghar Z (2014) Diabetic retinopathy risk factors: plasma erythropoietin as a risk factor for proliferative diabetic retinopathy. Korean J Ophthalmol 28:373
González de Vega R, García M, Fernández-Sánchez ML, González-Iglesias H, Sanz-Medel A (2018) Protective effect of selenium supplementation following oxidative stress mediated by glucose on retinal pigment epithelium. Metallomics 10:83–92
Group DRSR (1976) Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol 81:383-396
Haritoglou C, Gerss J, Sauerland C, Kampik A, Ulbig MW, Group CS (2009) Effect of calcium dobesilate on occurrence of diabetic macular oedema (CALDIRET study): randomised, double-blind, placebo-controlled, multicentre trial. Lancet 373:1364-1371
Haritoglou C, Gerss J, Hammes HP, Kampik A, Ulbig MW, RETIPON Study Group (2011) Alpha-lipoic acid for the prevention of diabetic macular edema. Ophthalmologica 226(3):127–137. https://doi.org/10.1159/000329470
Haurigot V, Villacampa P, Ribera A, Bosch A, Ramos D, Ruberte J, Bosch F (2012) Long-term retinal PEDF overexpression prevents neovascularization in a murine adult model of retinopathy. PLoS ONE 7:e41511
Huang S-Y, Jeng C, Kao S-C, Yu JJ-H, Liu D-Z (2004) Improved haemorrheological properties by Ginkgo biloba extract (Egb 761) in type 2 diabetes mellitus complicated with retinopathy. Clin Nutr 23:615–621
Huang Q, Liu Q, Ouyang D (2019) Sorbinil, an aldose reductase inhibitor, in fighting against diabetic complications. Med Chem 15:3–7
Hussein Z, Taher SW, Singh HKG, Swee WCS (2015) Diabetes care in Malaysia: problems, new models, and solutions. Annals Glob Health 81:851–862
Ichiyama Y, Sawada O, Mori T, Fujikawa M, Kawamura H, Ohji M (2016) The effectiveness of vitrectomy for diffuse diabetic macular edema may depend on its preoperative optical coherence tomography pattern. Graefes Arch Clin Exp Ophthalmol 254:1545–1551
Institute for Public Health (IPH) (2015) National health and morbidity survey 2015 (NHMS 2015) Vol. II: Non-communicable diseases, risk factors & other health problems. Minist Health Malays 2:185–186
Jeong SH, Han JI, Cho SW, Lee DW, Kim CG, Lee TG, Kim JW (2016) Effect of focal laser photocoagulation in eyes with mild to moderate non-proliferative diabetic retinopathy. Int J Ophthalmol 9:1439
PubMed PubMed Central Google Scholar
Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, Moromizato Y, Bursell S-E, Wiegand SJ, Rudge J (2002) Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol 160:1683–1693
Julius A, Hopper W (2019) A non-invasive, multi-target approach to treat diabetic retinopathy. Biomed Pharmacother 109:708–715
Kähler W, Kuklinski B, Rühlmann C, Plötz C (1993) Diabetes mellitus–a free radical-associated disease. Results of adjuvant antioxidant supplementation. Z Gesamte Inn Med 48:223–232
PubMed Google Scholar
Kaji Y, Usui T, Ishida S, Yamashiro K, Moore TC, Moore J, Yamamoto Y, Yamamoto H, Adamis AP (2007) Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol vis Sci 48:858–865
Kern TS, Engerman RL (2001) Pharmacological inhibition of diabetic retinopathy: aminoguanidine and aspirin. Diabetes 50:1636–1642
Koleva-Georgieva DN, Sivkova NP, Terzieva D (2011) Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (plovdiv) 53:44–50
Kowluru RA, Kanwar M (2007) Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (lond) 4:8
Kowluru RA, Menon B, Gierhart DL (2008) Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest Ophthalmol vis Sci 49:1645–1651
Kowluru RA, Chan PS (2007) Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007:43603. https://doi.org/10.1155/2007/43603
Kumar B, Gupta SK, Nag TC, Srivastava S, Saxena R (2012) Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res 47:103–108
Lanzetta P, Loewenstein A, Committee VAS (2017) Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti–vascular endothelial growth factor therapy of macular diseases. Graefe’s Arch Clin Exp Ophthalmol 255:1259–1273
Le Bagge S, Fotheringham AK, Leung SS, Forbes JM (2020) Targeting the receptor for advanced glycation end products (RAGE) in type 1 diabetes. Med Res Rev 40:1200–1219
Lee DH, Agron E, Keenan TD, Chew E (2020) Visual field changes in proliferative and severe non-proliferative diabetic retinopathy: natural history without treatment and after pan-retinal photocoagulation in the Diabetic Retinopathy Study (DRS) and Early Treatment Diabetic Retinopathy Study (ETDRS). Invest Ophthalmol vis Sci 61:3822–3822
Lee J, Kim KE, Choi D-K, Jang JY, Jung J-J, Kiyonari H, Shioi G, Chang W, Suda T, Mochizuki N (2013) Angiopoietin-1 guides directional angiogenesis through integrin αvβ5 signaling for recovery of ischemic retinopathy. Sci Transl Med 5:203ra127
Lip PL, Kolli H, Trivedi D (2020) Ultra-widefield fluorescein angiographic patterns, retinal microvascular anomalies and retinal ischemic index in branch retinal vein occlusions with established retinal neovascularization. Clin Ophthalmol 14:2965
Liu L, Zuo Z, Lu S, Liu A, Liu X (2017) Naringin attenuates diabetic retinopathy by inhibiting inflammation, oxidative stress and NF-κB activation in vivo and in vitro. Iran J Basic Med Sci 20:813
Gálvez MI (2011) Protein kinase C inhibitors in the treatment of diabetic retinopathy. Curr Pharm Biotechnol 12:386–391
Luo D, Fan Y, Xu X (2012) The effects of aminoguanidine on retinopathy in STZ-induced diabetic rats. Bioorg Med Chem Lett 22:4386–4390
Mahajan N, Arora P, Sandhir R (2019) Perturbed biochemical pathways and associated oxidative stress lead to vascular dysfunctions in diabetic retinopathy. Oxid Med Cell Longev 2019:8458472. https://doi.org/10.1155/2019/8458472
Mansour SE, Browning DJ, Wong K, Flynn HW Jr, Bhavsar AR (2020) The evolving treatment of diabetic retinopathy. Clin Ophthalmol 14:653
Medić A, Jukić T, Matas A, Vukojević K, Sapunar A, Znaor L (2017) Effect of preoperative topical diclofenac on intraocular interleukin-12 concentration and macular edema after cataract surgery in patients with diabetic retinopathy: a randomized controlled trial. Croat Med J 58(1):49–55. https://doi.org/10.3325/cmj.2017.58.49
Mehrzadi S, Motevalian M, Rezaei Kanavi M, Fatemi I, Ghaznavi H, Shahriari M (2018) Protective effect of melatonin in the diabetic rat retina. Fundam Clin Pharmacol 32:414–421
Millen AE, Klein R, Folsom AR, Stevens J, Palta M, Mares JA (2004) Relation between intake of vitamins C and E and risk of diabetic retinopathy in the atherosclerosis risk in Communities Study. Am J Clin Nutr 79:865–873
Mishra M, Zhong Q, Kowluru RA (2014) Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 55:7256–7265
Mishra S, Gupta A, Patyal S, Kumar S, Raji K, Singh A, Sharma V (2018) Intravitreal dexamethasone implant versus triamcinolone acetonide for macular oedema of central retinal vein occlusion: quantifying efficacy and safety. Int J Retina Vitr 4:1–8
Movahed A, Raj P, Nabipour I, Mahmoodi M, Ostovar A, Kalantarhormozi M, Netticadan T (2020) Efficacy and safety of resveratrol in type 1 diabetes patients: a two-month preliminary exploratory trial. Nutrients 12:161
Nakamura S, Tsuruma K, Shimazawa M, Hara H (2012) Candesartan, an angiotensin II type 1 receptor antagonist, inhibits pathological retinal neovascularization by downregulating VEGF receptor-2 expression. Eur J Pharmacol 685:8–14
Nambu H, Nambu R, Oshima Y, Hackett S, Okoye G, Wiegand S, Yancopoulos G, Zack D, Campochiaro P (2004) Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood–retinal barrier. Gene Ther 11:865–873
Nambu H, Umeda N, Kachi S, Oshima Y, Akiyama H, Nambu R, Campochiaro PA (2005) Angiopoietin 1 prevents retinal detachment in an aggressive model of proliferative retinopathy, but has no effect on established neovascularization. J Cell Physiol 204:227–235
Obeid A, Gao X, Ali FS, Talcott KE, Aderman CM, Hyman L, Ho AC, Hsu J (2018) Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF injections. Ophthalmology 125:1386–1392
Obrosova IG, Minchenko AG, Vasupuram R, White L, Abatan OI, Kumagai AK, Frank RN, Stevens MJ (2003) Aldose reductase inhibitor fidarestat prevents retinal oxidative stress and vascular endothelial growth factor overexpression in streptozotocin-diabetic rats. Diabetes 52:864–871
Obrosova IG, Kador PF (2011) Aldose reductase/polyol inhibitors for diabetic retinopathy. Curr Pharm Biotechnol 12:373–385
Ola MS, Nawaz MI, Khan HA, Alhomida AS (2013) Neurodegeneration and neuroprotection in diabetic retinopathy. Int J Mol Sci 14:2559–2572
Ortega ÁL (2021) Oxidative stress in diabetic retinopathy. Multidisciplinary Digital Publishing Institute
Parolini B, Prigione G, Romanelli F, Cereda MG, Sartore M, Pertile G (2010) Postoperative complications and intraocular pressure in 943 consecutive cases of 23-gauge transconjunctival pars plana vitrectomy with 1-year follow-up. Retina 30:107–111
Peng X, Ma J, Chen F, Wang M (2011) Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct 2:289–301
Pessoa B, Dias DA, Baptista P, Coelho C, Beirão JNM, Meireles A (2019) Vitrectomy outcomes in eyes with tractional diabetic macular edema. Ophthalmic Res 61:94–99
Pinna A, Blasetti F, Ricci GDA, Boscia F (2017) Bromfenac eyedrops in the treatment of diabetic macular edema: a pilot study. Eur J Ophthalmol 27:326–330
Qi JH, Anand-Apte B (2015) Tissue inhibitor of metalloproteinase-3 (TIMP3) promotes endothelial apoptosis via a caspase-independent mechanism. Apoptosis 20:523–534
Quattrini L, La Motta C (2019) Aldose reductase inhibitors: 2013-present. Expert Opin Ther Pat 29:199–213
Ramis R, Ortega-Castro J, Caballero C, Casasnovas R, Cerrillo A, Vilanova B, Adrover M, Frau J (2019) How does pyridoxamine inhibit the formation of advanced glycation end products? The role of its primary antioxidant activity. Antioxidants 8:344
Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A (2011) A potential role for angiopoietin 2 in the regulation of the blood–retinal barrier in diabetic retinopathy. Invest Ophthalmol vis Sci 52:3784–3791
Reddy SV, Husain D (2018) Panretinal photocoagulation: a review of complications. Semin Ophthalmol. Taylor & Francis, pp 83–88
Regillo CD, Callanan DG, Do DV, Fine HF, Holekamp NM, Kuppermann BD, Singer MA, Singh RP (2017) Use of corticosteroids in the treatment of patients with diabetic macular edema who have a suboptimal response to anti-VEGF: recommendations of an expert panel. Ophthalmic Surg Lasers Imaging Retina 48:291–301
Rey A, Jürgens I, Maseras X, Dyrda A, Pera P, Morilla A (2018) Visual outcome and complications of cataract extraction after pars plana vitrectomy. Clin Ophthalmol (Auckland, NZ) 12:989
Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, Miller-Arrevillaga G, Pacheco-Moisés FP, Román-Pintos LM, Miranda-Díaz AG (2016) The effect of ubiquinone and combined antioxidant therapy on oxidative stress markers in non-proliferative diabetic retinopathy: a phase IIa, randomized, double-blind, and placebo-controlled study. Redox Rep 21:155–163
Sadikan MZ, Abdul Nasir NA, Agarwal R, Mohd Ismail N (2020) Protective effect of Palm oil-derived tocotrienol-rich fraction against retinal neurodegenerative changes in rats with streptozotocin-induced diabetic retinopathy. Biomolecules 10(4):556. https://doi.org/10.3390/biom10040556
Sadikan MZ, Abdul Nasir NA, Ghani NA, Lambuk L, Iezhitsa I, Agarwal R (2021) The use of fiji image J as an image analysis tool for measuring retinal vessel diameter in rodent model of diabetic retinopathy. Asian J Med Biomed 5(1):61–66
Sadikan MZ, Abdul Nasir NA, Iezhitsa I, Agarwal R (2022) Antioxidant and anti-apoptotic effects of tocotrienol-rich fraction against streptozotocin-induced diabetic retinopathy in rats. Biomedicine & Pharmacotherapy 153:113533. https://doi.org/10.1016/j.biopha.2022.113533
Sadikan MZ, Abdul Nasir NA, Bakar NS, Iezhitsa I, Agarwal R (2023) Tocotrienol-rich fraction reduces retinal inflammation and angiogenesis in rats with streptozotocin-induced diabetes. BMC Complement Med Ther 23(1):1–9. https://doi.org/10.1186/s12906-023-04005-9
Safi SZ, Qvist R, Kumar S, Batumalaie K, Ismail ISB (2014) Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res Int 2014:1–18
Sankaramoorthy A, Roy S (2021) Vascular basement membrane thickening: basis of disease pathology in diabetic retinopathy. In: Prakash G, Iwata T (eds) Advances in vision research, volume III. Essentials in ophthalmology. Springer, Singapore. https://doi.org/10.1007/978-981-15-9184-6_20
Sarao V, Veritti D, Boscia F, Lanzetta P (2014) Intravitreal steroids for the treatment of retinal diseases. Sci World J 2014:1–14
Schemmel KE, Padiyara RS, D’Souza JJ (2010) Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications 24:354–360
Schmidt I, Plange N, Rößler G, Schellhase H, Koutsonas A, Walter P, Mazinani B (2019) Long-term clinical results of vitrectomy and scleral buckling in treatment of rhegmatogenous retinal detachment. Sci World J 2019:1–7
Schreur V, Brouwers J, Van Huet RA, Smeets S, Phan M, Hoyng CB, de Jong EK, Klevering BJ (2020) Long-term outcomes of vitrectomy for proliferative diabetic retinopathy. Acta Ophthalmol (Copenh) 99:1–7
Senthilkumari S, Sharmila R, Chidambaranathan G, Vanniarajan A (2017) Epalrestat, an aldose reductase inhibitor prevents glucose-induced toxicity in human retinal pigment epithelial cells in vitro. J Ocul Pharmacol Ther 33:34–41
Shen J, Frye M, Lee BL, Reinardy JL, McClung JM, Ding K, Kojima M, Xia H, Seidel C, e Silva RL (2014) Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Investig 124:4564-4576
Simó R, Sundstrom JM, Antonetti DA (2014) Ocular anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 37:893–899
Singh Grewal A, Bhardwaj S, Pandita D, Lather V, Singh Sekhon B (2016) Updates on aldose reductase inhibitors for management of diabetic complications and non-diabetic diseases. Mini Rev Med Chem 16:120–162
Singh RP, Lehmann R, Martel J, Jong K, Pollack A, Tsorbatzoglou A, Staurenghi G, Cervantes GC, Alpern L, Modi S, Svoboda L (2017) Nepafenac 0.3% after cataract surgery in patients with diabetic retinopathy: results of 2 randomized phase 3 studies. Ophthalmology 124(6):776–785
Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, Bainbridge J, Tudor-Edwards R, Hopkins D, Hykin P (2017) Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 389:2193–2203
Sjølie et al., 2008 Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Chaturvedi N, Group DPS (2008) Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet 372:1385-1393
Song Y-S, Nagaoka T, Omae T, Yokota H, Takahashi A, Yoshida A (2016) Systemic risk factors in bilateral proliferative diabetic retinopathy requiring vitrectomy. Retina 36:1309–1313
Stefanini FR, Badaró E, Falabella P, Koss M, Farah ME, Maia M (2014) Anti-VEGF for the management of diabetic macular edema. J Immunol Res 2014:632307
Steven Ferrucci O (2020) Pipeline: Potential new therapy coming for macular disease. Optom Times 12:1–18
Stewart MW (2017) Future treatments of diabetic retinopathy: pharmacotherapeutic products under development. Eur Med J Diabetes 5:93–103
Sun JK, Glassman AR, Beaulieu WT, Stockdale CR, Bressler NM, Flaxel C, Gross JG, Shami M, Jampol LM, Network DRCR (2019) Rationale and application of the protocol S anti–vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology 126:87–95
Suzuma K, Takahara N, Suzuma I, Isshiki K, Ueki K, Leitges M, Aiello LP, King GL (2002) Characterization of protein kinase C β isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci 99:721–726
Testa R, Bonfigli AR, Prattichizzo F, La Sala L, De Nigris V, Ceriello A (2017) The “metabolic memory” theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients 9:437
Thornalley PJ (2003) Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys 419:31–40
Toyoda F, Kakehashi A, Takano H, Kinoshita N, Shimmura-Tomita M, Ota A, Tanaka Y, Matsumoto T, Tsuji J (2014) Effect of ranirestat, a new aldose reductase inhibitor, on diabetic retinopathy in SDT rats. Invest Ophthalmol vis Sci 55:1060–1060
Tu L, Wang J-H, Barathi VA, Prea SM, He Z, Lee JH, Bender J, King AE, Logan GJ, Alexander IE (2018) AAV-mediated gene delivery of the calreticulin anti-angiogenic domain inhibits ocular neovascularization. Angiogenesis 21:95–109
Tuuminen R, Sahanne S, Haukka J, Loukovaara S (2016) Improved outcome after primary vitrectomy in diabetic patients treated with statins. Eur J Ophthalmol 26:174–181
Valacchi G, Virgili F, Cervellati C, Pecorelli A (2018) OxInflammation: from subclinical condition to pathological biomarker. Front Physiol 9:858
van der Wijk A-E, Vogels IM, van Noorden CJ, Klaassen I, Schlingemann RO (2017) TNFα-induced disruption of the blood–retinal barrier in vitro is regulated by intracellular 3′, 5′-cyclic adenosine monophosphate levels. Invest Ophthalmol Vis Sci 58:3496–3505
Wang B, Wang F, Zhang Y, Zhao SH, Zhao WJ, Yan SL, Wang YG (2015) Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta-analysis. . Lancet Diabetes Endocrinol 3(4):263–274
Wang J-H, Roberts GE, Liu G-S (2020) Updates on gene therapy for diabetic retinopathy. Curr Diab Rep 20:1–12
Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ (2015) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372:1193–1203
Whitcup SM, Cidlowski JA, Csaky KG, Ambati J (2018) Pharmacology of corticosteroids for diabetic macular edema. Invest Ophthalmol Vis Sci 59:1–12
Whitehead M, Osborne A, Widdowson PS, Yu-Wai-Man P, Martin KR (2019) Angiopoietins in diabetic retinopathy: current understanding and therapeutic potential. J Diabetes Res 2019:1–9
Wilkinson-Berka JL, Suphapimol V, Jerome JR, Deliyanti D, Allingham MJ (2019) Angiotensin II and aldosterone in retinal vasculopathy and inflammation. Exp Eye Res 187:107766
Willis LM, El-Remessy AB, Somanath PR, Deremer DL, Fagan SC (2011) Angiotensin receptor blockers and angiogenesis: clinical and experimental evidence. Clin Sci 120:307–319
Wu H, Xu G, Liao Y, Ren H, Fan J, Sun Z, Zhang M (2012) Supplementation with antioxidants attenuates transient worsening of retinopathy in diabetes caused by acute intensive insulin therapy. Graefe’s Arch Clin Expl Ophthalmol 250:1453–1458
Xu J, Chen L-J, Yu J, Wang H-J, Zhang F, Liu Q, Wu J (2018) Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell Physiol Biochem 48:705–717
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen S-J, Dekker JM, Fletcher A, Grauslund J (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564
Yu Z, Lu B, Sheng Y, Zhou L, Ji L, Wang Z (2015) Andrographolide ameliorates diabetic retinopathy by inhibiting retinal angiogenesis and inflammation. Biochim Biophys Acta (BBA)-Gen Subj 1850:824–831
Zareie M, Tangelder G-J, ter Wee PM, Hekking LH, van Lambalgen AA, Keuning ED, Schadee-Eestermans IL, Schalkwijk CG, Beelen RH, van den Born J (2005) Beneficial effects of aminoguanidine on peritoneal microcirculation and tissue remodelling in a rat model of PD. Nephrol Dial Transplant 20:2783–2792
Zeng K, Xu H, Chen K, Zhu J, Zhou Y, Zhang Q, Mantian M (2010) Effects of taurine on glutamate uptake and degradation in Müller cells under diabetic conditions via antioxidant mechanism. Mol Cell Neurosci 45:192–199
Zhang X, Wang N, Schachat A, Bao S, Gillies M (2014) Glucocorticoids: structure, signaling and molecular mechanisms in the treatment of diabetic retinopathy and diabetic macular edema. Curr Mol Med 14:376–384
Download references
Author information
Authors and affiliations.
Department of Pharmacology, Faculty of Medicine, Manipal University College Malaysia (MUCM), Bukit Baru, 75150, Malacca, Malaysia
Muhammad Zulfiqah Sadikan
Department of Medical Education, Faculty of Medicine, Universiti Teknologi MARA, 47000, Sungai Buloh, Selangor, Malaysia
Nurul Alimah Abdul Nasir
Centre for Neuroscience Research (NeuRon), Faculty of Medicine, Universiti Teknologi MARA, 47000, Sungai Buloh, Selangor, Malaysia
You can also search for this author in PubMed Google Scholar
Contributions
MZS performed literature search and drafted the manuscript. NAAN supervised and revised the manuscript. All authors contributed to the manuscript and approved the submitted version. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Correspondence to Nurul Alimah Abdul Nasir .
Ethics declarations
Ethical approval.
Not applicable.
Competing interests
The authors declare no competing interests.

Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Sadikan, M.Z., Abdul Nasir, N. . Diabetic retinopathy: emerging concepts of current and potential therapy. Naunyn-Schmiedeberg's Arch Pharmacol 396 , 3395–3406 (2023). https://doi.org/10.1007/s00210-023-02599-y
Download citation
Received : 02 April 2023
Accepted : 22 June 2023
Published : 04 July 2023
Issue Date : December 2023
DOI : https://doi.org/10.1007/s00210-023-02599-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetic retinopathy
- Current management
- Pharmacological treatment
- Potential therapy
- Underlying pathways
- Find a journal
- Publish with us
- Track your research
- Frontiers in Endocrinology
- Clinical Diabetes
- Research Topics
Advances in the Research of Diabetic Retinopathy
Total Downloads
Total Views and Downloads
About this Research Topic
Diabetic Retinopathy is a common complication affecting patients with diabetes and refers to microvascular retinal damage as a result of hyperglycaemia. Without intervention, damage can progress through the stages of background, pre-proliferative and proliferative retinopathy, potentialling resulting diabetic ...
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, recent articles, submission deadlines.
Submission closed.
Participating Journals
Total views.
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Mapping research trends in diabetic retinopathy from 2010 to 2019
A bibliometric analysis.
Editor(s): Wane., Daryle
a Tianjin Eye Hospital, Tianjin Key Lab of Ophthalmology and Visual Science, Tianjin Eye Institute, Clinical College of Ophthalmology Tianjin Medical University
b Ophthalmology Department, Baodi Clinical College of Tianjin Medical University, Tianjin Baodi Hospital, Tianjin, China.
∗Correspondence: Yi Dong, Tianjin Eye Hospital, Tianjin Key Lab of Ophthalmology and Visual Science, Tianjin Eye Institute, Clinical College of Ophthalmology Tianjin Medical University, Gansu Road 4, Tianjin 300020, China (e-mail: [email protected] ).
Abbreviations: AMD = age-related macular degeneration, BRB = blood-retinal barrier, DME = diabetic macular edema, DR = diabetic retinopathy, FA = fluorescein angiography, FAZ = foveal avascular zone, MAs = microaneurysms, MKD = mapping knowledge domain, OCT = optic coherence tomography, OCTA = optical coherence tomography angiography, PDR = proliferative diabetic retinopathy, PRP = panretinal photocoagulation, VA = visual acuity, VEGF = vascular endothelial growth factor, WoS = Web of Science, WoSCC = Web of Science Core Collection.
How to cite this article: Dong Y, Liu Y, Yu J, Qi S, Liu H. Mapping research trends in diabetic retinopathy from 2010 to 2019: a bibliometric analysis. Medicine . 2021;100:3(e23981).
YD and YL contributed equally to this work
The authors declare no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial License 4.0 (CCBY-NC), where it is permissible to download, share, remix, transform, and buildup the work provided it is properly cited. The work cannot be used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc/4.0
Background:
Although many publications in diabetic retinopathy (DR) have been reported, there is no bibliometric analysis.
Purpose:
To perform a bibliometric analysis in the field of diabetic retinopathy (DR) research, to characterize the current international status of DR research, to identify the most effective factors involved in this field, and to explore research hotspots in DR research.
Methods:
Based on the Web of Science Core Collection (WoSCC), a bibliometric analysis was conducted to investigate the publication trends in research related to DR. Knowledge maps were constructed by VOSviewer v.1.6.10 to visualize the publications, the distribution of countries, international collaborations, author productivity, source journals, cited references and keywords, and research hotspots in this field.
Results:
In total, 11,839 peer-reviewed papers were retrieved on DR from 2010 to 2019, and the annual research output increased with time. The United States ranks highest among countries with the most publications. The most active institution is the University of Melbourne. Wong, TY contributed the largest number of publications in this field. Investigative Ophthalmology & Visual Science was the most prolific journal in DR research. The top-cited references mainly investigated the use of anti-vascular endothelial growth factor (VEGF) medications in the management of DR, and the keywords formed 6 clusters:
- 1. pathogenesis of DR;
- 2. epidemiology and risk factors for DR;
- 3. treatments for DR;
- 4. screening of DR;
- 5. histopathology of DR; and
- 6. diagnostic methods for DR.
Discussion:
With the improvement of living standard, DR has gradually become one of the important causes of blindness, and has become a hot spot of public health research in many countries. The application of deep learning and artificial intelligence in diabetes screening and anti-VEGF medications in the management of DR have been the research hotspots in recent 10 years.
Conclusions:
Based on data extracted from the WoSCC, this study provides a broad view of the current status and trends in DR research and may provide clinicians and researchers with insight into DR research and valuable information to identify potential collaborators and partner institutions and better predict their dynamic directions.
1 Introduction
Diabetic retinopathy (DR) is an important complication of diabetes that affects blood vessels in the retina and can cause vision loss and blindness. [1] It is quickly becoming a worldwide public health challenge. [2,3] A large number of research papers related to DR have been published in academic journals in recent decades. In recent decades, many reviews on the pathology, metabolomics, imaging, biomarkers, and treatment of DR have been published. [1,4–8]
However, to our knowledge, the global research trend and other related topics in DR have not yet been well studied. It is difficult to read all the publications. Therefore, there is a need to use a method and tool to investigate the global status of the research in DR. Bibliometric methods and mapping knowledge domain (MKD) methods have been used in various fields to visually highlight the most influential countries, authors, journals, publications, and identify main research topics. [9] Bibliometric analysis is a method for analyzing the literature and its accompanying citation counts over time with mathematical statistics. The MKD method provides a new way to conduct literature mining and reveal the core structure of scientific knowledge. It also enables researchers to determine the range of research topics and identify new topics and assists them in planning their research direction and predicting research trends. [10] This study aimed to use bibliometric tools to analyze DR articles retrieved from the Web of Science (WoS) (Thomson Reuters Company) database and assess the research development status of DR throughout the world. This analysis could help us to uncover the current status and global trends of DR. It was hoped that our research results could provide meaningful help to the current researchers of DR. Recently, some systematic reviews have been conducted to evaluate the efficacy of PD Other systematic reviews have analyzed the adverse events in patients treated with PD However, the status of research in the area of PD-1 and PD-L1 in the cancer field and other related topics have not been investigated.
The remainder of the paper is structured as follows. The data collection and analytical methods are described in Section 2. The distribution of publications, countries, research organizations, journals, and research hotspots are presented in Sections 3. Global trends, document citation analysis, and research frontiers are discussed in Section 4. The final section, Section 5, summarizes the findings and concludes the paper.
2 Materials and methods
This study followed the tenets of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Tianjin Eye Hospital and Tianjin Baodi Hospital. The search for papers to be included in this study was carried out on April 20, 2020 using the Science Citation Index Expanded (SCI-EXPANDED) database via the Web of Science Core Collection (WoSCC) provided by Thomson Reuters (Philadelphia, PA, USA). The database was searched using the term “diabetic retinopathy” in terms of “topic” (title, abstract, author's keywords, and WoS-assigned keywords, called Keywords Plus) to retrieve all articles where the expression “diabetic retinopathy” appeared, as well as other relevant expressions (e.g., diabetic retinopathies). The time span was set to between 2000 and 2019. Only articles were included as document types (nonarticle documents such as reviews, meeting abstracts, editorial materials, proceedings papers, letters were excluded). Journal articles were used for the analysis because they accounted for the majority of document types that also included complete research ideas and results. Data were downloaded from the WoS in “Full record and cited references” formats.
Visualization software can produce node-link maps that allow us to intuitively observe the publication outputs, hotspots, and other aspects of a research field. In this study, the data were imported into VOSviewer v.1.6.10 and analyzed systematically. VOSviewer ( www.vosviewer.com ), developed by van Eck and Waltman, is a literature visualization software that has the advantages of displaying cluster analysis results. [11] In the knowledge maps generated using VOSviewer, items are represented as nodes and links. The nodes and their labels, such as countries, organizations, authors, co-citation literature, and keywords, are proportional to the weight of the analysis components. The links between the nodes reflect the relationship between the components. CiteSpace IV (Drexel University, Philadelphia, PA) was used to capture keywords with strong citation bursts, which could be considered as predictors of research frontiers.
3.1 Yearly quantitative distribution of publications
According to the selection criteria, we identified and included 11,839 publications on DR that were indexed in the WoSCC from 2010 to 2020. The number of publications showed a gradually increasing trend over time, from 857 in 2010 to 1573 in 2019 ( Fig. 1 A). Through keyword burst detection analysis ( Fig. 1 B), we detected 28 keywords that represented citation bursts; among these keywords, “machine learning” showed citation bursts in 2019, which is consistent with the increase in published papers.

3.2 Distribution of productive countries in DR
According to the retrieved results, the 11,839 articles originated from 128 countries. As presented in Table 1 , the top 10 countries engaged in DR research published 10,419 articles, accounting for 88.0% of the total number of publications. The United States contributed the most publications (3280, 27.7%), followed by China (2222, 18.8%) and Japan (811, 6.9%). According to citation analysis, the United States had 79,761 citations, followed by China (26,304 citations) and Japan (15,670 citations).
| Rank | Country | Count (%) | Citations | Total link strengthen |
| 1 | United States | 3280 (27.7) | 79,761 | 2108 |
| 2 | China | 2222 (18.8) | 26,304 | 798 |
| 3 | Japan | 811 (6.9) | 15,670 | 297 |
| 4 | England | 752 (6.4) | 18,476 | 1002 |
| 5 | Germany | 657 (5.5) | 15,655 | 821 |
| 6 | Australia | 640 (5.4) | 17,188 | 966 |
| 7 | India | 578 (4.97) | 9666 | 394 |
| 8 | South Korea | 560 (4.7) | 6779 | 188 |
| 9 | Italy | 544 (4.6) | 12,545 | 575 |
| 10 | Spain | 375 (3.2) | 6439 | 343 |
Country co-authorship analysis reflects the degree of communication between countries as well as the most influential countries in this field. The larger nodes represent the more influential countries; the thickness and distance of the links between nodes represent the strength of the cooperative relationships among countries. Figure 2 shows that the United States intensely cooperated with many countries in the DR field, such as England, Australia, Germany, France, and Denmark. Although China has published a large number of articles, there is little cooperation with other countries. This indicates that geographical distance is not the primary influencing factor of cooperative relationships.

3.3 Distribution of main research organizations
According to the retrieved results, 11,839 articles were published by 8642 organizations. The top 10 organizations published 1852 articles, accounting for 15.64% of the total number of publications ( Table 2 ). Based on co-authorship analysis, Figure 3 displays the knowledge domain map of the research organizations’ distribution in DR research. The size of the node corresponds to the number of published articles. The links between nodes represent the collaborations. The thicker and longer the node-link, the closer the collaboration is between the 2 organizations.
| Rank | Organization | Country | Count (%) | Citations |
| 1 | University of Melbourne | Australia | 2.13 | 7499 |
| 2 | Shanghai Jiao Tong University | China | 1.82 | 2416 |
| 3 | Johns Hopkins University | USA | 1.76 | 8658 |
| 4 | University of Sydney | Australia | 1.72 | 7147 |
| 5 | National University of Singapore | Singapore | 1.71 | 5809 |
| 6 | University of Wisconsin | USA | 1.57 | 5893 |
| 7 | Sun Yat-Sen University | China | 1.39 | 2644 |
| 8 | Capital Medical University | China | 1.25 | 1707 |
| 9 | Harvard university | USA | 1.21 | 5152 |
| 10 | Singapore National Eye Center | Singapore | 1.09 | 2766 |

3.4 Distribution of authors and co-authorship of research groups
According to the retrieved results, over 111,933 authors contributed to DR research. Among all authors, Wong Tienyin (91 publications) ranked first, followed by Wong Tieny (82 publications) and Klein Ronald (79 publications), indicating their productive contribution to DR research. Information on author co-citations was analyzed as well. Among all co-cited authors, Klein, R (3199 co-citations) ranked first, followed by Kowluru, RA (1618 co-citations), and Aiello, LP (1423 co-citations), indicating their relative influence on DR research ( Table 3 ).
| Rank | Author | Count | Co-cited author | Count |
| 1 | Wong, TY | 173 | Klein, R | 3199 |
| 2 | Klein, R | 79 | Kowluru, RA | 1628 |
| 3 | Lamoureux, EL | 73 | Aiello, LP | 1423 |
| 4 | Mitchell, P | 59 | Wong, TY | 1370 |
| 5 | Bandello, F | 59 | Barber, AJ | 1113 |
| 6 | Kowluru, RA | 58 | Cheung, N | 1060 |
| 7 | Peto, T | 57 | Antonetti, DA | 984 |
| 8 | Simo, R | 56 | Joussen, AM | 885 |
| 9 | Wang, JJ | 55 | Yau, JWY | 785 |
| 10 | Kern, TS | 53 | Spaide, RF | 764 |
According to the co-authorship analysis, Figure 4 displays the knowledge domain map of the authors in DR research. The size of the node corresponds to the number of published articles. The links between nodes represent the cooperative relationship between authors.

The greater the link strength, the greater the density of cooperation was between the linked authors.
3.5 Distribution of source journals
Based on the retrieved results, articles on DR research were published in 1524 journals. The top 10 journals that publish on this topic are listed in Table 4 . Investigative Ophthalmology & Visual Science published the greatest number of articles (782, 6.6%), followed by PLOS ONE (464, 3.9%) and Retina-The Journal of Retinal and Vitreous Diseases (455, 3.8%). Articles published in these 3 journals accounted for 14.36% of all publications included in this study.
| Rank | Journal | Country | Count | % of 11,839 |
| 1 | Investigative ophthalmology & Visual Science | United States | 782 | 6.61 |
| 2 | PLOS ONE | United States | 464 | 3.92 |
| 3 | Retina-The Journal of Retinal and Vitreous Diseases | United States | 455 | 3.84 |
| 4 | Ophthalmology | United States | 283 | 2.39 |
| 5 | Acta ophthalmologica | Den Mark | 245 | 2.07 |
| 6 | Graefe's Archives for Clinical and Experimental Ophthalmology | United States | 216 | 1.83 |
| 7 | British Journal of Ophthalmology | England | 201 | 1.70 |
| 8 | American Journal of Ophthalmology | United States | 186 | 1.57 |
| 9 | Scientific Reports | England | 185 | 1.56 |
| 10 | International journal of ophthalmology | China | 176 | 1.49 |
3.6 Distribution of cited references: knowledge bases of DR research
Through co-citation analysis of the cited references, a knowledge base of DR research can be efficiently constructed. The minimum number of citations for a cited reference was set to 200. Of the 337,162 cited references, 305 met the threshold. The top 10 co-cited references are presented in Table 5 .
| Rank | Title | Author | Cluster | Citations |
| 1 | A survey on deep learning in medical image analysis | Litjens, Geert | 4 | 1402 |
| 2 | Global Prevalence and Major Risk Factors of Diabetic Retinopathy | Yau, Joanne W. Y. | 2 | 1389 |
| 3 | Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs | Gulshan, Varun | 4 | 1077 |
| 4 | Diabetic retinopathy | Cheung, Ning | 4 | 1020 |
| 5 | Randomized Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema | Elman, Michael J. | 3 | 776 |
| 6 | The RESTORE Study Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy for Diabetic Macular Edema | Mitchell, Paul | 3 | 740 |
| 7 | Ranibizumab for Diabetic Macular Edema Results from 2 Phase III Randomized Trials: RISE and RIDE | Quan Dong Nguyen | 3 | 737 |
| 8 | Effects of Medical Therapies on Retinopathy Progression in Type 2 Diabetes. | Chew, Emily Y. | 3 | 660 |
| 9 | Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema | Wells, John A | 3 | 592 |
| 10 | Inflammation in diabetic retinopathy | Tang, Johnny | 1 | 499 |
3.7 Distribution of keywords hotspots of DR research
Through the co-occurrence analysis of high-frequency keywords, the research hotspots of DR were identified. The minimum number of co-occurrences of a keyword was set to 20. Of the 13,415 extracted keywords involved in DR, 226 met the threshold. Based on the network, the keywords with similarities were clustered, and the 6 main clusters were denoted using the colors red, green, brown, yellow, purple, and blue, respectively ( Fig. 5 ). The top 10 keywords for each cluster are listed in Table 6 .

| Cluster 1 Red | Cluster 2 Green | Cluster 3 Brown | Cluster 4 Yellow | Cluster 5 Purple | Cluster 6 Blue |
| diabetes (675) | diabetic retinopathy (2940) | diabetic macular edema (838) | glaucoma (311) | vascular endothelial growth factor (269) | optical coherence tomography (854) |
| retina (524) | diabetes mellitus (497) | proliferative diabetic retinopathy (518) | screening (261) | age-related macular degeneration (195) | optical coherence tomography angiography (229) |
| inflammation (272) | retinopathy (343) | bevacizumab (541) | telemedicine (139) | ranibizumab (179) | cataract (228) |
| angiogenesis (243) | type 2 diabetes (311) | macular edema (459) | ophthalmology (106) | visual acuity (137) | fluorescein angiography (153) |
| oxidative stress (225) | type 2 diabetes mellitus (203) | vitrectomy (292) | diabetic retinopathy (dr) (51) | neovascularization (104) | foveal avascular zone (105) |
| vegf (222) | diabetic nephropathy (179) | anti-vegf (232) | deep learning (95) | choroidal thickness (72) | imaging (104) |
| apoptosis (200) | type 1 diabetes (167) | panretinal photocoagulation (126) | macula (106) | oct (71) | phacoemulsification (83) |
| polymorphism (83) | risk factors (138) | diabetic macular edema (125) | eye (103) | retinal neovascularization (60) | retinal thickness (83) |
| hypoxia (78) | epidemiology (109) | intravitreal injection (177) | retinal imaging (66) | choroidal neovascularization (58) | epiretinal membrane (71) |
| Neurodegeneratio (76) | nephropathy (84) | retinal vein occlusion (161) | classification (70) | aflibercept (54) | retinal vasculature (73) |
4 Discussion
4.1 global trends in research on dr.
The variation in the number of academic papers is an important research index that can reflect the development trend of the corresponding field. As shown in Figure 1 , a total of 11,839 papers were retrieved on DR from 2010 to 2019, and the annual research output increased with time. In the analysis of the most productive countries shown in Table 1 , the United States accounted for 27.7% of publications and ranked first in the number of publications. This indicates that the United States is the international scientific center of DR research.
Through the analysis of the distribution of research organizations, the most productive organizations and cooperation within the groups in a certain field can be identified. As shown in Table 2 , the most productive research institution was the University of Melbourne (252 documents), followed by Shanghai Jiao Tong University (216 documents) and Johns Hopkins University (208 documents), indicating that these research organizations are at the core of the entire research network. In terms of the number of links, the National University of Singapore presented the highest number (406 links), followed by the University of Melbourne (386 links), which indicated that these organizations are key nodes in the collaboration network (shown in Fig. 3 ).
The establishment of a co-authorship network knowledge map can provide valuable information to individual researchers seeking collaboration opportunities. The co-authorship groups are shown in Figure 4 : the red-colored group has Professor Klein as the center; the green-colored group has Professor Kowluru as the center; the blue-colored group has Professor Aiello as the center, and the yellow-colored group has Professor Spaide as the center.
A distribution analysis of academic journals helps determine the core journals in a certain research field. To this end, Investigative Ophthalmology & Visual Science, which has published the highest number of articles, is the most prolific journal on DR research.
4.2 Intellectual base
Based on the premise that high-quality research will be extensively cited, citation parameters were used to describe related topics within the selected articles. As shown in Table 5 , “A survey on deep learning in medical image analysis” ranked first in both citations and link strength. Through co-citation analysis, a large number of cited references can effectively show the background of a study. Therefore, we conducted a cluster analysis to explore the main topics in DR research. As shown in Table 5 , the 4 co-cited references list various clinical trials that mainly investigated the use of anti-VEGF medications in the management of DR. The publications entitled “A survey on deep learning in medical image analysis” and “Global Prevalence and Major Risk Factors of Diabetic Retinopathy” ranked in the top 2 in both frequency count and link weight, respectively, and are thus considered the core position of the whole knowledge map.
4.3 Research frontiers
The co-occurrence analysis of keywords is a common bibliometric research method in which the assigned keywords are considered to represent the search theme. Thus, the internal structure of the related literature and the frontier discipline can be revealed. As shown in Table 6 , DR topics mainly formed 6 clusters, and keywords in the same cluster showed greater similarity to a specific research topic than keywords in different clusters. Combined with the characteristics and current status of DR research, the 6 clusters are described as follows:
Cluster #1 (red) represents keywords mainly related to the pathogenesis of DR. The extracted co-occurrence keywords include “inflammation”, “angiogenesis”, “oxidative stress”, “apoptosis”, “hypoxia”, and “neurodegeneration”. Chronic hyperglycemia leads to increased inflammation and oxidative stress in the retina, which seems causally related to the development of at least diabetes-induced leakage and the degeneration of retinal capillaries. The incubation of retinal cells in high glucose causes the upregulation of proinflammatory factors, such as Inducible nitric oxide synthase, cyclooxygenase-2, and leukotrienes. [12–16] Inflammatory processes play an important role in the development of early and possibly later stages of DR, and the inflammatory pathogenesis of DR is based on the molecular characteristics of inflammation, as opposed to the classical cellular definition of inflammation. [17] Diabetes-induced oxidative stress plays a role in the development of inflammatory processes in the retina. [18,19] Two months of diabetes in rats significantly increased retinal levels of interleukin-1β and nuclear factor kappa-B, and antioxidants inhibited those increases. [20] Other research has shown that inhibition of interleukin -6 trans-signaling significantly reduces diabetes-induced oxidative damage in the retina. [21] Early proinflammatory changes, such as the appearance of microglia, the formation of advanced glycation endproducts, and the overproduction of VEGF, can directly cause hypoxia in the retina and not necessarily via reactive oxygen species. [22] VEGF is known to be a proinflammatory molecule whose vitreal levels are highly correlated with retinal neovascularization and edema. Many studies have evaluated the association of enzymes or gene polymorphisms with DR; for example, the nitric oxide synthase 3 gene rs869109213 polymorphism alone or in combination with the endothelin receptor B gene rs10507875 polymorphism may be associated with DR in Slovenian patients with type 2 diabetes mellitus, [23] and the methylenetetrahydrofolate reductase C677T polymorphism may contribute to DR development in multiethnic groups. [24] Researching the pathogenesis of DR could provide new therapeutic targets for inhibiting or preventing retinopathy.
Cluster #2 (green) represents keywords related to the epidemiology of and risk factors for DR. Age-standardized to the 2010 population, there are approximately 93 million people with DR, 17 million with proliferative DR, and 21 million with diabetic macular edema (DME). The overall prevalence is 34.6% for any DR, 6.96% for proliferative DR, 6.81% for DME, and 10.2% for vision-threatening DR. [25] The most common risk factors for DR are longer diabetes duration and poorer glycemic and blood pressure control. [26,27] Moreover, the overall prevalence is higher in people with type 1 diabetes than in those with type 2 diabetes. [25] In China in 2010, the pooled prevalence rates of any DR, nonproliferative DR, and proliferative DR were 1.14%, 0.90%, and 0.07% in the general population and 18.45%, 15.06%, and 0.99% in people with diabetes, respectively. A total of 13.16 million Chinese individuals aged 45 years and above live with DR, and the risk factors include residing in rural China, insulin treatment, elevated fasting blood glucose levels, and higher glyeosylated hemoglobin concentrations. [2] Other risk factors for DR include poor blood pressure and lipid control, high body mass index, puberty, pregnancy, and cataract surgery. There are weaker associations with some genetic and inflammatory markers. DR has become a serious global public health problem. [28] Diabetic nephropathy is another major public health problem with social and economic burdens. The prevalence of nephropathy among individuals with retinopathy is 35.6%, and there is a significant association between nephropathy and the development of retinopathy. Two abso ute risk factors for DR are nephropathy and hypertension. [29]
Cluster #3 (brown) represents keywords related to treatments for DR, such as “intravitreal injections” of “anti-VEGF”, “vitrectomy” and “panretinal photocoagulation” (PRP). DME is very common in proliferative diabetic retinopathy (PDR) and is characterized by metamorphopsia and loss of visual acuity (VA). Anti-VEGF intravitreal injections can benefit most patients. Aflibercept, bevacizumab, and ranibizumab are 3 commonly used anti-VEGF agents whose molecular structure and properties differ. [30] Many clinical trials have been conducted to determine the optimal anti-VEGF drug among the 3 listed above, as well as to elucidate their efficacy and guide their administration frequency for patients with DME. The Diabetic Retinopathy Clinical Research Network conducted a comparative effectiveness study for center-involved DME for all 3 drugs at a 2-year follow-up visit. Among eyes with better VA at baseline, no difference was identified in vision outcomes through the 2-year follow-up. For the eyes with worse VA at baseline, the advantage of aflibercept over bevacizumab for mean VA gain persisted through the 2 years, although the difference at 2 years was diminished. The VA difference between aflibercept and ranibizumab for eyes with worse VA at baseline that was noted at 1 year had decreased at 2 years. [31,32] The disadvantages are frequent injection, high medical costs, and poor results in some patients after multiple injections. Compared with anti-VEGF drugs, dexamethasone implants significantly improve anatomical outcomes. However, this does not translate to improve VA, which may be due to the progression of cataracts. Therefore, the dexamethasone implant may be recommended as the first choice for select cases, such as for pseudophakic eyes, anti-VEGF-resistant eyes, or patients reluctant to receive frequent intravitreal injections. [33] PDR is the worst stage of DR. For decades, PRP and vitrectomy have been the standard of care for the treatment of PDR. Recently, anti-VEGF has provided a new standard of care in PDR. [34] Tractional macular detachment occurs in 10% of eyes after anti-VEGF agent pretreatment before vitrectomy for complicated PDR. The main risk factors are days between anti-VEGF injection and vitrectomy, vitreous hemorrhage, and age. [35] However, preoperative intravitreal injections of anti-VEGF agents are effective and safe for complicated PDR. [36,37]
Cluster #4 (yellow) represents keywords related to the screening of DR. DR results in vision loss if not treated early. However, the interpretation of retinal images requires specialized knowledge and expertise in DR, and capital- and labor-intensive screening programs are difficult to rapidly scale up and expand to meet the needs of this growing global epidemic. Many artificial intelligence and deep learning-based methods have been developed from large image datasets in the assessment of retinal photographs for the detection and screening of DR as well as in the segmentation and assessment of optic coherence tomography (OCT) images for the diagnosis and screening of DME. [38] A hospital-based cross-sectional study showed that non-mydriatic funduscopic screening photography was practical and useful for the detection of DR in patients with type 1 and type 2 diabetes. [39] Telemedicine services facilitate the evaluation, diagnosis, and management of remote patients. In ophthalmology, telemedicine is in its infancy, particularly in its application to DR, as current models are largely performed via “store and forward” methods, but remote monitoring and interactive modalities exist. Telemedicine has the potential to improve access to care, decrease the cost of care, and improve adherence to evidence-based protocols. [40]
Cluster #5 (purple) represents keywords related to the histopathology of DR. The blood-retinal barrier (BRB) is a particularly tight and restrictive physiological barrier that regulates ion, protein, and water flux into and out of the retina. It consists of inner and outer components, the inner BRB being formed of tight junctions between retinal capillary endothelial cells and the outer BRB of tight junctions between retinal pigment epithelial cells. OCT is widely used to evaluate the BRB. DR and age-related macular degeneration (AMD) are the 2 most frequent and relevant retinal diseases that are directly associated with alterations of the BRB. [41] DR is a result of retinal neovascularization, and wet AMD is initiated by choroidal neovascularization. [41] It is well known that intravitreal injections of anti-VEGF agents are effective and safe in minimizing neovascularization. [42] Aflibercept and ranibizumab can both reduce macular edema and improve the VA of patients; these drugs are more commonly used in DME. [32] Swept-source OCT demonstrates a significant reduction in choroidal thickness of eyes with PDR compared with that of controls. In the foveal region, the choroid appears to be thinner in DR eyes than in diabetic eyes without retinopathy. [43]
Cluster #6 (blue) represents diagnostic methods for DR, such as “OCT”, “optical coherence tomography angiography (OCTA)”, and “fluorescein angiography (FA)”. FA is a dye-based ocular angiography and has been used in clinical practice for over 50 years. Only the superficial vascular plexus can be seen with it, and it provides limited information about the choroidal circulation. Despite these limitations, FA imaging offers many other advantages, including dynamic information regarding the transit of blood as well as identification of dye leakage from disruptions of BRB by disease. OCT constitutes one of the greatest advances in ophthalmic imaging and is capable of showing structural images of the retina and choroid. Building on this platform, OCTA provides depth-resolved images of blood flow in the retina and choroid with levels of detail far exceeding those obtained with FA. OCTA can generate high contrast, well-defined images of the microvasculature. Besides, OCTA images can be viewed in cross-section to confirm the depth location of vascular pathologies. Finally, OCTA can be performed much more rapidly than FA or indocyanine green angiography, streamlining the clinical workflow. At the same time, OCTA has important limitations. First, the fields of view that can be imaged by OCTA are smaller than those imaged by FA. Second, OCTA signals have a limited dynamic range. [44] Many studies have been conducted to compare OCTA with FA in patients with DR. [45–47]
These studies have found good agreement for the size of the foveal avascular zone (FAZ) and weak agreement regarding the number of microaneurysms (MAs) in both imaging modalities. It is better to assess the FAZ with OCTA and MAs with FA. Complementary use of FA and OCTA is the best diagnostic approach. [46] Diabetic macular ischemia grade, the size of the FAZ on OCTA, ellipsoid zone disruption, and disorganization of the retinal inner layers with OCT are associated with VA. The use of OCTA and OCT can predict VA in DR. [48] With the development of newer, wide-angle imaging technologies, wide-angle OCT, wide-angle OCTA, ultrawide-field FA, longitudinal wide-field swept-source OCTA, and en face OCTA have gradually been used in DR. [49–51] The multimodal imaging approach keeps the findings of any one modality in perspective by integrating that information with potentially useful data obtained by other imaging methods, which allows clinicians to gain the most information from each modality and thereby optimize patient care. [52]
5 Conclusions
We constructed a series of science maps of the annual number of publications, the distribution of countries, international collaborations, author productivity, source journals, cited references, and keywords in DR research. The results of this study may be helpful for ophthalmologists in choosing appropriate journals for publication and organizations or authors for collaborations. The extracted keywords enable researchers to identify new topics and assist them in predicting research directions. However, some limitations should be considered. First, the publications were extracted from the WoSCC between 2010 and 2019, which may not sufficiently represent all of the topics in DR research. Second, the primary data were extracted from WoSCC, which is a database more suited for performing citation analysis. Third, because most publications in the WoSCC were in English, a linguistic bias may exist. Last but not least, the collaboration network analysis successfully displayed the co-occurrence (distance between the 2 nodes/items) and the co-authorship of the institutions (the strength of the links). However, the strength of each pair of linked items is not shown in the final exported file, and VOSviewer was unable to generate a geographical map of co-authorship. Thus, a visualization of geographical location and co-authorship cannot be generated, and an understanding of the relationship thereof cannot be determined.
6 Future studies
Future studies may consider exploring a specific aspect of DR research, such as medicine and surgery. We will use different document databases and other bibliometric methods (such as bibliographic coupling analysis) to study other aspects of DR. In addition, Altmetrics, a new and comprehensive bibliometric method for evaluating the academic and social influences of research outputs, can also be applied in combination with scientometric analysis to better understand the trends and new areas of research in the field. It can be predicted that there will be an increasing number of papers in the coming year. In particular, studies about medicine and imaging will be the next popular hotspots and should receive more attention in the future.
Acknowledgments
The authors would like to thank all reviewers for their valuable comments.
Author contributions
Conceptualization: Yi Dong, Shixin Qi.
Data curation: Yi Dong, Huijuan Liu.
Formal analysis: Yi Dong.
Investigation: Yi Dong, Jianguo Yu.
Methodology: Yi Dong, Yanli Liu, Shixin Qi.
Project administration: Yi Dong, Yanli Liu, Jianguo Yu, Huijuan Liu.
Resources: Yi Dong, Yanli Liu.
Software: Yi Dong.
Supervision: Yi Dong, Jianguo Yu.
Validation: Yi Dong, Shixin Qi.
Visualization: Yi Dong.
Writing – original draft: Yanli Liu.
Writing – review & editing: Yi Dong, Yanli Liu.
- Cited Here |
- PubMed | CrossRef |
- Google Scholar
- View Full Text | PubMed | CrossRef |
bibliometric analysis; citespace; diabetic retinopathy; vosviewer
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Reporting quality of polycystic ovary syndrome practice guidelines based on the ..., therapeutic effect of percutaneous vertebroplasty and nonoperative treatment on ..., baihu jia renshen decoction for type 2 diabetic mellitus: a protocol for..., are parents’ statements reliable for diagnosis of serious bacterial infection..., zusanli (st36) acupoint injection for acute diarrhea in children under 5 years....
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Journal Proposal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
A systematic literature review on diabetic retinopathy using an artificial intelligence approach.

1. Introduction
1.1. applications of ai in retina images.
- Classification: Categorization cases are commonly used in binary or multi-class retinal image analysis, such as automated screenings or detecting of the stage of disease or type. ML and DL methods are applicable here based on the level of understandability required or the quantity of the dataset provided.
- Segmentation: The fundamental goal of segmentation-based approaches is to subdivide the objects in a picture. The primary purpose of all these techniques is to investigate morphological features or retrieve a meaningful pattern or feature of relevance from a snapshot, such as borders in 2D or 3D imaging. Segmentation of pigment epithelial detachment (PED) is used to diagnose chorioretinal diseases.
- Prediction: Most predicted situations are alarmed with illness development, future treatment outcomes based on an image, etc. The prediction approach can also be used to depict the local retention region.
1.2. Diabetic Retinopathy (DR)
- Hemorrhages (HM) appear as patches on the retina which can be 125 μm in diameter with an uneven edge. Its two categories are flames (superficial HM) and blot (deep HM) [ 23 ].
- Hard exudates: Hard exudates, which typically can be seen as bright yellow areas on the eye, are caused by hemolysis. These were also found in the eye’s coastal parts and had clear boundaries.
- Soft exudates: White spots on the eye generated from nerve fiber swelling are called soft exudates (cotton wool). These are ovular or circular. Soft or hard secretions constitute white lesions, whereas MA and HM were red growths (EX). A sample image of various stages of DR is provided in Figure 4 . DR is classified as non-proliferative DR (NPDR) and proliferative DR (PDR). Further, NPDR is classified as mild, moderate, and severe, as shown in Figure 5 .
Click here to enlarge figure
1.3. Evolution of DR Using AI
1.4. prior research.
- Datastores in the discipline of DR detection are accessible online, as well as the existence of DR datasets.
- An exhaustive survey of widely used ML and DL methodologies for DR detection is discussed.
- Feature extraction and classification techniques used in DR are discussed.
- Future research concepts such as domain adaptation, multitask learning, and explainable AI in DR detection are discussed
1.5. Motivation
1.6. research goals, 1.7. contribution of the study.
- To exploring available data sets which have been used for detecting DR.
- To investigate artificial intelligence strategies that have been employed in the literature for DR detection.
- To explore feature extraction and classification.
- To study multiple assessment metrics to analyze DR detection and categorization.
- To highlight the scope of future research, concepts such as domain adaptation, multitask learning, and explainable AI in DR detection techniques used in DR.
2. Research Mechanism of Study
Paradigms for inclusion and exclusion.
- RC1: Recommendations and results must be included in research articles.
- RC2: Scientific data must be included in scientific papers to support their conclusions.
- RC3: The aims and findings of the research must be expressed.
- RC4: For scientific studies, citations must be proper and adequate.
3. RQ1 Artificial Intelligence for DR Detection
3.1. machine learning techniques in dr detection, 3.2. deep learning in dr screening, 3.3. transfer learning in dr, 4. rq2 feature extraction techniques for dr, 4.1. explicit or traditional feature extraction methods, 4.2. direct methods, 5. rq3 datasets available for dr, 6. rq4 evaluation measures used for dr detection, 6.1. false positive rate (fpr), 6.2. false negative rate (fnr), 6.3. accuracy [ 89 ], 6.4. specificity, 6.5. sensitivity/recall rate, 6.6. f-score, 6.8. positive predictive value (ppv), 6.9. negative predictive value (npv), 6.10. false discovery rate (fdr), 6.11. confusion matrix.
- True Negative: when the model’s predicted and the actual value is No.
- True Positive: when the model’s predicted and the actual value is Yes.
- False Negative: when the model’s predicted value is Yes, and the actual value is No. It is also known as a Type-II mistake.
- False Positive: when the model’s predicted value is No, and the actual value is Yes. A type-I mistake is another name for it.
6.12. Kappa Value
7. emr and biomarkers in dr.
- Genetics: The investigation of genes associated with the development of advanced DR, vascular endothelial growth factor (VEGF), lipoproteins, and inflammation. There have been genome-wide association studies and single nucleotide polymorphisms (SNPs) linked to an enhanced danger of sight-threatening retinopathy [ 164 ].
- Epigenetics: It is the study of how environmental variables interact with genes. DNA methylation, histone modification, and microRNAs are among the biomarkers being investigated [ 165 , 166 ].
- Proteomics: It is the study of protein structure and function research in cultured cells and tissues. A current study shows that diabetic patients have higher levels of transport proteins (vitamin D binding protein), arginine N-methyltransferase 5, and inflammatory proteins (leucine-rich alpha-2-glycoprotein) [ 167 , 168 ].
- Metabolomics: The study of chemical traces left by biological activities. Data on increased metabolite cytidine, cytosine, and thymidine found in DR patients using mass spectrometry is included in the studies. These nucleotide concentrations may be relevant in monitoring DR progression and evaluating therapy [ 169 ].
8. RQ5 Challenges and Future Research Directions
9. discussion, 10. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Khatri, M. Diabetes Complications. Available online: https://www.webmd.com/diabetes/diabetes-complications (accessed on 18 May 2022).
- Chakrabarti, R.; Harper, C.A.; Keeffe, J.E. Diabetic Retinopathy Management Guidelines. Expert Rev. Ophthalmol. 2012 , 7 , 417–439. [ Google Scholar ] [ CrossRef ]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs- an extension of the modified Airlie House classification. Ophthalmology 2020 , 127 , S99–S119. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Scanlon, P.H.; Wilkinson, C.P.; Aldington, S.J.; Matthews, D.R. A Practical Manual of Diabetic Retinopathy Management , 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 1–214. [ Google Scholar ] [ CrossRef ]
- Ravelo, J.L. Aging and Population Growth, Challenges for Vision Care: WHO Report. 2019. Available online: https://www.devex.com/news/aging-and-population-growth-challenges-for-vision-care-who-report-95763 (accessed on 3 January 2022).
- WHO. World Report on Vision, 2019. Available online: https://www.who.int/publications/i/item/9789241516570 (accessed on 3 January 2022).
- Kumar, R.; Pal, R. India achieves WHO recommended doctor population ratio: A call for a paradigm shift in public health discourse! J. Fam. Med. Prim. Care 2018 , 7 , 841–844. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- WHO. Global Data on Visual Impairment. 2010. Available online: http://www.who.int/blindness/GLOBALDATAFINALforweb.pdf (accessed on 5 May 2022).
- Centers for Disease Control and Prevention. Common Eye Disorders and Diseases. 2020. Available online: https://www.cdc.gov/visionhealth/basics/ced/index.html (accessed on 10 May 2022).
- Malik, U. Most Common Eye Problems—Signs, Symptoms and Treatment Options. 2021. Available online: https://irisvision.com/most-common-eye-problems-signs-symptoms-and-treatment/ (accessed on 3 April 2022).
- Stoitsis, J.; Valavanis, I.; Mougiakakou, S.G.; Golemati, S.; Nikita, A.; Nikita, K.S. Computer aided diagnosis based on medical image processing and artificial intelligence methods. Nucl. Instrum. Methods Phys. Res. Sect. A 2006 , 569 , 591–595. [ Google Scholar ] [ CrossRef ]
- Mushtaq, G.; Siddiqui, F. Detection of diabetic retinopathy using deep learning methodology. IOP Conf. Ser. Mater. Sci. Eng. 2021 , 1070 , 012049. [ Google Scholar ] [ CrossRef ]
- Taylor, R.; Batey, D. Handbook of Retinal Screening in Diabetes: Diagnosis and Management. In Handbook of Retinal Screening in Diabetes: Diagnosis and Management , 2nd ed.; Wiley-Blackwell: Chichester, UK, 2012; pp. 89–103. [ Google Scholar ]
- Gupta, A.; Chhikara, R. Diabetic Retinopathy: Present and Past. Procedia Comput. Sci. 2018 , 132 , 1432–1440. [ Google Scholar ] [ CrossRef ]
- Ishtiaq, U.; Kareem, S.A.; Abdullah, E.R.M.F.; Mujtaba, G.; Jahangir, R.; Ghafoor, H.Y. Diabetic retinopathy detection through artificial intelligent techniques: A review and open issues. Multimedia Tools Appl. 2019 , 79 , 15209–15252. [ Google Scholar ] [ CrossRef ]
- Lin, J.; Yu, L.; Weng, Q.; Zheng, X. Retinal image quality assessment for diabetic retinopathy screening: A survey. Multimedia Tools Appl. 2020 , 79 , 16173–16199. [ Google Scholar ] [ CrossRef ]
- Qureshi, I. Glaucoma Detection in Retinal Images Using Image Processing Techniques: A Survey. Int. J. Adv. Netw. Appl. 2015 , 7 , 2705–2718. Available online: http://www.ijana.in/papers/V7I2-10.pdf (accessed on 5 April 2022).
- Wang, Z.; Yin, Y.; Shi, J.; Fang, W.; Li, H.; Wang, X. Zoom-in-Net: Deep Mining Lesions for Diabetic Retinopathy Detection. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Quebec City, QC, Canada, 11–13 September 2017; pp. 267–275. [ Google Scholar ]
- Scotland, G.S.; McNamee, P.; Fleming, A.D.; Goatman, K.A.; Philip, S.; Prescott, G.J.; Sharp, P.F.; Williams, G.J.; Wykes, W.; Leese, G.P.; et al. Costs and consequences of automated algorithms versus manual grading for the detection of referable diabetic retinopathy. Br. J. Ophthalmol. 2010 , 94 , 712–719. [ Google Scholar ] [ CrossRef ]
- Difference between Normal Vision and DR Vision. Available online: https://www.researchgate.net/publication/350930649_DRISTI_a_hybrid_deep_neural_network_for_diabetic_retinopathy_diagnosis/figures?lo=1 (accessed on 20 May 2022).
- Li, T.; Gao, Y.; Wang, K.; Guo, S.; Liu, H.; Kang, H. Diagnostic assessment of deep learning algorithms for diabetic retinopathy screening. Inf. Sci. 2019 , 501 , 511–522. [ Google Scholar ] [ CrossRef ]
- Pratt, H.; Coenen, F.; Broadbent, D.M.; Harding, S.P.; Zheng, Y. Convolutional neural networks for diabetic retinopathy. Procedia Comput. Sci. 2016 , 90 , 200–205. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Alyoubi, W.L.; Shalash, W.M.; Abulkhair, M.F. Diabetic retinopathy detection through deep learning techniques: A review. Inform. Med. Unlocked 2020 , 20 , 100377. [ Google Scholar ] [ CrossRef ]
- Arrigo, A.; Teussink, M.; Aragona, E.; Bandello, F.; Parodi, M.B. MultiColor imaging to detect different subtypes of retinal microaneurysms in diabetic retinopathy. Eye 2020 , 35 , 277–281. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yasin, S.; Iqbal, N.; Ali, T.; Draz, U.; Alqahtani, A.; Irfan, M.; Rehman, A.; Glowacz, A.; Alqhtani, S.; Proniewska, K.; et al. Severity Grading and Early Retinopathy Lesion Detection through Hybrid Inception-ResNet Architecture. Sensors 2021 , 21 , 6933. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Guo, S.; Li, T.; Kang, H.; Li, N.; Zhang, Y.; Wang, K. An end-to-end unified framework for multi-lesion segmentation offundus images. Neurocomput 2019 , 349 , 52–63. [ Google Scholar ] [ CrossRef ]
- Li, B.; Li, H.K. Automated Analysis of Diabetic Retinopathy Images: Principles, Recent Developments, and Emerging Trends. Curr. Diabetes Rep. 2013 , 13 , 453–459. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mishra, A.; Singh, L.; Pandey, M. Short Survey on machine learning techniques used for diabetic retinopathy detection. In Proceedings of the IEEE 2021 International Conference on Computing, Communication, and Intelligent Systems (ICCCIS), Greater Noida, India, 19–20 February 2021; pp. 601–606. [ Google Scholar ] [ CrossRef ]
- Oh, K.; Kang, H.M.; Leem, D.; Lee, H.; Seo, K.Y.; Yoon, S. Early detection of diabetic retinopathy based on deep learning and ultra-wide-field fundus images. Sci. Rep. 2021 , 11 , 1–9. [ Google Scholar ] [ CrossRef ]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.S.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell 2018 , 172 , 1122–1131.e9. [ Google Scholar ] [ CrossRef ]
- Mansour, R.F. Deep-learning-based automatic computer-aided diagnosis system for diabetic retinopathy. Biomed. Eng. Lett. 2017 , 8 , 41–57. [ Google Scholar ] [ CrossRef ]
- Pepose, J.S. A prospective randomized clinical evaluation of 3 presbyopia-correcting intraocular lenses after cataract extraction. Am. J. Ophthalmol. 2014 , 3–9. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Boudry, C.; Denion, E.; Mortemousque, B.; Mouriaux, F. Trends and topics in eye disease research in PubMed from 2010 to 2014. PeerJ 2016 , 4 , e1557. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Gardner, G.G.; Keating, D.; Williamson, T.H.; Elliott, A.T. ORIGINAL ARTICLES-Clinical science Automatic detection of diabetic retinopathy using an artificial neural network: A screening tool. Br. J. Ophthalmol. 1996 , 80 , 940–944. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Shi, C.; Lee, J.; Wang, G.; Dou, X.; Yuan, F.; Zee, B. Assessment of image quality on color fundus retinal images using the automatic retinal image analysis. Sci. Rep. 2022 , 12 , 1–11. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Franklin, S.W.; Rajan, S.E. An automated retinal imaging method for the early diagnosis of diabetic retinopathy. Technol. Health Care 2013 , 21 , 557–569. [ Google Scholar ] [ CrossRef ]
- Li, S.; Zhao, R.; Zou, H. Artificial intelligence for diabetic retinopathy. Chin. Med. J. 2021 , 135 , 253–260. [ Google Scholar ] [ CrossRef ]
- Pragathi, P.; Rao, A.N. An effective integrated machine learning approach for detecting diabetic retinopathy. Open Comput. Sci. 2022 , 12 , 83–91. [ Google Scholar ] [ CrossRef ]
- Zhang, W.; Zhong, J.; Yang, S.; Gao, Z.; Hu, J.; Chen, Y.; Yi, Z. Automated identification and grading system of diabetic retinopathy using deep neural networks. Knowl. Based Syst. 2019 , 175 , 12–25. [ Google Scholar ] [ CrossRef ]
- Asiri, N.; Hussain, M.; Al Adel, F.; Alzaidi, N. Deep learning based computer-aided diagnosis systems for diabetic retinopathy: A survey. Artif. Intell. Med. 2019 , 99 , 101701. [ Google Scholar ] [ CrossRef ]
- Kamal, M.M.; Shanto, M.H.I.; Mirza Mahmud Hossan, M.; Hasnat, A.; Sultana, S.; Biswas, M. A Comprehensive Review on the Diabetic Retinopathy, Glaucoma and Strabismus Detection Techniques Based on Machine Learning and Deep Learning. Eur. J. Med. Health Sci. 2022 , 24–40. [ Google Scholar ] [ CrossRef ]
- Aoun, S.M.; Breen, L.J.; Oliver, D.; Henderson, R.D.; Edis, R.; O’Connor, M.; Howting, D.; Harris, R.; Birks, C. Family carers’ experiences of receiving the news of a diagnosis of Motor Neurone Disease: A national survey. J. Neurol. Sci. 2017 , 372 , 144–151. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khade, S.; Ahirrao, S.; Phansalkar, S.; Kotecha, K.; Gite, S.; Thepade, S.D. Iris Liveness Detection for Biometric Authentication: A Systematic Literature Review and Future Directions. Inventions 2021 , 6 , 65. [ Google Scholar ] [ CrossRef ]
- Grzybowski, A.; Brona, P.; Lim, G.; Ruamviboonsuk, P.; Tan, G.S.W.; Abramoff, M.; Ting, D.S.W. Artificial intelligence for diabetic retinopathy screening: A review. Eye 2020 , 34 , 451–460. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ribeiro, L.; Oliveira, C.M.; Neves, C.; Ramos, J.D.; Ferreira, H.; Cunha-Vaz, J. Screening for Diabetic Retinopathy in the Central Region of Portugal. Added Value of Automated ‘Disease/No Disease’ Grading. Ophthalmologica 2015 , 233 , 96–103. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ipp, E.; Liljenquist, D.; Bode, B.; Shah, V.N.; Silverstein, S.; Regillo, C.D.; Lim, J.I.; Sadda, S.; Domalpally, A.; Gray, G.; et al. Pivotal Evaluation of an Artificial Intelligence System for Autonomous Detection of Referrable and Vision-Threatening Diabetic Retinopathy. JAMA Netw. Open 2021 , 4 , e2134254. [ Google Scholar ] [ CrossRef ]
- Larsen, N.; Godt, J.; Grunkin, M.; Lund-Andersen, H.; Larsen, M. Automated Detection of Diabetic Retinopathy in a Fundus Photographic Screening Population. Investig. Opthalmology Vis. Sci. 2003 , 44 , 767–771. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ting, D.S.W.; Cheung, C.Y.-L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; Yeo, I.Y.S.; Lee, S.Y.; et al. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images from Multiethnic Populations With Diabetes. JAMA 2017 , 318 , 2211–2223. [ Google Scholar ] [ CrossRef ]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016 , 316 , 2402–2410. [ Google Scholar ] [ CrossRef ]
- Abràmoff, M.D.; Lavin, P.T.; Birch, M.; Shah, N.; Folk, J.C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit. Med. 2018 , 39 , 1–8. [ Google Scholar ] [ CrossRef ]
- Dong, X.; Du, S.; Zheng, W.; Cai, C.; Liu, H.; Zou, J. Evaluation of an Artificial Intelligence System for the Detection of Diabetic Retinopathy in Chinese Community Healthcare Centers. Front. Med. 2022 , 9 , 840024. [ Google Scholar ] [ CrossRef ]
- Wu, J.; Xin, J.; Hong, L.; You, J.; Zheng, N. New hierarchical approach for microaneurysms detection with matched filter and machine learning. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: Milan, Italy, 2015; pp. 4322–4325. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Biyani, R.S.; Patre, B.M. A clustering approach for exudates detection in screening of diabetic retinopathy. In Proceedings of the 2016 International Conference on Signal and Information Processing (IConSIP), Nanded, India, 6–8 October 2016; pp. 1–5. [ Google Scholar ] [ CrossRef ]
- Naqvi, S.A.G.; Zafar, M.F.; Haq, I.U. Referral system for hard exudates in eye fundus. Comput. Biol. Med. 2015 , 64 , 217–235. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rahimy, E. Deep learning applications in ophthalmology. Curr. Opin. Ophthalmol. 2018 , 29 , 254–260. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sisodia, D.S.; Nair, S.; Khobragade, P. Diabetic Retinal Fundus Images: Preprocessing and Feature Extraction for Early Detection of Diabetic Retinopathy. Biomed. Pharmacol. J. 2017 , 10 , 615–626. [ Google Scholar ] [ CrossRef ]
- Xiao, Z.; Zhang, X.; Geng, L.; Zhang, F.; Wu, J.; Tong, J.; Ogunbona, P.O.; Shan, C. Automatic non-proliferative diabetic retinopathy screening system based on color fundus image. Biomed. Eng. Online 2017 , 16 , 122. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Srivastava, R.; Duan, L.; Wong, D.W.; Liu, J.; Wong, T.Y. Detecting retinal microaneurysms and hemorrhages with robustness to the presence of blood vessels. Comput. Methods Programs Biomed. 2017 , 138 , 83–91. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vanithamani, R.R.C.R.; Renee Christina, R. Exudates in detection and classification of diabetic retinopathy. In International Conference on Soft Computing and Pattern Recognition ; Springer: Cham, Germany, 2016; pp. 252–261. Available online: https://books.google.co.in/books?id=hFNuDwAAQBAJ&pg=PA108&lpg=PA108&dq=Vanithamani+R,+Renee+Christina+R+(2018)+Exudates+in+detection+and+classification+of+diabetic+retinopathy:+252–261&source=bl&ots=CWbiXEy9bP&sig=ACfU3U1vfBvwrh06MvSJbSKzMp8Sl2Cm4w&hl=en& (accessed on 5 April 2022).
- Wang, S.; Tang, H.L.; Al Turk, L.I.; Hu, Y.; Sanei, S.; Saleh, G.M.; Peto, T. Localizing Microaneurysms in Fundus Images Through Singular Spectrum Analysis. IEEE Trans. Biomed. Eng. 2016 , 64 , 990–1002. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nijalingappa, P.; Sandeep, B. Machine learning approach for the identification of diabetes retinopathy and its stages. In Proceedings of the 2015 International Conference on Applied and Theoretical Computing and Communication Technology (iCATccT), Davangere, India, 29–31 October 2016; pp. 653–658. [ Google Scholar ] [ CrossRef ]
- Xiao, D.; Yu, S.; Vignarajan, J.; An, D.; Tay-Kearney, M.-L.; Kanagasingam, Y. Retinal hemorrhage detection by rule-based and machine learning approach. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Republic of Korea, 11–15 July 2017; pp. 660–663. [ Google Scholar ] [ CrossRef ]
- Almotiri, J.; Elleithy, K.; Elleithy, A. Retinal Vessels Segmentation Techniques and Algorithms: A Survey. Appl. Sci. 2018 , 8 , 155. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Bui, T.; Maneerat, N.; Watchareeruetai, U. Detection of cotton wool for diabetic retinopathy analysis using neural network. In Proceedings of the IEEE 10th International Workshop on Computational Intelligence and Applications, Hiroshima, Japan, 11–12 November 2017; pp. 203–206. [ Google Scholar ] [ CrossRef ]
- Franklin, S.W.; Rajan, S.E. Computerized screening of diabetic retinopathy employing blood vessel segmentation in retinal images. Biocybern. Biomed. Eng. 2014 , 34 , 117–124. [ Google Scholar ] [ CrossRef ]
- Hanđsková, V.; Pavlovičova, J.; Oravec, M.; Blaško, R. Diabetic Rethinopathy Screening by Bright Lesions Extraction from Fundus Images. J. Electr. Eng. 2013 , 64 , 311–316. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Kavitha, M.; Palani, S. Hierarchical classifier for soft and hard exudates detection of retinal fundus images. J. Intell. Fuzzy Syst. 2014 , 27 , 2511–2528. [ Google Scholar ] [ CrossRef ]
- Paing, M.P.; Choomchuay, S.; Yodprom, M.D.R. Detection of lesions and classification of diabetic retinopathy using fundus images. In Proceedings of the 2016 9th Biomedical engineering international conference (BMEiCON), Laung Prabang, Laos, 7–9 December 2016; pp. 1–5. [ Google Scholar ] [ CrossRef ]
- Zhou, W.; Wu, C.; Chen, D.; Yi, Y.; Du, W. Automatic Microaneurysm Detection Using the Sparse Principal Component Analysis-Based Unsupervised Classification Method. IEEE Access 2017 , 5 , 2563–2572. [ Google Scholar ] [ CrossRef ]
- Kusakunniran, W.; Wu, Q.; Ritthipravat, P.; Zhang, J. Hard exudates segmentation based on learned initial seeds and iterative graph cut. Comput. Methods Programs Biomed. 2018 , 158 , 173–183. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khade, S.; Gite, S.; Thepade, S.D.; Pradhan, B.; Alamri, A. Detection of Iris Presentation Attacks Using Feature Fusion of Thepade’s Sorted Block Truncation Coding with Gray-Level Co-Occurrence Matrix Features. Sensors 2021 , 21 , 7408. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wen, L. Algorithms: A Comparative Study of Bagging, Boosting and Stacking Techniques. Remote Sens 2020 , 12 , 1683. [ Google Scholar ] [ CrossRef ]
- Brownlee, J. Stacking Ensemble Machine Learning with Python. In Machine Learning Mastery ; Machine Learning Mastery: San Francisco, CA, USA, 2020; Available online: https://machinelearningmastery.com/stacking-ensemble-machine-learning-with-python/ (accessed on 21 May 2022).
- Mane, V.M.; Jadhav, D.V.; Shirbahadurkar, S.D. Hybrid classifier and region-dependent integrated features for detection of diabetic retinopathy. J. Intell. Fuzzy Syst. 2017 , 32 , 2837–2844. [ Google Scholar ] [ CrossRef ]
- Fraz, M.M.; Jahangir, W.; Zahid, S.; Hamayun, M.M.; Barman, S.A. Multiscale segmentation of exudates in retinal images using contextual cues and ensemble classification. Biomed. Signal Process. Control. 2017 , 35 , 50–62. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Prentašić, P.; Lončarić, S. Detection of exudates in fundus photographs using deep neural networks and anatomical landmark detection fusion. Comput. Methods Programs Biomed. 2016 , 137 , 281–292. [ Google Scholar ] [ CrossRef ]
- Bala, M.P.; Vijayachitra, S. Early detection and classification of microaneurysms in retinal fundus images using sequential learning methods. Int. J. Biomed. Eng. Technol. 2014 , 15 , 128. [ Google Scholar ] [ CrossRef ]
- Sopharak, A.; Uyyanonvara, B.; Barman, S.; Williamson, T. Comparative Analysis of Automatic Exudate Detection between Machine Learning and Traditional Approaches. IEICE Trans. Inf. Syst. 2009 , 92 , 2264–2271. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Srinivasan, R.; Surya, J.; Ruamviboonsuk, P.; Chotcomwongse, P.; Raman, R. Influence of Different Types of Retinal Cameras on the Performance of Deep Learning Algorithms in Diabetic Retinopathy Screening. Life 2022 , 12 , 1610. [ Google Scholar ] [ CrossRef ]
- Valarmathi, S.; Vijayabhanu, R. A Survey on Diabetic Retinopathy Disease Detection and Classification using Deep Learning Techniques. In Proceedings of the 2021 IEEE 7th International Conference on Bio Signals, Images and Instrumentation, ICBSII, Chennai, India, 25–27 March 2021; pp. 1–4. [ Google Scholar ] [ CrossRef ]
- Wang, X.; Lu, Y.; Wang, Y.; Chen, W.-B. Diabetic Retinopathy Stage Classification Using Convolutional Neural Networks. In Proceedings of the 2018 IEEE 19th International Conference on Information Reuse and Integration for Data Science, IRI, Salt Lake City, UT, USA, 7–9 July 2018; pp. 465–471. [ Google Scholar ] [ CrossRef ]
- Chudzik, P.; Majumdar, S.; Calivá, F.; Al-Diri, B.; Hunter, A. Microaneurysm detection using fully convolutional neural networks. Comput. Methods Programs Biomed. 2018 , 158 , 185–192. [ Google Scholar ] [ CrossRef ]
- Yan, Y.; Gong, J.; Liu, Y. A Novel Deep Learning Method for Red Lesions Detection Using Hybrid Feature. In Proceedings of the 31st Chinese Control and Decision Conference, CCDC, Nanchang, China, 3–5 June 2019; pp. 2287–2292. [ Google Scholar ] [ CrossRef ]
- Guo, Y.; Liu, Y.; Oerlemans, A.; Lao, S.; Wu, S.; Lew, M.S. Deep learning for visual understanding: A review. Neurocomputing 2016 , 187 , 27–48. [ Google Scholar ] [ CrossRef ]
- Abbas, Q.; Ibrahim, M.E.A.; Jaffar, M.A. Video scene analysis: An overview and challenges on deep learning algorithms. Multimed. Tools Appl. 2017 , 77 , 20415–20453. [ Google Scholar ] [ CrossRef ]
- Gurcan, O.F.; Beyca, O.F.; Dogan, O. A Comprehensive Study of Machine Learning Methods on Diabetic Retinopathy Classification. Int. J. Comput. Intell. Syst. 2021 , 14 , 1132–1141. [ Google Scholar ] [ CrossRef ]
- Khade, S.; Gite, S.; Pradhan, B. Iris Liveness Detection Using Multiple Deep Convolution Networks. Big Data Cogn. Comput. 2022 , 6 , 67. [ Google Scholar ] [ CrossRef ]
- Ketkar, N.; Moolayil, J. Deep Learning with Python ; Manning Publications: New York, NY, USA, 2021. [ Google Scholar ] [ CrossRef ]
- Olivas, E.S.; Guerrero, J.D.M.; Martinez-Sober, M.; Magdalena-Benedito, J.R.; Serrano, L. Magdalena-Benedito, Handbook of Research on Machine Learning Applications and Trends: Algorithms, Methods, and Techniques ; IGI Global: Pennsylvania, PA, USA, 2010. [ Google Scholar ]
- Masood, S.; Luthra, T.; Sundriyal, H.; Ahmed, M. Identification of diabetic retinopathy in eye images using transfer learning. In Proceedings of the International Conference on Computing, Communication and Automation (ICCCA), Greater Noida, India, 5–6 May 2017; pp. 1183–1187. [ Google Scholar ] [ CrossRef ]
- Xu, X.; Lin, J.; Tao, Y.; Wang, X. An Improved DenseNet Method Based on Transfer Learning for Fundus Medical Images. In Proceedings of the 2018 7th international conference on digital home (ICDH), Guilin, China, 30 November–1 December 2018; pp. 137–140. [ Google Scholar ] [ CrossRef ]
- Lian, C.; Liang, Y.; Kang, R.; Xiang, Y. Deep Convolutional Neural Networks for Diabetic Retinopathy Classification. ACM Int. Conf. Proceeding Ser. 2018 , 72 , 68–72. [ Google Scholar ] [ CrossRef ]
- Blakely, M. ‘The Importance of Sight and Vision,’ Marvel Optics. 2015. Available online: https://www.marveloptics.com/blog/the-importance-of-sight-and-vision-molly-blakely/ (accessed on 7 April 2022).
- Quellec, G.; Charrière, K.; Boudi, Y.; Cochener, B.; Lamard, M. Deep image mining for diabetic retinopathy screening. Med. Image Anal. 2017 , 39 , 178–193. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Oliveira, A.; Pereira, S.; Silva, C. Retinal vessel segmentation based on Fully Convolutional Neural Networks. Expert Syst. Appl. 2018 , 112 , 229–242. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Orlando, J.I.; Prokofyeva, E.; del Fresno, M.; Blaschko, M.B. An ensemble deep learning based approach for red lesion detection in fundus images. Comput. Methods Programs Biomed. 2018 , 153 , 115–127. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Mahendran, G.; Dhanasekaran, R. Investigation of the severity level of diabetic retinopathy using supervised classifier algorithms. Comput. Electr. Eng. 2015 , 45 , 312–323. [ Google Scholar ] [ CrossRef ]
- Wu, D.; Zhang, M.; Liu, J.-C.; Bauman, W. On the Adaptive Detection of Blood Vessels in Retinal Images. IEEE Trans. Biomed. Eng. 2006 , 53 , 341–343. [ Google Scholar ] [ CrossRef ]
- Sánchez, C.I.; García, M.; Mayo, A.; Lopez, M.I.; Hornero, R. Retinal image analysis based on mixture models to detect hard exudates. Med. Image Anal. 2009 , 13 , 650–658. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- García, M.; Sánchez, C.I.; López, M.I.; Abásolo, D.; Hornero, R. Neural network based detection of hard exudates in retinal images. Comput. Methods Programs Biomed. 2009 , 93 , 9–19. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Sánchez, C.I.; Hornero, R.; López, M.I.; Aboy, M.; Poza, J.; Abásolo, D. A novel automatic image processing algorithm for detection of hard exudates based on retinal image analysis. Med. Eng. Phys. 2008 , 30 , 350–357. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Quellec, G.; Lamard, M.; Abramoff, M.; Decencière, E.; Lay, B.; Erginay, A.; Cochener, B.; Cazuguel, G. A multiple-instance learning framework for diabetic retinopathy screening. Med. Image Anal. 2012 , 16 , 1228–1240. [ Google Scholar ] [ CrossRef ]
- Köse, C.; Şevik, U.; Ikibaş, C.; Erdöl, H. Simple methods for segmentation and measurement of diabetic retinopathy lesions in retinal fundus images. Comput. Methods Programs Biomed. 2012 , 107 , 274–293. [ Google Scholar ] [ CrossRef ]
- Giancardo, L.; Meriaudeau, F.; Karnowski, T.P.; Li, Y.; Garg, S.; Tobin, K.W.; Chaum, E. Exudate-based diabetic macular edema detection in fundus images using publicly available datasets. Med. Image Anal. 2012 , 16 , 216–226. [ Google Scholar ] [ CrossRef ]
- Zhang, B.; Karray, F.; Li, Q.; Zhang, L. Sparse Representation Classifier for microaneurysm detection and retinal blood vessel extraction. Inf. Sci. 2012 , 200 , 78–90. [ Google Scholar ] [ CrossRef ]
- Qureshi, R.J.; Kovacs, L.; Harangi, B.; Nagy, B.; Peto, T.; Hajdu, A. Combining algorithms for automatic detection of optic disc and macula in fundus images. Comput. Vis. Image Underst. 2012 , 116 , 138–145. [ Google Scholar ] [ CrossRef ]
- Noronha, K.; Acharya, U.R.; Nayak, K.P.; Kamath, S.; Bhandary, S.V. Decision support system for diabetic retinopathy using discrete wavelet transform. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2012 , 227 , 251–261. [ Google Scholar ] [ CrossRef ]
- Gharaibeh, N.; Al-Hazaimeh, O.M.; Abu-Ein, A.; Nahar, K.M. A Hybrid SVM NAÏVE-BAYES Classifier for Bright Lesions Recognition in Eye Fundus Images. Int. J. Electr. Eng. Inform. 2021 , 13 , 530–545. [ Google Scholar ] [ CrossRef ]
- Al Hazaimeh, O.M.; Nahar, K.M.; Al Naami, B.; Gharaibeh, N. An effective image processing method for detection of diabetic retinopathy diseases from retinal fundus images. Int. J. Signal Imaging Syst. Eng. 2018 , 11 , 206. [ Google Scholar ] [ CrossRef ]
- Akram, M.U.; Khalid, S.; Khan, S.A. Identification and classification of microaneurysms for early detection of diabetic retinopathy. Pattern Recognit. 2013 , 46 , 107–116. [ Google Scholar ] [ CrossRef ]
- Harini, R.; Sheela, N. Feature extraction and classification of retinal images for automated detection of Diabetic Retinopathy. In Proceedings of the 2016 Second International Conference on Cognitive Computing and Information Processing (CCIP), Mysuru, India, 12–13 August 2016; Nagabhusan, T.N., Sundararajan, N., Suresh, S., Eds.; Sri Jayachamarajendra College of Engineering, JSS TI Campus: Mysuru, India, 2016; p. 570006. [ Google Scholar ]
- Umapathy, A.; Sreenivasan, A.; Nairy, D.S.; Natarajan, S.; Rao, B.N. Image Processing, Textural Feature Extraction and Transfer Learning based detection of Diabetic Retinopathy. In Proceedings of the 2019 9th International Conference on Bioscience, Biochemistry and Bioinformatics, Singapore, 7–9 January 2019; pp. 17–21. [ Google Scholar ] [ CrossRef ]
- Zago, G.T.; Andreão, R.V.; Dorizzi, B.; Salles, E.O.T. Diabetic retinopathy detection using red lesion localization and convolutional neural networks. Comput. Biol. Med. 2019 , 116 , 103537. [ Google Scholar ] [ CrossRef ]
- Jiang, H.; Yang, K.; Gao, M.; Zhang, D.; Ma, H.; Qian, W. An Interpretable Ensemble Deep Learning Model for Diabetic Retinopathy Disease Classification. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 2045–2048. [ Google Scholar ] [ CrossRef ]
- Abràmoff, M.D.; Garvin, M.K.; Sonka, M. Retinal Imaging and Image Analysis. IEEE Rev. Biomed. Eng. 2010 , 3 , 169–208. [ Google Scholar ] [ CrossRef ]
- Gargeya, R.; Leng, T. Automated Identification of Diabetic Retinopathy Using Deep Learning. Ophthalmology 2017 , 124 , 962–969. [ Google Scholar ] [ CrossRef ]
- Doshi, D.; Shenoy, A.; Sidhpura, D.; Gharpure, P. Diabetic retinopathy detection using deep convolutional neural networks. In Proceedings of the 2016 International Conference on Computing, Analytics and Security Trends (CAST), Pune, India, 11 July 2016; pp. 261–266. [ Google Scholar ]
- Ghosh, R.; Ghosh, K.; Maitra, S. Automatic detection and classification of diabetic retinopathy stages using CNN. In Proceedings of the 2017 4th International Conference on Signal Processing and Integrated Networks (SPIN), Delhi, India, 26–27 August 2017; pp. 550–554. [ Google Scholar ] [ CrossRef ]
- Gondal, W.M.; Kohler, J.M.; Grzeszick, R.; Fink, G.A.; Hirsch, M. Weakly-supervised localization of diabetic retinopathy lesions in retinal fundus images. In Proceedings of the 2017 IEEE international conference on image processing (ICIP), Beijing, China, 17–20 September 2017; pp. 2069–2073. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Jiang, Y.; Wu, H.; Dong, J. Automatic Screening of Diabetic Retinopathy Images with Convolution Neural Network Based on Caffe Framework. In Proceedings of the 1st International Conference on Medical and Health Informatics 2017, Taichung city, Taiwan, 20–22 May 2017; pp. 90–94. [ Google Scholar ] [ CrossRef ]
- Prentasic, P.; Loncaric, S. Weighted ensemble based automatic detection of exudates in fundus photographs. IEEE 2014 , 2014 , 138–141. [ Google Scholar ] [ CrossRef ]
- Roy, P.; Tennakoon, R.; Cao, K.; Sedai, S.; Mahapatra, D.; Maetschke, S.; Garnavi, R. A novel hybrid approach for severity assessment of Diabetic Retinopathy in colour fundus images. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, Australia, 18–21 April 2017; pp. 1078–1082. [ Google Scholar ] [ CrossRef ]
- Xu, K.; Feng, D.; Mi, H. Deep Convolutional Neural Network-Based Early Automated Detection of Diabetic Retinopathy Using Fundus Image. Molecules 2017 , 22 , 2054. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Yang, Y.; Li, T.; Li, W.; Wu, H.; Fan, W.; Zhang, W. Lesion Detection and Grading of Diabetic Retinopathy via Two-Stages Deep Convolutional Neural Networks. In International Conference on Medical Image Computing and Computer-Assisted Intervention ; Springer: Cham, Germany, 2017; pp. 533–540. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- van Grinsven, M.J.J.P.; van Ginneken, B.; Hoyng, C.B.; Theelen, T.; Sanchez, C.I. Fast Convolutional Neural Network Training Using Selective Data Sampling: Application to Hemorrhage Detection in Color Fundus Images. IEEE Trans. Med. Imaging 2016 , 35 , 1273–1284. [ Google Scholar ] [ CrossRef ]
- Budak, U.; Şengür, A.; Guo, Y.; Akbulut, Y. A novel microaneurysms detection approach based on convolutional neural networks with reinforcement sample learning algorithm. Health Inf. Sci. Syst. 2017 , 5 , 14. [ Google Scholar ] [ CrossRef ]
- Zhou, W.; Wu, C.; Chen, D.; Wang, Z.; Yi, Y.; Du, W. Automatic Microaneurysms Detection Based on Multifeature Fusion Dictionary Learning. Comput. Math. Methods Med. 2017 , 2017 , 1–11. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Barkana, B.D.; Sariçiçek, I.; Yildirim, B. Performance analysis of descriptive statistical features in retinal vessel segmentation via fuzzy logic, ANN, SVM, and classifier fusion. Knowl. Based Syst. 2017 , 118 , 165–176. [ Google Scholar ] [ CrossRef ]
- Dasgupta, A.; Singh, S. A fully convolutional neural network based structured prediction approach towards the retinal vessel segmentation. In Proceedings of the 14th International Symposium on Biomedical Imaging (ISBI), Melbourne, VIC, Australia, 18–21 April 2017; pp. 248–251. [ Google Scholar ]
- Mo, J.; Zhang, L. Multi-level deep supervised networks for retinal vessel segmentation. Int. J. Comput. Assist. Radiol. Surg. 2017 , 12 , 2181–2193. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tan, J.H.; Acharya, U.R.; Bhandary, S.V.; Chua, K.C.; Sivaprasad, S. Segmentation of optic disc, fovea, and retinal vasculature using a single convolutional neural network. J. Comput. Sci. 2017 , 20 , 70–79. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Vega, R.; Sanchez-Ante, G.; Falcon-Morales, L.E.; Sossa, H.; Guevara, E. Retinal vessel extraction using lattice neural networks with dendritic processing. Comput. Biol. Med. 2015 , 58 , 20–30. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, S.; Yin, Y.; Cao, G.; Wei, B.; Zheng, Y.; Yang, G. Hierarchical retinal blood vessel segmentation based on feature and ensemble learning. Neurocomputing. Neurocomputing 2015 , 149 , 708–717. [ Google Scholar ] [ CrossRef ]
- Choi, J.Y.; Yoo, T.K.; Seo, J.G.; Kwak, J.; Um, T.T.; Rim, T.H. Multi-categorical deep learning neural network to classify retinal images: A pilot study employing small database. PLoS ONE 2017 , 12 , 16. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Mumtaz, R.; Hussain, M.; Sarwar, S.; Khan, K.; Mumtaz, S.; Mumtaz, M. Automatic detection of retinal hemorrhages by exploiting image processing techniques for screening retinal diseases in diabetic patients. Int. J. Diabetes Dev. Ctries. 2017 , 38 , 80–87. [ Google Scholar ] [ CrossRef ]
- Santhi, D.; Manimegalai, D.; Parvathi, S.; Karkuzhali, S. Segmentation and classification of bright lesions to diagnose diabetic retinopathy in retinal images. Biomed. Eng. Biomed. Tech. 2016 , 61 , 443–453. [ Google Scholar ] [ CrossRef ]
- Li, G.; Zheng, S.; Li, X. Exudate Detection in Fundus Images via Convolutional Neural Network. In International Forum on Digital TV and Wireless Multimedia Communications ; Springer: Singapore, 2018; pp. 193–202. [ Google Scholar ] [ CrossRef ]
- Bala, P.; Vijayachitra, S. A Sequential learning method for detection and classification of exudates in retinal images to assess diabetic retinopathy. J. Biol. Syst. 2014 , 22 , 413–428. [ Google Scholar ] [ CrossRef ]
- Rahim, S.S.; Jayne, C.; Palade, V.; Shuttleworth, J. Automatic detection of microaneurysms in colour fundus images for diabetic retinopathy screening. Neural Comput. Appl. 2015 , 27 , 1149–1164. [ Google Scholar ] [ CrossRef ]
- Omar, M.; Khelifi, F.; Tahir, M.A. Detection and classification of retinal fundus images exudates using region based multiscale LBP texture approach. In Proceedings of the 2016 International Conference on Control, Decision and Information Technologies (CoDIT), Saint Julian’s, Malta, 6–8 April 2016; pp. 227–232. [ Google Scholar ] [ CrossRef ]
- Abbas, Q.; Fondon, I.; Sarmiento, A.; Jiménez, S.; Alemany, P. Automatic recognition of severity level for diagnosis of diabetic retinopathy using deep visual features. Med. Biol. Eng. Comput. 2017 , 55 , 1959–1974. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ouyang, W.; Luo, P.; Zeng, X.; Qiu, S.; Tian, Y.; Li, H.; Yang, S.; Wang, Z.; Xiong, Y.; Qian, C.; et al. Deepid-net: Multi-stage and deformable deep convolutional neural networks for object detection. arXiv 2014 , arXiv:1409.3505. [ Google Scholar ]
- Shan, J.; Li, L. A deep learning method for microaneurysm detection in fundus images. In Proceedings of the IEEE First International Conference on Connected Health: Applications, Systems, and Engineering Technologies, Washington, DC, USA, 27–29 June 2016; pp. 357–358. [ Google Scholar ]
- Shirbahadurkar, S.D.; Mane, V.M.; Jadhav, D.V. Early Stage Detection of Diabetic Retinopathy Using an Optimal Feature Set. In Advances in Intelligent Systems and Computing ; Springer: Berlin/Heidelberg, Germany, 2017; Volume 678, pp. 15–23. [ Google Scholar ] [ CrossRef ]
- SK, S. A Machine Learning Ensemble Classifier for Early Prediction of Diabetic Retinopathy. J. Med. Syst. 2017 , 41 , 201. [ Google Scholar ] [ CrossRef ]
- Abràmoff, M.D.; Lou, Y.; Erginay, A.; Clarida, W.; Amelon, R.; Folk, J.C.; Niemeijer, M. Improved Automated Detection of Diabetic Retinopathy on a Publicly Available Dataset Through Integration of Deep Learning. Investig. Ophthalmol. Vis. Sci. 2016 , 57 , 5200–5206. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Antal, B.; Hajdu, A. An ensemble-based system for automatic screening of diabetic retinopathy. Knowl. Based Syst. 2014 , 60 , 20–27. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Carrera, E.V.; Gonzalez, A.; Carrera, R. Automated detection of diabetic retinopathy using SVM. In Proceedings of the IEEE XXIV International Conference on Electronics, Electrical Engineering and Computing (INTERCON), Cusco, Peru, 15–18 August 2017; pp. 1–4. [ Google Scholar ] [ CrossRef ]
- Gegundez-Arias, M.E.; Marin, D.; Ponte, B.; Alvarez, F.; Garrido, J.; Ortega, C.; Vasallo, M.J.; Bravo, J.M. A tool for automated diabetic retinopathy pre-screening based on retinal image computer analysis. Comput. Biol. Med. 2017 , 88 , 100–109. [ Google Scholar ] [ CrossRef ]
- Li, X.; Pang, T.; Xiong, B.; Liu, W.; Liang, P.; Wang, T. Convolutional neural networks based transfer learning for diabetic retinopathy fundus image classification. In Proceedings of the 10th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Shanghai, China, 14–16 October 2017. [ Google Scholar ]
- Arunkumar, R.; Karthigaikumar, P. Multi-retinal disease classification by reduced deep learning features. Neural Comput. Applic. 2017 , 28 , 329–334. [ Google Scholar ] [ CrossRef ]
- Tan, J.H.; Fujita, H.; Sivaprasad, S.; Bhandary, S.V.; Rao, A.K.; Chua, K.C.; Acharya, U.R. Automated segmentation of exudates, hemorrhages, and microaneurysms using a single convolutional neural network. Inf. Sci. 2017 , 420 , 66–76. [ Google Scholar ] [ CrossRef ]
- Takahashi, H.; Tampo, H.; Arai, Y.; Inoue, Y.; Kawashima, H. Applying artificial intelligence to disease staging: Deep learning for the improved staging of diabetic retinopathy. PLoS ONE 2017 , 12 , e0179790. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Hemanth, D.J.; Anitha, J.; Indumathy, A. Diabetic Retinopathy Diagnosis in Retinal Images Using Hopfield Neural Network. IETE J. Res. 2016 , 62 , 893–900. [ Google Scholar ] [ CrossRef ]
- Sharma, S.; Singh, G.; Sharma, M. A comprehensive review and analysis of supervised-learning and soft computing techniques for stress diagnosis in humans. Comput. Biol. Med. 2021 , 134 , 104450. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lakshminarayanan, V.; Kheradfallah, H.; Sarkar, A.; Balaji, J.J. Automated Detection and Diagnosis of Diabetic Retinopathy: A Comprehensive Survey. J. Imaging 2021 , 7 , 165. [ Google Scholar ] [ CrossRef ]
- Shultz, T.R.; Fahlman, S.E.; Craw, S.; Andritsos, P.; Tsaparas, P.; Silva, R.; Drummond, C.; Lanzi, P.L.; Gama, J.; Wiegand, R.P. Confusion Matrix. Encycl. Mach. Learn. 2011 , 61 , 209. [ Google Scholar ]
- Wikipedia, F. Cohen Kappa. 2006. Available online: https://thenewstack.io/cohens-kappa-what-it-is-when-to-use-it-and-how-to-avoid-its-pitfalls (accessed on 12 March 2022).
- Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; Gibson, J.; et al. The Usefulness of Serum Biomarkers in the Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. J. Clin. Med. 2020 , 9 , 1233. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jacoba, C.M.P.; Celi, L.A.; Silva, P.S. Biomarkers for Progression in Diabetic Retinopathy: Expanding Personalized Medicine through Integration of AI with Electronic Health Records. Semin. Ophthalmol. 2021 , 36 , 250–257. [ Google Scholar ] [ CrossRef ]
- Records, H. HHS Public Access. Biomarkers 2022 , 36 , 250–257. [ Google Scholar ]
- Control, D.; Trial, C. Progression of Retinopathy with Intensive versus Conventional Treatment in the Diabetes Control and Complications Trial. Ophthalmology 1995 , 102 , 647–661. [ Google Scholar ] [ CrossRef ]
- Group, A.S.; Group, A.E.S.; Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; et al. Effects of Medical Therapies on Retinopathy Progression in Type 2 Diabetes. N. Engl. J. Med. 2010 , 363 , 233–244. [ Google Scholar ] [ CrossRef ]
- Kuo, J.Z.; Wong, T.Y.; Rotter, J.I. Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 2014 , 132 , 96–107. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Mastropasqua, R.; Toto, L.; Cipollone, F.; Santovito, D.; Carpineto, P.; Mastropasqua, L. Role of microRNAs in the modulation of diabetic retinopathy. Prog. Retin. Eye Res. 2014 , 43 , 92–107. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cooper, M.E.; El-Osta, A. Epigenetics. Circ. Res. 2010 , 107 , 1403–1413. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Torok, Z.; Peto, T.; Csosz, E.; Tukacs, E.; Molnar, A.M.; Berta, A.; Tozser, J.; Hajdu, A.; Nagy, V.; Domokos, B.; et al. Combined Methods for Diabetic Retinopathy Screening, Using Retina Photographs and Tear Fluid Proteomics Biomarkers. J. Diabetes Res. 2015 , 2015 , 1–8. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Lu, C.-H.; Lin, S.-T.; Chou, H.-C.; Lee, Y.-R.; Chan, H.-L. Proteomic analysis of retinopathy-related plasma biomarkers in diabetic patients. Arch. Biochem. Biophys. 2013 , 529 , 146–156. [ Google Scholar ] [ CrossRef ]
- Xia, J.-F.; Wang, Z.-H.; Liang, Q.-L.; Wang, Y.-M.; Li, P.; Luo, G.-A. Correlations of six related pyrimidine metabolites and diabetic retinopathy in Chinese type 2 diabetic patients. Clin. Chim. Acta 2011 , 412 , 940–945. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hussain, F.; Hussain, R.; Hossain, E. Explainable Artificial Intelligence (XAI): An Engineering Perspective. arXiv 2021 , arXiv:2101.03613. Available online: http://arxiv.org/abs/2101.03613 (accessed on 21 March 2022).
- Jang, S.I.; Girard, M.J.; Thiéry, A.H. Thiery, Explainable diabetic retinopathy classification based on neural-symbolic learning. CEUR Workshop Proc. 2021 , 2986 , 104–113. [ Google Scholar ]
- Deshpande, N.M.; Gite, S.; Pradhan, B.; Assiri, M.E. Explainable Artificial Intelligence–A New Step towards the Trust in Medical Diagnosis with AI Frameworks: A Review. Comput. Model. Eng. Sci. 2022 , 133 , 1–30. [ Google Scholar ] [ CrossRef ]
- Leopold, H.A.; Singh, A.; Sengupta, S.; Zelek, J.S.; Lakshminarayanan, V. Recent advances in deep learning applications for retinal diagnosis using OCT. 2020. In State of the Art in Neural Networks ; Elsevier: New York, NY, USA, 2020. [ Google Scholar ]
- Liu, R.; Li, Q.; Xu, F.; Wang, S.; He, J.; Cao, Y.; Shi, F.; Chen, X.; Chen, J. Application of artificial intelligence-based dual-modality analysis combining fundus photography and optical coherence tomography in diabetic retinopathy screening in a community hospital. Biomed. Eng. Online 2022 , 21 , 1–11. [ Google Scholar ] [ CrossRef ]
- Nguyen, D.M.H.; Mai, T.T.N.; Than, N.T.T.; Prange, A.; Sonntag, D. Self-supervised Domain Adaptation for Diabetic Retinopathy Grading Using Vessel Image Reconstruction. In German Conference on Artificial Intelligence (Künstliche Intelligenz) ; Springer: Cham, Germany, 2021; pp. 349–361. [ Google Scholar ] [ CrossRef ]
- Song, R.; Cao, P.; Yang, J.; Zhao, D.; Zaiane, O.R. A Domain Adaptation Multi-instance Learning for Diabetic Retinopathy Grading on Retinal Images. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Republic of Korea, 16–19 December 2020; pp. 743–750. [ Google Scholar ] [ CrossRef ]
- Crawshaw, M. Multi-task learning with deep neural networks: A survey. arXiv 2020 , arXiv:2009.09796. [ Google Scholar ]
- Foo, A.; Hsu, W.; Lee, M.L.; Lim, G.; Wong, T.Y. Multi-Task Learning for Diabetic Retinopathy Grading and Lesion Segmentation. Proc. Conf. AAAI Artif. Intell. 2020 , 34 , 13267–13272. [ Google Scholar ] [ CrossRef ]
| Ref No | Objectives and Topic | Discussions | Type |
|---|---|---|---|
| [ ] | Datasets, picture preparation methods, ML-based methods, DL-based strategies, and evaluation metrics are presented as five components of DR screening methodologies. | Did not follow the PRISMA approach. Studies that were released between January 2013 and March 2018 are considered in this study. | Review |
| [ ] | It discusses DeepDR, an automated DR identification, and grading system. DeepDR uses transfer learning and ensemble learning to detect the presence and severity of DR in fundus images. | Did not follow the PRISMA approach. Experiment results indicate the importance and effectiveness of the ideal number and combinations of component classifiers in model performance. | Review |
| [ ] | It discusses an integrated ML approach that incorporates support vector machines (SVMs), principal component analysis (PCA), and moth flame optimization approaches for DR. | Did not follow the PRISMA approach. Utilizing the PCA technique to reduce the dimensions has had a detrimental impact on the performance of the majority of ML algorithms. | Review |
| [ ] | It presents the latest DL algorithms used in DR detection, highlighting the contributions and challenges of recent research papers. | Did not follow the PRISMA approach. Robust deep-learning methods must be developed to give satisfactory performance in cross-database evaluation, i.e., trained with one dataset and tested with another. | Review |
| [ ] | It presents a comprehensive survey of automated eye diseases detection systems using available datasets, techniques of image preprocessing, and deep learning models. | Studies that did not follow the PRISMA approach are considered from January 2016 to June 2021. | Review |
| RQ. No. | Research Question | Objective/Discussion |
|---|---|---|
| 1 | What are the most common artificial intelligence-based methods for DR detection? | It assists in determining the most relevant artificial intelligence algorithms for DR diagnosis applications nowadays. |
| 2 | What are the various Features Extraction Techniques for DR? | List various feature extraction techniques used for DR. |
| 3 | What are the relevant datasets for DR? | Discovers several publicly available datasets that may be used as benchmarks to compare and assess the performance of various methodologies, as well as gives new researchers a head start. |
| 4 | What are the various evaluation measures used for DR detection? | The most used standards and metrics for DR detection are reviewed. |
| 5 | What are the potential solutions for a robust and reliable DR detection system? | It makes it easier to find significant research areas to be studied. |
| Fundamental Keyword | “Diabetic Retinopathy” | |||
|---|---|---|---|---|
| Direct Keyword | “Artificial Intelligence” | “Machine Learning” | “Deep Learning” | |
| Indirect Keyword | “Ophthalmology” | “Fundus Images” | “DR Stages” | “OCT” |
| Database | Query | Initial Outcome |
|---|---|---|
| Scopus | (Diabetic AND Retinopathy AND Artificial AND Intelligence AND Machine AND Learning AND Deep AND Learning) | 149 |
| Web of Science | 79 |
| Inclusion Criteria |
|---|
| Rather than reviews or survey pieces, scientific papers should be primary research papers. |
| Scholarly articles that appeared between 2014 and April 2022. |
| Query terms must be included in the titles, abstracts, or whole body of peer-reviewed publications. |
| Articles that address at least one research question. |
| The developed solution should aim at resolving issues with diabetic retinopathy detection using AI. |
| Articles that are written in languages other than English. |
| Studies published that are identical. |
| Complete scientific papers are not always available. |
| Research papers that are not related to diabetic retinopathy using AI. |
| Software | Sample Size | Only DR OR Controls | Device | Grading/ Mechanism | Limitation | Software Mechanism | Used | Accuracy |
|---|---|---|---|---|---|---|---|---|
| Bosch [ ] | 1128 | DR with age of 18+. | Bosch Mobile Eye Care fundus camera. Single field non-mydriatic. | ETDRS. | In some of the eyes diagnosed as normal, the other eye may have had early evidence. Further, while the study notes the findings of DR, it would be useful to know how accurate this software is for individual lesions, such as exudates, microaneurysms, and macular edema. | CNN-based AI software. | For DR screening in India. | Sensitivity—91%. Specificity—96%. Positive predicted value (PPV)—94%. Negative predictive value (NPV)—95%. |
| Retmarker DR [ ] | 45,148 | Screening diabetic patients. | Used non-mydriatic cameras. Canon CR6-45NM with a Sony DXC-950P 3CCD color video camera other cameras, such as Nidek AFC-330 and CSO Cobra, have been used temporarily. | Coimbra Ophthalmology. Reading Centre (CORC). | The short duration of the study (2 years) and the lack of more detailed information on systemic parameters, such as lipid stratification. | Feature-based ML algorithms. | Used in local DR screening in Portugal, Aveiro, Coimbra, Leiria, Viseu, Castelo Branco, and Cova da Beira. | R0—71.5%, RL—22.7%, M—2.2%, RP—0.1%, NC—3.5%. Human grading burden reduction of 48.42%. |
| Eye Art [ ] | 78,685 | A cross-sectional diagnostic study of individuals with diabetes. | Two-field undilated fundus photograph. Two-field retinal CFP images (one disc-centered and one macula-centered) were taken for each eye (Canon CR-2 AF or Canon CR-2 Plus AF; Canon USA Inc.). | ETDRS. | A limitation of the study is that optical coherence tomography was not used to determine clinically significant macular edema. Color fundus photographs CFP is known to be an accurate, sufficient, and widely accepted clinical reference standard, including by the FDA. | AI Algo. | Used in Canada for detection of both mtmDR and vtDR without physician assistance. | Sensitivity—91.7%. Specificity—91.5%. |
| Retinalyze [ ] | 260 | Retrospective cross-sectional study of diabetic patients attending routine. | Mydriatic 60° fundus photography on 35-mm color transparency film was used, with a single fovea-centered field fundus camera (CF-60UV; Canon Europa NV, Amstelveen, The Netherlands) set. | Routine grading was based on a visual examination of slide-mounted transparencies. Reference grading was performed with specific emphasis on achieving high sensitivity. | Commercially unavailable for a long time until reintroduced into its web-based form with DL improvements. | Deep learning based. | Used in Europe to a greater extent. | Sensitivity 93.1% and specificity 71.6%. |
| Singapore SERI-NUS [ ] | 76,370 SIDRP between 2010 and 2013 (SIDRP 2010–2013) | With diabetes. | FundusVue, Canon, Topcon, and Carl Zeiss nonmydriatic. | Grading was completed by a certified ophthalmologist and retina specialist. | Identification of diabetic macular edema from fundus photographs may not identify all cases appropriately without clinical examination and optical coherence tomography. | Using a deep learning system. | Singapore. | Sensitivity 90.5% and specificity 91.6%. AUC—0.936 |
| Google [ ] | 128,175 Aravind Eye Hospital, Sankara Nethralaya, and Narayana Nethralaya | Macula-centered retinal fundus images were retrospectively obtained from EyePACS in the United States and three eye hospitals in India among patients presenting for diabetic retinopathy screening. | Two sets of 9963 Eyepacs images from Centervue DRS, Optovue iCam, Canon CR1/DGi/CR2, and Topcon NW using 45° FOV and 40% acquired with pupil dilation. Images from a 1748- Messidor-2 from a Topcon TRC NW6 nonmydriatic camera and 45° FOV with 44% pupil dilation. | DR severity (none, mild, moderate, severe, or proliferative) was graded according to the International Clinical Diabetic Retinopathy scale. | Further research is necessary to determine the feasibility of applying this algorithm in the clinical setting and to determine whether the use of the algorithm could lead to improved care and outcomes compared with current ophthalmologic assessment. | CNN based. Inception-v3 architecture. | Used in North Carolina to a greater extent. | Sensitivity—97.5%. Specificity—93.4%. |
| IDx-DR [ ] | 900 | With no history of DR. | Widefield stereoscopic photography mydriatic. | FPRC Wisconsin Fundus Photograph Reading Center, and ETDRS. | The prevalence of referable retinopathy in this population is small, which limits the comparison to other populations with higher disease prevalence. | AI-based logistic regression model. | Dutch diabetic Care system-1410. | Sensitivity—87.2%. Specificity—90.7%. |
| Comprehensive Artificial Intelligence Retinal Expert (CARE)system [ ] | 443 subjects (848 eyes) | Previously diagnosed diabetic patients. | One-field color fundus photography (CFP) (macula-centered with a 50◦ field of vision) was taken for both eyes using a nonmydriatic fundus camera (RetiCam 3100, China) by three trained ophthalmologists in dark rooms. | International Clinical Diabetic Retinopathy (ICDR) classification criteria. | This technique has drawbacks when it comes to detecting severe PDR and DME. (1) Poor imaging results from fundus such as ghost images and fuzzy lesions, in leukoplakia, lens opacity, and tiny pupils. Cases create difficulty in AI identification. (2) The difference in the results was caused by the study’s insufficient sample size. (3) Some lesions were overlooked during the 50-degree fundus photography focused on the macula. | AI-based. | Chinese community health care centers. | Sensitivity—75.19%. Specificity 93.99%. |
| Ref. No | Authors | Feature Selected | Features and Classifiers (Technique) | Weakness | Database | (Performance Analysis) |
|---|---|---|---|---|---|---|
| [ ] | Di Wu, Wu, Zhang, Liu, and Bauman, 2006. | To find out blood vessels in the retina. | Gabor filters. | Requires high-performance time with greater feature vector dimension. | STARE. | Tested 20 images. For normal images, TPR—80 to 91% and FPR—2.8 to 5.5%. For abnormal images, TPR—73.8–86.5% FPR—2.1–5.3%. |
| [ ] | Sanchez et al. (2009). | To detect hard exudates from cotton wool spots and other artifacts. | Edge detection and mixture models. | The diversity of brightness and size makes it difficult to detect the hard exudates, hence method may fail when they appear very few in the retina. | Eighty retinal images with variable color, brightness, and quality. | A sensitivity of 90.2% and a positive predictive value of 96.8% for an image-based classification accuracy sensitivity of 100% and a specificity of 90%. |
| [ ] | Garcia, Sanchez, Lopez, Abasolo, and Hornero (2009). | Red lesions image and shape features. | Neural networks with multilayer perceptron (MLP), radial basis function (RBF), and support vector machine (SVM). | The black box nature of ANN and more accuracy requires more amount of data. | The database was composed of 117 images with variable color, brightness, and quality. 50 were used for training and 67 for testing. | Using lesion-based sensitivity and positive prediction values in percent. MLP—88.1, 80.722. RBF—88.49, 77.41. SVM—87.61, 83.51. Using image-based sensitivity and specificity in percent. MLP—100, 92.59. RBF—100, 81.48. SVM—100, 77.78. |
| [ ] | Sanchez et al. (2008). | Hard exudates. | Color information and Fisher’s linear discriminant analysis. | When there are only a few very faint HEs in the retina, this proposed algorithm may have limited performance. More images are required for better results. | Fifty-eight retinal images with variable color, brightness, and quality from the Instituto de Oftalmobiología Aplicadaat University of Valladolid, Spain. | Using a lesion-based performance sensitivity of 88% with a mean number of 4.83 ± 4.64 false positives per image. Using Image-based sensitivity-100 and Specificity of 100% is achieved. |
| [ ] | Quellec et al. (2012). | Abnormal patterns in fundus images. | Multiple-instance learning. | The training procedure is complex and takes a lot of time. | Messidor (1200 images) and e-optha (25,000 images). | In the Messidor dataset, the proposed framework achieved an area under the ROC curve of A = 0.881 and e-optha A = 0.761. |
| [ ] | Kose, ¸SEvik, ˙IKiba¸s, and Erdo¨l (2012). | Image pixel information. | Inverse segmentation using region growing, adaptive region growing, and Bayesian approaches. | Difficult to choose the correct way to select a prior. | A total of 328 images with 760 X 570 resolution from the Department of Ophthalmology at the Faculty of Medicine at Karadeniz Technical University were used. | This approach successfully identifies and localizes over 97% of ODs and segments around 95% of DR lesions. |
| [ ] | Giancardo et al. (2012). | Exudates in fundus images. | Feature cector generated using an exudate probability map, the color analysis, and the wavelet analysis. Exudate probability map and wavelet analysis. | Intensive calculation. | HEI-MED, Messidor, and DIARETDB1. | AUC is between 0.88 and 0.94, depending on the dataset/features used. |
| [ ] | Zhang, Karray, Li, and Zhang (2012). | Microaneurysms and blood vessel detection. | Locate MAs using multi-scale Gaussian correlation filtering (MSC) with dictionary learning and sparse representation classifier (SRC). | Dictionaries for vessel extraction are artificially generated using Gaussian functions which can cause a low discriminative ability for SRC. Additionally, a larger dataset is required. | STARE and DRIVE. | For STARE: FPR—0.00480. TPR—0.73910. PPV—0.740888. For DRIVE: FPR—0.0028. TPR—0.5766. PPV—0.8467. |
| [ ] | Qureshi et al. (2012). | Identifying macula and optic disk (OD). | Ensemble combined algorithm of edge detectors, Hough transform, and pyramidal decomposition. | It is difficult to determine which one is the best approach because good results were reported for healthy retinas but less precise on a difficult data set. | Diaretdb0, Diaretdb1, and DRIVE 40% of the images from each benchmark are used for training and 60% of the images are used for testing. | The average detection rate of macula is 96.7 and OD is 98.6. |
| [ ] | Noronha and Nayak (2013). | Two energy features and six energy values in three orientations. | Wavelet transforms and support vector machine (SVM) kernels. | The performance depends on factors such size and quality of the training features, the robustness of the training, and the features extracted. | Fundus images were used. | Accuracy, sensitivity, and specificity of more than 99% are achieved. |
| [ ] | Gharaibeh N (2021). | Cotton wool spots, exudates. Nineteen features were extracted from the fundus image. | Unsupervised particle swarm optimization based relative reduct algo (US-PSO-RR), SVM, and naïve-Bayes classifiers. | Detection and elimination of optic discs from fundus images are difficult, hence lesion detection is challenging. | Image-Ret. | Obtained a sensitivity of 99%, A specificity of 99% and a high accuracy of 98.60%. |
| [ ] | Gharaibeh N (2018). | Microaneurysm, hemorrhage, and exudates. | Co-occurrence matrix and SVM. | Can be tried on larger datasets. | DIARETDB1. | Obtained a sensitivity of 99%, a specificity of 96%, and an accuracy of 98.4%. |
| [ ] | Akram, Khalid, and Khan (2013). | Image shape and statistics. | Gaussian mixture models and support vector machine and Gabor filter bank. | Need to work on a large dataset. | Four hundred and thirty-eight Fundus images. | An accuracy of 99.4%, a sensitivity of 98.64%, and a specificity of 99.40% are achieved. |
| [ ] | Harini R and Sheela N (2016). | Blood vessels, microaneurysms, and exudates. | The gray level co-occurrence matrix (GLCM) is utilized to extract textural features the classification is completed using SVM. | Problem working with large datasets since training requires more time with SVMs. | Seventy-five Fundus images were considered, forty-five were used for training, and thirty for testing | An accuracy of 96.67%, a sensitivity of fundus of 100%, and a specificity of 95.83% are achieved. |
| [ ] | Anjana Umapathy, Anusha Sreenivasan, Divya S. Nairy (2019). | Exudates and red lesions in the fundus image. | Decision tree classifier. | Requires more time for training and persistent overfitting. | STARE, HRF, MESSIDOR, and a novel dataset created from Retina Institute of Karnataka. | The approach achieved an accuracy of 94.4%. |
| Sr. No | Dataset Name | Description | References | Availability | Link |
|---|---|---|---|---|---|
| 1 | Kaggle | EyePACS has supplied this dataset for the DR detection challenge. There are 88,702 photos in this collection (35,126 for training and 53,576 for testing) [ ]. | [ , , , , , , , , , , , , , ] | Free | (accessed on 2 May 2022). |
| 2 | ROC (Retinopathy Online Challenge) | There are 100 photos in this collection. Canon CR5-45NM, Topcon NW 100, and NW 200 cameras were used. | [ , , , , , , ] | Free | (accessed on 2 May 2022) |
| 3 | DRIVE | This dataset contains 40 photos from a DR program in Holland (split into training and testing, 20 images each). The camera was a Canon CR5 non-mydriatic 3CCD with a 45-degree field of view (FOV). | [ , , , , , , , ] | Free | (accessed on 2 May 2022) |
| 4 | STARE | There are 400 photos in total in this dataset. The fundus camera used was a Topcon TRV-50 with a 35-degree field of view. | [ , , , , , , , ] | Free | (accessed on 3 May 2022) |
| 5 | E-Optha | The OPHDIAT telemedical network created this dataset. E-Ophtha MA and E-Ophtha EX are the two datasets that make up this collection. Both have 381 and 82 photos in them, respectively. | [ , , , , , , , ] | Free | (accessed on 3 May 2022) |
| 6 | DIARETDB0 | There are 130 photos in this dataset (normal images = 20, images with DR symptoms = 110). The photos were obtained with a fundus camera with a field of view of 50 degrees. | [ , , , ] | Free | (accessed on 3 May 2022) |
| 7 | DIARETDB1 | There are 89 photos in this dataset (standard images = 5, images with at least mild DR = 84). The photos were obtained with a fundus camera with a field of view of 50 degrees. | [ , , , , , , , , , , , , , , , , , , , , , , , , ] | Free | (accessed on 4 May 2022) |
| 8 | Messidor-2 | This dataset includes 1748 photos collected with a Topcon TRC NW6 non-mydriatic fundus camera with a 45-degree field of view. | [ ] | On-demand | (accessed on 3 May 2022) |
| 9 | Messidor | This dataset includes 1748 photos collected with a Topcon TRC NW6 non-mydriatic fundus camera with a 45-degree field of view. | [ , , , , , , , , , , , ] | Free | (accessed on 3 May 2022) |
| 10 | DRiDB | This dataset, which includes 50 photos, is accessible upon request. | [ , ] | On-demand | (accessed on 3 May 2022) |
| 11 | DR1 | The Department of Ophthalmology of the Federal University of Sao Paulo created this dataset. (UNIFESP). It contains 234 images captured with TRX-50X, the mydriatic camera having 45 degrees FOV. | [ , ] | Free | (accessed on 4 May 2022) |
| 12 | DR2 | The Department of Ophthalmology at the Federal University of Sao Paulo also contributed to this dataset (UNIFESP). It contains 520 photographs taken with the TRC-NW8, a non-mydriatic camera with a 45-degree field of view. | [ ] | Free | (accessed on 3 May 2022) |
| 13 | ARIA | This dataset contains 143 images. The camera used was a Zeiss FF450+ fundus camera with a 50-degree field of view. | [ ] | Free | (accessed on 5 May 2022) |
| 14 | FAZ (Foveal Avascular Zone) | There are 60 photos in this dataset (25 images that are normal and 35 images with DR). | [ ] | Free | (accessed on 5 May 2022) |
| 15 | CHASE-DB1 | There are 28 photos of 14 children included in this dataset (consisting of one image/eye). CHASE-DB1 deals with Child Heart and Health Study (CHASE) in England. | [ ] | Free | (accessed on 5 May 2022) |
| 16 | Tianjin Medical University Metabolic Diseases Hospital | This dataset contains 414 fundus images. | [ ] | Not publicly available | (accessed on 5 May 2022) |
| 17 | Moorfields Eye Hospital | Data from countries such as Kenya, Botswana, Mongolia, China, Saudi Arabia, Italy, Lithuania, and Norway are collected at Moorfields Eye Hospital in London. | [ ] | Not publicly available | (accessed on 5 May 2022) |
| 18 | CLEOPATRA | The CLEOPATRA collection consists of 298 fundus images. It includes images from 15 hospitals across the United Kingdom to diagnose DR. | [ ] | Not publicly available | Not available |
| 19 | Jichi Medical University | There are 9939 posterior pole fundus images of diabetic patients in this dataset. The camera used was a NIDEK Co., Ltd., Aichi, Japan, AFC-230, with a 45-degree field of view. | [ ] | Not publicly available | (accessed on 5 May 2022) |
| 20 | Singapore National DR Screening Program | This dataset was collected during the Singapore National Diabetic Screening Program (SIDRP) between 2010 and 2013; a total of 197,085 retinal images were collected. | [ ] | Not publicly available | Not available |
| 21 | Lotus Eye Care Hospital Coimbatore, India | It contains 122 fundus images (normal = 28, DR = 94). A Canon non-mydriatic Zeiss fundus camera with a FOV of 90 degrees was used. | [ , , ] | Not publicly available | (accessed on 5 May 2022) |
| 22 | Department of Ophthalmology, Kasturba Medical College, Manipal, India | This dataset contains 340 images (normal = 170, with retinopathy = 170). Non-mydriatic retinal camera, namely, TOPCON, was used | [ ] | Not publicly available | (accessed on 5 May 2022) |
| 23 | HUPM, Cádiz, Spain | Fundus photos from Hospital Puerta del Mar in Spain were taken, including 250 photos (50 normal and 200 with DR symptoms). | [ ] | Not publicly available | (accessed on 5 May 2022) |
| N = Total Predictions | Actual: NO | Actual: Yes |
|---|---|---|
| Predicted: No | True Negative | False Positive |
| Predicted: Yes | False Negative | True Positive |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Bidwai, P.; Gite, S.; Pahuja, K.; Kotecha, K. A Systematic Literature Review on Diabetic Retinopathy Using an Artificial Intelligence Approach. Big Data Cogn. Comput. 2022 , 6 , 152. https://doi.org/10.3390/bdcc6040152
Bidwai P, Gite S, Pahuja K, Kotecha K. A Systematic Literature Review on Diabetic Retinopathy Using an Artificial Intelligence Approach. Big Data and Cognitive Computing . 2022; 6(4):152. https://doi.org/10.3390/bdcc6040152
Bidwai, Pooja, Shilpa Gite, Kishore Pahuja, and Ketan Kotecha. 2022. "A Systematic Literature Review on Diabetic Retinopathy Using an Artificial Intelligence Approach" Big Data and Cognitive Computing 6, no. 4: 152. https://doi.org/10.3390/bdcc6040152
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Diabetic retinopathy
Showing results 1 - 10 of 92
Sorted by most recent
17 September 2024
28 August 2024
22 August 2024
21 May 2024
21 March 2024
12 February 2024
30 January 2024
24 October 2023
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Endocrinol (Lausanne)
- PMC10516549
Editorial: Advances in the research of diabetic retinopathy, volume II
Mohd imtiaz nawaz.
1 Department of Ophthalmology, College of Medicine, King Saud University, Riyadh, Saudi Arabia
2 Dr. Nasser Al-Rashid Research Chair in Ophthalmology, Abdulaziz University Hospital, Riyadh, Saudi Arabia
Diabetic retinopathy (DR) is a progressive disease of the retina. Diabetic retinopathy occurs because of long-term accumulated functional and structural impairments in the diabetic retina. The global prevalence of any DR form among diabetic patients is estimated to be around 27.0% ( 1 ). It takes several years before any clinical signs of DR appear in a diabetic patient, making it difficult to properly evaluate and diagnose the patient at an early stage of the disease. Diabetic retinopathy is a multifactorial disease arising from the complex interplay between dysregulated biochemical and metabolic pathways. Diabetic retinopathy begins as non-proliferative retinal abnormalities and progresses to proliferative diabetic retinopathy (PDR), characterized by a persistent low grade of inflammation and neovascularization ( 2 , 3 ). The implication of several inflammatory pathways and angiogenesis processes complicates the pathology through the initiation of retinal neovascularization, vitreous hemorrhage, and/or tractional retinal detachment, which are hallmark features of PDR ( 4 ).
Clinically, the use of photocoagulation and vitrectomy remains the standard of care for treating severe complications of PDR. However, these treatments are either destructive or their successful implementation approaches are limited. Additional PDR treatment strategies involve surgical procedures to remove a thin epiretinal membrane from the surface of the retina, which further allows the retina to remodel and reattach. Despite dramatic developments, vitreoretinal surgery for epiretinal membranes is often dissatisfying both anatomically and functionally ( 5 , 6 ).
The discovery of the role of the potential angiogenic modulator vascular endothelial growth factor (VEGF) in PDR has led to the development of anti-VEGF agents as therapies. However, limitations to anti-VEGF interventions exist that include a short duration of action, the presence of adverse side effects, and a poor response in a significant percentage of patients ( 7 , 8 ). Furthermore, various pro-inflammatory and angiogenic factors other than VEGF may play a role in PDR (reviewed in ( 2 , 9 )), causing resistance to anti-VEGF interventions.
This observation, therefore, suggests that PDR-associated pathogenesis is multifactorial and that factors beyond VEGF may be playing a role in the initiation and progression of the disease. Thus, more theoretical, or clinical insight into the pathogenesis of diabetic retinopathy is warranted. More profound knowledge could help in developing novel approaches to target dysregulated molecular pathways, or increasing target affinity, and shortening treatment durability for the management of PDR.
Given the success of the first edition of the Research Topic Advances in the Research of Diabetic Retinopathy ( 10 ), and the continuing advancement in the field, we aimed to launch Volume II of the edition. The aim of Volume II was to seek more research articles exploring new paradigms toward understating the pathological mechanisms that are involved in early retinal vascular damage in patients with diabetic retinopathy. To meet this demand, Volume II of the edition was also overwhelmed by the publication of many exciting articles, including original research as well as reviews. Articles addressing or discussing new therapeutic implications for the early management of diabetic retinopathy were given equal space.
The editor of this topic strongly believes that articles published in volume II of the Research Topic could have added some knowledge to improve our understanding of the pathogenesis associated with diabetic retinopathy.
Likewise, the work by Su et al. reported that a genetically higher hip circumference is associated with a lower risk of DR. Serum levels of acylcarnitine 8:0 ( Jin et al. ) and cellular communication network factor-1 ( Xiang et al. ) can serve as predictive biomarkers for DR identification at an early stage of the disease. Using a confocal scanning laser ophthalmoscope, Song et al. demonstrated that the retinal branch arterial tortuosity may be a direct and specific indicator for early detection or assessment of DR severity. Recent scientific advancements in the use of scanning swept-source optical coherence tomography angiography (SS-OCTA) devices ( Zeng et al. , Qi et al. , Xu et al. , Lin et al. , and Li et al. ) could be an important clinical tool in assessing the early diabetes-induced changes in choroidal or retinal capillaries in DR patients. Furthermore, using OCT, Yao et al. showed preclinical DR may be more severe in diabetic nephropathy (DN) individuals in regard to microvascular and microstructural impairments. Similarly, Xiaodong et al. demonstrated that peripheral blood inflammatory biomarkers and OCT retinal macular imaging indexes have important value for risk prediction and diagnosis of DN in combination with DR. Hsieh et al. showed partial inner segment-outer segment layers are predictive of better response, whereas the presence of epiretinal membrane is a significant predictor of poor response to anti-VEGF treatment in eyes with diabetic macular edema.
Nevertheless, the use of artificial intelligence, or machine learning, and risk nomogram prediction models has been finding its place as a training aid system for assessing the degree of DR pathogenesis in type 2 diabetic patients. Accordingly, using fundus images from real-world diabetics, Qian et al. discussed the AI-based system for high diagnostic accuracy for the detection of DR. Wang et al. developed a predictive risk nomogram using retinal vascular geometry parameters and clinical information with no blood test requirements to facilitate risk stratification and early detection of DR. Independent common or potential predictors were tested to establish and validate a predictive model for DR ( Yang et al. , Wang et al. ). Such a quick screening model can assist clinicians and researchers, based on a minimal amount of clinical data, to quickly determine if a diabetic patient is prone to developing DR.
Among the review topics discussing advancements in PDR treatment strategies, Lin et al. discussed targeted retinal photocoagulation, an emerging laser technology, in combination with anti-VEGF for the management of retinal diseases. A network meta-analysis review article by Wang et al. concluded that there are no distensible effects of intraoperative intravitreal conbercept (IVC) on PDR, but preoperative, except for very long intervals, is an effective adjuvant to par-plana vitrectomy for treating PDR. The analysis was indeed confirmed in an original article by Yang et al. showing that an IVC treatment that was administered 7 days preoperatively was associated with better effectiveness and a lower vitreous VEGF concentration than its administration at other time points.
A growing piece of evidence suggests that various pro-inflammatory and angiogenic factors, other than VEGF may play a role in the progression of pathogenesis associated with PDR. Accordingly, a review by Xu et al. highlights the therapeutic roles of pigment epithelium-derived factors and their receptors in the diagnosis and management of retinal diseases, including PDR. Lastly, two review articles report the significance of oral Chinese patent medicines in improving visual acuity and fundus lesions in non-PDR ( Liu et al. ) and PDR ( Huai et al. ) patients. However, the relevant clinical trials on the use of many such Chinese medicines are few, and more high-quality clinical trials await to determine their effectiveness and safety. Towards this, a study by Kim et al. suggests that a 60% edible ethanolic and catechin 7-O-b-Dapiofuranoside extract of Ulmus davidiana could be a potential therapeutic agent for reducing vascular leakage by preventing pericyte apoptosis in DR.
In conclusion, Volume II of the Research Topic brings new insights and novel data toward understanding the early retinal vascular damage or pathological mechanism involved in the initiation and progression of diabetic retinopathy. The pool of data obtained using an ultrawide SS-OCTA device or predictive nomogram model provides a wealth of knowledge regarding the early assessment or pathological grading of DR. A few research articles dedicated to understanding the role of potential biomarkers could open new therapeutic avenues for the early management of diabetic retinopathy. Nevertheless, the adjuvant effects of conbercept or oral Chinese medicine could be a game changer for the management of diabetic retinopathy.
The editor of this Research Topic strongly feels that this set of articles could be a benchmark and may add some clinical knowledge in the field of diabetic retinopathy. Last but not least, the editor invites more interdisciplinary research towards early assessment and development of treatment strategies for the management of diabetic retinopathy.
Author contributions
MN: Conceptualization, Writing – original draft, Writing – review & editing.
Acknowledgments
The author would like to thank the hundreds of researchers and scientists who contributed to this Research Topic. The success of this Research Topic would not be completed without the experts in the field, so the author would also like to thank all the reviewers. Lastly, the author extends a sincere thanks to the topic editor Professor Rajashekhar Gangaraju from the University of Tennessee Health Science Center (UTHSC) Memphis, who equally participated in the success of this Research Topic.
Funding Statement
This work was supported by the Nasser Al-Rasheed Research Chair in Ophthalmology (Abu El-Asrar A.M.), Department of Ophthalmology, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
Diabetic retinopathy (DR) is a significant global health concern, with its prevalence and severity increasing alongside the rising incidence of diabetes. DR is a leading cause of vision impairment among working-age adults, resulting in substantial economic and healthcare burdens. This article explores the epidemiology and pathophysiology of DR ...
Diabetic retinopathy (DR) research has had significant advancements over the past decades. We analysed the impact and characteristics of the top 100 (T100) most-cited articles in DR research. The Scopus database was searched for articles published from 1960 to June 2020 by two independent investigators. The T100 DR articles were published between 1961 and 2017 with median citations of 503 ...
Diabetic retinopathy (DR) is a major complication of diabetes mellitus (DM), which remains a leading cause of visual loss in working-age populations. The diagnosis of DR is made by clinical manifestations of vascular abnormalities in the retina. Clinically, DR is divided into two stages: non-proliferative diabetic retinopathy (NPDR) and ...
Diabetic retinopathy is a leading cause of both moderate and severe vision loss worldwide. Over the past decade, advances in technology have dramatically improved the evaluation, treatment, and vis...
Diabetic retinopathy (DR) is the leading cause of preventable blindness in the working-age population. The disease progresses slowly, and we can roughly differentiate two stages: early-stage (ESDR), in which there are mild retinal lesions and visual acuity ...
A new treatment for diabetic retinopathy. NovaGo Therapeutics is developing a first-in-class fully human antibody therapy to treat diabetic retinopathy. With its novel disease-modifying mode of ...
Abstract Diabetic retinopathy (DR) research has had significant advancements over the past decades. We analysed the impact and characteristics of the top 100 (T100) most-cited articles in DR research. The Scopus database was searched for articles published from 1960 to June 2020 by two independent investigators.
As the leading cause of vision loss in working-age adults, diabetic retinopathy requires routinely retinal screening. Here the authors develop a deep learning system that can facilitate the ...
Diabetic retinopathy may lead to vision-threatening damage to the retina, eventually leading to blindness; it is the most common and severe ocular complication. Poor glycemic control, uncontrolled hypertension, dyslipidemia, nephropathy, male sex, and obesity are associated with worsening diabetic retinopathy.
Diabetic retinopathy (DR) is one of the leading causes of permanent central blindness worldwide. Despite the complexity and inadequate understanding of DR pathogenesis, many of the underlying pathways are currently partially understood and may offer potential targets for future treatments. Anti-VEGF medications are currently the main medication for this problem. This article provides an ...
Diabetic Retinopathy is a common complication affecting patients with diabetes and refers to microvascular retinal damage as a result of hyperglycaemia. Without intervention, damage can progress through the stages of background, pre-proliferative and proliferative retinopathy, potentialling resulting diabetic macular edema and the loss of vision. As a leading cause of visual impairment and ...
Diabetic Retinopathy (DR) is a leading cause of visual impairment in the United States. The CDC estimates that the prevalence of DR will triple from 2005 to 2050.The report summarizes major past advances in diabetes research and their impact on clinical ...
Dear Colleagues, Diabetic retinopathy (DR) is a common complication of diabetes and remains a leading cause of severe loss of visual acuity and blindness, thus representing a significant economic burden for the health-care systems. Experimental research has notably evolved in recent years and the classic definition of DR as a merely microangiopathic complication of diabetes has been replaced ...
1 Introduction Diabetic retinopathy (DR) is an important complication of diabetes that affects blood vessels in the retina and can cause vision loss and blindness. [1] It is quickly becoming a worldwide public health challenge. [2,3] A large number of research papers related to DR have been published in academic journals in recent decades.
Abstract. This overview introduces contributions to a special issue on causes of vision loss from diabetes mellitus, focusing on the retina and also the cornea. Diabetic retinopathy is the most common and leading cause of vision loss among people with diabetes. Research to detect early symptoms, understand mechanisms leading to diabetic eye ...
Diabetic retinopathy occurs due to long-term diabetes with changing blood glucose levels and has become the most common cause of vision loss worldwide. It has become a severe problem among the working-age group that needs to be solved early to avoid vision loss in the future. Artificial intelligence-based technologies have been utilized to detect and grade diabetic retinopathy at the initial ...
British Journal of Ophthalmology's topic collection on the subject of diabetic retinopathy
The risk of vision loss from diabetic retinopathy has fallen dramatically over the past 3 decades with improvements in diabetes and blood pressure treatments, and with advances in laser surgery and intraocular drug delivery. Nevertheless, diabetes remains ...
Diabetic Retinopathy (DR) is a common complication of diabetes mellitus, which causes lesions on the retina that effect vision. If it is not detected early, it can lead to blindness. Unfortunately, DR is not a reversible process, and treatment only sustains vision. DR early detection and treatment can significantly reduce the risk of vision loss. The manual diagnosis process of DR retina ...
Diabetic retinopathy (DR) is a progressive disease of the retina. Diabetic retinopathy occurs because of long-term accumulated functional and structural impairments in the diabetic retina. The global prevalence of any DR form among diabetic patients is estimated to be around 27.0% ( 1 ). It takes several years before any clinical signs of DR ...
A retrospective cohort study, using data from the Sight Outcomes Research Collaborative (SOURCE) Ophthalmology Repository, found that among a sample population of 37,397 adults with diabetes, those with pre-existing diabetic retinopathy who are of Black or Hispanic race/ethnicity are less likely to receive routine dilated fundus examinations ...