- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions

Thyroid Cancer : A Review
- 1 Department of Medicine, Division of Endocrinology, Memorial Sloan Kettering Cancer Center, New York, New York
- 2 Department of Head and Neck Surgery, University of Texas MD Anderson Cancer Center, Houston, Texas
- 3 Department of Endocrine Neoplasia and Hormonal Disorders, University of Texas MD Anderson Cancer Center, Houston, Texas
- US Preventive Services Task Force USPSTF Recommendation: Screening for Thyroid Cancer US Preventive Services Task Force; Kirsten Bibbins-Domingo, PhD, MD, MAS; David C. Grossman, MD, MPH; Susan J. Curry, PhD; Michael J. Barry, MD; Karina W. Davidson, PhD, MASc; Chyke A. Doubeni, MD, MPH; John W. Epling Jr, MD, MSEd; Alex R. Kemper, MD, MPH, MS; Alex H. Krist, MD, MPH; Ann E. Kurth, PhD, RN, MSN, MPH; C. Seth Landefeld, MD; Carol M. Mangione, MD, MSPH; Maureen G. Phipps, MD, MPH; Michael Silverstein, MD, MPH; Melissa A. Simon, MD, MPH; Albert L. Siu, MD, MSPH; Chien-Wen Tseng, MD, MPH, MSEE JAMA
- Research Letter Changes in Trends in Thyroid Cancer Incidence in the United States, 1992 to 2016 Ann E. Powers, BA; Andrea R. Marcadis, MD; Mark Lee, BS, BA; Luc G. T. Morris, MD, MSc; Jennifer L. Marti, MD JAMA
- Comment & Response A Review of Thyroid Cancer—Reply Laura Boucai, MD; Mark Zafereo, MD; Maria E. Cabanillas, MD JAMA
- Comment & Response A Review of Thyroid Cancer Samineh Beheshtirouy, PharmD; Ali Shayanfar, PharmD, PhD JAMA
- Comment & Response A Review of Thyroid Cancer Salvatore Sciacchitano, MD; Massimo Rugge, MD; Armando Bartolazzi, MD JAMA
- JAMA Patient Page Patient Information: What Is Thyroid Cancer? Rebecca Voelker, MSJ JAMA
- Original Investigation Global Burden of Thyroid Cancer From 1990 to 2017 YuJiao Deng, PhD; HongTao Li, MD; Meng Wang, MD; Na Li, PhD; Tian Tian, MD; Ying Wu, MD; Peng Xu, MD; Si Yang, MD; Zhen Zhai, MD; LingHui Zhou, MD; Qian Hao, MD; DingLi Song, MD; TianBo Jin, PhD; Jun Lyu, PhD; ZhiJun Dai, PhD JAMA Network Open
Importance Approximately 43 720 new cases of thyroid carcinoma are expected to be diagnosed in 2023 in the US. Five-year relative survival is approximately 98.5%. This review summarizes current evidence regarding pathophysiology, diagnosis, and management of early-stage and advanced thyroid cancer.
Observations Papillary thyroid cancer accounts for approximately 84% of all thyroid cancers. Papillary, follicular (≈4%), and oncocytic (≈2%) forms arise from thyroid follicular cells and are termed well-differentiated thyroid cancer. Aggressive forms of follicular cell-derived thyroid cancer are poorly differentiated thyroid cancer (≈5%) and anaplastic thyroid cancer (≈1%). Medullary thyroid cancer (≈4%) arises from parafollicular C cells. Most cases of well-differentiated thyroid cancer are asymptomatic and detected during physical examination or incidentally found on diagnostic imaging studies. For microcarcinomas (≤1 cm), observation without surgical resection can be considered. For tumors larger than 1 cm with or without lymph node metastases, surgery with or without radioactive iodine is curative in most cases. Surgical resection is the preferred approach for patients with recurrent locoregional disease. For metastatic disease, surgical resection or stereotactic body irradiation is favored over systemic therapy (eg, lenvatinib, dabrafenib). Antiangiogenic multikinase inhibitors (eg, sorafenib, lenvatinib, cabozantinib) are approved for thyroid cancer that does not respond to radioactive iodine, with response rates 12% to 65%. Targeted therapies such as dabrafenib and selpercatinib are directed to genetic mutations ( BRAF , RET , NTRK , MEK ) that give rise to thyroid cancer and are used in patients with advanced thyroid carcinoma.
Conclusions Approximately 44 000 new cases of thyroid cancer are diagnosed each year in the US, with a 5-year relative survival of 98.5%. Surgery is curative in most cases of well-differentiated thyroid cancer. Radioactive iodine treatment after surgery improves overall survival in patients at high risk of recurrence. Antiangiogenic multikinase inhibitors and targeted therapies to genetic mutations that give rise to thyroid cancer are increasingly used in the treatment of metastatic disease.
Read More About
Boucai L , Zafereo M , Cabanillas ME. Thyroid Cancer : A Review . JAMA. 2024;331(5):425–435. doi:10.1001/jama.2023.26348
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Correspondence
- Open access
- Published: 27 August 2024
Global burden of thyroid cancer from 1990 to 2021: a systematic analysis from the Global Burden of Disease Study 2021
- Tianjiao Zhou 1 , 2 , 3 na1 ,
- Xiaoting Wang 1 , 2 , 3 na1 ,
- Jingyu Zhang 1 , 2 , 3 na1 ,
- Enhui Zhou 1 , 2 , 3 na1 ,
- Chen Xu 1 , 2 , 3 ,
- Ying Shen 4 ,
- Jianyin Zou 1 , 2 , 3 ,
- Wen Lu 1 , 2 , 3 ,
- Kaiming Su 1 , 2 , 3 ,
- Weijun Huang 1 , 2 , 3 ,
- Hongliang Yi 1 , 2 , 3 &
- Shankai Yin 1 , 2 , 3
Journal of Hematology & Oncology volume 17 , Article number: 74 ( 2024 ) Cite this article
24 Accesses
Metrics details
Thyroid cancer (TC) is a significant global healthcare burden. However, the lack of comprehensive data has impeded our understanding of its global impact. We aimed to examine the burden of TC and its trends at the global, regional, and national levels using data stratified by sociodemographic index (SDI), sex, and age. Data on TC, including incidence, mortality, and disability-adjusted life-years (DALYs) from 1990 to 2021, were obtained from the Global Burden of Disease Study 2021. Estimated annual percentage changes (EAPCs) were calculated to assess the incidence rate, mortality, and DALYs trends. The incidence, mortality, and DALYs of TC in 2021 were 249,538 (95% uncertainty interval: 223,290–274,638), 44,799 (39,925–48,541), and 646,741 (599,119–717,357), respectively. The age-standardized incidence rate (ASIR) in 2021 was 2.914 (2.607–3.213), with an EAPC of 1.25 (1.14–1.37) compared to 1990. In 2021, the age-standardized death rate (ASDR) was 0.53 (0.47–0.575) and age-standardized DALYs rate was 14.571 (12.783–16.115). Compared with 1990, the EAPCs of ASDR and age-standardized DALYs rate showed decreasing trends, at − 0.24 (− 0.27 to − 0.21) and − 0.14 (− 0.17 to − 0.11), respectively. Low SDI regions showed the highest ASDR and age-standardized DALYs rate, at 0.642 (0.516–0.799) and 17.976 (14.18–23.06), respectively. Low-middle SDI regions had the highest EAPCs for ASDR and age-standardized DALYs rate, at 0.74 (0.71–0.78) and 0.67 (0.63–0.7), respectively. Females exhibited decreasing trend in ASDR and age-standardized DALYs rate, with EAPCs of − 0.58 (− 0.61 to − 0.55) and − 0.45 (− 0.47 to − 0.42), respectively. In contrast, males showed an increasing trend in ASDR and age-standardized DALYs rate, with EAPCs of 0.41 (0.35–0.46) for both. In high-income regions, most countries with decreased annual changes in deaths experience increasing age-related deaths. Over the past few decades, a notable increase in TC incidence and decreased mortality has been observed globally. Regions characterized by lower SDI, male sex, and an aging population exhibited no improvement in TC mortality. Effective resource allocation, meticulous control of risk factors, and tailored interventions are crucial for addressing these issues.
To the editor
Thyroid cancer (TC) is a common endocrine malignancy with high incidence of lymph node metastasis [ 1 ]. The availability of medical resources within regions often dictates the standardization of postoperative follow-up and adjuvant therapies, which consequently affects TC recurrence and metastasis rates [ 2 ]. Therefore, holistic understanding of recent TC disease burden and trends requires a global perspective. The Global Burden of Disease (GBD) database provides valuable TC data [ 3 ]. We used the latest GBD data (1990–2021) to evaluate TC burden, to provide insight on personalized approaches to alleviate its global impact.
We initially investigated the global TC burden and trends. The incidence, mortality, and DALYs of TC in 2021 were 249,538 (95% uncertainty interval: 223,290–274,638), 44,799 (39,925–48,541), and 646,741 (599,119–717,357), respectively (Table 1 ). The age-standardized incidence rate (ASIR) in 2021 was 2.914 (2.607–3.213), with an estimated annual percentage change (EAPC) of 1.25 (1.14–1.37). In 2021, the ASDR was 0.53 (0.47–0.575) and age-standardized DALYs rate was 14.571 (12.783–16.115). Both EAPCs for ASDR (− 0.24; − 0.27 to − 0.21) and age-standardized DALYs rate (− 0.14; − 0.17 to − 0.11) showed decreasing trends. High-income North America had the highest ASIR (5.303; 5.075–5.526), whereas Saudi Arabia had the highest overall ASIR (7.131; 5.395–9.331) (Fig. 1 A, Table 1 and Table S1 ). In summary, the 1990–2021 period witnessed a global increase in TC incidence coupled with decreased mortality rates, although this trend exhibited regional differences.
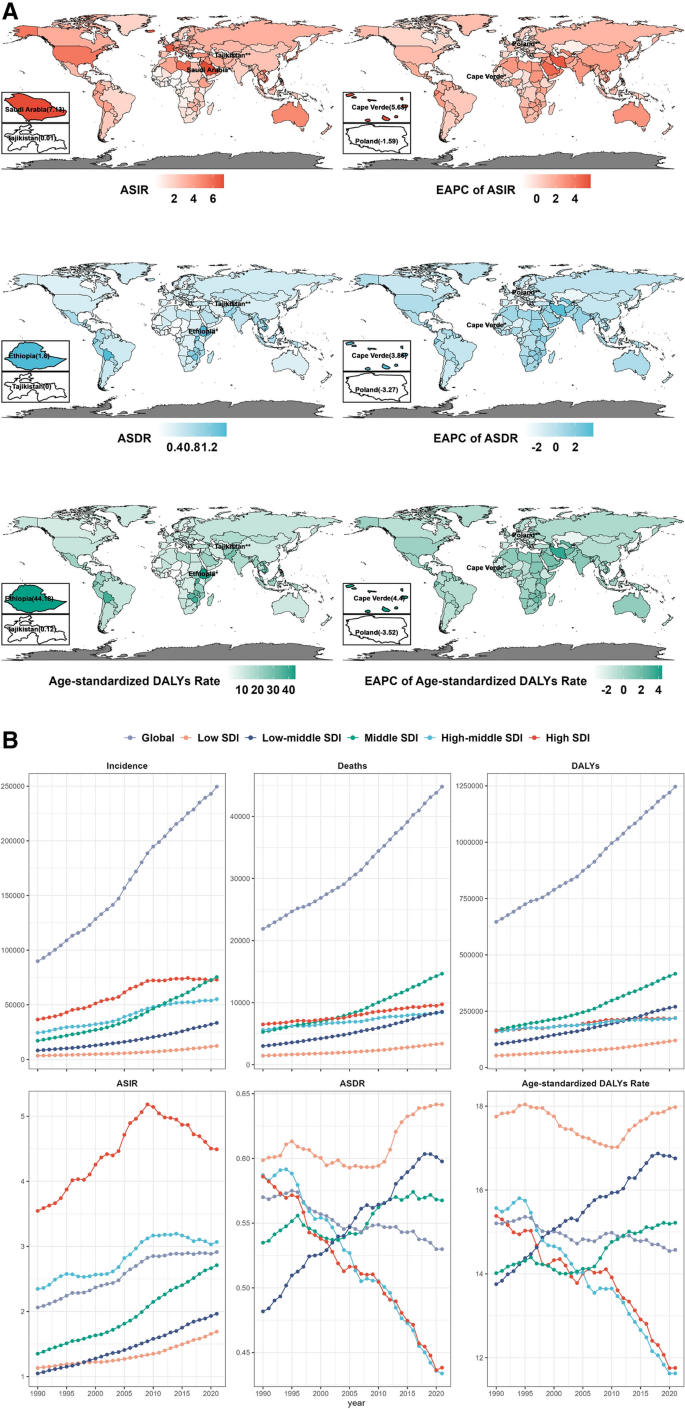
Burden and trends of thyroid cancer at the global, regional, and national levels by sociodemographic index (SDI), sex and age from 1990 to 2021. A Age-standardized disease burden and EAPCs of thyroid cancer across 204 countries and territories from 1990 to 2021. B Trends in the incidence, deaths, DALYs, ASIR, ASDR and age-standardized DALYs rate of thyroid cancer from 1990 to 2021 by different SDI level regions. C The correlation between SDI and thyroid cancer burden across 204 countries in 2021. (Different colors indicate GBD super-regions. Point size represents the numbers of incidence, deaths, and DALYs. "R" represents the Pearson correlation coefficient). D Thyroid cancer burden across different age groups (5 year intervals) by sex in 2021. E Age-related thyroid cancer burden ratio between 2000 and 2021 in 7 GBD super-regions.(Point size represents the absolute annual change of age-related thyroid cancer burden rate from 2000 to 2021. Points filled by grey color represent an annual change less than zero.The values in parentheses represent ratios of the age-related thyroid cancer burden rate between 2000 and 2021). ASIR, age-standardized incidence rate; ASDR, age-standardized death rate; DALYs, disability-adjusted life years.EAPC, estimated annual percentage change.SDI, socio-demographic index.Data are represented as mean ± SEM.
We first analyzed these regional differences using the sociodemographic index (SDI). From 1990 to 2021, the incidence, mortality, and DALYs of TC increased across all SDI regions, similar to the 2019 findings (Fig. 1 B) [ 4 ]. High and high-middle SDI regions showed higher ASIR than other regions, and the ASIR of 204 countries were positively correlated with the SDI (r = 0.57, p < 0.05) (Fig. 1 C, Figure S1, S2). Low SDI regions showed the highest ASDR (0.642; 0.516–0.799) and age-standardized DALYs rate (17.976; 14.18–23.06). Low-middle SDI regions had the highest EAPCs for ASDR (0.74; 0.71–0.78) and age-standardized DALYs rate (0.67; 0.63–0.7) (Table 1 ). Therefore, healthcare departments in low and middle SDI countries need to be prepared for the potential increase in TC burden as their SDI improves [ 5 ].
For sex-based analysis, the incidence, mortality, and DALYs ratio between females and males was 2.03, 1.49, and 1.47 in 2021, respectively (Table S2). From 1990 to 2021, only males showed increasing trends in ASDR and age-standardized DALYs rate, with EAPCs of 0.41 (0.35–0.46) (Table S2, Figure S3). The median male-to-female burden rate ratios of TC for ages 5–70 years increased significantly ( p < 0.05) every 10 years across 204 countries (Figure S4). Although TC incidence was lower in males, mortality rates did not improve. Therefore, increased emphasis should be placed on monitoring male patients with TC in the future.
From an age-specific viewpoint, the highest TC incidence was observed in 55–59-years-old, with 19,600.77 (17,266.17–22,578.22) in females and 11,440.96 (9759.37–13,132.14) in males (Fig. 1 D). Elderly patients with TC face unique challenges, including declining immunity, comorbidities, and changes in cancer pathology, necessitating further investigation into age-related trends in TC burden [ 6 ]. We defined the ratio of disease burden in those aged ≥ 65 years to that in those aged < 15 years as the aging-related disease burden [ 7 ]. In 2021, the countries with the lowest rates of age-related incidence, deaths, and DALYs ratio were all from Sub-Saharan Africa (Fig. 1 E). Most countries experiencing decreased annual change are located in high-income regions (Fig. 1 E, Figure S5). The rising TC incidence and death rates due to aging highlight the need for targeted strategies to manage the increasing burden in older patients, especially in regions with high SDI [ 8 ]. The present study is limited by its reliance on global disease data sources, which vary in quality control standards across different regions and statistical agencies [ 9 ]. Moreover, the increase in the global population also influenced the number of TC reported in 2021.
In summary, the 1990–2021 period witnessed increased TC incidence coupled with decreased mortality rates. In particular, regions characterized by lower SDI, male sex, and an aging population have emerged as significant contributors to the currently rising TC burden. Thus, targeted international collaborative research is essential to identify the relevant influencing factors and effectively manage the global disease burden of TC.
Availability of data and materials
The data used for these analyses are all publicly available at http://ghdx.healthdata.org/gbd-2021 .
Chen DW, Lang BHH, McLeod DSA, Newbold K, Haymart MR. Thyroid cancer. Lancet (London, England). 2023;401(10387):1531–44.
Article PubMed Google Scholar
Gubbi S, Al-Jundi M, Foerster P, et al. The effect of thyrotropin suppression on survival outcomes in patients with differentiated thyroid cancer: a systematic review and meta-analysis. Thyroid: Off J Am Thyroid Assoc. 2024;34:674.
Article Google Scholar
GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet (London, England). 2024;403(10440):2100–32.
Zhou T, Huang W, Wang X, et al. Global burden of head and neck cancers from 1990 to 2019. iScience. 2024;27(3):109282.
Article PubMed PubMed Central Google Scholar
Chaudhri E, Fathi W, Hussain F, Hashmi SK. The increasing trends in cases of the most common cancers in Saudi Arabia. J Epidemiol Global Health. 2020;10(4):258–62.
Zeng PYF, Prokopec SD, Lai SY, et al. The genomic and evolutionary landscapes of anaplastic thyroid carcinoma. Cell Rep. 2024;43(3): 113826.
Article CAS PubMed PubMed Central Google Scholar
GBD 2021 Demographics Collaborators. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: a comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet (London, England) . 2024;403(10440). Available at https://pubmed.ncbi.nlm.nih.gov/38484753/ .
Tota JE, Engels EA, Lingen MW, et al. Inflammatory tongue conditions and risk of oral tongue cancer among the US elderly individuals. J Clin Oncol Off J Am Soc Clin Oncol. 2024;42(15):1745–53.
Article CAS Google Scholar
GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet (London, England). 2024;403(10440):2162–203.
Download references
Acknowledgements
We sincerely appreciate the exceptional cooperation of our study team members and gratefully acknowledge the significant contributions of the Global Burden of Disease Study.
This study was supported by grants from the National STI2030-Major Projects of China (2021ZD0201900), National Natural Science Foundation of China (Grant Nos. 81970869, 82171125, and 82371131), Hospital Level Scientific Research Fund Program of Shanghai Sixth People's Hospital (ynts202404 and hlyjkt202325), Shanghai Municipal Commission of Science and Technology (18DZ2260200), Shanghai Science and Technology Innovation Program of Science and Technology Commission (20Y11902100), and Shanghai Shen-Kang Hospital Management Center Project (Grant Nos. SHDC2020CR2044B and SHDC2020CR3056B).
Author information
Tianjiao Zhou, Xiaoting Wang, Jingyu Zhang and Enhui Zhou have contributed equally to this work.
Authors and Affiliations
Department of Otorhinolaryngology Head and Neck Surgery, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Tianjiao Zhou, Xiaoting Wang, Jingyu Zhang, Enhui Zhou, Chen Xu, Jianyin Zou, Wen Lu, Kaiming Su, Weijun Huang, Hongliang Yi & Shankai Yin
Shanghai Key Laboratory of Sleep Disordered Breathing, Shanghai, China
Otolaryngology Institute of Shanghai Jiao Tong University, Shanghai, China
Department of Otolaryngology, Shanghai Tenth People’s Hospital, School of Medicine, Tongji University, Shanghai, China
You can also search for this author in PubMed Google Scholar
Contributions
The corresponding authors are responsible for the authenticity of the data. All authors made a significant contribution to the work reported (i.e., in the conception design or execution of the study, acquisition, analysis, or interpretation of the data, or in all of these areas). All authors contributed to the drafting of the work or revised the article critically for important intellectual content.All authors approved the final version of the manuscript to be published. TJZ and EHZ confirmed the accuracy of the data sources. TJZ and JYZ processed the raw data using software, and TJZ and XTW visualized the data. TJZ and WJH reviewed and edited the manuscript. KMS, HLY and SKY contributed to managing the overall research enterprise.
Corresponding authors
Correspondence to Weijun Huang , Hongliang Yi or Shankai Yin .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., supplementary material 3., supplementary material 4., supplementary material 5., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zhou, T., Wang, X., Zhang, J. et al. Global burden of thyroid cancer from 1990 to 2021: a systematic analysis from the Global Burden of Disease Study 2021. J Hematol Oncol 17 , 74 (2024). https://doi.org/10.1186/s13045-024-01593-y
Download citation
Received : 25 June 2024
Accepted : 06 August 2024
Published : 27 August 2024
DOI : https://doi.org/10.1186/s13045-024-01593-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Global burden of disease study
- Thyroid cancer
- Disability-adjusted life-years
Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Thyroid Cancer
If you have thyroid cancer or are close to someone who does, knowing what to expect can help you cope. Here you can find out all about thyroid cancer, including risk factors, symptoms, how it is found, and how it is treated.
About Thyroid Cancer
Causes, risk factors, and prevention, early detection, diagnosis, and staging, treating thyroid cancer, after treatment, easy reading, if you have thyroid cancer.
If you or someone you know has been diagnosed with thyroid cancer, this guide can help.
Downloadable PDFs
- About and Key Statistics [PDF]
- Causes, Risks, Prevention [PDF]
- Detection, Diagnosis, Staging [PDF]
- Treatment and Side Effects [PDF]
- Next Steps After Treatment [PDF]
This information is possible thanks to people like you.
We depend on donations to keep our cancer information available for the people who need it most.
More Resources

Understanding Your Diagnosis

Treatments & Side Effects

Survivorship: During & After Treatment

Programs & Services
News & stories.

Latest Cancer News

Stories of Hope

ACS Research News
Help us end cancer as we know it, for everyone..

If this was helpful, donate to help fund patient support services, research, and cancer content updates.
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
Home > Patients, Caregivers, and Advocates > About Cancer > Cancers > Thyroid Cancer
Thyroid Cancer

Thyroid cancer is cancer of the butterfly-shaped gland at the base of the throat. The thyroid uses iodine, a mineral found in some foods and in iodized salt, to help make several hormones that control heart rate, body temperature, metabolism, and the amount of calcium in the blood.
There are four main types of thyroid cancer: papillary thyroid cancer, follicular thyroid cancer, medullary thyroid cancer, and anaplastic thyroid cancer. According to the National Cancer Institute , approximately 44,020 people in the United States will be diagnosed with thyroid cancer in 2024, and about 2,170 will die of the disease. Thyroid cancer is highly treatable, and the five-year relative survival rate is estimated at 98.4 percent.
Thyroid cancer is much more common among women than it is among men. Age and exposure to radiation are also risk factors.
Source: National Cancer Institute

You can support the AACR's mission by contacting your representatives and senators and advocating for increased funding for lifesaving cancer research.

FDA Approvals in Oncology: April-June 2024
Accessing a Clinical Trial During the Pandemic

What It’s Like to Get a “C” in Graduate School
- September is Thyroid Cancer Awareness Month
- ThyCa: Thyroid Cancer Survivors’ Association
- Vulvar Cancer

Sinai Health / Sinai Health News and Media / Sinai Health
Research points to improved detection of thyroid cancer
Aug 28, 2024 | Sinai Health

A new study involving researchers from Sinai Health and the University of Toronto has uncovered new insights into how thyroid cancer may be more effectively treated, avoiding unnecessary surgeries.
For the study, researchers looked at thyroid tumor tissues and thyroid nodule biopsies from 620 patients at Mount Sinai Hospital, from 2016 to 2022.
The research – recently published in JAMA Network – involved doctors Guodong (David) Fu and Ronald Chazen of the Lunenfeld-Tanenbaum Research Institute and the Alex and Simona Shnaider Research Laboratory in Molecular Oncology at Mount Sinai Hospital, as well as Christina MacMillan , a pathologist at Sinai Health and an assistant professor in the Temerty Faculty of Medicine’s Department of Pathology and Laboratory Medicine, and Ian Witterick , Surgeon-in-Chief at Sinai health and a professor in Temerty Medicine’s Department of Otolaryngology-Head and Neck Surgery.
Dr.Fu says research that assists with precision thyroid cancer detection is important for many reasons, including that some patients who seek treatment for thyroid tumours end up finding out their tumours are benign after diagnostic surgery. The findings could help medical practitioners differentiate low-risk tumours from high-risk ones, he says, and help avoid unneeded surgical procedures.
Researchers examined whether differences in the patient’s RAS genomic variants were reflected in the status of their tumours. They also investigated the presence of the variant BRAF V600E, and TERT promoter variants, in the patient’s samples.
Ultimately, researchers concluded that “discrimination of interpatient differences in RAS in combination with BRAF V600E and TERT promoter variants” could lead to more accurate cancer diagnosis, by doing molecular assays of cellular biopsies from patients with thyroid nodules.
“The findings help promote understanding of the interpatient differences in genomic variation among patients who carry the same genetic mutation, thereby facilitating individualized treatment based on the extent of the mutation present in the patient,” says Dr. Fu.
For the study, Dr. Fu says researchers developed novel molecular assays using digital polymerase chain reaction, a technique that means they could sensitively quantify the genetic mutation level of the patient materials.
The paper notes that there has been a sharp increase in papillary thyroid cancer since the 1980s, and notes that in 30 per cent of cases where a fine-needle aspiration biopsy of a suspected nodule takes place, there is an indeterminate diagnosis, which may lead to a diagnostic surgery.
“(This finding) enhances the preoperative diagnostic accuracy for patients, in order to avoid unnecessary surgery for benign thyroid nodules,” says Dr. Fu.
Dr. Witterick agrees and says the research is important because identifying differences in genomic variants between patients can enhance precision in cancer detection, especially diagnosing malignancies before surgery and distinguishing low-risk cancers from more aggressive ones.
This article appears courtesy of the University of Toronto’s Temerty Faculty of Medicine. It has been edited for clarity and length. You can view the original version here .
Filter by Category
- Circle of Care
- Hennick Bridgepoint Hospital
- Lunenfeld-Tanenbaum Research Institute
- Media Releases
- Mount Sinai Hospital
- Renew Sinai
- Sinai Health
- Support Sinai
Filter by Date
- February (7)
- January (4)
- December (8)
- November (9)
- October (4)
- September (5)
- January (6)
- December (4)
- November (6)
- October (5)
- September (4)
- January (8)
- November (7)
- October (13)
- September (9)
- August (11)
- January (9)
- December (7)
- October (9)
- September (15)
- February (15)
- January (26)
- December (17)
- November (14)
- October (22)
- September (11)
- August (13)
- February (13)
- January (20)
- December (26)
- November (25)
- October (26)
- September (13)
- August (17)
- February (14)
- January (18)
- September (3)
- February (1)
- January (3)
- December (1)
- November (3)
- October (1)
- September (1)
- Open access
- Published: 29 August 2024
Pathology diagnosis of intraoperative frozen thyroid lesions assisted by deep learning
- Tingting He 1 ,
- Shanshan Shi 1 ,
- Yiqing Liu 1 ,
- Lianghui Zhu 1 ,
- Yani Wei 2 ,
- Fenfen Zhang 2 ,
- Huijuan Shi 2 ,
- Yonghong He 1 &
- Anjia Han 2
BMC Cancer volume 24 , Article number: 1069 ( 2024 ) Cite this article
Metrics details
Thyroid cancer is a common thyroid malignancy. The majority of thyroid lesion needs intraoperative frozen pathology diagnosis, which provides important information for precision operation. As digital whole slide images (WSIs) develop, deep learning methods for histopathological classification of the thyroid gland (paraffin sections) have achieved outstanding results. Our current study is to clarify whether deep learning assists pathology diagnosis for intraoperative frozen thyroid lesions or not.
We propose an artificial intelligence-assisted diagnostic system for frozen thyroid lesions that applies prior knowledge in tandem with a dichotomous judgment of whether the lesion is cancerous or not and a quadratic judgment of the type of cancerous lesion to categorize the frozen thyroid lesions into five categories: papillary thyroid carcinoma, medullary thyroid carcinoma, anaplastic thyroid carcinoma, follicular thyroid tumor, and non-cancerous lesion. We obtained 4409 frozen digital pathology sections (WSI) of thyroid from the First Affiliated Hospital of Sun Yat-sen University (SYSUFH) to train and test the model, and the performance was validated by a six-fold cross validation, 101 papillary microcarcinoma sections of thyroid were used to validate the system’s sensitivity, and 1388 WSIs of thyroid were used for the evaluation of the external dataset. The deep learning models were compared in terms of several metrics such as accuracy, F1 score, recall, precision and AUC (Area Under Curve).
We developed the first deep learning-based frozen thyroid diagnostic classifier for histopathological WSI classification of papillary carcinoma, medullary carcinoma, follicular tumor, anaplastic carcinoma, and non-carcinoma lesion. On test slides, the system had an accuracy of 0.9459, a precision of 0.9475, and an AUC of 0.9955. In the papillary carcinoma test slides, the system was able to accurately predict even lesions as small as 2 mm in diameter. Tested with the acceleration component, the cut processing can be performed in 346.12 s and the visual inference prediction results can be obtained in 98.61 s, thus meeting the time requirements for intraoperative diagnosis. Our study employs a deep learning approach for high-precision classification of intraoperative frozen thyroid lesion distribution in the clinical setting, which has potential clinical implications for assisting pathologists and precision surgery of thyroid lesions.
Peer Review reports
Pathological diagnosis provides a theoretical and practical basis for the diagnosis, treatment and prevention of diseases through the study of the causes and pathogenesis of diseases and the morphological structure of the diseased organism during the disease process. The production of hematoxylin and eosin (H&E) sections usually takes about three days to get the diagnosis result from the initial taking of specimen [ 1 ]. The number of pathologists is limited globally [ 2 ], and the lack of pathologists in hospitals has resulted in senior pathology doctors completing residents’ work. Nevertheless, the surgeon needs to know the information about the lesion as soon as possible to adjust the surgical plan. This requires rapid sectioning to obtain efficient diagnostic results from pathologists [ 3 ], and intraoperative frozen sectioning is the most widely used method [ 4 ]. It is a procedure in which the tissue cut during surgery is frozen in a frozen sectioning machine to allow for rapid cooling of the tissue through low-temperature conditions and then for production. Frozen sections are obtained during the process and are a significant guide to surgical treatment such as benign or malignancy for the patient’s lesion which might not match the original diagnostic surgical plan, and then affect the operation procedure [ 5 ]. On the contrary, many primary care hospitals have limited clinical experience in issuing frozen pathology reports because of a severe shortage of pathologists, which often makes it difficult to meet clinical needs.
Over recent years, the application of deep learning artificial intelligence approaches in digital pathology has shown excellent results, both in the broad application of different tissue diseases such as liver cancer [ 6 , 7 ], breast cancer [ 8 , 9 , 10 ] and esophageal cancer [ 11 , 12 ] and in the optimization and updating of cellular tissues in detection [ 13 ], segmentation [ 14 ] and classification [ 15 ] methods, all of which show great potential. For example, deep learning methods enable networks for Digital whole slide images (WSIs) lymphocyte measurement and segmentation tasks simultaneously [ 14 ]; artificial intelligence approaches for lung cancer histopathology classification by supervised or weakly supervised strategies [ 16 ]; deep learning for prediction from H&E images of follicular thyroid tumors [ 17 ]; and differential diagnosis of follicular thyroid tumors based on histopathological images using deep learning techniques [ 18 ]. However, existing deep learning methods for histopathological classification of thyroid cancer have been studied with paraffin-embedded H&E sections WSIs, but ignore the significance of intraoperative frozen sections. In this study, we attempt to develop a deep learning-based five-class classifier for identifying a broader and more detailed range of thyroid lesions. We refined the frozen sections’ analysis into five classification problem classes through differences in the histological characteristics of different cancers, including papillary thyroid carcinoma (PTC), follicular thyroid tumor (FTT), medullary thyroid carcinoma (MTC), anaplastic thyroid carcinoma (ATC), and non-cancerous thyroid lesion (NTC). ResNet [ 19 ], multi-processing, TensorRT [ 20 ], and graphics processing units (GPUs) were used to improve efficiency. To validate the performance, efficiency, and sensitivity of clinical applications, comparative model experiments, prior knowledge cascade experiments, time-cost acceleration analyses, and visualization of the effects of applications were carried out.
Due to the high resolution of digitized frozen slices, which can reach 100,000 × 100,000 pixels, direct complete WSIs processing with large-scale down-sampling loses many detailed features, which is not conducive to a classification approach regarding morphological features. Also training a convolutional neural network (CNN) on the whole slide tissue image (WSI) is computationally tricky to achieve. Cropping WSIs into tiles for analysis is a standard solution for studying histopathological WSIs by training a tiles-level classifier and later performing tiles fusion in an Expectation-Maximization (EM) manner to summarize the prediction results and obtain an effect map of the whole slice prediction [ 21 ]. Wei et al. [ 22 ] used sliding windows over the entire slide to generate small tiles and classified each tile by a neural network. They used heuristics to determine the primary and secondary histological patterns across the slide so that labels for different regions could be obtained.
In this study, the high accuracy performance and interpretability of our proposed system served as our primary goal. Against this backdrop, we ensured the accuracy of the tissue regions of candidate training tiles by double manual annotation of regions of interest (ROIs) by pathologists and pathologists. Then we segmented the localized tissue regions into small tiles and simulate real clinical diagnostic scenarios by a cascade diagnostic system to diagnose the presence of a cancerous lesion and subdivide the type of the cancerous lesion to predict the category of each tidbit separately. The final classification criterion was a fusion recovery of the entire tiles of the pathology slices, using a heuristic to identify the significant and minor histological patterns across the WSI. In addition, we confirmed the performance sensitivity of our system with test results of papillary thyroid microcarcinoma and different scale thermograms with accelerated plug-ins to speed up the diagnostic inference of the system. Our system not only allows for a more detailed classification of thyroid lesions but also allows for the system’s sensitivity to localize suspicious lesions, saving diagnostic time and cost and assisting the pathologist in making a diagnosis in copious ways. The main contributions of this paper can be summarized as follows:
A tile-supervised WSI-label learning-based classification and diagnosis system for frozen thyroid lesions is designed. We propose a Two-step cascade diagnostic system (TSCD), which utilizes a priori knowledge cascading into a five-classification diagnostic system that fits the clinical diagnostic scenarios, and for the first time achieves fine-grained classification of lesions, realizes a high-accuracy classification task and demonstrating advanced performance.
We conducted a systematic working test on papillary thyroid microcarcinoma alone. To ensure the accuracy of clinical application, we strictly selected cases with lesion sizes within 1 cm, and the results showed that a lesion size of 2 mm could be accurately localized.
A diagnostic acceleration component is designed, this acceleration component accelerates the processing from multi-processing of data to TensorRT acceleration of model inference, reducing consumption time to 729.37% of the original speedup. In addition, this component can be embedded in all tiles-based classification inference models for broad applicability.
A multi-scale thermographic visualization of the system is proposed to test the sensitivity, which is reflected by the selection of different scales of lesion areas from different cases to visualize the system’s identification results due to the high resolution of the WSI.
For the consideration of the clinical application of the diagnostic system, a heuristic strategy of tumor prioritization based on thresholds is proposed to clarify the diagnostic process by thresholding the WSI primary and secondary tissues in a heuristic manner to promote the development of deep learning methods for the clinical application of frozen histopathological images.
A database of frozen thyroid digital pathology sections was established, and the developability of deep learning in frozen sections was confirmed by designing a deep learning model to analyze data and assist in diagnosing pathology library data.
In this section, we will describe the preparation of data, the selection of deep learning models, composition of acceleration components, and the generation of the system for the final frozen diagnosis of thyroid lesions from several perspectives.
Slides collection and categorization
We collected frozen thyroid sections from the First Affiliated Hospital of Sun Yat-sen University to form a library of frozen thyroid digital pathology sections Four certified pathologists independently reviewed the organized frozen thyroid sections. The diagnosis was determined with the aid of the results of paraffin-embedded H&E sections and immunohistochemistry staining sections. In order to ensure the accuracy of the data, three rounds of screening were conducted sequentially through diagnostic pathology reports, four pathologists, and two pathologists in the process of selecting the data, and finally the data that were all approved were selected. Figure 1 shows the morphology of five types of typical thyroid sections, Fig. 2 shows their high magnification histopathology, and Table 5 lists the clinical management of different thyroid lesions. It can be seen that differentiating the types of thyroid cancer can facilitate efficient and targeted clinical diagnosis and help patients heal better. This study was approved by the Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University.
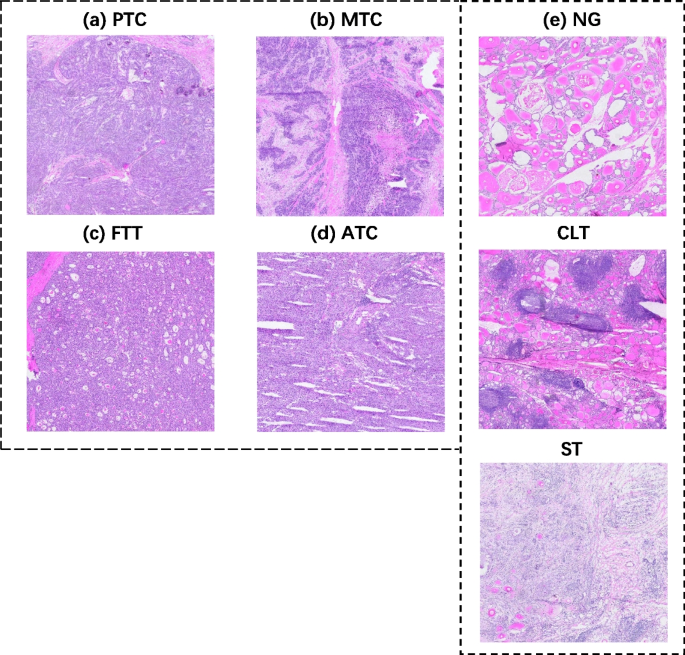
Frozen section examples WSI, A for papillary thyroid carcinoma nodule, B for medullary thyroid carcinoma nodule, C for follicular tumor nodule, D for anaplastic carcinoma nodule, and E for all non-cancerous frozen section slides WSI, from top to bottom, for nodular goiter, chronic lymphocytic thyroiditis, and subacute thyroiditis
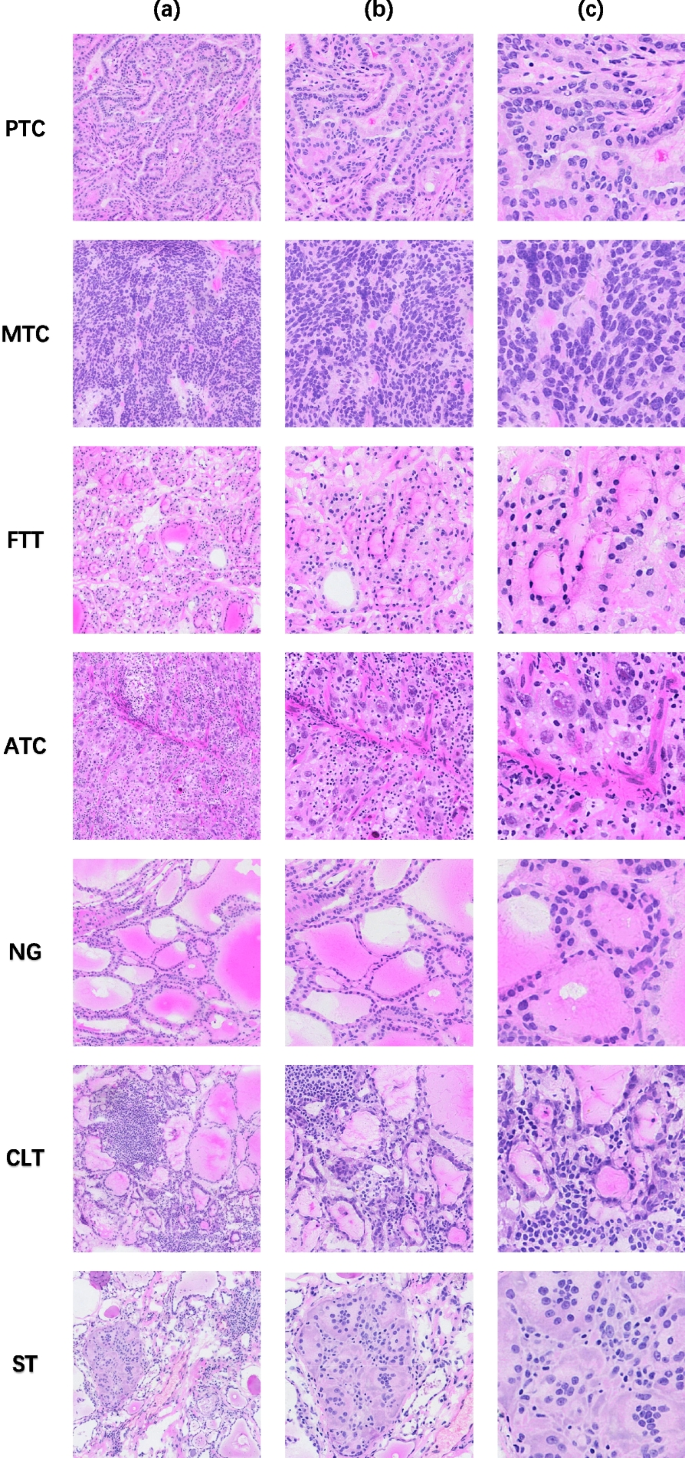
High-magnification histopathology, a listed as WSI at 20x magnification, b listed as WSI at 40x magnification, and c listed as WSI at 80x magnification, from top to bottom, PTC, MTC, FTT, ATC, NG, CLT, ST
Thyroid cancer is divided into papillary thyroid cancer, medullary thyroid cancer, anaplastic thyroid cancer and follicular thyroid cancer. However, if a follicular thyroid tumor is determined to be benign and less than 1 cm in diameter, it can be treated with an observation instead of follow-up surgery [ 23 ]. Follicular thyroid cancer which is malignant follicular thyroid tumor requires demonstration of capsular and/or vascular invasion. Ultrasound, puncture, intraoperative frozen pathology, and even postoperative paraffin-embedded pathology may only partially guarantee accuracy [ 24 ]. So there exists a tendency for various findings to be benign but may be malignant, so it is not easy to fully determine whether it is benign or malignant. Therefore, our classification tends to be divided into five parts: papillary carcinoma, follicular tumor, medullary carcinoma, anaplastic carcinoma, and non-cancer lesion, where the noncancer frozen section consists of data from nodular goiter, chronic lymphocytic thyroiditis (CLT), and subacute thyroiditis (ST) together.
WSI datasets
The database of frozen thyroid WSIs we created consisted of 4409 frozen thyroid sections from the First Hospital of Sun Yat-sen University (SYSUFH) and the First Affiliated Hospital of Sun Yat-sen University (FAH-SYSU), covering 3873 cases diagnosed as PTC, FTT, ATC, MTC or NTC, and the inclusion criteria for the dataset were that each frozen section was able to be included in the above categories. Before WSI annotation, all sections were observed by two senior pathologists at SYSUFH through microscopy, including immunohistochemically stained Sects. [ 25 , 26 ] used as an aid to diagnosis. The diagnostic reports of all cases were also checked as a way to determine the accuracy of the collected data. Slides were then scanned with the Slide Scan Imaging System SQS-600P scanner at 40x magnification and digitized into SDPC format. For an unbiased evaluation, classification was performed according to the 5th edition of WHO thyroid tumor classification criteria. For the scanned digitized pathology slides, four pathologists first annotated WSI for regional ROI, and two pathologists then checked the annotated slides with at least seven years of clinical experience in the pathology department of SYSUFH to ensure the accuracy of the annotated information.
We collected three batches of frozen thyroid slices for database development, and all the data in the library were used in our system’s training validation and testing. It contained 4409 thyroid frozen sections (PTC, 2300; FTT, 225; MTC, 49; ATC, 7; NTC, 1904) for model construction, randomly divided into training and testing 11 groups in a 10:1 ratio. Of these, 2894 (PTC, 1479; FTT, 224; MTC, 49; ATC, 7; NTC, 1135) were used to construct the model, 127 (PTC, 127) were used to evaluate the sensitivity of the model to small cancers, and 1388 (PTC, 694; FTT, 1; NTC, 769) were used in the external test set to evaluate the system’s generalization ability. The number of follicular variant of papillary thyroid carcinoma (FVPTC) cases was 135, containing 163 WSIs, or 3.49% of 3873 cases, and 3.70% of 4409 WSIs, and non- invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) The number of cases was 11, containing 14 WSIs, representing 0.28% of 3873 cases and 0.32% of 4409 WSIs.
Based on the patch cropping method, 105622 papillary carcinoma patches, 42644 follicular tumor patches, 27491 medullary carcinoma patches, 8507 anaplastic carcinoma patches, and 215418 non-cancerous lesion patches were cropped from the training slides used for system construction. Of these, 78343 papillary carcinoma patches, 42407 follicular tumor patches, 27491 medullary carcinoma patches, 8507 anaplastic carcinoma patches, and 118895 non-cancerous lesion patches were cropped from the training slides used for model construction and the rest of the patches used for testing, as shown in Table 1 .
Data pre-processing
The pathology slide scanner used to obtain the WSIs was the SQS-600P slide scanning imaging system from Shenzhen Shengqiang Technology Co. (Shenzhen, China). The labeling software is also the digital pathology reading software ImageViewer provided by Shenzhen Sangqiang Technology Co., Ltd, version DPVIEW V2.0.1.0927. We use a supervised approach to train the model, which requires us to annotate the ROI region carefully.
The pathologists manually annotated the specific histopathological thyroid tissue types in each WSI by using colored irregular shapes, and the annotated WSIs were reviewed by two pathologists to ensure the accuracy of the annotation. The colored irregularly annotated regions were cut at the tile level after ROI region annotation to provide the tissue details of the cut patches, as shown in Fig. 3 . Different categories of tiles obtained by cropping are shown in Fig. 4 . All processing was performed at a magnification of 40x, based on a pixel scale of 0.2065 μm. We traversed all manual annotations by reading the SDPL file (the file generated by manual annotation). Use the multiplicity of the rectangle marked in the blank area as the reference coordinate system, and calculate the ratio of the reference coordinate system to the absolute coordinate system using the screen position of the colored outline, and then deflating the annotated points to the reference coordinate system, taking the center point of the annotation as the distribution of tiles is described in Table 1 .
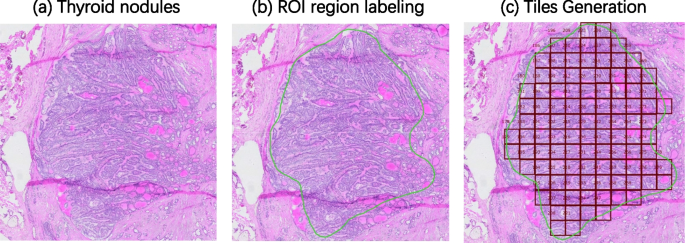
Diagram of the process of cropping WSI into tiles. a shows the WSI of the original frozen thyroid nodule, and b shows the annotation of the contours by the pathologist, the green contour is the ROI area, i.e., the area with lesions and non-cancerous areas can also be present in the slides with lesions. figure ( c ) shows the tiles obtained by cropping from the annotated section, the number of patches obtained by cropping is proportional to the area of the annotated area
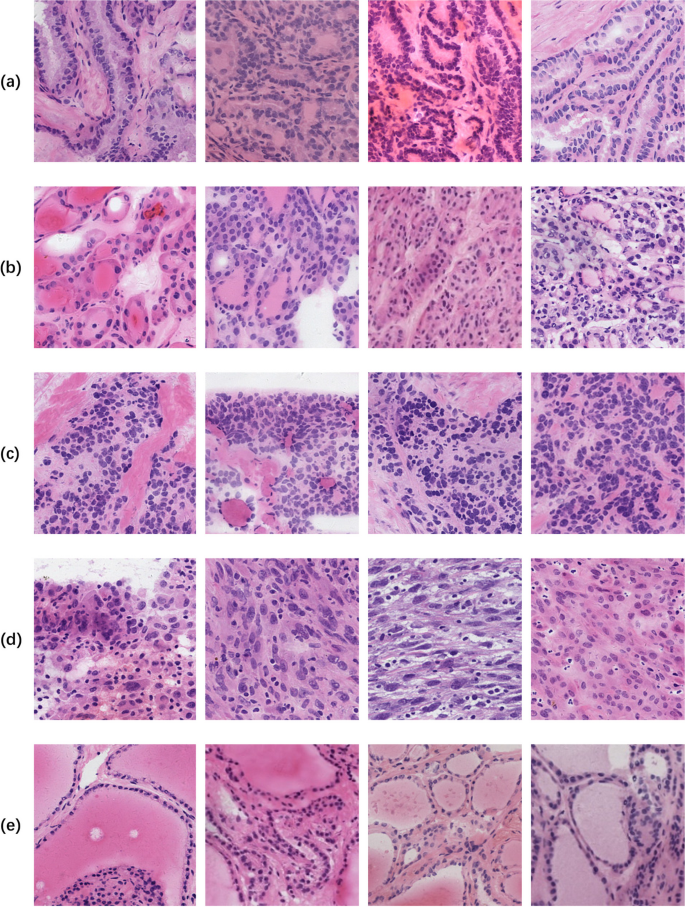
Different categories of tiles obtained by cropping, A for PTC, B for FTT, C for MTC, D for ATC, and E for NTC, with a pixel size of 224 × 224
Deep neural networks
According to the research content and its applicability, what we need is a neural network framework with high accuracy while ensuring low cost. The more commonly used neural network backbones include ResNet 19 , DenseNet [ 27 ], EfficientNet [ 28 ], ShuffleNet [ 29 ], etc. In addition, with the extension of the transformer to the computer vision (CV) field, the generic backbone network Swin-Transformer [ 30 ] is also showing superior performance in vision tasks such as image generation, medical image segmentation, etc. performance. We choose ResNet101 as the backbone network for histopathology classification tasks. The residual structure of ResNet is proposed to solve the problem of network degradation, and the gradient disappearance or explosion has been translated by normalized initialization, etc. The model can make learning less and more manageable, thus ensuring sure accuracy while reducing the cost. And Resnet101 is used for our histopathology classification task by replacing the last fully connected layer with five outputs representing papillary thyroid carcinoma, medullary carcinoma, follicular tumor, anaplastic carcinoma, and non-carcinoma lesion, respectively. For training, testing and optimization, we divided all the datasets into a training set, a validation set, and a test set. Although we used more than 399682 patches of data for system development, we still trained the thyroid patch model by transfer learning from models pre-trained on the ImageNet recognition task. By initializing the pre-trained model, the patch classifier can achieve better performance. At the same time, we also fine-tune models such as Swin Transformer, EfficientNet-B5, and GoogLeNet [ 31 ] using the same data and settings as Resnet101 and also test the models using the same test set to make qualitative comparisons among different kinds of model results.
A priori knowledge and classification cascade
In image classification tasks, a priori knowledge can be obtained by pre-training on large-scale image datasets [ 32 ]. Through pre-training, the model can learn the basic features and patterns of the images, and the introduction of a priori knowledge as pre-training weights can effectively improve the accuracy and efficiency of image classification. Modeled after the actual diagnostic process of clinical thyroid freezing, the TSCD system was designed as a categorization cascade of two classification cascades for the presence of cancerous lesions and a categorization cascade of four classification cascades for subdivided lesion types. However, the pre-training dataset ImageNet [ 33 ] used now has a feature extraction method that does not fit the pathology image, and the training data of the two parts of the cascade model is not as much as that of the five-classification model, which is insufficiently fine-grained for the classification task. In order to make the cascade model have similar feature extraction ability as the five-classification model and fit the pathological images of thyroid lesions, the five-classification baseline model’s is used as the pretrain model of the cascade model.
Network training and implementation details
Our training, validation and testing data are not publicly available due to strict controls on medical management and strict policies on sample privacy. Even though we have acquired several 399062 tiles for the model training process, we still apply transfer learning to our network model training to obtain better performance. By initializing the network with the default weights transferred from the ImageNet dataset, the entire network is fine-tuned to fit our data target better. The initial learning rate is set to 0.00003 and the optimizer is Adam. Momentum and attenuation are both set to 0.9. dynamic data enhancement, including horizontal flip or in the vertical direction, color dithering includes automatic adjustment of luminance in the range of (0.65, 1.35), automatic adjustment of contrast in the field of (0.5, 1.5), automatic adjustment of automatic saturation adjustment, and 0.3 size adjustment for hue to increase the data variation. To improve the learning characteristics of convergence, pixels were rescaled from 0 to 255 to 0–1 by dividing by 255, and the Z-score was normalized using the mean (0.485, 0.456, 0.406) and STD (0.229, 0.224, 0.225). The training process lasts 40 epochs, and the optimization model with the most negligible loss is saved and used. We used the deep learning framework PyTorch 1.10.2 to implement all CNN models, and an NVIDIA GeForce RTX 3090 GPU with 24 GB of memory was used for CNN model training and evaluation. Training ResNet18, ResNet34 models took about 32 h, and Resnet50 model took about 44 h, and ResNet101 model took about 48 h. GoogLeNet model took about 28 h. Swin Transformer model took about 67 h. EfficienNet-B5 took about 47 h. Vision-Transformer took about 88 h. AlexNet, DenseNet121, VGGNet-16, ShuffleNet-v2 models took about 16 h, 36 h, 43 h and 25 h.
Tile splitting and prediction
For the sake of achieving the integrity of tissue details and clarity of morphological features, we cut the labeled area into non-overlapping tiles of 224*224 pixels in size. Tiles were filtered with the criterion of 50% or more of class-labeled components to obtain a dataset with a high degree of lesion fit from both WSI and tile-level perspectives. We use our deep learning system to perform classification prediction for these tiles from manually labeled regions. After obtaining the initial tile-level class labels, we perform WSI fusion according to our tiles fusion strategy to get the WSI-level label prediction, which is the auxiliary lesion diagnosis result. Figure 5 illustrates the diagnostic flow chart of a whole frozen thyroid section.
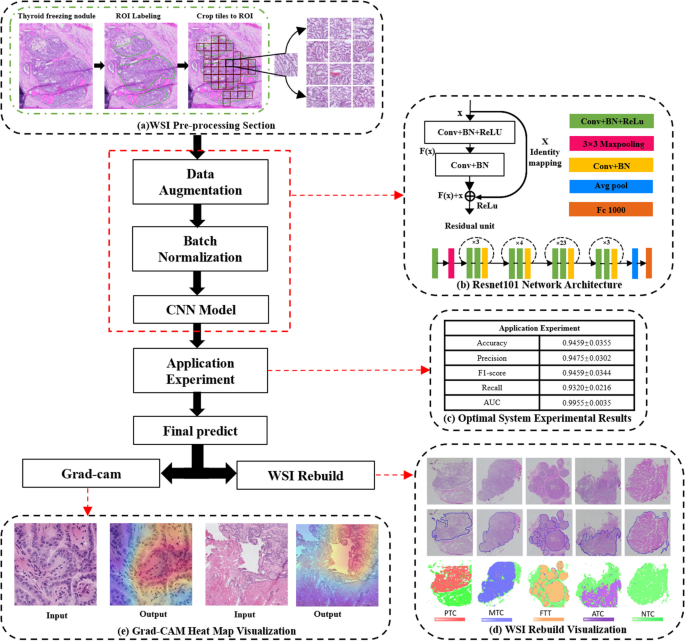
Deep learning-based diagnostic workflow for frozen thyroid lesions. The pipeline contains five modules: a WSI pre-processing section, b Resnet101 network structure, c optimal system experimental results, d WSI reconstruction visualization, and e Grad-CAM (Gradient-weighted Class Activation Mapping) heat map visualization
Whole-slide label inferencing with tiles fusion
Since the network outputs are tile-level predictions, the final diagnosis must be integrated into WSI-level forecasts. In general, the prediction results of slice-level WSI are aggregated to decide the classification based on tiles, which depend on the category to which the maximum probability belongs. Classical integration methods include averaging [ 34 ] and voting [ 35 ] procedures, which use more straightforward strategies to combine the predictions of individual learners. The majority voting method [ 36 ] is based on the classification results of a single classification model. It uses the principle of minority rule to determine the category labels predicted by the model, that is, by the number of tiles in each category and assigning the entire WSI to the one label with the highest number of corresponding types. The other is the average pooling method, where the probabilities of each category are summed and the slide labels are derived from the maximum average class probabilities [ 37 ].
In our data, tissue components of non-cancerous categories and other tumors may coexist in one WSI. For example, papillary thyroid cancer and non-cancerous parts are distributed in different regions of the same WSI. Still, the final label of one WSI is attributed to only one category. Inspired by the work of Li et al. [ 38 ], we propose a threshold-based approach to compound the results based on the majority voting method incorporating a threshold division of pathological tissue priorities. When we encounter multiple tiles class labels co-located in a single WSI, equal treatment of tumor-containing and non-cancerous WSIs will result in the neglect of microscopic cancerous lesions and serious misclassification of the patient’s diagnosis, such that the results we predict will not fit the requirements for putting into practical clinical use. Therefore, to improve the priority level of tumor-containing WSIs and enhance the sensitivity of the system to tumor regions, we propose a heuristic strategy based on threshold tumor priority, as shown in Fig. 6 . Firstly, we set the threshold value according to the severity of lesions, because PTC, ATC, FTT, and MTC are all tumor types except the NTC category, so the threshold value of NTC is set to 95%. In contrast, all the rest of tumors are not differentiated by a threshold value. In addition, the pixel size of the tiles we selected was 224 × 224. Such a size is small even when compared with the lesion diameter of papillary thyroid microcarcinoma. As shown in Fig. 6 , in our work for the prediction of WSI of papillary thyroid microcarcinoma with a lesion diameter ≤ 10 mm, the size of the lesion in the visualization result can be cropped to 20 tiles with continuity. Therefore, the category of this WSI was determined as NTC only when the weight of NTC exceeded a threshold value of 95% and the number of regional continuity tiles (RCT) was less than 20. In addition to the above, if there was a coexistence of NTC and other categories, all different categories were used as the main tissue component, and the same was valid for label selection. The task classification we implemented in this study is based on the visual features observable at the cellular or partial tissue level on the image tiles scale, so this strategy of fusing tiles is used.
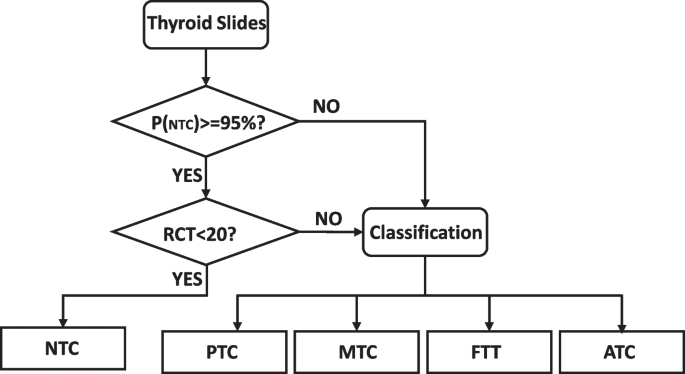
Athreshold-based tumor-first frozen section diagnostic method for the thyroid is proposed
Acceleration components
Due to the time-specific nature of intraoperative diagnosis, efficient diagnostic results need to be given in a short period of time, so we designed an acceleration component to enhance the diagnostic efficiency, and the component includes multiprocessing for cutting tiles and TensorRT to accelerate the inference.
Multiprocessing for cutting tiles
In the program of cutting tiles, multiple processes [ 39 ] can be run at the same time through the multi-process module, each process has its own independent execution space and resources, and can handle multiple tasks at the same time, here we use 9 processes, compared to the original time to get a single tile can now be obtained 9 tiles, in order to improve the concurrency and response speed of the system.
Diagnostic reasoning acceleration
PyTorch [ 40 ] is an open-source deep learning framework that can be used to build and train neural network models. ONNX (Open Neural Network Exchange) [ 41 ] is an open deep learning model exchange format that can be used to share and convert models between different deep learning frameworks. TensorRT [ 42 ] is an NVIDIA provided high-performance deep learning inference engine that can be used to accelerate the inference process of deep learning models. Our initial system was trained to obtain PyTorch models, and in model inference, to speed up the inference process, we converted Pytorch to ONNX files via the torch.onnx.export() function, after which we used the TensorRT Python API to build the TensorRT engine with ONNX models, as depicted in Fig. 7 conversion flow so that high performance inference can be achieved in TensorRT using PyTorch-trained models.

TensorRT accelerates the PyTorch model inference process, which consists of three steps:(1) export the PyTorch model to an ONNX file, (2) build the TensorRT Engine, and (3) deploy the TensorRT Engine
Evaluation Metrics
In our experiments, the accuracy, precision, recall, and F1 score were used to evaluate the performance of our proposed method and the state-of-the-art techniques [ 43 ]. In the assay method, the actual category value and the prediction were the same and both were positive, then TP (True Positive); if both were negative, then TN (True Negative); the actual category value did not agree with the prediction and the prediction was positive, then false positive (FP) if the forecast was negative, then false negative (FN). Based on these basic definitions, additional evaluation metrics (Accuracy [A], Precision [P], F1-Score [F1], Recall [R], Specificity[S], TPR [True Positive Rate], FPR [False Positive Rate]) of the frozen thyroid tissue diagnostic test can be introduced as follows.
Patch-based classification
In the twelve experimental ones of the Convolutional Neural Networks (CNN) model, the model’s performance is optimized by fine-tuning the model. Among them, Resnet34, Resnet50 and Resnet101 models perform close to each other in the fine-tuned training mode, but Resnet101 shows a slight lead. Table 2 ; Fig. 8 offer a qualitative comparison between the system backbone network Resnet and other classical deep learning models. These results compare and validate the superior results of selecting the Resnet34, Resnet50 and Resnet101 models for the classification of frozen thyroid lesions by transfer learning, and the final visualization of the whole slice prediction is in excellent agreement with the ground. The absolute consistency of the entire prophecy is also extremely high.
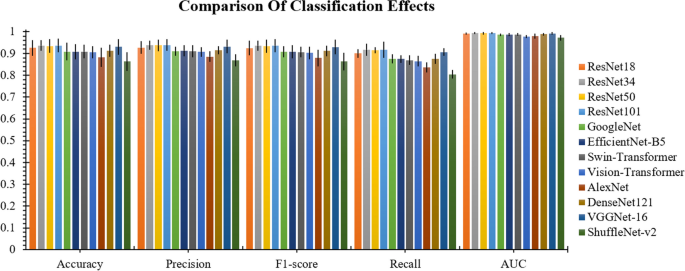
Comparison of classification effects. It can be seen from the figure that Resnet34, Resnet50, Resnet101 are higher than other models in all four evaluation indexes
Three backbone models were selected for the cascade test since the performance differences on the five-category baseline of Resnet34, Resnet50 and Resnet101model were small. Figure 9 compares the evaluation of Accuracy [A], Precision [P], F1-score [F], and Recall [R] of the direct cascade with ImageNet as the pre-training model and the TSCD system with the five base classifications of thyroid lesions as the pre-training model under the three models metrics. The experimental results show that whether it is Resnet34, Resnet50 or Resnet101 model, the evaluation indexes from BASE classification to direct cascade to pretrain cascade system are incremental effect, as this can prove the validity of our proposed cascade system modeled on clinical diagnosis. Since the cascade of the five classifications of thyroid lesions as a pretrained model yielded more accurate results than the direct cascade using ImageNet as a pretrained model, and the overall performance of the cascade of Resnet101 outperformed that of the other models in the system, we ultimately chose Resnet101 as the model that constitutes the backbone of our TSCD system for diagnosing frozen thyroid lesions. The final test results of our TSCD system were 94.59% ± 3.55% in Accuracy, 94.75% ± 3.02% in Precision, 94.59% ± 3.44% in F1-score, 93.20% ± 2.16% in Recall, and the value of AUC was 99.55% ± 0.35% (Table 3 ).
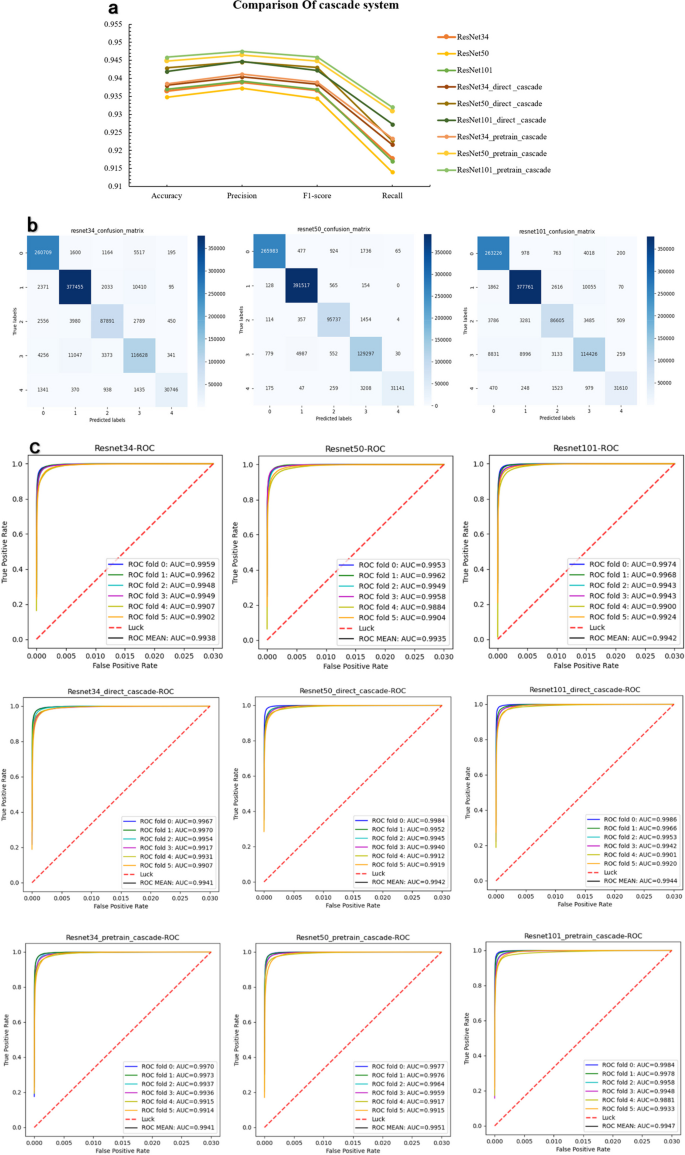
Better performing comparison in cascade system. The top three comprehensive performances of the above models are selected for a more visual comparison. a Comparison of the metrics performance of the three selected models on base classification and TSCD system. b Confusion matrix at the tile level predicted by the basis of the three models. c ROC curves and their AUC values for the three models on the base categorization and the TSCD system under cross-validation. It can be seen that Resnet101_pretrain_cascade is superior to other models in every index
Internal and external test sets
Internal and external test sets were selected to evaluate the generalization ability of the diagnostic system. The external test set mainly focuses on the two categories of papillary thyroid cancer and non-cancer, which is due to the imbalance in the distribution of thyroid lesions themselves, with papillary cancer accounting for a much larger proportion than other lesions. Therefore, we selected the binary classification model for the presence of cancer in the first part of the TSCD system to be used to test the effectiveness of the diagnostic system on an external test set. At the same time, we divided the internal data originally used to test the five-classification diagnostic system into two categories for the comparison test between the internal and external test sets. The test results are shown in Fig. 10 . The accuracy of our model on the external test set reaches 99.37% ± 0.15%, and the accuracy of the internal test data is slightly lower than the external data set because the complexity of the internal data is greater than that of the external test set, but the overall effect is still as high as 97.62% ± 0.58%. The accuracy of our diagnostic system is 97.62%±0.58% for the binary classification of cancer or not, and 99.37%±0.15% for the external test set, which shows the strong generalization ability of our system.

The first part of the TSCD system, i.e., the dichotomous classification of the presence or absence of cancer, was selected for the test. The first row shows the effects on the internal test set originally used to construct the diagnostic system, and the second row shows the effects on the external test set obtained from the East Hospital of the First Affiliated Hospital of Sun Yat-sen University. The effects on the four metrics Accuracy [A], Precision [P], F1-score [F], Recall [R], the confusion matrix, and the AUC performance are shown from left to right
Visualizing predictions with heatmaps
Grad-CAM (Gradient-weighted Class Activation Mapping) can assist in analyzing the regions of interest of the system for a given class, and we also verify whether we have learned the correct features or information through the regions of interest of the network in turn. We use Grad-CAM to draw the heat map of Fig. 11 . From these heat maps, we can find that our system achieves accurate localization and recognition of the regions that we classify for attention.
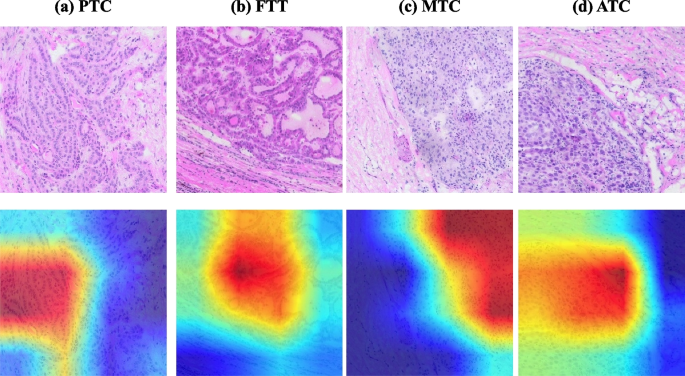
Grad-cam heat map visualization of tumor classification, a tile level visualization for papillary carcinoma, b tile level visualization for follicular tumor, c tile level visualization for medullary carcinoma, and d tile level visualization for anaplastic carcinoma
Slide-level performance
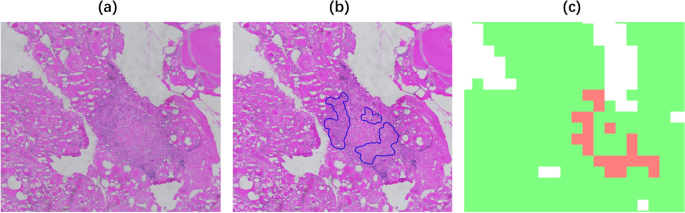
To obtain the predictions on the whole slide image, we mapped the system predicted tile-level results onto the original frozen sections, with different color blocks representing different categories. Figure 12 selected slices with different labels, the original WSI, the pathologist annotated WSI and the system slice-level prediction results are plotted, which visually shows the high agreement between our system classification prediction area and the pathologist annotated area.
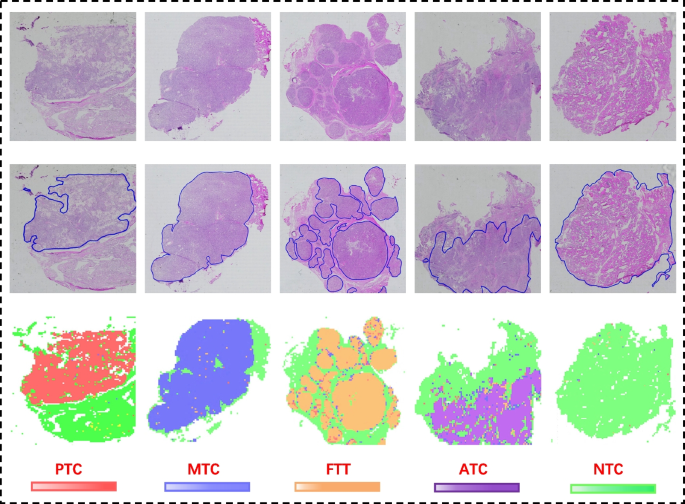
From left to right, the slide-level visualization (performance) of PTC, MTC, FTT, ATC, and NTC tissue predictions are shown. The first row shows the original slides, the second row shows the annotated WSIs with closed blue curves depicting the ROIs annotated by the pathologist, and the last row shows the corresponding WSI prediction visualization
Test on papillary thyroid microcarcinoma
To test the sensitivity of the system and for more accurate results in clinical use, we developed a sensitivity testing strategy for papillary thyroid microcarcinoma. we screened papillary thyroid microcarcinoma by lesion diameter and used them to test the system’s sensitivity. We screened 101 frozen WSIs of papillary thyroid microcarcinoma, whose lesion diameters were all within the range of 1 cm, and obtained more than 3000 tiles by cropping them and inputting them into the system for testing. And obtained visualization results displayed in Fig. 13 , which can accurately identify the cancerous regions, verifying the accuracy of the system.
Test of papillary thyroid microcarcinoma, the example of papillary thyroid microcarcinoma with lesion size of 2 mm in diameter, A original WSI, B manually labeled ROI, C Slide-level visualization display, the result can accurately identify the cancerous area
As can be seen from Slide-level diagnosis Fig. 13 , our system can accurately identify cancerous lesions even in the face of lesions as small as 2 mm in diameter when testing papillary thyroid microcarcinoma cases.
Multi-scale Thermographic visualization
We proposed a multi-scale heat map visualization, which, due to the high resolution of WSIs, the lesion areas of different cases and different scales were selected, as in Fig. 14 , we chose the WSIs of four papillary carcinoma cases and cropped the four WSIs at different scales, and the pixel sizes obtained were 224 × 224, 512 × 512, 1120 × 1120, 2240 × 2240 in order, and the results of the system in different scale tiles for grad-cam heat map visualization [ 44 ], reflecting the sensitivity of the system. Figure 14 shows the classification effect of our deep neural network system in the form of a Grad-CAM visual thermal map. Even when faced with tiles of different scales, the diagnostic system was able to pinpoint the area where the tumor was located.
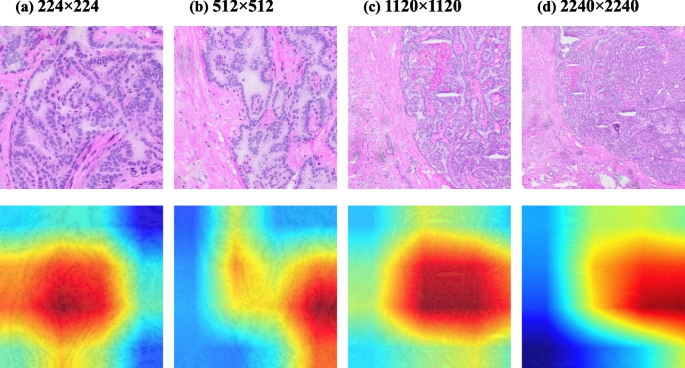
Grad-cam heat map visualization of different scales of tumor classification, illustration of papillary thyroid carcinoma. The pixel size of figure ( a ) is 224×224, figure ( b ) is 512×512, figure ( c ) is 1120×1120, and figure ( d ) is 2240×2240
Accelerated component results in diagnostic time cost
After the optimization, excluding the necessary time to read and store the image brought by the hardware, the average time to cut the whole WSI is reduced from 2899.89s to 346.12s, which is an 837.83% speed improvement. The average inference time of the model’s individual WSIs is reduced from 343.84s to 98.61s, which is a 348.69% speed improvement. The system’s overall inference and diagnosis time is reduced from 3243.73 s to 444.73s, an improvement of 729.37%, as shown in the details in Table 4 . As intraoperative freezing has very strict time requirements, doctors need to accurately grasp the freezing time to ensure the safety and effectiveness of the procedure. The shortened time helps the effective intraoperative diagnosis, thus further improving the efficiency and success rate of the surgery.
The primary time cost of WSI analysis lies in the data processing and prediction results of the WSI and its visualization generation. the level down-sampling factor in the WSI pyramidal storage structure is 2, and the annotated ROI size is one-fourth of the slide height and width, Then the size of the ROI region is 10,000 × 10,000 pixels in the level 2 slide image, which corresponds to 40,000 × 40,000 in level 0 of the WSI, and the size of the tiles extracted by the CNN model is 224 × 224.A frozen slide was scanned using a slide scanning imaging system SQS-600P scanner, and the time from getting the frozen slide to scanning to obtain the WSI was within two minutes. The scanned WSI can be directly processed by our system first by cropping the whole WSI into tiles of 224 × 224 size, and the cropped tiles are directly input for the entire slide prediction module in the system for visualizing the effect, the whole process is strictly controlled within 10 min, which can well assist the pathologist’s diagnosis.
Up to now, histopathology is still the gold standard for pathology doctors to diagnose diseases, and its development from slides under the light microscope to digital pathology images shows the significance of artificial intelligence in medical treatment. As the incidence of thyroid cancer is increasing year by year worldwide, the advancement of AI-assisted approaches to update it has received widespread attention.
Reasons for classification task selection
In recent years, there has been good development in the field of research on the artificial intelligence-assisted diagnosis of the thyroid, but almost the vast majority of the findings have focused on paraffin Sects. [ 45 , 46 , 47 , 48 ], and few studies have been performed on frozen sections of thyroid cancer in clinical surgery [ 38 , 49 ]. The only articles that are available only address the benignity and malignancy of nodules in thyroid sections, and the management of different thyroid cancers in clinical surgery is exceptionally variable [ 50 , 51 ], as shown in Table 5 .
Due to time constraints in surgery, protocols for different thyroid cancers must be adjusted and determined as soon as possible. Therefore, to solve the practical clinical problem, we designed a five-class classifier for frozen thyroid lesions, through which we can cover a broader range of thyroid lesions and assist pathologists in obtaining efficient results. Since thyroid cancers are morphologically distinct, such as papillary thyroid carcinoma nuclei showing variations in size and shape, such as enlargement, lengthening, overlapping and crowding, our deep learning model centers around morphological features for the classification task.
Development of a database of frozen thyroid WSIs
We built a Frozen Thyroid WSIs Database with 3873 cases, 4409 frozen thyroid slices containing WSIs for papillary thyroid cancer, medullary carcinoma, anaplastic carcinoma, follicular tumor, nodular goiter, chronic lymphocytic thyroiditis, and subacute thyroiditis. All the data in the database were used in our diagnostic system, of which 2894 were used for system building. The creation of the Frozen Thyroid WSIs Database lays the foundation for research into rapid intraoperative diagnosis, introducing deep learning into the surgical process. In addition, the database broadens the idea of integrating artificial intelligence into clinical care and confirms the potential of deep learning approaches in the freezing field. We will also continue to collect samples by enriching the database in terms of sample types and numbers in the future.
Distinction between FVPTC and NIFTP
In the classification diagnosis, distinguishing between NIFTP and FVPTC is crucial for the improvement of model accuracy. FVPTC and NIFTP have partial similarity in pathological features, but still have obvious distinguishing features. Nuclei in FVPTC are usually larger, heteromorphic, and irregular, whereas nuclei in NIFTP are more regular, smaller, and relatively homogeneous in shape. The follicular structure in FVPTC may appear disrupted, misshapen, or atypically arranged and have more microstructural variations. The follicular structure in NIFTP usually remains relatively regular and organized and lacks significant disruption or heterogeneity [ 52 , 53 , 54 , 55 ]. These pathological feature differences can be effectively learned and utilized by deep learning models to achieve better results in classification tasks.
Patch size selection
Our proposed system is a neural network model based on tiles classification so the selection of tiles has a particular influence on the model results to a large extent. We use tiles of 224 × 224 pixels in size, which firstly ensure that there is no significant loss of image information, and secondly, our model is mainly based on the extraction of morphological features for classification learning, and the selected tiles are also as detailed as possible to show each morphological feature to assist in making a reliable diagnosis. By comparing the performance of the benign and malignant classification work done by Li et al. [ 38 ], the size of the tiles selected by them is 2392 × 2392, and the average accuracy of our results presents better results under the same model action, which is also reflected in the tiles-based classification model, where our tiles size selection can better capture the morphological features of the lesion. In addition, the chosen tiles size allows the model to diagnose a single thyroid slide within 5 min in most cases, satisfying the time requirement. Therefore, considering the perspective of clinical application, the size of this tile can fulfill both the model feature extraction needs and the diagnostic time limit, thus better diagnosing from the standpoint of simulated pathologists.
As the number of cases of different lesions is relatively unbalanced, this may have some impact on the generalizability of the study’s findings and the limitations of its practical application. One of the goals of the study is to generalize to a wider population or context to strengthen the validity and reliability of its practical application. We will continue to collect samples, firstly, to contain a relatively small number of instances, such as the part of anaplastic carcinoma; secondly, to collect some external data sets, and combine the two aspects with improving the robustness and generalization ability of the model.
Assistant to pathologists
Our work demonstrates that deep learning can have a fine-grained aid to the intraoperative frozen section classification diagnostic work, which helps pathologists to develop surgical strategies efficiently. On the one hand, our system can analyze digital pathology sections while the pathologist is studying frozen sections under a light microscope, visualizing the distribution of lesions and areas of cancer by a grad-cam heat map or tiles fusion. The two complement each other and work together to improve the accuracy of the pathologist’s judgmental findings. On the other hand, our system can also complement the pathologist’s diagnosis with specific details, for example, in areas easily missed, thus complementing each other. However, the deep learning approach is used only to aid the pathologist’s diagnosis and cannot directly replace the pathologist’s independent work.
In this paper, we propose a deep learning model classification system based on tiles to build a Frozen Thyroid WSIs Database, which is the first work on the fine classification of frozen thyroid carcinoma slices and the first digital database for frozen slices. We finally used the Resnet101 model as the final development model by comparing multiple models. We tested our data through the diagnostic system and obtained a high accuracy result of 94.59%±3.55%. Meanwhile, we proposed sensitivity tests for both systems to reflect the system’s accurate control of cancer diagnosis by pooling multiple scales. In addition, to consider the clinical application, we developed a threshold-based tumor-first heuristic strategy for dividing WSI primary and secondary tissues by double thresholds, which meets the actual clinical needs. Finally, our study can diagnose typical WSIs images within 10 min, confirming the efficient performance of our system. Our work is consistent with the application to clinical scenarios in terms of the breadth of classification coverage of frozen thyroid sections, the accuracy of model results, the efficiency of diagnosis and the consistency with experienced pathologists, and it has significant implications for clinical diagnosis. In future work, we will also enrich the number of frozen digital databases and extend them to other tissue-frozen sections to build a multi-tissue system for intraoperative rapid frozen sections.

Availability of data and materials
The pathological images used in this paper is not publicly available. However, they can be obtained by contacting the corresponding author ([email protected]) for scientific research purposes.
Abbreviations
Artificial intelligence
Convolutional neural network
Graphics processing unit
Hematoxylin and eosin
Papillary thyroid carcinoma
Follicular thyroid carcinoma
Thyroid follicles tumors MTC: medullary thyroid carcinoma
Anaplastic thyroid carcinoma
Non-carcinoma lesion
Papillary thyroid microcarcinoma
Region of interest
Sun Yat-sen University dataset
Sun Yat-sen University First Affiliated Hospital
whole slide image
Computer Vision
True Positive
False Negative
False Positive
True Negative
World Health Organization
Chronic lymphocytic thyroiditis
Subacute thyroiditis
Nodular goiter
Regional continuity tiles
Receiver Operating Characteristic
Area Under Curve
True Positive Rate
False Positive Rate
Gradient-weighted Class Activation Mapping
Two-step cascade diagnostic
Non-invasive follicular thyroid neoplasm with papillary-like nuclear features
Follicular variant of papillary thyroid carcinoma
Rodig SJ. Preparing Paraffin Tissue Sections for Staining. Cold Spring Harb Protoc. 2021;2021(3). https://doi.org/10.1101/PDB.prot099663 . PMID: 33649119.
Märkl B, Füzesi L, Huss R, Bauer S, Schaller T. Number of pathologists in Germany: comparison with European countries, USA, and Canada. Virchows Arch. 2021;478(2):335–41. https://doi.org/10.1007/s00428-020-02894-6 . Epub 2020 Jul 27. PMID: 32719890; PMCID: PMC7969551.
Article PubMed Google Scholar
Kraemer BB. Frozen section diagnosis and the thyroid. Semin Diagn Pathol. 1987;4(2):169–89. PMID: 3313602.
CAS PubMed Google Scholar
Novis DA, Gephardt GN, Zarbo RJ. Interinstitutional comparison of frozen section consultation in small hospitals: a college of American pathologists Q-probes study of 18532 frozen section consultation diagnoses in 233 small hospitals. Arch Pathol Lab Med. 1996;120(12):1087.
Albores-Saavedra J, Wu J. The many faces and mimics of papillary thyroid carcinoma. Endocr Pathol. 2006;17(1):1–18.
Kiani A, Uyumazturk B, Rajpurkar P, et al. Impact of a deep learning assistant on the histopathologic classification of liver cancer[J]. NPJ Digit Med. 2020;3(1):1–8.
Article Google Scholar
Chaudhary K, Poirion OB, Lu L, et al. Deep learning–based Multi-omics Integration robustly predicts Survival in Liver CancerUsing Deep Learning to Predict Liver Cancer Prognosis[J]. Clin Cancer Res. 2018;24(6):1248–59.
Article CAS PubMed Google Scholar
Han Z, Wei B, Zheng Y, et al. Breast cancer multi-classification from histopathological images with structured deep learning model[J]. Sci Rep. 2017;7(1):1–10.
Google Scholar
Wang D, Khosla A, Gargeya R, et al. Deep learning for identifying metastatic breast cancer[J]. arXiv preprint arXiv:1606.05718. 2016.
Bejnordi BE, Veta M, Van Diest PJ, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer[J]. JAMA. 2017;318(22):2199–210.
Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning[J]. Nat Med. 2018;24(10):1559–67.
Article CAS PubMed PubMed Central Google Scholar
Sun W, Zheng B, Qian W. Computer aided lung cancer diagnosis with deep learning algorithms[C]//Medical imaging 2016: computer-aided diagnosis. SPIE. 2016;9785:241–8.
Liu KL, Wu T, Chen PT, et al. Deep learning to distinguish pancreatic cancer tissue from non-cancerous pancreatic tissue: a retrospective study with cross-racial external validation[J]. Lancet Digit Health. 2020;2(6):e303–13.
Zhang X, Zhu X, Tang K, Zhao Y, Lu Z, Feng Q. DDTNet: a dense dual-task network for tumor-infiltrating lymphocyte detection and segmentation in histopathological images of breast cancer, Medical Image Analysis, 78,2022, 102415, ISSN 1361–8415, https://doi.org/10.1016/j.media.2022.102415
Wang S, Yang DM, Rong R, et al. Pathology image analysis using segmentation deep learning algorithms[J]. Am J Pathol. 2019;189(9):1686–98.
Article PubMed PubMed Central Google Scholar
Yang H, Chen L, Cheng Z, Yang M, Wang J, Lin C, Wang Y, Huang L, Chen Y, Peng S, Ke Z, Li W. Deep learning-based six-type classifier for lung cancer and mimics from histopathological whole slide images: a retrospective study. BMC Med. 2021;19(1):80. https://doi.org/10.1186/s12916-021-01953-2 . PMID: 33775248; PMCID: PMC8006383.
Dolezal JM, Trzcinska A, Liao CY, et al. Deep learning prediction of BRAF-RAS gene expression signature identifies noninvasive follicular thyroid neoplasms with papillary-like nuclear features. Mod Pathol. 2021;34:862–74. https://doi.org/10.1038/s41379-020-00724-3 .
Nojima S, Kadoi T, Suzuki A, Kato C, Ishida S, Kido K, Fujita K, Okuno Y, Hirokawa M, Terayama K, Morii E. Deep learning-based Differential diagnosis of follicular thyroid tumors using histopathological images. Mod Pathol. 2023;36(11):100296. Epub 2023 Jul 31. PMID: 37532181.
He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. 2016 IEEE Conf Comput Vis Pattern Recognit (CVPR). 2016;770–8. https://doi.org/10.1109/CVPR.2016.90 .
NVIDIA TensorRT, Accessed. Mar. 23, 2021. Available: https://developer.nvidia.com/tensorrt/
Hou L, Samaras D, Kurc TM, Gao Y, Davis JE, Saltz JH. Patch-based convolutional neural network for whole slide tissue image classification. Proceedings of the IEEE conference on computer vision and pattern recognition 2016:2424–33. https://doi.org/10.1109/CVPR.2016.266
Wei JW, Tafe LJ, Linnik YA, Vaickus LJ, Tomita N, Hassanpour S. Pathologist-level classification of histologic patterns on resected lung adenocarcinoma slides with deep neural networks. Sci Rep. 2019;9(1):3358. https://doi.org/10.1038/s41598-019-40041-7 . PMID: 30833650; PMCID: PMC6399447.
Rau JV, Fosca M, Graziani V, et al. Proof-of-concept Raman spectroscopy study aimed to differentiate thyroid follicular patterned lesions. Sci Rep. 2017;7:14970. https://doi.org/10.1038/s41598-017-14872-1 .
Ito Y, Hirokawa M, Hayashi T, Kihara M, Onoda N, Miya A, et al. Clinical outcomes of follicular tumor of uncertain malignant potential of the thyroid: real-world data. Endocr J. 2022;69(7):757–61. https://doi.org/10.1507/endocrj.EJ21-0723 . Epub 2022 Jan 26. PMID: 35082189.
Cheung C, Ezzat S, Freeman J, et al. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol. 2001;14:338–42. https://doi.org/10.1038/modpathol.3880312 .
Haines DM, Chelack BJ. Technical considerations for developing enzyme immunohistochemical staining procedures on Formalin-fixed paraffin-embedded tissues for Diagnostic Pathology. J Vet Diagn Invest. 1991;3(1):101–12. https://doi.org/10.1177/104063879100300128 .
Huang G, Liu Z, Van Der Maaten L, et al. Densely connected convolutional networks[C]//Proceedings of the IEEE conference on computer vision and pattern recognition. 2017. p. 4700–8.
Tan M, Le Q. Efficientnet: Rethinking model scaling for convolutional neural networks[C]//International conference on machine learning. PMLR; 2019. p. 6105–14.
Zhang X, Zhou X, Lin M, et al. ShuffleNet: An Extremely Efficient Convolutional Neural Network for Mobile Devices. 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition. Salt Lake City: IEEE; 2018. p. 6848–56. https://doi.org/10.1109/CVPR.2018.00716 .
Liu Z, Lin Y, Cao Y, et al. Swin transformer: Hierarchical vision transformer using shifted windows[C]//Proceedings of the IEEE/CVF International Conference on Computer Vision. 2021. p. 10012–22.
Szegedy C, Liu W, Jia Y, et al. Going deeper with convolutions. 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). Boston: IEEE; 2015. p. 1–9. https://doi.org/10.1109/CVPR.2015.7298594 .
Kieffer B, Babaie M, Kalra S, Tizhoosh HR. Convolutional neural networks for histopathology image classification: Training vs. Using pre-trained networks, 2017 Seventh International Conference on Image Processing Theory, Tools and Applications (IPTA), Montreal, QC, Canada, 2017, pp. 1–6, https://doi.org/10.1109/IPTA.2017.8310149
Krizhevsky A, Sutskever I, Hinton GE. Imagenet classification with deep convolutional neural networks[C]//Advances in neural information processing systems. 2012. p. 1097–105.
Jazar RN. Averaging Method. Perturbation methods in Science and Engineering. Cham: Springer; 2021. https://doi.org/10.1007/978-3-030-73462-6_8 .
Kangas A, Kurttila M, Hujala T, Eyvindson K, Kangas J. Voting methods. Decision support for Forest Management. Managing Forest ecosystems. Volume 30. Cham: Springer; 2015. https://doi.org/10.1007/978-3-319-23522-6_10 .
Mahdavi S, Rahnamayan S, Mahdavi A. Majority voting for discrete population-based optimization algorithms. Soft Comput. 2019;23:1–18. https://doi.org/10.1007/s00500-018-3530-1 .
Xiaomeng Wu G, Irie K, Hiramatsu, Kashino K, Weighted Generalized Mean Pooling for Deep Image, Retrieval., IEEE Signal Processing Society SigPort, 2018. Available: https://sigport.org/documents/weighted-generalized-mean-pooling-deep-image-retrieval . Accessed: Dec. 11, 2022.
Li Y, Chen P, Li Z, Su H, Yang L, Zhong D. Rule-based automatic diagnosis of thyroid nodules from intraoperative frozen sections using deep learning, Artificial Intelligence in Medicine, 108,2020,101918, ISSN 0933–3657, https://doi.org/10.1016/j.artmed.2020.101918
Aziz A, Naseradeen Abdulqader Z, Sallow D, A. B., Khalid Omer H. Python parallel Processing and Multiprocessing: a Rivew. Acad J Nawroz Univ. 2021;10(3):345–54. https://doi.org/10.25007/ajnu.v10n3a1145 .
Meta. From research to production. Online at https://pytorch.org/ .
The Linux Foundation. Open neural network exchange. Online at https://onnx.ai/ .
Zhou Y, Yang K. Exploring TensorRT to Improve Real-Time Inference for Deep Learning, 2022 IEEE 24th Int Conf on High Performance Computing & Communications; 8th Int Conf on Data Science & Systems; 20th Int Conf on Smart City; 8th Int Conf on Dependability in Sensor, Cloud & Big Data Systems & Application (HPCC/DSS/SmartCity/DependSys), Hainan, China, 2022, pp. 2011–2018, https://doi.org/10.1109/HPCC-DSS-SmartCity-DependSys57074.2022.00299
Ting KM. Confusion Matrix. In: Sammut C, Webb GI, editors. Encyclopedia of machine learning. Boston, MA: Springer; 2011. https://doi.org/10.1007/978-0-387-30164-8_157 .
Chapter Google Scholar
Selvaraju RR, Cogswell M, Das A et al. Grad-cam: Visual explanations from deep networks via gradient-based localization. 2017 IEEE International Conference on Computer Vision (ICCV). Venice: IEEE; 2017: 618–26. https://doi.org/10.1109/ICCV.2017.74 .
Gupta N, Sarkar C, Singh R, et al. Evaluation of diagnostic efficiency of computerized image analysis based quantitative nuclear parameters in papillary and follicular thyroid tumors using paraffin-embedded tissue sections. Pathol Oncol Res. 2001;7:46–55. https://doi.org/10.1007/BF03032605 .
Nafe R, Fritsch RS, Soudan B, Hammann A, Choritz H. Histomorphometryin paraffin sections of thyroid tumors, Pathology - Research and Practice, Volume 188, Issue 8, 1992, Pages 1042–1048, ISSN 0344 – 0338, https://doi.org/10.1016/S0344-0338(11)81249-5
Sui Peng Y, Liu W, Lv L, Liu Q, Zhou H, Yang et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: a multicentre diagnostic study, The Lancet Digital Health,Volume 3, Issue 4, 2021, Pages e250-e259, ISSN 2589–7500, https://doi.org/10.1016/S2589- 7500(21)00041 – 8.
Buddhavarapu VG, Jothi AA. An experimental study on classification of thyroid histopathology images using transfer learning. Pattern Recognit Lett. 2020;140. https://doi.org/10.1016/j.patrec.2020.09.020 . Pages 1–9, ISSN 0167–8655.
Chen P, Shi X, Liang Y, Li Y, Yang L, Gader PD. Interactive thyroid whole slide image diagnostic system using deep representation, computer methods and programs in Biomedicine, 195, 2020, 105630, ISSN 0169–2607, https://doi.org/10.1016/j.cmpb.2020.105630
National Cancer Institute. Physician Data Query (PDQ). Thyroid Cancer Treatment. 02/06/2019. Accessed at https://www.cancer.gov/types/thyroid/hp/thyroid-treatmentpdq#_313_toc . on February 20, 2019. 42.
Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2021;31(3):337–386. DOI: 10.1089/thy.2020.0944. Erratum in: Thyroid. 2021;31(10):1606–1607. PMID: 33728999; PMCID: PMC8349723.
Turan G, Sevgiye Kaçar Özkara. Pathological findings of the retrospective diagnosis of NIFTP (non-invasive follicular thyroid neoplasm with papillary-like nuclear features) in 84 cases from Turkey and systematic review. Annals Diagn Pathol. 2021;53:151764.
Tunca F, et al. Comparison of histopathological features and prognosis of classical and follicular variant papillary thyroid carcinoma. J Endocrinol Investig. 2015;38:1327–34.
Article CAS Google Scholar
Jug R, Jiang X. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: an evidence-based nomenclature change. Patholog Res Int. 2017;2017(1):1057252.
PubMed PubMed Central Google Scholar
Maletta F, et al. Cytological features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features and their correlation with tumor histology. Hum Pathol. 2016;54:134–42.
Download references
National Science Foundation of China (61875102 and 61975089), Natural Science Foundation of Guangdong province (2021A1515012379 and 2022A1515012550), Natural Science Foundation of Shenzhen city (JCY120200109110606054 and WDZC2020200821141349001).
Author information
Authors and affiliations.
Institute of Biopharmaceutical and Health Engineering, Tsinghua Shenzhen International Graduate School, Shenzhen, Guangdong, China
Tingting He, Shanshan Shi, Yiqing Liu, Lianghui Zhu & Yonghong He
Department of Pathology, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China
Yani Wei, Fenfen Zhang, Huijuan Shi & Anjia Han
You can also search for this author in PubMed Google Scholar
Contributions
A.J.H., T.T.H., Y.H.H., and H.J.S. conceived the study and designed the experiments. T.T.H., S.S.S completed all codes of data processing, model training and testing. T.T.H., Y.N.W., Y.Q.L., L.H.Z. and F.F.Z. performed the experiments analysis. T.T.H. prepared the manuscript. Y.H.H., A.J.H., revised the manuscript. All authors have read and agreed to publish the paper.
Corresponding authors
Correspondence to Huijuan Shi , Yonghong He or Anjia Han .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of First Affiliated Hospital of Sun Yat-sen University, approval number [2023] C-061. The requirement for informed consent was waived by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
He, T., Shi, S., Liu, Y. et al. Pathology diagnosis of intraoperative frozen thyroid lesions assisted by deep learning. BMC Cancer 24 , 1069 (2024). https://doi.org/10.1186/s12885-024-12849-8
Download citation
Received : 27 February 2024
Accepted : 26 August 2024
Published : 29 August 2024
DOI : https://doi.org/10.1186/s12885-024-12849-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Deep learning
- Thyroid cancer
- Frozen section
- Whole slide image
- Histopathological classification
- Acceleration
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 07 September 2020
Understanding the ever-changing incidence of thyroid cancer
- Cari M. Kitahara 1 &
- Julie A. Sosa 2
Nature Reviews Endocrinology volume 16 , pages 617–618 ( 2020 ) Cite this article
7972 Accesses
71 Citations
25 Altmetric
Metrics details
- Cancer epidemiology
- Thyroid cancer
- Thyroid diseases
This Comment article provides a behind-the-scenes perspective and update of our 2016 Review, which discussed possible factors contributing to thyroid cancer incidence trends worldwide. We also highlight promising research directions that are improving the understanding of thyroid cancer aetiology.
In 2016, we co-authored a Review for Nature Reviews Endocrinology , entitled ‘The changing incidence of thyroid cancer’ 1 . In this Review (cited 332 times according to Google Scholar, as of 18 August 2020), we discussed the rising incidence of thyroid cancer in the USA and much of the rest of the world and the probable reasons behind the changes. The timing of our Review coincided with major shifts in thinking about the epidemiology, aetiology and clinical management of thyroid cancer, and we covered many of the key points raised at the 2015 Endocrine Society conference symposium on this topic (C.M.K. was a speaker and J.A.S. was the moderator). Bringing together our different areas of expertise (epidemiology, clinical thyroidology and endocrine surgery), we sought to provide a balanced, comprehensive discussion on the topic from both a public health and clinical perspective. In addition to discussing the important role of overdiagnosis in the rising rates of thyroid cancer worldwide, we also highlighted factors, such as obesity, that might have contributed to a true increase in the occurrence of this disease, particularly in the USA.
By 2016, the contribution of overdiagnosis to thyroid cancer incidence trends was well recognized. In the USA, the incidence of thyroid cancer, particularly papillary thyroid cancer (PTC), increased rapidly for several decades, beginning in the early 1980s, with an increasing proportion of smaller and early-stage versus larger and more advanced PTCs 1 . These trends were largely attributed to the more widespread use of diagnostic imaging and fine-needle aspiration biopsies of thyroid nodules since the 1980s and 1990s. Despite this shift towards diagnosis of tumours at reduced risk of disease recurrence or disease-specific mortality, there was also evidence of an increasing proportion of patients being treated aggressively with thyroidectomy along with radioactive iodine (RAI) ablation or RAI therapy 2 , 3 . At the same time, the substantial adverse psychological, financial and health consequences of overdiagnosis and overtreatment of thyroid cancer were becoming increasingly apparent.
Recognizing the urgent need to reverse these trends, between 2009 and 2017, the American College of Radiology, the American Thyroid Association (ATA) and the US Preventive Services Task Force issued strong recommendations against biopsy of very small thyroid nodules and those lacking suspicious features and screening of thyroid cancer in the asymptomatic population 4 , 5 . For the first time, the 2015 ATA guidelines discussed use of molecular testing for nodules with indeterminate cytology to circumvent diagnostic surgery. For tumours lacking suspicious or aggressive features, alternative ‘less is more’ management strategies were called out, including active surveillance for papillary thyroid microcarcinomas, thyroid lobectomy as an alternative to total thyroidectomy for low-risk differentiated thyroid cancer and use of reduced administered activities (doses) of RAI 5 . In 2017, the ATA recommended a terminology change for noninvasive encapsulated follicular variant of PTC, from a malignant to an in situ neoplasm, owing to its excellent prognosis 6 . The extent to which changing clinical guidelines have or will influence trends in thyroid cancer incidence and mortality in the USA and elsewhere might not be fully apparent for several more years; however, the statistically significant decline in thyroid cancer incidence in the USA from 2015 to 2017 suggests that these efforts have already had a major effect 7 .
Our Review also summarized evidence suggesting that enhanced detection, or overdiagnosis, could explain only about half of the rise in PTC incidence in countries such as the USA and Australia, due to the trends observed for larger and more advanced PTCs, as well as thyroid cancer mortality 1 . As a follow-up to this discussion, in 2017, our research team published a comprehensive analysis of US cancer registry data, which confirmed that the increasing incidence of PTCs between the mid-1970s and mid-2010s was not exclusive to tumours that were small and localized at diagnosis and that the increase in incidence was accompanied by a more subtle but statistically significant increase in thyroid cancer mortality 8 . We also found that the increase in thyroid cancer mortality was exclusive to patients diagnosed with PTC and was most apparent for those diagnosed with advanced PTC. Thus, in addition to overdiagnosis, we concluded that there appeared to be a true, concurrent increase in the occurrence of thyroid cancer. These results lent further support to the hypothesis that changes in the prevalence of modifiable risk factors might have at least partially contributed to observed trends in thyroid cancer in the USA, and possibly other countries as well.
We also discussed potential environmental and lifestyle-related factors that might have contributed to observed thyroid cancer incidence and mortality trends 1 . Since the publication of our Review, the evidence supporting an association between obesity and increased risk of thyroid cancer, particularly PTC and anaplastic thyroid cancer, has continued to grow. For example, studies have shown that obesity might be more strongly associated with the risk of PTC characterized by suspicious or aggressive tumour features than PTC lacking these features 9 . Combining information from cohort, survey and cancer registry data, our research team estimated that about 16% of all PTCs (63% of large PTCs) diagnosed in 2013–2015 in the USA were attributable to overweight and obesity 10 . In the absence of overweight and obesity, PTC incidence trends would have been attenuated by about 14% (58% for large PTCs) (Fig. 1 ). These results suggest that public health measures aimed at minimizing excess weight might help to prevent the development of PTC, among numerous other health benefits.
The rates of papillary thyroid cancer are shown as overall rates and have been stratified as attributable to overweight or obesity or as being unrelated to overweight or obesity. Annual population attributable fractions are based on relative risk estimates from the NIH–AARP Diet and Health Study and prevalence estimates of overweight and obesity among US adults aged ≥50 years from the National Health Interview Survey (1985−2005). The arrows represent the hypothetical reduction in the incidence rates for papillary thyroid cancer in the absence of overweight and obesity in the 10 years before the date of diagnosis. Please note the 10-fold difference in scale of the y axes. Adapted with permission from ref. 10 , Oxford University Press.
Further progress towards a better understanding of thyroid cancer aetiology will require overcoming some of the inherent challenges of existing observational studies and original, outside-the-box thinking. Large, well-powered and carefully designed case–control and cohort studies are needed to precisely quantify associations between exposures and thyroid cancer risk; however, few such studies currently offer the ability to distinguish between true risk factors versus risk factors for overdiagnosis. One exception is the Queensland Thyroid Cancer Study 9 , in which clinical records, pathology data and tumour tissues were collected to obtain information about the method of initial thyroid cancer detection and diagnosis and characteristics of the tumours. This level of diagnostic information enables separate evaluation of exposure–disease relationships for higher-risk versus lower-risk thyroid cancers. Novel aetiological factors might yet be discovered through exploration of rich, unique data resources and by investigating exposures occurring during potentially susceptible periods in the life course. Laboratory-based studies and observational studies with pre-diagnostic biomarkers of exposure are also valuable in understanding biological pathways underlying observed associations. Finally, comprehensive descriptive epidemiological studies continue to play an understated but pivotal role, not only in guiding future clinical recommendations but in generating hypotheses regarding factors that might be contributing to changing incidence and mortality trends. Such studies could also be used to quantify the effect of the novel coronavirus (COVID-19) pandemic on thyroid cancer diagnostic and treatment practices in 2020 and beyond.
In conclusion, we thank the editors of Nature Reviews Endocrinology for the opportunity to express our views on this topic, as well as the four peer reviewers who provided valuable feedback on our original Review. We hope that our previous and current commentaries inspire future descriptive, aetiological and clinical research on thyroid cancer and illustrate the value of multidisciplinary collaborations among researchers sharing the common goals of thyroid cancer prevention and improved patient outcomes.
Kitahara, C. M. & Sosa, J. A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 12 , 646–653 (2016).
Article Google Scholar
Sosa, J. A. et al. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery 154 , 1420–1426 (2013).
Haymart, M. R. et al. Use of radioactive iodine for thyroid cancer. JAMA 306 , 721–728 (2011).
Article CAS Google Scholar
Haugen, B. R. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer 123 , 372–381 (2017).
US Preventive Services Task Force. Screening for thyroid cancer: US Preventive Services Task Force Recommendation Statement. JAMA 317 , 1882–1887 (2017).
Haugen, B. R. et al. American Thyroid Association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid 27 , 481–483 (2017).
Lee, M. et al. Letter to the Editor: reversal in thyroid cancer incidence trends in the United States, 2000–2017. Thyroid 30 , 1226–1227 (2020).
Lim, H. et al. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317 , 1338–1348 (2017).
Rahman, S. T. et al. Obesity is associated with BRAF V600E -mutated thyroid cancer. Thyroid https://doi.org/10.1089/thy.2019.0654 (2020).
Article PubMed Google Scholar
Kitahara, C. M. et al. Impact of overweight and obesity on US papillary thyroid cancer incidence trends (1995–2015). J. Natl Cancer Inst. 112 , 810–817 (2020).
Download references
Acknowledgements
The authors acknowledge the support of the Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
Author information
Authors and affiliations.
Radiation Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA
Cari M. Kitahara
Department of Surgery, University of California San Francisco, San Francisco, CA, USA
Julie A. Sosa
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Cari M. Kitahara .
Ethics declarations
Competing interests.
J.A.S. is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by GlaxoSmithKline, Novo Nordisk, AstraZeneca and Eli Lilly. J.A.S. receives institutional research funding from Exelixis and Eli Lilly. C.M.K. declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Kitahara, C.M., Sosa, J.A. Understanding the ever-changing incidence of thyroid cancer. Nat Rev Endocrinol 16 , 617–618 (2020). https://doi.org/10.1038/s41574-020-00414-9
Download citation
Published : 07 September 2020
Issue Date : November 2020
DOI : https://doi.org/10.1038/s41574-020-00414-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Predictive biomarkers in thyroid cancer in the current molecular-morphology paradigm.
- Humberto Carvalho Carneiro
- Rodrigo de Andrade Natal
- Ana Amélia Fialho de Oliveira Hoff
Surgical and Experimental Pathology (2024)
Organochlorine pesticides and risk of papillary thyroid cancer in U.S. military personnel: a nested case-control study
- Jennifer A. Rusiecki
- Jordan McAdam
- Yawei Zhang
Environmental Health (2024)
TERT RNAscope analysis of sub-centimetric papillary thyroid carcinomas and synchronous lymph node metastases
- Marie-Lisa Eich
- Wiebke Jeske
- Anne M. Schultheis
Thyroid Research (2024)
m6A reader IGF2BP2 promotes lymphatic metastasis by stabilizing DPP4 in papillary thyroid carcinoma
- Wenlong Wang
Cancer Gene Therapy (2024)
YTHDC2 Retards Cell Proliferation and Triggers Apoptosis in Papillary Thyroid Cancer by Regulating CYLD-Mediated Inactivation of Akt Signaling
- Guangying Zhou
- Shasha Wang
Applied Biochemistry and Biotechnology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Global burden of thyroid cancer from 1990 to 2021: a systematic analysis from the Global Burden of Disease Study 2021
Affiliations.
- 1 Department of Otorhinolaryngology Head and Neck Surgery, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China.
- 2 Shanghai Key Laboratory of Sleep Disordered Breathing, Shanghai, China.
- 3 Otolaryngology Institute of Shanghai Jiao Tong University, Shanghai, China.
- 4 Department of Otolaryngology, Shanghai Tenth People's Hospital, School of Medicine, Tongji University, Shanghai, China.
- 5 Department of Otorhinolaryngology Head and Neck Surgery, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China. [email protected].
- 6 Shanghai Key Laboratory of Sleep Disordered Breathing, Shanghai, China. [email protected].
- 7 Otolaryngology Institute of Shanghai Jiao Tong University, Shanghai, China. [email protected].
- 8 Department of Otorhinolaryngology Head and Neck Surgery, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China. [email protected].
- 9 Shanghai Key Laboratory of Sleep Disordered Breathing, Shanghai, China. [email protected].
- 10 Otolaryngology Institute of Shanghai Jiao Tong University, Shanghai, China. [email protected].
- 11 Department of Otorhinolaryngology Head and Neck Surgery, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China. [email protected].
- 12 Shanghai Key Laboratory of Sleep Disordered Breathing, Shanghai, China. [email protected].
- 13 Otolaryngology Institute of Shanghai Jiao Tong University, Shanghai, China. [email protected].
- PMID: 39192360
- DOI: 10.1186/s13045-024-01593-y
Thyroid cancer (TC) is a significant global healthcare burden. However, the lack of comprehensive data has impeded our understanding of its global impact. We aimed to examine the burden of TC and its trends at the global, regional, and national levels using data stratified by sociodemographic index (SDI), sex, and age. Data on TC, including incidence, mortality, and disability-adjusted life-years (DALYs) from 1990 to 2021, were obtained from the Global Burden of Disease Study 2021. Estimated annual percentage changes (EAPCs) were calculated to assess the incidence rate, mortality, and DALYs trends. The incidence, mortality, and DALYs of TC in 2021 were 249,538 (95% uncertainty interval: 223,290-274,638), 44,799 (39,925-48,541), and 646,741 (599,119-717,357), respectively. The age-standardized incidence rate (ASIR) in 2021 was 2.914 (2.607-3.213), with an EAPC of 1.25 (1.14-1.37) compared to 1990. In 2021, the age-standardized death rate (ASDR) was 0.53 (0.47-0.575) and age-standardized DALYs rate was 14.571 (12.783-16.115). Compared with 1990, the EAPCs of ASDR and age-standardized DALYs rate showed decreasing trends, at - 0.24 (- 0.27 to - 0.21) and - 0.14 (- 0.17 to - 0.11), respectively. Low SDI regions showed the highest ASDR and age-standardized DALYs rate, at 0.642 (0.516-0.799) and 17.976 (14.18-23.06), respectively. Low-middle SDI regions had the highest EAPCs for ASDR and age-standardized DALYs rate, at 0.74 (0.71-0.78) and 0.67 (0.63-0.7), respectively. Females exhibited decreasing trend in ASDR and age-standardized DALYs rate, with EAPCs of - 0.58 (- 0.61 to - 0.55) and - 0.45 (- 0.47 to - 0.42), respectively. In contrast, males showed an increasing trend in ASDR and age-standardized DALYs rate, with EAPCs of 0.41 (0.35-0.46) for both. In high-income regions, most countries with decreased annual changes in deaths experience increasing age-related deaths. Over the past few decades, a notable increase in TC incidence and decreased mortality has been observed globally. Regions characterized by lower SDI, male sex, and an aging population exhibited no improvement in TC mortality. Effective resource allocation, meticulous control of risk factors, and tailored interventions are crucial for addressing these issues.
Keywords: Death; Disability-adjusted life-years; Global burden of disease study; Incidence; Thyroid cancer; Trend.
© 2024. The Author(s).
PubMed Disclaimer
- Chen DW, Lang BHH, McLeod DSA, Newbold K, Haymart MR. Thyroid cancer. Lancet (London, England). 2023;401(10387):1531–44. - DOI - PubMed
- Gubbi S, Al-Jundi M, Foerster P, et al. The effect of thyrotropin suppression on survival outcomes in patients with differentiated thyroid cancer: a systematic review and meta-analysis. Thyroid: Off J Am Thyroid Assoc. 2024;34:674. - DOI
- GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet (London, England). 2024;403(10440):2100–32. - DOI
- Zhou T, Huang W, Wang X, et al. Global burden of head and neck cancers from 1990 to 2019. iScience. 2024;27(3):109282. - DOI - PubMed - PMC
- Chaudhri E, Fathi W, Hussain F, Hashmi SK. The increasing trends in cases of the most common cancers in Saudi Arabia. J Epidemiol Global Health. 2020;10(4):258–62. - DOI
Publication types
- Search in MeSH
Grants and funding
- hlyjkt202325/Hospital Level Scientific Research Fund Program of Shanghai Sixth People's Hospital
- ynts202404/Hospital Level Scientific Research Fund Program of Shanghai Sixth People's Hospital
- 2021ZD0201900/National STI2030-Major Projects of China
- 81970869/National Natural Science Foundation of China
LinkOut - more resources
Miscellaneous.
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

- Events & Education
- ATA Publications
- ATA Guidelines & Statements
- Research Grants
- Thyroid Cancer Patient Information
- Trainees Corner
- Corporate Leadership Council
- ATA Career Center
- Laboratory Services Library
- Scientific & Professional Interest
- Thyroid Cancer Staging Calculator
- (CEA) Doubling Time Calculator
- Change In Thyroid Nodule Volume Calculator
- Thyroid Patient Information
- Find an Endocrinology – Thyroid Specialist
- Patient Support Links
- Clinical Thyroidology for the Public
- Friends of the ATA Newsletter
- ATA Practice Guidelines
- Clinical Trials
- ATA Research Accomplishments
- Member Benefits
- Become an ATA Member
- Renew Your Membership
- Member Guidelines & Categories
- Society Committees
- Member Directory
- Trainee Membership
- Meet our Members
- Women in Thyroidology
- Thyroid Online Access
- Clinical Thyroidology Online
- Video Endocrinology
- Leadership & Staff
- Committees & Workgroups
- Diversity, Equity, Inclusion
- Awards & Recognition
- Our History
- Give Online
- Valerie Anne Galton Fund
- Samuel Refetoff Fund
- Ridgway Legacy Fund
- Memorial or Tribute Gift Donation
- Workplace Giving
- Estate and Planned Giving
- Donate by Mail/Fax/Phone
- Research Accomplishments
Clinical Thyroidology®: Levothyroxine Dosage and the Increased Risk of Second Primary Malignancy in Thyroid Cancer Survivors
- No Comments
Home » Clinical Thyroidology®: Levothyroxine Dosage and the Increased Risk of Second Primary Malignancy in Thyroid Cancer Survivors
Levothyroxine Dosage and the Increased Risk of Second Primary Malignancy in Thyroid Cancer Survivors Young Joo Park
Comments are closed.
Thyroid News Information
Thyroid posts by category, ata resources.

THYROID NEWS
Publications
About the ata.
© 2024 American Thyroid Association.
You need to enable JavaScript to run this app.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Surg Oncol
- v.13(1); 2022 Mar

Thyroid Cancer: Global Burden and Trends
Jessica b. shank.
Division of Surgical Oncology, Department of Surgery, University of Nebraska Medical Center, 986880 Nebraska Medical Center, Omaha, NE 68198 USA
Chandrakanth Are
Chelsea d. wenos, associated data.
Publicly accessible GLOBOCAN database.
Not applicable.
The incidence of thyroid cancer continues to increase, representing the 5th most common cancer type in the USA today (Sherman, Lancet 361(9356):501–11, 2003). The current study sought to analyze the global burden of thyroid cancer utilizing the publicly accessible GLOBOCAN database. An estimated 586,202 cases of thyroid cancer were reported in 2020, making thyroid cancer the 10th most common cancer worldwide. The majority of thyroid cancer cases occurred in countries with a high or very high Human Development Index (HDI), accounting for 91% of new cases. With respect to the World Health Organization (WHO) regions, the Western Pacific had the highest incidence of thyroid cancer accounting for 47.6% of cases despite representing only 25.4% of the world’s population. Thyroid cancer incidence and mortality are expected to increase by 29.9% and 67%, respectively, by the year 2040. The African region is projected to experience the highest increase in both incidence (84.3%) and mortality (100.3%) over this time period. The results of our study demonstrate that the incidence and mortality of thyroid cancer vary by the geographic location and socio-economic status. Although the incidence was noted to be the highest in very high HDI countries and the Western Pacific region, mortality was noted to be disproportionately higher in the low HDI countries and African region. This may be due to discrepancies in access to care and/or environmental exposures such as ionizing radiation and iodine deficiency. Further measures are required to improve the outcomes from thyroid cancer regardless of the geographic location or socio-economic status.
Introduction
The incidence of thyroid cancer around the world continues to increase [ 1 ]. In the USA, thyroid cancer remains the 5th most common cancer type [ 1 ]. Differentiated thyroid cancer, including papillary and follicular thyroid cancer, is the most common subtype constituting greater than 90% of cases. The prognosis remains excellent and life expectancy of patients treated for differentiated thyroid cancer does not differ significantly from the general population [ 2 ].
Based on the classification outlined by the World Health Organization (WHO) [ 2 ], papillary cancer consists of more than ten subtypes with variable risk stratifications. Some histopathologic variants such as tall cell, columnar cell, and hobnail are associated with more unfavorable outcomes [ 3 ].
Some of the risk factors for the development of thyroid cancer include exposure to ionizing radiation via radiotherapy or nuclear fallout. Familial thyroid carcinoma and other familial syndromes increase the likelihood of thyroid cancer development. PTEN, Cowden’s disease, and multiple endocrine neoplasia type 2 (MEN2) are all syndromes associated with thyroid cancer.
Pre-operative evaluation of thyroid cancer includes a thorough neck ultrasound with imaging of the thyroid gland as well as the central and lateral neck compartments. Any concerning lymph nodes warrant a biopsy to confirm the diagnosis of locoregional metastatic disease. Surgery is the mainstay of therapy, but recommendations differ based on the size and extent of thyroid cancer. According to the latest American Thyroid Association (ATA) guidelines, patients with primary tumor size greater than 4 cm, lymph node involvement, distant metastases or extrathyroidal extension (ETE) are recommended to undergo a total thyroidectomy [ 3 ]. A compartmental lymph node dissection is indicated for positive lymph node involvement. For cancers between the size of 1 and 4 cm lacking evidence of ETE or lymph node involvement, the appropriate initial operation may be thyroid lobectomy or total thyroidectomy [ 3 ]. Thyroid cancer under 1 cm in size may be adequately treated with a thyroid lobectomy, unless clear indication for removal of contralateral lobe exists [ 3 ]. Additional treatment with radioactive iodine (RAI) depends on the risk of recurrence, but is specifically indicated for residual remnant ablation and metastatic thyroid cancer treatment.
Although the incidence of thyroid cancer is increasing, the level of increase may vary depending on the geographic location and prevalence of imaging modality utilization. Previous research has revealed that 50% of thyroid cancer global burden resides in Southern and Eastern Asia [ 4 ]. Countries with a high sociodemographic index harbor one-third of patients with thyroid cancer [ 4 ]. Further geographic evaluation may provide insight into region-based differences in thyroid cancer incidence and mortality that may improve health care resource allocation with regard to treatments and policies.
This study sought to analyze the global burden of thyroid cancer with a focus on variations in the burden of disease based on geographic location and socio-economic development.
GLOBOCAN provides access to comprehensive data on global and regional cancer burden, which is further stratified by various metrics such as geographic location and socio-economic development [ 5 ]. The accuracy of data is based on the availability of data in each country or region, which sometimes may be limited in certain low- and middle-income countries (LMICs) [ 5 ]. The sources utilized to gather data vary by individual country, and may include observational rates or country- or region-specific cancer registries. In some countries where none of the above is available, estimates are arrived based on comparable projections from other countries within geographic proximity of the index country [ 5 ]. Some of the population-based cancer registries accessed by GLOBOCAN include the International Association of Cancer Registries (IACR) and the Global Initiative for Cancer Registry Development (GICR) [ 5 ].
The publicly available GLOBOCAN database 2.0 was accessed to obtain data regarding the global burden of thyroid cancer. Intrinsic variables within the GLOBOCAN database were extracted to provide datapoints that were used to generate the results. The GLOBOCAN project combines data from 185 countries or territories around the world. It includes data on specific cancer types such as estimates of incidence, mortality, and prevalence by age and sex [ 5 ]. Details on the data source and methods have been previously described [ 5 , 6 ]. The methodology of stratification based on socio-economic standing or the Human Development Index (HDI) and World Health Organization (WHO) regions has been described previously [ 7 ].
Age-standardized rate (ASR) is utilized by GLOBOCAN and this corrects for variations in burden of cancer based on age for different geographic populations. The purpose of ASR is to correct for differences in ages among countries, since age significantly impacts the risk of development of cancer and its outcomes. ASR is calculated as an average of age-specific rates per 100,000 persons over a given period of time [ 5 ].
Human Development Index (HDI) accounts for socio-economic status of various countries by summarizing three areas of human development including life expectancy at birth and literacy rates among adults as well as tertiary education enrollment rates and gross domestic product per head adjusted for purchasing-power parity [ 8 ]. Based on the HDI, individual countries are divided into four categories, namely low HDI-LHDI (HDI < 0.550), medium HDI-MHDI (HDI ≥ 0.550), high HDI-HHDI (HDI ≥ 0.700), and very high HDI-VHHDI (HDI ≥ 0.800) [ 8 ]. The countries included within each category are again available on the GLOBOCAN website [ 5 , 8 ].
A list of cancer registries that contributed to the GLOBOCAN data can be found on their website [ 5 ]. The methods used to estimate the age- and sex-specific incidence and mortality rates by cancer are based on the specific country and are summarized on the GLOBOCAN website [ 5 ]. The prevalence estimates were calculated using a formula provided on the GLOBOCAN website for review [ 5 , 9 ].
An estimated 586,000 cases of thyroid cancer were reported worldwide in 2020 (Table (Table1), 1 ), which makes thyroid cancer the 10th most common cancer (Fig. 1 ). Female incidence was estimated at 449,000 compared to male incidence of 137,000, making thyroid cancer three times more common among females (Table (Table1). 1 ). However, mortality rates remain low, but differ by gender. Mortality among males totaled 15,900 compared to 27,700 deaths in females (Table (Table1). 1 ). Compared another way, cumulative risk for females is estimated at 0.05 compared to males at 0.04, making male mortality from thyroid cancer disproportionate to the incidence (Table (Table1 1 ).
Projected incidence of thyroid cancer from 2020 to 2040 [ 13 , 14 ]
| Thyroid | 2020 | 2040 | % increase |
|---|---|---|---|
| World incidence | 586,000 | 761,000 | 29.9 |
| Males | 137,000 | 185,000 | 34.8 |
| Females | 449,000 | 576,000 | 28.4 |
| World mortality | 43,600 | 72,900 | 67 |
| Males | 15,900 | 26,700 | 67.9 |
| Females | 27,700 | 46,200 | 66.5 |
| World incidence | 18,100,000 | 28,000,000 | 56.7 |
| Males | 9,340,000 | 15,000,000 | 62.5 |
| Females | 8,750,000 | 13,000,000 | 50.3 |
| World mortality | 9,890,000 | 16,200,000 | 63.7 |
| Males | 5,490,000 | 9,150,000 | 66.8 |
| Females | 4,400,000 | 7,030,000 | 59.8 |

Incidence of the ten most prevalent cancers worldwide in 2020 [ 13 , 14 ]
Thyroid cancer was responsible for approximately 43,600 (previously recorded as 41,071 in 2018) deaths in 2020 (Table (Table1). 1 ). The majority of thyroid cancer cases occurred in countries with high or very high HDI with incidence rates of 321,625 (compared to 276,499 in 2018) and 212,643 (220,219 in 2018), respectively (Tables (Tables2 2 and and3). 3 ). The vast majority of worldwide case numbers are represented by these countries with high HDI status at 91% (previously 88% in 2018). However, the mortality from thyroid cancer in countries with high and very high HDI accounts for only 73% (previously 66% in 2018) of the deaths (Tables (Tables2 2 and and3 3 ).
Incidence and mortality of thyroid cancer in 2020 by the WHO region, continent, and HDI status [ 13 , 14 ]
| Thyroid cancer incidence | Thyroid cancer mortality | 5-year prevalence number | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Rank | % | Cum Risk | Number | Rank | % | Cum Risk | Number | |
| African region (AFRO) | 11,756 | 16 | 1.4 | 0.18 | 3233 | 25 | 0.59 | 0.07 | 28,747 |
| Americas region (PAHO) | 125,624 | 5 | 3.1 | 1.03 | 6826 | 24 | 0.48 | 0.05 | 453,429 |
| East Mediterranean region (EMRO) | 21,882 | 12 | 3 | 0.34 | 3403 | 21 | 0.74 | 0.07 | 58,014 |
| Europe region (EURO) | 104,193 | 14 | 2.2 | 0.83 | 7637 | 26 | 0.35 | 0.04 | 359,139 |
| South-East Asia region (SEARO) | 43,651 | 16 | 1.9 | 0.21 | 8619 | 25 | 0.6 | 0.05 | 122,887 |
| Western Pacific region (WPRO) | 279,035 | 9 | 4.2 | 1.04 | 13,923 | 23 | 0.35 | 0.04 | 929,077 |
| World | 586,202 | 10 | 3 | 0.68 | 43,646 | 25 | 0.44 | 0.05 | 1,984,927 |
| Africa | 18,457 | 15 | 1.7 | 0.22 | 4443 | 22 | 0.62 | 0.07 | 47,595 |
| Latin America/Caribbean | 63,368 | 6 | 4.3 | 0.86 | 4406 | 24 | 0.62 | 0.06 | 209,541 |
| Northern America | 62,256 | 12 | 2.4 | 1.26 | 2420 | 25 | 0.35 | 0.03 | 243,888 |
| Asia | 349,897 | 10 | 3.7 | 0.65 | 25,668 | 24 | 0.44 | 0.05 | 1,139,172 |
| Europe | 87,162 | 15 | 2 | 0.83 | 6399 | 27 | 0.33 | 0.04 | 325,708 |
| Oceania | 5062 | 10 | 2 | 1 | 310 | 24 | 0.45 | 0.05 | 19,023 |
| Low HDI | 10,508 | 16 | 1.6 | 0.19 | 3220 | 22 | 0.73 | 0.08 | 21,835 |
| Medium HDI | 41,225 | 17 | 1.8 | 0.18 | 8368 | 25 | 0.55 | 0.05 | 111,316 |
| High HDI | 321,625 | 9 | 4.4 | 0.88 | 18,934 | 23 | 0.42 | 0.05 | 1,056,960 |
| Very high HDI | 212,643 | 14 | 2.4 | 1 | 13,117 | 25 | 0.38 | 0.04 | 794,816 |
Projected incidence and mortality of thyroid cancer by continent from 2020 to 2040 [ 13 , 14 ]
| 2020 | 2040 | |||
|---|---|---|---|---|
| Continent | Incidence | Mortality | Incidence (% increase) | Mortality (% increase) |
| Asia | 349,897 | 25,668 | 442,929 (26.6) | 45,639 (77.8) |
| Europe | 87,162 | 6399 | 85,490 (− 1.9) | 8264 (29.1) |
| Latin America and Caribbean | 63,368 | 4406 | 83,361 (31.6) | 7987 (81.3) |
| Northern America | 62,256 | 2420 | 71,055 (14.1) | 3625 (49.8) |
| Africa | 18,457 | 4443 | 34,006 (84.2) | 8901 (100.3) |
| Oceania | 5062 | 310 | 6635 (31.1) | 486 (56.8) |
ASR incidence in 2020 was the highest in the Republic of Korea at 26.6. For comparison, the USA ranks 14th in terms of estimated ASR incidence rate in 2020, which equates to a value of 11.8 (Table (Table4). 4 ). When comparing mortality, however, the US rates are equivalent to the Republic of Korea at 0.3 (Table (Table4). 4 ). Both countries are considered very high HDI countries. The highest ranking mortality with an ASR of 2.7 is the country of Samoa, a Polynesia island group, which has a corresponding ASR incidence of 8.9 (Table (Table4). 4 ). Samoa is considered a high HDI country. Disparities may exist between countries in terms of stage of disease at diagnosis and access to intervention for cure.
Incidence and mortality of thyroid cancer in 2020 by continent and select countries [ 13 , 14 ]
| Thyroid cancer incidence | Thyroid cancer mortality | |||
|---|---|---|---|---|
| Continent | Number | ASR | Number | ASR |
| Asia | 349,897 | 6.4 | 25,668 | 0.44 |
| Republic of Korea | 17,788 | 26.6 | 386 | 0.30 |
| Europe | 87,162 | 8.3 | 6399 | 0.33 |
| Latin America and the Caribbean | 63,368 | 8.6 | 4406 | 0.53 |
| North America | 62,256 | 12.4 | 2420 | 0.30 |
| USA | 52,912 | 11.8 | 2161 | 0.30 |
| Africa | 18,457 | 2.0 | 4443 | 0.62 |
| Ethiopia | 3203 | 4.3 | 927 | 1.5 |
| Egypt | 2661 | 2.9 | 472 | 0.61 |
| Oceania | 5062 | 9.7 | 310 | 0.45 |
| Samoa | 15 | 8.9 | 4 | 2.7 |
Breakdown of thyroid cancer by the WHO region revealed the Western Pacific region to have the highest rates of thyroid cancer diagnoses in 2020 at 279,035 making it the 9th most common cancer type in that area (Table (Table2). 2 ). Compared to population percentage, the Western Pacific region accounts for 25.4% of the world’s population as of 2018, but accounts for 47.6% of thyroid cancer cases and 31.9% of deaths attributed to the disease in 2020 (Table (Table2). 2 ). The African region had the highest percentage of cases resulting in mortality (number of deaths due to thyroid cancer divided by prevalence of thyroid cancer cases multiplied by 100) at 11.25%. This was followed by the South-East Asia region at 7.01% with single digit percentages for the remaining regions (5.87%, 2.13%, 1.51%, and 1.49% for the East Mediterranean region, Europe region, Americas region, and Western Pacific region, respectively).
Although not entirely explicable by income status, the Africa region has the lowest gross domestic product (GDP) value per capita of the WHO regions. Economic status also appears to play a role in the outcomes of patients with thyroid cancer based on HDI status. Although the greatest number of cases by incidence rates is noted in the high to very high HDI groups, when comparing prevalence to mortality, the low HDI group has the highest ratio (mortality divided by prevalence × 100). The highest proportion of thyroid cancer–related mortality compared to prevalence was in the low HDI group at 14.75 followed by the medium HDI group at 7.52 (Table (Table2 2 ).
Overall, the estimated number of incident cases of all types of cancer from 2020 to 2040 is expected to increase by 56.7% (Table (Table1). 1 ). Thyroid cancer, in particular, is projected to increase 29.9% over the same time period (Table (Table1; 1 ; Fig. 2 a). Mortality from thyroid cancer, however, is estimated to increase by 67% for both sexes (Fig. 2 b). Again, thyroid cancer incidence is projected to increase by the greatest percentage in the WHO Africa region at 84.2 (Table (Table3). 3 ). This also corresponds to projected increase in mortality due to thyroid cancer in the same region at 100.3%, which is the most significant predicted increase among all WHO regions (Table (Table3). 3 ). Thyroid cancer’s increase in incidence, which is partially attributed to increased utilization of imaging modalities, may begin to eventually plateau. However, the steady increase in mortality may be due to extraneous factors which are thought to contribute to the development of thyroid cancer such as environmental exposures and obesity. Additional studies may reveal causal relationships that would potentially influence development of the disease and outcomes.

a Projected incidence of thyroid cancer by sex from 2020 to 2040 [ 1 , 2 ]. b Projected mortality of thyroid cancer by sex from 2020 to 2040 [ 1 , 2 ]
The aim of this study was to evaluate the trends and analyze the burden of thyroid cancer around the globe. The burden of disease is projected to increase, as is the associated malignancy-related death rate. The incidence of disease does not uniformly impact all areas of the world and varies by socio-economic development. This may point to decreased access to imaging modalities, such as ultrasound, and access to care in general. As a counter point, thyroid cancer may be over-diagnosed in high-income countries, which may not contribute to a large burden on mortality.
It has been suggested that geographic areas across the globe with previously high mortality rates secondary to thyroid carcinoma included regions with iodine deficiency [ 10 ]. Iodine deficiency is associated with benign thyroid disease, which is a known risk factor for the development of thyroid carcinoma, such as iodine-deficient goiter [ 11 ].
Another known risk factor for the development of thyroid cancer is ionizing radiation. Improved control of nuclear waste may be contributing to decline in thyroid cancer mortality [ 12 ].
Limitations
Limitations from this study include those from data source collection and also from thyroid cancer knowledge gaps, in general. Thyroid cancer development likely has multiple contributing factors ranging from environmental exposures to diet and lifestyle. Many of these remain undefined and future studies are required to elicit true contributions.
GLOBOCAN data does not distinguish thyroid cancer subtypes, but rather groups thyroid cancer as a whole. Thyroid cancer subtypes are not clarified and therefore, understanding mortality risk is limited. In particular, distribution of high-risk subtypes of thyroid cancer is unknown, which may alter mortality rates.
Additional limitations are inherently based on the GLOBOCAN database, which have previously been described [ 7 ]. Data collected based on cancer registries or estimates from neighboring country’s cancer registries may limit application of these results. Compared with developed countries, less developed countries may have incomplete population and mortality estimates, affecting the accuracy of rates. Additionally, cancer registries in less developed countries are likely representative of metropolis regions, thereby not a true representation of the country as a whole.
This study is not intended to be utilized to determine causality or factors influencing mortality of thyroid cancer. Results may reveal geographic disparities that allude to potential environmental exposures that encourage development of thyroid cancer and may be helpful for future studies. In summary, thyroid cancer disease burden has and is increasing around the globe, but certain geographic locations and socio-economic factors may influence the disparities demonstrated in this study.
Conclusions
The majority of thyroid cancers have an overall excellent prognosis. Revealing underlying discrepancies between geographic areas in terms of mortality may be beneficial in order to level the mortality rates by improving cancer care. Given the highlighted anticipated continued increase in thyroid cancer incidence regardless of geography, an importance must be placed on outcomes. In particular, low HDI groups and the WHO Africa region have unexpectedly high mortality to prevalence ratios, indicating an area in need of improvement. This may be due to inherently prevalent environmental exposures and development of specific thyroid cancer subtypes; however, it may also be due to problems with access to care and early cancer detection. Additional studies evaluating timing and stage of disease at diagnosis may elicit the cause of the prevalence to mortality discrepancy based on geographic region. Environmental exposure studies would aid in proper risk stratifying in particular areas at risk. This important work has lent toward exposing underlying differences based on population groups that hopefully initiates the development of future studies, which will narrow the profound treatment gap in thyroid cancer treatment and thereby improve outcomes globally.
Author Contribution
Data availability, code availability, declarations.
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Advertisement

- Previous Article
- Next Article
Tumor Classification
Descriptive epidemiology, modifiable (or potentially modifiable) risk factors, nonmodifiable risk factors, primary prevention, future directions, authors' disclosures, acknowledgments, epidemiology of thyroid cancer.
Cancer Epidemiol Biomarkers Prev 2022;31:1284–97
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
- Version of Record July 1 2022
Cari M. Kitahara , Arthur B. Schneider; Epidemiology of Thyroid Cancer. Cancer Epidemiol Biomarkers Prev 1 July 2022; 31 (7): 1284–1297. https://doi.org/10.1158/1055-9965.EPI-21-1440
Download citation file:
- Ris (Zotero)
- Reference Manager
The incidence of thyroid cancer has risen dramatically in the United States over the past four decades, with similar patterns observed internationally. Thyroid cancer currently ranks as the 13th most common cancer diagnosis overall and the 6th most common among women. Because the greatest increases in incidence have been observed for small and localized tumors having the highest rate of survival, the incidence trends have been primarily attributed to overdiagnosis, resulting from more widespread use of diagnostic imaging and more sensitive diagnostic tools. Increasing incidence of large and advanced thyroid cancers, as well as thyroid cancer mortality, suggests that etiologic factors may have contributed to rising incidence of the disease, albeit to a lesser extent than overdiagnosis. Until recently, childhood exposure to ionizing radiation was considered the only established modifiable risk factor for thyroid cancer. Obesity has emerged as another important risk factor, although the underlying biological mechanisms remain poorly understood. The potential influence of endocrine-disrupting chemicals and thyroid dysfunction on thyroid cancer development has been a focus of recent etiologic studies. Important recent advances in identifying molecular subtypes of thyroid cancer and genetic susceptibility factors provide insights regarding the etiology of this disease.
Of the major histologic types, about 90% are papillary thyroid carcinomas (PTC), 4% follicular thyroid carcinomas (FTC), 2% Hürthle cell carcinomas, 2% medullary thyroid carcinomas (MTC), and 1% anaplastic thyroid carcinomas (ATC; ref. 1 ). MTCs are neuroendocrine tumors arising from calcitonin-producing C cells (parafollicular cells). Other rare thyroid cancers, with some exceptions (e.g., squamous cell, lymphoma, mesenchymal tumors), arise from follicular cells.
Thyroid cancers are also classified according to genetic drivers. The 2014 Cancer Genome Atlas Research Network landmark publication reported work that led to the identification of driver mutations in over 95% of nearly 500 PTCs ( 2 ). Subsequent work using a similar approach has identified Hürthle cell thyroid cancers as a separate entity from PTCs and FTCs ( 3 ). PTCs can now be distinguished according to those with somatic mutations related to the BRAF mutations in the MAPK pathway (BRAF-like) and those related to RAS mutations (RAS-like). BRAF V600E mutation has been associated with disease recurrence and mortality in patients with PTC, particularly when co-occurring with TERT promotor mutations ( 4, 5 ). BRAF and RAS mutations appear to play an important role in the development of poorly differentiated and anaplastic thyroid cancers, with BRAF V600E being an early molecular event and TP53 mutations contributing to the progression of PTCs to poorly differentiated and anaplastic types ( 5 ). TP53 mutations are present in about 26% of poorly differentiated cancers and 80% of ATCs, but rarely occur in PTCs ( 5 ). In general, the TERT promotor mutation and TP53 mutation, especially if co-occurring or co-occurring with other mutations, are strongly linked to higher degrees of dedifferentiation, disease aggressiveness, and poor outcomes ( 5 ). RET/PTC rearrangements are another distinct and common molecular event in the development of PTC; these occur more frequently in individuals exposed to radiation in childhood where they act through the MAPK pathway (ref. 6 ; see the “Ionizing radiation” section). RET mutations are the most common somatic mutations in MTCs, following by H-RAS and K-RAS ( 5 ).
Incidence and mortality
Thyroid cancer incidence varies substantially by geographic location ( Fig. 1A ), especially in women ( 7 ). In general, the highest incidence is observed in higher-income countries, including the Republic of Korea, Canada, Italy, France, Israel, Croatia, Austria, and the United States, as well as some middle- to upper–middle-income countries, such as Turkey, Brazil, Costa Rica, and China ( 7, 8 ). Incidence is also high in some island nations and territories, including Cyprus, Cabo Verde, French Polynesia, New Caledonia, and Puerto Rico ( 7 ). This variation is thought to be mainly attributable to geographic differences in access to care and diagnostic practices, although environmental exposures may also play a role ( 9 ). Compared with incidence, thyroid cancer mortality rates tend to be much lower and vary much less geographically ( Fig. 1B ).

A and B, Estimated age-standardized rates (ASR) for thyroid cancer incidence ( A ) and thyroid cancer mortality ( B ) worldwide. Data source and graph production: GLOBOCAN 2020, International Agency for Research on Cancer, World Health Organization ( 7 ).
In the United States, thyroid cancer is estimated to be the 13th most commonly diagnosed cancer, accounting for nearly 44,000 new cancer diagnoses in 2022 (2.3% of the total), and the 6th most commonly diagnosed cancer among women ( 10 ). Thyroid cancer incidence is approximately 3-fold higher in women (22.8 per 100,000 per year in 2014–2018) than in men (8.0 per 100,000 per year; Fig. 2A ; ref. 1 ). Thyroid cancer incidence increases from adolescence through middle age, peaking around 55 years in women and 65 years in men, and subsequently declining with older age ( Fig. 2B ; ref. 11 ). Currently, one in 55 U.S. women and one in 149 U.S. men are expected to be diagnosed with thyroid cancer during their lifetime ( 10 ). Thyroid cancer mortality is very low relative to incidence (approximately 0.5 deaths per 100,000 per year) with less evidence of a sex disparity ( 7 ).

A and B, Thyroid cancer incidence in U.S. men and women, by calendar year at diagnosis (SEER-9, 1975–2018) ( A ) and age at diagnosis (SEER-21, 2014–2018) ( B ), (all races). Data source and graph production: SEER, Bethesda, MD ( 11 ). 1 Estimates based on <16 cases are suppressed.
The prognosis for thyroid cancer is typically excellent, as most cases are PTCs and are localized to the thyroid gland at diagnosis ( 1 ). In the United States, the 5-year relative survival rate is 98.6% overall, 99.9% for localized, 98.3% for regional, and 54.9% for distant metastatic disease ( 1 ). Relative survival rates are highest for PTC and FTC compared with other histologic types owing to the slow-growing nature of these tumors and effective therapies. Most are effectively managed with total or partial removal of the thyroid or, in some cases, even by prospective observation. In cases that are more advanced, surgery is usually followed by radioactive iodine for the destruction of any remaining thyroid cells or tissue. ATCs, poorly differentiated thyroid cancers, and some other uncommon variants are highly aggressive and less amenable to treatment, although individualized medications, kinase inhibitors and others, have been developed ( 12, 13 ). During 1974–2013, ATC accounted for only 1% of thyroid cancer diagnoses but 30% of thyroid cancer deaths ( 14 ).
Trends in incidence and the role of overdiagnosis
From the early-1980s to mid-2010s, the incidence of thyroid cancer in the United States nearly tripled, rising at a faster rate than any other cancer type, before stabilizing and then declining during the mid-to-late-2010s ( Fig. 2A ; refs. 11, 15, 16 ). The upward trend was driven primarily by PTC, with the greatest rate of increase observed for small, early-stage PTCs ( 14 ). Although very modest relative increases in thyroid cancer mortality have been observed during this time (rising, on average, ∼1% per year), in absolute terms, mortality has remained very low and stable over time relative to incidence ( 14 ). The rising incidence coincided with the introduction and increasingly widespread use since the 1980s to 1990s of medical imaging techniques, including thyroid ultrasonography, and sensitive diagnostic tools ( 17, 18 ), resulting in the incidental detection and diagnosis of cancers that would previously not have been detected. For these reasons, the rising incidence of thyroid cancer has been referred to as an “epidemic of overdiagnosis” ( 19, 20 ). Overdiagnosis is defined as the diagnosis of a condition that would not have caused harm to the individual over their lifetime if left undetected. Similar trends in incidence have been observed in nearly every region of the world, including some lower-resource countries, without clear corresponding increases in mortality ( 17 ). As an extreme example, thyroid cancer incidence increased 15-fold between 1993 and 2011 in the Republic of Korea following the launch of a national campaign to promote cancer screening, whereas thyroid cancer mortality remained stable ( 21 ). Although thyroid cancer incidence subsequently declined after 2014 following major efforts to reverse this trend ( 22 ), the country continues to have the highest incidence of thyroid cancer in the world ( 7 ).
Clinical practice guidelines around the management of thyroid cancer have been modified over the last 15 years, with the goal of reducing the potential for overdiagnosis and resulting overtreatment. For instance, biopsying small nodules is no longer recommended by the American Thyroid Association (ATA; refs. 23, 24 ). In 2017, the ATA recommended reclassification of noninvasive encapsulated follicular variant subtype of PTCs (NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features), known for their indolent behavior, from a malignant to an in situ carcinoma ( 16, 25 ). These efforts appear to have contributed to the recent reduction in total PTC incidence, driven by declining incidence of small PTCs and the PTC follicular variant ( 16 ). On the other hand, the incidence of larger and advanced-stage PTCs continues to increase rapidly ( 16 ). Therefore, it is likely that diagnostic practices largely, but not fully, account for the changing trends in thyroid cancer incidence. This raises the question, discussed below, of whether and to what extent lifestyle and environmental risk factors may have played a role ( 14, 26–28 ).
Disparities
Thyroid cancer is susceptible to socioeconomic disparities in incidence because it is often detected during routine physical examinations or incidentally during work-up of other conditions. Greater access to and utilization of healthcare increases the likelihood of overdiagnosis and, thus, overdiagnosis and overtreatment. For example, in the United States, PTC patients with private health insurance are more likely to be diagnosed with early-stage disease compared with uninsured patients or those with Medicare or Medicaid ( 29 ). They are also more likely to undergo extensive treatment (total thyroidectomy, lymphadenectomy, and/or radioactive iodine). In contrast, those without private insurance may be susceptible to delayed diagnosis and treatment, potentially leading to worse outcomes.
Sex disparities
Questions have been raised about whether the higher female–male ratio in thyroid cancer incidence is real or artifactual ( 30 ). Autopsy studies have suggested a high prevalence (>10%) of subclinical PTC in the population, with no apparent changes in prevalence over time and no evidence of sex differences ( 30, 31 ). This contrasts with registry data showing a 4-fold higher incidence of small (≤2 cm), localized PTC in women versus men and two-fold higher incidence of all other types of PTC ( 30 ). As mentioned above, no sex disparities are observed for thyroid cancer mortality. Women may have more opportunities for incidental detection of thyroid nodules in clinical settings, via palpation or imaging, and overdiagnosis may be more likely during the reproductive years and around menopause; this may account for the more exaggerated age-at-diagnosis curves in women than men over time ( Fig. 2B ; ref. 19 ). On the other hand, the higher incidence in women than in men has been observed for decades and consistently across nearly every region of the world, including regions less affected by overdiagnosis ( 7 ). In the United States, a 2-fold higher incidence of thyroid cancer in women than in men was observed in the 1970s, prior to the introduction of thyroid ultrasonography and fine-needle aspiration biopsy ( 11 ). Finally, thyroid screening studies in Chernobyl and Fukushima area residents showed a slightly higher prevalence of thyroid nodules and cancer in females than in males, with sex ratios of 1.4 to 1.6 ( 32–34 ). Thus, overdiagnosis appears to have inflated the observed sex disparity in thyroid cancer incidence beyond biological reasons for these differences.
Racial/ethnic disparities
In the United States, thyroid cancer incidence is highest in non-Hispanic whites and lowest in Blacks and Native American/Alaskan Natives ( Fig. 3A ; ref. 11 ). Non-Hispanic whites also have a higher incidence of small, localized PTCs, suggesting more overdiagnosis, whereas no racial/ethnic differences are observed for large or advanced PTCs ( 35 ). Five-year relative survival is similar by race/ethnicity, although survival for metastatic thyroid cancer is slightly lower in Black patients versus other race/ethnic groups ( Fig. 3B ). A study using California Cancer Registry data showed that Black, Hispanic, and Asian/Pacific Islander patients received care at hospitals with lower-quality evaluations for thyroid cancer treatment than white patients after controlling for socioeconomic and insurance status ( 36 ). Treatment of thyroid cancer in lower-quality hospitals was associated with worse overall and disease-specific survival ( 37 ).
Substantial heterogeneity in incidence exists across Asian/Pacific Islander ethnic subgroups. Filipino-Americans have higher rates of PTC than non-Hispanic whites and other Asian/Pacific Islander groups and are more likely to be diagnosed with advanced disease ( 38 ), differences that persist after adjustment for sociodemographic factors ( 39 ). As higher incidence has been observed in first- versus subsequent-generation Filipinos and not other Asian/Pacific Islander groups ( 40 ), environmental exposures are considered to play a role (ref. 38 ; see “Other environmental exposures” below).

A and B, Race/ethnicity differences in thyroid cancer incidence trends (SEER-21, 2000–2018, delay-adjusted) ( A ) and five-year relative survival by stage at diagnosis (SEER-18, 2011–2017) ( B ). Data source and graph production: SEER, Bethesda, MD ( 11 ). 1 Estimates based on <16 cases are suppressed.
Identifying true etiologic (causal) risk factors has been a major challenge in thyroid cancer epidemiologic research. A high proportion of these tumors are indolent and detected incidentally, through thyroid screening, imaging for unrelated reasons, or diagnostic work-up of benign thyroid conditions. Thus, the term “risk factor” in the context of thyroid cancer may mean any characteristic or exposure that increases the likelihood of having a thyroid cancer diagnosis. Where possible, we use the term “causal risk factor” or “etiologic factor” to denote any factor that appears to fulfill most or all of the main criteria for causation: temporality, strength of association, consistency, biological gradient, specificity, biological plausibility, and coherence ( 41 ). Modifiable risk factors are those that can, in theory, be changed to increase or lower an individual's risk of the outcome (thyroid cancer). Evidence regarding modifiable (or potentially modifiable) risk factors is summarized in Table 1 .
Summary of epidemiologic evidence on risk factors for thyroid cancer.
| . | Association direction . | Evidence of consistency across studies and dose–response . | Bias potential (epidemiologic studies) . |
|---|---|---|---|
| Ionizing radiation | Consistent findings; evidence of dose–response | Some; exposure, if known, may be associated with thyroid screening | |
| Benign thyroid conditions | Consistent findings; some evidence of dose–response for thyroid function parameters | Substantial; conditions likely associated with thyroid screening | |
| Dietary factors | Inconsistent findings; no clear evidence of dose–response (except iodine and follicular thyroid cancer) | Moderate (iodine); confounding likely in ecological studies | |
| Metabolic factors | Consistent findings; evidence of dose–response (obesity/excess adiposity) | Some; conditions may be associated with thyroid screening | |
| Reproductive factors/sex steroid hormones | Inconsistent findings; no clear evidence of dose–response with hormonal exposures | Moderate; reproductive events or hormone use may be associated with thyroid screening | |
| Perinatal and exposures | Consistent findings and evidence of dose–response (birth weight); evidence on maternal and congenital thyroid conditions based on only one study | Moderate; maternal/paternal characteristics and medical conditions may be associated with thyroid screening | |
| Cigarette smoking and alcohol consumption | Consistent findings; evidence of dose–response | Some; behavior may be associated with thyroid screening | |
| Environmental contaminants | Inconsistent findings; no clear evidence of dose–response | Some; exposure, if known, may be associated with thyroid screening |
| . | Association direction . | Evidence of consistency across studies and dose–response . | Bias potential (epidemiologic studies) . |
|---|---|---|---|
| Ionizing radiation | Consistent findings; evidence of dose–response | Some; exposure, if known, may be associated with thyroid screening | |
| Benign thyroid conditions | Consistent findings; some evidence of dose–response for thyroid function parameters | Substantial; conditions likely associated with thyroid screening | |
| Dietary factors | Inconsistent findings; no clear evidence of dose–response (except iodine and follicular thyroid cancer) | Moderate (iodine); confounding likely in ecological studies | |
| Metabolic factors | Consistent findings; evidence of dose–response (obesity/excess adiposity) | Some; conditions may be associated with thyroid screening | |
| Reproductive factors/sex steroid hormones | Inconsistent findings; no clear evidence of dose–response with hormonal exposures | Moderate; reproductive events or hormone use may be associated with thyroid screening | |
| Perinatal and exposures | Consistent findings and evidence of dose–response (birth weight); evidence on maternal and congenital thyroid conditions based on only one study | Moderate; maternal/paternal characteristics and medical conditions may be associated with thyroid screening | |
| Cigarette smoking and alcohol consumption | Consistent findings; evidence of dose–response | Some; behavior may be associated with thyroid screening | |
| Environmental contaminants | Inconsistent findings; no clear evidence of dose–response | Some; exposure, if known, may be associated with thyroid screening |
Note: Association with thyroid cancer risk, based on overall evidence: ↑ = positive; ↓ = inverse; − = null.
Ionizing radiation
Ionizing radiation exposure in childhood is currently the most well-established modifiable risk factor for thyroid cancer. Based on astute clinical observation, Duffy and Fitzgerald were the first to draw attention to the relationship between radiation exposure and thyroid cancer ( 42 ). This was confirmed by a groundbreaking study in Rochester, NY, which prospectively followed a cohort of people exposed to thymus-directed radiation treatments as children ( 43 ). The third landmark occurred in the 1995 publication of a pooled analysis of seven cohort studies showing that the association between external radiation exposure and thyroid cancer risk follows a linear dose–response pattern, and that the association is much stronger for individuals exposed as young children ( 44 ).
Perhaps the strongest evidence so far comes from the recent update and expansion of the pooled analysis ( 45–47 ). At one time, it was thought that very high radiation exposure to the thyroid, characteristic of cancer therapy, destroys thyroid tissue rendering malignant transformation of cells impossible. However, the updated pooled analysis showed that the risk of thyroid cancer continues to increase until very high doses before curving downward, but never returning to baseline ( 46 ). Another long-standing question relates to the existence of a “threshold” effect of radiation exposure on thyroid cancer risk, meaning a level of exposure below which the risk of thyroid cancer is zero. The pooled analysis focused on the dose–response relation at the lowest dose range, finding that 40 mGy is associated with a statistically elevated relative risk, lower than the 100 mGy found in the earlier pooled analysis ( 47 ). The data also support a minimum latency period of 5 to 10 years; this is the minimum time after an exposure that its effects on an outcome may be clinically observable. Finally, it showed that the risk increases for 30 to 40 years before decreasing, although not to the baseline. The dose–response estimates from this study can be used as a benchmark for assessing the potential for bias in other epidemiologic studies of radiation-exposed populations ( Table 1 ), particularly in the context of the estimated radiation exposure levels and age and time since exposure ( 48 ).
There has been long-standing debate about whether thyroid cancer risk is influenced by internal sources of radiation exposure (i.e., from radiopharmaceuticals, such as 131 I targeting the thyroid gland) in the same way and to the same degree as with external sources of exposure. 131 I and other iodine isotopes are used in the diagnosis and treatment of some thyroid disorders, such as hyperthyroidism. Data from the Chernobyl accident recently confirmed that the association of childhood exposure to 131 I and thyroid cancer risk is linear and compatible with findings from populations exposed to external radiation, at least at low to moderate doses ( 49 ). However, other factors, especially iodine deficiency in the region of the Chernobyl accident, could affect this comparison. It remains unclear whether 131 I treatment for hyperthyroidism influences thyroid cancer risk, although most patients are treated at older ages, and the very high treatment doses (∼100 Gy) used in this therapy may have cell killing effects similar to that of radiotherapy for cancer ( 45, 50 ).
Whether ionizing radiation exposure may have contributed to population-level trends in thyroid cancer incidence is unclear. Although unique biomarkers of radiation exposure have not yet been identified (to determine with complete certainty that ionizing radiation was the cause of a specific PTC), RET/PTC chromosomal rearrangements are much more prevalent in radiation-exposed cases, and a recent large-scale integrated genomic landscape analysis of PTCs in individuals exposed in utero or in childhood following the Chernobyl accident showed that these carcinogenic events are linearly associated with radiation doses up to 1 Gy ( 51, 52 ). Moreover, the DNA-damaging effects of radiation are more apparent for individuals exposed at younger age ( 52 ). However, single-institution studies have shown that the proportion of PTC cases with RET/PTC rearrangements appears to decline over time, whereas the proportion of PTCs with BRAF and RAS point mutations remains stable or increase ( 53, 54 ). These observations suggest that exposures capable of inducing BRAF or RAS mutations may have had greater influence on population-level trends in PTC incidence than those causing RET/PTC rearrangements. On the other hand, radiation exposure to the general population has increased in recent decades, especially in the United States, owing to a dramatic increase in use of diagnostic imaging, particularly computed tomography (CT; ref. 55 ). In 2016, CT scans constituted 63% of the total radiation exposure to the U.S. population from all medical sources ( 56 ). Between 5% and 15% of all CT scans are conducted in children, and a single head or neck CT scan in children can deliver a dose to the thyroid of about 10 to 20 mGy, although the dose can vary widely depending on the scan type, age of the individual, and other factors ( 57 ). Repeated CT scans are common, and each scan contributes to greater cumulative thyroid dose and, thereby, greater risk of thyroid cancer, although it remains uncertain whether the effects of repeated exposures are additive effects or subadditive due to DNA repair mechanisms. From the pooled analysis, cumulative radiation doses between 50 and 100 mGy were estimated to increase thyroid cancer risk by 50% to 100% ( 46 ). Consistent with this, a large Australian cohort found that a single head CT scan before age 20 was associated with a 33% to 53% increased risk of thyroid cancer accounting for a one-year exposure lag ( 58 ). Thus, it is conceivable that the increased use of CT imaging in children has had at least a small influence on population-level trends in thyroid cancer incidence since the 1980s.
Benign thyroid disease
Thyroid cancer is often preceded by benign thyroid disease (e.g., thyroid nodules, goiter, hyperthyroidism, hypothyroidism, and thyroiditis), but it remains unclear whether benign thyroid diseases cause the development or progression of thyroid cancer. Diagnostic work-up of benign thyroid conditions may lead to the incidental detection of thyroid cancer. The impact of this bias may be reduced by excluding thyroid cancers occurring in the first few months or years after benign disease. Studies that have done this, including a large international pooled analysis of case–control studies ( 59 ), typically have shown elevated (albeit attenuated) thyroid cancer risks in relation to goiter and benign thyroid nodules, more modest positive associations for hyperthyroidism [characterized by high thyroid hormone and low thyroid stimulating hormone (TSH) levels] and hypothyroidism (low thyroid hormone and high TSH levels), and weak or null associations for autoimmune thyroiditis ( 60–62 ). Nonetheless, it is not fully possible to eliminate detection bias as an explanation for these results, as diagnosis of one or more thyroid conditions may mean many years or even decades of regular thyroid hormone testing or more intense monitoring of the thyroid gland. It has also been difficult to disentangle whether an association for a particular benign disease is due to the disease itself or its treatment (e.g., in the case of 131 I-therapy for hyperthyroidism).
Prospective cohort studies incorporating prediagnostic measures of thyroid hormones, TSH, and/or thyroid autoantibodies, and with long-term duration of follow-up for thyroid cancer incidence, provide an opportunity to evaluate the relation between thyroid disorders and thyroid cancer risk, while also minimizing the potential for the biases highlighted above. For instance, such studies may exclude individuals with a prior thyroid disease diagnosis, allowing more direct focus on whether normal variation in thyroid function or autoimmunity may influence thyroid cancer risk. Two separate prospective studies of individuals in the normal (euthyroid) range of thyroid function demonstrated an inverse association between prediagnostic TSH and differentiated thyroid cancer risk, whereas no association was observed for thyroid hormones ( 63, 64 ). These findings were surprising considering that TSH has been shown in experimental studies to promote growth and proliferation of thyroid cancer cells and has long been hypothesized to play an important role in thyroid cancer etiology ( 65 ). On the other hand, the inverse association of TSH is somewhat consistent with the positive association of hyperthyroidism and thyroid cancer risk, described above, and the finding that thyroid cancer risk alleles located near the FOXE1 and NKX2-1 genes are associated with low TSH levels ( 66 ). Additional research may be needed to evaluate whether the underlying genetic, autoimmune-related, dietary, or environmental causes of overt or structural thyroid disorders contribute to thyroid cancer development.
Iodine is a trace element essential for the formation of thyroid hormones and found primarily in and around coastal areas ( 67 ). Iodine deficiency is a major risk factor for several types of benign thyroid diseases, including goiter and hypothyroidism, whereas iodine excess can induce thyroid dysfunction in patients with certain risk factors, such as preexisting thyroid disease, the elderly, fetuses, and newborns ( 67 ). However, it has been difficult to determine whether iodine insufficiency or excess causes thyroid cancer, owing to the lack of data from prospective cohort studies and reliance on self-reported dietary surveys, which provide an unreliable estimate of iodine consumption ( 68 ). A comprehensive review of the animal and human data has supported the view that iodine deficiency increases the risk of FTC and possibly ATC ( 69 ). Ecological studies have demonstrated that the introduction of iodine supplementation into areas of iodine deficiency increases the ratio of PTC to FTC, raising the question of whether excessive iodine intake is a risk factor for PTC ( 70–72 ), although such studies are strongly confounded by changes over time in thyroid imaging and diagnostic practices. Lee and colleagues performed a meta-analysis of 16 studies to address the role of iodine intake and thyroid cancer risk ( 73 ). Rather than settling the question, however, the report highlighted the weaknesses in the available evidence. For example, the two studies with urinary iodine measurements that showed evidence of a positive association between urinary iodine and PTC had low quality scores ( 74, 75 ).
Few epidemiologic studies have attempted to address the association between iodine intake and thyroid cancer risk using dietary intake instruments (food frequency questionnaires) to approximate iodine intake or to evaluate associations for individual iodine-rich food items, including fortified foods (bread, dairy, and salt), seaweed/kelp, and fish/shellfish. In an international pooled analysis of case–control studies, fish intake was inversely associated with thyroid cancer in endemic goiter areas, and intake of cruciferous vegetables (which contain goitrogens, thyroid function-disrupting substances that result in the stimulation of TSH production) was inversely associated with thyroid cancer in iodine-rich and endemic goiter regions ( 76, 77 ). In a Japanese cohort study, seaweed consumption (the primary source of dietary iodine in the population) was positively associated with risk of PTC (HR = 1.71; 95% CI, 1.01–2.90 for daily consumption versus ≤2 days/week; ref. 78 ). However, this finding was not confirmed in a separate Japanese cohort ( 79 ). In a large U.S. cohort of middle-to-older adults, higher adolescent intakes of canned tuna and mid-life intake of broccoli were positively associated with thyroid cancer risk among males, providing some support for an association of iodine-rich or goitrogen-containing foods, respectively ( 80 ); however, errors in recall of diet during adolescence may have been substantial. No association was observed for middle-to-older adulthood consumption of fish or shellfish in the large European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study ( 81 ). Further insights about the role of iodine in thyroid cancer development could come from large prospective cohort studies with prediagnostic measures of iodine, such as in urine, and the ability to separately assess risks according to thyroid cancer histologic type.
Other dietary factors
Selenium has been hypothesized to play a protective role in thyroid carcinogenesis owing to its antioxidant properties and role in thyroid hormone metabolism, but results from epidemiologic studies have been inconsistent ( 82 ). Similarly, the hypothesis that nitrates and nitrites influence thyroid cancer risk owing to their ability to inhibit iodide intake and disrupt thyroid homeostasis has not been consistently supported by results from epidemiologic studies ( 83–85 ).
In large cohort studies, positive associations of starch intake and glycemic index with thyroid cancer have been observed in individuals who were overweight or obese (possibly because of their greater insulin resistance), with inverse associations in normal-weight individuals ( 86 ). Inverse associations of polyunsaturated fat intake ( 86 ) and positive associations of fruit juice intake ( 87, 88 ) have been observed. Results from studies evaluating tea consumption and thyroid cancer risk have been mixed ( 88–90 ), whereas coffee intake has not been associated with risk ( 89, 90 ). Reported evidence on the relation between polyphenol intake and thyroid cancer risk has been mixed ( 88, 91, 92 ). However, foods and beverages rich in polyphenols, including tea, wine, and citrus fruit, also contain other vitamins and nutrients and have other properties that may independently influence thyroid cancer risk. This, and the potential for substantial measurement error in dietary assessment from food frequency questionnaires, are some of the challenges faced when attempting to investigate individual dietary risk or protective factors for thyroid cancer.
In recent decades, the global prevalence of overweight and obesity, including morbid obesity [a body mass index (BMI) above 40], has increased substantially ( 93 ). The impact of these trends may not be fully realized for decades, although it is well understood that excess body fat leads to development of adverse metabolic conditions, including insulin resistance, hormonal fluctuations, and inflammation, and is a cause of diabetes, cardiovascular disease, and several types of cancers ( 94 ). The parallel increasing trends in overweight and obesity and PTC incidence since at least the 1980s has led many to question as to whether a direct relationship exists between excess adiposity and thyroid cancer development ( 95 ).
Until about a decade ago, most epidemiologic studies on overweight and obesity were cross-sectional or case–control in design. A large international pooled case–control study revealed a positive association between BMI and thyroid cancer confined to women; however, the number of cases in men was small ( 96 ). The earliest prospective studies provided some evidence that higher BMI was associated with increased risk of differentiated thyroid cancer, not only in women but also in men ( 62, 97–99 ). A pooled analysis of five prospective U.S. cohorts demonstrated that BMI was positively associated with risk of thyroid cancer ( 95 ). Overall, study subjects who were obese (BMI ≥30 kg/m 2 ) at study entry had about a 53% greater risk of thyroid cancer than those with normal weight. The positive association was observed for all major histologic types of thyroid cancer, apart from MTC, and appeared to be strongest for ATC. Similar patterns by histologic type were observed in a Norwegian cohort study of over two million individuals ( 98 ). An expanded, international pooled analysis of 22 prospective cohort studies confirmed these results and showed positive associations for additional measures of adiposity, including waist circumference (a better measure of central, or visceral, adiposity) and adulthood weight gain (a more accurate measure of excess adiposity than weight or BMI at one point in time; ref. 100 ). In addition, greater adiposity was more strongly associated with thyroid cancer mortality than incidence, providing further evidence that excess adiposity may directly influence tumor promotion and progression. Similar findings were observed in a recent case–control study in Australia; BMI was positively associated with PTCs characterized by the BRAF V600E mutation, whereas no association was observed for BRAF -negative PTC ( 101 ).
Although some researchers have argued that much, if not all, of the observed association could be explained by greater likelihood of thyroid function testing and imaging in obese, versus, normal-weight individuals ( 102 ), the associations were not limited to indolent tumors, as one would expect in the presence of major detection bias. There is also little evidence that method of initial detection of differentiated thyroid cancer (palpation, imaging, incidental) differs by obesity status ( 103 ). Associations of childhood BMI and thyroid cancer risk are less likely to be influenced by detection bias than those of adulthood BMI. In a large Danish study, childhood BMI (based on annual measurements of height and weight) was positively associated with risk of adult thyroid cancer ( 104 ). Also, the magnitude of the association appeared to be stronger than that for adulthood BMI, based on published results from other studies. Due to the mounting evidence from epidemiologic studies, a panel of experts convening at the International Agency for Research on Cancer in 2016 added thyroid cancer to a growing list cancers having sufficient evidence of a causal relationship with excess adiposity ( 105 ).
A provocative question, therefore, has been whether and to what extent overweight and obesity have contributed to rising thyroid cancer incidence trends. Kitahara and colleagues estimated that the rising prevalence of overweight and obesity in the United States was responsible for about 14% of the rise in PTC incidence between 1995 and 2015 and 58% of the rise in large (>4 cm) PTCs ( 106 ). Hypothetically, if all of the individuals in the overweight or obese categories had been of normal weight, the PTC incidence would have increased by an average of 5.1% per year during 1995–2015, as opposed to 5.9% per year ( Fig. 4 ), and the incidence of the larger PTCs would have increased, on average, 1.9% per year, as opposed to 4.5% per year. Comparable estimates of population-attributable risk were obtained in an Australian study using similar methods ( 107 ).

Trends in the incidence of papillary thyroid cancer in the United States, overall and stratified by overweight and obesity-related and -unrelated cases, 1995–2015. The trendlines represent the incidence in each calendar-year period, and the gray bars represent the population-attributable fraction. Adapted from Kitahara et al., J Natl Cancer Inst 2020 ( 106 ).
Biological mechanisms underlying the association between obesity and thyroid cancer have been proposed, partly based on the more developed knowledge of the role of obesity in development of other cancer types, with inflammation and insulin resistance being the most well-studied factors ( 108 ). Epidemiologic evidence linking diabetes with thyroid cancer has been mixed, with some studies suggesting a positive association ( 109 ), and many others finding no or inconsistent evidence of an association ( 61, 110–113 ). Some studies have shown a reduced risk of thyroid cancer in individuals treated with metformin ( 111, 114 ). Findings from studies evaluating prediagnostic markers of insulin resistance, dyslipidemia, and blood pressure in relation to thyroid cancer risk also have been mixed ( 115, 116 ). In the EPIC cohort, some but not all prediagnostic markers of inflammation were associated with risk of differentiated thyroid cancer ( 117 ). Interestingly, however, a doubling in prediagnostic concentrations of insulin-like growth factor (IGF)-I was associated with a 48% increase in risk (95% CI, 6%–108%; ref. 118 ). A future study investigating IGF-I levels in younger individuals in relation to thyroid cancer risk could provide further insights, considering the separate findings regarding childhood BMI (described above; ref. 104 ) and larger birth size ( 119–121 ; see also “Perinatal and in utero exposures”).
Reproductive and hormonal factors
The higher incidence of thyroid cancer in women than in men ( 7 ), along with evidence from laboratory-based studies that estrogen stimulates proliferation of thyroid cancer cells ( 122 ), suggests a direct role of sex steroid hormones in thyroid cancer development. However, evidence from case–control and cohort studies evaluating traditional “proxies” for lifetime estrogen exposure, including parity, ages at menarche and menopause, and use of oral contraceptives and menopausal hormone therapy, in relation to thyroid cancer risk has been weak and inconsistent ( 62, 123–131 ). Several explanations have been proposed for the mixed findings: (i) strong confounding by medical surveillance or access to care inhibits the ability to identify any “true” effects of reproductive or hormonal factors on thyroid cancer risk; (ii) the proxy indicators evaluated in those studies do not accurately capture estrogen exposure during the etiologically relevant time windows for thyroid cancer; and/or (iii) the higher incidence of thyroid cancer risk in women than in men is largely explained by other factors that are more prevalent in women, such as autoimmunity ( 68, 132 ). Some of the more consistent epidemiologic findings (i.e., for infertility and use of infertility drugs; refs. 125, 133 ), recent pregnancy ( 125, 128 ), irregular menstrual cycles ( 128 ), and hysterectomy or surgical menopause ( 125, 127, 134 ) could provide etiologic clues, including the importance of specific exposures and exposure time windows, or they may be explained by detection bias ( 30, 125 ); it is also possible that both scenarios are true. Detection bias does not seem to be a likely explanation for some observations, such as the modest reduction in risk observed with greater duration of breastfeeding ( 125, 126 ); findings such as this warrant further exploration.
Perinatal and in utero exposures
Whether certain perinatal or in utero exposures influence risk of thyroid cancer in mothers or their offspring is an intriguing question considering the rising incidence of pediatric thyroid cancer ( 135 ), the higher incidence of thyroid cancer in women than men starting at young ages ( 132 ), and the elevated risk of thyroid cancer shortly after pregnancy ( 125, 128 ).
As discussed above, younger age at exposure to ionizing radiation is much more strongly associated with thyroid cancer risk than older age at exposure ( 46 ); however, the influence of in utero radiation exposure on thyroid cancer risk remains unclear. Based on just eight cases of thyroid cancer, including one case of a Hürthle cell carcinoma, in utero exposure to 131 I at the time of the Chernobyl accident was associated with a nonsignificant increased odds ratio of 11.66 per Gy ( 136 ).
In a large Swedish cohort study, greater birthweight and fetal growth (birthweight standardized by gestational age and infant sex) were associated with higher maternal risk of PTC and FTC ( 121 ). These associations were adjusted for maternal age, height, weight, smoking, and sociodemographic factors. The authors hypothesize that IGF-I may underlie these associations due to its procarcinogenic properties and association with fetal growth.
Two large registry-based studies in California and the Nordic countries were conducted to evaluate a variety of factors including maternal and paternal characteristics, pregnancy complications, and birth outcomes in relation to thyroid cancer risk in offspring ( 119, 120 ). Both studies found positive associations between birthweight and risk of thyroid cancer (particularly PTC), with a 10% to 20% increase in risk for every 1 kg, and inverse associations for male sex and birth order. In the California study, birth order was inversely associated with PTC and positively associated with FTC ( 120 ), whereas in the Nordic study, associations trended in the inverse direction for across all major histologic types except MTC ( 119 ). Birth order serves as a crude proxy for a wide range of childhood exposures, including childhood exposure to infectious diseases and exposure to maternal hormones and environmental contaminants in utero and during breastfeeding ( 120 ). Other findings from the California study included positive associations for Hispanic ethnicity (with PTC only) and higher maternal education ( 120 ). Other findings from the Nordic study included very strong positive associations for neonatal diagnosis of congenital hypothyroidism (OR = 4.55; 95% CI, 1.58–13.08) and maternal history of most benign thyroid disorders (with ORs ranging from 11.91 for maternal hyperthyroidism to 67.36 for maternal goiter); the magnitude of these associations was generally stronger for FTC than PTC, suggesting a possible underlying role of iodine deficiency ( 119 ). Maternal diabetes diagnosis before pregnancy and postpartum hemorrhage were modestly positively associated with thyroid cancer risk. No associations were observed for other maternal characteristics examined, including marital status, age at birth, smoking, or other comorbid conditions or pregnancy complications apart from those listed above. Paternal age at birth and other birth characteristics (multiple births, preterm birth, Cesarian section birth) were also not associated with offspring thyroid cancer risk. There was no evidence that smoking or prepregnancy BMI biased the associations observed in the Nordic study. Detection bias is a plausible explanation for some of these findings. For instance, offspring of mothers of higher socioeconomic standing and those with comorbid conditions, especially benign thyroid diseases, may be more likely to undergo routine thyroid monitoring throughout life leading to more incidental detection of thyroid nodules and cancer. However, the authors found that many of the associations held after restricting the outcome to advanced thyroid cancers, which are more likely to be detected due to symptoms.
Smoking and alcohol
Case–control studies and cohort studies have consistently shown that cigarette smoking and alcohol consumption are inversely associated with risk of thyroid cancer ( 137–141 ). Current smokers were found to have a 40% to 50% reduction in risk of thyroid cancer compared with never smokers ( 141 ). The associations appeared to be independent and not modified by other thyroid cancer risk factors, including obesity. Risks associated with both smoking and alcohol consumption appear to be dose dependent with regard to duration and frequency of use ( 137–139, 141 ), although a dose-dependent association of smoking and thyroid cancer was not observed in a Korean cohort of 10 million adults ( 140 ). The potential underlying mechanisms remain unclear, although alterations in TSH, thyroid hormones, and thyroid autoantibodies, as well as sex steroid hormones, have been suggested to play a role ( 137 ). Although it is possible that detection bias could confound these results, as current smokers and heavier drinkers may be less likely to undergo thyroid imaging or screening, a case–control study in Australia found that the inverse association of current smoking and thyroid cancer became stronger after adjusting for detection bias, and smoking was more strongly inversely associated with thyroid cancers harboring BRAF V600E mutations ( 142 ).
Other environmental exposures
Interest in environmental contaminants and thyroid cancer arises because some classes of chemicals may influence thyroid homeostasis, although the exact mechanisms of action are likely complex and not entirely understood. Some of these mechanisms include adverse effects on thyroid hormone metabolism and inhibition of iodine uptake by follicular thyroid cells. Also, because many of these chemicals have similar molecular structure to thyroid hormones, they can alter the ability of thyroid hormone to bind to receptors and transport proteins and contribute to the development of subclinical hypothyroidism ( 143 ).
Use of flame retardants chemicals in commercial and household items, including furniture, electronics, and construction materials, increased substantially since the 1970s, and household exposure to these chemicals is now ubiquitous. A recent case–control study evaluated whether 12 flame retardants measured from household dust and 27 polybrominated diphenyl ethers (PBDEs) measured in serum were associated with occurrence of PTC ( 144 ). Higher concentrations of decabromodiphenyl ether (BDE-209) and tris(2-chloroethyl) phosphate (TCEP) were found in the dust samples of PTC cases compared with controls. TCEP was associated with aggressive tumor features, including extrathyroidal extension, greater size, and nodal metastasis, whereas BDE-209 was associated only with smaller, low-stage PTCs. However, serum PBDEs were not associated with PTC risk in that study nor in a large, nested case–control study in the United States ( 145 ).
Evidence linking pesticide exposures with thyroid cancer risk has been mixed ( 146–148 ). In a U.S. cohort of male licensed pesticide applicators (mostly farmers), use of the fungicide metalaxyl and the organochlorine insecticide lindane were associated with a 2-fold and 74% increased risk of thyroid cancer, respectively, whereas use of the herbicide chlorimuron-ethyl and insecticide carbaryl were inversely associated with risk ( 148 ). Exposure to organophosphate insecticides by the spouses of the cohort participants was associated with increased risks of thyroid, breast, and ovarian cancers ( 147 ). Malathion, the most used organophosphate, was positively associated with risk, although the number of exposed cases was small. In a Norwegian nested case–control study, prediagnostic concentrations of several polychlorinated biphenyl (PCB) congeners and organochlorine pesticide analytes were inversely associated with thyroid cancer risk ( 149 ); positive associations for certain PCBs and chlordane metabolites were observed but only in the most recent birth cohort (1943–1957).
Exposure to trace elements associated with volcanic activity, including vanadium, sulfur, thiocyanates, zinc, and cadmium, has been suggested to contribute to the relatively high thyroid cancer incidence in the Phillipines, Hawaii, Iceland, New Caledonia, French Polynesia, and the Catania providence of Sicily (refs. 150–152 ; see “Disparities,” above). However, exposures to these specific trace elements and thyroid cancer have not been investigated in well-controlled epidemiologic studies, and the potential underlying biological mechanisms remain unclear ( 152 ).
Other potential modifiable risk factors
Other potential risk (or protective) factors for thyroid cancer have been explored, mainly centered around exposures influencing thyroid hormone synthesis or with estrogen-like or inflammatory properties. Several medical conditions, other than the ones described above, have been linked with an increased risk of thyroid cancer, including end-stage renal and liver disease in solid organ transplant recipients ( 153 ), benign breast disease ( 62, 123, 154 ), breast cancer ( 155 ), uterine fibroids ( 124 ), asthma ( 62 ), and autoimmune conditions such as lupus and Sjögren's syndrome ( 156, 157 ). Positive associations have been observed for artificial light at night ( 158 ), and inverse associations have been observed for ultraviolet radiation exposure ( 159 ). Although several of these findings are novel and compelling, they require replication in other studies before drawing strong conclusions.
Familial clusters and germline mutations
That developing thyroid cancer is affected by genetic factors is supported by at least two types of evidence. First, the importance of genetic risk factors in thyroid cancer is evidenced by studies using national cancer registry data showing a substantial familial risk ( 160, 161 ). In some cases, the occurrence of nonsyndromic familial clusters of thyroid carcinomas, referred to as “familial non-medullary thyroid carcinoma” (FNMTC), can be clinically identified. However, although the existence of FNMTC has been well documented, not all clusters have a genetic cause, especially in view of the recent increase of incidentally diagnosed cases. Based on a statistical analysis, it is generally accepted that three first-degree relatives with thyroid cancer are usually necessary to conclude that a genetic factor is causing a particular family cluster ( 162 ). Specific genetic factors have been identified in only a minority of family clusters ( 163 ).
Second, many studies have identified germline polymorphisms found in the general population that contribute to the susceptibility of developing thyroid cancer. The first to be identified, initially among people living in Iceland, were variants in the gene FOXE1 ( 66 ). This finding has been duplicated in a variety of other populations and remains the strongest risk factor. Several polymorphisms have been identified in this thyroid transcription factor that are related to its role in thyroid carcinogenesis ( 164 ). Many other genetic polymorphisms, with weaker effects, have been identified ( 165 ). A recent effort combining 10 polymorphisms found that their contribution to genetic predisposition is 8% ( 166 ).
Screening for thyroid cancer can be performed by palpation of the thyroid, as is done in a general physical examination, or by imaging using thyroid ultrasound. Based on epidemiologic and clinical observation, the U.S. Preventative Services Task Force and other clinical practice guidelines strongly recommend against thyroid cancer screening in asymptomatic people without demonstrable risk factors ( 167, 168 ). The effects of widespread thyroid cancer screening were clearly demonstrated in the example set by the Republic of Korea ( 21 ), as discussed above (see “Trends in incidence and the role of overdiagnosis”). At the other end of the spectrum is screening for genetic syndromes. The clearest example is related to MTC ( 169 ). Every patient with MTC should be screened for a genetic basis. For people known to have a syndrome that includes MTC, often identified in relatives of an MTC patient, screening is indicated. In some forms of the syndrome, prophylactic thyroid surgery is recommended ( 170 ).
In familial clusters of thyroid cancer, as defined above, some suggest that screening family members should be carried out, but “with caution” ( 171 ). However, aside from routine palpation, no guidelines recommend screening ( 172 ). Screening is suggested for people with genetic syndromes associated with a high risk of thyroid cancer, although it is less clear whether screening should be by palpation or by ultrasound ( 173 ).
Whether to screen people exposed to radiation at a young age is unclear, although it is difficult to envision scenarios where the benefits of screening (ideally, reduced morbidity and mortality) outweigh the risks. Screening after the Chernobyl accident found many radiation-related cases and may have improved the outcomes for some individuals ( 174 ). However, screening after the Fukushima accident, which yielded much lower radiation doses to the population, identified many thyroid cancers that were highly unlikely to be radiation-related, resulting in unnecessary psychological distress over a cancer diagnosis and subjecting some individuals to unnecessary treatment ( 48 ). Childhood and young adult cancer survivors who received therapeutic doses of radiation exposing the thyroid and survived their cancer diagnosis are clearly at increased risk of developing thyroid nodules and second primary thyroid cancer, especially those exposed at very young ages ( 45 ). Many papers report the sensitivity of thyroid ultrasound in detecting thyroid cancer in these individuals, and some recommend thyroid ultrasound as routine ( 175 ). However, those that take into account the potential for overdiagnosis are cautious about following this recommendation. In fact, the International Late Effects of Childhood Cancer Guideline Harmonization Group found that the available data were insufficient to recommend for or against screening cancer survivors ( 176 ).
As summarized above (see “Trends in incidence and the role of overdiagnosis” and “Disparities”), trends and disparities in thyroid cancer are explained largely, but not completely, by diagnostic practices. Greater awareness of the extent and problems associated with thyroid cancer overdiagnosis, clinical recommendations against thyroid cancer screening, introduction of the NIFTP terminology for tumors previously considered malignant, and higher tumor size thresholds for fine-needle aspiration biopsy have been among the most effective measures of reducing the incidence of thyroid cancer ( 15, 16 ).
Epidemiologic research on potential modifiable risk factors raises the possibility of identifying other specific targets for primary prevention. Together with clinical efforts to avoid overdiagnosis and overtreatment, further advancement in thyroid cancer epidemiologic research has the potential to substantially reduce the total disease burden in the population. However, few modifiable risk factors have been identified to date apart from ionizing radiation and obesity.
With increasing evidence supporting a linear relationship between ionizing radiation exposure and thyroid cancer risk (except at very high levels; ref. 46 ), minimizing exposure to the thyroid to the extent possible, especially in children, is likely to be effective for thyroid cancer prevention. A concerted effort to minimize medical radiation exposure to children has been operationalized by the slogan and educational campaign “Image Gently” ( 177 ), initiated by the Society of Pediatric Radiology in collaboration with the American Association of Physicists in Medicine, American College of Radiology, and the American Society of Radiologic Technologists, with many other professional organizations subsequently joining the effort. The campaign encourages the use of pediatric-specific imaging protocols, avoidance of multiphase scanning, and consideration of nonradiation imaging procedures whenever possible. Although thyroid radiation from dental radiology has decreased over time, and suggestions have been made to decrease this even further ( 178 ), radiation exposures from dental radiologic procedures represent a tiny fraction of the total radiation exposure from medical sources ( 57 ). Reduction in the use of CT imaging to the head and neck in children would have a far greater impact on thyroid cancer prevention ( 57 ).
Minimizing radiation exposure to the thyroid is especially important in young children due to their much higher radiosensitivity compared with adults ( 46 ). High-dose radiotherapy for most benign conditions have been long abandoned due to concerns over the late effects ( 179 ), and treatment of hyperthyroidism with 131 I has been declining in favor of long-term antithyroid drugs ( 180 ). Alternatives to radiotherapy are unavailable for several types of cancers, but modalities and collimation have improved over time, reducing off-target effects.
Another potential source of radiation exposure is the release of radioactive iodine isotopes from nuclear power plant accidents, not unlike the one that occurred in Chernobyl. Should a similar accident occur, it is to be expected that the food pathway would be quickly controlled. However, this may not be complete, and people very near the accident would be subject to thyroid exposure through the inhalation pathway. If the age-specific potential dose threshold is exceeded, ingestion of potassium iodide will be advised by public health officials. The ATA has reviewed the issues related to this dose-reduction measure ( 181 ). The World Health Organization, the U.S. Food and Drug Administration, and others have published guidelines for the use of potassium iodide in a radiologic emergency ( 182, 183 ).
Efforts to promote weight loss in individuals who are overweight or obese and avoidance of excess weight gain throughout adulthood may help to prevent the occurrence of thyroid cancer or the promotion of existing thyroid cancers ( 106 ). Considering the immense difficulty that many adults face in achieving or maintaining a healthy body weight, studies that contribute to a better understanding of the biological mechanisms underlying the association between obesity and thyroid cancer may lead to the identification of more specific, and potentially more effective, targets for prevention. On the other hand, widespread interventions to promote healthy body weight or weight loss have the potential for numerous and extensive health benefits, including the prevention and improvement of a spectrum of metabolic diseases apart from thyroid cancer.
There remains a critical need for prospective studies with objective prediagnostic measures of exposure, which would help to avoid measurement error, minimize detection bias, and allow for assessment of dose–response. Such studies are needed to better understand the biological mechanisms underlying commonly observed associations for benign thyroid conditions, obesity, smoking, and alcohol consumption, for instance. More confirmatory studies or in-depth investigations of some of the more novel or compelling findings to date (e.g., associations for benign thyroid diseases, iodine consumption, infertility medications, and endocrine-disrupting chemicals, to name a few) are also needed. Studies that evaluate exposures that are unique or more prevalent among women, including certain cosmetic products, particularly those with endocrine-disrupting properties, and certain medical conditions and their associated therapies (autoimmune conditions, benign thyroid disorders), could help to improve understanding around the higher incidence in women than men. Additional studies investigating environmental and lifestyle-related exposures and screening practices across racial/ethnic subgroups and immigration status may provide further etiologic clues. In general, there is a major need for large case–control and prospective studies with detailed diagnostic information, including markers of tumor aggressiveness and pathways to diagnosis (i.e., how the tumor was initially detected), which would enable researchers to better distinguish incidentally detected thyroid cancers from those considered clinically relevant. There is great potential for advancing current understanding of thyroid cancer epidemiology by designing epidemiologic studies that characterize thyroid cancer cases according to their molecular profiles, as in the recent example of radiation and thyroid cancer in Chernobyl (see “Ionizing Radiation”; ref. 52 ).
A.B. Schneider reports personal fees from UpToDate outside the submitted work. No disclosures were reported by the other author.
This work was funded by the Intramural Research Program of the NCI (C.M. Kitahara).
Citing articles via
Email alerts.
- Online First
- Online ISSN 1538-7755
- Print ISSN 1055-9965
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Information on Advertising & Reprints
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account

COMMENTS
This page highlights some of the latest research in thyroid cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies. ... Thyroid Cancer Research Results. Thyroid Cancer Diagnosis. Over the last two decades, a major concern ...
Thyroid Cancer: A Review. ImportanceApproximately 43 720 new cases of thyroid carcinoma are expected to be diagnosed in 2023 in the US. Five-year relative survival is approximately 98.5%. This review summarizes current evidence regarding pathophysiology, diagnosis, and management of early-stage and advanced thyroid cancer.
Latest Research and Reviews. Thyroid nodules: diagnosis and management. ... Medullary thyroid cancer (MTC) is a neuroendocrine tumor that originates from thyroid parafollicular cells, generally ...
The past 5-10 years have brought in a new era in the care of patients with thyroid cancer, with the introduction of transformative diagnostic and management options. Several international ultrasound-based thyroid nodule risk stratification systems have been developed with the goal of reducing unnecessary biopsies. Less invasive alternatives to surgery for low-risk thyroid cancer, such as ...
Posted: May 6, 2016. After rising steadily since the 1990s, the incidence of thyroid cancer in the United States may be leveling off, according to an analysis of data from NCI's SEER program. FDA Approves Lenvatinib for Radioactive Iodine-Refractory Thyroid Cancer. Posted: March 2, 2015. The FDA has approved lenvatinib (Lenvima) to treat some ...
Thyroid cancer is the most common endocrine malignancy with almost one million people living with thyroid cancer in the United States. Although early-stage well-differentiated thyroid cancers account for the majority of thyroid cancers on diagnosis and have excellent survival rates, the incidence of advanced-stage disease has increased over the past few years and confers poorer prognosis ...
Importance: Approximately 43 720 new cases of thyroid carcinoma are expected to be diagnosed in 2023 in the US. Five-year relative survival is approximately 98.5%. This review summarizes current evidence regarding pathophysiology, diagnosis, and management of early-stage and advanced thyroid cancer. Observations: Papillary thyroid cancer ...
Genomic data from patients with thyroid cancer, combined with information on mutation-specific mechanisms from experimental models, is transforming the thyroid cancer research field. This Review ...
Read the latest Research articles in Thyroid cancer from Nature Reviews Endocrinology ... A new analysis of thyroid cancer incidence and mortality trends over the period 1974-2013 in the USA ...
Thyroid carcinoma or thyroid cancer (TC) is a malignant tumor of the thyroid gland. It is the most common malignancy of the endocrine (hormonal) system, but is very rare, accounting for about 1% of all malignant tumors, however, it has been increasing in frequency in recent years. The latest Global Cancer Observatory survey from 2020 reports ...
Guidelines from multiple societies across the world reflect these changes, which focus on taking a more individualized approach to clinical management. In this review, we discuss the current more personalized approach to patients with follicular cell-derived thyroid cancer and point toward areas of future research still needed in the field.
In the U.S., thyroid cancer is estimated to be the 13 th most commonly diagnosed cancer, accounting for nearly 44,000 new cancer diagnoses in 2022 (2.3% of the total), and the 6 th most commonly diagnosed cancer among women ().Thyroid cancer incidence is approximately three-fold higher in women (22.8 per 100,000 per year in 2014-2018) than in men (8.0 per 100,000 per year) (Fig. 2a) ().
Thyroid cancer is the most common endocrine cancer. The discovery of new biomarkers for thyroid cancer has significantly improved the understanding of the molecular pathogenesis of thyroid cancer, thus allowing more personalized treatments for patients with thyroid cancer. ... ATC is refractory to current conventional treatments with ...
In many papillary thyroid cancers, the cells have changes in the BRAF gene, which helps them grow. Drugs that target cells with BRAF gene changes, such as vemurafenib (Zelboraf), dabrafenib (Tafinlar), and selumetinib, are now being studied in thyroid cancers with this gene change. In one study, giving selumetinib to patients with thyroid ...
Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-322. Crossref. PubMed. ISI. Google Scholar. 2. ... Cancer Genome Atlas Research Network. Integrated ...
Thyroid cancer (TC) is a significant global healthcare burden. However, the lack of comprehensive data has impeded our understanding of its global impact. We aimed to examine the burden of TC and its trends at the global, regional, and national levels using data stratified by sociodemographic index (SDI), sex, and age. Data on TC, including incidence, mortality, and disability-adjusted life ...
Guidelines from multiple societies across the world reflect these changes, which focus on taking a more individualized approach to clinical management. In this review, we discuss the current more personalized approach to patients with follicular cell-derived thyroid cancer and point toward areas of future research still needed in the field.
Introduction. As in any patient with cancer, the aims of initial treatment in patients with differentiated thyroid cancer (DTC) are to prolong life expectancy with a good quality of life and to ...
Help us end cancer as we know it, for everyone. Cancer information, answers, and hope. Available every minute of every day. Find out all about thyroid cancer, including risk factors, symptoms, how it's found, and how it's treated.
According to the National Cancer Institute, approximately 44,020 people in the United States will be diagnosed with thyroid cancer in 2024, and about 2,170 will die of the disease. Thyroid cancer is highly treatable, and the five-year relative survival rate is estimated at 98.4 percent. Thyroid cancer is much more common among women than it is ...
Several studies have utilized artificial intelligence and machine learning and their findings have substantially added to the current knowledge of imaging, genetic, and molecular features of thyroid nodules and thyroid cancer [106-110]. The constant improvements in these technologies can give rise to more advanced methods which may ...
A new study involving researchers from Sinai Health and the University of Toronto has uncovered new insights into how thyroid cancer may be more effectively treated, avoiding unnecessary surgeries. For the study, researchers looked at thyroid tumor tissues and thyroid nodule biopsies from 620 patients at Mount Sinai Hospital, from 2016 to 2022.
Background Thyroid cancer is a common thyroid malignancy. The majority of thyroid lesion needs intraoperative frozen pathology diagnosis, which provides important information for precision operation. As digital whole slide images (WSIs) develop, deep learning methods for histopathological classification of the thyroid gland (paraffin sections) have achieved outstanding results. Our current ...
One exception is the Queensland Thyroid Cancer Study 9, in which clinical records, pathology data and tumour tissues were collected to obtain information about the method of initial thyroid cancer ...
Thyroid cancer (TC) is a significant global healthcare burden. However, the lack of comprehensive data has impeded our understanding of its global impact. We aimed to examine the burden of TC and its trends at the global, regional, and national levels using data stratified by sociodemographic index (SDI), sex, and age.
Clinical Thyroidology®: Levothyroxine Dosage and the Increased Risk of Second Primary Malignancy in Thyroid Cancer Survivors By ATA August 25, 2024 Clinical Thyroidology , Corporate News , Featured
Known previous or current serious ophthalmic disorders, including history of glaucoma, history of retinal vein occlusion (RVO) or current risk factors for RVO, history of retinal pathology or evidence of retinal pathology. Active skin disorder requiring systemic treatment ≤3 months prior to start of study treatment.
The current study sought to analyze the global burden of thyroid cancer utilizing the publicly accessible GLOBOCAN database. An estimated 586,202 cases of thyroid cancer were reported in 2020, making thyroid cancer the 10th most common cancer worldwide. The majority of thyroid cancer cases occurred in countries with a high or very high Human ...
The incidence of thyroid cancer has risen dramatically in the United States over the past four decades, with similar patterns observed internationally. Thyroid cancer currently ranks as the 13th most common cancer diagnosis overall and the 6th most common among women. Because the greatest increases in incidence have been observed for small and localized tumors having the highest rate of ...
Recent statistics from the International Agency for Research on Cancer indicate that lung cancer is ... Epigenetic predictive biomarkers for response or outcome to platinum-based chemotherapy in non-small cell lung cancer, current state-of-art. ... Legoffic A, et al. Positive thyroid transcription factor 1 staining strongly correlates with ...