What Is the Hygiene Hypothesis?
Many parents believe that their children must be kept in an environment that is as clean as possible, but some research suggests that being exposed to what many would call unclean conditions is good for a child's immune system. Research has indicated that children who are kept in very clean environments have a higher rate of hay fever, asthma and a wide range of other conditions. This is what is called the hygiene hypothesis.
The hygiene hypothesis was first introduced in the late 1980s by David P. Strachan, a professor of epidemiology, in the British Medical Journal. Strachan found that children in larger households had fewer instances of hay fever because they are exposed to germs by older siblings. This finding led to further research that suggests a lack of early childhood exposure to less than pristine conditions can increase the individual's susceptibility to disease.
For example, in the late 1990s, Dr. Erika von Mutius, a health researcher, compared the rates of allergies and asthma in East Germany and West Germany, which unified in 1999. Her initial hypothesis was that East German children, who grew up in dirtier and generally less healthful conditions, would have more allergies and suffer more from asthma than their Western counterparts. However, her research found the opposite: children in the polluted areas of East Germany had lower allergic reactions and fewer cases of asthma than children in West Germany.
Further research has found that children in developing areas of the world are less likely to develop allergies and asthma compared with children in the developed world.

Building the immune system
The idea is simple. When babies are inside the womb they have a very weak immune system because they are given protection by their mother's antibodies. When they exit the womb, though, the immune system must start working for itself. For the immune system to work properly, it is thought that the child must be exposed to germs so that it has a chance to strengthen, according to the U.S. Food and Drug Administration (FDA).
The idea is similar to the training of a body builder. For a body builder to be able to lift heavy objects, the muscles must be trained by lifting heavier and heavier objects. If the body builder never trains, then he will be unable to lift a heavy object when asked. The same is thought to be true for the immune system. In able to fight off infection, the immune system must train by fighting off contaminants found in everyday life. Systems that aren't exposed to contaminants have trouble with the heavy lifting of fighting off infections.
Mutius hypothesized that the reason children who are not exposed to germs and bacteria are sicklier is due to how the human immune system evolved. She thinks there are two types of biological defenses. If one of the defense systems isn't trained or practiced enough to fight off illness, the other system overcompensates and creates an allergic reaction to harmless substances like pollen.
Research by other scientists has found similar results. Exposure to germs triggered an internal inflammatory response in children who were raised in cleaner environments, leading to ailments such as asthma, according to a 2002 article in Science magazine.
One researcher has personal experience has leads him to back the hygiene hypothesis. "I believe that there is a role in the development of a child's immunity exposure to various germs and a vast microbiome diversity," said Dr. Niket Sonpal, an assistant professor of clinical medicine at Touro College of Osteopathic Medicine, Harlem Campus. "I was born in India but moved to the U.S. and went to college in Virginia and medical school in Europe. I am sure that the vast change in environment has played a role in my immunity. How has it? I don't think we know just yet."
In 1997, some began to question if there is a correlation between the hygiene hypothesis and vaccinations. The number of children getting vaccinations was going up, but so were the number of children afflicted with allergies, eczema and other problems. Could depriving the developing immune system of infections using vaccines cause the immune system to eventually attack itself and cause autoimmune diseases like asthma and diabetes? This is a highly contested issue.
Three studies conducted in the 1990s showed that vaccines had no correlation with children developing allergies and other ailments later in life. In fact, vaccinations may help prevent asthma and other health problems other than the diseases they were intended to prevent, according to The National Center for Immunization Research and Surveillance . The idea that vaccinations can cause health problems does not consider the fact that children, whether vaccinated or not, are still exposed to pathogens that help build the immune system. These pathogens also have no relation to the diseases that the vaccines prevent.
The conflict between cleanliness and exposure can leave parents feeling confused. There are many microbes that can make children very sick, such as such as respiratory syncytial virus (RSV), E.coli and salmonella. So cleaning the home is still very important. What should children be exposed to and what should they be protected from?
The CDC recommends regularly cleaning and disinfecting surfaces in the home, especially when surfaces have been contaminated by fecal matter or meat or have come in contact with those who have a virus. Children are also encouraged, though, to play outside , even if they may get dirty in the process. This balancing act may prove to help children stay healthy while still developing a healthy immune system.
Sonpal thinks that the healthy growth of the immune system isn't just about coming in contact with dirt. It also has to do with what foods are consumed, what kind of environments the person grows up in and intrinsic genetics coupled with physical activity levels. Harvard Medical School noted that getting plenty of sleep, avoiding cigarette smoke, drinking in moderation and controlling blood pressure also all play a part in a healthy immune system.
Additional Resources
- Clinical & Experimental Immunology: The 'Hygiene Hypothesis' for Autoimmune and Allergic Diseases: An Update
- Mayo Clinic: Early germ exposure prevents asthma?
- U.S. National Library of Medicine: The Hygiene Hypothesis and home hygiene
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

The same genetic mutations behind gorillas' small penises may hinder fertility in men
In a 1st, HIV vaccine triggers rare and elusive antibodies in human patients
Scientists grow diamonds from scratch in 15 minutes thanks to groundbreaking new process
Most Popular
- 2 James Webb telescope confirms there is something seriously wrong with our understanding of the universe
- 3 Massive study of 8,000 cats reveals which breeds live longest
- 4 Some of the oldest stars in the universe found hiding near the Milky Way's edge — and they may not be alone
- 5 Newfound 'glitch' in Einstein's relativity could rewrite the rules of the universe, study suggests
- 2 Creepy Deep-Sea Anglerfish Captured in Rare Video
- 3 Can a commercial airplane do a barrel roll?
- 4 Mysterious L-shaped structure found near Egyptian pyramids of Giza baffles scientists
- 5 In a 1st, HIV vaccine triggers rare and elusive antibodies in human patients
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Vaccines, Blood & Biologics
- Resources for You (Biologics)
- Consumers (Biologics)
Asthma: The Hygiene Hypothesis
What do clean houses have in common with childhood infections.
One of the many explanations for asthma being the most common chronic disease in the developed world is the “hygiene hypothesis.” This hypothesis suggests that the critical post-natal period of immune response is derailed by the extremely clean household environments often found in the developed world. In other words, the young child’s environment can be “too clean” to pose an effective challenge to a maturing immune system.
According to the “hygiene hypothesis,” the problem with extremely clean environments is that they fail to provide the necessary exposure to germs required to “educate” the immune system so it can learn to launch its defense responses to infectious organisms. Instead, its defense responses end up being so inadequate that they actually contribute to the development of asthma.
Scientists based this hypothesis in part on the observation that, before birth, the fetal immune system’s “default setting” is suppressed to prevent it from rejecting maternal tissue. Such a low default setting is necessary before birth—when the mother is providing the fetus with her own antibodies. But in the period immediately after birth the child’s own immune system must take over and learn how to fend for itself.
The “hygiene hypothesis” is supported by epidemiologic studies demonstrating that allergic diseases and asthma are more likely to occur when the incidence and levels of endotoxin (bacterial lipopolysaccharide, or LPS) in the home are low. LPS is a bacterial molecule that stimulates and educates the immune system by triggering signals through a molecular “switch” called TLR4, which is found on certain immune system cells.
The science behind the hygiene hypothesis
The Inflammatory Mechanisms Section of the Laboratory of Immunobiochemistry is working to better understand the hygiene hypothesis, by looking at the relationship between respiratory viruses and allergic diseases and asthma, and by studying the respiratory syncytial virus (RSV) in particular.
What does RSV have to do with the hygiene hypothesis?
- RSV is often the first viral pathogen encountered by infants.
- RSV pneumonia puts infants at higher risk for developing childhood asthma. (Although children may outgrow this type of asthma, it can account for clinic visits and missed school days.)
- RSV carries a molecule on its surface called the F protein, which flips the same immune system “switch” (TLR4) as do bacterial endotoxins.
It may seem obvious that, since both the RSV F protein and LPS signal through the same TLR4 “switch,” they both would educate the infant’s immune system in the same beneficial way. But that may not be the case.
The large population of bacteria that normally lives inside humans educates the growing immune system to respond using the TLR4 switch. When this education is lacking or weak, the response to RSV by some critical cells in the immune system’s defense against infections—called “T-cells”—might inadvertently trigger asthma instead of protecting the infant and clearing the infection. How this happens is a mystery that we are trying to solve.
In order to determine RSV’s role in triggering asthma, our laboratory studied how RSV blocks T-cell proliferation.
Studying the effect of RSV on T-cells in the laboratory, however, has been very difficult. That’s because when RSV is put into the same culture as T-cells, it blocks them from multiplying as they would naturally do when they are stimulated. To get past this problem, most researchers kill RSV with ultraviolet light before adding the virus to T-cell cultures. However we did not have the option of killing the RSV because that would have prevented us from determining the virus’s role in triggering asthma.
Our first major discovery was that RSV causes the release from certain immune system cells of signaling molecules called Type I and Type III interferons that can suppress T-cell proliferation (Journal of Virology 80:5032-5040; 2006).
The hygiene hypothesis suggests that a newborn baby’s immune system must be educated so it will function properly during infancy and the rest of life. One of the key elements of this education is a switch on T cells called TLR4. The bacterial protein LPS normally plays a key role by flipping that switch into the “on” position.
Prior research suggested that since RSV flips the TLR4 switch, RSV should “educate” the child’s immune system to defend against infections just like LPS does.
But it turns out that RSV does not flip the TLR switch in the same way as LPS. This difference in switching on TLR, combined with other characteristics of RSV, can prevent proper education of the immune system.
One difference in the way that RSV flips the TLR4 switch may be through the release of interferons, which suppresses the proliferation of T-cells. We still do not know whether these interferons are part of the reason the immune system is not properly educated or simply an indicator of the problem. Therefore, we plan to continue our studies about how RSV can contribute to the development of asthma according to the hygiene hypothesis.
Further research
This finding that Type I and Type III interferons can mediate the suppression of T-cells caused by RSV generated two significant questions that our laboratory is now addressing:
- Interferons are important molecules that enhance inflammation, so why--in the context of RSV--do they suppress T-cells?
- Interferons are clearly not the only way RSV suppresses T-cells. What are the other mechanisms that may depend upon T-cells coming in direct contact and communicating with other immune cells?
Related Research
- Assessing the Mechanism of Immunotherapy for Allergy and Allergic Asthma: Effect of Viral Respiratory Infections on Pathogenesis and Clinical Course of Asthma and Allergy Ronald Rabin, MD
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
Is the Hygiene Hypothesis True?
Did Covid shutdowns stunt kids' immune systems?
Caitlin Rivers
The hygiene hypothesis is the idea that kids need to be exposed to germs in order to develop healthy immune systems. We know that many common viruses did not circulate as widely during the pandemic, thanks to social distancing, masking, and other COVID mitigation measures. Are there downsides to those missed infections?
In this Q&A, Caitlin Rivers speaks with Marsha Wills-Karp, PhD, MHS , professor and chair of Environmental Health and Engineering , about the role of household microbiomes, birth, and vaccines in the development of kids’ immune systems—and whether early exposure really is the best medicine.
This Q&A is adapted from Rivers’ Substack blog, Force of Infection .
I think there’s some concern among parents who have heard about the hygiene hypothesis that there is a downside to all those stuffy noses that didn’t happen [during the COVID-19 pandemic]. Are there any upsides to viral infections? Do they help the immune system in some meaningful way?
I don’t think so.
You mentioned the hygiene hypothesis, which was postulated back in the ‘80s. German scientists noticed that families with fewer children tended to have more allergic disease. This was interpreted [to mean] that allergic disease was linked to experiencing fewer infections. I have explored this idea in my research for a couple of decades now.
This phenomenon has helped us to understand the immune system, but our interpretation of it has grown and expanded—particularly with respect to viruses. Almost no virus is protective against allergic disease or other immune diseases. In fact, infections with viruses mostly either contribute to the development of those diseases or worsen them.
The opposite is true of bacteria. There are good bacteria and there are bad bacteria. The good bacteria we call commensals . Our bodies actually have more bacterial cells than human cells. What we’ve learned over the years is that the association with family life and the environment probably has more to do with the microbiome. So one thing I would say is sanitizing every surface in your home to an extreme is probably not a good thing. Our research team showed in animals that sterile environments don’t allow the immune system to develop at all. We don’t want that.
What does contribute to the development of the immune system, if not exposure to viruses?
There are a number of factors that we’ve associated with the hygiene hypothesis over the last 20 years, and these exposures start very early in life. Cesarean sections, which do not allow the baby to travel through the birth canal and get exposed to the mother’s really healthy bacterial content, is a risk factor for many different immune diseases. Getting that early seeding with good bacteria is critical for setting up the child going forward. Breastfeeding also contributes to the development of a healthy immune system.
There are other factors. Our diets have changed dramatically over the years. We eat a lot of processed food that doesn’t have the normal components of a healthy microbiome, like fiber. These healthy bacteria in our gut need that fiber to maintain themselves. They not only are important for our immune system but they’re absolutely critical to us deriving calories and nutrients from our food. All these things contribute to a healthy child.
We’ve also noticed that people who live on farms have fewer of these diseases because they’re exposed to—for lack of a better term—the fecal material of animals. And what we have found is that it’s due to these commensal bacteria. That is one of the components that help us keep a healthy immune system. Most of us will probably not adopt farm life. But we can have a pet, we can have a dog.
I think all the pet lovers out there will be pleased to hear that.
There’s a lot of evidence that owning a pet in early childhood is very protective.
What about the idea that you need to be exposed to viruses in early life because if you get them as an adult, you’ll get more severely ill? We know that’s true for chickenpox, for example. Do you have any concerns about that?
We should rely on vaccines for those exposures because we can never predict who is going to be susceptible to severe illness, even in early childhood. If we look back before vaccines, children under 4 often succumbed to infections. I don’t think we want to return to that time in history.
Let me just give you one example. There’s a virus called RSV, it’s a respiratory virus. Almost all infants are positive for it by the age of 2. But those who get severe disease are more likely to develop allergic disease and other problems. So this idea that we must become infected with a pathogenic virus to be healthy is not a good one.
Even rhinovirus, which is the common cold, most people recover fine. But there’s a lot of evidence that for somebody who is allergic, rhinovirus exposures make them much worse. In fact, most allergic or asthmatic kids suffer through the winter months when these viruses are more common.
And that’s particularly salient because there is a lot of rhinovirus and enterovirus circulating right now.
From my point of view, right now, avoiding flu and COVID-19 is a priority. Those are not going to help you develop a healthy immune response, and in fact, they can do a lot of damage to the lungs during that critical developmental time. Data [show] that children that have more infections in the first 6 months to a year of life go on to have more problems.
It’s always surprising to me when I look at the data of the fraction of time that young children spend with these common colds—and this is pre-pandemic—it’s not uncommon for kids to be sick 50% of the time. That feels right as a parent, but it’s startling.
The other thing people don’t know is that the GI tract is where you get tolerized to all of your foods, allergens and things. Without those healthy bacteria in your gut, you can’t tolerate common allergens.
How does that relate to the guidance that’s changed over the years—that you should withhold peanuts in early life and now you’re supposed to offer them in early life?
The guidance to delay exposure to peanuts didn’t consider the fact that oral exposure to peanuts was not the only exposure kids were getting. There were peanut oils in all kinds of skin creams and other things. So kids got exposed through their skin, but they had no gut protection—and the GI tract is important for a tolerant system. If you have a healthy immune response, you get tolerized in early life.
This concept is a little bit different for those families who may already have a predisposition to allergies. But for the general public, exposure is key to protecting them in early life.
I think some parents look at the guidance that you should now offer peanuts in early life and say, “Are we not doing that with rhinovirus by masking kids or improving ventilation?” How should people think about the development of the immune system for food allergies compared to infections?
The thing about rhinoviruses is that after recovering, you’re not protected from the next infection. There is no real immune protection there. Most of us suffer from colds throughout our whole life. Like I said, bacterial exposure is what’s key to priming the immune response.
Also, we forget that a lot of kids die from the flu. Unlike COVID-19, where younger kids are not quite as susceptible to severe illness, that’s not true for flu. RSV, too, can be quite severe in young children and older adults.
Caitlin Rivers, PhD, MPH , is a senior scholar at the Johns Hopkins Center for Health Security and an assistant professor in Environmental Health and Engineering at the Johns Hopkins Bloomberg School of Public Health.
- Study Finds That Children’s Antibody Responses to COVID-19 Are Stronger Than Adults’
- Back to School: COVID, CDC Guidance, Monkeypox, and More
- A New Shot Prevents Serious Illness from RSV
Related Content

The Hunger Gap

What to Know About COVID FLiRT Variants

Rotavirus the Leading Cause of Diarrheal Deaths Among Children Under 5, New Analysis Finds

What We Know (and Don’t) About Nicotine Pouches

To Protect Human Health, We Must Protect the Earth’s Health
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 16 October 2017
The hygiene hypothesis in autoimmunity: the role of pathogens and commensals
- Jean-François Bach 1 , 2 , 3
Nature Reviews Immunology volume 18 , pages 105–120 ( 2018 ) Cite this article
19k Accesses
303 Citations
264 Altmetric
Metrics details
- Autoimmune diseases
- Toll-like receptors
The initial application of the hygiene hypothesis for autoimmune diseases proposed in the early 2000s has been confirmed and consolidated by a wealth of published data in both animal models and human autoimmune conditions.
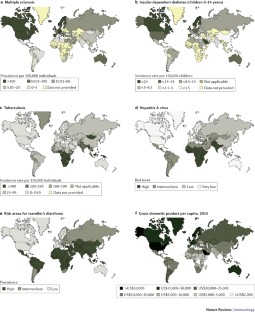
The hygiene hypothesis probably explains the uneven geographical distribution of autoimmune diseases in the world. Individuals migrating from countries with low incidence of autoimmune diseases to countries with high incidence develop the disease with the frequency of the host country, provided that migration occurred at a young age and under a threshold that varies according to the disease.
Pathogenic bacteria, viruses and parasites are often endowed with strong protective effects on autoimmunity even when infection occurs late after birth.
Gut commensal bacteria may also have a protective role in autoimmunity when administered early in life.
Pathogens, parasites and commensals essentially act by stimulating immune regulatory pathways, implicating the innate and the adaptive immune system. Importantly, the effect is seen with both living organisms and their derivatives or purified extracts.
Both pathogens and commensals stimulate pattern recognition receptors, including Toll-like receptors (TLRs) to protect against autoimmunity. This effect may be mimicked by TLR agonists acting through pharmacological stimulation or desensitization of the target receptor.
The incidence of autoimmune diseases has been steadily rising. Concomitantly, the incidence of most infectious diseases has declined. This observation gave rise to the hygiene hypothesis, which postulates that a reduction in the frequency of infections contributes directly to the increase in the frequency of autoimmune and allergic diseases. This hypothesis is supported by robust epidemiological data, but the underlying mechanisms are unclear. Pathogens are known to be important, as autoimmune disease is prevented in various experimental models by infection with different bacteria, viruses and parasites. Gut commensal bacteria also play an important role: dysbiosis of the gut flora is observed in patients with autoimmune diseases, although the causal relationship with the occurrence of autoimmune diseases has not been established. Both pathogens and commensals act by stimulating immunoregulatory pathways. Here, I discuss the importance of innate immune receptors, in particular Toll-like receptors, in mediating the protective effect of pathogens and commensals on autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Microbiota in health and diseases

A guide to vaccinology: from basic principles to new developments

Periportal macrophages protect against commensal-driven liver inflammation
Strachan, D. P. Hay fever, hygiene, and household size. BMJ 299 , 1259–1260 (1989). This is a visionary epidemiological study that paved the way for the hygiene hypothesis in atopic diseases.
CAS PubMed PubMed Central Google Scholar
Strachan, D. P. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 55 (Suppl. 1), S2–S10 (2000).
PubMed PubMed Central Google Scholar
Ege, M. J. et al. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 364 , 701–709 (2011).
CAS PubMed Google Scholar
Greenwood, B. M., Herrick, E. M. & Voller, A. Suppression of autoimmune disease in NZB and (NZB x NZW) F1 hybrid mice by infection with malaria. Nature 226 , 266–267 (1970).
Greenwood, B. M., Herrick, E. M. & Voller, A. Can parasitic infection suppress autoimmune disease? Proc. R. Soc. Med. 63 , 19–20 (1970).
Rook, G. A. & Stanford, J. L. Give us this day our daily germs. Immunol. Today 19 , 113–116 (1998).
Sewell, D. L., Reinke, E. K., Hogan, L. H., Sandor, M. & Fabry, Z. Immunoregulation of CNS autoimmunity by helminth and mycobacterial infections. Immunol. Lett. 82 , 101–110 (2002).
Oldstone, M. B. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science 239 , 500–502 (1988). This seminal study demonstrates the protective effect of a viral infection on the development of spontaneous autoimmune IDDM in NOD mice.
Oldstone, M. B., Ahmed, R. & Salvato, M. Viruses as therapeutic agents. II. Viral reassortants map prevention of insulin-dependent diabetes mellitus to the small RNA of lymphocytic choriomeningitis virus. J. Exp. Med. 171 , 2091–2100 (1990).
Bach, J. F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347 , 911–920 (2002).
PubMed Google Scholar
Bach, J. F. Protective role of infections and vaccinations on autoimmune diseases. J. Autoimmun. 16 , 347–353 (2001).
Deckers, I. A. et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS ONE 7 , e39803 (2012).
Kotz, D., Simpson, C. R. & Sheikh, A. Incidence, prevalence, and trends of general practitioner-recorded diagnosis of peanut allergy in England, 2001 to 2005. J. Allergy Clin. Immunol. 127 , 623–630.e1 (2011).
Patterson, C. C. et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia 55 , 2142–2147 (2012).
Karvonen, M., Pitkaniemi, J. & Tuomilehto, J. The onset age of type 1 diabetes in Finnish children has become younger. The Finnish Childhood Diabetes Registry Group. Diabetes Care 22 , 1066–1070 (1999).
Koch-Henriksen, N. & Sorensen, P. S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 9 , 520–532 (2010).
Mackenzie, I. S., Morant, S. V., Bloomfield, G. A., MacDonald, T. M. & O'Riordan, J. Incidence and prevalence of multiple sclerosis in the UK 1990-2010: a descriptive study in the General Practice Research Database. J. Neurol. Neurosurg. Psychiatry 85 , 76–84 (2014).
Grytten, N., Torkildsen, O. & Myhr, K. M. Time trends in the incidence and prevalence of multiple sclerosis in Norway during eight decades. Acta Neurol. Scand. 132 , 29–36 (2015).
Houzen, H. et al. Increased prevalence, incidence, and female predominance of multiple sclerosis in northern Japan. J. Neurol. Sci. 323 , 117–122 (2012).
Li, X. H. et al. A nine-year prospective study on the incidence of childhood type 1 diabetes mellitus in China. Biomed. Environ. Sci. 13 , 263–270 (2000).
Handel, A. E., Handunnetthi, L., Ebers, G. C. & Ramagopalan, S. V. Type 1 diabetes mellitus and multiple sclerosis: common etiological features. Nat. Rev. Endocrinol. 5 , 655–664 (2009).
Stewart, A. W., Mitchell, E. A., Pearce, N., Strachan, D. P. & Weiland, S. K. The relationship of per capita gross national product to the prevalence of symptoms of asthma and other atopic diseases in children (ISAAC). Int. J. Epidemiol. 30 , 173–179 (2001).
Patterson, C. C., Carson, D. J. & Hadden, D. R. Epidemiology of childhood IDDM in Northern Ireland 1989-1994: low incidence in areas with highest population density and most household crowding. Diabetologia 39 , 1063–1069 (1996).
Paalanen, L., Prattala, R., Palosuo, H., Helakorpi, S. & Laatikainen, T. Socio-economic differences in the use of dairy fat in Russian and Finnish Karelia, 1994–2004. Int. J. Publ. Health 55 , 325–337 (2010).
Google Scholar
Kondrashova, A. et al. A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann. Med. 37 , 67–72 (2005).
Laatikainen, T. et al. Allergy gap between Finnish and Russian Karelia on increase. Allergy 66 , 886–892 (2011).
Kondrashova, A. et al. Signs of beta-cell autoimmunity in nondiabetic schoolchildren: a comparison between Russian Karelia with a low incidence of type 1 diabetes and Finland with a high incidence rate. Diabetes Care 30 , 95–100 (2007).
Kuehni, C. E., Strippoli, M. P., Low, N. & Silverman, M. Asthma in young south Asian women living in the United Kingdom: the importance of early life. Clin. Exp. Allergy 37 , 47–53 (2007).
Bodansky, H. J., Staines, A., Stephenson, C., Haigh, D. & Cartwright, R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ 304 , 1020–1022 (1992).
Feltbower, R. G. et al. Trends in the incidence of childhood diabetes in south Asians and other children in Bradford. UK. Diabet. Med. 19 , 162–166 (2002).
Dean, G. & Elian, M. Age at immigration to England of Asian and Caribbean immigrants and the risk of developing multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 63 , 565–568 (1997).
Gale, C. R. & Martyn, C. N. Migrant studies in multiple sclerosis. Prog. Neurobiol. 47 , 425–448 (1995).
Kostic, A. D. et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 17 , 260–273 (2015). This is a remarkable study reporting the detailed follow-up of the gut microbiota composition in children at risk of developing IDDM from birth to the onset of hyperglycaemia.
Ball, T. M. et al. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N. Engl. J. Med. 343 , 538–543 (2000).
Cardwell, C. R. et al. Birth order and childhood type 1 diabetes risk: a pooled analysis of 31 observational studies. Int. J. Epidemiol. 40 , 363–374 (2011).
Almeida, M. C. et al. The effect of antihelminthic treatment on subjects with asthma from an endemic area of schistosomiasis: a randomized, double-blinded, and placebo-controlled trial. J. Parasitol. Res. 2012 , 296856 (2012).
Fleming, J. O. et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult. Scler. 17 , 743–754 (2011).
Larson, J. D. et al. Murine gammaherpesvirus 68 infection protects lupus-prone mice from the development of autoimmunity. Proc. Natl Acad. Sci. USA 109 , E1092–E1100 (2012).
Alyanakian, M. A. et al. Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes 55 , 179–185 (2006).
Finlay, C. M., Walsh, K. P. & Mills, K. H. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol. Rev. 259 , 206–230 (2014).
Gause, W. C. & Maizels, R. M. Macrobiota -— helminths as active participants and partners of the microbiota in host intestinal homeostasis. Curr. Opin. Microbiol. 32 , 14–18 (2016). This study reports that the helminth H. polygyrus produces a TGFβ mimic that fully reproduces the effect of this immunoregulatory cytokine on the host immune system.
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504 , 451–455 (2013).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107 , 14691–14696 (2010).
Lin, A. et al. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS ONE 8 , e53838 (2013).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 , 559–563 (2014).
Schmidt, B. et al. Establishment of normal gut microbiota is compromised under excessive hygiene conditions. PLoS ONE 6 , e28284 (2011). This study provides direct confirmation of the role of hygiene on gut microbiota composition in an original experimental model using piglets reared in conventional or clean conditions.
Vatanen, T. et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165 , 842–853 (2016). This is a comparative study of the gut microbiota composition in individuals from Finland and Karelia, two neighbouring countries with a substantial difference in the incidence of IDDM. The study demonstrates structural differences in LPS produced by 'non-protective' versus 'protective' commensals in the Finnish and the Karelian microbiota, respectively.
Alam, C. et al. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia 54 , 1398–1406 (2011).
Candon, S. et al. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE 10 , e0125448 (2015).
Yurkovetskiy, L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39 , 400–412 (2013).
Brown, K. et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 10 , 321–332 (2016).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479 , 538–541 (2011).
Lee, Y. K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4615–4622 (2011).
Goverman, J. et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell 72 , 551–560 (1993).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139 , 485–498 (2009).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31 , 677–689 (2009).
Barclay, W. & Shinohara, M. L. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Brain Pathol. 27 , 213–219 (2017).
Dumas, A. et al. The inflammasome pyrin contributes to pertussis toxin-induced IL-1β synthesis, neutrophil intravascular crawling and autoimmune encephalomyelitis. PLoS Pathog. 10 , e1004150 (2014).
Aumeunier, A. et al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS ONE 5 , e11484 (2010). This is the first systematic comparative study of the protective effect of different TLR agonists on autoimmunity and experimental asthma, which shows that distinct mechanisms underlie the therapeutic activity, depending on the TLR agonist used.
Calcinaro, F. et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia 48 , 1565–1575 (2005).
Falcone, M. et al. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei . J. Diabetes Res. 105 , 643–649 (1997).
Lavasani, S. et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 5 , e9009 (2010).
Markle, J. G. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339 , 1084–1088 (2013).
Wen, L. et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455 , 1109–1113 (2008).
Peng, J. et al. Long term effect of gut microbiota transfer on diabetes development. J. Autoimmun 53 , 85–94 (2014).
Cardwell, C. R. et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia 51 , 726–735 (2008).
Clausen, T. D. et al. Prelabor cesarean section and risk of childhood type 1 diabetes: a nationwide register-based cohort study. Epidemiology 27 , 547–555 (2016).
Maghzi, A. H. et al. Cesarean delivery may increase the risk of multiple sclerosis. Mult. Scler. 18 , 468–471 (2012).
Knights, D. et al. Use of antibiotics in childhood and risk of Type 1 diabetes: a population-based case-control study. Nat. Microbiol. 34 , 272–277 (2017).
Boursi, B., Mamtani, R., Haynes, K. & Yang, Y. X. The effect of past antibiotic exposure on diabetes risk. Eur. J. Endocrinol. 172 , 639–648 (2015).
Clausen, T. D. et al. Broad-spectrum antibiotic treatment and subsequent childhood type 1 diabetes: a nationwide Danish cohort study. PLoS ONE 11 , e0161654 (2016).
Hviid, A. & Svanstrom, H. Antibiotic use and type 1 diabetes in childhood. Am. J. Epidemiol. 169 , 1079–1084 (2009).
Livanos, A. E. et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol. 1 , 16140 (2016).
Alonso, A., Jick, S. S., Jick, H. & Hernan, M. A. Antibiotic use and risk of multiple sclerosis. Am. J. Epidemiol. 163 , 997–1002 (2006).
Ljungberg, M., Korpela, R., Ilonen, J., Ludvigsson, J. & Vaarala, O. Probiotics for the prevention of beta cell autoimmunity in children at genetic risk of type 1 diabetes — the PRODIA study. Ann. NY Acad. Sci. 1079 , 360–364 (2006).
Uusitalo, U. et al. Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr. 170 , 20–28 (2016).
Pelucchi, C. et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology 23 , 402–414 (2012).
Liacopoulos, P. & Ben-Efraim, S. Antigenic competition. Prog. Allergy 18 , 97–204 (1975).
Pross, H. F. & Eidinger, D. Antigenic competition: a review of nonspecific antigen-induced suppression. Adv. Immunol. 18 , 133–168 (1974).
Buus, S., Sette, A., Colon, S. M., Miles, C. & Grey, H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science 235 , 1353–1358 (1987).
Guillet, J. G. et al. Immunological self, nonself discrimination. Science 235 , 865–870 (1987).
Almeida, A. R., Rocha, B., Freitas, A. A. & Tanchot, C. Homeostasis of T cell numbers: from thymus production to peripheral compartmentalization and the indexation of regulatory T cells. Semin. Immunol. 17 , 239–249 (2005).
Surh, C. D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29 , 848–862 (2008).
Qin, H. Y., Sadelain, M. W., Hitchon, C., Lauzon, J. & Singh, B. Complete Freund's adjuvant-induced T cells prevent the development and adoptive transfer of diabetes in nonobese diabetic mice. J. Immunol. 150 , 2072–2080 (1993).
Qin, H. Y. & Singh, B. BCG vaccination prevents insulin-dependent diabetes mellitus (IDDM) in NOD mice after disease acceleration with cyclophosphamide. J. Autoimmun. 10 , 271–278 (1997).
Lee, I. F., Qin, H., Trudeau, J., Dutz, J. & Tan, R. Regulation of autoimmune diabetes by complete Freund's adjuvant is mediated by NK cells. J. Immunol. 172 , 937–942 (2004).
Tian, B. et al. Upregulating CD4 + CD25 + FOXP3 + regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy. Transplantation 87 , 198–206 (2009).
Serreze, D. V. et al. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J. Immunol. 166 , 1352–1359 (2001).
Mori, Y., Kodaka, T., Kato, T., Kanagawa, E. M. & Kanagawa, O. Critical role of IFN-γ in CFA-mediated protection of NOD mice from diabetes development. Int. Immunol. 21 , 1291–1299 (2009).
Salomon, B. et al. B7/CD28 costimulation is essential for the homeostasis of the CD4 + CD25 + immunoregulatory T cells that control autoimmune diabetes. Immunity 12 , 431–440 (2000).
Caramalho, I. et al. Regulatory T cells contribute to diabetes protection in lipopolysaccharide-treated non-obese diabetic mice. Scand. J. Immunol. 74 , 585–595 (2011).
Tian, J. et al. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J. Immunol. 167 , 1081–1089 (2001).
Fillatreau, S., Sweenie, C. H., McGeachy, M. J., Gray, D. & Anderton, S. M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3 , 944–950 (2002).
Mauri, C., Gray, D., Mushtaq, N. & Londei, M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 197 , 489–501 (2003).
Mizoguchi, A., Mizoguchi, E., Takedatsu, H., Blumberg, R. S. & Bhan, A. K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16 , 219–230 (2002).
Shen, P. & Fillatreau, S. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 15 , 441–451 (2015).
Shen, P. et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507 , 366–370 (2014).
Shen, P. & Fillatreau, S. Suppressive functions of B cells in infectious diseases. Int. Immunol. 27 , 513–519 (2015).
Filippi, C. M., Estes, E. A., Oldham, J. E. & von Herrath, M. G. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J. Clin. Invest. 119 , 1515–1523 (2009).
Cooke, A. et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 21 , 169–176 (1999).
Zaccone, P. et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol. 33 , 1439–1449 (2003).
Grainger, J. R. et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 207 , 2331–2341 (2010).
Ince, M. N. et al. Role of T cell TGF-β signaling in intestinal cytokine responses and helminthic immune modulation. Eur. J. Immunol. 39 , 1870–1878 (2009).
Liu, Q. et al. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect. Immun. 77 , 5347–5358 (2009).
Walk, S. T., Blum, A. M., Ewing, S. A., Weinstock, J. V. & Young, V. B. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus . Inflamm. Bowel Dis. 16 , 1841–1849 (2010).
Ramanan, D. et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 352 , 608–612 (2016).
Hubner, M. P., Stocker, J. T. & Mitre, E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3 + regulatory T cells. Immunology 127 , 512–522 (2009).
Hubner, M. P. et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β. J. Immunol. 188 , 559–568 (2012).
Lund, M. E. et al. Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS ONE 9 , e86289 (2014).
Finlay, C. M. et al. Helminth products protect against autoimmunity via innate type 2 cytokines IL-5 and IL-33, which promote eosinophilia. J. Immunol. 196 , 703–714 (2016).
Cording, S., Medvedovic, J., Aychek, T. & Eberl, G. Innate lymphoid cells in defense, immunopathology and immunotherapy. Nat. Immunol. 17 , 755–757 (2016).
Dolpady, J. et al. Oral probiotic VSL#3 prevents autoimmune diabetes by modulating microbiota and promoting indoleamine 2,3-dioxygenase-enriched tolerogenic intestinal environment. J. Diabetes Res. 2016 , 7569431 (2016).
Kobayashi, T. et al. Probiotic upregulation of peripheral IL-17 responses does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis mouse models. Immunopharmacol. Immunotoxicol. 34 , 423–433 (2012).
Ochoa-Reparaz, J. et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183 , 6041–6050 (2009).
Ochoa-Reparaz, J. et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3 , 487–495 (2010). This is an important study describing a constituent of the commensal organism B. fragilis (that is, PSA) with remarkable protective activity in models of EAE and colitis.
Mazmanian, S. K., Round, J. L. & Kasper, D. L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453 , 620–625 (2008).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3 + regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107 , 12204–12209 (2010).
Chinen, T., Volchkov, P. Y., Chervonsky, A. V. & Rudensky, A. Y. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J. Exp. Med. 207 , 2323–2330 (2010).
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4 , 337–349 (2008).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331 , 337–341 (2011).
Nagano, Y., Itoh, K. & Honda, K. The induction of Treg cells by gut-indigenous Clostridium . Curr. Opin. Immunol. 24 , 392–397 (2012).
Gagliani, N. et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19 , 739–746 (2013).
Apetoh, L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Brain Pathol. 11 , 854–861 (2010).
CAS Google Scholar
Weiner, H. L., da Cunha, A. P., Quintana, F. & Wu, H. Oral tolerance. Immunol. Rev. 241 , 241–259 (2011).
Pistoia, V. & Raffaghello, L. Mesenchymal stromal cells and autoimmunity. Int. Immunol. 29 , 49–58 (2017).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8 , 726–736 (2008).
Sica, A. & Massarotti, M. Myeloid suppressor cells in cancer and autoimmunity. J. Autoimmun. http://dx.doi.org/10.1016/j.jaut.2017.07.010 (2017).
Karumuthil-Melethil, S., Perez, N., Li, R. & Vasu, C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J. Immunol. 181 , 8323–8334 (2008).
Karumuthil-Melethil, S. et al. TLR2- and Dectin 1-associated innate immune response modulates T-cell response to pancreatic β-cell antigen and prevents type 1 diabetes. Diabetes 64 , 1341–1357 (2015).
Serreze, D. V., Hamaguchi, K. & Leiter, E. H. Immunostimulation circumvents diabetes in NOD/Lt mice. J. Autoimmun. 2 , 759–776 (1989).
Quintana, F. J., Rotem, A., Carmi, P. & Cohen, I. R. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: modulation of spontaneous 60-kDa heat shock protein autoimmunity. J. Immunol. 165 , 6148–6155 (2000).
Round, J. L. et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332 , 974–977 (2011).
Wang, Y. et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat. Commun. 5 , 4432 (2014).
Filippi, C. M. et al. TLR2 signaling improves immunoregulation to prevent type 1 diabetes. Eur. J. Immunol. 41 , 1399–1409 (2011).
Xiong, Y. et al. Endotoxin tolerance inhibits Lyn and c-Src phosphorylation and association with Toll-like receptor 4 but increases expression and activity of protein phosphatases. J. Innate Immun. 8 , 171–184 (2016).
Freudenberg, M. A. & Galanos, C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect. Immun. 56 , 1352–1357 (1988).
Medvedev, A. E., Kopydlowski, K. M. & Vogel, S. N. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and Toll-like receptor 2 and 4 gene expression. J. Immunol. 164 , 5564–5574 (2000). This is a comprehensive work on changes in intracellular signalling in macrophages that lead to LPS (endotoxin) tolerance, which highlights the fundamental role of the activation of various phosphatases.
Kim, D. H. et al. Inhibition of autoimmune diabetes by TLR2 tolerance. J. Immunol. 187 , 5211–5220 (2011).
Anstadt, E. J., Fujiwara, M., Wasko, N., Nichols, F. & Clark, R. B. TLR tolerance as a treatment for central nervous system autoimmunity. J. Immunol. 197 , 2110–2118 (2016). This is an interesting report demonstrating that low doses of two different TLR2 ligands attenuate adoptively transferred EAE through receptor desensitization. One of these TLR2 ligands is a human microbiome product, which has significantly decreased serum levels in patients with multiple sclerosis compared with unaffected controls.
Hayashi, T. et al. Treatment of autoimmune inflammation by a TLR7 ligand regulating the innate immune system. PLoS ONE 7 , e45860 (2012).
Schuijs, M. J. et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 349 , 1106–1110 (2015). This is a study highlighting the potential in vivo relevance of TLR desensitization by LPS in humans. The novel finding is that the LPS-induced TLR4 desensitization targets the lung epithelium and requires the ubiquitin-modifying enzyme A20.
Siebeneicher, S. et al. Epicutaneous immune modulation with Bet v 1 plus R848 suppresses allergic asthma in a murine model. Allergy 69 , 328–337 (2014).
Aryan, Z. & Rezaei, N. Toll-like receptors as targets for allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 15 , 568–574 (2015).
Burrows, M. P., Volchkov, P., Kobayashi, K. S. & Chervonsky, A. V. Microbiota regulates type 1 diabetes through Toll-like receptors. Proc. Natl Acad. Sci. USA 112 , 9973–9977 (2015). This is a unique study that uses a genetic approach and proposes a distinct role for single TLRs in their capacity to modulate autoimmunity.
Bras, A. & Aguas, A. P. Diabetes-prone NOD mice are resistant to Mycobacterium avium and the infection prevents autoimmune disease. Immunology 89 , 20–25 (1996).
Lee, J., Reinke, E. K., Zozulya, A. L., Sandor, M. & Fabry, Z. Mycobacterium bovis bacille Calmette-Guerin infection in the CNS suppresses experimental autoimmune encephalomyelitis and Th17 responses in an IFN-γ-independent manner. J. Immunol. 181 , 6201–6212 (2008).
Newland, S. A. et al. PD-L1 blockade overrides Salmonella typhimurium -mediated diabetes prevention in NOD mice: no role for Tregs. Eur. J. Immunol. 41 , 2966–2976 (2011).
Drescher, K. M., Kono, K., Bopegamage, S., Carson, S. D. & Tracy, S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329 , 381–394 (2004).
Tracy, S. et al. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 76 , 12097–12111 (2002).
Davydova, B. et al. Coxsackievirus immunization delays onset of diabetes in non-obese diabetic mice. J. Med. Virol. 69 , 510–520 (2003).
Richer, M. J., Straka, N., Fang, D., Shanina, I. & Horwitz, M. S. Regulatory T-cells protect from type 1 diabetes after induction by coxsackievirus infection in the context of transforming growth factor-β. Diabetes 57 , 1302–1311 (2008).
Hermitte, L. et al. Paradoxical lessening of autoimmune processes in non-obese diabetic mice after infection with the diabetogenic variant of encephalomyocarditis virus. Eur. J. Immunol. 20 , 1297–1303 (1990).
Takei, I. et al. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J. Autoimmun. 5 , 665–673 (1992).
Wilberz, S., Partke, H. J., Dagnaes-Hansen, F. & Herberg, L. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia 34 , 2–5 (1991).
Smith, K. A., Efstathiou, S. & Cooke, A. Murine gammaherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J. Immunol. 179 , 7325–7333 (2007).
Mishra, P. K., Patel, N., Wu, W., Bleich, D. & Gause, W. C. Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol. 6 , 297–308 (2013).
Saunders, K. A., Raine, T., Cooke, A. & Lawrence, C. E. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 75 , 397–407 (2007).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16 , 407–420 (2016).
Inoue, M., Williams, K. L., Gunn, M. D. & Shinohara, M. L. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 109 , 10480–10485 (2012).
Inoue, M. et al. Interferon-β therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci. Signal. 5 , ra38 (2012).
Cuda, C. M., Pope, R. M. & Perlman, H. The inflammatory role of phagocyte apoptotic pathways in rheumatic diseases. Nat. Rev. Rheumatol. 12 , 543–558 (2016).
de Souza, H. S. & Fiocchi, C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13 , 13–27 (2016).
von Moltke, J., Ayres, J. S., Kofoed, E. M., Chavarria-Smith, J. & Vance, R. E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 31 , 73–106 (2013).
Rzepecka, J. et al. Prophylactic and therapeutic treatment with a synthetic analogue of a parasitic worm product prevents experimental arthritis and inhibits IL-1beta production via NRF2-mediated counter-regulation of the inflammasome. J. Autoimmun. 60 , 59–73 (2015).
Patterson, C. et al. Diabetes in the young — a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res. Clin. Pract. 103 , 161–175 (2013).
Giongo, A. et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5 , 82–91 (2011).
Brown, C. T. et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 6 , e25792 (2011).
de Goffau, M. C. et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes 62 , 1238–1244 (2013).
Murri, M. et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 11 , 46 (2013).
Soyucen, E. et al. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr. Int. 56 , 336–343 (2014).
Davis-Richardson, A. G. et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 5 , 678 (2014).
Endesfelder, D. et al. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes 63 , 2006–2014 (2014).
Mejia-Leon, M. E., Petrosino, J. F., Ajami, N. J., Dominguez-Bello, M. G. & de la Barca, A. M. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 4 , 3814 (2014).
Alkanani, A. K. et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 64 , 3510–3520 (2015).
Qi, C. J. et al. Imbalance of fecal microbiota at newly diagnosed type 1 diabetes in Chinese children. Chin. Med. J. 129 , 1298–1304 (2016).
Maffeis, C. et al. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. Diabetes Metab. Res. Rev. 32 , 700–709 (2016).
Stewart, C. J. et al. Gut microbiota of Type 1 diabetes patients with good glycaemic control and high physical fitness is similar to people without diabetes: an observational study. Diabet Med. 34 , 127–134 (2017).
Miyake, S. et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS ONE 10 , e0137429 (2015).
Tremlett, H. et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur. J. Neurol. 23 , 1308–1321 (2016).
Chen, J. et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6 , 28484 (2016).
Hevia, A. et al. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio 5 , e01548–e01514 (2014).
Wilson, C. S., Elizer, S. K., Marshall, A. F., Stocks, B. T. & Moore, D. J. Regulation of B lymphocyte responses to Toll-like receptor ligand binding during diabetes prevention in non-obese diabetic (NOD) mice. J. Diabetes 8 , 120–131 (2016).
Zhang, Y. et al. TLR9 blockade inhibits activation of diabetogenic CD8 + T cells and delays autoimmune diabetes. J. Immunol. 184 , 5645–5653 (2010).
Manicassamy, S. et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat. Med. 15 , 401–409 (2009).
Manoharan, I. et al. TLR2-dependent activation of beta-catenin pathway in dendritic cells induces regulatory responses and attenuates autoimmune inflammation. J. Immunol. 193 , 4203–4213 (2014).
Li, H. et al. Low dose zymosan ameliorates both chronic and relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 254 , 28–38 (2013).
Dillon, S. et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116 , 916–928 (2006).
Barrat, F. J. & Coffman, R. L. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol. Rev. 223 , 271–283 (2008).
Barrat, F. J., Meeker, T., Chan, J. H., Guiducci, C. & Coffman, R. L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 37 , 3582–3586 (2007).
Wong, F. S. et al. The role of Toll-like receptors 3 and 9 in the development of autoimmune diabetes in NOD mice. Ann. NY Acad. Sci. 1150 , 146–148 (2008).
Gulden, E. et al. Toll-like receptor 4 deficiency accelerates the development of insulin-deficient diabetes in non-obese diabetic mice. PLoS ONE 8 , e75385 (2013).
Prinz, M. et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Invest. 116 , 456–464 (2006).
Cohen, S. J., Cohen, I. R. & Nussbaum, G. IL-10 mediates resistance to adoptive transfer experimental autoimmune encephalomyelitis in MyD88 −/− mice. J. Immunol. 184 , 212–221 (2010).
Marta, M., Andersson, A., Isaksson, M., Kampe, O. & Lobell, A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 38 , 565–575 (2008).
Miranda-Hernandez, S. et al. Role for MyD88, TLR2 and TLR9 but not TLR1, TLR4 or TLR6 in experimental autoimmune encephalomyelitis. J. Immunol. 187 , 791–804 (2011). This is a comprehensive study that examines the impact of TLR invalidation in the development of EAE.
Reynolds, J. M. et al. Toll-like receptor 2 signaling in CD4 + T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity 32 , 692–702 (2010).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25 , 417–428 (2006).
Atlas of MS 2013. https://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf
Global tuberculosis report 2016. http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1
Screening for hepatitis during the domestic medical examination for newly arrived refugees. https://www.cdc.gov/immigrantrefugeehealth/pdf/domestic-hepatitis-screening-guidelines.pdf
Caisse des Français de l'Étranger. https://www.cfe.fr/pages/votre-sante/guidespatho.php?id=126 [French]
International Monetary Fund. World Economic Outlook Database. https://www.imf.org/external/pubs/ft/weo/2015/01/weodata/index.aspx
Download references
Acknowledgements
The laboratory of the author was supported by an advanced grant from the European Research Council (ERC, Hygiene N°: 250290).
Author information
Authors and affiliations.
Université Paris Descartes, Sorbonne Paris Cité, Paris, France
Jean-François Bach
INSERM U1151, Institut Necker-Enfants Malades, Hôpital Necker-Enfants Malades, Paris, France
CNRS UMR 8253, Institut Necker-Enfants Malades, Hôpital Necker-Enfants Malades, Paris, France
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jean-François Bach .
Ethics declarations
Competing interests.
The author declares no competing financial interests.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for table 1, powerpoint slide for table 2, powerpoint slide for table 3.
A genetic predisposition to the cumulative development of common allergies, for example, atopic dermatitis and allergic asthma. Atopy involves phenomena of cutaneous or general hypersensitivity to allergens.
A hypothesis that postulates that an increased frequency of infections contributes to a decrease in autoimmune and allergic diseases.
An inbred mouse line that spontaneously develops an autoimmune syndrome including insulin-dependent diabetes mellitus (IDDM or type 1 diabetes).
A digestive tract disorder provoked by eating contaminated food or drinking contaminated water. In the context of our discussion, it is a self-limited pathology that illustrates the presence of a basic health environment.
Autoantibodies to various β-cell-specific autoantigens that are markers of the destruction of insulin-producing β-cells, which is the hallmark of insulin-dependent diabetes mellitus (IDDM or type 1 diabetes).
An imbalance of the microbial flora that most frequently affects the digestive tract. Dysbiosis can also be detected in other 'barrier' organs such as the skin, the lungs or the vagina.
The metabolome consists of all signalling molecules (for example, metabolites and hormones) detected in a biological sample. The metabolome thus defines a given physiological or pathological state and is therefore dynamic.
Mice born by hysterectomy under sterile conditions and raised in isolators to guarantee an environment totally devoid of pathogenic and commensal germs.
(EAE). A demyelinating allergic encephalomyelitis produced by the injection of brain tissue or purified proteins of the nervous system or their derived peptides in the presence of an adjuvant.
Germ-free mice whose intestinal microflora is reconstituted by a single commensal bacterium (monocolonized mice).
Gut commensal bacteria available as single or combined species delivered orally and putatively endowed with a health benefit.
The competition for recognition of the cognate antigen for soluble factors (cytokines) driving the proliferation and differentiation of antigen-specific lymphocytes.
Islet transplants between syngeneic (genetically identical) donor and recipient individuals, which therefore does not give rise to allograft rejection. These grafts performed in diabetic non-obese diabetic mice provide a robust model to test for recurrence of the autoimmune disease.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Bach, JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 18 , 105–120 (2018). https://doi.org/10.1038/nri.2017.111
Download citation
Published : 16 October 2017
Issue Date : February 2018
DOI : https://doi.org/10.1038/nri.2017.111
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Are We Over-Sanitizing? The Hygiene Hypothesis
How modern norms of cleanliness may be stunting immune system development..
Posted August 27, 2021 | Reviewed by Tyler Woods
- The hygiene hypothesis was first advanced more than 30 years ago and has been altered and refined since then.
- Children who grow up in sterile environments may have deficits in their immune systems.
- The significance of gut bacteria on mental and physical well-being is still being intensely studied.
While many new parents believe that it’s best to keep their children in a pristine, clean environment, some research suggests that being exposed to a variety of microorganisms early in life is good for a child’s immune system. This research indicates that children who are raised in very sterile environments are more likely to develop hay fever, asthma, and certain food allergies. The has been dubbed the " hygiene hypothesis ."
The hygiene hypothesis was first proposed more than 30 years ago by David P. Strachan, a professor of epidemiology, in the British Medical Journal. Since then, it has been challenged by new scientific developments, adapted, expanded, and fine-tuned. Among the changes, additional research has found that some stress -related psychiatric disorders, such as depression and anxiety , may also be on the rise in developed countries, like the U.S., perhaps in part, due to a changing microbial environment.
Although it may seem strange in the age of Covid-19 to suggest that not using antibacterial soap and hand sanitizer is actually better for your health, evidence suggests that changes to the microbial environment brought on by such products can disrupt our immuno-regulatory circuitry and lead to ongoing inflammation in the body, which can impact our health in ways we’re only now discovering.
The Developing Immune System
The hygiene hypothesis is a very rich and complex argument that can be broken down into simple terms. When a fetus is inside the womb, it has a relatively weak immune system because it is protected by the mother’s antibodies. Once the baby is born, the immune system must start working for itself and it takes time to develop to full capacity. The hygiene hypothesis suggests that this development requires being exposed to the “right” germs at the right time.
If an infant’s immune system is not exposed to certain contaminants found in everyday life, the infant may have an underdeveloped immune system. The child would then have a harder time fighting off infections later in life when it inevitably comes into contact with these contaminants. For example, one study found that exposure to germs triggered an internal inflammatory response in children raised in cleaner environments, leading to ailments such as asthma and auto-immune conditions (e.g., inflammatory bowel disease, type 1 diabetes).
So where do psychiatric disorders come into the picture? Just as incidents of allergies, asthma, and auto-immune conditions are on the rise around the world, especially in developed countries, so too are incidents of major depressive disorder. The rate of increase is too rapid to be attributed to genetic changes.
One possible explanation for the increase in both physical and mental conditions is that our obsession with cleanliness in modern society has disrupted our immune systems’ ability to shut down inflammation in the body. The loss of this ability, according to the hypothesis, has led to an increase of both autoimmune and allergic diseases and also plausibly contributes to an increase in the incidence of mood disorders because of the link between these conditions and bodily inflammation.
Further, patients suffering from affective and anxiety disorders present with features that mirror inflammatory conditions such as:
- Pro-inflammatory cytokines (proteins secreted by cells to communicate with other cells) in the blood and central nervous system (CNS).
- Elevated levels of circulating C-reactive protein (CRP) (an indication of inflammation in the body)
- Activation of lymphocytes (a type of white blood cell that fights infection and the main type found in lymph)
- Inflammatory cellular signaling pathways.

The Hygiene Hypothesis and Gut Microbiota
In a previous article , I outlined the gut-brain connection and discussed how the treatment of gastrointestinal issues may also improve mood disorder symptoms. Combining gut microbiota studies with the above research into the hygiene hypothesis directs clinicians to new areas of research. The hygiene hypothesis itself touches on immunological, microbiological, and evolutionary science. Thus, examining the conclusions requires a holistic approach explaining the broader impact of our modern lifestyle on human beings.

For individuals who are genetically susceptible to developing depression and anxiety, for example, it’s possible that disruption to the microbiota or the lack of exposure to vital microorganisms in infancy may contribute to symptoms of depression later in life. Although most scientists agree that more research needs to be done into both the hygiene hypothesis and gut microbiota, a future where clinicians can use microbiota profiling as a diagnostic tool for psychiatric patients seems to be just around the corner.
So the next time you worry about your toddler eating something off of the floor, let the hygiene hypothesis offer you some comfort.
Garn, H., Potaczek, D. P., & Pfefferle, P. I. (2021, March 18). The Hygiene Hypothesis and New Perspectives—Current Challenges Meeting an Old Postulate. Frontiers In Immunology. https://doi.org/10.3389/fimmu.2021.637087
Rook, G. A., & W., Lowry, C. (2008, April). The Hygiene Hypothesis and Psychiatric Disorders. Trends in Immunology. 29(4), 150-8. https://doi.org/10.1016/j.it.2008.01.002
Sehrawat, S., & Rouse, B. T., (2020, October). Does the Hygiene Hypothesis Apply to COVID-19 Susceptibility? Microbes and Infection, 22(9), 400–402. https://doi.org/10.1016/j.micinf.2020.07.002
Yazdanbakhsh, M., Kremsner, P. G., & van Ree, R. (2002, April 19). Allergy, Parasites, and the Hygiene Hypothesis. Science, 296(5567), 490-494. Retrieved from: https://science.sciencemag.org/content/296/5567/490.abstract

Deana Goldin, Ph.D., DNP, APRN, is an associate clinical professor at Florida International University and an integrative psychiatric and family nurse practitioner.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wiley Open Access Collection

Too clean, or not too clean: the Hygiene Hypothesis and home hygiene
Sf bloomfield.
* London School of Hygiene and Tropical Medicine, London, UK
R Stanwell-Smith
† Royal Institute of Public Health, London, UK
‡ Safety and Environmental Assurance Centre, Unilever, Bedford, UK
§ Consultant in Scientific Issues, Bridgnorth, Shropshire, UK
The ‘hygiene hypothesis’ as originally formulated by Strachan, proposes that a cause of the recent rapid rise in atopic disorders could be a lower incidence of infection in early childhood, transmitted by unhygienic contact with older siblings. Use of the term ‘hygiene hypothesis’ has led to several interpretations, some of which are not supported by a broader survey of the evidence. The increase in allergic disorders does not correlate with the decrease in infection with pathogenic organisms, nor can it be explained by changes in domestic hygiene. A consensus is beginning to develop round the view that more fundamental changes in lifestyle have led to decreased exposure to certain microbial or other species, such as helminths, that are important for the development of immunoregulatory mechanisms. Although this review concludes that the relationship of the hypothesis to hygiene practice is not proven, it lends strong support to initiatives seeking to improve hygiene practice. It would however be helpful if the hypothesis were renamed, e.g. as the ‘microbial exposure’ hypothesis, or ‘microbial deprivation’ hypothesis, as proposed for instance by Bjorksten. Avoiding the term ‘hygiene’ would help focus attention on determining the true impact of microbes on atopic diseases, while minimizing risks of discouraging good hygiene practice.
Introduction
When a disease, or group of diseases, rises rapidly without a specific explanation, it stimulates investigation to identify the cause, so that preventive measures can be devised. An example of this is the rise in the incidence and prevalence of atopic disorders that has occurred over the last 30–40 years.
Although genetic predisposition is a fundamental factor governing susceptibility to atopic disease, the rise in atopy has occurred within too short a time frame to be explained by a genetic shift in the population, thus pointing to environmental or lifestyle changes. Although some increase in exposure to allergens to sensitize and then trigger attacks has been observed, such as increased exposure to house dust mites in modern, centrally heated houses, these changes do not seem adequately to explain the rise. This has led to the search for other factors to explain increased phenotypic expression of atopic disease in genetically predisposed individuals.
The ‘hygiene hypothesis’ was first formulated in 1989 by an epidemiologist, Dr Strachan [ 1 ] who reported an inverse relationship between family size and development of atopic disorders, and proposed that a lower incidence of infection in early childhood, transmitted by unhygienic contact with older siblings or acquired pre-natally could be a cause of the rise in allergic diseases. Subsequently, as the concept was further explored by specialists in allergy and immunology, it evolved into the broader notion that declining microbial exposure is a major causative factor in the increasing incidence of atopy in recent years. A wide range of factors which might have resulted in altered microbial exposure have been examined such as clean water and food, sanitation, antibiotics and vaccines, birth practices, as well as incidental factors such as the move from farm to urban living.
Most recently, a further aspect of Strachan's hypothesis has received considerable attention, particularly from the media, namely his proposition that the reason why this key exposure no longer occurs, or occurs to an insufficient extent, is the trend not only towards smaller family sizes but also ‘improved household amenities and higher standards of personal cleanliness’– in effect, cleaner homes.
Until recently, discussion of the hypothesis has been relatively compartmentalized, with most research focusing on the nature of the possible link between atopy and microbial exposure. Research into other factors that could explain the atopy trends, such as changes in diet and other shifts in lifestyle have continued in parallel. It is only recently that infectious disease (ID) specialists have entered the debate, concerned that publicizing the idea that we might be ‘too clean’ could have a detrimental impact on the public's perception of ID risks in the home and elsewhere, and of the importance of controlling such risks.
In response to these latter concerns the International Scientific Forum on Home Hygiene ( www.ifh-homehygiene.org ) commissioned a review of the hypothesis, in order to consider the implications it might have for hygiene, particularly hygiene in the domestic setting. It sought to do this by addressing two distinct questions:
- How clear is the evidence of a causal link between a decline in microbial exposure and the recent rises in atopic disease?
- To what extent might cleaning and hygiene, as distinct from other influences on microbial exposure, be a significant factor?
This review is a summary of the main findings from this report together with more recently published data [ 2 ].
The rise in atopic disease
The rapid increase in allergic asthma and other atopic disorders in the industrialized world is usually considered to have started between 1960 and 1970 with progressive rises during the 1980s and ‘90 s, although Isolauri suggests that there has been a steady rise in sensitization to aeroallergens since the 1920s [ 3 ]. Asthma prevalence, for example, increased by about 1% a year from around 1980 [ 4 ]. Among children 5–18 years of age, the increase has predominantly been among allergic individuals [ 5 ] and recent UK studies confirm that atopic, rather than non-atopic, asthma is responsible for much of the rise [ 6 , 7 ]. A comparison of two British birth cohorts (1958 and 1970) showed increases from 3.1% to 6.4% for eczema and from 12% to 23.3% for hayfever at age 16 years.
Both incidence and prevalence of atopy generally remain much lower in many, but not all the developing regions of the world. The International Study of Asthma and Allergies in Childhood (ISAAC) showed that the prevalence of self-reported asthma ranged from 2–3% in developing countries to 20–40% of the responding population of 13–14-year olds in industrialized countries, depending on the questionnaire used (written or video) [ 8 ]. The prevalence of other atopic disorders varied over a similarly wide range, with countries showing similar, but not identical rankings. Variations also appear between areas within countries.
The reunification of Germany provided some new insights into the influence of lifestyle on atopic disease within relatively homogeneous populations. Von Mutius et al. [ 9 ] showed that hayfever and atopic sensitization among children in the former East Germany both significantly increased between 1991–1992 and 1995–1996, raising the issue of ‘Western living’ influences on children, as previous studies had shown lower rates in East Germany compared with West Germany.
Some recent data suggests that the rise in incidence of atopic disease may be halting. A UK survey published in 2000 suggests that the number of new GP consultations for asthma is now falling, with declining reported episodes of asthma and acute bronchitis since 1993 [ 10 ]. Similarly, the third in a series of cross-sectional studies in Italy [ 11 ] showed prevalence to be stable among children born after 1985, in contrast to a threefold rise of prevalence between 1974 and 1992. The authors concluded that ‘the progressive induction of asthma symptoms in genetically predisposed subjects has probably come to an end’. Most recently, Z ö llner et al. [ 12 ] studying trends among children in SW Germany, have suggested the allergy epidemic may have reached a plateau.
Evidence for and against a relationship between atopy and reduced microbial exposure
The proposition that reduced infection and/or changed microbial exposure may have driven the rapid rise in atopic disease requires evaluation on several levels. These include biological plausibility, the strength of the epidemiological data, the temporal correlation between trends in atopy and trends in ID, prevention measures and hygiene practice. Biological plausibility is one of the greatest strengths of the hygiene hypothesis. The hypothesis generally fits with current knowledge about maturation and disorders of immune system e.g. dysregulation of T helper type 1 (Th1), Th2 and regulatory T cell pathways, although the immunological models are constantly being revised and refined. It is not the aim of this paper to assess current immunological knowledge and closeness of fit with the hypothesis. Both have been reviewed elsewhere [ 13 , 14 ] and, for the purpose of this review, biological plausibility is accepted.
Epidemiological studies of the relationship between prevalence of atopy and measures of infection and microbial exposure
The hygiene hypothesis originated not from observations about infection, but from data suggesting a relationship between atopy, family size and birth order. The link to infection was an assumption that these factors could be proxy measures of infection.
As the hypothesis has evolved, the range of potentially significant exposures under consideration has widened beyond those that result in clinical infection to include sub-clinical infection, colonization or even seemingly inconsequential exposure. Similarly, the range of microbes postulated as responsible has widened to include not only pathogens, capable of causing infection, but also non-pathogenic types or strains (commensals and environmental strains), and components of microbes such as bacterial endotoxins. There has also been a growing appreciation of the diversity of contributions by different microbes. Studies investigating the relationship between direct measures of infection or microbial exposure rates and atopy necessarily rely on epidemiological data. Even for confirmed clinical infection, accurate measures of infection rates are difficult to obtain retrospectively, especially for common, relatively minor, childhood infections. Surveillance systems are geared to monitoring diseases that are serious enough to be notifiable, and the vast majority of common infections go unrecorded. Measuring sub-clinical infection or the extent of colonization with environmental strains is even more difficult.
Many investigations have thus used other proxy measures of microbial exposure such as farm living, bed sharing and attendance at day nurseries. These studies will be reviewed first, before turning to studies that have examined more direct measures of microbial exposure and/or infection.
Family size and structure and atopy
Associations between atopy and family structure have been found in many studies, although the associations are less consistent for individual atopic diseases, and sub-divisions such as birth order, sibship size and gender.
An inverse association between atopy and family size was found in studies using hayfever, skin prick positivity and specific IgE levels as markers [ 15–17 ]. For asthma, Haby et al. [ 18 ] reported a protective effect of three or more older siblings on children aged 3–5 years, using a broad definition of asthma as a clinical diagnosis, cough or wheeze in the previous 12 months. A case–control study of clinically diagnosed asthma compared with healthy controls [ 19 ] also showed a small family size effect for cases diagnosed between 3 and 4 years of age.
The protective effect in large families was found to be stronger from older siblings [ 20 ] and from brothers compared with sisters [ 21 , 22 ]. Bodner et al. [ 23 ], however, found that older siblings decreased the probability of hayfever and eczema, whereas the risk of asthma was reduced by the presence of younger siblings. Analysis of allergies among children of 11 042 women enrolled in the ALSPAC study [ 15 ] found a protective effect from brothers for inhalant allergy, but no significant trends for overall family size. Seaton and Devereux [ 24 ], using a cohort followed up since a primary school study in 1964, found membership of a large family reduced risks of hayfever and eczema, but offered no significant protection against asthma. Furthermore, the effect of a large family was not explained by infections the child had suffered: in contrast, the larger the number of infections, the greater the likelihood of later asthma, with the exception of a modest protective effect of measles. Genetic predisposition to allergy as indicated by an atopic parent also complicates the picture: Mattes et al. [ 25 ] found a sibship relationship only in children of atopic fathers, whereas in the European study by Svanes et al. [ 21 ], family size influence on prevalence of specific IgE was restricted to children with no parental history of allergy.
Differences related to gender and birth order thus remain unexplained. There is little temporal relationship between family size and the rise in atopy: the major decline in family size dates from the early 20th century in the UK and in most other industrialized countries where atopic disorders have increased. Wickens et al. [ 26 ] estimated that declining family size could account for only a small proportion of the rise in atopy between 1961 and 1991, for example only 1% of the rise in asthma and hayfever in England and Wales during this period.
The epidemiological studies show that although these factors are related to the incidence of atopic disease, they do not explain how the relationship operates. A wholly different explanation to the hygiene hypothesis has been suggested by workers focussing on changes in the mother, rather than in the environment of the offspring. Karmaus et al. [ 27 ] suggests that the sibling effect originates in utero and that ‘the negative association of infections and atopic manifestation is not causal but more likely to be spurious’. In a study of 981 newborn babies in the Isle of Wight, England, they found that levels of cord blood IgE reduce with increasing birth order. Subsequent studies [ 28 ] showed that maternal IgE decreases with birth order, and suggest that the decrease in cord serum is an indirect effect of the reduction in maternal IgE. This could explain why younger siblings have less later atopy. Similarly, Devereux et al. [ 29 ] compared Th cell proliferative responses in cord blood samples from a cohort of 2000 births, according to birth order, maternal smoking and maternal dietary intake. The magnitude of the Th cell responses to allergens decreased with birth order and high maternal vitamin E intake, but increased with a family history of atopy or maternal smoking. They conclude that birth order, diet and smoking are the risk factors in the maternal environment for subsequent atopy.
Other proxy measures and atopy: microbial exposures in households, day nurseries, rural environments, farms and places of work
(i) Close contact: bed sharing and day nurseries. In the European Community Respiratory Health Survey [ 21 ], sharing a bedroom as a child, a factor more likely in large families, had a protective effect on the subsequent risk of atopic disease. As Strachan [ 30 ] observes, this accords with the hygiene hypothesis, in providing more opportunity for exposure to microbes or infection.
Because of the likelihood of increased exposure, an association between infections such as the common cold and day care attendance would be expected. This has been confirmed, e.g. by the Tucson Children's Respiratory Study, a prospective study in large day care facilities in the USA [ 31 ]: the children had more frequent colds at year 2 and fewer colds at the ages 6–11, than those cared for at home. There was no evident protective effect against colds by the time they had reached 13 years of age, and no protective effect was observed for children in small day care facilities. Results are inconsistent for studies investigating whether early exposure to large day care facilities protects against atopic disease.
Some studies report reduced risk of atopy [ 32–34 ] while others show no protective effect [ 35 , 19 ]. The effects on asthma and on other respiratory symptoms may be in different directions at different points in time. Using data from the Tucson Children's Respiratory Study, Ball et al. [ 31 ] reported that asthma was less frequent in children with one or more siblings at home or who had attended day care during the first 6 months of life. However, children with more exposure to other children at home or in day care were more likely to have frequent wheezing at the age of two, but had a decreased risk from the age of 6–13 years. Increased risk of transient childhood asthma associated with day care attendance was reported for Canadian children [ 19 ], although factors such as short duration of breastfeeding contributed to the raised risk; and the risk was decreased for persistent cases (6-year follow-up) with a history of day care attendance.
(ii) Farm and other rural exposure. Differences between people living in the towns or the country have long aroused curiosity. Recent studies have isolated the farm, rather than the general rural environment as the factor conveying a protective effect against atopy. For example, several studies report a reduced incidence of hayfever in children of farmers, as compared with other rural dwellers [ 36–39 ].
In the 16-year serological and questionnaire survey reported by Gassner-Bachman and Wuthrich [ 37 ], a statistically significant increase in the incidence and severity of hayfever and asthma, and of sero-prevalence of sensitization, was found in rural children with no direct contact with agriculture. By contrast, farming children had less atopic disease and lower levels of seroprevalence to a wide range of allergens, including those to which they had high exposure. An inverse dose–response relationship was identified, with children who had intermittent farm contact showing intermediate results.
Several factors have been associated with the lower prevalence of some allergies but not others: contact with animals as a child, exposure to stables under the age of 1 year, and consumption of farm milk (presumably raw/ unpasteurized) [ 38–40 ]. However the specific exposure has not been fully characterized. Much recent interest has focussed more specifically on bacterial endotoxin as the possible protective factor.
Most of the farm studies rely on serological measures of atopy or on skin prick tests (SPTs): the recent results from the ISAAC study [ 8 ] suggest that this may be an unreliable way of testing for differences in atopy and asthma between population groups. Although Leynaert et al. [ 41 ] found adults who had lived on a farm as a child had lower levels of cat sensitization and a lower risk of nasal symptoms in the presence of pollen, they found no consistent association between living on a farm in childhood and the risk of asthma or wheeze: a similar proportion had ‘atopic asthma’ in the farm and non-farm groups.
There is, however, no epidemiological evidence that working on a farm per se is associated with reduced risk of adult atopy. All the available evidence about the protective effects of exposure to farm environments discussed above relates to early childhood exposure. Farmers have a higher risk of occupational allergic disease and long-term exposure to endotoxins can harm lung function [ 42 ]. Similar hazards (but no evidence of protection against atopy) are reported for waste disposal and sewage workers [ 43 ].
More direct measures of microbial exposure and/or infection
Despite the difficulties outlined previously, several studies have attempted to evaluate the link between atopy and more direct markers of microbial exposure and/or infection.
Food-borne and gastrointestinal disease:
A retrospective case–control study in Italy among male air force cadets by Matricardi et al. [ 47 ], found that cadets with atopy had significantly lower serum levels than non-atopic controls of antibodies to Toxoplasma gondii, Helicobacter pylori , as well as HAV, although independent effects of particular infections were not assessed. The investigators concluded that early exposure to microbes via orofaecal and food-borne routes protect against respiratory allergy and that ‘hygiene and a westernized, semi-sterile diet may facilitate atopy by influencing the overall pattern of commensals and pathogens that stimulate the gut-associated lymphoid tissue’. However, some populations show no association between HAV or H. pylori status and atopic disease [ 48 , 49 ].
An important question is whether HAV acts at a molecular level, or is merely a marker for poor orofaecal hygiene and exposure to other gastrointestinal organisms. McIntire et al. [ 50 ] showed that infection by HAV may have a specific protective effect from atopy for individuals who carry a particular variant of the gene that encodes TIM-1 (also known as HAVcr-1), the cell-surface receptor used by HAV to infect human cells. In contrast, Matricardi emphasizes the importance of general (i.e. non-specific) exposure to infections during the ‘window’ period of immune system development in early life [ 51 , 52 ].
Studies of Sardinian children found that children hospitalized with non-typhoid salmonellosis were less likely to develop hayfever or asthma than children hospitalized with non-bacterial enteritis and the authors speculate that clinical or sub-clinical infection by Salmonella may contribute to the atopy protective effect of the farming environment [ 53 ].
Other studies looking at endotoxin variations in non-farm environments, [ 56 ] have found that higher levels of endotoxin in house dust are associated with less allergen sensitization in infants aged 9–24 months, as measured by skin prick testing. Böttcher [ 57 ] found endotoxin levels were higher in house dust in Estonia, where there is a low prevalence of allergy, than in Sweden, where allergy is high. The levels were inversely related to the development of atopic disease during the first 2 years of life in Swedish, but not in Estonian, children.
Other workers [ 58 , 59 ] however, have sounded notes of caution about the potential role of endotoxins in immune priming, not least about whether endotoxin per se is implicated or whether it is just a convenient, measurable marker for some other microbiological agent.
Strachan [ 30 ] suggests that the key effect of farm living may be early programming of intestinal microflora, and several authors have suggested that endotoxins may have a ‘gut priming’ role.
It has been suggested that ‘probiotics’ preparations commonly comprising lactic acid bacteria, such as Lactobacillus or Bifidobacterium may help prime or maintain normal gut flora and preserve intestinal mucosal integrity, which in turn may be beneficial to atopy and other immune-related conditions, including the response to infection. Probiotics may be protective against eczema more than other forms of atopic disorder [ 64 ]. Recent studies [ 65–68 ] suggest that probiotics may have a role in preventing respiratory and diarrhoeal diseases in children in situations where they are at increased risk of infection, e.g in day care facilities.
- Intestinal parasites. Various studies have suggested that intestinal infestation with helminths, particularly heavy and chronic infection, protects against atopy [ 69 ]. Self-reported wheeze was found to be lower in rural subsistence areas of Ethiopia than in the urban population [ 70 ]: a follow up study in the same population suggested that high degrees of parasite infection, particularly hookworm, prevented asthma symptoms in atopic individuals [ 71 ]. These findings are at first surprising as helminth infections promote a Th2 bias, and they were first explained by a ‘blocking’ mechanism whereby available cellular receptors for IgE were saturated with polyclonal IgE antibodies [ 69 ]. Helminth infections are also immuno-suppressive, however, and more recent explanations of the protective effect propose that this stems from promotion of a strong immunoregulatory network and anti-inflammatory effects that suppress symptoms in individuals otherwise predisposed to allergy [ 69 , 72 ]. Other data, however, paint a more complex picture, indicate a suppressive effect on SPT reactivity, but not asthma or allergic rhinitis [ 73 ].
Respiratory and other non-gastrointestinal diseases and atopy:
- Measles. An inverse relationship between wild measles infection and atopy has been cited as evidence for the hygiene hypothesis, most particularly the evidence from a longitudinal study of children in Guinea-Bissau following an epidemic of wild measles [ 74 ]. An important feature of this ‘natural experiment’ was the high child fatality rate (25%) introducing possible survivor bias to the results. In contrast, no evidence of a protective effect from wild measles infection was found in the 1970 UK birth cohort [ 75 ], or in a Finnish survey of children exposed to measles in the early 1980s [ 76 ]. Although immunological mechanisms by which measles might protect against atopy can be postulated, the totality of epidemiological evidence is conflicting, and there is some evidence that measles is more likely to be harmful than protective in terms of the risk of atopic disorders [ 77 ].
A study of 867 children in Japan aged 6 or 12 years at the time of routine tuberculin tests prior to BCG vaccination [ 78 ] suggested a protective effect of natural exposure to Mycobacterium tuberculosis . Others, however, have suggested this may reflect host effects rather than any causal effect between infection and atopy [ 79 ]. Alternatively, the benefits may be restricted to healthy subjects who do not develop tuberculous disease. Other retrospective analyses of BCG immunization programmes in relation to atopy have produced conflicting results [ 80–82 ].
In a study in which tuberculosis notification rates in different countries were matched to ISAAC data on the prevalence of atopic symptoms von Mutius et al. [ 55 ] concluded that there is a trend to less atopy in countries with high TB prevalence, although this again could simply reflect the theory that the healthy individuals in those communities have enhanced Th1 responses and consequently less atopy.
Mycobacteria may be of particular relevance to the hygiene hypothesis, since it has been suggested that early exposure to mycobacteria with low or no pathogenicity may protect against later atopic disease [ 83 , 84 ]. The authors postulate that mycobacteria have a key role in ensuring proper regulation of the immune system by acting as stimulators for induction of regulatory T cells. They suggest that exposure to mainly innocuous mycobacteria in soil and water has been greatly reduced by water treatment and sanitation in Western urban environments.
Investigators are now interested in the possibility that RSV may be an important primer of the immune system during the second half of pregnancy or in early infancy [ 89 ], although this is based on immunological mechanisms rather than epidemiological studies. Similarly, although early evidence suggested that respiratory infections could exacerbate existing asthma, recent studies have focused on the possibility that viral respiratory infections could have a protective or immune system ‘priming’ role [ 90–92 , 39 ].
- Malaria. A recent study in Gabon found that children with a positive skin test for atopy had a history of less infections and lower incidence of malaria than children who tested negative [ 93 ]. While these results could help to explain the lower prevalence of most forms of atopy in developing countries, this could also be a survival effect whereby children who survive malaria may have less genetic susceptibility both to infection and to atopy.
A recent cohort study [ 92 ] produced evidence of a protective relationship: the total burden of infections was inversely related to subsequent development of asthma, with specific associations for repeated runny nose and herpes-like infections, but not bacterial, fungal or gastrointestinal infections. Matricardi and Bonini [ 52 ] found that the protective effect was confined to infections other than those affecting the lower respiratory tract. Conversely, other studies point to an increased rather than reduced risk of atopy following a combination of early infections [ 24 , 97 ].
The temporal relationship between the rise in atopy and trends in infectious disease
If the trends in atopy are related to infection then, unless the mechanisms involved defer effects to later generations, the data should show a reduction in the incidence of ID just prior to the start of the rapid rise in the incidence of atopy in the 1960s/1970s. Infections should also have continued to decline during the first couple of decades, at least, of the observed rise. Also, if the window of opportunity for immune system ‘priming’ is pregnancy and early infancy, there should be evidence of a dramatic decline in ID 3–6 years before the steep rise in atopy.
The assumption that there has been a decline in ID in recent decades in countries with a notable rise in atopic disease, such as the UK, is understandable since the majority of data on ID trends derives from national surveillance data, the decline in hospital admissions for infection and the success of vaccination programmes and antibiotics. However, these data focus on serious, systemic infection and infection mortality, while the continuing burden of general ID, particularly sporadic or non-notifiable cases, tends to be overlooked. Even more neglected is the proportion of ID acquired in the home environment.
The decline in serious infections such as cholera and typhoid, associated with improvements in water, sanitation and personal hygiene, occurred in the late 19th and early 20th century in industrialized countries, much too early to be associated with the rise in atopy. For other serious notifiable disease, the decline in specific infections such as measles, mumps, rheumatic fever, tuberculosis and HAV dates from the 1940s onwards following the introduction of antibiotics and a comprehensive range of vaccines. These infections were cited recently as showing an inverse relationship with the incidence of immune disorders [ 98 ]. Yet in the UK, a country with high incidence and prevalence of atopic disorders and one of those showing the rapid post-1970 rise, there is no convincing relationship between trends in atopy and of infections as reported to national surveillance. The decline in TB started much earlier, and has reversed recently in London and other metropolitan areas. The decline in measles in the UK dates from the introduction of the combined measles, mumps and rubella vaccine in 1988. Similarly, a decline in HAV since 1994 in the UK following the introduction of an effective vaccine post-dates the rise in atopic disease. The longer term decline in HAV since the 1940s also shows no convincing temporal relationship with trends in atopic disorders.
Data indicate that, during the critical period relating to the rise in atopy, there has been a rise rather than a decline in many gastrointestinal infections. For example, food poisoning notifications increased from the early 1970s. Salmonella infections in England and Wales reached a peak in 1997. Although overall food poisoning figures for Salmonella are now declining, the commonest cause of bacterial gastrointestinal infection, Campylobacter , shows a continuing rise [ 99 ].
A study by Wheeler et al in 1995–6 confirmed the long accepted view that that national surveillance significantly underestimates the true incidence of infectious intestinal disease (IID) and revealed the extent of that underestimate for England and Wales [ 100 ]. This showed that many minor cases are not reported to general practitioners, and that the true figure is nearer to 9.5 million cases, suggesting that one fifth of this population suffer from one episode of IID every year. According to the UK Food Standards Agency about 50% of these infections are foodborne, the remainder being due to person to person transmission. Since many cases are ‘sporadic’ (not part of a recognized outbreak), and are not picked up by surveillance, it is reasonable to assume that a significant proportion of these infections occur in the community and in the home. The most recent WHO survey [ 99 ] suggests that about 40% of all foodborne infections across the WHO European region are caused by consumption of food in the home. The general trend for several IIDs such as rotavirus and norovirus infections is an increase over the past 20 years [ 101 ]. Wheeler [ 100 ] indicated that for every one case of rotavirus and norovirus reported to surveillance, at least 35 and 1562 additional cases respectively go unreported in the community.
Respiratory tract infections have also shown no overall decline, with the exception of those controllable by immunization, such as measles. The true incidence of respiratory tract infections is also likely to be much higher than the relatively few infections ascertained in laboratory reporting systems or by statutory notification. This particularly applies to the milder types of upper respiratory tract infection, such as the common cold and influenza-like illness. Consultation rates for influenza and influenza-like illness are well documented in the UK: a peak was noted in the winter of 1998–1999, of 200–400 per 100 000 population [ 99 ]: although influenza epidemics show a cyclic pattern and vary in severity, and thus also in the level detected by surveillance, there was no overall change preceding or during the rapid rise of atopic disorders. Similar patterns of influenza have been reported from other European countries. While RSV has declined slightly since 1997 [ 102 ], there was no recorded decline during the rapid rise of atopic disorders. For the common cold, there is no evidence of any change in incidence in recent decades or indeed over the last century. The evidence suggesting differences in frequency of colds and other minor infections related to day nursery attendance and other exposures, as reviewed earlier, may indicate differences in susceptibility between population subgroups, but it cannot be used to infer any overall increase or decrease in these infections in the population.
Although it is generally accepted that, in the US and Europe, helminth infections have steadily declined [ 103 ] there is no data available to show whether there might be a temporal trend suggesting a link between reductions in helminth infections and the rise in atopy in the developed world. However Asato et al reported rapid reductions in Ascariasis and Trichuriasis and hook worm infections during the 1950s and 60s, because of improved sanitation and improved living standards [ 104 ].
The relationship between the rise in atopy and measures taken to prevent and control infectious disease
A wide range of public health, medical and other changes have occurred over the past century such as clean water and food, sanitation, antibiotics and vaccines, all of which are likely to have resulted in significant alterations in microbial exposure and infection in the community:
Sanitation, water treatment and food quality
The evidence gives no support for a relationship between provision of treated water supplies and sanitation, and the rise in atopy over the last 30 years. Filtration and chlorination of municipal water dates from the late 19th and early 20th century in most developed countries: only a small proportion of consumers depended on untreated private water supplies after this era, particularly in the UK. Chlorination of mains water was introduced in 1908 in the USA [ 105 ] and 1910 in the UK [ 106 ]. Progress in sanitation followed the first connection of house drains and cesspools to sewers in 1848 in England. It was nearly fifty years before such systems were common. While the ‘sanitation revolution’ continued throughout the first part of the 20th century in developed countries, there appears to be no temporal association with the rapid rise in atopy over the last 30 years.
Changes in food and water quality designed to reduce our exposure to pathogens are likely to have altered our exposure to commensal and environmental strains as well as pathogens; removal of pathogens in modern water treatment inevitably also removes most benign microbial bacterial contamination, such as environmental mycobacteria. However, there is no direct temporal relationship with the rapid rise in atopy. Similarly changes in food preferences are likely to have altered the microbial content of our diet. Since foods are only controlled for pathogens, there are no available data to indicate what trends might have occurred in the broad microbial content of our diet during the period critical for the rise in atopy.
Immunization/vaccination
As vaccination involves the administration of attenuated or killed microorganisms, or selected components from them, in order to induce an immune response, the hygiene hypothesis predicts that it should influence susceptibility to atopic disease. However, epidemiological studies provide no consistent support for either a beneficial or adverse effect of vaccination/immunization on atopic tendency. The Guinea-Bissau study by Shaheen [ 74 ] suggested measles vaccination might increase the tendency to atopy, but this finding is not supported by other epidemiological studies. In a trial of pertussis vaccine in Sweden, Henderson [ 107 ] and Nilsson [ 108 ] found no convincing evidence of an effect on atopy.
Hurwitz [ 109 ] reported a twofold increase in asthma in a US study comparing vaccinated with unvaccinated children, but various authors have cautioned that, in countries with high vaccine coverage, families who choose not to immunize their children are unusual and that both increased risk of atopy and a protective effect could be demonstrated, depending on the population examined [ 110 , 30 ]. In contrast, in a carefully controlled study, Ring and colleagues [ 111 ] found that former East Germans, whose take-up of pertussis vaccination was high, experienced lower rates of asthma than their West German counterparts, many of whom shunned this vaccine.
Although Rook and Stanford [ 83 ] suggested that early exposure to mycobacteria with low or no pathogenicity may protect against later atopic disease, several studies have reported a protective effect against immune disorders for BCG [ 82 , 112–114 ]. The most supportive evidence comes from animal studies such as those showing suppression of allergen-induced eosinophilia in mice by infection with Mycobacterium bovis [ 115 ] and from promising initial results of trials with mycobacterial vaccines for the treatment of some diseases associated with immune dysregulation [ 116 , 117 ].
Antibiotic therapy
The possibility of a relationship between antibiotic use and later asthma or other atopic disease is difficult to disentangle from the potential confounders such as whether the key exposure relates to the infection or the antibiotic [ 118 ]. A study in Germany by von Mutius [ 119 ] showed that six or more courses of antibiotics in the first year of life were associated with a later excess of hayfever and eczema, but SPTs did not indicate an increase in the prevalence of atopy. Similarly, a study in UK [ 120 ] reported that any course of an antibiotic before the age of 2 years was associated with a doubling of the risk of hayfever and eczema, particularly if the antibiotics contained cephalosporins and macrolides.
Bjőrkstén [ 121 ] has proposed that the antibiotic effect could be linked to the influence on the bacterial colonization of the gut in early years of development. In line with this a study with laboratory mice [ 122 ] showed that antibiotic-induced changes in the gastrointestinal tract can affect how the immune system responds to common allergens in the lungs. A recent study presented at the 2003 European Respiratory Society conference by Christine Cole Johnson, of the Henry Ford Hospital, Detroit, US (unpublished) showed that children who received antibiotics within their first 6 months of birth were up to three times more likely to develop allergies to pets ragweed, grass and dust mites by age 7. Of the 448 children studied, 49% had received antibiotics in the first 6 months of life. However, recent analysis of the relationship between antibiotics sales and the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema in 99 centres from 28 countries [ 123 ] found there was a positive association between per capita antibiotics sales and the prevalence of symptoms for asthma, rhinitis, and eczema, but the associations generally became negative once the analyses had been adjusted for GNP. Another recent study [ 124 ] alternatively suggests that, rather than antibiotic use in early life being associated with subsequent development of asthma, frequent antibiotic use in early life is more common amongst asthmatic children.
Breastfeeding
The well-established protective effect of breastfeeding against infection is mediated by transfer of maternal antibodies and by constituents affecting the infant's gut. Breastfeeding protects against intestinal pathogens [ 125 , 126 ] and respiratory infections [ 127 , 128 ], and it is somewhat paradoxical, therefore, that in some studies the reported benefits of breastfeeding include prevention of later asthma or atopic disorders [ 18 , 129–132 ]. Breastfeeding may, however, also transfer active maternal viral infections such as HIV and Hepatitis [ 133 ].
The duration of breastfeeding appears to be an important factor, with little or no protective effect against atopy for short periods of breastfeeding, e.g. less than 3 months [ 134 , 74 , 135 ]. A dose-response effect was indicated in a retrospective longitudinal study of Canadian children aged 1–2 years: a higher risk of asthma occurred in children breastfed for up to 9 months, than in those breastfed for longer. The trend to shorter periods of breastfeeding, was also indicated in this study: 44% of the children were breastfed for only 2 months.
Auto-immune and other immune-related diseases
Several workers have looked for possible links between reduced microbial exposure and rises in certain auto-immune and other immune-related diseases which have accompanied the rise in atopic disorders. A variety of studies have found positive associations between various potential indicators of microbial exposure and inflammatory disorders regarded as Th1 mediated such as childhood diabetes [ 136–141 ], juvenile arthritis [ 142 ], Crohn's disease [ 143 ] and with childhood leukaemia [ 144–146 ]. However, as in the case of atopic disease, other studies find no association with these indicators [ 147–151 , 144 ], while others suggest that certain infections may cause the disease [ 151–155 ].
Other explanations for the rise in atopy, not linked to microbial exposure
The epidemiological evidence supporting the hygiene hypothesis as an explanation for the recent rapid rise in atopic disorders needs to be viewed in the context of other possible explanations. Indeed, it seems likely that the rise results from several factors, either operating independently, or perhaps with some measure of synergy. The observation that atopy is more prevalent among higher socio-economic groups has been cited as evidence for the hygiene hypothesis [ 30 ], although it could equally be a proxy measure for other, non-microbial factors, such as diet. Poverty has also been identified as a risk factor for asthma and other atopic disease [ 5 , 156 ], and studies in the inner cities of the USA [ 157 ] suggest that the previously observed socio-economic gradient may be levelling out.
The effects of increased contact with house-dust mites, cockroach allergen, pollen and fungal spores (for example in damp housing) are primarily seen as triggering attacks or exacerbating symptoms of atopic disease rather than increasing susceptibility to atopy [ 158 ]. However, some studies show correlation between exposure to fungi for example and sensitization, positive skin-prick tests and IgE antibodies [ 159 , 160 ]. The current balance of opinion about the effects of pollutants is similar, although perhaps moving further towards identifying situations where effects of exposure may go beyond triggering and exacerbation. For example, traffic emissions were reported to increase atopic sensitization for nine-year-old children living near major roads and in suburban areas in West Germany [ 161 ]. Ozone in urban air has been implicated as associated with increased risk of asthma [ 162 ] and heavy exercise in high ozone environments has been found to increase the asthma risk in children with no history of the disease [ 163 ].
One area of possibly more fundamental predisposition to atopy is that of nutrition and diet. Observed correlations between birth head circumference (which reflects increased birth weight [ 164 ] and raised IgE levels [ 165 ] have given rise to the suggestion that increased fetal growth trajectory resulting from improved nutrition in pregnancy and infancy may be achieved at the expense of immune system development. It is possible that changes in diet could mediate an effect, through changes in gut flora. Attempts have been made to alter the gut flora in infants [ 166 ], where manipulation is much easier than in the adult [ 167 ].
Obesity, now a major problem in children in developed countries, may be another factor in the rise in atopy. The body mass index (BMI) is positively associated with the risk of adult-onset asthma [ 168 ]. In a prevalence study by von Mutius [ 169 ], increasing BMI correlated with an increased risk of childhood asthma and atopy and BMI remained significant after adjustment for other factors. The consistency of the relationship with obesity, the temporal association and the dose response curve has prompted the theory that obesity causes asthma [ 170 ]. This is not yet a widely held view, since the biological mechanisms remain unclear [ 171 ]. Platts-Mills et al. [ 5 , 172 ] suggests that the key changed factor, related to obesity, is less exercise.
In a study in Saudi Arabia, Hijazi et al showed that diet was more directly implicated as a predisposing factor for atopy [ 173 ]. As in other changing societies, asthma has increased in urban areas of Saudi Arabia. Hijazi et al. showed that diet may be the most important change in such societies, and most likely to explain the recent trends in atopic disease. The dietary factors involved include eating at fast food outlets, as well as lower intake of milk, vegetables, fibre and foods rich in Vitamin E. Dietary vitamin E was reported to inhibit IgE responses to allergic stimuli in a random sample of 2633 adults [ 174 ]. In a study of adult-onset wheeze in Aberdeen, intakes of vitamin E and plasma levels of ascorbate (a measure of Vitamin C) were inversely related to adult wheeze in the manual social class and among current smokers [ 175 ].
Changing diet, as an explanation of trends in atopic disorders, may not only have a ‘microbial’ effect by altering the gut flora (see above) but also has biological plausibility via non-microbial mechanisms e.g. in terms of the nutrients needed for healthy immune system development, such as polyunsaturated fatty acids and antioxidants [ 173 ]. The dietary hypothesis has also been coupled with the notion that there has been a shift in the population susceptibility to atopic disease [ 176 ], possibly also linked to increasing population mixing and greater genetic diversity [ 177 ]. In an investigation of whether reduction in childhood infections or change in diet could explain increases in asthma and atopic disease, Seaton and Devereux [ 178 ] found that low intake of vitamin C was associated with a sevenfold increase in the risk of bronchial hyper-reactivity; by contrast, those with the lowest intake of saturated fats had a tenfold lower risk. In a separate study, the lowest intake of vitamin E was associated with a fivefold increase in adult-onset wheezy illness, while the lowest intake of vitamin C doubled the risk. Dietary intakes were confirmed by measurement of vitamins and triglycerides in plasma. Seaton and Devereux concluded that changes in the diet of pregnant women may have resulted in the birth of cohorts of children predisposed to atopy and asthma.
The possibility that maternal influences or changes may be important is reflected in several studies. For example, a greater risk of atopic disease has been reported in children of older mothers [ 179 ]. The risk of childhood asthma is also greater for infants with a maternal compared with a paternal history of asthma [ 180 ], indicating the possible importance of maternal imprinting (preferential expression of maternal genes in the fetus) [ 181 , 182 ], as well as the influence of the maternal-fetal interface [ 171 ]. Grandmaternal, as well as maternal, smoking has also been found to be associated with increased asthma risk in children, suggesting influences may extend beyond the parental generation [ 183 ].
For the protective factors discussed previously, such as large family size, farm life or breastfeeding, an important issue to consider is whether it is greater infection and/or greater microbial exposure, or other related ‘non-microbial factors’ which are the key. Family size undoubtedly influences the potential for case-to-case spread of infection by both aerosol and other routes, but it cannot be assumed that a large family inevitably causes increased spread of infection, or poor hygienic conditions: much depends on socio-economic factors such as overcrowding, sharing a bedroom, bed-sharing or awareness of how to prevent infections. The protective factors in farm life could include private, often untreated, water supply and non-microbial factors such as diet, exercise and outdoor activity.
Evidence for and against the proposition that the reduced microbial exposure critical for immune development relates to improved hygiene practice, household amenities and personal cleanliness
The second proposition of Strachan's original hypothesis was that the reduced microbial exposure causing the rise in atopy was due to ‘improved household amenities and higher standards of personal cleanliness’. A popular view is that the microbial or infection exposure required for the development of a balanced immune system has been reduced as a result of the more ‘rigorous’ cleaning and hygiene practices which we now use not only to counter our fear of exposure to ‘germs’ but also to create a more aesthetically pleasing dirt-free environment in which to live. Evidence in relation to this question derives from the following sources:
Epidemiological studies of the relationship between the proxy measures of cleanliness and hygiene and the rise in atopy
Hygiene practices in relation to atopy
In two recent studies, Sherriff and co-workers [ 184 , 185 ] examined the link between ‘hygiene behaviour’ and atopy by devising hygiene scores for personal hygiene practices, and looking for associations with wheezing and atopic eczema. The data was derived from the Avon Longitudinal Study of Parents and Children (ALSPAC). In self-completed questionnaires for 10 970 children aged 15 months, parents were asked how often in a normal day they wiped the child's face and hands, whether hands were wiped before meals and how often the child was given a bath or shower. The cumulative hygiene score derived from these responses was examined statistically in relation to socio-economic and peri-natal factors. These data suggested a ‘cleanliness norm’ for the majority of the children comprising washing face and hands 3–4 times a day, hands cleaned before meals, and a daily bath or shower. The hygiene scores ranged from 2 (least hygienic) to 14 (most hygienic). A score of > 10 was independently associated with maternal smoking during pregnancy, low maternal educational achievement, living in local authority housing and increased use of chemical household products (the latter based on a score derived from reported use of disinfectant, bleach and aerosols). High scores were also associated with higher maternal parity (2 or more children), short duration or no breastfeeding, overcrowded accommodation and little contact with dogs or other furry pets. In their follow up questionnaire study asking about atopic symptoms, Sherriff [ 184 ] reported that high scores for children aged 15 months were independently associated with increased risk of wheezing and eczema when this group of children reached 30 and 42 months of age. Mothers with a history of eczema were less likely to have children with a high score than non-atopic mothers, suggesting that knowledge of factors that exacerbate eczema influenced frequency of child bathing: no such association was found for mothers with a history of asthma.
While the responders in this long-standing ALSPAC study would be accustomed to answering questions, the association of high scores with social disadvantage (low educational attainment and overcrowded living conditions) and maternal smoking is surprising and raises the possibility of an association between social disadvantage and a tendency to exaggerate about personal cleanliness, particularly where this relates to childcare. Alternatively, these social factors may influence hygiene practice: for example, in younger, poorer or less educated mothers. The study also contains no measures of microbial exposure or how effective individual hygiene practices may have been in terms of reducing microbial exposure. Some studies [ 186 ] report that both asthma prevalence and severity are associated with social disadvantage. The findings from the ALSPAC study may thus reflect a non-causal association between asthma and hygiene behaviour.
Use of household cleaning products and atopy
A comparison of soap and detergent consumption with data on prevalence of atopic disease [ 187 ] showed no evidence of a relationship; plots of per capita consumption of soap, detergents and cleaning products in 1994 for 12 European countries against reported prevalence of asthma, hayfever and eczema as reported in the ISAAC study [ 8 ] showed no correlation. Nor was any correlation apparent between individual diseases and individual product types such as fabric washing detergents, dishwashing detergents, toilet soaps and hard surface cleaners. Consumption of household bleach, a highly effective disinfectant that is widely used by consumers, varies greatly across Europe but again shows no correlation with prevalence of atopic disease. Bleach consumption per capita is highest in Spain and other countries of southern Europe, which have relatively low incidence of atopy, whereas in Scandinavia, where bleach use is limited, some 30 times lower per capita than in Spain, atopy rates are relatively high.
Temporal relationship between the rise in atopy and trends in hygiene practice
As far as personal and other hygiene practices in the home are concerned, widespread access to clean water, soap and chemicals to aid cleaning dates back, with only a few exceptions to the end of the 19th Century, and thus significantly predate the rise in atopy. During the last half century availability of household amenities and appliances and the range and effectiveness of household cleaning products has increased. Water use has increased in all industrialized countries, including use both for personal cleanliness and for kitchen appliances such as dishwashers and washing machines.
Although the discovery of the transmission of infection by microorganisms led to much greater emphasis on hygiene in the home, this occurred mainly around the turn of the 19th/20th century. Prior to the 20th century, home hygiene was largely focussed on food preparation and storage. During the first half of the 20th century there was increasing emphasis on cleanliness in the home, with advice on regularly cleaning walls, ceilings and other areas, partly prompted by the fear of infection before the antibiotic era. An increasing number of products and equipment were developed for home cleaning during the last century, but other social changes during the latter part of the century changed the approach to housework and its extent:
- Domestic help became less available and more expensive;
- Women increasingly worked outside the home and had less time for housework;
- Vaccination and antibiotic therapy for treatment of infectious enemies, such as diphtheria and typhoid fever reduced perception that hygiene was important.
These changes led to a more superficial approach to home cleaning, with speed and aesthetic factors more important than hygiene and disease prevention. The trend towards the modern pattern of frequent bathing and laundering in the USA and UK dates from 1890 to 1915. Soap manufacture in the USA more than doubled between 1904 (700 000 tonnes (8.4 kg per capita)) and 1919 (1 700 000 tonnes, 16 kg per capita) [ 188 ]. The increasing popularity of showers in the USA occurred between the 1940s and 1960s, with the proportion of American homes with bathtubs and/or showers increasing from 61% in 1940 to 87% in 1960 [ 188 ]. Similar rises in showers, although slightly later, have occurred in European countries but the rise in atopy occurred at much the same time throughout the industrialized world.
Many cleaning agents used since the 1960s/70s are formulations of chemicals that have been available for a considerable time, such as bleach or phenolic disinfectants. The first detergent powders were introduced at the beginning of the 20th century. Usage of soap, detergents and cleaning products has continued to rise over the last 50 years, although at a lower rate than observed in the first quarter of the 20th century.
The data in Table 1 suggests a steady rise in consumption of soaps and detergents to a peak in the late 80s, followed by a decline to 1994. However, changes in product classification as well as formulation distort the picture such that only broad assessments are possible. For example, the rapid growth of liquid fabric washing and rinse conditioning products in the 1980s meant that the total consumption of product increased, but not the ‘cleaning power’ deployed. Conversely, the introduction of more concentrated liquid and powder products in the late 80s and early 90s will have had the opposite effect . Taking into account these formulation trends [ 187 ] it is estimated that the real increase in cleaning product usage across the 12 European countries over the 25 year period would have been of the order of 50%. Such changes are considerably smaller than the variation in usage between countries, which range from 8 kg/cap in Finland to 30 kg/cap in Spain, although different national formulation preferences again distort this picture.
Per capita consumption (kg) of soaps and detergents
With regard to temporal trends for particular product types, the greatest increases have been in use of dishwashing products and hard surface cleaners, although the rise in the latter was substantially offset by the decline of hard soap and scouring products previously used for this purpose. Detergents based on synthetic surfactants, rather than soap, came into general use in the 1950s, thus pre-dating the rise in atopy.
In assessing whether the altered microbial exposure which may be responsible for the current trends in atopy bears a relationship to changes in our cleaning and hygiene habits, it is also necessary to evaluate studies of the impact of hygiene on microbial exposure in the community, and the various case control studies etc. assessing the impact on infection rates. These are discussed in the next part of this section.
Hygiene practice and microbial exposure and infection in the home
Microbial contamination and infection in the home
If we compare our modern centrally-heated, double-glazed, sealed homes, with homes of the 1950s where coal fires, urban pollution and open windows meant that housewives waged a constant war against dust and dirt, it is easy to make the assumption that modern homes are ‘cleaner’. In the immediate post-war years, lack of resources meant that European homes were less well maintained; cracked tiles, damaged flooring etc. all added to giving homes the appearance of being ‘dirty’.
In recent years a range of studies have been published, as reviewed by Beumer et al. [ 189 ], showing that pathogenic, commensal and environmental microbial species are all introduced continually into the home by people, food, water, pets, and sometimes via the air, and that our modern home environments, despite their clean appearance still offer constant opportunities for microbial exposure.
As far as food is concerned, exposure to food-borne microorganisms (both pathogens and commensals) during food handling in the home must be a fairly common occurrence. Surveys of chickens in the UK between 1979 and 1998 indicated contamination rates between 25% and 79% for Salmonella [ 190 ] and 80–90% for Campylobacter [ 191 , 192 ]. While recent data [ 193 ] suggest that these rates have now fallen, data on the high chicken consumption in the UK suggest that at least one in 25 UK homes prepare a meal with contaminated chicken every day of the year. Similar high rates of contamination are reported from other European countries, such as the Netherlands, France, Italy and Germany [ 194 ]. Cattle and sheep are important sources of Escherichia coli O157; Chapman et al. [ 195 ] showed that 0.4–0.8% of meat products purchased from UK butchers were positive for E. coli O157. Domestic cats and dogs in the home can act as reservoirs and shedders of Salmonella, Campylobacter and other enteric pathogens as well as commensal strains [ 196 ]. In a recent study [ 197 ], 19 species of Campylobacter , including C. upsaliensis and C. jejuni were isolated from 100 faecal specimens obtained from a London cattery.
Exposure to microorganisms from these human, animal, food and environmental sources can occur either by direct contact or by transfer involving inhalation of infected aerosols, consumption of contaminated food or water, or indirect transfer via hands or other surfaces into the nose, mouth or eyes, or into open cuts or wounds etc. Laboratory-based studies [ 198–203 ] show that bacteria and viruses spread to environmental surfaces from an infected or carrier source can survive in significant numbers for periods of several hours and in some cases days particularly on surfaces where moisture is present but also on dry surfaces. Other studies show that, when surfaces become contaminated, the organisms are readily spread via hands, cleaning cloths, and hand and food contact surfaces around the home, providing continual opportunities for human exposure [ 200 , 204–206 ]. Using a bacteriophage φX174 as a model, Rheinbaben et al. [ 207 ] showed that, following contact (handshaking) with a volunteer whose hands were contaminated from touching a virus-contaminated door handle, successive transmission from one person to another could be followed up to the sixth person. Similarly, Cogan et al. [ 200 ] demonstrated that, following preparation of Salmonella- and Campylobacter- contaminated chickens in domestic kitchens, these species could be isolated from 17.3% of the hands, and hand and food contact surfaces sampled.
Although raw food is probably the main source of microbes in kitchens, there is evidence for a contributory role of surfaces such as draining boards, sinks, U-tubes, nappy buckets, dishcloths and cleaning utensils: wet sites, in particular, may act as permanent sources or reservoirs of free-living bacterial populations. In the bathroom or toilet, enteric bacteria probably originate from the toilet or directly from people, but permanent reservoirs of bacteria readily survive in baths, basins, cleaning cloths and face cloths [ 209 ]. Most species isolated in these studies are not normally pathogenic, but the evidence suggests an abundant population of microorganisms in the home. An evaluation of dust samples in 20 US homes showed high levels of endotoxin on kitchen and bedroom floors [ 208 ]. These and other data [ 209–212 ] confirm that all types of microbes, are found in all areas of the home environment, and that patterns or levels of microbial contamination have not significantly altered in the 20 years between the earliest and most recent of these studies.
The extent to which exposure to food-borne pathogens still occurs in the home is suggested by community-based [ 100 ] estimates that, each year, one in five of the population in England and Wales suffers a bout of IID. While contaminated food accounts for about 75% of reported infections attributed to bacteria, enteric viral infections are spread mainly by person-to-person contact or via aerosolized particles resulting from vomiting or fluid diarrhoea. During and after viral infections (both respiratory and enteric viral infections), virus particles are shed in large numbers in body fluids including blood, faeces, saliva and nasal secretions. There is relatively little data on the occurrence of viruses in the home environment, but a recent study in five US homes where there was an influenza-infected child showed the presence of influenza A virus on between 20% and 100% of the surfaces sampled, which included telephone receivers, door knobs, toilet handles and computer surfaces [ 213 ]. Other studies show that surface to finger/surface to mouth transmission of rotavirus readily occurs [ 214 , 215 ]; in a laboratory study, Ward et al. [ 214 ] showed that 13 out of 14 adult subjects became infected after consuming Rotavirus (10 3 particles). The potential for norovirus transmission from person-to-person via hands and surfaces is indicated by the recurrent infection outbreaks in successive cohorts of guests in hotels and cruise ships [ 216 , 217 ]. Epidemiological studies suggest that, whereas aerosols are the main route of dissemination within a cohort of people, contaminated surfaces are responsible for ongoing outbreaks by forming the link between successive groups. A recent study [ 218 ] shows the ease of spread of norovirus via hands, cloths and other surfaces. There is now growing evidence that respiratory infections such as rhinovirus and RSV infections can also be spread via hands and surfaces such as handkerchiefs, tissues, door handles and telephones; indications are that the virus is transferred via the fingers to the nasal mucosa or conjunctiva; self-inoculation with rhinovirus by rubbing nasal mucosa or the eye can lead to infection [ 219 , 220 ].
A key questions with regard to the hygiene hypothesis, which remains to be addressed, is how big the critical microbial exposure needs to be. Must exposure be sufficient to cause clinical infection or at least asymptomatic colonization, or is ‘subclinical exposure’ adequate to produce the required immune response? The evidence (see review by Beumer et al. [ 189 ] suggests that, for some pathogens, the ‘infectious dose’ may be as little as 1–100 cells or particles, while for others, exposure to several thousands of units may be required to elicit clinical infection.
The impact of cleaning and hygiene practices on cross contamination and microbial exposure in the home
When people set about ‘cleaning’ their home, or their hands, or body surface, visual observation is used to decide whether ‘cleanliness’ has been achieved. The assumption that visibly clean means ‘free from microbes’ is a misconception. In practice a hygienically clean surface, i.e a surface free from microbes, can be achieved using soap or detergent and water, but, as this involves mechanical removal of the microbes, to be effective it must be properly applied, and used in conjunction with a thorough rinsing process under running water. The alternative method is to apply a disinfectant product which kills the microbes in situ .
A range of ‘in-homes’ studies, as reviewed by Beumer et al. [ 189 ] demonstrate that soap or detergent-based cleaning routines, as they are currently practised in the home, may have only a limited effect in reducing exposure to microbes. Thus for example, Cogan et al. [ 200 ] demonstrated that, following preparation of Salmonella- and Campylobacter- contaminated chickens in domestic kitchens, 15.3% of hands, and hand and food contact surfaces still showed evidence of contamination even after participants had carried out a washing-up routine with detergent and hot water in a washing-up bowl and then used the cloth to wipe surfaces ‘clean’. A separate study involving the hands, cloths and chopping board showed that, where surfaces were cleaned using the bowl-wash routine but then rinsed under running water, a more significant reduction was achieved, but 23% of 60 sites sampled were still contaminated with Salmonella . A recent study [ 218 ] showed that, where surfaces contaminated with a faecal suspension infected with norovirus were cleaned using detergent solution applied with a cloth, the virus was not eliminated from the surface. Detergent-based cleaning was insufficient even where the cloth was rinsed in clean water and the surface re-wiped. These and other studies [ 200 , 218 , 221 ] clearly demonstrate that, where a hygiene procedure fails to eliminate contamination from a surface and the cleaning cloth or mop is then used to wipe another surface, the contamination is transferred to that surface, and to the hands of the person handling the cloth.
Preventing microbial transfer in the home depends not only on the effectiveness of the hygiene procedure, but also on when it is applied. The critical influence of these factors on microbial exposure is rarely appreciated. An ‘in homes’ study to evaluate the effects of routine day-to-day home cleaning [ 222 ], showed that, before cleaning, one in five (20%) of 10 selected hand and food contact and other sites in the kitchen, bathroom and toilet could be considered as hygienically clean (<10 colony forming units per 25 cm 2 ). After detergent-based cleaning, the proportion of contaminated sites was actually increased to 68%. Although disinfectant products were effective in reducing microbial contamination levels, the effects were relatively short lived. After a relatively limited period (90 min to 3 h), most sites and surfaces become substantially re-contaminated. This is probably because of reuse, redisposition from the air or, for wet sites or surfaces (e.g. for damp cloths), regrowth of residual survivors not destroyed by the hygiene process. In other studies where effects were monitored over longer periods (3 days to 9 months [ 211 , 222 ] the authors concluded that casual use of disinfectant cleaners for daily or weekly cleaning is unlikely to reduce the risk of exposure to pathogens.
The ineffectiveness of non-specific routine cleaning activities in reducing infection exposure is supported by a recent study of home hygiene practices and infection in 398 households of an inner city population [ 223 ]. Only two specific practices, using a communal laundry and not using bleach in communal laundering, were found to be predictive of increased risk of infection. Other general cleaning practices such as daily personal bathing or showering, daily cleaning of bathrooms and toilets, frequent changing of dish-sponges, or use/non use of antimicrobial cleaning products for these activities, showed no significant correlation with infection rates.
In arguing the proposition that reduced exposure to microbial pathogens has resulted from changes in domestic cleaning and hygiene practices in recent years, this begs an assumption that patterns of hygiene behaviour in the home are of a type and quality that reduces pathogen exposure. Although there is good evidence to show that handwashing and other hygiene interventions in the home can have a significant impact in reducing the incidence of infection [ 224 ] a number of observational studies suggest that compliance with hygiene practices that specifically protect us from pathogen exposure is relatively poor. A study of 108 UK participants to estimate the risk of food poisoning following domestic food preparation showed that only a small proportion of consumers (4.6%) fully implemented appropriate food safety measures while 3.7% prepared food in a way that seriously violated recommended practice and exposed them to a high level of risk [ 225 ]. These results complement the studies of Cogan et al. [ 200 , 226 ] described previously, which showed the extent to which pathogens are spread, from a contaminated chicken during food preparation in a domestic kitchen. Similarly Curtis et al. [ 227 ] studied the hygiene practices of mothers with young children who had recently been vaccinated for polio, and were consequently shedding the virus in their faeces. Only 43% of childcarers washed their hands after changing a nappy compared with 76% who washed them after toilet visits. Nappy changing took place mainly in the living room and contact with living room surfaces and objects during nappy changing was frequent. Not surprisingly, poliovirus was detected on 12% of living room surfaces, 10% of kitchen surfaces and 15% of bathroom sites. Hand contact sites were most frequently contaminated, such as bathroom taps, toilet flushes and door handles, soap dispensers and nappy changing equipment.
Discussion of the hygiene hypothesis and the implications for hygiene practice
The link between atopy, and microbial exposure and infection
In the first part of this paper the evidence for a causal link between the sharp rise in atopy over the past 30 years and the possibility of a reduction in our level of exposure to microbes was reviewed. Although many of the studies cited in support of the hygiene hypothesis are based on proxy measures of microbial exposure, some provide striking evidence supporting such a link. A consistent finding is the inverse relation between atopy, family size and birth order. There is also an apparent protective effect for children living on a farm. In addition however, there are numerous contradictory studies, and the totality of the evidence remains inconclusive.
Some proponents of the hygiene hypothesis suggest that the infection exposure necessary for immune priming should be ‘intense’ [ 228 ] or at least produce clinical disease [ 69 ]. In this review, data covering the past 100 years was examined in order to look for infection trends which might correlate inversely with trends in atopy, supporting the hypothesis, and might provide clues as to the nature of the critical exposure. The data show that the decline in serious infections such as cholera and typhoid, mumps, rheumatic fever and tuberculosis occurred too early to be associated with the rise in atopic disease in the late 20th century, unless the mechanisms are such that the effects are manifest only in subsequent generations. There is conflicting evidence regarding an inverse relationship between atopy and exposure to infections such as measles, HAV and Mycobacterium tuberculosis . While ‘old’ infections with high mortality have declined, this has, to an extent, been offset by the emergence of new infections with high mortality, or those which have re-surfaced (e.g. tuberculosis). Introduction of measures designed to reduce the burden of ID, such as improved housing, sanitation and clean drinking water, correlate with the decline in life threatening enteric disease during the first part of the 20th century, rather than the later rise in atopy. Reduced consumption of food-borne pathogens is also an unlikely candidate as the incidence of food poisoning rose during the critical period of the rise in atopic disorders.
Intuitively the idea that exposure to invasive infection, with all the attendant risks might be needed to protect against atopy seems inefficient in evolutionary terms. A more plausible explanation is that the critical change involves less severe endemic infections. As far as ID morbidity is concerned, there is no evidence of a general decline across the broad range of gastro-intestinal, respiratory and other endemic infections, even in developed countries. Additionally, although the findings of a recent large scale study of 24 341 mother–child pairs [ 229 ] confirmed that larger family sizes, early childcare, pet keeping and farm living correlates with decreased risk of atopic dermatitis (AD) in children before 18 months, the results suggested that experience of ID in early life is associated with increased, rather than reduced, risk of AD.
An alternative possibility is that, rather than infection, ‘background’ exposure to ‘subclinical’ doses of pathogens, or to commensal or environmental microbes, particularly those with low invasiveness or virulence such as the rapid growing saprophytic strains of Mycobacteria , or perhaps to endotoxins could be the key. In a recent review paper, Rook [ 13 ] proposes that the immune dysregulation associated with increased risk of atopy is a consequence of decreased exposure to certain microbes that are ‘old friends’, because of their continuous presence throughout mammalian evolution. He proposes that organisms such as saprophytic mycobacteria, helminths and lactobacilli are recognized by the immune system as harmless, and act as adjuvants for immune regulation. This has prompted work on the development of mycobacterial vaccines for the treatment of some diseases associated with immune dysregulation, with promising initial results [ 116 , 117 ]. The apparent protective effect against atopy of living on a farm in childhood is consistent with the possibility that ‘background exposure’ may be the critical factor.
Thus, despite some good evidence supporting a link between microbial exposure and susceptibility to atopic disease, clear evidence is still lacking as to the nature of the critical changes that might have occurred, whether it is the general level of exposure which is important, or exposure to specific microbes, whether exposure is only important at certain times of life, or whether the route of exposure is important etc.
The link between atopy, microbial exposure and hygiene practice in the home
The second key question for this review is whether the microbial exposure that is vital for the development of the immune system might no longer occur, or might occur to an insufficient extent, is a result of modern trends in hygiene and personal cleanliness.
Evidence of a link between atopy and domestic cleaning and hygiene is weak at best. Data published since the 1980s, as reviewed in this paper, show that our modern homes, whatever their visual appearance, still abound with a rich mixture of bacteria, viruses, fungi and moulds, as well as dust mites and other insects, and that our opportunities for exposure to these are quite likely to have increased rather than decreased, since a rising proportion of time is spent indoors [ 5 ]. The evidence shows that human, animal and food-borne microbes are continuously brought into the home and that transmission from these and other sources via hands, hand contact surfaces, food preparation surfaces and cloths during normal daily activities provide ample opportunities for exposure to foodborne pathogens or pathogens from infected people or pets, as well as exposure to commensals and environmental microbes.
Although consumption of cleaning products has increased over time, consumption overall or for individual product types for individual European countries shows no correlation with levels of atopy. In reality routine daily or weekly cleaning habits actually have little effect in reducing exposure to microbes beyond the levels that have probably prevailed throughout the rise in atopy, even where they involve use of a disinfectant. Re-colonization of surfaces rapidly occurs and many species are adapted to survival, even on apparently dry surfaces. Contrary to perception, domestic cleaning practices can actually increase the distribution of microbes in the home. While ‘hygiene’ practice (i.e. the specific actions we take to prevent transmission of disease) has been shown to be associated with reduced infection rates, observational studies indicate that consumer adherence to basic hygiene rules remains poor. Although the pattern of microbial exposure in the home may have changed, there is no evidence that our modern preoccupation for cleanliness has resulted in a decline in overall microbial exposure.
The suggestion that trends towards more frequent showering and bathing show a temporal correlation with the rise in atopy is superficially consistent with the results of the ALSPAC Study [ 184 , 185 ], but requires further investigation. In this study the emphasis was towards ‘routine’ rather than ‘targeted’ hygiene i.e. ‘parents were asked how often in a day they wiped the child's face and hands, whether hands were wiped before meals and how often the child was given a bath or shower’. Only 0.4% of children had the highest hygiene score, an insufficient proportion to account for the several fold rise in atopy that has been seen across the whole population.
From the evidence linking atopy to declining family size, it can be argued that, regardless of hygiene behaviour, a decrease in the number of people in the home inevitably decreases opportunities for person-to-person transfer of human commensals, or case-to-case spread of infections via direct or indirect contact or airborne transmission, although much depends on socio-economic factors such as overcrowding, bed-sharing and education level. However, if exposure to childhood infections or commensals is important, it should be found that the effects of decline in family size are offset by increased opportunities for exposure resulting from attendance at day nursery. Although there is some supporting data, other studies show no evidence of a protective effect. The strong evidence for a link between farm living and reduced risk of atopy also supports the possibility that exposure to environmental microbes (and possibly helminths) in our outdoor environment could be the key factor.
Quite apart from hygiene, there are a number of other lifestyle, medical and public health trends which could equally well have caused incidental changes in microbial exposure, manifesting as increased risk of atopy. For example, changes must have occurred in the non-pathogenic microbial flora of water or foods consequent on changing technologies of water purification and food production etc, but as food and water is only routinely monitored for pathogen content there are no data to show what these trends might have been. Alternatively the changes may have been generated by the introduction of antibiotics. This might have operated either by changing the nature, intensity and duration of exposure to pathogens, or by altering the normal balance of commensal microbes such as the gut flora. Although this fits well with the rise in atopy in temporal terms, the supporting data is inconsistent. The balance of evidence is also against vaccination as a causative factor. More important perhaps is the significant evidence supporting a range of ‘non-microbial’ factors, such as diet, obesity and lack of exercise which may be causative factors in the rise in atopy.
The implications for hygiene practice
On the basis of current evidence, relaxing hygiene standards seems neither justified, nor rational. On the contrary, current concerns about ID, and the key role that hygiene plays in controlling ID, provides compelling reasons why we should not do this.
As discussed previously, the global burden of ID is still a major concern, accounting for over 18 million deaths annually: while the majority of deaths occur in the developing world, infection also causes around 4% of deaths in developed countries [ 230 ]. Although ID mortality is declining in the developed world, trends in morbidity suggest a change in the pattern of ID rather than declining rates. This is partly associated with emergence of new infections, such as E. coli O157 and the re-emergence of old pathogens; tuberculosis has increased in Europe, including more invasive and antibiotic-resistant strains, while in the former soviet block countries, diphtheria cases rose 50-fold from 1989 to 1995 [ 230 ]. The globalization of infection risks has also increased because of global food markets, increased travel and refugee movements: thus pathogens can more readily reach areas where there is little or no innate resistance:
In recent decades, our attitudes to ID in developed countries have become more relaxed, nurtured by the evidence that improved quality of water, sanitation, housing and nutrition have produced a marked decline in infection mortality from infections such as typhoid fever and cholera. Antimicrobial therapy and advances in immunization have supplemented this trend. Yet several factors are combining to make it likely that the threat of ID will increase in coming years, rather than decrease. One such factor is the rising proportion of the population who are more vulnerable to infection. Such ‘vulnerable groups’ include the elderly: it is estimated that, by 2025, more than 800 million people will be aged over 65 years in the world, two-thirds of these in developing countries [ 231 ]. Other groups include neonates, people with chronic or degenerative illness; and immuno-compromised patients discharged from hospital. All of these groups, together with family members who are carriers of HIV, are increasingly cared for at home. Currently, about one in six persons in the UK belongs to an ‘at risk’ group, and it is likely that the same applies in most European countries [ 232 ].
In addition to the threat posed by acute infections, pathogens are increasingly implicated as causative or as co-factors in cancers and some degenerative diseases. Examples include Hepatitis B virus [ 233 ], C. jejuni [ 234 ] and H. pylori [ 235 ]. Foodborne illness has been estimated to result in chronic sequelae in 2–3% of cases [ 236 ]; a more recent report from the European Commission [ 237 ] cited evidence of chronic disease, such as reactive arthritis, following 5% of Salmonella cases, with 5% also of E. coli O157 cases progressing to the serious and often fatal complication of uraemic syndrome.
Antibiotic resistance is a global problem with resistant strains such as MRSA spreading into communities [ 238 , 239 ]. The need for improved hygiene to reduce the spread of antibiotic resistance has been recommended by recent working parties in Europe [ 240 ]. Reduced rates of infection and antibacterial resistance have been demonstrated where an approach combining good hygiene and reduced prescribing has been evaluated [ 241 , 242 ].
Measures such as water purification, sanitation, waste disposal and hygiene have played an essential role over the past two centuries in reducing the burden of ID, most markedly in developed countries. For developing countries, where the ID burden remains high, it is now apparent that health gains commensurate with investment in programmes of water and sanitation will only be achieved if steps are also taken to improve standards of hygiene practice. A review of intervention studies [ 224 ] suggests that, even in the developed world, improved standards of personal and environmental hygiene could reduce infection transmission by over 20%. The overall conclusion is that hygiene is a key cornerstone in the control of ID, and that a significant increase in morbidity and mortality from infection would result from any attempt to reduce the integrated practices of sanitation, clean water provision and hygiene practice.
Developing a rational approach to home hygiene
Regardless of whether the hygiene hypothesis is correct, the popular interpretation that ‘dirt is good for us’ [ 243 ] has considerably influenced attitudes, and caused loss of confidence among the public regarding home hygiene. One positive benefit however is a recognition by public health professionals of the need to provide clearer guidance. One of the concepts which we need to clarify is the difference between ‘dirt’ and ‘germs’, and between ‘cleanliness’ and ‘hygiene’. Without knowing the nature of the microbial exposure which may be critical for immune priming, it is difficult to reformulate hygiene policy, in favour of improving immune function without compromising protection against ID. Even if we had the correct information, selective targeting of hygiene interventions, as a means of maintaining beneficial microbial exposure, would only be an option if their mode of transmission were significantly different from that for pathogens. If it were proved that intense infection is an essential factor, the evidence suggests that encouraging such exposures would cause significant morbidity and mortality; if the main effect was, for example, a reduction in hayfever, with little or no impact on asthma, the ‘trade off’ would represent a very poor bargain. If it turns out that more general ‘background’ exposure is needed, e.g. organisms with low invasiveness or virulence, such as the rapid growing saprophytic strains of Mycobacteria , the idea of ‘right’ and ‘wrong’ types of microbial exposure is academic, unless we could engineer the ‘right’ exposure, without introducing dangerous organisms. As untreated water may contain up to 10 9 mycobacteria per litre, the difficulty is how to preserve the ‘friendly’ species while removing those likely to cause disease. One option that is already being pursued is an attenuated vaccine containing the ‘right’ type of microbes (e.g. saprophytic mycobacteria), and there is evidence of efficacy of a vaccine strategy in animal studies [ 244 ] and in some of human trials [ 245 , 246 ]. With vaccine strategies, there is no conflict with hygiene.
If background exposure is proved to be the important factor, this opens up the way to promote an approach to hygiene which focuses on preventing exposure to infectious doses of pathogens, but is more relaxed about other exposures. As part of its work to promote better understanding of hygiene and better hygiene practice, the International Scientific Forum on Home Hygiene (IFH) has produced guidance documents on home hygiene practice [ 247 , 248 ]. The key feature of the guidelines is that they are based on the concept of risk assessment and risk prevention. [ 249 , 250 ]. Risk assessment (also known as HACCP or Hazard Analysis Critical Control Point) is now the accepted approach for controlling microbial risks in food and other manufacturing environments. Applied to the home this has come to be known as ‘targeted hygiene’.
The IFH guidelines start from the premise that homes always contain potentially harmful microbes (from people, pets, food, etc.) and that ID prevention is not about eradication, but about targeting measures in the places and at the times that matter, in order to limit risks of exposure. Fundamental to developing infection prevention policies is the need to recognize that the home is an environment where all human activities occur including food hygiene, personal hygiene (particularly hands) and hygiene related to care of vulnerable groups, all of which are based on the same underlying microbiological principles. Hygienic cleanliness (reducing the level of contamination to a level that does not pose a significant risk) is required only where the infection risk is significant e.g. after contact with excreta, during food preparation etc. The level of risk varies according to occupants of the home (e.g. presence of children, pets, ill people) and their immune status.
Although this review concludes that the relationship of the hygiene hypothesis to hygiene practice has not been proved, it lends strong support to initiatives which seek to improve hygiene practice. Whatever the reality regarding atopy and microbial exposure, ‘targeted hygiene’ with its emphasis on selective hygiene intervention when and where risks of infection are greatest makes sense on its own merits because it seeks to maximize protection against the harmful effects of ID, while retaining the beneficial effects which microbes may have on our human and natural environment.
The term ‘hygiene hypothesis’ initially raised awareness of the role of microbes and their products in immune regulation. It has also stimulated a considerable amount of research into the aetiology of atopic disease. This research has highlighted that the term has probably now outlived its usefulness. Several of its proponents have suggested renaming the hypothesis as the ‘microbial deprivation hypothesis’ (Bjorksten [ 251 ]) or the ‘old friends hypothesis’ (Rook [ 13 ]). Avoiding the term ‘hygiene’ would help focus attention on determining the true impact of microbes on atopic diseases, while minimizing risks of discouraging good hygiene practice.
MINI REVIEW article
The hygiene hypothesis – learning from but not living in the past.

- 1 Comprehensive Biobank Marburg, Medical Faculty, Philipps University of Marburg, Comprehensive Biobank Marburg, Marburg, Germany
- 2 German Center for Lung Research (DZL), Marburg, Germany
- 3 German Biobank Alliance, Marburg, Germany
- 4 Institute for Pathology, Medical Faculty, Institute for Pathology, Philipps University of Marburg, Marburg, Germany
- 5 Translational Inflammation Research Division & Core Facility for Single Cell Multiomics, Medical Faculty, Biochemical Pharmacological Center, Philipps University of Marburg, Marburg, Germany
Postulated by Strachan more than 30 years ago, the Hygiene Hypothesis has undergone many revisions and adaptations. This review journeys back to the beginnings of the Hygiene Hypothesis and describes the most important landmarks in its development considering the many aspects that have refined and generalized the Hygiene Hypothesis over time. From an epidemiological perspective, the Hygiene Hypothesis advanced to a comprehensive concept expanding beyond the initial focus on allergies. The Hygiene Hypothesis comprise immunological, microbiological and evolutionary aspects. Thus, the original postulate developed into a holistic model that explains the impact of post-modern life-style on humans, who initially evolved in close proximity to a more natural environment. Focusing on diet and the microbiome as the most prominent exogenous influences we describe these discrepancies and the resulting health outcomes and point to potential solutions to reestablish the immunological homeostasis that frequently have been lost in people living in developed societies.
Last year we celebrated the 30th anniversary of the Hygiene Hypothesis. Since Strachan framed the Hygiene Hypothesis in 1989 ( 1 ) his fundamental idea to explain the origins of allergic diseases development has survived the test of time. The basic idea of how humans, their microbiota, and a continuously modernizing environment have interacted to drive immune dysregulation has persisted and become part of the popular imagination. Here, we aim to provide an editorial overview on the history of the Hygiene Hypothesis and related topics to offer a framework for the articles collected in the special edition research topic “The Hygiene Hypothesis and its Immunological Implications.”
A Chronological Overview
The epidemiological basis for the Hygiene Hypothesis became apparent long before the Hygiene Hypothesis was postulated. Two simple observations were made in the 1960s and in the 1970s. First, a Swedish study described differences in the prevalence of asthma and socio-medical conditions between populations living in urban or rural sites ( 2 ). A few years later, in a population-based study conducted in Saskatchewan, Canada, showed that allergies were less frequent in native tribes living traditionally in rural sites compared to Caucasian Canadians living in urban habitats ( 3 ). Moreover, the authors postulated that frequent bacterial infections in childhood might be responsible for the inverse association with allergic diseases. Strachan's observations made in the late 80s in a British population corroborated these findings and he later named this concept “Hygiene Hypothesis” in 2000. Briefly, Strachan suggested that transfer of early childhood infections between siblings is associated with protection against allergies later in life ( 4 ).
The hypothesis was further substantiated and extended by studies that compared asthma and allergy prevalence directly after the “Fall of the Iron Curtain” between Western and Eastern Germany, a decade later ( 5 , 6 ). Interestingly, these studies triggered a paradigm shift in allergy research. Until then, environmental pollution was broadly regarded as the leading force for allergy development. Environmental data clearly indicated a higher level of pollution by industrial emissions in Eastern Germany compared to the Western part and the study team therefore hypothesized that the prevalence of allergic diseases was higher in children from Eastern Germany. Surprisingly, the researchers found their hypothesis disproved, as children in Western Germany showed a higher prevalence of allergies. Hence, it was postulated that other exposures than pollutants influence the development of atopic diseases. Socio-demographic and –economic factors, as well as household hygiene turned out to be further discriminatory factors between both parts of German population. Improved sanitation and hygiene were positively associated with atopic diseases. Another decade later a follow-up further validated this hypothesis and found life-style differences and the prevalence of atopic diseases began to equilibrate within 10 years after the reunification. In consequence, the Hygiene Hypothesis became the leading postulate to explain underlying relationships and mechanisms for the development of allergic diseases in a societal context ( 6 ).
Based on this paradigm shift, Rook published the “Old Friends-Hypothesis” which argues that infectious diseases have a long co-evolutionary history with human development, and appropriate levels of exposure to these microorganisms early in life might protect against immune deviation and allergic diseases. These early-life exposures to potential pathogens might educate the developing immune system from a type-2-dominated in utero -milieu toward a more defensive T helper (h)1 response ( 7 ).
The next milestone involved findings obtained from the so-called “Alpine farm studies” conducted at the turn of the millennium. Von Mutius and Braun-Fahrländer recognized the unique situation that the Alpine traditional farming environment represents a socio-cultural and ecological niche which significantly differs from the post-modern and urbanized life-style. In a number of epidemiological studies they identified traditional farming characteristics such as consumption of unprocessed farm milk and close contact with farm animals to act allergoprotective and found these parameters to be associated with a higher microbial load. These Alpine farm studies added substantial evidence to Strachan's basic idea and led to a broader view and understanding of the relationship between human health and (early life) exposure to microbes ( 8 , 9 ).
Further evidence was added by studies conducted in Northern Europe. In the late 1990s studies conducted in Scandinavian und Baltic children described microbial factors to be associated with a lower prevalence of allergic diseases in the Eastern countries ( 10 – 12 ). Next, the Karelia Study, conducted on both sides of the Finnish-Russian border, addressed the impact of the environmental microbial burden on the development of allergic diseases in Finnish and Russian Karelian children that share the same ethnic background but have different life-styles ( 13 ). These studies corroborated the Alpine farm studies and point to the microbial environment as a major factor in allergy development.
Furthermore, these studies demonstrate that the diversity and the richness of an immune-stimulating microbial world in human habitats is crucial to establish a competent, tolerogenic and defensive immune system configuration while absence or depletion of those stimuli as found in post-modern environments foster immune deviation and development of allergic diseases ( 14 ).
Moreover, two relevant studies [the cross-sectional study “Prevention of Allergy Risk factors for Sensitization In children related to Farming and Anthroposophic Lifestyle (PARSIFAL)” and the multi-center, pregnancy/birth cohort study “Protection against Allergy: Study in Rural Environments (PASTURE)”] support the idea that the “window of opportunity” in which the appropriate education of the immune system starts already in the mother's womb ( 15 – 17 ). The PARSIFAL Study demonstrated that maternal exposure to a farm environment rich in microbial compounds is inversely associated with the development of atopic sensitization and correlated with an upregulation of receptors of the innate immune system in the offspring at school age ( 15 ). Further, maternal farm activities during pregnancy were shown to modulate cord blood cytokines and allergen-specific immunoglobulin responses toward a Th1 pattern ( 16 , 17 ). These findings are in line with the Barker theory ( 18 ), postulating that pathological pathways occurring in adolescence and adulthood are paved already in prenatal life.
The Hygiene Hypothesis and the Bacterial World
Even before high-throughput sequencing techniques were established that allow a deeper view into the microbial world on our body surfaces, Noverr and Hufnagle proclaimed the “Microbiota Hypothesis” by which they claimed the microbiota to be indispensable for developing and maintaining a tolerogenic immune status ( 19 ). A similar idea concept was proposed earlier by Holt, Sly and Björkstén ( 20 ). The rediscovery of the microbiota and its powerful metabolic and immunologic interplay with the mucosal surfaces of the host underlined and complemented the principals of this basic idea ( 21 , 22 ). Microbiome research has made significant achievements over the past 15 years; here we can emphasize only a few aspects that might be relevant in the context of the Hygiene Hypothesis and the development of allergies.
Phylogenetic Impacts
An intriguing concept to better understand the complex symbiotic interplay at organ surfaces was suggested by McFall-Ngai in 2007. In her evolutionary perspective she shed light on findings made in invertebrates which not only lack an endoskeleton but also an adaptive immune system. Thus, invertebrates have to exclusively rely on their innate immune system, which to our current understanding, lacks an immunological memory. Analyses of the intestinal microbiota in such animals have shown—in contrast to vertebrates—a rather low diversity in the community of their microbial residents. Only a handful of strains could be identified as stable colonizers on the gastrointestinal surfaces while most bacteria travel through as transient visitors. Some invertebrates, like insects, separate bacterial colonies from epithelial host cells by a peritrophic matrix composed of chitin and other compounds ( 24 ). During the course of evolution, the microbial colonization of epithelia started to get more complex and in turn the host was challenged to develop new strategies to manage these diversifying communities. To permanently recognize a specific bacterium as beneficial or harmful, an adaptive immune response that provides an immunological memory over generations of immune cells was needed. Mutual adaption of both partners, the bacterial community, as well as the complex network of adaptive immune cells, led to a sophisticated metabolic and immunologic interplay with a highly digestive and defensive performance. This symbiosis is based on early education of the host's immune cells by a diverse microbial community to successfully discriminate dangerous pathogens from beneficial symbionts and own healthy cells. Finally McFall-Ngai stated, that complex systems might be prone to failure and allergies and autoimmune disorders might be a consequence of this ( 23 ).
Ontogenetic Impacts
A number of recently published reports substantiated the impact of the early life microbiota on immune maturation [recently reviewed in ( 25 )] and the development of allergic disorders in early infancy [recently reviewed in ( 26 )]. The developmental starting point of the infant gut microbiota is still unknown, but undoubtedly, the process of delivery seems to be a key point in the development of the neonatal microbiota ( 27 ). Meconium, the neonate's first intestinal discharge, was shown to contain various bacterial strains indicating that the perinatal gut is colonized by bacteria ( 28 , 29 ). In a landmark study, Dominguez-Bello et al. reported that the neonatal microbiota differs between vaginally born infants and neonates delivered by Caesarian (C)-section. The authors found a high abundance of Bacteroides, Bifidobacterium , and Lactobacillus spec . in meconium samples obtained from vaginally delivered newborns, while Staphylococcus, Streptococcus, Corynebacterium , and Propionibacterium spp . were found predominantly in meconium samples of C-section born neonates ( 30 ).
Colonization of the neonate's colon by Lactobacilli and Bifidobacteria transferred from the maternal vaginal compartment during vaginal passage might provide advantages for the newborn due to the metabolic properties of these bacteria that foster the adaptation to milk-based feeding. These bacteria are capable of metabolizing breast milk-derived lactose and human milk oligosaccharides (HMOS) ( 31 ) and were shown to provide immune-modulating short chain fatty acids (SCFAs) ( 32 ) and conjugated trans-linoleic acids (tCLAs) ( 33 ), which are shown to reduce pro-inflammatory eicosanoid production by regulating the transcription of cyclooxygenase 2 (COX-2) ( 34 ) and to induce anti-inflammatory M2-macrophage differentiation ( 35 ).
However, how sustainable and decisive are these mode of delivery-associated differences beyond the neonatal age? Chu et al. recently showed that function and composition of the microbiota significantly diversifies in all body sites within the first 6 weeks of life, resembling the corresponding maternal body site microbiota at this time point. Infant's mode of delivery or other prenatal factors seems to have no impact on this development ( 36 ). Data from the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC 2010 ) cohort underlined the importance of the maturation of the microbiota on the further development of the gut microbiome and the risk of asthma later in life. In that study, Stockholm et al. compared the gut microbiome of vaginally and C-section delivered infants from birth to 1 year of life in the context of asthma development at school age. Marked differences between C-section and vaginally delivered infants were observed by 1 week and by 1 month of life, but only minor differences between these groups were found by 1 year of age. An increased risk for school-age asthma was only observed in a subgroup of C-section-born infants that maintained the C-section-associated composition for at least 1 year. The authors conclude that vaginal delivery and/or subsequent maturation of the infant microbiota might support a more robust and stable microbiota in the offspring that is more adaptive to the challenges later in life ( 37 ). Further exposure to the maternal microbiota ( 38 ), as well as nutritional impacts (e.g., cessation of breastfeeding) ( 39 ) within the first month of life, might foster the maturation of the gut microbiome in early infancy.
Nutritional Impacts
How is the microbiota linked to the rising atopic epidemic observed in the recent decades? A recently published study conducted in indigenous tribes living in the Brazilian Amazonas-Orinoco Basin may help to answer this question ( 40 ). In this study the gut microbiome of the semi-nomadic gatherer/hunter people of the Yanomami who maintained a primitive close-to-nature life-style was compared to subjects representing populations that are characterized by a westernized or non-ancestral life-style in rural and urban settings. The Yanomami microbiota was significantly more diverse than those of the westernized counterparts. Moreover, an additional study comparing Venezuelan with Brazilian Yanomami indicated a high level of adaptability to specific environmental conditions of the microbiota in these peoples. While a high taxonomic diversity was found in both sub-tribes, the composition of microbiota was significantly different ( 41 ). These findings point to environmental and life-style factors that influence the composition of the microbiota the absence of which may thus foster the loss of taxonomic and metabolic diversity in westernized societies ( 42 ).
Diet is one of the most prominent environmental factors that differ between modern and ancient life-styles. While dietary habits in indigenous people such as the Yanomami strongly depend on the sometimes limited food supply due to seasonal cycling, people living in developed societies have access to high in calories food ready at any time and in abundance. Moreover, diet in indigenous cultures is often based on high-fiber products derived from plants that are easy to culture such as plantain, manioc or sweet potatoes, all rich in inulin ( 43 ). High-fiber diet and, in particular inulin, is known as an effective enhancer of beneficial bacteria such as Bifidobacteria in the colon that stabilize gut homeostasis ( 44 ). Translating these findings into a clinical approach, McLoughlin et al. applied soluble inulin to asthmatics in a short-term placebo-controlled-trial and could report an array of beneficial effects in patients orally treated with inulin. In comparison to the placebo group, inulin-treated patients displayed a significantly reduced number of eosinophils in the sputum and, overall, reported a significantly improved asthma control. Inhibition of histone deacetylase 9 (HDAC9) in sputum cells upon a combined application of inulin and a multi-strain probiotic mixture of Lactobacillus acidophilus, Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis indicated that epigenetic pathways are involved in the mechanisms by which lactic acid bacteria modulate host responses in combination with the prebiotic gavage ( 45 ).
A number of recently recognized metabolites released by beneficial symbiotic bacteria convey immunomodulatory effects, mainly in the gut but also on other mucosal surfaces ( 46 ).
In particular, SCFAs derived from dietary fibers and released in the lumen of the colon contribute to immune modulation and inhibition of pro-inflammatory cytokines when absorbed by gut epithelial cells ( 47 ). By binding to chemoattractant G protein 43 receptor, SCFAs are capable of regulating inflammatory responses ( 48 ) as shown for intestinal inflammation ( 49 ). Tryptophan, an amino acid produced by an array of beneficial microorganisms, is degraded to indole derivatives which may bind to the aryl hydrocarbon receptor (AHR) and by this regulate the activity of immune cells at the epithelial barrier. That involves AHR-dependent differentiation of regulatory T cells associated with anti-inflammatory IL-10 expression. Further, Th2-cells are inhibited on the transcription factor level in favor of a Th1 response ( 50 ).
A number of beneficial bacteria contribute to the orchestration of T cell subsets at the gut epithelial barrier. Bacteroides sp . and Clostridium clusters IV and XIVa colonizing the gut epithelium are known to stimulate intestinal epithelial cells to release thymic stromal lymphopoietin (TSLP), transforming growth factor (TGF)-ß and interleukin (IL)-25 which in combination may induce tolerogenic effects in dendritic cells (DCs) ( 51 ), e.g., by secretion of TGF-ß and retinoic acid. Both factors initiate differentiation of naïve T cells to regulatory T cells upon activation of the nuclear transcription factor forkhead box P (FoxP3) ( 52 ). These regulatory mechanisms are challenged by “pathobionts” or other damage factors. In presence of these stressors, overexpansion of Th1, Th2 and Th17 effector cell subsets might result in an inflammatory response in the infected organ or, by migration of these cells, at distant sites. Namely, Clostridium difficile , which is associated with wheezing and atopic sensitization, was shown to initially disturb the intestinal balance when acquired early in childhood ( 53 ).
Traveling from the gastrointestinal to the respiratory tract the microbiota established in the lung might also play a role in the development of allergic disorders, namely of allergic asthma. Though the gut is known to play a major role in establishing and regulating immune defense mechanisms, the “gut-lung axis” alone might not completely explain the rise of allergic asthma ( 54 ). As many studies focused on the lung microbiome, it has become clear that there is a strong relationship between frequently inhaled environmental microbes, microbial colonization of the respiratory tract, and the prevalence of allergic asthma ( 55 ). For example, results from the “Multidisciplinary Study to Identify the Genetic and Environmental Causes of Asthma in the European Community (GABRIEL) Advanced Studies (GABRIELA)” study suggested a transfer of built-environment-associated bacteria into the respiratory tract. Indoor dust samples from farm houses and nasal swabs from farm children displayed a higher bacterial diversity than those samples collected in rural non-farm children ( 56 ). New evidence was added recently by studies conducted in the Finnish part of the PASTURE-study. Kirjavainen et al. reported that the ecological diversity of the so-called “indoor microbiota” is inversely linked to the prevalence of allergic asthma. Substantiating former farm studies, this report further validated the hypothesis that microbial diversity and composition in the natural environment is linked to a reduced risk of early-onset allergic asthma and that traditional farming is a proxy for this effect ( 57 ).
But what are the cellular and molecular mechanisms associated with high microbial diversity? Interestingly, the farm studies consistently showed an inverse association between a highly diverse environmental microbiota and allergic asthma, but this did not account to other allergic manifestations such as hay fever or atopic sensitization. On the other hand, endotoxin exposure protects against atopy but fosters the risk of non-allergic asthma and early onset of wheeze when inhaled in higher concentrations. These findings derived from the farm studies still challenge the Hygiene Hypothesis and might point out that microbial colonization and exposure to microbial compounds have to be considered separately ( 58 ). Integration of beneficial environmental bacteria into the microbial community of the respiratory tract leads to a tolerogenic mucosal symbiosis that establishes a local T-cell balanced anti-inflammatory milieu at the epithelium, probably enhanced by a well-balanced gut microbiota. Endotoxins are potent activators of innate TLR-signaling and can attenuate B cell driven sensitization and formation of IgE-antibodies ( 59 ). Already in 2003, Vercelli postulated a switch from Th2-driven allergic responses at low endotoxin exposure to a pronounced Th1 response in the lung under high levels of environmental endotoxin. This might explain the elevated prevalence of non-allergic asthma in environments overloaded with endotoxin ( 60 ).
Conclusions
The many aspects and facets of the Hygiene Hypothesis have been supported by concepts and findings coming from a variety of scientific disciplines such as epidemiology, immunology, microbiology and anthropology. Within the last three decades we obtained a multiplicity of new insights into the complexity and plasticity of T cell networks which led us to recognize the complexity and significance of a powerful and well-regulated adaptive immune response in relation to exogenous factors ( 61 ). Early developmental findings characterizing pre and postnatal life events highlighted the initial role of the innate immune system as an early warning system that orchestrates, educates and shapes subsequent immune responses ( 62 , 63 ). Evidence from evolutionary biology and anthropology enabled us to understand how host-environment interactions are refined throughout evolutionary adaption ( 58 , 64 ). Microbiology added fundamental knowledge about the micro-ecosystem that is established throughout the human body as a unique symbiosis between humans and microbes. And finally, coming back to the introductory statement, epidemiological observations such as those initially made by Strachan and von Mutius about 30 years ago still challenge and refine the hypothesis.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was funded by the Library of the Philipps-University of Marburg.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Strachan DP. Hay fever, hygiene, and household size. BMJ. (1989) 299:1259–60. doi: 10.1136/bmj.299.6710.1259
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Irnell L, Kiviloog J. Bronchial asthma and chronic bronchitis in a Swedish urban and rural population. With special reference to prevalence, respiratory function and socio-medical condition. Scand J Respir Dis Suppl. (1968) 66:1–86.
PubMed Abstract | Google Scholar
3. Gerrard J. Serum IgE levels in white and metis communities in saskatchewan. Ann Allergy. (1976) 37:91–100.
4. Strachan DP. Family size, infection and atopy: the first decade of the “Hygiene Hypothesis”. Thorax. (2000) 55(Suppl. 1):S2–10. doi: 10.1136/thorax.55.suppl_1.S2
5. Weiland SK, von Mutius E, Hirsch T, Duhme H, Fritzsch C, Werner B, et al. Prevalence of respiratory and atopic disorders among children in the East and West of Germany five years after unification. Eur Respir J. (1999) 14:862–70. doi: 10.1034/j.1399-3003.1999.14d23.x
6. Heinrich J, Hoelscher B, Frye C, Meyer I, Wjst M, Wichmann HE. Trends in prevalence of atopic diseases and allergic sensitization in children in Eastern Germany. Eur Respir J. (2002) 19:1040–6. doi: 10.1183/09031936.02.00261802
7. Rook GA, Brunet LR. Microbes, immunoregulation, and the gut. Gut. (2005) 54:317–20. doi: 10.1136/gut.2004.053785
CrossRef Full Text | Google Scholar
8. Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. (2001) 358:1129–33. doi: 10.1016/S0140-6736(01)06252-3
9. Braun-Fahrländer C, Gassner M, Grize L, Neu U, Sennhauser FH, Varonier HS, et al. Prevalence of hay fever and allergic sensitization in farmer's children and their peers living in the same rural community. SCARPOL team. swiss study on childhood allergy and respiratory symptoms with respect to air pollution. Clin Exp Allergy . (1999) 29:28–34. doi: 10.1046/j.1365-2222.1999.00479.x
10. Björkstén B, Dumitrascu D, Foucard T, Khetsuriani N, Khaitov R, Leja M, et al. Prevalence of childhood asthma, rhinitis and eczema in Scandinavia and Eastern Europe. Eur Resp J. (1998) 12:432–7. doi: 10.1183/09031936.98.12020432
11. Bråbäck L, Breborowicz A, Julge K, Knutsson A, Riikjärv M, Vasar M, et al. Risk factors for respiratory symptoms and atopic sensitization in the Baltic area. Arch Dis Child. (1995) 72:487–93. doi: 10.1136/adc.72.6.487
12. Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic estonian and swedish 2-year old children. Clin Exper Allergy . (1999) 29:342–6. doi: 10.1046/j.1365-2222.1999.00560.x
13. Seiskari T, Kondrashova A, Viskari H, Kaila M, Haapala AM, Aittoniemi J, et al. Allergic sensitization and microbial load–a comparison between Finland and Russian Karelia. Clin Exp Immunol. (2007) 148:47–52. doi: 10.1111/j.1365-2249.2007.03333.x
14. Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. (2011) 364:701–9. doi: 10.1056/NEJMoa1007302
15. Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. (2006) 117:817–23. doi: 10.1016/j.jaci.2005.12.1307
16. Pfefferle PI, Sel S, Ege MJ, Büchele G, Blümer N, Krauss-Etschmann S, et al. Cord blood allergen-specific IgE is associated with reduced IFN-gamma production by cord blood cells: the protection against allergy-study in rural environments (pasture) study. J Allergy Clin Immunol. (2008) 122:711–16. doi: 10.1016/j.jaci.2008.06.035
17. Pfefferle PI, Büchele G, Blümer N, Roponen M, Ege MJ, Krauss-Etschmann S, et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: the PASTURE study. J Allergy Clin Immunol. (2010) 125:108–15.e1-3. doi: 10.1016/j.jaci.2009.09.019
18. Barker DJ. The fetal and infant origins of disease. Eur J Clin Invest. (1995) 25:457–63. doi: 10.1111/j.1365-2362.1995.tb01730.x
19. Noverr MC, Huffnagle GB. The ‘microflora hypothesis' of allergic diseases. Clin Exp Allergy. (2005) 35:1511–20. doi: 10.1111/j.1365-2222.2005.02379.x
20. Holt P, Sly P, Björkstén B. Atopic versus infectious disease in childhood: a question of balance? Pediatr Allergy Immunol. (1997) 8:1–5 doi: 10.1111/j.1399-3038.1997.tb00145.x
21. Fyhrquist N. The human microbiota and its relationship with allergies. Gastroenterol Clin North Am. (2019) 48:377–87. doi: 10.1016/j.gtc.2019.04.005
22. von Mutius E. A fascinating look at the world with a new microscope. J Allergy Clin Immunol. (2012) 129:1202–3. doi: 10.1016/j.jaci.2011.12.994
23. McFall-Ngai M. Adaptive immunity: care for the community. Nature. (2007) 445:153. doi: 10.1038/445153a
24. Wong ACN, Vanhove AS, Watnick PI. The interplay between intestinal bacteria and host metabolism in health and disease: lessons from drosophila melanogaster. Dis Model Mech. (2016) 9:271–81. doi: 10.1242/dmm.023408
25. Al Nabhani Z, Eberl G. Imprinting of the immune system by the microbiota early in life. Mucosal Immunol. (2020) 13:183–18. doi: 10.1038/s41385-020-0257-y
26. Peroni DG, Nuzzi G, Trambusti I, Di Cicco ME, Comberiati P. Microbiome composition and its impact on the development of allergic diseases. Front Immunol. (2020) 11:700. doi: 10.3389/fimmu.2020.00700
27. Mesa MD, Loureiro B, Iglesia I, Fernandez Gonzalez S, Llurba Olivé E, García Algar O, et al. The evolving microbiome from pregnancy to early infancy: a comprehensive review. Nutrients. (2020) 12:133–54. doi: 10.3390/nu12010133
28. Jimenez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, et al. Is meconium from healthy newborns actually sterile? Res Microbiol. (2008) 159:187–93. doi: 10.1016/j.resmic.2007.12.007
29. Hansen R, Scott KP, Khan S, Martin JC, Berry SH, Stevenson M. First-pass meconium samples from healthy term vaginally-delivered neonates: an analysis of the microbiota. PLoS ONE. (2015) 10:e0133320. doi: 10.1371/journal.pone.0133320
30. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. (2010) 107:11971–75. doi: 10.1073/pnas.1002601107
31. Plaza-Díaz J, Fontana L, Gil A. Human milk oligosaccharides and immune system development. Nutrients. (2018) 10:1038–55. doi: 10.3390/nu10081038
32. Rivière A, Selak M, Lantin D. Butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. (2016) 7:979–80. doi: 10.3389/fmicb.2016.00979
33. Kishino S, Takeuchi M, Park SB, Hirata A, Kitamura N, Kunisawa J. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci USA. (2013) 110:17808–13. doi: 10.1073/pnas.1312937110
34. Ringseis R, Müller A, Herter C, Gahler S, Steinhart H, Eder K, et al. CLA isomers inhibit TNFalpha-induced eicosanoid release from human vascular smooth muscle cells via a PPARgamma ligand-like action. Biochim Biophys Acta. (2006) 1760:290–300. doi: 10.1016/j.bbagen.2005.12.002
35. Ohue-Kitano R, Yasuoka Y, Goto T, Kitamura N, Park SB, Kishino S, et al. α-Linolenic acid-derived metabolites from gut lactic acid bacteria induce differentiation of anti-inflammatory M2 macrophages through G protein-coupled receptor 40. FASEB J. (2018) 32:304–18. doi: 10.1096/fj.201700273R
36. Chu DM, Ma J, Prince A, Antony KM, Sefrovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. (2017) 23:314–26. doi: 10.1038/nm.4272
37. Stokholm J, Thorsen J, Blaser MJ, Rasmussen MA, Hjelmsø M, Shah S, et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci Transl Med. (2020) 12:eaax9929. doi: 10.1126/scitranslmed.aax9929
38. Mortensen MS, Rasmussen MA, Stokholm J, Brejnrod AD, Balle C, Thorsen J, et al. Modeling transfer of vaginal microbiota from mother to infant in early life. Elife. (2021) 10:e57051. doi: 10.7554/eLife.57051
39. Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. (2015) 17:690–703. doi: 10.1016/j.chom.2015.04.004
40. Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, et al. The microbiome of uncontacted amerindians Sci Adv . (2015) 1:e1500183. doi: 10.1126/sciadv.1500183
41. Conteville LC, Oliveira-Ferreira J, Vicente ACP. Gut microbiome biomarkers and functional diversity within an amazonian semi-nomadic hunter-gatherer group. Front Microbiol. (2019) 10:1743. doi: 10.3389/fmicb.2019.01743
42. Moeller AH. The shrinking human gut microbiome. Curr Opin Microbiol. (2017) 38:30–5. doi: 10.1016/j.mib.2017.04.002
43. Lizot J. Resources and warfare among the Yanomami. Man New Ser. (1977) 12:497–17. doi: 10.2307/2800552
44. Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. (1995) 108:975–82. doi: 10.1016/0016-5085(95)90192-2
45. McLoughlin R, Berthon BS, Rogers GB, Baines KJ, Lex EX, Leong LEX, et al. Soluble fibre supplementation with and without a probiotic in adults with asthma: a 7-day randomised, double blind, three way cross-over trial. EBioMedicine. (2019) 46:473–85. doi: 10.1016/j.ebiom.2019.07.048
46. O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. (2016) 13:691–706. doi: 10.1038/nrgastro.2016.165
47. Smith PM, Howitt MR, Panikoc N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science. (2013) 341 569–73. doi: 10.1126/science.1241165
48. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. (2009) 461:1282–86. doi: 10.1038/nature08530
49. Sina C, Gavrilova O, Förster M, Till A, Derer S, Hildebrand F, et al. G protein- coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. (2009) 183:7514–22. doi: 10.4049/jimmunol.0900063
50. Hao N, Whitelaw ML. The emerging roles of AhR in physiology and immunity. Biochem Pharmacol. (2013) 86:561–70. doi: 10.1016/j.bcp.2013.07.004
51. Brown EM, Arrieta MC, Finlay BB. A fresh look at the hygiene hypothesis: how intestinal microbial exposure drives immune effector responses in atopic disease. Semin Immunol. (2013) 25:378–87. doi: 10.1016/j.smim.2013.09.003
52. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. (2011) 331:337–4. doi: 10.1126/science.1198469
53. van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. (2011) 128:948–55. doi: 10.1016/j.jaci.2011.07.027
54. Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. (2015) 12(Suppl. 2):S150–6. doi: 10.1513/AnnalsATS.201503-133AW
55. Fabbrizzi A, Amedei A, Lavorini F, Renda T, Fontana G. The lung microbiome: clinical and therapeutic implications. Intern Emerg Med. (2019) 14:1241–50. doi: 10.1007/s11739-019-02208-y
56. Birzele LT, Depner M, Ege MJ, Engel M, Kublik S, Bernau C, et al. Environmental and mucosal microbiota and their role in childhood asthma. Allergy. (2017) 72:109–19. doi: 10.1111/all.13002
57. Kirjavainen PV, Karvonen AM, Adams RI, Täubel M, Roponen M, Tuoresmäki P, et al. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat Med. (2019) 25:1089–95. doi: 10.1038/s41591-019-0469-4
58. Ege MJ. The hygiene hypothesis in the age of the microbiome. Ann Am Thorac Soc. (2017) 14(Suppl. S3):48–53. doi: 10.1513/AnnalsATS.201702-139AW
59. Vatanen T, Kostic AD, d'Hennezel E, Siljander H, Franzosa EA, Yassour M, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. (2016) 165:1551. doi: 10.1016/j.cell.2016.05.056
60. Vercelli D. Learning from discrepancies: CD14 polymorphisms, atopy and the endotoxin switch. Clin Exp Allergy. (2003) 33:153–5. doi: 10.1046/j.1365-2222.2003.01606.x
61. Haspeslagh E, Heyndrickx I, Hammad H, Lambrecht BN. The hygiene hypothesis: immunological mechanisms of airway tolerance. Curr Opin Immunol. (2018) 54:102–8. doi: 10.1016/j.coi.2018.06.007
62. Conrad ML, Ferstl R, Teich R, Brand S, Blümer N, Yildirim AO, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe acinetobacter lwoffii F78. J Exp Med. (2009) 206:2869–77. doi: 10.1084/jem.20090845
63. Pivniouk V, Gimenes Junior JA, Honeker LK, Vercelli D. The role of innate immunity in asthma development and protection: lessons from the environment. Clin Exp Allergy. (2020) 50:282–90. doi: 10.1111/cea.13508
64. Parker W, Ollerton J. Evolutionary biology and anthropology suggest biome reconstitution as a necessary approach toward dealing with immune disorders. Evol Med Public Health. (2013) 2013:89–103. doi: 10.1093/emph/eot008
Keywords: hygiene hypothesis, allergy, asthma, immune tolerance, T cell-response, microbiome
Citation: Pfefferle PI, Keber CU, Cohen RM and Garn H (2021) The Hygiene Hypothesis – Learning From but Not Living in the Past. Front. Immunol. 12:635935. doi: 10.3389/fimmu.2021.635935
Received: 30 November 2020; Accepted: 17 February 2021; Published: 16 March 2021.
Reviewed by:
Copyright © 2021 Pfefferle, Keber, Cohen and Garn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petra I. Pfefferle, petraina.pfefferle@uni-marburg.de
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
The Hygiene Hypothesis - Learning From but Not Living in the Past
Affiliations.
- 1 Comprehensive Biobank Marburg, Medical Faculty, Philipps University of Marburg, Comprehensive Biobank Marburg, Marburg, Germany.
- 2 German Center for Lung Research (DZL), Marburg, Germany.
- 3 German Biobank Alliance, Marburg, Germany.
- 4 Institute for Pathology, Medical Faculty, Institute for Pathology, Philipps University of Marburg, Marburg, Germany.
- 5 Translational Inflammation Research Division & Core Facility for Single Cell Multiomics, Medical Faculty, Biochemical Pharmacological Center, Philipps University of Marburg, Marburg, Germany.
- PMID: 33796103
- PMCID: PMC8007786
- DOI: 10.3389/fimmu.2021.635935
Postulated by Strachan more than 30 years ago, the Hygiene Hypothesis has undergone many revisions and adaptations. This review journeys back to the beginnings of the Hygiene Hypothesis and describes the most important landmarks in its development considering the many aspects that have refined and generalized the Hygiene Hypothesis over time. From an epidemiological perspective, the Hygiene Hypothesis advanced to a comprehensive concept expanding beyond the initial focus on allergies. The Hygiene Hypothesis comprise immunological, microbiological and evolutionary aspects. Thus, the original postulate developed into a holistic model that explains the impact of post-modern life-style on humans, who initially evolved in close proximity to a more natural environment. Focusing on diet and the microbiome as the most prominent exogenous influences we describe these discrepancies and the resulting health outcomes and point to potential solutions to reestablish the immunological homeostasis that frequently have been lost in people living in developed societies.
Keywords: T cell-response; allergy; asthma; hygiene hypothesis; immune tolerance; microbiome.
Copyright © 2021 Pfefferle, Keber, Cohen and Garn.
Publication types
- Historical Article
- Research Support, Non-U.S. Gov't
- Adaptive Immunity*
- Asthma / immunology
- Asthma / microbiology
- Bacteria / immunology*
- Bacteria / pathogenicity
- Diet / adverse effects
- Evolution, Molecular
- Gastrointestinal Microbiome / immunology*
- History, 20th Century
- History, 21st Century
- Host-Pathogen Interactions
- Hygiene Hypothesis* / history
- Immune Tolerance
- Immunity, Innate*
- T-Lymphocytes / immunology*
- T-Lymphocytes / metabolism
- T-Lymphocytes / microbiology

IMAGES
VIDEO
COMMENTS
Study with Quizlet and memorize flashcards containing terms like Hygiene Hypothesis (definition), Who was the first to suggest the hygiene hypothesis?, What actions can humans take in regard to their environment to strengthen their immune system? and more.
Study with Quizlet and memorize flashcards containing terms like What is the hygiene hypothesis? a) The idea that childhood exposure to microbes contributes to lower microbiome diversity and disease prevalence in developed countries. b) The idea that hygiene is the main way to prevent communicable diseases in crowded, overdeveloped urban areas. c) The idea that childhood exposure to ...
1. Introduction. The hygiene hypothesis was coined by Strachan (1989). It was formulated to describe how the increased susceptibility to allergic diseases (through immunomodulation) is related to a lack of exposure to infectious agents, symbiotic microorganisms, and parasites during childhood. Rook and Stanford (1998) first thought this only ...
Study with Quizlet and memorize flashcards containing terms like Briefly describe the hygiene hypothesis / how parasites benefit host, what differences hygiene hypothesis predicts between people born and raised in developed versus undeveloped countries., Hook worm characteristics and more.
Study with Quizlet and memorize flashcards containing terms like hygiene hypothesis, old friends theory, What patterns did the hygiene hypothesis attempt to explain? and more.
The Hygiene Hypothesis. explain the allergy epidemic? >30%of children have allergies around 10% have allergic asthma and/or allergic rhinitis 5-7% have food allergies asthma has increased between 1950 and 1990 food allergies have increased since 1990 and hay fever started to increase around 1870 allergies are x20 more common in westernised ...
Study with Quizlet and memorize flashcards containing terms like What is the "hygiene hypothesis" for autoimmune and allergic disease? Who first proposed this hypothesis and based on what observations?, What are the epidemiologic trends of autoimmune diseases and infectious diseases, respectively? What do migration studies demonstrate about this?, Describe one animal study that supports the ...
In medicine, the hygiene hypothesis states that early childhood exposure to particular microorganisms (such as the gut flora and helminth parasites) protects against allergies by strengthening the immune system. [1] [2] In particular, a lack of such exposure is thought to lead to poor immune tolerance. [1] The time period for exposure begins ...
The hygiene hypothesis states that early exposure to germs helps a child's immune system develop resistance to infections. Studies suggest that a lack of exposure results in higher rates of ...
Conclusion. The hygiene hypothesis suggests that a newborn baby's immune system must be educated so it will function properly during infancy and the rest of life. One of the key elements of this ...
The hygiene hypothesis is the idea that kids need to be exposed to germs in order to develop healthy immune systems. We know that many common viruses did not circulate as widely during the pandemic, thanks to social distancing, masking, and other COVID mitigation measures. Are there downsides to those missed infections? In this Q&A, Caitlin ...
The hygiene hypothesis postulates that an increased frequency of infections contributes to a decrease in autoimmune and allergic diseases. Here, Bach summarizes the epidemiological and ...
They decided the name has to go ( 15 ). "The trouble is, as soon as you use the words 'hygiene hypothesis,' the word hygiene prejudges what the cause is," says Bloomfield. To the public, "hygiene" is interpreted as personal cleanliness: washing hands, keeping food clean and fresh, sanitizing the home.
The hygiene hypothesis emerged as a tentative explanation to a massive shift in the human disease spectrum from infections to allergies ().It borrowed its name from a hypothesis originally proposed in the 1980s to explain the appendicitis epidemic of the early 20th century by an immune system not adequately trained for infections ().Against the then prevailing idea that viruses triggered ...
The epidemiological basis for the Hygiene Hypothesis became apparent long before the Hygiene Hypothesis was postulated. Two simple observations were made in the 1960s and in the 1970s. First, a Swedish study described differences in the prevalence of asthma and socio-medical conditions between populations living in urban or rural sites .
The old friends hypothesis, proposed by Rook et al, notes the co-evolution of microorganisms and macroorganisms, such as parasitic helminths, with the development of the human immune system. 22 Similar to the hygiene hypothesis, it suggests that these organisms are required for normal immune system development. 22, 24 For example, a study in ...
The hygiene hypothesis was first advanced more than 30 years ago and has been altered and refined since then. Children who grow up in sterile environments may have deficits in their immune systems ...
Keywords: hygiene hypothesis, allergy, asthma, non-communicable inflammatory diseases, chronic inflammation. Go to: Throughout its history, the Hygiene Hypothesis has shown itself to be adaptable and flexible whenever it has been challenged by innovation in science ( 1 ). A number of new findings need to be considered in this ongoing revisiting ...
Abstract. According to the 'hygiene hypothesis', the decreasing incidence of infections in western countries and more recently in developing countries is at the origin of the increasing incidence of both autoimmune and allergic diseases. The hygiene hypothesis is based upon epidemiological data, particularly migration studies, showing that ...
The 'hygiene hypothesis' as originally formulated by Strachan, proposes that a cause of the recent rapid rise in atopic disorders could be a lower incidence of infection in early childhood, transmitted by unhygienic contact with older siblings. Use of the term 'hygiene hypothesis' has led to several interpretations, some of which are ...
The epidemiological basis for the Hygiene Hypothesis became apparent long before the Hygiene Hypothesis was postulated. Two simple observations were made in the 1960s and in the 1970s. First, a Swedish study described differences in the prevalence of asthma and socio-medical conditions between populations living in urban or rural sites .
The "hygiene hypothesis", proposed by Strachan in 1989, aimed to explain this peculiar generational rise in immune dysregulation. However, research over the past 10 years provides evidence connecting the commensal and symbiotic microbes (intestinal microbiota) and parasitic helminths with immune development, expanding the hygiene hypothesis ...
The Hygiene Hypothesis comprise immunological, microbiological and evolutionary aspects. Thus, the original postulate developed into a holistic model that explains the impact of post-modern life-style on humans, who initially evolved in close proximity to a more natural environment. Focusing on diet and the microbiome as the most prominent ...