- Search by keyword
- Search by citation
Page 1 of 36

DNA methylation and type 2 diabetes: a systematic review
DNA methylation influences gene expression and function in the pathophysiology of type 2 diabetes mellitus (T2DM). Mapping of T2DM-associated DNA methylation could aid early detection and/or therapeutic treatm...
- View Full Text
Machine learning unveils an immune-related DNA methylation profile in germline DNA from breast cancer patients
There is an unmet need for precise biomarkers for early non-invasive breast cancer detection. Here, we aimed to identify blood-based DNA methylation biomarkers that are associated with breast cancer.
DNA methylation signatures of youth-onset type 2 diabetes and exposure to maternal diabetes
Youth-onset type 2 diabetes (T2D) is physiologically distinct from adult-onset, but it is not clear how the two diseases differ at a molecular level. In utero exposure to maternal type 2 diabetes (T2D) is know...
Hyper-physiologic mechanical cues, as an osteoarthritis disease-relevant environmental perturbation, cause a critical shift in set points of methylation at transcriptionally active CpG sites in neo-cartilage organoids
Osteoarthritis (OA) is a complex, age-related multifactorial degenerative disease of diarthrodial joints marked by impaired mobility, joint stiffness, pain, and a significant decrease in quality of life. Among...
Decitabine as epigenetic priming with CLAG induce improved outcome of relapsed or refractory acute myeloid leukemia in children
Decitabine (DAC), a DNA methyltransferase inhibitor, has shown efficacy combined with chemotherapy for relapsed or refractory (R/R) acute myeloid leukemia (AML) in adults, but less is known about its efficacy ...
Novel 14q32.2 paternal deletion encompassing the whole DLK1 gene associated with Temple syndrome
Temple syndrome (TS14) is a rare imprinting disorder caused by maternal UPD14, imprinting defects or paternal microdeletions which lead to an increase in the maternal expressed genes and a silencing the patern...
Epigenetics of the non-coding RNA nc886 across blood, adipose tissue and skeletal muscle in offspring exposed to diabetes in pregnancy
Diabetes in pregnancy is associated with increased risk of long-term metabolic disease in the offspring, potentially mediated by in utero epigenetic variation. Previously, we identified multiple differentially...
Genome- and epigenome-wide association studies identify susceptibility of CpG sites and regions for metabolic syndrome in a Korean population
While multiple studies have investigated the relationship between metabolic syndrome (MetS) and its related traits (fasting glucose, triglyceride, HDL cholesterol, blood pressure, waist circumference) and DNA ...
Correction: Improvements in lung function following vitamin C supplementation to pregnant smokers are associated with buccal DNA methylation at 5 years of age
The original article was published in Clinical Epigenetics 2024 16 :35
DNA methylation of imprint control regions associated with Alzheimer’s disease in non-Hispanic Blacks and non-Hispanic Whites
Alzheimer’s disease (AD) prevalence is twice as high in non-Hispanic Blacks (NHBs) as in non-Hispanic Whites (NHWs). The objective of this study was to determine whether aberrant methylation at imprint control...
Evaluating the link between DIO3 -FA27 promoter methylation, biochemical indices, and heart failure progression
Heart failure (HF) is a disease that poses a serious threat to individual health, and DNA methylation is an important mechanism in epigenetics, and its role in the occurrence and development of the disease has...
Discovery and technical validation of high-performance methylated DNA markers for the detection of cervical lesions at risk of malignant progression in low- and middle-income countries
Cervical cancer remains a leading cause of death, particularly in developing countries. WHO screening guidelines recommend human papilloma virus (HPV) detection as a means to identify women at risk of developi...
DNMT1/miR-152-3p/SOS1 signaling axis promotes self-renewal and tumor growth of cancer stem-like cells derived from non-small cell lung cancer
CSLCs(Cancer stem cell-like cells), which are central to tumorigenesis, are intrinsically influenced by epigenetic modifications. This study aimed to elucidate the underlying mechanism involving the DNMT1/miR-...
Polycomb repressive complex 2 and its core component EZH2: potential targeted therapeutic strategies for head and neck squamous cell carcinoma
The polycomb group (PcG) comprises a set of proteins that exert epigenetic regulatory effects and play crucial roles in diverse biological processes, ranging from pluripotency and development to carcinogenesis...
Meta-analysis of epigenetic aging in schizophrenia reveals multifaceted relationships with age, sex, illness duration, and polygenic risk
The study of biological age acceleration may help identify at-risk individuals and reduce the rising global burden of age-related diseases. Using DNA methylation (DNAm) clocks, we investigated biological aging...
Epigenetics in diabetic cardiomyopathy
Diabetic cardiomyopathy (DCM) is a critical complication that poses a significant threat to the health of patients with diabetes. The intricate pathological mechanisms of DCM cause diastolic dysfunction, follo...
Targeting histone demethylases JMJD3 and UTX: selenium as a potential therapeutic agent for cervical cancer
The intriguing connection between selenium and cancer resembles a captivating puzzle that keeps researchers engaged and curious. While selenium has shown promise in reducing cancer risks through supplementatio...
Nucleosome reorganisation in breast cancer tissues
Nucleosome repositioning in cancer is believed to cause many changes in genome organisation and gene expression. Understanding these changes is important to elucidate fundamental aspects of cancer. It is also ...
Refining risk prediction in pediatric acute lymphoblastic leukemia through DNA methylation profiling
Acute lymphoblastic leukemia (ALL) is the most prevalent cancer in children, and despite considerable progress in treatment outcomes, relapses still pose significant risks of mortality and long-term complicati...
Low expression of miR-182 caused by DNA hypermethylation accelerates acute lymphocyte leukemia development by targeting PBX3 and BCL2: miR-182 promoter methylation is a predictive marker for hypomethylation agents + BCL2 inhibitor venetoclax
miR-182 promoter hypermethylation frequently occurs in various tumors, including acute myeloid leukemia, and leads to low expression of miR-182. However, whether adult acute lymphocyte leukemia (ALL) cells hav...
Head and neck cancer of unknown primary: unveiling primary tumor sites through machine learning on DNA methylation profiles
The unknown tissue of origin in head and neck cancer of unknown primary (hnCUP) leads to invasive diagnostic procedures and unspecific and potentially inefficient treatment options for patients. The most commo...
Epigenetic scores of blood-based proteins as biomarkers of general cognitive function and brain health
Epigenetic Scores (EpiScores) for blood protein levels have been associated with disease outcomes and measures of brain health, highlighting their potential usefulness as clinical biomarkers. They are typicall...
A validated restriction enzyme ddPCR cg05575921 ( AHRR ) assay to accurately assess smoking exposure
In this study, a novel restriction enzyme (RE) digestion-based droplet digital polymerase chain reaction (ddPCR) assay was designed for cg005575921 within the AHRR gene body and compared with matching results obt...
Mother adversity and co-residence time impact mother–child similarity in genome-wide and gene-specific methylation profiles
The effects of adverse life events on physical and psychological health, with DNA methylation (DNAm) as a critical underlying mechanism, have been extensively studied. However, the epigenetic resemblance betwe...
Correction: Increased CpG methylation at the CDH1 locus in inflamed ileal mucosa of patients with Crohn disease
The original article was published in Clinical Epigenetics 2024 16 :28
Identification of miR-20b-5p as an inhibitory regulator in cardiac differentiation via TET2 and DNA hydroxymethylation
Congenital heart disease (CHD) is a prevalent congenital cardiac malformation, which lacks effective early biological diagnosis and intervention. MicroRNAs, as epigenetic regulators of cardiac development, pro...
Epigenetic age acceleration and risk of aortic valve stenosis: a bidirectional Mendelian randomization study
Aortic valve stenosis (AVS) is the most prevalent cardiac valve lesion in developed countries, and pathogenesis is closely related to aging. DNA methylation-based epigenetic clock is now recognized as highly a...

MAL expression downregulation through suppressive H3K27me3 marks at the promoter in HPV16-related cervical cancers is prognostically relevant and manifested by the interplay of novel MAL antisense long noncoding RNA AC103563.8, E7 oncoprotein and EZH2
MAL (T-lymphocyte maturation-associated protein) is highly downregulated in most cancers, including cervical cancer (CaCx), attributable to promoter hypermethylation. Long noncoding RNA genes (lncGs) play pivo...
Novel histone post-translational modifications in Alzheimer’s disease: current advances and implications
Alzheimer’s disease (AD) has a complex pathogenesis, and multiple studies have indicated that histone post-translational modifications, especially acetylation, play a significant role in it. With the developme...
An EWAS of dementia biomarkers and their associations with age, African ancestry, and PTSD
Large-scale cohort and epidemiological studies suggest that PTSD confers risk for dementia in later life but the biological mechanisms underlying this association remain unknown. This study examined this quest...
Cell-free DNA methylation reveals cell-specific tissue injury and correlates with disease severity and patient outcomes in COVID-19
The recently identified methylation patterns specific to cell type allows the tracing of cell death dynamics at the cellular level in health and diseases. This study used COVID-19 as a disease model to investi...
DNA methylation may partly explain psychotropic drug-induced metabolic side effects: results from a prospective 1-month observational study
Metabolic side effects of psychotropic medications are a major drawback to patients’ successful treatment. Using an epigenome-wide approach, we aimed to investigate DNA methylation changes occurring secondary ...
Improvements in lung function following vitamin C supplementation to pregnant smokers are associated with buccal DNA methylation at 5 years of age
We previously reported in the “Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function” randomized clinical trial (RCT) that vitamin C (500 mg/day) supplementation to pregnant smokers...
The Correction to this article has been published in Clinical Epigenetics 2024 16 :59
Prediction of methylation status using WGS data of plasma cfDNA for multi-cancer early detection (MCED)
Cell-free DNA (cfDNA) contains a large amount of molecular information that can be used for multi-cancer early detection (MCED), including changes in epigenetic status of cfDNA, such as cfDNA fragmentation pro...
Reduced representative methylome profiling of cell-free DNA for breast cancer detection
Whole-genome methylation sequencing of cfDNA is not cost-effective for tumor detection. Here, we introduce reduced representative methylome profiling (RRMP), which employs restriction enzyme for depletion of A...
Epigenetic aging in older people living with HIV in Eswatini: a pilot study of HIV and lifestyle factors and epigenetic aging
People living with HIV (PLHIV) on effective antiretroviral therapy are living near-normal lives. Although they are less susceptible to AIDS-related complications, they remain highly vulnerable to non-communica...
Abnormal DNA methylation within HPA-axis genes years after paediatric critical illness
Critically ill children suffer from impaired physical/neurocognitive development 2 years later. Glucocorticoid treatment alters DNA methylation within the hypothalamus–pituitary–adrenal (HPA) axis which may im...
Epigenetic regulation and factors that influence the effect of iPSCs-derived neural stem/progenitor cells (NS/PCs) in the treatment of spinal cord injury
Spinal cord injury (SCI) is a severe neurological disorder that causes neurological impairment and disability. Neural stem/progenitor cells (NS/PCs) derived from induced pluripotent stem cells (iPSCs) represen...
Epigenome-wide association study of dietary fatty acid intake
Dietary intake of n-3 polyunsaturated fatty acids (PUFA) may have a protective effect on the development of cardiovascular diseases, diabetes, depression and cancer, while a high intake of n-6 PUFA was often r...
Increased CpG methylation at the CDH1 locus in inflamed ileal mucosa of patients with Crohn disease
E-cadherin, a major actor of cell adhesion in the intestinal barrier, is encoded by the CDH1 gene associated with susceptibility to Crohn Disease (CD) and colorectal cancer. Since epigenetic mechanisms are suspec...
The Correction to this article has been published in Clinical Epigenetics 2024 16 :43
Kidney-specific methylation patterns correlate with kidney function and are lost upon kidney disease progression
Chronological and biological age correlate with DNA methylation levels at specific sites in the genome. Linear combinations of multiple methylation sites, termed epigenetic clocks, can inform us the chronologi...
Molecular map of disulfidptosis-related genes in lung adenocarcinoma: the perspective toward immune microenvironment and prognosis
Disulfidptosis is a recently discovered form of programmed cell death that could impact cancer development. Nevertheless, the prognostic significance of disulfidptosis-related genes (DRGs) in lung adenocarcino...
Tissue of origin prediction for cancer of unknown primary using a targeted methylation sequencing panel
Cancer of unknown primary (CUP) is a group of rare malignancies with poor prognosis and unidentifiable tissue-of-origin. Distinct DNA methylation patterns in different tissues and cancer types enable the ident...
The interaction between DNA methylation and tumor immune microenvironment: from the laboratory to clinical applications
DNA methylation is a pivotal epigenetic modification that affects gene expression. Tumor immune microenvironment (TIME) comprises diverse immune cells and stromal components, creating a complex landscape that ...
Correction: A transgenic mice model of retinopathy of cblG‑type inherited disorder of one‑carbon metabolism highlights epigenome‑wide alterations related to cone photoreceptor cells development and retinal metabolism
The original article was published in Clinical Epigenetics 2023 15 :158
Self-control is associated with health-relevant disparities in buccal DNA-methylation measures of biological aging in older adults
Self-control is a personality dimension that is associated with better physical health and a longer lifespan. Here, we examined (1) whether self-control is associated with buccal and saliva DNA-methylation (DN...
Per-cell histone acetylation is associated with terminal differentiation in human T cells
Epigenetic remodeling at effector gene loci has been reported to be critical in regulating T cell differentiation and function. However, efforts to investigate underlying epigenetic mechanisms that control T c...
Epigenetics of prenatal stress in humans: the current research landscape
Fetal exposure to prenatal stress can have significant consequences on short- and long-term health. Epigenetic mechanisms, especially DNA methylation (DNAm), are a possible process how these adverse environmen...
Identification of DNA methylation biomarkers for evaluating cardiovascular disease risk from epigenome profiles altered by low-dose ionizing radiation
Environmental exposure, medical diagnostic and therapeutic applications, and industrial utilization of radionuclides have prompted a growing focus on the risks associated with low-dose radiation (< 100 mGy). C...
METTL3 promotes osteoblast ribosome biogenesis and alleviates periodontitis
Periodontitis is a highly prevalent oral disease characterized by bacterium-induced periodontal inflammation and alveolar bone destruction. Osteoblast function is impaired in periodontitis with a global proteo...
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Contact Support for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
- Follow us on Facebook
- ISSN: 1868-7083 (electronic)
Clinical Epigenetics
ISSN: 1868-7083
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Broadening the epigenetic horizon of abiotic stress response in plants
- Review Paper
- Published: 11 May 2024
Cite this article

- Himani Chhatwal 1 ,
- Jogindra Naik 1 ,
- Ashutosh Pandey 1 &
- Prabodh Kumar Trivedi ORCID: orcid.org/0000-0001-6463-1731 2
162 Accesses
4 Altmetric
Explore all metrics
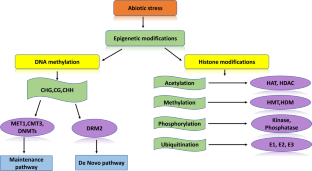
Plants, unlike animals, cannot move from one place to another and have to face different climatic disturbances wherever they are growing. So, they have innumerable built-in mechanisms to adapt to various abiotic stressful conditions like drought, heat, cold, and salinity. The changing environmental conditions influence the expression patterns of genes. Epigenetics involves heritable changes in DNA bases or histone proteins, which ultimately create different conformational states of chromatin. The regulatory enzymes of epigenetic modifications are grouped as writers, readers and erasers, which add, recognize and remove the epigenetic marks, respectively. Here, we provide a comprehensive overview of the mechanism of DNA methylation by the RdDM pathway, its maintenance and removal, and different histone modification categories like acetylation, methylation, phosphorylation and ubiquitination. This review further discusses in detail the crucial role these modifications play in adapting to major abiotic stresses and how plants preserve these experiences as stress memory to respond to recurring stresses. It emphasizes the role of epigenetic modifications as a crucial mechanism for building plant’s tolerance and how it can be an important research priority to improve plant growth and development under abiotic stress conditions.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Drought Tolerance Strategies in Plants: A Mechanistic Approach

Drought Stress in Plants: An Overview

Nitrogen in plants: from nutrition to the modulation of abiotic stress adaptation
Abbreviations.
Chromomethylase
Domains rearranged methyltransferase 2
Methyltransferase 1
RNA-directed DNA methylation
Histone acetyltransferase
Histone deactylase
Antunez-Sanchez J, Naish M, Ramirez-Prado JS, Ohno S, Huang Y, Dawson A, Opassathian K, Manza-Mianza D, Ariel F, Raynaud C, Wibowo A (2020) A new role for histone demethylases in the maintenance of plant genome integrity. Elife 9:e58533. https://doi.org/10.7554/eLife.58533
Article CAS PubMed PubMed Central Google Scholar
Aranda S, Mas G, Di Croce L (2015) Regulation of gene transcription by polycomb proteins. Sci Adv 1:e1500737. https://doi.org/10.1126/sciadv.1500737
Article PubMed PubMed Central Google Scholar
Ashapkin VV, Kutueva LI, Aleksandrushkina NI, Vanyushin BF (2020) Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int J Mol Sci 21:7457. https://doi.org/10.3390/ijms21207457
Baek D, Jiang J, Chung J-S, Wang B, Chen J, Xin Z, Shi H (2011) Regulated AtHKT1 gene expression by a distal enhancer element and DNA methylation in the promoter plays an important role in salt tolerance. Plant Cell Physiol 52:149–161. https://doi.org/10.1093/pcp/pcq182
Article CAS PubMed Google Scholar
Bäurle I, Trindade I (2020) Chromatin regulation of somatic abiotic stress memory. J Exp Bot 71:5269–5279. https://doi.org/10.1093/jxb/eraa098
Bobde RC, Kumar A, Vasudevan D (2022) Plant-specific HDT family histone deacetylases are nucleoplasmins. Plant Cell 34:4760–4777. https://doi.org/10.1093/plcell/koac275
Buszewicz D, Archacki R, Palusiński A, Kotliński M, Fogtman A, Iwanicka-Nowicka R, Sosnowska K, Kuciński J, Pupel P, Olędzki J, Dadlez M (2016) HD2C histone deacetylase and a SWI/SNF chromatin remodelling complex interact and both are involved in mediating the heat stress response in Arabidopsis . Plant Cell Environ 39:2108–2122. https://doi.org/10.1111/pce.12756
Carter B, Bishop B, Ho KK, Huang R, Jia W, Zhang H, Pascuzzi PE, Deal RB, Ogas J (2018) The chromatin remodelers PKL and PIE1 act in an epigenetic pathway that determines H3K27me3 homeostasis in Arabidopsis . Plant Cell 30:1337–1352. https://doi.org/10.1105/tpc.17.00867
Chandrika NNP, Sundaravelpandian K, Yu S, Schmidt W (2013) ALFIN-LIKE 6 is involved in root hair elongation during phosphate deficiency in Arabidopsis . New Phytol 198:709–720. https://doi.org/10.1111/nph.12194
Article PubMed Google Scholar
Chen X, Schönberger B, Menz J, Ludewig U (2018) Plasticity of DNA methylation and gene expression under zinc deficiency in Arabidopsis roots. Plant Cell Physiol 59:1790–1802. https://doi.org/10.1093/pcp/pcy100
Chinnusamy V, Gong Z, Zhu J (2008) Abscisic acid-mediated epigenetic processes in plant development and stress responses. J Integ Plant Biol 50:1187–1195. https://doi.org/10.1111/j.1744-7909.2008.00727.x
Article CAS Google Scholar
Cong W, Miao Y, Xu L, Zhang Y, Yuan C, Wang J, Zhuang T, Lin X, Jiang L, Wang N (2019) Transgenerational memory of gene expression changes induced by heavy metal stress in rice ( Oryza sativa L.). BMC Plant Biol 19:1–14. https://doi.org/10.1186/s12870-019-1887-7
Cuerda-Gil D, Slotkin RK (2016) Non-canonical RNA-directed DNA methylation. Nat Plants 2:1–8
Article Google Scholar
Ding Y, Fromm M, Avramova Z (2012) Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis . Nat Commun 3:740. https://doi.org/10.1038/ncomms1732
Ding Y, Wang W, Zhuang Q, Luo Y (2020) Adaptation of paddy rice in China to climate change: the effects of shifting sowing date on yield and irrigation water requirement. Agric Water Manag 228:105890. https://doi.org/10.1016/j.agwat.2019.105890
Du J, Zhong X, Bernatavichute YV, Stroud H, Feng S, Caro E, Vashisht AA, Terragni J, Chin HG, Tu A (2012) Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell 151:167–180
Duan CG, Zhu JK, Cao X (2018) Retrospective and perspective of plant epigenetics in China. J Genet Genom 45:621–638. https://doi.org/10.1016/j.jgg.2018.09.004
Earley KW, Shook MS, Brower-Toland B, Hicks L, Pikaard CS (2007) In vitro specificities of Arabidopsis co-activator histone acetyltransferases: implications for histone hyperacetylation in gene activation. Plant J 52:615–626. https://doi.org/10.1111/j.1365-313X.2007.03264.x
Fan H, Zhang Z, Wang N, Cui Y, Sun H, Liu Y, Wu H, Zheng S, Bao S, Ling H (2014) SKB1/PRMT 5-mediated histone H 4 R 3 dimethylation of I b subgroup bHLH genes negatively regulates iron homeostasis in Arabidopsis thaliana . Plant J 77:209–221. https://doi.org/10.1111/tpj.12380
Fina JP, Masotti F, Rius SP, Crevacuore F, Casati P (2017) HAC1 and HAF1 histone acetyltransferases have different roles in UV-B responses in Arabidopsis . Front Plant Sci 8:1179. https://doi.org/10.3389/fpls.2017.01179
Finnegan EJ, Peacock WJ, Dennis ES (1996) Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc Natl Acad Sci 93:8449–8454. https://doi.org/10.1073/pnas.93.16.8449
Friedrich T, Faivre L, Bäurle I, Schubert D (2019) Chromatin-based mechanisms of temperature memory in plants. Plant Cell Environ 42:762–770. https://doi.org/10.1111/pce.13373
Goldberg AD, Allis CD, Bernstein E (2007) Epigenetics: a landscape takes shape. Cell 128:635–638. https://doi.org/10.1016/j.cell.2007.02.006
Gu T, Han Y, Huang R, McAvoy RJ, Li Y (2016) Identification and characterization of histone lysine methylation modifiers in Fragaria vesca . Sci Rep 6:23581. https://doi.org/10.1038/srep23581
Hammond CM, Strømme CB, Huang H, Patel DJ, Groth A (2017) Histone chaperone networks shaping chromatin function. Nat Rev Molecul Cell Biol 18:141–158. https://doi.org/10.1038/nrm.2016.159
Hsieh TF, Ibarra CA, Silva P, Zemach A, Eshed-Williams L, Fischer RL, Zilberman D (2009) Genome-wide demethylation of Arabidopsis endosperm. Science 324:1451–1454. https://doi.org/10.1126/science.1172417
Kim J, Kim JH, Richards EJ, Chung KM, Woo HR (2014) Arabidopsis VIM proteins regulate epigenetic silencing by modulating DNA methylation and histone modification in cooperation with MET1. Mol Plant 7:1470–1485
Kim JM, Sasaki T, Ueda M, Sako K, Seki M (2015) Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front Plant Sci 6:114. https://doi.org/10.3389/fpls.2015.00114
Kourani M, Mohareb F, Rezwan FI, Anastasiadi M, Hammond JP (2022) Genetic and physiological responses to heat stress in Brassica napus . Front Plant Sci 13:832147. https://doi.org/10.3389/fpls.2022.832147
Lämke J, Bäurle I (2017) Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genom Biol 18:1–11. https://doi.org/10.1186/s13059-017-1263-6
Lämke J, Brzezinka K, Altmann S, Bäurle I (2016) A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. The EMBO J. https://doi.org/10.15252/embj.201592593
Lang-Mladek C, Popova O, Kiok K, Berlinger M, Rakic B, Aufsatz W, Jonak C, Hauser MT, Luschnig C (2010) Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis . Mol plant 3(3):594–602
Law JA, Jacobsen SE (2010) Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 11:204–220. https://doi.org/10.1038/nrg2719
Liu J, He Z (2020) Small DNA methylation, big player in plant abiotic stress responses and memory. Front Plant Sci 11:595603. https://doi.org/10.3389/fpls.2020.595603
Liu R, Lang Z (2020) The mechanism and function of active DNA demethylation in plants. J Integr Plant Biol 62:148–159. https://doi.org/10.1111/jipb.12879
Liu P, Zhang S, Zhou B, Luo X, Zhou XF, Cai B, Jin YH, Niu D, Lin J, Cao X, Jin JB (2019) The histone H3K4 demethylase JMJ16 represses leaf senescence in Arabidopsis . Plant Cell 31:430–443. https://doi.org/10.3389/fpls.2020.595603
Mehdi S, Derkacheva M, Ramström M, Kralemann L, Bergquist J, Hennig L (2016) The WD40 domain protein MSI1 functions in a histone deacetylase complex to fine-tune abscisic acid signaling. Plant Cell 28:42–54. https://doi.org/10.1105/tpc.15.00763
Moglia A, Gianoglio S, Acquadro A, Valentino D, Milani AM, Lanteri S, Comino C (2019) Identification of DNA methyltransferases and demethylases in Solanum melongena L., and their transcription dynamics during fruit development and after salt and drought stresses. PLoS ONE 14:e0223581. https://doi.org/10.1371/journal.pone.0223581
Negrão S, Schmöckel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11. https://doi.org/10.1093/aob/mcw191
Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22:53–65. https://doi.org/10.1016/j.tplants.2016.08.015
Ortega-Galisteo AP, Morales-Ruiz T, Ariza RR, Roldán-Arjona T (2008) Arabidopsis DEMETER-LIKE proteins DML2 and DML3 are required for appropriate distribution of DNA methylation marks. Plant Mol Biol 67:671–681. https://doi.org/10.1007/s11103-008-9346-0
Pandey N, Pandey RS (2015) Deciphering UV-B-induced variation in DNA methylation pattern and its influence on regulation of DBR2 expression in Artemisia annua L. Planta 242:869–879. https://doi.org/10.1007/s00425-015-2323-3
Pandey R, MuÈller A, Napoli CA, Selinger DA, Pikaard CS, Richards EJ, Bender J, Mount DW, Jorgensen RA (2002) Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res 30:5036–5055. https://doi.org/10.1093/nar/gkf660
Park J, Lim CJ, Shen M, Park HJ, Cha JY, Iniesto E, Rubio V, Mengiste T, Zhu JK, Bressan RA (2018) Epigenetic switch from repressive to permissive chromatin in response to cold stress. Proc Natl Acad Sci 115:E5400–E5409. https://doi.org/10.1073/pnas.172124111
Popova OV, Dinh HQ, Aufsatz W, Jonak C (2013) The RdDM pathway is required for basal heat tolerance in Arabidopsis . Mol Plant 6:396–410. https://doi.org/10.1093/mp/sst023
Ricketts MD, Frederick B, Hoff H, Tang Y, Schultz DC, Singh Rai T, Grazia Vizioli M, Adams PD, Marmorstein R (2015) Ubinuclein-1 confers histone H3. 3-specific-binding by the HIRA histone chaperone complex. Nat Commun 6:7711. https://doi.org/10.1038/ncomms8711
Sani E, Herzyk P, Perrella G, Colot V, Amtmann A (2013) Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biol 14:1–24. https://doi.org/10.1186/gb-2013-14-6-r59
Secco D, Wang C, Shou H, Schultz MD, Chiarenza S, Nussaume L, Ecker JR, Whelan J, Lister R (2015) Stress induced gene expression drives transient DNA methylation changes at adjacent repetitive elements. Elife 4:e09343. https://doi.org/10.7554/eLife.09343
Secco D, Whelan J, Rouached H, Lister R (2017) Nutrient stress-induced chromatin changes in plants. Curr Plant Biol 39:1–7. https://doi.org/10.1016/j.pbi.2017.04.001
Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Dindaroglu T, Abdul-Wajid HH, Battaglia ML (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10:259. https://doi.org/10.3390/plants10020259
Shen Y, De Jonge J, Forsberg SK, Pettersson ME, Sheng Z, Hennig L, Carlborg Ö (2014) Natural CMT2 variation is associated with genome-wide methylation changes and temperature seasonality. PLoS Genet 10:e1004842. https://doi.org/10.1371/journal.pgen.1004842
Singh RK, Jaishankar J, Muthamilarasan M, Shweta S, Dangi A, Prasad M (2016) Genome-wide analysis of heat shock proteins in C4 model, foxtail millet identifies potential candidates for crop improvement under abiotic stress. Sci Rep 6:32641. https://doi.org/10.1038/srep32641
Singh J, Mishra V, Wang F, Huang HY, Pikaard CS (2019) Reaction mechanisms of Pol IV, RDR2, and DCL3 drive RNA channeling in the siRNA-directed DNA methylation pathway. Mol Cell 75:576–589. https://doi.org/10.1016/j.molcel.2019.07.008
Song X, Zhang Y, Zhong Q, Zhan K, Bi J, Tang J, Xie J, Li B (2020) Identification and functional characterization of methyl-CpG binding domain protein from Tribolium castaneum . Genomics 112:2223–2232. https://doi.org/10.1016/j.ygeno.2019.12.018
Srikant T, Yuan W, Berendzen KW, Contreras-Garrido A, Drost HG, Schwab R, Weigel D (2022) Canalization of genome-wide transcriptional activity in Arabidopsis thaliana accessions by MET1-dependent CG methylation. Genome Biol 23:263. https://doi.org/10.1186/s13059-022-02833-5
Tang Z, Kang Y, Wang P (2016) Zhao FJ (2016) Phytotoxicity and detoxification mechanism differ among inorganic and methylated arsenic species in Arabidopsis thaliana . Plant Soil 401(243–257):243–257. https://doi.org/10.1007/s11104-015-2739-3
Trujillo M (2018) News from the PUB: plant U-box type E3 ubiquitin ligases. J Exp Bot 69:371–384. https://doi.org/10.1093/jxb/erx411
Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y (2006) Histone demethylation by a family of JmjC domain-containing proteins. Nature 43:811–816. https://doi.org/10.1038/nature04433
Tu YT, Chen CY, Huang YS, Chang CH, Yen MR, Hsieh JWA, Chen PY, Wu K (2022) HISTONE DEACETYLASE 15 and MOS4-associated complex subunits 3A/3B coregulate intron retention of ABA-responsive genes. Plant Physiol 19:882–897. https://doi.org/10.1093/plphys/kiac271
Ueda M, Seki M (2020) Histone modifications form epigenetic regulatory networks to regulate abiotic stress response. Plant Physiol 182:15–26. https://doi.org/10.1104/pp.19.00988
Van Dijk K, Ding Y, Malkaram S, Riethoven JJ, Liu R, Yang J, Laczko P, Chen H, Xia Y, Ladunga I, Avramova Z (2010) Dynamic changes in genome-wide histone H3 lysine 4 methylation patterns in response to dehydration stress in Arabidopsis thaliana . BMC Plant Biol 10:1–2. https://doi.org/10.1186/1471-2229-10-238
Virlouvet L, Ding Y, Fujii H, Avramova Z, Fromm M (2014) ABA signaling is necessary but not sufficient for RD 29 B transcriptional memory during successive dehydration stresses in Arabidopsis thaliana . The Plant J 79:150–161
Wang X, Vignjevic M, Jiang D, Jacobsen S, Wollenweber B (2014) Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat ( Triticum aestivum L.) var. Vinjett. J Exp Bot 65:6441–6456. https://doi.org/10.1093/jxb/eru362
Wang Z, Casas-Mollano JA, Xu J, Riethoven JJ, Zhang C, Cerutti H (2015) Osmotic stress induces phosphorylation of histone H3 at threonine 3 in pericentromeric regions of Arabidopsis thaliana . Proc Natl Acad Sci 112:8487–8492. https://doi.org/10.1073/pnas.1423325112
Wang Q, Liu P, Jing H, Zhou XF, Zhao B, Li Y, Jin JB (2021) JMJ27-mediated histone H3K9 demethylation positively regulates drought-stress responses in Arabidopsis . New Phytol 232:221–236. https://doi.org/10.1111/tpj.12548
Waqas MA, Kaya C, Riaz A, Farooq M, Nawaz I, Wilkes A, Li Y (2019) Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front Plant Sci 10:1336. https://doi.org/10.3389/fpls.2019.01336
Widiez T, El Kafafi ES, Girin T, Berr A, Ruffel S, Krouk G, Vayssières A, Shen W-H, Coruzzi GM, Gojon A (2011) High nitrogen insensitive 9 (HNI9)- mediated systemic repression of root NO3− uptake is associated with changes in histone methylation. Proc Natl Acad Sci 108:13329–13334. https://doi.org/10.1073/pnas.101786310
Xiao J, Zhang H, Xing L, Xu S, Liu H, Chong K, Xu Y (2013) Requirement of histone acetyltransferases HAM1 and HAM2 for epigenetic modification of FLC in regulating flowering in Arabidopsis . J Plant Physiol 170:444–451. https://doi.org/10.1016/j.jplph.2012.11.007
Xie H, Sun Y, Cheng B, Xue S, Cheng D, Liu L, Meng L, Qiang S (2019) Variation in ICE1 methylation primarily determines phenotypic variation in freezing tolerance in Arabidopsis thaliana . Plant Cell Physiol 60:152–165. https://doi.org/10.1093/pcp/pcy197
Xing J, Wang T, Liu Z, Xu J, Yao Y, Hu Z, Peng H, Xin M, Yu F, Zhou D (2015) GENERAL CONTROL NONREPRESSED PROTEIN5-mediated histone acetylation of FERRIC REDUCTASE DEFECTIVE3 contributes to iron homeostasis in Arabidopsis . Plant Physiol 168:1309–1320. https://doi.org/10.1104/pp.15.00397
Xu Y, Zhang S, Lin S, Guo Y, Deng W, Zhang Y, Xue Y (2017) WERAM: a database of writers, erasers and readers of histone acetylation and methylation in eukaryotes. Nucleic Acids Res. https://doi.org/10.1093/nar/gkw1011
Xu J, Zhou S, Gong X, Song Y, van Nocker S, Ma F, Guan Q (2018) Single-base methylome analysis reveals dynamic epigenomic differences associated with water deficit in apple. Plant Biotechnol J 16:672–687. https://doi.org/10.1111/pbi.12820
Yang R, Zheng Z, Chen Q, Yang L, Huang H, Miki D, Wu W, Zeng L, Liu J, Zhou JX (2017) The developmental regulator PKL is required to maintain correct DNA methylation patterns at RNA-directed DNA methylation loci. Genome Biol 18:1–18. https://doi.org/10.1186/s13059-017-1226-y
Yang DL, Zhang G, Wang L, Li J, Xu D, Di C, Tang K, Yang L, Zeng L, Miki D, Duan CG (2018) Four putative SWI2/SNF2 chromatin remodelers have dual roles in regulating DNA methylation in Arabidopsis . Cell Discov 4:55. https://doi.org/10.1038/s41421-018-0056-8
Yang R, Hong Y, Ren Z, Tang K, Zhang H, Zhu JK, Zhao C (2019) A role for PICKLE in the regulation of cold and salt stress tolerance in Arabidopsis . Front Plant Sci 10:900
Yolcu S, Ozdemir F, Güler A, Bor M (2016) Histone acetylation influences the transcriptional activation of POX in Beta vulgaris L. and Beta maritima L. under salt stress. Plant Physiol Biochem 100:37–46. https://doi.org/10.1016/j.plaphy.2015.12.019
Zhang H, Lang Z, Zhu JK (2018) Dynamics and function of DNA methylation in plants. Nat Rev Mol Cell Biol 19:489–506. https://doi.org/10.1038/s41580-018-0016-z
Zhang W, Wang N, Yang J, Guo H, Liu Z, Zheng X, Li S, Xiang F (2020) The salt-induced transcription factor GmMYB84 confers salinity tolerance in soybean. Plant Sci 291:110326
Zhao F, Zhang H, Zhao T, Li Z, Jiang D (2021) The histone variant H3. 3 promotes the active chromatin state to repress flowering in Arabidopsis . Plant Physiol 186:2051–2063. https://doi.org/10.1093/plphys/kiab224
Zheng X, Chen L, Xia H, Wei H, Lou Q, Li M, Li T, Luo L (2017) Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci Rep 7:39843. https://doi.org/10.1038/srep39843
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324
Zubko E, Gentry M, Kunova A, Meyer P (2012) De novo DNA methylation activity of METHYLTRANSFERASE 1 (MET1) partially restores body methylation in Arabidopsis thaliana . Plant J 71:1029–1037. https://doi.org/10.1111/j.1365-313X.2012.05051.x
Download references
Acknowledgements
This work was supported by the core grant of National Institute of Plant Genome Research and research grant from Department of Biotechnology (BT/PR36694/NNT/281722/2020) to AP. HC and JN acknowledge University Grants Commission and Council of Scientific and Industrial Research, Government of India for Junior and Senior Research Fellowships, respectively. PKT acknowledges Science and Engineering Research Board, New Delhi for JC Bose National Fellowship (JCB/2021/000036). The authors are thankful to DBT-eLibrary Consortium (DeLCON) for providing access to e-resources. CSIR-CIMAP Publication Number: CIMAP/PUB/ 2024/58.
Funding was provided by CIMAP (Grant No. JCB/2021/000036).
Author information
Authors and affiliations.
National Institute of Plant Genome Research, Aruna Asaf Ali Marg, New Delhi, 110067, India
Himani Chhatwal, Jogindra Naik & Ashutosh Pandey
CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, 226 015, India
Prabodh Kumar Trivedi
You can also search for this author in PubMed Google Scholar
Contributions
AP and PKT conceived the idea. HC, JN and AP wrote the first draft. AP and PKT finalized the manuscript. All authors read and approved the manuscript.
Corresponding authors
Correspondence to Ashutosh Pandey or Prabodh Kumar Trivedi .
Ethics declarations
Competing interests.
The authors have not disclosed any competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Chhatwal, H., Naik, J., Pandey, A. et al. Broadening the epigenetic horizon of abiotic stress response in plants. Plant Growth Regul (2024). https://doi.org/10.1007/s10725-024-01152-y
Download citation
Received : 01 January 2024
Accepted : 20 April 2024
Published : 11 May 2024
DOI : https://doi.org/10.1007/s10725-024-01152-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Abiotic stress
- Epigenetics
- Euchromatin
- Heterochromatin
- Histone modification
- Methylation
- Stress memory
- Find a journal
- Publish with us
- Track your research
- Infographics
Epigenetics and Child Development: How Children’s Experiences Affect Their Genes
For more information about epigenetics, please scroll down below the infographic .

New scientific research shows that environmental influences can actually affect whether and how genes are expressed. In fact, scientists have discovered that early experiences can determine how genes are turned on and off and even whether some are expressed at all. Thus, the old ideas that genes are “set in stone” or that they alone determine development have been disproven. Nature vs. Nurture is no longer a debate—it’s nearly always both!
More Information on Epigenetics Deep Dive: Gene-Environment Interaction Learn more about the physical and chemical processes that take place as part of the creation of the epigenome. Working Paper 10: Early Experiences Can Alter Gene Expression and Affect Long-Term Development This in-depth working paper explains how genes and the environment interact, and gives recommendations for ways that caregivers and policymakers can effectively respond to the science.
During development, the DNA that makes up our genes accumulates chemical marks that determine how much or little of the genes is expressed. This collection of chemical marks is known as the “ epigenome .” The different experiences children have rearrange those chemical marks. This explains why genetically identical twins can exhibit different behaviors, skills, health, and achievement.
Correcting Popular Misrepresentations of Science
Until recently, the influences of genes were thought to be set, and the effects of children’s experiences and environments on brain architecture and long-term physical and mental health outcomes remained a mystery. That lack of understanding led to several misleading conclusions about the degree to which negative and positive environmental factors and experiences can affect the developing fetus and young child. The following misconceptions are particularly important to set straight.
- Contrary to popular belief, the genes inherited from one’s parents do not set a child’s future development in stone. Variations in DNA sequences between individuals certainly influence the way in which genes are expressed and how the proteins encoded by those genes will function. But that is only part of the story—the environment in which one develops , before and soon after birth, provides powerful experiences that chemically modify certain genes which, in turn, define how much and when they are expressed. Thus, while genetic factors exert potent influences, environmental factors have the ability to alter the genes that were inherited.
- Although frequently misunderstood, adverse fetal and early childhood experiences can—and do—lead to physical and chemical changes in the brain that can last a lifetime. Injurious experiences , such as malnutrition, exposure to chemical toxins or drugs, and toxic stress before birth or in early childhood are not “forgotten,” but rather are built into the architecture of the developing brain through the epigenome. The “biological memories” associated with these epigenetic changes can affect multiple organ systems and increase the risk not only for poor physical and mental health outcomes but also for impairments in future learning capacity and behavior.
- Despite some marketing claims to the contrary, the ability of so-called enrichment programs to enhance otherwise healthy brain development is not known. While parents and policymakers might hope that playing Mozart recordings to newborns will produce epigenetic changes that enhance cognitive development, there is absolutely no scientific evidence that such exposure will shape the epigenome or enhance brain function. What research has shown is that specific epigenetic modifications do occur in brain cells as cognitive skills like learning and memory develop, and that repeated activation of brain circuits dedicated to learning and memory through interaction with the environment, such as reciprocal “ serve and return ” interaction with adults, facilitates these positive epigenetic modifications. We also know that sound maternal and fetal nutrition , combined with positive social-emotional support of children through their family and community environments, will reduce the likelihood of negative epigenetic modifications that increase the risk of later physical and mental health impairments.
The epigenome can be affected by positive experiences, such as supportive relationships and opportunities for learning, or negative influences, such as environmental toxins or stressful life circumstances, which leave a unique epigenetic “signature” on the genes. These signatures can be temporary or permanent and both types affect how easily the genes are switched on or off. Recent research demonstrates that there may be ways to reverse certain negative changes and restore healthy functioning, but that takes a lot more effort, may not be successful at changing all aspects of the signatures, and is costly. Thus, the very best strategy is to support responsive relationships and reduce stress to build strong brains from the beginning, helping children grow up to be healthy, productive members of society.
For more information: Early Experiences Can Alter Gene Expression and Affect Long-Term Development: Working Paper No. 10 .

Full Text of the Graphic
“Epigenetics” is an emerging area of scientific research that shows how environmental influences—children’s experiences—actually affect the expression of their genes.
This means the old idea that genes are “set in stone” has been disproven. Nature vs. Nurture is no longer a debate. It’s nearly always both!
During development, the DNA that makes up our genes accumulates chemical marks that determine how much or little of the genes is expressed. This collection of chemical marks is known as the “epigenome.” The different experiences children have rearrange those chemical marks. This explains why genetically identical twins can exhibit different behaviors, skills, health, and achievement.
Epigenetics explains how early experiences can have lifelong impacts.
The genes children inherit from their biological parents provide information that guides their development. For example, how tall they could eventually become or the kind of temperament they could have.
When experiences during development rearrange the epigenetic marks that govern gene expression, they can change whether and how genes release the information they carry.
Thus, the epigenome can be affected by positive experiences, such as supportive relationships and opportunities for learning, or negative influences, such as environmental toxins or stressful life circumstances, which leave a unique epigenetic “signature” on the genes. These signatures can be temporary or permanent and both types affect how easily the genes are switched on or off. Recent research demonstrates that there may be ways to reverse certain negative changes and restore healthy functioning. But the very best strategy is to support responsive relationships and reduce stress to build strong brains from the beginning.
Young brains are particularly sensitive to epigenetic changes.
Experiences very early in life, when the brain is developing most rapidly, cause epigenetic adaptations that influence whether, when, and how genes release their instructions for building future capacity for health, skills, and resilience. That’s why it’s crucial to provide supportive and nurturing experiences for young children in the earliest years.
Services such as high-quality health care for all pregnant women, infants, and toddlers, as well as support for new parents and caregivers can—quite literally— affect the chemistry around children’s genes. Supportive relationships and rich learning experiences generate positive epigenetic signatures that activate genetic potential.
Related Topics: brain architecture , lifelong health , mental health
Explore related resources.
- Reports & Working Papers
- Tools & Guides
- Presentations

Videos : InBrief: Early Childhood Mental Health

Reports & Working Papers : Establishing a Level Foundation for Life: Mental Health Begins in Early Childhood

Reports & Working Papers : From Best Practices to Breakthrough Impacts
Reports & Working Papers : The Foundations of Lifelong Health Are Built in Early Childhood

Reports & Working Papers : Children’s Emotional Development Is Built into the Architecture of Their Brains

Reports & Working Papers : Connecting the Brain to the Rest of the Body: Early Childhood Development and Lifelong Health Are Deeply Intertwined

Tools & Guides : El lugar es importante: Guía para la aplicación de políticas

Infographics : El lugar importa: lo que nos rodea nos define

Briefs : Health and Learning Are Deeply Interconnected in the Body: An Action Guide for Policymakers

Videos : How Early Childhood Experiences Affect Lifelong Health and Learning

Briefs : How to Support Children (and Yourself) During the COVID-19 Outbreak

Briefs : InBrief: Early Childhood Mental Health

Reports & Working Papers : Maternal Depression Can Undermine the Development of Young Children

Reports & Working Papers : Persistent Fear and Anxiety Can Affect Young Children’s Learning and Development
Tools & Guides : Place Matters: An Action Guide for Policy

Reports & Working Papers : Place Matters: The Environment We Create Shapes the Foundations of Healthy Development
Infographics : Place Matters: What Surrounds Us Shapes Us

Podcasts : The Brain Architects Podcast: COVID-19 Special Edition: A Different World

Podcasts : The Brain Architects Podcast: COVID-19 Special Edition: Creating Communities of Opportunity

Podcasts : The Brain Architects Podcast: COVID-19 Special Edition: Mental Health in a Locked-Down World

Podcasts : The Brain Architects Podcast: COVID-19 Special Edition: Self-Care Isn’t Selfish

Infographics : The Brain Circuits Underlying Motivation: An Interactive Graphic

Infographics : What Is COVID-19? And How Does It Relate to Child Development?

Tools & Guides , Partner Resources : Training Module: Health Care Practitioner Module and Resources
Study explores role of epigenetics, environment in differing Alzheimer's risk between Black and white communities
A study from North Carolina State University has found that environmentally caused alterations to specific areas of the genome -- known as imprint control regions -- during early development may contribute to the risk of developing Alzheimer's disease, and that Black people may be more affected than white people. The work adds to our understanding of the ways in which environmental factors can contribute to genetic alterations and disease susceptibility.
"In terms of genetics and disease, I always think of Dr. Kenneth Olden's analogy: genetics loads the gun and the environment pulls the trigger," says Cathrine Hoyo, professor of biological sciences at NC State and co-corresponding author of the research.
"In fact, the Institute of Medicine has estimated that epigenetic response to the environment -how our genes respond to the environment -- contributes between 70% to 90% of chronic disease risk. And we know that in the case of Alzheimer's disease, only about 5% of cases are familial, or inherited.
"We also know that the risk of developing non-familial, or sporadic, Alzheimer's differs according to race -- Black people have twice the incidence of white people," Hoyo continues. "So we wanted to see if we could identify stable epigenetic features -- parts of the epigenome that are unlikely to change once established -- that distinguished Alzheimer's brains from those without the disease."
Specifically, the research team used the imprintome -- the imprint control regions (ICRs) in the human genome that regulate the expression of imprinted genes -- to identify stable epigenetic features that distinguished people with Alzheimer's disease from those without.
Imprinted genes differ from other genes because only one parental copy of an imprinted gene is active. The other copy is methylated, or silenced, early in development. Additionally with these genes, the methylation marks that control their expression are susceptible to environmental influences.
"With imprinted genes, there isn't a backup copy in the event of mutation," says Randy Jirtle, professor of epigenetics at NC State and co-corresponding author of the research. "ICRs control the expression of these genes -- in other words, they tell imprinted genes where, when and how to work through DNA methylation. And these methylation marks in ICRs don't normally change unless altered early in development, either at conception or shortly thereafter."
For the study, the team had brain tissue samples from 17 donors -- eight normal brains and nine with Alzheimer's. Each group was divided between non-Hispanic white and non-Hispanic Black donors (the Alzheimer's group had five samples from Black donors and four from white donors).
The team sequenced the entire genome for each sample, then looked for ICRs in the Alzheimer's brains that were either over- or under-methylated compared to the healthy brains.
They found 120 differently methylated ICRs in the Alzheimer's brains. Forty were found in the combined Black and white populations; however, 81 ICRs were found only in the Black population, and 27 were found only in the white population.
The differently methylated ICRs common to both populations are associated with (MEST/MESTIT1), a paternally expressed imprinted gene, and NLRP1, a predicted imprinted gene involved in brain inflammation.
"The importance of finding the common ICRs is that it could help us develop universal tests for potential disease markers," says Hoyo. "But it was very puzzling to discover that the Black population had almost three times as many affected ICRs as the white population.
"When you see that level of difference, and you know that the changes you're finding are likely caused early by environmental interactions, one possible explanation is that there are unique or different stressors in that population, and those epigenetic effects are being passed along."
The researchers hope the work could lead to testing and targeted early interventions to prevent Alzheimer's disease.
"We know that targeted prevention over long periods can alter risk," Hoyo says. "So if you can alert people early on about their risk and apply targeted interventions, you could prevent disease onset."
"Epigenetics is the science of hope," Jirtle says. "You can't necessarily reverse genetic mutations, but when you know disease risks result from changes in the epigenome you can potentially negate them."
The work appears in Clinical Epigenetics and was supported by the National Institutes of Health under grants R01HD098857, R01MD017696, R01MD011746, P30ES025128, and R01ES032462. Brain tissue samples were provided by Duke University School of Medicine. Former NC State Ph.D. student Sebnam Cevik is first author. Other NC State contributors were David Skaar, associate research professor of biology; Antonio Planchart, associate professor of biology; and Ph.D. student Dereje Jima. Dr. Andy Liu, Dr. Truls Østbye and Dr. Heather E. Whitson of Duke University School of Medicine also contributed to the work.
- Alzheimer's Research
- Healthy Aging
- Parkinson's Research
- Alzheimer's
- Racial Issues
- Disorders and Syndromes
- Alzheimer's disease
- Human skin color
- Yellow fever
- Dementia with Lewy bodies
- Illusion of control
- Vaccination
Story Source:
Materials provided by North Carolina State University . Original written by Tracey Peake. Note: Content may be edited for style and length.
Journal Reference :
- Sebnem E. Cevik, David A. Skaar, Dereje D. Jima, Andy J. Liu, Truls Østbye, Heather E. Whitson, Randy L. Jirtle, Cathrine Hoyo, Antonio Planchart. DNA methylation of imprint control regions associated with Alzheimer’s disease in non-Hispanic Blacks and non-Hispanic Whites . Clinical Epigenetics , 2024; 16 (1) DOI: 10.1186/s13148-024-01672-4
Cite This Page :
Explore More
- Record Low Antarctic Sea Ice: Climate Change
- Brain 'Assembloids' Mimic Blood-Brain Barrier
- 'Doomsday' Glacier: Catastrophic Melting
- Blueprints of Self-Assembly
- Meerkat Chit-Chat
- First Glimpse of an Exoplanet's Interior
- High-Efficiency Photonic Integrated Circuit
- Life Expectancy May Increase by 5 Years by 2050
- Toward a Successful Vaccine for HIV
- Highly Efficient Thermoelectric Materials
Trending Topics
Strange & offbeat.
- Research and Innovation
- The Abstract
- Audio Abstract Podcast
- Centennial Campus
- Campus Life
- Faculty and Staff
- Awards and Honors
- HR and Finance
- Resilient Pack
- We Are the Wolfpack
- Service and Community
- Red Chair Chats
- News Releases
- In the News
- NC State Experts on 2022 Elections
- NC State Experts Available on Climate
- NC State Experts on Roe v. Wade
- NC State Supply Chain Experts
- Experts on COVID-19
- Hurricane Experts
- About NC State News
- Faculty Support
- Training Program
Study Explores Role of Epigenetics, Environment in Differing Alzheimer’s Risk Between Black and White Communities

For Immediate Release
A study from North Carolina State University has found that environmentally caused alterations to specific areas of the genome – known as imprint control regions – during early development may contribute to the risk of developing Alzheimer’s disease, and that Black people may be more affected than white people. The work adds to our understanding of the ways in which environmental factors can contribute to genetic alterations and disease susceptibility.
“In terms of genetics and disease, I always think of Dr. Kenneth Olden’s analogy: genetics loads the gun and the environment pulls the trigger,” says Cathrine Hoyo, professor of biological sciences at NC State and co-corresponding author of the research.
“In fact, the Institute of Medicine has estimated that epigenetic response to the environment –how our genes respond to the environment – contributes between 70% to 90% of chronic disease risk. And we know that in the case of Alzheimer’s disease, only about 5% of cases are familial, or inherited.
“We also know that the risk of developing non-familial, or sporadic, Alzheimer’s differs according to race – Black people have twice the incidence of white people,” Hoyo continues. “So we wanted to see if we could identify stable epigenetic features – parts of the epigenome that are unlikely to change once established – that distinguished Alzheimer’s brains from those without the disease.”
Specifically, the research team used the imprintome – the imprint control regions (ICRs) in the human genome that regulate the expression of imprinted genes – to identify stable epigenetic features that distinguished people with Alzheimer’s disease from those without.
Imprinted genes differ from other genes because only one parental copy of an imprinted gene is active. The other copy is methylated, or silenced, early in development. Additionally with these genes, the methylation marks that control their expression are susceptible to environmental influences.
“With imprinted genes, there isn’t a backup copy in the event of mutation,” says Randy Jirtle, professor of epigenetics at NC State and co-corresponding author of the research. “ICRs control the expression of these genes – in other words, they tell imprinted genes where, when and how to work through DNA methylation. And these methylation marks in ICRs don’t normally change unless altered early in development, either at conception or shortly thereafter.”
For the study, the team had brain tissue samples from 17 donors – eight normal brains and nine with Alzheimer’s. Each group was divided between non-Hispanic white and non-Hispanic Black donors (the Alzheimer’s group had five samples from Black donors and four from white donors).
The team sequenced the entire genome for each sample, then looked for ICRs in the Alzheimer’s brains that were either over- or under-methylated compared to the healthy brains.
They found 120 differently methylated ICRs in the Alzheimer’s brains. Forty were found in the combined Black and white populations; however, 81 ICRs were found only in the Black population, and 27 were found only in the white population.
The differently methylated ICRs common to both populations are associated with (MEST/MESTIT1), a paternally expressed imprinted gene, and NLRP1, a predicted imprinted gene involved in brain inflammation.
“The importance of finding the common ICRs is that it could help us develop universal tests for potential disease markers,” says Hoyo. “But it was very puzzling to discover that the Black population had almost three times as many affected ICRs as the white population.
“When you see that level of difference, and you know that the changes you’re finding are likely caused early by environmental interactions, one possible explanation is that there are unique or different stressors in that population, and those epigenetic effects are being passed along.”
The researchers hope the work could lead to testing and targeted early interventions to prevent Alzheimer’s disease.
“We know that targeted prevention over long periods can alter risk,” Hoyo says. “So if you can alert people early on about their risk and apply targeted interventions, you could prevent disease onset.”
“Epigenetics is the science of hope,” Jirtle says. “You can’t necessarily reverse genetic mutations, but when you know disease risks result from changes in the epigenome you can potentially negate them.”
The work appears in Clinical Epigenetics and was supported by the National Institutes of Health under grants R01HD098857, R01MD017696, R01MD011746, P30ES025128, and R01ES032462. Brain tissue samples were provided by Duke University School of Medicine. Former NC State Ph.D. student Sebnam Cevik is first author. Other NC State contributors were David Skaar, associate research professor of biology; Antonio Planchart, associate professor of biology; and Ph.D. student Dereje Jima. Dr. Andy Liu, Dr. Truls Østbye and Dr. Heather E. Whitson of Duke University School of Medicine also contributed to the work.
Note to editors: An abstract follows.
“DNA methylation of imprint control regions associated with Alzheimer’s disease in non-Hispanic Blacks and non-Hispanic Whites”
DOI: 10.1186/s13148-024-01672-4
Authors: Sebnem E. Cevik, David A. Skaar, Dereje D. Jima, Randy L. Jirtle, Cathrine Hoyo, Antonio Planchart, North Carolina State University; Andy J. Liu, Truls Østbye, Heather E. Whitson, Duke University Published: April 25, 2024 in Clinical Epigenetics
Abstract: Alzheimer’s disease (AD) prevalence is twice as high in non-Hispanic Blacks (NHBs) as in non-Hispanic Whites (NHWs). The objective of this study was to determine whether aberrant methylation at imprint control regions (ICRs) is associated with AD. Differentially methylated regions (DMRs) were bioinformatically identified from whole-genome bisulfite sequenced DNA derived from brain tissue of 9 AD (5 NHBs and 4 NHWs) and 8 controls (4 NHBs and 4 NHWs). We identified DMRs located within 120 regions defined as candidate ICRs in the human imprintome (https://genome.ucsc.edu/s/imprintome/hg38.AD.Brain_track). Eighty-one ICRs were differentially methylated in NHB-AD, and 27 ICRs were differentially methylated in NHW-AD, with two regions common to both populations that are proximal to the inflammasome gene, NLRP1, and a known imprinted gene, MEST/MESTIT1. These findings indicate that early developmental alterations in DNA methylation of regions regulating genomic imprinting may contribute to AD risk and that this epigenetic risk differs between NHBs and NHWs.
- Bulletin Board
- Designing Healthy and Resilient Societies
- Harnessing Data for Decision-Making
- Innovative Outcomes
- biological sciences
- college of sciences
- news release
- research news
More From NC State News

Study Sheds Light on Bacteria Associated With Pre-Term Birth
New Technique Improves Finishing Time for 3D Printed Machine Parts

Daylight Saving Time Spells Bad News for Healthy Habits
- Reference Manager
- Simple TEXT file
People also looked at
Review article, epigenetics across the human lifespan.

- Epigenetics Laboratory, Department of Anatomy, Howard University, Washington, DC, USA
Epigenetics has the potential to explain various biological phenomena that have heretofore defied complete explication. This review describes the various types of endogenous human developmental milestones such as birth, puberty, and menopause, as well as the diverse exogenous environmental factors that influence human health, in a chronological epigenetic context. We describe the entire course of human life from periconception to death and chronologically note all of the potential internal timepoints and external factors that influence the human epigenome. Ultimately, the environment presents these various factors to the individual that influence the epigenome, and the unique epigenetic and genetic profile of each individual also modulates the specific response to these factors. During the course of human life, we are exposed to an environment that abounds with a potent and dynamic milieu capable of triggering chemical changes that activate or silence genes. There is constant interaction between the external and internal environments that is required for normal development and health maintenance as well as for influencing disease load and resistance. For example, exposure to pharmaceutical and toxic chemicals, diet, stress, exercise, and other environmental factors are capable of eliciting positive or negative epigenetic modifications with lasting effects on development, metabolism and health. These can impact the body so profoundly as to permanently alter the epigenetic profile of an individual. We also present a comprehensive new hypothesis of how these diverse environmental factors cause both direct and indirect epigenetic changes and how this knowledge can ultimately be used to improve personalized medicine.
Introduction
The literal meaning of the term epigenetic is “on top of or in addition to genetics.” The series of chemical tags that modify DNA and its associated structures constitute the epigenome, and include any genetic expression modifier independent of the DNA sequence of a gene. The genome defines the complete set of genetic information contained in the DNA, residing within the cells of each organism. The epigenome, on the other hand, comprises the complex modifications associated with genomic DNA, imparting a unique cellular and developmental identity.
The epigenome integrates the information encoded in the genome with all the molecular and chemical cues of cellular, extracellular, and environmental origin. Along with the genome, the epigenome instructs the unique gene expression program of each cell type to define its functional identity during development or disease ( Rivera and Ren, 2013 ).
The epigenome also, in some sense, represents the ability of an organism to adapt and evolve through expression of a set of characteristics or phenotypes developed in response to environmental stimuli.
Thus, in contrast to the consistency of the genome, the epigenome is characterized by a dynamic and flexible response to intra- and extra-cellular stimuli, through cell-cell contact, by neighboring cells, by physiology, or entirely by the environment that the organism is exposed to Figure 1 . Cytokines, growth factors, alterations in hormonal levels as well as release of stress-response and neurotropic factors are some examples of molecules that are modulated by the environment and which come under the category of epigenome modifiers. Ultimately, the environment presents these various factors to the individual that influence the epigenome, and the unique epigenetic and genetic profile of each individual also modulates the specific response to these factors (Figure 1 ).
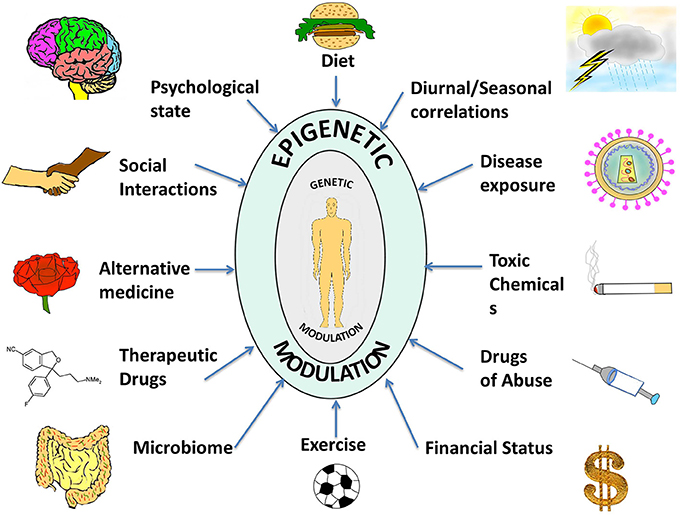
Figure 1. A compilation of epigenetic influences on humans . The figure represents a compilation of the various epigenetic influences on humans by different sources present in the environment. While some of these might be beneficial for health and behavior, others might be harmful and interfere with the body and mind creating an imbalance, which might manifest as a disease or psychological disorder. Some of the beneficial influences listed are exercise, microbiome (beneficial intestinal bacteria), and alternative medicine whereas harmful influences include exposure to toxic chemicals and drugs of abuse. Factors such as diet, seasonal changes, financial status, psychological state, social interactions, therapeutic drugs, and disease exposure might have beneficial or harmful effects depending on the specific nature of the influence. The environment thus complements and shapes human health. With the help of extended research in the field, we might be able to steer these influences in a positive way.
Enzymatic activity in response to the environment promotes addition or removal of epigenetic tags on DNA and/or chromatin, sparking a cavalcade of changes that affect cellular memory transiently, permanently or with a heritable alteration. Some of the molecular mechanisms involved in this process are explained in more detail below.
Mechanisms Underlying Epigenetics
As explained, every cell in the organism carries an identical genome, however, despite the stability of these instructions, the terminal phenotype within an organism is not fixed and deviation is caused by gene expression changes in response to environmental cues. DNA methylation, histone modification and RNA-associated silencing are the major ways these changes are controlled, which are described in more detail below.
DNA Methylation
The methylome is the genomic distribution of methylated DNA sequence present in a cell and is capable of undergoing modification with respect to the environment or the developmental stage. DNA methylation involves the covalent addition of a methyl group at position 5 of the pyrimidine ring of cytosine that is represented as 5-methyl C or C Me . Transcription of most protein coding genes in mammals is initiated at promoters rich in CG sequences, where cytosine is positioned next to a guanine nucleotide linked by a phosphate called a CpG site. Such short stretches of CpG-dense DNA are known as CpG islands. In the human genome 60–80% of 28 million CpG dinucleotides are methylated ( Lister et al., 2009 ; Ziller et al., 2013 ). Chromatin structure adjacent to CpG island promoters facilitates transcription, while methylated CpG islands impart a tight compaction to chromatin that prevents onset of transcription and therefore, gene expression.
In CpG islands active C's are normally unmethylated and when an unmethylated cytosine spontaneously deaminates to uridine, it is converted back to cytosine by DNA repair mechanisms, thus preserving CpG sequences through evolution. The presence of 5-methyl C in a CpG island denotes an inactive promoter owing to the condensation of chromatin triggered by DNA methylation.
CpG sites are methylated by one of three enzymes called DNA methyltransferases (DNMTs). A variety of DNMTs are responsible for DNA methylation patterns established during embryogenesis. One type of DNA methyltransferase, DNMT1, is responsible for maintaining normal methylation patterns by copying them exactly between cell generations during replication. DNMT2 is associated with embryonic stem cells and potential RNA methylation. DNMT3a and DNMT3b are involved in de novo DNA methylation at CpG sites ( Clouaire and Stancheva, 2008 ; Singh and Li, 2012 ).
Histone Modification
Histones are the core protein components of chromatin complexes, and they provide the structural backbone around which DNA wraps at regular intervals generating chromatin. The nucleosome represents the first level of chromatin organization and is composed of two of each of histones H2A, H2B, H3, and H4, assembled in an octameric core with DNA tightly wrapped around the octamer ( Luger et al., 1997 ). Histones regulate DNA packaging with immense influence on the degree of chromatin compaction, influencing transcriptional activity as well as transcriptional silencing.
Histone modifications are post-translational changes on the histone tails, that are flexible stretches of N or C terminal residues extending from the globular histone octamer. Modifications of histones include acetylation of lysine residues, methylation of lysine and arginine residues, phosphorylation of serine and threonine residues, and ubiquitination of lysine residues present on histone tails, as well as sumoylation and ADP ribosylation. All of these changes influence DNA transcription. Addition or removal of methyl groups on DNA (see above) and histones and acetyl groups on histones are the prime mechanisms of changing the epigenetic landscape ( Cedar and Bergman, 2009 ).
Histone acetylation is carried out by enzymes called histone acetyltransferases (HATs), that are responsible for adding acetyl groups to lysine residues on histone tails while histone deacetylases (HDACs) are those that remove acetyl groups from acetylated lysines. Generally, presence of acetylated lysine on histone tails leads to a relaxed chromatin state that promotes transcriptional activation of selected genes; in contrast, deacetylation of lysine residues leads to chromatin compaction and transcriptional inactivation.
RNA Silencing
RNA-associated silencing is a type of post-transcriptional gene modification during which the expression of one or more genes is downregulated or suppressed by small non-coding stretches of RNA, sometimes called microRNAs (miRNA) and small interfering RNAs (siRNA). Although microRNAs only represent 1% of the genome they have been estimated to target 30% of genes ( Lewis et al., 2005 ). These RNAs can act as switches and modulators, exerting extensive influence within the cell and beyond. These RNAs fine-tune the gene expression as they act as specific modulators based on cell-type specificity of the organism during development as well as pathological conditions ( Giraldez et al., 2005 ; Girardot et al., 2012 ; Baer et al., 2013 ). Also, miRNAs have been known to play a role in tumor suppression, apoptosis, cellular proliferation and cell movement which suggests that they can be manipulated in treating epigenetic diseases like cancer ( Kala et al., 2013 ).
Putative mechanisms of RNA silencing include the ability of non-coding RNA to negatively regulate expression of target genes at the posttranscriptional level by binding to 3′-untranslated regions of target mRNAs resulting in their degradation ( Singh et al., 2008 ).
All genes in every cell type are activated or silenced by an underlying interplay between these described epigenetic mechanisms. And as explained in the Introduction, exogenous epigenetic forces modify the endogenous inherited epigenetic pattern.
Endogenous and Exogenous Epigenetic Regulation of Genes
In order to illustrate the endogenous epigenetic regulation of a gene, we will use the example of OCT4. OCT4 is the master pluripotency gene, which is regulated through different stages of human development, and its activation is necessary for maintaining pluripotency, whereas it must be silenced in order for a cell to differentiate ( Kellner and Kikyo, 2010 ) (Figure 2 ). OCT4 is thus active in embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) as well as in cancer cells, but is silenced in differentiated cell types. The three types of epigenetic modifications explained above i.e., DNA methylation, histone modification and RNA silencing are responsible for such regulation of OCT4 gene expression. This has been illustrated in detail along with the various epigenetic tags involved in its regulation in Figure 2 .
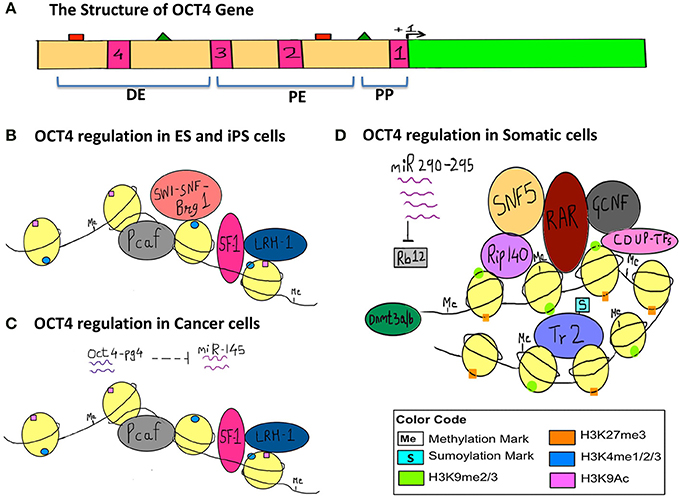
Figure 2. Epigenetic regulation of OCT4 in stem cells, cancer cells and somatic cells . The figure represents the various epigenetic mechanisms involved in regulating the gene expression of OCT4. (A) This section represents the structure of the OCT4 gene. The green bar represents the coding region. The beige bar represents the upstream region containing regulatory sequences. The four pink bars represent conserved regions; CR1 (−129 to −1), CR2 (−1510 to −1315), CR3 (−1956 to −1852), CR4 (−2557 to −2425) (data for human OCT4 gene). The green triangles represent the sites for binding of activators and the red squares represent the sites for binding of the repressors and these sites lie within the enhancers and promoter. +1 denotes transcription start site (TSS). (DE, Distal Enhancer −2057 to −1955; PE, Proximal Enhancer −1152 to −901; PP, Proximal Promoter −240 to +1) (This data is for the OCT4 gene from mouse ESCs since information for human OCT4 was insufficient). (B) This section represents the regulation of OCT4 in ESCs and iPSCs. OCT4 is highly expressed in these cells. Binding of transcriptional machinery induces OCT4 expression. This can be attributed to the hypomethylated promoter, enhancer region, which promotes binding of transcriptional activators and co-activators like Pcaf, LRH−1, SF−1, and Brg1 sub-unit of the SWI-SNF nucleosome-remodeling complex, which in turn facilitates the binding of RNA polymerase II. Activating histone marks like H3K4me and H3K9Ac are found on the histones surrounding the regulatory regions of the gene and they enhance the recruitment and binding of transcription factors thus inducing gene expression. (C) This section represents the regulation of the OCT4 gene in cancer cells, where similar to embryonic-like cells, it is highly expressed. In the case of hepatocellular carcinoma, an additional layer of epigenetic regulation is seen with the activity of the OCT4-pseudogene (pg)4, a non-coding RNA that protects the OCT4 transcript from inhibition by miRNA-145. Thus, OCT4-pg4 indirectly enhances the expression of OCT4 in cancer cells by acting as a protective shell for nascent OCT4 transcripts. The activity of the Brg1 sub-unit of the SWI-SNF complex is not necessary for OCT4 expression in cancer cells. (D) This section represents the regulation of OCT4 in somatic cells, which are differentiated and have a specialized function. Here OCT4 expression is repressed because of the hypermethylated promoter region and the repressive H3K27me and H3K9me marks. This results in compaction of the regulatory regions and facilitates the recruitment of other transcriptional repressors like RAR, GCNF, COUP-TFs, further compacting the regulatory regions, and making it inaccessible to the transcriptional machinery. SNF5, a core sub-unit of the BAF complex (SWI-SNF) acts as a switch between pluripotency and differentiation and promotes differentiation by binding to regulatory regions of OCT4. In addition, the sumyolation of orphan nuclear receptor Tr2 at Lys 238 results in its release from promyelocytic (PML) nuclear bodies. (In ESCs and iPSCs Tr2 is bound to PML nuclear bodies and acts as an activator). This in turn results in an exchange of co-repressor Rip-140 for co-activator Pcaf, making Tr2 a repressor. Also, miR-290 through miR-295 enhance methylation as they inhibit Rb12, which would otherwise inhibit the activity of de novo methyl transferase Dnmt 3a/3b.
In a similar way, let us consider the effect of exogenous epigenetic forces on the expression of OCT4. Vitrification, which is a commonly used method for cryopreservation, has been documented to alter the methylation patterns of the OCT4 gene. In two such cases, vitrification resulted in decreased methylation of the OCT4 promoter causing reduced gene expression in mouse blastocysts and the same was observed for mouse oocytes that underwent vitrfication followed by in vitro maturation ( Milroy et al., 2011 ; Zhao et al., 2012 ). This explains how the external environment, in this case temperature, can lead to alteration of the epigenetic profile of one or many genes, ultimately causing differential gene expression.
How do Cellular Biochemical Changes Cause Epigenetic Changes? A Hypothetical Mechanism
The effects of an epigenetic factor can be manifested as a global change in DNA methylation affecting multiple genes, or modified expression of very specific genes. The mechanisms and cellular pathways that are involved in the creation of these global or specific epigenetic changes are currently obscure. Below we describe a working hypothesis on how these changes might occur.
We propose that an epigenetic factor can act through either a direct or an indirect mechanism (Figure 3 ). A direct effect could happen in two ways; which we term Type 1 and Type 2. Type 1 direct effect occurs when the epigenetic factor directly alters the state of epigenetic enzymes—either by binding to them and preventing them from carrying out their normal function, damaging them in some other way, or upregulating them. The altered bioavailability of epigenetic enzymes then results in aberrant recruitment of epigenetic tags to promoters and enhancers on a genome-wide scale. Such a direct effect would be effective across the entire genome, not affecting any specific gene but resulting in a randomly altered epigenome. An example of a directly acting epigenetic factor is the antihypertensive hydralazine, which inhibits DNA methylation.
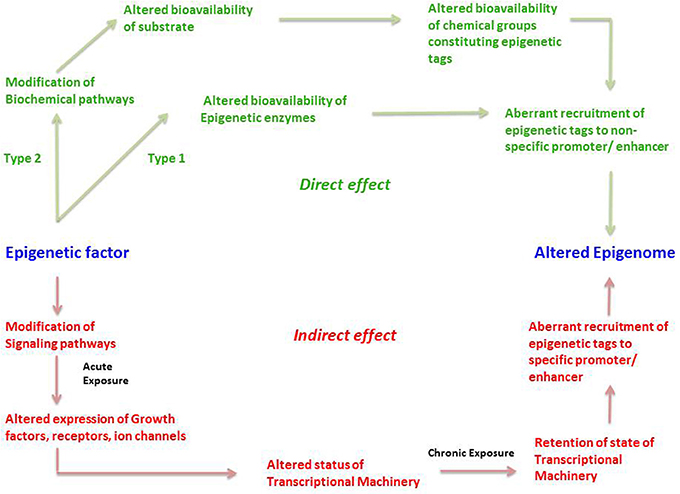
Figure 3. The direct and indirect epigenetic pathway . The figure represents two different routes through which an epigenetic factor can modify the epigenome leading to altered gene expression. Epigenetic effects exerted by an external factor or intrinsic environment can lead to direct and indirect effects on the epigenome. Green represents direct effects of an epigenetic factor and red represents indirect effects of an epigenetic factor. The direct pathway can operate in two different ways; namely Type1 and Type 2. In the Type 1 direct pathway, the epigenetic factor directly exerts an effect on the epigenetic enzymes such as DNMTs, HDACs, HATs, HMTs, HDMs, etc. such that there is an altered bioavailability of these enzymes in the cell. A Type 2 direct effect is when an epigenetic factor interferes with a biochemical pathway such that there is altered availability of a metabolite required for constituting an epigenetic tag. Both cases can result in aberrant or inadequate recruitment of epigenetic tags in a random fashion to non-specific promoters, ultimately establishing an altered epigenetic profile. In the indirect pathway, the epigenetic factor indirectly exerts an effect on the epigenome by first interfering with any signaling pathways of the cell. An acute exposure to the epigenetic factor can cause altered expression of growth factors, receptors, ion channels and so on resulting in non-homeostatic cellular processes. This in turn might lead to an altered status of transcriptional machinery (bound or unbound to the promoter/enhancer) and its bioavailability in a cell. A chronic exposure to the epigenetic factor might lead to retention of such state of transcriptional machinery (bound or unbound to the promoter/enhancer) causing altered gene expression as well as aberrant recruitment of epigenetic enzymes, leading to permanent addition or removal of epigenetic tags to specific promoter/enhancers. This consequently establishes an altered epigenetic profile.
Type 2 direct effects occur when an epigenetic factor causes a change in a biochemical process that results in an altered availability of a substrate, intermediate, by-product or any other metabolite participating in the biochemical pathway, that is used to make up epigenetic tags (for example acetate). This in turn leads to altered availability of epigenetic tags, in this case acetyl groups on histones, which leads non-specific modification of the epigenome (Figure 3 ).
The second major way that factors can cause epigenetic changes is by what we term indirect mechanisms (Figures 3 , 4 ). A biphasic mechanism is postulated for indirect effects in which acute exposure to a factor influences cellular signaling pathways that leads to altered expression of growth factors, receptors and ion channels, which in turn alter transcription factor activity at gene promoters. With more chronic exposure, the transcription factors and other gene regulatory proteins, in addition to altering gene expression activity, actually recruit or repel epigenetic enzymes to/from the associated chromatin, resulting in the addition or removal of epigenetic tags (Figure 4 ). In this way cells adapt to the persistent gene expression changes by causing permanent modifications to DNA methylation and chromatin structure, leading to enduring alteration of the affected epigenetic network (Figures 3 , 4 ). An example of an indirectly acting factor is the drug isotretinoin, which has transcription factor activity.
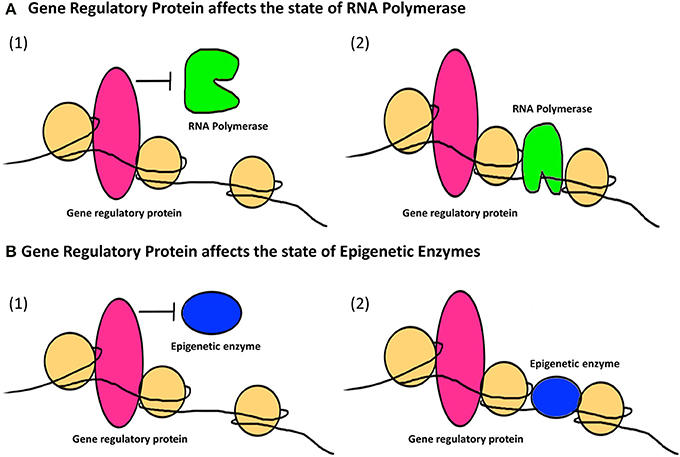
Figure 4. The indirect epigenetic pathway . An epigenetic factor operating through an indirect pathway interferes with transcriptional machinery. Chronic exposure to an epigenetic factor can lead to the retention of an already altered state of transcriptional machinery. The transcriptional machinery (bound or unbound to the gene regulatory regions i.e., promoters/enhancers) includes a number of proteins like transcription factors, activators and co-activators, repressors and co-repressors and nucleosome or chromatin remodeling complexes. For simplicity, these proteins are Fectively termed as “Gene regulatory protein” for this figure. (A) A gene regulatory protein might affect the status of RNA Polymerase (1) By inhibiting it from binding to a transcriptional apparatus or forming one or (2) by facilitating the binding of RNA Polymerase as well as formation of a transcriptional apparatus essential for the initiation of transcription. (B) A gene regulatory protein might also affect the status of epigenetic enzymes like DNMTs, HDACs, HATs, HMTs, HDMs, etc., which are responsible for the addition or removal of epigenetic tags (methyl group on DNA or histone, acetyl group on histones) on gene regulatory regions. This can be (1) By inhibiting epigenetic enzymes from binding to the gene regulatory regions and hence prohibiting the addition or removal of epigenetic tags (2) By facilitating the binding of epigenetic enzymes to gene regulatory regions and hence allowing the addition or removal of epigenetic tags. Such retention of a gene regulatory protein due to chronic exposure to an epigenetic factor might result in a permanent change in the epigenetic profile and/or gene expression of a specific gene affected by that epigenetic factor through an indirect pathway.
Epigenetic factors known to cause such direct and indirect effects are well-documented but their exact mechanism has not been accurately elucidated. For example, nutritional interventions in adulthood like caloric restriction can induce epigenetic changes that have the potential to alleviate age-related diseases ( Bacalini et al., 2014 ). In caloric restriction food intake is intentionally reduced by 30–50% ( Zakhari, 2013 ) and has been shown to delay the aging process in mice by decreasing the levels of histone deacetylase 2 (HDAC2) which otherwise increases during the normal aging process ( Chouliaras et al., 2013 ). Thus, this is an example of direct effect by an epigenetic influence (caloric restriction).
Another example is curcumin, a polyphenol found in turmeric, which can be regarded as a dietary epigenetic factor. It is an inhibitor of the Histone acetlytransferase p300/CBP (co-activator) and GATA4 (a zinc finger transcription factor), which leads to decrease in nuclear acetylation induced during myocardial cell hypertrophy ( Morimoto et al., 2008 ). The possible mechanisms through which curcumin exerts such an effect might be through allosteric regulation of p300 or through interfering with nuclear signaling pathways like transcriptional activation by NF-κB ( Morimoto et al., 2008 ). Hence the epigenetic effect of curcumin is an example of a combined direct and indirect effect.
Epigenetics Across the Human Lifespan
In this main section of the paper, we describe the major epigenetic milestones in the human lifespan, integrated chronologically with the various environmental factors that affect the human epigenome and the interaction with these milestones (Figure 6 ).
In differentiated cells, signals fine-tune cell functions through changes in gene expression across the lifespan. A flexible epigenome allows us to adjust to changes in the world around us, and to “learn” from our experiences. In many ways, epigenetic expression can be thought of as the “software” of the genome and directs embryogenesis and development, as well as influences the development of an individual's body and brain after birth. Unique sets of genes are induced or silenced epigenetically during different stages of life and these are responsible for the development and maturation of the individual through orchestrated events in combination with input from the environment. Any kind of epigenetic factors influencing genes or gene expression networks during life stages can result in an imbalance in the regulation process, and might have a life-long effect on the individual. While such flexibility gives rise to beneficial adaptability to environmental conditions, it likewise allows weaknesses to integrate and exert negative and diseased outcomes on both individual and evolutionary scales.
We have used data from human studies in most cases in this review, but in some cases where such data is sparse or unavailable, we have supported our explanations with data from studies on rodent and/or other animal models.
From the Periconceptional Environment to Birth
For most genes, total reprogramming is necessary very soon after conception in order to start with an epigenetic “clean slate,” which then allows all of the specialized cells derived from the egg and sperm to develop with stable cell-specific gene expression profiles and remain properly differentiated. This happens in the fertilized egg: global DNA demethylation is followed by remethylation to reprogram the maternal and paternal genomes for efficient gene expression regulation. As a fertilized egg develops into a human baby, signals received cause steady changes in gene expression patterns. Epigenetic tags physically record the cell's experiences on the DNA, and stabilize gene expression. Each signal activates some genes, and inactivates others, as the cell develops toward its final fate. Early in development, most signals come from within cells or from neighboring cells. Different experiences cause the epigenetic profiles of each cell type to grow increasingly different over time. Eventually, hundreds of cell types form, each with a distinct identity and specialized function. Specifc genes are turned on and off at certain time intervals, and any disruption of this finely-tuned DNA methylation regulation may persistently alter gene expression. The fetal epigenome is most susceptible during this developmental period to epigenetic modifiers in the maternal environment. An error during such a crucial time might lead to an abnormal phenotypic outcome in the offspring.
Assisted reproductive technologies (ARTs) like IntraCytoplasmic Sperm Injection (ICSI) and in vitro feritlization (IVF), which are used in case of male or female sub-fertility, cause epigenetic changes in offspring such that there is differential allelic expression in the early embryo ( Kohda and Ishino, 2013 ). While comparing the two ARTs, ICSI, and IVF, both result in epigenetic errors owing to aberrant DNA methylation, but neither one has an increased effect as compared to the other ( Santos et al., 2010 ). It has been shown that the use of ICSI results in hypermethylation of the Small Nuclear RiboNucleoprotein Polypeptide N gene (SNRNP) indicating likely mRNA splicing errors, which might result in such offspring ( Whitelaw et al., 2014 ) (Figure 6 ).
Studies have also shown that ART can increase the risk of adverse pregnancy outcomes and imprinting errors resulting in incorrectly silenced genes ( Bowdin et al., 2007 ; Reddy et al., 2007 ). As already mentioned in Section Endogenous and Exogenous Epigenetic Regulation of Genes, vitrification, which is also used in cryopreservation of embryos, leads to decreased methylation of OCT4 as well as other genes ( Milroy et al., 2011 ; Zhao et al., 2012 ) further suggesting that ART is capable of altering the epigenetic profile, which might cause serious complications in future progeny.
Maternal influences
Maternal health can predict childhood development, health outcome and disease consequences. More specifically, fetal programming describes how the in utero environment impacts molecular development in the fetus via epigenetic remodeling ( Barker and Clark, 1997 ; Schuz, 2010 ). Specific examples include the observation that reprogramming of the developing zygote involves conversion of 5-methylcytosine to 5-hydroxymethylcytosine ( Wossidlo et al., 2011 ).
Maternal diet. A mother's diet and stress influence the fetus in utero and can cause epigenetic changes as well. A study based on maternal folic acid supplementation showed that the offspring of mice fed with folic acid had a distinct global DNA methylation pattern compared to that of the offspring of mice which received a low folic acid dosage, involving genes associated with autism spectrum disorder (ASD) pathogenesis ( Barua et al., 2014 ). The data supports the studies on the complexity of epigenetic regulation of genes GAD1 and RELN in ASD ( Zhubi et al., 2014 ).
Also, experiments wherein maternal diet modification was the only variable were able to improve health outcome and increase longevity in offspring without changing DNA sequence. For example pregnant agouti mice, which are a strain predisposed to severe obesity, when fed diets supplemented with genistein, give birth to totally normal pups ( Dolinoy et al., 2006 ).
Epigenetic effects of periconceptional diet on the DNA methylation status of rural Gambians shows that altered nutritional status of mothers during seasonal (weather) changes results in epigenetic variation in the three germ layers of offspring born during different seasons, including differential birth weight, and these changes persist through adulthood ( Waterland and Jirtle, 2003 ).
Maternal smoking. Other maternal effects include cigarette smoking, which during pregnancy can cause altered DNA methylation and micro RNA expression ( Knopik et al., 2012 ).
Maternal mental health and social environment. Maternal psychological health also exerts a powerful influence over the epigenetic outcome in offspring. In rats, prenatal stress during late gestation has been shown to modify epigenetic signatures that are linked to neurological disease during the critical period of fetal brain development ( Zucchi et al., 2013 ). Also, domestic violence triggers stress in pregnant women that results in epigenetic changes in the DNA of the cortisol receptor in offspring observed during adolescence ( Radtke et al., 2011 ).
Paternal influences
Environmentally induced epigenetic variation is also driven by paternal factors, and they are as important as their maternal counterparts in influencing epigenetic outcome in offspring ( Carone et al., 2010 ; Hughes, 2014 ). During embryogenesis and fetal growth the insulin–like growth factor 2 (IGF2) gene is regulated by DNA methylation. Imprint marks are erased in primordial germ cells early in the process and new methylation patterns are created. Only paternal IGF2 is transcribed in normal tissues ( Dechiara et al., 1991 ). New evidence indicates that paternal obesity is associated with a decrease in DNA methylation at IGF2 ( Soubry et al., 2013 ).
Also, DNA methylation in sperm can be influenced by paternal alcohol consumption, and paternal exposure to toxic chemicals such as vinclozolin and chromium chloride likewise alters the germ line epigenome ( Cheng et al., 2004 ; Anway et al., 2005 ; Ouko et al., 2009 ; Stouder and Paoloni-Giacobino, 2010 ). Also, folate deficiency in male mice affects sperm function involving differential DNA methylation compared to control and the male offspring of such folate deficient mice show an altered gene expression compared to offspring born to control mice ( Lambrot et al., 2013 ).
Developmental/in utero influences
Imprinted genes have trans-generationally stable DNA methylation patterns oblivious to the normal resetting that occurs early in normal development. These imprinted epigenetic marks are passed from parent to progeny on gametes, evading the normal epigenetic purging process that occurs during gamete formation, as described above. If a particular epigenetic profile is to pass to the next generation, the epigenetic tags associated with it must avoid erasure during reprogramming. Typically, a small minority of genes possesses epigenetic tags that survive the reprogramming process and pass unchanged from one generation to the next.
One such example is the neurological epigenetic disease called Angelman syndrome, that results from epigenetic silencing of the paternal UBE3 allele combined with a mutation or deletion in the maternal UBE3 allele, resulting in loss of E3 ubiquitin ligase, leading to multiple neuronal defects like epilepsy, ataxia, etc. ( Rudenko and Tsai, 2014 ). Such epigenetic alterations based on comparison with a normal epigenetic profile can be considered useful biological markers for investigating neuropsychiatric symptoms of mental disorders with the help of new technologies like MeD-Chip sequencing ( Fass et al., 2014 ).
Imprinted genes possess molecular memory of their germ line, associated with a variety of allelic DNA methylation patterns affecting genotype. But imprinted genes are still subject to epigenetic reprogramming following environmental exposures. Exposure to certain dioxin compounds induces DNA methylation in imprinted genes ( Surani, 2001 ). Similarly, certain mammals respond to a hormonally triggered type of diabetes during pregnancy, known as gestational diabetes. Maternal gestational diabetes causes the developing fetus to be exposed to high levels of glucose. The high glucose in turn triggers epigenetic changes in the progeny DNA, often resulting in gestational diabetes in the next generation. Studies in rats with the proximal promoter region of Pdx1 , a duodenal and pancreatic specific homeobox transcription factor reveal that onset of diabetes was associated with permanent silencing of the locus ( Park et al., 2008 ).
In females, random X-chromosome inactivation is initiated during gastrulation in the epiblast through the X-inactive specific transcript (Xist) gene, which encodes a long non-coding RNA that silences the X-chromosome transcribing it ( Reik, 2007 ).
Thus, as a fertilized egg develops into an embryo to fetus to a baby, numerous signals over the course of development cause incremental changes in expression of the genetic profile (Figure 6 ). Even in differentiated cells, signals will fine-tune cellular function by altering gene expression. Ultimately the flexibility inherent in the epigenome allows adjustments in response to the changing environment and the ability for future generations to successfully evolve and learn from earlier experience ( Barlow and Bartolomei, 2014 ).
Perinatal Effects
Epigenetic influences continue to shape an individual after birth. Even at birth, the type of delivery seems to have an effect on the offspring being born. For example, offspring born from ceasarian section have shown to have global hypermethylation in leucocytes as compared to those born vaginally ( Schlinzig et al., 2009 ).
Infancy and childhood
After birth and as life continues, a wider variety of environmental factors begin to play a role. As in early development, signals from within the body continue to be important for many processes, including physical growth and learning, but gradually more and more external environmental and social influences begin to take effect. For example, groundbreaking studies on the ability of behavior to modify the epigenome were conducted and demonstrated the important interplay between social and physical processes ( Weaver et al., 2004 ). Early life positive and negative experiences like maternal care, stress adaptation, and early life adversities contribute to a biological memory, and epigenetic modifications of DNA are responsible for imprinting such influences in to the neuronal circuits of the developing brain which can have life-long impacts ( Hoffmann and Spengler, 2014 ).
Maternal care and interaction
During infancy and childhood maternal care and social environment shape a child's psychology ( Fagiolini et al., 2009 ). Maternal bonding has a profound effect on the physical and psychological welfare of children. Epigenetic mechanisms interact with and impact the hypothalamic-pituitary-adrenal axis of the stress response in the brain. For example, in mice, absence of maternal grooming epigenetically modifies DNA methylation, which alters glucocorticoid receptor expression. This leads to increased cortisol production by the adrenal glands and increases the stress response in pups ( Plotsky and Meaney, 1993 ).
Also, murine maternal care like licking and grooming (LG) and arched back nursing (ABN) resulted in increased levels of hippocampal N-methyl D-aspartate receptor (NMDAR) and brain-derived neurotrophic factor (BDNF) in infant rats leading to increased neural synaptogenesis and enhanced spatial learning in adulthood ( Meaney, 2001 ). Also pups experiencing frequent licking and grooming by their mother exhibit decrease in stress and anxiety through adulthood as a result of epigenetic changes at cortisol receptors ( Weaver et al., 2004 ). Maternal care in rats has demonstrated decreased methylation of the offspring Grm1 gene encoding metabotropic glutamate receptor (mGluR1) denoting epigenetic control of genes involved in glutamatergic synaptic signaling thereby influencing hippocampal function and cognitive performance ( Bagot et al., 2012 ).
Licking by mother rats of their pups activates the NRC31 gene in the hippocampus of the brain and as a result protects the pups against stress ( Oberlander et al., 2008 ; Bromer et al., 2013 ) (Figure 4 ). This epigenetically induced modification of DNA observed in rats is reversible, with remethylation of glucocorticoid receptors occurring in response to methionine injection ( Waris and Ahsan, 2006 ). Presumably, therefore, it may be possible to prevent or reverse the onset of similar damaging epigenetic modifications in human children through behavioral therapy such as hugging, cuddling and other nurturing and stress-alleviating activities.
On the other hand, increased levels of corticotropin- release factor (CRF) in pups as a result of maternal separation was shown to be associated with serious mood disorders in adulthood ( Meaney, 2001 ) There is similar evidence that suicide victims with a history of domestic violence and childhood abuse showed increased DNA methylation of the glucocorticoid receptor (NR3C1) gene leading to increased HPA (hypothalmis-pitutary-adrenal axis) activity resulting in elevated stress levels, also suggesting that suicide has a developmental origin ( McGowan et al., 2009 ) (Figure 6 ).
Poverty and neglect have direct negative impacts upon future development ( McGowan et al., 2009 ). The quality of family life including maternal care continues to influence the physiology and psychology of the child such that persistent neglect, emotional or sexual abuse hamper growth and intellectual development and increase risk of disorders like obesity during adulthood ( Meaney, 2001 ).
More positively, epigenetic analysis of the serotonin transporter gene can be used for screening soldiers and identifying those at a greater risk for Post-traumatic stress disorder (PTSD) based on childhood trauma and thus specific epigenetic signatures can help in improvement of training regimens of such soldiers at a higher risk in order to avoid PSTD ( Miller, 2011 ).
Adolescence
The transition from childhood to adolescence is accompanied by temperamental and behavioral changes including emergence of sexual behavior which is driven by underlying hormonal changes that can also be influenced by environmental factors ( Laviola et al., 2003 ). Puberty is a primary event of adolescence and is itself a major development event of human life. The HPG (hypothalamic-pitutary-gonadal) axis, which is dormant during childhood, is now activated ( Seminara et al., 2003 ) which results in a sustained increase in gonadotropin-releasing hormone (GnRH) ( Ojeda et al., 2010 ).
Puberty involves the maturation of certain regions of the pre-frontal cortex in the brain, and it has been suggested that environmental influences like stress can trigger neuropsychiatric diseases via epigenetic mechanisms during such vulnerable plastic development ( Morrison et al., 2014 ). Puberty in females involves maturation of specific brain regions along with the increased expression of genes like KISS-1 (kisspeptin), NKB (neurokinnin B), GPR54, TAC3 along with decreased expression of two genes Eed and Cbx7 (polycomb group proteins) leading to the beginning of estrous cycle ( Lomniczi et al., 2013 ; Morrison et al., 2014 ).
Puberty in females also leads to menarche i.e., the beginning of the menstrual cycle in females. The menstrual cycle is an orchestrated event that operates through a 28 day period during which the endometrium undergoes cyclic morphological transformations of growth, differentiation and regression ( Munro et al., 2010 ). We have illustrated the epigenetic fluctuations (global histone acetylation) governing the gene expression in the endometrium through this cycle (Figure 5 ).
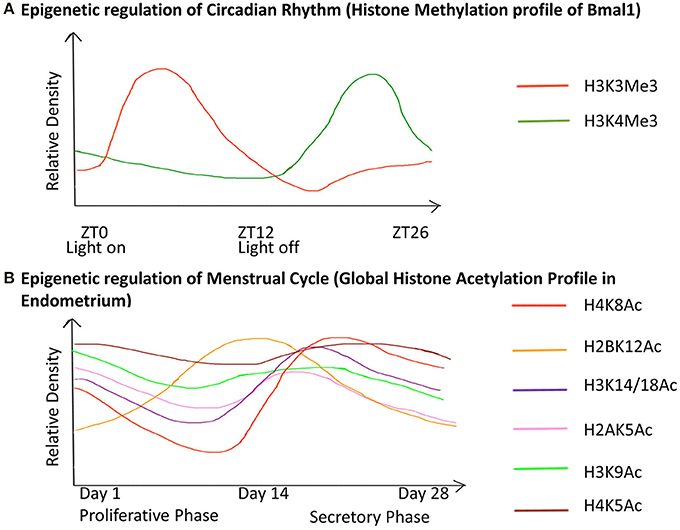
Figure 5. Chronology of epigenetic regulation during the circadian rhythm and menstrual cycle . The regulation of biological cycles like the Circadian rhythm and Menstrual cycle in females has been shown to work through epigenetic regulatory mechanisms. (The figure is relative and not accurate to scale). (A) The gene Bmal1 is the master regulator of the circadian rhythm. The graph represents the relative density of the two active histone marks H3K3me and H3K4me on Bmal1 through a 26-hour period. ZT is Zeitgeber Time where ZT0 corresponds to light on and ZT12 to light off. The peaks represent the maxima of the two-histone marks with respect to ZT, similarly, the dips represent the minima. This shows differential expression of the gene with respect to time, which has an epigenetic regulatory basis. (B) The graph represents the global acetylation profile from endometrial tissue through the 28-day menstrual cycle. The peaks represent the maxima and the dips represent the minima of the histone marks with respect to time. Global changes in the histone acetylation profile show that these marks contribute to the induction of different genes during different stages of the cycle. Hence they are responsible for the variable morphology of the endometrium through the 28-day period. These epigenetic changes are thus the basis for changes in the endometrial morphology like regeneration (1–7 days), proliferation (7–14 days), ovulation (13–17 days), differentiation (15–22 days), degradation (22–28 days) during the proliferative phase and secretory phase.
Early life experiences modify the neurobiology of development and such influences continue to affect behavioral patterns and psychological outcomes in adulthood ( Roth, 2012 ). In addition, various external epigenetic factors modulate the biology of an individual at a physical and emotional level. Some of the most important exogenous factors influencing human health are described hereafter. We have described these influences in the “Adulthood” section for the sake of narrative simplicity, although they actually apply across the entire lifespan.
Studies in genomic imprinting have revealed how DNA methylation patterns are influenced by diet, and how epigenomic sensitivity to environmental cues and specifically diet can be used to influence disease susceptibility ( Waterland and Jirtle, 2003 ; Carone et al., 2010 ; Jennings and Willis, 2014 ).
Nutrients extracted from the diet enter metabolic pathways and are transformed into useful molecules. These nutrients are known to have epigenetic targets in cells such that they can be used to modify the epigenome in order to correct abnormally activated or silenced genes and can be combined into an “epigenetic diet” useful as a therapeutic or chemopreventive measure ( Hardy and Tollefsbol, 2011 ). During this transitory phase methyl groups are formed from key nutrients including folic acid, B vitamins and s-adenosyl methionine (SAMe), and these methyl groups, as described earlier, comprise important epigenetic marks for gene silencing. Diets high in such methyl rich nutrients may significantly alter gene expression and offer protective health benefits. Deficiencies in folate and methionine, both of which are involved in cellular processes that supply methyl groups needed for DNA methylation, can change the expression (imprinting) of growth factor genes such as (IGF1).
Folic acid. As explained earlier, DNA methylation can be used to distinguish a healthy CpG methylation profile from its inverted counterpart in tumor cells. Certain DNA repair genes including MGMT and MLH1 are prone to transcriptional silencing by promoter hypermethylation ( Jones and Baylin, 2002 ; Esteller, 2007 ). Variables including diet are capable of influencing epigenetic programing by altering DNA methylation and interfering with gene expression. Such potentially heritable modifications can be regulated by diet-responsive methylation. Specifically, deficient levels of folic acid lead to epigenetic alterations by inhibiting remethylation of s-adenosyl homocysteine (SAH) and s-adenosylmethionine (SAM) which results in demethylation and chromosome instability ( Dulthie, 2011 ). Thus, not only can dietary folate bolster a healthy locus-specific and global DNA methylation program, but can also direct proper uracil incorporation, inhibit DNA breakage, and foster DNA repair via thymidine and purine biosynthesis ( Ingrosso et al., 2003 ). Dietary folate present in a variety of green vegetables including broccoli, zucchini, brussels sprouts, green beans and spinach participates in maintaining a healthy DNA methylation profile and even reverses accrued damage ( Bhusari et al., 2010 ; Jennings and Willis, 2014 ).
Antioxidants and phytochemicals. Disruption in the balance between reactive oxygen species and antioxidants may result in harmful health effects caused by DNA damage due to the genotoxic effects of oxidative stress ( Waris and Ahsan, 2006 ). Foods are known to alter epigenetic expression in rats on different diets. Chemopreventive agents that target the epigenome include micronutrients found in folate, retinoic acid, selenium compounds, polyphenols from green tea, apples, coffee, black raspberries, and other dietary sources. Similar compounds are present in foods containing genistein and soy isoflavones, curcumin, and resveratrol to name a few ( Gerhauser, 2013 ). While certain food components epigenetically increase the levels of DNA repair enzymes such as MGMT and MLH1, others such as soy, isoflavones and bilberry anthocyanins actively decrease DNA damage ( Davis et al., 2001 ; Djuric et al., 2001 ; Olaharski et al., 2005 ; Fang et al., 2007 ; Kikuno et al., 2008 ; Burdge et al., 2011 ; Kropat et al., 2013 ). Anthocyanin is an effective antioxidant for humans that is found in plants and are easily identified by its potent red or purple pigment. It is found in plants such as eggplant, plums, pomegranate, red onion, cranberries, blueberries, kidney beans and cherries which all possess anthocyanins. This flavonoid serves as a powerful antioxidant that contributes to scavenging of DNA-damaging free radicals. While the direct fate of anthocyanins in vivo following digestion may be less than 5% (the majority being rapidly excreted), the potent residual antioxidant property remains in blood following consumption of anthocyanin-rich foods due to metabolic breakdown of the flavonoids and resultant increase in uric acid levels ( Williams et al., 2004 ; Lotito and Frei, 2006 ).
Another example is the polyphenol epigallocatechin-3-gallate (EGCG), which is contained in green tea and has been shown to retard carcinogenesis ( Fang et al., 2003 ). The pathway is similar to that used by other foods such as genistein in soy and involves regulation of DNA methylation at key genes to elicit positive epigenetic outcomes. Others like sulfopropanes from cruciferous vegetables and green tea can be used to treat age-related diseases and cancer since they are capable of reverting an aberrant epigenetic profile ( Tollefsbol, 2014 ). Other like sulfopropane form Owing to the numerous advantages that can be obtained through foods containing beneficial components and from probiotics, nutri- and microbial epigenetics is growing in importance because these bioactive molecules have been shown to be involved in regulating chromatin receptors and epigenome-related pathways ( Shenderov and Midtvedt, 2014 ).
Social interaction and behavior
There is no sharp edge dividing the biological from the behavioral aspects of epigenetic research. In fact there is a great deal of overlap. However, many interesting social perspectives contribute to the role of behavior on epigenetic modifications, and as such warrant a separate discussion since behavior is more readily controlled than biology. When mice were subjected to odor-fear conditioning prior to conception, increased behavioral sensitivity was observed in offspring in subsequent two generations ( Dias and Ressler, 2014 ). Epigenetic modifications complement the genome in that they do not change the DNA code directly, but influence it in such a way as to present it to the factors that read it and translate it into a final product. The ability to create, process and recall memory is reliant in part upon epigenetic modifications such as DNA remodeling ( Lattal and Wood, 2013 ). Nucleosome remodeling where histone-dependent nucleosomes are repositioned to expose DNA for gene expression is a factor in both healthy memory formation and cognitive impairment ( Malvaez et al., 2013 ; Vogel-Ciernia et al., 2013 ).
Exercise is one way that an individual can modify their epigenome in order to preserve and prolong life. Exercise has been shown to induce positive changes in DNA methylation within adipose tissue and regulate metabolism in both healthy and diseased individuals ( Ronn et al., 2013 ). Increased DNA methylation of genes Hdac4 and Ncor2 has also shown to increase lipogenesis following exercise ( Ronn et al., 2013 ). Exercise also leads to beneficial changes in DNA methylation patterns in skeletal muscle ( Nitert et al., 2012 ). Not only is obesity an indicator for diseases such as type 2 diabetes and cardiovascular disease, but also puts additional stress on the system which can itself negatively impact health ( Ronti et al., 2006 ). Acute exercise is associated with DNA hypomethylation of the entire genome in skeletal muscle cells of sedentary individuals and high intensity exercise tends to cause reduction in promoter methylation of certain genes ( Ntanasis-Stathopoulos et al., 2013 ). Exercise is also known to positively influence the expression patterns of miRNAs in leukocyte cells ( Radom-Aizik et al., 2010 ). The health benefits of physical exercise, especially on a long term and strenuous basis, has a positive effect on epigenetic mechanisms and ultimately may reduce incidence and severity of disease ( Sanchis-Gomar et al., 2012 ).
Pharmaceutical drugs
Pharmacoepigenetics is the study of inter-individual variations in epigenetic modifications as a result of prescribed pharmaceutical drug use. One working hypothesis is that exposure to therapeutic drugs may cause persistent epigenetic changes, possibly manifesting as permanent adverse side-effects ( Csoka and Szyf, 2009 ). There is a long list of drugs for which clinical or experimental evidence exists documenting either direct or indirect epigenetic effects (as described above). Direct effects are caused by drugs that interfere directly with the normal controls of DNA and/or histone methylation, resulting in aberrant gene expression. Indirect effects cause epigenetic changes by interaction with a cell surface receptor, enzyme, or other protein, which thereby alters expression of said receptors, and subsequently alters expression of transcription factors, which in turn change epigenetic regulation. Several epigenomic screening protocols are in place to identify drugs whose epigenetic power has therapeutic benefit and to isolate other drugs whose negative epigenetic impact outweighs potential benefit.
Drugs of abuse
Many drugs are used to enhance or alter the perception of reality and reward pleasure centers in the brain, but oftentimes these substances increase the risk of acute and/or chronic disease. Generally speaking, recreational drugs like cocaine but also including opiates, amphetamines, alcohol and nicotine modify the epigenome by altering methylation patterns in areas such as the nucleus accumbens of the brain, the major pleasure reward center ( Renthal and Nestler, 2008 ; Maze and Nestler, 2011 ; Doehring et al., 2013 ). Recreational drugs such as cocaine induce epigenetic changes in many ways, such as increasing histone acetylation on c-fos and fosb gene promoters ( Kumar et al., 2005 ). In terms of histone methylation, the epigenetic mark H3K9 been associated with chronic cocaine use, as well as with the process of cocaine addiction. Cocaine-induced plasticity is associated with the reduction in H3K9 methylation marks due to the repression of HMT G9a in the nucleus accumbens region of the brain ( Maze et al., 2010 ). Similarly, it has been demonstrated that pretreatment with histone deacetylase (HDAC) inhibitors helped in curbing cocaine addiction in animal models ( Romieu et al., 2011 ).
Smoking causes epigenetic changes such as DNA methylation changes, which alter gene expression. For example cigarette smoke induces demethylation of metastatic genes in lung cancer cells by downregulating DNMT3B ( Liu et al., 2007 ). DNA methylation is a type of epigenetic change that can result in tumor suppressor genes being inactivated, and methylation of the tumor suppressor gene p16 has frequently been associated with the development of cancer. When p16 is methylated, this gene's tumor suppressing function undergoes inactivation. This is why smoking is a major trigger for carcinogenesis. Of particular interest is the finding that not only maternal smoking but also grandmaternal smoking is linked to pediatric disease as a result of epigenetic changes to DNA and histones ( Rehan et al., 2012 ; Leslie, 2013 ) (See below for description of trans-generational influences).
Alcohol. The epigenetic effects of alcohol on hepatic and neuronal tissue are well documented ( Shukla et al., 2008 ). Ethanol is known to cause site-selective methylation, acetylation and phosphorylation of histones and DNA hypomethylation due to reduction of tissue SAM ( Shukla et al., 2008 ; Zakhari, 2013 ). One carbon metabolism (OCM) is a major methyl group donor, contributing toward DNA methylation and alcohol's interference with OCM leads to reduced availability of methyl groups and hence aberrant DNA methylation and gene expression in alcohol consumers ( Kruman and Fowler, 2014 ). A study with young mice showed that chronic alcohol exposure reduced global DNA hydroxymethylation in the liver as compared to control ( Tammen et al., 2014 ). Alcohol induced changes at the gastrointestinal- hepatic level like steatosis, carcinogenesis, endotexemia are also a result of epigenetic alterations ( Shukla and Lim, 2013 ).
Alcohol induced neuro-adaptations like tolerance and dependence are a result of epigenetic modulation at the neurobiological level ( Finegersh and Homanics, 2014a ). Acute ethanol exposure in mice leads to decreased expression of GAD1, HDAC2, HDAC11 associated with decreased histone acetylation at GAD1, HDAC2 promoters and increased expression of MT1, MT2, EGR1 associated with increased levels of H3K4me3 at MT2 promoter and decreased level of H3K27me3 at the MT1 promoter in the cerebral cortex ( Finegersh and Homanics, 2014a ).
Paternal alcohol exposure, prior to mating, has shown to induce increased sensitivity to anxiolytic and motor effects of alcohol, reduced alcohol preference and consumption exclusively in male offspring in mice ( Finegersh and Homanics, 2014b ). An increased expression of BDNF in the ventral tegmental area of such male offspring was also observed along with DNA hypomethyaltion of the BDNF promoter in the sire's germ cells and male and female offspring ( Finegersh and Homanics, 2014b ). Such effects are epigenetically transmitted through the male lineage and are capable of being a trans-generational influence. (See below for description of trans-generational influences.) Recent efforts toward elucidation of exact interactions between alcohol and the epigenome will help in developing treatments for alcohol-related disorders including fetal alcohol spectrum disorders, alcohol addiction and organ damage ( Shukla and Zakhari, 2013 ).
In recent years the interest in the identification of epigenetic marks associated with alcohol dependence has been growing ( Starkman et al., 2012 ). A major obstacle in this research is the limited number of candidate structural genes that might be participating in these pathways. However, DNA methylation in relation to alcohol use disorders has lately become a burning topic of research; a significant association between loss of control over drinking behavior and DNA methylation at multiple CpG sites has been observed, specifically methylation at CpG sites near the ALDH1A2 gene was found to be associated with rate of intoxication and inclination toward alcohol ( Harlaar et al., 2014 ).
Alternative medicine
Alternative medicine (AM) approaches like ayurverda, homeopathy, yoga, taichi, reiki, acupuncture, body massages, naturopathy, and hypnotherapy offer a promising approach toward improving human health and lifestyle by mediating beneficial environment-epigenome interactions (Figure 1 ). AM includes a variety of health practices, knowledge, beliefs and moreover therapeutic as well as spiritual techniques, which can be used to prevent or treat health conditions ( Report, 2001 ). One of the well-known forms of AM is Ayurveda, which has emphasized personalized health care for over 5000 years. Ayurvedic science advocates the use of herbs and spices in addition to foods for their medicinal properties, and their epigenetic effects have been identified. For example, tulsi and ginger regulate histone H3 acetylation and others spices such as turmeric and cinnamon possess similar effects ( Shim et al., 2011 ). Acupuncture, another form of AM, has been noted for its beneficial effects on cardiac health. Acupuncture therapy has been used for prevention of myocardial infarction (MI), strengthening of cardiovascular function and angiogenesis, and protection from further injury and apoptosis to myocardial cells after an MI ( He et al., 2014 ). This suggests how alternative medicine may operate through epigenetic regulation of regeneration, or cellular apoptosis in case of injury or degenerative diseases.
Environmental chemicals
Encounters with pesticides, toxins and synthetic compounds can methylate genes in adults and also promulgate diseases decades later in offspring following in utero exposures.
Heavy metals including arsenic, nickel and cadmium are widespread environmental contaminants capable of disrupting DNA methylation and histone acetylation and as a result have been associated with a number of diseases including cancer, neurological disorders and autoimmune diseases. Putative mechanisms may involve the fact that metals act as catalysts in the oxidative deterioration of biological macromolecules to produce free radicals and induce epigenetic changes ( Leonard et al., 2004 ; Babar et al., 2008 ; Monks et al., 2006 ).
Arsenic undergoes metabolic detoxification via methylation by S -adenosyl methionine (SAM), a universal methyl donor for methyltransferases (including DNMTs). Exposure to arsenic both decreases the activity of DNMTs and down regulates DNMT gene expression ( Reichard et al., 2007 ). Arsenic exposure induces hypermethylation of tumor suppressor genes ( Jensen et al., 2008 ), dysregulation of histone acetylation ( Hou et al., 2012 ), and promotion of micro RNA expression ( Marsit et al., 2006 ). Nickel is capable of compacting chromatin and methylating the DNA of tumor suppressor genes ( Lee et al., 1995 ). Exposure to nickel affects histone acetylation causing gene silencing and ultimately cell transformation ( Broday et al., 2000 ; Zhang and Zhu, 2012 ). Exposure to cadmium, mercury, lead, and chromium cause similar epigenetic modifications ( Takahashi et al., 2005 ; Huang et al., 2008 ; Sun et al., 2009 ; Wright et al., 2010 ; Arai et al., 2011 ).
Bisphenol A (BPA) and phthalates are plastics that leach into the environment. Not only are they estrogenic and known endocrine disruptors, but these chemicals and their metabolites also induce epigenetic modification in animal models ( Singh and Li, 2012 ). When ingested by humans, diethylhexyl phthalate (DEHP) is converted by intestinal lipases to mono-(2-ethylhexyl) phthalate (MEHP), which is then preferentially absorbed ( Morgan et al., 1999 ). Specifically, BPA disrupts DNA methylation by decreasing CpG methylation in the mouse agouti gene. This effect is reversible through dietary supplementation with a source of methyl donor groups such as folic acid and the phytoestrogen genistein (found in fava beans, soy beans, and coffee) ( Dolinoy et al., 2006 ).
Seasonal and diurnal changes
The operation of the circadian rhythm in humans is a result of temporal expression of clock controlled genes (CCGs) ( Sahar and Sassone-Corsi, 2012 ). Chromatin remodeling is the basis for differential expression of such CCGs through a 24-hour cycle, for example the epigenetic regulation Bmal1 (Figure 5 ). The drastic changes in lifestyle through the last century and higher incidence of modern day diseases like obesity, Type 2 diabetes and a spectrum of mental disorders has been linked to disruption of the internal circadian clock, a mechanism of homeostatic regulation of physiological processes and metabolic functions, and this has been attributed to exposure to epigenetic factors ( Orozco-Solis and Sassone-Corsi, 2014 ).
It is known that, unlike humans, the changes in season affect the reproductive behavior of seasonal breeding vertebrates as a physiological response to an altered photoperiod exposure. A study with Siberian hamsters showed that nocturnal pineal melatonin (MEL) protein is involved in DNA methylation changes of the promoter of dio3 gene, which is responsible for photoperiodic time measurement, and undergoes reversible epigenetic alterations for physiological orientation in time ( Stevenson and Prendergast, 2013 ).
Menopause is an event in a female's life that results in termination of her reproductive capability and is a gradual process accompanied by the deregulation of hormones ( Ubeda et al., 2014 ). Many genes are known to be epigenetically regulated during this process, like those implicated in DNA repair (EXO1, HELQ, UIMC1, FAM175A, FANCI, TLK1, POLG, and PRIM1) and immune function (IL11, NLRP11, and PRRC2A) ( Stolk et al., 2012 ). ENO1 is downregulated after menopause whereas TRIB2 and IGSF4 are up regulated ( Kosa et al., 2009 ) (Figure 6 ).
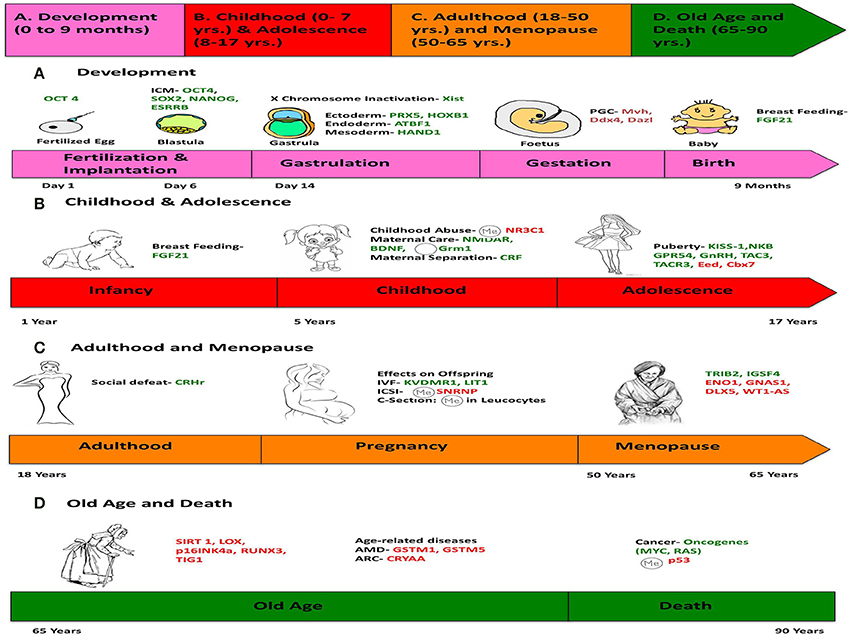
It is known that the DNA methylation status of Polycomb group target (PCGT) genes in endometrial tissue can be used as a biomarker to predict the risk for cancer development in premenopausal women ( Widschwendter et al., 2009 ). One of the members of the PCGT group, namely HOXA10, is highly expressed during implantation and is important for the process and its expression is reduced in patients with endometriosis ( Bagot et al., 2000 ; Wu et al., 2005 ). It was found that the global DNA methylation of eutopic endometrium in patients with endometriosis was higher as compared to ectopic endometrium of patients with endometriosis and a control group ( Andersson et al., 2014 ). Overall, aberrant DNA methylation leading to aberrant gene expression plays an important role in disease states of the endometrium and can cause additional changes to the process of menopause in females.
Aging and Age-Related Disease
Aging is a multifactorial process that results in a progressive loss of regenerative capacity through a decline in cell-proliferation and tissue functionality ( Campisi, 2013 ). It induces global and complex changes in the human DNA methylation landscape ( Xu and Taylor, 2014 ). Additional epigenetic changes have been described which provide clues to understanding the aging process. For example, senescent cells are characterized by formation of a specialized heterochromatin called senescence-associated heterochromatin foci that exhibit noticeable histone modifications ( Narita et al., 2003 ). These foci contribute to senescence-associated proliferation arrest that may silence the expression of proliferation-promoting genes ( Sedivy et al., 2008 ). Shortened telomeres, a hallmark of aging, may have reduced heterochromatin ( Benetti et al., 2007 ).
Genome-Wide DNA Methylation Changes
Generally, during the aging process, global hypomethylation of DNA occurs in a repetitive sequence pattern that may promote genomic instability ( Heyn et al., 2012 ). Not only is aging correlated with hypomethylation of proto-oncogenes, but also with hypermethylation of tumor suppressor genes, potentially leading to increased risk of cancer and other diseases ( Coppede, 2014 ).
Some methylation changes are so predictable that age-related DNA methylation patterns have been used to predict an individual's actual chronological age, such that DNA methylation can be used as a measure of age or age acceleration, as so-called “aging clock” ( Horvath, 2013 ).
Epigenetic changes in disease are not always focal, but can be global and encompass large chromosomal regions. For example the aberrant expression of micro RNAs has been linked to various age-related diseases such as Alzheimer's disease, cardiac disease and many cancers including leukemia and lymphoma ( Natarajan et al., 1992 ; Fabbri et al., 2009 ; Cheng and Zhang, 2010 ; Montgomery and Van Rooij, 2010 ; Provost, 2010 ), as described below.
Alzheimer's Disease
Alzheimer's disease is a neurodegenerative disorder characterized by β-Amyloid, inflammatory, oxidative and vascular damage. DNA hypomethylation of the entorhinal region of the cerebral cortex, plus hypermethylation and consequent overexpression of the hTERT gene have been reported in Alzheimer's research ( Silva et al., 2008 ; Mastroeni et al., 2010 ). Animal studies suggest that overexpression of histone deacetylase 2 (HDAC 2) may decrease synaptic plasticity and impair memory formation ( Guan et al., 2009 ; Urdinguio et al., 2009 ). Also, DNA methylation deficiency can accelerate age-related diseases and it has been shown that Dnmt1 haploinsufficiency can impair learning and memory function causing cognitive disorders with age ( Liu et al., 2011 ).
The epigenetic basis of osteoarthritis (OA) is seen in the fact that OA chondrocytes express genes involved in degradation of cartilage as a result of their hypomethylated promoters under the influence of cytokines like IL-1β and TNF-α, as opposed to normal chondrocytes ( Haseeb et al., 2014 ).
Cardiovascular Disease
Heart disease is often attributed to genetic predisposition, but epigenetic marks that vary between cell types and respond to endogenous and exogenous stimuli likely share culpability ( Ordovas and Smith, 2010 ). DNA methylation is critical for the development of atherosclerosis and cardiovascular disease; recently hypermethylation has been shown in differentially methylated genomic regions of patients suffering with coronoary artery disease (CAD) ( Sharma et al., 2014 ). DNA methyltransferases ( DNMT s) exhibit hypomethylation in mice, an observation associated with increased expression of inflammatory mediators ( Makar and Wilson, 2004 ). Also, DNA hypermethylation has been observed in the estrogen receptor genes ESR1 and ESR2 of vascular smooth muscle which contributes to atherosclerosis ( Lund and Zaina, 2011 ).
Not only is DNA methylation a primary regulator of inflammation, but also controls leukocyte functions related to cardiovascular risk ( Baccarelli et al., 2010 ). Removal of epigenetic signatures of oxidative and inflammatory genes has been proposed as a promising therapeutic option to prevent endothelial dysfunction and vascular complications in diabetic people ( Paneni et al., 2013 ).
Histone modification has also been implicated in various aspects of cardiovascular disease such as angiogenesis and myocardial infarction ( Webster et al., 2013 ). Histones are important for the normal regulation of the NOS3 gene. This gene codes for the protein eNOS that catalyzes formation of NO, a vasodilating factor in blood vessels that operates in the regulation of healthy cardiovascular tissue and can inhibit the ability of KDM3a protein to remove histone methyl groups ( Hickok et al., 2013 ).
Cancer has long been considered a disease of genetic origin, but an historic link between cancer and epigenetics was identified when DNA of colorectal cancer patients was observed to be hypomethylated ( Feinberg and Vogelstein, 1983 ). The normally hypermethylated and silent regions of the genome which contain repetitive sequences become demethylated. Conversely, hypermethylation of CpG islands in certain cancers is correlated with abnormal gene activity, such as deactivation of tumor suppressor genes ( Esteller, 2002 ). Hypermethylation of DNA also damages repetitive sequences of DNA called mircosatellites ( Oki et al., 1999 ); hypermethylation of the MLH1 promoter (a DNA repair gene) disfigures microsatellites, a phenomenon present in many cancers including colorectal and ovarian ( Jones and Baylin, 2002 ). These observations of promoter hypermethylation ( Coppede, 2014 ) and heterochromatinization ( Rideout et al., 1994 ) are compelling evidence of the dramatic influence of epigenetics on tumorigenesis.
A major locus susceptible to transcriptional silencing as a result of promoter hypermethylation is the INK4 locus located on chromosome 9 ( Kim and Sharpless, 2006 ). INK4 represents a family of cyclin-dependent kinase inhibitors. This gene encodes several proteins that are often targeted early during malignant progression, including p14, p15, and p16. Histone modification is another epigenetically important mechanism prevalent during carcinogenic transformation. Transcriptional control of p16 is interrupted when chromatin domains are lost and discrete histone structure is destroyed, in breast cancer cells for example ( Witcher and Emerson, 2009 ).
Epigenetic changes differ from their genetic counterparts such as gene mutations in that epigenetic hypermethylation affects many genes within a single cancer cell ( Coppede, 2014 ). For example, PIAS1, a transcriptional repressor, is involved in the progression of breast cancer and operates on an epigenetic level through gene silencing by recruting DNMTs ( Liu et al., 2014 ). Still, many researchers view the link between cancer and epigenetics with optimism, since epigenetic modifications are potentially reversible. For example, it is thought that cells harboring gene mutations must be killed or removed to prevent uncontrolled propagation of the damaged code. But advances in epigenetic technology may soon allow repair of defective epigenetic modifications by a variety of therapeutics. For example the drug azacitidine, the first FDA-approved epigenomic drug, treats leukemia by reactivating tumor suppressor genes and similar drugs are now in development ( Braiteh et al., 2008 ; Phillips, 2008 ).
Trans-Generational Influences
Many transgenerational phenotypic inheritance profiles cannot be explained by the normal human mutation rate of 2.3 × 10 −8 per nucleotide per generation ( Arnheim and Calabrese, 2009 ). The reason for this discrepancy is that epigenetic changes most likely contribute to the majority of these transgenerational effects.
Transgenerational epigenetic inheritance was first observed in plants ( Manning et al., 2006 ), however, it has also been reported in rodents and humans ( Carone et al., 2010 ). Heritability of epigenetic expression is demonstrable where multiple generations are simultaneously exposed to the same environmental conditions that include diet, toxins, hormones, etc. ( Curley et al., 2011 ; Vassoler et al., 2013 ). In such a model, the mother = first generation, the fetus = 2nd generation, the reproductive cells of fetus = 3rd generation, and the future offspring of fetus = 4th generation. In order to provide a convincing case for trans-generational stability, an epigenetic change must be observed in the 4th generation ( Hughes, 2014 ). Recent studies have shown that maternal methyl-donor supplementation affects not only the mother, but is also inherited in the F2 generation through germline epigenetic modifications ( Duhl et al., 1994 ; Wolff et al., 1998 ; Morgan et al., 1999 ; Cropley et al., 2006 ). Transgenerational epigenetic influence of longevity has also been shown in C. elegans ( Greer et al., 2011 ).
Epigenetics in Translational and Personalized Medicine
For the past several decades genetics has been at the forefront in terms of understanding human disease. A recent addition to genetics has been epigenetics, which includes the role of the environment, both social and natural, including day-to-day habits, lifestyle and personal experiences on human health. Epigenetics establishes a scientific basis for how external factors and the environment can shape an individual both physically and mentally.
The knowledge that environment and lifestyle can alter health brings with it awareness that habits, social environment, diet and other factors shape health beyond our acquired genetic traits. Moreover, despite the risk presented by inherited genes and mutations, epigenetic factors play a decisive role in the actual development of disease. Research into epigenetics could lead to insights into how factors like diet and exercise can be customized to an individual in concordance with their naturally inherited genome in order to minimize the risk of developing a disease to which he/she is naturally predisposed.
Advances in epigenetic profiling technology such as genome-scale DNA methylation analysis points to the future possibilities of how epigenetic profiling can help in determining the risk of an individual with a particular type of genetic makeup developing a specific disease. Also the same epigenetic profile, along with knowledge of the genomic sequence, can help to determine which medications or alternative medicine approaches would be effective in preventing or curing a particular disease. Such approaches toward improving health and lifestyle and reducing the risk of developing an otherwise inevitable disease might be possible in the near future. It will require extensive knowledge of the biochemical and physiological mechanisms of epigenetics, and we are still on this challenging path, but we have good reasons to be optimistic.
Conclusions
We have presented a comprehensive narrative of the role of both endogenous and exogenous epigenetic influences on the human lifespan.
The future of epigenetics holds tremendous promise for understanding the complexities involved in genetic regulation, cellular differentiation, aging and disease; and a more complete and comprehensive understanding of the mechanisms that underlie the formation and erasure of epigenetic marks could allow us to commandeer the process and possibly fine tune the human epigenome.
Ultimately, continued efforts to determine how and when epigenetic switches regulate gene function will elucidate the interplay between the genome, the epigenome, and the environment and facilitate the development and optimization of novel therapeutic tools. In terms of future application, full understanding of these mechanisms will ultimately revolutionize personalized medicine.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
The Authors would like to thank Dr. Donald Orlic for critical review of the manuscript.
Andersson, K. L., Bussani, C., Fambrini, M., Polverino, V., Taddei, G. L., Gemzell-Danielsson, K., et al. (2014). DNA methylation of HOXA10 in eutopic and ectopic endometrium. Hum. Reprod . 29, 1906–1911. doi: 10.1093/humrep/deu161
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Anway, M. D., Cupp, A. S., Uzumcu, M., and Skinner, M. K. (2005). Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469. doi: 10.1126/science.1108190
Arai, Y., Ohgane, J., Yagi, S., Ito, R., Iwasaki, Y., Saito, K., et al. (2011). Epigenetic assessment of environmental chemicals detected in maternal peripheral and cord blood samples. J. Reprod. Dev . 57, 507–517. doi: 10.1262/jrd.11-034A
Arnheim, N., and Calabrese, P. (2009). Understanding what determines the frequency and pattern of human germline mutations. Nat. Rev. Genet . 10, 478–488. doi: 10.1038/nrg2529
Babar, I. A., Slack, F. J., and Weidhaas, J. B. (2008). miRNA modulation of the cellular stress response. Future Oncol . 4, 289–298. doi: 10.2217/14796694.4.2.289
Bacalini, M. G., Friso, S., Olivieri, F., Pirazzini, C., Giuliani, C., Capri, M., et al. (2014). Present and future of anti-ageing epigenetic diets. Mech. Ageing. Dev . 136–137, 101–115. doi: 10.1016/j.mad.2013.12.006
Baccarelli, A., Rienstra, M., and Benjamin, E. J. (2010). Cardiovascular epigenetics: basic concepts and results from animal and human studies. Circ. Cardiovasc. Genet . 3, 567–573. doi: 10.1161/CIRCGENETICS.110.958744
Baer, C., Claus, R., and Plass, C. (2013). Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res . 73, 473–477. doi: 10.1158/0008-5472.CAN-12-3731
Bagot, C. N., Troy, P. J., and Taylor, H. S. (2000). Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther . 7, 1378–1384. doi: 10.1038/sj.gt.3301245
Bagot, R. C., Zhang, T. Y., and Wen, X. (2012). Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor1 expression and hippocampal function in the rat. Proc. Natl. Acad. Sci. U.S.A . 109(Suppl. 2), 17200–17207. doi: 10.1073/pnas.1204599109
Barker, D. J., and Clark, P. M. (1997). Fetal undernutrition and disease in later life. Rev. Reprod . 2, 105–112. doi: 10.1530/ror.0.0020105
Barlow, D. P., and Bartolomei, M. S. (2014). Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol . 6:a018382. doi: 10.1101/cshperspect.a018382
Barua, S., Kuizon, S., Chadman, K. K., Flory, M. J., Brown, W. T., and Junaid, M. A. (2014). Single-base resolution of mouse offspring brain methylome reveals epigenome modifications caused by gestational folic acid. Epigenetics Chromatin 7:3. doi: 10.1186/1756-8935-7-3
Benetti, R., Garcia-Cao, M., and Blasco, M. A. (2007). Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nat. Genet . 39, 243–250. doi: 10.1038/ng1952
Bhusari, S. S., Dobosy, J. R., Fu, V., Almassi, N., Oberley, T., and Jarrard, D. F. (2010). Superoxide dismutase 1 knockdown induces oxidative stress and DNA methylation loss in the prostate. Epigenetics 5, 402–409. doi: 10.4161/epi.5.5.11853
Bowdin, S., Allen, C., Kirby, G., Brueton, L., Afnan, M., Barratt, C., et al. (2007). A survey of assisted reproductive technology births and imprinting disorders. Hum. Reprod . 22, 3237–3240. doi: 10.1093/humrep/dem268
Braiteh, F., Soriano, A. O., Garcia-Manero, G., Hong, D., Johnson, M. M., Silva Lde, P., et al. (2008). Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin. Cancer Res . 14, 6296–6301. doi: 10.1158/1078-0432.CCR-08-1247
Broday, L., Peng, W., Kuo, M. H., Salnikow, K., Zoroddu, M., and Costa, M. (2000). Nickel compounds are novel inhibitors of histone H4 acetylation. Cancer Res . 60, 238–241.
Pubmed Abstract | Pubmed Full Text
Bromer, C., Marsit, C. J., Armstrong, D. A., Padbury, J. F., and Lester, B. (2013). Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Dev. Psychobiol . 55, 673–683. doi: 10.1002/dev.21061
Burdge, G. C., Hoile, S. P., Uller, T., Thomas, N. A., Gluckman, P. D., Hanson, M. A., et al. (2011). Progressive, transgenerational changes in offspring phenotype and epigenotype following nutritional transition. PLoS ONE 6:e28282. doi: 10.1371/journal.pone.0028282
Campisi, J. (2013). Aging, cellular senescence, and cancer. Annu. Rev. Physiol . 75, 685–705. doi: 10.1146/annurev-physiol-030212-183653
Carone, B. R., Fauquier, L., Habib, N., Shea, J. M., Hart, C. E., Li, R., et al. (2010). Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096. doi: 10.1016/j.cell.2010.12.008
Cedar, H., and Bergman, Y. (2009). Linking DNA methylation and histone modification: patterns and paradigms. Nat. Rev. Genet . 10, 295–304. doi: 10.1038/nrg2540
Cheng, R. Y., Hockman, T., Crawford, E., Anderson, L. M., and Shiao, Y. H. (2004). Epigenetic and gene expression changes related to transgenerational carcinogenesis. Mol. Carcinog . 40, 1–11. doi: 10.1002/mc.20022
Cheng, Y., and Zhang, C. (2010). MicroRNA-21 in cardiovascular disease. J. Cardiovasc. Transl. Res . 3, 251–255. doi: 10.1007/s12265-010-9169-7
Chouliaras, L., Van Den Hove, D. L., Kenis, G., Draanen, M., Hof, P. R., Van Os, J., et al. (2013). Histone deacetylase 2 in the mouse hippocampus: attenuation of age-related increase by caloric restriction. Curr. Alzheimer Res . 10, 868–876. doi: 10.2174/1567205011310080009
Clouaire, T., and Stancheva, I. (2008). Methyl-CpG binding proteins: specialized transcriptional repressors or structural components of chromatin? Cell. Mol. Life Sci . 65, 1509–1522. doi: 10.1007/s00018-008-7324-y
Coppede, F. (2014). Epigenetic biomarkers of colorectal cancer: focus on DNA methylation. Cancer Lett . 342, 238–247. doi: 10.1016/j.canlet.2011.12.030
Cropley, J. E., Suter, C. M., Beckman, K. B., and Martin, D. I. (2006). Germ-line epigenetic modification of the murine Avy allele by nutritional supplementation. Proc. Natl. Acad. Sci. U.S.A . 103, 17308–17312. doi: 10.1073/pnas.0607090103
Csoka, A. B., and Szyf, M. (2009). Epigenetic side-effects of common pharmaceuticals: a potential new field in medicine and pharmacology. Med. Hypotheses 73, 770–780. doi: 10.1016/j.mehy.2008.10.039
Curley, J. P., Mashoodh, R., and Champagne, F. A. (2011). “Transgenerational epigenetics,” in The Handbook of Epigenetics , ed T. O. Tollefsbol (London: Academic Press), 13.
Davis, J. N., Kucuk, O., Djuric, Z., and Sarkar, F. H. (2001). Soy isoflavone supplementation in healthy men prevents NF-kappa B activation by TNF-alpha in blood lymphocytes. Free Radic. Biol. Med . 30, 1293–1302. doi: 10.1016/S0891-5849(01)00535-4
Dechiara, T. M., Robertson, E. J., and Efstratiadis, A. (1991). Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64, 849–859. doi: 10.1016/0092-8674(91)90513-X
Dias, B. G., and Ressler, K. J. (2014). Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci . 17, 89–96. doi: 10.1038/nn.3594
Djuric, Z., Chen, G., and Doerge, D. R. (2001). Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett . 172, 1–6. doi: 10.1016/S0304-3835(01)00627-9
Doehring, A., Oertel, B. G., Sittl, R., and Lotsch, J. (2013). Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain 154, 15–23. doi: 10.1016/j.pain.2012.06.011
Dolinoy, D. C., Weidman, J. R., Waterland, R. A., and Jirtle, R. L. (2006). Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect . 114, 567–572. doi: 10.1289/ehp.8700
Duhl, D. M., Vrieling, H., Miller, K. A., Wolff, G. L., and Barsh, G. S. (1994). Neomorphic agouti mutations in obese yellow mice. Nat. Genet . 8, 59–65. doi: 10.1038/ng0994-59
Dulthie, S. J. (2011). Folate and cancer: how DNA damage and repair and methylationn impact on colon carcinogenesis. J. Inherit. Metab. Dis . 34, 101–109. doi: 10.1007/s10545-010-9128-0
Esteller, M. (2002). CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene 21, 5427–5440. doi: 10.1038/sj.onc.1205600
Esteller, M. (2007). Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet . 8, 286–298. doi: 10.1038/nrg2005
Fabbri, M., Croce, C. M., and Calin, G. A. (2009). MicroRNAs in the ontogeny of leukemias and lymphomas. Leuk. Lymphoma 50, 160–170. doi: 10.1080/10428190802535114
Fagiolini, M., Jensen, C. L., and Champagne, F. A. (2009). Epigenetic influences on brain development and plasticity. Curr. Opin. Neurobiol . 19, 207–212. doi: 10.1016/j.conb.2009.05.009
Fang, M., Chen, D., and Yang, C. S. (2007). Dietary polyphenols may affect DNA methylation. J. Nutr . 137, 223S–228S.
Fang, M. Z., Wang, Y., Ai, N., Hou, Z., Sun, Y., Lu, H., et al. (2003). Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res . 63, 7563–7570.
Fass, D. M., Schroeder, F. A., Perlis, R. H., and Haggarty, S. J. (2014). Epigenetic mechanisms in mood disorders: targeting neuroplasticity. Neuroscience 264, 112–130. doi: 10.1016/j.neuroscience.2013.01.041
Feinberg, A. P., and Vogelstein, B. (1983). Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 301, 89–92. doi: 10.1038/301089a0
Finegersh, A., and Homanics, G. E. (2014a). Acute ethanol alters multiple histone modifications at model gene promoters in the cerebral cortex. Alcohol. Clin. Exp. Res . 38, 1865–1873. doi: 10.1111/acer.12465
Finegersh, A., and Homanics, G. E. (2014b). Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS ONE 9:e99078. doi: 10.1371/journal.pone.0099078
Gerhauser, C. (2013). Cancer chemoprevention and nutriepigenetics: state of the art and future challenges. Top. Curr. Chem . 329, 73–132. doi: 10.1007/128_2012_360
Giraldez, A. J., Cinalli, R. M., Glasner, M. E., Enright, A. J., Thomson, J. M., Baskerville, S., et al. (2005). MicroRNAs regulate brain morphogenesis in zebrafish. Science 308, 833–838. doi: 10.1126/science.1109020
Girardot, M., Cavaille, J., and Feil, R. (2012). Small regulatory RNAs controlled by genomic imprinting and their contribution to human disease. Epigenetics 7, 1341–1348. doi: 10.4161/epi.22884
Greer, E. L., Maures, T. J., Ucar, D., Hauswirth, A. G., Mancini, E., Lim, J. P., et al. (2011). Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans . Nature 479, 365–371. doi: 10.1038/nature10572
Guan, J. S., Haggarty, S. J., Giacometti, E., Dannenberg, J. H., Joseph, N., Gao, J., et al. (2009). HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459, 55–60. doi: 10.1038/nature07925
Hardy, T. M., and Tollefsbol, T. O. (2011). Epigenetic diet: impact on the epigenome and cancer. Epigenomics 3, 503–518. doi: 10.2217/epi.11.71
Harlaar, N., Bryan, A. D., Thayer, R. E., Karoly, H. C., Oien, N., and Hutchison, K. E. (2014). Methylation of a CpG site near the ALDH1A2 gene is associated with loss of control over drinking and related phenotypes. Alcohol. Clin. Exp. Res . 38, 713–721. doi: 10.1111/acer.12312
Haseeb, A., Makki, M. S., and Haqqi, T. M. (2014). Modulation of Ten-Eleven Translocation 1 (TET1), Isocitrate Dehydrogenase (IDH) expression, alpha-Ketoglutarate (alpha-KG), and DNA hydroxymethylation levels by Interleukin-1beta in primary human chondrocytes. J. Biol. Chem . 289, 6877–6885. doi: 10.1074/jbc.M113.512269
He, S. Y., Lu, S. F., and Zhu, B. M. (2014). [Progress of researches on mechanisms of acupuncture therapy underlying improving myocardial ischemia and the future approach for in-depth study on its mechanisms from epigenetics]. Zhen Ci Yan Jiu 39, 73–78.
Heyn, H., Li, N., and Ferreira, H. J. (2012). Distinct DNA methylomes of newborns and centenarians. Proc. Natl. Acad. Sci. U.S.A . 109, 10522–10527. doi: 10.1073/pnas.1120658109
Hickok, J. R., Vasudevan, D., and Antholine, W. E. (2013). Role of nitric oxide in regulating histone methylation: nitric oxide modifies global histone methylation by inhibiting Jumonji C domain-containing demethylases. J. Biol. Chem . 288, 16004–16015. doi: 10.1074/jbc.M112.432294
Hoffmann, A., and Spengler, D. (2014). DNA memories of early social life. Neuroscience 264, 64–75. doi: 10.1016/j.neuroscience.2012.04.003
Horvath, S. (2013). DNA methylation age of human tissues and cell types. Genome Biol . 14, R115. doi: 10.1186/gb-2013-14-10-r115
Hou, L., Zhang, X., Wang, D., and Baccarelli, A. (2012). Environmental chemical exposures and human epigenetics. Int. J. Epidemiol . 41, 79–105. doi: 10.1093/ije/dyr154
Huang, D., Zhang, Y., Qi, Y., Chen, C., and Ji, W. (2008). Global DNA hypomethylation, rather than reactive oxygen species (ROS), a potential facilitator of cadmium-stimulated K562 cell proliferation. Toxicol. Lett . 179, 43–47. doi: 10.1016/j.toxlet.2008.03.018
Hughes, V. (2014). Epigenetics: the sins of the father. Nature 507, 22–24. doi: 10.1038/507022a
Ingrosso, D., Cimmino, A., Perna, A. F., Masella, L., De Santo, N. G., De Bonis, M. L., et al. (2003). Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet 361, 1693–1699. doi: 10.1016/S0140-6736(03)13372-7
Jennings, B. A., and Willis, G. (2014). How folate metabolism affects colorectal cancer development and treatment, a story of heterogeneity and pleiotropy. Cancer Lett . doi: 10.1016/j.canlet.2014.02.024. [Epub ahead of print].
Jensen, T. J., Novak, P., Eblin, K. E., Gandolfi, A. J., and Futscher, B. W. (2008). Epigenetic remodeling during arsenical-induced malignant transformation. Carcinogenesis 29, 1500–1508. doi: 10.1093/carcin/bgn102
Jones, P. A., and Baylin, S. B. (2002). The fundamental role of epigenetic events in cancer. Nat. Rev. Genet . 3, 415–428. doi: 10.1038/nrg816
Kala, R., Peek, G. W., Hardy, T. M., and Tollefsbol, T. O. (2013). MicroRNAs: an emerging science in cancer epigenetics. J. Clin. Bioinforma . 3:6. doi: 10.1186/2043-9113-3-6
Kellner, S., and Kikyo, N. (2010). Transcriptional regulation of the Oct4 gene, a master gene for pluripotency. Histol. Histopathol . 25, 405–412.
Kikuno, N., Shiina, H., Urakami, S., Kawamoto, K., Hirata, H., Tanaka, Y., et al. (2008). Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int. J. Cancer 123, 552–560. doi: 10.1002/ijc.23590
Kim, W. Y., and Sharpless, N. E. (2006). The regulation of INK4/ARF in cancer and aging. Cell 127, 265–275. doi: 10.1016/j.cell.2006.10.003
Knopik, V. S., Maccani, M. A., Francazio, S., and McGeary, J. E. (2012). The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev. Psychopathol . 24, 1377–1390. doi: 10.1017/S0954579412000776
Kohda, T., and Ishino, F. (2013). Embryo manipulation via assisted reproductive technology and epigenetic asymmetry in mammalian early development. Philos. Trans. R. Soc. Lond. B Biol. Sci . 368, 20120353. doi: 10.1098/rstb.2012.0353
Kosa, J. P., Balla, B., Speer, G., Kiss, J., Borsy, A., Podani, J., et al. (2009). Effect of menopause on gene expression pattern in bone tissue of nonosteoporotic women. Menopause 16, 367–377. doi: 10.1097/gme.0b013e318188b260
Kropat, C., Mueller, D., Boettler, U., Zimmermann, K., Heiss, E. H., Dirsch, V. M., et al. (2013). Modulation of Nrf2-dependent gene transcription by bilberry anthocyanins in vivo . Mol. Nutr. Food Res . 57, 545–550. doi: 10.1002/mnfr.201200504
Kruman, I. I., and Fowler, A. K. (2014). Impaired one carbon metabolism and DNA methylation in alcohol toxicity. J. Neurochem . 129, 770–780. doi: 10.1111/jnc.12677
Kumar, N., Choi, K. H., and Renthal, W. (2005). Chromatin remodelling is a key mechanism underlying coccaine- induced plasticity in striatum. Neuon 48, 12. doi: 10.1016/j.neuron.2005.09.023
Lambrot, R., Xu, C., Saint-Phar, S., Chountalos, G., Cohen, T., Paquet, M., et al. (2013). Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun . 4, 2889. doi: 10.1038/ncomms3889
Lattal, K. M., and Wood, M. A. (2013). Epigenetics and persistent memory: implications for reconsolidation and silent extinction beyond the zero. Nat. Neurosci . 16, 124–129. doi: 10.1038/nn.3302
Laviola, G., Macri, S., Morley-Fletcher, S., and Adriani, W. (2003). Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev . 27, 19–31. doi: 10.1016/S0149-7634(03)00006-X
Lee, Y. W., Klein, C. B., Kargacin, B., Salnikow, K., Kitahara, J., Dowjat, K., et al. (1995). Carcinogenic nickel silences gene expression by chromatin condensation and DNA methylation: a new model for epigenetic carcinogens. Mol. Cell. Biol . 15, 2547–2557.
Leonard, S. S., Bower, J. J., and Shi, X. (2004). Metal-induced toxicity, carcinogenesis, mechanisms and cellular responses. Mol. Cell. Biochem . 255, 3–10. doi: 10.1023/B:MCBI.0000007255.72746.a6
Leslie, F. M. (2013). Multigenerational epigenetic effects of nicotine on lung function. BMC Med . 11:27. doi: 10.1186/1741-7015-11-27
Lewis, B. P., Burge, C. B., and Bartel, D. P. (2005). Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20. doi: 10.1016/j.cell.2004.12.035
Lister, R., Pelizzola, M., Dowen, R. H., Hawkins, R. D., Hon, G., Tonti-Filippini, J., et al. (2009). Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462, 315–322. doi: 10.1038/nature08514
Liu, B., Tahk, S., Yee, K. M., Yang, R., Yang, Y., Mackie, R., et al. (2014). PIAS1 regulates breast tumorigenesis through selective epigenetic gene silencing. PLoS ONE 9:e89464. doi: 10.1371/journal.pone.0089464
Liu, H., Zhou, Y., Boggs, S. E., Belinsky, S. A., and Liu, J. (2007). Cigarette smoke induces demethylation of prometastatic oncogene synuclein-gamma in lung cancer cells by downregulation of DNMT3B. Oncogene 26, 5900–5910. doi: 10.1038/sj.onc.1210400
Liu, L., Van Groen, T., Kadish, I., Li, Y., Wang, D., James, S. R., et al. (2011). Insufficient DNA methylation affects healthy aging and promotes age-related health problems. Clin. Epigenetics 2, 349–360. doi: 10.1007/s13148-011-0042-6
Lomniczi, A., Loche, A., Castellano, J. M., Ronnekleiv, O. K., Bosch, M., Kaidar, G., et al. (2013). Epigenetic control of female puberty. Nat. Neurosci . 16, 281–289. doi: 10.1038/nn.3319
Lotito, S. B., and Frei, B. (2006). Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic. Biol. Med . 41, 1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033
Luger, K., Mader, A. W., Richmond, R. K., Sargent, D. F., and Richmond, T. J. (1997). Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389, 251–260. doi: 10.1038/38444
Lund, G., and Zaina, S. (2011). Atherosclerosis: an epigenetic balancing act that goes wrong. Curr. Atheroscler. Rep . 13, 208–214. doi: 10.1007/s11883-011-0174-3
Makar, K. W., and Wilson, C. B. (2004). DNA methylation is non-redundant repressor of the effector program. J. Immunol . 173, 4402–4406. doi: 10.4049/jimmunol.173.7.4402
Malvaez, M., McQuown, S. C., and Rogge, G. A. (2013). HDAC3 selective inhibitor enhances exinction of coccaine-seeking behaviour in persistent manner. Proc. Natl. Acad. Sci. U.S.A . 110, 2647. doi: 10.1073/pnas.1213364110
Manning, K., Tor, M., Poole, M., Hong, Y., Thompson, A. J., King, G. J., et al. (2006). A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet . 38, 948–952. doi: 10.1038/ng1841
Marsit, C. J., Eddy, K., and Kelsey, K. T. (2006). MicroRNA responses to cellular stress. Cancer Res . 66, 10843–10848. doi: 10.1158/0008-5472.CAN-06-1894
Mastroeni, D., Grover, A., and Delvaux, E. (2010). Epigenetic changes in Alzheimer's disease: decrements in DNA methylation. Neurobiol. Aging 31, 13. doi: 10.1016/j.neurobiolaging.2008.12.005
Maze, I., Covington, H. E. 3rd., Dietz, D. M., Laplant, Q., Renthal, W., Russo, S. J., et al. (2010). Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 327, 213–216. doi: 10.1126/science.1179438
Maze, I., and Nestler, E. J. (2011). The epigenetic landscape of addiction. Ann. N. Y. Acad. Sci . 1216, 99–113. doi: 10.1111/j.1749-6632.2010.05893.x
McGowan, P. O., Sasaki, A., D'alessio, A. C., Dymov, S., Labonte, B., Szyf, M., et al. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci . 12, 342–348. doi: 10.1038/nn.2270
Meaney, M. J. (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci . 24, 1161–1192. doi: 10.1146/annurev.neuro.24.1.1161
Miller, G. (2011). Mental health. Predicting the psychological risks of war. Science 333, 520–521. doi: 10.1126/science.333.6042.520
Milroy, C., Liu, L., Hammoud, S., Hammoud, A., Peterson, C. M., and Carrell, D. T. (2011). Differential methylation of pluripotency gene promoters in in vitro matured and vitrified, in vivo -matured mouse oocytes. Fertil. Steril . 95, 2094–2099. doi: 10.1016/j.fertnstert.2011.02.011
Monks, T. J., Xie, R., and Tikoo, K. (2006). ROS- induced histone modification and their role in cell survival and cell death. Drug Metab. Rev . 38, 755–767. doi: 10.1080/03602530600959649
Montgomery, R. L., and Van Rooij, E. (2010). MicroRNA regulation as a therapeutic stratergy for cardiovascular disease. Curr. Drug Targets 11, 936–942. doi: 10.2174/1389210204156534501
Morgan, H. D., Sutherland, H. G., and Martin, D. I. (1999). Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet . 23, 314–328. doi: 10.1038/12595
Morimoto, T., Sunagawa, Y., Kawamura, T., Takaya, T., Wada, H., Nagasawa, A., et al. (2008). The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Invest . 118, 868–878. doi: 10.1172/JCI33160
Morrison, K. E., Rodgers, A. B., Morgan, C. P., and Bale, T. L. (2014). Epigenetic mechanisms in pubertal brain maturation. Neuroscience 264, 17–24. doi: 10.1016/j.neuroscience.2013.11.014
Munro, S. K., Farquhar, C. M., Mitchell, M. D., and Ponnampalam, A. P. (2010). Epigenetic regulation of endometrium during the menstrual cycle. Mol. Hum. Reprod . 16, 297–310. doi: 10.1093/molehr/gaq010
Narita, M., Nunez, S., Heard, E., Narita, M., Lin, A. W., Hearn, S. A., et al. (2003). Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716. doi: 10.1016/S0092-8674(03)00401-X
Natarajan, A. T., Vermeulen, S., Darroudi, F., Valentine, M. B., Brent, T. P., Mitra, S., et al. (1992). Chromosomal localization of human O6-methylguanine-DNA methyltransferase (MGMT) gene by in situ hybridization. Mutagenesis 7, 83–85. doi: 10.1093/mutage/7.1.83
Nitert, M. D., Dayeh, T., Volkov, P., Elgzyri, T., Hall, E., Nilsson, E., et al. (2012). Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes 61, 3322–3332. doi: 10.2337/db11-1653
Ntanasis-Stathopoulos, J., Tzanninis, J. G., Philippou, A., and Koutsilieris, M. (2013). Epigenetic regulation on gene expression induced by physical exercise. J. Musculoskelet. Neuronal Interact . 13, 133–146.
Oberlander, T. F., Weinberg, J., Papsdorf, M., Grunau, R., Misri, S., and Devlin, A. M. (2008). Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 3, 97–106. doi: 10.4161/epi.3.2.6034
Ojeda, S. R., Dubay, C., Lomniczi, A., Kaidar, G., Matagne, V., Sandau, U. S., et al. (2010). Gene networks and the neuroendocrine regulation of puberty. Mol. Cell. Endocrinol . 324, 3–11. doi: 10.1016/j.mce.2009.12.003
Oki, E., Oda, S., Maehara, Y., and Sugimachi, K. (1999). Mutated gene-specific phenotypes of dinucleotide repeat instability in human colorectal carcinoma cell lines deficient in DNA mismatch repair. Oncogene 18, 2143–2147. doi: 10.1038/sj.onc.1202583
Olaharski, A. J., Rine, J., Marshall, B. L., Babiarz, J., Zhang, L., Verdin, E., et al. (2005). The flavoring agent dihydrocoumarin reverses epigenetic silencing and inhibits sirtuin deacetylases. PLoS Genet . 1:e77. doi: 10.1371/journal.pgen.0010077
Ordovas, J. M., and Smith, C. E. (2010). Epigenetics and cardiovascular disease. Nat. Rev. Cardiol . 7, 510–519. doi: 10.1038/nrcardio.2010.104
Orozco-Solis, R., and Sassone-Corsi, P. (2014). Epigenetic control and the circadian clock: linking metabolism to neuronal responses. Neuroscience 264, 76–87. doi: 10.1016/j.neuroscience.2014.01.043
Ouko, L. A., Shantikumar, K., Knezovich, J., Haycock, P., Schnugh, D. J., and Ramsay, M. (2009). Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes: implications for fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res . 33, 1615–1627. doi: 10.1111/j.1530-0277.2009.00993.x
Paneni, F., Costantino, S., Volpe, M., Luscher, T. F., and Cosentino, F. (2013). Epigenetic signatures and vascular risk in type 2 diabetes: a clinical perspective. Atherosclerosis 230, 191–197. doi: 10.1016/j.atherosclerosis.2013.07.003
Park, J. H., Stoffers, D. A., and Nicholls, R. D. (2008). Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Invest . 118, 2316–2324. doi: 10.1172/JCI33655
Phillips, T. (2008). The role of methylation in gene expression. Nat. Educ . 1:116.
Plotsky, P. M., and Meaney, M. J. (1993). Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol. Brain Res . 18, 195–200. doi: 10.1016/0169-328X(93)90189-V
Provost, P. (2010). Interpretation and applicability of microRNA data to the context of Alzheimer's and age-related diseases. Aging (Albany NY) 2, 166–169.
Radom-Aizik, S., Zaldivar, F. Jr., Oliver, S., Galassetti, P., and Cooper, D. M. (2010). Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J. Appl. Physiol . 109, 252–261. doi: 10.1152/japplphysiol.01291.2009
Radtke, K. M., Ruf, M., Gunter, H. M., Dohrmann, K., Schauer, M., Meyer, A., et al. (2011). Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl. Psychiatry 1:e21. doi: 10.1038/tp.2011.21
Reddy, U. M., Wapner, R. J., Rebar, R. W., and Tasca, R. J. (2007). Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet. Gynecol . 109, 967–977. doi: 10.1097/01.AOG.0000259316.04136.30
Rehan, V. K., Liu, J., Naeem, E., Tian, J., Sakurai, R., Kwong, K., et al. (2012). Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med . 10:129. doi: 10.1186/1741-7015-10-129
Reichard, J. F., Schnekenburger, M., and Puga, A. (2007). Long term low-dose arsenic exposure induces loss of DNA methylation. Biochem. Biophys. Res. Commun . 352, 188–192. doi: 10.1016/j.bbrc.2006.11.001
Reik, W. (2007). Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447, 425–432. doi: 10.1038/nature05918
Renthal, W., and Nestler, E. J. (2008). Epigenetic mechanisms in drug addiction. Trends Mol. Med . 14, 341–350. doi: 10.1016/j.molmed.2008.06.004
Report, W. (2001). Legal Status of Traditional Medicine and Complementary/Alternative Medicine: a Worldwide Review . Geneva: World Health Organisation.
Rideout, W. M. 3rd., Eversole-Cire, P., Spruck, C. H. 3rd., Hustad, C. M., Coetzee, G. A., Gonzales, F. A., et al. (1994). Progressive increases in the methylation status and heterochromatinization of the myoD CpG island during oncogenic transformation. Mol. Cell. Biol . 14, 6143–6152. doi: 10.1128/MCB.14.9.6143
Rivera, C. M., and Ren, B. (2013). Mapping human epigenomes. Cell 155, 39–55. doi: 10.1016/j.cell.2013.09.011
Romieu, P., Deschatrettes, E., Host, L., Gobaille, S., Sandner, G., and Zwiller, J. (2011). The inhibition of histone deacetylases reduces the reinstatement of cocaine-seeking behavior in rats. Curr. Neuropharmacol . 9, 21–25. doi: 10.2174/157015911795017317
Ronn, T., Volkov, P., Davegardh, C., Dayeh, T., Hall, E., Olsson, A. H., et al. (2013). A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet . 9:e1003572. doi: 10.1371/journal.pgen.1003572
Ronti, T., Lupattelli, G., and Mannarino, E. (2006). The endocrine function of adipose tissue: an update. Clin. Endocrinol. (Oxf.) 64, 355–365. doi: 10.1111/j.1365-2265.2006.02474.x
Roth, T. L. (2012). Epigenetics of neurobiology and behavior during development and adulthood. Dev. Psychobiol . 54, 590–597. doi: 10.1002/dev.20550
Rudenko, A., and Tsai, L. H. (2014). Epigenetic regulation in memory and cognitive disorders. Neuroscience 264, 51–63. doi: 10.1016/j.neuroscience.2012.12.034
Sahar, S., and Sassone-Corsi, P. (2012). Circadian rhythms and memory formation: regulation by chromatin remodeling. Front. Mol. Neurosci . 5:37. doi: 10.3389/fnmol.2012.00037
Sanchis-Gomar, F., Garcia-Gimenez, J. L., and Perez-Quilis, C. (2012). Physical exercise as an epigenetic modulator: eustress, the postive stress, as an effector of gene expression. J. Strength Cond. Res . 26, 3469–3472. doi: 10.1519/JSC.0b013e31825bb594
Santos, F., Hyslop, L., Stojkovic, P., Leary, C., Murdoch, A., Reik, W., et al. (2010). Evaluation of epigenetic marks in human embryos derived from IVF and ICSI. Hum. Reprod . 25, 2387–2395. doi: 10.1093/humrep/deq151
Schlinzig, T., Johansson, S., Gunnar, A., Ekstrom, T. J., and Norman, M. (2009). Epigenetic modulation at birth - altered DNA-methylation in white blood cells after Caesarean section. Acta Paediatr . 98, 1096–1099. doi: 10.1111/j.1651-2227.2009.01371.x
Schuz, L. C. (2010). The Dutch hunger winter and the developmental origins of health and disease. Proc. Natl. Acad. Sci. U.S.A . 107, 16757–16758. doi: 10.1073/pnas.1012911107
Sedivy, J. M., Banumathy, G., and Adams, P. D. (2008). Aging by epigenetics–a consequence of chromatin damage? Exp. Cell Res . 314, 1909–1917. doi: 10.1016/j.yexcr.2008.02.023
Seminara, S. B., Messager, S., Chatzidaki, E. E., Thresher, R. R., Acierno, J. S. Jr., Shagoury, J. K., et al. (2003). The GPR54 gene as a regulator of puberty. N. Engl. J. Med . 349, 1614–1627. doi: 10.1056/NEJMoa035322
Sharma, P., Garg, G., Kumar, A., Mohammad, F., Kumar, S. R., Tanwar, V. S., et al. (2014). Genome wide DNA methylation profiling for epigenetic alteration in coronary artery disease patients. Gene 541, 31–40. doi: 10.1016/j.gene.2014.02.034
Shenderov, B. A., and Midtvedt, T. (2014). Epigenomic programing: a future way to health? Microb. Ecol. Health Dis . 25:24145. doi: 10.3402/mehd.v25.24145
Shim, S., Kim, S., Choi, D. S., Kwon, Y. B., and Kwon, J. (2011). Anti-inflammatory effects of [6]-shogaol: potential roles of HDAC inhibition and HSP70 induction. Food Chem. Toxicol . 49, 2734–2740. doi: 10.1016/j.fct.2011.08.012
Shukla, S. D., and Lim, R. W. (2013). Epigenetic effects of ethanol on the liver and gastrointestinal system. Alcohol Res . 35, 47–55.
Shukla, S. D., Velazquez, J., French, S. W., Lu, S. C., Ticku, M. K., and Zakhari, S. (2008). Emerging role of epigenetics in the actions of alcohol. Alcohol. Clin. Exp. Res . 32, 1525–1534. doi: 10.1111/j.1530-0277.2008.00729.x
Shukla, S. D., and Zakhari, S. (2013). Epigenetics–new frontier for alcohol research. Alcohol Res . 35, 1–2.
Silva, P. N., Gigek, C. O., Leal, M. F., Bertolucci, P. H., De Labio, R. W., Payao, S. L., et al. (2008). Promoter methylation analysis of SIRT3, SMARCA5, HTERT and CDH1 genes in aging and Alzheimer's disease. J. Alzheimers Dis . 13, 173–176.
Singh, S. K., Pal Bhadra, M., and Girschick, H. J. (2008). Micro-RNA- micro in size but macro in function. FEBS J . 275, 16. doi: 10.1111/j.1742-4658.2008.06624.x
Singh, S., and Li, S. S. (2012). Epigenetic effects of environmental chemicals bisphenol a and phthalates. Int. J. Mol. Sci . 13, 10143–10153. doi: 10.3390/ijms130810143
Soubry, A., Schildkraut, J. M., and Murtha, A. (2013). Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a newborn epigenetics study (NEST) cohort. BMC Med . 11:29. doi: 10.1186/1741-7015-11-29
Starkman, B. G., Sakharkar, A. J., and Pandey, S. C. (2012). Epigenetics-beyond the genome in alcoholism. Alcohol. Res . 34, 293–305.
Stevenson, T. J., and Prendergast, B. J. (2013). Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc. Natl. Acad. Sci. U.S.A . 110, 16651–16656. doi: 10.1073/pnas.1310643110
Stolk, L., Perry, J. R., Chasman, D. I., He, C., Mangino, M., Sulem, P., et al. (2012). Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat. Genet . 44, 260–268. doi: 10.1038/ng.1051
Stouder, C., and Paoloni-Giacobino, A. (2010). Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction 139, 373–379. doi: 10.1530/REP-09-0340
Sun, H., Zhou, X., Chen, H., Li, Q., and Costa, M. (2009). Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol. Appl. Pharmacol . 237, 258–266. doi: 10.1016/j.taap.2009.04.008
Surani, M. A. (2001). Reprogramming of genome function through epigenetic inheritance. Nature 414, 122–128. doi: 10.1038/35102186
Takahashi, Y., Kondo, K., Hirose, T., Nakagawa, H., Tsuyuguchi, M., Hashimoto, M., et al. (2005). Microsatellite instability and protein expression of the DNA mismatch repair gene, hMLH1, of lung cancer in chromate-exposed workers. Mol. Carcinog . 42, 150–158. doi: 10.1002/mc.20073
Tammen, S. A., Dolnikowski, G. G., Ausman, L. M., Liu, Z., Sauer, J., Friso, S., et al. (2014). Aging and alcohol interact to alter hepatic DNA hydroxymethylation. Alcohol. Clin. Exp. Res . 38, 2178–2185. doi: 10.1111/acer.12477
Tollefsbol, T. O. (2014). Dietary epigenetics in cancer and aging. Cancer Treat. Res . 159, 257–267. doi: 10.1007/978-3-642-38007-5_15
Ubeda, F., Ohtsuki, H., and Gardner, A. (2014). Ecology drives intragenomic conflict over menopause. Ecol. Lett . 17, 165–174. doi: 10.1111/ele.12208
Urdinguio, R. G., Sanchez-Mut, J. V., and Esteller, M. (2009). Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol . 8, 1056–1072. doi: 10.1016/S1474-4422(09)70262-5
Vassoler, F. M., White, S. L., Schmidt, H. D., Sadri-Vakili, G., and Pierce, R. C. (2013). Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci . 16, 42–47. doi: 10.1038/nn.3280
Vogel-Ciernia, A., Matheos, D. P., and Barrett, R. M. (2013). The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat. Neurosci . 16, 552–561. doi: 10.1038/nn.3359
Waris, G., and Ahsan, H. (2006). Reactive oxygen species: role in development of cancer and various chronic conditions. J. Carcinog . 5:14. doi: 10.1186/1477-3163-5-14
Waterland, R. A., and Jirtle, R. L. (2003). Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol . 23, 5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003
Weaver, I. C., Cervoni, N., Champagne, F. A., D'alessio, A. C., Sharma, S., Seckl, J. R., et al. (2004). Epigenetic programming by maternal behavior. Nat. Neurosci . 7, 847–854. doi: 10.1038/nn1276
Webster, A. L., Yan, M. S., and Marsden, P. A. (2013). Epigenetics and cardiovascular disease. Can. J. Cardiol . 29, 46–57. doi: 10.1016/j.cjca.2012.10.023
Whitelaw, N., Bhattacharya, S., Hoad, G., Horgan, G. W., Hamilton, M., and Haggarty, P. (2014). Epigenetic status in the offspring of spontaneous and assisted conception. Hum. Reprod . 29, 1452–1458. doi: 10.1093/humrep/deu094
Widschwendter, M., Apostolidou, S., Jones, A. A., Fourkala, E. O., Arora, R., Pearce, C. L., et al. (2009). HOXA methylation in normal endometrium from premenopausal women is associated with the presence of ovarian cancer: a proof of principle study. Int. J. Cancer 125, 2214–2218. doi: 10.1002/ijc.24599
Williams, R. J., Spencer, J. P., and Rice-Evans, C. (2004). Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med . 36, 838–849. doi: 10.1016/j.freeradbiomed.2004.01.001
Witcher, M., and Emerson, B. M. (2009). Epigenetic silencing of the p16(INK4a) tumor suppressor is associated with loss of CTCF binding and a chromatin boundary. Mol. Cell 34, 271–284. doi: 10.1016/j.molcel.2009.04.001
Wolff, G. L., Kodell, R. L., Moore, S. R., and Cooney, C. A. (1998). Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J . 12, 949–957.
Wossidlo, M., Nakamura, T., and Lepikhov, K. (2011). 5-hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat. Commun . 2:241. doi: 10.1038/ncomms1240
Wright, R. O., Schwartz, J., Wright, R. J., Bollati, V., Tarantini, L., Park, S. K., et al. (2010). Biomarkers of lead exposure and DNA methylation within retrotransposons. Environ. Health Perspect . 118, 790–795. doi: 10.1289/ehp.0901429
Wu, Y., Halverson, G., Basir, Z., Strawn, E., Yan, P., and Guo, S. W. (2005). Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol . 193, 371–380. doi: 10.1016/j.ajog.2005.01.034
Xu, Z., and Taylor, J. A. (2014). Genome-wide age-related DNA methylation changes in blood and other tissues relate to histone modification, expression and cancer. Carcinogenesis 35, 356–364. doi: 10.1093/carcin/bgt391
Zakhari, S. (2013). Alcohol metabolism and epigenetics changes. Alcohol Res . 35, 6–16.
Zhang, H., and Zhu, J. K. (2012). Active DNA demethylation in plants and animals. Cold Spring Harb. Symp. Quant. Biol . 77, 161–173. doi: 10.1101/sqb.2012.77.014936
Zhao, X. M., Du, W. H., Hao, H. S., Wang, D., Qin, T., Liu, Y., et al. (2012). Effect of vitrification on promoter methylation and the expression of pluripotency and differentiation genes in mouse blastocysts. Mol. Reprod. Dev . 79, 445–450. doi: 10.1002/mrd.22052
Zhubi, A., Chen, Y., Dong, E., Cook, E. H., Guidotti, A., and Grayson, D. R. (2014). Increased binding of MeCP2 to the GAD1 and RELN promoters may be mediated by an enrichment of 5-hmC in autism spectrum disorder (ASD) cerebellum. Transl. Psychiatry 4, e349. doi: 10.1038/tp.2013.123
Ziller, M. J., Gu, H., Muller, F., Donaghey, J., Tsai, L. T., Kohlbacher, O., et al. (2013). Charting a dynamic DNA methylation landscape of the human genome. Nature 500, 477–481. doi: 10.1038/nature12433
Zucchi, F. C., Yao, Y., Ward, I. D., Ilnytskyy, Y., Olson, D. M., Benzies, K., et al. (2013). Maternal stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring. PLoS ONE 8:e56967. doi: 10.1371/journal.pone.0056967
Keywords: epigenetics, human lifespan, disease, environment, diet, development
Citation: Kanherkar RR, Bhatia-Dey N and Csoka AB (2014) Epigenetics across the human lifespan. Front. Cell Dev. Biol . 2 :49. doi: 10.3389/fcell.2014.00049
Received: 20 June 2014; Accepted: 22 August 2014; Published online: 09 September 2014.
Reviewed by:
Copyright © 2014 Kanherkar, Bhatia-Dey and Csoka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonei B. Csoka, Epigenetics Laboratory, Department of Anatomy, Howard University, 520 W St. NW, Mudd 431, Washington, DC 20059, USA e-mail: [email protected]
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Genetics Basics
- Family Health History
- About Cascade Testing
Epigenetics, Health, and Disease
What to know.
Epigenetics refers to the way your behaviors and environment can cause changes that affect the way your genes work. Epigenetics turns genes "on" and "off." Your epigenetics change as you age, both as part of normal development and aging and because of exposure to environmental factors that happen over the course of your life. Epigenetic changes can affect your health in different ways.
Your genes play an important role in your health, but so do your behaviors and environment, such as what you eat and how physically active you are. Epigenetics refers to how your behaviors and environment can cause changes that affect the way your genes work. Unlike genetic changes (mutations), epigenetic changes are reversible and do not change the sequence of DNA bases, but they can change how your body reads a DNA sequence.
Gene expression refers to the process of making proteins using the instructions from genes. A person's DNA includes many genes. Each gene includes instructions for making proteins. Additionally, there are other sections of DNA that are not part of any gene but are important for making sure the genes work properly. These DNA sections provide directions about where in the body the protein is made, when it is made, and how much is made.
While changes to the genes (mutations) can change the protein that is made, epigenetic changes affect gene expression to turn genes "on" and "off." This can mean that genes make proteins in cells and tissues where or when they normally would not, or that genes don't make proteins where and when they normally would. It can also mean that genes make more or less of a protein than they normally would.
There are several ways an environmental factor can cause an epigenetic change to occur. One of the most common ways is by causing changes to DNA methylation. DNA methylation works by adding a chemical (known as a methyl group) to DNA. This chemical can also be removed from the DNA through a process called demethylation. Typically, methylation turns genes off and demethylation turns genes on. Thus, environmental factors can impact the amount of protein a cell makes. Less protein might be made if an environmental factor causes an increase in DNA methylation, and more protein might be made if a factor causes an increase in demethylation.
Epigenetics and age
Your epigenetics change as you age as part of normal development.
Epigenetics and development
Epigenetic changes begin before you are born. All your cells have the same genes but look and act differently. As you grow and develop, epigenetics helps determine which function a cell will have—for example, whether it will become a heart cell, nerve cell, muscle cell, or skin cell.
EXAMPLE: Nerve cell and muscle cell. Your nerve cells and muscle cells have the same DNA, but they work differently. A nerve cell transports information to other cells in your body. A muscle cell has a structure that aids in your body's ability to move. Epigenetics allows the muscle cell to turn on genes to make proteins important for its job and turn off genes important for a nerve cell's job.
Your epigenetics change throughout your life. Your epigenetics at birth are not the same as your epigenetics during childhood or adulthood.
EXAMPLE: A newborn, 26-year-old, and 103-year-old. Scientists measured DNA methylation at millions of sites in a newborn, 26-year-old, and 103-year-old. The level of DNA methylation decreased with age. The newborn had the highest level of DNA methylation, the 103-year-old had the lowest level of DNA methylation, and the 26-year-old had a DNA methylation level that was between that of the newborn and the 103-year-old. 1
Epigenetics and exposures
Your epigenetics can change in response to your behaviors and environment.
Nutrition during pregnancy
A pregnant person's environment and behavior during pregnancy, such as whether they eat healthy food, can change the baby's epigenetics. Some of these changes can remain for decades and might make the child more likely to get certain diseases.
EXAMPLE: Dutch Hunger Winter famine (1944 – 1945). People whose mothers were pregnant with them during the famine were more likely to develop certain diseases, such as heart disease, schizophrenia, and type 2 diabetes. 2 Around 60 years after the famine, researchers looked at DNA methylation levels in people whose mothers were pregnant with them during the famine. These people had increased DNA methylation at some genes and decreased DNA methylation at other genes, compared with their siblings who were not exposed to famine before birth. 3 4 5 These differences in DNA methylation could help explain why these people had an increased likelihood for certain diseases later in life. 2 5 6 7
Exposures such as smoking can cause epigenetic changes. However, these epigenetic changes can be reversible in some cases.
EXAMPLE: Smokers, nonsmokers, and former smokers. Smoking can result in epigenetic changes. For example, at certain parts of the AHRR gene, smokers tend to have less DNA methylation than nonsmokers. The difference is greater for heavy smokers and long-term smokers. After quitting smoking, former smokers can begin to have increased DNA methylation at this gene. Eventually, they can reach levels similar to those of nonsmokers. In some cases, this can happen in less than a year, but the length of time depends on how long and how much someone smoked before quitting. 8
Epigenetics and diseases
Certain diseases can change your epigenetics. In addition, some epigenetic changes can make you more likely to develop certain diseases, such as cancer.
Germs can change your epigenetics to weaken your immune system. This helps the germ survive.
EXAMPLE: Tuberculosis. Mycobacterium tuberculosis causes tuberculosis. Infections with these germs can cause epigenetic changes in some of your immune cells that result in turning off the IL-12B gene. Turning off the IL-12B gene weakens your immune system and improves the survival of Mycobacterium tuberculosis. 9
Certain mutations make you more likely to develop cancer. Likewise, some epigenetic changes increase your cancer risk. For example, having a mutation in the BRCA1 gene that prevents it from working properly makes you more likely to get breast and other cancers. Similarly, increased DNA methylation that results in decreased BRCA1 gene expression raises your risk for breast and other cancers. 10 While cancer cells have increased DNA methylation at certain genes, overall DNA methylation levels are lower in cancer cells compared with normal cells.
Different types of cancer that seem similar can have different DNA methylation patterns. Epigenetics can be used to help determine which type of cancer a person has or can help to find hard-to-detect cancers earlier. Epigenetics alone cannot diagnose cancer. Cancers would need to be confirmed with further screening tests.
EXAMPLE: Colorectal cancer. Colorectal cancers have abnormal DNA methylation near certain genes, which affects expression of these genes. Some commercial colorectal cancer screening tests (for example, Cologuard ® ) use stool samples to look for this abnormal DNA methylation. It is important to know that if you have one of these tests and the result is positive or abnormal, you will need to have a colonoscopy, which is a procedure to check your colon for cancer. 11
- Learn. Genetics: Genetic Science Learning Center at the University of Utah provides a detailed explanation and interactive tutorial about epigenetics.
- National Human Genomic Research Institute: Epigenomics Fact Sheet provides answers to questions about the epigenome.
- National Institute of Environmental Health Sciences: Epigenetics provides information about epigenetics, epigenetic research, and a video about epigenetics.
- Distinct DNA methylomes of newborns and centenarians . Proc Natl Acad Sci U S A 2012; 109:10522-7. Heyn H, Li N, Ferreira H, et al.
- Epidemiological evidence for the developmental origins of health and disease: effects of prenatal undernutrition in humans . J Endocrinol 2019. 242:T135-T144. Roseboom T.
- Persistent epigenetic differences associated with prenatal exposure to famine in humans . Proc Natl Acad Sci U S A 2008; 105: 17046-17049. Heijmans B, Tobi E, Stein A, et al.
- DNA Methylation Differences After Exposure to Prenatal Famine Are Common and Timing- And Sex- Specific . Hum Mol Genet 2009; 18:4046-53. Tobi E, Lumey L, Talens R, et al.
- DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood . Sci Adv 2018; 4:eaao4364. Tobi E, Slieker R, Luijk R, et al.
- DNA Methylation of Loci Within ABCG1 and PHOSPHO1 in Blood DNA is Associated With Future Type 2 Diabetes Risk . Epigenetics 2016; 7: 482-8. Dayeh T, Tuomi T, Almgren P, et al.
- Epigenetic and genetic variation at the IGF2/H19 imprinting control region on 11p15.5 is associated with cerebellum weight . Epigenetics 2012; 7:155-163. Pidsley R, Dempster E, Troakes C, et al.
- Epigenetic signatures of starting and stopping smoking . EBioMedicine 2018; 37:214-220. McCartney D, Stevenson A, Hillary R, et al.
- Mycobacterium tuberculosis Infection Induces HDAC1-Medicated Suppression of IL-12B Gene Expression in Macrophages . Front Cell Infect Microbiol 2015; 5:90. Chandran A, Antony C, Jose L, et al.
- Blood-based DNA methylation as biomarker for breast cancer: a systematic review . Clin Epigenetics 2016; 8: 115. Tang Q, Cheng J, Cao X, et al.
- Advances in tests for colorectal cancer screening and diagnosis . Expert Rev Mol Diagn 2022; 22: 449-460. Chan SCH, Liang JQ.
Genomics and Your Health
Learn more about genomics and its importance for your health
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Genetics, epigenetic mechanism.
Nora M. Al Aboud ; Connor Tupper ; Ishwarlal Jialal .
Affiliations
Last Update: August 14, 2023 .
- Introduction
Epigenetics is the study of heritable and stable changes in gene expression that occur through alterations in the chromosome rather than in the DNA sequence. [1] Despite not directly altering the DNA sequence, epigenetic mechanisms can regulate gene expression through chemical modifications of DNA bases and changes to the chromosomal superstructure in which DNA is packaged.
Briefly, negatively charged DNA is packaged around a positively charged histone protein octamer, which contains 2 copies of histone proteins H2A, H2B, H3, and H4. [2] This nucleoprotein complex is a nucleosome, the basic unit of chromatin. [3] The nucleosomes of a continuous DNA polymer are connected by linker DNA and the complex is stabilized by histone protein H1. The aggregation of chromatin results in the formation of a chromosome. The chromatin of a chromosome exists as either loose, transcriptionally active euchromatin or dense, transcriptionally inactive heterochromatin. [4] Chemical alterations to histone proteins can induce the formation of either the open euchromatin state, which facilitates gene expression by allowing transcription factors and enzymes to interact with the DNA, or the closed heterochromatin state, which suppresses gene expression by preventing initiation of transcription.
In addition to histone changes, DNA methylation is an epigenetic mechanism associated with gene silencing when the methylation occurs in CpG islands of promoter sequences. [5] Further, non-coding RNA sequences have shown to play a key role in the regulation of gene expression. [6] These epigenetic modifications can be induced by several factors including age, diet, smoking, stress, and disease state. [7] [8] Epigenetic modifications are reversible, but they rarely remain through generations in humans despite persisting through multiple cycles of cell replication. [9]
Epigenetic mechanisms form a layer of control within a cell that regulates gene expression and silencing. This control varies between tissues and plays an important role in cell differentiation. [10] Additionally, differences in gene expression between cells, which are driven by epigenetic modifications, result in the unique function of specific cell types. [11] Genome-wide patterns of DNA and histone modifications are established during early development and are maintained throughout multiple cell divisions. In cancer, the normal epigenetic patterns are disrupted resulting in the expression of anti-apoptotic and pro-proliferative genes and silencing of tumor suppressor genes like CDKN2A. [12]
Three different epigenetic mechanisms have been identified: DNA methylation, histone modification, and non-coding RNA (ncRNA)-associated gene silencing. Catalyzed by DNA methyltransferase enzymes, DNA methylation involves the addition of a methyl group directly to a cytosine nucleotide within a cytosine-guanine sequence (CpG), which are often surrounded by other CpG’s forming a CpG island. CpG islands are common targets for epigenetic DNA methylation, notably the CpG islands within promoter regions. Indeed, it has been reported that around 70% of gene promotor regions lie within CpG islands. [13] Methylated cytosines within a promoter region recruit gene suppressor proteins and reduce interaction between the DNA and transcription factors. [14] Cytosine methylation also drives the formation of heterochromatin, so the nucleosome tightening prevents transcriptional machinery from interacting with the DNA. [15] As such, DNA methylation within promoter regions results in gene silencing. Cancers often show marked hypermethylation of tumor suppressor genes and hypomethylation of proto-oncogenes, both of which contribute to tumor carcinogenesis. [15] This epigenetic mechanism also plays an important role in tissue-specific gene regulation, genomic imprinting, and X chromosome inactivation. [14]
The second epigenetic mechanism is post-translational modifications to histone proteins. These modifications include enzyme-catalyzed acetylation, methylation, phosphorylation, and ubiquitylation, each of which alters the DNA-histone interactions in nucleosomes. [16] Histone acetylation often occurs at positively charged lysine residues which weakens the DNA-histone interactions, thus opening the chromatin and facilitating transcription. [17] For example, acetylation of lysine 9 and lysine 27 on histone 3 (H3K9ac and H3K27ac, respectively) correlates with transcription activation. Histone methylation is more complex as it does not change the histone protein charge and can include the addition of 1-3 methyl groups to lysine and 1-2 methyl groups to arginine. [17] For example, methylation of lysine 4 on histone 3 (H3K4me) is associated with transcription activation while trimethylation of lysine 27 on histone 3 (H3K27me3) correlates with transcription repression. [18] Histone phosphorylation involves the addition of a negative phosphate group to the histone tail, but less is known of its function aside from phosphorylation of H2A(X) playing a role in the response to DNA damage and its subsequent repair. [19] Histone ubiquitylation involves the addition of a large ubiquitin molecule to lysine residues. Examples of histone ubiquitylation include H2AK119ub, which is associated with gene silencing, and H2BK123ub, which is involved in transcription. [17] Aside from the relatively straightforward effect of histone acetylation on gene expression, the effects of other histone modifications are complex and greatly influenced by the state of nearby DNA molecules.
The most recently elucidated epigenetic mechanism is non-coding RNA-associated gene silencing. A non-coding RNA (ncRNA) is a functional RNA molecule that is transcribed but not translated into proteins. Once regarded as waste of the genome, recent insight suggests the ncRNA molecules harbor a crucial role in epigenetic gene expression and likely account for the great difference in phenotype between species and within human populations despite such similarity in encoded proteins. [6] [18] Notable ncRNA molecules include microRNAs (miRNA) and short interfering RNAs (siRNA), which include less than 30 nucleotides, and long non-coding RNAs (lncRNA), which are 200 nucleotides or longer. Though the full extent of their role in epigenetics is still being determined, there is evidence suggesting that ncRNAs participate in DNA methylation and histone modifications in addition to gene silencing. [20] siRNAs and lncRNAs both have been shown to regulate gene expression by the formation of heterochromatin. [6] [21]
- Clinical Significance
As people age, the largest influence on the epigenome is the environment. Direct influencers such as diet can affect one's epigenome, as determined by the Dutch famine studies. [22] [23] Other environmental stressors include smoking and psychological stress. Epigenetic changes in utero are particularly sensitive as the epigenetic profile of the fetus is forming and developing rapidly during this time. [24] Indeed, teratogens like cigarette smoke, alcohol, and specific minerals have shown to induce in utero epigenetic changes. [24] [25]
Perhaps the most studied clinical application of epigenetic mechanisms is cancer. One of the first reports of epigenetics involved in cancer reported hypomethylation of DNA in cancer cell genomes, which caused overexpression of genes within that cell. [26] Since this report, great strides have been made toward understanding the role of epigenetics in carcinogenesis. For example, the degree of DNA methylation continues to decrease as a benign tumor cell progresses to invasive cancer. [27] Other studies have shown hypomethylation of pro-proliferative genes like BAX2 that are suppressed in normal cells. [28] Other reports show hypermethylation of tumor suppressor genes, like Rb, BCRA1, and CDKN2A, in cancer cells. [29] [30] [31] Despite the wealth of knowledge present on the relationship between epigenetics and carcinogenesis, treatment development is still very much in the preliminary phase for most cancers.
Epigenetics is a promising field of research because of the potential to regulate gene expression without changing the DNA sequence, which may likely cause safety and ethical concerns if performed in humans. The most promising way to treat diseases through epigenetic regulation has been through pharmacology. Previous clinical trials for drugs formulated to block epigenetic modifications associated with cancers have proved successful. The FDA has approved a number of these drugs which target epigenetic regulators to treat various cancers including azacytidine and decitabine for myelodysplastic syndrome, panobinostat for multiple myeloma, and romidepsin for cutaneous T cell lymphoma. [32] More drugs are likely to be approved in the coming years as a number of clinical trials for DNA methylation inhibitors and histone modification inhibitors are underway.
In addition to cancers, many conditions associated with genomic imprinting are the result of malfunctioning epigenetic mechanisms. Epigenetic mechanisms can induce disease, but they are also necessary for normal cell function, specifically in imprinted genes where only one parental chromosome is expressed. For genomic imprinting to successfully occur, the other parental chromosome must be silenced, which occurs through DNA methylation. Noteworthy conditions associated with abnormalities in gene imprinting include Prader-Willi syndrome, Angelman syndrome, Beckwith-Wiedemann syndrome, Russell-Silver syndrome, and Rubenstein-Taybi syndrome. [33] [34] Recent studies have shown positive results for epigenetic-based therapies for imprinting disorders, which may be a field of increased focus in the coming years in search of better treatments. [35] [36]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Nora Al Aboud declares no relevant financial relationships with ineligible companies.
Disclosure: Connor Tupper declares no relevant financial relationships with ineligible companies.
Disclosure: Ishwarlal Jialal declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Al Aboud NM, Tupper C, Jialal I. Genetics, Epigenetic Mechanism. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Epigenomics in stress tolerance of plants under the climate change. [Mol Biol Rep. 2023] Review Epigenomics in stress tolerance of plants under the climate change. Kumar M, Rani K. Mol Biol Rep. 2023 Jul; 50(7):6201-6216. Epub 2023 Jun 9.
- Genetics, DNA Packaging. [StatPearls. 2024] Genetics, DNA Packaging. Simpson B, Tupper C, Al Aboud NM. StatPearls. 2024 Jan
- Genetics, Histone Code. [StatPearls. 2024] Genetics, Histone Code. Shahid Z, Simpson B, Miao KH, Singh G. StatPearls. 2024 Jan
- Review Analytical and therapeutic profiles of DNA methylation alterations in cancer; an overview of changes in chromatin arrangement and alterations in histone surfaces. [Horm Mol Biol Clin Investig. 2...] Review Analytical and therapeutic profiles of DNA methylation alterations in cancer; an overview of changes in chromatin arrangement and alterations in histone surfaces. Norollahi SE, Vahidi S, Shams S, Keymoradzdeh A, Soleymanpour A, Solymanmanesh N, Mirzajani E, Jamkhaneh VB, Samadani AA. Horm Mol Biol Clin Investig. 2023 Sep 1; 44(3):337-356. Epub 2023 Feb 17.
- Review Integrative Chemical Biology Approaches to Deciphering the Histone Code: A Problem-Driven Journey. [Acc Chem Res. 2021] Review Integrative Chemical Biology Approaches to Deciphering the Histone Code: A Problem-Driven Journey. Li X, Li XD. Acc Chem Res. 2021 Oct 5; 54(19):3734-3747. Epub 2021 Sep 23.
Recent Activity
- Genetics, Epigenetic Mechanism - StatPearls Genetics, Epigenetic Mechanism - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 20 May 2024
In vitro reconstitution of epigenetic reprogramming in the human germ line
- Yusuke Murase 1 , 2 na1 ,
- Ryuta Yokogawa ORCID: orcid.org/0000-0002-7033-2794 1 , 2 na1 ,
- Yukihiro Yabuta 1 , 2 ,
- Masahiro Nagano 1 , 2 ,
- Yoshitaka Katou 1 , 2 ,
- Manami Mizuyama ORCID: orcid.org/0009-0003-3971-2996 1 , 2 ,
- Ayaka Kitamura 1 , 2 ,
- Pimpitcha Puangsricharoen 1 , 2 ,
- Chika Yamashiro ORCID: orcid.org/0000-0001-7412-6341 2 ,
- Bo Hu 1 , 2 ,
- Ken Mizuta ORCID: orcid.org/0000-0002-3922-1009 1 , 2 ,
- Kosuke Ogata ORCID: orcid.org/0000-0002-0634-3990 3 ,
- Yasushi Ishihama ORCID: orcid.org/0000-0001-7714-203X 3 &
- Mitinori Saitou ORCID: orcid.org/0000-0002-2895-6798 1 , 2 , 4
Nature ( 2024 ) Cite this article
Metrics details
We are providing an unedited version of this manuscript to give early access to its findings. Before final publication, the manuscript will undergo further editing. Please note there may be errors present which affect the content, and all legal disclaimers apply.
- Embryonic germ cells
- Germline development
Epigenetic reprogramming resets parental epigenetic memories and differentiates primordial germ cells (PGCs) into mitotic pro-spermatogonia or oogonia, ensuring sexually dimorphic germ-cell development for totipotency 1 . In vitro reconstitution of epigenetic reprogramming in humans remains a fundamental challenge. Here, we establish a robust strategy for inducing epigenetic reprogramming and differentiation of pluripotent stem cell (PSC)-derived human PGC-like cells (hPGCLCs) into mitotic pro-spermatogonia or oogonia, coupled with their extensive amplification (~>10 10 -fold). Strikingly, bone morphogenetic protein (BMP) signalling is a key driver of these processes: BMP-driven hPGCLC differentiation involves an attenuation of the mitogen-activated protein kinase/extracellular-regulated kinase (MAPK/ERK) pathway and both de novo and maintenance DNA methyltransferase (DNMT) activities, likely promoting replication-coupled, passive DNA demethylation. On the other hand, hPGCLCs deficient in tens-eleven translocation (TET) 1, an active DNA demethylase abundant in human germ cells 2,3 , differentiate into extraembryonic cells, including amnion, with de-repression of key genes bearing bivalent promoters; these cells fail to fully activate genes vital for spermatogenesis and oogenesis, with their promoters remaining methylated. Our study elucidates the framework of epigenetic reprogramming in humans, making a fundamental advance in human biology, and through the generation of abundant mitotic pro-spermatogonia and oogonia-like cells, represents a milestone for human in vitro gametogenesis (IVG) research and its potential translation into reproductive medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Author information
These authors contributed equally: Yusuke Murase, Ryuta Yokogawa
Authors and Affiliations
Institute for the Advanced Study of Human Biology (ASHBi), Kyoto University, Yoshida-Konoe-cho, Sakyo-ku, Kyoto, Japan
Yusuke Murase, Ryuta Yokogawa, Yukihiro Yabuta, Masahiro Nagano, Yoshitaka Katou, Manami Mizuyama, Ayaka Kitamura, Pimpitcha Puangsricharoen, Bo Hu, Ken Mizuta & Mitinori Saitou
Department of Anatomy and Cell Biology, Graduate School of Medicine, Kyoto University, Yoshida-Konoe-cho, Sakyo-ku, Kyoto, Japan
Yusuke Murase, Ryuta Yokogawa, Yukihiro Yabuta, Masahiro Nagano, Yoshitaka Katou, Manami Mizuyama, Ayaka Kitamura, Pimpitcha Puangsricharoen, Chika Yamashiro, Bo Hu, Ken Mizuta & Mitinori Saitou
Department of Molecular Systems BioAnalysis, Graduate School of Pharmaceutical Sciences, Kyoto University, Kyoto, Japan
Kosuke Ogata & Yasushi Ishihama
Center for iPS Cell Research and Application (CiRA), Kyoto University, 53 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto, Japan
Mitinori Saitou
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mitinori Saitou .
Supplementary information
Supplementary information.
This file contains Supplementary Figures 1-3 and Supplementary Discussions 1&2.
Reporting Summary
Peer review file, supplementary tables.
This folder contains Supplementary Tables 1-12.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Murase, Y., Yokogawa, R., Yabuta, Y. et al. In vitro reconstitution of epigenetic reprogramming in the human germ line. Nature (2024). https://doi.org/10.1038/s41586-024-07526-6
Download citation
Received : 21 May 2023
Accepted : 07 May 2024
Published : 20 May 2024
DOI : https://doi.org/10.1038/s41586-024-07526-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Abstract. Epigenetic research has accelerated rapidly in the twenty-first century, generating justified excitement and hope, but also a degree of hype. Here we review how the field has evolved ...
INTRODUCTION. Classical definition by Conrad Waddington in the 1950s states "an epigenetic trait is a stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence"[].Based on our understanding of epigenetics, actual epigenetic definitions express that the whole DNA content is exactly the same in somatic cells of one species, while gene ...
Over the past few decades, epigenetics has evolved from a collection of curious biological phenomena to a functionally dissected research field. In this article, the authors provide a personal ...
The definition of epigenetics refers that molecular modifications on DNA that can regulate gene activity are independent of DNA sequence and mitotically stable. Notably, epigenetics studies have grown exponentially in the past few years. Recent progresses that lead to exciting discoveries and groundbreaking nature of this area demand thorough ...
These questions were recently discussed at the symposium Epigenetic Inheritance: Impact for Biology and Society organized every 2 years in Zürich, Switzerland. This review provides a summary of the research presented during the symposium and discusses current important questions, perspectives and challenges for the field in the future.
Epigenetics is the regulation of gene expression through alterations in DNA or associated factors (other than the DNA sequence). These factors control the diverse manifestations of diseases. Insigh...
Epigenetics of prenatal stress in humans: the current research landscape. Fetal exposure to prenatal stress can have significant consequences on short- and long-term health. Epigenetic mechanisms, especially DNA methylation (DNAm), are a possible process how these adverse environmen...
Read the latest research news on epigenetics, epigenetic influences on disease risk and gene silencing. ... Apr. 24, 2024 — A research team has discovered that cancer, ...
This article highlights the origin of epigenetics, the major phases of epigenetic research, and the changes in the meaning of the term. It also calls into question some of the far-reaching claims that have accompanied the recent rise of epigenetics. Previous article in issue; Next article in issue; Keywords.
More research in this area is needed, including designs that can disentangle the independent and joint influences of genetic, epigenetic, social, and environmental factors on cross-generational ...
Abstract. Epigenetics has the potential to explain various biological phenomena that have heretofore defied complete explication. This review describes the various types of endogenous human developmental milestones such as birth, puberty, and menopause, as well as the diverse exogenous environmental factors that influence human health, in a ...
Research Article Article Transmission of reduced levels of miR-34/449 from sperm to preimplantation embryos is a key step in the transgenerational epigenetic inheritance of the effects of paternal chronic social instability stress
In this article, we summarize methylation establishment, maintenance, and removal, different genome histone modifications, and how these epigenetic changes contribute to developing abiotic stress tolerance. The stable and reversible nature of chromatin alterations has a huge advantage to the dynamic epigenetic network.
National Institute ofEnvironmental Health Sciences. Last Reviewed: September 12, 2023. For decades, scientists have known the basic structure of our DNA, the building blocks that make up our genes. Epigenetics is a rapidly growing area of science that focuses on the processes that help direct when individual genes are turned on or off.
Full Text of the Graphic. "Epigenetics" is an emerging area of scientific research that shows how environmental influences—children's experiences—actually affect the expression of their genes. This means the old idea that genes are "set in stone" has been disproven. Nature vs. Nurture is no longer a debate. It's nearly always both!
The work appears in Clinical Epigenetics and was supported by the National Institutes of Health under grants R01HD098857, R01MD017696, R01MD011746, P30ES025128, and R01ES032462.
"With imprinted genes, there isn't a backup copy in the event of mutation," says Randy Jirtle, professor of epigenetics at NC State and co-corresponding author of the research. "ICRs control the expression of these genes - in other words, they tell imprinted genes where, when and how to work through DNA methylation.
Among all the epigenetics research conducted so far, the most extensively studied disease is cancer, and the evidence linking epigenetic processes with cancer is becoming "extremely compelling," says Peter Jones, director of the University of Southern California's Norris Comprehensive Cancer Center. Halfway around the world, Toshikazu ...
Epigenetics has the potential to explain various biological phenomena that have heretofore defied complete explication. This review describes the various types of endogenous human developmental milestones such as birth, puberty, and menopause, as well as the diverse exogenous environmental factors that influence human health, in a chronological epigenetic context.
Epigenetics accepts original research articles, reviews and brief reports. The journal operates a single anonymized peer review policy. Read the Instructions for Authors for information on how to submit your article. *Please note that Epigenetics converted to a full Open Access journal from Volume 18 (2023). Previous volumes will continue to ...
Epigenetics refers to how your behaviors and environment can cause changes that affect the way your genes work. Unlike genetic changes (mutations), epigenetic changes are reversible and do not change the sequence of DNA bases, but they can change how your body reads a DNA sequence. Gene expression refers to the process of making proteins using ...
Epigenetics articles within Nature. Featured. News & Views | 14 May 2024. ... Research articles News Opinion Research Analysis Careers ...
Ketogenic Diet Alters the Epigenetic and Immune Landscape of Prostate Cancer to Overcome Resistance to Immune Checkpoint Blockade Therapy ... Boler-Parseghian Center for Rare and Neglected Diseases, Harper Cancer Research Institute, University of Notre Dame, Notre Dame, Indiana. 2 Integrated Biomedical Sciences Graduate Program, University of ...
Firstly, the concept of resilience in epigenetic research should be studied in its own right, rather than simply as a disease-free state. 2 While some studies solely study people that have experienced adversity, 23 or consider the severity of adversity, 92 others do not. Therefore, identified epigenetic differences may reflect true resilience ...
The University of Florida Research Foundation has named 34 of the university's most productive and promising faculty members as UFRF Professors for 2024, among them, PRICE affiliates Larisa Cavallari, Stephen Anton and Debra Lyon. ... Congratulations! New Article on Food Insecurity and Epigenetic Aging. Epigenetic aging has to do with DNA and ...
Epigenetics is a promising field of research because of the potential to regulate gene expression without changing the DNA sequence, which may likely cause safety and ethical concerns if performed in humans. The most promising way to treat diseases through epigenetic regulation has been through pharmacology. Previous clinical trials for drugs ...
Epigenetic reprogramming resets parental epigenetic memories and differentiates primordial germ cells (PGCs) into mitotic pro-spermatogonia or oogonia, ensuring sexually dimorphic germ-cell ...