

Recent Advances in Animal Nutrition and Metabolism
- © 2022
- Guoyao Wu 0
Department of Animal Science, Texas A&M University, College Station, USA
You can also search for this editor in PubMed Google Scholar
- Covers hot topics in the nutrition and metabolism of terrestrial and aquatic animals
- Addresses the use of new genome-editing biotechnologies to generate animals as bioreactors
- Highlights the use of animals as models in biomedical research to prevent and treat human diseases
Part of the book series: Advances in Experimental Medicine and Biology (AEMB, volume 1354)
50k Accesses
250 Citations
8 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
This book covers hot topics in the nutrition and metabolism of terrestrial and aquatic animals, including the interorgan transport and utilization of water, minerals, amino acids, glucose, and fructose; the development of alternatives to in-feed antibiotics for animals (e.g., swine and poultry); and metabolic disorders (or diseases) resulting from nutrient deficiencies. It enables readers to understand the crucial roles of nutrients in the nutrition, growth, development, and health of animals. Such knowledge has important implications for humans.
Readers will also learn from well-written chapters about the use of new genome-editing biotechnologies to generate animals (e.g., cows and swine) as bioreactors that can produce large amounts of pharmaceutical proteins and other molecules to improve the health and well-being of humans and other animals, as well as the growth and productivity of farm animals. Furthermore, the book provides usefulinformation on the use of animals (e.g., cattle, swine, sheep, chickens, and fish) as models in biomedical research to prevent and treat human diseases, develop infant formulas, and improve the cardiovascular and metabolic health of offspring with prenatal growth restriction.
Editor of this book is an internationally recognized expert in nutrition and metabolisms. He has about 40 years of experience with research and teaching at world-class universities in the subject matters. He has published more than 660 papers in peer-reviewed journals, 90 chapters in books, and authored two text/reference books, with a very high H-index of 127 and more than 66,000 citations in Google Scholar.
This publication is a useful reference for nutrition and biomedical professionals, as well as undergraduate and graduate students in animal science, aquaculture, zoology, wildlife, veterinary medicine, biology, biochemistry, food science, nutrition, pharmacology, physiology, toxicology, and other related disciplines. In addition, all chapters provide general and specific references to nutrition and metabolism for researchers and practitioners in animal agriculture (including aquaculture), dietitians, animal and human medicines, and for government policy makers.
Similar content being viewed by others

Amino Acid Nutrition for Optimum Growth, Development, Reproduction, and Health of Zoo Animals
Nutrigenomics in livestock—recent advances
Amino acids in swine nutrition and production.
- Animal Production
Table of contents (17 chapters)
Front matter, nutrition and metabolism: foundations for animal growth, development, reproduction, and health, insights into the regulation of implantation and placentation in humans, rodents, sheep, and pigs.
- Claire Stenhouse, Heewon Seo, Guoyao Wu, Gregory A. Johnson, Fuller W. Bazer
A Role for Fructose Metabolism in Development of Sheep and Pig Conceptuses
- Robyn M. Moses, Avery C. Kramer, Heewon Seo, Guoyao Wu, Gregory A. Johnson, Fuller W. Bazer
Nutritional Regulation of Embryonic Survival, Growth, and Development
- Lawrence P. Reynolds, Kyle J. McLean, Kacie L. McCarthy, Wellison J. S. Diniz, Ana Clara B. Menezes, J. Chris Forcherio et al.
Phosphate, Calcium, and Vitamin D: Key Regulators of Fetal and Placental Development in Mammals
- Claire Stenhouse, Larry J. Suva, Dana Gaddy, Guoyao Wu, Fuller W. Bazer
Nutritional and Physiological Regulation of Water Transport in the Conceptus
- Cui Zhu, Zongyong Jiang, Gregory A. Johnson, Robert C. Burghardt, Fuller W. Bazer, Guoyao Wu
Amino Acids in Microbial Metabolism and Function
- Zhaolai Dai, Zhenlong Wu, Weiyun Zhu, Guoyao Wu
Potential Replacements for Antibiotic Growth Promoters in Poultry: Interactions at the Gut Level and Their Impact on Host Immunity
- Christina L. Swaggerty, Cristiano Bortoluzzi, Annah Lee, Cinthia Eyng, Gabriela Dal Pont, Michael H. Kogut
Microbiomes in the Intestine of Developing Pigs: Implications for Nutrition and Health
- Chunlong Mu, Yu Pi, Chuanjian Zhang, Weiyun Zhu
L-Arginine Nutrition and Metabolism in Ruminants
- Guoyao Wu, Fuller W. Bazer, M. Carey Satterfield, Kyler R. Gilbreath, Erin A. Posey, Yuxiang Sun
Hepatic Glucose Metabolism and Its Disorders in Fish
- Xinyu Li, Tao Han, Shixuan Zheng, Guoyao Wu
Protein-Sourced Feedstuffs for Aquatic Animals in Nutrition Research and Aquaculture
- Sichao Jia, Xinyu Li, Wenliang He, Guoyao Wu
Functional Molecules of Intestinal Mucosal Products and Peptones in Animal Nutrition and Health
- Peng Li, Guoyao Wu
Use of Genome Editing Techniques to Produce Transgenic Farm Animals
- Alayna N. Hay, Kayla Farrell, Caroline M. Leeth, Kiho Lee
Cows as Bioreactors for the Production of Nutritionally and Biomedically Significant Proteins
- P. S. Monzani, P. R. Adona, S. A. Long, M. B. Wheeler
Use of Agriculturally Important Animals as Models in Biomedical Research
- Brandon I. Smith, Kristen E. Govoni
Pigs ( Sus Scrofa ) in Biomedical Research
- Werner G. Bergen
Back Matter
Editors and affiliations, department of animal science, texas a&m university, college station, usa, about the editor.
Guoyao Wu is Distinguished Professor, University Faculty Fellow, and AgriLife Research Senior Faculty Fellow in the Department of Animal Science. He also holds appointments with the Graduate Faculty of Nutrition, the Departments of Systems Biology and Translational Medicine and Veterinary Integrative Biosciences.
Dr. Wu teaches graduate courses in protein metabolism and nutritional biochemistry. He conducts research in protein and amino acid metabolism at molecular, cellular, andwhole body levels . The animal models used in his research include cattle, chicks, pigs, rats, mice, fish, shrimp, and sheep.
Professional memberships include the American Society of Animal Science, the American Society for Nutritional Sciences, Society for Study of Reproduction, American Association for the Advancement of Sciences, and American Heart Association. Dr. Wu currently serves on the Editorial Board of the Journal of Nutritional Biochemistry. He is Editor of “Amino Acids” and “Frontiers in Bioscience.”
Bibliographic Information
Book Title : Recent Advances in Animal Nutrition and Metabolism
Editors : Guoyao Wu
Series Title : Advances in Experimental Medicine and Biology
DOI : https://doi.org/10.1007/978-3-030-85686-1
Publisher : Springer Cham
eBook Packages : Biomedical and Life Sciences , Biomedical and Life Sciences (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2022
Hardcover ISBN : 978-3-030-85685-4 Published: 23 November 2021
Softcover ISBN : 978-3-030-85688-5 Published: 24 November 2022
eBook ISBN : 978-3-030-85686-1 Published: 22 November 2021
Series ISSN : 0065-2598
Series E-ISSN : 2214-8019
Edition Number : 1
Number of Pages : VI, 346
Number of Illustrations : 16 b/w illustrations, 27 illustrations in colour
Topics : Biomedical Engineering/Biotechnology , Biochemistry, general , Animal Physiology , Agriculture
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Nutrigenomics in livestock sector and its human-animal interface-a review
Zulfqar ul haq, afnan saleem, azmat alam khan, mashooq ahmad dar, abdul majeed ganaie, yasir afzal beigh, heena hamadani, syed mudasir ahmad.
- Author information
- Article notes
- Copyright and License information
Corresponding author. [email protected]
Collection date 2022 Sep.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Nutrigenomics unfolds the link between nutrition and gene expression for productivity.expression profile of intramuscular.
Nutrigenomics helps scientists discover genes and DNA in each animal's cell or tissue by assisting them in selecting nutrients.
It brings out the importance of micronutrition for increasing animal production.
Nutrigenomics integrates nutrition, molecular biology, genomics, bioinformatics, molecular medicine, and epidemiology.
Keywords: Nutrigenomics, Humans, Livestock, Proteomics, Metabolomics, Lipidomics
Noncommunicable diseases such as cardiovascular disease, obesity, diabetes, and cancer now outnumber all other health ailments in humans globally due to abrupt changes in lifestyle following the industrial revolution. The industrial revolution has also intensified livestock farming, resulting in an increased demand for productivity and stressed animals. The livestock industry faces significant challenges from a projected sharp increase in global food and high animal protein demand. Nutrition genomics holds great promise for the future as its advances have opened up a whole new world of disease understanding and prevention. Nutrigenomics is the study of the interactions between genes and diet. It investigates molecular relationships between nutrients and genes to identify how even minor modifications could potentially alter animal and human health/performance by using techniques like proteomics, transcriptomics, metabolomics, and lipidomics. Dietary modifications mostly studied in livestock focus mainly on health and production traits through protein, fat, mineral, and vitamin supplementation changes. Nutrigenomics meticulously selects nutrients for fine-tuning the expression of genes that match animal/human genotypes for better health, productivity, and the environment. As a step forward, nutrigenomics integrates nutrition, molecular biology, genomics, bioinformatics, molecular medicine, and epidemiology to better understand the role of food as an epigenetic factor in the occurrence of these diseases. This review aims to provide a comprehensive overview of the fundamental concepts, latest advances, and studies in the field of nutrigenomics, emphasizing the interaction of diet with gene expression, and how it relates to human and animal health along with its human-animal interphase.
1. Introduction
The production of an adequate amount of nutritious animal protein for the world's growing population is a challenge faced globally. By 2050, the world's population is expected to reach 9.4 billion ( Fukase & Martin, 2020 ; Molotoks, et al., 2021 ), and the global food supply will also increase at an annual rate of 2.3% to meet the growing demand of the increasing population ( Tian et al., 2021 ). Animal protein makes up an essential part of the human diet globally, but its demand, especially in developed countries, is expected to increase by many folds with the increase in the GDP of these countries ( Sun & Guan, 2018 ). An 11% increase in the global population is expected to support a 14% increase in global meat consumption by 2030 (Aduli et al., 2022). Furthermore, the livestock industry has been using antibiotics indiscriminately to increase production, which has led to the emergence of antimicrobial-resistant bacteria and the spread of antimicrobial resistance genes, a global health concern ( Kimura et al., 2022 ). Therefore, the only logical way out appears by increasing per unit plant and animal productivity, which will allow us to meet the growing nutrient demand of our population ( Barros-Rodríguez, et al., 2021 ).
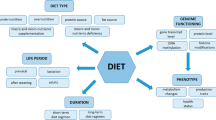
Nutrigenomics is a short-term assessment of genome activity in response to dietary changes, while nutrigenetics is the study of population diversity ( Malgwi, Halas, Grünvald, Schiavon & Jócsák, 2022 ). Interactions using a variety of genome sequences include microarray (transcriptomics), proteomics, metabolomics, and epigenomics ( Fig. 1 ) ( Pal, 2022 ).
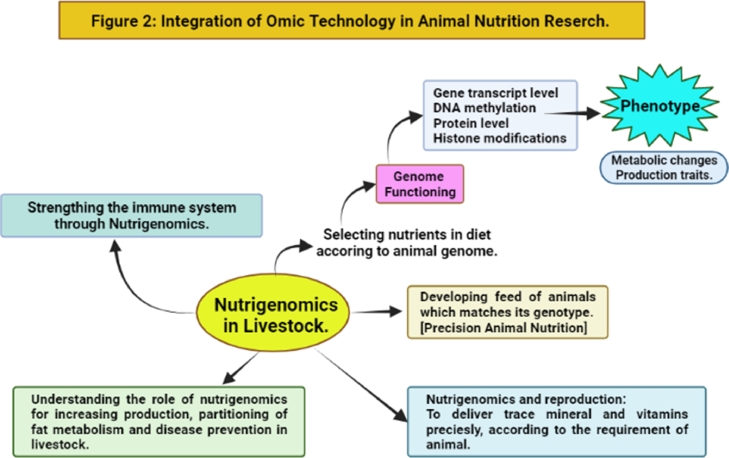
Integration of omic technology in animal nutrition research.
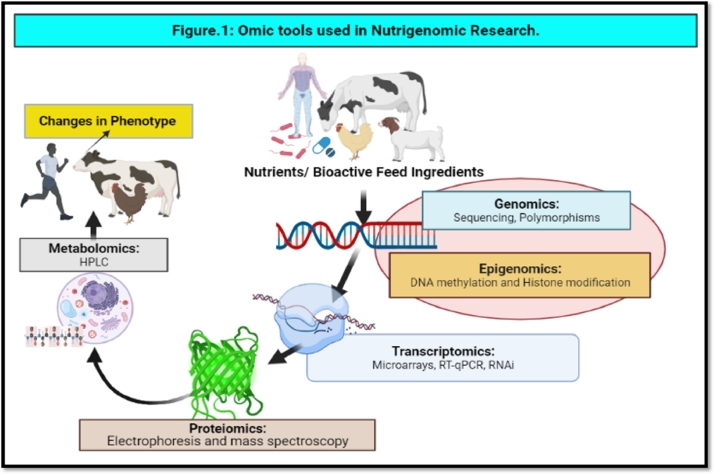
Omic tools used in nutrigenomic research.
However, this requires introducing modern production technology to improve animal husbandry, such as precision animal husbandry using molecular methods ( Hasan, Feugang & Liao, 2019 ). As we all know, the animal kingdom is a sequence of gene expression pathways. Nutrition is the driving force behind these processes, including the fact that they are genetic. Therefore, animal nutrition study aims to thoroughly comprehend how nutrients influence animals' genetic programming throughout their lifetimes ( Wu, 2022 ). Nutrigenomics (molecular animal nutrition) is a relatively recent topic that deals with animal nutrition on a molecular level ( Pal, 2022 ). The goal is to use contemporary molecular biological sciences and technology to examine animal biological processes at the gene expression level or to examine the interactions between nutrients and genes ( Malgwi et al., 2022 ). In the end, it is about studying the complex bi-directional process interactions between nutrients and genes that will clarify the fundamental molecular mechanisms that regulate the expression of nutrients in the diet of the animal genes, the biochemical reaction, as well as the physiological and phenotypic processes ( Ahluwalia, 2021 ).
Nutrigenomics has effect on dietary ingredients that results changes in gene expression patterns and epigenetic modifications, such as histone modifications and DNA methylation ( Bordoni & Gabbianelli, 2019 ; Mosca, Leheup & Dreumont, 2019 ). Like genomic imprinting, they rely on epigenetic pathways that are very sensitive to dietary changes. For example, developmental traits such as insulin-like growth factor 2 (IGF2) genes are printed on pigs which promote increased muscle mass and fat storage ( Ding et al., 2022 ). In the past decade, the number of microRNA precursors found in miRBase of cattle has increased three times ( Ibeagha-Awemu & Zhao, 2015 ).
Nutrigenomics studies were conducted in humans and mice to study the molecular base of diseases like obesity, cardiovascular diseases, and cancer to understand them through interactions between genes and diet ( Ahluwalia, 2021 ; Pal, 2022 ). Conventionally, rodent models have been used in research to examine the effects of diet on genome function. Cross-generational studies and measurement of specific life stages (such as pregnancy) have also been performed on mice and rodents. The hypothesis that the mother's diet will affect the genome structure of the offspring has recently been studied in mice ( Chmurzynska et al., 2018 ). Although similar farm animal methods are still in their infancy, nutrigenomics research is booming, especially in the meat industry, due to health reasons as meat products directly impact human health (Nowacka-Woszuk, 2020).
Nutrigenomics is not a new subject, but a technology with such a promise for the future warrants periodic reviews and updates to inspire thinking and novel approaches. This review aims to provide a comprehensive overview of the fundamental concepts, latest advances, studies in the field of nutrigenomics, emphasizing the interaction of diet with gene expression, how nutritional intervention can change the tissue composition of animals, as well as how it relates to human health along with its human-animal interphase.
2. Nutrigenomics in the livestock sector
The livestock industry is one of the primary sources of livelihood for the farmers in India. Around 60–70% of the total inputs for livestock production are in the form of animal feed. Thus, the livestock industry economics largely depends upon the feed utilization efficiency of the animals ( Rauw et al., 2020 ). The complex and highly diversified rumen microbial ecosystem act symbiotically and synergistically for efficient bio-fermentation within the rumen. In the rumen, efficient anaerobic systems exist to degrade lignocellulosic feeds. However, rumen microbes can extract only a fraction of potential energy from lignocellulosic feeds ( Liang et al., 2020 ). Losing feed energy leads to significant economic losses for the livestock industry. Rumen microbiologists and nutritionists have been making efforts to minimize the losses and decode the complexity of the microbial ecosystem. The molecular era and advanced molecular techniques apply to resolve un-explained quarries of nutrition at the gene level. The field of nutrigenomics paves a way by which nutritional diseases/ metabolic disorders can be prevented or clinically managed. Differential gene expression studies will allow the identification of candidate genes and the pathways accountable for economically important traits. In the changing nutrition scenario, nutrigenomics examines the impact of nutrition on gene expression or regulatory systems that could be linked to various biological processes affecting animal health and production ( Asmelash, Mahlet & Brhane, 2018 ).
For years, functional genomic methods like transcriptomics, proteomics, and metabolomics have been hailed as having immense potential for advancing nutritional science by offering a new understanding of diet-gene interactions and discovery of novel nutritional biomarkers ( Akanbi, 2019 ). It can be said that genomics summarizes the instructions given by DNA, whereas transcriptomics is concerned with the pattern of gene expression. The study of complex protein products and their interactions is called proteomics, whereas a step toward comprehending an organism's entire metabolism is called metabolomics ( Muthubharathi, Gowripriya & Balamurugan, 2021 ). Technologies available for practical genomic testing are constantly evolving and improving. The field of nutrigenomic research is continually expanding due to this development. The relationship between nutrition and genes can be assessed using DNA microarray methods, real-time quantitative polymerase chain reaction (PCR), and DNA sequencing. Chip technology now enables the sequencing of many genes and the development of an accurate understanding of changes in gene expression patterns ( Zduńczyk et al., 2009 ).
Contrary to expensive transgenesis ( Bruh et al., 2017 ), nutrigenomics can be successfully and cheaply used to increase animal production, growth rate, milk production, feed utilization, disease resistance, and fertility (Figure: 2). Genomics can help bridge the gap between the genetic map of animal husbandry and feeding, improving transformation efficiency and productivity. In the past, nutrigenomics studies have used approaches like Differential Display PCR and Real-Time Quantitative PCR to monitor the expression of some genes. Modern advances in genome analysis offer more effective solutions to a wide variety of biological problems. Thanks to the discovery of microarrays and massive transcriptome sequencing (RNAseq), it is now possible to analyze gene expression on a large scale ( Benítez, Núñez, Óvilo & Ovilo, 2017 ). These innovative technologies were based on the idea of accumulating high-throughput data by automating and parallelizing protein and DNA/RNA synthesis. The most extensively utilized omics methods for gathering proteomics, transcriptomic, and metabolomic data have been created. However, scientists may now use current systemic methodologies to examine interactions occurring within living animals using developing bioinformatic tools in tandem with biological data provided by genomics and transcriptomics ( Manzoni et al., 2018 ). Nutrigenomics investigates the effects and interactions of nutrition on gene expression using large-scale gene expression methods ( Hasan et al., 2019 ). Researchers can learn more about the molecular basis of observed phenotypic differences within organisms by studying gene expression in animals subjected to different nutritional conditions and the metabolic pathways directly involved in regulating tissue structure, thus contributing to tissue quality.
2.1. Transcriptomics
With technical advancements in the microarrays and RNA sequences, the expression of virtually all the transcribed genes in a sample could be quantified, hence the transcriptome. A transcriptome is a snapshot of all the transcripts present in a cell at a particular point in time ( Lowe, Shirley, Bleackley, Dolan & Shafee, 2017 ). Transcriptome research has improved our understanding of the RNA-based gene regulatory network as next-generation high-throughput sequencing technology. For the first time in small ruminants, a nutrigenomic analysis of goat mammal transcriptome responses was performed in feed-deprived goats in 2007 ( Osorio, Vailati-Riboni, Palladino, Luo & Loor, 2017 ). This research showed the genes responsible for reductions of milk fat, lactose, and protein, genes responsible for the impediment in the proliferation and differentiation of mammalian cells, and the increase in programmed cell deaths contribute to the early mammalian involution. RNA sequencing has been in use since 2011 and has recently replaced microarray systems in omics science. This technology was used to study the transcriptome and the micro RNAome from small ruminants such as goats and sheep ( Martyniuk et al., 2020 ).
RNA-seq with high throughput has become the method of choice for animal nutritionists to set up a framework for developing feeds or feed additives, promoting animal growth, health, and production. This method is being used to investigate global gene expression patterns in tissues linked to economically significant features like feed effectiveness ( Alexandre et al., 2015 ) or quantitative determination of the genes or transcripts, which can be used as potential biomarkers for production traits ( Han, Gao, Muegge, Zhang & Zhou, 2015 ). Indeed, recent advances in farm animal genome sequencing, such as swine, cattle, and sheep, have given essential reference genomes for aligning RNA-Seq short reads to assess changes in gene expression in response to dietary nutrients ( Hasan et al., 2019 ; Santos, Blanck & de Freitas, 2014 ; Wickramasinghe, Rincon, Islas-Trejo & Medrano, 2012 ; Wang et al., 2009). In conclusion, even though its application faces some technical challenges in both experimental and computational aspects, the novel RNA-Seq technology for transcriptomics analyses of living organisms is considered a powerful approach to better understanding molecular mechanisms in terms of nutrient–gene interactions.
2.2. Proteomics
The word "proteomics" was coined in 1995, describing it as the broad-scale characterization of cell lines, the tissue's or organism's entire protein complement, simply defining it as a study of proteins, chiefly their function and structure ( Hossain et al., 2020 ). Proteomics is a crucial component of nutrigenomics, which aims to comprehend how our genome is expressed in response to nutrition on a holistic level. The main objective of proteomics is to achieve a more global and integrated view of biology by analyzing all of a cell's proteins at a time. The application of proteomics in livestock has been limited in past due to a lack of optimized protocols and high cost ( Baykalir, Baykalir & Simsek, 2018 ). However, new computational tools and analytical methods for the analysis of proteomic data have led to an increased understanding of animal health, production, and reproduction efficiency ( Kaya et al., 2022 ; Ye et al., 2022 ).
Proteomics-based approaches are used in a variety of research environments, identifying multiple diagnostic targets, vaccine candidates, pathogenicity factors, interpreting functioning protein pathways in various disorders, as well as variations in expression patterns in response to diverse signals ( De Sousa et al., 2018 ; Katsarou et al., 2021 ). The proteomic scope has advanced from protein recognition and characterization to protein-protein interactions, protein structure, and function since the human genome sequencing. The technologies used in proteomic research include mass spectrometry (MALDI-MS, ESI-MS, LC-MS/MS, MALDI-TOF MS, etc.) measuring mass to charge (m:z ratio) of ionized peptides for protein identification, yeast two-hybrid screens, one-dimensional (ID) gel electrophoresis, 2-dimensional (2D) gel electrophoresis, Chromatography (gas and liquid) and computational prediction programs ( Sharma, Ray & Mahapatra, 2022 ).
The use of proteomic approaches for studying reproductive problems has dramatically increased in the past decade ( Peddinti, Memili & Burgess, 2011 ). Targeting robust protein markers is useful in disease monitoring, surveillance, health monitoring, and overall well-being of the animal which will help assess pharmacologic response to therapeutics ( Muhanguzi et al., 2022 ). Proteomics is used to identify genetic variants with traits desirable for breeding and selection for various animal products post-harvest like milk, meat, cheese, etc. (Almedia et al., 2015).
Proteomic approaches to farm animals have been applied to improve animal welfare to prevent a negative effect on animal production and human health besides clinical management & treatment of human diseases ( Ceciliani, Eckersall, Burchmore & Lecchi, 2014 ). Since the animal genome has not been fully sequenced as in humans, the serum proteome analysis and characterization in most farm animals are in the preliminary phase ( Di Girolamo et al., 2014 ). Protein and metabolites level expression measurement provides new ways and knowledge to determine and manage the nutrient balance in farm animals. Hypotheses for such studies can be translated from similar human studies which help to optimize diet composition and dietary nutrient supply in defined populations of livestock species or individual animals ( Asmelash et al., 2018 ).
In farm animals, proteomics could help evaluate the risk of zoonosis, increase productivity and fertility, and improve the safety and quality of animal products thus, reducing financial losses as well. The proteomic investigation has been applied to veterinary and animal sciences over the last decade but it has still not reached its full potential in animal health and production. Quantitative proteomic investigation of biofluids and tissue samples from cattle, pigs, sheep, chickens, cats, and dogs has helped to identify potential novel biomarkers like in an experimental model of Streptococcus uberis mastitis of dairy cows. Milk proteins have potential as disease biomarkers, like haptoglobin, cathelicidin, and mammary-associated serum amyloid A3. These markers can help diagnose subclinical as well as clinical mastitis. On similar lines, proteomic analysis of chicken plasma following stimulation of the inflammatory response to E. coli LPS reveals major changes in the plasma proteome ( Eckersall, 2020 ).
Proteomics also enables the identification of candidate protein markers of fertility for molecular breeding. In a recent study on Tibetan pigs having heritable adaptation to hypoxic environments, eight fertility-related proteins were overexpressed by LC-MS/MS analysis ( Zhao et al., 2021 ). Analysis of seminal plasma proteins by LC-MS/MS found a significant correlation between proteins with fertility ( Willforss et al., 2021 ). The application of proteomics can help identify fertile bulls to increase productivity and reduce the non-return rate (NRR). For selective breeding, proteomic tools are utilized to identify superior genetic variants. Mullins et al. (2021) have identified protein markers by proteomics which will help in the selection of genetic variants, accelerate genetic gain and increase profitability ( Mullins et al., 2021 ).
The last decade has marked proteomics as the main forum for interviewing plasma/serum proteomics for exploration of next-generation biomarkers in the veterinary and human sectors, with introducing standard pre-analytical sample therapies, MS-based systems, and data processing systems for data management ( Castelli et al., 2021 ; Ghodasara, Satake, Sadowski, Kopp & Mills, 2022 ). Thanks to the vast number of serum proteins that can be investigated concurrently, the proteomic approach is quicker than the ELISA and PCR methods. However, the high initial cost needed to purchase the equipment is a significant barrier to the broader application of the technique. However, the costs incurred have quickly decreased, thus preferring their procurement ( Nicholson et al., 2012 ). The discovery of next-generation biomarkers that help a range of human and farm animal disease diagnoses and new proteomic technologies can significantly improve surveillance.
2.3. Metabolomics
Metabolomics deals with screening the small molecule metabolites in biological samples like animals, plants, and microbes ( Baharum et al., 2018; ; Bishai et al., 2021 ). By comparing the metabolome profiles (metabolic phenotypes or 'metabotypes'), one can establish patterns of variation among different groups, i.e., control versus treated, healthy versus diseased. Metabolomics in livestock has made significant advancements to livestock welfare, reproduction, and development, as well as the detection of biomarkers for transformation diseases ( Sun et al., 2015 ), production traits in dairy ( Goldansaz et al., 2017 ), and beef cattle ( Miggiels, Wouters, van Westen, Dubbelman & Hankemeier, 2019 ), all to incorporate prognostic techniques in animal health and improve prediction accuracies. In addition, many bio-fluids have been investigated for metabolomics research, including metabolic profiling of plasma, milk, urine, and serum, which has abridged animal welfare concerns ( Gowda et al., 2008 ).
Currently, two fundamental techniques are being used in metabolomics, i.e., nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). However, mass spectrometry is often coupled together with a separation technique, such as gas chromatography (GC), liquid chromatography (LC), or capillary electrophoresis (CE). Nuclear magnetic resonance (NMR) is now more advanced than mass spectrometry (MS) for high-throughput analysis in terms of analysis time and cost per sample. The NMR spectrum usually takes 5 to 8 min, while the LC analysis takes 5 to 30 min Wishart, Feunang and Marcu (2018) . Due to its high sensitivity, mass spectrometry (MS) has a broader range of coverage. It has potential performance advantages in terms of metabolites per minute, which explains why more innovative technologies than NMR have been developed in this field ( Zampieri, Sekar, Zamboni & Sauer, 2017 ). For metabolomics, there have been recent technical advancements and movements toward quicker and more comprehensive processing using a smaller number of samples at a low cost-per-sample (more for less) ( Wishart et al., 2018 ). Increasing data exchange initiatives and standard data reporting will make it easier to implement fully automated (open source) data analysis systems to analyze the vast amounts of data collected from omics studies to provide novel biological insights ( Wang, Yang & Harris, 2016 ).
The most formidable challenge in metabolomics research is integrating data with other omics and phenotypic data ( Balkir, Kemahlioglu & Yucel, 2021 ). A more conscientious approach in the design and implementation of metabolome studies would be to investigate a wider variety of samples such as saliva, urine, amniotic fluid, and semen, along with expanding the types and number of animal breeds ( Goldansaz et al., 2017 ).
2.4. Lipidomics
Meat production worldwide has recently concentrated on improving meat quality in fatty acid profile and marbling. Induced marbling requires a better understanding of the pathways that regulate fat formation ( Ladeira et al., 2018 ). Vitamin A has recently been discovered to positively impact almost all adipogenesis stages in animals. Retinoic acid is the active metabolite of vitamin A, which can induce retinoic acid receptor (RAR) and retinoid X receptor (RXR), leading to epigenetic mutations in the central regulatory genes of adipogenesis ( Chen, 2021 ). Retinoic acid, vitamin D, and folate receptors regulate adipogenesis and reduce lipid accumulation. Vitamin D interacts with retinoic acid receptor signals to change the development and differentiation of adipose tissue ( Szymczak-Pajor, Miazek, Selmi, Balcerczyk & Śliwińska, 2022 ). Further, dietary manipulation during specific periods of pregnancy, like in the prenatal period, nutrients acts not only on the developing fetus but also on the primary germ cells that give rise to the next generation ( Dimofski, Meyre, Dreumont & Leininger-Muller, 2021 ). Thus, feeding specific nutrients will have both short-term and long-term consequences.
By using these newly developed methods, functional lipidomics can help profile and understand the unique functions of each lipid subtype ( Ferguson et al., 2016 ). Lipidomics can be applied directly to human plasma, potentially identifying novel and predictive disease biomarkers such as coronary disease progression and responsiveness to treatment. Considering that many specific lipid species are synthesized in vivo from dietary precursors, lipidomics provides a promising method for investigating the relationship between dietary lipids and cardiometabolic risk, allowing the identification of lipid biomarkers.
3. Impact of the relationship between nutrients and genome in animal health and production
There has been a prodigious increase in facing the substantial challenges from a steep projected increase in global demand for food and high-quality animal proteins, besides adapting to environmental stresses. Nutrigenomics research aims to explain the molecular interactions between genes and diet and identify necessary molecular signatures to develop effective nutritional strategies. Most nutrigenomics studies have concentrated on human nutrition and its consequent effects on disease etiology ( Reddy, Palika, Ismail, Pullakhandam & Reddy, 2018 ). Studies of gene expression analysis are carried out in response to dietary regimens because the biochemical parameters depend on the particular gene transcripts ( Mierziak et al., 2021 ). In dairy cattle, the experiments are focused on milk yield and composition.
In contrast, in beef cattle, experiments are focused on obtaining the fatty acid profile in muscle tissue and thus modulating specific diets. Animal health is essential in both pigs and poultry, so supplements such as amino acids, vitamins, and prebiotics are used, affecting the transcription of genes and playing a vital role in improving the immune system function ( Alagawany et al., 2021 ).
The effects of diet on genome functioning have usually been performed on experimental models like pigs, rats, and mice. Studies of specific life periods (pregnancy) have been conducted in rats and mice ( Chmurzynska et al., 2018 ), and the phenomenon of fetal programming, wherein maternal nutrition affects progeny genome functioning, has also been studied in rodents ( Reynolds & Vickers, 2018 ). Similar approaches to farm animals are still in their infancy stage. However, the nutrigenomics concepts have gained much importance, especially in the meat production industry and in improving the health of animals. A recent study by Kang, Madkour and Kuenzel (2017) demonstrated that psychological and early life nutritional stress on birds modulated vital regulators of DNA methylation. In another study, a link between nutrients and bovine genotype and the enteric biome in acidosis challenging conditions has been seen. Several putative QTL were identified for metabolites like butyrate and lactate, acidosis, and host-microbiome interactions ( Golder, Thomson, Denman, McSweeney & Lean, 2018 ). In broilers, a diet rich in Morinda Citrifolia (Noni) was reported to alleviate heat stress by modulating hypothalamic orexin-AMPK-mTOR pathways ( Rajaei-Sharifabadi et al., 2017 ). These studies suggested the ability of nutrition-genome interaction in modulating and changing the future of dietary guidelines by identifying key molecular markers for personalized nutrition approaches.
4. Epigenetic control on livestock production
The study of nutritional epigenetics relates to gene-diet interactions ( Lucini et al., 2020 ). Nutritional epigenetics in livestock production is still in its infancy stage. However, a foundation of nutritional epigenetics research has been established and validated. Diet might play a regulatory role in the epigenetic makeup of the first-generation consumer, but it also affects progeny performance. Due to the rapid reproductive cycles of swine and poultry and the large number of offspring produced may be a landmark for the industry ( Roberts et al., 2019 ).
There are multiple genes and environment interactions while considering the phenotypic variation. One of the critical environmental players in these phenotypes is nutrient quality and quantity. For example, nutrient-induced epigenetic events can be found in honeybees and mice (Kucharski et al., 2008).
Nutritional phenotypes are primarily studied from the growth, feed intake, and efficiency perspectives. In livestock and genome-wide nutritional epigenomics studies ( Shin et al., 2014 ), associations between epigenetic modifications and nutritional phenotypes have been well documented ( Shin et al., 2014 ). Associations between epigenetic mechanisms, genetic variation, and subsequently economically important phenotypes have been identified in livestock. One of the overarching goals is to investigate the economically essential traits to determine the extent to which phenotypic variation can be accounted for by genetic variation in the livestock genomics community. Nevertheless, to comprehend a mechanistic link between phenotype and genotype, tools must be generated that will allow us to interrogate the epigenetic modifications. In the past, epigenetic studies in livestock animals have utilized DNA methylation-based next-generation sequencing methods to investigate the role of methylation in the phenotypic variation of their economically important trait ( Boddicker et al., 2016 ). A high-resolution DNA methylation array has been used in humans ( Bibikova et al., 2011 ). Using the same technology for animals will permit EWAS and ultimately enable epigenetic modifications for livestock production and breeding ( Rolf et al., 2014 ).
4.1. Epigenetic control of Adipose tissue
Reversible inhibition of lineage-specific genes allows stem cells and progenitor cells to retain pluripotency or multipotency while promoting gene expression involved in stem cell self-renewal. However, lineage-specific genes are released during separation, while pluripotency and multipotency genes are inhibited ( Reik et al., 2007 ). A transcription factor and lineage-specific gene are triggered to enable progenitor cells to commit to a particular lineage when a central development gene is activated ( Aloia, Di Stefano & Di Croce, 2013 ).
Zfp423 is one of the leading CpG-rich genes in the development and is primarily controlled by epigenetic modifications ( Yang et al., 2013 ). Histone modifications and DNA methylation are also examples of epigenetic modifications. Polycomb inhibition complex (PRC) is responsible for reversible gene suppression by catalyzing histone methylation. PRC1 and PRC2 are two PRCs that have been thoroughly studied. The PRC2senhancerZeste 2 (EZH2) is a crucial component ( Qi et al., 2012 ) that mediates the trimethylation of the gene silencing marker histone 3-lysine 27 (H3K27me3) ( Mendenhall, Koche & Truong, 2010 ). It has not been proven that PRC2 has specific DNA binding properties, but it prefers to bind to promoters with many CpG sites to attract PRC1 binding. In the absence of PRC release stimulation, these promoters are usually methylated in DNA (Shankar et al., 2020 Shankar et al., 2020 ; Mackin et al., 2019 ).
Environmental and genetic factors influence the complexities of these epigenetics of regulation in critical developing genes. Gene polymorphisms in essential developed genes modify the binding of epigenetic modulation complexes and influence the cell lineage interplay of the progenitor during development ( Murdoch, Murdoch, Greenwood & McKay, 2016 ). In addition, environmental stimuli and indications, such as nutrients influence animal development by modifying pathologies for cellular signage or by recruiting epigenetic transcription factors, such as adipogenesis.
4.2. Role of B-complex vitamins and methyl donors
Epigenetic changes are histone and methylation of DNA, but unless methyl donors help, methylation is unlikely to happen. Using S-adenosyl methionine, DNA's methyl methionine (SAM) adds a methyl group to DNA (SAM). Following transfer to DNA or histones, SAM is converted into S-adenosylhomocysteine (SAH). This operation is supported by folic acid, vitamin B12, choline, and concrete nutrients. Food methylation, folic acid, vitamin B12, and choline, betaine promote DNA and histone methylation of genes reliably ( Bellner et al., 2015 ). Folic acid and Vitamin B12 therapy inhibit adipogenic development in 3T3-L1 cells via modulating Wingless and Int (Wnt) 10b ( Cordero, Gomez-Uriz, Campion, Milagro & Martinez, 2013 ). Burton et al., 2019 suggested the influence of methyl donors on adipogenesis through epigenetic alterations in processing genes.
5. Adipogenesis and the role of vitamin A, retinoic acid, and vitamin D receptors
Various lipid signaling components, such as steroid hormones, retinoids, thyroid hormones, and Vitamin D metabolites activate intracellular nuclear receptors. Ligand-activated transcription factors bind to their corresponding DNA components to stimulate gene expression. Adipogenesis is summarized in the following section by discussing the effects of Vitamin D and retinoids. All-trans retinoic acid is produced when dietary vitamin A is absorbed and extracted. Retinoic acid acts as a ligand for retinoic acid receptors (RAR). They will bind to retinoic acid reaction elements (RAREs) on target genes via retinoid X receptors (RXR). By activating the orphan receptor PPAR, retinoic acid promotes cell proliferation, lipid breakdown, and lipid oxidation ( Osz et al., 2020 ; Rochette-Egly 2020 Rochette-Egly et al., 2020 ). The partitioning of retinoic acid between RAR and PPAR will determine the biological consequences of retinoic acid. CRABPII and fatty acid-binding protein type 5 (FABP5) are cellular retinoic acid-binding proteins that govern retinoic acid partitioning, with CRABPII delivering retinoic acid to RAR and FABP5 supplying retinoic acid to PPAR/. A high CRABP-II/FABP5 is expressed in adipogenic progenitor cells, leading to RAR signaling dominance. CRABP-II and RAR down-regulate during adipogenic differentiation, whereas FABP5 and PPAR/ up-regulate, activating PPAR/signaling in mature adipocytes. Because of the stage-specific expression of associated transcription factors, retinoic acid has differing effects on progenitor cells and mature adipocytes ( Abdelhamed, El-Dawla, Karadag, Agamia & Melnik, 2021 ; Ladeira et al., 2018 ).
Vitamin D is considered to control adipogenesis and its roles in calcium balance and bone metabolism. In obesity, vitamin D has been reported to affect insulin secretion, tissue sensitivity to insulin, and ultimately, systemic inflammation. The direct and paracrine effects of vitamin D led to VDR activation in pancreatic beta-cells, CYP27B1 expression, and local synthesis of 1,25(OH)2D ( Maestro, Dávila, Carranza & Calle, 2003 ). Polymorphisms reduce obesity and diabetes in genes involved in Vitamin D production and signaling. In both in vivo and in vitro investigations, vitamin D has been shown to decrease adipogenesis. The active metabolite of vitamin D, 1,25-dihydroxy vitamin D, suppresses 3T3-L1 preadipocyte development and downregulates adipogenic gene expression in a dose-dependent manner ( Blumberg et al., 2006 ). The 1,25-dihydroxy vitamin D receptor, which has been identified to be activated by 1,25-dihydroxy vitamin D, regulates adipogenesis (VDR). VDR suppresses adipogenesis by downregulating C/EBP expression in the presence of 1,25-dihydroxy vitamin D ( Lu, Taylor & Körner, 2018 ; Miao et al., 2020 ).
6. Summing up the role of nutrigenomic in nutrients
Vitamin A, during the early adipocyte development, triggers adipocyte generation during the fattening phase and thus, increases marbling. Conversely, vitamin D metabolites curtail adipocyte production. Vitamin D through VDR binding, binds to the RXR, competing with PPARG ligands and their receptors, which bind to the RXR, which otherwise is required for adipogenesis ( Wang et al., 2016 ). Adipogenesis may also be affected by methyl-donors that regulate epigenetic changes during early adiposis. On the other side, vitamin A supplementation decreases adipocyte hypertrophy and marbling by promoting lipid degradation in feedstock beef cattle. Suppose vitamin A is supplemented from birth until weaning ( Peng, Smith & Lee, 2021 ). In that case, there may be a greater production of intramuscular adipocytes, resulting in more intramuscular pre-adipocytes capable of storing lipids (hypertrophy) throughout the fattening stage, promoting marbling without increasing carcass fatness.
The path to creating healthy meat should be paved with adequate balanced feeding, with alternative nutrients being thoroughly researched for short and long-term impacts. The gene expression studies are carried out concerning the dietary regimes because any change in the biochemical parameter will depend on the particular gene transcript. Even though transcriptome studies have been carried out in farm animals, sophisticated investigations involving protein levels should be carried out. Furthermore, in farm animals, the impact of nutrition on epigenetic mechanisms is still unknown (Nowacka-Woszuk, 2020). Therefore, a complete strategy should be used during nutrigenomic investigations, including genome functioning, breeding aims, and physical characteristics. Innovative technology is already being used extensively in rodent and human nutrigenomic investigations and will almost definitely be employed more often in other species.
7. Nutrigenomics in poultry
In poultry, the intestinal tract (GIT) expands allometrically compared to the rest of the body during the first week after hatching. Because of this rapid expansion rate, supplemental feeding during the first 96 h of hatching has a long-term impact on the animal ( Van Every, 2021 ). For these animals to reach their genetic potential, they must have a stable digestive system. Intensive poultry rearing causes stress in birds, resulting in a lowered immune response that enhances intestinal colonization of pathogens and gastrointestinal disturbances ( Arif et al., 2021 ). Scientists have used modern nutrigenomic approaches to investigate how the diet interacts with the gut immune system to further understand the processes and efficacy of various food therapy strategies. Cinnamaldehyde, carvacrol, oleoresin from Capsicum spp., and turmeric (Abd El-Hack et al., 2022 ; Khodadadi, Sheikhi, Nazarpak & Brujeni, 2021 ; Pirgozliev, 2019 ), according to studies, are all effective on the GIT immune response. Plant-derived phytochemicals have been shown to control the gene expression involved in physiology and immunity (e.g., protein and energy metabolism), implying that they can help chickens become more immune. Eimeria acervulina induces intestinal damage in commercial poultry production. It has been tested for its efficacy against anethole, turmeric, and garlic metabolites ( Nahed et al., 2022 ; Abdelli, Solà-Oriol & Pérez, 2021 ).
The expression of oxidative phosphorylation genes and other cellular stress response genes in the jejunum was improved by prebiotics, such as yeast cell walls. Gene expression profiles in yeast cell-wall supplemented broilers demonstrated that biological processes and pathways connected to enhanced health and metabolism were activated compared to a common antibiotic (bacitracin) ( Jha & Mishra, 2021 ). According to the findings of this microarray study, birds that were provided yeast cell walls underwent genomic changes that corresponded to slower gut cell turnover and, as a result, improved energy utilization for growth ( Khan et al., 2018 ). Probiotics have also been studied with the use of omics techniques. Among the enterocyte proteome of broilers fed Enterococcus faecium , some differentially expressed proteins were associated with antioxidant and immune processes, suggesting that the chickens used fewer nutrients and energy to cope with antioxidant and immune stress ( Khan et al., 2018 ). In addition, trace minerals like chromium have been utilized for weight gain to improve feed conversion ratio and muscle development and modify the immune response in broilers ( Haq et al., 2016 ; Wang et al., 2022 ). The latest nutrigenomics findings explicitly open up new opportunities for developing effective drug-free disease prevention strategies for poultry infectious diseases.
The effect of micronutrient supplementation on transcriptional profiles of chicks was also studied including vitamins and minerals in the diet of hens. Supplementation increased the gene mRNA level of intestinal cells and proliferation, which corresponded to the number of villi proliferating cells and the transitional area of the supplemented population. As a result, gene expression has been modified and could affect feed absorption, metabolism, and the gut immune system ( Gilani et al., 2021 ). Chromium is a mineral needed to metabolize the body's nucleic acids, lipids, and carbohydrates. Complete chromium supplementation of serum proteins and sugar triglycerides has affected the action of insulin. Out of the 57 skeletal muscle development and growth microRNAs, 16 have been shown to have modified muscle tissue expression (Vilar et al., 2020).
The recent increase in mycotoxin content in feedstuffs due to shifting climatic conditions has sparked curiosity about their effect on the liver genome. At concentrations as low as 2 to 2.5 mg/kg of feed, aflatoxin B1 and deoxynivalenol may affect this organ. In addition, these toxins can affect hepatocytes, nutritional and immune status. Adsorbents such as clays are used as a tactile solution to reduce the consumption of mycotoxins ( Dietrich, Neuenschwander, Bucher & Wenk, 2012 ). In chicks fed aflatoxin B1, turmeric blocked the biotransformation expression of antioxidants and immune system genes ( Muhammad et al., 2018 ). Dietary aflatoxin B1 (AFB1) alters serum biochemical parameters, decreases liver anti-oxidative enzymes, suppresses Nrf2 and phase-II enzymes involved in AFB1 detoxification, and significantly increases CYP enzyme expression levels involved in AFB1 biotransformation ( Yang et al., 2022 ).
In contrast, dietary curcumin significantly reversed AFB1-induced alterations in serum biochemical parameters and enhanced liver antioxidant enzyme activities. Curcumin also decreased CYP enzyme-mediated AFB1 bio-activation and increased Nrf2 defense system activity, including GST enzyme activity ( Muhammad et al., 2018 ). Mycotoxin adsorbents have been widely used in animal feed as one of the most widespread intervention methods. Clay minerals like Bentonite, adsorbents like aluminosilicates, and esterified glucomannan derived from the cell wall of Saccharomyces cerevisiae are examples of mycotoxin binders. These adsorbing macromolecules bind mycotoxin molecules, preventing them from absorbing through the intestinal epithelium ( Bhatti et al., 2018 ). Implementing a single mycotoxins decontamination/control technique rarely warrants protection against a chemically diverse range of mycotoxins. The presence of multi-mycotoxins and a range of fungi in food and feed needs the application of several decontamination techniques at the same time for effective management. On the other hand, decontamination of poultry feeds can be accomplished using chemical, physical, and biological means. Some bacteria that detoxify mycotoxins in the gut have been put into feeds to avoid their absorption ( Adebo, Njobeh, Gbashi, Nwinyi & Mavumengwana, 2017 ).
8. Nutrigenomics of meat and its human perspective
Marbling is an essential characteristic of ruminant meat consistency because it affects juiciness and flavor. Adding together, since the fatty acid content of lipids in meat is critical for human health, this issue has gotten much attention in recent decades. Nutrigenomic research has aided researchers in better understanding the cellular pathways that affect fatty acid profile and meat marbling in this way. This information could benefit the livestock industry by encouraging it to produce substances or chemical compounds that can modulate the expression of genes, resulting in better meat quality ( Aiken & Ozanne, 2014 ). Nutritionists will now be able to use feedstuffs and other foods due to this experience. Adipogenesis and marbling are programmed in the womb. Adipogenesis occurs in the womb and has long-term implications for meat quality and fat deposition ( Desoye & Herrera, 2021 ). It's necessary to look into the effect of dam nutrition on offspring adipogenesis through nutrients influencing gene expression in the fetus until we can look into the direct impact of nutrients on genes during the finishing phase. This influence is related to the idea of fetal programming. It's difficult to say how the dam's diet affects the offspring. It has been suggested that dams may affect progeny phenotypes by providing half of the fetal genes in addition to epigenetic marks via somatic epigenetic reprogramming, through ooplasmic contribution to the fetus, and by providing an intrauterine atmosphere ( Vahmani et al., 2015 ).
There is a concern about manipulating the fatty acid profile of meat due to their potential beneficial or harmful effects on human health. Conjugated linoleic acid C9, t11-C18:2 reduces the risk of cancer, hyperlipidemia, and diabetes, and certain saturated fatty acids (SFA) increase high-density lipoprotein (HDL)-cholesterol ( Berton, Fonseca & Gimenez, 2016 ). PUFA is involved in many biological processes vital to human health ( Wood, Richardson & Nute, 2004 ). Because of the observed hypercholesterolemia, lauric and palmitic fatty acids are hypercholesterolemic. Conversely, because of the pragmatic increase in low-density lipoprotein content in the blood, lauric and palmitic fatty acids are hypercholesterolemic ( McPherson et al., 2020 ).
9. Human nutrigenetics
Nutrigenetics studies how genetic factors determine an individual's disease risk, nutritional requirements, and metabolic response ( Surendran & Vimaleswaran, 2021 ). The human genome is 99.9% identical in all people. Single nucleotide polymorphisms (SNPs) account for nearly 90% of the human variation between individuals and account for the remaining 0.1% of the variation. SNPs are DNA sequence variations when one nucleotide in the genome sequence changes. Each gene is thought to differ from the standard gene by about ten differences (or polymorphisms) in its code. However, because SNPs can be found in both coding and non-coding regions of the human genome, not all polymorphisms have a functional impact ( Roche, 2006 ). Given many potential SNPs for candidate genes, it's critical to distinguish between functional and non-functional SNPs to include only functional genetic variants in genetic association studies.
Nutritional genomics aims to find the key candidate genes involved in the most common diet-related disorders (obesity, metabolic syndrome, T2DM, CVD, etc.). This is a complicated process due to the complex polygenic nature of many diseases, which involve disruptions in multiple metabolic pathways ( Sikalidis, 2019 ). Metabolic syndrome symptoms include insulin resistance, abnormal lipoprotein metabolism, hypertension, and obesity. Over 50 candidate genes have been implicated in lipid metabolism, with new candidate genes emerging due to ongoing research in this field. As a result, many genes and polymorphisms are likely to play a role in the pathogenesis and progression of major dietary disorders (Laddu & Hauser, 2019).
Nutrigenetics will be a very intriguing subject of study in the future decades. To understand the impact of gene nutrient interactions, huge data-rich cohorts will be required. Well-characterized phenotypes based on extensive clinical food intake data and sensitive biomarkers of dietary intake data will be necessary to establish the impact of various nutrient exposures in the setting of common polygenic, diet-related illnesses. Robust bioinformatic techniques capable of determining the influence of many genes interacting with nutritional exposures will be necessary to tackle these prevalent but complicated diet-related disorders.
10. Impact of nutrigenomics on humans
Nutrigenomics involves studying how food affects a person's genes and how genes affect a person's response to food. Nutrigenomics explores how genes and diet may affect health and the risk of developing diseases like cancer, diabetes, atherosclerosis, etc. Several micronutrients (vitamins), macronutrients (protein, carbohydrate), and naturally occurring chemicals (flavonoids, coumarins, carotenoids) have been shown to influence gene regulation ( Lundstrom, 2020 ). Tumors and coronary disorders (CVD) are the most noticeable pathologies caused by lifestyle changes ( Popovic et al., 2022 ). Therefore, recognizing nutritional risk factors is essential, and it will undoubtedly aid in reducing their effect on the population.
10.1. Cancer treatment
The most current developments in molecular biology approaches have provided researchers with new insights into many health-related topics, including cancer. Unlike normal cells, cancer cells produce energy by glycolysis accompanied by lactic acid fermentation. Therefore, glucose plays a critical function in cancer cells, and changes in the expression of glucose-responsive genes such as pyruvate kinase type M2 and phosphofructokinase1 may treat cancer ( Rodríguez-Enríquez et al., 2019 ). Fruits and vegetables are high in vitamins and minerals to help prevent cancer. Folic acid in fruit is transformed by several chemical reactions into 5-methyltetrahydrofolate and is then a methionine precursor, which helps mutilate DNA. This leads to DNA replication and cancer in a folic acid deficit diet. The active form of vitamin D, 1,25-dihydroxy vitamin D3, regulates a range, including cell proliferation, of body functions through the Polymorphic Vitamin D Receptor (VDR) ( Nicastro, Trujillo & Milner, 2012 ; Rahman, Chakraborty & Kabir, 2020 ). According to Nicastro et al. (2012) , colorectal cancer may be affected by a Fok2 mutation (polymorphic VDR variant). According to several studies, PUFA has been linked to cell proliferation-related gene expression (PTEN, cyclin, p53, Wnt) and angiogenesis-related gene expression (VEGF, MMP-2, PDGF) and that can modulate tumorigenesis ( Phillips et al., 2013 ).
10.2. Obesity
Obesity is related to an energy imbalance, but it is also linked to several metabolic and endocrine diseases. Indeed, while hereditary factors are likely to set the scene for obesity, nutrition, exercise, and lifestyle all play a role in determining the severity of the condition. Dietary nutrient intake is an important environmental factor in the development of obesity. Intervention studies on the effect of quantity and quality of nutrients in the development of obesity are inconsistent. However, nutrigenomics is being extensively used for researching obesity as well as diet-related disorders. For the effective management of obesity, nutrigenomics can provide the edge through “personalized” nutritional advice and consultation with individuals’ genetic profiles. A combination of environmental and genetic causes, according to investigators, is responsible for many metabolic syndromes (Pena-Romero et al., 2018). The concept of "gene-nutrient interaction" has been established in nutrition science, with gene expression being a significant element determining obesity risk and development. Specific signaling molecules such as insulin, leptin, and cholecystokinin and their receptors can help treat obesity by influencing gene expression and food consumption ( Pathak, Flatt & Irwin, 2018 ). Several studies have examined the interactions between genetic variations (SNPs) and dietary nutrient intake involved in obesity-related variables. Sonestedt et al. (2009) examined the interactions between energy-adjusted fat or carbohydrate intake and a variant of the FTO gene on BMI in a cross-sectional study among participants with an intake of high fat or low carbohydrate. Genetic variations are identified all over the world which affects the development of obesity in individuals; however, there exist unique differences in the genetic variations on obesity by races or populations. Further investigations will still be required as a source of personalized nutrition on obesity for various races or populations.
10.3. Cardiovascular diseases
Individual dietary patterns have changed dramatically in recent years, resulting in many health issues. Obesity, stroke, and thrombosis are signs of cardiovascular disease (CVD) which is a chronic illness caused by a combination of abnormal conditions. Apolipoprotein A-1, which is related to HDL, is present in the liver and intestine (HDL). The A-1 HDL complex takes part in cholesterol transfer and has been associated with a lower risk of heart failure (Sacks et al., 2018 Sacks et al., 2018 ). The APOA1 gene, which codes for Apo A-1, is polymorphic and has been extensively studied ( Sampath et al., 2005 ). Variation in this gene significantly affects cholesterol transfer, which contributes to CVD. Polyunsaturated fatty acids (PUFA) can activate the APOA1 gene. In a study on PUFA in cholesterol transport, people with the A allele in the APOA1 gene had higher HDL levels as their PUFA intake increased ( Sampath et al., 2005 ).
11. Role of nutrigenomics in human-livestock interface
The expanding number of research on humans, animals, and cell cultures indicates that nutrients and other bioactive chemicals in the diet can affect the expression of genes in a variety of ways ( Felisbino, Granzotti, Bello-Santos & Guiloski, 2021 ). Thus, studying the molecular mechanisms of nutrients and other bioactive food ingredients is necessary to understand the effect of diet on health. Because of the intricacy of human behavior and the human genome, studying animals is a helpful way to understand genetic implications on food selection. As a result of the applied study of animal nutrition, the use of animals for food consumption studies was a natural development. A recent study in mice and humans has been conducted in this field to understand the molecular basis of diseases such as cardiovascular disease, obesity, and cancer due to gene/diet type interactions ( Mondal & Panda, 2020 ). These nutrigenomic studies in domestic animals are much less common. They focus on a minimal number of genes that are specifically related to a specific treatment, which is usually associated with the energy content of the diet or the content of some of its components, such as polyunsaturated fatty acids (PUFA), protein, or l -carnitine ( Hamill, Aslan, Mullen, O'Doherty & McBryan, 2013 ). Nutrigenomics in the future will help create a genotype-matched animal feed, by selecting nutrients that are fine-tuned to a particular animal's genes, improving health and productivity significantly. Research advances made in lab animals like pigs, mice, and rats can be implemented in humans for further research.
12. Current progress
With the help of omics approaches, such as metabolomics, transcriptomics, and proteomics, molecular genetics technology can apply to inheritable traits like growth rate, body weight, carcass quality, and feed intake ( Kasper et al., 2020 ; Manzoni et al., 2018 ). Nutrigenomics promotes animal growth by focusing on an animal's physiological and nutritional state and adjusting metabolism to nutrient status in bodily fluids such as blood and saliva and excretion (feces, urine, and breath). Guo et al. (2015) discovered fatty liver etiology gene expression in dairy cows at calving and gluconeogenesis gene expression in early lactation in cows, and a transcriptome study of bovine muscle revealed prospective indicators for skeletal muscle development rate and cell types. Detecting nutrient imbalances and using other omics approaches in livestock feeding research gives new means and more significant knowledge to assess and regulate the balance of nutrients in farm animals ( Hasan et al., 2019 ). The same human investigations may translate the hypotheses of this research. Nutrigenomics helps scientists discover genes and DNA in each animal's cell or tissue by assisting them in selecting nutrients. As a result, nutrigenomics is a technique for creating genotype-matched animal feed/food, selecting fine-tuned nutrients for animal genes, and understanding how nutritional management influences animal performance ( Malgwi et al., 2022 ).
13. Conclusion
Humans and animals have a complex relationship (the human-animal interphase) by which improvements in animal health and products will bring positive changes to human life such as access to good protein for equitable development globally and low cholesterol meat for reducing cardiovascular problems in society. In the future, we may feed livestock and poultry differently so that the diet is based upon genotypes and nutrients influence genotype expression. Improving these relationships and adapting them to improve health and competitiveness for people of all ages will be the most challenging task. There have been many nutrigenomic studies in mice and humans, but information regarding nutrigenomics in food-producing organisms is still nascent.
On the other hand, the detection of novel non-coding RNAs, such as microRNA, in animals is rapidly progressing, with the number of microRNA precursors in the miRbase increasing three to four times for both animals and pigs, respectively. Several transcriptomic experiments have been performed on livestock animals, but the effects of nutrients at the proteome stage are still poorly understood. To better understand the interactions between nutrition and genes in food-producing animals, epigenetic alterations regulated by nutrients must be clarified. A comprehensive investigation is needed to gain a complete understanding of the mechanistic basis for dietary control of gene expression via histone modifications, DNA methylation, and noncoding RNA interactions. Recent advances in nutrigenomics research have led to several high-performance tools being developed for livestock like full-genome sequencing, global RNA sequencing, and chromatin immunoprecipitation sequencing.
Ethical statement
We declare there no ethical issue is involved with the submitting review article “Nutrigenomics in Livestock Sector and its Human-Animal Interface-A Review.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The help and suggestion provided by the Indian Council of Medical Research (ICMR) are highly acknowledged.
- Abd El-Hack E.M., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird's health and production. Poultry Science. 2022 doi: 10.1016/j.psj.2022.101696. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Abdelhamed A., El-Dawla R.E., Karadag A.S., Agamia N.F., Melnik B.C. The impact of isotretinoin on the pituitary-ovarian axis: An interpretative review of the literature. Reproductive Toxicology. 2021;104:85–95. doi: 10.1016/j.reprotox.2021.06.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- Abdelli N., Solà-Oriol D., Pérez J.F. Phytogenic feed additives in poultry: Achievements, prospective and challenges. Animals. 2021;11(12):3471. doi: 10.3390/ani11123471. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Adebo O.A., Njobeh P.B., Gbashi S., Nwinyi O.C., Mavumengwana V. Review on microbial degradation of aflatoxins. Critical Reviews In Food Science And Nutrition. 2017;57(15):3208–3217. doi: 10.1080/10408398.2015.1106440. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ahluwalia M.K. Nutrigenetics and nutrigenomics—A personalized approach to nutrition. Advances in Genetics. 2021;108:277–340. doi: 10.1016/bs.adgen.2021.08.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- Aiken C.E., Ozanne S.E. Transgenerational developmental programming. Human Reproduction Update. 2014;20(1):63–75. doi: 10.1093/humupd/dmt043. [ DOI ] [ PubMed ] [ Google Scholar ]
- Akanbi O.M. Animal nutrigenomics: opportunities and challenges for sustainable animal products in Nigeria. Emergency Medicine and Trauma Care Journal: EMTCJ-100007, 2019. 2019;(02) [ Google Scholar ]
- Alagawany M., Elnesr S.S., Farag M.R., Tiwari R., Yatoo M.I., Karthik K., et al. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health–A comprehensive review. Veterinary Quarterly. 2021;41(1):1–29. doi: 10.1080/01652176.2020.1857887. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alexandre P.A., Kogelman L.J., Santana M.H., Passarelli D., Pulz L.H., Fantinato-Neto P., et al. Liver transcriptomic networks reveal main biological processes associated with feed efficiency in beef cattle. BMC genomics. 2015;16(1):1–13. doi: 10.1186/s12864-015-2292-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Aloia L., Di Stefano B., Di Croce L. Polycomb complexes in stem cells and embryonic development. Development (Cambridge, England) 2013;140(12):2525–2534. doi: 10.1242/dev.091553. [ DOI ] [ PubMed ] [ Google Scholar ]
- Arif M., Akteruzzaman M., Islam S.S., Das B.C., Siddique M.P., Kabir S.L. Dietary supplementation of Bacillus-based probiotics on the growth performance, gut morphology, intestinal microbiota and immune response in low biosecurity broiler chickens. Veterinary and Animal Science. 2021;14 doi: 10.1016/j.vas.2021.100216. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Asmelash B., Mahlet D., Brhane H. Livestock nutrigenomics applications and prospects. J. Vet. Sci. Technol. 2018;9:1–4. [ Google Scholar ]
- Baharum S.N., Azizan K.A. Omics applications for systems biology. Springer; Cham: 2018. Metabolomics in systems biology; pp. 51–68. [ DOI ] [ PubMed ] [ Google Scholar ]
- Balkir P., Kemahlioglu K., Yucel U. Foodomics: A new approach in food quality and safety. Trends in Food Science & Technology. 2021;108:49–57. [ Google Scholar ]
- Barros-Rodríguez A., Rangseekaew P., Lasudee K., Pathom-Aree W., Manzanera M. Impacts of agriculture on the environment and soil microbial biodiversity. Plants. 2021;10(11):2325. doi: 10.3390/plants10112325. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Baykalir, Y., Baykalir, B.G., .& Simsek, U.G. (.2018). Application of some proteome analysis techniques in animal reproduction. In New insights into theriogenology . IntechOpen.
- Bellner, L., Nichols, A., Pandey, V., Vanella, L., Gilliam, C., Gupte, R. et al. (2015). Effect of Vitamin B12 and Nutrients on Adipogenesis-adipogenic Markers in 3T3 Cells. The FASEB Journal , 29, 996-2.
- Benítez R., Núñez Y., Óvilo C., Ovilo C. Nutrigenomics in farm animals. Journal of Investigative Genomics. 2017;4:1. [ Google Scholar ]
- Berton M.P., Fonseca L.F., Gimenez D.F., et al. Gene expression profile of intramuscular muscle in Nellore cattle with extreme values of fatty acid. BMC genomics. 2016;17:972. doi: 10.1186/s12864-016-3232-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bhatti S.A., Khan M.Z., Hassan Z.U., Saleemi M.K., Saqib M., Khatoon A., et al. Comparative efficacy of Bentonite clay, activated charcoal and Trichosporon mycotoxinivorans in regulating the feed-to-tissue transfer of mycotoxins. Journal of the Science of Food and Agriculture. 2018;98(3):884–890. doi: 10.1002/jsfa.8533. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bibikova M., Barnes B., Tsan C., Ho V., Klotzle B., Le J.M., et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98:288–295. doi: 10.1016/j.ygeno.2011.07.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bishai J.D., Palm N.W. Small molecule metabolites at the host–microbiota interface. The Journal of Immunology. 2021;207(7):1725–1733. doi: 10.4049/jimmunol.2100528. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Blumberg J.M., Tzameli I., Astapova I., Lam F.S., Flier J.S., Hollenberg A.N. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. Journal of Biological Chemistry. 2006;281(16):11205–11213. doi: 10.1074/jbc.M510343200. [ DOI ] [ PubMed ] [ Google Scholar ]
- Boddicker R.L., Koltes J.E., Fritz-Waters E.R., Koesterke L., Weeks N., Yin T., et al. Genome-wide methylation profile following prenatal and postnatal dietary omega-3 fatty acid supplementation in pigs. Animal genetics. 2016;47(6):658–671. doi: 10.1111/age.12468. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bordoni L., Gabbianelli R. Primers on nutrigenetics and nutri (epi) genomics: Origins and development of precision nutrition. Biochimie. 2019;160:156–171. doi: 10.1016/j.biochi.2019.03.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bruh A., Haіom B. Gene transfer in eukaryotic cells: Current 8. applications and implication. International Research Journal of Biological Sciences. 2017;6:1–7. [ Google Scholar ]
- Burton M.A., Lillycrop K.A. Nutritional modulation of the epigenome and its implication for future health. Proceedings of the Nutrition Society. 2019;78(3):305–312. doi: 10.1017/S0029665119000016. [ DOI ] [ PubMed ] [ Google Scholar ]
- Castelli F.A., Rosati G., Moguet C., Fuentes C., Marrugo-Ramírez J., Lefebvre T., Junot C. Metabolomics for personalized medicine: The input of analytical chemistry from biomarker discovery to point-of-care tests. Analytical and Bioanalytical Chemistry. 2021:1–31. doi: 10.1007/s00216-021-03586-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ceciliani F., Eckersall D., Burchmore R., Lecchi C. Proteomics in veterinary medicine: Applications and trends in disease pathogenesis and diagnostics. Veterinary pathology. 2014;51(2):351–362. doi: 10.1177/0300985813502819. [ DOI ] [ PubMed ] [ Google Scholar ]
- Chen G. The interactions of insulin and Vitamin A signaling systems for the regulation of hepatic glucose and lipid metabolism. Cells. 2021;10(8):2160. doi: 10.3390/cells10082160. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chmurzynska A., Mlodzik M.A., Radziejewska A., Szwengiel A., Malinowska A.M., Nowacka-Woszuk J. Caloric restriction can affect one-carbon metabolism during pregnancy in the rat: A transgenerational model. Biochimie. 2018;152:181–187. doi: 10.1016/j.biochi.2018.07.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cordero P., Gomez-Uriz A.M., Campion J., Milagro F.I., Martinez J.A. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes & Nutrition. 2013;8(1):105–113. doi: 10.1007/s12263-012-0300-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- De Sousa, C.S., .Hassan, S.S., .Pinto, A.C., .Silva, W.M., .De Almeida, S.S., .Soares, S.D.C. et al. (2018). Microbial omics: Applications in biotechnology. In Omics technologies and bio-engineering (pp. 3–20). Academic Press.
- Desoye G., Herrera E. Adipose tissue development and lipid metabolism in the human fetus: The 2020 perspective focusing on maternal diabetes and obesity. Progress in Lipid Research. 2021;81 doi: 10.1016/j.plipres.2020.101082. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dietrich B., Neuenschwander S., Bucher B., Wenk C. Fusarium mycotoxin-contaminated wheat containing deoxynivalenol alters the gene expression in the liver and the jejunum of broilers. Animal : an international journal of animal bioscience. 2012;6:278–291. doi: 10.1017/S1751731111001601. [ DOI ] [ PubMed ] [ Google Scholar ]
- Di Girolamo F., D'Amato A., Lante I., Signore F., Muraca M., Putignani L. Farm animal serum proteomics and impact on human health. International journal of molecular sciences. 2014;15(9):15396–15411. doi: 10.3390/ijms150915396. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dimofski P., Meyre D., Dreumont N., Leininger-Muller B. Consequences of paternal nutrition on offspring health and disease. Nutrients. 2021;13(8):2818. doi: 10.3390/nu13082818. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ding R., Zhuang Z., Qiu Y., Ruan D., Wu J., Ye J., Yang J. Identify known and novel candidate genes associated with backfat thickness in Duroc pigs by large-scale genome-wide association analysis. Journal of Animal Science. 2022;100(2):skac012. doi: 10.1093/jas/skac012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eckersall D. 321 ASAS-EAAP Talk: Quantitative proteomics in animal and veterinary science: A pipeline for exploiting advanced analytical technology. Journal of Animal Science. 2020;98(Supplement_4):55–56. [ Google Scholar ]
- Felisbino K., Granzotti J.G., Bello-Santos L., Guiloski I.C. Nutrigenomics in regulating the expression of genes related to type 2 diabetes mellitus. Frontiers in physiology. 2021:1109. doi: 10.3389/fphys.2021.699220. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ferguson J.F., Allayee H., Gerszten R.E., Ideraabdullah F., Kris-Etherton P.M., Ordovás J.M., et al. Nutrigenomics, the microbiome, and gene-environment interactions: New directions in cardiovascular disease research, prevention, and treatment: A scientific statement from the American Heart Association. Circulation: Cardiovascular Genetics. 2016;9(3):291–313. doi: 10.1161/HCG.0000000000000030. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fukase E., Martin W. Economic growth, convergence, and world food demand and supply. World Development. 2020;132:12. 104954. [ Google Scholar ]
- Ghodasara P., Satake N., Sadowski P., Kopp S., Mills P.C. Investigation of cattle plasma proteome in response to pain and inflammation using next generation proteomics technique, SWATH-MS. Molecular Omics. 2022;18(2):133–142. doi: 10.1039/d1mo00354b. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gilani S.M.H., Rashid Z., Galani S., Ilyas S., Sahar S., Al-Ghanim K., Al-Mulham N. Growth performance, intestinal histomorphology, gut microflora and ghrelin gene expression analysis of broiler by supplementing natural growth promoters: A nutrigenomics approach. Saudi Journal of Biological Sciences. 2021;28(6):438–3447. doi: 10.1016/j.sjbs.2021.03.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Goldansaz S.A., Guo A.C., Sajed T., Steele M.A., Plastow G.S., Wishart D.S. Livestock metabolomics and the livestock metabolome: A systematic review. PloS.One. 2017;12 doi: 10.1371/journal.pone.0177675. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Golder H.M., Thomson J.M., Denman S.E., McSweeney C.S., Lean I.J. Genetic markers are associated with the ruminal microbiome and metabolome in grain and sugar challenged dairy heifers. Frontiers in Genetics. 2018;9:62. doi: 10.3389/fgene.2018.00062. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gowda G.N., Zhang S., Gu H., Asiago V., Shanaiah N., Raftery D. Metabolomics-based methods for early disease diagnostics. Expert Review Of Molecular Diagnostics. 2008;8(5):617–633. doi: 10.1586/14737159.8.5.617. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Guo B., Greenwood P.L., Café L.M., Zhou G., Zhang W., Dalrymple B.P. Transcriptome analysis of cattle muscle identifies potential markers for skeletal muscle growth rate and major cell types. BMC genomics. 2015;16:177. doi: 10.1186/s12864-015-1403-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hamill R., Aslan M.O., Mullen A.M., O'Doherty J.V., McBryan J., et al. Transcriptome analysis of porcine M. semimembranosus divergent in intramuscular fat as a consequence of dietary protein restriction. BMC genomics. 2013;14:453. doi: 10.1186/1471-2164-14-453. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Han Y., Gao S., Muegge K., Zhang W., Zhou B. Advanced applications of RNA sequencing and challenges. Bioinformatics and Biology Insights. 2015;9:BBI–S28991. doi: 10.4137/BBI.S28991. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Haq Z., Jain R.K., Khan N., Dar M.Y., Ali S., Gupta M., et al. Recent advances in role of chromium and its antioxidant combinations in poultry nutrition: A review. Veterinary World. 2016;9(12):1392. doi: 10.14202/vetworld.2016.1392-1399. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hasan M.S., Feugang J.M., Liao S.F. A nutrigenomics approach using RNA sequencing technology to study nutrient–gene interactions in agricultural animals. Current Developments in Nutrition. 2019;3(8):82. doi: 10.1093/cdn/nzz082. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hossain M. Selected reaction monitoring mass spectrometry (SRM-MS) in proteomics. Springer; Cham: 2020. Selected Reaction Monitoring Mass Spectrometry; pp. 53–88. [ Google Scholar ]
- Ibeagha-Awemu E.M., Zhao X. Epigenetic marks: Regulators of livestock phenotypes and conceivable sources of missing variation in livestock improvement programs. Frontiers in Genetics. 2015;6:302. doi: 10.3389/fgene.2015.00302. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jha R., Mishra P. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: A review. Journal of Animal Science and Biotechnology. 2021;12(1):1–16. doi: 10.1186/s40104-021-00576-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kang S.W., Madkour M., Kuenzel W.J. Tissue-specific expression of DNA methyltransferases involved in early-life nutritional stress of chicken, gallus gallus. Frontiers in Genetics. 2017;8:204. doi: 10.3389/fgene.2017.00204. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kasper C., Ribeiro D., Almeida A.M.D., Larzul C., Liaubet L., Murani E. Omics application in animal science—A special emphasis on stress response and damaging behaviour in pigs. Genes. 2020;11(8):920. doi: 10.3390/genes11080920. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Katsarou E.I., Billinis C., Galamatis D., Fthenakis G.C., Tsangaris G.T., Katsafadou A.I. Applied proteomics in ‘One Health. Proteomes. 2021;9(3):31. doi: 10.3390/proteomes9030031. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kaya A., Dogan S., Vargovic P., Kutchy N.A., Ross P., Topper E., et al. Sperm proteins ODF2 and PAWP as markers of fertility in breeding bulls. Cell and Tissue Research. 2022;387(1):159–171. doi: 10.1007/s00441-021-03529-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- Khan A.A., Ganai A.M., ul Haq Z. Advances in nutrigenomics and its application in poultry. International Journal of Current Microbiology and Applied Sciences. 2018;7:2866–2872. [ Google Scholar ]
- Khodadadi M., Sheikhi N., Nazarpak H.H., Brujeni G.N. Effects of dietary turmeric (Curcuma longa) on innate and acquired immune responses in broiler chicken. Veterinary and Animal Science. 2021;14 doi: 10.1016/j.vas.2021.100213. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kimura J., Kudo H., Fukuda A., Yamada M., Makita K., Oka K., et al. Decreasing the abundance of tetracycline-resistant Escherichia coli in pig feces during nursery using flavophospholipol as a pig feed additive. Veterinary and Animal Science. 2022 doi: 10.1016/j.vas.2022.100236. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ladeira M.M., Schoonmaker J.P., Swanson K.C., Duckett S.K., Gionbelli M.P., Rodrigues L.M., et al. Nutrigenomics of marbling and fatty acid profile in ruminant meat. Animal : An International Journal Of Animal Bioscience. 2018;12(s2):s282–s294. doi: 10.1017/S1751731118001933. [ DOI ] [ PubMed ] [ Google Scholar ]
- Liang J., Nabi M., Zhang P., Zhang G., Cai Y., Wang Q., Ding Y. Promising biological conversion of lignocellulosic biomass to renewable energy with rumen microorganisms: A comprehensive review. Renewable and Sustainable Energy Reviews. 2020;134 [ Google Scholar ]
- Lowe, R., Shirley, N., Bleackley, M., Dolan, S., & Shafee, T. (2017). Transcriptomics technologies. PLoS computational biology . 13, e1005457. [ DOI ] [ PMC free article ] [ PubMed ]
- Lu M., Taylor B.V., Körner H. Genomic effects of the vitamin D receptor: Potentially the link between vitamin D, immune cells, and multiple sclerosis. Frontiers in immunology. 2018;9:477. doi: 10.3389/fimmu.2018.00477. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lucini, L., Marti-Quijal, F.J., .Barba, F.J., .Rocchetti, G., Quilez, F., Cuesta, L. et al. (2020). Nutrigenomics and public health. In Agri-Food industry strategies for healthy diets and sustainability (pp. 219–233). Academic Press.
- Lundstrom, K. (2020). Nutrition and disease: Prevention and therapy . Cambridge Scholars Publishing.
- Mackin, S.J. (.2019). Novel insights into the effects of manipulating DNA methyltransferase levels on the imprinted and non-imprinted regions of the genome (Doctoral dissertation, Ulster University).
- Maestro B., Dávila N., Carranza M.C., Calle C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. The Journal of Steroid Biochemistry and Molecular Biology. 2003;84(2–3):223–230. doi: 10.1016/s0960-0760(03)00032-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- Malgwi I.H., Halas V., Grünvald P., Schiavon S., Jócsák I. Genes related to fat metabolism in pigs and intramuscular fat content of pork: A focus on Nutrigenetics and Nutrigenomics. Animals. 2022;12:150. doi: 10.3390/ani12020150. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Manzoni C., Kia D.A., Vandrovcova J., Hardy J., Wood N.W., Lewis P.A., et al. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Briefings in bioinformatics. 2018;19(2):286–302. doi: 10.1093/bib/bbw114. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Martyniuk C.J. Perspectives on transcriptomics in animal physiology studies. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2020;250 doi: 10.1016/j.cbpb.2020.110490. [ DOI ] [ PubMed ] [ Google Scholar ]
- McPherson, R., & Spiller, G.A. (.2020). Effects of dietary fatty acids and cholesterol on cardiovascular disease risk factors in man. In Handbook of lipids in human nutrition (pp. 41–50). CRC Press.
- Mendenhall E.M., Koche R.P., Truong T., et al. GC-rich sequence elements recruit PRC2 in mammalian ES cells. PLoS Genetics. 2010;6 doi: 10.1371/journal.pgen.1001244. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miao, Z., Wang, S., Wang, Y., Guo, L., Zhang, J., Liu, Y. et al. (2020). A potential linking between vitamin D and adipose metabolic disorders. Canadian Journal of Gastroenterology and Hepatology , 2020. [ DOI ] [ PMC free article ] [ PubMed ]
- Mierziak J., Kostyn K., Boba A., Czemplik M., Kulma A., Wojtasik W. Influence of the bioactive diet components on the gene expression regulation. Nutrients. 2021;13(11):3673. doi: 10.3390/nu13113673. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miggiels P., Wouters B., van Westen G.J., Dubbelman A.C., Hankemeier T. Novel technologies for metabolomics: More for less. TrAC Trends in Analytical Chemistry. 2019;120 [ Google Scholar ]
- Molotoks A., Smith P., Dawson T.P. Impacts of land use, population, and climate change on global food security. Food and Energy Security. 2021;10(1):261. [ Google Scholar ]
- Mondal, S., & Panda, D. (2020). Nutrigenomics: An interface of gene-diet-disease interaction. In Mineral deficiencies-electrolyte disturbances, genes, diet and disease interface . intechopen.
- Mosca P., Leheup B., Dreumont N. Nutrigenomics and RNA methylation: Role of micronutrients. Biochimie. 2019;164:53–59. doi: 10.1016/j.biochi.2019.07.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- Muhammad I., Wang H., Sun X., Wang X., Han M., Lu Z., et al. Dual role of dietary curcumin through attenuating AFB1-induced oxidative stress and liver injury via modulating liver phase-I and phase-II enzymes involved in AFB1 bioactivation and detoxification. Frontiers in Pharmacology. 2018;9:554. doi: 10.3389/fphar.2018.00554. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Muhanguzi D., Ndekezi C., Nkamwesiga J., Kalayou S., Ochwo S., Vuyani M., et al. Anti-tick vaccines: Current advances and future prospects. Vaccine Design. 2022;2411:253–267. doi: 10.1007/978-1-0716-1888-2_15. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mullins Y., Keogh K., Blackshields G., Kenny D.A., Kelly A.K., Waters S.M. Transcriptome assisted label free proteomics of hepatic tissue in response to both dietary restriction and compensatory growth in cattle. Journal of Proteomics. 2021;232 doi: 10.1016/j.jprot.2020.104048. [ DOI ] [ PubMed ] [ Google Scholar ]
- Murdoch B.M., Murdoch G.K., Greenwood S., McKay S. Nutritional influence on epigenetic marks and effect on livestock production. Frontiers in Genetics. 2016;7:182. doi: 10.3389/fgene.2016.00182. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Muthubharathi B.C., Gowripriya T., Balamurugan K. Metabolomics: Small molecules that matter more. Molecular Omics. 2021;17(2):210–229. doi: 10.1039/d0mo00176g. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nahed A., El-Hack Abd M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A. Phytochemical control of poultry coccidiosis: A review. Poultry Science. 2022;101(1) doi: 10.1016/j.psj.2021.101542. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nicastro H.L., Trujillo E.B., Milner J.A. Nutrigenomics and cancer prevention. Current Nutrition Reports. 2012;1:37–43. doi: 10.1007/s13668-011-0007-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nicholson J.K., Holmes E., Kinross J.M., Darzi A.W., Takats Z., Lindon J.C. Metabolic phenotyping in clinical and surgical environments. Nature. 2012;491:384–392. doi: 10.1038/nature11708. [ DOI ] [ PubMed ] [ Google Scholar ]
- Osorio J.S., Vailati-Riboni M., Palladino A., Luo J., Loor J.J. Application of nutrigenomics in small ruminants: Lactation, growth, and beyond. Small Ruminant Research. 2017;154:29–44. [ Google Scholar ]
- Osz J., McEwen A.G., Bourguet M., Przybilla F., Peluso-Iltis C., Poussin-Courmontagne P., et al. Structural basis for DNA recognition and allosteric control of the retinoic acid receptors RAR–RXR. Nucleic Acids Research. 2020;48(17):9969–9985. doi: 10.1093/nar/gkaa697. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pal A. Springer; New York, NY: 2022. Nutrigenomics. in protocols in advanced genomics and allied techniques; pp. 559–569. [ Google Scholar ]
- Pathak V., Flatt P.R., Irwin N. Cholecystokinin (CCK) and related adjunct peptide therapies for the treatment of obesity and type 2 diabetes. Peptides. 2018;100:229–235. doi: 10.1016/j.peptides.2017.09.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- Peddinti D., Memili E., Burgess S.C. 13 Proteomics in animal reproduction and breeding. Methods in Animal Proteomics. 2011:369. [ Google Scholar ]
- Peng D.Q., Smith S.B., Lee H.G. Vitamin A regulates intramuscular adipose tissue and muscle development: Promoting high-quality beef production. Journal of Animal Science and Biotechnology. 2021;12(1):34. doi: 10.1186/s40104-021-00558-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Phillips C.M. Nutrigenetics and metabolic disease: Current status and implications for personalised nutrition. Nutrients. 2013;5:32–57. doi: 10.3390/nu5010032. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pirgozliev V., Mansbridge S.C., Rose S.P., Lillehoj H.S., Bravo D. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poultry Science. 2019;98(9):3443–3449. doi: 10.3382/ps/pey472. [ DOI ] [ PubMed ] [ Google Scholar ]
- Popovic, D., Bjelobrk, M., Tesic, M., Seman, S., Jayasinghe, S., Hills, A.P. et al. (2022). Defining the importance of stress reduction in managing cardiovascular disease-the role of exercise. Progress in cardiovascular diseases . [ DOI ] [ PubMed ]
- Qi W., Chan H., Teng L., Li L., Chuai S., Zhang R., et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proceedings of the National Academy of Sciences. 2012;109(52):21360–21365. doi: 10.1073/pnas.1210371110. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rahman, A., Chakraborty, S., & Kabir, Y. (2020). Harnessing personalized nutrigenomics for cancer prevention and treatment through diet-gene interaction. In Functional foods in cancer prevention and therapy (pp. 387–403). Academic Press.
- Rajaei-Sharifabadi H., Ellestad L., Porter T., Donoghue A., Bottje W.G., Dridi S. Noni (Morinda citrifolia) modulates the hypothalamic expression of stress-and metabolic-related genes in broilers exposed to acute heat stress. Frontiers in genetics. 2017;8:192. doi: 10.3389/fgene.2017.00192. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rauw W.M., Rydhmer L., Kyriazakis I., Øverland M., Gilbert H., Dekkers J.C., et al. Prospects for sustainability of pig production in relation to climate change and novel feed resources. Journal of the Science of Food and Agriculture. 2020;100(9):3575–3586. doi: 10.1002/jsfa.10338. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Reddy V.S., Palika R., Ismail A., Pullakhandam R., Reddy G.B. Nutrigenomics: Opportunities & challenges for public health nutrition. The Indian Journal of Medical Research. 2018;148(5):632. doi: 10.4103/ijmr.IJMR_1738_18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [ DOI ] [ PubMed ] [ Google Scholar ]
- Reynolds, C.M., .& Vickers, M.H. (.2018). Utility of small animal models of developmental programming. Investigations of early nutrition effects on long-term health , 145–163. [ DOI ] [ PubMed ]
- Roberts A.J. Multigenerational effects. Veterinary Clinics of North America. Food Animal Practice. 2019;35(2):355–364. doi: 10.1016/j.cvfa.2019.02.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- Roche H.M. Nutrigenomics—New approaches for human nutrition research. Journal of the Science of Food and Agriculture. 2006;86(8):1156–1163. [ Google Scholar ]
- Rochette-Egly C. Retinoic acid-regulated target genes during development: Integrative genomics analysis. The Biochemistry of Retinoid Signaling III. 2020:57–85. doi: 10.1007/978-3-030-42282-0_3. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rodríguez-Enríquez S., Marín-Hernández Á., Gallardo-Pérez J.C., Pacheco-Velázquez S.C., Belmont-Díaz J.A., Robledo-Cadena D.X., M.oreno-Sánchez R. Transcriptional regulation of energy metabolism in cancer cells. Cells. 2019;8(10):1225. doi: 10.3390/cells8101225. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rolf M.M., Decker J.E., McKay S.D., Tizioto P.C., Branham K.A., Whitacre L.K., et al. Genomics in the United States beef industry. Livestock Science. 2014;166:84–93. [ Google Scholar ]
- Sacks F.M., Jensen M.K. From high-density lipoprotein cholesterol to measurements of function: Prospects for the development of tests for high-density lipoprotein functionality in cardiovascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2018;38(3):487–499. doi: 10.1161/ATVBAHA.117.307025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sampath H., Ntambi J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annual Review of Nutrition. 2005;25(1):317–340. doi: 10.1146/annurev.nutr.25.051804.101917. [ DOI ] [ PubMed ] [ Google Scholar ]
- Santos C.A., Blanck D.V., de Freitas P.D. RNA-seq as a powerful tool for penaeid shrimp genetic progress. Frontiers in Genetics. 2014;5:298. doi: 10.3389/fgene.2014.00298. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shankar, R. (2020). The dynamic aspects of rna regulation. in rna-based regulation in human health and disease (pp. 85–115). Academic Press.
- Sharma S., Ray B., Mahapatra S.K. Genomic and proteomic: Their tools and application. Asian Journal of Research in Biosciences. 2022:48–61. [ Google Scholar ]
- Shin J.H., Xu L., Li R.W., Gao Y., Bickhart D., Liu G.E., et al. A high-resolution whole-genome map of the distinctive epigenomic landscape induced by butyrate in bovine cells. Animal genetics. 2014;45:40–50. doi: 10.1111/age.12147. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sikalidis A.K. From food for survival to food for personalized optimal health: A historical perspective of how food and nutrition gave rise to nutrigenomics. Journal of the American College of Nutrition. 2019;38(1):84–95. doi: 10.1080/07315724.2018.1481797. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sonestedt E., Roos C., Gullberg B., Ericson U., Wirfält E., Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. The American journal of clinical nutrition. 2009;90(5):1418–1425. doi: 10.3945/ajcn.2009.27958. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sun H.Z, Guan L., Luo Feedomics: Promises for food security with sustainable food animal production. Trends in analytical chemistry. 2018;107:130–141. [ Google Scholar ]
- Sun H.Z., Wang D.M., Wang B., Wang J.K., Liu H.Y., Guan L.L., et al. Metabolomics of four biofluids from dairy cows: Potential biomarkers for milk production and quality. Journal of proteome research. 2015;14(2):1287–1298. doi: 10.1021/pr501305g. [ DOI ] [ PubMed ] [ Google Scholar ]
- Surendran, S., & Vimaleswaran, K.S. (.2021). A nutrigenetic approach to examine the relationship between vitamin B12 status and cardio-metabolic traits in multiple ethnic groups–findings from the GeNuIne Collaboration.
- Szymczak-Pajor I., Miazek K., Selmi A., Balcerczyk A., Śliwińska A. The action of vitamin D in adipose tissue: is there the link between vitamin D deficiency and adipose tissue-related metabolic disorders? International Journal of Molecular Sciences. 2022;23(2):956. doi: 10.3390/ijms23020956. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tian X., Engel B.A., Qian H., Hua E., Sun S., Wang Y. Will reaching the maximum achievable yield potential meet future global food demand? Journal of Cleaner Production. 2021;294 [ Google Scholar ]
- Vahmani P., Mapiye C., Prieto N., Rolland D.C., McAllister T.A., Aalhus J.L., et al. The scope for manipulating the polyunsaturated fatty acid content of beef: A review. Journal of Animal Science and Biotechnology. 2015;6(1):1–13. doi: 10.1186/s40104-015-0026-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Van Every H.A., Schmidt C.J. Transcriptomic and metabolomic characterization of post-hatch metabolic reprogramming during hepatic development in the chicken. BMC genomics. 2021;22(1):1–21. doi: 10.1186/s12864-021-07724-w. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang B., Yang Q., Harris C.L., et al. Nutrigenomic regulation of adipose tissue development - role of retinoic acid: A review. Meat Science. 2016;120:100–106. doi: 10.1016/j.meatsci.2016.04.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang J., Ishfaq M., Miao Y., Liu Z., Hao M., Wang C., et al. Dietary administration of Bacillus subtilis KC1 improves growth performance, immune response, heat stress tolerance, and disease resistance of broiler chickens. Poultry Science. 2022;101(3) doi: 10.1016/j.psj.2021.101693. (b) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wickramasinghe S., Rincon G., Islas-Trejo A., Medrano J.F. Transcriptional profiling of bovine milk using RNA sequencing. BMC Genomics. 2012;13(1):1–14. doi: 10.1186/1471-2164-13-45. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Willforss J., Morrell J.M., Resjö S., Hallap T., Padrik P., Siino V., et al. STable bull fertility protein markers in seminal plasma. Journal of Proteomics. 2021;236 doi: 10.1016/j.jprot.2021.104135. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wishart D.S., Feunang Y.D., Marcu A., et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Research. 2018;46:D608–D617. doi: 10.1093/nar/gkx1089. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wood J.D., Richardson R.I., Nute G.R., et al. Effects of fatty acids on meat quality: A review. Meat Science. 2004;66:21–32. doi: 10.1016/S0309-1740(03)00022-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wu G. Recent advances in animal nutrition and metabolism. Springer; Cham: 2022. Nutrition and metabolism: Foundations for animal growth, development, reproduction, and health; pp. 1–24. [ DOI ] [ PubMed ] [ Google Scholar ]
- Yang H., Wang Y., Yu C., Jiao Y., Zhang R., Jin S., et al. Dietary resveratrol alleviates AFB1-induced ileum damage in ducks via the Nrf2 and NF-κB/NLRP3 signaling pathways and CYP1A1/2 expressions. Agriculture. 2022;12:54. [ Google Scholar ]
- Yang Q.Y., Liang J.F., Rogers C.J., Zhao J.X., Zhu M.J., Du M. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes. 2013;62:3727–3735. doi: 10.2337/db13-0433. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ye J., Yan X., Qin P., Gong X., Li H., Liu Y., et al. Proteomic analysis of hypothalamus in prepubertal and pubertal female goat. Journal of Proteomics. 2022;251 doi: 10.1016/j.jprot.2021.104411. [ DOI ] [ PubMed ] [ Google Scholar ]
- Zampieri M., Sekar K., Zamboni N., Sauer U. Frontiers of high-throughput metabolomics. Current Opinion in Chemical Biology. 2017;36:15–23. doi: 10.1016/j.cbpa.2016.12.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- Zduńczyk Z., Pareek C.S. Application of nutrigenomics tools in animal feeding and nutritional research. Journal of Animal and Feed Sciences. 2009;18(1):3–16. [ Google Scholar ]
- Zhao Y., Wang Y., Guo F., Lu B., Sun J., Wang J., et al. iTRAQ-based proteomic analysis of sperm reveals candidate proteins that affect the quality of spermatozoa from boars on plateaus. Proteome Science. 2021;19(1):9. doi: 10.1186/s12953-021-00177-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (2.0 MB)
- Collections

Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

Recent Articles
Open access
Effects of different rapeseed varieties on egg production performance, egg quality, hormone levels, follicle development, and thyroid function in hens
Available online 19 October 2024
Liping Zhu | Jianping Wang | Xuemei Ding | Shiping Bai | Qiufeng Zeng | Yue Xuan | Keying Zhang
This study was to evaluate the effects of rapeseed (Brassica napus L.) from China with different content of glucosinolate (Gls) and erucic acid (EA) on laying hens. A total of 600 laying hens at 33...
Share article
The role of milk urea nitrogen in nutritional assessment and its relationship with phenotype of dairy cows: A review
Xiaowei Zhao | Nan Zheng | Yangdong Zhang | Jiaqi Wang
Urea is a small molecule that can readily cross the blood-milk barrier into milk, leading to a strong correlation between blood urea nitrogen (BUN) and milk urea nitrogen (MUN) concentrations. Although...
Dependence of the hindgut disappearance of phosphorus in pigs on the quantity of phosphorus entering the hindgut based on a Meta-analysis
Available online 5 October 2024
Noa Park | Beob G. Kim
The objectives of the current study were to compare the difference between standardized ileal digestibility (SID) and standardized total tract digestibility (STTD) of phosphorus (P) in pigs using published...
Aflatoxin B1 decreased flesh flavor and inhibited muscle development in grass carp (Ctenopharyngodon idella)
September 2024
Xiang-Ning He | Zhen-Zhen Zeng | Wei-Dan Jiang | Pei Wu | Yang Liu | Sheng-Yao Kuang | Ling Tang | Shu-Wei Li | Lin Feng | Xiao-Qiu Zhou
In nature, aflatoxins, especially aflatoxin B1 (AFB1), are the common mycotoxins, which cause serious health problems for humans and animals. This paper aimed to study the effects of AFB1 on flesh flavor...
Microencapsulated Lactobacillus plantarum promotes intestinal development through gut colonization of layer chicks
Yaoming Cui | Yanxia Liu | Jing Yang | Haitao Duan | Peng Wang | Linna Guo | Yanjiao Guo | Suying Li | Yating Zhao | Jinrong Wang | Guanghai Qi | Junjun Guan
The effects of Lactobacillus plantarum in microencapsulation (LPM) on intestinal development in layer chicks were investigated in this study, as well as the colonization of L. plantarum in the gut....
Rumen microbiota succession throughout the perinatal period and its association with postpartum production traits in dairy cows: A review
Xiaowei Zhao | Yangdong Zhang | Ashikur Rahman | Meiqing Chen | Ning Li | Tao Wu | Yunxia Qi | Nan Zheng | Shengguo Zhao | Jiaqi Wang
The transition period for dairy cows usually refers to the 3 weeks pre-calving to the 3 weeks post-calving. During this period, dairy cows undergo metabolic and physiological adaptations because of...
Effect of changing the proportion of C16:0 and cis-9 C18:1 in fat supplements on rumen fermentation, glucose and lipid metabolism, antioxidation capacity, and visceral fatty acid profile in finishing Angus bulls
Haixin Bai | Lubo Wang | Modinat Tolani Lambo | Yang Li | Yonggen Zhang
This study evaluated the effects of different proportions of palmitic (C16:0) and oleic (cis-9 C18:1) acids in fat supplements on rumen fermentation, glucose (GLU) and lipid metabolism, antioxidant...
Dietary fishmeal replacement by Clostridium autoethanogenum protein meal influences the nutritional and sensory quality of turbot (Scophthalmus maximus) via the TOR/AAR/AMPK pathways
Zezheng Qi | Nan Bai | Qing Li | Shihui Pan | Min Gu
Clostridium autoethanogenum protein (CAP) is a promising protein source for aquaculture; however, how CAP influences fish quality is worth extensive research. We randomly allocated 630 turbot with initial...
The potential of glutamine supplementation in reduced-crude protein diets for chicken-meat production
Peter H. Selle | Shemil P. Macelline | Mehdi Toghyani | Sonia Yun Liu
This review explores the potential of including glutamine, a so-called non-essential amino acid, in the formulation of reduced-crude protein (CP) diets for broiler chickens. There is a precedent for...
Dietary copper improves intestinal structural integrity in juvenile grass carp (Ctenopharyngodon idella) probably related to its increased intestinal antioxidant capacity and apical junction complex
Rui Ma | Lin Feng | Pei Wu | Yang Liu | Hong-Mei Ren | Xiao-Wan Jin | Shu-Wei Li | Ling Tang | Xiao-Qiu Zhou | Wei-Dan Jiang
This research evaluated the effects of copper (Cu) on intestinal antioxidant capacity and apical junctional complex (AJC) in juvenile grass carp. A total of 1080 healthy juvenile grass carp (11.16 ± 0.01 g)...
Maternal supplementation with mulberry-leaf flavonoids improves the development of skeletal muscle in the offspring of chickens
Zhenwu Huang | Hongjian Dai | Simeng Li | Zhe Wang | Quanwei Wei | Zhonghua Ning | Yuming Guo | Fangxiong Shi | Zengpeng Lv
The development of skeletal muscle is a crucial factor in determining the meat yield and economic benefits of broiler production. Recent research has shown that mulberry leaves and their extracts can...
Dietary crude protein and protein solubility manipulation enhances intestinal nitrogen absorption and mitigates reactive nitrogen emissions through gut microbiota and metabolome reprogramming in sheep
Zhenbin Zhang | Yiquan Sun | Xinhuang Zhong | Jun Zhu | Sihan Yang | Yalan Gu | Xiang Yu | Yue Lu | Zhiqi Lu | Xuezhao Sun | Mengzhi Wang
Dietary nutrient manipulation (e.g. protein fractions) could lower the environmental footprints of ruminants, especially reactive nitrogen (N). This study investigated the impacts of dietary soluble...
Integrated multi-omics reveals the relationship between growth performance, rumen microbes and metabolic status of Hu sheep with different residual feed intakes
Yanzhen Zhang | Xiaowei Zhang | Dingren Cao | Jinyong Yang | Huiling Mao | Lingling Sun | Chong Wang
Residual feed intake (RFI) is a metric that provides a more accurate measure of feed efficiency. The lower the RFI, the higher the feed efficiency. The changes in the host microbiome and metabolome...
Rumen metagenome reveals the mechanism of mitigation methane emissions by unsaturated fatty acid while maintaining the performance of dairy cows
Zhantao Yang | Yuhui Zheng | Siyuan Liu | Tian Xie | Qianqian Wang | Zhonghan Wang | Shengli Li | Wei Wang
Dietary fat content can reduce the methane production of dairy cows; however, the relevance fatty acid (FA) composition has towards this inhibitory effect is debatable. Furthermore, in-depth studies...
Influences of lauric acid addition on performance, nutrient digestibility and proteins related to mammary gland development in dairy cows
Jing Zhang | Lijun Bu | Yapeng Liu | Wenjie Huo | Chengqiang Xia | Caixia Pei | Qiang Liu
Lauric acid (LA) has the possibility to improve milk production in dairy cows by improving mammary gland development, however, the mechanism by which it might regulate mammary gland development is unclear....
Activation of skeletal carbohydrate-response element binding protein (ChREBP)-mediated de novo lipogenesis increases intramuscular fat content in chickens
Peng Wang | Haihan Xiao | Tian Wu | Qinghua Fu | Xudong Song | Yameng Zhao | Yan Li | Jieping Huang | Ziyi Song
The intracellular lipids in muscle cells of farm animals play a crucial role in determining the overall intramuscular fat (IMF) content, which has a positive impact on meat quality. However, the mechanisms...
Effects of different ratios of soluble to insoluble dietary fiber on growth performance and intestinal health of piglets
Luya Feng | Zhenfu Luo | Jing Wang | Kunfu Wu | Wenliang Wang | Zhimou Liu | Juping Wen | Zhenbin Wang | Gregory J. Duns | Xiaokang Ma | Bi'e Tan
This study investigated the impact of different ratios of soluble to insoluble dietary fiber (SDF:IDF) formulations by sugar beet pulp (SBP) supplementation on piglet growth performance, nutrient digestibility,...
Marine red yeast supplementation improves laying performance by regulating small intestinal homeostasis in aging chickens
Yudian Zhao | Sujin Si | Yangguang Ren | Xing Wu | Zihao Zhang | Yixiang Tian | Jing Li | Yijie Li | Meng Hou | Xueyang Yao | Zhaoheng Xu | Ruirui Jiang | Xiangtao Kang | Yujie Gong | Qiang Li | Yadong Tian
Recent studies have shown that age-related aging evolution is accompanied by imbalances in intestinal homeostasis. Marine red yeast (MRY) is a functional probiotic that has been shown to have antioxidant,...
The evaluation of next-generation probiotics on broiler growth performance, gut morphology, gut microbiome, nutrient digestibility, in addition to enzyme production of Bacillus spp. in vitro
Jacoba I. Bromfield | Shahram Niknafs | Xiaojing Chen | Juhani von Hellens | Darwin Horyanto | Baode Sun | Lei Yu | Viet Hai Tran | Marta Navarro | Eugeni Roura
Considerable research has been conducted into the efficacy of individual probiotics in broiler production, however information on the most effective combinations of synergistic Bacillus probiotic is...
Dietary curcumin alleviates intestinal damage induced by ochratoxin A in juvenile grass carp (Ctenopharyngodon idella): Necroptosis and inflammatory responses
Piao Zhao | Wei-Dan Jiang | Pei Wu | Yang Liu | Hong-Mei Ren | Xiao-Wan Jin | Lin Feng | Xiao-Qiu Zhou
Ochratoxin A (OTA) is one of the most common pollutants in aquatic feed. As a first line of defense, intestinal barriers could be utilized against OTA in order to prevent disorders. Natural product...
Increasing concentrations of dietary threonine, tryptophan, and glycine improve growth performance and intestinal health with decreasing stress responses in broiler chickens raised under multiple stress conditions
Hyun Woo Kim | Jong Hyuk Kim | Gi Ppeum Han | Dong Yong Kil
The current study aimed to compare the effects of increasing concentrations of dietary threonine (Thr), tryptophan (Trp), and glycine (Gly) on growth performance, stress biomarkers, and intestinal function...
Artificial parasin I protein (API) supplementation improves growth performance and intestinal health in weaned piglets challenged with enterotoxigenic Escherichiacoli
Congzhi Zou | Wanxin Zhao | Shenggang Yin | Xiaoyu Xiang | Jiayong Tang | Gang Jia | Lianqiang Che | Guangmang Liu | Xiaoling Chen | Gang Tian | Jingyi Cai | Bo Kang | Hua Zhao
Diarrheas are common risks faced by piglets during the weaning period. This study investigated the alleviating effects of artificial parasin I protein (API) on growth performance and intestinal health...
Supplementation with dimethylglycine sodium salt improves lipid metabolism disorder in intrauterine growth-retarded pigs
Kaiwen Bai | Luyi Jiang | Tian Wang
This study aims to elucidate the mechanism of lipid metabolism disorder in intrauterine growth retardation (IUGR) pigs and the potential alleviating effects of dimethylglycine sodium salt (DMG-Na)....
Combined intestinal microbiota and transcriptomic analysis to investigate the effect of different stocking densities on the ability of Pacific white shrimp (Litopenaeus vannamei) to utilize Chlorella sorokiniana
Hang Yuan | Minghua Xie | Jian Chen | Naijie Hu | Honming Wang | Beiping Tan | Lili Shi | Shuang Zhang
Aiming to investigate the impact of different stocking densities on the ability of Pacific white shrimp (Litopenaeus vannamei) to utilize Chlorella sorokiniana (CHL), a 3 × 2 factorial design stocking...
Dietary saccharin sodium supplementation improves the production performance of dairy goats without residue in milk in summer
Xiongfei Zhang | Jirong Lv | Jingtao Hui | Ao Wu | Lichao Zhao | Linyu Feng | Lu Deng | Miao Yu | Feng Liu | Junhu Yao | Xinjian Lei
The purpose of this study was to investigate the effects of dietary saccharin sodium supplementation on production performance, serum biochemical indicators, and rumen fermentation of dairy goats in...
- Guide for Authors
- Abstracting and Indexing
Stay Informed
Register your interest and receive email alerts tailored to your needs. Sign up below.
- Stay up to date KeAi About us
Cookie notice
We use cookies to analyse and improve our service, to improve and personalise content, advertising and your digital experience. We also share information about your use of our site with our social media, advertising and analytics partners.
Your cookie preferences
We use cookies and similar technologies on our website. Cookies are text files containing small amounts of information, which your computer or mobile device downloads when you visit a website. When you return to websites, or visit websites that use the same cookies, they recognise these cookies and therefore your browsing device.
We use different types of cookies for different things, such as:
- Analysing how you use our website
- Giving you a better, more personalised experience
- Recognising when you’ve sign in
We split our cookies into three distinct categories. You can turn Functional and Performance cookies on and off right here. Strictly necessary cookies can’t be turned off.
Strictly necessary cookies
These cookies are necessary for the website to function and cannot be switched off in our systems. They are usually only set in response to actions made by you which amount to a request for services, such as setting your privacy preferences, logging in or filling in forms. You can set your browser to block or alert you about these cookies, but some parts of the site will not then work. These cookies do not store any personally identifiable information.
Performance cookies
These cookies allow us to count visits and traffic sources so we can measure and improve the performance of our site. They help us to know which pages are the most and least popular and see how visitors move around the site.
Functional cookies
These cookies enable the website to provide enhanced functionality and personalisation. They may be set by us or by third party providers whose services we have added to our pages. If you do not allow these cookies then some or all of these services may not function properly.
Targeting cookies
These cookies are set by a range of social media services that we have added to the site to enable you to share our content with your friends and networks. They are capable of tracking your browser across other sites and building up a profile of your interests. This may impact the content and messages you see on other websites you visit. If you do not allow these cookies you may not be able to use or see these sharing tools.
EDITORIAL article
Editorial: natural feed additives in animal nutrition—their potential as functional feed.

- 1 Centre of Biosciences of the Slovak Academy of Sciences—Institute of Animal Physiology, Košice, Slovakia
- 2 National Research Council of Italy—Institute of Sciences of Food Production (CNR-ISPA), Grugliasco, Italy
Editorial on the Research Topic Natural feed additives in animal nutrition—Their potential as functional feed
In recent years, natural products have become of great importance as growth-promoting agents to replace antibiotics. There is a social pressure on animal production industries to improve animal production performances, minimize economic losses, and ensure the safety of products for human consumption ( 1 ).
The potential negative consequences of the removal of antibiotics as growth promoters on gut health and growth performance have increased interest in finding alternatives to reduce the prevalence of bacterial infections and improve the quality of food products and animal performance by promoting gut health. In general, the term “gut health” represents the interaction between the microbiome, the intestinal wall barrier, and physiological and immune components, which allow different animals to cope with internal and external stressors ( 2 ).
Natural additives in animal feed are able to improve productivity and performance by enhancing digestibility and maintaining and stabilizing beneficial microflora in the gut; improving the quality of animal products can also positively influence the environment ( 3 ).
Natural feed additives such as prebiotics, beneficial microorganisms, bacteriocins, phytogenic compounds, and organic acids have potential as a research area for human or animal nutrition and health, and can satisfy the increasing consumer demand for natural substances. Since they are seen as novel valuable substances, their research is an ongoing discipline. Moreover, pharmacokinetics and the mechanism of action of phytogenic bioactive compounds are discussed and represent a novel approach ( 4 ).
Due to current restrictions on the use of antibiotics, particularly as feed additives, the objective of this Research Topic is to produce novel research results focused on the use of herbs, essential oils, prebiotics, probiotics, and postbiotics as alternative feed additives in animal nutrition.
This Research Topic aims to collect the latest research on the natural feed additives in animal nutrition. It covers a total of 21 articles (three reviews and 18 original researches) focused on three main topics: phytoadditives, probiotics/beneficial bacteria, and other natural additives as an alternative to antibiotic growth promoters with a beneficial impact on animal health. Most of the articles are related to monogastric animals, with more than half of the submissions focusing on poultry, while only two articles are related to ruminants.
Yang et al. examined in their study the effect of in ovo injection of Astragalus polysaccharide (APS) in broiler chickens and their results indicated that APS administered in this form did not affect hatchability but had the potential of promoting intestinal development and enhancing intestinal mucosal immunity in the early stage after hatching, which provides timely and effective protection for chickens.
Ghavipanje et al. determined the effect of pre- and post-partum supplementation of berberin (BBR) administrated in encapsulated form orally to goats. Taking all obtained results together, this study showed the potential of BBR as a novel strategy to mitigate oxidative stress and inflammation in dairy goats during the transition period.
To date, there have been few research studies on dietary supplementation with Elephantopus scaber L., used as a traditional herbal medicine in duck production. The authors of this study, Hu et al. , provided basic knowledge on E. scaber addition to produce high-quality duck meat for human consumption. Based on their study, which contributes to a better understanding of the role of E. scaber as a feed additive in ducks, they recommended E. scaber as an effective supplement with beneficial impact on meat quality and intestinal development.
Phesatcha et al. investigated liquid-containing phytonutrients in dairy cows as dietary additives to reduce rumen protein degradation and confirmed their hypothesis that mangosteen peel liquid-protected soybean meal is able to improve milk yield and milk quality in lactating dairy cows.
Chen et al. showed that the natural polyphenol, chlorogenic acid (CGA), can attenuate oxidative stress-induced growth retardation and intestinal mucosa disruption by increasing antioxidant capacity and improving intestinal barrier integrity in weaned pigs. The authors explored this effect in pigs who were exposed to oxidative stress. They investigated the effect on growth performance, antioxidant activity, and the structure and functions of intestinal mucosa.
Darvishi et al. found that Licorice ( Glycyrrhiza glabra ) in the diet of rainbow trout fingerlings can increase their resistance to Yersinis ruckeri infection and effectively boost their immune system.
Microcine C7 is an antimicrobial peptide produced by Escherichia coli . Dai et al. evaluated its effect on growth performance, immune functions, intestinal barrier and cecal microbiota of broiler chickens. Obtained results indicated that Microcine C7 can be used as a promising alternative to traditional antibiotics. As an immunomodulator, it can regulate intestinal immune function and also exhibit antimicrobial activity against pathogen invasion. The enhancement of gut health improved the serum index and growth performance. To verify the specific mechanism responsible for these positive observations, future studies are needed.
Agradi et al. in their mini-review study evaluated the effects of Goji berries, the fruit of Lycium barbarum , supplementation in the diet of rabbits on reproductive and productive performances, immune system, metabolic homeostasis, and meat quality. Goji berries could determine health benefits for animals and consequently for consumers and have a role in optimizing production as well as in reducing the use of drugs. To find the correct dosage and period of its administration, further research is needed.
Recently, the application of soy lecithin has been dominant in poultry production, and only a few studies have reported the application of its hydroxylated form. Wu et al. described the positive effect of hydroxylated lecithin on growth performance, serum enzyme activity, hormone levels related to lipid metabolism, and the meat quality of Jiangan White goslings and recommended this form of lecithin as a safe and reliable additive in livestock production.
Based on current literature, recommendations for the addition of Paeoniae radix alba extract in raccoon dog diets are scarce. Wang et al. decided to obtain more information for its recommendation as a feed additive for raccoon dogs and they suggest Paeoniae radix alba extract in a concentration of 1–2 g/kg as optimal to achieve the optimal performance of Ussuri raccoon dogs.
To improve stability and bioavailability of essential oils and their compounds, the nanoemulsion as a novel strategy is recommended. Ibrahim et al. found that formulation of eugenol into the nanoemulsion form efficiently controlled its release in the gastrointestinal tract and thus boosted its bioavailability and broilers' response to avian pathogenic Escherichia coli (APEC) strains, especially O78, which causes colibacillosis with major economic losses. Obtained results could provide new insights for controlling colisepticemia induced by APEC.
The objective of the study by Luo et al. was to confirm the hypothesis that dietary supplementation of yucca powder may alleviate heat stress and improve growth performance in growing broilers. They confirmed that yucca could attenuate the heat stress and improve antioxidant status and feed intake probably by down-regulating the cholecystokinin in plasma and hypothalamus.
Chang et al. compared the effect of various oligosaccharides like isomaltooligosaccharide, raffinose oligosaccharide, and chitooligosaccharide on growth performance, immune and antioxidant functions, intestinal morphology, and microbiota in broiler chickens, and they concluded that oligosaccharides represent a very strong alternative to antibiotics.
The study by Danladi et al. demonstrates positive effects of postbiotics and paraprobiotics produced from Lactiplantibacillus plantarum supplementation in the broiler chicken diet on the intestinal microbiota. Administered additives increased concentration of beneficial microbes like Firmicutes while they decreased harmful microbes like Proteobacteria . To achieve a healthier gut, the authors recommended postbiotics and paraprobiotics for modification of the microbiota in the intestine.
Numerous studies have investigated the potential of probiotics, prebiotics, synbiotics, and medicinal herbs as alternatives to antibiotics for the health management of aquaculture species. Wei et al. in their mini-review discuss the potential use of combinations of probiotics and medicinal herbs as prophylactic agents in aquaculture.
Ogbuewu et al. in their review indicated that Bacillus probiotics currently used in the broiler chicken industry are isolated from the gut, food, soil, and ponds. Probiotics may have achieved beneficial effects through various mechanisms of action. They are able to decrease pathogen proliferation in the gastrointestinal tract, increase production of organic acids leading to a decline in gut pH, improve production of antimicrobial compounds, improve oxidative stability and the immune system, and support the production and release of digestive enzymes. However, the potential of Bacillus probiotics in broiler chicken nutrition depends on a variety of factors such as Bacillus strain, species, dose level, and age of chicken. Further research is required to determine the optimum dose level before Bacillus probiotics could be considered as a substitute for antibiotics.
Chang et al. evaluated the potential of postbiotics originated from Lactiplantibacillus plantarum to modulate gut microbiota, mucin dynamics, and immune response in broiler chickens and recommended postbiotics as a good alternative to substitute antibiotic growth promoters in poultry.
The review by Damato et al. focuses on the characteristics and properties of clays as feed additives. It must be considered that clay minerals are not completely inert additives and can interfere with intestinal/ruminal metabolism with possible consequences on animal health. Further studies are needed, particularly on ruminants, to verify possible interferences of clays with rumen fermentations and metabolite uptake, which may affect animal metabolism and, possibly, milk characteristics. In particular, future studies should consider the effects of long-term administration or accumulation of clay minerals in organisms.
Azad et al. determined a beneficial replacement of corn-soybean meal with agricultural by-product cassava residue or fermented cassava residue, which could be a cost-effective dietary supplemental strategy for livestock production. These supplements increased the gut barrier function and beneficially altered gut microbiota composition.
Bai et al. confirmed that Dimethylglycine sodium salt (DMG-Na) can improve the redox status and relieve oxidative damage by scavenging the excessive generated free radicals in suckling piglets. They found that DMG- Na can act as a health-promoting additive in treating hepatic dysfunction in suckling piglets and is beneficial in improving their performance during the suckling period.
Guanidine acetic acid (GAA) is increasingly being considered as a nutritional growth promoter in monogastric animals. Whether or not such a response would exist in rapid-growing lambs is as yet unclear. Under a high-concentrate feed lotting pattern, the feed results obtained in the study of Li et al. indicated that daily gain presented a greater increase response to not only uncoated (GAA) but also coated (GAA) supplementation in young lambs with body weight from 13 to 30 kg, and such positive responses were more pronounced in oaten hay fed lambs in comparison with oaten hay plus wheat silage fed lambs.
In summary, the results of the above-mentioned research studies and reviews represent and summarize new relevant data on application of natural feed additives in animals. Despite all the existing research papers on this extremely important topic, the obtained information show that many aspects of bioavailability and beneficial doses of natural feed additives should be clarified. The presented and recommended future data on biological activity of natural substances in animal organisms could be suitable for pharmaceutical industries and the veterinary sectors.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kostadinović L, Lević J. Effect of phytoadditives in poultry and pig diseases. J Agron Technol Eng Manag. (2018) 1:1–7.
Google Scholar
2. Artuso-Ponte V, Pastor A, Andratsch M. The effects of plant extracts on the immune system of livestock: the isoquinoline alkaloids model. In:Florou-Paneri P, Christaki E, Giannenas I, editors. Feed Additives, Aromatic Plants and Herbs in Animal Nutrition and Health . United Kingdom: Elsevier (2020), p. 295–310. doi: 10.1016/B978-0-12-814700-9.00017-0
CrossRef Full Text | Google Scholar
3. Franz C, Baser KHC, Windisch W. Essential oils and aromatic plants in animal feeding—a European perspective. A review. Flavour Fragr J. (2010) 25:327–40. doi: 10.1002/ffj.1967
4. Placha I, Pogány Simonová M, Lauková A. Natural feed additives and novel approaches for healthy rabbit breeding. Animals. (2022) 12:2111. doi: 10.3390/ani12162111
PubMed Abstract | CrossRef Full Text | Google Scholar
Keywords: nutraceuticals, phytochemicals, probiotics, gut health, metabolism, oxidative stress, immunity
Citation: Placha I, Gai F and Pogány Simonová M (2022) Editorial: Natural feed additives in animal nutrition—Their potential as functional feed. Front. Vet. Sci. 9:1062724. doi: 10.3389/fvets.2022.1062724
Received: 06 October 2022; Accepted: 17 October 2022; Published: 09 November 2022.
Edited and reviewed by: Domenico Bergero , University of Turin, Italy
Copyright © 2022 Placha, Gai and Pogány Simonová. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iveta Placha, placha@saske.sk
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Our Program Divisions
- Our Three Academies
- Government Affairs
- Statement on Diversity and Inclusion
- Our Study Process
- Conflict of Interest Policies and Procedures
- Project Comments and Information
- Read Our Expert Reports and Published Proceedings
- Explore PNAS, the Flagship Scientific Journal of NAS
- Access Transportation Research Board Publications
- Coronavirus Disease 2019 (COVID-19)
- Diversity, Equity, and Inclusion
- Economic Recovery
- Fellowships and Grants
- Publications by Division
- Division of Behavioral and Social Sciences and Education
- Division on Earth and Life Studies
- Division on Engineering and Physical Sciences
- Gulf Research Program
- Health and Medicine Division
- Policy and Global Affairs
- Transportation Research Board
- National Academy of Sciences
- National Academy of Engineering
- National Academy of Medicine
- Publications by Topic
- Agriculture
- Behavioral and Social Sciences
- Biography and Autobiography
- Biology and Life Sciences
- Computers and Information Technology
- Conflict and Security Issues
- Earth Sciences
- Energy and Energy Conservation
- Engineering and Technology
- Environment and Environmental Studies
- Food and Nutrition
- Health and Medicine
- Industry and Labor
- Math, Chemistry, and Physics
- Policy for Science and Technology
- Space and Aeronautics
- Surveys and Statistics
- Transportation and Infrastructure
- Searchable Collections
- New Releases
VIEW LARGER COVER
Scientific Advances in Animal Nutrition
Promise for the new century: proceedings of a symposium.
The science of animal nutrition has made significant advances in the past century. In looking back at the discoveries of the 20th century, we can appreciate the tremendous impact that animal nutrition has had on our lives. From the discovery of vitamins and the sweeping shift in the use of oilseeds to replace animal products as dietary protein sources for animals during the war times of the 1900s-to our integral understanding of nutrients as regulators of gene expression today-animal nutrition has been the cornerstone for scientific advances in many areas.
At the milestone of their 70th year of service to the nation, the National Research Council's (NRC) Committee on Animal Nutrition (CAN) sought to gain a better understanding of the magnitude of recent discoveries and directions in animal nutrition for the new century we are embarking upon. With financial support from the NRC, the committee was able to organize and host a symposium that featured scientists from many backgrounds who were asked to share their ideas about the potential of animal nutrition to address current problems and future challenges.
RESOURCES AT A GLANCE
- Press Release
- Agriculture — Animal Health and Nutrition
- Agriculture — Aquaculture and Fisheries
Suggested Citation
National Research Council. 2001. Scientific Advances in Animal Nutrition: Promise for the New Century: Proceedings of a Symposium . Washington, DC: The National Academies Press. https://doi.org/10.17226/10299. Import this citation to: Bibtex EndNote Reference Manager
Publication Info
- Paperback: 978-0-309-08276-1
- Ebook: 978-0-309-16991-2
What is skim?
The Chapter Skim search tool presents what we've algorithmically identified as the most significant single chunk of text within every page in the chapter. You may select key terms to highlight them within pages of each chapter.
Copyright Information
The National Academies Press (NAP) has partnered with Copyright Clearance Center's Marketplace service to offer you a variety of options for reusing NAP content. Through Marketplace, you may request permission to reprint NAP content in another publication, course pack, secure website, or other media. Marketplace allows you to instantly obtain permission, pay related fees, and print a license directly from the NAP website. The complete terms and conditions of your reuse license can be found in the license agreement that will be made available to you during the online order process. To request permission through Marketplace you are required to create an account by filling out a simple online form. The following list describes license reuses offered by the NAP through Marketplace:
- Republish text, tables, figures, or images in print
- Post on a secure Intranet/Extranet website
- Use in a PowerPoint Presentation
- Distribute via CD-ROM
Click here to obtain permission for the above reuses. If you have questions or comments concerning the Marketplace service, please contact:
Marketplace Support International +1.978.646.2600 US Toll Free +1.855.239.3415 E-mail: [email protected] marketplace.copyright.com
To request permission to distribute a PDF, please contact our Customer Service Department at [email protected] .
What is a prepublication?
An uncorrected copy, or prepublication, is an uncorrected proof of the book. We publish prepublications to facilitate timely access to the committee's findings.
What happens when I pre-order?
The final version of this book has not been published yet. You can pre-order a copy of the book and we will send it to you when it becomes available. We will not charge you for the book until it ships. Pricing for a pre-ordered book is estimated and subject to change. All backorders will be released at the final established price. As a courtesy, if the price increases by more than $3.00 we will notify you. If the price decreases, we will simply charge the lower price. Applicable discounts will be extended.
Downloading and Using eBooks from NAP
What is an ebook.
An ebook is one of two file formats that are intended to be used with e-reader devices and apps such as Amazon Kindle or Apple iBooks.
Why is an eBook better than a PDF?
A PDF is a digital representation of the print book, so while it can be loaded into most e-reader programs, it doesn't allow for resizable text or advanced, interactive functionality. The eBook is optimized for e-reader devices and apps, which means that it offers a much better digital reading experience than a PDF, including resizable text and interactive features (when available).
Where do I get eBook files?
eBook files are now available for a large number of reports on the NAP.edu website. If an eBook is available, you'll see the option to purchase it on the book page.
View more FAQ's about Ebooks
Types of Publications
Proceedings: Proceedings published by the National Academies of Sciences, Engineering, and Medicine chronicle the presentations and discussions at a workshop, symposium, or other event convened by the National Academies. The statements and opinions contained in proceedings are those of the participants and are not endorsed by other participants, the planning committee, or the National Academies.

IMAGES
COMMENTS
all related to animal nutrition | Explore the latest full-text research PDFs, articles, conference papers, preprints and more on ANIMAL NUTRITION. Find methods information, sources, references or ...
An International Publication for Research Findings Related to Animal Nutrition. Animal Nutrition encompasses the full gamut of animal nutritional sciences and reviews including, but not limited to, fundamental aspects of animal nutrition such as nutritional requirements, metabolic studies, body composition, energetics, immunology, neuroscience, microbiology, genetics and molecular and cell ...
This book covers hot topics in the nutrition and metabolism of terrestrial and aquatic animals, including the interorgan transport and utilization of water, minerals, amino acids, glucose, and fructose; the development of alternatives to in-feed antibiotics for animals (e.g., swine and poultry); and metabolic disorders (or diseases) resulting from nutrient deficiencies.
Nutrigenomics (molecular animal nutrition) is a relatively recent topic that deals with animal nutrition on a molecular level . The goal is to use contemporary molecular biological sciences and technology to examine animal biological processes at the gene expression level or to examine the interactions between nutrients and genes ( Malgwi et al ...
Read the latest articles of Animal Nutrition at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature. ... Research article Open access ... [Animal Nutrition 12 (2023) 236-244]
Animal Nutriomics is an international, peer-reviewed, open-access journal that aims to provide timely updates on the latest research achievements, breakthrough advances, new technologies, methods, and analytical approaches in the field of animal nutrition science. The journal focuses primarily on molecular animal nutrition, omics technologies, and epigenetics, while also encompassing ...
Decades of development on the science of animal nutrition have allowed for remarkably precise estimations of the dietary needs of livestock animals; as our fundamental understanding of nutrients shifts to focus on bioactivity, molecular nutrition represents a new enticing frontier for animal scientists.This Research Topic aims to capture recent ...
The Journal of Animal Physiology and Animal Nutrition is a bi-monthly journal that publishes scientific research in the fields of animal physiology, biochemistry and physiology of nutrition, animal nutrition, and animal feed science and research (including feed technology and preservation). Specific research on farm and companion animals is encouraged, although work on exotic species is also ...
This Special Issue "Animal Nutrition and Productions: Series II" is a collection of 23 articles that capture the latest knowledge and innovations in animal nutrition and production and cover current topics through multi- and trans-disciplinary approaches, such as additives and novel feeds, animal productivity, reproductive efficiency, animal welfare and health, and product safety and quality.
Paternal transgenerational nutritional epigenetic effect: A new insight into nutritional manipulation to reduce the use of antibiotics in animal feeding Xinyi Li, Mengya Wang, Shimin Liu, Xiaodong Chen, ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Recent Advances in Animal ...
Animal Nutrition ousama alzahal. AB Vista. Marlborough, United Kingdom. Associate Editor. Animal Nutrition sonia andrés. Spanish National Research Council (CSIC) ... Research Topics See all (16) Learn more about Research Topics. Footer. Guidelines. Author guidelines; Editor guidelines; Policies and publication ethics ...
Recent research has shown that mulberry leaves and their extracts can... Share article Dietary crude protein and protein solubility manipulation enhances intestinal nitrogen absorption and mitigates reactive nitrogen emissions through gut microbiota and metabolome reprogramming in sheep
This Research Topic aims to collect the latest research on the natural feed additives in animal nutrition. It covers a total of 21 articles (three reviews and 18 original researches) focused on three main topics: phytoadditives, probiotics/beneficial bacteria, and other natural additives as an alternative to antibiotic growth promoters with a ...
Read the latest articles of Animal Nutrition at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature
At the milestone of their 70th year of service to the nation, the National Research Council's (NRC) Committee on Animal Nutrition (CAN) sought to gain a better understanding of the magnitude of recent discoveries and directions in animal nutrition for the new century we are embarking upon.
Animal nutrition is a section in the open access journal Animals focusing on nutritional research, which is crucial for the health, well-being, and productivity of various animal species including farm and companion animals, wildlife, and aquatic species.The section explores dietary requirements, nutrient metabolism, and feeding practices to optimize feed formulations and management strategies ...
Archives of Animal Nutrition is an international journal covering the biochemical and physiological basis of animal nutrition. Emphasis is laid on original papers on protein and amino acid metabolism, energy transformation, mineral metabolism, vitamin metabolism, nutritional effects on intestinal and body functions in combination with performance criteria, respectively.
A project collection of Animals (ISSN 2076-2615). This project collection belongs to the section "Animal Nutrition". Papers displayed on this page all arise from the same project. Editorial decisions were made independently of project staff and handled by the Editor-in-Chief or qualified Editorial Board members. Viewed by 31479
The Recent Advances in Animal Nutrition in Australia (RAAN-A) conference in 2023 continued its tradition of fostering the sharing of the latest research in the field of animal nutrition across the most economically significant animal species, including poultry, pigs, sheep and cattle, and also companion animals and aquaculture. RAAN-A is