An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

National Institute of Environmental Health Sciences
Your environment. your health., what is ethics in research & why is it important, by david b. resnik, j.d., ph.d..
December 23, 2020
The ideas and opinions expressed in this essay are the author’s own and do not necessarily represent those of the NIH, NIEHS, or US government.

When most people think of ethics (or morals), they think of rules for distinguishing between right and wrong, such as the Golden Rule ("Do unto others as you would have them do unto you"), a code of professional conduct like the Hippocratic Oath ("First of all, do no harm"), a religious creed like the Ten Commandments ("Thou Shalt not kill..."), or a wise aphorisms like the sayings of Confucius. This is the most common way of defining "ethics": norms for conduct that distinguish between acceptable and unacceptable behavior.
Most people learn ethical norms at home, at school, in church, or in other social settings. Although most people acquire their sense of right and wrong during childhood, moral development occurs throughout life and human beings pass through different stages of growth as they mature. Ethical norms are so ubiquitous that one might be tempted to regard them as simple commonsense. On the other hand, if morality were nothing more than commonsense, then why are there so many ethical disputes and issues in our society?
Alternatives to Animal Testing
Alternative test methods are methods that replace, reduce, or refine animal use in research and testing
Learn more about Environmental science Basics
One plausible explanation of these disagreements is that all people recognize some common ethical norms but interpret, apply, and balance them in different ways in light of their own values and life experiences. For example, two people could agree that murder is wrong but disagree about the morality of abortion because they have different understandings of what it means to be a human being.
Most societies also have legal rules that govern behavior, but ethical norms tend to be broader and more informal than laws. Although most societies use laws to enforce widely accepted moral standards and ethical and legal rules use similar concepts, ethics and law are not the same. An action may be legal but unethical or illegal but ethical. We can also use ethical concepts and principles to criticize, evaluate, propose, or interpret laws. Indeed, in the last century, many social reformers have urged citizens to disobey laws they regarded as immoral or unjust laws. Peaceful civil disobedience is an ethical way of protesting laws or expressing political viewpoints.
Another way of defining 'ethics' focuses on the disciplines that study standards of conduct, such as philosophy, theology, law, psychology, or sociology. For example, a "medical ethicist" is someone who studies ethical standards in medicine. One may also define ethics as a method, procedure, or perspective for deciding how to act and for analyzing complex problems and issues. For instance, in considering a complex issue like global warming , one may take an economic, ecological, political, or ethical perspective on the problem. While an economist might examine the cost and benefits of various policies related to global warming, an environmental ethicist could examine the ethical values and principles at stake.
See ethics in practice at NIEHS
Read latest updates in our monthly Global Environmental Health Newsletter

Many different disciplines, institutions , and professions have standards for behavior that suit their particular aims and goals. These standards also help members of the discipline to coordinate their actions or activities and to establish the public's trust of the discipline. For instance, ethical standards govern conduct in medicine, law, engineering, and business. Ethical norms also serve the aims or goals of research and apply to people who conduct scientific research or other scholarly or creative activities. There is even a specialized discipline, research ethics, which studies these norms. See Glossary of Commonly Used Terms in Research Ethics and Research Ethics Timeline .
There are several reasons why it is important to adhere to ethical norms in research. First, norms promote the aims of research , such as knowledge, truth, and avoidance of error. For example, prohibitions against fabricating , falsifying, or misrepresenting research data promote the truth and minimize error.
Join an NIEHS Study
See how we put research Ethics to practice.
Visit Joinastudy.niehs.nih.gov to see the various studies NIEHS perform.

Second, since research often involves a great deal of cooperation and coordination among many different people in different disciplines and institutions, ethical standards promote the values that are essential to collaborative work , such as trust, accountability, mutual respect, and fairness. For example, many ethical norms in research, such as guidelines for authorship , copyright and patenting policies , data sharing policies, and confidentiality rules in peer review, are designed to protect intellectual property interests while encouraging collaboration. Most researchers want to receive credit for their contributions and do not want to have their ideas stolen or disclosed prematurely.
Third, many of the ethical norms help to ensure that researchers can be held accountable to the public . For instance, federal policies on research misconduct, conflicts of interest, the human subjects protections, and animal care and use are necessary in order to make sure that researchers who are funded by public money can be held accountable to the public.
Fourth, ethical norms in research also help to build public support for research. People are more likely to fund a research project if they can trust the quality and integrity of research.
Finally, many of the norms of research promote a variety of other important moral and social values , such as social responsibility, human rights, animal welfare, compliance with the law, and public health and safety. Ethical lapses in research can significantly harm human and animal subjects, students, and the public. For example, a researcher who fabricates data in a clinical trial may harm or even kill patients, and a researcher who fails to abide by regulations and guidelines relating to radiation or biological safety may jeopardize his health and safety or the health and safety of staff and students.
Codes and Policies for Research Ethics
Given the importance of ethics for the conduct of research, it should come as no surprise that many different professional associations, government agencies, and universities have adopted specific codes, rules, and policies relating to research ethics. Many government agencies have ethics rules for funded researchers.
- National Institutes of Health (NIH)
- National Science Foundation (NSF)
- Food and Drug Administration (FDA)
- Environmental Protection Agency (EPA)
- US Department of Agriculture (USDA)
- Singapore Statement on Research Integrity
- American Chemical Society, The Chemist Professional’s Code of Conduct
- Code of Ethics (American Society for Clinical Laboratory Science)
- American Psychological Association, Ethical Principles of Psychologists and Code of Conduct
- Statement on Professional Ethics (American Association of University Professors)
- Nuremberg Code
- World Medical Association's Declaration of Helsinki
Ethical Principles
The following is a rough and general summary of some ethical principles that various codes address*:

Strive for honesty in all scientific communications. Honestly report data, results, methods and procedures, and publication status. Do not fabricate, falsify, or misrepresent data. Do not deceive colleagues, research sponsors, or the public.

Objectivity
Strive to avoid bias in experimental design, data analysis, data interpretation, peer review, personnel decisions, grant writing, expert testimony, and other aspects of research where objectivity is expected or required. Avoid or minimize bias or self-deception. Disclose personal or financial interests that may affect research.

Keep your promises and agreements; act with sincerity; strive for consistency of thought and action.

Carefulness
Avoid careless errors and negligence; carefully and critically examine your own work and the work of your peers. Keep good records of research activities, such as data collection, research design, and correspondence with agencies or journals.

Share data, results, ideas, tools, resources. Be open to criticism and new ideas.
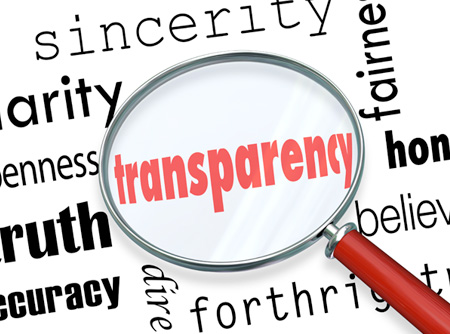
Transparency
Disclose methods, materials, assumptions, analyses, and other information needed to evaluate your research.

Accountability
Take responsibility for your part in research and be prepared to give an account (i.e. an explanation or justification) of what you did on a research project and why.

Intellectual Property
Honor patents, copyrights, and other forms of intellectual property. Do not use unpublished data, methods, or results without permission. Give proper acknowledgement or credit for all contributions to research. Never plagiarize.

Confidentiality
Protect confidential communications, such as papers or grants submitted for publication, personnel records, trade or military secrets, and patient records.

Responsible Publication
Publish in order to advance research and scholarship, not to advance just your own career. Avoid wasteful and duplicative publication.

Responsible Mentoring
Help to educate, mentor, and advise students. Promote their welfare and allow them to make their own decisions.

Respect for Colleagues
Respect your colleagues and treat them fairly.

Social Responsibility
Strive to promote social good and prevent or mitigate social harms through research, public education, and advocacy.

Non-Discrimination
Avoid discrimination against colleagues or students on the basis of sex, race, ethnicity, or other factors not related to scientific competence and integrity.

Maintain and improve your own professional competence and expertise through lifelong education and learning; take steps to promote competence in science as a whole.

Know and obey relevant laws and institutional and governmental policies.

Animal Care
Show proper respect and care for animals when using them in research. Do not conduct unnecessary or poorly designed animal experiments.

Human Subjects protection
When conducting research on human subjects, minimize harms and risks and maximize benefits; respect human dignity, privacy, and autonomy; take special precautions with vulnerable populations; and strive to distribute the benefits and burdens of research fairly.
* Adapted from Shamoo A and Resnik D. 2015. Responsible Conduct of Research, 3rd ed. (New York: Oxford University Press).
Ethical Decision Making in Research
Although codes, policies, and principles are very important and useful, like any set of rules, they do not cover every situation, they often conflict, and they require interpretation. It is therefore important for researchers to learn how to interpret, assess, and apply various research rules and how to make decisions and act ethically in various situations. The vast majority of decisions involve the straightforward application of ethical rules. For example, consider the following case:
The research protocol for a study of a drug on hypertension requires the administration of the drug at different doses to 50 laboratory mice, with chemical and behavioral tests to determine toxic effects. Tom has almost finished the experiment for Dr. Q. He has only 5 mice left to test. However, he really wants to finish his work in time to go to Florida on spring break with his friends, who are leaving tonight. He has injected the drug in all 50 mice but has not completed all of the tests. He therefore decides to extrapolate from the 45 completed results to produce the 5 additional results.
Many different research ethics policies would hold that Tom has acted unethically by fabricating data. If this study were sponsored by a federal agency, such as the NIH, his actions would constitute a form of research misconduct , which the government defines as "fabrication, falsification, or plagiarism" (or FFP). Actions that nearly all researchers classify as unethical are viewed as misconduct. It is important to remember, however, that misconduct occurs only when researchers intend to deceive : honest errors related to sloppiness, poor record keeping, miscalculations, bias, self-deception, and even negligence do not constitute misconduct. Also, reasonable disagreements about research methods, procedures, and interpretations do not constitute research misconduct. Consider the following case:
Dr. T has just discovered a mathematical error in his paper that has been accepted for publication in a journal. The error does not affect the overall results of his research, but it is potentially misleading. The journal has just gone to press, so it is too late to catch the error before it appears in print. In order to avoid embarrassment, Dr. T decides to ignore the error.
Dr. T's error is not misconduct nor is his decision to take no action to correct the error. Most researchers, as well as many different policies and codes would say that Dr. T should tell the journal (and any coauthors) about the error and consider publishing a correction or errata. Failing to publish a correction would be unethical because it would violate norms relating to honesty and objectivity in research.
There are many other activities that the government does not define as "misconduct" but which are still regarded by most researchers as unethical. These are sometimes referred to as " other deviations " from acceptable research practices and include:
- Publishing the same paper in two different journals without telling the editors
- Submitting the same paper to different journals without telling the editors
- Not informing a collaborator of your intent to file a patent in order to make sure that you are the sole inventor
- Including a colleague as an author on a paper in return for a favor even though the colleague did not make a serious contribution to the paper
- Discussing with your colleagues confidential data from a paper that you are reviewing for a journal
- Using data, ideas, or methods you learn about while reviewing a grant or a papers without permission
- Trimming outliers from a data set without discussing your reasons in paper
- Using an inappropriate statistical technique in order to enhance the significance of your research
- Bypassing the peer review process and announcing your results through a press conference without giving peers adequate information to review your work
- Conducting a review of the literature that fails to acknowledge the contributions of other people in the field or relevant prior work
- Stretching the truth on a grant application in order to convince reviewers that your project will make a significant contribution to the field
- Stretching the truth on a job application or curriculum vita
- Giving the same research project to two graduate students in order to see who can do it the fastest
- Overworking, neglecting, or exploiting graduate or post-doctoral students
- Failing to keep good research records
- Failing to maintain research data for a reasonable period of time
- Making derogatory comments and personal attacks in your review of author's submission
- Promising a student a better grade for sexual favors
- Using a racist epithet in the laboratory
- Making significant deviations from the research protocol approved by your institution's Animal Care and Use Committee or Institutional Review Board for Human Subjects Research without telling the committee or the board
- Not reporting an adverse event in a human research experiment
- Wasting animals in research
- Exposing students and staff to biological risks in violation of your institution's biosafety rules
- Sabotaging someone's work
- Stealing supplies, books, or data
- Rigging an experiment so you know how it will turn out
- Making unauthorized copies of data, papers, or computer programs
- Owning over $10,000 in stock in a company that sponsors your research and not disclosing this financial interest
- Deliberately overestimating the clinical significance of a new drug in order to obtain economic benefits
These actions would be regarded as unethical by most scientists and some might even be illegal in some cases. Most of these would also violate different professional ethics codes or institutional policies. However, they do not fall into the narrow category of actions that the government classifies as research misconduct. Indeed, there has been considerable debate about the definition of "research misconduct" and many researchers and policy makers are not satisfied with the government's narrow definition that focuses on FFP. However, given the huge list of potential offenses that might fall into the category "other serious deviations," and the practical problems with defining and policing these other deviations, it is understandable why government officials have chosen to limit their focus.
Finally, situations frequently arise in research in which different people disagree about the proper course of action and there is no broad consensus about what should be done. In these situations, there may be good arguments on both sides of the issue and different ethical principles may conflict. These situations create difficult decisions for research known as ethical or moral dilemmas . Consider the following case:
Dr. Wexford is the principal investigator of a large, epidemiological study on the health of 10,000 agricultural workers. She has an impressive dataset that includes information on demographics, environmental exposures, diet, genetics, and various disease outcomes such as cancer, Parkinson’s disease (PD), and ALS. She has just published a paper on the relationship between pesticide exposure and PD in a prestigious journal. She is planning to publish many other papers from her dataset. She receives a request from another research team that wants access to her complete dataset. They are interested in examining the relationship between pesticide exposures and skin cancer. Dr. Wexford was planning to conduct a study on this topic.
Dr. Wexford faces a difficult choice. On the one hand, the ethical norm of openness obliges her to share data with the other research team. Her funding agency may also have rules that obligate her to share data. On the other hand, if she shares data with the other team, they may publish results that she was planning to publish, thus depriving her (and her team) of recognition and priority. It seems that there are good arguments on both sides of this issue and Dr. Wexford needs to take some time to think about what she should do. One possible option is to share data, provided that the investigators sign a data use agreement. The agreement could define allowable uses of the data, publication plans, authorship, etc. Another option would be to offer to collaborate with the researchers.
The following are some step that researchers, such as Dr. Wexford, can take to deal with ethical dilemmas in research:
What is the problem or issue?
It is always important to get a clear statement of the problem. In this case, the issue is whether to share information with the other research team.
What is the relevant information?
Many bad decisions are made as a result of poor information. To know what to do, Dr. Wexford needs to have more information concerning such matters as university or funding agency or journal policies that may apply to this situation, the team's intellectual property interests, the possibility of negotiating some kind of agreement with the other team, whether the other team also has some information it is willing to share, the impact of the potential publications, etc.
What are the different options?
People may fail to see different options due to a limited imagination, bias, ignorance, or fear. In this case, there may be other choices besides 'share' or 'don't share,' such as 'negotiate an agreement' or 'offer to collaborate with the researchers.'
How do ethical codes or policies as well as legal rules apply to these different options?
The university or funding agency may have policies on data management that apply to this case. Broader ethical rules, such as openness and respect for credit and intellectual property, may also apply to this case. Laws relating to intellectual property may be relevant.
Are there any people who can offer ethical advice?
It may be useful to seek advice from a colleague, a senior researcher, your department chair, an ethics or compliance officer, or anyone else you can trust. In the case, Dr. Wexford might want to talk to her supervisor and research team before making a decision.
After considering these questions, a person facing an ethical dilemma may decide to ask more questions, gather more information, explore different options, or consider other ethical rules. However, at some point he or she will have to make a decision and then take action. Ideally, a person who makes a decision in an ethical dilemma should be able to justify his or her decision to himself or herself, as well as colleagues, administrators, and other people who might be affected by the decision. He or she should be able to articulate reasons for his or her conduct and should consider the following questions in order to explain how he or she arrived at his or her decision:
- Which choice will probably have the best overall consequences for science and society?
- Which choice could stand up to further publicity and scrutiny?
- Which choice could you not live with?
- Think of the wisest person you know. What would he or she do in this situation?
- Which choice would be the most just, fair, or responsible?
After considering all of these questions, one still might find it difficult to decide what to do. If this is the case, then it may be appropriate to consider others ways of making the decision, such as going with a gut feeling or intuition, seeking guidance through prayer or meditation, or even flipping a coin. Endorsing these methods in this context need not imply that ethical decisions are irrational, however. The main point is that human reasoning plays a pivotal role in ethical decision-making but there are limits to its ability to solve all ethical dilemmas in a finite amount of time.
Promoting Ethical Conduct in Science

Do U.S. research institutions meet or exceed federal mandates for instruction in responsible conduct of research? A national survey

Read about U.S. research instutuins follow federal manadates for ethics in research
Learn more about NIEHS Research
Most academic institutions in the US require undergraduate, graduate, or postgraduate students to have some education in the responsible conduct of research (RCR) . The NIH and NSF have both mandated training in research ethics for students and trainees. Many academic institutions outside of the US have also developed educational curricula in research ethics
Those of you who are taking or have taken courses in research ethics may be wondering why you are required to have education in research ethics. You may believe that you are highly ethical and know the difference between right and wrong. You would never fabricate or falsify data or plagiarize. Indeed, you also may believe that most of your colleagues are highly ethical and that there is no ethics problem in research..
If you feel this way, relax. No one is accusing you of acting unethically. Indeed, the evidence produced so far shows that misconduct is a very rare occurrence in research, although there is considerable variation among various estimates. The rate of misconduct has been estimated to be as low as 0.01% of researchers per year (based on confirmed cases of misconduct in federally funded research) to as high as 1% of researchers per year (based on self-reports of misconduct on anonymous surveys). See Shamoo and Resnik (2015), cited above.
Clearly, it would be useful to have more data on this topic, but so far there is no evidence that science has become ethically corrupt, despite some highly publicized scandals. Even if misconduct is only a rare occurrence, it can still have a tremendous impact on science and society because it can compromise the integrity of research, erode the public’s trust in science, and waste time and resources. Will education in research ethics help reduce the rate of misconduct in science? It is too early to tell. The answer to this question depends, in part, on how one understands the causes of misconduct. There are two main theories about why researchers commit misconduct. According to the "bad apple" theory, most scientists are highly ethical. Only researchers who are morally corrupt, economically desperate, or psychologically disturbed commit misconduct. Moreover, only a fool would commit misconduct because science's peer review system and self-correcting mechanisms will eventually catch those who try to cheat the system. In any case, a course in research ethics will have little impact on "bad apples," one might argue.
According to the "stressful" or "imperfect" environment theory, misconduct occurs because various institutional pressures, incentives, and constraints encourage people to commit misconduct, such as pressures to publish or obtain grants or contracts, career ambitions, the pursuit of profit or fame, poor supervision of students and trainees, and poor oversight of researchers (see Shamoo and Resnik 2015). Moreover, defenders of the stressful environment theory point out that science's peer review system is far from perfect and that it is relatively easy to cheat the system. Erroneous or fraudulent research often enters the public record without being detected for years. Misconduct probably results from environmental and individual causes, i.e. when people who are morally weak, ignorant, or insensitive are placed in stressful or imperfect environments. In any case, a course in research ethics can be useful in helping to prevent deviations from norms even if it does not prevent misconduct. Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. Many of the deviations that occur in research may occur because researchers simply do not know or have never thought seriously about some of the ethical norms of research. For example, some unethical authorship practices probably reflect traditions and practices that have not been questioned seriously until recently. If the director of a lab is named as an author on every paper that comes from his lab, even if he does not make a significant contribution, what could be wrong with that? That's just the way it's done, one might argue. Another example where there may be some ignorance or mistaken traditions is conflicts of interest in research. A researcher may think that a "normal" or "traditional" financial relationship, such as accepting stock or a consulting fee from a drug company that sponsors her research, raises no serious ethical issues. Or perhaps a university administrator sees no ethical problem in taking a large gift with strings attached from a pharmaceutical company. Maybe a physician thinks that it is perfectly appropriate to receive a $300 finder’s fee for referring patients into a clinical trial.
If "deviations" from ethical conduct occur in research as a result of ignorance or a failure to reflect critically on problematic traditions, then a course in research ethics may help reduce the rate of serious deviations by improving the researcher's understanding of ethics and by sensitizing him or her to the issues.
Finally, education in research ethics should be able to help researchers grapple with the ethical dilemmas they are likely to encounter by introducing them to important concepts, tools, principles, and methods that can be useful in resolving these dilemmas. Scientists must deal with a number of different controversial topics, such as human embryonic stem cell research, cloning, genetic engineering, and research involving animal or human subjects, which require ethical reflection and deliberation.
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Activities /
Ensuring ethical standards and procedures for research with human beings
Research ethics govern the standards of conduct for scientific researchers. It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants. As such, all research involving human beings should be reviewed by an ethics committee to ensure that the appropriate ethical standards are being upheld. Discussion of the ethical principles of beneficence, justice and autonomy are central to ethical review.
WHO works with Member States and partners to promote ethical standards and appropriate systems of review for any course of research involving human subjects. Within WHO, the Research Ethics Review Committee (ERC) ensures that WHO only supports research of the highest ethical standards. The ERC reviews all research projects involving human participants supported either financially or technically by WHO. The ERC is guided in its work by the World Medical Association Declaration of Helsinki (1964), last updated in 2013, as well as the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS 2016).
WHO releases AI ethics and governance guidance for large multi-modal models
Call for proposals: WHO project on ethical climate and health research
Call for applications: Ethical issues arising in research into health and climate change
Research Ethics Review Committee

Standards and operational guidance for ethics review of health-related research with...

WHO tool for benchmarking ethics oversight of health-related research involving human...
Related activities
Developing normative guidance to address ethical challenges in global health
Supporting countries to manage ethical issues during outbreaks and emergencies
Engaging the global community in health ethics
Building ethics capacity
Framing the ethics of public health surveillance
Related health topics
Global health ethics
Human genome editing
Related teams
Related links
- International ethical guidelines for biomedical research involving human subjects Council for International Organizations of Medical Sciences. pdf, 1.55Mb
- International ethical guidelines for epidemiological studies Council for International Organizations of Medical Sciences. pdf, 634Kb
- World Medical Association: Declaration of Helsinki
- European Group on Ethics
- Directive 2001/20/ec of the European Parliament and of the Council pdf, 152Kb
- Council of Europe (Oviedo Convention - Protocol on biomedical research)
- Nuffield Council: The ethics of research related to healthcare in developing countries
Ethical Considerations In Psychology Research
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
Ethics refers to the correct rules of conduct necessary when carrying out research. We have a moral responsibility to protect research participants from harm.
However important the issue under investigation, psychologists must remember that they have a duty to respect the rights and dignity of research participants. This means that they must abide by certain moral principles and rules of conduct.
What are Ethical Guidelines?
In Britain, ethical guidelines for research are published by the British Psychological Society, and in America, by the American Psychological Association. The purpose of these codes of conduct is to protect research participants, the reputation of psychology, and psychologists themselves.
Moral issues rarely yield a simple, unambiguous, right or wrong answer. It is, therefore, often a matter of judgment whether the research is justified or not.
For example, it might be that a study causes psychological or physical discomfort to participants; maybe they suffer pain or perhaps even come to serious harm.
On the other hand, the investigation could lead to discoveries that benefit the participants themselves or even have the potential to increase the sum of human happiness.
Rosenthal and Rosnow (1984) also discuss the potential costs of failing to carry out certain research. Who is to weigh up these costs and benefits? Who is to judge whether the ends justify the means?
Finally, if you are ever in doubt as to whether research is ethical or not, it is worthwhile remembering that if there is a conflict of interest between the participants and the researcher, it is the interests of the subjects that should take priority.
Studies must now undergo an extensive review by an institutional review board (US) or ethics committee (UK) before they are implemented. All UK research requires ethical approval by one or more of the following:
- Department Ethics Committee (DEC) : for most routine research.
- Institutional Ethics Committee (IEC) : for non-routine research.
- External Ethics Committee (EEC) : for research that s externally regulated (e.g., NHS research).
Committees review proposals to assess if the potential benefits of the research are justifiable in light of the possible risk of physical or psychological harm.
These committees may request researchers make changes to the study’s design or procedure or, in extreme cases, deny approval of the study altogether.
The British Psychological Society (BPS) and American Psychological Association (APA) have issued a code of ethics in psychology that provides guidelines for conducting research. Some of the more important ethical issues are as follows:
Informed Consent
Before the study begins, the researcher must outline to the participants what the research is about and then ask for their consent (i.e., permission) to participate.
An adult (18 years +) capable of being permitted to participate in a study can provide consent. Parents/legal guardians of minors can also provide consent to allow their children to participate in a study.
Whenever possible, investigators should obtain the consent of participants. In practice, this means it is not sufficient to get potential participants to say “Yes.”
They also need to know what it is that they agree to. In other words, the psychologist should, so far as is practicable, explain what is involved in advance and obtain the informed consent of participants.
Informed consent must be informed, voluntary, and rational. Participants must be given relevant details to make an informed decision, including the purpose, procedures, risks, and benefits. Consent must be given voluntarily without undue coercion. And participants must have the capacity to rationally weigh the decision.
Components of informed consent include clearly explaining the risks and expected benefits, addressing potential therapeutic misconceptions about experimental treatments, allowing participants to ask questions, and describing methods to minimize risks like emotional distress.
Investigators should tailor the consent language and process appropriately for the study population. Obtaining meaningful informed consent is an ethical imperative for human subjects research.
The voluntary nature of participation should not be compromised through coercion or undue influence. Inducements should be fair and not excessive/inappropriate.
However, it is not always possible to gain informed consent. Where the researcher can’t ask the actual participants, a similar group of people can be asked how they would feel about participating.
If they think it would be OK, then it can be assumed that the real participants will also find it acceptable. This is known as presumptive consent.
However, a problem with this method is that there might be a mismatch between how people think they would feel/behave and how they actually feel and behave during a study.
In order for consent to be ‘informed,’ consent forms may need to be accompanied by an information sheet for participants’ setting out information about the proposed study (in lay terms), along with details about the investigators and how they can be contacted.
Special considerations exist when obtaining consent from vulnerable populations with decisional impairments, such as psychiatric patients, intellectually disabled persons, and children/adolescents. Capacity can vary widely so should be assessed individually, but interventions to improve comprehension may help. Legally authorized representatives usually must provide consent for children.
Participants must be given information relating to the following:
- A statement that participation is voluntary and that refusal to participate will not result in any consequences or any loss of benefits that the person is otherwise entitled to receive.
- Purpose of the research.
- All foreseeable risks and discomforts to the participant (if there are any). These include not only physical injury but also possible psychological.
- Procedures involved in the research.
- Benefits of the research to society and possibly to the individual human subject.
- Length of time the subject is expected to participate.
- Person to contact for answers to questions or in the event of injury or emergency.
- Subjects” right to confidentiality and the right to withdraw from the study at any time without any consequences.
Debriefing after a study involves informing participants about the purpose, providing an opportunity to ask questions, and addressing any harm from participation. Debriefing serves an educational function and allows researchers to correct misconceptions. It is an ethical imperative.
After the research is over, the participant should be able to discuss the procedure and the findings with the psychologist. They must be given a general idea of what the researcher was investigating and why, and their part in the research should be explained.
Participants must be told if they have been deceived and given reasons why. They must be asked if they have any questions, which should be answered honestly and as fully as possible.
Debriefing should occur as soon as possible and be as full as possible; experimenters should take reasonable steps to ensure that participants understand debriefing.
“The purpose of debriefing is to remove any misconceptions and anxieties that the participants have about the research and to leave them with a sense of dignity, knowledge, and a perception of time not wasted” (Harris, 1998).
The debriefing aims to provide information and help the participant leave the experimental situation in a similar frame of mind as when he/she entered it (Aronson, 1988).
Exceptions may exist if debriefing seriously compromises study validity or causes harm itself, like negative emotions in children. Consultation with an institutional review board guides exceptions.
Debriefing indicates investigators’ commitment to participant welfare. Harms may not be raised in the debriefing itself, so responsibility continues after data collection. Following up demonstrates respect and protects persons in human subjects research.
Protection of Participants
Researchers must ensure that those participating in research will not be caused distress. They must be protected from physical and mental harm. This means you must not embarrass, frighten, offend or harm participants.
Normally, the risk of harm must be no greater than in ordinary life, i.e., participants should not be exposed to risks greater than or additional to those encountered in their normal lifestyles.
The researcher must also ensure that if vulnerable groups are to be used (elderly, disabled, children, etc.), they must receive special care. For example, if studying children, ensure their participation is brief as they get tired easily and have a limited attention span.
Researchers are not always accurately able to predict the risks of taking part in a study, and in some cases, a therapeutic debriefing may be necessary if participants have become disturbed during the research (as happened to some participants in Zimbardo’s prisoners/guards study ).
Deception research involves purposely misleading participants or withholding information that could influence their participation decision. This method is controversial because it limits informed consent and autonomy, but can provide otherwise unobtainable valuable knowledge.
Types of deception include (i) deliberate misleading, e.g. using confederates, staged manipulations in field settings, deceptive instructions; (ii) deception by omission, e.g., failure to disclose full information about the study, or creating ambiguity.
The researcher should avoid deceiving participants about the nature of the research unless there is no alternative – and even then, this would need to be judged acceptable by an independent expert. However, some types of research cannot be carried out without at least some element of deception.
For example, in Milgram’s study of obedience , the participants thought they were giving electric shocks to a learner when they answered a question wrongly. In reality, no shocks were given, and the learners were confederates of Milgram.
This is sometimes necessary to avoid demand characteristics (i.e., the clues in an experiment that lead participants to think they know what the researcher is looking for).
Another common example is when a stooge or confederate of the experimenter is used (this was the case in both the experiments carried out by Asch ).
According to ethics codes, deception must have strong scientific justification, and non-deceptive alternatives should not be feasible. Deception that causes significant harm is prohibited. Investigators should carefully weigh whether deception is necessary and ethical for their research.
However, participants must be deceived as little as possible, and any deception must not cause distress. Researchers can determine whether participants are likely distressed when deception is disclosed by consulting culturally relevant groups.
Participants should immediately be informed of the deception without compromising the study’s integrity. Reactions to learning of deception can range from understanding to anger. Debriefing should explain the scientific rationale and social benefits to minimize negative reactions.
If the participant is likely to object or be distressed once they discover the true nature of the research at debriefing, then the study is unacceptable.
If you have gained participants’ informed consent by deception, then they will have agreed to take part without actually knowing what they were consenting to. The true nature of the research should be revealed at the earliest possible opportunity or at least during debriefing.
Some researchers argue that deception can never be justified and object to this practice as it (i) violates an individual’s right to choose to participate; (ii) is a questionable basis on which to build a discipline; and (iii) leads to distrust of psychology in the community.
Confidentiality
Protecting participant confidentiality is an ethical imperative that demonstrates respect, ensures honest participation, and prevents harms like embarrassment or legal issues. Methods like data encryption, coding systems, and secure storage should match the research methodology.
Participants and the data gained from them must be kept anonymous unless they give their full consent. No names must be used in a lab report .
Researchers must clearly describe to participants the limits of confidentiality and methods to protect privacy. With internet research, threats exist like third-party data access; security measures like encryption should be explained. For non-internet research, other protections should be noted too, like coding systems and restricted data access.
High-profile data breaches have eroded public trust. Methods that minimize identifiable information can further guard confidentiality. For example, researchers can consider whether birthdates are necessary or just ages.
Generally, reducing personal details collected and limiting accessibility safeguards participants. Following strong confidentiality protections demonstrates respect for persons in human subjects research.
What do we do if we discover something that should be disclosed (e.g., a criminal act)? Researchers have no legal obligation to disclose criminal acts and must determine the most important consideration: their duty to the participant vs. their duty to the wider community.
Ultimately, decisions to disclose information must be set in the context of the research aims.
Withdrawal from an Investigation
Participants should be able to leave a study anytime if they feel uncomfortable. They should also be allowed to withdraw their data. They should be told at the start of the study that they have the right to withdraw.
They should not have pressure placed upon them to continue if they do not want to (a guideline flouted in Milgram’s research).
Participants may feel they shouldn’t withdraw as this may ‘spoil’ the study. Many participants are paid or receive course credits; they may worry they won’t get this if they withdraw.
Even at the end of the study, the participant has a final opportunity to withdraw the data they have provided for the research.
Ethical Issues in Psychology & Socially Sensitive Research
There has been an assumption over the years by many psychologists that provided they follow the BPS or APA guidelines when using human participants and that all leave in a similar state of mind to how they turned up, not having been deceived or humiliated, given a debrief, and not having had their confidentiality breached, that there are no ethical concerns with their research.
But consider the following examples:
a) Caughy et al. 1994 found that middle-class children in daycare at an early age generally score less on cognitive tests than children from similar families reared in the home.
Assuming all guidelines were followed, neither the parents nor the children participating would have been unduly affected by this research. Nobody would have been deceived, consent would have been obtained, and no harm would have been caused.
However, consider the wider implications of this study when the results are published, particularly for parents of middle-class infants who are considering placing their young children in daycare or those who recently have!
b) IQ tests administered to black Americans show that they typically score 15 points below the average white score.
When black Americans are given these tests, they presumably complete them willingly and are not harmed as individuals. However, when published, findings of this sort seek to reinforce racial stereotypes and are used to discriminate against the black population in the job market, etc.
Sieber & Stanley (1988) (the main names for Socially Sensitive Research (SSR) outline 4 groups that may be affected by psychological research: It is the first group of people that we are most concerned with!
- Members of the social group being studied, such as racial or ethnic group. For example, early research on IQ was used to discriminate against US Blacks.
- Friends and relatives of those participating in the study, particularly in case studies, where individuals may become famous or infamous. Cases that spring to mind would include Genie’s mother.
- The research team. There are examples of researchers being intimidated because of the line of research they are in.
- The institution in which the research is conducted.
salso suggest there are 4 main ethical concerns when conducting SSR:
- The research question or hypothesis.
- The treatment of individual participants.
- The institutional context.
- How the findings of the research are interpreted and applied.
Ethical Guidelines For Carrying Out SSR
Sieber and Stanley suggest the following ethical guidelines for carrying out SSR. There is some overlap between these and research on human participants in general.
Privacy : This refers to people rather than data. Asking people questions of a personal nature (e.g., about sexuality) could offend.
Confidentiality: This refers to data. Information (e.g., about H.I.V. status) leaked to others may affect the participant’s life.
Sound & valid methodology : This is even more vital when the research topic is socially sensitive. Academics can detect flaws in methods, but the lay public and the media often don’t.
When research findings are publicized, people are likely to consider them fact, and policies may be based on them. Examples are Bowlby’s maternal deprivation studies and intelligence testing.
Deception : Causing the wider public to believe something, which isn’t true by the findings, you report (e.g., that parents are responsible for how their children turn out).
Informed consent : Participants should be made aware of how participating in the research may affect them.
Justice & equitable treatment : Examples of unjust treatment are (i) publicizing an idea, which creates a prejudice against a group, & (ii) withholding a treatment, which you believe is beneficial, from some participants so that you can use them as controls.
Scientific freedom : Science should not be censored, but there should be some monitoring of sensitive research. The researcher should weigh their responsibilities against their rights to do the research.
Ownership of data : When research findings could be used to make social policies, which affect people’s lives, should they be publicly accessible? Sometimes, a party commissions research with their interests in mind (e.g., an industry, an advertising agency, a political party, or the military).
Some people argue that scientists should be compelled to disclose their results so that other scientists can re-analyze them. If this had happened in Burt’s day, there might not have been such widespread belief in the genetic transmission of intelligence. George Miller (Miller’s Magic 7) famously argued that we should give psychology away.
The values of social scientists : Psychologists can be divided into two main groups: those who advocate a humanistic approach (individuals are important and worthy of study, quality of life is important, intuition is useful) and those advocating a scientific approach (rigorous methodology, objective data).
The researcher’s values may conflict with those of the participant/institution. For example, if someone with a scientific approach was evaluating a counseling technique based on a humanistic approach, they would judge it on criteria that those giving & receiving the therapy may not consider important.
Cost/benefit analysis : It is unethical if the costs outweigh the potential/actual benefits. However, it isn’t easy to assess costs & benefits accurately & the participants themselves rarely benefit from research.
Sieber & Stanley advise that researchers should not avoid researching socially sensitive issues. Scientists have a responsibility to society to find useful knowledge.
- They need to take more care over consent, debriefing, etc. when the issue is sensitive.
- They should be aware of how their findings may be interpreted & used by others.
- They should make explicit the assumptions underlying their research so that the public can consider whether they agree with these.
- They should make the limitations of their research explicit (e.g., ‘the study was only carried out on white middle-class American male students,’ ‘the study is based on questionnaire data, which may be inaccurate,’ etc.
- They should be careful how they communicate with the media and policymakers.
- They should be aware of the balance between their obligations to participants and those to society (e.g. if the participant tells them something which they feel they should tell the police/social services).
- They should be aware of their own values and biases and those of the participants.
Arguments for SSR
- Psychologists have devised methods to resolve the issues raised.
- SSR is the most scrutinized research in psychology. Ethical committees reject more SSR than any other form of research.
- By gaining a better understanding of issues such as gender, race, and sexuality, we are able to gain greater acceptance and reduce prejudice.
- SSR has been of benefit to society, for example, EWT. This has made us aware that EWT can be flawed and should not be used without corroboration. It has also made us aware that the EWT of children is every bit as reliable as that of adults.
- Most research is still on white middle-class Americans (about 90% of research is quoted in texts!). SSR is helping to redress the balance and make us more aware of other cultures and outlooks.
Arguments against SSR
- Flawed research has been used to dictate social policy and put certain groups at a disadvantage.
- Research has been used to discriminate against groups in society, such as the sterilization of people in the USA between 1910 and 1920 because they were of low intelligence, criminal, or suffered from psychological illness.
- The guidelines used by psychologists to control SSR lack power and, as a result, are unable to prevent indefensible research from being carried out.
American Psychological Association. (2002). American Psychological Association ethical principles of psychologists and code of conduct. www.apa.org/ethics/code2002.html
Baumrind, D. (1964). Some thoughts on ethics of research: After reading Milgram’s” Behavioral study of obedience.”. American Psychologist , 19 (6), 421.
Caughy, M. O. B., DiPietro, J. A., & Strobino, D. M. (1994). Day‐care participation as a protective factor in the cognitive development of low‐income children. Child development , 65 (2), 457-471.
Harris, B. (1988). Key words: A history of debriefing in social psychology. In J. Morawski (Ed.), The rise of experimentation in American psychology (pp. 188-212). New York: Oxford University Press.
Rosenthal, R., & Rosnow, R. L. (1984). Applying Hamlet’s question to the ethical conduct of research: A conceptual addendum. American Psychologist, 39(5) , 561.
Sieber, J. E., & Stanley, B. (1988). Ethical and professional dimensions of socially sensitive research. American psychologist , 43 (1), 49.
The British Psychological Society. (2010). Code of Human Research Ethics. www.bps.org.uk/sites/default/files/documents/code_of_human_research_ethics.pdf
Further Information
- MIT Psychology Ethics Lecture Slides
BPS Documents
- Code of Ethics and Conduct (2018)
- Good Practice Guidelines for the Conduct of Psychological Research within the NHS
- Guidelines for Psychologists Working with Animals
- Guidelines for ethical practice in psychological research online
APA Documents
APA Ethical Principles of Psychologists and Code of Conduct
Public Health Notes
Your partner for better health, research ethics: definition, principles and advantages.
October 13, 2020 Kusum Wagle Epidemiology 0

Table of Contents
What is Research Ethics?
- Ethics are the set of rules that govern our expectations of our own and others’ behavior.
- Research ethics are the set of ethical guidelines that guides us on how scientific research should be conducted and disseminated.
- Research ethics govern the standards of conduct for scientific researchers It is the guideline for responsibly conducting the research.
- Research that implicates human subjects or contributors rears distinctive and multifaceted ethical, legitimate, communal and administrative concerns.
- Research ethics is unambiguously concerned in the examination of ethical issues that are upraised when individuals are involved as participants in the study.
- Research ethics committee/Institutional Review Board (IRB) reviews whether the research is ethical enough or not to protect the rights, dignity and welfare of the respondents.
Objectives of Research Ethics:
- The first and comprehensive objective – to guard/protect human participants, their dignity, rights and welfare .
- The second objective – to make sure that research is directed in a manner that assists welfares of persons, groups and/or civilization as a whole.
- The third objective – to inspect particular research events and schemes for their ethical reliability, considering issues such as the controlling risk, protection of privacy and the progression of informed consent.
Principles of Research Ethics:
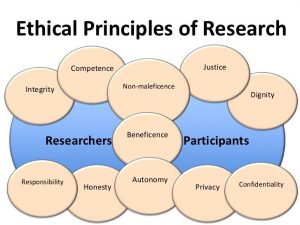
The general principles of research ethics are:
|
| Being honest with the beneficiaries and respondents. Being honest about the findings and methodology of the research. Being honest with other direct and indirect stakeholders. |
| Ensuring honesty and sincerity. Fulfilling agreements and promises. Do not create false expectations or make false promises. | |
|
| Avoiding bias in experimental design, data analysis, data interpretation, peer review, and other aspects of research. |
| It includes: | |
| Maximize the benefits of the participants. Ethical obligation to maximize possible benefits and to minimize possible harms to the respondents. | |
| Do no harm. Minimize harm/s or risks to the human. Ensure privacy, autonomy and dignity. | |
| Responsibly publishing to promote and uptake research or knowledge. No duplicate publication. | |
| It means keeping the participant anonymous. It involves not revealing the name, caste or any other information about the participants that may reveal his/her identity.
| |
| Protecting confidential information, personnel records. It includes information such as: | |
| Avoid discrimination on the basis of age, sex, race, ethnicity or other factors that are violation of human rights and are not related to the study. | |
| Be open to sharing results, data and other resources. Also accept encouraging comments and constructive feedback. | |
| Be careful about the possible error and biases. Give credit to the intellectual property of others. Always paraphrase while referring to others article, writing. Never plagiarize. | |
| The obligation to distribute benefits and burdens fairly, to treat equals equally, and to give reasons for differential treatment based on widely accepted criteria for just ways to distribute benefits and burdens. |
Broad Categorization of Principles of Research Ethics:
Broadly categorizing, there are mainly five principles of research ethics:
1. MINIMIZING THE RISK OF HARM
It is necessary to minimize any sort of harm to the participants. There are a number of forms of harm that participants can be exposed to. They are:
- Bodily harm to contributors.
- Psychological agony and embarrassment.
- Social drawback.
- Violation of participant’s confidentiality and privacy.
In order to minimize the risk of harm, the researcher/data collector should:
- Obtain informed consent from participants.
- Protecting anonymity and confidentiality of participants.
- Avoiding misleading practices when planning research.
- Providing participants with the right to withdraw .
2. OBTAINING INFORMED CONSENT
One of the fundamentals of research ethics is the notion of informed consent .
Informed consent means that a person knowingly, voluntarily and intelligently gives consent to participate in a research.
Informed consent means that the participants should be well-informed about the:
- Introduction and objective of the research
- Purpose of the discussion
- Anticipated advantages, benefits/harm from the research (if any)
- Use of research
- Their role in research
- Methods which will be used to protect anonymity and confidentiality of the participant
- Freedom to not answer any question/withdraw from the research
- Who to contact if the participant need additional information about the research
3. PROTECTING ANONYMITY AND CONFIDENTIALITY
Protecting the anonymity and confidentiality of research participants is an additionally applied constituent of research ethics.
Protecting anonymity: It means keeping the participant anonymous. It involves not revealing the name, caste or any other information about the participants that may reveal his/her identity.
Maintaining confidentiality: It refers to ensuring that the information given by the participant are confidential and not shared with anyone, except the research team. It is also about keeping the information secretly from other people.
4. AVOIDING MISLEADING PRACTICES
- The researcher should avoid all the deceptive and misleading practices that might misinform the respondent.
- It includes avoiding all the activities like communicating wrong messages, giving false assurance, giving false information etc.
5. PROVIDING THE RIGHT TO WITHDRAW
- Participants have to have the right to withdraw at any point of the research.
- When any respondent decides on to withdraw from the research, they should not be stressed or forced in any manner to try to discontinue them from withdrawing.
Apart from the above-mentioned ethics, other ethical aspects things that must be considered while doing research are:
Protection of vulnerable groups of people:
- Vulnerability is one distinctive feature of people incapable to protect their moralities and wellbeing. Vulnerable groups comprise captive populations (detainees, established, students, etc.), mentally ill persons, and aged people, children, critically ill or dying, poor, with learning incapacities, sedated or insensible.
- Their participation in research can be endorsed to their incapability to give an informed consent and to the need for their further safety and sensitivity from the research/researcher as they are in a greater risk of being betrayed, exposed or forced to participate.
Skills of the researcher:
- Researchers should have the basic skills and familiarity for the specific study to be carried out and be conscious of the bounds of personal competence in research.
- Any lack of knowledge in the area under research must be clearly specified.
- Inexperienced researchers should work under qualified supervision that has to be revised by an ethics commission.
Advantages of Research Ethics:
- Research ethics promote the aims of research.
- It increases trust among the researcher and the respondent.
- It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants.
- Researchers can be held accountable and answerable for their actions.
- Ethics promote social and moral values.
- Promote s the ambitions of research, such as understanding, veracity, and dodging of error.
- Ethical standards uphold the values that are vital to cooperative work , such as belief, answerability, mutual respect, and impartiality.
- Ethical norms in research also aid to construct public upkeep for research. People are more likely to trust a research project if they can trust the worth and reliability of research.
Limitations of Research Ethics:
For subjects:
- Possibilities to physical integrity, containing those linked with experimental drugs and dealings and with other involvements that will be used in the study (e.g. measures used to observe research participants, such as blood sampling, X-rays or lumbar punctures).
- Psychological risks: for example, a questionnaire may perhaps signify a risk if it fears traumatic events or happenings that are especially traumatic.
- Social, legal and economic risks : for example, if personal information collected during a study is unintentionally released, participants might face a threat of judgment and stigmatization.
- Certain tribal or inhabitant groups may possibly suffer from discrimination or stigmatization, burdens because of research, typically if associates of those groups are recognized as having a greater-than-usual risk of devouring a specific disease.
- The research may perhaps have an influence on the prevailing health system: for example, human and financial capitals dedicated to research may distract attention from other demanding health care necessities in the community.
How can we ensure ethics at different steps of research?
The following process helps to ensure ethics at different steps of research:
- Collect the facts and talk over intellectual belongings openly
- Outline the ethical matters
- Detect the affected parties (stakeholders)
- Ascertain the forfeits
- Recognize the responsibilities (principles, rights, justice)
- Contemplate your personality and truthfulness
- Deliberate innovatively about possible actions
- Respect privacy and confidentiality
- Resolve on the appropriate ethical action and be willing to deal with divergent point of view.
References and For More Information:
http://dissertation.laerd.com/principles-of-research-ethics.php
https://researchethics.ca/what-is-research-ethics/
https://www.who.int/ethics/Ethics_basic_concepts_ENG.pdf
https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
https://research.ku.edu/sites/research.ku.edu/files/docs/EESE_EthicalDecisionmakingFramework.pdf
https://www.who.int/ethics/research/en/
https://www.ufrgs.br/bioetica/cioms2008.pdf
https://www.who.int/ethics/research/en/#:~:text=WHO%20Research%20Ethics%20Review%20Committee,financially%20or%20technically%20by%20WHO .
https://www.who.int/reproductivehealth/topics/ethics/review_bodies_guide_serg/en/
https://www.who.int/ethics/indigenous_peoples/en/index13.html
https://www.who.int/bulletin/archives/80(2)114.pdf
https://www.who.int/about/ethics
https://www.slideshare.net/uqudent/introduction-to-research-ethics
https://libguides.library.cityu.edu.hk/researchmethods/ethics#:~:text=Methods%20by%20Subject-,What%20is%20Research%20Ethics%3F,ensure%20a%20high%20ethical%20standard .
https://www.apa.org/monitor/jan03/principles
https://www.hsj.gr/medicine/what-are-the-major-ethical-issues-in-conducting-research-is-there-a-conflict-between-the-research-ethics-and-the-nature-of-nursing.php?aid=3485
https://www.skillsyouneed.com/learn/research-ethics.html
- advantages of research ethics
- difference between confidentiality and anonymity in research
- minimizing the risk of harm in research
- obtaining informed consent in research
- principles of research ethics
- PROTECTING ANONYMITY AND CONFIDENTIALITY
- what are the advantages of research ethics
- what are the limitations of research ethics
- what are the principles of research ethics
- what is obtaining informed consent in research
- what is research ethics
- what is right to withdraw in research
- what is ROTECTING ANONYMITY AND CONFIDENTIALITY in research
Copyright © 2024 | WordPress Theme by MH Themes

This page has been archived and is no longer being updated regularly.
Cover Story
Five principles for research ethics
Cover your bases with these ethical strategies
By DEBORAH SMITH
Monitor Staff
January 2003, Vol 34, No. 1
Print version: page 56
13 min read
- Conducting Research
Not that long ago, academicians were often cautious about airing the ethical dilemmas they faced in their research and academic work, but that environment is changing today. Psychologists in academe are more likely to seek out the advice of their colleagues on issues ranging from supervising graduate students to how to handle sensitive research data , says George Mason University psychologist June Tangney, PhD.
"There has been a real change in the last 10 years in people talking more frequently and more openly about ethical dilemmas of all sorts," she explains.
Indeed, researchers face an array of ethical requirements: They must meet professional, institutional and federal standards for conducting research with human participants, often supervise students they also teach and have to sort out authorship issues, just to name a few.
Here are five recommendations APA's Science Directorate gives to help researchers steer clear of ethical quandaries:
1. Discuss intellectual property frankly
Academe's competitive "publish-or-perish" mindset can be a recipe for trouble when it comes to who gets credit for authorship . The best way to avoid disagreements about who should get credit and in what order is to talk about these issues at the beginning of a working relationship, even though many people often feel uncomfortable about such topics.
"It's almost like talking about money," explains Tangney. "People don't want to appear to be greedy or presumptuous."
APA's Ethics Code offers some guidance: It specifies that "faculty advisors discuss publication credit with students as early as feasible and throughout the research and publication process as appropriate." When researchers and students put such understandings in writing, they have a helpful tool to continually discuss and evaluate contributions as the research progresses.
However, even the best plans can result in disputes, which often occur because people look at the same situation differently. "While authorship should reflect the contribution," says APA Ethics Office Director Stephen Behnke, JD, PhD, "we know from social science research that people often overvalue their contributions to a project. We frequently see that in authorship-type situations. In many instances, both parties genuinely believe they're right." APA's Ethics Code stipulates that psychologists take credit only for work they have actually performed or to which they have substantially contributed and that publication credit should accurately reflect the relative contributions: "Mere possession of an institutional position, such as department chair, does not justify authorship credit," says the code. "Minor contributions to the research or to the writing for publications are acknowledged appropriately, such as in footnotes or in an introductory statement."
The same rules apply to students. If they contribute substantively to the conceptualization, design, execution, analysis or interpretation of the research reported, they should be listed as authors. Contributions that are primarily technical don't warrant authorship. In the same vein, advisers should not expect ex-officio authorship on their students' work.
Matthew McGue, PhD, of the University of Minnesota, says his psychology department has instituted a procedure to avoid murky authorship issues. "We actually have a formal process here where students make proposals for anything they do on the project," he explains. The process allows students and faculty to more easily talk about research responsibility, distribution and authorship.
Psychologists should also be cognizant of situations where they have access to confidential ideas or research, such as reviewing journal manuscripts or research grants, or hearing new ideas during a presentation or informal conversation. While it's unlikely reviewers can purge all of the information in an interesting manuscript from their thinking, it's still unethical to take those ideas without giving credit to the originator.
"If you are a grant reviewer or a journal manuscript reviewer [who] sees someone's research [that] hasn't been published yet, you owe that person a duty of confidentiality and anonymity," says Gerald P. Koocher, PhD, editor of the journal Ethics and Behavior and co-author of "Ethics in Psychology: Professional Standards and Cases" (Oxford University Press, 1998).
Researchers also need to meet their ethical obligations once their research is published: If authors learn of errors that change the interpretation of research findings, they are ethically obligated to promptly correct the errors in a correction, retraction, erratum or by other means.
To be able to answer questions about study authenticity and allow others to reanalyze the results, authors should archive primary data and accompanying records for at least five years, advises University of Minnesota psychologist and researcher Matthew McGue, PhD. "Store all your data. Don't destroy it," he says. "Because if someone charges that you did something wrong, you can go back."
"It seems simple, but this can be a tricky area," says Susan Knapp, APA's deputy publisher. "The APA Publication Manual Section 8.05 has some general advice on what to retain and suggestions about things to consider in sharing data."
The APA Ethics Code requires psychologists to release their data to others who want to verify their conclusions, provided that participants' confidentiality can be protected and as long as legal rights concerning proprietary data don't preclude their release. However, the code also notes that psychologists who request data in these circumstances can only use the shared data for reanalysis; for any other use, they must obtain a prior written agreement.
2. Be conscious of multiple roles
APA's Ethics Code says psychologists should avoid relationships that could reasonably impair their professional performance or could exploit or harm others. But it also notes that many kinds of multiple relationships aren't unethical--as long as they're not reasonably expected to have adverse effects.
That notwithstanding, psychologists should think carefully before entering into multiple relationships with any person or group, such as recruiting students or clients as participants in research studies or investigating the effectiveness of a product of a company whose stock they own.
For example, when recruiting students from your Psychology 101 course to participate in an experiment, be sure to make clear that participation is voluntary. If participation is a course requirement, be sure to note that in the class syllabus, and ensure that participation has educative value by, for instance, providing a thorough debriefing to enhance students' understanding of the study. The 2002 Ethics Code also mandates in Standard 8.04b that students be given equitable alternatives to participating in research.
Perhaps one of the most common multiple roles for researchers is being both a mentor and lab supervisor to students they also teach in class. Psychologists need to be especially cautious that they don't abuse the power differential between themselves and students, say experts. They shouldn't, for example, use their clout as professors to coerce students into taking on additional research duties.
By outlining the nature and structure of the supervisory relationship before supervision or mentoring begins, both parties can avoid misunderstandings, says George Mason University's Tangney. It's helpful to create a written agreement that includes both parties' responsibilities as well as authorship considerations, intensity of the supervision and other key aspects of the job.
"While that's the ideal situation, in practice we do a lot less of that than we ought to," she notes. "Part of it is not having foresight up front of how a project or research study is going to unfold."
That's why experts also recommend that supervisors set up timely and specific methods to give students feedback and keep a record of the supervision, including meeting times, issues discussed and duties assigned.
If psychologists do find that they are in potentially harmful multiple relationships, they are ethically mandated to take steps to resolve them in the best interest of the person or group while complying with the Ethics Code.
3. Follow informed-consent rules
When done properly, the consent process ensures that individuals are voluntarily participating in the research with full knowledge of relevant risks and benefits.
"The federal standard is that the person must have all of the information that might reasonably influence their willingness to participate in a form that they can understand and comprehend," says Koocher, dean of Simmons College's School for Health Studies.
APA's Ethics Code mandates that psychologists who conduct research should inform participants about:
The purpose of the research, expected duration and procedures.
Participants' rights to decline to participate and to withdraw from the research once it has started, as well as the anticipated consequences of doing so.
Reasonably foreseeable factors that may influence their willingness to participate, such as potential risks, discomfort or adverse effects.
Any prospective research benefits.
Limits of confidentiality, such as data coding, disposal, sharing and archiving, and when confidentiality must be broken.
Incentives for participation.
Who participants can contact with questions.
Experts also suggest covering the likelihood, magnitude and duration of harm or benefit of participation, emphasizing that their involvement is voluntary and discussing treatment alternatives, if relevant to the research.
Keep in mind that the Ethics Code includes specific mandates for researchers who conduct experimental treatment research. Specifically, they must inform individuals about the experimental nature of the treatment, services that will or will not be available to the control groups, how participants will be assigned to treatments and control groups, available treatment alternatives and compensation or monetary costs of participation.
If research participants or clients are not competent to evaluate the risks and benefits of participation themselves--for example, minors or people with cognitive disabilities--then the person who's giving permission must have access to that same information, says Koocher.
Remember that a signed consent form doesn't mean the informing process can be glossed over, say ethics experts. In fact, the APA Ethics Code says psychologists can skip informed consent in two instances only: When permitted by law or federal or institutional regulations, or when the research would not reasonably be expected to distress or harm participants and involves one of the following:
The study of normal educational practices, curricula or classroom management methods conducted in educational settings.
Anonymous questionnaires, naturalistic observations or archival research for which disclosure of responses would not place participants at risk of criminal or civil liability or damage their financial standing, employability or reputation, and for which confidentiality is protected.
The study of factors related to job or organization effectiveness conducted in organizational settings for which there is no risk to participants' employability, and confidentiality is protected.
If psychologists are precluded from obtaining full consent at the beginning--for example, if the protocol includes deception, recording spontaneous behavior or the use of a confederate--they should be sure to offer a full debriefing after data collection and provide people with an opportunity to reiterate their consent, advise experts.
The code also says psychologists should make reasonable efforts to avoid offering "excessive or inappropriate financial or other inducements for research participation when such inducements are likely to coerce participation."
4. Respect confidentiality and privacy
Upholding individuals' rights to confidentiality and privacy is a central tenet of every psychologist's work. However, many privacy issues are idiosyncratic to the research population, writes Susan Folkman, PhD, in " Ethics in Research with Human Participants " (APA, 2000). For instance, researchers need to devise ways to ask whether participants are willing to talk about sensitive topics without putting them in awkward situations, say experts. That could mean they provide a set of increasingly detailed interview questions so that participants can stop if they feel uncomfortable.
And because research participants have the freedom to choose how much information about themselves they will reveal and under what circumstances, psychologists should be careful when recruiting participants for a study, says Sangeeta Panicker, PhD, director of the APA Science Directorate's Research Ethics Office. For example, it's inappropriate to obtain contact information of members of a support group to solicit their participation in research. However, you could give your colleague who facilitates the group a letter to distribute that explains your research study and provides a way for individuals to contact you, if they're interested.
Other steps researchers should take include:
Discuss the limits of confidentiality. Give participants information about how their data will be used, what will be done with case materials, photos and audio and video recordings, and secure their consent.
Know federal and state law. Know the ins and outs of state and federal law that might apply to your research. For instance, the Goals 2000: Education Act of 1994 prohibits asking children about religion, sex or family life without parental permission.
Another example is that, while most states only require licensed psychologists to comply with mandatory reporting laws, some laws also require researchers to report abuse and neglect. That's why it's important for researchers to plan for situations in which they may learn of such reportable offenses. Generally, research psychologists can consult with a clinician or their institution's legal department to decide the best course of action.
Take practical security measures. Be sure confidential records are stored in a secure area with limited access, and consider stripping them of identifying information, if feasible. Also, be aware of situations where confidentiality could inadvertently be breached, such as having confidential conversations in a room that's not soundproof or putting participants' names on bills paid by accounting departments.
Think about data sharing before research begins. If researchers plan to share their data with others, they should note that in the consent process, specifying how they will be shared and whether data will be anonymous. For example, researchers could have difficulty sharing sensitive data they've collected in a study of adults with serious mental illnesses because they failed to ask participants for permission to share the data. Or developmental data collected on videotape may be a valuable resource for sharing, but unless a researcher asked permission back then to share videotapes, it would be unethical to do so. When sharing, psychologists should use established techniques when possible to protect confidentiality, such as coding data to hide identities. "But be aware that it may be almost impossible to entirely cloak identity, especially if your data include video or audio recordings or can be linked to larger databases," says Merry Bullock, PhD, associate executive director in APA's Science Directorate.
Understand the limits of the Internet. Since Web technology is constantly evolving, psychologists need to be technologically savvy to conduct research online and cautious when exchanging confidential information electronically. If you're not a Internet whiz, get the help of someone who is. Otherwise, it may be possible for others to tap into data that you thought was properly protected.
5. Tap into ethics resources
One of the best ways researchers can avoid and resolve ethical dilemmas is to know both what their ethical obligations are and what resources are available to them.
"Researchers can help themselves make ethical issues salient by reminding themselves of the basic underpinnings of research and professional ethics," says Bullock. Those basics include:
The Belmont Report. Released by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research in 1979, the report provided the ethical framework for ensuing human participant research regulations and still serves as the basis for human participant protection legislation (see Further Reading).
APA's Ethics Code , which offers general principles and specific guidance for research activities.
Moreover, despite the sometimes tense relationship researchers can have with their institutional review boards (IRBs), these groups can often help researchers think about how to address potential dilemmas before projects begin, says Panicker. But psychologists must first give their IRBs the information they need to properly understand a research proposal.
"Be sure to provide the IRB with detailed and comprehensive information about the study, such as the consent process, how participants will be recruited and how confidential information will be protected," says Bullock. "The more information you give your IRB, the better educated its members will become about behavioral research, and the easier it will be for them to facilitate your research."
As cliché as it may be, says Panicker, thinking positively about your interactions with an IRB can help smooth the process for both researchers and the IRBs reviewing their work.
Further reading
American Psychological Association. (2002). Ethical principles of psychologists and code of conduct. American Psychologist, 57 (12).
Sales, B.D., & Folkman, S. (Eds.). (2000). Ethics in research with human participants . Washington, DC: American Psychological Association.
APA's Research Ethics Office in the Science Directorate; e-mail ; Web site: APA Science .
The National Institutes of Health (NIH) offers educational materials on human subjects .
NIH Bioethics Resources Web site .
The Department of Health and Human Services' (DHHS) Office of Research Integrity Web site .
DHHS Office of Human Research Protections Web site .
The 1979 Belmont Report on protecting human subjects .
Association for the Accreditation of Human Research Protection Programs Web site: www.aahrpp.org .
Related Articles
- Ethics in research with animals
Letters to the Editor
- Send us a letter

Ethical Issues in Research: Perceptions of Researchers, Research Ethics Board Members and Research Ethics Experts
- Published: 12 August 2022
- Volume 21 , pages 269–292, ( 2023 )
Cite this article

- Marie-Josée Drolet ORCID: orcid.org/0000-0001-8384-4193 1 ,
- Eugénie Rose-Derouin 2 ,
- Julie-Claude Leblanc 2 ,
- Mélanie Ruest 2 &
- Bryn Williams-Jones 3
33k Accesses
12 Citations
4 Altmetric
Explore all metrics
In the context of academic research, a diversity of ethical issues, conditioned by the different roles of members within these institutions, arise. Previous studies on this topic addressed mainly the perceptions of researchers. However, to our knowledge, no studies have explored the transversal ethical issues from a wider spectrum, including other members of academic institutions as the research ethics board (REB) members, and the research ethics experts. The present study used a descriptive phenomenological approach to document the ethical issues experienced by a heterogeneous group of Canadian researchers, REB members, and research ethics experts. Data collection involved socio-demographic questionnaires and individual semi-structured interviews. Following the triangulation of different perspectives (researchers, REB members and ethics experts), emerging ethical issues were synthesized in ten units of meaning: (1) research integrity, (2) conflicts of interest, (3) respect for research participants, (4) lack of supervision and power imbalances, (5) individualism and performance, (6) inadequate ethical guidance, (7) social injustices, (8) distributive injustices, (9) epistemic injustices, and (10) ethical distress. This study highlighted several problematic elements that can support the identification of future solutions to resolve transversal ethical issues in research that affect the heterogeneous members of the academic community.
Similar content being viewed by others

Ethical Research? Examining Knotty, Moment-to-Moment Challenges Throughout the Research Process

Approaching Research in a Prepared, Mindful and Ethical Manner

Conceptualising Ethical Issues in the Conduct of Research: Results from a Critical and Systematic Literature Review
Explore related subjects.
- Artificial Intelligence
- Medical Ethics
Avoid common mistakes on your manuscript.
Introduction
Research includes a set of activities in which researchers use various structured methods to contribute to the development of knowledge, whether this knowledge is theoretical, fundamental, or applied (Drolet & Ruest, accepted ). University research is carried out in a highly competitive environment that is characterized by ever-increasing demands (i.e., on time, productivity), insufficient access to research funds, and within a market economy that values productivity and speed often to the detriment of quality or rigour – this research context creates a perfect recipe for breaches in research ethics, like research misbehaviour or misconduct (i.e., conduct that is ethically questionable or unacceptable because it contravenes the accepted norms of responsible conduct of research or compromises the respect of core ethical values that are widely held by the research community) (Drolet & Girard, 2020 ; Sieber, 2004 ). Problematic ethics and integrity issues – e.g., conflicts of interest, falsification of data, non-respect of participants’ rights, and plagiarism, to name but a few – have the potential to both undermine the credibility of research and lead to negative consequences for many stakeholders, including researchers, research assistants and personnel, research participants, academic institutions, and society as a whole (Drolet & Girard, 2020 ). It is thus evident that the academic community should be able to identify these different ethical issues in order to evaluate the nature of the risks that they pose (and for whom), and then work towards their prevention or management (i.e., education, enhanced policies and procedures, risk mitigation strategies).
In this article, we define an “ethical issue” as any situation that may compromise, in whole or in part, the respect of at least one moral value (Swisher et al., 2005 ) that is considered socially legitimate and should thus be respected. In general, ethical issues occur at three key moments or stages of the research process: (1) research design (i.e., conception, project planning), (2) research conduct (i.e., data collection, data analysis) and (3) knowledge translation or communication (e.g., publications of results, conferences, press releases) (Drolet & Ruest, accepted ). According to Sieber ( 2004 ), ethical issues in research can be classified into five categories, related to: (a) communication with participants and the community, (b) acquisition and use of research data, (c) external influence on research, (d) risks and benefits of the research, and (e) selection and use of research theories and methods. Many of these issues are related to breaches of research ethics norms, misbehaviour or research misconduct. Bruhn et al., ( 2002 ) developed a typology of misbehaviour and misconduct in academia that can be used to judge the seriousness of different cases. This typology takes into consideration two axes of reflection: (a) the origin of the situation (i.e., is it the researcher’s own fault or due to the organizational context?), and (b) the scope and severity (i.e., is this the first instance or a recurrent behaviour? What is the nature of the situation? What are the consequences, for whom, for how many people, and for which organizations?).
A previous detailed review of the international literature on ethical issues in research revealed several interesting findings (Beauchemin et al., 2021 ). Indeed, the current literature is dominated by descriptive ethics, i.e., the sharing by researchers from various disciplines of the ethical issues they have personally experienced. While such anecdotal documentation is relevant, it is insufficient because it does not provide a global view of the situation. Among the reviewed literature, empirical studies were in the minority (Table 1 ) – only about one fifth of the sample (n = 19) presented empirical research findings on ethical issues in research. The first of these studies was conducted almost 50 years ago (Hunt et al., 1984 ), with the remainder conducted in the 1990s. Eight studies were conducted in the United States (n = 8), five in Canada (n = 5), three in England (n = 3), two in Sweden (n = 2) and one in Ghana (n = 1).
Further, the majority of studies in our sample (n = 12) collected the perceptions of a homogeneous group of participants, usually researchers (n = 14) and sometimes health professionals (n = 6). A minority of studies (n = 7) triangulated the perceptions of diverse research stakeholders (i.e., researchers and research participants, or students). To our knowledge, only one study has examined perceptions of ethical issues in research by research ethics board members (REB; Institutional Review Boards [IRB] in the USA), and none to date have documented the perceptions of research ethics experts. Finally, nine studies (n = 9) adopted a qualitative design, seven studies (n = 7) a quantitative design, and three (n = 3) a mixed-methods design.
More studies using empirical research methods are needed to better identify broader trends, to enrich discussions on the values that should govern responsible conduct of research in the academic community, and to evaluate the means by which these values can be supported in practice (Bahn, 2012 ; Beauchemin et al., 2021 ; Bruhn et al., 2002 ; Henderson et al., 2013 ; Resnik & Elliot, 2016; Sieber 2004 ). To this end, we conducted an empirical qualitative study to document the perceptions and experiences of a heterogeneous group of Canadian researchers, REB members, and research ethics experts, to answer the following broad question: What are the ethical issues in research?
Research Methods
Research design.
A qualitative research approach involving individual semi-structured interviews was used to systematically document ethical issues (De Poy & Gitlin, 2010 ; Hammell et al., 2000 ). Specifically, a descriptive phenomenological approach inspired by the philosophy of Husserl was used (Husserl, 1970 , 1999 ), as it is recommended for documenting the perceptions of ethical issues raised by various practices (Hunt & Carnavale, 2011 ).
Ethical considerations
The principal investigator obtained ethics approval for this project from the Research Ethics Board of the Université du Québec à Trois-Rivières (UQTR). All members of the research team signed a confidentiality agreement, and research participants signed the consent form after reading an information letter explaining the nature of the research project.
Sampling and recruitment
As indicated above, three types of participants were sought: (1) researchers from different academic disciplines conducting research (i.e., theoretical, fundamental or empirical) in Canadian universities; (2) REB members working in Canadian organizations responsible for the ethical review, oversight or regulation of research; and (3) research ethics experts, i.e., academics or ethicists who teach research ethics, conduct research in research ethics, or are scholars who have acquired a specialization in research ethics. To be included in the study, participants had to work in Canada, speak and understand English or French, and be willing to participate in the study. Following Thomas and Polio’s (2002) recommendation to recruit between six and twelve participants (for a homogeneous sample) to ensure data saturation, for our heterogeneous sample, we aimed to recruit approximately twelve participants in order to obtain data saturation. Having used this method several times in related projects in professional ethics, data saturation is usually achieved with 10 to 15 participants (Drolet & Goulet, 2018 ; Drolet & Girard, 2020 ; Drolet et al., 2020 ). From experience, larger samples only serve to increase the degree of data saturation, especially in heterogeneous samples (Drolet et al., 2017 , 2019 ; Drolet & Maclure, 2016 ).
Purposive sampling facilitated the identification of participants relevant to documenting the phenomenon in question (Fortin, 2010 ). To ensure a rich and most complete representation of perceptions, we sought participants with varied and complementary characteristics with regards to the social roles they occupy in research practice (Drolet & Girard, 2020 ). A triangulation of sources was used for the recruitment (Bogdan & Biklen, 2006 ). The websites of Canadian universities and Canadian health institution REBs, as well as those of major Canadian granting agencies (i.e., the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and the Social Sciences and Humanities Research Council of Canada, Fonds de recherche du Quebec), were searched to identify individuals who might be interested in participating in the study. Further, people known by the research team for their knowledge and sensitivity to ethical issues in research were asked to participate. Research participants were also asked to suggest other individuals who met the study criteria.
Data Collection
Two tools were used for data collecton: (a) a socio-demographic questionnaire, and (b) a semi-structured individual interview guide. English and French versions of these two documents were used and made available, depending on participant preferences. In addition, although the interview guide contained the same questions, they were adapted to participants’ specific roles (i.e., researcher, REB member, research ethics expert). When contacted by email by the research assistant, participants were asked to confirm under which role they wished to participate (because some participants might have multiple, overlapping responsibilities) and they were sent the appropriate interview guide.
The interview guides each had two parts: an introduction and a section on ethical issues. The introduction consisted of general questions to put the participant at ease (i.e., “Tell me what a typical day at work is like for you”). The section on ethical issues was designed to capture the participant’s perceptions through questions such as: “Tell me three stories you have experienced at work that involve an ethical issue?” and “Do you feel that your organization is doing enough to address, manage, and resolve ethical issues in your work?”. Although some interviews were conducted in person, the majority were conducted by videoconference to promote accessibility and because of the COVID-19 pandemic. Interviews were digitally recorded so that the verbatim could be transcribed in full, and varied between 40 and 120 min in duration, with an average of 90 min. Research assistants conducted the interviews and transcribed the verbatim.
Data Analysis
The socio-demographic questionnaires were subjected to simple descriptive statistical analyses (i.e., means and totals), and the semi-structured interviews were subjected to qualitative analysis. The steps proposed by Giorgi ( 1997 ) for a Husserlian phenomenological reduction of the data were used. After collecting, recording, and transcribing the interviews, all verbatim were analyzed by at least two analysts: a research assistant (2nd author of this article) and the principal investigator (1st author) or a postdoctoral fellow (3rd author). The repeated reading of the verbatim allowed the first analyst to write a synopsis, i.e., an initial extraction of units of meaning. The second analyst then read the synopses, which were commented and improved if necessary. Agreement between analysts allowed the final drafting of the interview synopses, which were then analyzed by three analysts to generate and organize the units of meaning that emerged from the qualitative data.
Participants
Sixteen individuals (n = 16) participated in the study, of whom nine (9) identified as female and seven (7) as male (Table 2 ). Participants ranged in age from 22 to 72 years, with a mean age of 47.5 years. Participants had between one (1) and 26 years of experience in the research setting, with an average of 14.3 years of experience. Participants held a variety of roles, including: REB members (n = 11), researchers (n = 10), research ethics experts (n = 4), and research assistant (n = 1). As mentioned previously, seven (7) participants held more than one role, i.e., REB member, research ethics expert, and researcher. The majority (87.5%) of participants were working in Quebec, with the remaining working in other Canadian provinces. Although all participants considered themselves to be francophone, one quarter (n = 4) identified themselves as belonging to a cultural minority group.
With respect to their academic background, most participants (n = 9) had a PhD, three (3) had a post-doctorate, two (2) had a master’s degree, and two (2) had a bachelor’s degree. Participants came from a variety of disciplines: nine (9) had a specialty in the humanities or social sciences, four (4) in the health sciences and three (3) in the natural sciences. In terms of their knowledge of ethics, five (5) participants reported having taken one university course entirely dedicated to ethics, four (4) reported having taken several university courses entirely dedicated to ethics, three (3) had a university degree dedicated to ethics, while two (2) only had a few hours or days of training in ethics and two (2) reported having no knowledge of ethics.
- Ethical issues
As Fig. 1 illustrates, ten units of meaning emerge from the data analysis, namely: (1) research integrity, (2) conflicts of interest, (3) respect for research participants, (4) lack of supervision and power imbalances, (5) individualism and performance, (6) inadequate ethical guidance, (7) social injustices, (8) distributive injustices, (9) epistemic injustices, and (10) ethical distress. To illustrate the results, excerpts from verbatim interviews are presented in the following sub-sections. Most of the excerpts have been translated into English as the majority of interviews were conducted with French-speaking participants.
Ethical issues in research according to the participants
Research Integrity
The research environment is highly competitive and performance-based. Several participants, in particular researchers and research ethics experts, felt that this environment can lead both researchers and research teams to engage in unethical behaviour that reflects a lack of research integrity. For example, as some participants indicated, competition for grants and scientific publications is sometimes so intense that researchers falsify research results or plagiarize from colleagues to achieve their goals.
Some people will lie or exaggerate their research findings in order to get funding. Then, you see it afterwards, you realize: “ah well, it didn’t work, but they exaggerated what they found and what they did” (participant 14). Another problem in research is the identification of authors when there is a publication. Very often, there are authors who don’t even know what the publication is about and that their name is on it. (…) The time that it surprised me the most was just a few months ago when I saw someone I knew who applied for a teaching position. He got it I was super happy for him. Then I looked at his publications and … there was one that caught my attention much more than the others, because I was in it and I didn’t know what that publication was. I was the second author of a publication that I had never read (participant 14). I saw a colleague who had plagiarized another colleague. [When the colleague] found out about it, he complained. So, plagiarism is a serious [ethical breach]. I would also say that there is a certain amount of competition in the university faculties, especially for grants (…). There are people who want to win at all costs or get as much as possible. They are not necessarily going to consider their colleagues. They don’t have much of a collegial spirit (participant 10).
These examples of research misbehaviour or misconduct are sometimes due to or associated with situations of conflicts of interest, which may be poorly managed by certain researchers or research teams, as noted by many participants.
Conflict of interest
The actors and institutions involved in research have diverse interests, like all humans and institutions. As noted in Chap. 7 of the Canadian Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS2, 2018),
“researchers and research students hold trust relationships, either directly or indirectly, with participants, research sponsors, institutions, their professional bodies and society. These trust relationships can be put at risk by conflicts of interest that may compromise independence, objectivity or ethical duties of loyalty. Although the potential for such conflicts has always existed, pressures on researchers (i.e., to delay or withhold dissemination of research outcomes or to use inappropriate recruitment strategies) heighten concerns that conflicts of interest may affect ethical behaviour” (p. 92).
The sources of these conflicts are varied and can include interpersonal conflicts, financial partnerships, third-party pressures, academic or economic interests, a researcher holding multiple roles within an institution, or any other incentive that may compromise a researcher’s independence, integrity, and neutrality (TCPS2, 2018). While it is not possible to eliminate all conflicts of interest, it is important to manage them properly and to avoid temptations to behave unethically.
Ethical temptations correspond to situations in which people are tempted to prioritize their own interests to the detriment of the ethical goods that should, in their own context, govern their actions (Swisher et al., 2005 ). In the case of researchers, this refers to situations that undermine independence, integrity, neutrality, or even the set of principles that govern research ethics (TCPS2, 2018) or the responsible conduct of research. According to study participants, these types of ethical issues frequently occur in research. Many participants, especially researchers and REB members, reported that conflicts of interest can arise when members of an organization make decisions to obtain large financial rewards or to increase their academic profile, often at the expense of the interests of members of their research team, research participants, or even the populations affected by their research.
A company that puts money into making its drug work wants its drug to work. So, homeopathy is a good example, because there are not really any consequences of homeopathy, there are not very many side effects, because there are no effects at all. So, it’s not dangerous, but it’s not a good treatment either. But some people will want to make it work. And that’s a big issue when you’re sitting at a table and there are eight researchers, and there are two or three who are like that, and then there are four others who are neutral, and I say to myself, this is not science. I think that this is a very big ethical issue (participant 14). There are also times in some research where there will be more links with pharmaceutical companies. Obviously, there are then large amounts of money that will be very interesting for the health-care institutions because they still receive money for clinical trials. They’re still getting some compensation because its time consuming for the people involved and all that. The pharmaceutical companies have money, so they will compensate, and that is sometimes interesting for the institutions, and since we are a bit caught up in this, in the sense that we have no choice but to accept it. (…) It may not be the best research in the world, there may be a lot of side effects due to the drugs, but it’s good to accept it, we’re going to be part of the clinical trial (participant 3). It is integrity, what we believe should be done or said. Often by the pressure of the environment, integrity is in tension with the pressures of the environment, so it takes resistance, it takes courage in research. (…) There were all the debates there about the problems of research that was funded and then the companies kept control over what was written. That was really troubling for a lot of researchers (participant 5).
Further, these situations sometimes have negative consequences for research participants as reported by some participants.
Respect for research participants
Many research projects, whether they are psychosocial or biomedical in nature, involve human participants. Relationships between the members of research teams and their research participants raise ethical issues that can be complex. Research projects must always be designed to respect the rights and interests of research participants, and not just those of researchers. However, participants in our study – i.e., REB members, researchers, and research ethics experts – noted that some research teams seem to put their own interests ahead of those of research participants. They also emphasized the importance of ensuring the respect, well-being, and safety of research participants. The ethical issues related to this unit of meaning are: respect for free, informed and ongoing consent of research participants; respect for and the well-being of participants; data protection and confidentiality; over-solicitation of participants; ownership of the data collected on participants; the sometimes high cost of scientific innovations and their accessibility; balance between the social benefits of research and the risks to participants (particularly in terms of safety); balance between collective well-being (development of knowledge) and the individual rights of participants; exploitation of participants; paternalism when working with populations in vulnerable situations; and the social acceptability of certain types of research. The following excerpts present some of these issues.
Where it disturbs me ethically is in the medical field – because it’s more in the medical field that we’re going to see this – when consent forms are presented to patients to solicit them as participants, and then [these forms] have an average of 40 pages. That annoys me. When they say that it has to be easy to understand and all that, adapted to the language, and then the hyper-technical language plus there are 40 pages to read, I don’t understand how you’re going to get informed consent after reading 40 pages. (…) For me, it doesn’t work. I read them to evaluate them and I have a certain level of education and experience in ethics, and there are times when I don’t understand anything (participant 2). There is a lot of pressure from researchers who want to recruit research participants (…). The idea that when you enter a health care institution, you become a potential research participant, when you say “yes to a research, you check yes to all research”, then everyone can ask you. I think that researchers really have this fantasy of saying to themselves: “as soon as people walk through the door of our institution, they become potential participants with whom we can communicate and get them involved in all projects”. There’s a kind of idea that, yes, it can be done, but it has to be somewhat supervised to avoid over-solicitation (…). Researchers are very interested in facilitating recruitment and making it more fluid, but perhaps to the detriment of confidentiality, privacy, and respect; sometimes that’s what it is, to think about what type of data you’re going to have in your bank of potential participants? Is it just name and phone number or are you getting into more sensitive information? (participant 9).
In addition, one participant reported that their university does not provide the resources required to respect the confidentiality of research participants.
The issue is as follows: researchers, of course, commit to protecting data with passwords and all that, but we realize that in practice, it is more difficult. It is not always as protected as one might think, because professor-researchers will run out of space. Will the universities make rooms available to researchers, places where they can store these things, especially when they have paper documentation, and is there indeed a guarantee of confidentiality? Some researchers have told me: “Listen; there are even filing cabinets in the corridors”. So, that certainly poses a concrete challenge. How do we go about challenging the administrative authorities? Tell them it’s all very well to have an ethics committee, but you have to help us, you also have to make sure that the necessary infrastructures are in place so that what we are proposing is really put into practice (participant 4).
If the relationships with research participants are likely to raise ethical issues, so too are the relationships with students, notably research assistants. On this topic, several participants discussed the lack of supervision or recognition offered to research assistants by researchers as well as the power imbalances between members of the research team.
Lack of Supervision and Power Imbalances
Many research teams are composed not only of researchers, but also of students who work as research assistants. The relationship between research assistants and other members of research teams can sometimes be problematic and raise ethical issues, particularly because of the inevitable power asymmetries. In the context of this study, several participants – including a research assistant, REB members, and researchers – discussed the lack of supervision or recognition of the work carried out by students, psychological pressure, and the more or less well-founded promises that are sometimes made to students. Participants also mentioned the exploitation of students by certain research teams, which manifest when students are inadequately paid, i.e., not reflective of the number of hours actually worked, not a fair wage, or even a wage at all.
[As a research assistant], it was more of a feeling of distress that I felt then because I didn’t know what to do. (…) I was supposed to get coaching or be supported, but I didn’t get anything in the end. It was like, “fix it by yourself”. (…) All research assistants were supposed to be supervised, but in practice they were not (participant 1). Very often, we have a master’s or doctoral student that we put on a subject and we consider that the project will be well done, while the student is learning. So, it happens that the student will do a lot of work and then we realize that the work is poorly done, and it is not necessarily the student’s fault. He wasn’t necessarily well supervised. There are directors who have 25 students, and they just don’t supervise them (participant 14). I think it’s really the power relationship. I thought to myself, how I saw my doctorate, the beginning of my research career, I really wanted to be in that laboratory, but they are the ones who are going to accept me or not, so what do I do to be accepted? I finally accept their conditions [which was to work for free]. If these are the conditions that are required to enter this lab, I want to go there. So, what do I do, well I accepted. It doesn’t make sense, but I tell myself that I’m still privileged, because I don’t have so many financial worries, one more reason to work for free, even though it doesn’t make sense (participant 1). In research, we have research assistants. (…). The fact of using people… so that’s it, you have to take into account where they are, respect them, but at the same time they have to show that they are there for the research. In English, we say “carry” or take care of people. With research assistants, this is often a problem that I have observed: for grant machines, the person is the last to be found there. Researchers, who will take, use student data, without giving them the recognition for it (participant 5). The problem at our university is that they reserve funding for Canadian students. The doctoral clientele in my field is mostly foreign students. So, our students are poorly funded. I saw one student end up in the shelter, in a situation of poverty. It ended very badly for him because he lacked financial resources. Once you get into that dynamic, it’s very hard to get out. I was made aware of it because the director at the time had taken him under her wing and wanted to try to find a way to get him out of it. So, most of my students didn’t get funded (participant 16). There I wrote “manipulation”, but it’s kind of all promises all the time. I, for example, was promised a lot of advancement, like when I got into the lab as a graduate student, it was said that I had an interest in [this particular area of research]. I think there are a lot of graduate students who must have gone through that, but it is like, “Well, your CV has to be really good, if you want to do a lot of things and big things. If you do this, if you do this research contract, the next year you could be the coordinator of this part of the lab and supervise this person, get more contracts, be paid more. Let’s say: you’ll be invited to go to this conference, this big event”. They were always dangling something, but you have to do that first to get there. But now, when you’ve done that, you have to do this business. It’s like a bit of manipulation, I think. That was very hard to know who is telling the truth and who is not (participant 1).
These ethical issues have significant negative consequences for students. Indeed, they sometimes find themselves at the mercy of researchers, for whom they work, struggling to be recognized and included as authors of an article, for example, or to receive the salary that they are due. For their part, researchers also sometimes find themselves trapped in research structures that can negatively affect their well-being. As many participants reported, researchers work in organizations that set very high productivity standards and in highly competitive contexts, all within a general culture characterized by individualism.
Individualism and performance
Participants, especially researchers, discussed the culture of individualism and performance that characterizes the academic environment. In glorifying excellence, some universities value performance and productivity, often at the expense of psychological well-being and work-life balance (i.e., work overload and burnout). Participants noted that there are ethical silences in their organizations on this issue, and that the culture of individualism and performance is not challenged for fear of retribution or simply to survive, i.e., to perform as expected. Participants felt that this culture can have a significant negative impact on the quality of the research conducted, as research teams try to maximize the quantity of their work (instead of quality) in a highly competitive context, which is then exacerbated by a lack of resources and support, and where everything must be done too quickly.
The work-life balance with the professional ethics related to work in a context where you have too much and you have to do a lot, it is difficult to balance all that and there is a lot of pressure to perform. If you don’t produce enough, that’s it; after that, you can’t get any more funds, so that puts pressure on you to do more and more and more (participant 3). There is a culture, I don’t know where it comes from, and that is extremely bureaucratic. If you dare to raise something, you’re going to have many, many problems. They’re going to make you understand it. So, I don’t talk. It is better: your life will be easier. I think there are times when you have to talk (…) because there are going to be irreparable consequences. (…) I’m not talking about a climate of terror, because that’s exaggerated, it’s not true, people are not afraid. But people close their office door and say nothing because it’s going to make their work impossible and they’re not going to lose their job, they’re not going to lose money, but researchers need time to be focused, so they close their office door and say nothing (participant 16).
Researchers must produce more and more, and they feel little support in terms of how to do such production, ethically, and how much exactly they are expected to produce. As this participant reports, the expectation is an unspoken rule: more is always better.
It’s sometimes the lack of a clear line on what the expectations are as a researcher, like, “ah, we don’t have any specific expectations, but produce, produce, produce, produce.” So, in that context, it’s hard to be able to put the line precisely: “have I done enough for my work?” (participant 3).
Inadequate ethical Guidance
While the productivity expectation is not clear, some participants – including researchers, research ethics experts, and REB members – also felt that the ethical expectations of some REBs were unclear. The issue of the inadequate ethical guidance of research includes the administrative mechanisms to ensure that research projects respect the principles of research ethics. According to those participants, the forms required for both researchers and REB members are increasingly long and numerous, and one participant noted that the standards to be met are sometimes outdated and disconnected from the reality of the field. Multicentre ethics review (by several REBs) was also critiqued by a participant as an inefficient method that encumbers the processes for reviewing research projects. Bureaucratization imposes an ever-increasing number of forms and ethics guidelines that actually hinder researchers’ ethical reflection on the issues at stake, leading the ethics review process to be perceived as purely bureaucratic in nature.
The ethical dimension and the ethical review of projects have become increasingly bureaucratized. (…) When I first started working (…) it was less bureaucratic, less strict then. I would say [there are now] tons of forms to fill out. Of course, we can’t do without it, it’s one of the ways of marking out ethics and ensuring that there are ethical considerations in research, but I wonder if it hasn’t become too bureaucratized, so that it’s become a kind of technical reflex to fill out these forms, and I don’t know if people really do ethical reflection as such anymore (participant 10). The fundamental structural issue, I would say, is the mismatch between the normative requirements and the real risks posed by the research, i.e., we have many, many requirements to meet; we have very long forms to fill out but the research projects we evaluate often pose few risks (participant 8). People [in vulnerable situations] were previously unable to participate because of overly strict research ethics rules that were to protect them, but in the end [these rules] did not protect them. There was a perverse effect, because in the end there was very little research done with these people and that’s why we have very few results, very little evidence [to support practices with these populations] so it didn’t improve the quality of services. (…) We all understand that we have to be careful with that, but when the research is not too risky, we say to ourselves that it would be good because for once a researcher who is interested in that population, because it is not a very popular population, it would be interesting to have results, but often we are blocked by the norms, and then we can’t accept [the project] (participant 2).
Moreover, as one participant noted, accessing ethics training can be a challenge.
There is no course on research ethics. […] Then, I find that it’s boring because you go through university and you come to do your research and you know how to do quantitative and qualitative research, but all the research ethics, where do you get this? I don’t really know (participant 13).
Yet, such training could provide relevant tools to resolve, to some extent, the ethical issues that commonly arise in research. That said, and as noted by many participants, many ethical issues in research are related to social injustices over which research actors have little influence.
Social Injustices
For many participants, notably researchers, the issues that concern social injustices are those related to power asymmetries, stigma, or issues of equity, diversity, and inclusion, i.e., social injustices related to people’s identities (Blais & Drolet, 2022 ). Participants reported experiencing or witnessing discrimination from peers, administration, or lab managers. Such oppression is sometimes cross-sectional and related to a person’s age, cultural background, gender or social status.
I have my African colleague who was quite successful when he arrived but had a backlash from colleagues in the department. I think it’s unconscious, nobody is overtly racist. But I have a young person right now who is the same, who has the same success, who got exactly the same early career award and I don’t see the same backlash. He’s just as happy with what he’s doing. It’s normal, they’re young and they have a lot of success starting out. So, I think there is discrimination. Is it because he is African? Is it because he is black? I think it’s on a subconscious level (participant 16).
Social injustices were experienced or reported by many participants, and included issues related to difficulties in obtaining grants or disseminating research results in one’s native language (i.e., even when there is official bilingualism) or being considered credible and fundable in research when one researcher is a woman.
If you do international research, there are things you can’t talk about (…). It is really a barrier to research to not be able to (…) address this question [i.e. the question of inequalities between men and women]. Women’s inequality is going to be addressed [but not within the country where the research takes place as if this inequality exists elsewhere but not here]. There are a lot of women working on inequality issues, doing work and it’s funny because I was talking to a young woman who works at Cairo University and she said to me: “Listen, I saw what you had written, you’re right. I’m willing to work on this but guarantee me a position at your university with a ticket to go”. So yes, there are still many barriers [for women in research] (participant 16).
Because of the varied contextual characteristics that intervene in their occurrence, these social injustices are also related to distributive injustices, as discussed by many participants.
Distributive Injustices
Although there are several views of distributive justice, a classical definition such as that of Aristotle ( 2012 ), describes distributive justice as consisting in distributing honours, wealth, and other social resources or benefits among the members of a community in proportion to their alleged merit. Justice, then, is about determining an equitable distribution of common goods. Contemporary theories of distributive justice are numerous and varied. Indeed, many authors (e.g., Fraser 2011 ; Mills, 2017 ; Sen, 2011 ; Young, 2011 ) have, since Rawls ( 1971 ), proposed different visions of how social burdens and benefits should be shared within a community to ensure equal respect, fairness, and distribution. In our study, what emerges from participants’ narratives is a definite concern for this type of justice. Women researchers, francophone researchers, early career researchers or researchers belonging to racialized groups all discussed inequities in the distribution of research grants and awards, and the extra work they need to do to somehow prove their worth. These inequities are related to how granting agencies determine which projects will be funded.
These situations make me work 2–3 times harder to prove myself and to show people in power that I have a place as a woman in research (participant 12). Number one: it’s conservative thinking. The older ones control what comes in. So, the younger people have to adapt or they don’t get funded (participant 14).
Whether it is discrimination against stigmatized or marginalized populations or interest in certain hot topics, granting agencies judge research projects according to criteria that are sometimes questionable, according to those participants. Faced with difficulties in obtaining funding for their projects, several strategies – some of which are unethical – are used by researchers in order to cope with these situations.
Sometimes there are subjects that everyone goes to, such as nanotechnology (…), artificial intelligence or (…) the therapeutic use of cannabis, which are very fashionable, and this is sometimes to the detriment of other research that is just as relevant, but which is (…), less sexy, less in the spirit of the time. (…) Sometimes this can lead to inequities in the funding of certain research sectors (participant 9). When we use our funds, we get them given to us, we pretty much say what we think we’re going to do with them, but things change… So, when these things change, sometimes it’s an ethical decision, but by force of circumstances I’m obliged to change the project a little bit (…). Is it ethical to make these changes or should I just let the money go because I couldn’t use it the way I said I would? (participant 3).
Moreover, these distributional injustices are not only linked to social injustices, but also epistemic injustices. Indeed, the way in which research honours and grants are distributed within the academic community depends on the epistemic authority of the researchers, which seems to vary notably according to their language of use, their age or their gender, but also to the research design used (inductive versus deductive), their decision to use (or not use) animals in research, or to conduct activist research.
Epistemic injustices
The philosopher Fricker ( 2007 ) conceptualized the notions of epistemic justice and injustice. Epistemic injustice refers to a form of social inequality that manifests itself in the access, recognition, and production of knowledge as well as the various forms of ignorance that arise (Godrie & Dos Santos, 2017 ). Addressing epistemic injustice necessitates acknowledging the iniquitous wrongs suffered by certain groups of socially stigmatized individuals who have been excluded from knowledge, thus limiting their abilities to interpret, understand, or be heard and account for their experiences. In this study, epistemic injustices were experienced or reported by some participants, notably those related to difficulties in obtaining grants or disseminating research results in one’s native language (i.e., even when there is official bilingualism) or being considered credible and fundable in research when a researcher is a woman or an early career researcher.
I have never sent a grant application to the federal government in English. I have always done it in French, even though I know that when you receive the review, you can see that reviewers didn’t understand anything because they are English-speaking. I didn’t want to get in the boat. It’s not my job to translate, because let’s be honest, I’m not as good in English as I am in French. So, I do them in my first language, which is the language I’m most used to. Then, technically at the administrative level, they are supposed to be able to do it, but they are not good in French. (…) Then, it’s a very big Canadian ethical issue, because basically there are technically two official languages, but Canada is not a bilingual country, it’s a country with two languages, either one or the other. (…) So I was not funded (participant 14).
Researchers who use inductive (or qualitative) methods observed that their projects are sometimes less well reviewed or understood, while research that adopts a hypothetical-deductive (or quantitative) or mixed methods design is better perceived, considered more credible and therefore more easily funded. Of course, regardless of whether a research project adopts an inductive, deductive or mixed-methods scientific design, or whether it deals with qualitative or quantitative data, it must respect a set of scientific criteria. A research project should achieve its objectives by using proven methods that, in the case of inductive research, are credible, reliable, and transferable or, in the case of deductive research, generalizable, objective, representative, and valid (Drolet & Ruest, accepted ). Participants discussing these issues noted that researchers who adopt a qualitative design or those who question the relevance of animal experimentation or are not militant have sometimes been unfairly devalued in their epistemic authority.
There is a mini war between quantitative versus qualitative methods, which I think is silly because science is a method. If you apply the method well, it doesn’t matter what the field is, it’s done well and it’s perfect ” (participant 14). There is also the issue of the place of animals in our lives, because for me, ethics is human ethics, but also animal ethics. Then, there is a great evolution in society on the role of the animal… with the new law that came out in Quebec on the fact that animals are sensitive beings. Then, with the rise of the vegan movement, [we must ask ourselves]: “Do animals still have a place in research?” That’s a big question and it also means that there are practices that need to evolve, but sometimes there’s a disconnection between what’s expected by research ethics boards versus what’s expected in the field (participant 15). In research today, we have more and more research that is militant from an ideological point of view. And so, we have researchers, because they defend values that seem important to them, we’ll talk for example about the fight for equality and social justice. They have pressure to defend a form of moral truth and have the impression that everyone thinks like them or should do so, because they are defending a moral truth. This is something that we see more and more, namely the lack of distance between ideology and science (participant 8).
The combination or intersectionality of these inequities, which seems to be characterized by a lack of ethical support and guidance, is experienced in the highly competitive and individualistic context of research; it provides therefore the perfect recipe for researchers to experience ethical distress.
Ethical distress
The concept of “ethical distress” refers to situations in which people know what they should do to act ethically, but encounter barriers, generally of an organizational or systemic nature, limiting their power to act according to their moral or ethical values (Drolet & Ruest, 2021 ; Jameton, 1984 ; Swisher et al., 2005 ). People then run the risk of finding themselves in a situation where they do not act as their ethical conscience dictates, which in the long term has the potential for exhaustion and distress. The examples reported by participants in this study point to the fact that researchers in particular may be experiencing significant ethical distress. This distress takes place in a context of extreme competition, constant injunctions to perform, and where administrative demands are increasingly numerous and complex to complete, while paradoxically, they lack the time to accomplish all their tasks and responsibilities. Added to these demands are a lack of resources (human, ethical, and financial), a lack of support and recognition, and interpersonal conflicts.
We are in an environment, an elite one, you are part of it, you know what it is: “publish or perish” is the motto. Grants, there is a high level of performance required, to do a lot, to publish, to supervise students, to supervise them well, so yes, it is clear that we are in an environment that is conducive to distress. (…). Overwork, definitely, can lead to distress and eventually to exhaustion. When you know that you should take the time to read the projects before sharing them, but you don’t have the time to do that because you have eight that came in the same day, and then you have others waiting… Then someone rings a bell and says: “ah but there, the protocol is a bit incomplete”. Oh yes, look at that, you’re right. You make up for it, but at the same time it’s a bit because we’re in a hurry, we don’t necessarily have the resources or are able to take the time to do things well from the start, we have to make up for it later. So yes, it can cause distress (participant 9). My organization wanted me to apply in English, and I said no, and everyone in the administration wanted me to apply in English, and I always said no. Some people said: “Listen, I give you the choice”, then some people said: “Listen, I agree with you, but if you’re not [submitting] in English, you won’t be funded”. Then the fact that I am young too, because very often they will look at the CV, they will not look at the project: “ah, his CV is not impressive, we will not finance him”. This is complete nonsense. The person is capable of doing the project, the project is fabulous: we fund the project. So, that happened, organizational barriers: that happened a lot. I was not eligible for Quebec research funds (…). I had big organizational barriers unfortunately (participant 14). At the time of my promotion, some colleagues were not happy with the type of research I was conducting. I learned – you learn this over time when you become friends with people after you enter the university – that someone was against me. He had another candidate in mind, and he was angry about the selection. I was under pressure for the first three years until my contract was renewed. I almost quit at one point, but another colleague told me, “No, stay, nothing will happen”. Nothing happened, but these issues kept me awake at night (participant 16).
This difficult context for many researchers affects not only the conduct of their own research, but also their participation in research. We faced this problem in our study, despite the use of multiple recruitment methods, including more than 200 emails – of which 191 were individual solicitations – sent to potential participants by the two research assistants. REB members and organizations overseeing or supporting research (n = 17) were also approached to see if some of their employees would consider participating. While it was relatively easy to recruit REB members and research ethics experts, our team received a high number of non-responses to emails (n = 175) and some refusals (n = 5), especially by researchers. The reasons given by those who replied were threefold: (a) fear of being easily identified should they take part in the research, (b) being overloaded and lacking time, and (c) the intrusive aspect of certain questions (i.e., “Have you experienced a burnout episode? If so, have you been followed up medically or psychologically?”). In light of these difficulties and concerns, some questions in the socio-demographic questionnaire were removed or modified. Talking about burnout in research remains a taboo for many researchers, which paradoxically can only contribute to the unresolved problem of unhealthy research environments.
Returning to the research question and objective
The question that prompted this research was: What are the ethical issues in research? The purpose of the study was to describe these issues from the perspective of researchers (from different disciplines), research ethics board (REB) members, and research ethics experts. The previous section provided a detailed portrait of the ethical issues experienced by different research stakeholders: these issues are numerous, diverse and were recounted by a range of stakeholders.
The results of the study are generally consistent with the literature. For example, as in our study, the literature discusses the lack of research integrity on the part of some researchers (Al-Hidabi et al., 2018 ; Swazey et al., 1993 ), the numerous conflicts of interest experienced in research (Williams-Jones et al., 2013 ), the issues of recruiting and obtaining the free and informed consent of research participants (Provencher et al., 2014 ; Keogh & Daly, 2009 ), the sometimes difficult relations between researchers and REBs (Drolet & Girard, 2020 ), the epistemological issues experienced in research (Drolet & Ruest, accepted; Sieber 2004 ), as well as the harmful academic context in which researchers evolve, insofar as this is linked to a culture of performance, an overload of work in a context of accountability (Berg & Seeber, 2016 ; FQPPU; 2019 ) that is conducive to ethical distress and even burnout.
If the results of the study are generally in line with those of previous publications on the subject, our findings also bring new elements to the discussion while complementing those already documented. In particular, our results highlight the role of systemic injustices – be they social, distributive or epistemic – within the environments in which research is carried out, at least in Canada. To summarize, the results of our study point to the fact that the relationships between researchers and research participants are likely still to raise worrying ethical issues, despite widely accepted research ethics norms and institutionalized review processes. Further, the context in which research is carried out is not only conducive to breaches of ethical norms and instances of misbehaviour or misconduct, but also likely to be significantly detrimental to the health and well-being of researchers, as well as research assistants. Another element that our research also highlighted is the instrumentalization and even exploitation of students and research assistants, which is another important and worrying social injustice given the inevitable power imbalances between students and researchers.
Moreover, in a context in which ethical issues are often discussed from a micro perspective, our study helps shed light on both the micro- and macro-level ethical dimensions of research (Bronfenbrenner, 1979 ; Glaser 1994 ). However, given that ethical issues in research are not only diverse, but also and above all complex, a broader perspective that encompasses the interplay between the micro and macro dimensions can enable a better understanding of these issues and thereby support the identification of the multiple factors that may be at their origin. Triangulating the perspectives of researchers with those of REB members and research ethics experts enabled us to bring these elements to light, and thus to step back from and critique the way that research is currently conducted. To this end, attention to socio-political elements such as the performance culture in academia or how research funds are distributed, and according to what explicit and implicit criteria, can contribute to identifying the sources of the ethical issues described above.
Contemporary culture characterized by the social acceleration
The German sociologist and philosopher Rosa (2010) argues that late modernity – that is, the period between the 1980s and today – is characterized by a phenomenon of social acceleration that causes various forms of alienation in our relationship to time, space, actions, things, others and ourselves. Rosa distinguishes three types of acceleration: technical acceleration , the acceleration of social changes and the acceleration of the rhythm of life . According to Rosa, social acceleration is the main problem of late modernity, in that the invisible social norm of doing more and faster to supposedly save time operates unchallenged at all levels of individual and collective life, as well as organizational and social life. Although we all, researchers and non-researchers alike, perceive this unspoken pressure to be ever more productive, the process of social acceleration as a new invisible social norm is our blind spot, a kind of tyrant over which we have little control. This conceptualization of the contemporary culture can help us to understand the context in which research is conducted (like other professional practices). To this end, Berg & Seeber ( 2016 ) invite faculty researchers to slow down in order to better reflect and, in the process, take care of their health and their relationships with their colleagues and students. Many women professors encourage their fellow researchers, especially young women researchers, to learn to “say No” in order to protect their mental and physical health and to remain in their academic careers (Allaire & Descheneux, 2022 ). These authors also remind us of the relevance of Kahneman’s ( 2012 ) work which demonstrates that it takes time to think analytically, thoroughly, and logically. Conversely, thinking quickly exposes humans to cognitive and implicit biases that then lead to errors in thinking (e.g., in the analysis of one’s own research data or in the evaluation of grant applications or student curriculum vitae). The phenomenon of social acceleration, which pushes the researcher to think faster and faster, is likely to lead to unethical bad science that can potentially harm humankind. In sum, Rosa’s invitation to contemporary critical theorists to seriously consider the problem of social acceleration is particularly insightful to better understand the ethical issues of research. It provides a lens through which to view the toxic context in which research is conducted today, and one that was shared by the participants in our study.
Clark & Sousa ( 2022 ) note, it is important that other criteria than the volume of researchers’ contributions be valued in research, notably quality. Ultimately, it is the value of the knowledge produced and its influence on the concrete lives of humans and other living beings that matters, not the quantity of publications. An interesting articulation of this view in research governance is seen in a change in practice by Australia’s national health research funder: they now restrict researchers to listing on their curriculum vitae only the top ten publications from the past ten years (rather than all of their publications), in order to evaluate the quality of contributions rather than their quantity. To create environments conducive to the development of quality research, it is important to challenge the phenomenon of social acceleration, which insidiously imposes a quantitative normativity that is both alienating and detrimental to the quality and ethical conduct of research. Based on our experience, we observe that the social norm of acceleration actively disfavours the conduct of empirical research on ethics in research. The fact is that researchers are so busy that it is almost impossible for them to find time to participate in such studies. Further, operating in highly competitive environments, while trying to respect the values and ethical principles of research, creates ethical paradoxes for members of the research community. According to Malherbe ( 1999 ), an ethical paradox is a situation where an individual is confronted by contradictory injunctions (i.e., do more, faster, and better). And eventually, ethical paradoxes lead individuals to situations of distress and burnout, or even to ethical failures (i.e., misbehaviour or misconduct) in the face of the impossibility of responding to contradictory injunctions.
Strengths and Limitations of the study
The triangulation of perceptions and experiences of different actors involved in research is a strength of our study. While there are many studies on the experiences of researchers, rarely are members of REBs and experts in research ethics given the space to discuss their views of what are ethical issues. Giving each of these stakeholders a voice and comparing their different points of view helped shed a different and complementary light on the ethical issues that occur in research. That said, it would have been helpful to also give more space to issues experienced by students or research assistants, as the relationships between researchers and research assistants are at times very worrying, as noted by a participant, and much work still needs to be done to eliminate the exploitative situations that seem to prevail in certain research settings. In addition, no Indigenous or gender diverse researchers participated in the study. Given the ethical issues and systemic injustices that many people from these groups face in Canada (Drolet & Goulet, 2018 ; Nicole & Drolet, in press ), research that gives voice to these researchers would be relevant and contribute to knowledge development, and hopefully also to change in research culture.
Further, although most of the ethical issues discussed in this article may be transferable to the realities experienced by researchers in other countries, the epistemic injustice reported by Francophone researchers who persist in doing research in French in Canada – which is an officially bilingual country but in practice is predominantly English – is likely specific to the Canadian reality. In addition, and as mentioned above, recruitment proved exceedingly difficult, particularly amongst researchers. Despite this difficulty, we obtained data saturation for all but two themes – i.e., exploitation of students and ethical issues of research that uses animals. It follows that further empirical research is needed to improve our understanding of these specific issues, as they may diverge to some extent from those documented here and will likely vary across countries and academic research contexts.
Conclusions
This study, which gave voice to researchers, REB members, and ethics experts, reveals that the ethical issues in research are related to several problematic elements as power imbalances and authority relations. Researchers and research assistants are subject to external pressures that give rise to integrity issues, among others ethical issues. Moreover, the current context of social acceleration influences the definition of the performance indicators valued in academic institutions and has led their members to face several ethical issues, including social, distributive, and epistemic injustices, at different steps of the research process. In this study, ten categories of ethical issues were identified, described and illustrated: (1) research integrity, (2) conflicts of interest, (3) respect for research participants, (4) lack of supervision and power imbalances, (5) individualism and performance, (6) inadequate ethical guidance, (7) social injustices, (8) distributive injustices, (9) epistemic injustices, and (10) ethical distress. The triangulation of the perspectives of different members (i.e., researchers from different disciplines, REB members, research ethics experts, and one research assistant) involved in the research process made it possible to lift the veil on some of these ethical issues. Further, it enabled the identification of additional ethical issues, especially systemic injustices experienced in research. To our knowledge, this is the first time that these injustices (social, distributive, and epistemic injustices) have been clearly identified.
Finally, this study brought to the fore several problematic elements that are important to address if the research community is to develop and implement the solutions needed to resolve the diverse and transversal ethical issues that arise in research institutions. A good starting point is the rejection of the corollary norms of “publish or perish” and “do more, faster, and better” and their replacement with “publish quality instead of quantity”, which necessarily entails “do less, slower, and better”. It is also important to pay more attention to the systemic injustices within which researchers work, because these have the potential to significantly harm the academic careers of many researchers, including women researchers, early career researchers, and those belonging to racialized groups as well as the health, well-being, and respect of students and research participants.
Al-Hidabi, Abdulmalek, M. D., & The, P. L. (2018). Multiple Publications: The Main Reason for the Retraction of Papers in Computer Science. In K. Arai, S. Kapoor, & R. Bhatia (eds), Future of Information and Communication Conference (FICC): Advances in Information and Communication, Advances in Intelligent Systems and Computing (AISC), Springer, vol. 886, pp. 511–526
Allaire, S., & Deschenaux, F. (2022). Récits de professeurs d’université à mi-carrière. Si c’était à refaire… . Presses de l’Université du Québec
Aristotle (2012). Aristotle’s Nicomachean Ethics . Chicago: The University of Chicago Press
Google Scholar
Bahn, S. (2012). Keeping Academic Field Researchers Safe: Ethical Safeguards. Journal of Academic Ethics , 10 , 83–91. https://doi.org/10.1007/s10805-012-9159-2
Article Google Scholar
Balk, D. E. (1995). Bereavement Research Using Control Groups: Ethical Obligations and Questions. Death Studies , 19 , 123–138
Beauchemin, É., Côté, L. P., Drolet, M. J., & Williams-Jones, B. (2021). Conceptualizing Ethical Issues in the Conduct of Research: Results from a Critical and Systematic Literature Review. Journal of Academic Ethics , Early Online. https://doi.org/10.1007/s10805-021-09411-7
Berg, M., & Seeber, B. K. (2016). The Slow Professor . University of Toronto Press
Birchley, G., Huxtable, R., Murtagh, M., Meulen, R. T., Flach, P., & Gooberman-Hill, R. (2017). Smart homes, private homes? An empirical study of technology researchers’ perceptions of ethical issues in developing smart-home health technologies. BMC Medical Ethics , 18 (23), 1–13. https://doi.org/10.1186/s12910-017-0183-z
Blais, J., & Drolet, M. J. (2022). Les injustices sociales vécues en camp de réfugiés: les comprendre pour mieux intervenir auprès de personnes ayant séjourné dans un camp de réfugiés. Recueil annuel belge d’ergothérapie , 14, 37–48
Bogdan, R. C., & Biklen, S. K. (2006). Qualitative research in education: An introduction to theory and methods . Allyn & Bacon
Bouffard, C. (2000). Le développement des pratiques de la génétique médicale et la construction des normes bioéthiques. Anthropologie et Sociétés , 24 (2), 73–90. https://doi.org/10.7202/015650ar
Bronfenbrenner, U. (1979). The Ecology of Human development. Experiments by nature and design . Harvard University Press
Bruhn, J. G., Zajac, G., Al-Kazemi, A. A., & Prescott, L. D. (2002). Moral positions and academic conduct: Parameters of tolerance for ethics failure. Journal of Higher Education , 73 (4), 461–493. https://doi.org/10.1353/jhe.2002.0033
Clark, A., & Sousa (2022). It’s time to end Canada’s obsession with research quantity. University Affairs/Affaires universitaires , February 14th. https://www.universityaffairs.ca/career-advice/effective-successfull-happy-academic/its-time-to-end-canadas-obsession-with-research-quantity/?utm_source=University+Affairs+e-newsletter&utm_campaign=276a847f 70-EMAIL_CAMPAIGN_2022_02_16&utm_medium=email&utm_term=0_314bc2ee29-276a847f70-425259989
Colnerud, G. (2015). Ethical dilemmas in research in relation to ethical review: An empirical study. Research Ethics , 10 (4), 238–253. DOI: https://doi.org/10.1177/1747016114552339
Davison, J. (2004). Dilemmas in Research: Issues of Vulnerability and Disempowerment for the Social Workers/Researcher. Journal of Social Work Practice , 18 (3), 379–393. https://doi.org/10.1080/0265053042000314447
DePoy, E., & Gitlin, L. N. (2010). Introduction to Research . St. Louis: Elsevier Mosby
Drolet, M. J., & Goulet, M. (2018). Travailler avec des patients autochtones du Canada ? Perceptions d’ergothérapeutes du Québec des enjeux éthiques de cette pratique. Recueil annuel belge francophone d’ergothérapie , 10 , 25–56
Drolet, M. J., & Girard, K. (2020). Les enjeux éthiques de la recherche en ergothérapie: un portrait préoccupant. Revue canadienne de bioéthique , 3 (3), 21–40. https://doi.org/10.7202/1073779ar
Drolet, M. J., Girard, K., & Gaudet, R. (2020). Les enjeux éthiques de l’enseignement en ergothérapie: des injustices au sein des départements universitaires. Revue canadienne de bioéthique , 3 (1), 22–36. https://www.erudit.org/fr/revues/bioethics/2020-v3-n1-bioethics05237/1068761ar/
Drolet, M. J., & Maclure, J. (2016). Les enjeux éthiques de la pratique de l’ergothérapie: perceptions d’ergothérapeutes. Revue Approches inductives , 3 (2), 166–196
Drolet, M. J., Pinard, C., & Gaudet, R. (2017). Les enjeux éthiques de la pratique privée: des ergothérapeutes du Québec lancent un cri d’alarme. Ethica – Revue interdisciplinaire de recherche en éthique , 21 (2), 173–209
Drolet, M. J., & Ruest, M. (2021). De l’éthique à l’ergothérapie: un cadre théorique et une méthode pour soutenir la pratique professionnelle . Québec: Presses de l’Université du Québec
Book Google Scholar
Drolet, M. J., & Ruest, M. (accepted). Quels sont les enjeux éthiques soulevés par la recherche scientifique? In M. Lalancette & J. Luckerhoff (dir). Initiation au travail intellectuel et à la recherche . Québec: Presses de l’Université du Québec, 18 p
Drolet, M. J., Sauvageau, A., Baril, N., & Gaudet, R. (2019). Les enjeux éthiques de la formation clinique en ergothérapie. Revue Approches inductives , 6 (1), 148–179. https://www.erudit.org/fr/revues/approchesind/2019-v6-n1-approchesind04618/1060048ar/
Fédération québécoise des professeures et des professeurs d’université (FQPPU). (2019). Enquête nationale sur la surcharge administrative du corps professoral universitaire québécois. Principaux résultats et pistes d’action . Montréal: FQPPU
Fortin, M. H. (2010). Fondements et étapes du processus de recherche. Méthodes quantitatives et qualitatives . Montréal, QC: Chenelière éducation
Fraser, D. M. (1997). Ethical dilemmas and practical problems for the practitioner researcher. Educational Action Research , 5 (1), 161–171
Fraser, N. (2011). Qu’est-ce que la justice sociale? Reconnaissance et redistribution . La Découverte
Fricker, M. (2007). Epistemic Injustice: Power and the Ethics of Knowing . Oxford University Press
Giorgi, A. (1997). De la méthode phénoménologique utilisée comme mode de recherche qualitative en sciences humaines: théories, pratique et évaluation. In J. Poupart, L. H. Groulx, J. P. Deslauriers, et al. (Eds.), La recherche qualitative: enjeux épistémologiques et méthodologiques (pp. 341–364). Boucherville, QC: Gaëtan Morin
Giorgini, V., Mecca, J. T., Gibson, C., Medeiros, K., Mumford, M. D., Connelly, S., & Devenport, L. D. (2016). Researcher Perceptions of Ethical Guidelines and Codes of Conduct. Accountability in Research , 22 (3), 123–138. https://doi.org/10.1080/08989621.2014.955607
Glaser, J. W. (1994). Three realms of ethics: Individual, institutional, societal. Theoretical model and case studies . Kansas Cuty, Sheed & Ward
Godrie, B., & Dos Santos, M. (2017). Présentation: inégalités sociales, production des savoirs et de l’ignorance. Sociologie et sociétés , 49 (1), 7. https://doi.org/10.7202/1042804ar
Hammell, K. W., Carpenter, C., & Dyck, I. (2000). Using Qualitative Research: A Practical Introduction for Occupational and Physical Therapists . Edinburgh: Churchill Livingstone
Henderson, M., Johnson, N. F., & Auld, G. (2013). Silences of ethical practice: dilemmas for researchers using social media. Educational Research and Evaluation , 19 (6), 546–560. https://doi.org/10.1080/13803611.2013.805656
Husserl, E. (1970). The crisis of European sciences and transcendental phenomenology . Evanston, IL: Northwestern University Press
Husserl, E. (1999). The train of thoughts in the lectures. In E. C. Polifroni, & M. Welch (Eds.), Perspectives on Philosophy of Science in Nursing . Philadelphia, PA: Lippincott. 247 – 62. 43
Hunt, S. D., Chonko, L. B., & Wilcox, J. B. (1984). Ethical problems of marketing researchers. Journal of Marketing Research , 21 , 309–324
Hunt, M. R., & Carnevale, F. A. (2011). Moral experience: A framework for bioethics research. Journal of Medical Ethics , 37 (11), 658–662. https://doi.org/10.1136/jme.2010.039008
Jameton, A. (1984). Nursing practice: The ethical issues . Englewood Cliffs, Prentice-Hall
Jarvis, K. (2017). Dilemmas in International Research and the Value of Practical Wisdom. Developing World Bioethics , 17 (1), 50–58. DOI: https://doi.org/10.1111/dewb.12121
Kahneman, D. (2012). Système 1, système 2: les deux vitesses de la pensée . Paris: Flammarion
Keogh, B., & Daly, L. (2009). The ethics of conducting research with mental health service users. British Journal of Nursing , 18 (5), 277–281. https://doi.org/10.12968/bjon.2009.18.5.40539
Lierville, A. L., Grou, C., & Pelletier, J. F. (2015). Enjeux éthiques potentiels liés aux partenariats patients en psychiatrie: État de situation à l’Institut universitaire en santé mentale de Montréal. Santé mentale au Québec , 40 (1), 119–134
Lynöe, N., Sandlund, M., & Jacobsson, L. (1999). Research ethics committees: A comparative study of assessment of ethical dilemmas. Scandinavian Journal of Public Health , 27 (2), 152–159
Malherbe, J. F. (1999). Compromis, dilemmes et paradoxes en éthique clinique . Anjou: Éditions Fides
McGinn, R. (2013). Discernment and denial: Nanotechnology researchers’ recognition of ethical responsibilities related to their work. NanoEthics , 7 , 93–105. https://doi.org/10.1007/s11569-013-0174-6
Mills, C. W. (2017). Black Rights / White rongs. The Critique of Racial Liberalism . Oxford University Press
Miyazaki, A. D., & Taylor, K. A. (2008). Researcher interaction biases and business ethics research: Respondent reactions to researcher characteristics. Journal of Business Ethics , 81 (4), 779–795. DOI https://doi.org/10.1007/s10551-007-9547-5
Mondain, N., & Bologo, E. (2009). L’intentionnalité du chercheur dans ses pratiques de production des connaissances: les enjeux soulevés par la construction des données en démographie et santé en Afrique. Cahiers de recherche sociologique , 48 , 175–204. https://doi.org/10.7202/039772ar
Nicole, M., & Drolet, M. J. (in press). Fitting transphobia and cisgenderism in occupational therapy, Occupational Therapy Now
Pope, K. S., & Vetter, V. A. (1992). Ethical dilemmas encountered by members of the American Psychological Association: A national survey. The American Psychologist , 47 (3), 397–411
Provencher, V., Mortenson, W. B., Tanguay-Garneau, L., Bélanger, K., & Dagenais, M. (2014). Challenges and strategies pertaining to recruitment and retention of frail elderly in research studies: A systematic review. Archives of Gerontology and Geriatrics , 59 (1), 18–24. https://doi.org/10.1016/j.archger.2014.03.006
Rawls, J. (1971). A Theory of Justice . Harvard University Press
Resnik, D. B., & Elliott, K. C. (2016). The Ethical Challenges of Socially Responsible Science. Accountability in Research , 23 (1), 31–46. https://doi.org/10.1080/08989621.2014.1002608
Rosa, H. (2010). Accélération et aliénation. Vers une théorie critique de la modernité tardive . Paris, Découverte
Sen, A. K. (2011). The Idea of Justice . The Belknap Press of Harvard University Press
Sen, A. K. (1995). Inegality Reexaminated . Oxford University Press
Sieber, J. E. (2004). Empirical Research on Research Ethics. Ethics & Behavior , 14 (4), 397–412. https://doi.org/10.1207/s15327019eb1404_9
Sigmon, S. T. (1995). Ethical practices and beliefs of psychopathology researchers. Ethics & Behavior , 5 (4), 295–309
Swazey, J. P., Anderson, M. S., & Lewis, K. S. (1993). Ethical Problems in Academic Research. American Scientist , 81 (6), 542–553
Swisher, L. L., Arsalanian, L. E., & Davis, C. M. (2005). The realm-individual-process-situation (RIPS) model of ethical decision-making. HPA Resource , 5 (3), 3–8. https://www-s3-live.kent.edu/s3fs-root/s3fs-public/file/RIPS_DecisionMaking_0.pdf
Tri-Council Policy Statement (TCPS2) (2018). Ethical Conduct for Research Involving Humans . Government of Canada, Secretariat on Responsible Conduct of Research. https://ethics.gc.ca/eng/documents/tcps2-2018-en-interactive-final.pdf
Thomas, S. P., & Pollio, H. R. (2002). Listening to Patients: A Phenomenological Approach to Nursing Research and Practice . New York: Springer Publishing Company
Wiegand, D. L., & Funk, M. (2012). Consequences of clinical situations that cause critical care nurses to experience moral distress. Nursing Ethics , 19 (4), 479–487. DOI https://doi.org/10.1177/0969733011429342
Williams-Jones, B., Potvin, M. J., Mathieu, G., & Smith, E. (2013). Barriers to research on research ethics review and conflicts of interest. IRB: Ethics & Human Research , 35 (5), 14–20
Young, I. M. (2011). Justice and the Politics of difference . Princeton University Press
Download references
Acknowledgements
The team warmly thanks the participants who took part in the research and who made this study possible. Marie-Josée Drolet thanks the five research assistants who participated in the data collection and analysis: Julie-Claude Leblanc, Élie Beauchemin, Pénéloppe Bernier, Louis-Pierre Côté, and Eugénie Rose-Derouin, all students at the Université du Québec à Trois-Rivières (UQTR), two of whom were active in the writing of this article. MJ Drolet and Bryn Williams-Jones also acknowledge the financial contribution of the Social Sciences and Humanities Research Council of Canada (SSHRC), which supported this research through a grant. We would also like to thank the reviewers of this article who helped us improve it, especially by clarifying and refining our ideas.
Author information
Authors and affiliations.
Department of Occupational Therapy (OT), Université du Québec à Trois-Rivières (UQTR), Trois-Rivières (Québec), Canada
Marie-Josée Drolet
Bachelor OT program, Université du Québec à Trois-Rivières (UQTR), Trois-Rivières (Québec), Canada
Eugénie Rose-Derouin, Julie-Claude Leblanc & Mélanie Ruest
Department of Social and Preventive Medicine, School of Public Health, Université de Montréal, Montréal (Québec), Canada
Bryn Williams-Jones
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Marie-Josée Drolet .
Ethics declarations
Competing interests and funding.
As noted in the Acknowledgements, this research was supported financially by the Social Sciences and Humanities Research Council of Canada (SSHRC).
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Drolet, MJ., Rose-Derouin, E., Leblanc, JC. et al. Ethical Issues in Research: Perceptions of Researchers, Research Ethics Board Members and Research Ethics Experts. J Acad Ethics 21 , 269–292 (2023). https://doi.org/10.1007/s10805-022-09455-3
Download citation
Received : 24 March 2022
Revised : 13 July 2022
Accepted : 13 July 2022
Published : 12 August 2022
Issue Date : June 2023
DOI : https://doi.org/10.1007/s10805-022-09455-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Researchers
- Research Ethics Board Members
- Research Ethics experts
- Find a journal
- Publish with us
- Track your research
Frequently asked questions
What are ethical considerations in research.
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
Frequently asked questions: Methodology
Attrition refers to participants leaving a study. It always happens to some extent—for example, in randomized controlled trials for medical research.
Differential attrition occurs when attrition or dropout rates differ systematically between the intervention and the control group . As a result, the characteristics of the participants who drop out differ from the characteristics of those who stay in the study. Because of this, study results may be biased .
Action research is conducted in order to solve a particular issue immediately, while case studies are often conducted over a longer period of time and focus more on observing and analyzing a particular ongoing phenomenon.
Action research is focused on solving a problem or informing individual and community-based knowledge in a way that impacts teaching, learning, and other related processes. It is less focused on contributing theoretical input, instead producing actionable input.
Action research is particularly popular with educators as a form of systematic inquiry because it prioritizes reflection and bridges the gap between theory and practice. Educators are able to simultaneously investigate an issue as they solve it, and the method is very iterative and flexible.
A cycle of inquiry is another name for action research . It is usually visualized in a spiral shape following a series of steps, such as “planning → acting → observing → reflecting.”
To make quantitative observations , you need to use instruments that are capable of measuring the quantity you want to observe. For example, you might use a ruler to measure the length of an object or a thermometer to measure its temperature.
Criterion validity and construct validity are both types of measurement validity . In other words, they both show you how accurately a method measures something.
While construct validity is the degree to which a test or other measurement method measures what it claims to measure, criterion validity is the degree to which a test can predictively (in the future) or concurrently (in the present) measure something.
Construct validity is often considered the overarching type of measurement validity . You need to have face validity , content validity , and criterion validity in order to achieve construct validity.
Convergent validity and discriminant validity are both subtypes of construct validity . Together, they help you evaluate whether a test measures the concept it was designed to measure.
- Convergent validity indicates whether a test that is designed to measure a particular construct correlates with other tests that assess the same or similar construct.
- Discriminant validity indicates whether two tests that should not be highly related to each other are indeed not related. This type of validity is also called divergent validity .
You need to assess both in order to demonstrate construct validity. Neither one alone is sufficient for establishing construct validity.
- Discriminant validity indicates whether two tests that should not be highly related to each other are indeed not related
Content validity shows you how accurately a test or other measurement method taps into the various aspects of the specific construct you are researching.
In other words, it helps you answer the question: “does the test measure all aspects of the construct I want to measure?” If it does, then the test has high content validity.
The higher the content validity, the more accurate the measurement of the construct.
If the test fails to include parts of the construct, or irrelevant parts are included, the validity of the instrument is threatened, which brings your results into question.
Face validity and content validity are similar in that they both evaluate how suitable the content of a test is. The difference is that face validity is subjective, and assesses content at surface level.
When a test has strong face validity, anyone would agree that the test’s questions appear to measure what they are intended to measure.
For example, looking at a 4th grade math test consisting of problems in which students have to add and multiply, most people would agree that it has strong face validity (i.e., it looks like a math test).
On the other hand, content validity evaluates how well a test represents all the aspects of a topic. Assessing content validity is more systematic and relies on expert evaluation. of each question, analyzing whether each one covers the aspects that the test was designed to cover.
A 4th grade math test would have high content validity if it covered all the skills taught in that grade. Experts(in this case, math teachers), would have to evaluate the content validity by comparing the test to the learning objectives.
Snowball sampling is a non-probability sampling method . Unlike probability sampling (which involves some form of random selection ), the initial individuals selected to be studied are the ones who recruit new participants.
Because not every member of the target population has an equal chance of being recruited into the sample, selection in snowball sampling is non-random.
Snowball sampling is a non-probability sampling method , where there is not an equal chance for every member of the population to be included in the sample .
This means that you cannot use inferential statistics and make generalizations —often the goal of quantitative research . As such, a snowball sample is not representative of the target population and is usually a better fit for qualitative research .
Snowball sampling relies on the use of referrals. Here, the researcher recruits one or more initial participants, who then recruit the next ones.
Participants share similar characteristics and/or know each other. Because of this, not every member of the population has an equal chance of being included in the sample, giving rise to sampling bias .
Snowball sampling is best used in the following cases:
- If there is no sampling frame available (e.g., people with a rare disease)
- If the population of interest is hard to access or locate (e.g., people experiencing homelessness)
- If the research focuses on a sensitive topic (e.g., extramarital affairs)
The reproducibility and replicability of a study can be ensured by writing a transparent, detailed method section and using clear, unambiguous language.
Reproducibility and replicability are related terms.
- Reproducing research entails reanalyzing the existing data in the same manner.
- Replicating (or repeating ) the research entails reconducting the entire analysis, including the collection of new data .
- A successful reproduction shows that the data analyses were conducted in a fair and honest manner.
- A successful replication shows that the reliability of the results is high.
Stratified sampling and quota sampling both involve dividing the population into subgroups and selecting units from each subgroup. The purpose in both cases is to select a representative sample and/or to allow comparisons between subgroups.
The main difference is that in stratified sampling, you draw a random sample from each subgroup ( probability sampling ). In quota sampling you select a predetermined number or proportion of units, in a non-random manner ( non-probability sampling ).
Purposive and convenience sampling are both sampling methods that are typically used in qualitative data collection.
A convenience sample is drawn from a source that is conveniently accessible to the researcher. Convenience sampling does not distinguish characteristics among the participants. On the other hand, purposive sampling focuses on selecting participants possessing characteristics associated with the research study.
The findings of studies based on either convenience or purposive sampling can only be generalized to the (sub)population from which the sample is drawn, and not to the entire population.
Random sampling or probability sampling is based on random selection. This means that each unit has an equal chance (i.e., equal probability) of being included in the sample.
On the other hand, convenience sampling involves stopping people at random, which means that not everyone has an equal chance of being selected depending on the place, time, or day you are collecting your data.
Convenience sampling and quota sampling are both non-probability sampling methods. They both use non-random criteria like availability, geographical proximity, or expert knowledge to recruit study participants.
However, in convenience sampling, you continue to sample units or cases until you reach the required sample size.
In quota sampling, you first need to divide your population of interest into subgroups (strata) and estimate their proportions (quota) in the population. Then you can start your data collection, using convenience sampling to recruit participants, until the proportions in each subgroup coincide with the estimated proportions in the population.
A sampling frame is a list of every member in the entire population . It is important that the sampling frame is as complete as possible, so that your sample accurately reflects your population.
Stratified and cluster sampling may look similar, but bear in mind that groups created in cluster sampling are heterogeneous , so the individual characteristics in the cluster vary. In contrast, groups created in stratified sampling are homogeneous , as units share characteristics.
Relatedly, in cluster sampling you randomly select entire groups and include all units of each group in your sample. However, in stratified sampling, you select some units of all groups and include them in your sample. In this way, both methods can ensure that your sample is representative of the target population .
A systematic review is secondary research because it uses existing research. You don’t collect new data yourself.
The key difference between observational studies and experimental designs is that a well-done observational study does not influence the responses of participants, while experiments do have some sort of treatment condition applied to at least some participants by random assignment .
An observational study is a great choice for you if your research question is based purely on observations. If there are ethical, logistical, or practical concerns that prevent you from conducting a traditional experiment , an observational study may be a good choice. In an observational study, there is no interference or manipulation of the research subjects, as well as no control or treatment groups .
It’s often best to ask a variety of people to review your measurements. You can ask experts, such as other researchers, or laypeople, such as potential participants, to judge the face validity of tests.
While experts have a deep understanding of research methods , the people you’re studying can provide you with valuable insights you may have missed otherwise.
Face validity is important because it’s a simple first step to measuring the overall validity of a test or technique. It’s a relatively intuitive, quick, and easy way to start checking whether a new measure seems useful at first glance.
Good face validity means that anyone who reviews your measure says that it seems to be measuring what it’s supposed to. With poor face validity, someone reviewing your measure may be left confused about what you’re measuring and why you’re using this method.
Face validity is about whether a test appears to measure what it’s supposed to measure. This type of validity is concerned with whether a measure seems relevant and appropriate for what it’s assessing only on the surface.
Statistical analyses are often applied to test validity with data from your measures. You test convergent validity and discriminant validity with correlations to see if results from your test are positively or negatively related to those of other established tests.
You can also use regression analyses to assess whether your measure is actually predictive of outcomes that you expect it to predict theoretically. A regression analysis that supports your expectations strengthens your claim of construct validity .
When designing or evaluating a measure, construct validity helps you ensure you’re actually measuring the construct you’re interested in. If you don’t have construct validity, you may inadvertently measure unrelated or distinct constructs and lose precision in your research.
Construct validity is often considered the overarching type of measurement validity , because it covers all of the other types. You need to have face validity , content validity , and criterion validity to achieve construct validity.
Construct validity is about how well a test measures the concept it was designed to evaluate. It’s one of four types of measurement validity , which includes construct validity, face validity , and criterion validity.
There are two subtypes of construct validity.
- Convergent validity : The extent to which your measure corresponds to measures of related constructs
- Discriminant validity : The extent to which your measure is unrelated or negatively related to measures of distinct constructs
Naturalistic observation is a valuable tool because of its flexibility, external validity , and suitability for topics that can’t be studied in a lab setting.
The downsides of naturalistic observation include its lack of scientific control , ethical considerations , and potential for bias from observers and subjects.
Naturalistic observation is a qualitative research method where you record the behaviors of your research subjects in real world settings. You avoid interfering or influencing anything in a naturalistic observation.
You can think of naturalistic observation as “people watching” with a purpose.
A dependent variable is what changes as a result of the independent variable manipulation in experiments . It’s what you’re interested in measuring, and it “depends” on your independent variable.
In statistics, dependent variables are also called:
- Response variables (they respond to a change in another variable)
- Outcome variables (they represent the outcome you want to measure)
- Left-hand-side variables (they appear on the left-hand side of a regression equation)
An independent variable is the variable you manipulate, control, or vary in an experimental study to explore its effects. It’s called “independent” because it’s not influenced by any other variables in the study.
Independent variables are also called:
- Explanatory variables (they explain an event or outcome)
- Predictor variables (they can be used to predict the value of a dependent variable)
- Right-hand-side variables (they appear on the right-hand side of a regression equation).
As a rule of thumb, questions related to thoughts, beliefs, and feelings work well in focus groups. Take your time formulating strong questions, paying special attention to phrasing. Be careful to avoid leading questions , which can bias your responses.
Overall, your focus group questions should be:
- Open-ended and flexible
- Impossible to answer with “yes” or “no” (questions that start with “why” or “how” are often best)
- Unambiguous, getting straight to the point while still stimulating discussion
- Unbiased and neutral
A structured interview is a data collection method that relies on asking questions in a set order to collect data on a topic. They are often quantitative in nature. Structured interviews are best used when:
- You already have a very clear understanding of your topic. Perhaps significant research has already been conducted, or you have done some prior research yourself, but you already possess a baseline for designing strong structured questions.
- You are constrained in terms of time or resources and need to analyze your data quickly and efficiently.
- Your research question depends on strong parity between participants, with environmental conditions held constant.
More flexible interview options include semi-structured interviews , unstructured interviews , and focus groups .
Social desirability bias is the tendency for interview participants to give responses that will be viewed favorably by the interviewer or other participants. It occurs in all types of interviews and surveys , but is most common in semi-structured interviews , unstructured interviews , and focus groups .
Social desirability bias can be mitigated by ensuring participants feel at ease and comfortable sharing their views. Make sure to pay attention to your own body language and any physical or verbal cues, such as nodding or widening your eyes.
This type of bias can also occur in observations if the participants know they’re being observed. They might alter their behavior accordingly.
The interviewer effect is a type of bias that emerges when a characteristic of an interviewer (race, age, gender identity, etc.) influences the responses given by the interviewee.
There is a risk of an interviewer effect in all types of interviews , but it can be mitigated by writing really high-quality interview questions.
A semi-structured interview is a blend of structured and unstructured types of interviews. Semi-structured interviews are best used when:
- You have prior interview experience. Spontaneous questions are deceptively challenging, and it’s easy to accidentally ask a leading question or make a participant uncomfortable.
- Your research question is exploratory in nature. Participant answers can guide future research questions and help you develop a more robust knowledge base for future research.
An unstructured interview is the most flexible type of interview, but it is not always the best fit for your research topic.
Unstructured interviews are best used when:
- You are an experienced interviewer and have a very strong background in your research topic, since it is challenging to ask spontaneous, colloquial questions.
- Your research question is exploratory in nature. While you may have developed hypotheses, you are open to discovering new or shifting viewpoints through the interview process.
- You are seeking descriptive data, and are ready to ask questions that will deepen and contextualize your initial thoughts and hypotheses.
- Your research depends on forming connections with your participants and making them feel comfortable revealing deeper emotions, lived experiences, or thoughts.
The four most common types of interviews are:
- Structured interviews : The questions are predetermined in both topic and order.
- Semi-structured interviews : A few questions are predetermined, but other questions aren’t planned.
- Unstructured interviews : None of the questions are predetermined.
- Focus group interviews : The questions are presented to a group instead of one individual.
Deductive reasoning is commonly used in scientific research, and it’s especially associated with quantitative research .
In research, you might have come across something called the hypothetico-deductive method . It’s the scientific method of testing hypotheses to check whether your predictions are substantiated by real-world data.
Deductive reasoning is a logical approach where you progress from general ideas to specific conclusions. It’s often contrasted with inductive reasoning , where you start with specific observations and form general conclusions.
Deductive reasoning is also called deductive logic.
There are many different types of inductive reasoning that people use formally or informally.
Here are a few common types:
- Inductive generalization : You use observations about a sample to come to a conclusion about the population it came from.
- Statistical generalization: You use specific numbers about samples to make statements about populations.
- Causal reasoning: You make cause-and-effect links between different things.
- Sign reasoning: You make a conclusion about a correlational relationship between different things.
- Analogical reasoning: You make a conclusion about something based on its similarities to something else.
Inductive reasoning is a bottom-up approach, while deductive reasoning is top-down.
Inductive reasoning takes you from the specific to the general, while in deductive reasoning, you make inferences by going from general premises to specific conclusions.
In inductive research , you start by making observations or gathering data. Then, you take a broad scan of your data and search for patterns. Finally, you make general conclusions that you might incorporate into theories.
Inductive reasoning is a method of drawing conclusions by going from the specific to the general. It’s usually contrasted with deductive reasoning, where you proceed from general information to specific conclusions.
Inductive reasoning is also called inductive logic or bottom-up reasoning.
A hypothesis states your predictions about what your research will find. It is a tentative answer to your research question that has not yet been tested. For some research projects, you might have to write several hypotheses that address different aspects of your research question.
A hypothesis is not just a guess — it should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations and statistical analysis of data).
Triangulation can help:
- Reduce research bias that comes from using a single method, theory, or investigator
- Enhance validity by approaching the same topic with different tools
- Establish credibility by giving you a complete picture of the research problem
But triangulation can also pose problems:
- It’s time-consuming and labor-intensive, often involving an interdisciplinary team.
- Your results may be inconsistent or even contradictory.
There are four main types of triangulation :
- Data triangulation : Using data from different times, spaces, and people
- Investigator triangulation : Involving multiple researchers in collecting or analyzing data
- Theory triangulation : Using varying theoretical perspectives in your research
- Methodological triangulation : Using different methodologies to approach the same topic
Many academic fields use peer review , largely to determine whether a manuscript is suitable for publication. Peer review enhances the credibility of the published manuscript.
However, peer review is also common in non-academic settings. The United Nations, the European Union, and many individual nations use peer review to evaluate grant applications. It is also widely used in medical and health-related fields as a teaching or quality-of-care measure.
Peer assessment is often used in the classroom as a pedagogical tool. Both receiving feedback and providing it are thought to enhance the learning process, helping students think critically and collaboratively.
Peer review can stop obviously problematic, falsified, or otherwise untrustworthy research from being published. It also represents an excellent opportunity to get feedback from renowned experts in your field. It acts as a first defense, helping you ensure your argument is clear and that there are no gaps, vague terms, or unanswered questions for readers who weren’t involved in the research process.
Peer-reviewed articles are considered a highly credible source due to this stringent process they go through before publication.
In general, the peer review process follows the following steps:
- First, the author submits the manuscript to the editor.
- Reject the manuscript and send it back to author, or
- Send it onward to the selected peer reviewer(s)
- Next, the peer review process occurs. The reviewer provides feedback, addressing any major or minor issues with the manuscript, and gives their advice regarding what edits should be made.
- Lastly, the edited manuscript is sent back to the author. They input the edits, and resubmit it to the editor for publication.
Exploratory research is often used when the issue you’re studying is new or when the data collection process is challenging for some reason.
You can use exploratory research if you have a general idea or a specific question that you want to study but there is no preexisting knowledge or paradigm with which to study it.
Exploratory research is a methodology approach that explores research questions that have not previously been studied in depth. It is often used when the issue you’re studying is new, or the data collection process is challenging in some way.
Explanatory research is used to investigate how or why a phenomenon occurs. Therefore, this type of research is often one of the first stages in the research process , serving as a jumping-off point for future research.
Exploratory research aims to explore the main aspects of an under-researched problem, while explanatory research aims to explain the causes and consequences of a well-defined problem.
Explanatory research is a research method used to investigate how or why something occurs when only a small amount of information is available pertaining to that topic. It can help you increase your understanding of a given topic.
Clean data are valid, accurate, complete, consistent, unique, and uniform. Dirty data include inconsistencies and errors.
Dirty data can come from any part of the research process, including poor research design , inappropriate measurement materials, or flawed data entry.
Data cleaning takes place between data collection and data analyses. But you can use some methods even before collecting data.
For clean data, you should start by designing measures that collect valid data. Data validation at the time of data entry or collection helps you minimize the amount of data cleaning you’ll need to do.
After data collection, you can use data standardization and data transformation to clean your data. You’ll also deal with any missing values, outliers, and duplicate values.
Every dataset requires different techniques to clean dirty data , but you need to address these issues in a systematic way. You focus on finding and resolving data points that don’t agree or fit with the rest of your dataset.
These data might be missing values, outliers, duplicate values, incorrectly formatted, or irrelevant. You’ll start with screening and diagnosing your data. Then, you’ll often standardize and accept or remove data to make your dataset consistent and valid.
Data cleaning is necessary for valid and appropriate analyses. Dirty data contain inconsistencies or errors , but cleaning your data helps you minimize or resolve these.
Without data cleaning, you could end up with a Type I or II error in your conclusion. These types of erroneous conclusions can be practically significant with important consequences, because they lead to misplaced investments or missed opportunities.
Data cleaning involves spotting and resolving potential data inconsistencies or errors to improve your data quality. An error is any value (e.g., recorded weight) that doesn’t reflect the true value (e.g., actual weight) of something that’s being measured.
In this process, you review, analyze, detect, modify, or remove “dirty” data to make your dataset “clean.” Data cleaning is also called data cleansing or data scrubbing.
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information—for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
In multistage sampling , you can use probability or non-probability sampling methods .
For a probability sample, you have to conduct probability sampling at every stage.
You can mix it up by using simple random sampling , systematic sampling , or stratified sampling to select units at different stages, depending on what is applicable and relevant to your study.
Multistage sampling can simplify data collection when you have large, geographically spread samples, and you can obtain a probability sample without a complete sampling frame.
But multistage sampling may not lead to a representative sample, and larger samples are needed for multistage samples to achieve the statistical properties of simple random samples .
These are four of the most common mixed methods designs :
- Convergent parallel: Quantitative and qualitative data are collected at the same time and analyzed separately. After both analyses are complete, compare your results to draw overall conclusions.
- Embedded: Quantitative and qualitative data are collected at the same time, but within a larger quantitative or qualitative design. One type of data is secondary to the other.
- Explanatory sequential: Quantitative data is collected and analyzed first, followed by qualitative data. You can use this design if you think your qualitative data will explain and contextualize your quantitative findings.
- Exploratory sequential: Qualitative data is collected and analyzed first, followed by quantitative data. You can use this design if you think the quantitative data will confirm or validate your qualitative findings.
Triangulation in research means using multiple datasets, methods, theories and/or investigators to address a research question. It’s a research strategy that can help you enhance the validity and credibility of your findings.
Triangulation is mainly used in qualitative research , but it’s also commonly applied in quantitative research . Mixed methods research always uses triangulation.
In multistage sampling , or multistage cluster sampling, you draw a sample from a population using smaller and smaller groups at each stage.
This method is often used to collect data from a large, geographically spread group of people in national surveys, for example. You take advantage of hierarchical groupings (e.g., from state to city to neighborhood) to create a sample that’s less expensive and time-consuming to collect data from.
No, the steepness or slope of the line isn’t related to the correlation coefficient value. The correlation coefficient only tells you how closely your data fit on a line, so two datasets with the same correlation coefficient can have very different slopes.
To find the slope of the line, you’ll need to perform a regression analysis .
Correlation coefficients always range between -1 and 1.
The sign of the coefficient tells you the direction of the relationship: a positive value means the variables change together in the same direction, while a negative value means they change together in opposite directions.
The absolute value of a number is equal to the number without its sign. The absolute value of a correlation coefficient tells you the magnitude of the correlation: the greater the absolute value, the stronger the correlation.
These are the assumptions your data must meet if you want to use Pearson’s r :
- Both variables are on an interval or ratio level of measurement
- Data from both variables follow normal distributions
- Your data have no outliers
- Your data is from a random or representative sample
- You expect a linear relationship between the two variables
Quantitative research designs can be divided into two main categories:
- Correlational and descriptive designs are used to investigate characteristics, averages, trends, and associations between variables.
- Experimental and quasi-experimental designs are used to test causal relationships .
Qualitative research designs tend to be more flexible. Common types of qualitative design include case study , ethnography , and grounded theory designs.
A well-planned research design helps ensure that your methods match your research aims, that you collect high-quality data, and that you use the right kind of analysis to answer your questions, utilizing credible sources . This allows you to draw valid , trustworthy conclusions.
The priorities of a research design can vary depending on the field, but you usually have to specify:
- Your research questions and/or hypotheses
- Your overall approach (e.g., qualitative or quantitative )
- The type of design you’re using (e.g., a survey , experiment , or case study )
- Your sampling methods or criteria for selecting subjects
- Your data collection methods (e.g., questionnaires , observations)
- Your data collection procedures (e.g., operationalization , timing and data management)
- Your data analysis methods (e.g., statistical tests or thematic analysis )
A research design is a strategy for answering your research question . It defines your overall approach and determines how you will collect and analyze data.
Questionnaires can be self-administered or researcher-administered.
Self-administered questionnaires can be delivered online or in paper-and-pen formats, in person or through mail. All questions are standardized so that all respondents receive the same questions with identical wording.
Researcher-administered questionnaires are interviews that take place by phone, in-person, or online between researchers and respondents. You can gain deeper insights by clarifying questions for respondents or asking follow-up questions.
You can organize the questions logically, with a clear progression from simple to complex, or randomly between respondents. A logical flow helps respondents process the questionnaire easier and quicker, but it may lead to bias. Randomization can minimize the bias from order effects.
Closed-ended, or restricted-choice, questions offer respondents a fixed set of choices to select from. These questions are easier to answer quickly.
Open-ended or long-form questions allow respondents to answer in their own words. Because there are no restrictions on their choices, respondents can answer in ways that researchers may not have otherwise considered.
A questionnaire is a data collection tool or instrument, while a survey is an overarching research method that involves collecting and analyzing data from people using questionnaires.
The third variable and directionality problems are two main reasons why correlation isn’t causation .
The third variable problem means that a confounding variable affects both variables to make them seem causally related when they are not.
The directionality problem is when two variables correlate and might actually have a causal relationship, but it’s impossible to conclude which variable causes changes in the other.
Correlation describes an association between variables : when one variable changes, so does the other. A correlation is a statistical indicator of the relationship between variables.
Causation means that changes in one variable brings about changes in the other (i.e., there is a cause-and-effect relationship between variables). The two variables are correlated with each other, and there’s also a causal link between them.
While causation and correlation can exist simultaneously, correlation does not imply causation. In other words, correlation is simply a relationship where A relates to B—but A doesn’t necessarily cause B to happen (or vice versa). Mistaking correlation for causation is a common error and can lead to false cause fallacy .
Controlled experiments establish causality, whereas correlational studies only show associations between variables.
- In an experimental design , you manipulate an independent variable and measure its effect on a dependent variable. Other variables are controlled so they can’t impact the results.
- In a correlational design , you measure variables without manipulating any of them. You can test whether your variables change together, but you can’t be sure that one variable caused a change in another.
In general, correlational research is high in external validity while experimental research is high in internal validity .
A correlation is usually tested for two variables at a time, but you can test correlations between three or more variables.
A correlation coefficient is a single number that describes the strength and direction of the relationship between your variables.
Different types of correlation coefficients might be appropriate for your data based on their levels of measurement and distributions . The Pearson product-moment correlation coefficient (Pearson’s r ) is commonly used to assess a linear relationship between two quantitative variables.
A correlational research design investigates relationships between two variables (or more) without the researcher controlling or manipulating any of them. It’s a non-experimental type of quantitative research .
A correlation reflects the strength and/or direction of the association between two or more variables.
- A positive correlation means that both variables change in the same direction.
- A negative correlation means that the variables change in opposite directions.
- A zero correlation means there’s no relationship between the variables.
Random error is almost always present in scientific studies, even in highly controlled settings. While you can’t eradicate it completely, you can reduce random error by taking repeated measurements, using a large sample, and controlling extraneous variables .
You can avoid systematic error through careful design of your sampling , data collection , and analysis procedures. For example, use triangulation to measure your variables using multiple methods; regularly calibrate instruments or procedures; use random sampling and random assignment ; and apply masking (blinding) where possible.
Systematic error is generally a bigger problem in research.
With random error, multiple measurements will tend to cluster around the true value. When you’re collecting data from a large sample , the errors in different directions will cancel each other out.
Systematic errors are much more problematic because they can skew your data away from the true value. This can lead you to false conclusions ( Type I and II errors ) about the relationship between the variables you’re studying.
Random and systematic error are two types of measurement error.
Random error is a chance difference between the observed and true values of something (e.g., a researcher misreading a weighing scale records an incorrect measurement).
Systematic error is a consistent or proportional difference between the observed and true values of something (e.g., a miscalibrated scale consistently records weights as higher than they actually are).
On graphs, the explanatory variable is conventionally placed on the x-axis, while the response variable is placed on the y-axis.
- If you have quantitative variables , use a scatterplot or a line graph.
- If your response variable is categorical, use a scatterplot or a line graph.
- If your explanatory variable is categorical, use a bar graph.
The term “ explanatory variable ” is sometimes preferred over “ independent variable ” because, in real world contexts, independent variables are often influenced by other variables. This means they aren’t totally independent.
Multiple independent variables may also be correlated with each other, so “explanatory variables” is a more appropriate term.
The difference between explanatory and response variables is simple:
- An explanatory variable is the expected cause, and it explains the results.
- A response variable is the expected effect, and it responds to other variables.
In a controlled experiment , all extraneous variables are held constant so that they can’t influence the results. Controlled experiments require:
- A control group that receives a standard treatment, a fake treatment, or no treatment.
- Random assignment of participants to ensure the groups are equivalent.
Depending on your study topic, there are various other methods of controlling variables .
There are 4 main types of extraneous variables :
- Demand characteristics : environmental cues that encourage participants to conform to researchers’ expectations.
- Experimenter effects : unintentional actions by researchers that influence study outcomes.
- Situational variables : environmental variables that alter participants’ behaviors.
- Participant variables : any characteristic or aspect of a participant’s background that could affect study results.
An extraneous variable is any variable that you’re not investigating that can potentially affect the dependent variable of your research study.
A confounding variable is a type of extraneous variable that not only affects the dependent variable, but is also related to the independent variable.
In a factorial design, multiple independent variables are tested.
If you test two variables, each level of one independent variable is combined with each level of the other independent variable to create different conditions.
Within-subjects designs have many potential threats to internal validity , but they are also very statistically powerful .
Advantages:
- Only requires small samples
- Statistically powerful
- Removes the effects of individual differences on the outcomes
Disadvantages:
- Internal validity threats reduce the likelihood of establishing a direct relationship between variables
- Time-related effects, such as growth, can influence the outcomes
- Carryover effects mean that the specific order of different treatments affect the outcomes
While a between-subjects design has fewer threats to internal validity , it also requires more participants for high statistical power than a within-subjects design .
- Prevents carryover effects of learning and fatigue.
- Shorter study duration.
- Needs larger samples for high power.
- Uses more resources to recruit participants, administer sessions, cover costs, etc.
- Individual differences may be an alternative explanation for results.
Yes. Between-subjects and within-subjects designs can be combined in a single study when you have two or more independent variables (a factorial design). In a mixed factorial design, one variable is altered between subjects and another is altered within subjects.
In a between-subjects design , every participant experiences only one condition, and researchers assess group differences between participants in various conditions.
In a within-subjects design , each participant experiences all conditions, and researchers test the same participants repeatedly for differences between conditions.
The word “between” means that you’re comparing different conditions between groups, while the word “within” means you’re comparing different conditions within the same group.
Random assignment is used in experiments with a between-groups or independent measures design. In this research design, there’s usually a control group and one or more experimental groups. Random assignment helps ensure that the groups are comparable.
In general, you should always use random assignment in this type of experimental design when it is ethically possible and makes sense for your study topic.
To implement random assignment , assign a unique number to every member of your study’s sample .
Then, you can use a random number generator or a lottery method to randomly assign each number to a control or experimental group. You can also do so manually, by flipping a coin or rolling a dice to randomly assign participants to groups.
Random selection, or random sampling , is a way of selecting members of a population for your study’s sample.
In contrast, random assignment is a way of sorting the sample into control and experimental groups.
Random sampling enhances the external validity or generalizability of your results, while random assignment improves the internal validity of your study.
In experimental research, random assignment is a way of placing participants from your sample into different groups using randomization. With this method, every member of the sample has a known or equal chance of being placed in a control group or an experimental group.
“Controlling for a variable” means measuring extraneous variables and accounting for them statistically to remove their effects on other variables.
Researchers often model control variable data along with independent and dependent variable data in regression analyses and ANCOVAs . That way, you can isolate the control variable’s effects from the relationship between the variables of interest.
Control variables help you establish a correlational or causal relationship between variables by enhancing internal validity .
If you don’t control relevant extraneous variables , they may influence the outcomes of your study, and you may not be able to demonstrate that your results are really an effect of your independent variable .
A control variable is any variable that’s held constant in a research study. It’s not a variable of interest in the study, but it’s controlled because it could influence the outcomes.
Including mediators and moderators in your research helps you go beyond studying a simple relationship between two variables for a fuller picture of the real world. They are important to consider when studying complex correlational or causal relationships.
Mediators are part of the causal pathway of an effect, and they tell you how or why an effect takes place. Moderators usually help you judge the external validity of your study by identifying the limitations of when the relationship between variables holds.
If something is a mediating variable :
- It’s caused by the independent variable .
- It influences the dependent variable
- When it’s taken into account, the statistical correlation between the independent and dependent variables is higher than when it isn’t considered.
A confounder is a third variable that affects variables of interest and makes them seem related when they are not. In contrast, a mediator is the mechanism of a relationship between two variables: it explains the process by which they are related.
A mediator variable explains the process through which two variables are related, while a moderator variable affects the strength and direction of that relationship.
There are three key steps in systematic sampling :
- Define and list your population , ensuring that it is not ordered in a cyclical or periodic order.
- Decide on your sample size and calculate your interval, k , by dividing your population by your target sample size.
- Choose every k th member of the population as your sample.
Systematic sampling is a probability sampling method where researchers select members of the population at a regular interval – for example, by selecting every 15th person on a list of the population. If the population is in a random order, this can imitate the benefits of simple random sampling .
Yes, you can create a stratified sample using multiple characteristics, but you must ensure that every participant in your study belongs to one and only one subgroup. In this case, you multiply the numbers of subgroups for each characteristic to get the total number of groups.
For example, if you were stratifying by location with three subgroups (urban, rural, or suburban) and marital status with five subgroups (single, divorced, widowed, married, or partnered), you would have 3 x 5 = 15 subgroups.
You should use stratified sampling when your sample can be divided into mutually exclusive and exhaustive subgroups that you believe will take on different mean values for the variable that you’re studying.
Using stratified sampling will allow you to obtain more precise (with lower variance ) statistical estimates of whatever you are trying to measure.
For example, say you want to investigate how income differs based on educational attainment, but you know that this relationship can vary based on race. Using stratified sampling, you can ensure you obtain a large enough sample from each racial group, allowing you to draw more precise conclusions.
In stratified sampling , researchers divide subjects into subgroups called strata based on characteristics that they share (e.g., race, gender, educational attainment).
Once divided, each subgroup is randomly sampled using another probability sampling method.
Cluster sampling is more time- and cost-efficient than other probability sampling methods , particularly when it comes to large samples spread across a wide geographical area.
However, it provides less statistical certainty than other methods, such as simple random sampling , because it is difficult to ensure that your clusters properly represent the population as a whole.
There are three types of cluster sampling : single-stage, double-stage and multi-stage clustering. In all three types, you first divide the population into clusters, then randomly select clusters for use in your sample.
- In single-stage sampling , you collect data from every unit within the selected clusters.
- In double-stage sampling , you select a random sample of units from within the clusters.
- In multi-stage sampling , you repeat the procedure of randomly sampling elements from within the clusters until you have reached a manageable sample.
Cluster sampling is a probability sampling method in which you divide a population into clusters, such as districts or schools, and then randomly select some of these clusters as your sample.
The clusters should ideally each be mini-representations of the population as a whole.
If properly implemented, simple random sampling is usually the best sampling method for ensuring both internal and external validity . However, it can sometimes be impractical and expensive to implement, depending on the size of the population to be studied,
If you have a list of every member of the population and the ability to reach whichever members are selected, you can use simple random sampling.
The American Community Survey is an example of simple random sampling . In order to collect detailed data on the population of the US, the Census Bureau officials randomly select 3.5 million households per year and use a variety of methods to convince them to fill out the survey.
Simple random sampling is a type of probability sampling in which the researcher randomly selects a subset of participants from a population . Each member of the population has an equal chance of being selected. Data is then collected from as large a percentage as possible of this random subset.
Quasi-experimental design is most useful in situations where it would be unethical or impractical to run a true experiment .
Quasi-experiments have lower internal validity than true experiments, but they often have higher external validity as they can use real-world interventions instead of artificial laboratory settings.
A quasi-experiment is a type of research design that attempts to establish a cause-and-effect relationship. The main difference with a true experiment is that the groups are not randomly assigned.
Blinding is important to reduce research bias (e.g., observer bias , demand characteristics ) and ensure a study’s internal validity .
If participants know whether they are in a control or treatment group , they may adjust their behavior in ways that affect the outcome that researchers are trying to measure. If the people administering the treatment are aware of group assignment, they may treat participants differently and thus directly or indirectly influence the final results.
- In a single-blind study , only the participants are blinded.
- In a double-blind study , both participants and experimenters are blinded.
- In a triple-blind study , the assignment is hidden not only from participants and experimenters, but also from the researchers analyzing the data.
Blinding means hiding who is assigned to the treatment group and who is assigned to the control group in an experiment .
A true experiment (a.k.a. a controlled experiment) always includes at least one control group that doesn’t receive the experimental treatment.
However, some experiments use a within-subjects design to test treatments without a control group. In these designs, you usually compare one group’s outcomes before and after a treatment (instead of comparing outcomes between different groups).
For strong internal validity , it’s usually best to include a control group if possible. Without a control group, it’s harder to be certain that the outcome was caused by the experimental treatment and not by other variables.
An experimental group, also known as a treatment group, receives the treatment whose effect researchers wish to study, whereas a control group does not. They should be identical in all other ways.
Individual Likert-type questions are generally considered ordinal data , because the items have clear rank order, but don’t have an even distribution.
Overall Likert scale scores are sometimes treated as interval data. These scores are considered to have directionality and even spacing between them.
The type of data determines what statistical tests you should use to analyze your data.
A Likert scale is a rating scale that quantitatively assesses opinions, attitudes, or behaviors. It is made up of 4 or more questions that measure a single attitude or trait when response scores are combined.
To use a Likert scale in a survey , you present participants with Likert-type questions or statements, and a continuum of items, usually with 5 or 7 possible responses, to capture their degree of agreement.
In scientific research, concepts are the abstract ideas or phenomena that are being studied (e.g., educational achievement). Variables are properties or characteristics of the concept (e.g., performance at school), while indicators are ways of measuring or quantifying variables (e.g., yearly grade reports).
The process of turning abstract concepts into measurable variables and indicators is called operationalization .
There are various approaches to qualitative data analysis , but they all share five steps in common:
- Prepare and organize your data.
- Review and explore your data.
- Develop a data coding system.
- Assign codes to the data.
- Identify recurring themes.
The specifics of each step depend on the focus of the analysis. Some common approaches include textual analysis , thematic analysis , and discourse analysis .
There are five common approaches to qualitative research :
- Grounded theory involves collecting data in order to develop new theories.
- Ethnography involves immersing yourself in a group or organization to understand its culture.
- Narrative research involves interpreting stories to understand how people make sense of their experiences and perceptions.
- Phenomenological research involves investigating phenomena through people’s lived experiences.
- Action research links theory and practice in several cycles to drive innovative changes.
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics. It is used by scientists to test specific predictions, called hypotheses , by calculating how likely it is that a pattern or relationship between variables could have arisen by chance.
Operationalization means turning abstract conceptual ideas into measurable observations.
For example, the concept of social anxiety isn’t directly observable, but it can be operationally defined in terms of self-rating scores, behavioral avoidance of crowded places, or physical anxiety symptoms in social situations.
Before collecting data , it’s important to consider how you will operationalize the variables that you want to measure.
When conducting research, collecting original data has significant advantages:
- You can tailor data collection to your specific research aims (e.g. understanding the needs of your consumers or user testing your website)
- You can control and standardize the process for high reliability and validity (e.g. choosing appropriate measurements and sampling methods )
However, there are also some drawbacks: data collection can be time-consuming, labor-intensive and expensive. In some cases, it’s more efficient to use secondary data that has already been collected by someone else, but the data might be less reliable.
Data collection is the systematic process by which observations or measurements are gathered in research. It is used in many different contexts by academics, governments, businesses, and other organizations.
There are several methods you can use to decrease the impact of confounding variables on your research: restriction, matching, statistical control and randomization.
In restriction , you restrict your sample by only including certain subjects that have the same values of potential confounding variables.
In matching , you match each of the subjects in your treatment group with a counterpart in the comparison group. The matched subjects have the same values on any potential confounding variables, and only differ in the independent variable .
In statistical control , you include potential confounders as variables in your regression .
In randomization , you randomly assign the treatment (or independent variable) in your study to a sufficiently large number of subjects, which allows you to control for all potential confounding variables.
A confounding variable is closely related to both the independent and dependent variables in a study. An independent variable represents the supposed cause , while the dependent variable is the supposed effect . A confounding variable is a third variable that influences both the independent and dependent variables.
Failing to account for confounding variables can cause you to wrongly estimate the relationship between your independent and dependent variables.
To ensure the internal validity of your research, you must consider the impact of confounding variables. If you fail to account for them, you might over- or underestimate the causal relationship between your independent and dependent variables , or even find a causal relationship where none exists.
Yes, but including more than one of either type requires multiple research questions .
For example, if you are interested in the effect of a diet on health, you can use multiple measures of health: blood sugar, blood pressure, weight, pulse, and many more. Each of these is its own dependent variable with its own research question.
You could also choose to look at the effect of exercise levels as well as diet, or even the additional effect of the two combined. Each of these is a separate independent variable .
To ensure the internal validity of an experiment , you should only change one independent variable at a time.
No. The value of a dependent variable depends on an independent variable, so a variable cannot be both independent and dependent at the same time. It must be either the cause or the effect, not both!
You want to find out how blood sugar levels are affected by drinking diet soda and regular soda, so you conduct an experiment .
- The type of soda – diet or regular – is the independent variable .
- The level of blood sugar that you measure is the dependent variable – it changes depending on the type of soda.
Determining cause and effect is one of the most important parts of scientific research. It’s essential to know which is the cause – the independent variable – and which is the effect – the dependent variable.
In non-probability sampling , the sample is selected based on non-random criteria, and not every member of the population has a chance of being included.
Common non-probability sampling methods include convenience sampling , voluntary response sampling, purposive sampling , snowball sampling, and quota sampling .
Probability sampling means that every member of the target population has a known chance of being included in the sample.
Probability sampling methods include simple random sampling , systematic sampling , stratified sampling , and cluster sampling .
Using careful research design and sampling procedures can help you avoid sampling bias . Oversampling can be used to correct undercoverage bias .
Some common types of sampling bias include self-selection bias , nonresponse bias , undercoverage bias , survivorship bias , pre-screening or advertising bias, and healthy user bias.
Sampling bias is a threat to external validity – it limits the generalizability of your findings to a broader group of people.
A sampling error is the difference between a population parameter and a sample statistic .
A statistic refers to measures about the sample , while a parameter refers to measures about the population .
Populations are used when a research question requires data from every member of the population. This is usually only feasible when the population is small and easily accessible.
Samples are used to make inferences about populations . Samples are easier to collect data from because they are practical, cost-effective, convenient, and manageable.
There are seven threats to external validity : selection bias , history, experimenter effect, Hawthorne effect , testing effect, aptitude-treatment and situation effect.
The two types of external validity are population validity (whether you can generalize to other groups of people) and ecological validity (whether you can generalize to other situations and settings).
The external validity of a study is the extent to which you can generalize your findings to different groups of people, situations, and measures.
Cross-sectional studies cannot establish a cause-and-effect relationship or analyze behavior over a period of time. To investigate cause and effect, you need to do a longitudinal study or an experimental study .
Cross-sectional studies are less expensive and time-consuming than many other types of study. They can provide useful insights into a population’s characteristics and identify correlations for further research.
Sometimes only cross-sectional data is available for analysis; other times your research question may only require a cross-sectional study to answer it.
Longitudinal studies can last anywhere from weeks to decades, although they tend to be at least a year long.
The 1970 British Cohort Study , which has collected data on the lives of 17,000 Brits since their births in 1970, is one well-known example of a longitudinal study .
Longitudinal studies are better to establish the correct sequence of events, identify changes over time, and provide insight into cause-and-effect relationships, but they also tend to be more expensive and time-consuming than other types of studies.
Longitudinal studies and cross-sectional studies are two different types of research design . In a cross-sectional study you collect data from a population at a specific point in time; in a longitudinal study you repeatedly collect data from the same sample over an extended period of time.
| Longitudinal study | Cross-sectional study |
|---|---|
| observations | Observations at a in time |
| Observes the multiple times | Observes (a “cross-section”) in the population |
| Follows in participants over time | Provides of society at a given point |
There are eight threats to internal validity : history, maturation, instrumentation, testing, selection bias , regression to the mean, social interaction and attrition .
Internal validity is the extent to which you can be confident that a cause-and-effect relationship established in a study cannot be explained by other factors.
In mixed methods research , you use both qualitative and quantitative data collection and analysis methods to answer your research question .
The research methods you use depend on the type of data you need to answer your research question .
- If you want to measure something or test a hypothesis , use quantitative methods . If you want to explore ideas, thoughts and meanings, use qualitative methods .
- If you want to analyze a large amount of readily-available data, use secondary data. If you want data specific to your purposes with control over how it is generated, collect primary data.
- If you want to establish cause-and-effect relationships between variables , use experimental methods. If you want to understand the characteristics of a research subject, use descriptive methods.
A confounding variable , also called a confounder or confounding factor, is a third variable in a study examining a potential cause-and-effect relationship.
A confounding variable is related to both the supposed cause and the supposed effect of the study. It can be difficult to separate the true effect of the independent variable from the effect of the confounding variable.
In your research design , it’s important to identify potential confounding variables and plan how you will reduce their impact.
Discrete and continuous variables are two types of quantitative variables :
- Discrete variables represent counts (e.g. the number of objects in a collection).
- Continuous variables represent measurable amounts (e.g. water volume or weight).
Quantitative variables are any variables where the data represent amounts (e.g. height, weight, or age).
Categorical variables are any variables where the data represent groups. This includes rankings (e.g. finishing places in a race), classifications (e.g. brands of cereal), and binary outcomes (e.g. coin flips).
You need to know what type of variables you are working with to choose the right statistical test for your data and interpret your results .
You can think of independent and dependent variables in terms of cause and effect: an independent variable is the variable you think is the cause , while a dependent variable is the effect .
In an experiment, you manipulate the independent variable and measure the outcome in the dependent variable. For example, in an experiment about the effect of nutrients on crop growth:
- The independent variable is the amount of nutrients added to the crop field.
- The dependent variable is the biomass of the crops at harvest time.
Defining your variables, and deciding how you will manipulate and measure them, is an important part of experimental design .
Experimental design means planning a set of procedures to investigate a relationship between variables . To design a controlled experiment, you need:
- A testable hypothesis
- At least one independent variable that can be precisely manipulated
- At least one dependent variable that can be precisely measured
When designing the experiment, you decide:
- How you will manipulate the variable(s)
- How you will control for any potential confounding variables
- How many subjects or samples will be included in the study
- How subjects will be assigned to treatment levels
Experimental design is essential to the internal and external validity of your experiment.
I nternal validity is the degree of confidence that the causal relationship you are testing is not influenced by other factors or variables .
External validity is the extent to which your results can be generalized to other contexts.
The validity of your experiment depends on your experimental design .
Reliability and validity are both about how well a method measures something:
- Reliability refers to the consistency of a measure (whether the results can be reproduced under the same conditions).
- Validity refers to the accuracy of a measure (whether the results really do represent what they are supposed to measure).
If you are doing experimental research, you also have to consider the internal and external validity of your experiment.
A sample is a subset of individuals from a larger population . Sampling means selecting the group that you will actually collect data from in your research. For example, if you are researching the opinions of students in your university, you could survey a sample of 100 students.
In statistics, sampling allows you to test a hypothesis about the characteristics of a population.
Quantitative research deals with numbers and statistics, while qualitative research deals with words and meanings.
Quantitative methods allow you to systematically measure variables and test hypotheses . Qualitative methods allow you to explore concepts and experiences in more detail.
Methodology refers to the overarching strategy and rationale of your research project . It involves studying the methods used in your field and the theories or principles behind them, in order to develop an approach that matches your objectives.
Methods are the specific tools and procedures you use to collect and analyze data (for example, experiments, surveys , and statistical tests ).
In shorter scientific papers, where the aim is to report the findings of a specific study, you might simply describe what you did in a methods section .
In a longer or more complex research project, such as a thesis or dissertation , you will probably include a methodology section , where you explain your approach to answering the research questions and cite relevant sources to support your choice of methods.
Ask our team
Want to contact us directly? No problem. We are always here for you.
- Email [email protected]
- Start live chat
- Call +1 (510) 822-8066
- WhatsApp +31 20 261 6040

Our team helps students graduate by offering:
- A world-class citation generator
- Plagiarism Checker software powered by Turnitin
- Innovative Citation Checker software
- Professional proofreading services
- Over 300 helpful articles about academic writing, citing sources, plagiarism, and more
Scribbr specializes in editing study-related documents . We proofread:
- PhD dissertations
- Research proposals
- Personal statements
- Admission essays
- Motivation letters
- Reflection papers
- Journal articles
- Capstone projects
Scribbr’s Plagiarism Checker is powered by elements of Turnitin’s Similarity Checker , namely the plagiarism detection software and the Internet Archive and Premium Scholarly Publications content databases .
The add-on AI detector is powered by Scribbr’s proprietary software.
The Scribbr Citation Generator is developed using the open-source Citation Style Language (CSL) project and Frank Bennett’s citeproc-js . It’s the same technology used by dozens of other popular citation tools, including Mendeley and Zotero.
You can find all the citation styles and locales used in the Scribbr Citation Generator in our publicly accessible repository on Github .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Understanding Ethical Issues of Research Participation from the Perspective of Participating Children and Adolescents: A Systematic Review
Stacey crane.
Predoctoral Fellow, Indiana University School of Nursing, 1111 Middle Dr., NU345, Indianapolis, IN, 46202, 513-508-3936
Marion E. Broome
Ruby F. Wilson Distinguished Professor and Dean, Vice-Chancellor for Nursing Affairs, Duke University School of Nursing, 307 Trent Drive, Durham, NC, 27710, 919-684-9444
Associated Data
TABLE S2. Articles Included in Sample: Methodologies Used
TABLE S3. Articles Included in Sample: Demographics of Participating Children and Adolescents
The past twenty years have seen distinct shifts in the way the participation of children and adolescents in research is viewed. This has been emphasized by the growing pediatric research enterprise. Additional information on children’s and adolescents’ experiences during research participation is needed to better inform researchers on the ethical conduct of research with this vulnerable population.
The objective of this analysis was to examine ethical issues in research with children and adolescents from their perspective as participants, including: assent, parental consent, risk perception, impact of research participation, and incentives.
This systematic review was conducted per the Long et al. framework by means of an iterative searching process. Using the key words ‘research ethics’ and ‘child or pediatric or adolescent’, PubMed, CINAHL, and EBSCOhost databases were searched to identify articles. Limitations placed on the original searches were: English language, year of publication between 2003–2014, humans, abstract available, and age birth–18 years.
Twenty-three empiric studies were identified and formed the sample. Included studies represented a diverse range of areas of research, methods, settings, sample demographics, authors, and journals.
Even young children demonstrated the ability to understand essential elements of research, although there is variability in children’s level of understanding. Trust was a significant contributing factor to children’s and adolescents’ participation in research, and also shaped their assessments of risk. Research participation was mainly beneficial for children and adolescents. Incentives were mainly viewed positively, although concerns of possible undue influence were expressed.
Linking Evidence to Action
This systematic review highlights the importance of including the perspectives of children and adolescents and provides researchers and nurse clinicians with best practices for involving children in research.
Introduction
In 2004, the Institute of Medicine published a report, ‘Ethical Conduct of Clinical Research Involving Children’, the purpose of which was to review federal regulations, reports and research and make recommendations about ethical research involving children ( Institute of Medicine, 2004 ). Themes of the report included the need for:
- Well-designed and executed research with children to improve the health of children and future generations worldwide;
- Children to not be either burdened or excluded from participation in research;
- A robust system for protecting child and adolescent research participants, including additional resources like experts in physiology and development, to recognize and address unique ethical issues.
In the ensuing years, researchers have continued to recognize the need to balance the inherent vulnerability of children and adolescents with the necessity to research their unique needs and perspectives ( Broome, Kodish, Geller, & Siminoff, 2003 ; Hurst, 2008 ; Levine et al., 2004 ; Solomon, 2013 ). There have been new research investigations with child and adolescent participants, outside of traditional clinical research settings and those using more novel designs. More child/adolescent populations have been identified as vulnerable within the research context; indeed the current prevalence of vulnerable populations has compromised our understanding of the unique needs of any particular vulnerable population ( Levine et al., 2004 ). As the research enterprise continues to evolve it is important to better inform researchers about the unique needs that should inform the ethical conduct of research with children and adolescents.
Much of our understanding of the conduct of ethical research with children and adolescents has been formulated based on general ethical principles, without consideration of the heterogeneity of children and adolescents research participants ( Carter, 2009 ; Hurst, 2008 ; Levine et al., 2004 ). It is unclear what, if any, voice children and adolescents have had in the development of these ethical guidelines. The objective of this systematic review was to examine ethical issues surrounding research with children and adolescents from their perspective as participants. Specific questions that guided this review were:
- What research methods have been used to understand children’s and adolescents’ experiences of participating in research?
- What has been learned from children and adolescents about assent and parental consent for research participation?
- How do children and adolescents perceive the risks inherent with research participation?
- What impact have children and adolescents identified as a result of their research participation?
- What has been learned from child and adolescent research participants regarding the use of incentives?
This systematic review was conducted using the framework described by Long, Godfrey, Randall, Brettle, and Grant (2002) . An iterative searching process was used including three stages: scoping, refinement and confirmation ( Long et al., 2002 ). After a broad search and relevance check on initially identified studies, inclusion and exclusion criteria were refined and questions refocused. Searches were then rerun. References cited in included studies were reviewed to identify further relevant studies. A PRISMA flowchart was used to graphically represent search procedures ( Moher, Liberati, Tetzlaff, & Altman, 2009 ).
Search Methods
Using the key words ‘research ethics’ and ‘child or pediatric or adolescent’, PubMed, CINAHL, and EBSCOhost (including Academic Search Premier, Health Source, PsycINFO, SocINDEX, Family & Society Studies Worldwide, MasterFILE Premier, Biomedical Reference Collection, Applied Science & Technology Source, Historical Abstracts) databases were searched to identify potentially relevant published articles. Limitations placed on the original searches were: English language, year of publication between 2003 and 2014, humans, abstract available, and age birth – 18 years. Due to the high volume of articles initially retrieved (PubMed = 2,240 and EBSCOhost = 3,313) additional limitations were placed on searches. Explicitly, in PubMed a limitation was added for the MeSH search term ‘Ethics, research’ and in EBSCOhost a limitation was placed using ‘Research ethics’ as a major concept. Using this process, these searches yielded a combined total of 1,424 potentially relevant published articles (including 56 duplicates).
Search Outcomes
Articles were then reviewed to select those that met criteria for inclusion. Inclusion criteria included: 1) empiric research studies from the child/adolescent perspective; 2) articles considering ethical issues in research with children/adolescents or child/adolescent perspectives of research participation; and 3) ethical issues as the primary focus of the paper. Exclusion criteria included articles that focused solely on: 1) broad medical ethics or bioethics, or 2) research procedures or regulatory approval processes. From the original search 19 articles met these criteria. Manual searching yielded four further articles meeting the criteria for inclusion. In the end, 23 articles provided the sample for this analysis. Table 1 lists the included articles. Figure 1 illustrates the search results and screening procedures.

PRISMA Diagram of Search Results and Screening
Articles Included in Sample
| Article | Purpose/Aims |
|---|---|
| ( ) | To evaluate factors that would influence children and adolescents’ decision-making for research participation, in terms of the impact of monetary and other incentives. |
| ( ) | To describe the ethical challenges and acceptability of the cold pressor test from the perspective of researchers, children and parents. |
| ( ) | To determine adolescents’ perceptions of participation in research involving the collection of biomarkers via blood, saliva and/or urine samples. |
| ( ) | To examine the capacity of 4th, 7th, and 10th graders, as well as college students, to understand their rights in research and the extent to which this capacity can be enhanced following exposure to The Research Participants’ Bill of Rights. |
| ( ) | To maximize the amount of information children and adolescents understand about the risks and benefits associated with participation in a biomedical research study. |
| ( ) | To compare the effects of research participation on children who have experienced traumatic events with children who have not, in their perception of the risks and benefits of research participation and their understanding of assenting to participate. |
| ( ) | To explore the factors that influenced adolescents’ decisions to participate in an ED-based research study about youth violence, and to determine the feelings elicited by being a research subject. |
| ( ) | To evaluate children’s perceptions of completing a research survey about their exposure to violence. |
| ( ) | To describe the experiences and perspectives of homeless young people as participants in research, including their perspectives and advice on how to handle ethical challenges posed by such research. |
| ( ) | To define an appropriate process for providing research results to participants in pediatric oncology clinical trials, based on participants’ needs and attitudes. |
| ( ) | To empirically examine generational and ethnic variations about ethical issues in youth drug use and suicide survey research in order to: a) evaluate risks and benefits, b) establish guardian permission requirements, c) develop disclosure and confidentiality policies, and d) identify appropriate incentives for recruitment. |
| ( ) | To examine distress related to answering personal survey questions about drug use, suicidal behavior, and physical and sexual abuse in multiple convenience samples of adolescents. |
| ( ) | To investigate children’s and teachers’ perceptions of emotional responses to sociometric testing, and whether children understood their research rights as participants. Also to measure both quantitative and qualitative aspects of the sociometric experience. |
| ( ) | To determine older adolescents’ responses after learning that they were participants in a research study that involved identification of participants using Facebook. |
| ( ) | To explore parent and children’s views of anonymity and the intrinsic link to the ethic of confidentiality with the objective of questioning the taken- for-granted nature of the ethic of anonymity. |
| ( ) | To increase our understanding of how diabetic and at-risk adolescents (i.e., those who are obese and/or have a family history of diabetes) and their parents perceive risks and make decisions about research participation. |
| ( ) | To explore what views children 10–12 years of age express about medical research and participation in such research. |
| ( ) | To explore 10- to 13-year-old children’s views on medical research, trust, information, decision making, and their views on data sampling and risk identification. |
| ( ) | To examine factors influencing informed assent, initial involvement, and ongoing involvement in HIV- focused community based participatory research for African American children. |
| ( ) | To assess what children aged 7 to 18 with cancer understand about research, their research-related treatment, and their preferences for inclusion in decision-making. |
| ( ) | To examine the extent to which parents and adolescents participating in the Treatment for Adolescents With Depression Study understood key aspects of the study. |
| ( ) | To prospectively assess youths’ and their parents’ attitudes and experiences about participation in clinical treatment research. |
| ( ) | To detail how parents as well as children view and assess the risks to involving children in health research. This paper focuses on one of the factors, a matter of trust, that shaped Canadian parents’ and children’s perceptions and assessments of risk in child health research. |
Quality Appraisal
All articles in the sample were evaluated using an instrument to assess overall quality; the Evaluative Tool for Quantitative Research Studies ( Long et al., 2002 ) or the Critical Review Form for Qualitative Research ( Letts et al., 2007 ). Mixed methods studies were assessed using both instruments. These tools were used to ensure the overall quality of studies within the sample, to summarize study findings, and as a method for ensuring inter-rater agreement between the two authors. Both authors independently completed assessments of 14 articles in the sample, three articles at a time, comparing results until substantive agreement was achieved. The nine remaining articles were then assessed by the first author, with the second author performing a secondary confirmation. All articles included in the sample met a minimum of 80% of the instruments’ criteria. The potential for bias in the quality appraisal was minimal as both authors are trained in research ethics and experienced in conducting research with pediatric and adolescent participants.
Data Abstraction and Synthesis
Data abstracted from the articles included: journal, country of publication, purpose, approach, method, data collection techniques, research context, sample size, sample characteristics (ages, health, and research experience), method of child assent and parent consent, and findings.
For further details of the articles in the sample, including their findings, journal and country of publication, research methodologies used, and demographics of the children and adolescents studied in each article, see Tables S1 through S3 online.
Characteristics of Reviewed Studies
The sample reflected a variety of different methods and settings. Of the 23 articles, 11 used a descriptive, quantitative design, eight used a descriptive qualitative design, two used mixed methods, and two used a quantitative design that involved testing an intervention. Eleven of the articles involved retrospective reflection on a research experience and one involved a prospective, longitudinal design. The remaining 11 articles considered participants’ current views on research issues. In terms of setting, 10 of the articles involved studies conducted at or through a hospital, nine at schools, three were community based, and one did not indicate the study site.
Children and adolescents were asked to either reflect on their personal experience as a research participant, on research in general, or on a specific hypothetical research situation. Hypothetical scenarios reflected a variety of medical treatments, clinical trials, diagnostic procedures, collection of laboratory samples and/or descriptive research studies.. In other studies children were asked to reflect on their experiences as part of a study they just participated in including those studying exposure to violence, depression, oncology, pain, drug testing, sociometric testing, and health screening. In most cases these reflections were obtained at the end of their participation in the study. In five of the studies children were asked to reflect on their overall impressions of research participation.
Methods for data collection varied. Four of the studies involved either semi-structured or unstructured individual interviews, one involved a focus group, and two studies used a combination of focus groups and interviews. Eight of the studies used written instruments completed at home, school, hospital, or juvenile justice program settings. Seven studies involved structured quantitative instruments that were completed in individual interviews. One study involved a structured quantitative survey conducted orally in a classroom.
Obtaining Assent and Parental Consent
Per the Declaration of Helsinki, “when a potential research subject who is deemed incapable of giving informed consent is able to give assent to decisions about participation in research, the [researcher] must seek that assent in addition to the consent of the legally authorized representative” ( World Medical Association, 2013 ). When conducting research with children and adolescents, when and how to obtain assent versus informed consent (for adolescents) and parental consent has been ethically debated ( Giesbertz, Bredenoord, & van Delden, 2014 ; Lambert & Glacken, 2011 ). In general, literature and policies support researchers seeking assent from all child and adolescent participants in addition to parental consent. In this sample of 23 articles, seven indicated assent was obtained from the participating children/adolescents although the method was not discussed, seven obtained assent using a written form, and three obtained assent verbally. Two articles took the completion of written survey instruments as an indication of implied consent, and the remaining seven articles did not discuss assent procedures.
The majority of child and adolescent research participants were able to comprehend the purpose and nature of research, research risks and benefits, and the voluntary nature of research participation. Comprehension increased with grade level, with 15 – 16 year olds having a similar understanding of research as adults ( Bruzzese & Fisher, 2003 ; Unguru, Sill, & Kamani, 2010 ). However, while even young children could understand complex concepts like research risks and benefits, there remained a significant minority of children under around the age of 10 who had difficulties understanding research concepts ( Bruzzese & Fisher, 2003 ; Burke, Abramovitch, & Zlotkin, 2005 ). In addition, cultural factors, including race, influenced children’s interpretation of research information ( Traube, Cederbaum, Kerkorian, Bhupali, & McKay, 2013 ; Unguru et al., 2010 ).
Noteworthy challenges to children’s comprehension, including their inability to specify any risks associated with previously collected data or samples, were found in a multi-year longitudinal study. This highlights the need to view assent as an ongoing process to be reaffirmed in studies with research activities at more than one time point ( Swartling, Hansson, Ludvigsson, & Nordgren, 2011 ). In addition, challenges were found in children’s ability to understand clinical trials despite researchers’ explanations; with children’s expectations for clinical improvement as a result of clinical trial participation sometimes being unrealistically high ( Unguru et al., 2010 ; Wagner, Martinez, & Joiner, 2006 ).
Most children preferred a shared decision-making model when deciding to participate in research, where they were actively involved and supported by parents, doctors, and researchers ( Swartling, Helgesson, Ludvigsson, Hansson, & Nordgren, 2014 ; Unguru et al., 2010 ). One challenging situation, however, was indicated by children with cancer who did not feel free to dissent to clinical trial enrollment ( Unguru et al., 2010 ). In another, homeless adolescents felt strongly that they should be able to independently consent to participate in research, without requiring parental approval ( Ensign, 2006 ).
An important consideration is the type of parental consent processes that should be used in research with children and adolescents. Three studies specified that parental consent was obtained but did not indicate a method, eight of the articles obtained consent from parents using a written form, three obtained verbal parental consent, and three articles did not discuss parental consent processes. Other processes used included passive parental consent (1), implied parental consent (1), and not obtaining any parental consent (2). Of the two articles that did not obtain any parental consent one was conducted with homeless adolescents, and the other involved a survey completed at school ( Ellonen & Pösö, 2011 ; Ensign, 2006 ). For this latter study, the children aged 12–16 years decided whether they wished to participate in the study and parents were notified afterwards regarding their child’s participation. Participation rates were lower when active parental consent processes were used in school-based research ( Langhinrichsen-Rohling, Arata, O’Brien, Bowers, & Klibert, 2006 ). One article demonstrated that an active vs. passive parental consent procedure did not reduce the risk of adolescents completing a survey of high-risk behavior feeling upset ( Langhinrichsen-Rohling et al., 2006 ). However, other findings suggested that whether parents were present during the assent discussion impacted whether adolescents perceived their decision to participate in research to be autonomous ( Cohn, Ginsburg, Kassam-Adams, & Fein, 2005 ).
Specific suggestions from children and adolescents on improving assent processes included: for researchers to speak directly to children about research participation – not simply through their parents, ensuring written materials are written in a way that is appealing and understandable, and providing written information instead of using e-mails or websites ( Brawner, Volpe, Stewart, & Gomes, 2013 ; Burke et al., 2005 ; Swartling et al., 2011 ; Swartling et al., 2014 ; Unguru et al., 2010 ). Specific tools that were demonstrated to enhance children’s and adolescents’ comprehension of research included an assent quiz, and a specific lesson on research rights ( Bruzzese & Fisher, 2003 ; Chu, DePrince, & Weinzierl, 2008 ).
Perception of Research Risks
The presence or absence of trust was perceived by children as a contributing factor to being involved in research, and also shaped their assessments of risk ( Brawner et al., 2013 ; Traube et al., 2013 ; Woodgate & Edwards, 2010 ). Findings in the sample of articles suggested that children and adolescents are most willing to participate in research when they feel safe ( Brawner et al., 2013 ; O’Reilly, Karim, Taylor, & Dogra, 2012 ; Swartling et al., 2011 ; Traube et al., 2013 ). Most children and adolescents expected that researchers and their parents would protect them during their research participation ( Brawner et al., 2013 ; Woodgate & Edwards, 2010 ). Children tended to believe that if a researcher behaved unethically or caused harm, the researcher would suffer consequences professionally and personally ( Traube et al., 2013 ). When asked in one study to identify factors that shaped their perceptions and assessments of risks in research children identified: the potential for harm to the child; the potential for good for the child and children in general; the burden to the child and family; and the trust experienced by the child and their parents ( Woodgate & Edwards, 2010 ).
However, weighing of risks and benefits may be age dependent in children, with young children more likely to choose options they are familiar with, and older children paying more attention to the advantages and disadvantages of each option ( Burke et al., 2005 ). In addition, Traube et al. (2013) found that both race and relationship of the researchers to participants impacted children’s trust of researchers, with African-American children having more trust in researchers who weren’t from their own community, and the most trust in Caucasian researchers who had no connection to their community.
There was also evidence that research participants interpreted and used risk information subjectively, based on their personal experiences, and may overlook risk probability information ( Reynolds & Nelson, 2007 ). One article demonstrated that adolescents and adults evaluate risks using a similar process where first the magnitude of the risks are considered, followed by consideration of the probability of the risks ( Reynolds & Nelson, 2007 ). If the magnitude was acceptable, adolescents were willing to tolerate the stated or perceived risks of a research procedure, regardless of probability.
Adolescents were very concerned regarding how their information and samples would be used, and in particular whether information would be shared with their parents ( Brawner et al., 2013 ). Adolescents and parents were found to have different opinions about research disclosures; parents often wanted to receive their children’s research information, but adolescents reported wanting to withhold private and sensitive findings ( Brawner et al., 2013 ). Specific suggestions adolescents made to researchers included allowing their parents and/or friends to attend data collection visits, being able to participate in research along with their friends, and in research where blood, urine, or other biological samples are taken to explicitly inform the adolescent whether or whether not pregnancy, sexually transmitted disease, or drug testing would be performed ( Brawner et al., 2013 ).
Impact of Research Participation Experiences
Twelve studies examined children’s feelings about their own research participation. When asked how the participation affected them, overall the majority of ratings and reports were positive. Specific benefits reported by children/adolescents included: 1) learned something new, 2) helped others, 3) helped other people learn something new, 4) felt ‘empowered’, 5) liked talking about themselves to someone else, 6) enjoyed the procedures, 7) thought filling out forms was ‘fun’, 8) trusted in researchers, 9) thought they experienced ‘clinical improvement’, and 10) would be willing to participate in another study ( Bruzzese & Fisher, 2003 ; Chu et al., 2008 ; Ensign, 2006 ; Fernandez et al., 2009 ; Reynolds & Nelson, 2007 ; Swartling et al., 2014 ; Wagner et al., 2006 ).
In the studies asking about positive aspects of participation there were always some children who did not report positive experiences. The percentages in those studies who reported negative experiences ranged from 4% – 6.1% ( Cohn et al., 2005 ; Ellonen & Pösö, 2011 ). Negative reports associated with research participation included: anxiety, feeling upset, being bored, worry about being identified as high risk for disease, and inconvenient or painful (i.e. blood draws) procedures ( Bruzzese & Fisher, 2003 ; Wagner et al., 2006 ). In some cases, those who rated aspects of research experiences more negatively also rated other aspects positively, indicating an overall positive cost-benefit ratio of research participation ( Cohn et al., 2005 ). Interestingly, of the few studies that examined factors associated with negative or positive appraisals of research participation, only one found that a demographic variable, namely the child’s level of emotional problems, was associated with their appraisals (i.e. there was a positive association between emotional problems and negative feelings towards research participation) ( Cohn et al., 2005 ; Ellonen & Pösö, 2011 ; Swartling et al., 2011 ). No other demographic variables –including age, gender, data collection strategy, type of precipitating event that lead to inclusion in the study (i.e. violence), or anxiety - were reported as significantly associated with children’s appraisals.
Use of Incentives
In seven of the articles a cash incentive of between $10 and $50 was provided to participants. Other incentives used included: small tokens or prizes, $10 phone cards, movie gift certificates, psychology course credits, and a raffle for a gift certificate). Three articles did not provide incentive to participants. Twelve articles did not discuss whether participants were provided with incentives. One article where studies were conducted at schools provided the school with $1 per participant recruited to the study.
There was an interesting range of opinions expressed by children and adolescents about the usefulness of incentives. In five of the studies, at least some of the participants thought cash incentives were a preferred form of incentive ( Brawner et al., 2013 ; Bruzzese & Fisher, 2003 ; Ensign, 2006 ; Fernandez et al., 2009 ; Langhinrichsen-Rohling et al., 2006 ). Rationale for this included: justice, pleasure associated with receiving cash, and compensation for time spent, discomfort experienced, and effort expended. In a few studies the issue of ‘how much is too much’, in terms of cash incentives as a coercive factor, was explored with children and adolescents. In one study, some adolescents voiced concerns that disproportionately large amounts of cash could be coercive for homeless youth ( Ensign, 2006 ). In another, ethnic minority children were concerned that financial incentives could potentially undermine altruistic motivations, or even tempt youth into providing false information ( Mayeux, Underwood, & Risser, 2007 ). In another study, adolescents felt that cash was not coercive for older children who had a better understanding of the role of incentives ( Vitiello et al., 2007 ).
Although parental consent for participation remains the first step in involving children and adolescents in most research, researchers and human research ethics committees are now taking assent to participate in children aged 7–12 and consent from adolescents aged 13–18 far more seriously. This systematic review confirms that obtaining children’s and adolescents’ assent to participate in research is valid and important, as even young children have demonstrated the ability to understand the essential elements of research ( Burke et al., 2005 ; Unguru et al., 2010 ). Some of the variability in levels of understanding reported in studies with children likely reflects how assent forms were written, as opposed to developmental differences ( Burke et al., 2005 ). The challenge for researchers is to find better ways to get information across to children. This problem, while relevant for all research participants, is especially pertinent in younger children who have less life experience and are challenged with a less developed ability to understand new experiences ( Brawner et al., 2013 ; Swartling et al., 2014 ). Assent processes and instruments need to be created with the assistance of child development specialists and piloted with children before being used in a research study ( Burke et al., 2005 ). Researchers should also consider using quizzes to assess children’s understanding of assent information. In addition to setting an empirical standard for assessing understanding, assent quizzes also provide additional opportunities to interact with children about assent information ( Chu et al., 2008 ).
The decision-making model used in research consent and assent processes needs to reflect the context. With the adolescent population, in particular, there is a need to balance the desire for privacy and autonomy with inclusion of parents ( Cohn et al., 2005 ; Fisher, 2003 ). Both adolescents and parents are sympathetic to the ethical dilemmas researchers face when conducting sensitive research with adolescents ( Fisher, 2003 ). A priori consultation with representative adolescents and parents can provide guidance for the selection of consent and assent procedures within challenging contexts ( Fisher, 2003 ). While this type of consultation may seem burdensome for researchers, it demonstrates that researchers respect local norms regarding parental decision-making and do not wish to inadvertently undermine the parent–child relationship ( Fisher, 2003 ).
A key gap in the findings of this review is consideration of whether children and adolescents, beyond simply understanding their research rights, are capable of applying this knowledge and of actually exerting their research rights ( Bruzzese & Fisher, 2003 ; Unguru et al., 2010 ). For example, is a child truly capable of asserting and following through on their desire to stop participating in a research study or dissenting to participate?
Findings of this systematic review demonstrate that children and adolescents have a realistic perception of research risks, and that trust - towards both parents and researchers - is essential in establishing a safe environment for children and adolescents to participate in research. As a result, researchers must establish a mutual respect with child and adolescent participants; if their trust is eroded this could have implications for both the researcher-child and the parent-child relationship ( Woodgate & Edwards, 2010 ). In addition, when performing sensitive research with adolescents, researchers should consider obtaining a federal-wide assurance to protect all data collected for use solely in research, and in particular to prevent data from being used in legal proceedings ( Langhinrichsen-Rohling et al., 2006 ).
Researchers need to appreciate that the assessment of risk is an ongoing process throughout a research study, beyond simply explanations provided when obtaining assent, and that ethnicity and context play important roles in children’s and adolescents’ perceptions of risk. In a study of African-American children, it was found that children were more likely to trust researchers from outside their neighborhood, in particular Caucasian researchers, over and above researchers they knew from their own community ( Traube et al., 2013 ). Children in this study seemed to be fearful that a researcher from their own neighborhood might tell their parents what they shared ( Traube et al., 2013 ). This is particularly interesting as the same sample of children reported that during consent processes they believed the veracity of information provided from African-American researchers over that of Caucasian researchers ( Traube et al., 2013 ).
Impact of Research Participation
Findings from this review provide evidence that research participation can be beneficial for children and adolescents. The overwhelming majority of participants confirmed a willingness to participate in research again. Children and adolescents also identified reasons for participation in research as including altruistic motivations to help others and their own learning.
This review confirmed that most children prefer to be involved in the decision-making of whether they will participate in research. In addition, soliciting children’s and adolescents’ opinions about their involvement in illness management decision-making has been found to be related to adherence to care regimens ( Miller & Jawad, 2014 ). This decision-making involvement could also be important in intervention research where children’s participation is important to the integrity of the intervention.
All research with children and adolescents could benefit from inclusion of a short interview or survey with participants to gauge their degree of satisfaction with the study. If this were done it should be formative rather than summative. That is, the responses from participants early in the study could help shape aspects of the study to enhance the experience for future participants. Talking with children and adolescents about the process of research participation, beyond the actual study, may also be useful in enhancing engagement and future participation in research.
In this review, a common reason children and adolescents gave for participating in research was incentives. Incentives for the most part were viewed positively, although some children did share that the amount of cash incentives should be carefully considered by researchers. Based on findings from this review the age of the child and their vulnerability status needs to be considered when developing plans for incentives. Younger children do not have the same understanding of monetary incentives and could be better suited to more age appropriate incentives such as toys, books, and movie passes. Researchers could consider consulting parents or adolescents from the target population when designing a study to obtain their perceptions about the timing, type, and amount of incentives. Children and adolescents who are homeless or very financially disadvantaged require special consideration, as these participants may weigh incentives differently from those who are more advantaged. For all children and adolescents, per the findings of this integrative review, when obtaining assent/consent researchers should include non-monetary elements as a benefit of study participation including: helping other children learn things, learning something new themselves, and making others’ lives better.
Conclusions
Although there have been many studies on obtaining informed consent and assent when conducting research with ill children, historically there has been much less emphasis placed on children who are involved in research in other contexts. In addition, few studies have focused on the perspectives of children and adolescents themselves. The objective of this systematic review was to examine ethical issues in research with children and adolescents from their perspective as participants, related to assent, parental consent, risk perception, impact of research participation, and incentives. This systematic review highlights the importance of including the voice of children and adolescents in the debate regarding the ethical conduct of research. Children and adolescents are a vulnerable population in the research context, formed of diverse individuals with unique, varying needs. The wide variety of strategies used in the studies described herein exemplifies that in addition to primary research studies of children’s and adolescents’ perspectives of research participation, secondary objectives related to examining their experience as participants can feasibly be added into any pediatric research study. This analysis highlights how researchers and nurses working with children and adolescents enrolled in research can expand their voice and encourage the children to share their experiences in terms of benefits, risks and challenges. It is through linking the evidence found in these studies with their own practice that researchers can improve the experience of and benefits to child and adolescent research participants.
- Assent processes and instruments need to be created with the assistance of child development specialists and piloted with children before being used.
- A priori consultation with representative adolescents and parents can provide guidance for developing consent and assent procedures within challenging contexts.
- A key gap is consideration of whether children and adolescents, beyond simply understanding their research rights, are capable of applying this knowledge and of actually exerting their research rights.
- Researchers need to appreciate that the assessment of risk is an ongoing process throughout a research study, beyond simply explanations provided when obtaining assent and/or consent.
- All research with children and adolescents could benefit from inclusion of a short, formative, off-study interview or survey with participants to gauge their experience in the study.
- The age of the child and their vulnerability status needs to be considered when selecting incentives.
Supplementary Material
Supplemental tables.
TABLE S1. Articles Included in Sample: Summary and Characteristics
Contributor Information
Stacey Crane, Predoctoral Fellow, Indiana University School of Nursing, 1111 Middle Dr., NU345, Indianapolis, IN, 46202, 513-508-3936.
Marion E. Broome, Ruby F. Wilson Distinguished Professor and Dean, Vice-Chancellor for Nursing Affairs, Duke University School of Nursing, 307 Trent Drive, Durham, NC, 27710, 919-684-9444.
- Bagley SJ, Reynolds WW, Nelson RM. Is a “Wage-Payment” model for research participation appropriate for children? Pediatrics. 2007; 119 (1):46. doi: 10.1542/peds.2006-1813. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Birnie KA, Noel M, Chambers CT, von Baeyer CL, Fernandez CV. The cold pressor task: Is it an ethically acceptable pain research method in children? Journal of Pediatric Psychology. 2011; 36 (10):1071–1081. doi: 10.1093/jpepsy/jsq092. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brawner BM, Volpe EM, Stewart JM, Gomes MM. Attitudes and beliefs toward biobehavioural research participation: Voices and concerns of urban adolescent females receiving outpatient mental health treatment. Annals of Human Biology. 2013; 40 (6):485–495. doi: 10.3109/03014460.2013.806590. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Broome ME, Kodish E, Geller G, Siminoff LA. Children in research: New perspectives and practices for informed consent. IRB: Ethics and Human Research, Suppl. 2003; 25 (5):S20–S25. [ PubMed ] [ Google Scholar ]
- Bruzzese JM, Fisher CB. Assessing and enhancing the research consent capacity of children and youth. Applied Developmental Science. 2003; 7 (1):13–26. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=8699738&site=ehost-live . [ Google Scholar ]
- Burke TM, Abramovitch R, Zlotkin S. Children’s understanding of the risks and benefits associated with research. J Med Ethics. 2005; 31 (12):715–720. doi: 10.1136/jme.2003.003228. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carter B. Tick box for child? The ethical positioning of children as vulnerable, researchers as Barbarians and reviewers as overly cautious. International Journal of Nursing Studies. 2009; 46 (6):858–864. doi: 10.1016/j.ijnurstu.2009.01.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chu AT, DePrince AP, Weinzierl KM. Children’s perception of research participation: Examining trauma exposure and distress. Journal of Empirical Research on Human Research Ethics. 2008; 3 (1):49–58. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2008-03332-005&site=ehost-live ; [email protected]. [ PubMed ] [ Google Scholar ]
- Cohn JM, Ginsburg KR, Kassam-Adams N, Fein JA. Adolescent decisional autonomy regarding participation in an emergency department youth violence interview. American Journal of Bioethics. 2005; 5 (5):70–74. doi: 10.1080/15265160500246319. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ellonen N, Pösö T. Children’s experiences of completing a computer-based violence survey: ethical implications. Children & Society. 2011; 25 (6):470–481. doi: 10.1111/j.1099-0860.2010.00292.x. [ CrossRef ] [ Google Scholar ]
- Ensign BJ. Perspectives and experiences of homeless young people. Journal of Advanced Nursing. 2006; 54 (6):647–652. doi: 10.1111/j.1365-2648.2006.03853.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fernandez CV, Gao J, Strahlendorf C, Moghrabi A, Pentz RD, Barfield RC, … Kodish E. Providing research results to participants: Attitudes and needs of adolescents and parents of children with cancer. Journal of Clinical Oncology. 2009; 27 (6):878–883. doi: 10.1200/JCO.2008.18.5223. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fisher CB. Adolescent and parent perspectives on ethical issues in youth drug use and suicide survey research. Ethics Behav. 2003; 13 (4):303–332. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=2004184880&site=ehost-live . [ PubMed ] [ Google Scholar ]
- Giesbertz NAA, Bredenoord AL, van Delden JJM. Clarifying assent in pediatric research. European Journal of Human Genetics. 2014; 22 (2):266–269. doi: 10.1038/ejhg.2013.119. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hurst SA. Vulnerability in research and health care; Describing the elephant in the room? Bioethics. 2008; 22 (4):191–202. [ PubMed ] [ Google Scholar ]
- Institute of Medicine. The ethical conduct of clinical research involving children. Washington, DC: National Academies Press; 2004. [ PubMed ] [ Google Scholar ]
- Lambert V, Glacken M. Engaging with children in research: Theoretical and practical implications of negotiating informed consent/assent. Nurs Ethics. 2011; 18 (6):781–801. doi: 10.1177/0969733011401122. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Langhinrichsen-Rohling J, Arata C, O’Brien N, Bowers D, Klibert J. Sensitive research with adolescents: Just how upsetting are self-report surveys anyway? Violence & Victims. 2006; 21 (4):425–444. Retrieved from http://media.proquest.com/media/pq/classic/doc/1096430801/fmt/pi/rep/NONE?hl=&cit%3Aauth=Langhinrichsen-Rohling%2C+Jennifer%3BArata%2C+Catalina%3BO%27Brien%2C+Natalie%3BBowers%2C+David%3BKlibert%2C+Jeffrey&cit%3Atitle=Sensitive+Research+With+Adolescents%3A+Just+How+Upsetting+Are+Self-Report+Surveys+Anyway%3F&cit%3Apub=Violence+and+Victims&cit%3Avol=21&cit%3Aiss=4&cit%3Apg=425&cit%3Adate=Aug+2006&ic=true&cit%3Aprod=ProQuest&_a=ChgyMDE0MTAwOTIyMDI0MTM2Mjo5OTgwNjUSBjExNDA1MBoKT05FX1NFQVJDSCIOMTQwLjE4Mi42Ny4yMTcqBTQ1NjE5MgkyMDg1MzE2NTA6DURvY3VtZW50SW1hZ2VCATBSBk9ubGluZVoCRlRiA1BGVGoKMjAwNi8wOC8wMXIKMjAwNi8wOC8zMXoAggExUC0xMDA3MDI1LTczOTgtQ1VTVE9NRVItMTAwMDAwMzkvMTAwMDAxNDctMTA0NjkxMZIBBk9ubGluZcoBB0VuZE5vdGXSARJTY2hvbGFybHkgSm91cm5hbHOaAgdQcmVQYWlkqgIoT1M6RU1TLVBkZkRvY1ZpZXdCYXNlLWdldE1lZGlhVXJsRm9ySXRlbcoCD0FydGljbGV8RmVhdHVyZdICAVniAgFO6gIA8gIA&_s=ucBTFWGYfgRjWNZzoaaQAoiq3pQ%3D . [ PubMed ] [ Google Scholar ]
- Letts L, Wilkins S, Law M, Stewart D, Bosch J, Westmorland M. Guidelines for critical review form: Qualitative studies (version 2.0) 2007 Retrieved from http://www.srs-mcmaster.ca/Portals/20/pdf/ebp/qualreview_version2.0.pdf Retrieved from Retrieved from http://www.srs-mcmaster.ca/Portals/20/pdf/ebp/qualreview_version2.0.pdf .
- Levine C, Faden R, Grady C, Hammerschmidt D, Eckenwiler L, Sugarman J The Consortium to Examine Clinical Research Ethics. The limitations of “vulnerability” as a protection for human research participants. The American Journal of Bioethics. 2004; 4 (3):44–49. [ PubMed ] [ Google Scholar ]
- Long AF, Godfrey M, Randall T, Brettle A, Grant M. Developing evidence based social care policy and practice. Part 3: Feasibility of undertaking systematic reviews in social care. 2002 Retrieved from http://usir.salford.ac.uk/13071/ Retrieved from Retrieved from http://usir.salford.ac.uk/13071/
- Mayeux L, Underwood MK, Risser SD. Perspectives on the ethics of sociometric research with children. Merrill-Palmer Quarterly. 2007; 53 (1):53–78. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2007-08527-003&site=ehost-live ; [email protected]; [email protected]. [ Google Scholar ]
- Miller VA, Jawad AF. Relationship of youth involvement in diabetes-related decisions to treatment adherence. Journal of clinical psychology in medical settings. 2014; 21 (2):183–189. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of internal medicine. 2009; 151 (4):264–269. [ PubMed ] [ Google Scholar ]
- Moreno MA, Grant A, Kacvinsky L, Moreno P, Fleming M. Older adolescents’ views regarding participation in Facebook research. Journal of Adolescent Health. 2012; 51 (5):439–444. doi: 10.1016/j.jadohealth.2012.02.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- O’Reilly M, Karim K, Taylor H, Dogra N. Parent and child views on anonymity: ‘I’ve got nothing to hide’ International Journal of Social Research Methodology: Theory & Practice. 2012; 15 (3):211–223. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2012-12510-003&site=ehost-live ; [email protected]. [ Google Scholar ]
- Reynolds WW, Nelson RM. Risk perception and decision processes underlying informed consent to research participation. Social Science & Medicine. 2007; 65 (10):2105–2115. doi: 10.1016/j.socscimed.2007.06.021. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Solomon SR. Protecting and respecting the vulnerable: Existing regulations or further protections? Theor Med Bioeth. 2013; 34 (1):17–28. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Swartling U, Hansson MG, Ludvigsson J, Nordgren A. “My parents decide if I can. I decide if I want to.” Children’s views on participation in medical research. Journal of Empirical Research on Human Research Ethics. 2011; 6 (4):68–75. doi: 10.1525/jer.2011.6.4.68. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Swartling U, Helgesson G, Ludvigsson J, Hansson MG, Nordgren A. Children’s views on long-term screening for type 1 diabetes. Journal of Empirical Research on Human Research Ethics. 2014; 9 (4):1–9. doi: 10.1177/1556264614544456. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Traube DE, Cederbaum JA, Kerkorian D, Bhupali C, McKay MM. African American children’s perceptions of HIV-focused community-based participatory research. Journal of Empirical Research on Human Research Ethics. 2013; 8 (1):79–90. doi: 10.1525/jer.2013.8.1.79. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Unguru Y, Sill AM, Kamani N. The experiences of children enrolled in pediatric oncology research: Implications for assent. Pediatrics. 2010; 125 (4):e876–e883. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=49753789&site=ehost-live . [ PubMed ] [ Google Scholar ]
- Vitiello B, Kratochvil CJ, Silva S, Curry J, Reinecke M, Pathak S, … May DE. Research knowledge among the participants in the Treatment for Adolescents with Depression Study (TADS) Journal of the American Academy of Child and Adolescent Psychiatry. 2007; 46 (12):1642–1650. [ PubMed ] [ Google Scholar ]
- Wagner KD, Martinez M, Joiner T. Youths’ and their parents’ attitudes and experiences about participation in psychopharmacology treatment research. Journal of Child & Adolescent Psychopharmacology. 2006; 16 (3):298–307. [ PubMed ] [ Google Scholar ]
- Woodgate RL, Edwards M. Children in health research: A matter of trust. J Med Ethics. 2010; 36 (4):211–216. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=ovftk&AN=00005004-201004000-00006 ; http://jme.bmj.com/content/36/4/211.full.pdf . [ PubMed ] [ Google Scholar ]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. Journal of the American Medical Association. 2013; 310 (20):2191–2194. doi: 10.1001/jama.2013.281053. [ PubMed ] [ CrossRef ] [ Google Scholar ]

COMMENTS
Abstract. Ethics, as an integral component of human decision-making, undeniably shape the landscape of scientific research. This article delves deeply into the nuanced realm of ethical ...
To summarize research ethics review experiences in a study about the research ethics review process: Online feedback form of researcher's data: ... and to know how to face one's own limitations (Bishop & Shepherd, 2011; Guillemin & Guillan, 2015). Reflexivity and ethical mindfulness are interdependent concepts, with the first being a tool ...
Revised on May 9, 2024. Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective treatments ...
Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. Many of the deviations that occur in research may occur because researchers simply do not know or have never thought seriously about some of the ethical norms of research. For ...
The concept of 'ethics in practice' provides a useful framework for researchers when considering their role in responding to unexpected ethical challenges that arise from interactions with research participants, and it has been used by researchers in discussions about appropriate ethical behaviour in research on a wide range of subjects (e.g., Fletcher, 2022; McEvoy et al., 2017; Robinson ...
Introduction. Research includes a set of activities in which researchers use various structured methods to contribute to the development of knowledge, whether this knowledge is theoretical, fundamental, or applied (Drolet & Ruest, accepted).University research is carried out in a highly competitive environment that is characterized by ever-increasing demands (i.e., on time, productivity ...
Informed consent has been recognized as an integral part of ethics in research carried out in different fields. For qualitative researchers, it is of the utmost importance to specify in advance which data will be collected and how they are to be used . The principle of informed consent stresses the researcher's responsibility to completely ...
Introduction. Ethics are a guiding principle that shapes the conduct of researchers. It influences both the process of discovery and the implications and applications of scientific findings 1.Ethical considerations in research include, but are not limited to, the management of data, the responsible use of resources, respect for human rights, the treatment of human and animal subjects, social ...
Research ethics guide researchers conducting any research, educate, ... This code also emphasizes the balance between risk and benefit of involving humans in research. This code's key limitation was the self-regulation of the research that could be abused. All declarations followed prohibited nontherapeutic research, but Helsinki's ...
Keywords: Ethical limitations, ethics, moral guidelines, ... Research ethics is an essential aspect of human behaviour, guiding interactions and activities in various fields (Blumberg
Typically, the field of research ethics concerns itself with matters of risk, harm, justice, consequences, benefits, and care (Israel, 2015).Mitigating the risk of psychological (distress) and social (discriminatory judgment) harm is a key consideration in research ethics (Resnik, 2018).Researchers and ethics committees are accustomed to assessing these aspects of proposed research and ...
It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants. As such, all research involving human beings should be reviewed by an ethics committee to ensure that the appropriate ethical standards are being upheld. Discussion of the ethical principles of beneficence, justice and ...
Institutional Ethics Committee (IEC): for non-routine research. External Ethics Committee (EEC): for research that s externally regulated (e.g., NHS research). ... They should make the limitations of their research explicit (e.g., 'the study was only carried out on white middle-class American male students,' 'the study is based on ...
Research ethics is a foundational principle of modern medical research across all disciplines. The overarching body, the IRB, is intentionally comprised of experts across various disciplines, including ethicists, social workers, physicians, nurses, other scientific researchers, counselors, mental health professionals, and advocates for ...
Limitations of Research Ethics: For subjects: Possibilities to physical integrity, containing those linked with experimental drugs and dealings and with other involvements that will be used in the study (e.g. measures used to observe research participants, such as blood sampling, X-rays or lumbar punctures).
In the conduct of research, several key principles and actions must be observed and preserved. These include freedom from harm, right to self-determination, right to privacy, and right to anonymity and confidentiality (See Figure 2). Freedom from physical or mental harm or discomfort should be of utmost concern to the researcher.
Others argue that local development of research ethics is crucial to ensuring buy-in and avoiding bioethical imperialism [11,12]. Acknowledging the past abuses that took place under the guise of research, Western ethical thought now insists on voluntary consent, with special protections for traditionally vulnerable populations such as children ...
4. Respect confidentiality and privacy. Upholding individuals' rights to confidentiality and privacy is a central tenet of every psychologist's work. However, many privacy issues are idiosyncratic to the research population, writes Susan Folkman, PhD, in "Ethics in Research with Human Participants" (APA, 2000).
Research Design. A qualitative research approach involving individual semi-structured interviews was used to systematically document ethical issues (De Poy & Gitlin, 2010; Hammell et al., 2000).Specifically, a descriptive phenomenological approach inspired by the philosophy of Husserl was used (Husserl, 1970, 1999), as it is recommended for documenting the perceptions of ethical issues raised ...
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication. Scientists and researchers must always adhere to a certain code of conduct when collecting data ...
In this paper the authors, one a research ethicist and one an experienced action researcher, will consider the challenges faced when seeking ethical approval for AR. This paper will consider these challenges from the perspective of both the researcher and the research ethics committee (REC). Whilst these experiences are drawn from the Health ...
Due to the high volume of articles initially retrieved (PubMed = 2,240 and EBSCOhost = 3,313) additional limitations were placed on searches. Explicitly, in PubMed a limitation was added for the MeSH search term 'Ethics, research' and in EBSCOhost a limitation was placed using 'Research ethics' as a major concept.
Email: [email protected]. This paper reports a literature review on the topic of ethical issues in in-depth interviews. The review returned three types of article: general discussion, issues in particular studies, and studies of interview-based research ethics. Whilst many of the issues discussed in these articles are generic to research ...
Starting from the recognition of the limits of today's common essentialist and axiological understandings of data and ethics, in this article we make the case for an ecosystemic understanding of data ethics (for the city) that accounts for the inherent value-laden entanglements and unintended (both positive and negative) consequences of the development, implementation, and use of data-driven ...