Essay on Smoking
500 words essay on smoking.
One of the most common problems we are facing in today’s world which is killing people is smoking. A lot of people pick up this habit because of stress , personal issues and more. In fact, some even begin showing it off. When someone smokes a cigarette, they not only hurt themselves but everyone around them. It has many ill-effects on the human body which we will go through in the essay on smoking.


Ill-Effects of Smoking
Tobacco can have a disastrous impact on our health. Nonetheless, people consume it daily for a long period of time till it’s too late. Nearly one billion people in the whole world smoke. It is a shocking figure as that 1 billion puts millions of people at risk along with themselves.
Cigarettes have a major impact on the lungs. Around a third of all cancer cases happen due to smoking. For instance, it can affect breathing and causes shortness of breath and coughing. Further, it also increases the risk of respiratory tract infection which ultimately reduces the quality of life.
In addition to these serious health consequences, smoking impacts the well-being of a person as well. It alters the sense of smell and taste. Further, it also reduces the ability to perform physical exercises.
It also hampers your physical appearances like giving yellow teeth and aged skin. You also get a greater risk of depression or anxiety . Smoking also affects our relationship with our family, friends and colleagues.
Most importantly, it is also an expensive habit. In other words, it entails heavy financial costs. Even though some people don’t have money to get by, they waste it on cigarettes because of their addiction.
How to Quit Smoking?
There are many ways through which one can quit smoking. The first one is preparing for the day when you will quit. It is not easy to quit a habit abruptly, so set a date to give yourself time to prepare mentally.
Further, you can also use NRTs for your nicotine dependence. They can reduce your craving and withdrawal symptoms. NRTs like skin patches, chewing gums, lozenges, nasal spray and inhalers can help greatly.
Moreover, you can also consider non-nicotine medications. They require a prescription so it is essential to talk to your doctor to get access to it. Most importantly, seek behavioural support. To tackle your dependence on nicotine, it is essential to get counselling services, self-materials or more to get through this phase.
One can also try alternative therapies if they want to try them. There is no harm in trying as long as you are determined to quit smoking. For instance, filters, smoking deterrents, e-cigarettes, acupuncture, cold laser therapy, yoga and more can work for some people.
Always remember that you cannot quit smoking instantly as it will be bad for you as well. Try cutting down on it and then slowly and steadily give it up altogether.
Get the huge list of more than 500 Essay Topics and Ideas
Conclusion of the Essay on Smoking
Thus, if anyone is a slave to cigarettes, it is essential for them to understand that it is never too late to stop smoking. With the help and a good action plan, anyone can quit it for good. Moreover, the benefits will be evident within a few days of quitting.
FAQ of Essay on Smoking
Question 1: What are the effects of smoking?
Answer 1: Smoking has major effects like cancer, heart disease, stroke, lung diseases, diabetes, and more. It also increases the risk for tuberculosis, certain eye diseases, and problems with the immune system .
Question 2: Why should we avoid smoking?
Answer 2: We must avoid smoking as it can lengthen your life expectancy. Moreover, by not smoking, you decrease your risk of disease which includes lung cancer, throat cancer, heart disease, high blood pressure, and more.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 24 March 2022
Tobacco and nicotine use
- Bernard Le Foll 1 , 2 ,
- Megan E. Piper 3 , 4 ,
- Christie D. Fowler 5 ,
- Serena Tonstad 6 ,
- Laura Bierut 7 ,
- Lin Lu ORCID: orcid.org/0000-0003-0742-9072 8 , 9 ,
- Prabhat Jha 10 &
- Wayne D. Hall 11 , 12
Nature Reviews Disease Primers volume 8 , Article number: 19 ( 2022 ) Cite this article
35k Accesses
70 Citations
104 Altmetric
Metrics details
- Disease genetics
- Experimental models of disease
- Preventive medicine
Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will die prematurely from tobacco-related complications. In addition, people who smoke have a significant reduction in their quality of life. Neurobiological findings have identified the mechanisms by which nicotine in tobacco affects the brain reward system and causes addiction. These brain changes contribute to the maintenance of nicotine or tobacco use despite knowledge of its negative consequences, a hallmark of addiction. Effective approaches to screen, prevent and treat tobacco use can be widely implemented to limit tobacco’s effect on individuals and society. The effectiveness of psychosocial and pharmacological interventions in helping people quit smoking has been demonstrated. As the majority of people who smoke ultimately relapse, it is important to enhance the reach of available interventions and to continue to develop novel interventions. These efforts associated with innovative policy regulations (aimed at reducing nicotine content or eliminating tobacco products) have the potential to reduce the prevalence of tobacco and nicotine use and their enormous adverse impact on population health.
Similar content being viewed by others

Distinct µ-opioid ensembles trigger positive and negative fentanyl reinforcement

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls
Introduction.
Tobacco is the second most commonly used psychoactive substance worldwide, with more than one billion smokers globally 1 . Although smoking prevalence has reduced in many high-income countries (HICs), tobacco use is still very prevalent in low-income and middle-income countries (LMICs). The majority of smokers are addicted to nicotine delivered by cigarettes (defined as tobacco dependence in the International Classification of Diseases, Tenth Revision (ICD-10) or tobacco use disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)). As a result of the neuro-adaptations and psychological mechanisms caused by repeated exposure to nicotine delivered rapidly by cigarettes, cessation can also lead to a well-characterized withdrawal syndrome, typically manifesting as irritability, anxiety, low mood, difficulty concentrating, increased appetite, insomnia and restlessness, that contributes to the difficulty in quitting tobacco use 2 , 3 , 4 .
Historically, tobacco was used in some cultures as part of traditional ceremonies, but its use was infrequent and not widely disseminated in the population. However, since the early twentieth century, the use of commercial cigarettes has increased dramatically 5 because of automated manufacturing practices that enable large-scale production of inexpensive products that are heavily promoted by media and advertising. Tobacco use became highly prevalent in the past century and was followed by substantial increases in the prevalence of tobacco-induced diseases decades later 5 . It took decades to establish the relationship between tobacco use and associated health effects 6 , 7 and to discover the addictive role of nicotine in maintaining tobacco smoking 8 , 9 , and also to educate people about these effects. It should be noted that the tobacco industry disputed this evidence to allow continuing tobacco sales 10 . The expansion of public health campaigns to reduce smoking has gradually decreased the use of tobacco in HICs, with marked increases in adult cessation, but less progress has been achieved in LMICs 1 .
Nicotine is the addictive compound in tobacco and is responsible for continued use of tobacco despite harms and a desire to quit, but nicotine is not directly responsible for the harmful effects of using tobacco products (Box 1 ). Other components in tobacco may modulate the addictive potential of tobacco (for example, flavours and non-nicotine compounds) 11 . The major harms related to tobacco use, which are well covered elsewhere 5 , are linked to a multitude of compounds present in tobacco smoke (such as carcinogens, toxicants, particulate matter and carbon monoxide). In adults, adverse health outcomes of tobacco use include cancer in virtually all peripheral organs exposed to tobacco smoke and chronic diseases such as eye disease, periodontal disease, cardiovascular diseases, chronic obstructive pulmonary disease, stroke, diabetes mellitus, rheumatoid arthritis and disorders affecting immune function 5 . Moreover, smoking during pregnancy can increase the risk of adverse reproductive effects, such as ectopic pregnancy, low birthweight and preterm birth 5 . Exposure to secondhand cigarette smoke in children has been linked to sudden infant death syndrome, impaired lung function and respiratory illnesses, in addition to cognitive and behavioural impairments 5 . The long-term developmental effects of nicotine are probably due to structural and functional changes in the brain during this early developmental period 12 , 13 .
Nicotine administered alone in various nicotine replacement formulations (such as patches, gum and lozenges) is safe and effective as an evidence-based smoking cessation aid. Novel forms of nicotine delivery systems have also emerged (called electronic nicotine delivery systems (ENDS) or e-cigarettes), which can potentially reduce the harmful effects of tobacco smoking for those who switch completely from combustible to e-cigarettes 14 , 15 .
This Primer focuses on the determinants of nicotine and tobacco use, and reviews the neurobiology of nicotine effects on the brain reward circuitry and the functioning of brain networks in ways that contribute to the difficulty in stopping smoking. This Primer also discusses how to prevent tobacco use, screen for smoking, and offer people who smoke tobacco psychosocial and pharmacological interventions to assist in quitting. Moreover, this Primer presents emerging pharmacological and novel brain interventions that could improve rates of successful smoking cessation, in addition to public health approaches that could be beneficial.
Box 1 Tobacco products
Conventional tobacco products include combustible products that produce inhaled smoke (most commonly cigarettes, bidis (small domestically manufactured cigarettes used in South Asia) or cigars) and those that deliver nicotine without using combustion (chewing or dipping tobacco and snuff). Newer alternative products that do not involve combustion include nicotine-containing e-cigarettes and heat-not-burn tobacco devices. Although non-combustion and alternative products may constitute a lesser risk than burned ones 14 , 15 , 194 , no form of tobacco is entirely risk-free.
Epidemiology
Prevalence and burden of disease.
The Global Burden of Disease Project (GBDP) estimated that around 1.14 billion people smoked in 2019, worldwide, increasing from just under a billion in 1990 (ref. 1 ). Of note, the prevalence of smoking decreased significantly between 1990 and 2019, but increases in the adult population meant that the total number of global smokers increased. One smoking-associated death occurs for approximately every 0.8–1.1 million cigarettes smoked 16 , suggesting that the estimated worldwide consumption of about 7.4 trillion cigarettes in 2019 has led to around 7 million deaths 1 .
In most populations, smoking prevalence is much higher among groups with lower levels of education or income 17 and among those with mental health disorders and other co-addictions 18 , 19 . Smoking is also more frequent among men than women (Figs 1 – 3 ). Sexual and/or gender minority individuals have disproportionately high rates of smoking and other addictions 17 , 20 . In addition, the prevalence of smoking varies substantially between regions and ethnicities; smoking rates are high in some regions of Asia, such as China and India, but are lower in North America and Australia. Of note, the prevalence of mental health disorders and other co-addictions is higher in individuals who smoke compared with non-smokers 18 , 19 , 21 . For example, the odds of smoking in people with any substance use disorder is more than five times higher than the odds in people without a substance use disorder 19 . Similarly, the odds of smoking in people with any psychiatric disorder is more than three times higher than the odds of smoking in those without a psychiatric diagnosis 22 . In a study in the USA, compared with a population of smokers with no psychiatric diagnosis, subjects with anxiety, depression and phobia showed an approximately twofold higher prevalence of smoking, and subjects with agoraphobia, mania or hypomania, psychosis and antisocial personality or conduct disorders showed at least a threefold higher prevalence of smoking 22 . Comorbid disorders are also associated with higher rates of smoking 22 , 23 .
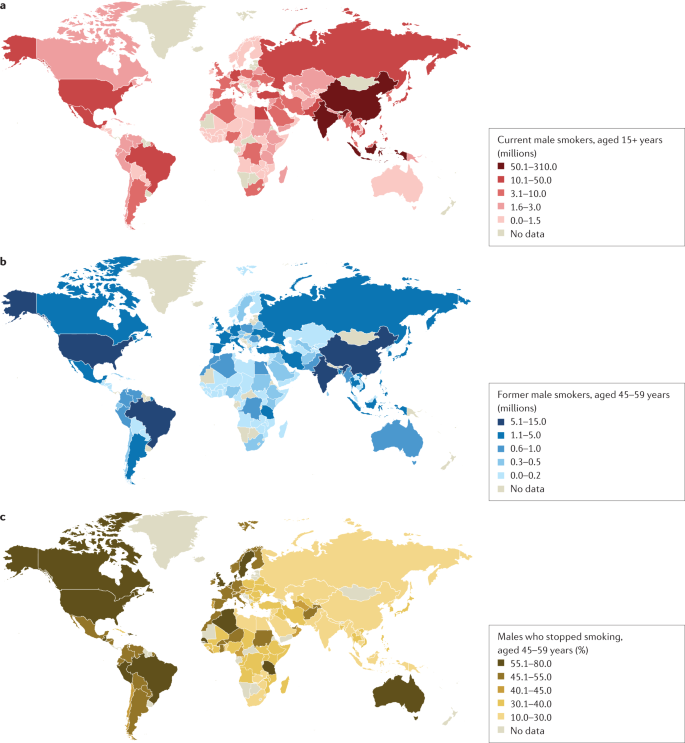
a | Number of current male smokers aged 15 years or older per country expressed in millions. b | Former male smokers aged 45–59 years per country expressed in millions. c | Former male smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for male smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among males is less variable than among females. Data from ref. 1 .
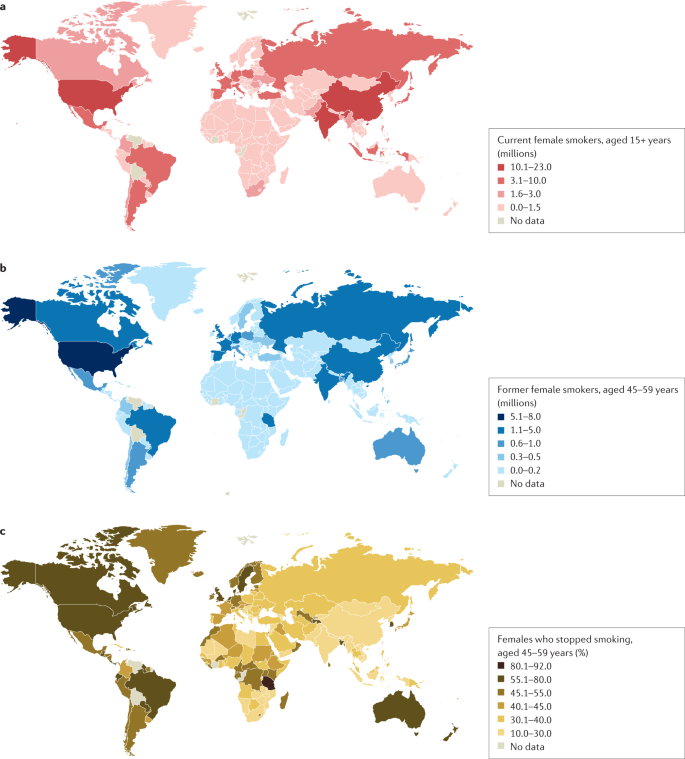
a | Number of current female smokers aged 15 years or older per country expressed in millions. b | Former female smokers aged 45–59 years per country expressed in millions. c | Former female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for female smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among females is much lower in East and South Asia than in Latin America or Eastern Europe. Data from ref. 1 .
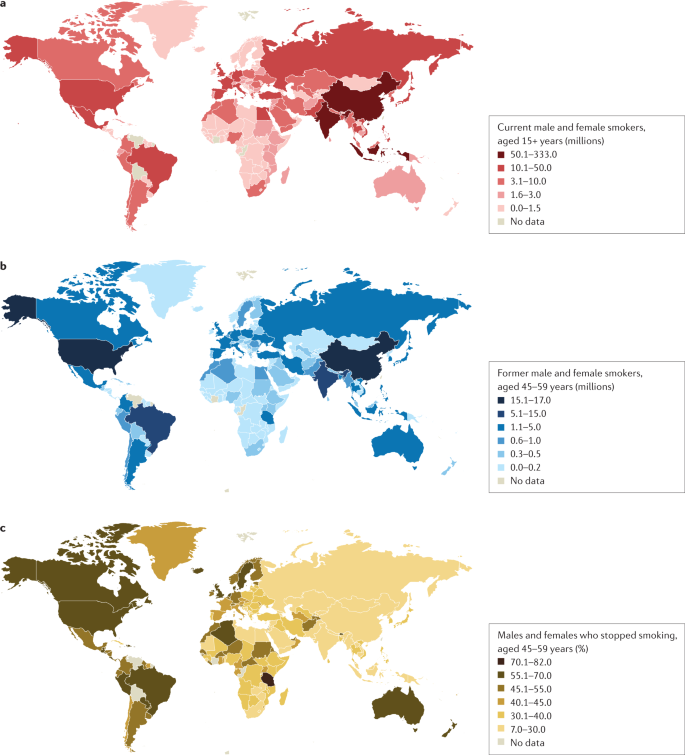
a | Number of current male and female smokers aged 15 years or older per country expressed in millions. b | Former male and female smokers aged 45–59 years per country expressed in millions. c | Former male and female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for the period 2015–2019 from countries with direct smoking surveys. Cessation rates are higher in high-income countries, but also notably high in Brazil. Cessation is far less common in South and East Asia and Russia and other Eastern European countries, and also low in South Africa. Data from ref. 1 .
Age at onset
Most smokers start smoking during adolescence, with almost 90% of smokers beginning between 15 and 25 years of age 24 . The prevalence of tobacco smoking among youths substantially declined in multiple HICs between 1990 and 2019 (ref. 25 ). More recently, the widespread uptake of ENDS in some regions such as Canada and the USA has raised concerns about the long-term effects of prolonged nicotine use among adolescents, including the possible notion that ENDS will increase the use of combustible smoking products 25 , 26 (although some studies have not found much aggregate effect at the population level) 27 .
Smoking that commences in early adolescence or young adulthood and persists throughout life has a more severe effect on health than smoking that starts later in life and/or that is not persistent 16 , 28 , 29 . Over 640 million adults under 30 years of age smoke in 22 jurisdictions alone (including 27 countries in the European Union where central efforts to reduce tobacco dependence might be possible) 30 . In those younger than 30 years of age, at least 320 million smoking-related deaths will occur unless they quit smoking 31 . The actual number of smoking-related deaths might be greater than one in two, and perhaps as high as two in three, long-term smokers 5 , 16 , 29 , 32 , 33 . At least half of these deaths are likely to occur in middle age (30–69 years) 16 , 29 , leading to a loss of two or more decades of life. People who smoke can expect to lose an average of at least a decade of life versus otherwise similar non-smokers 16 , 28 , 29 .
Direct epidemiological studies in several countries paired with model-based estimates have estimated that smoking tobacco accounted for 7.7 million deaths globally in 2020, of which 80% were in men and 87% were current smokers 1 . In HICs, the major causes of tobacco deaths are lung cancer, emphysema, heart attack, stroke, cancer of the upper aerodigestive areas and bladder cancer 28 , 29 . In some lower income countries, tuberculosis is an additional important cause of tobacco-related death 29 , 34 , which could be related to, for example, increased prevalence of infection, more severe tuberculosis/mortality and higher prevalence of treatment-resistant tuberculosis in smokers than in non-smokers in low-income countries 35 , 36 .
Despite substantial reductions in the prevalence of smoking, there were 34 million smokers in the USA, 7 million in the UK and 5 million in Canada in 2017 (ref. 16 ), and cigarette smoking remains the largest cause of premature death before 70 years of age in much of Europe and North America 1 , 16 , 28 , 29 . Smoking-associated diseases accounted for around 41 million deaths in the USA, UK and Canada from 1960 to 2020 (ref. 16 ). Moreover, as smoking-associated diseases are more prevalent among groups with lower levels of education and income, smoking accounts for at least half of the difference in overall mortality between these social groups 37 . Any reduction in smoking prevalence reduces the absolute mortality gap between these groups 38 .
Smoking cessation has become common in HICs with good tobacco control interventions. For example, in France, the number of ex-smokers is four times the number of current smokers among those aged 50 years or more 30 . By contrast, smoking cessation in LMICs remains uncommon before smokers develop tobacco-related diseases 39 . Smoking cessation greatly reduces the risks of smoking-related diseases. Indeed, smokers who quit smoking before 40 years of age avoid nearly all the increased mortality risks 31 , 33 . Moreover, individuals who quit smoking by 50 years of age reduce the risk of death from lung cancer by about two-thirds 40 . More modest hazards persist for deaths from lung cancer and emphysema 16 , 28 ; however, the risks among former smokers are an order of magnitude lower than among those who continue to smoke 33 .
Mechanisms/pathophysiology
Nicotine is the main psychoactive agent in tobacco and e-cigarettes. Nicotine acts as an agonist at nicotinic acetylcholine receptors (nAChRs), which are localized throughout the brain and peripheral nervous system 41 . nAChRs are pentameric ion channels that consist of varying combinations of α 2 –α 7 and β 2 –β 4 subunits, and for which acetylcholine (ACh) is the endogenous ligand 42 , 43 , 44 . When activated by nicotine binding, nAChR undergoes a conformational change that opens the internal pore, allowing an influx of sodium and calcium ions 45 . At postsynaptic membranes, nAChR activation can lead to action potential firing and downstream modulation of gene expression through calcium-mediated second messenger systems 46 . nAChRs are also localized to presynaptic membranes, where they modulate neurotransmitter release 47 . nAChRs become desensitized after activation, during which ligand binding will not open the channel 45 .
nAChRs with varying combinations of α-subunits and β-subunits have differences in nicotine binding affinity, efficacy and desensitization rate, and have differential expression depending on the brain region and cell type 48 , 49 , 50 . For instance, at nicotine concentrations found in human smokers, β 2 -containing nAChRs desensitize relatively quickly after activation, whereas α 7 -containing nAChRs have a slower desensitization profile 48 . Chronic nicotine exposure in experimental animal models or in humans induces an increase in cortical expression of α 4 β 2 -containing nAChRs 51 , 52 , 53 , 54 , 55 , but also increases the expression of β 3 and β 4 nAChR subunits in the medial habenula (MHb)–interpeduncular nucleus (IPN) pathway 56 , 57 . It is clear that both the brain localization and the type of nAChR are critical elements in mediating the various effects of nicotine, but other factors such as rate of nicotine delivery may also modulate addictive effects of nicotine 58 .
Neurocircuitry of nicotine addiction
Nicotine has both rewarding effects (such as a ‘buzz’ or ‘high’) and aversive effects (such as nausea and dizziness), with the net outcome dependent on dose and others factors such as interindividual sensitivity and presence of tolerance 59 . Thus, the addictive properties of nicotine involve integration of contrasting signals from multiple brain regions that process reward and aversion (Fig. 4 ).
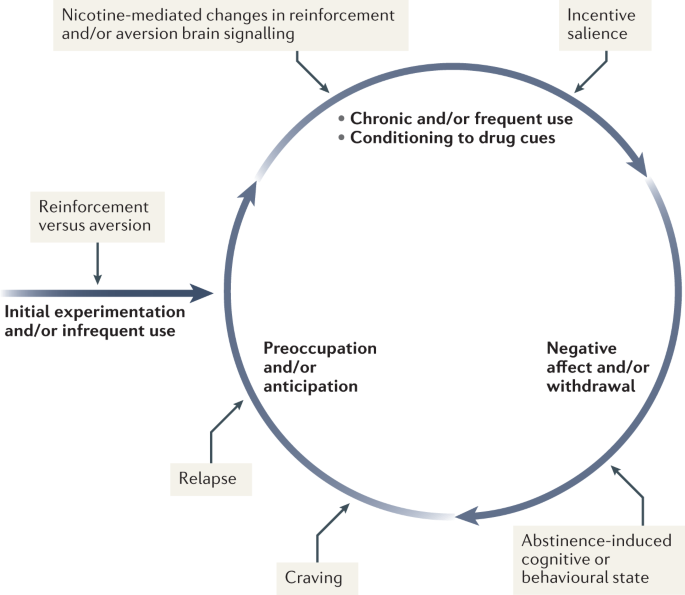
During initial use, nicotine exerts both reinforcing and aversive effects, which together determine the likelihood of continued use. As the individual transitions to more frequent patterns of chronic use, nicotine induces pharmacodynamic changes in brain circuits, which is thought to lead to a reduction in sensitivity to the aversive properties of the drug. Nicotine is also a powerful reinforcer that leads to the conditioning of secondary cues associated with the drug-taking experience (such as cigarette pack, sensory properties of cigarette smoke and feel of the cigarette in the hand or mouth), which serves to enhance the incentive salience of these environmental factors and drive further drug intake. When the individual enters into states of abstinence (such as daily during sleep at night or during quit attempts), withdrawal symptomology is experienced, which may include irritability, restlessness, learning or memory deficits, difficulty concentrating, anxiety and hunger. These negative affective and cognitive symptoms lead to an intensification of the individual’s preoccupation to obtain and use the tobacco/nicotine product, and subsequently such intense craving can lead to relapse.
The rewarding actions of nicotine have largely been attributed to the mesolimbic pathway, which consists of dopaminergic neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens and prefrontal cortex 60 , 61 , 62 (Fig. 5 ). VTA integrating circuits and projection regions express several nAChR subtypes on dopaminergic, GABAergic, and glutamatergic neurons 63 , 64 . Ultimately, administration of nicotine increases dopamine levels through increased dopaminergic neuron firing in striatal and extrastriatal areas (such as the ventral pallidum) 65 (Fig. 6 ). This effect is involved in reward and is believed to be primarily mediated by the action of nicotine on α 4 -containing and β 2 -containing nAChRs in the VTA 66 , 67 .
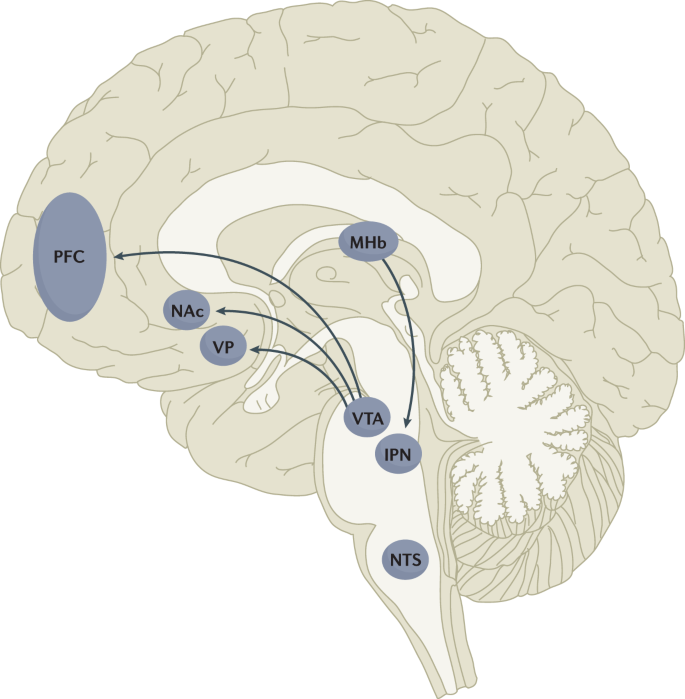
Multiple lines of research have demonstrated that nicotine reinforcement is mainly controlled by two brain pathways, which relay predominantly reward-related or aversion-related signals. The rewarding properties of nicotine that promote drug intake involve the mesolimbic dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). By contrast, the aversive properties of nicotine that limit drug intake and mitigate withdrawal symptoms involve the fasciculus retroflexus projection from the medial habenula (MHb) to the interpeduncular nucleus (IPN). Additional brain regions have also been implicated in various aspects of nicotine dependence, such as the prefrontal cortex (PFC), ventral pallidum (VP), nucleus tractus solitarius (NTS) and insula (not shown here for clarity). All of these brain regions are directly or indirectly interconnected as integrative circuits to drive drug-seeking and drug-taking behaviours.
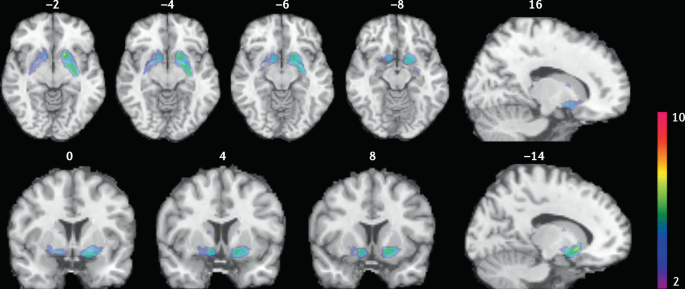
Smokers received brain PET scans with [ 11 C]PHNO, a dopamine D 2/3 PET tracer that has high sensitivity in detecting fluctuations of dopamine. PET scans were performed during abstinence or after smoking a cigarette. Reduced binding potential (BP ND ) was observed after smoking, indicating increased dopamine levels in the ventral striatum and in the area that corresponds to the ventral pallidum. The images show clusters with statistically significant decreases of [ 11 C]PHNO BP ND after smoking a cigarette versus abstinence condition. Those clusters have been superimposed on structural T1 MRI images of the brain. Reprinted from ref. 65 , Springer Nature Limited.
The aversive properties of nicotine are mediated by neurons in the MHb, which project to the IPN. Studies in rodents using genetic knockdown and knockout strategies demonstrated that the α 5 -containing, α 3 -containing and β 4 -containing nAChRs in the MHb–IPN pathway mediate the aversive properties of nicotine that limit drug intake, especially when animals are given the opportunity to consume higher nicotine doses 68 , 69 , 70 , 71 , 72 . In addition to nAChRs, other signalling factors acting on the MHb terminals in the IPN also regulate the actions of nicotine. For instance, under conditions of chronic nicotine exposure or with optogenetic activation of IPN neurons, a subtype of IPN neurons co-expressing Chrna5 (encoding the α 5 nAChR subunit) and Amigo1 (encoding adhesion molecule with immunoglobulin-like domain 1) release nitric oxide from the cell body that retrogradely inhibits MHb axon terminals 70 . In addition, nicotine activates α 5 -containing nAChR-expressing neurons that project from the nucleus tractus solitarius to the IPN, leading to release of glucagon-like peptide-1 that binds to GLP receptors on habenular axon terminals, which subsequently increases IPN neuron activation and decreases nicotine self-administration 73 . Taken together, these findings suggest a dynamic signalling process at MHb axonal terminals in the IPN, which regulates the addictive properties of nicotine and determines the amount of nicotine that is self-administered.
Nicotine withdrawal in animal models can be assessed by examining somatic signs (such as shaking, scratching, head nods and chewing) and affective signs (such as increased anxiety-related behaviours and conditioned place aversion). Interestingly, few nicotine withdrawal somatic signs are found in mice with genetic knockout of the α 2 , α 5 or β 4 nAChR subunits 74 , 75 . By contrast, β 2 nAChR-knockout mice have fewer anxiety-related behaviours during nicotine withdrawal, with no differences in somatic symptoms compared with wild-type mice 74 , 76 .
In addition to the VTA (mediating reward) and the MHb–IPN pathway (mediating aversion), other brain areas are involved in nicotine addiction (Fig. 5 ). In animals, the insular cortex controls nicotine taking and nicotine seeking 77 . Moreover, humans with lesions of the insular cortex can quit smoking easily without relapse 78 . This finding led to the development of a novel therapeutic intervention modulating insula function (see Management, below) 79 , 80 . Various brain areas (shell of nucleus accumbens, basolateral amygdala and prelimbic cortex) expressing cannabinoid CB 1 receptors are also critical in controlling rewarding effects and relapse 81 , 82 . The α 1 -adrenergic receptor expressed in the cortex also control these effects, probably through glutamatergic afferents to the nucleus accumbens 83 .
Individual differences in nicotine addiction risk
Vulnerability to nicotine dependence varies between individuals, and the reasons for these differences are multidimensional. Many social factors (such as education level and income) play a role 84 . Broad psychological and social factors also modulate this risk. For example, peer smoking status, knowledge on effect of tobacco, expectation on social acceptance, exposure to passive smoking modulate the risk of initiating tobacco use 85 , 86 .
Genetic factors have a role in smoking initiation, the development of nicotine addiction and the likelihood of smoking cessation. Indeed, heritability has been estimated to contribute to approximatively half of the variability in nicotine dependence 87 , 88 , 89 , 90 . Important advances in our understanding of such genetic contributions have evolved with large-scale genome-wide association studies of smokers and non-smokers. One of the most striking findings has been that allelic variation in the CHRNA5 – CHRNA3 – CHRNB4 gene cluster, which encodes α 5 , α 3 and β 4 nAChR subunits, correlates with an increased vulnerability for nicotine addiction, indicated by a higher likelihood of becoming dependent on nicotine and smoking a greater number of cigarettes per day 91 , 92 , 93 , 94 , 95 . The most significant effect has been found for a single-nucleotide polymorphism in CHRNA5 (rs16969968), which results in an amino acid change and reduced function of α 5 -containing nAChRs 92 .
Allelic variation in CYP2A6 (encoding the CYP2A6 enzyme, which metabolizes nicotine) has also been associated with differential vulnerability to nicotine dependence 96 , 97 , 98 . CYP2A6 is highly polymorphic, resulting in variable enzymatic activity 96 , 99 , 100 . Individuals with allelic variation that results in slow nicotine metabolism consume less nicotine per day, experience less-severe withdrawal symptoms and are more successful at quitting smoking than individuals with normal or fast metabolism 101 , 102 , 103 , 104 . Moreover, individuals with slow nicotine metabolism have lower dopaminergic receptor expression in the dopamine D2 regions of the associative striatum and sensorimotor striatum in PET studies 105 and take fewer puffs of nicotine-containing cigarettes (compared with de-nicotinized cigarettes) in a forced choice task 106 . Slower nicotine metabolism is thought to increase the duration of action of nicotine, allowing nicotine levels to accumulate over time, therefore enabling lower levels of intake to sustain activation of nAChRs 107 .
Large-scale genetic studies have identified hundreds of other genetic loci that influence smoking initiation, age of smoking initiation, cigarettes smoked per day and successful smoking cessation 108 . The strongest genetic contributions to smoking through the nicotinic receptors and nicotine metabolism are among the strongest genetic contributors to lung cancer 109 . Other genetic variations (such as those related to cannabinoid, dopamine receptors or other neurotransmitters) may affect certain phenotypes related to smoking (such as nicotine preference and cue-reactivity) 110 , 111 , 112 , 113 , 114 , 115 .
Diagnosis, screening and prevention
Screening for cigarette smoking.
Screening for cigarette smoking should happen at every doctor’s visit 116 . In this regard, a simple and direct question about a person’s tobacco use can provide an opportunity to offer information about its potential risks and treatments to assist in quitting. All smokers should be offered assistance in quitting because even low levels of smoking present a significant health risk 33 , 117 , 118 . Smoking status can be assessed by self-categorization or self-reported assessment of smoking behaviour (Table 1 ). In people who smoke, smoking frequency can be assessed 119 and a combined quantity frequency measure such as pack-year history (that is, average number of cigarettes smoked per day multiplied by the number of years, divided by 20), can be used to estimate cumulative risk of adverse health outcomes. The Association for the Treatment of Tobacco Use and Dependence recommends that all electronic health records should document smoking status using the self-report categories listed in Table 1 .
Owing to the advent of e-cigarettes and heat-not-burn products, and the popularity of little cigars in the US that mimic combustible cigarettes, people who use tobacco may use multiple products concurrently 120 , 121 . Thus, screening for other nicotine and tobacco product use is important in clinical practice. The self-categorization approach can also be used to describe the use of these other products.
Traditionally tobacco use has been classified according to whether the smoker meets criteria for nicotine dependence in one of the two main diagnostic classifications: the DSM 122 (tobacco use disorder) and the ICD (tobacco dependence) 123 . The diagnosis of tobacco use disorder according to DSM-5 criteria requires the presence of at least 2 of 11 symptoms that have produced marked clinical impairment or distress within a 12-month period (Box 2 ). Of note, these symptoms are similar for all substance use disorder diagnoses and may not all be relevant to tobacco use disorder (such as failure to complete life roles). In the ICD-10, codes allow the identification of specific tobacco products used (cigarettes, chewing tobacco and other tobacco products).
Dependence can also be assessed as a continuous construct associated with higher levels of use, greater withdrawal and reduced likelihood of quitting. The level of dependence can be assessed with the Fagerström Test for Nicotine Dependence, a short questionnaire comprising six questions 124 (Box 2 ). A score of ≥4 indicates moderate to high dependence. As very limited time may be available in clinical consultations, the Heaviness of Smoking Index (HSI) was developed, which comprises two questions on the number of cigarettes smoked per day and how soon after waking the first cigarette is smoked 125 . The HSI can guide dosing for nicotine replacement therapy (NRT).
Other measures of cigarette dependence have been developed but are not used in the clinical setting, such as the Cigarette Dependence Scale 126 , Hooked on Nicotine Checklist 127 , Nicotine Dependence Syndrome Scale 128 , the Wisconsin Inventory of Smoking Dependence Motives (Brief) 129 and the Penn State Cigarette Dependence Index 130 . However, in practice, these are not often used, as the most important aspect is to screen for smoking and encourage all smokers to quit smoking regardless of their dependence status.
Box 2 DSM-5 criteria for tobacco use disorder and items of the Fagerström Test for nicotine dependence
DSM-5 (ref. 122 )
Taxonomic and diagnostic tool for tobacco use disorder published by the American Psychiatric Association.
A problematic pattern of tobacco use leading to clinically significant impairment or distress as manifested by at least two of the following, occurring within a 12-month period.
Tobacco often used in larger amounts or over a longer period of time than intended
A persistent desire or unsuccessful efforts to reduce or control tobacco use
A great deal of time spent in activities necessary to obtain or use tobacco
Craving, or a strong desire or urge to use tobacco
Recurrent tobacco use resulting in a failure to fulfil major role obligations at work, school or home
Continued tobacco use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of tobacco (for example, arguments with others about tobacco use)
Important social, occupational or recreational activities given up or reduced because of tobacco use
Recurrent tobacco use in hazardous situations (such as smoking in bed)
Tobacco use continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by tobacco use
Tolerance, defined by either of the following.
A need for markedly increased amounts of tobacco to achieve the desired effect
A markedly diminished effect with continued use of the same amount of tobacco
Withdrawal, manifesting as either of the following.
Withdrawal syndrome for tobacco
Tobacco (or a closely related substance, such as nicotine) taken to relieve or avoid withdrawal symptoms
Fagerström Test for Nicotine Dependence 124
A standard instrument for assessing the intensity of physical addiction to nicotine.
How soon after you wake up do you smoke your first cigarette?
Within 5 min (scores 3 points)
5 to 30 min (scores 2 points)
31 to 60 min (scores 1 point)
After 60 min (scores 0 points)
Do you find it difficult not to smoke in places where you should not, such as in church or school, in a movie, at the library, on a bus, in court or in a hospital?
Yes (scores 1 point)
No (scores 0 points)
Which cigarette would you most hate to give up; which cigarette do you treasure the most?
The first one in the morning (scores 1 point)
Any other one (scores 0 points)
How many cigarettes do you smoke each day?
10 or fewer (scores 0 points)
11 to 20 (scores 1 point)
21 to 30 (scores 2 points)
31 or more (scores 3 points)
Do you smoke more during the first few hours after waking up than during the rest of the day?
Do you still smoke if you are so sick that you are in bed most of the day or if you have a cold or the flu and have trouble breathing?
A score of 7–10 points is classified as highly dependent; 4–6 points is classified as moderately dependent; <4 points is classified as minimally dependent.
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Young people who do not start smoking cigarettes between 15 and 25 years of age have a very low risk of ever smoking 24 , 131 , 132 . This age group provides a critical opportunity to prevent cigarette smoking using effective, evidence-based strategies to prevent smoking initiation and reduce escalation from experimentation to regular use 131 , 132 , 133 , 134 , 135 .
Effective prevention of cigarette uptake requires a comprehensive package of cost-effective policies 134 , 136 , 137 to synergistically reduce the population prevalence of cigarette smoking 131 , 135 . These policies include high rates of tobacco taxation 30 , 134 , 137 , 138 , widespread and rigorously enforced smoke-free policies 139 , bans on tobacco advertising and promotions 140 , use of plain packaging and graphic warnings about the health risks of smoking 135 , 141 , mass media and peer-based education programmes to discourage smoking, and enforcement of laws against the sale of cigarettes to young people below the minimum legal purchase age 131 , 135 . These policies make cigarettes less available and affordable to young people. Moreover, these policies make it more difficult for young people to purchase cigarettes and make smoking a much less socially acceptable practice. Of note, these policies are typically mostly enacted in HICs, which may be related to the declining prevalence of smoking in these countries, compared with the prevalence in LMICs.
Pharmacotherapy
Three evidence-based classes of pharmacotherapy are available for smoking cessation: NRT (using nicotine-based patches, gum, lozenges, mini-lozenges, nasal sprays and inhalers), varenicline (a nAChR partial agonist), and bupropion (a noradrenaline/dopamine reuptake inhibitor that also inhibits nAChR function and is also used as an antidepressant). These FDA-approved and EMA-approved pharmacotherapies are cost-effective smoking cessation treatments that double or triple successful abstinence rates compared with no treatment or placebo controls 116 , 142 .
Combinations of pharmacotherapies are also effective for smoking cessation 116 , 142 . For example, combining NRTs (such as the steady-state nicotine patch and as-needed NRT such as gum or mini-lozenge) is more effective than a single form of NRT 116 , 142 , 143 . Combining NRT and varenicline is the most effective smoking cessation pharmacotherapy 116 , 142 , 143 . Combining FDA-approved pharmacotherapy with behavioural counselling further increases the likelihood of successful cessation 142 . Second-line pharmacotherapies (for example, nortriptyline) have some potential for smoking cessation, but their use is limited due to their tolerability profile.
All smokers should receive pharmacotherapy to help them quit smoking, except those in whom pharmacotherapy has insufficient evidence of effectiveness (among adolescents, smokeless tobacco users, pregnant women or light smokers) or those in whom pharmacotherapy is medically contraindicated 144 . Table 2 provides specific information regarding dosing and duration for each FDA-approved pharmacotherapy. Extended use of pharmacotherapy beyond the standard 12-week regimen after cessation is effective and should be considered 116 . Moreover, preloading pharmacotherapy (that is, initiating cessation medication in advance of a quit attempt), especially with the nicotine patch, is a promising treatment, although further studies are required to confirm efficacy.
Cytisine has been used for smoking cessation in Eastern Europe for a long time and is available in some countries (such as Canada) without prescription 145 . Cytisine is a partial agonist of nAChRs and its structure was the precursor for the development of varenicline 145 . Cytisine is at least as effective as some approved pharmacotherapies for smoking cessation, such as NRT 146 , 147 , 148 , and the role of cytisine in smoking cessation is likely to expand in the future, notably owing to its much lower cost than traditional pharmacotherapies. E-cigarettes also have the potential to be useful as smoking cessation devices 149 , 150 . The 2020 US Surgeon General’s Report concluded that there was insufficient evidence to promote cytisine or e-cigarettes as effective smoking cessation treatments, but in the UK its use is recommended for smoking cessation (see ref. 15 for regularly updated review).
Counselling and behavioural treatments
Psychosocial counselling significantly increases the likelihood of successful cessation, especially when combined with pharmacotherapy. Even a counselling session lasting only 3 minutes can help smokers quit 116 , although the 2008 US Public Health Service guidelines and the Preventive Services Task Force 151 each concluded that more intensive counselling (≥20 min per session) is more effective than less intensive counselling (<20 min per session). Higher smoking cessation rates are obtained by using behavioural change techniques that target associative and self-regulatory processes 152 . In addition, behavioural change techniques that will favour commitment, social reward and identity associated with changed behaviour seems associated with higher success rates 152 . Evidence-based counselling focuses on providing social support during treatment, building skills to cope with withdrawal and cessation, and problem-solving in challenging situations 116 , 153 . Effective counselling can be delivered by diverse providers (such as physicians, nurses, pharmacists, social workers, psychologists and certified tobacco treatment specialists) 116 .
Counselling can be delivered in a variety of modalities. In-person individual and group counselling are effective, as is telephone counselling (quit lines) 142 . Internet and text-based intervention seem to be effective in smoking cessation, especially when they are interactive and tailored to a smoker’s specific circumstances 142 . Over the past several years, the number of smoking cessation smartphone apps has increased, but there the evidence that the use of these apps significantly increases smoking cessation rates is not sufficient.
Contingency management (providing financial incentives for abstinence or engagement in treatment) has shown promising results 154 , 155 but its effects are not sustained once the contingencies are removed 155 , 156 . Other treatments such as hypnosis, acupuncture and laser treatment have not been shown to improve smoking cessation rates compared with placebo treatments 116 . Moreover, no solid evidence supports the use of conventional transcranial magnetic stimulation (TMS) for long-term smoking cessation 157 , 158 .
Although a variety of empirically supported smoking cessation interventions are available, more than two-thirds of adult smokers who made quit attempts in the USA during the past year did not use an evidence-based treatment and the rate is likely to be lower in many other countries 142 . This speaks to the need to increase awareness of, and access to, effective cessation aids among all smokers.
Brain stimulation
The insula (part of the frontal cortex) is a critical brain structure involved in cigarette craving and relapse 78 , 79 . The activity of the insula can be modulated using an innovative approach called deep insula/prefrontal cortex TMS (deep TMS), which is effective in helping people quit smoking 80 , 159 . This approach has now been approved by the FDA as an effective smoking cessation intervention 80 . However, although this intervention was developed and is effective for smoking cessation, the number of people with access to it is limited owing to the limited number of sites equipped and with trained personnel, and the cost of this intervention.
Quality of life
Generic instruments (such as the Short-Form (SF-36) Health Survey) can be used to evaluate quality of life (QOL) in smokers. People who smoke rate their QOL lower than people who do not smoke both before and after they become smokers 160 , 161 . QOL improves when smokers quit 162 . Mental health may also improve on quitting smoking 163 . Moreover, QOL is much poorer in smokers with tobacco-related diseases, such as chronic respiratory diseases and cancers, than in individuals without tobacco-related diseases 161 , 164 . The dimensions of QOL that show the largest decrements in people who smoke are those related to physical health, day-to-day activities and mental health such as depression 160 . Smoking also increases the risk of diabetes mellitus 165 , 166 , which is a major determinant of poor QOL for a wide range of conditions.
The high toll of premature death from cigarette smoking can obscure the fact that many of the diseases that cause these deaths also produce substantial disability in the years before death 1 . Indeed, death in smokers is typically preceded by several years of living with the serious disability and impairment of everyday activities caused by chronic respiratory disease, heart disease and cancer 2 . Smokers’ QOL in these years may also be adversely affected by the adverse effects of the medical treatments that they receive for these smoking-related diseases (such as major surgery and radiotherapy).
Expanding cessation worldwide
The major global challenge is to consider individual and population-based strategies that could increase the substantially low rates of adult cessation in most LMICs and indeed strategies to ensure that even in HICs, cessation continues to increase. In general, the most effective tools recommended by WHO to expand cessation are the same tools that can prevent smoking initiation, notably higher tobacco taxes, bans on advertising and promotion, prominent warning labels or plain packaging, bans on public smoking, and mass media and educational efforts 29 , 167 . The effective use of these policies, particularly taxation, lags behind in most LMICs compared with most HICs, with important exceptions such as Brazil 167 . Access to effective pharmacotherapies and counselling as well as support for co-existing mental health conditions would also be required to accelerate cessation in LMICs. This is particularly important as smokers living in LMICs often have no access to the full range of effective treatment options.
Regulating access to e-cigarettes
How e-cigarettes should be used is debated within the tobacco control field. In some countries (for example, the UK), the use of e-cigarettes as a cigarette smoking cessation aid and as a harm reduction strategy is supported, based on the idea that e-cigarette use will lead to much less exposure to toxic compounds than tobacco use, therefore reducing global harm. In other countries (for example, the USA), there is more concern with preventing the increased use of e-cigarettes by youths that may subsequently lead to smoking 25 , 26 . Regulating e-cigarettes in nuanced ways that enable smokers to access those products whilst preventing their uptake among youths is critical.
Regulating nicotine content in tobacco products
Reducing the nicotine content of cigarettes could potentially produce less addictive products that would allow a gradual reduction in the population prevalence of smoking. Some clinical studies have found no compensatory increase in smoking whilst providing access to low nicotine tobacco 168 . Future regulation may be implemented to gradually decrease the nicotine content of combustible tobacco and other nicotine products 169 , 170 , 171 .
Tobacco end games
Some individuals have proposed getting rid of commercial tobacco products this century or using the major economic disruption arising from the COVID-19 pandemic to accelerate the demise of the tobacco industry 172 , 173 . Some tobacco producers have even proposed this strategy as an internal goal, with the idea of switching to nicotine delivery systems that are less harmful ( Philip Morris International ). Some countries are moving towards such an objective; for example, in New Zealand, the goal that fewer than 5% of New Zealanders will be smokers in 2025 has been set (ref. 174 ). The tobacco end-game approach would overall be the best approach to reduce the burden of tobacco use on society, but it would require coordination of multiple countries and strong public and private consensus on the strategy to avoid a major expansion of the existing illicit market in tobacco products in some countries.
Innovative interventions
The COVID-19 pandemic has shown that large-scale investment in research can lead to rapid development of successful therapeutic interventions. By contrast, smoking cessation has been underfunded compared with the contribution that it makes to the global burden of disease. In addition, there is limited coordination between research teams and most studies are small-scale and often underpowered 79 . It is time to fund an ambitious, coordinated programme of research to test the most promising therapies based on an increased understanding of the neurobiological basis of smoking and nicotine addiction (Table 3 ). Many of those ideas have not yet been tested properly and this could be carried out by a coordinated programme of research at the international level.
GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397 , 2337–2360 (2021). This study summarizes the burden of disease induced by tobacco worldwide .
Google Scholar
West, R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol. Health 32 , 1018–1036 (2017).
PubMed PubMed Central Google Scholar
West, R. The multiple facets of cigarette addiction and what they mean for encouraging and helping smokers to stop. COPD 6 , 277–283 (2009).
PubMed Google Scholar
Fagerström, K. Determinants of tobacco use and renaming the FTND to the Fagerström test for cigarette dependence. Nicotine Tob. Res. 14 , 75–78 (2012).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung; preliminary report. Br. Med. J. 2 , 739–748 (1950).
CAS PubMed PubMed Central Google Scholar
Royal College of Physicians. Smoking and health. Summary of a report of the Royal College of Physicians of London on smoking in relation to cancer of the lung and other diseases (Pitman Medical Publishing, 1962).
Henningfield, J. E., Smith, T. T., Kleykamp, B. A., Fant, R. V. & Donny, E. C. Nicotine self-administration research: the legacy of Steven R. Goldberg and implications for regulation, health policy, and research. Psychopharmacology 233 , 3829–3848 (2016).
Le Foll, B. & Goldberg, S. R. Effects of nicotine in experimental animals and humans: an update on addictive properties. Hand. Exp. Pharmacol. https://doi.org/10.1007/978-3-540-69248-5_12 (2009).
Article Google Scholar
Proctor, R. N. The history of the discovery of the cigarette–lung cancer link: evidentiary traditions, corporate denial, global toll. Tob. Control. 21 , 87–91 (2012).
Hall, B. J. et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol. Biochem. Behav. 120 , 103–108 (2014).
Musso, F. et al. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology 191 , 159–169 (2007).
CAS PubMed Google Scholar
Goriounova, N. A. & Mansvelder, H. D. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb. Perspect. Med. 2 , a012120 (2012).
Fagerström, K. O. & Bridgman, K. Tobacco harm reduction: the need for new products that can compete with cigarettes. Addictive Behav. 39 , 507–511 (2014).
Hartmann-Boyce, J. et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 9 , CD010216 (2021).
Jha, P. The hazards of smoking and the benefits of cessation: a critical summation of the epidemiological evidence in high-income countries. eLife https://doi.org/10.7554/eLife.49979 (2020).
Article PubMed PubMed Central Google Scholar
Palipudi, K. M. et al. Social determinants of health and tobacco use in thirteen low and middle income countries: evidence from Global Adult Tobacco Survey. PLoS ONE 7 , e33466 (2012).
Goodwin, R. D., Pagura, J., Spiwak, R., Lemeshow, A. R. & Sareen, J. Predictors of persistent nicotine dependence among adults in the United States. Drug Alcohol. Depend. 118 , 127–133 (2011).
Weinberger, A. H. et al. Cigarette use is increasing among people with illicit substance use disorders in the United States, 2002-14: emerging disparities in vulnerable populations. Addiction 113 , 719–728 (2018).
Evans-Polce, R. J., Kcomt, L., Veliz, P. T., Boyd, C. J. & McCabe, S. E. Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates. Am. J. Psychiatry 177 , 1073–1081 (2020).
Hassan, A. N. & Le Foll, B. Survival probabilities and predictors of major depressive episode incidence among individuals with various types of substance use disorders. J. Clin. Psychiatry https://doi.org/10.4088/JCP.20m13637 (2021).
Article PubMed Google Scholar
Smith, P. H., Mazure, C. M. & McKee, S. A. Smoking and mental illness in the U.S. population. Tob. Control. 23 , e147–e153 (2014).
Bourgault, Z., Rubin-Kahana, D. S., Hassan, A. N., Sanches, M. & Le Foll, B. Multiple substance use disorders and self-reported cognitive function in U.S. adults: associations and sex-differences in a nationally representative sample. Front. Psychiatry https://doi.org/10.3389/fpsyt.2021.797578 (2022).
Reitsma, M. B. et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990-2019. Lancet Public Health 6 , e472–e481 (2021).
Warner, K. E. How to think–not feel–about tobacco harm reduction. Nicotine Tob. Res. 21 , 1299–1309 (2019).
Soneji, S. et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 171 , 788–797 (2017).
Levy, D. T. et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob. Control. 28 , 629–635 (2019).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking — 50 years of progress: a report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Jha, P. & Peto, R. Global effects of smoking, of quitting, and of taxing tobacco. N. Engl. J. Med. 370 , 60–68 (2014). This review covers the impact of tobacco, of quitting smoking and the importance of taxation to impact prevalence of smoking .
Jha, P. & Peto., R. in Tobacco Tax Reform: At the Crossroads of Health and Development . (eds Marquez, P. V. & Moreno-Dodson, B.) 55–72 (World Bank Group, 2017).
Jha, P. et al. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 368 , 341–350 (2013).
Banks, E. et al. Tobacco smoking and all-cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Med. 13 , 38 (2015).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381 , 133–141 (2013).
Jha, P. et al. A nationally representative case-control study of smoking and death in India. N. Engl. J. Med. 358 , 1137–1147 (2008).
Chan, E. D. et al. Tobacco exposure and susceptibility to tuberculosis: is there a smoking gun? Tuberculosis 94 , 544–550 (2014).
Wang, M. G. et al. Association between tobacco smoking and drug-resistant tuberculosis. Infect. Drug Resist. 11 , 873–887 (2018).
Jha, P. et al. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet 368 , 367–370 (2006).
Jha, P., Gelband, H, Irving, H. & Mishra, S. in Reducing Social Inequalities in Cancer: Evidence and Priorities for Research (eds Vaccarella, S et al.) 161–166 (IARC, 2018).
Jha, P. Expanding smoking cessation world-wide. Addiction 113 , 1392–1393 (2018).
Jha, P. Avoidable global cancer deaths and total deaths from smoking. Nat. Rev. Cancer 9 , 655–664 (2009).
Wittenberg, R. E., Wolfman, S. L., De Biasi, M. & Dani, J. A. Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction. Neuropharmacology 177 , 108256 (2020).
Boulter, J. et al. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc. Natl Acad. Sci. USA 84 , 7763–7767 (1987).
Couturier, S. et al. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 5 , 847–856 (1990).
Picciotto, M. R., Addy, N. A., Mineur, Y. S. & Brunzell, D. H. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol. 84 , 329–342 (2008).
Changeux, J. P. Structural identification of the nicotinic receptor ion channel. Trends Neurosci. 41 , 67–70 (2018).
McKay, B. E., Placzek, A. N. & Dani, J. A. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 74 , 1120–1133 (2007).
Wonnacott, S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 20 , 92–98 (1997).
Wooltorton, J. R., Pidoplichko, V. I., Broide, R. S. & Dani, J. A. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 23 , 3176–3185 (2003).
Gipson, C. D. & Fowler, C. D. Nicotinic receptors underlying nicotine dependence: evidence from transgenic mouse models. Curr. Top. Behav. Neurosci. 45 , 101–121 (2020).
Hamouda, A. K. et al. Potentiation of (α4)2(β2)3, but not (α4)3(β2)2, nicotinic acetylcholine receptors reduces nicotine self-administration and withdrawal symptoms. Neuropharmacology 190 , 108568 (2021).
Lallai, V. et al. Nicotine acts on cholinergic signaling mechanisms to directly modulate choroid plexus function. eNeuro https://doi.org/10.1523/ENEURO.0051-19.2019 (2019).
Benwell, M. E., Balfour, D. J. & Anderson, J. M. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J. Neurochem. 50 , 1243–1247 (1988).
Perry, D. C., Davila-Garcia, M. I., Stockmeier, C. A. & Kellar, K. J. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharmacol. Exp. Ther. 289 , 1545–1552 (1999).
Marks, M. J. et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 12 , 2765–2784 (1992).
Le Foll, B. et al. Impact of short access nicotine self-administration on expression of α4β2* nicotinic acetylcholine receptors in non-human primates. Psychopharmacology 233 , 1829–1835 (2016).
Meyers, E. E., Loetz, E. C. & Marks, M. J. Differential expression of the beta4 neuronal nicotinic receptor subunit affects tolerance development and nicotinic binding sites following chronic nicotine treatment. Pharmacol. Biochem. Behav. 130 , 1–8 (2015).
Zhao-Shea, R., Liu, L., Pang, X., Gardner, P. D. & Tapper, A. R. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr. Biol. 23 , 2327–2335 (2013).
Jensen, K. P., Valentine, G., Gueorguieva, R. & Sofuoglu, M. Differential effects of nicotine delivery rate on subjective drug effects, urges to smoke, heart rate and blood pressure in tobacco smokers. Psychopharmacology 237 , 1359–1369 (2020).
Villanueva, H. F., James, J. R. & Rosecrans, J. A. Evidence of pharmacological tolerance to nicotine. NIDA Res. Monogr. 95 , 349–350 (1989).
Corrigall, W. A., Coen, K. M. & Adamson, K. L. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 653 , 278–284 (1994).
Nisell, M., Nomikos, G. G., Hertel, P., Panagis, G. & Svensson, T. H. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 22 , 369–381 (1996).
Rice, M. E. & Cragg, S. J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 7 , 583–584 (2004).
Mameli-Engvall, M. et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50 , 911–921 (2006).
Picciotto, M. R., Higley, M. J. & Mineur, Y. S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76 , 116–129 (2012).
Le Foll, B. et al. Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-+-PHNO PET study in humans. Neuropsychopharmacology 39 , 415–424 (2014). This brain imaging study identified the brain areas in which smoking elevates dopamine levels .
Maskos, U. et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436 , 103–107 (2005). This article discusses the implication of the β 2 - containing nAChRs in the VTA in mammalian cognitive function .
Picciotto, M. R. et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391 , 173–177 (1998). This article discusses the implication of the β 2 - containing nAChRs in addictive effects of nicotine .
Fowler, C. D., Lu, Q., Johnson, P. M., Marks, M. J. & Kenny, P. J. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471 , 597–601 (2011). This article discusses the implication of the α5 nicotinic receptor located in the MHb in a mechanism mediating the aversive effects of nicotine .
Elayouby, K. S. et al. α3* Nicotinic acetylcholine receptors in the habenula-interpeduncular nucleus circuit regulate nicotine intake. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.0127-19.2020 (2020).
Ables, J. L. et al. Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference. Proc. Natl Acad. Sci. USA 114 , 13012–13017 (2017).
Frahm, S. et al. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70 , 522–535 (2011).
Jackson, K. J. et al. Role of α5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther. 334 , 137–146 (2010).
Tuesta, L. M. et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat. Neurosci. 20 , 708–716 (2017).
Salas, R., Pieri, F. & De Biasi, M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J. Neurosci. 24 , 10035–10039 (2004).
Salas, R., Sturm, R., Boulter, J. & De Biasi, M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 29 , 3014–3018 (2009).
Jackson, K. J., Martin, B. R., Changeux, J. P. & Damaj, M. I. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 325 , 302–312 (2008).
Le Foll, B. et al. Translational strategies for therapeutic development in nicotine addiction: rethinking the conventional bench to bedside approach. Prog. Neuropsychopharmacol. Biol. Psychiatry 52 , 86–93 (2014).
Naqvi, N. H., Rudrauf, D., Damasio, H. & Bechara, A. Damage to the insula disrupts addiction to cigarette smoking. Science 315 , 531–534 (2007). This article discusses the implication of the insular cortex in tobacco addiction .
Ibrahim, C. et al. The insula: a brain stimulation target for the treatment of addiction. Front. Pharmacol. 10 , 720 (2019).
Zangen, A. et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 20 , 397–404 (2021). This study validated the utility of deep insula/prefrontal cortex rTMS for smoking cessation .
Le Foll, B., Forget, B., Aubin, H. J. & Goldberg, S. R. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict. Biol. 13 , 239–252 (2008).
Kodas, E., Cohen, C., Louis, C. & Griebel, G. Cortico-limbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology 194 , 161–171 (2007).
Forget, B. et al. Noradrenergic α1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35 , 1751–1760 (2010).
Garrett, B. E., Dube, S. R., Babb, S. & McAfee, T. Addressing the social determinants of health to reduce tobacco-related disparities. Nicotine Tob. Res. 17 , 892–897 (2015).
Polanska, K., Znyk, M. & Kaleta, D. Susceptibility to tobacco use and associated factors among youth in five central and eastern European countries. BMC Public Health 22 , 72 (2022).
Volkow, N. D. Personalizing the treatment of substance use disorders. Am. J. Psychiatry 177 , 113–116 (2020).
Li, M. D., Cheng, R., Ma, J. Z. & Swan, G. E. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 98 , 23–31 (2003).
Carmelli, D., Swan, G. E., Robinette, D. & Fabsitz, R. Genetic influence on smoking–a study of male twins. N. Engl. J. Med. 327 , 829–833 (1992).
Broms, U., Silventoinen, K., Madden, P. A. F., Heath, A. C. & Kaprio, J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res. Hum. Genet. 9 , 64–72 (2006).
Kendler, K. S., Thornton, L. M. & Pedersen, N. L. Tobacco consumption in Swedish twins reared apart and reared together. Arch. Gen. Psychiat 57 , 886–892 (2000).
Saccone, N. L. et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 69 , 6848–6856 (2009).
Bierut, L. J. et al. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165 , 1163–1171 (2008). This study demonstrates that nAChR gene variants are important in nicotine addiction .
Bierut, L. J. et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum. Mol. Genet. 16 , 24–35 (2007).
Berrettini, W. et al. α-5/α-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatry 13 , 368–373 (2008).
Sherva, R. et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction 103 , 1544–1552 (2008).
Thorgeirsson, T. E. et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 42 , 448–453 (2010).
Ray, R., Tyndale, R. F. & Lerman, C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J. Neurogenet. 23 , 252–261 (2009).
Bergen, A. W. et al. Drug metabolizing enzyme and transporter gene variation, nicotine metabolism, prospective abstinence, and cigarette consumption. PLoS ONE 10 , e0126113 (2015).
Mwenifumbo, J. C. et al. Identification of novel CYP2A6*1B variants: the CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clin. Pharmacol. Ther. 83 , 115–121 (2008).
Raunio, H. & Rahnasto-Rilla, M. CYP2A6: genetics, structure, regulation, and function. Drug Metab. Drug Interact. 27 , 73–88 (2012).
CAS Google Scholar
Patterson, F. et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin. Pharmacol. Ther. 84 , 320–325 (2008).
Rodriguez, S. et al. Combined analysis of CHRNA5, CHRNA3 and CYP2A6 in relation to adolescent smoking behaviour. J. Psychopharmacol. 25 , 915–923 (2011).
Strasser, A. A., Malaiyandi, V., Hoffmann, E., Tyndale, R. F. & Lerman, C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob. Res. 9 , 511–518 (2007).
Liakoni, E. et al. Effects of nicotine metabolic rate on withdrawal symptoms and response to cigarette smoking after abstinence. Clin. Pharmacol. Ther. 105 , 641–651 (2019).
Di Ciano, P. et al. Influence of nicotine metabolism ratio on [11C]-(+)-PHNO PET binding in tobacco smokers. Int. J. Neuropsychopharmacol. 21 , 503–512 (2018).
Butler, K. et al. Impact of Cyp2a6 activity on nicotine reinforcement and cue-reactivity in daily smokers. Nicotine Tob. Res. https://doi.org/10.1093/ntr/ntab064 (2021).
Benowitz, N. L., Swan, G. E., Jacob, P. 3rd, Lessov-Schlaggar, C. N. & Tyndale, R. F. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin. Pharmacol. Ther. 80 , 457–467 (2006).
Liu, M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 51 , 237–244 (2019).
McKay, J. D. et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet. 49 , 1126–1132 (2017).
Chukwueke, C. C. et al. The CB1R rs2023239 receptor gene variant significantly affects the reinforcing effects of nicotine, but not cue reactivity, in human smokers. Brain Behav. 11 , e01982 (2021).
Ahrens, S. et al. Modulation of nicotine effects on selective attention by DRD2 and CHRNA4 gene polymorphisms. Psychopharmacology 232 , 2323–2331 (2015).
Harrell, P. T. et al. Dopaminergic genetic variation moderates the effect of nicotine on cigarette reward. Psychopharmacology 233 , 351–360 (2016).
Lerman, C. et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 31 , 231–242 (2006).
Le Foll, B., Gallo, A., Le Strat, Y., Lu, L. & Gorwood, P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav. Pharmacol. 20 , 1–17 (2009).
Chukwueke, C. C. et al. Exploring the role of the Ser9Gly (rs6280) dopamine D3 receptor polymorphism in nicotine reinforcement and cue-elicited craving. Sci. Rep. 10 , 4085 (2020).
The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff A clinical practice guideline for treating tobacco use and dependence: 2008 update: a U.S. Public Health Service report. Am. J. Prev. Med. 35 , 158–176 (2008).
Hackshaw, A., Morris, J. K., Boniface, S., Tang, J. L. & Milenković, D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 360 , j5855 (2018).
Qin, W. et al. Light cigarette smoking increases risk of all-cause and cause-specific mortality: findings from the NHIS cohort study. Int. J. Env. Res. Public Health https://doi.org/10.3390/ijerph17145122 (2020).
Rodu, B. & Plurphanswat, N. Mortality among male smokers and smokeless tobacco users in the USA. Harm Reduct. J. 16 , 50 (2019).
Kasza, K. A. et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N. Engl. J. Med. 376 , 342–353 (2017).
Richardson, A., Xiao, H. & Vallone, D. M. Primary and dual users of cigars and cigarettes: profiles, tobacco use patterns and relevance to policy. Nicotine Tob. Res. 14 , 927–932 (2012).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders 5th edn (American Psychiatric Association, 2013).
World Health Organization. Tobacco fact sheet. WHO https://www.who.int/news-room/fact-sheets/detail/tobacco (2021).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C. & Fagerström, K. O. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br. J. Addict. 86 , 1119–1127 (1991).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., Rickert, W. & Robinson, J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 84 , 791–799 (1989).
Etter, J. F., Le Houezec, J. & Perneger, T. V. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology 28 , 359–370 (2003).
DiFranza, J. R. et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch. Pediatr. Adolesc. Med. 156 , 397–403 (2002).
Shiffman, S., Waters, A. & Hickcox, M. The Nicotine Dependence Syndrome Scale: a multidimensional measure of nicotine dependence. Nicotine Tob. Res. 6 , 327–348 (2004).
Smith, S. S. et al. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob. Res. 12 , 489–499 (2010).
Foulds, J. et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob. Res. 17 , 186–192 (2015).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing tobacco use among youth and young adults: a report of the Surgeon General (Centers for Disease Control and Prevention, 2012).
World Health Organization. Tobacco control to improve child health and development. Thematic brief (WHO, 2021).
Lantz, P. M. et al. Investing in youth tobacco control: a review of smoking prevention and control strategies. Tob. Control. 9 , 47–63 (2000).
Leão, T., Kunst, A. E. & Perelman, J. Cost-effectiveness of tobacco control policies and programmes targeting adolescents: a systematic review. Eur. J. Public Health 28 , 39–43 (2018).
Royal College of Physicians. Smoking and health 2021: a coming of age for tobacco control? (RCP, 2021).
Higashi, H. et al. Cost effectiveness of tobacco control policies in Vietnam: the case of population-level interventions. Appl. Health Econ. Health Policy 9 , 183–196 (2011).
Ranson, M. K., Jha, P., Chaloupka, F. J. & Nguyen, S. N. Global and regional estimates of the effectiveness and cost-effectiveness of price increases and other tobacco control policies. Nicotine Tob. Res. 4 , 311–319 (2002).
International Agency for Research on Cancer. I ARC Handbooks of Cancer Prevention: Tobacco control Vol. 14 (IARC, 2011).
Frazer, K. et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. 2 , CD005992 (2016).
Hoffman, S. J. & Tan, C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC Public Health 15 , 744 (2015).
McNeill, A. et al. Tobacco packaging design for reducing tobacco use. Cochrane Database Syst. Rev. 4 , CD011244 (2017).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking cessation: a report of the Surgeon General (Department of Health and Human Services, 2020).
Lindson, N. et al. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 4 , CD013308 (2019).
Krist, A. H. et al. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA 325 , 265–279 (2021).
Tutka, P. & Zatonski, W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacol. Rep. 58 , 777–798 (2006).
Courtney, R. J. et al. Effect of cytisine vs varenicline on smoking cessation: a randomized clinical trial. JAMA 326 , 56–64 (2021).
Walker, N. et al. Cytisine versus nicotine for smoking cessation. N. Engl. J. Med. 371 , 2353–2362 (2014). This study validated the utility of cytisine for smoking cessation .
West, R. et al. Placebo-controlled trial of cytisine for smoking cessation. N. Engl. J. Med. 365 , 1193–1200 (2011).
Hajek, P. et al. E-cigarettes compared with nicotine replacement therapy within the UK Stop Smoking Services: the TEC RCT. Health Technol. Assess. 23 , 1–82 (2019).
Walker, N. et al. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Respir. Med. 8 , 54–64 (2020).
Siu, A. L., U.S. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 163 , 622–634 (2015).
Black, N. et al. Behaviour change techniques associated with smoking cessation in intervention and comparator groups of randomized controlled trials: a systematic review and meta-regression. Addiction 115 , 2008–2020 (2020).
Center for Substance Abuse and Treatment. Detoxification and Substance Abuse Treatment (Center for Substance Abuse and Treatment, 2006).
Cahill, K., Hartmann-Boyce, J. & Perera, R. Incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub5 (2015).
Secades-Villa, R., Aonso-Diego, G., García-Pérez, Á. & González-Roz, A. Effectiveness of contingency management for smoking cessation in substance users: a systematic review and meta-analysis. J. Consult. Clin. Psychol. 88 , 951–964 (2020).
Cahill, K. & Perera, R. Competitions and incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub4 (2011).
Trojak, B. et al. Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: a randomized controlled trial. Brain Stimul. 8 , 1168–1174 (2015).
Wing, V. C. et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 6 , 221–230 (2013).
Dinur-Klein, L. et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76 , 742–749 (2014).
Goldenberg, M., Danovitch, I. & IsHak, W. W. Quality of life and smoking. Am. J. Addict. 23 , 540–562 (2014).
Heikkinen, H., Jallinoja, P., Saarni, S. I. & Patja, K. The impact of smoking on health-related and overall quality of life: a general population survey in Finland. Nicotine Tob. Res. 10 , 1199–1207 (2008).
Moayeri, F., Hsueh, Y. A., Dunt, D. & Clarke, P. Smoking cessation and quality of life: insights from analysis of longitudinal Australian data, an application for economic evaluations. Value Health 24 , 724–732 (2021).
Taylor, G. M. et al. Smoking cessation for improving mental health. Cochrane Database Syst. Rev. 3 , CD013522 (2021).
López-Nicolás, Á., Trapero-Bertran, M. & Muñoz, C. Smoking, health-related quality of life and economic evaluation. Eur. J. Health Econ. 19 , 747–756 (2018).
Morris, A. Linking nicotine addiction and T2DM. Nat. Rev. Endocrinol. 16 , 6 (2020).
Willi, C., Bodenmann, P., Ghali, W. A., Faris, P. D. & Cornuz, J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama 298 , 2654–2664 (2007).
World Health Organization. WHO report on the global tobacco epidemic (WHO, 2019).
Donny, E. C. et al. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 373 , 1340–1349 (2015). This study tested the impact of reducing the quantity of nicotine present in cigarettes on smoking .
Benowitz, N. L. & Henningfield, J. E. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N. Engl. J. Med. 331 , 123–125 (1994).
Benowitz, N. L. & Henningfield, J. E. Reducing the nicotine content to make cigarettes less addictive. Tob. Control. 22 , i14–i17 (2013).
Gottlieb, S. & Zeller, M. A nicotine-focused framework for public health. N. Engl. J. Med. 377 , 1111–1114 (2017).
Hall, W. & West, R. Thinking about the unthinkable: a de facto prohibition on smoked tobacco products. Addiction 103 , 873–874 (2008).
Ioannidis, J. P. A. & Jha, P. Does the COVID-19 pandemic provide an opportunity to eliminate the tobacco industry? Lancet Glob. Health 9 , e12–e13 (2021).
Smokefree. Smokefree 2025. Smokefree https://www.smokefree.org.nz/smokefree-in-action/smokefree-aotearoa-2025 (2021).
Morgan, C. J., Das, R. K., Joye, A., Curran, H. V. & Kamboj, S. K. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict. Behav. 38 , 2433–2436 (2013).
Elsaid, S., Kloiber, S. & Le Foll, B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 167 , 25–75 (2019).
Butler, K. & Le Foll, B. Novel therapeutic and drug development strategies for tobacco use disorder: endocannabinoid modulation. Expert Opin. Drug Discov. 15 , 1065–1080 (2020).
D’Souza, D. C. et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 6 , 35–45 (2019).
Robinson, J. D. et al. Pooled analysis of three randomized, double-blind, placebo controlled trials with rimonabant for smoking cessation. Addict. Biol. 23 , 291–303 (2018).
Gueye, A. B. et al. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int. J. Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyw068 (2016).
Yammine, L. et al. Exenatide adjunct to nicotine patch facilitates smoking cessation and may reduce post-cessation weight gain: a pilot randomized controlled trial. Nicotine Tob. Res. 23 , 1682–1690 (2021).
Eren-Yazicioglu, C. Y., Yigit, A., Dogruoz, R. E. & Yapici-Eser, H. Can GLP-1 be a target for reward system related disorders? A qualitative synthesis and systematic review analysis of studies on palatable food, drugs of abuse, and alcohol. Front. Behav. Neurosci. 14 , 614884 (2020).
Vanderkam, P. et al. Effectiveness of drugs acting on adrenergic receptors in the treatment for tobacco or alcohol use disorders: systematic review and meta-analysis. Addiction 116 , 1011–1020 (2021).
Sokoloff, P. & Le Foll, B. The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 45 , 2–19 (2017).
David, S. P., Lancaster, T., Stead, L. F., Evins, A. E. & Prochaska, J. J. Opioid antagonists for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003086.pub3 (2013).
Ray, L. A. et al. Efficacy of combining varenicline and naltrexone for smoking cessation and drinking reduction: a randomized clinical trial. Am. J. Psychiatry 178 , 818–828 (2021).
Mooney, M. E. et al. Bupropion and naltrexone for smoking cessation: a double-blind randomized placebo-controlled clinical trial. Clin. Pharmacol. Ther. 100 , 344–352 (2016).
Justinova, Z., Le Foll, B., Redhi, G. H., Markou, A. & Goldberg, S. R. Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology 233 , 1791–1800 (2016).
Le Foll, B., Wertheim, C. E. & Goldberg, S. R. Effects of baclofen on conditioned rewarding and discriminative stimulus effects of nicotine in rats. Neurosci. Lett. 443 , 236–240 (2008).
Franklin, T. R. et al. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol. Depend. 103 , 30–36 (2009).
Lotfy, N., Elsawah, H. & Hassan, M. Topiramate for smoking cessation: systematic review and meta-analysis. Tob. Prev. Cessat. 6 , 14 (2020).
Shanahan, W. R., Rose, J. E., Glicklich, A., Stubbe, S. & Sanchez-Kam, M. Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine Tob. Res. 19 , 944–951 (2017).
Higgins, G. A., Fletcher, P. J. & Shanahan, W. R. Lorcaserin: a review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol. Ther. 205 , 107417 (2020).
Stead, L. F. & Lancaster, T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD005231.pub2 (2007).
Download references
Acknowledgements
B.Le F. is supported by a clinician-scientist award from the Department of Family and Community Medicine at the University of Toronto and the Addiction Psychiatry Chair from the University of Toronto. The funding bodies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The authors thank H. Fu (University of Toronto) for assistance with Figs 1–3.
Author information
Authors and affiliations.
Translational Addiction Research Laboratory, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada
Bernard Le Foll
Departments of Family and Community Medicine, Psychiatry, Pharmacology and Toxicology, University of Toronto, Toronto, Ontario, Canada
Department of Medicine, University of Wisconsin, Madison, WI, USA
Megan E. Piper
University of Wisconsin Center for Tobacco Research and Intervention, Madison, WI, USA
Department of Neurobiology and Behaviour, University of California Irvine, Irvine, CA, USA
Christie D. Fowler
Section for Preventive Cardiology, Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway
Serena Tonstad
Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA
Laura Bierut
Institute of Mental Health, Peking University Sixth Hospital, Peking University, Beijing, China
National Institute on Drug Dependence, Peking University Health Science Center, Beijing, China
Centre for Global Health Research, Unity Health Toronto, University of Toronto, Toronto, Ontario, Canada
- Prabhat Jha
National Centre for Youth Substance Use Research, The University of Queensland, St Lucia, Queensland, Australia
Wayne D. Hall
Queensland Alliance for Environmental Health Sciences, The University of Queensland, Woolloongabba, Queensland, Australia
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (B.Le F.); Epidemiology (P.J. and W.D.H.); Mechanisms/pathophysiology (C.D.F., L.B., L.L. and B.Le F.); Diagnosis, screening and prevention (P.J., M.E.P., S.T. and B.Le F.); Management (M.E.P., S.T., W.D.H., L.L. and B.Le F.); Quality of life (P.J. and W.D.H.); Outlook (all); Conclusions (all). All authors contributed substantially to the review and editing of the manuscript.
Corresponding author
Correspondence to Bernard Le Foll .
Ethics declarations
Competing interests.
B.Le F. has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator-initiated projects. B.Le F. has received some in-kind donations of cannabis product from Aurora and medication donation from Pfizer and Bioprojet and was provided a coil for TMS study from Brainsway. B.Le F. has obtained industry funding from Canopy (through research grants handled by CAMH or the University of Toronto), Bioprojet, ACS, Indivior and Alkermes. B.Le F. has received in-kind donations of nabiximols from GW Pharma for past studies funded by CIHR and NIH. B.Le F. has been an advisor to Shinoghi. S.T. has received honoraria from Pfizer the manufacturer of varenicline for lectures and advice. All other authors declare no competing interests.
Peer review
Peer reviewer information.
Nature Reviews Disease Primers thanks Jamie Brown, Elisardo Iglesias and Ming Li for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CTRI website: https://d3futrf33lk36a.cloudfront.net/wp-content/uploads/sites/240/2019/04/Meds-Chart.pdf
Philip Morris International: https://www.pmi.com/
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Le Foll, B., Piper, M.E., Fowler, C.D. et al. Tobacco and nicotine use. Nat Rev Dis Primers 8 , 19 (2022). https://doi.org/10.1038/s41572-022-00346-w
Download citation
Accepted : 07 February 2022
Published : 24 March 2022
DOI : https://doi.org/10.1038/s41572-022-00346-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Integrated mrna- and mirna-sequencing analyses unveil the underlying mechanism of tobacco pollutant-induced developmental toxicity in zebrafish embryos.
- Jiasheng Chen
- Xiaoping Zhong
Journal of Translational Medicine (2024)
Influence of substance use on male reproductive health and offspring outcomes
- Jamie O. Lo
- Jason C. Hedges
- Charles A. Easley
Nature Reviews Urology (2024)
Reductions in smoking due to ratification of the Framework Convention for Tobacco Control in 171 countries
- Guillermo Paraje
- Mauricio Flores Muñoz
Nature Medicine (2024)
Impact of benzoic acid and 2,2’-methylenebis (6-tert-butyl-4-methylphenol) on the metabolome of flue-cured tobacco and rhizosphere microbial communities: implications for continuous cropping obstacles
- Qiulian Peng
- Jiayan Zhang
Plant and Soil (2024)
Nicotinic regulation of microglia: potential contributions to addiction
- Alexa R. Soares
- Marina R. Picciotto
Journal of Neural Transmission (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Alcoholism and Alcohol Abuse
Relaxation techniques for stress relief.
- Emotional Eating and How to Stop It
Choosing an Alcohol Rehab Treatment Program
Staying social when you quit drinking.
- Vaping: The Health Risks and How to Quit
Women and Alcohol
- Binge Drinking: What it is, the Effects, and How to Stop
- Online Therapy: Is it Right for You?
- Mental Health
- Health & Wellness
- Children & Family
- Relationships
Are you or someone you know in crisis?
- Bipolar Disorder
- Eating Disorders
- Grief & Loss
- Personality Disorders
- PTSD & Trauma
- Schizophrenia
- Therapy & Medication
- Exercise & Fitness
- Healthy Eating
- Well-being & Happiness
- Weight Loss
- Work & Career
- Illness & Disability
- Heart Health
- Childhood Issues
- Learning Disabilities
- Family Caregiving
- Teen Issues
- Communication
- Emotional Intelligence
- Love & Friendship
- Domestic Abuse
- Healthy Aging
- Aging Issues
- Alzheimer’s Disease & Dementia
- Senior Housing
- End of Life
- Meet Our Team
Why is quitting so hard?
Your personal stop smoking plan, identify your smoking triggers, coping with nicotine withdrawal symptoms, manage cigarette cravings, preventing weight gain after you stop smoking, medication and therapy to help you quit, what to do if you slip or relapse, helping a loved one to stop smoking, how to quit smoking.
Ready to stop smoking? These tips will help you kick the cigarette habit for good.

We all know the health risks of smoking, but that doesn’t make it any easier to kick the habit. Whether you’re an occasional teen smoker or a lifetime pack-a-day smoker, quitting can be really tough.
Smoking tobacco is both a physical addiction and a psychological habit. The nicotine from cigarettes provides a temporary—and addictive—high. Eliminating that regular fix of nicotine causes your body to experience physical withdrawal symptoms and cravings. Because of nicotine’s “feel good” effect on the brain, you may turn to cigarettes as a quick and reliable way to boost your outlook, relieve stress, and unwind. Smoking can also be a way of coping with depression, anxiety, or even boredom. Quitting means finding different, healthier ways to cope with those feelings.
Smoking is also ingrained as a daily ritual. It may be an automatic response for you to smoke a cigarette with your morning coffee, while taking a break at work or school, or on your commute home at the end of a hectic day. Or maybe your friends, family, or colleagues smoke, and it’s become part of the way you relate with them.
To successfully stop smoking, you’ll need to address both the addiction and the habits and routines that go along with it. But it can be done. With the right support and quit plan, any smoker can kick the addiction—even if you’ve tried and failed multiple times before.
Speak to a Licensed Therapist
BetterHelp is an online therapy service that matches you to licensed, accredited therapists who can help with depression, anxiety, relationships, and more. Take the assessment and get matched with a therapist in as little as 48 hours.
While some smokers successfully quit by going cold turkey, most people do better with a tailored plan to keep themselves on track. A good quit plan addresses both the short-term challenge of stopping smoking and the long-term challenge of preventing relapse. It should also be tailored to your specific needs and smoking habits.
Questions to ask yourself
Take the time to think of what kind of smoker you are, which moments of your life call for a cigarette, and why. This will help you to identify which tips, techniques, or therapies may be most beneficial for you.
Are you a very heavy smoker (more than a pack a day)? Or are you more of a social smoker? Would a simple nicotine patch do the job?
Are there certain activities, places, or people you associate with smoking? Do you feel the need to smoke after every meal or whenever you break for coffee?
Do you reach for cigarettes when you’re feeling stressed or down? Or is your cigarette smoking linked to other addictions, such as alcohol or gambling ?
Start your stop smoking plan with START
S = Set a quit date.
Choose a date within the next two weeks, so you have enough time to prepare without losing your motivation to quit. If you mainly smoke at work, quit on the weekend, so you have a few days to adjust to the change.
T = Tell family, friends, and co-workers that you plan to quit.
Let your friends and family in on your plan to quit smoking and tell them you need their support and encouragement to stop. Look for a quit buddy who wants to stop smoking as well. You can help each other get through the rough times.
A = Anticipate and plan for the challenges you’ll face while quitting.
Most people who begin smoking again do so within the first three months. You can help yourself make it through by preparing ahead for common challenges, such as nicotine withdrawal and cigarette cravings.
R = Remove cigarettes and other tobacco products from your home, car, and work.
Throw away all of your cigarettes, lighters, ashtrays, and matches. Wash your clothes and freshen up anything that smells like smoke. Shampoo your car, clean your drapes and carpet, and steam your furniture.
T = Talk to your doctor about getting help to quit.
Your doctor can prescribe medication to help with withdrawal symptoms. If you can’t see a doctor, you can get many products over the counter at your local pharmacy, including nicotine patches, lozenges, and gum.
One of the best things you can do to help yourself quit is to identify the things that make you want to smoke, including specific situations, activities, feelings, and people.
Keep a craving journal
A craving journal can help you zero in on your patterns and triggers. For a week or so leading up to your quit date, keep a log of your smoking. Note the moments in each day when you crave a cigarette:
- What time was it?
- How intense was the craving (on a scale of 1-10)?
- What were you doing?
- Who were you with?
- How were you feeling?
- How did you feel after smoking?
Do you smoke to relieve unpleasant feelings?
Many of us smoke to manage unpleasant feelings such as stress, depression, loneliness, and anxiety. When you have a bad day, it can seem like cigarettes are your only friend. As much comfort as cigarettes provide, though, it’s important to remember that there are healthier and more effective ways to keep unpleasant feelings in check. These may include exercising, meditating, relaxation strategies , or simple breathing exercises.
For many people, an important aspect of giving up smoking is to find alternate ways to handle these difficult feelings without turning to cigarettes. Even when cigarettes are no longer a part of your life, the painful and unpleasant feelings that may have prompted you to smoke in the past will still remain. So it’s worth spending some time thinking about the different ways you intend to deal with stressful situations and the daily irritations that would normally have you lighting up.
Tips for avoiding common triggers
Alcohol. Many people smoke when they drink . Try switching to non-alcoholic drinks or drink only in places where smoking inside is prohibited. Alternatively, try snacking on nuts, chewing on a cocktail stick or sucking on a straw.
Other smokers. When friends, family, and co-workers smoke around you, it can be doubly difficult to give up or avoid relapse. Talk about your decision to quit so people know they won’t be able to smoke when you’re in the car with them or taking a coffee break together. In your workplace, find non-smokers to have your breaks with or find other things to do, such as taking a walk.
End of a meal. For some smokers, ending a meal means lighting up, and the prospect of giving that up may appear daunting. However, you can try replacing that moment after a meal with something else, such as a piece of fruit, a healthy dessert, a square of chocolate, or a stick of gum.
Once you stop smoking, you’ll likely experience a number of physical symptoms as your body withdraws from nicotine. Nicotine withdrawal begins quickly, usually starting within an hour of the last cigarette and peaking two to three days later. Withdrawal symptoms can last for a few days to several weeks and differ from person to person.
Common nicotine withdrawal symptoms include:
- Cigarette cravings
- Irritability, frustration, or anger
- Anxiety or nervousness
- Difficulty concentrating Restlessness
- Increased appetite
- Increased coughing
- Constipation or upset stomach
- Decreased heart rate
As unpleasant as these withdrawal symptoms may be, it’s important to remember that they are only temporary. They will get better in a few weeks as the toxins are flushed from your body. In the meantime, let your friends and family know that you won’t be your usual self and ask for their understanding.
While avoiding smoking triggers will help reduce your urge to smoke, you probably can’t avoid cigarette cravings entirely. Fortunately, cravings don’t last long—typically, about 5 or 10 minutes. If you’re tempted to light up, remind yourself that the craving will soon pass and try to wait it out. It helps to be prepared in advance by having strategies to cope with cravings.
Distract yourself. Do the dishes, turn on the TV, take a shower, or call a friend. The activity doesn’t matter as long as it gets your mind off smoking.
Remind yourself why you quit. Focus on your reasons for quitting, including the health benefits (lowering your risk for heart disease and lung cancer, for example), improved appearance, money you’re saving, and enhanced self-esteem.
Get out of a tempting situation. Where you are or what you’re doing may be triggering the craving. If so, a change of scenery can make all the difference.
Reward yourself. Reinforce your victories. Whenever you triumph over a craving, give yourself a reward to keep yourself motivated.
Coping with cigarette cravings in the moment
Find an oral substitute – Keep other things around to pop in your mouth when cravings hit. Try mints, carrot or celery sticks, gum, or sunflower seeds. Or suck on a drinking straw.
Keep your mind busy – Read a book or magazine, listen to some music you love, do a crossword or Sudoku puzzle, or play an online game.
Keep your hands busy – Squeeze balls, pencils, or paper clips are good substitutes to satisfy that need for tactile stimulation.
Brush your teeth – The just-brushed, clean feeling can help banish cigarette cravings.
Drink water – Slowly drink a large glass of water. Not only will it help the craving pass, but staying hydrated helps minimize the symptoms of nicotine withdrawal.
Light something else – Instead of lighting a cigarette, light a candle or some incense.
Get active – Go for a walk, do some jumping jacks or pushups, try some yoga stretches, or run around the block.
Try to relax – Do something that calms you down, such as taking a warm bath, meditating, reading a book, or practicing deep breathing exercises.
Go somewhere smoking is not permitted – Step into a public building, store, mall, coffee shop, or movie theatre, for example.
Smoking acts as an appetite suppressant, so gaining weight is a common concern for many of us when we decide to give up cigarettes. You may even be using it as a reason not to quit. While it’s true that many smokers put on weight within six months of stopping smoking, the gain is usually small—about five pounds on average—and that initial gain decreases over time. It’s also important to remember that carrying a few extra pounds for a few months won’t hurt your heart as much as smoking does. However, gaining weight is NOT inevitable when you stop smoking.
Smoking dampens your sense of smell and taste, so after you quit food will often seem more appealing. You may also gain weight if you replace the oral gratification of smoking with eating unhealthy comfort foods. Therefore, it’s important to find other, healthy ways to deal with unpleasant feelings such as stress, anxiety, or boredom rather than mindless, emotional eating .
Nurture yourself. Instead of turning to cigarettes or food when you feel stressed, anxious, or depressed, learn new ways to quickly soothe yourself . Listen to uplifting music, play with a pet, or sip a cup of hot tea, for example.
Eat healthy, varied meals. Eat plenty of fruit, vegetables, and healthy fats . Avoid sugary food , sodas, fried, and convenience food.
Learn to eat mindfully. Emotional eating tends to be automatic and virtually mindless. It’s easy to polish off a tub of ice cream while zoning out in front of the TV or staring at your phone. But by removing distractions when you eat, it’s easier to focus on how much you’re eating and tune into your body and how you’re really feeling. Are you really still hungry or eating for another reason?
Drink lots of water. Drinking at least six to eight 8 oz. glasses will help you feel full and keep you from eating when you’re not hungry. Water will also help flush toxins from your body.
Take a walk. Not only will it help you burn calories and keep the weight off , but it will also help alleviate feelings of stress and frustration that accompany smoking withdrawal.
Snack on guilt-free foods. Good choices include sugar-free gum, carrot and celery sticks, or sliced bell peppers or jicama.
There are many different methods that have successfully helped people to kick the smoking habit. While you may be successful with the first method you try, more likely you’ll have to try a number of different methods or a combination of treatments to find the ones that work best for you.
Medications
Smoking cessation medications can ease withdrawal symptoms and reduce cravings. They are most effective when used as part of a comprehensive stop smoking program monitored by your physician. Talk to your doctor about your options and whether an anti-smoking medication is right for you. The U.S. Food and Drug Administration (FDA) approved options are:
Nicotine replacement therapy. Nicotine replacement therapy involves “replacing” cigarettes with other nicotine substitutes, such as nicotine gum, patch, lozenge, inhaler, or nasal spray. It relieves some of the withdrawal symptoms by delivering small and steady doses of nicotine into your body without the tars and poisonous gases found in cigarettes. This type of treatment helps you focus on breaking your psychological addiction and makes it easier to concentrate on learning new behaviors and coping skills.
Non-nicotine medication. These medications help you stop smoking by reducing cravings and withdrawal symptoms without the use of nicotine. Medications such as bupropion (Zyban) and varenicline (Chantix, Champix) are intended for short-term use only.
What you need to know about e-cigarettes (vaping)
While some people find that vaping can help them to stop smoking, the FDA has not approved vaping as a method of smoking cessation. And recent news reports have even linked vaping to severe lung disease, prompting many questions about the safety of vaping. Here’s what you need to know:
- In the United States, the FDA does not regulate e-cigarette products.
- The FDA warns that vaping is “not safe for youth, young adults, pregnant women, or adults who do not currently use tobacco products.”
- It’s hard to always know exactly what’s in e-cigarettes.
- The liquid used in some e-cigarettes contains nicotine, which has many negative health effects. It can lead to high blood pressure and diabetes and can be especially dangerous to the developing brains of children and teens.
- There is no information available about the long-term effects vaping can have on your health.
- Until more is known, federal and state authorities recommend avoiding all vaping.
To learn more, read: Vaping: The Health Risks and How to Quit
Alternative therapies
There are several things you can do to stop smoking that don’t involve nicotine replacement therapy, vaping, or prescription medications. These include:
Hypnosis – This is a popular option that has produced good results for many smokers struggling to quit. Forget anything you may have seen from stage hypnotists, hypnosis works by getting you into a deeply relaxed state where you are open to suggestions that strengthen your resolve to stop smoking and increase your negative feelings toward cigarettes.
Acupuncture – One of the oldest known medical techniques, acupuncture is believed to work by triggering the release of endorphins (natural pain relievers) that allow the body to relax. As a smoking cessation aid, acupuncture can be helpful in managing smoking withdrawal symptoms.
Behavioral Therapy – Nicotine addiction is related to the habitual behaviors or rituals involved in smoking. Behavior therapy focuses on learning new coping skills and breaking those habits.
Motivational Therapies – Self-help books and websites can provide a number of ways to motivate yourself to give up smoking. One well known example is calculating the monetary savings. Some people have been able to find the motivation to quit just by calculating how much money they will save. It may be enough to pay for a summer vacation.
Smokeless or spit tobacco is NOT a healthy alternative to smoking
Smokeless tobacco, otherwise known as spit or chewing tobacco, is not a safe alternative to smoking cigarettes. It contains the same addictive chemical, nicotine, contained in cigarettes. In fact, the amount of nicotine absorbed from smokeless tobacco can be 3 to 4 times the amount delivered by a cigarette.
Most people try to stop smoking several times before they kick the habit for good, so don’t beat yourself up if you slip up and smoke a cigarette. Instead, turn the relapse into a rebound by learning from your mistake. Analyze what happened right before you started smoking again, identify the triggers or trouble spots you ran into, and make a new stop-smoking plan that eliminates them.
It’s also important to emphasize the difference between a slip and a relapse. If you start smoking again, it doesn’t mean that you can’t get back on the wagon. You can choose to learn from the slip and let it motivate you to try harder or you can use it as an excuse to go back to your smoking habit. But the choice is yours. A slip doesn’t have to turn into a full-blown relapse.
You’re not a failure if you slip up. It doesn’t mean you can’t quit for good.
Don’t let a slip become a mudslide. Throw out the rest of the pack. It’s important to get back on the non-smoking track as soon as possible.
Look back at your quit log and feel good about the time you went without smoking.
Find the trigger. Exactly what was it that made you smoke again? Decide how you will cope with that issue the next time it comes up.
Learn from your experience. What has been most helpful? What didn’t work?
Are you using a medicine to help you quit? Call your doctor if you start smoking again. Some medicines cannot be used if you’re smoking at the same time.
It’s important to remember that you cannot make a friend or loved one give up cigarettes; the decision has to be theirs. But if they do make the decision to stop smoking, you can offer support and encouragement and try to ease the stress of quitting. Investigate the different treatment options available and talk them through with the smoker; just be careful never to preach or judge. You can also help a smoker overcome cravings by pursuing other activities with them, and by keeping smoking substitutes, such as gum, on hand.
If a loved one slips or relapses, don’t make them feel guilty. Congratulate them on the time they went without cigarettes and encourage them to try again. Your support can make all the difference in helping your loved one eventually kick the habit for good.
Helping a teen to quit
Most smokers try their first cigarette around the age of 11, and many are addicted by the time they turn 14. The use of e-cigarettes (vaping) has also soared dramatically in recent years. While the health implications of vaping aren’t yet fully known, the FDA warns that it’s not safe for teens and we do know that teens who vape are more likely to begin smoking cigarettes.
[Read: Vaping: The Health Risks and How to Quit]
This can be worrying for parents, but it’s important to appreciate the unique challenges and peer pressure teens face when it comes to quitting smoking (or vaping). While the decision to give up has to come from the teen smoker him- or herself, there are still plenty of ways for you to help.
Tips for parents of teens who smoke or vape
- Find out why your teen is smoking or vaping; they may want to be accepted by their peers or be seeking attention from you. Rather than making threats or ultimatums, talk about what changes can be made in their life to help them stop smoking.
- If your child agrees to quit, be patient and supportive as they go through the process.
- Set a good example by not smoking yourself. Parents who smoke are more likely to have kids who smoke.
- Know if your kids have friends that smoke or vape. Talk with them about how to refuse a cigarette or e-cigarette.
- Explain the health dangers and the unpleasant side effects smoking can have on their appearance (such as bad breath, discolored teeth and nails).
- Establish a smoke-free policy in your home. Don’t allow anyone to smoke or vape indoors at any time.
Hotlines and support
Visit Smokefree.gov or call the quitline at 1-800-784-8669.
Take steps NOW to stop smoking or call the helpline at 0300 123 1044.
Visit Health Canada or call the helpline at 1-866-366-3667.
QuitNow or call 13 7848.
Nicotine Anonymous offers a 12-Step program modeled after Alcoholics Anonymous with meetings in many different countries.
More Information
- Join Freedom From Smoking - Smoking cessation program. (American Lung Association)
- How to Quit Using Tobacco - Dealing with both the mental and physical addiction. (American Cancer Society)
- How to Help Someone Quit Smoking - General hints for friends and family supporting someone who is quitting. (American Cancer Society)
- Substance-Related and Addictive Disorders. (2013). In Diagnostic and Statistical Manual of Mental Disorders . American Psychiatric Association. Link
- Lopez-Quintero, C., Pérez de los Cobos, J., Hasin, D. S., Okuda, M., Wang, S., Grant, B. F., & Blanco, C. (2011). Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug and Alcohol Dependence, 115(1–2), 120–130. Link
- Quit Smoking | Smokefree. (n.d.). Retrieved August 2, 2021, from Link
- US Preventive Services Task Force. (2021). Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Persons: US Preventive Services Task Force Recommendation Statement. JAMA, 325(3), 265–279. Link
- Leone, F. T., Zhang, Y., Evers-Casey, S., Evins, A. E., Eakin, M. N., Fathi, J., Fennig, K., Folan, P., Galiatsatos, P., Gogineni, H., Kantrow, S., Kathuria, H., Lamphere, T., Neptune, E., Pacheco, M. C., Pakhale, S., Prezant, D., Sachs, D. P. L., Toll, B., … Farber, H. J. (2020). Initiating Pharmacologic Treatment in Tobacco-Dependent Adults. An Official American Thoracic Society Clinical Practice Guideline. American Journal of Respiratory and Critical Care Medicine, 202(2), e5–e31. Link
- Complementary Health Approaches for Smoking Cessation: What the Science Says. (n.d.). NCCIH. Retrieved August 2, 2021, from Link
- Miller, Jacqueline W., Timothy S. Naimi, Robert D. Brewer, and Sherry Everett Jones. Binge Drinking and Associated Health Risk Behaviors among High School Students. Pediatrics 119, no. 1 (January 2007): 76–85. Link
- O’Brien, Charles P. Evidence-Based Treatments of Addiction. FOCUS 9, no. 1 (January 1, 2011): 107–17. Link
More in Addiction
Recognizing the signs and symptoms of a drinking problem

The power of the relaxation response to reduce stress and boost mood


Emotional Eating
How to recognize and stop emotional and stress eating

A guide to alcohol addiction treatment services

Cutting down on alcohol doesn’t have to mean losing your social life

The health risks in young people and how to quit

The hidden risks of drinking

Binge Drinking
Help if you have trouble stopping drinking once you start

Professional therapy, done online
BetterHelp makes starting therapy easy. Take the assessment and get matched with a professional, licensed therapist.
Help us help others
Millions of readers rely on HelpGuide.org for free, evidence-based resources to understand and navigate mental health challenges. Please donate today to help us save, support, and change lives.
Smoking Isn't the Only Source of Nicotine Addiction in Town
— emerging data suggest pharmacological interventions may help patients quit vaping.
by Neal L. Benowitz, MD May 29, 2024

E-cigarettes were introduced in the early 2000s as a way for smokers to manage their nicotine addiction without exposing themselves to the well-documented harms associated with the toxins and carcinogens in combustible cigarette smoke. These harms include increased risks of cancer, cardiovascular disease, chronic lung disease, and a number of other debilitating or life-threatening diseases. While many people have used e-cigarettes to quit smoking, other non-smokers have taken up e-cigarette use, particularly young adults.
However, the use of e-cigarettes, commonly known as vaping, is not without harm. Based on a review of the published data available at the time, an expert committee of the National Academies of Sciences, Engineering, and Medicine issued a report in 2018 finding conclusive evidence that although substituting e-cigarettes for combustible cigarettes reduced exposure to numerous toxins and carcinogens, e-cigarette use is not risk-free.
What are some of the harms? People who vape continue to be exposed to nicotine, which is highly addictive, making quitting e-cigarettes difficult for some. In addition, vapers may be subject to social stigma, and they incur expenses for purchasing vaping devices and liquids. Many people who have taken up vaping, either to help themselves quit smoking or as a primary form of recreational drug use, would like to quit. Furthermore, some healthcare professionals are supportive of the use of e-cigarettes to quit smoking but would like their patients to stop use when they are confident they will not relapse to smoking, due to the possible risks of nicotine use.
A study recently published in JAMA Internal Medicine is the first randomized, placebo-controlled trial to report a successful e-cigarette cessation benefit with pharmacological treatment. As a member of the Data and Safety Monitoring Committee for this study and an author on the publication, I believe the findings are an important step in addressing a relatively new source of nicotine addiction. This publication provides an opportunity for clinicians and the public health community to revisit how we think and talk about the harms associated with smoking, vaping, and nicotine addiction, and to reconsider how we can best take action to help reduce these harms.
Help or Harm: How Do We Discuss Vaping With Patients?
The fact that vaping can be associated with a high degree of dependence and a possible risk to health creates a challenge in counseling patients about their use of e-cigarettes. Given the extreme dangers associated with cigarette smoking, I believe that vaping can be a useful tool for harm reduction. I also believe that overcoming nicotine addiction -- in any form -- provides health, social, and economic benefits to those who can quit.
Healthcare professionals are solutions-oriented, and we strive to help patients set and achieve goals that can improve their physical and mental health and their quality of life. The lack of effective vaping cessation medications is an important missing piece of the puzzle when discussing the relative risks and benefits of smoking, vaping, and overcoming nicotine dependence. Survey data show that more than half of adults vaping nicotine plan to quit using e-cigarettes, and approximately 15% had already tried to quit in the prior year. Some succeeded, but additional tools are likely needed to help others. Is it realistic to counsel patients to quit vaping without addressing the very real biochemical basis of their nicotine addiction?
Hope for an Effective Vaping Cessation Therapy
Our data in JAMA Internal Medicine suggest we may be moving closer to finding an important intervention for e-cigarette-related nicotine dependence. The Phase II randomized clinical trial ORCA-V1 found that e-cigarette cessation rates were significantly higher for participants receiving 12 weeks of cytisinicline plus behavioral support compared with those receiving behavioral support alone. Cytisinicline is a partial agonist at α4β2 nicotinic acetylcholine receptors that mediate nicotine dependence and has shown efficacy for cigarette smoking cessation.
In the study, 160 participants were randomized 2:1 to 3 mg cytisinicline taken three times daily or placebo for 12 weeks. All participants received weekly behavioral support. The primary endpoint was biochemically verified continuous abstinence from nicotine e-cigarette use, measured during the last 4 weeks of treatment. Participants receiving 12 weeks of cytisinicline treatment had 2.6 times higher odds of having quit vaping during the last 4 weeks of treatment compared with subjects who received placebo, with vaping cessation rates during weeks 9-12 of 31.8% and 15.1%, respectively. No treatment-related serious adverse events were reported, and rates of adverse events were similar between the cytisinicline (50.9%) and placebo (54.7%) arms.
Acting Today While Awaiting Tomorrow's Advances
Although additional studies are needed to confirm the efficacy and safety of cytisinicline as a vaping cessation therapy, the ORCA-V1 results show that pharmacological tools may help to address the complex role that vaping plays in the continuum of reducing harms from cigarette smoking while sustaining nicotine addiction. These findings are particularly encouraging given the increasing prevalence of e-cigarette use among adults -- especially young adults -- in the U.S. Moreover, as a plant-based alkaloid, cytisinicline may appeal to those who prefer natural products as well as those who have tried and failed to quit smoking or vaping using other pharmacologic agents.
As we await additional data supporting the use of cytisinicline as a vaping cessation therapy, those of us who counsel patients who smoke cigarettes or vape nicotine should not shy away from having candid discussions about smoking, vaping, and overcoming nicotine addiction. We should be able to explain the risks and benefits of vaping and our patients' ability to make decisions that meet their personal health and lifestyle goals. With additional research, I hope those discussions will one day soon include the availability of safe and effective vaping cessation therapy.
Neal L. Benowitz, MD, is professor emeritus in the Department of Medicine at the University of California San Francisco.
Disclosures
Benowitz reported personal fees from Achieve Life Sciences while the JAMA Internal Medicine study was being conducted, as well as personal fees as a tobacco litigation expert witness in litigation against tobacco companies.

World No Tobacco Day 2024: Preventing Tobacco Addiction in Children and Youth
Patricio v. marquez.

The theme for this year’s World No Tobacco Day, "Protecting Children from Tobacco Industry Interference," underscores the critical need to prevent future generations from falling prey to the harmful impact of tobacco use.
About 8 million people die each year from tobacco-related diseases , accounting for 13 percent of deaths worldwide. The industry's strategic targeting of youth through marketing tactics such as appealing packaging, flavors, and advertising is designed to entice new, younger users. These practices, part of the “economics of deception and manipulation” as described in the acclaimed book by Nobel Prize in Economics laureates, George Akerlof and Robert J. Shiller, undermine public health efforts and pose a significant threat to the well-being of young people, who are more susceptible to addiction and the long-term ill health consequences of tobacco use.
The significance of protecting youth for human capital development
Protecting children from tobacco use is vital for supporting human capital development . By safeguarding young people from the influence of tobacco, we ensure that they are less likely to develop tobacco-attributable-diseases that hinder their educational attainment and professional achievements. Healthy, tobacco-free youth are more likely to perform better academically, which translates into a more skilled and capable workforce in the future. This fosters innovation, economic growth, and societal progress, as individuals can fully realize their lifetime potential without the ill health burden of tobacco use.
These efforts also contribute to reducing healthcare costs associated with treating tobacco-attributable diseases, such as cancer, cardiovascular diseases, and respiratory illnesses. This not only improves individual quality of life but also reduces the strain on healthcare systems.
Tobacco taxation is a highly effective measure to control tobacco use
One effective strategy in protecting children from tobacco industry interference is the implementation of higher taxes on tobacco products to increase their prices and lower their affordability. Research has consistently shown that young people are more price-sensitive than adults, meaning that higher tobacco prices can significantly reduce their likelihood of purchasing these products.
In addition to fiscal measures, regulatory policies can also play an important role in protecting children and adolescents from relentless advertising and promotion by the tobacco industry. Comprehensive bans on tobacco advertising, promotion, and sponsorship, as well as plain packaging laws, can reduce the social acceptability of tobacco use. By creating environments where tobacco use is less visible and less glamorized, we can deter youth from starting to use tobacco in the first place.
Despite the potential of tobacco taxes, they remain an underused policy
Taxing tobacco is a highly effective but underused policy to control tobacco use. The 2024 Tobacconomics Cigarette Tax Scorecard reveals that governments worldwide have made insufficient progress in leveraging tobacco taxation to combat tobacco use as one of the leading causes of preventable death. Despite the potential of tobacco taxes to both save lives and increase government revenues, most countries have not effectively utilized this policy tool. The Scorecard finds that the global average cigarette tax score dropped down to 1.99 out of 5.00 points in 2022 following a modest increase from 1.89 in 2014 to 2.25 in 2020. Overall scores improved in only 31 countries from 2020 to 2022, while scores worsened in 76 countries and stayed the same in 55 countries. Only 68 countries of the 170 for which data are available scored 2.50 or higher. As a result, cigarettes are affordable in most countries and becoming more affordable in too many.
Advancing the tobacco taxation agenda
A World Bank Group assessment of country experiences suggested that to move forward with tobacco taxation, leaders need to adopt bold and decisive strategies. One critical step is to implement substantial tax rate increases early in the process, prioritizing health gains over fiscal benefits. Successful tax strategies should focus on reducing tobacco product affordability. Combining large initial tax hikes with recurrent increases can help ensure that prices are adjusted not only for inflation, but rise faster than real income growth, thus curbing consumption.
Effective communication is also crucial in managing public expectations. Governments must convey that tax hikes are not one-off events but part of a sustained effort to keep tobacco prices increasing to motivate current smokers to quit and deter young people from starting. Simplifying tax rates and basing them on quantity rather than price can prevent tobacco users from switching to cheaper brands. Additionally, "soft earmarking" of tax revenues—linking increased taxes to increased health spending—can garner grassroots support for tax hikes. Regional collaboration can also enhance the impact of these measures, as seen in the European Union , by minimizing cross-border smuggling and fostering shared goals among countries.
There is no time to waste
The COVID-19 pandemic exposed the potential devastating impact of unattended public health, social, and environmental risks and their spillover effects in a fast-changing, crowded, and interconnected world. That sobering experience clearly illustrated the high price societies pay for inaction in dealing with global challenges, old and new . Tobacco use is a decades-old pandemic that needs to end once and for all, across the world, as an imperative to sustainable development.
To receive weekly articles, sign-up here

Former World Bank Group (WBG) Lead Public Health Specialist
Join the Conversation
- Share on mail
- comments added

Essay on Teenage Smoking
Students are often asked to write an essay on Teenage Smoking in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Teenage Smoking
What is teenage smoking.
Teenage smoking means when young people, usually between 13 and 19 years old, start to smoke cigarettes. It is a big problem because it can harm their health very badly. Smoking can cause diseases like cancer and heart problems.
Why Do Teenagers Start Smoking?
Many teenagers start smoking because they see their friends doing it or they think it makes them look cool. Sometimes, they are under a lot of stress and think smoking will help them relax.
Effects of Smoking on Teenagers
Smoking can make teenagers sick. It can reduce their lung function and make it hard for them to breathe. It also increases the risk of getting sick with diseases like bronchitis and pneumonia.
Stopping Teenage Smoking
To stop teenagers from smoking, adults should talk to them about the dangers of smoking. Schools can also help by teaching students about the risks of smoking and how to say no to cigarettes.
250 Words Essay on Teenage Smoking
Teenage smoking refers to young people, usually between the ages of 13 and 19, who use cigarettes. This is a serious issue because smoking can harm their health. Many teenagers start smoking due to peer pressure or because they see adults in their lives doing it.
One main reason teenagers start smoking is peer pressure. They see their friends doing it and don’t want to feel left out. Some teenagers think smoking makes them look cool or grown-up. Others might start smoking because they are curious or because they see family members smoking.
Smoking is very harmful to anyone’s health, but it is especially bad for teenagers because their bodies are still growing. Smoking can lead to serious health problems like lung cancer, heart disease, and breathing problems. It also affects how they look, causing bad breath, yellow teeth, and a greater risk of getting sick.
Stopping teenage smoking is important. Parents, teachers, and communities can help by teaching teenagers about the dangers of smoking. They can also set a good example by not smoking themselves. Programs that encourage teenagers to stay away from cigarettes and offer support to those who want to quit are also very helpful.
In conclusion, teenage smoking is a problem that affects the health and future of young people. By understanding why teenagers start smoking and the effects it has, we can work together to help stop it.
500 Words Essay on Teenage Smoking
Teenage smoking: a grave threat to young lives.
Smoking among teenagers has become a pressing concern, posing significant risks to their health and overall well-being. It’s crucial to understand the harmful effects of smoking and take proactive measures to prevent and discourage teenagers from engaging in this dangerous habit.
Health Hazards of Teenage Smoking
Smoking cigarettes exposes teenagers to a multitude of health hazards. The chemicals present in cigarettes can damage the lungs, heart, and other vital organs. Smoking increases the risk of developing lung cancer, heart disease, stroke, and chronic obstructive pulmonary disease (COPD). Additionally, it can lead to addiction, respiratory problems, and premature aging.
Negative Impact on Physical Development
Smoking interferes with the normal growth and development of teenagers. It can stunt their physical growth, delay puberty, and weaken their immune system, making them more susceptible to illnesses. Smoking also affects bone health, increasing the risk of osteoporosis in later life.
Social and Psychological Effects
Teenage smoking has detrimental social and psychological consequences. It can lead to isolation, peer pressure, and impaired social skills. Smokers are more likely to engage in risky behaviors, such as alcohol consumption and drug abuse. Moreover, smoking can negatively impact academic performance, concentration, and memory.
Preventing Teenage Smoking
Preventing teenage smoking requires a multifaceted approach involving parents, educators, healthcare providers, and policymakers. Parents should have open and honest conversations with their children about the dangers of smoking. Schools should implement comprehensive tobacco education programs to inform students about the health risks associated with smoking. Healthcare providers should counsel teenagers about the importance of avoiding tobacco use and offer support to those who want to quit. Additionally, policymakers should enact and enforce strict laws and regulations to restrict tobacco sales to minors and reduce youth access to cigarettes.
Teenage smoking is a serious public health issue that demands immediate attention. It’s essential to raise awareness about the harmful effects of smoking and empower teenagers with the knowledge and skills to resist tobacco use. By working together, we can create a smoke-free environment for our youth, ensuring their health and well-being for a brighter future.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Teenage Pregnancy Reflection
- Essay on Teenage Pregnancy In The Philippines
- Essay on Teenage Pregnancy Awareness
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

How Smoking Is Harmful to Your Health Essay
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Introduction
The impact of smoking on cardiovascular system, the impact of smoking on oral system, the impact of smoking on mental health.
More and more people become addicted to smoking every year. However, few of them are aware of the health damages all smokers may face in a long-term perspective. Smoking causes such incurable diseases as cancer, diabetes, asthma, or blindness. That is why many people nowadays substitute tobacco cigarettes with something they consider less damageable, for instance, electronic cigarettes or vapes. Although, there is almost no difference between smoking tobacco cigarettes and vapes or electronic cigarettes (Rofles et al., 2020). All of them are equally harmful to people’s health and may cause severe diseases. The primary purpose of the present speech is to inform the audience about the detrimental effects of smoking. The speech is divided into three parts. First, I will discuss the impact of tobacco on the cardiovascular system. Then I will proceed with the discussion of oral system diseases caused by smoking. Finally, I will finish the speech by speaking about the mental health problems that smoking causes.
The first system of the human body that suffers from cigarettes is the cardiovascular system. Almost one-third of smoke-related deaths worldwide are caused by cardiovascular disease (Kondo et al., 2019). Even people who smoke not so often may face cardiovascular problems. However, they have significantly lower chances of suffering from cardio diseases than those who smoke from 5 to 20 cigarettes on a daily basis. Both active and passive cigarette-smokers consume such dangerous chemicals as nicotine or carbon monoxide that may cause high blood pressure, atherosclerosis, or atrial fibrillation. The diseases in question carry the risk of a sudden heart attack both for men and women leading to hospitalization or death.
Besides, excessive consumption of nicotine and carbon monoxide causes congestive heart failure. In that case, the heart is unable to send blood around the body at a proper speed. Cognitive heart failure is also called heart aging, which means that a 30-year-old smoker may have the heart of a 50 or 60-year-old. That is why it is hard sometimes for people who smoke to do some physical activities, as their hearts cannot endure high tension.
Tobacco cigarettes smoke is proven to behave like a toxin or even drug. Interestingly, if at least one toxic chemical that tobacco smoke contains is excluded, the smoke becomes less damageable for the cardiovascular system (Kondo et al., 2019). That is why many people substitute tobacco cigarettes with electronic cigarettes because the latest contain such toxic chemicals as carbon monoxide, reactive oxygen species, carbonyls, and polyaromatic hydrocarbons to a lower degree than tobacco cigarettes. However, the nicotine level of electronic cigarettes remains unreasonably high, and the toxin affects all body systems and elevates cardiovascular risks.
However, smoking impacts not only the cardiovascular system of humans’ bodies. Now I am determined to discuss its influence on the oral system. The oral cavity system is the first one that meets toxic chemicals that cigarettes contain. Hence, it is logical to suppose that most of them remain on people’s teeth and tongues. However, mucosal cells of the oral cavity are susceptible to nicotine and other substances (Yu et al., 2017). That is why smokers often either do not notice some signs of oral cavity diseases or just do not relate them to smoking.
Apart from that, high temperature of cigarettes traumatizes the oral cavity. The temperature of smoldering tobacco is about 300°C and is higher than the average temperature of the oral cavity, so the smoke harms capillaries in the mouth.
Tobacco smoking causes changes in the pH of saliva (Yu et al., 2017). Cigarettes provoke oral dysbiosis and may become the reason for emerging unpleasant scents (, which may also be a consequence of dehydration of the oral cavity. The last often causes the loss of antibacterial and antiviral functions of saliva, which weakens oral mucosal immunity.
Refreshing toothpaste, mouthwash or chewing gum help to reduce unpleasant smells for some time, but they do not clean the oral cavity completely.
Weak oral immunity may also result in teeth disruption and discoloration. If not properly treated, these symptoms may cause parodontitis or complete teeth loss.
Now it is time to discuss what impact smoking has on mental health. Many people, especially the younger generation, see cigarettes as a means that helps to reduce stress and anxiety. It is true because nicotine, as one of the psychoactive substances stimulates adrenaline and dopamine release, provoking happiness and reducing stress (Chambers, 2017).
That is why people with mental disorders are more susceptible to smoking than anyone else. There are several reasons for that. First, people with mental illnesses seek all the possible ways to lessen stress, and smoking becomes for them the only source of dopamine. Second, culture itself associates depression and mental disorders in general with smoking, as in many books and movies, people suffering from mental illnesses are portrayed as smokers.
However, nicotine does not cause mental disorders. Cigarette addiction may be the result of depression or another mental illness. Nicotine, in that case, not only releases dopamine and adrenaline but also increases the risks of irrational behavior (Hefner et al., 2019). That is why many smokers are incredibly nervous and have trembling hands if they do not smoke at least for an hour. According to Hefner et al., nicotine addiction may be compared to alcohol addiction and cause far more damage.
It is necessary to remember that not only people with mental disorders may become addicted to smoking. For people who do not suffer from mental illnesses, nicotine addiction may shorten the attention span, cause problems with concentration and irrational behavior.
Many people nowadays are addicted to smoking. However, not all of them realize to which consequences it may lead. It is essential to assess all possible damages and be considerate of your health.
All the issues mentioned above compose only a tiny part of negative effects smoking causes on people’s health. It requires a lot of time and effort to number all of them. There were only three aspects of cigarettes’ harmful influence on people’s health discussed today, but there are far more of them. Concerning the present speech, it is possible to conclude that the harmful effects of smoking may not be noticeable when a person only starts doing it. However, in a long-term perspective, cigarettes pose a considerable threat to people’s physical and mental health.
Agarwal, N., Huq, S. M. & Dorji, C. (2018). The fatal link between tobacco smoking and cardiovascular diseases. The WHO South-Asia region.
Chambers, M. (Ed.). (2017). Psychiatric and mental health nursing: the craft of caring. Taylor & Francis.
Hefner, K.R., Sollazzo, A., Mullaney, S., Coker, K. L. & Sofuoglu, M. (2019). E-cigarettes, alcohol use, and mental health: Use and perceptions of e-cigarettes among college, by alcohol use and mental health status. Addict Behav. 91, 12-20.
Kondo, T., Nakano Y., Adachi , S. & Murohara, T. (2019). Effects of tobacco smoking on cardiovascular disease. Circulation Journal, 83, 1980-1985.
Naveed, A., Sohalib, A., Syed, N. B., Karobari, M. I., Anand, M., Charu, M., M., Pratibha, T., Pietro, M., Chan, Y., Y. & Scardina, G. A. (2021). Smoking a dangerous addiction: a systematic review on an underrated risk factor for oral diseases . I nt. J. Environ. Res. Public Health , 18 (21). Web.
Smoking and mental health. (n.d.). Web.
Yu, G., Philips, S., Gail, M. H., Goedert, J.J., Humphrys, M. S., Ravel, J., Ren, Y. & Caporaso, N. E. (2017). The effect of cigarette smoking on the oral and nasal microbiota. Microbiome, 5 (3).
- Health Promotion Plan: Smokers in Mississippi
- Causes and Effects of Smoking
- A Critical Examination of the Link between Nicotine Dependence and Schizophrenia
- Interoperability, Data Dictionary, and Communications Draft
- The "Death Stalks a Continent" Article Review
- Importance of Marketing Program in Organizations
- Tobacco Use as Health Issue in Georgia
- Health Disparities and Ways to Address Them
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, November 17). How Smoking Is Harmful to Your Health. https://ivypanda.com/essays/how-smoking-is-harmful-to-your-health/
"How Smoking Is Harmful to Your Health." IvyPanda , 17 Nov. 2022, ivypanda.com/essays/how-smoking-is-harmful-to-your-health/.
IvyPanda . (2022) 'How Smoking Is Harmful to Your Health'. 17 November.
IvyPanda . 2022. "How Smoking Is Harmful to Your Health." November 17, 2022. https://ivypanda.com/essays/how-smoking-is-harmful-to-your-health/.
1. IvyPanda . "How Smoking Is Harmful to Your Health." November 17, 2022. https://ivypanda.com/essays/how-smoking-is-harmful-to-your-health/.
Bibliography
IvyPanda . "How Smoking Is Harmful to Your Health." November 17, 2022. https://ivypanda.com/essays/how-smoking-is-harmful-to-your-health/.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

Smoking a Dangerous Addiction: A Systematic Review on an Underrated Risk Factor for Oral Diseases
Naveed ahmed.
1 Department of Medical Microbiology and Parasitology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Kota Bharu 16150, Malaysia; moc.liamg@882kilaman
Sohaib Arshad
2 Periodontics Unit, School of Dental Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Kota Bharu 16150, Malaysia; moc.liamg@399biahosdahsra
Syed Nahid Basheer
3 Department of Restorative Dental Sciences, College of Dentistry, Jazan University, Jazan 45142, Saudi Arabia; as.ude.unazaj@reehsabns
Mohmed Isaqali Karobari
4 Conservative Dentistry Unit, School of Dental Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Kota Bharu 16150, Malaysia
5 Department of Conservative Dentistry & Endodontics, Saveetha Dental College & Hospitals, Saveetha Institute of Medical and Technical Sciences University, Chennai 600077, India
Anand Marya
6 Department of Orthodontics, University of Puthisastra, Phnom Penh 12211, Cambodia; hk.ude.artsasihtup@ayrama
7 Department of Orthodontics, Saveetha Dental College & Hospitals, Saveetha Institute of Medical and Technical Sciences University, Chennai 600077, India
Charu Mohan Marya
8 Department of Public Health Dentistry, Sudha Rustagi College of Dental Sciences and Research, Faridabad 121002, India; ku.oc.oohay@mcayram (C.M.M.); moc.liamg@3ajenatahbitarp (P.T.)
Pratibha Taneja
Pietro messina.
9 Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, 90133 Palermo, Italy; [email protected]
Chan Yean Yean
Giuseppe alessandro scardina, associated data.
Any type of data used in this systematic review can be assessed upon the request email to the corresponding author.
Despite growing knowledge of the adverse effects of cigarette smoking on general health, smoking is one of the most widely prevalent addictions around the world. Globally, about 1.1 billion smokers and over 8 million people die each year because of cigarette smoking. Smoking acts as a source for a variety of oral and systemic diseases. Various periodontal issues such as increased pocket depth, loss of alveolar bone, tooth mobility, oral lesions, ulcerations, halitosis, and stained teeth are more common among smokers. This systematic review was conducted according to the guidelines from PRISMA, and research articles were retrieved from the Web database sources on 31 May 2021. The quality of research articles was ensured by the type of evidence from combined schema incorporating as schema-13 evidence type description, Cochrane health promotion and public health field (CHPPHF), and the health gains notation framework-14 screening question for quality assessment of qualitative and quantitative studies. Smokers have been found to have bleeding on probing, periodontal pockets, and clinical attachment loss compared to nonsmokers. Oral and respiratory cancers are among the most lethal known diseases caused by cigarette smoking and other commonly occurring sequelae such as stained teeth, periodontal diseases, etc.
1. Introduction
Oral diseases appear to be a global problem that should be addressed as a matter of global health concern. Oral health issues include various behavioral and social features such as habits, oral health knowledge, practices, availability, modifiable risk factors, and accessibility to oral health treatments [ 1 ]. Health is considered as a significant factor in making life valuable [ 2 ]. In general, lifestyles and behavioral patterns are continuously changing, making people more susceptible to oral disorders. Common preventable risk factors for oral diseases include consuming great amounts of sugary food and alcohol and smoking excessively [ 3 ]. Back in 2015, untreated oral disorders crippled over half of the world’s population (age-standardized prevalence: 48.0 percent), affecting 3.5 million individuals worldwide [ 4 , 5 ].
Oral and orofacial problems can affect children and adolescents, affecting physical functioning and psychosocial well-being [ 6 ]. One of the scientific theories used to influence human health-related behaviors is the knowledge, attitude, and practices (KAP) theory. According to the KAP theory, healthy knowledge is the foundation for developing an optimistic and healthy lifestyle, attitudes are the motivating factor behind changing behavior, and the goal is to promote oral health [ 7 ]. This is why oral health professionals play a critical role in disease prevention and diagnosis through screening and raising awareness [ 8 ]. Recently, a shift of focus in health care has been noticed, signaling a transition from biological to a more complete and broader biopsychosocial concept of health [ 9 ].
The oral cavity is a speculum for a person’s current health issues. Some of the modifiable risk factors for poor oral hygiene include cigarette smoking, betel quid chewing, and alcohol consumption. Despite the fact that there is growing knowledge of the adverse effects of cigarette smoking on general health, smoking is one of the widely prevalent addictions around the world [ 10 ]. Globally, about 1.1 billion smokers and over 8 million people die each year due to cigarette smoking [ 11 ]. Smoking acts as a source for a variety of diseases, including cardiovascular diseases (CVD), chronic obstructive pulmonary diseases (COPD), cancer, and periodontal disease (POD), as one of the five top risk factors for the global burden of the disease [ 12 , 13 , 14 ]. According to the alcohol and drugs survey, 15% of people currently smoke cigarettes, with 17% of men and 13% of women. Teenagers aged 15–19 years have been found to smoke at an estimated rate of 8%, with 10% of males and 6% of females being current smokers. The frequency was 16 percent among people aged 20–24 years and 25 years and older [ 15 ].
Tobacco smoking has numerous and well-documented negative consequences. The oral cavity is the first to get exposed to cigarette smoke, wherein the soft and hard tissues come in direct contact, making it the first area of confrontation [ 16 ]. Tobacco smoking, particularly in the form of cigarettes, has been proved to be a significant risk factor for periodontitis ( Figure 1 ) [ 17 ]. Other than plaque, smoking has been identified as an important risk factor for POD. It also affects the prevalence of POD, severity, progression, and treatment response. According to epidemiological research, smokers have a much higher risk of POD than nonsmokers, and the increased risk is proportionate to the duration and rate of smoking [ 18 , 19 ]. Various gingival and periodontal issues such as gingivitis, increased pocket depth, loss of alveolar bone, tooth mobility, oral lesions, ulcerations, halitosis, and stained teeth are more common among smokers [ 20 ].

Diagrammatic presentation of possible effects of smoking on oral health.
According to a meta-analysis, exposure to cigarette smoke in the environment relates to a considerably increased risk of lung cancer [ 21 ]. Cigarette smoking has also been linked to several other oral cancers. Kumar, A. et al. presented a clinic-pathological investigation that showed that 29.4% of people with established oral cancer cases chewed only tobacco, 25.5% only smoked, 42.2% chewed both types of tobacco (smoke and nonsmokers), and 2.9% did not chew tobacco. 83.3% of those who solely chewed tobacco had oral cavity malignancies, with 6.7% having malignancies of the oropharynx and hypopharynx. Of those who only smoked tobacco, 69.2% of individuals had the disease [ 22 ]. This predicts that there is a high chance of developing cancer regardless of how you use tobacco (smoking, chewing, etc.).
Rationale: The most critical risk factor associated with the onset of various gingival and periodontal diseases is tobacco smoking. It reduces the quality of life of patients and poses a risk to oral health. It has been demonstrated that oral health among smokers is compromised in comparison to nonsmokers. Thus, this study is aimed at reviewing the literature to evaluate the effect of smoking on oral health.
Objectives: In this systematic review, we aim to examine the effects of cigarette smoking on oral health, present the major oral diseases caused by cigarette smoking, and determine if there is any possibility of bacterial or fungal infections among smokers.
2. Material and Methods
2.1. protocol and registration.
This systematic review was conducted according to the guidelines from PRISMA ( http://www.prisma-statement.org accessed on 4 October 2021). Research articles were retrieved from the Web database sources on 31 May 2021. The study has been registered on PROSPERO with registration number CRD42021273462. Initially, articles were assessed from MDPI, PubMed, Scopus, and Web of Science (WOS).
2.2. Research Questions
The research questions of this systematic review are as follows: What are the effects of cigarette smoking on oral health? What are the major oral diseases caused by cigarette smoking? Is there any possibility of bacterial or fungal infections among smokers?
2.3. Data Sources
Relevant articles for inclusion in the review were found through a search of electronic databases. Keywords included “smoking and or oral health”, “cigarette smoking”, “Smoking effects and or oral health”, and “smoking and or tobacco use”.
2.4. Search Strategies
Electronic databases were searched for articles according to the selected keywords such as smoking, cigarette smoking, and tobacco use following the MeSH strategy, which evaluates the effects of cigarette smoking on oral health, published between 2011 to 2021. The number of articles retrieved from each database is shown in Table 1 .
Search strategy for study-related articles.
2.5. Study Selection and Criteria for Eligibility of Articles
A detailed search of research articles from Web sources was performed to find out the studies that examined the effects of cigarette smoking on oral health and were published between 2011 to 2021. Two researchers assessed possibly relevant research papers against the previously specified inclusion and exclusion criteria to validate the selection approach, as shown in Table 2 .
Criteria for inclusion and exclusion of research studies.
2.6. Quality Assessment
The quality of research articles was ensured by the type of evidence from combined schema incorporating as schema-13 evidence type description, Cochrane health promotion and public health field (CHPPHF), and the health gains notation framework-14 screening question for quality assessment of qualitative and quantitative studies.
The CHPPHF quality assessment tool was utilized to assess the quality of articles. This instrument scored the criteria for allocation bias, selection bias, intervention integrity, blinding, withdrawals and dropouts, confounding, data collecting procedures, and statistical analysis for internal and external validity.
Qualitative research was evaluated and scored for quality using questions adapted from the CHPPHF’s Critical Appraisal Skills Programme. There were 19 Type V evidence articles among 3696 articles that have not been further rated for quality. The studies were categorized as weak, moderate, or strong evidence based on their quality.
The quality of published evidence was categorized as I, II-1, II-2, II-3, and III. The articles that had at least evidence from the one proper RCT were categorized as “I”. Articles with data from well-designed controlled trials that were not randomized were classed as “II-1”. Evidence from well-designed case-control analytic investigations or cohort, ideally by many centers or research groups, was graded as “II-2”. This included evidence from time or place comparisons with or without the intervention. “II-3” was used to describe dramatic results from uncontrolled studies. While renowned experts’ judgments were based on clinical experience, descriptive research or expert committee reports were designated as “III.”
A, B, C, D, and E were the categorized grades based on recommendations. Research having sufficient evidence to support the suggestion that the condition is included in a periodic health examination (PHE) were categorized as “A”. Reports with sufficient evidence to recommend that the condition be specially examined in a PHE were categorized as “B”. Reports that were given the “C” grade had too little evidence to support the inclusion or exclusion of a disease from a PHE; recommendations may be made on other reasons. Reports with sufficient evidence to indicate that a condition is expressly omitted from consideration in a PHE were categorized as “D”. When there was adequate information found to support the suggestion that the condition is expressly excluded from PHE, it was considered as “E”.
2.7. Data Extraction
Two researchers (S.A. and N.A.) performed the independent sampling and extraction of required data from the papers included in the current analysis after reading the entire text. The authors of the study, year of publication, study duration, business, group of references, country, number of samples, type of samples, and smoking effects on oral health were all extracted and reported in Table 3.
3.1. Study Selection Results
A total of 3696 studies were retrieved from the PubMed, Scopus (2271), WOS (1822), and MDPI (711) according to the inclusion/exclusion criteria as listed in Table 2 . Data were extracted from 19 studies that purely met the eligibility criteria. Figure 2 and Figure 3 shows the results of the total studies evaluated. Initially, a total of 3696 articles were screened from PubMed, 711 from MDPI and 4093 form other databases (Scopus and WOS) as per the search criteria described in Table 1 . After identifying duplicate articles, 972 were excluded from PubMed, 657 from Scopus, 507 from WOS, and 120 from MDPI. After removal of duplicates, the remaining 3315 articles were screened by reading the title and abstracts, after which 2929 articles were excluded. The remaining 47 articles were then fully read and assessed for the inclusion/exclusion criteria and quality assessment. After reading the full text, 28 studies were excluded due to reasons including cigarette cessation studies with no oral effects, prevalence of cigarette smoking among different population reporting no oral effects, questionnaire-based studies, and studies with chewable tobacco or other oral tobacco products. The finally selected articles were finalized to proceed further for data extraction. Based on the quality assessment of the research studies, these 19 articles were screened for the present study. A meta-analysis of the studies included in this study was not performed due to the methodological heterogeneity of the findings.

Flowchart of articles retrieved from different Web sources.

Articles included in the current study (MDPI and PubMed).
From the total retrieved articles (MDPI, PubMed, Scopus, and Web of Sciences), only MDPI and PubMed articles were further included in this study.
3.2. Study Features
A total of 19 studies were included in this systematic review. The studies were conducted in different countries; many researchers cited different time durations. The number of citations for each of the study was observed from Google Scholar. Each of the included studies was published in reputed and indexed journals. Table 3 summarizes the sample type, total sample size, adopted methodology, results, and the conclusion of each study. Different types of samples were collected from the patients to observe the effects of smoking on oral health, including biopsies, blood, buckle cells, teeth, saliva, etc. In the included studies, a total of 26,236 samples were observed.
Methodology, outcomes, and conclusion of articles included in the current study.
3.3. Quality Assessment of Research Articles
All of the articles were shortlisted and screened based on the inclusion and exclusion criteria, titles, and abstract. Full texts were read one by one after the screening process and assessed for the quality of material using the CHPPHF recommendations. CHPPHF assessed articles for internal and external validity and rated the criteria for allocation biases, selection biases, intervention integrity, blinding, withdrawals and dropouts, confounding, data collection methods, and statistical analysis. No statistical assessment of publishing bias was carried out for the included studies as there were limited experimental techniques. A total of eight RCTs were included in the present systematic review.
Bias assessment was conducted according to the Cochrane tool of bias risk assessment. Overall, four included RCTs were at higher risk of bias, four were at lower risk of bias, and many items according to Cochrane tool of bias risk assessment were unclear in eight included RCTs. Selection bias was observed in two of the eight included RCTs, as was performance bias in two studies, detection bias in one study, attrition bias in one study, reporting bias in two studies, and other concerns in one study ( Figure 4 ).

Risk of bias summary: review authors judgment about each risk of bias item for each included RCT.
4. Discussion
Cigarette smoking has been linked to a variety of health problems. When comparing current smokers to nonsmokers, the rate of mortality from any cause was two to three times higher [ 42 , 43 ]. In many smoking-related studies, the duration of smoking, a quantity of cigarettes smoked per day, brand of cigarettes smoked, cigarette type, and topographical factors related to smoking all are linked to the severity of tobacco consumption [ 19 ]. Because of tobacco use, other lifestyle risk factors, and poor dental care usage, smokers are at a higher risk for many oral diseases. As many oral health problems go unrecognized and untreated, the lack of regular dental care becomes particularly problematic [ 8 , 44 ].
Oral diseases are one of the most frequent chronic diseases, and they are significant public health issues due to their prevalence, effect on people and society, and treatment costs [ 33 , 45 ]. Oral disease determinants are well understood. Oral hygiene, smoking, drinking, hazardous behaviors, and stress are all risk factors for various chronic diseases, and efficient public health interventions to prevent oral diseases [ 7 ]. Smoking is one of the most common risk factors for oral diseases [ 3 ].
Dental caries and periodontal disease and the probable consequences of both (tooth loss) are serious dental public health issues that affect people all over the world [ 6 , 21 , 46 ]. Individuals’ quality of life and general health are negatively impacted by poor oral health and untreated oral illnesses [ 9 ]. A significant positive association between tobacco smoking and higher risk for periodontitis has been found in prospective longitudinal studies.
The elevations in interleukin (IL)-1 and IL-6 associated with smoking levels upregulate bone resorption through the increase in the ratio between the receptor activator of nuclear factor-κβ ligand (RANKL) and its inhibitor osteoprotegerin (OPG). In addition, higher concentrations of elastase and matrix metalloproteinase (MMP)-8 and MMP-9 with proteolytic activity and decreased levels of protease inhibitors, such as alpha-2-macroglobulin and α-1-antitrypsin, may compromise periodontal healing.
If smoking were eliminated in this population, the risk of periodontitis would be reduced by approximately 14% as calculated using the population attributable risk fraction. In underdeveloped countries, the burden of oral diseases is significantly higher [ 4 , 47 , 48 ]. Among smokers and nonsmokers, in the treatment of chronic periodontitis (CP), Al-Ahmari et al. (2019) checked the effectiveness of scaling & root planning (SRP) with and without the adjunct antimicrobial photodynamic therapy (aPDT). Bleeding on probing (BOP), plaque index (PI), clinical attachment loss (CAL), and probing pocket depth (PD) 4 mm were all assessed at baseline, one month, and three months of follow-up. Smokers and nonsmokers had similar BOP, PI, PD, and clinical AL at the start of the study. PD, PI, and clinical AL were shown to be greater in smokers than nonsmokers after a one-month and three-month follow-up. At the one-month and three-month follow-ups, all nonsmokers’ BOP, PI, clinical AL, and PD were equivalent [ 34 ]. In similar research, Al-Bayaty et al. (2013) conducted a study to observe the effects of cigarette smoking on gingival bleeding, to measure the serum haptoglobin, cotinine, and alpha 1-antitrypsin concentrations in Malaysian smokers. BOP levels were determined to be low, whereas PI values were high. Smokers had considerably more significant levels of serum haptoglobin, cotinine, and alpha 1-antitrypsin than nonsmokers. There was a strong connection between PI and smoking duration (years) and blood cotinine levels [ 24 ].
Even though tobacco use has decreased in many high-income countries such as the United States and the United Kingdom, it is growing in many low- and middle-income countries [ 11 , 35 ]. According to the World Health Organization (WHO), there are more than 1.1 billion smokers throughout the world, with more than 80% of them residing in low- and middle-income countries [ 49 ]. After nonsurgical periodontal treatment, Varghese et al. (2020) studied the salivary 8-hydroxyguanosine (8-OHdG) levels in smokers and nonsmokers with CP. Clinical periodontal markers (PI, GI, PD, and CLI) were assessed at the start of the study. SRP was performed on patients with CPs (CP smokers) and CPns (CP nonsmokers) [ 40 ]. In a three-month follow-up period, all of the clinical measures and salivary collections were repeated. At the baseline period, the PI, GI, PD, and CAL values in the CPs and CPns groups were significantly higher as compared to the CHns and CHs groups. At baseline, salivary levels of 8-OHdG were found significantly higher in the CPs group than the other groups. All of the clinical measures in the CP group improved by the follow-up interval at the third month. However, the salivary levels of 8-OHdG in the CP smoker category were still higher values than the CPns [ 40 ]. Haswell et al. (2014) analyzed the biomarkers of biological effect (BOBE) and demonstrated the difference between smokers, nonsmokers, and ex-smokers. The levels of biomarkers were compared, and it was seen that there were 27 possible biomarkers evaluated in all, 14 of which were substantially different between smokers and nonsmokers, and 12 of which were able to discriminate between smokers and former smokers, indicating the possibility of reversibility [ 26 ].
The maxillary antrum, submandibular region, salivary glands, and tongue are commonly affected by cervicofacial actinomycosis [ 35 ]. The mandible is affected in about half of the cases, with the chin (15%), cheek (15%), and submaxillary ramus and angle (15%). The paranasal sinuses, tongue, larynx, middle ear, thyroid gland, and lachrymal pathways are all nonodontogenic orofacial regions that may also be get affected by cervicofacial actinomycosis [ 22 ]. Abduljabbar et al. (2017) worked on a project and wanted to see how effective aPDT was at preventing oral fungus colonization in smokers and nonsmokers suffering from denture stomatitis (DS). Among smokers, a statistically significant decrease in the mean fungal CFU/mL was seen at the 3-month follow-up compared to their respective baseline values of CFU/mL. When compared to their individual baseline values, nonsmokers’ mean levels were lower. After a 3-month follow-up, smokers’ fungal CFU/mL levels were statistically substantially higher than nonsmokers [ 30 ].
Study Limitations
In this systematic review, we have searched the data from a limited number of significant Web sources with specific final publication periods. The articles which have been published in any other sources may be overlooked. We have included articles that were published in the English language; as a result, articles which were published in other languages may also be overlooked.
5. Conclusions
Cigarette smoking has well-known hazardous effects on oral health and throughout the respiratory tract. Because of the impairment in oral health, it can also lead to problems in other parts of the body, such as the gastrointestinal tract system. Oral and respiratory cancers are among the most lethal known diseases caused by cigarette smoking, which can also cause plaque, dental caries, and other periodontal diseases. There is also an increased risk for bacterial and fungal infections in the oral cavity. This conclusion is based on a limited number of research studies.
Author Contributions
Project administration and supervision, M.I.K.; conceptualization, M.I.K. and A.M.; methodology, N.A. and C.Y.Y.; formal analysis, N.A. and C.Y.Y.; data curation, N.A.; validation, M.I.K., S.N.B., and P.M.; writing the original draft, N.A., S.A., A.M., and M.I.K.; writing—review and editing, C.M.M., P.T., S.A., N.A., A.M., G.A.S., and M.I.K.; funding acquisition, G.A.S. and P.M. The final manuscript has been read and approved by all the authors. All authors have read and agreed to the published version of the manuscript.
Any external funding was not received for this systematic review.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors have declared no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nicotine Addiction From Vaping Is a Bigger Problem Than Teens Realize
March 19, 2019

Data show clearly that young people are vaping in record numbers. And despite the onslaught of reports and articles highlighting not only its dangers but the marketing tactics seemingly aimed to hook teens and young adults, the number of vaping users continues to climb.
These teens may be overlooking (or underestimating) a key ingredient in the vapors they inhale: nicotine. Though it’s possible to buy liquid or pod refills without nicotine, the truth is you have to look much harder to find them. Teens may not realize that nicotine is deeply addictive. What’s more, studies show that young people who vape are far likelier to move on to cigarettes, which cause cancer and other diseases.
So, why is nicotine so addictive for teens?
Nicotine can spell trouble at any life stage, but it is particularly dangerous before the brain is fully developed, which happens around age 25.
“Adolescents don’t think they will get addicted to nicotine, but when they do want to stop, they find it’s very difficult,” says Yale neuroscientist Marina Picciotto, PhD, who has studied the basic science behind nicotine addiction for decades. A key reason for this is that “the adolescent brain is more sensitive to rewards,” she explains.
The reward system, called the mesolimbic dopamine system, is one of the more primitive parts of the brain. It developed as a positive reinforcement for behavior we need to survive, like eating. Because the mechanism is so engrained in the brain, it is especially hard to resist.
When a teen inhales vapor laced with nicotine, the drug is quickly absorbed through the blood vessels lining the lungs. It reaches the brain in about 10 seconds. There, nicotine particles fit lock-and-key into a type of acetylcholine receptor located on neurons (nerve cells) throughout the brain.
The unique attributes that make nicotine cravings persist
“Nicotine, alcohol, heroin, or any drug of abuse works by hijacking the brain’s reward system,” says Yale researcher Nii Addy, PhD, who specializes in the neurobiology of addiction. The reward system wasn’t meant for drugs—it evolved to interact with natural neurotransmitters already present in the body, like acetylcholine. This neurotransmitter is used to activate muscles in our body. The reason nicotine fits into a receptor meant for acetylcholine is because the two have very similar shapes, biochemically speaking, Addy explains.
Once nicotine binds to that receptor, it sends a signal to the brain to release a well-known neurotransmitter—dopamine—which helps create a ‘feel-good’ feeling. Dopamine is part of the brain’s feedback system that says “whatever just happened felt good” and trains the brain to repeat the action. But nicotine, unlike other drugs such as alcohol, quickly leaves the body once it is broken down by the liver. Once it’s gone, the brain craves nicotine again.
When an addicted teen tries to quit nicotine, the problem of cravings is of course tied to the drug that causes the dopamine rush, Addy says. What’s more, recent animal study research and human brain imaging studies have shown that “environmental cues, especially those associated with drug use, can change dopamine concentrations in the brain,” he says. This means that simply seeing a person you vape with, or visiting a school restroom—where teens say they vape during the school day—can unleash intense cravings. “In the presence of these cues, it’s difficult not to relapse,” Addy says.
Physical changes caused by nicotine
Nicotine can also cause physical changes in the brain, some temporary, and others that some researchers, like Picciotto, worry could be long-lasting.
Decades of cigarette smoking research have shown that, in the short term, the number of acetylcholine receptors in the brain increases as the brain is continuously exposed to nicotine. The fact that there are more of these receptors may make nicotine cravings all the more intense. However, those same studies found that the number of receptors decreases after the brain is no longer exposed to nicotine, meaning that these changes can be reversed.
But animal studies show nicotine also can cause issues with brain function, leading to problems with focus, memory, and learning—and these may be long-lasting. In animals, nicotine can cause a developing brain to have an increased number of connections between cells in the cerebral cortex region, says Picciotto. “If this is also true for humans, the increased connections would interfere with a person’s cognitive abilities,” Picciotto says.
To illustrate how this might work, Picciotto gives an example. A student sitting in a noisy classroom, with traffic passing by the window, needs to be able to focus her attention away from the distracting sounds so she can understand what the teacher says. “Brains not exposed to nicotine learn to decrease connections, and refinement within the brain can happen efficiently,” Picciotto says. “But when you flood the system with nicotine, this refinement doesn’t happen as efficiently.”
“There’s hope that the current vaping epidemic won’t lead to major health problems like lung cancer or pulmonary disease,” Picciotto says. “But we may still see an epidemic of cognitive function problems and attention problems. The changes made in the brain could persist.”
Vaping vs. regular cigarettes
Weighing the pros and cons of vaping versus smoking is difficult to do. On the one hand, e-cigarettes likely do not produce 7,000 chemicals—some of which cause cancer—when they are activated, like regular combustible cigarettes do. However, the aerosol from a vape device has not been proven safe. Studies have found that it contains lead and volatile organic compounds, some of which are linked to cancer. Researchers are still gathering data on the possible long-term health effects from vaping. It’s notable that e-cigarettes have not been approved by the Food and Drug Administration (FDA) as smoking cessation devices. However, e-cigarettes may be a better choice for adult smokers if they completely replace smoking, according to the Centers for Disease Control and Prevention (CDC).
But where nicotine levels are concerned, a newer and popular type of vape device, called a “pod mod,” outcompetes many other e-cigarette devices. The form of nicotine in these pods is estimated to be 2 to 10 times more concentrated than most free-base nicotine found in other vape liquids. A single pod from one vape manufacturer contains 0.7 mL of nicotine, which is about the same as 20 regular cigarettes.
Despite its extremely addictive nature, people can successfully quit using nicotine with personalized approaches, especially under the guidance of physicians who understand addiction.
For young people, intervening early in a vaping habit could make an important difference in the quality of life they have throughout their adult years. It could also mean they won’t become part of next year’s statistics.
More news from Yale Medicine


Scientists Reveal Why Women Are More Easily Addicted to Cigarettes
A newly discovered brain circuit may explain why women tend to become hooked on nicotine more quickly than men.
Smoking remains a leading cause of preventable disease and death in the United States, with more than 480,000 Americans killed by smoking every year, according to the U.S. Centers for Disease Control and Prevention. As of 2021, roughly 11 percent of American adults reported smoking cigarettes, with men slightly more likely to smoke than women.
However, while fewer women might smoke on average, previous studies have shown that women are more likely to develop a nicotine addiction and tend to become addicted more quickly with lower nicotine exposures.
"Studies show that women have a higher propensity to develop addiction to nicotine than men and are less successful at quitting ," Sally Pauss, a doctoral student at the University of Kentucky College of Medicine in Lexington who led the project under the supervision of associate professor Terry Hinds Jr., said in a statement.
"Our work aims to understand what makes women more susceptible to nicotine use disorder to reduce the gender disparity in treating nicotine addiction."
The Role of Estrogen
One clear difference between men and women is their production of estrogen. Therefore, the researchers trawled through a large library of genes known to be activated by this hormone, specifically those expressed in our brains. Only one class of candidate genes fit this criteria: those coding for a group of proteins called olfactomedins, which play a diverse range of roles in the early development and functional development of the nervous system.
The researchers then performed a series of studies with human uterine cells and rats to better understand the interactions between olfactomedins, estrogen and nicotine. Through their experiments, an interesting feedback loop emerged: estrogen activates olfactomedins, which, in turn, are suppressed in the presence of nicotine in areas of the brain involved in reward and addiction. In other words, this olfactomedin intermediary could be driving individuals to seek out nicotine to satisfy these reward circuits.
Making It Easier for Women To Quit Smoking
"If we can confirm that estrogen drives nicotine seeking and consumption through olfactomedins, we can design drugs that might block that effect by targeting the altered pathways," Pauss said. "These drugs would hopefully make it easier for women to quit nicotine."
The researchers said that this knowledge could be particularly useful for those taking estrogen in the form of oral contraceptives or hormone replacement therapy, which might increase the risk of developing a nicotine use disorder.
The findings will be presented at the annual meeting of the American Society for Biochemistry and Molecular Biology on March 25 in San Antonio.
Is there a health problem that's worrying you? Do you have a question about quitting smoking? Let us know via [email protected]. We can ask experts for advice, and your story could be featured in Newsweek.
Related Articles
- Theory About Why Millennials Age Better Than Gen X Has Internet Convinced
- Vaping, Like Smoking, May Cause 'Similar Damaging Changes' to DNA
- 'Frequent' Cannabis Use Linked to Heart Attacks and Strokes
Start your unlimited Newsweek trial

- / Miscellaneous
Cigarette Addiction
By: July • Essay • 283 Words • March 1, 2010 • 1,198 Views
Join now to read essay Cigarette Addiction
Literature Review
Introduction: There has been an increasingly negative stigma surrounding cigarette smoking following public education efforts with regards to the detrimental effects of the habit. This social dynamic has produced an environment of increasing costs for cigarette companies in the form of higher specific taxes as well as mandated anti-smoking campaigns. This paper will research whether increases in cigarettes prices due to increased costs faced by the manufacturers will decrease demand significantly or whether the addiction to cigarettes is too strong.
The answer to this question is very interesting because there have been many advocates of the idea of taxing cigarettes heavily in an attempt to augment the demand function of cigarette smoking consumers, with the end result of less cigarette consumption in America. Every year millions of citizens suffer from health care problems ranging from minor health care maladies to death, many of these which would be non-existent with the absence of cigarette smoking. These problems are not solely dealt with by the consenting smoker, however, but also by non-consenting
World No-Tobacco Day 2024: 8 foods that help fight tobacco addiction

About the Author
The TOI Lifestyle Desk is a dynamic team of dedicated journalists who, with unwavering passion and commitment, sift through the pulse of the nation to curate a vibrant tapestry of lifestyle news for The Times of India readers. At the TOI Lifestyle Desk, we go beyond the obvious, delving into the extraordinary. Consider us your lifestyle companion, providing a daily dose of inspiration and information. Whether you're seeking the latest fashion trends, travel escapades, culinary delights, or wellness tips, the TOI Lifestyle Desk is your one-stop destination for an enriching lifestyle experience. Read More
Visual Stories

Viktor Hovland receives the trophy from Jack Nicklaus after winning in a playoff over Denny McCarthy at the 2023 Memorial Tournament at Muirfield Village Golf Club.
Of course, another great feat Nicklaus accomplished was winning the U.S. Amateur and the NCAA Tournament while with Ohio State in 1961. Nicklaus was happy to see the Buckeyes tie for third last week at the NCAA Championship in Carlsbad, California.
“(Coach Jay) Moseley has done a good job with it. They’ve got a good golf course to play and practice on, to develop a golf game,” he said, referencing the Scarlet course. “I think a lot of guys shy from coming north to play.”
Not Nicklaus, who grew up in Upper Arlington.
“I had offers from a lot of schools to play elsewhere, and I wanted to come to Ohio State,” he said. “I loved going to the football games, the basketball games and being part of fraternity life and school life. That was as important to me as playing on the golf team.”
[email protected] @rollerCD
Check out the best equipment you can buy: Best drivers for 2024 | Best irons for 2024 | Best putters for 2024 | Best golf balls for 2024
Most Popular
Golfweek's best 2024: top public-access golf courses in every state, ranked, golfweek's best 2024: top private golf courses in every state, ranked, photos: rory mcilroy, peyton manning, chris pratt and augusta national chairman fred ridley headline 2024 memorial tournament pro-am, jim furyk announces u.s. president's cup captain's assistants, and one is a match-play bulldog, titleist gt2, gt3 and gt4 woods debut at 2024 memorial tournament, the memorial tournament 2024 odds, course history and picks to win, golf's longest day: see who survived 2024 u.s. open qualifying to advance to pinehurst.
LGBTQI+ People and Substance Use
- Research has found that sexual and gender minorities, including lesbian, gay, bisexual, transgender, queer, and intersex people (LGBTQI+), have higher rates of substance misuse and substance use disorders than people who identify as heterosexual. People from these groups are also more likely to enter treatment with more severe disorders.
- People in LGBTQI+ communities can face stressful situations and environments like stigma and discrimination , harassment, and traumatic experiences . Coping with these issues may raise the likelihood of a person having substance use problems.
- NIDA supports research to help identify the particular challenges that sexual and gender minority people face, to prevent or reduce substance use disorders among these groups, and to promote treatment access and better health outcomes.
Latest from NIDA

A Plan to Address Racism in Addiction Science
Find more resources on lgbtqi+ health.
- Hear the latest approaches in treatment and care from experts in the fields of HIV and SUD in this NIDA video series, “ At the Intersection .”
- See the Stigma and Discrimination Research Toolkit from the National Institute of Mental Health.

IMAGES
VIDEO
COMMENTS
Summary. Tobacco addiction can cause significant harm to the human body and health. It is necessary to treat the symptoms of smoking to combat it and the psychological force of the habit. Scientists have identified a gene in the standard DNA strand that increases the likelihood of developing nicotine addiction and, as a result, lung cancer.
500 Words Essay On Smoking. One of the most common problems we are facing in today's world which is killing people is smoking. A lot of people pick up this habit because of stress, personal issues and more. In fact, some even begin showing it off. When someone smokes a cigarette, they not only hurt themselves but everyone around them.
Introduction. Tobacco use is a global epidemic among young people. As with adults, it poses a serious health threat to youth and young adults in the United States and has significant implications for this nation's public and economic health in the future (Perry et al. 1994; Kessler 1995).The impact of cigarette smoking and other tobacco use on chronic disease, which accounts for 75% of ...
INTRODUCTION. Smoking is known as one of the top 3 disease-causing factors worldwide, along with hypertension and air pollution [].The World Health Organization reported that tobacco claims half of smokers' lives and that smoking causes about six million deaths every year [].Among these deaths, over 600 000 occur in non-smokers exposed to secondhand smoke.
Smoking is the practice of inhaling smoke from burning plant material. Nicotine works on your brain to create a relaxing, pleasurable feeling that makes it tough to quit. But smoking tobacco puts you at risk for cancer, stroke, heart attack, lung disease and other health issues. Nicotine replacements and lifestyle changes may help you quit.
Views of addiction have continued to evolve and change, driven variously by societal trends, medical opinion, and research findings. This article examines the question of whether addiction impairs or even destroys free will, based on a review of the research literature on smoking cigarettes.
Abstract. Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will ...
Regular use of tobacco products leads to addiction in many users. Nicotine is a drug that occurs naturally in tobacco and it's thought to be as addictive as heroin or cocaine. How nicotine affects you. Nicotine and other chemicals in tobacco smoke are easily absorbed into the blood through the lungs. From there, nicotine quickly spreads ...
Smoking tobacco is both a physical addiction and a psychological habit. The nicotine from cigarettes provides a temporary—and addictive—high. Eliminating that regular fix of nicotine causes your body to experience physical withdrawal symptoms and cravings. Because of nicotine's "feel good" effect on the brain, you may turn to ...
The Biological Aspects of Nicotine Addiction Nicotine is the critical factor in tobacco smoke that dictates addiction and continued use of tobacco (Stolerman and Shoaib 1991; Belfour and Fagerstrom 1996; Benowita 1996; Rose and Corrigall 1997). Markou (2008) stated that "nicotine is one of the main psychoactive ingredients in tobacco that ...
Essay On Electronic Cigarettes. According to Statistics, "Tobacco kills almost 6 million people each year, according to the World Health Organization (Senthilingam).". The use of tobacco increases the user's risk for a large range of cancers, including lung, mouth, larynx, stomach, kidney and much more.
1-3-1 ESSAY Did you know three cigarettes could get one addicted? Every time one takes a puff of a cigarette they're playing a dangerous game with life. I always wondered, Why are cigarettes so deadly, what is in cigarettes, if people know they're deadly then why do they still smoke them, a...
The addiction to tobacco, nicotine, and smoking is something the humans have embraced and battled since the early 1800's. With more and more people falling into the habit and becoming addicted, many detrimental health effects on the body caused people to question what was going on and what was causing these negative reactions in the body.
Smoking Addiction Essay. Smoking is an extremely addictive habit that usually forms in the early teen years. We should be targeting our children from the time they enter elementary school to prepare them for this temptation and encourage them to steer clear of this problem (Schoebel 287). There is no sure cure for smoking, and every method ...
E-cigarettes were introduced in the early 2000s as a way for smokers to manage their nicotine addiction without exposing themselves to the well-documented harms associated with the toxins and ...
The theme for this year's World No Tobacco Day, "Protecting Children from Tobacco Industry Interference," underscores the critical need to prevent future generations from falling prey to the harmful impact of tobacco use.. About 8 million people die each year from tobacco-related diseases, accounting for 13 percent of deaths worldwide.The industry's strategic targeting of youth through ...
The chemicals present in cigarettes can damage the lungs, heart, and other vital organs. Smoking increases the risk of developing lung cancer, heart disease, stroke, and chronic obstructive pulmonary disease (COPD). Additionally, it can lead to addiction, respiratory problems, and premature aging. Negative Impact on Physical Development
Introduction. More and more people become addicted to smoking every year. However, few of them are aware of the health damages all smokers may face in a long-term perspective. Smoking causes such incurable diseases as cancer, diabetes, asthma, or blindness. That is why many people nowadays substitute tobacco cigarettes with something they ...
Kumar, A. et al. presented a clinic-pathological investigation that showed that 29.4% of people with established oral cancer cases chewed only tobacco, 25.5% only smoked, 42.2% chewed both types of tobacco (smoke and nonsmokers), and 2.9% did not chew tobacco. 83.3% of those who solely chewed tobacco had oral cavity malignancies, with 6.7% ...
A single pod from one vape manufacturer contains 0.7 mL of nicotine, which is about the same as 20 regular cigarettes. Despite its extremely addictive nature, people can successfully quit using nicotine with personalized approaches, especially under the guidance of physicians who understand addiction.
However, while fewer women might smoke on average, previous studies have shown that women are more likely to develop a nicotine addiction and tend to become addicted more quickly with lower ...
In 2017, the U.S. Food and Drug Administration (FDA) announced a regulatory plan to reduce the nicotine content of cigarettes. This study examines the association of exposure to industry-sponsored corrective statements on perceptions of the addictiveness of low-nicotine cigarettes relative to typical cigarettes within the general US population.
Join now to read essay Cigarette Addiction. Issac Literature Review Introduction: There has been an increasingly negative stigma surrounding cigarette smoking following public education efforts with regards to the detrimental effects of the habit. This social dynamic has produced an environment of increasing costs for cigarette companies in the ...
This year's World No Tobacco Day focuses on youth, with a campaign that will provide a platform for young people across the world to urge governments to stop predatory tobacco marketing tactics. The industry has long targeted youth, hoping to create a new wave of addiction and never-ending profits. Evidence shows that tobacco companies seek to attract children and youth, including with ...
Substance abuse and addiction cost the country more than $740 billion annually in terms of healthcare expenses, crime-related costs, and lost productivity In 2017, approximately 4 % of the teens aged between 12 and 17 suffered from a drug use disorder
World No-Tobacco Day, created by the WHO in 1987, aims to highlight health risks of tobacco use globally. Scroll down to learn about the foods that can help fight tobacco addiction among youths.
"Well, '77 was the second year of the tournament and I spent most of my time on the golf course picking up papers and cigarette butts," Nicklaus said Tuesday at Muirfield Village Golf Club in his annual press conference before the 2024 Memorial. Nicklaus' caddie, Angelo Argea, had to empty the pockets of his bib every 3-4 holes because ...
IN AN EMERGENCY: Are you or someone you know experiencing severe symptoms or in immediate danger? Please seek immediate medical attention by calling 9-1-1 or visiting an Emergency Department.Poison control can be reached at 1-800-222-1222 or www.poison.org.; Are you or someone you know experiencing a substance use and/or mental health crisis or any other kind of emotional distress?