- Open access
- Published: 01 June 2021

The value of the patient and public contribution to cancer research UK’s review of covid-19 impact on its clinical research portfolio
- Anne Croudass ORCID: orcid.org/0000-0001-6584-7999 1 &
- Richard Stephens 2
Research Involvement and Engagement volume 7 , Article number: 35 ( 2021 ) Cite this article
2904 Accesses
1 Citations
25 Altmetric
Metrics details
In July 2020 Cancer Research UK undertook a rapid review of the studies in its clinical research portfolio to assess the impact of the Covid-19 pandemic. The review examined over 160 research studies funded by the charity, and in keeping with its usual practice, the charity involved patient/public contributors in the review process.
Cancer Research UK (CRUK) spends over £450 million pa on research, including clinical trials, tissue collections, laboratory science and biomarker studies. It has involved patient/public contributors in clinical research funding decisions for ten years, recruiting volunteers from the National Cancer Research Institute’s (NCRI) Consumer Forum. The NCRI is a partnership of funders, including the 4 UK governments and major charities such as CRUK. Its Consumer Forum is a group of volunteers with personal experience of cancer as patients or carers, who are trained for and experienced in working on national strategic bodies as well as on individual research studies.
The CRUK whole-portfolio review was held over a two-week period in a series of online meetings. A pair from the team of patient/public contributors was included in each meeting, and they made comments on every application reviewed as well as participating in reaching decisions.
Conclusions
The process not only demonstrated CRUK’s continued commitment to involving patient/public contributors in their funding decisions, but also provided an opportunity for these contributors to take a holistic view of processes to inform future patient/public contribution in the charity’s work, as well as to influence the decisions about the individual studies being reviewed.
Plain English summary
The process not only demonstrated CRUK’s continued commitment to involving patients and the public in their funding decisions, but also provided an opportunity for these contributors to take a holistic view of processes to inform future patient/public contributionin the charity’s work, as well as to influence the decisions about the individual studies being reviewed.
Peer Review reports
Clinical trials play an essential role in determining the effectiveness and safety of new cancer drugs, and in the process provide patients with access to potentially life-saving new treatments still early in development. Cancer Research UK (CRUK) funds nearly 200 clinical studies at any one time in order to make progress in achieving the charity’s ambition that by 2034, 3 in 4 patients will survive their cancer by ten or more years [ 1 ].
In July 2020 Cancer Research UK undertook a rapid review of the studies in its clinical research portfolio. This review was in response to the challenges posed by the initial impact of the Covid-19 pandemic, which included an almost blanket suspension of recruitment to non-Covid clinical trials by sponsors, investigators and study sites, redeployment of National Health Service (NHS) and laboratory staff, the closure of university laboratories, some changes to standard of care and for many patients, reduced access to resources and services. The purpose of the review was to assess the impact of Covid-19 on these clinical trials and on the infrastructure supporting them and to establish whether they would still be viable in a “post-Covid” world.
Whilst it was not a criteria for judgements in the review, the Covid-19 pandemic had already significantly affected charitable income. Therefore it was important to understand which studies would be able to complete recruitment, how much delay there might be, and if the finished study would then still contribute to CRUKs strategic aims within the context of a more limited budget to support research in the short-term future.
Since 2011 CRUK has routinely involved patient/public contributors in the funding decisions made by its Clinical Research Committee (CRC) and various feeder panels (Fig. 1 ), and the rapid portfolio review was no exception. With the potential impact of Covid 19 on cancer patients, care and clinical research, having the patient’s perspective was essential to the portfolio review process, as decisions made as part of the review could potentially have an impact on those patients already recruited to trials, and might also affect future cancer patients.
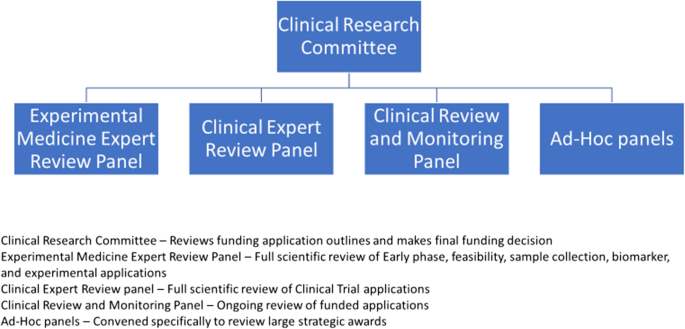
CRC and associated panels
Cancer Research UK
CRUK is the world’s largest charity dedicated to saving lives through research. In 2019/20 CRUK spent £455 million on research, including laboratory based science, prevention, clinical trials and infrastructure awards. CRUK works with over 150 hospital trusts, supports over 4000 Researchers, Doctors and Nurses and funds up to 200cancer research studiesacross all cancer sites [ 2 ].
The review process
The review included 163 current clinical studies of the 180 funded or endorsed through the CRC (Table 1 ). These included clinical trials sample collections, experimental medicine awards and biomarker awards. The 17 studies that were not included had either completed all follow up and were in the write-up stage or were biomarker projects that were funded by CRUK and did not rely on NHS resources. At the time of the review, these were deemed unlikely to be directly affected by the impact of Covid-19.
Review meetings were held using the Microsoft Teams platform, and focused on the progress or otherwise of the studies up to the end of May 2020 (ie 4–5 weeks before the review), their likely new reporting dates and the continued relevance of the findings at that point. The review was held over several days, with studies being grouped to reflect the topic areas of the National Cancer Research Institute (NCRI) Research Groups (RG) (Table 2 ).
Each review session was undertaken by a panel of between 12 and 15 members, including scientists, clinicians, statisticians and patient/public contributors, as is usual for CRUK clinical research funding committees. In addition, for this whole-portfolio review the NCRI RG Chairs or their nominated representative attended relevant sessions, so that the recommendations of the panel took account of the particular NCRI RG’s strategic priorities for their national portfolios.
The role of patient and public contributors
CRUK has had patient/public representation on its funding committees for over 10 years. Initially there was one contributor, on the main funding committee only, and the role was as an observer to ensure due process. The role and remit have evolved so that there are now two patient/public contributors on each panel and committee. They are full voting members, and as such they have equal scoring rights, are bound by the same confidentiality, governance and conflict requirements and are offered the same honoraria as other members. They comment on each application under consideration. They provide the perspective of people affected by cancer on applications, considering areas including but not limited to, the patient acceptability of study design, the value of the study aims to the intended patient populations and whether there are any potential unexpected adverse effects from the point of view of the participants. They also provide insight and advice on the level of patient and public involvement in the applications received.
All the current CRUK patient/public committee members are, or have previously been, active members of the NCRI Consumer Forum (Table 3 ). This membership is extremely beneficial as it confers the level of understanding, training and professionalism required to contribute fully at CRUK (and other) strategic research meetings. The patient/public contributors involved in the rapid portfolio review were those who also sit on the Panels and Committees shown in Fig. 1 .
This whole-portfolio review was a new experience for the team at CRUK and for the patient/public contributors. Working with their mentor (CRUK’s lead research nurse), the contributors were assigned in pairs to cover up to 3 of the 6 review meetings each. For the Paediatric/Teenager and Young Adult review, patient/public contributors with specific experience in this field were recruited from the NCRI Consumer Forum. For the other 5 review meetings, all the contributors had had previous experience of working with CRUK funding committees or other CRUK research initiatives. The contributors knew each other and had worked together previously, which for this task was another benefit.
Debbie Keatley, PPI representative stated,
“To be honest, the request from CRUK for public members to be involved in the reviews that took place in summer 2020 felt daunting. This felt very different to funding and monitoring meetings in the course of an ordinary year and I was honoured to take part but under the circumstances it could not be anything but a difficult process.”
As this review was different to the standard funding committee meetings, a new template form was developed by CRUK and the patient/public contributors to guide and capture their feedback (Table 5 in Appendix ). This reflected the questions asked of those submitting the trial paperwork for review and provided consistency across each meeting. For each study discussed, 3 lead reviewers were nominated; a clinician, a statistician and a patient/public contributor, ensuring that the patient view was given equal consideration to the scientific views. After each meeting there was opportunity for a debrief with theirmentor, where the contributors could reflect on the meeting and make suggestions that would improve their experience of the process for subsequent meetings.
Involving patient/public contributors in this review demonstrates CRUKs commitment to putting patients at the heart of all that the charity undertakes. It was evident to CRUK staff and participating researchers that the patient/public contributors were adding a unique and essential expertise. Without exception they were well prepared and engaged. They were flexible and accommodating to the tight schedules, new technological requirements and evolving time frames, giving concise, thoughtful and objective feedback throughout. Most importantly the patient voice was not only heard but carried equivalent weight to that of other panel members.
Mat Baker commented,
“I was delighted to have the opportunity to contribute to this research review and to the future of so many potentially practice changing trials. It was important to ensure that the patient perspective was clearly articulated, and I was pleased that its value in the decision-making process was so positively acknowledged.”
The value of a pool of patient/public contributors with appropriate skills to respond at short notice and to contribute effectively to the review validated the CRUK stance that for this type of strategic meeting and decision-making process, patient/public contributors representatives should ideally have a background understanding of the research environment, such as that provided by NCRI involvement.
Ian Walker, Director of Research Funding, Communications and Partnerships said,
“As always, the comments from our patient contributors were insightful, thoughtful and added great value to the discussions. The insights and intelligence we have gathered through the process will provide us with really important data to support both our research agenda and our policy priorities going forward.”
The portfolio review had to make difficult decisions about the future of clinical trials. Involving patient/public contributors in these decisions gives credibility to those decision and outcomes, for the cancer community as a whole and in particular for people affected by cancer, especially those participating in research studies.
Paula Ghaneh, Professor of Surgery, University of Liverpool and Chair of the Upper GI and Colorectal review meeting commented,
“The patient contribution to the portfolio review was and continues to be extremely valuable. In all manner of committee meetings, they always manage to sum up the key issues in a clear and precise way. With the ethos of CRUK at the centre of their arguments, they remind us of all the people who raise the money for CRUK and what really matters for patients. They always give us the perspective to make the best decisions even if they are difficult or tough.”
A further benefit of involving patient/public contributors in the review was the identification of cross cutting themes. Four of the contributors attended at least three review sessions each, whereas the majority of the other panellists attended only one or two. Moreover, the patient/public contributors worked together informally during the review and more formally afterwards to identify themes for CRUK and for other patient/public contributors to consider for the future. These included
the need for robust remote assessment processes
the involvement of primary care in delivering protocol-led care
the need to update patient information to reflect impact of Covid-19 and the opportunity to incorporate electronic consenting procedures.
The patient/public contributors also collated a report for their NCRI Consumer colleagues. As well as providing an overview of the process and outcomes, it included reflections on how their involvement in this review could benefit and inform wider NCRI consumer activity (Table 4 ). By circulating the report to all 100 members of the NCRI Consumer Forum, they encouraged other patient/public contributors to discuss, debate and disseminate the information in the report.
This was a further demonstration of the value of having patient/public contributors linked into NCRI consumer activities and thence to their own national, international, local and online networks of patient representatives and groups. Mat Baker observed,
“CRUK have once again demonstrated that they are at the forefront of good practice in involving patients and carers at the heart of the research decision making process. A necessary corollary is that patients and carers possess the knowledge and skills to contribute effectively at this level. Fortunately the NCRI Consumer Forum, through its collaborative ethos and exacting standards, enables patients and carers such as myself to step up and to forge partnerships with the leading teams in cancer research.”
The novel nature of the review for both CRUK staff and committee members provided equal opportunity for patient/public contribution to the discussions and in the decisions. This increased the levels of engagement and responsibility of the patient/public contributors,, demonstrated their ability to provide useful and relevant input and kept patients at the centre of the process. As Debbie Keatley said,
“This was an extraordinary series of reviews, brought about by extraordinary events but at the end of it all were real patients, and for some, taking part in *their* trial offered access to otherwise unavailable treatments and not being able to take part carried real consequences. It was sobering to absorb how hard research teams had worked to keep trials open wherever possible, to adjust protocols, to attempt to keep as much valuable work and learning as possible and to restart as soon as possible. It was clear that CRUK staff too had worked extremely hard to support trials, and us - a resource intensive process, providing us with rich information and context. The impact of COVID-19 on clinical research will be felt for a long time but we found many examples of good practice under very difficult conditions and the recommendations we made were taken together, equally, with outcomes for current and future patients held firmly in mind.”
This review has provided CRUK with a further opportunity to develop the patient/public contributor role in funding committees and has prompted a review of the patient/public contribution to funding committee practices. This will lead to a piece of work to further strengthen the patient/public contribution, including increasing the number of patient/public contributors involved, and will be developed jointly by staff and patient/public contributors.
CRUK would like to thank all the patient/public contributors, Mat Baker, Debbie Keatley, Angela Polanco, Janette Rawlinson, Richard Stephens and Max Williamson for their valuable input to the review.
Availability of data and materials
Not Applicable.
Abbreviations
Clinical Research Committee
National Cancer Research Institute
National Health Service
Research Group
https://www.cancerresearchuk.org/about-us/our-organisation/our-strategy-to-beat-cancer-sooner Accessed 31 Mar 2021.
https://www.cancerresearchuk.org/ Accessed 6 Jan 2021.
https://www.ncri.org.uk/ Accessed 6 Jan 2021.
https://www.ncri.org.uk/groups/ Accessed 6 Jan 2021.
https://www.ncri.org.uk/how-we-work/patients-carers/ncri-consumer-forum/ Accessed 6 Jan 2021.
https://www.ncri.org.uk/wp-content/uploads/NCRI-Group-Membership-Consumer-Recruitment-Pack-UPDATED-17.12.pdf Accessed 2 Apr 2021.
Download references
AC is an employee of Cancer Research UK.
RS received an honorarium for his contribution to the portfolio review, but no payment for his authorship of this article.
Author information
Authors and affiliations.
CRUK Lead Research Nurse, 2 Redman Place, London, E20 1JQ, UK
Anne Croudass
Patient and Public Representative, 18 Russell Close, Stevenage, PB, SG2 8, UK
Richard Stephens
You can also search for this author in PubMed Google Scholar
Contributions
AC and RS were co-authors on this paper. As such, both have contributed equally, and have read and approved the final manuscript.
Corresponding author
Correspondence to Anne Croudass .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
AC has no competing interests.
RS is co-editor in chief of the Research Engagement and Involvement journal.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
For trials in set up
Study name | |
Can the study continue as originally planned? Please consider the impact of the pandemic on patients’ safety, Inclusion/Exclusions criteria, deliverability in post-COVID19 NHS. | |
Do you think the potential impact of the study for cancer care and cancer patients has changed? Will this be practice changing? | |
Do you think the study will still be of interest to patients, and is it likely to recruit? | |
Will the study need revised/additional PPI to start? | |
What would be the ethical considerations/ implications for patients if the study did not go ahead? | |
Is this study still value for money in light of budget reduction? | |
Any other comments |
For trials open to recruitment
Study name | |
Could this trial restart as per protocol? Please consider the impact of the pandemic on patients’ safety, Inclusion/Exclusion criteria, changes to standard of care arms if applicable, and deliverability in post-COVID19 NHS | |
Impact of Covid on recruitment. | |
Do you think the study will still be of interest to patients, and is it likely to continue recruiting? | |
Has the potential impact of the study for cancer care and cancer patients been affected? Is this practice changing? | |
Will the study need revised/additional PPI to restart? | |
What would be the ethical considerations/ implications for patients if the study was discontinued? Would anything be lost if this study closed and reported now on the data available? | |
Is the study still value for money in light of budget reduction | |
Any other comments |
For trials in follow up
Will the follow up schedule for this study need to change? Please consider patients’ safety, alternative methods of obtaining follow-up data, financial implications of changing the follow-up schedule. | |
Could the study follow-up be stopped ow? | |
Is any additional PPI input required? | |
What would be the cost/benefit of completing follow up? | |
What would be the ethical considerations/ implications for patients if the study stopped early? | |
Any other comments |
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Croudass, A., Stephens, R. The value of the patient and public contribution to cancer research UK’s review of covid-19 impact on its clinical research portfolio. Res Involv Engagem 7 , 35 (2021). https://doi.org/10.1186/s40900-021-00279-w
Download citation
Received : 11 February 2021
Accepted : 03 May 2021
Published : 01 June 2021
DOI : https://doi.org/10.1186/s40900-021-00279-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Patient/public contributors
- Clinical trials
Research Involvement and Engagement
ISSN: 2056-7529
- General enquiries: [email protected]
Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Policy & Insight
- Charity News
Improving research with registered reports
16 May 2022
We're launching a new scheme, in collaboration with the University of Bristol and a consortium of journals, to streamline the way researchers can publish their research, irrespective of the findings. Here we chat to Professor Marcus Munafò – a member of one of our funding committees – to find out what a Registered Report is, how you can get involved, and why this relatively small change in the way research is done could have a real impact on reproducibility issues and even patient outcomes.
So, first up – what exactly are Registered Reports?
Registered Reports are an article type offered by an increasing number of journals. When published, these articles look no different to a conventional article. What is different is the process they’ve been through.
Registered Reports are reviewed in two stages. At stage 1, the study protocol is reviewed – roughly, the introduction and methods of what will eventually become the full article – before any data have been collected. The focus is on whether the research question is important and the methodology robust. If the article passes stage 1 review it is given ‘in-principle’ acceptance, which means that it will be published once the data have been collected regardless of the eventual outcome.
The stage 2 review is a light touch check. In principle, this approach ensures that studies are designed to be informative regardless of the nature of the eventual results and removes incentives to focus on positive results only.
Will this way of reporting their work involve more work for researchers and what benefits could they see by participating in the pilot?
It’s more a matter of the work being in a different place, with much of the writing and the peer review happening before data collection rather than afterwards. Since the focus is on reviewing the study before data collection, reviewers can recommend changes at the point where these changes can still be incorporated.
There are therefore reasons to believe that the quality of Registered Reports may be higher than conventional articles. In addition, once ‘in-principle’ acceptance is offered the authors can be confident that their work will be published, and rapidly, once data collection is complete. It’s important to note that deviations from the stage 1 submission are permissible, but these need to be justified and described clearly as such. Sometimes this requires additional review. But overall, the hope is that Registered Reports will support higher quality work and more rapid publication.
Registered Reports have been offered from a number of journals for a while, why is involving the funders an important step?
It’s great that journals offer Registered Reports, but it’s important that there are incentives for researchers to try this format. By joining up journals and a funder to create a single, coordinated process, the hope is that applicants for funding will be encouraged to try Registered Reports.
The rationale is that the study protocol required for stage 1 submission is similar to a grant proposal, and the logical next step after funding has been awarded is to develop the study protocol. It’s a small step from there to submitting the protocol for consideration as a Registered Report. There is also scope for greater efficiency – for example, if some of those who reviewed the grant also review the stage 1 submission, this will be a relatively small amount of work compared with seeing the submission for the first time.
Do you think every area of cancer research is equally applicable to this way of working?
Registered Reports work very well for some study designs – particularly those where new data are being collected to test a focal hypothesis. But they can be surprisingly flexible – the basic approach has been used for multi-experiment studies, observational studies, secondary data analyses, and qualitative research. It’s really for researchers themselves to decide whether the format works for them, but the format isn’t intended to be prescriptive. The principle of the methodology being reviewed before data collection is the important thing, as well as the publication decision not being dependent on the nature of the results, but rather on the importance of the question and the robustness of the methodology. This can certainly be applied to different types of research.
This way of doing ‘open science’ could really help with aspects of the ‘reproducibility crisis’ – do you think it’ll be enough?
I don’t really like the “crisis” narrative. What we’re seeing across the sector are lots of innovative approaches to how we conduct our research being developed and piloted. The focus, for me, is thinking about how we can continuously reflect on, and improve, our working practices to ensure our research is of the highest quality possible. This focus on improving quality should, hopefully, also mean that the knowledge we generate translates into clinical or societal benefit more rapidly. No single approach – including Registered Reports – will be a silver bullet though.
In general, I think transparency in research is valuable for a number of reasons, and Registered Reports are part of this. But it’s also important to remember that we will have to evidence these new approaches to see whether they do in fact work as intended, and check that they don’t have any unintended consequences. Hence the need for meta-research – or research on research.
Could we even go so far as to suggest this could help with speeding up the patent benefit of research?
There are good reasons to believe that focusing on improving the quality of research outputs should also speed up the translation of that knowledge into patient benefit. Science self-corrects, eventually, but it will do so more efficiently (and have less need to!) if we generate more robust knowledge in the first place. We’ll always need to balance the need to generate robust evidence with the need to take risk and pursue new directions, but in my view that balance isn’t quite right at the moment.
Ultimately, this is an empirical question we can answer by collecting and evaluating evidence. With the variety of innovative approaches to research and publication that we’re seeing emerging – including Registered Reports – we should eventually be able to determine whether these new ways of working do in fact produce more robust findings, and in turn whether this speed up translation into patient benefit.
Want to find out more about Registered Reports? Join our webinar on 19 May
You can find out more about Registered Reports here and our pilot scheme here

He is currently Editor-in-Chief of Nicotine and Tobacco Research, and a member of the Cancer Research UK Prevention and Population Research Committee.
Highlighted content
34,000 cancer deaths could be prevented if we make this election a turning point for cancer, thousands of nhs patients to access trials of personalised cancer 'vaccines', sarah harding's legacy: finding women who may have higher breast cancer risks, at-home saliva test could help diagnose prostate cancer sooner, asco 2024: data on weight loss drugs and a melanoma vaccine, a test that can predict breast cancer relapse, and more, one to one with rhian: volunteering special 2 - that cancer conversation podcast, general election 2024: what does this mean for the tobacco and vapes bill, skin cancer cases reach all-time high, an animal's guide to staying safe in the sun.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Journal Information
On this page: Aims & Scope | Subject Categories | Journal metrics | Indexing | Newsfeeds | Journal details | Submissions video
Aims and Scope
The British Journal of Cancer ( BJC ) is one of the most cited general cancer journals, it is committed to publishing cutting edge discovery, translational and clinical cancer research. The BJC aims to provide a global platform to disseminate important research within the broad spectrum of oncology. The journal welcomes research across all cancer types and has a focus on: metastasis, microenvironment, immunology and immunotherapy, targeted and next-generation therapeutics, chemotherapy and radiotherapy, mechanisms of resistance, clinical trials, genomics, epigenomics and precision medicine, epidemiology, metabolism and state-of-the art diagnostic approaches.
The BJC is published in association with Cancer Research UK , the world’s leading independent cancer charity dedicated to saving lives through research.
Submit your next manuscript to BJC and benefit from:
- International and highly respected editorial team
- Rapid decision and publication times
- High exposure and article visibility via nature.com
- Funder-compliant open access options available
- Engaged and growing Twitter following at @BrJCancer
- Share your article through SharedIt: Springer Nature’s content-sharing initiative allowing authors and subscribers to share links to view-only, full-text articles from this journal
Subject Categories
BJC publishes across the following six subject categories:
- Clinical Studies
- Translational Therapeutics
- Molecular Diagnostics
- Genetics and Genomics
- Cellular and Molecular Biology
- Epidemiology
Translational Therapeutics Translational Therapeutics is dedicated to scientific work that has possible direct applicability in clinical uses or opens new avenues for treatments. The type of manuscript ranges from basic research to early informative clinical trials with extensive concomitant biomaterial analyses. Translational concepts include new computational or wet lab Technologies as well.
Molecular Diagnostics The Molecular Diagnostic subject category considers studies that examine genomic, transcriptomic, epigenomic, and proteomic biomarkers in the context of cancer diagnosis, staging, prognosis, therapeutic prediction, and radiographic and pathologic characteristics. We are particularly interested in papers that explore novel or evolving biomarkers with direct clinical applicability in cancer care.
Genetics and Genomics This subject category covers the use of genetic epidemiology and bioinformatics, together with molecular and cell biology, to identify and characterise the genetic and epigenetic factors underlying tumour development and progression. We are interested in papers which focus on the mechanistic consequences of genetic variance, and adequately powered studies of how genetic and epigenetic variation impacts patient risk and outcome phenotypes, in particular where these point to new clinical approaches. Wherever possible, bioinformatics analyses should be supported by experimental results.
Cellular and Molecular Biology The Cellular and Molecular Biology subject category considers studies that provide novel insight into basic cellular and molecular mechanisms of cancer, but are not biomarker, methodology, or pathology focused. Studies considered for this category will generally go beyond in vitro and/or in silico evaluation of basic cellular and molecular mechanisms of cancer, and usually include the use of appropriate in vivo model systems. Findings of studies considered for this category should be clinically and/or therapeutically applicable.
Epidemiology The Epidemiology subject category focuses on prevention, early detection, and factors that influence the risk and prognosis of cancer.
To explore the type of articles that the BJC publishes, please browse our subject pages .
Journal Metrics
Article metrics such as number of downloads, citations and online attention are available from each article page, and provide an overview of the attention received by a paper.
2022 Citation Metrics
2-year Impact Factor*: 8.8 5-year Impact Factor*: 8.4 Immediacy index*: 1.2 Eigenfactor® score*: 0.03984 Article influence score*: 2.4 Journal Citation Indicator*: 1.38 SNIP**: 1.928 SJR***: 2.867
*2022 Journal Citation Reports® Science Edition (Clarivate Analytics, 2023) **Source-normalized Impact per Paper (Scopus) ***SCImago Journal Rank (Scopus)
2023 Peer Review Metrics
Submission to first editorial decision: 12 days Submission to Accept: 188 days
2023 Usage Metrics
Downloads: 4,588,727 Altmetric mentions: 7,049
All articles published in the BJC are included in:
EBSCO Discovery Service Google Scholar Medline Science Citation Index Science Citation Index Expanded (SciSearch) Current Contents / Clinical Medicine Current Contents / Life Sciences BIOSIS OCLC Summon by ProQuest SCOPUS EBSCO Academic Search PubMed Central EBSCO Advanced Placement Source EBSCO Biomedical Reference Collection EBSCO CINAHL EBSCO STM Source EBSCO TOC Premier INIS Atomindex
The BJC now provides its latest table of contents as an RSS web feed. This allows users with an RSS reader to receive automatic updates whenever new content is added to these pages.
Receive the BJC 's current issue table of contents.
Follow us on Twitter @BrJCancer
Journal details
ISSN and eISSN
ISSN: 0007-0920 eISSN:1532-1827.
Submissions video
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Online ISSN : 2631-5297 -->
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
Peer Review Process
All manuscripts submitted to Mak Periodical Library are subject to single blind peer review process (reviewers know the author’s identity, but not vice versa) prior to publication.
What is Peer review Process? How does peer review work?
Peer review is the central pillar of trust of researchers by which the quality of research is judged. It is designed to assess the validity, quality and often the originality of articles for publication. All submitted manuscripts are read by the editorial staff to ensure that it conforms to the submission criteria, before being forwarded to the Editor. To save time for Editors, peer-reviewers and authors, only those papers that seem most likely to meet our editorial criteria are sent for the formal review process. Usually the peer review process is done by one Editor and two or more reviewers. Those papers judged by the editors to be of insufficient general interest (or) otherwise inappropriate are rejected promptly without external review. The Editor (or) Chief Editor reserves the right to reject or to return the manuscript to the author(s) for additional changes.
The Peer reviewer: Reviewers are selected based on their expertise and experience. We check with potential reviewers before sending them manuscripts to review. Anonymous peer reviewers are the best way to get honest opinions on manuscripts. We recommend our authors, editors and reviewers to disclose any conflicts of interest (COIs) that may skew the fairness of the publishing process.
Reviewer selection: Editors usually select researchers that are experts in the same subject area as the paper. We check with potential reviewers before sending them manuscripts to review.
Editors usually select reviewers based on a few criteria:
Qualifications (Masters/PhD – depending on subject area)
The number of papers they have published in their given area of expertise
How well those papers have been cited
Recommendations from other researchers/reviewers they know or have worked with
On their reputations in the specific field of the article
We may ask authors to recommend suitable reviewers on submission of their manuscript. When recommending reviewers, the following points should be considered:
Authors should not recommend reviewers with whom they have a conflict of interest, for example, a close collaborator or colleague.
Recommended reviewers should not be at the same institute as any of the authors listed on the manuscript.
Institutional email addresses should be provided for recommended reviewers, wherever possible.
Once enough reviewers have been selected, then the manuscript will move onto the next stage. Reviewers should treat the review process as being stricter and confidential.
Reviewers Guidelines:
If you receive a manuscript that does not match to your research profile please notify the editor
Reviewers are requested to disclose the Conflicts of Interest (COIs)
Should not be discussed with anyone not directly involved in the review process
Please do not contact the author directly.
Should not disclose the reviewer’s identities to the authors or to other colleagues since they may be asked to comment on the criticisms of other referees and may then find it difficult to be objective.
A reviewer should treat a manuscript sent for review as a confidential document
Reviewers should not use or disclose unpublished information, arguments, or interpretations contained in a manuscript under consideration, except with the consent of the author
Please contact the editorial office if you require an extension to the review deadline
Possible Outcomes of Peer Review:
After the reviewers receive a paper from the editor, they read it closely and provide individual critiques, usually within the time frame. Each reviewer may recommend that the paper be rejected, revised and resubmitted, or accepted.
Accept without any changes (acceptance): The journal will publish the paper in its original form
Accept with minor revisions (acceptance): The journal will publish the paper and asks the author to make small corrections. This is typically the best outcome that authors should hope for
Accept after major revisions (conditional acceptance): The journal will publish the paper provided the authors make the changes suggested by the reviewers and/or editors
Revise and resubmit (conditional rejection): The journal is willing to reconsider the paper in another round of decision making after the authors make major changes
Reject the paper (outright rejection): The journal will not publish the paper or reconsider it even if the authors make major revisions
Peer reviewers’ comments and recommendations are an essential guide to inform the editor’s decision on a manuscript. If the reviewers suggest that the paper should be revised and resubmitted, they will leave detailed comments concerning the revisions that should be made. When the reviewers have all returned their comments (made directly on the manuscript or in a separate document), the editor makes a preliminary decision about the manuscript. The editor strips out the identity of the reviewers and sends their review comments about the manuscript to the author.
When a Revised Paper is Received:
Minor changes will usually be assessed directly by the editor
If significant revisions were requested, the editor will usually return the manuscript to the original reviewers (unless they opted out of this)
Rarely, the editor may invite comments from a new reviewer – the editor should explain why this fresh review is sought. It is important new reviewers respect previous review comments and the efforts the author has made to revise the paper
The revised manuscript should be accompanied by a Response to Reviewers Comments Letter that includes a point-by-point response to reviewer’s comments and an explanation of how the manuscript has been revised. If the manuscript was accepted with modifications, it is then up to the author to make changes until the editor is satisfied that the reviewers’ reservations are met.
When the editors have reached a final decision on the paper, they notify the corresponding author by email.
Fast Publication Process:
Our few journals are offering Fast Track Review Process. We aim to offer you the fastest possible speed of publication, without compromising on the quality of our peer-review process. Our Fast Track service is the fastest way to publish, quicker than any competitor; Support and guidance from our expert editors, every step of the way. If authors are interested in our Fast Track Review Process they can choose it. In recognition of the time constraints of the authors we are offering a Fast Track Review by paying a remuneration to the reviewer on completion of their review. Online Publication of the article is relying on the author’s response to the Galley Corrections.
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

How does Cancer Research UK evaluate research?
- We review the latest research on the causes of cancer and look at how well the research has been done.
- The health information on our website is based on lots of quality research carried out over many years.
- Make sure you use reliable information sources when it comes to cancer.
We continually review new research (scientific studies) on the causes of cancer. This is to make sure our information is up to date and based on the best quality evidence. We develop our information by looking at lots of research carried out over many years. Although new research comes out all the time, it is unlikely that one individual study or paper would change our position on a topic.
How to read a scientific paper
Some studies are better than others for telling us what affects cancer risk. These are some of the things we look at when evaluating evidence:
Did the study look at cells, animals or people? Studies in animals and cells can help scientists understand how cancer works. But they can’t always tell us how it’s relevant to humans, so we focus on studies in people.
How big was the study and how long did it go on for? Studies in a small number of people aren’t as reliable, because results are more likely to happen by chance. Studies that only follow people for a short amount of time can miss long-term effects. So we mainly look at studies that follow thousands of people over many years.
Did the study consider other factors that can affect cancer risk? There are lots of things that can affect a person’s risk of cancer. These are called risk factors. Researchers should consider known risk factors when designing a study, to make sure they don’t impact the results. For example, a study looking at air pollution and lung cancer should record whether participants smoke. This is because smoking is the biggest cause of lung cancer.
Was the study peer-reviewed and who funded it? It’s important to see if a study is published in a peer-reviewed scientific journal. This means that other experts (peers) have assessed the quality of the research and have checked the results. It’s also important to know who funded the study, as this can affect how the findings are presented. For example, Cancer Research UK disregards research funded by the tobacco industry.
How to find accurate information on cancer
Sometimes news outlets exaggerate stories about cancer. So it isn’t always clear what is supported by good evidence. You can use some of the questions above to judge a news story. Read more about spotting fake news on cancer on our blog.
The most important thing is to get your information from a trusted source. For example our website and the NHS .
You can trust health information that the Patient Information Forum (PIF) has approved.
The Patient Information Forum tick looks like this:
logo_pif.png
When you see this symbol it shows that the information is based on reliable, up to date and high-quality evidence. Cancer Research UK is a trusted information creator and our health and patient information is approved by PIF. Read more about how we write and produce our information .
Last reviewed
Rate this page:.

- Study Protocol
- Open access
- Published: 07 June 2024
The OVAREX study: Establishment of ex vivo ovarian cancer models to validate innovative therapies and to identify predictive biomarkers
- Lucie Thorel 1 , 2 ,
- Jordane Divoux 1 , 2 , 3 ,
- Justine Lequesne 4 ,
- Guillaume Babin 1 , 5 ,
- Pierre-Marie Morice 1 , 2 ,
- Romane Florent 1 , 2 , 3 ,
- Guillaume Desmartin 2 , 3 ,
- Lucie Lecouflet 2 , 3 ,
- Chloé Marde Alagama 1 ,
- Alexandra Leconte 4 ,
- Bénédicte Clarisse 4 ,
- Mélanie Briand 1 , 6 ,
- Roman Rouzier 5 ,
- Léopold Gaichies 5 ,
- Sandrine Martin-Françoise 5 ,
- Jean-François Le Brun 5 ,
- Christophe Denoyelle 1 , 2 ,
- Nicolas Vigneron 1 , 2 , 7 ,
- Corinne Jeanne 8 ,
- Cécile Blanc-Fournier 1 , 6 , 8 ,
- Raphaël Leman 9 ,
- Dominique Vaur 9 ,
- Martin Figeac 10 ,
- Matthieu Meryet-Figuiere 1 , 2 ,
- Florence Joly 4 ,
- Louis-Bastien Weiswald 1 , 2 , 3 ,
- Laurent Poulain 1 , 3 , 6 na1 &
- Enora Dolivet 1 , 5 na1
BMC Cancer volume 24 , Article number: 701 ( 2024 ) Cite this article
Metrics details
Ovarian cancer is the first cause of death from gynecological malignancies mainly due to development of chemoresistance. Despite the emergence of PARP inhibitors, which have revolutionized the therapeutic management of some of these ovarian cancers, the 5-year overall survival rate remains around 45%. Therefore, it is crucial to develop new therapeutic strategies, to identify predictive biomarkers and to predict the response to treatments. In this context, functional assays based on patient-derived tumor models could constitute helpful and relevant tools for identifying efficient therapies or to guide clinical decision making.
The OVAREX study is a single-center non-interventional study which aims at investigating the feasibility of establishing in vivo and ex vivo models and testing ex vivo models to predict clinical response of ovarian cancer patients. Patient-Derived Xenografts (PDX) will be established from tumor fragments engrafted subcutaneously into immunocompromised mice. Explants will be generated by slicing tumor tissues and Ascites-Derived Spheroids (ADS) will be isolated following filtration of ascites. Patient-derived tumor organoids (PDTO) will be established after dissociation of tumor tissues or ADS, cell embedding into extracellular matrix and culture in specific medium. Molecular and histological characterizations will be performed to compare tumor of origin and paired models. Response of ex vivo tumor-derived models to conventional chemotherapy and PARP inhibitors will be assessed and compared to results of companion diagnostic test and/or to the patient’s response to evaluate their predictive value.
This clinical study aims at generating PDX and ex vivo models (PDTO, ADS, and explants) from tumors or ascites of ovarian cancer patients who will undergo surgical procedure or paracentesis. We aim at demonstrating the predictive value of ex vivo models for their potential use in routine clinical practice as part of precision medicine, as well as establishing a collection of relevant ovarian cancer models that will be useful for the evaluation of future innovative therapies.
Trial registration
The clinical trial has been validated by local research ethic committee on January 25th 2019 and registered at ClinicalTrials.gov with the identifier NCT03831230 on January 28th 2019, last amendment v4 accepted on July 18, 2023.
Peer Review reports
Ovarian cancer: epidemiology and therapeutic management
Ovarian cancers are responsible for over 207.000 deaths worldwide in 2022, and in 80% of epithelial ovarian carcinoma cases the diagnosis is made at an advanced stage (FIGO III/IV), making it the first cause of death from gynecological malignancies [ 1 , 2 ]. Optimal surgery and platinum-based chemotherapy are the basis of the treatment of epithelial ovarian cancers. The treatment timeline will be based on the stage, resectability of the carcinomatosis, histological type and comorbidities of the patients. Even if first-line carboplatine/paclitaxel combination achieves response rates close to 80%, among patients whose tumors were initially sensitive to treatment, 75% relapse within 18 months, eventually developing chemoresistance [ 3 ]. The introduction of new treatments and the evolution of protocols over the last thirty years have only marginally improved overall survival, which remains around 45% at 5 years [ 4 ]. In ovarian cancers, innovative treatments are struggling to become established, and the only recognized and used prognostic factors (i.e. impacting management modalities) are stage of dissemination, residual tumor mass after excision, histology and the homologous recombination (RH) status. The development of new therapeutic strategies likely to overcome chemoresistance therefore remains a major challenge.
Over the years, targeted therapies such as antiangiogenic treatments and PARP inhibitors (PARPi) have been developed first as a treatment for recurrences before being recommended in first line, thanks to their effectiveness. Anti-angiogenic therapies (bevacizumab) have found their place in the management of these cancers with a real benefit in terms of quality of life, but very modest in terms of overall survival [ 5 , 6 ]. However, it still showed greater effectiveness in at-risk groups (inoperable stage III, unable to be debulked to < 1 cm maximum disease, and stage IV disease) [ 5 , 7 ]. In the other hand, PARPi have revolutionized the therapeutic management of epithelial ovarian cancers (EOC) [ 8 ]. All the different trials showed a significant improvement of progression-free survival in patients with EOC, in first-line and second-line or later maintenance therapy. However, PARPi provided the greatest clinical benefit in patient tumor carrying BRCA1/BRCA2 mutation or exhibiting homologous recombination deficiency (HRD). Indeed, PARP enzymes play a role in DNA repair and their inhibition leads to an accumulation of single and then double-strand breaks that will cause synthetic lethality in an HRD context. Although there is no companion test for carboplatin or bevacizumab, some have been developed for PARPi such as Myriad test or GIScar based on the HRD signature [ 9 , 10 ]. The development of a companion test is a key step in the development of new therapies to enable personalized medicine: having a suitable treatment for presumed sensitive tumors and avoiding unnecessary and potentially toxic treatment for patients. Functional tests could therefore be used to improve HR status profiling and accurately identify HRD tumors, as well as enabling the implementation of companion tests for other treatments [ 11 ].
Predictive functional assays
Functional precision medicine is a strategy whereby live tumor cells from patients are directly exposed to drugs to provide translatable, personalized information to guide therapy [ 12 ]. This approach generates dynamic, functional data that may highlight key vulnerabilities not necessarily driven by genomic alterations. Predictive functional assays rely on the ex vivo (or in vivo) modelling of a patient tumor from pathologically-qualified samples obtained during a medical procedure such as diagnosis biopsy, primary tumor or metastasis resection, blood containing circulating tumor, ascites, etc… Tumor samples are generally processed to primary cultures retaining the original features of the tumor cells of the patient and exposed to treatments of interest. This allows to determine their functional profile (sensitivity/resistance to treatment, ability to repair DNA, mitochondrial apoptotic priming, etc…) using different methods (viability/cytotoxicity assays, real-time imaging, histology/immunohistochemistry, BH3 profiling…). This profile can be used afterwards for predictive purposes and thus guide clinical decision making [ 12 ]. Such predictive functional assay can be performed on various biological materials and tumor models as detailed thereafter.
Tumor models
Developing functional precision medicine requires advanced experimental models to properly predict the behavior of a complex system such as cancer. In the past decades, much progress has been made in developing representative cancer models using in vitro, ex vivo and in vivo approaches that mirror cancer pathogenesis, tumor heterogeneity and angiogenesis [ 13 ]. Among others, they include ex vivo models such as patient-derived tumor organoids (PDTO) [ 14 ], spheroids from ascites [ 15 ] and tissue slices [ 16 , 17 , 18 ] or in vivo models such as patient-derived xenograft (PDX) models [ 19 ].
PDX models are established by transplanting human tumors into immune-deficient mice and then maintained by passaging from mouse to mouse. These models retain accurately the genetic, histological, and molecular characteristics of the original tumor and their response to treatments is correlated with clinical response [ 20 ]. However, they have some limitations, such as a low success rate of establishment for some tumor types, the long time required for the establishment, the time-consuming and costly process of their use, as well as the ethical issues associated to animal experimentation [ 21 ]. They offer therefore a suitable tumor model for testing innovative therapies but the above-mentioned limitations could restrict the use of these models for predictive purposes. However, their predictive value is currently tested in some clinical trials, as well as ex vivo models [ 12 ].
Among the ex vivo approaches, the technique of explants (or tumor slices) derives from the originally described technique of floating brain sections [ 22 ]. This model is obtained by cutting fresh tumor samples into slices 250 to 350 μm thick using a vibratome, and cultured ex vivo at 37 °C. The use of tumor slices maintains tumor-stroma interactions while preserving a tissue architecture that mimics the reality of the tumor in the short term. Despite a lack of reproducibility due to tumor heterogeneity, a study demonstrated the value of this model for predicting patient’s response to different anticancer agents [ 17 ] or for identifying predictive signature [ 16 ].
Ascites-derived spheroids (ADS) could offer as well a promising cancer model to guide clinical decision making. Ascites is most frequently associated with ovarian, pancreatic, colorectal, liver cancers, and provides a unique opportunity to easily sample tumor cells from these cancer patients. In the ascites, tumor cells shed from the primary tumor or visceral and parietal peritoneal carcinosis, forming free-floating spheroids [ 23 ]. These spheroids are poorly described and their predictive value has not been investigated so far. These samples can be used to perform ex vivo assays to assess their sensitivity to treatments [ 24 ] and therefore represent a particularly interesting alternative to explants, since the cells are abundant and can be collected at various time during the therapeutic management.
Finally, patient-derived tumor organoids (PDTO) have emerged more recently, as preclinical models that have the potential to predict an individual patient’s response to treatment. They are developed from patient tumor cells following embedding in basement membrane matrix and cultured in a medium supplemented with a cocktail of growth factors and inhibitors of signaling pathways to recapitulate in vivo niche conditions and allow long term growth [ 14 ]. These models are able to closely reproduce the genetic and morphological heterogeneous composition of the cancer cells in the original tumor. They can be rapidly grown from small amount of tumor cells, such as needle biopsy, with a high success rate compared to other models [ 14 ]. More importantly, despite the lack of stromal cells, there are more and more evidence that PDTO can recapitulate clinical response of patients [ 25 , 26 ], including ovarian cancer patients, although most of the studies were based on small sample size.
Therefore, it is crucial to develop relevant patient-derived tumor models (PDX, PDTO, explants and ADS) to evaluate new therapeutic strategies, identify predictive molecular signatures and to determine predictive value of ex vivo models in clinical studies based on larger patient cohorts. In this regard, our study will evaluate the feasibility of establishing these models and performing functional assay for drug testing and to compare their response to treatments to the clinical response of ovarian cancer patients.
Method/Design
The OVAREX study is a single-center non-interventional study conducted at Comprehensive Cancer Centre François Baclesse (Caen, France) to investigate the feasibility of establishing and testing ex-vivo tumor models from ovarian cancer to predict clinical response of the patient (Fig. 1 ).

OVAREX study design (created with Biorender.com)
Study objectives and endpoints
The main objective of the study is to assess the feasibility of developing ex-vivo tumor models that can be used for functional predictive assays.
The secondary objectives are to: (i) evaluate the effectiveness of ex vivo functional assays to predict the response to treatment; (ii) identify predictive biomarkers in tumor and serum samples; (iii) compare the ex vivo response of tumor models to clinical response; (iv) establish PDX models from ovarian cancer samples; (v) develop co-cultures of PDTO with autologous immune cells allowing the evaluation of anticancer effects of immunotherapy.
Study population
Eligibility criteria are described in Table 1 . The OVAREX study focuses on patients with proven cancer of the ovary, fallopian tube, and peritoneum, all FIGO stages (I-IV) who undergo laparoscopic or laparotomy surgery at our institution.
Study assessment
The study was approved by the “East III” ethical committee (IDRCB: 2018-A02152-53). Clinicians will inform all patients enrolled in the study that their biological samples could be used for this study (specific information letter will be given to patients) and they will express their non-opposition. Moreover, we will obtain written informed consent from patients for the use of their biological samples for research purposes.
Medical data collection
In order to correlate the biological data obtained on the initial tumor with the response to ex vivo treatments and the response observed in the clinic, the patients’ clinical data will be routinely collected from medical records by the Calvados Cancer Registry, which also checks for data completeness and consistency, and will be transmitted for enrolled patients. The collected data are summarized in Table 2 .
This collection will be carried out from an already existing database which has been the subject of a prior declaration to the establishment’s French data protection authority (CNIL) representative. Indeed, a collection of samples annotated in terms of clinico-pathological parameters has been set up at the Centre François Baclesse in order to allow a correlation between the profile sensitivity to chemotherapy (conventional or innovative) and the parameters studied. The OVAREX project will therefore use pseudonymized data collected by our biological resource center for studies correlation between results obtained ex vivo and clinical data. The samples and associated data will be retrospectively collected at the Centre François Baclesse and stored in the Biological Resource Center (BRC) OvaRessources (NF-S 96,900 quality management, AFNOR No. 2016: 72860.5). All biological collections are declared to the MESR (Ministry of Education, Health and Research, France, No. DC-2010-1243).
Collection of tumor and blood samples
A laparoscopic surgery will be performed as part of the patient’s care and tumor sample will be collected for anatomopathological diagnosis. Tumor sample which is excess to diagnostic purposes will be sent directly to the laboratory in sterile vials filled with cold culture medium supplemented with a Rho-kinase inhibitor (Y-27632).
As ascites can also be punctured during the surgery or outpatient hospitalization, excess fluid unneeded for anatomopathological evaluation will be collected in sterile jars and transferred to the laboratory.
Blood sampling will be realized before surgical intervention as part of the blood test included in the patient’s care. No blood draw will be done specifically for this study. Two dry tubes of 5 mL and 7 EDTA tubes of 5 mL will be collected and processed at the laboratory for serum analysis and peripheral blood mononuclear cells (PBMC) isolation.
Biological sample processing
Tumor sample processing.
Different procedures will be carried out on tumor samples: for future characterization, two pieces will be snap frozen and stored at -80 °C for molecular analyses and one piece will be fixed in paraformaldehyde for paraffin embedding and subsequent histopathological analysis and immunohistochemistry. The rest of the tumor will be processed to establish different models as described hereafter. All tumor samples will be stored in the BRC ‘OvaRessources’. Histology of all samples will be confirmed by a certified pathologist.
Isolation of PBMC
PBMC will be isolated from blood by density gradient centrifugation using Ficoll-Paque in Leucosep tubes. Cells will be resuspended in cold culture media, and counted. PBMC will be then resuspended in freezing solution (10% DMSO, 90% FBS), aliquoted (about 5 cryovials, 4.10 6 cells/cryovial), and frozen with gradually decreasing temperatures (1 °C/min) to -80 °C before long-term storage at liquid nitrogen temperatures and stored in the BRC TCBN.
Establishment and culture of PDTO, PDX, explants and ADS
Pdto establishment.
Tumor samples and ascites will be processed as previously described [ 27 ]. Briefly, samples are mechanically and/or enzymatically dissociated to obtain single cells or small cell clusters. Cells will then be embedded in extracellular matrix BME2 and cultured in an enriched medium [Advanced DMEM (Gibco) supplemented with 100 UI/mL of penicillin and streptomycin (Gibco), 1% GlutaMAX (Gibco), 1X B27 (Gibco), 10 mM Nicotinamide (Sigma-Aldrich), 1.25 mM N-Acetyl-L-Cysteine (Sigma-Aldrich), 50 µg/mL Primocin (InvivoGen), 5 µM Y27632 (Interchim), 20 ng/mL FGF-10 (PeproTech), 500 nM A-83–01 (PeproTech), 50 ng/mL EGF (PeproTech), 1 ng/ml FGF-basic (PeproTech), 1 µM SB202190 (PeproTech), 1 µM PGE2 (Sigma-Aldrich), 10% RSPO1- conditioned media (Cultrex HA-R-Spondin1-Fc 293 T, Amsbio) and 50% L-WRN- conditioned media (Cultrex L-WRN, Amsbio)]. Culture medium will be changed every 3–4 days and PDTO passaged every 2–4 week in order to expand them. PDTO lines will be considered as established when they will be cultured for more than 3 passages. For each established PDTO line, samples will be kept frozen for DNA/RNA/protein analysis, others will be embedded in paraffin for histopathological analysis and dissociated cells will be biobanked at -150 °C.
PDX establishment
Immediately following patient’s surgery, tumor fragments will be subcutaneously engrafted into the scapular area of anaesthetized nude mice as previously described [ 26 ]. Tumor growth will be measured twice a week and serial fragment grafts of each tumor will be conducted on 3 to 5 athymic nude mice. When the tumors reach a volume of 800 to 1000 mm 3 , tumors will be harvested, one fragment will be fixed for paraffin embedding and histopathological/immunochemistry analyses, two pieces will be snap frozen and stored at -150 °C for DNA/RNA extractions and three pieces will be used for passage, residual fragments will be frozen in 10% (v/v) dimethylsulfoxid (DMSO) and 90% (v/v) fetal bovine serum (FBS).
As described by Lheureux et al. [ 16 ], vibratome-sliced nodes (300–400 μm) will be fixed with 3% paraformaldehyde, frozen at -80 °C for immunoblotting or transferred into sterile prewarmed complete culture medium (RPMI 1640 supplemented with 2 mM GlutamaxTM, 25 mM HEPES, 10% fetal calf serum, 33 mM sodium bicarbonate (Fisher Scientific Bioblock, Illkirch, France) and 1% antibiotic).
ADS culture
Following patient paracentesis, ascites will be centrifugated at 1300 g for 7 min, the supernatant will then be filtered using a 300 μm and a 50 μm sieves to retrieve the spheroids contained in ascites. Spheroids will be fixed in 3% PFA, frozen at -80 °C, biobanked at -150 °C or cultured in agarose-coated plate with the ascites supernatant obtained after filtration.
Coculture of PDTO with immune cells
PDTO specific autologous T cells will be induced according to modified version of the protocol described in Dijkstra et al. [ 28 ]. Briefly, PBMC will be activated with the corresponding PDTO lysate and specific T cells clones will be isolated based on their expression of CD154 and CD137 markers using flow cytometry sorting. Once isolated and their purity controlled, specific T cells will be amplified by the use of a stimulation matrix and then cryopreserved. A quality control will be performed before cryopreservation by flow cytometry to check for reactivity against PDTO using CD107a expression and cytokines production after antigen re-stimulation. Once produced and checked for antigen specificity, PDTO-specific T cells will be cocultured with PDTO to produce iPDTO for the evaluation of response to immunotherapy.
Evaluation of the response of tumor-derived models to treatment
Pdto treatment.
When PDTO reached the size of 75–150 μm in diameter, they will be collected and resuspended in PDTO treatment medium (PDTO culture medium lacking primocin, Y-27,632 and N-acetylcysteine) with 2% BME2. 200 PDTO per well will be seeded in 100 µL volume in a previously coated (1:1 PDTO treatment medium/BME2) white clear bottom 96-well plates (Greiner). Drug solutions will then be prepared in a 2% BME2/PDTO treatment medium, added to each well and plates will be transferred to a humidified 37 °C/5% CO2 incubator. During the treatment, PDTO will be monitored using IncuCyte S3 ZOOM (Sartorius). One week later, ATP levels will be measured by CellTiter-Glo 3D assay (Promega) and luminescence will be quantified using GloMax Discover Microplate Reader (Promega). The half-maximal inhibitory concentration (IC50) and the area under the dose-response curve (AUC) will be computed for each PDTO model.
PDX treatment
PDX fragments will be subcutaneously implanted into nude mice as described above. On the first day of treatment, the animals bearing 100 to 200 mm 3 tumors will be randomly distributed to the various treatment and control groups (8–10 mice per group). Drugs will be administered intraperitoneally. Mice will be weighed and tumor volumes will be determined once or twice weekly from two-dimensional caliper measurements using the equation: Tumor volume (mm 3 ) = [length (mm) x width (mm) 2 ]/2. After 28 days of treatment, the mice will be euthanized and the tumors will be harvested for analysis. These experiments will be performed under guidelines from the European Community Council (2010/63/EU) and are approved by the protocol APAFIS #9577 validated by the French ethics committee “Comité d’éthique de Normandie en matière d’expérimentation animale” (CENOMEXA).
Explants treatment
After the transfer into sterile prewarmed complete culture medium, slices will be treated in complete medium for 6 to 48 h in a 5% CO2 humidified atmosphere at 37 °C. Slices will then be fixed in PFA 3% and paraffin-embedded for further analyses including the immunohistochemical detection of cleaved caspase-3 in order to quantify apoptosis.
ADS treatment
Directly after filtration and spheroids seeding in agarose-coated plates, the spheroid will be exposed to treatments for 6 to 96 h. As for PDTO, ADS will be monitored using IncuCyte S3 ZOOM (Sartorius) and viability will be assessed using CellTiter-Glo 3D assay (Promega).

Evaluation of PDTO model relevance and identification of potential predictive biomarkers
Transcriptomic analysis.
RNA analysis will be performed according to the protocol described in Perréard et al. [ 29 ]. Briefly, total RNA will be extracted using the Nucleospin RNA kit (Macherey Nagel, Hoerdt) and libraries will be made with the QuantSeq 3’RNA Library Kit. Once produced, the final library will be purified and deposed on High sensitivity DNA chip to be controlled on Agilent bioanalyzer 2100 and sequenced on NovaSeq 6000 (Illumina). Elimination of poor-quality regions and poly(A) of reads will be done through the use of the fastp program. Read alignments will be performed using the program STAR with the human genome reference (GRCh38) and the Ensembl reference gene annotations. Reads counts will be obtained using FeatureCount and statistical analysis will be realized with the R/bioconductor package DESeq2.
Copy number variation (CNV) analysis by low-pass whole genome sequencing (WGS)
WGS will be performed using Illumina DNA PCR Free prep kit, starting with 500ng of DNA. Data will be analyzed with HMMcopy and ichorCNA.
Transcriptome and CNV analysis
Analysis of intra reproducibility and differences between original tumors or ascites and PDTO will be assessed by principal component analysis and unsupervised hierarchical clustering as described in Perréard et al. [ 29 ].
Panel BRCAness
In order to assess tumors’ homologous recombination (HR) status, tumors will be sequenced with a 127-genes panel including 15 h genes ( BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L ). The sequencing data will be also used to determine a genomic instability score (GIS) as described by Leman et al. [ 10 ].
Statistical consideration
Sample size determination.
To estimate the PDTO establishment rate, assumed around 90%, with a 95% confidence interval of 10% width, 141 tumor samples will be required. Anticipating non-assessable samples, it is planned to include 250 patients.
Statistical analyses
Qualitative variables will be described using the sample numbers and percentages. Quantitative variables will be described using the mean (+/- standard deviation) or the median and the range if normality hypothesis is not verified. The significative threshold is set to 5% for all statistical analysis and confidence interval.
To address the primary objective, the rate of successful PDTO establishment, i.e., the rate of tumor samples usable for predictive functional assays based on PDTO, will be estimated with its 95% confidence interval. Then, association between PDTO response to treatment and clinical response will be measured by the Chi2 test. Associations between biological parameters and clinical response will be assessed by one-way analysis of variance (or the non-parametric Kruskal-Wallis test, if necessary). Receiver Operating Characteristic (ROC) curves and a logistic regression model will also be used to identify predictive factors of clinical response. Survival curves will be estimated by using the Kaplan-Meier method; median survival and survival rates at different times will be provided with their confidence intervals.
Current approaches to precision oncology are mainly based on the detection of genomic alterations. Unfortunately, many patients still do not benefit from these approaches despite the presence of an actionable alteration [ 12 ]. The use of personalized tumor models, such as PDX, PDTO, spheroids or explants, is rapidly emerging as a strategy to complement the use of genomics. These models could be used for drug testing as part of a predictive functional assay to guide clinical decision making, as well as to test innovative therapeutic strategies and identify predictive biomarkers. Interestingly, responses of some ex vivo models to treatments have been positively correlated to patient responses [ 17 , 25 ], including ovarian cancer patients [ 30 ]. However, these studies were based on small sample size and it is therefore crucial to determine if the response of these different personalized models to treatments recapitulate clinical response on large patient cohorts.
In this clinical study, we propose to establish PDX and ex vivo models (PDTO, ascites-derived spheroids, and explants) from tumors or ascites of ovarian cancer patients who will undergo surgical procedure or paracentesis. We aim at demonstrating the predictive value of the ex vivo models for their potential use in routine clinical practice as part of precision medicine. In the meantime, we want to establish tumor model collection of PDTO and PDX for new therapeutic compounds/strategies testing as well as for the identification of predictive biomarkers. Special attention will be given to immunotherapy testing using co-culture of PDTO with immune cells. This study will allow the establishment of a collection of relevant ovarian cancer models of various histology including rare ovarian cancer types and could demonstrate the interest of ex vivo models to predict the response to treatments or to identify innovative therapeutic strategies. In the event that one (or several) model(s) could faithfully predict patient response in a clinical-adapted manner (high success rate of establishment, results available within clinically relevant time frames, etc.), a prospective randomized clinical trial could be designed. The implementation of such predictive functional assay could thus allow individualizing cancer care and enabling physicians to select the most effective treatment for their patients.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Ascites-Derived Spheroids
Area Under the Curve
Basement Membrane Extract
Biological Resource Center
Breast Cancer
Copy Number Variations
Dulbecco’s Modifed Eagle Medium
DeoxyriboNucleic Acid
EthyleneDiamineTetraacetic Acid
Epidermal Growth Factor
Epithelial Ovarian Cancer
Fetal Bovine Serum
Fibroblast Growth Factor
Federation of Gynecology and Obstetrics
Homologous Recombination Deficiency
Half maximal inhibitory concentration
ImmunoHisto Chemistry
Next-Generation Sequencing
Poly (ADP-Ribose) Polymerase Inhibitor
Peripheral Blood Mononuclear Cells
Patient-Derived Tumor Organoid
Patient-Derived Xenograft
ParaFormAldehyde
RiboNucleic Acid
Receiver Operating Characteristic
Whole Genome Sequencing
Cabasag CJ, Butler J, Arnold M, Rutherford M, Bardot A, Ferlay J, et al. Exploring variations in ovarian cancer survival by age and stage (ICBP SurvMark-2): a population-based study. Gynecol Oncol avr. 2020;157(1):234–44.
Article Google Scholar
Torres D, Wang C, Kumar A, Bakkum-Gamez JN, Weaver AL, McGree ME, et al. Factors that influence survival in high-grade serous ovarian cancer: a complex relationship between molecular subtype, disease dissemination, and operability. Gynecol Oncol août. 2018;150(2):227–32.
Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280–304.
Article PubMed Google Scholar
Marth C, Abreu MH, Andersen KK, Aro KM, de Lurdes Batarda M, Boll D, et al. Real-life data on treatment and outcomes in advanced ovarian cancer: an observational, multinational cohort study (RESPONSE trial). Cancer 15 août. 2022;128(16):3080–9.
CAS Google Scholar
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 29 déc. 2011;365(26):2484–96.
Rossi L, Verrico M, Zaccarelli E, Papa A, Colonna M, Strudel M, et al. Bevacizumab in ovarian cancer: a critical review of phase III studies. Oncotarget 14 févr. 2017;8(7):12389–405.
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 29 déc. 2011;365(26):2473–83.
Mateo J, Lord CJ, Serra V, Tutt A, Balmaña J, Castroviejo-Bermejo M, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol Sept. 2019;30(9):1437–47.
Article CAS Google Scholar
Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus Bevacizumab as First-Line maintenance in Ovarian Cancer. N Engl J Med 19 déc. 2019;381(25):2416–28.
Leman R, Muller E, Legros A, Goardon N, Chentli I, Atkinson A, et al. Validation of the clinical use of GIScar, an academic-developed genomic instability score Predicting sensitivity to maintenance Olaparib for Ovarian Cancer. Clin Cancer Res 1 nov. 2023;29(21):4419–29.
Morice PM, Coquan E, Weiswald LB, Lambert B, Vaur D, Poulain L. Identifying patients eligible for PARP inhibitor treatment: from NGS-based tests to 3D functional assays. Br J Cancer Juill. 2021;125(1):7–14.
Letai A, Bhola P, Welm AL. Functional precision oncology: testing tumors with drugs to identify vulnerabilities and novel combinations. Cancer Cell 10 janv. 2022;40(1):26–35.
Alkema NG, Wisman GBA, van der Zee AGJ, van Vugt MATM, de Jong S. Studying platinum sensitivity and resistance in high-grade serous ovarian cancer: different models for different questions. Drug Resist Updat janv. 2016;24:55–69.
Thorel L, Florent R, Perréard M, Vincent A, Poulain L, Weiswald LB. [Patient-derived tumor organoids (or tumoroid), a growing preclinical model for oncology]. Med Sci (Paris) Nov. 2022;38(11):880–7.
Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia janv. 2015;17(1):1–15.
Lheureux S, N’Diaye M, Blanc-Fournier C, Dugué AE, Clarisse B, Dutoit S, et al. Identification of predictive factors of response to the BH3-mimetic molecule ABT-737: an ex vivo experiment in human serous ovarian carcinoma. Int J Cancer 1 mars. 2015;136(5):E340–350.
Majumder B, Baraneedharan U, Thiyagarajan S, Radhakrishnan P, Narasimhan H, Dhandapani M, et al. Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nat Commun 27 févr. 2015;6:6169.
Vaira V, Fedele G, Pyne S, Fasoli E, Zadra G, Bailey D, et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci U S 4 mai. 2010;107(18):8352–6.
Zanella ER, Grassi E, Trusolino L. Towards precision oncology with patient-derived xenografts. Nat Rev Clin Oncol Nov. 2022;19(11):719–32.
Izumchenko E, Paz K, Ciznadija D, Sloma I, Katz A, Vasquez-Dunddel D, et al. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol 1 oct. 2017;28(10):2595–605.
Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev févr. 2014;24:68–73.
Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol oct. 2007;8(10):839–45.
Ford CE, Werner B, Hacker NF, Warton K. The untapped potential of ascites in ovarian cancer research and treatment. Br J Cancer Juill. 2020;123(1):9–16.
Latifi A, Luwor RB, Bilandzic M, Nazaretian S, Stenvers K, Pyman J, et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS ONE. 2012;7(10):e46858.
Article CAS PubMed PubMed Central Google Scholar
Veninga V, Voest EE. Tumor organoids: opportunities and challenges to guide precision medicine. Cancer Cell 13 sept. 2021;39(9):1190–201.
Thorel L, Morice PM, Paysant H, Florent R, Babin G, Thomine C, et al. Comparative analysis of response to treatments and molecular features of tumor-derived organoids versus cell lines and PDX derived from the same ovarian clear cell carcinoma. J Exp Clin Cancer Res. oct 2023;7(1):260.
Florent R, Weiswald LB, Lambert B, Brotin E, Abeilard E, Louis MH, et al. Bim, Puma and Noxa upregulation by Naftopidil sensitizes ovarian cancer to the BH3-mimetic ABT-737 and the MEK inhibitor Trametinib. Cell Death Dis 18 mai. 2020;11(5):1–16.
Google Scholar
Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, et al. Generation of Tumor-reactive T cells by co-culture of Peripheral Blood lymphocytes and Tumor Organoids. Cell. sept 2018;6(6):1586–e159812.
Perréard M, Florent R, Divoux J, Grellard JM, Lequesne J, Briand M, et al. ORGAVADS: establishment of tumor organoids from head and neck squamous cell carcinoma to assess their response to innovative therapies. BMC Cancer déc. 2023;23(1):1–9.
de Witte CJ, Espejo Valle-Inclan J, Hami N, Lõhmussaar K, Kopper O, Vreuls CPH et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Reports. 16 juin. 2020;31(11):107762.
Download references
Acknowledgements
The ORGAPRED core facility ‘Tumor organoids for research and predicting response to treatment’ is supported by the Normandy County Council, the European Union within the framework of the Operational Programme ERDF/ESF 2014–2020 and the French state which was conducted as part of the planning contract 2015–2020 between the State and the Lower Normandie Region (ORGAPRED, POLARIS and EquipInnovCaen2022-PLATONUS ONE projects). We thank the donors, the Lions clubs of Normandy, Vaincrabe and the other foundations for their support of the projects carried out by our teams on PDTO. We are grateful to Inserm, University of Caen Normandy and the Comprehensive Cancer Center François Baclesse for their support in the implementation of these activities.
This work is supported by Cancéropôle Nord-Ouest (“ORGRAFT” project), Ligue contre le Cancer (Calvados’s commitee), Fondation de l’Avenir (#AP-RM-19-020), the “Fondation ARC pour la recherche sur le cancer” (#PJA20191209649). “ORGATHEREX” project is co-funded by the Normandy County Council, the European Union within the framework of the Operational Programme ERDF/ESF 2014–2020 which was conducted as part of the planning contract 2015–2020 between the French State and the Normandy Region. The funders had no participation in study design, data management, or publication management.
Author information
Laurent Poulain and Enora Dolivet contributed equally to this work.
Authors and Affiliations
INSERM U1086 ANTICIPE (Interdisciplinary Research Unit for Cancers Prevention and Treatment), BioTICLA Laboratory (Precision Medicine for Ovarian Cancers), Université de Caen Normandie, Caen, France
Lucie Thorel, Jordane Divoux, Guillaume Babin, Pierre-Marie Morice, Romane Florent, Chloé Marde Alagama, Mélanie Briand, Christophe Denoyelle, Nicolas Vigneron, Cécile Blanc-Fournier, Matthieu Meryet-Figuiere, Louis-Bastien Weiswald, Laurent Poulain & Enora Dolivet
Comprehensive Cancer Center François Baclesse, UNICANCER, Caen, France
Lucie Thorel, Jordane Divoux, Pierre-Marie Morice, Romane Florent, Guillaume Desmartin, Lucie Lecouflet, Christophe Denoyelle, Nicolas Vigneron, Matthieu Meryet-Figuiere & Louis-Bastien Weiswald
ORGAPRED Core Facility, US PLATON, Université de Caen Normandie, Caen, France
Jordane Divoux, Romane Florent, Guillaume Desmartin, Lucie Lecouflet, Louis-Bastien Weiswald & Laurent Poulain
Clinical Research Department, Comprehensive Cancer Center François Baclesse, UNICANCER, Caen, France
Justine Lequesne, Alexandra Leconte, Bénédicte Clarisse & Florence Joly
Department of Surgery, Comprehensive Cancer Center François Baclesse, UNICANCER, Caen, France
Guillaume Babin, Roman Rouzier, Léopold Gaichies, Sandrine Martin-Françoise, Jean-François Le Brun & Enora Dolivet
Biological Resource Center ‘OvaRessources’, US PLATON, Université de Caen Normandie, Caen, France
Mélanie Briand, Cécile Blanc-Fournier & Laurent Poulain
Calvados General Tumor Registry, Comprehensive Cancer Center François Baclesse, UNICANCER, Caen, France
Nicolas Vigneron
Department of Pathology, Comprehensive Cancer Center François Baclesse, UNICANCER, Caen, France
Corinne Jeanne & Cécile Blanc-Fournier
Department of Cancer Biology and Genetics, U1245 “Cancer and Brain Genomics”, Comprehensive Cancer Center François Baclesse, UNICANCER, Caen, France
Raphaël Leman & Dominique Vaur
US 41 - UAR 2014 - PLBS, University of Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, Lille, France
Martin Figeac
You can also search for this author in PubMed Google Scholar
Contributions
L.T., J.D., LB.W., L.P. and E.D. wrote the manuscript. J.D., J.L., G.B., PM.M., A.L., B.C., M.B., C.D., N.V., C.BF., D.V., F.J., LB.W., L.P. and E.D. devised the study concept and design. J.L. was responsible for overseeing the statistical section. L.T., J.D., J.L., G.B., PM.M., R.F., G.D., L.L., C.MA., A.L., B.C., M.B., R.R., L.G., S.MF., JF.LB., C.D., N.V., C.J., C.BF., R.L., D.V., M.F., M.MF., F.J., LB.W., L.P. and E.D. contributed to the study protocol, read and approved the final manuscript. Each author has been sufficiently involved in the work to take public responsibility for appropriate portions of the content. Figures and illustrations were designed and created by L.T., J.D. and LB.W. Funding was obtained by LB.W. and L.P.
Corresponding authors
Correspondence to Louis-Bastien Weiswald , Laurent Poulain or Enora Dolivet .
Ethics declarations
Ethics approval and consent to participate.
This study has received ethical approval from the Comité de Protection des Personnes Est IV (N°EudraCT: 2018-A02152-53) in January 2019 with last amendment (V4) accepted in March 2023. This committee is independent and not related with any affiliation of the authors. Any subsequent will of modification of the protocol would be submitted to agreement of the committee. The clinical trial has been registered at ClinicalTrials.gov with the identifier NCT03831230 on January 28th, 2019. The study will be explained to the patients by the surgeons or the oncologists, who will give them an information file. All patients will consent to be part of the study by giving their informed non-opposition.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Thorel, L., Divoux, J., Lequesne, J. et al. The OVAREX study: Establishment of ex vivo ovarian cancer models to validate innovative therapies and to identify predictive biomarkers. BMC Cancer 24 , 701 (2024). https://doi.org/10.1186/s12885-024-12429-w
Download citation
Received : 24 April 2024
Accepted : 24 May 2024
Published : 07 June 2024
DOI : https://doi.org/10.1186/s12885-024-12429-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ovarian cancer
- Patient-derived tumor organoids
- Patient-derived tumor xenografts
- Predictive functional assays.
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancer Control
- v.28; Jan-Dec 2021
Cancer Biology, Epidemiology, and Treatment in the 21st Century: Current Status and Future Challenges From a Biomedical Perspective
Patricia piña-sánchez.
1 Oncology Research Unit, Oncology Hospital, Mexican Institute of Social Security, Mexico
Antonieta Chávez-González
Martha ruiz-tachiquín, eduardo vadillo, alberto monroy-garcía, juan josé montesinos, rocío grajales.
2 Department of Medical Oncology, Oncology Hospital, Mexican Institute of Social Security, Mexico
Marcos Gutiérrez de la Barrera
3 Clinical Research Division, Oncology Hospital, Mexican Institute of Social Security, Mexico
Hector Mayani
Since the second half of the 20th century, our knowledge about the biology of cancer has made extraordinary progress. Today, we understand cancer at the genomic and epigenomic levels, and we have identified the cell that starts neoplastic transformation and characterized the mechanisms for the invasion of other tissues. This knowledge has allowed novel drugs to be designed that act on specific molecular targets, the immune system to be trained and manipulated to increase its efficiency, and ever more effective therapeutic strategies to be developed. Nevertheless, we are still far from winning the war against cancer, and thus biomedical research in oncology must continue to be a global priority. Likewise, there is a need to reduce unequal access to medical services and improve prevention programs, especially in countries with a low human development index.
Introduction
During the last one hundred years, our understanding of the biology of cancer increased in an extraordinary way. 1 - 4 Such a progress has been particularly prompted during the last few decades because of technological and conceptual progress in a variety of fields, including massive next-generation sequencing, inclusion of “omic” sciences, high-resolution microscopy, molecular immunology, flow cytometry, analysis and sequencing of individual cells, new cell culture techniques, and the development of animal models, among others. Nevertheless, there are many questions yet to be answered and many problems to be solved regarding this disease. As a consequence, oncological research must be considered imperative.
Currently, cancer is one of the illnesses that causes more deaths worldwide. 5 According to data reported in 2020 by the World Health Organization (WHO), cancer is the second cause of death throughout the world, with 10 million deaths. 6 Clearly, cancer is still a leading problem worldwide. With this in mind, the objective of this article is to present a multidisciplinary and comprehensive overview of the disease. We will begin by analyzing cancer as a process, focusing on the current state of our knowledge on 4 specific aspects of its biology. Then, we will look at cancer as a global health problem, considering some epidemiological aspects, and discussing treatment, with a special focus on novel therapies. Finally, we present our vision on some of the challenges and perspectives of cancer in the 21 st century.
The Biology of Cancer
Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and epigenomic alterations that lead to cell transformation, the cells where these changes occur, and the processes of invasion and metastasis that, to an important degree, determine tumor aggressiveness.
Cancer Genomics
The genomics of cancer can be defined as the study of the complete sequence of DNA and its expression in tumor cells. Evidently, this study only becomes meaningful when compared to normal cells. The sequencing of the human genome, completed in 2003, was not only groundbreaking with respect to the knowledge of our gene pool, but also changed the way we study cancer. In the post-genomic era, various worldwide endeavors, such as the Human Cancer Genome Project , the Cancer Genome ATLAS (TCGA), the International Cancer Genome Consortium, and the Pan-Cancer Analysis Working Group (PCAWG), have contributed to the characterization of thousands of primary tumors from different neoplasias, generating more than 2.5 petabytes (10 15 ) of genomic, epigenomic, and proteomic information. This has led to the building of databases and analytical tools that are available for the study of cancer from an “omic” perspective, 7 , 8 and it has helped to modify classification and treatment of various neoplasms.
Studies in the past decade, including the work by the PCAWG, have shown that cancer generally begins with a small number of driving mutations (4 or 5 mutations) in particular genes, including oncogenes and tumor-suppressor genes. Mutations in TP53, a tumor-suppressor gene, for example, are found in more than half of all cancer types as an early event, and they are a hallmark of precancerous lesions. 9 - 12 From that point on, the evolution of tumors may take decades, throughout which the mutational spectrum of tumor cells changes significantly. Mutational analysis of more than 19 000 exomes revealed a collection of genomic signatures, some associated with defects in the mechanism of DNA repair. These studies also revealed the importance of alterations in non-coding regions of DNA. Thus, for example, it has been observed that various pathways of cell proliferation and chromatin remodeling are altered by mutations in coding regions, while pathways, such as WNT and NOTCH, can be disrupted by coding and non-coding mutations. To the present date, 19 955 genes that codify for proteins and 25 511 genes for non-coding RNAs have been identified ( https://www.gencodegenes.org/human/stats.html ). Based on this genomic catalogue, the COSMIC (Catalogue Of Somatic Mutations In Cancer) repository, the most robust database to date, has registered 37 288 077 coding mutations, 19 396 fusions, 1 207 190 copy number variants, and 15 642 672 non-coding variants reported up to August 2020 (v92) ( https://cosmic-blog.sanger.ac.uk/cosmic-release-v92/ ).
The genomic approach has accelerated the development of new cancer drugs. Indeed, two of the most relevant initiatives in recent years are ATOM (Accelerating Therapeutics for Opportunities in Medicine), which groups industry, government and academia, with the objective of accelerating the identification of drugs, 13 and the Connectivity Map (CMAP), a collection of transcriptional data obtained from cell lines treated with drugs for the discovery of functional connections between genes, diseases, and drugs. The CMAP 1.0 covered 1300 small molecules and more than 6000 signatures; meanwhile, the CMAP 2.0 with L1000 assay profiled more than 1.3 million samples and approximately 400 000 signatures. 14
The genomic study of tumors has had 2 fundamental contributions. On the one hand, it has allowed the confirmation and expansion of the concept of intratumor heterogeneity 15 , 16 ; and on the other, it has given rise to new classification systems for cancer. Based on the molecular classification developed by expression profiles, together with mutational and epigenomic profiles, a variety of molecular signatures have been identified, leading to the production of various commercial multigene panels. In breast cancer, for example, different panels have been developed, such as Pam50/Prosigna , Blue Print , OncotypeDX , MammaPrint , Prosigna , Endopredict , Breast Cancer Index , Mammostrat, and IHC4 . 17
Currently, the genomic/molecular study of cancer is more closely integrated with clinical practice, from the classification of neoplasms, as in tumors of the nervous system, 18 to its use in prediction, as in breast cancer. 17 Improvement in molecular methods and techniques has allowed the use of smaller amounts of biological material, as well as paraffin-embedded samples for genomic studies, both of which provide a wealth of information. 19 In addition, non-invasive methods, such as liquid biopsies, represent a great opportunity not only for the diagnosis of cancer, but also for follow-up, especially for unresectable tumors. 20
Research for the production of genomic information on cancer is presently dominated by several consortia, which has allowed the generation of a great quantity of data. However, most of these consortia and studies are performed in countries with a high human development index (HDI), and countries with a low HDI are not well represented in these large genomic studies. This is why initiatives such as Human Heredity and Health in Africa (H3Africa) for genomic research in Africa are essential. 21 Generation of new information and technological developments, such as third-generation sequencing, will undoubtedly continue to move forward in a multidisciplinary and complex systems context. However, the existing disparities in access to genomic tools for diagnosis, prognosis, and treatment of cancer will continue to be a pressing challenge at regional and social levels.
Cancer Epigenetics
Epigenetics studies the molecular mechanisms that produce hereditable changes in gene expression, without causing alterations in the DNA sequence. Epigenetic events are of 3 types: methylation of DNA and RNA, histone modification (acetylation, methylation, and phosphorylation), and the expression of non-coding RNA. Epigenetic aberrations can drive carcinogenesis when they alter chromosome conformation and the access to transcriptional machinery and to various regulatory elements (promoters, enhancers, and anchors for interaction with chromatin, for example). These changes may activate oncogenesis and silence tumor-suppressor mechanisms when they modulate coding and non-coding sequences (such as micro-RNAs and long-RNAs). This can then lead to uncontrolled growth, as well as the invasion and metastasis of cancer cells.
While genetic mutations are stable and irreversible, epigenetic alterations are dynamic and reversible; that is, there are several epigenomes, determined by space and time, which cause heterogeneity of the “epigenetic status” of tumors during their development and make them susceptible to environmental stimuli or chemotherapeutic treatment. 22 Epigenomic variability creates differences between cells, and this creates the need to analyze cells at the individual level. In the past, epigenetic analyses measured “average states” of cell populations. These studies revealed general mechanisms, such as the role of epigenetic marks on active or repressed transcriptional states, and established maps of epigenetic composition in a variety of cell types in normal and cancerous tissue. However, these approaches are difficult to use to examine events occurring in heterogeneous cell populations or in uncommon cell types. This has led to the development of new techniques that permit marking of a sequence on the epigenome and improvement in the recovery yield of epigenetic material from individual cells. This has helped to determine changes in DNA, RNA, and histones, chromatin accessibility, and chromosome conformation in a variety of neoplasms. 23 , 24
In cancer, DNA hypomethylation occurs on a global scale, while hypermethylation occurs in specific genomic loci, associated with abnormal nucleosome positioning and chromatin modifications. This information has allowed epigenomic profiles to be established in different types of neoplasms. In turn, these profiles have served as the basis to identify new neoplasm subgroups. For example, in triple negative breast cancer (TNBC), 25 and in hepatocellular carcinoma, 26 DNA methylation profiles have helped to the identification of distinct subgroups with clinical relevance. Epigenetic approaches have also helped to the development of prognostic tests to assess the sensitivity of cancer cells to specific drugs. 27
Epigenetic traits could be used to characterize intratumoral heterogeneity and determine the relevance of such a heterogeneity in clonal evolution and sensitivity to drugs. However, it is clear that heterogeneity is not only determined by genetic and epigenetic diversity resulting from clonal evolution of tumor cells, but also by the various cell populations that form the tumor microenvironment (TME). 28 Consequently, the epigenome of cancer cells is continually remodeled throughout tumorigenesis, during resistance to the activity of drugs, and in metastasis. 29 This makes therapeutic action based on epigenomic profiles difficult, although significant advances in this area have been reported. 30
During carcinogenesis and tumor progression, epigenetic modifications are categorized by their mechanisms of regulation ( Figure 1A ) and the various levels of structural complexity ( Figure 1B ). In addition, the epigenome can be modified by environmental stimuli, stochastic events, and genetic variations that impact the phenotype ( Figure 1C ). 31 , 32 The molecules that take part in these mechanisms/events/variations are therapeutic targets of interest with potential impact on clinical practice. There are studies on a wide variety of epidrugs, either alone or in combination, which improve antitumor efficacy. 33 However, the problems with these drugs must not be underestimated. For a considerable number of epigenetic compounds still being under study, the main challenge is to translate in vitro efficacy of nanomolar (nM) concentrations into well-tolerated and efficient clinical use. 34 The mechanisms of action of epidrugs may not be sufficiently controlled and could lead to diversion of the therapeutic target. 35 It is known that certain epidrugs, such as valproic acid, produce unwanted epigenetic changes 36 ; thus the need for a well-established safety profile before these drugs can be used in clinical therapy. Finally, resistance to certain epidrugs is another relevant problem. 37 , 38

Epigenetics of cancer. (A) Molecular mechanisms. (B) Structural hierarchy of epigenomics. (C) Factors affecting the epigenome. Modified from Refs. 31 and 32 .
As we learn about the epigenome of specific cell populations in cancer patients, a door opens to the evaluation of sensitivity tests and the search for new molecular markers for detection, prognosis, follow-up, and/or response to treatment at various levels of molecular regulation. Likewise, the horizon expands for therapeutic alternatives in oncology with the use of epidrugs, such as pharmacoepigenomic modulators for genes and key pathways, including methylation of promoters and regulation of micro-RNAs involved in chemoresponse and immune response in cancer. 39 There is no doubt that integrated approaches identifying stable pharmagenomic and epigenomic patterns and their relation with expression profiles and genetic functions will be more and more valuable in our fight against cancer.
Cancer Stem Cells
Tumors consist of different populations of neoplastic cells and a variety of elements that form part of the TME, including stromal cells and molecules of the extracellular matrix. 40 Such intratumoral heterogeneity becomes even more complex during clonal variation of transformed cells, as well as influence the elements of the TME have on these cells throughout specific times and places. 41 To explain the origin of cancer cell heterogeneity, 2 models have been put forward. The first proposes that mutations occur at random during development of the tumor in individual neoplastic cells, and this promotes the production of various tumor populations, which acquire specific growth and survival traits that lead them to evolve according to intratumor mechanisms of natural selection. 42 The second model proposes that each tumor begins as a single cell that possess 2 functional properties: it can self-renew and it can produce several types of terminal cells. As these 2 properties are characteristics of somatic stem cells, 43 the cells have been called cancer stem cells (CSCs). 44 According to this model, tumors must have a hierarchical organization, where self-renewing stem cells produce highly proliferating progenitor cells, unable to self-renew but with a high proliferation potential. The latter, in turn, give rise to terminal cells. 45 Current evidence indicates that both models may coexist in tumor progression. In agreement with this idea, new subclones could be produced as a result of a lack of genetic stability and mutational changes, in addition to the heterogeneity derived from the initial CSC and its descendants. Thus, in each tumor, a set of neoplastic cells with different genetic and epigenetic traits may be found, which would provide different phenotypic properties. 46
The CSC concept was originally presented in a model of acute myeloid leukemia. 47 The presence of CSCs was later proved in chronic myeloid leukemia, breast cancer, tumors of the central nervous system, lung cancer, colon cancer, liver cancer, prostate cancer, pancreatic cancer, melanoma, and cancer of the head and neck, amongst others. In all of these cases, detection of CSCs was based on separation of several cell populations according to expression of specific surface markers, such as CD133, CD44, CD24, CD117, and CD15. 48 It is noteworthy that in some solid tumors, and even in some hematopoietic ones, a combination of specific markers that allow the isolation of CSCs has not been found. Interestingly, in such tumors, a high percentage of cells with the capacity to start secondary tumors has been observed; thus, the terms Tumor Initiating Cells (TIC) or Leukemia Initiating Cells (LIC) have been adopted. 46
A relevant aspect of the biology of CSCs is that, just like normal stem cells, they can self-renew. Such self-renewal guarantees the maintenance or expansion of the tumor stem cell population. Another trait CSCs share with normal stem cells is their quiescence, first described in chronic myeloid leukemia. 49 The persistence of quiescent CSCs in solid tumors has been recently described in colorectal cancer, where quiescent clones can become dominant after therapy with oxaliplatin. 50 In non-hierarchical tumors, such as melanoma, the existence of slow-cycling cells that are resistant to antimitogenic agents has also been proved. 51 Such experimental evidence supports the idea that quiescent CSCs or TICs are responsible for both tumor resistance to antineoplastic drugs and clinical relapse after initial therapeutic success.
In addition to quiescence, CSCs use other mechanisms to resist the action of chemotherapeutic drugs. One of these is their increased numbers: upon diagnosis, a high number of CSCs are observed in most analyzed tumors, making treatment unable to destroy all of them. On the other hand, CSCs have a high number of molecular pumps that expulse drugs, as well as high numbers of antiapoptotic molecules. In addition, they have very efficient mechanisms to repair DNA damage. In general, these cells show changes in a variety of signaling pathways involved in proliferation, survival, differentiation, and self-renewal. It is worth highlighting that in recent years, many of these pathways have become potential therapeutic targets in the elimination of CSCs. 52 Another aspect that is highly relevant in understanding the biological behavior of CSCs is that they require a specific site for their development within the tissue where they are found that can provide whatever is needed for their survival and growth. These sites, known as niches, are made of various cells, both tumor and non-tumor, as well as a variety of non-cellular elements (extracellular matrix [ECM], soluble cytokines, ion concentration gradients, etc.), capable of regulating the physiology of CSCs in order to promote their expansion, the invasion of adjacent tissues, and metastasis. 53
It is important to consider that although a large number of surface markers have been identified that allow us to enrich and prospectively follow tumor stem cell populations, to this day there is no combination of markers that allows us to find these populations in all tumors, and it is yet unclear if all tumors present them. In this regard, it is necessary to develop new purification strategies based on the gene expression profiles of these cells, so that tumor heterogeneity is taken into account, as it is evident that a tumor can include multiple clones of CSCs that, in spite of being functional, are genetically different, and that these clones can vary throughout space (occupying different microenvironments and niches) and time (during the progression of a range of tumor stages). Such strategies, in addition to new in vitro and in vivo assays, will allow the development of new and improved CSC elimination strategies. This will certainly have an impact on the development of more efficient therapeutic alternatives.
Invasion and Metastasis
Nearly 90% of the mortality associated with cancer is related to metastasis. 54 This consists of a cascade of events ( Figure 2 ) that begins with the local invasion of a tumor into surrounding tissues, followed by intravasation of tumor cells into the blood stream or lymphatic circulation. Extravasation of neoplastic cells in areas distant from the primary tumor then leads to the formation of one or more micrometastatic lesions which subsequently proliferate to form clinically detectable lesions. 4 The cells that are able to produce metastasis must acquire migratory characteristics, which occur by a process known as epithelial–mesenchymal transition (EMT), that is, the partial loss of epithelial characteristics and the acquirement of mesenchymal traits. 55

Invasion and metastasis cascade. Invasion and metastasis can occur early or late during tumor progression. In either case, invasion to adjacent tissues is driven by stem-like cells (cancer stem cells) that acquire the epithelial–mesenchymal transition (EMT) (1). Once they reach sites adjacent to blood vessels, tumor cells (individually or in clusters) enter the blood (2). Tumor cells in circulation can adhere to endothelium and extravasation takes place (3). Other mechanisms alternative to extravasation can exist, such as angiopelosis, in which clusters of tumor cells are internalized by the endothelium. Furthermore, at certain sites, tumor cells can obstruct microvasculature and initiate a metastatic lesion right there. Sometimes, a tumor cells that has just exit circulation goes into an MET in order to become quiescent (4). Inflammatory signals can activate quiescent metastatic cells that will proliferate and generate a clinically detectable lesion (5).
Although several of the factors involved in this process are currently known, many issues are still unsolved. For instance, it has not yet been possible to monitor in vivo the specific moment when it occurs 54 ; the microenvironmental factors of the primary tumor that promote such a transition are not known with precision; and the exact moment during tumor evolution in which one cell or a cluster of cells begin to migrate to distant areas, is also unknown. The wide range of possibilities offered by intra- and inter-tumoral heterogeneity 56 stands in the way of suggesting a generalized strategy that could resolve this complication.
It was previously believed that metastasis was only produced in late stages of tumor progression; however, recent studies indicate that EMT and metastasis can occur during the early course of the disease. In pancreatic cancer, for example, cells going through EMT are able to colonize and form metastatic lesions in the liver in the first stages of the disease. 52 , 57 Metastatic cell clusters circulating in peripheral blood (PB) are prone to generate a metastatic site, compared to individual tumor cells. 58 , 59 In this regard, novel strategies, such as the use of micro-RNAs, are being assessed in order to diminish induction of EMT. 60 It must be mentioned, however, that the metastatic process seems to be even more complex, with alternative pathways that do not involve EMT. 61 , 62
A crucial stage in the process of metastasis is the intravasation of tumor cells (alone or in clusters) towards the blood stream and/or lymphatic circulation. 63 These mechanisms are also under intensive research because blocking them could allow the control of spreading of the primary tumor. In PB or lymphatic circulation, tumor cells travel to distant parts for the potential formation of a metastatic lesion. During their journey, these cells must stand the pressure of blood flow and escape interaction with natural killer (NK) cells . 64 To avoid them, tumor cells often cover themselves with thrombocytes and also produce factors such as VEGF, angiopoietin-2, angiopoietin-4, and CCL2 that are involved in the induction of vascular permeability. 54 , 65 Neutrophils also contribute to lung metastasis in the bloodstream by secreting IL-1β and metalloproteases to facilitate extravasation of tumor cells. 64
The next step in the process of metastasis is extravasation, for which tumor cells, alone or in clusters, can use various mechanisms, including a recently described process known as angiopellosis that involves restructuring the endothelial barrier to internalize one or several cells into a tissue. 66 The study of leukocyte extravasation has contributed to a more detailed knowledge of this process, in such a way that some of the proposed strategies to avoid extravasation include the use of integrin inhibitors, molecules that are vital for rolling, adhesion, and extravasation of tumor cells. 67 , 68 Another strategy that has therapeutic potential is the use of antibodies that strengthen vascular integrity to obstruct transendothelial migration of tumor cells and aid in their destruction in PB. 69
Following extravasation, tumor cells can return to an epithelial phenotype, a process known as mesenchymal–epithelial transition and may remain inactive for several years. They do this by competing for specialized niches, like those in the bone marrow, brain, and intestinal mucosa, which provide signals through the Notch and Wnt pathways. 70 Through the action of the Wnt pathway, tumor cells enter a slow state of the cell cycle and induce the expression of molecules that inhibit the cytotoxic function of NK cells. 71 The extravasated tumor cell that is in a quiescent state must comply with 2 traits typical of stem cells: they must have the capacity to self-renew and to generate all of the cells that form the secondary tumor.
There are still several questions regarding the metastatic process. One of the persisting debates at present is if EMT is essential for metastasis or if it plays a more important role in chemoresistance. 61 , 62 It is equally important to know if there is a pattern in each tumor for the production of cells with the capacity to carry out EMT. In order to control metastasis, it is fundamental to know what triggers acquisition of the migratory phenotype and the intrinsic factors determining this transition. Furthermore, it is essential to know if mutations associated with the primary tumor or the variety of epigenetic changes are involved in this process. 55 It is clear that metastatic cells have affinity for certain tissues, depending on the nature of the primary tumor (seed and soil hypothesis). This may be caused by factors such as the location and the direction of the bloodstream or lymphatic fluid, but also by conditioning of premetastatic niches at a distance (due to the large number of soluble factors secreted by the tumor and the recruitment of cells of the immune system to those sites). 72 We have yet to identify and characterize all of the elements that participate in this process. Deciphering them will be of upmost importance from a therapeutic point of view.
Epidemiology of Cancer
Cancer is the second cause of death worldwide; today one of every 6 deaths is due to a type of cancer. According to the International Agency for Research on Cancer (IARC), in 2020 there were approximately 19.3 million new cases of cancer, and 10 million deaths by this disease, 6 while 23.8 million cases and 13.0 million deaths are projected to occur by 2030. 73 In this regard, it is clear the increasing role that environmental factors—including environmental pollutants and processed food—play as cancer inducers and promoters. 74 The types of cancer that produce the greatest numbers of cases and deaths worldwide are indicated in Table 1 . 6
Total Numbers of Cancer Cases and Deaths Worldwide in 2020 by Cancer Type (According to the Global Cancer Observatory, IARC).
| Cases | ||
|---|---|---|
| Both sexes | Women | Men |
| Breast (2.26 million) | Breast (2.26 million) | Lung (1.43 million) |
| Lung (2.20 million) | Colorectal (865 000) | Prostate (1.41 million) |
| Colorectal (1.93 million) | Lung (770 000) | Colorectal (1.06 million) |
| Prostate (1.41 million) | Cervical (604 000) | Stomach (719 000) |
| Stomach (1.08 million) | Thyroid (448 000) | Liver (632 000) |
| Deaths | ||
|---|---|---|
| Both sexes | Women | Men |
| Lung (1.79 million) | Breast (684 000) | Lung (1.18 million) |
| Colorectal (935 000) | Lung (607 000) | Liver (577 000) |
| Liver (830 000) | Colorectal (419 000) | Colorectal (515 000) |
| Stomach (768 000) | Cervical (341 000) | Stomach (502 000) |
| Breast (684 000) | Stomach (266 000) | Prostate (375 000) |
Data presented on this table were obtained from Ref. 6.
As shown in Figure 3 , lung, breast, prostate, and colorectal cancer are the most common throughout the world, and they are mostly concentrated in countries of high to very high human development index (HDI). Although breast, prostate, and colorectal cancer have a high incidence, the number of deaths they cause is proportionally low, mostly reflecting the great progress made in their control. However, these data also reveal the types of cancer that require further effort in prevention, precise early detection avoiding overdiagnosis, and efficient treatment. This is the case of liver, lung, esophageal, and pancreatic cancer, where the difference between the number of cases and deaths is smaller ( Figure 3B ). Social and economic transition in several countries has had an impact on reducing the incidence of neoplasms associated with infection and simultaneously produced an increase in the types related to reproductive, dietary, and hormonal factors. 75

Incidence and mortality for some types of cancer in the world. (A) Estimated number of cases and deaths in 2020 for the most frequent cancer types worldwide. (B) Incidence and mortality rates, normalized according to age, for the most frequent cancer types in countries with very high/& high (VH&H; blue) and/low and middle (L&M; red) Human Development Index (HDI). Data include both genders and all ages. Data according to https://gco.iarc.fr/today , as of June 10, 2021.
In the past 3 decades, cancer mortality rates have fallen in high HDI countries, with the exception of pancreatic cancer, and lung cancer in women. Nevertheless, changes in the incidence of cancer do not show the same consistency, possibly due to variables such as the possibility of early detection, exposure to risk factors, or genetic predisposition. 76 , 77 Countries such as Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the United Kingdom have reported a reduction in incidence and mortality in cancer of the stomach, colon, lung, and ovary, as well as an increase in survival. 78 Changes in modifiable risk factors, such as the use of tobacco, have played an important role in prevention. In this respect, it has been estimated that decline in tobacco use can explain between 35% and 45% of the reduction in cancer mortality rates, 79 while the fall in incidence and mortality due to stomach cancer can be attributed partly to the control of Helicobacter pylori infection. 80 Another key factor in the fall of mortality rates in developed countries has been an increase in early detection as a result of screening programs, as in breast and prostate cancer, which have had their mortality rates decreased dramatically in spite of an increase in their incidence. 76
Another important improvement observed in recent decades is the increase in survival rates, particularly in high HDI countries. In the USA, for example, survival rates for patients with prostate cancer at 5 years after initial diagnosis was 28% during 1947–1951; 69% during 1975–1977, and 100% during 2003–2009. Something similar occurred with breast cancer, with a 5-year survival rate of 54% in 1947–1951, 75% in 1975–1977, and 90% in 2003–2009. 81 In the CONCORD 3 version, age-standardize 5-year survival for patients with breast cancer in the USA during 2010–2014 was 90%, and 97% for prostate cancer patients. 82 Importantly, even among high HDI countries, significant differences have been identified in survival rates, being stage of disease at diagnosis, time for access to effective treatment, and comorbidities, the main factors influencing survival in these nations. 78 Unfortunately, survival rates in low HDI countries are significantly lower due to several factors, including lack of information, deficient screening and early detection programs, limited access to treatment, and suboptimal cancer registration. 82 It should be noted that in countries with low to middle HDI, neoplasms with the greatest incidence are those affecting women (breast and cervical cancer), which reflects not only a problem with access to health services, but also a serious inequality issue that involves social, cultural, and even religious obstacles. 83
Up to 42% of incident cases and 47% of deaths by cancer in the USA are due to potentially modifiable risk factors such as use of tobacco, physical activity, diet, and infection. 84 It has been calculated that 2.4 million deaths by cancer, mostly of the lung, can be attributed to tobacco. 73 In 2020, the incidence rate of lung cancer in Western Africa was 2.2, whereas in Polynesia and Eastern Asia was 37.3 and 34.4, respectively. 6 In contrast, the global burden of cancer associated with infection was 15.4%, but in Sub-Saharan Africa it was 30%. 85 Likewise, the incidence of cervical cancer in Eastern Africa was 40.1, in contrast with the USA and Canada that have a rate of 6.2. This makes it clear that one of the challenges we face is the reduction of the risk factors that are potentially modifiable and associated with specific types of cancer.
Improvement of survival rates and its disparities worldwide are also important challenges. Five-year survival for breast cancer—diagnosed during 2010-2014— in the USA, for example, was 90%, whereas in countries like South Africa it was 40%. 82 Childhood leukemia in the USA and several European countries shows a 5-year survival of 90%, while in Latin-American countries it is 50–76%. 86 Interestingly, there are neoplasms, such as pancreatic cancer, for which there has been no significant increase in survival, which remains low (5–15%) both in developed and developing countries. 82
Although data reported on global incidence and mortality gives a general overview on the epidemiology of cancer, it is important to note that there are great differences in coverage of cancer registries worldwide. To date, only 1 out of every 3 countries reports high quality data on the incidence of cancer. 87 For the past 50 years, the IARC has supported population-based cancer registries; however, more than one-third of the countries belonging to the WHO, mainly countries of low and middle income (LMIC), have no data on more than half of the 18 indicators of sustainable development goals. 88 High quality cancer registries only cover 4% of the population in Africa, 8% in Asia, and 7% in Latin America, contrasting with 83% in the USA and Canada, and 33% in Europe. 89 In response to this situation, the Global Initiative for Cancer Registry Development was created in 2012 to generate improved infrastructure to permit greater coverage and better quality registries, especially in countries with low and middle HDI. 88 It is expected that initiatives of this sort in the coming years will allow more and better information to guide strategies for the control of cancer worldwide, especially in developing regions. This will enable survival to be measured over longer periods of time (10, 15, or 20 years), as an effective measure in the control of cancer. The WHO has established as a target for 2025 to reduce deaths by cancer and other non-transmissible diseases by 25% in the population between the ages of 30–69; such an effort requires not only effective prevention measures to reduce incidence, but also more efficient health systems to diminish mortality and increase survival. At the moment, it is an even greater challenge because of the effects of the COVID-19 pandemic which has negatively impacted cancer prevention and health services. 90
Oncologic Treatments
A general perspective.
At the beginning of the 20th century, cancer treatment, specifically treatment of solid tumors, was based fundamentally on surgical resection of tumors, which together with other methods for local control, such as cauterization, had been used since ancient times. 91 At that time, there was an ongoing burst of clinical observations along with interventions sustained on fundamental knowledge about physics, chemistry, and biology. In the final years of the 19 th century and the first half of the 20th, these technological developments gave rise to radiotherapy, hormone therapy, and chemotherapy. 92 - 94 Simultaneously, immunotherapy was also developed, although usually on a smaller scale, in light of the overwhelming progress of chemotherapy and radiotherapy. 95
Thus began the development and expansion of disciplines based on these approaches (surgery, radiotherapy, chemotherapy, hormone therapy, and immunotherapy), with their application evolving ever more rapidly up to their current uses. Today, there is a wide range of therapeutic tools for the care of cancer patients. These include elements that emerged empirically, arising from observations of their effects in various medical fields, as well as drugs that were designed to block processes and pathways that form part of the physiopathology of one or more neoplasms according to knowledge of specific molecular alterations. A classic example of the first sort of tool is mustard gas, originally used as a weapon in war, 96 but when applied for medical purposes, marked the beginning of the use of chemicals in the treatment of malignant neoplasms, that is, chemotherapy. 94 A clear example of the second case is imatinib, designed specifically to selectively inhibit a molecular alteration in chronic myeloid leukemia: the Bcr-Abl oncoprotein. 97
It is on this foundation that today the 5 areas mentioned previously coexist and complement one another. The general framework that motivates this amalgam and guides its development is precision medicine, founded on the interaction of basic and clinical science. In the forecasts for development in each of these fields, surgery is expected to continue to be the fundamental approach for primary tumors in the foreseeable future, as well as when neoplastic disease in the patient is limited, or can be limited by applying systemic or regional elements, before and/or after surgical resection, and it can be reasonably anticipated for the patient to have a significant period free from disease or even to be cured. With regards to technology, intensive exploration of robotic surgery is contemplated. 98
The technological possibilities for radiotherapy have progressed in such a way that it is now possible to radiate neoplastic tissue with an extraordinary level of precision, and therefore avoid damage to healthy tissue. 99 This allows administration of large doses of ionizing radiation in one or a few fractions, what is known as “radiosurgery.” The greatest challenges to the efficacy of this approach are related to radio-resistance in certain neoplasms. Most efforts regarding research in this field are concentrated on understanding the underlying biological mechanisms of the phenomenon and their potential control through radiosensitizers. 100
“Traditional” chemotherapy, based on the use of compounds obtained from plants and other natural products, acting in a non-specific manner on both neoplastic and healthy tissues with a high proliferation rate, continues to prevail. 101 The family of chemotherapeutic drugs currently includes alkylating agents, antimetabolites, anti-topoisomerase agents, and anti-microtubules. Within the pharmacologic perspective, the objective is to attain a high concentration or activity of such molecules in specific tissues while avoiding their accumulation in others, in order to achieve an increase in effectiveness and a reduction in toxicity. This has been possible with the use of viral vectors, for example, that are able to limit their replication in neoplastic tissues, and activate prodrugs of normally nonspecific agents, like cyclophosphamide, exclusively in those specific areas. 102 More broadly, chemotherapy also includes a subgroup of substances, known as molecular targeted therapy, that affect processes in a more direct and specific manner, which will be mentioned later.
There is no doubt that immunotherapy—to be explored next—is one of the therapeutic fields where development has been greatest in recent decades and one that has produced enormous expectation in cancer treatment. 103 Likewise, cell therapy, based on the use of immune cells or stem cells, has come to complement the oncologic therapeutic arsenal. 43 Each and every one of the therapeutic fields that have arisen in oncology to this day continue to prevail and evolve. Interestingly, the foreseeable future for the development of cancer treatment contemplates these approaches in a joint and complementary manner, within the general framework of precision medicine, 104 and sustained by knowledge of the biological mechanisms involved in the appearance and progression of neoplasms. 105 , 106
Immunotherapy
Stimulating the immune system to treat cancer patients has been a historical objective in the field of oncology. Since the early work of William Coley 107 to the achievements reached at the end of the 20 th century, scientific findings and technological developments paved the way to searching for new immunotherapeutic strategies. Recombinant DNA technology allowed the synthesis of cytokines, such as interferon-alpha (IFN-α) and interleukin 2 (IL-2), which were authorized by the US Food and Drug Administration (FDA) for the treatment of hairy cell leukemia in 1986, 108 as well as kidney cancer and metastatic melanoma in 1992 and 1998, respectively. 109
The first therapeutic vaccine against cancer, based on the use of autologous dendritic cells (DCs), was approved by the FDA against prostate cancer in 2010. However, progress in the field of immunotherapy against cancer was stalled in the first decade of the present century, mostly due to failure of several vaccines in clinical trials. In many cases, application of these vaccines was detained by the complexity and cost involved in their production. Nevertheless, with the coming of the concept of immune checkpoint control, and the demonstration of the relevance of molecules such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), and programmed cell death molecule-1 (PD-1), immunotherapy against cancer recovered its global relevance. In 2011, the monoclonal antibody (mAb) ipilimumab, specific to the CTLA-4 molecule, was the first checkpoint inhibitor (CPI) approved for the treatment of advanced melanoma. 110 Later, inhibitory mAbs for PD-1, or for the PD-1 ligand (PD-L1), 111 as well as the production of T cells with chimeric receptors for antigen recognition (CAR-T), 112 which have been approved to treat various types of cancer, including melanoma, non-small cell lung cancer (NSCLC), head and neck cancer, bladder cancer, renal cell carcinoma (RCC), and hepatocellular carcinoma, among others, have changed the paradigm of cancer treatment.
In spite of the current use of anti-CTLA-4 and anti-PD-L1 mAbs, only a subgroup of patients has responded favorably to these CPIs, and the number of patients achieving clinical benefit is still small. It has been estimated that more than 70% of patients with solid tumors do not respond to CPI immunotherapy because either they show primary resistance, or after responding favorably, develop resistance to treatment. 113 In this regard, it is important to mention that in recent years very important steps have been taken to identify the intrinsic and extrinsic mechanisms that mediate resistance to CPI immunotherapy. 114 Intrinsic mechanisms include changes in the antitumor immune response pathways, such as faulty processing and presentation of antigens by APCs, activation of T cells for tumor cell destruction, and changes in tumor cells that lead to an immunosuppressive TME. Extrinsic factors include the presence of immunosuppressive cells in the local TME, such as regulatory T cells, myeloid-derived suppressor cells (MDSC), mesenchymal stem/stromal cells (MSCs), and type 2 macrophages (M2), in addition to immunosuppressive cytokines.
On the other hand, classification of solid tumors as “hot,” “cold,” or “excluded,” depending on T cell infiltrates and the contact of such infiltrates with tumor cells, as well as those that present high tumor mutation burden (TMB), have redirected immunotherapy towards 3 main strategies 115 ( Table 2 ): (1) Making T-cell antitumor response more effective, using checkpoint inhibitors complementary to anti-CTLA-4 and anti-PD-L1, such as LAG3, Tim-3, and TIGT, as well as using CAR-T cells against tumor antigens. (2) Activating tumor-associated myeloid cells including monocytes, granulocytes, macrophages, and DC lineages, found at several frequencies within human solid tumors. (3) Regulating the biochemical pathways in TME that produce high concentrations of immunosuppressive molecules, such as kynurenine, a product of tryptophan metabolism, through the activity of indoleamine 2,3 dioxygenase; or adenosine, a product of ATP hydrolysis by the activity of the enzyme 5’nucleotidase (CD73). 116
Current Strategies to Stimulate the Immune Response for Antitumor Immunotherapy.
| Strategies | T cells | Myeloid cells | TME |
|---|---|---|---|
| Lymph node | Anti-CTLA4 | TNF-α | |
| To improve tumor antigen presentation by APCs | Anti-CD137 | IFN-α | |
| To optimize effector T-cell activation | Anti-OX40 | IL-1 | |
| Anti-CD27/CD70 | GM-CSF | ||
| HVEM | CD40L/CD40 | ||
| GITR | CDN | ||
| L-2 | ATP | ||
| IL-12 | HMGB1 | ||
| TLR | |||
| STING | |||
| RIG-1/MDA-5 | |||
| Blood vessel | CX3CL1 | ||
| To improve T-cell traffic to tumors | CXCL9 | ||
| To favor T-cell infiltration into tumors | CXCL10 | ||
| Transference of T cells bearing antigen-specific receptor | CCL5 | ||
| LFA1/ICAM1 | |||
| Selectins | |||
| CAR-T cell | |||
| TCR-T cell | |||
| Tumor | Anti-PD-L1 | Anti-CSF1/CSF1R | Anti-VEGF |
| To improve tumor antigen uptake by APCs | Anti-CTLA-4 | Anti-CCR2 | Inhibitors of IDO anti-CD73 |
| To improve recognition and killing of tumor cells by T cells | Anti-LAG-3 | PI3Kγ | ARs antagonists |
| Anti-TIM-3 | |||
| Anti-TIGIT | |||
| TNFR-agonists | |||
| IL-2 | |||
| IL-10 |
Abbreviations: TME, tumor microenvironment; IL, interleukin; TNF, Tumor Necrosis Factor; TNFR, TNF-receptor; CD137, receptor–co-stimulator of the TNFR family; OX40, member number 4 of the TNFR superfamily; CD27/CD70, member of the TNFR superfamily; CD40/CD40L, antigen-presenting cells (APC) co-stimulator and its ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; STING, IFN genes-stimulator; RIG-I, retinoic acid inducible gene-I; MDA5, melanoma differentiation-associated protein 5; CDN, cyclic dinucleotide; ATP, adenosine triphosphate; HMGB1, high mobility group B1 protein; TLR, Toll-like receptor; HVEM, Herpes virus entry mediator; GITR, glucocorticoid-induced TNFR family-related gene; CTLA4, cytotoxic T lymphocyte antigen 4; PD-L1, programmed death ligand-1; TIGIT, T-cell immunoreceptor with immunoglobulin and tyrosine-based inhibition motives; CSF1/CSF1R, colony-stimulating factor-1 and its receptor; CCR2, Type 2 chemokine receptor; PI3Kγ, Phosphoinositide 3-Kinase γ; CXCL/CCL, chemokine ligands; LFA1, lymphocyte function-associated antigen 1; ICAM1, intercellular adhesion molecule 1; VEGF, vascular endothelial growth factor; IDO, indolamine 2,3-dioxigenase; TGF, transforming growth factor; LAG-3, lymphocyte-activation gene 3 protein; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; CD73, 5´nucleotidase; ARs, adenosine receptors; Selectins, cell adhesion molecules; CAR-T, chimeric antigen receptor T cell; TCR-T, T-cell receptor engineered T cell.
Apart from the problems associated with its efficacy (only a small group of patients respond to it), immunotherapy faces several challenges related to its safety. In other words, immunotherapy can induce adverse events in patients, such as autoimmunity, where healthy tissues are attacked, or cytokine release syndrome and vascular leak syndrome, as observed with the use of IL-2, both of which lead to serious hypotension, fever, renal failure, and other adverse events that are potentially lethal. The main challenges to be faced by immunotherapy in the future will require the combined efforts of basic and clinical scientists, with the objective of accelerating the understanding of the complex interactions between cancer and the immune system, and improve treatment options for patients. Better comprehension of immune phenotypes in tumors, beyond the state of PD-L1 and TME, will be relevant to increase immunotherapy efficacy. In this context, the identification of precise tumor antigenicity biomarkers by means of new technologies, such as complete genome sequencing, single cell sequencing, and epigenetic analysis to identify sites or subclones typical in drug resistance, as well as activation, traffic and infiltration of effector cells of the immune response, and regulation of TME mechanisms, may help define patient populations that are good candidates for specific therapies and therapeutic combinations. 117 , 118 Likewise, the use of agents that can induce specific activation and modulation of the response of T cells in tumor tissue, will help improve efficacy and safety profiles that can lead to better clinical results.
Molecular Targeted Therapy
For over 30 years, and based on the progress in our knowledge of tumor biology and its mechanisms, there has been a search for therapeutic alternatives that would allow spread and growth of tumors to be slowed down by blocking specific molecules. This approach is known as molecular targeted therapy. 119 Among the elements generally used as molecular targets there are transcription factors, cytokines, membrane receptors, molecules involved in a variety of signaling pathways, apoptosis modulators, promoters of angiogenesis, and cell cycle regulators. 120
Imatinib, a tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia, became the first targeted therapy in the final years of the 1990s. 97 From then on, new drugs have been developed by design, and today more than 60 targeted therapies have been approved by the FDA for the treatment of a variety of cancers ( Table 3 ). 121 This has had a significant impact on progression-free survival and global survival in neoplasms such as non-small cell lung cancer, breast cancer, renal cancer, and melanoma.
FDA Approved Molecular Targeted Therapies for the Treatment of Solid Tumors.
| Drug | Therapeutic target | Indications | Biomarkers |
|---|---|---|---|
| Abemaciclib | CDK4/6 inhibitor | Breast cancer | ER+/PR+ |
| Abiraterone | Anti-androgen | Prostate cancer | AR+ |
| Afatinib | TKI anti-ErbB, EGFR (ErbB1), HER2 (ErbB2), ErbB3, ErbB4 | NSCLC | EGFR mutated |
| Deletion of exon 19 | |||
| Substitution in exon 21 (L858R) | |||
| Aflibercept | Anti-VEGF fusion protein | Colorectal cancer | |
| Alectinib | Anti-ALK TKI | NSCLC | ALK+ |
| Alpelisib | PI3K inhibitor | Breast cancer | PI3K mutated |
| Apalutamide | Anti-androgen | Prostate cancer | AR+ |
| Atezolizumab | Anti-PD-L1 mAb | Breast cancer | PD-L1 |
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Bladder cancer | |||
| Avapritinib | Kinase inhibitor | GIST | PDGFRA mutated in exon 18 (D842V) |
| Avelumab | Anti-PD-L1 mAb | Renal cancer | PD-L1 |
| Bladder cancer | |||
| Neuroendocrine tumors | |||
| Axitinib | Anti-VEGF TKI | Renal cancer | |
| Bevacizumab | Anti-VEGF mAb | CNS tumors | |
| Ovarian cancer | |||
| Cervical cancer | |||
| Colorectal cancer | |||
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Renal cancer | |||
| Brigatinib | Anti-ALK TKI | NSCLC | ALK+ |
| Cabozantinib | TKR inhibitor: anti-MET, anti-VEGF, anti-RET, ROS1, MER, KIT | Renal cancer | |
| Hepatocellular carcinoma | |||
| Thyroid cancer | |||
| Ceritinib | Anti-ALK TKI | NSCLC | ALK+ |
| Cetuximab | Anti-EGFR mAb | Colorectal cancer | KRAS |
| Head and Neck cancer | EGFR+ | ||
| Crizotinib | Anti-ALK TKI | NSCLC | ALK+, ROS1+ |
| Dabrafenib | BRAF inhibitor | NSCLC | BRAF-V600E, V600K |
| Thyroid cancer | |||
| Melanoma | |||
| Dacomitinib | Anti-EGFR TKI | NSCLC | EGFR+ |
| Darolutamide | Anti-androgen | Prostate cancer | AR+ |
| Durvalumab | Anti-PD-L1 mAb | NSCLC | PD-L1 |
| Bladder cancer | |||
| Encorafenib | BRAF inhibitor | Colorectal cancer | BRAF-V600E |
| Melanoma | |||
| Entrectinib | Anti-ROS1 TKI | NSCLC | ROS1+ |
| Enzalutamide | Anti-androgen | Prostate cancer | AR+ |
| Erdafitinib | Anti-FGFR-1 TKI | Bladder cancer | |
| Erlotinib | Anti-EGFR TKI | NSCLC | EGFR mutated |
| Pancreatic caner | Deletion of exon 19 | ||
| Substitution in exon 21 (L858R) | |||
| Everolimus | mTOR inhibitor | CNS tumors | |
| Pancreatic cancer | |||
| Breast cancer | |||
| Renal cancer | |||
| Fulvestrant | ER antagonist | Breast cancer | ER+/PR+ |
| Gefitinib | Anti-EGFR TKI | NSCLC | EGFR mutated |
| Deletion of exon 19 | |||
| Substitution in exon 21 (L858R) | |||
| Imatinib | Anti-KIT TKI | GIST | KIT+ |
| Dermatofibroma protuberans | |||
| Ipilimumab | Anti-CTLA-4 mAb | Colorectal cancer | |
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Melanoma | |||
| Renal cancer | |||
| Lapatinib | TKI: anti-EGFR, anti-HER2 | Breast cancer | ERBB2 over-expression or amplification |
| Lenvatinib | TKR: anti-VEGF, VEGFR1 (FLT1), VEGFR2 (KDR) y VEGFR3 (FLT4); (FGF) FGFR1, 2, 3 y 4, PDGF, PDGFRA, KIT, RET | Endometrial cancer | |
| Hepatocellular carcinoma | |||
| Renal cancer | |||
| Thyroid cancer | |||
| Lorlatinib | TKI: anti-ALK, anti-ROS2 | NSCLC | ALK+, ROS1+ |
| Necitumumab | Anti-EGFR mAb | NSCLC | EGFR+ |
| Neratinib | Anti-HER2 TKI | ||
| Anti-EGFR | Breast cancer | ERBB2 over-expression or amplification | |
| Niraparib | PARP inhibitor | Ovarian cancer | BRCA1/2 mutations |
| Fallopian tube cancer | Homologous recombination deficiency | ||
| Peritoneal cancer | |||
| Nivolumab | Anti-PD-1 mAb | Colorectal cancer | PD-1 |
| Esophageal cancer | |||
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Melanoma | |||
| Renal cancer | |||
| Bladder cancer | |||
| Head and Neck cancer | |||
| Olparib | PARP inhibitor | Breast cancer | BRCA1/2 mutations |
| Ovarian cancer | |||
| Pancreatic cancer | |||
| Prostate cancer | |||
| Osimertinib | Anti-EGFR TKI | NSCLC | EGFR-T790M |
| Palbociclib | CDK4/6 inhibitor | Breast cancer | RE+/RP+ |
| Pantitumumab | Anti-EGFR mAb | Colorectal cancer | KRAS |
| EGFR+ | |||
| Pazopanib | TKI: Anti-VEGF, anti-PDGFR, anti-FGFR, anti-cKIT | Renal cancer | |
| Soft tissues sarcoma | |||
| Pembrolizumab | PD-1 inhibitor | Cervical cancer | PD-1 |
| Endometrial cancer | |||
| Esophageal cancer | |||
| Gastric cancer | |||
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Bladder cancer | |||
| Head and Neck cancer | |||
| Pertuzumab | Anti-HER2 mAb | Breast cancer | ERBB2 over-expression or amplification |
| Ramucirumab | Anti-VEGF mAb | Colorectal cancer | |
| Esophageal cancer | |||
| Gastric cancer | |||
| Hepatocellular carcinoma | |||
| NSCLC | |||
| Regorafenib | Anti-cKIT TKI | Colorectal cancer | KIT+ |
| Hepatocellular carcinoma | |||
| GIST | |||
| Ribociclib | CDK4/6 inhibitor | Breast cancer | ER+/PR+ |
| Ripretinib | TKI: anti-KIT, anti-PDGFR | GIST | KIT+ |
| Rucaparib | PARP inhibitor | Prostate cancer | BRCA1/2 mutations |
| Ovarian cancer | |||
| Fallopian tube cancer | |||
| Peritoneal cancer | |||
| Sacituzumab-Govitecan | Conjugated Ab anti-trop-2 | Breast cancer | RE- RP- HER2- |
| Selpercatinib | Kinase inhibitor | NSCLC | RET+ |
| Thyroid cancer | |||
| Sorafenib | Multi-kinase inhibitor: anti-PDGFR, VEGFR, cKIT, TKR | Renal cancer | |
| Hepatocellular carcinoma | |||
| Thyroid cancer | |||
| Sunitinib | Multi-kinase inhibitor: anti-PDGFR, VEGFR, cKIT, TKR | Renal cancer | |
| Pancreatic cancer | |||
| GIST | |||
| Tamoxifeno | SERM | Breast cancer | ER+/PR+ |
| Talazoparib | PARP inhibitor | Breast cancer | BRCA1/2 mutations |
| Temsirolimus | mTOR inhibitor | Renal cancer | |
| Trametinib | BRAF inhibitor | NSCLC | BRAF-V600E, V600K |
| Thyroid cancer | |||
| Melanoma | |||
| Trastuzumab | Anti-HER2 mAb | Gastric cancer | ERBB2 over-expression of amplification |
| Gastro-esophageal junction cancer | |||
| Breast cancer | |||
| Trastuzumab-Deruxtecan | Anti-HER2 conjugated Ab | Breast cancer | ERBB2 over-expression of amplification |
| Trastuzumab-Emtansine | Anti-HER2 conjugated Ab | Breast cancer | ERBB2 over-expression of amplification |
| Tucatinib | Anti-HER2 TKI | Breast cancer | ERBB2 over-expression of amplification |
| Vandetanib | TKI: anti-VEGF, anti-EGFR | Thyroid cancer | EGFR+ |
| Vemurafenib | BRAF inhibitor | Melanoma | BRAF-V600E |
Abbreviations: mAb, monoclonal antibody; ALK, anaplastic lymphoma kinase; CDK, cyclin-dependent kinase; CTLA-4, cytotoxic lymphocyte antigen-4; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stroma tumor; mTOR, target of rapamycine in mammal cells; NSCLC, non-small cell lung carcinoma; PARP, poli (ADP-ribose) polimerase; PD-1, programmed death protein-1; PDGFR, platelet-derived growth factor receptor; PD-L1, programmed death ligand-1; ER, estrogen receptor; PR, progesterone receptor; TKR, tyrosine kinase receptors; SERM, selective estrogen receptor modulator; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor. Modified from Ref. [ 127 ].
Most drugs classified as targeted therapies form part of 2 large groups: small molecules and mAbs. The former are defined as compounds of low molecular weight (<900 Daltons) that act upon entering the cell. 120 Targets of these compounds are cell cycle regulatory proteins, proapoptotic proteins, or DNA repair proteins. These drugs are indicated based on histological diagnosis, as well as molecular tests. In this group there are multi-kinase inhibitors (RTKs) and tyrosine kinase inhibitors (TKIs), like sunitinib, sorafenib, and imatinib; cyclin-dependent kinase (CDK) inhibitors, such as palbociclib, ribociclib and abemaciclib; poli (ADP-ribose) polimerase inhibitors (PARPs), like olaparib and talazoparib; and selective small-molecule inhibitors, like ALK and ROS1. 122
As for mAbs, they are protein molecules that act on membrane receptors or extracellular proteins by interrupting the interaction between ligands and receptors, in such a way that they reduce cell replication and induce cytostasis. Among the most widely used mAbs in oncology we have: trastuzumab, a drug directed against the receptor for human epidermal growth factor-2 (HER2), which is overexpressed in a subgroup of patients with breast and gastric cancer; and bevacizumab, that blocks vascular endothelial growth factor and is used in patients with colorectal cancer, cervical cancer, and ovarian cancer. Other mAbs approved by the FDA include pembolizumab, atezolizumab, nivolumab, avelumab, ipilimumab, durvalumab, and cemiplimab. These drugs require expression of response biomarkers, such as PD-1 and PD-L1, and must also have several resistance biomarkers, such as the expression of EGFR, the loss of PTEN, and alterations in beta-catenin. 123
Because cancer is such a diverse disease, it is fundamental to have precise diagnostic methods that allow us to identify the most adequate therapy. Currently, basic immunohistochemistry is complemented with neoplastic molecular profiles to determine a more accurate diagnosis, and it is probable that in the near future cancer treatments will be based exclusively on molecular profiles. In this regard, it is worth mentioning that the use of targeted therapy depends on the existence of specific biomarkers that indicate if the patient will be susceptible to the effects of the drug or not. Thus, the importance of underlining that not all patients are susceptible to receive targeted therapy. In certain neoplasms, therapeutic targets are expressed in less than 5% of the diagnosed population, hindering a more extended use of certain drugs.
The identification of biomarkers and the use of new generation sequencing on tumor cells has shown predictive and prognostic relevance. Likewise, mutation analysis has allowed monitoring of tumor clone evolution, providing information on changes in canonic gene sequences, such as TP53, GATA3, PIK3CA, AKT1, and ERBB2; infrequent somatic mutations developed after primary treatments, like SWI-SNF and JAK2-STAT3; or acquired drug resistance mutations such as ESR1. 124 The study of mutations is vital; in fact, many of them already have specific therapeutic indications, which have helped select adequate treatments. 125
There is no doubt that molecular targeted therapy is one of the main pillars of precision medicine. However, it faces significant problems that often hinder obtaining better results. Among these, there is intratumor heterogeneity and differences between the primary tumor and metastatic sites, as well as intrinsic and acquired resistance to these therapies, the mechanisms of which include the presence of heterogeneous subclones, DNA hypermethylation, histone acetylation, and interruption of mRNA degradation and translation processes. 126 Nonetheless, beyond the obstacles facing molecular targeted therapy from a biological and methodological point of view, in the real world, access to genomic testing and specific drugs continues to be an enormous limitation, in such a way that strategies must be designed in the future for precision medicine to be possible on a global scale.
Cell Therapy
Another improvement in cancer treatment is the use of cell therapy, that is, the use of specific cells as therapeutic agents. This clinical procedure has 2 modalities: the first consists of replacing and regenerating functional cells in a specific tissue by means of stem/progenitor cells of a certain kind, 43 while the second uses immune cells as effectors to eliminate malignant cells. 127
Regarding the first type, we must emphasize the development of cell therapy based on hematopoietic stem and progenitor cells. 128 For over 50 years, hematopoietic cell transplants have been used to treat a variety of hematologic neoplasms (different forms of leukemia and lymphoma). Today, it is one of the most successful examples of cell therapy, including innovative modalities, such as haploidentical transplants, 129 as well as application of stem cells expanded ex vivo . 130 There are also therapies that have used immature cells that form part of the TME, such as MSCs. The replication potential and cytokine secretion capacity of these cells make them an excellent option for this type of treatment. 131 Neural stem cells can also be manipulated to produce and secrete apoptotic factors, and when these cells are incorporated into primary neural tumors, they cause a certain degree of regression. They can even be transfected with genes that encode for oncolytic enzymes capable of inducing regression of glioblastomas. 132
With respect to cell therapy using immune cells, several research groups have manipulated cells associated with tumors to make them effector cells and thus improve the efficacy and specificity of the antitumor treatment. PB leckocytes cultured in the presence of IL-2 to obtain activated lymphocytes, in combination with IL-2 administration, have been used in antitumor clinical protocols. Similarly, infiltrating lymphocytes from tumors with antitumor activity have been used and can be expanded ex vivo with IL-2. These lymphocyte populations have been used in immunomodulatory therapies in melanoma, and pancreatic and kidney tumors, producing a favorable response in treated patients. 133 NK cells and macrophages have also been used in immunotherapy, although with limited results. 134 , 135
One of the cell therapies with better projection today is the use of CAR-T cells. This strategy combines 2 forms of advanced therapy: cell therapy and gene therapy. It involves the extraction of T cells from the cancer patient, which are genetically modified in vitro to express cell surface receptors that will recognize antigens on the surface of tumor cells. The modified T cells are then reintroduced in the patient to aid in an exacerbated immune response that leads to eradication of the tumor cells ( Figure 4 ). Therapy with CAR-T cells has been used successfully in the treatment of some types of leukemia, lymphoma, and myeloma, producing complete responses in patients. 136

CAR-T cell therapy. (A) T lymphocytes obtained from cancer patients are genetically manipulated to produce CAR-T cells that recognize tumor cells in a very specific manner. (B) Interaction between CAR molecule and tumor antigen. CAR molecule is a receptor that results from the fusion between single-chain variable fragments (scFv) from a monoclonal antibody and one or more intracellular signaling domains from the T-cell receptor. CD3ζ, CD28 and 4-1BB correspond to signaling domains on the CAR molecule.
Undoubtedly, CAR-T cell therapy has been truly efficient in the treatment of various types of neoplasms. However, this therapeutic strategy can also have serious side effects, such as release of cytokines into the bloodstream, which can cause different symptoms, from high fever to multiorgan failure, and even neurotoxicity, leading to cerebral edema in many cases. 137 Adequate control of these side effects is an important medical challenge. Several research groups are trying to improve CAR-T cell therapy through various approaches, including production of CAR-T cells directed against a wider variety of tumor cell-specific antigens that are able to attack different types of tumors, and the identification of more efficient types of T lymphocytes. Furthermore, producing CAR-T cells from a single donor that may be used in the treatment of several patients would reduce the cost of this sort of personalized cell therapy. 136
Achieving wider use of cell therapy in oncologic diseases is an important challenge that requires solving various issues. 138 One is intratumor cell heterogeneity, including malignant subclones and the various components of the TME, which results in a wide profile of membrane protein expression that complicates finding an ideal tumor antigen that allows specific identification (and elimination) of malignant cells. Likewise, structural organization of the TME challenges the use of cell therapy, as administration of cell vehicles capable of recognizing malignant cells might not be able to infiltrate the tumor. This results from low expression of chemokines in tumors and the presence of a dense fibrotic matrix that compacts the inner tumor mass and avoids antitumor cells from infiltrating and finding malignant target cells.
Further Challenges in the 21st Century
Beyond the challenges regarding oncologic biomedical research, the 21 st century is facing important issues that must be solved as soon as possible if we truly wish to gain significant ground in our fight against cancer. Three of the most important have to do with prevention, early diagnosis, and access to oncologic medication and treatment.
Prevention and Early Diagnosis
Prevention is the most cost-effective strategy in the long term, both in low and high HDI nations. Data from countries like the USA indicate that between 40-50% of all types of cancer are preventable through potentially modifiable factors (primary prevention), such as use of tobacco and alcohol, diet, physical activity, exposure to ionizing radiation, as well as prevention of infection through access to vaccination, and by reducing exposure to environmental pollutants, such as pesticides, diesel exhaust particles, solvents, etc. 74 , 84 Screening, on the other hand, has shown great effectiveness as secondary prevention. Once population-based screening programs are implemented, there is generally an initial increase in incidence; however, in the long term, a significant reduction occurs not only in incidence rates, but also in mortality rates due to detection of early lesions and timely and adequate treatment.
A good example is colon cancer. There are several options for colon cancer screening, such as detection of fecal occult blood, fecal immunohistochemistry, flexible sigmoidoscopy, and colonoscopy, 139 , 140 which identify precursor lesions (polyp adenomas) and allow their removal. Such screening has allowed us to observe 3 patterns of incidence and mortality for colon cancer between the years 2000 and 2010: on one hand, an increase in incidence and mortality in countries with low to middle HDI, mainly countries in Asia, South America, and Eastern Europe; on the other hand, an increase in incidence and a fall in mortality in countries with very high HDI, such as Canada, the United Kingdom, Denmark, and Singapore; and finally a fall in incidence and mortality in countries like the USA, Japan, and France. The situation in South America and Asia seems to reflect limitations in medical infrastructure and a lack of access to early detection, 141 while the patterns observed in developed countries reveal the success, even if it may be partial, of that which can be achieved by well-structured prevention programs.
Another example of success, but also of strong contrast, is cervical cancer. The discovery of the human papilloma virus (HPV) as the causal agent of cervical cancer brought about the development of vaccines and tests to detect oncogenic genotypes, which modified screening recommendations and guidelines, and allowed several developed countries to include the HPV vaccine in their national vaccination programs. Nevertheless, the outlook is quite different in other areas of the world. Eighty percent of the deaths by cervical cancer reported in 2018 occurred in low-income nations. This reveals the urgency of guaranteeing access to primary and secondary prevention (vaccination and screening, respectively) in these countries, or else it will continue to be a serious public health problem in spite of its preventability.
Screening programs for other neoplasms, such as breast, prostate, lung, and thyroid cancer have shown outlooks that differ from those just described, because, among other reasons, these neoplasms are highly diverse both biologically and clinically. Another relevant issue is the overdiagnosis of these neoplasms, that is, the diagnosis of disease that would not cause symptoms or death in the patient. 142 It has been calculated that 25% of breast cancer (determined by mammogram), 50–60% of prostate cancer (determined by PSA), and 13–25% of lung cancer (determined by CT) are overdiagnosed. 142 Thus, it is necessary to improve the sensitivity and specificity of screening tests. In this respect, knowledge provided by the biology of cancer and “omic” sciences offers a great opportunity to improve screening and prevention strategies. All of the above shows that prevention and early diagnosis are the foundations in the fight against cancer, and it is essential to continue to implement broader screening programs and better detection methods.
Global Equity in Oncologic Treatment
Progress in cancer treatment has considerably increased the number of cancer survivors. Nevertheless, this tendency is evident only in countries with a very solid economy. Indeed, during the past 30 years, cancer mortality rates have increased 30% worldwide. 143 Global studies indicate that close to 70% of cancer deaths in the world occur in nations of low to middle income. But even in high-income countries, there are sectors of society that are more vulnerable and have less access to cancer treatments. 144 Cancer continues to be a disease of great social inequality.
In Europe, the differences in access to cancer treatment are highly marked. These treatments are more accessible in Western Europe than in its Eastern counterpart. 145 Furthermore, highly noticeable differences between high-income countries have been detected in the cost of cancer drugs. 146 It is interesting to note that in many of these cases, treatment is too costly and the clinical benefit only marginal. Thus, the importance of these problems being approached by competent national, regional, and global authorities, because if these new drugs and therapeutic programs are not accessible to the majority, progress in biomedical, clinical and epidemiological research will have a limited impact in our fight against cancer. We must not forget that health is a universal right, from which low HDI countries must not be excluded, nor vulnerable populations in nations with high HDI. The participation of a well-informed society will also be fundamental to achieve a global impact, as today we must fight not only against the disease, but also against movements and ideas (such as the anti-vaccine movement and the so-called miracle therapies) that can block the medical battle against cancer.
Final Comments
From the second half of the 20th century to the present day, progress in our knowledge about the origin and development of cancer has been extraordinary. We now understand cancer in detail in genomic, molecular, cellular, and physiological terms, and this knowledge has had a significant impact in the clinic. There is no doubt that a patient who is diagnosed today with a type of cancer has a better prospect than a patient diagnosed 20 or 50 years ago. However, we are still far from winning the war against cancer. The challenges are still numerous. For this reason, oncologic biomedical research must be a worldwide priority. Likewise, one of the fundamental challenges for the coming decades must be to reduce unequal access to health services in areas of low- to middle income, and in populations that are especially vulnerable, as well as continue improving prevention programs, including public health programs to reduce exposure to environmental chemicals and improve diet and physical activity in the general population. 74 , 84 Fostering research and incorporation of new technological resources, particularly in less privileged nations, will play a key role in our global fight against cancer.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Hector Mayani https://orcid.org/0000-0002-2483-3782
- Open access
- Published: 11 March 2021
Evaluating cancer research impact: lessons and examples from existing reviews on approaches to research impact assessment
- Catherine R. Hanna ORCID: orcid.org/0000-0002-0907-7747 1 ,
- Kathleen A. Boyd 2 &
- Robert J. Jones 1
Health Research Policy and Systems volume 19 , Article number: 36 ( 2021 ) Cite this article
6693 Accesses
6 Citations
10 Altmetric
Metrics details
Performing cancer research relies on substantial financial investment, and contributions in time and effort from patients. It is therefore important that this research has real life impacts which are properly evaluated. The optimal approach to cancer research impact evaluation is not clear. The aim of this study was to undertake a systematic review of review articles that describe approaches to impact assessment, and to identify examples of cancer research impact evaluation within these reviews.
In total, 11 publication databases and the grey literature were searched to identify review articles addressing the topic of approaches to research impact assessment. Information was extracted on methods for data collection and analysis, impact categories and frameworks used for the purposes of evaluation. Empirical examples of impact assessments of cancer research were identified from these literature reviews. Approaches used in these examples were appraised, with a reflection on which methods would be suited to cancer research impact evaluation going forward.
In total, 40 literature reviews were identified. Important methods to collect and analyse data for impact assessments were surveys, interviews and documentary analysis. Key categories of impact spanning the reviews were summarised, and a list of frameworks commonly used for impact assessment was generated. The Payback Framework was most often described. Fourteen examples of impact evaluation for cancer research were identified. They ranged from those assessing the impact of a national, charity-funded portfolio of cancer research to the clinical practice impact of a single trial. A set of recommendations for approaching cancer research impact assessment was generated.
Conclusions
Impact evaluation can demonstrate if and why conducting cancer research is worthwhile. Using a mixed methods, multi-category assessment organised within a framework, will provide a robust evaluation, but the ability to perform this type of assessment may be constrained by time and resources. Whichever approach is used, easily measured, but inappropriate metrics should be avoided. Going forward, dissemination of the results of cancer research impact assessments will allow the cancer research community to learn how to conduct these evaluations.
Peer Review reports
Cancer research attracts substantial public funding globally. For example, the National Cancer Institute (NCI) in the United States of America (USA) had a 2020 budget of over $6 billion United States (US) dollars. In addition to public funds, there is also huge monetary investment from private pharmaceutical companies, as well as altruistic investment of time and effort to participate in cancer research from patients and their families. In the United Kingdom (UK), over 25,000 patients were recruited to cancer trials funded by one charity (Cancer Research UK (CRUK)) alone in 2018 [ 1 ]. The need to conduct research within the field of oncology is an ongoing priority because cancer is highly prevalent, with up to one in two people now having a diagnosis of cancer in their lifetime [ 2 , 3 ], and despite current treatments, mortality and morbidity from cancer are still high [ 2 ].
In the current era of increasing austerity, there is a desire to ensure that the money and effort to conduct any type of research delivers tangible downstream benefits for society with minimal waste [ 4 , 5 , 6 ]. These wider, real-life benefits from research are often referred to as research impact. Given the significant resources required to conduct cancer research in particular, it is reasonable to question if this investment is leading to the longer-term benefits expected, and to query the opportunity cost of not spending the same money directly within other public sectors such as health and social care, the environment or education.
The interest in evaluating research impact has been rising, partly driven by the actions of national bodies and governments. For example, in 2014, the UK government allocated its £2 billion annual research funding to higher education institutions, in part based on an assessment of the impact of research performed by each institution in an assessment exercise known as the Research Excellence Framework (REF). The proportion of funding dependent on impact assessment will increase from 20% in 2014, to 25% in 2021[ 7 ].
Despite the clear rationale and contemporary interest in research impact evaluation, assessing the impact of research comes with challenges. First, there is no single definition of what research impact encompasses, with potential differences in the evaluation approach depending on the definition. Second, despite the recent surge of interest, knowledge of how best to perform assessments and the infrastructure for, and experience in doing so, are lagging [ 6 , 8 , 9 ]. For the purposes of this review, the definition of research impact given by the UK Research Councils is used (see Additional file 1 for full definition). This definition was chosen because it takes a broad perspective, which includes academic, economic and societal views of research impact [ 10 ].
There is a lack of clarity on how to perform research impact evaluation, and this extends to cancer research. Although there is substantial interest from cancer funders and researchers [ 11 ], this interest is not accompanied by instruction or reflection on which approaches would be suited to assessing the impact of cancer research specifically. In a survey of Australian cancer researchers, respondents indicated that they felt a responsibility to deliver impactful research, but that evaluating and communicating this impact to stakeholders was difficult. Respondents also suggested that the types of impact expected from research, and the approaches used, should be discipline specific [ 12 ]. Being cognisant of the discipline specific nature of impact assessment, and understanding the uniqueness of cancer research in approaching such evaluations, underpins the rationale for this study.
The aim of this study was to explore approaches to research impact assessment, identify those approaches that have been used previously for cancer research, and to use this information to make recommendations for future evaluations. For the purposes of this study, cancer research included both basic science and applied research, research into any malignant disease, concerning paediatric or adult cancer, and studies spanning nursing, medical, public health elements of cancer research.
The study objectives were to:
Identify existing literature reviews that report approaches to research impact assessment and summarise these approaches.
Use these literature reviews to identify examples of cancer research impact evaluations, describe the approaches to evaluation used within these studies, and compare them to those described in the broader literature.
This approach was taken because of the anticipated challenge of conducting a primary review of empirical examples of cancer research impact evaluation, and to allow a critique of empirical studies in the context of lessons learnt from the wider literature. A primary review would have been difficult because examples of cancer research impact evaluation, for example, the assessment of research impact on clinical guidelines [ 13 ], or clinical practice [ 14 , 15 , 16 ], are often not categorised in publication databases under the umbrella term of research impact. Reasons for this are the lack of medical subject heading (MeSH) search term relating to research impact assessment and the differing definitions for research impact. In addition, many authors do not recognise their evaluations as sitting within the discipline of research impact assessment, which is a novel and emerging field of study.
General approach
A systematic search of the literature was performed to identify existing reviews of approaches to assess the impact of research. No restrictions were placed on the discipline, field, or scope (national/global) of research for this part of the study. In the second part of this study, the reference lists of the literature reviews identified were searched to find empirical examples of the evaluation of the impact of cancer research specifically.
Data sources and searches
For the first part of the study, 11 publication databases and the grey literature from January 1998 to May 2019 were searched. The electronic databases were Medline, Embase, Health Management and Policy Database, Education Resources Information Centre, Cochrane, Cumulative Index of Nursing and Allied Health Literature, Applied Social Sciences Index and Abstract, Social Services Abstracts, Sociological Abstracts, Health Business Elite and Emerald. The search strategy specified that article titles must contain the word “impact”, as well as a second term indicating that the article described the evaluation of impact, such as “model” or “measurement” or “method”. Additional file 1 provides a full list of search terms. The grey literature was searched using a proforma. Keywords were inserted into the search function of websites listed on the proforma and the first 50 results were screened. Title searches were performed by either a specialist librarian or the primary researcher (Dr. C Hanna). All further screening of records was performed by the primary researcher.
Following an initial title screen, 800 abstracts were reviewed and 140 selected for full review. Articles were kept for final inclusion in the study by assessing each article against specific inclusion criteria (Additional file 1 ). There was no assessment of the quality of the included reviews other than to describe the search strategy used. If two articles drew primarily on the same review but contributed a different critique of the literature or methods to evaluate impact, both were kept. If a review article was part of a grey literature report, for example a thesis, but was also later published in a journal, the journal article only was kept. Out of 140 articles read in full, 27 met the inclusion criteria and a further 13 relevant articles were found through reference list searching from the included reviews [ 17 ].
For the second part of the study, the reference lists from the literature reviews were manually screened [ 17 ] ( n = 4479 titles) by the primary researcher to identify empirical examples of assessment of the impact of cancer research. Summary tables and diagrams from the reviews were also searched using the words “cancer” and “oncology” to identify relevant articles that may have been missed by reference list searching. After removal of duplicates, 57 full articles were read and assessed against inclusion criteria (Additional file 1 ). Figure 1 shows the search strategy for both parts of the study according to the guidelines for preferred reporting items for systematic reviews and meta-analysis (PRISMA) [ 18 ].
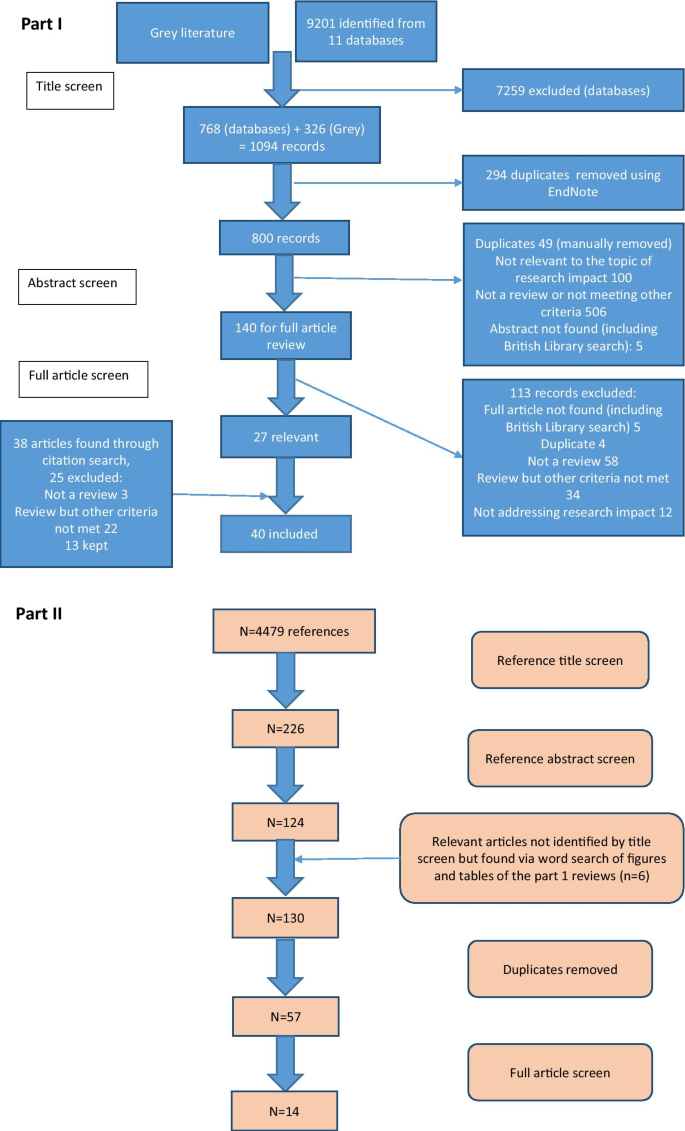
Search strategies for this study
Data extraction and analysis
A data extraction form produced in Microsoft ® Word 2016 was used to collect details for each literature review. This included year of publication, location of primary author, research discipline, aims of the review as described by the authors and the search strategy (if any) used. Information on approaches to impact assessment was extracted under three specific themes which had been identified from a prior scoping review as important factors when planning and conducting research impact evaluation. These themes were: (i) categorisation of impact into different types depending on who or what is affected by the research (the individuals, institutions, or parts of society, the environment), and how they are affected (for example health, monetary gain, sustainability) (ii) methods of data collection and analysis for the purposes of evaluation, and (iii) frameworks to organise and communicate research impact. There was space to document any other key findings the researcher deemed important. After data extraction, lists of commonly described categories, methods of data collection and analysis, and frameworks were compiled. These lists were tabulated or presented graphically and narrative analysis was used to describe and discuss the approaches listed.
For the second part of the study, a separate data extraction form produced in Microsoft ® Excel 2016 was used. Basic information on each study was collected, such as year of publication, location of primary authors, research discipline, aims of evaluation as described by the authors and research type under assessment. Data was also extracted from these empirical examples using the same three themes as outlined above, and the approaches used in these studies were compared to those identified from the literature reviews. Finally, a set of recommendations for future evaluations of cancer research impact were developed by identifying the strengths of the empirical examples and using the lists generated from the first part of the study to identify improvements that could be made.
Part one: Identification and analysis of literature reviews describing approaches to research impact assessment
Characteristics of included literature reviews.
Forty literature reviews met the pre-specified inclusion criteria and the characteristics of each review are outlined in Table 1 . A large proportion (20/40; 50%) were written by primary authors based in the UK, followed by the USA (5/40; 13%) and Australia (5/40; 13%), with the remainder from Germany (3/40; 8%), Italy (3/40; 8%), the Netherlands (1/40; 3%), Canada (1/40; 3%), France (1/40; 3%) and Iran (1/40; 3%). All reviews were published since 2003, despite the search strategy dating from 1998. Raftery et al. 2016 [ 19 ] was an update to Hanney et al. 2007 [ 20 ] and both were reviews of studies assessing research impact relevant to a programme of health technology assessment research. The narrative review article by Greenhalgh et al. [ 21 ] was based on the same search strategy used by Raftery et al. [ 19 ].
Approximately half of the reviews (19/40; 48%) described approaches to evaluate research impact without focusing on a specific discipline and nearly the same amount (16/40; 40%) focused on evaluating the impact of health or biomedical research. Two reviews looked at approaches to impact evaluation for environmental research and one focused on social sciences and humanities research. Finally, two reviews provided a critique of impact evaluation methods used by different countries at a national level [ 22 , 23 ]. None of these reviews focused specifically on cancer research.
Twenty-five reviews (25/40; 63%) specified search criteria and 11 of these included a PRISMA diagram. The articles that did not outline a search strategy were often expert reviews of the approaches to impact assessment methods and the authors stated they had chosen the articles included based on their prior knowledge of the topic. Most reviews were found by searching traditional publication databases, however seven (7/40; 18%) were found from the grey literature. These included four reports written by an independent, not-for-profit research institution (Research and Development (RAND) Europe) [ 23 , 24 , 25 , 26 ], one literature review which was part of a Doctor of Philosophy (Ph.D) thesis [ 27 ], a literature review informing a quantitative study [ 28 ] and a review that provided background information for a report to the UK government on the best use of impact metrics [ 29 ].
Key findings from the reviews: approaches to research impact evaluation
Categorisation of impact for the purpose of impact assessment
Nine reviews attempted to categorise the type of research impact being assessed according to who or what is affected by research, and how they are affected. In Fig. 2 , colour coding was used to identify overlap between impact types identified in these reviews to produce a summary list of seven main impact categories.
The first two categories of impact refer to the immediate knowledge produced from research and the contribution research makes to driving innovation and building capacity for future activities within research institutions. The former is often referred to as the academic impact of research. The academic impact of cancer research may include the knowledge gained from conducting experiments or performing clinical trials that is subsequently disseminated via journal publications. The latter may refer to securing future funding for cancer research, providing knowledge that allows development of later phase clinical trials or training cancer researchers of the future.
The third category identified was the impact of research on policy. Three of the review articles included in this overview specifically focused policy impact evaluation [ 30 , 31 , 32 ]. In their review, Hanney et al. [ 30 ] suggested that policy impact (of health research) falls into one of three sub-categories: impact on national health policies from the government, impact on clinical guidelines from professional bodies, and impact on local health service policies. Cruz Rivera and colleagues [ 33 ] specifically distinguished impact on policy making from impact on clinical guidelines, which they described under health impact. This shows that the lines between categories will often blur.
Impact on health was the next category identified and several of the reviews differentiated health sector impact from impact on health gains. For cancer research, both types of health impact will be important given that it is a health condition which is a major burden for healthcare systems and the patients they treat. Economic impact of research was the fifth category. For cancer research, there is likely to be close overlap between healthcare system and economic impacts because of the high cost of cancer care for healthcare services globally.
In their 2004 article, Buxton et al. [ 34 ] searched the literature for examples of the evaluation of economic return on investment in health research and found four main approaches, which were referenced in several later reviews [ 19 , 25 , 35 , 36 ]. These were (i) measuring direct cost savings to the health-care system, (ii) estimating benefits to the economy from a healthy workforce, (iii) evaluating benefits to the economy from commercial development and, (iv) measuring the intrinsic value to society of the health gain from research. In a later review [ 25 ], they added an additional approach of estimating the spill over contribution of research to the Gross Domestic Product (GDP) of a nation.
The final category was social impact. This term was commonly used in a specific sense to refer to research improving human rights, well-being, employment, education and social inclusion [ 33 , 37 ]. Two of the reviews which included this category focused on the impact of non-health related research (social sciences and agriculture), indicating that this type of impact may be less relevant or less obvious for health related disciplines such as oncology. Social impact is distinct from the term societal impact, which was used in a wider sense to describe impact that is external to traditional academic benefits [ 38 , 39 ]. Other categories of impact identified that did not show significant overlap between the reviews included cultural and technological impact. In two of the literature reviews [ 33 , 40 ], the authors provided a list of indicators of impact within each of their categories. In the review by Thonon et al. [ 40 ], only one (1%) of these indicators was specific to evaluating the impact of cancer research.
Methods for data collection and analysis
In total, 36 (90%) reviews discussed methods to collect or analyse the data required to conduct an impact evaluation. The common methods described, and the strengths and weaknesses of each approach, are shown in Additional file 2 : Table S1. Many authors advocated using a mixture of methods and in particular, the triangulation of surveys, interviews (of researchers or research users), and documentary analysis [ 20 , 30 , 31 , 32 ]. A large number of reviews cautioned against the use of quantitative metrics, such as bibliometrics, alone [ 29 , 30 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ]. Concerns included that these metrics were often not designed to be comparable between research programmes [ 49 ], their use may incentivise researchers to focus on quantity rather than quality [ 42 ], and these metrics could be gamed and used in the wrong context to make decisions about researcher funding, employment and promotion [ 41 , 43 , 45 ].
Several reviews explained that the methods for data collection and analysis chosen for impact evaluation depended on both the unit of research under analysis and the rationale for the impact analysis [ 23 , 24 , 26 , 31 , 36 , 50 , 51 ]. Specific to cancer research, the unit of analysis may be a single clinical trial or a programme of trials, research performed at a cancer centre or research funded by a specific institution or charity. The rationale for research impact assessment was categorised in multiple reviews under four headings (“the 4 As”): advocacy, accountability, analysis and allocation [ 19 , 20 , 23 , 24 , 30 , 31 , 32 , 33 , 36 , 46 , 52 , 53 ]. Finally, Boaz and colleagues found that there was a lack of information on the cost-effectiveness of research impact evaluation methods but suggested that pragmatic, but often cheaper approaches to evaluation, such as surveys, were least likely to give in depth insights into the processes through which research impact occurred [ 31 ].
Using a framework within a research impact evaluation
Applied to research impact evaluation, a framework provides a way of organising collected data, which encourages a more objective and structured evaluation than would be possible with an ad hoc analysis. In total, 27 (68%) reviews discussed the use of a framework in this context. Additional file 2 : Table S2 lists the frameworks mentioned in three or more of the included reviews. The most frequently described framework was the Payback Framework, developed by Buxton and Hanney in 1996 [ 54 ], and many of the other frameworks identified reported that they were developed by adapting key elements of the Payback framework. None of the frameworks identified were specifically developed to assess the impact of cancer research, however several were specific to health research. The unit of cancer research being evaluated will dictate the most suitable framework to use in any evaluation. The unit of research most suited to each framework is outlined in Additional file 2 : Table S2.
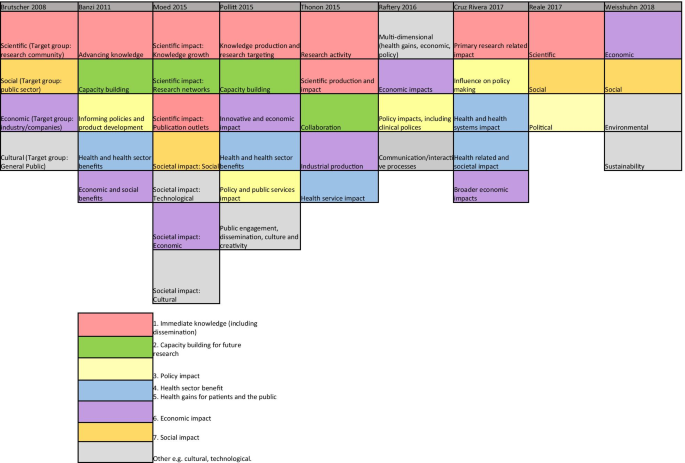
Categories of impact identified in the included literature reviews
Additional findings from the included reviews
The challenges of research impact evaluation were commonly discussed in these reviews. Several mentioned that the time lag [ 24 , 25 , 33 , 35 , 38 , 46 , 50 , 53 , 55 ] between research completion and impact occurring should influence when an impact evaluation is carried out: too early and impact will not have occurred, too late and it is difficult to link impact to the research in question. This overlapped with the challenge of attributing impact to a particular piece of research [ 24 , 26 , 33 , 34 , 35 , 37 , 38 , 39 , 46 , 50 , 56 ]. Many authors argued that the ability to show attribution was inversely related to the time since the research was carried out [ 24 , 25 , 31 , 46 , 53 ].
Part II: Empirical examples of cancer research impact evaluation
Study characteristics.
In total, 14 empirical impact evaluations relevant to cancer research were identified from the references lists of the literature reviews included in the first part of this study. These empirical studies were published between 1994–2015 by primary authors located in the UK (7/14; 50%), USA (2/14; 14%), Italy (2/14; 14%), Canada (2/14; 14%) and Brazil (1/14; 14%). Table 2 lists these studies with the rationale for each assessment (defined using the “4As”), the unit of analysis of cancer research evaluated and the main findings from each evaluation. The categories of impact evaluated, methods of data collection and analysis, and impact frameworks utilised are also summarised in Table 2 and discussed in more detail below.
Approaches to cancer research impact evaluation used in empirical studies
Categories of impact evaluated in cancer research impact assessments
Several of the empirical studies focused on academic impact. For example, Ugolini and colleagues evaluated scholarly outputs from one cancer research centre in Italy [ 57 ] and in a second study looked at the academic impact of cancer research from European countries [ 58 ]. Saed et al. [ 59 ] used submissions to an international cancer conference (American Society of Clinical Oncology (ASCO)) to evaluate the dissemination of cancer research to the academic community, and Lewison and colleagues [ 60 , 61 , 62 , 63 ] assessed academic, as well as policy impact and dissemination of cancer research findings to the lay media.
The category of the health impact was also commonly evaluated, with particular focus on the assessment of survival gains. Life years gained or deaths averted [ 64 ], life expectancy gains [ 65 ] and years of extra survival [ 66 ] were all used as indicators of the health impact attributable to cancer research. Glover and colleagues [ 67 ] used a measure of health utility, the quality adjusted life year (QALY), which combines both survival and quality of life assessments. Lakdawalla and colleagues [ 66 ] considered the impact of both research on cancer screening and treatments, and concluded that survival gains were 80% attributable to treatment improvement. In contrast, Glover and colleagues [ 67 ] acknowledged the importance of improved cancer therapies due to research but also highlight the major impacts from research around smoking cessation, as well as cervical and bowel cancer screening. Several of these studies that assessed health impact, also used the information on health gains to assess the economic impact of the same research [ 64 , 65 , 66 , 67 ].
Finally, two studies [ 68 , 69 ] performed multi-dimensional research impact assessments, which incorporated nearly all of the seven categories of impact identified from the previous literature (Fig. 2 ). In their assessment of the impact of research funded by one breast cancer charity in Australia, Donovan and colleagues [ 69 ] evaluated academic, capacity building, policy, health, and wider economic impacts. Montague and Valentim [ 68 ] assessed the impact of one randomised clinical trial (MA17) which investigated the use of a hormonal medication as an adjuvant treatment for patients with breast cancer. In their study, they assessed the dissemination of research findings, academic impact, capacity building for future trials and international collaborations, policy citation, and the health impact of decreased breast cancer recurrence attributable to the clinical trial.
Methods of data collection and analysis for cancer research impact evaluation
Methods for data collection and analysis used in these studies aligned with the categories of impact assessed. For example, studies assessing academic impact used traditional bibliometric searching of publication databases and associated metrics. Ugolini et al. [ 57 ] applied a normalised journal impact factor to publications from a cancer research centre as an indicator of the research quality and productivity from that centre. This analysis was adjusted for the number of employees within each department and the scores were used to apportion 20% of future research funding. The same bibliometric method of analysis was used in a second study by the same authors to compare and contrast national level, cancer research efforts across Europe [ 58 ]. They assessed the quantity and the mean impact factor of the journals for publications from each country and compared this to the location-specific population and GDP. A similar approach was used for the manual assessment of 10% of cancer research abstracts submitted to an international conference (ASCO) between 2001–2003 and 2006–2008 [ 59 ]. These authors examined if the location of authors affected the likelihood of the abstract being presented orally, as a face-to-face poster or online only.
Lewison and colleagues, who performed four of the studies identified [ 60 , 61 , 62 , 63 ], used a different bibliometric method of publication citation count to analyse the dissemination, academic, and policy impact of cancer research. The authors also assigned a research level to publications to differentiate if the research was a basic science or clinical cancer study by coding the words in the title of each article or the journal in which the paper was published. The cancer research types assessed by these authors included cancer research at a national level for two different countries (UK and Russia) and research performed by cancer centres in the UK.
To assess policy impact these authors extracted journal publications from cancer clinical guidelines and for media impact they looked at publications cited in articles stored within an online repository from a well-known UK media organisation (British Broadcasting Co-operation). Interestingly, most of the cancer research publications contained in guidelines and cited in the UK media were clinical studies whereas a much higher proportion published by UK cancer centres were basic science studies. These authors also identified that funders of cancer research played an critical role as commentators to explain the importance of the research in the lay media. The top ten most frequent commentators (commenting on > 19 media articles (out of 725) were all representatives from the UK charity CRUK.
A combination of clinical trial findings and documentary analysis of large data repositories were used to estimate health system or health impact. In their study, Montague and Valentim [ 68 ] cited the effect size for a decrease in cancer recurrence from a clinical trial and implied the same health gains would be expected in real life for patients with breast cancer living in Canada. In their study of the impact of charitable and publicly funded cancer research in the UK, Glover et al. [ 67 ] used CRUK and Office for National Statistics (ONS) cancer incidence data, as well as national hospital databases listing episodes of radiotherapy delivered, number of cancer surgeries performed and systemic anti-cancer treatments prescribed, to evaluate changes in practice attributable to cancer research. In their USA perspective study, Lakdawalla et al. [ 66 ] used the population-based Surveillance, Epidemiology and End Results Program (SEER) database to evaluate the number of patients likely to be affected by the implementation of cancer research findings [ 66 ]. Survival calculations from clinical trials were also applied to population incidence estimates to predict the scale of survival gain attributable to cancer research [ 64 , 66 ].
The methods of data collection and analysis used for economic evaluations aligned with the categories of assessment identified by Buxton in their 2004 literature review [ 34 ]. For example, three studies [ 65 , 66 , 67 ] estimated direct healthcare cost savings from implementation of cancer research. This was particularly relevant in one ex-ante assessment of the potential impact of a clinical trial testing the equivalence of using less intensive follow up for patients following cancer surgery [ 65 ]. These authors assessed the number of years it would take (“years to payback”) of implementing the hypothetical clinical trial findings to outweigh the money spent developing and running the trial. The return on investment calculation was performed by estimating direct cost savings to the healthcare system by using less intensive follow up without any detriment to survival.
The second of Buxton’s categories was an estimation of productivity loss using the human capital approach. In this method, the economic value of survival gains from cancer research are calculated by measuring the monetary contribution from patients surviving longer who are of working age. This approach was used in two studies [ 64 , 66 ] and in both, estimates of average income (USA) were utilised. Buxton’s fourth category, an estimation of an individual’s willingness to pay for a statistical life, was used in two assessments [ 65 , 66 ], and Glover and colleagues [ 67 ] adapted this method, placing a monetary value on the opportunity cost of QALYs forgone in the UK health service within a fixed budget [ 70 ]. One of the studies that used this method identified that there may be differences in how patients diagnosed with distinct cancer types value the impact of research on cancer specific survival [ 66 ]. In particular, individuals with pancreatic cancer seemed to be willing to spend up to 80% of their annual income for the extra survival attributable to implementation of cancer research findings, whereas this fell to below 50% for breast and colorectal cancer. Only one of the studies considered Buxton’s third category of benefits to the economy from commercial development [ 66 ]. These authors calculated the gain to commercial companies from sales of on-patent pharmaceuticals and concluded that economic gains to commercial producers were small relative to gains from research experienced by cancer patients.
The cost estimates used in these impact evaluations came from documentary analysis, clinical trial publications, real-life data repositories, surveys, and population average income estimates. For example, in one study, cost information from NCI trials was supplemented by using a telephone phone survey to pharmacies, historical Medicare documents and estimates of the average income from the 1986 US Bureau of the Census Consumer Income [ 64 ]. In their study, Coyle et al. [ 65 ] costed annual follow up and treatment for cancer recurrence based on the Ontario Health Insurance plan, a cost model relevant to an Ottawa hospital and cost estimates from Statistics Canada [ 71 ]. The data used to calculate the cost of performing cancer research was usually from funding bodies and research institutions. For example, charity reports and Canadian research institution documents were used to estimate that it costs the National Cancer Institute in Canada $1500 per patient accrued to a clinical trial [ 65 ]. Government research investment outgoings were used to calculate that $300 billion was spent on cancer research in the USA from 1971 to 2000, 25% of which was contributed by the NCI [ 66 ] and that the NCI spent over $10 million USD in the 1980s to generate the knowledge that adjuvant chemotherapy was beneficial to colorectal cancer patients [ 64 ]. Charity and research institution spending reports, along with an estimation of the proportion of funds spent specifically on cancer research, were used to demonstrate £15 billion of UK charity and public money was spent on cancer research between 1970 and 2009 [ 67 ].
Lastly, the two studies [ 68 , 69 ] which adopted a multi-category approach to impact assessment used the highest number and broadest range of methods identified from the previous literature (Additional file 2 : Table S1). The methods utilised included surveys and semi-structured telephone interviews with clinicians, documentary analysis of funding and project reports, case studies, content analysis of media release, peer review, bibiliometrics, budget analysis, large data repository review, and observations of meetings.
Frameworks for cancer research impact evaluation
Only two of the empirical studies identified used an impact framework. Unsurprisingly, these were also the studies that performed a multi-category assessment and used the broadest range of methods within their analyses. Donovan et al. [ 69 ] used the Payback framework (Additional file 2 : Table S2) to guide the categories of impact assessed and the questions in their researcher surveys and interviews. They also reported the results of their evaluation using the same categories: from knowledge production, through capacity building to health and wider economic impacts. Montague and Valentim [ 68 ] used the Canadian Academy Health Services (CAHS) Framework (Additional file 2 : Table S2). Rather than using the framework in it is original form, they arranged impact indicators from the CAHS framework within a hierarchy to illustrate impacts occurring over time. The authors distinguished short term, intermediate and longer-term changes resulting from one clinical cancer trial, aligning with the concept of categorising impacts based on when they occur, which was described in one of the literature reviews identified in the first part of this study [ 33 ].
Lastly, the challenges of time lags and attribution of impact were identified and addressed by several of these empirical studies. Lewison and colleagues tracked the citation of over 3000 cancer publications in UK cancer clinical guidelines over time [ 61 ], and in their analysis Donovan et al. [ 69 ] explicitly acknowledged that the short time frame between their analysis and funding of the research projects under evaluations was likely to under-estimate the impact achieved. Glover et al. [ 67 ] used bibliometric analysis of citations in clinical cancer guidelines to estimate the average time from publication to clinical practice change (8 years). They added 7 years to account for the time between funding allocation and publication of research results giving an overall time lag from funding cancer research to impact of 15 years. The challenge of attribution was addressed in one study by using a time-line to describe impacts occurring at different time-points but linking back to the original research in question [ 68 ]. The difficultly of estimating time lags and attributing impact to cancer research were both specifically addressed in a companion study [ 72 ] to the one conducted by Glover and colleagues. In this study, instead of quantifying the return on cancer research investment, qualitative methods of assessment were used. This approach identified factors that enhanced and accelerated the process of impact occurring and helped to provide a narrative to link impacts to research.
This study has identified several examples of the evaluation of the impact of cancer research. These evaluations were performed over three decades, and mostly assessed research performed in high-income countries. Justification for the approach to searching the literature used in this study is given by looking at the titles of the articles identified. In only 14% (2/14) was the word “impact” included, suggesting that performing a search for empirical examples of cancer research impact evaluation using traditional publication databases would have been challenging. Furthermore, all the studies identified were included within reviews of approaches to research impact evaluation, which negated the subjective decision of whether the studies complied with a particular definition of research impact.
Characteristics of research that were specifically relevant to cancer studies can be identified from these impact assessments. Firstly, many of these evaluations acknowledged the contribution of both basic and applied studies to the body of cancer research, and several studies categorised research publications based on this distinction. Second, the strong focus on health impact and the expectation that cancer research will improve health was not surprising. The focus on survival in particular, especially in economic studies looking at the value of health gains, reflects the high mortality of cancer as a disease entity. This contrasts with similar evaluations of musculoskeletal or mental health research, which have focused on improvements in morbidity [ 73 , 74 ]. Third, several studies highlighted the distinction between research looking at different aspects of the cancer care continuum; from screening, prevention and diagnosis, to treatment and end of life care. The division of cancer as a disease entity by the site of disease was also recognised. Studies that analysed the number of patients diagnosed with cancer, or population-level survival gains, often used site-specific cancer incidence and other studies evaluated research relating to only one type of cancer [ 64 , 65 , 68 , 69 ]. Lastly, the empirical examples of cancer research impact identified in this study confirm the huge investment into cancer research that exists, and the desire by many research organisations and funders to quantify a rate of return on that investment. Most of these studies concluded that cancer research investment far exceeded expectations of the return on investment. Even using the simple measure of future research grants attracted by researchers funded by one cancer charity, the monetary value of these grants outweighed the initial investment [ 69 ].
There were limitations in the approaches to impact evaluation used in these studies which were recognised by reflecting on the findings from the broader literature. Several studies assessed academic impact in isolation, and studies using the journal impact factor or the location of authors on publications were limited in the information they provided. In particular, using the journal impact factor (JIF) to allocate funding research which was used in one study, is now outdated and controversial. The policy impact of cancer research was commonly evaluated by using clinical practice guidelines, but other policy types that could be used in impact assessment [ 30 ], such as national government reports or local guidelines, were rarely used. In addition, using cancer guidelines as a surrogate for clinical practice change and health service impact could have drawbacks. For example, guidelines can often be outdated, irrelevant or simply not used by cancer clinicians and in addition, local hospitals often have their own local clinical guidelines, which may take precedent over national documents. Furthermore, the other aspects of policy impact described in the broader literature [ 30 ], such as impact on policy agenda setting and implementation, were rarely assessed. There were also no specific examples of social, environmental or cultural impacts and very few of the studies mentioned wider economic benefits from cancer research, such as spin out companies and patents. It may be that these types of impact were less relevant to cancer research being assessed, however unexpected impacts may have be identified if they were considered at the time of impact evaluation.
Reflecting on how the methods of data collection and analysis used in these studies aligned with those listed in Additional file 2 : Table S1 bibliometrics, alternative metrics (media citation), documentary analysis, surveys and economic approaches were often used. Methods less commonly adopted were interviews, using a scale and focus groups. This may have been due to the time and resource implications of using qualitative techniques and more in depth analysis, or a lack of awareness by authors regarding the types of scales that could be used. An example of a scale that could be used to assess the impact of research on policy is provided in one of the literature reviews identified [ 30 ]. The method of collecting expert testimony from researchers was utilised in the studies identified, but there were no obvious examples of testimony about the impact of cancer research from stakeholders such as cancer patients or their families.
Lastly, despite the large number of examples identified from the previous literature, a minority of the empirical assessments used an impact framework. The Payback Framework, and an iteration of the CAHS Framework were used with success and these studies are excellent examples of how frameworks can be used for cancer research impact evaluation in future. Other frameworks identified from the literature (Additional file 2 : Table S2) that may be appropriate for the assessment of cancer research impact in future include Anthony Weiss’s logic model [ 75 ], the research impact framework [ 76 ] and the research utilisation ladder [ 77 ]. Weiss’s model is specific to medical research and encourages evaluation of how clinical trial publication results are implemented in practice and lead to health gain. He describes an efficacy-efficiency gap [ 75 ] between clinical decision makers becoming aware of research findings, changing their practice and this having impact on health. The Research Impact Framework, developed by the Department of Public Health and Policy at the UK London School of Hygiene and Tropical Medicine [ 76 ], is an aid for researchers to self-evaluate their research impact, and offers an extensive list of categories and indicators of research which could be applied to evaluating the impact of cancer research. Finally, Landry’s Research Utilisation Ladder [ 77 ] has similarities to the hierarchy used in the empirical study by Montegue and Valentim [ 68 ], and focuses on the role of the individual researcher in determining how research is utilised and its subsequent impact.
Reflecting on the strengths and limitations of the empirical approaches to cancer research impact identified in this study, Fig. 3 outlines recommendations for the future. One of these recommendations refers to improving the use of real-life data to assess the actual impact of research on incidence, treatment, and outcomes, rather than predicting these impacts by using clinical trial results. Databases for cancer incidence, such as SEER (USA) and the Office of National Statistics (UK), are relatively well established. However, those that collect data on treatments delivered and patient outcomes are less so, and when they do exist, they have been difficult to establish and maintain and often have large quantities of missing data [ 78 , 79 ]. In their study, Glover et al. [ 67 ] specifically identified the lack of good quality data documenting radiotherapy use in the UK in 2012.
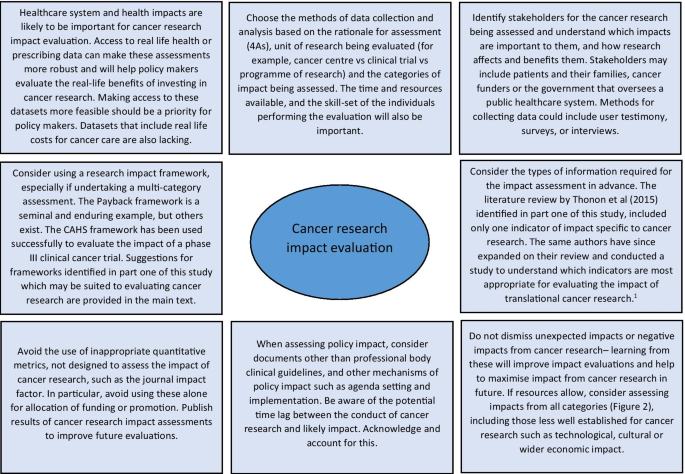
1 Thonon F, Boulkedid R, Teixeira M, Gottot S, Saghatchian M, Alberti C. Identifying potential indicators to measure the outcome of translational cancer research: a mixed methods approach. Health Res Policy Syst. 2015;13:72
Suggestions for approaching cancer research impact evaluation.
The recommendations also suggest that impact assessment for cancer and other health research could be made more robust by giving researchers access to cost data linked to administrative datasets. This type of data was used in empirical impact assessments performed in the USA [ 64 , 66 ] because the existing Medicare and Medicaid health service infrastructure collects and provides access to this data. In the UK, hospital cost data is collected for accounting purposes but this could be unlocked as a resource for research impact assessments going forward. A good example of where attempts are being made to link resource use to cost data for cancer care in the UK is through the UK Colorectal Cancer Intelligence Hub [ 80 ].
Lastly, several empirical examples highlighted that impact from cancer research can be increased when researchers or research organisations advocate, publicise and help to interpret research findings for a wider audience [ 60 , 72 ]. In addition, it is clear from these studies that organisations that want to evaluate the impact of their cancer research must also appreciate that research impact evaluation is a multi-disciplinary effort, requiring the skills and input from individuals with different skill sets, such as basic scientists, clinicians, social scientists, health economists, statisticians, and information technology analysts. Furthermore, the users and benefactors from cancer research, such as patients and their families, should not be forgotten, and asking them which impacts from cancer research are important will help direct and improve future evaluations.
The strengths of this study are the broad, yet systematic approach used to identify existing reviews within the research impact literature. This allowed a more informed assessment of cancer research evaluations than would have been possible if a primary review of these empirical examples had been undertaken. Limitations of the study include the fact that the review protocol was not registered in advance and that one researcher screened the full articles for review. The later was partly mitigated by using pre-defined inclusion criteria.
Impact assessment is a way of communicating to funders and patients the merits of undertaking cancer research and learning from previous research to develop better studies that will have positive impacts on society in the future. To the best of our knowledge, this is the first review to consider how to approach evaluation of the impact of cancer research. At the policy level, a lesson learned from this study for institutions, governments, and funders of cancer research, is that an exact prescription for how to conduct cancer research impact evaluation cannot be provided, but a multi-disciplinary approach and sufficient resources are required if a meaningful assessment can be achieved. The approach to impact evaluation used to assess cancer research will depend on the type of research being assessed, the unit of analysis, rationale for the assessment and the resources available. This study has added to an important dialogue for cancer researchers, funders and patients about how cancer research can be evaluated and ultimately how future cancer research impact can be improved.
Availability of data and materials
Additional files included. No primary research data analysed.
Abbreviations
National Cancer Institute
United States of America
United States
United Kingdom
Cancer Research UK
Medical subject heading
Preferred reporting items for systematic reviews and meta-analysis
Gross Domestic Product
American Society of Clinical Oncology
Surveillance, Epidemiology and End Results Program
Journal impact factor
Research evaluation framework
Health Technology Assessment
Doctor of Philosophy
Research and Development
Quality adjusted life year
Canadian Academy of Health Sciences
Office for National Statistics
UK CR. CRUK: "Current clinical trial research". https://www.cancerresearchuk.org/our-research-by-cancer-topic/our-clinical-trial-research/current-clinical-trial-research . Accessed 20th May 2019.
UK CR. CRUK: "Cancer statistics for the UK.". https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk . Accessed 20th May 2019.
Cancer Research UK. Cancer risk statistics 2019. https://www.cancerresearchuk.org/health-professional/cancer-statistics/risk . Accessed 20th Dec 2019.
Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gulmezoglu AM, et al. How to increase value and reduce waste when research priorities are set. Lancet. 2014;383(9912):156–65.
Article PubMed Google Scholar
Ioannidis JPA, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383(9912):166–75.
Article PubMed PubMed Central Google Scholar
Richard S. BMJ blogs. In: Godlee F, editor. 2018. https://blogs.bmj.com/bmj/2018/07/30/richard-smith-measuring-research-impact-rage-hard-get-right/ . Accessed 31st July 2019.
Department for the Economy, Higher Education Funding Council for Wales, Research England, Scottish Funding Council. Draft guidance on submissions REF 2018/01 2018 July 2018. Report No.
Gunn A, Mintrom M. Social science space network blogger. 2017. https://www.socialsciencespace.com/2017/01/five-considerations-policy-measure-research-impact/ . Accessed 2019.
Callaway E. Beat it, impact factor! Publishing elite turns against controversial metric. Nature. 2016;535(7611):210–1.
Article CAS PubMed Google Scholar
Research Councils UK. Excellence with impact 2018. https://webarchive.nationalarchives.gov.uk/20180322123208/http://www.rcuk.ac.uk/innovation/impact/ . Accessed 24th Aug 2020.
Cancer Research UK. Measuring the impact of research 2017. https://www.cancerresearchuk.org/funding-for-researchers/research-features/2017-06-20-measuring-the-impact-of-research . Accessed 24th Aug 2020.
Gordon LG, Bartley N. Views from senior Australian cancer researchers on evaluating the impact of their research: results from a brief survey. Health Res Policy Syst. 2016;14:2.
Article CAS PubMed PubMed Central Google Scholar
Thompson MK, Poortmans P, Chalmers AJ, Faivre-Finn C, Hall E, Huddart RA, et al. Practice-changing radiation therapy trials for the treatment of cancer: where are we 150 years after the birth of Marie Curie? Br J Cancer. 2018;119(4):389–407.
Downing A, Morris EJ, Aravani A, Finan PJ, Lawton S, Thomas JD, et al. The effect of the UK coordinating centre for cancer research anal cancer trial (ACT1) on population-based treatment and survival for squamous cell cancer of the anus. Clin Oncol. 2015;27(12):708–12.
Article CAS Google Scholar
Tsang Y, Ciurlionis L, Kirby AM, Locke I, Venables K, Yarnold JR, et al. Clinical impact of IMPORT HIGH trial (CRUK/06/003) on breast radiotherapy practices in the United Kingdom. Br J Radiol. 2015;88(1056):20150453.
South A, Parulekar WR, Sydes MR, Chen BE, Parmar MK, Clarke N, et al. Estimating the impact of randomised control trial results on clinical practice: results from a survey and modelling study of androgen deprivation therapy plus radiotherapy for locally advanced prostate cancer. Eur Urol Focus. 2016;2(3):276–83.
Horsley T DO, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database Syst Rev. 2011(8).
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open medicine : a peer-reviewed, independent, open-access journal. 2009;3(3):e123–30.
Google Scholar
Raftery JH, Greenhalgh S, Glover T, Blatch-Jones MA. Models and applications for measuring the impact of health research: update of a systematic review for the Health Technology Assessment programme. Health Technol Assess. 2016;20(76):1–254.
Hanney SB, Buxton M, Green C, Coulson D, Raftery J. An assessment of the impact of the NHS Health Technology Assessment Programme. Health Technol Assess. 2007;11(53):82.
Article Google Scholar
Greenhalgh T, Raftery J, Hanney S, Glover M. Research impact: a narrative review. BMC Med. 2016;14:78.
Coryn CLS, Hattie JA, Scriven M, Hartmann DJ. Models and mechanisms for evaluating government-funded research—an international comparison. Am J Eval. 2007;28(4):437–57.
Philipp-Bastian Brutscher SW, Grant J. Health research evaluation frameworks: an international comparison. RAND Europe. 2008.
Guthrie S, Wamae W, Diepeveen S, Wooding S, Grant J. Measuring Research. A guide to research evaluation frameworks and tools. RAND Europe Cooperation 2013.
Health Economics Research Group OoHE, RAND Europe. Medical research: what’s it worth? Estimating the economic benefits from medical research in the UK. London: UK Evaluation Forum; 2008.
Marjanovic SHS, Wooding Steven. A historical reflection on research evaluation studies, their recurrent themes and challenges. Technical report. 2009.
Chikoore L. Perceptions, motivations and behaviours towards 'research impact': a cross-disciplinary perspective. University of Loughborough; 2016.
Pollitt A, Potoglou D, Patil S, Burge P, Guthrie S, King S, et al. Understanding the relative valuation of research impact: a best-worst scaling experiment of the general public and biomedical and health researchers. BMJ Open. 2016;6(8).
Wouters P, Thelwall M, Kousha K, Waltman L, de Rijcke S, Rushforth A, Franssen T. The metric tide literature review (Supplementary report I to the independent review of the role of metrics in research assessment and management). 2015.
Hanney SR, Gonzalez-Block MA, Buxton MJ, Kogan M. The utilisation of health research in policy-making: concepts, examples and methods of assessment. Health Res Policy Syst. 2003;1(1):2.
Boaz A, Fitzpatrick S, Shaw B. Assessing the impact of research on policy: a literature review. Sci Public Policy. 2009;36(4):255–70.
Newson R, King L, Rychetnik L, Milat A, Bauman A. Looking both ways: a review of methods for assessing research impacts on policy and the policy utilisation of research. Health Res Policy Syst. 2018;16(1):54.
Cruz Rivera S, Kyte DG, Aiyegbusi OL, Keeley TJ, Calvert MJ. Assessing the impact of healthcare research: a systematic review of methodological frameworks. PLoS Med. 2017;14(8):e1002370.
Buxton M, Hanney S, Jones T. Estimating the economic value to societies of the impact of health research: a critical review. Bull World Health Organ. 2004;82(10):733–9.
PubMed PubMed Central Google Scholar
Yazdizadeh B, Majdzadeh R, Salmasian H. Systematic review of methods for evaluating healthcare research economic impact. Health Res Policy Syst. 2010;8:6.
Hanney S. Ways of assessing the economic value or impact of research: is it a step too far for nursing research? J Res Nurs. 2011;16(2):151–66.
Banzi R, Moja L, Pistotti V, Facchini A, Liberati A. Conceptual frameworks and empirical approaches used to assess the impact of health research: an overview of reviews. Health Res Policy Syst. 2011;9:26.
Bornmann L. What is societal impact of research and how can it be assessed? A literature survey. J Am Soc Inform Sci Technol. 2013;64(2):217–33.
Pedrini M, Langella V, Battaglia MA, Zaratin P. Assessing the health research’s social impact: a systematic review. Scientometrics. 2018;114(3):1227–50.
Thonon F, Boulkedid R, Delory T, Rousseau S, Saghatchian M, van Harten W, et al. Measuring the outcome of biomedical research: a systematic literature review. PLoS ONE. 2015;10(4):e0122239.
Article PubMed PubMed Central CAS Google Scholar
Raftery J, Hanley S, Greenhalgh T, Glover M, Blatch-Jones A. Models and applications for measuring the impact of health research: Update of a systematic review for the Health Technology Assessment Programme. Health Technol Assess. 2016;20(76).
Ruscio J, Seaman F, D'Oriano C, Stremlo E, Mahalchik K. Measuring scholarly impact using modern citation-based indices. Meas Interdiscip Res Perspect. 2012;10(3):123–46; 24.
Carpenter CR, Cone DC, Sarli CC. Using publication metrics to highlight academic productivity and research impact. Acad Emerg Med. 2014;21(10):1160–72.
Patel VM, Ashrafian H, Ahmed K, Arora S, Jiwan S, Nicholson JK, et al. How has healthcare research performance been assessed? A systematic review. J R Soc Med. 2011;104(6):251–61.
Smith KM, Crookes E, Crookes PA. Measuring research "Impact" for academic promotion: issues from the literature. J High Educ Policy Manag. 2013;35(4):410–20; 11.
Penfield T, Baker M, Scoble R, Wykes M. Assessment, evaluations and definitions of research impact: a review. Res Eval. 2014;23(1):21–32.
Agarwal A, Durairajanayagam D, Tatagari S, Esteves SC, Harlev A, Henkel R, et al. Bibliometrics: tracking research impact by selecting the appropriate metrics. Asian J Androl. 2016;18(2):296–309.
Braithwaite J, Herkes J, Churruca K, Long J, Pomare C, Boyling C, et al. Comprehensive researcher achievement model (CRAM): a framework for measuring researcher achievement, impact and influence derived from a systematic literature review of metrics and models. BMJ Open. 2019;9(3):e025320.
Moed HF, Halevi G. Multidimensional assessment of scholarly research impact. J Assoc Inf Sci Technol. 2015;66(10):1988–2002.
Milat AJ, Bauman AE, Redman S. A narrative review of research impact assessment models and methods. Health Res Policy Syst. 2015;13:18.
Weißhuhn P, Helming K, Ferretti J. Research impact assessment in agriculture—a review of approaches and impact areas. Res Eval. 2018;27(1):36-42; 7.
Deeming S, Searles A, Reeves P, Nilsson M. Measuring research impact in Australia’s medical research institutes: a scoping literature review of the objectives for and an assessment of the capabilities of research impact assessment frameworks. Health Res Policy Syst. 2017;15(1):22.
Peter N, Kothari A, Masood S. Identifying and understanding research impact: a review for occupational scientists. J Occup Sci. 2017;24(3):377–92.
Buxton M, Hanney S. How can payback from health services research be assessed? J Health Serv Res Policy. 1996;1(1):35–43.
Bornmann L. Measuring impact in research evaluations: a thorough discussion of methods for, effects of and problems with impact measurements. High Educ Int J High Educ Res. 2017;73(5):775–87; 13.
Reale E, Avramov D, Canhial K, Donovan C, Flecha R, Holm P, et al. A review of literature on evaluating the scientific, social and political impact of social sciences and humanities research. Res Eval. 2017;27(4):298–308.
Ugolini D, Bogliolo A, Parodi S, Casilli C, Santi L. Assessing research productivity in an oncology research institute: the role of the documentation center. Bull Med Libr Assoc. 1997;85(1):33–8.
CAS PubMed PubMed Central Google Scholar
Ugolini D, Casilli C, Mela GS. Assessing oncological productivity: is one method sufficient? Eur J Cancer. 2002;38(8):1121–5.
Saad ED, Mangabeira A, Masson AL, Prisco FE. The geography of clinical cancer research: analysis of abstracts presented at the American Society of Clinical Oncology Annual Meetings. Ann Oncol. 2010;21(3):627–32.
Lewison G, Tootell S, Roe P, Sullivan R. How do the media report cancer research? A study of the UK’s BBC website. Br J Cancer. 2008;99(4):569–76.
Lewison GS, Sullivan R. The impact of cancer research: how publications influence UK cancer clinical guidelines. Br J Cancer. 2008;98(12):1944–50.
Lewison G, Markusova V. The evaluation of Russian cancer research. Res Eval. 2010;19(2):129–44.
Sullivan R, Lewison G, Purushotham AD. An analysis of research activity in major UK cancer centres. Eur J Cancer. 2011;47(4):536–44.
Brown ML, Nayfield SG, Shibley LM. Adjuvant therapy for stage III colon cancer: economics returns to research and cost-effectiveness of treatment. J Natl Cancer Inst. 1994;86(6):424–30.
Coyle D, Grunfeld E, Wells G. The assessment of the economic return from controlled clinical trials. A framework applied to clinical trials of colorectal cancer follow-up. Eur J Health Econ. 2003;4(1):6–11.
Lakdawalla DN, Sun EC, Jena AB, Reyes CM, Goldman DP, Philipson TJ. An economic evaluation of the war on cancer. J Health Econ. 2010;29(3):333–46.
Glover M, Buxton M, Guthrie S, Hanney S, Pollitt A, Grant J. Estimating the returns to UK publicly funded cancer-related research in terms of the net value of improved health outcomes. BMC Med. 2014;12:99.
Montague S, Valentim R. Evaluation of RT&D: from ‘prescriptions for justifying’ to ‘user-oriented guidance for learning.’ Res Eval. 2010;19(4):251–61.
Donovan C, Butler L, Butt AJ, Jones TH, Hanney SR. Evaluation of the impact of National Breast Cancer Foundation-funded research. Med J Aust. 2014;200(4):214–8.
Excellence NIfHaC. Guide to the methods of technology appraisal: 2013. 2013.
Government of Canada. Statistics Canada 2020. https://www.statcan.gc.ca/eng/start . Accessed 31st Aug 2020.
Guthrie S, Pollitt A, Hanney S, Grant J. Investigating time lags and attribution in the translation of cancer research: a case study approach. Rand Health Q. 2014;4(2):16.
Glover M, Montague E, Pollitt A, Guthrie S, Hanney S, Buxton M, et al. Estimating the returns to United Kingdom publicly funded musculoskeletal disease research in terms of net value of improved health outcomes. Health Res Policy Syst. 2018;16(1):1.
Wooding S, Pollitt A, Castle-Clarke S, Cochrane G, Diepeveen S, Guthrie S, et al. Mental health retrosight: understanding the returns from research (lessons from schizophrenia): policy report. Rand Health Q. 2014;4(1):8.
Weiss AP. Measuring the impact of medical research: moving from outputs to outcomes. Am J Psychiatry. 2007;164(2):206–14.
Kuruvilla S, Mays N, Pleasant A, Walt G. Describing the impact of health research: a research impact framework. BMC Health Serv Res. 2006;6:134.
Landry RA, Lamari NM. Climbing the ladder of research utilization. Evidence from social science research. Sci Commun. 2001;22(4):396–422.
Eisemann N, Waldmann A, Katalinic A. Imputation of missing values of tumour stage in population-based cancer registration. BMC Med Res Methodol. 2011;11(1):129.
Morris EJ, Taylor EF, Thomas JD, Quirke P, Finan PJ, Coleman MP, et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut. 2011;60(6):806–13.
University of Leeds. CORECT-R 2020. https://bci.leeds.ac.uk/applications/ . Accessed 24th Aug 2020.
Download references
Acknowledgements
We would like to acknowledge the help of Ms Lorraine MacLeod, specialist librarian from the Beatson West of Scotland Cancer Network in NHS Greater Glasgow and Clyde for her assistance in formulating the search strategy. We would like to acknowledge that Professor Stephen Hanney provided feedback on an earlier version of this review.
Dr. Catherine Hanna has a CRUK and University of Glasgow grant. Grant ID: C61974/A2429.
Author information
Authors and affiliations.
CRUK Clinical Trials Unit, Institute of Cancer Sciences, University of Glasgow, Glasgow, United Kingdom
Catherine R. Hanna & Robert J. Jones
Health Economics and Health Technology Assessment, Institute of Health and Wellbeing, University of Glasgow, Glasgow, United Kingdom
Kathleen A. Boyd
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the concept and design of the study. CH was responsible for the main data analysis and writing of the manuscript. KAB and RJJ responsible for writing, editing and final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Catherine R. Hanna .
Ethics declarations
Ethics approval and consent to participate.
No ethical approval required.
Consent for publication
No patient level or third party copyright material used.
Competing interests
Nil declared.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Research Council UK Impact definition, summary of search terms for part one, and inclusion criteria for both parts of the study.
Additional file 2: Table S1
(List of methods for research impact evaluation) and Table S2 (List if frameworks for research impact evaluation).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hanna, C.R., Boyd, K.A. & Jones, R.J. Evaluating cancer research impact: lessons and examples from existing reviews on approaches to research impact assessment. Health Res Policy Sys 19 , 36 (2021). https://doi.org/10.1186/s12961-020-00658-x
Download citation
Received : 05 March 2020
Accepted : 09 November 2020
Published : 11 March 2021
DOI : https://doi.org/10.1186/s12961-020-00658-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Health Research Policy and Systems
ISSN: 1478-4505
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Doctor is struck off...
Doctor is struck off for trying to sell unproven treatments to patient with advanced cancer
- Related content
- Peer review
A doctor in private practice who tried to sell unproven treatments to a patient with advanced cancer has been struck off the UK medical register after the patient’s daughter, herself a doctor, complained to the General Medical Council (GMC).
The conduct of Julian Kenyon, who was medical director at the Dove Clinic for Integrated Medicine in Hampshire, was “wholly unacceptable, morally culpable and disgraceful,” said Aaminah Khan, chairing the medical practitioners tribunal.
Kenyon, who offered various alternative medicine treatments, was consulted in May 2022 by a man named only as Patient A, who had had stage IV metastatic prostate cancer diagnosed in December 2019. He had already received androgen deprivation therapy, chemotherapy, and radiotherapy and had recently been started on enzalutamide, a relatively new hormone treatment.
Patient A consulted Kenyon about supplementing this with ozone therapy, but Kenyon instead offered him a treatment plan consisting of cannabidiol, Claricell, …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £33 / $40 / €36 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article

IMAGES
COMMENTS
Peer review forms the basis of funding decisions at CRUK. When you apply to us for research funding, your application will be taken through a thorough peer review assessment process. Depending on the funding you've applied for, this can include assessment by an expert review panel or an interview. Final funding decisions are made by our ...
Find out more about our peer review process. Peer Review Week is a community-led yearly global event celebrating the essential role that peer review plays in maintaining scientific quality. The event brings together individuals, institutions, and organisations committed to sharing the central message that good peer review, whatever shape or ...
Cancer Research UK puts out somewhere between 3,000 and 5,000 written peer review requests a year, and we're not the only people asking for peer reviews. If you look at the growth in scientific publishing in the last 10 years, that's leading to more peer review requests - the system's exhausted!
By agreeing to peer review an application for Cancer Research UK you agree to declare any conflicts of interest to Cancer Research UK and to treat the information contained within the application as confidential. If you are unsure whether or not you have a conflict of interest in relation to the application, please contact us. Cancer Research UK agrees that it will not disclose your name to ...
Purpose This policy sets out Cancer Research UK's (CRUK) position on how researcher communities and Host Institutions that receive CRUK funding are expected to maintain good research conduct and. ... Peer review is a primary control route for maintaining good research practice. Regular meetings should be held to allow peers and group leaders to ...
Background In July 2020 Cancer Research UK undertook a rapid review of the studies in its clinical research portfolio to assess the impact of the Covid-19 pandemic. The review examined over 160 research studies funded by the charity, and in keeping with its usual practice, the charity involved patient/public contributors in the review process. Main body Cancer Research UK (CRUK) spends over £ ...
This policy sets out Cancer Research UK's position on open access publications and how researchers and Host Institutions that receive CRUK funding are expected to provide open and unrestricted access to published research articles. ... We believe that the peer review process adds significant value to research articles. Researchers must make ...
UKRI will undertake a review of how they use expert peer review - CRUK has recently changed the way we peer review to improve the way we make funding decisions and reduce the burden of written peer review on ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey ...
Expert review panels don't represent a shift away from independent peer review - they're just a different way of doing it, and we think having in-person discussions is generally a more valuable way of reviewing research applications than asking for written reviews. ... Cancer Research UK is a registered charity in England and Wales ...
As we mark peer review week - it's good to take time to reflect on how pivotal the process is in maintaining the quality of research. This year's theme, Research Integrity: creating and supporting trust in research, is particularly important for Cancer Research UK.Last year we deepened our commitment to good research practice by becoming a signatory of the Concordat to Support Research ...
Primary, peer-reviewed literature: from searches on ... NHS Breast Screening Programme Annual Review NHS Cancer Screening Programmes, 2010 ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee.
Registered Reports are reviewed in two stages. At stage 1, the study protocol is reviewed - roughly, the introduction and methods of what will eventually become the full article - before any data have been collected. The focus is on whether the research question is important and the methodology robust. If the article passes stage 1 review ...
The BJC is published in association with Cancer Research UK, ... 2023 Peer Review Metrics. Submission to first editorial decision: 12 days Submission to Accept: 188 days.
We have prepared some key insights which reports latest data across the UK (and where possible for the devolved nations) on screening, urgent suspected referrals, diagnostic tests and treatment. Cancer Intelligence Team, Cancer Research UK. Performance measures across the cancer pathway: Key Stats Updated January 2024.
Online Publication of the article is relying on the author's response to the Galley Corrections. Online ISSN : 2631-5297. Discover cutting-edge cancer research, diagnosis, genetics, predictive markers, prevention, chemotherapy, radiation therapy, immunotherapy, and next-gen therapeutics in the British Journal of Cancer Research.
This means that other experts (peers) have assessed the quality of the research and have checked the results. It's also important to know who funded the study, as this can affect how the findings are presented. For example, Cancer Research UK disregards research funded by the tobacco industry.
Objective To examine and interpret trends in UK cancer incidence and mortality for all cancers combined and for the most common cancer sites in adults aged 35-69 years. Design Retrospective secondary data analysis. Data sources Cancer registration data, cancer mortality and national population data from the Office for National Statistics, Public Health Wales, Public Health Scotland, Northern ...
Peer review; Preprints; Clinical. Specialty collections; The Lancet Clinic ... We defined SPC sites using the ICD-10 code groups employed by Cancer Research UK. 11. Cancer Research UK ... Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: a systematic review and meta ...
The COVID-19 pandemic necessitated remote cancer care delivery via the internet and telephone, rapidly accelerating an already growing care delivery model and associated research. This scoping review of reviews characterised the peer-reviewed literature reviews on digital health and telehealth interventions in cancer published from database inception up to May 1, 2022, from PubMed, Cumulated ...
Ovarian cancer: epidemiology and therapeutic management. Ovarian cancers are responsible for over 207.000 deaths worldwide in 2022, and in 80% of epithelial ovarian carcinoma cases the diagnosis is made at an advanced stage (FIGO III/IV), making it the first cause of death from gynecological malignancies [1, 2].Optimal surgery and platinum-based chemotherapy are the basis of the treatment of ...
Since 1980, the UK and USA have seen substantial growth in their respective cancer research outputs as assessed by publication volume (figure 1A).In the past decade (2011-20), the UK's cancer research output (peer-reviewed publications) of 63 722 papers represented 9·4% of the total UK biomedical research output, and 5·7% of the world's total.
The UK's National Cancer Research Institute (NCRI) announced unexpectedly in June 2023 that it will be permanently "winding down" activities because of ongoing uncertainties in the wider economic and research environment. 1 2 The failure of the UK's public research funders to ensure the sustainability and continuity of the NCRI has drawn widespread condemnation from patients, doctors ...
The Biology of Cancer. Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and ...
Depression affects up to 20%, and anxiety 10%, of patients with cancer, compared with figures of 5% and 7% for past-year prevalence in the general population. Poor recognition of depression and anxiety is associated with reduced quality of life and survival. Some cancers, such as pancreatic and lung, can release chemicals that are thought to ...
By agreeing to peer review an application for Kidney Cancer UK you must agree to declare any conflicts of interest to Kidney Cancer UK and must treat the information contained within any application reviewed as confidential. ... Independent chair of research grant award and peer review committee Consultant Medical Oncologist. Dr. Paul Nathan MB ...
Performing cancer research relies on substantial financial investment, and contributions in time and effort from patients. It is therefore important that this research has real life impacts which are properly evaluated. The optimal approach to cancer research impact evaluation is not clear. The aim of this study was to undertake a systematic review of review articles that describe approaches ...
Peer review; Preprints; Clinical. Specialty collections; The Lancet Clinic; Commissions; Series; Picture Quiz; Global health. Global health Global health overview; ... This work was supported by Cancer Research UK C33493/A29678. Funding IIG_FULL_2020_033 was obtained from World Cancer Research Fund (WCRF UK), as part of the World Cancer ...
Grounds for optimism but warning signs must not be ignored Cancer is a major public health problem in the UK, and in most high income countries. The disease is the leading cause of death in both men and women, and one in four people die prematurely from it at ages 30-69 years.1 A comprehensive assessment of the evolution of cancer incidence and mortality rates over time is not straightforward ...
A doctor in private practice who tried to sell unproven treatments to a patient with advanced cancer has been struck off the UK medical register after the patient's daughter, herself a doctor, complained to the General Medical Council (GMC). The conduct of Julian Kenyon, who was medical director at the Dove Clinic for Integrated Medicine in Hampshire, was "wholly unacceptable, morally ...
The UK's National Health Service (NHS)—in partnership with biotechnology company BioNTech SE and hospitals across the country—is to grant thousands of patients with cancer in England fast-track access to trials of personalised cancer vaccines. Ahead of the American Society of Clinical Oncology's annual conference in Chicago (ASCO, May 31 ...