A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Guidelines and Guidance Library
- Core Practices
- Isolation Precautions Guideline
- Disinfection and Sterilization Guideline
- Environmental Infection Control Guidelines
- Hand Hygiene Guidelines
- Multidrug-resistant Organisms (MDRO) Management Guidelines
- Catheter-Associated Urinary Tract Infections (CAUTI) Prevention Guideline
- Tools and resources
- Evaluating Environmental Cleaning
- Show All Home

CDC's Core Infection Prevention and Control Practices for Safe Healthcare Delivery in All Settings
At a glance.
Core Infection Prevention and Control Practices for Healthcare
Introduction
Adherence to infection prevention and control practices is essential to providing safe and high quality patient care across all settings where healthcare is delivered
This document concisely describes a core set of infection prevention and control practices that are required in all healthcare settings, regardless of the type of healthcare provided. The practices were selected from among existing CDC recommendations and are the subset that represent fundamental standards of care that are not expected to change based on emerging evidence or to be regularly altered by changes in technology or practices, and are applicable across the continuum of healthcare settings. The practices outlined in this document are intended to serve as a standard reference and reduce the need to repeatedly evaluate practices that are considered basic and accepted as standards of medical care. Readers should consult the full texts of CDC healthcare infection control guidelines for background, rationale, and related infection prevention recommendations for more comprehensive information.
The core practices in this document should be implemented in all settings where healthcare is delivered. These venues include both inpatient settings (e.g., acute, long-term care) and outpatient settings (e.g., clinics, urgent care, ambulatory surgical centers, imaging centers, dialysis centers, physical therapy and rehabilitation centers, alternative medicine clinics). In addition, these practices apply to healthcare delivered in settings other than traditional healthcare facilities, such as homes, assisted living communities, pharmacies, and health fairs.
Healthcare personnel (HCP) referred to in this document include all paid and unpaid persons serving in healthcare settings who have the potential for direct or indirect exposure to patients or infectious materials, including body substances, contaminated medical supplies, devices, and equipment; contaminated environmental surfaces; or contaminated air.
CDC healthcare infection control guidelines 1-17 were reviewed, and recommendations included in more than one guideline were grouped into core infection prevention practice domains (e.g., education and training of HCP on infection prevention, injection and medication safety). Additional CDC materials aimed at providing general infection prevention guidance outside of the acute care setting 18-20 were also reviewed. HICPAC formed a workgroup led by HICPAC members and including representatives of professional organizations (see Contributors in archives for full list). The workgroup reviewed and discussed all of the practices, further refined the selection and description of the core practices and presented drafts to HICPAC at public meeting and recommendations were approved by the full Committee in July 2014. In October 2022, the Core Practices were reviewed and updated by subject matter experts within the Division of Healthcare Quality Promotion at CDC. The addition of new practices followed the same methodology employed by the Core Practices Workgroup but also included review of pathogen-specific guidance documents 21-22 that were created or updated after July 2014. These additions were presented to HICPAC at the November 3, 2022 meeting. Future updates to the Core Practices will be guided by the publication of new or updated CDC infection prevention and control guidelines.
Core Practices Table
Infection control.
CDC provides information on infection control and clinical safety to help reduce the risk of infections among healthcare workers, patients, and visitors.
For Everyone
Health care providers, public health.

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Introduction to Infection Prevention and Control Practices
Infection prevention and control refers to practices that can prevent or reduce the risk of transmission of microorganisms. Evidence-based best practices for infection prevention and control provide guidelines to healthcare providers to ensure safe, quality care is provided to clients, visitors, healthcare providers, and the healthcare environment.
When infection prevention and control practices are used consistently, the transfer of healthcare-associated infections (HAIs) can be prevented in healthcare settings. HAIs are infections that occur when a person is infected with a pathogen during their care in a healthcare setting. A survey conducted by the Canadian Nosocomial Infection Surveillance Program (2020) found that participating Canadian hospitals estimated that 7.9% of clients had at least one HAI. Hand hygiene is considered the most important and effective measure to prevent HAIs. HAIs will be discussed further in Chapter 3 .
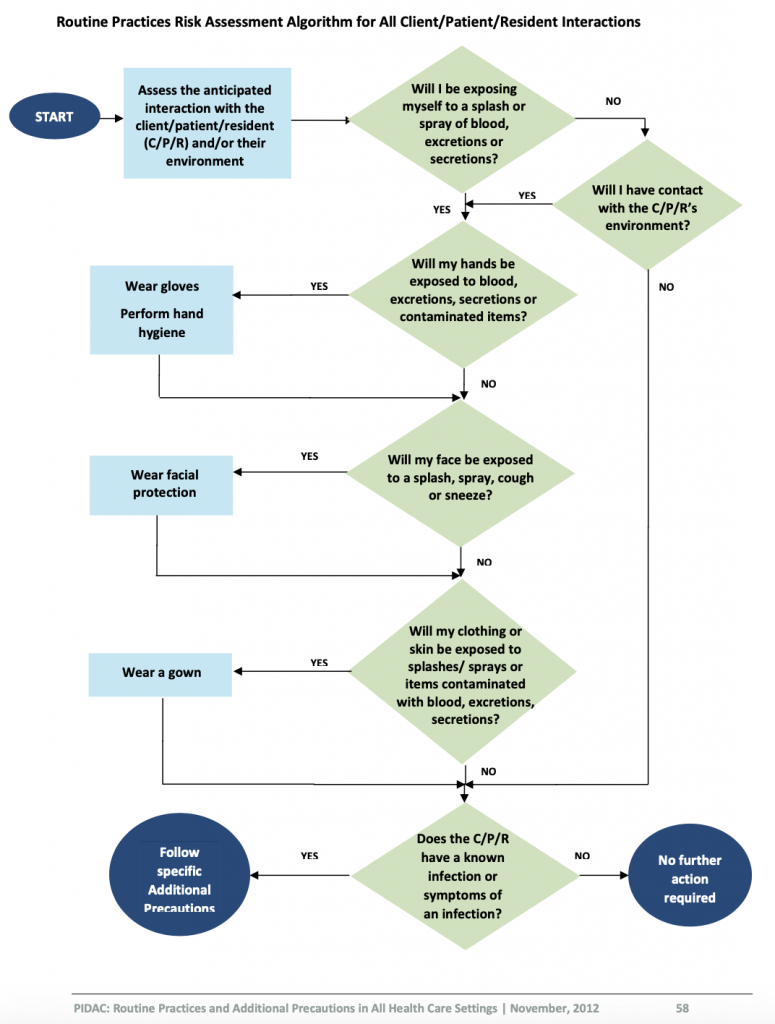
Prior to providing care, healthcare providers must perform a point-of-care risk assessment of the environment before every interaction with clients to ensure safe care and determine the potential risk for exposure to infections. Risks include exposure to blood, body fluids, mucous membranes, non-intact skin, contaminated surfaces or soiled items, and even airborne particles. Once you have completed a risk assessment, you need to assess how to decrease your risk of exposure, determine the infection prevention and control practices required to minimize your risk (e.g., hand hygiene, required PPE) and how to prevent the risk transmission to others (Provincial Infectious Diseases Advisory Committee [PIDAC], 2012).
Performing a risk assessment is foundational in the prevention of infection transmission. Public Health Ontario outlines how to perform a risk assessment related to routine practices and additional precautions ( https://www.publichealthontario.ca/-/media/documents/r/2012/rpap-risk-assessment.pdf?la=en ).
When performing the risk assessment, you need to ask yourself a series of questions prior to providing care for every client. The answers from the risk assessment will help you to identify and determine which infection prevention and control strategies you need to implement to reduce the risk of transmission of microorganisms.
Performing a Risk Assessment
Review Public Health Ontario (2012) decision trees Performing a Risk Assessment related to Routine Practices and Additional Precautions to determine the steps required by healthcare providers to assess their risk.
Digital Story with Czarielle Dela Cruz
Test your Knowledge on Performing a Risk Assessment
Medical and sterile asepsis.
Sterile asepsis, or sterile technique, is a strict technique to eliminate all microorganisms from an area (Potter et al., 2019). Examples include using steam, hydrogen peroxide, or other sterilizing agents to clean surgical tools.
Routine Practices
Routine practices include performing a point-of-care risk assessment, hand hygiene, wearing the appropriate personal protective equipment (PPE) when needed, respiratory etiquette, safe handling of sharps, controlling the surrounding environment, using avoidance procedures and actions, and following environmental cleaning and disinfecting protocols. To decrease the risk of infections, it is your responsibility to ensure that you understand and consistently follow routine practices with all clients, with every interaction, and in every healthcare setting to prevent and control the transmission of microorganisms (PIDAC, 2012). The principles of routine practices are based on the assumption that all clients are potentially infectious, even when asymptomatic. Infection prevention and control routine practices should be used to prevent exposure to blood, body fluids, secretions, excretions, mucous membranes, non-intact skin, or soiled items (PIDAC, 2012).
All clients can potentially be infectious; thus, it is important to consider which routine practices to follow and why. Routine practices refer to minimum practices that should be used with all clients. Routine practices will prevent transmission of microorganisms from client to client, client to healthcare provider, healthcare provider to client, and healthcare provider to healthcare provider.
Routine Practices include:
Additional precautions.

Certain types of infectious microorganisms require additional precautions in addition to routine practices. Additional precautions include contact, droplet, and airborne precautions or a combination of these precautions. The mode of transmission of the infectious agent will determine which additional precautions are required. The client may have a suspected infection according to their clinical signs and symptoms, or could have an infection confirmed with a test result. Healthcare providers must follow the additional precaution guidelines according to the healthcare setting policies.
Additional precautions can include PPE, specialized equipment (e.g., N95 respirator), specialized accommodation and signage, client-dedicated equipment, advanced cleaning protocols, limited movement of the client and specific environmental protocols (e.g., client placement, negative-pressure-engineered rooms). Additional precautions will be discussed further in Chapter 6 .
Routine Practices and Additional Precautions
Test your knowledge.
Attribution
This page was remixed with our own original content and adapted from:
Clinical Procedures for Safer Patient Care — Thompson Rivers University Edition by Renée Anderson, Glynda Rees Doyle, and Jodie Anita McCutcheon is used under a CC BY 4.0 Licence . This book is an adaptation of Clinical Procedures of Safer Patient Care by Glynda Rees Doyle and Jodie Anita McCutcheon, which is under a CC BY 4.0 Licence . A full list of changes and additions made by Renée Anderson can be found in the About the Book section.
Physical Examination Techniques: A Nurse’s Guide by Jennifer Lapum, Michelle Hughes, Oona St-Amant, Wendy Garcia, Margaret Verkuyl, Paul Petrie, Frances Dimaranan, Mahidhar Pemasani, and Nada Savicevic is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Introduction to Infection Prevention and Control Practices for the Interprofessional Learner Copyright © by Michelle Hughes; Audrey Kenmir; Oona St-Amant; Caitlin Cosgrove; and Grace Sharpe is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Infection control.
Yacob Habboush ; Siva Naga S. Yarrarapu ; Nilmarie Guzman .
Affiliations
Last Update: September 4, 2023 .
- Continuing Education Activity
Infection control refers to the policy and procedures implemented to control and minimize the dissemination of infections in hospitals and other healthcare settings with the main purpose of reducing infection rates. Infection control as a formal entity was established in the early 1950s in the United States. By the late 1950s and 1960s, a small number of hospitals began to recognize healthcare-associated infections (HAIs) and implemented some of the infection control concepts. This activity reviews the types of infection control methods and their indications and highlights the role of the interprofessional team in following principles of infection control to improve outcomes.
- Identify the single most effective and least expensive way for providers to prevent the spread of infection.
- Summarize standard precautions, contact precautions, droplet precautions, and airborne precautions.
- Review the types of precautions required for a patient with tuberculosis versus a patient with Clostridium difficile.
- Outline interprofessional team strategies for ensuring proper infection control measures are being followed to prevent the spread of infection in healthcare institutions.
- Introduction
Infection control refers to the policy and procedures implemented to control and minimize the dissemination of infections in hospitals and other healthcare settings with the main purpose of reducing infection rates. Infection control as a formal entity was established in the early 1950s in the United States. By the late 1950s and 1960s, a small number of hospitals began to recognize healthcare-associated infections (HAIs) and implemented some of the infection control concepts. The primary purpose of infection control programs was to focus on the surveillance for HAIs and in-cooperate the basic understandings of epidemiology to elucidate risk factors for HAIs [1] . However, most of the infection control programs were organized and managed by large academic centers rather than public health agencies which lead to sporadic efficiency and suboptimal outcomes. It was not until the late 19th and early 20th century when the new era in infection control was started through three pivotal events. These events included the Institute of Medicine’s 1999 report on errors in health care [2] , the 2002 Chicago Tribune representation on HAIs [3] , and the 2004/2006 publications of the significant reductions in bloodstream infection rate through the standardization of central venous catheter insertion process [4] . This new era in healthcare epidemiology is characterized by consumer demands for more transparency and accountability, increasing scrutiny and regulation, and expectations for rapid reductions in HAIs rates [5] . The role of infection control is to prevent and reduce the risk for hospital-acquired infections. This can be achieved by implementing infection control programs in the forms of surveillance, isolation, outbreak management, environmental hygiene, employee health, education, and infections prevention policies and management.
- Indications
Infection control program has the main purpose of preventing and stopping the transmission of infections. Specific precautions are needed to prevent infection transmission depending on the microorganism.
The following are examples of indications for transmission-based precautions:
- Standard precautions: Used for all patient care. It includes hand hygiene, personal protective equipment, appropriate patient placement, clean and disinfects patient care equipment, textiles and laundry management, safe injection practices, proper disposal of needles and other sharp objects.
- Contact precaution: Used for patients with known or suspected infections that can be transmitted through contact. For those patients, standard precautions are needed, plus limit transport and movement of patients, use disposable patient care equipment, and thorough cleaning and disinfection strategies. Patients with acute infectious diarrhea such as Clostridium difficile , vesicular rash, respiratory tract infection with a multidrug-resistant organism, abscess or draining wound that cannot be covered need to be under contact precautions.
- Droplet precautions: Used for patients with known or suspected infections that can transmit by air droplets through the mechanism of a cough, sneeze, or by talking. In such cases, it is vital to control the source by placing a mask on the patient, use standard precautions plus limitation on transport and movement. Patients with respiratory tract infection in infants and young children, petechial or ecchymotic rash with fever, and meningitis are placed under droplet precautions.
- Airborne precautions: Use for patients with known or suspected infections that can be transmitted by the airborne route. Those patients require to be in an airborne infection isolation room with all the previously mentioned protections. The most important pathogens that need airborne precautions are tuberculosis, measles, chickenpox, and disseminated herpes zoster. Patients with suspected vesicular rash, cough/fever with pulmonary infiltrate, maculopapular rash with cough/coryza/fever need to be under airborne precaution.
Multiple of those indications might require more than one precaution to ensure efficient standard and transmission-based precautions. For example, patients with suspected C. difficile need to be under contract and standard precautions, tuberculosis need to be under airborne, contact, and standard precautions.
Healthcare facilities must have the necessary equipment to implement the standard precautions for all patient. The most significant precaution that is effective in preventing infection transmission is hand hygiene. This is achieved by washing hands with soap and warm water and/or by hand rubbing with alcohol or nonalcohol based hand sanitizer. Gloves can also be used as a standard precaution, new gloves have to be used for each patient and must be disposed of after each patient interaction. Other personal protective equipment includes facial protection (procedure/surgical masks, goggles, face shield) and gown before entering the patient's room. Infection control equipment also includes the housekeeping tools where adequate and routine disinfection of surfaces and floors are implemented. Also, linens have to be handled and transported in a manner which prevents skin and mucous exposure by using the appropriate personal protective equipment.
Hospitals need to attain hospital epidemiologists, infection preventionists, and an infection control committee to organize a well-structured and implemented infection control program. The hospital epidemiologist is required to interface with many of the hospital departments and administrators to discuss their responsibilities, expectations, and available resources. The epidemiologist generally oversees the infection prevention program and in some cases the quality improvement program. A physician with a subspecialty in infectious disease usually holds the position [6] . A registered nurse with a background in clinical practice, epidemiology, and basic microbiology typically hold the infection preventionist title. Hospitals can have multiple infection preventionists depending on the number of beds available, mix of patients, and the Center for Disease Contol and Prevention (CDC) recommendations [7] . The last aspect of a functioning infection control program is the infection control committee, which consists of an interprofessional group of clinicians, nurses, administrators, epidemiologist, infection preventionists and other representatives from the laboratory, pharmacy, operating rooms, and central services. The responsibilities of this committee are to generate, implement, and maintain policies related to infection control [7] .
- Technique or Treatment
To achieve a successful and functioning infection control program, a hospital can implement the following measures:
Surveillance: The primary aim of surveillance programs is to assess the rate of infections and endemic likelihood. Generally, hospitals target surveillance for HAIs in areas where the highest rate of infection is, including intensive care units (ICUs), hematology/oncology, and surgery units. However, surveillance has expanded in the recent years to include a hospital-wide based surveillance as it is becoming a mandatory requirement by the public health authorities in multiple states [8] . This change has also been empowered by the wide implementation of the electronic health records in most hospitals in the United States, and now it is easy for any medical provider to access the electronic records at patients’ bedside and assess risks and surveillance data for each patient. Most hospitals have developed sophisticated algorithms in their electronic health systems that could streamline surveillance and identify patients at highest risk for HAIs. Hence, a hospital-wide surveillance targeting a specific infection could be implemented relatively easily. Public health agencies require that hospitals report some specific infections to strengthen the public health surveillance system [9] .
Isolation: The main purpose of isolation is to prevent the transmission of microorganisms from infected patients to others. Isolation is an expensive and time-consuming process, therefore, should only be utilized if necessary. On the other hand, if isolation is not implemented then we risk the increase in morbidity and mortality, henceforth, increasing overall healthcare cost. Hospitals that operate based on single-patient per room can implement isolation efficiently, however, significant facilities still have a substantial number of double-patient rooms which is challenging for isolation. [10] . The CDC and the Healthcare Infection Control Practice Advisory Committee have issued a guideline to outline the approaches to enhance isolation. These guidelines are based on standard and transmission-based precautions. The standard precaution refers to the assumption that all patients are possibly colonized or infected with microorganisms, therefore, precautions are applied to all patients, at all times and all departments. The main elements for standard precautions include hand hygiene (before and after patient contact), personal protective equipment (for contact with any body fluid, mucous membrane, or nonintact skin), and safe needle practices (use one needle per single dose medication per single time, then dispose of it is a safe container) [11] . Other countries such as the United Kingdom have also adopted the bare below the elbows initiative that requires all healthcare providers to wear short-sleeved garments with no accessories including rings, bracelets, and wrist watches. As for the transmission-based precautions, a cohort of patients is selected based on their clinical presentations, diagnostic criteria, or confirmatory tests with specific indication of infection or colonization of microorganisms to be isolated. In these cases, a requirement for airborne/droplet/contact precautions is necessary. These precautions are designed to prevent the transmission of disease based on the type of microorganism [12] .
Outbreak Investigation and Management: Microorganisms outbreaks can be identified through the surveillance system. Once a particular infection monthly rate crosses the 95% confidence interval threshold, an investigation is warranted for a possible outbreak. Also, clusters of infections can be reported by the healthcare providers of laboratory staff which should be followed by an initial investigation to assess if this cluster is indeed an outbreak. Usually, clusters of infections involve a common microorganism which can be identified by using the pulsed-field gel electrophoresis or the whole-genome sequencing which provides a more detailed tracking of the microorganism. Most outbreaks are a result of direct or indirect contact involving multidrug-resistant organism. Infected patients have to be separated, isolated if needed, and implementation of the necessary contact precautions, depending on what the suspected cause of infection is, have to be enforced to control such outbreaks [13] .
Education: Healthcare professionals need to be educated and periodically reinforce their knowledge through seminars and workshops to ensure high understanding of how to prevent communicable diseases transmission. The hospital might develop infection prevention liaison program by appointing a healthcare professional who could reach out and disseminate the infection prevention information to all members of the hospital.
Employee Health: It is essential for the infection control program to work closely with employee health service. Both teams need to address important topics related to the well-being of employees and infection prevention, including management of exposure to bloodborne communicable diseases and other communicable infections. Generally, all new employees undergo a screening by the employee health service to ensure that they are up-to-date with their vaccinations and have adequate immunity against some of the common communicable infections such as hepatitis B, rubella, mumps, measles, tetanus, pertussis, and varicella. Moreover, healthcare employees should always be encouraged to take the annual influenza vaccination. Also, periodic test for latent tuberculosis should be performed assess for any new exposure. Employ health service should develop proactive campaigns and policies to engage employees in their wellbeing and prevent infections.
Antimicrobial Stewardship: Antimicrobials are widely used in the inpatient and outpatient settings. Antimicrobial usage widely varies between hospitals, commonly, a high percentage of patients admitted to hospitals are administered with antibiotics. Increasingly, hospitals are adapting antimicrobial stewardship programs to control antimicrobial resistance, improve outcomes, and reduce healthcare costs. Antimicrobial stewardship should be programmed to monitor antimicrobial susceptibility profiles to anticipate and assess any new antimicrobial resistance patterns. These trends need to be correlated with the antimicrobial agents used to evaluate susceptibility [14] . Antimicrobial stewardship programs can be designed to be active and/or passive and can target pre-prescription or post-prescription periods. In the pre-prescription period, an active program includes prescriptions restrictions and preauthorization, while passive initiative includes education, guidelines, and antimicrobial susceptibility reports. On the other hand, an active post-prescription program would focus on a real-time feedback provision to physicians regarding antibiotic usage, dose, bioavailability, and susceptibility with automatic conversion of intravenous to oral formulations, while passive post-prescription involves the integration of the electronic medical records to generate alerts for prolonged prescriptions and antibiotic-microorganism mismatch [15] .
Policy and Interventions: The main purpose of the infection control program is to develop, implement, and evaluate policies and interventions to minimize the risk for HAIs. Policies are usually developed by the hospital’s infections control committee to enforce procedures that are generalizable to the hospital or certain departments. These policies are developed based on the hospital’s needs and evidence-based practice. Interventions that impact infection control can be categorized into two categories; vertical and horizontal interventions. The vertical intervention involves the reduction of risk from a single pathogen. For example, the surveillance cultures and subsequent isolation of patients infected with Methicillin-resistant Staphylococcus aureus (MRSA). Whereas, horizontal intervention targets multiple different pathogens that are transmitted in the same mechanism such as the handwashing hygiene, where clinicians are required to wash their hands before and after any patient contact which will prevent the transmission of multiple different pathogens. Vertical and horizontal interventions can be implemented simultaneously and are not mutually exclusive. However, vertical interventions might be more expensive and would not impact the other drug-resistant pathogens, while horizontal intervention might be a more affordable option with more impactful results if implemented appropriately [16] .
Environmental Hygiene: As the inpatient population becomes more susceptible to infections the emphasize on environmental hygiene has increased. Hospital decontamination through the traditional cleaning methods is notoriously inefficient. Newer methods including steam, antimicrobial surfaces, automated dispersal systems, sterilization techniques and disinfectants have a better effect in limiting transmission of pathogens through the surrounding environment [17] . The CDC has published guidelines that emphasize the collaboration between federal agencies and hospital engineers, architectures, public health and medical professionals to manage a safe and clean environment within hospitals which include air handling, water supply, and construction [18] .
- Clinical Significance
Infection control clinically translates to identifying and containing infections to minimize its dissemination. Clinicians play a significant role in infection control by identifying patients' signs and symptoms suspicious for a transmissible infection such as tuberculosis. Precaution orders have to be placed and implemented even before a confirmatory diagnosis is reached to avoid the possible transmission of the infectious pathogen. Clinically, an efficient infection control program results into fewer infection rates and lower risk for the development of multidrug-resistant pathogens. Hospital-acquired infections are one of the most common healthcare complications. Therefore simple standard precautions such as hand hygiene can prove to be highly effective. In fact, the most effective and least expensive way for clinicians to also apply infection control principles is by washing hands before and after any patient interaction [19] . Hence, hospitals need to promote and enable handwashing by providing reminders at all bedsides and having sinks or hand sanitizer stations available at the entrance to each room in the hospital. Another simple measure can be to educate patient always to try to use their forearm to block their cough or sneeze to avoid the transmission of droplets and the direct contamination of their hands by which pathogens can be transferred to other surfaces.
- Enhancing Healthcare Team Outcomes
Infection control has many challenges especially with the increasing number of hospitalized patients, a greater prevalence of invasive technologies, and a higher prevalence of immunocompromised patients [20] . Poor infection control programs lead to increased rates of infections, increase the likelihood of multidrug-resistant bacterias, and increases the risk of outbreaks in specific departments that might disseminate to the entire hospital and community. Resources are one of the major limitations in achieving an optimal infection control program; hospital epidemiologists should consider the balance between cost, clinical outcomes, patient satisfaction, and economic impact when considering new interventions. Hospital epidemiologists also need to assess the latest evidence-based literature to make certain that all infection control policies are up-to-date and to monitor the newly emerging multidrug-resistant pathogens. The major direct complication of an inappropriately managed infection control program is infection risk for the patient. Patients might be at risk for bacterial, viral, fungal, or parasitic infection. If the infection is severe, it can spread to the bloodstream leading to sepsis and possible septic shock which are life-threatening. All healthcare workers have a duty to prevent infection and maintain an aseptic environment when possible. Nursing is on the front lines of this issue, since they routinely have the highest level of contact with the patient, and have access to all aspects of the facility; their observations and recommendations should be taken seriously by all members of the interprofessional healthcare team. The most basic preventive method is by washing hands.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Yacob Habboush declares no relevant financial relationships with ineligible companies.
Disclosure: Siva Naga Yarrarapu declares no relevant financial relationships with ineligible companies.
Disclosure: Nilmarie Guzman declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Habboush Y, Yarrarapu SNS, Guzman N. Infection Control. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- New York State Infection Control. [StatPearls. 2024] New York State Infection Control. Habboush Y, Benham MD, Louie T, Noor A, Sprague RM. StatPearls. 2024 Jan
- Medical Error Reduction and Prevention. [StatPearls. 2024] Medical Error Reduction and Prevention. Rodziewicz TL, Houseman B, Vaqar S, Hipskind JE. StatPearls. 2024 Jan
- Review Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 6: Prevention of Healthcare–Associated Infections) [ 2007] Review Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 6: Prevention of Healthcare–Associated Infections) Ranji SR, Shetty K, Posley KA, Lewis R, Sundaram V, Galvin CM, Winston LG. 2007 Jan
- Prospective surveillance of device-associated health care-associated infection in an intensive care unit of a tertiary care hospital in New Delhi, India. [Am J Infect Control. 2018] Prospective surveillance of device-associated health care-associated infection in an intensive care unit of a tertiary care hospital in New Delhi, India. Kumar S, Sen P, Gaind R, Verma PK, Gupta P, Suri PR, Nagpal S, Rai AK. Am J Infect Control. 2018 Feb; 46(2):202-206. Epub 2017 Oct 16.
- Review Hospital Infection Prevention: How Much Can We Prevent and How Hard Should We Try? [Curr Infect Dis Rep. 2019] Review Hospital Infection Prevention: How Much Can We Prevent and How Hard Should We Try? Bearman G, Doll M, Cooper K, Stevens MP. Curr Infect Dis Rep. 2019 Feb 2; 21(1):2. Epub 2019 Feb 2.
Recent Activity
- Infection Control - StatPearls Infection Control - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Discussions
- Certificates
- Collab Space
- Course Details
- Announcements
Infection prevention and control (IPC) is an essential component of healthcare quality and patient safety. In this module you will learn how and why healthcare-associated infections (HAIs) occur and how IPC reduces their risk and spread.
Course contents
Introduction to ipc:, enroll me for this course, certificate requirements.
- Gain a Record of Achievement by earning at least 70% of the maximum number of points from all graded assignments.
Safety and Infection Control NCLEX Practice Quiz (75 Questions)

Welcome to your NCLEX practice quiz on Safety and Infection Control. According to the NCLEX-RN test plan , about 9 to 15% of questions will come from this subcategory that includes content about the “ nurse ‘s ability required to protect clients, families, and healthcare personnel from health and environmental hazards.” Good luck, and hope you will learn a lot from this quiz.
Safety and Infection Control Nursing Test Banks
For this nursing test bank , we have included 75 NCLEX practice questions related to the Safety and Infection Control subcategory divided into three sets. Patient safety and infection control are essential and vital components of quality nursing care. A nurse’s ability to think critically and use this knowledge in the delivery of nursing care is essential to the well-being of the patients.
Quiz Guidelines
Before you start, here are some examination guidelines and reminders you must read:
- Practice Exams : Engage with our Practice Exams to hone your skills in a supportive, low-pressure environment. These exams provide immediate feedback and explanations, helping you grasp core concepts, identify improvement areas, and build confidence in your knowledge and abilities.
- You’re given 2 minutes per item.
- For Challenge Exams, click on the “Start Quiz” button to start the quiz.
- Complete the quiz : Ensure that you answer the entire quiz. Only after you’ve answered every item will the score and rationales be shown.
- Learn from the rationales : After each quiz, click on the “View Questions” button to understand the explanation for each answer.
- Free access : Guess what? Our test banks are 100% FREE. Skip the hassle – no sign-ups or registrations here. A sincere promise from Nurseslabs: we have not and won’t ever request your credit card details or personal info for our practice questions. We’re dedicated to keeping this service accessible and cost-free, especially for our amazing students and nurses. So, take the leap and elevate your career hassle-free!
- Share your thoughts : We’d love your feedback, scores, and questions! Please share them in the comments below.
Quizzes included in this guide are:
Recommended Resources
Recommended books and resources for your NCLEX success:
Disclosure: Included below are affiliate links from Amazon at no additional cost from you. We may earn a small commission from your purchase. For more information, check out our privacy policy .
Saunders Comprehensive Review for the NCLEX-RN Saunders Comprehensive Review for the NCLEX-RN Examination is often referred to as the best nursing exam review book ever. More than 5,700 practice questions are available in the text. Detailed test-taking strategies are provided for each question, with hints for analyzing and uncovering the correct answer option.

Strategies for Student Success on the Next Generation NCLEX® (NGN) Test Items Next Generation NCLEX®-style practice questions of all types are illustrated through stand-alone case studies and unfolding case studies. NCSBN Clinical Judgment Measurement Model (NCJMM) is included throughout with case scenarios that integrate the six clinical judgment cognitive skills.

Saunders Q & A Review for the NCLEX-RN® Examination This edition contains over 6,000 practice questions with each question containing a test-taking strategy and justifications for correct and incorrect answers to enhance review. Questions are organized according to the most recent NCLEX-RN test blueprint Client Needs and Integrated Processes. Questions are written at higher cognitive levels (applying, analyzing, synthesizing, evaluating, and creating) than those on the test itself.

NCLEX-RN Prep Plus by Kaplan The NCLEX-RN Prep Plus from Kaplan employs expert critical thinking techniques and targeted sample questions. This edition identifies seven types of NGN questions and explains in detail how to approach and answer each type. In addition, it provides 10 critical thinking pathways for analyzing exam questions.

Illustrated Study Guide for the NCLEX-RN® Exam The 10th edition of the Illustrated Study Guide for the NCLEX-RN Exam, 10th Edition. This study guide gives you a robust, visual, less-intimidating way to remember key facts. 2,500 review questions are now included on the Evolve companion website. 25 additional illustrations and mnemonics make the book more appealing than ever.

NCLEX RN Examination Prep Flashcards (2023 Edition) NCLEX RN Exam Review FlashCards Study Guide with Practice Test Questions [Full-Color Cards] from Test Prep Books. These flashcards are ready for use, allowing you to begin studying immediately. Each flash card is color-coded for easy subject identification.

Recommended Links
An investment in knowledge pays the best interest. Keep up the pace and continue learning with these practice quizzes:
- Nursing Test Bank: Free Practice Questions UPDATED ! Our most comprehenisve and updated nursing test bank that includes over 3,500 practice questions covering a wide range of nursing topics that are absolutely free!
- NCLEX Questions Nursing Test Bank and Review UPDATED! Over 1,000+ comprehensive NCLEX practice questions covering different nursing topics. We’ve made a significant effort to provide you with the most challenging questions along with insightful rationales for each question to reinforce learning.
11 thoughts on “Safety and Infection Control NCLEX Practice Quiz (75 Questions)”
In # 5 the correct answer should be letter B right?
Please review question #5 in Safety and Infection Control NCLEX Practice Exam (Set 2: 25 Questions) I chose the correct answer which was cool air dryer and it was marked wrong. But in the rationales it had it correct. Thank you
Corrected! Thank you for letting us know.
in the first set of questions #16 about doing CPR. your rational for the answer being what it is states “The nurse should use the heel of one hand at the center of the chest, then place the heel of the other hand on top of the first hand and lace fingers together and give 30 compressions that are about 1” to 1½” deep.”
this is actually incorrect. per the American Heart Association, depth of compression on children is about 2 inches. answer C is more correct than D. just think the answers should be worded better and rationale should be corrected in the depth.
Question 19 regarding the child in foster care. Foster Parents do not have permission to sign informed consent on invasive procedures and need Social Worker permission. Most often the hospital/clinic will request a signature from Social Worker. The child is in the CUSTODY of the state and the CARE of the foster parent.
Thank you very much. It is a great site, very helpful. Please kindly review the answers of the following: Safety and Infection Control NCLEX Practice Exam | Quiz #2: Question 25 The posted answers were not correspond to the Question #25. Thanks again.
Thanks Kumari, this has been fixed! :)
For Q#25 for Safety and Infection Control NCLEX Practice Exam Quiz #2, the rationale given does not match the question & its answer choices. Can you fix this, please?
Fixed. Thanks for letting us know! :)
the test was completed how i can get the certificate or anything elses to be done
Hello Piyanee, Thanks for completing the test! Just to let you know, currently, we don’t offer certificates for the quizzes on our platform. They’re mainly designed for self-assessment and practice. However, you’re welcome to try out other quizzes we have available to further strengthen your knowledge. It’s a great way to keep testing your skills and learning! If you have any other questions or need guidance on specific topics, feel free to reach out.
Leave a Comment Cancel reply

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
9.6 Preventing Infection
Open Resources for Nursing (Open RN)
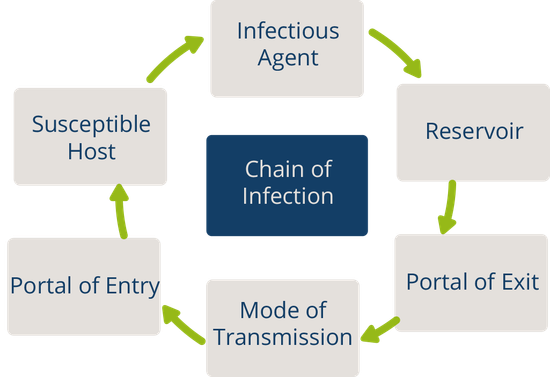
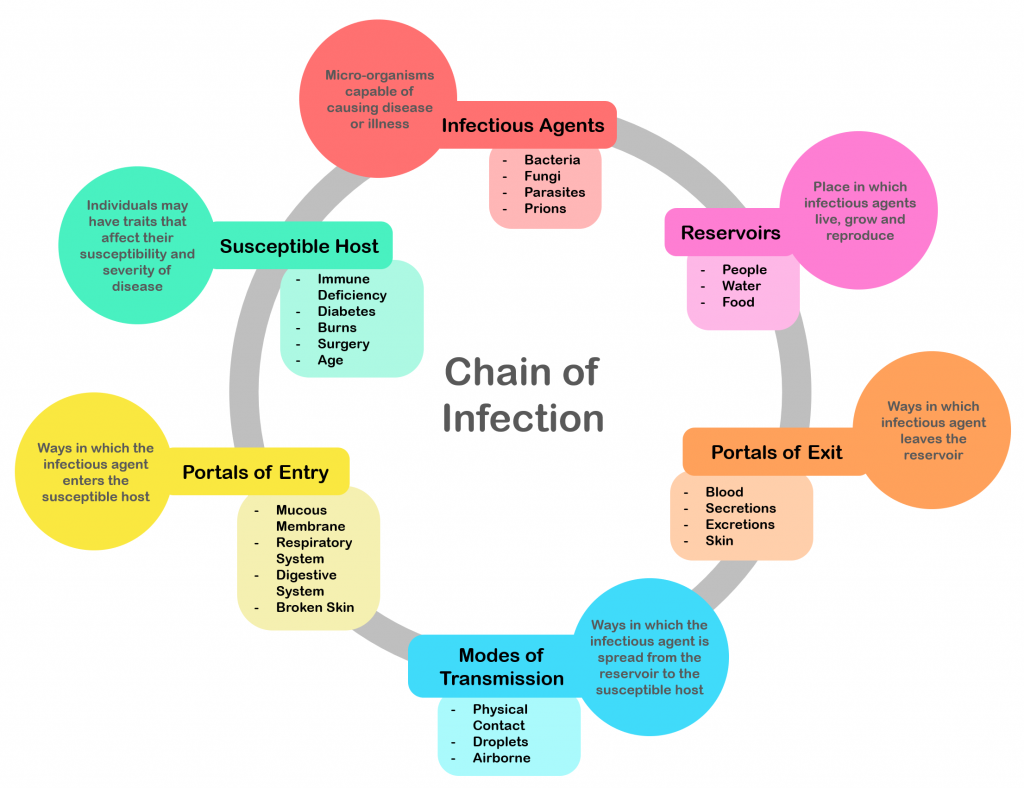
In addition to recognizing signs of infection and educating patients about the treatment of their infection, nurses also play an important role in preventing the spread of infection. A cyclic process known as the chain of infection describes the transmission of an infection. By implementing interventions to break one or more links in the chain of infection, the spread of infection can be stopped. See Figure 9.16 [1] for an illustration of the links within the chain of infection. These links are described as the following:
- Infectious Agent: A causative organism, such as bacteria, virus, fungi, parasite.
- Reservoir: A place where the organism grows, such as in blood, food, or a wound.
- Portal of Exit: The method by which the organism leaves the reservoir, such as through respiratory secretions, blood, urine, breast milk, or feces.
- Mode of Transmission: The vehicle by which the organism is transferred such as physical contact, inhalation, or injection. The most common vehicles are respiratory secretions spread by a cough, sneeze, or on the hands. A single sneeze can send thousands of virus particles into the air.
- Portal of Entry: The method by which the organism enters a new host, such as through mucous membranes or nonintact skin.
- Susceptible Host: The susceptible individual the organism has invaded. [2]
For a pathogen to continue to exist, it must put itself in a position to be transmitted to a new host, leaving the infected host through a portal of exit. Similar to portals of entry, the most common portals of exit include the skin and the respiratory, urogenital, and gastrointestinal tracts. Coughing and sneezing can expel thousands of pathogens from the respiratory tract into the environment. Other pathogens are expelled through feces, urine, semen, and vaginal secretions. Pathogens that rely on insects for transmission exit the body in the blood extracted by a biting insect. [3]
The pathogen enters a new individual via a portal of entry, such as mucous membranes or nonintact skin. If the individual has a weakened immune system or their natural defenses cannot fend off the pathogen, they become infected.
Interventions to Break the Chain of Infection
Infections can be stopped from spreading by interrupting this chain at any link. Chain links can be broken by disinfecting the environment, sterilizing medical instruments and equipment, covering coughs and sneezes, using good hand hygiene, implementing standard and transmission-based precautions, appropriately using personal protective equipment, encouraging patients to stay up-to-date on vaccines (including the flu shot), following safe injection practices, and promoting the optimal functioning of the natural immune system with good nutrition, rest, exercise, and stress management.
Disinfection and Sterilization
Disinfection and sterilization are used to kill microorganisms and remove harmful pathogens from the environment and equipment to decrease the chance of spreading infection. Disinfection is the removal of microorganisms. However, disinfection does not destroy all spores and viruses. Sterilization is a process used on equipment and the environment to destroy all pathogens, including spores and viruses. Sterilization methods include steam, boiling water, dry heat, radiation, and chemicals. Because of the harshness of these sterilization methods, skin can only be disinfected and not sterilized. [4]

Standard and Transmission-Based Precautions
To protect patients and health care workers from the spread of pathogens, the CDC has developed precautions to use during patient care that address portals of exit, methods of transmission, and portals of entry. These precautions include standard precautions and transmission-based precautions.
Standard Precautions
Standard precautions are used when caring for all patients to prevent healthcare-associated infections. According to the Centers for Disease Control and Prevention (CDC), standard precautions are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered. These precautions are based on the principle that all blood, body fluids (except sweat), nonintact skin, and mucous membranes may contain transmissible infectious agents. These standards reduce the risk of exposure for the health care worker and protect the patient from potential transmission of infectious organisms. [5] See Figure 9.17 [6] for an image of some of the components of standard precautions.
Current standard precautions according to the CDC include the following:
- Appropriate hand hygiene
- Use of personal protective equipment (e.g., gloves, gowns, masks, eyewear) whenever infectious material exposure may occur
- Appropriate patient placement and care using transmission-based precautions when indicated
- Respiratory hygiene/cough etiquette
- Proper handling and cleaning of environment, equipment, and devices
- Safe handling of laundry
- Sharps safety (i.e., engineering and work practice controls)
- Aseptic technique for invasive nursing procedures such as parenteral medication administration [7]
![]“hand-disinfection-4954840_960_720.jpg” by KlausHausmann is licensed under CC0 Image showing hand sanitizer, gloves, and a surgical mask](https://wtcs.pressbooks.pub/app/uploads/sites/31/2021/02/hand-sanitizer-1024x685.png)
Hand Hygiene
Hand hygiene, although simple, is still the best and most effective way to prevent the spread of infection. The 2021 National Patient Safety Goals from The Joint Commission encourages infection prevention strategy practices such as implementing the hand hygiene guidelines from the Centers for Disease Control. [8] Accepted methods for hand hygiene include using either soap and water or alcohol-based hand sanitizer. It is essential for all health care workers to use proper hand hygiene at the appropriate times, such as the following:
- Immediately before touching a patient
- Before performing an aseptic task or handling invasive devices
- Before moving from a soiled body site to a clean body site on a patient
- After touching a patient or their immediate environment
- After contact with blood, body fluids, or contaminated surfaces (with or without glove use)
- Immediately after glove removal [9]
Hand hygiene also includes health care workers keeping their nails short with tips less than 0.5 inches and no nail polish. Nails should be natural, and artificial nails or tips should not be worn. Artificial nails and chipped nail polish have been associated with a higher level of pathogens carried on the hands of the nurse despite hand hygiene. [10]
Respiratory Hygiene/Cough Etiquette
Respiratory hygiene is targeted at patients, accompanying family members and friends, and staff members with undiagnosed transmissible respiratory infections. It applies to any person with signs of illness, including cough, congestion, or increased production of respiratory secretions when entering a health care facility. The elements of respiratory hygiene include the following:
- Education of health care facility staff, patients, and visitors
- Posted signs, in language(s) appropriate to the population served, with instructions to patients and accompanying family members or friends
- Source control measures for a coughing person (e.g., covering the mouth/nose with a tissue when coughing and prompt disposal of used tissues, or applying surgical masks on the coughing person to contain secretions)
- Hand hygiene after contact with one’s respiratory secretions
- Spatial separation, ideally greater than 3 feet, of persons with respiratory infections in common waiting areas when possible [11]
Health care personnel are advised to wear a mask and use frequent hand hygiene when examining and caring for patients with signs and symptoms of a respiratory infection. Health care personnel who have a respiratory infection are advised to stay home or avoid direct patient contact, especially with high-risk patients. If this is not possible, then a mask should be worn while providing patient care. [12]
Personal Protective Equipment
Personal Protective Equipment (PPE) includes gloves, gowns, face shields, goggles, and masks used to prevent the spread of infection to and from patients and health care providers. See Figure 9.18 [13] for an image of a nurse wearing PPE. Depending upon the anticipated exposure and type of pathogen, PPE may include the use of gloves, a fluid-resistant gown, goggles or a face shield, and a mask or respirator. When used while caring for a patient with transmission-based precautions, PPE supplies are typically stored in an isolation cart next to the patient’s room.

Transmission-Based Precautions
In addition to standard precautions, transmission-based precautions are used for patients with documented or suspected infection of highly-transmissible pathogens, such as C. difficile (C-diff), Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant enterococci (VRE), Respiratory Syncytial Virus (RSV), measles, and tuberculosis (TB). For patients with these types of pathogens, standard precautions are used along with specific transmission-based precautions. [14]
There are three categories of transmission-based precautions: contact precautions, droplet precautions, and airborne precautions. Transmission-based precautions are used when the route(s) of transmission of a specific disease are not completely interrupted using standard precautions alone.
Some diseases, such as tuberculosis, have multiple routes of transmission so more than one transmission-based precaution category must be implemented. See Table 9.6 outlining the categories of transmission precautions with associated PPE and other precautions. When possible, patients with transmission-based precautions should be placed in a single occupancy room with dedicated patient care equipment (e.g., blood pressure cuffs, stethoscope, and thermometer stay in the patient’s room). A card is posted outside the door alerting staff and visitors to required precautions before entering the room. See Figure 9.19 [15] for an example of signage used for a patient with contact precautions. Transport of the patient and unnecessary movement outside the patient room should be limited. When transmission-based precautions are implemented, it is also important for the nurse to make efforts to counteract possible adverse effects of these precautions on patients, such as anxiety, depression, perceptions of stigma, and reduced contact with clinical staff. [16]
Table 9.6 Transmission-Based Precautions [17]

Patient Transport
Several principles are used to guide transport of patients requiring transmission-based precautions. In the inpatient and residential settings, these principles include the following:
- Limit transport for essential purposes only, such as diagnostic and therapeutic procedures that cannot be performed in the patient’s room
- When transporting, use appropriate barriers on the patient consistent with the route and risk of transmission (e.g., mask, gown, covering the affected areas when infectious skin lesions or drainage is present)
- Notify health care personnel in the receiving area of the impending arrival of the patient and of the precautions necessary to prevent transmission [18]
Enteric Precautions
Enteric precautions are used when there is the presence, or suspected presence, of gastrointestinal pathogens such as Clostridium difficile (C-diff) or norovirus. These pathogens are present in feces, so health care workers should always wear a gown in the patient room to prevent inadvertent fecal contamination of their clothing from contact with contaminated surfaces.
In addition to contact precautions, enteric precautions include the following:
- Using only soap and water for hand hygiene. Do not use hand sanitizer because it is not effective against C-diff.
- Using a special disinfecting process. Special disinfecting should be used after patient discharge and includes disinfection of the mattress.
Reverse Isolation
Reverse isolation, also called neutropenic precautions, is used for patients who have compromised immune systems and low neutrophil levels. This type of isolation protects the patient from pathogens in their environment. In addition to using contact precautions to protect the patient, reverse isolation precautions include the following:
- Meticulous hand hygiene by all visitors, staff, and the patient
- Frequently monitoring for signs and symptoms of infection and sepsis
- Not allowing live plants, fresh flowers, fresh raw fruits or vegetables, sushi, deli foods, or cheese into the room due to bacteria and fungi
- Placement in a private room or a positive pressure room
- Limited transport and movement of the patient outside of the room
- Masking of the patient for transport with a surgical mask [19]
Psychological Effects of Isolation
Although the use of transmission-based precautions is needed to prevent the spread of infection, it is important for nurses to be aware of the potential psychological impact on the patient. Research has shown that isolation can cause negative impact on patient mental well-being and behavior, including higher scores for depression, anxiety, and anger among isolated patients. It has also been found that health care workers spend less time with patients in isolation, resulting in a negative impact on patient safety. [20]
Patient and family education at the time of instituting transmission-based precautions is a critical component of the process to reduce anxiety and distress. Patients often feel stigmatized when placed in isolation, so it is important for them to understand the rationale of the precautions to keep themselves and others free from the spread of disease. Preparing patients emotionally will also help decrease their anxiety and help them cope with isolation. [21] It is also important to provide distractions from boredom, such as music, television, video games, magazines, or books, as appropriate.
Aseptic and Sterile Techniques
In addition to using standard precautions and transmission-based precautions, aseptic technique (also called medical asepsis) is used to prevent the transfer of microorganisms from one person or object to another during a medical procedure. For example, a nurse administering parenteral medication or performing urinary catheterization uses aseptic technique. When performed properly, aseptic technique prevents contamination and transfer of pathogens to the patient from caregiver hands, surfaces, and equipment during routine care or procedures. It is important to remember that potentially infectious microorganisms can be present in the environment, on instruments, in liquids, on skin surfaces, or within a wound. [22]
There is often misunderstanding between the terms aseptic technique and sterile technique in the health care setting. Both asepsis and sterility are closely related with the shared concept being the removal of harmful microorganisms that can cause infection. In the most simplistic terms, aseptic technique involves creating a protective barrier to prevent the spread of pathogens, whereas sterile technique is a purposeful attack on microorganisms. Sterile technique (also called surgical asepsis) seeks to eliminate every potential microorganism in and around a sterile field while also maintaining objects as free from microorganisms as possible. Sterile fields are implemented during surgery, as well as during nursing procedures such as the insertion of a urinary catheter, changing dressings on open wounds, and performing central line care. See Figure 9.20 [23] for an image of a sterile field during surgery. Sterile technique requires a combination of meticulous hand washing, creating and maintaining a sterile field, using long-lasting antimicrobial cleansing agents such as Betadine, donning sterile gloves, and using sterile devices and instruments. [24]
Read additional information about aseptic and sterile technique in the “ Aseptic Technique ” in Open RN Nursing Skills .
Read a continuing education article about Sterile Technique and surgical scrubbing.
Other Hygienic Patient Care Interventions
In addition to implementing standard and transmission-based precautions and utilizing aseptic and sterile technique when performing procedures, nurses implement many interventions to place a patient in the best health possible to prevent an infection or treat infection. These interventions include actions like encouraging rest and good nutrition, teaching stress management, providing good oral care, encouraging daily bathing, and changing linens. It is also important to consider how gripper socks, mobile devices, and improper glove usage can contribute to the transmission of pathogens.
Patient hygiene is important in the prevention and spread of infection. Although oral care may be given a low priority, research has found that poor oral care is associated with the spread of infection, poor health outcomes, and poor nutrition. Oral care should be performed in the morning, after meals, and before bed. [25]
Daily Bathing
Daily bathing is another intervention that may be viewed as time-consuming and receive low priority, but it can have a powerful impact on decreasing the spread of infection. Studies have shown a significant decrease in healthcare-associated infections with daily bathing using chlorhexidine gluconate (CHG) wipes or solution. The use of traditional soap and water baths do not reduce infection rates as significantly as CHG products, and wash basins have also been shown to be a reservoir for pathogens. [26]
Changing bed linens, towels, and a gown regularly eliminates potential reservoirs of bacteria. Fresh linens also promote patient comfort.
Gripper Socks
Have you ever thought about what happens to the bed linens when a patient returns from a walk in the hallway with gripper socks and gets back into bed with these socks? Research demonstrates that pathogens from the floor are transferred to the patient bed linens from the gripper socks. Nurses should remove gripper socks that were used for walking before patients climb into bed. They should also throw the socks away when the patient is discharged instead of sending them home. [27]
Cellular Phones and Mobile Devices
Research has shown that cell phones and mobile devices carry many pathogens and are dirtier than a toilet seat or the bottom of a shoe. Patients, staff, and visitors routinely bring these mobile devices into health care facilities, which can cause the spread of disease. Nurses should frequently wipe mobile devices with disinfectant. They should encourage patients and visitors to disinfect phones frequently and avoid touching the face after having touched a mobile device. [28]
Although gloves are used to prevent the spread of infection, they can also contribute to the spread of infection if used improperly. For example, research has shown that hand hygiene opportunities are being missed because of the overuse of gloves. For example, a nurse may don gloves to suction a patient but neglect to remove them and perform hand hygiene before performing the next procedure on the same patient. This can potentially cause the spread of secondary infection. The World Health Organization (WHO) states that gloves should be worn when there is an expected risk of exposure to blood or body fluids or to protect the hands from chemicals and hazardous drugs, but hand hygiene is the best method of disease prevention and is preferred over wearing gloves when the exposure risk is minimal. Nurses have the perception that wearing gloves provides extra protection and cleanliness. However, the opposite is true. Nonsterile gloves have a high incidence of contamination with a range of bacteria, which means that a gloved hand is dirtier than a washed hand. Research has shown that nearly 40% of the times that gloves are used in patient care, there is cross contamination. The most striking example of cross contamination includes situations when gloves are used for toileting a patient and not being removed before touching other surfaces or the patient. [29] , [30] , [31]
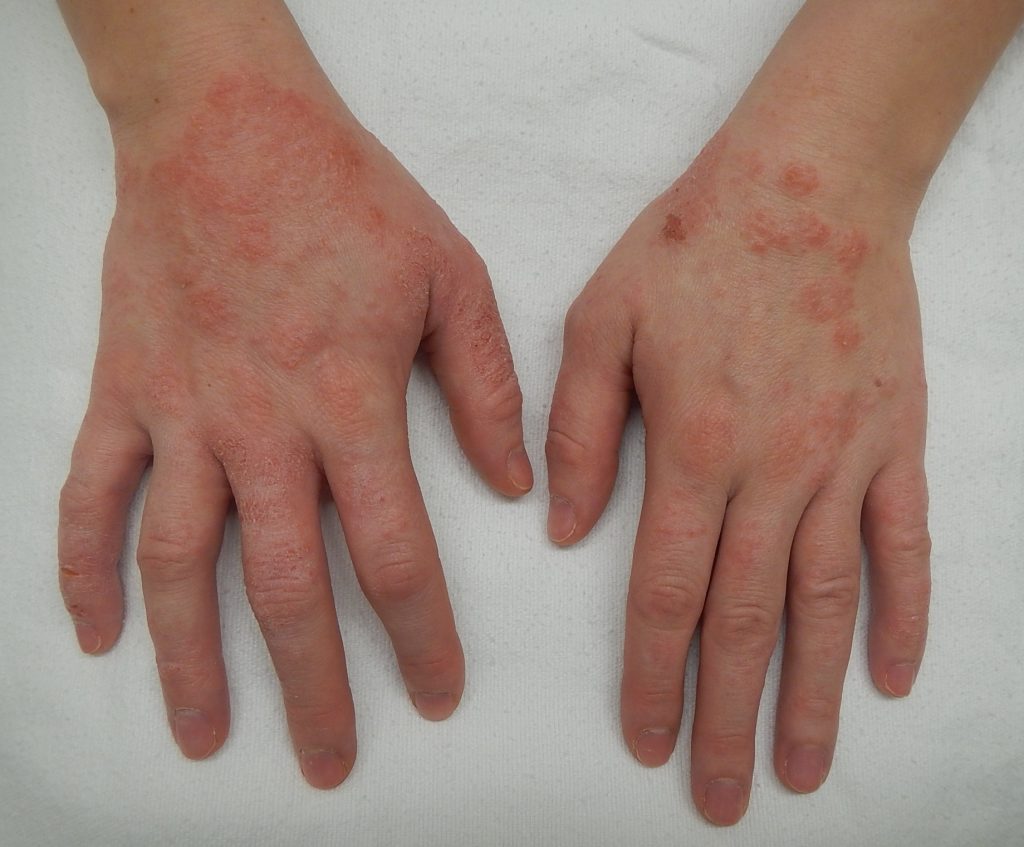
Glove-related contact dermatitis has also become an important issue in recent years as more and more nurses are experiencing damage to the hands. Contact dermatitis can develop from repeated use of gloves and develops as dry, itchy, irritated areas on the skin of the hands. See Figure 9.21 [32] for an image of contact dermatitis from gloves. Because the skin is the first line of defense in preventing pathogens from entering the body, maintaining intact skin is very important to prevent nurses from exposure to pathogens.
- “ Chain_of_Infection.png ” by Genieieiop is licensed under CC BY-SA 4.0 ↵
- Centers for Disease Control and Prevention. (2012, May 18). Lesson 1: Introduction to epidemiology. https://www.cdc.gov/csels/dsepd/ss1978/lesson1/section10.html ↵
- This work is a derivative of Microbiology by OpenStax and is licensed under CC BY 4.0 . Access for free at https://openstax.org/books/microbiology/pages/1-introduction ↵
- Centers for Disease Control and Prevention. (2019, May 24). Disinfection and sterilization . https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html ↵
- Centers for Disease Control and Prevention. (2016, January 26). Standard precautions for all patient care . https://www.cdc.gov/infectioncontrol/basics/standard-precautions.html ↵
- “ hand-disinfection-4954840_960_720.jpg ” by KlausHausmann is licensed under CC0 ↵
- Centers for Disease Control and Prevention. (2019, April 29). Hand hygiene in healthcare settings . https://www.cdc.gov/handhygiene/ ↵
- Blackburn, L., Acree, K., Bartley, J., DiGiannantoni, E., Renner, E., & Sinnott, L. T. (2020). Microbial growth on the nails of direct patient care nurses wearing nail polish. Nursing Oncology Forum, 47 (2), 155-164. https://doi.org/10.1188/20.onf.155-164 ↵
- “ U.S. Navy Doctors, Nurses and Corpsmen Treat COVID Patients in the ICU Aboard USNS Comfort (49825651378).jpg ” by Navy Medicine is licensed under CC0 . ↵
- Siegel, J. D., Rhinehart, E., Jackson, M., Chiarello, L., & Healthcare Infection Control Practices Advisory Committee. (2019, July 22). 2007 guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings . https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html ↵
- “ Contact_Precautions_poster.pdf ” by U.S. Centers for Disease Control and Prevention is in the Public Domain ↵
- Centers for Disease Control and Prevention. (n.d.). What you need to know: Neutropenia and risk for infection. https://www.cdc.gov/cancer/preventinfections/pdf/neutropenia.pdf ↵
- U.S. Department of Health and Human Services. (2020, January 15). Health care-associated infections. https://health.gov/our-work/health-care-quality/health-care-associated-infections ↵
- Centers for Disease Control and Prevention. (2020, August 10). Glossary of terms for infection prevention and control in dental settings. https://www.cdc.gov/oralhealth/infectioncontrol/glossary.htm ↵
- " 226589236-huge.jpg " by TORWAISTUDIO is used under license from Shutterstock.com ↵
- This work is a derivative of StatPearls by Tennant and Rivers and is licensed under CC BY 4.0 . ↵
- Ackley, B., Ladwig, G., Makic, M. B., Martinez-Kratz, M., & Zanotti, M. (2020). Nursing diagnosis handbook: An evidence-based guide to planning care (12th ed.). Elsevier. pp. 546-552, 828-832. ↵
- Salamone, K., Yacoub, E., Mahoney, A. M., & Edward, K. L. (2013). Oral care of hospitalised older patients in the acute medical setting. Nursing Research and Practice, 2013 , 827670. https://doi.org/10.1155/2013/827670 ↵
- Welle, M. K., Bliha, M., DeLuca, J., Frauhiger, A., & Lamichhane-Khadka, R. Bacteria on the soles of patient-issued nonskid slipper socks: An overlooked pathogen spread threat? Orthopedic Nursing, 38 (1), 33-40. https://doi.org/10.1097/nor.0000000000000516 ↵
- Morubagal, R. R., Shivappa, S. G., Mahale, R. P., & Neelambike, S. M. (2017). Study of bacterial flora associated with mobile phones of healthcare workers and non-healthcare workers. Iranian Journal of Microbiology, 9 (3), 143–151. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5719508/ ↵
- Burdsall, D. P., Gardner, S. E., Cox, T., Schweizer, M., Culp, K. R., Steelman, V. M., & Herwaldt, L. A. (2017). Exploring inappropriate certified nursing assistant glove use in long-term care. American Journal of Infection Control, 45 (9), 940-945. https://doi.org/10.1016/j.ajic.2017.02.017 ↵
- Jain, S., Clezy, K., & McLaws, M. L. Glove: Use for safety or overuse? American Journal of Infection Control, 45 (12), 1407-1410. https://doi.org/10.1016/j.ajic.2017.08.029 ↵
- “ Dermatitis2015.jpg ” by James Heilman, MD is licensed under CC BY-SA 4.0 ↵
Removal of organisms from inanimate objects and surfaces.
A process used to destroy all pathogens from inanimate objects, including spores and viruses.
The minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where healthcare is delivered.
Gloves, gowns, face shields, goggles, and masks used to prevent the spread of infection to and from patients and health care providers.
The purposeful reduction of pathogens to prevent the transfer of microorganisms from one person or object to another during a medical procedure.
A process, also called surgical asepsis, used to eliminate every potential microorganism in and around a sterile field while also maintaining objects as free from microorganisms as possible.
Nursing Fundamentals Copyright © by Open Resources for Nursing (Open RN) is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book
Acknowledgements
We would like to thank the hospitals and research participants involved in this learning report and the research team: Professor Alison Holmes, Professor Mary Dixon-Woods, Dr Raheelah Ahmad, Dr Elizabeth Brewster, Dr Enrique Castro-Sánchez, Dr Federica Secci and Dr Walter Zingg. With input from Susan Burnett, Professor Ewan Ferlie, Tracey Galletly, Fran Husson, Claire Kilpatrick, Dr Reda Lebcir and the Patients Association.
Alison Holmes Professor of Infectious Diseases, Imperial College London [email protected]
It is with pleasure that I write the foreword for this concise learning report on infection prevention and control (IPC), which builds on the large body of work conducted by researchers at Imperial College London and the University of Leicester. The Health Foundation has brought together a strong multidisciplinary team to review activity and interventions to reduce health care associated infections (HCAI) in English acute care organisations over the last 15 years with the aim of ensuring that key learning can be collectively reflected upon and to shape further national and local activity.
Continuous learning is a key Health Foundation principle and is particularly supported by one of the overriding lessons highlighted in this report: namely, the importance of ensuring that any major interventions, such as local antimicrobial resistance (AMR) action plans, are supported by a planned analysis which should examine impact and implementation as well as cost-effectiveness. Such an understanding would form a solid basis for informing further interventions. There has been much interest internationally in some of the UK’s significant successes in HCAI reduction, but there is little material available for shared learning in the absence of adequate studies on national interventions.
The key lessons in this report are being well heeded and are being discussed with relevant partners to ensure actions are put in place to address them. These actions should build upon this learning report, recognising that whilst IPC behaviours of those working at the front line are critical, they must also be actively supported and positively reinforced by a hospital environment that supports best IPC practice and minimises risk. Several of the findings and key lessons particularly in relation to the need to address AMR are also reinforced by the Chief Medical Officer’s report UK 5-year Antimicrobial Resistance (AMR) Strategy (2013–2018). I am also pleased to acknowledge that the Health Foundation is becoming involved in improving antibiotic prescribing behaviours, which is a key aspect of IPC.
I am delighted that the views and input from our patients and the public have been particularly invited and explored in this report and that there will be ongoing work to better understand public and patient engagement and involvement.
Even though the scope of this commissioned report was confined to acute care and to HCAI prevention, the authors make two fundamental recommendations that should be considered by policymakers. These are, firstly, that addressing the threat of AMR must be more effectively integrated with the delivery of strong IPC and, secondly, that tackling any transmissible organism must be addressed comprehensively across the whole patient journey. Organisms do not respect boundaries between community, primary and secondary care, so a whole health care economy approach is vital.
As Chair of the Department of Health advisory committee on antimicrobial resistance and healthcare associated infection (ARHAI), I fully endorse the lessons within this learning report. I am delighted that ARHAI will discuss actions to address the report’s findings with the Health Foundation in the coming year.
Professor Mike Sharland
Chair of the Department of Health advisory committee on antimicrobial resistance and healthcare associated infection (ARHAI)
Glossary of common abbreviations
Introduction.
Infection control has been high on the political agenda and on the agenda of the NHS in England for the last 15 years. During this time there have been many successes, not least the reduction in MRSA bloodstream infections (BSIs) and cases of Clostridium difficile infection. Though these successes should be celebrated, it is important not to become complacent. Other health care associated infections (HCAIs) that have not been monitored as rigorously are growing in incidence. New infections, including the growing number of more resistant strains of bacteria, are in danger of spreading. As a result, infection control needs to remain central to the work of the NHS and it is essential to continue building on the achievements of recent years to reduce mortality, morbidity and health care costs.
This report considers what has been learned from the infection prevention and control (IPC) work carried out over the last 15 years in hospitals in England and looks at how these lessons should be applied in future. Should the NHS continue to respond in the same way to infection threats or should new approaches be adopted? Of all the interventions made during this period, what has worked best? Is it even possible to tell? How have all the infection control procedures and practices impacted on the front line in clinical care and on service users?
The lessons the report sets out are drawn from the findings of a large research study that identified and consolidated published evidence from the UK about national IPC initiatives and interventions and synthesised this with findings from qualitative case studies in two large NHS hospitals, including the perspectives of service users recorded during consultation events.
The report begins with an overview of HCAIs, especially those linked to hospitals. With this background, the landscape of infection control in English hospitals is set out, including recent successes and the challenges ahead. The report goes on to draw out lessons to help inform future work to maintain hospitals as safe places.
What are health care associated infections?
Health care associated infections are infections that develop as a direct result of medical or surgical treatment or contact in a health care setting. , They can occur in hospitals and in health or social care settings in the community and can affect both patients and health care workers. An infection occurs when a germ (an organism such as a bacterium, virus or fungus) enters the body and attacks or causes damage to it. Every individual is covered with bacteria on their body and also carries trillions of them in their gut. Any medical or surgical procedure that breaks the skin or any mucous membrane, introduces any foreign material or reduces immunity creates a risk of infection. Some infections can enter the bloodstream and become generalised throughout the body. This is known as a bacteraemia or bloodstream infection (BSI).
In this report we focus particularly on those infections that arise during hospital care. Meticillin-resistant Staphylococcus aureus (MRSA) BSIs and C. difficile associated diarrhoea ( C. difficile infection) are two well-known HCAIs that have been the focus of attention in England, but there are many more. MRSA BSIs are particularly associated with intravenous devices (eg central lines) and C. difficile with antibiotic exposure. Hand hygiene, environmental hygiene, the capacity to isolate patients and assuring optimal antibiotic prescribing are all critical aspects of prevention of both.
Other HCAIs include BSIs caused by other organisms, urinary tract infections related to urinary catheters (CAUTIs), respiratory tract infections such as pneumonia related to being ventilated (VAP), or wound or surgical site infections (SSIs). Microorganisms come from droplets that are sneezed or coughed (eg flu), from air (eg tuberculosis), or from water (eg legionnaires’ disease); they are passed within the hospital or they can come in from the community (eg norovirus). Germs causing HCAIs may have different modes of transmission and different sources, and patients may have different profiles of risk factors making them more vulnerable to an HCAI, but some core infection prevention principles remain the same.
In hospital, infections can generally be classified as either transmission-dependent or as arising from a patient’s own microbial flora (endogenous transmission). Transmission-dependent infections involve the acquisition of the pathogen from the health care environment, for example person-to-person (for instance, through inadequate hand hygiene) or from contaminated equipment, devices and environments (exogenous transmission). Infections from a patient’s own microbial flora may arise post-surgically or post-insertion of invasive devices such as intravenous lines. Prevention here relies on skin cleansing and, for some procedures, prophylactic antibiotics. , ,
The landscape of infection control in England since 2000
A 2000 National Audit Office (NAO) report was highly critical of the strategic management of HCAIs in England. Suggesting that infection control was the Cinderella of the health service, the report criticised the lack of information about the infections and the limited resources allocated to infection control teams. A key problem was that the size and scope of HCAIs was simply unknown. A voluntary scheme for reporting BSIs had existed during the 1990s, but suffered from problems of completeness and comparability. More broadly, the report suggested that HCAIs had come to be seen as an intractable problem, regarded by those working in hospitals (clinicians and managers) as an inevitable consequence of providing health care. Such infections were thus regrettable, but were to a large extent tolerated.
In 2001, mandatory reporting of MRSA BSI cases in hospitals was introduced, with a few other selected infections included in the surveillance programme in subsequent years. Further national reports in 2003 and 2004 suggested some improvement, including evidence of hospital trusts giving higher priority to infection control. , But criticism remained of the failure of the NHS to ‘get a grip’ on both the extent and the cost of HCAIs.
At the same time, HCAIs became a frequent and often vivid topic in the media , and a focus of huge public concern and political attention. Three recurring themes were evident: the vulnerability to infection and corresponding fear felt by patients; dirty wards, which, it was claimed, often occurred when cleaning contracts were outsourced and the standards of cleaning dropped; and a demand to ‘bring back matron’, seen as the solution to nurses’ poor compliance with prevention measures. Analysis of newspaper reporting at the time shows a steady increase in stories of ‘hospital superbugs’, peaking in the run-up to the 2005 election, when hospital hygiene became a highly publicised issue. A separate analysis of reporting about MRSA in 12 newspapers between 1994 and 2005 identified that the momentum was fuelled in part by celebrity stories and some fictional media. Interestingly, the role of the pressure of antibiotic use in driving this increase was rarely discussed. By 2008, a BBC poll was reporting that the risk of acquiring an infection was the main fear of the public about inpatient care.
The combination of critical reports and media publicity produced important agenda-setting effects, converting HCAI from a problem that had become rather neglected and under-resourced into a social problem , demanding a solution in the face of intense public and political pressure. High-profile policy interventions followed in close succession.
Progress and successes, 2000 to 2015
The mandatory surveillance of MRSA BSI cases was introduced in April 2001. It was managed by the Health Protection Agency (HPA) on behalf of the Department of Health. A new target was introduced to NHS acute trusts in April 2005: the year-on-year reduction of MRSA BSI rates. The data show striking progress towards this target, with MRSA bloodstream infection rates (expressed as cases per 100,000 bed days) in steady decline in England since 2004 (Figure 1).
Figure 1: Annual rates of MRSA BSIs for NHS trusts in England per 100,000 bed days, 2001/02–2014/15 ,
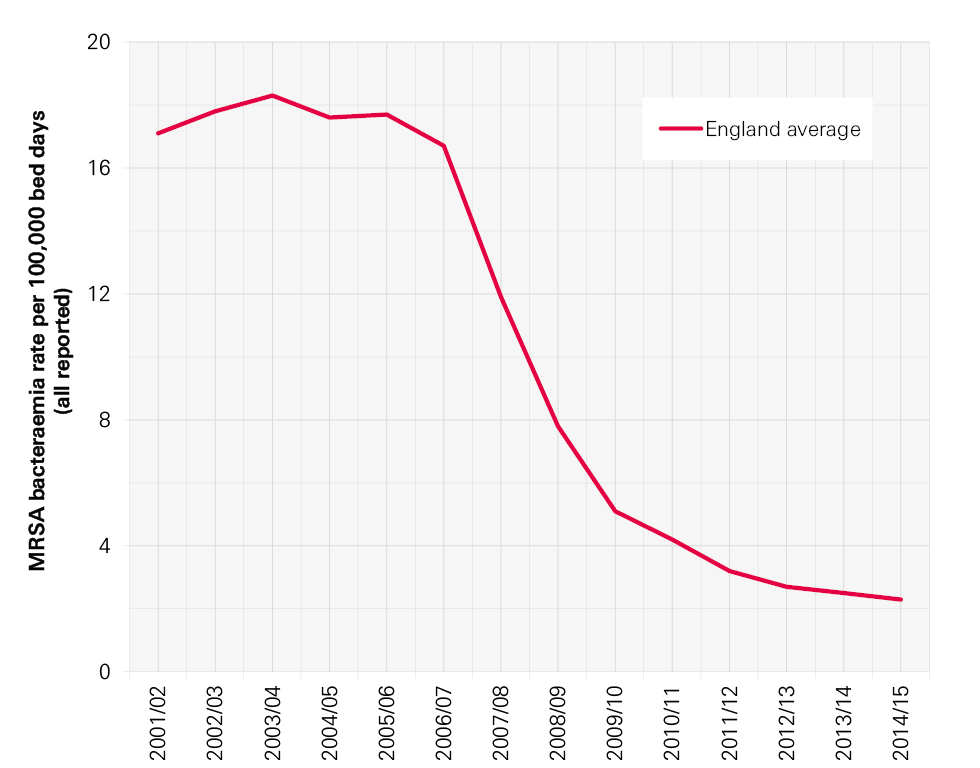
Mandatory surveillance was extended to glycopeptide-resistant Enterococcal (GRE) BSI in October 2003, C. difficile infection (CDI) for people aged over 65 in January 2004, meticillin-sensitive S. aureus (MSSA) BSI in January 2011 and Escherichia coli ( E. coli ) BSI in June 2011. The reporting of CDI was extended to everyone over the age of two in 2007 after the inquiry into the C. difficile outbreaks at Stoke Mandeville (where the focus on MRSA alone was cited as a contributory factor to the CDI outbreak). Trust apportioned rates show a decline since 2007/08 (see Figure 2).
Figure 2: Annual C. difficile rates in England, 2007/08–2014/15 ,
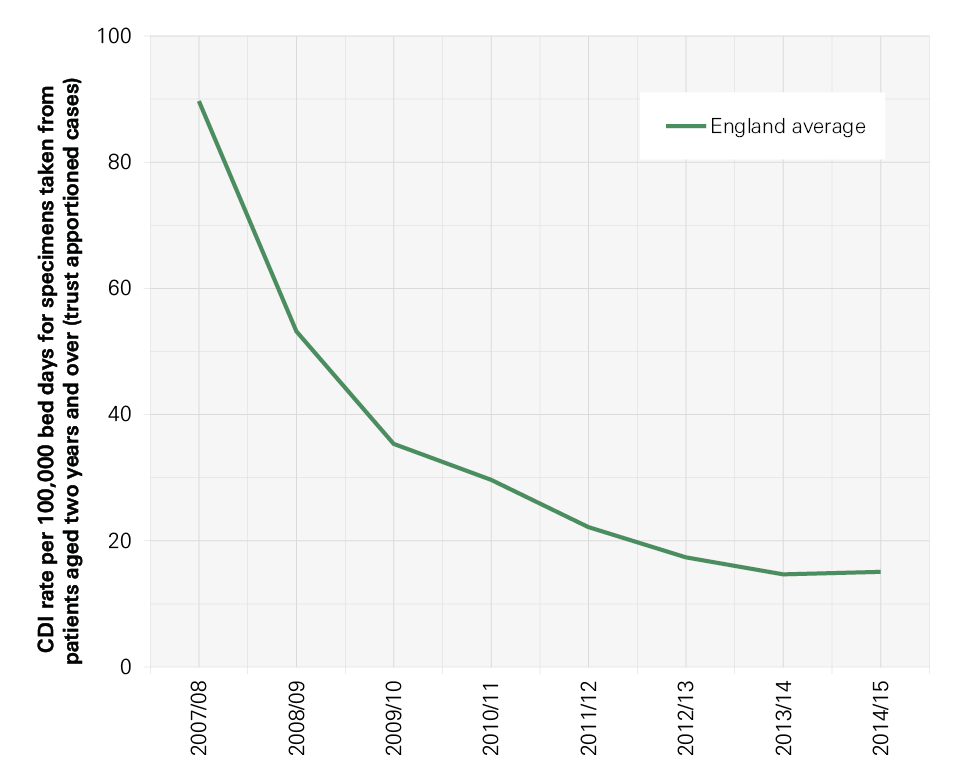
Not all progress has been in the right direction, however. E. coli represents the most rapidly increasing and most common BSI, accounting for 36% of the BSIs seen nationally, compared with 1.6% caused by MRSA (see Figure 3, overleaf).23
Figure 3: All reported rates England average: MRSA BSI, C. difficile infection, MSSA BSI, E. coli BSI, 2001/02–2013/14 , , , , ,
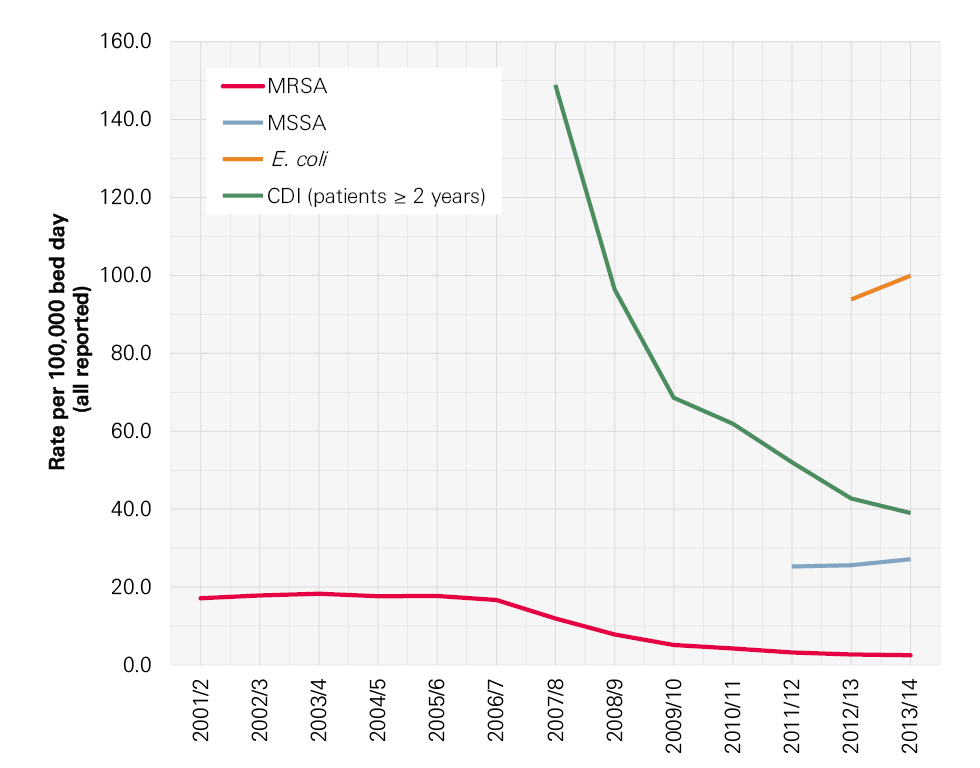
Health care associated infections in 2015 and challenges ahead
The most recent studies report that between 5.1% and 11.6% of hospitalised patients will acquire at least one HCAI, with the risk of HCAI greatest in intensive care units (ICUs), where the prevalence is 23.4% (with a 95% confidence interval of 17.3–31.8). , The best estimates available for attributable deaths from HCAI are those attributable to Staphylococcus aureus BSI or C. difficile infections. These combined estimates have been recorded as causing 9,000 deaths per year. ,
Figure 5 : Timeline of selected interventions to reduce HCAIs and improve IPC
For details of the references in this timeline, see www.health.org.uk/hcai
The types of infections occurring have also shifted, with far fewer MRSA bloodstream and C. difficile infections, but increasing numbers of BSIs caused by E. coli and other bacteria increasingly resistant to the effects of antibiotics or the actions of the body’s immune system.
Figure 4 shows the trends in infections in the population as a whole rather than in people in hospital. This shows how infections in hospital mirror those in the wider community. For example, the spike in C. difficile in the financial year 2006/07 and the current rise in E. coli BSIs are being experienced in the population as a whole, and, inevitably, in hospitals too.
Figure 4: Trends in C. difficile infection, MRSA, MSSA and E. coli BSIs (England 2002–2014, calendar years)
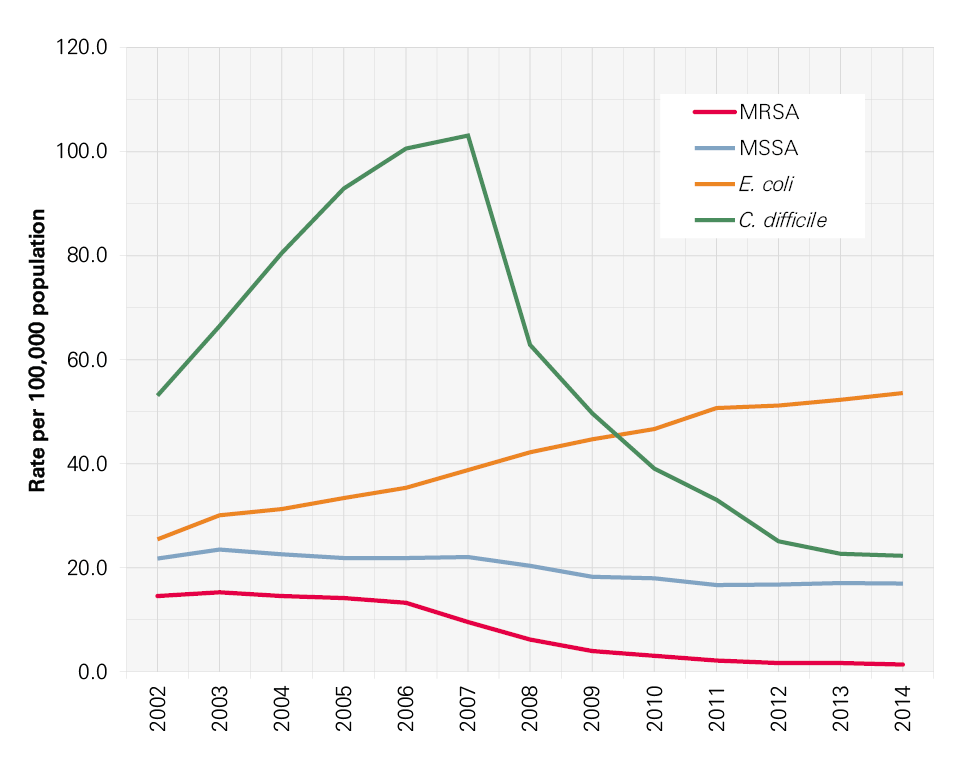
In her annual report in 2011, the Chief Medical Officer set out the challenges ahead for infections in the UK and in particular for the joint challenges of antibiotic resistance, infection prevention and HCAI:
- The host : as medicine becomes more successful in keeping us alive, more and more people in the population are immunocompromised. This includes the increase in the numbers of older people; more babies (neonates) surviving pre-term; more people with lifestyle risks (obesity, smoking, excess alcohol consumption); and more people on immunosuppressant drugs for conditions such as cancer and renal transplant.
- The environment : as hospitals get busier, the ease with which infections can spread increases. The risk may be increased by overcrowding; poor cleaning due to areas being too busy; inadequate facilities for hand hygiene and poor aseptic technique.
- The pathogens : many more pathogens are becoming resistant to antibiotics and new pathogens are now coming into hospitals.
To truly address infection prevention and antimicrobial resistance (AMR), action must not be confined within the walls of a hospital; it must consider all aspects of patient care and pathways. That includes community, primary and hospital care and includes the health care professionals and patients within and across each of these settings – ie across the whole health care economy.
Lessons from research about acute care in England
Many lessons can be learned from the tremendous amount of work undertaken in England over the last 15 years to improve infection prevention and control (IPC). In this section, we set out a brief summary of findings and conclusions from our research. Our research methods are set out in more detail in Appendix 1.
Briefly, we used a mixed-method approach incorporating literature reviews, qualitative case studies including interviews with members of staff from ward to board in two acute hospitals in England, and a user consultation event to hear the views of patients and the public. We also undertook a scoping exercise to identify the availability and use of data to monitor HCAI and indicators of IPC performance in UK hospital trusts. Data from three previous studies were used: one, the Lining Up ethnographic study of intensive care units’ attempts to reduce central venous catheter bloodstream infections, and two large-scale innovation adoption studies (Commissioned by the Department of Health; and funded by the National Institute for Health Research, Health Services and Delivery Research ).
Evaluating campaigns and initiatives for infection prevention and control
The timeline set out in Figure 5 shows the concentration of interventions targeting HCAI in hospitals since 2000. These range from mandatory reporting of selected infections, regulatory interventions, national guidelines and national campaigns that were often running in parallel. Perhaps the most influential intervention was the early introduction of mandatory surveillance of MRSA BSIs. This quantified the problem and made it clear that action was needed across the NHS. Following on from this, in 2004 the national cleanyourhands campaign was launched. Among many changes introduced in this campaign, the most visible are the alcohol hand-rub dispensers that can be seen throughout hospitals. Further initiatives have been based on the use of ‘care bundles’, which group together practices that should be performed consistently. Programmes that used bundles included Saving Lives High Impact Interventions , Patient Safety First and Matching Michigan . These initiatives were aimed at improving reliability in the use of procedures known to prevent surgical site infections, central venous catheter infections and infections linked to ventilators in intensive care units.
HCAI rates have declined over this period, but our research showed that it is impossible to attribute success to any one initiative, nor is it possible to say which components of any one programme were critical. We do know that multimodal interventions work. We also know that having the backing of a national campaign gains the attention of trust executives, bringing focus and resource to IPC where needed and providing strong external reinforcement. , , But much learning about what works in large-scale IPC programmes has been lost because so little research and evaluation was conducted to determine effectiveness or to identify mechanisms of change and contextual influences. A key lesson here is that future campaigns would benefit from a programme of evaluation running in parallel to help understand what works and why. Also important for future campaigns will be a requirement for a systems-wide focus across entire health economies, rather than confining efforts and resources to the acute sector.
Figure 5: Timeline of selected interventions to reduce HCAIs and improve IPC
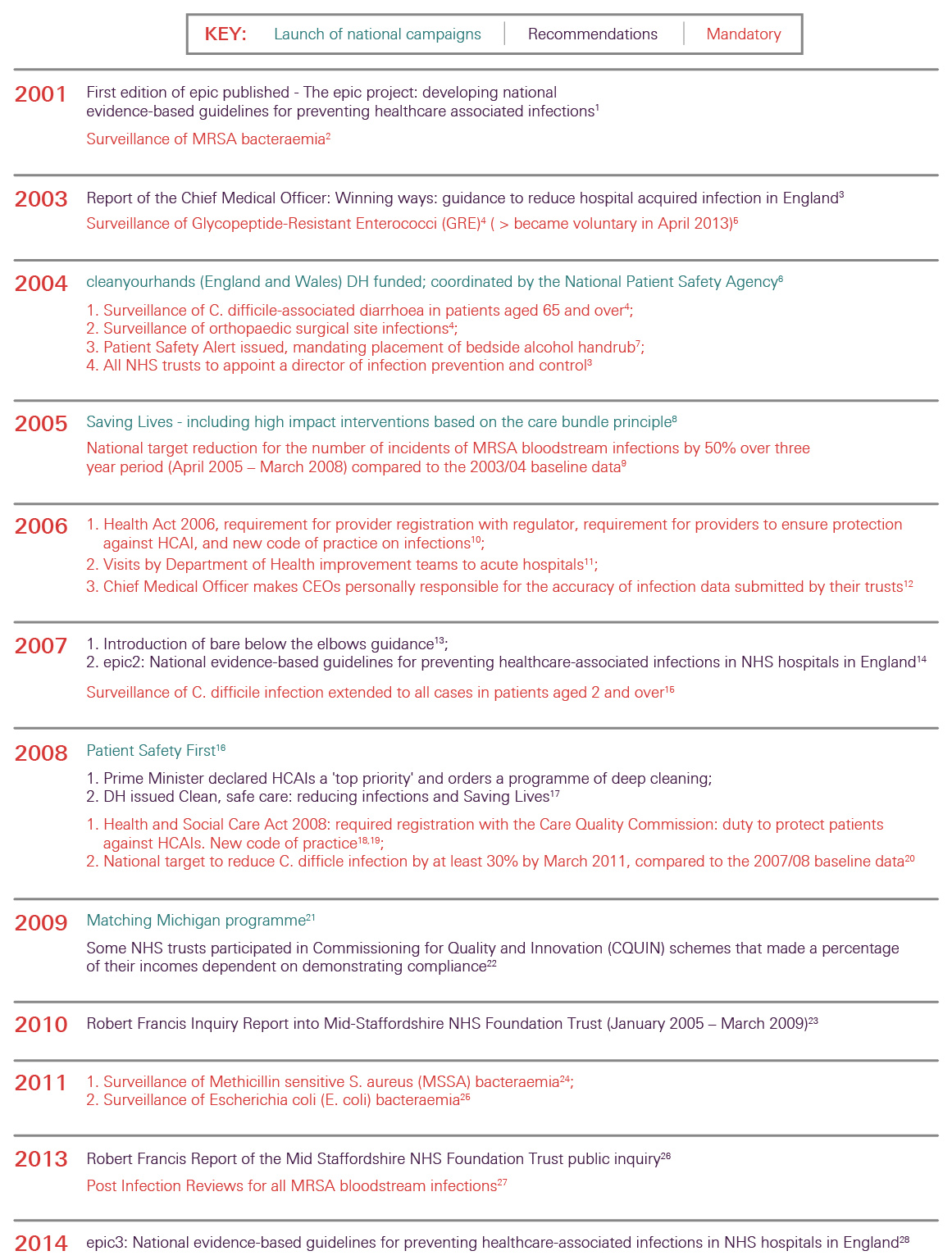
The effective organisation of infection prevention and control at trust level
In our research, it was clear that organisations may aspire to best practice in IPC. Executive team members in our case study hospitals commented that IPC was on the agenda for every board meeting and was integrated into trust strategy. IPC interventions were ‘very visible’. Many of those working on the front line felt that IPC was a high priority in the area in which they worked and that colleagues generally understood what good IPC involved.
Despite the high priority given to IPC, often it was not considered part of wider patient safety and quality improvement work in hospitals. This has meant that at the front line, IPC work may be seen as competing with other patient safety and quality strategies. Trusts need to be aware of this, and where possible bring the improvement work together in an integrated way at the clinical front line while maintaining the necessary expert input.
A key lesson is that trusts must continue to have both the structural and cultural capacity to deliver effective IPC. They should recognise and understand the impact of a positive organisational culture since it has implications for staff turnover and motivation and is important when looking at the sustainability of approaches to IPC.
Participants in our research stressed the need for strong leadership to support activities to prevent and control infection within the organisation. They also emphasised the need for support for IPC to be fully aligned, from the trust board, executive team and specialist infection control staff through to all those working on the front line (both clinical and non-clinical). Two views were expressed on leadership: one stressed the need for leadership and support from specialist infection control staff; the other emphasised the need for clinicians working on the front line to ‘own’ and lead themselves.
Since 2004, all NHS trusts have been required to have a director of infection prevention and control (DIPC) to provide a direct line of accountability to the CEO and the board. The DIPC is intended to lead and champion IPC at multiple levels within the organisation, ensuring that a consistent messages and best practice are embedded and continuously improved in directorates, groups, teams and networks. Many other clinical and managerial roles in hospitals now have IPC built into their responsibilities, including matrons, clinical directors, directorate managers, ward managers, pharmacists and others.
A key lesson here is that leadership for IPC needs to be distributed throughout an organisation, with clinical champions identified in all areas. , , This is especially important as we found that, at the sharp end of care, health care workers had to address competition between immediate priorities and best IPC practice on a day-to-day basis.
The role of measurement and monitoring in infection prevention and control
Our research found that there can be little doubt about the value of mandatory surveillance systems for specific infections. Surveillance, involving the measurement and reporting of infections according to agreed definitions and with timely feedback, makes problems visible and hence actionable. Surveillance is now part of day-to-day life in clinical areas. For organisations, data on their particular infection rates and their own problems are critical in stimulating action, particularly when teams can identify that they are performing poorly in comparison with similar trusts.
The available data suggest that some infections (such as MRSA bloodstream infections) have shown a welcome reduction, but at the same time others have risen in number. Since these latter infections are not in the national spotlight, their rise has almost gone unnoticed, particularly at the hospital level. Care needs to be taken to ensure that all locally relevant HCAIs are monitored in hospitals, not just those subject to mandatory surveillance. At the national level, measures also need to be appropriate and able to change in response to the shifting epidemiology of infectious diseases.
We also know now that at the trust level, understanding IPC needs a wider set of data. Recent research has identified that a suite of organisational process and outcome indicators need to be assessed in order to monitor IPC performance (Table 1). These indicators cover such issues as the effective organisation of IPC at a hospital level; measures of how crowded or busy a hospital is from data on bed occupancy and staffing levels; how many temporary members of staff are employed; levels and take-up of education and training in IPC; the findings from regular audits against agreed guidelines; and – recognising the role of a positive organisational culture in good IPC – some regular measures of safety culture.
Table 1: Recommended organisational components for effective Infection prevention and control
- Effective organisation of IPC at a hospital level.
- Effective bed occupancy, appropriate staffing and workload, minimal use of pool/ agency nurses.
- Sufficient availability of and easy access to materials and equipment, optimisation of ergonomics.
- Use of guidelines in combination with practical education and training.
- Education and training (involves front-line staff and is team- and task-oriented).
- Organising audits as a standardised and systematic review of practice with timely feedback.
- Participating in prospective surveillance and offering active feedback, preferably as part of a network.
- Implementing IPC programmes following a multimodal strategy, including tools such as bundles and checklists developed by multidisciplinary teams, and taking into account local conditions (and principles of behavioural change).
- Identifying and engaging champions in the promotion of intervention strategies.
- Promoting positive organisational culture by fostering working relationships and communication across units and staff groups.
IPC is now embedded in the regulatory structures governing NHS care in England, with oversight by the Care Quality Commission. It was clear in our research that this external regulatory pressure has been felt at all levels in NHS organisations and is seen by many in the case study organisations as having stimulated action that has improved care for patients. The external emphasis on HCAI was reported to have brought about a new shared acceptance of the importance of IPC and the need for it to be a collective responsibility. Given the figures for infection in the wider population (see section 1), in the context of the restructured NHS in England, this shared ownership now needs to extend beyond the hospital setting to primary care and the clinical commissioning groups.
Regulation has been important in keeping IPC central to the NHS agenda, particularly for trust boards. However, too narrow a focus or too harsh a regime can have unintended consequences, including the neglect of other important infections; for this reason, future inspection and regulation needs to be designed well, for example, taking into account specific local infection risks that are relevant for that trust.
Keeping patients and the public informed
The national media has continued to take an interest in infection prevention and in infections more generally. The influence of the media on patient perceptions has been reported in many research papers. In our interviews, members of staff discussed both the positive and negative impacts of the media on IPC practices in their hospital. Some felt that media coverage of infections and outbreaks was unhelpful, but some also reported that the raised profile of IPC helped to drive up standards. Our research found that patients took a more holistic view of safety in hospitals than just MRSA rates when able to access this information.
Care bundles
A key principle underpinning the government’s strategy on HCAIs is that clinical procedures performed on patients should be done correctly and with appropriate infection control on every occasion. The care bundle approach, which involves assembling evidence-based or logical actions into a group of tasks that should all be performed consistently for specific activities, has been seen as key to this and has been widely adopted.
Research has shown that using care bundles has benefits when used as part of a wider multimodal improvement programme involving all aspects of what has been shown to work.
In our research hospitals, participants appreciated the clarity about expected practices that care bundles bring, but only if the practices are evidence-based and continue to be updated as new knowledge is discovered.
Screening and vaccination strategies
Research suggests that universal admission screening for MRSA is associated with decreased colonisation, MRSA BSI and mortality. These studies indicate that effective infection prevention approaches need to consider the entire health care economy in order to be effective and sustainable. Comprehensive strategies to reduce carriage and the clinical reservoir of MRSA are required if progress towards zero preventable BSIs for all MRSA BSIs is to be achieved.
Screening on or prior to admission is beneficial but can require a lot of resources. If screening is to be carried out routinely for organisms of particular concern, then it is crucial that effective structures are introduced within the organisation to minimise the impact on nurses and infection control practitioners. , In parallel, the isolation capacity of the hospital must be considered and managed, as the screening will increase the isolation demand. This is becoming a particularly pertinent issue for acute trusts, as the NHS is expected to deliver on the Public Health England toolkit for the management and control of Carbapenemase-producing Enterobacteriaceae (CPE), a multi-resistant bacteria of significant global concern.
Vaccination is important for IPC, both for patients and people working in health care. It prevents important infections such as measles, which can be very dangerous to some vulnerable patients. Hepatitis B vaccination can protect people working in health care from acquiring the virus from patients they care for and flu vaccination reduces staff sickness and resulting ward staffing absences during flu outbreaks. Vaccination also plays an important role in reducing antibiotic usage.
The future of infection prevention and control
Drawing on what the research found about the successes and challenges in infection prevention and control (IPC) over the last 15 years, we now move on to look at the lessons learned and offer examples for future directions for effective IPC.
Measures for infection prevention and control need to be appropriate and responsive.
Surveillance has allowed us to understand the extent of the problem of infection in hospitals and has provided motivation to trust boards and clinical teams to engage in IPC. In future, measures of progress need to be appropriate and responsive over time, taking into account new infection threats in hospitals and where infection threats have reduced significantly.
For example:
- National mandatory surveillance must continue for specific infections, but must take into account and respond to new and emerging infection threats.
- Care needs to be taken to ensure that local surveillance is appropriate and all relevant HCAIs are monitored in hospitals, not just those subject to mandatory surveillance, which can skew efforts away from infections that are on the rise or those of local importance. All health care associated BSIs relevant to the local trust should be monitored (for example in neonatal, paediatric or adult ICUs, in haematology or in haemodialysis), and the local surveillance of surgical site infections should reflect the surgery performed at the trust, not just the mandatory surveillance of orthopaedic joint replacement surgery.
Infection prevention and control should remain central to inspection and regulation.
Future inspection and regulation needs to be well designed. It should consider the local infection profile and the local vulnerable patient groups, specialities or risk procedures of a trust and its community, and also include antibiotic stewardship. It must also consider managerial responses to minimising risk so that unintended consequences can be anticipated.
- Strong management support for IPC, including surveillance support, maximising environmental hygiene and isolation capacity should be tangible and clearly evident.
- Antibiotic stewardship activities should be included in any IPC review.
- Local IPC programmes must address HCAI risks of local patient population, specialities and procedures.
All national-level campaigns require an explicit framework underpinning how the campaign is intended to work and must be accompanied by an evaluation strategy.
National campaigns have had an impact across the NHS, but it is impossible to say for certain what component has worked or which aspects have been particularly important in reducing infections in hospitals. It is important that future campaigns are evaluated in order to learn and to be confident in what works and why.
- Future campaigns must address the basics of what is already known to work for IPC.
- Future campaigns must involve the whole health economy.
- All future campaigns must only be launched when there is an evaluation strategy in place.
Hospitals must have the structural and cultural capacity to deliver effective infection prevention and control and antibiotic usage.
Effective IPC requires the underpinning of a healthy organisation with the capacity and capability to learn and to improve on a range of fronts (see Table 1: Recommended organisational components for effective infection prevention and control). There should be a move to introduce indicators of a healthy organisation and these must be linked to the outcomes of IPC.
- A range of process and outcome measures should be used to monitor effective IPC.
- A positive organisational culture should be fostered to ensure IPC is maintained. More work is needed at the trust level to measure and understand this.
Trusts need to ensure that the goals for infection prevention and control and patient safety are integrated and aligned at the clinical front line.
We found that at times those working at the front line were overwhelmed with the requirements for IPC, patient safety and quality improvement initiatives. This can demotivate the exact people who need to remain engaged in IPC. Clearly the goals for IPC and patient safety need to be integrated and aligned so that ‘doing the right thing, in the safest possible way’ is the easiest thing for people to do.
- Trust boards should integrate and align goals for IPC, safety and quality so that ‘doing the right thing’ is clear to all.
Clinical and managerial leaders of infection prevention and control are needed at all levels in the organisation.
The challenges ahead for IPC mean that it must remain central to the NHS agenda and the work in all health care organisations. As such, clinical and managerial leaders of IPC are needed at all levels with demonstrable managerial and clinical commitment. This must also be supported by champions of good practice who lead by example.
- Leaders of IPC should be identified at all levels in a trust and supported appropriately.
- Champions of IPC who lead by example are needed.
Define the role of the public before they become patients.
In the context of often high media coverage, health care organisations need to understand better how the public and patients make sense of publicly available indicators and information. IPC education, awareness of hand hygiene and the optimal use of antibiotics need to be instilled in the wider public before they become patients, since studies have suggested that patients are less likely to get involved at the point of care.
- Patient education needs to start with the wider public understanding and using meaningful indicators and supporting campaigns.
- People working in hospitals should also be able to discuss what infection indicators mean with patients.
A whole health economy approach is needed for infection prevention and control in future.
Bugs don’t differentiate between primary and secondary care or between hospital and home, yet most of the IPC focus to date has been on the hospital. It is time now to move to a whole health economy approach. This will require measures of HCAI that span health sector boundaries and look at the whole patient pathway. Economic analysis using a public health approach in the wider community is needed to fully understand the impact of HCAIs and to enable interventions to be developed that will have most impact for their investment.
- The future focus of IPC should be across the whole health economy, promoting joined up working.
- Measures of HCAI prevention activity and antibiotic stewardship should be developed that span health sector boundaries.
- Economic analysis of IPC needs to have a system-level approach in order to understand the full impact of HCAIs and to target interventions.
In this report we have set out the findings from our research study and have drawn out the lessons for the future of infection prevention and control (IPC). Tremendous progress in IPC has been made in hospitals since 2000, but there are many areas that still need to be understood better. For example, how to improve screening strategies and how to design and implement patient pathways that minimise infection transmission risk in hospitals. A new approach to research in IPC is needed, with analysis aiming to explicitly consider multimodal factors. Importantly, we must learn from other countries – not just about what works in IPC, but also what doesn’t.
The central message is that we cannot take our eye off the IPC ball. Many challenges lie ahead, and IPC must remain central to clinical care and to the priorities of the NHS. Many of the successes to date have been hard won and if the focus on IPC was reduced in any way, it would not be long before the numbers would rise again. Furthermore, while attention has been focused on MRSA BSI and CDI, the numbers of other infections, such as those caused by increasingly resistant bacteria and E. coli , have been rising. Therefore, effective antibiotic stewardship activity needs to be part of any IPC programme. It is vital to continuously monitor and be alert, responsive and adaptive to all infections in hospitals, and across all health and social care settings and the community, while retaining best practice in IPC.
Appendix 1: Research methods
This report is based on a mixed-method study comprising reviews of the literature, qualitative case studies and a user consultation event. An integrated deductive-inductive approach to analysis was used. A multi-stakeholder advisory board provided guidance throughout the study process. Members included: patients’ representatives, health care practitioners and experts in IPC, health systems, psychology, sociology and organisational analysis.
The literature review was designed to examine peer-reviewed UK literature aimed at reducing HCAIs and improving health care workers’ practices relating to IPC in hospitals, during the period between January 2000 and January 2013. It focused on practices of IPC, and therefore excluded consideration of antibiotic stewardship and studies that were primarily basic science, microbiological or epidemiological in character, with no implications for practice. An extensive search of databases using specially designed search strategies was conducted. All types of study design were included. These searches identified 343 candidate articles which were screened according to the inclusion and exclusion criteria, and quality assessed, resulting in 47 articles available for analysis. A scoping review of the international peer-reviewed and grey literature provided the range of indicators recommended to assess IPC performance. This informed a scoping exercise to identify the availability and use of data to monitor HCAI and of indicators of IPC performance in UK hospital trusts.
The case studies included two purposively sampled NHS trusts, one in the north and one in the south of England. The two trusts provided different contexts: a teaching hospital NHS trust and a university hospital foundation trust. Interviews with 41 members of staff were conducted in the two hospitals, with the aim of understanding the views and experiences of those with responsibility for IPC from front-line staff to executive roles. The qualitative analysis followed an integrated approach where a ‘start-up’ list of themes from the reviewed literature was used followed by an inductive exploration of the data.50 The analysis also drew on data from previous research conducted by the project team, specifically the Lining Up study (Health Foundation), and two large-scale innovation adoption studies (Department of Health, and Health Services and Delivery Research). This previous research sample included the two case study sites sampled here, allowing for a longitudinal perspective.
User views were sought through a public consultation event facilitated by the research team in collaboration with Opinion Leader and the Patients Association. A sample of 15 carers and 26 patients was recruited from across London. Recruitment was by quota sampling on ethnicity, and satisfaction level with care received by self or in capacity as a carer. Data collection was via group interviews, self-completion questionnaires and notes from direct observation. Group discussions were audio recorded, transcribed and analysed by the research team and the patient representative on the steering group (who also attended the event as an observer).
For more information, including details of all the papers included in the literature review, see www.health.org.uk/hcai
- Department of Health. What is a healthcare associated infection? Department of Health, 2012. Available from: http://webarchive.nationalarchives.gov.uk/20120118164404/http://hcai.dh.gov.uk/reducinghcais/hcai/ (accessed 29.09.2015).
- Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control 1988;16(3):128–140.2.
- Emori TG, Edwards JR, Culver DH, Sartor C , Stroud LA , Gaunt EE , et al. Accuracy of reporting nosocomial infections in intensive-care-unit patients to the National Nosocomial Infections Surveillance System: a pilot study. Infect Control Hosp Epidemiol. 1998;19(5):308–316.3.
- Anderson DJ, Podgorny K, Berríos-Torres SI, Bratzler DW, Dellinger EP, Greene L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infection Control and Hospital Epidemiology. 2014;35(06);605–627.
- Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32(7):687–699.
- Report by the Comptroller and Auditor General – HC 230 session 1999–2000. The management and control of hospital acquired infection in acute NHS Trusts in England. London: The Stationery Office, 2000. Available from: www.nao.org.uk/wp-content/uploads/2000/02/9900230.pdf (accessed 29.09.2015).
- Department of Health. Winning ways: working together to reduce healthcare associated infection in England. London: Department of Health, 2003. Available from: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4064689.pdf (accessed 29.09.2015).
- Department of Health. Towards cleaner hospitals and lower rates of infection: a summary of action. London: Department of Health, 2004. Available from: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4085861.pdf (accessed 29.09.2015).
- Taylor K. Improving patient care by reducing the risk of hospital acquired infection: a progress report by the National Audit Office. Br J Infect Control. 2004;5(5):4-5.
- BBC News. MRSA target ‘likely to be missed’. 11 July 2007. Available from: http://news.bbc.co.uk/1/hi/6249149.stm (accessed 29.09.2015).
- MailOnline. MRSA – It’s even worse than you think. 15 January 2007. Available from: www.dailymail.co.uk/health/article-429027/MRSA--Its-worse-think.html#ixzz3nDGg7lXE (accessed 29.09.2015).
- Templeton, S-K, Leonard S. Health secretary Reid admits ward bug killed his mother. The Sunday Times. London: Timesonline. 17 April 2005. Available from: www.thesundaytimes.co.uk/sto/news/uk_news/article88938.ece (accessed 29.09.2015).
- Washer P, Joffe H. The ‘hospital superbug’: social representations of MRSA. Soc Sci Med. 63(8):2141-2152.
- Boyce T, Murray E, Holmes A. What are the drivers of the UK media coverage of meticillin-resistant Staphylococcus aureus, the inter-relationships and relative influences? J Hosp Infect. 2009;73( 4):400-407. doi: 10.1016/j.jhin.2009.05.022
- Kingdon JW. Agendas, Alternatives, and Public Policies. Boston: Little, Brown, 1984.
- Dixon-Woods M, Bosk CL, Aveling EL, Goeschel CA, Pronovost PJ. Explaining Michigan: developing an ex-post theory of a quality improvement program. Milbank Q. 2011;89(2):167-205.
- Public Health England (formerly Health Protection Agency). Results from the mandatory surveillance of MRSA bacteraemia. Public Health England, 2014. Available from: http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1233906819629 (accessed 29.09.2015)
- Public Health England. Statistics – MRSA bacteraemia: annual data. Gov.uk, 2015. Available from: https://www.gov.uk/government/statistics/mrsa-bacteraemia-annual-data (accessed 29.09.2015).
- Chief Medical Officer, Deputy NHS Chief Executive. Extension of mandatory surveillance to E. coli bloodstream Infections – June 2011. London: Department of Health, 2011. Available from: http://webarchive.nationalarchives.gov.uk/20140714084352/https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215619/dh_126221.pdf (accessed 29.09.2015).
- Healthcare Commission. Investigation into outbreaks of Clostridium difficile at Stoke Mandeville Hospital, Buckinghamshire Hospitals NHS Trust. London: Commission for Healthcare Audit and Inspection, 2006. Available from: www.buckinghamshirehospitals.nhs.uk/healthcarecommision/HCC-Investigation-into-the-Outbreak-of-Clostridium-Difficile.pdf (accessed 29.09.2015).
- Public Health England (formerly Health Protection Agency). Results from the mandatory Clostridium difficile reporting scheme. Public Health England, 2014. Available from: http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1195733750761 (accessed 29.09.2015).
- Public Health England. Statistics – Clostridium difficile infection: annual data. Gov.uk, 2015. Available from: www.gov.uk/government/statistics/clostridium-difficile-infection-annual-data (accessed 29.09.2015).
- Davies SC. Annual report of the Chief Medical Officer, Volume Two, 2011, Infections and the rise of antimicrobial resistance. London: Department of Health, 2013. Available from: www.gov.uk/government/uploads/system/uploads/attachment_data/file/138331/CMO_Annual_Report_Volume_2_2011.pdf
- Public Health England. Statistics – MSSA bacteraemia: annual data. Gov.uk, 2015. Available from: www.gov.uk/government/statistics/mssa-bacteraemia-annual-data (accessed 29.09.2015).
- Public Health England. Statistics – Escherichia coli (E. coli) bacteraemia: annual data. Gov.uk, 2015. Available from: www.gov.uk/government/statistics/escherichia-coli-e-coli-bacteraemia-annual-data (accessed 29.09.2015)
- World Health Organization. The burden of health care-associated infection worldwide: a summary. Geneva: World Health Organization, 2010. Available from: www.who.int/gpsc/country_work/summary_20100430_en.pdf (accessed 29.09.2015).
- Health Protection Agency. English national point prevalence survey on healthcare-associated infections and antimicrobial use, 2011: preliminary data. London: Health Protection Agency, 2012. Available from: www.gov.uk/government/uploads/system/uploads/attachment_data/file/331871/English_National_Point_Prevalence_Survey_on_Healthcare_associated_Infections_and_Antimicrobial_Use_2011.pdf (accessed 29.09.2015).
- Report by the Comptroller and Auditor General – HC 560 session 2008–2009. Reducing healthcare associated infection in hospitals in England. London: The Stationery Office, 2009. Available from: www.nao.org.uk/wp-content/uploads/2009/06/0809560.pdf (accessed 29.09.2015).
- Dixon-Woods M, Leslie M, Tarrant C, Bion J. Lining up: how is harm measured? London: Health Foundation, 2013. Available from: www.health.org.uk/sites/default/files/LiningUpHowIsHarmMeasured.pdf (accessed 29.09.2015).
- Kyratsis Y, Ahmad R, Holmes A. Technology adoption and implementation in organisations: comparative case studies of 12 English NHS trusts. BMJ Open. 2012;2: e000872. doi:10.1136/bmjopen-2012-000872.
- Kyratsis Y, Ahmad R, Hatzaras K, Iwami M, Holmes A. Making sense of evidence in management decisions: the role of research-based knowledge on innovation adoption and implementation in healthcare. Health Serv Deliv Res. 2014;2(6). doi: 10.3310/hsdr02060.
- Patient Safety First. About Patient Safety First. Available from: www.patientsafetyfirst.nhs.uk/Content.aspx?path=/About-the-campaign/ (accessed 29.09.2015).
- Stone SP, Fuller C, Savage J, Cookson B, Hayward A, Cooper B, et al. Evaluation of the national Cleanyourhands campaign to reduce Staphylococcus aureus bacteraemia and Clostridium difficile infection in hospitals in England and Wales by improved hand hygiene: four year, prospective, ecological, interrupted time series study. BMJ. 2012;344: e3005. doi: http://dx.doi.org/10.1136/bmj.e3005.
- Benning A, Dixon-Woods M, Nwulu U, Ghaleb M, Dawson J, Barber N, et al. Multiple component patient safety intervention in English hospitals: controlled evaluation of second phase. BMJ. 2011;342:d199. doi:10.1136/bmj.d199.
- Curran E, Harper P, Loveday H, Gilmour H, Jones S, Benneyan J, et al. Results of a multicentre randomised controlled trial of statistical process control charts and structured diagnostic tools to reduce ward-acquired meticillin-resistant Staphylococcus aureus: the CHART Project. J Hosp Infect. 2008;70(2):127-135. doi:10.1016/j.jhin.2008.06.013.
- Koteyko N, Carter R. Discourse of ‘transformational leadership’ in infection control. Health. 2008;12(4):479-499. doi:10.1177/1363459308094421.
- Ahmad R, Brewster L, Castro-Sanchez E, Dixon-Woods M, Holmes A, Zingg W. Leading for sustained change in infection control – do we need external censure or distributed leadership? Health Services Research Network (HSRN) conference – Poster presentation. Nottingham, June 2014.
- Hendy J, Barlow J. The role of the organizational champion in achieving health system change. Soc Sci Med. 2012;74(3):348-355. doi: 10.1016/j.socscimed.2011.02.009.
- Septimus E, Weinstein RA, Perl TM, Goldmann DA, Yokoe DS. Approaches for preventing healthcare-associated infections: go long or go wide? Infect Control Hosp Epidemiol. 2014; 35(7):797–801.
- Zingg W, Holmes A, Dettenkofer M, Goetting T, Secci F, Clack L, et al. Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. Lancet Infect Dis. 2015;15(2):212–224.
- Ahmad R, Iwami M, Castro-Sánchez E, Husson F, Taiyari K, Zingg W, et al. Defining the user role in infection control. Journal of Hospital Infection. 2015, In press.
- Duerden B, Fry C, Johnson AP, Wilcox MH. The control of methicillin-resistant Staphylococcus aureus blood stream infections in England. Open Forum Infectious Diseases 03/2015; 2(2). doi: 10.1093/ofid/ofv035.
- Duerden BI. MRSA: why have we got it and can we do anything about it? Eye 2012; 26: 218–221.
- NHS England. Guidance on the reporting and monitoring arrangements and post infection review process for MRSA bloodstream infections from April 2014 version 2. London: NHS England, 2014. Available from: www.england.nhs.uk/wp-content/uploads/2014/04/mrsa-pir-guid-april14.pdf (accessed 29.09.2015).
- Lee YJ, Chen JZ, Lin HC, Liu HY, Lin SY, Lin HH, et al. Impact of active screening for methicillin-resistant Staphylococcus aureus (MRSA) and decolonization on MRSA infections, mortality and medical cost: a quasi-experimental study in surgical intensive care unit. Crit Care. 2015;19(1):143. doi: 10.1186/s13054-015-0876-y.
- Chaberny IF, Schwab F, Ziesing S, Suerbaum S, Gastmeier P. Impact of routine surgical ward and intensive care unit admission surveillance cultures on hospital-wide nosocomial methicillin-resistant Staphylococcus aureus infections in a university hospital: an interrupted time-series analysis. J. Antimicrob Chemother. 2008;62(6):1422-1429. doi:10.1093/jac/dkn373.
- Vella V, Gharbi M, Moore LSP, Robotham J, Davies F, Brannigan E, et al. Screening suspected cases for carbapenemase-producing Enterobacteriaceae, inclusion criteria and demand. J Infect. 2015;71(4):493-495. doi: http://dx.doi.org/10.1016/j.jinf.2015.06.002 .
- Public Health England. Acute trust toolkit for the early detection, management and control of carbapenemase-producing Enterobacteriaceae. London: Public Health England, 2013. Available from: www.gov.uk/government/uploads/system/uploads/attachment_data/file/329227/Acute_trust_toolkit_for_the_early_detection.pdf (accessed 29.09.2015).
- Frieden T. Safe and sound: guarding against health care associated infections. Mediaplanet, 2015. Available from: www.futureofpersonalhealth.com/advocacy/safe-and-sound-guarding-against-health-care-associated-infections . (accessed 29.09.2015).
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758–1772. DOI: 10.1111/j.1475-6773.2006.00684.x.
- Zingg W Castro-Sánchez E Secci F, Edwards R, Drumright L, Sevdalis N, Holmes. A Innovative tools for quality assessment: Integrated Quality Criteria for Review of Multiple Study Designs (ICROMS). Public Health. 2015, in press.
- Fontana A, Frey J. Interviewing: the art of science. In: Denzin NK, Lincoln YS, editor. The handbook of qualitative research. Thousand Oaks, CA: Sage Publications; 1994. pp. 361–376. Available from: http://jan.ucc.nau.edu/~pms/cj355/readings/fontana%26frey.pdf (accessed 29.09.2015).
Share this chapter
- International
- Education Jobs
- Schools directory
- Resources Education Jobs Schools directory News Search

BTEC L3 Health and Social Care - Unit 9 Infection Prevention and Control Resource Pack
Subject: Vocational studies
Age range: 16+
Resource type: Unit of work
Last updated
5 April 2023
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

Complete Unit 9 (Infection Prevention and Control) resource pack including:
- One 117-page workbook which covers the entire unit and all 3 assignments
- Powerpoint slides that follow the workbook (including all learning aims and assignments)
- Assignment guidance, templates and examples
Updated resource pack contains a wide range of research embedded into the powerpoint slides, including examples which can be used throughout all assignments, as required on the assessment criteria.
Tes paid licence How can I reuse this?
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
How can I email you to request your scheme of work?
bethlitchfield
Thank you for your great review, I hope you find it all useful in your lessons. I am happy to send you my scheme of work if you can provide me with an email address?
My email is [email protected]
Empty reply does not make any sense for the end user
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:
- Padres Showing Strong Interest In Garrett Crochet
- White Sox Activate Luis Robert, Place Tommy Pham On IL, Designate Zach Remillard
- Major League Baseball Closes Investigation Involving Shohei Ohtani, Ippei Mizuhara
- Marlins To Designate Avisail Garcia For Assignment
- MLB Issues Lifetime Ban To Tucupita Marcano For Betting On Baseball, Announces Four Other One-Year Suspensions
- Tucupita Marcano Under Investigation For Betting On Baseball
- Hoops Rumors
- Pro Football Rumors
- Pro Hockey Rumors
MLB Trade Rumors
Marlins Outright Eli Villalobos
By Darragh McDonald | June 1, 2024 at 10:55am CDT
TODAY : The Marlins outrighted Villalobos to Triple-A after he cleared waivers, as per MLB.com’s official transactions page. It isn’t yet known if Villalobos will accept the assignment to opt into free agency.
MAY 27 : The Marlins announced that infielder Xavier Edwards has been reinstated from the 60-day injured list and optioned to Triple-A Jacksonville. To open up a spot for him on the 40-man roster, right-hander Eli Villalobos has been designated for assignment.
Edwards, 24, battled a foot infection during Spring Training and began the season on the injured list. He has been playing in rehab games for over a week now and is healthy enough to be activated, though he only played seven games on his rehab so the club will keep him on optional assignment for regular playing time in Jacksonville. Though he won’t be joining the active roster, the Fish needed to make a corresponding 40-man move since Edwards was on the 60-day injured list, which will nudge Villalobos off his spot.
The right-handed Villalobos is about a month away from his 27th birthday. The Marlins claimed him off waivers from the Pirates last June but then passed him through waivers about a week later. He got his 40-man roster spot back earlier this month and was able to make his major league debut. He made three appearances for the Marlins, allowing one earned run in 4 1/3 innings, before being optioned back to Jacksonville about two weeks ago.
In addition to that small sample of big league action, Villalobos has also thrown 18 innings over 13 Triple-A appearances this year with a 4.50 earned run average. He has struck out 26.5% of batters faced at that level and kept 44.7% of batted balls on the ground, but he’s also walked 13.3% of hitters that have stepped to the plate.
That has generally been the recipe for Villalobos. Dating back to the start of 2021, he has tossed 196 innings in the minors with a 3.72 ERA. His 29.4% strikeout rate in that stretch is quite strong but he’s also given free passes at a 12.2% rate.
The Marlins will have a week to trade Villalobos or pass him through waivers. He can still be optioned for the rest of this year and one additional season, which could perhaps give him appeal for a club that is intrigued by the strikeouts and willing to wait to see if the control improves. If Villalobos were to pass through waivers unclaimed, he would have the right to elect free agency by virtue of his previous outright.
10 Comments
Huizinga backed off and unloaded his ’97ws champs when they still couldn’t draw fans in to watch. That team was mostly bought and paid for FA, not brought up talent and yeah.. I’ve always had a liking for the fish since they became a new franchise, less than handful of years before the title.
Alex fernandez was the only SP on that team (worth 2c) who Miami brought up. Pen was more of the same. Lineup included (best players) Sheffield, bobby bonilla, moises Alou.
best “home” talent wasn’t from the milb system they bad, but still wildly popular ‘the niner”.
that ’97 team was an NL version of george steinbrenner, buying every FA out there, then breaking it down less than a yr later in selloffs.
One of the most disapointing things was huizinga selling off a WS winner, into a team in ’98 barely winning 50g.
Always been hard to watch/root for this team because of lousy ownership and several awful gm’s along the way. That castoff title in ’97 has never been anything to brag about.

lol you literally just danced around the fact that the best pitcher in the Marlins rotation during that 97 WS run was Livan Hernandez, a homegrown player. As was Tony Saunders. Alex Hernandez didn’t even play in the playoffs. Most of their bullpen were players they either signed or acquired well before the player debuted in the majors. Even Nenn they acquired when he was an unknown.
They “bought their World Series” as much as any team in the modern era has bought theirs. Their 2003 WS team was a thing of beauty. I like the Marlins. I hate their ownerships and front offices. But I like the team and the players.
Like the fish as well, moreso than local TB Rays franchise, partly because thought huizinga was going to attempt turning them into long term winners. Only fans never saw that. Odd (2) titles, spread out 6y and totally different players.
Hernandez and saunders did both have nice post season runs, but were mostly mia for the regular season. kevin brown and alex fernandez drove that pitching thru 162g, not the to be traded and 1yr back end starter/rest of career quad A player tony saunders.
This also wasn’t some team, like ’07 rockies which went on a close to 2m streak of beating everyone. They were solid throughout that magical ’97y and guys, like fernandez and brown reason they make the post season.
Hutton and rich walz 9when he was there) used to tell things straight. Now, with hutton back after 5y layoff has become more of a cheerleader than honest on the air. disapointed in him, tho will agree when the jeter run team and ownership took over? it was a dark stain on that franchise. no wonder the nyy never gave him a job inside their front office.. it was way beyond him.
Id like to hear harry carry pronounce Villalobos on air
Fan base doesn’t show up to games unless the weather is fair
Funny stuff citazen. how many teams are ate up with fair weather fans? Miami never had much hope of drawing, especially (then) playing in joe robbie aka awful football stadium.
Tampa same way, only no matter win/lose they draw 10-15k max.
Cubs, giants, Yankees Red Sox draw well no Matter what record. Miami can’t even give tickets away in the new stadium.
If Eli Villalobos is related to Heitor Villa-Lobos, maybe he can make a living playing classical guitar once his MLB career is over. And maybe if he listens enough to Bachianas Brazileiras and finds inner vibrational roots, Eli can improve his control and extend his MLB tenure.
(I do not expect anyone to understand my comment. Just showing gratitude to my Music History mentors and to the resonances of beisbol.)
One of these teams should move to Nashville!!
Eli’s going, hide your heart girl
Leave a Reply Cancel reply
Please login to leave a reply.
Log in Register
- Feeds by Team
- Commenting Policy
- Privacy Policy
MLB Trade Rumors is not affiliated with Major League Baseball, MLB or MLB.com

Username or Email Address
Remember Me

COMMENTS
Methods. CDC healthcare infection control guidelines 1-17 were reviewed, and recommendations included in more than one guideline were grouped into core infection prevention practice domains (e.g., education and training of HCP on infection prevention, injection and medication safety). Additional CDC materials aimed at providing general infection prevention guidance outside of the acute care ...
Infection prevention and control (IPC) is a practical, evidence-based approach preventing patients and health workers from being harmed by avoidable infections. Effective IPC requires constant action at all levels of the health system, including policymakers, facility managers, health workers and those who access health services. IPC is unique in the field of patient safety and quality of care ...
Actions to ensure reliable improvements in infection prevention and control (IPC) practices Aide-memoire Environmental cleaning, waste and linen management. Actions to ensure reliable improvements in infection prevention and control (IPC) practices What success might look like after using the aide-memoires Additional associated resources Annex ...
Practice Implications. The ability to put knowledge into action is one way to define competence. Core Infection Prevention Control Practices for Safe Healthcare Delivery in All Settings [], is organized into eight distinct, yet interrelated, infection prevention and control domains.A list of Core Practices is shown in Table 1.The first four domains relate to the organizational infrastructure ...
Infection prevention and control routine practices should be used to prevent exposure to blood, body fluids, secretions, excretions, mucous membranes, non-intact skin, or soiled items (PIDAC, 2012). All clients can potentially be infectious; thus, it is important to consider which routine practices to follow and why.
Infection control refers to the policy and procedures implemented to control and minimize the dissemination of infections in hospitals and other healthcare settings with the main purpose of reducing infection rates. Infection control as a formal entity was established in the early 1950s in the United States. By the late 1950s and 1960s, a small number of hospitals began to recognize healthcare ...
Introduction to infection prevention & control 4 Each year, hundreds of millions of people contract an infection related to healthcare : HAI rates range from 5-7 % in high income countries in Europe to an estimated 10-20 % in low-resources settings. The most frequent HAI are: • Surgical Site Infections (SSI)
Overview: Infection prevention and control (IPC) is an applied discipline that affects all patient care activities in healthcare settings. IPC, including prevention of antimicrobial resistance (AMR), is an essential component of healthcare quality and patient safety. In this module you will learn how and why healthcare-associated infections ...
Shaheen Mehtar (Infection Control Africa Network, South Africa), Nico T. Mutters (University of Freiburg, ... IPC is not the primary assignment of this professional but, among others, he/she may undertake tasks in support to IPC, including for example supporting implementation of IPC practices;
ANA and the Centers for Disease Control and Prevention (CDC) have teamed up with a number of Nursing Specialty Organizations to educate and train nurses on infection control. The goals of the training programs developed through the NICE Network are to improve adherence to infection prevention and control practices and enhance the confidence of nurses to care for patients with Ebola and other ...
Welcome to your NCLEX practice quiz on Safety and Infection Control. According to the NCLEX-RN test plan, about 9 to 15% of questions will come from this subcategory that includes content about the "nurse's ability required to protect clients, families, and healthcare personnel from health and environmental hazards." Good luck, and hope you will learn a lot from this quiz.
Page 58 of the CDC document discusses sterilization. Provide a definition of sterilization. -Involves the use of an agent capable of killing bacterial spores and all other microorganisms when used in sufficient concentration under suitable conditions. Define high-level disinfection (page 99 in the glossary) Study with Quizlet and memorize ...
Infection Control Levels of Prevention Delegation The performance of good and frequent hand washing is a very important step to help prevent the spread of pathogens. Keeping the body and the environment clean helps to prevent tje spread of germs. Nurse to perform frequent and effective hand-hygiene before and after care
The order it should be performed is as follows: Wet hands and wrists under running water; lather hands with liquid soap; wash hands, including fingers and palms, as well as wrists, with a firm circular motion and friction; wash for 15 to 30 seconds; rinse wrists and hands completely; and dry hands thoroughly with paper towels.
Knowledge Assignment: Infection Control It is important for the nurse to provide information and clarity to the patient and family. The nurse must update the family and patient about their health and how the patient has been doing. According to Healthy People, "1 out of every 25 hospitalized patients are affected by a healthcare associated ...
Infection Prevention and Control. Assignment 2. Hand hygiene is an important practice to prevent the spread of infection. For 1 day (away from school or work), make a list of the times and places you wash your hands. At the end of the day, make a second list of times you should have washed your hands and did not.
According to the Centers for Disease Control and Prevention (CDC), standard precautions are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered. These precautions are based on the principle that all blood ...
Infection control has been high on the political agenda and on the agenda of the NHS in England for the last 15 years. During this time there have been many successes, not least the reduction in MRSA bloodstream infections (BSIs) and cases of Clostridium difficile infection. Though these successes should be celebrated, it is important not to become complacent.
AJIC covers key topics and issues in infection control and epidemiology.Infection control professionals, including physicians, nurses, and epidemiologists, rely on AJIC for peer-reviewed articles covering clinical topics as well as original research.As the official publication of the Association for Professionals in Infection Control and Epidemiology (APIC) Opens in new window AJIC is the ...
Complete Unit 9 (Infection Prevention and Control) resource pack including: One 117-page workbook which covers the entire unit and all 3 assignments; Powerpoint slides that follow the workbook (including all learning aims and assignments) Assignment guidance, templates and examples
Assignment Under supervision, with latitude for independent judgment, performs supervisory and/or moderately ... Maintains infection control files (rounds) and generates correspondence and follows ...
Find Medicare-approved providers near you & compare care quality for nursing homes, doctors, hospitals, hospice centers, more. Official Medicare site.
Knowledge Assignment: Infection Control As a nurse it is my responsibility to provide answers for the patient and the patient's family about their care in the hospital and any changes that might happen throughout their stay in the hospital. According to Healthy People, "1 out of every 25 hospitalized patients are affected by a healthcare ...
To open up a spot for him on the 40-man roster, right-hander Eli Villalobos has been designated for assignment. Edwards, 24, battled a foot infection during Spring Training and began the season on ...
She presented an optional assignment. Professor d'Aquino said that DePaul violated her academic freedom with the termination. Classroom instruction comes with a particular set of academic freedom considerations. Teaching is a fundamental part of being a faculty member, and the classroom is an arena they're supposed to control day-to-day.
A third person in the US has tested positive for H5 bird flu in connection to an ongoing outbreak in dairy cattle, the Michigan Department of Health and Human Services said Thursday. This is the ...