Featured Topics
Featured series.
A series of random questions answered by Harvard experts.

Explore the Gazette
Read the latest, how to fight depression faster..

Plastics are everywhere, even in our bodies

Threat of mosquito-borne diseases rises in U.S. with global temperature

“When my son was diagnosed [with Type 1], I knew nothing about diabetes. I changed my research focus, thinking, as any parent would, ‘What am I going to do about this?’” says Douglas Melton.
Kris Snibbe/Harvard Staff Photographer
Breakthrough within reach for diabetes scientist and patients nearest to his heart
Harvard Correspondent
100 years after discovery of insulin, replacement therapy represents ‘a new kind of medicine,’ says Stem Cell Institute co-director Douglas Melton, whose children inspired his research
When Vertex Pharmaceuticals announced last month that its investigational stem-cell-derived replacement therapy was, in conjunction with immunosuppressive therapy, helping the first patient in a Phase 1/2 clinical trial robustly reproduce his or her own fully differentiated pancreatic islet cells, the cells that produce insulin, the news was hailed as a potential breakthrough for the treatment of Type 1 diabetes. For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the challenges ahead, and the personal side of his research. The interview was edited for clarity and length.
Douglas Melton
GAZETTE: What is the significance of the Vertex trial?
MELTON: The first major change in the treatment of Type 1 diabetes was probably the discovery of insulin in 1920. Now it’s 100 years later and if this works, it’s going to change the medical treatment for people with diabetes. Instead of injecting insulin, patients will get cells that will be their own insulin factories. It’s a new kind of medicine.
GAZETTE: Would you walk us through the approach?
MELTON: Nearly two decades ago we had the idea that we could use embryonic stem cells to make functional pancreatic islets for diabetics. When we first started, we had to try to figure out how the islets in a person’s pancreas replenished. Blood, for example, is replenished routinely by a blood stem cell. So, if you go give blood at a blood drive, your body makes more blood. But we showed in mice that that is not true for the pancreatic islets. Once they’re removed or killed, the adult body has no capacity to make new ones.
So the first important “a-ha” moment was to demonstrate that there was no capacity in an adult to make new islets. That moved us to another source of new material: stem cells. The next important thing, after we overcame the political issues surrounding the use of embryonic stem cells, was to ask: Can we direct the differentiation of stem cells and make them become beta cells? That problem took much longer than I expected — I told my wife it would take five years, but it took closer to 15. The project benefited enormously from undergraduates, graduate students, and postdocs. None of them were here for 15 years of course, but they all worked on different steps.
GAZETTE: What role did the Harvard Stem Cell Institute play?
MELTON: This work absolutely could not have been done using conventional support from the National Institutes of Health. First of all, NIH grants came with severe restrictions and secondly, a long-term project like this doesn’t easily map to the initial grant support they give for a one- to three-year project. I am forever grateful and feel fortunate to have been at a private institution where philanthropy, through the HSCI, wasn’t just helpful, it made all the difference.
I am exceptionally grateful as well to former Harvard President Larry Summers and Steve Hyman, director of the Stanley Center for Psychiatric Research at the Broad Institute, who supported the creation of the HSCI, which was formed specifically with the idea to explore the potential of pluripotency stem cells for discovering questions about how development works, how cells are made in our body, and hopefully for finding new treatments or cures for disease. This may be one of the first examples where it’s come to fruition. At the time, the use of embryonic stem cells was quite controversial, and Steve and Larry said that this was precisely the kind of science they wanted to support.
GAZETTE: You were fundamental in starting the Department of Stem Cell and Regenerative Biology. Can you tell us about that?
MELTON: David Scadden and I helped start the department, which lives in two Schools: Harvard Medical School and the Faculty of Arts and Science. This speaks to the unusual formation and intention of the department. I’ve talked a lot about diabetes and islets, but think about all the other tissues and diseases that people suffer from. There are faculty and students in the department working on the heart, nerves, muscle, brain, and other tissues — on all aspects of how the development of a cell and a tissue affects who we are and the course of disease. The department is an exciting one because it’s exploring experimental questions such as: How do you regenerate a limb? The department was founded with the idea that not only should you ask and answer questions about nature, but that one can do so with the intention that the results lead to new treatments for disease. It is a kind of applied biology department.
GAZETTE: This pancreatic islet work was patented by Harvard and then licensed to your biotech company, Semma, which was acquired by Vertex. Can you explain how this reflects your personal connection to the research?
MELTON: Semma is named for my two children, Sam and Emma. Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that’s when I changed my research plan. And my daughter, who’s four years older than my son, became diabetic about 10 years later, when she was 14.
When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs develop. I changed my research focus, thinking, as any parent would, “What am I going to do about this?” Again, I come back to the flexibility of Harvard. Nobody said, “Why are you changing your research plan?”
GAZETTE: What’s next?
MELTON: The stem-cell-derived replacement therapy cells that have been put into this first patient were provided with a class of drugs called immunosuppressants, which depress the patient’s immune system. They have to do this because these cells were not taken from that patient, and so they are not recognized as “self.” Without immunosuppressants, they would be rejected. We want to find a way to make cells by genetic engineering that are not recognized as foreign.
I think this is a solvable problem. Why? When a woman has a baby, that baby has two sets of genes. It has genes from the egg, from the mother, which would be recognized as “self,” but it also has genes from the father, which would be “non-self.” Why does the mother’s body not reject the fetus? If we can figure that out, it will help inform our thinking about what genes to change in our stem cell-derived islets so that they could go into any person. This would be relevant not just to diabetes, but to any cells you wanted to transplant for liver or even heart transplants. It could mean no longer having to worry about immunosuppression.
Share this article
You might like.
Hope flags when medications fail, isolating and endangering patients. Backed by a major grant, 2 Harvard scientists are focused on reducing the distance between diagnosis and recovery.
We ingest equivalent of credit card per week — how worried should we be? In ‘Harvard Thinking,’ experts discuss how to minimize exposure, possible solutions.

Experts fear more cases of West Nile virus, EEE (and possibly Zika, Dengue fever) as warm seasons get longer, wetter
What happened when a meteorite the size of four Mount Everests hit Earth?
Giant impact had silver lining for life, according to new study
How to apply cool-headed reason to red-hot topics
Michael J. Sandel brings back wildly popular ‘Justice’ course amid time of strained discourse on college campuses
Do phones belong in schools?
Banning cellphones may help protect classroom focus, but school districts need to stay mindful of students’ sense of connection, experts say.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Diabetes articles from across Nature Portfolio
Diabetes describes a group of metabolic diseases characterized by high blood sugar levels. Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes) or by insulin resistance (cells do not respond to insulin; type 2 diabetes).
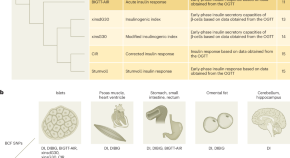
Genetics brings new insight to β-cell function
A meta-analysis of genome-wide association study for eight traits related to pancreatic β-cell function, based on 26,000 individuals, identified 55 independent association signals mapping to 44 loci. This study highlighted new effectors of β-cell function.
- Amélie Bonnefond
- Philippe Froguel
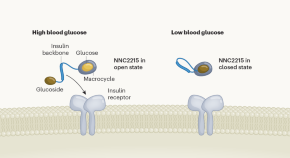
Smart insulin switches itself off in response to low blood sugar
Scientists have engineered a modified insulin that reduces its activity at low glucose levels. This glucose-responsive insulin could prevent people with diabetes from experiencing dangerously low blood glucose.
- David B. Sacks
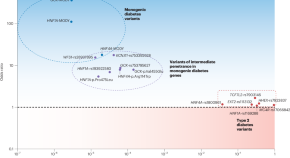

Parsing the spectrum of allelic architectures in diabetes
Distinguishing ordinary diabetes from its monogenic forms has been one of the challenges in optimally managing the disease. Using high-quality imputation of rare variants and large databases, a study now defines the gray zone between the two and lays down a blueprint for objectively evaluating the related variants.
- Constantin Polychronakos
Related Subjects
- Diabetes complications
- Diabetes insipidus
- Gestational diabetes
- Type 1 diabetes
- Type 2 diabetes
Latest Research and Reviews
Metformin in gestational diabetes: physiological actions and clinical applications
This Review outlines the complex actions of metformin in metabolic regulation, providing a plausible basis for its benefits in the management of gestational diabetes mellitus. The authors also critically evaluate the safety profile of metformin use during pregnancy in patients with gestational diabetes mellitus.
- Taitum Mason
- Simon Alesi
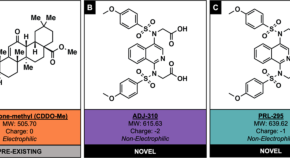
Non-electrophilic NRF2 activators promote wound healing in human keratinocytes and diabetic mice and demonstrate selective downstream gene targeting
- May Barakat
- Luisa A. DiPietro
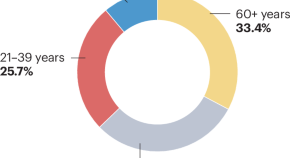
The epidemiology of type 1 diabetes mellitus in older adults
This Review discusses the incidence, prevalence and disease burden of type 1 diabetes mellitus in older adults in diverse geographical regions. The challenges involved in defining and diagnosing type 1 diabetes mellitus in this population and the implications of these challenges for epidemiological studies of this disease are also addressed.
- Dunya Tomic
- Jessica L. Harding
- Dianna J. Magliano
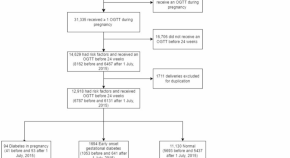
Early pregnancy hyperglycaemia among pregnant women with risk factors for gestational diabetes increases the risk of pregnancy complications
- Ka Wang Cheung
- Tiffany Sin-Tung Au
- Mimi Tin Yan Seto
Diabetic microvascular complications among adults with type 2 diabetes in Adama, central Ethiopia
- Yohannes Mekuria Negussie
- Midekso Sento
- Nesra Mohammed Fati
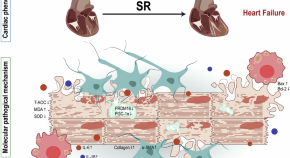
Sleep restriction exacerbates cardiac dysfunction in diabetic mice by causing cardiomyocyte death and fibrosis through mitochondrial damage
- Jingyi Zhang
- Qingfeng Du
News and Comment

‘Smart’ insulin prevents diabetic highs — and deadly lows
In animals, the molecule automatically reduced blood-sugar levels without causing them to dip too much.
Stem cells reverse woman’s diabetes — a world first
Patient is the first person with type 1 diabetes to receive this kind of transplant.
- Smriti Mallapaty

Brain goop that traps hunger neurons drives obesity
A mechanism for metabolic disease is traced to a defective cellular scaffolding that holds together the brain’s hunger cells.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

COMMENTS
In this trial involving participants with type 2 diabetes of less than 10 years’ duration who were receiving metformin and had glycated hemoglobin levels of 6.8 to 8.5%, we compared the...
Type 2 diabetes affects more than 34 million U.S. adults and significantly increases the risk of cardiovascular events, microvascular disease, and premature death. 1 Tight glycemic,...
For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease.
The world has failed to understand the social nature of diabetes and underestimated the true scale and threat the disease poses. The GBD 2021 estimates and the Lancet Global Inequity in Diabetes Series are an urgent call to course correct.
Type 2 diabetes affects approximately 8 percent of adults in the United States. Some risk factors — elevated plasma glucose concentrations in the fasting state and after an oral glucose load,...
Diabetes describes a group of metabolic diseases characterized by high blood sugar levels. Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes) or by insulin resistance...