

Advancing health through innovation
We are transforming the face of pharmacy by leading the convergence of science, healthcare and policy.
The USC Advantage
Forging new frontiers.
Since our founding in 1905, the school has fueled pioneering initiatives in research, education and practice while fostering new generations of leaders. Our impact includes groundbreaking laboratory discoveries, novel regulatory approaches, meaningful health policy reforms, and transformative collaborations in medication management and chronic disease prevention. Curricular advances include launching the first PharmD, regulatory science doctorate and healthcare decision analysis programs, among many others.
8th most innovative university, citing research from the school’s Papadopoulos Lab (ranked by Reuters)
4 american pharmacists awards pinnacle awards (a national record).

Lifelong, Worldwide
The moment you join the school, you become part of the renowned Trojan Family—a vast, supportive international network. Our alumni hold leadership roles at major pharmaceutical companies, government agencies, top medical centers, NASA and Jet Propulsion Lab, and many other dynamic environments—and are dedicated to helping USC students and fellow graduates. We give you a world of opportunity.
20K+ global USC pharmacy alumni
32 usc pharmacy global partners, 8 usc international offices.

Building a Healthier Society
Through health fairs, community clinic partnerships, public health campaigns and unparalleled collaborations to manage chronic disease, the school’s students, faculty and alumni demonstrate an ongoing commitment to improving the health of the community we serve. And through our Science, Technology and Research (STAR) program, we help new generations of underrepresented high school students pursue careers in the life sciences.
50+ annual health fairs
600+ graduates of our star program, 10 fotonovelas in multiple languages on public health issues.

Full Spectrum
Our programs span the entire pharmaceutical continuum — from discovery and development to regulation and from translation to patient care and outcomes.
Planning for a New USC Pharmacy in South Los Angeles
Soon after Vassilios Papadopoulos became dean of the USC School of Pharmacy in October 2016, he approached Raffi Svadjian, executive director of community pharmacies, with a bold idea. What about launching a new pharmacy in South Los Angeles, an underserved area for pharmaceutical care?

Our school by the numbers
Private Pharmacy School
Different Degree Programs
Our school by the numbers.

In the Heart of Los Angeles
Our location on a major academic medical center campus in the heart of a vibrant city offers extraordinary opportunities for internships, community engagement and mentoring. With 140 countries represented and 224 languages spoken, Los Angeles is filled with diverse cuisine, cultural institutions and recreational offerings. L.A. is home to more theaters, music venues and museums than any other U.S. city, and you can ski, hike and surf in nearby beaches and mountains.
USC Mann RegSci Gathering at DIA Global Annual Meeting
Tuesday June 18, 2024
Moving Targets 2024: Computational Data-Driven Drug Discovery & Development
Thursday August 22, 2024
2024 USC Mann White Coat Ceremony
Friday August 23, 2024

How did your time at the USC Mann School impact you?
“The scholastic discipline instilled at USC was instrumental in forging my career. Always think of patients first—but with a commercial hat on as well. [The Trojan Family] is a connection that’s always there, and comes into light in the strangest of places and the most fanciful of situations.”
Peter Lassoff
Senior vice president, head of regulatory, medical writing and regulatory intelligence, syneos health, united kingdom.

“The rigorous training I received through the USC Pharmaceutical Sciences PhD program equipped me with a strong foundation in natural product chemistry, microbial genetics and analytical chemistry. This comprehensive background has proven invaluable in my research on unraveling the molecular mechanisms underlying microbe-host interactions.”
Chun-Jun (CJ) Guo
Phd pharmaceutical sciences '14, assistant professor, weill cornell graduate school of medical sciences.

“My USC experiences and mentors enhanced both my technical and soft skills, priming me for my professional career. Their impact on me, professionally and personally, is priceless.”
Meleeneh DerHartunian
Phd, molecular and cellular biology '10, certificate in clinical trial design and management, regulatory science, regulatory documentation team leader, product development, genetech.

“My experiences at USC equipped me with the leadership and clinical skills to propel my career. The lifelong friends and colleagues I made continue to show up in my personal and professional life.”
Clinical Pharmacy Manager and PGY-2 Oncology Residency Program Director Dana-Farber Cancer Institute

It’s all about your health. Four locations to serve you.


Where discoveries are delivered. SM

Groundbreaking Research and Inspired Teaching

Enhancing Knowledge and Discovery

Interdisciplinary Activities Lead to Unsurpassed Discoveries

Healthcare Teams Committed to Outstanding Patient Care

Creating a healthier world, one life at a time, through new science, new pharmacy and new cures
#12 in the nation for best pharmacy school by u.s. news & world report, #1 school of pharmacy in california by cpje pass rates, #1 school of pharmacy in california for residency match rates (phase i), #2 in california #19 nationally by naplex® first-time pass rates, named #1 pharmacy school in the nation by pharmacy technician guide, quick links.

Applications and Important Admissions Dates →

News Archive
View Calendar

Students recite the Oath of a Pharmacist at a white coat ceremony held at Broad Lawn on the USC Health Sciences Campus. Photo by Isaac Mora.
Non-Degree Programs
- Courses of Instruction
Founded in 1905, the USC Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences is the oldest and foremost pharmacy school in Southern California. The school is a national leader known for its progressive curriculum and research excellence. Approximately 50 percent of the practicing pharmacists in Southern California are graduates of USC. The school has an average student body of 755 full-time students in the PharmD program and 417 students pursuing MS, PhD, DRSc and undergraduate degrees in pharmacology and toxicology, pharmaceutical sciences, health economics, regulatory science, healthcare decision analysis and biopharmaceutical marketing. There are 77 full-time faculty and more than 300 part-time and volunteer faculty at the school.
The school occupies state-of-the-art facilities on the USC Health Sciences Campus in metropolitan Los Angeles, adjacent to the Los Angeles General Medical Center (one of the largest teaching hospitals in the country), the USC Norris Cancer Hospital and the Keck Hospital of USC. USC Mann School students receive clinical training at these facilities and many other affiliated hospitals, healthcare clinics, skilled nursing facilities, home healthcare agencies and pharmacies in the Southern California region.
Recognized as one of the most innovative schools of pharmacy, the USC Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences serves as a model for other progressive schools. In 1950, USC was the first to establish a Doctor of Pharmacy program. Additional national “firsts” that distinguish the school include: first clinical pharmacy program (1968); first PharmD/MBA dual degree program (1988); first MS and PhD programs in pharmaceutical economics and policy (1994) and first professional doctorate in regulatory science (2008).
Consistently the top private pharmacy school nationwide, the school is a member of the American Association of Colleges of Pharmacy, and the PharmD program is accredited by the Accreditation Council for Pharmacy Education, 135 S. LaSalle Street, Suite 4100, Chicago, IL 60603-4810, phone: (312) 664-3575; fax: (312) 664-4652 or (312) 664-7008.
USC Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences 1985 Zonal Avenue Los Angeles, CA 90089-9121 (323) 442-1369 (phone) (323) 442-1681 (fax)
pharmacyschool.usc.edu Office of Admission and Student Affairs (PharmD) (323) 442-1466 Email: [email protected] Email: [email protected] pharmacyschool.usc.edu/apply/admission
Office of Graduate Affairs (PhD, MS) (323) 442-1474 (323) 442-2258 (fax) Email: [email protected] pharmgradprograms.usc.edu
Healthcare Decision Analysis 635 Downey Way Verna & Peter Dauterive Hall, VPD 312 Los Angeles, CA 90089-3333 (213) 821-6478 Email: [email protected] hcda.usc.edu
Biopharmaceutical Marketing 635 Downey Way Verna & Peter Dauterive Hall, VPD 312 Los Angeles, CA 90089-3333 (213) 821-6478 Email: [email protected] bpmk.usc.edu
Regulatory and Quality Sciences 1540 Alcazar St., CHP 140 Los Angeles, CA 90089 (323) 442-3102 Email: [email protected] regulatory.usc.edu
Administration
Vassilios Papadopoulos, DPharm, PhD, DSc (hon), Dean
Steven W. Chen, PharmD, Associate Dean for Clinical Affairs
Daryl L. Davies, PhD, Associate Dean of Undergraduate Education
Kari L. Franson, PharmD, PhD, Associate Dean for Academic and Student Affairs
Irving Steinberg, PharmD, Associate Dean for Faculty Affairs
Annie Wong-Beringer, PharmD, Associate Dean for Research Affairs
Curtis T. Okamoto, PhD, Associate Dean for Graduate Education and Postdoctoral Studies
Paul Beringer, PharmD, Chair, Titus Family Department of Clinical Pharmacy
Geoffrey Joyce, PhD, Chair, Department of Pharmaceutical and Health Economics
Eunjoo Pacifici, PhD, Chair, Department of Regulatory and Quality Sciences
Clay C.C. Wang, PhD, Chair, Department of Pharmacology and Pharmaceutical Sciences
John Stauffer Dean’s Chair in Pharmaceutical Sciences: Vassilios Papadopoulos, DPharm, PhD
William A. and Josephine A. Heeres, Endowed Chair in Community Pharmacy : Steven Chen, PharmD
University Professor and Boyd P. and Elsie D. Welin Professor in Pharmaceutical Sciences: Jean Chen Shih, PhD
Distinguished Professor of Pharmacy, Public Policy, and Economics and Leonard D. Schaeffer Director’s Chair : Dana Goldman, PhD
John A. Biles Professor in Pharmaceutical Sciences: Julio A. Camarero, PhD
Gavin Herbert Professorship in Pharmaceutical Sciences: Andrew MacKay, PhD
Charles Krown/Pharmacy Alumni Professor in Pharmaceutical Sciences: Enrique Cadenas, MD, PhD
Emeritus Professor and Dean: Timothy M. Chan, PhD
Emeritus Professors : Eric J. Lien, PhD; Bradley R. Williams, PharmD; Wei-Chiang Shen, PhD
Department of Pharmacology and Pharmaceutical Sciences
Professors of Pharmacology and Pharmaceutical Sciences: Enrique Cadenas, MD, PhD; Julio A. Camarero, PhD; Sarah F. Hamm-Alvarez, PhD; Vassilios Papadopoulos, DPharm, PhD; Jean C. Shih, PhD; Bangyan Stiles, PhD; Clay C.C. Wang, PhD
Associate Professors of Pharmacology and Pharmaceutical Sciences: Martine Culty, PhD; Roger F. Duncan, PhD; Ian S. Haworth, PhD; J. Andrew MacKay, PhD; Curtis T. Okamoto, PhD; Jennica Zaro, PhD
Assistant Professors of Pharmacology and Pharmaceutical Sciences: Zhipeng Lu, PhD; Paul Seidler, PhD; Jianming Xie, PhD; Yong (Tiger) Zhang, PhD
Lecturers: Rebecca Romero, PhD; Angel Tabancay, PhD
Titus Department of Clinical Pharmacy
Professors of Clinical Pharmacy: Melvin F. Baron, PharmD, MPA; Paul M. Beringer, PharmD; Steven Chen, PharmD; Daryl Davies, PhD; Julie A. Dopheide, PharmD; Kari L. Franson, PharmD, PhD; Stanley G. Louie, PharmD; Tien M.H. Ng, PharmD; Fred G. Weissman, PharmD, JD; Annie Wong-Beringer, PharmD
Associate Professors of Clinical Pharmacy: Melissa Durham, PharmD; Kevin L. Forrester, PharmD; Lisa W. Goldstone, PharmD; William C. Gong, PharmD; Cynthia L.L. Lieu, PharmD; Emi Minejima, PharmD; Edith Mirzaian, PharmD; Dima M. Qato, PharmD, PhD, MPH; Irving Steinberg, PharmD; Fred G. Weissman, PharmD, JD
Assistant Professors of Clinical Pharmacy: Houda Alachkar, PharmD, PhD; Carla Blieden, PharmD; Amanda Burkhardt, PhD; Michelle Chu, PharmD; David Dadiomov, PharmD; Richard Dang, PharmD; Tatyana Gurvich, PharmD; Emily Han, PharmD; Connie Kang, PharmD; Kum Ja Lee, PharmD; Serghei Mangul, PhD; Scott Mosley, PharmD; Rory O’Callaghan-Kim, PharmD; Tam Phan, PharmD; Raffi Svadjian, PharmD, MBA; Patrick Tabon, PharmD; Ying Wang, PharmD; Paul J. Wong, PharmD; Maryann Wu, EdD
Research Professor: Jing Liang, MD, PhD
Research Assistant Professor of Clinical Pharmacy: Liana Asatryan, PhD
Department of Pharmaceutical and Health Economics
Professors of Pharmaceutical and Health Economics : Dana Goldman, PhD; Joel W. Hay, PhD; Darius N. Lakdawalla, PhD
Associate Professors of Pharmaceutical and Health Economics : Geoffrey Joyce, PhD; Grant D. Lawless, MD, RPh; Jeffrey S. McCombs, PhD; John Romley, PhD; Seth Seabury, PhD; Daniel Tomaszewski, PharmD, PhD; Ken S. Wong, PharmD, MPH
Assistant Professor s of Pharmaceutical and Health Economics : William Padula, PhD; Erin Trish, PhD
Research Assistant Professors of Pharmaceutical and Health Economics : Bo Zhou, PhD; Steven Fox, MD
Department of Regulatory and Quality Sciences
Professor, Department of Regulatory and Quality Sciences: Frances J. Richmond, PhD
Associate Professor, Department of Regulatory and Quality Sciences: Eunjoo Pacifici, PharmD, PhD
Assistant Professors, Department of Regulatory and Quality Sciences: Susan Bain, DRSc; Terry David Church, DRSc; C. Benson Kuo, PhD; Nancy Pire-Smerkanich, DRSc
The Mann School offers curricula leading to the Doctor of Pharmacy (PharmD) and Doctor of Regulatory Science (DRSc) degrees and graduate degrees through the Graduate School including: Doctor of Philosophy (PhD) in Health Economics; and the PhD Programs in Pharmaceutical and Translational Sciences which is a one-year umbrella program after which students select one of the following three tracks to complete their PhD degree in Pharmaceutical Sciences, Molecular Pharmacology and Toxicology, or Clinical and Experimental Therapeutics. The School also offers Master of Science (MS) in Pharmaceutical Economics and Policy; Master of Science (MS) in Pharmaceutical Sciences; Master of Science (MS) in Molecular Pharmacology and Toxicology; Master of Science (MS) in Clinical and Experimental Therapeutics; Master of Science (MS) in Healthcare Decision Analysis; Master of Science (MS) in Biopharmaceutical Marketing; Master of Science (MS) in Regulatory Science; Master of Science (MS) in Regulatory Management; Master of Science (MS) in Management of Drug Development; and Master of Science (MS) in Medical Product Quality. Eight dual degree programs are also offered, including: PharmD/PhD, PharmD/JD, PharmD/MBA, PharmD/MPH, PharmD/MS in Regulatory Science, PharmD/MS in Gerontology, PharmD/MS in Global Medicine, PharmD/MS in Healthcare Decision Analysis, and a joint degree in Pharmaceutical Economics and Policy (PhD). Graduate certificates include advanced pharmacy practice, biopharmaceutical marketing, clinical research design and management, food safety, healthcare analytics and operations, healthcare decision analysis, medical product quality, preclinical drug development, patient and product safety, and regulatory and clinical affairs.
The Mann School also offers a Bachelor of Arts (BA) and a Bachelor of Science (BS) in Pharmacology and Drug Development; a Bachelor of Arts (BA) and a Bachelor of Science (BS) in Biopharmaceutical Sciences; and minors in Biopharmaceutical Business, Science and Management of Biomedical Therapeutics, and Foundation in Regulatory Sciences.
Doctor of Pharmacy (PharmD) Program
Admission requirements for the doctor of pharmacy (pharmd) program.
Applicants should possess a bachelor’s degree from an accredited college or university. A cumulative grade point average of 3.0 and a prerequisite grade point average of 3.0 or higher is strongly recommended. All students should complete the prerequisite courses that are required by the USC Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences before they start the program. An interview is mandatory and required of all students prior to admission. International students are required to complete the TOEFL and may request a virtual interview.
Acceptance criteria will be assessed on a case-by-case basis. The admission committee reviews each student holistically, taken all application materials and the interview process into consideration when making an admission decision. Along with being academically prepared, having the soft skills to communicate, problem solve, build relationships and show your ability to think critically are important characteristics to demonstrate.
Tuition and Fees (Estimated)
Tuition for the PharmD at USC Mann School degree programs is charged at a flat rate (which differs from standard USC tuition). See the Tuition and Fees section for fee information. These fees are subject to change.
Doctor of Pharmacy students must pay a $1,000 non-refundable acceptance deposit that is applicable toward tuition. For deposit information in other degree programs in the USC Mann School, please consult appropriate offices.
Honor Societies
Theta chapter of Rho Chi, the academic honor society in pharmacy, was established at USC in 1925. Eligibility for membership is based on high attainment in scholarship, character, personality and leadership. All candidates selected for membership must have completed three semesters of the pharmacy program (or post-qualifying exam for PhD students), and they must be approved by the dean of the USC Alfred E. Mann School of Pharmacy and Pharamceutical Sciences.
Phi Lambda Sigma
The Phi Lambda Sigma chapter was established at USC in 1988. This national pharmacy leadership society is devoted to identifying, supporting and recognizing the contribution of pharmacy students to their colleges, their classmates, their campuses, their communities and to their chosen profession.
Undergraduate Honors Program
The undergraduate honors in pharmacology and drug development (post code 1681) or biopharmaceutical sciences is awarded through successful completion of the senior capstone project. The project is a demonstration of knowledge in the student’s chosen area of interest which results in a product/project, research data, research paper, or portfolio of work, and a presentation. This experience encourages students to use a variety of skills in the areas of writing, speaking, research, and documentation, which distinguishes them as scholars and future leaders in pharmacy. Students will register in RXRS/BPSI - 493, Senior Honors Seminar I and RXRS/BPSI - 494 Senior Honors Seminar II.
Student Housing and Service Facility, Health Sciences Campus
There are no university-managed accommodations on the Health Sciences Campus. Currie Hall is privately owned, has a state-of-the-art fitness center, 24-hour academic success center, pool, wi-fi and fully furnished apartments with enhanced-privacy floor plans. For more information about Currie Hall, call (213) 784-7558 or visit the Currie Hall website .
For bookstore information, call (323) 442-2674. Students may also live in student housing on the University Park Campus, located about eight miles from the Health Sciences Campus.
Student Health Services, Health Sciences Campus
Services of the Student Health Center, covered by the mandatory student health fee, include the ambulatory care health services provided by the Student Health Center nursing staff. The Student Health Center is located in the USC Health Care Consultation Center, 1500 San Pablo Street, Suite 104, adjacent to the USC University Hospital, one block northeast of the USC Mann School. The telephone number is (323) 442-5980. In addition to the student health fee, all students must have major medical insurance coverage from the USC Student Health Plan. A student may request a waiver of the USC Student Health Plan if covered by a personal medical plan that meets criteria established by the Health Insurance Office.
Graduate Degrees
The USC Alfred E. Mann School of Pharmacy and Pharamceutical Sciences, through the Graduate School, offers curricula leading to the MS and PhD degrees in clinical and experimental therapeutics, molecular pharmacology and toxicology, pharmaceutical sciences and health economics, as well as a doctorate in Regulatory Sciences (DRSc). The Pharmaceutical and Translational Sciences (PHTS) PhD Program is a one-year umbrella program after which students select a particular track to complete their PhD in pharmaceutical sciences, molecular pharmacology and toxicology, or clinical and experimental therapeutics. The school also offers interdisciplinary MS degrees in regulatory science, in regulatory management, in the management of drug development, in medical product quality, in healthcare decision analysis and in biopharmaceutical marketing. The MS degree in pharmaceutical economics and policy is offered jointly with the USC Price School of Public Policy and the Department of Economics. In addition, the school offers dual degrees with the schools of law, business, gerontology and medicine as well as other programs. Instructions given in the Admission section of this catalogue are to be followed. An online application is required. See the Graduate Admission application page. Additional information may be obtained by calling (323) 442-1474 or sending an email to [email protected] .
Admission Requirements for the Master of Science in Clinical and Experimental Therapeutics
Applicants should possess a bachelor’s degree or equivalent from an accredited college or university. Applicants with graduate or professional degrees are encouraged to apply. A minimum grade point average of 3.0 (A = 4.0) is required. Special attention is given to the grades achieved in science courses relevant to the program (e.g., chemistry, biology, biochemistry, pharmacology and mathematics). Students who have research experience and/or work experience in the pharmaceutical arena are encouraged to apply.
Acceptance criteria for those individuals will be assessed on a case-by-case basis. English proficiency is essential. Students will be selected for admission, whenever possible, after interviews with one or more members of faculty.
Admission Requirements for the Master of Science in Molecular Pharmacology and Toxicology and Master of Science in Pharmaceutical Sciences
Applicants should possess a bachelor’s or master’s degree in pharmacy, chemistry, biology or other related disciplines from an accredited college or university. A minimum grade point average of 3.0 is required. Special attention is given to the grades achieved in science courses relevant to the program (e.g., chemistry, biology, biochemistry, pharmacology and mathematics).
Applicants must have demonstrated proficiency in verbal and written English and in fundamental scientific areas such as organic and physical chemistry, biochemistry, biology, mathematics, statistics and computer science. Three letters from faculty knowledgeable about the student’s ability and capability are required. These letters should provide a thorough assessment of the student’s experience in laboratory research, ability to communicate in verbal and written English, motivation and creativity, and other qualities in the student’s academic performance.
Applications for admission are reviewed by the Pharmacology and Pharmaceutical Sciences Graduate Admissions Committee of the USC Mann School and are evaluated primarily on the basis of academic excellence.
Admission Requirements for Programs in Pharmaceutical and Translational Sciences: Doctor of Philosophy in Clinical and Experimental Therapeutics, Doctor of Philosophy in Molecular Pharmacology and Toxicology, and Doctor of Philosophy in Pharmaceutical Sciences
All prospective students will apply through the single umbrella program in Pharmaceutical and Translational Sciences and become enrolled in one of the three participating PhD programs after having successfully completed the first year’s course work and laboratory rotations. Application materials will be reviewed by a joint admission committee, with equal representation of faculty from each track, evaluating applications on the basis of academic excellence and scientific research commitment.
Applicants must have a baccalaureate degree in the natural sciences, or sufficient courses in mathematics and the life sciences. This is required to provide a strong background for studies in biomedical and biological research. Appropriate undergraduate degrees include biology, physiology, engineering, chemistry or computer science. A student currently enrolled in the PharmD program may pursue a PharmD/PhD by following the admission procedure in the Catalogue.
Applicants should have a strong record of academic achievement. A minimum grade point average of 3.0 is required and previous research experience is expected.
In addition to the application for admission, three letters of recommendation from faculty knowledgeable of the student’s ability and capability are required. These letters should provide a thorough assessment of the student’s experience in laboratory research, ability to communicate in verbal and written English, motivation, creativity and other qualities in the student’s academic performance. The student’s research and professional experience should be well described within the application and include a personal statement summarizing career objectives and research interests.
Admission Requirements for the Master of Science in Pharmaceutical Economics and Policy
Applicants for admission must have achieved a minimum 3.0 GPA in an undergraduate or professional school and adequate scores on the GRE. In addition, applicants will be required to have completed upper-division courses in statistical methods, calculus and microeconomics.
Admission Requirements for the Doctor of Philosophy in Health Economics
Candidates with a bachelor’s, master’s or PharmD degree are invited to apply. Applicants must have demonstrated proficiency in verbal and written English and aptitude in economics, mathematics, statistics and computer science. Deficiencies in economics and statistical background can be addressed through preliminary course work after admission to the program.
A minimum grade point average of at least 3.0 (A = 4.0) is required. Special attention is given to the grades achieved in economics, statistics and mathematics courses relevant to the program. A qualifying score on the GRE in verbal and quantitative areas is required. There is no set minimum score required for admission, and GRE scores are considered in conjunction with all other parts of the application.
Admission Requirements for the Master of Science in Healthcare Decision Analysis
Applicants should possess a bachelor’s degree or equivalent from an accredited college or university. Applicants with graduate or professional degrees are encouraged to apply. A minimum grade point average of 3.0 is required. The program encourages the participation of part-time students with work experience. Acceptance criteria for those individuals will be assessed on a case-by-case basis. English proficiency is essential. Additional requirements for international students are outlined by university regulations under Admission of International Students.
Admission Requirements for the Master of Science in Biopharmaceutical Marketing
Applicants should possess a bachelor’s degree or equivalent from an accredited college or university. Applicants with graduate or professional degrees are encouraged to apply. A minimum grade point average of 3.0 and qualifying scores on the GRE or GMAT examinations are required. The program encourages the participation of part-time students with work experience. Acceptance criteria for those individuals will be assessed on a case-by-case basis. English proficiency is essential. Additional requirements for international students are outlined by university regulations under Admission of International Students.
Admission Requirements for the Doctorate of Science in Regulatory Science
The program is designed for individuals with strong professional experience and demonstrated intellectual and leadership capabilities. Applicants are expected to have a GPA of 3.0 on university-level course work and ten or more years of professional experience. Admission requirements include university transcripts, a résumé or curriculum vitae, at least three letters of reference, and a one-page personal statement that outlines the background, a topic of interest for the dissertation and goals of the applicant. Students are encouraged even at this early stage to identify areas in which they are interested in conducting research. Additional requirements for international students are outlined by university regulations under Admission of International Students. (See Admission and Orientation .) Students are not required to provide GRE scores unless indicated by the program director. Applicants will be selected for admission, whenever possible, after interviews with one or more members of faculty, current student and/or alumnus of the program.
Students with an appropriate graduate or professional degree may use some previous graduate courses as transfer units toward the overall credit requirements of the Doctor of Regulatory Science program with the approval of the program director and under the policies of the university. Students who have graduated from the Master of Science program in Regulatory Science at USC are eligible to apply all of the previously taken course work toward the doctoral degree. Students with graduate degrees from outside of the Regulatory Science program are required to take a minimum of 32 units of course work and 4 units of dissertation research to complete the requirements for graduation. The course work requirements will be determined on an individual basis in consultation with the program director and student’s advisers.
Admission Requirements for the Master of Science in Regulatory Science
Applicants should possess a bachelor’s degree or equivalent from an accredited college or university. Applicants with graduate or professional degrees are encouraged to apply. A minimum grade point average of 3.0 and applicants are not required to provide GRE scores unless indicated by the program director. The program encourages the participation of part-time students with work experience.
Acceptance criteria for those individuals will be assessed on a case-by-case basis. English proficiency is essential.
Admission Requirements for the Master of Science in Management of Drug Development
Applicants should possess a bachelor’s degree or equivalent from an accredited college or university. Applicants with graduate or professional degrees are encouraged to apply. A minimum grade point average of 3.0 and applicants are not required to provide GRE scores unless indicated by the program director. The program encourages the participation of part-time students with work experience. Acceptance criteria for those individuals will be assessed on a case-by-case basis. English proficiency is essential.
Admission Requirements for the Master of Science in Medical Product Quality
Applicants should possess a bachelor’s degree or equivalent from an accredited college or university. Applicants with graduate or professional degrees are encouraged to apply. A minimum grade point average of 3.0 and applicants are not required to provide GRE scores unless indicated by the program director. The program encourages the participation of part-time students who are already working in the industry as well as students who have recently completed or are about to complete an undergraduate program.
Acceptance criteria will be assessed on a case-by-case basis. English proficiency is essential. Applicants who do not meet all the specific requirements indicated above, but who show unique potential, may be considered for admission with conditions, which may be fulfilled during the first semester of enrollment.
Admission Requirements for the Master of Science in Regulatory Management
Applicants should possess a bachelor’s degree or equivalent from an accredited college or university. Applicants should also possess a conferred doctoral degree or equivalent from an accredited college or university. A minimum grade point average of 3.0 and applicants are not required to provide GRE scores unless indicated by the program director. Acceptance criteria for those individuals will be assessed on a case-by-case basis. English proficiency is essential. The program encourages the participation of part-time students who are already working in the industry as well as students who have recently completed or are about to complete a doctoral program.
Acceptance criteria will be assessed on a case-by-case basis. English proficiency is essential. Applicants who do not meet all the specific requirements indicated above, but who show unique potential, may be considered for admission with conditions, which may be fulfilled during the first two semesters of enrollment.
Admission of International Students to Graduate Degree Programs
All requirements described in this section are also applicable to the admission of international students. In addition, special application and admission procedures are required of international students. Refer to the section on Admission of International Students in this catalogue.
Degree Requirements
These degrees are under the jurisdiction of the USC Alfred E. Mann School of Pharmacy and Pharamceutical Sciences and/or jointly with the Graduate School. Students should also refer to the Requirements for Graduation section and The Graduate School section of this catalogue for general regulations. All courses applied toward the degrees must be courses accepted by the Graduate School.
- Master of Science in Biopharmaceutical Marketing
- Master of Science in Clinical and Experimental Therapeutics
- Master of Science in Healthcare Decision Analysis
- Master of Science in Management of Drug Development
- Master of Science in Medical Product Quality
- Master of Science in Molecular Pharmacology and Toxicology
- Master of Science in Pharmaceutical Economics and Policy
- Master of Science in Pharmaceutical Sciences
- Master of Science in Regulatory Management
- Master of Science in Regulatory Science
- Doctor of Philosophy in Clinical and Experimental Therapeutics
- Doctor of Philosophy in Health Economics
- Doctor of Philosophy in Molecular Pharmacology and Toxicology
- Doctor of Philosophy in Pharmaceutical Sciences
- Doctor of Philosophy in Pharmaceutical Economics and Policy
- Doctor of Regulatory Science
- PharmD/Juris Doctor
- PharmD/Master of Business Administration Dual Degree Program
- PharmD/Master of Science, Gerontology
- PharmD/Master of Science, Global Medicine
- PharmD/Master of Science, Healthcare Decision Analysis
- PharmD/Master of Public Health
- PharmD/Master of Science, Regulatory Science
- PharmD/Doctor of Philosophy
Office of Continuing Professional Development 1985 Zonal Avenue Los Angeles, CA 90089-9121 (323) 442-2403 FAX: (323) 442-3600 Email: [email protected] pharmacyschool.usc.edu/programs/ce/
Continuing Education
The USC Alfred E. Mann School of Pharmacy and Pharamceutical Sciences, Office of Continuing Professional Development, is a recognized provider of continuing pharmacy education accredited by the Accreditation Council for Pharmacy Education (ACPE) and recognized by the California State Board of Pharmacy and throughout the United States.
The school serves as a primary educational resource for pharmacists in California and as a supplementary resource for other health professionals and pharmacists, nationally and internationally.
Programs are designed to educate pharmacists about current issues in pharmaceutical care, practice management, therapeutics and other topics of professional interest. Continuing education programs are held at the USC Mann School and other locations.
For information concerning continuing education programs contact the Office of Continuing Professional Development.
Bachelor’s Degree
- • Biopharmaceutical Sciences (BA)
- • Biopharmaceutical Sciences (BS)
- • Pharmacology and Drug Development (BA)*
- • Pharmacology and Drug Development (BS)
- • Biopharmaceutical Business Minor
- • Foundation in Regulatory Sciences Minor
- • Science and Management of Biomedical Therapeutics Minor
Joint Degree
- • Pharmaceutical Economics and Policy (PhD)
Master’s Degree
- • Biopharmaceutical Marketing (MS)
- • Clinical and Experimental Therapeutics (MS)
- • Healthcare Decision Analysis (MS)
- • Management of Drug Development (MS)
- • Medical Product Quality (MS)
- • Molecular Pharmacology and Toxicology (MS)
- • Pharmaceutical Economics and Policy (MS)
- • Pharmaceutical Sciences (MS)
- • Regulatory Management (MS)
- • Regulatory Science (MS)
Dual Degree
- • Doctor of Pharmacy/Doctor of Philosophy (PharmD/PhD)
- • Doctor of Pharmacy/Juris Doctor (PharmD/JD)
- • Doctor of Pharmacy/Master of Business Administration (PharmD/MBA)
- • Doctor of Pharmacy/Master of Public Health (PharmD/MPH)
- • Doctor of Pharmacy/Master of Science, Gerontology (PharmD/MS)
- • Doctor of Pharmacy/Master of Science, Global Medicine (PharmD/MS)
- • Doctor of Pharmacy/Master of Science, Regulatory Science (PharmD/MS)
- • Healthcare Decision Analysis (PharmD/MS)
Graduate Certificate
- • Advanced Pharmacy Practice Graduate Certificate
- • Biopharmaceutical Marketing Certificate
- • Clinical Research Design and Management Certificate
- • Food Safety Certificate
- • Healthcare Analytics and Operations Certificate
- • Healthcare Decision Analysis Certificate
- • Medical Product Quality Graduate Certificate
- • Patient and Product Safety Certificate
- • Preclinical Drug Development Certificate
- • Regulatory and Clinical Affairs Certificate
Doctoral Degree
- • Clinical and Experimental Therapeutics (PhD)
- • Health Economics (PhD)
- • Molecular Pharmacology and Toxicology (PhD)
- • Pharmaceutical Sciences (PhD)
- • Pharmacy (PharmD)
- • Regulatory Science (DRSc)
Biopharmaceutical Marketing
- • BPMK 500 Biopharmaceutical Marketing Management
- • BPMK 501 Healthcare Payers, Insurance and Coverage Policy
- • BPMK 502 Biopharmaceutical Product Development and Marketing
- • BPMK 503 Biopharmaceutical Advertising and Communication
- • BPMK 504 Market Access and Reimbursement Strategy
- • BPMK 505 Product Health Economics and Valuation
- • BPMK 506 Biopharmaceutical Product Pricing and Competition
- • BPMK 508 Biopharmaceutical Marketing Research and Analytics
- • BPMK 509 Seminars in Biopharmaceutical Marketing
- • BPMK 510 Capstone I: Biopharmaceutical Management Project
- • BPMK 511 Capstone II: Biopharmaceutical Management Project
- • BPMK 599 Special Topics
Biopharmaceutical Sciences
- • BPSI 402 Biopharmaceutics I
- • BPSI 403 Biopharmaceutics II
- • BPSI 405 Organ Systems Physiology, Drug Delivery and Drug Action
- • BPSI 406 Drug Safety Pharmacology and Toxicology
- • BPSI 407 Pharmaceutical and Health Economics
- • BPSI 408 Biologics and Vaccines
- • BPSI 410 Biopharmaceutical Product Development and Brand Planning
- • BPSI 411 Biopharmaceutical Marketing Analysis and Strategy
- • BPSI 412 Targeted and Precision Medicines
- • BPSI 413 Rigor, Resources and Reproducibility
- • BPSI 414 Pharmacoethics
- • BPSI 415 Science Talk
- • BPSI 490x Directed Research
- • BPSI 493 Senior Honors Seminar I
- • BPSI 494 Senior Honors Seminar II
Clinical and Experimental Therapeutics
- • CXPT 501 Biomedical Data Sciences
- • CXPT 590 Directed Research
- • CXPT 594a Master’s Thesis
- • CXPT 594b Master’s Thesis
- • CXPT 594z Master’s Thesis
- • CXPT 596 Internship for Curricular Practical Training
- • CXPT 599 Special Topics
- • CXPT 609 Preclinical Experimental Drug Therapeutic Development
- • CXPT 610 Experimental and Clinical Drug Metabolism and Transport
- • CXPT 664 Clinical Problem Solving
- • CXPT 790 Research
- • CXPT 794a Doctoral Dissertation
- • CXPT 794b Doctoral Dissertation
- • CXPT 794c Doctoral Dissertation
- • CXPT 794d Doctoral Dissertation
- • CXPT 794z Doctoral Dissertation
Health Care Decision Analysis
- • HCDA 501 Fundamentals of Healthcare Insurance Design
- • HCDA 502 Comparative International Healthcare Systems
- • HCDA 503 Competitive Healthcare Intelligence
- • HCDA 506 Foundations of Insurance and Global Access
- • HCDA 507 Foundations of Product Development and Commercialization
- • HCDA 510 Business Implications of Healthcare Policy
- • HCDA 515 Healthcare Decision Analysis and Modeling
- • HCDA 520 Health Economic and Outcomes Methodology
- • HCDA 525 Healthcare Literature Analysis and Applications
- • HCDA 530 Total Product Development: Benchtop to Launch
- • HCDA 540 Executive Leadership and Healthcare Marketing
- • HCDA 550 Healthcare Innovation: Creativity to Value
- • HCDA 553 Advanced Pricing Strategies
- • HCDA 560 Managing Effective Partnerships and Mergers
- • HCDA 570 Asia Pacific: Access, Delivery and Reimbursement
- • HCDA 572 Introduction to Healthcare Data Analytics
- • HCDA 580 Seminars in Healthcare Decision Analysis
- • HCDA 589 Healthcare Consulting Enterprise Team Project
- • HCDA 590 Directed Research
- • HCDA 596 Internship for Curricular Practical Training
- • HCDA 599 Special Topics
Molecular Pharmacology and Toxicology
- • MPTX 500 Molecular Pharmacology and Toxicology I
- • MPTX 501 Molecular Pharmacology and Toxicology II
- • MPTX 502 Pharmacology
- • MPTX 510 Topics in Pharmacology: the Other Side of Drugs
- • MPTX 511 Introduction to Medical Product Regulation
- • MPTX 512 Regulation of Pharmaceutical and Biological Products
- • MPTX 513 Regulation of Medical Devices and Diagnostics
- • MPTX 514 Regulation of Food and Dietary Supplements
- • MPTX 515 Quality Systems and Standards
- • MPTX 516 Medical Products and the Law
- • MPTX 517 Structure and Management of Clinical Trials
- • MPTX 518 Writing Regulatory Drug Submissions
- • MPTX 519 Global Regulation of Medical Products
- • MPTX 520 Toxicology and the Media
- • MPTX 522 Introduction to Clinical Trial Design and Statistics
- • MPTX 524 Introduction to Food Science and Technology
- • MPTX 526 Chemistry Manufacturing and Controls
- • MPTX 531 Cell Biology
- • MPTX 561 Molecular Biology
- • MPTX 571 Biochemistry
- • MPTX 572 Medical Physiology I
- • MPTX 573 Medical Physiology II
- • MPTX 590 Directed Research
- • MPTX 594a Master’s Thesis
- • MPTX 594b Master’s Thesis
- • MPTX 594z Master’s Thesis
- • MPTX 596 Internship for Curricular Practical Training
- • MPTX 599 Special Topics
- • MPTX 602 Science, Research and Ethics
- • MPTX 630 Directed Field-Research Project
- • MPTX 700 Seminar in Molecular Pharmacology and Toxicology
- • MPTX 790 Research
- • MPTX 794a Doctoral Dissertation
- • MPTX 794b Doctoral Dissertation
- • MPTX 794c Doctoral Dissertation
- • MPTX 794d Doctoral Dissertation

UCI School of Pharmacy & Pharmaceutical Sciences
Offering undergraduate, graduate and professional degrees, the UCI School of Pharmacy & Pharmaceutical Sciences spans a continuum from discovery, development, and delivery of new drugs, devices, and diagnostics all the way to optimizing medication therapy in patients and patient populations.
Our school encompasses two departments: Pharmaceutical Sciences (established in 2007) and Clinical Pharmacy Practice (established in 2020).
Our Commitment to Diversity, Equity, and Inclusion
The mission of the UCI School of Pharmacy & Pharmaceutical Sciences is to be a driving force for the advancement of health and wellness of individuals and society. We believe that plurality of experiences, accomplishments, and values leads to greater innovation and creativity.
We, therefore, strive to maximize opportunities and inclusion for all our members and affiliates so that we can use our potential to achieve academic, scientific, and clinical excellence. Our goal is to serve all communities in the region, the State and beyond.

Sign up for our newsletter!
Receive updates on our school, faculty research, and outstanding students.
Pharmacy Graduate Programs in California
1-15 of 15 results
USC Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences (Doctor of Pharmacy Program)
- Los Angeles, CA ·
- University of Southern California ·
- Graduate School
- · Rating 4.78 out of 5 9 reviews
Blue checkmark.
University of Southern California ,
Graduate School ,
LOS ANGELES, CA ,
9 Niche users give it an average review of 4.8 stars.
Featured Review: Doctoral Student says The USC Mann School of Pharmacy and Pharmaceutical Sciences offers a great pharmacy program. It contains a diverse faculty and student population and they encourage diversity, equity, and inclusion.... .
Read 9 reviews.
Susan and Henry Samueli College of Health Sciences
- Irvine, CA ·
- University of California - Irvine ·
- · Rating 5 out of 5 1 review
University of California - Irvine ,
IRVINE, CA ,
1 Niche users give it an average review of 5 stars.
Featured Review: Niche User says what I like about university of California-irvine is that it has a really good nursing program.I like that it has a good nursing because that's a major I'm looking forward to major in nursing. .
Read 1 reviews.
Skaggs School of Pharmacy and Pharmaceutical Sciences
- La Jolla, CA ·
- University of California - San Diego ·
- · Rating 5 out of 5 3 reviews
University of California - San Diego ,
LA JOLLA, CA ,
3 Niche users give it an average review of 5 stars.
Featured Review: Doctoral Student says The class sizes are really small and you really get the chance to connect with your professors and learn a lot .
Read 3 reviews.
San Francisco Bay University
- Graduate School ·
- FREMONT, CA
- · Rating 3 out of 5 2
College of Health and Social Sciences - San Francisco State University
- San Francisco State University ·
- SAN FRANCISCO, CA
- · Rating 4 out of 5 1
School of Christian Leadership - William Jessup University
- Jessup University ·
- ROCKLIN, CA
Chapman University School of Pharmacy
- Orange, CA ·
- Chapman University ·
Chapman University ,
ORANGE, CA ,
Thomas J. Long School of Pharmacy
- Stockton, CA ·
- University of the Pacific ·
- · Rating 5 out of 5 2 reviews
University of the Pacific ,
STOCKTON, CA ,
2 Niche users give it an average review of 5 stars.
Featured Review: Doctoral Student says It is a new curriculum but so far I feel like I am still learning everything that I need to learn. Like any other school, you have to put in the time to be successful. .
Read 2 reviews.
Marshall B Ketchum University
- Fullerton, CA ·
- · Rating 5 out of 5 8 reviews
FULLERTON, CA ,
8 Niche users give it an average review of 5 stars.
Featured Review: Doctoral Student says I am currently enrolled in the Pharm D program at Marshall B Ketchum University. The program is a hybrid program as some lectures require mandatory in -person attendance while other are attendance optional. The course curricular is designed to gradually introduce the student to pharmacy practice. Efficiently starting off with skills that enable the student to get into pharmacy practice immediately as an... The faculty is completely dedicated to student progress, and structure lectures and assessments in a manner that enhances learning. As a P1 student at the end of my first year, my experiences so far have been very positive. .
Read 8 reviews.
- Find college scholarships
College of Pharmacy - Western University of Health Sciences
- Pomona, CA ·
- Western University of Health Sciences ·
Western University of Health Sciences ,
POMONA, CA ,
Featured Review: Doctoral Student says The faculty is great. They are very caring and always want students to succeed. They are always available. .

West Coast University - Center for Graduate Studies
- · Rating 4.65 out of 5 26 reviews
26 Niche users give it an average review of 4.7 stars.
Featured Review: Master's Student says Hello Future PA Students, Congratulations on considering joining a Physician Assistant program! This amazing journey will demand dedication, resilience, and a passion for patient care. Expect a rigorous curriculum, diverse... .
Read 26 reviews.
School of Pharmacy - University of California - San Francisco
- San Francisco, CA ·
- University of California - San Francisco ·
University of California - San Francisco ,
SAN FRANCISCO, CA ,
College of Pharmacy - Touro University - California
- Vallejo, CA ·
- Touro University - California ·
Touro University - California ,
VALLEJO, CA ,
School of Pharmacy - Loma Linda University
- Loma Linda, CA ·
- Loma Linda University ·
Loma Linda University ,
LOMA LINDA, CA ,
College of Pharmacy - California Health Sciences University
- Clovis, CA ·
- California Health Sciences University ·
California Health Sciences University ,
CLOVIS, CA ,
- Sponsored Find Student Loan Options
- Law Schools
- Public Administration Graduate Programs
American University of Health Sciences
- Signal Hill, CA ·
- · Rating 4.5 out of 5 6 reviews
SIGNAL HILL, CA ,
6 Niche users give it an average review of 4.5 stars.
Featured Review: Other says Excited to start school in Jan 2024. Everyone in the administration team are very warm and kind with the enrollment process although the tuition fee is a lot. I feel that it is a great school to be... .
Read 6 reviews.
California Northstate University
- Elk Grove, CA ·
- · Rating 5 out of 5 12 reviews
ELK GROVE, CA ,
12 Niche users give it an average review of 5 stars.
Featured Review: Doctoral Student says My academic experience thus far with California Northstate University has been well. Staff has been extremely helpful, kind and always available for help. I would recommend California Northstate to... .
Read 12 reviews.
School of Pharmacy and Health Sciences - Keck Graduate Institute
- Claremont, CA ·
- Keck Graduate Institute (KGI) ·
Keck Graduate Institute (KGI) ,
CLAREMONT, CA ,
College of Pharmacy - Thomas Jefferson University
- Thomas Jefferson University ·
- PHILADELPHIA, PA
- · Rating 5 out of 5 1
Thomas Jefferson University
- · Rating 4.68 out of 5 73
Rowan University Graduate
- GLASSBORO, NJ
- · Rating 4.38 out of 5 29
Showing results 1 through 15 of 15
Featured Searches
- Clinical Trials
- Find a Doctor
- Cancer Care
- Heart Surgery
- Health Professional Schools
- UCH Leadership
- Diversity, Equity and Inclusion
- Mission, Vision & Values
University of California Schools of Pharmacy
Want to redefine the role of the pharmacist in tomorrow’s health care workforce? We’re ready for you.

Pharmacists are among the most visible health care professions in the nation. Patients visit pharmacies dozens of times a year for new prescriptions, refills and consultations. For many pharmacists, the frequent interaction with patients is one of the most rewarding aspects of their career.
Pharmacy is a rapidly changing field. Pharmacy faces the introduction of new medications, increasingly personalized drug selection and dosing for individuals based on their genetics, and the rising cost of drugs—to name only a few challenges.
Now is the time for pharmacists to practice at the top of their profession as never before. As a system with a culture of collaboration, innovation and service, UC prepares our pharmacy students to meet today’s demanding health care needs.
And, as the pharmacy profession continues to evolve, our schools and faculty are making sure you’re prepared for what’s next.
Leading California — and the Nation
Our pharmacy schools are among the top in the U.S. and the world, according to U.S. News & World Report . As public institutions, they are affordable, high quality options. In fact, the UCI School of Pharmacy & Pharmaceutical Sciences is listed as the most affordable pharmacy degree program in the United States by the College Affordability Guide.
UC San Diego
#18 in Pharmacy (tie)
#2 in Pharmacy
Degree Programs Offered Across the UC System for Pharmacy
*UCI Department of Pharmaceutical Sciences was founded in 2007. In 2020, the University of California Regents approved the School of Pharmacy and Pharmaceutical Sciences. The first class will enroll in fall 2021.
Our Schools of Pharmacy
Our Schools of Pharmacy offer a variety of innovative, rigorous programs to meet your professional goals and advance the pharmaceutical field. Whether you want to be a pharmacist in a hospital setting or a retail pharmacy, or pursue pharmaceutical science, our schools can support your desired career path.
UC San Diego Skaggs School of Pharmacy and Pharmaceutical Sciences
UC San Diego Skaggs School of Pharmacy and Pharmaceutical Sciences routinely has an above 90 percent pass rate for graduates taking the North American Pharmacist Licensure Examination (NAPLEX), including 100 percent in 2017, 2018 and 2019. It also had the highest percentage among California schools for the California Pharmacist Licensure Exam (CPJE) pass rate in 2020.

- Degree Programs
- Patient Care
UCI School of Pharmacy & Pharmaceutical Sciences
UCI School of Pharmacy and Pharmaceutical Sciences comprises the departments of Pharmaceutical Sciences and Clinical Pharmacy Practice. Pharmaceutical Sciences provides undergraduate and graduate students training for careers in pharmacy, medicine and biomedical research. Clinical Pharmacy Practice trains students to serve as competent health care providers and future-ready pharmacists.

UCSF School of Pharmacy
Science is at the foundation of the UCSF School of Pharmacy’s pioneering research, education, and patient care. The School has been number one in research funding from the National Institutes of Health (NIH) every year for over four decades. The doctor of pharmacy (PharmD) degree program, with its unique emphasis on scientific thinking, prepares students to be critical thinkers and leaders in health care.

Systemwide Expertise Across California
In California, there is a growing recognition that pharmacists can play crucial roles as members of interprofessional care teams and in settings that focus on public health issues. As a system with six highly respected academic health centers, our pharmacy students have opportunities to collaborate with their health professional peers in our UC Schools of Medicine and more. With peers across the UC family, pharmacy students can draw from a rich alumni network throughout their careers.
Explore patient care within our six academic health centers

Using Research to Inform Patient Care
At UCH, research continually improves our patient care. In fact, one in seven National Institutes of Health grants is typically given to the University of California. This emphasis on research is exemplified in the pharmaceutical investigation of methodologies to identify individualized therapeutics that are safest and most effective, affordable, and accessible for all patients.
See how University of California Health is challenging the status quo through innovative research
- Quick Links
Tools & Resources
- Events Calendar
- Strauss Health Sciences Library
- Department A-Z Directory
- Campus Directory
- Faculty & Staff Resources
- Supporter & Alumni Resources
- Student Resources
- Mental Health Resources
- University Policies
CU Campuses
Cu anschutz medical campus.
- CU Colorado Springs
- School of Dental Medicine
- Graduate School
- School of Medicine
- College of Nursing
Skaggs School of Pharmacy and Pharmaceutical Sciences
- Colorado School of Public Health
PhD in Pharmaceutical Outcomes Research
Pharmaceutical Outcomes Research is a PhD program in the graduate program in Clinical Pharmacy. This program is housed in the Center for Pharmaceutical Outcomes Research (CePOR, SEE-por), a school-wide center in the Skaggs School of Pharmacy. Our doctoral curriculum is designed to provide competent and highly skilled researchers in the study of patient, provider, or population-level health care and health system interventions. We most often focus on economic, clinical, and humanistic outcomes such as clinical or cost effectiveness or safety. Areas of focus available to students undertaking this course of study include pharmacoeconomics, pharmacoepidemiology, health services research, and drug policy.
Core faculty are methodological experts and provide collaborative linkage to clinical experts in all pharmacy, medicine, nursing, and public health. Programs of study are tailored to student interests in disease or drug areas, such as cardiology, psychiatry, neurology, and cancer. Opportunities exist to link to more basic science colleagues depending on your topic of interests. For example, students interested in drug safety might link with toxicology faculty, or in gene-environment interactions might link with pharmacogenomics faculty.
Applications for all doctoral programs are submitted electronically through the Graduate School of the University of Colorado Denver. After signing up for an account, select 'PhD' under the 'Academic Interests' menu and scroll down to 'Skaggs School of Pharmacy and Pharmaceutical Sciences' and select "PhD in Pharmaceutical Outcomes Research."
Application requirements are:
- A completed Graduate School application and $50.00 application fee (Domestic) $75.00 application fee (International)
- A baccalaureate degree of arts or science from an accredited college or university with a minimum GPA of 3.0.** One (1) official transcript of all academic work completed to date with awarded baccalaureate degree. University transcripts from other countries must include a transcript evaluation from World Education Services ( WES ). Applicants who complete a transcript evaluation with WES will have their application fee waived automatically.
- All applicants for the program should complete a year of study in the following subjects: general chemistry, organic chemistry, calculus, biology, English and physics. In addition, courses in the following subjects are highly recommended to supplement the student's background: physiology, biochemistry, statistics, cell biology, physical chemistry, and computer science.
- Three (3) letters of recommendation from professors or research supervisors familiar with your aptitude for graduate study
Additionally:
- The GRE (Graduate Record Examination) is not required but is optional.
- The TOEFL is required of applicants for whom English is not their first language, Duolingo and IELTS also accepted (more information on this here )
- Please use 4875 as the Institution Code so that the test results will be sent directly to our institution
- Under special circumstances, deficiencies in important areas may be made up within the first year after entrance into the program. Normally, admission to the program will be based on an undergraduate GPA of 3.0 or better. However, applicants' recommendations, research experience and additional individual accomplishments will also be considered in the admissions process.
Application opens September 1, 2023. Applications will not be reviewed until all required materials have been received. The application deadline for Fall 2024 admission is December 1, 2023 for all students.
Admission to the program may include financial support via a stipend awarded on a 12-month basis.
Although a priority of the School of Pharmacy is to provide financial support to its graduate students, payment of stipend, tuition and any fees by the School of Pharmacy or by grants, contracts or gifts to the School of Pharmacy faculty is contingent upon availability of funding, satisfactory academic progress (as defined by the UCD Graduate School, Graduate Student Handbook) and completion of required teaching duties, core courses, and examinations. The School of Pharmacy also reserves the right to review and adjust its funding policies at any time. All students are expected to work full-time toward program requirements for 12 months of the year.
Generally, the first year of financial support will be in the form of stipend support for working as a teaching assistant. Depending on availability, teaching assistantships may be offered beyond one year to students. Faculty may choose to offer research assistant scholarships to students as well. Other funding opportunities in the form of external student grants and awards also exist. Students are encouraged to talk to the faculty about funding and scholarship opportunities. Funds for travel to one meeting where students are presenting a poster or giving a podium presentation are limited to $500 per fiscal year.
Students who do not remain in good graduate standing (3.0 GPA or above) or maintain satisfactory academic progress are placed on academic probation. Probation and suspension policies are described in the UCD Graduate School, Graduate Student Handbook. Payment of stipend, tuition, insurance and fees for a student while on academic probation is at the discretion of the graduate program committee.
What does "pharmaceutical outcomes research" mean?
What kind of students should pursue a phd degree in pharmaceutical outcomes research why should you apply to this program.
We are looking for students who want to influence healthcare but do not want to be a provider. With the belief that the research done will allow for the application of new knowledge towards health improvement.
What makes the Pharmaceutical Outcomes Research PhD program at the University of Colorado different from other PhD programs?
The benefits of this program are multi-faceted. Housed on a major medical campus, students will be able to collaborate with the schools of pharmacy, medicine, nursing, and public health. Also the University of Colorado Hospital, Children's Hospital, and the Veterans Affairs Medical Center are located on campus.
By having a small group of graduate students, they are allowed more time with the faculty members. With two faculty members from each component, students will be able to have the support necessary to complete their degree.
What are the job prospects for a graduate with a PhD degree in the pharmaceutical sciences? What can you do with this career?
Graduates of the program will have many career options within these areas:
- Pharmaceutical industry
- Government agencies
- Contract research organization (CROs)
- Organized healthcare systems
There is a critical need for individuals who are able to conduct rigorous, credible, and relevant population and patient-based research within stringent ethical and regulatory guidelines; the demand for such researchers is expected to grow given the developing health care reform and the investment in federal development and expansion on comparative effectiveness research.
Past graduates have gone on to be an interim dean at a Regis University and the director of pharmacy at the University of Colorado Hospital
How are current students doing?
Our students often win awards at regional and national symposium (such as Julia Slejko at ISPOR or SMDM). One holds a prestigious pre-doctoral dissertation award in health outcomes from the PhRMA Foundation. Two have completed comprehensive exams are working on defending their dissertation proposals. All these more advanced students have published manuscripts in peer-reviewed journals as first authors with the mentorship of the CePOR faculty.
Faculty comments on the program.
Heather Anderson, PhD What's great about this campus is that we are able to collaborate with other schools such as public health, nursing, and medicine. While many Pharmaceutical Outcomes Research programs have a major focus on economics, we do that and more. Our program has a strong focus on epidemiology and policy too. I actually got my PhD in epidemiology from our School of Public health and can link up students with the best courses and advise on exciting local opportunities for research assistantships.
Kelly Anderson, PhD Training at a world-class medical campus allows PhD students in the Center for Pharmaceutical Outcomes Research to engage with faculty in the center with expertise in outcomes research, drug pricing, economics, epidemiology, and health policy, and also have the opportunity to learn from and collaborate with faculty throughout the Schools of Pharmacy, Public Health, Medicine, Dentistry, and Nursing. For anyone who loves big data, our faculty also work with numerous large data sets: Medicare claims, linked EHR-claims data, and all-payer data just to name a few. As a lot of my work is focused on payment policy, I welcome the opportunity to engage students as they think about the real-world implications of their research for policy makers, health insurers, patients, clinicians, and drug companies and disseminate their findings to these key stakeholders.
R. Brett McQueen, PhD Pharmaceutical outcomes research includes aspects of multiple disciplines including math, economics, and epidemiology. I joined the faculty at CU to contribute to comparative- and cost-effectiveness research and to education both for the PhD and the PharmD programs. Our PhD program emphasizes quality over quantity. We maintain a very favorable student to faculty ratio, we offer competitive student financial support, and we strive to graduate scientific leaders in the field of outcomes research.
Kavita V. Nair, PhD Our expectations for graduate students are high and we have structured the education and training requirements to help you meet these goals. I will require a lot of you as a student but will also be your strongest advocate!
Robert Valuck, PhD, RPh I believe that the strengths of our program are the skills and the diversity of the faculty, and size and connectedness of our program with others on campus and in the state and region. With a smaller number of graduate students in our program, they are able to spend more time with faculty members. Our program is well connected with others on the Anschutz campus, and students have opportunities to collaborate both across campus, and with state agencies, provider groups, and others that have an interest in outcomes research and its applications to patient care and policy.
Advance the science of pharmaceutical outcomes research by training scientists who generate and synthesize evidence to inform practice and policy.
The goal of the PhD n pharmaceutical outcomes research is to develop methodological experts. Graduates will have the knowledge and extensive skills necessary to conduct pharmacoeconomic, pharmacoepidemiologic, health services, and drug policy research. We train individuals who can contribute to T3-T4 clinical translational pharmacy and pharmaceutical sciences, specifically on effective, population health, and policy studies. These contributions should ultimately benefit pharmacists and society with safe, effective, and efficient use of pharmaceutical care.
The Pharmaceutical Outcomes Research PhD program trains graduate students to become proficient and successful investigators who are able to:
- demonstrate an in-depth knowledge of central concepts in Pharmaceutical Outcomes Research, including the areas of pharmacoeconomics, pharmacoepidemiology, and/or drug policy.
- critically appraise existing literature and sources of information.
- formulate hypotheses based on current concepts in the field and accurately and correctly design, conduct, and interpret their own research projects.
- present research results in peer-reviewed publications and in a dissertation.
- perform research that adheres to the principles and guidelines of ethical conduct.
- communicate research results effectively through oral presentations at scientific seminars, conferences, and other venues
The program’s strengths in outcomes research are emphasized in 35 credits of several areas:
- Biostatistics
- Epidemiology
- Health Policy
- Research/Study Design
- Doctoral Thesis
The program has experience in accessing a multitude of data such as MEPS, PHARMetrics, University Health-System, Consortium and MarketScan. Students are encouraged to utilize these datasets as well as primary data collection. Students may enroll in courses not listed (e.g courses in downtown campuses and/or newly developed courses) by consulting with the program director.
Pharmacoepidemiology

Heather Anderson PhD

Robert Valuck PhD, RPh
Pharmacoeconomics.

Mike J. DiStefano PhD, MBE

Kelly Anderson PhD, MPP

R. Brett McQueen PhD
Pharmaceutical and drug related policy.

Kavita Nair PhD

Antal Zemplényi
Antal Zemplényi, PhD, is an Associate Professor at the Center for Health Technology Assessment at the University of Pécs and a senior researcher at the Syreon Research Institute, an international research corporation specializing in health policy, health economic modeling, and technology assessment. He has experience in value assessment, HTA, health economics and outcomes research, and real-world data analysis. He is the past president of the ISPOR Hungary Chapter. Antal is currently a Fulbright Scholar at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences working as a visiting research associate in the Pharmaceutical Value (pValue) initiative.

Monica Bianchini
Monica Bianchini is originally from Indianapolis, Indiana. She received a PharmD and MPH from the University of Wisconsin-Madison in 2017. She subsequently completed a hospital pharmacy residency (PGY-1) and Infectious Diseases PGY-2 at Henry Ford Hospital in Detroit, Michigan. Monica joined CePOR in 2019 and currently works as a clinical inpatient pharmacist. Her dissertation will evaluate opportunities to decrease syphilis rates and improve syphilis care in Colorado. Outside of work, she enjoys reading, live music, cooking, and anything outside (running, hiking, backpacking, skiing).

Sue is a fifth year PhD student at CePOR. Her dissertation focuses on characterizing the burden of MS and treatment strategies using real-world claims data. Prior to grad school, she studied Neuroscience in Pomona College. In her free time, Sue enjoys taking her puppies on adventures.

Mahesh Maiyani
Mahesh Maiyani was born and raised in India. He earned his Bachelor of Pharmacy (BPharm) from India in 2006 and then he came to the US to pursue his Master’s in Business administration. He completed his MBA from The University of Findlay in Ohio. He has work experience in clinical trials and heath care research. He joined the Pharmaceutical Outcomes Research PhD program in Fall-2021. His research interests are focused around cost effectiveness in real-world clinical settings. Mahesh enjoys hiking and spending time with family and friends.

Nick Mendola
Nick Mendola was born and raised in Buffalo, New York. He graduated from The University of Akron with a BS in Exercise Science in 2016. He then moved to Washington D.C. to attend The George Washington University Milken Institute School of Public Health, where he obtained his MPH in Epidemiology, in 2018. During this time, he worked researching the impact of the pharmaceutical industry’s marketing to healthcare professionals, and its influence on prescribing practices and population level drug utilization. Nick joined the Pharmaceutical Outcomes Research program in the fall of 2018. Nick’s current work with Dr. Robert Brett McQueen, explores the use of Multi-Criteria Decision Analysis (MCDA) as a novel health technology value assessment tool. His work specifically explores MCDA in evaluating treatments for neuromyelitis optica spectrum disorder (NMOSD), a rare neurological disorder.

Vanessa Paul Patterson
Vanessa Patterson is originally from Kansas City. She graduated from Tulane University School of Public Health and Tropical Medicine with an MPH concentrating in Epidemiology and Maternal and Child Health in 2012. Vanessa went on to work as an applied epidemiologist for government public health agencies for six years. She joined the Pharmaceutical Outcomes Research program at CU in the fall of 2018. Working under the mentorship of Dr. Heather Anderson, Vanessa is currently a PhRMA Foundation Predoctoral Fellow and a PhD candidate. Her dissertation focuses on the utilization of cardioprotective medications among women with a history of breast cancer. In her free time, Vanessa enjoys making pottery and spending time outdoors with family and friends.

Nai-Chia (Sammi) Chen
Sammi is originally from Taiwan. She holds a bachelor’s degree in Pharmacy from Kaohsiung Medical University. After graduation, she had worked in pharmaceutical companies and clinical research organizations for several years before coming to the US. She completed her Master’s training at the University of Pittsburgh, Pharmaceutical Outcomes and Policy Research, in 2022. And then she joined Pharmaceutical Outcomes Research PhD program at CU Anschutz in Fall 2022. Her research interest lies in the intersection of pharmacoeconomics, real-world evidence, and pharmaceutical outcomes. Outside the schoolwork, she enjoys cooking, baking, and snowboarding/skiing in winter.

Mouna Dardouri
Mouna was born and raised in Tunisia. She graduated with a PharmD from the University of Pharmacy of Monastir in 2016. She then worked for two years at a consulting company focusing on pharmaceuticals’ Pricing, Reimbursement and Market Access (PRMA) in Europe. After that, she was awarded with the Fulbright Foreign student scholarship and moved to Colorado, where she obtained her MPH in Global Health Systems, management, and Policy in 2022. Mouna joined the Pharmaceutical Outcomes Research program in the fall of 2022. Her research interests include evaluating the use of Health Technology Assessment in the context of low- and middle- income countries and developing tools that permit equitable patients’ access to cost-effective technologies. In her free time, Mouna enjoys learning new languages, improv theatre and cooking.
Why CU's Pharmaceutical Outcomes Research PhD Program?
“One key reason I chose to join CePOR at CU was the tight-knit group of faculty and students. Given the program is smaller, the faculty has a better opportunity to stay in touch with all the students and provide support for everyone's research, regardless if they're on the student's committee or not. The student group is also very close as we are together for weekly seminars and enjoy out-of-school gatherings when possible. Another draw of CU's POR program is the diverse expertise of our faculty and alumni. Our current faculty have a range of expertise including: pharmacoepidemiology using big data sources, pharmacoeconomics and drug pricing, rare diseases, opioid use disorder and treatment, and Medicare payment models. Recent alumni have found work in a variety of different fields from consulting to academia to the pharmaceutical industry and the public health department. The wide range of backgrounds and areas of expertise covered by our faculty and alumni provide so many resources for mentorship and future career planning. Finally, there are so many unique opportunities within CePOR to work with different data sources (e.g. electronic health records, national claims data, Medicaid claims data) and different methodology experts, so I am confident that our program could be a great fit for prospective PhD students of all backgrounds.” – Monica Bianchini, PharmD, MPH
“CU's Pharmaceutical Outcomes Research Program produces robust interdisciplinary research that spans from pharmacoepidemiology to pharmaceutical economics with a variety of collaborators, such as Institute for Clinical and Economic Review (ICER) and Colorado Department of Public Health & Environment (CDPHE).” – Sue Kwon, BA
“I joined the POR program because my previous research was focused on population drug utilization and the pharmaceutical industry’s impact on prescribing practices, and the POR program seemed like a natural fit for me to be able to keep learning about the areas of pharmacoepidemiology and drug related policy. What I like most about the program so far has been the core faculty in our program. They seem to truly care about student success and how we progress both academically and professionally. ” – Nick Mendola, MPH
PhD Student Research Projects
- Comparative Effectiveness of Rare Disease Therapies Using Multi-Criteria Decision Analysis: Case Example in Neuromyelitis Optica Spectrum Disorder, a Rare Neurological Disorder
- Characterizing Real-world Burden of Multiple Sclerosis and Treatment Strategies in a Colorado-representative Population
- Utilization of Cardioprotective Medication Strategies Among Women with a History of Breast Cancer
- Opportunities to Improve Syphilis Care in Colorado
Mission: To educate, increase awareness and promote growth within the 'Pharmacoeconomics and Outcomes Research' field in general and to increase the CU Denver presence among the international society ISPOR. To collaborate across different sciences on campus and different departments worldwide.
Description: Promote pharmacoeconomics and outcomes research education by holding regular seminars on current issues in the field and presenting research at least once a year at the annual meeting in the US-Canada region.
Membership requirements: We expect members to be passionate about the kind of research that is involved related to public health, epidemiology, pharmacoeconomics, and policy. Also, attending our regular educational seminars/webinars is highly encouraged.
Activities: Details will be emailed to members soon!
Benefits: The opportunity to present research, network and collaborate with faculty from different universities around the world, professionals from industry and research organizations at a global level.
Julia Slejko, PhD ('12) Associate Professor Practice, Sciences, and Health Outcomes Research University of Maryland School of Pharmacy
R. Brett McQueen, PhD ('13) Associate Professor Department of Clinical Pharmacy, Skaggs School of Pharmacy and Pharmaceutical Sciences University of Colorado Anschutz Medical Campus
William Padula, PhD ('13) Assistant Professor of Pharmaceutical and Health Economics, School of Pharmacy Fellow, Leonard D. Schaeffer Center for Health Policy & Economics University of Southern California
David Tabano, PhD ('18) Principal Health Economist Evidence for Access (E4A) | Public Affairs & Access Genentech, Inc.
Katie Sullivan, PhD ('18) Prescription Drug Epidemiologist Colorado Department of Public Health and Environment
Angela Czaja, MD, PhD ('19) Associate Professor Pediatrics-Critical Care Medicine Children’s Hospital Colorado Anschutz Medical Campus
Chong Kim, PhD ('20) Associate Director Global Value & Access | HEOR Gilead Sciences
Katia Hannah, PhD ('21) Lead HEOR Specialist Dexcom
Kimberly Deininger, PhD ('22) HEOR Manager Amgen
For questions regarding graduate school programs contact:
Isabella Jaramillo Email: [email protected] Phone: 303.724.7263

Kelly Anderson, PHD, MPP
Assistant Professor; Director, Pharmaceutical Outcomes Research PhD Program Email: [email protected] Phone: 434-466-1990
CU Anschutz
Pharmacy and Pharmaceutical Sciences Building
12850 East Montview Boulevard
Aurora, CO 80045
303-724-2882
- Pharmacy Directory
- Continuing Education
- Academic Calendar
- Request information
- Virtual Advising
- Scholarships & Financial Aid
- Career Services
- Experiential Program
- CORE for Students
- UCD-Access Portal
- Zoom Web Conferencing
- Payroll and Benefits
- Campuswide Directory
- University Laws and Policies
- CORE for Preceptors, Faculty, and Staff
Your browser is unsupported
We recommend using the latest version of IE11, Edge, Chrome, Firefox or Safari.
College of Pharmacy - Chicago | Rockford
Pharmd/phd program.
The joint PharmD/PhD trains students for careers in academic pharmacy and bench science research. Students participate in the PharmD curriculum and pursue original doctoral research projects in the laboratories of faculty in the Department of Pharmaceutical Sciences .
By counting some course requirements toward the completion of both degrees, the joint program can reduce the total time of earning the degrees by about two years. Both degrees are awarded at the end of the training period and neither degree can be received before the other is completed.
The PharmD/PhD program is for exceptional, highly motivated and achieving students ready to meet the challenge of increased academic load and independent research project.
Admission Requirements and Application Process Heading link Copy link
Students may apply to the joint PharmD/PhD degree program at the same time as when applying to the PharmD program or within the first two years after their acceptance into the PharmD program. Students must be accepted by both programs. Please note : the PharmD/PhD program is not offered in the Department of Pharmacy Systems, Outcomes, and Policies.
Apply Concurrently
Minimum requirements for admission include:
- a baccalaureate degree from an accredited college or university
Application has 3 components:
- Submit an application to the PharmD program via PharmCAS and indicate your interest in the joint degree.
- Submit the Supplemental Application ( PSTP form ) via e-mail to [email protected] .
- Submit an application to the Pharmaceutical Sciences PhD program following the program’s specific guidelines.
Apply for the joint degree after acceptance to PharmD
- Submit the Supplemental Application ( PSTP form ) via e-mail to [email protected]
- Submit an application to the Pharmaceutical Sciences PhD program following the program’s specific guidelines during your first two years of enrollment in the PharmD.
- If you are accepted by the PhD program and have a baccalaureate degree, you will be accepted to the joint program immediately. Contact [email protected] to notify the College of your acceptance into the PhD program.
- Students without a baccalaureate degree are not eligible for the program, as it is a requirement of the PhD program application.
Program Timeline
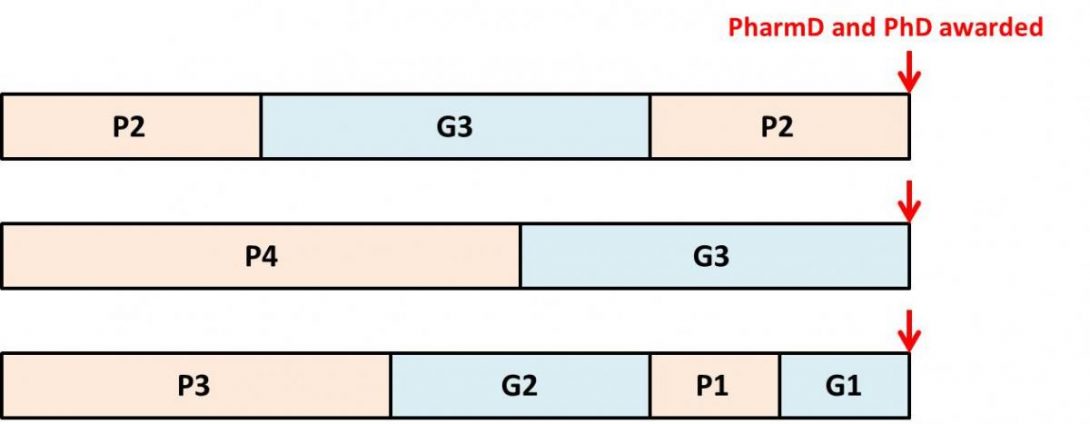
Students admitted to the joint program can begin requirements for both degrees upon admission. Summers can be used for research and laboratory rotations. The first two to three years of the program are used to complete the P-1 through P-3 didactic PharmD curriculum with some PhD courses as electives. Choice of a permanent thesis advisor can take place at any point before moving to the graduate focused years (G-1 through G-3). Following completion of the PhD phase of the program, students rejoin other PharmD students to complete PharmD didactic and/or clerkship requirements.
The program is flexible and actual timeline will depend on the requirements of the specific PhD program and the PhD thesis advisor. Sample timelines used by our current PharmD/PhD students are below (“P” refers to PharmD portions of the joint degree program, “G” refers to graduate segments of the joint program. The numbers indicate years spent in each segment. However, it should be understood that some research will be going on in years labeled as “P”.)
Financial Aid
Students enrolled in the joint degree program are eligible for the graduate level tuition for the PharmD portion of their study, which results in significant cost reduction. You may compare the cost of professional and graduate tuition and fees at the Office of the Registrar website . Additional financial benefits may apply. When a student becomes a full-time graduate student, student may receive a tuition waiver and a stipend.

Lindsey McQuade, PhD Heading link Copy link
Director, research & graduate resources.
Website Navigation
Colleges & programs, degrees & programs.
Welcome to the Xavier University of Louisiana. Each of our programs at Xaiver provides meaningful, quality graduate experiences in a personalized environment!
College of Arts & Science
Our progressive degree programs provide natural synergy between student academic exploration and career readiness, making Xavier a unique HBCU undergraduate experience in New Orleans.
Xavierites study
- Fine Arts, Humanities
- Biological and Applied Health Sciences
- Social Behavior Sciences
- Education and Counseling
- Mathematical and Physical Sciences
- Visit the College of Arts & Sciences
- Visit The Office of Graduate Programs
Graduate Programs
Whether you're looking for a certificate, master's or doctorate, our graduate degree programs integrate independent study, research and professional development.
The Center for Equity, Justice, and the Human Spirit
The Center for Equity, Justice, and the Human Spirit at Xavier University of Louisiana will serve as an anchor, a magnet, a beacon of hope, and a catalyst for change reflecting and furthering our calling, as a Historically Black and Catholic university: to create a more just and humane society.
College of Pharmacy
- Learn more about the Doctor of Pharmacy Program
- Learn more about the Physician Assistant Program
- Learn more about the Master's in Pharmaceutical Sciences
- For More Information
Submit Your Feedback

IMAGES
VIDEO
COMMENTS
The PSCI PhD Program provides training that emphasizes basic as well as applied research through advanced coursework in contemporary pharmaceutical sciences. ... "I only applied to pharmacy schools that advertised a dual degree program; plus USC is the number-one private pharmacy school in the country. ... University of Southern California ...
Fall 2025 Admission Deadline: TBD PhD in Pharmacological Sciences UC Irvine's PhD in Pharmacological Sciences program provides a unique opportunity for those interested in any scientific discipline represented by the Pharmaceutical Sciences faculty to have a year of broad, interdisciplinary training and self-selected lab rotations followed by focused doctoral research in the Pharmaceutical ...
First year pharmacy students can pursue the PharmD/PhD degree by conducting 3 research rotations with faculty on research topics of PSDD during years 1-2 of the pharmacy curriculum. Pharmacy students in their 2nd year can apply for admissions for the PhD program of the Biomedical Sciences graduate program at UCSD (see previous paragraph).
Contact Leslie Carstensen Floren, PharmD, PhD, MA for more information about the dual degree program. This program is a unique joint effort between the UCSF School of Pharmacy's PharmD program and the Pharmaceutical Sciences and Pharmacogenomics (PSPG) PhD program from the UCSF Graduate Division. The overall goal of this dual-degree.
About the program. The Pharmaceutical Sciences and Pharmacogenomics (PSPG) Graduate Program at the University of California, San Francisco (UCSF) focuses on how to develop effective drug therapies for patients that have a minimum of adverse effects. To do this we give our graduate students solid training in the pharmaceutical-related basic ...
The School of Pharmacy at UC San Francisco (UCSF) is a prominent academic institution, founded in 1872, that today pioneers the health sciences graduate-level education, biomedical research, and patient care needed to further the development and best use of precise therapeutics—medications, medical devices, and diagnostic tests—to improve the health of people everywhere. The School is an ...
One of the top pharmacy schools nationwide, USC Mann continues its century-long reputation for innovative programming, practice and collaboration. ... "The rigorous training I received through the USC Pharmaceutical Sciences PhD program equipped me with a strong foundation in natural product chemistry, microbial genetics and analytical ...
Deadline: Sunday, December 1, 2024. The Pharmaceutical Sciences and Pharmacogenomics Graduate Program at UCSF provides a welcoming environment for a wide range of diversity in its student population, including students with disabilities. Typically, qualified applicants will have diverse backgrounds with an undergraduate degree in the biological sciences, genetics, chemistry, bioinformatics ...
Review USC Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences (Doctor of Pharmacy Program) Rating 5 out of 5 My academic experience was a good mix of challenging and interesting coursework, along with out-of-classroom experience, allowing us to become more familiar with pharmacy as a profession.
The PSPG program office is located at the Mission Bay campus. Visit the program website for more information. The PSPG program is offered by the UCSF Graduate Division, administered by the UCSF School of Pharmacy, and delivered by faculty members in the UCSF schools of pharmacy and medicine.
Graduate Student Organizations; UCSD Student-Run Free Clinic Project; Admissions. Admissions: General Information ... Ph.D. Program in 'Pharmaceutical Sciences and Drug Development' ... #1 School of Pharmacy in California by CPJE Pass Rates #1 School of Pharmacy in California for Residency Match Rates (Phase I) ...
All candidates selected for membership must have completed three semesters of the pharmacy program (or post-qualifying exam for PhD students), and they must be approved by the dean of the USC Alfred E. Mann School of Pharmacy and Pharamceutical Sciences. Phi Lambda Sigma. The Phi Lambda Sigma chapter was established at USC in 1988.
The University of California, Irvine School of Pharmacy and Pharmaceutical Sciences' Doctor of Pharmacy program has been granted Candidate status by the Accreditation Council for Pharmacy Education, 190 South LaSalle Street, Suite 2850, Chicago, Illinois 60603-3499, 312-644-3575; FAX 866-228-2631; website: www.acpe-accredit.org.
Offering undergraduate, graduate and professional degrees, the UCI School of Pharmacy & Pharmaceutical Sciences spans a continuum from discovery, development, and delivery of new drugs, devices, and diagnostics all the way to optimizing medication therapy in patients and patient populations. Our school encompasses two departments ...
Transcript. University of California San Francisco School of Pharmacy's Doctor of Pharmacy program is accredited by the Accreditation Council for Pharmacy Education, 190 South LaSalle Street, Suite 2850, Chicago, IL 60603, 312/664-3575; FAX, 312/664-4652, web site www.acpe-accredit.org.
University of Southern California ·. Graduate School. ·. 9 reviews. Doctoral Student: The USC Mann School of Pharmacy and Pharmaceutical Sciences offers a great pharmacy program. It contains a diverse faculty and student population and they encourage diversity, equity, and inclusion. The school offers a variety of opportunities for students ...
Training options. Residency. Fellowship. Advanced practice. MBA. PhD. MPH. …more. No matter where they choose to use the skills they learn here—and learn after they graduate-UCSF PharmDs are transforming health care through pharmacy, and always with the patient first in mind.
Our pharmacy schools are among the top in the U.S. and the world, according to U.S. News & World Report. As public institutions, they are affordable, high quality options. In fact, the UCI School of Pharmacy & Pharmaceutical Sciences is listed as the most affordable pharmacy degree program in the United States by the College Affordability Guide.
Program Description. The UCSF PharmD degree program prepares graduates to be academically and professionally ready for careers in pharmacy practice as caring, patient-centered experts in the safe and effective use of medicines. Admission Requirements. Minimum GPA of 2.8; Minimum completion of 88 quarter units or 59 semester units
About the Program. Pharmaceutical Outcomes Research is a PhD program in the graduate program in Clinical Pharmacy. This program is housed in the Center for Pharmaceutical Outcomes Research (CePOR, SEE-por), a school-wide center in the Skaggs School of Pharmacy. Our doctoral curriculum is designed to provide competent and highly skilled ...
PharmD/PhD Program. Program. The joint PharmD/PhD trains students for careers in academic pharmacy and bench science research. Students participate in the PharmD curriculum and pursue original doctoral research projects in the laboratories of faculty in the Department of Pharmaceutical Sciences.. By counting some course requirements toward the completion of both degrees, the joint program can ...
The College of Pharmacy houses two health profession programs: the Doctor of Pharmacy (Pharm.D.) Program and the Physician Assistant (PA) Program. In keeping with our missions, our programs aim to improve the healthcare of the diverse communities that we serve. Our graduates are knowledgeable across their respective disciplines, prepared to ...
Total Cost: $2,995. Program Length: 12 months. Program Overview: Ed2go partners with various accredited colleges, including Texas Tech and several California State University schools, to offer its ...
How Much Do Online Ph.D. Programs and Doctorates Cost? The cost of an online doctorate can add up, especially when you're typically spending at least three years in your doctoral program. On average, graduate tuition and fees cost around $20,510 in 2021-2022, according to the National Center for Education Statistics (NCES).
Students from the UCSF schools of medicine, dentistry, and pharmacy traveled to Sacramento to advocate for expansion of UC PRIME Program, which aims to recruit and support students who are interested in working in underserved geographic areas to improve health care. Many participants come from diverse backgrounds similar to the places they often end up serving.
The Alumni Association of UC San Francisco (AAUCSF) has named the UCSF Alumni Achievement Awards winners for 2024. These awards honor alumni across UCSF's four schools - Dentistry, Medicine, Nursing, and Pharmacy - and the Graduate Division for their extraordinary contributions to clinical practice, research, entrepreneurship, philanthropy, service, and mentorship, as well as early ...
UC Davis. With a history dating back to 1905, the University of California, Davis, is a public institution that serves more than 40,000 students. Among the school's standout programs is an online PMHNP certificate program that students can complete in just 12 months. PMHNP certificate students must enroll full-time and complete the majority ...
Graduate Programs in Education (GPE) has over 1000 graduate students registered in seven (7) specializations. GPE is proud of the high-quality graduate programs delivered in both online and on campus formats. Its programs are characterized by a strong learner focus, flexibility, technological innovation, and responsiveness to community desire ...