Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science Fair Project Ideas for Kids, Middle & High School Students ⋅

Paper Chromatography Science Projects With a Hypothesis

Chemicals in Dry-Erase Markers
Paper chromatography analyzes mixtures by separating the chemical contents onto paper. For instance, chromatography is used in forensic science to separate chemical substances such as drugs in urine and blood samples. Students can perform paper chromatography projects using ink to understand how scientists are able to determine the presence of different chemicals.
Separate Ink Colors
Form an experiment to separate ink colors using paper chromatography. Hypothesize that regular black ink will show colors on the paper chromatography more noticeably than permanent ink. Set up the experiment using coffee filters and washable and permanent markers. Cut the coffee filters into long strips for each pen. Form a loop by stapling the ends of the strips together. Place a dot of ink on the bottoms of the coffee filter strips. Label each strip using a pencil, specifying the type of pen. Place the strips into a glass, then add water until it touches the bottom of the paper. Observe the strip. Compare your results between permanent marker and washable marker ink. The washable marker colors should spread out onto the paper, while the permanent marker does not because of its permanent ink.
Water vs. Rubbing Alcohol
Create an experiment to separate permanent marker ink colors using paper chromatography in water and rubbing alcohol. Hypothesize that rubbing alcohol will separate the ink colors in permanent markers, while water will not. Set up the experiment using coffee filters and permanent markers. Cut the coffee filters into long strips for each pen. Form a loop by stapling the ends of each strip together. Place a dot of ink on the bottom of the coffee filter strips. Place one strip into a glass of water and place another strip into a glass of rubbing alcohol until the fluid touches the bottom of the paper. Observe the strips. Compare your results between the water and rubbing alcohol solution. The colors should separate on the strip dipped in the rubbing alcohol, but won’t separate when using water.
Different Solvents
Conduct a paper chromatography project to find out if different types of solvents separate ink differently. Set up the experiment using coffee filters and permanent markers. Cut the coffee filters into long strips. Form a loop by stapling the ends of each strip together. Place a dot of ink on the bottom of the coffee filter strips. Place a strip each into a glass of water, rubbing alcohol, vinegar and nail polish remover. Make sure to only add liquid to touch the bottom of the strip. Observe the strips and compare results. Indicate which solvent separated the ink colors the best.
Use a Black Light
Perform an ink paper chromatography test and use a black light to determine if there are any more components visible on the paper than in regular light. Hypothesize that more components will be seen under black light, because some chemicals are invisible under white light. Make sure to look at the paper the same day the paper chromatography test was conducted in order to assure there is no fading on the paper.
Related Articles
How to separate the components of ink, how can parts of a solution be separated by chromatography, why does chromatography work, methods on how to determine ph in ph paper, cool science experiments for teens, what is contained in a permanent marker, how does paper chromatography work & why do pigments..., 5th grade solubility experiment, how-to science experiments for kids with iodine and..., what color would a tester ph paper turn if is dipped..., chalk and vinegar science projects, how to make disappearing ink reappear, how to use litmus papers, what turns ph paper green, food coloring & science projects, food coloring experiments, science fair projects & ideas on art, what is the difference between a solution and a suspension, how to test for hydrochloric acid.
- Science Buddies; Paper Chromatography: Basic Version; Amber Hess; April 2008
About the Author
Based in Huntington Beach, Calif., Dana Schafer has been writing environmental articles and grant proposals since 2006. Schafer has written for Grace Unlimited Corporation and Youth Have Vision. Schafer is in the process of receiving a Master of Science in biology from California State University, Long Beach.
Photo Credits
Comstock/Comstock/Getty Images
Find Your Next Great Science Fair Project! GO

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

paper chromatography
Our editors will review what you’ve submitted and determine whether to revise the article.
- Open Library Publishing Platform - DRAFT – Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry - Thin Layer (TLC) And Paper Chromatography (PC)
- Chemistry LibreTexts Library - Paper Chromatography - Separation and Identification of Five Metal Cations
- Oregon State University - College of Engineering - Paper chromatography
- Academia - Paper Chromatography
- Journal of Emerging Technologies and Innovative Research - Paper Chromatography: A Review

paper chromatography , in analytical chemistry , technique for separating dissolved chemical substances by taking advantage of their different rates of migration across sheets of paper. It is an inexpensive but powerful analytical tool that requires very small quantities of material.
The method consists of applying the test solution or sample as a spot near one corner of a sheet of filter paper. The paper is initially impregnated with some suitable solvent to create a stationary liquid phase . An edge of the paper close to the test spot is then immersed in another solvent in which the components of the mixture are soluble in varying degrees. The solvent penetrates the paper by capillary action and, in passing over the sample spot, carries along with it the various components of the sample. The components move with the flowing solvent at velocities that are dependent on their solubilities in the stationary and flowing solvents. Separation of the components is brought about if there are differences in their relative solubilities in the two solvents. Before the flowing solvent reaches the farther edge of the paper, both solvents are evaporated , and the location of the separated components is identified, usually by application of reagents that form coloured compounds with the separated substances. The separated components appear as individual spots on the path of the solvent. If the solvent flowing in one direction is not able to separate all the components satisfactorily, the paper may be turned 90° and the process repeated using another solvent.
Paper chromatography has become standard practice for the separation of complex mixtures of amino acids , peptides , carbohydrates , steroids , purines , and a long list of simple organic compounds . Inorganic ions can also readily be separated on paper. Compare thin-layer chromatography .
Chemistry Learner
It's all about chemistry.
- Chemical Bonds
- Chemical Reactions
- Materials Chemistry
- Organic Chemistry
- Periodic Trends
- Periodic Table Groups
- How to Read Periodic Table
- Naming Covalent Compounds Worksheets
- Net Ionic Equation Worksheets
- Types of Chemical Reactions Worksheets
- Word Equations Worksheets
- Valence Electrons Worksheets
- Graphing Periodic Trends Worksheets
- Periodic Trends Ionization Energy Worksheets
- Atomic Structure And Isotopes Worksheets
Paper Chromatography
How does paper chromatography work, stationary and mobile phases, paper chromatography experiment, applications.
Paper chromatography is a simple and cost-effective separation technique that separates and identifies different components in a mixture. [1-4]
In paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. The mixture to be analyzed is applied to the paper. As the solvent moves up through capillary action, it carries along the individual components of the mixture at different rates based on their solubility and affinity for the stationary phase.
The principle behind paper chromatography lies in the differential partitioning of compounds between the stationary and mobile phases. The stationary phase typically consists of cellulose fibers embedded in filter paper or thin-layer chromatography plates. These fibers provide an adsorbent surface for compounds to interact with.
Understanding the mechanism behind paper chromatography requires knowledge of several key processes. [1-4]
The first process is capillary action, which refers to the ability of liquids to flow through narrow spaces against gravity. In paper chromatography, capillary action allows the solvent to move up the paper strip due to its attraction to the fibers in the paper. As the solvent moves up, it carries the solutes in the analyzed mixture. This migration of solutes is driven by two main mechanisms: adsorption and partitioning.
Adsorption occurs when solute molecules adhere to the fibers or other surfaces within the paper. It can be influenced by polarity and molecular size, with more polar or larger molecules having stronger interactions with the stationary phase.
Conversely, partitioning involves solute molecules distributing themselves between two immiscible phases – in this case, between the stationary phase (paper) and mobile phase (solvent). The extent of partitioning depends on factors such as solute polarity and affinity for either phase.
As solutes migrate up through capillary action, they may experience different degrees of adsorption and partitioning along their journey. This results in their separation based on their characteristics. By analyzing how far each component migrates on a chromatogram – a visual representation of separated components – scientists can determine properties such as retention factor (R f ) values and identify unknown substances based on known reference compounds.
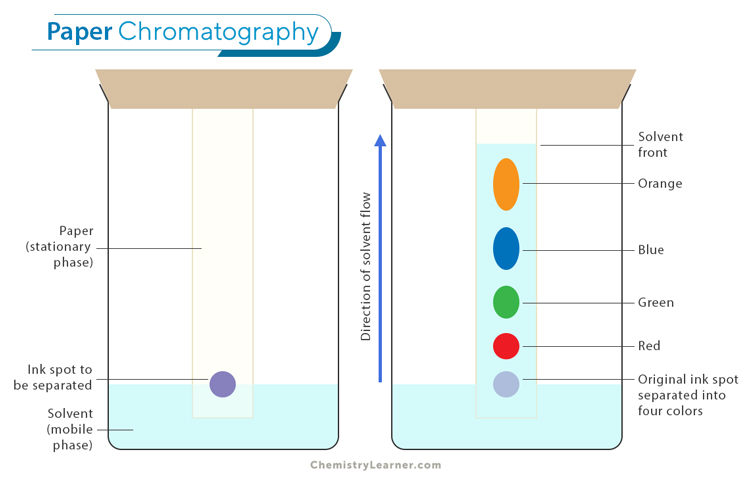
Stationary and mobile phases play crucial roles in separating components of a mixture. [1-4]
The stationary phase refers to the absorbent material fixed on the chromatography paper. It can be made of cellulose or other materials with high absorbency. The stationary phase acts as a substrate for the sample mixture to interact with during separation.
On the other hand, the mobile phase is the solvent or liquid that moves through the stationary phase, carrying the sample components. The mobile phase must have good solubility with the components of interest. It should be able to flow easily through the paper.
As the mobile phase moves through the stationary phase, it interacts differently with each mixture component based on their solubility and affinity for both phases. This differential interaction leads to separation as different components travel at different rates along the paper.
Choosing an appropriate combination of stationary and mobile phases is important for effective separation in paper chromatography. Factors such as polarity, viscosity, and compatibility between phases must be considered to achieve optimal results.
Performing a paper chromatography experiment involves several essential steps to ensure accurate results. The process begins with preparing samples for paper chromatography, then spotting the sample on the paper strip, and finally, developing the chromatogram. [1-4]
Preparing the samples is crucial in obtaining reliable data. It involves selecting appropriate substances to analyze and ensuring they are suitable for chromatography. Samples can be liquid or solid and must be dissolved or crushed into a solution before application.
Next, spotting the sample on the paper strip is done carefully to ensure accurate separation. A small spot of the prepared sample is placed near one end of a designated area on the filter paper strip. It is essential to use a capillary tube or micropipette for precise and consistent application.
Once all samples are spotted on the filter paper strip, it is time for the development of the chromatogram. This step involves placing one end of the strip into a solvent traveling up through capillary action. The choice of solvent depends on factors such as solubility and desired separation distance.
As the solvent moves up through the filter paper strip, it carries different components in each sample. These components separate based on their affinity for stationary (filter paper) and mobile (solvent) phases. The separation occurs due to differences in molecular size, polarity, or other physical properties.
Throughout this process, it is important to maintain controlled conditions such as temperature and humidity to ensure reproducibility. Further analysis can be conducted once an optimal separation has been achieved, which can take several minutes or hours depending on various factors, including solvent choice and sample composition.
The diverse applications of paper chromatography across various fields are listed below. [1-4]
- It plays a crucial role in forensic analysis by separating and identifying different components in complex mixtures, such as blood or ink samples.
- Aids in the analysis of crime scene evidence, allowing forensic scientists to determine the presence of specific substances and identify potential suspects based on chromatographic patterns
- Enables the separation of different dyes used in food coloring, helping to ensure compliance with regulatory standards and quality control measures
- Determines the authenticity and safety of food products by identifying and quantifying specific components present in complex food matrices
- Separate and identify active ingredients, impurities, and by-products in pharmaceutical formulations.
- Chem.libretexts.org
- Swe.mit.edu
- Chemlab.truman.edu
Trending Topics
© 2024 ( Chemistry Learner )
Chemistry Dictionary
A complete A-Z dictionary of chemistry terms.
Are you a chemistry student? Visit A-Level Chemistry to download comprehensive revision materials - for UK or international students!
Paper chromatography
Introduction to paper chromatography.
Paper chromatography is a chromatography technique used to separate mixture of chemical substances into its individual compounds. Paper chromatography is used to teach TLC or other chromatography as it is very similar to TLC.
Principles of paper chromatography
All chromatography follow the same principle. Paper Chromatography consists of two phases: one mobile phase and one contiguous stationery phase. The stationery phase a paper and the mobile gas is solvent. The compound mixture moves along with the mobile phase through stationery phase and separates depending on the different degree of adhesion (on the paper) of each component in the sample or the compound mixture.
Explanation
The stationery phase.
The paper chromatography is very similar to Thin layer chromatography. Difference is, instead of using a thin layer of silica on metal, it uses a special type of chromatography paper as stationery phase. This paper is made of cellulose. Cellulose is a polymer of simple sugar, glucose.
Cellulose contains -OH group similar to the silica or alumina on the TLC plate. The surface of cellulose is thus very polar. So the compounds can form hydrogen bond or can interact by van der waals dispersion forces and dipole dipole forces.
Paper chromatography works in few steps:
Step 1: A horizontal line is drawn near one end (about 1.5 cm from the bottom edge) of the paper. In figure below 6 is the horizontal line.
![Paper chromatography 13 By No machine-readable author provided. Dubaj~commonswiki assumed (based on copyright claims). [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/0/0a/Paper.jpg)
Step 2: The sample needs to be separated is placed as a small drop or line on to the paper using capillary tube. Labelling the drop by a pencil with an alphabet or number help to identify the compound later. In figure above 3 and 4 are the drops labelled. The drops are then soaked on the paper and dried.
Step 3: The paper is then placed into a sealed container with a swallow layer of suitable solvent. The solvent level must be lower than the pencil line or drop on it. The container need to be covered to stop the solvent to evaporate.

Step 4: The solvent rises up the paper chromatography taking each component of the sample with it. The components travel with the solvent depends on three things:
- The polarity of the sample molecule. The non polar components travel faster than the polar component.
- The attraction between the sample molecule and the solvent or solvent mixture.
- The attraction between the sample and the silica.
Suppose any sample compound mixture contains three colored molecules green, blue and red. According to their polarity, the order of these compounds is green<blue<red. Thus the most non polar green will travel first along with the mobile phase. Then blue and at last most polar compound the red one.
Step 5: When the solvent rises near the end of the paper then the paper should be taken out from sealed container and air dried. The paper with separated bands of components are then observed under UV-light.
![Paper chromatography 15 By L26 (Own work) [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/c/c2/TLC_under_UV_lump.jpg)
The compounds in the sample travels along with solvent to give separate bands on the paper. The distance travelled by same compound with respect to the solvent is always constant. Thus the ratio of the distance that the compound travelled and the distance that the solvent travelled is denoted as R f . And mathematically expressed as:
- Paper chromatography is an chromatography technique used to separate mixture of chemical substances into its individual compounds.
- Paper chromatography consists of two phases: one mobile phase and one contiguous stationery phase.
- Paper used in paper chromatography is made of cellulose.
- A suitable solvent (mobile phase) is moved along with a compound mixture through the paper according to the polarity and the degree of adhesion of each component on the stationery phase.
- The ratio of the distance that the compound travelled and the distance that the solvent travelled is denoted as R f .
Please rate these notes

Microbe Notes
Paper Chromatography- Definition, Types, Principle, Steps, Uses
Table of Contents
Interesting Science Videos
What is Paper Chromatography?
Paper chromatography (PC) is a type of planar chromatography whereby chromatography procedures are run on a specialized paper.
PC is considered to be the simplest and most widely used of the chromatographic techniques because of its applicability to isolation, identification, and quantitative determination of organic and inorganic compounds.
It was first introduced by German scientist Christian Friedrich Schonbein (1865).
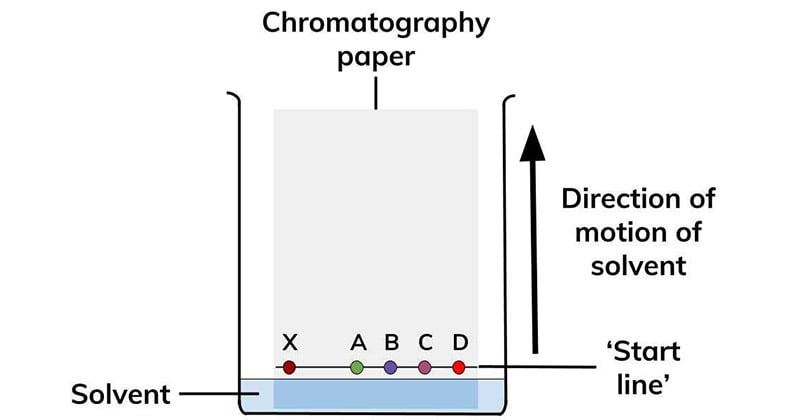
Types of Paper chromatography
Paper adsorption chromatography.
Paper impregnated with silica or alumina acts as adsorbent (stationary phase) and solvent as mobile phase.
Paper Partition Chromatography
Moisture / Water present in the pores of cellulose fibers present in filter paper acts as stationary phase & another mobile phase is used as solvent In general paper chromatography mostly refers to paper partition chromatography.
Principle of Paper chromatography
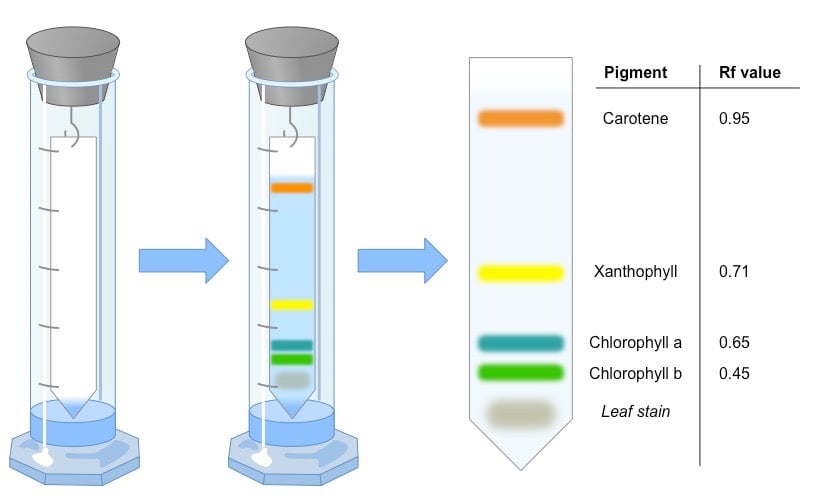
The principle of separation is mainly partition rather than adsorption. Substances are distributed between a stationary phase and a mobile phase. Cellulose layers in filter paper contain moisture which acts as a stationary phase. Organic solvents/buffers are used as mobile phase. The developing solution travels up the stationary phase carrying the sample with it. Components of the sample will separate readily according to how strongly they adsorb onto the stationary phase versus how readily they dissolve in the mobile phase.
Instrumentation of Paper chromatography
- Stationary phase & papers used
- Mobile phase
- Developing Chamber
- Detecting or Visualizing agents
1. STATIONARY PHASE AND PAPERS
- Whatman filter papers of different grades like No.1, No.2, No.3, No.4, No.20, No.40, No.42 etc
- In general the paper contains 98-99% of α-cellulose, 0.3 – 1% β -cellulose.
Other modified papers
- Acid or base washed filter paper
- Glass fiber type paper.
- Hydrophilic Papers – Papers modified with methanol, formamide, glycol, glycerol etc.
- Hydrophobic papers – acetylation of OH groups leads to hydrophobic nature, hence can be used for reverse phase chromatography.
- Impregnation of silica, alumna, or ion exchange resins can also be made.
2. PAPER CHROMATOGRAPHY MOBILE PHASE
- Pure solvents, buffer solutions or mixture of solvents can be used.
Hydrophilic mobile phase
- Isopropanol: ammonia:water 9:1:2
- Methanol : water 4:1
- N-butanol : glacial acetic acid : water 4:1:5
Hydrophobic mobile phases
- dimethyl ether: cyclohexane kerosene : 70% isopropanol
- The commonly employed solvents are the polar solvents, but the choice depends on the nature of the substance to be separated.
- If pure solvents do not give satisfactory separation, a mixture of solvents of suitable polarity may be applied.
3. CHROMATOGRAPHIC CHAMBER
- The chromatographic chambers are made up of many materials like glass, plastic or stainless steel . Glass tanks are preferred most.
- They are available invarious dimensional size depending upon paper length and development type.
- The chamber atmosphere should be saturated with solvent vapor.
Steps in Paper Chromatography
In paper chromatography, the sample mixture is applied to a piece of filter paper, the edge of the paper is immersed in a solvent, and the solvent moves up the paper by capillary action. The basic steps include:
Selection of Solid Support
Fine quality cellulose paper with defined porosity, high resolution, negligible diffusion of the sample, and favoring good rate of movement of solvent.
Selection of Mobile Phase
Different combinations of organic and inorganic solvents may be used depending on the analyte.
Example. Butanol: Acetic acid: Water (12:3:5) is a suitable solvent for separating amino acids.
Saturation of Tank
The inner wall of the tank is wrapped with filter paper before the solvent is placed in the tank to achieve better resolution.
Sample Preparation and Loading
If the solid sample is used, it is dissolved in a suitable solvent. Sample (2-20ul) is added on the baseline as a spot using a micropipette and air dried to prevent the diffusion.
Development of the Chromatogram
Different types of development techniques can be used:
ASCENDING DEVELOPMENT
- Like conventional type, the solvent flows against gravity.
- The spots are kept at the bottom portion of paper and kept in a chamber with mobile phase solvent at the bottom.
DESCENDING TYPE
- This is carried out in a special chamber where the solvent holder is at the top.
- The spot is kept at the top and the solvent flows down the paper.
- In this method solvent moves from top to bottom so it is called descending chromatography.
ASCENDING – DESCENDING DEVELOPMENT
- A hybrid of above two techniques is called ascending-descending chromatography.
- Only length of separation increased, first ascending takes place followed by descending.
CIRCULAR / RADIAL DEVELOPMENT
- Spot is kept at the centre of a circular paper.
- The solvent flows through a wick at the centre & spreads in all directions uniformly.
Drying of Chromatogram
After the development, the solvent front is marked and left to dry in a dry cabinet or oven.
Colorless analytes were detected by staining with reagents such as iodine vapor, ninhydrin, etc.
Radiolabeled and fluorescently labeled analytes were detected by measuring radioactivity and fluorescence respectively.
Some compounds in a mixture travel almost as far as the solvent does; some stay much closer to the baseline . The distance traveled relative to the solvent is a constant for a particular compound as long as other parameters such as the type of paper and the exact composition of the solvent are constant. The distance traveled relative to the solvent is called the Rf value.
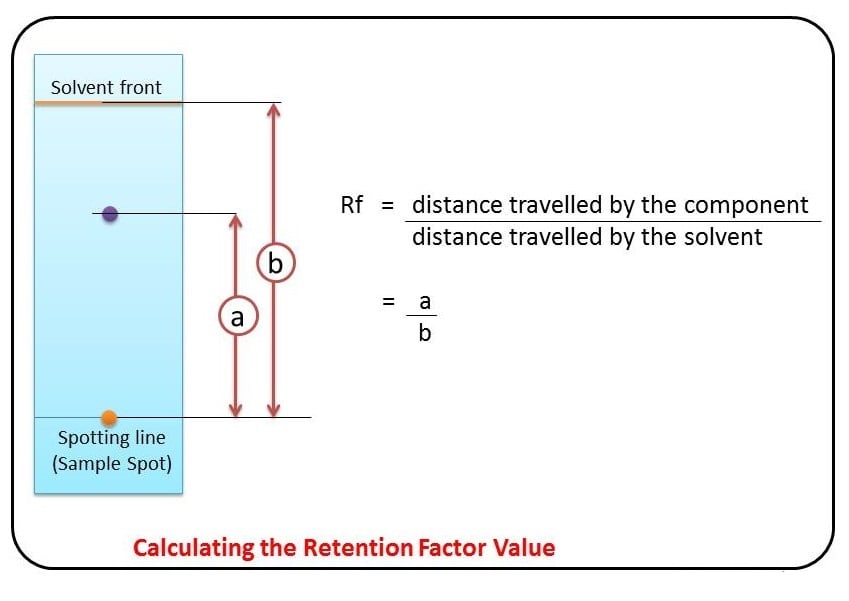
Thus, in order to obtain a measure of the extent of movement of a component in a paper chromatography experiment, “Rf value” is calculated for each separated component in the developed chromatogram. An Rf value is a number that is defined as the distance traveled by the component from the application point.
Applications of Paper Chromatography
- To check the control of purity of pharmaceuticals,
- For detection of adulterants,
- Detect the contaminants in foods and drinks,
- In the study of ripening and fermentation,
- For the detection of drugs and dopes in animals & humans
- In analysis of cosmetics
- Analysis of the reaction mixtures in biochemical labs.
Advantages of Paper Chromatography
- Paper Chromatography requires very less quantitative material.
- Paper Chromatography is cheaper compared to other chromatography methods.
- Both unknown inorganic as well as organic compounds can be identified by paper chromatography method.
- Paper chromatography does not occupy much space compared to other analytical methods or equipments.
- Excellent resolving power
Limitations of Paper Chromatography
- Large quantity of sample cannot be applied on paper chromatography.
- In quantitative analysis paper chromatography is not effective.
- Complex mixture cannot be separated by paper chromatography.
- Less Accurate compared to HPLC or HPTLC
- http://frndzzz.com/Advantages-and-Disadvantages-of-Paper-Chromatography
- https://www.slideshare.net/shaisejacob/paper-chromatography-pptnew?next_slideshow=1
- https://www.slideshare.net/shaisejacob/paper-chromatography-ppt-new
- https://www.biochemden.com/paper-chromatography/
- http://web.engr.oregonstate.edu/~rochefow/K-12%20Outreach%20Activities/Microfluidics%20&%20Pregnancy%20Test%20Kit%20Lab/paper%20chromatography_Chemguide.pdf
- https://pubs.acs.org/doi/abs/10.1021/ac60051a002
About Author
Sagar Aryal
6 thoughts on “Paper Chromatography- Definition, Types, Principle, Steps, Uses”
Examples of substances that can be effectively separated by paper chromatography is necessary.
I enjoy this write up, but the definition of Rf Values just mention distance traveled by the solute from the point from the point of application of the sample, how about the total distance traveled by the solvent? Some examples of how Rf value can be calculated is necessary.
Pls how is charging a disadvantage of filter paper in chromatography
Hi! I enjoyed reading it. Hmm just wanna know somepoints.
Why it is best to use the farthest distance traveled by a sugar if and when the solvent went over the paper and what is the purpose of heating the chromatographic paper after running the procedure?
This links are so much useful and it’s very helpful also….
Thanks for sharing important details like this. I enjoyed reading your site, and love to know the latest updates.
Paper Chromatography
What is paper chromatography.
Chromatography technique that uses paper sheets or strips as the adsorbent being the stationary phase through which a solution is made to pass is called paper chromatography. It is an inexpensive method of separating dissolved chemical substances by their different migration rates across the sheets of paper. It is a powerful analytical tool that uses very small quantities of material. Paper chromatography was discovered by Synge and Martin in the year 1943.
Table of Contents
Paper chromatography principle, paper chromatography diagram, paper chromatography procedure, paper chromatography applications.
- Types of Paper Chromatography
- Frequently Asked Questions – FAQs
The principle involved can be partition chromatography or adsorption chromatography. Partition chromatography because the substances are partitioned or distributed between liquid phases. The two phases are water held in pores of the filter paper and the other phase is a mobile phase which passes through the paper. When the mobile phase moves, the separation of the mixture takes place. The compounds in the mixture separate themselves based on the differences in their affinity towards stationary and mobile phase solvents under the capillary action of pores in the paper. Adsorption chromatography between solid and liquid phases, wherein the solid surface of the paper is the stationary phase and the liquid phase is the mobile phase.
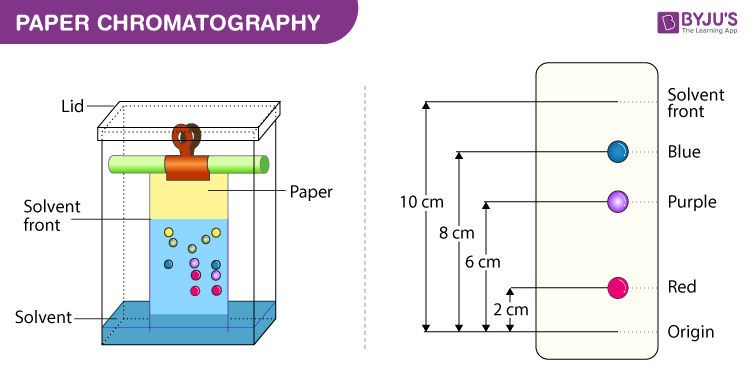
Below we have explained the procedure to conduct Paper Chromatography Experiment for easy understanding of students.
- Selecting a suitable type of development: It is decided based on the complexity of the solvent, paper, mixture, etc. Usually ascending type or radial paper chromatography is used as they are easy to perform. Also, it is easy to handle, the chromatogram obtained is faster and the process is less time-consuming.
- Selecting a suitable filter paper : Selection of filter paper is done based on the size of the pores and the sample quality.
- Prepare the sample: Sample preparation includes the dissolution of the sample in a suitable solvent (inert with the sample under analysis) used in making the mobile phase.
- Spot the sample on the paper: Samples should be spotted at a proper position on the paper by using a capillary tube.
- Chromatogram development: Chromatogram development is spotted by immersing the paper in the mobile phase. Due to the capillary action of paper, the mobile phase moves over the sample on the paper.
- Paper drying and compound detection : Once the chromatogram is developed, the paper is dried using an air drier. Also, detecting solution can be sprayed on the chromatogram developed paper and dried to identify the sample chromatogram spots.
There are various applications of paper chromatography . Some of the uses of Paper Chromatography in different fields are discussed below:
- To study the process of fermentation and ripening.
- To check the purity of pharmaceuticals.
- To inspect cosmetics.
- To detect the adulterants.
- To detect the contaminants in drinks and foods.
- To examine the reaction mixtures in biochemical laboratories.
- To determine dopes and drugs in humans and animals.
Types of paper chromatography:
- Ascending Paper Chromatography – The techniques goes with its name as the solvent moves in an upward direction.
- Descending Paper Chromatography – The movement of the flow of solvent due to gravitational pull and capillary action is downwards, hence the name descending paper chromatography.
- Ascending – Descending Paper Chromatography – In this version of paper chromatography, movement of solvent occurs in two directions after a particular point. Initially, the solvent travels upwards on the paper which is folded over a rod and after crossing the rod it continues with its travel in the downward direction.
- Radial or Circular Paper Chromatography – The sample is deposited at the centre of the circular filter paper. Once the spot is dried, the filter paper is tied horizontally on a Petri dish which contains the solvent.
- Two Dimensional Paper Chromatography – Substances which have the same r f values can be resolved with the help of two-dimensional paper chromatography.
Frequently Asked Questions – FAQs
What are the advantages of paper chromatography.
Paper Chromatography Has Many Benefits Simple and rapid Paper chromatography necessitates a minimal amount of quantitative material. Paper chromatography is less expensive than other chromatography methods. The paper chromatography method can identify both unknown inorganic and organic compounds. Paper chromatography takes up little space when compared to other analytical methods or equipment. Outstanding resolving power
Why water is not used in paper chromatography?
It is preferable to use a less polar solvent, such as ethanol, so that the non-polar compounds will travel up the paper while the polar compounds will stick to the paper, separating them.

What are the limitations of Paper Chromatography?
Limitations of Paper Chromatography are as follows- Paper chromatography cannot handle large amounts of sample. Paper chromatography is ineffective in quantitative analysis. Paper chromatography cannot separate complex mixtures. Less Accurate than HPLC or HPTLC
What is the importance of paper chromatography?
Paper chromatography has traditionally been used to analyse food colours in ice creams, sweets, drinks and beverages, jams and jellies. Only edible colours are permitted for use to ensure that no non-permitted colouring agents are added to the foods. This is where quantification and identification come into play.
Is paper chromatography partition or adsorption?
A type of partition chromatography is paper chromatography.
To learn more about the different types of paper chromatography from the experts, register with BYJU’S now!
Other important links:

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
It is so easy to understand by students Explained with applications also.
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School
Paper Chromatography Experiment
March 17, 2021 By Emma Vanstone Leave a Comment
This simple felt tip pen paper chromatography experiment is a great way to learn about this particular method of separating mixtures .
WHAT IS CHROMATOGRAPHY?
Chromatography is a technique used to separate mixtures. Information from a chromatography investigation can also be used to identify different substances.
In chromatography, the mixture is passed through another substance, in this case, filter paper. The different-coloured ink particles travel at different speeds through the filter paper, allowing the constituent colours of the pen ink to be seen.
All types of chromatography have two phases: a mobile phase where the molecules can move and a stationary phase where they can’t move. In the case of paper chromatography, the stationary phase is the filter paper, and the mobile phase is the solvent ( water ).
The more soluble the ink molecules, the further they are carried up the paper.
The video below shows chromatography in action.
You’ll need:
Filter paper or paper towel
Felt tip pens – not washable or permanent
A container – glass, jar or plate
Instructions
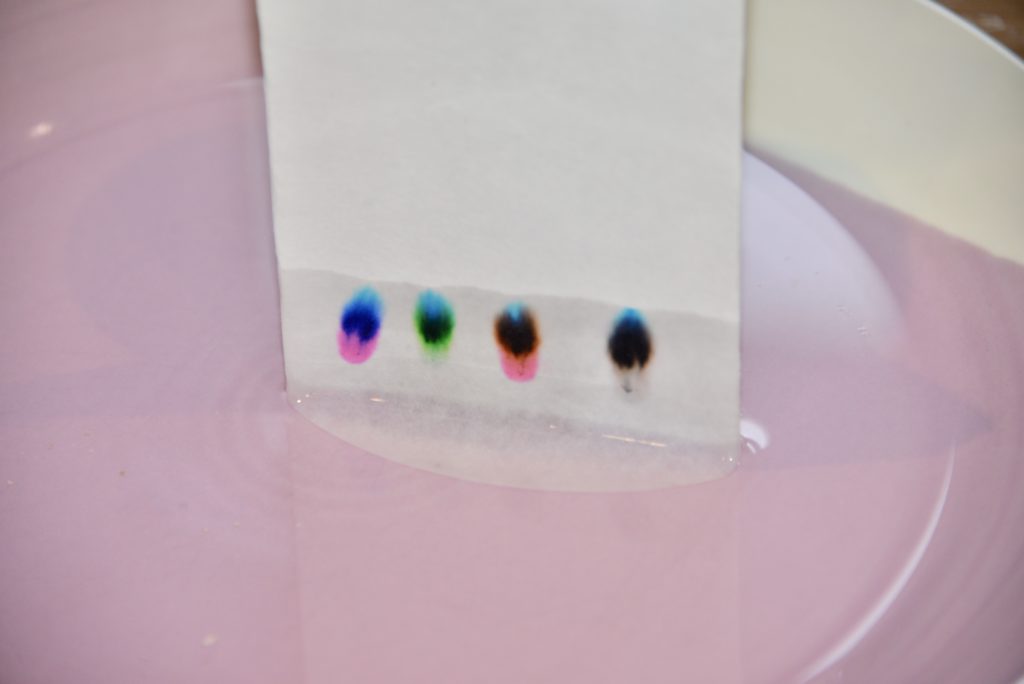
Pour a small amount of water onto a plate or into the bottom of a jar.
Find a way to suspend the filter paper over the water so that just the very bottom touches the water. If you do the experiment in a jar, the easiest way to do this is to wrap the top of the filter paper around a pencil, clip it in place, and suspend it over the top of the jar.
Our LEGO holder worked well, too!
Use the felt tip pens to draw a small circle about 1cm from the bottom of the filter paper with each colour pen you want to test.
Suspend the filter paper in the water and watch as the ink moves up the filter paper.
You should end up with something like this! The end result is called a chromatogram.

What happens if you use washable pens?
If the inks are washable, they tend to contain just one type of ink, so there is no separation of colour.
Below, only a couple of the inks have separated compared to the non-washable pens above.

Why does chromatography work?
When the filter paper containing the ink spots is placed in the solvent ( in this case, water ), the dyes travel through the paper.
Different dyes in ink travel through the chromatography filter paper at different speeds. The most soluble colours dissolve and travel further and faster than less soluble dyes, which stick to the paper more.
I’ve created a free instruction sheet and chromatography experiment write up to make the activity even easier.

Extension task
Experiment with different types and colours of pens. Depending on the type of ink used, some will work better than others.
Try chromatography with sweets .
Steamstational also has a great leaf chromatography investigation.
More separation experiments
Clean up water by making your own filter .

Separate water and sand by evaporation .
Make colourful salt crystals by separating salt and water.
Separate liquid mixtures with a bicycle centrifuge .

Last Updated on May 20, 2024 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Leaf Chromatography Experiment – Easy Paper Chromatography

Leaf chromatography is paper chromatography using leaves. Paper chromatography is a separation technique. When applied to leaves, it separates the pigment molecules mostly according to their size. The main pigment molecule in green leaves is chlorophyll, which performs photosynthesis in the plant. Other pigments also occur, such as carotenoids and anthocyanins. When leaves change color in the fall , the amount and type of pigment molecules changes. Leaf chromatography is a fun science project that lets you see these different pigments.
Leaf Chromatography Materials
You only need a few simple materials for the leaf chromatography project:
- Rubbing alcohol (isopropyl alcohol)
- Coffee filters or thick paper towels
- Small clear jars or glasses with lids (or plastic wrap to cover the jars)
- Shallow pan
- Kitchen utensils
You can use any leaves for this project. A single plant leaf contains several pigment molecules, but for the most colors, use a variety of leaves. Or, collect several of each kind of leaf and compare them to each other. Good choices are colorful autumn leaves or chopped spinach.
Perform Paper Chromatography on Leaves
The key steps are breaking open the cells in leaves and extracting the pigment molecule and then separating the pigment using the alcohol and paper.
- Finely chop 2-3 leaves or several small leaves. If available, use a blender to break open the plant cells. The pigment molecules are in the chloroplasts of the cells, which are organelles encased within the plant cell walls. The more you break up the leave, the more pigment you’ll collect.
- Add enough alcohol to just cover the leaves.
- If you have more samples of leaves, repeat this process.
- Cover the container of leaves and alcohol and set it in a shallow pan filled with enough hot tap water to surround and heat the container. You don’t want water getting into your container of leaves.
- Replace the hot water with fresh water as it cools. Swirl the container of leaves around from time to time to aid the pigment extraction into the alcohol. The extraction is ready when the alcohol is deeply colored. The darker its color, the brighter the resulting chromatogram.
- Cut a long strip of coffee filter or sturdy paper towel for each chromatography jar. Paper with an open mesh (like a paper towel) works quickly, but paper with a denser mesh (like a coffee filter) is slower but gives a better pigment separation.
- Place a strip of paper into jar, with one end in the leaf and alcohol mixture and the other end extending upward and out of the jar.
- The alcohol moves via capillary action and evaporation, pulling the pigment molecules along with it. Ultimately, you get bands of color, each containing different pigments. After 30 to 90 minutes (or whenever you achieve pigment separation), remove the paper strips and let them dry.
How Leaf Chromatography Works
Paper chromatography separates pigments in leaf cells on the basis of three criteria:
- Molecule size
Solubility is a measure of how well a pigment molecule dissolves in the sol vent. In this project, the solvent is alcohol . Crushing the leaves breaks open cells so pigments interact with alcohol. Only molecules that are soluble in alcohol migrate with it up the paper.
Assuming a pigment is soluble, the biggest factor in how far it travels up the paper is particle size. Smaller molecules travel further up the paper than larger molecules. Small molecules fit between fibers in the paper more easily than big ones. So, they take a more direct path through the paper and get further in less time. Large molecules slowly work their way through the paper. In the beginning, not much space separates large and small molecules. The paper needs to be long enough that the different-sized molecules have enough time to separate enough to tell them apart.
Paper consists of cellulose, a polysaccharide found in wood, cotton, and other plants. Cellulose is a polar molecule . Polar molecules stick to cellulose and don’t travel very far in paper chromatography. Nonpolar molecules aren’t attracted to cellulose, so they travel further.
Of course, none of this matters if the solvent doesn’t move through the paper. Alcohol moves through paper via capillary action . The adhesive force between the liquid and the paper is greater than the cohesive force of the solvent molecules. So, the alcohol moves, carrying more alcohol and the pigment molecules along with it.
Interpreting the Chromatogram
- The smallest pigment molecules are the ones that traveled the greatest distance. The largest molecules are the ones that traveled the least distance.
- If you compare chromatograms from different jars, you can identify common pigments in their leaves. All things being equal, the lines made by the pigments should be the same distance from the origin as each other. But, usually conditions are not exactly the same, so you compare colors of lines and whether they traveled a short or long distance.
- Try identifying the pigments responsible for the colors.
There are three broad classes of plant pigments: porphyrins, carotenoids, and flavonoids. The main porphyrins are chlorophyll molecules. There are actually multiple forms of chlorophyll, but you can recognize them because they are green. Carotenoids include carotene (yellow or orange), lycopene (orange or red), and xanthophyll (yellow). Flavonoids include flavone and flavonol (both yellow) and anthocyanin (red, purple, or even blue).
Experiment Ideas
- Collect leaves from a single tree or species of tree as they change color in the fall. Compare chromatograms from different colors of leaves. Are the same pigments always present in the leaves? Some plants produce the same pigments, just in differing amounts. Other plants start producing different pigments as the seasons change.
- Compare the pigments in leaves of different kinds of trees.
- Separate leaves according to color and perform leaf chromatography on the different sets. See if you can tell the color of leaves just by looking at the relative amount of different pigments.
- The solvent you use affects the pigments you see. Repeat the experiment using acetone (nail polish remover) instead of alcohol.
- Block, Richard J.; Durrum, Emmett L.; Zweig, Gunter (1955). A Manual of Paper Chromatography and Paper Electrophoresis . Elsevier. ISBN 978-1-4832-7680-9.
- Ettre, L.S.; Zlatkis, A. (eds.) (2011). 75 Years of Chromatography: A Historical Dialogue . Elsevier. ISBN 978-0-08-085817-3.
- Gross, J. (1991). Pigments in Vegetables: Chlorophylls and Carotenoids . Van Nostrand Reinhold. ISBN 978-0442006570.
- Haslam, Edwin (2007). “Vegetable tannins – Lessons of a phytochemical lifetime.” Phytochemistry . 68 (22–24): 2713–21. doi: 10.1016/j.phytochem.2007.09.009
- McMurry, J. (2011). Organic chemistry With Biological Applications (2nd ed.). Belmont, CA: Brooks/Cole. ISBN 9780495391470.
Related Posts

BIOLOGY JUNCTION
Test And Quizzes for Biology, Pre-AP, Or AP Biology For Teachers And Students
Paper Chromatography Report
Introduction The purpose of this experiment is to observe how chromatography can be used to separate mixtures of chemical substances. Chromatography serves mainly as a tool for the examination and separation of mixtures of chemical substances. Chromatography is using a flow of solvent or gas to cause the components of a mixture to migrate differently from a narrow starting point in a specific medium, in the case of this experiment, filter paper. It is used for the purification and isolation of various substances. A chromatographically pure substance is the result of the separation. Because purification of substances is required to determine their properties, chromatography is an indispensable tool in the sciences concerned with chemical substances and their reactions.
Chromatography is also used to compare and describe chemical substances. The chromatographic sequence of sorbed substances is related to their atomic and molecular structures. A change in a chemical substance produced by a chemical or biological reaction often alters the solubility and migration rate. With this knowledge, alterations or changes can be detected in the substance.
In all chromatographic separations, there is an important relationship between the solvent, the chromatography paper, and the mixture. For a particular mixture, the solvent and the paper must be chosen so the solubility is reversible and be selective for the components of the mixture. The main requirement, though, of the solvent is to dissolve the mixture needing to be separated. The porous paper used must also absorb the components of the mixtures selectively and reversibly. For the separation of a mixture, the substances making up the mixture must be evenly dispersed in a solution, a vapor, or a gas. Once all of the above criteria have been met, chromatography can be a simple tool for separating and comparing chemical mixtures.
Hypothesis Paper can be used to separate mixed chemicals.
Materials The materials used for this lab are paper, pencil, eraser, filter paper, test tube, rubber stopper, paper clip, metric ruler, black felt-tip pen, and a computer.
Methods The first step of the method is to bend a paper clip so that it is straight with a hook at one end. Push the straight end of the paper clip into the bottom of the rubber stopper. Next, you hang a thin strip of filter paper on the hooked end of the paper clip. Insert the paper strip into the test tube. The paper should not touch the sides of the test tube and should almost touch the bottom of the test tube. Now you will remove the paper strip from the test tube. Draw a solid 5-mm-wide band about 25 mm from the bottom of the paper, using the black felt-tip pen. Use a pencil to draw a line across the paper strip 10 cm above the black band.
Pour about 2 mL of water into the test tube. The water will act as a solvent. Put the filter paper back into the test tube with the bottom of the paper in the water and the black band above the water. Observe what happens as the liquid travels up the paper. Record the changes you see. When the solvent has reached the pencil line, remove the paper from the test tube. Measure how far the solvent traveled before the strip dries. Finally, let the strip dry on the desk. With the metric ruler, measure the distance from the starting point to the top edge of each color. Record this data in a data table. Calculate a ratio for each color by dividing the distance the color traveled by the distance the solvent traveled.
Results The results of the experiment are shown in a chart and a graph.
| 70 mm | 111 mm | .63 | |
| 82 mm | 111 mm | .74 | |
| 101 mm | 111 mm | .91 | |
| 110 mm | 111 mm | .99 | |
| 111 mm | 111 mm | 1.0 |
1. How many colors separated from the black ink? Five colors separated from the black ink: yellow, pink, red, purple, and blue.
2. What served as the solvent for the ink? Water served as the solvent for the ink. As the solvent traveled up the paper, which color of ink appeared first? The color orange first appeared as the solvent traveled up the paper.
3. List the colors in order, from top to bottom, which separated from the black ink. The colors separated in this order, from top to bottom: blue, purple, red, pink, and then yellow.
4. In millimeters, how far did the solvent travel? The solvent traveled 111 mm.
5. From your results, what can you conclude is true about black ink? Black ink is a mixture of several different colors.
6 . Why did the inks separate? The inks separated because the black ink was a mixture of different pigments with different molecular characteristics. These differences allow for different rates of absorption by the filter paper.
7. Why did some inks move a greater distance? The ink least readily absorbed by the paper would then travel the farthest from the starting mark. You can conclude from this information that the different pigments were absorbed at different rates.
Error Analysis Possible errors could include inaccurate measurements of the distances traveled by the inks and mistakes when calculating the ratio traveled by the water and colors. If a longer test tube was used, a longer strip of filter paper could have been used. This may have changed the ratios. Another color may have been present, but not detected because of the filter paper length.
Conclusion The proposed hypothesis was correct. The paper chromatography did show that black ink could be separated into various colors. The black ink gets its color from a mixture of various colored inks blended together. The first color of ink to appear on the filter paper was yellow followed by pink, red, purple then blue. The colors separated the way they did because of the differences in their molecular characteristics, specifically, their solubility in water and their rate of absorption by the paper. The most soluble and readily absorbed ink color was the yellow. The least soluble and least absorbable ink color was the blue.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
Leaf chromatography
In association with Nuffield Foundation
- Five out of five
- No comments
Try this class practical using paper chromatography to separate and investigate the pigments in a leaf
Most leaves are green due to chlorophyll. This substance is important in photosynthesis (the process by which plants make their food). In this experiment, students investigate the different pigments present in a leaf, from chlorophyll to carotenes, using paper chromatography.
The experiment takes about 30 minutes and can be carried out in groups of two or three students.
- Eye protection
- Pestle and mortar
- Chromatography paper
- Beaker, 100 cm 3
- Small capillary tube (see note 1)
- Cut-up leaves, or leaves and scissors (see note 2)
- Propanone (HIGHLY FLAMMABLE, IRRITANT), supplied in a small bottle fitted with a teat pipette (see note 3)
Equipment notes
- The capillary tubing can be ‘home-made’ from lengths of ordinary glass tubing (diameter: 3–4 mm) using a Bunsen burner fitted with a flame-spreading (‘fish-tail’) jet.
- A variety of leaves can be used. Best results are obtained from trees or bushes with dark green leaves, eg holly.
- Preferably use teat pipettes that do not allow squirting, eg those fitted to dropper bottles of universal indicator.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Propanone, CH 3 COCH 3 (l), (HIGHLY FLAMMABLE, IRRITANT) – see CLEAPSS Hazcard HC085A . The vapour of propanone is HIGHLY FLAMMABLE. Do not have any source of ignition nearby.
- Finely cut up some leaves and fill a mortar to about 2 cm depth.
- Add a pinch of sand and about six drops of propanone from the teat pipette.
- Grind the mixture with a pestle for at least three minutes.
- On a strip of chromatography paper, draw a pencil line 3 cm from the bottom.
- Use a fine glass tube to put liquid from the leaf extract onto the centre of the line. Keep the spot as small as possible.
- Allow the spot to dry, then add another spot on top. Add five more drops of solution, letting each one dry before putting on the next. The idea is to build up a very concentrated small spot on the paper.
- Attach the paper to the pencil using sellotape so that when placed in the beaker, the paper is just clear of its base.
- Place no more than about 10 cm 3 of propanone in the beaker and hang the paper so it dips in the propanone. Ensure the propanone level is below the spot.
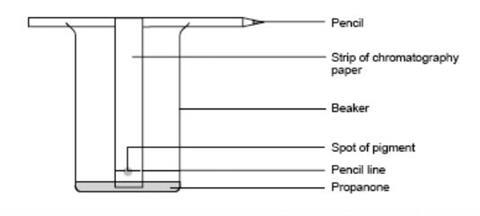
Source: Royal Society of Chemistry
The equipment required for using paper chromatography to separate the different pigments in leaves
- Avoid moving the beaker in any way once the chromatography has started.
- Leave the experiment until the propanone has soaked near to the top, and then remove the paper from the beaker.
- Mark how high the propanone gets on the paper with a pencil and let the chromatogram dry.
Teaching notes
This experiment works very well providing care is taken over preparing the spot on the chromatography paper. It should be as small and as concentrated as possible. Encourage students to be patient and to wait until each application is dry before adding the next.
At least three spots should be obtained, and one of these should be yellow due to carotenes.
The extent to which any particular component moves up the paper is dependent not only on its solubility in propanone but also on its attraction for the cellulose in the chromatography paper. The yellow carotene spot (with a higher RF value) tends to move up the paper the furthest.
More resources
Add context and inspire your learners with our short career videos showing how chemistry is making a difference .
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology .
© Nuffield Foundation and the Royal Society of Chemistry
- 11-14 years
- 14-16 years
- Practical experiments
- Chromatography
Specification
- 2. Develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation.
- Chromatography as a separation technique in which a mobile phase carrying a mixture is caused to move in contact with a selectively absorbent stationary phase.
- 6 Investigate how paper chromatography can be used to separate and tell the difference between coloured substances. Students should calculate Rf values.
- Chromatography involves a stationary phase and a mobile phase. Separation depends on the distribution of substances between the phases.
- The ratio of the distance moved by a compound (centre of spot from origin) to the distance moved by the solvent can be expressed as its Rf value: Rf = (distance moved by substance / distance moved by solvent)
- Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. These physical processes do not involve chemical reactions and no new substances are made.
- Recall that chromatography involves a stationary and a mobile phase and that separation depends on the distribution between the phases.
- Interpret chromatograms, including measuring Rf values.
- Suggest chromatographic methods for distinguishing pure from impure substances.
- 12 Investigate how paper chromatography can be used to separate and tell the difference between coloured substances. Students should calculate Rf values.
- 2.11 Investigate the composition of inks using simple distillation and paper chromatography
- 2.9 Describe paper chromatography as the separation of mixtures of soluble substances by running a solvent (mobile phase) through the mixture on the paper (the paper contains the stationary phase), which causes the substances to move at different rates…
- C2.1g describe the techniques of paper and thin layer chromatography
- 2.9 Describe paper chromatography as the separation of mixtures of soluble substances by running a solvent (mobile phase) through the mixture on the paper (the paper contains the stationary phase), which causes the substances to move at different rates o…
- C5.1.4 recall that chromatography involves a stationary and a mobile phase and that separation depends on the distribution between the phases
- 3 Using chromatography to identify mixtures of dyes in a sample of an unknown composition
- C3 Using chromatography to identify mixtures of dyes in a sample of an unknown composition
- 1.9.5 investigate practically how mixtures can be separated using filtration, crystallisation, paper chromatography, simple distillation or fractional distillation (including using fractional distillation in the laboratory to separate miscible liquids…
- 1.9.7 interpret a paper chromatogram including calculating Rf values;
- carry out paper and thin-layer chromatography and measure the Rf values of the components and interpret the chromatograms;
Related articles

Solubility | Review my learning worksheets | 14–16 years
By Lyn Nicholls
Identify learning gaps and misconceptions with this set of worksheets offering three levels of support

How to teach chromatography at post-16
2024-03-11T04:00:00Z By Andy Markwick
Everything you need to help your students master the fundamentals of this analytical technique

Chromatography challenge | 16–18 years
By Andy Markwick
Explore analytical techniques and their applications with a chromatography investigation and research activity
No comments yet
Only registered users can comment on this article., more experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud

IMAGES
VIDEO
COMMENTS
Conduct a paper chromatography project to find out if different types of solvents separate ink differently. Set up the experiment using coffee filters and permanent markers. Cut the coffee filters into long strips. Form a loop by stapling the ends of each strip together. Place a dot of ink on the bottom of the coffee filter strips.
Figure 1: Completed paper chromatography containing only 1 dye. In this experiment, students will measure the values of several dyes in 3 different solvent systems. Students will also analyze an unknown mixture of dyes in order to identify the dyes present in the mixture. The three different solvent systems are 1) laboratory water, 2) an ...
Paper chromatography, in analytical chemistry, a technique for separating dissolved chemical substances by taking advantage of their different rates of migration across sheets of paper. It is an inexpensive but powerful analytical tool that requires very small quantities of material.
Paper chromatography is a simple and cost-effective separation technique that separates and identifies different components in a mixture. [1-4] Principle. In paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. The mixture to be analyzed is applied to the paper.
E. Paper Chromatography. Chromatography is used to separate mixtures of substances into their components. All forms of chromatography work on the same principle. They all have a stationary phase (a solid, or a liquid supported on a solid) and a mobile phase (a liquid or a gas). The mobile phase flows through the stationary phase and carries the ...
Process. Paper chromatography works in few steps: Step 1: A horizontal line is drawn near one end (about 1.5 cm from the bottom edge) of the paper. In figure below 6 is the horizontal line. Step 2: The sample needs to be separated is placed as a small drop or line on to the paper using capillary tube.
Paper chromatography (PC) is a type of planar chromatography whereby chromatography procedures are run on a specialized paper. PC is considered to be the simplest and most widely used of the chromatographic techniques because of its applicability to isolation, identification, and quantitative determination of organic and inorganic compounds.
Further analysis may be needed to confirm this hypothesis. If the known and the unknown have different retention times, then it is not likely that the two components are identical. ... Lay the chromatography paper on a paper towel and immediately mark the solvent front with a pencil. Pour the used eluting solvent into the waste container ...
Paper chromatography is an analytical method used to separate coloured chemicals or substances. [1] It is now primarily used as a teaching tool, having been replaced in the laboratory by other chromatography methods such as thin-layer chromatography (TLC).. The setup has three components. The mobile phase is a solution that travels up the stationary phase by capillary action.
Figure 4. A marker or pen should be used to put a single spot of black ink in the middle of the origin line on the chromatography strip. Make a 45% isopropyl alcohol solution to use as your chromatography solvent. Pour 20 milliliters (mL) of 90% isopropyl alcohol into the 100 mL beaker.
Paper Chromatography Principle. The principle involved can be partition chromatography or adsorption chromatography. Partition chromatography because the substances are partitioned or distributed between liquid phases. The two phases are water held in pores of the filter paper and the other phase is a mobile phase which passes through the paper.
Fill a capillary tube by placing it in the leaf extract (it will fill by capillary action). Keep your finger off the end of the capillary tube. Apply the extract to the center of the dot (e) on the paper by quickly touching the end of the TLC applicator to the plate. Allow to dry (you can gently blow on the strip).
PAPER CHROMATOGRAPHY. This page is an introduction to paper chromatography - including two way chromatography. Chromatography is used to separate mixtures of substances into their components. All forms of chromatography work on the same principle. They all have a stationary phase (a solid, or a liquid supported on a solid) and a mobile phase (a ...
This video introduces the general ideas behind chromatography and separation by polarity, describes how to report the conditions and results of a chromatogra...
Instructions. Pour a small amount of water onto a plate or into the bottom of a jar. Find a way to suspend the filter paper over the water so that just the very bottom touches the water. If you do the experiment in a jar, the easiest way to do this is to wrap the top of the filter paper around a pencil, clip it in place, and suspend it over the ...
to handle paper as little as possible. 1. Cut a piece of Whatman #1 filter paper or chromatography paper to the dimensions of 12 cm X 14 cm. Edges must be straight. 2. With a pencil lightly make a line 1.5 - 2 cm from the bottom edge of the paper which measures 14 cm. 3. Select 2 large dark green spinach leaves and blot dry with paper towels.
Perform Paper Chromatography on Leaves. The key steps are breaking open the cells in leaves and extracting the pigment molecule and then separating the pigment using the alcohol and paper. Finely chop 2-3 leaves or several small leaves. If available, use a blender to break open the plant cells.
Let excess eluent drip into the beaker. Gently remove the tape and lay the chromatogram on a piece of paper towel in the hood. Leave the paper in the fume hood, where it will dry completely. If needed, use a heat lamp (in the fume hood) to dry the chromatogram; if using the heat lamp, allow 5-10 minutes to dry.
Conclusion The proposed hypothesis was correct. The paper chromatography did show that black ink could be separated into various colors. The black ink gets its color from a mixture of various colored inks blended together. The first color of ink to appear on the filter paper was yellow followed by pink, red, purple then blue.
Add a pinch of sand and about six drops of propanone from the teat pipette. Grind the mixture with a pestle for at least three minutes. On a strip of chromatography paper, draw a pencil line 3 cm from the bottom. Use a fine glass tube to put liquid from the leaf extract onto the centre of the line. Keep the spot as small as possible.