- Skip to main menu
- Skip to user menu

How To Become A Clinical Research Associate - A New Scientist Careers Guide
- Finding a job
- Career guides
What does a clinical research associate do?
Clinical research associates (CRAs) are responsible for running clinical research, which consists of trials designed to test new or current drugs/immunisations and analyse their effectiveness, risks, benefits and safety of use.
CRAs play an important role in the healthcare industry and public health development by helping to design and test new medications, vaccinations and other therapeutic agents.
CRAs can be involved at any stage of a drug development trial, including planning, coordinating and supervising. It is their responsibility to ensure a drug has been appropriately examined and all its risks have been evaluated before it is released to be used publicly.
CRAs most commonly work for pharmaceutical companies or contract research organisations. They might also have to spend some time working in a hospital setting to collect data about the drugs they are analysing. They might also work for universities or public/global health organisations.
As a CRA, you may have a range of responsibilities depending on your employer, project and level of experience. Typically, CRAs will need to complete tasks such as:
- Designing and writing trial protocols and standard operating procedures
- Presenting protocols and procedures to steering committees
- Designing data collection forms
- Requesting ethics approvals and working with ethics committees
- Liaising with staff conducting the trials, such as doctors or consultants
- Training local staff based on trial-specific standards
- Monitoring operations during clinical trial data collection
- Collecting completed data collection forms
- Performing data management and analysis, and discussing the results
- Closing trials and finalising reports with the help of a statistician
CRAs will work in a team of other research professionals, including contract organisation or sponsor staff, principal investigators and clinical research coordinators.
How to become a clinical research associate
To become a CRA, you need to obtain a degree in medical sciences, life sciences or nursing. This can be in subjects such as biomedical science , anatomy, physiology, immunology , pharmacology or broader degree subjects like chemistry and biology .
Alternatively, you can access a career as a CRA by acquiring a higher national diploma (HND). This is a qualification equivalent to the second year of a bachelor’s degree. HNDs can be beneficial to those who want to enter more practical fields, clinical research included.
Occasionally, you can enter a CRA role from an administrative background. For instance, if you begin working as a clinical trials administrator/assistant and decide you would prefer the role of a CRA, you can complete additional qualifications to do this. However, this will take some time and can be difficult.
Most employees view undergraduate qualifications as sufficient, but in some cases a postgraduate degree may be beneficial. Master’s degrees and PhDs can gain you an advantage when applying for competitive positions, and help you gain more experience in research .
Work experience is key to securing a clinical research job. You can get this at any point in your training, and some universities may help with this. The types of work experience most useful for a CRA role include:
- Academic research
- Pharmaceutical research (e.g. via a pharmaceutical industry placement during your degree)
- Laboratory work
- Nursing or care work
- Work in a pharmacy or medical sales
- Other, similar activities
How long does it take to become a clinical research associate?
Becoming a CRA will usually take around three to four years, depending on the access pathway you choose.
If you opt to complete an undergraduate degree, this can take three to four years. You can then apply to job positions as a CRA straight away. However, if you don’t have sufficient work experience, you may need to start at a lower-level position such as a more administrative job. From here, you can gain more experience and reapply for a higher-level position.
If you choose to obtain a HND qualification, this will take two years. Provided you have sufficient experience, you can then apply for a graduate post as a CRA, but again you may need to gain some extra experience/qualifications in some cases.
If you opt to do a postgraduate degree first, this may take an additional one to three years depending on whether it is a master’s degree or a PhD. Many CRA job positions also allow for completion of postgraduate qualifications alongside the job.
A day in the life of a clinical research associate
Most CRAs work about 40 hours a week, during weekdays. There may be an out-of-hours commitment, for instance if working in a hospital setting monitoring a new drug, but this is dependent on your employer and role.
CRAs can work on multiple trials at a time, in multiple different sites. This will depend on the complexity of each trial and what stage each of the trials is in. Therefore, the role may entail some travelling at times, while other times most of your work will be concentrated on one site.
As a CRA, you will carry out a wide variety of tasks. Some days may be spent writing reports. On other days, you will work on site with healthcare staff , or you might go into your office and attend meetings.
No matter your experience level or how senior your role is, as a CRA, you will need to work in a team with other research and healthcare professionals. You will be communicating with research nurses, doctors, health consultants, investigators and managerial and administrative staff from the company requesting the trial.
The role requires good communication skills, as well as good time management and organisation skills, because carrying out a few different studies at once may mean some tasks clash with one another and you need to prioritise the most important ones.
Clinical research associate: Career options
As with most clinical roles, CRAs undergo lots of continuing professional development (CPD) within their role. There are many training courses available to CRAs to build on their existing skills and develop new competencies.
Most training courses are organised by external bodies, and many are paid for by the employer. One of the organisations that runs training courses is the Institute of Clinical Research (ICR). It provides training in areas such as effective project management for clinical trials and advanced clinical trial monitoring, among several others.
The ICR also offers certificates and a diploma that you can complete to evidence your skills in clinical research. Becoming an ICR member and obtaining courses and qualifications from it can also be beneficial to career development, as you will meet other prominent professionals in your field through interacting with this organisation.
You can also opt to complete a postgraduate qualification, such as a PhD or master’s degree in several different areas, including clinical research and clinical pharmacology .
As mentioned, you may need to climb up the professional ladder to become a CRA, and many people start off as clinical trial administrators or junior CRAs. Within these roles, you might complete tasks such as handling documentation and correspondence or helping to set up trial sites.
From here, you can move on to becoming a senior CRA as you gain experience. At this point, you will have more advanced responsibilities, such as project management of whole trials and designing case report forms.
If you develop sufficient experience and gain contacts in the field, there is a possibility of self-employment if you want to become a freelance CRA.
Salary: How much does a clinical research associate earn in the UK and US?
In the UK, starting salaries for CRAs range between £26,000 and £34,000 per year. As a more senior CRA, you might earn between £35,000 and £50,000, and in the most senior positions involving managerial tasks, you might earn upwards of £55,000.
Salaries will vary between regions and employers, as well as depending on your level of experience and responsibilities. Some companies offer additional benefits.
In the US, the average salary for a CRA is $70,000 per year. The range is between $60,200 and $80,900. This can vary depending on the region you work in, your education and experience levels and any additional qualifications you have.
Salaries will also be different as a freelance CRA, and this will depend on the number of clients you have and any business-related expenses you need to cover.
- Prospects. Clinical research associate. Available from: https://www.prospects.ac.uk/job-profiles/clinical-research-associate (accessed Apr 2024)
- CK Group. Clinical research associate (CRA) job profile. Available from: https://ckgroup.co.uk/candidate/job-profiles/clinical-research-associate-cra-job-profile/ (accessed Apr 2024)
- Nikolova, T. The CRA Wizard. How to become a CRA for dummies in 7 steps (or less). Available from: https://www.thecrawizard.com/how-to-become-a-cra-for-dummies (accessed Apr 2024)
- Glassdoor. Clinical research associate career. Available from: https://www.glassdoor.co.uk/Career/how-to-become-clinical-research-associate_KO14,41.htm (accessed Apr 2024)
- Coursera. How to become a clinical research associate. Published Nov 2023. Available from: https://www.coursera.org/articles/clinical-research-associate
- Walters, L. Pharmiweb.jobs. 8 ways to advance your career as a clinical research associate (CRA). Published Sept 2023. Available from: https://www.pharmiweb.jobs/article/8-ways-to-advance-your-career-as-a-clinical-research-associate-cra- (accessed Apr 2024)
- Salary.com. Clinical research assistant salary in the United States. Available from: https://www.salary.com/research/salary/alternate/clinical-research-assistant-salary (available from Apr 2024)
Share this article
Related articles

How to Become a Data Analyst? - A New Scientist Careers Guide

What Jobs Can You Get With A Computer Science Degree? - A New Scientist Careers Guide

What does a Clinical Physiologist in Neurophysiology do??
Latest articles.
- Conference Coverage
- CRO/Sponsor
- 2023 Salary Survey

- Publications
- Conferences
An Examination of the Role of the Clinical Research Associate and Factors Impacting Performance and Experience
- Robert Howie
- Kenneth Getz, MBA
Study by Tufts CSDD uncovers potential reasons behind CRA shortages, turnover, experience requirements, and more.
The COVD-19 pandemic and changing global economies have impacted the clinical research workforce resulting in attrition, shortages, and staffing challenges. Current workforce shortages have posed numerous hurdles for clinical research stakeholders, including sponsors, investigative sites, contract research organizations (CROs), and industry associations. 1 The role of the clinical research associate (CRA) has been especially affected as high rates of turnover and vacancies proliferate. CRA turnover rates have been reported as high as 30% in the US with similar rates noted globally. 2
Recent research has examined current approaches to CRA training, performance assessment, and experience. The results of a global survey of 661 clinical research professionals, including 52 CRAs, assessing their own competencies indicated that respondents with more post-secondary education reported higher levels of self-confidence in their research skills and those professionally certified by the Association of Clinical Research Professionals (ACRP) and/or Society of Clinical Research Associates (SOCRA) compared to those who were not. Self-confidence also increased with the level of academic degree. 3 Researchers used a Joint Task Force (JTF) for Clinical Trial Competency Framework to assess respondents on eight competency domains. The results are consistent with prior research conducted in 2016. 4
The JTF for Clinical Trial Competency framework as discussed by ACRP “objectively defines the knowledge, skills, and attitudes necessary for conducting safe, ethical and high-quality research.” 5 Although common practice is currently to hire CRAs with two years of experience, high demands, vacancies, and attrition in the industry have led researchers to recognize a need to address the two years of required experience for those entering the clinical research workforce. Proposed solutions include conducting outreach to raise awareness of the profession, capturing measures of experience and transferable skills as well as add to competency-based training and innovative recruitment and retention approaches to attract candidates to the field. 5
An examination of the entry-level shortage of CRAs cites several factors including the requirement of a minimum of two years of work experience, high CRA turnover, increasing numbers of open CRA positions, and clinical trial growth and complexity. An assessment of a monitoring simulation of 579 CRAs from a global CRO indicates no difference in performance regardless of CRA seniority and years of experience. 6,7 The research also discusses that addressing the requirement of two years of experience for CRA roles would alleviate the current shortages in the workforce.
Given the impact of the changing clinical research industry on CRAs, Tufts CSDD investigated the current role of the CRA and critical issues including shortages and turnover, and approaches to CRA training and required experience. Also, the study examined the industry practice of two years’ experience as a hiring requirement and its relationship to CRA performance and recruitment and retention practices.*
*This research study was funded by Virb, Inc.
Methodology
The research used a dual methodology comprised of a web survey of pharmaceutical, biotechnology and CRO executives and an executive roundtable with industry experts. Tufts CSDD conducted an online survey prior to the meeting to gauge perceptions and insights about the role of the CRA based on a small group of clinical research professionals from the US Areas examined included years of experience required to become a CRA and type and effectiveness of training provided. In addition, the survey gathered perceptions about CRA shortages and attrition, most successful recruitment approaches and frequently used retention strategies. CRA assessment and performance were also investigated.
A roundtable meeting was held in October 2022 with 33 participants from biotechnology and pharmaceutical companies, CROs, academia, consulting, and technology. Participants provided their insights and perceptions across a broad range of topics including CRA assessment and training and the industry standard of requiring two years of work experience as a prerequisite to hiring. Other areas explored included recruitment and retention practices; the investigative site perspective; and the impact of outsourcing models, technology, and economic factors on the CRA role.
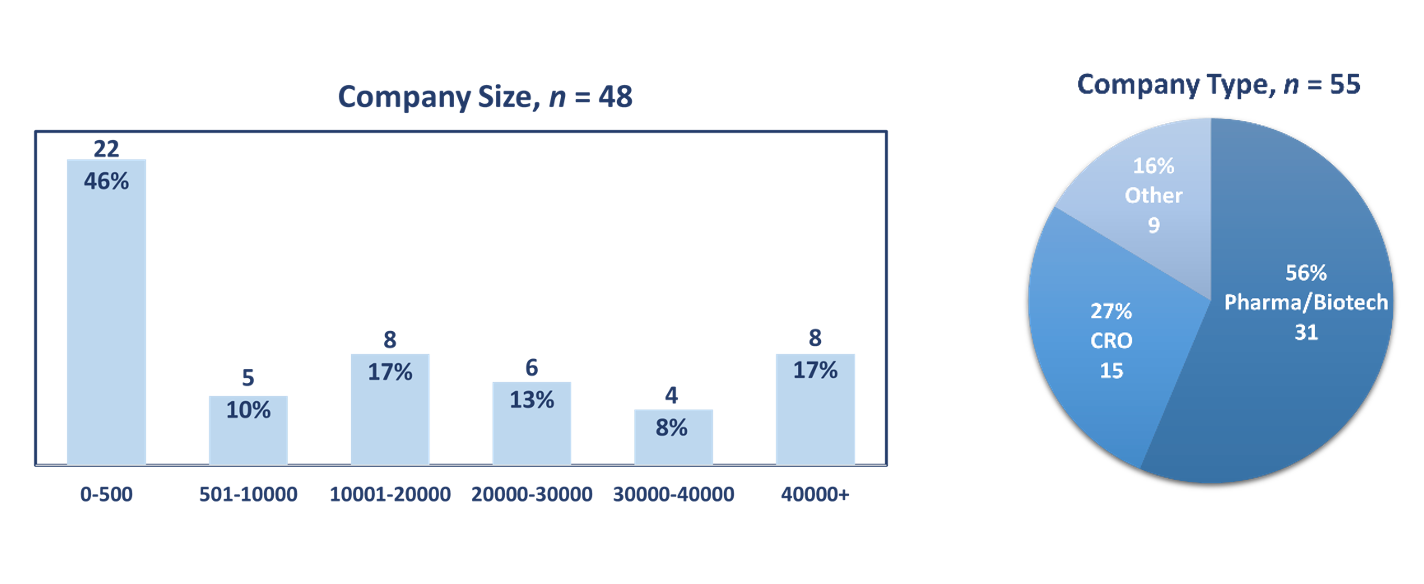
CRA survey demographics
The survey was distributed via a link to an e-mail invitation using Qualtrics software to industry executives in the US primarily working in clinical operations or clinical development. A total of 55 responses were collected between August and September 2022. Respondents represented a mix of company types with over half (56%, n=31) representing pharmaceutical or biotechnology companies; more than one-fourth (27%, n=15) from CROs and 16% (n=9) from other company types. The mean number of company employees was 17,581 with companies ranging in size from 4 to 95,000 employees. Respondents had 21 years of experience on average and an overwhelming majority (91%, n=50) identified their functional area as clinical operations or clinical development.
What Can ClinOps Learn from Pre-Clinical?
Dr. Hanne Bak, Senior Vice President of Preclinical Manufacturing and Process Development at Regeneron speaks about her role at the company as well as their work with monoclonal antibodies, the regulatory side of manufacturing, and more.

DPHARM 2024: Improving Patient Recruitment Outcomes
The presentation expresses the importance of putting the patient at the center of patient recruitment.

Decentralized Clinical Trials: Bust or Breakthrough?
Five industry leaders share their perspectives on the evolution of DCTs.

Sustainability in Clinical Trials: Purposeful Digitalization is Key
Its integration is opening up more opportunities for sustainable study practices.

Innovating Beyond the Lab: The Critical Role of Contracting in Research
Biotechs can successfully overcome bottlenecks in time-to-market for new drugs by embracing contracting innovations with the same passion applied to research breakthroughs.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


The Ultimate Guide to Becoming a Clinical Research Associate

A clinical research associate (CRA) plays a vital role in the development of new medical treatments. They ensure that clinical trials are conducted ethically and according to protocols, monitoring the progress from start to finish. CRAs are essential for ensuring data integrity and regulatory compliance.
The demand for CRAs is increasing due to the growth of the pharmaceutical industry and more clinical research studies. This field offers many opportunities for career growth with competitive salaries and benefits.
To become a successful clinical research associate, it's important to have a strong understanding of various aspects of the job like GCP monitoring of clinical trials and familiarity with EU clinical trials registry.
To gain this knowledge and expertise, consider enrolling in specialized online certification courses offered by CCRPS . They provide flexible learning options designed specifically for individuals aiming to excel in clinical research.
What Does a Clinical Research Associate (CRA) Do?
A Clinical Research Associate (CRA) plays an integral role in the clinical research process, ensuring that clinical trials are conducted ethically, safely, and efficiently. CRAs are responsible for monitoring clinical trials and ensuring compliance with regulatory requirements and study protocols.
Key Responsibilities of a CRA:
Study design and protocol development.
CRAs assist in the development of study protocols , which outline the objectives, design, methodology, statistical considerations, and organization of a clinical trial. This ensures that the study is scientifically sound and regulatory compliant.
Site Selection and Initiation
They identify suitable sites for conducting clinical trials, assessing their qualifications and infrastructure. Once selected, CRAs initiate the sites by training site staff on study protocols and procedures.
Monitoring Visits
Regular site visits to monitor the progress of clinical trials are a key responsibility. During these visits, CRAs ensure data integrity by verifying source documents against case report forms (CRFs) and checking for protocol adherence.
Regulatory Compliance
Ensuring that all activities related to the trial comply with local and international regulations is critical. CRAs keep up-to-date with guidelines such as Good Clinical Practice (GCP) to maintain high standards throughout the research process.
Data Management
CRAs oversee data collection processes to ensure accuracy and completeness. They resolve any data discrepancies promptly to maintain data quality.
Safety Reporting
Monitoring participant safety is paramount. CRAs ensure that adverse events (AEs) and serious adverse events (SAEs) are reported in accordance with regulatory requirements, following pharmacovigilance practices .
Communication
Acting as a liaison between the sponsor, clinical sites, and regulatory bodies is another essential duty. Effective communication helps address issues swiftly and keeps all stakeholders informed about trial progress.
Documentation
Maintaining meticulous records of all aspects of the trial is crucial. CRAs document everything from visit reports to correspondence with investigative sites, ensuring transparency and traceability throughout the trial process.
These responsibilities highlight the importance of a CRA in maintaining the integrity of clinical trials. By managing these tasks effectively, CRAs contribute significantly to the advancement of medical knowledge and patient care.
Why Should You Consider a Career as a Clinical Research Associate?
High demand for cras.
The pharmaceutical industry is experiencing rapid growth, resulting in an increased number of clinical research studies. Consequently, there is a significant demand for skilled Clinical Research Associates (CRAs). These professionals play a vital role in overseeing clinical trials, ensuring adherence to regulatory guidelines and generating reliable data.
Opportunities for Career Advancement
A career as a Clinical Research Associate offers numerous opportunities for advancement. CRAs can transition into various management positions or specialize in specific research areas. Some potential career paths include:
Clinical Trial Manager : Overseeing multiple clinical trials and managing teams of CRAs.
Regulatory Affairs Specialist : Ensuring compliance with local and international regulations.
Project Manager : Coordinating all aspects of a clinical trial from start to finish.
These roles not only offer professional growth but also enable CRAs to make significant contributions to medical science.
Attractive Salary Packages and Benefits
CRAs receive competitive salaries and comprehensive benefits. Reports indicate that entry-level CRAs can earn attractive salaries which increase with experience and certifications.
Some benefits commonly provided to CRAs include:
Health Insurance : Comprehensive medical coverage.
Retirement Plans : Contributions towards retirement savings.
Paid Time Off : Generous leave policies.
The combination of financial rewards and benefits makes this career option appealing for individuals who seek fulfilling work while also achieving their personal financial goals.
For those interested in the field, it's worth exploring the resources available at CCRPS for insights on the latest trends, as well as the opportunities offered by contract research organizations in India.

The Path to Becoming a Successful Clinical Research Associate
1. assessing your suitability for the role.
Before you embark on a career as a Clinical Research Associate (CRA), it's important to evaluate whether this path is right for you. Take some time to assess your interests, skills, and qualifications to determine if becoming a CRA aligns with your aspirations.
Exploring Your Interests
Passion for Science and Medicine : A strong interest in medical research and scientific inquiry is crucial. CRAs work closely with clinical trials that aim to improve healthcare outcomes.
Attention to Detail : The role involves meticulous documentation and adherence to protocols. If you enjoy tasks that require careful attention to detail, this could be a good fit.
Problem-Solving Skills : CRAs often face unexpected challenges during clinical trials. Having a knack for problem-solving can make the job more rewarding.
Evaluating Your Skills
Communication Skills : Effective communication with various stakeholders, including researchers, sponsors, and regulatory bodies, is vital.
Organizational Abilities : CRAs juggle multiple tasks at once, so strong organizational skills are necessary.
Technical Proficiency : Familiarity with data management systems and clinical trial software is beneficial.
Reviewing Your Qualifications
Educational Background : Most CRAs have degrees in life sciences or related fields. Consider if your educational background aligns with these requirements.
Experience in Clinical Research : Prior experience in clinical research roles, such as internships or volunteer work, can be advantageous.
For those seeking additional insights on gaining relevant experience, you may find it helpful to explore resources on how to get clinical trial experience here. Additionally, for those interested in advancing their knowledge in specific areas, there are management forums that offer courses like the Management Forum Advanced Pharmacovigilance.
By conducting this thorough self-assessment, you can gain clarity on whether pursuing a career as a CRA aligns with your goals and strengths. This foundational step sets the stage for acquiring the necessary education and skills needed to succeed in this field.
2. Acquiring the Necessary Education and Skills
Importance of an academic degree.
Starting a career as a CRA requires a solid educational foundation. Obtaining an academic degree in a relevant field such as Life Sciences, Biology, or Health Sciences is essential.
This not only demonstrates your commitment to the field but also equips you with the fundamental knowledge needed to understand complex clinical research processes.
Medical Terminology Knowledge
A strong grasp of medical terminology is crucial for anyone aspiring to become a CRA. Clinical research involves intricate medical details that require precise understanding and communication. Developing this knowledge through coursework or specialized training programs is beneficial.
Courses offered by CCRPS provide comprehensive training in medical terminology specific to clinical research, which can greatly enhance your medical terminology proficiency.
Understanding Regulations
Clinical trials are governed by strict local and international regulations. Familiarizing yourself with these regulations is critical for ensuring compliance and maintaining the integrity of research data.
Training programs often include modules on regulatory affairs, helping you stay updated on guidelines such as ICH GCP (International Council for Harmonisation Good Clinical Practice).
Education and skill development are foundational steps in becoming a successful CRA. By focusing on obtaining relevant academic qualifications, mastering medical terminology, and understanding regulatory requirements, you set yourself up for a rewarding career in clinical research.
3. Gaining Experience in the Field of Clinical Research
Gaining practical experience is crucial for anyone looking to build a career as a Clinical Research Associate (CRA). This hands-on exposure not only enhances your resume but also provides you with invaluable insights into the daily responsibilities and challenges of the role.
Internships, Volunteer Work, and Certificate Courses
Taking up internships or volunteer positions in research organizations or healthcare facilities can offer a solid foundation. These opportunities allow you to:
Observe real-world clinical trials
Interact with experienced professionals
Understand regulatory requirements
Certificate courses in areas such as pharmacovigilance and pharmacoepidemiology offered by reputable institutions like CCRP can also significantly enhance your knowledge and make you more competitive in the field.
Volunteering can demonstrate your commitment to the field, making you a more attractive candidate for future employers.
Entry-Level Positions
Starting in entry-level roles such as Clinical Trial Assistant or Data Coordinator is another effective strategy. These positions often involve:
Monitoring data quality
Assisting in patient recruitment
Supporting CRAs in their tasks
Such roles help you build relevant experience while gaining an understanding of the intricacies involved in clinical research.
Personal Assessment
Before diving into these experiences, conducting a personal assessment can be beneficial. Ask yourself:
Do I have strong organizational skills?
Am I detail-oriented?
Can I handle multiple tasks efficiently?
This self-assessment helps ensure that a career as a CRA aligns with your personal strengths and career aspirations.
Investing time in gaining practical experience through internships, volunteer work, or entry-level positions not only strengthens your resume but also prepares you for the complexities of a CRA role.
Benefits of Becoming Certified
Getting certified as a CRA comes with several advantages:
Increased Job Prospects : Employers often prefer candidates who are certified, as it assures them of the applicant's competence and dedication to the field. This can make your job applications more competitive.
Professional Credibility : Certification provides an official acknowledgement of your skills and knowledge, enhancing your credibility among peers and employers.
Career Advancement : With certification, you position yourself for advanced roles within clinical research, opening doors to management positions or specialized areas of interest.
Investing in certification is a strategic move for those serious about a career as a CRA. It not only boosts your qualifications but also instills confidence in potential employers regarding your capability to manage complex clinical trials effectively.
For those looking to deepen their understanding of medical efficacy definitions or find clinical trials for specific conditions like cancer, additional resources can be found through CCRPS, an organization that offers valuable insights and expertise in the field.
They also provide specific resources like a comprehensive medical efficacy definition and guidance on how to find clinical trials for cancer .
Certifications are more than just credentials; they are gateways to numerous opportunities and professional growth in the dynamic world of clinical research.
5. Nailing the CRA Job Application Process
Finding and applying for CRA positions requires strategic planning and preparation. Here are some essential steps to enhance your job application process:
Strategies for Finding and Applying for CRA Positions
Leverage Online Job Portals :
Websites like Indeed, Glassdoor, and LinkedIn are great places to start.
Set up job alerts to receive notifications about new postings.
Networking :
Join professional groups on LinkedIn and attend industry conferences.
Connect with professionals in the field through platforms like CCRPS's alumni network.
Creating a Strong Resume and Cover Letter :
Resume Tips :
Highlight relevant education, certifications, and experience.
Use keywords from the job description to pass Applicant Tracking Systems (ATS).
Quantify achievements where possible (e.g., "Monitored over 50 clinical trials with a 98% compliance rate").
Cover Letter Tips :
Tailor each cover letter to the specific job and company.
Demonstrate your understanding of the role and how your skills align.
Mention any relevant training or certifications from CCRPS.
Preparing for Interviews with Potential Employers
Research the Company :
Understand their mission, values, and recent projects.
Familiarize yourself with their clinical trial focuses, such as cancer drug trials .
Showcase Your Knowledge :
Be prepared to discuss clinical trial phases, Good Clinical Practice (GCP), and regulatory requirements.
Share examples of how you've applied your knowledge in practical settings.
Behavioral Interview Questions :
Practice responses to questions about teamwork, problem-solving, and conflict resolution.
Use the STAR method (Situation, Task, Action, Result) to structure your answers.
Technical Skills Demonstration :
You might be asked about specific tools or software used in clinical research.
Mention any hands-on experience you have with Electronic Data Capture (EDC) systems or other relevant technologies.
By following these strategies, you'll position yourself as a strong candidate in your career as a CRA. 6. Continuing Your Professional Development as a CRA
Staying updated on industry advancements is vital for a successful career as a Clinical Research Associate (CRA). Engaging in continuous professional development ensures that you remain knowledgeable about the latest trends, regulations, and best practices in clinical research.
Participation in Workshops and Conferences
Workshops : Attending workshops allows CRAs to gain hands-on experience and practical skills. These sessions often cover new methodologies, regulatory updates, and emerging technologies in clinical trials.
Conferences : Industry conferences provide valuable networking opportunities. They bring together professionals from various sectors of clinical research, fostering knowledge exchange and professional growth.
Ongoing Training Opportunities
Online Courses : Enrolling in online courses can be a flexible way to stay current with industry standards. CCRPS offers a range of specialized certification courses like CRA, CRC, and ICH GCP that are designed to enhance your expertise.
In-House Training Programs : Many organizations offer internal training programs tailored to their specific needs. Participating in these programs can help CRAs stay aligned with their employer's expectations and protocols.
Self-Assessment and Personal Growth
Regular self-assessment helps CRAs identify areas for improvement and set career goals. Exploring personal interests and strengths can guide you towards specialized areas within clinical research, such as data management or regulatory affairs.
For more insights on maintaining industry relevance, consider exploring resources like our clinical trial monitoring plan SOPs or understanding salary trends through our clinical trial assistant salary guide .
These guides can offer additional context for your ongoing professional development.
Embracing continuous learning not only enhances your competency but also boosts your career prospects. As the clinical research landscape evolves, being proactive about professional growth ensures you remain a valuable asset to any research team.

How CCRPS Can Help You Become a Clinical Research Associate Faster
Comprehensive online certification courses.
CCRPS offers specialized online certification courses tailored to the needs of aspiring Clinical Research Associates (CRAs). These courses include:
Clinical Research Associate (CRA) Certification
Clinical Research Coordinator (CRC) Certification
ICH Good Clinical Practice (GCP) Certification
Benefits of Choosing Online Learning
Opting for online certification through CCRPS brings multiple advantages:
Flexibility: Learn at your own pace, fitting coursework around your existing schedule. This is particularly beneficial for those balancing current jobs or academic commitments.
Accessibility: Access course materials from anywhere in the world. No need to relocate or commute, making it easier to integrate learning into your daily life.
Online learning also allows you to revisit course materials as needed, ensuring you fully grasp each topic before moving on.
Ensuring Industry Relevance Through Updated Curriculam
CCRPS ensures its courses reflect the latest industry standards and best practices. The curriculum is continually updated, incorporating new developments in clinical research techniques and regulatory guidelines.
This commitment to excellence ensures that graduates are well-prepared for the evolving demands of the field.
Graduates from CCRPS often share success stories, highlighting how these courses have facilitated their transition into CRA roles.
For instance, many have noted that the comprehensive nature of CCRPS training gave them a competitive edge in job applications and interviews.
Recognition and Trust in the Field
CCRPS is widely recognized as a credible training provider within the clinical research community. Many leading research organizations and agencies prefer candidates who have completed certification programs from CCRPS.
This recognition not only enhances your resume but also increases your professional credibility.
Choosing CCRPS for your certification means aligning yourself with a trusted institution known for producing competent and knowledgeable CRAs.
The industry trust in CCRPS graduates is a testament to the quality and relevance of their training programs.
By selecting CCRPS for your CRA certification, you are making a strategic decision to boost your career prospects and prepare for a successful future in clinical research.

Choosing a career as a Clinical Research Associate (CRA) opens doors to many opportunities for growth, competitive salaries, and the satisfaction of contributing to medical advancements. The path to becoming a CRA may seem challenging, but with hard work and the right guidance, you can achieve your goals.
CCRPS is here to support you throughout this journey. We offer comprehensive online certification courses designed specifically for clinical research roles, providing you with:
Flexibility : Learn at your own pace through our accessible online modules.
Up-to-date Knowledge : Stay informed about the latest practices and standards in clinical research.
Professional Recognition : Earn certifications that are widely acknowledged by top research organizations and agencies.
With CCRPS's resources, you can turn your dreams into reality. Join thousands of successful graduates who have advanced their careers in clinical research through our programs.
Contact us today and become a part of the dynamic field of clinical research associates.
FAQs (Frequently Asked Questions)
What is the role of a clinical research associate (cra).
A Clinical Research Associate (CRA) is responsible for overseeing and monitoring clinical trials conducted by pharmaceutical companies, contract research organizations (CROs), or academic medical centers. Their role involves ensuring that the trials are conducted in compliance with regulatory standards, protocols, and good clinical practice (GCP). CRAs also verify the accuracy of data collected during the trials and communicate with the study site staff to address any issues that may arise.
Why should I consider a career as a Clinical Research Associate?
Becoming a Clinical Research Associate offers numerous opportunities for career growth and development. The pharmaceutical industry's continuous expansion and the rise in clinical research studies have created a high demand for CRAs. This demand translates into attractive salary packages, benefits, and opportunities for advancement into management roles or specialized areas of research.
How can I become a successful Clinical Research Associate?
Becoming a successful Clinical Research Associate involves several key steps. These include assessing your suitability for the role by exploring your interests, skills, and qualifications; acquiring the necessary education and skills in fields such as Life Sciences and medical terminology; gaining relevant experience through internships or entry-level positions; considering certification programs offered by reputable organizations CCRPS nailing the job application process through effective resume crafting and interview preparation; and continuing your professional development through ongoing training opportunities.
What does CCRPS offer to help me become a Clinical Research Associate?
CCRPS provides comprehensive online certification courses tailored to individuals aspiring to become Clinical Research Associates. These courses cover essential knowledge areas such as CRA, CRC, and ICH GCP. By choosing online learning with CCRPS, you can benefit from flexibility and accessibility while gaining industry-relevant skills. Additionally, CCRPS ensures that their curricula reflect the latest industry standards and best practices, thereby accelerating your journey towards becoming a successful CRA.
How does CCRPS ensure industry relevance through its courses?
CCRPS maintains industry relevance by enhancing its course curricula to reflect the latest standards and best practices in clinical research. Graduates of CCRPS courses have successfully transitioned into CRA roles, showcasing the effectiveness of CCRPS training in preparing individuals for careers in clinical research.
Why should I trust CCRPS as a training provider for Clinical Research Associate certification?
CCRPS is recognized as a trusted training provider by leading research organizations and agencies within the field of clinical research. The credibility of CCRPS is evidenced by its success stories of graduates who have excelled in their careers as CRAs after completing the specialized online certification courses offered by CCRPS.
Clinical Trial Management System
Do you know what clinical trials and studies can offer.
- Technical Help
- CE/CME Help
- Billing Help
- Sales Inquiries
Premier Episode of On Research Podcast – What is a Clinical Research Associate?
Season 1 – Episode 1 – What is a Clinical Research Associate?
A Clinical Research Associate (CRA) is a professional within a healthcare setting who oversees research activities, typically related to clinical trials. CRAs can be employed and work in a variety of sectors, including government agencies, pharmaceutical companies, research institutions, and in similar settings. Most CRAs possess an academic degree from a higher education institution in a field related to the healthcare industry. The overall role and tasks of a CRA are outlined and defined by good clinical practice guidelines for clinical trials.
Episode Transcript
Darren Gaddis : From CITI Program, I’m Darren Gaddis and this is On Research. Today, what is the difference between a clinical research associate and a clinical research professional? The skills and training needed to be a clinical research associate and general misconceptions about clinical research associates. I spoke with Elizabeth Waddell, owner and CEO of the CRA Helper.
She has been in the clinical research industry for over 20 years. Elizabeth began her career as an in-house clinical research associate and later transitioned to an on-site clinical research associate. After 14 years as a monitor, she transitioned from a senior clinical research associate to a line manager role focused on training new CRAs.
As a reminder, this podcast is for educational purposes only. It is not intended to provide legal advice or guidance. You should consult with your organization’s attorneys if you have questions or concerns about relevant laws and regulations discussed in this podcast. Additionally, the views expressed in this podcast are solely those of the presenter.
Hi Elizabeth. Thank you for joining me today.
Elizabeth Waddell : Hi Darren. Thank you so much for having me. I’m excited to be here.
Darren Gaddis : To get us started, what is a clinical research associate or CRA?
Elizabeth Waddell : So a clinical research associate, like you said, A CRA, and they’re also known as a monitor. I think the best way to explain what it is, is to start with why is it even required.
So per FDA regulations and GCPs, the sponsor must ensure that the clinical trials are properly monitored and this is to ensure the rights and the wellbeing of the human participants are protected, and they have to make sure the patients are safe. And on the flip side, they need to make sure, and ensure that the data from the clinical trial is accurate, complete and verifiable from source documents. And that the study’s being conducted in compliance with the protocol GCPs and reg requirements. So there’s a lot they have to monitor in a trial and that’s where we come in, that’s where the CRAs come in.
We must monitor the studies on behalf of the sponsor. And our two major obligations are ensuring subject safety and data integrity. And there’s even guidance regarding the selection of CRAs, as we must be qualified by the training and experience to monitor the progress of the trial. So it’s a very important role, as keeping patients safe and protected is so huge. And also verifying the validity of the data is huge, because for example, in a pharmaceutical trial, the FDA is reviewing this data to evaluate if a drug is safe. They’re evaluating if the drug is effective and that’s going to go into all that decision-making if a drug is approved or not. So it’s very important, monitoring the progress of a trial.
Darren Gaddis : And from that definition, what is the difference between a clinical research associate and clinical research professional?
Elizabeth Waddell : So a clinical research professional to me, is anyone in the clinical research industry. So a CRA would be an example of a clinical research professional. It’s one of the roles in the industry. There are so many roles as you’ll see from project managers to auditors to clinical trial associates, and then you have roles at the site level as well.
Darren Gaddis : From your own experience, could you describe the training and skills that one needs, to be successful as a CRA ?
Elizabeth Waddell : So I’ll start with the training. When we would review CVs, a curriculum vitae, it’s like a resume in the research world. When we would review CVS in order to interview a potential CRA candidate, we primarily looked at their education, as well as their clinical research experience.
So taking a CRA training per se, wasn’t something that was required. Again, we focused on the clinical research experience. Now, although a CRA training course is not required though, can it help build confidence? Absolutely.
So for example, when I was assigned to a study that was a new therapeutic area, in addition to training at our company, I would take extra trainings to build confidence when monitoring the disease under study. So it’s the same thing with a CRA training course that may not be a requirement to get the job, but if it can help you learn and build the confidence to perform the job better or even interview better, it is so worth it.
And the same thing, even certification being a certified CRA, it wasn’t necessarily a requirement for me, I didn’t have to be certified and actually I did my certification, or obtained it when I was a senior CRA.
My company offered, they were like, “Hey, we’ll pay for it, you have the time. Do you want to go for it?” And I’m like, “Oh my gosh, yeah that would be awesome!” So I did do it, and it’s an accomplishment for sure, and I’m glad to have it and I’m glad to have the credentials. But did I have to be certified to get a CRA job? I did not. So I do like to point that out. Definitely, clinical research experience on a CV speaks volumes, but again, you do the training more for yourself to help build that confidence.
As far as skills required, definitely, attention to detail. You will go through documents like a fine tooth comb, you definitely need to have those eagle eyes, attention to detail, and definitely critical thinking skills, because in research you’ll find that not a lot of things are black and white. It takes critical thinking, problem-solving, sometimes escalating things to different study team members.
I’ve been in research for 20 plus years and I still send emails like, “Hey, I’ve noticed this. This is what I’m thinking of, how to resolve the issue, what are your thoughts?”
And sometimes you have to consult with different departments. Not being afraid. Do not be afraid to ask questions. Again, like I talked about, you’re going to confirm things with different departments sometimes. And to me, asking questions is not a sign of weakness, it’s a sign of strength, because you want to do your job, you want to confirm things for your study.
And you definitely need to have time management skills, there are a lot of deadlines and metrics that you must meet, so definitely time management, being able to prioritize, and then being able to reprioritize, because there’s many times things are changing in clinical research, you may get an email that totally changes your day. So you’ll have to reprioritize what needs to be performed for that day. And like I mentioned, being able to adapt, being flexible, so these are some soft skills that you’ll need to have as well, as deal with different personalities.
Very important, as you’ll be not only with your study team, but you’re going to be going to many different sites and interacting with different people and things, so that’s important to have as well as being able to be organized, being able to work independently because again, you’re going to many sites working by yourself there, monitoring documents, as well as sometimes when you’re not on the road, you may be working remotely, at home in your office.
So you have to be able to still meet your deliverables, work independently and be able to communicate effectively. And not just verbally, but written communications is very important. Lots of trip report completion, email communication, lots of documentation as a CRA, so definitely written communication is so important. And I know there are many more skills I could probably point out, but I would say this is a good start of important skills to have.
Darren Gaddis : And with all of this in mind, would you be willing to share your own journey to becoming A CRA ?
Elizabeth Waddell : Yeah, sure. I graduated with a bachelor’s in clinical research, and while I was doing my internship, I interviewed to work in the lab actually, at a company that manufactured vaccines. And I was offered the job, but at the same time I received a call from a recruiter with an opportunity as an entry level in-house CRA, and I was like, “Oh wow, this is clinical research…”
My heart was just being tugged in that direction, and it ended up that I chose the in-house CRA opportunity and it was definitely a blessing. I first was contract, then I went permanent, and after about a year of being an in-house CRA, a CRA 1, I was promoted to a CRA 2, and then moved to the clinical department and started onsite monitoring. And the rest is history, here we are 20 years later.
Darren Gaddis : And from your own tenure, what is one general misconception about CRAs?
Elizabeth Waddell : In my experience, I would say one of the misconceptions about CRAs I would see, is people would picture, “Okay, CRAs, they come to a site and their role is to go to the site and pick them apart.”
And although sometimes I would, I’d be at a site and I’d see another monitor come in, and maybe they would have this air about them, like high and mighty. And they wouldn’t nicely point out issues that they observe. Sometimes that happens…
But the truth is we are a team with the site. So even though I may go to a site and I’m monitoring the study and I’m reviewing charts and reg docs and study drugs… And yes, I may discover or observe an issue, there’s a way that we treat our sites, and a respectful way to point out observations and also reeducate them because we want to help them, we’re there to help.
We also should work together with a site as a team, having that same goal of subject safety and data integrity. So that’s definitely a misconception that people have because there’s not one role that’s higher than the other. You have the site level and then you have a CRA that’s either with a sponsor or a CRO, and again, we’re all a team doing this clinical trial together.
Darren Gaddis : How could someone get their start as a CRA?
Elizabeth Waddell : Well, most CRA positions require at least a bachelor’s degree and usually in a scientific area or a scientific discipline is preferred. This of course would depend on the company. In addition, most entry level CRA roles require about one to three years of clinical research experience. Again, that’s going to depend on the company, but clinical research experience, like I mentioned, speaks volumes on a CV, so some ways that people may gain this experience in order to apply for an entry level CRA position, they can gain this clinical research experience like me, maybe starting out as an entry level in-house CRA, and then move to onsite monitoring. Or they may gain experience as a clinical trial associate or a project specialist or project coordinator. And some may gain experience at the site level, as a research assistant, or a clinical research coordinator, and they’re also known as a study coordinator.
And I always say that in my experience, study coordinators make great CRAs. So some people gain experience that way, and others may start with even a clinical research internship and start getting that experience under their belt that way. So unless a company has a position where they have their own training program and they do not require previous research experience, which there’s not many that I’ve seen, most of the time the companies will require at least one to three years of that previous clinical research experience. So it’s definitely important to have clinical research experience under your belt.
Darren Gaddis : And what else would you like to share with us about CRAs?
Elizabeth Waddell : Clinical research is a blessing, and to be a part of, and being a clinical research monitor, CRA is so rewarding. And also there are so many roles that it can lead to in the industry, like becoming a lead CRA or project manager, or even if you want to manage CRAs like becoming a line manager or QA auditor, or like me managing and training new CRAs, which I absolutely love. So there is so much growth in the industry. So that’s another great thing about it.
Darren Gaddis : Elizabeth, thank you for joining me today. Be sure to follow, like and subscribe to On Research with CITI Program to stay in the know.
I also invite you to review our content offerings regularly, as we are continually adding new courses and webinars that may be of interest to you. All of our content is available to you anytime through organizational and individual subscriptions. You may also be interested in CITI Program’s, Clinical Research Coordinator courses. Please visit CITI Program’s website to learn more about all of our offerings.
How to Listen and Subscribe to the Podcast
You can find On Research with CITI Program available from several of the most popular podcast services. Subscribe on your favorite platform to receive updates when episodes are newly released. You can also subscribe to this podcast, by pasting “ https://feeds.buzzsprout.com/1896915 .rss” into your your podcast apps.

Meet the Guest

Elizabeth Waddell, BS, CCRA – The CRA Helper Owner/CEO of The CRA Helper. I provide online training based on 20+ years of clinical research experience in order to help others pursue a career as a Clinical Research Associate.
Meet the Host

Darren Gaddis , Host, On Campus Podcast – CITI Program
He is the host of the CITI Program’s higher education podcast. Mr. Gaddis received his BA from University of North Florida, MA from The George Washington University, and is currently a doctoral student at Florida State University.

- What CITI Program is Reading – October 24, 2024
- NIH and Voices For Our Fathers Legacy Foundation Commemorate Tuskegee Study Victims
- Understanding the CHIPS and Science Act’s Research Security Training Requirement
- On Campus Podcast – The Shifting Tide: Trends in College Enrollment
- Human Research Protection Program (HRPP) Analyst
- SALES COORDINATOR
- Research Scientist 4 – Research Administrator
- IRB Review Specialist (open rank)
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Training needs of clinical research associates
Samyuktha ajay.
- Author information
- Copyright and License information
Address for correspondence: Dr. Samyuktha Ajay, Clinical Operations, Feasibility and Site ID, Quintiles India, 5th Floor, Leela Business Park, M. V. Road, Andheri (E), Mumbai - 400 059, India. E-mail: [email protected]
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Clinical research is a relatively new field in our country that has seen very rapid growth in the last few years. Availability of personnel appropriately trained to the specific requirements of the role they will perform in clinical research is critical for capacity expansion. Our study attempts to understand the specific areas of knowledge and skills that are important for the role of a clinical research associate. The survey was conducted among clinical research professionals from industry and academia who had more than five years of clinical research experience and held important decision making positions in clinical research (stakeholders). The survey questionnaire was designed as a matrix of various clinical research roles on the y-axis and six knowledge modules and eight skills on the x-axis. Respondents were asked to rate the importance of the knowledge /skills to the role of clinical research associates on a three point scale. In discussing results, a significant response was considered to be 50% or greater positive response from the total group. The significant findings were that general, ethics and clinical trial execution modules were rated as critical for the role of clinical research associate. Regulatory module was rated as important for the role. The other significant responses were that three of the sub-topics in the methodology module - framing a research proposal/protocol and experimental design, designing case report forms and EDCs and conducting PK studies - were rated as important and one sub topic in the data management and statistics module was rated as not important. All the skills except leadership skills were rated as critical for the role. The findings of our survey were in general on the lines of expectations of performance of the role. The general, ethics and clinical trial execution modules are critical knowledge areas for the role of a clinical research associate. No clear trends emerged for some of the other modules. Leadership skills were not rated as critical to the role. This kind of a survey gives a good direction when training curriculum has to be designed for specific roles in clinical research. However, there is a need to expand the sample size to fine-tune the knowledge and skills areas.
Keywords: Clinical research associates, clinical research training, modules, performance, roles, topics
INTRODUCTION
GATT compliance in 2005 and the positive regulatory changes that ensued have made India an increasingly attractive location for clinical research. We have witnessed a rapid growth in this sector in the last few years[ 1 ] with projections for increased requirements for personnel in the various roles like clinical research associate (CRA), investigators, site coordinators, data management personnel, statisticians etc.[ 2 ] These roles are very well differentiated in terms of their performance expectations and availability of training tailored to the specific role requirements will be critical to capacity expansion.[ 3 ]
The CRA has the very critical role of ‘monitoring’ a clinical trial, which includes that the rights and well-being of human subjects are protected, the reported trial data are accurate, complete, and verifiable from source documents and that the conduct of the trial is in compliance with the currently approved protocol/amendment(s), with GCP, and with the applicable regulatory requirement(s). About the training of monitors, the GCP guideline mentions only that monitors should be appropriately trained, and should have the scientific and/or clinical knowledge needed to monitor the trial adequately and that a monitor’s qualifications should be documented.
Since clinical research itself is a relatively new field in India, we considered it is important to understand the requirements of knowledge and skills for the diverse roles. This paper describes the survey findings of the knowledge and skills needed for the role of a clinical research associate. We conducted a survey among key stakeholders in clinical research professionals in the industry, who by virtue of their positions had directly or indirectly employed or worked with CRAs.
MATERIALS AND METHODS
We chose to have the opinion of important stakeholders in clinical research on what they think are important knowledge and skill areas for a person performing the role of a clinical research associate. We looked for people who had more than five years of clinical research experience and held important decision making positions in clinical research (stakeholders). Forty eight such people were identified and they formed the population for this survey. The survey questionnaire was designed as a matrix of various clinical research roles on the y-axis and the areas of knowledge and skills on the x-axis.
Knowledge areas were further classified into six broad categories with sub-topics in each module as follows-
Regulations
Methodology
Data management and statistics
Clinical trial execution
Skills were classified as-
Negotiation
Conflict management
Interpersonal skills
Presentation skills
Communication skills
Each respondent graded the importance of the area of knowledge/skill for the role by grading it as:
3 - Critical - Performance of the role is not possible without knowledge of this area
2 - Important - Knowledge is important, but not critical to performance
1 - Not important - Knowledge is not important for performance in the current role
In discussing results, descriptive statistics will be used. A significant response was considered to be 50% or greater positive response from the total group as described by Stonier and Gabby in a similar study conducted by them.[ 4 ]
Of the 48 questionnaires sent, we obtained 31 responses. Fifteen were from Indian CROs, six from multinational pharma companies, five from multinational CROs, three from Indian pharma companies and two from academic medical institutions [ Table 1 ].
Role profile of respondents
The work experience distribution was as follows:
Knowledge modules
A frequency distribution of grades for knowledge areas and skills are given in Tables 2 and 3 [ Figure 1 ].
Clinical research knowledge areas for clinical research associates
Clinical research skill areas for clinical research associates
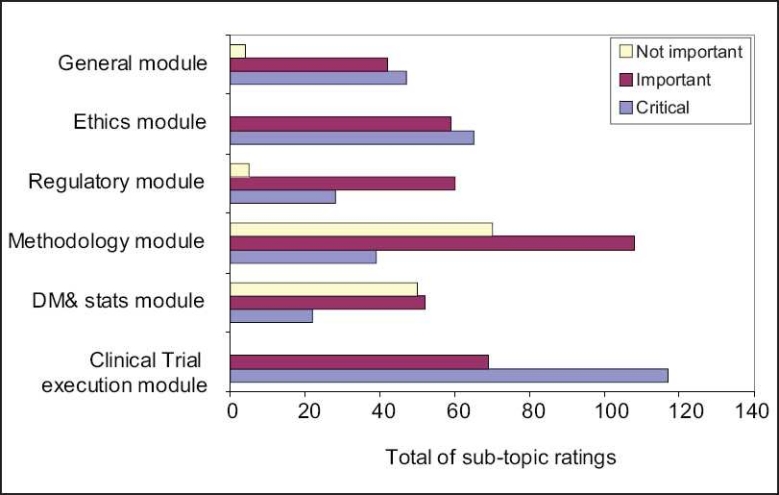
Responses for the role of clinical research associate (n=31)
The key findings for knowledge and skills were as follows:
Knowledge areas
There were 31 responses for knowledge areas and 26 responses were considered as evaluable for skills areas.
The general, ethics and execution modules were rated as ‘critical’ by 50% and more respondents. All the sub-topics in the clinical trial execution module were rated as critical.
The regulatory module and all the sub topics within were rated as “important” by 50% or more respondents.
The other significant responses were –
Sub topics - Framing a research proposal/protocol and experimental design, designing case report forms and EDCs and conducting PK studies were rated as ‘important’
Data coding and cleaning were rated as “not important’ by 50% or more respondents.
Ratings for the other modules and sub topics did not reach significance level.
Data of 26 responders were considered as evaluable for the skills. All the skills except leadership skills were rated as ‘critical’ by more than 50% of respondents. Team work and conflict management received the highest number of “critical’ responses closely followed by negotiation, interpersonal, presentation and communication skills.
Our survey suggests that for the CRA role, knowledge of the subtopics within the general, ethics and clinical trial execution modules is critical. These modules included most of the sub-topics that directly impact his critical role of ‘monitoring’ a clinical trial- including the ethical aspects of the rights and well-being of human subjects and the execution aspects that the reported trial data are accurate, complete, and verifiable.
There was no clear trend otherwise, with the regulatory module and all the sub topics within it being rated as ‘important’. This is rather surprising to find as compliance with GCP, and with the applicable regulatory requirement(s) is also an important aspect of the monitoring role. It was also interesting to note that while significant number of stakeholders rated the execution aspects of pharmacovigilance and safety management as ‘critical’, the regulations pertaining to the same were rated as ‘important’.
The only other survey done in India to understand training needs was done in 2003, to give direction to the curriculum development for courses proposed by the Academy for Clinical Excellence (ACE).[ 5 ] We were involved in this survey as executive curriculum committee members and active contributors for the set-up of curriculum for ACE. The population surveyed included investigators, CRO and pharmaceutical industry professionals and academia. The questionnaire captured the response as a rating of the importance of training in four modules of clinical research: clinical research regulations and environment, clinical research administration, clinical research methodology and applied clinical research. The methodology module received the highest rating, with 38% respondents rating it as important. However, this survey did not cover specific knowledge subtopics or skills for different roles.
The CRA is the central point of communication between sites and the sponsor and it was as expected to find communication skills, presentation skills, computing, interpersonal, conflict management, negotiation and team work rated as critical. It was surprising to find no significant trends in leadership skills for this role, which was probably because of the understanding and perception of the term leadership.
Although our survey had a small sample size, the trends that emerged match the performance expectations of the role, validating the study data. The three point grading we used to grade the importance of the areas of knowledge and skills requirements was probably not discriminatory enough. Hence there was equal weight given to critical and important grades for some subtopics for the CRA role. It was probable that the grading of ‘critical’ and ‘important’ were directionally similar and not characterized enough to avoid interchangeable use. Besides, we had a larger number of industry respondents and under-representation of academia, probably skewing the data to industry expectations. Our respondents belonged to organizations of various sizes and types and their own experiences would have colored their perception of training requirements of this role. Also, there were few areas that were grouped as knowledge areas, which could probably be better classified as competencies, eg, writing investigators’ brochure or designing case report forms and EDCs. Another limitation of our study was that we had not added behavioral indicators to describe each of the skills terms.
Survey was launched in 2006 and since then the clinical research industry has grown exponentially, with much technological advancement –e.g. most studies are now using e-CRFs. The role of the CRA has also evolved from the traditional role of compliance monitor to a new one of site relationship builder is being discussed with different competencies.[ 6 ] Zimmerman includes skills in using and troubleshooting hardware- computers, peripherals and other office apparatus and knowledge of multiple software programs- standard word processing database, project management, productivity programs and custom designed intranet software program as important competencies in preparing CRAs for the 21st century.[ 7 ]
The challenge to define the training requirements of this role can be understood from the predicament that even the association of clinical research professionals (ACRP), the leading certifier of CRAs for the last several years, is still trying to define what the minimum education and training requirements for CRAs ought to be.[ 8 ]
This kind of a survey gives a good direction when training curriculum has to be designed for specific roles in clinical research. However, there is a need to expand the sample size to fine-tune the knowledge and skills areas.
Disclaimer: All opinions expressed herewith are those of the authors and do not reflect the views of their organizations.
- 1. Gambrill SA. CenterWatch. 2006. New Era begins for India’s clinical research market; pp. 6–15. [ Google Scholar ]
- 2. Making the right choice. Express Pharma Pulse Jan 2009. Available from: http://www.expresspharmaonline.com/200901131/pharmalife01.shtml [last cited on 2009 Feb 12]
- 3. Bhatt A. Pharma Bio World. 2005. The Challenge of Growth in clinical Research: Training gap analysis; pp. 56–8. [ Google Scholar ]
- 4. Stonier PD, Gabby FJ. Training requirements in relation to the scope of pharmaceutical medicine- a survey of practitioners. J Pharmaceut Med. 1992;2:263–72. [ Google Scholar ]
- 5. ACE. Available from: http//www.aceindia.org [last accessed on 2010]
- 6. Harper B, Neuer A. Good site and sponsor relationships pay off. Applied clinical trials. 2008. Available from: http://www.appliedclinicaltrialsonline.findpharma.com/appliedclinicaltrials/CRO%2FSponsor/Good-Site-and-Sponsor-Relationships-Pay-Off/ArticleStandard/Article/detail/483705 [last cited on 2008 Mar 22]
- 7. Zimmerman JF. Preparing clinical research associates for the 21st century. 2008. Available from: http://www.impactcg.com/docs/ACRP_Sp99_CRAs.pdf [last cited on 2008 Dec 13]
- 8. ACRP 2008 Panel Discussion on Accreditation.doc and DIA 2008 Session on CR Accreditation Standards. doc. 2008. Available from: http://cedric.mghihp.edu/JSPWiki/Wiki.jsp?page=ConsortiumOfAcademicProgramsInClinicalResearch [last cited on 2009 Jan 12]
- View on publisher site
- PDF (2.1 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- MTS Health Sciences How to Become a Clinical Research Associate
- Laboratory Technology
- Natural & Clinical Science
- Medical IT & Administrative
- Patient-Facing Technology
- Cytologist (Cytotechnologist)
- Dental Lab Technician
- Histotechnologist
- Medical Lab Assistant
- Medical Lab Technician
- Medical Laboratory Scientist
- Biological Sciences
- Biomedical Science
- Biotechnology
- Health Sciences
- Infection Preventionist
- Nutritionist & Dietitian
- Pathologists' Assistant (PathA)
- Pre-Vet (Veterinarian)
- Biomedical Equipment Technician
- Biomedical Informatics
- Health Informatics
- Health Information Management
- Health Information Technology
- Healthcare Administration
- Medical Billing & Coding
- Nursing Informatics
- Sterile Processing Technician
- Anesthesia Technician & Technologist
- Audiologist & SLP
- Cardiovascular Technologist
- Dental Assistant
- Dental Hygienist
- Diagnostic Medical Sonographer
- Dialysis Technician
- EKG Technician
- EMT & Paramedic
- Kinesiologist
- Mammography Technologist
- Medical Assistant
- MRI Technologist
- Neurodiagnostic Technologist
- Nuclear Medicine Technologist
- Ophthalmic Technician
- Pharmacy Technician
- Phlebotomist
- Physical Therapist Assistant & Aide
- Psychiatric & Mental Health Technician
- Radiation Therapist
- Radiologic Technologist
- Rehabilitation Technician
- Respiratory Therapist
- Surgical Technologist
Certification Guides
Career guides, interviews & features, how to become a clinical research associate (cra), search for schools.
When you click on a sponsoring school or program advertised on our site, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
Scientists, researchers, and doctors make discoveries about drugs, surgical procedures, behavioral therapies, or medical devices through their work in laboratories and healthcare settings. This is only the beginning of the journey for pharmaceuticals, therapies, and devices, as bringing the findings from the lab to the street requires a vigorous scientific process known as a clinical trial. Clinical research associates (CRAs) are the professionals responsible for ensuring that clinical trials move forward following established guidelines and regulations for ethics, safety, and reporting.
Clinical research associates, also known as “monitors,” work on behalf of sponsors funding clinical trials for the new or existing drug, device, surgery, or behavioral intervention. Working directly for the sponsor or through a contract research organization, the main task of a CRA is to monitor the progress of an ongoing clinical trial.
Through in-person site visits or remote monitoring systems, a CRA serves as the central point of contact between a sponsor and testing sites; ensures that the trial is being administered per approved protocols; verifies that the clinical trial is being conducted ethically at all sites; and confirms the validity and accuracy of all data being collected and reported at test sites.
In addition to reading, interpreting, and understanding medical technology, clinical research associates must have excellent interpersonal and communication skills. The ability to understand best clinical practices, design protocols, and data standards requires CRAs to have outstanding attention to detail, analytical skills, and the capacity to deliver constructive feedback to participating research sites on their performance.
Although not a requirement, many CRAs travel between multiple research sites for study oversight, which may require a valid driver’s license, the physical capacity to travel, and/or willingness to fly or drive regularly.
This detailed guide explores the education and credentials required to become a clinical research associate (CRA).
Arizona State University
Johns hopkins university (aap), university of west florida, steps to become a clinical research associate (cra).
The pathways to becoming a clinical research associate are numerous and available to anyone with a high school diploma or higher. While formal education is not technically required to enter the field, having a bachelor’s degree or higher can make potential candidates much more competitive.
Certification in the field is also not required, but obtaining certification from the Society of Clinical Research Associates (SOCRA) or the Association of Clinical Research Professionals (ACRP) can result in more opportunities and even more competitive salaries.
Finally, all aspiring CRAs are advised to check out the International Conference on Harmonisation’s (ICH) guidelines for Good Clinical Practice (GCP) to get a feel for the professional expectations and responsibilities.
Here is how to become a CRA depending on one’s level of education. Please note that in the United States, there are two major certification bodies for CRAs: the Society of Clinical Research Associates (SOCRA) and the Association of Clinical Research Professionals (ACRP). Each pathway includes the eligibility requirements to pursue credentialing through either of these entities.
PATH 1: Earn a High School Diploma and Gain Experience
Perhaps the most strenuous route to this career is becoming a certified CRA with a high school diploma and between 3,000 and 3,500 hours of qualifying work experience (depending on the certification entity).
These candidates often start out in support positions assisting a more experienced or certified CRA with mundane tasks. An entry-level worker can earn increased responsibilities through a demonstrated capacity to learn the regulations, protocols, and ethical considerations. To qualify for the following CRA certification exams, high school graduates must:
SOCRA Category 1
- Complete two full-time years of CRA work within five years, or 3,500 hours of part-time work
ACRP CCRA (Certified Clinical Research Associate)
- Complete 3,000 hours performing essential duties
- Submit a resume documenting and demonstrating job performance
Please note that in some cases, additional education can be used to substitute for work experience hours. Please see credentialing websites for details.

PATH 2: Earn an Associate Degree and Gain Experience
Depending on the program, an associate’s degree of applied science (AAS) in clinical research can be a standalone degree or a stepping-stone to a bachelor’s or master’s. Licensed vocational or practical nurse (LVN or LPN) programs are designed specifically for practical, job-ready skills and may qualify aspiring CRAs for the ACRP certification.
Similar to the path taken by those with a high school diploma, having an associate degree, LPN, or LVN can open the door to some entry-level jobs in the industry. At this level, some prospective CRAs assist more experienced CRAs or some engage independently in entry-level tasks related to study monitoring. Those working as CRAs with an associate’s degree, LPN, or LVN can qualify for certification after working a certain number of hours in the field.
To qualify for the following CRA certification exams, associate degree graduates must:
SOCRA Category 2
- Hold a “clinical research” degree
- Complete one full-time year as a CRA or 1,750 hours part-time
ACRP Option 2 (Also for LVN, LPN)
- Hold a “clinical research degree” or complete 1,500 hours performing essential duties
PATH 3: Earn a Bachelor’s Degree and Gain Experience
Most entry-level clinical research associate positions require candidates to have a bachelor’s of science (BS) in a health-related field from an accredited four-year university. In some cases, programs are designed to add practical hours needed to qualify for certification tests.
Those interested in becoming a CRA can study nursing, health sciences, biological sciences, clinical research, clinical research administration, clinical research management, medical technology, or life sciences, among many other subjects. Because many entry-level positions are looking for those with previous work in the field, those earning a BS should seek internships, part-time work, and/or fellowships involving participation in research, if possible.
To qualify for the following CRA certification exams, bachelor’s degree graduates must:
SOCRA Category 3
- Hold a “clinical research” undergraduate degree
ACRP Options 1 & 2
- Complete 3,000 hours performing essential job duties or 1,500 hours of equivalent work experience requirements through ACRP certifications or approved clinical research degree programs accredited by the Council for Higher Education
PATH 4: Earn a Master’s Degree for Opportunities in Management
A master’s program in clinical research is generally designed for those already working as CRAs to expand their skills or to advance into management or supervisory roles within the field. However, for those with non-health science bachelor’s degrees who want to become CRAs, seeking a master’s of science in clinical research or a master’s of science in clinical research management could be a pathway to breaking into the field.
Because many of these programs are offered online, earning a degree is possible for even those students who need full flexibility of schedule to complete the degree. Although requirements for admission into master’s programs vary, those looking to gain admission into a master’s of science for clinical research commonly need the following:
- A bachelor’s degree
- Official transcripts demonstrating specific coursework in science
- A statement of purpose
- Letters of Recommendation or Reference
- A resume or CV
- An application fee
- TOEFL or IELTS scores (international students only)
Clinical Research Associate (CRA) Degree Programs
There is a range of formalized training programs that prepare professionals for this key role in ensuring the safe, and ethical development of medical technologies. Below you will find examples of programs at a range of educational levels available to those interested in a career as a CRA.
Durham Tech – AAS Program
Durham tech, located in Durham, North Carolina, offers a 71-credit hybrid on-campus and online clinical trials research associate (CTRA) associate of applied science (AAS) program. Durham’s CTRA AAS prepares graduates to work on any side of clinical research in an assistant’s role.
While most programs require the student to attend on-campus courses, there are several courses that are offered completely online. The program takes 20 to 21 months and includes coursework in research site management; clinical research management; research protocol design; an introduction to ethics; anatomy and physiology; an introduction to clinical data; pathophysiology; and clinical research terminology.
Graduates of the program may be eligible to sit for national certification examinations and will be prepared for opportunities at medical centers, pharmaceutical industries, hospitals, research facilities, clinics, physicians’ offices, and device companies.
- Location: Durham, NC
- Accreditation: Southern Association of Colleges and Schools Commission on Colleges (SACSCOC); Commission on Accreditation of Allied Health Education Programs (CAAHEP)
- Expected Time to Completion: 20 to 21 months
- Estimated Tuition: $5,396
Washington University in St.Louis University College – BS, MS, Certificates
Washington University in St. Louis, Missouri, has various degree options for CRAs at all stages of their career to work as monitors. Students can enhance their current skills and knowledge in clinical research management, as well as gain a deep mastery regarding how to best move clinical research forward in an ethical, compliant, and safe way.
Those with at least six units of transferable coursework qualify to apply to the 120-credit-hour bachelor of science in clinical research management to start their careers. Anyone with any educational background can pursue University College’s 21-credit undergraduate certificate in clinical research management to enhance career skills or make a resume more competitive.
Students who already have a BA or BS also have options at Washington University. Experienced professionals in the clinical research field who wish to seek formalized training can earn a 21-credit advanced certificate in clinical research management or a 30-credit master of science (MS) in clinical research management. Those with a non-healthcare bachelor’s degree who wish to become high-level CRAs can up their skills and knowledge by choosing the combined bachelor’s and master’s degree options.
Although the coursework in each program varies to suit the level of education, themes across all the programs include the fundamentals of clinical research management; research ethics and regulatory affairs; compliance, legal and regulatory issues; and data and information management in health sciences.
- Location: St. Louis, MO
- Accreditation: Higher Learning Commission (HLC)
- Expected Time to Completion: BS (up to 48 months); certificate (12 months); MS (24 months)
- Estimated Tuition: Undergraduate courses ($695 to 895 per credit); Graduate courses ($665 to 995 per credit)
Barnett International – Online Seminar
Designed for CRAs with two years of experience or less, this online clinical research associate onboarding program by Barnett International prepares entry-level employees to monitor clinical trials at high levels appropriate to industry standards.
Over ten weeks of synchronous online coursework lasting three hours per week, participants will learn topics including informed consent, investigational product accountability, safety definitions and reporting requirements, and regulatory compliance and quality assurance: audits and inspections. Participants receive 30 hours (3.0 CEUs) of continuing education credits.
- Location: Needham, MA
- Accreditation: Accreditation Council for Pharmacy Education
- Expected Time to Completion: Ten weeks
- Estimated Tuition: By Early Bird Deadline ($1,795); After Early Bird Deadline ($1,995); June 10 is the early bird deadline
Continuing Education for Clinical Research Associates (CRAs)
Both CRA certification bodies require continuing education to maintain active certification status.
SOCRA requires recertification every three years. It calls for 45 hours of CE to be completed over the course of the first three years beyond passing the initial test. Twenty-two CE units must be related to clinical research; the remainder can be in the professional or therapeutic area in which one works or specializes. In addition, those looking to maintain or renew certification must complete a “recertification continuing competence learning module.”
The ACRP expects certified CRAs to engage in continuing education (CE) and continuing involvement (CI) to maintain certifications. Continuing education should include coursework in research and healthcare, and continuing involvement requires candidates to engage in activities such as authorship, participating in investigator meetings, or working as a peer reviewer, among other opportunities. Notably, ACRP utilizes an ongoing point system for professionals to maintain their certifications.
CRA Career and Salary
Clinical trials and the objectivity they bring to advances in treatment are extremely important. In an increasingly globalized society, diseases spread across borders, and in an age of increased antibiotic resistance, new ways to fight bacteria will be needed. Furthermore, with an aging U.S. population comes increased rates of chronic conditions and the subsequent reliance on pharmaceuticals to improve people’s quality of life.
It’s not surprising that the Bureau of Labor Statistics (2022) predicted a 7 percent increase in openings for medical and clinical laboratory technicians between 2021 and 2031, much more than the average growth anticipated across all U.S. occupations during that same decade (5 percent). As far as the salaries are concerned, here are the salary percentiles for clinical laboratory technologists and technicians in the US ( BLS May 2022):
Lastly, while the BLS doesn’t track salaries for CRAs, PayScale.com (June 2023)—a site that relies on self-reported data—found that the median annual salary for a CRA was $72,393. Among the 1,391 CRAs reporting their annual salaries, Payscale found these percentiles:
- 10th percentile: $48,000
- 50th percentile (median): $72,393
- 90th percentile: $101,000
Specialized skills in CRA that increased salaries included medical devices (37 percent pay increase over average), team leadership (35 percent), and writing procedures & documentation (20 percent).
Years of experience, predictably, also have an impact on salary. Entry-level CRAs earn 15 percent below the average, while experienced CRAs (ten to 19 years) earn 16 percent above the average and late-career CRAs (20+ years) earn 25 percent above the average.
It is important to note that these figures also vary based on the data source. For illustration, Indeed.com (June 2023) found an average annual salary of $80,957 among United States clinical research associates.

Becca Brewer is building a better future on a thriving earth by healing herself into wholeness, divesting from separation, and walking the path of the loving heart. Previously to her journey as an adventurer for a just, meaningful, and regenerative world, Becca was a formally trained sexuality educator with a master of education.
Related Articles
- Upskilling in the Allied Health Professions
- Single-use Plastics in Medicine Raise Concerns About Sustainability
- Clinical Research Certification - CCRA, CCRC, CPI
- Online Master’s in Clinical and Translational Research Programs
- Online Bachelor’s in Health Science Programs (BSHS Degree)
- Online Master's Programs in Clinical Management and Leadership
- Clinical Lab Science vs. Clinical Research
- BSHS (Health Sciences) vs BSHA (Health Administration) Programs
- Clinical vs Translational Research
- Guide to Health Science Careers
Related Programs
- Skip to main menu
- Skip to user menu
10 Clinical Research Career Paths
- Industry Features
- General Careers Advice
In 2020, the global Clinical Trials market was estimated at $44.3 billion, and this is expected to grow at an annual rate of 5.7% between 2021 and 2028. The National Institute for Health Research (NIHR) also recorded that between April 2020 and March 2021, 1,390,483 participants took part in Clinical Research across England, which is almost double the numbers from the previous year.
In this article, we look at 10 different career paths within Clinical Research, with an outline of some of the most common responsibilities for each role…
Clinical Trials Manager / Administrator
Clinical Trials Managers / Administrators are responsible for the administrative aspects of clinical trials. Their duties often include:
- Preparing essential documents and ensuring documentation is kept private and confidential.
- Attending safety and study start-up meetings and coordinating investigator meetings.
- Managing clinical trial supplies.
- Reviewing trial protocols and identifying any protocol issues.
- Processing and tracking payments to investigator sites.
More information on the role of a Clinical Trials Manager can be found here.
Clinical Research Associate (CRA)
CRAs are responsible for organising and administering clinical trials and are typically involved in all stages of a trial, from identifying investigator sites to closing down the trial. The responsibilities of a CRA can include:
- Identifying suitable facilities to be used as trial sites and selecting an investigator to be responsible for the site.
- Briefing trial investigators and instructing clinicians on how the trial should be conducted.
- Writing up clinical trial methodologies and designing trial materials.
- Monitoring the progress of clinical trials and preparing final reports.
- Designing and authenticating data collection forms and managing regulatory applications/approvals.
More information on the role of a Clinical Research Associate can be found here.
Clinical Project Manager
Clinical Project Managers are responsible for managing the workers involved in clinical research projects, ensuring protocol compliance whilst coordinating projects to meet clinical objectives. The main responsibilities of a Clinical Project Manager may include:
- Overseeing the enrolment of subjects into clinical trials by assessing the eligibility of potential subjects and tracking the enrolment status of suitable participants.
- Ensuring compliance with protocols and informing investigators of any protocol issues.
- Monitoring study activities to ensure the study remains on schedule and is kept within allocated budgets.
- Maintaining records of study activity, including records of side effect data.
More information on the role of a Clinical Project Manager can be found here.
Pharmacovigilance / Drug Safety Officer
Pharmacovigilance Officers, also known as Drug Safety Officers, are responsible for ensuring that new and existing drugs on the market are safe for patients, and for identifying any issues with these drugs. They may be responsible for:
- Monitoring the effectiveness of new drugs and pharmaceutical products already on the market.
- Monitoring adverse effects to new or existing drugs and flag any early warning signs of these to minimise risk.
- Conducting interviews with patients and healthcare professionals.
- Completing safety update reports and conducting safety audits.
Study Start Up Associate
Study Start Up Associates are integral in making sure that clinical research sites are well prepared to begin a new trial. They can be involved in the following:
- Executing start-up activities before site activation including preparing consent forms, identifying new investigator sites, allocating study budgets, and supporting patient recruitment and retention.
- Ensuring physicians working at research sites are prepared to begin trials.
- Obtaining appropriate ethics and regulatory approvals and ensuring research operations comply with protocols.
- Analysing study start-up metrics to ensure efficiency and identifying areas for development, including in terms of start-up timelines.
More information on the role of a Study Start Up Associate can be found here.
Clinical Research Nurse
Clinical Research Nurses help to improve patient care by supporting patients through their treatment, ensuring they are both safe and fully informed of the study activities. Some of their main responsibilities could include:
- Helping to develop new treatments and care pathways for patients.
- Aiding data collection activities.
- Ensuring patients give full consent prior to being enrolled in clinical trials and making sure patients fully understand all aspects of the study before doing so.
- Assisting the principal investigator with pre-study preparation and study start-up activities, including preparing protocols for regulatory and ethical approval, and attending investigator meetings.
- Arranging appointments for potential and enrolled trial participants.
More information on the role of a Clinical Research Nurse can be found here.
Clinical Research Scientist
Clinical Research Scientists are responsible for undertaking medical research in research labs to find more effective ways of diagnosing and curing a variety of illnesses. They may also be responsible for:
- Interacting with patients taking experimental treatments to understand the effectiveness of these treatments and to investigate new ways of improving their wellbeing.
- Working with other medical staff to advise on how to use products and equipment already on or coming to the market.
- Analysing data to further develop treatments and test any new methods of diagnosis and treatment.
Clinical Investigator
Clinical Investigators ensure that the investigation is meeting research expectations and is conducted in line with the investigator statement, investigational plan, and all necessary regulations. By doing so, they protect the welfare of clinical trial participants as well as the integrity of the resulting data. Their responsibilities can include:
- Meeting specific guidelines and/or requirements set by applicable regulatory and ethical bodies.
- Conducting or supervising research to ensure the investigational plan and corresponding study protocols are being followed.
- Notifying relevant bodies of any changes in research activity, including any unanticipated obstacles that may introduce risk to study participants.
- Ensuring informed consent has been obtained from all participants.
- Maintaining records of the clinical studies and preparing reports to be sent to investigation sponsors and other relevant bodies.
Patient Recruitment Specialist
Patient Recruitment Specialists are responsible for recruitment-related activities. Their main responsibilities include:
- Recruiting participants in line with protocol-specific inclusion and exclusion criteria.
- Tracking recruitment progress and developing new and existing recruitment strategies.
- Contacting potential participants to assess eligibility and to schedule site visits.
- Ensure patient information is accurately collected and entered into the relevant database and is protected.
Biostatistician
Biostatisticians provide statistical support to clinical studies and work across all study phases. Typically, their work can include:
- Obtaining clinical data from the Clinical Data Manager to undertake necessary statistical analyses. Interpreting the meanings of statistical outputs resulting from different analyses.
- Assisting the Clinical Trial Manager in writing up the final technical paper for the study, sharing findings from statistical analyses.
- Analysing safety and efficacy data and applying statistical methods to develop the science of data analysis.
More information on the role of a Biostatistician can be found here.
Current Opportunities in Clinical Research…
Take a look at current opportunities in Clinical Research here and set up job alerts to be notified of the latest opportunities in the industry.
* Article updated March 2024
Related links
- Jobs in Clinical Research
- More Careers Advice
Share this article
Related articles

U.S. CDC and ACIP Recommend Additional COVID-19 Vaccine Dose for Older Individuals and Those Who Are Immunocompromised

Abbott Initiates New Clinical Trial to Improve Outcomes in Patients with Advanced Heart Failure

Merck’s KEYTRUDA® (pembrolizumab) Receives 30th Approval From European Commission With Two New Indications in Gynecologic Cancers
Latest articles, about proqr therapeutics.

A Day in the Life of a Clinical Research Associate
For the 2023-2024 academic year, we have 112 schools in our MHAOnline.com database and those that advertise with us are labeled “sponsor”. When you click on a sponsoring school or program, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
Every pill, cream, tonic, and capsule a pharmacist passes over the counter to an individual has taken a long journey from concept to the point of sale. In fact, many medications that do not require a prescription, such as those found in the allergy, cold, and flu aisle, also go through the same arduous process.
Before a drug can be prescribed and sold, it must be proven safe and effective. Pharmaceutical companies, universities, and health organizations put drugs through clinical trials or clinical study processes to obtain this proof. In the clinical trials world, the company that provides financial support to a research group selected to put the drug through clinical trials is known as a contract research organization or CRO. Primarily, CRO organizations offer financial aid for pharmaceutical development and biotechnology for agricultural and medical device industries.
In the case of pharmaceuticals, drugs that have not yet been approved for sale are administered to vetted participants in a controlled manner during a clinical trial. Clinical trials involve a great deal of documentation, analysis, observation, and organization. A team of professionals is engaged in administering a clinical trial, including a clinical research associate (CRA).
The CRA acts as a liaison between the study’s sponsor CRO (e.g., pharmaceutical company) and the clinics where the study occurs. Because the clinical trial results must be kept entirely transparent and not influenced by the sponsor’s interests, this is a critical role. Therefore, a successful CRA will be detail-oriented, highly educated, and communicate clearly with sponsors and clinical representatives.
THANK YOU FOR YOUR INTEREST IN Southern New Hampshire University Online MS - Construction Management
Work environment of clinical research associates.
A clinical research associate works both at clinical sites and sponsor locations. During a trial, the CRA conducts regular site visits—virtually and physically—to ensure good progress and record-keeping on the clinical site.
CRAs are often responsible for multiple trials at one time, meaning significant amounts of travel between these sites. Sometimes, a CRA may be assigned to a specific geographical region, limiting travel.
Clinical Team of a Clinical Research Associate
Typically, a clinical research associate needs to have direct contact with the participants involved in the study. However, CRAs must work in a collaborative environment, coming into frequent contact with the clinical team at the study site and the supervisors from the study sponsor. Thus, clinical research associates work in the middle of the chain of command, which begins at the top with:
- Contract research organization (CRO) or sponsor
- Principal investigator (PI)
- Clinical research associate (CRA)
- Clinical research coordinators (CRC)
In short, a CRA is focused more on data, accuracy, and quality control while a CRC collects data and interacts with patients. Dan Sfera , in his YouTube channel titled The Clinical Trials Guru, summarizes the CRA profession with this description: “CROs select various PIs across the country, and PIs make sure that sites follow good clinical practices and protocols. They ensure this by hiring CRAs who look at all the recorded data and do not interact with patients when they are completing a site visit.”
Typical Daily Responsibilities of Clinical Research Associates
The daily responsibilities of a CRA are mainly dependent on the stage of the trial they are supervising. As such, below is a breakdown of the typical duties of a CRA during the beginning, middle, and end of a clinical study.
Before a Study Begins
Every clinical study must take place in an appropriately equipped clinical location. The CRA plays a critical role in selecting a site for a clinical study and may even be asked to suggest sites based on their previous experiences. CRAs may also evaluate the applications of sites that self-select as eligible for a particular study. A pool of potential study sites is narrowed down by having sites complete and submit a feasibility survey.
When the pool of sites has been narrowed down, the clinical research associate conducts site selection visits with the chosen locations. During these visits, the CRA spends up to half a day confirming the validity of the feasibility study, meeting with the team (mainly the assigned coordinator), and observing the capabilities and equipment at the facility.
Upon completion of the site visit, the CRA compiles a report for the study sponsor and presents their findings and recommendations for proceeding with the study.
During a Study
Once a site has been selected, the CRA is now responsible for ensuring that the site knows the sponsor’s required protocol and is appropriately set up to conduct the study. In addition, the clinical research associate conducts site visits at regular intervals throughout the study to ensure that protocols are followed and data is effectively collected.
Depending on the study, CRAs may conduct in-person site visits as well as virtual visits. In recent years, the use of remote visit technology has allowed CRAs to review paperwork online, for instance, and reserve in-person visits for necessary personal interactions. During site visits, the CRA ensures that the study is always proceeding with good clinical practices. More details on the specific methods are available below.
Ultimately, since the CRA is a liaison, developing and maintaining positive relationships is an essential part of the job. During the trial, the CRA must communicate effectively and help the clinical staff appropriately to ensure the study progresses smoothly.
When a Study Ends
The CRA typically conducts a closeout visit after a study or when it becomes necessary to end a study—e.g. when enrollment is too low.
During the closeout visit, the CRA verifies that all paperwork is in order and that all obligations have been met on both sides. For example, the verification process may mean ensuring the trial drugs are returned or destroyed, completing and adequately filing all documentation, and compiling all information necessary to complete a final report for the study sponsor.
Required Skills & Knowledge of Clinical Research Associates
Based on the responsibilities outlined above, it should be no surprise that CRAs must have a knack for detail. Proper documentation, filing, and storage are all critical parts of the job description. It is up to the CRA to ensure that the sponsor and clinic understand their obligations at every point of the study. All policies and procedures are being followed to ensure successful data collection.
In addition to being detail-oriented, a good CRA also pays heed to the ethics of the position. For example, clinical trials and studies can have extensive consequences for the organizations that sponsor them, as well as for the trial participants, and eventually, for consumers at large. Therefore, CRAs act as vital monitors for ethical issues and should be able to stand up to any perceived transgressions.
Above all, it is essential that a CRA understands and can implement good clinical practices (GCP) successfully. The GCP guidelines are an internationally developed set of standards from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) that details ethically and effectively conducting drug trials with human subjects.
The ICH topics are divided into four categories: quality, efficacy, safety, and multidisciplinary. CRAs can find the guidelines in more detail on the ICH website. The YouTube Channel, GCP Mindset , outlines the different roles and responsibilities of CRAs. In short, upholding the GCP standards is the most critical part of the CRA job, meaning that any CRA must be familiar with GCP and recognize and enforce these practices in a clinical setting.
Certification for Clinical Research Associates
While certification is not a legal requirement to work as a clinical research associate, it can provide an important differentiator when looking for employment or career advancement in many professions.
Two main bodies offer certification for CRAs: the Association of Clinical Research Professionals (ACRP) and the Society of Clinical Research Associates (SOCRA).
Association of Clinical Research Professionals (ACRP) – Certified Clinical Research Associate (CCRA) Certification
To earn the Certified Clinical Research Associate (CCRA) credential from ACRP, applicants have two available pathways:
- Pathway 1 – Clinical research professionals with 3,000 hours of verifiable work experience are eligible to sit for the CCRA Exam.
- Pathway 2 – Clinical research professionals with 1,500 hours of verifiable work experience and a clinical research degree are eligible to sit for the CCRA Exam.
Applicants also have to submit proof of their current job description and resume and pass the CCRA exam. In addition, CCRAs must complete at least 24 hours of continuing education credits and be recertified every two years to maintain certification. Beyond the CCRA certification, ACRP also offers certifications for project managers, research coordinators, and principal investigators, and an umbrella certification for clinical professionals.
Society of Clinical Research Associates (SOCRA) – Certified Clinical Research Professional (CCRP) Certification
To earn the Certified Clinical Research Professional (CCRP) certification from SOCRA, applicants must either:
- Have at least two years of full-time experience as clinical research professionals or 3,500 hours of part-time experience in the past five years
- Have a degree in clinical research plus at least one year of full-time experience (or 1,750 hours part-time during the previous two years)
- Have a certificate in clinical research (undergraduate or graduate), a degree in science, health science, pharmacy, or a related field (associate’s or bachelor’s), plus at least one year of full-time experience (or 1,750 hours part-time during the previous two years)
Applicants must also pass the CCRP exam and be recertified every three years. Recertification requires 45 total hours of continuing education credits.
The critical difference between the SOCRA and ACRP certifications is that ACRP expressly certifies CRAs, while the SOCRA certification can apply to other clinical research professionals.
Matt Zbrog is a writer and researcher from Southern California. Since 2018, he’s written extensively about emerging issues in healthcare administration and public health, with a particular focus on progressive policies that empower communities and reduce health disparities. His work centers around detailed interviews with researchers, professors, and practitioners, as well as with subject matter experts from professional associations such as the American Health Care Association / National Center for Assisted Living (AHCA/NCAL) and the American College of Health Care Executives (ACHCA).
Related Programs
- 1 Online Master’s in Clinical Research Administration Programs
- 2 Online Doctor of Health Sciences (DHS)
- 3 Online MS in Health Science Programs – MSHS Degrees
- 4 Online Master’s Degrees in Food Safety – Regulatory Affairs & Quality Assurance
- 5 Online Master’s in Bioinformatics Programs
Related FAQs
- 1 Clinical Significance vs. Statistical Significance
- 2 How Do I Become a Clinical Trials Research Nurse?
- 3 How Do You Become a Clinical Research Coordinator (CRC)?
- 4 What Can You Do with a Degree in Clinical Research Administration?
- 5 What is Clinical Data Management (CDM)?
Related Posts
A day in the life of a clinical data analyst.
Every day, health organizations like hospitals, clinics, and physician offices collect data about their patients. This information is used to make data-driven decisions in order to provide the highest level of care to their patients as well as reduce expenses and errors. From the outcome of a particular treatment to a large-scale clinical trial, health data is a critical part of the modern healthcare process. Clinical data analysts help to make sense of the extensive data that is at their fingertips, creating stories that turn numbers into actionable intelligence to improve healthcare outcomes.
Healthcare Debates: Does Costly US Healthcare Fund R&D and Medical Research?
This article explores research and development in the context of greater healthcare expenditures, including looking into the main drivers of healthcare spending, influences on pricing, and where R&D fits into the whole ecosystem.
Pharmaceutical Quality Director – A Day in the Life
Consumers and corporations need to know that the drugs dispensed by pharmaceutical companies are manufactured safely and efficiently. Pharmaceutical quality directors ensure that quality control protocols are followed within the industry’s manufacturing, testing, and inspection procedures.
Closing the Gap: Women Leading Healthcare
Women drive healthcare. They are the industry's biggest consumers and workers. They serve as caregivers in their homes and make most decisions for their family's health. Why is it, then, that of the 40 Fortune 500 healthcare companies, not a single one is helmed by a woman?
Clinical Application Analyst – A Day in the Life
Electronic health records (EHR) and other clinical software systems are the new industry standard in healthcare. However, plenty of clinics, hospitals, and private practices have yet to make the full migration. That’s where clinical application analysts come in.
- Thought Leadership
THE INTERESTING LIFE AND CRITICAL ROLE OF A CLINICAL RESEARCH ASSOCIATE (CRA)
Clinical trials leverage the expertise of people in a wide variety of roles — researchers, project managers, clinical data managers, etc. One of the positions with which people may be less familiar is that of the clinical research associate (CRA). CRAs play a critical, if somewhat unheralded, role in the success of a trial.
What is a Clinical Research Associate?
A CRA is an observer, documenter, and conductor of a clinical trial. However, the CRA’s responsibilities and title can vary widely depending on the need of the sponsor. Some of the titles that identify CRAs are:
- Clinical Monitor
- Clinical Trial Specialist
- Site Manager
CRAs need to be prepared to travel regularly because they visit study sites frequently. They need to thrive while working independently, as they often work by themselves, and they must have well-honed skills in planning, organization, and preparation to maximize the effectiveness of their site visits. In addition, CRAs need to be flexible and creative, as the role is one that involves juggling many tasks, being the intermediary for different study participants or stakeholders, and, in some cases, meeting tight deadlines.
CRAs must have a comprehensive understanding of a trial and the people involved, as they may be required to interact with anyone on the team. This can include project managers, data managers, investigators, pharmacists, biostatisticians, safety experts, CRA managers, and others.
What Tasks do Clinical Research Associates Perform?
CRAs have responsibilities that cover the full lifecycle of a clinical trial, which starts with pre-study qualification visits (PSQVs). The CRA travels to study sites to assess whether the site has the necessary experience and resources for working on the trial, an acceptable number of eligible patients, appropriate and available clinic space, the equipment that may be necessary for the trial, security for keeping records inaccessible to non-study personnel, and regulated drug storage areas. Next, site initiation visits (SIVs) take place as part of the kick-off of the trial. At these visits, the CRA trains the team members at the site to understand what their responsibilities will be, and to officially green-light the start of the trial.
Over the course of a study there will be periodic interim monitoring visits (IMVs) at the participating sites. Generally, the goal is to have the CRA onsite at least once every four to eight weeks, depending on the rate of enrollment and complexity of the study objectives and assessments. The CRA will review study progress, the quality and accuracy of data collection, compliance of patients to trial visits and assessments, and investigational product accountability, and will ensure good clinical practices are maintained throughout the trial and offer direction when needed. At the end of the trial, a close out visit (COV) occurs at the site to address any unresolved study issues, ensure the site has properly informed the ethics committees of the completion of the trial, help document the end of a site’s participation, and collect study-related items for a sponsor when the study is complete.
Regardless of the type of visit, when a CRA is at a trial site there are many study-related activities that may occur. Some specific examples of monitoring tasks include:
- Enrollment process and standards. Is the site enrolling qualified participants?
- Informed consent procedure. What process is being used to obtain and manage properly-completed consent forms?
- Protocol adherence. Are any deviations from the protocol taking place?
- Data entry. Is the site entering data completely, accurately, and in a timely manner?
- Medication distribution. Is the site carefully managing and distributing the study medication?
- Regimen compliance. Are subjects complying with the medication regimen that they have been assigned?
- Query response. Does the site answer questions promptly?
- Regulatory compliance. Are the necessary legal and regulatory documents completed correctly and filed?
- Adverse event reporting. Are all adverse events being properly logged and reported? Are serious adverse events being sent to the Institutional Review Board (IRB)?
When not onsite, CRAs have a wide range of tasks they must complete related to a trial. For example, they write pre-study visit letters, visit reports, and follow-up letters for each site visit that detail the progress at that site and list any recommendations for improvement. Remote monitoring is done to ensure the site is keeping up with data entry and is resolving queries in a timely manner. Listings are reviewed to look for data trends that may be missed during rigorous source-to-CRF data comparisons.
Ultimately, CRAs must wear many hats to help ensure the study produces valid and meaningful results. On any given day, they may serve as a negotiator, drug auditor, communicator, trainer, and motivator, just to name a few of the roles. The importance of the CRA position in the community of professionals working in clinical trials cannot be overstated.
Challenges in an Evolving Industry
As our industry evolves, CRAs face many new challenges. Reporting is one area where changing expectations are requiring CRAs to adapt. Sponsors increasingly want more visit details in the body of a report, even though the FDA may not require them. This is a change from the past, when sponsors had fewer requirements. Unfortunately, these new requirements can affect efficiency and impact the cost of the trial.
The move toward electronic content and the idea of an “eRegulatory” approach is also relatively recent. Historically, each trial site had a binder that held all the administrative study documents. Today there are software packages that allow sponsors to collect and review regulatory, and other, trial documents instantly. While technology has its advantages, there will likely always be a need for paper documentation, in some capacity. CRAs must learn to merge the two approaches and become adept at using electronic trial management files (eTMFs) and records information management (RIM) procedures.
Another change is that sponsors and clinical research organizations (CROs) are building electronic forms that allow for more legible communication compared to handwritten documents. The use of electronic forms also allows senders to precisely track the receipt of a form, which might have been lost in the past when information was communicated via documents that were faxed or emailed.
Onsite monitoring versus remote monitoring is an additional area of evolution in our industry that affects CRAs. As more hospitals develop online portals that site monitors can use to access data, there is a move toward this more efficient web-based form of observation. However, it remains to be seen whether the efficiency gains justify the loss of the human connection that comes from face-to-face visits.
The Glue that Holds a Clinical Trial Together
While it may not be the most flattering metaphor, it is fairly accurate to think of CRAs as the “glue” that holds studies together. A CRA’s contribution to the success of a clinical trial may not be the most visible, but it is certainly significant. It is important that sponsors, CROs, and study sites recognize the key role these team members play and strive to give them the time, resources, and support they need to help ensure that the study program achieves its objectives effectively.
Share This Article
How to Become a Clinical Research Associate

Step 1: Understand the job description and responsibilities of a Clinical Research Associate
What does a clinical research associate do.
A Clinical Research Associate participates in the design, administration and monitoring of clinical trials. Analyzes and evaluates clinical data gathered during research. Being a Clinical Research Associate ensures compliance with protocol and overall clinical objectives. Knowledge of FDA regulatory requirements is required. Additionally, Clinical Research Associate may require ACRP or SOCRA Clinical Research Professional exam completion. Requires a master's degree in science or equivalent. Typically reports to a supervisor or manager. The Clinical Research Associate occasionally directed in several aspects of the work. Gaining exposure to some of the complex tasks within the job function. To be a Clinical Research Associate typically requires 2-4 years of related experience.
A clinical research associate typically assists in designing the clinical trial and may be responsible for communicating the findings to the research communities.
CRAs typically make sure that the clinical trials are conducted according to government regulations and ethical standards, as well.
In addition to monitoring the trial, a clinical research associate may also be responsible for recruiting and screening people to take part in clinical trials.
Two years of experience in pharmaceutical research, medical research, or nursing may also be required.
The ability to convey information effectively when speaking to others, knowledge of mathematics and science, and the ability to teach others could also be helpful for a career as a clinical research associate.
Step 2: Learn best tips to become a Clinical Research Associate
Best tips for those who want to become a clinical research associate.
Here are some tips to become a Clinical Research Associate.
Through work experience and exposure to different companies to get experience in a variety of therapeutic areas and also through recruitment companies and by replying to job offers.
Just a small networking club held at university.
develop a competitive compensation package.
identify the top skills for clinical research associates.
write an eye-catching job description.
Step 3: View best colleges and universities for Clinical Research Associate
Best colleges and universities for clinical research associate.
- Butler University
- Carroll College
- High Point University
- Princeton University
- Providence College
- Rollins College
Step 4: Think about whether is it worth to be a Clinical Research Associate
Is being a clinical research associate worth it.
Research shows ACRP results in higher enrollment rates, lower protocol deviations, fewer 483 Warning Letters, improved regulatory compliance, and better inspection performance.
You may see your interview for a clinical research associate position as a high-stress ordeal or daunting challenge.
Look at your prior and future research, weaving them together into a coherent story of your career to present during the interview.
Come to the interview prepared to discuss your role in current and future research.
Weave your past research into a coherent story that meshes with the clinical research you will be performing.
Step 5: Prepare relevant skills for being a Clinical Research Associate
What skills do you need to be a clinical research associate.
There are skills required to succeed in every role, and this one is no different. Strategic knowledge of the follow skills will be required: Clinical Data Analysis, Clinical Data Management, Clinical Research, Medical Writing, Microscopy, Research and Development, Research Design. For success, a grasp of the following is key: Clinical Trial Management Software. Your ability to stand out from the competition depends on these skills, as well as your resume, interview, and other factors.
Develop a comprehensive knowledge of clinical study protocol and other relevant documents.
Maintain required regulatory files for all clinical trials to ensure regulatory compliance.
Train study site personnel on the clinical protocol, study procedures, and electronic data capture as required.
Trained to administer ADOS for clinical practice and research reliability at University of Michigan Autism and Communications Disorders Center.
While each individual clinical research associate position will list a unique combination of desired skills and experiences, certain qualifications are fairly universal.
Step 6: View average salary for Clinical Research Associate
How much does a clinical research associate make.
The average salary range for a Clinical Research Associate is from $69,534 to $91,215. The salary will change depending on your location, job level, experience, education, and skills.
Average salary for Clinical Research Associate jobs
- Clinical Research Associate I
- Clinical Research Associate II
- Senior Clinical Research Associate
Step 7: Find relevant Clinical Research Associate jobs, and apply.
Looking for clinical research associate jobs.
Here are some Clinical Research Associate jobs in the United States.
Step 8: Explore Career Path of Clinical Research Associate
Clinical Researcher
Being an Independent Contractor: Seeking Success in the Workforce
Clinical Researcher August 16, 2021

Clinical Researcher—August 2021 (Volume 35, Issue 6)
PEER REVIEWED
Jerry M. Stein, PhD, ACRP-CP, ACRP-MDP, FACRP; Suheila Abdul-Karrim, CCRT, CCRA, ACRP-MDP, FACRP; Ernest Allen
The model has changed. Millions of people have exited full-time positions to work as independent contractors (ICs) in hundreds of industries. Experts say it is better for the economy. Do I care? Or what if I really need to know whether it is best for me, but it’s too soon to know? This article provides guidance on career choices, essential business practices, and tools for ICs involved with clinical research to help you survive while working under this competitive model.
Who Are Independent Contractors?
For the purposes of this article, independent clinical research contractors include any clinical monitor, clinical project manager, statistician, medical monitor, data manager, and medical writer hired for a specific project or time period and not dedicated on a long-term basis to one employer.
If you fit into any of these job titles and if your relationship to your employer matches this situation, congratulations! You have joined a growing segment of freelance professionals. In addition, you are now a small business owner with one employee: you. You are responsible for everything; there are no more support services down the hall or a phone call away. This includes the business development (finding clients), legal (contract negotiations), payroll and benefits (taxes, 401(k)), information technology (equipment, help desk), purchasing (office supplies, toner cartridges), and insurance (liability) departments. It is all you.
While the change from employee to contractor is often an individual decision, there have been significant changes across the entire workforce. A U.S. government study estimated 22% of workers received income as ICs, with the largest growth during the 2001 to 2016 period occurring among individuals identified as primary earners.{1} Thus, this is not just a “side-hustle” phenomenon among secondary earners. The same report found that the largest number of ICs (both men and women) were in the “professional, scientific, technical” sector followed by the “healthcare and social assistance” sector—two categories that likely include clinical monitors, study coordinators, and other clinical researchers. However, specific statistics regarding the number of clinical research ICs could not be found.
Forty years ago, most large sponsors employed everyone necessary to discover, test, manufacture, market, and support new drugs and medical devices. This A-to-Z approach required a long-term commitment to lots of employees and entailed significant financial risk to the companies.
Prominently beginning in the 1990s, the industry experienced downsizing that was triggered by economic downturns, mergers, and new corporate strategies.{2–4} Today, a large portion of research and development risk has been transferred to independent clinical contractors and small companies. In various forms, employees who traditionally worked for sponsors are now either employed by contract research organizations (CROs)—some embedded at large sponsors—or hired as contractors directly from a marketplace filled with ICs.
The required workforce expands and contracts using individuals who serve on-demand in response to additions or losses of contracts, projects, or changes in scope. Many companies only hire contractors when there is a high probability that the company will be paid or will successfully develop a new product.
In some cases, hiring an IC with unique expertise or skills is essential for project success. In other instances, hiring the IC only occurs when internal resources are limited and it would be risky to hire full-time, “permanent” employees. It is not too dissimilar to situations in which sponsors employ investigational study sites on an as-needed basis. However, while income from clinical studies is typically a side business for most sites outside academic medical centers, working for a sponsor is the primary source of income for most ICs.
What Are the Benefits of Being an Independent Contractor?
There are significant financial and non-tangible benefits to being a clinical IC (see Figure 1) and many of these features have been previously reported.{5–7} Foremost is control of your schedule. While contracts and teams can significantly vary, it is often the case that a strict 9-to-5, mandatory schedule is not an expectation of most contract assignments. Although 50- or 60-hour work weeks are not uncommon, these high-pressure periods are often transitory, predictable, and may be voluntary.
Figure 1: Advantages of Being an Independent Contractor
- Flexibility
- Getting to choose projects
- Not getting caught up in company/office “politics”
- Working from your own office where YOU control the environment
- A wider range of experiences in different phases of clinical trials, with different therapeutic areas and at different companies
The contractor has the benefit of focus, flexibility, and choice. Being a contractor liberates you from many meetings that are not specific to your immediate job responsibilities and allows more time to focus on your assigned project. For parents with young kids, the work-life balance can be much better: drop the kids off at school, work six hours, pick up the kids, attend after-school activities, put the kids to sleep, and work some more when the house is quiet. For those with elderly family members, work can be planned around their treatments and schedules.
Contractors working across time zones can plan personal chores around team meeting schedules. Planning a two-week, international vacation? While there are no paid vacations, you can adapt by working longer hours in advance, delegating key responsibilities to other team members while you are unavailable, and/or refusing to take on an assignment that creates a conflict. The key to success is maintaining good communication with the company that hired you and the entire team. Almost every obstacle has a work-around.
There are also significant emotional benefits when you are not dependent on one supervisor or employer. You may realize a significant increase in self-esteem. You will likely develop significant pride in being a self-sufficient, independent business owner. Finally, you get to say “no, thank you” much more often. Perhaps anticipated, project-related air travel is too extensive. Perhaps you expect database lock pressures will be too much, the protocol too boring, or the therapeutic area beyond your comfort zone. Perhaps a better opportunity is on the horizon. Saying “yes” or “no” is your choice.
Finally, the existence of a significant number of clinical ICs has a particular benefit in the age of COVID-19 and in anticipation of future epidemics. While many employers faced challenges moving their employees from central offices to home settings, the ICs were already working from home offices and were ready to go,{8} which was a win-win for both contractors and sponsors.
Money Matters: Income
Improved compensation has been cited as one of the reasons individuals aspire to change from traditional employment to contracting.{6} The authors of the current article caution that this is not a guarantee—especially for newcomers.
The first step is a smart negotiation strategy, including knowing your marketplace value. How much are companies currently paying someone with your set of skills and experience? Can you justify a higher rate of pay by promising better responsiveness, efficiency, or skills?
When negotiating your compensation terms, remember that the company does not have to provide benefits frequently paid to so-called permanent employees (e.g., health insurance, disability, paid time off, and retirement). Traditional, full-time employee salaries are often 60% to 80% of total compensation budgets. Thus, a contractor’s higher hourly rate of pay is somewhat misleading.
Of course, in a competitive marketplace, you may decide to sell your services for significantly less than your peers in order to more easily land a contract. However, you may regret the decision with every hour you add to your timesheet, and your perceived value to this employer will be forever diminished.
Developing a network of peers has an impact on your compensation and job satisfaction. Networking—in person or electronically—is not simply a social function. Information about customary compensation, as well as common job expectations, is often only available through word-of-mouth sources. This will require an investment of your time. Professional meetings and other educational opportunities are also excellent forums for learning your value.
What About Taxes and Insurance?
Although a contractor’s income is generally higher compared to that of a full-time employee, you must be prepared to pay expenses and for financial surprises to pop up. The category of anticipated expenses includes insurance (general liability), income taxes (U.S.), value-added taxes (in some international markets), office supplies, travel considerations, professional dues, and training events.
Taxes vary from country-to-country and state-to-state. We will concentrate on the U.S. tax situation for simplicity, recognizing that the vast majority of our readers reside in the U.S. If you are paid as an IC, the company that hired you will typically not withhold federal, state, or local taxes. These tax payments are your responsibility, and some must be paid quarterly to avoid penalties. This requires planning and discipline.
Contractors must also pay for their own medical insurance and consider their options for paying for dental and vision care services. Planning for a comfortable retirement requires lots of time and, often, some short-term sacrifices. Finally, not all companies pay promptly; receiving a check 30 or 60 days after submission of an invoice is not unusual.
Most major companies require clinical ICs to have professional liability insurance, and some require workman’s compensation insurance. Premiums will vary with your role and responsibilities. For example, medical writers frequently document actions that have occurred already and are outside their control. Therefore, liability insurance (e.g., errors and omission insurance) for medical writers should be relatively inexpensive.
In contrast, if you are making decisions that directly impact the health of patient volunteers, higher premiums may be required. Although the chances of being sued are very small, securing these type of insurance policies is a common requirement based on our experience. Companies often require a minimum dollar level of insurance, and you must decide on your tolerance for risk. If you are successful and, hopefully, your net worth has increased, your risk tolerance may change. You may have more to lose if sued and might be considered a larger target for a lawsuit.
Individuals with higher net worth should consider umbrella liability insurance to economically protect their assets. The highest risk to your income stream might be your health. Health insurance might pay your medical bills, but will not feed your checkbook. A disability insurance policy will provide a portion of your lost income when you are unable to work under certain conditions.
Finally, setting up a limited liability company (LLC) or the equivalent will help separate your personal finances from your business activities. The Internal Revenue Service and your accountant will thank you.
What is Considered a Business Expense?
Deducting legitimate business expenses from your gross business income is allowed and is essential for your business to survive. The list of business expenses is extensive and includes, but is not limited to travel (air, automobile mileage, lodging, out-of-town meals), home office (toner, paper, office supplies, telephone, internet), professional dues, payments to sub-contractors, insurance, and continuing education. Maintaining certification through ACRP or other professional organizations is also an expense. Deduct it!
Itemizing business expenses also requires tools and discipline. Keep a log (hard or electronic copy) on everything you spend related to the business. Be sure to document a date and business reason for all expenditures. You should open a business checking account and obtain a credit card to use exclusively for business expenses.
While you might want to hire a bookkeeper and/or an accountant, Quickbooks and TurboTax are simpler to use when compared to reading standard operating procedures or regulatory documents. Of course, the authors of this article are not accountants or tax experts. Please consult with your Certified Public Accountant or the government tax office concerning all tax issues.
What Are Helpful Business Development Strategies?
If you are new to clinical research, your chances of finding a well-paying contract situation will be limited. Competition abounds and sponsors are looking for maturity, education, and experience in hopes of minimizing risk to their projects. While there are no firm, industry-wide education or experience requirements, professional certification expectations, or government-mandated qualifications, most sponsors are looking for individuals with three to five years of experience working for established companies.
Certifications (e.g., the ACRP-Certified Professional [ACRP-CP]) and academic/clinical training achievements (e.g., earning an RN, PA, MD, or PhD) offer advantages but are not guarantees of being taken seriously as an IC. Exceptions might include unique educational backgrounds, experiences, and therapeutic area expertise.
Someday, when you have a proven track record, clients will reach out to you spontaneously through word-of-mouth and there will be lots of repeat business Someday, you will be able to say “no” when approached to accept a job with a rate of pay lower than you expected or a project with unrealistic timelines. You might be able to pick your next employer/client from among several who are hoping to work with you at the same time (see Figures 2 and 3). However today, especially if you are just starting out, it is more likely that you will say “yes” to some unfavorable job offers. Building your experience and reputation is your short-term goal. Be careful not to over-commit, and only take on what you know you can manage and deliver on-time in a quality manner.
Figure 2: Picking Your Employer/Client
Big clients will probably:
- Know what they want, and your voice may not be heard
- Be less flexible on all matters (contracts, processes, time)
- Have a larger team
- Have established standard operating procedures and processes in place
- Have more stringent continuing education requirements
- Pay more slowly
Small clients will probably:
- Let you influence their decisions to a greater extent
- Have more flexibility on all matters
- Have a smaller team
- Have no or fewer standard operating procedures or processes
- Have fewer continuing education requirements
- Pay more quickly
Figure 3: Key Questions to Ask About a Proposed Clinical Project
- This may impact communication and travel expectations.
- A team? An individual?
- Good communication and knowing expectations are essential.
- How soon after an invoice is received is payment processed?
- What is the turnaround time for reimbursement of expenses?
Where Do You Look for Jobs?
Our experience indicates that submitting your resume to most large staffing services will not lead to a job. It will be tempting to respond to every advertisement for a monitor, study coordinator, or other position. Unfortunately, each recruiter receives hundreds of resumes or CVs, and the odds are against you.
It is highly likely that your responses to too many job postings will simply consume your time, elevate your hopes, and lead to frustration. Occasionally, you will get a positive response—a phone call from the agency, human resources department, or hiring manager—but not very often. This is a classical intermittent, positive-reinforcement regimen that is strongly addictive, similar to putting coins in slot machines. Do not do it!
There are superior strategies for finding jobs that are much more productive and less frustrating (see Figure 4). By far, the best path to employment involves your network: business colleagues, ex-supervisors, principal investigators, site personnel, friends, and family. Personal referrals significantly influence the decisions of hiring managers and cultivating them is a highly recommended practice. Attend meetings, contact individuals employed in positions similar to what you are seeking, and get out there to “press the flesh” in person or virtually.
Figure 4: Business Tools, Tips, and Strategies
- Don’t put all your eggs in one basket. Have two or more clients (just in case!)
- Be flexible—clients like someone who is willing to adapt or accommodate.
- Be disciplined—especially when working from a home office.
- Develop a webpage
- Develop one or more CVs
- Plan your office supply needs
- Build a network, both in person and through social media (LinkedIn, Facebook, Twitter)
- Friends, friends, friends (essential)
- Professional recruiters and job post sites (worthless?)
What Are the Disadvantages of Being an Independent Contractor?
Being a clinical IC has several disadvantages (see Figure 5). First, to be fair, there are many excellent employers who treat their dedicated employees very well: providing long-term employment, excellent benefits, flexible workhours, team spirit, training, and career growth. If you are working in this type of situation, do not leave the nest without lots of planning and good reasons—the grass is not always greener in the next situation.
Figure 5: Disadvantages of Being an Independent Contractor
- You are not a “company man or woman”—you may not participate in company successes. You are the outsider.
- No bonuses, annual compensation increases, health benefits, profit sharing, etc.
- Once a contract ends, there is no guarantee of continued employment—you will have to find a new contract.
- If working for start-up company, there are risks of company funding issues, bankruptcy, lack of payment.
- Late or delayed payment is common.
Some individuals thrive in the IC marketplace while others fail financially or emotionally. You might not want to be a small business owner with record-keeping responsibilities or the occasional 50- or 60-hour week, mentioned earlier, that comes without overtime or a thank you. A contractor may experience significant gaps in employment and periods when income is insufficient. A contractor is likely to find both good companies and bad ones.
Finally, even if you are working as a clinical IC for a company with a great reputation, it is likely that you will still be considered an outsider and not part of the core clinical research team. You will likely be excluded from training opportunities outside the immediate scope of work related to your assigned project. You might never know the results of a study that consumed your time for months or the outcome of a registration submission. The emotional aspects of employment are often very important.
Conclusions
There are significant advantages and disadvantages to being a clinical IC. Individuals are encouraged to consider each of the factors presented in this article, speak to experienced contractors about their careers, and plan carefully. Improved long-term compensation, flexible schedules, and strengthened self-esteem are among the many benefits that might make this a good career choice for you.
- Lim K, Miller A, Risch M, Wiking E. 2019. Independent contractors in the U.S.: new trends from 15 years of administrative tax data. U.S. Department of the Treasury. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjer_6h_d3yAhXxKVkFHQhgBlsQFnoECAMQAQ&url=https%3A%2F%2Fwww.irs.gov%2Fpub%2Firs-soi%2F19rpindcontractorinus.pdf&usg=AOvVaw3JVi-jVzRUg5X_GXo1nkQT
- Katz LF, Krueger AB. The rise and nature of alternate work arrangements in the United States, 1995–2015. https://scholar.harvard.edu/lkatz/publications/rise-and-nature-alternative-work-arrangements-united-states-1995-2015
- Shultz AE. 2002. The IRS vs. Independent Contractors. https://www.pharmavoice.com/article/2002-09-the-irs-vs-independent-contractors
- Gautam A, Pan X. 2016. The changing model of big pharma: impact of key trends. Drug Discovery Today 21(3):379–84.
- Tesar N. 2017. The clinical research BOSS: built on self success. Clinical Researcher. https://acrpnet.org/2017/12/12/clinical-research-boss-built-self-success/
- Roberts A. 2013. The pros and cons of being an independent consultant. CRA Sources . https://clinical-cra.com/pros-cons-independent-consultant
- Noguchi Y. 2018. Freelanced: the rise of the contract workforce. National Public Radio . https://www.npr.org/2018/01/22/578825135/rise-of-the-contract-workers-work-is-different-now
- Methia J. 2020. COVID-19 creates urgent need for remote monitoring in clinical trials. Clinical Researcher 34(5). https://acrpnet.org/2020/05/12/covid-19-creates-urgent-need-for-remote-monitoring-in-clinical-trials/

Jerry M. Stein, PhD, ACRP-CP, ACRP-MDP, FACRP, is President and Owner of Summer Creek Consulting, LLC in Fort Worth, Texas, an ACRP Fellow, and a member of the ACRP Content Advisory Committee.

Suheila Abdul-Karrim, CCRT, CCRA, ACRP-MDP, FACRP, is a Freelance Consultant based in Johannesburg, South Africa.

Ernest Allen is a Clinical Research Consultant based in Verona, Wis.
Sorry, we couldn't find any jobs that match your criteria.

Optimizing Clinical Trial Education in Academia

A Case Study on Training Initiatives to Support Clinical Researchers with Electronic Medical Records

Back to Basics with Research Education
- Skip to main menu
- Skip to user menu
Sign up for our regular email newsletter

How To Become A Clinical Research Associate - A New Scientist Careers Guide
- Finding a job
- Career guides
What does a clinical research associate do?
Clinical research associates (CRAs) are responsible for running clinical research, which consists of trials designed to test new or current drugs/immunisations and analyse their effectiveness, risks, benefits and safety of use.
CRAs play an important role in the healthcare industry and public health development by helping to design and test new medications, vaccinations and other therapeutic agents.
CRAs can be involved at any stage of a drug development trial, including planning, coordinating and supervising. It is their responsibility to ensure a drug has been appropriately examined and all its risks have been evaluated before it is released to be used publicly.
CRAs most commonly work for pharmaceutical companies or contract research organisations. They might also have to spend some time working in a hospital setting to collect data about the drugs they are analysing. They might also work for universities or public/global health organisations.
As a CRA, you may have a range of responsibilities depending on your employer, project and level of experience. Typically, CRAs will need to complete tasks such as:
- Designing and writing trial protocols and standard operating procedures
- Presenting protocols and procedures to steering committees
- Designing data collection forms
- Requesting ethics approvals and working with ethics committees
- Liaising with staff conducting the trials, such as doctors or consultants
- Training local staff based on trial-specific standards
- Monitoring operations during clinical trial data collection
- Collecting completed data collection forms
- Performing data management and analysis, and discussing the results
- Closing trials and finalising reports with the help of a statistician
CRAs will work in a team of other research professionals, including contract organisation or sponsor staff, principal investigators and clinical research coordinators.
How to become a clinical research associate
To become a CRA, you need to obtain a degree in medical sciences, life sciences or nursing. This can be in subjects such as biomedical science , anatomy, physiology, immunology , pharmacology or broader degree subjects like chemistry and biology .
Alternatively, you can access a career as a CRA by acquiring a higher national diploma (HND). This is a qualification equivalent to the second year of a bachelor’s degree. HNDs can be beneficial to those who want to enter more practical fields, clinical research included.
Occasionally, you can enter a CRA role from an administrative background. For instance, if you begin working as a clinical trials administrator/assistant and decide you would prefer the role of a CRA, you can complete additional qualifications to do this. However, this will take some time and can be difficult.
Most employees view undergraduate qualifications as sufficient, but in some cases a postgraduate degree may be beneficial. Master’s degrees and PhDs can gain you an advantage when applying for competitive positions, and help you gain more experience in research .
Work experience is key to securing a clinical research job. You can get this at any point in your training, and some universities may help with this. The types of work experience most useful for a CRA role include:
- Academic research
- Pharmaceutical research (e.g. via a pharmaceutical industry placement during your degree)
- Laboratory work
- Nursing or care work
- Work in a pharmacy or medical sales
- Other, similar activities
How long does it take to become a clinical research associate?
Becoming a CRA will usually take around three to four years, depending on the access pathway you choose.
If you opt to complete an undergraduate degree, this can take three to four years. You can then apply to job positions as a CRA straight away. However, if you don’t have sufficient work experience, you may need to start at a lower-level position such as a more administrative job. From here, you can gain more experience and reapply for a higher-level position.
If you choose to obtain a HND qualification, this will take two years. Provided you have sufficient experience, you can then apply for a graduate post as a CRA, but again you may need to gain some extra experience/qualifications in some cases.
If you opt to do a postgraduate degree first, this may take an additional one to three years depending on whether it is a master’s degree or a PhD. Many CRA job positions also allow for completion of postgraduate qualifications alongside the job.
A day in the life of a clinical research associate
Most CRAs work about 40 hours a week, during weekdays. There may be an out-of-hours commitment, for instance if working in a hospital setting monitoring a new drug, but this is dependent on your employer and role.
CRAs can work on multiple trials at a time, in multiple different sites. This will depend on the complexity of each trial and what stage each of the trials is in. Therefore, the role may entail some travelling at times, while other times most of your work will be concentrated on one site.
As a CRA, you will carry out a wide variety of tasks. Some days may be spent writing reports. On other days, you will work on site with healthcare staff , or you might go into your office and attend meetings.
No matter your experience level or how senior your role is, as a CRA, you will need to work in a team with other research and healthcare professionals. You will be communicating with research nurses, doctors, health consultants, investigators and managerial and administrative staff from the company requesting the trial.
The role requires good communication skills, as well as good time management and organisation skills, because carrying out a few different studies at once may mean some tasks clash with one another and you need to prioritise the most important ones.
Clinical research associate: Career options
As with most clinical roles, CRAs undergo lots of continuing professional development (CPD) within their role. There are many training courses available to CRAs to build on their existing skills and develop new competencies.
Most training courses are organised by external bodies, and many are paid for by the employer. One of the organisations that runs training courses is the Institute of Clinical Research (ICR). It provides training in areas such as effective project management for clinical trials and advanced clinical trial monitoring, among several others.
The ICR also offers certificates and a diploma that you can complete to evidence your skills in clinical research. Becoming an ICR member and obtaining courses and qualifications from it can also be beneficial to career development, as you will meet other prominent professionals in your field through interacting with this organisation.
You can also opt to complete a postgraduate qualification, such as a PhD or master’s degree in several different areas, including clinical research and clinical pharmacology .
As mentioned, you may need to climb up the professional ladder to become a CRA, and many people start off as clinical trial administrators or junior CRAs. Within these roles, you might complete tasks such as handling documentation and correspondence or helping to set up trial sites.
From here, you can move on to becoming a senior CRA as you gain experience. At this point, you will have more advanced responsibilities, such as project management of whole trials and designing case report forms.
If you develop sufficient experience and gain contacts in the field, there is a possibility of self-employment if you want to become a freelance CRA.
Salary: How much does a clinical research associate earn in the UK and US?
In the UK, starting salaries for CRAs range between £26,000 and £34,000 per year. As a more senior CRA, you might earn between £35,000 and £50,000, and in the most senior positions involving managerial tasks, you might earn upwards of £55,000.
Salaries will vary between regions and employers, as well as depending on your level of experience and responsibilities. Some companies offer additional benefits.
In the US, the average salary for a CRA is $70,000 per year. The range is between $60,200 and $80,900. This can vary depending on the region you work in, your education and experience levels and any additional qualifications you have.
Salaries will also be different as a freelance CRA, and this will depend on the number of clients you have and any business-related expenses you need to cover.
- Prospects. Clinical research associate. Available from: https://www.prospects.ac.uk/job-profiles/clinical-research-associate (accessed Apr 2024)
- CK Group. Clinical research associate (CRA) job profile. Available from: https://ckgroup.co.uk/candidate/job-profiles/clinical-research-associate-cra-job-profile/ (accessed Apr 2024)
- Nikolova, T. The CRA Wizard. How to become a CRA for dummies in 7 steps (or less). Available from: https://www.thecrawizard.com/how-to-become-a-cra-for-dummies (accessed Apr 2024)
- Glassdoor. Clinical research associate career. Available from: https://www.glassdoor.co.uk/Career/how-to-become-clinical-research-associate_KO14,41.htm (accessed Apr 2024)
- Coursera. How to become a clinical research associate. Published Nov 2023. Available from: https://www.coursera.org/articles/clinical-research-associate
- Walters, L. Pharmiweb.jobs. 8 ways to advance your career as a clinical research associate (CRA). Published Sept 2023. Available from: https://www.pharmiweb.jobs/article/8-ways-to-advance-your-career-as-a-clinical-research-associate-cra- (accessed Apr 2024)
- Salary.com. Clinical research assistant salary in the United States. Available from: https://www.salary.com/research/salary/alternate/clinical-research-assistant-salary (available from Apr 2024)
Share this article
Related articles

What Jobs Can You Get With A Computer Science Degree? - A New Scientist Careers Guide

What does a Clinical Physiologist in Neurophysiology do??

What Jobs Can You Get With A Physics Degree? - A New Scientist Careers Guide
Latest articles.

IMAGES
VIDEO
COMMENTS
With the end goal of reaching a sustainable environment of high-quality, inclusive, compliant, and impactful studies and research roles, the August 2024 issue of Clinical Researcher invites you to explore the theme of "New Destinations in Clinical Research: You CAN Get There from Here."Eye-opening layovers and side quests along the way will consider adventures in career development, hiring ...
Salary: How much does a clinical research associate earn in the UK and US? In the UK, starting salaries for CRAs range between £26,000 and £34,000 per year. As a more senior CRA, you might earn ...
The JTF for Clinical Trial Competency framework as discussed by ACRP "objectively defines the knowledge, skills, and attitudes necessary for conducting safe, ethical and high-quality research." 5 Although common practice is currently to hire CRAs with two years of experience, high demands, vacancies, and attrition in the industry have led ...
A clinical research associate (CRA) plays a vital role in the development of new medical treatments. They ensure that clinical trials are conducted ethically and according to protocols, monitoring the progress from start to finish. CRAs are essential for ensuring data integrity and regulatory compliance. The demand for CRAs is increasing due to ...
In a forthcoming peer-reviewed article for ACRP's Clinical Researcher journal, Anthony Chew, a clinical trial operations professional and recent graduate of the MS program in Medical Product Development Management at San Jose State University, describes CRAs as serving as "the primary liaison between the sponsor and the site by monitoring and verifying data to ensure accuracy and adherence ...
She had been a Clinical Research Associate (CRA) for a major pharmaceutical company for a couple of years and seemed very happy. ... A Guide to Careers in Clinical Research," is produced by Ingenix Pharmaceutical Services and can be ordered free from their Web site. doi: 10.1126/article.62927 About the author ...
Data Management in Clinical Trials. Diane C. St Germain, Marjorie J. Good, in Principles and Practice of Clinical Research (Fourth Edition), 2018 Clinical Research Associate. CRAs are responsible for data collection and documentation as well as are often responsible for managing trial-related supplies and other research-related support tasks. 7 The person in this role should be adept with ...
Here are some steps you can take to pursue a career as a clinical research associate: 1. Pursue a bachelor's degree in a health science-related field. Most clinical research associate positions require candidates to have a bachelor's degree in a health science-related field. For those interested in a position as a clinical research associate ...
A Clinical Research Associate (CRA) is a professional within a healthcare setting who oversees research activities, typically related to clinical trials. CRAs can be employed and work in a variety of sectors, including government agencies, pharmaceutical companies, research institutions, and in similar settings.
Training needs of clinical research associates - PMC. Journal List. Perspect Clin Res. v.1 (4); Oct-Dec 2010. PMC3043360. As a library, NLM provides access to scientific literature. Inclusion in an NLM database does not imply endorsement of, or agreement with, the contents by NLM or the National Institutes of Health.
Abstract: Research coordinators may transition to clinical research associates/monitors during their careers.This article provides an overview of how to determine whether it is the right time to make this transition, how to evaluate current competencies and gaps that must be filled in order to make this transition, and how to address needs during the on-boarding process.
ACRP CCRA (Certified Clinical Research Associate) Complete 3,000 hours performing essential duties. Submit a resume documenting and demonstrating job performance. Please note that in some cases, additional education can be used to substitute for work experience hours. Please see credentialing websites for details.
Share this article. In this article, we've rounded up 10 career paths within the Clinical Research industry, highlighting key responsibilities of roles such as Clinical Trials Manager, Clinical Research Associate, Clinical Trials Administrator, and more.
Clinical trials involve a great deal of documentation, analysis, observation, and organization. A team of professionals is engaged in administering a clinical trial, including a clinical research associate (CRA). The CRA acts as a liaison between the study's sponsor CRO (e.g., pharmaceutical company) and the clinics where the study occurs.
Clinical trials leverage the expertise of people in a wide variety of roles — researchers, project managers, clinical data managers, etc. One of the positions with which people may be less familiar is that of the clinical research associate (CRA). CRAs play a critical, if somewhat unheralded, role in the success of a trial.
Clinical research associates are an essential component of the development (R&D) function at cutting-edge pharma and life sciences companies today — and bear in mind, a ton of interpersonal interaction is baked into the role. As a result, some of the soft skills that might be key differentiators for aspiring clinical research associates include:
To be a Clinical Research Associate typically requires 2-4 years of related experience. People's Opinions on Clinical Research Associate responsibilities. A clinical research associate typically assists in designing the clinical trial and may be responsible for communicating the findings to the research communities. 10/29/2019: Shreveport, LA.
Clinical Researcher—August 2021 (Volume 35, Issue 6) PEER REVIEWED Jerry M. Stein, PhD, ACRP-CP, ACRP-MDP, FACRP; Suheila Abdul-Karrim, CCRT, CCRA, ACRP-MDP, FACRP; Ernest Allen The model has changed. Millions of people have exited full-time positions to work as independent contractors (ICs) in hundreds of industries. Experts say it is better for the economy. Do I care? Or what if I really ...
The Office of Human Research (OHR) supports The George Washington University research community in the conduct of innovative and ethical research by providing guidance, education, and oversight for the protection of human subjects.The IRB Analyst II reports to the Assistant Director and manages the review of research study applications at all levels and stages of review and approval to ensure ...
How to become a clinical research associate. To become a CRA, you need to obtain a degree in medical sciences, life sciences or nursing. This can be in subjects such as biomedical science, anatomy, physiology, immunology, pharmacology or broader degree subjects like chemistry and biology. Alternatively, you can access a career as a CRA by ...
Research.com is an editorially independent organization with a carefully engineered commission system that's both transparent and fair. ... This article aims to alleviate that uncertainty by presenting the 2024 Most Valuable Nursing Degree Programs Ranking in Scranton, PA, meticulously crafted by the Research.com team of data scientists ...