
AARON SAGUIL, MD, MPH, SHAWN F. KANE, MD, REBECCA LAUTERS, MD, AND MICHAEL G. MERCADO, MD
Am Fam Physician. 2019;100(7):408-414
Author disclosure: No relevant financial affiliations.
Hand-foot-and-mouth disease is caused by human enteroviruses and coxsackieviruses. Outbreaks can occur in the spring to fall and are common in North America, and most cases occur in patients younger than 10 years. Hand-foot-and-mouth disease is transmitted by fecal-oral, oral-oral, and respiratory droplet contact. Patients present with a low-grade fever, a maculopapular or papulovesicular rash on the hands and soles of the feet, and painful oral ulcerations. Lesions usually resolve in seven to 10 days; however, in rare cases, patients may have neurologic or cardiopulmonary complications. The differential diagnosis for childhood rashes and oral enanthems is broad and includes erythema multiforme, herpes, measles, and varicella. Treatment is supportive and directed toward hydration and pain relief as needed with acetaminophen or ibuprofen. Oral lidocaine is not recommended, and antiviral treatment is not available. The best methods to prevent the spread of hand-foot-and-mouth disease are handwashing and disinfecting potentially contaminated surfaces and fomites.
Hand-foot-and-mouth disease is a common viral disease that presents in primary care. This article presents a brief summary and review of the etiology, clinical features, diagnosis, prognosis, and evidence for the care of patients with hand-foot-and-mouth disease.
| Expert opinion from the Centers for Disease Control and Prevention | ||
| , but . , , | Consensus opinion (acetaminophen/ibuprofen); small randomized controlled trial and case report (lidocaine) | |
| , | Disease-oriented, retrospective studies |

Epidemiology
Hand-foot-and-mouth disease was first described after an outbreak in Canada in the 1950s. 1 It is caused by picornaviruses, specifically human enteroviruses and coxsackieviruses. 2
The most common viruses that cause hand-foot-and-mouth disease are enterovirus 71 and coxsackievirus A16. 2 Currently, hand-foot-and-mouth disease is not listed as a notifiable condition in the United States by the Centers for Disease Control and Prevention; however, it has been a reportable illness in the Western Pacific region, where there are more severe outbreaks. 3 – 5
Coxsackievirus A6 can cause severe disease manifestations with atypical lesions such as vesicles, bullae, and scabs on the trunk, extremities, and face. 6
Spring to fall seasonal outbreaks of hand-foot-and-mouth disease are typical in North America and temperate zones. 7 , 8 Years can pass between cyclical epidemics, during which time the pool of unexposed children increases. 1
Outbreaks of hand-foot-and-mouth disease are possible during the winter, and some are associated with coxsackievirus A6. 2 Year-round outbreaks are common in tropical zones. 8
Most cases occur in patients younger than 10 years, 1 and the largest incidence is within the first five years of life. 9
Health care professionals working with children are at risk of contracting hand-foot-and-mouth disease, and males and females are equally affected. 2
Hand-foot-and-mouth disease has a low fatality rate in uncomplicated cases in the United States (0.06% to 0.11%). 10 However, there were 10.7 million cases in China between May 2008 and June 2014, with 3,046 deaths attributed to neurologic and cardiopulmonary complications. 5 Patients with more severe disease are more likely to have been infected with enterovirus 71. 5
Transmission
Humans are the only carrier for hand-foot-and-mouth disease–causing viruses. 1 The disease is spread by fecal-oral, oral-oral, and respiratory droplet contact. 10
The patient is most infectious during the first week of illness 7 ; however, an active virus may be present in the stool for up to four to eight weeks. 10 Therefore, the household transmission rate for hand-foot-and-mouth disease enterovirus 71 is 52% to 84%. 10
Incubation range is estimated to be three to six days. 8
Lack of access to clean water partially explains the burden of disease in the developing world and Asia, where hand-foot-and-mouth disease is a significant public health threat. 2
Clinical Features
Hand-foot-and-mouth disease is a clinical diagnosis based on the presentation of a low-grade fever with a maculopapular or papulovesicular rash on the hands ( Figure 1 11 ) and soles of the feet ( Figure 2 11 ) and by painful oral ulcerations. 7 If the diagnosis is unclear, serologic and polymerase chain reaction studies may be obtained to detect enterovirus or coxsackievirus. 1 , 4 , 5 , 12
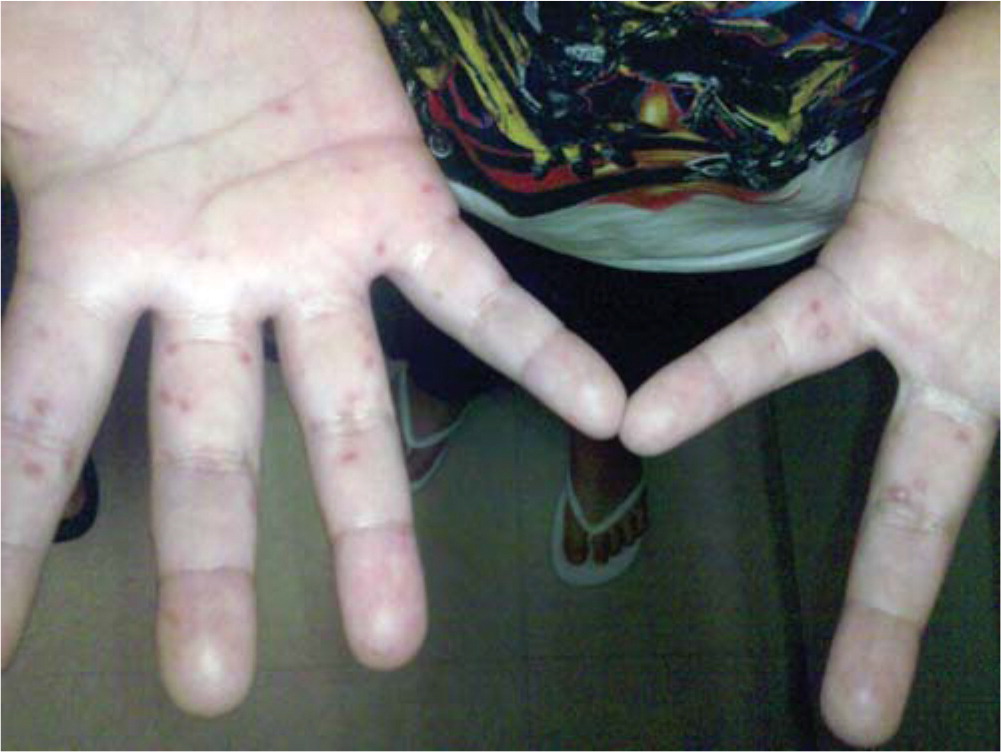
Skin lesions are typically 2 mm to 6 mm in diameter, have an erythematous halo, and evolve into vesicles that rupture and leave painless shallow ulcers that do not scar. 4
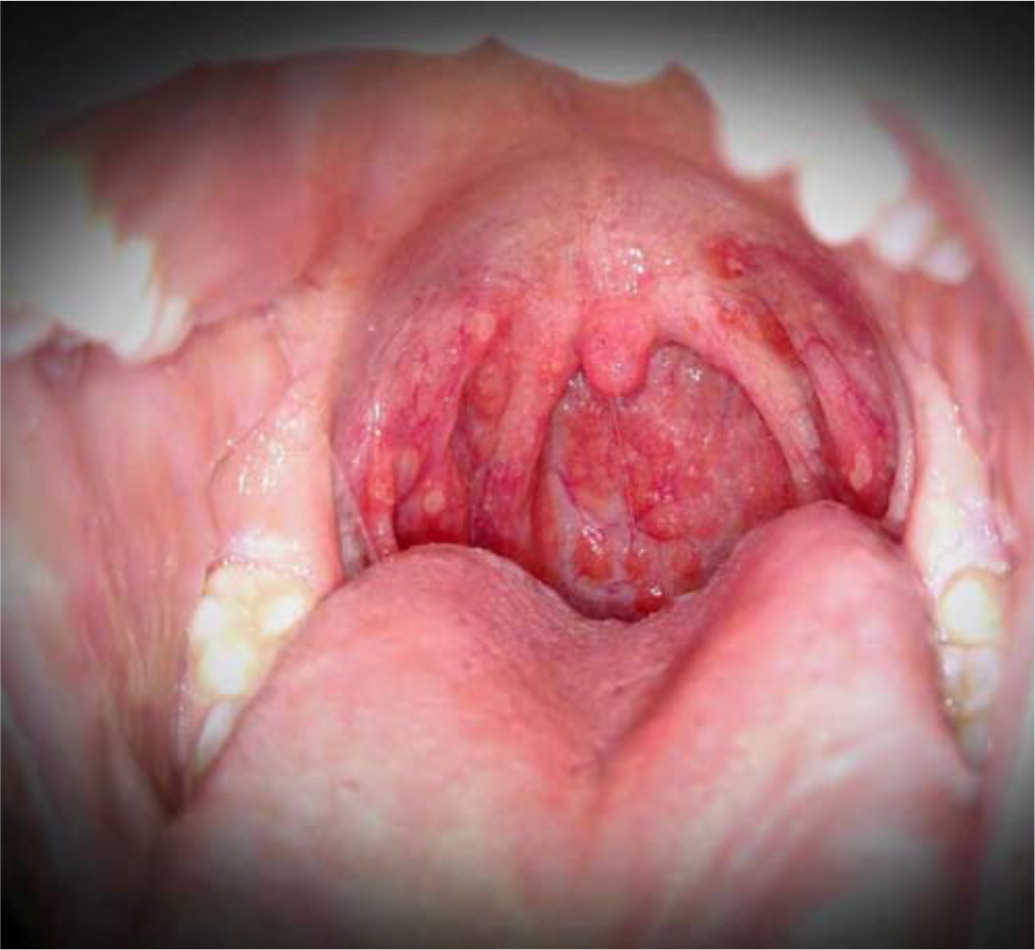
Oral enanthems of painful ulcerations typically affect the posterior oral cavity, including the soft palate. Lesions may also affect the tongue and buccal mucosa, and pain may cause dehydration 4 ( Figure 3 ) .
Lesions resolve in seven to 10 days. 5
Patients may have atypical skin lesions, including hemorrhagic or purpuric lesions; bullae and pustules; trunk, cheek, or genital involvement; palm and sole of the feet desquamation; and accentuation in areas of atopic dermatitis (eczema coxsackium). 7 , 13
The disease may be associated with delayed nail separation or horizontal nail ridges or grooves. 1
Rare neurologic complications can occur such as aseptic meningitis, acute flaccid paralysis, and encephalomyelitis, especially with enterovirus 71. 5
Other rare complications include pulmonary edema, pulmonary hemorrhage, and cardiorespiratory failure. 4
Differential Diagnosis
Differential diagnosis includes diseases that feature maculopapular or papulovesicular rashes and/or oral lesions ( Table 1 14 – 38 ) .
Aphthous ulcers and herpetic gingivostomatitis are typically limited to the oral cavity or surrounding skin. 14 , 19
Herpangina caused by the same agents as hand-foot-and-mouth disease is limited to the oral cavity without skin involvement. 18
Pemphigus vulgaris and Behçet syndrome include oral lesions and involve multiple systems. Both require recognition, further investigation, and treatment. 17 , 22
Herpes and varicella rashes have characteristic vesicles and erythema. 30 , 38
Atopic dermatitis is usually recurrent and has typical age-related distribution of lesions. 24
Scabies is intensely pruritic and associated with a linear distribution of lesions attributed to mite burrows. 35
Erythema multiforme major presents as target lesions on the face and limbs. 27
Bullous impetigo causes flaccid bullae that affect the trunk and extremities. 26
HIV should be considered with skin rash or oral lesions if risk factors are present.
| Aphthous ulcers | Unknown | Shallow, round, painful ulcers, measuring up to 1 cm, with surrounding erythema and pseudomembrane Simple aphthae resolve in one to two weeks, not associated with skin lesions Complex aphthae tend to be larger, occur more frequently, and may indicate systemic disease (e.g., gluten sensitive enteropathy), HIV, cyclic neutropenia, systemic lupus erythematosus, inflammatory bowel disease, periodic fever, aphthous stomatitis, pharyngitis, or cervical adenitis syndrome | Simple aphthae: supportive care Complex aphthae: treat underlying cause Pain relief: chlorhexidine (Peridex) mouthwash, lidocaine spray or ointment, anti-inflammatory or corticosteroid pastes or mouthwashes , | |||
| Behçet syndrome | Unclear etiology, associations with human leukocyte antigen-B51 allele, postulated environmental triggers | Oral aphthae, genital ulcerations, or recurrent uveitis May have arthralgia, vascular or neurologic lesions Oral lesions are painful, round, with an erythematous border, and are 1 cm to 3 cm in diameter or larger | Corticosteroids, azathioprine (Imuran), cyclophosphamide, methotrexate, interferon alpha, ustekinumab (Stelara), infliximab (Remicade), etanercept (Enbrel), adalimumab (Humira) | |||
| Herpangina | Coxsackievirus, echovirus | Oral vesicles that form ulcers with associated inflammation Coxsackievirus A subtypes 1–6, 8, 10, and 22 Thought to be on a continuum with hand-foot-and-mouth disease | Supportive care | |||
| Herpetic gingivostomatitis | Herpes simplex virus 1 and 2 | Fever, anorexia, lymphadenopathy, oral erythema and small, oral vesicles on the palate, tongue, gingiva, and oral mucosa that form ulcers that may become confluent; vesicles may be present on lips; Tzanck cells may be present, diagnosis can be made by culture or immunologic assay , | Supportive care; acyclovir started in the first 72 hours resulted in faster resolution of oral lesions | |||
| Pemphigus vulgaris | Caused by desmosome autoantibodies | Oral mucosal bullae and erosions of lips, tongue, and oropharynx; may affect eyes and genital area; potentially life-threatening Diagnostic testing with direct immunofluorescence microscopy or serum testing | Corticosteroids, azathioprine, cyclophosphamide, intravenous immunoglobulin | |||
| Atopic dermatitis | Genetic, immunologic, and environmental factors | Erythematous plaques and vesicular lesions, excoriation, dry skin Younger children with lesions on extensor surfaces, cheeks; older children lesions on flexor surfaces; lesions on hands and feet common | Avoid triggers (e.g., cold weather, frequent hot baths, fragrances, detergents) Emollient creams, topical corticosteroids ; oral agents for severe cases | |||
| Bullous impetigo | Superficial vesicles progress to flaccid bullae that rupture; collarette of scale surrounding blister at periphery of lesion; tends to affect trunk, extremities and moist, intertriginous areas; does not scar, systemic symptoms uncommon | Topical mupirocin (Bactroban) or retapamulin (Altabax); for more extensive disease or inability to tolerate topical therapy, may use amoxicillin/clavulanate (Augmentin), cephalexin (Keflex), dicloxacillin, doxycycline, or trimethoprim/sulfamethoxazole | ||||
| Erythema multiforme | Immune mediated, often secondary to infection (specifically herpes simplex virus and ), may also be secondary to drugs and other causes | Trunk, limb, and face distribution, erythema multiforme minor limited to the skin, erythema multiforme major involves mucosal membranes; skin lesions < 3 cm in diameter; two concentric, colored rings surround dusky central zone; affects < 10% of body surface area, often elevated C-reactive protein level | Supportive care; if caused by a drug, discontinue that agent; if secondary to herpes simplex virus, consider antiviral therapy; corticosteroids may be used in severe cases, although controlled studies are lacking | |||
| Herpes | Herpes simplex virus 1 and 2 | Fever, pruritus, maculopapular and vesicular rash , ; lesions may appear on areas in contact with oral herpes (e.g., herpetic whitlow), in areas prone to bodily contact (e.g., herpes gladiatorum), or on sites of previous atopy (e.g., eczema herpeticum ) | Acyclovir, famciclovir, or valacyclovir (Valtrex) | |||
| Measles | Measles virus | Respiratory spread; presents with fever, cough, coryza; Koplik spots (white papules) may present on buccal mucosa before maculopapular rash that starts on head and spreads distally Complications include pneumonia, keratoconjunctivitis, encephalomyelitis | Supportive treatment; vitamin A supplementation; measles may be prevented with routine childhood immunization; measles cause 100,000 deaths per year, worldwide | |||
| Rocky Mountain spotted fever | , transmitted by infected tick (e.g., American dog tick, Rocky Mountain wood tick) | History of a tick bite (50% to 60% of patients), headaches, fever, fatigue, nausea, photophobia; rash starts with blanching, erythematous macules and papules on wrist and ankles, spreads centripetally; may ulcerate Complications include congestive heart failure, dysrhythmia, seizures, nerve palsies | Doxycycline; preventive measures include avoiding tick-infested habitats, tick repellant, full body skin examinations after exposure to areas with ticks | |||
| Scabies | hominis | Linear distribution of papules corresponding with mite burrows; typical distribution includes hands, feet, skinfolds, genitalia; intense pruritus, worse at night; mites can be visualized in skin scrapings by microscope | Permethrin cream 5% (Elimite); wash all clothing, bedding, and towels in hot water; treat close contacts | |||
| Stevens-Johnson syndrome | Delayed-type hypersensitivity reaction usually associated with drugs | Fever, malaise prodrome; painful skin and mucous membrane (i.e., eye, mouth, and genital) lesions; erythematous skin with blister formation and flat atypical target lesions; pulmonary, renal, and hepatic involvement common; < 10% of skin surface area involved | Discontinue causative drug; refer to specialized units (e.g., burn centers); may consider corticosteroids, intravenous immunoglobulin, and/or cyclosporine A | |||
| Varicella (chickenpox) | Varicella zoster virus | Generalized, itchy, vesicular rash; fever, malaise; may cause pneumonitis, hepatitis, encephalitis, skin rash may become secondarily infected ; rash starts on face and trunk and spreads to rest of body; starts with macules and progresses to papules and vesicles; lesions visible in all stages at the same time as each other; symptoms last four to seven days | May use acyclovir within 24 hours of rash onset, or later in severe cases or in patients who are immunocompromised ; prevent with vaccination; avoid aspirin, may consider corticosteroids | |||
Management is supportive and directed toward the relief of pain, lowering of fever, and adequate oral hydration because of the self-limiting nature of hand-foot-and-mouth disease.
Discomfort because of pain or fever can be treated with weight-based acetaminophen or ibuprofen. 7
Oral application of topical lidocaine is not recommended for use in children because of the lack of benefit 39 and the potential for harm. 40
Antiviral treatments are not available. One clinical trial of acyclovir (n = 13) reported a reduction of fever and skin changes within 24 hours; however, more evidence is needed. 41
Indications for hospitalization include a failure to maintain adequate hydration or the development of neurologic or cardiopulmonary complications. 4
Intravenous immunoglobulin is not recommended. In Asia, intravenous immunoglobulin is used in severe cases because of the potential benefit in stopping the progression to cardiopulmonary failure based on retrospective data; however, more prospective evidence is needed. 4
Handwashing stops the spread of hand-foot-and-mouth disease, specifically after diaper changes and toileting, and before eating. 7 , 42 , 43
In China, children who “always wash” hands before meals were less likely to contract the disease. 8
Disinfect surfaces and fomites (e.g., toys), avoiding close contact and the sharing of personal items such as utensils and cups with infected persons. 7 , 43
Breastfeeding does not impact the incidence of hand-foot-and-mouth disease. Mothers do not need to stop breastfeeding to prevent transmission of disease. 8
There are no vaccines or chemoprophylaxis agents available to prevent hand-foot-and-mouth disease and herpangina. 7 , 44
In the United States, exclusion from childcare does not reduce the spread of the disease and is not recommended unless the child is unable to participate or staff are unable to care for the child without compromising the care of other children. 45
Data Sources: Sources consulted for this article include PubMed from the National Library of Medicine, Essential Evidence Plus, the Cochrane Database of Systematic Reviews, the Centers for Disease Control and Prevention, and the World Health Organization. Search terms included hand-foot-and-mouth disease, herpangina, and maculopapular exanthems. Search dates: October 2018, January 2019, and June 2019.
Editor's Note: Dr. Saguil is a contributing editor for AFP.
The views expressed in this article are the authors' own and do not necessarily reflect the views of the U.S. Army, U.S. Navy, U.S. Air Force, the Department of Defense, or the U.S. government.
Nassef C, Ziemer C, Morrell DS. Hand-foot-and-mouth disease: a new look at a classic viral rash. Curr Opin Pediatr. 2015;27(4):486-491.
Repass GL, Palmer WC, Stancampiano FF. Hand, foot, and mouth disease: identifying and managing an acute viral syndrome. Cleve Clin J Med. 2014;81(9):537-543.
Centers for Disease Control and Prevention. 2019 National Notifiable Conditions. Accessed March 25, 2019. https://wwwn.cdc.gov/nndss/conditions/notifiable/2019/
World Health Organization. Hand, foot and mouth disease. Accessed January 14, 2019. https://www.who.int/westernpacific/emergencies/surveillance/archives/hand-foot-and-mouth-disease
Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis. 2018;37(3):391-398.
Centers for Disease Control and Prevention. Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6 - Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep. 2012;61(12):213-214.
Centers for Disease Control and Prevention. Hand, foot, and mouth disease (HFMD). Accessed January 14, 2019. https://www.cdc.gov/hand-foot-mouth
Koh WM, Bogich T, Siegel K, et al. The epidemiology of hand, foot and mouth disease in Asia: a systematic review and analysis. Pediatr Infect Dis J. 2016;35(10):e285-e300.
Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42(4):517-567.
Ventarola D, Bordone L, Silverberg N. Update on hand-foot-and-mouth disease. Clin Dermatol. 2015;33(3):340-346.
Pillai AS, Medina D. Rash in an eight-year-old boy. Am Fam Physician. 2012;86(12):1141-1142. Accessed July 26, 2019. https://www.aafp.org/afp/2012/1215/p1141.html
Korman AM, Alikhan A, Kaffenberger BH. Viral exanthems: an update on laboratory testing of the adult patient. J Am Acad Dermatol. 2017;76(3):538-550.
Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132(1):e149-e157.
Lehman JS, Rogers RS. Acute oral ulcers. Clin Dermatol. 2016;34(4):470-474.
Bischoff EW, Uijen A, van der Wel M. Aphthous ulcers. BMJ. 2009;339:b2382.
Stoopler ET, Sollecito TP. Recurrent oral ulcers. JAMA. 2015;313(23):2373-2374.
Greco A, De Virgilio A, Ralli M, et al. Behçet's disease: new insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. 2018;17(6):567-575.
Puenpa J, Mauleekoonphairoj J, Linsuwanon P, et al. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS One. 2014;9(6):e98888.
Clarkson E, Mashkoor F, Abdulateef S. Oral viral infections: diagnosis and management. Dent Clin North Am. 2017;61(2):351-363.
Mohan RP, Verma S, Singh U, et al. Acute primary herpetic gingivostomatitis. BMJ Case Rep. 2013;2013:bcr2013200074.
Goldman RD. Acyclovir for herpetic gingivostomatitis in children. Can Fam Physician. 2016;62(5):403-404.
Mullick S, Pan YF, Desai A, et al. Recurrent oral ulcers in a refugee. Am Fam Physician. 2018;97(6):411-412. Accessed July 26, 2019. https://www.aafp.org/afp/2018/0315/p411.html
Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338-351.
Allmon A, Deane K, Martin KL. Common skin rashes in children. Am Fam Physician. 2015;92(3):211-216. Accessed July 26, 2019. https://www.aafp.org/afp/2015/0801/p211.html
Sidbury R, Kodama S. Atopic dermatitis guidelines: diagnosis, systemic therapy, and adjunctive care. Clin Dermatol. 2018;36(5):648-652.
Hartman-Adams H, Banvard C, Juckett G. Impetigo: diagnosis and treatment. Am Fam Physician. 2014;90(4):229-235. Accessed July 26, 2019. https://www.aafp.org/afp/2014/0815/p229.html
Siedner-Weintraub Y, Gross I, David A, et al. Paediatric erythema multiforme: epidemiological, clinical and laboratory characteristics. Acta Derm Venereol. 2017;97(4):489-492.
Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, et al. Current perspectives on erythema multiforme. Clin Rev Allergy Immunol. 2018;54(1):177-184.
Keighley CL, Saunderson RB, Kok J, et al. Viral exanthems. Curr Opin Infect Dis. 2015;28(2):139-150.
Usatine RP, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82(9):1075-1082. Accessed July 26, 2019. https://www.aafp.org/afp/2010/1101/p1075.html
Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377(7):e9.
Moss WJ. Measles. Lancet. 2017;390(10111):2490-2502.
Gottlieb M, Long B, Koyfman A. The evaluation and management of Rocky Mountain Spotted Fever in the emergency department: a review of the literature. J Emerg Med. 2018;55(1):42-50.
Engelman D, Fuller LC, Steer AC International Alliance for the Control of Scabies Delphi panel. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12(5):e0006549.
Tarbox M, Walker K, Tan M. Scabies. JAMA. 2018;320(6):612.
Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, et al. Current perspectives on Stevens-Johnson Syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. 2018;54(1):147-176.
Cohen J, Breuer J. Chickenpox: treatment. BMJ Clin Evid. 2015;2015:0912.
Centers for Disease Control and Prevention. Chickenpox (varicella). Accessed January 14, 2019. https://www.cdc.gov/chickenpox/index.html
Hopper SM, McCarthy M, Tancharoen C, et al. Topical lidocaine to improve oral intake in children with painful infectious mouth ulcers: a blinded, randomized, placebo-controlled trial. Ann Emerg Med. 2014;63(3):292-299.
Hess GP, Walson PD. Seizures secondary to oral viscous lidocaine. Ann Emerg Med. 1988;17(7):725-727.
Shelley WB, Hashim M, Shelley ED. Acyclovir in the treatment of hand-foot-and-mouth disease. Cutis. 1996;57(4):232-234.
Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898-e904.
Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Enterovirus (nonpoliovirus). In: Red Book: 2018 Report of the Committee on Infectious Diseases . 31st ed. American Academy of Pediatrics; 2018:331.
Li R, Liu L, Mo Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370(9):829-837.
Aronson SS, Shope TR, eds. Hand-foot-and-mouth disease. In: Managing Infectious Diseases in Child Care and Schools: A Quick Reference Guide . 4th ed. American Academy of Pediatrics; 2017:97–98.
Continue Reading
More in AFP
More in pubmed.
Copyright © 2019 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- A to Z Guides
Coxsackievirus

What is coxsackievirus?
Coxsackievirus is one of the four types of viruses known as enteroviruses. Enteroviruses are made up of a single strand of genetic material called ribonucleic acid (RNA). Coxsackievirus causes illnesses such as hand, foot, and mouth disease (HFMD), muscle infections, heart infections, and meningitis, which is an infection of the brain and spinal cord.
Coxsackievirus got its name from the town of Coxsackie, located south of Albany in New York, where it was first found.
Coxsackievirus in Adults
Although it’s more common for children to get coxsackievirus, anyone can catch it. Adults are more likely to have infections in the heart because of coxsackievirus but can also get HFMD and other infections.
The risk of complications from coxsackievirus is low for most adults. Like all infections, it can cross your blood-brain barrier, the protective layer of cells that acts as a filter to help keep certain substances away from your brain. It’s also possible for coxsackievirus to infect your heart.
The infection is riskiest for people who have weak immune systems, such as the elderly and people with cancer or other diseases that weaken the body’s germ defenses. Pregnant women who get the virus are at a higher risk of stillbirth, wherein the baby dies in the womb before birth. Your baby can also catch coxsackievirus late in your pregnancy even if you aren’t infected.
Coxsackievirus in Babies
Infants and young kids are at especially high risk of getting coxsackievirus infections that cause symptoms. Typically, it causes only a fever in babies. It’s most common for babies to get coxsackievirus in the summer or early fall.
Sometimes, complications can happen when the infection reaches the heart, causing heart failure or even sudden death, but this is rare.
If newborns get coxsackievirus in their first 2 weeks of life, it can turn severe and lead to liver failure and internal bleeding, which can be fatal.
Coxsackievirus Infections
Coxsackievirus disease causes a variety of infections, depending on which type of virus you catch.
Types of coxsackievirus
There are two categories of coxsackieviruses: type A and type B.
Type A viruses cause:
- Herpangina (sores in the throat)
- Hand, foot, and mouth disease (HFMD)
Type B viruses cause:
- Muscle infections that cause spasms of the stomach and chest muscles
Other infections caused by subtypes of both group A and B include:
- Meningitis (infection of the membranes that protect the brain and spinal cord)
- Myopericarditis (inflammation of the heart)
- Encephalitis (inflammation of the brain)
Coxsackievirus Symptoms
About 90% of coxsackievirus infections don't cause symptoms or cause only a fever. The symptoms you get depend on the illness your infection causes.
- HFMD causes painful blisters in your mouth, on the palms of your hands, and on the bottoms of your feet. It goes away on its own but can cause complications if you or your child can't drink or eat because of pain.
- Herpangina causes a sore throat and may give you a high fever and headache.
- Muscle infections cause periods of sharp spasms between your ribs and upper part of the belly that last 15 to 30 minutes.
- Meningitis infection causes symptoms such as a stiff neck, headache , vomiting, and sensitivity to bright light.
- Heart infections cause chest pain, shortness of breath, and abnormal heart rhythms.
Are coxsackievirus infections contagious?
You can get coxsackievirus through droplets sneezed or coughed into the air, from touching surfaces with the virus, or from coming into contact with fecal matter (poop) that has the virus in it.
You can help prevent coxsackievirus from spreading with good handwashing, especially after changing a baby’s diaper. Kids and adults with the virus should stay home from work and school for at least a few days until its symptoms go away.
How long does a coxsackievirus infection last?
After you come into contact with the virus , symptoms show up in about 3 to 6 days, if you have symptoms. In most cases, fever lasts about 2 to 3 days. Mouth sores tend to last longer, around 7 days. The rashes on your hands and feet last the longest, usually fading after 10 days. These rashes usually peel before going away.
Your child can go back to school once their fever is gone. However, if they have many blisters on their body, they may need to wait until those have dried up.
Coxsackievirus Treatment
There is no treatment for coxsackievirus itself, only the symptoms. One type of antiviral drug (pleconaril) is in testing but isn’t available in the U.S. Researchers are also exploring a type of treatment called intravenous immune globulin (IVIG) to help those who have weakened immune systems . This therapy may especially help treat heart and brain infections caused by coxsackievirus.
To treat symptoms, your doctor may suggest pain medications and fever-reducing medications.
When to Call Your Doctor
Call your doctor if you or your child have symptoms such as stiff neck or neck pain, chest pain, difficulty breathing, or any other signs of coxsackievirus. If your child has a fever for more than 24 hours or mouth sores that make it hard for them to swallow, contact your pediatrician.
Top doctors in ,
Find more top doctors on, related links.
- Health A-Z News
- Health A-Z Reference
- Health A-Z Slideshows
- Health A-Z Quizzes
- Health A-Z Videos
- WebMDRx Savings Card
- Coronavirus (COVID-19)
- Hepatitis C
- Diabetes Warning Signs
- Rheumatoid Arthritis
- Morning-After Pill
- Breast Cancer Screening
- Psoriatic Arthritis Symptoms
- Heart Failure
- Multiple Myeloma
- Types of Crohn's Disease
Coxsackie Viruses
- First Online: 15 February 2024
Cite this chapter

- Suhaib Alqudah 5 , 6
89 Accesses
Hand, foot, and mouth disease (HFMD) and herpangina (HA) are two common childhood viral infections that are characterised by febrile illness, maculopapular rash, and vesicles affecting the mouth, hands, and feet. They are caused by coxsackieviruses which exclusively infect humans and are primarily transmitted via faecal -oral or respiratory droplets routes. This chapter summarises the clinical presentation of HFMD and HA.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Recommended Reading
Corsino CB, Ali R, Linklater DR. Herpangina. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
Google Scholar
Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis. 2018;37(3):391–8.
Saguil A, Kane SF, Lauters R, Mercado MG. Hand-Foot-and-Mouth Disease: Rapid Evidence Review. Am Fam Physician. 2019;100(7):408–14.
Yu H, Li XW, Liu QB, Deng HL, Liu G, Jiang RM, et al. Diagnosis and treatment of herpangina: Chinese expert consensus. World J Pediatr. 2020;16(2):129–34.
Download references
Author information
Authors and affiliations.
Melbourne Dental School, The University of Melbourne, Carlton, VIC, Australia
Suhaib Alqudah
Faculty of Dentistry, Jordan University of Science and Technology, Irbid, Jordan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Suhaib Alqudah .
Editor information
Editors and affiliations.
UWA Dental School, The University of Western Australia, Nedlands, WA, Australia
Ramesh Balasubramaniam
Sydney Dental School, The University of Sydney, Camperdown, NSW, Australia
Sue-Ching Yeoh
School of Dentistry, The University of Queensland, Herston, QLD, Australia
S.R. Prabhu
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Alqudah, S. (2023). Coxsackie Viruses. In: Balasubramaniam, R., Yeoh, SC., Yap, T., Prabhu, S. (eds) Oral Medicine - A Clinical Guide. Springer, Cham. https://doi.org/10.1007/978-3-031-36797-7_7
Download citation
DOI : https://doi.org/10.1007/978-3-031-36797-7_7
Published : 15 February 2024
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-36796-0
Online ISBN : 978-3-031-36797-7
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
infectious disease health center / infectious disease a-z list / coxsackie virus article
Coxsackievirus
- Medical Author: Charles Patrick Davis, MD, PhD
- Medical Author: Karthik Kumar, MBBS
- Medical Editor: Melissa Conrad Stöppler, MD
What is coxsackievirus?
What are the types and causes of coxsackievirus, is coxsackievirus contagious, what are coxsackievirus symptoms, diagnosis of coxsackievirus, what is the treatment for coxsackievirus, when should you see a doctor for a coxsackie rash, what is the prognosis for coxsackievirus, is it possible to prevent coxsackievirus, is there a vaccine for coxsackievirus, frequently asked questions.

Coxsackievirus is a member of the Picornaviridae family of viruses in the genus termed Enterovirus . Coxsackieviruses are subtype members of Enterovirus that have a single strand of ribonucleic acid (RNA) for their genetic material. The enteroviruses are also referred to as picornaviruses (pico means "small," so "small RNA viruses").
Coxsackievirus was first isolated from human feces in the town of Coxsackie, N.Y., in 1948 by G. Dalldorf. Coxsackievirus is also written as coxsackie virus in some publications.
Coxsackieviruses are separated into two groups, A (CVA) and B (CVB), which are based on their effects on newborn mice (coxsackievirus A results in muscle injury, paralysis, and death; coxsackievirus B results in organ damage but less severe outcomes.) There are over 24 different serotypes of the virus (having distinct proteins on the viral surface). Coxsackieviruses infect host cells and cause them to break open (lyse).
Type A viruses cause coxsackievirus herpangina (painful blisters in the mouth, throat, hands, feet , or in all these areas). Hand, foot, and mouth disease ( HFMD ) is the common name of this viral infection. Coxsackievirus A16 (CVA16) causes the majority of HFMD infections in the U.S. It usually occurs in children (age 10 and under), but coxsackievirus can also affect adults. This childhood disease should not be confused with the "foot and mouth disease" usually found in animals with hooves (for example, cattle, pigs, and deer). Type A viruses also cause inflammation of the eyelids and white area of the eye ( conjunctivitis ). Coxsackievirus A6 (CVA6) has caused herpangina symptoms, such as mouth blisters, in infants.
Type B viruses cause epidemic pleurodynia ( fever , lung and abdominal pain with headache that lasts about 2-12 days and resolves). Epidemic pleurodynia is also termed Bornholm disease. There are six serotypes of coxsackievirus B (1-6, with B 4 considered by some researchers as a possible cause of diabetes in a number of individuals).
Both types of viruses (A and B) can cause meningitis , myocarditis , and pericarditis , but these occur infrequently from coxsackievirus infections.
Enterovirus 71, like coxsackievirus, also causes HFMD. In Asia in July 2012, particularly in Cambodia, children infected with enterovirus 71 (EV-71) had a high mortality rate due to encephalitis and acute polio -like paralysis. This epidemic mainly affected babies , toddlers, and children under 2 years of age.
Infection is usually spread by fecal-oral contamination, although occasionally the virus is spread by droplets expelled by infected individuals. Items like utensils, diaper -changing tables, and toys that come in contact with body fluids containing the virus may also transmit them to other individuals. Although people of any age, including adults, can get infected, the majority of patients with coxsackievirus infection are young children. Pregnant women can pass coxsackievirus to their newborns, which may cause serious problems for the newborn. So during pregnancy , women need to notify their obstetrician if they exhibit symptoms of the infection, especially if they are near their delivery date.
Yes, coxsackieviruses are contagious and can spread from person to person. These viruses are transmitted mainly by the fecal-oral route and by respiratory aerosols. Droplets containing viruses that land on objects like toys or utensils may occasionally transmit the viruses indirectly to uninfected individuals.
Coxsackieviruses are most contagious during the first week of symptoms. However, viable virus microbes have been found in respiratory tracts for up to 3 weeks and then in feces up to 8 weeks after initial infection, but during this time, the viruses are less contagious.
The incubation period for coxsackievirus infections is relatively short; it lasts about 1-2 days with a range of about 1-5 days.
Risk factors for coxsackievirus infection include physical contact with any individuals with HFMD symptoms. Other risk factors include rural living conditions, association with children in child care centers, and a large number of children in the family. Infectious viruses can be found in feces, saliva, fluid in blisters, and nasal secretions. Even patients who have recovered and have no symptoms may still shed infectious virus for weeks. A fetus or newborn is at risk if their mother becomes infected near the delivery date. Pregnant women should avoid contact with HFMD patients. They should contact their OB/GYN physician if they develop any symptoms of HFMD.

The most common signs and symptoms of coxsackievirus infections are initially fever, a poor appetite, and respiratory illness, including sore throat , cough , and malaise (feeling tired). This incubation period lasts about 1-2 days.
Sore areas in the mouth develop about a day or two after the initial fever and develop into small blisters that often ulcerate. Many infected people (usually children 10 years of age and younger) go on to develop a coxsackie rash that itches on the palms of the hands and the soles of the feet. Other areas such as the buttocks and genitals may be involved. Some patients develop conjunctivitis .
These symptoms usually last about 7-10 days, and the person usually recovers completely. Individuals are most contagious for about a week after symptoms begin, but because the virus can be shed by the infected individual sometimes for weeks after the symptoms have gone away, the person may be mildly contagious for several weeks.

Occasionally, the infection may result in temporary fingernail or toenail loss (termed onychomadesis) and chest or abdominal muscle pain . Rarely, the disease may progress to cause viral meningitis (headache , stiff neck), myocarditis ( heart muscle infection), pericarditis (inflammation/fluid collection of the tissue surrounding the heart), or encephalitis (brain inflammation).
Infection with EV-71 results in a higher incidence of neurologic involvement with symptoms such as polio-like syndrome, meningitis, encephalitis, Guillain-Barré syndrome, and/or ataxia .
Health News
- Could Ozempic Help Prevent Opioid Overdoses?
- Trial Confirms 'Life-Changing' Impact of Gene Therapy for Hemophilia B
- Most Pregnant Women Will Become Iron Deficient, Study Finds
- Airports Take Big Toll on Sleep of Those Living Nearby
- One More Death Tied to Listeria From Boar's Head Deli Meats
Patients are usually diagnosed by their clinical appearance. Clinically, blisters that are painful usually on the hands, feet, and mouth in a child with fever are considered diagnostic of coxsackievirus infection. However, in rare instances, viral tests can be done to identify the virus, but the tests are expensive, usually need to be sent to a specialized viral diagnostic laboratory that uses RT- PCR , and often take about two weeks to get a result. This testing is almost never done since most infections are self-limited and typically mild. RT-PCR testing can distinguish between many viral genera, species, and subtypes. Distinguishing coxsackievirus strains from adenoviruses , other enterovirus types, echoviruses, and viruses causing mononucleosis and other viral diseases may become necessary in the future.
In most instances, if treatment is needed, it is done by the patient's pediatrician and/or primary care physician. However, in severe cases, specialists in pediatric critical care and infectious diseases may be consulted. If severe complications develop (for example, carditis or pleurodynia), others like lung or cardiac specialists may be consulted.
There is no specific treatment for this typically self-limited disease (the symptoms resolve without specific antiviral treatment in about 2-10 days). However, symptomatic over-the-counter treatment ( acetaminophen [ Tylenol ]) that reduces fever and discomfort is currently recommended. Mouthwashes and sprays may lessen the oral discomfort. Fluids are also suggested to prevent dehydration ; however, acidic juices may irritate mouth ulcers. Home remedies like cold milk may soothe the oral discomfort. Some physicians use topical diphenhydramine ( Benadryl )-containing gel or liquids to treat the hand and foot discomfort.
The relatively rare complications of coxsackievirus infections (for example, heart or brain infection) require special individualized treatments (possibly human immune globulin or specific antivirals, although such treatments are rare and have not yet been proven to be safe and effective with serious HFMD infections). These treatments are often administered by an infectious-disease doctor.
You should see a doctor for a coxsackie rash if you or your child experience any of the following symptoms or complications:
- A fever lasting longer than 24 hours
- Severe symptoms such as a stiff neck, headache , vomiting , or sensitivity to bright light, which could indicate meningitis, encephalitis, or other serious complications
- Difficulty breathing
- Difficulty swallowing
- Weakened immune system
- Blisters or sores that don’t heal
- Symptoms that worsen after 7-10 days
If your child is younger than 6 months and has symptoms, it's crucial to consult a doctor immediately due to the increased risk of complications.
In general, it's always a good idea to consult a doctor if you or your child are experiencing any unusual or concerning symptoms, especially if you're unsure about the severity or progression of the infection.
Subscribe to MedicineNet's General Health Newsletter
By clicking Submit, I agree to the MedicineNet's Terms & Conditions & Privacy Policy and understand that I may opt out of MedicineNet's subscriptions at any time.
Are there long-term effects of a coxsackievirus infection? Only rarely do patients suffer poor outcomes with complications of meningitis, pericarditis, or encephalitis. Unfortunately, infants and young children infected with EV-71 have a prognosis that may vary from good to poor.
The prevention of coxsackievirus infections is difficult but possible. With children, keeping strict hygienic precautions is almost impossible, but good practices such as hand washing after diaper changing or touching infected skin may reduce viral transmission to other family members. Attempts to regularly clean items that children contact, especially toys, pacifiers, and any items they may place in their mouths, may also reduce viral transmission. Hand washing, in general, is the best prevention technique. Currently, there is no commercial vaccine available.
Pregnant women should avoid contact with children (or adults) with HFMD because some studies suggest that coxsackievirus may cause developmental and other defects in the fetus.
Although infection and resolution of the disease usually render the person immune to reinfection with the viral type that initiated the disease, the person is not immune to other coxsackievirus types. For example, a person may become immune to coxsackievirus type B4 but still would be susceptible to all of the other coxsackievirus types (for example, CVA16). In addition, other viruses such as enterovirus 71 and enteric cytopathic human orphan (ECHO) viruses can produce HFMD symptoms. Consequently, it is possible for some people to have multiple infections with HFMD symptoms even though repeated infections occur infrequently.
Currently, there is no vaccine to prevent coxsackievirus infections. This is because different coxsackievirus B (CVB) serotypes can affect different organs or cause similar diseases with different severities. However, there are a few vaccines in development that are being evaluated for prevention of this infection.
Is coxsackievirus more severe in adults than in children?
While the infection is commonly mild and self-limited in children, certain strains, such as coxsackievirus A6, can cause more severe illness in both children and adults. Reports have highlighted cases of a severe form of hand, foot, and mouth disease (HFMD) associated with coxsackievirus A6 in adults, leading to hospitalization for supportive care. This severe variant can present with fevers , joint pains, and painful eruptions.
Although HFMD is rare in adults, the rising incidence of adult cases may be expected due to factors such as climate change and viral evolution.
Can coxsackievirus in adults cause chronic health issues?
In most cases, coxsackievirus infections in adults cause mild symptoms that resolve on their own without causing long-term health issues. However, in some cases, coxsackievirus infections can lead to more severe conditions such as viral meningitis or myocarditis, which can have lasting effects, especially in individuals with weakened immune systems or underlying health conditions.
Infectious Disease Resources
- Is It a Cold, Strep, or Tonsillitis?
Featured Centers
- What Are the Best PsA Treatments for You?
- Understanding Biologics
- 10 Things People With Depression Wish You Knew
Top Coxsackie Virus Related Articles

Abdominal Pain

acetaminophen

diphenhydramine

Enterovirus (Non-Polio Enterovirus Infection)

Hand-Foot-and-Mouth Disease in Mouth Picture 1

Hand, Foot, and Mouth Disease (HFMD)

Myocarditis

Pericarditis

Septic Arthritis

Sore Throat
Sore throat (throat pain) usually is described as pain or discomfort in the throat area. A sore throat may be caused by bacterial infections, viral infections, toxins, irritants, trauma, or injury to the throat area. Common symptoms of a sore throat include a fever, cough, runny nose, hoarseness, earaches, sneezing, and body aches. Home remedies for a sore throat include warm soothing liquids and throat lozenges. OTC remedies for a sore throat include OTC pain relievers such as ibuprofen or acetaminophen. Antibiotics may be necessary for some cases of sore throat.

What's a Virus?

Coxsackieviruses Workup
- Author: Eric Wu, MD; Chief Editor: Michael Stuart Bronze, MD more...
- Sections Coxsackieviruses
- Pathophysiology
- Epidemiology
- Patient Education
- Physical exam findings
- Complications
- Approach Considerations
- Laboratory Studies
- Imaging Studies
- Other Tests
- Histologic Findings
- Medical Care
- Surgical Care
- Consultations
- Long-Term Monitoring
- Further Outpatient Care
- Further Inpatient Care
- Inpatient & Outpatient Medications
- Questions & Answers
Enteroviruses can be excreted in human feces for up to 3 months after infection. However, a clinically identifiable syndrome correlates with the acute phase of infection, during which time virus can be found in the throat, blood, and various organs.
There are no confirmatory laboratory tests, procedures, or imaging that are used in routine clinical practice for HFMD or herpangina. Diagnosis for these conditions mainly is based on clinical presentation and assessment.
Definitive diagnosis of coxsackievirus infection can be made based on isolation of the virus in cell culture. Cytopathic effect usually can be seen within 2 to 6 days. Samples normally are taken from the stool or rectal swabs; the virus also can be isolated from the oropharynx early in the disease course. However, given improved sensitivity and faster turn-around time, polymerase chain reaction (PCR) has emerged as the most prominent diagnostic tool used for enteroviral detection. Serology is available as a diagnostic modality but can be difficult to interpret. Traditionally, enteroviral infections are diagnosed after a rise in neutralizing antibody titer (at least a 4-fold rise in titer between acute and convalescent phase).

Aseptic meningitis
Before a diagnosis of aseptic meningitis can be made, bacterial meningitis should be considered and excluded. Empiric antibiotics typically are required during this time period. Diagnosis requires cerebrospinal fluid (CSF) evaluation, which tends to show a lymphocytic predominance, normal-to-decreased glucose levels, and normal-to-slightly elevated protein levels. The virus can be isolated via PCR (sensitivity, 66-90%) and, much less commonly, cell culture (sensitivity, 30-35%). A recent study in infants reported that routine CSF PCR for enteroviruses resulted in shorter hospital stays (by 1.54 days) and a decreased duration of antibiotic use (by 33%).
Encephalitis
Diagnostic workup requires a lumbar puncture (LP) with CSF evaluation, which yields findings similar to those of aseptic meningitis.
Electroencephalography (EEG) can be considered in some patients, particularly for the evaluation of nonconvulsive or subclinical seizures. Enteroviral and other causes of viral encephalitis typically appear as diffuse background slowing on EEG, but epileptiform activity may be present as well. [ 20 ]
Please see section on Imaging Studies below for further recommendations
Myopericarditis
Laboratory tests generally are circumstantial, with evidence of infection based upon positive PCR tests from the oropharynx or feces, or upon serological testing.
Acute hemorrhagic conjunctivitis (AHC)
Diagnosis requires conjunctival swabs or scrapings, which are 90% successful. A rising antibody titer also can theoretically be used to confirm a diagnosis.
Computed tomographic (CT) scanning of the brain can be obtained upon initial presentation of patients with suspected meningitis and/or encephalitis to evaluate for hemorrhage, increased intracranial pressure, or mass lesions.
Magnetic Resonance Imaging (MRI) of the brain can show hyperintense signal uptake in the posterior brain stem, substantia nigra, dentate nucleus, and anterior horns of the spinal cord. [ 21 ]
Echocardiography should be used to evaluate cardiac function and valvular disease in patients with myopericarditis and/or heart failure.
Cardiovascular Magnetic Resonance (CMR) can be used to identify imaging features characteristic of myocarditis such as necrosis, scarring, and myocardial hyperemia and edema. [ 22 ]
Depending upon the clinical presentation, a throat culture can be obtained to evaluate for possible streptococcal pharyngitis and/or tonsillitis.
HIV testing can be considered in patients who present with nonspecific febrile illness or rashes, depending on the epidemiologic history.
ECG changes in myopericarditis include ST-segment elevations or nonspecific ST segment and/or T-wave abnormalities, arrhythmia, and heart block.
In select instances in which viral myocarditis is being considered as the etiological cause for new-onset heart failure, endomyocardial biopsy might be indicated.
Lumbar puncture is crucial in the evaluation of suspected meningitis and/or encephalitis.
Skin biopsy rarely may be helpful in the evaluation of nonspecific exanthems.
Intracytoplasmic viral particles may be observed, especially with skin lesions and/or rashes of HFMD.
Tariq N, Kyriakopoulos C. Group B Coxsackie Virus. 2022 Jan. [QxMD MEDLINE Link] . [Full Text] .
Nikonov OS, Chernykh ES, Garber MB, Nikonova EY. Enteroviruses: Classification, Diseases They Cause, and Approaches to Development of Antiviral Drugs. Biochemistry (Mosc) . December 2017. (82)13:1615-1631.
Issacs S, Foskett D, Maxwell A, Ward E, Faulkner C, Luo J, et al. Viruses and type-1 diabetes: from enteroviruses to the virome. Microorganisms . 2021. 9:
Tyring S. Hand foot and mouth disease: enteroviral load and disease severity. EBioMedicine . 2020. 62:
Elrick M, Pekosz A, Duggal P. Enterovirus D68 molecular and cellular biology and pathogenesis. Journal Biol Chem . 2021. 296:
Olsen S, Winn A, Budd A, Prill M, Steel J, Midgley, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic-United States, 2020-2021. Morbidity and Mortality Weekly Report . July 23, 2021. 70:1013-1019.
Brotons P, Jordan I, Bassat Q, Henares D, Fernandez de Sevilla M, Ajanovic S, et al. The positive rhinovirus/enterovirus detection and SARS-CoV-2 persistence beyond the acute infection phase: an intra-household surveillance study. Viruses . 2021. 13:
Olsen SJ, Winn AK, Budd AP, et al. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic — United States, 2020–2021. MMWR Morb Mortal Wkly Rep . July 23, 2021. 70:1013-1019. [Full Text] .
Chow Eric J, Uyeki Timothy M, Chu Helen Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol . October 17, 2022. 1-16. [Full Text] .
Rotbart HA, McCracken GH Jr, Whitley RJ, Modlin JF, Cascino M, Shah S, et al. Clinical significance of enteroviruses in serious summer febrile illnesses of children. Pediatr Infect Dis J . 1999. 10:869-74.
Kogon A, Spigland I, Frothingham TE, Elveback L, Williams C, Hall CE, et al. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VII. Observations on viral excretion, seroimmunity, intrafamilial spread and illness association in coxsackie and echovirus infections. Am J Epidemiol . Jan 1968. 1:51-61.
Posnakoglou L, Tatsi E, Chatzichristou P, Siahanidou T, Kanaka-Gantenbein C, Syriopoulou V, et al. Molecular epidemiology of enterovirus in children with central nervous system infections. Viruses . 2021. 13:
Oikarinen S, Krogvold L, Edwin B, Buanes T, Korsgren O, Laiho J, et al. Characterisation of enterovirus RNA detected in the pancreas and other specimens of live patients with newly diagnosed type 1 diabetes in the DiViD study. Diabetologia . 2021 Nov. 64:2491-2501.
Nekoua MP, Alidjinou EK, Hober D. Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol . June 1, 2022. 18(8):503-516. [Full Text] .
Alhazmi A, Nekoua MP, Michaux H, Sane F, Halouani A, Engelmann I, et al. Effect of Coxsackievirus B4 Infection on the Thymus: Elucidating Its Role in the Pathogenesis of Type 1 Diabetes. Microorganisms . May 29, 2021. 9(6):1177. [Full Text] .
Horsten H. Eczema Coxsackium caused by Coxsackievirus A6. Pediatric Dermatology . 2016. 33:e230-e231.
Perez V, Melnick L, Whittier S, Dayan P, Garzon M, Morel K, et al. The use of respiratory pathogen panel nasal polymerase chain reaction testing in predicting cutaneous enteroviral infections in the pediatric population. Pediatric Dermatology . 2021. 38:602-605.
Baggen J, Hurdiss DL, Zocher G, Mistry N, Roberts RW, Slager JJ, et al. Role of enhanced receptor engagement in the evolution of a pandemic acute hemorrhagic conjunctivitis virus. Proc Natl Acad Sci U S A . January 9, 2018. 115(2):397-402.
Zheng M, Wang H, Tang J, He Y, Xiong T, Li W, et al. Clinical characteristics of severe neonatal enterovirus infection: a systematic review. BMC Pediatrics . 2021. 21:127.
Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology . May 25, 2004. 10:1743-8. [Full Text] .
Mohamed Saied Abdelgawad, Abd El-Aziz El-Nekidy, Rania A.M. Abouyoussef, Amr El-Fatary. MRI findings of enteroviral encephalomyelitis. The Egyptian Journal of Radiology and Nuclear Medicine . 2016. 47:1031-6.
Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging . November 2018. 11:1583-1590.
Anasir M, Zarif F, Poh C. Antivirals blocking entry of enteroviruses and therapeutic potential. Journal of Biomedical Science . 2021. 28:
Pevear DC, Tull TM, Seipel ME, Groarke JM. Activity of pleconaril against enteroviruses. Antimicrob Agents Chemother . September 1999. 43(9):2109-15. [Full Text] .
Rotbart HA, Webster AD; Pleconaril Treatment Registry Group. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis . January 15, 2001. 32(2):228-35. [Full Text] .
Desmond RA, Accortt NA, Talley L, Villano SA, Soong SJ, Whitley RJ. Enteroviral meningitis: natural history and outcome of pleconaril therapy. Antimicrob Agents Chemother . July 2006. 50(7):2409-14. [Full Text] .
Wagner JN, Leibetseder A, Troescher A, Panholzer J, von Oertzen TJ. Characteristics and therapy of enteroviral encephalitis: case report and systematic literature review. Int J Infect Dis . December 2021. 113:93-102.
Schiff GM, Sherwood JR. Clinical activity of pleconaril in an experimentally induced coxsackievirus A21 respiratory infection. J Infect Dis . 2000 Jan. 181(1):20-6. [QxMD MEDLINE Link] .
Brunetti L, DeSantis ER. Treatment of viral myocarditis caused by coxsackievirus B. Am J Health Syst Pharm . 2008 Jan 15. 65(2):132-7. [QxMD MEDLINE Link] .
Yue-Chun L, LiSha G, Jiang-Hua R, Peng-Lin Y, Jia-Feng L, Ji-Fei T, et al. Protective effects of carvedilol in murine model with the coxsackievirus B3-induced viral myocarditis. J Cardiovas Pharmacol . Jan/2008. 51:92-98. [QxMD MEDLINE Link] .
Shi L, Xiong H, He J, et al. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol . 2007. 152(8):1447-55. [QxMD MEDLINE Link] .
Cheng D, Chiu YW, Huang SW, Lien YY, Chang CL, Tsai HP, et al. Genetic and Cross Neutralization Analyses of Coxsackievirus A16 Circulating in Taiwan from 1998 to 2021 Suggest Dominant Genotype B1 can Serve as Vaccine Candidate. Viruses . October 20, 2022. 14(10):2306. [Full Text] .
Mao QY, Wang Y, Bian L, Xu M, Liang Z. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev Vaccines . January 14, 2016. 15(5):599-606. [Full Text] .
PROtocol for Coxsackievirus VaccinE in Healthy VoluNTteers (PROVENT). ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT04690426 . December 30, 2020; Accessed: July 25, 2022.
CDC. Non-Polio Enterovirus: Outbreaks & Surveillance. CDC. Available at https://www.cdc.gov/non-polio-enterovirus/outbreaks-surveillance.html . November 14, 2018; Accessed: December 6, 2019.
CDC. Non-Polio Enterovirus: For Health Care Professionals. CDC. Available at https://www.cdc.gov/non-polio-enterovirus/hcp.html . November 14, 2018; Accessed: December 6, 2019.
CDC. Acute Flaccid Myelitis: AFM Investigation. CDC. Available at https://www.cdc.gov/acute-flaccid-myelitis/afm-investigation.html . November 4, 2019; Accessed: December 6, 2019.

Contributor Information and Disclosures
Eric Wu, MD Academic Hospitalist, Michael E DeBakey VA Medical Center; Assistant Professor, Baylor College of Medicine Disclosure: Nothing to disclose.
Rajeev Balchandani, MD Assistant Professor of Medicine, Baylor College of Medicine; Hospitalist Physician, Medicine Care Line, Michael E DeBakey VA Medical Center Rajeev Balchandani, MD is a member of the following medical societies: American Medical Association , Society of Hospital Medicine Disclosure: Nothing to disclose.
Prathit A Kulkarni, MD Assistant Professor, Department of Medicine, Section of Infectious Diseases, Associate Program Director, Infectious Diseases Fellowship Program, Baylor College of Medicine; Assistant Chief of Medicine, Medical Care Line, Associate Site Director, Internal Medicine Residency Program, Michael E DeBakey Veterans Affairs Medical Center Prathit A Kulkarni, MD is a member of the following medical societies: Alpha Omega Alpha , American College of Physicians , Gold Humanism Honor Society, Infectious Diseases Society of America , Society for Healthcare Epidemiology of America Disclosure: Received research grant from: Vessel Health, Inc.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference Disclosure: Received salary from Medscape for employment. for: Medscape.
John W King, MD Professor of Medicine, Chief, Section of Infectious Diseases, Director, Viral Therapeutics Clinics for Hepatitis, Louisiana State University School of Medicine in Shreveport; Consultant in Infectious Diseases, Overton Brooks Veterans Affairs Medical Center John W King, MD is a member of the following medical societies: American Association for the Advancement of Science , American College of Physicians , American Federation for Medical Research , American Society for Microbiology , Association of Subspecialty Professors, Infectious Diseases Society of America , Sigma Xi, The Scientific Research Honor Society Disclosure: Nothing to disclose.
Michael Stuart Bronze, MD David Ross Boyd Professor and Chairman, Department of Medicine, Stewart G Wolf Endowed Chair in Internal Medicine, Department of Medicine, University of Oklahoma Health Science Center; Master of the American College of Physicians; Fellow, Infectious Diseases Society of America; Fellow of the Royal College of Physicians, London Michael Stuart Bronze, MD is a member of the following medical societies: Alpha Omega Alpha , American College of Physicians , American Medical Association , Association of Professors of Medicine , Infectious Diseases Society of America , Oklahoma State Medical Association , Southern Society for Clinical Investigation Disclosure: Nothing to disclose.
Maria D Mileno, MD Associate Professor of Medicine, Division of Infectious Diseases, The Warren Alpert Medical School of Brown University Maria D Mileno, MD is a member of the following medical societies: Alpha Omega Alpha , American College of Physicians , American Society of Tropical Medicine and Hygiene , Infectious Diseases Society of America , International Society of Travel Medicine , Sigma Xi, The Scientific Research Honor Society Disclosure: Nothing to disclose.
Martha L Muller, MD, MPH Professor of Pediatrics, Division of Infectious Diseases, University of New Mexico School of Medicine Disclosure: Nothing to disclose.
Mashiul H Chowdhury, MD Assistant Professor, Department of Medicine, Division of Infectious Disease, Program Director, Infectious Disease Fellowship, Director, TravelHealth Center, Drexel University College of Medicine
Disclosure: Nothing to disclose.
Nhat M Doan, MD Fellow, Department of Internal Medicine, Division of Infectious Diseases, Washington Hospital Center
Parul Kaushik, MD, MPH Fellow, Department of Medicine, Division of Infectious Disease, Drexel University College of Medicine
Michael Rajnik, MD Associate Professor, Department of Pediatrics, Program Director, Pediatric Infectious Disease Fellowship Program, Uniformed Services University of the Health Sciences
Michael Rajnik is a member of the following medical societies: American Academy of Pediatrics , Armed Forces Infectious Disease Society , Infectious Diseases Society of America , and Pediatric Infectious Diseases Society .
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Trending Clinical Topic: Respiratory Syncytial Virus (RSV)
- Pediatric Pneumonia
- Respiratory Syncytial Virus Infection
- Fast Five Quiz: Respiratory Syncytial Virus (RSV)
- Fast Five Quiz: Respiratory Syncytial Virus
- Pediatric Bocavirus
- Fast Five Quiz: Overview of Pediatric Respiratory Syncytial Virus
- Taking on Inequities in Pediatric Respiratory Care
- Adenosine Safe for Pediatric Tachyarrhythmia
- Clear Association Between Vaping and Respiratory Symptoms

- Drug Interaction Checker
- Pill Identifier
- Calculators

- 2001/viewarticle/taking-inequities-pediatric-respiratory-care-2024a1000gvcnews news Taking on Inequities in Pediatric Respiratory Care

- 2010/viewarticle/1001505 Fast Five Quiz: Common Back-to-School Concerns for Primary Care
- 2010/viewarticle/1000784 Reducing the risk of RSV illness through active immunisation with vaccines
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature
Affiliations.
- 1 Center for Clinical Studies, Houston, TX, USA; Department of Dermatology, Tufts Medical Center, Boston, MA, USA.
- 2 Center for Clinical Studies, Houston, TX, USA.
- 3 Department of Dermatology, University of Texas Health Science Center at Houston, Houston, TX, USA.
- 4 Division of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
- 5 Center for Clinical Studies, Houston, TX, USA; Department of Dermatology, University of Texas Health Science Center at Houston, Houston, TX, USA. Electronic address: [email protected].
- PMID: 24932735
- DOI: 10.1016/j.jcv.2014.04.023
- J Clin Virol. 2015 Jan;62:123
Expression of concern in
- “Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature” [J. Clin. Virol. 60 (2014) 381–386]. [No authors listed] [No authors listed] J Clin Virol. 2015 Jan;62:122. J Clin Virol. 2015. PMID: 25692203 No abstract available.
Background: Hand, foot, and mouth disease (HFMD) is generally considered a rare illness in adults. Classically, HFMD has been strongly associated with coxsackievirus strain A16 and enterovirus 71. The coxsackievirus A6 (CVA6) strain has been linked to severe worldwide outbreaks since 2008. CVA6 is associated with a more severe and profound course of disease, affecting both children and adults.
Objectives: To present a series of five adult patients diagnosed with HFMD due to CVA6. We investigate method of diagnosis and compare clinical presentation of adult cases to those in children.
Study design: Each patient underwent a full-body skin exam as well as inspection of the oral cavity. Rapid plasma reagin (RPR) and serologic assays by complement fixation against coxsackievirus B (1-6) and A (2,4,7,9,10,16) were performed as indicated. As standard serological testing does not detect CVA6, real-time reverse transcription-polymerase chain reaction (qRT-PCR) of serum, buccal swabs, and skin scrapings were performed by the Centers for Disease Control and Prevention (CDC).
Results: Each patient had clinical findings consistent with various stages of HFMD. One patient presented with delayed onychomadesis and desquamation of the palms and soles. RPR and serologic assays by complement fixation against CVB (1-6) and CVA (2,4,7,9,10,16) were mostly negative, although elevated in two patients due to cross-reactivity. qRT-PCR identified CVA6 genetic material in samples from all patients.
Conclusion: This series demonstrates that there is a wide array of disease presentation of CVA6 associated HFMD in adults.
Keywords: Coxsackievirus A6; Enterovirus; Hand foot and mouth disease.
Copyright © 2014 Elsevier B.V. All rights reserved.
PubMed Disclaimer
Similar articles
- Prevalence of Coxsackievirus A6 and Enterovirus 71 in Hand, Foot and Mouth Disease in Nanjing, China in 2013. Hu YQ, Xie GC, Li DD, Pang LL, Xie J, Wang P, Chen Y, Yang J, Cheng WX, Zhang Q, Jin Y, Duan ZJ. Hu YQ, et al. Pediatr Infect Dis J. 2015 Sep;34(9):951-7. doi: 10.1097/INF.0000000000000794. Pediatr Infect Dis J. 2015. PMID: 26090576
- Development of single-step multiplex real-time RT-PCR assays for rapid diagnosis of enterovirus 71, coxsackievirus A6, and A16 in patients with hand, foot, and mouth disease. Puenpa J, Suwannakarn K, Chansaenroj J, Vongpunsawad S, Poovorawan Y. Puenpa J, et al. J Virol Methods. 2017 Oct;248:92-99. doi: 10.1016/j.jviromet.2017.06.013. Epub 2017 Jun 27. J Virol Methods. 2017. PMID: 28662914
- Clinical features of hand, foot and mouth disease caused by Coxsackievirus A6 in Xi'an, China, 2013-2019: A multicenter observational study. Li M, Li Y, Du J, Zhang Y, Xi M, Yan K, Liu R, Wang X, Xu P, Yuan J, Deng H. Li M, et al. Acta Trop. 2024 Sep;257:107310. doi: 10.1016/j.actatropica.2024.107310. Epub 2024 Jun 30. Acta Trop. 2024. PMID: 38955319
- Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Bian L, Wang Y, Yao X, Mao Q, Xu M, Liang Z. Bian L, et al. Expert Rev Anti Infect Ther. 2015;13(9):1061-71. doi: 10.1586/14787210.2015.1058156. Epub 2015 Jun 25. Expert Rev Anti Infect Ther. 2015. PMID: 26112307 Review.
- Hand-foot-and-mouth disease caused by coxsackievirus A6 on the rise. Kimmis BD, Downing C, Tyring S. Kimmis BD, et al. Cutis. 2018 Nov;102(5):353-356. Cutis. 2018. PMID: 30566537 Review.
- Development of a robust cell-based potency assay for a coxsackievirus A21 oncolytic virotherapy. Chamcha V, He L, Jenny Xu, Swartz AR, Green-Trexler E, Gurney K, McNeely T. Chamcha V, et al. Heliyon. 2024 Mar 20;10(7):e28414. doi: 10.1016/j.heliyon.2024.e28414. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38560158 Free PMC article.
- Construction of a novel kinetic model for the production process of a CVA6 VLP vaccine in CHO cells. Xing Z, Nguyen TB, Kanai-Bai G, Yamano-Adachi N, Omasa T. Xing Z, et al. Cytotechnology. 2024 Feb;76(1):69-83. doi: 10.1007/s10616-023-00598-8. Epub 2023 Oct 27. Cytotechnology. 2024. PMID: 38304624 Free PMC article.
- Hand, Foot, and Mouth Disease in Adults. Afonso C, Almeida A. Afonso C, et al. Cureus. 2023 Nov 6;15(11):e48387. doi: 10.7759/cureus.48387. eCollection 2023 Nov. Cureus. 2023. PMID: 38060762 Free PMC article.
- Identification of Multiple Novel Viruses in Fecal Samples of Black-Necked Cranes Using Viral Metagenomic Methods. Zhao Q, Zhao R, Sun Y, Ji L, Xi Y, Wang X, Shen Q, Ji L, Wang Y, You Z, Yang S, Zhang W. Zhao Q, et al. Viruses. 2023 Oct 9;15(10):2068. doi: 10.3390/v15102068. Viruses. 2023. PMID: 37896845 Free PMC article.
- Current status of hand-foot-and-mouth disease. Zhu P, Ji W, Li D, Li Z, Chen Y, Dai B, Han S, Chen S, Jin Y, Duan G. Zhu P, et al. J Biomed Sci. 2023 Feb 24;30(1):15. doi: 10.1186/s12929-023-00908-4. J Biomed Sci. 2023. PMID: 36829162 Free PMC article. Review.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- ClinicalKey
- Elsevier Science
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations
Research Materials
- NCI CPTC Antibody Characterization Program
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Help & FAQ
Coxsackievirus A6 associated hand, foot and mouth disease in adults: Clinical presentation and review of the literature
Research output : Contribution to journal › Article › peer-review
Background: Hand, foot, and mouth disease (HFMD) is generally considered a rare illness in adults. Classically, HFMD has been strongly associated with coxsackievirus strain A16 and enterovirus 71. The coxsackievirus A6 (CVA6) strain has been linked to severe worldwide outbreaks since 2008. CVA6 is associated with a more severe and profound course of disease, affecting both children and adults. Objectives: To present a series of five adult patients diagnosed with HFMD due to CVA6. We investigate method of diagnosis and compare clinical presentation of adult cases to those in children. Study design: Each patient underwent a full-body skin exam as well as inspection of the oral cavity. Rapid plasma reagin (RPR) and serologic assays by complement fixation against coxsackievirus B (1-6) and A (2,4,7,9,10,16) were performed as indicated. As standard serological testing does not detect CVA6, real-time reverse transcription-polymerase chain reaction (qRT-PCR) of serum, buccal swabs, and skin scrapings were performed by the Centers for Disease Control and Prevention (CDC). Results: Each patient had clinical findings consistent with various stages of HFMD. One patient presented with delayed onychomadesis and desquamation of the palms and soles. RPR and serologic assays by complement fixation against CVB (1-6) and CVA (2,4,7,9,10,16) were mostly negative, although elevated in two patients due to cross-reactivity. qRT-PCR identified CVA6 genetic material in samples from all patients. Conclusion: This series demonstrates that there is a wide array of disease presentation of CVA6 associated HFMD in adults.
| Original language | English (US) |
|---|---|
| Pages (from-to) | 381-386 |
| Number of pages | 6 |
| Journal | |
| Volume | 60 |
| Issue number | 4 |
| DOIs | |
| State | Published - Aug 2014 |
| Externally published | Yes |
- Coxsackievirus A6
- Enterovirus
- Hand foot and mouth disease
ASJC Scopus subject areas
- Infectious Diseases
Access to Document
- 10.1016/j.jcv.2014.04.023
Other files and links
- Link to publication in Scopus
Fingerprint
- Clinical Presentation Keyphrases 100%
- Clinical Review Keyphrases 100%
- Foot-and-mouth Disease Keyphrases 100%
- CVA6 Keyphrases 100%
- Enterovirus Immunology and Microbiology 100%
- Foot Immunology and Microbiology 100%
- Mouth Immunology and Microbiology 100%
- Hand, Foot and Mouth Disease Medicine and Dentistry 100%
T1 - Coxsackievirus A6 associated hand, foot and mouth disease in adults
T2 - Clinical presentation and review of the literature
AU - Downing, Christopher
AU - Ramirez-Fort, Marigdalia K.
AU - Doan, Hung Q.
AU - Benoist, Frances
AU - Oberste, M. Steven
AU - Khan, Farhan
AU - Tyring, Stephen K.
N1 - Copyright: Copyright 2019 Elsevier B.V., All rights reserved.
PY - 2014/8
Y1 - 2014/8
N2 - Background: Hand, foot, and mouth disease (HFMD) is generally considered a rare illness in adults. Classically, HFMD has been strongly associated with coxsackievirus strain A16 and enterovirus 71. The coxsackievirus A6 (CVA6) strain has been linked to severe worldwide outbreaks since 2008. CVA6 is associated with a more severe and profound course of disease, affecting both children and adults. Objectives: To present a series of five adult patients diagnosed with HFMD due to CVA6. We investigate method of diagnosis and compare clinical presentation of adult cases to those in children. Study design: Each patient underwent a full-body skin exam as well as inspection of the oral cavity. Rapid plasma reagin (RPR) and serologic assays by complement fixation against coxsackievirus B (1-6) and A (2,4,7,9,10,16) were performed as indicated. As standard serological testing does not detect CVA6, real-time reverse transcription-polymerase chain reaction (qRT-PCR) of serum, buccal swabs, and skin scrapings were performed by the Centers for Disease Control and Prevention (CDC). Results: Each patient had clinical findings consistent with various stages of HFMD. One patient presented with delayed onychomadesis and desquamation of the palms and soles. RPR and serologic assays by complement fixation against CVB (1-6) and CVA (2,4,7,9,10,16) were mostly negative, although elevated in two patients due to cross-reactivity. qRT-PCR identified CVA6 genetic material in samples from all patients. Conclusion: This series demonstrates that there is a wide array of disease presentation of CVA6 associated HFMD in adults.
AB - Background: Hand, foot, and mouth disease (HFMD) is generally considered a rare illness in adults. Classically, HFMD has been strongly associated with coxsackievirus strain A16 and enterovirus 71. The coxsackievirus A6 (CVA6) strain has been linked to severe worldwide outbreaks since 2008. CVA6 is associated with a more severe and profound course of disease, affecting both children and adults. Objectives: To present a series of five adult patients diagnosed with HFMD due to CVA6. We investigate method of diagnosis and compare clinical presentation of adult cases to those in children. Study design: Each patient underwent a full-body skin exam as well as inspection of the oral cavity. Rapid plasma reagin (RPR) and serologic assays by complement fixation against coxsackievirus B (1-6) and A (2,4,7,9,10,16) were performed as indicated. As standard serological testing does not detect CVA6, real-time reverse transcription-polymerase chain reaction (qRT-PCR) of serum, buccal swabs, and skin scrapings were performed by the Centers for Disease Control and Prevention (CDC). Results: Each patient had clinical findings consistent with various stages of HFMD. One patient presented with delayed onychomadesis and desquamation of the palms and soles. RPR and serologic assays by complement fixation against CVB (1-6) and CVA (2,4,7,9,10,16) were mostly negative, although elevated in two patients due to cross-reactivity. qRT-PCR identified CVA6 genetic material in samples from all patients. Conclusion: This series demonstrates that there is a wide array of disease presentation of CVA6 associated HFMD in adults.
KW - Coxsackievirus A6
KW - Enterovirus
KW - Hand foot and mouth disease
UR - http://www.scopus.com/inward/record.url?scp=84903560571&partnerID=8YFLogxK
UR - http://www.scopus.com/inward/citedby.url?scp=84903560571&partnerID=8YFLogxK
U2 - 10.1016/j.jcv.2014.04.023
DO - 10.1016/j.jcv.2014.04.023
M3 - Article
C2 - 24932735
AN - SCOPUS:84903560571
SN - 1386-6532
JO - Journal of Clinical Virology
JF - Journal of Clinical Virology
Are you a healthcare professional
GO TO DERMNET PRO
Common skin conditions

Join DermNet PRO
Quick links
Skin checker
Try our skin symptom checker
Hand, foot, and mouth disease
Hand, foot, and mouth disease — extra information.
Infections Rashes
August 2022
Author: Dr Stanley Leong, Paediatric Registrar; Dr Caroline Mahon, Dermatologist, Christchurch Hospital, New Zealand Previous contributors: A/Prof Amanda Oakley, Dermatologist (1998 and 2016); Dr Jannet Gomez (2016) Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction Demographics Causes Clinical features Variation in skin types Complications Diagnosis Differential diagnoses Treatment Advice on school leave Outcome
What is hand, foot, and mouth disease?
Hand, foot, and mouth disease (HFMD or HFM) is a common, self-limiting , viral infection that causes blisters on the hands, feet, and inside or around the mouth. It mainly affects children under the age of 5 years.
HFMD, also called enteroviral vesicular stomatitis , occurs sporadically worldwide. Epidemics are most common during warm weather, usually in the late summer or early autumn.
It is important to note that HFMD is NOT related to foot and mouth disease of animals.
Click here for images
Who gets hand, foot, and mouth disease?
HFMD mostly occurs in children under 10 years of age with 95% of the cases occurring in toddlers aged under 5 years. However, it can also affect older children/adolescents. Adults, especially those who are immunocompromised , may also be affected. However, HFMD only rarely affects healthy adults.
Hand, foot, and mouth disease is very common. The average annual incidence of HFMD has been reported as 90–2400 cases per 100,000 people in some countries.
What causes hand, foot, and mouth disease?
Enteroviral vesicular stomatitis (HFMD) is usually caused by the Coxsackie virus, most commonly the A16 subtype. It may also be caused by other viruses such as:
- Coxsackie A virus (5, 6, 7, 9, 10)
- Coxsackie B virus (2, 5)
- Enterovirus 71
- Echoviruses.
Enterovirus 71 infection is associated with more severe infections that may involve the heart, lungs, and can also cause inflammation of the lining of the brain ( meningitis ).
Transmission occurs via direct contact with blister fluid or droplets spread from the mouth. It can spread very rapidly among family members or within a school. The virus can be shed in faeces and saliva for several weeks.
What are the clinical features of hand, foot, and mouth disease?
The illness usually begins with one or all of the following: fever , sore throat, loss of appetite, and lethargy. However, many children remain well in themselves despite the rash. The blisters usually appear 1–2 days following the fever.
The incubation period is typically 3–6 days and children remain infectious until the blisters have ruptured and healed (usually 7–10 days).
Skin findings typically include:
- Feel tender
- Evolve over time from flat pink macules to small, elongated, red-greyish blisters
- Are often oval rather than round
- Peel off within a week, without leaving a scar.
- Small blisters ( vesicles ) and ulcers may develop in and/or around the lips and mouth and the back of the throat. These can sometimes be very painful. Oral intake may be significantly impacted, especially in infants and younger children.
- In children with eczema , or past eczema , blisters, flat red macules and papules may develop over other areas of the skin, especially the buttocks and sometimes on the arms, legs, and genital skin.
Atypical HFMD can result in a more widespread rash and blistering. Features may include:
- Red, crusted macules and papules without blistering
- Large blisters ( bulla )
- Targetoid ( bulls-eye , or target-shaped) lesions
- Nail shedding
- Involvement of atypical or unusual sites such as the ears.
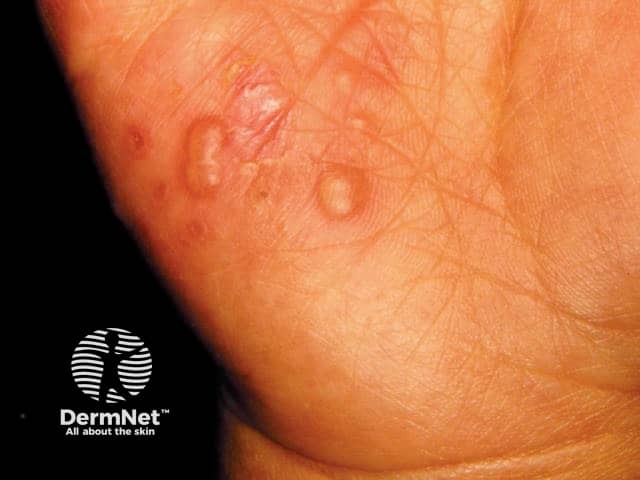
Hand, foot, and mouth disease
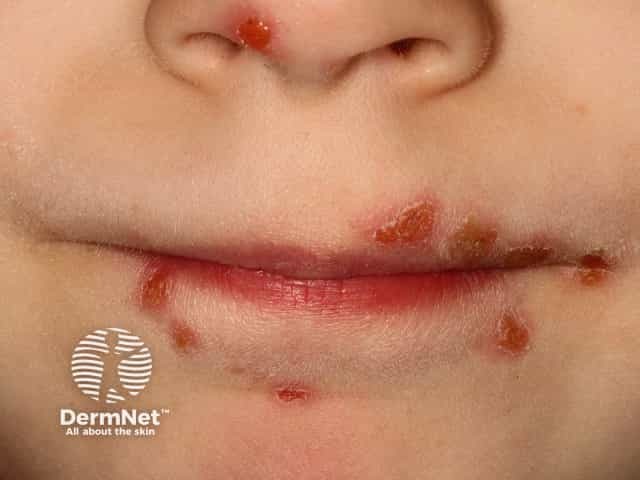
Stomatitis in HFMD
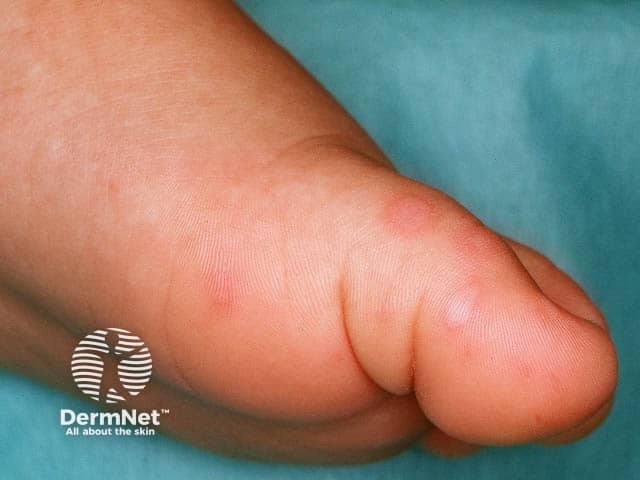
Oval vesicles on the sole in hand, foot and mouth disease
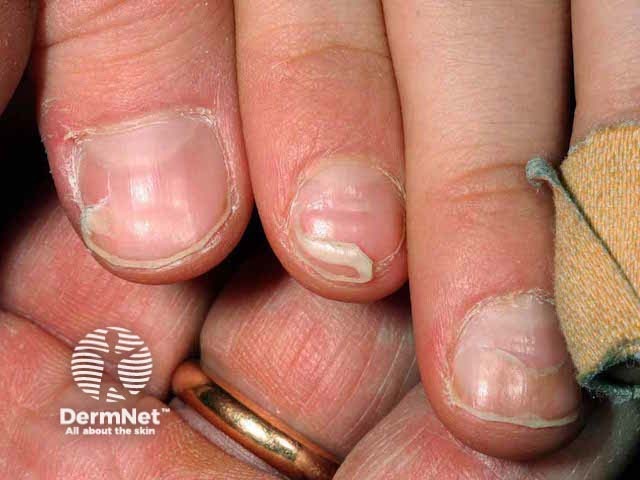
Nail changes noted 6 weeks after hand and mouth blister resolution
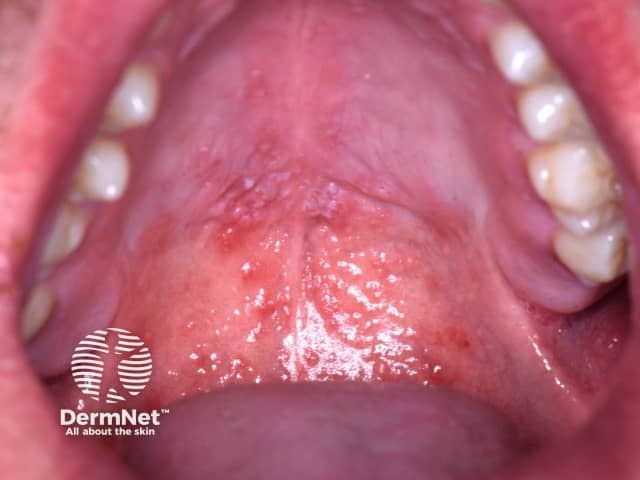
Oral hand, foot, and mouth disease
Click here for more images
How do clinical features vary in differing types of skin?
In children with pre-existing eczema ( atopic dermatitis ), HFMD lesions may be localised in eczematous areas ( eczema coxsackium ).
What are the complications of hand, foot, and mouth disease?
Severe complications are very uncommon in people that are otherwise healthy. They include:
- Dehydration due to inadequate fluid intake. This can cause significant problems in younger children.
- Transverse lines in the nail plate that slowly move outwards
- Onychomadesis (nail shedding) may occur about 2 months after the illness, however, eventually the nails return to normal.
Serious enteroviral infection can lead to:
- Widespread blistering
- Myocarditis
- Inflammation of the brain and or the lining of the brain (meningoencephalitis)
- Loss of nerve function in a limb ( acute flaccid paralysis )
- Pulmonary oedema and pneumonia
- Haemorrhagic conjunctivitis
- In pregnancy, viruses that cause HFMD can cause first trimester spontaneous miscarriage or intrauterine growth restriction
- Meningoencephalitis, thrombocytopenia , disseminated intravascular coagulopathy , cardiomyopathy and hepatitis in the newborn have rarely been described.
How is hand, foot, and mouth disease diagnosed?
HFMD is usually diagnosed clinically. Cutaneous lesions are typically distributed symmetrically over common sites of the skin such as the hands, feet, and in and around the mouth in a child.
Other diagnostic tools include:
- Viral DNA may be detected from nasopharyngeal , (throat or nose), swabs and stool specimens.
- Analysis of blood, cerebrospinal fluid (CSF), and faeces samples can confirm the diagnosis, but are rarely needed except in atypical or severe cases.
- Very rarely indicated.
- Shows acral skin with lymphocytic infiltrates at the epidermis.
- The infiltrate is associated with keratinocyte apoptosis in early lesions.
See hand, foot, and mouth disease pathology for more information.
What is the differential diagnosis for hand, foot, and mouth disease?
- Bacterial infections: such as Group A Streptococcus and Staphylococcus aureus , may cause similar blistering skin lesions, eg, bullous impetigo .
- Other viral infections such as human parechoviruses, herpes simplex virus , adenoviruses, varicella zoster virus , Epstein-Barr virus , and human herpesvirus 6 and 7.
- Bullous insect bite reactions may also present on the hands and feet in children.
- Pompholyx eczema.
What is the treatment for hand, foot, and mouth disease?
Specific treatment is not usually required for HFMD, and the focus is symptomatic care. HFMD rarely causes serious complications. Antibiotics do not work and should not be given to children with HFMD.
No vaccines or specific antiviral medications are available.
General measures
Pain relief
- Simple analgesia such as paracetamol or ibuprofen as needed.
- Antiseptic mouthwashes or topical soothing agents (eg, lignocaine) can be used in children with painful oral/palatal ulcers.
- Aspirin should not be used routinely due to the risk of Reye syndrome.
- Constantly offer the child sips of water/juice to prevent dehydration.
- If oral intake is poor, nasogastric or intravenous fluids may be indicated.
Blister care
- Leave blisters to dry naturally.
- Do not pierce/rupture the blisters to reduce contagion.
- Keep the blisters clean and apply non-adherent dressings to erosions.
Should children with hand, foot, and mouth disease stay home from school?
In the vast majority of cases, HFMD is a mild illness and there is no need to keep children from school once they are well enough to attend.
The blisters remain infective until they have dried, which is usually within a few days. However, the virus sheds through faecal stools and these remain infective for up to a month after the illness. Therefore, it is impractical to keep children who are well away from school.
General preventative measures include:
- This includes touching their blisters, helping them to blow their nose and changing nappies or helping with toileting.
- Minimise sharing personal items such as cutlery, drinking cups, towels, toothbrushes, and clothing.
What is the outcome for hand, foot, and mouth disease?
HFMD infection is usually mild and complete resolution is seen within 7–10 days. Infection often results in long-term immunity to the specific virus, however a second episode can occur following infection with a different member of the enterovirus group.
Bibliography
- Fong SY, Mori D, Rundi C et al. A five-year retrospective study on the epidemiology of hand, foot and mouth disease in Sabah, Malaysia. Scientific Reports, 2021. doi: https://doi.org/10.1038/s41598-021-96083-3. PubMed Central
- Frydenberg A, Starr M. Theme: School contagions: Hand, foot and mouth disease. Australian Family Physician, Vol. 32, No.8, August 2003. Journal
- Hong J, Liu F, Qi H, et al. Changing epidermiology of hand, foot and mouth disease in China, 2013-2019: a population-based study. The Lancet Regional Health Western Pacific, 2022. doi:https://doi.org/10.1016/j.lanwpc.2021.100370. Journal
- Saguil A, Kane SF, Lauters R, Mercado MG. Hand-Foot-and-Mouth Disease: Rapid Evidence Review. Am Fam Physician. 2019;100(7):408–414. Journal
- Hand, foot, and mouth disease pathology
- Hand, foot, and mouth disease images
- Enteroviral infections
- Eczema coxsackium
- Blistering skin conditions
- Herpes simplex
- Viral skin infections
Other websites
- Hand, foot and mouth disease — Royal Children's Hospital Melbourne
- Hand, Foot, and Mouth Disease (HFMD) — Centers for Disease Control and Prevention
- Hand, foot and mouth disease — raisingchildren.net.au
- Hand, foot and mouth disease — Health Navigator New Zealand
Books about skin diseases
- Books about the skin
- Dermatology Made Easy - second edition
Other recommended articles
Select a Community
- MB 1 Preclinical Medical Students
- MB 2/3 Clinical Medical Students
- ORTHO Orthopaedic Surgery
Are you sure you want to trigger topic in your Anconeus AI algorithm?
You are done for today with this topic.
Would you like to start learning session with this topic items scheduled for future?
Coxsackievirus
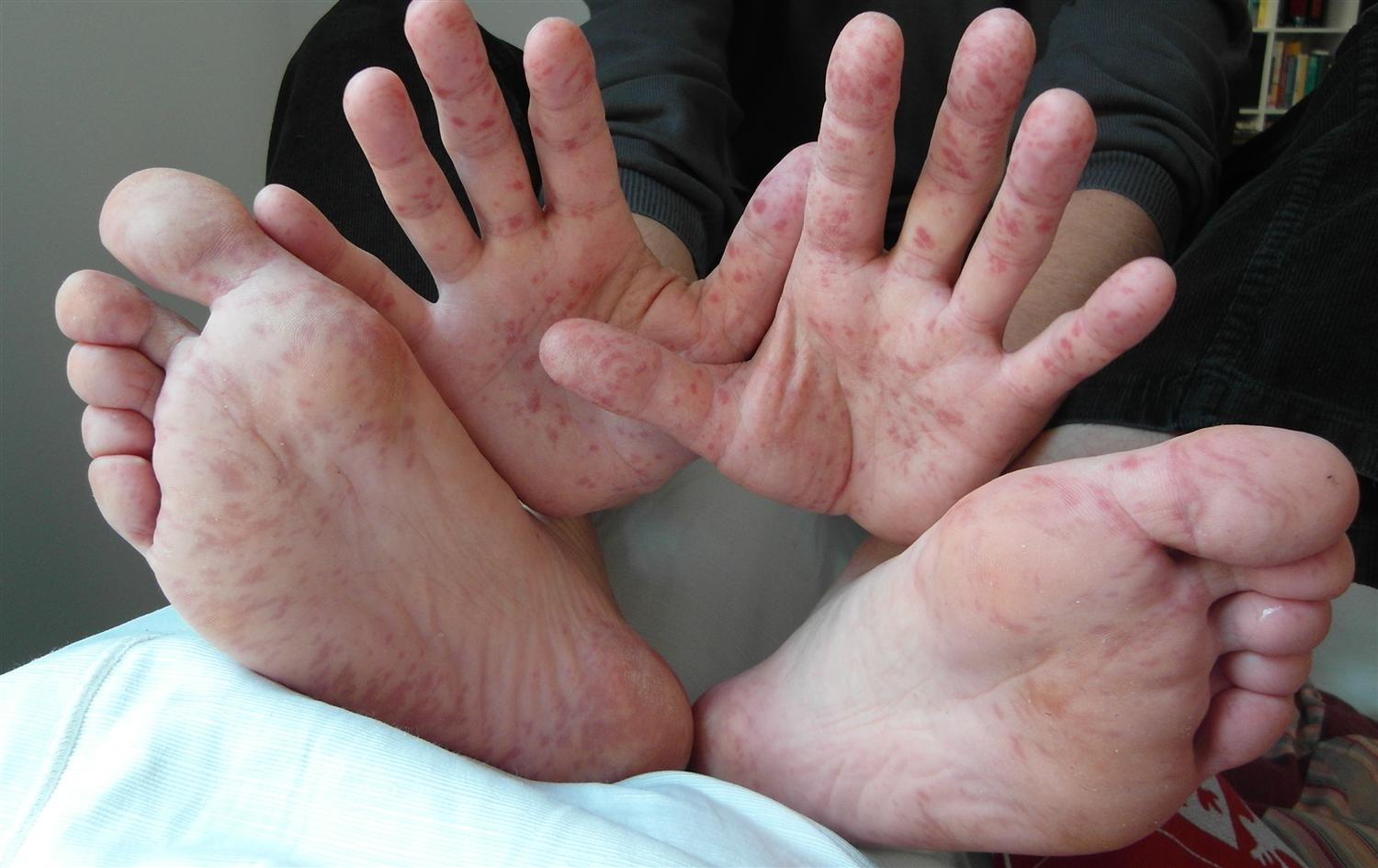
- A 16-year-old boy presents to the emergency room for chest pain and shortness of breath. He has no history of congenital heart disease. He recently attended a month-long camp with other teenagers, several of whom had viral illnesses. On physical exam, he has marked tachycardia and a low-grade fever. An echo shows left ventricular dysfunction and a cardiac magnetic resonance imaging reveals myocarditis. Serology confirms that the etiology is viral.
- an enterovirus that belongs to the picornavirus family
- non-enveloped, icosahedral capsid, linear, single-stranded, positive-sense RNA virus
- transmission via oral secretions or feces
- hand, foot, and mouth disease
- most commonly causes nonspecific prodrome
- myocarditis
- pericarditis
- common in children
- more common in children
- exposure to others with the virus
- daycare centers
- poor hygiene
- finger sucking
- once infected, the virus will travel to the lymph nodes and incubate, causing a prodrome
- typically spontaneously resolves
- vesicles on the palms and soles
- may also be erythematous papules
- vesicles and ulcers in oral mucosa
- ulcers and vesicles in oral mucosa
- heart failure
- arrhythmias
- tachycardia out of proportion to fever
- relieved by sitting up and forward
- friction rub on exam
- coxsackievirus-specific immunoglobulin A
- viral culture
- based on clinical presentation but may be confirmed with laboratory studies
- also presents with rash on palms and soles but is often not macular and not vesicular in nature
- itchy vesicular rash that typically does not occur on the palms, soles, and oral mucosa
- mainstay of treatment is supportive care and treating any organ failure (such as heart failure in myocarditis)
- all patients
- antipyretics
- Aseptic meningitis
- Guillain-Barre syndrome
| Action | Numeric Key | Letter Key | Function Key |
|---|---|---|---|
| Show Bullets | S | Enter (frontside only) | |
| 20% | 1 | N | |
| 40% | 2 | H | |
| 60% | 3 | F | Enter (backside only) |
| 80% | 4 | E | |
| 100% | 5 | M | |
| Previous Card | Left Arrow | ||
| Next Card | N | Right Arrow | |
| Toss | 0 | T |
| Action | Numeric Key | Letter Key | Function Key |
|---|---|---|---|
| Choose 1 | 1 | ||
| Choose 2 | 2 | ||
| Choose 3 | 3 | ||
| Choose 4 | 4 | ||
| Choose 5 | 5 | ||
| Submit Response | Enter | ||
| Previous Question | Left Arrow | ||
| Next Question | N | Right Arrow | |
| Open/Close Bookmode | C | ||
| Open Image | Spacebar |
Please Login to add comment
VisualDx requires the use of cookies. We are checking your browser for cookie support. Please wait a moment while we redirect you.
Investor Relations
News Details
Darzalex faspro®-based quadruplet regimen significantly improves minimal residual disease negativity for newly diagnosed multiple myeloma patients for whom transplant is not planned.
Study of the first and only subcutaneous quadruplet regimen demonstrates 60.9 percent improvement in minimal residual disease (MRD)-negativity and 43 percent reduction in the risk of progression or death
Phase 3 CEPHEUS study results presented in late-breaking oral presentation at the International Myeloma Society (IMS) Annual Meeting
RIO DE JANEIRO , Sept. 27, 2024 /PRNewswire/ -- Johnson & Johnson (NYSE:JNJ) announced today results from the Phase 3 CEPHEUS study demonstrating a significant clinical improvement with DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) in combination with bortezomib, lenalidomide and dexamethasone (D-VRd) in the treatment of patients with newly diagnosed multiple myeloma (NDMM) who are transplant ineligible (TIE) or for whom transplant was not planned as initial therapy (transplant deferred). The data showing significant improvement in minimal residual disease (MRD) negativity rate, progression-free survival (PFS) and complete response (CR) or better rate, were featured as a late-breaking oral presentation at the 2024 International Myeloma Society (IMS) Annual Meeting (Abstract #OA — 63).

CEPHEUS is an ongoing, multicenter, randomized, open-label, Phase 3 study evaluating the efficacy and safety of D-VRd compared to bortezomib, lenalidomide and dexamethasone (VRd) for NDMM patients for whom transplant was not planned as initial therapy (TIE or deferred). Results show that treatment with D-VRd resulted in deeper responses, including MRD-negativity, compared with VRd. At a median follow-up of 58.7 months, the primary endpoint was met, with overall MRD-negativity rate at a sensitivity of 10 -5 (no cancer cells detected within 100,000 bone marrow cells) of 60.9 percent for patients receiving D-VRd and 39.4 percent for VRd (OR [odds ratio], 2.37; 95% confidence interval [CI], 1.58-3.55; P <0.0001). The proportion of patients achieving sustained MRD-negativity of ≥ 12 months almost doubled with D-VRd vs VRd (48.7 percent vs 26.3 percent; P< 0.0001). The study also demonstrated that D-VRd significantly reduced the risk of progression or death by 43 percent (HR [hazard ratio], 0.57; 95% CI, 0.41-0.79; P< 0.0005) vs VRd. The median PFS was not reached for D-VRd vs 52.6 months for VRd. 1
"The CEPHEUS study results show that 60 percent of patients achieved MRD negativity, which is clinically important for physicians treating patients with multiple myeloma and, in general, a strong predictor of improved long-term outcomes, including progression free survival and overall survival," said Saad Z. Usmani, M.D., F.A.C.P., Chief, Myeloma Service, Memorial Sloan Kettering Cancer Center and study investigator.* "The subcutaneous daratumumab-based quadruplet regimen has compelling efficacy characterized by deep, durable responses and reduced risk of disease progression in the frontline population of patients not undergoing transplant, supporting the potential of this quadruplet to become a new regimen in this treatment setting."
The DARZALEX FASPRO ® -based quadruplet regimen, compared to VRd, also significantly increased the depth of response with higher rates of CR or better. The CR or better rate was 81.2 percent with D-VRd vs 61.6 percent with VRd ( P <0.0001). Overall survival data are not yet mature. The overall safety profile of D-VRd was consistent with the known safety profiles for DARZALEX FASPRO ® and VRd. The most common (>10 percent) Grade 3/4 hematologic and non-hematologic adverse events with D-VRd vs VRd were neutropenia (44.2 percent vs 29.7 percent), thrombocytopenia (28.4 percent vs 20.0 percent), anemia (13.2 percent vs 11.8 percent), peripheral neuropathies (8.1 percent vs 8.2 percent), diarrhea (12.2 percent vs 9.2 percent), and COVID-19 (11.2 percent vs 4.6 percent). 1
"Data from PERSEUS and now CEPHEUS add to the body of evidence illustrating how the DARZALEX FASPRO ® -based quadruplet regimen has the potential to be a foundational frontline therapy across all patient types during first-line treatment, regardless of transplant eligibility status," said Robin Carson, M.D., Global Head, Oncology, Innovative Medicine, Johnson & Johnson. "We look forward to continuing to advance this potential new quadruplet therapy and deliver on our commitment to transforming outcomes for people with multiple myeloma."
About the CEPHEUS Study CEPHEUS ( NCT03652064 ) is an ongoing, multicenter, randomized, open-label, Phase 3 study comparing the efficacy and safety of D-VRd vs VRd in patients with newly diagnosed multiple myeloma for whom autologous stem cell transplant (ASCT) is not planned as initial therapy. The primary endpoint is minimal residual disease (MRD)-negativity rate at a 10 -5 sensitivity threshold. Key secondary endpoints include overall complete response (CR) or better rate, progression-free survival (PFS), and sustained MRD-negative rate at 1 year. The trial has enrolled 396 patients in 13 countries.
About the PERSEUS Study The PERSEUS study ( NCT03710603 ) is being conducted in collaboration with the European Myeloma Network as the sponsor. PERSEUS is an ongoing, randomized, open-label, Phase 3 study comparing the efficacy and safety of D-VRd during induction and consolidation versus VRd during induction and consolidation in patients with NDMM eligible for autologous stem cell transplant (ASCT). Following consolidation, patients received an investigational treatment regimen for maintenance that included DARZALEX FASPRO ® in combination with lenalidomide or lenalidomide alone. The trial was not designed to isolate the effect of DARZALEX FASPRO ® in the maintenance phase of treatment. The efficacy of DARZALEX FASPRO ® in combination with lenalidomide for maintenance has not been established. The primary endpoint is PFS, and secondary endpoints include overall CR or better rate, and overall MRD-negativity (in patients with CR or better). The median age is 61.0 (range, 32-70) years for patients in the D-VRd arm and 59.0 (range, 31-70) years for patients in the VRd arm. The study is being conducted in 14 countries in Europe and Australia.
About Multiple Myeloma Multiple myeloma is a blood cancer that affects a type of white blood cell called plasma cells, which are found in the bone marrow. 2 In multiple myeloma, these malignant plasma cells proliferate and replace normal cells in the bone marrow. 3 Multiple myeloma is the second most common blood cancer worldwide and remains an incurable disease. 4 In 2024, it is estimated that more than 35,000 people will be diagnosed with multiple myeloma in the U.S. and more than 12,000 will die from the disease. 5 People with multiple myeloma have a 5-year survival rate of 59.8 percent. 5 While some people diagnosed with multiple myeloma initially have no symptoms, most patients are diagnosed due to symptoms that can include bone fracture or pain, low red blood cell counts, tiredness, high calcium levels, kidney problems or infections. 6 , 7
About DARZALEX FASPRO ® DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) received U.S. FDA approval in May 2020 and is approved for nine indications in multiple myeloma, four of which are for frontline treatment in newly diagnosed patients who are transplant eligible or ineligible. 1 It is the only subcutaneous CD38-directed antibody approved to treat patients with multiple myeloma. DARZALEX FASPRO ® is co-formulated with recombinant human hyaluronidase PH20 (rHuPH20), Halozyme's ENHANZE ® drug delivery technology.
In August 2012 , Janssen Biotech, Inc. and Genmab A/S entered a worldwide agreement, which granted Janssen an exclusive license to develop, manufacture and commercialize daratumumab.
For more information, visit https://www.darzalexhcp.com .
DARZALEX FASPRO ® INDICATIONS AND IMPORTANT SAFETY INFORMATION
INDICATIONS
DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) is indicated for the treatment of adult patients with multiple myeloma:
- In combination with bortezomib, lenalidomide, and dexamethasone for induction and consolidation in newly diagnosed patients who are eligible for autologous stem cell transplant
- In combination with bortezomib, melphalan, and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant
- In combination with lenalidomide and dexamethasone in newly diagnosed patients who are ineligible for autologous stem cell transplant and in patients with relapsed or refractory multiple myeloma who have received at least one prior therapy
- In combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients who are eligible for autologous stem cell transplant
- In combination with pomalidomide and dexamethasone in patients who have received at least one prior line of therapy including lenalidomide and a proteasome inhibitor (PI)
- In combination with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy
- In combination with bortezomib and dexamethasone in patients who have received at least one prior therapy
- As monotherapy in patients who have received at least three prior lines of therapy including a PI and an immunomodulatory agent or who are double refractory to a PI and an immunomodulatory agent
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS DARZALEX FASPRO ® is contraindicated in patients with a history of severe hypersensitivity to daratumumab, hyaluronidase, or any of the components of the formulation.
WARNINGS AND PRECAUTIONS
Hypersensitivity and Other Administration Reactions Both systemic administration-related reactions, including severe or life-threatening reactions, and local injection-site reactions can occur with DARZALEX FASPRO ® . Fatal reactions have been reported with daratumumab-containing products, including DARZALEX FASPRO ® .
Systemic Reactions In a pooled safety population of 1249 patients with multiple myeloma (N=1056) or light chain (AL) amyloidosis (N=193) who received DARZALEX FASPRO ® as monotherapy or in combination, 7% of patients experienced a systemic administration-related reaction (Grade 2: 3.2%, Grade 3: 0.7%, Grade 4: 0.1%). Systemic administration-related reactions occurred in 7% of patients with the first injection, 0.2% with the second injection, and cumulatively 1% with subsequent injections. The median time to onset was 2.9 hours (range: 5 minutes to 3.5 days). Of the 165 systemic administration-related reactions that occurred in 93 patients, 144 (87%) occurred on the day of DARZALEX FASPRO ® administration. Delayed systemic administration-related reactions have occurred in 1% of the patients.
Severe reactions included hypoxia, dyspnea, hypertension, tachycardia, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Other signs and symptoms of systemic administration-related reactions may include respiratory symptoms, such as bronchospasm, nasal congestion, cough, throat irritation, allergic rhinitis, and wheezing, as well as anaphylactic reaction, pyrexia, chest pain, pruritus, chills, vomiting, nausea, hypotension, and blurred vision.
Pre-medicate patients with histamine-1 receptor antagonist, acetaminophen, and corticosteroids. Monitor patients for systemic administration-related reactions, especially following the first and second injections. For anaphylactic reaction or life-threatening (Grade 4) administration-related reactions, immediately and permanently discontinue DARZALEX FASPRO ® . Consider administering corticosteroids and other medications after the administration of DARZALEX FASPRO ® depending on dosing regimen and medical history to minimize the risk of delayed (defined as occurring the day after administration) systemic administration-related reactions.
Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with daratumumab-containing products. If ocular symptoms occur, interrupt DARZALEX FASPRO ® and seek immediate ophthalmologic evaluation prior to restarting DARZALEX FASPRO ® .
Local Reactions In this pooled safety population, injection-site reactions occurred in 7% of patients, including Grade 2 reactions in 0.8%. The most frequent (>1%) injection-site reaction was injection-site erythema. These local reactions occurred a median of 5 minutes (range: 0 minutes to 6.5 days) after starting administration of DARZALEX FASPRO ® . Monitor for local reactions and consider symptomatic management.
Neutropenia Daratumumab may increase neutropenia induced by background therapy. Monitor complete blood cell counts periodically during treatment according to manufacturer's prescribing information for background therapies. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX FASPRO ® until recovery of neutrophils. In lower body weight patients receiving DARZALEX FASPRO ® , higher rates of Grade 3-4 neutropenia were observed.
Thrombocytopenia Daratumumab may increase thrombocytopenia induced by background therapy. Monitor complete blood cell counts periodically during treatment according to manufacturer's prescribing information for background therapies. Consider withholding DARZALEX FASPRO ® until recovery of platelets.
Embryo-Fetal Toxicity Based on the mechanism of action, DARZALEX FASPRO ® can cause fetal harm when administered to a pregnant woman. DARZALEX FASPRO ® may cause depletion of fetal immune cells and decreased bone density. Advise pregnant women of the potential risk to a fetus. Advise females with reproductive potential to use effective contraception during treatment with DARZALEX FASPRO ® and for 3 months after the last dose.
The combination of DARZALEX FASPRO ® with lenalidomide, thalidomide, or pomalidomide is contraindicated in pregnant women because lenalidomide, thalidomide, and pomalidomide may cause birth defects and death of the unborn child. Refer to the lenalidomide, thalidomide, or pomalidomide prescribing information on use during pregnancy.
Interference With Serological Testing Daratumumab binds to CD38 on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test). Daratumumab-mediated positive indirect antiglobulin test may persist for up to 6 months after the last daratumumab administration. Daratumumab bound to RBCs masks detection of antibodies to minor antigens in the patient's serum. The determination of a patient's ABO and Rh blood type are not impacted.
Notify blood transfusion centers of this interference with serological testing and inform blood banks that a patient has received DARZALEX FASPRO ® . Type and screen patients prior to starting DARZALEX FASPRO ® .
Interference With Determination of Complete Response Daratumumab is a human immunoglobulin G (IgG) kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein. This interference can impact the determination of complete response and of disease progression in some DARZALEX FASPRO ® -treated patients with IgG kappa myeloma protein.
ADVERSE REACTIONS In multiple myeloma, the most common adverse reaction (≥20%) with DARZALEX FASPRO ® monotherapy is upper respiratory tract infection. The most common adverse reactions with combination therapy (≥20% for any combination) include fatigue, nausea, diarrhea, dyspnea, insomnia, headache, pyrexia, cough, muscle spasms, back pain, vomiting, hypertension, upper respiratory tract infection, peripheral neuropathy, peripheral sensory neuropathy, constipation, pneumonia, edema, peripheral edema, musculoskeletal pain, and rash.
The most common hematology laboratory abnormalities (≥40%) with DARZALEX FASPRO ® are decreased leukocytes, decreased lymphocytes, decreased neutrophils, decreased platelets, and decreased hemoglobin.
Please click here to read full Prescribing Information for DARZALEX FASPRO ® .
About Johnson & Johnson At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more at https://www.jnj.com/ or at www.janssen.com/johnson-johnson-innovative-medicine . Follow us at @JanssenUS and @JNJInnovMed . Janssen Research & Development, LLC and Janssen Biotech, Inc. are both Johnson & Johnson companies.
Cautions Concerning Forward-Looking Statements This press release contains "forward-looking statements" as defined in the Private Securities Litigation Reform Act of 1995 regarding product development and the potential benefits and treatment impact of DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) . The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Janssen Research & Development, LLC, Janssen Biotech, Inc. and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; manufacturing difficulties and delays; competition, including technological advances, new products and patents attained by competitors; challenges to patents; product efficacy or safety concerns resulting in product recalls or regulatory action; changes in behavior and spending patterns of purchasers of health care products and services; changes to applicable laws and regulations, including global health care reforms; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, including in the sections captioned "Cautionary Note Regarding Forward-Looking Statements" and "Item 1A. Risk Factors," and in Johnson & Johnson's subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov , www.jnj.com or on request from Johnson & Johnson. None of Janssen Research & Development, LLC, Janssen Biotech, Inc., nor Johnson & Johnson undertake to update any forward-looking statement as a result of new information or future events or developments.
*Dr. Saad Z. Usmani has provided consulting, advisory, and speaking services to Johnson & Johnson; he has not been paid for any media work.
1 Usmani, S., et al. Daratumumab SC + Bortezomib/Lenalidomide/Dexamethasone in Patients With Transplant-ineligible or Transplant-deferred Newly Diagnosed Multiple Myeloma: Results of the Phase 3 CEPHEUS Study. IMS 2024. September 27, 2024. 2 Rajkumar SV. Multiple Myeloma: 2020 Update on Diagnosis, Risk-Stratification and Management . Am J Hematol . 2020;95(5):548-5672020;95(5):548-567. http://www.ncbi.nlm.nih.gov/pubmed/32212178 3 National Cancer Institute. Plasma Cell Neoplasms. Accessed July 2024. Available at: https://www.cancer.gov/types/myeloma/patient/myeloma-treatment-pdq 4 Multiple Myeloma. City of Hope, 2022. Multiple Myeloma: Causes, Symptoms & Treatments. Accessed July 2024. Available at: https://www.cancercenter.com/cancer-types/multiple-myeloma 5 American Cancer Society. Myeloma Cancer Statistics. Accessed July 2024. Available at: https://cancerstatisticscenter.cancer.org/types/myeloma 6 American Cancer Society. What is Multiple Myeloma? Accessed July 2024. Available at: https://www.cancer.org/cancer/multiple-myeloma/about/what-is-multiple-myeloma.html 7 American Cancer Society. Multiple Myeloma Early Detection, Diagnosis, and Staging. Accessed July 2024. Available at: https://www.cancer.org/cancer/types/multiple-myeloma/detection-diagnosis-staging/detection.html
SOURCE Johnson & Johnson
Multimedia Files:
Contact Investor Relations
Questions please contact us:.
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Viral infections of the oral mucosa.
Coral A. Ruiz-Mojica ; Melina Brizuela .
Affiliations
Last Update: March 19, 2023 .
- Continuing Education Activity
The oral cavity is particularly susceptible to the manifestations of viral illnesses. The clinical aspect of viral lesions and the possible causative microorganism is diverse. Thorough knowledge of the physical findings is essential to establish an accurate diagnosis, as timely diagnosis and management limit potential complications. Identifying oral lesions that may be the initial signs of an immunocompromised state is vital and warrant further workup. This activity reviews the evaluation and treatment of oral lesions of viral etiology and highlights the role of the healthcare team in managing patients with such conditions.
- Describe the etiology of common oral manifestations due to viral illness.
- Review the physical exam findings associated with viral lesions of the oral mucosa.
- Summarize the common complications of common viral infections of the oral mucosa.
- Outline the treatment of viral infections of the oral mucosa.
- Introduction
The oral cavity is particularly susceptible to the manifestations of viral illnesses. [1] Viral infections often have a subclinical course, and clinical manifestations in the form of oral lesions result from viral cellular destruction or an immune response to viral proteins. [2]
Oral lesions associated with viral conditions are encountered in daily practice by a wide range of healthcare providers, including general practitioners, dentists, otolaryngologists, and dermatologists. [3] Such lesions may pose a diagnostic challenge, as the clinical presentation and the possible causative microorganisms are extensive. [4] DNA viruses, including members of the Herpesviridae , Papillomaviridae , and Poxviridae families, are known to cause oral lesions. RNA viruses, including enteroviruses and paramyxoviruses, can also affect the oral cavity.
Establishing a definitive diagnosis is sometimes tricky. [2] Timely recognition of the lesions reduces the risk of complications. This is particularly important in specific patient populations, like those with HIV or AIDS, as the incidence of oral lesions often correlates with the progression of the disease. [5]
DNA Viruses
Herpesviridae
The viruses that belong to the Herpesviridae family are the most common cause of viral infections of the oral cavity. [6] All of the viruses within this family can remain latent and subsequently reactivate to develop a secondary infection. [3] More specifically, eight serotypes are known to produce disease in human populations. These include herpes simplex virus (HSV-1 and HSV-2), varicella-zoster virus (VZV or HHV-3), cytomegalovirus (CMV or HHV-5), human herpes viruses (HHV-6, HHV-7 or HHV-8) and Epstein-Barr virus (HHV-4). [7]
Primary Herpetic Gingivostomatitis (PHGS)
Primary herpetic gingivostomatitis is the primary form of infection with herpes simplex viruses 1 and 2 (HSV-1 and HSV-2). Both HSV-1 and HSV-2 are double-stranded DNA viruses that cause mucocutaneous lesions on the oral and genital mucosa. [7] [2] Although HSV-2 is classically known to cause genital infection, it may also manifest in the oral cavity. [3] [8] The most common form of transmission is contact with either oral secretions or mucocutaneous lesions. [9] [4] Viral shedding may occur despite the absence of physical lesions. [10]
Herpes Labialis
Herpes labialis is the secondary infection with herpes simplex viruses, occurring in about 40% of infected individuals. [1] It results from the reactivation of the dormant virus in the trigeminal ganglion, [3] which can be precipitated by trauma, stress, immunosuppression, and sunlight exposure. [6]
Chicken Pox
Chickenpox is the primary infection associated with the varicella-zoster virus (VZV or HHV-3). [6] The condition is more often seen in pediatric populations. [9] Transmission of viral particles usually occurs via respiratory droplets or contact with infected lesions. [6]
Shingles is caused by a secondary infection with the varicella-zoster virus. It occurs due to the reactivation of the dormant virus at the dorsal root ganglion of the spinal nerves, which may occur in immunosuppressed states. [2] [3]
Infectious Mononucleosis
Mononucleosis is caused by the Epstein-Barr virus (EBV or HHV-4). The Epstein-Barr virus is associated with primary infections but also neoplastic processes. [11] The pathogenesis of mononucleosis primarily involves the infection of B-cells in the oropharyngeal mucosa, where the virus may remain latent. [8] [2] [4]
The primary transmission mode is via close contact with oral secretions due to viral shedding in saliva. [2] The most common clinical manifestation of infectious mononucleosis, also referred to as "kissing disease" or "glandular fever," is most often seen in adolescent patients. [3]
Oral Hairy Leukoplakia
Oral hairy leukoplakia is the most common oral lesion caused by the Epstein-Barr virus in patients with AIDS - usually observed with CD4 counts lower than 200 to 300/mm^3. [5] [12] It usually occurs in men and may represent the first sign of HIV infection. [4] [12] It is thought to be caused by the replication of viral particles in the mucosal keratinocytes. [13] [5]
Cytomegalovirus
Cytomegalovirus is also a member of the Herpesviridae family and is otherwise known as HHV-5. [5] Viral transmission occurs through the exchange of body fluids or infected blood products. It may cross the placental barrier and, thus, lead to congenital disease. Most cases are asymptomatic, particularly in immunocompetent hosts. [5] However, some immunocompromised individuals may experience chronic oral mucosal ulcerations. [14] [5]
Kaposi Sarcoma
Kaposi sarcoma is the most prevalent malignancy seen in untreated HIV patients, and it is associated with the human herpesvirus 8 (HHV-8). [15] It is important to note that it is most often observed in immunocompromised patients. [2]
Papillomaviridae
Human Papilloma Virus
The human papillomavirus (HPV) is a double-stranded DNA virus that may lead to benign, premalignant, or malignant manifestations in the oral mucosa. [16] Approximately 25 strains have been demonstrated to affect the oral mucosa; however, most subtypes have a low risk of oncogenesis. [1] Transmission occurs primarily through oral or genital contact. [1]
Verruca Vulgaris
Verruca vulgaris is a benign lesion also referred to as the common wart, and it is often caused by HPV subtypes 2 and 57. [8]
Oral Squamous Papilloma
Oral squamous papillomas are the most common growth in the oral cavity. [1] They are benign lesions associated with infection with HPV subtypes 6 and 11. [1] They are often indistinguishable clinically from verruca vulgaris, and thus, differentiation often relies on HPV subtyping. [3] These lesions may be transmitted between sites by autoinoculation. [3]
Condyloma Acuminatum
Condyloma acuminata, also known as genital warts, are caused by the human papillomavirus, mainly subtypes 6 and 11. [17] These lesions are transmitted by sexual contact; hence, they are primarily seen in the genitalia. Oral lesions are due to genital-oral transmission or autoinoculation. [16]
Heck Disease
Heck disease, also known as focal or multifocal epithelial hyperplasia, is caused by HPV infection with subtypes 13 and 32. [14] It is a benign condition that may be seen in adults and children. [3] [11]
Molluscum Contagiosum
Molluscum contagiosum is caused by a DNA virus known as Poxvirus . [8] It is often seen in immunocompromised patients and leads to characteristic intraoral lesions accompanying systemic symptoms. [8]
RNA Viruses
Enteroviruses
Enteroviruses, particularly coxsackievirus A and B, are the most common cause of viral infections of the oropharynx. [3] These viral infections usually affect children and cause epidemics every couple of years during summertime. [12]
Herpangina is caused by coxsackievirus A, serotypes explicitly 1-10, 16, and 22. [3] The spread of viral particles occurs through contact with contaminated oral secretions or fecal matter. [3]
Hand, Foot, and Mouth Disease
Hand, foot, and mouth disease is caused by the coxsackie virus A16 or enterovirus 71 and is most often found in children younger than ten. [18] [19] The infection is characterized by a seasonal pattern, with outbreaks in the summer months. [18] The primary transmission mode is through the spread of airborne particles or fecal-oral contamination. [19]
Acute Lymphonodular Pharyngitis
Acute lymphonodular pharyngitis is caused by the coxsackie virus type A serotype 10. [9] The infection is characterized by fever and oral mucosal eruption.
Paramyxoviruses
Measles or Rubeola
Measles, also known as rubeola, is caused by an enveloped RNA virus whose transmission occurs through respiratory droplets. However, the prevalence of measles has significantly decreased due to widespread vaccination. [4]
- Epidemiology
Primary Herpetic Gingivostomatitis (PHGS) and Herpes Labialis
The worldwide infection rates for herpes simplex viruses range between 60 to 90%. [7] The incidence of herpes is slightly increased in populations with low socioeconomic status. [20] Infection with non-genital herpes simplex virus, more commonly HSV-1, has decreased somewhat since the eighties. [21]
The infection is usually acquired in childhood by asymptomatic shedders. [22] Viral shedding in oral secretions is estimated to occur in about 5 to 10% of individuals.
The widespread vaccination for varicella has significantly reduced the incidence of chickenpox and its associated morbidity. [23] In 2014, the World Health Organization estimated that about 4.2 million cases of infection developed significant complications, and 4,200 disease-related deaths occurred annually. [24] The vast majority of infections are seen in children. [25]
The lifetime incidence of shingles is estimated to be about 30% for the general population, increasing slightly in patients above the age of 85. [26] Important patient factors that increase the risk of infection include patients older than 50, systemic immunosuppression, and stress. [27]
Infectious Mononucleosis
Acquisition of the Epstein-Barr virus usually occurs early in childhood and leads to latent infection. It is estimated that greater than 90% of the global population has acquired EBV. [28] The primary infection, also known as infectious mononucleosis, usually presents during adolescence. [29] About 90% of mononucleosis cases are due to EBV infection. [29]
Oral hairy leukoplakia caused by EBV is almost exclusively seen in patients with immunocompromised states, such as those with HIV. [30]
The reported rate of seropositivity to CMV in the United States is estimated to be 50%. A higher prevalence is seen with increasing age, particularly in developing countries where rates may be as high as 100%. [31] CMV is often acquired during childhood but may also be obtained by vertical transmission. [32]
Four variants of Kaposi sarcoma are recognized, each with specific disease prevalence amongst different patient populations. A classic variant is often seen in males of Mediterranean or Eastern European descent. Patient-specific risk factors, such as chronic steroid use and diabetes, may place them at higher risk. [33] An aggressive variant, African-endemic Kaposi Sarcoma, is most often seen in patients from sub-Saharan Africa and is a frequent carcinoma amongst HIV-negative individuals from this geographic location. [33]
As its name suggests, immunosuppression-related Kaposi Sarcoma is seen in patients with chronic immunosuppression, particularly solid-organ transplant recipients. [33] Finally, AIDS-related Kaposi Sarcoma is most often observed in HIV-infected men. However, after the introduction of antiretroviral therapy, the incidence of the disease has decreased. The infection's risk increases as the immunosuppression become more severe, often seen with lower CD4 counts. [33]
Verruca Vulgaris, Oral Squamous Papillomas, Condyloma Acuminatum, and Heck's Disease
The estimated global prevalence of HPV infection is approximately 10%. It is considered the most common sexually transmitted disease, and the highest infection rate is observed in women in their twenties. The prevalence of the disease subsequently decreases with age. [34]
The majority of HPV infections of the oral cavity lead to benign lesions. Squamous papillomas or common warts are most frequently observed in children. Additionally, adults between 30 to 50 years old may also develop oral papillomas. Specific syndromes, such as Down syndrome and Cowden syndrome, have been associated with multiple oral papillomas. [35]
It is worth noting that focal epithelial hyperplasia is associated with a genetic predisposition and is considered an autosomal recessive condition often seen in Native American populations. [35]
Molluscum contagiosum
Molluscum contagiosum mainly develops in pediatric patients. [36] In the US, children between 1 to 4 years old are the most affected by the poxvirus infection. [37] Infection in adults has also been described and, in most cases, occurs in the genital region; therefore considered to be acquired by sexual transmission. It most often occurs in immunocompromised individuals. The infection rate in HIV patients is estimated to be 20%. [38]
RNA Viruses
Herpangina, Hand, Foot, and Mouth Disease, and Acute Lymphonodular Pharyngitis
Enteroviruses are responsible for various clinical conditions, including HFMD, herpangina, and acute lymphonodular pharyngitis. The patient's age may affect the infection's severity and associated complications. The majority of infections are seen in pediatric patients. [39] Epidemics of Enterovirus infections occur most often in the summer and fall; however, they may happen sporadically year-round too. A higher incidence of viral infection is seen in tropical regions. [39]
The prevalence of measles has reduced significantly since implementing routine childhood vaccination with the MMR (measles, mumps, and rubella) jab. This resulted in disease eradication in 2000. [40] However, recent anti-vaccination movements have led to a resurgence of rubeola infection, with hundreds of cases reported in 2019, primarily in unvaccinated individuals. [40]
- History and Physical
As previously stated, PHGS is the primary infection with the herpes simplex virus (HSV-1 and HSV-2). Symptoms usually develop after five to ten days of incubation. [8] [4] [8] In some patients, the infection is subclinical. [1] [13] When oral manifestations become apparent, the classic finding is a generalized inflammation of the gingiva and associated oral tenderness. [1]
The characteristic oral lesions are small vesicles that break and transform into shallow, painful, gray-yellow ulcers. [2] [3] [6] [12] These lesions often involve the gingiva and buccal mucosa. [4] Intact vesicles are a rare physical exam finding due to constant intraoral friction that leads to rupture. [11]
Oral lesions often accompany systemic symptoms, including fever, sore throat, chills, and lymphadenopathy. [2] [3] [4]
Herpes labialis is the secondary infection with the herpes simplex virus due to the reactivation of the dormant virus. [1] The lesions develop in the perioral region, particularly in the skin of the lips and a vermillion border. [2] However, the keratinized oral mucosa, including the gingiva and palate, may also be affected. [2] See Image. Herpes Labialis.
A burning sensation may precede the eruption. [9] The patient will then subsequently develop areas of erythema and vesicle, which break down and form crusted lesions often referred to as cold sores. [3] [6] [3]
Chickenpox is the primary infection with the varicella-zoster virus. The classic skin lesions are pruritic maculopapular and vesicular eruptions with an erythematous base on the trunk that then spread to extremities. [2] The cutaneous eruption is often suggested to have a "dew drop on a rose petal" appearance. [6] The cutaneous manifestations can be preceded by painless blistering on the palate, uvula, and tonsillar pillars. [2] [4] See Image. Chickenpox (Varicella).
Early signs of shingles are pain or paresthesia affecting a specific dermatome due to the involvement of sensory nerves. [2] This is followed by an eruption of vesicular lesions that often ulcerate and develop overlying crusting. [3] The lesions appear in a specific dermatome with unilateral and linear distributions - characteristic of the condition. [3] Most often, they involve the thoracic or lumbar regions.
Oral involvement is seen when the infection affects the maxillary or mandibular branches of the trigeminal nerve. [4] [6] [12] The prodrome symptom of oral pain caused by shingles infection may be confused with odontalgia leading to incorrect diagnosis or unwarranted medical therapy. [4]
Moreover, a unilateral vesicular eruption on the oral mucosa and external ear may occur and is referred to as Ramsay Hunt Syndrome. It may be accompanied by unilateral facial nerve palsy due to geniculate ganglion involvement. [2] [6]
Infectious Mononucleosis
Even though the infection is asymptomatic in most cases, some patients may experience a triad of fever, reactive adenopathy, and pharyngitis. [2] The oral lesions range from petechia and erythema to necrotizing ulcerative gingivitis. [4] See Image. Infectious Mononucleosis.
Oral hairy leukoplakia is an asymptomatic, white patch with a "hairy" appearance that cannot be scraped off. It usually develops on the lateral borders of the tongue. [5] [4] [8]
Cytomegalovirus
Most cases of cytomegalovirus infection are asymptomatic, particularly in immunocompetent hosts. [5] However, some individuals may develop hepatosplenomegaly, thrombocytopenia, and jaundice. [2] Central nervous system (CNS) involvement has also been described.
Some patients may develop non-specific oral mucosal ulcerations, particularly in cases of coinfection with HSV or immunocompromised status. The ulcerations may become chronic and involve the lips, buccal mucosa, and oropharynx. [14] [5] Usually, these lesions are seen in patients with CD4 counts <100cells/mm3. [12]
The clinical presentation of HHV-8 is characterized by red, blue, or purple macules, nodules, or plaques on the gingiva, hard palate, or tongue's dorsum. The lesions can be single or multiple. [5] [2] [4] Ulceration and bleeding have been described. [5]
Human Papillomavirus
Verruca vulgaris or common warts are benign oral lesions. They are sessile and papillomatous lesions that classically affect the lips, palate, and gingiva. [8]
Papillomas are the most common growth in the oral cavity. [1] They are benign pedunculated, exophytic lesions with a "cauliflower" appearance that may involve the palate, including the uvula and lips. [3] It is clinically indistinguishable from verruca vulgaris. [3]
Condyloma acutimatum lesions are transmitted sexually and have been reported in the oral cavity. [4] [8] Oral lesions present as white-pink papules or plaques with a pebbled appearance involving labial mucosa and palate. [4] [8]
Heck's Disease
Heck's disease is a benign condition that often presents as multiple, painless, white, well-circumscribed papules or plaques in the tongue, labial, and buccal mucosa. [3] [4]
Molluscum contagiosum infection often occurs in immunocompromised individuals. [8] The associated oral lesions are clustered flesh-toned, smooth papules with central umbilication. [8]
Enteroviruses
Herpangina presents as oral vesicular lesions or pseudomembranous ulcers, classically in the posterior oropharynx involving the soft palate and tonsillar pillar. Patients usually report a sore throat. [3]
Hand, foot, and mouth disease is characterized by mucocutaneous lesions and flu-like symptoms, including low-grade fever and generalized malaise. [19] Vesicular lesions or bullae appear within the oral cavity, which may subsequently ulcerate. The oral lesions may precede the skin lesions and involve any site of the oral cavity but tend to affect the buccal mucosa, palate, and tongue. [18] The skin lesions begin as macular erythema and progress to vesicles. The vesicles may burst, ulcerate and become painful or coalesce to form larger lesions. Cutaneous lesions classically appear on the palms and soles in a linear distribution. [18] See Image. Hand, Foot, and Mouth Virus, Lesions.
Acute Lymphonodular Pharyngitis
Acute lymphonodular pharyngitis is usually associated with other symptoms such as fever and sore throat. The oral lesions are often described as yellow or pink nodules in the posterior oropharynx. [18]
Measles is characterized by three stages. The symptoms of the first stage usually include cough and conjunctivitis. Additionally, patients may develop red macules with a blue or white center located on the buccal mucosa, commonly referred to as Koplik spots (see Image. Kiplik Spots). These may precede the second stage by 48 hours. [4] The second stage brings about a cutaneous eruption of a maculopapular rash that spreads centrifugally. Finally, the resolution is seen in the third stage of the disease. [4]
Herpetoviridae
Primary herpetic gingivostomatitis (PHGS)
Diagnosis of primary herpetic gingivostomatitis is based on history and physical exam findings. [3] The lesions often heal spontaneously within two weeks without scarring. [8] If the lesions fail to resolve within ten days, an alternative diagnosis, such as erythema multiforme or disease recurrence, should be considered. [3]
Recurrence in immunocompetent individuals is rare, and underlying conditions such as acute leukemia should be investigated. [3] Although diagnosis is usually clinical, rises in antibody titers to HHV-1 are confirmatory. [11] Additional modalities for diagnostic testing include polymerase chain reaction (PCR) and Tzanck testing. Tzanck smears detect cytopathologic changes in epithelial cells highly suggestive of viral infection. [11] A viral culture may also be used and is often considered the gold standard for diagnosis. However, it is not routinely implemented as it causes delays. [11]
Similar to primary herpetic gingivostomatitis, the diagnosis of herpes labialis is based on clinical findings. The anatomical location of herpes labialis allows for clinical distinction from recurrent aphthous ulcers. [11] However, if the diagnosis is questionable, an additional diagnostic evaluation can be performed, including histologic examination, viral culture, polymerase chain reaction, direct immunofluorescence, and in-situ hybridization. [4]
Diagnosis is usually based on a classic clinical presentation and physical exam findings.
Reactivated herpes zoster infections are diagnosed based on clinical findings. The most typical clinical sign is the unilateral distribution of the lesions along a specific dermatome. [11] Viral cultures, PCR, or serologic testing may allow definitive confirmation if the diagnosis is questionable. [1]
The diagnosis of infectious mononucleosis is established when there is EBV-specific IgM in the serum; this is often performed via a mononuclear spot test (heterophile antibody). [3] A peripheral blood smear may be part of ancillary testing demonstrating abnormal lymphocytes. [3]
Diagnosis of oral hairy leukoplakia is often established clinically. The histopathological evaluation of the lesion may also detect EBV. It may be performed with PCR, direct immunofluorescence, or in situ hybridization techniques. [4] [5] [8]
Cytomegalovirus infection is usually seen in patients with HIV/AIDS with CD4 counts of <100cells/mm3. [12] Diagnosis of CMV infection is generally established with PCR or serologic testing. [4] [11]
Kaposi sarcoma is diagnosed via biopsy, and a PCR identifies the HHV-8. [4] [11]
Oral lesions associated with HPV infection, including verruca vulgaris, squamous papilloma, condyloma acuminatum, and Heck's disease, usually require a biopsy to establish the diagnosis. Specific HPV subtypes are identified with in situ hybridization DNA techniques. [11]
Molluscum contagiosum is diagnosed histopathologically. The lesions demonstrate Henderson-Paterson bodies and inclusion bodies in cytoplasm, which are classic findings. Viral identification in the specimen is then performed with in situ hybridization. [11] [4]
Herpangina, acute lymphonodular pharyngitis, and hand, foot, and mouth disease (HFMD) are diagnosed clinically. Serum antibodies may be present and detected on serologic testing. If the diagnosis is questionable, the virus may be cultured from samples of intact vesicles. [11]
Infection is usually diagnosed according to clinical findings. Confirmation with serologic testing may be helpful if the diagnosis is questionable. [4]
- Treatment / Management
Primary herpetic gingivostomatitis self-resolves after ten to fourteen days and treatment is directed at alleviating symptoms. Patients should be recommended to bed rest, have a soft diet, and increase fluid intake. Oral pain is managed with systemic or topical analgesics, and secondary infection of the lesions can be prevented with chlorhexidine mouthwashes. Immunocompromised or severely unwell patients must be indicated systemic antivirals. [2] Therapy usually consists of acyclovir 200 mg orally five times daily for five days, initiated within 24 to 48 hours of vesicle eruption. [6] [3]
Herpes labialis lesions often heal within two weeks without scar formation. However, topical antiviral medication such as penciclovir or acyclovir 5% cream can be indicated during the prodrome stage to reduce the duration of clinical disease. [2] [3] In immunosuppressed populations, acyclovir is the treatment of choice. Intravenous foscarnet is an alternative therapy for acyclovir-resistant strains. [4]
Treatment of chickenpox consists of supportive measures: over-the-counter analgesia, increased fluid intake, and a healthy lifestyle. Aspirin should be avoided due to the risk of Reye syndrome. [9] The lesions are no longer contagious five to ten days after the initial presentation once complete crusting has occurred. [41]
Shingles infection is treated with supportive measures, and ulcers are expected to heal in approximately three weeks. [12] In immunocompromised patients, treatment for primary infection may require high-dose oral acyclovir. [12]
Infectious Mononucleosis
In most cases, infectious mononucleosis resolves spontaneously; thus, treatment mainly supports patient-specific symptoms. It is essential to avoid oral antibiotics such as amoxicillin or ampicillin due to the risk of developing a cutaneous eruption. [12]
Treatment of oral hairy leukoplakia involves using topical agents such as 0.1% vitamin A and podophyllum. [4] A combination of 25% podophyllin and 5% acyclovir cream is used without lesion recurrence. [42] In HIV patients, antiretroviral therapy must be initiated or adjusted to optimize the immune status. [4]
The recommended antimicrobial therapy for CMV infection is usually intravenous ganciclovir. Foscarnet and cidofovir can also be indicated. [2] Similarly to oral hairy leukoplakia, improving immune status with antiretroviral therapy is essential.
Kaposi sarcoma lesions vary in size and location. Treatment depends on the lesion's specific features and ranges from excision, laser destruction, and sclerosing agents to the use of intralesional chemotherapy and radiation. [5] [4]
Lesions of the oral cavity due to the human papillomavirus , including verruca vulgaris, oral papilloma, and condylomas, require surgical removal. This may be performed with scalpel incision, laser excision, or cryosurgery. [3] [11] The vast majority are likely to recur if an appropriate margin is not obtained. [3] Thus, common warts and oral papillomas are usually excised along the base.
Lesions of focal epithelial hyperplasia or Heck's disease may recur in immunocompromised patients. The recommended therapy includes topical imiquimod 5% cream, cidofovir gel, podofilox solution, or intralesional injection of interferon-alpha. [1]
Lesions associated with molluscum contagiosum are usually removed surgically with cryotherapy or chemical destruction with cantharidin. [43]
The management of most coxsackie virus A infections is mainly supportive. Symptoms are self-limiting and usually resolve spontaneously within seven to ten days. [19]
The incidence of measles infection has significantly reduced after widespread vaccination. [3] However, in cases of infection, the management is mainly supportive.
Treatment with immune serum globulin may be necessary for high-risk populations, including pregnant women, young children, and immunocompromised individuals. It is most effective when administered within six days of exposure to the virus. [4]
- Differential Diagnosis
- Squamous cell carcinoma
- Candidiasis
- Primary or secondary syphilis
- Tuberculosis
- Bacterial pharyngitis
- Pemphigus vulgaris
- Geographic tongue
Most viral infections in the oral cavity resolve spontaneously and are managed with supportive therapy. However, severe untreated conditions may lead to significant comorbidity. Recognizing specific high-risk viral organisms that may impact patients' prognoses is vital. For example, HPV and herpes viruses are synergistic in developing oral malignancy. [2]
Specifically, EBV, HHV-8, and CMV are associated with malignant neoplasms, including lymphomas, nasopharyngeal or gastric carcinomas, Kaposi's sarcoma, and Castleman's disease. [2]
- Complications
Primary herpetic gingivostomatitis (PHGS) and herpes labialis can potentially recur, mainly in immunocompromised patients. The untreated cases lead to the dissemination of the infection. [2]
Additionally, reactivation of HSV-1, particularly in the geniculate ganglion, may lead to Bell palsy. [2] However, the exact mechanism by which the virus causes facial nerve damage remains unclear.
In cases of severe chickenpox infection, patients may experience severe complications, including Reye's syndrome, encephalitis, and Guillan Barre. [2] [3]
Long-term complications of shingles infection include postherpetic neuralgia and severe pain in affected areas after the resolution of the infection. This is due to scarring of the affected sensory nerve. Additionally, patients may experience hyperpigmentation and scarring of affected areas. [2] [3] [1]
Several complications associated with EBV infection are recognized. These include hepatitis, aplastic anemia, or splenic rupture. [2] EBV infection has also been associated with developing African-endemic Burkitt's lymphoma, non-Hodgkins lymphoma, and nasopharyngeal in immunocompromised populations. [14] [5]
Oral hairy leukoplakia is a benign lesion, and malignant transformation has not been described. However, due to the location of lesions and potential size, patients may experience mild complications such as oral burning or pain. [30]
Cytomegalovirus may lead to complications in at-risk populations, e.g., infection in pregnancy can cause fetal transmission and illness. In immunocompromised patients, the infection may disseminate and cause retinitis and blindness. [8]
Potential complications of Kaposi sarcoma include ulceration and associated bleeding. [5]
Human papillomavirus infection and its long-term implications are still not completely understood. Most benign lesions are associated with low-risk HPV subtypes, usually cleared from the oral mucosa with time. [35] Common complications include oral discomfort, accidental bite injuries, and cosmetic concerns. However, a significant potential long-term complication is the persistence of the virus in the oral cavity.
A high-risk subtype, HPV-16, is known to remain in the oral and oropharyngeal mucosa and contribute to HPV-positive oropharyngeal malignancy. The risk of HPV persistence in oral mucosa is particularly increased in smokers. [35]
Generally, the disease resolves within six months, but cases where the condition has remained for several years, have been described. [44] Patients with molluscum contagiosum may experience mild complications such as erythema and swelling of the lesions, known as the BOTE sign. [44]
Additionally, patients with immunodeficiency experience larger and refractory lesions with more extensive involvement of the oral mucosa. Some may also develop eczema molluscorum: eczematous plaques around the lesions. [44]
Oral lesions and pain caused by coxsackie virus infection may result in dehydration. Additionally, those with severe illness may experience neurologic involvement, like encephalitis or aseptic meningitis. [19]
Complications of primary measles infection are rare due to decreased disease prevalence; however, several complications were described before vaccination. These included pneumonia, keratoconjunctivitis leading to blindness, and CNS infections, including acute disseminated encephalomyelitis, measles inclusion body encephalitis, and subacute sclerosing panencephalitis. [45] Such complications were primarily seen in patients with predisposing factors like immunodeficiency or vitamin A deficiency. [45]
- Deterrence and Patient Education
As the information provided in this article suggests, the majority of viral infections of the oral mucosa are benign and self-limiting. However, some conditions may progress and lead to potentially life-threatening complications. Moreover, the development of specific oral lesions of viral nature may suggest undiagnosed immunodeficiency and increase the risk of malignancy.
For this reason, patients should be discouraged from disregarding benign-appearing lesions or mild symptoms. This will allow for adequate treatment initiation, appropriate specialist referral, and optimal disease prevention as warranted.
- Enhancing Healthcare Team Outcomes
Viral infections have a preference for targeting the oral mucosa. [1] The oral lesions are, in many cases, the initial manifestation or even the only sign of disease. Additionally, some oral lesions may suggest more serious underlying conditions such as severe immunodeficiency, and a subgroup of viruses, e.g., HPV 16, may place patients at risk of more severe infections or neoplastic processes. [35]
Patients may experience oral pain and systemic symptoms such as fever, lymphadenopathy, and generalized malaise. These symptoms prompt patients to seek medical attention, usually through a primary care physician, dentist, or otolaryngologist. Potential complications of these conditions, including decreased oral intake and dehydration, may lead to visits to the emergency department.
In immunocompetent individuals, most viral infections of the oral mucosa resolve after two weeks with symptomatic treatment. However, in some cases, an interprofessional team of physicians may be needed to establish a definitive diagnosis, initiate therapy and refer high-risk patients appropriately. This team involves several clinicians (MDs, DOs, NPs, and PAs), including primary physicians, dentists, otolaryngologists, pathologists, nurses, and pharmacists.
While clinicians will determine the overall course of diagnosis and management, nursing will help coordinate communication between the various clinical entities, provide patient counsel, and assist in examinations and monitoring patient progress. Pharmacists will verify appropriate agent selection and dosing, as well as perform medication reconciliation. An infectious disease specialty pharmacist may be necessary in more challenging cases to optimize treatment. Open communication between all interprofessional team members is crucial to optimizing the management of these infections.
Proper coordination and communication of the interprofessional healthcare team will minimize morbidity associated with prolonged, severe, or untreated disease.[Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Chickenpox (Varicella). Chickenpox is seen in an unvaccinated child. Public Health Image Library, Public Domain, Centers for Disease Control and Prevention
Herpes Labialis. Doc James, Public Domain, via Wikimedia Commons
Infectious Mononucleosis. Welleschik, Public Domain, via Wikimedia Commons
Koplik Spots. Lämpel, Public Domain, via Wikimedia Commons
Hand, Foot, and Mouth Virus, Lesions. MidgleyDJ, Public Domain, via Wikimedia Commons
Disclosure: Coral Ruiz-Mojica declares no relevant financial relationships with ineligible companies.
Disclosure: Melina Brizuela declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Ruiz-Mojica CA, Brizuela M. Viral Infections of the Oral Mucosa. [Updated 2023 Mar 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Mechanisms of expression of HHV8, EBV and HPV in selected HIV-associated oral lesions. [Oral Dis. 2002] Review Mechanisms of expression of HHV8, EBV and HPV in selected HIV-associated oral lesions. Hille JJ, Webster-Cyriaque J, Palefski JM, Raab-Traub N. Oral Dis. 2002; 8 Suppl 2:161-8.
- Review Oral manifestations of recently described viral infections, including AIDS. [Curr Opin Dent. 1991] Review Oral manifestations of recently described viral infections, including AIDS. Reichart PA. Curr Opin Dent. 1991 Aug; 1(4):377-83.
- Oral manifestations of AIDS in a heterosexual population in a Zaire hospital. [J Oral Pathol Med. 1990] Oral manifestations of AIDS in a heterosexual population in a Zaire hospital. Tukutuku K, Muyembe-Tamfum L, Kayembe K, Odio W, Kandi K, Ntumba M. J Oral Pathol Med. 1990 May; 19(5):232-4.
- Review [Malpighian epithelia infected by DNA viruses and Langerhans cells]. [Pathol Biol (Paris). 1989] Review [Malpighian epithelia infected by DNA viruses and Langerhans cells]. Chardonnet Y, Viac J, Schmitt D. Pathol Biol (Paris). 1989 Oct; 37(8):927-36.
- Seropositive Neuromyelitis Optica in a Case of Undiagnosed Ankylosing Spondylitis: A Neuro-Rheumatological Conundrum. [Qatar Med J. 2022] Seropositive Neuromyelitis Optica in a Case of Undiagnosed Ankylosing Spondylitis: A Neuro-Rheumatological Conundrum. Ghosh Md R, Roy D, León-Ruiz M, Das S, Dubey S, Benito-León J. Qatar Med J. 2022; 2022(3):29. Epub 2022 Jul 7.
Recent Activity
- Viral Infections of the Oral Mucosa - StatPearls Viral Infections of the Oral Mucosa - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

COMMENTS
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children but can affect adults. The infection usually involves the hands, feet, mouth, and sometimes, even the genitals and buttocks. The cause of hand, foot, and mouth disease is coxsackievirus A type 16 in most cases, but the infection can also be caused by many other strains of coxsackievirus. See ...
History. More than 90% of coxsackieviruses infections are asymptomatic or cause nonspecific febrile illnesses. In neonates, they are the most common cause of febrile illnesses during the summer and fall months. As measured yearround, thirteen percent of newborns with fever in the first month of life were noted to have an enteroviral infection.
Hand-foot-and-mouth disease is a clinical diagnosis based on the presentation of a low-grade fever with a maculopapular or papulovesicular rash on the hands (Figure 1 11) and soles of the feet ...
INTRODUCTION. Hand, foot, and mouth disease (HFMD) is a clinical syndrome characterized by an oral enanthem and a macular, maculopapular, or vesicular rash of the hands and feet (and possibly other locations) [1]. HFMD is one of the most recognizable viral exanthems in children and adults [2]. HFMD was first described in a summer outbreak that ...
Coxsackievirus Group B is a member of the family Picornaviridae, genus Enterovirus. The enterovirus (EV) is a positive-sensed, single-stranded RNA virus named for their enteric, or gastrointestinal route of transmission.[1] Before being reclassified as EV A-D, the enteroviruses were categorized according to their pathogenesis in humans and laboratory animals into four groups, polioviruses ...
Clinical Presentation References. Tariq N, Kyriakopoulos C. Group B Coxsackie Virus. 2022 Jan. [QxMD MEDLINE Link]. . Nikonov OS, Chernykh ES, Garber MB, Nikonova EY. ... Nekoua MP, Alidjinou EK, Hober D. Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. June 1, 2022. 18(8):503-516.
Herpangina causes a sore throat and may give you a high fever and headache. Muscle infections cause periods of sharp spasms between your ribs and upper part of the belly that last 15 to 30 minutes ...
Discussion. HFMD is an uncommon viral disease in adults as a result of cross-immunity with other enteroviruses and immunological memory. However, an increase in cases with atypical clinical presentation and a troublesome diagnosis has been observed in this age group in recent years. 5 Unlike international publications, the cases reported in the present publication were caused by Coxsackievirus ...
Abstract. Hand, foot, and mouth disease (HFMD) and herpangina (HA) are two common childhood viral infections that are characterised by febrile illness, maculopapular rash, and vesicles affecting the mouth, hands, and feet. They are caused by coxsackieviruses which exclusively infect humans and are primarily transmitted via faecal -oral or ...
Coxsackievirus A6 (CV-A6) has been associated with increasingly occurred sporadic hand-foot-mouth disease (HFMD) cases and outbreak events in many countries. ... A review and meta-analysis of the epidemiology and clinical presentation of coxsackievirus A6 causing hand-foot-mouth disease in China and global implications Rev Med Virol. 2020 Mar ...
Coxsackievirus is a member of the Picornaviridae family of viruses in the genus termed Enterovirus.Coxsackieviruses are subtype members of Enterovirus that have a single strand of ribonucleic acid (RNA) for their genetic material. The enteroviruses are also referred to as picornaviruses (pico means "small," so "small RNA viruses").
Diagnosis for these conditions mainly is based on clinical presentation and assessment. Next: Laboratory Studies. Definitive diagnosis of coxsackievirus infection can be made based on isolation of the virus in cell culture. Cytopathic effect usually can be seen within 2 to 6 days. Samples normally are taken from the stool or rectal swabs; the ...
This series demonstrates that there is a wide array of disease presentation of CVA6 associated HFMD in adults. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature ... in adults. Classically, HFMD has been strongly associated with coxsackievirus strain A16 and enterovirus 71 ...
Background: Hand, foot, and mouth disease (HFMD) is generally considered a rare illness in adults. Classically, HFMD has been strongly associated with coxsackievirus strain A16 and enterovirus 71. The coxsackievirus A6 (CVA6) strain has been linked to severe worldwide outbreaks since 2008.
- Enanthem in Coxsackie infection - Hand-foot-and-mouth disease - lip and hand - Hand-foot-and-mouth disease - Buccal - Hand-foot-and-mouth disease - Foot - Hand-foot-and-mouth disease - Tongue RELATED TOPICS. Acute flaccid myelitis; Acute pericarditis: Clinical presentation and diagnosis; Acute viral encephalitis in children: Treatment and prevention
Coxsackievirus Infection and Associated Diseases. Coxsackieviruses (CV) are ubiquitous and widespread single-stranded RNA viruses belonging to the Picornaviridae family and the genus Enterovirus, which also includes poliovirus (PV), the best known of the enteroviruses (EV). They are mainly transmitted by the fecal-oral route and are a major ...
Hand, foot, and mouth disease (HFMD or HFM) is a common, self-limiting, viral infection that causes blisters on the hands, feet, and inside or around the mouth. It mainly affects children under the age of 5 years. HFMD, also called enteroviral vesicular stomatitis, occurs sporadically worldwide. Epidemics are most common during warm weather ...
Classification. coxsackievirus. an enterovirus that belongs to the picornavirus family. non-enveloped, icosahedral capsid, linear, single-stranded, positive-sense RNA virus. transmission via oral secretions or feces. coxsackievirus type A. hand, foot, and mouth disease. herpangina. coxsackievirus type B.
The most common clinical presentation is an undifferentiated febrile illness. All age groups are affected, but neonatal infection with these viruses may progress to severe organ dysfunction and death. ... Most cases of coxsackievirus infection are benign and self-limited, with resolution of fevers within 2-4 days and resolution of rashes within ...
A review and meta-analysis of the epidemiology and clinical presentation of coxsackievirus A6 causing hand-foot-mouth disease in China and global implications. Tian-Shuo Zhao, Tian-Shuo Zhao. ... (CV-A6) has been associated with increasingly occurred sporadic hand-foot-mouth disease (HFMD) cases and outbreak events in many countries. In order ...
1. Introduction. Coxsackievirus group B belongs to the family Picornaviridae and the genus enterovirus. Enteroviruses (EVs) are positive-sense, single-stranded RNA viruses, named for their gastrointestinal route of transmission [].These viruses are categorized based on their pathogenesis in humans and laboratory animals into four groups: polioviruses, coxsackie A viruses (CA), coxsackie B ...
Enterovirus A71 (EV-A71) and Coxsackie virus A16 (CV-A16) are the most common enterovirus serotypes causing HFMD. EV-A71 ... A review and meta-analysis of the epidemiology and clinical presentation of coxsackievirus A6 causing hand-foot-mouth disease in China and global implications. Rev Med Virol. 2020; 30, e2087. Crossref. Scopus (30) Google ...
Study of the first and only subcutaneous quadruplet regimen demonstrates 60.9 percent improvement in minimal residual disease (MRD)-negativity and 43 percent reduction in the risk of progression or death Johnson Johnson (NYSE:JNJ) announced today results from the Phase 3 CEPHEUS study demonstrating a significant clinical improvement with DARZALEX FASPRO® (daratumumab and hyaluronidase-fihj ...
Clinical features of coxsackievirus A4 (CA4), B3 (CB3) and B4 (CB4) infections in children have not been comprehensively described. ... Herpangina (74.8%) was the most common presentation for children with CA4 infection, aseptic meningitis (26.7%) and young infant with fever (23.7%) for those with CB3 infection, and herpangina (32.3%) and ...
فيروس كوكساكي أ (بالإنجليزية: Coxsackie A virus) هو فيروس كوكساكي محلل للخلايا من عائلة الفيروسات البيكورناوية، وهو فيروس معوي (مجموعة تحتوي على فيروسات شلل الأطفال، وفيروسات كوكساكي، وفيروسات ...
The clinical presentation of HHV-8 is characterized by red, blue, or purple macules, nodules, or plaques on the gingiva, hard palate, or tongue's dorsum. ... The management of most coxsackie virus A infections is mainly supportive. Symptoms are self-limiting and usually resolve spontaneously within seven to ten days.