- Corpus ID: 169555558

Technology Transfer from a Technical University: A Case Study of IIT Delhi
- P. Bhattacharya
- Published 1 September 2005
- Engineering, Business
11 Citations
Foundation for innovation and technology transfer (fitt): a case study on industry–academia interface in india, the roadmap for enhancing university–industry research collaboration in india, science, technology, innovation and ip in india: new directions and prospects, towards an integrated model for academia- industry interface in india, university-industry technology transfer: issues and probable remedies, melbourne institute working paper series working.
- Highly Influenced
Indian Universities and their Involvement in Patenting Activity
Recognize the role of academia and industry to match between demand and supply of quality manpower, structural equation modeling ( sem ) approach to identify critical success factors of technology transfer : an empirical analysis from indian context, determining design patent similarity based on the ordinary observer test, one reference, technology transfer and the intellectual property issues emerging from it – an analysis from a developing country perspective, related papers.
Showing 1 through 3 of 0 Related Papers
Tata Electronics and Powerchip Semiconductor Manufacturing Corporation (PSMC) Complete Landmark Agreement for Technology Transfer to Build India's First Semiconductor Fab
News provided by
26 Sep, 2024, 18:41 IST
Share this article
NEW DELHI , Sept. 26, 2024 /PRNewswire/ -- Tata Electronics, a wholly owned subsidiary of Tata Sons Pvt. Ltd, a pioneer in India's electronics manufacturing sector, has announced a significant step forward in its journey to establish India's first semiconductor Fab in Dholera, Gujarat. In a landmark move towards establishing semiconductor manufacturing in India , Tata Electronics has completed the Definitive Agreement with Powerchip Semiconductor Manufacturing Corporation (PSMC) of Taiwan . This pivotal agreement is a cornerstone of Tata Electronics' strategy to bring sophisticated semiconductor manufacturing technology and best practices to the shores of India and serve global customers with a robust and resilient supply chain.
As per the agreement, PSMC will provide design and construction support to build India's first AI-enabled state-of-the-art greenfield Fab in Gujarat, license a broad portfolio of technologies and provide engineering support to successfully transfer licensed technologies to the Gujarat Fab. This Fab will have manufacturing capacity of up to 50,000 wafers per month and will include next-generation factory automation capabilities deploying data analytics and machine learning to achieve industry-best factory efficiency. The new semiconductor Fab will manufacture chips for applications such as power management IC, display drivers, microcontrollers (MCU) and high-performance computing logic, addressing the growing demand in markets such as AI, automotive, computing and data storage, and wireless communication.
This pioneering agreement between Tata Electronics and PSMC will bring to India a portfolio of cutting-edge semiconductor technologies, advanced skill set and talent, and a network of semiconductor manufacturing suppliers and ecosystem partners, setting the foundation for an indigenous semiconductor ecosystem in India . The agreement marks a seminal moment in positioning India as a trusted partner in the global semiconductor supply chain and accelerates the country's journey towards 'Make in India , For the World' as it transforms into a global semiconductor manufacturing hub.
With a total investment of up to INR 91,000 crores ( ~US$11bn ), the Fab will create over 20,000 direct and indirect skilled jobs. With this Fab, India for the first time will be able to address the growing chip demand of domestic and global customers. Tata Group's multi-fab vision for Dholera is projected to create over 1,00,000 skilled jobs.
N Chandrasekaran, Chairman, Tata Sons , said, "We are pleased to partner with PSMC, whose technology and expertise will significantly accelerate our roadmap to pioneer semiconductor manufacturing in India . This collaboration is a key milestone, and I am confident that our comprehensive technology partnership with PSMC will pave the way for innovation, drive growth, and strengthen the global semiconductor supply chain. It will position us to play a key role in the growing semiconductor market to serve global customers."
Dr Frank Huang , Chairman of Powerchip Group and CEO of PSMC, said, "We are excited to collaborate with Tata Electronics on this pioneering initiative to establish India's first semiconductor Fab in Gujarat. It reflects our commitment to providing cutting-edge technology and expertise, helping Tata Electronics create a state-of-the-art facility that will catalyze India's semiconductor landscape. This partnership represents a win-win situation, as it positions PSMC and the Taiwanese ecosystem to gain a significant first-mover advantage in the rapidly expanding Indian market , while helping India achieve self-reliance in semiconductor manufacturing. I strongly believe that our partnership will be foundational to the India - Taiwan collaboration in semiconductors and will inspire more commercial and strategic tie-ups between the two sides."
Notably, Tata Electronics has already engaged two esteemed design firms from Taiwan to create a top-tier Fab that adheres to global standards of quality, safety, and sustainability. The collaboration between Tata Electronics, PSMC and the Taiwanese ecosystem is poised to strengthen the resilience of the global supply chain and effectively cater to the needs of global customers.
About the Tata Group
Founded by Jamsetji Tata in 1868, the Tata Group is a global enterprise headquartered in India , comprising 30 companies across ten verticals. The group operates in more than 100 countries across six continents, with a mission 'To improve the quality of life of the communities we serve globally, through long-term stakeholder value creation based on Leadership with Trust.'
Tata Sons is the principal investment holding company and promoter of Tata companies. Sixty-six percent of the equity share capital of Tata Sons is held by philanthropic trusts, which support education, health, livelihood generation and art and culture.
In 2023-24, the revenue of Tata companies, taken together, was $165 billion . These companies collectively employ over 1 million people.
Each Tata company or enterprise operates independently under the guidance and supervision of its own board of directors. There are 26 publicly listed Tata enterprises with a combined market capitalisation of $365 billion as of March 31, 2024 .
About Powerchip Semiconductor Manufacturing Corporation
Powerchip Semiconductor Manufacturing Corporation (PSMC) is the world's seventh-largest pure-play foundry, with four 12-inch and two 8-inch fabs in Taiwan , capable of producing over 2.1 million 12-inch equivalent wafers annually. Since its establishment in 1994, the company transitioned successfully from DRAM manufacturing to advanced foundry services for memory and logic chips. Ranked seventh in global semiconductor ESG evaluations, PSMC demonstrates strong governance and environmental commitment. In May 2024 , PSMC's new 12-inch fab in Taiwan's Tongluo Science Park began operations with a planned capacity of 1.2 million wafers annually, using advanced 28nm and wafer stacking technologies.
About Tata Electronics Private Limited.
Tata Electronics Pvt. Ltd. is a prominent global player in the electronics manufacturing industry, boasting growing capabilities in Electronics Manufacturing Services, Semiconductor Assembly & Test, Semiconductor Foundry, and Design Services. Established in 2020 as a new initiative of the Tata Group, the company aims to enhance its global customer service through integrated offerings across a trusted electronics and semiconductor value chain. With a rapidly expanding workforce, the company currently employs over 45,000 individuals and has significant operations in Gujarat, Assam, Tamil Nadu, and Karnataka, India. Tata Electronics is committed to creating a socio-economic footprint by employing a large number of women in its workforce and actively supporting local communities through initiatives in healthcare, hygiene, and education.
Contact:
Pooja Rajput [email protected] +91 99102 78452
Modal title
Also from this source, tata group to build the nation's first fab in dholera.
In a significant step towards creating an indigenous ('Make in India, For the World') semiconductor ecosystem in India, Government of India has...

Semiconductors

Computer & Electronics

Electronic Components

The Economic Times daily newspaper is available online now.
Tata electronics and psmc complete agreement for tech transfer to build india’s first semiconductor fab.
The company said the new semiconductor fab will manufacture chips for applications such as power management IC, display drivers, microcontrollers (MCU) and high-performance computing logic, addressing the growing demand in markets such as AI, automotive, computing and data storage, and wireless communication.
Artificial Intelligence(AI)
Java Programming with ChatGPT: Learn using Generative AI
By - Metla Sudha Sekhar, Developer and Lead Instructor

Basics of Generative AI : Unveiling Tomorrow's Innovations

Generative AI for Dynamic Java Web Applications with ChatGPT

Mastering C++ Fundamentals with Generative AI: A Hands-On

Master in Python Language Quickly Using the ChatGPT Open AI

Office Productivity
Zero to Hero in Microsoft Excel: Complete Excel guide 2024

Vastu Shastra Course
By - Sachenkumar Rai, Vastu Shashtri

Data Science
SQL for Data Science along with Data Analytics and Data Visualization

Web Development
A Comprehensive ASP.NET Core MVC 6 Project Guide for 2024

Mastering Microsoft Office: Word, Excel, PowerPoint, and 365

Digital marketing - Wordpress Website Development
By - Shraddha Somani, Digital Marketing Trainer, Consultant, Strategiest and Subject Matter expert
Mastering Full Stack Development: From Frontend to Backend Excellence

Financial Literacy i.e Lets Crack the Billionaire Code
By - CA Rahul Gupta, CA with 10+ years of domain experience, trainer

Business Storytelling Masterclass
By - Ameen Haque, Founder of Storywallahs
Future of Marketing & Branding Masterclass
By - Dr. David Aaker, Professor at Haas School of Business

Human Potential and the Future of Employment
By - Lynda Gratton, Co-chair of the World Economic Forum Council on Work, Wages and Job Creation, Professor of Management Practice

ESG and Business Sustainability Strategy
By - Vipul Arora, Partner, ESG & Climate Solutions at Sattva Consulting Author I Speaker I Thought Leader

Financial Reporting and Analytics
By - Dr. C.P. Gupta, Professor: Department of Finance and Business Economics, University of Delhi
Read More News on

Silver prices are trending higher. Here’s what it means for the long-term investors

How a little-known Delhi-based company bought Nasdaq-listed Ebix out of bankruptcy

Why your SIP investment may work, until one day, it won’t.

The importance of tackling money laundering and terror financing through offshore gaming platforms

Taken to duck Houthi attacks, Cape of Good Hope now gives shipping firms a sinking feeling

5 problems SpiceJet needs to fix to be competitive again
Find this comment offensive?
Choose your reason below and click on the Report button. This will alert our moderators to take action
Reason for reporting:
Your Reason has been Reported to the admin.

To post this comment you must
Log In/Connect with:
Fill in your details:
Will be displayed
Will not be displayed
Share this Comment:
Uh-oh this is an exclusive story available for selected readers only..
Worry not. You’re just a step away.

Prime Account Detected!
It seems like you're already an ETPrime member with
Login using your ET Prime credentials to enjoy all member benefits
Log out of your current logged-in account and log in again using your ET Prime credentials to enjoy all member benefits.
To read full story, subscribe to ET Prime
₹34 per week
Billed annually at ₹2499 ₹1749
Super Saver Sale - Flat 30% Off
On ET Prime Membership
Unlock this story and enjoy all members-only benefits.
Offer Exclusively For You
Save up to Rs. 700/-
ON ET PRIME MEMBERSHIP
Get 1 Year Free
With 1 and 2-Year ET prime membership
Get Flat 40% Off
Then ₹ 1749 for 1 year
ET Prime at ₹ 49 for 1 month
Market Savvy Offer
Get flat 20% off on ETPrime
90 Days Prime access worth Rs999 unlocked for you

Exclusive Economic Times Stories, Editorials & Expert opinion across 20+ sectors
Stock analysis. Market Research. Industry Trends on 4000+ Stocks
Get 1 Year Complimentary Subscription of TOI+ worth Rs.799/-
Stories you might be interested in
- Help & FAQ
Academia-industry technology transfer – a detailed study on indian scenario at global platform
- Department of Pharmaceutical Quality Assurance, Manipal College of Pharmaceutical Sciences, Manipal
- Department of Pharmacy Management, Manipal College of Pharmaceutical Sciences, Manipal
- Technology Transfer Office
Research output : Contribution to journal › Review article › peer-review
Translation of research and innovation from universities or academic institutions to market for societal benefits demand substantial and persistent efforts. This could be achieved through various means and one of the most preferred method of translating university research into commercially viable product is through technology transfer or technology licensing. This urges on establishing technology transfer offices in universities/academia comprising a group of experts from various fields focusing on protecting and commercializing inventions disclosed by faculty members, students or other stakeholders of the organization. This paper discusses various successful strategies and technology transfer models between industry and academia in developed nations as well as a few important success factors. It also provides an insight on evolution of technology transfer practices (governing Acts and legislation) and current status of technology transfer in India.
| Original language | English |
|---|---|
| Pages (from-to) | 4981-4989 |
| Number of pages | 9 |
| Journal | |
| Volume | 13 |
| Issue number | 10 |
| DOIs | |
| Publication status | Published - 12-10-2020 |
All Science Journal Classification (ASJC) codes
- Pharmacology, Toxicology and Pharmaceutics (miscellaneous)
- Pharmacology (medical)
Access to Document
- 10.5958/0974-360X.2020.00873.2
Other files and links
- Link to publication in Scopus
- Link to the citations in Scopus
Fingerprint
- india INIS 100%
- industry INIS 100%
- technology transfer INIS 100%
- Indians Social Sciences 100%
- Technology Transfer Social Sciences 100%
- Specific Industry Economics, Econometrics and Finance 100%
- universities INIS 50%
- Success Factor Economics, Econometrics and Finance 50%
T1 - Academia-industry technology transfer – a detailed study on indian scenario at global platform
AU - Ravi, Ramya
AU - Janodia, Manthan D.
N1 - Funding Information: Collaborate for opportunities for funding to work on their research ideas, entrepreneurship and utilization of resources. Funding Information: (a) Contract research sponsored by industry: Publisher Copyright: © RJPT All right reserved.
PY - 2020/10/12
Y1 - 2020/10/12
N2 - Translation of research and innovation from universities or academic institutions to market for societal benefits demand substantial and persistent efforts. This could be achieved through various means and one of the most preferred method of translating university research into commercially viable product is through technology transfer or technology licensing. This urges on establishing technology transfer offices in universities/academia comprising a group of experts from various fields focusing on protecting and commercializing inventions disclosed by faculty members, students or other stakeholders of the organization. This paper discusses various successful strategies and technology transfer models between industry and academia in developed nations as well as a few important success factors. It also provides an insight on evolution of technology transfer practices (governing Acts and legislation) and current status of technology transfer in India.
AB - Translation of research and innovation from universities or academic institutions to market for societal benefits demand substantial and persistent efforts. This could be achieved through various means and one of the most preferred method of translating university research into commercially viable product is through technology transfer or technology licensing. This urges on establishing technology transfer offices in universities/academia comprising a group of experts from various fields focusing on protecting and commercializing inventions disclosed by faculty members, students or other stakeholders of the organization. This paper discusses various successful strategies and technology transfer models between industry and academia in developed nations as well as a few important success factors. It also provides an insight on evolution of technology transfer practices (governing Acts and legislation) and current status of technology transfer in India.
UR - http://www.scopus.com/inward/record.url?scp=85109259252&partnerID=8YFLogxK
UR - http://www.scopus.com/inward/citedby.url?scp=85109259252&partnerID=8YFLogxK
U2 - 10.5958/0974-360X.2020.00873.2
DO - 10.5958/0974-360X.2020.00873.2
M3 - Review article
AN - SCOPUS:85109259252
SN - 0974-3618
JO - Research Journal of Pharmacy and Technology
JF - Research Journal of Pharmacy and Technology
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
Exploring University-Industry Technology Transfer in India: Two Models

2016, Journal of Research Innovation and Management Science
The purpose of this paper is to understand the influence of policy environment on development of technology transfer in university industry linkage in India. This study reviews literature on design perspectives of university spin offs including large scale survey of Indian universities, cross national comparisons and analysis of documents from professional bodies. There is evidence that policy environment is composed of structures that influence the implementation of a design. There is a policy shift that favoured indigenous state led technology transfer to private partnership in technology transfer in India. The opening of the Indian economy introduced policy environment favouring entrepreneurship. Two models of technology transfer in university-industry are proposed. The type I model is a technology push process that results in an IPR based regime where as the type II is a business pull model that favours university spin offs. Unlike the linear model of growth of technology transfer in the West, there has been a persistent divide between the sub systems of intellectual property and entrepreneurship in India. Research into the environment that designs a policy outcome in academic entrepreneurship may offer a template for a system that co-opts both IPR and entrepreneurship. Indian universities have been analysed for performance based on their traditional role in academics. The non traditional roles like technology transfer have been evaluated only through comparative case studies. This research fills the gap by giving an overview of the Indian scene and proposes theoretical models to understand them.
Related papers
Journal of the Knowledge Economy, 2021
In recent years, there is a great emphasis on transferring inventions and technologies originating from academia to industry through technology transfer/licensing or commercialization. The efforts of the Government of India (GOI) aim to create socially useful innovation through university-industry technology transfer. The objective of the study is to examine and understand enabling factors and barriers for technology transfer among Indian universities. The study covers three key aspects: (1) the awareness and practice of patents and research commercialization among Indian academia, (2) comprehending strategies adapted to commercialize research activities, and (3) barriers in university-industry technology transfer (TT). This paper is an attempt to answer the research question whether current dynamics within Indian universities create an environment for enabling knowledge transfer/commercialization and propose plausible suggestions to enable academia-industry technology transfer. A s...
The Journal of Technology Transfer, 2000
Journal of Entrepreneurship and Management, 2017
Universities acting as catalyst for entrepreneurial activities are central to the phenomenon of academic entrepreneurship. Lack of appropriate academic entrepreneurship models has hindered the smooth transfer of knowledge/technology from university to industry in India. The study explores the synergy between the academia and industry through academic entrepreneurship which is crucial to develop a world class higher education system. This paper attempts to understand the processes and stages of academic entrepreneurship activities besides focussing on education and entrepreneurship in India. Further, it discusses a few models and frameworks which integrate academic entrepreneurship, economic development and education in developing countries. The paper concludes with the argument that it is essential to restructure education/research to enable its integration within the economy which nurtures entrepreneurship.
Asian Journal of Innovation and Policy
Seoul Journal of Economics, 2009
Journal of Entrepreneurship, Management and Innovation (JEMI), Volume 8, Issue 1, 2012: p. 68-83, 2012
In developed countries, the academic entrepreneurship makes up a very important element of academic environment activities. For some time, the increase in the role of technology transfer and knowledge commercialisation has been also promoted in Poland. Strong connections between the scholarship and the economy (in the future, within the university of the third generation) have a chance to build an economy based on knowledge in our country. The flow of knowledge and the introduction of new solutions (results of scholarly research) in enterprises take place through the intermediary of various methods of transfer and commercialisation paths. Independent of the manner, each fulfils an important role in the public life and economy. This is confirmed by the experience of the States that are recognised as innovation leaders, and presented in the paper as examples of Polish scholars’ academic entrepreneurship.
Journal of technology management & innovation, 2012
IAEME PUBLICATION, 2020
The objective of this paper is to study the various Technology Transfers and its commercialization followed by various public funded Research and Development institutions in India. More specifically, this paper also examined and highlighted the challenges faced by the Research and Development institutions and industries. Technology Transfer is the complete process which enables the disclosure of advancement in Science and Technology. Research institutions and their industry partnership in the field of science and technology is complex and needs to be developed through customized model and mechanisms. The protection and the licensing mechanism of intellectual property rights at such institutions are the focus of attention of the policy makers at all levels. Over the last 50 years, Government Research institutions (GRES) have played a major role in developing science and technology in the country. They have supported a variety of government initiatives and functions by proving basic and applied research, technological development and engineering solutions. Successful technological development requires a deep understanding of the technical context of applications and relies on the organizations culture and environment in which scientists, engineers and technicians often coming from different disciplines work and interact. Therefore, an organization’s learning base is not only the sum of its individual employees, there is also addition by the “organizational knowhow’’. In view of the above, a detailed commercialization process followed by Indian Research and Development institutions has been studied and the success of such technology transfer analysis and commercialization has been reviewed with reference to controlling parameters. The effort of technology development is complete only when it is adopted or adapted by the end user as a product suitable for commercial applications. It is most important that the research organization needs to understand industry or work in close collaboration with the industry. To enhance linkages between research institutions and industry, one should augment market driven research and assign value to innovative research. Universities and Research and Development institutions should have initiated technology transfer and commercialization efforts. Public Sector Research and Development institutions should take special initiative to bring forth a multitude of benefits to society and generate a model that needs to be promulgated in emerging economies especially utilizing our indigenous resources. This paper presents major five Indian public funded Research and Development institutions, their Technology Transfer models involving their unique approaches for commercialization of products that assured overall benefit to the society while simultaneously promoting the Indian economy
Referred Chapter in Academic Entrepreneurship and Technological Innovation: A Business Management Perspective
arXiv (Cornell University), 2023
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
CURRENT SCIENCE, 2014
GALAXY INTERNATIONAL INTERDISCIPLINARY RESEARCH JOURNAL (GIIRJ), 2021
Advances in Management and Applied Economics
SSRN Electronic Journal, 2000
Review of International …, 2011
Management and Economics Research Journal, 2019
The Journal of Technology Transfer, 2011
Space and Culture, India
Environmental Economics, 2017
Small Business Economics
Technology Innovation Management Review
Related topics
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Faculty & Research

NEC - India: The Opportunity in the Indian Growth Story (Abridged Version)

V. (Paddy) Padmanabhan
Professor of Marketing

Professor of Strategy

Our website has a lot of features which will not display correctly without Javascript.
Please enable Javascript in your browser
Here how you can do it: http://enable-javascript.com
- Technology Transfer
FDI, Technology Transfer and Spillover —A Case Study of India
- January 2010

- Jawaharlal Nehru University
- This person is not on ResearchGate, or hasn't claimed this research yet.
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Biniam Zemene

- C.H. Sahyaja
- G. Rajeshkumar
- K.S. Sekhararao

- N K Taneja Arjun

- Chi-Mei Lin

- Fredrik Sjoholm

- Ruben Tansini
- Mario Zejan

- Håkan Persson

- Xiaoying Li

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- General publications
Technology Transfer in India
Description.
EU SMEs interested in venturing the Indian market by way of technology transfer arrangement must ensure drawing up a clear and unambiguous contract.

- 21 OCTOBER 2022
Share this page
Browse Econ Literature
- Working papers
- Software components
- Book chapters
- JEL classification
More features
- Subscribe to new research
RePEc Biblio
Author registration.
- Economics Virtual Seminar Calendar NEW!

FDI, Technology Transfer and Spillover —A Case Study of India
- Author & abstract
- 19 References
- 2 Citations
- Most related
- Related works & more
Corrections
(Centre for International Trade and development)
Suggested Citation
Download full text from publisher, references listed on ideas.
Follow serials, authors, keywords & more
Public profiles for Economics researchers
Various research rankings in Economics
RePEc Genealogy
Who was a student of whom, using RePEc
Curated articles & papers on economics topics
Upload your paper to be listed on RePEc and IDEAS
New papers by email
Subscribe to new additions to RePEc
EconAcademics
Blog aggregator for economics research
Cases of plagiarism in Economics
About RePEc
Initiative for open bibliographies in Economics
News about RePEc
Questions about IDEAS and RePEc
RePEc volunteers
Participating archives
Publishers indexing in RePEc
Privacy statement
Found an error or omission?
Opportunities to help RePEc
Get papers listed
Have your research listed on RePEc
Open a RePEc archive
Have your institution's/publisher's output listed on RePEc
Get RePEc data
Use data assembled by RePEc
Advertisement
Factors Affecting Technology Transfer and Commercialization of University Research in India: a Cross-sectional Study
- Open access
- Published: 24 February 2021
- Volume 13 , pages 787–803, ( 2022 )
Cite this article
You have full access to this open access article

- Ramya Ravi 1 &
- Manthan D. Janodia ORCID: orcid.org/0000-0003-0000-9673 2
9745 Accesses
20 Citations
25 Altmetric
Explore all metrics
In recent years, there is a great emphasis on transferring inventions and technologies originating from academia to industry through technology transfer/licensing or commercialization. The efforts of the Government of India (GOI) aim to create socially useful innovation through university-industry technology transfer. The objective of the study is to examine and understand enabling factors and barriers for technology transfer among Indian universities. The study covers three key aspects: (1) the awareness and practice of patents and research commercialization among Indian academia, (2) comprehending strategies adapted to commercialize research activities, and (3) barriers in university-industry technology transfer (TT). This paper is an attempt to answer the research question whether current dynamics within Indian universities create an environment for enabling knowledge transfer/commercialization and propose plausible suggestions to enable academia-industry technology transfer. A self- assessed structured methodology is contemplated and applied. Convenience sampling methods were adopted. Administrators of 25 universities overseeing research and development activities/patent cell/incubation cell or industrial collaboration of universities were approached to participate in the study. Indian universities are categorized as (i) public funded universities and (ii) private institutes for the purpose of the study. It is interesting to understand that public funded universities have an advantage in terms of receiving funds and licensing the research to potential industrial partners. The authors further conclude that research undertaken in academia is far from the demands of the industry. Even though the relevant supporting system for enhancing university-industry collaboration is in place, such as establishing technology transfer office (TTO) in the university, they hardly channelize the resources for socially useful innovation. It is important for Indian academia to undertake commercially viable research for the benefit of society.
Similar content being viewed by others

University-Industry Technology Transfer in India: a Plausible Model Based on Success Stories from the USA, Japan, and Israel
Identifying the key barriers and their interrelationships impeding the university technology transfer in taiwan: a multi-stakeholder perspective.

University technology transfer efficiency in a factor driven economy: the need for a coherent policy in Egypt
Explore related subjects.
- Artificial Intelligence
Avoid common mistakes on your manuscript.
Introduction
Innovation is the direct outcome of structured and planned scheme of work, referred to as research. The competition among the industry is exceedingly independent of the research undertaken by public funded or private research organizations and universities. The main advantage of universities are the faculty members and students who constantly enter the system, bringing fresh concepts, ideas that eventually leads to research and innovation. Accessibility to results obtained from research is the core challenge to rebuild the ideas to innovation and further convert to commercially viable product (European commission, 2009 ). Thus, dissemination of innovation from universities and research institutions to parties capable of commercialization is defined as technology transfer (TT). The main objectives of technology commercializing include leveraging R&D outcome and intellectual assets, raise the accessibility of scientific outcome to broad range of consumers, development of new services and products ready for commercialization, and last but not the least, to intensify industrial competition. The transfer can originate either due to technology push (through research) or market pull (through industry). The international scope of technology commercialization may encompass developed nations, developing nations and other countries with economy transition (Thompson, 2015 ). In the current trend, universities must position themselves as authentic players to raise the chance of success in R&D probably by two aspects: increase in knowledge transfer by using scientific knowledge and raise the business value of knowledge transfer by introducing cutting edge breakthrough technologies (Baron, 2020 ). Though there is enough literature discussing cases of technology transfer from research institutions in developed nations, there are very few instances of knowledge transfer between universities and industries in India. Very few articles discuss technology transfer in Indian academia. Hyndman et al. studied and compared knowledge commercialization practices of Massachusetts Institute of Technology (MIT) with Indian academia. The author emphasized the lack of policy to support commercializing technology in India (Hyndman et al., 2005 ). Comprehending knowledge transfer models, practiced in the USA and Germany, Rath et al. proposed a model suited to Indian scenario that supports fiscal incentives to encourage large-scale industry-academia partnerships. Further, the proposed model emphasized on channelizing the profit, obtained from commercialization of technology originating from university, to R&D (Rath et al., 2014 ). A study by Srivastava et al. stressed upon the importance of creation of companies around academic technologies and job creation to promote the economic growth. Further, the study suggests that one commercialization model may not be successful in all universities, as there are differences in culture, resources, environment, and priorities among universities (Srivastava & Chandra, 2012 ). A review by Pagar et al. explained the significance of continuous exchange of information between industry and academia to maintain the quality of the product transferred (Pagar et al., 2014 ). In the past decade there has been an emphasis on Indian academia to generate revenue from research outcomes, and hence, knowledge transfer/technology transfer becomes an integral part of discussion about Indian academia.
As the availability of literature on the commercialization of technology in Indian academia is limited, this paper explores the state of knowledge exchange by presenting data on Indian universities regarding intellectual property and technology transfer practices in both public and private universities. The NIRF data for the year 2018 has also been used for the purpose of this study. The study covers three key aspects: (1) the awareness and practice of patents and research commercialization among Indian academia, (2) comprehending strategies adapted to commercialize research activities, and (3) barriers in university-industry technology transfer . The paper is an attempt to answer the research question whether the current dynamics within Indian universities create an environment for enabling knowledge transfer/commercialization and proposes plausible suggestions to enable academia-industry technology transfer. The study further aims to comprehend the scenario and recommend strategies to support technology transfer in Indian academia. The method used for the study proposes and implies a self-assessment concerning the activities of innovation and research commercialization capacity. Relevant results obtained from the respondents were cross verified with the publically available data for a few universities through their organization webpage. This allows a qualitative and semi- quantitative approach to the study.
Literature Review
Evolution of technology innovation in india.
The past five decades looks progressive in terms of technology evolution in India, including innovation and research. The first scientific policy, enacted in 1958, emphasized on importance of technology in India. India has a history of success stories among a few research and development organizations. One such initiative is from National Chemical Laboratory (NCL innovations)—a chief laboratory of The Council of Scientific and Industrial Research (CSIR) under the central government of India. In its 80 years of existence, NCL has an impressive history of commercializing technology both within India and abroad in collaboration with industry. In 1950s, NCL was successful in launching organic chemicals and manufacturers of dye for the first time. In Green Revolution during 1960s, NCL played a significant role in establishing various agro- chemical based companies. In 1970s after the launch of Patent Act in India, NCL has worked with various pharma companies and developed manufacturing processes for drugs (Nandagopal et al., 2011 ). In 1988, the Technology Information Forecasting and Assessment Council (TIFAC), an autonomous body owned by Government of India under Department of Science and Technology, was established to provide financial support for infrastructure and to develop and commercialize technologies under “Home Grown Technology” Scheme (Kumar & Jain, 2002 ).
Emphasizing the importance of promoting local goods, “Make in India” initiative launched in 2014 by Government of India includes the salient features to facilitate inventions, protect Intellectual Property, foster innovation, and build best manufacturing infrastructure in the country. In May 2016, first national IPR policy was released. Through this policy, GOI aimed to promote, create awareness, and enforce intellectual property. Moreover, the government prioritized to bring the administration of IP laws under Department of Industrial Promotion and Policy (DIPP) (Joseph, 2016 ).
The objectives of the policy were as follows:
Emphasize the significance of intellectual property among all sectors in the society
Stimulate creation of intellectual property by undertaking appropriate measures
Have stringent IP laws, consistent with international obligations
Modernize and strength IP administration and catalyze the commercialization of IP rights
Strengthen the enforcement on combating IP violations
Capacity development by strengthening and expanding human resources, institutions for training, research and skill building in IP (Joseph, 2016 )
Adding to the initiatives promoting IP, “Cell for IPR Promotion and Management” (CIPAM) under the aegis of DIPP for promotion, creation and commercialization of IP assets were constituted. CIPAM enforced a nationwide promotion scheme titled “Creative India; Innovative India” to create awareness on the benefits of the new IPR policy. The duration for the scheme was for 3 years (April 2017 to March 2020). The primary objective of the scheme was to conduct IP awareness workshops/seminars in collaboration with industry organizations, academic institutions, and other stakeholders across the country (Department of Industrial Policy and Promotion, Ministry of Commerce and Industry and Government of India, 2016 ).
Positioning of Indian Universities in Terms of IP Generation and Technology Commercialization
The inception of formal technical education in India dates back to the mid-nineteenth century. In 1945, an All India Council for Technical Education (AICTE) was set at a national advisory body to facilitate the infrastructure for technical education in India.
Universities in India are governed by Department of Higher Education under the Ministry of Human Resources Development (now Ministry of Education), Government of India. Indian universities are categorized as public funded and private (state private or deemed to be) universities based on the University Grants Commission (UGC) Act 1956. Later in 1987, All India Council for Technical Education Act was constituted to regulate and sustain the standards in technical education system in India. In the past decade, there have been several amendments in legislative framework for the public universities under various states governance in India.
In 2008, The Protection and Utilization of Public Funded Intellectual Property Bill (PUPFIP) was proposed to address the challenges in the university-industry technology transfer. The provisions of the Bill were to provide incentives to the universities through public funded research wherein:
Ownership of patents remains with the academic institute on inventions from government funded projects.
Institute creating an invention must inform the funding agency within 60 days of the creation.
Research institute must inform the government agency about the intention to patent the invention within 90 days; if they fail to inform, under defined prior Acts, the agency will acquire the title of patent.
Bill had the provision of 30% of royalties given to the inventor.
On receiving the government aided funds, the research institute must frame an intellectual management committee to process the innovation in terms of assignment of rights, potential for marketing the invention in concern, licensing agreements.
There were a few concerns expressed with implementation of PUPFIP. The major mission of the bill was to commercialize the invention, which lead to fear of ignoring public concerns and priorities. Other major concern of the bill was non-disclosure requirement insisted by the government on the research institutes, thus increasing bureaucracy and suppressing innovations and academic exchanges. The Ministry of Science and Technology had created a board of authorities to support the entrepreneur generation and support through National Science and Technology Entrepreneurship Development Board (NSTEDB) in 2009. The NSTEDB had supported the creation of Technology Business Incubators (TBI) and Technology Parks. TBIs were created at IIT-Delhi, Chennai, Mumbai, Kanpur, Delhi University and Banaras Hindu University (BHU), Varanasi. Despite the initiatives, the research commercializing at Indian Universities are sub-par compared with developed nations (Srivastava & Chandra, 2012 ).
In 2018, AICTE had initiated a program to empower faculty members by organizing a cell in all technical institutions, universities, deemed to be universities, and other institutions for training, known as AICTE Training and Learning (ATAL) academy. The main objective of ATAL academy is to impart quality technical education in India, support institutions in fostering research, innovation, and entrepreneurship through training. The training sessions are conducted through online portal in the form of workshops, orientations, learning communities, or faculty development programs. Government of India has announced to establish more than eleven such academies throughout the country (AICTE Training And Learning ( ATAL) Academy & Govt. of India, 2020 ). Ministry of Human Resource Development (MHRD), Government of India, established MHRD’s Innovation Cell (MIC) in 2019 to systematically foster the culture of innovation among all Higher Education Institutions (HEIs) in the country. The primary focus of MIC is to encourage, inspire, and nurture young students by exposing them to new ideas. Major programs under MIC include the following:
Smart India Hackathon (SIH) 2019, a nationwide initiative, to inculcate a culture of product innovation and a mindset of problem solving (AICTE-India, 2019 )
Institution Innovation Councils (IIC) to create local innovation ecosystem and support scouting ideas and pre-incubation of ideas
Atal Ranking of Institutions on Innovation Achievements (ARIIA) is an initiative in India to rank all major higher educational institutions and universities in India on indicators related to “Innovation and Entrepreneurship Development” amongst students and faculty members. ARIIA is set to channelize the institutions towards becoming competent in global platform and be forefront in innovations. The major indications for consideration of the HEIs to be ranked under ARIIA are as follows: Budget and Funding Support, Infrastructure and Facilities, Awareness, Promotions and support for Idea Generation and Innovation, Promotion and Support for Entrepreneurship Development, Innovative Learning Methods and Courses, Intellectual Property Generation, Technology Transfer and Commercialization, and Innovation in Governance of the Institution.(MHRD’s innovation council & Govt. of India. ( 2019 ).
National Innovation and Start-up Policy for Students and Faculty provides the framework on intellectual property ownership, revenue sharing mechanisms and norms for technology transfer, and commercialization (MHRD’s innovation council, Ministry of Human Resource Development & AICTE, 2019 ).
Earlier in India, the focus was on research and development, whereas now emphasis is also on creation of intellectual property and technology commercialization. Several universities have created facilities, infrastructure, and human resources to foster innovation, generate intellectual property, and commercialize academic research. This paper is an attempt to understand the barriers in commercializing academic research in India.
Research Methodology
The two main parameters underlying the study framework of research technology commercialization of the Indian universities includes the following:
Details on patent activities carried out in the universities in the past 5 years (2013–2018)
Revenue generated during the past 5 years (2013–2018)
The study framework for this research include qualitative and semi-quantitative analysis. The variables addressed in the study are represented in Table 1 . Convenient sampling was adopted.
Type of study: Cross-sectional study
Duration of study: 2 years (June 2018 to June 2020)
Sampling method: Convenience sampling
Sampling unit: Administrators of universities responsible for research/intellectual property/ technology commercialization/incubation cell
Sample size: 40 universities/institutes
Type of questionnaire: structured questionnaire
Data collection: in person where feasible, or through Google Form.
Data analysis
The questionnaire was designed by adapting the concept proposed by Association of University Technology Managers (AUTM) and Brazilian Survey of Technology transfer (Livesey, 2014 ). The questions in the questionnaire include descriptive, multiple choice, dichotomous, and scaled responses.
Results and Discussion
A total of 40 universities were approached for study of which only 25 responded with a response rate of 62.5%. Among other 25 universities/institutes (one respondent did not have technology transfer/IP office and hence excluded from analysis), four organizations were central government institutions, seven organizations were state private universities/institutions, five organizations were deemed to be universities, while eight others were private research organizations. IP policy was implemented in 17 universities, whereas six of them mentioned that the policy was under preparation and would be implemented within 6 months. The responses help to understand that IP policy has become an integral part of the research system in Indian universities.
As shown in Fig. 1 , 41% respondents had 2–5 years of experience in IP/TT, whereas 17% have more than 10 years of experience and 21% had less than a year or more than 6–10 years of experience.

Number of years of experience in IP/TT
The university/institute practiced customized strategies to transfer technology as shown in Fig. 2 . As there was no specific model practiced for TT, personal contacts in industry were the most preferred approach to license the invention, whereas the other approach was to organize innovation exhibitions. All the organizations adapted a combination of strategies as needed.

Strategies adapted by universities to attract potential licensee
Invention disclosure could be one of the important metric to assess innovation potential. In our study, it was observed that 33% of the universities had less than ten invention disclosures, whereas, 29% had more than 30 invention disclosures by the researchers in the past 5 years. Public universities correspond to higher number of invention disclosure due to higher quantum of funding received from the government. About 71% of the universities had entrepreneurship policy applicable to foster entrepreneurship among faculty members.
The number of patents granted to universities in past 5 years is shown in Fig. 3 . As is visible, the number of patents granted to the universities improved from 2016 to 2017. The data obtained through questionnaire was cross-verified for a few universities with the details publically available on their website. On analyzing the results, the universities governed under state or central government are consistently performing well in terms of patent applications filed and number of patents granted.

Number of patents granted to universities in the past 5 years
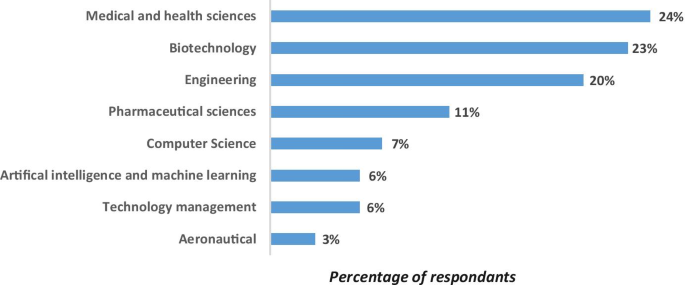
We tried to understand the scientific fields that are considered having maximum potential for technology commercialization. Though many universities have expertise in various fields, medical sciences and biotechnology domains are considered having maximum potential for technology commercialization as shown in Fig. 4
Domains deriving maximum inventions potential for commercialization
The budget allocation of the universities for IP/TT activities in the past 5 years is shown in Fig. 5 . The respondents stated that there was no separate budget allocated for IP/TT activities, but was included under research budget. The budget allocated in the last 5 years remains mostly unchanged.

Budget allocation for research /IP/TT activities in the past 5 years
We also tried to correlate budget allocated and expenditure on TT activities. The data on budget and expenditure is shown in Fig. 6 . In case of a few universities, the expenditure exceeded the budget allocated. The anomaly could be due to the fact that a few universities received funds for research from government funding agencies, which was not considered as a part of intramural research budget. The researchers of the universities/institutes were aware of patenting, but the concept of commercializing the invention is not well entrenched in their minds. The initiatives of establishing an IP/TT cell among universities is yet to strategize regarding use of resources for technology commercialization. The primary focus of TT/IP cell in the organization was related to technology evaluation (49%), licensing the invention (42%), business management (6%), and marketing (3%). Most of the universities focus on all the above listed activities based on the requirements.
Expenditure on university R&D activities in past 5 years
The number of active industrial collaboration was high (more than 30) among public universities. As shown in Fig. 7 , the concept of technology commercialization is far behind among Indian universities compared with their counterparts in the developed countries.

Number of technologies transferred, licensed, sold in past 5 years
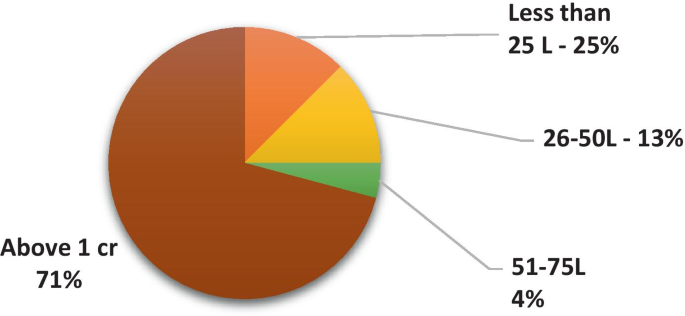
As shown in Fig. 8 , 13% of the universities generated a revenue of more than INR 1 Crore through technology transfer activities, whereas other universities/organization/institutes claim to have initiated discussion with industrial partners.

Approximate revenue generated from transfer/sale from IP/TT in pipeline of IP/TT
There is paucity of data and studies about university-industry technology transfer in Indian context. As a reference it would be interesting to compare it with other regions.
The Malaysian government under the 10th Malaysian Plan (2011–2015) had increased their R&D budget to RM 741 million among the universities as a part of research grants. The 2011 report states that among the total number of 313 inventions identified with potential of commercialization, only 58 research products were commercialized from 16 public funded universities. The critical factor identified as a barrier to commercialize R&D results was lack of absorptive capacity and entrepreneurial skills among the researchers (Latif et al., 2016 ). According to a study on commercializing research findings at schools of agriculture in Iran, it was found that research activities carried out at universities are independent from the research priorities that are in demand. The study noted that the mechanism of delivering a technology are not adequately tested or have been ineffective (Mostoufi & Highway, 2016 ).
The survey conducted in government research organizations in Sri Lanka reveals that there were 239 technological innovations, 11 new processes, and 11 new products, derived from the research organizations between the 2001 and 2008. Technology transfer was 80–90% successful, whereas technology commercialization rate was 40%. The study concluded that the policies were not adequate to support technology commercialization and the attempts of transfer were a self-activated endeavor that lacked coordination (Perera et al., 2015 ). Further, the quantitative findings about universities with University Technology Transfer Office (UTTO) focus on commercializing technology, some strategies that were employed include: (a) agenda of research commercialization to be included in the mission statement, (b) allocated funding for internal research commercialization as a part of UTTO’s budget, and (c) the funding for multi-purpose commercialization activities including prototype and business development. The qualitative study suggested the Australian UTTO is governed by panel of experts, with strong management support and most of all access to resources and staff with potential knowledge on commercialization (Alhomayden, 2017 ).
In the Republic of Serbia, research system is regulated under Law on Scientific and Research Activity and the innovation system is under Law of Innovation activity. As research organizations and institutes are not an integral part of innovation system, academia do not possess a strategic approach in research management creating innovation with a focus on commercialization (Belgrade, 2016 ). A survey conducted in three countries of Africa (Ghana, Kenya, and Zambia) suggested that dedicated Research and Development centers in Africa are limited and has shown a decline in past decades, as nearly 80% of budget allocated to top research centers goes toward salary for the staff. Moreover, the awareness of technology transfer at institutional level is negligible. Some of the research centers have expressed concerns on lack of multidisciplinary approach. It was recommended that relevant policies are framed and attempts made to recruit personnel trained in technology transfer (Africa U. N. E. C., 2013 ).
Our study focuses on three key aspects taking into account the challenges in university-industry technology transfer across regions: (1) the awareness and practice of patents and research commercialization among Indian academia, (2) comprehending strategies adapted to commercialize research activities, and (3) barriers in university-industry technology transfer. In terms of patents and technology transfer, Indian universities can be broadly classified into four categories: central government aided universities, state government funded universities, deemed to be universities, and state private universities/institutes. The most important findings are focused around the issues of patenting and technology transfer, not their strategic approach. The general outcome emerging from the study is discussed below.
Key aspect 1. The awareness and practice of patents and research commercialization among Indian academia
Compilation of the activities on patenting (inventions disclosed, patents filed and granted) and research commercialization was undertaken based on self- assessment. The word “declared” is associated with the realistic data provided by the university authorities which is emphasized in the study. The self-assessment of the analysis is shown in Table 2 . Considering the number of patents filed in the past 5 years, there has been consistency in the filing pattern among the public funded universities/institutes, whereas there has been increased awareness in the pattern of filing among private and deemed to be universities. This could be due to the central government funds received by central and state universities. A general trend observed gives a positive correlation between financial support received by the public funded universities from the funding agencies such as Department of Science and Technology (DST) or Department of Biotechnology (DBT) and other government funding agencies under GOI. Similarly, active industrial collaboration among the government aided universities/institutes are relatively high compared with other universities/institutions. On the contrary, less than five technologies are transferred/licensed/sold by the public funded universities that corresponds to a large proportion (75–86%) of public funded universities. The private organizations have higher number of active industrial collaborations yet minimal research commercialization activities. The revenue generated from the knowledge commercialization is comparatively high among the centrally funded universities with limited industrial partnership.
It is evident that with minimal industrial collaborations, high value is generated by the government universities/ institutes through knowledge transfer. Among private organizations despite high number of collaborations with the industrial partners, the value generated from the knowledge transfer is observed to be low. The results could lead to further studies exploring this inverse correlation.
Key aspect 2. Comprehending strategies adapted to commercialize research activities
Unlike in developed nations, there is no specific model proposed or practiced to commercialize research by Indian academia. As shown in Fig. 4 , inventions in the field of medical and health sciences, biotechnology, and engineering derive maximum potential for commercialization. The common strategy adopted to attract potential licensee is based on individual contacts of faculty with industry partners. The study emphasizes that the research undertaken in many universities/institutes are far from meeting the industrial needs. The probable solutions could be to create centralized repositories of technologies available at universities on a platform maintained by Government of India to provide the required assistance, something similar to government e- Marketplace (GEM) (Vihar, 2019 ).
Key aspect 3. Barriers in university-industry technology transfer encountered by the faculty members are (1) lack of adequate resources and infrastructure; (2) lack of creativity and critical thinking in curricula; (3) over emphasis on publications due to lack of awareness on patenting, publishing, and commercializing the research; (4) IP cell or similar offices are established merely to meet statutory requirements; (5) lack of qualified people to manage IP/TT activities; and (6) conflict between commercially viable and academic research.
The survey results reveal that the practice of IP generation and technology transfer is underdeveloped in the country. In order to make technology transfer more relevant:
Universities/institutes should leverage the expertise either in specific domains or pursue interdisciplinary research to attain high value through knowledge commercialization.
Universities should have people with the required skill sets manning the IP and tech transfer offices. Currently, in many universities, the responsibility is given to an individual who does not have required experience, expertise, and skills. This leads to a halo and the required outcomes expected by universities are not fulfilled. Additionally, the institutes should also promote interdisciplinary research to leverage domain strengths.
(2) Focus on commercially viable research
Universities should focus on outside-in approach where researchers are sensitized to work on commercially viable research. At present, in academia researchers follow an inside-out approach which leads to a knowledge gap. The inside-out approach is where researchers work on a problem that may address their inquisitiveness but does not lead to a research outcome that does not have commercial potential.
identify mechanisms to reach out to and collaborate with industry through exhibitions, conference and research partnerships
Universities/institutions should develop a mechanism wherein they showcase their research outcomes to industry with an intention to collaborate or license their invention. Current mechanisms to reach out to industry by academic institutions are inefficient and there exist no structure to strengthen industry-academia collaborations. These solutions though are not a guarantee to successful technology transfer but can help shape strategies to improve chances of stronger industry academia collaboration/technology commercialization among Indian universities.
Our current research has a few limitations. The data is collected from a small sample size. The respondent bias also cannot be ruled out while providing responses to the questionnaire.
In order to extract more meaningful data for analysis, it is required that a large-scale study involving a larger sample size with varying size and structure of universities, geographical spread, management systems be carried out.
Africa, U. N. E. C. for. (2013). National experiences in the transfer of publicly funded technologies in Africa: Ghana, Kenya and Zambia.
AICTE Training And Learning ( ATAL ) Academy. (2020). Government of India. 410917. https://www.aicte-india.org/sites/default/files/ATAL/atal%20vision%20mission.pdf
AICTE - India. (2019). Smart - India Hackaton. https://www.aicte-india.org/Initiatives/smart-india-hackathon
Alhomayden, R. S. R. (2017). University Technology Transfer Performance in Australia. Tez .
Baron, M. (2020). Open innovation capacity of the polish universities. Journal of the Knowledge Economy , (October 2015). https://doi.org/10.1007/s13132-017-0515-8
Belgrade. (2016). Research for innovation: strategy on scientific and technological development of The Republic of Serbia for the period 2016–2020. Ssrn . https://doi.org/10.2139/ssrn.2478304
Department of industrial policy and promotion, Ministry of Commerce & Industry; Govt. of India. (2016). CIPAM: Cell for IPR Promotion and Management, 1–7. http://cipam.gov.in/wp-content/uploads/2017/07/Scheme-IPR-Awareness.pdf
European commission. (2009). Expert GroupK nowledge Transfer. (2009), (November). http://www.desca-agreement.eu/fileadmin/content/New_DESCA_Website/2009Expertgroup_on_knowledge_transfer_final_report.pdf
Hyndman, K. G., Gruskin, S. M., & Iyer, C. S. (2005). Technology transfer: what India can learn from the United States. Journal of Intellectual Property Rights, 10 (September), 399–405.
Google Scholar
Joseph, R. K. (2016). Who will Gain from the National IPRs policy, Research gate (July2016). http://www.desca-agreement.eu/fileadmin/content/New_DESCA_Website/2009Expertgroup_on_knowledge_transfer_final_report.pdf
Kumar, V., & Jain, P. K. (2002). Commercializing new technologies in India: a perspective on policy initiatives. Technology in Society, 24 (3), 285–298. https://doi.org/10.1016/S0160-791X(02)00009-X
Latif, N. S. A., Abdullah, A., & Jan, N. M. (2016). A pilot study of entrepreneurial orientation towards commercialization of university research products. Procedia Economics and Finance, 37 (16), 93–99. https://doi.org/10.1016/s2212-5671(16)30098-3
Livesey, F. (2014). Report on survey of Brazilian Technology Transfer Offices (TTOs), (June). https://www.inova.unicamp.br/sites/default/files/images/FCO_BrazilTTOsurveyReport_0.pdf
MHRD’s innovation council & Govt. of India. (2019). Institution innovation council. https://www.mic.gov.in/iic.php#
MHRD’s innovation council, Ministry of Human Resource Development, AICTE. (2019). National innovation and startup policy 2019 for students and faculty. https://mic.gov.in/assets/doc/startup_policy_2019.pdf
Mostoufi, A., & Highway, A. (2016). Findings in Schools of Agriculture in Iran : a Qualitative, 5–14.
Nandagopal, M., Gala, K., & Premnath, V. (2011). Improving technology commercialization at research institutes: Practical insights from NCL Innovations. Innovation Educators’ Conference (IEC), Indian School of Business, Hyderabad, 1–12. https://www.venturecenter.co.in/pdfs/ISB-Conf-Paper-ver04.pdf
Pagar, S., Khivansara, A., Pagar, P., Gandhi, M., & Jondhale, S. (2014). Review pilot study of entrepreneurial orientation towards commercialization of university research products . International Journal of Pure & Applied Bioscience, 2 (3), 145–153.
Perera, H., Mudalige, D., & Liyanage, C. (2015). A case study of technology transfer process in a government research organization in Sri Lanka. Researchgate.Net , (March). Retrieved from. http://www.researchgate.net/profile/Darshana_Mudalige/publication/273629620_A_Case_Study_of_Technology_Transfer_Process_in_a_Government_Research_Organization_in_Sri_Lanka/links/5506d3250cf2d60c0e6db0d1.pdf
Rath, S., Nathani, A., Patel, D., Kulkarni, P., & Gota, V. (2014). Status of technology transfer in India—the much needed Magic Remedy. Current Science, 106 (8), 1058–1060.
Srivastava, P., & Chandra, S. (2012). Technology commercialization: Indian university perspective. Journal of Technology Management and Innovation, 7 (4), 121–131. https://doi.org/10.4067/S0718-27242012000400010
Thompson, D. (2015). Tech transfer and commercialisation, (May). https://scienceportal.org.by/upload/2015/June/Inconet%20EaP%20-%20Presentation/8%20Thompson_Tech%20Transfer%20and%20Commercialisation.pdf
Vihar, J. (2019). guideline on government e- MARKETPLACE ( GeM ). https://www.suniv.ac.in/docs/Guide-line-on-GeM-SU.pdf
Download references
Open access funding provided by Manipal Academy of Higher Education, Manipal

Author information
Authors and affiliations.
Department of Pharmaceutical Quality Assurance, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, 576104, Karnataka, Manipal, India
Department of Pharmacy Management, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, 576104, Karnataka, Manipal, India
Manthan D. Janodia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Manthan D. Janodia .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Ravi, R., Janodia, M.D. Factors Affecting Technology Transfer and Commercialization of University Research in India: a Cross-sectional Study. J Knowl Econ 13 , 787–803 (2022). https://doi.org/10.1007/s13132-021-00747-4
Download citation
Received : 21 February 2020
Accepted : 19 January 2021
Published : 24 February 2021
Issue Date : March 2022
DOI : https://doi.org/10.1007/s13132-021-00747-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Technology transfer
- Commercialization
- Industry-academia partnership
- Find a journal
- Publish with us
- Track your research
Loading metrics
Open Access
Peer-reviewed
Research Article
‘Our project, your problem?’ A case study of the WHO’s mRNA technology transfer programme in South Africa
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Faculty of Medicine, Department of Pharmacology, Dalhousie University, Halifax, Canada, Health Justice Institute, Schulich School of Law, Dalhousie University, Halifax, Canada
Roles Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing
Affiliations Program of Ethics, Politics and Economics, Yale University, New Haven, Connecticut, United States of America, Information Society Project, Yale Law School, New Haven, Connecticut, United States of America
- Matthew Herder,
- Ximena Benavides

- Published: September 23, 2024
- https://doi.org/10.1371/journal.pgph.0003173
- Peer Review
- Reader Comments
In June 2021 the World Health Organization (WHO) and the Medicines Patent Pool (MPP) launched an mRNA technology transfer programme. With a South African consortium serving as the hub, the programme aimed to increase vaccine manufacturing capacity in low- and middle-income countries (LMICs) in view of the “vaccine apartheid” that was observed during COVID-19. Following Clarke’s “situational analysis,” the present study assessed whether the mRNA programme differs from the approach and practices that comprise current biopharmaceutical production. Numerous documentary sources, including legal agreements underpinning the programme, funding agreements, and patent filings, were reviewed. Semi-structured interviews with 35 individuals, ranging from the programme’s architects and university scientists to representatives from LMIC vaccine manufacturers taking part in the programme were also conducted. While the mRNA programme may improve the sharing of knowledge, other design features, in particular, weak conditionalities around product affordability, participants’ freedom to contract with third parties, and acceptance of market-based competition, are in line with the status quo. Further, WHO and MPP’s tight control over the programme evokes the dynamics that are often in play in global health, to the detriment of empowering LMIC-based manufacturers to generate mRNA products in response to local health needs.
Citation: Herder M, Benavides X (2024) ‘Our project, your problem?’ A case study of the WHO’s mRNA technology transfer programme in South Africa. PLOS Glob Public Health 4(9): e0003173. https://doi.org/10.1371/journal.pgph.0003173
Editor: Roojin Habibi, University of Ottawa Faculty of Law - Common Law, CANADA
Received: February 7, 2024; Accepted: August 22, 2024; Published: September 23, 2024
Copyright: © 2024 Herder, Benavides. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: In order to preserve the confidentiality of people who participated in this research, but do not wish their identities to be disclosed we are unable to share interview transcripts in their entirety. The research ethics board at Dalhousie University approved this research provided that participants' identities would remain confidential. Inquiries about data availability related to this project can be sent to [email protected] .
Funding: One author (MH) holds a Chair in Applied Public Health, funded by the Canadian Institutes of Health Research (CIHR) and Public Health Agency of Canada (PHAC). This Chair carries a salary award as well as funding for research activities. However, neither CIHR nor PHAC played any role whatsoever in the design of the present study, data collection, analysis or writing process.
Competing interests: We have read the journal’s policy and the authors of this manuscript have the following competing interests: Matthew Herder was a member of the Patented Medicine Prices Review Board (PMPRB), Canada’s national drug pricing regulator, and received honoraria for his public service, June 2018 – February 2023. The PMPRB had no role whatsoever in the design or conduct of this research. Ximena Benavides worked for GAVI - The Vaccine Alliance, COVAX Facility, from May to October of 2021, as a Yale Institute for Global Health fellow.
Introduction
In June 2021 Afrigen Biologics, a for-profit company based in Cape Town, South Africa set out to change the global landscape of biopharmaceutical production. Chosen by the World Health Organization (WHO) to serve as the hub of an mRNA technology transfer programme, Afrigen’s initial task was to make an mRNA COVID-19 vaccine against SARS-CoV-2 and distribute the technology to manufacturers located in other low- and middle-income countries (LMICs). The motivation was plain: established makers of COVID-19 vaccines, especially mRNA vaccines, had largely neglected populations in LMICs [ 1 , 2 ]. In view of that “vaccine apartheid,”[ 3 ] building capacity to make vaccines locally for local populations became imperative. The WHO turned to a model of knowledge-sharing that was previously deployed in response to concerns that the global influenza virus sharing network was under-serving people in LMICS [ 4 – 6 ]. Another Geneva-based organization, the Medicines Patent Pool (MPP), was charged with managing the mRNA programme’s fundraising and legal needs.
Within six months Afrigen succeeded in producing its own mRNA COVID-19 candidate, “AfriVac 2121 [ 7 ].”. The programme has the potential to be transformative as a model of vaccine production [ 8 ], encompassing both upstream research and development (R&D) and ‘end-to-end’ vaccine manufacturing. Still, the initiative faces several risks, including precarious levels of funding, the looming threat of patent litigation by established mRNA vaccine manufacturers, and a range of governance issues that have complicated the work of an organization created out of dire need—all the while trying to develop the technical capacity to produce high-quality mRNA-based technologies that protect against not only COVID-19 but also tuberculosis (TB), malaria, human immunodeficiency virus (HIV), and other diseases that disproportionately afflict people in LMICs.
We set out to study, using qualitative research methods, to what extent the WHO/MPP-managed mRNA programme differs from the approach and practices that comprise current biopharmaceutical production. We describe the key features of the status quo as a basis for comparison with the mRNA programme under our findings below. Combining insights from documentary sources, including the legal architecture underpinning the programme, patent filings related to mRNA products, and data from semi-structured interviews with 35 individuals involved in the programme, we find that the design of this initiative is largely in line with dominant approaches to vaccine production, steeped in the neocolonial dynamics that are often in play in the sphere of global health [ 9 – 14 ], and at risk of failing to enhance equitable access even if it ultimately succeeds in one day making mRNA vaccines.
A ‘situational analysis’ of the mRNA programme amidst global power imbalances
Our research followed a “situational analysis” approach—a form of grounded theory, which develops theories through observations and multiple sources of data [ 15 ]. Under situational analysis, data collection and analysis occur in parallel, requiring constant comparison between new sources of data and the preliminary, but evolving, analysis. We describe the multiple sources of data incorporated into our situational analysis below, which has been applied by social scientists to gain insight into complex systems, comprising a variety of actors with diverging interests [ 16 , 17 ]. At the same time, we were cognizant of the power imbalances that afflict global health from the study’s inception [ 13 , 18 – 21 ]. Attention to power differences among the variety of actors and institutions engaged in the mRNA programme, and the multiple drivers of power imbalances, was central to our data collection and analysis.
Document analysis
Multiple types of documents were analyzed by both researchers; a few minor inconsistencies in interpretation occurred but were resolved through discussion. The first type of document was a range of legal documents that codify the relationships between different actors in the mRNA programme, which, pursuant to a memorandum of understanding, the WHO tasked MPP with drafting and implementing. These “programme agreements [ 22 ]” are in place between MPP and the three principal members of the South African “consortium”, that is, Afrigen, another Cape Town-based vaccine manufacturer called Biovac, and the South African Medical Research Council (SAMRC). The “technology transfer template agreement,” which served as the basis for negotiations with LMIC manufacturer partners to the hub, as well as the finalized agreements between MPP and thirteen of the fifteen programme “partners” that have signed a technology transfer agreement, all of which are publicly available from MPP’s website (accessed: March 30, 2024), were also analyzed. (Only Bio-Manguinhos (Brazil) and BiovaX (Kenya) have not signed such an agreement). Additionally, research agreements shared by interview participants were analyzed, including a funding agreement between scientists at the University of Cape Town and the SAMRC, a research collaboration agreement between the United States’ (US) National Institute of Allergy and Infectious Diseases (NIAID, a component of the National Institutes of Health (NIH)) and Afrigen, as well as sample clauses from Afrigen’s collaboration agreements with entities outside the programme. Powerpoint presentations and other information shared at the programme’s inaugural meeting held in Cape Town, April 17–21, 2023, as well as a regional meeting in Bangkok, Thailand, October 31 –November 1, 2023 were also incorporated into the study. To gain insight into the relationship between countries sponsoring the programme and WHO/MPP, an access to information (ATI) request was filed with the Canadian government, which is the second highest funder of the mRNA programme. Our ATI request yielded 153 pages of correspondence, agreements, and other documentation that we incorporated into our analysis. ( see S1 Letter and S1 Document for further details about our request and the corresponding disclosure package) Finally, a dataset of patent applications as well as withdrawn and granted patents, compiled and made publicly available by MPP [ 23 ] was analyzed to understand the evolving patent landscape related to mRNA technologies in South Africa and other LMICs tied to the programme.
Semi-structured interviews
We used a purposive sampling strategy, contacting individuals that hold leadership positions within their respective organizations or who have relevant experience, for example, in a relevant scientific field. Within the consortium (n = 12), we interviewed executives of Afrigen (3) and Biovac (1), as well as officials from SAMRC and other parts of the South African government (3), and university-based scientists (5). We also interviewed WHO (3) and MPP (4) officials, which we refer to as the ‘programme’s architects’ (n = 7), and representatives from vaccine manufacturers based in Argentina (2), Brazil (2), Serbia (1), India (1), Bangladesh (2), and another LMIC (2), which are now described as programme partners (n = 10). Finally, we interviewed scientists from the global North and other outside experts, businesses, and organizations (n = 6) that have supported or taken part in the programme in some fashion or work in the field of epidemic preparedness. Only one individual (of 36 that we contacted) declined to participate in an interview. The majority of interview participants (29 of 35 that chose to participate) agreed to be interviewed ‘on the record’, allowing quotations to be attributed to them by name. ( Table 1 ) One researcher (MH) traveled to Geneva, Cape Town, Chicago, and Bangkok to recruit and run interviews in person (n = 16). Nineteen interviews took place virtually and usually involved both researchers (MH, XB).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgph.0003173.t001
Research ethics statement
We received ethics approval to conduct this study from Dalhousie University’s Social Sciences and Humanities Research Ethics Board (REB# 2022–6457) and Yale University’s Institutional Review Board (IRB#2000034524). After discussing the purpose, benefits and risks associated with our research, all individuals we interviewed provided verbal consent to participate in the study at the outset of each interview. Consent was thus recorded as part of each interview transcript. All interviews occurred between February 2023 and January 2024.
Data coding and analysis
Consistent with situational analysis, data collection and analysis occurred in parallel. We created memos summarizing key exchanges or text, interpreting both interview and documentary data to identify lines of inquiry and points to follow up during future interviews. MH and XB generated a list of concept areas, in turn, developing a set of situational maps to define relationships between all the entities involved in the mRNA programme as well as key dynamics (e.g., influence of funding organizations; competing institutional priorities) that are often operative in the field of global health and access to medicines. We followed a constant comparative method throughout our research process, and met regularly to discuss uncertainties, unresolved questions, and points of divergence among interview participants.
Review by independent equity advisory committee
Our research process, data analysis, and preliminary findings were developed in consultation with an Independent Equity Advisory Committee (IEAC). Comprised of six members with diverse expertise in clinical trials, global health policy, access to medicines, and bioethics, the IEAC has extensive experience working with or inside organizations, such as WHO, the South Centre, Universities Allied for Essential Medicines, Médecins Sans Frontières, and the Health Justice Initiative. While the IEAC had no direct involvement in data collection, access to interview data, or control over our analysis, it played an essential role in helping to identify potential participants and critically appraising our preliminary findings and, at bottom, ensuring that our approach was attentive to the larger social and political context in which our research is situated.
We first examine the mRNA programme’s origins (2020–2021) and then compare its design to the four paradigmatic features of global biopharmaceutical production, which we abstracted from a review of literature and evidence from numerous scholarly disciplines; namely, 1) weak conditionalities attached to publicly funded science; 2) secret, transactional R&D partnerships; 3) a high degree of financialization; and, 4) market-based governance. Below, we elaborate upon, and juxtapose these four features against, our findings about the mRNA programme following an examination of the political context and policy choices that were made early on during the pandemic yet, as we show, continue to constrain the programme’s approach and practices.
Politicized origins: Building the mRNA technology transfer programme
Foreseeing access challenges from the start of the pandemic [ 2 , 24 , 25 ], WHO became home to several attempts to improve access to COVID-19 vaccines and other needed interventions in LMICs. Each differed markedly in terms of their approach to mitigating access challenges and the actors involved. The first, the “Access to COVID-19 Tools Accelerator” (ACT-A), was launched in April 2020 by a mix of public and private actors, including WHO, the government of France, the European Commission, the Bill and Melinda Gates Foundation, and three biopharmaceutical industry associations [ 25 ]. The vaccine-focused arm of ACT-A, COVID-19 Vaccines Global Access or “COVAX” (governed by Gavi, the Vaccine Alliance, the Coalition for Epidemic Preparedness Innovations (CEPI), and WHO), was intended to procure vaccines for LMICs by leveraging the collective purchasing power of high-income countries (HICs). With HICs prioritizing domestic populations at the expense of equitable global distribution, however, COVAX’s charitable approach failed to secure vaccines for LMICs [ 26 – 28 ]. A second initiative, the COVID-19 Technology Access Pool (C-TAP), created by WHO, the government of Costa Rica, and other member states, followed in May 2020 [ 29 ]. In contrast to ACT-A’s charity-based approach, C-TAP sought to distribute control of the intellectual property (IP), data, and knowledge related to COVID-19 interventions. Pooling a variety of technologies through voluntary licenses, vaccine and other product manufacturers could in-license technologies to address population needs in LMICs [ 30 ] rather than depending on vaccine donations from HICs—a move applauded by civil society but fiercely contested by industry, its allies, and the Gates Foundation [ 31 , 32 ].
Meanwhile, individuals inside and adjacent to WHO began crafting a third proposal, predicated on building capacity to manufacture vaccines in LMICs for LMICs. Martin Friede, the WHO’s lead coordinator for vaccine research, and Marie-Paule Kieny, the Chair of MPP’s Governance Board and formerly an Assistant Director-General at WHO were especially influential. Drawing upon a “hub and spoke” model of vaccine manufacturing that WHO deployed once before [ 5 , 33 , 34 ], they envisioned a centralized knowledge sharing system with a view to enhancing local vaccine production capacity in LMICs. Friede recalls how they arrived at this model in the context of influenza vaccines:
[I]t was very easy finding experts in terms of the vaccines because the world is full of retired people used to making influenza vaccines, but […] we realized these were generally quite old gentlemen and they got very tired going around the world teaching the same process at each facility […]. And this is when the concept was born of us creating a central hub, again, a corporate direction of interest. (MF)
Several crucial questions about the design of the model, in the context of COVID-19, nevertheless remained: How would it fit within WHO and the organization’s other newly launched COVID-19 access initiatives? Who would oversee its operations? Would there be one central hub or several spread across different regions? What vaccine platform(s) should command its focus for technology transfer purposes?
According to the lead of WHO’s IP Unit, Erika Dueñas Loayza, the original plan was to embed the COVID-19 hub within C-TAP. On behalf of WHO, Loayza’s team was actively seeking voluntary licenses from COVID-19 vaccine manufacturers.(EDL) Any new IP generated by the hub or its spokes would in turn become part of C-TAP’s pool, thus distributing control to LMIC-based manufacturers as their productive capacity increased. However, as industry opposition to C-TAP grew because of the threat that it posed to IP-holders’ control over COVID-19 interventions [ 32 ], then-WHO assistant director general (ADG) of access to medicines and health products, Dr. Mariângela Simão, and then-WHO Chief Scientist Dr. Soumya Swaminathan opted to “move the mRNA [programme] away from C-TAP to the ACT-[Accelerator]”—an outcome that MPP’s Kieny also favoured.(EDL) Although CEPI was the nominal lead for the “development and manufacturing” workstream within COVAX [ 25 ], it was the Kieny-led MPP that would later assume, in concert with WHO, responsibility for the design, day-to-day oversight, and fundraising for the hub.(CG)
Once positioned inside ACT-A, WHO issued a call for expressions of interest for “one or more” technology transfer hubs in April 2021 [ 35 ]. Afrigen’s chief executive Petro Terblanche remembers recognizing the opportunity: “We are small, but we know tech transfer.”(PT) Terblanche’s strategy of assembling a “consortium” together with Biovac and SAMRC for the WHO application proved wise. Friede describes the decision-making process inside WHO, which culminated in the selection of the Afrigen-led proposal on July 21, 2021:
[T]he WHO’s PDVAC, which is the production and development of vaccines advisory committee, decided that mRNA was the first platform to go for first because of its flexibility, potentially lower cost, and speed with which you could look at different antigens and whether they work or not. […] Then WHO put out a call for expressions of interest to be the hub initially, and a number of companies applied. South Africa came with a consortium, which is the only one that did come with a consortium, consisting of Afrigen as the hub, Biovac as the first spoke and the South African Medical Research Council as the research institute to feed into potentially new pathogens, potentially second-generation technologies and so on. And so that was attractive because they came as a consortium, and clearly also the fact that it was in Africa was attractive to them because Africa was the standout continent of inequity and access.(MF)
Brazil’s Bio-Manguinhos, a non-profit, state-owned company that is part of the Oswaldo Cruz Foundation, submitted a competing bid. Their proposal contemplated building ‘end-to-end’ mRNA manufacturing capacity, that is, the complete production process—from antigen design to producing the drug substance, drug product, and the fill and finish phase of inputting doses into vials—and then transferring the know-how from one LMIC manufacturer to another.(PN) Sotiris Missailidis, then head of vaccine innovation at Bio-Manguinhos, details how things shifted in the months that followed the Afrigen announcement in June 2021:
Africa was announced first, but I think it’s important to say that, in the beginning, at least, what we had understood […] was that the model was going to be a decentralized model. So there were going to be two hubs in Africa [and] there were going to be two hubs, regional hubs in Latin America. There were going to be two regional hubs in Asia. […] And each of them then would have spokes, potentially, that would be partners that had an interest in producing and accepting the technology. So we applied to be original hub. We didn’t apply to be a spoke, ever. And we got selected. […] What I didn’t know was that, at some stage, […] there was a decision taken from WHO or whoever, that as there was increasing political and financial pressure, many people wanted to come in. […] So the decision was taken to have one central hub and everybody else would be spokes.(SM)
It remains unclear why the change in plans occurred. Bio-Manguinhos learned of their ‘spoke status’ when they visited Afrigen in April 2022—six months after WHO indicated they would be a regional hub.(SM,PN) [ 36 ] The minutes from WHO’s PDVAC meetings show that multiple hubs were still being contemplated as late as November 2021 [ 37 ]. According to Patricia Neves, project manager for Bio-Manguinhos’ center for vaccines using mRNA, MPP officials queried “why are you here [in Cape Town visiting Afrigen] if you are developing your own technology?”(PN) At the time, MPP was focused on securing a voluntary license from Moderna or another more established mRNA manufacturer even though WHO had previously tried and failed to secure such a license.(EDL) MPP’s track record shows that it adheres to the norms of market-based competition and contract-based solutions even though voluntary licenses frequently exclude countries with strong manufacturing capacity, such as Brazil [ 38 , 39 ]. Perhaps this organizational philosophy explains why MPP appeared to be unsupportive of Bio-Manguinhos’ plan to establish end-to-end manufacturing capacity, at least in early 2022. Yet, Missailidis explains why mastering every step of the production process is critical to national health security:
We don’t do fill and finish. We need to have all the technology transferred up to… Well in the traditional vaccines, the master cell bank and everything. And we need to be able to produce [the active pharmaceutical ingredient] 100% properly. This is a condition for any tech transfer we’ve ever done […] because of guaranteed national production in Brazil. […] [H]istory shows that when the epidemics or pandemics or whatever else, you can’t guarantee that you have the vaccine when you want it. […] So, when we spoke, for example, to Moderna for COVID, didn’t even speak with us. Pfizer did, but they were not eager to do a tech transfer, they wanted to do fill and finish. Which we said ‘Look, you know we’re not doing that. This is not our motto. That’s not how we work.’(SM)
Two years on, the mRNA programme continues to evolve. The programme currently encompasses a diverse array of actors, including the South African consortium and fourteen other LMIC-based spokes ( Fig 1 ), which are now referred to as ‘partners’ because of the negative connotations of the term ‘spoke’.(CG) The programme’s architects have also come around to the idea of creating the end-to-end manufacturing capacity first espoused by Bio-Manguinhos and echoed by Afrigen not long after it became the hub. At a meeting in Bangkok in the fall of 2023, WHO and MPP officials outlined potential sub-consortia—engaging partners both inside and outside of the programme—focused on R&D around pathogens of shared, regional interest. In this way, Bio-Manguinhos or other manufacturers that assume the lead for a particular sub-consortium might become de facto hubs for a given target.(CN) Expanding the programme’s focus upstream is also seen as crucial to its overall sustainability given that demand for COVID-19 vaccines is now limited.(PT,CG,AK, MF, CN) Yet, as we show in the sections that follow, a number of choices made by its architects about what commitments participation in the programme entails, what kinds of support should be provided to Afrigen and others, and how the programme is governed, may limit the programme’s potential as a collaborative effort to improve equitable access to mRNA interventions in LMICs. Our analysis reveals that the programme’s relatively weak commitments to access and affordability, preservation of companies’ respective freedom to contract, consolidation of control by powerful actors in Geneva, and deference to the market as the ultimate arbiter of which entities will survive, both resembles the status quo and risks fragmentation within the programme, to the potential detriment of equitable access in LMICs.
Notes : (1) Two participating manufacturers, Biovac (South Africa) and Biovaccines Nigeria, are shown on the boundary between public and private ownership because each entity is a public-private partnership. All other entities depicted in the figure are state-controlled enterprises (i.e., publicly owned) or private companies. (2) For the purposes of this figure, the term ‘experienced’ refers to entities that have produced at least one vaccine that has been licensed for clinical use. Several manufacturers that fall into the ‘experienced’ half of the figure have produced more than one vaccine. The entities in the ‘inexperienced’ sphere have not yet fully developed a vaccine; however, some have generated sales through other products.
https://doi.org/10.1371/journal.pgph.0003173.g001
Critical inputs from publicly funded science, weak conditionalities & measured charity
The first defining feature of biopharmaceutical production concerns the limited quid pro quo that the public sector receives in exchange for supplying private actors with financing, biopharmaceutical R&D, and product leads. Despite significant government investments in, and publicly funded researchers’ extensive contributions to the development of vaccines and other products [ 40 – 45 ], weak conditionalities tend to be attached to government and philanthropic funding of biopharmaceutical R&D. Generally, public funding (whether in the form of research grants, collaborative research agreements, or advance procurement agreements) stipulates an obligation of data transparency (e.g., publishing studies). Also, the right of university scientists to continue to conduct research with the technology in question is usually included in IP agreements. But clauses that stipulate where manufacturing should occur, when and where products can be distributed, or how resulting goods are to be priced, are not standard [ 46 – 49 ]. Instead, conventional wisdom is to grant maximum discretion to recipients of public funding, including universities and government laboratories, as well as private actors about how to commercialize biopharmaceuticals. Under this logic, informed by the dominant approach that maximization of profits encourages innovation, the state’s role is not to shape—but simply subsidize—biopharmaceutical innovation [ 47 ].
Consistent with most early-stage biopharmaceutical R&D, the funding for the mRNA programme comes solely from governmental sources. Charged with the responsibility of fundraising, MPP secured financial commitments from France, the European Commission, Germany, Norway, Belgium, and Canada alongside the government of South Africa and the African Union [ 50 ]. To date, these donors have committed USD 117 million to the programme (with USD 89 million received so far (CN), the majority (73%) of which has been allocated to the consortium, including Afrigen, with the remainder (27%) supporting LMIC partners. According to MPP, which holds the bulk of the funds in Geneva, the USD 117 million is “seed money.” By 2026 the programme is expected to be “self-sustaining [ 50 ].” Still, MPP is continuing to seek additional funding, in particular, from the US government, which, in contrast to the WHO’s influenza technology transfer hub [ 51 ], has yet to offer any direct financial support for the mRNA programme.
Countries donating funds—or contemplating doing so—have shaped the programme in multiple ways. Germany, for example, earmarked a portion of its funding for a staff position at the hub. With only German or French nationals deemed eligible for the role by the funder, however, Afrigen was unable to fill the position.(PT) The government records obtained through an access to information request reveal that Canada, the second largest donor country, has stipulated that its funding be allocated to the hub in Cape Town and four select countries hosting manufacturers participating in the programme: Senegal, Nigeria, Kenya, and Bangladesh [ 52 ]. Further, according to one interview participant, while HICs are supportive of transferring technology to LMICs, they would prefer that such transfers do not extend to the more upstream inputs into mRNA vaccine production, including novel LNPs and antigens. Nevertheless, researchers at a number of publicly funded institutions located in South Africa as well as others abroad, including the University of Witwatersrand (Wits), the University of Cape Town and the North-West University, the University of Pennsylvania, as well as the US’ NIH/NIAID, have already made substantial contributions to various aspects of mRNA product development at Afrigen.(CF,PT,PA,CdK,XB)
At the start of the manufacturing process the aim is developing an ‘antigen’ that will provoke an immune response, conferring protection against a given pathogen. For Afrigen’s AfriVac 2121, the lab of Patrick Arbuthnot at Wits drew upon information already in the public domain to design a plasmid, a circular piece of DNA which can be propagated efficiently in bacteria and then prepared in larger amounts to use as a template, and shared it with Afrigen.(PA) NIAID’s Vaccine Research Center (VRC) similarly contributed to the plasmid construction and purification steps in line with current “good manufacturing practices” (cGMP) standards set by regulatory authorities.(XB,CF) After entering into a Research Collaboration Agreement with Afrigen in March 2022, the VRC shared its knowledge and hosted Afrigen scientists for onsite training [ 53 , 54 ]. Demonstrating the value of being part of a consortium, Afrigen will pull in more contributions from publicly funded researchers at Wits, the University of Cape Town, and other South African universities, as it increases its focus on the development of second-generation technologies, such as novel lipid nanoparticles (LNPs),(CdK) and new disease targets like TB, malaria, and HIV.
The critical question is whether the funding that has been secured for the programme and supporting the development of these second-generation mRNA technologies has been leveraged into a shared set of commitments geared towards improving equitable access. The relationships among the actors involved in the mRNA programme are defined by a set of legal agreements crafted by MPP. ( see S1 Table ) Under the technology transfer template agreement and all but one finalized technology transfer agreement involving MPP and LMIC partners, the latter are granted a “non-exclusive, royalty-free, non-sublicensable, non-transferable, irrevocable, fully paid-up, royalty-free licence” to the technology as well as any rights held by Afrigen and the Biovac “to make, or have made, use, offer for sale, sell, have sold, export or import” in their respective territories and other LMICs (as defined by the World Bank) [ 22 ]. In return, LMIC partners must grant to MPP upfront a “worldwide” non-exclusive, royalty-free license to “practice and have practiced the data and the Inventions for the purposes of fulfilling its mission to facilitate the development and equitable access of health technologies” that is “non-transferable, but sub-licensable.” As WHO’s Friede explains, the programme is akin to an IP sharing club comprised of the South African consortium as well as the thirteen other LMIC manufacturers that have signed an agreement to date:
[T]he key objective here is that for us, open means open for LMICs. It does not mean open for [HICs]. So, if Wits can generate some revenue providing a license for use and sale within [the] US, Canada, Europe, Australia, good for them, on condition that for all of the LMICs, there is a fully paid-up, royalty-free license available.(MF)
The programme’s pooled, multilateral approach to knowledge production is rare in the biopharmaceutical sector. MPP’s head legal counsel, Chan Park, notes that this deviation from standard practice stems from the fact that MPP was in a fundamentally different position compared to when it is attempting to secure a voluntary license from a multinational pharmaceutical company to an existing therapy:
When we’re negotiating with big pharma on a drug that they have already developed and are commercializing, our leverage is far lower. We can ask nicely for it and if they say no, we can ask again, and if they say no again, we just have to live with it. But here we’re building it from the ground up. We’re providing the funding, and so we can say, ‘This is a project for [LMICs] and that’s it.’(CP)
Still, there are a number of notable incongruities embedded in the programme’s underlying legal architecture, which run the risk of fragmenting the larger, collective enterprise of improving equitable access to mRNA products in LMICs. To start, some of the partners have yet to sign on. According to Bio-Manguinhos’ Missailidis, the Brazilian manufacturer cannot sign such an agreement because of its pre-existing, exclusive technology transfer agreement with AstraZeneca.(SM) His colleague leading Bio-Manguinhos’ mRNA vaccine project, Patricia Neves, also intimates that the idea that technology developed by Bio-Manguinhos, using funding from the Brazillian government (as opposed to funding from the mRNA programme) would flow to manufacturers from participating LMICs, which in some cases, are for-profit commercial entities, without anything in return is an “injustice.”(PN)
The issue of royalties also proved to be a sticking point within the South African consortium. According to a South African government official, a lot of back and forth with MPP was required:
[I]f somebody has spent 20 years developing a piece of IP, it’s really hard for them to say take it and go and do what you like with it. And a manufacturer can make a markup of 15%, but I’m going to get nothing from it. So that to us was a disconnect that we had quite a lot of discussion around. […] And we can’t just have somebody else outside the country making money offered, but we have to balance that with affordability and access. And that’s the balance we’re constantly struggling to achieve.(XX)
An unevenness between LMIC partners and the South African university laboratories funded by the SAMRC, where the former must share their IP royalty-free and the latter may expect a return, is thus embedded in the programme. (CP,XX) None of the executed technology transfer agreements between MPP and LMIC partners state this; on the contrary, the license granted from MPP to partners is framed as “royalty free.” However, the Grant Agreement with SAMRC grants MPP a “non-exclusive, transferable, sublicensable, irrevocable, worldwide, license to practice and have practiced the data and Inventions for the purposes of fulfilling its mission to facilitate the development and affordable and equitable access of mRNA technologies in low- and middle-income countries (as defined by the World Bank), which license may include a royalty sacrifice.” ( see S1 Table ) Thus, inventions patented by SAMRC-funded researchers, including second-generation mRNA technologies such as the novel LNP, may be rewarded with royalties whereas new IP generated by partners using mRNA technology will not.
A second IP-related incongruity in the programme’s legal architecture concerns the territorial limitations imposed upon IP licenses among different actors involved in the mRNA programme. As the central intermediary, MPP is granted a “worldwide” license to IP that is generated by both members of the South African consortium as well as LMIC partners. In turn, partners (with the exception of Indonesia’s BioFarma) are entitled to receive IP via MPP but only for use, sale, export or import within LMICs. For its part, BioFarma managed to negotiate a “worldwide, non-sublicensable” license to the IP it receives from MPP; it can therefore use, sell, and export such IP globally, but it cannot sub-license it to other entities in LMICs or HICs. That flexibility of licensing IP they generated to companies based in HICs only extends to members of the South African consortium (excluding Biovac as one of the partners). ( see S1 Table )
Notwithstanding the leverage the programme’s funding conferred, MPP also stopped short of requiring that resulting mRNA products be priced affordably for populations in need outside of a “Public Health Emergency of International Concern” (PHEIC). If an mRNA product developed by one or more LMIC partners targets a PHEIC, they cannot charge more than the cost of production plus a twenty-percent mark-up [ 22 ]. ( see S1 Table ) However, none of the pathogens being targeted by the programme’s partners—from TB to respiratory syncytial virus (RSV), malaria, and other infectious diseases—are currently designated as a PHEIC. Thus, consistent with industry norms, the mRNA programme does not contractually constrain partners’ pricing decisions. Rather, the assumption is that by targeting LMIC markets, the price of the final product will, of necessity, be affordable; otherwise LMIC governments will simply not pay for it. “Traditionally,” Charles Gore recalls, “MPP has not interfered in pricing. Our model is based on competition, and clearly we are potentially giving this to 15 companies around the world.”(CG, emphasis added)
In contrast, researchers in South Africa who receive funding through the SAMRC must, under the terms of the funding, ensure that any “resulting products”—regardless of whether they target a PHEIC or not—will “be made available and accessible at an affordable price to people most in need within [LMICs].” Revealing differential treatment among participants in the programme, SAMRC-funded researchers and partners with products targeting a PHEIC have agreed to some form of pricing constraint whereas Afrigen has no such obligation unless and until it is in receipt of funding from SAMRC.
Significant questions about the enforceability of affordability clauses exist. Although they have included such clauses for “many, many years,” one South African government official emphasizes, “it’s really an aspirational clause” because “we haven’t had to yet really test that.”(XX) Other funders in the field of infectious diseases, notably CEPI and the Gates Foundation, are experimenting with various pricing commitments, such as “costs of manufacturing plus” a set percentage and tiered pricing (where products are priced lower in LMICs than HICs through confidential discounts) [ 55 ]. (JC) In contrast, MPP appears to be comfortable relying on free-market competition among LMIC-based manufacturers instead of imposing affordability clauses when it comes to products generated by virtue of participating in the mRNA programme.
In effect, the programme’s approach reduces the pursuit of equitable access to the task of fostering more localized production. This is a logical step towards addressing local population health needs. But localized access is never guaranteed, particularly with initiatives that are expected to be “self-sustaining” businesses. Whether local manufacturers ultimately develop and sell their wares to local populations at an affordable price assumes, first, that those same manufacturers will maintain control over how their products are designed, where they will be launched and at what price; and, second, that local manufacturers’ own business models and resource constraints will not compromise their pursuit of localized access and affordability. As we explain next, the web of transactional relationships that Afrigen and other programme participants have entered into may complicate that mission.
Transactional R&D: Testing the limits of voluntary licensing
Under the dominant model of biopharmaceutical production, partnerships among the multiple actors engaged in the development of a biopharmaceutical product—from publicly funded labs to start-up companies, providers of research materials and equipment, contract research organizations (CROs), and large multinational manufacturers—tend to be secret and transactional in nature [ 56 – 59 ]. Whether the aim is to secure research materials such as reagents or lipids, a license to use IP, assistance with recruiting participants for a clinical trial, or purchase outright a medium-sized company with a promising therapeutic candidate, agreements are generally actioned under conditions of confidentiality between two partners, with one typically acquiring the asset of interest from the other. Thus, enclosed , bilateral partnerships — often short in duration—dominate biopharmaceutical R&D. More open and continuous knowledge-sharing arrangements through multilateral collaboration are, in contrast, relatively uncommon [ 33 ].
The original budget the South African consortium submitted to WHO was predicated on receiving technology transfer from an established mRNA manufacturer.(PT) Securing voluntary licenses to use IP is at the core of MPP’s work and philosophy [ 38 ]. The organization “has no intention […] of infringing any patents,” MPP wrote while seeking funding from the Canadian government, “not least because MPP’s key partners for licensing are pharmaceutical companies [ 52 ].” However, none of the HIC-based mRNA companies—Pfizer, BioNTech, Moderna, or CureVac—were interested in sharing their technology with the mRNA programme: “They didn’t even want to talk.”(PT) As a result, “the project turned into a green fields vaccine innovation,” that is, “product development from nothing,”(PT) just as Bio-Manguinhos had proposed in 2021.(PN)
Looking to scale up rapidly but “wisely,”(CF) Afrigen began enhancing its own in-house capabilities where possible while outsourcing other elements of the manufacturing process. In order to make the drug substance and then formulate it into a product with the addition of an LNP, Afrigen purchased off the shelf a microfluidic device from Precision Nanosystems, a Canadian firm, to assist with the LNP encapsulation process.(CF) Fenner, Afrigen’s scientific director, details how Afrigen overcame the key hurdle only to change plans in order to streamline costs for its LMIC-based programme partners down the road:
[W]e knew that it was difficult to do LNP formulations and we saw all the skepticism and everything from everywhere else. […] But for us we were like, ‘Well, what was all the hype about really? We have been able to do it.’ So we did use the Precision NanoSystems [PNI] machine, it’s not that difficult to use. […] And if you don’t have access to lipids to do the actual formulation, the company themselves have a lipids mix which is proprietary to the company that they make available to their customers. And so that you can then formulate the mRNA into an LNP. […] Having said that, we decided to not scale up on the [PNI] machine for the actual manufacturing. [T]he reason why we chose [another] machine is because that we thought that it is more simple to operate and that it has a lower running cost associated with it, which would be more appropriate for [LMICs].(CF)
While Fenner noted that Precision NanoSystems was acquired by Danaher Corporation after Afrigen began using its PNI machine, it was not clear whether Danaher’s record of acquiring products and increasing prices, including for a TB diagnostic test [ 60 , 61 ], factored into Afrigen’s decision to shift to another microfluidic device.
To demonstrate that AfriVac 2121 was ‘non-inferior’ to the Moderna and Pfizer/BioNTech’s vaccines, it is necessary to perform preclinical testing in one or more animal models. The architects of the mRNA programme decided that aspect would be done by Xavier de Lamballerie’s lab in Marseille, France, given that lab’s experience using a hamster model to conduct SARS-CoV-2 challenge studies [ 62 ]. Marie-Paule Kieny, chair of the MPP’s board, explains:
[W]e wanted to have this in a center where the model has been validated internationally. So if Xavier de Lamballerie publishes that these results are equivalent, everybody will believe it. If somebody in Afrigen is saying that it’s the same, ‘Uh-uh.’ So, he’s neutral, he’s independent, he has no skin in the game. So he’s testing the system. And now that we have this, so he has a lot of other studies to do, he will do neutralization of variants and so on and so forth, so this will be one package. And now he is also starting immunization of another batch of hamsters with the Afrigen product, the Moderna product, the Pfizer product, and this hamster will be challenged.(MPK)
When Lamballerie’s preclinical studies of AfriVac 2121 are complete, the hamster model will be transferred to South Africa.(MPK,CF) As a result, “the local university [in Cape Town] is actually being capacitated…there’s essentially a transfer of knowledge and protocols between the two so that in the future we would be able to do it in South Africa.”(CF)
At each turn of the manufacturing process knowledge gaps are thus identified and addressed, often with the help of outsiders. Terblanche reports that Afrigen has at least nine different “cooperative research and development agreements” (CRADAs) at the “active implementation stage.”(PT) [ 63 – 66 ] In some cases, the outsider’s contribution is coupled with a commitment to assist Afrigen or another consortium member in gaining internal capacity, such as the hamster challenge model or performing GLP compliant toxicology studies, which Afrigen has outsourced to a “one stop shop” in India.(CF) In other instances, it is not clear whether the bilateral agreements will precipitate sustained collaboration around a shared set of goals. Meanwhile, mRNA programme partners are striking new deals and funding arrangements of their own. Bangladesh’s Incepta, for example, has partnerships in the works or already in place with the University of Pennsylvania, Afrigen, Imperial College London, US NIH, and the Belgian company Quantoom.(MMA,MK)
It is notable that all of these bilateral CRADAs, funding agreements, and other contracts are the product of the programme’s design. WHO and MPP, as the programme’s architects, have chosen to place minimal constraints upon programme participants’ ability to enter into bilateral agreements with external actors. The only stipulation under MPP’s technology transfer template agreement is, if a partner of the mRNA programme obtains access to IP of a third party, the partner undertakes to “use reasonable efforts to negotiate a licence to MPP for such” third party IP. According to Terblanche, Afrigen has carried those access commitments through all of its CRADAs; where potential partners have balked at those terms, Afrigen has backed away from the deal.(PT) None of Afrigen’s bilateral deals, nor those of programme partners, are publicly available, however.
Participating in the programme is a business opportunity. Serbia’s Torlak Institute, for instance, has offered to sell reagents used during the production of influenza vaccines to other partners during the first programme-wide meeting held in Cape Town in April 2023.(LD) “I think this is the interesting part that we have,” Bio-Manguinhos Missailidis explains, “you create a network that eventually there will be bilateral agreements within the network of people interested in some of our projects.”
Outside actors engaged in the mRNA space have also increased their deal flow by virtue of their connections with the programme. According to Jose Castillo, head of Quantoom, which is known for its machines that automate an early part of the mRNA production process, already counts seven of the fifteen partners as its “customers” and is in active discussions with the other partners as well.(JC) Quantoom’s contracts with the programme’s partners moreover run deeper than simply selling its machines. Castillo recounts how he “knock[s] on the door talking about tech, but the contract I sign is a collaboration agreement”(JC). In return for assisting a partner to design an antigen against a pathogen of interest, Quantoom acquires a non-exclusive license to any project-related IP the partner in question generates.(JC) With agreements in place with many of the programme’s participants, Quantoom stands to add substantially to its IP assets, rendering it an attractive target for acquisition by a larger entity. Castillo’s previous company, Artelis, was ultimately acquired by Danaher in 2015 [ 67 ].
The programme’s architects are thus walking a fine line between trying to seed collaboration within and on the margins of the programme and trusting all involved to thread the commitments to IP access throughout that evolving web of relationships. Terblanche and Castillo appear steadfast in their commitment to the programme’s stated objectives, yet cognizant of their respective organizations’ vulnerability to market forces. Terblanche shares her thinking:
I have a very strong, purpose driven, public health orientation. But I’m not stupid, I know my company needs to be financially viable to deliver on that promise. But greed is not my sin. Okay? I think that’s the difference. But I can’t tell you […] what will be the next CEO’s orientation? If Avacare [Afrigen’s primary shareholder] dilute or sell[s] us […] I have no control. So the only control I now have is agreements of care, which is public access. And these agreements will survive shareholders’ ownership. That’s the only thing I can do.(PT)
The decision to rely, to a significant extent, on private actors, banded together through CRADAs and other contracts, to build and share mRNA manufacturing capacities in LMIC settings is a signature feature of the programme. It is also reflective of the demonstrated preferences of its main architects, especially MPP, which has ascended in prominence in the sphere of global health as a result of the programme’s development.
Financialization’s intermediaries: MPP as a rising ‘power broker’ in global health
A third defining feature of the biopharmaceutical sector today meriting comparison with the mRNA programme stems from the industry’s highly financialized character. While the financialization of an industrial sector can manifest in several ways, the concept has generally been used to refer to the “increasing role of financial motives, financial markets, financial actors and financial institutions in the operation” of both domestic and international economies [ 68 ]. In the biopharmaceutical sector, the shift toward financialization is evident in the move by most major firms to become publicly traded on one or more stock exchanges (as opposed to family-owned businesses reliant solely on product sales for revenues) since the mid-twentieth century, the increasing importance assigned to maximizing shareholder value by financial actors with a controlling interest in many biopharmaceutical firms, and the growing reliance upon the tools of the financial sector, including mergers and acquisitions, and share buybacks and dividends, as the primary means to generate revenues [ 57 , 69 , 70 ].
The consequences of biopharmaceutical financialization are also several-fold. With financial companies, such as banks, venture capital firms, and asset management groups today often owning a controlling interest in any given biopharmaceutical firm, the strategic direction of those firms tends to become “more short-term oriented seeking to maximise immediate shareholder returns instead of making investments that look to the long-term health of the company [ 69 ].” Financialized biopharmaceutical companies may also increase prices for products already on the market to offset the cost of share buybacks and dividends, allocate greater resources towards marketing and advertising instead of R&D, and outsource R&D and manufacturing activities to countries, including LMICs, where labor costs are lower to the detriment of companies’ in-house capabilities [ 69 , 70 ]. In fact, many biopharmaceutical firms actually outsource R&D and manufacturing activities to third-party CROs rather than perform the work in-house [ 71 ]. Outsourcing R&D has, in turn, created a space for a variety of intermediaries and consultants to develop business models of their own, connecting large firms with CROs and other service suppliers in exchange for a fee, claims to IP, and reputational capital that flows from bridging various steps in the R&D process.
There is no indication that any of the mRNA programme’s direct participants mirror the financialization that is evident among more established biopharmaceutical companies. Neither Afrigen, nor its primary shareholder Avacare, are publicly traded companies. The same is true of the other private companies partnering with the programme. None of the companies involved appear to be using the tools of finance such as share buybacks as a means to generate returns. While some inputs into the manufacturing process have been secured from outside entities, Afrigen, Biovac, and programme partners in other LMICs are invested in developing their R&D and manufacturing capacities in-house in an effort to increase local production in LMICs. The Belgium company Quantoom, which has locked in partnerships with most LMIC manufacturers in the programme, may be amassing an interest in each partner’s IP. But Quantoom’s machines, which automate the in vitro transcription step of mRNA production, seem to contribute meaningfully to the manufacturing process.
While MPP’s position within the programme is not a symptom of financialization, the role that the foundation plays is analogous to the intermediaries that link together biopharmaceutical R&D and production supply chains. Like the industry’s many intermediaries, MPP’s presence simultaneously adds value to, and imposes a drain upon, the mRNA programme.
Through the programme’s legal architecture, MPP has positioned itself as the central intermediary for technology transfer. Afrigen is the nominal hub for partners to receive training and it has provided a 3-day course about mRNA manufacturing to most participating manufacturers. However, it is MPP—at times, with the assistance of outside consultants (MMA, CN)—that has assumed the role of conducting site visits, assessing the needs and capabilities of each partner, and conveying the knowledge, data, and IP generated by the consortium. Awaiting for MPP to visit its facilities, representatives from one partner noted that receiving the transfer directly from Afrigen, as the producer of the drug substance, might be more efficient. But the partner cautions that its technology transfer agreement is with MPP, not Afrigen. As a result, “all the information comes from MPP.” That is, “Afrigen to MPP and MPP to the spoke, [i.e.] to the technology transfer recipient.”(XZ,XA)
Having MPP as a ‘middleperson’ is not the optimal way to provide technology transfer.(AK,EW,PN,PT) Typically, technology is transferred from one party, which has direct experience utilizing it, to another, through both sharing of hands-on know-how and detailed documentation such as ‘Standard Operating Procedures.’ Even in that two-party scenario mistakes occur, for example, when experimental protocols are not sufficiently delineated. Introducing an intermediary into the process, which lacks hands-on experience practicing the technology in question, increases the chances that the transfer will be unsuccessful. Nevertheless, over the course of 2022–2023, MPP created its own technology transfer unit, comprised of five individuals with doctoral and master degrees in chemical engineering and other fields as well as varying levels of experience in the pharmaceutical industry [ 72 ], to manage technology transfer within the mRNA programme. According to some interview participants, this has precipitated frustration with MPP’s approach to technology transfer:
The MPP is spending gobs of money on creating a group that is supposed to be doing tech transfer. I’ve worked for more than 30 years in the industry. You do not have a remote group that does tech transfer. If a group is going to do tech transfer, it needs to be in the facility that’s sending the technology out. They need to be the detailed subject matter experts. So they seem to be building this empire that’s going to do what, I don’t know. And they seem to be micromanaging and want to be in charge of everything.(AK)
In that participant’s view, “if [the programme] doesn’t succeed, it’s going to be because of that kind of dynamic, not because the science doesn’t work.”(AK) Altering that dynamic, moreover, requires tact:
MPP now takes the funding for that [technology transfer] part and it goes to MPP because they have the team. So I don’t want to be seen, now I want all the money for Afrigen. It’s a […] sensitive thing because people I think, they go sometimes, “because oh, you’re Afrigen, you just want to dominate and control.”(PT)
Afrigen will, according to Terblanche, assume responsibilities for the technology transfer in 2024. But whether the Cape Town company’s people will be resourced to travel, train, and transfer technology to partners remains “under negotiations.”(PT)
MPP clearly has the potential to add value to the programme in the IP domain. Its core expertise lies in evaluating patent landscapes for legal risk and negotiating licensing agreements with IP holders. MPP has compiled the patent landscape associated with mRNA technologies; the findings to date are worrisome. Of the nine LMICs linked to the programme through a partner for which patent data is available, patent applications have, according to MPP’s dataset, increased markedly since a PHEIC was declared in January 2020 [ 23 ]. (see S2 Table ) More than a third (56 of 159) of all patenting activity related to mRNA since 2006 in the 15 countries connected to the programme (concentrated mainly in South Africa, Brazil, India and Serbia) occurred between 2020 to 2022. Further, there are reportedly an increasing number of “use patents” (purporting to claim IP on the use of mRNA technology against a given disease, e.g., malaria) being filed in South Africa and other LMICs. WHO’s Friede says such patents could block Afrigen and partners’ R&D plans, “especially […] in countries like South Africa where there’s no substantive examination [of patent applications to ensure they meet standards of novelty, non-obviousness, and utility].”(MF)
Notably, MPP’s work on the IP dimension of the programme does not extend to providing the South African consortium or other LMIC-based manufacturing partners with legal opinions about their respective “freedom to operate,” (FTO) i . e ., to conduct research and product development without undue risk of patent infringement litigation. The memorandum of understanding between WHO and MPP states that MPP will “provide IP analysis and commission [FTO] assessments for the Partners, as necessary [ 22 ].” However, the technology transfer agreements entered into with each partner refer only to providing “IP analysis…as practicable and appropriate” rather than supporting FTO assessments. MPP has reportedly provided funding for some partners to pay for FTO analyses by local law firms.(FL) Afrigen, however, has had to seek out independent legal advice using its own funding.(AK,PT) At least one partner is under the impression that the patent data that MPP has prepared and presented at programme meetings amounts to FTO guidance.(XZ,XA) But presenting a high-level summary of all the patent documents that exist is not the same as an in-depth interpretation of the scope of each patent granted in a jurisdiction—the core inquiry involved in crafting an FTO opinion.
The IP strategy behind the programme appears instead to be that the programme will meet incumbents’ IP positions with IP of its own. Glaudina Loots of South Africa’s department of science and innovation emphasises that the university scientists funded by the programme are keeping “the patent lawyers quite happy.”(GL) At least two patent applications have been filed to date, one pertaining to antigen design and another on a novel LNP technology, with each listing inventors from the University of Witwatersrand, including Arbuthnot and chemist Charles de Koning. Arbuthnot contextualizes the importance of these new patent applications in light of the programme’s goals of not just manufacturing a COVID-19 vaccine but also producing second generation mRNA technologies:
The lipid nanoparticle part of an mRNA vaccination technology is very, very important, so we’ve been doing a lot of work on that, and it’s a particularly interesting project, actually, with some synthetic organic chemists here in Johannesburg at Wits as well, who are using a bio-renewable source for the manufacturer of the important ionizable lipids, they’re called. […] We’re very excited about this because the lipids that are made to put into the vaccines that are used by, say, Moderna and Pfizer, are based on petroleum chemistry, so they’re not bio-renewable products at all. If we are able to get products that can work as lipid nanoparticles, this is potentially something that could be quite big, and we’re very excited and enthusiastic and working quite hard at trying to do that. That’s an example of something that would enable us to have freedom to operate where we wouldn’t have, wouldn’t be dependent on the [IP] of anybody else.(PA)
While the experimental work to determine whether the bio-renewable lipid works is ongoing, “the medicines patent pool guys in Geneva,” de Koning emphasises, “are telling us, ‘You need to patent the stuff’” to ensure the hub has FTO.(CdK) Underscoring the potential strategic value of these patents in the event that the consortium or programme partners are confronted by a competitor down the road, WHO’s Friede offers that “if that IP stands up to be really powerful, and we run into problems with certain companies, that IP might be a bargaining chip.”(MF) In other words, a strategy of “defensive patenting” could help shield the programme from threats of patent infringement from outsiders while also sharing knowledge among participating manufacturers [ 73 , 74 ]. The success of that strategy will depend on whether the patents are ultimately granted.
In short, MPP’s philosophy around IP, FTO, and contractual, negotiation-based solutions is imprinted on the programme; and, through its control over the programme, MPP has grown significantly in size and stature in the world of access to medicines. In the five years leading up to the pandemic, MPP’s annual budget was in the range of CHF 5–6 million [ 75 ]. Since the pandemic began and the mRNA programme was launched, MPP’s annual income has increased roughly fourfold (to CHF 23 and CHF 19 million in 2021 and 2022, respectively), the number of staff has jumped from 25 to 44, and their budget for external consultants has doubled [ 75 ]. Yet, it is not clear that MPP’s presence and philosophy will work to the advantage of the programme’s different participants. For instance, other go-to sources of funding in the field of infectious diseases, including the US NIH, the Gates Foundation and CEPI, have steered away from funding the programme as a whole. Gates recently provided USD 40 million spread between Cape Town-based Biovac, L’Institut Dakar in Senegal, and Quantoom, which has agreements with several programme partners [ 76 ]. CEPI has likewise supplied funding to four manufacturers taking part in the mRNA programme,(CN) including Indonesia’s BioFarma [ 77 ]. None of these awards will flow through MPP, however. “I don’t understand” why MPP and Gates are not working together, Quantoom’s chief executive Castillo explains, and I have “that conversation with MPP and the same conversation with Gates, and not only with Gates, but with Mr. Gates.”(JC)
Institutions engaged in the field of global health have long competed for resources, goodwill, and influence [ 78 – 81 ]. To the extent that there is fallout between actors vying for influence in Geneva, its impact upon the mRNA programme will not be uniform. Having expanded its portfolio of work to the realm of technology transfer, developed funding relationships with several HIC governments, opened a local chapter in South Africa, and launched a new strategic plan [ 82 ], MPP’s place as a new “power broker” [ 81 ] within the sphere of global health appears assured. In contrast, the business at the heart of the programme, Afrigen, has thus far been unable to secure funding from Gates and other sources, and is, according to its head executive, increasingly “vulnerable” to financial strain.(PT)
The sustainability question: Market-based governance
The fourth and final feature of the status quo is that control over the direction of biopharmaceutical production is concentrated in the hands of powerful, private actors that are, at bottom, governed by the market . Established firms calculate the ‘net present value’ (NPV) of one disease target versus another, which systematically devalues diseases that are endemic in LMICs and thus offer lesser financial returns [ 83 – 85 ]. The entrance of the Gates Foundation and other philanthropic organizations has supplied new research funding for TB, HIV, malaria, and many other “neglected diseases.” However, it is far from clear that these dominant organizations are prepared to rethink IP-intensive R&D practices or enforce access commitments in the service of public health [ 32 , 81 , 86 , 87 ]. They, too, are private actors, wielding significant influence over governments and entire scientific fields outside—and unaccountable to—the broader public.
Afrigen has targeted eleven potential diseases for mRNA product development. Unlike the standard approach to R&D, Terblanche suggests that the prospect of financial gains has not shaped the hub’s priorities to date:
Somebody asked me, […] ‘Your pipeline of 11. How did you get to it?’ I said, ‘I look at the unmet need, I look at the region. I look at whether there are vaccines or not, and I look whether the mRNA is suitable for it. And I look at whether we have access to antigens.’ And they said, ‘And what about the market?’ I said, ‘I’ve not included the market in my decision-making. I’m driving a need. And I’m not driving a profit, I’m driving a need, I’m driving sustainability.’ (PT)
Multiple proposals focusing on Lassa fever, RSV, and other disease targets, have since been turned down by a variety of funders, leaving Terblanche to concede that her “hand will now be forced to prioritize” in light of market rewards.(PT)
The architects of the programme appear to have accepted Afrigen’s precarity from the start, anticipating that the Cape Town firm might not survive in the hyper-competitive mRNA marketplace. The absence of pricing commitments parallel to those imposed upon programme partners or a commitment to allocate up to ten percent of “real-time production” capacity in the event of a PHEIC within the four corners of Afrigen’s Grant Agreement with MPP [ 22 ] ( see also S2 Table ) could be interpreted as an incentive for Afrigen to commercialize its technology. Read in conjunction with the views of the programme’s architects, the omission of these terms from the Grant Agreement likely suggests that the architects did not contemplate that Afrigen would ever generate mRNA products of its own. Recalling that some of the companies that took part in the influenza hub later shut down production, WHO’s Friede estimates that if a handful of LMIC manufacturers manage to make mRNA vaccines, the newer programme will be an overall success.(MF) Charles Gore of MPP notes, “we are funding [Afrigen] to develop and then shift [the technology] out,” but “they don’t [yet] have a business model.”(CG) Like any private enterprise engaged in biopharmaceutical innovation, MPP’s Marie-Paule Kieny speculates that Afrigen will, in the end, probably yield to market forces:
[W]ill the hub always be a necessity? I would argue no. […] The hub is really there to establish a first platform and improvement, and to help with an early pipeline. After that we are fully aware that Afrigen is a private company, at one point they will try to find somebody to buy them out and to get the benefit. I don’t know, what can we do? (MPK)
The near inevitability of Afrigen’s exit in the eyes of those who designed the programme speaks to an underlying failure of imagination concerning how the mRNA programme is governed. During the pandemic, calls for more “inclusive and decentralized” governance structures have grown [ 88 ] in order to shield initiatives such as the mRNA programme from the risks and constraints posed by dominant market actors. At a minimum, a more inclusive and decentralized structure would entail two key changes to the programme. First, representatives from participating LMICs capable of steering the programme’s R&D towards local population would need to be directly involved in the programme’s overall governance and day-to-day decision-making. Second, multiple actors would need to serve as regional mRNA hubs—as originally planned—in order to mitigate the risk that one organization’s failure (or acquisition by an outside actor) might compromise the programme as a whole.
Instead, WHO and MPP internalized programme decision-making within two hand-picked committees, leaned on private actors like Afrigen to play crucial roles, preserved their discretion about what projects and partnerships to pursue, and limited input from LMIC governments and civil society during the programme’s first two-plus years of operation. Members of the South African consortium, including scientists funded by the mRNA programme, also registered concerns about the lack of transparency surrounding the allocation of resources inside the programme. (ALW,ER)
The privatized approach to governance preserves the programme architects’ control over the programme. The function of the “Scientific and Technical Review Committee” (or “STeRCO”) is mainly to advise WHO and validate “high-level funding envelopes,” including “the funding for the transfer of technology, the hamster model from Marseille to South Africa” and the purchase of large GMP equipment for preparation of mRNA for Afrigen.(MPK) For its part, the mRNA Scientific Advisory Committee (mSAC), which includes several notable experts with ties to industry, HIC governments, and academia, plays a more technical role, for example, evaluating the funding proposals submitted by South African researchers connected to the consortium. Reflecting their close working relationship, MPP’s Kieny chairs the STeRCO while WHO’s Friede chairs mSAC; the WHO is the secretariat for STeRCO while MPP supports mSAC’s meetings and deliberations; and, the decisions by both serve to validate the use of funds from one source or the other ( Fig 2 ). Missing altogether from this governance structure is any direct representation from LMIC governments whose needs and interests the programme is meant to serve.
Notes : (1) This figure provides a schematic representation of the organizations and actors involved in the mRNA programme, and the inter-relationships between them. We contrast how the mRNA programme has been described in principle (Panel A) where Afrigen is described as the “hub” and manufacturers in LMICs are partners (or spokes), versus how the mRNA programme appears to work in practice based upon our findings (Panel B). (2) There are several important differences between Panel A and Panel B, including the fact that all of the technology transfer agreements are between partnering manufacturers and MPP (represented by double-arrowed lines). The only direct collaboration between Afrigen and a partner is with Bio-Manguinhos; the two organizations are in negotiations regarding a partnership focused on RSV (represented by the dashed line). Panel B also shows that all of the funding that has been secured for the programme flows through MPP (and WHO to a lesser extent). Members of the South African consortium as well as programme partners must negotiate access to funding from MPP, which, together with WHO, has delegated decision-making to the mSAC and STeRCo committees. Finally, Panel B also highlights other actors involved in the programme, including researchers at the University of Witwatersrand, the University of Cape Town, and North-West University (represented as Wits, UCT, and North-West, respectively), and the Belgium-based company, Quantoom, which has formed bilateral relationships with Afrigen and the majority of LMIC-based manufacturing partners. (3) While the figure’s details derive from our research findings, they are not intended to provide a comprehensive picture of the mRNA programme. For example, recently the programme’s architects have put into place a “Funders Forum” for countries and organizations that have provided financial support for the mRNA programme, and four new R&D consortia involving programme partners and outside actors were announced in March 2024 [ 89 ]. In order to limit the complexity of the figure, these new structures are not represented here.
https://doi.org/10.1371/journal.pgph.0003173.g002
Collectively, these choices reflect the programme’s alignment with the dominant, market-drive approach to biopharmaceutical production. By design, the programme cannot stop Afrigen or other private companies embedded in the programme (e.g., Quantoom) from capitalizing upon the IP they amass through the programme or, if needed to satisfy their shareholders, sell their stake when the right offer materializes. Importantly, such a transaction would not mean that LMIC partners would be deprived of access to IP generated by Afrigen or other parts of the South African consortium. Similarly, a partner that patents an improvement of an mRNA technology that they receive from the programme cannot claim exclusive ownership over that new IP. Rather, the terms of the various agreements underpinning the programme pre-empt those possibilities. “[I]t’s a self-executing grant-back obligation,” MPP’s head lawyer Park stresses, so “if a spoke partner invents something, patents it, the license automatically comes back to us.”(CP) Notwithstanding this safeguard, however, the prospect of Afrigen exiting the space altogether or other parts of the consortium selling (on a non-exclusive basis) its IP assets to an entity based in an HIC, is in tension with the programme’s stated goals of building local mRNA manufacturing capacity in LMICs.
The foregoing findings raise questions about the design choices embedded into the mRNA programme, how best to empower LMIC-based manufacturers, and what additional steps need to be taken to ensure that this initiative enhances equitable access. We explore each in turn by way of conclusion.
A facsimile of the status quo in biopharmaceutical production?
Assessed against the dominant mode of biopharmaceutical production, we find that the mRNA programme nuances—but does not substantially depart from—at least three of the four key features of the status quo. Funding sources wield considerable influence over the mRNA programme, and university-based researchers and publicly funded institutions have made critical contributions to manufacturing processes and second-generation mRNA technologies. Yet, apart from requiring IP sharing inside the programme, the architects of the programme opted not to stipulate terms of affordability upon partners’ products (outside of the context of a PHEIC) on the strength of the assumption that market competition (either between the actors involved in the programme or with other mRNA manufacturers) will naturally yield that outcome. Further, the programme preserves every players’ freedom to contract with third parties. Thus, the programme’s commitment to IP sharing rests on each organization’s willingness to carry that condition through its various bilateral relationships, which have grown during the course of the programme. It is unclear if the programme provides oversight with respect to these bilateral transactions and, unlike the various legal agreements underpinning the programme, bilateral transactions between members of the South African consortium or manufacturing partners in other LMICs and third parties are also not publicly available. Finally, even though the entities involved are not embracing financialization in the manner of HIC-based biopharmaceutical companies, it appears that global market forces, rather than representatives of LMICs or local health needs, will ultimately decide what pathogens are prioritized for product development and whether Afrigen, the company at the heart of the programme, has a sustainable business model. To their credit, WHO and MPP are trying to seed collaboration within the programme, match-making participants around pathogens of shared interest and regional need [ 90 ]. But with trust among the diverse players still a work-in-progress, asymmetries in the programme’s legal architecture in terms of who stands to earn IP royalties and where products generated with the help of the programme can be sold, and funding shortfalls on the horizon for the hub in Cape Town,(PT) it is far from clear that these collaborations within the programme will materialize before difficult decisions about licensing IP to HIC contexts will need to be made in the name of sustainability.
Rather than forming a collective enterprise, the relationships among local producers engaged in the programme are fragile and participants appear just as vulnerable to market forces as they were before the programme was created in 2021. This outcome is a by-product of how the programme has been designed, in particular, a number of choices made by MPP while constructing the programme. MPP’s pursuit of a voluntary license from Moderna or another mRNA manufacturer arguably made sense initially given the state of the global health emergency when the programme began. However, MPP’s preference for a voluntary deal with an HIC-based manufacturer also appears to have undercut Bio-Manguinhos’ bid to become one of the mRNA hubs back in 2021. Bio-Manguinhos proposed building up end-to-end mRNA manufacturing capacity and ‘South-to-South’ technology transfer—a vision that the programme is now coming around to two-plus years later. The moment that a voluntary license from an established entity was not forthcoming, a deeper rethink of the programme’s architecture and norms was needed. Instead, most of the features of status quo biopharmaceutical production have been carried through the mRNA programme and MPP continues to court voluntary agreements from Moderna and other HIC-based biopharmaceutical manufacturers [ 91 ].
From remotely managed tech transfer to LMIC manufacturer empowerment
The mRNA programme’s design reflects both the resource constraints that (in the absence of more funding) the programme must operate under as well as MPP’s own institutional preferences around IP, competition, and solving access problems through voluntary (as opposed to mandatory) mechanisms. Without more funding from HIC governments, philanthropic organizations, or other sources, MPP was likely hard-pressed to demand that participating manufacturers agree to pricing constraints for mRNA products outside of a PHEIC. The money to make mRNA vaccines, in short, has to come from somewhere. Still, focusing exclusively on that economic reality obscures the high degree of control that the programme’s architects have maintained over all decision-making, resource allocation, and technology transfer activities.
Representatives from LMICs, including South Africa, are largely excluded from the programme’s governing structures. Likewise, civil society organizations from LMICs are not meaningfully represented on the programme’s STeRCo or mSAC committees. All of the funding for the programme sits in Geneva; only SteRCo and mSAC can decide if and when funding should be allocated. When Afrigen or another entity that is part of the programme is in need of additional funds, for instance, for the purposes of scaling up the next phase of technology transfer, or to pay for manufacturing equipment, it must engage in negotiations with MPP in order to access funding. Technology transfer, too, has to date been managed almost entirely by MPP’s new technology transfer unit notwithstanding the inefficiencies that this approach may carry.
It remains to be seen whether MPP, which has ascended in the sphere of global health during the pandemic as a result of its role as the central power broker for the entire mRNA programme, will over time cede some of its control and take the steps necessary to truly empower LMIC manufacturers. The move to create R&D consortia as part of the programme, constructed around pathogens of shared interest, holds promise. Providing FTO opinions to LMIC manufacturers when needed, advising LMIC governments about when and how to invoke compulsory licenses, stepping back from micromanaging technology transfer among programme participants—all of those actions stand to re-distribute control and decision-making authority to the researchers, organizations, and governments in LMICs. Yet, compulsory licensing runs counter to MPP’s way of doing business with established manufacturers and relinquishing control over the exchange of mRNA technology is in tension with the foundation’s newfound mantle as the go-to facilitator of technology transfer within the sphere global health.
Far from unique to the mRNA programme, conflicts around power and control are commonly in play within multilateral initiatives. In theory, “multistakeholder models of governance promise greater participation of different stakeholders;” yet, in practice, “these models can also undermine the authority of intergovernmental organizations, while expanding opportunities for powerful private actors to exert influence over governing structures, and concentrating power among parties with less democratic accountability to poorer countries and populations [ 12 ].” MPP’s rise has added to the “forum shifting”, turf wars, and competition that preoccupies a number of global health institutions claiming, or already commanding, political capital from Geneva [ 78 , 81 ]. Too often in the past initiatives with the stated intention of combatting neglected diseases in LMICs have remained under the control of organizations rooted in HICs [ 92 ]. Claiming ownership over the mRNA programme, its architects see themselves as “giving” mRNA technology to LMIC partners.(MF,CG,MPK) Many partners in turn express gratitude to be involved but, as often occurs when power imbalances exist, remain subjugated by the gift [ 81 ]. If the mRNA programme’s “decolonial aspirations” are to be realized [ 93 ], participating manufacturers in LMICs must be more empowered to collaborate South-to-South, build technological capacity, and generate mRNA interventions that are responsive to the health needs of, and affordable for, local populations.
Translating investments in the mRNA programme into LMIC-centered scientific infrastructure, participatory rights, and power
The mRNA programme has in one sense already succeeded. Producing an mRNA COVID-19 candidate with limited assistance from outside actors in a six-month timeframe demonstrates the South African hub’s technical capacity. From another perspective, the programme’s success should not be construed exclusively in terms of product development, but its potential as a lasting safeguard of local production capacity in LMICs. Afrigen and the programme’s partners are moving on to target other pathogens. Even if those efforts do not yield safe and effective mRNA vaccines against TB, RSV, malaria, and other priority diseases, the programme can still have a lasting impact by generating an accessible body of scientific knowledge, tools, and people with the know-how to apply them—provided that those outputs remain within the reach of, and are responsive to, LMIC health needs.
The idea of linking public funding for biopharmaceutical innovation to public scientific infrastructure, participatory rights, and power gained renewed attention during COVID-19 [ 47 , 88 , 94 – 96 ]. However, most governments demanded little to nothing in terms of IP sharing, equitable access, and reasonable pricing commitments from biopharmaceutical companies in exchange for the massive public investments that were made towards mRNA vaccines, among other interventions [ 45 , 49 , 96 ]. The mRNA programme improves on this state of affairs by requiring sharing of IP among the South African universities and LMIC partners who have signed onto the programme. Yet it does not stop SAMRC-funded university labs from also licensing their IP to actors in HICs or LMIC partners from entering into bilateral deals that capitalize upon the IP and know-how they have gained through the programme. Quantoom, the Belgium-based company that is formally outside the programme and thus not subject to its IP sharing commtiments, has struck partnerships with the majority of the programme’s manufacturers. Afrigen, for its part, can be sold to a third party if and when its primary shareholder decides that that course of action offers greater return than continued participation in the mRNA programme. All of these flexibilities that were built into the programme’s design introduce a risk that the knowledge, tools, and know-how generated by the mRNA programme may be exploited or extracted by outside corporations that do not share the goal of improving equitable access in LMICs.
To mitigate that risk and ensure that the programme’s outputs remain grounded in LMICs, a number of changes to the programme’s legal architecture and governance structures are worthy of consideration. First, the programme’s commitment to transparency should be extended throughout its operations. In particular, there must be greater transparency within the programme, such that Afrigen and other manufacturers can participate in, and have an understanding of, the decisions that are made by STeRCo and mSAC as well as the expectations of funders, including HIC governments. Similarly, the effort to make the programme’s legal architecture transparent should encompass any bilateral deals that members of the South African consortium or LMIC manufacturing partners secure. Making those transactions transparent will help guard against the risk that the programme’s collective IP and know-how will be captured by commercial outsiders. Second, representatives from LMICs taking part in the programme as well as civil society organizations from LMIC regions must have a stronger voice in the mRNA programme’s decision-making structures. Running forums with LMICs and civil society after decisions have already been made by STeRCo or mSAC is insufficient. It distances the work of the programme from the very people it is intended to serve. Third, the prospect of Afrigen—the hub at the centre of the programme—being acquired by an outside entity, or dissolved altogether due to financial challenges, must be more proactively addressed. The architects of the mRNA programme contend that outcome is essentially inevitable. However, steps can be taken to ensure that the publicly funded R&D infrastructure, product leads, and scientific know-how that Afrigen has amassed are not lost in the event of a private acquisition or insolvency. In return for additional funding, WHO and MPP should require Afrigen to make all of its IP and know-how available to the other members of the programme. Further, WHO and MPP should engage with the government of South Africa to see if corporate restructuring, acquisition by the state, or some other strategic investment offers a means to preserve public control over the knowledge and assets that Afrigen, in collaboration with others inside and outside the consortium, has developed during the course of the programme.
Public investment intended to improve equitable access to health interventions, such as mRNA vaccines, requires policy innovation in the form of participatory rights for LMIC communities and a protected stake in the scientific infrastructure and knowledge that that investment generates [ 96 , 97 ]. Revised with those fundamental goals in mind, the mRNA programme can position LMICs to lead where many HICs fell short in the context of COVID-19.
The provision of a technological solution, including vaccines, is no safeguard for equitable access. Attention to social context and structural challenges is needed to realize technology’s emancipatory potential. Our situational analysis of the WHO’s technology transfer mRNA programme, including semi-structured interviews with 35 individuals involved to varying degrees in the programme, suggests that the needs and perspectives of LMICs are not sufficiently centered in the programme. Further, the architects of the programme are working within the existing system of biopharmaceutical production and, at the same time, preserving their own control over the programme’s design and preferred measures meant to remedy shortfalls in equitable access to mRNA-based interventions. In particular, MPP continues to champion voluntary IP licensing as the optimal means to improve local production capacity in LMICs even though that mechanism did not attract collaboration from more established mRNA manufacturers in the context of COVID-19 and slowed adoption of a more transformative end-to-end approach to R&D and manufacturing. The technological outcomes of the mRNA programme remain uncertain. Absent significant reform and concerted effort to redistribute not just IP, but agency to LMIC actors, there is a significant risk that the programme, which is claimed by WHO and MPP as a collective effort to improve manufacturing capacity in LMICs for LMICs, will not solve the problem of equitable access to biopharmaceutical innovation.
Supporting information
S1 letter. response from global affairs canada to access to information request..
https://doi.org/10.1371/journal.pgph.0003173.s001
S1 Document. Disclosure Package from Global Affairs Canada.
https://doi.org/10.1371/journal.pgph.0003173.s002
S1 Table. Comparison of Legal Agreements Underlying the mRNA Programme.
https://doi.org/10.1371/journal.pgph.0003173.s003
S2 Table. mRNA Related Patent Filings in Countries with Participants in the mRNA Programme.
https://doi.org/10.1371/journal.pgph.0003173.s004
Acknowledgments
The authors express our gratitude to Morris Odeh, PhD student at Schulich School of Law, Dalhousie University, for research assistance on mRNA patent data in South Africa and LMICs; to the members of the IEAC (Fatima Hassan, Françoise Baylis, Jason Nickerson, Reshma Ramachandran, Srinivas Murthy, and Viviana Munoz) for their contributions and insightful feedback to this work and conversations that helped shape the form and substance of this manuscript and research project at large; to the Health Justice Initiative (HJI) for their support and engagement throughout this process; and to Els Torreele, Amy Maxmen, Melissa Barber, and Amy Kapczynski for their feedback, suggestions, and assistance during the research and writing process. Any errors or inconsistencies are solely the responsibility of the authors. Finally, we are especially grateful to the many individuals who took part in interviews in connection with this research project. We hope our findings will play a constructive role as the work of the mRNA programme continues. All views in this paper are the authors and do not represent the views of the institutions they are affiliated with.
- View Article
- PubMed/NCBI
- Google Scholar
- 10. Farmer P, Kim JY, Kleinman A, Basilico M. Reimagining Global Health: An Introduction. Univ of California Press; 2013. 508 p.
- 15. Clarke A. Situational Analysis: Grounded Theory After The Postmodern Turn. SAGE; 2005. 412 p.
- 36. Pan-American Health Organization [Internet]. 2021 [cited 2024 Jan 8]. PAHO selects centers in Argentina, Brazil to develop COVID-19 mRNA vaccines—PAHO/WHO | Pan American Health Organization. Available from: https://www.paho.org/en/news/21-9-2021-paho-selects-centers-argentina-brazil-develop-covid-19-mrna-vaccines .
- 37. WHO PDVAC [Internet]. [cited 2023 Nov 28]. 2021 meetings: WHO Product Development for Vaccines Advisory Committee (PDVAC). Available from: https://www.who.int/news-room/events/detail/2021/04/15/default-calendar/pdvac-2021 .
- 64. Abi. Development of First African-Owned COVID-19 Vaccine [Internet]. Quantoom. 2022 [cited 2023 Oct 24]. Available from: https://quantoom.com/new-agreement-paves-way-for-development-of-first-african-owned-covid-19-vaccine/ .
- 66. Victoria And South Africa To Partner On mRNA Vaccines | Premier of Victoria [Internet]. [cited 2023 Oct 24]. Available from: http://www.premier.vic.gov.au/victoria-and-south-africa-partner-mrna-vaccines .
- 67. Holly. Introducing Quantoom Biosciences—Medicines & Autonomy Bioproduction [Internet]. Quantoom. 2021 [cited 2023 Nov 28]. Available from: https://quantoom.com/ntroducing-quantoom-biosciences-a-univercells-company-with-the-mission-to-improve-access-to-essential-medicines-and-autonomy-in-bioproduction-through-innovation/ .
- 71. Benavides X. Private Equity Firms and the Financialization of Digital Clinical Trials [Internet]. Rochester, NY; 2023 [cited 2024 Jun 2]. Available from: https://papers.ssrn.com/abstract=4565316 .
- 81. McGoey L. No Such Thing as a Free Gift: The Gates Foundation and the Price of Philanthropy. Reprint edition. London New York: Verso; 2016. 304 p.
- 94. PAHO. Landmark report charts route for reorienting economies to deliver health for all—PAHO/WHO | Pan American Health Organization [Internet]. 2023 [cited 2024 May 19]. Available from: https://www.paho.org/en/news/24-5-2023-landmark-report-charts-route-reorienting-economies-deliver-health-all .
- 97. Kapczynski A, Michaels J. Administering a Democratic Industrial Policy [Internet]. Rochester, NY; 2024 [cited 2024 Jun 2]. Available from: https://papers.ssrn.com/abstract=4711216 .
Your profile information is incomplete. Please click below to complete it.
This feature is restricted to eTISC’s member users. Please sign in or create a WIPO account.
- Reports by name
- Reports by coverage
- Map view with filters
- Table overview
- Detailed jurisdiction files
- WIPO Patent Landscape Reports (PLR)
- PLR Database
- WIPO Technology Trends
- PLR Guidelines
- Patent Analytics Open Source Manual
- Patent Analytics Handbook
- Technology Transfer Organizations
- Technology Transfer Agreements
- IP Valuation
- IP Policies for Universities
- IP Policies Database
- Academic Intellectual Assets
- Managing Technology Transfer
- Ask the expert
WIPO eTISC is a social platform which provides a secure space for the Technology and Innovation Support Center (TISC) community and intellectual property (IP) users to interact with each other and share knowledge and ideas.
Sign into eTISC
To access the full features and services of eTISC, please sign in with a WIPO account or create an account.
By accessing/using the eTISC platform, the user is agreeing to be bound by these Terms of Use .
Technology Transfer in Action case studies
Technology transfer in action case studies go back.
From inception to market diffusion to commercialization: Technology transfer is a collaborative process that supports the flow of scientific findings, knowledge, and intellectual property (IP) from creators to public and private users. But how does it work in practice? Whether through ideathons, the development of IP policies or the creation of university networks, tech transfer can take many forms.
See for yourself: https://lnkd.in/eUrmrjPB
Visit the link here: https://www.linkedin.com/feed/update/urn:li:activity:6964876203929255936
Licensing And Technology Transfer: A Glance On Indian Scenario
Contributor.

INTRODUCTION
Technology Transfer (also called Transfer of Technology (TOT) and Technology Commercialization) 1 are the processes by which the information or knowledge related to the technological aspects travel within the group or between the organizations or entity. Taking this to the broader scenario, give rise to International technology transfer in which the knowledge travels in between the countries, which is not only limited to the Knowledge and information, rather includes skill transferring, methods of manufacturing, physical assets, know-how, and other technical aspects, and henceforth helps in further development of the technology and innovation, by effectively utilizing the technology transferred and finally incorporating it.
Technology transfer has been used in the movements of technology from the laboratory to industry or from one application to another domain application or taking developing countries into consideration technology transfer helps in growing access to technologies which are related to other developed countries and henceforth helps in approaching towards the newer technologies and inventions i.e. from Developed to developing countries.
On the other hand licensing is allowance granted by the patent owner to another person or organization for using the patented invention on agreed terms and conditions, while the patent owner continues maintaining his ownership to the patent and hereafter becomes the source of income by receiving the predetermined royalties or as per the condition.
So by combining the concept of the technology transfer with the licensing one can help in taking the benefit of the technology research that has been done previously, as licensing creates the permissible structure for the transfer of the technology to a larger assembly of researchers and engineers, which will help in saving the expenses of conducting the research and the costs of maintaining development activities or facilities and hence will help in the further development of the technology which has already been done.
As now a days in the era of the advancement in the technologies there are many technologies which with the combination with the other technologies is giving birth to the other new advent technologies. so here the licensing do play the important role in providing the legal platform to utilize the combination of the technologies made or discovered by the other persons or the organization which has been created earlier, and hereafter prevents from wastage of the time and the research cost incurred in developing the earlier inventions.
FORMS OF TECHNOLOGY TRANSFER:
Technology transfer can be classified into vertical and horizontal technology transfer 2
Vertical transfer refers to transfer of technology where transmission of new technologies is done from the generation of new technology during the research and development programs into the science and technology organizations, for instance, to the application related to the industrial and agricultural sectors, or we can say that vertical transfer is the technology transfer commencing from basic research to applied research, from applied research to development followed by development to production.
While the horizontal technology transfer is the movement of a well-known technology from one equipped environment to another (from one company to another) or say refers to the transfer and use of technology used in one place or organization to another place or organization.
As discussed above generally developed countries follow the route:-
Research -> Development -> Design -> Production
While less advanced and developing countries follow the route:-
Production -> Design -> Development -> Research
Generally there are the reverse trends in the developing countries because the path to be followed depends upon the transfer, absorption, and adaptation of existing technology
(Habibie (1990), often referred to as the architect of the Indonesian aircraft industry, states that, "technology receivers must be prepared to implement manufacturing plans on a step-by step basis, with the ultimate objective of eventually matching the added-value percentage obtained by the technology transferring firm." He refers to such an approach as "progressive manufacturing" and popularized the slogan, "begin at the end and end at the beginning" implying that a transferee firm should start with production and move backwards to research.)
Today in the era of advent in technology one could choose any of the routes of the technology transfer which depends upon how the technology advancement chains of the transferor and transferee are associated.
ADVANTAGES RELATED TO TECHNOLOGY TRANSFER:
The advantages related to technology transfer comprises of the essential gain to the public who benefits from the manufactured goods that get to the market and ultimately the availability of the jobs which results from the improvement and sale of the products so formed. And hence it encourages use of technology developed and the benefiting to the society development which comes from the revenue of the tax payers. And escalating visibility to researchers and allows researcher to generate and earn royalty income and henceforth attaining financial profits for the government and the employees from royalty payments for those technology transfers that involve patent licenses.
Moreover resulting in commercialization of the researches and the discoveries made, which was the course of the investment done for the development and being protected by the patent. Hereafter all the Investments done in the course of the development in intellectual property are returned to the public through products made for the public, opportunity of more employment, and revenue in the form of taxes.
Technology transfer strengthens industry by identifying new business opportunities which contributes to enhancing the know-how and competitiveness of the technology providers, which ultimately results in broadening the business area and re-focusing to the technologies and systems to serve several different fields. In addition, technology transfer promotes the wider use and awareness of technology and systems.
Technology transfer brings economic benefits by increasing revenues for both technology donors and receiver's benefits with new and better products, processes, and services that lead to increased efficiency and effectiveness, greater market share and increased profits.
Moreover technology transfer helps in earning rewards which is above and beyond the regular salary which is received through patents, licenses, and other technology transfer awards which help in benefiting intellectually and professionally through working collaboratively with their peers in the industrial sector.
DISADVANTAGES RELATED TO TECHNOLOGY TRANSFER
As technology transfer is keen or meant for the business oriented activity, hence forth there can be the chances to have financial or commercial risk, as we are well aware that Licences can generate the income, but patent application which are not licensed will only cost money.
Even when the transfer programme related to the technology transfer is successful or in particular after technology transfer institutional tensions may arise within the organization which may be in between the recipient of licensing income and those who know they will never make utilizable inventions. For the sake of remedy in those circumstances Institutional policies can be made aiming to have partial rearrangement of income received by license between all research groups but, using this strategy may not eradicate the problem rather in most of the cases discoverer will be frustrated or disappointed because the income that they have earned is given to other groups. Technology transfer activities may put researchers in conflict of interest situations, especially when the transfer involves the creation of the spin- off company, hence Institutions should be aware of these possible dangers.
Moreover problem can be because of non performance of licensee. And may be the licensee has limited chances beyond the license scope unless future enhancements to patent included in initial agreement and Unrealistic expectations and demands from licensor.
INDIAN SCENARIO REGARDING THE LICENSING AND TECHNOLOGY TRANSFER
Technology in India is growing exponentially and has played an important role in all round development and growth of economy in the country, India has opted for a wise mix of original and imported technology. Henceforth "Technology transfer" plays a very important role and is generally covered by a technology transfer agreement.
Developing countries like India generally not follow the usual path for development with regard to technologies but use their advantage in the cutting edge technology options which is now available and put the tools to use this modern technology.
Technology transfer is assumed to get benefits from R&D which is shared with the developing and underdeveloped countries , so taking this to the point of consideration National research laboratories is been constructed by the Indian government for the purpose of R&D which is yet to be commenced by the private sectors.
India generally comprises of Small and medium enterprises and is growing since liberalization, which has resulted in growth of The multinational enterprises, which in turn is competing with the international companies which has enhanced the confidence of India. Not only confined to the pharmaceuticals but is broadly categorized in other areas too such as agriculture, dairy and other technologies.
Government of India is in the verge to open Technology Transfer Offices, Universities, institutions which will be funded by central government and will acts as mechanism for transferring or exporting the research conducted and its outcome to the desired place.
Though some of the Indian Institutes have been already commercializing their research and are successful in technology transfer in which they have been licensed as technologies to industry. Moreover, numerous cases of technology transfer are seen in India by various well-known institutions.
Technology transfer and its licensing have played a crucial role in all round development and the advent of the technology which in results help in the development of the economy of the country. Hence forth helps in creating the wealth to the country.
India as a developing country need to work on the technology development and technology transfer and needs to make a building strategy comprising of the construction of new offices related to technology transfer and to make youngsters aware to the benefits related to the technology transfer, by establishing the specified universities and henceforth increasing the pace of the technology transfer and technical research and development in technical perspective.
Finally as discussed we can conclude that there is the possible advantage and disadvantage of the technology transfer. But we have to see this in the broader aspect so that our country as well as the citizen of our country should be benefited.
1. http://en.wikipedia.org/wiki/Technology_transfer
2. http://www.businessasia.net/Pdf_Pages/Guidebook%20on%20Technology%20Transfer%20Mechanisms/ An%20overview%20of%20TT%20and%20TT%20Models.pdf
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

Intellectual Property
Mondaq uses cookies on this website. By using our website you agree to our use of cookies as set out in our Privacy Policy.

A comprehensive study on the physicochemical characteristics of faecal sludge from septic tank and single pit latrine facilities in a typical semi-urban Indian town: a case study of Rajasthan, India

First published on 16th September 2024
| The Swachh Bharat Mission (SBM) in India, launched in 2014, built 110 million toilets (including public and individual household toilets) to eradicate open defecation. In India, 90% of the population relies on onsite sanitation (OSS). In addition to that, due to the SBM, there was a rapid increase in the usage of OSS. Consequently, if untreated, the rise in faecal sludge (FS) from OSS poses a significant pollution risk to surface and groundwater. This case study characterized FS to aid sanitation stakeholders in designing treatment systems in Indian towns and comparable nations globally, ensuring efficient treatment and resource recovery and protecting water quality, directly contributing to achieving SDG6. |
1. Introduction
Wastewater is water generated from domestic, commercial, and industrial sources containing chemicals and heavy metals. FS is entirely different from usual wastewater since FS is a sludge that accumulates in OSS systems, such as septic tanks, pit latrines, and composting toilets. Thus, the characteristics of wastewater and FS differ widely, even if they are produced in the same geographical location. The overall concentrations of total solids, organic content, ammonia, total nitrogen, and helminth eggs are 10–100 times more in FS than they are in wastewater sludge. 2,3,6 Thus, FS treatment is an essential aspect of FSM for its safe disposal and resource recovery from FS. Co-treatment with sewer-based wastewater treatment technology is one option for treating FS. However, most wastewater treatment plants in low-income nations have failed because of improper loading rates and greater FS strength compared to municipal wastewater. 7 Thus, faecal sludge treatment plants (FSTPs) are essential for treating FS from OSS for safe disposal, especially in low-income countries. FS characterization plays a vital role in choosing/designing FS treatment technologies. However, FS raw data are highly location-specific and less uniform compared to wastewater data. 8
FS is higher in chemical oxygen demand (COD), biochemical oxygen demand (BOD), total solids (TS), total nitrogen (TN), nutrients, and pathogen content. The large variability in FS and various OSS in use, such as pit latrines, septic tanks, and dry toilets, makes it difficult to assess the FS generation rate and the average FS characteristics. 9 For example, a study in Bangangte, Cameroon 10 found that the TS value of FS was higher in pit latrines (15.90 g l −1 ) compared to septic tank FS (1.92 g l −1 ). Similarly, another study in Vadgaon Maval, Maharashtra 11 analysed septic tank FS samples by age and found that the TS and COD in FS increase with age. A study in Chennai, India 12 found that the total solid content of FS is 1.6 times more in the winter than in the summer. The cleaning frequency of OSS is influenced by demographic factors like population density, household size, socioeconomic status, and urbanization levels, which affect the volume of FS generated and the size of the OSS system. Inputs like excreta, blackwater, greywater, and additives, factors like the type of containment (septic tanks, single pits, cesspool, dry toilets, etc. ), demographic factors (urban, rural, cleaning frequency), and environmental factors (climate, topography) affect the FS characterization. 13
As of 2023, India remains the most populous country, and many Indians face serious health issues due to contaminated soil and water resulting from inadequate sanitation practices. 14 The Sustainable Development Goals (SDG) of UN 2015 include SDG 6, which aims to provide everyone with clean water and safely managed sanitation systems. The Swachh Bharat Mission (SBM) is a country-wide campaign by the Government of India to eradicate open defecation and make open-defecation-free towns and villages. Under the SBM, 110 million toilets (including public and individual household toilets) have been built in towns and villages nationwide to combat open defecation. 15 The stages in constructing 110 million toilets nationwide under the SBM are shown in Fig. 1 .
| Stages of the SBM in providing toilets to households. | ||
1.1. Septic tank and single-pit latrine
2. materials and methodology, 2.1. study area information.
| Study area, Pilani located in the state of Rajasthan, India. | ||
2.2. Sample collection
| (1) |
| Sample collection locations in the study area. | ||
2.3. Questionnaire
2.4. sample preservation, 2.5. sample preparation, 2.6. fs characterization.
| S. no | Parameters | Analysis methods/instruments | Standardization methods |
|---|---|---|---|
| 1 | Temperature, pH & EC | pH meter and electrical conductivity meter | Calibration standard solutions |
| 2 | Total dissolved solids | Benchtop meter | Calibration standard solutions |
| 3 | Total solids | Volumetric and gravimetric methods by oven drying | Analysis protocol: the oven was maintained at 105 to 110 °C. The crucible was preheated and dried before testing |
| 4 | Total suspended solids | Oven drying method/digital meter | |
| 5 | Chemical oxygen demand | Closed reflux titrimetric method | Potassium hydrogen phthalate (KHP) stock solution with a theoretical COD value of 400 mg l |
| 6 | Biochemical oxygen demand | Winkler's method/5-day method | Titration of sodium thiosulfate with standard potassium iodate and Millipore water solution results in consistent and reproducible results of less than 0.05 ml |
| 7 | Total nitrogen | Total nitrogen analysers | Standard calibration curve |
| 8 | Total phosphorus | Vanadomolybdate yellow color method | Standard phosphorus stock solutions |
| 9 | Faecal coliform | Sample ready culture medium-coliform count plates | — |
| 10 | Capillary suction time (CST) | Capillary suction timer | Calibrated by the manufacturer |
| Methodology of the case study. | ||
3. Results and discussion
3.1. questionnaire results.
| FS samples | Sample set number | Type of OSS | Type of building | Dimensions of OSS | Age of FS sample | Type of sample | No. of people in the household | Remarks |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Single pit | House | 0.9 m × 0.9 m × 8 m | >1 year | Yellowish liquid | 7 | Lined pit |
| 2 | FS + blackwater | |||||||
| 3 | ||||||||
| 2 | 4 | Single pit | House | 4 m depth with 0.6 m diameter | 1.5 years | Yellowish liquid to slurry | 6 | Lined pit |
| 5 | FS + blackwater | |||||||
| 3 | 6 | Two-chamber septic tank | House | 1 m × 1.4 m × 1.8 m | 2 years | Greenish-black liquid | 5 | FS + blackwater |
| 7 | ||||||||
| 8 | ||||||||
| 4 | 9 | Single pit | House | 4 m depth with 0.7 m diameter | 2 years | Yellowish-black liquid | 6 | Lined pit |
| 10 | FS + blackwater | |||||||
| 11 | ||||||||
| 5 | 12 | Square | House | 3.5 m depth with 3 m × 3 m surface area | 2 years | Brownish-yellow thick slurry | 7 | Lined pit |
| 13 | Single pit | FS + blackwater | ||||||
| 14 | ||||||||
| 6 | 15 | Two-chamber septic tank | House | 2 m × 1.7 m × 1.6 m | 2 years | Black liquid | 5 | FS + blackwater |
| 16 | ||||||||
| 17 | ||||||||
| 7 | 18 | Single pit | House | 4 m depth with 1 m diameter | 2.5 years | Greenish black slurry | 2 | FS + blackwater |
| 19 | ||||||||
| 20 | ||||||||
| 8 | 21 | Two-chamber septic tank | Hotel | 2 m × 2.7 m × 2.5 m | 3 years | Dark black liquid | 15 workers + moving population | FS + blackwater + greywater |
| 22 | ||||||||
| 23 | ||||||||
| 9 | 24 | Two-chamber septic tank | Bakery | 1.5 m × 2.5 m × 2.1 m | 3 years | Light yellow liquid | 5 | FS + bakery wastewater |
| 25 | ||||||||
| 26 | ||||||||
| 10 | 27 | Septic tank | House | 2 m × 3.1 m × 1.5 m | 3 years | Yellow liquid | 3 | FS + blackwater |
| 28 | ||||||||
| 29 | ||||||||
| 11 | 30 | Two-chamber septic tank | Sweet shop | 2 m × 1 m ×1.8 m | 3.5 years | Yellowish-black liquid sample | 5 workers | FS + blackwater + greywater |
| 31 | ||||||||
| 12 | 32 | Two-chamber septic tank | House | 1.8 m × 1.6 m × 2 m | 3.5 years | Yellowish black liquid | 8 | FS + blackwater |
| 33 | ||||||||
| 34 | ||||||||
| 13 | 35 | Two-chamber septic tank | Hotel | 2.1 m × 3.1 m × 1.5 m | 4 years | Light yellow liquid | 10 workers + moving population | FS + blackwater + kitchen wastewater |
| 36 | ||||||||
| 37 | ||||||||
| 14 | 38 | Single pit | House | 5 m depth with 1 m diameter | 4 years | Dark green slurry | 4 | Unlined pit |
| 39 | FS + blackwater | |||||||
| 40 | ||||||||
| 15 | 41 | Single pit | House | 7 m depth with 0.6 m diameter | 5 years | Dark yellowish-brown slurry | 6 | Unlined pit |
| 42 | FS + blackwater | |||||||
| 43 | ||||||||
| 16 | 44 | Two-chamber septic tank | Complex shops | 2.2 m × 3.1 m × 2 m | 6 years | Dark brown slurry | 5 | FS + blackwater |
| 45 | ||||||||
| 46 | ||||||||
| 17 | 47 | Two-chamber septic tank | Shop | 2.1 m × 1.8 m × 1.9 m | 6 years | Yellowish black slurry | — | FS + blackwater + greywater |
| 48 | ||||||||
| 49 | ||||||||
| 18 | 50 | Single pit | House | 4.5 m depth with 0.8 m diameter | 6 years | Greenish slurry | 4 | FS + blackwater |
| 51 | ||||||||
| 52 | ||||||||
| 19 | 53 | Single pit | House | 7 m depth with 0.8 m diameter | 7 years | Yellowish brown slurry | 5 | Unlined pit |
| 54 | FS + blackwater | |||||||
| 55 | ||||||||
| 20 | 56 | Composite sample | — | — | Composite sample of 7 years and 1 year | Greenish yellow slurry | — | FS + blackwater + greywater |
| 57 | ||||||||
| 58 | ||||||||
| 21 | 59 | Single chamber septic tank | House | 1.5 m × 1.5 m × 1 m | 8 years | Dark green slurry | 6 | Unlined tank |
| 60 | FS + blackwater | |||||||
| 61 | ||||||||
| 22 | 62 | Single chamber septic tank | House | 1.8 m × 1.5 m × 1.2 m | 8 years | Greenish black slurry | 8 | FS + blackwater |
| 63 | ||||||||
| 64 | ||||||||
| 23 | 65 | Composite sample | — | — | Composite samples of 9 years and 1 year | Greenish-yellow slurry | — | FS + blackwater |
| 66 | ||||||||
| 67 | ||||||||
| 24 | 68 | Single pit | House | 5 m depth with 0.9 m diameter | 9 years | Dark blackish slurry | 7 | FS + blackwater |
| 69 | ||||||||
| 70 | ||||||||
| 25 | 71 | Single pit | House | 10 m depth with 0.8 m diameter | 10 years | Greenish-yellow slurry | 4 | Unlined pit |
| 72 | FS + blackwater | |||||||
| 73 | ||||||||
| 26 | 74 | Single pit | House | 6 m depth with 1 m diameter | 10 years | Dark greenish colour, thick slurry | 9 | Unlined pit |
| 75 | FS + blackwater | |||||||
| 76 | ||||||||
| 27 | 77 | Composite sample | — | — | Composite samples of 11 years and 8 years | Greenish-black slurry | — | FS + blackwater + greywater |
| 78 | ||||||||
| 79 | ||||||||
| 28 | 80 | Two-chamber septic tank | House | 2.2 m × 1.8 m × 1.5 m | 12 years | Brownish black liquid | 10 | FS + blackwater |
| 81 | ||||||||
| 82 | ||||||||
| 29 | 83 | Two-chamber septic tank | House | 2.6 m × 2.6 m × 2 m | 13 years | Yellowish-brown slurry | 3 | FS + blackwater |
| 84 | ||||||||
| 30 | 85 | Single pit | House | 12.1 m depth with 0.7 m diameter | 16 years | Dark black slurry | 4 | Unlined pit |
| 86 | FS + blackwater |
3.2. Physical examination of faecal sludge samples
| Stages of FS decomposition (by physical examination interpretation). | ||
3.3. Temperature, pH, and electrical conductivity
| Temperature, pH, and EC of FS samples collected from Pilani, Rajasthan. | ||
3.4. Total solids
| TS, TSS, and TDS of FS samples collected from Pilani, Rajasthan. | ||
| EC–TDS correlation of FS samples collected from Pilani, Rajasthan. | ||
3.5. Chemical oxygen demand (COD) and biochemical oxygen demand (BOD)
| COD, COD & TS correlation and BOD/COD ratio of FS samples from Pilani, Rajasthan. | ||
| COD & TS correlation of FS samples from Pilani, Rajasthan. | ||
| COD & BOD correlation of FS samples from Pilani, Rajasthan. | ||
3.6. Faecal coliform
| Faecal coliform count, TN concentration, and TP concentration in FS samples from Pilani, Rajasthan. | ||
3.7. Total nitrogen
3.8. total phosphorus, 3.9. capillary suction time (cst).
| CST apparatus and CST values measured for FS samples from Pilani, Rajasthan. | ||
4. FS treatment options
| FS treatment methodology. | ||
4.1. Site-specific FS treatment system
Settling and Imhoff tanks are other types of dewatering techniques in which FS treatment starts by separating solid FS and liquid parts using settling and thickening tanks. In Imhoff tanks, the mechanism involved is anaerobic digestion and settling; these principles combine to treat FS. 31 Mechanical dewatering consists of a belt filter press, screw press, and centrifuge. This equipment removes water from sludge and produces a thick, dried sludge cake. The removal efficiencies and loading rates of various dewatering techniques available from the literature are given in Table 3 .
| Dewatering methodology | Sludge loading rate | Removal efficiency |
|---|---|---|
| Belt filter press | 218–272 kg TS h m | 80–90% TS removal |
| Unplanted drying beds | 196 to 321 kg TS m y | 80% TS, 69% COD and 76% BOD removal |
| Settling tank | 0.16 m m | 60–70% of TSS removal |
| Planted drying bed | 300 kg TS m y | 90% BOD and 77% COD removal |
In the Pilani context, a semi-urban, arid tier-III town, an effective dewatering method can be a drying bed. Mechanical dewatering involves the establishment of high-cost equipment along with power motors to dewater the sludge, which cannot be suitable for the Pilani context because of more initial investments. Operation and maintenance costs will also be high due to the high electricity requirement and skillful labor. Settling and thickening tanks require an initial construction cost and more land, which is unsuitable for dense tier-III towns. Pilani is an arid region where the maximum temperature can reach around 45–48 °C, so drying beds can be a viable and sustainable option for dewatering in Pilani because more sunny days can increase the efficiency of drying beds. Also, planted/unplanted drying beds involve direct dumping of FS on the top surface, so electricity and motors are not required for the functioning of drying beds, which indicates less operation and maintenance cost.
In Pilani's local context, composting can be a viable option since it is a cheaper and more efficient method. Agriculture is a significant occupation in the local context of most tier-III Indian towns, so producing manure from FS makes a sustainable FSM model.
The treatment system suggested based on the characterization of FS for treating FS in the local context of Pilani and other tier-III towns can be hybridization of a drying bed, composting, and coagulation, as shown in Fig. 15 . A zero FS discharge model can be achieved in which treated FS can be used as manure and treated leachate can be used for domestic water consumption. Zero waste discharge can make the FSM service chain safe and sustainable.
| Suggested line of treatment for FS in this case study. | ||
| S. no | Parameters | Minimum | Maximum | Lower quartile | Upper quartile | Median | Mean | Standard deviation |
|---|---|---|---|---|---|---|---|---|
| 1 | Temperature (°C) | 20.6 | 27.5 | 22.425 | 26 | 24.1 | 24.15 | 1.916 |
| 2 | pH | 4.64 | 7.93 | 7.352 | 7.737 | 7.54 | 7.316 | 0.702 |
| 3 | EC (mS cm ) | 1.857 | 6.315 | 3.696 | 4.915 | 4.346 | 4.305 | 1.064 |
| 4 | Total solids (mg l ) | 3430 | 95 | 18 | 66 | 34 | 42 | 27 |
| 5 | TSS (mg l ) | 1098 | 90 | 16 | 62 | 30 | 38 | 26 |
| 6 | TDS (mg l ) | 1773 | 6807 | 3432.5 | 4767 | 4100.5 | 4111.25 | 1154.66 |
| 7 | COD (mg l ) | 4406 | 160 | 20 | 96 | 44 | 58 | 42 |
| 8 | BOD (mg l ) | 780 | 16 | 5550 | 12 | 7000 | 8409.886 | 4132.499 |
| 9 | BOD/COD | 0.0095 | 0.4375 | 0.12857 | 0.225 | 0.14586 | 0.19136 | 0.0889 |
| 10 | Escherichia coli (CFU ml ) | 1.2 × 10 | 1.6 × 10 | 2 × 10 | 5.5 × 10 | 9.5 × 10 | 3.24 × 10 | 4.75 × 10 |
| 11 | Klebsiella pneumoniae (CFU ml ) | 4.4 × 10 | 4 × 10 | 2.3 × 10 | 1.5 × 10 | 10 | 1.03 × 10 | 1.51 × 10 |
| 12 | Serotype enteritidis (CFU ml ) | 7 × 10 | 10 | 8 × 10 | 3 × 10 | 8 × 10 | 2.38 × 10 | 3.29 × 10 |
| 13 | Total nitrogen (mg l ) | 81.7 | 709.2 | 192.7 | 364.9 | 248.8 | 297.894 | 148.917 |
| 14 | Total phosphorus (mg l ) | 285 | 4471 | 996.7 | 1957.281 | 1362.43 | 1590.437 | 840.3370 |
| 15 | CST (s) | 149 | 1256.8 | 248.4 | 661.55 | 442.6 | 503.6531 | 272.0384 |
| Study description | COD (mg l ) | BOD (mg l ) | Total solids (mg l ) | Faecal coliforms |
|---|---|---|---|---|
| FS characteristics in Ghana | 49 | 7600 | 52 | — |
| FS characteristics in Thailand | 39 | 8240–123 | ||
| FS characteristics in Ghana | 201 | 56 | 132 × 10 CFU ml | |
| FS (septage) characteristics in India | 960–6080 | — | 1000–123 | Total coliform of 10 –10 No L |
| Septage characteristics in India | 6656 | 1896 | 17 | — |
| FS characteristics in Ghana | 48 | 5280 | 55 | — |
| FS characteristics in Burkina Faso | 12 | 2126 | 13 | |
| This present case study of Pilani | 4406–160 | 780–16 | 3430–95 | E. coli – 3.24 × 10 CFU ml |
| K. pneumoniae – 1.03 × 10 CFU ml | ||||
| S. enteritidis – 2.38 × 10 CFU ml |
5.1. Factors influencing the variations in faecal sludge characteristics
From the ANOVA test, it is also observed that COD and total solids also vary based on the OSS type with p -values of 0.044 and 0.002, respectively, indicating that the OSS type significantly affects the FS characteristics. The OSS type also affects the BOD and total nitrogen, which can be observed from p -values of 0.007 and 0.016, respectively. Surprisingly, the OSS system did not affect pH, possibly due to the same anaerobic conditions observed in Pilani among all OSS. Also, the BOD/COD ratio was not affected by the OSS type, which suggests that, irrespective of the OSS type, as the age of the FS increases, the BOD/COD ratio tends to decrease because of the less biodegradable organic matter due to mineralization. Greywater inclusion into the OSS also affects the FS characteristics, mainly because FS dilution reduces the total solids ( p = 0.011). It is observed that the pH value was also affected due to the inclusion of greywater because of the mixing of acidic kitchen wastewater with the OSS ( p < 0.01). In assessing differences in FS characteristic parameters with independent variables, the FS age, OSS type, and greywater content of FS significantly affected at least some of the FS characteristic parameters, as shown in Table 6 of p -values from the one-way ANOVA test. The statistically significant p -values ( p < 0.05) are highlighted in bold.
| Variables compared | pH | Temperature | TS | COD | BOD | BOD/COD | TN | CST | EC | TP |
|---|---|---|---|---|---|---|---|---|---|---|
| Age of FS (1–16 years) | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Type of OSS system (septic tank vs. single-pit) | 0.458 | 0.001 | 0.002 | 0.044 | 0.007 | 0.844 | 0.016 | 0.624 | 0.699 | 0.963 |
| Grey water inclusion (with or without greywater) | <0.001 | 0.406 | 0.011 | 0.223 | 0.045 | 0.517 | 0.033 | 0.064 | 0.554 | 0.097 |
6. Discussions and suggestions
6.1. fs age, 6.2. type of oss containment, 6.3. water input to oss, 6.4. addition of water during emptying, 6.5. other factors, 6.6. socio-economic aspects, 6.7. suggestions specific to the study area, 6.8. challenges associated with recommendations, 6.9. role of faecal sludge management in achieving sdg6.
| Contribution of FSM to SDG6: clean water and sanitation. | ||
7. Limitations of the study
8. conclusion, disclosures and declarations, ethics approval and consent to participate, availability of data and material, disclosure statement, data availability, author contributions, conflicts of interest, acknowledgements.
- L. Strande, L. Schoebitz, F. Bischoff, D. Ddiba, F. Okello, M. Englund, B. J. Ward and C. B. Niwagaba, Methods to reliably estimate faecal sludge quantities and qualities for the design of treatment technologies and management solutions, J. Environ. Manage. , 2018, 223 , 898–907 CrossRef PubMed .
- Faecal sludge management: systems approach for implementation and operation , ed. L. Strande and D. Brdjanovic, IWA publishing, 2014 Search PubMed .
- S. Singh, N. Hariteja, T. R. Prasad, N. J. Raju and C. Ramakrishna, Impact assessment of faecal sludge on groundwater and river water quality in Lucknow environs, Uttar Pradesh India, Groundw. Sustain. Dev. , 2020, 11 , 100461 CrossRef .
- K. Velkushanova, D. Brdjanovic, T. Koottatep, L. Strande, C. Buckley and M. Ronteltap, Methods for faecal sludge analysis , IWA publishing, 2021 Search PubMed .
- World Health Organization, Guidelines on sanitation and health , Geneva: World Health Organization (WHO), 2018, Contract No.: Licence: CC BY-NC-SA.3.0 IGO, https://iris.who.int/bitstream/handle/10665/274939/9789241514705-eng.pdf?sequence=25 Search PubMed .
- M. Jain, M. Upadhyay, A. K. Gupta and P. S. Ghosal, A review on the treatment of septage and faecal sludge management: a special emphasis on constructed wetlands, J. Environ. Manage. , 2022, 315 , 115143 CrossRef PubMed .
- C. M. Lopez-Vazquez, B. Dangol, C. M. Hooijmans and D. Brdjanovic, Co-treatment of faecal sludge in municipal wastewater treatment plants, Faecal Sludge Management—Systems Approach Implementation and Operation , IWA Publishing, London, UK, 2014, pp. 177–198 Search PubMed .
- U. Heinss, S. A. Larmie and M. Strauss, Characteristics of faecal sludges and their solids-liquid separation , EAWAG/SANDEC, Duebendorf, Switzerland, 1999 Search PubMed .
- C. B. Niwagaba, M. Mbéguéré and L. Strande, Faecal sludge quantification, characterisation and treatment objectives , IWA publishing, London, 2014 Search PubMed .
- C. Wanda, E. S. Kengne, G. V. Wafo, W. A. Nzouebet, P. Nbendah, Y. A. Ngandjui, L. Zapfack and I. M. Noumsi, Quantification and characterisation of faecal sludge from on-site sanitation systems prior the design of a treatment plant in Bangangte, West Region of Cameroon, Environ. Challenges , 2021, 5 , 100236 CrossRef .
- N. Chandana and B. Rao, Assessing inter and intra-variation in the characteristics of faecal sludge from Vadgaon Maval, Maharashtra: For better faecal sludge management in India, J. Environ. Manage. , 2021, 300 , 113634 CrossRef PubMed .
- D. Krithika, A. R. Thomas, G. R. Iyer, M. Kranert and L. Philip, Spatio-temporal variation of septage characteristics of a semi-arid metropolitan city in a developing country, Environ. Sci. Pollut. Res. , 2017, 24 , 7060–7076 CrossRef CAS PubMed .
- K. Velkushanova and L. Strande, Faecal sludge properties and considerations for characterisation, Methods for faecal sludge analysis , 2021, pp. 15–54 Search PubMed .
- Government of India, Faecal sludge and septage management , Government of India, Ministry of Urban Development National policy, 2017 Search PubMed .
- Government of India. Ministry of Jal Shakti, Press release. Government of India, 2023, Available from: https://www.pib.gov.in/PressReleasePage.aspx?PRID=1907510#:~:text=Under%20SBM(G)%2C%20so,in%20having%20access%20to%20toilets.
- EAI. Energy Alternatives India Energy, 2011, Available from: https://www.eai.in/ref/ae/wte/typ/clas/fecal_sludge.html.
- Centre for Science and Environment (CSE). Uttar Pradesh State Policy on Faecal Sludge and Septage Management, CSE, New Delhi, 2019, Available from: https://cdn.cseindia.org/attachments/0.86741500_1562063305_Draft-UP-State-FSSM-Policy.pdf.
- Standard methods for the examination of water and wastewater , ed. E. W. Rice, R. B. Baird and A. D. Eaton, American Public Health Association, Washington, DC, 23rd edn, 2017, ISBN: 9780875532875 Search PubMed .
- M. Bassan, T. Tchonda, L. Yiougo, H. Zoellig, I. Mahamane, M. Mbéguéré and L. Strande, Characterization of faecal sludge during dry and rainy seasons in Ouagadougou , Burkina Faso, 2013 Search PubMed .
- W. G. Cochran, Sampling techniques , John Wiley& Sons, 1977 Search PubMed .
- World Health Organization (WHO), United Nations Children's Emergency Fund (UNICEF). Joint Monitoring Programme for Water Supply, Sanitation and Hygiene (JMP) , WHO and UNICEF, Geneva, 2022, Available from: https://washdata.org/data/household#!/dashboard/new Search PubMed .
- K. Velkushanova, M. Reddy, T. Zikalala, B. Gumbi, C. Archer, B. J. Ward, N. Andriessen, S. Sam and L. Strande, Laboratory procedures and methods for characterisation of faecal sludge, Methods for Faecal Sludge, Analysis , 2021 Search PubMed .
- American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF), Standard methods for the examination of water and wastewater , APHA, Washington, DC, 23rd edn, 2017 Search PubMed .
- Faecal Sludge and Septage Management (FSSM). Rajasthan Urban Infrastructure Development Project. Government of Rajasthan, 2018, Available from: https://scbp.niua.org/sites/default/files/FSSM_Policy_Rajasthan_.pdf.
- Y. Han, S. Sung and R. R. Dague, Temperature-phased anaerobic digestion of wastewater sludges, Water Sci. Technol. , 1997, 36 (6–7), 367–374 CrossRef CAS .
- C. F. Liu, X. Z. Yuan, G. M. Zeng, W. W. Li and J. Li, Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste, Bioresour. Technol. , 2008, 99 (4), 882–888 CrossRef CAS PubMed .
- H. Jothinathan and A. P. Singh, Fecal sludge characterization, treatment, and resource recovery options: a state-of-the-art review on fecal sludge management, Environ. Sci. Pollut. Res. , 2023, 30 (57), 119549–119567 CrossRef PubMed .
- R. G. Feachem, R. G. Feachem, D. J. Bradley, H. Garelick and D. D. Mara, Sanitation and disease: health aspects of excreta and wastewater management , Wiley, New York, 1983 Search PubMed .
- J. O. Drangert, Urine blindness and the use of nutrients from human excreta in urban agriculture, GeoJournal , 1998, 45 , 201–208 CrossRef .
- B. J. Ward, J. Traber, A. Gueye, B. Diop, E. Morgenroth and L. Strande, Evaluation of conceptual model and predictors of faecal sludge dewatering performance in Senegal and Tanzania, Water Res. , 2019, 167 , 115101 CrossRef PubMed .
- P. H. Dodane and M. Ronteltap, Unplanted drying beds, Faecal sludge management: systems approach for implementation and operation , 2014, pp. 141–154 Search PubMed .
- O. O. Cofie, S. Agbottah, M. Strauss, H. Esseku, A. Montangero, E. Awuah and D. Kone, Solid–liquid separation of faecal sludge using drying beds in Ghana: Implications for nutrient recycling in urban agriculture, Water Res. , 2006, 40 (1), 75–82 CrossRef .
- S. B. Joceline, M. Koné, O. Yacouba and Y. H. Arsène, Planted sludge drying beds in treatment of faecal sludge from Ouagadougou: case of two local plant species, J. Water Resour. Prot. , 2016, 8 (07), 697 CrossRef .
- I. M. Kengne, E. S. Kengne, A. Akoa, N. Bemmo, P. H. Dodane and D. Koné, Vertical-flow constructed wetlands as an emerging solution for faecal sludge dewatering in developing countries, J. Water, Sanit. Hyg. Dev. , 2011, 1 (1), 13–19 CrossRef .
- B. Kim, T. Bel, P. Bourdoncle, J. Dimare, S. Troesch and P. Molle, Septage unit treatment by sludge treatment reed beds for easy management and reuse: performance and design considerations, Water Sci. Technol. , 2018, 77 (2), 279–285 CrossRef CAS .
- J. Nikiema and O. O. Cofie, Technological options for safe resource recovery from fecal sludge , Resource Recovery and Reuse Series, 2014 Search PubMed .
- D. Koné and M. Strauss, Low-cost options for treating faecal sludges (FS) in developing countries–Challenges and performance, in 9th International IWA Specialist Group Conference on Wetlands Systems for Water Pollution Control and to the 6th International IWA Specialist Group Conference on Waste Stabilisation Ponds , Avignon, France, 2004, vol. 27 Search PubMed .
- S. Singh, R. R. Mohan, S. Rathi and N. J. Raju, Technology options for faecal sludge management in developing countries: Benefits and revenue from reuse, Environ. Technol. Innovation , 2017, 7 , 203–218 CrossRef .
- E. G. Nartey, P. Amoah, G. K. Ofosu-Budu, A. Muspratt and S. K. Pradhan, Effects of co-composting of faecal sludge and agricultural wastes on tomato transplant and growth, Int. J. Recycl. Org. Waste Agric. , 2017, 6 , 23–36 CrossRef .
- M. Manga, B. E. Evans, T. M. Ngasala and M. A. Camargo-Valero, Recycling of faecal sludge: nitrogen, carbon and organic matter transformation during co-composting of faecal sludge with different bulking agents, Int. J. Environ. Res. Public Health , 2022, 19 (17), 10592 CrossRef CAS PubMed .
- M. Ronteltap, P. H. Dodane and M. Bassan, Overview of treatment technologies, Faecal Sludge Management-Systems Approach Implementation and Operation , IWA Publishing, London, UK, 2014, pp. 97–120 Search PubMed .
- K. Samal, S. Moulick, B. G. Mohapatra, S. Samanta, S. Sasidharan, B. Prakash and S. Sarangi, Design of faecal sludge treatment plant (FSTP) and availability of its treatment technologies, Energy Nexus , 2022, 7 , 100091 CrossRef .
- E. C. Mrimi, F. J. Matwewe, C. C. Kellner and J. M. Thomas, Safe resource recovery from faecal sludge: evidence from an innovative treatment system in rural Tanzania, Environ. Sci.: Water Res. Technol. , 2020, 6 (6), 1737–1748 RSC .
- Z. Daud, H. Awang, N. Nasir, M. B. Ridzuan and Z. Ahmad, Suspended solid, color, COD and oil and grease removal from biodiesel wastewater by coagulation and flocculation processes, Procedia Soc. Behav. Sci. , 2015, 195 , 2407–2411 CrossRef .
- A. L. Ahmad, S. Sumathi and B. H. Hameed, Coagulation of residue oil and suspended solid in palm oil mill effluent by chitosan, alum and PAC, Chem. Eng. J. , 2006, 118 (1–2), 99–105 CrossRef CAS .
- I. Ahmed, D. Ofori-Amanfo, E. Awuah and F. Cobbold, A comprehensive study on the physicochemical characteristics of faecal sludge in greater Accra region and analysis of its potential use as feedstock for green energy, J. Renewable Energy , 2019, 2019 (1), 8696058 Search PubMed .
- E. Appiah–Effah, G. A. Duku, B. Dwumfour–Asare, I. Manu and K. B. Nyarko, Toilet chemical additives and their effect on faecal sludge characteristics, Heliyon , 2020, 6 (9), e04998 CrossRef .
- T. Koottatep, N. Surinkul, R. Paochaiyangyuen, W. Suebsao, M. Sherpa, C. Liangwannaphorn and A. Panuwatvanich, Assessment of faecal sludge rheological properties - Final report , Environmental Engineering Program, School of Environment, Resources and Development Asian Institute of Technology, Thailand, 2012, https://www.susana.org/_resources/documents/default/2-1661-fs-final-report31-01-12.pdf Search PubMed .
- P. Prasad, N. Andriessen, A. Moorthy, A. Das, K. Coppens, R. Pradeep and L. Strande, Methods for estimating quantities and qualities (Q&Q) of faecal sludge: field evaluation in Sircilla, India, J. Water, Sanit. Hyg. Dev. , 2021, 11 (3), 494–504 CrossRef .
- S. Singh, M. A. Ibrahim, S. Pawar and D. Brdjanovic, Public perceptions of reuse of faecal sludge co-compost in Bhubaneswar, India, Sustainability , 2022, 14 (8), 4489 CrossRef .
- N. Chandana and B. Rao, Evaluating the physicochemical, nutrient, and pathogenic characteristics of fecal sludge for fertilizer application: case from vadgaon maval, Maharashtra, India, J. Environ. Eng. , 2021, 147 (3), 04021003 CrossRef CAS .
- P. Simha, C. Lalander, B. Vinnerås and M. Ganesapillai, Farmer attitudes and perceptions to the re–use of fertiliser products from resource–oriented sanitation systems–The case of Vellore, South India, Sci. Total Environ. , 2017, 581 , 885–896 CrossRef PubMed .
- C. S. Prasad and I. Ray, When the pits fill up:(in) visible flows of waste in urban India, J. Water Sanit. Hyg. Dev. , 2019, 9 (2), 338–347 CrossRef .
- Z. Burt, C. S. Sharada Prasad and S. Drechsel, The cultural economy of human waste reuse: perspectives from peri-urban Karnataka, India, J. Water Sanit. Hyg. Dev. , 2021, 11 (3), 386–397 CrossRef .

IMAGES
VIDEO
COMMENTS
This technology licensed to M/s Innovative Engitech (P) Ltd, pertains to development of an objective evaluation system of piling, using image processing techniques. A continuous scene of pilled 416. fabric sample is converted to digital image and stored in a memory by the image acquisition element.
successful technology transfer - through formal technical collaboration as well as through trade in equipment and raw materials embodying technology. This is based on an analysis of six case study firms. The next section reviews the existing literature of technology transfer and its links 147 A. Banik et al., Foreign Capital Inflows to China ...
This paper presents a case study of an Indian higher technical institution to show how to develop effective technology transfer process to transfer technologies to industry. The case analysis clearly supports the critical role played by an intellectual property management system in enhancing the effectiveness of the technology transfer.
The case study approach was selected as the methodology for this research that investigated a contemporary live phenomenon (TT processes in a higher technical institution from India). The case study approach facilitated deriving a broader applicability to review both intra and interorganizational interactions and relations towards TT.23 IIT ...
Industry in India-A Case study of IIT Bombay. ... (2010). Utilising patent portf olios for effective technology transfer A case learning in IIT Bom-bay. 31-37. Retreived April 4, 2021, from, ...
Patenting and technology commercialization activities are rapidly gaining momentum in Indian academia. Currently, there is paucity of data suggesting technology commercialization activities among Indian academia. This study aims to examine issues regarding technology commercialization among Indian academics. The objectives of this study are to (1) understand the policy implications of ...
Technology Transfer from Higher Technical Institutions to the Industry in India - A Case study of IIT Bombay @inproceedings{Arumugam2012TechnologyTF, title={Technology Transfer from Higher Technical Institutions to the Industry in India - A Case study of IIT Bombay}, author={Arumugam and Karuna Jain and Shailesh J. Mehta}, year={2012}, url ...
The paper touches upon the global perspective of technology transfer process from technical universities and academic institutions. The role of FITT (Foundation for Innovation and Technology Transfer), the technology transfer office of IIT, Delhi, is described. The different components of technology transfer process, some successful case studies from the institute are described. With ...
This study aims to exam-ine issues regarding technology commercialization among Indian academics. The objectives of this study are to (1) understand the policy implications of university-industry technology transfer and (2) propose a conceptual model for technology transfer suitable for Indian scenario. The data included for our analysis is drawn
It reflects our commitment to providing cutting-edge technology and expertise, helping Tata Electronics create a state-of-the-art facility that will catalyze India's semiconductor landscape.
Tata Electronics on Thursday said it has completed the Definitive Agreement with Powerchip Semiconductor Manufacturing Corporation (PSMC) wherein the Taiwanese major will provide design and construction support to build India's first AI-enabled greenfield fab in Gujarat. In addition, PSMC will license a broad portfolio of technologies and provide engineering support to successfully transfer ...
This paper discusses various successful strategies and technology transfer models between industry and academia in developed nations as well as a few important success factors. It also provides an insight on evolution of technology transfer practices (governing Acts and legislation) and current status of technology transfer in India.",
The non traditional roles like technology transfer have been evaluated only through comparative case studies. This research fills the gap by giving an overview of the Indian scene and proposes theoretical models to understand them. ... Discussion and Conclusion From the review of technology transfer in India, it emerges that India has a strong ...
A Case Study of the Pharmaceutical Industry in India Biswajit Dhar and C. Niranjan Rao, with inputs by Veena Gupta United Nations New York and Geneva 2002 . Transfer of Technology Note This paper is part of the series of case studies on Transfer of Technology for Successful Integration into the Global Economy carried out by the Investment ...
NEC-India started out as an OEM of products, mostly from NEC-Japan and NEC-Singapore, supplied to master system integrators (MSI) in India. In Case A (2017 - 2020), NEC-India responds to HQ's mandate to focus more on creating value for Indian customers by aligning its technology and assets with local needs by becoming an MSI.
The Indian R&D system is defi The different modes of transmission of technology sectors4. cient in the following. technology across various domains in At present, India spends 1% of its 1. Minimal private sector involvement clude publications, conferences, consul GDP on research, which is 3.7% of in R&D support programmes. 2.
110067,INDIA. Email: [email protected] ; [email protected] . FDI, Technology Transfer and Spillover —A Case Study of India. One of the major changes in the international arena in the last ...
EU SMEs interested in venturing the Indian market by way of technology transfer arrangement must ensure drawing up a clear and unambiguous contract. Files. 21 OCTOBER 2022. Case Study: Technology Transfer in India. English (383.9 KB - HTML) Download. Share this page IP Helpdesk. This site is managed by:
This study for India shows that this technology transfer is more likely to be achieved by the presence of foreign firms rather than by simple purchase of foreign technology. ... "undated". "FDI, Technology Transfer and Spillover —A Case Study of India," Centre for International Trade and Development, Jawaharlal Nehru University, New Delhi ...
In recent years, there is a great emphasis on transferring inventions and technologies originating from academia to industry through technology transfer/licensing or commercialization. The efforts of the Government of India (GOI) aim to create socially useful innovation through university-industry technology transfer. The objective of the study is to examine and understand enabling factors and ...
In June 2021 the World Health Organization (WHO) and the Medicines Patent Pool (MPP) launched an mRNA technology transfer programme. With a South African consortium serving as the hub, the programme aimed to increase vaccine manufacturing capacity in low- and middle-income countries (LMICs) in view of the "vaccine apartheid" that was observed during COVID-19. Following Clarke's ...
The technology transfer case studies illustrate how patents facilitate technology transfer from R&D-conducting organisations and promote market success. The examples cover a range of economic sectors, countries and types of technology transfer. Each case study provides key takeaways for stakeholders in universities, other public research ...
OBJECTIVE. Technology Without Borders presents case studies of successful transfer of climate-friendly technology and practices. It explores the causes for success and draws the lessons learned. Key messages are presented for the fight against climate destabilisation.
Technology Transfer in Action case studies. Submitted by Efua Halm on Tue, 16/08/2022 - 13:02. From inception to market diffusion to commercialization: Technology transfer is a collaborative process that supports the flow of scientific findings, knowledge, and intellectual property (IP) from creators to public and private users.
INTRODUCTION. Technology Transfer (also called Transfer of Technology (TOT) and Technology Commercialization) 1 are the processes by which the information or knowledge related to the technological aspects travel within the group or between the organizations or entity. Taking this to the broader scenario, give rise to International technology transfer in which the knowledge travels in between ...
"Technology-Transfer-Case Studies ... Hyder, Road traffic injuries in India: A review of the literature, Scandinavian Journal of Public Health, 2006; 34: 100-109 *Assuming 60% of 9M cases of limb injuries in RTA in 2010 **Assuming 15% of number of pts from Non RTA injuries -
This study explores why large-scale biogas plants are not widely installed in India despite the wealth of biomass resources. The methodology includes an extensive literature review and surveyed biogas experts in different sectors, such as private, public, and academic, to identify and rank key obstacles using the Analytical Hierarchy Process (AHP) and Fuzzy-AHP techniques.
A comprehensive study on the physicochemical characteristics of faecal sludge from septic tank and single pit latrine facilities in a typical semi-urban Indian town: a case study of Rajasthan, India. Harishvar Jothinathan and Ajit Pratap Singh * Civil Engineering Department, Birla Institute of Technology and Science, Pilani-333031, India.