- Articles in Press
- Current Issue

Journal Archive
Join our Community on:
Biofuel Research Journal`s Official Channel on WeChat (in Mandarin Chinese)

Latest News
Biofuel Research Journal
has been accepted to be indexed by Inspec!

"Inspec, produced by The
Institution of Engineering
and Technology , has been
the definitive engineering
and physics research database
for over 50 years"
Read more about Inspec on Clarivate TM
has been accepted to be indexed by Ei Compendex (Engineering Village)!
Read more here !
Read the Editor`s Pick: Biogas Collection here !
is now indexed by ProQuest!

We are delighted to announce .... Read more here !
Celebrating a year of growth
for Biofuel Research Journal !

This year marks a year of growth .... Read more here !
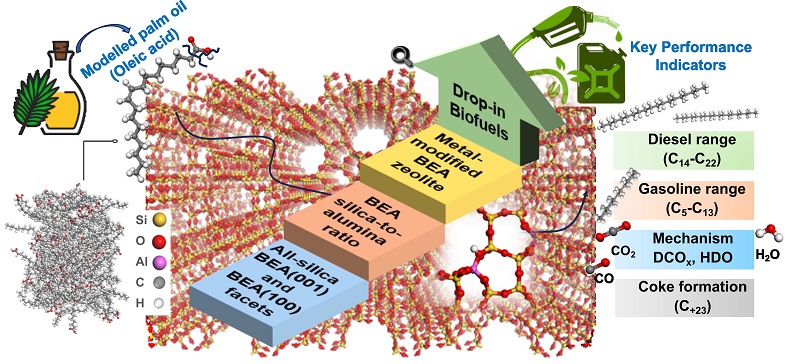
| Advances in biofuel technology are crucial as the world transitions from fossil fuels to sustainable energy sources. Central to this effort is the continuous improvement of catalysts, which enhance biofuel yield and minimize unwanted by-products that can impact sustainability. Researchers from the UAE have made significant progress by using advanced computational modeling to optimize beta zeolite (BEA) catalysts specifically for oleic acid upgrading—a critical process in biofuel production ( ). By fine-tuning the silica-to-aluminium ratios within BEA zeolites, they identified conditions that balance high fuel yields with reduced coke deposition. Additionally, Cu-dopped BEA catalysts have shown promise in lowering environmental impacts and costs, making them strong candidates for future biofuel innovations. This research pushes the boundaries of catalyst design and offers valuable insights for creating more efficient and sustainable biofuel processes. |

Biofuel Research Journal (BRJ) defines “biofuel” in both a specific and generalized context. In the specific sense, BRJ focuses on traditional biofuels and bioproducts derived from biomass. This includes biofuels such as biodiesel, bioethanol, biogas, and algal biofuels, as well as bioproducts like bio-based smart materials, biocomposites, and bio-based chemicals. In a generalized sense, BRJ extends the definition of “biofuel” to include any bio-based technologies, innovations, and strategies that contribute to reducing carbon emissions and fueling the transition toward a sustainable bioeconomy. Here, "biofuel" includes efforts that drive the shift from a carbon-intensive economy to a resilient, bio-based economy. Through this dual approach, BRJ aims to highlight the comprehensive role that both specific biofuels and generalized bio-based innovations play in fostering a sustainable future. The journal welcomes original articles, review papers, case studies, short communications, and hypotheses on the following topics:
- Biofuels and Bioproducts: Traditional biofuels such as biodiesel, bioethanol, biogas, algal biofuels, and emerging biofuel sources, as well as bioproducts like bio-based smart materials, biocomposites, and their applications in industries like food, pharmaceuticals, and others. These developments contribute to the traditional understanding of biofuels and bioproducts, and the broader bioeconomy.
- Innovative Bio-based Strategies: Exploring innovative technologies and strategies that contribute to carbon reduction and sustainability, supporting the journal’s broader definition of biofuel as a driver of the bioeconomy. These strategies are integral to BRJ’s generalized definition of biofuels as enablers of the bioeconomy.
- Biomass Valorization: Research into efficient biomass conversion methods, biorefineries, and bioprocesses aimed at maximizing energy output and value-added products, aligning with the shift toward a bio-based economy. This aligns with both specific biofuel production and broader efforts to transition to a bioeconomy.
- Biomass-Derived Materials for Energy and Storage Systems: Developing biomass-derived materials for use in energy systems, including fuel cells, batteries, supercapacitors, and photovoltaics, contributing to sustainable energy solutions.
- Biomass-Derived Materials for Environmental Sustainability: Investigating biomass-derived materials for carbon capture, pollution mitigation, and other environmental sustainability applications that mitigate climate change and promote circularity.
- Sustainable Applications in Food and Medicine: Utilizing biomass-derived materials in sustainable packaging, functional food ingredients, drug delivery systems, tissue engineering, and regenerative medicine to support a circular bioeconomy. These applications contribute both to the bio-based economy and a circular economy.
- Catalytic Applications of Biomass-Derived Materials: Advancing green manufacturing processes through biomass-derived catalysts and sustainable chemical transformations.
- Techno-Economic and Environmental Assessments: Evaluating the techno-economic feasibility and sustainability of biofuels, bioproducts, and biomass-derived material pathways (life cycle assessment, exergy, emergy, risk assessment), ensuring compliance with global sustainability standards. These analyses ensure the sustainability of bio-based innovations across different scales.
- Climate Change and Bioeconomy Integration: Examining the role of biofuels and bio-based innovations in mitigating climate change and promoting the transition to a low-carbon bioeconomy. These innovations not only reduce greenhouse gas emissions but also align with global sustainability goals.
- Integrated Biofuel and Bioproduct Processing Systems: Highlighting novel and integrated approaches to biofuel and bioproduct processing that optimize efficiency and resource use, key drivers for both specific biofuel production and the broader bioeconomy transition.
- Artificial Photosynthesis for Biofuel Production: Exploring research on artificial photosynthesis as an emerging, sustainable pathway for biofuel production, reinforcing the journal's focus on next-generation bio-based energy solutions.
- Biofuels and Bioproducts in Developing Economies: Encouraging the promotion and adoption of biofuels, bioproducts, and bio-based technologies in developing economies, contributing to local economic development, sustainability, and global climate mitigation.
- Circular Economy and Resource Efficiency: Investigating the role of biofuels and bioproducts in circular economy frameworks, with an emphasis on resource efficiency, waste valorization, and sustainable biomass utilization as part of broader efforts to build a resilient bioeconomy.
BRJ supports interdisciplinary collaboration and invites contributions from researchers, policymakers, and industry leaders to accelerate the transition to a sustainable bioeconomy through innovative bio-based solutions. The journal is committed to maintaining the highest standards of peer review and editorial integrity, ensuring that only high-quality and impactful research is published. As an open-access journal, BRJ is completely free-of-charge, allowing unrestricted access to cutting-edge research for researchers, policymakers, and industry leaders alike. Biofuel Research Journal is indexed in Scopus and Web of Science . BRJ currently has no fees. Learn More . Editor-in-Chief: Vijai Kumar Gupta > View Full Editorial Board
Editorial Board
10.18331/BRJ2024.11.3.1
- PDF 226.38 K
Research Paper
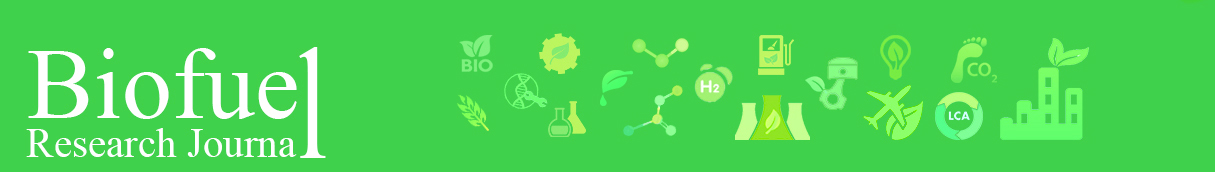
Cascading valorization of defatted rice bran for lactic acid fermentation and biogas production.
Pages 2146-2167
10.18331/BRJ2024.11.3.2
Christiane Herrmann; Raj Shekhar Bose; Anna-Katrin Neu; Roland Schneider; Maria Alexandri
- View Article
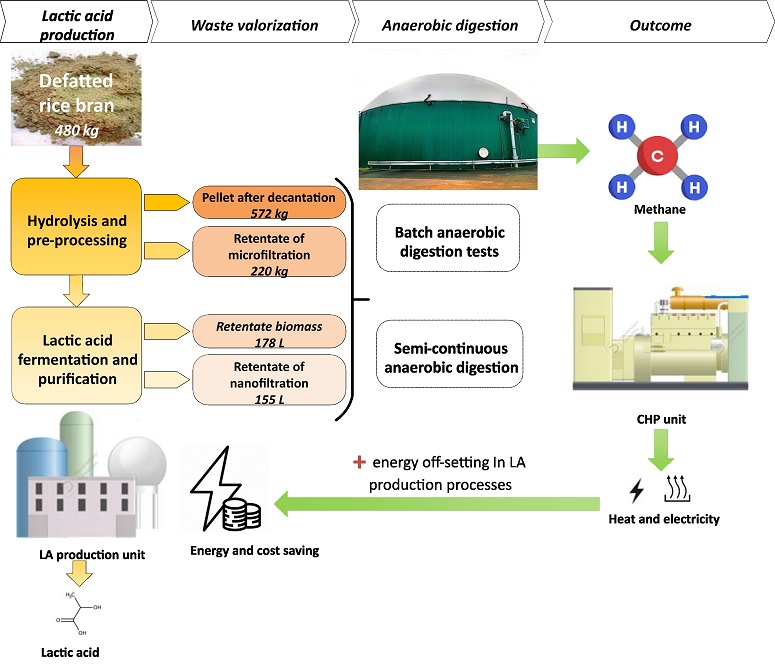
Sustainable catalytic pathways for biofuel precursors: quantitative conversion of glucose to gluconic acid using Pt-Zn biochar catalyst
Pages 2168-2180
10.18331/BRJ2024.11.3.3
Hengyu Hao; Haixin Guo; Bingkun Chen; Richard Lee Smith Jr; Shen Feng
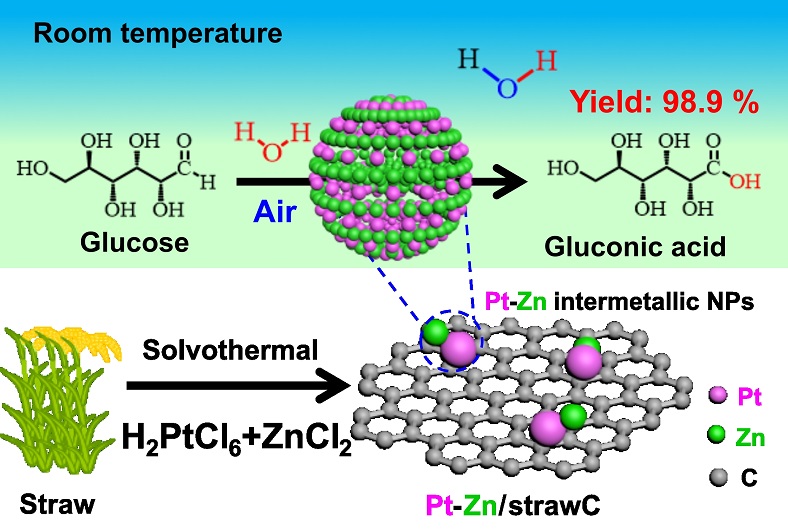
Beyond tradition: charting a greener future for cassava starch industry using multi-criteria decision-making
Pages 2181-2193
10.18331/BRJ2024.11.3.4
Varshini Ravichandran; Deepak Kumar; Sivakumar Mani; Karthik Rajendran
Advanced computational modelling for biofuel catalyst optimization: enhancing beta zeolite acidity for oleic acid upgrading
Pages 2194-2210
10.18331/BRJ2024.11.3.5
Seba AlAreeqi; Daniel Bahamon; Ismail I.I. Alkhatib; Kyriaki Polychronopoulou; Lourdes F. Vega
Publication Information
Indexing and abstracting.

Advertisement
Biofuels: present and future
- S.I. : Biofuels
- Published: 08 May 2024
Cite this article

- Richard Vincent Asase ORCID: orcid.org/0009-0002-7712-5955 1 ,
- Queency N. Okechukwu 1 &
- Maria N. Ivantsova 1
516 Accesses
9 Altmetric
Explore all metrics
Biofuels represent a promising departure from conventional fossil fuels, presenting viable remedies for both energy security and environmental apprehensions. This review intricately examines the various realms of biofuels, encompassing their historical progression, present status, obstacles, and outlook. Commencing with an in-depth exploration of their historical antecedents and developmental milestones, this paper navigates through the spectrum of biofuel variants, encompassing first, second, and third-generation iterations. It meticulously scrutinizes the methodologies of production, advantages, limitations, and ecological implications associated with each variant, providing a nuanced comprehension seldom found in singular sources. A pivotal emphasis is placed on technological innovations propelling the biofuel industry’s advancement, shedding light on recent breakthroughs such as nanotechnologies and mathematical models in biofuel production. Moreover, the paper thoroughly assesses the current global landscape of biofuel production, dissecting the health, environmental, and socioeconomic ramifications of their utilization. The findings reveal that the field of biofuels is still developing and possesses a lot of opportunities toward environmental sustainability. Only a small part of the world is experiencing the production and utilization of biofuels, hence the need for its campaign to engulf the entire world. Also, most of the modern technologies identified in this work are on a laboratory basis and require the needed scalability skills for industrial production. Finally, this work of varied viewpoints seeks to serve as a significant scholarly resource for stakeholders, policymakers, academics, and professionals within the industry, offering insights to grasp and influence the trajectory of biofuels.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Global Conclusions and Future Perspectives

Biofuels: An Overview

Introduction
Explore related subjects.
- Environmental Chemistry
Data availability
Not applicable.
Adewale, P., Dumont, M. J., & Ngadi, M. (2015). Recent trends of biodiesel production from animal fat wastes and associated production techniques. Renewable and Sustainable Energy Reviews , 45 , 574–588. https://doi.org/10.1016/j.rser.2015.02.039 .
Article CAS Google Scholar
Ahmed, S. F., Debnath, J. C., Mehejabin, F., Islam, N., Tripura, R., Mofijur, M., Hoang, A. T., Rasul, M. G., & Vo, D. V. N (2023a). Utilization of nanomaterials in accelerating the production process of sustainable biofuels. Sustainable Energy Technologies and Assessments , 55 , 102894. https://doi.org/10.1016/j.seta.2022.102894 .
Article Google Scholar
Ahmed, S. F., Debnath, J. C., Mehejabin, F., Islam, N., Tripura, R., Mofijur, M., Hoang, A. T., Rasul, M. G., & Vo, D. V. N (2023b). Utilization of nanomaterials in accelerating the production process of sustainable biofuels. Sustainable Energy Technologies and Assessments , 55 , 102894. https://doi.org/10.1016/j.seta.2022.102894 .
Alam, F., Mobin, S., & Chowdhury, H. (2015). Third generation biofuel from algae. Procedia Engineering , 105 , 763–768. https://doi.org/10.1016/j.proeng.2015.05.068 .
Anderson, J. E., DiCicco, D. M., Ginder, J. M., Kramer, U., Leone, T. G., Raney-Pablo, H. E., & Wallington, T. J. (2012). High octane number ethanol–gasoline blends: Quantifying the potential benefits in the United States. Fuel , 97 , 585–594. https://doi.org/10.1016/j.fuel.2012.03.017 .
Araújo, K. (2018). Low Carbon Energy transitions . Oxford University Press. https://doi.org/10.1093/oso/9780199362554.001.0001 .
Arora, A., Nandal, P., Singh, J., & Verma, M. L. (2020). Nanobiotechnological advancements in lignocellulosic biomass pretreatment. Materials Science for Energy Technologies , 3 , 308–318. https://doi.org/10.1016/j.mset.2019.12.003 .
Asomaning, J., Omidghane, M., Chae, M., & Bressler, D. C. (2016). Thermal processing of algal biomass for biofuel production. Current Opinion in Green and Sustainable Chemistry , 2 , 1–5. https://doi.org/10.1016/j.cogsc.2016.08.005 .
Atabani, A. E., Silitonga, A. S., Badruddin, I. A., Mahlia, T. M. I., Masjuki, H. H., & Mekhilef, S. (2012). A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renewable and Sustainable Energy Reviews , 16 (4), 2070–2093. https://doi.org/10.1016/j.rser.2012.01.003 .
Balasubramanian, R., Abishek, A., Gobinath, S., & Jaivignesh, K. (2022). Alternative fuel: Hydrogen and its thermodynamic behaviour. Journal of Human Earth and Future , 3 (2), 195–203. https://doi.org/10.28991/HEF-2022-03-02-05 .
Bera, S., Banerjee, T., & Samanta, A. (2021). Evaluation of enzymatic delignification of rice straw residues for bioethanol production. International Journal of Renewable Energy Technology , 12 (2), 99. https://doi.org/10.1504/IJRET.2021.115276 .
Bhatia, S. K., Jagtap, S. S., Bedekar, A. A., Bhatia, R. K., Patel, A. K., Pant, D., Banu, R., Rao, J., Kim, C. V., Y.-G., & Yang, Y. H. (2020). Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresource Technology , 300 , 122724. https://doi.org/10.1016/j.biortech.2019.122724 .
Bidir, M. G., Millerjothi, N. K., Adaramola, M. S., & Hagos, F. Y. (2021). The role of nanoparticles on biofuel production and as an additive in ternary blend fuelled diesel engine: A review. Energy Reports , 7 , 3614–3627. https://doi.org/10.1016/j.egyr.2021.05.084 .
Bogaerts, A., Neyts, E., Gijbels, R., & van der Mullen, J. (2002). Gas discharge plasmas and their applications. Spectrochimica Acta Part B: Atomic Spectroscopy , 57 (4), 609–658. https://doi.org/10.1016/S0584-8547(01)00406-2 .
Botella, L., Stankovikj, F., Sánchez, J. L., Gonzalo, A., Arauzo, J., & Garcia-Pérez, M. (2018). Bio-oil hydrotreatment for enhancing solubility in biodiesel and the oxydation stability of resulting blends. Frontiers in Chemistry , 6 . https://doi.org/10.3389/fchem.2018.00083 .
Brar, A., Kumar, M., Soni, T., Vivekanand, V., & Pareek, N. (2021). Insights into the genetic and metabolic engineering approaches to enhance the competence of microalgae as biofuel resource: A review. Bioresource Technology , 339 , 125597. https://doi.org/10.1016/j.biortech.2021.125597 .
Cameron, D. E., Bashor, C. J., & Collins, J. J. (2014). A brief history of synthetic biology. Nature Reviews Microbiology , 12 (5), 381–390. https://doi.org/10.1038/nrmicro3239 .
Carboué, Q., Claeys-Bruno, M., Bombarda, I., Sergent, M., Jolain, J., & Roussos, S. (2018). Experimental design and solid state fermentation: A holistic approach to improve cultural medium for the production of fungal secondary metabolites. Chemometrics and Intelligent Laboratory Systems , 176 , 101–107. https://doi.org/10.1016/j.chemolab.2018.03.011 .
Cheah, W. Y., Sankaran, R., Show, P. L., Tg. Ibrahim, T. N. B., Chew, K. W., Culaba, A., & Chang, J. S. (2020). Pretreatment methods for lignocellulosic biofuels production: Current advances, challenges and future prospects. Biofuel Research Journal , 7 (1), 1115–1127. https://doi.org/10.18331/BRJ2020.7.1.4 .
Chowdury, K. H., Nahar, N., & Deb, U. K. (2020). The growth factors involved in microalgae cultivation for biofuel production: A review. Computational Water Energy and Environmental Engineering , 09 (04), 185–215. https://doi.org/10.4236/cweee.2020.94012 .
Clauser, N. M., Felissia, F. E., Area, M. C., & Vallejos, M. E. (2021). A framework for the design and analysis of integrated multi-product biorefineries from agricultural and forestry wastes. Renewable and Sustainable Energy Reviews , 139 , 110687. https://doi.org/10.1016/j.rser.2020.110687 .
Dahman, Y., Syed, K., Begum, S., Roy, P., & Mohtasebi, B. (2019). Biofuels. In Biomass, Biopolymer-Based Materials, and Bioenergy (pp. 277–325). Elsevier. https://doi.org/10.1016/B978-0-08-102426-3.00014-X .
Dave, N., Varadavenkatesan, T., Selvaraj, R., & Vinayagam, R. (2021). Modelling of fermentative bioethanol production from indigenous ulva prolifera biomass by saccharomyces cerevisiae NFCCI1248 using an integrated ANN-GA approach. Science of the Total Environment , 791 , 148429. https://doi.org/10.1016/j.scitotenv.2021.148429 .
Demirbas, A., & Fatih Demirbas, M. (2011). Importance of algae oil as a source of biodiesel. Energy Conversion and Management , 52 (1), 163–170. https://doi.org/10.1016/j.enconman.2010.06.055 .
Devi, A., Singh, A., Bajar, S., & Owamah, H. I. (2021). Nanomaterial in liquid biofuel production: Applications and current status. Environmental Sustainability , 4 (2), 343–353. https://doi.org/10.1007/s42398-021-00193-7 .
Dutta, K., Daverey, A., & Lin, J. G. (2014). Evolution retrospective for alternative fuels: First to fourth generation. Renewable Energy , 69 , 114–122. https://doi.org/10.1016/j.renene.2014.02.044 .
Elgarahy, A. M., Hammad, A., El-Sherif, D. M., Abouzid, M., Gaballah, M. S., & Elwakeel, K. Z. (2021). Thermochemical conversion strategies of biomass to biofuels, techno-economic and bibliometric analysis: A conceptual review. Journal of Environmental Chemical Engineering , 9 (6), 106503. https://doi.org/10.1016/j.jece.2021.106503 .
Erdei, B., Frankó, B., Galbe, M., & Zacchi, G. (2012). Separate hydrolysis and co-fermentation for improved xylose utilization in integrated ethanol production from wheat meal and wheat straw. Biotechnology for Biofuels , 5 (1), 12. https://doi.org/10.1186/1754-6834-5-12 .
Ghag, S. B., Vavilala, S. L., & D’Souza, J. S. (2019). Metabolic engineering and genetic manipulation of novel biomass species for biofuel production. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts (pp. 13–34). Elsevier. https://doi.org/10.1016/B978-0-12-817941-3.00002-4 .
Godbole, V., Pal, M. K., & Gautam, P. (2021). A critical perspective on the scope of interdisciplinary approaches used in fourth-generation biofuel production. Algal Research , 58 , 102436. https://doi.org/10.1016/j.algal.2021.102436 .
Gonzales, R. R., Sivagurunathan, P., & Kim, S. H. (2016). Effect of severity on dilute acid pretreatment of lignocellulosic biomass and the following hydrogen fermentation. International Journal of Hydrogen Energy , 41 (46), 21678–21684. https://doi.org/10.1016/j.ijhydene.2016.06.198 .
Graham-Rowe, D. (2011). Agriculture: Beyond food versus fuel. Nature , 474 (7352), S6–S8. https://doi.org/10.1038/474S06a .
Guo, M. (2020). The Global Scenario of Biofuel Production and Development (pp. 29–56). https://doi.org/10.1007/978-81-322-3965-9_3 .
Hellsmark, H., Mossberg, J., Söderholm, P., & Frishammar, J. (2016). Innovation system strengths and weaknesses in progressing sustainable technology: The case of Swedish biorefinery development. Journal of Cleaner Production , 131 , 702–715. https://doi.org/10.1016/j.jclepro.2016.04.109 .
Heredia-Olea, E., Pérez-Carrillo, E., Montoya-Chiw, M., & Serna-Saldívar, S. O. (2015). Effects of extrusion pretreatment parameters on sweet sorghum bagasse enzymatic hydrolysis and its subsequent conversion into bioethanol. BioMed Research International , 2015 , 1–10. https://doi.org/10.1155/2015/325905 .
Hossain, M. N., Bin, Basu, J. K., & Mamun, M. (2015). The production of ethanol from micro-algae spirulina. Procedia Engineering , 105 , 733–738. https://doi.org/10.1016/j.proeng.2015.05.064 .
Hosseinzadeh, G., Sajjadi, S. M., Mostafa, L., Yousefi, A., Vafaie, R. H., & Zinatloo-Ajabshir, S. (2023). Synthesis of novel direct Z-scheme heterojunction photocatalyst from WO3 nanoplates and SrTiO3 nanoparticles with abundant oxygen vacancies. Surfaces and Interfaces , 42 , 103349. https://doi.org/10.1016/j.surfin.2023.103349 .
Huang, H., & Tang, L. (2007). Treatment of organic waste using thermal plasma pyrolysis technology. Energy Conversion and Management , 48 (4), 1331–1337. https://doi.org/10.1016/j.enconman.2006.08.013 .
Ingle, A. P., Chandel, A. K., Antunes, F. A. F., Rai, M., & da Silva, S. S. (2019). New trends in application of nanotechnology for the pretreatment of lignocellulosic biomass. Biofuels Bioproducts and Biorefining , 13 (3), 776–788. https://doi.org/10.1002/bbb.1965 .
Ishizaki, H., & Hasumi, K. (2014). Ethanol production from biomass. In Research Approaches to Sustainable Biomass Systems (pp. 243–258). Elsevier. https://doi.org/10.1016/B978-0-12-404609-2.00010-6 .
Jambo, S. A., Abdulla, R., Mohd Azhar, S. H., Marbawi, H., Gansau, J. A., & Ravindra, P. (2016). A review on third generation bioethanol feedstock. Renewable and Sustainable Energy Reviews , 65 , 756–769. https://doi.org/10.1016/j.rser.2016.07.064 .
Jatoi, A. S., Abbasi, S. A., Hashmi, Z., Shah, A. K., Alam, M. S., Bhatti, Z. A., Maitlo, G., Hussain, S., Khandro, G. A., Usto, M. A., & Iqbal, A. (2023). Recent trends and future perspectives of lignocellulose biomass for biofuel production: A comprehensive review. Biomass Conversion and Biorefinery , 13 (8), 6457–6469. https://doi.org/10.1007/s13399-021-01853-8 .
Jeswani, H. K., Chilvers, A., & Azapagic, A. (2020). Environmental sustainability of biofuels: a review. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences , 476 (2243). https://doi.org/10.1098/rspa.2020.0351 .
Jiang, D., Ge, X., Zhang, T., Chen, Z., Zhang, Z., He, C., Zhang, Q., & Li, Y. (2020). Effect of alkaline pretreatment on photo-fermentative hydrogen production from giant reed: Comparison of NaOH and ca(OH)2. Bioresource Technology , 304 , 123001. https://doi.org/10.1016/j.biortech.2020.123001 .
Johansson, M. T., & Ilic, D. D. (2018). Review of sustainable development of the road transport sector – are there geographical differences? WEENTECH Proceedings in Energy , 4 (2), 67–87. https://doi.org/10.32438/WPE.8918 .
Keasling, J., Garcia Martin, H., Lee, T. S., Mukhopadhyay, A., Singer, S. W., & Sundstrom, E. (2021). Microbial production of advanced biofuels. Nature Reviews Microbiology , 19 (11), 701–715. https://doi.org/10.1038/s41579-021-00577-w .
Kong, H., Yang, X., Gu, Z., Li, Z., Cheng, L., Hong, Y., & Li, C. (2018). Heat pretreatment improves the enzymatic hydrolysis of granular corn starch at high concentration. Process Biochemistry , 64 , 193–199. https://doi.org/10.1016/j.procbio.2017.09.021 .
Kucharska, K., Hołowacz, I., Konopacka-Łyskawa, D., Rybarczyk, P., & Kamiński, M. (2018). Key issues in modeling and optimization of lignocellulosic biomass fermentative conversion to gaseous biofuels. Renewable Energy , 129 , 384–408. https://doi.org/10.1016/j.renene.2018.06.018 .
Kucharska, K., Słupek, E., Cieśliński, H., & Kamiński, M. (2020). Advantageous conditions of saccharification of lignocellulosic biomass for biofuels generation via fermentation processes. Chemical Papers , 74 (4), 1199–1209. https://doi.org/10.1007/s11696-019-00960-1 .
Li, Y., Zhang, R., Liu, G., Chen, C., He, Y., & Liu, X. (2013). Comparison of methane production potential, biodegradability, and kinetics of different organic substrates. Bioresource Technology , 149 , 565–569. https://doi.org/10.1016/j.biortech.2013.09.063 .
Li, C., Zhao, X., Wang, A., Huber, G. W., & Zhang, T. (2015). Catalytic transformation of lignin for the production of chemicals and fuels. Chemical Reviews , 115 (21), 11559–11624. https://doi.org/10.1021/acs.chemrev.5b00155 .
Li, J., Tao, J., Yan, B., Jiao, L., Chen, G., & Hu, J. (2021). Review of microwave-based treatments of biomass gasification tar. Renewable and Sustainable Energy Reviews , 150 , 111510. https://doi.org/10.1016/j.rser.2021.111510 .
Liu, Y., Cruz-Morales, P., Zargar, A., Belcher, M. S., Pang, B., Englund, E., Dan, Q., Yin, K., & Keasling, J. D. (2021). Biofuels for a sustainable future. Cell , 184 (6), 1636–1647. https://doi.org/10.1016/j.cell.2021.01.052 .
Liu, X., Chen, Z., Lu, S., Xu, B., Cheng, D., Wei, W., Shen, Y., & Ni, B. J. (2023). Heterogeneous photocatalytic conversion of biomass to biofuels: A review. Chemical Engineering Journal , 476 , 146794. https://doi.org/10.1016/j.cej.2023.146794 .
Lopes, M. L., Paulillo, S. C., de Godoy, L., Cherubin, A., Lorenzi, R. A., Giometti, M. S., Bernardino, F. H. C., Neto, C. D. A., H. B., & Amorim, H. V. (2016). de. Ethanol production in Brazil: a bridge between science and industry. Brazilian Journal of Microbiology , 47 , 64–76. https://doi.org/10.1016/j.bjm.2016.10.003 .
López-Linares, J. C., Lucas, S., García-Cubero, M. T., Jiménez, J. J., & Coca, M. (2020). A biorefinery based on brewer’s spent grains: Arabinoxylans recovery by microwave assisted pretreatment integrated with butanol production. Industrial Crops and Products , 158 , 113044. https://doi.org/10.1016/j.indcrop.2020.113044 .
Luque, R., Menéndez, J. A., Arenillas, A., & Cot, J. (2012). Microwave-assisted pyrolysis of biomass feedstocks: The way forward? Energy & Environmental Science , 5 (2), 5481–5488. https://doi.org/10.1039/C1EE02450G .
Malode, S. J., Gaddi, S. A. M., Kamble, P. J., Nalwad, A. A., Muddapur, U. M., & Shetti, N. P. (2022). Recent evolutionary trends in the production of biofuels. Materials Science for Energy Technologies , 5 , 262–277. https://doi.org/10.1016/j.mset.2022.04.001 .
Mohammadi, M., Shafiei, M., Abdolmaleki, A., Karimi, K., Mikkola, J. P., & Larsson, C. (2019). A morpholinium ionic liquid for rice straw pretreatment to enhance ethanol production. Industrial Crops and Products , 139 , 111494. https://doi.org/10.1016/j.indcrop.2019.111494 .
Monlau, F., Sambusiti, C., Barakat, A., Guo, X. M., Latrille, E., Trably, E., Steyer, J. P., & Carrere, H. (2012). Predictive models of biohydrogen and biomethane production based on the compositional and structural features of lignocellulosic materials. Environmental Science & Technology , 46 (21), 12217–12225. https://doi.org/10.1021/es303132t .
Mulyaningtyas, A., & Sediawan, W. B. (2019). Effect of combined pretreatment of lignocellulose and the kinetics of its subsequent bioconversion by aspergillus niger. Biocatalysis and Agricultural Biotechnology , 21 , 101292. https://doi.org/10.1016/j.bcab.2019.101292 .
Mushtaq, F., Mat, R., & Ani, F. N. (2014). A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renewable and Sustainable Energy Reviews , 39 , 555–574. https://doi.org/10.1016/j.rser.2014.07.073 .
Mutsengerere, S., Chihobo, C. H., Musademba, D., & Nhapi, I. (2019). A review of operating parameters affecting bio-oil yield in microwave pyrolysis of lignocellulosic biomass. Renewable and Sustainable Energy Reviews , 104 , 328–336. https://doi.org/10.1016/j.rser.2019.01.030 .
Nagendranatha Reddy, C., Kondaveeti, S., Mohanakrishna, G., & Min, B. (2022). Application of bioelectrochemical systems to regulate and accelerate the anaerobic digestion processes. Chemosphere , 287 , 132299. https://doi.org/10.1016/j.chemosphere.2021.132299 .
Neag, E., Stupar, Z., Maicaneanu, S. A., & Roman, C. (2023). Advances in biodiesel production from microalgae. Energies , 16 (3), 1129. https://doi.org/10.3390/en16031129 .
Nizami, A. S., & Rehan, M. (2018). Towards nanotechnology-based biofuel industry. Biofuel Research Journal , 5 (2), 798–799. https://doi.org/10.18331/BRJ2018.5.2.2 .
Nizamuddin, S., Baloch, H. A., Griffin, G. J., Mubarak, N. M., Bhutto, A. W., Abro, R., Mazari, S. A., & Ali, B. S. (2017). An overview of effect of process parameters on hydrothermal carbonization of biomass. Renewable and Sustainable Energy Reviews , 73 , 1289–1299. https://doi.org/10.1016/j.rser.2016.12.122 .
OECD-FAO Agricultural (2023). Outlook 2023–2032 . OECD. https://doi.org/10.1787/08801ab7-en .
Orugba, H. O., Oghenejoboh, K. M., Oghenejoboh, U. M., & Ohimor, O. E. (2021). Production of biodiesel from a novel combination of Raphia Africana kernel oil and turtle shell (Centrochelys Sulcata) Heterogenous Catalyst. Journal of Human Earth and Future , 2 (3), 258–268. https://doi.org/10.28991/HEF-2021-02-03-07 .
Ou, L., Thilakaratne, R., Brown, R. C., & Wright, M. M. (2015). Techno-economic analysis of transportation fuels from defatted microalgae via hydrothermal liquefaction and hydroprocessing. Biomass and Bioenergy , 72 , 45–54. https://doi.org/10.1016/j.biombioe.2014.11.018 .
Pathak, C., Mandalia, H. C., & Rupala, Y. M. (2012). Biofuels: Indian energy scenario. Research Journal of Recent Sciences , 1 (4), 88–90.
Google Scholar
Phwan, C. K., Ong, H. C., Chen, W. H., Ling, T. C., Ng, E. P., & Show, P. L. (2018). Overview: Comparison of pretreatment technologies and fermentation processes of bioethanol from microalgae. Energy Conversion and Management , 173 , 81–94. https://doi.org/10.1016/j.enconman.2018.07.054 .
Puligundla, P., Oh, S. E., & Mok, C. (2016). Microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to sugars and ethanol: A review. Carbon Letters , 17 (1), 1–10. https://doi.org/10.5714/CL.2016.17.1.001 .
Punnathanam, V., & Shastri, Y. (2020). Efficient optimization of a large-scale biorefinery system using a novel decomposition based approach. Chemical Engineering Research and Design , 160 , 175–189. https://doi.org/10.1016/j.cherd.2020.05.023 .
Rana, M. S., Bhushan, S., Sudhakar, D. R., & Prajapati, S. K. (2020). Effect of iron oxide nanoparticles on growth and biofuel potential of Chlorella spp. Algal Research , 49 , 101942. https://doi.org/10.1016/j.algal.2020.101942 .
Reilly, M., Dinsdale, R., & Guwy, A. (2015). Enhanced biomethane potential from wheat straw by low temperature alkaline calcium hydroxide pre-treatment. Bioresource Technology , 189 , 258–265. https://doi.org/10.1016/j.biortech.2015.03.150 .
Rezayeenik, M., Mousavi-Kamazani, M., & Zinatloo-Ajabshir, S. (2023). CeVO4/rGO nanocomposite: Facile hydrothermal synthesis, characterization, and electrochemical hydrogen storage. Applied Physics A , 129 (1), 47. https://doi.org/10.1007/s00339-022-06325-y .
Robles-Medina, A., González-Moreno, P. A., Esteban-Cerdán, L., & Molina-Grima, E. (2009). Biocatalysis: Towards ever greener biodiesel production. Biotechnology Advances , 27 (4), 398–408. https://doi.org/10.1016/j.biotechadv.2008.10.008 .
Rouleau, M., Egyed, M., Taylor, B., Chen, J., Samaali, M., Davignon, D., & Morneau, G. (2013). Human Health impacts of biodiesel use in on-road heavy duty diesel vehicles in Canada. Environmental Science & Technology , 47 (22), 13113–13121. https://doi.org/10.1021/es4023859 .
Roy, R., Rahman, M. S., & Raynie, D. E. (2020). Recent advances of greener pretreatment technologies of lignocellulose. Current Research in Green and Sustainable Chemistry , 3 , 100035. https://doi.org/10.1016/j.crgsc.2020.100035 .
Ruiz, H. A., Galbe, M., Garrote, G., Ramirez-Gutierrez, D. M., Ximenes, E., Sun, S. N., Lachos-Perez, D., Rodríguez-Jasso, R. M., Sun, R. C., Yang, B., & Ladisch, M. R. (2021). Severity factor kinetic model as a strategic parameter of hydrothermal processing (steam explosion and liquid hot water) for biomass fractionation under biorefinery concept. Bioresource Technology , 342 , 125961. https://doi.org/10.1016/j.biortech.2021.125961 .
Sandesh, K., & Ujwal, P. (2021). Trends and perspectives of liquid biofuel – process and industrial viability. Energy Conversion and Management: X , 10 , 100075. https://doi.org/10.1016/j.ecmx.2020.100075 .
Santos, L. C., dos, Adarme, O. F. H., Baêta, B. E. L., Gurgel, L. V. A., & de Aquino, S. F. (2018). Production of biogas (methane and hydrogen) from anaerobic digestion of hemicellulosic hydrolysate generated in the oxidative pretreatment of coffee husks. Bioresource Technology , 263 , 601–612. https://doi.org/10.1016/j.biortech.2018.05.037 .
Santos, F., Eichler, P., de Queiroz, J. H., & Gomes, F. (2020). Production of second-generation ethanol from sugarcane. In Sugarcane Biorefinery, Technology and Perspectives (pp. 195–228). Elsevier. https://doi.org/10.1016/B978-0-12-814236-3.00011-1 .
Saravanan, A., Senthil Kumar, P., Jeevanantham, S., Karishma, S., & Vo, D. V. N. (2022). Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresource Technology , 344 , 126203. https://doi.org/10.1016/j.biortech.2021.126203 .
Sarip, H., Hossain, M. S., N., M. A. M. N., & Allaf, K. (2016). A review of the thermal pretreatment of lignocellulosic biomass towards glucose production: Autohydrolysis with DIC technology. Bioresources , 11 (4), 10625–10653. https://doi.org/10.15376/biores.11.4.Sarip .
Savi, E. L., Herculano, L. S., Lukasievicz, G. V. B., Torquato, A. S., Baesso, M. L., Astrath, N. G. C., & Malacarne, L. C. (2017). Evaluation of thermo-oxidative stability of biodiesel. Energy & Fuels , 31 (7), 7132–7137. https://doi.org/10.1021/acs.energyfuels.7b00696 .
Scarlat, N., Dallemand, J. F., Monforti-Ferrario, F., & Nita, V. (2015). The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environmental Development , 15 , 3–34. https://doi.org/10.1016/j.envdev.2015.03.006 .
Scovronick, N., & Wilkinson, P. (2014). Health impacts of liquid biofuel production and use: A review. Global Environmental Change , 24 , 155–164. https://doi.org/10.1016/j.gloenvcha.2013.09.011 .
Shah, A., Darr, M., Khanal, S., & Lal, R. (2017). A techno-environmental overview of a corn stover biomass feedstock supply chain for cellulosic biorefineries. Biofuels , 8 (1), 59–69. https://doi.org/10.1080/17597269.2016.1200864 .
Shahbeik, H., Peng, W., Kazemi Shariat Panahi, H., Dehhaghi, M., Guillemin, G. J., Fallahi, A., Amiri, H., Rehan, M., Raikwar, D., Latine, H., Pandalone, B., Khoshnevisan, B., Sonne, C., Vaccaro, L., Nizami, A. S., Gupta, V. K., Lam, S. S., Pan, J., Luque, R., & Aghbashlo, M. (2022a). Synthesis of liquid biofuels from biomass by hydrothermal gasification: A critical review. Renewable and Sustainable Energy Reviews , 167 , 112833. https://doi.org/10.1016/j.rser.2022.112833 .
Shahbeik, H., Peng, W., Kazemi Shariat Panahi, H., Dehhaghi, M., Guillemin, G. J., Fallahi, A., Amiri, H., Rehan, M., Raikwar, D., Latine, H., Pandalone, B., Khoshnevisan, B., Sonne, C., Vaccaro, L., Nizami, A. S., Gupta, V. K., Lam, S. S., Pan, J., Luque, R., & Aghbashlo, M. (2022b). Synthesis of liquid biofuels from biomass by hydrothermal gasification: A critical review. Renewable and Sustainable Energy Reviews , 167 , 112833. https://doi.org/10.1016/j.rser.2022.112833 .
Shahid, M. K., Kim, J. Y., Shin, G., & Choi, Y. (2020). Effect of pyrolysis conditions on characteristics and fluoride adsorptive performance of bone char derived from bone residue. Journal of Water Process Engineering , 37 , 101499. https://doi.org/10.1016/j.jwpe.2020.101499 .
Shahid, M. K., Batool, A., Kashif, A., Nawaz, M. H., Aslam, M., Iqbal, N., & Choi, Y. (2021). Biofuels and biorefineries: Development, application and future perspectives emphasizing the environmental and economic aspects. Journal of Environmental Management , 297 , 113268. https://doi.org/10.1016/j.jenvman.2021.113268 .
Sheikh, M. M. I., Kim, C., Park, H., Kim, S., Kim, G., Lee, J., Sim, S., & Kim, J. W. (2013). Effect of torrefaction for the pretreatment of rice straw for ethanol production. Journal of the Science of Food and Agriculture , 93 (13), 3198–3204. https://doi.org/10.1002/jsfa.6155 .
Shokravi, Z., Shokravi, H., Aziz, M. A., & Shokravi, H. (2019). The fourth-generation biofuel: A systematic review on nearly two decades of research from 2008 to 2019. Fossil Free Fuels (pp. 213–251). CRC. https://doi.org/10.1201/9780429327773-12 .
Singh, J., & Gu, S. (2010). Commercialization potential of microalgae for biofuels production. Renewable and Sustainable Energy Reviews , 14 (9), 2596–2610. https://doi.org/10.1016/j.rser.2010.06.014 .
Srivastava, N., Singh, R., Srivastava, M., Mohammad, A., Harakeh, S., Singh, P., Pal, R., Haque, D. B., Tayeb, S., Moulay, H. H., M., & Gupta, K., V (2023). Impact of nanomaterials on sustainable pretreatment of lignocellulosic biomass for biofuels production: An advanced approach. Bioresource Technology , 369 , 128471. https://doi.org/10.1016/j.biortech.2022.128471 .
Suali, E., & Sarbatly, R. (2012). Conversion of microalgae to biofuel. Renewable and Sustainable Energy Reviews , 16 (6), 4316–4342. https://doi.org/10.1016/j.rser.2012.03.047 .
Tabatabaeinejad, S. M., Zinatloo-Ajabshir, S., Amiri, O., & Salavati-Niasari, M. (2021). Magnetic Lu 2 Cu 2 O 5 -based ceramic nanostructured materials fabricated by a simple and green approach for an effective photocatalytic degradation of organic contamination. RSC Advances , 11 (63), 40100–40111. https://doi.org/10.1039/D1RA06101A .
Talebnia, F., Karakashev, D., & Angelidaki, I. (2010). Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresource Technology , 101 (13), 4744–4753. https://doi.org/10.1016/j.biortech.2009.11.080 .
Tareen, W. U. K., Dilbar, M. T., Farhan, M., Ali Nawaz, M., Durrani, A. W., Memon, K. A., Mekhilef, S., Seyedmahmoudian, M., Horan, B., Amir, M., & Aamir, M. (2019). Present status and potential of biomass energy in Pakistan based on existing and future renewable resources. Sustainability , 12 (1), 249. https://doi.org/10.3390/su12010249 .
Usmani, R. A., Mohammad, A. S., & Ansari, S. S. (2023). Comprehensive biofuel policy analysis framework: A novel approach evaluating the policy influences. Renewable and Sustainable Energy Reviews , 183 , 113403. https://doi.org/10.1016/j.rser.2023.113403 .
Van Dyk, J. S., & Pletschke, B. I. (2012). A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—factors affecting enzymes, conversion and synergy. Biotechnology Advances , 30 (6), 1458–1480. https://doi.org/10.1016/j.biotechadv.2012.03.002 .
Velmurugan, R., & Incharoensakdi, A. (2016). Proper ultrasound treatment increases ethanol production from simultaneous saccharification and fermentation of sugarcane bagasse. RSC Advances , 6 (94), 91409–91419. https://doi.org/10.1039/C6RA17792A .
Velvizhi, G., Jacqueline, P. J., Shetti, N. P., Mohanakrishna, K. L., G., & Aminabhavi, T. M. (2023). Emerging trends and advances in valorization of lignocellulosic biomass to biofuels. Journal of Environmental Management , 345 , 118527. https://doi.org/10.1016/j.jenvman.2023.118527 .
Verardi, A., Lopresto, C. G., Blasi, A., Chakraborty, S., & Calabrò, V. (2020). Bioconversion of lignocellulosic biomass to bioethanol and biobutanol. In Lignocellulosic Biomass to Liquid Biofuels (pp. 67–125). Elsevier. https://doi.org/10.1016/B978-0-12-815936-1.00003-4 .
Voloshin, R. A., Rodionova, M. V., Zharmukhamedov, S. K., Veziroglu, T. N., & Allakhverdiev, S. I. (2019). Review: Biofuel production from plant and algal biomass. Alternative Energy and Ecology (ISJAEE) , 7-9 , 12–31. https://doi.org/10.15518/isjaee.2019.07-09.012-031 .
Yang, C., Wang, S., Yang, J., Xu, D., Li, Y., Li, J., & Zhang, Y. (2020). Hydrothermal liquefaction and gasification of biomass and model compounds: A review. Green Chemistry , 22 (23), 8210–8232. https://doi.org/10.1039/D0GC02802A .
Zhang, S., Yan, Y., Li, T., & Ren, Z. (2005). Upgrading of liquid fuel from the pyrolysis of biomass. Bioresource Technology , 96 (5), 545–550. https://doi.org/10.1016/j.biortech.2004.06.015 .
Zhang, S., Wang, J., Zhu, S., Liu, X., Xiong, Y., & Zhang, H. (2020). Effects of MgCl2 and mg(NO3)2 loading on catalytic pyrolysis of sawdust for bio-oil and MgO-impregnated biochar production. Journal of Analytical and Applied Pyrolysis , 152 , 104962. https://doi.org/10.1016/j.jaap.2020.104962 .
Zheng, T., Jiang, J., & Yao, J. (2021). Surfactant-promoted hydrolysis of lignocellulose for ethanol production. Fuel Processing Technology , 213 , 106660. https://doi.org/10.1016/j.fuproc.2020.106660 .
Zhou, Z., Liu, D., & Zhao, X. (2021). Conversion of lignocellulose to biofuels and chemicals via sugar platform: An updated review on chemistry and mechanisms of acid hydrolysis of lignocellulose. Renewable and Sustainable Energy Reviews , 146 , 111169. https://doi.org/10.1016/j.rser.2021.111169 .
Zhu, J. Q., Zong, Q. J., Li, W. C., Chai, M. Z., Xu, T., Liu, H., Fan, H., Li, B. Z., & Yuan, Y. J. (2020). Temperature profiled simultaneous saccharification and co-fermentation of corn stover increases ethanol production at high solid loading. Energy Conversion and Management , 205 , 112344. https://doi.org/10.1016/j.enconman.2019.112344 .
Zhuang, X., Liu, J., Wang, C., Zhang, Q., & Ma, L. (2022). A review on the stepwise processes of hydrothermal liquefaction (HTL): Recovery of nitrogen sources and upgrading of biocrude. Fuel , 313 , 122671. https://doi.org/10.1016/j.fuel.2021.122671 .
Zinatloo-Ajabshir, S., & Salavati-Niasari, M. (2016). Facile route to synthesize zirconium dioxide (ZrO2) nanostructures: Structural, optical and photocatalytic studies. Journal of Molecular Liquids , 216 , 545–551. https://doi.org/10.1016/j.molliq.2016.01.062 .
Zinatloo-Ajabshir, S., Morassaei, M. S., Amiri, O., & Salavati-Niasari, M. (2020). Green synthesis of dysprosium stannate nanoparticles using Ficus carica extract as photocatalyst for the degradation of organic pollutants under visible irradiation. Ceramics International , 46 (5), 6095–6107. https://doi.org/10.1016/j.ceramint.2019.11.072 .
Ziolkowska, J. R. (2018). Introduction to Biofuels and Potentials of Nanotechnology (pp. 1–15). https://doi.org/10.1007/978-3-319-75052-1_1 .
Download references
Acknowledgements
The research funding from the Ministry of Science and Higher Education of the Russian Federation (Ural Federal University Program of Development within the Priority-2030 Program) is gratefully acknowledged.
The study received no external funding.
Author information
Authors and affiliations.
Institute of Chemical Engineering, Ural Federal University Named after the First President of Russia B. N. Yeltsin, Yekaterinburg, Russia
Richard Vincent Asase, Queency N. Okechukwu & Maria N. Ivantsova
You can also search for this author in PubMed Google Scholar
Contributions
Writing-original draft RVA; Writing- reviewing and editing, QNO; Conceptualization and supervision, MNI.
Corresponding author
Correspondence to Richard Vincent Asase .
Ethics declarations
Competing interests.
No competing interest.
Ethical approval
Additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Asase, R.V., Okechukwu, Q.N. & Ivantsova, M.N. Biofuels: present and future. Environ Dev Sustain (2024). https://doi.org/10.1007/s10668-024-04992-w
Download citation
Received : 10 October 2022
Accepted : 29 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1007/s10668-024-04992-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Renewable energy
- Technological innovations
- Socioeconomic impacts
- Environmental impacts
- Find a journal
- Publish with us
- Track your research
Loading metrics
Open Access
Essays articulate a specific perspective on a topic of broad interest to scientists.
See all article types »
The potential of biofuels from first to fourth generation
Contributed equally to this work with: Philipp Cavelius, Selina Engelhart-Straub
Roles Conceptualization, Data curation, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation Werner Siemens-Chair of Synthetic Biotechnology, TUM School of Natural Sciences, Technical University of Munich (TUM), Garching, Germany
Roles Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing
Roles Conceptualization, Data curation, Supervision, Writing – review & editing
Affiliation Chair of Technical Chemistry II, TUM School of Natural Sciences, Technical University of Munich (TUM), Garching, Germany
Roles Conceptualization, Data curation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected] (DA); [email protected] (TB)
- Philipp Cavelius,
- Selina Engelhart-Straub,
- Norbert Mehlmer,
- Johannes Lercher,
- Dania Awad,
- Thomas Brück

Published: March 30, 2023
- https://doi.org/10.1371/journal.pbio.3002063
- Reader Comments
The steady increase in human population and a rising standard of living heighten global demand for energy. Fossil fuels account for more than three-quarters of energy production, releasing enormous amounts of carbon dioxide (CO 2 ) that drive climate change effects as well as contributing to severe air pollution in many countries. Hence, drastic reduction of CO 2 emissions, especially from fossil fuels, is essential to tackle anthropogenic climate change. To reduce CO 2 emissions and to cope with the ever-growing demand for energy, it is essential to develop renewable energy sources, of which biofuels will form an important contribution. In this Essay, liquid biofuels from first to fourth generation are discussed in detail alongside their industrial development and policy implications, with a focus on the transport sector as a complementary solution to other environmentally friendly technologies, such as electric cars.
Citation: Cavelius P, Engelhart-Straub S, Mehlmer N, Lercher J, Awad D, Brück T (2023) The potential of biofuels from first to fourth generation. PLoS Biol 21(3): e3002063. https://doi.org/10.1371/journal.pbio.3002063
Copyright: © 2023 Cavelius et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was supported by the German Federal Ministry of Education and Research (BMBF) (031B0853A to NM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: EEA, European Environment Agency; EIC, European Innovation Council; GHG, greenhouse gas; GMO, genetically modified organism; ILUC, indirect land use change; IPCC, Intergovernmental Panel on Climate Change; IRENA, International Renewable Energy Agency; RED, Renewable Energy Directive
Introduction
For decades, global energy demand is on the rise due to economic growth and a rapidly growing world population. Additionally, the standard of living is increasing worldwide, in most cases correlating with increased energy consumption, as energy is needed in almost every aspect of our lives, including land, water, and air transport as well as in agriculture, commercial, industrial, and domestic sectors [ 1 ]. To date, fossil fuels account for around 80% of the world’s energy demand [ 2 ], despite being a major instigator for global warming, representing roughly 89% of total greenhouse gas (GHG) emissions in 2020 [ 3 ]. Additionally, fossil fuels are predicted to deplete with the steadily increasing energy demands. As petroleum demand is constantly on the rise, estimations predict a shortage by 2070 to 2080 [ 4 ]. To that end, distinct biofuel types such as liquid and biogas should be methodologically and strategically developed as a preventive measure against predicted energy shortages, all while reducing the anthropogenic climate impact and preserving the environment.
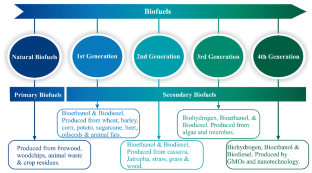
Currently, biofuels are categorized as first to fourth generation, depending on feedstock and/or biosynthetic platform (i.e., genetic engineering). In this Essay, we present comparative advantages and disadvantages among these categories, as well as fossil sources. Furthermore, the development of biofuel technologies hinges on the socioeconomic and political landscape, which can greatly benefit from policy recommendations by respective regulatory bodies. At present, the European Union has the most stringent biofuel legislation and the most ambitious climate impact goals. Hence, we focus on EU-centered development with respect to current biofuel technology platforms at various stages of industrial deployment, the legislative framework implemented in the EU, as well as policy recommendations that would accelerate academic breakthroughs toward industrial implementation. Although, our recommendations are EU-centric, many are also applicable on a global level.
The four generations of biofuels
One alternative to fossil fuels are biofuels, which originate from organic matter and therefore can be regrown and are termed renewable. Biofuels emit less GHGs and are in general more eco-friendly (non-toxic, sulfur-free, biodegradable) than their fossil fuel predecessors [ 5 ]. Biofuels contribute to the achievement of Sustainable Development Goals 7 (affordable and clean energy) and 13 (climate action) of the United Nations [ 6 ]. Global demand for biofuels is set to grow by 41 to 53 billion liters, or 28%, over 2021 to 2026 [ 7 ]. Typically, one can find four main types of biofuel discussed in the context of fermentation: biogas, bioethanol, biobutanol, and biodiesel. The physiochemical properties of these biofuels are compared to fossil-based fuels in Table 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pbio.3002063.t001
Biogas formation is a fairly simple process that has been utilized for several decades. It includes four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. Mixed microorganisms consortia and waste streams are combined in a sealed fermentation system in the absence of oxygen. During the biogas production process, microorganisms hydrolyze waste materials into sugars, peptides and amino acids, fatty acids, and to some part into acetate and hydrogen. Afterwards, acidogenic bacteria convert those intermediate products into organic acids, mainly constituting acetic acid. In addition, they produce carbon dioxide and hydrogen. In the third step, acetogenesis, acetate is formed from hydrogen and carbon dioxide produced in the previous stage. Lastly, methanogenesis follows, creating methane from the products of acetogenesis and acidogenesis [ 8 ]. These gases can then be transformed into hydrogen and/or electricity, or can be stored as biomethane in existing geological reservoirs [ 9 ]. Since the Ukraine crisis began, the resulting lack of fossil fuel availability in the EU has led to biogas being politically pushed as a substitute to natural gas [ 10 ].
Compared to gas (biogas/hydrogen), liquid fuels offer higher energy density and simplified transport and storage. This renders them more compatible with current engine and turbine technologies [ 11 ]. Most engines and turbines are designed and built for the use of liquid fuels, which makes liquid biofuels an easy drop-in solution without the need for modifying present engine technologies or infrastructure [ 5 , 12 ]. These gaseous fuels pose a significant safety hazard as they ignite at lower energies and are flammable over a range of concentrations, hydrogen to higher extent, requiring high level of safety procedures [ 13 ]. The low boiling point and high octane number of bioethanol allow blending with gasoline to a certain extent. The added benefits include a more complete combustion and reduced tailpipe emissions, boosting the engine performance and reducing CO 2 emissions. It is, however, inapt for blending with diesel. Diesel engines require hydrocarbons of higher chain length and low autoignition temperature. However, biodiesel, being of similar chemical constitution, can be blended with fossil-based diesel and hence constitutes a major energy-dense liquid biofuel. A third increasingly attractive biofuel is biobutanol, which holds high promise as it displays superior properties to bioethanol such as higher energy density (25% more energy than ethanol) and usually lower water content due to increased hydrophobicity. Biobutanol is less volatile and possesses less corrosive properties, making it easier and safer to use and store [ 11 , 14 – 19 ]. More importantly, it can be blended with both gasoline, fossil-based or biodiesel at any ratio without the need of new engine technologies and might even allow complete substitution of gasoline, while the use of ethanol is only possible as additive [ 11 , 18 ].
While the classification of biofuel technologies somewhat varies in the literature, products can generally be classed as first to fourth generation, depending on the type of feedstock and conversion process that was applied ( Fig 1 ) [ 5 ].
https://doi.org/10.1371/journal.pbio.3002063.g001
First-generation biofuels
Biofuels of the first generation are mainly divided into bioethanol and biodiesel. Bioethanol production of the first generation is based on microbial fermentation of edible feedstocks, rich in starch and sucrose, such as wheat, corn, and sugarcane in Europe, North America, and South America, respectively. Commercial strains include but are not limited to Saccharomyces cerevisiae , S . stipites , and S . pombe . Bioethanol production is not limited to first-generation biofuels; depending on the feedstock and production strain, bioethanol can also be categorized as second and third generation [ 32 – 35 ]. Biodiesel is mainly obtained from food-grade rapeseed, soy, or palm oil sourced from Europe, South America, and Asia, respectively. In contrast to bioethanol, it is only partially biosynthesized as its production includes chemically catalyzed steps such as transesterification of the lipids with alcohols. Enzymatic catalysis currently only exists on a lab scale [ 36 , 37 ]. Although biobutanol production is also possible by sugar fermentation from sugar cane, corn, wheat, and other food crops, it is limited by lower productivity and yields, product inhibition, and high costs [ 11 , 16 , 18 , 38 ].
During the global food demand crisis in 2007/2008, crops used for biofuel became more important to be used as food, giving rise to the “food versus fuel” debate that persists to date. Additionally, an increased demand for crops (e.g., corn) for fuel production yielded an increased market price for those foods [ 5 ]. Models predict that massive agricultural areas would be needed for fuel production and still could supply only limited amounts of fuel compared to the overall demand. It is estimated that more than two times the globally available area of arable land would needed to meet the global market demand for biodiesel when produced from rapeseed oil [ 39 ]. Furthermore, increased market values of palm oil and other biofuel cultures prompted extended deforestation of tropical rainforests for biofuel crop plantations, which releases more CO 2 than the emission saved by those biofuels. In 2008, Fargione and colleagues estimated that it would take 319 years to repay the biofuel carbon debt resulting from clearing of tropical rainforest in Brazil and subsequent conversion to soybean plantations [ 40 ].
Second-generation biofuels
As a result of the issues of the first generation, second-generation biofuels were developed, utilizing lignocellulosic biomass from agricultural and woodland residues as well as other waste streams (for example, from food industry like wheat bran, animal fats, or wastes of cooking and frying oil). Other non-food plants like the drought-resistant shrub or tree Jatropha curcas , which can also be grown in wastelands, might yet be a different promising source for second-generation biofuels [ 41 ]. Hence, second-generation biofuels circumvent the need for agricultural land use change and do not compete with food resources. However, often second-generation waste streams represent more complex feedstocks than sugarcane or palm oil, potentially containing compounds able to reduce fermentation efficiency, such as lignin. Therefore, application of additional pretreatment steps are common, increasing process time and costs [ 5 , 42 , 43 ].
For the most part, biofuels of the first and in the vast majority of the second generation are commercially produced, around 4% and 96% in 2019, respectively [ 44 ]. One example is the commercially available sunliquid from Clariant, which is a cellulosic ethanol from currently underutilized agricultural residues, such as straw. The first commercial ethanol plant in Romania started production in 2022, with plans to convert 250,000 tons of locally sourced agricultural residues to 50,000 tons of ethanol per year. After enzyme production, which hydrolyses cellulose and hemicellulose to sugar monomers, optimized microorganisms are used in fermentation to produce ethanol. These microorganisms can utilize various carbon sources like glucose and xylose, ensuring higher yields and enabling high efficiency and flexibility in waste valorization as more building blocks of waste streams can be converted to product [ 45 ]. Alongside ethanol producers, the production of second-generation biodiesel is possible from microbial lipids produced by organisms, such as Cutaneotrichosporon oleaginosus , a yeast capable of producing up to 90% (w/w) lipids per biomass in a fermentation process, which can be grown on residue streams (e.g., wheat bran hydrolysate medium) [ 46 – 49 ]. Second-generation biodiesel can also be sourced from waste oils via catalytic cracking and hydrogenation. Drawbacks of this process include incomplete conversion and coke formation, which leads to the deactivation of the catalyst. [ 50 , 51 ]. Biobutanol production on lignocellulose biomass and other waste streams is most commonly based on Clostridia fermentation, as it is one of the oldest and best-established fermentative processes for butanol production. Many Clostridia are natural butanol producers and possess the ability to metabolize a variety of different substrates. However, similar to its first-generation predecessor, the process is limited by low butanol titers and product inhibition [ 11 , 16 , 18 , 38 ]. Typically, butanol is produced via ABE fermentation, which results in solvents in ratio of 3 parts acetone, 6 parts butanol, and 1 part ethanol, and butanol refinement is not an energetically favorable solution. Other drawbacks also include cell toxicity at low concentration [ 52 , 53 ]. To that end, cell-free isobutanol biosynthesis using a designed artificial metabolic pathway has been developed [ 54 ]. At present, this approach remains costly for commercialization.
Various carbonaceous compounds can be transformed to syngas by gasification. Commonly, it is a gaseous waste stream from industrial processes such as steel manufacture, in which fossil fuels are burned in the process. Syngas is a mixture mainly consisting of carbon monoxide (CO), CO 2 , and hydrogen. It can be derived from biomass, including lignocellulosic compounds, coal, animal or municipal solid waste, and industrial CO-rich gases. This gas can be metabolized by strictly anaerobic, methanogenic archaea as well as by acetogenic bacterial genera such as Acetobacterium or Clostridium , often used in syntrophic fermentations. The process is mostly focused on biosynthesis of organic acids and alcohol compounds such as acetate, ethanol, and butanol [ 55 – 57 ]. Advantages of syngas fermentation compared to other second-generation approaches are high feedstock flexibility as well as high rates of energy and carbon capture. Complicated pretreatments of second-generation feedstocks can be replaced with gasification, using all components of the biomass, including lignin and other recalcitrant compounds [ 58 ]. LanzaTech developed a process converting feedstocks including industrial waste streams to fuel and chemicals utilizing bacteria. They estimate a total product capacity of 600,000 metric tons as well as 1,000,000 metric tons of captured carbon per year, for all their plants combined [ 59 ]. Since 2022, a demonstration plant in Japan has turned municipal solid waste to ethanol, with a production target of 20 tons of ethanol per day [ 60 ].
More than half of the biologically stored carbon is bound in marine biomass, especially macroalgae and seagrass. Detached seagrass material is seasonally washed on beaches and shore lines; due to low biological degradation and herbivore consumption, an excess of it accumulates as waste. Estimations of up to 40 million tons of dry seagrass biomass, which can be used for biofuel production, are given. Through enzymatic hydrolysis, the carbohydrate content of the seagrass can be used in a fermentation medium for microorganisms, additionally offering low nitrogen and phosphorus content, which is typically required for lipid production [ 61 ].
Despite the highly favorable ability to valorize waste streams, second-generation biofuels by themselves will not be sufficient to supply energy for the current worldwide demand. As is the case for food crops with first-generation biofuels, biomass used in these processes is available in limited amounts. Therefore, second-generation biofuels must be combined with other technologies to ensure sufficient provision of fuels. This prompted research on third-generation biofuels. However, scientific estimations predict second-generation biofuels could supply up to 30% of the world’s transportation energy [ 5 ].
Third-generation biofuels
Third-generation biofuels are mainly derived from microalgae and cyanobacteria biomass, which can be used to naturally generate alcohols and lipids to transform into biodiesel or any other high energy fuel product. Algae exhibit 2- to 4-fold higher photosynthesis rates than terrestrial plants, resulting in faster biomass formation [ 62 ]. Algae do not require arable land or fresh water for cultivation. Many cultures can be grown using waste water, brackish or salt water, which is cost efficient and circumvents competition with agricultural activity [ 63 , 64 ]. Most importantly, efficient algae cultivation requires a direct CO 2 supply, which can be derived from industrial emitters or by atmospheric carbon capture. In conventional cultivation systems, around 70% of supplied CO 2 is used for photosynthesis and therefore biomass production [ 65 ]. Hence, algae biofuels potentially could have a negative carbon footprint as they directly bind the GHG in their biomass. One of the most prominent third-generation processes is the production of biodiesel or other energy density biofuels, such as biokerosene, using oleaginous microalgae [ 66 , 67 ].
One of the most economically critical and versatile operations in algal biofuel production is algae cultivation. Algal bioreactors ( Fig 2 ) are independent of location and climate, therefore can be operated almost irrespective of these factors. For low price, high volume products, such as biofuels, algae are commonly cultivated in open ponds. Open pond reactors are significantly cheaper in their construction and operation but have drawbacks like high loss of water through evaporation and lack of temperature control, which lowers biomass productivity. The alternative, preferred for high price, low volume products, such as cosmetic ingredients, is a closed photobioreactor, where process parameters can be precisely controlled, which often leads to higher productivity [ 63 , 68 ]. These bioreactors also enable a three-dimensional mode of cultivation, significantly increasing the productivity per area. In contrast to second-generation biofuels, the third-generation processes completely decouple biofuel production from the need for agricultural land. Additionally, algal-based oil production is likely greater than that in higher plants, as lipids mainly accumulate in specific parts of the plant (e.g., in rape seeds), while in algae, each cell can contain high amount of lipids, making the process more mass efficient. One bottleneck in production is harvesting, as the low size and density of the microalgal cells combined with the sensitivity of the cells to changes in pH render it challenging. [ 66 ]. Furthermore, downstream processing for algal biofuels is commonly more energy intensive than other biofuel productions [ 63 , 69 ]. Araújo and colleagues mapped 447 algae and cyanobacteria Spirulina production units in 2021 in the EU [ 70 ]. Most of these companies directed their biomass to the production of food, feed, and related uses; commercial application of biofuels only had a very small share. Further technological developments in upscaling and reduction of production costs are necessary for commercialization.
This image showcases the open algae cultivation systems located at Technical University of Munich, Ottobrunn.
https://doi.org/10.1371/journal.pbio.3002063.g002
Fourth-generation biofuels
The latest biofuel generation, termed fourth-generation biofuels, encompasses the use of genetic engineering to increase desired traits of organisms used in biofuel production. This applies to a variety of traits from utilizing multiple types of sugars (e.g., pentoses and hexoses), to higher lipid synthesis or increased photosynthesis and carbon fixation. For model organisms, such as Escherichia coli and Saccharomyces cerevisiae , a wide variety of tools for genetically engineering the regulation of endogenous pathways or inserting new pathways are reported. Unfortunately, for most native producers of biofuels, the genetic engineering toolbox is far more limited.
Currently, two different approaches have been adopted: engineering of pathways in native producers (optimizing growth rates, utilization of different carbon sources, directing the metabolic flux toward biofuel production and increased production titers) and reconstruction of pathways identified in natural producers in more genetically accessible model organisms. A wide variety of microorganisms can be used as heterologous hosts for the production of biofuels, including bacteria, yeast, and algae. Their metabolic versatility enables the use of various substrates to produce a wide range of biofuels. For example, butanol pathway genes from Clostridia were introduced into E . coli , Pseudomonas putida , and Bacillus subtilis strains [ 14 , 16 , 19 ]. While the introduction of heterologous genes is well established, a major challenge is the disruption of competing metabolic fluxes. Another obstacle for high product titers can be toxicity of large amounts of product on the cell. To enable increased accumulation of biofuels, the cellular stress response can be modified through genetic engineering, for example, with cell membrane modifications. Through the overexpression of certain membrane transporters, biofuel molecules can be secreted into the medium thereby circumventing accumulation as well as toxicity while simultaneously simplifying product recovery. In E . coli , membrane transporters have been used successfully to excrete n-alkanes, such as n-octane [ 71 , 72 ]. However, the overexpression of transporters is challenging as it modifies the membrane composition, creating a metabolic burden as well as potentially overloading the cellular import and export, thereby disabling the cells ability to regulate its internal environment/homeostasis [ 71 ].
Genetically modified algae can offer higher product yields and a variety of other improvements compared to wild-type algae. In order to enhance photosynthetic efficiency, the antennae systems of algae capable of absorbing a broader range of the light spectrum could be transferred to more suitable production organisms [ 44 , 73 ]. With respect to genetic engineering, CRIPSR/Cas9 is a frequently used tool, as it offers a simple design with efficient transfection and targeted gene disruption [ 74 ].
In fourth-generation biofuel processes that focus on genetically optimized cyanobacteria, the production of ethanol, as well as other fuel products such as butanol, isobutanol, and modified fatty acids have been realized successfully [ 75 , 76 ]. While 1-butanol production reached titers of 300 mg/L, bioethanol titers of up to 5.5 g/L were reported [ 77 – 79 ].
For the efficient optimization of native producers, systems biology can offer many insights. The availability of whole-genome sequences is essential, as this information allows for the annotation of genes to their respective function and reconstruction of the innate metabolic pathways, which can subsequently be modified. Recent advances have been made in the field of genome sequencing allowing for a more rapid and cost-efficient collection of data [ 19 ], while the gene expression patterns in different growth environments can be analyzed by transcriptomics and protein products identified by proteomics.
With genetic engineering tools, the quantity and quality of biofuels can be controlled and increased but will need political acceptance and support to be widely adopted [ 5 ]. There is a controversial debate around genetic engineering in agriculture and medicine, especially in Europe; therefore, similar concerns can be anticipated surrounding the use in biofuel production. A European-based study came to the conclusion that genetically engineered algae for biofuel production would be accepted by the majority of consumers, when the safety of the systems can be guaranteed [ 80 ]. However, with proper containment methods and carefully selected locations, such risks could be drastically minimized. Therefore, closed production systems with high security standards are expected to be built [ 80 ]. Additional biocontainment methods can be directly based on genetic changes inside the production cells such as auxotrophies or kill switches, significantly decreasing the risk of genetically modified organism (GMO) escape [ 44 , 81 ].
One alternative to targeted genetic engineering is random mutagenesis, which can be described as accelerated evolution. Microorganisms and products generated by this approach are not subjected to GMO regulations. Furthermore, this technique can be performed with little knowledge about the production organism and production pathway. Random mutagenesis can be achieved by a variety of methods such as UV light, chemical agents, or fast neutron irradiation. For the first time, the latter was applied on C . oleaginosus , resulting in mutants with elevated lipid titers suitable for biodiesel applications. It is noteworthy that biodiesel from prominent oleaginous yeast platforms, such as Yarrowia lipolytica , C . oleaginosus , Rhodosporidium toruloides , and Lipomyces starkeyi , are compliant with international biodiesel standards, including US ASTM D6751 and EU standard EN 14214 [ 82 , 83 ].
A new, more experimental approach to fourth-generation biofuels is the production of electrobiofuels. These are based on the approach to establish new-to-nature hybrid systems, which are able to use renewable electricity and carbon sources directly for the production of commodity chemicals and biofuels, thereby enabling the conversion of solar energy into storable liquid fuel. Such a process could combine the higher photon efficiency of modern photovoltaic systems (compared to photosynthesis) with the sustainability of biofuel production, increasing overall process effectiveness [ 84 ].
Economics of biofuels in transportation
Apart from reducing GHG emissions and air pollution, biofuel industries can contribute to energy security on a local and national scale, as it is not reliant on local reservoirs of fossil oil. Additionally, the creation of new employment and economic growth, especially in rural locations, should positively impact the social environment as well. However, to fully exploit all the positive traits of biofuels, further research and investments are necessary, as the production of biofuels requires more processing steps compared with the conventional methods of drilling into the ground to obtain crude oil, followed by refining. Therefore, at present, biofuels commonly exceed fossil fuel production costs. Furthermore, raw materials for biofuel production do not compare to crude oil in energy density, requiring far greater amounts of biomass for the same energy output compared to fossil sources. The infrastructure required for the sector of biofuel production has to be extensively developed as well. One example is the primary energy needed to run the process, which should be obtained through sustainable operations. Candidates for that include solar and wind energy among others. Thus, by reducing the overall production cost and increasing process efficiency, biofuels could become more competitive to fossil fuels. Furthermore, by-products of biofuel production should be efficiently utilized in a circular economy, which could increase cost efficiency of such processes.
Transportation is one of the most socioeconomically sensitive sectors for the use of liquid biofuels ( Fig 3 ). It contributes about 17% of global CO 2 emissions [ 85 , 86 ], and so far, sustainable solutions are not fully developed. Due to their limitations, current technologies for biofuels are not likely to completely replace fossil fuels in their entirety but can offer new routes for waste stream valorization in a circular economy and contribute significantly to minimize our dependency on fossil fuels one step at a time. A complementary approach to this goal is electric cars, which have zero tailpipe emissions, although CO 2 emissions are associated with the production of the car and the source of the electricity. Essential in electric vehicle batteries are metals like lithium, cobalt, nickel, and manganese. The demand for these metals is surging, while at the same time toxic waste electronics are accumulating all over the world. Traditional recycling/extraction methods require high temperatures and strong acids. This is a high energy process involving toxic chemicals. One alternative is bioleaching or biomining, which employs microbes such as Acidithiobacillus ferrooxidans that can bind and recover metals, bypassing the need for high temperatures and toxic chemicals [ 87 – 90 ]. This emerging technology offers an eco-friendly approach to recycling but still requires extensive research and development. Additionally, a new infrastructure must be put into place, supporting millions of electric cars at the same time. To that point, a combination of synthetic and biofuels in synergy with electric cars might be an optimal solution for the years to come, partially substituting fossil fuels, thereby drastically reducing CO 2 output of transportation.
The transport sector, specifically, results in 17% of emissions. Adapted from Ritchie and colleagues (2020), Carbon Leadership Forum 2020 [ 85 , 86 ].
https://doi.org/10.1371/journal.pbio.3002063.g003
EU policy recommendations
In order to promote the use of clean and sustainable energy at the industrial, retail, and consumer level, a cohesive framework of policies is imperative. The European Commission and European Environment Agency (EEA) have cooperated with the International Renewable Energy Agency (IRENA) and the Intergovernmental Panel on Climate Change (IPCC) in leading the efforts for clean energy transition through a number of directives and legislations since the 1990s [ 91 – 94 ]. These efforts manifest as a commitment by EU countries to lower GHG emissions and increase the use of renewable energy. Most notable is the Renewable Energy Directive (RED), which came into force in 2009. Through this directive, EU countries set targets for renewable energy consumption, including a subtarget mandating 10% of energy used in transport to be produced from renewables. It is noteworthy that the deployment of renewable energy has continuously grown, exceeding 22% in 2020 [ 92 ]. The legislation also mandates GHG reduction targets for fuel suppliers, requiring a reduction in GHG intensity of the fuel mix by 6% in 2020 [ 92 ]. In 2018, the commission revised the legislative proposal and the European Parliament and the EU Council proposed amendments as RED II. Therewith, the EU aims to increase the share of renewable energy to 32% and in transport to at least 14%, including a minimum share of 3.5% of advanced biofuels (second- and third-generation biofuels). The latter streamlines waste residues, such as agricultural waste (e.g., straw), and also encompasses renewable electricity in road and rail transport [ 95 ].
At present, the industrial biofuel production is dominated by first- and second-generation processes, respectively. Nevertheless, RED II and indirect land use change (ILUC) proposals have initiated the gradual shift toward second- and third-generation processes, which are associated with significant changes in feedstock supply and logistics, as well as technology deployment (e.g., market penetration of advanced biofuels). ILUC qualifies first-generation biofuels based on the unintended consequences of releasing carbon emissions as a result of land use changes [ 96 , 97 ]. While technical process development for third- and fourth-generation biofuels is advancing rapidly in academic and start-up settings, large-scale industrial implementation remains lagging. This indicates a profound gap in transferring technologies from a pilot scale (TRL 5) to an industrial scale (TRL 8). To that end, clear and implementable criteria remain to be addressed by legislators for industrial technology transition toward advanced biofuels with a notable climate impact. Table 2 summarizes our policy recommendations aimed at advancing biofuels implementation as well as their respective expected results and acting entity.
https://doi.org/10.1371/journal.pbio.3002063.t002
First and foremost, legislators need to create stable policies and regulatory frameworks based on measurable cradle-to-cradle sustainability performance indicators. In the past, one of the greatest barriers for industry to adopt new biofuel technologies, at least in the EU, was the constantly changing regulatory and provisions framework, which ultimately led to waves of market and company consolidation for first-generation fuels such as crop-based biodiesel, corn and sugar beet-based bioethanol, and, more recently, corn-based biogas products. Therefore, it is of the utmost importance that policy makers provide clearly formulated, long-term stable policies, provisions, and regulatory frameworks to allow industrial transition to advanced biofuel technologies with clear climate impact.
With respect to sustainability, measurable criteria can be categorized as agriculture biomass, forest biomass with respect to biodiversity, and carbon stocks and emissions. Biofuel ILUC factors could be included in the biannual reports of fuel suppliers and EU countries. Accordingly, biofuel produced from palm oil and soy should carry a high ILUC factor and phasing out these feedstocks could be achieved by encouraging the diversification of feedstock. Reports estimate that 130,000 to 210,000 hectares of deforestation, which has detrimental effect on biodiversity and soil quality, could be avoided by limiting the demand of EU countries for palm oil biofuels [ 98 ]. Land requirement and fresh water use, carbon trading, and carbon offsets should also be factored in upcoming legislations. The criteria should also include GHG emissions that take the levels of methane, nitric oxides, and sulfur oxides into account in addition to levels of CO 2 . Legislation criteria should also take into consideration end-use performance, whereby industry sector, energy efficiency, and socioeconomic impact could represent qualifying measures. Risk determination and possible exceptions could be evaluated for specific industries, such as security and electricity. With respect to energy efficiency, it should be considered that distinct biofuels differ in their output. For example, ethanol yields 25% more energy than that invested in its production, while biodiesel yields 93% more [ 99 ]. To that end, performance-based renewable energy policies are needed. Finally, a reliable system that verifies compliance and reporting is eminent to putting these proposals into practice. In that respect, a mass balance system that observes the global carbon inventory and defines optimal distribution of energy profiles (first to fourth generation) and mixtures (e.g., E10 petrol/ethanol) to ensure minimal climate impact is in order. This system could integrate a range of parameters, including flexible distribution channels, demand management, storage, and price signals in real time [ 97 , 100 ]. Independent auditing services could further ensure compliance, which could also be extended to trading partners of the EU countries at a later stage.
As the implementation of industrial biofuel production sites are associated with immense capital investments, it is crucial to shed light on the financial aspect linked to these policies, primarily, multilevel incentives schemes, investment risk reduction, and infrastructure and logistics. On an EU level, specific funding mechanisms such as European Innovation Council (EIC) pathfinder, EIC Transition, and EIC Accelerator that aim to enable and accelerate the scaling trajectory of new technologies toward market entry already exist. While this is an initial step toward implementing new biofuel technologies, these measures do not translate into national actions and legislation on a member state level, which impedes the regional mobilization of capital, leading to a slow uptake and implementation of new technologies. Hence, a significant step toward rapid technology adoption and implementation would be the regional implementation of funding and capital mobilization as already practiced on the EU level.
An integral element in promoting advanced biofuels could be incentivizing biofuel processes that show favorable sets of sustainability parameters and end-use performance by a higher cost of CO 2 certificates, which realistically should be in the order of 500 to 1,000 Euros/ton CO 2 . Consolidated long-term measures would also provide companies and investors with valuable tools to calculate return of investment and hence de-risk decision-making for iterative technology transition. To enable more efficient technology transfer from academia toward industrial technology deployment, additional factors need to be considered. To that end, academic projects should receive sequential, stage-gated extended funding periods of 4 to 8 years that commonly go beyond a single governmental administration period. This would allow ideas to be developed toward a proof of concept stage, where they can be translated to spin-outs or industry partners. Governments should incentivize start-up formation derived from academic units using focused funding measures, such as the EXIST funding program in Germany [ 101 ]. As technology development from proof of concept (TRL 2 to 4) in academic settings to pilot plant level often requires time periods exceeding 5 to 7 years, synergistic midterm private funding resources also have to be mobilized. To that end, technology familiarity, better understanding of time frames for solid technology development, and proper risk assessment are essential for private capital investors. In order to motivate private capital in the EU to accept development risks and extended time frames for return of investment in biofuel start-up companies, governments could implement tax write-offs for spent risk capital. This legislatively guided de-risking of capital investment into new technologies is already implemented in the United States of America and the United Kingdom, as well as in other, less compliance-driven, financial markets. Hence, the EU has to rapidly implement such legislative tax reliefs to secure innovation on the biofuels and other innovation and sustainability-driven sectors for added economic value and a vibrant job sector.
Capital is also short at the infrastructure and logistics level. Investments are required to construct dedicated pilot plants that allow industrial scale validation and optimization of new technologies, independent of any large-scale industrial partner. In that respect, multiple regionally decentralized pilot plants could provide dedicated instrumental parks that house state of the art fermentation and downstream processing equipment. In the case of gas fermentation, these parks could be associated with significant security measures and demand special regulatory approval and regular inspection. Accordingly, construction and operation by large national research organizations, such as Fraunhofer institutions in Germany, or private–public partnerships is recommended. Governmentally driven funding actions that enable access and use of these pilot plant facilities by innovators in the biofuels sector could further accelerate industrial deployment and market entry. In parallel to technology market readiness, the implementation of biofuels in industrial processes requires a secured feedstock supply.
Contrary to Nordic countries that are the forefront of advanced biofuel processes development, most industrialized countries in the EU with a high population density do not have sufficient land or biomass availability for large-scale biofuel production [ 100 ]. Hence, the location and feedstock supply require strategic positioning. Two routes for biofuels production are viable in the EU: a large production plant located in a region with abundant, long-term feedstock/biomass supply or secured trade routes; or a network of smaller, decentralized production facilities. In the latter case, a farm-integrated production facility with secured access to local residue streams can be envisioned. To optimize the economics of the production facilities, its location should be leveraged with maximal carbon credits in order to meet fuel market prices. To make an informed decision on the location and mode of production, a global carbon inventory map would be extremely beneficial. While we have a good overview of regional carbon emissions, there is little information on correlative carbon storage, which is mostly limited to terrestrial biomass. To that end, other carbon storage mechanisms should be considered, such as existing geological carbon (CO 2 ) capture activities and marine biomass. Considering that 68% of the world population is projected to live in urban areas by 2050, it is sensible to consider urban waste streams, such as sewage sludge and food waste, as yet underutilized biomass feedstocks for biofuel production processes [ 102 ]. More generally, a map of the carbon flux resolved on a country-specific level would enable a more informed decision on the selection of process feedstock (biomass residues/CO 2 ) and trading partners that could secure operation of large-scale production facilities for third- and fourth-generation biofuels. Currently, the major trading partners of the EU are Argentina, Brazil, USA, Indonesia, and Malaysia [ 97 ]. These trading practices do not ensure level field sustainability over the long term. To that end, future trading legislation should consider balanced trade between the global North and global South to ensure long-term beneficial socioeconomic impact on the stability and sustainability of feedstock and biofuel production.
Conclusions
In this Essay, we laid out the reasoning for biofuel production as immediate and long-term measures to limit and eliminate energy and mobility-related GHG emissions. In that regard, biofuels will not be the only solution but an essential building block in a network with other physical (i.e., wind power, photovoltaic systems [ 103 – 105 ]) and chemical technologies (i.e., Sabatier process, Power to X [ 106 , 107 ]) that together can provide carbon neutral or even carbon negative energy and mobility solutions. In regard to transportation, biofuels should act in synergy with other technologies, such as electrified vehicles. In addition to biofuel manufacturing, similar processes could also be implemented in other applications. Here, algal and yeast oil can be transformed into building materials such as carbon fibers and cement additives. Via these routes, atmospheric CO 2 can be absorbed from the environment and stored for very long periods of time. Such technologies could complement materials derived from fossil fuels or that generate large amounts of CO 2 during the manufacturing process (e.g., steel, aluminum and concrete) [ 108 ].
We are convinced that, in the last decades, mankind has been generally too hesitant to adopt climate-centered technologies, which has put the world on a perilous pathway toward catastrophic climate change [ 109 – 111 ]. The destructive outcomes of this scenario have been documented in the scientific literature and are subject to numerous high level reports [ 112 – 117 ].
As time for action is already overdue, it is essential to act now by implementing the tools and technologies we have at hand at the present time. It is our opinion, that the only path to enable climate effective energy security and mobility is to deploy available technologies at a global scale right now. The global implementation of large-scale production infrastructure for sustainable (bio)technologies to kick-start production of renewable energy carriers and sustainable commodities is imperative in this timely development scenario. Once production with a base process has commenced, these processes can be iteratively refined or modulated at scale to evolve toward the next technology generation. This approach demands close, long-term academic and industry partnerships.
This fundamental transition toward sustainable bio-based technologies will require long-sighted, fact-driven legislative guidance and immense capital investments across the private and governmental sectors. However, it will be the only route to limit climate change effects and provide a livelihood for future societies.
With respect to governments, this means that neither ideology nor demagogically driven decision-making will protect any society from the effects of climate change. There are just no simple answers to complex, global problems. What is needed are global governmental alliances that make technocratically oriented long-sighted decisions, aiming for definitively set climate-centered outcomes even if the communication of the measures that have to be taken may not be popular on first sight.
Even outside the scientific communities, people are ready to accept change of the status quo in order to curb climate change effects and transition to a sustainable society. The question remains if the global political elites are ready to communicate and implement this change. Time is running out to maintain the global ecosystems as we know it.
Acknowledgments
The authors dedicate this manuscript to Dr. Christian Patermann (former EU Program Director Biotechnology, Agriculture, and Food) and Dr. Günther von Au (Chairman of the Board of Directors of Clariant AG), each being outstanding political and industrial visionaries, influencers, and decision-makers in the field of sustainable (bio)technologies and the bioeconomy, respectively.
- View Article
- Google Scholar
- PubMed/NCBI
- 7. IEA. Renewables 2021. Paris: IEA; 2021.
- 59. LanzaTech. Annual Report 2021. 2021. Available from: https://lanzatech.com/wp-content/uploads/2022/08/LanzaTech_2021_Annual_Report.pdf .
- 93. European Environment Agency. 19.01.2023. Available from: https://www.eea.europa.eu/ .
Inexpensive, carbon-neutral biofuels are finally possible

When it comes to making fuel from plants, the first step has always been the hardest — breaking down the plant matter. A new study finds that introducing a simple, renewable chemical to the pretreatment step can finally make next-generation biofuel production both cost-effective and carbon neutral.

For biofuels to compete with petroleum, biorefinery operations must be designed to better utilize lignin.
Lignin is one of the main components of plant cell walls. It provides plants with greater structural integrity and resiliency from microbial attacks. However, these natural properties of lignin also make it difficult to extract and utilize from the plant matter, also known as biomass.
“Lignin utilization is the gateway to making what you want out of biomass in the most economical and environmentally friendly way possible,” said UC Riverside associate research professor Charles Cai. “Designing a process that can better utilize both the lignin and sugars found in biomass is one of the most exciting technical challenges in this field.”
To overcome the lignin hurdle, Cai invented CELF, which stands for co-solvent enhanced lignocellulosic fractionation. It is an innovative biomass pretreatment technology.
“CELF uses tetrahydrofuran or THF to supplement water and dilute acid during biomass pretreatment. It improves overall efficiency and adds lignin extraction capabilities,” Cai said. “Best of all, THF itself can be made from biomass sugars.”
A landmark Energy & Environmental Science paper details the degree to which a CELF biorefinery offers economic and environmental benefits over both petroleum-based fuels and earlier biofuel production methods.

The paper is a collaboration between Cai’s research team at UC Riverside, the Center for Bioenergy Innovation managed by Oak Ridge National Laboratories, and the National Renewable Energy Laboratory, with funding provided by the U.S. Department of Energy’s Office of Science. In it, the researchers consider two main variables: what kind of biomass is most ideal and what to do with the lignin once it’s been extracted.
First-generation biofuel operations use food crops like corn, soy, and sugarcane as raw materials, or feedstocks. Because these feedstocks divert land and water away from food production, using them for biofuels is not ideal.
Second-generation operations use non-edible plant biomass as feedstocks. An example of biomass feedstocks includes wood residues from milling operations, sugarcane bagasse, or corn stover, all of which are abundant low-cost byproducts of forestry and agricultural operations.
According to the Department of Energy , up to a billion tons per year of biomass could be made available for the manufacture of biofuels and bioproducts in the US alone, capable of displacing 30 percent of our petroleum consumption while also creating new domestic jobs.
Because a CELF biorefinery can more fully utilize plant matter than earlier second-generation methods, the researchers found that a heavier, denser feedstock like hardwood poplar is preferable over less carbon-dense corn stover for yielding greater economic and environmental benefits.
Using poplar in a CELF biorefinery, the researchers demonstrate that sustainable aviation fuel could be made at a break-even price as low as $3.15 per gallon of gasoline equivalent. The current average cost for a gallon of jet fuel in the U.S. is $5.96.

The U.S. government issues credits for biofuel production in the form of renewable identification number credits, a subsidy meant to bolster domestic biofuel production. The tier of these credits issued for second-generation biofuels, the D3 tier, is typically traded at $1 per gallon or higher. At this price per credit, the paper demonstrates that one can expect a rate of return of over 20 percent from the operation.
“Spending a little more for a more carbon-rich feedstock like poplar still yields more economic benefits than a cheaper feedstock like corn stover, because you can make more fuel and chemicals from it,” Cai said.
The paper also illustrates how lignin utilization can positively contribute to overall biorefinery economics while keeping the carbon footprint as low as possible. In older biorefinery models, where biomass is cooked in water and acid, the lignin is mostly unusable for more than its heating value.
“The older models would elect to burn the lignin to supplement heat and energy for these biorefineries because they could mostly only leverage the sugars in the biomass - a costly proposition that leaves a lot of value off the table,” said Cai.
In addition to better lignin utilization, the CELF biorefinery model also proposes to produce renewable chemicals. These chemicals could be used as building blocks for bioplastics and food and drink flavoring compounds. These chemicals take up some of the carbon in the plant biomass that would not get released back into the atmosphere as CO2.
“Adding THF helps reduce the energy cost of pretreatment and helps isolate lignin, so you wouldn’t have to burn it anymore. On top of that, we can make renewable chemicals that help us achieve a near-zero global warming potential,” Cai said. “I think this moves the needle from Gen 2 biofuels to Gen 2+.”
In light of the team’s recent successes, the Department of Energy’s Bioenergy Technology Office has awarded the researchers a $2 million grant to build a small-scale CELF pilot plant at UC Riverside. Cai hopes that demonstrating the pilot plant will lead to larger-scale investment in the technology, as harnessing energy from fossil fuels adds to global warming and hurts the planet.
“I began this work more than a decade ago because I wanted to make an impact. I wanted to find a viable alternative to fossil fuels and my colleagues and I have done that,” Cai said. “Using CELF, we have shown it is possible to create cost-effective fuels from biomass and lignin and help curb our contribution of carbon emissions into the atmosphere.”
Keep reading

UCLA and Equatic to build world’s largest ocean-based plant…
The $20 million system in Singapore will be capable of removing 3,650 metric tons of CO2 per year.

New canal project expands on UC Merced solar research
A new project to place solar panels on the water in the Delta-Mendota Canal, part of a $19 million investment through President Joe Biden’s Inflation Reduction Act, was announced by the Department of…
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 25 June 2021
Microbial production of advanced biofuels
- Jay Keasling 1 , 2 , 3 , 4 , 5 , 6 ,
- Hector Garcia Martin ORCID: orcid.org/0000-0002-4556-9685 1 , 4 , 7 , 8 , 9 ,
- Taek Soon Lee 1 , 4 ,
- Aindrila Mukhopadhyay ORCID: orcid.org/0000-0002-6513-7425 1 , 4 , 9 ,
- Steven W. Singer 1 , 4 &
- Eric Sundstrom 4 , 10
Nature Reviews Microbiology volume 19 , pages 701–715 ( 2021 ) Cite this article
12k Accesses
143 Citations
37 Altmetric
Metrics details
- Applied microbiology
- Metabolic engineering
Concerns over climate change have necessitated a rethinking of our transportation infrastructure. One possible alternative to carbon-polluting fossil fuels is biofuels produced by engineered microorganisms that use a renewable carbon source. Two biofuels, ethanol and biodiesel, have made inroads in displacing petroleum-based fuels, but their uptake has been limited by the amounts that can be used in conventional engines and by their cost. Advanced biofuels that mimic petroleum-based fuels are not limited by the amounts that can be used in existing transportation infrastructure but have had limited uptake due to costs. In this Review, we discuss engineering metabolic pathways to produce advanced biofuels, challenges with substrate and product toxicity with regard to host microorganisms and methods to engineer tolerance, and the use of functional genomics and machine learning approaches to produce advanced biofuels and prospects for reducing their costs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
WLTC and real-driving emissions for an autochthonous biofuel from wine-industry waste
Physiological limitations and opportunities in microbial metabolic engineering
Towards carbon-neutral and clean propulsion in heavy-duty transportation with hydroformylated Fischer–Tropsch fuels
US Energy Information Administration. International energy outlook 2019: with projections to 2050 (EIA, 2019).
US Environmental Protection Agency. Sources of greenhouse gas emissions. EPA https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (2020).
Kircher, M. Sustainability of biofuels and renewable chemicals production from biomass. Curr. Opin. Chem. Biol. 29 , 26–31 (2015).
Article CAS PubMed Google Scholar
Hughes, S. R. & Jones, M. A. in Green Energy to Sustainability: Strategies for Global Industries (eds Vertès, A. A., Qureshi, N., Blaschek, H. P. & Yukawa, H.) 137–156 (Wiley, 2020).
Liu, Y. et al. Biofuels for a sustainable future. Cell 184 , 1636–1647 (2021).
Field, J. L. et al. Robust paths to net greenhouse gas mitigation and negative emissions via advanced biofuels. Proc. Natl Acad. Sci. USA 117 , 21968–21977 (2020).
Article CAS PubMed PubMed Central Google Scholar
Baral, N. et al. Techno-economic analysis and life-cycle greenhouse gas mitigation cost of five routes to bio-jet fuel blendstocks. Energy Environ. Sci. 12 , 807–824 (2018).
Article Google Scholar
Hannon, J. R. et al. Technoeconomic and life-cycle analysis of single-step catalytic conversion of wet ethanol into fungible fuel blendstocks. Proc. Natl Acad. Sci. USA 117 , 12576–12583 (2020).
Baral, N. R. et al. Approaches for more efficient biological conversion of lignocellulosic feedstocks to biofuels and bioproducts. ACS Sustain. Chem. Eng. 7 , 9062–9079 (2019).
Article CAS Google Scholar
Yang, M., Baral, N. R., Anastasopoulou, A., Breunig, H. M. & Scown, C. D. Cost and life-cycle greenhouse gas implications of integrating biogas upgrading and carbon capture technologies in cellulosic biorefineries. Environ. Sci. Technol. 54 , 12810–12819 (2020).
Langholtz, M. H., Stokes, B. J. & Eaton, L. M. Billion-ton report: advancing domestic resources for a thriving bioeconomy, volume 1: economic availability of feedstocks (Oak Ridge National Laboratory, 2016).
Global Bioenergy Association. Global Bioenergy Statistics 2019 . http://www.worldbioenergy.org/uploads/191129%20WBA%20GBS%202019_HQ.pdf (Global Bioenergy Association, 2019).
Pattrick, C. A. et al. Proteomic profiling, transcription factor modeling, and genomics of evolved tolerant strains elucidate mechanisms of vanillin toxicity in Escherichia coli. mSystems 4 , e00163-19 (2019).
Article PubMed PubMed Central Google Scholar
Carroll, A. & Somerville, C. Cellulosic biofuels. Annu. Rev. Plant Biol. 60 , 165–182 (2009).
Blanch, H. W., Simmons, B. A. & Klein-Marcuschamer, D. Biomass deconstruction to sugars. Biotechnol. J. 6 , 1086–1102 (2011).
Dale, B. E. & Ong, R. G. Energy, wealth, and human development: why and how biomass pretreatment research must improve. Biotechnol. Prog. 28 , 893–898 (2012).
Klein-Marcuschamer, D., Oleskowicz-Popiel, P., Simmons, B. A. & Blanch, H. W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 109 , 1083–1087 (2012).
Lee, S. Y., Kim, H. M. & Cheon, S. Metabolic engineering for the production of hydrocarbon fuels. Curr. Opin. Biotechnol. 33 , 15–22 (2015). A comprehensive review of metabolic engineering for biofuel production .
Adom, F., Dunn, J. B., Han, J. & Sather, N. Life-cycle fossil energy consumption and greenhouse gas emissions of bioderived chemicals and their conventional counterparts. Environ. Sci. Technol. 48 , 14624–14631 (2014).
Biddy, M. J. et al. The techno-economic basis for coproduct manufacturing to enable hydrocarbon fuel production from lignocellulosic biomass. ACS Sustain. Chem. Eng. 4 , 3196–3211 (2016).
Brooks, K. P. et al. in Biofuels for Aviation: Feedstocks, Technology and Implementation (ed. Chuck, C.) 109–150 (Academic, 2016).
George, K. W., Alonso-Gutierrez, J., Keasling, J. D. & Lee, T. S. Isoprenoid drugs, biofuels, and chemicals-artemisinin, farnesene, and beyond. Adv. Biochem. Eng. Biotechnol. 148 , 355–389 (2015).
CAS PubMed Google Scholar
Li, M. et al. Recent advances of metabolic engineering strategies in natural isoprenoid production using cell factories. Nat. Prod. Rep. 37 , 80–99 (2020).
Rodríguez-Concepción, M. Plant isoprenoids: a general overview. Methods Mol. Biol. 1153 , 1–5 (2014).
Article PubMed CAS Google Scholar
Gao, Y., Honzatko, R. B. & Peters, R. J. Terpenoid synthase structures: a so far incomplete view of complex catalysis. Nat. Prod. Rep. 29 , 1153–1175 (2012).
Peralta-Yahya, P. P. et al. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2 , 483 (2011).
Harrison, K. W. & Harvey, B. G. Renewable high density fuels containing tricyclic sesquiterpanes and alkyl diamondoids. Sustain. Energy Fuels 1 , 467–473 (2017).
Zebec, Z. et al. Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr. Opin. Chem. Biol. 34 , 37–43 (2016).
George, K. W. et al. Correlation analysis of targeted proteins and metabolites to assess and engineer microbial isopentenol production. Biotechnol. Bioeng. 111 , 1648–1658 (2014).
Meadows, A. L. et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 537 , 694–697 (2016). A good demonstration of microbial engineering for biofuel-producing yeast, especially at industrial scale .
Kang, A. et al. Isopentenyl diphosphate (IPP)-bypass mevalonate pathways for isopentenol production. Metab. Eng. 34 , 25–35 (2016).
Kang, A. et al. Optimization of the IPP-bypass mevalonate pathway and fed-batch fermentation for the production of isoprenol in Escherichia coli. Metab. Eng. 56 , 85–96 (2019).
Lennen, R. M. & Pfleger, B. F. Engineering Escherichia coli to synthesize free fatty acids. Trends Biotechnol. 30 , 659–667 (2012).
Budin, I. et al. Viscous control of cellular respiration by membrane lipid composition. Science 362 , 1186–1189 (2018).
Marella, E. R., Holkenbrink, C., Siewers, V. & Borodina, I. Engineering microbial fatty acid metabolism for biofuels and biochemicals. Curr. Opin. Biotechnol. 50 , 39–46 (2018).
Qiao, K. et al. Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica . Metab. Eng. 29 , 56–65 (2015).
Steen, E. J. et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463 , 559–562 (2010).
Zhou, Y. J. et al. Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat. Commun. 7 , 11709 (2016).
Krivoruchko, A., Zhang, Y., Siewers, V., Chen, Y. & Nielsen, J. Microbial acetyl-CoA metabolism and metabolic engineering. Metab. Eng. 28 , 28–42 (2015).
Qiao, K., Wasylenko, T. M., Zhou, K., Xu, P. & Stephanopoulos, G. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat. Biotechnol. 35 , 173–177 (2017).
Zhu, Z. et al. Expanding the product portfolio of fungal type I fatty acid synthases. Nat. Chem. Biol. 13 , 360–362 (2017).
Schirmer, A., Rude, M. A., Li, X., Popova, E. & del Cardayre, S. B. Microbial biosynthesis of alkanes. Science 329 , 559–562 (2010).
Rude, M. A. et al. Terminal olefin (1-alkene) biosynthesis by a novel p450 fatty acid decarboxylase from Jeotgalicoccus species. Appl. Environ. Microbiol. 77 , 1718–1727 (2011).
Youngquist, J. T. et al. Production of medium chain length fatty alcohols from glucose in Escherichia coli . Metab. Eng. 20 , 177–186 (2013).
Goh, E.-B., Baidoo, E. E. K., Keasling, J. D. & Beller, H. R. Engineering of bacterial methyl ketone synthesis for biofuels. Appl. Environ. Microbiol. 78 , 70–80 (2012).
Javidpour, P. et al. Investigation of proposed ladderane biosynthetic genes from anammox bacteria by heterologous expression in E. coli . PLoS ONE 11 , e0151087 (2016).
Article PubMed PubMed Central CAS Google Scholar
Czerwiec, Q. et al. Optimization of cyclopropane fatty acids production in Yarrowia lipolytica . Yeast 36 , 143–151 (2019).
Rabinovitch-Deere, C. A., Oliver, J. W. K., Rodriguez, G. M. & Atsumi, S. Synthetic biology and metabolic engineering approaches to produce biofuels. Chem. Rev. 113 , 4611–4632 (2013).
Bond-Watts, B. B., Bellerose, R. J. & Chang, M. C. Y. Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways. Nat. Chem. Biol. 7 , 222–227 (2011).
Atsumi, S., Hanai, T. & Liao, J. C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451 , 86–89 (2008).
Sherkhanov, S. et al. Isobutanol production freed from biological limits using synthetic biochemistry. Nat. Commun. 11 , 4292 (2020). A good demonstration of the cell-free system for biofuel (isobutanol) production with high TRY .
Rodriguez, G. M., Tashiro, Y. & Atsumi, S. Expanding ester biosynthesis in Escherichia coli . Nat. Chem. Biol. 10 , 259–265 (2014).
Lee, J.-W. & Trinh, C. T. Microbial biosynthesis of lactate esters. Biotechnol. Biofuels 12 , 226 (2019).
Yuzawa, S., Keasling, J. D. & Katz, L. Insights into polyketide biosynthesis gained from repurposing antibiotic-producing polyketide synthases to produce fuels and chemicals. J. Antibiot. 69 , 494–499 (2016).
Cai, W. & Zhang, W. Engineering modular polyketide synthases for production of biofuels and industrial chemicals. Curr. Opin. Biotechnol. 50 , 32–38 (2018).
Liu, Q. et al. Engineering an iterative polyketide pathway in Escherichia coli results in single-form alkene and alkane overproduction. Metab. Eng. 28 , 82–90 (2015).
Poust, S. et al. Divergent mechanistic routes for the formation of gem-dimethyl groups in the biosynthesis of complex polyketides. Angew. Chem. Int. Ed. 54 , 2370–2373 (2015).
Srirangan, K. et al. Engineering Escherichia coli for Microbial Production of Butanone. Appl. Environ. Microbiol. 82 , 2574–2584 (2016).
Yuzawa, S. et al. Short-chain ketone production by engineered polyketide synthases in Streptomyces albus . Nat. Commun. 9 , 4569 (2018).
Zargar, A. et al. Leveraging microbial biosynthetic pathways for the generation of “drop-in” biofuels. Curr. Opin. Biotechnol. 45 , 156–163 (2017).
Smith, K. M., Cho, K.-M. & Liao, J. C. Engineering Corynebacterium glutamicum for isobutanol production. Appl. Microbiol. Biotechnol. 87 , 1045–1055 (2010).
Lin, P. P. et al. Consolidated bioprocessing of cellulose to isobutanol using Clostridium thermocellum . Metab. Eng. 31 , 44–52 (2015).
Yan, Q. & Pfleger, B. F. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals. Metab. Eng. 58 , 35–46 (2020).
Hanko, E. K. R. et al. Engineering β-oxidation in Yarrowia lipolytica for methyl ketone production. Metab. Eng. 48 , 52–62 (2018).
Kim, H. M., Chae, T. U., Choi, S. Y., Kim, W. J. & Lee, S. Y. Engineering of an oleaginous bacterium for the production of fatty acids and fuels. Nat. Chem. Biol. 15 , 721–729 (2019).
Sasaki, Y. et al. Engineering Corynebacterium glutamicum to produce the biogasoline isopentenol from plant biomass hydrolysates. Biotechnol. Biofuels 12 , 41 (2019).
Yaegashi, J. et al. Rhodosporidium toruloides : a new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol. Biofuels 10 , 241 (2017).
Sundstrom, E. et al. Demonstrating a separation-free process coupling ionic liquid pretreatment, saccharification, and fermentation with Rhodosporidium toruloides to produce advanced biofuels. Green Chem. 20 , 2870–2879 (2018).
Zhuang, X. et al. Monoterpene production by the carotenogenic yeast Rhodosporidium toruloides . Microb. Cell Fact. 18 , 54 (2019).
Miao, R., Xie, H. & Lindblad, P. Enhancement of photosynthetic isobutanol production in engineered cells of Synechocystis PCC 6803. Biotechnol. Biofuels 11 , 267 (2018).
Nguyen, A. D., Kim, D. & Lee, E. Y. Unlocking the biosynthesis of sesquiterpenoids from methane via the methylerythritol phosphate pathway in methanotrophic bacteria, using α-humulene as a model compound. Metab. Eng. 61 , 69–78 (2020).
Krieg, T., Sydow, A., Faust, S., Huth, I. & Holtmann, D. CO 2 to terpenes: autotrophic and electroautotrophic α-humulene production with Cupriavidus necato r. Angew. Chem. Int. Ed. 57 , 1879–1882 (2018).
Grenz, S. et al. Exploiting Hydrogenophaga pseudoflava for aerobic syngas-based production of chemicals. Metab. Eng. 55 , 220–230 (2019).
Mukhopadhyay, A. Tolerance engineering in bacteria for the production of advanced biofuels and chemicals. Trends Microbiol. 23 , 498–508 (2015).
Niu, F.-X., He, X., Wu, Y.-Q. & Liu, J.-Z. Enhancing production of pinene in Escherichia coli by using a combination of tolerance, evolution, and modular co-culture engineering. Front. Microbiol. 9 , 1623 (2018).
Mendez-Perez, D. et al. Production of jet fuel precursor monoterpenoids from engineered Escherichia coli . Biotechnol. Bioeng. 114 , 1703–1712 (2017).
Chong, H. et al. Enhancing E. coli isobutanol tolerance through engineering its global transcription factor cAMP receptor protein (CRP). Biotechnol. Bioeng. 111 , 700–708 (2014).
Mukhopadhyay, A., Hillson, N. J. & Keasling, J. D. in Microbial Stress Tolerance for Biofuels (ed. Liu, Z. L.) 209–238 (Springer, 2012).
Park, J. I. et al. A thermophilic ionic liquid-tolerant cellulase cocktail for the production of cellulosic biofuels. PLoS ONE 7 , e37010 (2012).
Yu, C., Simmons, B. A., Singer, S. W., Thelen, M. P. & VanderGheynst, J. S. Ionic liquid-tolerant microorganisms and microbial communities for lignocellulose conversion to bioproducts. Appl. Microbiol. Biotechnol. 100 , 10237–10249 (2016).
Thorwall, S., Schwartz, C., Chartron, J. W. & Wheeldon, I. Stress-tolerant non-conventional microbes enable next-generation chemical biosynthesis. Nat. Chem. Biol. 16 , 113–121 (2020).
Sandoval, N. R. & Papoutsakis, E. T. Engineering membrane and cell-wall programs for tolerance to toxic chemicals: beyond solo genes. Curr. Opin. Microbiol. 33 , 56–66 (2016).
Basler, G., Thompson, M., Tullman-Ercek, D. & Keasling, J. A Pseudomonas putida efflux pump acts on short-chain alcohols. Biotechnol. Biofuels 11 , 136 (2018).
Chen, B., Ling, H. & Chang, M. W. Transporter engineering for improved tolerance against alkane biofuels in Saccharomyces cerevisiae . Biotechnol. Biofuels 6 , 21 (2013).
Chen, Y. et al. Reverse engineering of fatty acid-tolerant Escherichia coli identifies design strategies for robust microbial cell factories. Metab. Eng. 61 , 120–130 (2020).
Otoupal, P. B. & Chatterjee, A. CRISPR gene perturbations provide insights for improving bacterial biofuel tolerance. Front. Bioeng. Biotechnol. 6 , 122 (2018).
Kurgan, G. et al. Bioprospecting of native efflux pumps to enhance furfural tolerance in ethanologenic Escherichia coli . Appl. Environ. Microbiol. 85 , e02985-18 (2019).
Song, H.-S. et al. Increase in furfural tolerance by combinatorial overexpression of NAD salvage pathway enzymes in engineered isobutanol-producing E. coli . Bioresour. Technol. 245 , 1430–1435 (2017).
Frederix, M. et al. Development of an E. coli strain for one-pot biofuel production from ionic liquid pretreated cellulose and switchgrass. Green Chem. 18 , 4189–4197 (2016).
Eng, T. et al. Restoration of biofuel production levels and increased tolerance under ionic liquid stress is enabled by a mutation in the essential Escherichia coli gene cydC. Microb. Cell Fact. 17 , 159 (2018).
Ruegg, T. L. et al. Jungle Express is a versatile repressor system for tight transcriptional control. Nat. Commun. 9 , 3617 (2018).
Wang, S. et al. NaCl enhances Escherichia coli growth and isoprenol production in the presence of imidazolium-based ionic liquids. Bioresour. Technol. Rep. 6 , 1–5 (2019).
Nikel, P. I. & de Lorenzo, V. Pseudomonas putida as a functional chassis for industrial biocatalysis: from native biochemistry to trans-metabolism. Metab. Eng. 50 , 142–155 (2018).
Yang, S. et al. Zymomonas mobilis as a model system for production of biofuels and biochemicals. Microb. Biotechnol. 9 , 699–717 (2016).
Stella, R. G., Wiechert, J., Noack, S. & Frunzke, J. Evolutionary engineering of Corynebacterium glutamicum . Biotechnol. J. 14 , e1800444 (2019).
Castro, A. R., Rocha, I., Alves, M. M. & Pereira, M. A. Rhodococcus opacus B4: a promising bacterium for production of biofuels and biobased chemicals. AMB. Express 6 , 35 (2016).
Thompson, M. G. et al. Fatty acid and alcohol metabolism in Pseudomonas putida : functional analysis using random barcode transposon sequencing. Appl. Environ. Microbiol. 86 , e01665-20 (2020).
Sandberg, T. E., Salazar, M. J., Weng, L. L., Palsson B. O. & Feist, A. M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 56 , 1–16 (2019).
Lim, H. G. et al. Generation of ionic liquid tolerant Pseudomonas putida KT2440 strains via adaptive laboratory evolution. Green Chem. 22 , 5677–5690 (2020).
Price, M. N. et al. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature 557 , 503–509 (2018). A study showing the power of the RB-TnSeq approach in elucidating gene function in a vast number of microorganisms, which is not only valuable for identifying gene targets for strain engineering but is also broadly useful for improving GSMMs .
Li, W.-J. et al. Unraveling 1,4-butanediol metabolism in Pseudomonas putida KT2440. Front. Microbiol. 11 , 382 (2020).
Phaneuf, P. V., Gosting, D., Palsson, B. O. & Feist, A. M. ALEdb 1.0: a database of mutations from adaptive laboratory evolution experimentation. Nucleic Acids Res. 47 , D1164–D1171 (2019).
Article PubMed Google Scholar
Zhang, F., Carothers, J. M. & Keasling, J. D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat. Biotechnol. 30 , 354–359 (2012).
DeLoache, W. C., Russ, Z. N. & Dueber, J. E. Towards repurposing the yeast peroxisome for compartmentalizing heterologous metabolic pathways. Nat. Commun. 7 , 11152 (2016).
Hu, T. et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast. Metab. Eng. 60 , 87–96 (2020).
McCloskey, D. et al. Adaptation to the coupling of glycolysis to toxic methylglyoxal production in tpiA deletion strains of Escherichia coli requires synchronized and counterintuitive genetic changes. Metab. Eng. 48 , 82–93 (2018).
Gubellini, F. et al. Physiological response to membrane protein overexpression in E. coli . Mol. Cell. Proteom. 10 , M111.007930 (2011).
Baumgarten, T., Ytterberg, A. J., Zubarev, R. A. & de Gier, J.-W. Optimizing recombinant protein production in the Escherichia coli periplasm alleviates stress. Appl. Environ. Microbiol. 84 , e00270-18 (2018).
Boyarskiy, S., Davis López, S., Kong, N. & Tullman-Ercek, D. Transcriptional feedback regulation of efflux protein expression for increased tolerance to and production of n -butanol. Metab. Eng. 33 , 130–137 (2016).
Henard, C. A., Freed, E. F. & Guarnieri, M. T. Phosphoketolase pathway engineering for carbon-efficient biocatalysis. Curr. Opin. Biotechnol. 36 , 183–188 (2015).
Lin, P. P. et al. Construction and evolution of an Escherichia coli strain relying on nonoxidative glycolysis for sugar catabolism. Proc. Natl Acad. Sci. USA 115 , 3538–3546 (2018). A very detailed and extensive demonstration of engineering non-oxidative glycolysis .
Bogorad, I. W., Lin, T.-S. & Liao, J. C. Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature 502 , 693–697 (2013).
Fleige, C., Kroll, J. & Steinbüchel, A. Establishment of an alternative phosphoketolase-dependent pathway for fructose catabolism in Ralstonia eutropha H16. Appl. Microbiol. Biotechnol. 91 , 769–776 (2011).
Chubukov, V., Mukhopadhyay, A., Petzold, C. J., Keasling, J. D. & Martín, H. G. Synthetic and systems biology for microbial production of commodity chemicals. NPJ Syst. Biol. Appl. 2 , 16009 (2016).
Tian, T., Kang, J. W., Kang, A. & Lee, T. S. Redirecting metabolic flux via combinatorial multiplex CRISPRi-mediated repression for isopentenol production in Escherichia coli . ACS Synth. Biol. 8 , 391–402 (2019).
George, K. W. et al. Metabolic engineering for the high-yield production of isoprenoid-based C 5 alcohols in E. coli . Sci. Rep. 5 , 11128 (2015).
Strucko, T. et al. Laboratory evolution reveals regulatory and metabolic trade-offs of glycerol utilization in Saccharomyces cerevisiae . Metab. Eng. 47 , 73–82 (2018).
Caspeta, L. et al. Biofuels. Altered sterol composition renders yeast thermotolerant. Science 346 , 75–78 (2014).
Mohamed, E. T. et al. Generation of a platform strain for ionic liquid tolerance using adaptive laboratory evolution. Microb. Cell Fact. 16 , 204 (2017).
Lennen, R. M. et al. Adaptive laboratory evolution reveals general and specific chemical tolerance mechanisms and enhances biochemical production. Preprint at bioRxiv https://doi.org/10.1101/634105 (2019).
Shepelin, D., Hansen, A. S. L., Lennen, R., Luo, H. & Herrgård, M. J. Selecting the best: evolutionary engineering of chemical production in microbes. Genes 9 , 249 (2018). An excellent review on growth coupling .
Article PubMed Central CAS Google Scholar
Fong, S. S. et al. In silico design and adaptive evolution of Escherichia coli for production of lactic acid. Biotechnol. Bioeng. 91 , 643–648 (2005).
Zhang, X., Jantama, K., Moore, J. C., Shanmugam, K. T. & Ingram, L. O. Production of L-alanine by metabolically engineered Escherichia coli . Appl. Microbiol. Biotechnol. 77 , 355–366 (2007).
Shen, C. R. et al. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli . Appl. Environ. Microbiol. 77 , 2905–2915 (2011).
Machado, H. B., Dekishima, Y., Luo, H., Lan, E. I. & Liao, J. C. A selection platform for carbon chain elongation using the CoA-dependent pathway to produce linear higher alcohols. Metab. Eng. 14 , 504–511 (2012).
Reyes, L. H., Gomez, J. M. & Kao, K. C. Improving carotenoids production in yeast via adaptive laboratory evolution. Metab. Eng. 21 , 26–33 (2014).
Tai, Y.-S. et al. Engineering nonphosphorylative metabolism to generate lignocellulose-derived products. Nat. Chem. Biol. 12 , 247–253 (2016).
Hädicke, O. & Klamt, S. Computing complex metabolic intervention strategies using constrained minimal cut sets. Metab. Eng. 13 , 204–213 (2011).
Harder, B.-J., Bettenbrock, K. & Klamt, S. Model-based metabolic engineering enables high yield itaconic acid production by Escherichia coli . Metab. Eng. 38 , 29–37 (2016).
von Kamp, A. & Klamt, S. Growth-coupled overproduction is feasible for almost all metabolites in five major production organisms. Nat. Commun. 8 , 15956 (2017).
Banerjee, D. et al. Genome-scale metabolic rewiring improves titers rates and yields of the non-native product indigoidine at scale. Nat. Commun. 11 , 5385 (2020).
King, Z. A. et al. BiGG models: a platform for integrating, standardizing and sharing genome-scale models. Nucleic Acids Res. 44 , D515–D522 (2016).
Landon, S., Rees-Garbutt, J., Marucci, L. & Grierson, C. Genome-driven cell engineering review: in vivo and in silico metabolic and genome engineering. Essays Biochem. 63 , 267–284 (2019).
Yim, H. et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 7 , 445–452 (2011).
Ng, C. Y., Jung, M.-Y., Lee, J. & Oh, M.-K. Production of 2,3-butanediol in Saccharomyces cerevisiae by in silico aided metabolic engineering. Microb. Cell Fact. 11 , 68 (2012).
Izallalen, M. et al. Geobacter sulfurreducens strain engineered for increased rates of respiration. Metab. Eng. 10 , 267–275 (2008).
Fowler, Z. L., Gikandi, W. W. & Koffas, M. A. G. Increased malonyl coenzyme A biosynthesis by tuning the Escherichia coli metabolic network and its application to flavanone production. Appl. Environ. Microbiol. 75 , 5831–5839 (2009).
Chemler, J. A., Fowler, Z. L., McHugh, K. P. & Koffas, M. A. G. Improving NADPH availability for natural product biosynthesis in Escherichia coli by metabolic engineering. Metab. Eng. 12 , 96–104 (2010).
Asadollahi, M. A. et al. Enhancing sesquiterpene production in Saccharomyces cerevisiae through in silico driven metabolic engineering. Metab. Eng. 11 , 328–334 (2009).
Brochado, A. R. et al. Improved vanillin production in baker’s yeast through in silico design. Microb. Cell Fact. 9 , 84 (2010).
Choi, H. S., Lee, S. Y., Kim, T. Y. & Woo, H. M. In silico identification of gene amplification targets for improvement of lycopene production. Appl. Environ. Microbiol. 76 , 3097–3105 (2010).
Becker, J., Zelder, O., Häfner, S., Schröder, H. & Wittmann, C. From zero to hero–design-based systems metabolic engineering of Corynebacterium glutamicum for L-lysine production. Metab. Eng. 13 , 159–168 (2011).
Li, S., Huang, D., Li, Y., Wen, J. & Jia, X. Rational improvement of the engineered isobutanol-producing Bacillus subtilis by elementary mode analysis. Microb. Cell Fact. 11 , 101 (2012).
Xu, P., Ranganathan, S., Fowler, Z. L., Maranas, C. D. & Koffas, M. A. G. Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab. Eng. 13 , 578–587 (2011).
Ranganathan, S. et al. An integrated computational and experimental study for overproducing fatty acids in Escherichia coli . Metab. Eng. 14 , 687–704 (2012). An illustrative example of using GSMMs to guide bioengineering .
Otero, J. M. et al. Industrial systems biology of Saccharomyces cerevisiae enables novel succinic acid cell factory. PLoS ONE 8 , e54144 (2013).
Ghosh, A. et al. 13 C metabolic flux analysis for systematic metabolic engineering of S. cerevisiae for overproduction of fatty acids. Front. Bioeng. Biotechnol. 4 , 76 (2016).
d’Espaux, L. et al. Engineering high-level production of fatty alcohols by Saccharomyces cerevisiae from lignocellulosic feedstocks. Metab. Eng. 42 , 115–125 (2017).
Lawson, C. E. et al. Machine learning for metabolic engineering: a review. Metab. Eng . 63 , 34–60. A good introduction to machine learning for the metabolic engineer .
Alonso-Gutierrez, J. et al. Principal component analysis of proteomics (PCAP) as a tool to direct metabolic engineering. Metab. Eng. 28 , 123–133 (2015).
Ohtake, T. et al. Metabolomics-driven approach to solving a CoA imbalance for improved 1-butanol production in Escherichia coli . Metab. Eng. 41 , 135–143 (2017).
Xu, P., Rizzoni, E. A., Sul, S.-Y. & Stephanopoulos, G. Improving metabolic pathway efficiency by statistical model-based multivariate regulatory metabolic engineering. ACS Synth. Biol. 6 , 148–158 (2017).
Zhou, Y. et al. MiYA, an efficient machine-learning workflow in conjunction with the YeastFab assembly strategy for combinatorial optimization of heterologous metabolic pathways in Saccharomyces cerevisiae . Metab. Eng. 47 , 294–302 (2018).
Costello, Z. & Martin, H. G. A machine learning approach to predict metabolic pathway dynamics from time-series multiomics data. NPJ Syst. Biol. Appl. 4 , 19 (2018).
Opgenorth, P. et al. Lessons from two design-build-test-learn cycles of dodecanol production in Escherichia coli aided by machine learning. ACS Synth. Biol. 8 , 1337–1351 (2019).
Jervis, A. J. et al. Machine learning of designed translational control allows predictive pathway optimization in Escherichia coli . ACS Synth. Biol. 8 , 127–136 (2019). An excellent application of machine learning to transcriptional control .
HamediRad, M. et al. Towards a fully automated algorithm driven platform for biosystems design. Nat. Commun. 10 , 5150 (2019). A fantastic example of the promise of combining machine learning, synthetic biology and automation .
Zhang, J. et al. Combining mechanistic and machine learning models for predictive engineering and optimization of tryptophan metabolism. Nat. Commun. 11 , 880 (2020).
CAS Google Scholar
Radivojević, T., Costello, Z., Workman, K. & Garcia Martin, H. A machine learning automated recommendation tool for synthetic biology. Nat. Commun. 11 , 4879 (2020).
Yadav, V. G., De Mey, M., Lim, C. G., Ajikumar, P. K. & Stephanopoulos, G. The future of metabolic engineering and synthetic biology: towards a systematic practice. Metab. Eng. 14 , 233–241 (2012).
Carbonell, P., Radivojevic, T. & García Martín, H. Opportunities at the intersection of synthetic biology, machine learning, and automation. ACS Synth. Biol. 8 , 1474–1477 (2019).
Dietrich, J. A., McKee, A. E. & Keasling, J. D. High-throughput metabolic engineering: advances in small-molecule screening and selection. Annu. Rev. Biochem. 79 , 563–590 (2010).
Crater, J. S. & Lievense, J. C. Scale-up of industrial microbial processes. FEMS Microbiol. Lett. 365 , fny138 (2018).
Article CAS PubMed Central Google Scholar
Davis, R. et al. Process design and economics for the conversion of lignocellulosic biomass to hydrocarbons: dilute-acid and enzymatic deconstruction of biomass to sugars and biological conversion of sugars to hydrocarbons (National Renewable Energy Laboratory, 2013).
Cruz Bournazou, M. N. et al. Online optimal experimental re-design in robotic parallel fed-batch cultivation facilities. Biotechnol. Bioeng. 114 , 610–619 (2017).
Tai, M., Ly, A., Leung, I. & Nayar, G. Efficient high-throughput biological process characterization: definitive screening design with the Ambr250 bioreactor system. Biotechnol. Prog. 31 , 1388–1395 (2015).
Wong, B. G., Mancuso, C. P., Kiriakov, S., Bashor, C. J. & Khalil, A. S. Precise, automated control of conditions for high-throughput growth of yeast and bacteria with eVOLVER. Nat. Biotechnol. 36 , 614–623 (2018).
Haringa, C. et al. Computational fluid dynamics simulation of an industrial P. chrysogenum fermentation with a coupled 9-pool metabolic model: towards rational scale-down and design optimization. Chem. Eng. Sci. 175 , 12–24 (2017).
Rugbjerg, P. & Sommer, M. O. A. Overcoming genetic heterogeneity in industrial fermentations. Nat. Biotechnol. 37 , 869–876 (2019).
Wehrs, M. et al. Investigation of Bar-seq as a method to study population dynamics of Saccharomyces cerevisiae deletion library during bioreactor cultivation. Microb. Cell Fact. 19 , 167 (2020).
Wang, O. & Coates, J. D. Biotechnological applications of microbial (per)chlorate reduction. Microorganisms 5 , 76 (2017).
Shaw, A. J. et al. Metabolic engineering of microbial competitive advantage for industrial fermentation processes. Science 353 , 583–586 (2016).
Dahl, R. H. et al. Engineering dynamic pathway regulation using stress-response promoters. Nat. Biotechnol. 31 , 1039–1046 (2013).
Dafoe, J. T. & Daugulis, A. J. In situ product removal in fermentation systems: improved process performance and rational extractant selection. Biotechnol. Lett. 36 , 443–460 (2014).
Xue, C. et al. Integrated butanol recovery for an advanced biofuel: current state and prospects. Appl. Microbiol. Biotechnol. 98 , 3463–3474 (2014).
Gaspar, D. Top ten blendstocks for turbocharged gasoline engines: bioblendstocks with potential to deliver the for highest engine efficiency (Pacific Northwest National Laboratory, 2019). A systematic analysis and down-election of petrol bioblendstock candidates based on fuel properties and engine performance .
Monroe, E. et al. Discovery of novel octane hyperboosting phenomenon in prenol biofuel/gasoline blends. Fuel 239 , 1143–1148 (2019).
Ignea, C. et al. Synthesis of 11-carbon terpenoids in yeast using protein and metabolic engineering. Nat. Chem. Biol. 14 , 1090–1098 (2018).
Huccetogullari, D., Luo, Z. W. & Lee, S. Y. Metabolic engineering of microorganisms for production of aromatic compounds. Microb. Cell Fact. 18 , 41 (2019).
Das, D. D., St. John, P. C., McEnally, C. S., Kim, S. & Pfefferle, L. D. Measuring and predicting sooting tendencies of oxygenates, alkanes, alkenes, cycloalkanes, and aromatics on a unified scale. Combust. Flame 190 , 349–364 (2018).
Huo, X. et al. Tailoring diesel bioblendstock from integrated catalytic upgrading of carboxylic acids: a “fuel property first” approach. Green Chem. 21 , 5813–5827 (2019).
Yang, M. et al. Accumulation of high-value bioproducts in planta can improve the economics of advanced biofuels. Proc. Natl Acad. Sci. USA 117 , 8639–8648 (2020).
Lin, C.-Y. & Eudes, A. Strategies for the production of biochemicals in bioenergy crops. Biotechnol. Biofuels 13 , 71 (2020).
Blombach, B. et al. Corynebacterium glutamicum tailored for efficient isobutanol production. Appl. Environ. Microbiol. 77 , 3300–3310 (2011).
Higashide, W., Li, Y., Yang, Y. & Liao, J. C. Metabolic engineering of Clostridium cellulolyticum for production of isobutanol from cellulose. Appl. Environ. Microbiol. 77 , 2727–2733 (2011).
Sawyer, R. F. Trends in auto emissions and gasoline composition. Environ. Health Perspect. 101 (Suppl. 6), 5–12 (1993).
Ghosh, P., Hickey, K. J. & Jaffe, S. B. Development of a detailed gasoline composition-based octane model. Ind. Eng. Chem. Res. 45 , 337–345 (2006).
Ghosh, P. & Jaffe, S. B. Detailed composition-based model for predicting the cetane number of diesel fuels. Ind. Eng. Chem. Res. 45 , 346–351 (2006).
ASTM International. ASTM D1655 — 20e1: standard specification for aviation turbine fuels (ASTM, 2020).
Download references
Acknowledgements
The authors thank C. Scown (Lawrence Berkeley National Laboratory) for helpful discussions on life cycle and technoeconomic analyses of biofuel production. This work was performed as part of the US Department of Energy (DOE) Joint BioEnergy Institute ( https://www.jbei.org ) supported by the DOE, Office of Science, Office of Biological and Environmental Research, and by the DOE, Energy Efficiency and Renewable Energy, Bioenergy Technologies Office, and as part of the Co-Optimization of Fuels & Engines project sponsored by the DOE, Office of Energy Efficiency and Renewable Energy, Bioenergy Technologies Office and Vehicle Technologies Office, under contract DEAC02-05CH11231 between the DOE and Lawrence Berkeley National Laboratory. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the US Government or any agency thereof. Neither the US Government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. The US Government retains and the publisher, by accepting the article for publication, acknowledges that the US Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of the manuscript, or allow others to do so, for US Government purposes. The DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan ( http://energy.gov/downloads/doe-public-access-plan ).
Author information
Authors and affiliations.
Joint BioEnergy Institute, Emeryville, CA, USA
Jay Keasling, Hector Garcia Martin, Taek Soon Lee, Aindrila Mukhopadhyay & Steven W. Singer
Department of Chemical & Biomolecular Engineering, University of California, Berkeley, Berkeley, CA, USA
Jay Keasling
Department of Bioengineering, University of California, Berkeley, Berkeley, CA, USA
Biological Systems and Engineering Division, Lawrence Berkeley National Laboratory, Berkeley, CA, USA
Jay Keasling, Hector Garcia Martin, Taek Soon Lee, Aindrila Mukhopadhyay, Steven W. Singer & Eric Sundstrom
Center for Biosustainability, Danish Technical University, Lyngby, Denmark
Center for Synthetic Biochemistry, Institute for Synthetic Biology, Shenzhen Institute of Advanced Technology, Shenzhen, China
DOE Agile BioFoundry, Emeryville, CA, USA
Hector Garcia Martin
BCAM,Basque Center for Applied Mathematics, Bilbao, Spain
Environmental Genomics and Systems Biology Division, Lawrence Berkeley National Laboratory, Berkeley, CA, USA
Hector Garcia Martin & Aindrila Mukhopadhyay
Advanced Biofuels and Bioproducts Process Development Unit, Emeryville, CA, USA
Eric Sundstrom
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Jay Keasling .
Ethics declarations
Competing interests.
J.K. has a financial interest in Amyris, Lygos, Demetrix, Napigen, Apertor Pharmaceuticals, Maple Bio, Ansa Biotechnologies, Berkeley Yeast and Zero Acre Farms. The other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Microbiology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A standard measure of an engine or aviation fuel capability against compression.
An indicator of the combustion speed of diesel fuel and compression needed for ignition.
(ILs). A highly efficient set of reagents for the depolymerization and deconstruction of a range of feedstocks.
One-carbon microbial substrates, including CO 2 , CH 4 , CO, HCO 2 − and CH 3 OH.
A mixture of CO, CO 2 and H 2 .
Single fuel components that are blended with additional components to produce a finished fuel.
Modelling approach that uses a polynomial of up to grade 2 to predict the response.
Applied statistics techniques that deal with planning, conducting, analysing and interpreting controlled tests to evaluate the factors that control the experimental output under study.
Modelling approach that takes the input of various different models and has them ‘vote’ for a particular prediction.
The exhaust gas stream exiting a bioreactor.
Development of the genetic tools necessary to allow metabolic engineering of a previously unengineered microorganism.
The degree to which a fuel mixture generates black carbon soot when combusted.
Petrol containing 10% ethanol by volume.
Fuel viscosity at low temperature; poor cold flow can lead to gelling and compromise engine operability in cold weather conditions.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Keasling, J., Garcia Martin, H., Lee, T.S. et al. Microbial production of advanced biofuels. Nat Rev Microbiol 19 , 701–715 (2021). https://doi.org/10.1038/s41579-021-00577-w
Download citation
Accepted : 13 May 2021
Published : 25 June 2021
Issue Date : November 2021
DOI : https://doi.org/10.1038/s41579-021-00577-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Bayesianssa: a bayesian statistical model based on structural sensitivity analysis for predicting responses to enzyme perturbations in metabolic networks.
- Shion Hosoda
- Hisashi Iwata
BMC Bioinformatics (2024)
Enhanced bioethanol production by evolved Escherichia coli LGE2-H in a microbial electrolysis cell system
- Dongdong Chang
- Zhisheng Yu
Bioresources and Bioprocessing (2024)
Increased CO2 fixation enables high carbon-yield production of 3-hydroxypropionic acid in yeast
Nature Communications (2024)
RhizoNet segments plant roots to assess biomass and growth for enabling self-driving labs
- Zineb Sordo
- Peter Andeer
- Daniela Ushizima
Scientific Reports (2024)
Sustainable power generation from sewage with engineered microorganisms as electrocatalysts
- Zhengyu Bai
Nature Sustainability (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Journal Proposal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Biofuels production: a review on sustainable alternatives to traditional fuels and energy sources.

1. Introduction
2. biomass energy.
- Energy crop-based: Biomass from different energy crops such as herbaceous crops, woody, industrial crops, agricultural and aquatic crops are used for energy purposes.
- Agricultural residues and waste: Agriwaste (rice and wheat straw) and animal farm waste.
- Forestry waste and residues: Various mill wood waste, logging residues, trees and shrub residues etc.
- Industrial and municipal wastes: Municipal solid waste (MSW), sewage sludge and different industrial waste.
3. Types of Biofuels
3.1. conventional/traditional biofuels, 3.2. advanced biofuels.
- Enzymatic dry milling
- Dry fractionation
- Ammoniation process in the wet mill
- Continuous membrane reactor for starch hydrolysis
- High-gravity fermentation
- Improved and efficient yeast (recombinant DNA techniques)
- Conversion of pentose and hexoses sugars to ethanol
- New enzymes for liquefaction and saccharification
- Optimized systems/conditions
4. Biofuels Generations/Classifications
4.1. first-generation biofuels, 4.2. second-generation (2g) biofuels, 4.3. third generations of biofuels, 4.4. fourth generations of biofuels, 5. biomass conversion technologies for biofuel production.
- Briquetting
- Pelletization
- Agrochemical route/processes
- Combustion in excess air
- Carbonization
- Very little air/no oxygen added
- 750 °F to 1500 °F
- Some air/oxygen used but less than for incineration
- Begins at 1300 °F
- Bacteria breaks down feedstock
- Anaerobic process
- Microbes used to produce ethanol

6. Comparison between Biofuels and Fossil Fuels
7. future prospective for biofuels production, 8. national policy on biofuels.
- To meet the energy needs of India’s rural population and create employment opportunities;
- To address global concerns by tightening automotive vehicle emission standards to curb air pollution;
- To reduce the dependence on the import of fossil fuels, provide a higher degree of national energy security;
- To derive biofuels from non-edible feedstock on degraded soils or wastelands unsuited to agriculture, avoiding a possible conflict between food and fuel.
9. Major Challenges in Biofuels Production
10. conclusions, author contributions, data availability statement, acknowledgments, conflicts of interest.
- Dale, B. Biofuels: Thinking Clearly About the Issues. J. Agric. Food Chem. 2008 , 56 , 3885–3891. [ Google Scholar ] [ CrossRef ]
- Wang, T.; Zhang, D.; Dai, L.; Chen, Y.; Dai, X. Effects of Metal Nanoparticles on Methane Production from Waste-Activated Sludge and Microorganism Community Shift in Anaerobic Granular Sludge. Sci. Rep. 2016 , 6 , 25857–25862. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Srivastava, A.; Prasad, R. Triglycerides Based Diesel Fuels. Renew. Sustain. Energy Rev. 2000 , 4 , 111–133. [ Google Scholar ] [ CrossRef ]
- Hussein, A.K. Applications of Nanotechnology in Renewable Energies—A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015 , 42 , 460–476. [ Google Scholar ] [ CrossRef ]
- Palaniappan, K. An Overview of Applications of Nanotechnology in Biofuel Production. World Appl. Sci. J. 2017 , 35 , 1305–1311. [ Google Scholar ]
- Serrano, E.; Rus, G.; Garcia-Martinez, J. Nanotechnology for Sustainable Energy. Renew. Sustain. Energy Rev. 2009 , 13 , 2373–2384. [ Google Scholar ] [ CrossRef ]
- Ingle, A.; Paralikar, P.; Silva, D.S.S.; Rai, M. Nanotechnology-Based Developments in Biofuel Production: Current Trends and Appli. In Sustainable Biotechnology—Enzymatic Resources of Renewable Energy ; Singh, O.V., Chandel, K.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 289–305. [ Google Scholar ]
- Aransiola, E.F.; Betiku, E.; Ikhuomoregbe, D.; Ojumu, T.V. Production of Biodiesel from Crude Neem Oil Feedstock and Its Emissions from Internal Combustion Engines. Afr. J. Biotechnol. 2012 , 11 , 6178–6186. [ Google Scholar ] [ CrossRef ]
- Pugh, S.; McKenna, R.; Moolick, R.; Nielsen, D.R. Advances and Opportunities at the Interface Between Microbial Bioenergy and Nanotechnology. Can. J. Chem. Eng. 2011 , 89 , 2–12. [ Google Scholar ] [ CrossRef ]
- Puri, M.; Abraham, R.E.; Barrow, C.J. Biofuel Production: Prospects, Challenges and Feedstock in Australia. Renew. Sustain. Energy Rev. 2012 , 16 , 6022–6031. [ Google Scholar ] [ CrossRef ]
- AFDC; Alternative Fuels Data Center; US Department of Energy. Energy Efficiency and Renewable Energy (EERE). 2006. Available online: https://afdc.energy.gov/ (accessed on 1 March 2006).
- Demirbas, A. Political, Economic and Environmental Impacts of Biofuels: A review. Appl. Energy 2009 , 86 , 108–117. [ Google Scholar ] [ CrossRef ]
- Nizami, A.; Rehan, M. Towards Nanotechnology Based Biofuel Industry. Biofuel Res. J. 2018 , 18 , 798–799. [ Google Scholar ] [ CrossRef ]
- Bandyopadhyay, K.R. Biofuel Promotion in India for Transport: Exploring the Grey Areas ; The Energy and Resources Institute, TERI: Mithapur, India, 2015; Available online: https://www.teriin.org/policy-brief/biofuel-promotion-india-transport-exploring-grey-areas (accessed on 7 February 2015).
- Reddy, B.V.S.; Ramesh, S.; Kumar, A.A.; Wani, S.P. Biofuel Crops Research for Energy Security and Rural Development in Developing Countries. Bioenergy Res. 2008 , 1 , 248–258. [ Google Scholar ] [ CrossRef ]
- GOI (Government of India). Report of the Committee on Development of Bio-Fuel ; Planning Commission, Government of India: New Delhi, India, 2016; 130p. Available online: https://fas.usda.gov/ (accessed on 24 June 2016).
- Talukdar, D.; Verma, D.K.; Malik, K.; Mohapatra, B.; Yulianto, R. Sugarcane as a Potential Biofuel Crop. In Sugarcane Biotechnology ; Mohan, C., Ed.; Challenges and Prospects; Springer International Publishing: Cham, Switzerland, 2017; pp. 123–137. [ Google Scholar ]
- Shu, R.; Li, R.; Lin, B.; Wang, C.; Cheng, Z.; Chen, Y. A review on the catalytic hydrodeoxygenation of lignin-derived phenolic compounds and the conversion of raw lignin to hydrocarbon liquid fuels. Biomass Bioenergy 2020 , 132 , 105432. [ Google Scholar ] [ CrossRef ]
- Goldemberg, J. Biomass and Energy. Química Nova 2008 , 32 , 582–587. [ Google Scholar ] [ CrossRef ]
- Demirbas, A. Biofuels Securing the Planet’s Future Energy Needs. Energy Convers. Manag. 2009 , 50 , 2239–2249. [ Google Scholar ] [ CrossRef ]
- Čuček, L.; Klemeš, J.; Kravanja, Z. Carbon and nitrogen trade-offs in Biomass Energy Production. Clean Technol. Environ. Policy 2012 , 14 , 389–397. [ Google Scholar ] [ CrossRef ]
- DieCorrea, D.F.; Beyer, H.L.; Fargione, J.E.; Hill, J.D.; Possingham, H.P.; Thomas-Hall, S.R.; Schenka, P.M. Towards the Implementation of Sustainable Biofuel Production Systems. Renew. Sustain. Energy Rev. 2019 , 107 , 250–263. [ Google Scholar ]
- NREL. Biomass Research. 2009. Available online: https://www.nrel.gov/ (accessed on 1 August 2009).
- Cramer, J.; Wissema, E.; Lammers, E.; Dijk, D.; Jager, H.; Bennekom, S.; Breunesse, E.; Horster, R.; Leenders, C.; Wolters, W. Criteria for Sustainable Biomass Production. Final Report of the Project Group Sustainable Production of Biomass. 2006. Available online: https://www.globalbioenergy.org/bioenergyinfo/bioenergy-and-food-security/detail/en/c/1488/ (accessed on 2 September 2008).
- Ravindranath, N.H.; Sita Lakshmi, C.; Manuvie, R.; Balachandra, P. Biofuel Production and Implications for Land Use, Food Production and Environment in India. Energy Policy 2011 , 39 , 5737–5745. [ Google Scholar ] [ CrossRef ]
- Sivagurunathan, P.; Kumar, G.; Bakonyi, P.; Kim, S.H.; Kobayashi, T.; Nemestóthy, N.; Bélafi-Bakó, K. A Critical Review on Issues and Overcoming Strategies for the Enhancement of Dark Fermentative Hydrogen Production in Continuous Systems. Int. J. Hydrogen Energy 2016 , 41 , 3820–3836. [ Google Scholar ] [ CrossRef ]
- Singhvi, M.; Zinjarde, S.; Kim, B.S. Sustainable Strategies for the Conversion of Lignocellulosic Materials into Biohydrogen: Challenges and Solutions toward Carbon Neutrality. Energies 2022 , 15 , 8987. [ Google Scholar ] [ CrossRef ]
- Akram, H.A.; Imran, M.; Javaid, A.; Latif, S.; Rizvi, N.B.; Jesionowski, T.; Bilal, M. Pretreatment and catalytic conversion of lignocellulosic and algal biomass into biofuels by metal organic frameworks. Mol. Catal. 2023 , 539 , 112893. [ Google Scholar ] [ CrossRef ]
- Singhvi, M.; Kim, B.S. Green Hydrogen Production through Consolidated Bioprocessing of Lignocellulosic Biomass using Nanobiotechnology Approach. Bioresour. Technol. 2022 , 365 , 128108. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Singh, A.; Sevda, S.; Abu Reesh, I.M.; Vanbroekhoven, K.; Rathore, D.; Pant, D. Biohydrogen Production from Lignocellulosic Biomass: Technology and Sustainability. Energies 2015 , 8 , 13062–13080. [ Google Scholar ] [ CrossRef ]
- IEA. Technology Roadmap Biofuels for Transport ; International Energy Agency: Paris, France, 2011; p. 52. [ Google Scholar ]
- Available online: https://fuelsmarketnews.com/exploring-biofuels-conventional-and-advanced-biofuels-part-1 (accessed on 17 February 2021).
- Oh, Y.K.; Hwang, K.R.; Kim, C.; Kim, J.R.; Lee, J.S. Recent developments and key barriers to advanced biofuels: A short review. Bioresour Technol. 2018 , 257 , 320–333. [ Google Scholar ] [ CrossRef ]
- Singh, S.; Singh, S. Plant-Based Biofuels: An Overview. In Green Approach to Alternative Fuel for a Sustainable Future ; Elsevier: Amsterdam, The Netherlands, 2023; pp. 433–442. [ Google Scholar ]
- Sindhu, R.; Binod, P.; Pandey, A.; Ankaram, S.; Duan, Y.; Awasthi, M.K. Biofuel Production from Biomass. In Current Developments in Biotechnology and Bioengineering ; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–92. [ Google Scholar ]
- Dickson, R.; Liu, J.J. A strategy for advanced biofuel production and emission utilization from macroalgal biorefinery using superstructure optimization. Energy 2021 , 221 , 119883. [ Google Scholar ] [ CrossRef ]
- Khan, M.A.H.; Bonifacio, S.; Clowes, J.; Foulds, A.; Holland, R.; Matthews, J.C.; Percival, C.J.; Shallcross, D.E. Investigation of Biofuel as a Potential Renewable Energy Source. Atmosphere 2021 , 12 , 1289. [ Google Scholar ] [ CrossRef ]
- Kolakoti, A.; Prasadarao, B.; Satyanarayana, K.; Setiyo, M.; Köten, H.; Raghu, M. Elemental Thermal and Physicochemical Investigation of Novel Biodiesel from Wodyetia Bifurcata and Its Properties Optimization using Artificial Neural Network (ANN). Automot. Exp. 2022 , 5 , 3–15. [ Google Scholar ] [ CrossRef ]
- Sekhon, B.S. Nanotechnology in Agri-food Production: An Overview. Nanotechnol. Sci. Appl. 2014 , 7 , 31–53. [ Google Scholar ] [ CrossRef ]
- IEA. Energy Technology Perspectives 2012: Pathways to a Clean Energy System ; International Energy Agency: Paris, France, 2012; p. 690. [ Google Scholar ]
- Trindade, S.C. Nanotech Biofuels and Fuel Additives ; MADS; InTech: Berlin, Germany, 2011. [ Google Scholar ]
- Cavelius, P.; Engelhart-Straub, S.; Mehlmer, N.; Lercher, J.; Awad, D.; Bruck, T. The Potential of Biofuels from First to Fourth Generation. PloS Biol. 2023 , 21 , e3002063. [ Google Scholar ] [ CrossRef ]
- Mizik, T.; Gyarmati, G. Three pillars of advanced biofuels’ sustainability. Fuels 2022 , 3 , 607–626. [ Google Scholar ] [ CrossRef ]
- Gnanasekaran, L.; Priya, A.K.; Thanigaivel, S.; Tuan, K.A.H.; Moscoso, M.S. The conversion of biomass to fuels via cutting-edge technologies: Explorations from natural utilization systems. Fuel 2023 , 33 , 125668. [ Google Scholar ] [ CrossRef ]
- Hofsetz, K.; Silva, M.A. Brazilian Sugarcane Bagasse: Energy and Non-Energy Consumption. Biomass Bioenergy 2012 , 46 , 564–573. [ Google Scholar ] [ CrossRef ]
- Nanda, S.; Rana, R.; Sarangi, P.K.; Dalai, A.K.; Kozinski, J.A. A Broad Introduction to First, Second and Third Generation Biofuels. In Recent Advancements in Biofuels and Bioenergy Utilization ; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–25. [ Google Scholar ]
- Malik, K.; Anand, R.C.; Kadian, D.; Narula, A. Microalgae: A Promising Feedstock as Source for Third Generation Renewable Energy. In Microorganisms in Sustainable Agriculture, Food and the Environment ; Verma, D.K., Srivastav, P.P., Eds.; Apple Academic Press Inc.: Palm Bay, FL, USA, 2018; pp. 395–420. [ Google Scholar ]
- Lin, Y.; Tanaka, S. Ethanol Fermentation from Biomass Resources: Current state and Prospects. Appl. Microbiol. Biotechnol. 2006 , 69 , 627–642. [ Google Scholar ] [ CrossRef ]
- Sikarwal, V.S.; Zhao, M.; Fennell, P.; Shah, N.; Anthony, E.J. Progress in Biofuel Production from Gasification. Prog. Energy Combust. 2017 , 61 , 189–248. [ Google Scholar ] [ CrossRef ]
- Hayder, A.; Alalwan, A.; Alminshid, A.H.; Haydar, A.S. Promising Evolution of Biofuel Generations: A Review. Renew. Energy Focus 2019 , 28 , 127–139. [ Google Scholar ]
- Naik, S.N.; Goud, V.; Rout, P.K.; Dalai, A.K. Production of First- and Second-Generation Biofuels: A Comprehensive Review. Renew. Sustain. Energy Rev. 2010 , 14 , 578–597. [ Google Scholar ] [ CrossRef ]
- Koçar, G.; Civas, N. An Overview of Biofuels from Energy Crops: Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2013 , 28 , 900–916. [ Google Scholar ] [ CrossRef ]
- Rathmann, R.; Szklo, A.; Schaeffer, R. Land Use Competition for Production of Food and Liquid Biofuels: An Analysis of the Arguments in the Current Debate. Renew. Energy 2010 , 35 , 14–22. [ Google Scholar ] [ CrossRef ]
- Tuli, D.K. Opportunities in 2nd Generation Biofuel-Current Status ; Petrofed, Indian Oil Corporation Limited: New Delhi, India, 2011. [ Google Scholar ]
- El-Desouky, M.G.; Khalil, M.A.; El-Bindary, A.A.; El-Bindary, M.A. Biological, Biochemical and Thermochemical Techniques for Biofuel Production: An Updated Review. Biol. Interface Res. Appl. Chem. 2022 , 12 , 3034–3054. [ Google Scholar ]
- Bacovsky, D.; Michal, D.; Wörgetter, M. Status of 2nd Generation Biofuels Demonstration Facilities in June 2010 ; Report T39-P1b; IEA: Paris, France, 2010. [ Google Scholar ]
- Swanson, R.M.; Platon, A.; Satrio, J.A.; Brown, R.C. Techno-Economic Analysis of Biomass-to-Liquids Production Based on Gasification. Fuel 2010 , 89 , 11–19. [ Google Scholar ] [ CrossRef ]
- Kim, M.; Singhvi, M.S.; Kim, B.S. Eco-Friendly and Rapid One-Step Fermentable Sugar Production from Raw Lignocellulosic Biomass using Enzyme Mimicking Nanomaterials: A Novel Cost-Effective Approach to Biofuel Production. Chem. Eng. J. 2023 , 465 , 142879. [ Google Scholar ] [ CrossRef ]
- Demirbas, A. Social, Economic, Environmental and Policy Aspects of Biofuels. Energy Educ. Sci. Technol. Part B Soc. Educ. Study 2010 , 2 , 75–109. [ Google Scholar ]
- Sivasubramanian, V. Algal Biofuels: Indian Scenario. In Proceedings of the Sahyog Minisymposium and Twinning Workshop Developments in Sustainable Biomass Valorization EU-India R&D Collaboration in Biomass and Biowaste, Utrecht, The Netherlands, 28–29 October 2013. [ Google Scholar ]
- Um, B.H.; Kim, Y.S. Review: A Chance for Korea to Advance Algal-Biodiesel Technology. J. Ind. Eng. Chem. 2009 , 15 , 1–7. [ Google Scholar ] [ CrossRef ]
- Bastianoni, S.; Coppola, F.T.; Colacevicin, A.; Borghini, F.; Focardi, S. Biofuel Potential Production from the Orbetello Lagoon Macroalgae: A Comparision with Sunflower Feedstock. Biomass Bioenergy 2008 , 32 , 619–628. [ Google Scholar ] [ CrossRef ]
- Lang, X.; Dalai, A.K.; Bakshi, N.N. Preparation and Characterization of Biodiesels from Various Bio Oils. Biores. Technol. 2001 , 80 , 53–62. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007 , 25 , 294–306. [ Google Scholar ] [ CrossRef ]
- Rajvanshi, S.; Sharma, M.P. Microalgae: A Potential Source of Biodiesel. J. Sustain. Bioenergy Syst. 2012 , 2 , 49–59. [ Google Scholar ] [ CrossRef ]
- Nigam, P.S.; Singh, A. Production of Liquid Biofuels from Renewable Resources. Prog. Energy Combust. Sci. 2011 , 37 , 52–68. [ Google Scholar ] [ CrossRef ]
- Abdullah, B.; Syed, A.; Muhammad, E.S.; Mahmood, A.N. Fourth Generation Biofuel: A Review on Risks and Mitigation Strategies. Renew. Sust. Energy Rev. 2019 , 107 , 37–50. [ Google Scholar ] [ CrossRef ]
- Hallenbeck, P. Hydrogen Production by Cyanobacteria. Microbial Technologies. In Advanced Biofuels Production ; Hallenbeck, P.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 15–28. [ Google Scholar ]
- Fritsche, U.R.; Fehrenbach, H.; Koppen, S. Biofuels—What Role in the Future Energy Mix? Shell Deutschland Oil: Darmstadt, Germany, 2012; p. 42. [ Google Scholar ]
- Farghali, P.M.; Mayumi, M.; Syo, K.; Satoshi, A.; Takashima, S.; Moustaf, T.Y. Potential of Biogas Production from Manure of Dairy Cattle Fed on Natural Soil Supplement Rich in Iron Under Batch and Semi-Continous Anaerobic Digestion. Bioresour. Technol. 2020 , 309 , 123298. [ Google Scholar ] [ CrossRef ]
- Sohel, M.I.; Jack, M.W. Thermodynamic Analysis of Lignocellulosic Biofuel Production via a Biochemical Process: Guiding Technology Selection and Research Focus. Bioresour. Technol. 2011 , 102 , 2617–2622. [ Google Scholar ] [ CrossRef ]
- Malik, K.; Verma, D.K.; Srivastava, S.; Mehta, S.; Khushboo, N.K.; Verma, M.; Kumar, A.; Tiwari, A.K.; Singh, K.P. Sugarcane Production and It’s Utilization as Biofuel. In India: Current Policy and Status ; Verma, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 123–138. [ Google Scholar ]
- Mussatto, S.I.; Dragonea, G.; Guimarãesa, P.M.R.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicentea, A.; Domingues, L.; Teixeira, J.A. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010 , 28 , 817–830. [ Google Scholar ] [ CrossRef ]
- Pradoand, J.M.; Meireles, M.A.A. Production of Valuable Compounds by Supercritical Technology Using Residues from Sugarcane Processing. In Biorefinery Co-Products: Phytochemicals, Primary Metabolites and Value-Added Biomass Processing ; Bergeron, C., Carrier, D.J., Ramaswamy, S., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 133–151. [ Google Scholar ]
- Muhammad, U.L.; Shamsuddin, I.M.; Danjuma, A.; Musawa, R.S.; Dembo, U.A. Biofuels as the Starring Substitute to Fossil Fuels. Pet. Sci. Eng. 2018 , 2 , 44–49. [ Google Scholar ]
- Reijnders, L. The Life Cycle Emission of Greenhouse Gases Associated with Plant Oils used as Biofuel. Renew. Energy 2011 , 36 , 879–880. [ Google Scholar ] [ CrossRef ]
- Le, T.L.; van Ierland, E.C.; Zhu, X.; Wesseler, J. Energy and Greenhouse Gas Balances of Cassava-Based Ethanol. Biomass Bioenergy 2013 , 51 , 125–135. [ Google Scholar ] [ CrossRef ]
- Duer, H.; Christensen, P.O. Socio-Economic Aspects of Different Biofuel Development Pathways. Biomass Bioenergy 2010 , 34 , 237–243. [ Google Scholar ] [ CrossRef ]
- Nogueira, L.A. Does Biodiesel make Sense? Energy 2011 , 36 , 3659–3666. [ Google Scholar ] [ CrossRef ]
- IRENA. Global Energy Transformation: The REmap Transition Pathway ; Background Report to 2019 Edition; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019; Available online: https://www.irena.org/publications/2019/Apr/Global-energy-transformation-The-REmap-transition-pathway (accessed on 4 August 2020).
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; AL-Muhtaseb, A.H. Challenges and Perspectives on Innovative Technologies for Biofuel Production and Sustainable Environmental Management. Fuel 2022 , 325 , 124845. [ Google Scholar ] [ CrossRef ]
- Ambaye, T.G.; Mentore Vaccari, M.; Bonilla-Petriciolet, A.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Emerging technologies for biofuel production: A critical review on recent progress, challenges and perspectives. J. Environ. Manag. 2021 , 290 , 112627. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- De Araujo, B.M.C.; Costa, I.O.; de Brito, H.G.; Rios, N.S.; Dos, S. Enzyme technology in bioethanol production from lignocellulosic biomass: Recent trends with a focus on immobilized enzymes. Bioresources 2023 , 18 , 352023. [ Google Scholar ] [ CrossRef ]
- Ministry of New and Renewable Energy. National Policy on Biofuels ; Ministry of New and Renewable Energy: New Delhi, India, 2009. [ Google Scholar ]
- Available online: https://pib.gov.in/PressReleaseIframePage.aspx?PRID=1960491 (accessed on 25 September 2023).
- National Biofuel Policy. 2018. Available online: https://mnre.gov.in/file-manager/UserFiles/biofuel_policy.pdf (accessed on 20 September 2015).
- Mandade, P.P.; Yogesh, M.; Nimdeo, N. Techno-economic assessment of biofuel production using thermochemical pathways. In Biofuels Bioenergy ; Elsevier: Amsterdam, The Netherlands, 2022; pp. 653–671. [ Google Scholar ]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.T.; Show, P.L. Waste to Bioenergy: A Review on the Recent Conversion Technologies. BMC Energy 2019 , 1 , 4. [ Google Scholar ] [ CrossRef ]
- Prasad, S.; Singh, A.; Korres, N.E.; Rathore, D.; Pant, D. Sustainable utilization of crop residues for energy generation: A life cycle assessment (LCA) perspective. Bioresour. Technol. 2020 , 303 , 122964. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sie, S.W.; Riyang, S.; Zhang, J.; Liu, H.; Ning, Y. Downstream processing of lignin derived feedstock into end products. Chem. Soc. Rev. 2020 , 49 , 5510–5560. [ Google Scholar ]
Click here to enlarge figure
| Classifications | Product | Feedstock |
|---|---|---|
| First generation (food feedstocks) | Ethanol | Corn, sugar beet, sugarcane, cereals, sorghum, grains etc. |
| Biodiesel | Rapeseed, palm oil, soybean, waste oils, animal fats | |
| Second generation (biomass) | Cellulosic ethanol | Switch grass, wheat straw, jatropha, miscanthus, corn Stover, stalks, stubbles, leaves, seed pods, rice and wheat straw, sugarcane bagasse, fruit and vegetable wastes |
| Third generation | Bioethanol/ethanol | Algae |
| Fourth generation | Biodiesel/bioethanol | “Drop in” biofuels, genetically modified crops for biofuels, renewable solar fuel |
| Type of Biofuel | Product | Feedstock | Conversion Process/Technologies |
|---|---|---|---|
| Biodiesel | Biodiesel (methyl and ethyl esters of fatty acids) derived from energy crops | Oil crops (soybean, sunflower, rapeseed, palm, etc.) | Cold and warm pressing, drying, extraction, transesterification, purification, |
| Biodiesel from organic waste materials | Waste oil, cooking/frying oil | Hydrogenation | |
| Bioethanol | Conventional ethanol | Sugar beet, sugarcane | Direct fermentation of juice |
| Starchy ethanol | Corn, wheat and other grains | Enzymatic hydrolysis, fermentation |
| Substrate(s) | Fermentation Mode |
|---|---|
| Bioethanol | |
| Corn, potato, cassava, sorghum, grains, fruit and vegetables waste, sweet potato, sugar cane, sugar beets, etc. | Simultaneous saccharification and fermentation (SSF), separated hydrolysis and fermentation (SHF) |
| Lignocellulose materials, such as wheat and rice straw, corn stover, corn cobs, switchgrass, hardwood, sugarcane bagasse, etc. | Simultaneous saccharification and fermentation (SSF), simultaneous saccharification and fermentation with prehydrolysis time (PTSSF) |
| Alkaline-treated sugarcane bagasse | Consolidated bioprocessing (CBP) |
| Biohydrogen | |
| Agriculture residue (sugarcane bagasse, rice straw, leaves, etc.) | Dark and photofermentation |
| Fruits and vegetables waste | Photofermentation |
| Algae (macro and micro), cyanobacteria | Biophotolysis |
| Types of Biofuels | Biomass/Substrate | Conversion Process/ Technologies | Applications |
|---|---|---|---|
| Bioethanol | Corn, potatoes, sugarcane, sugar beet crops, Wheat, barley sorghum grains, Poplar and wood chips, agricultural waste (corn, sorghum, oat, barley, wheat, soybean, cotton, bagasse, and rice straws) and energy crops (hybrid sorghum, energy cane, miscanthus, switchgrass, eucalyptus, and pine) | Pre-treatment, Liquefaction, Enzymatic hydrolysis, saccharification and fermentation | Drop in fuel, blending for gasoline engine, alcohol to jet for aviation |
| Biodiesel | Rapeseed, sunflower, palm, soybean, canola, jatropha oil, algae, etc. | Pressing, Cultivation, Harvesting, extraction, purification and trans-esterification | Drop in fuel, blending for diesel engine |
| Biogas | Dung, agriwaste, sewage water and sludge, municipal solid waste, organic waste, industrial waste, etc. | Anaerobic digestion (AD) | Blended with natural gas for transportation, cooking |
| Biohydrogen | Lignocellulosic materials, bio-waste, macro and micro algae, industrial waste, etc. | Dark and photo fermentation, biophotolysis | Internal combustion engines |
| Characteristics | Biofuels | Fossil Fuels |
|---|---|---|
| Type | Renewable | Non-renewable |
| Impact on health | Nontoxic | Toxic ingredients, chemicals and by-products |
| State of industry | Growing | declining |
| Energy production | Provides a low amount of energy per unit biomass | Provides a high amount of energy per unit mass |
| Environmentally friendly | yes | no |
| production methods | Safer | unsafe |
| CO neutral process | Release net zero carbon dioxide | Increase the concentration of carbon dioxide |
| Sustainability | yes | no |
| Examples | Bioethanol, biodiesel, methanol, biobutanol, biogas, biohydrogen | Gasoline, ethane, diesel, methane, butane |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Malik, K.; Capareda, S.C.; Kamboj, B.R.; Malik, S.; Singh, K.; Arya, S.; Bishnoi, D.K. Biofuels Production: A Review on Sustainable Alternatives to Traditional Fuels and Energy Sources. Fuels 2024 , 5 , 157-175. https://doi.org/10.3390/fuels5020010
Malik K, Capareda SC, Kamboj BR, Malik S, Singh K, Arya S, Bishnoi DK. Biofuels Production: A Review on Sustainable Alternatives to Traditional Fuels and Energy Sources. Fuels . 2024; 5(2):157-175. https://doi.org/10.3390/fuels5020010
Malik, Kamla, Sergio C. Capareda, Baldev Raj Kamboj, Shweta Malik, Karmal Singh, Sandeep Arya, and Dalip Kumar Bishnoi. 2024. "Biofuels Production: A Review on Sustainable Alternatives to Traditional Fuels and Energy Sources" Fuels 5, no. 2: 157-175. https://doi.org/10.3390/fuels5020010
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Physical Sciences
- Renewable Energy
- Renewable Energy Technologies
- Energy-Generating Resources
Biofuel production: Challenges and opportunities
- International Journal of Hydrogen Energy 42(12)

- Russian Academy of Sciences

- Institute for Basic Science

- Wonkwang University School of Medicine and Hospital

- Institute of Plant Physiology, Russian Academy of Sciences, Russia, Moscow
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- BIOFUEL BIOPROD BIOR
- Taufiq Nawaz

- Dilshad Z. Ethen

- Zhiyuan Fan
- Dr S Julio Friedmann
- Amit Khatri

- INT J HYDROGEN ENERG
- Aditya Tiwari
- Kazuho Nakamura
- ENVIRON RES

- Nilima Shivale
- Sheetal Sonawdekar
- Simran Lilwani
- Priti Uchgaonkar
- BIOTECHNOL PROGR

- BIOTECHNOL BIOFUELS

- M. Lokhande

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- View PDF
- Download full issue
- View Open Manuscript
- Other access options
Review Biofuels for a sustainable future
- Previous article in issue
- Next article in issue
Cited by (0)
Argonne National Laboratory
Biofuel on the road to energy, cost savings.

Biofuel is closer to becoming a cost-competitive, climate-friendly solution for slashing carbon emissions in cars and trucks, according to two new studies.
The U.S. Department of Energy’s ( DOE ) Argonne National Laboratory collaborated with the DOE ’s National Renewable Energy Laboratory ( NREL ), Pacific Northwest National Laboratory ( PNNL ) and Idaho National Laboratory ( INL ) on the research. Results showed that biofuel combined with advanced engine design can reduce greenhouse gas ( GHG ) emissions by roughly 60% while improving fuel efficiency or reducing tailpipe emissions.
Argonne energy system analyst Pahola Thathiana Benavides, NREL process engineer Andrew W. Bartling and PNNL engineer Steven Phillips were lead analysts for the two studies published in ACS Sustainable Chemistry & Engineering.
“ The idea is to develop new biofuels blended with conventional fuels to improve engine performance. This means a gasoline car or truck could go further on the same amount of fuel or a diesel vehicle could meet more stringent emissions standards.” — Troy Hawkins, Argonne’s group manager, Fuels and Products Group
Biofuel has significant advantages over petroleum gasoline. But the engines themselves are also critical to energy efficiency. Designing low-carbon fuels and engines to work together can maximize energy use and vehicle performance.
“ We are at the intersection of new innovations in both engines and biofuel,” said Troy Hawkins, Argonne’s group manager, fuels and products group, an author on both ACS Sustainable Chemistry & Engineeringstudies.“Our goal was to develop new biofuels blended with conventional fuels to improve engine performance. This means a gasoline-powered car or truck could go further on the same amount of fuel. Or a diesel vehicle could meet more stringent emissions standards.”
In both studies, Argonne scientists worked with other national labs to identify promising fuels for different engine types. Researchers considered cost, environmental impact and potential for expanding to commercial markets.
The research is supported by the Co-Optimization of Fuels & Engines (Co-Optima) initiative jointly led by DOE ’s Office of Energy Efficiency and Renewable Energy, Bioenergy Technologies Office and Vehicle Technologies Office.
Argonne is part of Co-Optima’s consortium of nine national laboratories and over 20 university and industry partners. The consortium studies how simultaneous innovations in fuel and engines can boost fuel economy and vehicle performance while reducing emissions.
Scientists and experts at every DOE laboratory played an important role in each phase of the research, Hawkins said.
“ This research is a really good example of how laboratories can work together to help the DOE accomplish its mission,” Hawkins said.
Finding biofuel pathways
Co-Optima’s research builds on the goal to identify and understand bioblendstocks, or biofuel. Biofuel is produced from biomass — organic materials including plants, agricultural waste and wet waste. Biofuel can be blended with conventional fuel to reduce emissions and improve fuel and engine performance.
Collaborating with Co-Optima fuel experts, researchers used a screening process to develop a list of biofuels for their research, Benavides said.
Argonne scientists developed the list of biofuels working with experts including PNNL technical team manager and Co-Optima Leadership Team member Daniel Gaspar, NREL senior scientist Gina Fioroni, NREL senior research fellow Robert McCormick, and Anthe George, senior manager at DOE ’s Sandia National Laboratories ( SNL ).
“ We worked with other experts to use specific criteria to narrow many biofuel candidates down to a short list for our research. This list was developed based on the required properties and the engine’s combustion mode,” Benavides said.
Converting biomass to biofuel is a complex process involving variables in feedstock, conversion technologies and fuel types. It is especially challenging finding biofuel pathways that also meet economic, technology and energy goals.
One study was co-first-authored by Benavides. The team assessed 12 biofuel production pathways for optimizing multimode internal combustion engines. Multimode engines can deliver greater efficiency and cost savings by using different methods of ignition, combustion and/or fuel-preparation, depending on driving demands.
Researchers used renewable biomass feedstock found in forestry byproducts such as wood waste and agricultural byproducts such as corn stover. They used conversion technologies including either fermentation, catalysis under high heat and pressure, or a combination of both.
“ We found that not only can seven biofuels be produced cost-competitively, but that these seven are varied in terms of feedstock used and conversion technology,” Bartling said. “ This means that biorefineries can be more flexible in choosing where and how to build their facilities.”
NREL and PNNL researchers did a techno-economic assessment of the biofuel production pathways, analyzing cost and technology performance.
“ Our findings showed that many of the biofuels are competitive with the current cost of petroleum fuel,” Phillips said.
Researchers also analyzed environmental impact. A life cycle analysis of the pathways using Argonne’s GREET ® (Greenhouse Gases, Regulated Emissions, and Energy used in Technologies) model showed impressive results. Ten biofuels have the potential to reduce GHG emissions by 60% compared to petroleum gasoline. The list includes alcohols, furan mixtures and olefins.
Biofuel promising for diesel engines
The second study was co-first-authored by Bartling. Researchers analyzed 25 ways of producing biofuel optimized for a type of engine known as mixing-controlled compression ignition. This diesel engine is mainly used in freight transportation.
To develop biofuel production pathways, researchers used feedstocks ranging from plant materials such as wood chips or corn stover, to oils from soybean and cuphea, to wet wastes and recycled grease. They used conversion technologies including fermentation, gasification, and hydrothermal liquefaction.
“ The diverse set of biomass resources available in the U.S. has great potential to replace a portion of fuels and chemicals that now come from petroleum,” said Damon Hartley, INL ’s Operations Research and Analysis Group lead. “ However, one of the largest barriers is the wide variability in quality in the raw materials. This can have a large impact on how the material performs in conversion.”
As with the first study, most of the technologies performed well. Most of the biofuels were cost-competitive with current gas prices.
In terms of environmental impact, GHG emissions were reduced more than 60% in 12 of the 25 pathways, according to the GREET life cycle analysis.
“ We evaluated the life cycle GHG emissions for each mixing-controlled compression ignition engine pathway. This included not only the tailpipe emissions but also upstream emissions resulting from biomass cultivation, feedstock transportation, biofuel production and biofuel distribution,” Hawkins said.
Creating a biofuel playbook
Researchers did not intend to produce a definitive list of biofuels, Benavides said. Instead, the studies offer a guide for stakeholders on selecting biofuel pathways that best meet their needs.
“ We provide researchers and industry guidance on assessing biofuels based on a number of complex variables,” Benavides said. “ The life cycle and techno-economic analysis is important in guiding stakeholders as early as possible. We can’t tell stakeholders what choices to make but these tools can point them in the right direction from the beginning.”
While many of these biofuel pathways could potentially be cost-competitive, it is too soon to lock in prices in a constantly fluctuating gas market. “ The challenge is providing cost-competitive prices in the long term,” Hawkins said.
While these biofuel production pathways target cars and diesel trucks, Argonne researchers are also studying the potential for using these pathways in hard-to-electrify sectors like aviation and maritime industries. The goal is to bring biofuel to market across a range of industries as quickly as possible.
“ DOE is constantly working on sustainable solutions for decarbonizing the transportation sector. Biofuel is a big piece of that,” Hawkins said. “ We will continue to expand on Co-Optima’s important work.”
Along with Argonne, ORNL , NREL , PNNL , INL , and SNL , other U.S DOE national labs in the Co-Optima Initiative are Los Alamos, Lawrence Berkeley, and Lawrence Livermore national laboratories.
ACS Sustainable Chemistry & Engineering research authors:
“ Identification of key drivers of cost and environmental impact for biomass-derived fuel for advanced multimode engines based on techno-economic and life cycle analysis”: Pahola Thathiana Benavides, Argonne, Andrew W. Bartling, NREL , Steven D. Phillips, PNNL , Troy R. Hawkins, Argonne, Avantika Singh, NREL , George G. Zaimes, Argonne, Matthew Wiatrowski, NREL , Kylee Harris, NREL , Pralhad H. Burli, INL , Damon Hartley, INL , Teresa Alleman, NREL , Gina Fioroni, NREL , Daniel Gaspar, PNL .
“ Environmental, economic, and scalability considerations of selected bio-derived blendstocks for mixing-controlled compression ignition engines”: Andrew W. Bartling, NREL , Pahola Thathiana Benavides, Argonne, Steven D. Phillips, PNNL , Troy Hawkins, Argonne, Avantika Singh, NREL , Matthew Wiatrowski, NREL , Eric C. D. Tan, NREL , Christopher Kinchin, NREL , Longwen Ou, Argonne, Hao Cai, Argonne, Mary Biddy, NREL , Ling Tao, NREL , Andrew Young, NREL , Kathleen Brown, NREL , Shuyun Li, PNL , Yunhua Zhu, PNL , Lesley J. Snowden-Swan, PNL , Chirag R. Mevawala, PNL , Daniel J. Gaspar, PNL .
Argonne National Laboratory seeks solutions to pressing national problems in science and technology by conducting leading-edge basic and applied research in virtually every scientific discipline. Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science .
- Forum article
- Open access
- Published: 18 May 2017
Biofuel research: perceptions of power and transition
- Lena Partzsch 1
Energy, Sustainability and Society volume 7 , Article number: 14 ( 2017 ) Cite this article
14k Accesses
8 Citations
1 Altmetric
Metrics details
Whether biofuels represent a sustainable innovation, a creative alternative, or a gold rush, very much depends on our perception of power and change with regard to sustainability. This article provides an overview of existing understandings of power in the research on biofuels, including positive perceptions that often lead to more optimistic evaluations of biofuels. It exposes the diversity with which one can understand power through three ideal type concepts: “power with,” “power to,” and “power over”. Integrating these concepts in one power framework allows for examining how the three dimensions interrelate with each other and developing the contours of a power lens on biofuel governance and research. With the 2007–2008 food price crisis, critics re-politicized the governance of biofuels. Several farmer associations have completely turned against biofuels. The article argues that this rejection of biofuels is due to a limited perception of power as a coercion and manipulation (power over). While the current governance of biofuels basically reproduces systems and positions, we should start to more seriously and intensively ask questions of where, when, and how the governance of biofuels may also allow for “green” resistance (power to) and collective empowerment (power with).
Introduction
Whether biofuels represent a sustainable innovation, a creative alternative or a gold rush [ 1 ], very much depends on our perception of power and change with regard to sustainability. This leads to the challenge of how to conceptualize these understandings. I gather diverse perceptions of power and illustrate them for biofuel research. The aim is to initiate a broader, more comprehensive debate across ontological and epistemological differences in this field of research. To begin the discussion, I introduce key components of the debate by identifying different perceptions of power that are common to research on biofuels along three ideal type conceptions:
Power with means collective empowerment through convincing and learning with and from each other. It refers to processes of developing shared values, finding common ground, and generating collective strengths [ 2 ]. Based on this understanding of power, biofuels can potentially be a sustainable innovation that serves the common good (climate protection, energy security, regional development, etc.) (e.g., [ 3 , 4 ]).
Power to corresponds to the ability of agents “to get things done” [ 5 ]. While Pitkin [ 6 ] defines power to as non-relational, Barnett and Duvall [ 7 ] define power to as tied to social relations of constitution that define who the actors are, along with their capacities and practices. Footnote 1 Scholars, who take a perspective of power to, may highlight the agency of producing biofuels as a creative alternative in hitherto fossil fuel-dependent societies (e.g., [ 8 , 9 ]).
Power over describes the direct and indirect ability of powerful actors, structures, and discourses to influence the actions and even the thoughts of others. It is based on power concepts by Dahl [ 10 ], Bachrach and Baratz [ 11 ], and Lukes [ 12 ], among others. I also discuss concepts of discursive power under this category (e.g., [ 13 , 14 ]), while I am aware that these concepts partly fall under the category of power to [ 7 ]. From a perspective of power over, biofuels can be seen as a gold rush: While everybody expected sudden wealth in this new field, there are very few winners and many losers (e.g. [ 15 , 16 ]).
I chose this tripartite approach as a framework for my article, because it is most comprehensive and makes an extension of the power discussion on biofuels possible. At the same time, the framework allows for the discussion of the well-known grouping of the four “faces of power” under the category of power over [ 17 , 18 ]. I will argue that in the research on biofuels, the understandings of power as power with and power to tend to prevail, even when they are not made explicit. This means that scholars have overemphasized the potential of biofuels as a creative alternative to fossil fuels and sustainable innovation for rural development. Concepts of power over have only more recently been applied, specifically since research has started to explicitly issue power. This has, in particular, been used to explain why any process of governing biofuels (biofuel governance) did not lead to urgent sustainability transitions, and why the biofuel boom should rather be seen as a gold rush. Scholars have demonstrated that the development of biofuels markets benefitted large companies and conglomerates [ 19 ]. Critical and post-structuralist perspectives have helped to understand this development by exploring structures and discourses favoring them [ 20 ]. Scholars have used Foucault’s concepts to outline how scientific knowledge practices render the very essence of problems (and solutions) raised on the biofuel agenda [ 21 , 22 ].
This article involves first of all implicit and explicit understandings of power (how do biofuel researchers think and talk about power?). These understandings are expressed in empirical research, as I will demonstrate below, and they hence also allow for an illustration of the practice of biofuel governance (how is power exercised in and through biofuel governance?). This makes the article also relevant for political practice. We should understand, not only in theoretical but also in practical terms, how we effectuate or prevent changes towards a more sustainable supply of energy and transport fuel. As in analytical heuristics, it is not possible to offhand separate power with , power to , and power over in empirical research. These categories shine multiple lights on different aspects of the same empirical phenomena. In practice, these forms of power exercise are mostly interrelated. My less concern is to weigh and compare the pros and cons of each perspective, but rather to outline an agenda for a multidimensional analysis of all three mechanisms of power and their interrelations.
In order to get the full picture of how change happens, we should understand how different perspectives add on to each other (besides overlaps and contradictions). To do this, I will begin by describing each perspective in itself. Based on a survey on biofuel research, I will give references for each perspective. These references are only illustrative. Then, I will exemplify the interrelations between each of these perspectives with respect to biofuel research. I explain how power imbalances can affect processes of power with and power to . Again, scholars have demonstrated how large conglomerates have manipulated biofuel governance in their favor, and why therefore the biofuels boom should be considered as a gold rush. However, I argue that interrelations may also work the other way around, and this is particularly relevant to the main argument of this article. Biofuels as a creative alternative and a sustainable innovation may also provoke changes in existing relations of power over and contribute to address asymmetries and inequalities in agrifood and transport systems. We need a multidimensional power approach to explore these interrelations.
Biofuel: sustainable innovation (power with)
Research on biofuel governance and other studies in the field of sustainability are most often based on a positive perception of power in the sense of power with . Power with is a term that refers to processes of developing shared values, finding common ground, and generating collective strengths [ 2 ]. This conception does not necessarily refer to the diffusion of already existing (predefined) norms. Rather, power with implies learning processes that allow actors to question self-perceptions and to actively build up a new awareness of individuals or groups [ 23 , 24 ]. In this vein, with regard to biofuels, scholars have assumed that collective empowerment and solidarity are possible and that biofuel technologies as a “sustainable innovation” can pave the way to post-carbon societies [ 25 , 26 ].
Power with is often linked to Arendt’s definition of power [ 27 ]. Footnote 2 According to Arendt, power always refers to a group or to a collective of individuals:
Power corresponds to the human ability not just to act but to act in concert. Power is never the property of an individual; it belongs to a group and remains in existence only so long as the group keeps together. When we say of somebody that he is ‘in power’ we actually refer to his being empowered by a certain number of people to act in their name ([ 28 ]: 44). Footnote 3
Research on environmental leadership (e.g., [ 29 ]) in pioneer countries, such as Germany and France in the biofuel sector [ 3 , 30 ], most obviously reflects such an understanding of power. Leaders or pioneers are empowered to act in the name of others from this perspective (while they dominate others from a perspective of power over , see below). In this sense, (Young [ 31 ]: 285) defines leadership in the interest of common welfare:
Leadership (…) refers to the actions of individuals who endeavor to solve or circumvent the collective action problems that plague the efforts of parties seeking to reap joint gains in processes of institutional bargaining.
Leaders and pioneers do not enforce their own interests against or over others; rather they seek “to reap joint gains” of environmentalism. Environmental leadership studies, based on such an understanding of power, usually follow the discourse of Ecological Modernization that highlights flexible and cost-efficient problem solving. Ecological modernization outlines a win-win storyline of environmental protection that benefits green (biofuel) business as much as the environment [ 32 , 33 ]. From this perspective, those who are neither leaders nor pioneers are considered free-riders or laggards , rather than subordinates. Non-leaders also benefit, at least in the long run, from power (with), since biofuels are expected to tackle common problems, such as climate change, enhance energy security, and to contribute to regional development [ 3 , 34 ]. Policies promoting biofuels are hence per se seen to be desirable since, from this perspective, they serve everybody’s interest.
Scholars have extensively analyzed the emergence, diffusion, efficiency, and effectiveness of policies promoting biofuels, with the (at least implicit) aim to foster their adoption and implementation [ 30 , 35 ]. In this context, policy learning and experiments have been gaining momentum [ 9 , 26 ]. Deliberative processes, including third-party certification schemes, were initiated and observed with the aim to introduce sustainable biofuel production schemes that would integrate those formerly excluded stakeholders with new technology; in everyday practice, every actor in the field would then become a winner [ 4 , 36 ].
Scholars who share this perspective of power as power with do not think in dichotomies such as winners - losers or good-bad . Instead, they understand power (or similar concepts, such as leadership) as serving the common good (climate protection, energy security, and sustainability). As there are no subordinates from this power perspective, no imperative follows to empower or to resist. The empowerment of non-leaders is not an issue because scholars assume that, in principle, they are also interested in developing sustainable innovations and that they likewise benefit from respective leadership efforts.
Biofuel: creative alternative and “green” resistance (power to)
While power with pertains to collective empowerment and solidarity, power to refers to single actors and separate groups, such as farmers, co-operatives, and individual processors who were initially key players in pioneering biofuel regions [ 19 ]. Accordingly, biofuels are often seen as an opportunity to empower green ideas and values. Pitkin [ 6 ] emphasizes how power can be non-relational, since an actor may have the power to accomplish something all by him- or herself. This understanding of power is related to the development of an individual identity; self-confidence and consciousness raising [ 23 ]. It is here where Nussbaum’s and Sen’s [ 37 ] capability approach comes in, which defines power as “a capability to act upon one’s environment” [ 38 ]. For example, an individual farmer can simply start to produce and use biomass-based fuels without any permission or interference from another actor, such as the petrol industry. However, constructivist research has demonstrated how every actor or group is defined through socially constituted relations that, at least indirectly, shape the actions of individuals [ 7 ]: only a farmer who receives knowledge about alternative technologies may effectively implement them.
Power to can be linked to Parsons’ definition of power as the ability “to get things done” [ 5 ]. It highlights a productive agency, especially in the cases where actors’ goals are opposed or resisted. Biofuel research by small farmers and rural communities is often based on this perception of power [ 9 , 39 ]. Scholars highlight the potential of biofuels for rural development by providing new markets for agricultural production. They assume that through the introduction of radically new technologies in niches, farmers are able to empower themselves in an attempt of an “agro-ecological revolution” [ 8 ]. They highlight the self-empowering agency of hitherto marginalized people to become “energy sheiks” [ 40 ], based on biomass production.
Scholars, who take a perspective of power to , focus on the productive agency of the biofuel sector. They are interested in the empowerment of alternative ideas and values which, in the case of biofuels, allow for transforming fossil fuel-dependent societies. These alternative agents criticize the practices or the authority of the dominant, carbon-intense system and refuse to reproduce their own positions in this system. Their non-conformism is perceived to serve the common good as they develop alternative technologies required by everyone in a world beyond petrol. From a perspective of power to and in difference to a perspective of power with , there are only a limited number of transformational agents: not everybody in the field is assumed to be a “winner” in the first place; there are only a few “energy sheiks”. However, scholars see an imperative to act based on normatively prior “green” values, for example, climate protection and sustainability (and everybody benefits from the realization of these values).
Biofuel: gold rush (power over)
Scholars who explicitly issue power in the context of biofuels usually perceive power as asymmetric. Biofuel governance is seen as a zero-sum game which produces winners and losers. From this perspective, powerful actors, structures, and discourses in the field of biofuel governance influence the actions and even the thoughts of others. In the following, I will illustrate this perspective, further differentiating the “four faces” of power over (see Table 1 ): visible , hidden , invisible , and unconscious power [ 2 , 41 ]. (the fourth dimension does not understand power as a zero-sum game and can also be added to power to , see the first footnote.)
In the first dimension, agents exercise visible power when they directly influence political decision-makers based on their material and ideational resources [ 42 ]. What is visible is not the power as such, but rather its physical means such as lobbying activities, party financing, and armed force. (Dahl [ 10 ]: 201) defines: “A has power over B to the extent that he can get B to do something that B would not otherwise do” (emphasis added). Any kind of state force implementing objectives of sustainability by top downregulation means exercising direct power. Non-state actors may also play a role in this game. Coase [ 43 ] explains this for business firms. Also when Pilgrim and Harvey [ 44 ] demonstrate how NGO lobbying significantly affected biofuel policy changes and sustainability regulation in the UK and in Europe, they assume that NGOs enforce their ideas against others in an arena of obviously competing demands.
The second dimension of hidden power refers to power not obviously opposed by anyone. Bachrach and Baratz [ 11 ] speak of “two faces of power” emphasizing that some issues never even make it onto the political agenda and are dismissed before observable negotiations start. For a long time, the EU issued biofuels only in the context of climate change, completely neglecting aspects of competing food demands and land use change in the Global South [ 45 , 46 ]. Scholars demonstrating such hidden aspects apply this second dimension of power over to analyze biofuel governance.
The traditional conception of structural (hidden) power in international relations aims to address the coercion resulting from the capital mobility of transnational corporations. Threats to shift investments abroad do not even need to be voiced in order to influence policies in their favor [ 42 , 47 ]. More recent studies point to the fact that businesses also exercise structural power by self-regulation and public-private partnerships; these types of governance allow business actors to actively set rules, for example, for the “sustainable” production of biofuels at the expense of state actors [ 42 , 48 ]. In addition, as public authorities have faced challenges in facilitating the implementation of their sustainability criteria outside their jurisdictions, the EU has started to use these private schemes to verify compliance with sustainability criteria in biofuel production outside its own territory [ 49 , 50 ]. As a result, following this perspective, power in the global political economy has been diffused, leaving biofuel conglomerates with considerable power over others [ 51 ].
Further, scholars are increasingly focusing on power relations linked to latent conflicts of interest. In the third dimension, invisible power comes to play as a result of norms and ideas [ 41 ]. Research analyzes discourses, communication practices, cultural values and institutions, which all work to shape relevant thoughts and actions [ 12 ]. With regard to biofuels, Munro [ 22 ] has shown how, in the United States, a powerful coalition of agricultural interests manipulated the governance of biofuels by linking it to public concerns about climate change and energy security. In consequence, corn biofuel received political support, tax reductions, and subsidies. Likewise, Puttkammer and Grethe [ 52 ] have found a coalition of biofuel advocates to dominate the public discourse in Germany, while scientists who doubted the efficiency of biofuels could not make their voice heard. The discourse only shifted with the 2007–2008 food price crisis when scholars demystified the “ethanol bubble” [ 53 ] and outlined potentially devastating implications for global poverty and food security. Experts, NGOs, and business actors who have challenged the sustainability of biofuels on many fronts began to be heard [ 20 , 22 ].
For the most part, these discourse scholars blame other scholars who apply a perspective of power with for neglecting and postponing important questions of social justice linked to biofuel production [ 21 , 54 ]. Win-win rhetoric is demonstrated to manifest global power asymmetries rather than to contribute to more ecology and fairness [ 22 , 53 ]. From this perspective, pioneers and leaders, whose role Young [ 31 ] and Bernard and Prieur [ 30 ], among others, consider to be positive, only serve dominant interests and prevent a more fundamental social transformation to sustainability. With reference to the International Political Economy, most scholars deny a simple confrontation of biofuel proponents (or pioneers) and opponents (or laggards). In this vein, Levidow [ 55 ] outlines how the EU can continue “its global plunder of resources” because it pursues global leadership for sustainable biofuels. Silva-Castaneda [ 56 ] demonstrates how, in Indonesia, some NGOs decided to participate in the Roundtable on Sustainable Palm Oil (RSPO), a certification process initiated by the WWF, among others. The local NGOs managed to include important clauses regarding indigenous and land rights in the RSPO standard. In practice, however, auditors rarely recognize as valid evidence the forms of proof put forward by local communities, and global conglomerates could even use the standards to increase their primacy vis-à-vis local farmers [ 56 ]. These examples reveal power over within multi-stakeholder processes.
Studies demonstrate that the expansion of biofuels in countries of the Global South was only possible through the partial neglect (simplification) of their cultural and ecological diversity [ 57 ]. Nygren [ 58 ] illustrates how leading retailers, in negotiation with environmental organizations, have guided consumers’ expectations of certified Southern forest products by building images of Southern community forest producers as authentic and exotic others . She concludes that certification as a market-based form of governance has only had a limited impact on altering the unequal relationship characteristic of global networks of production and consumption.
With reference to Foucault [ 13 ] and Bourdieu [ 59 ], we can capture links between knowledge, power, and politics in a fourth dimension of power over [ 17 ]. Critical and (post-) structuralist approaches understand power in a way that everything is socially constructed. Scholars analyze the normative impact on (supposed) losers, such as farmers in the Global South, as well as on (supposed) winners, such as major agribusiness actors. All actors work to mainly reproduce systems and positions [ 60 ]. With regard to biofuels, several studies have highlighted the central role of knowledge and framing [ 15 , 16 , 21 ]. Drawing on Foucault, Kuchler and Linnér [ 21 ] have analyzed the discursive practices of the three major international organizations focused on food and agriculture, energy, and climate with regard to biofuels over the last 20 years: the UN Food and Agriculture Organization (FAO), the International Energy Agency (IEA), and the Intergovernmental Panel on Climate Change (IPCC). They found that, in contrast to pro and contra accounts, the arguments of all three organizations reflected a policy consensus based on the mainstream notion of industrial agricultural production, promoting the intensification and expansion of rural production. The biofuel discourse has further constituted a concatenation of the three issues of agricultural production, energy security, and climate change mitigation. When the discourse shifted with the 2007–2008 food price crisis, all the three major organizations adapted to this shift [ 21 ]. Instead of exercising power over by manipulating discourses on biofuels according to specific pro or contra interests, the organizations were found to rather reproduce hegemonic discourses and their own positions.
The gold rush metaphor is used a lot to describe the situation of biofuels from a power over perspective [ 1 ]. Biofuel production, like gold mining, is unprofitable for most farmers, just like it was for diggers and mine owners. Both biofuel production and gold mining can in addition have very negative environmental effects. While, however, people are made to believe that everyone can become abundantly wealthy (“energy sheiks”), only some few investors make large fortunes. Applying discursive approaches of power over , we can argue that even such investors and major businesses are subject to and not only conscious manipulators of discourses of agricultural intensification and economic growth. The analysis of power over helps to understand why change to more sustainable transport and agricultural systems does not happen. However, as I argue in this article, it falls short on explaining when and why there also sometimes is disruptive change and empowerment.
Power to change: interrelations between power with, power to, and power over
While the perspectives of power with and power to (over-) emphasize the potential for change with regard to biofuels, scholars with understandings of power over often exaggerate their negative impacts. The tripartite framework allows for the combining of different analytical perspectives and to examine their interrelations. While the three categories are first of all analytical heuristics, they also stand for different mechanisms of the exercise of power (see Fig. 1 ). Power over affects what is considered a “sustainable innovation” and “creative alternative”. Research has demonstrated this. However, I argue that it is also possible the other way round: there are situations in which power with and power to can address power imbalances and prevent a situation in which there are only a few winners and many losers as a result of biofuel governance.
Agent-based power
As shown in Fig. 1 , besides considering material and ideational sources of power, we also need to consider different mechanisms of power (over/to/with), since they lead to different results of power (leading to a new distribution of sources in a circular process, see the arrow at the bottom of Fig. 1 ). Biofuels per se are neither a sustainable innovation, a creative alternative nor a gold rush. The three metaphors exemplify three different results of power: the exercise of power over leads to a gold rush situation. So, if scholars only ask for power over , they will always find winners and losers. By contrast, if we ask for the exercise of power to , we may find that biofuels are creative alternative. Finally, the exercise of power with can be exemplified by a case of finding an agreement on sustainability criteria of biofuel production. To demonstrate overlaps, especially, in terms of the results of power, I used dashed lines in Fig. 1 .
When, in the field of biofuels, scholars explicitly issue power, they generally use concepts of power over to explain why governance and research in this field have a blind spot for power asymmetries [ 49 , 53 ]. Biofuel opponents may have accomplished a shift in the biofuel discourse after the 2007–2008 food price crisis [ 20 , 22 ]. However, overriding power asymmetries have prevented a structural change in both the energy/transport and the agricultural sectors. The trend is now definitely towards large companies and conglomerates [ 49 , 50 ].
However, the fact that biofuels have caused no structural change and have disadvantaged rather than empowered small farmers in the Global South, does not mean that a structural change is impossible. What I want to argue in this article is that exercising consensual forms of power (power with) as well as self-empowerment and resistance (power to) can also eclipse and overcome power asymmetries (power over). Empirical research on deliberative processes suggests that communication and common action never happen among equals and that they are never free from any form of power over [ 36 , 61 ]. Hence, we need to understand power with as a form of exercising power, which is strategic (bargaining) as well as communicative (arguing). A crucial part of this process is the orientation of agents involved in processes of biofuel governance. If actors are open to changing their positions and developing shared understandings, transitions to sustainability can follow from dialogues [ 61 , 62 ].
Following this perspective, even if small farmers in the Global South have fewer capabilities compared to conglomerates from the EU and the United States, this does not mean that they have no possibility to act independently from them. For example, sugar is costly to establish, and thus is economically most efficient at large plantation scales. However, Jatropha can more readily be produced through outgrower schemes as it is less capital intensive [ 9 , 49 ]. While currently almost all bio-ethanol is produced from grain or sugarcane and therefore competes with food purposes, other efficient and economically viable technologies for ethanol production are available [ 63 ]. The production of perennial energy crops, such as grasses and trees, and crop residues, such as straw, are seen to require fewer inputs and less prime land [ 64 ].
Under specific conditions, empowerment is possible; processes of power with and power to can have a (positive) impact on unwanted relations of power over . For example, processes of stakeholder dialogue and certification demonstrate that an agreement beyond the lowest common denominator is possible. In addition, they can weaken the perceived legitimacy of powerful actors that are producing biofuels unsustainably. The critical discourse on biomass certification has issued consumers’ accountability for harmful social and environmental effects in countries of production [ 55 , 65 ]. When the legitimacy of unconditional import as well as of private certification schemes was put into question [ 50 ], transnational conglomerates lost ideational and material resources on which their power over others was based. In the agrifood sector, we can clearly see that certification has become a new normative obligation [ 66 ].
We can observe various kinds of empowerment and resistance related to biofuels. While Nygren [ 58 ] argues that certification schemes reproduce (inferior) positions of southern producers as authentic and exotic others, she does not completely deny that certification had a positive impact on altering asymmetries in global networks of production and consumption. Silva-Castaneda’s [ 56 ] study discloses new ways in which local communities can legally prove their land rights, for instance, by video documentation to replace missing formal documents or destructed land marks.
Scholars have described movements, such as Via Campesina, in terms of exercising power over and opposing transnational agriculture corporations [ 67 ]. In terms of reducing and overcoming power asymmetries, however, what is most striking is the fact that small farmers within this movement exercise power to by doing healthy and sustainable agriculture independently of the major agribusinesses to which, from a power over perspective, they would only be subordinated. At the same time, when producing organically, small farmers do not reproduce the system of industrial agricultural production (and their inferior positions within that system). So, their way of farming can be considered as a creative alternative and as a way of resistance. Moreover, within this movement of Via Campesina, despite widely different internal cultures, farmers also exercise power with by (re-) constituting a new shared peasant identity. From a perspective of power with, we can argue that, in the long run, everybody, even from outside this movement, may benefit and share norms and values developed here such as sustainability in farming. The movement delegitimizes the acquisition of land by established conglomerates (“land grabbing”), whose ideational sources of power shrink in consequence. The visible result is a new, more equal, and just distribution of (power) resources through land reforms.
Conclusions
This article should not only encourage a debate on power issues with regard to biofuels, but moreover, develop the debate more comprehensively. When political power has been analyzed in the context of biofuels, this has happened so far through using confrontational or structuralist and discursive approaches that are based on an understanding of power over . Respective scholars have accused other researchers of neglecting “real power concentrations” in the biofuels industries. Often quite rightly: biofuel research has neglected the limits of win-win for a very long time. Scholars have taken sides and normatively inflated their own pro biofuel position, while they have dispatched their adversaries as laggards with regard to the future of transport and agriculture. Of course, not every (supposedly) sustainable innovation is necessarily good in the sense that it is completely uncontroversial (even if there is no visible opposition as in the case of biofuels for a long time). In this context, the question of power essentially addresses the re-politicization of decisions perceived to be urgent and without alternative. With the 2007–2008’s shift in discourse, critics re-politicized the governance of biofuels. Several farmer associations have completely turned against biofuels. I argue that this rejection of biofuels is due to a limited perception of power as power over .
Why does it make sense to complement such a perception of power over ? Why does a multidimensional power framework make more sense? Naming different perspectives, as done here, with one and the same term—“power”—means, first, to put them on one normative level. Gold rush (power over) is a term with strongly negative connotations, on the one hand, and leads to normatively inflating sustainable innovations (power with) and creative resistance (power to), on the other. This is often unjustified because the exercise of power with and power to are not per se more legitimate forms of achieving social change. For example, preventing greenhouse gas emissions “from above” can be quite legitimate.
Secondly, as illustrated in this article, all three conceptions of power are already used in research on biofuels (although sometimes only implicitly; this should change). My hope is that this article addresses diverse communities and overcomes boundaries between them with this multidimensional power approach (in particular, between those who still celebrate biofuels as a “sustainable innovation” and those scholars who completely condemn them because of related power asymmetries). Especially those whose research is (implicitly) based on understandings of power as power with and power to could take stronger reference to researchers taking a critical viewpoint on their studies (power over)—in particular, through showing how consensual forms of power exercise (power with) and resistance and empowerment (power to) not only reproduce power asymmetries but also help overcome them. If we look at the gold rush metaphor from a perspective of power to , we may see that there is a lot of entrepreneurship involved in the discovery of gold deposits. From the perspective of power with , we may also see that people in the field of gold mining as well as of biofuel production find common ground among diverse interests and organize with each other.
Third, convincing and learning (power with) as well as creative ability (power to) and coercion and manipulation (power over) do not completely capture concrete change processes. The analytical categories applied in this paper help to cluster the various understandings of power in biofuel research, but they also reflect different mechanisms of power in reality. Power with perspectives focus on the benefits of biofuels (sustainable innovation); power to focuses on how new actors develop alternatives to fossil (and nuclear)-based economies; power over points to the limits of change because of the dominance of specific actors, structures, and discourses. The common terminology allows that the three perspectives on power are not considered as mutually exclusive (different interpretations of the same phenomenon), but as supplementary (different aspects of a change process). It becomes possible to examine their interrelations and their supplementary potential. With this article, I hope to have given an impetus for further research in this direction. A comprehensive analysis of power in diverse parts of biofuel research and governance is definitely a prerequisite for more seriously and intensively exploring questions of where, when, and how the governance of biofuels may also allow for “green” resistance and collective empowerment.
If actors create (reproduce) discourses and structures, I call this power to . Most constructivist studies however deal with identifying dominant (hegemonic) structures and discourses over others that are unconsciously reproduced, i.e., power over .
Power with is not identical to Arendt’s understanding of power or its empirical operationalization hardly accomplishes Arendt’s demands. So deliberative theories of democracy build upon her understanding of power without finding it comprehensively implemented in reality [ 61 , 68 , 69 ]. In difference to deliberative processes, power with encompasses communicative as well as common action.
An example, to which Arendt refers in a footnote to her definition of power, is the student protests at Berkeley and elsewhere at the end of the 1960s. She contrasts the power of the students—“obviously the strongest power on every campus simply because of the students’ superior number” ([ 28 ]: 44)—to the violence of the university authorities. An individual student leader ‘in power’ would speak on behalf of the movement.
Balkema A, Pols AJ (2015) Biofuels: sustainable innovation or gold rush? Identifying responsibilities for biofuel innovations. In: Koops B-J, Oosterlaken I, Romijn H et al (eds) Responsible innovation 2: concepts, approaches, and applications. Springer, New York, pp 283–303
Google Scholar
Partzsch L (2015) Kein Wandel ohne Macht–Nachhaltigkeitsforschung braucht ein mehrdimensionales Machtverständnis. GAIA 24(1):48–56
Article Google Scholar
Sordaa G, Banseb M, Kemfert C (2010) An overview of biofuel policies across the world. Energy Policy 38(11):6977–6988
Vermeulen S, Dufey A, Vorley B (2008) Biofuels: making tough choices. The International Institute for Environment and Development (IIED) (2)., pp 1–2
Parsons T (1963) On the concept of political power. Proc Am Philos Soc 107(3):232–262
Pitkin HF (1985) Wittgenstein and justice: on the significance of Ludwig Wittgenstein for social and political thought. University of California Press, Berkeley
Barnett M, Duvall R (2005) Power in global governance. In: Barnett MN, Duvall R (eds) Power in global governance. Cambridge University Press, Cambridge, pp 1–32
Altieri MA, Toledo VM (2011) The agroecological revolution in Latin America: rescuing nature, ensuring food sovereignty and empowering peasants. J Peasant Stud 38(3):587–612
van Eijcka J, Romijn H (2008) Prospects for Jatropha biofuels in Tanzania: an analysis with strategic niche management. Energy Policy 36(1):311–325
Dahl RA (1957) The concept of power. Behav Sci 2(3):201–215
Bachrach P, Baratz MS (1962) Two faces of power. Am Polit Sci Rev 4(56):947–952
Lukes S (1974) Power: a radical view. Macmillan, London
Book Google Scholar
Foucault M (1982) The subject and power. Crit Inq 8(4):777–795
Laclau E, Mouffe C (1985) Hegemony and socialist strategy: towards a radical democatic politics. Verso, London
Baka J (2014) What wastelands? A critique of biofuel policy discourse in South India. Geoforum 54(6):315–323
Hunsberger C (2010) The politics of Jatropha-based biofuels in Kenya: convergence and divergence among NGOs, donors, government officials and farmers. J Peasant Stud 37(4):939–962
Digeser P (1992) The fourth face of power. J Polit 54(4):977–1007
Haugaard M (2012) Rethinking the four dimensions of power: domination and empowerment. J Polit Power 5(1):33–54
Hunt SC, Sawin JL, Stair P (2006) Cultivating renewable alternatives to oil. In: Worldwatch Institute (ed) State of the world 2006: Special focus: India and China. Earthscan, London, pp 61–77
Sengers F, Raven R, van Venrooij A (2010) From riches to rags: biofuels, media discourses, and resistance to sustainable energy technologies. Energy Policy 38(9):5013–5027
Kuchler M, Linnér B-O (2012) Challenging the food vs. fuel dilemma: genealogical analysis of the biofuel discourse pursued by international organizations. Food Policy 37(5):581–588
Munro B (2015) The lost innocenece of ethanol: Power, knowledge, discourse, and the U.S. biofuel policy: Doctoral dissertation. Kansas State University, Kansas
Eyben R, Harris C, Pettit J (2006) Introduction: exploring power for change. IDS Bull 37(6):1–10
Gaard G (2010) Women, water, energy: an ecofeminist approach. In: Brown PG, Schmidt JJ (eds) Water ethics: foundational readings for students and professionals. Island Press, Washington, DC, pp 59–75
Balat M, Balat H (2009) Recent trends in global production and utilization of bio-ethanol fuel. Appl Energy 86(11):2273–2282
Nill J, Kemp R (2009) Evolutionary approaches for sustainable innovation policies: from niche to paradigm? Res Policy 38(4):668–680
Allen A (1998) Rethinking power. Hypatia 13(1):21–40
Arendt H (1970) On violence. Harcourt, Brace & World, New York
Skodvin T, Andresen S (2006) Leadership revisited. Glob Environ Polit 6(3):13–27
Bernard F, Prieur A (2007) Biofuel market and carbon modeling to analyse French biofuel policy. Energy Policy 35:5991–6002
Young OR (1991) Political leadership and regime formation: on the development of institutions in international society. Int Organ 45(3):281–308
Huber J (2000) Towards industrial ecology: sustainable development as a concept of ecological modernization. J Environ Policy Plan 2(4):269–285
Jänicke M (2008) Ecological modernisation: new perspectives. J Clean Prod 16(5):557–565
Bomb C, Deurwaarder E, Kaberger T (2007) Biofuels for transport in Europe: lessons from Germany and the UK. Energy Policy 35(4):2256–2267
Foxona T, Pearson P (2008) Overcoming barriers to innovation and diffusion of cleaner technologies: some features of a sustainable innovation policy regime. J Clean Prod 16(1):S148–S161
Longstaff H, Secko DM (2014) Assessing the quality of a deliberative democracy mini-public event about advanced biofuel production and development in Canada. Public Understanding of Science (online)., pp 1–10
Nussbaum M, Sen AK (1987) Internal criticism and Indian rationalist traditions. World Institute for Development Economics Research of the United Nations University, Helsinki
Scholtes F (2009) Analysing and explaining power a capability perspective, Bonn
Malik US, Ahmed M, Sombilla MA et al (2009) Biofuels production for smallholder producers in the Greater Mekong Sub-region. Appl Energy 86(S1):S58–S68
Rathke G-W, Diepenbrock W (2006) Mit Biomasse zum ‘Energie-Scheich’?: Stand, Ertragspotenziale und Perspektiven. Chancen für Landwirtschaft und angrenzende Industriezweige. In: DLG (ed) (Deutsche Landwirtschafts-Gesellschaft) Zukunftsstandort Deutschland: Strategien Für Die Landwirtschaft. DLG-Verlag, Frankfurt a.M
Gaventa J (2006) Finding the spaces for change: a power analysis. IDS Bull 37(6):23–33
Fuchs D (2005) Understanding business power in global governance. Nomos, Baden-Baden
Coase RH (1988) The firm, the market, and the law. University of Chicago Press, Chicago
Pilgrim S, Harvey M (2010) Battles over biofuels in Europe: NGOs and the politics of markets. Sociol Res Online 13(3):4
Stattman SL, Gupta A (2015) Negotiating authority in the global biofuel governance: Brazil and the EU in the WTO. Glob Environ Polit 15(1):41–59
Afionis S, Stringer LC (2012) European Union leadership in biofuels regulation: Europe as a normative power? J Clean Prod 32(1):114–123
Altvater E, Mahnkopf B (1999) Grenzen der Globalisierung: Ökonomie, Ökologie und Politik in der Weltgesellschaft, 4th edn. Westfälisches Dampfboot, Münster
Levin K, Cashore B, Koppell J (2009) Can non-state certification systems bolster state-centered efforts to promote sustainable development through the clean development mechanism? Wake Forest Law Review 44(1):777–798
Dauvergne P, Neville KJ (2010) Forests, food, and fuel in the tropics: the uneven social and ecological consequences of the emerging political economy of biofuels. J Peasant Stud 37(4):631–660
Ponte S, Daugbjerg C (2015) Biofuel sustainability and the formation of transnational hybrid governance. Environ Polit 24(1):96–114
Henriksen LF (2015) The global network of biofuel sustainability standards-setters. Environ Polit 24(1):115–137
Puttkammer J, Grethe H (2015) The public debate on biofuels in Germany: who drives the discourse? Ger J Agric Econ 64(4):263–273
Runge CF, Senauer B (2007) How biofuels could starve the poor. Foreign Aff 86(3):41–53
Horlings LG, Marsden TK (2011) Towards the real green revolution? Exploring the conceptual dimensions of a new ecological modernisation of agriculture that could ‘feed the world’. Glob Environ Chang 21(2):441–452
Levidow L (2013) EU criteria for sustainable biofuels: accounting for carbon, depoliticizing plunder. Geoforum 44(1):211–223
Silva-Castaneda L (2012) A forest of evidence: third-party certification and multiple forms of proof—a case study of oil palm plantations in Indonesia. Agric Hum Values 29(3):361–370
Selfa T, Bain C, Moreno R et al (2015) Interrogating social sustainability in the biofuels sector in Latin America: tensions between global standards and local experiences in Mexico, Brazil, and Colombia. Environmental Management
Nygren A (2015) Governance and images: representations of certified southern producers in high-quality design markets. Environ Values 24(3):391–412
Bourdieu P (1987) Sozialer Sinn: Kritik der theoretischen Vernunft. Suhrkamp, Frankfurt a.M
Guzzini S (1993) Structural power: the limits of neorealist power analysis. Int Organ 47(3):443–478
Dryzek JS (2000) Deliberative democracy and beyond: liberals, critics, contestations. Oxford University Press, New York
Prittwitz V (ed) (1996) Verhandeln und Argumentieren. Leske + Budrich, Opladen
Mussatto SI, Dragone G, Guimarães PM et al (2010) Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv 28(6):817–830
Shortall O (2014) Rethinking bioenergy from an agricultural perspective: ethical issues raised by perennial energy crop and crop residue production for energy in the UK and Denmark: PhD thesis. University of Nottingham, Nottigham
Partzsch L (2011) The legitimacy of biofuel certification. Agric Hum Values 28(3):413–425
Kalfagianni A (2015) ‘Just food’: the normative obligations of private agrifood governance. Glob Environ Chang 31(1):174–186
Martínez-Torres ME, Rosset PM (2010) La Vía Campesina: the birth and evolution of a transnational social movement. J Peasant Stud 37(1):149–175
Habermas J (1998) Between facts and norms: contributions to a discourse theory of law and democracy. MIT Press, Cambridge
Young IM (2001) Activist challenges to deliberative democracy. Polit Theo 29(5):670–690
Partzsch L, Fuchs DA (2012) Philanthropy: power with in international relations. J Polit Power 5(3):359–376
Guzzini S (2007) The concept of power: a constructivist analysis. In: Berenskoetter F, Williams MJ (eds) Power in world politics. Routledge, New York, pp 23–42
Download references
Acknowledgements
The research received no funding.
Competing interests
The author declares that she has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
Sustainability Governance, Institute of Environmental Social Sciences and Geography, University of Freiburg, Tennenbacher Strasse 4, D-79106, Freiburg, Germany
Lena Partzsch
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Lena Partzsch .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Partzsch, L. Biofuel research: perceptions of power and transition. Energ Sustain Soc 7 , 14 (2017). https://doi.org/10.1186/s13705-017-0116-1
Download citation
Received : 01 February 2017
Accepted : 04 May 2017
Published : 18 May 2017
DOI : https://doi.org/10.1186/s13705-017-0116-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Energy, Sustainability and Society
ISSN: 2192-0567
- General enquiries: [email protected]
800 343 7475 [email protected] bio-techne.com
News / Events
- Company Information
- Financial Information
- SEC Filings
- Corporate Governance
- Press Releases
- IR Calendar
- Email Alerts
- Presentations
- Management Team
- Balance Sheet
- Income Statement
- Financial Results
- Annual Reports
- Historical Data
- All SEC Filings
- 10-K Filings
- Quarterly Reports
- Section 16 Filings
- Board of Directors
- Board Committees
- Governance Documents
LUNAPHORE PARTNERS WITH DISCOVERY LIFE SCIENCES TO BRING INNOVATIVE SPATIAL BIOLOGY SOLUTIONS TO CLINICAL RESEARCH
MINNEAPOLIS , Sept. 26, 2024 /PRNewswire/ -- Bio-Techne Corporation (NASDAQ: TECH) today announced that one of its spatial biology brands, Lunaphore , is partnering with Discovery Life Sciences (Discovery) to add Lunaphore COMET™ , a best-in-class spatial biology platform, to Discovery's global suite of biospecimen products and specialty lab services supporting customers with clinical research.
The strategic partnership leverages Lunaphore's COMET technology, the only fully-automated, high-throughput, hyperplex platform with superior tissue profiling capabilities, with Discovery's scientific expertise to advance immuno-oncology, immunology, neuroscience, and infectious disease research across all development stages at a new level of precision and speed. Discovery's Lunaphore COMET Hyperplex Immunofluorescence Services will facilitate the development of next-generation assays for clinical trials and provide multidimensional spatial biology insights to advance biomarker discovery, patient stratification, and translational medicine.
"We are excited to expand our specialty lab services offering to include Lunaphore COMET Hyperplex Immunofluorescence Services," said Discovery CEO Greg Herrema. "By integrating COMET with Discovery's molecular pathology expertise, this partnership is poised to drive biomedical research breakthroughs. This new service combined with our rigorous regulatory compliance processes will provide pharmaceutical companies with the ability to develop Clinical Laboratory Improvement Amendment (CLIA) compliant hyperplex tests."
"Discovery Life Sciences stands out as a pioneering global leader offering COMET's cutting-edge capabilities to biopharmaceutical clients across the U.S., Europe, and APAC," said Matt McManus, President of Bio-Techne's Diagnostics & Genomics Segment. "This strategic partnership furthers Bio-Techne's commitment to push the boundaries of research to transform patient care. I look forward to seeing COMET accelerate the adoption of spatial biology techniques in clinical trials."
About Bio-Techne
Bio-Techne Corporation (NASDAQ: TECH) is a global life sciences company providing innovative tools and bioactive reagents for the research and clinical diagnostic communities. Bio-Techne products assist scientific investigations into biological processes and the nature and progress of specific diseases. They aid in drug discovery efforts and provide the means for accurate clinical tests and diagnoses. With hundreds of thousands of products in its portfolio, Bio-Techne generated approximately $1.2 billion in net sales in fiscal 2024 and has approximately 3,100 employees worldwide. For more information on Bio-Techne and its brands, please visit https://www.bio-techne.com or follow the Company on social media at: Facebook , LinkedIn , Twitter or YouTube .
| Contact: | David Clair, Vice President, Investor Relations & Corporate Development | |
|
| ||
| 612-656-4416 |
About Lunaphore
Lunaphore Technologies S.A. – a Bio-Techne brand, is a Swiss company born in 2014 with the vision of enabling spatial biology in every laboratory. Lunaphore provides solutions based on a game-changing chip technology that can extract spatial proteomic and transcriptomic data from tumors and other tissues, transforming any assay into multiplex spatial biology through a streamlined and easily integrated process. Lunaphore empowers researchers in immunology, immuno-oncology, and neuroscience to push the boundaries of scientific discovery and drug development. Lunaphore's technology enables the identification of biomarker signatures with clinical relevance to support the development of diagnostic tools and streamline clinical trials, to ultimately improve patient outcomes. For further information on Lunaphore and its products, please visit https://lunaphore.com .
About COMET™
Lunaphore COMET™ is the only fully-automated, high-throughput, hyperplex platform ensuring scalability and reproducibility without the need to conjugate primary antibodies. COMET provides walk-away automation, integrating staining, imaging, and image preprocessing steps to obtain standard hyperplex images. The multiomics capability of COMET enables the simultaneous analysis of both RNA and protein data within the spatial context of tissues to enhance the understanding of cellular dynamics and disease processes. COMET generates highly robust and reproducible data with full tissue preservation, allowing researchers to perform downstream modalities such as H&E or transcriptomics using the same slide. Its superior tissue profiling capabilities facilitate the analysis of 40 different spatial markers in each automated run on a tissue slide. In contrast to other spatial biology solutions, COMET works with off-the-shelf, label-free primary antibodies, making panel design much more flexible and faster than any other hyperplex solution. COMET works with regular glass slides from standard histology workflows; it is validated for human and mouse samples and is compatible with any other animal sample. The platform can be used for a wide range of research applications, allowing for a dramatic improvement in the understanding of disease pathology. To learn more about the COMET platform, please visit: https://lunaphore.com/products/comet/
About Discovery Life Sciences
Discovery Life Sciences is a leading global provider of biospecimen products and specialty lab services. The company enables the discovery and development of therapies and diagnostics by providing pharmaceutical, biotech, and diagnostic companies with the highest quality biospecimens, in vitro preclinical solutions, and cell and gene therapy starting materials. Additionally, Discovery offers a complete suite of specialty lab services, including genomics, proteomics, molecular pathology, and cell biology, to support both prospective and retrospective clinical trials. For more information, visit dls.com .
For further information on Lunaphore:
Lindsey Rodger, Head of Communications [email protected]

View original content to download multimedia: https://www.prnewswire.com/news-releases/lunaphore-partners-with-discovery-life-sciences-to-bring-innovative-spatial-biology-solutions-to-clinical-research-302258200.html
SOURCE Bio-Techne Corporation
Released September 26, 2024
- RSS News Feed
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Proc Math Phys Eng Sci
Environmental sustainability of biofuels: a review
Harish k. jeswani.
1 Department of Chemical Engineering and Analytical Science, The University of Manchester, Manchester M13 9PL, UK
Andrew Chilvers
2 Royal Academy of Engineering, 3 Carlton House Terrace, London SW1Y 5DG, UK
Adisa Azapagic
Associated data.
This article does not contain any additional data.
Biofuels are being promoted as a low-carbon alternative to fossil fuels as they could help to reduce greenhouse gas (GHG) emissions and the related climate change impact from transport. However, there are also concerns that their wider deployment could lead to unintended environmental consequences. Numerous life cycle assessment (LCA) studies have considered the climate change and other environmental impacts of biofuels. However, their findings are often conflicting, with a wide variation in the estimates. Thus, the aim of this paper is to review and analyse the latest available evidence to provide a greater clarity and understanding of the environmental impacts of different liquid biofuels. It is evident from the review that the outcomes of LCA studies are highly situational and dependent on many factors, including the type of feedstock, production routes, data variations and methodological choices. Despite this, the existing evidence suggests that, if no land-use change (LUC) is involved, first-generation biofuels can—on average—have lower GHG emissions than fossil fuels, but the reductions for most feedstocks are insufficient to meet the GHG savings required by the EU Renewable Energy Directive (RED). However, second-generation biofuels have, in general, a greater potential to reduce the emissions, provided there is no LUC. Third-generation biofuels do not represent a feasible option at present state of development as their GHG emissions are higher than those from fossil fuels. As also discussed in the paper, several studies show that reductions in GHG emissions from biofuels are achieved at the expense of other impacts, such as acidification, eutrophication, water footprint and biodiversity loss. The paper also investigates the key methodological aspects and sources of uncertainty in the LCA of biofuels and provides recommendations to address these issues.
1. Introduction
Greenhouse gas (GHG) emissions from transport have been increasing at a faster rate than from any other sector [ 1 ]. The sector relies heavily on fossil fuels, which accounted for 96.3% of all transportation fuels in 2018 [ 2 ]. Transport is also responsible for 15% of the world's GHG emissions and 23% of total energy-related CO 2 emissions [ 1 ]. To reduce dependence on petroleum-based fuels, as well as to mitigate climate change, biofuels are viewed widely as promising alternative transportation fuels.
Biofuels have been used since the early days of the automotive industry. For instance, Rudolph Diesel tested his first engine on peanut oil [ 3 ] after pulverized coal was found to be unsuitable. Until the 1940s, biofuels were seen as viable transport fuels and bioethanol blends, such as Agrol, Discol and Monopolin, were commonly used in the USA, Europe and other regions [ 3 ]. Further development of bioethanol ceased after the Second World War as petroleum-derived fuel became cheaper. During the oil crisis in the 1970s, many countries showed renewed interest in production of commercial biofuels; however, only Brazil started to produce ethanol at a large scale as part of the National Ethanol Programme ‘Proálcool’ [ 4 ]. During the late 1990s, with the rise in crude oil prices and concerns over energy security, the USA and many nations in Europe developed policies in support of domestic biofuel industries [ 5 ]. The interest in biofuels further increased in the past decade with the development of policies on climate change mitigation and strategies to reduce GHG emissions from the transport sector. More than 60 countries have since launched biofuel programmes and set targets for blending biofuels into their fuel pools [ 6 ]. The most notable are Renewable Fuel Standard (RFS) [ 7 ] in the USA and the Renewable Energy Directive (RED) in Europe [ 8 ].
Owing to these policies, world bioethanol production has increased by 67%, from 67 to 110.4 billion litres, over the decade of 2008–2018 [ 2 ]. During the same period, biodiesel production increased more than threefold, from 12 to 41 billion litres. Currently, biofuels account for about 3.4% of total transportation fuels worldwide [ 2 ]. The global production of biofuels is dominated by the USA and Brazil—producing 69% of all biofuels in 2018—followed by Europe (EU-28) with 9% [ 9 ]. Production of bioethanol in the USA is almost exclusively from corn, whereas in Brazil, it is from sugarcane. In Europe, the main feedstocks are corn, wheat and sugar beet for bioethanol, while rapeseed and used cooking oil (UCO) are used for biodiesel production [ 10 ]. Argentina, Brazil and the USA also produce significant quantities of biodiesel, predominantly from soya bean, while Malaysia and Indonesia produce biodiesel from palm oil. Several international and national organizations have made mid- and long-term projections for global production of biofuels. These projections provide wide-ranging estimates of potential future increases in liquid biofuels for transport globally. The International Energy Agency (IEA) estimates that as much as one-third of all transportation fuel could come from biofuels by 2050 [ 11 ], while organizations, such as the OECD and BP, project approximately a 7% share of biofuels by 2030 [ 12 ]. A recent assessment [ 13 ] also suggests that the IEA projections could be impossible to achieve, estimating the maximum potential of transport biofuels by 2050 to be at least 30% lower than those projected by the IEA.
Biofuels can be differentiated according to a number of key characteristics, including feedstock type, conversion process, technical specification of the fuel and its use. Owing to this multitude of possible distinctions, various definitions are in use for biofuel types. Two commonly used typologies are ‘first, second and third generation’ and ‘conventional and advanced’ biofuels. Biofuels produced from food or animal feed crops are referred to as first-generation biofuels. Since first-generation biofuels are produced through well-established technologies and processes, such as fermentation, distillation and transesterification, they are also commonly referred to as ‘conventional biofuels'. A key characteristic for second-generation biofuels is that they are derived from non-food feedstocks, such as dedicated energy crops (e.g. Miscanthus , switchgrass, short rotation coppice (SRC) and other lignocellulosic plants), agricultural residues, forest residues and other waste materials (e.g. UCO and municipal solid waste). Biodiesel produced from microalgae through conventional transesterification or hydro-treatment of algal oil is commonly known as third-generation biofuel. Second- and third-generation biofuels are often referred to as ‘advanced biofuels’ as their production techniques or pathways are still in the research and development, pilot or demonstration phase. In this paper, the terminology ‘first, second and third generation’ has been selected and followed throughout. An overview of different biofuel types, their feedstocks and conversion routes can be seen in figure 1 .

An overview of feedstocks and production processes for different biofuels, also showing the life cycle of fuels from cradle to gate (well to tank) and cradle to grave (well to wheel). Adapted from [ 14 ]. The figure has been simplified and other feedstocks, production routes, products/by-products and uses are possible. The italic font denotes the focus of this review, i.e. bioethanol and biodiesel used for transportation. DDGS, dark distillers grain with solids. (Online version in colour.)
Biofuels offer both advantages and disadvantages in terms of environmental, economic and social sustainability [ 14 ]. On the one hand, reduction in GHG emissions, energy security and rural development are the most important drivers for biofuels globally. On the other hand, there are concerns related to increasing the production of biofuels, such as upward pressure on food prices, the risk of increase in GHG emissions through direct and indirect land-use change (LUC) from production of biofuel feedstocks, as well as the risks of degradation of land, forests, water resources and ecosystems [ 15 ]. The use of first-generation feedstocks, such as corn, has become a particularly contentious issue, largely owing to competition with food production and concerns over diverting agricultural land into fuel production. A growing demand for agricultural produce risks an increase in deforestation and use of land with a high biodiversity value to meet this demand, as well as associated usage of freshwater, fertilizers and pesticides, with negative consequences on the environment. Some of these issues could be addressed by using second-generation feedstocks; however, the economic viability of some second generation of biofuels remains doubtful in the current economic context, largely because of the low oil prices [ 16 – 18 ]. Third-generation (algal) biofuels could also avoid the issue of food competition and land use because microalgae can be grown on non-arable land and in wastewater, saline or brackish water and they grow extremely rapidly. However, the production of biofuels from microalgae is energy-intensive and at present economically unviable [ 19 ].
To encourage sustainable development of biofuels, regulatory policies, such as the RED and RFS, stipulate various sustainability criteria for biofuels. One of the main criteria is related to life cycle GHG emissions. The RED stipulates that biofuels should have at least 50% lower emissions than their fossil fuel alternatives for installations in operation before October 2015 and 60% for installations starting after this date, rising to 65% lower for biofuel plants commencing operation after 1 January 2021 [ 8 ]. RFS requires producers of advanced biofuels to reduce GHG emissions by at least 50%, while standard biofuels have to achieve a 20% reduction in GHG emissions [ 7 ]. The climate change impact related to GHG emissions and other sustainability aspects of biofuels should be evaluated on a life cycle basis via life cycle assessment (LCA) to avoid shifting burdens from one part of the life cycle or supply chain to another.
Numerous LCA studies have considered the potential of biofuels to achieve reductions in life cycle GHG emissions by estimating their potential impact on climate change. However, their findings are often conflicting, with a wide variation in the estimates. A number of review papers have also discussed LCA of biofuels, but these focused on a particular aspect, such as a region, feedstock or type of biofuel. For example, Shonnard et al . [ 20 ] reviewed LCA studies of biofuels in the Pan American region. Morales et al . [ 21 ] and Roy et al . [ 22 ] concentrated on LCA studies of lignocellulosic bioethanol, while Menten et al . [ 23 ] focused on advanced biofuels. Sieverding et al . [ 24 ] conducted a review of soya bean-based biodiesel and van Eijck et al . [ 25 ] reviewed issues pertaining to biodiesel from Jatropha .
However, a comprehensive review of all types of biofuels is not available in the literature. Besides, many more LCA studies on biofuels have been published since the publication of the above-mentioned reviews. Thus, this review paper aims to close this gap by analysing and synthesizing the latest information concerning LCA of biofuels. The main objective is to provide a greater clarity and understanding of the environmental sustainability of different liquid biofuels with the aim of informing future policy. A further objective is to examine the state-of-the-art knowledge on environmental issues associated with the production and consumption of biofuels. The next section provides an overview of the reviewed LCA studies in terms of their coverage with regard to biofuel type, geographical location and their approaches to handling critical methodological aspects in LCA. Section 3 presents results for the climate change impact, energy and water use and other environmental issues associated with different types of biofuels. The key methodological aspects and sources of uncertainty in assessing the environmental impacts of biofuels are investigated and discussed in detail in §4. The paper ends with conclusions and recommendations on addressing the key issues related to sustainability of biofuels.
2. Study methodology and coverage
A systematic literature search was performed in different databases (Science Direct, Web of Science, Scopus and relevant academic journals) to identify academic, peer-reviewed studies on the environmental sustainability of biofuels. To avoid outdated information, the review of the literature predominantly focused on the articles published in the period from 2009 to 2020. Some important earlier publications cited frequently in the literature were also taken into account. In total, 179 articles were primary (original) LCA studies, combining between them 613 assessments of different types of biofuels, all of which are included in this review. In addition, further publications focusing on environmental issues not usually included in LCA studies, such as water footprint, biodiversity and LUC, as well as discussing various methodological aspects were also reviewed. These studies covered a wide spectrum of first-, second- and third-generation biofuels produced from more than 20 types of feedstock. Table 1 provides an overview of the LCA studies related to the types of fuel, feedstock and geographical coverage. Among them, 52% assessed first generation, 38% considered second generation and the remaining 10% assessed third-generation biofuels. Regarding the type of biofuel, 56% of studies were for bioethanol and the rest for biodiesel. Geographically, 36% of studies were based in Europe, 26% in North America, 20% in Asia, 12% in South America, 4% in Africa and 1% in Australia. An overview of how different studies approached some critical LCA methodological aspects, including type of LCA, goal and scope of the study, definition of the functional unit, allocation methods and estimation of the impacts, is provided below.
Table 1.
An overview of the number of LCA studies by biofuel type, feedstock, location and land-use change.
| location | land-use change | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| fuel type/feedstock | Europe | North America | South America | Asia | Africa | Australia | without | with | total |
| corn | 6 | 23 | 0 | 1 | 0 | 0 | 16 | 14 | 30 |
| molasses | 4 | 12 | 0 | 25 | 3 | 4 | 30 | 18 | 48 |
| sugar beet | 19 | 1 | 0 | 0 | 1 | 0 | 14 | 7 | 21 |
| sugarcane | 0 | 4 | 32 | 1 | 1 | 0 | 28 | 10 | 38 |
| wheat | 39 | 0 | 0 | 0 | 0 | 0 | 28 | 11 | 39 |
| bagasse | 1 | 1 | 3 | 1 | 0 | 0 | 6 | 0 | 6 |
| forest residue | 16 | 7 | 0 | 0 | 0 | 0 | 23 | 0 | 23 |
| 14 | 9 | 0 | 0 | 0 | 0 | 16 | 7 | 23 | |
| short rotation coppice | 29 | 2 | 0 | 0 | 0 | 0 | 17 | 14 | 31 |
| stover | 12 | 18 | 0 | 0 | 0 | 0 | 27 | 3 | 30 |
| straw/husk | 27 | 1 | 0 | 9 | 0 | 0 | 32 | 5 | 37 |
| switchgrass | 2 | 17 | 1 | 0 | 0 | 0 | 18 | 2 | 20 |
| palm oil | 0 | 0 | 3 | 56 | 0 | 0 | 32 | 27 | 59 |
| rapeseed | 19 | 13 | 2 | 0 | 4 | 0 | 24 | 14 | 38 |
| soya bean | 3 | 10 | 18 | 5 | 3 | 0 | 29 | 10 | 39 |
| sunflower | 1 | 0 | 2 | 0 | 5 | 0 | 5 | 3 | 8 |
| 1 | 13 | 0 | 0 | 0 | 0 | 14 | 0 | 14 | |
| 0 | 0 | 7 | 8 | 7 | 0 | 18 | 4 | 22 | |
| used cooking oil/tallow | 17 | 1 | 3 | 5 | 1 | 0 | 27 | 0 | 27 |
| algae | 13 | 28 | 4 | 13 | 0 | 2 | 60 | 0 | 60 |
| total | 223 | 160 | 75 | 124 | 25 | 6 | 464 | 149 | 613 |
a The total number of studies or analyses, rather than the number of papers published, as some papers included several studies or analyses.
(a) Type of life cycle assessment: attributional versus consequential
Two general types of LCA studies are distinguished: attributional (ALCA) and consequential (CLCA). They address different questions and follow different methodologies, and will normally have very different results. ALCA accounts for impacts directly related to the system of interest, attributing them to the activities within the system; hence, the term ‘attributional’. For biofuels, it is used mainly as an ‘accounting’ tool for estimating environmental impacts of various activities in the supply chain, comparisons of alternative systems and identification of environmental hotspots that can be targeted for improvements. CLCA, in addition to direct, also examines potential indirect consequences of the system under study by considering various ‘what if’ scenarios that could arise owing to this system; examples include changes in demand for the product of interest or technological improvements. For instance, CLCA can consider potential impacts of biofuel feedstock cultivation on other land-using sectors and the effect this might have on the food production system and LUC elsewhere in the global economy [ 26 , 27 ]. As such, CLCA is more suited for policy applications.
CLCA is still under development and, consequently, most of the LCA studies on biofuels found in the literature are attributional. Nevertheless, both the ALCA and CLCA are considered in this review.
(b) Goal and scope of studies
Goal and scope definition is an important initial step in LCA studies as the specific methodological approaches depend strongly on the specific goal, scope and question being addressed. The goal and scope of the study influence the definition of the system boundary and determine what activities and life cycle stages will be considered [ 28 ]. LCA studies of biofuels have addressed a wide range of goals and research questions, including:
- — What are the environmental impacts of the biofuel system under examination?
- — How do biofuels compare with fossil fuels?
- — What are the environmental hotspots in the life cycle of particular biofuel systems under study?
- — What are the improvement options to optimize the supply chain under study?
- — What are the environmental implications of biofuel policies?
Although the ISO 14040 LCA standard [ 28 ] requires clear definition of the goal and scope, a lack of or unclear definition of goal and scope is a common problem in LCA studies of biofuels. This can also mean that the study method and rationale can be unclear, making comparability of results difficult [ 29 ].
Two types of system boundaries were used in the reviewed LCA studies of biofuels: ‘cradle to gate’ (or ‘well to tank’) and ‘cradle to grave’ (or ‘well to wheel’); figure 1 . However, the latter system boundary is more appropriate as it is important to include the use of fuels to enable comparisons of biofuels with their fossil substitutes, since the combustion performance and associated emissions of biofuels can significantly differ from their fossil substitutes for the same type of vehicle [ 30 , 31 ]. Around half (48%) of the LCA studies reviewed considered a cradle to grave system boundary to compare environmental impacts of biofuels with fossil fuels, while the rest were from cradle to gate. Other inconsistencies include the omission in some studies of various inputs (such as enzymes, pesticides, fertilizers, etc.) and co-products. These differences are often important enough to influence the results significantly.
(c) Functional unit
In LCA, the term ‘functional unit’ describes the function of the system under study and represents the unit of analysis on which the study is based. The choice of the functional unit is driven by the goal of the study and must be representative of the system(s) studied and their main purpose (function). Biofuels regulations, such as the RED [ 8 ] and RFS [ 7 ], use the energy content of biofuels (MJ) as the functional unit. While this functional unit was often used in the reviewed literature, others include the distance travelled by a vehicle (vehicle . km) [ 21 , 32 ], volume (litre) [ 33 , 34 ] and mass (kilogram or tonne) [ 17 , 35 ] of biofuels. Some studies also used the mass of biofuel feedstock [ 36 , 37 ], agricultural land area [ 38 , 39 ] and annual operation of refinery [ 40 ]. The use of such a wide array of functional units makes comparisons of LCA studies challenging.
(d) Allocation methods
Biofuel production processes often produce several co-products, such as animal feed, heat, electricity and biochemicals. Therefore, to determine the impacts from the biofuel of interest, it is necessary to allocate the impacts between the biofuel and its co-products. The ISO 14040 and 14044 standards recommend that, if possible, allocation should be avoided through subdivision of processes, or by system expansion. The latter involves expanding the system boundary to include alternative ways of producing the co-products. The production system is then credited for displacing production of the co-products in the alternative systems by subtracting their impacts from the impacts of the biofuel production system. Hence, this method is also known as ‘substitution’ or the ‘avoided burden’ approach. If allocation cannot be avoided, the impacts can be apportioned between the biofuel and the co-products using allocation factors based on physical or economic relationships. Mass and energy content of biofuels and co-products are often used to derive allocation factors based on physical relationships. Economic allocation is based on the assumptions that the market prices are the driver for the production process and the impacts are apportioned in proportion to the economic value (cost or price) of the biofuel and the co-products. In LCA of biofuels, the most common approaches used to allocate the impacts are system expansion and allocation by the energy content. This perhaps reflects the regulatory requirements in the USA and Europe: RFS [ 7 ] prefers system expansion, while the RED [ 8 ] favours allocation based on the energy content of biofuels.
(e) Land-use change
LUC is an important source of GHG emissions that contributed 660 ± 290 Gt CO 2 to the atmospheric CO 2 in the period from 1750 and 2011 [ 41 ]. The majority of LUC is driven by demand for food, fibre and fuel [ 42 ]. Converting natural vegetation or forest to cultivate biofuel feedstocks releases a significant amount of carbon from soil and plant biomass, creating a ‘carbon debt’ that can take years to repay [ 43 , 44 ]. Furthermore, cultivation of biofuel feedstocks on land that has high soil carbon content, such as peat land, leads to a considerable increase in GHG emissions [ 45 ]. Besides increasing GHG emissions, changes in land use can have other environmental consequences, such as soil erosion, nutrient depletion, water consumption and loss of biodiversity [ 46 ]. LUC related to biofuels can occur in two ways: direct (DLUC) or indirect (ILUC). DLUC refers to the direct transformation of previously uncultivated areas (such as grasslands and forests) into croplands for biofuel feedstock production. ILUC occurs when additional demand for biofuel feedstock induces displacement of food and feed crop production to new land areas previously not used for cultivation. Only 25% of the reviewed LCA studies took LUC into account.
(f) Environmental impacts
GHG emissions and savings in comparison to fossil fuels are the centre of attention in most LCA studies on biofuels. Other environmental impact categories considered in biofuel LCA studies include acidification, eutrophication, photochemical smog, human toxicity and eco-toxicity. However, the number of studies that have assessed a wider set of impact categories is still limited: of the 179 (primary) LCA studies reviewed, only 40% of such studies were found in the literature. These are discussed in the next section, starting with the climate change impact, or global warming potential (GWP) as often referred to in LCA, and following on to discuss energy and water use, biodiversity and other impacts.
3. Environmental impacts of biofuels
(a) global warming potential.
For this impact, the LCA studies present contradictory results, ranging from favourable to unfavourable, even for the same type of feedstock. This is a result of the differences in the assumptions, data sources, allocation methods and LUC. The influence of these aspects is discussed in detail in §4. The GWP of biofuels reported in the reviewed LCA studies is summarized in figures 2 – 6 and discussed below for different types of biofuel. For further details, see electronic supplementary material, figures S1–S7.

GWP of first-generation biofuels without land-use change. Based on data from [ 24 , 32 , 34 , 47 – 118 ]. For the box plot legend, see electronic supplementary material, figure S1 and for the data used to plot this graph, see electronic supplementary material, figure S2. ‘Fossil fuel (reference)’ is the average carbon intensity of petrol and diesel supplied in the EU (94 g CO 2 eq. MJ −1 ) as specified in the RED [ 8 ]. ‘ A ’ refers to the number of LCA articles found in the literature and ‘ n ’ denotes the total number of analyses. (Online version in colour.)

GWP of microalgae biodiesel. Based on data from [ 19 , 85 , 97 , 113 , 141 , 167 , 190 – 209 ]. The negative values are due to the credits for co-products and avoided processes, such as wastewater treatment. For the box plot legend, see electronic supplementary material, figure S1 and for the data used to plot this graph, see electronic supplementary material, figure S6. ‘Fossil fuel (reference)’ is the average carbon intensity of petrol and diesel supplied in the EU (94 g CO 2 eq. MJ −1 ) as specified in the RED [ 8 ]. ‘ A ’ refers to the number of LCA articles found in the literature and ‘ n ’ denotes the total number of analyses. (Online version in colour.)
(i) First-generation biofuels
As first-generation biofuels may involve LUC, which in turn can affect significantly the total GWP, the results reported in the literature are discussed first for the cases without and then with LUC.
GWP without land-use change . As can be observed in figure 2 , the GWP of first-generation bioethanol from different food crops vary considerably, ranging from 3 to 162 g CO 2 eq. MJ −1 (see electronic supplementary material, figure S2). Figure 2 also shows that the average GWP of bioethanol is lower than that of petrol for all the feedstocks (23–59 versus 94 g CO 2 eq. MJ −1 ). However, only bioethanol from sugarcane can meet the RED requirement of 60% reduction in GHG emissions relative to petrol. The average reductions in emissions from the other four feedstocks—corn, wheat, molasses and sugar beet—are not sufficient to meet this requirement. The main reasons that bioethanol from sugarcane can meet the 60% reduction requirement are relatively lower inputs of agro-chemicals and higher yields of sugarcane crops as well as the credits for electricity produced as a co-product in a biorefinery.
The GHG emissions for first-generation biodiesel also show a large variation across the LCA studies, with the GWP ranging between 3 and 111 g CO 2 eq. MJ −1 (electronic supplementary material, figure S2). However, as shown in figure 2 , the average GWP of biodiesel from all the feedstocks considered is lower than that of fossil diesel. Nevertheless, only biodiesel from palm oil meets the RED requirement for 60% reduction of the GWP relative to diesel (average value). Rapeseed and soya bean also come close to fulfilling this requirement, but sunflower biodiesel cannot meet even the 35% reduction.
The large variability in the GWP of first-generation biofuels shown in figure 2 is due to several reasons. For example, the LCA study on corn ethanol and soya bean biodiesel production in China found that the GWP of corn ethanol and soya bean biodiesel were 40 and 20% higher than petrol and diesel, respectively, owing to the relatively higher use of fertilizers, higher process energy consumption and the coal-dominated energy mix of China [ 47 ]. Low or no GHG savings (0–20%) compared to the fossil fuels were reported for South African sugar beet bioethanol as well as rapeseed, soya bean and sunflower biodiesel due to water scarcity affecting crop yields [ 48 , 49 ]. On the other hand, owing to higher yield and lower farming inputs, soya bean biodiesel produced in Brazil, the USA and Argentina achieve more than 60% GHG savings relative to the fossil fuels [ 50 ]. Studies on palm [ 51 , 52 ], rapeseed [ 53 , 54 ] and sunflower [ 55 ] biodiesel have also found the significant effect on GHG emissions of different locations, farming practices and usage of fertilizers. In the case of palm oil, the emissions vary with the options for handling of methane emissions from the treatment of palm oil milling effluent [ 56 ]. The influence of the fuel used in the biorefinery was noted in the case of wheat ethanol, with the GHG savings varying from 4 to 85% depending on whether straw or distillers' dried grains are used as a fuel [ 57 ]. Similarly, another study on molasses-based ethanol found that the use of bagasse instead of fuel oil would reduce GHG emissions of bioethanol from 112 to 51 g CO 2 eq. MJ −1 [ 58 ]. A number of studies on biofuels from different feedstocks have also found that the emissions are highly influenced by the utilization of by-products and the allocation method [ 52 , 59 – 62 ]. The effects of allocation methods and other factors are discussed in more detail in §4.
GWP with land-use change . As shown in figure 3 , if LUC is involved and considering the average GWP values, bioethanol cannot meet the 60% GHG reduction requirement regardless of the type of feedstock [ 62 , 63 , 119 , 120 ]. The increasing demand for bioethanol from sugarcane in Brazil has led to a continuous expansion of land used for sugarcane cultivation [ 44 , 210 ]. If this involves deforestation of tropical rainforest, the GWP of bioethanol from sugarcane can be up to 60% higher than that of petrol [ 58 ]. Similarly, expansion of soya bean cultivation in Central and South America is driving both direct and indirect LUC [ 121 , 211 ]. Furthermore, palm cultivation in Malaysia and Indonesia is associated with deforestation and drainage of peat lands. As a consequence, biodiesel from palm oil on peat and forest lands can have 3–40 times higher GHG emissions than diesel [ 64 ]. A recent study assessing the LUC impact of biofuels consumed in Europe [ 212 ] also found that the GWP of palm oil and soya bean diesel are almost two times higher than that of diesel. The same study also estimated the GWP of biodiesel from rapeseed and sunflower to be 20 to 40% higher than from conventional diesel ( figure 3 ).

GWP of first-generation biofuels with land-use change. Based on data from [ 32 , 35 , 48 , 51 , 56 , 58 , 62 – 64 , 66 – 72 , 79 , 86 , 87 , 91 , 108 , 116 , 119 – 127 ]. For the box plot legend, see electronic supplementary material, figure S1 and for the data used to plot this graph, see electronic supplementary material, figure S3. ‘Fossil fuel (reference)’ is the average carbon intensity of petrol and diesel supplied in the EU (94 g CO 2 eq. MJ −1 ) as specified in the RED [ 8 ]. ‘ A ’ refers to the number of LCA articles found in the literature and ‘ n ’ denotes the total number of analyses. (Online version in colour.)
The significant variability shown in figure 3 for the GWP related to LUC is due to several reasons. For instance, some studies considered only ILUC [ 62 , 63 , 119 , 120 ] or DLUC [ 58 , 65 – 67 ], while others included both [ 68 , 122 ]. Furthermore, some studies applied partial equilibrium models and counterfactual (what if) scenarios to estimate ILUC emissions [ 62 , 119 ], whereas others used ILUC factors recommended by the US EPA [ 120 ]. The former tended to obtain higher ILUC emissions (34–155 g CO 2 eq. MJ −1 ) [ 62 , 119 , 123 ] than the latter (5–16 g CO 2 eq. MJ −1 ) [ 63 , 68 , 122 ]. For DLUC, several studies focused only on soil organic carbon (SOC) changes [ 65 , 122 ], but others also considered changes in the carbon stock related to the removal of biomass, both above and below the ground [ 58 , 67 , 68 ]. DLUC emissions also depend on the type of converted land and its previous use. For example, studies assuming palm oil cultivation on tropical forest and/or peat land in Malaysia and Indonesia estimated DLUC emissions in the range of 150–530 g CO 2 eq. MJ −1 [ 64 , 69 , 70 , 124 ]. On the other hand, studies on palm oil in Colombia and Thailand considered increase in the carbon stock due to LUC, assuming that the expansion of oil palm cultivation would occur in shrublands, savannahs, paddy fields and other agricultural lands [ 52 , 56 , 71 ]. In the case of sugarcane, molasses and soy, LUC emission reported in the literature varied from 30 to 200 g CO 2 eq. MJ −1 depending on the previous land use [ 35 , 58 , 69 , 72 , 121 ].
(ii) Second-generation biofuels
Figures 4 and and5 5 indicate that in most of the studies, the GWP of second-generation biofuels is considerably lower than that of fossil fuels. However, there is a large variation among different studies and feedstocks, with the values ranging from −115 to 173 g CO 2 eq. MJ −1 for bioethanol and −88 to 150 g CO 2 eq. MJ −1 for biodiesel (see electronic supplementary material, figures S4 and S5). These variations reflect the diversity of feedstocks and production routes, technology assumptions and methodological differences. Furthermore, some studies also considered emissions from ILUC [ 119 ] and SOC sequestration [ 68 , 128 ] associated with the production of SRC and perennial grasses as well as the reductions in SOC with removal of agricultural residues used as biofuel feedstocks [ 68 , 129 ]; for further discussion on SOC, see §4f. It should also be noted that the uncertainties related to technologies plays a particularly important role in the assessment of advanced biofuels as these are yet to be fully commercialized. Therefore, the quality of the available data is not as robust as in the case of the well-established first-generation biofuels.

GWP of second-generation bioethanol. Based on data from [ 17 , 18 , 33 , 34 , 40 , 66 , 68 , 119 , 120 , 102 , 104 , 105 , 115 , 117 , 128 – 163 ]. The negative values are due to the credits for co-products, such as heat and chemicals. For the box plot legend, see electronic supplementary material, figure S1 and for the data used to plot this graph, see electronic supplementary material, figure S4. ‘Fossil fuel (reference)’ is the average carbon intensity of petrol and diesel supplied in the EU (94 g CO 2 eq. MJ −1 ) as specified in the RED [ 8 ]. ‘ A ’ refers to the number of LCA articles found in the literature and ‘ n ’ denotes the total number of analyses. (Online version in colour.)

GWP of second-generation biodiesel. Based on data from [ 20 , 47 , 89 , 90 , 97 , 108 , 164 – 189 ]. The negative values are due to the credits for co-products. For the box plot legend, see electronic supplementary material, figure S1 and for the data used to plot this graph, see electronic supplementary material, figure S5. ‘Fossil fuel (reference)’ is the average carbon intensity of petrol and diesel supplied in the EU (94 g CO 2 eq. MJ −1 ) as specified in the RED [ 8 ]. ‘ A ’ refers to the number of LCA articles found in the literature and ‘ n ’ denotes the total number of analyses. (Online version in colour.)
In general, lignocellulosic bioethanol from agricultural and forest residues has a lower GWP than bioethanol from energy crops ( figure 4 ). This is largely due to N 2 O emitted during the cultivation of energy crops, related to the use of fertilizers. The latter are avoided in the case of residues as they are assumed to have no environmental burdens, which are all allocated to the original crop from which the waste is derived. In lignocellulosic bioethanol studies, the residual lignin is assumed to co-generate heat and power to meet the energy needs of the process, with surplus electricity exported to the grid. The biofuel production system is thus credited for avoiding the GHG emissions from the equivalent amount of grid electricity. For some feedstocks (SRC, forest residue, straw and corn stover), the credits for electricity generation and other co-products are higher than the total emissions from the biofuel production. Consequently, these studies report negative GWP, indicating the avoidance (saving) of GHG emissions. Some studies on energy crops also considered the increase in the carbon stock on the land that was converted to produce these crops, which led to the total net-negative GHG emissions [ 68 , 119 ]. On the other hand, harvesting of agricultural and forest residues can result in reduction of the land carbon stock [ 213 – 215 ], thus increasing GHG emissions [ 214 , 215 ]; however, most of the studies did not account for these changes. In the case of bioethanol from agricultural residues, other factors, such as the consideration of agricultural emissions [ 115 ], pre-treatment methods [ 130 ] and source of energy for the biorefinery [ 33 , 131 ], have a significant influence on GHG emissions.
While the LCA literature on second-generation bioethanol covers a wide range of feedstocks, the studies of biodiesel are more limited, focusing largely on three feedstocks: Jatropha, Camelina and UCO/tallow. As can be seen in figure 5 , the GWP of Jatropha and Camelina varies widely because of variations in the yield in different regions and differences in processes and assumptions, especially with respect to co-product allocation. For example, the yield of Jatropha oil seeds varies in different studies by a factor of 30, from 0.4 to 12 t ha −1 yr −1 [ 25 ]. The influence of allocation is also significant: using system expansion according to the US EPA methodology results in the GWP of Jatropha biodiesel of −88 g CO 2 eq. MJ −1 , while energy allocation as per the RED approach leads to GHG emissions of 15–20 g CO 2 eq. MJ −1 [ 20 ].
Although a majority of the studies of biodiesel from UCO report that GHG savings are greater than the RED reduction target of 60%, some studies also estimate that the GHG savings from this type of biodiesel are not sufficient to meet the target ( figure 5 ). This is due to some specific assumptions. For example, Intarapong et al . [ 90 ] considered pyrolysis for conversion of UCO to biodiesel, which is more energy-intensive than transesterification. Similarly, another study [ 47 ] assumed only a 5% biodiesel production yield, which is very low compared to more than 90% considered in other studies. Furthermore, Escobar et al . [ 171 ], who used consequential LCA methodology, considered indirect impacts, such as changes in the production of palm oil, soya bean and animal feed, found that the GWP of UCO biodiesel would be only 25% lower than that of diesel if ILUC and other indirect market impacts are considered.
(iii) Third-generation biofuels
In total, 27 LCA studies have estimated the GWP of third-generation algal biodiesel. However, they have all used very different approaches, process designs, system boundaries, methodologies and assumptions for feedstocks, nutrients and co-product management. As a result of the variation in these choices, the GWP differs widely between the studies, ranging from −2400 to 2880 g CO 2 eq. MJ −1 ( figure 6 ; electronic supplementary material, figure S6). These results would suggest that microalgae diesel can either reduce or increase GHG emissions significantly, relative to diesel, depending on the assumptions. However, a majority of the studies conclude that, at present state of development, algal biodiesel has higher life cycle GHG emissions than that of fossil diesel. The main reasons for higher emissions include lower algal yield [ 19 , 190 ] and high energy use in the cultivation, harvesting and drying stages [ 191 – 193 ].
Some studies which reported the high savings of GHG in comparison to diesel are based on the best-case assumptions that may not be feasible for large-scale implementation. These include the use of CO 2 from cement plants as a feedstock [ 216 ], cane sugar as a nutrient/feedstock [ 217 ] and recycling of nutrients from anaerobic digestion plants [ 194 ] or wastewater [ 190 ].
(b) Energy use
Various indicators have been used in LCA studies to quantify energy use in the life cycle of biofuels, including fossil energy consumption, primary, secondary or cumulative energy demand and net energy ratio [ 218 ]. However, many focused on fossil energy consumption, given that energy security and reducing dependence on fossil fuels are key objectives of national policies on biofuels, in addition to climate change mitigation.
As indicated in figure 7 , most of the studies estimate that the fossil energy consumption for first- and second-generation biofuels is below 0.5 MJ MJ −1 . However, there is a wide variation across different types of biofuel, ranging from 0.04 to 0.86 MJ MJ −1 for first generation and from −0.57 to 0.87 MJ MJ −1 for second-generation biofuels (see electronic supplementary material, figure S7), where negative values are due to energy credits for co-products, such as electricity and heat. These variations are due to several factors, including differences in feedstock productivity, agricultural practices, conversion technologies and allocation methods. The results are also affected by the assumption on the type of energy (biomass or fossil) used in the conversion process.

Fossil energy use in the life cycle of biofuels. Based on data from [ 17 – 19 , 40 , 47 , 48 , 54 , 55 , 57 , 58 , 60 , 68 , 76 , 77 , 80 , 85 , 89 , 92 , 93 , 95 , 96 , 98 , 108 , 113 – 115 , 120 , 121 , 129 , 132 , 133 , 136 , 138 , 139 , 143 , 144 , 147 , 152 – 154 , 156 , 159 , 161 , 162 , 164 – 167 , 169 – 171 , 175 , 176 , 179 – 181 , 187 , 190 – 192 , 195 – 199 , 204 , 206 , 208 , 219 – 223 ]. For the box plot legend, see electronic supplementary material, figure S1 and for the data used to plot this graph, see electronic supplementary material, figure S7. The value for third-generation biodiesel should be multiplied by 10 to obtain the actual value. ‘ A ’ refers to the number of LCA articles found in the literature and ‘ n ’ denotes the total number of analyses. (Online version in colour.)
The range of estimates for fossil fuel demand in the life cycle of algal biodiesel is even wider, ranging from 0.15 to 40.5 MJ MJ −1 ( figure 7 ; electronic supplementary material, figure S7). Like the GWP, the reasons for these variations are technological uncertainties and the diversity of potential feedstocks and production systems. However, most studies agree that algal biofuels are not energetically viable because of high energy requirements for pumping, dewatering, lipid extraction and thermal drying [ 141 , 191 , 224 – 226 ]. In general, algae cultivation in raceway ponds has lower energy demand than photo-bioreactors, with some studies suggesting that the former can have energy demand below 1 MJ MJ −1 [ 225 ].
(c) Water use
Water use in the production of feedstocks can be high, particularly for first-generation biofuels [ 5 , 227 , 228 ]. This is of concern where requirements for irrigation water for certain feedstocks might compete with water used for other purposes, such as food production. With increased agricultural biomass production for biofuels, the total global water consumption could increase significantly by 2050 [ 229 ] and, in areas that are already water stressed, additional water demand has a potential to substantially increase the overall environmental impacts of biofuels.
Water use is usually not included in LCA studies of biofuels, but there are numerous studies that have specifically focused on this aspect of biofuels production. Most of these provide a volumetric usage of water, such as the amount of green (soil moisture) and blue (surface) water. This is not sufficient to assess local environmental impacts of water consumption as these are highly dependent on the level of water availability in the local area and the specific characteristics of the hydrological cycle, even if the quantity used is the same for a particular product [ 230 ]. Furthermore, consideration of green water results in very large total water use for most agricultural crops. Since the local hydrological cycle may in reality be affected little by the use of green water in agriculture, inclusion of green water could overestimate the actual impact of water use for biofuels [ 231 ].
A more recent study [ 232 ] that assessed the water footprint of first-generation biofuels consumed in Europe suggests that blue water consumption of biofuels is very diverse, depending on the underlying crop and country ( figure 8 ). Bioethanol from sugar beet and wheat has lower water consumption because many countries produce crops using little or no irrigation. By contrast, the production of bioethanol from corn in Portugal consumes 86 m 3 GJ −1 . Furthermore, while no irrigation is needed to cultivate crops for biodiesel in the UK, Poland and Germany, in Spain, on average 90 m 3 of irrigation water is consumed to produce 1 GJ of crop-based biodiesel [ 232 ].

Blue water consumption for biofuels consumed in Europe. Based on data from [ 232 ]. Data labels represent the average values. (Online version in colour.)
As indicated in figure 8 , the average blue water consumption of bioethanol and biodiesel consumed in Europe is 3.3 m 3 GJ −1 and 1.9 m 3 GJ −1 , respectively—this is 40 and 60 times higher compared to their respective fossil alternatives. If regional water stress is taken into account, as opposed to just the volume of water consumed, biofuels have water footprints a factor of 55–246 higher than fossil fuels [ 232 ]. This is a result of a large share of water consumption in the production of biofuels occurring in relatively water-stressed countries.
The blue water consumption of algae-based biofuels depends on the geographical location, production systems and conversion routes [ 233 ]. For example, the blue water consumption for biofuels produced in a closed photo-reactor in the Netherlands is estimated at 8 m 3 GJ −1 , while it can be as high as 193 m 3 GJ −1 if algae are cultivated in open pond systems in Hawaii [ 233 ]. There is also a difference between dry and wet conversion, with the blue water consumption being higher for the latter.
(d) Biodiversity
Biofuels have the potential to contribute to loss of biodiversity through habitat loss and degradation, excessive nutrient load and other forms of pollution, over-exploitation and unsustainable use of land, as well as the cultivation of invasive alien species used as feedstocks [ 234 ]. The impact of biofuel production on biodiversity depends on the feedstock used and scale of production, management practices and LUC [ 235 ].
Intensive cultivation and use of agro-chemicals in the feedstock production for first-generation biofuels can create direct threats for local biodiversity [ 236 , 237 ]. LUC resulting from increased biofuel production exacerbates the risk of losing biodiversity through the direct loss of wildlife habitats, such as tropical rainforests [ 5 , 15 ].
Compared to first generation, second-generation biofuels are considered to have fewer negative impacts on biodiversity and could even have a positive effect [ 238 ]. For plant-based lignocellulosic feedstocks, this is because of their long growth cycle, low requirement for fertilizers and pesticides and less human intervention needed during the growth period. For example, large-scale SRC willow can provide benefits for some bird species, butterflies and flowering plants [ 239 ]. Furthermore, if degraded land is used for cultivation of feedstocks, the diversity of species might be enhanced. Similarly, perennial grasslands used for biomass production may enhance avian diversity, including migratory species. However, large energy crop monocultures can be detrimental to local biodiversity, particularly through habitat loss and the expansion of invasive species [ 5 ]. Eucalyptus, switchgrass and some Miscanthus species exhibit some traits of invasiveness [ 240 ].
The use of forest and agricultural residues as biofuel feedstock is expected to have a lower negative impact on biodiversity than dedicated energy crops [ 238 ]. Some of the impact on biodiversity associated with the use of forestry residues includes reduction in the amount of decaying wood—a niche habitat—and disturbance of wildlife caused by increased forest access. Excessive removal of agricultural residue from fields would also be a concern as it may increase weed growth, which could lead to the increased use of herbicides and thus affect local biodiversity.
For algal biofuels, the impact on biodiversity is uncertain. The large-scale cultivation of algae can bring significant risk to coastal biodiversity through invasion by algal species of coastal shallow ecosystems, such as mud flats, salt marshes, mangroves, sea grass bed and coral reefs [ 236 ].
Although the biodiversity loss is identified as one of the current key environmental concerns, it is only seldom included as an impact category in LCA studies of bioenergy systems [ 241 ]. Preserving biodiversity or avoiding biodiversity loss from biofuels is one of the criteria in sustainability certification schemes. However, biodiversity loss is difficult to measure and there are no standard ways of identifying and measuring systems that promote biodiversity.
(e) Other environmental impacts
The LCA studies on biofuels have used different impact assessment methods to estimate the other environmental impacts. Therefore, it is difficult to compare them and provide a meaningful range of impacts for different biofuels. Furthermore, the studies differ in scope, with some considering the cradle-to-gate and others cradle-to-grave system boundary. Results of the latter studies also depend on assumptions regarding the type of vehicle in which biofuels are used. Nonetheless, several studies suggest that reduction in GHG emissions from biofuels compared to fossil fuels is carried out at the expense of other impacts, such as acidification and eutrophication [ 32 , 54 , 76 , 81 , 83 , 88 , 121 , 129 , 139 , 148 , 216 , 242 – 244 ].
These two impacts are compared in table 2 for different feedstocks relative to fossil fuels. As can be seen, first-generation bioethanol has up to three times higher acidification and 3–20 times higher eutrophication. Similarly, first-generation biodiesel has 30–70% higher acidification and 3–14 times greater eutrophication than the fossil alternative. These impacts are largely due to the use of fertilizers and associated emissions of acid gases and nutrients to air and water.
Table 2.
Acidification and eutrophication of biofuels relative to fossil fuels.
| biofuel type | feedstock | acidification | eutrophication | source |
|---|---|---|---|---|
| bioethanol | corn | 1.4–3 | 4.4–20 | [ ] |
| wheat | 3 | 5 | [ ] | |
| sugar beet | 1.4–1.8 | 6–15 | [ ] | |
| sugarcane | 2 | 2.8 | [ ] | |
| biodiesel | rape seed | 1.3–1.7 | 3.1–5 | [ , ] |
| soya bean | 1.3–1.7 | 4–5 | [ ] | |
| palm oil | 1.3 | 14 | [ ] | |
| bioethanol | short rotation coppice | 0.45 | 1.2 | [ ] |
| switchgrass | 1.1 | 3.2 | [ ] | |
| straw | 1.6–3 | 2–3.6 | [ ] | |
| biodiesel | used cooking oil | 0.2 | 0.63 | [ ] |
| 1 | 1 | [ ] | ||
| biodiesel | algae | 2.6–3 | 2.1–3.2 | [ ] |
a The values represent the ratio of impacts from biofuels over fossil fuels and are dimensionless.
Lignocellulosic bioethanol from SCR performs better for acidification, but bioethanol from switchgrass and straw is worse than petrol for both impact categories. However, biodiesel from UCO has lower acidification and eutrophication than fossil diesel. These two impacts are also higher for algal biodiesel than for the fossil equivalent [ 216 ]. However, as mentioned earlier, the absence of full-scale plant data, large variability in production parameters and various assumptions lead to high uncertainty in the LCA estimates for algal biofuels [ 197 ].
4. Discussion
While many studies on biofuels have examined multiple scenarios and conducted sensitivity analyses, only a few have conducted comprehensive uncertainty analyses [ 63 , 197 , 245 ], demonstrating that the variability in results can be large. It is also clear from the findings discussed above that the outcomes of LCA studies are highly situational and dependent on many factors, including assumptions, data variation and gaps, models, methodology and software tools used. The outcomes of the study are also affected by the choice of allocation method, system boundaries and the cut-off criteria for auxiliary inputs. Especially in relation to GWP, there are significant uncertainties in models for estimating soil N 2 O emissions, direct and indirect LUC and the extent and duration of changes in soil and vegetation carbon stocks. The effects of these aspects on LCA results are discussed below.
The problems related to data availability and quality are inherent to LCA. It is always preferable to use site-specific inventory data for developing LCA models of biofuels. However, data availability is often limited, particularly for second- and third-generation biofuels that, along with the associated process technologies, are still under development. As such, the use of unrepresentative data or assumptions to fill data gaps becomes a source of uncertainty [ 29 ]. There is also a great deal of technical, spatial and temporal variability associated with agronomic practices, such as fertilizer inputs, cultivation intensities and yields, as well as with biofuel conversion processes. LCA results are highly sensitive to variations in crop yields, use of nitrogen fertilizer and energy sources for biofuel conversion processes. Original and measured field data are still scarce and many studies rely on secondary data. There is also a room for improvement in existing LCA databases and a need to develop better, open access databases with common assumptions. Many data in common usage are reportedly out-of-date and finding new data is often difficult and time-consuming.
(b) Methodological approaches
As mentioned earlier, ALCA and CLCA are different techniques that follow different methodologies and will normally have very different results that must be interpreted carefully based on the goal and scope of the study. For example, Searchinger et al . [ 246 ] found that using ALCA resulted in a 20% saving in GHG emissions from US corn ethanol compared to petrol. However, following a CLCA approach and considering the increase in output required by the US Energy Independence and Security Act lead to a 47% increase in emissions relative to petrol. This increase was related to LUC induced by higher prices of corn, soya bean and other grains as a consequence of the additional demand for corn for ethanol production.
As also mentioned earlier, CLCA is more suited for policy applications. However, the use of CLCA for policy is still in infancy and its application to biofuels is controversial and subject to criticism [ 29 , 247 ]. One of the main reasons is that consequential analysis is highly complex, being dependent on future projections, formulation of possible ‘what if’ scenarios and counterfactual circumstances, economic models of relationships between demand for inputs, price elasticities, supply and market effects of co-products, all of which can be highly uncertain [ 26 , 248 , 249 ]. There is also a real challenge in defining meaningful scenarios for how the world would develop with a biofuels policy or production in place. This is true for individual feedstocks all the way up to the economic and energy system models incorporated into CLCA studies. Therefore, caution should be exercised with the interpretation of CLCA results [ 249 ]. Furthermore, unlike ALCA, there is still no internationally agreed methodology for CLCA, making it difficult to carry out and compare different studies.
(c) Allocation methods
Allocation is one of the most controversial issues in LCA. Both system expansion and allocation are subject to shortcomings: for system expansion, the difficulty is to estimate various substitution effects (similar to the related consequential issues in CLCA), while different allocation methods produce very different results. For instance, allocation by mass could result in the majority of impacts being allocated to the co-products rather than the biofuel which is the main (economic) product, while allocation by product cost/price leads to changes in the estimates of environmental impacts over time with variations in costs/prices without any other changes in the system. Several studies considered more than one allocation approach and found that the results were highly affected. For instance, some authors [ 17 , 132 ] showed that biofuels had significantly lower environmental impacts when using system expansion instead of allocation. In some cases, system expansion can lead to the negative values, suggesting net savings in environmental impacts, including in GHG emissions. However, studies assessing uncertainty in LCA of biofuels showed that system expansion also results in higher uncertainties [ 63 , 65 ]. Other authors found that environmental impacts were higher if economic allocation was used instead of mass and energy allocation [ 250 ]. For some biofuels, the co-products are sufficiently substantial that choice of allocation procedure can tip the balance between net benefit and net impact.
(d) Emissions of soil nitrous oxide
Emissions of N 2 O arise from application of nitrogen fertilizer and decomposition of organic matter in soil. N 2 O is a potent GHG with a GWP 265 times higher than CO 2 [ 41 ]; hence, its emission can have a significant effect on the GHG balance of biofuels. The N 2 O emissions are particularly significant for first-generation biofuel crops since fertilization rates are larger for these than for second-generation biofuels from perennial energy crops, which are usually grown without fertilizers, except during the initial establishment of the crop [ 251 , 252 ].
LCA studies often use the ‘Tier 1’ methodology developed by the Intergovernmental Panel for Climate Change (IPPC) to estimate N 2 O emissions from fertilizers [ 253 ]. According to this method, 1–1.5% of nitrogen in synthetic fertilizer applied to crops is emitted as N 2 O [ 253 ]. Since in reality, the occurrence and level of N 2 O emissions depend on many factors, including soil characteristics and local weather following fertilizer application on the soil, the default IPCC emission factors represent an uncertain estimate [ 23 ]. For example, a study by Crutzen et al . [ 254 ] suggested that N 2 O emissions in feedstock production can be three to five times higher than those estimated based on the IPCC methodology. Inclusion of these variable N 2 O rates leads to dramatically different estimates of GHG emissions in the life cycles of biofuels. For instance, for corn ethanol, the nitrogen conversion of 5% instead of 1.5% could change its GHG savings relative to petrol from around 40% to zero [ 255 ].
Conversely, a recent study in the UK concluded that N 2 O emissions averaged across arable land in the UK are below those determined by following the IPCC guidelines [ 256 ]. Compared to the default IPCC emissions factor of 1% (of the amount of nitrogen applied), direct N 2 O emissions from soil related to the use of fertilizers on crops for first-generation biofuels were estimated to be, on average, 0.46% of the nitrogen applied. However, the study noted that any one instance of fertilizer application is subject to interacting effects of rainfall and soil type, such that fertilizer-induced emissions could also be larger than the default IPCC emission factors in the wetter regions of the UK. Thus, in summary, the estimates of N 2 O emissions are highly variable and uncertain and should be treated with caution when interpreting the results.
An increasing global demand for biofuels highlighted the potential for the competition for land between cropland and natural ecosystems. Early LCA studies on biofuels, which excluded LUC, concluded that first-generation biofuels, such as corn ethanol, had lower GWP than petrol [ 257 ]. However, when attempts were made to account for the LUC effects of the expansion of first-generation biofuels, these findings came under question [ 43 , 246 ]. Since then, several other studies have cast doubt on the ability of first-generation biofuels to meet mandatory GHG savings targets if LUC is involved [ 119 , 258 ].
From an LCA perspective, DLUC is relatively straightforward and easy to include in the assessment, although the uncertainty remains high. However, estimating ILUC related to biofuels remains difficult, complex and highly uncertain [ 259 , 260 ]. The latter is exemplified by that fact that estimates of GHG emissions from ILUC range widely, from very small to very large [ 261 ]. For instance, a study on the ILUC associated with US corn ethanol found that the ILUC emissions varied from 10 to 340 g CO 2 eq. MJ −1 [ 262 ]. For these reasons, the effects of ILUC and how to account for them in assessing the sustainability of biofuels are key areas requiring further research and consensus building [ 42 , 260 ]. Part of the challenge is constructing and analysing credible counterfactual scenarios. Another challenge is the economic (equilibrium) models used for consequential modelling [ 247 , 263 ] and the assumed yield-price elasticities for crops [ 26 ]. ILUC models make various assumption to estimate how much indirect change might be induced up to 20 years into the future under prescribed scenarios. Therefore, such estimates would only apply for the assumed conditions and must be interpreted with caution [ 264 ]. The lack of transparency in ILUC models, many of which are proprietary, is also problematic.
There is an ongoing question about how policymakers should respond to the growing evidence on ILUC from biofuel production. The blanket application of ‘ILUC factors’ according to feedstock type is unpopular as it offers producers no opportunity to improve the performance of their individual supply chains [ 265 ]. Moreover, there are many other drivers of LUC besides biofuels, such as demand for food and timber, urban development and infrastructure, leading some to argue that it is unfair to consider ILUC only for biofuels [ 247 , 266 ].
(f) Soil organic carbon
SOC is one of the largest carbon pools in the terrestrial ecosystem [ 267 ]. Its balance is affected because of agricultural activities and LUC. Depending on various soil characteristics and agricultural practices, soil can act as either a sink or a source of carbon emissions. Soils may lose SOC by mineralization through cultivation, emitting CO 2 to the atmosphere. Alternatively, SOC may increase through cropping or from repeated addition of crop residues or organic manures [ 268 ].
When biomass is left to decay in the soil, a part of the carbon in the biomass is sequestered into soil. Therefore, assuming biomass would have otherwise been left to decay in the soil, harvesting it decreases SOC and this may affect significantly the GHG balance of a biofuel [ 269 , 270 ]. For example, a study that included the effects of the removal of corn residue across the US corn belt concluded that the GWP of corn stover ethanol may exceed that of conventional petrol [ 271 ]. Another study on wheat-straw ethanol suggested that there is only a 30% probability that its GHG emissions will be 35% lower than that of petrol if SOC changes are included in the analysis [ 272 ]. A study on sugarcane ethanol claimed that the GHG balance of sugarcane ethanol could be significantly higher if the impacts on SOC from pre-harvest burning were considered [ 210 ]. The burning of biomass in the field, which is often carried out prior to a sugarcane harvest to help manual harvest, means that far less crop residues are left on the land to be incorporated into the soil.
Changes in SOC can also have a major influence on GHG emissions from LUC associated with biofuel feedstock production [ 267 , 273 ]. For example, reversal of grassland, woodland and perennial crops back to arable lands could reduce soil carbon by 0.6–1.7 t C ha −1 yr −1 , which would be emitted to the atmosphere as CO 2 (2.2–6.2 t ha −1 yr −1 ). On the other hand, cultivation of perennial energy crops, such as SRC and Miscanthus , could sequester CO 2 from the atmosphere into the soil at the rate of 2.2 t CO 2 ha −1 yr −1 [ 267 ]. However, the sequestration potential is very site-specific and highly dependent on former and current agronomic practices, previous land use, as well as climate and soil characteristics [ 17 , 40 , 267 , 274 – 276 ]. Therefore, quantifying changes in SOC storage is an important factor in estimating GHG emissions of biofuels [ 277 ]. However, most LCA studies do not account for potential SOC changes from biomass cropping systems. This is probably due to inherent complexity of soil science, the high degree of intra- and inter-site variability, substantial data uncertainties and the challenges of linking biomass feedstock supply to specific soils [ 46 ]. Furthermore, there is no consensus in LCA on how to account for SOC change of agricultural activities and delayed GHG emissions [ 278 ]. However, the work on developing models to estimate SOC emissions related to biofuels is ongoing [ 273 – 275 ].
(g) Biogenic carbon
In the context of biofuels, the term biogenic carbon refers to CO 2 that is sequestered from the atmosphere during the growth of feedstocks and subsequently released during the combustion of the biofuel. ‘Carbon neutrality’ is achieved when CO 2 sequestered and subsequently released are in balance. However, carbon neutrality cannot be claimed if there is a potential imbalance or a time delay between the amount of CO 2 taken up during feedstock growth and the amount released through biofuel production and use. Since many bioenergy products—including annual crops and perennial grasses—have relatively short lifespans, carbon neutrality is commonly assumed in LCA standards and regulations. Hence, most LCA studies of biofuels assume that biogenic CO 2 emissions, both from end-use combustion and the burning biomass to produce energy for conversion processes, are fully balanced by CO 2 uptake during feedstock growth. While this assumption is reasonable for fuels from annual crops and perennial grass feedstocks, it is open to challenge in relation to biofuel production from feedstocks with harvest cycles of more than a few years—such as longer-lived lignocellulosic feedstocks from forestry [ 26 , 279 ]. For such feedstocks, it is important to consider the balance of carbon sequestered during feedstock growth versus that which is emitted during biofuel production and use, together with the overall time profile of biogenic carbon storage, emission and re-sequestration [ 279 ].
Different approaches to account for the temporal impact of carbon emissions are suggested in the literature; for example, carbon payback period, carbon discounting and time-integrated accounting of biogenic carbon [ 279 , 280 ]. Where accounting for the carbon storage in other, more long-lived bio-based products is required, there are various standards and methods [ 46 ] and these contain significant procedural differences. For example, GHG Protocol [ 281 ], PAS 2050 [ 282 ] and ISO 14067 [ 283 ] require reporting of emissions and removal of GHG emissions from biogenic carbon sources, while regulations the RED [ 8 ] and RFS [ 7 ] do not require such reporting. Furthermore, the time between the production of the product (storage of biogenic carbon) and its end of life (release of biogenic carbon), referred to as ‘delayed emissions’, varies among the standards. For instance, in PAS 2050 [ 282 ], all emissions that occur within a 100-year period are quantified and treated as if they occurred at the beginning of the time period. By contrast, ISO 14067 [ 283 ] makes a distinction between emissions released within and after the first 10 years.
(h) Other environmental impacts
Production and use of biofuel generate emissions of various air pollutants, including particulate matter (PM), carbon monoxide (CO), nitrogen oxides (NO x ), hydrocarbons and volatile organic compounds (VOCs). Unburned hydrocarbons, VOCs and NO x are precursors for the formation of summer smog and ground-level ozone. These pollutants are associated with increased morbidity and mortality from cardiovascular and respiratory diseases and certain cancers [ 284 , 285 ]. Air quality modelling studies show that life cycle emissions of some pollutants may be higher for biofuels when compared with fossil fuels, largely resulting from the emissions associated with feedstock production and biofuel processing [ 284 , 286 ]. For example, in the case of sugarcane ethanol in Brazil, burning of straw in fields is the common practice in certain areas and is the predominant source of PM [ 284 , 286 ]. Studies on health impacts of sugarcane ethanol in Brazil suggest that there is strong evidence that burning straw in sugarcane fields causes substantial respiratory diseases, such as asthma and pneumonia, in sugarcane fieldworkers and local populations [ 284 , 286 – 289 ]. These effects are often ignored in LCA studies.
In cradle-to-grave LCA studies, assessing impacts of vehicular exhaust emissions is another challenge as they are affected by many different parameters, including the type of engine and how it is run (the operational drive cycle), vehicle age and maintenance, the quality of the base fuel and exhaust after treatment [ 290 ]. Vehicular exhaust emissions of bioethanol blends vary with blend strength. However, in general, lower bioethanol blends (E5–E15) have lower CO and PM emissions compared to petrol [ 290 , 291 ]. Beer et al . [ 291 ] suggest that lower PM emissions from low-ethanol blends used in spark-ignition vehicles have slight health benefits over petrol. However, they lead to significantly higher emissions of acetaldehyde, which is one of the precursor VOCs involved in ground-level ozone formation. Similarly, higher ethanol blends (E85) lead to comparable, or slightly lower, levels of PM, NO x and CO emissions than petrol, but 5–10 times higher acetaldehyde emissions [ 290 , 292 , 293 ].
Compared to fossil diesel, biodiesel has generally lower exhaust emissions of PM, CO, hydrocarbons and VOCs, but higher NO x emissions [ 294 , 295 ]. These differences are small for 5–20% biodiesel blends and would lead to negligible or non-measurable impacts on air quality [ 294 ], but increase with higher blends [ 290 ]. On the other hand, Larcombe et al . [ 296 ] argue that, despite having lower PM emissions, biodiesel exhaust emissions could potentially be more harmful to human health because of higher proportion of ultra-fine particles (less than 100 nm diameter) compared to diesel exhaust. This is due to the fact that smaller particles remain suspended in the air for longer, are more easily inhaled and are able to penetrate more deeply into the lungs. However, other assessments on the potential human health implications of biodiesel suggest that the use of biodiesel fuel blends compared to fossil diesel results in minimal changes in health impacts [ 294 , 295 ]. Thus, the topic of human health impacts from biofuels remains open to debate, requiring further research and evidence.
Besides air pollution, production of liquid biofuels could affect human health directly through water and soil pollution and occupational hazards [ 284 ]. However, these effects are scarcely discussed in the literature and should be explored further to understand whether there are risks that need to be addressed.
5. Conclusion and recommendations
LCA is widely used as a tool to estimate GWP and other environmental impacts of biofuels. However, as evident from this review, the estimates vary widely among the studies owing to a wide range of methodological choices in LCA and various uncertainties. Despite this, the existing evidence base is instructive. Firstly, it shows that, if no LUC is involved, first-generation biofuels can—on average—have lower GHG emissions than fossil fuels, but GHG savings for most of the feedstocks are not sufficient to meet those required by the RED. Secondly, in general, second-generation biofuels have a greater potential than first generation to reduce GHG emissions, again provided there is no LUC. However, the development of second-generation biofuels will take time and is likely to depend on the continued support of first-generation fuels to give the industry the confidence to invest. Thirdly, it is also clear that, at present state of development, third-generation biofuels from algae are unlikely to make a contribution to the transport sector as their GHG emissions are higher than those from fossil fuels. Moreover, they are unproven and expensive to produce and, as such, the algal feedstock will continue to be restricted to high-value markets, such as cosmetics and dietary supplements.
LCA is a complex tool that lies at the interface between science, engineering and policy. Despite this complexity, it is often perceived as a tool that can give a definitive answer to multifaceted questions. As the findings in this review demonstrate clearly, there are no definitive answers. Even focusing only on the GWP of biofuels—one of the main drivers for their development—brings with it a host of uncertainties. Moreover, almost every aspect related to biofuels is dynamic in nature across different scales, which adds to the complexity. Examples include changes in soil carbon content over time (micro-scale); time needed to replace vegetation used as feedstock for biofuels (meso-scale); and development of global biofuel supply chains (macro-scale). Considering these dynamic aspects and their interconnections presents a considerable challenge. There are also significant uncertainties in the models for estimating direct and indirect LUC, changes in SOC stocks and N 2 O emissions. It is important to recognize these limitations and interpret the results accordingly.
In addition to the environmental impacts, there are many other sustainability issues that must be considered when assessing the sustainability of biofuels. These include: costs of production and competitiveness with fossil fuels; food, energy and water security; employment provision; rural development; and human health impacts. It is essential that the sustainability aspects of biofuels be evaluated on a life cycle basis across full supply chains to avoid shifting the burdens from one part of the life cycle or supply chain to another. It is also important to note that LCA and wider sustainability assessments are of little use if the results cannot be trusted. Therefore, strong auditing of biofuel supply chains is vital to prevent negative socio-economic effects as well as to ensure traceability of the fuels and to mitigate the risk of fraud. Moreover, improving transparency, data availability and sharing are key if LCA is to be trusted and useful for policy. This could be achieved through development of open national and global databases, in a similar way that national inventories have been developed for GHG reporting under the Kyoto Protocol. It is also important to ensure that the data and models from different disciplines that are used in LCA preserve reasonable levels of transparency, rigour and robustness to avoid misuse and misinterpretation.
ALCA studies, which account for the direct impacts, should follow the ISO 14040 and 14044 standards more rigorously. For CLCA, both methodological and practical aspects need improvements. For the former, further work is required towards the standardization of CLCA methodology. As part of that, there is a need to improve development of counterfactual (what if) scenarios and ILUC models. Involvement of multiple stakeholders can help to build consensus on the definition of the scenarios and to improve the transparency of ILUC models, their assumptions and the associated uncertainty. In addition to improving the CLCA methodology, much work is required in its application in practice. Specifically, there is a need to validate ILUC models with empirical evidence; empirical methods to test alternative hypotheses also require attention. Further work is also needed on the development of models and empirical evidence of changes in soil and plant carbon stocks as well as emissions of nitrous oxide related to the application of fertilizers. Research is also needed on estimations of biogenic carbon, particularly changes in the forest carbon stock that may be affected by an increase in biofuels demand.
It is also important to take into account that biofuels do not exist in isolation but are part of much wider systems, including energy, agriculture and forestry. Like other production systems with which they interact, biofuels impact on various ecosystem services, such as land, water and food. It is, therefore, essential to take an integrated, systems view to developing future policy to ensure that biofuels are not disadvantaged relative to other sectors or that progress made in this sector is not undone by unsustainable practices in others. Analysis and, ultimately, policies based on ecosystem services and natural capital at a landscape level are needed to make the best overall use of land. This would, in turn, optimize ecosystem services, such as carbon storage, biodiversity, reductions of agricultural run-off and increases in water quality and flood risk management. Complete value chains rather than single bioenergy products should be analysed together to understand the interactions across sectors and land uses with the goal of identifying opportunities where collective benefits can be realized.
Supplementary Material
Acknowledgements.
This review was originally carried out as part of the Royal Academy of Engineering study on Sustainability of liquid biofuels and subsequently fully updated as part of a Research Councils UK project (EP/K011820/1). The suggestions and comments by members of the Academy's expert working group, the Academy's Engineering Policy Committee as well as external stakeholders are gratefully acknowledged.
Data accessibility
Authors' contributions.
H.K.J.: literature review, analysis and presentation of results, paper writing. A.C.: literature reivew, paper drafting. A.A.: conceptualization, supervision, paper writing.
Competing interests
We declare we have no competing interests.
The study was funded by Department for Business, Energy and Industrial Strategy (BEIS), Department for Transport and Research Councils UK.
Aboriginal Heart Health Grants
Fostering Aboriginal innovation and building capacity in cardiovascular health research.
The Aboriginal Heart Health Grants fund high impact Aboriginal-led research. The $5 million in funding is part of the Cardiovascular Research Capacity Program, the NSW Government’s $150 million investment over 10 years into cardiovascular research.
The Aboriginal Heart Health Grants aim to:
- improve cardiovascular health outcomes for Aboriginal peoples
- increase the number of targeted Aboriginal cardiovascular research projects being undertaken in NSW, and
- build the capacity of Aboriginal communities and researchers in cardiovascular focused research.
Aboriginal Heart Health Grants 2024 – Expressions of Interest now open
EOI Applications close 13 December 2024 at 5pm AEDT.
Information webinar: 16 October 2024, 12pm – 1pm – Register here
Aboriginal Heart Health Grants have a three-year duration.
There are two funding streams:
Stream 1: Maximum grant of $750,000 to conduct a multidisciplinary project, which has undertaken a consultation process with Aboriginal communities to identify a need/priority and solution/ innovation/strategy to be tested in the cardiovascular space. Feasibility studies can be included in this stream. Research in this stream can use quantitative and/or qualitative methods.
Stream 2: Maximum grant of $250,000 for early-stage developmental/exploratory research project, where a consultation process is required with Aboriginal communities to determine an identified priority/need, better understand its causes, and suggest solution(s)/innovation(s)/strategy(ies) to address the need in the cardiovascular space. This type of research should provide the groundwork for a feasibility study. Research in this stream can use quantitative and/or qualitative methods e.g. yarning interviews or circles with community or research participants.
The grant requested should be appropriate for the type, stage and scale of research proposed.

‘A Heart for Health’ by Carissa Paglino. More details in the artist bio below. “The interwoven lines in the artwork show the connection between heart health and Aboriginal and Torres Strait Islander people as well as cardiovascular research. Various aspects of cardiology and the heart anatomy, such as veins, arteries and blood cells, are represented in the piece. The artwork calls for healthier communities, better heart health research and improved health outcomes. The colours are reflective of the circulatory system.”
Who can apply
All projects must be led by a Lead Chief Investigator who identifies as Aboriginal or Torres Strait Islander.
Applications are encouraged from Aboriginal Community Controlled Organisations, Aboriginal Medical Services, Aboriginal peak bodies, local health districts, specialty health networks and other public health organisations.
Researchers from universities, medical research institutes and not-for-profit organisations are also eligible to apply.
Support for applicants
Information webinar: 16 October 2024, 12pm – 1pm – Register h ere
The Office for Health and Medical Research will host an information webinar to help applicants understand NSW Health requirements and gain advice on how to prepare a high quality EOI. There will also be an opportunity for applicants to ask any questions about the grant round.
Registrations for Sax Support: 18 October 2024 – Register here
The Sax Institute is offering two optional services for Aboriginal Heart Health Grant applicants:
- a concierge service that links applicants to academic partners or researchers with appropriate methods expertise for the project
- support with the development of applications.
Further information about the Sax Institute support service is in the Grant Guidelines on page 12.
| Call for Expressions of Interest opens | September 2024 |
| Information webinar for Expressions of Interest | 16 October 2024 |
| Registration for Sax Support close | 18 October 2024 |
| Expressions of Interest close | 13 December 2024 (5pm AEDT) |
| Applicants notified of Expressions of Interest outcome Full Applications open | 23 May 2025 |
| Information webinar for Full Applications | 29 August 2025 (5pm AEST) |
| Full Applications close | 11 June 2025 |
| Applicants notified of Full Application outcome | By 19 December 2025 |
Depending on the number of expressions of interest received, this timeline may be shortened.
Application and submission process
Aboriginal heart health grant guidelines 2024, stream 1 expression of interest form.
DOCX - 2 MB
Stream 2 Expression of Interest Form
NSW Health invites eligible researchers to submit an Expression of Interest for Aboriginal Heart Health Grants.
The Application process includes two stages:
Stage 1: Expression of Interest
- All EOIs should be submitted by email to [email protected] by 5pm (AEDT) on 13 December 2024 .
- Applicants must use the Expression of Interest Form above and attach any supporting evidence. Please submit both a word and PDF version of the application.
- All email applications will receive an email to acknowledge receipt within 72 business hours. It is the applicant’s responsibility to follow up if no acknowledgement email is received.
- The maximum email size is 20MB . Larger emails will be rejected by the NSW Health server and you may not be notified that the email has been rejected.
Any queries regarding Aboriginal Heart Health Grants may be directed by email to: [email protected] .
Stage 2: Full application
Detailed instructions will be provided to applicants successful at EOI stage.
Further information around the application submission process is in the Grant Guidelines on page 14.
Additional resources
Researchers may find the following resources useful in developing their application.
Aboriginal Health Ethics Guidelines Key Principles – AHMRC
AIATSIS Code of Ethics for Aboriginal and Torres Strait Islander Research
AIATSIS Engaging with traditional owners
AIATSIS Principles for engagement in projects concerning Aboriginal and Torres Strait Islander peoples
Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities | NHMRC
NSW Aboriginal Health Plan 2024‑2034 – Aboriginal health
NSW Ministry of Health: Quick-Guide-on-Undertaking-Appropriate-Aboriginal-Health-Research
Pathways into Research Toolkit – Lowitja Institute
Carissa Paglino was born and raised in the city of Newcastle. She is a proud descendant of the Wanaruah nation. She has always had a great passion for art and design, with her art being featured in several exhibitions and winning various awards. From 2004 to 2015, she worked as a Graphic Designer for Miromaa Aboriginal Language & Technology Center. She has pursued a full-time career as a Freelance Graphic Designer since 2015. Although she practices many forms of art, her professional forte is contemporary Aboriginal Graphic Design. She designs symbols and motifs that are influenced by traditional Aboriginal art and present them in bold, new ways, creating her own unique style.
Updated 2 days ago
Popular Searches:
- clinical trial expertise
- clinical trial support
- clinical trials
- collaboration opportunities
- commercialisation
- early phase clinical trials
- establish a clinical trial
- ethics and governance
- facilitate resolution
- good clinical practice
- human research ethics committee
- key contacts
- motor neurone disease
- news articles
- problem solving
- publications
- solution service
- standard operating procedures
- start a clinical trial
- translational research
Projects Align with Administration’s New Goal of Decarbonizing the Aviation Sector by 2050
WASHINGTON, D.C. – As part of a White House roundtable to launch the Sustainable Aviation Fuels (SAF) Grand Challenge to decarbonize the aviation sector by 2050, the U.S. Department of Energy (DOE) today announced $64.7 million in funding for projects focused on producing cost-effective, low-carbon biofuels. These investments will advance technologies to create replacements for petroleum fuels used in heavy-duty forms of transportation, like airplanes and ships, and accelerate America’s path to a net-zero emissions economy by 2050.
“Decarbonizing transportation – particularly planes and ships that are difficult to electrify – is an essential part of the path to a net-zero carbon future,” said Secretary of Energy Jennifer M. Granholm. “These investments mobilize industries to join this effort, which will create new, good-paying jobs across the biofuels, chemical, and agricultural supply chains and boost economic activity in rural economies.”
As part of the SAF Grand Challenge, DOE also signed a memorandum of understanding with the U.S. Department of Transportation and U.S. Department of Agriculture to collaborate on the needed research, development, and demonstration (RD&D) to reach the goals of supplying at least 3 billion gallons of SAF per year by 2030 and sufficient SAF to meet 100% of aviation fuel demand – currently 35 billion gallons per year – by 2050.
These efforts seek to cut carbon emissions from the aviation and shipping industries, which – because of their size – are more challenging to electrify. Biofuels, which are produced by converting the renewable carbon from recently living organic materials like crop waste, food waste, and algae into a liquid fuel, can serve as a low-carbon equivalent to fossil-based fuels such as gasoline, jet, and diesel fuel.
“I’m thrilled that the Department of Energy has awarded a grant to Lignolix, a company with roots in Professor Thomas Epps’ lab at the University of Delaware, to support their work to transform plant waste into clean fuels and sustainable materials – another success story out of Delaware's entrepreneurship community,” said U.S. Senator Chris Coons (D-DE).
“We must cut emissions in aviation and commercial shipping if we’re going to meet our net-zero goals,” said U.S. Senator John Hickenlooper (CO). “This DOE research grant will put Colorado’s National Renewable Energy Research Lab at the center of those efforts and at the forefront of biofuel research.”
“I am proud that the National Renewable Energy Laboratory (NREL), located in my district, is helping lead the way to reduce emissions from the aviation industry. NREL’s work will help lower the price of cellulosic sugars, which can be used to make sustainable aviation fuel or other biofuels and bioproducts,” said U.S. Representative Ed Perlmutter (CO-07). “By reducing the price of biofuels, we can incentivize the use of more sustainable options in the aviation industry and help accelerate our path to a cleaner future.”
“Biofuels are a critical component of our nation’s energy portfolio and our agriculture economy,” said U.S. Representative Rodney Davis (IL-13). “They help reduce emissions while promoting American energy independence. This DOE grant will assist ADM and others in developing new innovations in the biofuels sector. I look forward to watching ADM utilize this grant and continuing to partner with industry stakeholders to further promote the production and use of biofuels.”
The 22 selected projects target high-impact bioenergy technology RD&D to bolster foundational knowledge and scale up systems to produce low-carbon biofuels at lower costs. Among the projects are:
- National Renewable Energy Laboratory (Golden, CO) will lower the cost and carbon intensity of producing a highly fermentable sugar from corn stover. (Award amount: $2,800,000)
- Archer Daniels Midland (Decatur, IL) will couple isobutanol (a precursor for sustainable aviation fuel) fermentation with a membrane separations system – reducing energy used in the separation process by 50%. (Award amount: $3,466,844)
- Alder Energy (Charleston, SC) will convert miscanthus, a highly promising biomass crop, to SAF through their advanced pyrolysis oil technology, a process that utilizes heat, pressure, and solvents to deconstruct the miscanthus into oils for conversion to SAF. (Award amount: $3,000,000)
- D3MAX (Grand Forks, ND) will design a pilot plant to validate technology for first generation ethanol plants to produce ethanol from corn stover, which is then converted to SAF. (Award amount: $499,988)
- T2C Energy (Pinellas Park, FL) was selected to design a demonstration scale plant that converts waste landfill gas to SAF or renewable diesel. (Award amount: $533,619)
- AVAPCO (Thomaston, GA) will demonstrate a production process for clean, affordable cellulosic sugars, which are derived from agricultural or woody waste residues and can help reduce greenhouse gas emissions for a variety of products including SAF, bioplastics, and biopolymers. (Award amount: $2,800,000)
- Quasar Energy Group (Independence, OH) will use an anaerobic digestor to convert food waste to SAF precursors. The microbes that digest the food waste produce a type of chemical called a volatile fatty acid, which can be converted to SAF. (Award amount: $3,500,000)
These investments are administered by DOE’s Bioenergy Technologies Office (BETO), which is focused on developing technologies that convert domestic biomass and other waste resources into low-carbon biofuels and bioproducts. BETO is increasing its emphasis on partnering with industry to demonstrate technologies at large scale in recognition of the urgent need to reduce risks and scale-up SAF production.
For more information about the selected projects, visit BETO’s website .

IMAGES
VIDEO
COMMENTS
Biofuels are fuels produced from hydrocarbon-rich living organisms (biomass) — such as plants or microalgae — by thermal, chemical or biochemical conversion processes. As with fuels, biofuels ...
DOE Office of Science: Contributions to Biofuel Research. The Department of Energy Office of Science, Biological and Environmental Research program provides support for research on advanced biofuels and bioproducts developed from non-food lignocellulosic plant biomass. These efforts take place in the four DOE Bioenergy Research Centers.
However, scientific research has shown that various biofuels differ massively in the greenhouse gas balance when compared with petrol despite the potential advantages. Based on the techniques used for processing the fuel and production of the feedstock, certain crops may also emit more greenhouse gases than fossil fuels do [52], [53].
Cover art by BiofuelResJ. ©2024. Biofuel Research Journal (BRJ) is a leading, peer-reviewed academic journal dedicated to publishing high-quality research on biofuels, bioproducts, and related biomass-derived materials and technologies. BRJ is an open-access online journal and completely free-of-charge, aiming to advance knowledge and ...
The third and fourth generation also provides a better option for generating biofuels, but the research expedition is time-consuming and requires expert handling. One of the significant limitations of biofuel production is its implication on energy, water and food nexus. The growing demand and utilization of biofuels has developed a direct ...
Biofuels represent a promising departure from conventional fossil fuels, presenting viable remedies for both energy security and environmental apprehensions. This review intricately examines the various realms of biofuels, encompassing their historical progression, present status, obstacles, and outlook. Commencing with an in-depth exploration of their historical antecedents and developmental ...
Biofuels are fossil fuel alternatives produced from agricultural biomass or other organic matter; considered sustainable, eco-friendly, and bioeconomic biofuels have come up as a topic of discussion for over a decade. Their practical use depends on the production methods, low cost-technology implementation, and substrate used.
The four generations of biofuels. One alternative to fossil fuels are biofuels, which originate from organic matter and therefore can be regrown and are termed renewable. Biofuels emit less GHGs and are in general more eco-friendly (non-toxic, sulfur-free, biodegradable) than their fossil fuel predecessors [5].
Inexpensive, carbon-neutral biofuels are finally possible. February 15, 2024. Jules Bernstein, UC Riverside. Credit: iStock/Dudaeva. When it comes to making fuel from plants, the first step has always been the hardest — breaking down the plant matter. A new study finds that introducing a simple, renewable chemical to the pretreatment step can ...
Biofuel has emerged as an alternative source of energy to reduce the emissions of greenhouse gases in the atmosphere and combat global warming. Biofuels are classified into first, second, third and fourth generations. Each of the biofuel generations aims to meet the global energy demand while minimizing environmental impacts.
Research is also needed on estimations of biogenic carbon, particularly changes in the forest carbon stock that may be affected by an increase in biofuels demand. It is also important to take into account that biofuels do not exist in isolation but are part of much wider systems, including energy, agriculture and forestry.
Innovations in biofuels research are leveraged today in transportation technologies and infrastructure. The clean energy future is enabled by the U.S. Department of Energy (DOE) Bioenergy Technologies Office (BETO) investment of $255 million enacted in Fiscal Year 2021 for biofuels research and development (R&D). With over 300 active R&D ...
Carbon-efficient biofuel production. An important aspect of microbial production of biofuels is the conservation of carbon that is converted from biomass substrates to fuel products. A challenge ...
Nowadays, biofuel is a tremendous research area in which various advanced technologies used for biofuel production have also been developed, but fossil fuels cannot be completely replaced, and numerous integrated approaches in biological and genetic engineering are still important to standardize biofuel production at a marketable scale ...
ETHANOL. Ethanol (CH3CH2OH) is a renewable fuel that can be made from various plant materials, collectively known as " biomass.". Ethanol is an alcohol used as a blending agent with gasoline to increase octane and cut down carbon monoxide and other smog-causing emissions. The most common blend of ethanol is E10 (10% ethanol, 90% gasoline ...
Predominantly, biofuels are produced from photosynthetic organisms. such as photosynthetic bacteria, micro- and macro-algae and vascular land plants. The. primary products of biofuel may be in a ...
Biofuel technology has evolved through several generations of significant advancements. The predominant problem with first-generation biofuels is that they are derived from food crops (e.g., corn and sugar cane), which require fertilization, water, and soil, and thus directly compete with food production. ... This is a novel research area that ...
Finding biofuel pathways . Co-Optima's research builds on the goal to identify and understand bioblendstocks, or biofuel. Biofuel is produced from biomass — organic materials including plants, agricultural waste and wet waste. Biofuel can be blended with conventional fuel to reduce emissions and improve fuel and engine performance.
Whether biofuels represent a sustainable innovation, a creative alternative, or a gold rush, very much depends on our perception of power and change with regard to sustainability. This article provides an overview of existing understandings of power in the research on biofuels, including positive perceptions that often lead to more optimistic evaluations of biofuels. It exposes the diversity ...
Biofuel is a fuel that is produced over a short time span from biomass, rather than by the very slow natural processes involved in the formation of fossil fuels such as oil. Biofuel can be produced from plants or from agricultural, domestic or industrial biowaste. [1] [2] Biofuels are mostly used for transportation, but can also be used for heating and electricity.
While fuels for transportation are the primary targets of biofuels research, many products today -- like plastics and paints -- are also made from petroleum, which can be replaced with new forms of biofuel. Photo courtesy of the California Center for Algae Biotechnology. 4. Robots are going to improve the plants used to make fuel.
About Bio-Techne. Bio-Techne Corporation (NASDAQ: TECH) is a global life sciences company providing innovative tools and bioactive reagents for the research and clinical diagnostic communities. Bio-Techne products assist scientific investigations into biological processes and the nature and progress of specific diseases.
Research is also needed on estimations of biogenic carbon, particularly changes in the forest carbon stock that may be affected by an increase in biofuels demand. ... Environmental life cycle assessment of bio-fuel production via fast pyrolysis of corn stover and hydroprocessing. Fuel 131, 36-42. ( 10.1016/j.fuel.2014.04.029) [Google Scholar ...
Stifel Nicolaus analyst Benjamin Burnett maintained a Buy rating on Cabaletta Bio (CABA - Research Report) today and set a price target of $26.00.The company's shares closed yesterday at $3.95
A marine research engine at the U.S. Department of Energy's (DOE) Oak Ridge National Laboratory (ORNL) is providing scientists with valuable insights into biofuel design for large ocean-going vessels (OGVs) in a multi-lab project focused on reducing total life-cycle carbon emissions from this vital transportation sector. When the International Maritime Organization (IMO) announced in 2023 ...
Research in this stream can use quantitative and/or qualitative methods e.g. yarning interviews or circles with community or research participants. The grant requested should be appropriate for the type, stage and scale of research proposed. 'A Heart for Health' by Carissa Paglino. More details in the artist bio below.
WASHINGTON, D.C. - As part of a White House roundtable to launch the Sustainable Aviation Fuels (SAF) Grand Challenge to decarbonize the aviation sector by 2050, the U.S. Department of Energy (DOE) today announced $64.7 million in funding for projects focused on producing cost-effective, low-carbon biofuels. These investments will advance technologies to create replacements for petroleum ...