- 501/514, Crossroad Building, Bhumkar square, Wakad, Pune 411057
- Mon - Sat 10.00 - 06.00

A leading global contract research organisation in Asia

NUTRACEUTICAL
Clinical research, make clinically, proven product.

Global Reach of MPREX
Claim validation of nutraceutical through clinical research.
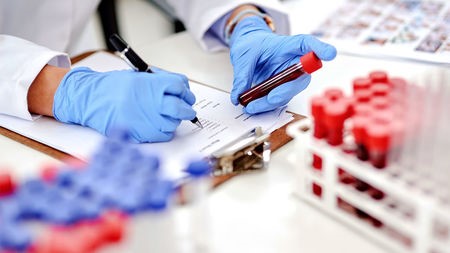
Claim validation for nutraceuticals involves rigorous scientific investigation to substantiate the health-related assertions associated with these products. It is important to validate nutraceuticals to guarantee that the products are of the highest caliber, fulfill all requirements, and are produced with the right procedures. Validation of the nutraceuticals helps to reduce the risks related to the products and processes. It is well accepted by the patients and clients if the product is validated.
Your benefits
- Regulatory compliance
- ASCI support
- Dosage performance assessment
- Proof for safety and efficacy
- Market Differentiation
- E-Com Support
- Consumer Confidence
- FSSAI Claim

Years Of Experience
About mprex, our infrastructure – reach and credibility, studies completed, investigators & sites, publications, countries presence, we offer services, we provide assistance in various directions, clinical trials.
Our clinical trial services range from Pilot studies, Proof of concept studies to large Multicentric clinical studies for evaluating efficacy and safety in humans. We offer customized study designs to validate all product-related health and marketing claims.
PHARMACOKINETIC STUDIES
We conduct bioavailability and pharmacokinetic (PK) studies for herbal actives, natural ingredients, protein supplements, etc. These studies can substantiate product claims and differentiate products from the non-standardized competition.
PRECLINICAL SERVICES
We are conducting preclinical and toxicity studies in GLP and non-GLP preclinical laboratories. We conduct proof of concept to establish the product’s mechanism of action. We assess pharmacokinetics and pharmacodynamics to validate efficacy and evaluate safety parameters to ensure regulatory compliance.
PROJECT MANAGEMENT
Our team offer services to ensure efficient planning, execution and completion of research projects. It involves coordinating timelines, managing budgets, maintaining communication to meet client objectives, regulatory requirement and quality standards.
IN VITRO STUDIES
In our laboratory, the products efficacy and safety is tested with outmost accuracy to provide better results. Our rigorous protocols and state-of-the-art equipment guarantee precise data collection for reliable analysis.
COSMETIC & DERMATOLOGY STUDIES
We conduct clinical studies in dermatology for dermatitis, psoriasis, acne, skin allergies, rashes, baby products, etc. We conduct clinical trials in anti-aging and other claim-substantiating studies for skin and hair care products.
Golden Journey Of MPREX
The inception of MPREX GROUP offering Site Management Services for clinical trials.
Added product development services, pathology labs and began specializing in Nutraceutical research.
We unfolded our wings and introduced preclinical, toxicity, in-vitro study services
The Mprex Foundation was registered and clinical trial sites expanded in variety of therapeutic areas.
Began conducting clinical trials for overseas clients as part of global expansion strategy for development.
Marked footprint in cosmetic clinical trials, and developed a skin and hair lab. Started Mprex Biotech, delivering research in the agriculture industry.
Developed Bioanalytical research facility and services for Nutraceuticals. Mprex is expanding at an exponential pace.
Operations Began started Mprex-USA Mpres-Biotech
What to write?
Specialized Clinical Research Needs.
Unveiling the science of clinical research in your nutraceuticals, whatsapp - 8554912644.

TESTIMONIALS
Happy clients of mprex.
“Explore our testimonials to uncover the success stories behind our innovative clinical trials, as shared by our valued partners.”
Third Testimonial
Mprex Healthcare provided exceptional care and tailored attention to my requirements, with consistently friendly and supportive staff who ensured my comfort and value as a client. Their profound expertise in healthcare services significantly contributed to my journey towards improved health. I am sincerely appreciative of the positive experience I encountered with Mprex Healthcare.
Ashley Foster
Mprex Healthcare Pvt. Ltd consistently delivers exceptional service, exceeding our expectations every time. Their dedication to excellence, timely delivery, and proactive communication have significantly contributed to our success. We highly recommend Mprex for their unparalleled commitment to customer satisfaction and quality healthcare solutions.
Mprex consistently offers outstanding service, presenting inventive solutions with the highest level of professionalism. Their commitment to ensuring client satisfaction shines through in every encounter. I wholeheartedly endorse them for their dependability and mastery in the field of Clinical Research.
Happy associates & clients of Mprex
Product transformation
Transform Your Product Potential with Mprex’s Clinical Trial Services

- [email protected]
- [email protected]
- Nyati Unitree, Yerwada, Pune
- Mon - Fri : 9:00 AM - 6:00 PM

Company Achievements and Recognition's
Our audits and vendor qualifications, our publications.

About Ardent Clinical Research Services Pvt Ltd.
Ardent Clinical Research Services Pvt Ltd is one of India’s leading providers of integrated clinical research services having operational facilities in Pune, MH, India. We have been successfully serving to a wide range of clientele comprising of biopharmaceutical, Herbal, Neutraceutical, Device companies and generic drug industry. Our broad spectrum of superior quality services are designed to meet our customers’ specific needs. The team of experts at Ardent offers management and leadership solutions to clinical researchers to ensure that their project starts on the right foot. Apart from a timely start, an efficient execution and delivery of clinical trial projects is ascertained. We’re equipped with ICH GCP, Standard Operating Procedures (SOPs) and all our clinical trial activities strictly comply with the regulatory principles and guidelines. As we conform to the highest standards of ethics, the patients under investigation can rest assured about their safety and confidentiality. With our exhaustive investigator database and technical expertise in diverse areas of therapeutic research, we enable faster patient recruitment, cost-effective trials and a world-class clinical support. But what really sets us apart from our peers is our quick turnaround times and the personal attention, we pay to our customers.
Ardent Health Values
Our mission is encapsulated in providing a conducive system for clinical trials, experiments & observational studies, tailored to specific requirements, to respond to rising burden of diseases & growing demand for innovation in drugs and medical devices.
We envision ourselves going beyond the development of new drugs, diagnostic tools & treatments, to contributing in planning of healthcare services, facilitating continuous evaluation & progress of medical care, with a thorough investigation of risk-factors.

Welcome To Ardent Clinical Research Services
Providing the best and quality services of clinical trials in the industry..
Ardent Clinical Research Services is western India’s one of the top-notch full-service clinical research organizations, operating from Pune, Maharashtra and Hyderabad India
- A - Attention to Detail
We at Ardent Clinical Research Services ensure that we carefully review and understand the client requirement.
- R - Responsibility
Concern for the Society and the people living in it is a Responsibility of a good organization
- D - Diversity
We at Ardent come from different backgrounds and different regions of India.
- E - Excellence
Striving for excellence is not doing different things its just doing things differently.
- N - Nobility
Nobility and Ethics are the values which we are proud to follow as an Organization.
- T - Teamwork
We believes in absolute teamwork. We support and encourage the participation of employees at all levels to give any kind of feedback.
Founder and Managing Director – Ardent Clinical Research Services
- Founder of the Company is Mr. Chandu Gangadhar Devanpally who holds Post Graduation in Bio-chemistry and Diploma in Clinical Research from Nagpur.
- He has started his career in the Department of Clinical Trials at Sushrut Hospital and Research Center, Nagpur in the year 2007.
- He is an experienced entrepreneur and has successfully launched various organizations for different industries.
- He is meticulous, goal-oriented entrepreneurial seasoned professional with hands-on real-time experience of End-to-End Clinical Trial Project Management, Data Management and Business Development for Clinical trials.
- A keen strategist with the ability to understand and articulate key opportunities in ever changing research industry.
- He has proficiency in planning, organizing, executing and training subordinates on protocol-based management and conduct of clinical trials.
- He had worked on various designations like CRC, CRA, Quality Manager and Operational Manager on various global and domestic trials.
- He is an effective communicator with excellent relationship management skills, problem solving and organizational abilities.
- His expertise is in Clinical Trial and Team Management, Business development, Project Management, Monitoring and Communications with various sponsors/vendors.

Different Types Of Departments
Medical writing.
Our medical writers compile new facts, ideas, thoughts & perceptions on drug & device-discoveries, which are nothing but a well-structured information to different audiences, including patients, consumers, physicians, drug regulators and other healthcare experts.
Regulatory Services
Our ability to assist sponsors obtaining regulatory approvals is supported by a deep understanding of, and experience in dealing with the Indian drug control regulations. We have the ability to provide services as a complete study management and/or as customized services.
Clinical Operations
Ardent Clinical Research Services (ACRS) Contract Research Organization’s Clinical Operation Department Helping You Design, Start-Up and Manage your entire Clinical Trials To Successful Completion, submission, and Regulatory Approvals.
Project Management
In our mechanism ensures that collaboration is maintained on each function of a project to manage all its operational aspects and quality, in terms of minimized risks & clean data, is delivered efficiently & timely, as per the GCP guidelines.
Safety Management
Through a team of dedicated and experienced Medical Monitors, we provide- highly credible inputs for the design of comprehensive study specific safety management plans, to ensure AE’s & SAEs are reported and processed accurately as per the regulatory guidelines.
Data Management
Ardent Clinical Research Services provides end to end comprehensive clinical data management services to the clients from Data Management Plan to Database Lock. The team works on a robust portfolio and strives to ensure the highest quality.Clinical Data Management Services Include :
Clinical trial monitoring
Clinical trial monitoring is a crucial part of trial conduct, improving the safety of the participants, the quality of the data and the trial integrity. Clinical trial monitoring is conducted by monitors, quality assurance teams and by trial managers
Medical monitoring
Medical monitoring is an essential component of the clinical research process. Medical monitors provide medical expertise and oversight for the entire clinical trial, from initial study design through final study close-out.
Site management
Site management in clinical research involves effective site monitoring and constant communication between the various stakeholders throughout all phases of the clinical study – study start-up, study conduct, and study closure.
Quality management
At ACRS, we understand the critical importance of quality in every aspect of the clinical trial we do. Our commitment to excellence is reflected in our comprehensive in-house Quality Management System (QMS),
Meet Our Team
Group of qualified and experienced team..
We are a team of qualified people who are having highly experience in clinical research and its management and have been working since last 10 years now.
Each one of our services is designed to meet our customers’ specific needs.
Ardent Clinical Research Services is western India’s one of the top-notch full-service clinical research organizations.
Deep technical expertise in diverse areas of therapeutic research.Access to ethnically diverse patient-population
OUR COLLABORATION
Ardent Clinical Research Services is one of India’s leading providers of personalized solutions in clinical development and consulting domain.
Actions (what is to be completed) Responsible person for each task Excellent training source for new employees and/or fellows.

OUR STRENGTHS
Our customers contribute to making us one of India’s most eminent providers of clinical development and consulting services.
OUR WORKING PRINCIPLES
The working procedures at Ardent Clinical Research Services are based on two-fold tenets but at the heart of these tenets, lie ethics, quality
OUR MEETING
it is a very positive sign when a potential sponsor agrees to meet with you. The situation is usually that you have sent your proposal to a potential sponsor.

Make An Appointment
Our Clients


Inspired Research
Better life needs better care, promising and advancing health care, company overview, purpose-built for biopharmaceutical acceleration.
Synergen Bio Private Limited is a DCGI(CDSCO) approved new age CRO with all necessary infrastructure to cater to the Pharmaceutical and Biotech industry, established by a group of enthusiastic professionalswith a vision to set-up a premium Clinical Research Organization known for its Quality, Knowledge and Expertise. Synergen Bio understands the importance of quality clinical research services and our significantly experienced team strives to provide the same to our sponsors at significant cost advantages. Representing a vertically integrated CRO, we offer customised solutions to suit sponsor requirements for the full spectrum of clinical research.

Full service Contract Research Organization
Synergen Bio is an Independent CRO which offers full spectrum clinical trial solutions to the biopharmaceutical and generic industry. Our mission is to become the preferred research partner in drug development process for our clients

- Nasal and Oral Inhalation drug BE study (MDI, DPI and nasal spray)
- Injectable Biosimilar drug BE studies
- Topical transdermal patch studies
- Intravenous Anesthetic drug BE study (e.g. Propofol)
- Topical Transdermal gel and cream based BE studies
- Patient PK studies
- Special population BE studies
- Population bio-equivalence
Our Services
Bioavailability & Bioequivalence

Bioanalytical

Clinical trials

Protocol Development & Medical Writing

Medical Services

Regulatory Affairs

Project Management

Quality Assurance

Pharmacokinetics & Biostatistics

Pharmacovigilance
- Company Insights
- New Registration
- Update Registration
- Phase 1 to 4 Clinical Trials
- Medical Monitoring
- Project Management
- Regulatory Affairs
- Site Selection
- Vendor Management
- Clinical Safety Services
- Clinical Operations
- Investigator Site Selection
- Site Selection and Management
- Patient Recruitment & Retention
- Data Management & Statistics
- Medical Writing
- Literature Services
- Signal detection and Report management
- Adverse Event Intake
- Case Processing
- Development and Implementation
Phase 1 to 4 Clinical Trial
Medical device clinical evaluation, software as a medical device clinical evaluation, bioequivalence clinical trials, biological clinical trials, herbal and nutraceutical clinical trials, in vitro diagnostics clinical evaluation, new drug and repurposed drug clinical trials, personalized medicine clinical trials, drug safety and pharmacovigilance services for us fda, drug safety and pharmacovigilance services for ema, drug safety and pharmacovigilance services for cdsco.
- CNS – Including Pain & Psychiatry
- Autoimmune / Inflammation
- Cardiovascular
- Opthalmology
- Endocrinology / Diabetology
Clinical Research Organization in US & India
Welcome to prorelix research cro, your drug development arm.
ProRelix Research is a Clinical Research Organization (CRO) with offices in the USA and India along with site networks in Australia, Thailand, Africa, and Europe supporting the clinical development of innovative new healthcare inventions and innovations to improve the health of patients. Every project is equally important to us. We first listen to you and strategize, aiming to a long-term partnership. We understand each project is unique on its own, and conscious clinical development, where efficiency is utmost and accuracy is supreme, is valuable and demanding to long-term success.
We provide services to help pharmaceutical, medical device, biopharmaceutical and nutraceutical/ herbal companies to transform scientific discoveries into new treatments. From clinical trial services, clinical data management services, pharmacovigilance services, medical writing services, drug regulatory affairs services, decentralized clinical trial services to leveraging real world insights, our therapeutic, technical, & operational ability is underpinned by a great conviction in what we do.
ProRelix Research has a global network of distinguished scientific leaders who are experts in a diverse range of therapeutic areas. We specialize in chronic diseases clinical trial services including oncology, respiratory, ophthalmology, endocrinology, immunology, dermatology, etc. Our reputation in clinical research services differentiates us from conventional clinical research organizations (CRO). We leverage our scientific leadership to establish and maintain the engagement of unique investigator networks for clinical research services.
Our Portfolio
Clinical trials play a pivotal role in the approval of new drugs as they most accurately reflect the effect of the drug in the human body. Since drug development is a costly and laborious process with the clinical trial phase...
Medical devices are an integral part of our lives from a simple thermometer to a life-saving pacemaker. Devising medical device clinical studies is complex and requires knowledge and experience-our experts at ProRelix Research can help you design studies depending upon...
The use of software to inform healthcare decisions is ubiquitous and is being utilized by patients, clinicians, and healthcare providers. Proper understanding, planning, and design of SaMD is essential for approval. ProRelix Research provides customized solutions and helps you adapt...
The success of a generic drug or biosimilar often hinges on the results of bioequivalence studies making it essential to partner with an experienced and reliable CRO to design and conduct bioequivalence studies. ProRelix Research offers full range service for...
ProRelix Research offers customized, end-to-end services for clinical trials of a broad range of biological products to help bring both innovator biologics and biosimilars to the market in a timely and cost-effective manner. We ensure compliance with regulatory requirements in...
The exponential growth in the natural product and dietary supplements field requires clinical trials to support safety and efficacy. ProRelix Research lends support at every step of your clinical trial journey to help you carve a niche in the dietary...
Diagnosis of diseases by clinicians using suitable tests guides treatment decisions and is necessary for improving and prolonging the health and well-being of patients. ProRelix Research partners with you to provide clinical trial support and ensure your device meets regulatory,...
ProRelix Research provides tailored end-to-end management of clinical trials keeping cost and patient safety at the forefront. We help you navigate the complex path of clinical trials by offering study support from patient recruitment to statistical interpretation to help you...
At ProRelix Research we understand that realization of the full potential of the emerging field of personalized medicine is dependent on the design and execution of clinical trials. We can help you circumvent the unique challenges associated with conducting precision...
ProRelix Research understands the utmost importance of protecting patient safety from clinical trials to real-world use of a new drug, biological or medical device. We help you develop appropriate pharmacovigilance plans and adverse event reporting systems in compliance with US...
Our experts at ProRelix research provide customized and comprehensive pharmacovigilance and safety monitoring programs in accordance with European Union (EU) regulations with an emphasis on patient well-being from research and development to post-market authorisation.
Patient safety and well-being lie at the heart of all our pharmacovigilance operations at ProRelix Research. We are with you every step of the way-from development of a pharmacovigilance system to regular reporting of safety and adverse events to regulatory...
How ProRelix Research can help you?
The successful execution and completion of a clinical trial are determined by several interconnected cross-functional expertise teams, including Medical Writing, Regulatory Affairs, Clinical Project Management, Clinical Data Management, Site Management, and Patient Recruitment, Faster Ethics Committee Approval, Pharmacovigilance, and much more.
A Quality driven Global Clinical Research Organization (CRO) that can truly understand your needs and help you to achieve your goals by minimizing the complexity across functional teams. We at ProRelix Research strive to bring forth the best clinical research services managed by therapeutically sound experts and professionals who are having over a decade of international regulatory experience in conducting complex clinical trials into simple ones.
Also, our extensive knowledge, quality work, and global recognition make us an ideal choice for the rising demand for conducting holistic clinical trials which include New/ Repurposed Pharmaceutical Products, Biological, Herbal, Nutraceutical, In Vitro Diagnostic Devices, Therapeutic Medical Devices, and more.
We are dedicated to follow the best ethical and clinical practices when conducting clinical studies while keeping the safety of all of our participant at core. Our motto is to provide our clients with prompt clinical study management, reliable clinical data with integrity, and ethical conduction of the clinical trials. The uniqueness of our services and functionality is that we not only design the ideal mechanism for better functionality of your trials but we also provide you a lending hand in all those processes and take you through them from the start till the end to reach your goal.
Our service portfolio includes Clinical Trial Project management, Phase 1 to 4 Clinical Trials, Drug Regulatory Affairs, Medical Writing, Clinical Data Management, Pharmacovigilance, Clinical Operations, etc . We are open to clients from all over the world and have a strong presence in USA and India and strategic partners in Europe, Australia. Let’s commit ourselves to better healthcare systems all around the world by ethical, prompt, and reliable clinical research studies!
Our Highlights
- Clinical Research Organization with Global Services Capability in USA, India, Europe, and Australia
- Phase 1, 2, 3, and 4 clinical trial services for (i) Pharmaceutical (ii) Medical Device (iii) Herbal/ Neutraceutical
- Organized Structure and Reputation
- Experienced and Best Service Portfolio
- Adaptability and Flexibility with Sponsor/Study Requirements
- Quality Assurance and Transparency
CRO Services
Clinical research services.
Our clinical research service focuses on the Quality, Ethics, and Well-being of the clinical trial subject… Read More
Data Management & Statistics Services
At ProRelix Research we are a team of experienced people who are well aware… Read More
Medical Writing Services
ProRelix Research’s medical writing service capabilities range from Clinical Research, Regulatory… Read More
Regulatory Affairs Services
ProRelix Research’s Regulatory Affairs services provide expert support in drug development programs. Read More
Recent Posts
Understanding biostatistical services in clinical trials: a comprehensive breakdown.
Biostatistics involves the application of statistical techniques for the design and analysis of clinical trial data. In addition to the conduct of trials which encompasses regulatory approval processes, informed consent, […]
Telemedicine: How Remote Monitoring is Changing Clinical Research
Clinical trials are undergoing a paradigm shift from a site-centric or traditional approach wherein the patients are required to go to central sites for consent processes, assessments, and safety monitoring […]
Putting Patients First: The Shift Towards Patient-Centric Clinical Trials
Although time, speed, and operational constraints have long plagued the progress of clinical trials, another factor that remains important and has been gaining prime importance as the centre of clinical […]
Testimonials
I would highly recommend Prorelix Research as a client‐focused company with a strong patient database that delivers on its promise to deal with patient recruitment.
Global Pharma Company
We selected the Prorelix Research because of its comprehensive nature, flexibility and reliability. We were happy as ProRelix Research proved to be a best solution to our clinical trial operations .
Indian Pharma Company
We are very happy to have found Prorelix Research, during our relationship, ProRelix Research has delivered best services for our medical device study project..
Global Medical Device Company
The quality of the service provided by ProRelix Research matches the sophistication of their services platform. Their services have helped us thoroughly and promptly.
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
Select Services Clinical Research Medical Writing Data Management & Statistics Pharmacovigilance Regulatory Affairs
Register Now
All the fields are mandatory
- 020 66434366
- [email protected]

The Leading Clinical Research Institute in Pune & India
Life -long partners in drug development.
Lifepoint is well known as the Best Clinical Research Institute in Pune & India . It has the best-in-the-class infrastructure for conducting clinical trials and a large and diverse therapy-naive patient pool to tap as well. We have well established research setup in 4500 square feet, which includes reception area, working station, Separate & access controlled pharmacy to meet all temperature needs (2 to 8 degree C, 15 to 25 degree C, -24 to -80 degree C), Monitoring rooms / Auditor Room/ Conference room. Research oriented qualified & experienced research Staff of 28 Study Coordinators, Site manager, quality manager, phlebotomists, Research Nurses & Other supporting staff.
Site has been inspected by:
- Local regulatory agency (DCGI) in May 2018 with no observations
- US FDA in June 2018 & July 2021 with no form 483
- WHO & AIFA in Dec 2019 with no major finding (we are now qualified site for WHO studies)
- In the last 7 years we have conducted 38 patient based bioequivalence studies, 90+ phase studies & 6 investigator initiated studies at this center in various indications.
- The Institutional Ethics Committee is Registered in CDSCO (ECR/751/INST/MH/2015/RR-21), also EC has been inspected for NABH.
- Site is registered with USFDA with FEI number- (3012051676) & (DUNS No. 650960482)
- Clinical laboratory is NABH & CAP certified & in the near vicinity with the turnaround time for getting reports 4 hours for Hematology & Biochemistry and within 24 hours for other tests.
- Management team having total domain experience of 13 Years and have successfully completed 400 plus trials with a variety of indications along with vaccine trials. We have closely worked with all the major domestic & Global Pharmaceutical companies .
Our Clinical Partners

We are ISO 9001:2015 Certified

Full Service SITE with phase 1, 2, 3, and 4 clinical trial services

Study Feasibility & Investigator Selection Site Qualification & Site Initiation visits Monitoring & Audit visits Correspondence with Ethics committee

Central/Local Lab logistics ICH GCP Trainings Site Closeouts
AE, SAE Reporting Completion of Paper/ e-CRFs and resolving Data Queries Document Maintenance, Updating & Retention

Assistance in ICF process Screening & Randomization of the subjects Subjects follow-up visits

Serving 15 Major Therapeutic Areas in Clinical Research

Gyn. & Obs.

Ophthalmology

Expert-Backed PG Diploma Program
The future of clinical research is bright and limitless. A PG Diploma Clinical Research Courses in Pune prepares students for careers in the healthcare field. In other words, this course provides the necessary skills and qualifications needed for the professional practice of Clinical Research Services in Pune. Students pursuing this course can make their career in the following:
- Healthcare and pharmaceuticals
- Nutrition and public health
- Bioinformatics, and drug designing
- Biomedical engineering
- Analytics,forensic sciences
- Food and beverages
Hear It From Our Students
Connect with us.
Cro/Sponsor Form
Investigator Form
Patient Form
Student Form
Recent Blog

Common Clinical Research Interview Questions
Common Clinical Research Interview Questions Clinical research provides invaluable insights into the patient’s experience, their illness, and how they respond to treatments. Preparing for a

Future Scope of Clinical Research
Future Scope of Clinical Research Clinical research is a growing, knowledge-intensive industry. It is one of the industries that is rapidly expanding and providing

Highlights Of Pharmacovigilance- Career Prospects and Salary
Highlights Of Pharmacovigilance- Career Prospects and Salary What is a Pharmacovigilance course? Pharmacovigilance is a branch of pharmacy concerned with identifying, quantifying, validating, and

- +917265092661
- +918329585189
- 020 66434366 ( For CRO )
- LIFEPOINT RESEARCH , Lifepoint Multispecialty Hospital, 145/1, Mumbai Bengluru Highway, near Hotel Sayaji, Wakad, Pune-411057
Quick Links
- Clinical Research
- PG Diploma Course
quick enquiry
©Copyright At LifePoint Research 2022 | Design & Developed By HMA | Privacy Policy | Terms and Conditions
- For appointments 022-69315555
- For appointments +91-8007006611 / 8000016611
- +91-8007006611
- 24/7 EMERGENCY: 105900
- English Marathi
- Find a Doctor
About Noble Hospital Research Center
We will provide you with the highest level of treatment!
Noble Hospital provides clinical research services for pharmaceutical and biotechnology product development from Phase I to Phase IV. Clinical trials must be scientifically conducted using appropriate methods based on strict observance of Good Clinical Practice and ethical considerations. This requirement places an increasingly heavy administrative burden on the medical institutions that perform clinical trials. A separate dedicated clinical research facility compliments the work of the investigators by providing them vital administrative and infrastructural support. Noble Hospital started its clinical research services in 2009. We have completed over 50 trials in various specialties and phases to date. Our team comprises Site Start-up specialists, QA manager and Clinical Research Coordinators (CRCs), headed by Dr. S. K. Raut, Director, Clinical Research.

Noble has the advantage of having efficient PI group Specialties in all major therapeutic areas like Oncology , Gynaecology, Cardiology, ENT, Ophthalmology, Orthopaedics, Dermatology, Gastroenterology, Urology & Nephrology.
The scope of our services includes
- Patient Screening and Recruitment : Our project management and study coordinators provide support to the investigators in the speedy recruitment of patients. A database of all patients admitted in the hospital and those availing out-patient facilities across the therapeutic area is maintained to aid in recruitment. This ensures that patients from various ethnic backgrounds and demographics are considered for the trial.
- Clinical Operations and Project Management : Our full-time staff of study co-ordinators ensures that the planning and execution of the clinical trials are smoothly managed from start to finish.
Clinical Research Facilities
- Dedicated space for clinical trials : Noble Hospital Clinical Research Department has a dedicated Space with 1200 sqft space within the hospital premises exclusively for conducting clinical trials. This includes clinics for consultants to see patients, sample collection room, data entry room, drug storage, conference hall and training hall facilities.
- State of the art drug storage : A separate fully automated temperature-controlled drug storage area for storage of the investigational product.
- Separate area for archival : A separate fire and water-resistant area has been made for archival of study documents.
- IT Infrastructure : Noble Hospital Clinical Research Department has a full-scale IT infrastructure with computers connected by LAN with a broadband connection to enable study coordinators to handle the data entry and data management requirements.
- Research Laboratory : We provide laboratory services for those sponsors who are interested in local labs instead of central labs.
Noble Hospital Institutional Ethics committee
Noble Hospital Institutional Ethics Committee (IEC) is approved by the Central Drugs Standard Control Organization (DCGI), under the Drugs and Cosmetics (Third Amendment) Rules 2013. Institutional Ethics Committee constituted as per ICH – GCP guidelines & Schedule – Y, has 11 members from various walks of life, with gender balance as per local statutory requirements. IEC meets every once in three months or whenever required and ensures that universal ethical values and international scientific standards are expressed in terms of local community values and customs. It especially has a focus to protect the safety, dignity, rights, and well-being of research participants.
The IEC attempts to ensure the protection of subjects by reviewing research protocols and related materials. The purpose of the IEC is to assure, both in advance and by periodic review, those appropriate steps are taken to protect the rights and welfare of humans participating as subjects in a research study. It promotes fully informed and voluntary participation by prospective subjects and seeks to maximize the safety of subjects. If required we seek expert opinion from various specialty and super specialty from our Hospital.
(DCGI Reg. NO. – ECR/259/Inst/MH/2013)
The Ethics committee is setup to ensure that the research protocols carried out:
- Are conducted according to the parameters of ICH–GCP as well as local regulatory requirements.
- Do not compromise the safety, rights and well being of the patients participating in the research study.
- Are conducted under the supervision of medical persons with the required experience/ expertise.
- Include solely patients who, through their legally acceptable representative have given informed consent for participation in the research study.
- The Committee can also be called upon to solve the ethical issues of the Noble Hospital on request.
- All Ethics Committee Members undergo a training program to update themselves about the current scenario in Clinical Research Also we send our Principal Investigators for ICH GCP Training programs.

About Noble
Message from Chairman & MD
Accreditations
Awards & Accolades
Covid 19 Screening
Quick Links
Noble Annex
Bio Medical Waste
Department of Nursing
Regulatory Compliance
Patients Rights & Responsibility
Accereditation
Insurance & TPA
Preventive Health Package
Noble Hospital
Multispecialty Clinic Magarpatta
Multispecialty Clinic Amanora
EON Polyclinic Kharadi
Samruddhi Diagnostic Centre - Sasanenagar
Modal title

- For appointments
- WhatsApp Us

raptim research pvt. ltd.

Operating with the latest COVID-19 guidelines

Raptim Research Private Limited (formerly known as Raptim Research Limited), is a global independent and internationally accredited Contract Research Organization (CRO), established in 2005 with state-of-the-art clinical research facilities in Mumbai and Gandhinagar (India).
Our Services

- Healthy Subject (Bioequivalence & Bioavailability) Studies
- Patient Based Studies
In-Vitro Studies

- In-vitro Release Rate Test (IVRT) & In-vitro Permeability Test (IVPT)
- In-vitro binding Studies
- BCS Biowaiver Studies
- In-vitro Feeding Tube Studies
Special Studies

- In Vivo Tape Stripping
- In Vivo Dermal Microdialysis
- Skin Blanching studies
- Skin Irritation and sensitization test
- Glucose clamp Studies
Clinical Trials

- Early Phase Clinical Trials
- Phase II-IV Clinical Trial & Post marketing studies
We apply innovative tools and processes to our clinical development and support end-to-end services under the Clinical Research domain for all Global Pharmaceutical companies. With our experience and enhanced capabilities, we are in a unique position to assist our Sponsors with unparalleled support and provide them a single, global research network.
ASSAY LIBRARY
Your Trusted Partner in Bioanalytical Method Development and Validation
With a proven track record of excellence, Raptim Research Lab stands as a premier provider of bioanalytical method development and validation services. Having served leading pharmaceutical companies both in India and abroad, we take pride in our portfolio of over 500 validated methods.
Our expertise lies in delivering tailored solutions to meet the unique needs of our clients, ensuring accuracy, reliability, and regulatory compliance every step of the way. Whether it’s method development for new compounds or optimization of existing protocols, our team of experienced scientists is dedicated to delivering results that exceed expectations.
Partner with Raptim Research Lab and unlock the full potential of your pharmaceutical research endeavours.

At Raptim Research Pvt. Ltd., we are committed to ensuring patient safety while minimizing disruption and keeping studies on track during this evolving time.
Our sites in Navi Mumbai and Gandhinagar are following the protocols necessary to conduct studies with highest standards of care and safety:
- Social Distancing Protocols have been applied throughout our sites
- Volunteer Areas such as bedding facilities are kept at a distance
- Across the facilities, hand wash zones are marked, and sanitizers are available
- Training has been conducted on new anti Covid-19 measures implemented
- In association with clients and partners, modifications are being made to studies to reduce volunteer visits, avoid exposure to immunosuppressive drugs and monitoring safety of the patients at different time during the study
- Enhanced screening for all studies are being carried out
- Procured kits to conduct testing for Covid-19 at the diagnostic laboratory
- All subjects to be oriented for social distancing measures at the facility along with being given protective gear
- Minimum contact is practiced
- An isolation area is created to separate any subject with influenza like symptoms immediately
We are complying with the FDA guidance for conducting clinical trials.
read fda guidelines here


Standing in the gap between what is and what can be… Siro can help navigate and find answers that empowers lifesavers.
READ ON >
Rediscover efficiency with our accurate solutioning and customer-focused approach.
Rediscover dependablity with our market leading data management services.
Since decades, Siro continues to deliver in the global healthcare industry. See the highlights of our journey here.
END TO END CLINICAL RESEARCH ORGANIZATION
One stop shop for services ranging from medical writing and clinical operations, to data management and biostatistics.
GLOBAL CLIENTS
Long term strategic partnerships with large pharmaceutical companies from USA, Europe and Japan.
THERAPEUTIC EXPERTISE
World class therapeutic experts specializing across all niche therapeutic areas and indications.
SOLUTIONS SIMPLIFIED
Striving to provide a unique and unchallengeable solution to complex drug development processes.
Our Services

Trial Supplies
Follow the buzz
Powered by juicer.

IT Consulting & Clinical Services
IVAAN is a Clinical Research Organization which provides services on Data Management, Biostatistics and SAS programming to our clients covering all phases and therapeutic areas of drug development including Bioavailability and Bioequivalence studies for the Pharmaceutical, Medical Device, Biopharmaceutical, and CRO industries.
Our Services
IVAAN is a Clinical Research Organization focussing on providing data parts of Submission package to FDA, Consulting Service of Data Standards from FDA, Data Standardization in CDISC (SDTM/ADAM) format, Data Management, Biostatistics and SAS programming services to pharmaceutical, health care, biotech and medical device industries.
BIOSTATISTICS
Delivering High-Quality Biostatistics Services in Support of Your Clinical Trials
SAS PROGRAMMING
Statistical services have always been a key component of IAneesh services as part of our heritage.
CLINICAL DATA MANAGEMENT
Our CDM team offer CRF design, database design, edit checks, discrepancy management, coding of AEs and medications...
MEDICAL WRITING
High-Quality Medical Writing to Support Your Studies
Training and Education
Comprehensive providers of post graduate training in clinical research, clinical statistical programming and a variety of other domains.
SAS Clinical Clinical SAS Programming CIDSC ADaM SDTM TLFs Table Listing Figures Statistics Biostatistics SDTM Specifications ADaM Specifications Graphs CDM clinical data Management
Clinical SAS Programming statistical software statistical Tool statistical Analysis Plan Reporting Analysis Plan Repotting tool Reports CIDSC Tool ADaM Tool SDTM Tool Table Tool Listing Tool Figures Tool Graphs Tool Dataset creation Tool SDTM Spec SDTM Specifications ADaM Specifications SAP Protocol CRO Clinical Research Organizations
Medicine Research IT software Software development Services Pharmaceutical Industries Analysis statistical model All models are wrong Conceptual model Design of experiments Deterministic model Effective theory Predictive model Scientific model Statistical inference Statistical model specification Statistical model validation Statistical theory Stochastic process Analysis of covariance regression ANCOVA ANOVA
Best India Pune
Ethics Committee
Associations.

On This Page
- The Genesis
- Competitive Advantage
- Mission & Vision
- USPs and Achievements
- Clinical Trials
- Investigator Initiated Research
- Our Research Team
The Genesis:
- Sahyadri Hospitals are the largest chain of hospitals in Maharashtra. It is the brainchild of Dr. Charudutt Apte, one of India’s most renowned Neurosurgeons and more importantly an ardent practitioner of ethical medical practices.
- Sahyadri Clinical Research and Development Center was established in 2006.
- Sahyadri Clinical Research and Development Center & excels in coordinating a research driven team which has a well designed infrastructure with all modern amenities providing a milieu conducive to the advancement of professional and personal excellence.
- Experience of more than 500+ Clinical Research Trials in various therapeutic areas like Haematology, Oncology, Cardiology, Endocrinology, Infectious disease, Vaccines, Rheumatology, Gastroenterology etc.
- Well defined SOPs.
- Qualified and research oriented investigators of all specialties in Sahyadri Super Speciality Hospitals across Pune.

What Makes Sahyadri Clinical Research And Development Center Have A Competitive Advantage
- Sahyadri Clinical Research and Development Center (SCRDC) is a vertical of Sahyadri Hospitals Pvt. Ltd which is the largest chain of hospitals in Maharashtra.
- It is a renowned chain of secondary tertiary and quaternary care hospitals.
- SCRDC has dedicated, experienced, proactive Investigators with subject expertise.
- SCRDC has received NABH accreditation for Ethics Committee.
- Sahyadri Super Speciality hospital is NABH accredited.
- Sahyadri Hospital has a well equipped NABL accredited laboratory.
- SCRDC has successfully completed two USFDA & DCGI inspections.
- Sahyadri group offers a unique proposition for clinical research by its network that consists of 08 hospitals, 900 beds including 200 ICU beds, 2000 clinicians, 2600 supporting staff and over 27 years of experience. 50 lacs lives touched.
Certificates Of Accreditation

Our Mission Vision
- To safeguard and promote the health and welfare of human research subjects by ensuring that their rights, safety and well-being are protected.
- To provide timely and high quality education, review and monitoring of human research studies.
- Medical care made available through accountable research.
Sahyadri Clinical Research and Development Center -USPs and Achievements:
- Institutional Ethics Committee has full accreditation of NABH.
- Previously accredited with international AAHRPP accreditation (Association for accreditation of Human Research protection program). We were the 4th site in India to receive this accreditation .
- Highest recruiting site for many studies.
- Sahyadri Hospitals Pvt. Ltd. Ethics Committee, Pune is registered under CDSCO with Reg. No. ECR/493/Inst/MH/2013/RR-19 valid upto Nov 2024 and it is accredited with NABH.
- Ethics Committee consisting of experienced members having passion for clinical research and ethics in general.
- Ethics Committee works as per The New Drugs and Clinical Trials Rules 2019, ICH-GCP, Schedule Y and ICMR guidelines & strictly adheres to approved Site SOP’s.
- Ethics Committee reviews and approves research activities with proper considerations of risks and anticipated benefits involving human participants.
- Ethics Committee has reviewed more than 500+ protocols.
- Ethics Committee committed to the safety of patients who take part in our trials, and uphold the highest ethical standards in all of our research initiatives.
Our Team of Ethics committee
Dr. ravindra ghooi.

Designation – Chairperson; Basic Scientist. Qualification – Phd. Medicine,M.Sc. Pharmacology.
Vast experience and expertise in Clinical Research for last 5 decades. Eminent member of various local, national and international research bodies.
Has wide knowledge and experience in teaching and training ethics in Clinical Research.
Dr. Deepa Divekar

Designation – Member Secretary; Clinician. Qualification – MD (Paediatric) Fellowship in Paediatric Neurology (London).
She is a Clinician and Pediatric Neurologist for more than 4 decades. Has a vast knowledge and expertise in research and ethics. She is associated with the hospital since its inception. She motivates and encourages her team members to be impartial and unbiased while making decision. She devotes adequate time to research and contribute largely to the quality of Ethics Committee decisions.
Dr. Mohini Barde

Designation – Clinical Pharmacologist. Qualification – MD Pharmacology.
Competent and knowledgeable Clinical Pharmacologist. Also provide consultancy to Biotech and Pharma companies. Very Resourceful with problem solving skills giving value addition in EC meetings.
Dr. Swati Sirsikar

Adv. Alka Patwardhan

Designation – Legal Expert. Qualification – LLM,LLB.
A school teacher and a advocate by profession for last 4 decades. Is very meticulous about the rights and safety of trial subjects. Takes a proactive approach when reviewing the clinical trial agreement, so that all the clauses pertaining to subjects’ rights are inclusive.
Dr. Swati Jadhav

Designation – Basic Scientist / Ethicist. Qualification – M Pharm, Phd Ethicist.
Has a Ph D in Bioethics in Clinical Research. Creative, enthusiastic health professional with 20 years’ experience pharma industry, academics, Clinical Research and Bioethics. Is very proactive with constructive participation in decision making a during EC meeting.
Mr. Trevor Martin

Mrs. Jayashree Jog

Designation – Lay Person. Qualification – Graduate in Nutrition.
Was actively involved in daily operation of family-initiated business. Nature lover, runs her own native plant nursery. Is a true representative of the community and always thinks and acts in a favor of research subjects. Contributor constructively to patient’s safety.
Adv. Dr. Deepa Paturkar

Designation – Legal Expert. Qualification – L.L.B, L.L.M. Ph D.
Vice principal and professor of law at ILS, Law college, Pune. Astute scholar, guide, researcher and academician with a rich experience of 3 decades in field of law spanning across college of repute. Advisor to corporates and hospitals on issues of ethics. Doctoral research in law relative to Human Organ Transplant. Her rich experience and expertise, contributes constructively to legal issues in Clinical Research.
Dr. Vaijayanti Kale

Collaborations
- Announcing alliance between Sahyadri Clinical Research and Development Center & Pfizer Ltd to push forward the research for future clinical trial developments.
- Sahyadri Clinical Research and Development Center has partnership with Sanofi Healthcare India Private Limited as preferred site in India.
Sponsors & CROs Associated with us
- Sanofi Health care India Private limited
- Paraxel International Clinical Research Pvt. Ltd.
- Boehringer Ingelheim
- JSS Research
- Dr. Reddy’s
- Reliance Life Sciences Pvt. Ltd.
- Celldex Therapeutics Pvt. Ltd.
- Sun Pharma Advanced Research Ltd.
- Cadila Healthcare Ltd.
- Glaxo Smithkline
- Johnson & Johnson
- Novonordisk
- Siro Clinpharm
- Manipal Accunova
- Semlar Research & Pvt. Ltd.
- Bristol Meyers Squibb
- Diagnosearch Life Sciences
- Lambda Therapeutics Ltd.
- Cangene Corporation
- Novartis Healthcare Pvt. Ltd.
- Novotech Clinical Research Pvt. LTd.
- Zydus Cadila
- Bharat Serum Pvt Ltd.
- Veeda Healthcare Pvt. Ltd.
Clinical Trials in Sahyadri Clinical Research and Development Center
Hematology trials.
- Haemophilia A & B
- Acute / Chronic myeloid leukaemia
- Invasive aspergillosis
- Multiple myeloma
- Patient with Factor VIII Inhibitor
- Hodgkin’s Lymphoma
- Myelodysplastic syndrome
- Afibrinogenemia
- Von Willebrand Disease
Oncology trials
- Ca Pancreas
- Ca Head & Neck
- Renal Cell carcinoma
- Glioblastoma Multiform
- Ca Colon /Rectum
- Urothelial Cancer

Neurology trials
- Multiple sclerosis
- Parkinson’s disease
- Fibromyalgia
- Paediatric Epilepsy
- Deep Vein Thrombosis
- Bariatric Surgery
- Stem Cell Transplant
- Hysterectomy
- Intra abdominal infections
Nephrology trials
- Chronic kidney disease
- IgA Nephropathy (IgAN)
- Erythropoiesis
Sahyadri Clinical Research And Development Center also conduct trial in different indication including Nephrology, Cardiology, Psychiatry, Internal Medicine, Gynecology, Dermatology, Urology, Orthopedic, Endocrinology, Paediatrics, Fertility Medicine etc.
Investigator Initiated Research
- Beside clinical trials Sahyadri Hospital Private limited conduct many in house trial, Research projects.
- SHPL has an established Scientific Committee for valuable assistance in new R & D projects.
- Additional Biomedical and Health Research Committee (BHRC) to review Investigator Initiated Research.
- This has added credibility and enhanced the research driven team of the institution.
You can contact us through
Our team of renowned principal investigator.

Dr. Shashikant Apte
Hematology & Bone Marrow
20 yrs in clinical research

Dr. Tushar Patil
10 yrs in clinical research

Dr. Shona Nag
20yrs of clinical experience

Dr. Uday Phadke
Endocrinology
15yrs of clinical research experience

Dr. Atul Sajgure

Dr. Suhas Hardas

Dr. Atul Joshi
Intensive Care and Medicine

Dr. Sheetal Mahajani
Gastroenterology
10 years in clinical research

Deccan , Pune

Plot No. 30-C, Erandvane, Karve Rd, Deccan Gymkhana, Pune – 411004
View Facilities >

Hadapsar, Pune
SN 163, Bhosale Garden Rd, beside Bhosale Nagar, Hadapsar, Pune 411028

Nagar Road, Pune
Near Hermes Heritage, Nagar Rd, Shastrinagar, Yerawada, Pune – 411006

Kothrud, Pune
Neena Co-op. Housing Society, Plot No. 9-B, Lokmanya Colony, Kothrud, Pune – 411038
Contact us:
Sahyadri Clinical Research and Development Center,
A Unit of Sahyadri Hospitals Private Ltd.
33/34B, Makarand Bhave Path, Erandwane, Pune-411004, Maharashtra, India.
Tel : +9120 6721 3120
E-mail id: [email protected]

c 2021, All Rights Reserved with Sahyadri Hospitals, Pune
Ask Your Query
Enquire now.
+91 888 690 4030

ADMISSION HELPLINE

Follow us at :
Admission Enquiry
Select Qualification B.Pharm M.Pharm Pharm.D M.Sc B.Sc BDS MBBS BHMS BAMS BE B.Tech Others
Select Branch Bangalore Pune Hyderabad Mumbai Delhi/Noida
Welcome to CLINI INDIA
India’s most trusted clinical research training institute, best clinical research training courses , career begins here...
Need help? Contact our support team Tell us about your query…

Students preparing with us.
Live Class Completed
Top Instructors
Latest Updates
Admissions Open for APCRM Programs..
CLINI INDIA provides hands on Training Program on Pharmacovigilance Software tool
CLINI INDIA join hands with CREDSURE to provide secure certification for all its Programs
CLINI INDIA Celebrating International Clinical Trials Day on 20th May 2024.

- Ring at : +91 888-690-4030
- Request A Call Back
CLINI INDIA-Clinical Research Institute
CLINI INDIA is India’s Premire Clinical Research Institute dedicated to nurturing the next generation of clinical research professionals and advancing the standards of research in the healthcare industry. Established with a vision to bridge the gap between theoretical knowledge and practical application, Clini India is committed to providing comprehensive and industry-oriented training programs.
Industry-Relevant Curriculum
Comprehensive training programs, a commitment to excellence, expert faculty, practical exposure, global outreach, industry faculties.

Clinical Research Institute Award
AdvnaceD Programs WITH-
Hands-on training ….
Hands-on training in clinical research is invaluable for preparing individuals to navigate the complexities of conducting research in a real-world setting, equipping them with the practical skills and knowledge necessary for success in the field.
What all can you learn in our workshops/Case Study based training-
Electronic Trial Master File (eTMF)
The TMF is essentially the “master file” that contains all the documentation and records pertaining to the planning, conduct, and results of a clinical trial.
eCTD Submissions
eCTD is a standardized format for submitting regulatory information for pharmaceuticals, biologics, and medical devices to regulatory agencies.
PV-Edge Software for Pharmacovigilance
Our aims to provide participants with a comprehensive understanding of PV-Edge software and its role in pharmacovigilance activities for monitoring drug safety and adverse events.
Introduction to CTMS Tool
CTMS tools play a critical role in improving the efficiency, compliance, and oversight of clinical trials, helping to accelerate the development of new treatments
What we offer
Courses/ programs, offline courses, online courses, offline course, advanced program in medical coding (apmc)™.
The skills one need to require to succeed is evolving. No matter where you are in your career...
Advanced Program in Pharma Regulatory Affairs (APPRA)™
This online program helps you to provide an overview on the general legal requirements to bring...
Advanced Program in Clinical Research & Management (APCRM)
This program is oriented towards candidates interested in joining the clinical research industry....
Call & Enroll over the phone with one of our representatives Ring at : +91 888-690-4030
Thousands of people are learning with Clini India…
Notice Board
Next masterclasses and alumni sessions: how clini india executive education can support your career growth, new apcrm batch commencing from monday., online live classes, one-day workshop on ectd submission., congratulations to recent placed students, upcoming events, clini india students are invited for sde on electronic trial master file (etmf), clinical trials day celebration 20th may 2024, upcoming batches, advance program in data science, medical coding course, clinical research course, offline classes, clinical research, programs in clinical research and other domains.

Clinical Trial Management
Clinical Data Management
Pharmacovigilance

Medical Writing

Regulatory Affairs
CRA Training Program
Our alumni work with 100+ leading cros, pharma and it companies.

Congratulations to recently placed students

Why Clini India ®
Why Student choose CLINI INDIA ®

Hands-on training
At CLINI INDIA we provide Hands-On training in different tools/Softwares related to Clinical Research Domain..

INDUSTRY ORIENTED
Program offered by Clini India, are Industry Oriented and helps participants to understand the insight of future employers.

BEST PLACEMENTS
At Clini India, Placement is an Ongoing process, Our Alumni are working with leading CROs, Pharma and IT Companies across the globe.

MOCK INTERVIEW
Get prepared Mock Interview is an Integral part of Training Program, to make students ready for Industry Standard.
HANDS-ON TRAINING HRS
online CLASS COMPLETED
Positive Google Reviews
TOP INSTRUCTORS
Best Institute awards
Some of our long-standing Associates and Partners are highlighted below.

Testimonials
What our students have to say.
Listen to the stories of our students, as they articulate the profound influence of our educational environment. Their testimonials encapsulate the enriching experiences and valuable insights gained throughout their educational endeavors.

Doing the CR program from CLINI INDIA was a life-changing experience for me. The practical skills backed by academic knowledge have given me the confidence to pursue a career in any domain in the Clinical Research Industry.

The quality of lecturers surpassed my expectations, and they represented a great blend of all modules in Clinical Research. I found the program is a milestone in my professional career.

My Training at CLINI INDIA was full of learning with the utmost knowledge. It made many positive frameworks in my attitude, Technical skills, work style, personality and behavior to shape best out of this Competitive world or Clinical Research. Thanks to Team CLINI INDIA for career support.
Anurag Shukla

Enrolling in CLINI INDIA was a valuable investment for me. In addition to broadening my perspective in the Clinical Research field, it has helped me to grow both professionally and personally. Thanks to CLINI INDIA, I am working with World’s Leading CRO in Bangalore
Prasanta Kumar Gouda

CLINI INDIA is a great institution. The staff and instructors are always helpful and are looking out for you. I have got placement before my course Completed. The atmosphere is great. I have made friends that I will have forever.
Golak Bihari Praharaj
Learn clinical research course online.
Embark on a transformative journey in the field of clinical research with our online courses. Dive into comprehensive modules crafted to impart industry-relevant knowledge and practical skills. Join our community of learners and elevate your career in clinical research through accessible and dynamic online education.
Join our community of learners and elevate your career in clinical research.
Year of Completion Prior to 2020 2021 2022 2023 2024
Preferred e-Campus Bangalore Pune Hyderabad Mumbai Delhi/Noida
Helpful Articles for You

Medical Coding Terminology
by Clini India | Sep 19, 2024

eCTD Workshop by CLINI INDIA
by Clini India | Sep 18, 2024
How to make ATS (Applicant Tracking System) friendly resume/CV
by Clini India | Jul 24, 2024

Regulatory Affairs Interview Questions Answers
Awards and certifications.

Published Resources/Books

Clinical Research Training Program
Campus connect.
Our Gallery


Life-changing therapies without a lifetime of development
No one who is working to help patients in need should be subjected to unnecessary delays during clinical development and commercialization. Precision’s integrated capabilities, responsive operations and deep scientific expertise are helping life science innovators accelerate research, development and manufacturing while reducing cost and increasing the probability of approval.
Specialized capabilities for every stage of clinical development
Leverage an integrated infrastructure that reduces the inefficiencies inherent in complex development. Integrate lab and trial execution to increase speed to market. Incorporate manufacturing expertise for advances therapies to ensure scalability. Precision brings a holistic view to your program's unique needs.
Global CRO Services
Award-winning CRO with deep oncology and rare disease expertise
Global Laboratory Services
Exceptional translational and biomarker sciences with global central lab services
Manufacturing Solutions
Pioneers in planning, building and maintaining complex manufacturing facilities for biotech and pharma

Biospecimens

Diagnostics & CDx

Data Intelligence

Commercialization

Precision for Medicine is a name and a movement
When our unusual name was chosen in 2012, it signaled our commitment to a new movement shaping medical innovation, a movement to bring precision to the development of novel treatments. Since our inception, we’ve brought precision to medicine with advanced biomarker capabilities, worldwide CRO services, technical manufacturing solutions and more. Unlike traditional approaches with scattered services, siloed expertise and ponderous processes, Precision has been purpose built to propel innovations to patients in need.
.webp?width=632&height=410&name=image%2037%20(1).webp)
Optimize early phase development
How can you successfully navigate the complexities of early phase development? Integrated clinical and laboratory sciences capabilities are vital. Over 25 years of experience in early oncology development helps anticipate and mitigate a diverse set of risks, including complicated kitting and sample management.
.webp?width=632&height=410&name=image%2037%20(2).webp)
Maximize late phase development
Congratulations if you are entering late phase development. Now you’ll need a partner that can recruit patients most likely to respond to treatment, manage complex protocols, anticipate global regulatory requirements, enable manufacturing, support commercial viability and more. Let us drive your innovation to market and empower access for patients.

Trusted partner to leading innovators in oncology and rare disease
Clinical development is growing more complicated. Progress can hinge on the ability to navigate a shifting pathway to approval. Your path may be unique from those who have gone before, but our experience will help inform success. Precision is a leader in oncology and rare disease research. Our deep experience in complex indications accelerates the ability to reach key milestones, drive positive outcomes and most importantly, help patients get access to innovative treatments.
Deep experience with debilitating disease and unique modalities
Like you, we understand that there’s a patient throughout the development pathway. Supporting our partners to bring innovative treatments to patients is our purpose. Leverage our experience in these therapeutic areas.

People first and patient-focused
One of our core values is service. Not just to our clients but to our communities. Volunteering at hospitals, supporting healthcare-related fund raisers and partnering with nonprofit organizations is an integral part of our culture. In doing so, we raise awareness of many life-altering diseases and our team gets closer to the actual patients that can be impacted by our work.
Precision for Medicine has been a wonderful partner in trying to identify the right doctors, the right patients, and collect all the data that will be necessary to analyze, to be able to unequivocally answer the question of safety and dose
Their experienced and qualified staff displayed resiliency, creativity and innovation to keep things moving in the face of huge obstacles.
The conversion of manual Excel sample tracking to automated QuartzBio reporting resulted in 70% resource saving and shortened study and site close timeline by weeks.
I recognize that relations between Sponsors and CRO vendors can be strained and difficult, often a blaming game; this has never been my experience with this group as it truly felt like a partnership.

Insights for the greater good
We feel privileged to work in an industry that can change lives. When knowledge is shared, it is amplified. Expanding our collective intelligence can bring remedies to market faster.

Clinical Trials - Oncology
Clinical Trial Trends: Non-Hodgkin’s Lymphoma

Manufacturing
US Crackdown on Chinese Clinical Trials: What’s at Stake for Biopharma?

Anshul Mangal

Clinical Trials
Safety isn’t a Milestone but a Mindset in Clinical Research

Renise Blythe

Life sciences innovators come to Precision to bring life-changing medicine to patients. How can we help you?
- ICH GCP (De)
- ICH GCP (En)
- ICH GCP (Es)
- ICH GCP (Fr)
- ICH GCP (It)
- ICH GCP (Pt)
- ICH GCP (Ru)
- AUSTRALIA (NHMRC)
- JAPAN (PMDA)
- US Clinical Trials Registry
- EU Clinical Trials Registry
- Pharmaceutical Companies
- Clinical Research Labs
- Service Companies
- Clinical Research Events
- Publications
- Researchers
Top 15 Contract Research Organizations (CROs) in 2024
Post-pandemic, as drug development businesses recover and grow, CROs around the world continue to undergo profound transformation. As in previous years, 2023 will be all about mergers and acquisitions, with big players getting even bigger and smaller companies surviving thanks to niche advantages such as technological know-how or brilliant scientists among their employees.
The top 15 CROs globally would be in the following order based on 2023 revenue :
- LabCorp (Covance) , with 15.05 billion USD (2023) and 75000 employees ( Labcorp , 2024). In February 2015, Labcorp completes its $6 billion purchase of Covance, Inc., creating the world’s leading health care diagnostics company. The combination of Covance’s drug development leadership and Labcorp’s medical testing expertise builds the market leader in central laboratory and bioanalysis services. Labcorp’s clinical trials companies, Labcorp Clinical Trials and Tandem Laboratories, align under the Covance brand.
- IQVIA , with 14.85 billion USD (2023) and 86000 employees (IQVIA, 2022). IQVIA, formerly Quintiles and IMS Health, Inc., is an American Fortune 500 and S&P 500 multinational company serving the combined industries of health information technology and clinical research. IQVIA (NYSE:IQV) is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. With approximately 86,000 employees, IQVIA conducts operations in more than 100 countries.
- ICON (PRA) , with 7.74 billion USD (2022) and 41160 employees (ICON, 2023). From a small team of 5 people in 1990, ICON now employs over 41,100 people. ICON has been recognised as one of the world’s leading Contract Research Organisations through a number of high-profile industry awards .
- Thermo Fisher Scientific (PPD) , with 7.02 billion USD (2023) and 35000 employees (Businesswire, 2023). Recognized as a global industry leader in accelerating promising medicines from early development through regulatory approval and market access, we serve pharma, biotech, medical device and government organizations with custom-tailored solutions, including full-service partnerships and functional service partnerships. As a strategic partner in clinical development and analytical services, we apply cutting edge technologies, therapeutic expertise and a firm commitment to quality to help customers deliver life-changing therapies.
- Lonza - 6.5 billion USD, 17896 employees. Though being a contract development and manufacturing organization (CDMO), rather than a CRO, takes the 10th position with the 5.9 billion USD in sales and over 17500 employees worldwide (2023), offering “proprietary line of in silico and in vitro services for manufacturability, immunogenicity, potency assessment, humanization and protein engineering” ( https://pharma.lonza.com , 2022). Founded in 1897 in the Swiss Alps, today, Lonza operates across five continents. With approximately 17,500 full-time employees, it comprises high-performing teams and individual talent that make a meaningful difference to our own business, as well as to the communities in which we operate. The business benefits from global supply chains, but we have worked to maintain the agility to address marketplace needs on a local level. CDMOs become reliable partners for Pharma companies as their “services reduce R&D costs while improving productivity and are essential to providing safe and effective treatment to patients” ( https://pharma.lonza.com , 2022.
- Wuxi AppTec with 5.8 billion USD revenue in 2021 and 44360 employees (Companiesmarketcap, 2022). As a global company with operations across Asia, Europe, and North America, WuXi AppTec provides a broad portfolio of R&D and manufacturing services that enable the pharmaceutical and healthcare industry around the world to advance discoveries and deliver groundbreaking treatments to patients. Through its unique business models, WuXi AppTec’s integrated, end-to-end services include chemistry drug CRDMO (Contract Research, Development and Manufacturing Organization), biology discovery, preclinical testing and clinical research services, and cell and gene therapies CTDMO (Contract Testing, Development and Manufacturing Organization), helping customers improve the productivity of advancing healthcare products through cost-effective and efficient solutions. WuXi AppTec received AA ESG rating from MSCI in 2023 and its open-access platform is enabling more than 6,000 customers from over 30 countries to improve the health of those in need – and to realize the vision that "every drug can be made and every disease can be treated."
Syneos Health® is a leading fully integrated biopharmaceutical solutions organization built to accelerate customer success. We translate unique clinical, medical affairs and commercial insights into outcomes to address modern market realities.
We bring together a talented team of professionals, who work across more than 110 countries, with a deep understanding of patient and physician behaviors and market dynamics. Together we share insights, use the latest technologies and apply advanced business practices to speed our customers’ delivery of important therapies to patients.
Cell and Gene Therapy CDMO Solutions has supported the development of 11 FDA approved cell and gene therapies and have conducted more than 900 studies in this field over the past year. Unsurpassed end-to-end offering results in enhanced access to scientific and regulatory expertise via multidisciplinary bench of experts to help you problem solve.
Parexel (EQT Private Equity and Goldman Sachs Asset Management) with 3.8 billion USD in 2022 and 21000 employees (Parexel, 2023). Parexel is among the world’s largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders, emerging innovators, and sites to design and deliver clinical trials with patients in mind, increasing access and participation to make clinical research a care option for anyone, anywhere.
- Medpace Holdings, Inc. with 1.135 billion USD revenue in 2021 and 5400 employees (Medpace, 2023). Integrating core clinical trial services delivers efficient and streamlined execution and higher quality results. Through its wholly-owned subsidiaries, Medpace offers clinical pharmacology, as well as supporting laboratory services including central labs, bioanalytical lab, ECG core lab, and imaging core labs.
Worldwide Clinical Trials – 0.6 billion USD , 3000 employees, 60 countries.
Worldwide Clinical Trials started out more than 30 years ago as a small science team with a big goal: To always provide authentic, personalized attention to our partners. And as the industry has grown, CROs have merged, and trials have grown increasingly complex, we haven’t forgotten what truly matters: People.
With more than 3,000 employees in more than 60 countries around the world, we’re still staying true to those roots. Our team is dedicated to staying accessible, flexible, and solution-focused to ensure our partners not only have the best possible outcomes – but they also know their Worldwide team is only a call away.
Allucent (CATO SMS and Pharm-Olam) – 0.36 billion USD, 1200+ employees .
Allucent originated with CATO SMS, itself created by the merger of Cato Research and SMS-oncology in 2019. Cato Research, founded in 1988, was known for its ability to design and execute successful development strategies and guide creative new products through the regulatory process.
Previous acquisitions by CATO SMS included Array Biostatistics, a full-service biostatistical and statistical programming CRO, and Nuventra Pharma Sciences, one of the industry’s leading providers of clinical pharmacology science and services. With these acquisitions, CATO SMS expanded its services to offer biostatistical consulting, analysis, programming, and cutting-edge modeling and simulation techniques to inform clinical trial designs and predict trial outcomes.
In early 2022, CATO SMS merged with Pharm-Olam, a global clinical research organization delivering clinical trial services to organizations around the world.
KCR – 0.15 billion USD, 700 employees
KCR is a clinical development solutions provider creating value for biotechnology and pharmaceutical organizations.
Founded in 1997, our expert teams support clients with full-service clinical development capabilities across three main areas: Trial Execution, Consulting, and Placement. KCR operates across North America, Europe, and Australia, with main office locations in Boston, US, Berlin, Germany, and Warsaw, Poland.
Our geographical coverage across 25+ countries, cutting-edge technical capabilities and tailored offerings allow for the optimized delivery of solutions to develop life-changing therapies. KCR offers access to an estimated population of 1.1 billion people.
Advanced Clinical – 0.1 billion USD , 1100+ employees
Today, Advanced Clinical has grown organically, with coverage across North America, Eastern Europe, Western Europe and Asia-Pacific, providing contract research organization (CRO), FSP and strategic resourcing solutions. A research services and strategic resourcing organization committed to providing a better clinical experience by delivering comprehensive Contract Research Organization (CRO), Functional Service Provider (FSP) and Strategic Resourcing services that optimize effectiveness for both patients and sponsors throughout the clinical research journey.
Using our decades of experience, we strive to improve the lives of everyone touched by clinical research and help our clients achieve better outcomes through candid conversations, foresight, resilience and innovative solutions.
CTI Clinical Trial and Consulting Services – 0.08 billion USD, 750 employees . CTI has grown consistently and significantly, becoming a global organization with associates in more than 60 countries across the world. We have worked on more than 10,000 projects, worked on every continent except Antarctica, and have contributed to more than 150 new drug and device approvals through global regulatory agencies such as the FDA, EMA, and others. Currently, we work with approximately 250 pharmaceutical and emerging biotechnology and medical device companies.
Among recent reasons for mergers and the whole new approach to conducting clinical research, apart from pursuit of new clients, talents, expertise, products, and markets, there have been notorious coronavirus crisis and military conflicts worldwide. We faced the advent of globalization, decentralization, remote monitoring, and personalized approach to drugs R&D. But we are now looking into the era of technology in clinical research. Processes that allow remote audits and monitoring, previously considered too risky, are now risk mitigators (Bahls, Christine, 2021), at least from that point of view. Clinical trial participants enrollment, and even participation itself, constantly moves into tech dimension.
Only few among the leading CROs escaped M&As for now, but this seems to be the matter of time. Savlovschi – Wicks, Theodora (2022) estimated that “in 2021, the global CRO market is expected to reach an impressive US$88 billion by 2028”. According to IQVIA , only in biopharma sector the total transaction value grew by 70% to reach 152 billion USD by the end of 2023, featuring prominent $43Bn Pfizer-Seagen deal, and other considerable M&As for BMS-Karuna ($14Bn), Merck-Prometheus ($10.2Bn), AbbVie-Immunogen ($10.1Bn), AbbVie-Cerevel ($8.7Bn), Biogen-Reata ($7.3Bn) and Roche-Telavant ($7.1Bn).
Among the CRO businesses the following M&As took place lately:
- Charles River Labs acquires MPI Research (2018), Citoxlab (2019), HemaCare (2020) and Cognate BioServices (2021)
- IQVIA (formerly Quintiles and IMS Health) acquired Clintec and 40% of Q2 Solutions (2021)
- ICON purchased PRA Health Services (2021)
- Thermo Fisher Scientific bought PPD (2021)
- Labcorp purchased Covance and Chiltern
- Inotiv acquired Envigo (2021)
- INC Research and inVentiv Health merged to later become Syneos Health
- Parexel become the property of EQT Private Equity and Goldman Sachs Asset Management
- CATO SMS and Pharm-Olam merged in 2022
- Triley Bidco acquired Clinigen (2022)
The article by Nataliia Vietchinkina, MS in Clinical Research Administration
University of Liverpool.
References:
- Christine Bahls (2021) ‘The Post-Pandemic CRO Landscape’, Applied Clinical Trials, Applied Clinical Trials-09-01-2021, Volume 30, Issue 9 [online] Available from: https://www.appliedclinicaltrialsonline.com/view/the-post-pandemic-cro-landscape
- Savlovschi – Wicks, Theodora (2022) ‘Top 10 CROs to watch in 2022’ [online]. Available from: https://www.proclinical.com/blogs/2022-3/top-10-cros-to-watch-in-2022
- Labcorp (2022) Labcorp Announces 2021 Fourth Quarter and Full-Year Results, 10 February 2022 [online]. Available from” https://ir.labcorp.com/news-releases/news-release-details/labcorp-announces-2021-fourth-quarter-and-full-year-results#:~:text=Revenue%20was%20%2416.12%20billion%20%2C%20an,by%20divestitures%20of%20(0.1%25)
- IQVIA (2022) IQVIA Reports Fourth-Quarter and Full-Year 2021 Results; Raises Full-Year 2022 Profit Guidance, 15 February 2022 [online]. Available from: https://ir.iqvia.com/press-releases/press-release-details/2022/IQVIA-Reports-Fourth-Quarter-and-Full-Year-2021-Results-Raises-Full-Year-2022-Profit-Guidance/default.aspx#:~:text=Revenue%20of%20%2413%2C874%20million%20for,12.4%20percent%20at%20constant%20currency
- ICON (2022) ICON Reports Fourth Quarter and Full Year 2021 Results, 2022 [online]. Available from: https://www.iconplc.com/news-events/press-releases/icon-reports-fourth-quarter-and-full-year-2021-results/
- Businesswire (2021) ‘PPD Reports Fourth Quarter and Full Year 2020 Results’, 23 February 2021 [online]. Available from: https://www.businesswire.com/news/home/20210223006094/en/PPD-Reports-Fourth-Quarter-and-Full-Year-2020-Results
- Macrotrends (2022) ‘Syneos Health Revenue 2012-2021 | SYNH’ [online]. Available from: https://www.macrotrends.net/stocks/charts/SYNH/syneos-health/revenue
- https://companiesmarketcap.com/ (2022) ‘Revenue for Charles River Laboratories (CRL)’ [online]. Available from: https://companiesmarketcap.com/charles-river-laboratories/revenue/
- Vinluan, Frank (2021) ‘CRO Parexel changes private equity hands again, this time for $8.5B’ [online]. Available from: https://medcitynews.com/2021/07/cro-parexel-changes-private-equity-hands-again-this-time-for-8-5b/
- Medpace (2021) ‘Medpace Holdings, Inc. Reports Third Quarter 2021 Results’ [online]. Available from: https://investor.medpace.com/news-releases/news-release-details/medpace-holdings-inc-reports-third-quarter-2021-results#:~:text=The%20Company%20forecasts%202021%20revenue,%24176.0%20million%20to%20%24180.0%20million%20 .
- Companiesmarketcap (2022) ‘Revenue for WuXi AppTec (2359.HK) Revenue in 2021 (TTM)’ [online]. Available from: https://companiesmarketcap.com/wuxi-apptec/revenue/
- https://pharma.lonza.com (2022) ‘Design, assess and optimize for clinical success’ [online]. Available from: https://pharma.lonza.com/offerings/early-development-services .
Clinical Research News
Upcoming clinical trials.
- Center for Innovative Public Health Research University of Miami; Ann and Robert H Lurie Childrens Hospital of Chicago Recruiting Testing a Gender-inclusive HIV Prevention Program HIV Prevention United States
- Takeda Not yet recruiting A Study to Explore Hereditary Angioedema (HAE) Symptoms and Treatment Patterns in Korean People (SPEAKUP) Hereditary Angioedema (HAE)
- Abbott Medical Devices Recruiting Abbott Structural Heart Device Registry (SH-Registry) Heart Diseases | Valvular Heart Disease | PFO - Patent Foramen Ovale | VSD - Muscular Ventricular Septal Defect | PIVSD - Post Infarct Muscular Ventricular Septal Defect | ASD - Atrial Septal Defect France, Germany
- Lisata Therapeutics, Inc. Not yet recruiting LSTA1 Phase 1b/2a Continuous Infusion Trial in mPDAC (FORTIFIDE) Pancreas Adenocarcinoma | Pancreatic Ductal Adenocarcinoma | Pancreas Cancer | Pancreatic Carcinoma | Metastatic Pancreatic Cancer | Unresectable Pancreatic Cancer | PDAC | PDAC - Pancreatic Ductal Adenocarcinoma
- Yonsei University Recruiting ATezolizumab and BEvacizumab With STereotactic Body Radiotherapy for Advanced Hepatocellular Carcinoma (ATBEST) Chemotherapy | Advanced Hepatocellular Carcinoma | Stereotactic Body Radiotherapy Korea, Republic of
- The First Affiliated Hospital with Nanjing Medical... Chinese Medical Association Not yet recruiting Effects of an Information-Based Discharge Service on Preterm Infants, Parents, and Hospitals Infant, Premature | Patient Discharge
- The First Affiliated Hospital with Nanjing Medical... Not yet recruiting Multi-Technology Integrated Total Mesorectal Excision Versus Conventional Total Mesorectal Excision for the Treatment of Middle and Distal Rectal Cancer. Rectal Cancer
- Guangzhou Medical University Recruiting Feasibility and Safety of Remote Robotic Bronchoscopy System in Diagnosis of Peripheral Pulmonary Lesions: a Multicenter, Randomized Controlled, Proof-of Concept Trial Pulmonary Nodule China
- The General Hospital of Western Theater Command Recruiting Clinical Study of Combined Platelet Transfusion Hematologic Diseases China
- Gerd Care Medical Ltd Recruiting Safety and Effectiveness of eGERD Device to Reduce Gastroesophageal Reflux Disease (GERD) Symptoms GERD (Gastroesophageal Reflux Disease) Israel
- Gazi University The Scientific and Technological Research Council of Turkey Recruiting The Effect of Mobile Application Use on Drug Administration Knowledge and Skill Level of Pediatric Nursing Students Nursing Caries | Nurse's Role Turkey
- Duke University Medical University of South Carolina; Eunice Kennedy Shriver National Institute... and other collaborators Not yet recruiting PK/PD of Digoxin in Infants With SVHD Infant, Newborn, Diseases | Infant, Premature, Diseases | Infant Conditions | Single-ventricle United States
Clinical Research Jobs
Sponsors and Collaborators
- Assiut University
- GlaxoSmithKline
- National Cancer Institute (NCI)
- Cairo University
- AstraZeneca
- Assistance Publique - Hôpitaux de Paris
- Mayo Clinic
- M.D. Anderson Cancer Center
- Novartis Pharmaceuticals
- National Institute of Allergy and Infectious Diseases (NIAID)
- Massachusetts General Hospital
- National Taiwan University Hospital
- Boehringer Ingelheim
- Hoffmann-La Roche
- Merck Sharp & Dohme LLC
- Stanford University
- University of California, San Francisco
- Eli Lilly and Company
- Duke University
- View Full List
Medical Conditions
- Breast Cancer
- Hypertension
- Prostate Cancer
- HIV Infections
- Coronary Artery Disease
- Heart Failure
- Diabetes Mellitus, Type 2
- Colorectal Cancer
- Lung Cancer
- Schizophrenia
- Cardiovascular Diseases
- Atrial Fibrillation
Drug Interventions
- Cyclophosphamide
- Carboplatin
- Dexamethasone
- Gemcitabine
- Capecitabine
- Bevacizumab
- Pembrolizumab
- Methotrexate
- Oxaliplatin
- Dexmedetomidine
- Fludarabine
- +91-7770040105
- [email protected]

Get A Quote
- Our Journey
- Awards & News
- Quality Assurance Unit (QAU)
- Test Item Control Office – TICO
Animal Research Facility (ARF)
- In-vitro Facilities
Environment, Health and Safety
- Toxicology Services
- Pathology Services
- Pharmacokinetics Services
- In-vitro & genotoxicity Services
- Biology Services
- Biocompatibility Services
- Risk Assessment Services
- Pharmacuetical
Medical Devices
- Agrochemical

- QUALITY ASSURANCE UNIT (QAU)

QAU at PRADO works as “A defined system, including personnel who are independent of studies being conducted and are committed to assure test facility management for maintenance of compliance with the principles of Good Laboratory Practice”.
Welcome To PRADO, Preclinical Research and Development Organization Private Limited
Where We Are Dedicated To Providing Notch Services That Cater To Your Research Needs
What Is PRADO
PRADO, Preclinical Research and Development Organization Private Limited is one of the emerging, global, independent market leaders in providing preclinical services. Following services are provided by PRADO – Toxicity Testing ( In-Vitro and In-Vivo ), Development of Animal Models for Human Diseases, Pharmacokinetic Studies, Biocompatibility Studies and Pathology Services. Determination of the Permitted Daily Exposure (PDE) and Occupational Exposure Level (OEL) of chemicals in compliance with EMA guidelines. Based at Pune India, PRADO is developing an outstanding reputation for providing excellent services and superior values. Our scientific data is of highest quality and our reports have excellent acceptance record with regulatory agencies, facilitating quick product registration and approval.
PRADO supports Pharmaceutical, Biopharma, Biotech, Medical Device, Pesticides and Agrochemicals, Food,Vaccine and Animal Health Industries by providing quality and cost effective preclinical services to meet their regulatory requirements. PRADO has all required technical expertise, research and development skills to provide excellent services. At PRADO, we understand how important in the on-time delivery for meeting the goals for regulatory submissions. We aim to work closely with our clients to understand their preclinical needs and develop program to meet their goals in timely manner. Thus, PRADO can be a strategic and scientific, yet responsive, flexible and trust worthy partner for your pre-clinical support.

Quality Policy

PRADO, Preclinical Research and Development Organization Private Limited is one of the emerging, global, independent market leaders in providing preclinical services. The following services are provided by PRADO – Toxicity Testing ( In-Vitro and In-Vivo ), Development of Animal Models for Human Diseases, Pharmacokinetic Studies, Biocompatibility Studies and Pathology Services. Determination of the Permitted Daily Exposure (PDE) and Occupational Exposure Level (OEL) of chemicals in compliance with EMA guidelines. Based in Pune India, PRADO is developing an outstanding reputation for providing excellent services and superior values. Our scientific data is of the highest quality and our reports have excellent acceptance records with regulatory agencies, facilitating quick product registration and approval.
PRADO supports Pharmaceutical, Biopharma, Biotech, Medical Devices, Pesticides and Agrochemicals, Food, Vaccine, and Animal Health Industries by providing quality and cost-effective preclinical services to meet their regulatory requirements. PRADO has all required technical expertise, research, and development skills to provide excellent services. At PRADO, we understand how important in the on-time delivery for meeting the goals for regulatory submissions. We aim to work closely with our clients to understand their preclinical needs and develop programs to meet their goals in a timely manner. Thus, PRADO can be a strategic and scientific, yet responsive, flexible, and trustworthy partner for your pre-clinical support.

Mission Statement
It is both our aspiration and commitment to place our values in everything we do to deliver the high quality integrated preclinical services building positive environment and sponsor satisfaction with achievement of safe and economic healthcare product development for mankind.
Our primary purpose and mission is to become a destination where all product development and registration for preclinical services available under one umbrella with international compliance to meet all our sponsor’s requirements.
We Build our reputation in preclinical excellence and wide scope speciality services at PRADO, we combine high quality research services at affordable cost. This is the reason why our sponsor’s have been entrusting values with us for their product development and registration work with confidence.

We are committed toquality as an internal part of our operations with the goal of achieving preclinical excellence and sponsor's satisfaction with consideration of safety by continuous improvement of our capabilities to identify, develop and provide services that adds value to our sponsors in Good Laboratory Practice in Preclinical Services.

Certifications

Services We Provide

Discovery and GLP Toxicity Studies. Acute, Subacute, Sub-chronic and Chronic Toxicity, Inhalation Toxicity, Genotoxicity Developmental and Reproductive Toxicity.

Clinical Pathology (Haematology, Clinical chemistry, Urine Analysis), Histopathology slide preparation and evaluation. Special Staining and Immunohistochemistry.
Pharmacokinetic
Pharmacokinetic and Tissue Distribution studies with single, multiple and cassette Dosing. Species- Rats, Mice, Rabbits, Pigs and Dogs (3 rd party collaboration).

Genotoxicity
Reverse Mutation Assay (Ames Assay, Mini Ames), Micronucleus Test (MNT), Chromosomal Aberration Assay, Cytotoxicity Tests, Hemocompatibility,…
Development of animal model of human diseases. E.g. Inflammation and Pain, Metabolic Disorders, Antipsychotic Activity, Osteoporosis, Wound Healing…

Biocompatibility
The interaction between a medical device and the human body need to remain effective and safe over the lifetime of both the device as well as its recipient.
Service we provide
Pharmacokinetic and Tissue Distribution studies with single, multiple and cassette Dosing. Species- Rats, Mice, Rabbits, Pigs and Dogs (3rd party collaboration).
Reverse Mutation Assay (Ames Assay, Mini Ames), Micronucleus Test (MNT), Chromosomal Aberration Assay, Cytotoxicity Tests, Hemocompatibility,...
Development of animal model of human diseases. E.g. Inflammation and Pain, Metabolic Disorders, Antipsychotic Activity, Osteoporosis, Wound Healing...
Clinical Pathology (Haematology, Clinical chemistry, Urine Analysis), Histopathology slide preparation and evaluation. Special Staining and Immunohistochemistry
Development of animal model of human diseases. E.g. Inflammation and Pain, Metabolic Disorders, Antipsychotic Activity, Osteoporosis, Wound Healing or any other model as per sponsor requirement.
Reverse Mutation Assay (Ames Assay, Mini Ames), Micronucleus Test (MNT), Chromosomal Aberration Assay, Cytotoxicity Tests, Hemocompatibility, Device Implantation, Irritation studies.
Industries We Serve

PHARMACUETICAL
The pharmaceutical industry discovers, develops and market the pharmaceutical drugs for use as medications to be administered to patients.

Biopharmaceuticals and Biotechnology are becoming increasingly importantas novel and less toxic therapeutics for human disease.
The determination of the safety or biocompatibility of a device in a biological host environment is critical step in the development of an entire...

AGROCHEMICAL
At PRADO, we understand the need for accurate and reliable data for regulatory submissions such as those of the Central Insecticide Board.

Biopharmaceuticals and Biotechnology are becoming increasingly important in the development of novel and less toxic
Infectious diseases cause an overwhelming number of deaths around the world and their clinical management is often hampered by the emergence...
Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using...

Risk Assessment
Setting health-based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities...
Food Biopharmaceuticals and Biotechnology are becoming increasingly important in the development of novel and less toxic Vaccine Infectious diseases cause an overwhelming number of deaths around the world and their clinical management is often hampered by the emergence... Stem Cell Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using... Risk Assessment Setting health-based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities... PHARMACUETICAL The pharmaceutical industry discovers, develops and market the pharmaceutical drugs for use as medications to be administered to patients. BIO-PHARMA Biopharmaceuticals and Biotechnology are becoming increasingly importantas novel and less toxic therapeutics for human disease. Medical Devices The determination of the safety or biocompatibility of a device in a biological host environment is critical step in the development of an entire... AGROCHEMICAL At PRADO, we understand the need for accurate and reliable data for regulatory submissions such as those of the Central Insecticide Board. Facilities
Quality assurance unit, test item control office, in vitro facility, prado’s animal research facility (arf) has been designed to meet the global standards of conducting experiments on small laboratory animals. the arf has approvals from cpcsea, ministry of environment and forests, government of india. know more, the pathology department at prado, have specially designed laboratory areas for histopathology and clinical pathology. a spacious necropsy room is available for blood collection, plasma separation, conduct of gross pathology examination, organ weights and organ collection. know more, at prado, quality assurance unit (qau) is separate department, established in accordance with the requirements of oecd principles of glp. qau conducts, study based, facility based and process-based inspections, as per the standard operating procedures (sops). know more, at prado, a designated, glp compliant test item control office (tico) is present. the tico area is separated based on different types of test items such as pharmaceutical, biopharmaceutical, stem cells, medical devices and agrochemical etc. know more, prado understands its corporate- social responsibility, towards this prado has established solar system to save the electricity and making use of natural renewable energy. for processing of facility waste, bio-digester system has been in place. know more, the safe and secure storage of the facility and study related data and material is required for oecd principles of glp. prado has suitably designed and constructed separate wet and dry archives. availability of ample space within the archives to accommodate archived records and material. archiving procedure are ensured through strict compliance with sops. know more, the in-vitro laboratories at prado are spread over 600 sqft and are designed for carrying out genotoxicity and biocompatibility studies, including cytotoxicity studies. know more.

PRADO’s Animal Research Facility (ARF) has been designed to meet the global standards of conducting experiments on small laboratory animals. The ARF has approvals from CPCSEA, Ministry of Environment and Forests, Government of India

The Pathology department at PRADO, have specially designed laboratory areas for histopathology and clinical pathology. A spacious necropsy room is available for blood collection, plasma separation, conduct of gross pathology examination, organ weights and organ collection.

At PRADO, Quality Assurance Unit (QAU) is separate department, established in accordance with the requirements of OECD principles of GLP. QAU conducts, study based, facility based and process-based inspections, as per the standard operating procedures (SOPs).

At PRADO, a designated, GLP compliant Test Item Control Office (TICO) is present. The TICO area is separated based on different types of test items such as pharmaceutical, biopharmaceutical, stem cells, medical devices and agrochemical etc.

PRADO understands its Corporate- social responsibility, towards this PRADO has established solar system to save the electricity and making use of natural renewable energy. For processing of facility waste, bio-digester system has been in place.
The safe and secure storage of the facility and study related data and material is required for OECD principles of GLP. PRADO has suitably designed and constructed separate Wet and Dry Archives. Availability of ample space within the archives to accommodate archived records and material. Archiving procedure are ensured through strict compliance with SOPs.

The in-vitro laboratories at PRADO are spread over 600 sqft and are designed for carrying out genotoxicity and biocompatibility studies, including cytotoxicity studies.
Certification & Awards
- PRADO received recognition by DSIR (Department Of Scientific & Industrial Research,Govt. Of India).
- PRADO has successfully received GLP certificate at our new location. Certificate No:GLP/C-168/2021
- We have successfully completed the relocation inspection of our Test Facility by NGCMA.
- PRADO received GLP certification at previous location - Certificate No: GLP/C-127/2018. We have relocated our test facility, awaiting renewal of GLP certificate.
- PRADO Received 'Fastest Growing Indian Company Excellence Award 2017' by International Achievers Conference at Bangkok, Thailand.
- Our CEO and Director received 'Indian Leadership Award for Industrial Development' from All India Achievers Foundation in 2017.
- Received CPCSEA certificate (1723/PO/RcBiBt/13/CPCSEA). Our new facility has also been inspected and approved by CPCSEA.

Certification & Awards
Our team members.

Dr. Pralhad Wangikar
Director and CEO

Ms. Ila Wangikar
Managing Director

Dr. Pradeep Deshmukh
Deputy Test Facility Management

- Phone: +1 (859) 254-6589
- Email: [email protected]


MOU signed with life sciences sector skill development council of india (lsssdc) in 2017
Awarded as “ best institute for clinical research in india ” in asia education summit & awards in 2017, certified as iso 9001:2015 company in 2016, “ certificate of commitment ” issued by central vigilance commission of india in 2016, agreements signed with multiple companies in uk, south africa, usa and malaysia for clinical research development segment in 2015-17, sister concern brand of prorelix research since 2017.
Students trained
Years of experience
Countries our student’s are from
Placement rate
Top Programs Online Mode
Ich gcp – good clinical practice course (paid).
Welcome to the course ICH GCP – Good Clinical Practice. This course gives an opportunity to get in depth knowledge of ICH GCP.
ICH GCP – Good Clinical Practice Course(Free)
Welcome to the Free course ICH GCP – Good Clinical Practice. This course gives an opportunity to get in depth knowledge of ICH GCP..
The beginner Course for Clinical Research
Nam dictum felis sed diam volutpat ultricies. Ut ultricies, dolor eu fermentum molestie, tortor libero pulvinar ipsum, nec congue leo elit at eros.
Top Programs Distance Mode
Professional certificate in pharmacovigilance.
This is to develop all pharmacy, medical, lifescience background students in the duration of 2 months without attending classroom training.
Professional Certificate In Clinical Data Management
Professional certificate in clinical trial, professional certificate in ich-gcp, ethics & regulations in clinical research, top programs up skilling, certification in crc.
Obtain your Certification in CRC from LSSSDC and achieve your professional clinical research coordinator NSQF level 5 certificate in just 15 days.
Certification for CRA
Obtain your Certification in CRA from LSSSDC and achieve your professional clinical research coordinator NSQF level 5 certificate in just 15 days.
Certification For Medical Writing
Obtain your Certification in Medical Writing from LSSSDC and achieve your professional clinical research coordinator NSQF level 5 certificate in just 15 days.
ProRelix Education Knowledge Hub
Career and Scope
Clinical Data Management
Clinical Research
Drug Regulatory Affairs
Pharmacovigilance
“The Secret Of Getting Ahead Is Getting Started.”
Happy STUDENT'S
What Student’s Say
Aishwarya nikam.
Trainee Clinical Data Coordinator (IQVIA)
I’ve completed my course at ProRelix Education. It was a wholesome experience of learning. The faculty is helpful and teaches very well, the trainers are available all time for query resolution.
Shweta Birajdar
Pharmacovigilance Trainee (TCS)
End-to-end query resolution, and placement assistance from the beginning. They also conduct group discussions, and quiz competitions to improve communication skills of students. Thanks for the opportunity!
Prerana Garje
Junior Data Analyst (IKS Health)
The courses are excellent and easy to understand; the trainers are knowledgeable and helpful; would recommend anyone learning about deep clinical research knowledge.
Neha Pachorkar
CRC (Sahyadri Hospital)
ProRelix Education provided us with the best study materials in both hard copies and digital formats. They made it easy for us to learn. Plus, they conduct multiple guest lectures from industry experts people, Thanks to their efforts as a result, I successfully secured a position as a Clinical Research Coordinator
Neha Mandrekar
Safety Data Analyst (Covance)
I started perusing clinical research in ProRelix Education in January. I got placement in Covance Bangalore in Pharmacovigilance department through ProRelix Education. The overall experience which I had in this institution was amazing. Trainers are really helpful and they got us through every difficulty. And I would recommend this course each and every one who wants to start their career in clinical research.
Indrajeet Patwardhan
Safety Data Analyst (Sciformix technology)
I enrolled in ProRelix Education for the Post Graduate Diploma in Clinical Research. This is a 6 months duration course and I got placement in Sciformix technology as a safety data analyst within 3 months of the post graduate course. I recommend all of you to join the ProRelix Education institute so just don’t see your dreams chase them and fulfill them because this institute has a well trained and qualified staff they are very supportive.
Industries Hiring
Embark on a journey in clinical research today.
Enroll today and embark on a fulfilling journey toward becoming a proficient and knowledgeable professional in the field of clinical research.
Select Course Full Time - Post Graduate Diploma In Clinical Research Online - ICH-GCP Good Clinical Pratice (Free) Online - ICH-GCP Good Clinical Pratice (Paid) Distance - Professional Certificate in Pharmacovigilance Distance - Professional Certificate In Clinical Data Management Distance - Professional Certificate In Clinical Trial Distance - Professional Certificate In Pharmacovigilance Distance - Professional Certificate In ICH-GCP Ethics & Regulations In Clinical Research Up Skilling - Certification In CRC Up Skilling - Certification For CRA Up Skilling - Certification For Medical Writing

IMAGES
VIDEO
COMMENTS
Looking For Top Clinical Trial Companies In India Mprex Is One Of The Best Clinical Research Organization In India At Pune. 501/514, Crossroad Building, Bhumkar square, Wakad, Pune 411057; Mon - Sat 10.00 - 06.00 ... Mprex Healthcare delivers advanced clinical research solutions and consultation through a broad suite of expert capabilities ...
Ardent Clinical Research Services is western India's one of the top-notch full-service clinical research organizations, operating from Pune, Maharashtra and Hyderabad India ... Pune- 411006, MH, India; Branch Office - Wework Krishe Emerald, Kondapur, Hyderabad, TS, India; [email protected]; 7507779564; @copyright 2012-2024 I ARDENT CLINICL ...
Synapse Labs is a DCGI approved CRO (Contract Research Organization) based in Pune, India. Synapse Labs is a leading, independent Contract Research Organization (CRO) founded in 2007. We are offering comprehensive Bioavailability / Bioequivalence (BA/BE),Clinical Trial service,Pharmacovigilance and Regualtory Services to global pharmaceutical ...
Synergen Bio Private Limited is a DCGI (CDSCO) approved new age CRO with all necessary infrastructure to cater to the Pharmaceutical and Biotech industry, established by a group of enthusiastic professionalswith a vision to set-up a premium Clinical Research Organization known for its Quality, Knowledge and Expertise.
ProRelix Research is a Clinical Research Organization (CRO) with offices in the USA and India along with site networks in Australia, Thailand, Africa, and Europe supporting the clinical development of innovative new healthcare inventions and innovations to improve the health of patients. Every project is equally important to us. We first listen to you and strategize, aiming to a long-term ...
Life -Long Partners In Drug Development. Lifepoint is well known as the Best Clinical Research Institute in Pune & India. It has the best-in-the-class infrastructure for conducting clinical trials and a large and diverse therapy-naive patient pool to tap as well. We have well established research setup in 4500 square feet, which includes ...
Noble Hospital provides clinical research services for pharmaceutical and biotechnology product development from Phase I to Phase IV. For appointments +91-8007006611 / 8000016611 24/7 EMERGENCY: 105900
Raptim Research is a global independent & internationally accredited Contract Research Organization (CRO) in Mumbai & Gandhinagar (India) since 2005. raptim research pvt. ltd. Operating with the latest COVID-19 guidelines ... (CRO), established in 2005 with state-of-the-art clinical research facilities in Mumbai and Gandhinagar (India). Our ...
SIRO - India's top CRO for clinical research services, operations, consulting, pharmacovigilance, and trials management. Trusted in Indian clinical research. ... END TO END CLINICAL RESEARCH ORGANIZATION. One stop shop for services ranging from medical writing and clinical operations, to data management and biostatistics.
Synapse Labs is a leading, independent Contract Research Organization (CRO) founded in 2007. Since its establishment, this visionary organization owns exceptional identity which resulted in naming it as 'Synapse'. ... Nr. Nyati Empire, Kharadi Bypass, Kharadi, Pune, Maharashtra 411014; Clinical Trials and Pharmacovigilance Office-Pride Icon ...
IVAAN is a Clinical Research Organization which provides services on Data Management, Biostatistics and SAS programming to our clients covering all phases and therapeutic areas of drug development including Bioavailability and Bioequivalence studies for the Pharmaceutical, Medical Device, Biopharmaceutical, and CRO industries. ... Pune. Contact ...
Innovate Research. Innovate Research stands as a leading Contract Research Organization (CRO) dedicated to providing comprehensive services in the dynamic field of clinical research, established in January 2014 With a strategic presence across the globe, our offices ar... 💻 Website ↗ 📞 +91 78277 58840 View all details.
Sahyadri Clinical Research and Development Center was established in 2006. Sahyadri Clinical Research and Development Center & excels in coordinating a research driven team which has a well designed infrastructure with all modern amenities providing a milieu conducive to the advancement of professional and personal excellence. Well defined SOPs.
Here are a few reasons why CLINI INDIA is recognized as the best clinical research institute in Pune: Cutting-Edge Curriculum: Our comprehensive curriculum is meticulously crafted to align with industry standards and equip our students with the latest knowledge and skills required for success in the dynamic field of clinical research. Expert ...
Clinical Research Services in Pune. Clinical research is a vital industry working on translating basic medical discoveries into working treatments. It is the study of data or samples to understand health and disease. Our Clinical Research Services in Pune helps to find new and better ways to detect, diagnose, treat, and prevent diseases.
Advance your career with clinical research training programs, offering comprehensive courses, develop expertise in clinical research industry. ... Magarpatta City, Hadapsar, Pune - 411013. 8886904030. Quick Links. Home; About Us; Eligibility; Placement Policy; Career & Jobs; Pay Fee; List of Clinical Research & Pharmacovigilance Companies;
Precision is a leader in oncology and rare disease research. Our deep experience in complex indications accelerates the ability to reach key milestones, drive positive outcomes and most importantly, help patients get access to innovative treatments. Contact us. 70 of clinical trials are in oncology. 2500 clinical trial and lab projects.
The top 15 CROs globally would be in the following order based on 2023 revenue: LabCorp (Covance), with 15.05 billion USD (2023) and 75000 employees (Labcorp, 2024). In February 2015, Labcorp completes its $6 billion purchase of Covance, Inc., creating the world's leading health care diagnostics company.
Experience: research: 2 years (Required) healthcare: 2 years (Required) Work Location: In person. 27 Clinical Research Organization jobs available in Pune, Maharashtra on Indeed.com.
Based in Pune India, PRADO is developing an outstanding reputation for providing excellent services and superior values. Our scientific data is of the highest quality and our reports have excellent acceptance records with regulatory agencies, facilitating quick product registration and approval. PRADO supports Pharmaceutical, Biopharma, Biotech ...
Associate Director, Sales (EMEA) Saama Technologies Inc. Remote in Hinjewadi, Pune, Maharashtra. 10+ years of experience in a market of the competitive landscape, find companies looking to scale in the clinical trial portfolio. Posted 30+ days ago ·.
ProRelix Education is an award-winning clinical research training institute in Pune, India that offers the best postgraduate diploma or professional skill enhancement courses in Clinical research, Hospital and pharma product management, Drug regulatory affairs, Pharmacovigilance, Clinical data management, Clinical trials, ICH GCP Ethics & Regulations, and much more.