- Case Report
- Open access
- Published: 25 November 2008

A case of PTSD presenting with psychotic symptomatology: a case report
- Georgios D Floros 1 ,
- Ioanna Charatsidou 1 &
- Grigorios Lavrentiadis 1
Cases Journal volume 1 , Article number: 352 ( 2008 ) Cite this article
30k Accesses
4 Citations
1 Altmetric
Metrics details
A male patient aged 43 presented with psychotic symptomatology after a traumatic event involving accidental mutilation of the fingers. Initial presentation was uncommon although the patient responded well to pharmacotherapy. The theoretical framework, management plan and details of the treatment are presented.
Recent studies have shown that psychotic symptoms can be a hallmark of post-traumatic stress disorder [ 1 , 2 ]. The vast majority of the cases reported concerned war veterans although there were sporadic incidents involving non-combat related trauma (somatic or psychic). There is a biological theoretical framework for the disease [ 3 ] as well as several psychological theories attempting to explain cognitive aspects [ 4 ].
Case presentation
A male patient, aged 43, presented for treatment with complaints tracing back a year ago to a traumatic work-related event involving mutilation of the distal phalanges of his right-hand fingers. Main complaints included mixed hallucinations, irritability, inability to perform everyday tasks and depressive mood. No psychic symptomatology was evident before the event to him or his social milieu.
Mental state examination
The patient was a well-groomed male of short stature, sturdy build and average weight. He was restless but not agitated, with a guarded attitude towards the interviewer. His speech pattern was slow and sparse, his voice low. He described his current mood as 'anxious' without being able to provide with a reason. Patient appeared dysphoric and with blunted affect. He was able to maintain a linear train of thought with no apparent disorganization or irrational connections when expressing himself. Thought content centred on his amputated fingers with a semi-compulsive tendency to gaze to his (gloved) hand. The patient was typically lost in ruminations about his accident with a focus on the precise moment which he experienced as intrusive and affectively charged in a negative and painful way. He could remember wishing for his fingers to re-attach to his hand almost as the accident took place. A trigger in his intrusive thoughts was the painful sensation of neuropathic pain from his half-mutilated fingers, an artefact of surgery.
He denied and thoughts of harming himself and demonstrated no signs of aggression towards others. Hallucinations had a predominantly depressive and ego-dystonic character. He denied any perceptual disturbances at the time of the examination. Their appearance was typically during nighttime especially in the twilight. Initially they were visual only, involving shapes and rocks tumbling down towards the patient, gradually becoming more complex and laden with significance. A mixed visual and tactile hallucination of burning rain came afterwards while in the time of examination a tall stranger clad in black and raiding a tall steed would threaten and ridicule the patient. He scored 21 on a MMSE with trouble in the attention, calculation and recall categories. The patient appeared reliable and candid to the extent of his self-disclosure, gradually opening up to the interviewer but displayed a marked difficulty on describing his emotions and memories of the accident, apparently independent of his conscious will. His judgement was adequate and he had some limited Insight into his difficulties, hesitantly attributing them to his accident.
He was married and a father of three (two boys and a girl aged 7–12) He had no prior medical history for mental or somatic problems and received no medication. He admitted to occasional alcohol consumption although his relatives confirmed that he did not present addiction symptoms. He had some trouble making ends meet for the past five years. Due to rampant unemployment in his hometown, he was periodically employed in various jobs, mostly in the construction sector. One of his children has a congenital deformity, underwent several surgical procedures with mixed results and, before the time of the patient's accident, it was likely that more surgery would be forthcoming. The patient's father was a proud man who worked hard but reportedly was victimized by his brothers, they reaping the benefits of his work in the fields by manipulating his own father. He suffered a nervous breakdown attributed to his low economic status after a failed economic endeavour ending in him being robbed of the profits, seven years before the accident. There was no other relevant family history.
Before the accident the patient was a lively man, heavily involved as a participant and organizer in important local social events from a young age. He was respected by his fellow villagers and felt his involvement as a unique source of pride in an otherwise average existence. Prior to his accident, the patient was repeatedly promised a permanent job as a labourer and fate would have it that his appointment was supposedly approved immediately after the accident only to be subsequently revoked. He viewed himself as an exploited man in his previous jobs, much the same way his father was, while he harboured an extreme bitterness over the unavailability of support for his long-standing problems. His financial status was poor, being in sick-leave from his previous job for the last four months following the accident and hoping to receive some compensation. Although his injuries were considered insufficient for disability pension he could not work to his full capacity since the hand affected was his primary one and he was a manual labourer.
Given that the patient clearly suffered a high level of distress as a result of his hallucinatory experiences he was voluntary admitted to the 2nd Psychiatric Department of the Aristotle University of Thessaloniki for further assessment, observation and treatment. A routine blood workup was ordered with no abnormalities. A Rorschach Inkblot Test was administered in order to gain some insight into patient's dynamics, interpersonal relations and underlying personality characteristics while ruling out any malingering or factitious components in the presentation as suggested in Wilson and Keane [ 5 ]. Results pointed to inadequate reality testing with slight disturbances in perception and a difficulty in separating reality from fantasy, leading to mistaken impressions and a tendency to act without forethought in the face of stress. Uncertainty in particular was unbearable and adjustment to a novel environment hard. Cognitive functions (concentration, attention, information processing, executive functions) were impaired possibly due to cognitive inability or neurological disease. Emotion was controlled with a tendency for impulsive behaviour; however there was difficulty in processing and expressing emotions in an adaptive manner. There were distinct patterns of aggression and anger towards others but expressing those patterns was avoided, switching to passivity and denial rather than succumbing to destructive urges or mature competitiveness. Self-esteem was low with feelings of inferiority and inefficiency.
A neurological examination revealed a left VI cranial nerve paresis, reportedly congenital, resulting in diplopia while gazing to the extreme left, which did not significantly affect the patient. The patient had a chronic complaint of occasional vertigo, to which he partly attributed his accident, although the symptoms were not of a persisting nature.
Initial diagnosis at this stage was 'Psychotic disorder NOS' and pharmacological treatment was initiated. An MRI scan of the brain with gadolinium contrast was ordered to rule out any focal neurological lesions. It was performed fifteen days later and revealed no abnormalities.
Patient was placed on ziprasidone 40 mg bid and lorazepam 1 mg bid. He reported an immediate improvement but when the attending physician enquired as to the nature of the improvement the patient replied that in his hallucinations he told the tall raider that he now had a tall doctor who would help him and the raider promptly left (sic). Apparently, the random assignment of a strikingly tall physician had an unexpected positive effect. Ziprasidone gradually increased to 80 mg bid within three days with no notable effect to the perceptual disturbances but with the development of akathisia for which biperiden was added, 1 mg tid. Duloxetine was added, 60 mg once-daily, in a hope that it could have a positive effect to his mood but also to this neuropathic pain which was frequent and demoralising. The patient had a tough time accommodating to the hospital milieu, although the grounds were extended and there was plenty of opportunity for walks and other activities. He preferred to stay in bed sometimes in obvious agony and with marked insomnia. He presented a strong fear for the welfare of his children, which he could not reason for. Due to the apparent inability of ziprasidone to make a dent in the psychotic symptomatology, medication was switched to amisulpride 400 mg bid and the patient was given a leave for the weekend to visit his home. On his return an improvement in his symptoms was reported by him and close relatives, although he still had excessive anxiety in the hospital setting. It was decided that his leave was to be extended and the patient would return for evaluation every third day. After three appointments he had a marked improvement, denied any psychotic symptoms while his sleep pattern improved. A good working relationship was established with his physician and the patient was with a schedule of follow-up appointments initially every fifteen days and following two months, every thirty days. His exit diagnosis was "Psychotic disorder Not Otherwise Specified – PTSD". He remained asymptomatic for five months and started making in-roads in a cognitively-oriented psychotherapeutic approach but unfortunately further trouble befell him, his wife losing a baby and his claim to an injury compensation rejected. He experienced a mood loss and duloxetine was increased to 120 mg per day to some positive effect. His status remains tenuous but he retains a strong will to make his appointments and work with his physician. A case conceptualization following a cognitive framework [ 6 ] is presented in Figure 1 .
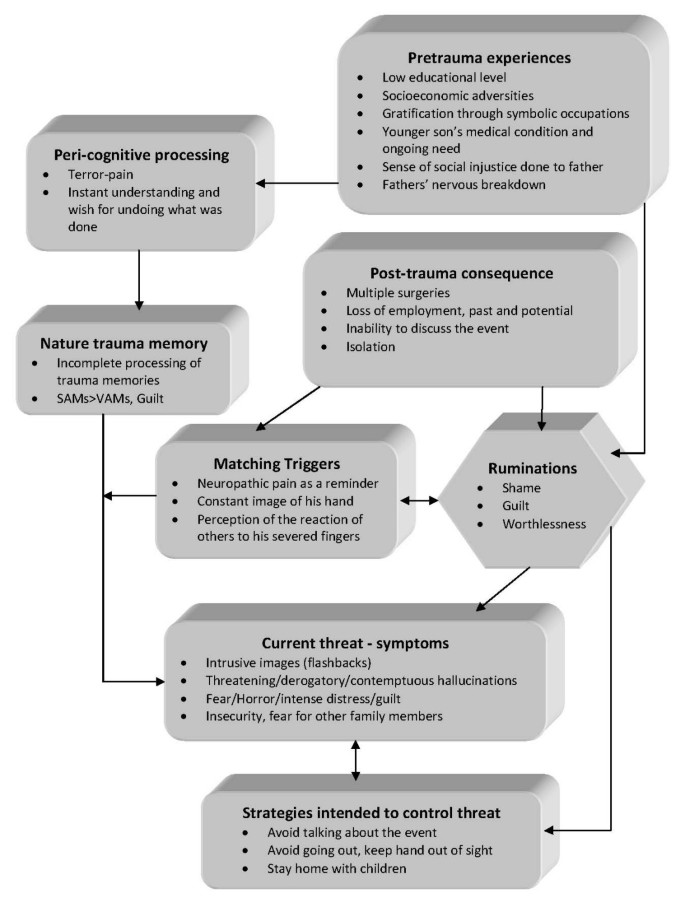
Case formulation – (Persistent PTSD, adapted from Ehlers and Clark [ 6 ] ) . Case formulation following the persistent PTSD model of Ehlers and Clark [ 6 ]. It is suggested that the patient is processing the traumatic information in a way which a sense of immediate threat is perpetuated through negative appraisals of trauma or its consequences and through the nature of the traumatic experience itself. Peri-traumatic influences that operate at encoding, affect the nature of the trauma memory. The memory of the event is poorly elaborated, not given a complete context in time and place, and inadequately integrated into the general database of autobiographical knowledge. Triggers and ruminations serve to re-enact the traumatic information while symptoms and maladaptive coping strategies form a vicious circle. Memories are encoded in the SAM rather than the VAM system, thus preventing cognitive re-appraisal and eventual overcoming of traumatic experience [ 4 ].
The value of a specialized formulation is made clear in complex cases as this one. There is a relationship between the pre-existing cognitive schemas of the individual, thought patterns emerging after the traumatic event and biological triggers. This relationship, best described as a maladaptive cognitive processing style, culminates into feelings of shame, guilt and worthlessness which are unrelated to similar feelings, which emerge during trauma recollection, but nonetheless acts in a positive feedback loop to enhance symptom severity and keep the subject in a constant state of psychotic turmoil. Its central role is addressed in our case formulation under the heading "ruminations" which best describes its ongoing and unrelenting character. The "what if" character of those ruminations may serve as an escape through fantasy from an unbearably stressful cognition. Past experience is relived as current threat and the maladaptive coping strategies serve as negative re-enforcers, perpetuating the emotional suffering.
The psychosocial element in this case report, the patient's involvement with a highly symbolic activity, demonstrates the importance of individualising the case formulation. Apparently the patient had a chronic difficulty in expressing his emotions and integrating into his social surroundings, a difficulty counter-balanced somewhat with his involvement in the local social events which gave him not only a creative way out from any emotional impasse but also status and recognition. His perceived inability to continue with his symbolic activities was not only an indicator of the severity of his troubles but also a stressor in its own right.
Complex cases of PTSD presenting with hallucinatory experiences can be effectively treated with pharmacotherapy and supportive psychotherapy provided a good doctor-patient relationship is established and adverse medication effects rapidly dealt with. A cognitive framework and a Rorschach test can be valuable in deepening the understanding of individuals and obtaining a personalized view of their functioning and character dynamics. A biopsychosocial approach is essential in integrating all aspects of the patients' history in a meaningful way in order to provide adequate help.
Patient's perspective
"My life situation can't seem to get any better. I haven't had any support from anyone in all my life. Leaving home to go anywhere nowadays is hard and I can't seem to be able to stay anyplace else for a long time either. Just getting to the hospital [where the follow-up appointments are held] makes me very nervous, especially the minute I walk in. Can't seem to stay in place at all, just keep pacing while waiting for my appointment. I am only able to open up somewhat to my doctor, whom I thank for his support. Staying in hospital was close to impossible; I was very stressed and particularly concerned for my children, not being able to be close to them. I still need to have them near-by. Getting the MRI scan was also a stressful experience, confined in a small space with all that noise for so long. I succeeded only after getting extra medication.
I hope that things will get better. I don't trust anyone for any help any more; they should have helped me earlier."
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
stands for 'Post Traumatic Stress Disorder'
for 'Verbally Accessible Memory'
for 'Situationally Accessible Memory'
Butler RW, Mueser KT, Sprock J, Braff DL: Positive symptoms of psychosis in posttraumatic stress disorder. Biological Psychiatry. 1996, 39: 839-844. 10.1016/0006-3223(95)00314-2.
Article CAS PubMed Google Scholar
Seedat S, Stein MB, Oosthuizen PP, Emsley RA, Stein DJ: Linking Posttraumatic Stress Disorder and Psychosis: A Look at Epidemiology, Phenomenology, and Treatment. The Journal of Nervous and Mental Disease. 2003, 191: 675-10.1097/01.nmd.0000092177.97317.26.
Article PubMed Google Scholar
Nutt DJ: The psychobiology of posttraumatic stress disorder. J Clin Psychiatry. 2000, 61: 24-29.
CAS PubMed Google Scholar
Brewin CR, Holmes EA: Psychological theories of posttraumatic stress disorder. Clinical Psychology Review. 2003, 23: 339-376. 10.1016/S0272-7358(03)00033-3.
Wilson JP, Keane TM: Assessing Psychological Trauma and PTSD. 2004, The Guilford Press
Google Scholar
Ehlers A, Clark DM: A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy. 2000, 38: 319-345. 10.1016/S0005-7967(99)00123-0.
Download references
Acknowledgements
The authors wish to acknowledge the valuable support and direction offered by the department's chair, Professor Ioannis Giouzepas who places the utmost importance in creating a suitable therapeutic environment for our patients and a superb learning environment for the SHO's and registrars in his department.
Author information
Authors and affiliations.
2nd Department of Psychiatry, Psychiatric Hospital of Thessaloniki, 196 Langada str., 564 29, Thessaloniki, Greece
Georgios D Floros, Ioanna Charatsidou & Grigorios Lavrentiadis
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Georgios D Floros .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
GF was the attending SHO and the major contributor in writing the manuscript. IC performed the psychological evaluation and Rorschach testing and interpretation. GL provided valuable guidance in diagnosis and handling of the patient. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Floros, G.D., Charatsidou, I. & Lavrentiadis, G. A case of PTSD presenting with psychotic symptomatology: a case report. Cases Journal 1 , 352 (2008). https://doi.org/10.1186/1757-1626-1-352
Download citation
Received : 12 September 2008
Accepted : 25 November 2008
Published : 25 November 2008
DOI : https://doi.org/10.1186/1757-1626-1-352
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ziprasidone
- Psychotic Disorder
- Amisulpride
- Hallucinatory Experience
Cases Journal
ISSN: 1757-1626
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 29 March 2022
Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits
- Kerry. J. Ressler ORCID: orcid.org/0000-0002-5158-1103 1 ,
- Sabina Berretta 1 ,
- Vadim Y. Bolshakov 1 ,
- Isabelle M. Rosso 1 ,
- Edward G. Meloni 1 ,
- Scott L. Rauch 1 &
- William A. Carlezon Jr 1
Nature Reviews Neurology volume 18 , pages 273–288 ( 2022 ) Cite this article
9209 Accesses
112 Citations
172 Altmetric
Metrics details
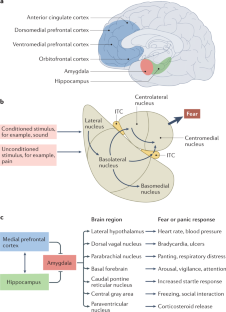
Post-traumatic stress disorder (PTSD) is a maladaptive and debilitating psychiatric disorder, characterized by re-experiencing, avoidance, negative emotions and thoughts, and hyperarousal in the months and years following exposure to severe trauma. PTSD has a prevalence of approximately 6–8% in the general population, although this can increase to 25% among groups who have experienced severe psychological trauma, such as combat veterans, refugees and victims of assault. The risk of developing PTSD in the aftermath of severe trauma is determined by multiple factors, including genetics — at least 30–40% of the risk of PTSD is heritable — and past history, for example, prior adult and childhood trauma. Many of the primary symptoms of PTSD, including hyperarousal and sleep dysregulation, are increasingly understood through translational neuroscience. In addition, a large amount of evidence suggests that PTSD can be viewed, at least in part, as a disorder that involves dysregulation of normal fear processes. The neural circuitry underlying fear and threat-related behaviour and learning in mammals, including the amygdala–hippocampus–medial prefrontal cortex circuit, is among the most well-understood in behavioural neuroscience. Furthermore, the study of threat-responding and its underlying circuitry has led to rapid progress in understanding learning and memory processes. By combining molecular–genetic approaches with a translational, mechanistic knowledge of fear circuitry, transformational advances in the conceptual framework, diagnosis and treatment of PTSD are possible. In this Review, we describe the clinical features and current treatments for PTSD, examine the neurobiology of symptom domains, highlight genomic advances and discuss translational approaches to understanding mechanisms and identifying new treatments and interventions for this devastating syndrome.
Post-traumatic stress disorder (PTSD) is a debilitating neuropsychiatric disorder, characterized by re-experiencing, avoidance, negative emotions and thoughts, and hyperarousal.
PTSD is frequently comorbid with neurological conditions such as traumatic brain injury, post-traumatic epilepsy and chronic headaches.
PTSD has a prevalence of approximately 6–8% in the general population and up to 25% among individuals who have experienced severe trauma.
Many of the neural circuit mechanisms that underlie the PTSD symptoms of fear-related and threat-related behaviour, hyperarousal and sleep dysregulation are becoming increasingly clear.
Key brain regions involved in PTSD include the amygdala–hippocampus–prefrontal cortex circuit, which is among the most well-understood networks in behavioural neuroscience.
Combining molecular–genetic approaches with a mechanistic knowledge of fear circuitry will enable transformational advances in the conceptual framework, diagnosis and treatment of PTSD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders
Impaired learning, memory, and extinction in posttraumatic stress disorder: translational meta-analysis of clinical and preclinical studies
Prefrontal cortex, amygdala, and threat processing: implications for PTSD
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Publishing, 2013).
Breslau, N. et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit area survey of trauma. Arch. Gen. Psychiatry 55 , 626–632 (1998).
Article CAS PubMed Google Scholar
Breslau, N., Peterson, E. L., Poisson, L. M., Schultz, L. R. & Lucia, V. C. Estimating post-traumatic stress disorder in the community: lifetime perspective and the impact of typical traumatic events. Psychol. Med. 34 , 889–898 (2004).
Bromet, E., Sonnega, A. & Kessler, R. C. Risk factors for DSM-III-R posttraumatic stress disorder: findings from the National Comorbidity Survey. Am. J. Epidemiol. 147 , 353–361 (1998).
McLaughlin, K. A. et al. Subthreshold posttraumatic stress disorder in the world health organization world mental health surveys. Biol. Psychiatry 77 , 375–384 (2015).
Article PubMed Google Scholar
Jovanovic, T., Kazama, A., Bachevalier, J. & Davis, M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62 , 695–704 (2011).
Article PubMed PubMed Central CAS Google Scholar
Jovanovic, T. & Ressler, K. J. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry 167 , 648–662 (2010).
Article PubMed PubMed Central Google Scholar
Morgan, C. A., Grillon, C., Southwick, S. M., Davis, M. & Charney, D. S. Fear-potentiated startle in posttraumatic stress disorder. Biol. Psychiatry 38 , 378–385 (1995).
Rauch, S. L. et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol. Psychiatry 47 , 769–776 (2000).
Shin, L. M. et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiatry 62 , 273–281 (2005).
Mellman, T. A., Pigeon, W. R., Nowell, P. D. & Nolan, B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J. Trauma. Stress 20 , 893–901 (2007).
Yehuda, R. & LeDoux, J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56 , 19–32 (2007).
Koenen, K. C., Goodwin, R., Struening, E., Hellman, F. & Guardino, M. Posttraumatic stress disorder and treatment seeking in a national screening sample. J. Trauma. Stress 16 , 5–16 (2003).
Koenen, K. C. et al. A high risk twin study of combat-related PTSD comorbidity. Twin Res. 6 , 218–226 (2003).
True, W. R. et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch. Gen. Psychiatry 50 , 257–264 (1993).
Stein, M. B. et al. Genome-wide association studies of posttraumatic stress disorder in 2 cohorts of US Army soldiers. JAMA Psychiatry 73 , 695–704 (2016).
Stein, M. B., Jang, K. L., Taylor, S., Vernon, P. A. & Livesley, W. J. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am. J. Psychiatry 159 , 1675–1681 (2002).
Duncan, L. E. et al. Largest GWAS of PTSD ( N =20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol. Psychiatry 23 , 666–673 (2018).
Reuveni, I. et al. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum. Brain Mapp. 37 , 589–599 (2015).
Porter, B., Bonanno, G. A., Frasco, M. A., Dursa, E. K. & Boyko, E. J. Prospective post-traumatic stress disorder symptom trajectories in active duty and separated military personnel. J. Psychiatr. Res. 89 , 55–64 (2017).
Ballenger, J. C. et al. Consensus statement update on posttraumatic stress disorder from the international consensus group on depression and anxiety. J. Clin. Psychiatry 65 (Suppl. 1), 55–62 (2004).
PubMed Google Scholar
Heim, C. & Nemeroff, C. B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry 49 , 1023–1039 (2001).
Kessler, R. C. et al. Trauma and PTSD in the WHO world mental health surveys. Eur. J. Psychotraumatol. 8 , 1353383 (2017).
Huckins, L. M. et al. Analysis of genetically regulated gene expression identifies a prefrontal PTSD gene, SNRNP35, specific to military cohorts. Cell Rep. 31 , 107716 (2020).
Article CAS PubMed PubMed Central Google Scholar
Koenen, K. C. et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 47 , 2260–2274 (2017).
Kornfield, S. L., Hantsoo, L. & Epperson, C. N. What does sex have to do with it? The role of sex as a biological variable in the development of posttraumatic stress disorder. Curr. Psychiatry Rep. 20 , 39 (2018).
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M. & Nelson, C. B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52 , 1048–1060 (1995).
Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15 , 353–375 (1992).
Davis, M. in The Amygdala , Second Edition: A Functional Analysis (ed. Aggleton, J. P.) 213–287 (Oxford Univ. Press, 2000).
LeDoux, J. E., Cicchetti, P., Xagoraris, A. & Romanski, L. M. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J. Neurosci. 10 , 1062–1069 (1990).
Maren, S. The amygdala, synaptic plasticity, and fear memory. Ann. NY Acad. Sci. 985 , 106–113 (2003).
Pitkanen, A. in The Amygdala, Second Edition: A Functional Analysis (ed. Aggleton, J. P.) 31–116 (Oxford Univ. Press, 2000).
Milad, M. R. & Quirk, G. J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420 , 70–74 (2002).
McCullough, K. M., Morrison, F. G. & Ressler, K. J. Bridging the gap: towards a cell-type specific understanding of neural circuits underlying fear behaviors. Neurobiol. Learn. Mem. 135 , 27–39 (2016).
Mobbs, D. et al. Viewpoints: approaches to defining and investigating fear. Nat. Neurosci. 22 , 1205–1216 (2019).
Ressler, K. J. Translating across circuits and genetics toward progress in fear- and anxiety-related disorders. Am. J. Psychiatry 177 , 214–222 (2020).
Fenster, R. J., Lebois, L. A. M., Ressler, K. J. & Suh, J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat. Rev. Neurosci. 19 , 535–551 (2018).
McAllister, T. W. Psychopharmacological issues in the treatment of TBI and PTSD. Clin. Neuropsychol. 23 , 1338–1367 (2009).
Strawn, J. R., Keeshin, B. R., DelBello, M. P., Geracioti, T. D. Jr. & Putnam, F. W. Psychopharmacologic treatment of posttraumatic stress disorder in children and adolescents: a review. J. Clin. Psychiatry 71 , 932–941 (2010).
Abdallah, C. G., Southwick, S. M. & Krystal, J. H. Neurobiology of posttraumatic stress disorder (PTSD): a path from novel pathophysiology to innovative therapeutics. Neurosci. Lett. 649 , 130–132 (2017).
Stojek, M. M., McSweeney, L. B. & Rauch, S. A. M. Neuroscience informed prolonged exposure practice: increasing efficiency and efficacy through mechanisms. Front. Behav. Neurosci. 12 , 281 (2018).
Kelmendi, B., Adams, T. G., Soutwick, S., Abdallah, C. G. & Krystal, J. H. Posttraumatic stress disorder: an integrated overview and neurobiological rationale for pharmacology. Clin. Psychol. 24 , 281–297 (2017).
Google Scholar
Krystal, J. H. et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol. Psychiatry 82 , e51–e59 (2017).
Stein, D. J. et al. Dissociation in posttraumatic stress disorder: evidence from the world mental health surveys. Biol. Psyhiatry 73 , 302–312 (2013).
Article Google Scholar
Lebois, L. A. M. et al. Large-scale functional brain network architecture changes associated with trauma-related dissociation. Am. J. Psychiatry 178 , 165–173 (2021).
Nicholson, A. A. et al. Dynamic causal modeling in PTSD and its dissociative subtype: bottom-up versus top-down processing within fear and emotion regulation circuitry. Hum. Brain Mapp. 38 , 5551–5561 (2017).
Stein, M. B. et al. Genome-wide analyses of psychological resilience in U.S. Army soldiers. Am. J. Med. Genet. B Neuropsychiatr. Genet. 180 , 310–319 (2019).
Nievergelt, C. M. et al. Genomic predictors of combat stress vulnerability and resilience in U.S. Marines: a genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology 51 , 459–471 (2015).
Thompson, N. J., Fiorillo, D., Rothbaum, B. O., Ressler, K. J. & Michopoulos, V. Coping strategies as mediators in relation to resilience and posttraumatic stress disorder. J. Affect. Disord. 225 , 153–159 (2018).
van Rooij, S. J. H. et al. Hippocampal activation during contextual fear inhibition related to resilience in the early aftermath of trauma. Behav. Brain Res. 408 , 113282 (2021).
Wingo, A. P., Ressler, K. J. & Bradley, B. Resilience characteristics mitigate tendency for harmful alcohol and illicit drug use in adults with a history of childhood abuse: a cross-sectional study of 2024 inner-city men and women. J. Psychiatr. Res. 51 , 93–99 (2014).
Wrenn, G. L. et al. The effect of resilience on posttraumatic stress disorder in trauma-exposed inner-city primary care patients. J. Natl Med. Assoc. 103 , 560–566 (2011).
Astill Wright, L., Horstmann, L., Holmes, E. A. & Bisson, J. I. Consolidation/reconsolidation therapies for the prevention and treatment of PTSD and re-experiencing: a systematic review and meta-analysis. Transl. Psychiatry 11 , 453 (2021).
Linnstaedt, S. D., Zannas, A. S., McLean, S. A., Koenen, K. C. & Ressler, K. J. Literature review and methodological considerations for understanding circulating risk biomarkers following trauma exposure. Mol. Psychiatry 25 , 1986–1999 (2020).
McLean, S. A. et al. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol. Psychiatry 25 , 283–296 (2020).
Iyadurai, L. et al. Preventing intrusive memories after trauma via a brief intervention involving Tetris computer game play in the emergency department: a proof-of-concept randomized controlled trial. Mol. Psychiatry 23 , 674–682 (2018).
Rothbaum, B. O. et al. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J. Clin. Psychiatry 75 , 1380–1387 (2014).
Seal, K. H. & Stein, M. B. Preventing the pain of PTSD. Sci. Transl Med. 5 , 188fs122 (2013).
Article CAS Google Scholar
Zohar, J. et al. Secondary prevention of chronic PTSD by early and short-term administration of escitalopram: a prospective randomized, placebo-controlled, double-blind trial. J. Clin. Psychiatry 79 , 16m10730 (2018).
Zohar, J. et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur. Neuropsychopharmacol. 21 , 796–809 (2011).
Myers, K. M. & Davis, M. Mechanisms of fear extinction. Mol. Psychiatry 12 , 120–150 (2007).
Ross, D. A. et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry 74 , 407–415 (2017).
LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E. & Phelps, E. A. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20 , 937–945 (1998).
LeDoux, J. The amygdala. Curr. Biol. 17 , R868–R874 (2007).
Rogan, M. T., Staubli, U. V. & LeDoux, J. E. Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390 , 604–607 (1997).
Myers, K. M. & Davis, M. Behavioral and neural analysis of extinction. Neuron 36 , 567–584 (2002).
Chhatwal, J. P., Myers, K. M., Ressler, K. J. & Davis, M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J. Neurosci. 25 , 502–506 (2005).
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454 , 600–606 (2008).
Lee, J., An, B. & Choi, S. Longitudinal recordings of single units in the basal amygdala during fear conditioning and extinction. Sci. Rep. 11 , 11177 (2021).
McCullough, K. M. et al. Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nat. Commun. 7 , 13149 (2016).
Jasnow, A. M. et al. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J. Neurosci. 33 , 10396–10404 (2013).
Hinrichs, R. et al. Increased skin conductance response in the immediate aftermath of trauma predicts PTSD risk. Chronic Stress 3 , 1–11 (2019).
Milad, M. R. et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 66 , 1075–1082 (2009).
Etkin, A. & Wager, T. D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164 , 1476–1488 (2007).
Steuber, E. R. et al. Thalamic volume and fear extinction interact to predict acute posttraumatic stress severity. Psychiatry Res. 141 , 325–332 (2021).
Kredlow, M. A., Fenster, R. J., Laurent, E. S., Ressler, K. J. & Phelps, E. A. Prefrontal cortex, amygdala and threat processing: implications for PTSD. Neuropsychopharmacology 47 , 247–259 (2022).
Maddox, S. A., Hartmann, J., Ross, R. A. & Ressler, K. J. Deconstructing the gestalt: mechanisms of fear, threat, and trauma memory encoding. Neuron 102 , 60–74 (2019).
Tsvetkov, E., Carlezon, W. A., Benes, F. M., Kandel, E. R. & Bolshakov, V. Y. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron 34 , 289–300 (2002).
Josselyn, S. A. et al. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 21 , 2404–2412 (2001).
Bremner, J. D. et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. Psychiatry 152 , 973–981 (1995).
Bremner, J. D. et al. The environment contributes more than genetics to smaller hippocampal volume in posttraumatic stress disorder (PTSD). J. Psychiatr. Res. 137 , 579–588 (2021).
Logue, M. W. et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatry 83 , 244–253 (2018).
Heldt, S. A., Stanek, L., Chhatwal, J. P. & Ressler, K. J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 12 , 656–670 (2007).
Ji, J. & Maren, S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn. Mem. 12 , 270–276 (2005).
Parsons, R. G. & Ressler, K. J. Implications of memory modulation for post-traumatic stress and fear disorders. Nat. Neurosci. 16 , 146–153 (2013).
Brown, E. S., Rush, A. J. & McEwen, B. S. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology 21 , 474–484 (1999).
Herrmann, L. et al. Long-lasting hippocampal synaptic protein loss in a mouse model of posttraumatic stress disorder. PLoS ONE 7 , e42603 (2012).
Hobin, J. A., Goosens, K. A. & Maren, S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J. Neurosci. 23 , 8410–8416 (2003).
Peters, J., Dieppa-Perea, L. M., Melendez, L. M. & Quirk, G. J. Induction of fear extinction with hippocampal-infralimbic BDNF. Science 328 , 1288–1290 (2010).
Milad, M. R. et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 62 , 446–454 (2007).
Milad, M. R. et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc. Natl Acad. Sci. USA 102 , 10706–10711 (2005).
Santhanam, P. et al. Decreases in white matter integrity of ventro-limbic pathway linked to post-traumatic stress disorder in mild traumatic brain injury. J. Neurotrauma 36 , 1093–1098 (2019).
Koch, S. B. J. et al. Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: a diffusion tensor imaging study. J. Psychiatry Neurosci. 42 , 331–342 (2017).
Kennis, M., van Rooij, S. J. H., Reijnen, A. & Geuze, E. The predictive value of dorsal cingulate activity and fractional anisotropy on long-term PTSD symptom severity. Depress. Anxiety 34 , 410–418 (2017).
Sotres-Bayon, F., Sierra-Mercado, D., Pardilla-Delgado, E. & Quirk, G. J. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76 , 804–812 (2012).
Lanius, R. A., Brand, B., Vermetten, E., Frewen, P. A. & Spiegel, D. The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress. Anxiety 29 , 701–708 (2012).
Mellman, T. A. & Hipolito, M. M. Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr. 11 , 611–615 (2006).
Neylan, T. C. et al. Prior sleep problems and adverse post-traumatic neuropsychiatric sequelae of motor vehicle collision in the AURORA study. Sleep 44 , zsaa200 (2021).
van Liempt, S., van Zuiden, M., Westenberg, H., Super, A. & Vermetten, E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety 30 , 469–474 (2013).
Pruiksma, K. E. et al. Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychol. Trauma. 8 , 697–701 (2016).
Bryant, R. A., O’Donnell, M. L., Creamer, M., McFarlane, A. C. & Silove, D. Posttraumatic intrusive symptoms across psychiatric disorders. J. Psychiatr. Res. 45 , 842–847 (2011).
Phelps, A. J. et al. Polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep 41 , zsx188 (2018).
Harb, G. C. et al. A critical review of the evidence base of imagery rehearsal for posttraumatic nightmares: pointing the way for future research. J. Trauma. Stress 26 , 570–579 (2013).
Norrholm, S. D. et al. Fear load: the psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int. J. Psychophysiol. 98 , 270–275 (2015).
Fani, N. et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med. 42 , 533–543 (2012).
Norrholm, S. D. et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry 69 , 556–563 (2011).
Colvonen, P. J., Straus, L. D., Acheson, D. & Gehrman, P. A review of the relationship between emotional learning and memory, sleep, and PTSD. Curr. Psychiatry Rep. 21 , 2 (2019).
van Liempt, S. et al. Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology 38 , 155–165 (2013).
Article PubMed CAS Google Scholar
Lipinska, G. & Thomas, K. J. F. Rapid eye movement fragmentation, not slow-wave sleep, predicts neutral declarative memory consolidation in posttraumatic stress disorder. J. Sleep. Res. 28 , e12846 (2019).
Onton, J. A., Matthews, S. C., Kang, D. Y. & Coleman, T. P. In-home sleep recordings in military veterans with posttraumatic stress disorder reveal less REM and deep sleep <1 Hz. Front. Hum. Neurosci. 12 , 196 (2018).
Wells, A. M. et al. Effects of chronic social defeat stress on sleep and circadian rhythms are mitigated by kappa-opioid receptor antagonism. J. Neurosci. 37 , 7656–7668 (2017).
Hasler, B. P., Insana, S. P., James, J. A. & Germain, A. Evening-type military veterans report worse lifetime posttraumatic stress symptoms and greater brainstem activity across wakefulness and REM sleep. Biol. Psychol. 94 , 255–262 (2013).
Nestler, E. J. & Carlezon, W. A. Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59 , 1151–1159 (2006).
McCullough, K. M. et al. Nucleus accumbens medium spiny neuron subtypes differentially regulate stress-associated alterations in sleep architecture. Biol. Psychiatry 89 , 1138–1149 (2021).
Sijbrandij, M., Engelhard, I. M., Lommen, M. J., Leer, A. & Baas, J. M. Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD). J. Psychiatr. Res. 47 , 1991–1997 (2013).
Rothbaum, B. O. & Davis, M. Applying learning principles to the treatment of post-trauma reactions. Ann. NY Acad. Sci. 1008 , 112–121 (2003).
Ressler, K. J. et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470 , 492–497 (2011).
Ladd, C. O., Plotsky, P. M., Davis, M. in Encyclopedia of Stress 2nd edn (ed. George Fink) 561–568 (Academic, 2007).
Davis, M. Sensitization of the acoustic startle reflex by footshock. Behav. Neurosci. 103 , 495–503 (1989).
Grillon, C., Ameli, R., Woods, S. W., Merikangas, K. & Davis, M. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 28 , 588–595 (1991).
Giardino, W. J. & Pomrenze, M. B. Extended amygdala neuropeptide circuitry of emotional arousal: waking up on the wrong side of the bed nuclei of stria terminalis. Front. Behav. Neurosci. 15 , 613025 (2021).
Hinrichs, R. et al. Mobile assessment of heightened skin conductance in posttraumatic stress disorder. Depress Anxiety 34 , 502–507 (2017).
Neylan, T. C., Schadt, E. E. & Yehuda, R. Biomarkers for combat-related PTSD: focus on molecular networks from high-dimensional data. Eur. J. Psychotraumatol. 5 , 23938 (2014).
Yehuda, R., Giller, E. L., Southwick, S. M., Lowy, M. T. & Mason, J. W. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol. Psychiatry 30 , 1031–1048 (1991).
Yehuda, R. et al. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am. J. Psychiatry 150 , 83–86 (1993).
Han, X. & Boyden, E. S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2 , e299 (2007).
Dunlop, B. W. et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol. Psychiatry 82 , 866–874 (2017).
Jovanovic, T. et al. Psychophysiological treatment outcomes: corticotropin-releasing factor type 1 receptor antagonist increases inhibition of fear-potentiated startle in PTSD patients. Psychophysiology 57 , e13356 (2020).
Galatzer-Levy, I. R. & Bryant, R. A. 636,120 ways to have posttraumatic stress disorder. Perspect. Psychol. Sci. 8 , 651–662 (2013).
Brewin, C. R. The nature and significance of memory disturbance in posttraumatic stress disorder. Annu. Rev. Clin. Psychol. 7 , 203–227 (2011).
Vasterling, J. J. & Arditte Hall, K. A. Neurocognitive and information processing biases in posttraumatic stress disorder. Curr. Psychiatry Rep. 20 , 99 (2018).
Stevens, J. S. & Jovanovic, T. Role of social cognition in post-traumatic stress disorder: a review and meta-analysis. Genes Brain Behav. 18 , e12518 (2019).
Mathias, J. L. & Mansfield, K. M. Prospective and declarative memory problems following moderate and severe traumatic brain injury. Brain Inj. 19 , 271–282 (2005).
Acosta, S. A. et al. Influence of post-traumatic stress disorder on neuroinflammation and cell proliferation in a rat model of traumatic brain injury. PLoS ONE 8 , e81585 (2013).
Elzinga, B. M. & Bremner, J. D. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect. Disord. 70 , 1–17 (2002).
Germine, L. T. et al. Neurocognition after motor vehicle collision and adverse post-traumatic neuropsychiatric sequelae within 8 weeks: initial findings from the AURORA study. J. Affect. Disord. 298 , 57–67 (2022).
Ben-Zion, Z. et al. Neuroanatomical risk factors for posttraumatic stress disorder in recent trauma survivors. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5 , 311–319 (2020).
Dark, H. E., Harnett, N. G., Knight, A. J. & Knight, D. C. Hippocampal volume varies with acute posttraumatic stress symptoms following medical trauma. Behav. Neurosci. 135 , 71–78 (2021).
van Rooij, S. J. H. et al. The role of the Hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biol. Psychiatry 84 , 106–115 (2018).
Girgenti, M. J. et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat. Neurosci. 24 , 24–33 (2021).
Wolf, E. J. et al. Klotho, PTSD, and advanced epigenetic age in cortical tissue. Neuropsychopharmacology 46 , 721–730 (2021).
Olive, I., Makris, N., Densmore, M., McKinnon, M. C. & Lanius, R. A. Altered basal forebrain BOLD signal variability at rest in posttraumatic stress disorder: a potential candidate vulnerability mechanism for neurodegeneration in PTSD. Hum. Brain Mapp. 42 , 3561–3575 (2021).
Mohlenhoff, B. S., O’Donovan, A., Weiner, M. W. & Neylan, T. C. Dementia risk in posttraumatic stress disorder: the relevance of sleep-related abnormalities in brain structure, amyloid, and inflammation. Curr. Psychiatry Rep. 19 , 89 (2017).
Miller, M. W. & Sadeh, N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol. Psychiatry 19 , 1156–1162 (2014).
Mohamed, A. Z., Cumming, P., Gotz, J. & Nasrallah, F., Department of Defense Alzheimer’s Disease Neuroimaging Initiative. Tauopathy in veterans with long-term posttraumatic stress disorder and traumatic brain injury. Eur. J. Nucl. Med. Mol. Imaging 46 , 1139–1151 (2019).
Kaplan, G. B., Vasterling, J. J. & Vedak, P. C. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav. Pharmacol. 21 , 427–437 (2010).
Mojtabavi, H., Saghazadeh, A., van den Heuvel, L., Bucker, J. & Rezaei, N. Peripheral blood levels of brain-derived neurotrophic factor in patients with post-traumatic stress disorder (PTSD): a systematic review and meta-analysis. PLoS One 15 , e0241928 (2020).
Licznerski, P. et al. Decreased SGK1 expression and function contributes to behavioral deficits induced by traumatic stress. PLoS Biol. 13 , e1002282 (2015).
de Kloet, C. S. et al. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J. Psychiatr. Res. 40 , 550–567 (2006).
Hawn, S. E. et al. GxE effects of FKBP5 and traumatic life events on PTSD: a meta-analysis. J. Affect. Disord. 243 , 455–462 (2019).
Zannas, A. S. & Binder, E. B. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 13 , 25–37 (2014).
Friend, S. F., Nachnani, R., Powell, S. B. & Risbrough, V. B. C-Reactive protein: marker of risk for post-traumatic stress disorder and its potential for a mechanistic role in trauma response and recovery. Eur. J. Neurosci. https://doi.org/10.1111/ejn.15031 (2020).
Michopoulos, V. et al. Association of prospective risk for chronic PTSD symptoms with low TNFα and IFNγ concentrations in the immediate aftermath of trauma exposure. Am. J. Psychiatry 177 , 58–65 (2020).
Michopoulos, V., Powers, A., Gillespie, C. F., Ressler, K. J. & Jovanovic, T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42 , 254–270 (2017).
Michopoulos, V. et al. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry 172 , 353–362 (2015).
Smith, A. K. et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 156B , 700–708 (2011).
Siegel, C. E. et al. Utilization of machine learning for identifying symptom severity military-related PTSD subtypes and their biological correlates. Transl. Psychiatry 11 , 227 (2021).
Wuchty, S. et al. Integration of peripheral transcriptomics, genomics, and interactomics following trauma identifies causal genes for symptoms of post-traumatic stress and major depression. Mol. Psychiatry 26 , 3077–3092 (2021).
Kuan, P. F. et al. PTSD is associated with accelerated transcriptional aging in World Trade Center responders. Transl. Psychiatry 11 , 311 (2021).
Logue, M. W. et al. An epigenome-wide association study of posttraumatic stress disorder in US veterans implicates several new DNA methylation loci. Clin. Epigenetics 12 , 46 (2020).
Sheerin, C. M. et al. Epigenome-wide study of posttraumatic stress disorder symptom severity in a treatment-seeking adolescent sample. J. Trauma. Stress 34 , 607–615 (2021).
Yang, R. et al. A DNA methylation clock associated with age-related illnesses and mortality is accelerated in men with combat PTSD. Mol. Psychiatry 26 , 4999–5009 (2020).
Smith, A. K. et al. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am. J. Med. Genet. B Neuropsychiatr. Genet. 168B , 36–44 (2015).
Nievergelt, C. M. et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat. Commun. 10 , 4558 (2019).
Gelernter, J. et al. Genome-wide association study of maximum habitual alcohol intake in >140,000 U.S. European and African American veterans yields novel risk loci. Biol. Psychiatry 86 , 365–376 (2019).
Stein, M. B. et al. Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the Million Veteran Program. Nat. Genet. 53 , 174–184 (2021).
Gelernter, J. et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat. Neurosci. 22 , 1394–1401 (2019).
Logue, M. W. et al. The Psychiatric Genomics Consortium Posttraumatic Stress Disorder Workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology 40 , 2287–2297 (2015).
Pape, J. C. et al. DNA methylation levels are associated with CRF 1 receptor antagonist treatment outcome in women with post-traumatic stress disorder. Clin. Epigenetics 10 , 136 (2018).
Mizuno, Y. More than 20 years of the discovery of Park2. Neurosci. Res. 159 , 3–8 (2020).
Shaltouki, A. et al. Mitochondrial alterations by PARKIN in dopaminergic neurons using PARK2 patient-specific and PARK2 knockout isogenic iPSC lines. Stem Cell Rep. 4 , 847–859 (2015).
Binder, E. B. et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299 , 1291–1305 (2008).
Heim, C., Owens, M. J., Plotsky, P. M. & Nemeroff, C. B. Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol. Bull. 33 , 185–192 (1997).
CAS PubMed Google Scholar
Bremner, J. D. et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatry 154 , 624–629 (1997).
Dias, B. G. & Ressler, K. J. PACAP and the PAC1 receptor in post-traumatic stress disorder. Neuropsychopharmacology 38 , 245–246 (2013).
Ross, R. A. et al. Circulating PACAP peptide and PAC1R genotype as possible transdiagnostic biomarkers for anxiety disorders in women: a preliminary study. Neuropsychopharmacology 45 , 1125–1133 (2020).
Bangasser, D. A. et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 15 , 896–904 (2010).
Jovanovic, T. et al. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol. Psychiatry 18 , 742–743 (2013).
Miles, O. W. & Maren, S. Role of the bed nucleus of the stria terminalis in PTSD: insights from preclinical models. Front. Behav. Neurosci. 13 , 68 (2019).
Stroth, N., Holighaus, Y., Ait-Ali, D. & Eiden, L. E. PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Ann. NY Acad. Sci. 1220 , 49–59 (2011).
Ramikie, T. S. & Ressler, K. J. Mechanisms of sex differences in fear and posttraumatic stress disorder. Biol. Psychiatry 83 , 876–885 (2018).
Mercer, K. B. et al. Functional evaluation of a PTSD-associated genetic variant: estradiol regulation and ADCYAP1R1. Transl. Psychiatry 6 , e978 (2016).
Klengel, T. et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 16 , 33–41 (2013).
Fani, N. et al. FKBP5 and attention bias for threat: associations with hippocampal function and shape. JAMA Psychiatry 70 , 392–400 (2013).
Galatzer-Levy, I. R. et al. A cross species study of heterogeneity in fear extinction learning in relation to FKBP5 variation and expression: implications for the acute treatment of posttraumatic stress disorder. Neuropharmacology 116 , 188–195 (2017).
Yun, J. Y., Jin, M. J., Kim, S. & Lee, S. H. Stress-related cognitive style is related to volumetric change of the hippocampus and FK506 binding protein 5 polymorphism in post-traumatic stress disorder. Psychol. Med. https://doi.org/10.1017/S0033291720002949 (2020).
Hartmann, J. et al. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 35 , 109185 (2021).
Herrmann, L. et al. Analysis of the cerebellar molecular stress response led to first evidence of a role for FKBP51 in brain FKBP52 expression in mice and humans. Neurobiol. Stress 15 , 100401 (2021).
Young, K. A., Thompson, P. M., Cruz, D. A., Williamson, D. E. & Selemon, L. D. BA11 FKBP5 expression levels correlate with dendritic spine density in postmortem PTSD and controls. Neurobiol. Stress 2 , 67–72 (2015).
Abdallah, C. G. et al. The neurobiology and pharmacotherapy of posttraumatic stress disorder. Annu. Rev. Pharmacol. Toxicol. 59 , 171–189 (2019).
Watts, B. V. et al. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J. Clin. Psychiatry 74 , e541–e550 (2013).
Krystal, J. H. et al. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. JAMA 306 , 493–502 (2011).
Han, C. et al. The potential role of atypical antipsychotics for the treatment of posttraumatic stress disorder. J. Psychiatr. Res. 56 , 72–81 (2014).
Cipriani, A. et al. Comparative efficacy and acceptability of pharmacological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol. Med. 48 , 1975–1984 (2018).
Adamou, M., Puchalska, S., Plummer, W. & Hale, A. S. Valproate in the treatment of PTSD: systematic review and meta analysis. Curr. Med. Res. Opin. 23 , 1285–1291 (2007).
Andrus, M. R. & Gilbert, E. Treatment of civilian and combat-related posttraumatic stress disorder with topiramate. Ann. Pharmacother. 44 , 1810–1816 (2010).
Hertzberg, M. A. et al. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Boil. Psychiatry 45 , 1226–1229 (1999).
Wang, H. R., Woo, Y. S. & Bahk, W. M. Anticonvulsants to treat post-traumatic stress disorder. Hum. Psychopharmacol. 29 , 427–433 (2014).
Davis, L. L. et al. Divalproex in the treatment of posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial in a veteran population. J. Clin. Psychopharmacol. 28 , 84–88 (2008).
Lindley, S. E., Carlson, E. B. & Hill, K. A randomized, double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. J. Clin. Psychopharmacol. 27 , 677–681 (2007).
Taylor, F. B. et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol. Psychiatry 63 , 629–632 (2008).
Raskind, M. A. et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol. Psychiatry 61 , 928–934 (2007).
Khachatryan, D., Groll, D., Booij, L., Sepehry, A. A. & Schütz, C. G. Prazosin for treating sleep disturbances in adults with posttraumatic stress disorder: a systematic review and meta-analysis of randomized controlled trials. Gen. Hosp. Psychiatry 39 , 46–52 (2016).
Brudey, C. et al. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309 , R315–R321 (2015).
Raskind, M. A. et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J. Clin. Psychiatry 61 , 129–133 (2000).
Porter-Stransky, K. A. et al. Noradrenergic transmission at alpha1-adrenergic receptors in the ventral periaqueductal gray modulates arousal. Biol. Psychiatry 85 , 237–247 (2019).
Mallick, B. N., Singh, S. & Pal, D. Role of alpha and beta adrenoceptors in locus coeruleus stimulation-induced reduction in rapid eye movement sleep in freely moving rats. Behav. Brain Res. 158 , 9–21 (2005).
Germain, A. et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J. Psychosom. Res. 72 , 89–96 (2012).
Raskind, M. A. et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am. J. Psychiatry 170 , 1003–1010 (2013).
Raskind, M. A. et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N. Engl. J. Med. 378 , 507–517 (2018).
Dutkiewics, S. Efficacy and tolerability of drugs for treatment of benign prostatic hyperplasia. Int. Urol. Nephrol. 32 , 423–432 (2001).
Lewis, C., Roberts, N. P., Andrew, M., Starling, E. & Bisson, J. I. Psychological therapies for post-traumatic stress disorder in adults: systematic review and meta-analysis. Eur. J. Psychotraumatol. 11 , 1729633 (2020).
Powers, M. B., Halpern, J. M., Ferenschak, M. P., Gillihan, S. J. & Foa, E. B. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin. Psychol. Rev. 30 , 635–641 (2010).
Pavlov, I. P. & Anrep, G. V. Conditioned Reflexes; An Investigation of the Physiological Activity of the Cerebral Cortex (Oxford Univ. Press, 1927).
Singewald, N., Schmuckermair, C., Whittle, N., Holmes, A. & Ressler, K. J. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol. Ther. 149 , 150–190 (2015).
Keynan, J. N. et al. Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience. Nat. Hum. Behav. 3 , 63–73 (2019).
Chen, B. K. et al. Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine. Neuropsychopharmacology 45 , 1545–1556 (2020).
Van’t Veer, A. & Carlezon, W. A. Jr. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology 229 , 435–452 (2013).
Smith, A. K. et al. Epigenome-wide meta-analysis of PTSD across 10 military and civilian cohorts identifies methylation changes in AHRR. Nat. Commun. 11 , 5965 (2020).
Mellon, S. H. et al. Metabolomic analysis of male combat veterans with post traumatic stress disorder. PLoS ONE 14 , e0213839 (2019).
Bremner, J. D. Neuroimaging in posttraumatic stress disorder and other stress-related disorders. Neuroimaging Clin. N. Am. 17 , 523–538 (2007).
Britton, J. C., Phan, K. L., Taylor, S. F., Fig, L. M. & Liberzon, I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol. Psychiatry 57 , 832–840 (2005).
Insel, T. R. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am. J. Psychiatry 171 , 395–397 (2014).
Hyman, S. Mental health: depression needs large human-genetics studies. Nature 515 , 189–191 (2014).
Bale, T. L. et al. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology 44 , 1349–1353 (2019).
Baker, J. T., Germine, L. T., Ressler, K. J., Rauch, S. L. & Carlezon, W. A. Jr. Digital devices and continuous telemetry: opportunities for aligning psychiatry and neuroscience. Neuropsychopharmacology 43 , 2499–2503 (2018).
Cakmak, A. S. et al. Classification and prediction of post-trauma outcomes related to PTSD using circadian rhythm changes measured via wrist-worn research watch in a large longitudinal cohort. IEEE J. Biomed. Health Inf. 25 , 2866–2876 (2021).
Tsanas, A., Woodward, E. & Ehlers, A. Objective characterization of activity, sleep, and circadian rhythm patterns using a wrist-worn actigraphy sensor: insights into posttraumatic stress disorder. JMIR Mhealth Uhealth 8 , e14306 (2020).
Thompson, R. S. et al. Repeated fear-induced diurnal rhythm disruptions predict PTSD-like sensitized physiological acute stress responses in F344 rats. Acta Physiol. 211 , 447–465 (2014).
Phillips, A. G., Geyer, M. A. & Robbins, T. W. Effective use of animal models for therapeutic development in psychiatric and substance use disorders. Biol. Psychiatry 83 , 915–923 (2018).
Van’t Veer, A., Yano, J. M., Carroll, F. I., Cohen, B. M. & Carlezon, W. A. Jr. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist JDTic. Neuropsychopharmacology 37 , 2809–2816 (2012).
Vogel, S. C. et al. Childhood adversity and dimensional variations in adult sustained attention. Front. Psychol. 11 , 691 (2020).
White, S. F. et al. Increased cognitive control and reduced emotional interference is associated with reduced PTSD symptom severity in a trauma-exposed sample: a preliminary longitudinal study. Psychiatry Res. Neuroimaging 278 , 7–12 (2018).
Beard, C. et al. Abnormal error processing in depressive states: a translational examination in humans and rats. Transl. Psychiatry 5 , e564 (2015).
Robble, M. A. et al. Concordant neurophysiological signatures of cognitive control in humans and rats. Neuropsychopharmacology 46 , 1252–1262 (2021).
Der-Avakian, A. et al. Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacology 234 , 1603–1614 (2017).
Lokshina, Y., Nickelsen, T. & Liberzon, I. Reward processing and circuit dysregulation in posttraumatic stress disorder. Front. Psychiatry 12 , 559401 (2021).
Lori, A. et al. Transcriptome-wide association study of post-trauma symptom trajectories identified GRIN3B as a potential biomarker for PTSD development. Neuropsychopharmacology 46 , 1811–1820 (2021).
Pacella, M. L., Hruska, B., Steudte-Schmiedgen, S., George, R. L. & Delahanty, D. L. The utility of hair cortisol concentrations in the prediction of PTSD symptoms following traumatic physical injury. Soc. Sci. Med. 175 , 228–234 (2017).
McCullough, K. M. et al. Genome-wide translational profiling of amygdala Crh-expressing neurons reveals role for CREB in fear extinction learning. Nat. Commun. 11 , 5180 (2020).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8 , 1263–1268 (2005).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446 , 633–639 (2007).
Chow, B. Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463 , 98–102 (2010).
Han, X. et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front. Syst. Neurosci. 5 , 18 (2011).
Luchkina, N. V. & Bolshakov, V. Y. Diminishing fear: optogenetic approach toward understanding neural circuits of fear control. Pharmacol. Biochem. Behav. 174 , 64–79 (2018).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141 , 154–165 (2010).
Coward, P. et al. Controlling signaling with a specifically designed Gi-coupled receptor. Proc. Natl Acad. Sci. USA 95 , 352–357 (1998).
Dong, S., Rogan, S. C. & Roth, B. L. Directed molecular evolution of DREADDs: a generic approach to creating next-generation RASSLs. Nat. Protoc. 5 , 561–573 (2010).
Roth, B. L. DREADDs for neuroscientists. Neuron 89 , 683–694 (2016).
Lin, M. Z. & Schnitzer, M. J. Genetically encoded indicators of neuronal activity. Nat. Neurosci. 19 , 1142–1153 (2016).
Download references
Acknowledgements
This work was supported by NIH awards P50-MH115874 (to W.C./K.J.R.), R01-MH108665 (to K.J.R.), R01-MH063266 (to W.C.), R01-MH123993 (to V.Y.B.), and the Frazier Institute at McLean Hospital (to K.J.R.). I.R. was partially supported by (R01-MH120400).
Author information
Authors and affiliations.
SPARED Center, Department of Psychiatry, McLean Hospital, Harvard Medical School, Boston, MA, USA
Kerry. J. Ressler, Sabina Berretta, Vadim Y. Bolshakov, Isabelle M. Rosso, Edward G. Meloni, Scott L. Rauch & William A. Carlezon Jr
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Kerry. J. Ressler .
Ethics declarations
Competing interests.
K.J.R. has received consulting income from Alkermes, Bionomics, Bioxcel and Jazz Pharmaceuticals, and is on scientific advisory boards for the Army STARRS Project, Janssen, the National Center for PTSD, Sage Therapeutics and Verily. He has also received sponsored research support from Brainsway and Takeda. He also serves on the Boards of ACNP and Biological Psychiatry. W.C. has received consulting income from Psy Therapeutics and has a sponsored research agreement with Cerevel Therapeutics. He is the editor-in-chief for Neuropsychopharmacology and serves on the board of ACNP. None of this work is directly related to the work presented here. S.L.R. receives compensation as a Board member of Community Psychiatry and for his role as Secretary of SOBP. He also serves on the Boards of ADAA and NNDC. He has received royalties from Oxford University Press and APPI.
Peer review
Peer review information.
Nature Reviews Neurology thanks Matthew Girgenti, Soraya Seedat and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A core feature of post-traumatic stress disorder (PTSD) that includes irritability, panic and disruptions in sleep and cognitive function.
A reflex that occurs rapidly and unconsciously in response to an external stimulus such as a noise burst.
A core feature of post-traumatic stress disorder (PTSD) characterized by a heightened state of active threat assessment.
A method of inducing alterations in gene expression involving the ability of the enzyme Cre-recombinase to induce site-specific recombination of genetic material.
A theoretical representation of a neural unit of memory storage.
A muscle located in the eyelid, activity of which is often an end point in human fear conditioning research.
Secondary phenotypes that reliably co-occur as a sub-feature of a broader primary phenotype.
Two or more biological processes that are modulated (activated, suppressed) in parallel by a common upstream factor.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Ressler, K.J., Berretta, S., Bolshakov, V.Y. et al. Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol 18 , 273–288 (2022). https://doi.org/10.1038/s41582-022-00635-8
Download citation
Accepted : 18 February 2022
Published : 29 March 2022
Issue Date : May 2022
DOI : https://doi.org/10.1038/s41582-022-00635-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Spatiotemporal dynamics of hippocampal-cortical networks underlying the unique phenomenological properties of trauma-related intrusive memories.
- Kevin J. Clancy
- Quentin Devignes
- Isabelle M. Rosso
Molecular Psychiatry (2024)
Trauma-related intrusive memories and anterior hippocampus structural covariance: an ecological momentary assessment study in posttraumatic stress disorder
Translational Psychiatry (2024)
Advancing preclinical chronic stress models to promote therapeutic discovery for human stress disorders
- Trevonn M. Gyles
- Eric J. Nestler
- Eric M. Parise
Neuropsychopharmacology (2024)
Differential effects of the stress peptides PACAP and CRF on sleep architecture in mice
- Allison R. Foilb
- Elisa M. Taylor-Yeremeeva
- William A. Carlezon
NPP—Digital Psychiatry and Neuroscience (2024)
Memory persistence: from fundamental mechanisms to translational opportunities
- Santiago Abel Merlo
- Mariano Andrés Belluscio
- Emiliano Merlo
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

- Create new account
- Request new password
Search form
Resource center, post traumatic stress disorder case study.

This case study presents information about post traumatic stress disorder in older adults. It is divided into different sections including the history, presentation and examination, diagnosis, case discussion, post traumatic stress disorder explained, take home points, and additional resources.
https://www.hopkinsmedicine.org/gec/studies/ptsd.html#history
- 3,698 Views
- 1 Collections
Related Resources
- Patrick and Gloria Lake- Unfolding Case
- Julia Morales and Lucy Grey- Unfolding Case
- Sherman "Red" Yoder- Unfolding Case
- Henry and Ertha Williams- Unfolding Case
- Judy and Karen Jones- Unfolding Case
User Collections
Related content, start the conversation.
Every registered user can comment on website content.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Post Traumatic Stress Disorder: PTSD Case Study: One Man's Journey

Abstract This paper is a case study on a client who has been diagnosed with Post Traumatic Stress Disorder (PTSD) from the Vietnam War. A narrative case description is included, which supports the clinical diagnosis and as well as an empirical treatment plan. The treatment plan has included the necessary identifying information with appropriate changes to shield the client’s real identity. The client was referred from the Veteran’s Administrative (VA) hospital in La Jolla, California. As part of the treatment plan the presenting problems will be identified and correlated to the criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition text revision (DSM-IV-TR) multi-axial diagnosis. This case study is based upon a holistic foundation, which includes the inter-connectedness of: the presenting problems, long-term goals, objectives, methods or interventions, treatment length, and measurement of potential outcomes. This paper concludes with a self-critique by the therapist regarding every aspect of the presented case study. Key Words: PTSD, Treatment Plan for Vietnam Vets, Holistic Foundation. Post-Traumatic Stress.
Related Papers
Journal of Traumatic Stress
Steven Silver
Journal of Clinical Psychology
Tracie Shea
Terry Keane
Journal of Consulting and Clinical Psychology
Paul Malloy
Croatian Medical Journal
Goran Arbanas
European Journal of Psychotraumatology
Aleksandra Stevanović
Journal of Aggression, Maltreatment & Trauma
Meredith Landy
Military medicine
Peter Yeomans
While the Veterans Health Administration continues to treat Vietnam War Veterans, approximately two million service men and women have returned from Iraq and Afghanistan. However, our treatments can only be as effective as the quality of our clinical assessment. Disclosure of trauma is facilitated when the type of trauma is present in the sociocultural environment of patient and clinician. Topics that once were deemed too shameful for inquiry, specifically, childhood abuse, domestic violence, sexual abuse, and military sexual trauma are now part of a standard assessment. Similarly, the standard clinical assessment of combat Veterans should include specific queries that address the darkest underside of wartime experiences.
Tracy Simpson
Clinical Psychological Science
Approximately two thirds of veterans with posttraumatic stress disorder (PTSD) remain with the disorder following treatment. Pinpointing the per-symptom effectiveness of treatments in real-world clinical settings can highlight relevant domains for treatment augmentation and development. Baseline and posttreatment assessments of PTSD and depression were performed in 709 veterans with PTSD. PTSD remission was 39.4%. Treatment was least effective for intrusion symptoms and had no effect on flashbacks or on poor recall of traumatic features. Of veterans who remitted, 72.8% still met diagnostic criteria for at least one cluster. Poor clinical effectiveness was noted for depression; only 4.1% of the patients remitted following treatment. Treatments for veterans with PTSD show limited overall effectiveness in real-world settings. Enhancing treatment response may require enhancing provider fidelity and patient compliance with extant treatments or the development of new treatments that speci...
RELATED PAPERS
Cheminformatics and its Applications [Working Title]
Dionisio Olmedo
Journal of Function Spaces
Iqra Shamas
Yuh-Lang Lin
Hagar Salamon
Philippe Hersant
Clinical cancer research : an official journal of the American Association for Cancer Research
Roberta Caputo
Water Quality Research Journal
Uday Chand Ghosh
Aysel Kekillioğlu
Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology
Fábio Everton Maciel
Etnográfica
Leonor Pires Martins
Molecular and Cellular Biology
Alfred Nordheim
Archivum immunologiae et therapiae experimentalis
ALEJANDRA SERRATO SANCHEZ
Comptes Rendus Chimie
Pierre Alphonse
Latin American Research Review
W. George Lovell
Martín cortés
Journal of Neurophysiology
Catherine Carr
The Journal of Immunology
Antonio Juretić
Journal of Fungi
Patrick Njobeh
Almufida : jurnal ilmu-ilmu keislaman
KHOIRUL SALEH HARAHAP
Journal of Engineering for Industry
Ray Edward Johnson
Frontiers in Marine Science
Joanna Flemming
Biodiversidade Brasileira
Francimeire Ferreira
실시간카지노 토토사이트
Journal of Landscape Architecture
Bianca Maria Rinaldi
Optica Applicata
Dr. S. Medhekar
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
CASE REPORT article
5-meo-dmt for post-traumatic stress disorder: a real-world longitudinal case study.

- 1 Integrated Research Literacy Group, Draper, UT, United States
- 2 Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom
- 3 Department of Psychology and Neuroscience, Duke University, Durham, NC, United States
- 4 Neuroscience Interdepartmental Program, University of California, Los Angeles, Los Angeles, CA, United States
- 5 Department of Family and Consumer Studies, University of Utah, Salt Lake City, UT, United States
- 6 School of Humanities and Creativity, Sheridan College, Oakville, ON, Canada
- 7 Department of Pediatrics, Division of Pediatric Psychology and Developmental Medicine, Medical College of Wisconsin, Milwaukee, WI, United States
- 8 Children’s Wisconsin, Milwaukee, WI, United States
- 9 The Mission Within, Baja California, Mexico
- 10 Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX, United States
- 11 Michael E. DeBakey Veterans Affairs Medical Center, Houston, TX, United States
- 12 Department of Psychiatry, Yale School of Medicine, New Haven, CT, United States
Psychedelic therapy is, arguably, the next frontier in psychiatry. It offers a radical alternative to longstanding, mainstays of treatment, while exciting a paradigm shift in translational science and drug discovery. There is particular interest in 5-methoxy- N,N -dimethyltryptamine (5-MeO-DMT)—a serotonergic psychedelic—as a novel, fast-acting therapeutic. Yet, few studies have directly examined 5-MeO-DMT for trauma- or stress-related psychopathology, including post-traumatic stress disorder (PTSD). Herein, we present the first longitudinal case study on 5-MeO-DMT for chronic refractory PTSD, in a 23-year-old female. A single dose of vaporized bufotoxin of the Sonoran Desert Toad ( Incilius alvarius ), containing an estimated 10−15 mg of 5-MeO-DMT, led to clinically significant improvements in PTSD, with next-day effects. This was accompanied by marked reductions in hopelessness and related suicide risk. Improvements, across all constructs, were sustained at 1-, 3-, 6-, and 12-months follow-up, as monitored by a supporting clinician. The subject further endorsed a complete mystical experience, hypothesized to underly 5-MeO-DMT’s therapeutic activity. No drug-related, serious adverse events occurred. Together, results showed that 5-MeO-DMT was generally tolerable, safe to administer, and effective for PTSD; however, this was not without risk. The subject reported acute nausea, overwhelming subjective effects, and late onset of night terrors. Further research is warranted to replicate and extend these findings, which are inherently limited, non-generalizable, and rely on methods not clinically accepted.
Introduction
5-methoxy- N,N -dimethyltryptamine (5-MeO-DMT) is a natural, short-acting psychoactive indolealkylamine ( 1 ). It was first synthesized in 1936 ( 2 ), later found in several plant (e.g., Dictyoloma incanescens ), fungi (e.g., Amanita citrina ), and animal (e.g., Incilius al var ius ) species ( 2 , 3 ). In humans, 5-MeO-DMT is likely endogenous, with trace amounts detected in urine (2 of 113 people), blood (20 of 39 people), and cerebrospinal fluid (40 of 136 people) ( 2 ). However, various studies contradict this finding ( 2 , 4 ); and its physiological role, if any, remains unclear. Based on ethnographic reports, 5-MeO-DMT may have been used by indigenous cultures, as part of plant extracts and botanical preparations (e.g., yopo or cohoba snuff), specifically for spiritual and ritualistic practices ( 5 , 6 ). These reports date back to ancient People of Mesoamerica ( 5 , 6 ). Yet, there is little evidence to support such claims. Further, contrary to published work, 5-MeO-DMT is not found in traditional or analog ayahuasca ( 7 , 8 ). This points to its use being a more recent phenomenon ( 9 , 10 ).
Regarding its pharmacology, 5-MeO-DMT is a nonselective serotonin (5-HT) receptor agonist ( 11 , 12 ). It also binds to other receptors, including dopamine and serotonin, as well as norepinephrine transporters ( 12 ). The entheogen mildly inhibits 5-HT reuptake, yet exerts no appreciable effects on monoamine release ( 13 ). 5-MeO-DMT has the highest affinity for 5-HT 1A (K i , < 10 nM) over 5-HT 2A (K i , >1,000 nM), with 300–1,000-fold greater selectivity ( 11 , 12 , 14 ). This is notable, given that most serotonergic psychedelics, like LSD and psilocybin, are mediated by 5-HT 2A activation ( 15 ). Other non-5-HT 2A receptors have not been studied as widely ( 16 ). Metabolically, 5-MeO-DMT is processed via oxidative deamination—catalyzed by monoamine oxidase A (MAO A )—into the active metabolite, bufotenine ( 17 ). Use of 5-MeO-DMT with MAO inhibitors (MAOIs), such as antidepressants, can augment and prolong neurochemical and behavioral effects, by blocking biotransformation of 5-MeO-DMT and increasing its exposure ( 18 ). Nonetheless, MAOIs can induce serotonergic toxicity ( 19 ), or ‘serotonin syndrome’, a potentially life-threatening drug reaction caused by excess serotonin in the brain ( 20 ). This can present as shivering or diarrhea, as well as muscle rigidity, high fever, and epileptic seizure. Combining 5-MeO-DMT with harmala alkaloids, short-term MAOIs found in ayahuasca, can also produce toxic interactions, and even death ( 21 ).
There are several routes for administering 5-MeO-DMT. This includes inhalation (~6–20 mg), intranasal (~10 mg), intravenous (~1–3 mg), sublingual (~10 mg), and oral (~30 mg) methods ( 18 , 22 ). Inhalation by vapor is most commonly reported, given its accessibility and relative ease of use, particularly in naturalistic settings ( 6 , 10 ). However, it can lead to intense rapid onset, relative to other dosage forms, like intramuscular injection. The onset, duration, and magnitude of subjective effects, occasioned by 5-MeO-DMT, are both route- and dose-dependent. For example, vaporization induces effects within ~10–15 s and peak experiences within ~2–5 min, resolving within ~25–30 min ( 6 , 22 , 23 ). Conversely, insufflation has a slower onset of action, due to delayed absorption, inducing effects within ~3–4 min and peak experiences within ~35–40 min, resolving within ~60–70 min ( 24 ). Irrespective of route, 5-MeO-DMT produces diverse subjective effects, including visual and auditory hallucinations, distorted time perception, and memory impairment ( 4 ). It also occasions peak mystical experiences comparable to high-dose psilocybin ( 25 ). Ego dissolution, a complete loss of self-identity, is frequently reported, as are profound near-death experiences ( 22 , 25 – 28 ). 5-MeO-DMT can, therefore, be challenging to navigate, with reports of fear, extreme anxiety, and paranoia ( 29 ). Users also describe perceptual isolation, seeing “all white” or “all black” ( 30 ). This contrasts to classic psychedelics, like N,N -DMT and LSD, that produce highly detailed, complex mental imagery. From a clinical standpoint, 5-MeO-DMT shows signals of benefit to mental health and well-being ( 3 , 4 ). However, there is a paucity of evidence in the field, particularly for trauma- and stress-related psychopathology.
Here, in accordance with CARE (CAse REport) guidelines ( 31 ), we present the first real-world, longitudinal case study on 5-MeO-DMT for post-traumatic stress disorder (PTSD). The subject provided written consent for publication and authorized disclosure of private health information. The data presented here were collected by the subject for their own interest and safety, and to monitor their progress over time. We then gained access to and analyzed the data retrospectively. To protect anonymity, the materials are not publicly available. This case study was exempt from ethics review and approval, in line with the Baylor College of Medicine Human Research Protections Manual, including Institutional Review Board procedures.

Subject information
A 23-year-old female presented with chronic refractory PTSD. She reported night terrors, trauma avoidance, negative affect, and hypervigilance. This developed from repeat sexual abuse, spanning six years as an adolescent. There was no relevant family history. Past interventions included variants of cognitive behavioral therapy (CBT), namely prolonged exposure (PE: 10 sessions), cognitive processing therapy (CPT: 12 sessions), and stress inoculation training (SIT: 8 sessions). These techniques targeted feared stimuli, maladaptive beliefs, and stress reactivity, respectively. However, each resulted in marginal improvements. She was then prescribed sertraline (Zoloft: 50 mg daily), a selective serotonin reuptake inhibitor (SSRI), following one week at 25 mg daily. This regimen adhered to pharmacotherapy guidelines for PTSD. Notwithstanding, the subject failed to respond adequately, reporting notable side effects, such as lethargy and disturbed sleep. She was, therefore, tapered off sertraline over the course of four weeks. This led to protracted symptoms and increased night terrors. Eventually, the subject was prescribed trazodone (Desyrel: 75 mg daily), a serotonin antagonist and reuptake inhibitor (SARI), for mixed insomnia. She experienced partial symptom relief and continued taking the medication, accordingly. The subject had no history of psychedelic use; however, she periodically smoked cannabis to manage her anxiety. See Figure 1 for a timeline of events.
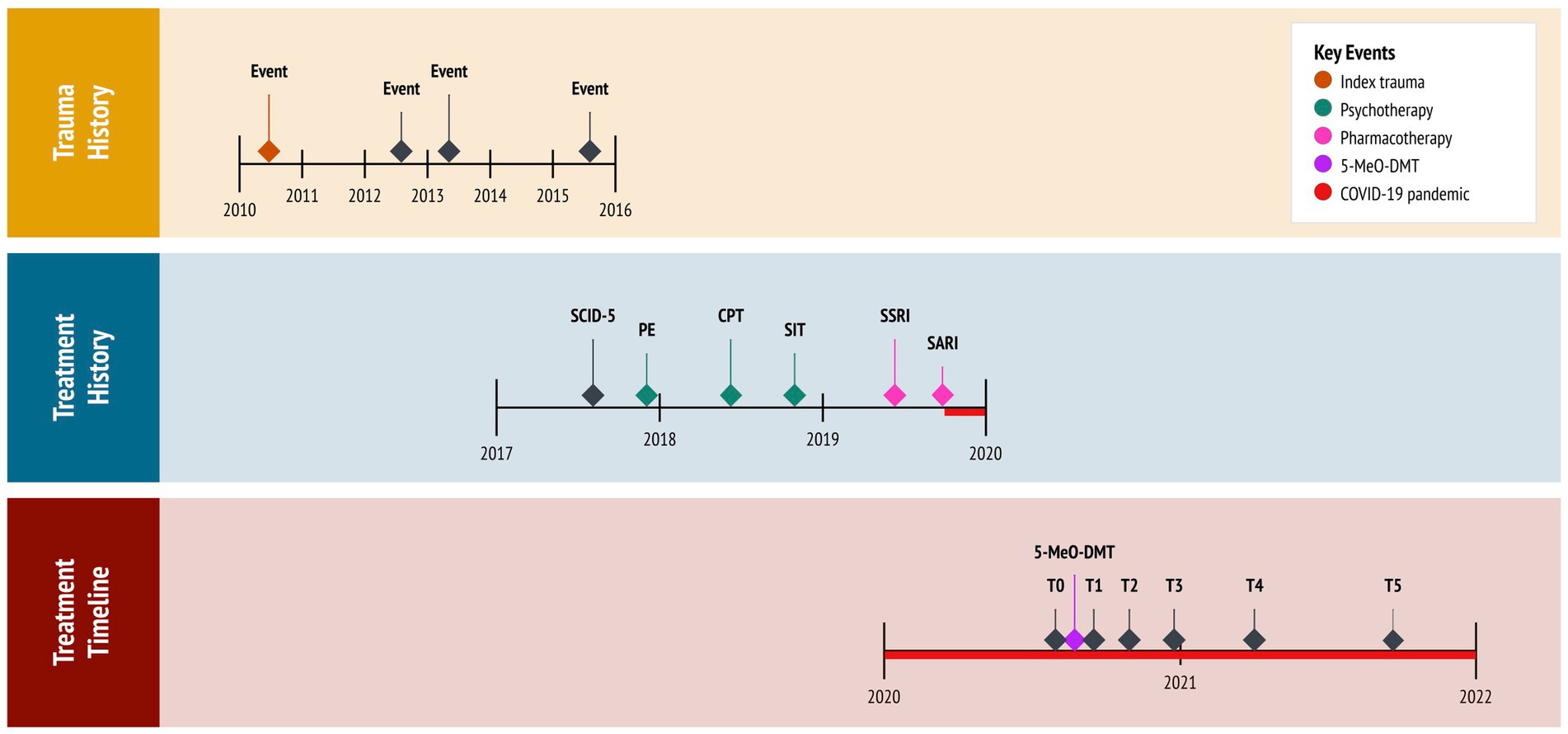
Figure 1 . Timeline of historical and clinical events. 5-MeO-DMT, 5-methoxy- N,N -dimethyltryptamine (experimental treatment); COVID-19, coronavirus disease pandemic; CPT, cognitive processing therapy (cognitive behavioral therapy); PE, prolonged exposure (cognitive behavioral therapy); SARI, serotonin antagonist and reuptake inhibitor (trazadone); SCID-5, Structured Clinical Interview for DSM-5 (diagnostic assessment); SIT, stress inoculation therapy (cognitive behavioral therapy); SSRI, selective serotonin reuptake inhibitor (sertraline); T0, baseline; T1, 24 h follow-up; T2, 1 month follow-up; T3, 3 months follow-up; T4, 6 months follow-up; T5, 12 months follow-up.
The coronavirus (COVID-19) pandemic, restricting social contact with friends and family, further aggravated the subject’s condition. Critically, she desired to end “intense emotional pain” and “chronic sadness.” Isolated in lockdown, desperate for help, and at risk of suicide, the subject pursued self-treatment with 5-MeO-DMT. This was motivated by (1) her resistance to first- and second-line therapies for PTSD, having attempted multiple interventions; (2) evidence on the potential benefits of 5-MeO-DMT for anxiety and trauma, acquired from reading news articles and research studies; (3) new legislation approved in her state (Oregon, Measure 110), which decriminalized the possession of controlled substances, including psychedelics; and (4) access to a trauma-informed 5-MeO-DMT facilitator, to whom a friend referred her to.
Diagnostic assessment
The subject was diagnosed with PTSD at 19 years of age. This was provided by her treating psychiatrist who, at the time, administered the Structured Clinical Interview for DSM-5 (SCID-5) ( 32 ), specifically the PTSD Module. The interview revealed a chronic course with severe PTSD symptoms and comorbid depression. Four years later, the subject pursued 5-MeO-DMT, independent from her psychiatrist, supported by a trauma-informed facilitator. The facilitator had extensive experience with 5-MeO-DMT, who advised on dosing and guided her experience. A licensed clinician, likewise, supported the subject in this pursuit. The clinician administered assessments, monitored her experience, and completed follow-ups. Assessments included the PTSD Checklist for DSM-5 [PCL-5; ( 33 )], the Beck Hopelessness Scale [BHS; ( 34 )] and the Clinical Global Impressions [CGI; ( 35 )] questionnaire. These were used to track the subject’s progress over time, administered prior to 5-MeO-DMT dosing (i.e., at baseline), and again 24 h-, 1 month-, 3 months-, 6 months-, and 12 months later (i.e., at follow-ups). For safety purposes, the clinician took vital signs before, during, and after 5-MeO-DMT dosing. This consisted of blood pressure (mmHg), heart rate (bpm), and peripheral oxygen saturation (SpO 2 ). To assess acute, subjective effects, the Mystical Experiences Questionnaire [MEQ-30; ( 36 )] was administered 3 h post-dosing. The clinician observed the subject for a total of 5 h after her 5-MeO-DMT experience, and conducted follow-ups via phone 24, 36, and 72 h later, before switching to once a month.
PTSD checklist for DSM-5
The PCL-5 is a 20-item measure of PTSD symptoms. It has excellent internal consistency (α = 0.94) ( 33 ), comprising four factors: ‘thought intrusion’, ‘stimuli avoidance’, ‘negative mood and cognitions’, and ‘altered reactivity’. Items are rated on a 5-point scale, with ‘not at all’ (0) and ‘extremely’ (4) as endpoints. The PCL-5 is scored by summing items within a given factor, as well as all items together. Total scores range from 0 to 80. Higher scores reflect greater symptom severity, with 31–33 typically used as the cut-off point for probabilistic PTSD. When monitoring symptoms, a 5–10-point difference indicates reliable change, not due to chance, whereas a 10–20-point difference indicates clinically significant change. The ‘past week’ version of the PCL-5 was utilized in this case study.
Impressions
At baseline, the subject’s total score was 72 of 80 (3.79 ± 0.42), meeting threshold criteria for ‘severe’ PTSD, and a provisional diagnosis. Regarding PCL-5 factors, she scored the highest on ‘altered reactivity’ (4.00 ± 0.00), followed by ‘thought intrusion’ (3.80 ± 0.45), ‘negative mood and cognitions’ (3.71 ± 0.49), and ‘stimuli avoidance’ (3.50 ± 0.71).
Beck hopelessness scale
The BHS is a 20-item measure of hopelessness. It has excellent internal consistency (α = 0.97) ( 37 ), comprising three factors: ‘feelings about the future’, ‘loss of motivation’, and ‘future expectations’. Items are rated on a 2-point scale, using dichotomous ‘true’ (0/1) and ‘false’ (0/1) statements. The BHS is scored by summing items within a given factor, as well as all items together. Total scores range from 0 to 20. Higher scores reflect greater hopelessness, categorized into four levels: ‘normal’ (0–3), ‘mild’ (4–8), ‘moderate’ (9–14), and ‘severe’ (>14). A cut-off score of 9 is frequently used to detect risk of suicidal ideation and behavior.
At baseline, the subject’s total score was 17 of 20 (0.85 ± 0.37), meeting threshold criteria for ‘severe’ hopelessness and suicide risk. Regarding BHS factors, she scored the highest on ‘feelings about the future’, (1.00 ± 0.00) and ‘future expectations’ (1.00 ± 0.00), followed by ‘loss of motivation’ (0.63 ± 0.52).
Clinical global impressions
The CGI is a 3-item measure of global functioning. It was developed for clinical trials, aimed at capturing change after initiating a study drug. The CGI includes three factors. The first factor measures ‘illness severity’, rated on a 7-point scale, anchored by ‘normal and not at all ill’ (1) and ‘among the most extremely ill’ (7). The second factor measures ‘global improvement’, also rated on a 7-point scale, with ‘very much improved’ (1) and ‘very much worse’ (7) as endpoints. Finally, the third factor measures ‘therapeutic response’, rated on a 5-point scale, anchored by ‘marked improvement and no side effects’ (0) and ‘unchanged or worse and side effects outweigh therapeutic effect’ (4). This third factor considers both therapeutic efficacy and drug-related adverse events. A zero is allocated if there is no assessment. Each factor is rated separately, yielding no total scores.
At baseline, the subject’s score for ‘illness severity’, regarding PTSD, was 6 of 7, meeting threshold criteria for ‘severely ill’. In particular, she exhibited disruptive trauma- and stress-related psychopathology, with symptoms considerably impairing her behavior and function. The other two factors, ‘global improvement’ and ‘therapeutic response’, were not assessed at baseline, as they measure changes after treatment.
Therapeutic intervention
5-MeO-DMT was obtained and administered by the subject. The experience occurred in the comfort of her home. Guided by the facilitator, she first set an intention for the experience. “I want to understand and accept the roots of my trauma.” This was designed to help navigate potentially difficult psychedelic states and material, by re-centering the subject’s attention. Next, she engaged in body scan meditation, a specific form of mindfulness practice. This involved deep breathing and mind–body awareness, aimed at relaxation. The subject then inhaled 50 mg of vaporized bufotoxin, derived from the Sonoran Desert Toad ( Incilius alvarius ), slowly and consistently. This was estimated to contain 10–15 mg of 5-MeO-DMT [20–30% of total dried weight ( 38 )]. Using a torch lighter, the bufotoxin was heated in a glass vial until its contents were vaporized. She held the dose for 10 s, exhaled slowly and consistently, and lied down with an eye mask on. Ambient music played in the background. The onset of effects was rapid (15–30 s), with peak effects lasting 10–15 min, resolving within 25–30 min. After the effects had subsided, the subject re-engaged in body scan meditation. She then discussed her experience with the facilitator, integrating newfound insights. Finally, the clinician reviewed the subject’s vital signs and asked about her experience, recording any undesirable reactions. Three hours later, the clinician administered the MEQ-30.
Follow-up and outcomes
5-MeO-DMT was generally tolerated by the subject. Mild nausea was reported, which resolved within 30 min. There were slight increases in systolic blood pressure (126.00 ± 3.54), diastolic blood pressure (89.00 ± 4.24), and heart rate (81.50 ± 4.95), whereas oxygen saturation (97.50 ± 0.71) remained stable. See Table 1 and Figure 2 . Overall, no drug-related, serious adverse events occurred. However, the subject reported “profoundly strong” subjective effects. She described being “instantly blasted” into another dimension. At first, colors were extremely vivid, then morphed into “complete whiteness.” The subject failed to make sense of psychedelic content, stating that visuals were “bright and god-like,” yet vague and fleeting. She also reported increased body temperature and euphoria. “I felt really warm, like my body was melting. It was calm and blissful.” This was accompanied by radical ego dissolution. “I had no identity. I was still alive, but my body was gone. It was quite overwhelming. I just had to surrender.”
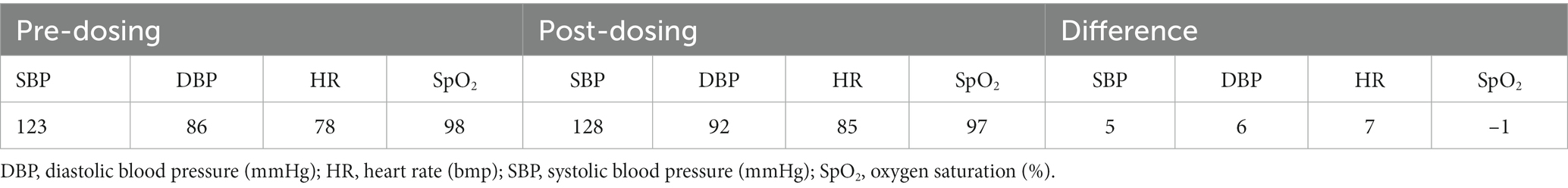
Table 1 . Clinician-reported vital signs.
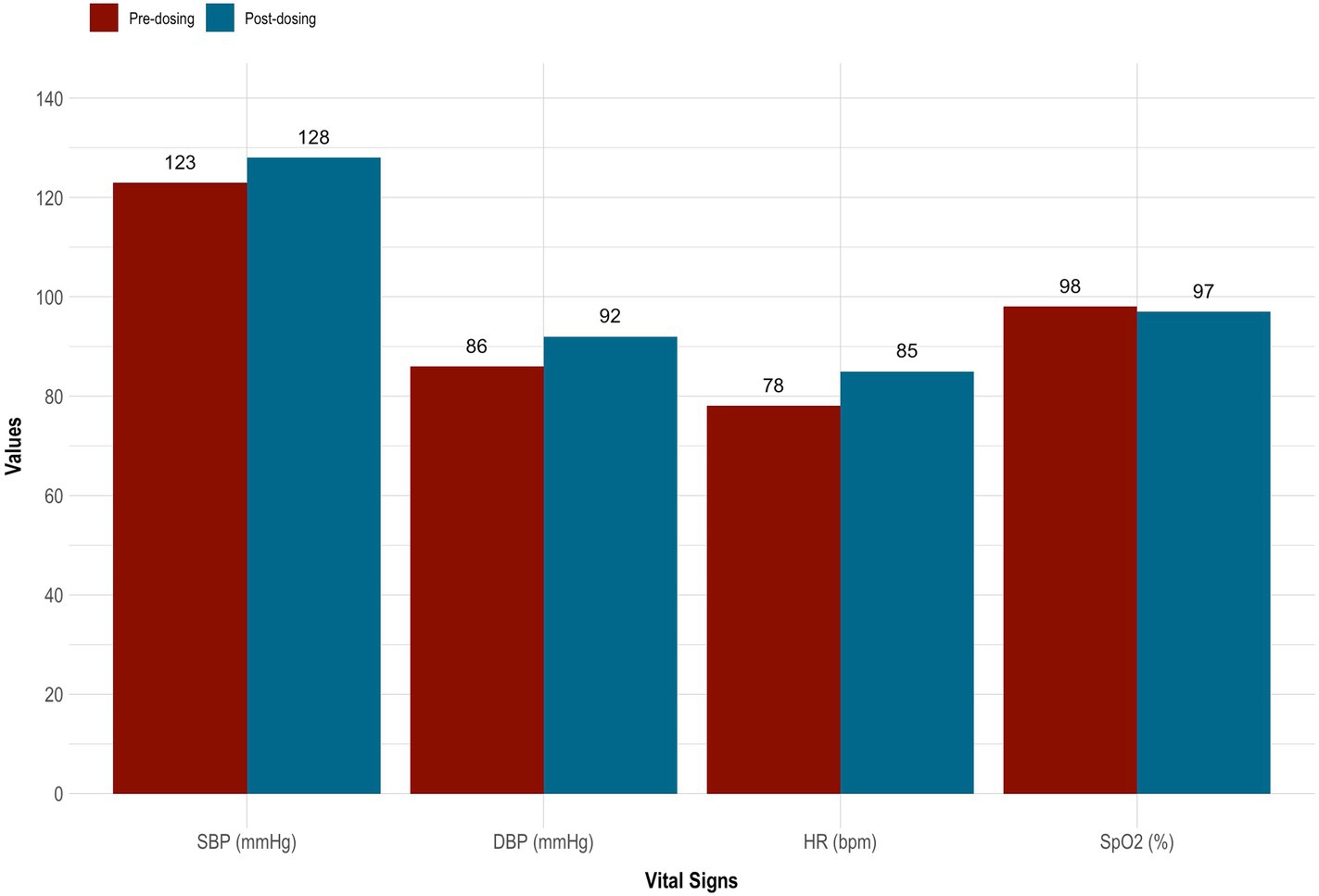
Figure 2 . Vital signs taken before and after 5-MeO-DMT dosing. 5-MeO-DMT, 5-methoxy- N,N -dimethyltryptamine; DBP, diastolic blood pressure (mmHg); HR, heart rate (bmp); SBP, systolic blood pressure (mmHg); SpO 2 , peripheral blood oxygenation (%).
On the MEQ-30, the subject endorsed strong mystical-like effects. Her total score was 135 of 150 (4.47 ± 0.62). She also met criteria for a ‘complete mystical experience’. This was evidenced by scoring ≥60% of the maximum possible scores on all four factors of the MEQ-30: ‘mysticism’ (4.47 ± 0.62 [89.3%]), ‘positive mood’ (4.33 ± 0.75 [86.7%]), ‘transcendence’ (4.67 ± 0.47 [93.3%]), and ‘ineffability; (4.33 ± 0.47 [86.7%]). See Figure 3 for details.
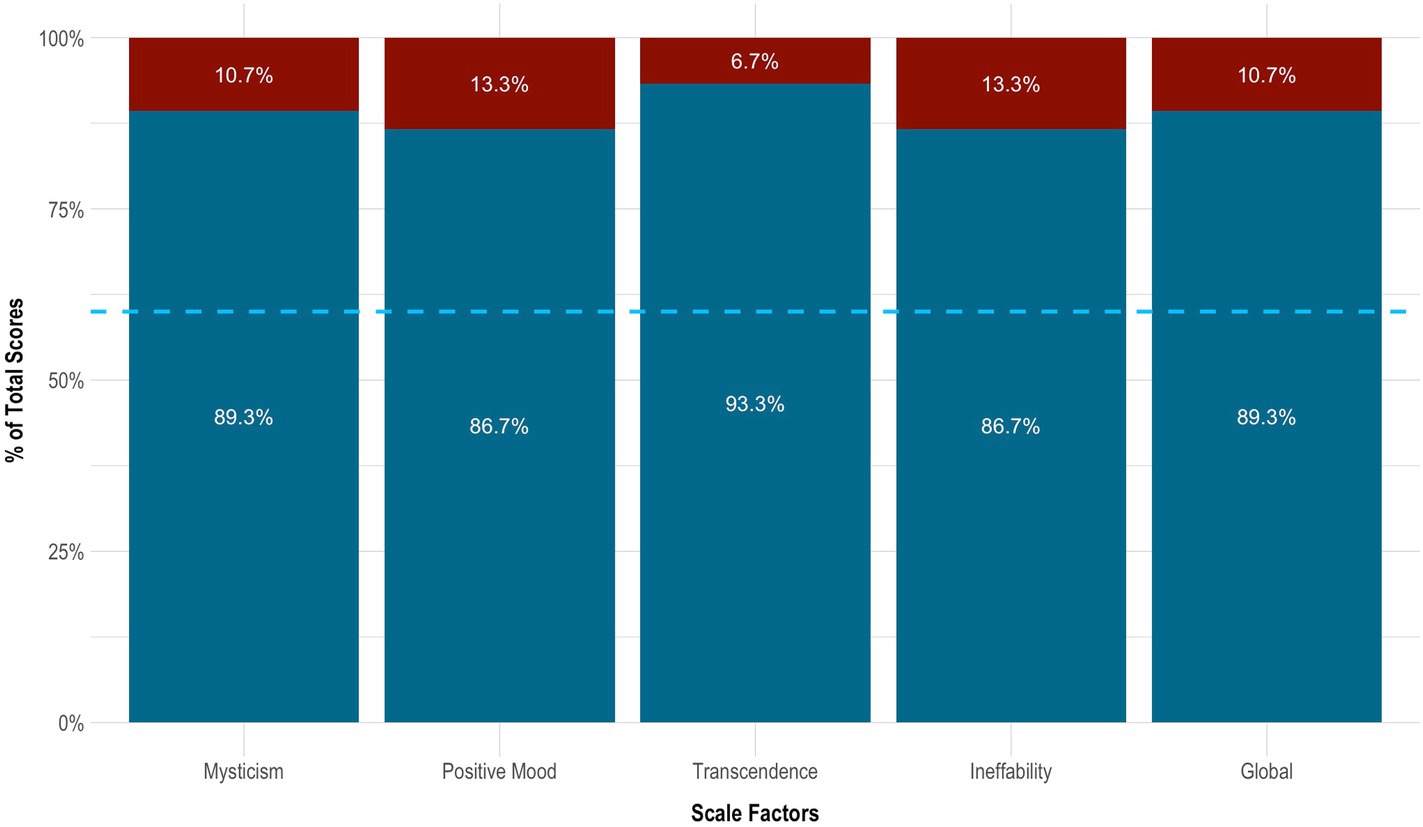
Figure 3 . Mystical effects of 5-MeO-DMT. Blue dotted line indicates the cut-off point for a complete mystical experience (≥60% of total scores across factors). 5-MeO-DMT, 5-methoxy- N,N -dimethyltryptamine; MEQ-30, Mystical Experience Questionnaire.
On the PCL-5, the subject had a clinically significant change in PTSD, which sustained across time. This was evidenced by a ≥ 10-point reduction in total scores from baseline to 24 h (−54 points), 1 month (−49 points), 3 months (−37 points), 6 months (−46 points), and 12 months (−50 points) follow-up. In particular, her symptoms decreased by 75.0% from baseline to 24 h (3.79 ± 0.42 vs. 0.95 ± 0.71), increased by 27.8% from 24 h to 1 month (0.95 ± 0.71 vs. 1.21 ± 0.63), increased by 52.2% from 1 month to 3 months (1.21 ± 0.63 vs. 1.84 ± 0.76), decreased by 25.7% from 3 months to 6 months (1.84 ± 0.76 vs. 1.37 ± 0.68), and finally decreased by 15.4% from 6 months to 12 months (1.37 ± 0.68 vs. 1.16 ± 0.60) follow-up. From baseline to 12 months follow-up, she experienced the greatest improvement in ‘negative mood and cognitions’ (3.71 ± 0.49 vs. 1.10 ± 0.69), followed by ‘thought intrusion’ (3.80 ± 0.45 vs. 1.00 ± 0.71), ‘altered reactivity’ (4.00 ± 0.00 vs. 1.40 ± 0.55), and ‘stimuli avoidance’ (3.50 ± 0.71 vs. 1.00 ± 0.00). See Table 2 and Figures 4A , B .
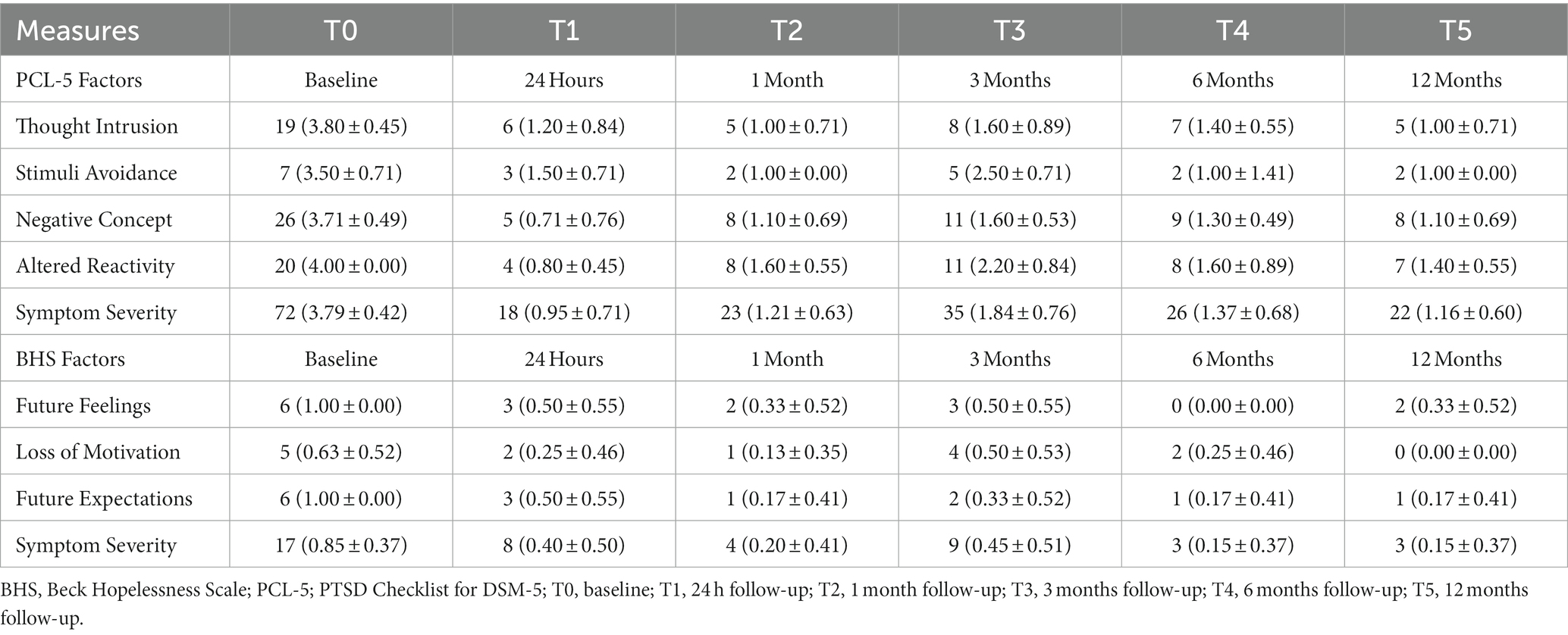
Table 2 . Self-reported outcome measures.
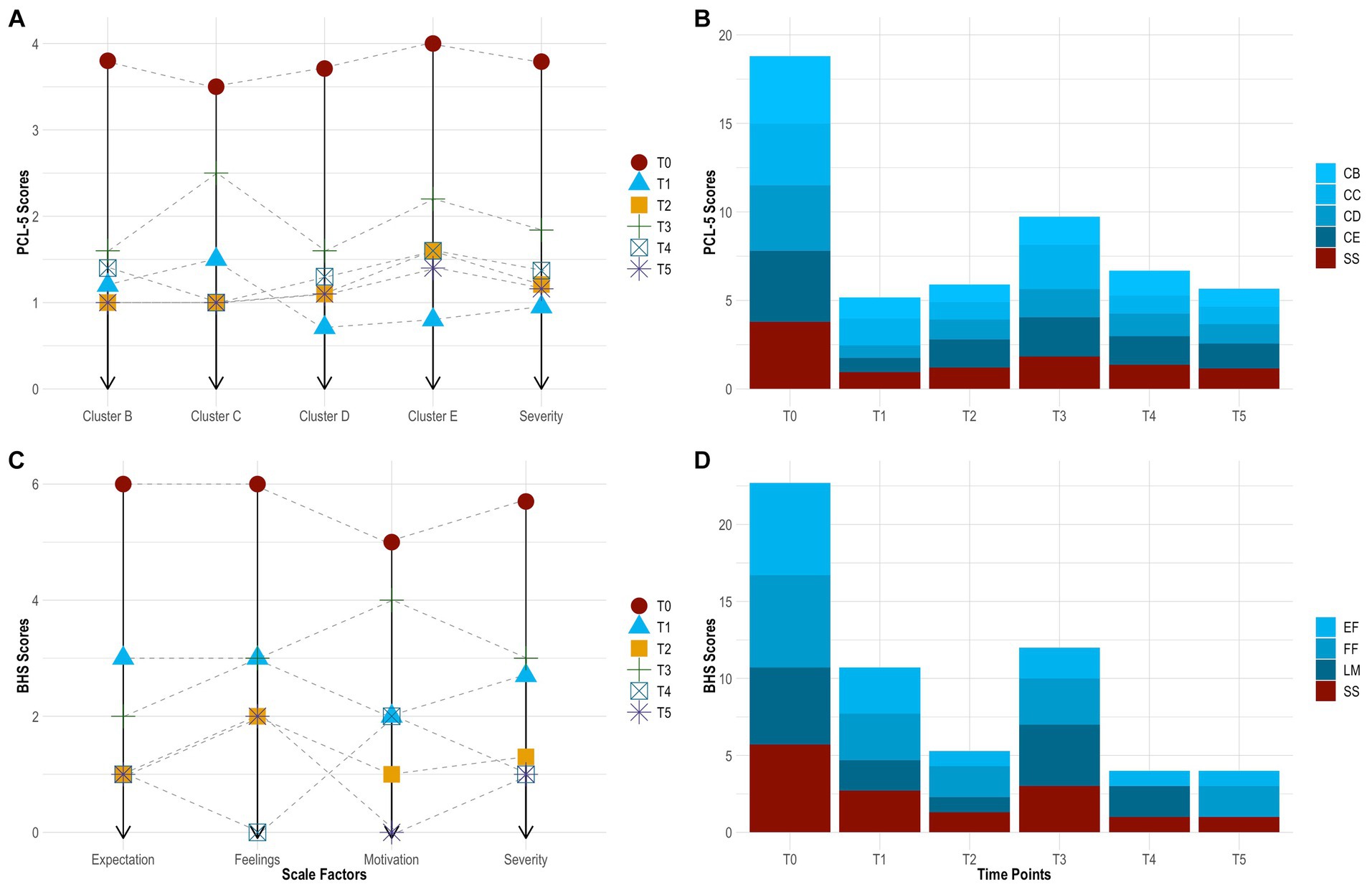
Figure 4 . Change in PTSD symptoms by time point [ (A) , line graph]. Change in PTSD symptoms across time [ (B) , bar chart]. Change in hopelessness symptoms by time point [ (C) line graph]. Change in hopelessness symptoms across time [ (D) , bar chart]. BHS, Beck Hopelessness Scale; CB, cluster B (thought intrusion); CC, cluster C (stimuli avoidance); CD, cluster D (negative mood and cognitions); CE, cluster E (altered reactivity); EF, expectations about the future (hopelessness); FF, feelings about the future (hopelessness); LM, loss of motivation (hopelessness); PCL-5, PTSD Checklist for DSM-5; PTSD, post-traumatic stress disorder; SS, symptom severity; T0, baseline; T1, 24 h follow-up; T2, 1 month follow-up; T3, 3 months follow-up; T4, 6 months follow-up; T5, 12 months follow-up.
On the BHS, the subject showed robust improvement in hopelessness. This also sustained across time. Her symptoms decreased by 52.9% from baseline to 24 h (0.85 ± 0.37 vs. 0.40 ± 0.50), decreased by 50.0% from 24 h to 1 month (0.40 ± 0.50 vs. 0.20 ± 0.41), increased by 125.0% from 1 month to 3 months (0.40 ± 0.50 vs. 0.45 ± 0.51), decreased by 66.7% from 3 months to 6 months (0.45 ± 0.51 vs. 0.15 ± 0.37), and remained stable from 6 months to 12 months (0.15 ± 0.37 vs. 0.15 ± 0.37) follow-up. From baseline to 12 months follow-up, she experienced the greatest improvement in ‘loss of motivation’ (0.63 ± 0.52 vs. 0.00 ± 0.00) and ‘future expectations’ (1.00 ± 0.00 vs. 0.17 ± 0.41), followed by ‘feelings about the future’ (0.63 ± 0.52 vs. 0.33 ± 0.52). Further, the subject had a clinically significant change in suicide risk. This was evidence by scoring ≤9 at 24 h (score = 8), 1 month (score = 4), 3 months (score = 9), 6 months (score = 3), and 12 months (score = 8) follow-up. See Table 2 and Figures 4C , D .
On the CGI, the clinician reported marked reductions in PTSD, which sustained across time. Rated at each time point, she presented as ‘severely ill’ at baseline (score = 6), ‘mildly ill’ at 24 h (score = 3), ‘borderline ill’ at 1 month (score = 2), ‘mildly ill’ at 3 months (score = 3), ‘not at all ill’ at 6 months (score = 1), and ‘not at all ill’ at 12 months (score = 1) follow-up. These ratings considered the clinician’s total experience treating PTSD. Relative to baseline, the subject’s global functioning changed from ‘much improved’ at 24 h post-dosing (score = 2), representing a significant change, with increased functioning and moderate symptoms, to ‘very much improved’ at 12 months follow-up (score = 1), indicating a substantial change, with good functioning and minimal symptoms. This was judged independent from any beliefs about 5-MeO-DMT. Finally, based on drug effect, her therapeutic response was ‘marked’ at 24 h post-dosing (score = 2), with side effects that did not significantly interfere with functioning, and ‘marked’ again at 12 months-follow-up (score = 1), with no side effects. See Table 3 .
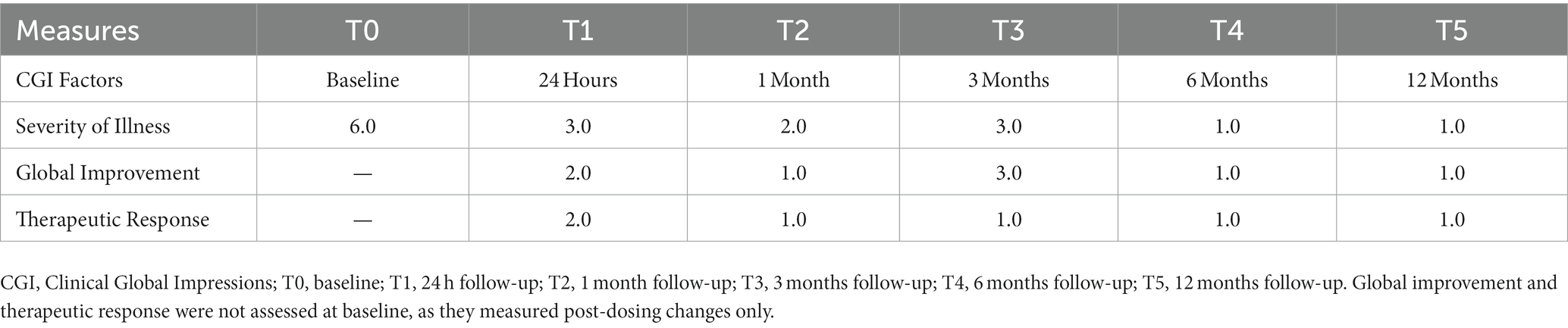
Table 3 . Clinician-reported outcome measures.
In this case study, a single dose of vaporized toad bufotoxin, containing 5-MeO-DMT, led to clinically significant improvements in PTSD, with next-day effects. These gains were sustained at 1-, 3-, 6-, and 12-months follow-up. Moreover, the subject showed striking reductions in hopelessness and related suicide risk. These changes were, likewise, durable across time. Self-reported improvements further reflected clinician-observed changes in global functioning. 5-MeO-DMT was generally tolerated. No drug-related, serious adverse events occurred. However, there were nominal increases in blood pressure and heart rate. This did not extend to oxygen saturation. Subjective effects were also overwhelming. Interestingly, 5-MeO-DMT produced more visual content than previously described ( 30 ). Colors appeared at the beginning of her experience, then faded into transcendent light; the latter being more consistent with literature ( 30 ). The subject’s dose and setting likely impacted her perceptual experience ( 38 ). Regardless, more data is needed to characterize the phenomenology of 5-MeO-DMT, and how this compares to other psychedelics. This is particularly important for optimizing facilitation and harm reduction practices, in helping patients navigate psychedelic states, as well as for targeting PTSD and chronic stress pathology.
Furthermore, the subject endorsed a strong and complete mystical experience. While the mechanism underlying her therapeutic response is unknown, it may be explained, in part, by the epistemological or ‘noetic quality’ of mystical states, occasioned by 5-MeO-DMT ( 39 ). These psychological states are characteristic of psychedelics, namely serotonergic compounds ( 39 ); have been shown to correlate, mediate, and predict therapeutic efficacy ( 40 ); and include feelings of transcendence, ego dissolution, and ineffability as well as unity, love, and peace ( 41 ). Thus, people have rated mystical experiences in their top five most important life events, in terms of personal meaning and spiritual significance, next to giving birth or losing a loved one ( 42 , 43 ). These effects can persist up to 30 years after taking a psychedelic ( 44 ). In the present case study, the subject described the mystical effects of 5-MeO-DMT as both substantial and enduring. “It was the most profound and frightening experience of my life. I saw bright colors. I was connected to all things. I disappeared into space. I smiled for the first time in a long time. I cried and screamed. I forgot about [my] pain and trauma… then relived it. My body had permission to heal. I moved on. It’s hard to put it into words… beautiful and challenging I guess…feeling everything and nothing at once. But it allowed me to view my trauma in a different way. Like a superpower. That insight has stayed with me.” Other possible mechanisms of change, from a psychological standpoint, include re-processing and transforming traumatic material.
This case study aligns with previous findings in the literature. For instance, in a retrospective, epidemiology survey on 5-MeO-DMT ( n = 515; M age = 35.4; male = 79%), 79% of participants with psychiatric disorders reported improved PTSD following 5-MeO-DMT use ( 22 ). Most participants (90%) had moderate-to-strong mystical experiences, while a significant proportion (37%) had challenging ones. In another retrospective, international survey ( n = 99; M age = 37.4; male = 74%), 79% of participants with past or present PTSD, who had used 5-MeO-DMT at least once in their lifetime, reported improved symptomatology ( 45 ). They also endorsed significantly stronger mystical experiences than those who did not experience symptom improvement or regressed. Most recently, Davis et al. ( 46 ) examined ibogaine and 5-MeO-DMT for trauma-related psychological and cognitive impairment, specifically among U.S. Special Operations Forces Veterans ( n = 51; M age = 40.0; male = 96%). They analyzed retrospective data collected 30 days before and 30 days after a clinical psychedelic program in Mexico. The results showed significant and large reductions in depression, suicidal ideation, anxiety, PTSD, and cognitive impairment. Participants additionally reported increased psychological flexibility, which was strongly associated with improvements in all constructs, excluding suicidality.
Other studies have investigated 5-MeO-DMT in naturalistic settings. For example, in an observational group study, using structured dosing protocols, researchers examined clinical correlates of 5-MeO-DMT ( 27 ). Among healthy participants ( n = 362; M age = 47.7; male = 55%), 80% with depression and 79% with anxiety reported spontaneous, unintended reductions in symptoms. This was associated with stronger mystical experiences, as well as higher ratings of spirituality and meaning in life. In another observational study, Uthaug et al. ( 47 ) investigated sub-acute and long-term effects of 5-MeO-DMT on affect and cognition. Among healthy participants ( n = 42; M age = 38.0; male = 60%), ratings of depression, anxiety, and stress decreased 24 h post-intake and reached significance at 4 weeks follow-up. Those who experienced high levels of ego dissolution or oceanic boundlessness, two markers of a mystical experience, displayed lower levels of depression and stress. However, this did not extend to anxiety.
Of note, the subject partially regressed at 3-months follow-up. She reported new onset of night terrors, the nature of which could not be recalled. These night terrors reflect higher scores across all measures at this time point, relative to the others. A phenomenon known as ‘reactivation’, similar to flashbacks, is commonly reported by 5-MeO-DMT users ( 22 , 48 ). This involves re-experiencing parts of a drug-induced state post-administration, which can occur days, weeks, or even months later ( 49 , 50 ). Additionally, the probability of 5-MeO-DMT reactivation increases with being female, dosing in a structured group format, and having a stronger mystical experience ( 48 ). All three of these factors applied to this case study. As such, the subject may have endured a reactivation event following 5-MeO-DMT, presenting as negatively-valenced night terrors. Alternatively, the benefits of 5-MeO-DMT may have only lasted for three months. Despite the partial regression, scores across all measures remained below clinical thresholds, with symptoms naturally remitting overtime. The onset of night terrors was not considered a ‘serious adverse event’, as its association with 5-MeO-DMT could not be definitively concluded. It neither was life-threatening, required intervention or hospitalization, resulted in persistent or significant disability, nor led to the subject’s death.
Strengths and limitations
The longitudinal nature of this case study serves as its primary strength, with repeated observations collected over a 1-year period. Findings are more robust, given the subject’s treatment resistance and disease chronicity, the complexity of this clinical population, and the limitation in available effective, evidence-based interventions. Further, the presence of psychiatric comorbidities, the lack of polypharmacy or medication washout, and the naturalistic setting better reflect patients in the real world. The use of well-validated measures, capturing both subject- and clinician-reported changes, is an additional strength. Notwithstanding, this case study is inherently limited.
First, it describes the presentation, treatment, and follow-up of a single person. Hence, the results cannot be generalized to others with PTSD. Second, the dose of 5-MeO-DMT was estimated by the subject, based on visual inspection. The precise amount cannot be determined, accordingly. Third, the source for obtaining toad bufotoxin, containing the 5-MeO-DMT, is unknown. The compound’s integrity may have been compromised as a result. Fourth, 5-MeO-DMT was self-administered by the subject. This is not considered a suitable clinical or pharmaceutical application, primarily due to safety reasons. Finally, there is no evidence that 5-MeO-DMT, in and of itself, produced therapeutic activity reported in this case study. Facilitation practices, like body scan meditation, for instance, may have confounded the results, magnifying or diminishing therapeutic effects. Findings should, therefore, be interpreted with caution, and only serve to catalyze future research. This is particularly important, as the field is far from establishing clinical efficacy, real-world effectiveness, and standard treatment protocols for 5-MeO-DMT in PTSD and beyond. Additionally, naturalistic psychedelic use has steadily increased over the past decade ( 51 ). This is likely due to media coverage, advances in research, and changes to legislation. It is, thus, critical to balance discussions on 5-MeO-DMT and other psychedelics with clear and careful acknowledgement of safety risks.
Looking ahead, the next logical step is to conduct pilot studies that explore the safety, tolerability, and preliminary efficacy of 5-MeO-DMT for PTSD, in larger and more diverse samples. Including a richer battery of psychometric instruments is highly encouraged. Results could then inform open-label, randomized, and adaptive trials to further characterize 5-MeO-DMT for this patient population; and to explore different therapeutic approaches, including adjunctive psychotherapy, which may augment patient adherence and therapeutic outcomes. Incorporating moderated mediation models, as statistical analyses, is also encouraged in future work. This would allow researchers to control for covariates, like age and gender, while examining potential underlying mechanisms, such as mystical experiences.
This case study is the first to report the longitudinal effects of 5-MeO-DMT for chronic refractory PTSD, complicated by hopelessness and suicidality. The results showed that 5-MeO-DMT offered fast-acting, robust, and sustained improvements in symptomatology, and was generally tolerable and safe to administer. However, this was not without risks, as evidenced by acute nausea, overwhelming subjective effects, and late onset of night terrors. Further research is warranted to replicate and extend these findings, which are inherently limited, non-generalizable, and rely on methods not clinically accepted. This can be achieved through clinical and naturalistic studies, in controlled and uncontrolled environments, to effectively converge on safety, efficacy, effectiveness, and durability of 5-MeO-DMT for PTSD. Evidence can then be leveraged to optimize therapeutic delivery, as well as develop standard clinical practice guidelines.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AR: Conceptualization, Formal analysis, Project administration, Visualization, Writing – original draft. RK: Investigation, Writing – original draft, Writing – review & editing. PS: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. LB: Writing – original draft, Writing – review & editing. RA: Writing – original draft, Writing – review & editing. MK: Writing – original draft, Writing – review & editing. NB: Validation, Writing – review & editing. LJ: Validation, Writing – review & editing. MG: Validation, Writing – review & editing. JB: Supervision, Validation, Writing – review & editing. LA: Conceptualization, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare that this study received funding from Cubed Biotech. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. All research in the Department of Psychiatry at the University of Cambridge was supported by the National Institute for Health and Care Research Cambridge Biomedical Research Centre (NIHR203312) and the National Institute for Health and Care Research Applied Research Collaboration East of England. The views expressed are those of the author(s) and not necessarily those of the National Institute for Health and Care Research or the Department of Health and Social Care. LA receives some salary support from the US Department of Veterans Affairs (IK2CX001873) and the American Foundation for Suicide Prevention (YIG-0-004-16).
Acknowledgments
We express our gratitude to the subject, facilitator, and clinician—described in this case study—for collecting and sharing their data; and for their valuable contributions to science, specifically in understanding the potential of 5-MeO-DMT for PTSD and beyond.
Conflict of interest
AR is the Founding Director of the Integrated Research Literacy Group. PS is the Director of Psychological Science at the Integrated Research Literacy Group. He also receives some salary/research support from Cubed Biotech. MK is the Director of Ethnographic Studies at the Integrated Research Literacy Group. JB is the Clinical Advisor to The Mission Within, Journey Colab, Beond, Kaivalya Kollective, Tandava Retreats, Kernel, Woven Science, Brain Health Restoration, and Lionheart Ventures. LA has served as a Consultant, Speaker and/or Advisory Board Member for Guidepoint, Transcend Therapeutics, Beond, Source Research Foundation, Reason for Hope, Beond, The Cohen Foundation, Ampelis, and is owner of NPSYT, PLLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer BS declared a shared research group 5 MEO Education with the author JB to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The authors do not condone the illegal use of any psychedelic substance, including 5-MeO-DMT.
1. Pachter, IJ, Zacharias, DE, and Ribeiro, O. Indole alkaloids of Acer saccharinum (the silver maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. J Org Chem . (1959) 24:1285–7. doi: 10.1021/jo01091a032
CrossRef Full Text | Google Scholar
2. Hoshino, T, and Shimodaira, K. Über die Synthese des Bufotenin-Methly-Äthers (5-Methoxyn-dimethyl-tryptamin) und Bufotenins (Synthesen in Der Indol-Gruppe. XV). Bull Chem Soc Jpn . (1936) 11:221–4. doi: 10.1246/bcsj.11.221
3. Ermakova, AO, Dunbar, F, Rucker, J, and Johnson, MW. A narrative synthesis of research with 5-MeO-DMT. J Psychopharmacol (Oxf) . (2022) 36:273–94. doi: 10.1177/02698811211050543
4. Reckweg, JT, Uthaug, MV, Szabo, A, Davis, AK, Lancelotta, R, Mason, NL, et al. The clinical pharmacology and potential therapeutic applications of 5‐methoxy‐N,N‐dimethyltryptamine (5‐MeO‐DMT). J Neurochem . (2022) 162:128–46. doi: 10.1111/jnc.15587
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Ott, J. Shamanic snuffs or entheogenic errhines . Solothrun, Switzerland: Entheobotanica (2001).
Google Scholar
6. Weil, AT, and Davis, W. Bufo Alvarius : a potent hallucinogen of animal origin. J Ethnopharmacol . (1994) 41:1–8. doi: 10.1016/0378-8741(94)90051-5
7. Lancelotta, R. 5-MeO-DMT has not been found in traditional ayahuasca preparations and the combination of 5-MeO-DMT with MAOIs is dangerous. Hum Psychopharmacol Clin Exp . (2022) 37:e2839. doi: 10.1002/hup.2839
8. Kaasik, H, Souza, RCZ, Zandonadi, FS, Tófoli, LF, and Sussulini, A. Chemical composition of traditional and analog Ayahuasca. J Psychoactive Drugs . (2021) 53:65–75. doi: 10.1080/02791072.2020.1815911
9. VICE TV. (2017). Hamilton Morris learns about the toad ceremonies of the Yaqui tribe. Available at: https://www.youtube.com/watch?v=M5U9D7Y5er4
10. Most, A. Bufo Alvarius : The psychedelic toad of the Sonoran Desert Venom Press (1984).
11. Halberstadt, AL, Nichols, DE, and Geyer, MA. Behavioral effects of α,α,β,β-tetradeutero-5-MeO-DMT in rats: comparison with 5-MeO-DMT administered in combination with a monoamine oxidase inhibitor. Psychopharmacology . (2012) 221:709–18. doi: 10.1007/s00213-011-2616-6
12. Ray, TS. Psychedelics and the human Receptorome. PLoS One . (2010) 5:e9019. doi: 10.1371/journal.pone.0009019
13. Berge, O, Chacho, D, and Hole, K. Inhibitory effect of 5-methoxy- N,N -dimethyltryptamine on the synaptosomal uptake of 5-hydroxytryptamine. Eur J Pharmacol . (1983) 90:293–6. doi: 10.1016/0014-2999(83)90253-4
14. Spencer, DG, Glaser, T, and Traber, J. Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy- N . N -dimethyltryptamine Psychopharmacology (Berl) . (1987) 93:158–66. doi: 10.1007/BF00179927
15. Nutt, D, Spriggs, M, and Erritzoe, D. Psychedelics therapeutics: what we know, what we think, and what we need to research. Neuropharmacology . (2023) 223:109257. doi: 10.1016/j.neuropharm.2022.109257
16. Halberstadt, AL, and Geyer, MA. Multiple receptors contribute to the behavioral effects of Indoleamine hallucinogens. Neuropharmacology . (2011) 61:364–81. doi: 10.1016/j.neuropharm.2011.01.017
17. Vigerelli, H, Sciani, JM, Eula, MAC, Sato, LA, Antoniazzi, MM, Jared, C, et al. Biological effects and biodistribution of Bufotenine on mice. Biomed Res Int . (2018) 2018:1–10. doi: 10.1155/2018/1032638
18. Shen, HW, Jiang, XL, Winter, J, and Yu, AM. Psychedelic 5-Methoxy- N,N -dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr Drug Metab . (2010) 11:659–66. doi: 10.2174/138920010794233495
19. Jiang, XL, Shen, HW, and Yu, AM. Potentiation of 5-methoxy- N,N -dimethyltryptamine-induced hyperthermia by harmaline and the involvement of activation of 5-HT1A and 5-HT2A receptors. Neuropharmacology . (2015) 89:342–51. doi: 10.1016/j.neuropharm.2014.10.013
20. Malcolm, B, and Thomas, K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology . (2022) 239:1881–91. doi: 10.1007/s00213-021-05876-x
21. Sklerov, J, Levine, B, Moore, KA, King, T, and Fowler, D. A fatal intoxication following the ingestion of 5-Methoxy- N,N -dimethyltryptamine in an Ayahuasca preparation*. J Anal Toxicol . (2005) 29:838–41. doi: 10.1093/jat/29.8.838
22. Davis, AK, Barsuglia, JP, Lancelotta, R, Grant, RM, and Renn, E. The epidemiology of 5-methoxy- N, N -dimethyltryptamine (5-MeO-DMT) use: benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J Psychopharmacol (Oxf) . (2018) 32:779–92. doi: 10.1177/0269881118769063
23. Uthaug, MV, Lancelotta, R, Ortiz Bernal, AM, Davis, AK, and Ramaekers, JG. A comparison of reactivation experiences following vaporization and intramuscular injection (IM) of synthetic 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting. J Psychedelic Stud . (2020) 4:104–13. doi: 10.1556/2054.2020.00123
24. Ott, J. Pharmepéna-Psychonautics: human intranasal, sublingual and Oral pharmacology of 5-Methoxy-N, N-Dimethyl-Tryptamine. J Psychoactive Drugs . (2001) 33:403–7. doi: 10.1080/02791072.2001.10399925
25. Barsuglia, JP, Polanco, M, Palmer, R, Malcolm, BJ, Kelmendi, B, and Calvey, T. A case report SPECT study and theoretical rationale for the sequential administration of ibogaine and 5-MeO-DMT in the treatment of alcohol use disorder. Progress Brain Res . (2018):121–58. doi: 10.1016/bs.pbr.2018.08.002
26. Barsuglia, J, Davis, AK, Palmer, R, Lancelotta, R, Windham-Herman, AM, Peterson, K, et al. Intensity of mystical experiences occasioned by 5-MeO-DMT and comparison with a prior psilocybin study. Front Psychol . (2018) 9:2459. doi: 10.3389/fpsyg.2018.02459
27. Davis, AK, So, S, Lancelotta, R, Barsuglia, JP, and Griffiths, RR. 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety. Am J Drug Alcohol Abuse . (2019) 45:161–9. doi: 10.1080/00952990.2018.1545024
28. Uthaug, MV, Lancelotta, R, Szabo, A, Davis, AK, Riba, J, and Ramaekers, JG. Prospective examination of synthetic 5-methoxy- N,N -dimethyltryptamine inhalation: effects on salivary IL-6, cortisol levels, affect, and non-judgment. Psychopharmacology . (2020) 237:773–85. doi: 10.1007/s00213-019-05414-w
29. Barsuglia, J, Davis, AK, Palmer, R, Lancelotta, R, Windham-Herman, M, Peterson, K, et al. Characterization of mystical experiences occasioned by 5-MeO-DMT- containing toad Bufotoxin and comparison with prior psilocybin studies [internet]. Poster presented at: 3rd International Psychedelic Science Conference. (2017). Oakland, CA.
30. Millière, R, Carhart-Harris, RL, Roseman, L, Trautwein, FM, and Berkovich-Ohana, A. Psychedelics, meditation, and self-consciousness. Front Psychol . (2018) 9:1475. doi: 10.3389/fpsyg.2018.01475
31. Gagnier, JJ, Kienle, G, Altman, DG, Moher, D, Sox, H, Riley, D, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep . (2013) 7:223. doi: 10.1186/1752-1947-7-223
32. First, MB, Williams, JBW, Karg, RS, and Spitzer, RL. The structured clinical interview for DSM-5®, clinician version (SCID-5-CV) . Washington DC, United States: The American Psychiatric Association (2016).
33. Blevins, CA, Weathers, FW, Davis, MT, Witte, TK, and Domino, JL. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress . (2015) 28:489–98. doi: 10.1002/jts.22059
34. Beck, AT, Weissman, A, Lester, D, and Trexler, L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol . (1974) 42:861–5. doi: 10.1037/h0037562
35. Guy, W. ECDEU Assessment Manual for Psychopharmacology . Rockville, MD: U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug abuse, and mental health administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs (1976).
36. MacLean, KA, Leoutsakos, JMS, Johnson, MW, and Griffiths, RR. Factor analysis of the mystical experience questionnaire: a study of experiences occasioned by the hallucinogen psilocybin. J Sci Study Relig . (2012) 51:721–37. doi: 10.1111/j.1468-5906.2012.01685.x
37. Bouvard, M, Charles, S, Guérin, J, Aimard, G, and Cottraux, J. Study of Beck’s hopelessness scale. Valid Factor Anal L’Encephale . (1992) 18:237–40.
PubMed Abstract | Google Scholar
38. Metzner, R. The toad and the jaguar: A field report of underground research on a visionary medicine: Bufo Alvarius and 5-methoxy-dimethyltryptamine . 1st ed. Berkeley, CA: Regent Press (2013).
39. Mosurinjohn, S, Roseman, L, and Girn, M. Psychedelic-induced mystical experiences: an interdisciplinary discussion and critique. Front Psych . (2023) 14:1077311. doi: 10.3389/fpsyt.2023.1077311
40. Ko, K, Knight, G, Rucker, JJ, and Cleare, AJ. Psychedelics, mystical experience, and therapeutic efficacy: a systematic review. Front Psych . (2022) 13:917199. doi: 10.3389/fpsyt.2022.917199
41. Pahnke, WN. The psychedelic mystical experience in the human encounter with death. Harv Theol Rev . (1969) 62:1–21. doi: 10.1017/S0017816000027577
42. Griffiths, RR, Richards, WA, McCann, U, and Jesse, R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology . (2006) 187:268–83. doi: 10.1007/s00213-006-0457-5
43. Griffiths, RR, Johnson, MW, Carducci, MA, Umbricht, A, Richards, WA, Richards, BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening Cancer: a randomized double-blind trial. J Psychopharmacol (Oxf) . (2016) 30:1181–97. doi: 10.1177/0269881116675513
44. Doblin, R. Pahnke’s “good Friday experiment”: a long-term follow-up and methodological critique. J Transpers Psychol . 23:1–28.
45. Cox, KE, Lancelotta, R, Barsuglia, J, and Davis, AK. 5-MeO-DMT and subjective improvements in post traumatic stress disorder. In: Annual conference of the Maryland Psychological Association . Baltimore, MD: (2018).
46. Davis, AK, Averill, LA, Sepeda, ND, Barsuglia, JP, and Amoroso, T. Psychedelic treatment for trauma-related psychological and cognitive impairment among US special operations forces veterans. Chronic Stress . (2020) 4:247054702093956. doi: 10.1177/2470547020939564
47. Uthaug, MV, Lancelotta, R, van Oorsouw, K, Kuypers, KPC, Mason, N, Rak, J, et al. A single inhalation of vapor from dried toad secretion containing 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms. Psychopharmacology . (2019) 236:2653–66. doi: 10.1007/s00213-019-05236-w
48. Ortiz Bernal, AM, Raison, CL, Lancelotta, RL, and Davis, AK. Reactivations after 5-methoxy- N,N -dimethyltryptamine use in naturalistic settings: an initial exploratory analysis of the phenomenon’s predictors and its emotional valence. Front Psych . (2022) 13:1049643. doi: 10.3389/fpsyt.2022.1049643
49. Heaton, RK, and Victor, RG. Personality characteristics associated with psychedelic flashbacks in natural and experimental settings. J Abnorm Psychol . (1976) 85:83–90. doi: 10.1037/0021-843X.85.1.83
50. Matefy, RE, and Krall, RG. An initial investigation of the psychedelic drug flashback phenomena. J Consult Clin Psychol . (1974) 42:854–60. doi: 10.1037/h0037523
51. Glynos, NG, Fields, CW, Barron, J, Herberholz, M, Kruger, DJ, and Boehnke, KF. Naturalistic psychedelic use: a world apart from clinical Care. J Psychoactive Drugs . (2023) 55:379–88. doi: 10.1080/02791072.2022.2108356
Keywords: 5-methoxy- N , N -dimethyltryptamine, 5-MeO-DMT, psychedelic therapy, post-traumatic stress disorder, PTSD, trauma, case report
Citation: Ragnhildstveit A, Khan R, Seli P, Bass LC, August RJ, Kaiyo M, Barr N, Jackson LK, Gaffrey MS, Barsuglia JP and Averill LA (2023) 5-MeO-DMT for post-traumatic stress disorder: a real-world longitudinal case study. Front. Psychiatry . 14:1271152. doi: 10.3389/fpsyt.2023.1271152
Received: 01 August 2023; Accepted: 03 November 2023; Published: 23 November 2023.
Reviewed by:
Copyright © 2023 Ragnhildstveit, Khan, Seli, Bass, August, Kaiyo, Barr, Jackson, Gaffrey, Barsuglia and Averill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anya Ragnhildstveit, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
5-MeO-DMT for post-traumatic stress disorder: a real-world longitudinal case study
Affiliations.
- 1 Integrated Research Literacy Group, Draper, UT, United States.
- 2 Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom.
- 3 Department of Psychology and Neuroscience, Duke University, Durham, NC, United States.
- 4 Neuroscience Interdepartmental Program, University of California, Los Angeles, Los Angeles, CA, United States.
- 5 Department of Family and Consumer Studies, University of Utah, Salt Lake City, UT, United States.
- 6 School of Humanities and Creativity, Sheridan College, Oakville, ON, Canada.
- 7 Department of Pediatrics, Division of Pediatric Psychology and Developmental Medicine, Medical College of Wisconsin, Milwaukee, WI, United States.
- 8 Children's Wisconsin, Milwaukee, WI, United States.
- 9 The Mission Within, Baja California, Mexico.
- 10 Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX, United States.
- 11 Michael E. DeBakey Veterans Affairs Medical Center, Houston, TX, United States.
- 12 Department of Psychiatry, Yale School of Medicine, New Haven, CT, United States.
- PMID: 38076677
- PMCID: PMC10710141
- DOI: 10.3389/fpsyt.2023.1271152
Psychedelic therapy is, arguably, the next frontier in psychiatry. It offers a radical alternative to longstanding, mainstays of treatment, while exciting a paradigm shift in translational science and drug discovery. There is particular interest in 5-methoxy- N,N -dimethyltryptamine (5-MeO-DMT)-a serotonergic psychedelic-as a novel, fast-acting therapeutic. Yet, few studies have directly examined 5-MeO-DMT for trauma- or stress-related psychopathology, including post-traumatic stress disorder (PTSD). Herein, we present the first longitudinal case study on 5-MeO-DMT for chronic refractory PTSD, in a 23-year-old female. A single dose of vaporized bufotoxin of the Sonoran Desert Toad ( Incilius alvarius ), containing an estimated 10-15 mg of 5-MeO-DMT, led to clinically significant improvements in PTSD, with next-day effects. This was accompanied by marked reductions in hopelessness and related suicide risk. Improvements, across all constructs, were sustained at 1-, 3-, 6-, and 12-months follow-up, as monitored by a supporting clinician. The subject further endorsed a complete mystical experience, hypothesized to underly 5-MeO-DMT's therapeutic activity. No drug-related, serious adverse events occurred. Together, results showed that 5-MeO-DMT was generally tolerable, safe to administer, and effective for PTSD; however, this was not without risk. The subject reported acute nausea, overwhelming subjective effects, and late onset of night terrors. Further research is warranted to replicate and extend these findings, which are inherently limited, non-generalizable, and rely on methods not clinically accepted.
Keywords: 5-MeO-DMT; 5-methoxy-N; N-dimethyltryptamine; PTSD; case report; post-traumatic stress disorder; psychedelic therapy; trauma.
Copyright © 2023 Ragnhildstveit, Khan, Seli, Bass, August, Kaiyo, Barr, Jackson, Gaffrey, Barsuglia and Averill.
Publication types
- Case Reports
Grants and funding
- IK2 CX001873/CX/CSRD VA/United States
The role of SKA2 on affective disorder, post-traumatic stress disorder and suicide behavior: systematic review and in silico analysis
- Review Article
- Published: 09 May 2024
Cite this article

- Thelma Beatriz González-Castro ORCID: orcid.org/0000-0001-8046-6908 1 ,
- Itzel Rodríguez-Fuentes 1 ,
- Carlos Alfonso Tovilla-Zárate ORCID: orcid.org/0000-0001-8170-8171 2 ,
- Isela Esther Juárez-Rojop ORCID: orcid.org/0000-0003-3760-7394 3 ,
- Yazmín Hernández-Díaz ORCID: orcid.org/0000-0003-1200-2292 1 ,
- María Lilia López-Narváez ORCID: orcid.org/0000-0002-7342-5988 4 ,
- Edith Elena Uresti-Rivera ORCID: orcid.org/0000-0003-4760-8841 5 , 6 &
- Jorge Luis Hernández-Vicencio 7
25 Accesses
Explore all metrics
Genes involved in the hypothalamic-pituitary-adrenal axis may be a robust biomarker of psychiatric disorders. Genetic polymorphisms of the SKA2 gene are associated with several behavioral disorders. In this study, we embarked on a systematic search of all possible reports of genetic association with SKA2 and affective disorder, post-traumatic stress disorder, and suicide behavior; the functional consequences of nsSNPs were explored through computational tools with an in silico analysis. Eight eligible articles were included. Our study identified that SKA2 did not show association with risk of Major Depression Disorder. Epigenetic variation at SKA2 mediates vulnerability to Post-Traumatic Stress Disorder. Studies provide strong preliminary evidence that alterations at the SKA2 gene covary with types of suicide behavior, including suicidal ideation, attempts, and completions. Results from in silico analysis predicted that I22S, I22G, I78T, A15L, D18R, R25L, N42I, Y21S, K14I, K14L, and L60R were the most structurally and functionally significant nsSNPs in SKA2. Amino acid conservation analysis revealed that the amino acids were highly conserved and some dissimilarities of mutant type amino acids from wild-type amino acids such as charge, size, and hydrophobicity were observed. In the future, SKA2 gene have the potential to be evaluated as prognostic biomarkers for diagnosis and research.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
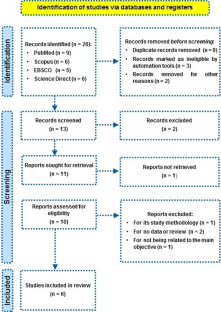
Similar content being viewed by others
Genome-wide association studies in suicidology: a review of recent achievements.

Genome-wide association analyses identify 95 risk loci and provide insights into the neurobiology of post-traumatic stress disorder

The PHF21B gene is associated with major depression and modulates the stress response
Data availability.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Berent D, Emilien G, Podgórski M, Kusideł E, Kulczycka-Wojdala D, Szymańska B,…. Pawłowska Z (2017) SSTR4, Childhood adversity, self-efficacy and suicide risk in alcoholics. Transl Neurosci 8:76–86. https://doi.org/10.1515/tnsci-2017-0013
Boks MP, Rutten BP, Geuze E, Houtepen LC, Vermetten E, Kaminsky Z, Vinkers CH (2016) SKA2 methylation is involved in cortisol stress reactivity and Predicts the Development of Post-Traumatic Stress Disorder (PTSD) after military deployment. Neuropsychopharmacology 41(5):1350–1356. https://doi.org/10.1038/npp.2015.286
Article CAS PubMed Google Scholar
Broom A, Jacobi Z, Trainor K, Meiering EM (2017) Computational tools help improve protein stability but with a solubility tradeoff. J Biol Chem 292(35):14349–14361. https://doi.org/10.1074/jbc.M117.784165
Article CAS PubMed PubMed Central Google Scholar
Chasman D, Adams RM (2001) Predicting the functional consequences of non-synonymous single nucleotide polymorphisms: structure-based assessment of amino acid variation. J Mol Biol 307(2):683–706. https://doi.org/10.1006/jmbi.2001.4510
Del Arco A, Mora F (2009) Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm (Vienna) 116(8):941–952. https://doi.org/10.1007/s00702-009-0243-8
Deller MC, Kong L, Rupp B (2016) Protein stability: a crystallographer’s perspective. Acta Crystallogr F Struct Biol Commun 72(Pt 2):72–95. https://doi.org/10.1107/s2053230x15024619
Di Iorio CR, Carey CE, Michalski LJ, Corral-Frias NS, Conley ED, Hariri AR, Bogdan R (2017) Hypothalamic-pituitary-adrenal axis genetic variation and early stress moderates amygdala function. Psychoneuroendocrinology 80:170–178. https://doi.org/10.1016/j.psyneuen.2017.03.016
González-Castro TB, Juárez-Rojop IE, Tovilla-Zárate CA, Ovando-Ricárdez JA, Hernández-Díaz Y, López-Narváez ML, … Rodríguez-Pérez C (2023). Gene-environment interaction between HPA-axis genes and trauma exposure in the suicide behavior: A systematic review. J Psychiatr Res 164:162–170. https://doi.org/10.1016/j.jpsychires.2023.06.011
Guintivano J, Brown T, Newcomer A, Jones M, Cox O, Maher BS, … Kaminsky ZA (2014) Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. Am J Psychiatry 171(12):1287–1296. https://doi.org/10.1176/appi.ajp.2014.14010008
Hernández-Díaz Y, Genis-Mendoza AD, González-Castro TB, Tovilla-Zárate CA, Juárez-Rojop IE, López-Narváez ML, Nicolini H (2021) Association and genetic expression between genes involved in HPA axis and suicide behavior: A systematic review. Genes (Basel) 12(10). https://doi.org/10.3390/genes12101608
Juruena MF, Eror F, Cleare AJ, Young AH (2020) The role of early life stress in HPA axis and anxiety. Adv Exp Med Biol 1191:141–153. https://doi.org/10.1007/978-981-32-9705-0_9
Kaminsky Z, Wilcox HC, Eaton WW, Van Eck K, Kilaru V, Jovanovic T, … Smith AK (2015) Epigenetic and genetic variation at SKA2 predict suicidal behavior and post-traumatic stress disorder. Transl Psychiatry 5(8):e627. https://doi.org/10.1038/tp.2015.105
Lira SS, Ahammad I (2021) A comprehensive in silico investigation into the nsSNPs of Drd2 gene predicts significant functional consequences in dopamine signaling and pharmacotherapy. Sci Rep 11(1):23212. https://doi.org/10.1038/s41598-021-02715-z
Mora C, Zonca V, Riva MA, Cattaneo A (2018) Blood biomarkers and treatment response in major depression. Expert Rev Mol Diagn 18(6):513–529. https://doi.org/10.1080/14737159.2018.1470927
Murphy F, Nasa A, Cullinane D, Raajakesary K, Gazzaz A, Sooknarine V, … Roddy DW (2022) Childhood trauma, the HPA axis and psychiatric illnesses: a targeted literature synthesis. Front Psychiatry 13:748372. https://doi.org/10.3389/fpsyt.2022.748372
Nakajima M, Hattori E, Yamada K, Iwayama Y, Toyota T, Iwata Y, … Yoshikawa T (2007) Association and synergistic interaction between promoter variants of the DRD4 gene in Japanese schizophrenics. J Hum Genet 52(1):86–91. https://doi.org/10.1007/s10038-006-0084-3
Navapour L, Mogharrab N (2021) In silico screening and analysis of nonsynonymous SNPs in human CYP1A2 to assess possible associations with pathogenicity and cancer susceptibility. Sci Rep 11(1):4977. https://doi.org/10.1038/s41598-021-83696-x
Ng QX, Yong BZJ, Ho CYX, Lim DY, Yeo WS (2018) Early life sexual abuse is associated with increased suicide attempts: An update meta-analysis. J Psychiatr Res 99:129–141. https://doi.org/10.1016/j.jpsychires.2018.02.001
Article PubMed Google Scholar
Nobile B, Ramoz N, Jaussent I, Dubois J, Guillaume S, Gorwood P, Courtet P (2020) Polymorphisms of stress pathway genes and emergence of suicidal ideation at antidepressant treatment onset. Transl Psychiatry 10(1):320. https://doi.org/10.1038/s41398-020-01003-0
O’Connell KS, Frei O, Bahrami S, Smeland OB, Bettella F, Cheng W, … Andreassen OA (2021) Characterizing the genetic overlap between psychiatric disorders and sleep-related phenotypes. Biol Psychiatry 90(9):621–631. https://doi.org/10.1016/j.biopsych.2021.07.007
Pandey GN, Rizavi HS, Zhang H, Bhaumik R, Ren X (2016) The expression of the suicide-associated gene SKA2 is decreased in the prefrontal cortex of suicide victims but not of nonsuicidal patients. Int J Neuropsychopharmacol 19(8). https://doi.org/10.1093/ijnp/pyw015
Pettersson E, Lichtenstein P, Larsson H, Song J, Agrawal A, Børglum AD, … Polderman TJC (2019) Genetic influences on eight psychiatric disorders based on family data of 4 408 646 full and half-siblings, and genetic data of 333 748 cases and controls. Psychol Med 49(7):1166–1173. https://doi.org/10.1017/s0033291718002039
Rice L, Waters CE, Eccles J, Garside H, Sommer P, Kay P, … Ray DW (2008) Identification and functional analysis of SKA2 interaction with the glucocorticoid receptor. J Endocrinol 198(3):499–509. https://doi.org/10.1677/joe-08-0019
Sabbagh JJ, Cordova RA, Zheng D, Criado-Marrero M, Lemus A, Li P, … Blair LJ (2018) Targeting the FKBP51/GR/Hsp90 complex to identify functionally relevant treatments for depression and PTSD. ACS Chem Biol 13(8):2288–2299. https://doi.org/10.1021/acschembio.8b00454
Sadeh N, Wolf EJ, Logue MW, Hayes JP, Stone A, Griffin LM, … Miller MW (2016) Epigenetic Variation at SKA2 predicts suicide phenotypes and internalizing psychopathology. Depress Anxiety 33(4):308–315. https://doi.org/10.1002/da.22480
Stoyanov DS (2021) Molecular pathway phenotypes and endophenotypes in psychiatry: Part I. Curr Top Med Chem 21(11):937. https://doi.org/10.2174/156802662111210719101559
Valderrama J, Macrynikola N, Miranda R (2022) Early life trauma, suicide ideation, and suicide attempts: The role of rumination and impulsivity. Arch Suicide Res 26(2):731–747. https://doi.org/10.1080/13811118.2020.1828208
Valenzuela-García LI, Ayala-García VM, Ramos-Rosales DF, Jacquez-Flores RE, Urtiz-Estrada N, Hernández EMM, Barraza-Salas M (2023) The rs7208505 polymorphism and differential expression of the SKA2 gene in the prefrontal cortex of suicide victims from the Mexican Population. Arch Suicide Res 1–12. https://doi.org/10.1080/13811118.2023.2209155
van Bodegom M, Homberg JR, Henckens M (2017) Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci 11:87. https://doi.org/10.3389/fncel.2017.00087
Witham S, Takano K, Schwartz C, Alexov E (2011) A missense mutation in CLIC2 associated with intellectual disability is predicted by in silico modeling to affect protein stability and dynamics. Proteins 79(8):2444–2454. https://doi.org/10.1002/prot.23065
Xie M, Bu Y (2019) SKA2/FAM33A: A novel gene implicated in cell cycle, tumorigenesis, and psychiatric disorders. Genes Dis 6(1):25–30. https://doi.org/10.1016/j.gendis.2018.11.001
Yin H, Galfalvy H, Pantazatos SP, Huang YY, Rosoklija GB, Dwork AJ, … Mann JJ (2016) Glucocorticoid receptor-related genes: genotype and brain gene expression relationships to suicide and major depressive disorder. Depress Anxiety 33(6):531–540. https://doi.org/10.1002/da.22499
Download references
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
División Académica Multidisciplinaria de Jalpa de Méndez, Universidad Juárez Autónoma de Tabasco, Jalpa de Méndez, Tabasco, México
Thelma Beatriz González-Castro, Itzel Rodríguez-Fuentes & Yazmín Hernández-Díaz
División Académica Multidisciplinaria de Comalcalco, Universidad Juárez Autónoma de Tabasco, Comalcalco, Tabasco, México
Carlos Alfonso Tovilla-Zárate
División Académica de Ciencias de la Salud, Universidad Juárez Autónoma de Tabasco, Villahermosa, Tabasco, México
Isela Esther Juárez-Rojop
Hospital Chiapas “Dr. Jesús Gilberto Gómez Maza”, Tuxtla Gutiérrez, Chiapas, México
María Lilia López-Narváez
Facultad de Ciencias Químicas, Universidad Autónoma de San Luis Potosí, San Luis Potosí, SLP, México
Edith Elena Uresti-Rivera
Centro de Investigación en Ciencias de la Salud y Biomedicina, Universidad Autónoma de San Luis Potosí, San Luis Potosí, SLP, México
Universidad Tecnológica de Tabasco, Centro, Tabasco, México
Jorge Luis Hernández-Vicencio
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Thelma Beatriz González-Castro, Itzel Rodríguez-Fuentes and Yazmín Hernández-Díaz. The first draft of the manuscript was written by Yazmin Hernández-Díaz and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Yazmín Hernández-Díaz .
Ethics declarations
Ethics approval.
Not applicable.
Consent to participate
Consent to publish, conflicting interests.
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
González-Castro, T.B., Rodríguez-Fuentes, I., Tovilla-Zárate, C.A. et al. The role of SKA2 on affective disorder, post-traumatic stress disorder and suicide behavior: systematic review and in silico analysis. Metab Brain Dis (2024). https://doi.org/10.1007/s11011-024-01346-3
Download citation
Received : 28 August 2023
Accepted : 04 May 2024
Published : 09 May 2024
DOI : https://doi.org/10.1007/s11011-024-01346-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Association
- Conservation
- Deleterious
- Find a journal
- Publish with us
- Track your research
Study shows heightened sensitivity to PTSD in autism
For the first time, researchers from the Queensland Brain Institute have proven that a mild stress is enough to trigger post-traumatic stress disorder (PTSD) in mouse models of autism spectrum disorder (ASD).
Dr Shaam Al Abed and Dr Nathalie Dehorter have demonstrated that the two disorders share a reciprocal relationship, identifying a predisposition to PTSD in ASD, and discovering that core autism traits are worsened when traumatic memories are formed.
While recent studies in humans have highlighted the co-occurrence of ASD and PTSD, the link between the disorders is often overlooked and remains poorly understood.
"We set out to determine the occurrence of traumatic stress in ASD, and to understand the neurobiological mechanisms underlying the reported predisposition to PTSD," said Dr Al Abed.
ASD and PTSD share common features, including impaired emotional regulation, altered explicit memory, and difficulties with fear conditioning.
"We demonstrated in four mouse models of ASD that a single mild stress can form a traumatic memory."
"In a control population, on the other hand, PTSD is triggered by extreme stress."
"We wanted to understand this unique perception of stress in ASD that leads to the formation of PTSD."
The prefrontal cortex is a highly specialised area in the front part of the brain that plays a crucial role in social cognition and behaviour.
According to Dr Dehorter, dysfunction in the prefrontal cortex has been linked to both disorders.
"We identified specific cortical circuit alterations that trigger the switch between the formation of a normal memory and a PTSD-like memory during stress," said Dr Dehorter.
The prefrontal cortex contains specialised cells called interneurons, which are crucial for adapted fear memorisation and normal sensory function and play a key role in stress-related disorders.
The formation of PTSD-like memories is triggered by over activation of the prefrontal cortex that is present in ASD and throws out the balance of these cortical circuits.
The capabilities of interneurons to respond to stress is altered in ASD. This alteration worsens autism traits following the formation of a traumatic memory.
"We didn't anticipate that forming a traumatic memory would aggravate the social and behavioural difficulties in ASD."
"What is really promising is once the traumatic memories are successfully recontextualised using behavioural therapy, the ASD traits that were worsened following the stress, are dramatically improved."
This discovery validates the assumption that the two disorders are closely linked and could change the way clinicians treat their patients.
An awareness of the PTSD predisposition and the success of behavioural therapy in treating it could shape the approach to managing stress in ASD.
This paper was published in iScience .
- Mental Health Research
- Psychology Research
- Nervous System
- Accident and Trauma
- Post-traumatic stress disorder
- Psychiatric service dog
- Autistic spectrum
- Mental illness
- Obsessive-compulsive disorder
- Stress (medicine)
Story Source:
Materials provided by University of Queensland . Note: Content may be edited for style and length.
Journal Reference :
- Alice Shaam Al Abed, Tiarne Vickie Allen, Noorya Yasmin Ahmed, Azza Sellami, Yovina Sontani, Elise Caitlin Rawlinson, Aline Marighetto, Aline Desmedt, Nathalie Dehorter. Parvalbumin interneuron activity in autism underlies susceptibility to PTSD-like memory formation . iScience , 2024; 27 (5): 109747 DOI: 10.1016/j.isci.2024.109747
Cite This Page :
Explore More
- Nature's 3D Printer: Bristle Worms
- Giant ' Cotton Candy' Planet
- A Young Whale's Journey
- No Inner Voice Linked to Poorer Verbal Memory
- Bird Flu A(H5N1) Transmitted from Cow to Human
- Universe's Oldest Stars in Our Galactic Backyard
- Polygenic Embryo Screening for IVF: Opinions
- VR With Cinematoghraphics More Engaging
- 2023 Was the Hottest Summer in 2000 Years
- Fastest Rate of CO2 Rise Over Last 50,000 Years
Trending Topics
Strange & offbeat.
FDA panel to consider MDMA for PTSD treatment
The Food and Drug Administration's panel of independent advisers will on June 4 deliberate whether they should recommend approval for the first MDMA-assisted therapy for post-traumatic stress disorder, Lykos Therapeutics said on Monday.
This would be the first FDA panel of outside experts to review a potential new PTSD treatment in 25 years.
PTSD is a disorder caused by very stressful events and can significantly disrupt patients’ lives.
Decades of studies has shown that psychoactive ingredients, whether derived from cannabis, LSD or magic mushrooms, have long captivated mental health researchers in their quest for treatments.
In support of its application, Lykos Therapeutics, formerly known as Multidisciplinary Association for Psychedelic Studies (MAPS), studied the party drug MDMA, more commonly called ecstacy or molly, in two late-stage studies.
More news on psychedelic research
- More high school students report using Delta-8 THC, also known as "weed lite."
- Effects of psychedelic drugs can last for days or weeks.
- High-potency marijuana highlights the risk of cannabis-induced psychiatric disorders.
The drug is intended to be used in combination with psychological intervention, which includes psychotherapy, or talk therapy, and other supportive services provided by a qualified healthcare provider.
No psychedelic-based therapy has been approved yet in the U.S., but MAPS and companies such as Compass Pathways are testing such drugs to find cures for a range of mental health disorders.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016.

Translational Research in Traumatic Brain Injury.
Chapter 16 post-traumatic stress disorder.
Jonathon R Howlett and Murray B Stein .
- INTRODUCTION
Post-traumatic stress disorder (PTSD) is characterized by a specific clinical syndrome including re-experiencing symptoms, avoidance, and alterations in arousal, cognition, and mood, resulting from exposure to severe traumatic events. PTSD was first officially recognized in the Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. (DSM III) in 1980, 1 and since that time a great deal of knowledge has accumulated about the characteristics of post-traumatic symptomatology, the epidemiology of PTSD, and assessment and treatment of individuals suffering from this disorder. Traumatic events that can result in PTSD fall into a number of categories, including military combat, rape, physical assault, natural disaster, and witnessing violence. 2
Since the beginning of the conflicts in Iraq and Afghanistan, it has become clear that PTSD has an important and complex relationship with traumatic brain injury (TBI). Both disorders are common in Iraq and Afghanistan veterans, and together they have been termed the “signature wounds” of these conflicts. 3 TBI has emerged as a clear and important risk factor for the development of PTSD, although the reasons for this association are not fully understood. 4 , 5 PTSD may also be an important mediator of the negative sequelae of TBI. 4 At the same time, the substantial overlap in symptoms of PTSD and postconcussive symptoms may result in considerable diagnostic confusion. 6 Finally, treatment of individuals with comorbid PTSD and TBI may present special challenges, and yet there is currently very little evidence base to guide pharmacologic and psychotherapeutic treatment in this population.
In this chapter, we begin with a brief overview of the epidemiology of PTSD, followed by a longer discussion of the epidemiology of comorbid PTSD and TBI, with special attention to the role of TBI as a risk factor for PTSD. We then consider diagnostic issues surrounding PTSD and TBI, including the substantial overlap in symptoms of PTSD and postconcussive syndrome and the resulting difficulty in attributing symptoms to either PTSD or TBI. Next, we briefly review evidence-based treatments for PTSD, including psychopharmacology and psychotherapy. Finally, we discuss special considerations in treating individuals with comorbid PTSD and TBI, as well as offer some preliminary recommendations in treating these patients while highlighting the need for more treatment studies in this population.
- EPIDEMIOLOGY OF POST-TRAUMATIC STRESS DISORDER AND TRAUMATIC BRAIN INJURY
Research into the epidemiology of PTSD can be divided into studies of combat veterans and studies of civilian populations. Surveys of combat-related PTSD have focused largely on veterans of the Vietnam War and of the conflicts in Iraq and Afghanistan. It is estimated that 18.7% of male Vietnam veterans developed PTSD, and that 9.1% still suffered from PTSD in the late 1980s when surveys were conducted. 7 Given that PTSD was not yet clinically defined during the Vietnam War, research into the epidemiology of the disorder in Vietnam veterans did not begin until years after the end of the conflict, which may affect the accuracy of the results. In contrast, research into PTSD in Iraq and Afghanistan veterans has been ongoing since soon after these conflicts began. 2 However, estimates of the prevalence of PTSD in these groups have varied widely, likely due in part to variations in sample populations and in how PTSD was defined. Estimates of the prevalence of PTSD in non-treatment-seeking samples of Iraq and Afghanistan veterans have ranged from 5% to 20%. 8 Risk of PTSD is positively associated with level of combat exposure. 2 , 8
As noted earlier, civilian populations can experience PTSD as a result of a variety of stressors including rape, assault, natural disasters, and motor vehicle accidents, among others. The lifetime prevalence of PTSD in the United States is estimated to be 6.8%. 9 Research has indicated that a large proportion of the general population has experienced at least one such stressor, but only a fairly small percentage go on to develop PTSD. For example, in a sample of adults from the Detroit metropolitan area, 89.2% of respondents had experienced at least one qualifying trauma based on DSM criteria, 10 yet only 9.2% of traumatic events resulted in PTSD. This rate varied depending on the type of traumatic event, with assaultive violence being most likely to lead to PTSD. Risk factors for the development of PTSD in individuals exposed to trauma include preexisting psychiatric disorders, family history of psychiatric disorders, poor social support, low IQ, and female gender. 2
Understanding of the relationship between PTSD and TBI has evolved over time and has grown considerably on the basis of studies of veterans of the conflicts in Iraq and Afghanistan. Prior to these conflicts, comorbid PTSD and TBI received relatively little attention, and was considered by some authors to be a rare phenomenon. In particular, it was argued that impairment or loss of consciousness that occurs with severe TBI may prevent PTSD by interfering with the encoding of trauma-related memories. 11 , 12 An early literature review concluded that PTSD could, indeed, occur after TBI even in the absence of explicit memories of trauma, through nondeclarative memories, fear conditioning occurring outside of awareness, and reconstructed memories of traumatic events. 13
Early epidemiological studies of veterans returning from Iraq and Afghanistan revealed high rates of comorbid PTSD and TBI, and that TBI was actually an important risk factor for PTSD. For example, a study of Iraq veterans found that 4.9% reported an injury with loss of consciousness, and an additional 10.3% reported an injury with altered mental status but without loss of consciousness. 4 The study found a strong association between TBI and PTSD: Of those who reported loss of consciousness, 43.9% developed PTSD, and of those who reported altered mental status, 27.3% developed PTSD. By contrast, in those with an injury without loss of consciousness or altered mental status, the rate of PTSD was 16.2%, and in those without an injury it was 9.1%. The relationship between TBI and PTSD remained significant after controlling for combat experiences. PTSD was also an important mediator of the relationship between TBI and poor physical health outcomes. 4 Another study of both Iraq and Afghanistan veterans found that 11% screened positive for PTSD and 12% reported a history consistent with mild TBI. 14 Mild TBI was associated with a 2.37-fold increase in the prevalence of PTSD. Of veterans with mild TBI, 34% also met criteria for PTSD. A survey of veterans performed by the RAND Corporation found that 19.5% of returned service members had sustained a probable TBI, and of these, about 34% were affected by probable PTSD (compared to an overall rate of 13.8% probable PTSD). 3
Subsequent research has further clarified the relationship between TBI and PTSD. A study of U.S. Army Special Operations Command personnel found that 12.7% of subjects suffered at least one mild TBI. 15 Of these, 28% reported clinical levels of PTSD symptoms. Not only was TBI associated with the development of PTSD, but there was a dose-response relationship between exposure to blast or combination blast-blunt mild TBI and levels of PTSD symptoms. Another study using retrospective chart review specifically compared blast and nonblast TBI in service members deployed to Iraq and Afghanistan. Blast TBI resulted in a higher rate of re-experiencing symptoms, but PTSD symptoms were otherwise equivalent between the two groups. 16 Overall, 37.3% of those who suffered TBI met criteria for clinically significant PTSD symptoms. A prospective study of Marines who were assessed before and after deployment to Iraq and Afghanistan further probed the role of TBI as a risk factor for PTSD by accounting for combat intensity and predeployment characteristics. The study found that 56.8% of participants reported a history of predeployment TBI, while 19.8% sustained a deployment-related TBI. 5 A regression analysis was performed to predict postdeployment symptoms of PTSD on the basis of predeployment symptoms, predeployment TBI, combat intensity, and deployment-related TBI. Both predeployment symptoms and combat intensity predicted postdeployment PTSD symptoms. However, even when controlling for these factors, deployment-related TBI was a strong predictor of PTSD symptoms. A deployment-related TBI approximately doubled the rate of PTSD for participants with low predeployment symptoms.
Several studies have investigated the relationship between TBI, PTSD, and suicide in military populations. A study of military personnel referred for evaluation of TBI in Iraq found that the number of TBIs was associated with suicidal thoughts and behaviors, depression, PTSD, and TBI symptom severity. 17 The number of TBIs was associated with suicide risk after controlling for depression, PTSD, and symptom severity. It is important to note that this study assessed suicide based on questionnaire data and did not measure actual suicides. Another study of Iraq and Afghanistan veterans used psychological and social risk factors to estimate suicide risk, and was unable to find clear evidence that veterans with PTSD and TBI were at greater risk for suicide than those with PTSD alone. 18 A study of veterans deployed to Iraq and Afghanistan found that, after controlling for psychiatric comorbidities, TBI increased the risk for current suicidal ideation in male but not female veterans. 19 A case-control study of suicides in the U.S. Armed Forces from 2001 to 2009 found that TBI and PTSD did not increase the odds for suicide (although mood disorders did). 20
Estimates of the connection between TBI and PTSD in civilian populations are variable; among those with TBI in cases of nonmilitary trauma, 14% to 56% have been found to have PTSD. 21 One study found no difference in rates of PTSD among those exposed to motor vehicle accidents with or without TBI. 22 However, a prospective cohort study of traumatically injured civilians found that those with traumatic brain injury were more likely to develop PTSD, with an odds ratio of 1.92. 23 Furthermore, patients with TBI were also more likely to develop other psychiatric illnesses including panic disorder, agoraphobia, and social anxiety compared to those with traumatic injuries but without TBI.
The aforementioned research has provided compelling evidence that TBI is a risk factor for PTSD. However, the causal mechanisms by which TBI increases the risk of PTSD remain unclear. Some authors have argued that damage to the brain may directly contribute to PTSD by compromising neural circuitry required to regulate fear. 24 , 25 Alternatively, TBI may increase the risk of PTSD by depleting the cognitive resources required to cope with stressors. 24 One possibility is that circumstances that surround TBI tend to be more emotionally traumatic than those that do not cause TBI. While studies have attempted to address this by controlling for combat severity or comparing TBI patients to those who have suffered other traumatic injuries, it is difficult to definitively control for all aspects of the traumatic situations. 5 It is also possible that in some cases, postconcussive symptoms (caused directly by trauma to the brain) are being mistaken for symptoms of PTSD, raising the probability of a PTSD diagnosis. 5 Overlap between postconcussive and PTSD symptoms will be discussed in the next section.
In summary, understanding of the epidemiologic relationship between TBI and PTSD has evolved rapidly over the past decade, and TBI has emerged as an important risk factor for the development of PTSD in both military and civilian populations. Several studies have shown that this relationship holds when controlling for preexisting psychiatric symptoms as well as when attempting to control for trauma severity. The role of TBI as a risk factor for psychiatric disorders appears not to be specific to PTSD but also to extend to other disorders. Given the high rates of comorbid TBI and PTSD, it has been difficult to disentangle the causal relationships between TBI and subsequent symptoms (i.e., which symptoms result from direct neurological insult and which are related to stress or emotional trauma). The diagnostic difficulties resulting from the overlap in symptoms of PTSD and postconcussive syndrome are discussed in the next section.
- DIAGNOSIS OF PTSD AND TBI
PTSD as described in the American Psychiatric Association’s DSM-5 requires a history of exposure to a traumatic event meeting certain criteria, along with symptoms from each of four symptoms clusters (see Table 16.1 ). 26 The first cluster is intrusion, which includes intrusive memories, nightmares, or flashbacks. The second symptom cluster involves avoidance of trauma-related thoughts and feelings or external reminders. The third symptom cluster consists of negative alterations in cognitions and mood, including dissociative amnesia; negative emotions such as fear, horror, and guilt; loss of interest in activities; and detachment from others. The final symptom cluster involves alterations in arousal and reactivity, which include irritability, reckless behavior, hypervigilance, difficulty concentrating, and sleep difficulties.
Diagnostic Criteria ( DSM 5 ) for Post-Traumatic Stress Disorder
There is significant overlap between PTSD and postconcussive symptoms. In particular, both syndromes can involve depressed mood, anxiety, insomnia, irritability, difficulty concentrating, fatigue, hyperarousal, and avoidance. 6 Emotional numbing, derealization, reduced awareness of one’s surroundings, depersonalization, and amnesia can also be related to either PTSD or TBI. 24 , 27 Symptoms that are more specific to PTSD include re-experiencing symptoms, shame, and guilt. Headache, sensitivity to light and sound, and dizziness are more specific to postconcussive syndrome. Beyond the overlap in symptoms, there may also be more complex interactions between the symptomatology of PTSD and TBI. For example, pain related to a traumatic injury may serve as a trigger for the re-experiencing symptoms of PTSD. 27 Another difficulty in diagnosis is the possible presence of other comorbid conditions. For example, depression is often comorbid with both TBI and PTSD, and can result in concentration problems, memory problems, irritability, reduced motivation, and fatigue. 24 The overlap in symptoms makes differential diagnosis difficult in patients at risk for both TBI and PTSD, and careful attention must be paid to the presentation and history in each individual case. It has also been noted that several symptoms characteristic of postconcussive syndrome are fairly nonspecific in general, including headaches, sleep difficulty, irritability, and memory problems. 24 In some individuals, these symptoms may be unrelated to either TBI or PTSD.
Researchers have debated whether postconcussive symptoms in general may be caused by emotional and psychological stress, direct neurological injury, or both. 28 A cross-sectional study of Iraq and Afghanistan veterans found that PTSD was the strongest factor associated with postconcussive symptoms, even after removing overlapping symptoms from the PTSD score. 14 A prospective study of patients admitted to a hospital for trauma found that acute postconcussive symptoms were not specific to mild traumatic brain injury but were predicted by prior affective or anxiety disorders. 29 A subsequent follow-up study found that mild TBI did not predict postconcussive symptoms at 3 months, but that PTSD was related to postconcussive symptoms. 30 A retrospective study of National Guard members using a self-report measure of postconcussive symptoms found that National Guard members with a TBI reported more postconcussive symptoms than those in a nonclinical group, but that those with PTSD reported higher postconcussive symptom severity than those with TBI. 31 Some studies have found an association between TBI and certain postconcussive symptoms after controlling for PTSD. For example, a large study found that TBI was associated with headaches after controlling for PTSD and depression. 4 Another study found that TBI with loss of consciousness was associated with headaches, memory problems, balance problems, and pain in the extremities after controlling for PTSD and depression. 32 One cross-sectional study found that PTSD and TBI were independently associated with postconcussive symptoms, and that those patients with both disorders were at greater risk of postconcussive symptoms than those with only PTSD, TBI, or neither. 33
Several studies have found a role for PTSD as a mediator between TBI and poor health outcomes and functional impairment. One cross-sectional study of combat veterans found that PTSD is an important mediator between TBI and poor health outcomes. 4 Similarly, another study found that the association between TBI and functional impairment disappeared after controlling for PTSD. 34 A study examining neuropsychological outcomes in Iraq and Afghanistan veterans with PTSD, TBI, or both, found that PTSD contributed to objective cognitive deficits, but TBI in and of itself did not. 35 However, another study found that PTSD was associated with functional impairment, but that TBI with loss of consciousness was also associated with functional impairment even after controlling for PTSD and depression. 36 In considering the literature on PTSD, TBI, postconcussive symptoms, and poor outcomes, it is important to note that biomechanical injury to the brain may contribute to PTSD itself. The role of PTSD as a mediator between TBI and postconcussive symptoms, health outcomes, and functional impairment therefore does not rule out a role for neurological injury, as opposed to emotional trauma. For this reason, the cause of postconcussive symptoms and poor outcomes in TBI patients remain difficult to disentangle, despite ongoing research.
Some authors have expressed concern that in some cases PTSD symptoms caused by emotional stress may be incorrectly attributed to physical brain injury, and that this misattribution may actually cause harm to patients by reducing their expectations of recovery. 24 This process has been termed “diagnosis threat.” 37 In order to avoid diagnosis threat, it may be advisable to be cautious when definitively attributing symptoms to neurological injuries rather than other causes, and also to emphasize that recovery from these symptoms is possible and that prognosis is not necessarily poor.
Because PTSD may contribute to postconcussive symptoms and poor outcomes, evaluation and treatment of comorbid PTSD is crucial in individuals with TBI. It should be noted that PTSD might be difficult to recognize in TBI patients, because there is evidence that some of these patients have a tendency to underreport symptoms. 28 TBI patients may have difficulties monitoring and summarizing their symptoms. It is important to be cognizant of the possibility of underreported symptoms when assessing PTSD symptoms in this population.
In summary, assessment of comorbid PTSD and TBI presents multiple diagnostic and conceptual challenges. There is significant overlap in diagnostic criteria between PTSD and postconcussive syndrome, although a few symptoms are more specific to each disorder. However, several symptoms of postconcussive syndrome are relatively nonspecific, and in some cases may be related to neither PTSD nor TBI. Despite ongoing research, there continues to be controversy over whether postconcussive symptoms are caused by biomechanical injury to the brain, by emotional and psychological stress, or both. PTSD also appears to be an important mediator between TBI and poor health outcomes and functional impairment in general. Some authors have argued that misattribution of PTSD symptoms to TBI may cause harm to patients by decreasing expectations for recovery. Given the probable role for PTSD in worsening postconcussive symptoms and more general outcomes in TBI patients, it is important to diagnose and treat PTSD in TBI patients. However, it should be noted that some TBI patients may be prone to underreporting symptoms of PTSD.
- PTSD TREATMENT
Treatment for PTSD can be categorized as pharmacologic and nonpharmacologic. Among pharmacologic treatments, the strongest evidence exists for selective serotonin reuptake inhibitors (SSRIs). The only FDA approved medications for PTSD are sertraline and paroxetine, which are both SSRIs and have been shown effective in randomized controlled trials. 38 – 40 There is also strong evidence for fluoxetine, another SSRI, and venlafaxine, a serotonin norepinephrine reuptake inhibitor (SNRI). 41 – 44 Prazosin, an alpha-1 adrenergic antagonist, has shown efficacy in decreasing nightmares associated with PTSD and may also decrease other PTSD symptoms. 45 – 47
Certain psychotherapies have been found effective for PTSD. Prolonged exposure (PE) is a type of trauma-focused cognitive behavioral therapy (CBT) that aims toward fear extinction through imaginal exposures (in which a patient repeatedly recounts memories of a trauma) and in vivo exposures (in which a patient is exposed to distressing situations in the present). PE has been shown to reduce symptoms of PTSD. 48 Another type of trauma-focused CBT, cognitive processing therapy (CPT) focuses on restructuring dysfunctional cognitions related to a traumatic event. It has also been found to be effective for PTSD. 49 , 50 Eye movement desensitization and reprocessing therapy (EMDR) is another evidence-based psychotherapy for PTSD that involves the use of bilateral eye movements in addition to other psychotherapy elements. 51 Within the Veterans Affairs system, both PE and CPT have been widely disseminated and are widely available. 52
- TREATMENT OF COMORBID PTSD AND TBI
Because PTSD is common in TBI patients and may be an important contributor to poor outcomes in these patients, it is important to identify and treat PTSD in this population. However, treatment of PTSD may present special challenges when comorbid with TBI. Unfortunately, there is currently little evidence to guide treatment in this population. Many PTSD treatment studies actually exclude individuals with TBI. 53 Therefore, treatment in this population must take account of the available evidence but also be adapted based on individualized considerations. Such considerations may include cognitive and other sequelae of TBI that may interfere in treatment, overlapping symptoms between PTSD and TBI, and possible tradeoffs when treatments are helpful for one condition but potentially harmful for the other.
Sequelae of TBI may require special attention in trauma-focused CBT for PTSD. For example, as discussed earlier, patients may sometimes attribute nonspecific symptoms to TBI and consequently assume that prognosis for recovery is poor. It may therefore be helpful to address these attributions and normalize the symptoms, reducing distress and increasing expectations for recovery. 24 It is also possible that cognitive dysfunction related to TBI may impair patients’ ability to understand, remember, and engage with material in the context of cognitive therapy. This may be addressed by simplifying the delivery of therapy, providing written instructions for exercises to compensate for memory problems, and using other strategies to minimize reliance on attentional focus. 27 , 28 Impulse control problems related to TBI may also make exposure exercises more difficult to tolerate. These patients may therefore benefit from greater emphasis on the rationale for these exercises in order to encourage engagement with treatment. 27 Amnesia related to TBI may interfere in imaginal exposures in PE, in which the patient repeatedly recounts a traumatic experience in order to facilitate fear extinction. In patients who cannot remember the trauma, imaginal exposures may focus on reconstructions of the traumatic experience rather than direct memories. Alternatively, therapy may emphasize in vivo exposure, in which patients are exposed to distressing situations in the present, rather than to distressing memories. 24 , 28 To date, no controlled trials have been performed on psychotherapy for PTSD with comorbid TBI. Two early case reports described treatment of individuals with PTSD and TBI using CBT with modifications, such as the use of personal digital assistants and cognitive breaks, 21 , 54 , 55 with improvements in mood and anxiety. More recently, a retrospective study of patients undergoing CPT for PTSD found similar treatment adherence in patients with or without mild TBI. 56 A case series of slightly modified PE treatment for individuals with comorbid PTSD and TBI found that the subjects significantly improved in symptoms of PTSD and depression from pre- to posttreatment. 57 Studies of a residential treatment program for comorbid PTSD and TBI using a modified version of CPT found a reduction in both PTSD and postconcussive symptoms over the course of treatment. 58 , 59 A controlled trial of CBT for acute stress disorder after TBI showed efficacy in preventing PTSD, providing further evidence that TBI patients are able to benefit from CBT. 60 Overall, more research is still needed to determine optimal approaches to psychotherapy in this population.
Given the overlapping domains of dysfunction in both TBI and PTSD, treatments that target symptoms common to both conditions may be especially helpful in this population. For example, SSRIs may be beneficial for both PTSD and TBI. 53 Additionally, stimulants may be prescribed for attentional dysfunctions, hypnotics for sleep difficulties, and anticonvulsants for affect dysregulation. 6 The risks of such treatments must also be taken into account (e.g., substance use disorder).
Some pharmacologic treatments for PTSD may carry special risk in TBI patients. For example, individuals with a history of TBI have a higher rate of seizures, gait and balance problems, and deficits in sensory processing. Certain psychotropic medications may worsen sensory or balance problems. Other medications, including buproprion, maprotiline, and amoxapine, can increase the risk for seizures. 53 These risks should be considered before starting psychotropic medications, and patients should be monitored for worsening of symptoms. Several types of medications, including antipsychotics, anticonvulsants, anxiolytics, and any anticholinergic medication, may contribute to cognitive deficits associated with TBI. 53 Medications often used for TBI may also worsen PTSD symptoms. For example, stimulants to combat fatigue and attentional problems related to TBI may theoretically worsen hyperarousal in PTSD, 53 although empirical evidence that this is commonly encountered as an adverse event is lacking. Finally, many psychotropic medications have complex effects on sleep, which is often impaired in both TBI and PTSD. 53 Considering the potential risks of psychotropic medications in these populations, it may be advisable to start at low doses and titrate slowly with careful monitoring for adverse effects.
The prevalence of comorbid TBI and PTSD, and the complexities of clinical care in this population, require special approaches in the delivery of care. The diversity of symptoms in this population, including both medical and mental health problems, means that coordination of care between specialists is crucial. 61 The need for coordination also extends to research, as better design and interpretation of studies in this population will benefit from expertise in psychiatry, neuropsychology, neurology, neurosurgery, and physical medicine and rehabilitation, among others. 6
In summary, there is currently very little evidence base for the treatment of comorbid PTSD and TBI. Treatment must be individualized based on the presentation of each patient. Cognitive sequelae of TBI may interfere with trauma-focused psychotherapy (though preliminary evidence suggests that many patients with mild-to-moderate cognitive deficits do benefit from such therapies), and therapy may need to be modified to account for cognitive impairment. Certain medications may carry benefit for both disorders, such as antidepressants, stimulants, anticonvulsants, and hypnotics. However, careful attention must be paid to the risks of each medication. In general, it is advisable to start at a low dose, titrate slowly, and monitor closely. This population also requires attention in terms of delivery of care, including special screening procedures and coordination between specialties. More research is clearly needed to determine the best treatments for comorbid PTSD and TBI.
- CONCLUSIONS
Comorbid PTSD and TBI is a common and challenging clinical situation that requires attention to develop better strategies for assessment and treatment. Accumulating evidence has identified TBI as an important risk factor for PTSD. The causal relationship between TBI and PTSD remains unclear, but may involve both neurological and psychological factors. Further, there is significant overlap in symptoms of PTSD and TBI, making differential diagnosis difficult. Multiple studies have shown that PTSD is associated with greater reporting of postconcussive symptoms, creating debate over whether these symptoms are related to neurological injury, psychological stress, or both. When assessing this evidence, it is important to note that PTSD itself may be related to neurological injury, and that the association between PTSD and post-concussive symptoms cannot definitively implicate a psychological cause for these symptoms. There are a number of effective pharmacologic and nonpharmacologic treatments for PTSD, but the treatment of comorbid PTSD and TBI may be complicated by several considerations. These include overlapping symptoms, cognitive and other sequelae of TBI that may interfere in treatment, and trade-offs in which a treatment for one condition may adversely affect the other condition. Unfortunately, there is currently very little research to guide treatment in this population, and there is a pressing need for treatment studies in this population.
- Cite this Page Howlett JR, Stein MB. Post-Traumatic Stress Disorder: Relationship to Traumatic Brain Injury and Approach to Treatment. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016. Chapter 16.
In this Page
Other titles in this collection.
- Frontiers in Neuroscience
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Combat TBI: History, Epidemiology, and Injury Modes. [Brain Neurotrauma: Molecular, ...] Review Combat TBI: History, Epidemiology, and Injury Modes. DePalma RG. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. 2015
- Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. [Am J Epidemiol. 2008] Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Schneiderman AI, Braver ER, Kang HK. Am J Epidemiol. 2008 Jun 15; 167(12):1446-52. Epub 2008 Apr 17.
- Examining the relation between combat-related concussion, a novel 5-factor model of posttraumatic stress symptoms, and health-related quality of life in Iraq and Afghanistan veterans. [J Clin Psychiatry. 2012] Examining the relation between combat-related concussion, a novel 5-factor model of posttraumatic stress symptoms, and health-related quality of life in Iraq and Afghanistan veterans. Tsai J, Whealin JM, Scott JC, Harpaz-Rotem I, Pietrzak RH. J Clin Psychiatry. 2012 Aug; 73(8):1110-8. Epub 2012 Jun 26.
- A cohort study examining headaches among veterans of Iraq and Afghanistan wars: Associations with traumatic brain injury, PTSD, and depression. [Headache. 2016] A cohort study examining headaches among veterans of Iraq and Afghanistan wars: Associations with traumatic brain injury, PTSD, and depression. Jaramillo CA, Eapen BC, McGeary CA, McGeary DD, Robinson J, Amuan M, Pugh MJ. Headache. 2016 Mar; 56(3):528-39. Epub 2015 Dec 21.
- Review Growing literature but limited evidence: A systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. [Brain Behav Immun. 2017] Review Growing literature but limited evidence: A systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brenner LA, Stearns-Yoder KA, Hoffberg AS, Penzenik ME, Starosta AJ, Hernández TD, Hadidi DA, Lowry CA. Brain Behav Immun. 2017 Oct; 65:57-67. Epub 2017 Jun 9.
Recent Activity
- Post-Traumatic Stress Disorder - Translational Research in Traumatic Brain Injur... Post-Traumatic Stress Disorder - Translational Research in Traumatic Brain Injury
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Bruxism is a recurring condition among people with post-traumatic stress disorder, finds study
A ccording to an article published in the journal Clinical Oral Investigations , people who suffer from post-traumatic stress disorder (PTSD) often report constant clenching or grinding of the teeth during the day, a condition known as awake (or diurnal) bruxism. Its prevalence in the general population varies from 8% to 30%.
The study, which included clinical examination of 76 patients and controls, highlights the importance of collaboration by dentists and psychiatrists to diagnose both health problems more accurately.
PTSD was first diagnosed in the United States among war veterans but has since been acknowledged in victims of urban violence as well. About 4% of people exposed to violent or accidental events such as combat, torture, imminent death, stray bullets, natural disasters, severe injuries, sexual abuse, kidnappings and so on are estimated to have PTSD.
"Considering that more than half the population of metropolitan São Paulo [in Brazil] has been exposed to some kind of urban trauma, a proportion comparable to those of populations in areas of civil conflict, it's very important to understand the possible psychological and physical manifestations of PTSD, which may go on for years after the trauma," said Yuan-Pang Wang, penultimate author of the article and a researcher in the Institute of Psychiatry at the University of São Paulo's Medical School (FM-USP).
The symptoms of PTSD include recurring flashbacks, a negative emotional state, self-destructive behavior, troubled sleep with nightmares, and dissociation (altered consciousness, memory, identity, emotion, perceptions of the environment and control of behavior), among others. There have not been many studies of orofacial pain and bruxism as symptoms of PTSD.
In this study, patients diagnosed with PSTD at FM-USP's Institute of Psychiatry were submitted to a clinical examination to assess their oral health. According to the researchers, besides self-reported bruxism, they were also found to have a lower pain threshold after the examination.
"Oral hygiene was not found to be associated with the problem," said Ana Cristina de Oliveira Solis, first author of the article. "Periodontal examination, which included measurement of bacterial plaque and gingival bleeding [or bleeding on probing], showed that patients with PTSD and controls had a similar level of oral health. However, the PTSD patients presented with more pain after probing."
Multidisciplinary treatment
According to the researchers, bruxism is no longer considered an isolated symptom but is seen as evidence of a larger problem. "Our study showed that PTSD can be manifested orally, in bruxism and a higher level of pain after a clinical dental examination. This requires joint action by psychiatrists, psychologists and dentists in screening and treatment of both health conditions," Solis said.
Dentists should take the patient's self-reported pain into account during clinical examinations and consider the possibility that the patient has undiagnosed psychiatric problems.
"If the patient has had a traumatic experience, they may be too embarrassed to talk about it or see a therapist. The habit of going to the dentist, on the other hand, is much more common and frequent. For this reason, psychiatric screening instruments should be used in routine patient care, and patients should be advised to seek therapeutic assistance," she said.
Psychiatrists can ask patients with PTSD about orofacial symptoms such as bruxism, muscle pain and temporomandibular joint pain, and if necessary, should refer them to a dentist so that multidisciplinary treatment can be provided and their quality of life improved.
More information: Ana Cristina de Oliveira Solis et al, Self-reported bruxism in patients with post-traumatic stress disorder, Clinical Oral Investigations (2024). DOI: 10.1007/s00784-024-05534-4
Provided by FAPESP


IMAGES
VIDEO
COMMENTS
Philip, a 60-year-old who was in a traffic accident (PDF, 294KB) This case example from the European Journal of Psychotraumatology details an assisted self-study application of cognitive therapy for PTSD. Philip developed PTSD and comorbid major depression following a traffic accident. He was treated in six sessions of cognitive therapy with ...
CASE STUDY Victor (post-traumatic stress disorder) Case Study Details. Victor is a 27-year-old man who comes to you for help at the urging of his fiancée. He was an infantryman with a local Marine Reserve unit who was honorably discharged in 2014 after serving two tours of duty in Iraq. His fiancé has told him he has "not been the same ...
Recent studies have shown that psychotic symptoms can be a hallmark of post-traumatic stress disorder [1, 2]. The vast majority of the cases reported concerned war veterans although there were sporadic incidents involving non-combat related trauma (somatic or psychic). ... Case formulation - (Persistent PTSD, adapted from Ehlers and Clark ).
Post-traumatic stress disorder (PTSD) is a severe stress reaction that develops in some people after traumatic events, such as accidents, disaster, or physical or sexual violence. ... In conclusion, this case study demonstrates the possibility of using self-study modules in the treatment of PTSD despite the memory and concentration problems ...
Post-traumatic stress disorder (PTSD) is a psychiatric condition which may develop after a terrifying event, usually involving a risk to physical integrity or to life, one's own or of other people. ... Our case indicates that peripheral post offices may also be at high risk. The second case is the victim of a terrible accident at work ...
This case-control study (n = 40) aimed to identify differences in peripheral blood biomarkers, and biomarker combinations, able to distinguish PTSD participants from controls, and examine in a biopsychosocial framework. ... Post-traumatic stress disorder (PTSD) is a debilitating psychiatric condition which may result following exposure to ...
Current treatment strategies for control of trauma-associated symptoms of Post Traumatic Stress Disorder (PTSD) have recently been updated by the Veterans Affairs (VA) and the Department of Defense (DoD, after over a decade of dedicated research. The most recent evidence is compelling that its use of trauma-focused therapies such as Cognitive ...
Post-traumatic stress disorder (PTSD) is one of the few neuropsychiatric disorders for which the timing and cause of onset are understood, facilitating research into the underlying mechanisms. In ...
Future case studies should consider the use of additional measures, such as the Post Traumatic Cognitions Inventory (PTCI; Foa et al., Reference Foa, Tolin, Ehlers, Clark and Orsillo 1999), and measures for grief response. Measuring grief, low mood and PTSD outcomes in parallel would enable a better understanding of the relationship between ...
Post-traumatic stress disorder (PTSD) is arguably the most common psychiatric disorder to arise after exposure to a traumatic event. Since its formal introduction in the DSM-III in 1980, knowledge has grown significantly regarding its causes, maintaining mechanisms and treatments. ... Subsequent longitudinal studies indicated that this ...
Background: Emotional Freedom Techniques (EFT) and Eye Movement Desensitization and Reprocessing (EMDR) have been empirically validated as effective psychotherapeutic interventions for treating Post Traumatic Stress Disorder (PTSD). This single subject design case study is of a survivor of the Twin Towers collapse who was treated for prolonged PTSD complicated by dissociated memories.
Abstract. The trends that are emerging from the literature point to a relationship between trauma exposure, posttraumatic stress, and schizophrenia. People with schizophrenia are at more risk of being exposed to a number of traumas and are more likely to experience the symptoms of post-traumatic stress disorder (PTSD).
This case study presents information about post traumatic stress disorder in older adults. It is divided into different sections including the history, presentation and examination, diagnosis, case discussion, post traumatic stress disorder explained, take home points, and additional resources.
Background: This systematic review aggregates research on psychotherapeutic interventions for Post-Traumatic Stress Disorder (PTSD) in children and adolescents. PTSD in this demographic presents differently from adults, necessitating tailored therapeutic approaches. In children and adolescents, PTSD arises from exposure to severe danger, interpersonal violence, or abuse, leading to significant ...
This paper concludes with a self-critique by the therapist regarding every aspect of the presented case study. Post Traumatic Case Study (PTSD): Case Study; Dick's Journey Descriptive Narrative Dick is a retired military man, in his late fifties, suffering from DSM-IV-TR chronic military-related posttraumatic stress disorder (PTSD).
This case study is emblematic for/of the assumption that very careful neurolinguistic analysis can detect neurological dysfunction linked to fine, discrete and diffuse lesions that might not be immediately detected by fRMI. ... 797-816. Sherin, J. E., Charles B. Nemeroff, (2011). Post-traumatic stress disorder: the neurobiological impact of ...
Introduction: Post-traumatic stress disorder (PTSD) is characterised by intrusive, anxious, and avoidant symptoms that are triggered after a stressful experience and affect the mood. The definition of a stressor that generates PTSD has been debated in recent years, as a clinical picture compatible with the disorder can occur after exposure to stressors that do not meet the criteria A1 of the ...
The case study of Rich (see Case Study 1) demonstrates that the body can be an ally and a catalyst in the process of healing from trauma. Such physical changes often help to resolve the habitual trauma-related responses and provide improved stability to handle future stressors (Ogden et al., 2006).
Yet, few studies have directly examined 5-MeO-DMT for trauma- or stress-related psychopathology, including post-traumatic stress disorder (PTSD). Herein, we present the first longitudinal case study on 5-MeO-DMT for chronic refractory PTSD, in a 23-year-old female.
Yet, few studies have directly examined 5-MeO-DMT for trauma- or stress-related psychopathology, including post-traumatic stress disorder (PTSD). Herein, we present the first longitudinal case study on 5-MeO-DMT for chronic refractory PTSD, in a 23-year-old female. A single dose of vaporized bufotoxin of the Sonoran Desert Toad ( Incilius ...
Post-traumatic stress disorder (PTSD) is a mental disorder that manifests after exposure to a stressful traumatic event, such as combat experience. ... et al. Association of genetic factors and gene-environment interactions with risk of developing posttraumatic stress disorder in a case-control study. Biol Res Nurs. 2015;17:364-372. https://doi ...
Posttraumatic stress disorder (PTSD) is a syndrome that results from exposure to real or threatened death, serious injury, or sexual assault. Following the traumatic event, PTSD is common and is one of the serious health concerns that is associated with comorbidity, functional impairment, and increased mortality with suicidal ideations and attempts. The Diagnostic and Statistical Manual of ...
Corpus ID: 18685612. Post-traumatic stress disorder in children. Overview and case study. S. Damian, A. Knieling, B. Ioan. Published 2011. Medicine, Psychology. Romanian Journal of Legal Medicine. TLDR. The authors' approach on PTSD in children starts with the overall data followed by an in depth presentation and analysis of a clinical case, in ...
Within socio-military history, the diagnosis of post-traumatic stress disorder has aroused a lot of attention, with many scholars claiming its existence in the pre-modern world while sceptics have challenged this claim in no uncertain terms. ... Post-traumatic Stress Disorder: An Ancient Greek Case Study in Retrospective Diagnosis Download book ...
Genes involved in the hypothalamic-pituitary-adrenal axis may be a robust biomarker of psychiatric disorders. Genetic polymorphisms of the SKA2 gene are associated with several behavioral disorders. In this study, we embarked on a systematic search of all possible reports of genetic association with SKA2 and affective disorder, post-traumatic stress disorder, and suicide behavior; the ...
A new study shows that a mild stress is enough to trigger post-traumatic stress disorder (PTSD) in mouse models of autism spectrum disorder (ASD). Researchers demonstrated that the two disorders ...
PTSD is a disorder caused by very stressful events and can significantly disrupt patients' lives. Decades of studies has shown that psychoactive ingredients, whether derived from cannabis, LSD ...
Post-traumatic stress disorder (PTSD) is characterized by a specific clinical syndrome including re-experiencing symptoms, avoidance, and alterations in arousal, cognition, and mood, resulting from exposure to severe traumatic events. PTSD was first officially recognized in the Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. (DSM III) in 1980,1 and since that time a great deal ...
More information: Ana Cristina de Oliveira Solis et al, Self-reported bruxism in patients with post-traumatic stress disorder, Clinical Oral Investigations (2024). DOI: 10.1007/s00784-024-05534-4 ...