April 10, 2014

Salty Science: Floating Eggs in Water
A density demonstration from Science Buddies
By Science Buddies
Key concepts Density Mass Volume Concentration Buoyancy Water Introduction Have you ever wondered why some objects float in water and others sink? It has to do with the density of the objects compared with the density of the water surrounding them. If an object is less dense than the water around it, it will float. Because salt water is denser than freshwater, some things float more easily in the ocean—or extremely salty bodies of the water, such as the Dead Sea. You can make your own dense water by adding salt to tap water. In fact, if you add enough salt, you can make the water so dense that an egg will actually float in it! Explore how this works in this science activity. Background If you put an egg in a cup of tap water, it will sink to the bottom. Why is this? Because the density of the egg is higher than the density of tap water, so it sinks. Density is the mass of a material per unit volume. For example, the density of freshwater under standard conditions is approximately one gram per cubic centimeter. But, if you add enough salt to the water, the egg will actually float back up to the surface! Adding salt to the water increases the density of the solution because the salt increases the mass without changing the volume very much. When enough salt is added to the water, the saltwater solution's density becomes higher than the egg's, so the egg will then float! The ability of something, like the egg, to float in water or some other liquid is known as buoyancy. But just how much salt is needed to make an egg float? In this science activity you'll figure that out by making solutions with varying concentrations of salt in them. Materials
Measuring cup
Large container, such as a large bowl or cooking pot (It must be able to hold at least three cups.)
One half cup of table salt
Five cups that hold at least 16 ounces each
Permanent marker (if you are using plastic cups) or masking tape and a pen (to label nondisposable cups)
Three spoons for mixing salty solutions
Soup spoon for egg transfers
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Preparation
Take the egg out of the refrigerator and allow it to warm to room temperature. Be sure to always wash your hands after handling uncooked eggs because they may carry salmonella.
Pour one and one half cups of water into your large container.
Add one half cup of salt to the large container and stir to dissolve some of the salt (it will not all dissolve yet).
Add one more cup of water to the large container (making two and one half cups total) and stir to dissolve the remaining salt. The salt should be completely dissolved before you go on to the next step. It may take several (five to 10) minutes of stirring, so you may need to be patient. Why do you think it's important to start out with a solution that has such a high concentration of salt?
Arrange the five cups on a surface, going in a line from left to right. Label the cups 1 to 5. If you are using plastic cups, you can use a permanent marker to label them. If you are using nondisposable cups, you can use masking tape and a pen to label them.
Add three quarters cup of the salty solution you prepared to cup 1.
Add three quarters cup of plain tap water to cups 2 through 5. (Cup 5 will be plain tap water.)
Add three quarters cup of the salty solution you prepared to cup 2 and mix it. What is the salt concentration in cup two compared with cup one?
Add three quarters cup of the salt solution from cup 2 to cup 3 and mix it. What is the salt concentration in cup 3 compared with cups 1 and 2?
Add three quarters cup of the salt solution from cup 3 to cup 4 and mix it. What is the salt concentration in cup 4 compared with the other cups?
Use a soup spoon to place an egg in cup 5. Does the egg float?
Use the spoon to take the egg out and place it in cup 4. Does the egg float?
Repeat this process with cups 3, 2 and then 1. In which cup does the egg first float? If the egg floated in more than one cup, did you notice any difference in how it floated? What does this tell you about the density of the egg?
Extra: In this science activity you figured out, within a factor of two, how much salt it takes to float an egg. You could narrow down the range further by testing additional saltwater solutions to try and determine the egg’s density. To do this, start your solution with the salt concentration in which the egg first floated and make a new dilution series, as you did before. Now in which cup does the egg first float? What does this tell you about the density of the egg?
Extra: Repeat this activity using several more eggs, possibly both hard-boiled and uncooked eggs. Do you get the same results with other eggs or is there some variation between different eggs? For testing hard-boiled versus raw eggs, you should test the same egg, first raw and then after hard-boiling it to investigate any differences.
Extra: Find out how much salt there is in seawater. From the results of your activity, do you think an egg would float or sink in seawater?
[break] Observations and results Did the egg float in cup 1 and 2, but not in cups 3, 4 or 5? You likely saw that the egg floated best in cup 1, floated a little less in cup 2 (but part of it was above the surface) and did not float in the other cups. Cup 1 had the undiluted salty solution that you originally prepared, which was one half cup of salt in two and one half cups water total. The concentrations of the salt solutions in cups 2 to 4 were halved as you increased in cup number; for example, the concentration of the salt in cup 2 was half that of cup 1, and the concentration of the salt in cup 3 was half again of cup 2. (Cup 5 had plain tap water.) The egg should have sunk in cups 3, 4 and 5 because the density of the egg was higher than the density of the solutions (or plain tap water) in those cups. Cups 1 and 2 had more salt in them than the other cups (with cup 1 having the most salt), which means these solutions were denser. The egg should have floated (with part of it above the water surface) in these two cups because the solutions were denser than the egg. The actual density of the egg is in between the density of the solution in cup 3 and that in cup 2. More to explore What Is Density? , from Charles E. Ophardt, Elmhurst College Why Is the Ocean Salty? , from Herbert Swenson, U.S. Geological Survey Publication Fun, Science Activities for You and Your Family , from Science Buddies How Salty Does the Sea Have to Be for an Egg to Float? , from Science Buddies
This activity brought to you in partnership with Science Buddies

- Free Resources
- Project Search
- Featured Projects
- Member Benefits
1059 Main Avenue, Clifton, NJ 07011
The most valuable resources for teachers and students

(973) 777 - 3113
1059 Main Avenue
Clifton, NJ 07011
07:30 - 19:00
Monday to Friday
123 456 789
Goldsmith Hall
New York, NY 90210

- Why We’re Unique
Egg Floatation, (Buoyancy)
Introduction: (initial observation).
Everyone has experienced the fact that things feel lighter under water than they do out of water. You may also have noticed that it is easier to float (swim) in salt water than fresh water.

This is due to a buoyant force upward. The buoyant force is equal to the weight of the liquid that the object displaces. If the liquid is denser, then the buoyant force is greater. Steel sinks in water, but floats in mercury.
Other possible titles for this project are:
1. Effects of Density 2. Visualize Density 3. Floatation Magic The third title is only good if you can successfully submerge the egg in the middle of the jar.
Dear This project guide contains information that you need in order to start your project. If you have any questions or need more support about this project, click on the “ Ask Question ” button on the top of this page to send me a message.
If you are new in doing science project, click on “ How to Start ” in the main page. There you will find helpful links that describe different types of science projects, scientific method, variables, hypothesis, graph, abstract and all other general basics that you need to know.
Project advisor
Information Gathering:
Find out about floatation. Read books, magazines or ask professionals who might know in order to learn about the factors that may cause an object float or submerge. Keep track of where you got your information from.
(The information you gather – along with what you already know- together form your background information. ) This is a sample.
What is buoyancy?
Buoyancy is the tendency or capacity to remain afloat in a liquid or rise in air or gas. Buoyant objects have a lower density than the liquid or gas they are in. For example a blimp has a lower density than air and wood has lower density than water. That is why wood floats on water and blimps rise in the air.
What is density?
Density is the ratio of mass to volume in metric system. you can also think of that as the mass of 1 cubic centimeter of anything. The following examples will help you to understand and calculate the density.
Q. 150 cc of water is 150 grams. What is the density of water? (or the mass of 1 cc water).
A. Density of water= 150/150 = 1 g/cm³
Q. A piece of oak wood masses 35 grams and has a volume of 50 cubic centimeter. What is the density of oak wood?
A. The density of oak = 35g / 50cm³ = 0.70 g/cm³
Q. A piece of iron masses 157 grams and has a volume of 20 cm3. What is the density of iron?
A. The density of iron = 157g / 20cm³ = 7.85 g/cm³
To find the density of any object, you need to know the Mass (grams) of the object, and its Volume (measured in mL or cm³). Divide the mass by the volume in order to get an object’s Density .
Please note that cc (cubic centimeter, cm³) and ml (milliliter) are the same volumes; however, ml is only used for liquids while cc is used both for solids and liquids.
How does the egg density compare to water?
Eggs normally sink in water. In other words an egg has a higher density than water. The density of pure water is 1. This means that one milliliter of water weights one gram. The density of an egg is slightly more than one. So one milliliter of an egg is heavier than one gram. If we want to have an egg to float in water, we must increase waters density. To do this we can dissolve some salt or sugar or any other water soluble substance that has a higher density into the water. For example, since salt has a higher density than water, salt water has a higher density than pure water.
Followings are properties of table salt (Na Cl, Sodium Chloride).
Salt Properties:
- Crystals or white crystalline powder
- Transparent and colorless in crystalline form, rather like ice Crystallizes in the isometric system, usually in the form of cubes Soluble in water (35.6g/100g at 0°C and 39.2g/100g at 100°) Slightly soluble in alcohol, but insoluble in concentrated hydrochloric acid
- Melts at 801°C and begins to vaporize at temperatures just slightly above this
- boiling point 1,413°C
- Hardness of 2.5 on the MHo scale of hardness
- Specific gravity of 2.165
- Non combustible – Low toxicity
- Hygroscopic – absorbs moisture from damp atmospheres above 75% relative humidity.
As you see the density of salt is 2.165 which is more than double the density of water.
Applications of Buoyancy: One of the useful applications of buoyancy and Archimedes’ principle are to the experimental determination of density. ( See how )
Buoyancy has many industrial applications. By knowing and understanding buoyancy you can sink and float the material as you wish. You can do this just by changing the density of liquid. This method is especially used for separation of minerals. For example most copper ores have only about 2% copper and copper compound in them is mixed with lots of soil. Buoyancy is used to bring the copper ore to the surface where it will be separated.
Buoyancy is also the main factor in the following:
- Ice on a lake
- Swim bladders in fish
- Scuba divers
- Aquatic plants (such as water hyacinth)
Floatation is also a mineral separation process, which takes place in a water-mineral slurry. In this method the difference in density is used to separate the pure minerals from unwanted soil that has a different density.
Question/ Purpose:
What do you want to find out? Write a statement that describes what you want to do. Use your observations and questions to write the statement. Following are some sample questions/ purposes for this project.
The purpose of this project is to understand the effect of salt on the density of water and floatation of objects. The main question for this project is:
How does the amount of salt in water affect the floatation of egg? (Experiment 2)
Some other related questions are:
- Does the size of an egg affect its ability to float?
- Does the color of egg affect its ability to float?
- Does the size of a glass jar filled with water affect the ability of egg to float?
Need a problem statement? This is a sample:
Materials may sink or float in water depending on their density. We need to have some control on this condition and be able to sink or float them as we need. This is especially important for us when we are separating a few different materials and we want some of them sink and some others float.
Note: This method of separation is already being used to separate minerals from each other and metals from soil. It is also used in recycling where plastics, papers and metals must be separated from each other.
How to measure the density of liquids?
To measure the density of any liquid (like water, saltwater, orange juice, alcohol,..) you will need 2 things. First you need a measuring tool to precisely measure the volume of liquid in milliliters. Then you need a balance scale or gram scale to measure the mass of the liquid in grams. (Mass is the same as weight at sea level. In reality balance scales measure mass, not weight). When you have these two values, then you divide the weight by volume. The result will be the density. For example if 50mL of liquid weights 53 grams, then the density is 53/50=1.06 g/ml.
To measure the volume of the liquid you may use a graduated cylinder, a graduated burette or a graduated pipette. For example if you have a 10mL pipette, you can fill it up to the 10mL marking and transfer the liquid to a cup or weighing dish. If you need 50ml you can repeat that 5 times.
How to make a 5% saltwater?
Weight 5 grams of salt and transfer it to a 100 ml graduated cylinder. Then add water up to the 100ml marking. Swirl the cylinder until the salt is fully dissolved.
Instead of a 100 mL graduated cylinder you can use any other measuring cup or beaker as long as it is marked for 100mL capacity.
With the same method you can make any other concentration of saltwater. For example if you start with 7 grams of salt and add water up to the 100mL marking, then your solution will be a 7% solution.
You can also increase the solute (salt) and solvent (water) at any ratio. For example in our 5% saltwater example you could use 50 grams of salt and add water up to the 1000mL marking.
Identify Variables:
When you think you know what variables may be involved, think about ways to change one at a time. If you change more than one at a time, you will not know what variable is causing your observation. Sometimes variables are linked and work together to cause something. At first, try to choose variables that you think act independently of each other. This is how you define the variables for the main question of this project (tested in experiment 2)
The independent variable (the one that we set; also known as manipulated variable) is the amount of salt in water.
The dependent variable (also known as responding variable) is the status of the egg in water (sink, submerge, float).
The control variable is water temperature. (We control the temperature because variations in temperature may cause variations in the density of water. Make sure all water or saltwater you use are at room temperature, and do all experiments in the same day and in the same room.)
Another way of defining the dependent variable is :
The dependent variable (also known as responding variable) is the density of salt-water.
Hypothesis:
Based on your gathered information, make an educated guess about what types of things affect the system you are working with. Identifying variables is necessary before you can make a hypothesis.
Following is a sample hypothesis for the above question.
Since the density of salt is more than the density of water, adding salt to water will increase the density of the mixture (solution). If the density of water becomes more than the density of the egg, then the egg will float. (Experiment 2)
Experiment Design:
Design an experiment to test each hypothesis. Make a step-by-step list of what you will do to answer each question. This list is called an experimental procedure. For an experiment to give answers you can trust, it must have a “control.” A control is an additional experimental trial or run. It is a separate experiment, done exactly like the others. The only difference is that no experimental variables are changed. A control is a neutral “reference point” for comparison that allows you to see what changing a variable does by comparing it to not changing anything. Dependable controls are sometimes very hard to develop. They can be the hardest part of a project. Without a control you cannot be sure that changing the variable causes your observations. A series of experiments that includes a control is called a “controlled experiment.”
Experiment 1: What is the density of egg?
Introduction: The density of pure water is 1. In other words the weight of 1ml water is 1 gram (ml=milliliter=1:1000 Liter) . Objects with a density of less than 1 will float on the water. Objects with a density of more than 1 will sink to the bottom of water. In this experiment we test the density of egg.
Place a 1000ml graduated cylinder on a scale and fill it up with 200ml of pure water. Record the total weight of the cylinder and water.
Carefully place an egg in the cylinder. Record the volume increase and weight increase.
Divide the weight increase by volume increase to find out the density of the egg.

In one experiment, the volume of the egg was 51ml and the weight was 57 grams. So the density of egg will be calculated as: Density of egg=57 : 51 = 1.176 g/cc
cc means cubic centimeter. cc is the same as milliliter.
What is the density of your egg?

Experiment 2: How much salt will it take to make an egg float ?
This is the main experiment for this project
Introduction:
In order to find the salt concentration that floats the egg, status of an egg in water samples with different amounts of salt can be examined.
For this experiment you will need:
- A plastic or glass jar,
- A measuring cup or graduated cylinder to measure the amount of water
- A gram scale to weigh the salt. Gram scale is a scale that can measure in grams. (Also see the Materials and Equipments section in this project guide)
- Fill 2/3rd of a clear plastic or glass jar with water. Measure and record the amount of water that you are using for this experiment. You will need either the volume or the mass (weight) of water.
- Carefully place an egg at the bottom of that jar. Egg will simply sink and remain at the bottom.
- Prepare some salt. For every 1000 grams of water (one liter), have about 500 grams salt. The salt that you are using must be in the form of fine crystals or powder so it can dissolve easily. Record the mass of salt that you are starting with.
- Start adding some salt and stir the solution carefully.
- Continue that until the egg starts to rise. Measure the amount of salt that is left over and use that to calculate the amount of salt that is used. (Subtract remaining amount from initial amount)
- Use the amount of water and the amount of salt that is used to calculate the concentration of salt water that can float an egg.
Concentration=(mass of salt)/(mass of salt + mass of water)
In other words first you add the mass of salt and the mass of water to calculate the total mass. You will then divide the mass of salt by total mass of salt water.
Need a Control Group?
Place a similar egg in another container of water, but don’t add any salt to that. That will be your control group. You will observe that the egg in the control group does not float, so you will be sure that the floatation of egg in your experimental container is due to the added salt.
Need a Data Table or Results Table?
The result of this experiment is one single value, so you will not need a data table. If you need a data table for your project, you can repeat your experiment 3 or 4 times and enter the results in a table. For example you may get a small white egg, a large white egg, a small brown egg, and a large brown egg. Try the experiment with each of these eggs and write the results in a table like this:
Make a bar graph:
You can use a bar graph to visually present the results in the above table. Make one vertical bar for each type of egg you try. Write the name or the type of egg under each bar. The height of each bar will represent the salt concentration that floated that egg. For example make a 21 cm tall bar to show the concentration of 21%.
This is a common question asked about this experiment:
1. How much salt will it take to make an egg float?
2. What’s my controls?
1. We don’t provide results. Keep adding salt until the egg floats. Keep track of the amount of salt you are adding.
2. Control is another container of water and egg that you do nothing with that. In other words you don’t add any salt. In this way when the egg starts to rise in the container that you are adding salt, you can be positive that adding salt caused the egg to rise, not an unknown environmental condition.
Experiment 3: How does salt affect the density of water?
In this experiment you will measure the density of water without salt and with different amounts of salt. (If you need a graph for your science project, this is the experiment that you need to do.)
For this experiment you need a metric scale that measures grams. You will also need a measuring cylinder to measure the volume of salt water.
Make different salt solutions starting from 1% (By weight or by volume; you choose!) salt and go up to 25% salt.
For each solution measure the density and record it in your results table.
To measure the density, measure the weight and the volume of the water and then divide the weight by volume. (Measure the weight in grams and measure the volume in milliliters or cubic centimeters).
Your results table may look like this:
How to make a 5% by weight salt solution? To make a 5% solution, you weight 5 grams of salt and then add water to that to make it 100 grams.
If you are good in math, you can also calculate the density of different salt solutions.
If you want to measure it and you don’t have much time, just measure the density of 5%, 10%, 15%, 20% and 25% salt solutions. Since the graph is linear (Straight line), it makes no difference how many different salt solutions you test.
You use the above table to make a graph. In the graph, you mark the point that the density of solution is the same as the density of egg. That is where the egg can remain submerged without sinking to the bottom or floating on the top.
This is a sample graph that shows the relation between the concentration of salt and the density of saltwater.
Materials and Equipment:
Material used for this project may vary based on the experiments that you choose and the equipment that are available to you. Following are a list of material and equipment used in the above experiments:
- 3 fresh (uncooked) eggs
- a bag of salt (2 lbs). Buy kosher salt or cooking salt from a local grocery store. water
- three beakers or any other clear jar
- 500 ML graduated cylinder MiniScience Part#AS2203
- Balance scale (gram scale). It is used to weigh the eggs.
See samples of balance scale at MiniScience.com or klk.com
If you cannot obtain a scale:
If you cannot obtain a scale for your experiments, you may try to use a measuring scoop instead. The results will not be very accurate if you use measuring scoops; however, they are good enough for you to complete your project. Make sure to write about your measuring method in your report in order to explain inaccuracy of results. To convert scoops to gram, use the following:
- 1/4 teaspoon tablesalt is almost 1.5 grams.
- One teaspoon tablesalt is almost 6 grams.
- One tablespoon tablesalt is almost 20 grams.
Results of Experiment (Observation):
Egg easily sinks in a drinking water (Right beaker) , but it floats in a concentrated salt water (Middle beaker) . We were also able to make a salty water that keeps the egg submerged (Left beaker) .
For the purpose of display, prepare three deferent jars. First jar will have pure water, Second jar will have saturated salt water and the third jar will have a salt water that has the same density of egg so the egg will remain in the middle.
When the density of the water is exactly the same as the density of egg, it would be difficult to make the egg stay in the middle. It may sometimes come up and sometimes go down. In the above picture, in order to force the left egg to stay in the middle, we filled 1/2 of the jar with saturated salt water. Then we added some pure water to the top of that without steering. Egg will sink in the top half and will stop as soon as it gets to the saturated salt water.
Calculations:
While you add salt to the water, record and calculate the amounts of water and salt for every condition. To do that, first add the mass of water to the mass of salt. For example if you used 700 grams of water and 150 grams of salt, the total is 850. This will be the total mass of the solution.
Then divide the mass of salt by the total mass of the solution. In this case you divide 150 by 850 and the results is 0.18 (or 18%). With this result you may conclude that salt water with concentration of 18% or more can float a fresh egg.
Numbers provided in this example are not real experiment results.
Summary of Results:
Summarize what happened. This can be in the form of a table of processed numerical data, or graphs. It could also be a written statement of what occurred during experiments.
It is from calculations using recorded data that tables and graphs are made. Studying tables and graphs, we can see trends that tell us how different variables cause our observations. Based on these trends, we can draw conclusions about the system under study. These conclusions help us confirm or deny our original hypothesis. Often, mathematical equations can be made from graphs. These equations allow us to predict how a change will affect the system without the need to do additional experiments. Advanced levels of experimental science rely heavily on graphical and mathematical analysis of data. At this level, science becomes even more interesting and powerful.
Conclusion:
Using the trends in your experimental data and your experimental observations, try to answer your original questions. Is your hypothesis correct? Now is the time to pull together what happened, and assess the experiments you did. In your conclusion you must write how much salt and how much water are required in order for an egg to float.
Related Questions & Answers:
What you have learned may allow you to answer other questions. Many questions are related. Several new questions may have occurred to you while doing experiments. You may now be able to understand or verify things that you discovered when gathering information for the project. Questions lead to more questions, which lead to additional hypothesis that need to be tested.
Following are samples of related questions:
- Can floatation experiment be performed with other materials such as beans and seeds and minerals?
- Can floatation be used as a separation method? (Can you separate sands from seeds or beans with this method?)
Possible Errors:
The temperature of water also effects its density. Warmer water has less density than cold water. The salt that you buy may not be pure salt. Salt manufacturers normally add other materials to the salt to absorb moisture. If your salt water appeared to be milky, leave it for a few hours until the white milky material added to the salt will precipitate. Then carefully transfer the clear salt solution to a new jar.
References:
Visit your local library and find some physics books with discussions in “Liquids”, “Density” and “Buoyancy”.
Following are some web resources:
http://www.iit.edu/~smile/ph9708.html
The Buoyancy Experiment
http://www.gpc.peachnet.edu/~pgore/Earth&Space/buoyancy.html
Q: Why you have used three different size of beakers shown in the picture? A: That is what we had available, but size of beaker or container has no effect on results.
Q: Where can I find, or what could be a substitute for the 500ml graduated cylinder and the balance scale (gram scale)?
A: You have many choices. Online they are available at MiniScience.com and other scientific suppliers. Locally, you may have a scientific supplier or photography supplier or teachers store that sell these. Balance scale is also available as kitchen supplies and in some pharmacies. Use a balance scale with precision of 0.1 gram or better.
Q: what is the problem statement of this project ?
A: This project does not have a problem statement. Instead it has a purpose. You can make up a problem statement if you wish. Any problem that is caused by low density of water can be used as the problem statement for this project. For example one problem is that many children each year drown in pools. Can adding salt to the pool increase buoyancy and reduce drowning? Is it better to fill up the swimming pool with fresh water or salty seawater?
Q: who is the first person to experiment egg buoyancy?
A: There are billions of objects in the world and egg is just one of them. No one will waste time to record who first did the buoyancy test in every one of these objects. Even if they do, it might be wrong. Buoyancy tests have been performed in many different seeds, many woods, plastics, metals, and minerals.
Q: What is the value of the project to society.?
A: The society benefits from the products that are made, filtered or improved by buoyancy method. Buoyancy is used to separate copper minerals, Zinc minerals and many others. It can also be used to clean seeds and beans from sand and other plant parts.
Q: How your findings can be used?
A: Your findings may be used to float eggs, beans or other materials with the purpose of separation and cleaning.
Q: I am having problems coming up with a good Hypothesis for this project the teacher wants my child to use the word buoyancy in her Hypothesis. How would I rewrite the problem statement and formulate a hypothesis based on what I have researched.
Problem: …………. Hypothesis: IF ……. Then …….. Because ……….
We are working with the experiment number 2 where would I find a gram scale.
A: Possible problem: We need to separate the eggs from stones by floatation. Possible Hypothesis: IF we add salt to the water THEN the buoyancy force of water will increase BECAUSE the density of salt is more than the density of water.
Gram scales are sold online and in some electronic stores and office suppliers.
It is always important for students, parents and teachers to know a good source for science related equipment and supplies they need for their science activities. Please note that many online stores for science supplies are managed by MiniScience.
Testimonials
" I called School Time and my husband and son came with me for the tour. We felt the magic immediately."
- Robby Robinson
" My husband and son came with me for the tour. We felt the magic immediately."
- Zoe Ranson
Contact Info
Our address, working hours.
Week Days: 07:00-19:00
Saturday: 09:00-15:00
Sunday: Closed
Science Project
It’s a wonderful world — and universe — out there.
Come explore with us!

Science News Explores
How salty does the sea have to be for an egg to float.
Try this eggs-periment to learn why some objects float in the ocean while others sink

Which glass holds the salty water? This activity will help you figure it out.
Arthit Pornpikanet/iStock/Getty Images Plus
Share this:
- Google Classroom
By Science Buddies
January 24, 2023 at 6:30 am
Objective : Determine what salt concentration will float an egg
Areas of science : Ocean sciences
Difficulty : Intermediate/Easy
Time required : ≤ 1 day
Prerequisites : None
Material availability : Readily available
Cost : Very low (under $20)
Safety : Always wash your hands after handling uncooked eggs because they may carry Salmonella .
Credits : Andrew Olson, PhD, Science Buddies; Sandra Slutz, PhD, Science Buddies
Did you know that if you put an egg in a cup of tap water, it will sink to the bottom? But, if you add enough salt, the egg will float back up to the surface! Why? Because the density of the egg is higher than the density of tap water, so it sinks.
Density (ρ), as shown in Equation 1, is the mass (m) of a material per unit volume (v). For example, the density of freshwater under standard conditions is approximately 1 gram (g) per cubic centimeter (cm 3 ). In other words, if you filled a 1-cm x 1-cm x 1-cm box with freshwater, the water inside the box would have a mass of 1 g.
Adding salt to the water increases the density of the water, because the salt increases the mass without changing the volume very much. With enough added salt, the saltwater solution density is higher than the egg’s, and the egg will then float, as shown in Figure 1. The ability of something, like the egg, to float in water or some other liquid is known as buoyancy .
Equation 1:
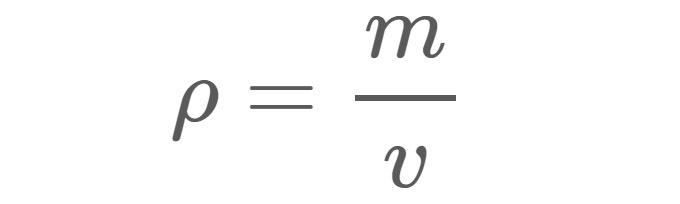
ρ = Density in whatever units are used for mass and volume. m = Mass in grams (g), kilograms (kg), or any other unit of weight. v = Volume in centimeters cubed (cm 3 ), meters cubed (m 3 ), or any other unit of volume.
But just how much salt is needed to make an egg float? In this science fair project, you will figure that out by placing an egg in cups with different salt concentrations . The concentration of a solution tells you how much of a compound is in a certain volume of a mixture.
In chemistry, the mass concentration is one way of expressing the concentration of a solution. The mass concentration is defined as the mass of a compound (in grams) in a certain solvent volume (in liters) and has the unit grams per liter (g/L). For example, in a solution with 750 grams of salt (sodium chloride or NaCl) in 1.5 liters of water, the mass concentration of salt is 750 g/1.5 L = 500 g/L.
In this project, you will be using the technique of making serial dilutions to create solutions with different salt concentrations. A serial dilution is a method for accurately diluting a solution in regular steps. You add a known amount of your starting, or stock, solution to a known amount of water and mix them. This process is called dilution. Diluting a solution means adding additional solvent (water in this project) to decrease the solution’s concentration. The new concentration of the diluted solution can be calculated using Equation 2.
Equation 2:
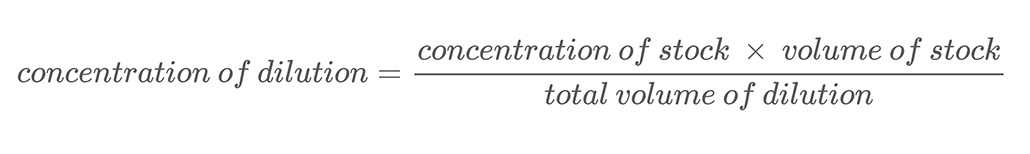
Here is an example calculation. Let’s say you have a salt solution with a mass concentration of 500 g/L. You dilute this solution by mixing 0.25 L of that salt solution with 0.25 L of water. This brings the total volume of your dilution to 0.5 liters (0.25 L + 0.25 L). To calculate the mass concentration of salt in the diluted salt solution you use Equation 2:
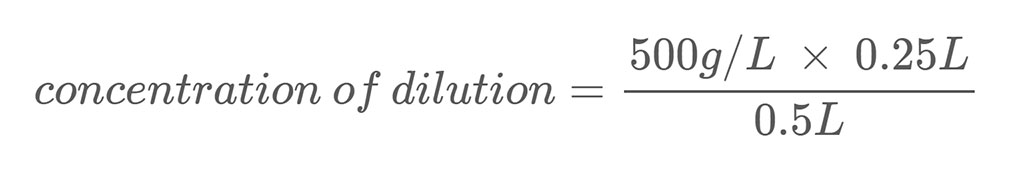
Solving the equation tells you that your dilution has a salt concentration of 250 g/L, which is half of your stock solution.
As a general rule, if the volume of stock solution and the volume of solvent (water) for your dilution are equal, you will be diluting the solution by half. This is called a two-fold dilution. A two-fold dilution means that with each dilution step, the new concentration of the dilution should be 50 percent of the original concentration.
If you want bigger steps, you should use relatively more water; if you want smaller steps, you should use relatively less water. By repeating the process, you can make a whole series of dilutions, which is how the method got its name. In this ocean science project, you will start out using two-fold dilutions to find out how much salt will cause an egg to float.
Terms and concepts
- Serial dilution
- Mass concentration
- Relative concentration
- Absolute concentration
- Why would an egg float in water with a lot of salt in it, but not in plain tap water?
- What happens to salt (sodium chloride or NaCl) molecules when dissolved in water?
- Why does adding salt to water increase its density?
Materials and equipment
- Permanent marker
- Table salt (1 cup)
- Measuring cup, liquid
- Large container, such as a large bowl or cooking pot. Must be able to hold at least five cups.
- Spoon for stirring
- Bag of clear 16-oz plastic cups
- Soup spoon for egg transfer
- Lab notebook
Experimental Procedure
- Science Made Simple, Inc. (n.d.). Metric conversions & US customary unit conversion calculator . Retrieved April 15, 2013.
- Take five eggs out of the refrigerator, use a permanent marker to label them 1-5, and allow them to warm to room temperature.
- Pour 3 cups of water into your large container.
- Add 1 cup of salt.
- Stir to dissolve some of the salt. It will not all dissolve yet.
- Add 2 more cups of water.
- This may take several (5 to 10) minutes of stirring, so you may need to be patient.
- Label five of the plastic cups 1-5. Cup 1 will be for the stock solution, cups 2-4 will be for the dilutions, and Cup 5 will be plain tap water.
- Add 3/4 cup of your stock salt solution to Cup 1.
- Add 3/4 cup plain tap water to cups 2-5.
- Measure out 3/4 cup stock solution, and add it to Cup 2. Mix.
- Measure out 3/4 cup of the solution from Cup 2 and add it to Cup 3. Mix.
- Measure out 3/4 cup of the solution from Cup 3 and add it to Cup 4. Mix.
- What are the absolute mass concentrations of salt in cups 1-4? (To calculate with metric units, use these conversions: 1 cup of salt is 292 grams [g], 1 cup of water is 237 milliliters [mL], and 3/4 cup of stock solution is 177.75 milliliters [mL]). Write these concentrations down in your lab notebook. Review the Introduction section if you need help with your calculations.
- What are the relative salt concentrations in cups 2-4 compared to the original stock solution? Use the absolute mass concentrations that you calculated in the previous step for your calculations. Example : Let’s assume that the original stock solution in Cup 1 has a salt concentration of 500 g/L. Cup 3 has a salt concentration of 125 g/L. The relative salt concentration can be calculated as the ratio of 125 g/L / 500 g/L, which is 0.25. Expressed as a percentage, this would be 25%. Therefore, Cup 3 has a relative salt concentration of 25% compared to Cup 1.
- Now, starting with Cup 5 and working your way up, test an egg in each solution to see if it will float. Use a soup spoon to lift the egg in and out of the cups.
- Be sure to record your results and observations in your lab notebook, including the egg’s number.
- Repeat steps 5-6 with four other eggs.
- Now you know, within a factor of 2, how much salt it takes to float an egg. How can you narrow down the range further to get a more precise estimate? By doing another serial dilution, of course.
- Figure out a new serial dilution with smaller steps. For example, you could try diluting the solution by 25 percent with each step. That means with each step, the new concentration should be 75 percent of the original concentration.
- Remember that you will need enough solution to more than cover the egg, which will probably be around 3/4 cup, and you probably cannot fit more than 2 cups of solution in each 16-oz cup.
- Hint: You may only be able to test the first few cups in a dilution series at a time unless you use larger cups.
- Tip: If you need additional help for making serial dilutions, check out the serial dilutions resource in the Bibliography in the Background section.
- Write up your new dilution procedure in your lab notebook, including the calculated relative and absolute salt concentrations for each cup.
- Make the new dilution series. Remember to start with the salt concentration where the egg first floated. (If you do not have enough solution from the original serial dilution, make some more by starting from the stock solution.)
- Repeat this step with the four other eggs.
- When you are done handling the eggs, wash your hands with soap and warm water. It is important to wash your hands after handling uncooked eggs because they may carry Salmonella .
- Hint: If the density of the saltwater is less than the egg’s density, the egg will sink, and if the density of the saltwater is greater than the egg’s density, the egg will float. So the density of the egg would be between these two absolute salt densities.
- Plot the densities for all five eggs on a chart, putting the egg’s number on the x-axis and its density on the y-axis. What is the density of the eggs? How much variation in density is there from egg to egg?
- Does a hard-boiled egg float at the same salt concentration as an uncooked one? Hint: You will need to measure the same egg before and after hard boiling and be very precise about your serial dilutions.
- Find out how much salt there is in sea water. From the results of your experiment, predict whether an egg would float or sink in sea water. (If you live close enough to the ocean, you can get collect some sea water and test your prediction!)
- Figure out another method of determining the density of an egg. Compare the density measurements for the same eggs using your method and this salt water float test.
This activity is brought to you in partnership with Science Buddies . Find the original activity on the Science Buddies website.

More Stories from Science News Explores on Physics

Thunderstorms churn up a ‘boiling pot’ of high-energy gamma rays

A Jurassic Park -inspired method can safely store data in DNA

Two AI trailblazers win the 2024 Nobel Prize in physics

Let’s learn about the Nobel Prize

Explainer: How cells use chemistry to make the electricity of life

Weirdly, mayo can help study conditions ripe for nuclear fusion

Science reveals the reasons behind painful paper cuts

Scientists Say: Kugelblitz
Science Fun

Floating Egg

- Salt (1 – 2 cups)
- A tall drinking glass
Instructions:
1. Pour water into the glass until it is about half full. 2. Place an egg in the glass of water and see if it sinks or floats (it should sink). 2. Stir in lots of salt. Start with 1 tablespoon and stir it until the salt dissolves. Keep adding more salt until the egg floats. 3. Next, carefully pour more fresh water until the glass is nearly full (be careful to not disturb or mix the salty water with the plain water). If you’re very careful, you can get the egg to float between the fresh and saltwater!
VIDEO COMING SOON BUT YOU CAN STILL ENJOY THESE AWESOME EXPERIMENTS!
How It Works:
The egg is denser than the fresh water (more molecules per square inch), this causes it to sink. When you start dissolving salt in the water, this is increasing the density (adding more molecules per square inch). Eventually the water becomes denser than the egg causing the egg to float. When you carefully add fresh water again, this fresh water is less dense than the salt water so it floats right on top!
Extra Experiments:
Are there other liquids you can add to make the egg sink or float? What else can you dissolve in the water to make the egg float?
EXPLORE TONS OF FUN AND EASY SCIENCE EXPERIMENTS!

SUBSCRIBE AND NEVER MISS A NEW SCIENCE FUN VIDEO!
previous experiment
Next experiment.

IMAGES
VIDEO
COMMENTS
Have you ever wondered why some objects float on top of the ocean, and other objects sink to the bottom? It has to do with the density of the objects compared to the density of the salt water surrounding them in the ocean. If you add salt to plain water, it increases the density of the water. In fact, if you add …
The Salt Water Egg Experiment explains why materials (such as an egg) float more in salt water than in fresh water.
In fact, if you add enough salt, you can make the water so dense that an egg will actually float in it! Explore how this works in this science activity. Background. If you put an egg in a...
Egg easily sinks in a drinking water (Right beaker), but it floats in a concentrated salt water (Middle beaker). We were also able to make a salty water that keeps the egg submerged (Left beaker) . For the purpose of display, prepare three …
Why does the egg sink in regular tap water, but float in saltwater? The answer lies in the density of water! Density is a measure of the mass per unit volume of a substance.
Why would an egg float in water with a lot of salt in it, but not in plain tap water? What happens to salt (sodium chloride or NaCl) molecules when dissolved in water? Why does adding salt to water increase its density?
What happens when you put an egg in a glass of regular water? This is a cool way to learn about density. Materials: One egg; Water; Salt (1 – 2 cups) A tall drinking glass; A spoon . Instructions: 1. Pour water into the glass until it is …