If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

Chemistry archive
Course: chemistry archive > unit 6.
- States of matter
- States of matter follow-up
- Specific heat and latent heat of fusion and vaporization
- Specific heat, heat of fusion and vaporization example
- Chilling water problem
- Change of state example
- Vapor pressure
Phase diagrams
- Representing solids, liquids, and gases using particulate models
- Crystalline and amorphous polymers
- Representing alloys using particulate models
- Structure of metals and alloys
- Solids, liquids, and gases
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript
Browse Course Material
Course info.
- Prof. Donald Sadoway
Departments
- Materials Science and Engineering
As Taught In
- Chemical Engineering
Learning Resource Types
Introduction to solid state chemistry, 33. unary phase diagrams.
« Previous | Next »
Session Overview
| Solid Solutions | |
| introduction to phase diagrams, basic definitions of phase, component, and equilibrium, one-component phase diagrams | |
| stability, pressure, temperature, vapor, liquid, solid, phase, equilibrium, component, triple point, melting, boiling, glass transition temperature, polymorph, sublimation, supercritical | |
| water (H O), zirconia (ZrO ), snowflake obsidian, aluminum (Al), silicon (Si), carbon dioxide (CO ), nitrogen (N), bismuth (Bi), sulfur (S), oxygen (O), argon (Ar), “dry ice”, carbon (C), mercury (Hg) | |
| failure analysis, cooking food at altitude and in pressure cooker, automobile engine cooling, ice skating, artificial diamonds, coffee decaffeination |
Prerequisites
Before starting this session, you should be familiar with:
- The Bonding and Molecules module, in particular Session 12: Intermolecular Forces
Looking Ahead
This is the first of three sessions on solid solutions and phase diagrams. Proceed to the next session, Session 34 .
Learning Objectives
After completing this session, you should be able to:
- Summarize the purpose of a phase diagram.
- Give a detailed interpretation of the phase diagram of water.
- Given a set of material properties data, draw the phase diagram.
- Given a phase diagram, describe some properties of the material, e.g. the relative densities of its solid and liquid forms.
Archived Lecture Notes #10 (PDF) , Part A
| Book Chapters | Topics |
|---|---|
| 11.6, “Critical Temperature and Pressure.” | Supercritical fluids; molten salts and ionic liquids |
| 11.7, “Phase Diagrams.” | General features of phase diagrams; phase diagrams of water and carbon dioxide |
Lecture Video
- Download video
- Download transcript
Lecture Slides (PDF - 3.0MB)
Lecture Summary
Phase stability is the basis for the solid state of matter. Knowledge of phase stability enables applications like:
- conducting failure analysis – recognizing phase “signatures” in the material
- cooking at altitude – adapting to different pressure/temperature relationships
- automobile engine cooling – exploiting pressure and chemical composition to control the coolant’s boiling point (a phase transition temperature)
Prof. Sadoway introduces phase diagrams as “stability maps” and “archives of pressure/temperature relationships.”
This lecture defines and gives examples of phase , one phase and two phase systems, equilibrium, component, and triple point . A detailed discussion of the most familiar one phase system, water, is followed by comparisons of the phase diagrams of aluminum and silicon.
Prof. Sadoway does some live demonstrations for the class:
- various objects are immersed in liquid nitrogen, going below their glass transition temperature
- properties of liquid oxygen
- sublimation of solid frozen CO 2 , aka ‘dry ice’
The phase diagram of zirconia illustrates the concept of changing a material’s composition to produce desired behaviors at particular temperature/pressure conditions. The phase diagram of carbon illustrates how artificial diamonds are made. Bisumth and sulfur phase diagrams – “phase diagrams from hell” – have many regions . Returning to water, Prof. Sadoway discusses the unusual phases of ice at very high pressures and very low temperatures.
As a final application note, the lecture ends with a quick summary of coffee decaffeination as a process employing sophisticated manipulation of phase transitions.
Problems (PDF)
Solutions (PDF)
More phase diagram homework problems will be provided for the next two sessions.
For Further Study
Queen. “‘Under Pressure’ (Live at Wembley).” June 16, 2009. YouTube. Accessed November 18, 2010. http://www.youtube.com/watch?v=SJCTgtDU-74 .
Alexander Calder, Mercury Fountain , 1937. Fundació Joan Miró. Barcelona, Spain.
Other OCW and OER Content
| Content | Provider | Level | Notes |
|---|---|---|---|
| MIT OpenCourseWare | Undergraduate (second-year) | See on single-component phase diagrams, plus associated recitation and assignment content |

You are leaving MIT OpenCourseWare
Chemistry Learner
It's all about chemistry.
- Chemical Bonds
- Chemical Reactions
- Materials Chemistry
- Organic Chemistry
- Periodic Trends
- Periodic Table Groups
- How to Read Periodic Table
- Naming Covalent Compounds Worksheets
- Net Ionic Equation Worksheets
- Types of Chemical Reactions Worksheets
- Word Equations Worksheets
- Valence Electrons Worksheets
- Graphing Periodic Trends Worksheets
- Periodic Trends Ionization Energy Worksheets
- Atomic Structure And Isotopes Worksheets
Phase Diagram
How to read a phase diagram, phase diagram example.
A phase diagram represents the various physical states or phases of matter at different pressures and temperatures. In other words, it summarizes the effect of pressure and temperature on the nature of a substance. A phase diagram is divided into three areas representing the substances’ solid, liquid, and gaseous phases [1-4] .
Several distinct lines separate these phases. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. A point on these lines indicates that multiple phases can coexist in thermodynamic equilibrium. Phase transitions occur along these lines.
A representative phase diagram of a pure substance is shown below.

A two-dimensional phase diagram shows temperature along the horizontal axis and pressure along the vertical axis. The pressure is plotted in the unit of atmosphere (atm) or Pascal (Pa), and the temperature is in Kelvin (K) or degrees Celcius ( o C). The lines of equilibrium represent a pressure-temperature combination where two phases can coexist in equilibrium. Low temperature and high pressure favor solid formation, and high temperature and low pressure favor gas formation. Liquid lies in between these two regions [1-6] .
Consider the horizontal dashed line XY as shown in the image below. As we move across the diagram from left to right, we pass through all three phases of matter. We start with the solid phase, cross the solid-liquid line (BD), and move into the liquid phase. We continue along the dashed line, cross the liquid-vapor line (BC), and finally reach the gaseous phase. In summary, if we increase the temperature of a substance at constant pressure, the substance transforms from solid to liquid to gas.
Along any line, two phases are in equilibrium with one another. It means that the rate at which a substance transforms from one phase to another is the same as when it transforms back to the first phase. Let us now analyze these phase boundaries.

Consider line AB, where the solid phase is in equilibrium with its gaseous counterpart. Every point on this line represents the condition under which the solid is in equilibrium with the gas. In other words, the rate at which the solid transforms into gas is the same as when the gas transforms back into solid.
The BC line contains all the conditions of pressure and temperature at which the liquid boils. Here, the liquid is in equilibrium with the gas. In other words, the boiling rate is equal to the condensation rate.
The BD line represents the equilibrium between solid and liquid. Each point on this line depicts the condition under which a solid melts into a liquid. The reverse also holds. The rate of melting is equal to the rate of freezing. The BD line is normally vertical for most pure substances because the melting point is not much affected by a change in pressure. For most compounds, the slope is slightly positive. It is slightly negative for water because ice melts upon applying pressure.
Triple Point and Critical Point
The phase diagram has two essential points – triple point and critical point. The point where the lines intersect is called the triple point. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium.
The critical point is the highest temperature and pressure at which a gas and a liquid can coexist at equilibrium. The critical point terminates the liquid-gas line and establishes the critical pressure and temperature. Beyond this point, the two phases become indistinguishable and form a phase known as a supercritical fluid. In the image above, point B is the triple point, and point C is the critical point. The supercritical fluid phase is indicated distinctly.
A well-known example of a phase diagram is that of water , as shown below. We know that water exists as ice, liquid water, and water vapor or steam. Water has a triple point corresponding to the temperature and pressure at which ice, liquid water, and vapor coexist in a stable equilibrium. It also has a critical point. It is that point at which water vapor cannot be forced back into water, no matter how much pressure is applied. Another example of a phase diagram is that of carbon dioxide, which is discussed in this article [1-6] .

The following table lists their values.
| 273.16 | 0.01 | 0.608 | 0.006 | |
| 647.14 | 373.99 | 22063.519 | 217.7 |
- Chem.libretexts.org
- Chemed.chem.purdue.edu
- Chemistrytalk.org
- Chemguide.co.uk
- Pressbooks-dev.oer.hawaii.edu
- Thoughtco.com
Related Articles

Sublimation

Graham’s Law

Crystal Field Theory

Van der Waals Equation
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Trending Topics
© 2024 ( Chemistry Learner )

- Planet Earth
- Strange News
How To Read Phase Diagrams Chemistry
Table of Contents:
phase diagrams of pure substances . An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water.
The phase diagram shows that the water would first freeze to form ice as it crossed into the solid area. When the pressure fell low enough, the ice would then sublime to give water vapour. In other words, the change is from liquid to solid to vapour. I find that satisfyingly bizarre!
Video advice: Phase diagrams
Understanding and interpreting phase diagrams

There are various guide-lines that you can use if you want to. For example, if the substance is commonly a liquid at or around room temperature, you tend to call what comes away from it a vapour. A slightly wider use would be to call it a vapour if the substance is below its critical point, and a gas if it is above it. Certainly it would be unusual to call anything a vapour if it was above its critical point at room temperature – oxygen or nitrogen or hydrogen, for example. These would all be described as gases.
Phase Diagrams
T.B. Massalski, D.E. Laughlin, in Reference Module in Materials Science and Materials Engineering, 2022.
- Publisher Summary
- Phase diagram:
- Viscosity measurement:
- Fluorescence measurement:
- Membrane dialysis:
Phase DiagramsT. B. Massalski, D.E. Laughlin, in Reference Module in Materials Science and Materials Engineering, 20177 Phase DiagramsPhase diagrams are graphical plots that are devised to show the relationships between the various phases that appear within the system under equilibrium conditions. As such, the diagrams are variously called constitutional diagrams, equilibrium diagrams, or phase diagrams. A single-component phase diagram can be simply a one- or two-dimensional plot, showing the phase changes in the given component (e. g., in iron) as temperature and/or pressure changes. Most diagrams, however, are two-dimensional plots describing the phase relationships in systems made up of two of more components. These usually contain fields (areas) consisting of multiple phase fields, as well as single-phase fields. Some typical examples are shown below. Read full chapterURL: sciencedirect. com/science/article/pii/B9780128035818032422Phase diagramsKLAUS HACK, in The SGTE Casebook (Second Edition), 2008Publisher SummaryThis chapter discusses a phase diagram, which is derivable from a projection of a property diagram.
Interpreting Phase Diagrams
- Learning Objective
Phase diagrams illustrate the effects selected variables of a system have on the state of matter. Phase diagrams are divided into three single phase regions that cover the pressure-temperature space over which the matter being evaluated exists: liquid, gaseous, and solid states. The lines that separate these single phase regions are known as phase boundaries. Along the phase boundaries, the matter being evaluated exists simultaneously in equilibrium between the two states that border the phase boundary.
33. Unary Phase Diagrams
This page contains materials for the solid solutions class session on unary phase diagrams. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study.
- Prerequisites
- Looking Ahead
- Learning Objectives
- Lecture Summary
- Other OCW and OER Content
For Further Study
« Previous | Next » Session Overview Modules Solid Solutions Concepts introduction to phase diagrams, basic definitions of phase, component, and equilibrium, one-component phase diagrams Keywords stability, pressure, temperature, vapor, liquid, solid, phase, equilibrium, component, triple point, melting, boiling, glass transition temperature, polymorph, sublimation, supercritical Chemical Substances water (H2O), zirconia (ZrO2), snowflake obsidian, aluminum (Al), silicon (Si), carbon dioxide (CO2), nitrogen (N), bismuth (Bi), sulfur (S), oxygen (O), argon (Ar), “dry ice”, carbon (C), mercury (Hg) Applications failure analysis, cooking food at altitude and in pressure cooker, automobile engine cooling, ice skating, artificial diamonds, coffee decaffeination Prerequisites Before starting this session, you should be familiar with: The Bonding and Molecules module, in particular Session 12: Intermolecular Forces Looking Ahead This is the first of three sessions on solid solutions and phase diagrams.
How do you read phase diagrams?
Correctly…. Let us look at a phase diagram of WATER and see if we make some sense from it….typically pressure is plotted against temperature….and we can read the resultant phase given the two coordinates. The scale of the x and y axes, representing temperature, and pressure respectively are fairly arbitrary….but they are useful. We see under conditions of high pressure we get a condensed phase, whereas under conditions of low pressure, and high temperature a gas is favoured. At P=218*atm and T=374*K we reach the critical point…and importantly at this point the densities of the water phase, and the gaseous phase become equal and we can no longer distinguish between the two phases, hence beyond this there is no designation of phase. At lower temperatures, say at 100 “”^@C liquid water expresses a vapour pressure of 1*atm…and thus we define the normal boiling point of water. At high pressures, and low temperatures we typically encounter a solid, and the graph reflects this. At much lower pressures, we find the triple point, the conditions of temperature and pressure at which ALL three phases are in equilibrium… Of special significance for the water molecule is that the SLOPE of the line differentiating the solid phase, and the liquid phase is NEGATIVE. This is very unusual….but we need further information from the Clapeyron equation to determine its significance….to put it in a nutshell, this means that the density of the solid phase is LESS than the density of the liquid phase, and so ice-bergs float…. Anyway, we are flying blind a bit here. If there is a specific issue or query raise it, and someone will address it. I would also consult your text for its account of phase diagrams.
The size from the #x# and #y# axes, representing temperature, and pressure correspondingly are fairly arbitrary.
Video advice: Chemistry Lecture: Phase Diagrams
http://mcatforme.com

A temperature‐pressure graph showing the various states of matter is a phase diagram. Phase refers to a single homogeneous physical state. Different phases have either different compositions or different physical states. In the preceding figure, there are three phases with the same composition in the solid, liquid, and gaseous states of matter.
Although the introductory example of H 2O mentioned changes of state caused by varying the temperature, it is known that variation of pressure can also produce such changes. In laboratory experiments, these two environmental factors—temperature and pressure—can each be varied or held constant; they are referred to as independent variables. Figure 1 assigns these variables to axes to form a plot that describes the physical condition at each point in the graph. The vertical axis is the pressure measured in atmospheres (atm).
Learn How To Read a Phase Diagram
A phase diagram is a graphical representation of pressure and temperature of a material. Learn about phase diagrams and how to read them.
A phase diagram is really a graphical representation of pressure and temperature of the material. Phase diagrams show the condition of matter at confirmed pressure and temperature. They reveal the limitations between phases and also the processes that occur once the pressure and/or temperatures are altered to mix these limitations. This short article outlines so what can be learned from the phase diagram and the way to read one.
Phases of Matter and Phase Diagrams
One of the properties of matter is its state. States of matter include solid, liquid or gas phases. At high pressures and low temperatures, the substance is in the solid phase. At low pressure and high temperature, the substance is in the gas phase. The liquid phase appears between the two regions. In this diagram, Point A is in the solid region. Point B is in the liquid phase and Point C is in the gas phase. The lines on a phase diagram correspond to the dividing lines between two phases. These lines are known as phase boundaries. At a point on a phase boundary, the substance can be in either one or the other phases that appear at either side of the boundary. These phases exist in equilibrium with one another. There are two points of interest on a phase diagram. Point D is the point where all three phases meet. When the material is at this pressure and temperature, it can exist in all three phases. This point is called the triple point. The other point of interest is when the pressure and temperature are high enough to be unable to tell the difference between the gas and liquid phases.
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist in equilibrium on the lines or curves.
Problems – Solid: A state of matter with tightly packed particles which do not change the shape or volume of the container that it is in. However, this does not mean that the volume of a solid is a constant. Solids can expand and contract when temperatures change. This is why when you look up the density of a solid, it will indicate the temperature at which the value for density is listed. Solids have strong intermolecular forces that keep particles in close proximity to one another. Another interesting thing to think about is that all true solids have crystalline structures. This means that their particles are arranged in a three-dimensional, orderly pattern. Solids will undergo phase changes when they come across energy changes.
Phase Diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium.
You are able to apply Le Chatelier’s Principle for this equilibrium just like whether it would be a chemical equilibrium. Should you boost the pressure, the equilibrium will move in a way regarding counter the modification you’ve just made. Whether it converted from liquid to solid, pressure would have a tendency to decrease again since the solid occupies slightly less space for many substances.
At its simplest, a phase can be just another term for solid, liquid or gas. If you have some ice floating in water, you have a solid phase present and a liquid phase. If there is air above the mixture, then that is another phase. But the term can be used more generally than this. For example, oil floating on water also consists of two phases – in this case, two liquid phases. If the oil and water are contained in a bucket, then the solid bucket is yet another phase. In fact, there might be more than one solid phase if the handle is attached separately to the bucket rather than moulded as a part of the bucket.
Consider a cylinder containing a mixture of liquid carbon dioxide in equilibrium with gaseous carbon dioxide at an initial pressure of 65 atm and a temperature of 20 °C. Sketch a plot depicting the change in the cylinder pressure with time as gaseous carbon dioxide is released at constant temperature.
Chemistry End of Chapter Exercises – In the previous module, the variation of a liquid’s equilibrium vapor pressure with temperature was described. Considering the definition of boiling point, plots of vapor pressure versus temperature represent how the boiling point of the liquid varies with pressure. Also described was the use of heating and cooling curves to determine a substance’s melting (or freezing) point. Making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, boiling points). A typical phase diagram for a pure substance is shown in (link).
The features of a phase change diagrams are thoroughly explained as well as its related terms and concepts, and the phase diagram of water.
- Phase Diagram Descriptions
- The Special Case of Water
- 3D Phase Diagrams
- Binary Mixtures
- Crystals and Polymorphs
What are Phase Chase Diagrams?
Core ConceptIn this tutorial, you will learn about the major features of phase diagrams, also called phase change diagrams, as well as other types of diagrams, and how to interpret and understand their importance. Topics Covered in Other ArticlesStates of MatterWhat is Pressure? Properties of solids, liquids, gasesIntermolecular ForcesPhysical vs. Chemical PropertiesVocabularyCondensation – when a substance goes from gaseous to liquid stateVaporization – when a substance goes from liquid to gaseous stateFusion – when a substance goes from liquid to solid stateMelting – when a substance goes from solid to liquid stateSublimation – when the substance goes directly from solid to gaseous stateDeposition – when a substance goes directly from gaseous to solid statePhase Change – when a substance changes from one state of matter to another (e. g. solid to liquid, or liquid to gas)What are Phase Chase Diagrams? A phase transition occurs when a substance changes from one state of matter to another state.
Video advice: Phase Diagrams of Water & CO2 Explained – Chemistry – Melting, Boiling & Critical Point
This chemistry video tutorial explains the concepts behind the phase diagram of CO2 / Carbon Dioxide and the phase diagram of water / H2O. This video contains plenty of examples and practice problems:

How do I read phase diagrams?
6:2012:47How to Read and Interpret Phase Diagrams - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo the first one says label the phases. Notice that the phases are not labeled. So if I go down hereMoreSo the first one says label the phases. Notice that the phases are not labeled. So if I go down here I only have two phases that you would transition from it to go to a point where horizontally.
What can you determine from a phase diagram?
A phase diagram is a graph which shows under what conditions of temperature and pressure distinct phases of matter occur . The simplest phase diagrams are of pure substances. These diagrams plot pressure on the y-axis and temperature on the x-axis.
How do you read density on a phase diagram?
The phase which has the higher density is the phase which exists under higher pressure and lower temperature. Thus, in a phase diagram showing pressure as a function of temperature, look for the phase which lies in the upper left region . Usually the solid phase is the densest.
What does each line in a phase diagram represents?
The lines in a phase diagram represent boundaries between different phases ; at any combination of temperature and pressure that lies on a line, two phases are in equilibrium.
How do you read an alloy phase diagram?
6:5823:25How to use phase diagrams and the lever rule to understand metal alloysYouTube.
Related Articles:
- How To Read Phase Diagrams Geology
- How To Read A Phase Diagram Geology
- How To Read A Ternary Phase Diagram Geology
- How To Draw Energy Level Diagrams Chemistry
- How To Read A Manometer Chemistry
- What Is Phase Rule In Chemistry
Science Journalist
Science atlas, our goal is to spark the curiosity that exists in all of us. We invite readers to visit us daily, explore topics of interest, and gain new perspectives along the way.
You may also like

What Jobs Can I Get With A Marine Science Degree
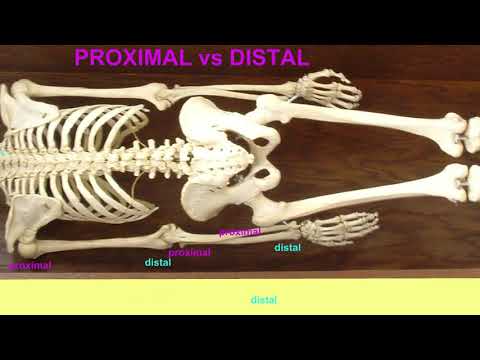

What Does Distal Versus Proximal Sections Geology Mean

What Is Evolution Definition Biology
Add comment, cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Recent discoveries

When Walmart Invested In Bossa Nova Robotics

When Is The Innovation Curve Used

How To Apply Dr Earth Fertilizer

Why Is Data Science Mainly About Teamwork
- Animals 3041
- Astronomy 8
- Biology 2281
- Chemistry 482
- Culture 1333
- Health 8466
- History 2152
- Physics 913
- Planet Earth 3239
- Science 2158
- Strange News 1230
- Technology 3625
Random fact

Which Of The Following Are Correct Associations Quizlet Geology
Just added to your cart

Chemistry Homework: Reading the Phase Diagram
Adding product to your cart
- Practice reading a phase diagram
- Identify and define the triple point and critical point on a phase diagram
- Relate temperature, energy, and pressure to phases changes
- Understand the concepts of phases of matter and how they relate to energy
- A good understanding of phase changes related to energy changes, changes in attractive forces, and particle movement and arrangement
- Choosing a selection results in a full page refresh.
- Press the space key then arrow keys to make a selection.
How to Read a Phase Diagrams
- Where the phases are
- Important points on the diagram
- Gibbs phase rule
Stack Exchange Network
Stack Exchange network consists of 183 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Q&A for work
Connect and share knowledge within a single location that is structured and easy to search.
Help reading a phase diagram? [closed]

- physical-chemistry
- 3 $\begingroup$ We have a policy which states that you should show your thoughts and/or efforts into solving the problem. It'll make us certain that we aren't doing your homework for you. Basically any question with the wording your question has is considered homework; it needn't be literally one. Self-study questions, puzzles etc. also count as homework. Don't worry, they're not banned. But, we require a minimal effort. Otherwise, this question may get closed. Please edit in your full reasoning or thoughts on this. See Homework $\endgroup$ – Poutnik Commented May 18, 2019 at 17:03
- $\begingroup$ What base are you using when applying the log? $log_e(10)=2.3$ whereas $log_{10}(10)=1$. Looks like you're using base 10. For sulfur dioxide the triple point is at a pressure in bar of $log_{10}(P)=-1.8$ $\endgroup$ – Buck Thorn ♦ Commented May 18, 2019 at 18:24
Here is how the y-axis works :

Not the answer you're looking for? Browse other questions tagged physical-chemistry or ask your own question .
- Featured on Meta
- Bringing clarity to status tag usage on meta sites
- We've made changes to our Terms of Service & Privacy Policy - July 2024
- Announcing a change to the data-dump process
Hot Network Questions
- Home Electrical DIY
- Are there jurisdictions where an uninvolved party can appeal a court decision?
- Connector's number of mating cycles
- Lookup value between two dates in excel
- Power line crossing data lines via the ground plane
- Sharing course material from a previous lecturer with a new lecturer
- What is a word/phrase that best describes a "blatant disregard or neglect" for something, but with the connotation of that they should have known?
- can a CPU once removed retain information that poses a security concern?
- Can you bring a cohort back to life?
- Do POVM and generalized measurements really describe all possible measurements we can do on a quantum system (open dynamics)?
- Did Avraham derive and keep the oral and written torah some how on his own before the yeshiva of Noach or only after?
- If Venus had a sapient civilisation similar to our own prior to global resurfacing, would we know it?
- Has the application of a law ever being appealed anywhere due to the lawmakers not knowing what they were voting/ruling?
- How is lost ammonia replaced aboard the ISS?
- Guitar amplifier placement for live band
- How do *Trinitarians* explain why Jesus didn't mention the Holy Spirit in Matthew 24:36 regarding his return?
- A study on the speed of gravity
- Do "Whenever X becomes the target of a spell" abilities get triggered by counterspell?
- How do you "stealth" a relativistic superweapon?
- Cardano full node disk space needed
- Can't figure out this multi-wire branch circuit
- DIN Rail Logic Gate
- What is this houseplant with a red fleshy stem and thick waxy leaves?
- Is a user considered liable if its user accounts on social networks are hacked and used to post illegal content?
- Rating Count
- Price (Ascending)
- Price (Descending)
- Most Recent
Phase diagram worksheet
Resource type.

Moon Phases Worksheet - Activity- Diagramming the Phases of the Moon

Mitosis Hands On Station Activity and Cell Cycle Worksheet with Phases Diagrams

Eclipses and Moon Phases Worksheet Review HIGHER RIGOR

Mitosis Phases Bundle with Diagrams , Games & Worksheets for Stages of Mitosis

Lunar Phase Diagrams and Worksheets

Phase Diagram worksheet

Chemistry Matter Unit Phase Changes Phase Diagrams Worksheet Set (w/ KEY)

Reading Phase Diagrams - Classwork Worksheet for in-class / extra practice

Chemistry: Phase Diagram Worksheet w/key

Interpreting Phase Diagrams : Printable Chemistry Worksheet

Phase Change Diagrams Reading Worksheet **Editable**

Phase Change Diagram worksheet

Create a Phase Change Diagram Worksheet

Phases of Mitosis Worksheet w Digital Diagrams of Mitosis Stages for 9th Grade

Moon Phase Diagram Worksheet

Phase Change Diagram Review Worksheet

Moon Phases Activities Phases of the Moon Worksheets Oreo Moon Phases Activity

Mitosis and Cell Cycle Practice Worksheet

Mitosis Guided Doodle Notes Worksheet - Mitosis Diagrams Cell Division Activity

2nd/3rd/4th/5th Phases of The Moon Diagram - Labeling and Coloring or Cookies

Phases of the moon craft: Includes solar and lunar eclipse

Moon Phases Diagram | Science Graphic Organizer Template

Lunar Cycle & Moon Phases Worksheets

Phase Change Task Cards |Changes in States of Matter Worksheet

- We're hiring
- Help & FAQ
- Privacy policy
- Student privacy
- Terms of service
- Tell us what you think

IMAGES
COMMENTS
This is a chemistry tutorial video that goes through how to read, analyze, and interpret a phase diagram. There are several examples of different questions y...
In the video here, Sal uses a horizontal line through the phase diagram. But, it doesn't have to be horizontal. Imagine a vertical line through this diagram-- for water, choose 100 degrees C. As long as you are at 100 C, you can change the phase by changing the pressure on the system.
With this homework page, students will practice reading a phase diagram. There are 3 different phase diagrams and 15 questions. A complete KEY is included. Objectives: Practice reading a phase diagram. Identify and define the triple point and critical point on a phase diagram. Relate temperature, energy, and pressure to phases changes.
This page contains materials for the solid solutions class session on unary phase diagrams. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study.
How to read the different features of a phase diagram, including boiling point, melting point, triple point, critical point, critical temperature, and critic...
How to Read a Phase Diagram. A two-dimensional phase diagram shows temperature along the horizontal axis and pressure along the vertical axis. The pressure is plotted in the unit of atmosphere (atm) or Pascal (Pa), and the temperature is in Kelvin (K) or degrees Celcius ( o C). The lines of equilibrium represent a pressure-temperature combination where two phases can coexist in equilibrium.
This homework page can be used for homework, class practice, or a quiz.With this homework page, students will practice reading a phase diagram. There are 3 different phase diagrams and 15 questions.A complete KEY is included.Objectives: Practice reading a phase diagramIdentify and define the triple point and critical point on a phase ...
In the field labeled Solid A and liquid, a two phase tie line intersects the Solid A side of the diagram and the liquidus. Note that each two phase tie line always intersects pure A but a liquid with a different bulk composition. The field of Solid B + liquid behaves in a similar fashion. The 4th field is labeled Solid A plus Solid B.
Figure Chapter4.1 C h a p t e r 4. 1: Free energy curves for Solid to Liquid Phase Transition. where Gl G l is the molar Gibbs free energy of the liquid phase and Gs G s is the molar Gibbs free energy of the solid phase. I will try to be as consistent as possible to keep liquid as blue color, like water.
This is a basic (emphasize on the basic) tutorial of reading phase diagrams and learning how to apply the diagram into various phase changes that occur. I us...
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium. You are able to apply Le Chatelier's Principle for this equilibrium just like whether it would be a chemical equilibrium.
This homework page is included in the lesson: Phase Changes, Phase Diagrams, & Heating/Cooling Curves. This homework page can be used for homework, class practice, or a quiz. With this homework page, students will practice reading a phase diagram. There are 3 different phase diagrams and 15 questions. A complete KE
How to Read a Phase Diagrams. Everybody remembers that the boiling point of water is 100°C, but that's only true at a certain pressure (1 bar). Boiling points, melting points, and sublimation points vary with pressure. A phase diagram helps show what phase exist at what temperature and pressure. What you need to know: Where the phases are.
View Graphs in Thermochemistry.pdf from CHEM 101 at Rowan-Cabarrus Community College. Reading the Phase Diagram Name: Homework Period: anthony Date: Reading the Phase Diagram Color and label the
It'll make us certain that we aren't doing your homework for you. Basically any question with the wording your question has is considered homework; it needn't be literally one. Self-study questions, puzzles etc. also count as homework. Don't worry, they're not banned. But, we require a minimal effort. Otherwise, this question may get closed.
How to use the sliders in WileyPLUS to get values off of phase diagrams for computation.
The moon phase diagrams are designed to display the moon in relationship the earth and sun in each of its eight phases. Their are three worksheets focusing on the phases of the moon. Other Astronomy activities: Astronomer Flipbook PowerPoint & Quiz Astronomy Unit Notes and Powerpoint Cool NASA Images Powerpoint Solar System Chart Solar System Chart and Powerpoint Solar System/Planets Quiz ...