- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
MRC Laboratory of Molecular Biology
One of the world's leading research institutes, our scientists are working to advance understanding of biological processes at the molecular level - providing the knowledge needed to solve key problems in human health.


Applications for the LMB PhD programme are now open. Deadline 3rd December 2024.
The MRC Laboratory of Molecular Biology (LMB) is a research institute dedicated to the understanding of important biological processes at the levels of atoms, molecules, cells and organisms. In doing so, we provide knowledge needed to solve key problems in human health.
Our scientists tackle fundamental, often difficult and long-term research problems. The LMB has made revolutionary contributions to science, such as pioneering X-ray crystallography and electron cryo-microscopy (cryo-EM) to determine protein structures, the sequencing of DNA and the development of monoclonal antibodies. Twelve Nobel Prizes have been awarded for work carried out by LMB scientists.
The LMB also promotes the application and exploitation of our research findings, both by collaboration with existing companies and the founding of new ones, helping to advance medical research and the translation and application of knowledge.
The LMB provides an unsurpassed environment for both young and established researchers, with state-of-the-art facilities and a unique scientific culture. The LMB has always been very diverse, with a truly international outlook. We currently employ men and women from over 50 countries, and LMB alumni work in research organisations across the world.
Insight on Research
Unprecedented heteromeric amyloid structure in neurodegenerative disease.
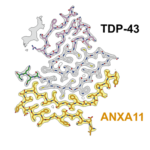
Surprising advance to our understanding of neurodegenerative diseases, as Diana Arseni and Benjamin Ryskeldi-Falcon in the LMB’s Neurobiology Division find that frontotemporal lobar degeneration Type C filaments include both annexin A11 and TDP-43 in a unique heteromeric structure.
How cells enter the germline at the right time and place during embryonic development
Marta Shahbazi’s group in the LMB’s Cell Biology Division has determined that contact with extracellular matrix proteins prevents embryonic cells from becoming germ cells.
Quick Links
- How to Find Us
- Current Vacancies
- Contact Directory
- PhD Programme
- LMB Nobel Prizes
- News & Events
- Useful Contacts
- Goals and Research Focus
- LMB Through the Years
- LMB Exhibitions
Latest News

Diana Arseni’s research group in the LMB’s Neurobiology Division will study mechanisms of brain health, ageing and disease focusing on the lysosomal protein TMEM106B. […]

The LMB is thrilled to announce that Poppy Marriott was recently appointed as the new Scientific Services and Sustainability Manager. […]
LMB in the News

LMB Alumni News

Latest Publications
- A Fragment-Based Competitive F LB-NMR Platform For Hotspot-Directed Ligand Profiling. McCarthy, WJ., et al. Angew Chem Int Ed Engl 63 (37): e202406846. (9th September 2024)
- Human coronaviruses activate and hijack the host transcription factor HSF1 to enhance viral replication. Pauciullo, S., et al. Cell Mol Life Sci 81 (1): 386. (7th September 2024)
- Integrin signaling in pluripotent cells acts as a gatekeeper of mouse germline entry. Makhlouf, A., Wang, A., Sato, N., Rosa, VS., Shahbazi, MN. Sci Adv 10 (36): eadk2252. (6th September 2024)
- Recipient tissue microenvironment determines developmental path of intestinal innate lymphoid progenitors. Clark, PA., et al. Nat Commun 15 (1): 7809. (6th September 2024)
- Engineering Pyrrolysine Systems for Genetic Code Expansion and Reprogramming. Dunkelmann, DL., Chin, JW. Chem Rev [Epub ahead of print]. (5th September 2024)
- Behavioral plasticity. Zhang, Y., Iino, Y., Schafer, WR. Genetics 228 (1). (4th September 2024)
- Using Organoids to Model Sex Differences in the Human Brain. Pavlinek, A., et al. Biol Psychiatry Glob Open Sci 4 (5): 100343. (4th September 2024)
Imperial College London Imperial College London
Latest news.

Neutrino discoveries and academic awards for excellence: News from Imperial

Imperial joins European discussion on accelerating innovation

Imperial to strengthen Transatlantic tech cooperation with new hub
- Institute of Clinical Sciences
- Faculty of Medicine
- Departments
MRC Laboratory of Medical Sciences
The MRC Laboratory of Medical Sciences (LMS) is a thriving biomedical research environment located within the heart of Hammersmith Hospital in partnership with Imperial College at its White City campus. The LMS forms a key component of the College's Institute of Clinical Sciences, and is a core-funded institute of the Medical Research Council.
Our scientific programmes are focused on three sections: Epigenetics, Genes and Metabolism, and Quantitative Biology. We also train and mentor the next generation of clinical and non-clinical scientists and strive to enhance the public’s interest, understanding and trust in science.
3 research groups
Epigenetics
Studying epigenetic processes in the cell, including stem cell development
Genes and metabolism
Exploring the molecular and cellular basis of disease
Quantitative biology
Investigating complexity with computational and experimental approaches
3 column colour block - News, PE, Students
Science news
Latest research news from MRC LMS

Institute news
Latest news from MRC LMS

Public engagement
Science for patients, the public and school children
AHSC and Study - 2

Our PhD Studentships, Clinical Research Fellowships and undergraduate summer placements offer you the chance to work within our community of international students, postdoctoral fellows and principle investigators on breakthrough research
Translating research
A critical part of the Institute's remit is translation. This is acheived through strategic partnerships and embedded clinical fellows, lecturers and principal investigators.

World first discoveries allow researchers to accurately diagnose prenatal exposure syndromes and birth disorders
Researchers at London Health Sciences Centre (LHSC) and Lawson Health Research Institute are using advanced technology and artificial intelligence (AI) to diagnose rare diseases and prenatal exposure-related birth abnormalities in two new studies.

Scientists develop new tool to detect consciousness in the intensive care unit

$1.2 million in federal funding to study women Veterans experiencing homelessness

New technique could lead to more organs being available for transplant

Café Scientifique

Celebrating health research excellence with the 2023 Lawson Impact Awards
Every day, hospital-based research in London, Ontario changes the lives of people around the world. Through close proximity to patients, scientists in the hospital can identify challenges and develop solutions effectively and then deliver them to care faster than anywhere else.
Lawson Health Research Institute has been driving innovation and helping to transform health care. Taking a “bench to bedside” approach to medical research, researchers focus their efforts on the development of new knowledge that can be applied directly to patient care. The fast pace of scientific discovery makes medical research an exciting place to be. Learn more about our current studies and how you can be a part of new groundbreaking research.

- News & Events
- Clinical Trials

- Accreditations
+44 208 961 4130
Welcome to HMR’s Central Laboratory
Clinical laboratory services in London, UK
Contract laboratory at Hammersmith Medicines Research
Central laboratory
- Click here for central laboratory services
- Click here for HMR careers
- Click here to enter phase 1 trial site
- Where to find us contact us
HMR’s medical laboratory in London, UK, offers a full range of tests and services, at competitive prices with fast turnaround times. We have a well established team of Biomedical Scientists who are experienced, have Health & Care Professions Council (HCPC) registration and participate in the Institute of Biomedical Scientists (IBMS) continuing professional development (CPD) programme.
We offer total pathology project management. You will have a dedicated project leader to facilitate communications on trial implementation, progress and reporting. We can prepare visit-specific kits containing all materials and instructions necessary for specimen collection and transport. Sample collection kits can be prepared quickly and distributed to trial sites. We can implement trial-specific requirements including result flagging, alert limits and blinding of data.
We are able to provide electronic transfer of data for daily results, interim reports for data review, and final trial data files to your specification.
For consistency of data, we use the same methodology and, whenever possible, the same reagent lot number for the life of the trial.
If you require a test that doesn’t appear on our list, we can set up and validate the method at your request.
Privacy Overview

- Treatment Resistant Depression
- Enquiry form
- Testimonials
- What happens next?
Cumberland Avenue London NW10 7EW
For urgent or weekend enquiries (not volunteering) 0800 783 8792
Welcome to London Trials
We’re arguably London’s leading research unit doing clinical trials with healthy volunteers
Register now
We’ve done more than 850 medical trials , with an exceptional record for safety and putting our volunteers first.
Discover who we are
“Very polite informative staff. HMR really sets the standard from medical right through to the end”
Register for Paid Clinical Trials
- Register now Trials for healthy volunteers
- Register now Trials for people with IPF and MS
- Register now Trials for people with asthma
- Register now Trials for people with depression
- Register now Trials for postmenopausal women
Who are we?

We’re arguably London’s leading medical research unit doing trials with healthy volunteers. We’ve done more than 850 trials, with an exceptional record for safety and putting our volunteers first.
We work mainly for ‘blue chip’ pharmaceutical companies like GSK, Eli Lilly, Merck, Sharpe & Dohme and Sanofi-Aventis. We have 230 highly trained staff, including 10 doctors, 40 nurses and over 120 graduates or PhDs. Our Medical Director has nearly 30 years’ experience of clinical trials. All our ward staff are enrolled in a unique, university-accredited, training programme, which lasts 18 months, and for which we received a National Training Award.
Since 1993, more than 13,000 volunteers have done a trial here. Many come back to repeat the experience. See what our trial participants said about us here.
Our phase 1 unit
HMR is one of the largest phase1 units in Europe. Our spacious new premises have first-class facilities for carrying out early trials of new medicines: 145 beds; a separate outpatients facility; laboratory; pharmacy; and offices.
We have outstanding new facilities for the volunteers who take part in our trials : restaurant-standard food cooked fresh every day; air-conditioned wards; separate toilets and showers for men and women; computers with wireless internet access; widescreen TVs, Sky and games consoles; range of DVDs, books, daily newspapers and board games; large recreation and dining area; secure storage for belongings; and short walk from underground stations.
Our clinical trials
We usually give a new medicine to healthy people aged between 18 and 45 first, to check it’s safe and to find out what the body does to it. If it’s going to be given to elderly patients, we may test it first in healthy people over 60. If the results in healthy people are encouraging, we then test it in patients who have the disease for which it’s being developed, to see if it’s effective.
We specialise in studies in healthy people but, we also do some of the first studies of new medicines in patients with conditions such as asthma , migraine and diabetes.
When we do our medical research studies, we follow strict laws and guidelines to make sure that we comply with Good Clinical Practice (GCP). They make sure that researchers who do studies of new medicines in people, such as in paid or unpaid clinical trials do them to high standards. Our facilities and staff meet the highest of standards for testing new medicines set by the Medicines and Healthcare products Regulatory Agency (MHRA) – the Government organisation that controls new medicines.
1993 – 4 beds in St Bartholomew’s Hospital, London 1994 – 24 beds in Central Middlesex Hospital, north-west London 1995 – Rubicon Award – New Business of the Year 1996 – 48 beds in Central Middlesex Hospital 1998 – Queen’s Award for Export Achievement 2002 – Queen’s Award for Enterprise: International Trade 2002 – 70 beds in Central Middlesex Hospital 2004 – National Training Award 2009 – 100 beds in purpose-designed clinical pharmacology unit 2013 – 145 beds – HMR becomes one of the largest phase 1 units in Europe
- The first phase 1 unit to get Phase 1 Supplementary Accreditation by the Medicines and Healthcare products Regulatory Agency (MHRA)
- The first phase 1 unit to get MHRA Manufacturer’s Authorisation [MIA(IMP) No 15140] to import, assemble, package and label non-sterile, sterile and radiopharmaceutical potential new medicines
- ISO 9001:2008 quality management system
- Pharmacy registration by the General Pharmaceutical Council
- University based course in GCP training
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| fr | 3 months | Facebook event tracking for analytic of how the content performs. |
Please enable JavaScript in your web browser to get the best experience.
We use cookies to track usage and preferences.
Primary navigation
- PhD students
- Exhibitions
- Translation
- Work experience
Discoveries to change lives
We're one of Europe's biggest labs and home to more than 1,500 scientists working to understand health, disease and how life works.
WE NEED THE EXHBITION TITLE HERE
Images of science.
Sixteen scientific images have been chosen to represent Crick research in a travelling international exhibition.
Latest news
Crick annual animal research numbers published, crick skylab opens as msd commence new research programmes, fabian fröhlich awarded erc starting grant, £2m awarded to study heart development in zebrafish, sign up for our newsletters.
Join our mailing lists to receive updates about our latest research and to hear about our free public events and exhibitions. If you would like to find out more about how we manage your personal information please see our privacy policy .
The Francis Crick Institute is a unique partnership between


MRC list of institutes, units and centres
The Medical Research Council (MRC) provides funding to the research institutes, units and centres listed below.
- Laboratory of Molecular Biology
- Laboratory of Medical Sciences
- Health Data Research UK
- UK Dementia Research Institute
- The Francis Crick Institute
Centres and Units
Infections and immunity board.
- MRC Translational Immune Discovery Unit at the University of Oxford
- MRC Unit The Gambia at London School of Hygiene and Tropical Medicine (LSHTM)
- MRC-University of Glasgow Centre for Virus Research
- MRC/UVRI and LSHTM Uganda Research Unit
- MRC Centre for Global Infectious Disease Analysis at Imperial College London
- MRC Centre for Medical Mycology at University of Exeter
- MRC Centre for Molecular Bacteriology and Infection at Imperial College London
Molecular and Cellular Medicine Board
- MRC Human Genetics Unit at the University of Edinburgh
- MRC Mitochondrial Biology Unit at the University of Cambridge
- MRC Molecular Haematology Unit at the University of Oxford
- MRC Prion Unit at University College London
- MRC Protein Phosphorylation and Ubiquitylation Unit at the University of Dundee
- MRC Toxicology Unit at the University of Cambridge
- MRC Centre for Environment and Health, Imperial College London
Strategic Partnerships:
- MRC Weatherall Institute of Molecular Medicine at the University of Oxford
- Research Complex at Harwell
Neurosciences and Mental Health Board
- MRC Brain Network Dynamics Unit
- MRC Cognition and Brain Sciences Unit
- MRC Centre for Neurodevelopmental Disorders
Population and Systems Medicine Board
- MRC Biostatistics Unit (BSU), Cambridge
- MRC Clinical Trials Unit (CTU), UCL
- MRC Epidemiology Unit (EU), University of Cambridge
- MRC Integrative Epidemiology Unit (IEU), University of Bristol
- MRC Unit for Lifelong Health and Ageing, UCL
- MRC Metabolic Diseases Unit (MDU), University of Cambridge
- MRC & CSO Social & Public Health Sciences Unit (SPHSU), University of Glasgow
- MRC/Versus Arthritis Centre for Musculoskeletal Ageing Research (CMAR), Birmingham/Nottingham
- MRC Lifecourse Epidemiology Centre (LEC), University of Southampton
- MRC Centre for Reproductive Health, University of Edinburgh .
Last updated: 23 August 2024
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .
- ICH GCP (De)
- ICH GCP (En)
- ICH GCP (Es)
- ICH GCP (Fr)
- ICH GCP (It)
- ICH GCP (Pt)
- ICH GCP (Ru)
- AUSTRALIA (NHMRC)
- JAPAN (PMDA)
- US Clinical Trials Registry
- EU Clinical Trials Registry
- Pharmaceutical Companies
- Clinical Research Labs
- Service Companies
- Clinical Research Events
- Publications
- Researchers
List of Contract Research Organizations in United Kingdom
Featured cros.

Clinicology - an FGK company
Clinicology - an FGK company is a bespoke CRO based near London, UK for Phase I-IV CTIMP and medical device studies. Our advantages are our speed and flexibility, with rapid project startup and team deployment with our network across Europe and the U...

Celero provides optimal returns to innovative biotechs & pharmaceutical companies by addressing unmet needs, accelerating clinical development and commercialising innovative healthcare products.Trial mismanagement is the main reason why 85%-90% o...

SanaClis is a full-service Global CRO with a fully integrated clinical supply chain, thereby offering a comprehensive range of end-to-end services for clinical trials. SanaClis was founded in 2000 by seasoned industry experts, all of whom have had ex...


Orci Trials Ltd
Orci Trials is a premier Contract Research Organization (CRO) with extensive experience in a broad range of therapeutic areas, including Oncology, Infectious Diseases, Respiratory, Gastroenterology, and Diseases of the Developing World. Having an off...
Local, small- and mid-size Contract Research Organizations in United Kingdom
We assist biotechnology, medical device and pharmaceutical start-ups and SMSEs to refine and fast-track their research goals through efficient study design, SAPs and protocol development and adaption, and careful statistical analysis of study endpoin... View full profile
- United Kingdom
APCER Life Sciences is committed to improving health in partnership with its clients. We bring together safety, medical, regulatory, and technology resources to ensure that patients receive the safest, most effective therapies possible. To achieve th... View full profile
- United States
AHRM Inc. is a full service Contract Research Organization (CRO) with a focus in Health Outcomes and Health Economics Research. By providing the ability to integrate both clinical and economic research into one protocol or study, AHRM Inc. can suppor... View full profile
Astron Research Limited, a leading IP oriented pharma contract research organization, is a fast growing Pharma Contract Research Organisation (CRO) with a strong quest to deliver best services to the rapidly growing Healthcare industry and has embark... View full profile
BBI Solutions has served the global diagnostics industry for over 50 years, as a leading developer and manufacturer of raw materials and finished test platforms for the in-vitro diagnostics market.Over the past 50 years, BBI has grown from a small sp... View full profile
James Neill and Rachel Foster of HNH Group, Jefferson House, 42 Queen Street, Belfast, Co. Antrim BT1 6HL were appointed as Joint Administrators of Bio-Kinetic Europe Limited on 19 October 2017. Any creditor of Bio-Kinetic Europe Limited with a claim... View full profile
Biolab Medical Unit, a medical referral laboratory specialising in nutritional and environmental medicine, is located in the heart of the West End of London. We are a nutritional biochemistry laboratory measuring vitamin and mineral levels, toxic met... View full profile
Welcome to the Biotrial website, where you will find extensive information on the services we offer, on our expertise and experience, and of course on how to contact us. At Biotrial, drug evaluation and pharmacology research is a tailor-made service.... View full profile
Carpathian Research Group supports a broad range of R&D and translation activities for biopharmaceutical and medical device industry. We have offices in Ukraine, the United Kingdom, and Poland.Our mission is to help our customers make most advant... View full profile
Chembiotech Laboratories has its roots in the scientific consultancy work undertaken by Prof. Kennedy since the early 1970's, both in his own name and under the trading name of Biotechnics. This practice continued until 1977 when Chembiotech Laborato... View full profile
CSSi was founded in 2005 as Clinical Site Services to serve individual sites with their patient recruitment needs, later expanding into a major global centralized patient recruitment company. A leader in the industry, CSSi delivers strategic patient... View full profile
Clinicology - an FGK company is a bespoke CRO based near London, UK for Phase I-IV CTIMP and medical device studies. Our advantages are our speed and flexibility, with rapid project startup and team deployment with our network across Europe and the U... View full profile
- United Arab Emirates
Clintec, established for 21 years, is an innovative, medium-sized global functional services provider with a depth of expertise in oncology and rare diseases. Clintec combines the agility and flexibility typical of smaller CROs with the global covera... View full profile
Congenix is an independent contract research organization offering its clients a full range of services, covering the organization and implementation of clinical trials on phases I to IV in the Russian Federation and former Soviet countries. Our miss... View full profile
Our headquarter is based in the UK, however, we operate in other European locations either with a very strong presence in Poland and other central European location, Germany, France, Netherlands, Spain, Italy and Switzerland. We are a global company... View full profile
DRK Pharma Solutions provides Clinical Research Services from Phase II-IV through its highly experienced Clinical Operations’ team in Pakistan. Also, we are the First Licensed Clinical Research Organization in Pakistan according to the Bio Study Rule... View full profile
- Switzerland
endpoint is the destination for people who are passionate about delivering the most innovative and high-quality IRT solutions for clinical trials. We find purpose through hard work and a commitment to excellence, resulting in a competitive advantage... View full profile
GenesisCare Clinical CRO is an independent company founded by GenesisCare, with people skilled across the full spectrum of traditional CRO service offerings yet connected through GenesisCare to additional research services and investigator sites. Why... View full profile
Described in our first routine GCP inspection by the MHRA in 2007, as a “centre of excellence” and providing “a superior service”, ESMS provide 24/7 information services for the following areas: Clinical Trials, Medical Information. View full profile
hVIVO was originally incorporated in 1988 as Retroscreen Virology (the name was changed to hVIVO in 2015). The company was spun-out from Queen Mary, University of London, to commercialise the academic research of Professor John Oxford in the field... View full profile
From site startup to study closeout (and everything in between), Imperial is ready to put 75 years of global operational expertise to work for you. We deliver all of this with a professional, personal touch, and a positive, hassle-free experience. View full profile
ICTA testifies of a 35-year long experience of successful international and local pre- and post-authorisation clinical studies. We’ll come up with the best solution whether the development is clinical, epidemiological or econometric!You will find at... View full profile
JRF Global offers comprehensive non clinical GLP research services for regulatory submissions, worldwide. We offer fast, transparent, cost-effective, and hassle-free services in Toxicology, Eco-toxicology, Chemistry, Environmental Fate and Metabolism... View full profile
Kinesis Pharma is a leading partner in early phase drug development which seamlessly integrates CMC, non-clinical development, clinical development, regulatory, quality and project management. Our experts in Drug Development have an extensive backgro... View full profile
- Netherlands
Lowden International Ltd is a consortium of experienced consultants who specialise in the qaulity management processes of pharmaceutical manufacturing. They have worked exstenively worldwide offering a wealth of knowledge in manufacturing practices,... View full profile
The Medicines Evaluation Unit (MEU) Ltd is one of the UK’s leading contract research organisations, and a facility where commitment to excellence is paramount. Possessing extensive pharmaceutical, scientific and clinical expertise, allied with fast v... View full profile
Mediconomics provides spot‑on professional CRO services. Mediconomics is a successful CRO and consultant for research and development projects of both Medicinal Products and Medical Devices. Headquartered in Hannover, Germany and with subsidiaries... View full profile
Medilife Ltd. is a UK based CRO specialising in medical device studies. We offer partnerships with medical device companies to provide end to end support to gain access to key UK and European markets, from clinical trial services, marketing and a sa... View full profile
Advancements in mesenchymal stem cell (MSC) research are shedding light on how these stem cells may someday be used in various clinical applications such as immunomodulatory therapies (i.e., prevention of graft-versus-host disease or treatment of Cro... View full profile
NeuroSolutions is a contract research organisation established in 2001 as a service provider of cutting edge electrophysiological and behavioural research in the field of neuroscience. Neurosolutions was founded to provide technically demanding elect... View full profile
NorthWise Services offers a range of services and products to the pharmaceutical and biotechnology industries, in support of the complete clinical trial process and beyond. Core services include data management, statistics and medical writing. These... View full profile
NovaBiotics Ltd is a leading clinical-stage biotechnology company focused on the design and development of first-in-class anti-infectives for difficult-to-treat, medically unmet diseases. View full profile
Orci Trials is a premier Contract Research Organization (CRO) with extensive experience in a broad range of therapeutic areas, including Oncology, Infectious Diseases, Respiratory, Gastroenterology, and Diseases of the Developing World. Having an off... View full profile
Navigating the CRO landscape can be difficult for biotech companies that vastly rely on outsourcing as the differentiation between CROs seems marginal and confusing at times. Orphan Reach is different: We are a boutique service provider solely focuse... View full profile
Paspigioni is a premier partner in the dynamic world of pharmaceutical and biotechnology research. As a leading full-service Contract Research Organization (CRO), we specialize in guiding esteemed pharmaceutical and biotech companies through every ph... View full profile
Perceptive Informatics, Inc., develops and offers a portfolio of innovative technology-based products and services that facilitate clinical drug development and are designed to decrease time to peak. View full profile
Pharm-Olam (is now Allucent) is a mid-size global full service contract research organization (CRO), founded in 1994, and over time, we have established a strong reputation of performing exceptionally in challenging international clinical trials. Our... View full profile
Pharmaceutical consulting services are used by many pharmaceutical and biotech companies. Often they are looking for an expert team experienced in scientific and management leadership. If your company requires a team who can confidently manage the en... View full profile
Pharmalys is a privately-owned regional Contract Research Organisation (CRO) established in 2008 in the UK. We have a strong presence in the UK and Republic of Ireland with our main European office located in London. Since 2012, we have expanded our... View full profile
PharSafer® is a specialist Global CRO focusing on Pharmacovigilance & Medical Services. Formed in 2002 and Headquartered in the UK, PharSafer® has enjoyed continued annual client growth, global expansion and increased staffing. As a privately ow... View full profile
Cancer is one the top 5 diseases in the world. It affects many lives worldwide, and we intend to do much about it. Since 2006, Releaf Research, a woman-owned CRO, is known to for exceeding client expectations. We consider every study we support a pot... View full profile
RenaSci provides an integrated blend of consultancy and experimental services to clients from all sectors of the pharmaceutical industry to help maximise research productivity and streamline drug development. Since the company was established in 2001... View full profile
Collaborating on all therapeutic modalities, Resolian fuses expertise from Alliance Pharma and Drug Development Solutions to provide world-class scientific solutions. View full profile
For over 15 years, Richmond Pharmacology has delivered clinical excellence from design to delivery of early phase clinical trials, going from strength to strength in a fiercely competitive market place. We have experience that is held by our long ser... View full profile
Selcia is a global contract research provider of integrated drug discovery, medicinal chemistry and 14C radiolabeled compounds. Established in 2001, the company has two divisions: Selcia Drug Discovery - with medicinal chemistry,biology ADME and a un... View full profile
Founded in 1925 and headquartered in Akron, Ohio, Smithers is a multinational provider of testing, consulting, information, and compliance services. With laboratories and operations in North America, Europe, and Asia, Smithers supports customers in t... View full profile
SMP is a small sized CRO with its main office in Brasov, Romania. Our main activity is to conduct clinical research in bioequivalence studies and clinical trials of phase II-IV in Romanian sites. Through our offices the SMP personnel provides full mo... View full profile
Source BioScience Limited is an international provider of integrated state of the art Laboratory Services and Products. Headquartered in the UK, with offices in UK, Europe and the USA, Source BioScience is privately held, having been acquired through... View full profile
The CLINICAL TRIAL Company Group™ (TCTC Group™) is a privately owned, full-service global Clinical Contract Research Organization providing professional support to pharmaceutical, biotechnology, medical device and academic businesses and institutions... View full profile
Full-service CRO with an unwavering focus delivering high quality, on-time and cost-effective clinical research. While our impeccable reputation in anti-infective research and development has been at the core of our growth, we have extended that repu... View full profile
- South Africa
We are Transcom Global, an ISO 9001:2015, ISO 17100:2015 and EN15038:2006 certified provider of translation, localization and QA services for the global life science industry. Our professional team understands your language and unique terminology. Fa... View full profile
Specialist Pharmaceutical ConsultancytranScrip is a highly specialist pharmaceutical consultancy that provides strategic expertise, therapeutic experience and operational excellence across the entire product lifecycle, supporting clients globally.We... View full profile
Global Contract Research Organizations in United Kingdom
Website: www.54gene.com Email: [email protected] Phone: +971 58 594 9926; +1 310-266-9926. 54gene’s Clinical Programs Group (CPG) is a contract research organization that provides innovative, customizable, and cost-effective clinical trials solutions... View full profile
Since 1975, ACM Global Laboratories has been a recognized leader in both medical diagnostic and global clinical trial testing services. With wholly owned facilities in New York, England, Shanghai China, Mumbai India and Singapore, we operate in more... View full profile
For more than 25 years, Aixial, the Contract Research Organisation (CRO) of the ALTEN Group, has been supporting pharmaceutical industry key accounts in Europe for various types of services: Insourcing & outsourcing. Aixial environments are vari... View full profile
- Czech Republic
With 15 years of experience, we have defined a process that consistently achieves success for our clients.RESEARCH that uncovers the real needs and delivers actionable insights.At the heart of any healthcare challenge lies a compelling narrative. To... View full profile
Axonal-Biostatem has been delivering top-notch management of clinical and epidemiological research projects in France and Europe for more than 30 years. Axonal-Biostatem is a full service CRO providing all services internally and able to integrally p... View full profile
BioReliance provides testing and manufacturing services to pharmaceutical and biopharmaceutical companies that span the product cycle from early pre-clinical development to licensed production. Our goal is to advance the development and delivery of h... View full profile
A global, full-service contract research organization. We take your trials personally. dMed Global, a full-service Clinical Contract Research Organization (CRO) based in Shanghai, China and Clinipace Incorporated, a full-service Clinical CRO with hea... View full profile
- South Korea
Celerion, a leader in early clinical research, delivers Applied Translational Medicine. In Applied Translational Medicine, Celerion applies our expertise and experience to translating information gained in research discoveries, to knowledge of drug a... View full profile
Celero provides optimal returns to innovative biotechs & pharmaceutical companies by addressing unmet needs, accelerating clinical development and commercialising innovative healthcare products.Trial mismanagement is the main reason why 85%-90% o... View full profile
- Philippines
At Charles River, we are passionate about our role in improving the quality of people’s lives. Our mission, our excellent science and our strong sense of purpose guides us in all that we do, and we approach each day with the knowledge that our work h... View full profile
Clinlogix provides the clinical, operational, regulatory, and niche therapeutic expertise — along with the passion, innovative spirit, and intellectual drive — that you need to fulfill the promise of your product. As drugs, devices, diagnostics, and... View full profile
Cmed specializes in complex disease areas, particularly oncology, immuno-oncology, cell therapy and other specialty therapeutics areas.Clinical trials in oncology, especially involving cell & gene therapy (e.g. CAR T cells) are increasingly compl... View full profile
CrownBio brings clarity to drug discovery around the world by helping biopharmaceutical companies solve some of today's most pressing challenges in oncology, inflammation, cardiovascular, and metabolic disease Drug Discovery. Our premier Translationa... View full profile
Established in 1999, Cyprotex Limited was acquired by Evotec AG (www.evotec.com) in 2016. Cyprotex, an Evotec company, has sites at Alderley Park near Macclesfield, UK and in Watertown near Boston, USA. The company has dedicated and highly qualified... View full profile
Address: 603-7 St Thomas Street Toronto, ON M5S 2B7, Canadadicentra is a full-service Contract Research Organization (CRO) and professional consulting firm that specializes in addressing all matters related to safety, quality, and compliance for all... View full profile
Established in 1995, Dokumeds has been expanding its coverage and services significantly over 25 years of operations. Gradual geographical and operational coverage expansion correlated with company staff growth and lowering of employee turnover to an... View full profile
Founded in Prague in 2004, EastHORN Clinical Services is today one of the leading CROs in Europe. We operate in over 20 countries in the region with an experienced team comprised largely of physicians and PhDs. EastHORN’s client-base ranges from the... View full profile
In clinical research, every detail matters. As trial complexity increases, new risks emerge. Missteps and inaccurate data can mean more expensive trials and delays in bringing life-saving treatments to the patients who need them most. We help you spo... View full profile
FGK Clinical Research provides full service for clinical studies to biotechnology, medical device and pharmaceutical companies. FGK has the right size to handle international multi-center studies with hundreds of patients or single country studies wi... View full profile
As a provider of comprehensive Phase I through IV clinical trial management, clinical pharmacology, patient access solutions and other enabling services, Fortrea partners with emerging and large biopharma and medical device and diagnostic companies t... View full profile
- New Zealand
FMD K&L is a global contract research organization supporting data management, biostatistics, statistical programming, clinical operations, regulatory affairs, safety, pharmacovigilance, toxicology, medical affairs, medical writing, quality, risk... View full profile
GreenLight Clinical is internationally recognised by sponsors and investigators as a Physician-led CRO of choice in Ophthalmic & Early-Phase Research. As a full-service CRO, we help customers bring innovative treatments and medical devices to mar... View full profile
Since our foundation in Dublin, Ireland in 1990, our mission has been to help our clients to accelerate the development of drugs and devices that save lives and improve quality of life. We are a global provider of consulting, and outsourced developme... View full profile
iNGENū is the FDA-centric Australian CRO championing disruptive, innovative biotech firms globally. Our core mission is to create access to high quality clinical research globally, for early to mid-stage biotechs by removing financial and other unnec... View full profile
IMS Health and Quintiles are now IQVIA, a world leader in using data, technology, advanced analytics and expertise to help customers drive healthcare - and human health - forward. Together with the companies we serve, we are enabling a more modern, m... View full profile
- Bosnia & Herzegovina
LINK Medical is a leading CRO and Regulatory service provider offering a wide range of experts, flexible services, and innovative technologies for the pharmaceutical and medical device industries across Northern Europe and beyond. With our transparent... View full profile
MAC Clinical Research is one of Europe’s largest contract research organisations (CRO), committed to providing full-service delivery of global clinical studies. Headquartered in the UK with offices globally, we conduct studies both through our fully-... View full profile
Our unique global partnering philosophy emphasizes an uncompromising commitment to clinical research and to the highest level of ethical standards and performance in our jobs. We are selective about the projects we engage in because we are devoted to... View full profile
NDA is a world leading drug development consultancy with a dedicated team of over 150 consultants supported by an expert network and a specialist Advisory Board. Our goal is to streamline drug development in order to accelerate patient access to impo... View full profile
OPIS was founded in 1998 as a small Italian CRO. Over the past 20 years we have worked with passion and dedication to conquer the trust of clients and the scientific community, learning from each project and challenge convinced that investing in know... View full profile
For over 35 years, PAREXEL has proven to be a trusted partner for the complex development journey required of biopharmaceutical and medical device companies. We’re also an astute guide, able to simplify that journey for our clients, so safe new produ... View full profile
Pivotal was born back in 2001 on the principle that strategic scientific and medical advice and support should be the backbone of all clinical trials and with the objective of becoming a reliable full-service CRO with proper and sufficient resources... View full profile
PPD is a leading global contract research organization providing comprehensive, integrated drug development, laboratory and lifecycle management services. Our clients and partners include pharmaceutical, biotechnology, medical device, academic and go... View full profile
PrimeVigilance, an Ergomed Group company, was established in 2008 by Dr. Miroslav Reljanovic together with co-founder Dr. Elliot Brown. PrimeVigilance offers holistic, top quality, cost-effective, innovative safety services for pharmaceutical, generic... View full profile
ProPharma Group partners with life science companies to solve their complex challenges. As an extension of your team, we care about not only the progression of your products through the development lifecycle, but also the safety of your products and... View full profile
Our mission is to be the best CRO in the world as measured by our employees, clients, investigators, and vendors. Our teams work tirelessly to ensure that we deliver on time and on budget. You will always know what's going on with your study when you... View full profile
With a data-driven approach and harnessing the power of AI, we support the optimization of clinical trials from design through delivery. In addition to end-to-end CRO services and supported by a well-developed local network, deep regulatory expertise... View full profile
Quanticate is a leading global data-focused Clinical Research Organization (CRO) which may also be known as a 'Biometric CRO'. Quanticate is primarily focused on the management, analysis and reporting of data from clinical trials and post-marketing s... View full profile
Rede Optimus is a medical device CRO with a global outreach to USA, EU and Brazil. Rede Optimus was founded by a group of physicians dedicated to the medical device field which focusses on collaborative research and traditional CRO work. With our fo... View full profile
RTI Health Solutions is the place you turn when you need substantiated, authoritative evidence and advice. We are scientists first—academically-trained, credentialed researchers who know how and when to apply the appropriate research methodologies to... View full profile
SanaClis is a full-service Global CRO with a fully integrated clinical supply chain, thereby offering a comprehensive range of end-to-end services for clinical trials. SanaClis was founded in 2000 by seasoned industry experts, all of whom have had ex... View full profile
Life sciences services from SGS – optimize your development timelines to get medicines and medical devices to market quickly and safely. There is no other area of business that is more heavily regulated than the development, testing and distribution... View full profile
- Burkina Faso
- Congo, DR of
- Cote d'Ivoire
- Dominican Republic
- El Salvador
- Equatorial Guinea
- Papua New Guinea
- Saint Lucia
- Saudi Arabia
- Trinidad & Tobago
- Turkmenistan
The best technology succeeds in the background. Signant Health provides solutions that simplify every step of the patient journey to make it easier for people to participate in, and for sites and study teams to run, clinical trials. Signant unites eC... View full profile
Simbec-Orion was created in 2014 by the merger of two very experienced CROs: Simbec Research, a specialist CRO in Early Stage Clinical Development and Orion Clinical, the specialist CRO in Late Stage Clinical Development to create an international, f... View full profile
The Smerud Medical Research Group (SMERUD) is a full-service clinical Contract Research Organisation operating in the Northern European area with head office in Norway and subsidiary offices in Denmark, Finland, Sweden, United Kingdom, Germany, Austr... View full profile
Syneos Health (Nasdaq:SYNH) is the only fully integrated biopharmaceutical solutions organization purpose-built to accelerate customer success. We lead with a product development mindset, strategically blending clinical development, medical affairs a... View full profile
Synexus is a company dedicated to conducting clinical studies and have been investigating the effectiveness of new medicines and treatments for more than 20 years. We provide a friendly, relaxed environment where you have the chance to help shape the... View full profile
TAKE Solutions delivers domain-intensive services in Life Sciences and Supply Chain Management. In the fast-growing Life Sciences space, TAKE offers clients a unique combination of full-service Clinical, Regulatory and Safety services backed by uniqu... View full profile
Venn Life Sciences is an Integrated Drug Development Partner offering a unique combination of drug development expertise, clinical trial design and execution services. This enables us to create, plan and execute drug and medical device development pr... View full profile
Worldwide is the first customer-centric CRO. Founded by physicians dedicated to advancing medical science and built on an unwavering commitment to operational excellence, we are able to strategically balance science, medicine, operations, and commerc... View full profile
ZEINCRO is a contract research organization (CRO) that provides its services to more than 21 countries in Europe, with special focus in the region of Central- and South-Eastern Europe. Zeincro was established in 1998 and currently is positioned as a... View full profile
List of CROs by location

UCL Division of Medicine
- Amyloidosis
- Clinical Kidney Disease
- Computational Medicine
- Dialysis and Physiology
- Experimental Inflammation
- Experimental Nephrology
- Genetics and Genomics
- Human Health and Performance
- Immunosenescence and Ageing
- Intensive Care Medicine
- Liver and Pancreaticobiliary Cancer
- Metabolism and Inflammation
- Obesity and Metabolism
- Precision Healthcare
- Radiochemistry
- Rheumatology
- Tissue Engineering
- Tissue Repair
- Transplantation
- Urological Biology

Chronic kidney disease progresses faster in hot countries
Chronic kidney disease patients living in the hottest countries experienced an additional 8% drop in kidney function each year compared to those living in temperate climates, finds a new study.
Treatments for rare diseases are needed to beat kidney failure
Focusing on rare conditions could reduce the burden of kidney disease on both patients and the NHS, according to a new study led by UCL and the UK Kidney Association.
Patients with rare heart condition given lifeline
People diagnosed with a life-threatening cardiac condition have been given new hope, thanks to a ground-breaking new drug developed by UCL and the Royal Free Hospital.

New app helping liver disease patients abstain from alcohol
The new AlcoChange app which helps patients with alcohol-related liver disease to stay sober could help save hundreds of lives every year.
Immune cells could offer new avenues for treating respiratory diseases
Healthy lung development hinges on communication between immune cells and cells that line the airways, according to new research.
Advanced Biomedical Imaging (Dept. Imaging)
Amyloidosis (Dept. Inflammation & Rare Diseases)
Cirrhosis (Inst. Liver & Digestive Health)
Clinical Kidney Disease (Dept. Renal Medicine)
Computational Medicine (Dept. Imaging)
Dialysis and Physiology (Dept. Renal Medicine)
Experimental Inflammation (Ageing, Rheumatology & Regenerative Medicine)
Experimental Nephrology (Dept. Renal Medicine)
Fibrosis and Tissue Repair (Inst. Liver & Digestive Health)
Genetics and Genomics (Dept. Renal Medicine)
Human Health and Performance (Ageing, Rheumatology & Regenerative Medicine)
Immunosenescense and Ageing (Ageing, Rheumatology & Regenerative Medicine)
Intensive Care Medicine (Ageing, Rheumatology & Regenerative Medicine)
Liver and Pancreaticobiliary Cancer (Inst. Liver & Digestive Health)
Liver Tissue Engineering (Inst. Liver & Digestive Health)
Medical Imaging (Dept. Imaging)
Metabolism and Inflammation (Dept. Inflammation & Rare Diseases)
Nuclear Medicine (UCL Respiratory)
Obesity and Metabolism (Ageing, Rheumatology & Regenerative Medicine)
Precision Healthcare (Ageing, Rheumatology & Regenerative Medicine)
Radiochemistry (Dept. Imaging)
Rheumatology (Dept. Inflammation & Rare Diseases / ARR)
Transplantation (Dept. Renal Medicine)
Urological Biology (Dept. Renal Medicine)
Our research departments
Ageing, Rheumatology & Regenerative Medicine
Inflammation and Rare Diseases
Institute for Liver and Digestive Health
Renal Medicine
Respiratory Medicine
Funding opportunities.
Guidance on submitting funding applications for PIs (PDF)
Links to funding bodies (PDF)
Fellowship allocation tips (PDF)
Graduate funding (including Student Conference Fund)
UK Research and Innovation Example Interview Questions for Candidates (PDF)

Research Excellence Framework 2021 results
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

Our research in London

- 34,100 people are diagnosed with cancer each year.
- 50-59% of cancers are diagnosed early.
- We spent over £136m in 2021/22.
We receive no government funding for our research. Our life-saving work relies on the money you give us.
Last year, Cancer Research UK (CRUK) spent over £136m in London on cancer research to find better ways to prevent, diagnose and treat cancer, where 34,100 people are diagnosed with cancer annually.
We fund ground breaking work at the Francis Crick Institute , where researchers are looking into the fundamental biology underlying health and disease to uncover new ways to prevent, diagnose and treat illnesses.
We also fund two major research centres in London:
City of London Centre
Covergence Science Centre
Connecting research in London
Across the capital, Cancer Research UK is fostering relationships between researchers to tackle cancer from all angles, as well as funding initiatives throughout London to help beat cancer.
What we're doing now

Personalising cancer medicine
Professor Charlie Swanton at the Francis Crick Institute and Mariam Jamal-Hanjani of University College London are leading TRACERx , the largest genetic study to see how lung cancer evolves to help personalise treatment.
Investigating Barrett's oesophagus
Dr Stuart McDonald at Barts Cancer Institute , is investigating why some people develop oesophageal cancer from Barrett’s oesophagus . His team are looking at how the cells in the food pipe differ between patients with Barrett’s and those without, and he’s hoping to shed light on why some patients with Barrett’s go on to develop cancer and others don’t.
Accelerating immunotherapies
Dr Sara Ghorashian is bringing together the best minds from across the world to turbo-charge research into immunotherapies. By combining their expertise, this network of researchers hope to accelerate the development of new, more powerful immunotherapies.
Finding clues for rare cancers
Dr Richard Gilson is looking for molecular clues, known as biomarkers, to detect anal cancer at the early stages. His team will use their findings to develop a new screening tool that could be offered to those most at risk of developing the rare disease.

Investigating ROCK
Professor Victoria Sanz-Moreno is investigating a particular molecule called ROCK in melanoma. She is looking at how ROCK works as cancer grows, spreads and becomes resistant to treatments. This will suggest whether it is a good target for future melanoma treatments.

How we've made a difference so far
Scientists in london developed new cancer drugs.
Our scientists developed a molecule that later became the drug abiraterone , which can be used to extend the lives of men with prostate cancer. UK’s National Institute of Clinical Excellence (NICE), approved the drug in 2012. Our scientists also helped develop and trial the drug temozolomide, which can treat patients with glioblastoma brain tumours. NICE approved the drug in 2001 for treating patients whose disease has returned after standard treatment. In 2007, NICE then approved it as a treatment for newly diagnosed patients with glioblastoma.
Scientists in London Improved Cancer Screening
Our scientists at Imperial College London discovered an innovative way to screen for bowel cancer , which could reduce an individual’s chance of developing this disease by 33% through the removal of precancerous ‘polyps.’
Our scientists studied the impact of cervical screening as part of crucial work in the development of the UK national cervical screening programme , which saves thousands of lives each year.
Scientists in London Introduced a New Field of Cancer Research
Our scientists discovered what role the molecule EGFR plays in cancer development , overturning widely held ideas on the causes of cancer in the scientific community. This discovery also led to the development of several cancer drugs that are being used today to treat cancer patients.
Volunteer in London
The energy, passion and support of our volunteers is helping make sure that 3 in 4 people survive cancer by 2034. Join us and help beat cancer sooner.
Our strategy to beat cancer

For the past 120 years, we’ve been making discoveries that have saved countless lives. But we have so much more to do. Our strategy sets out how we'll accelerate progress towards a better future.
Read the latest cancer news from Cancer Research UK and around the world.
Humanity Age - Longevity analysis
This service goes beyond mere numbers, interpreting your blood markers in terms of your biological age. Understand the real implications of your results.
Discover if you're aging slower or faster and by how much. Gain actionable insights to manage your health and longevity effectively. Add this analysis to any compatible blood test you order.
Results same day as blood test results.
General Health
Unlock your full potential and take charge of your overall health now with our range of tests designed with your unique health needs in mind.
Fast delivery | Next day results
Fitness Health
Optimize your fitness health with our blood tests and unlock your full potential!
Sexual Health
Take control of your sexual health now with our range of confidential and comprehensive testing options.
Long-Term Health
Gain valuable insights into your long-term health with our DNA Genotype product, identify potential health risks and get access to your DNA data.
Don`t let tiredness hold you back.Take charge of your energy levels today!

How it works
Give your sample.
At home, or in one of our partner clinics.
Send us your sample
We provide pre-paid postage for your at home samples and an overnight courier service for phlebotomy samples.
Receive your results
We provide a secure link to your results and our doctor’s comprehensive report.
Which blood test is right for you?
It isn’t always easy to detect underlying health issues. Whether you have noticed changes in how you feel or are just curious about what’s happening inside your body, we have the test for you.
Why use London Medical Laboratory?
Concerned about your health.
If you’re concerned about fatigue, tiredness, or any other symptoms, a blood test is the most effective way of detecting underlying health issues.
Want to monitor a health condition?
If you’re concerned or living with a disorder or illness, a blood test can be an effective way to detect or monitor an ongoing health issue.
Want to lift your performance?
If you’re training for an event or keen to monitor your fitness progress, a blood test is a fantastic place to start.
Sample collection available at our partner clinics

Our team of experts
Our team of physicians is made up of some of the best in the field, including consultant pathologists, microbiologists, biochemists, haematologists and general practitioners who work together to provide you with a clear picture of your current health. High ethical standards and a commitment to excellence are at the heart of their work at London Medical Laboratory.
Experienced doctors
Certified laboratory, omnichannel nationwide convenience.

- Central Middlesex Hospital
- testimonials
- trial design
- subject recruitment
- clinical research
- project management
- data management
- medical writing
- computer systems validation (CSV) and IT
- quality services
- adaptive studies
- bioavailability and bioequivalence
- bridging trials
- drug-drug interactions
- first-in-human healthy volunteer trials – SAD & MAD
- first-in-human patient trials
- food effect
- phase 2 patient studies
- thorough QT trials
- analysis of drug concentrations
- microdose trials
- polysomnography
- precision ECG
- cardiovascular
- central nervous system
- dermatological
- gastrointestinal
- haematological
- respiratory
- biologicals and biosimilars
- radiolabelled
- small molecules
- publications
Hammersmith Medicines Research Cumberland Avenue London NW10 7EW
For enquiry 020 8961 4130
Working at HMR
Unlike other companies, for many of our graduate posts we don’t require experience of related work. That’s because we have a dedicated training team and an award-winning, university-accredited training programme. For many of our new starters, it’s their first scientific job. We seek out candidates with exceptional potential. Below are some examples of our entry-level posts. Even when we’re not actively recruiting, we’re happy to receive your CV. If it looks promising, we’ll keep it on file until we’re next recruiting, or for up to 3 months.
Work experience or work shadowing
We can’t currently offer unpaid experience or work shadowing.
Recruitment & Screening Associates recruit and screen volunteers for our clinical trials. Duties include maintaining a database of potential subjects, interviewing them on the telephone, liaising with GPs, and taking physiological measurements: blood pressure, ECG, etc. Recruitment & Screening Associates work office hours, Monday to Friday. Once trained in clinical procedures, they can work overtime on our wards in the evenings or at weekends if they wish.
We usually start new people on casual contracts, with the aim of making them permanent after 8–12 months. Recruitment staff can progress to Senior and Principal Recruitment & Screening Associates. Those with exceptional ability have progressed to managerial roles such as Deputy Team Leader, Team Leader or Head of Recruitment and Screening.
We require excellent communication skills and an ‘A’ level (or equivalent) in Biology, and ideally some experience of telephone working. A customer services background, or experience in a scientific or clinical environment, is useful, but we give full training.
Application details
We advertise in the Metro and Evening Standard, and on Indeed.
Clinical Trials Associates take physiological measurements and blood samples from participants in our clinical trials. We train staff to be ‘trial champions’ who take responsibility for running designated trials: ensuring that everything is in place before the trial starts, preparing work instructions, schedules and case record forms; liaising with sponsor representatives over trial progress; and ensuring that all the paperwork is complete when the trial ends. Clinical Trials Associates work on a rota including evening, night and weekend shifts. However, most ward activity occurs between 0800–1200 h.
Clinical Trials Associates are enrolled on our University Certificate in Clinical Pharmacology Practice, funded by us and run in partnership with the University of West London . We prefer to promote people from within the company rather than recruit externally, so some of our Clinical Trials Associates have progressed to become Data Managers, Biomedical Scientists, Quality Research Associates, or Clinical Project Managers. Others have developed within the ward teams to become Senior or Principal Clinical Trials Associates, or to take on managerial roles such as Deputy Team Leader or Team Leader.
First and foremost, we look for graduates with an excellent academic record, who have the potential to become exceptional Clinical Trials Associates. An aptitude for accurate paperwork and attention to detail are essential, so we look very carefully at the presentation of applicants’ CVs and their covering e-mails.
We’re most likely to advertise on Indeed or in the New Scientist.
Currently about 12% of our work comes from Japan. We specialise in ‘bridging trials’, where medicines are tested in Japanese subjects and the results compared with other ethnic groups. Therefore, we need fluent Japanese-speaking Recruitment & Screening Associates to recruit and screen healthy Japanese subjects, and Research Associates to monitor them when they’re resident on our wards, and to take blood samples and physiological measurements – blood pressure, ECG, etc. Japanese-speaking Recruitment & Screening Associates work office hours, Monday to Friday, whereas Research Associates work on a rota including evening, night and weekend shifts.
Both posts require fluency in both English and Japanese. Recruitment & Screening Associates are usually graduates (from any discipline) with experience of telephone work or health screening. Research Associates are usually nurses registered in Japan, or science graduates. As with nurses and technicians, we offer opportunities for promotion, to Senior Recruitment & Screening Associate, and to Senior or Principal Research Associate. There’s also the potential for managerial responsibility as Deputy Team Leader or Team Leader.
We advertise in the national press or on MixB.
There’s a lot of overlap between the Clinical Trials Associate and Research Nurse roles. Nurses also take on the role of ‘trial champion’ (see Clinical Trials Associate above). Nurses have additional responsibilities for the health and wellbeing of our trial subjects, and take the lead role in dosing. Nurses also lead our emergency scenarios, and carry a bleep as part of the resuscitation team. They take charge of our wards of dosed subjects, and care for unwell subjects when necessary. However, there’s much less nursing care required here than in a hospital environment, because subjects have to be healthy to take part in our trials. There’s a greater emphasis on data collection and scientific aspects of drug development. Research Nurses work on a rota including evening, night and weekend shifts. However, most ward activity occurs between 0800–1200 h.
Our nurses are enrolled on a CPPD course in Clinical Pharmacology Practice, run in partnership with the University of West London . The course is funded by us and can be used to meet PREPP requirements. Our nurses can progress to Senior Research Nurse or Clinical Nurse Specialist, or to take on managerial responsibilities such as Deputy Team Leader or Team Leader. Those with nurse specialisations such as asthma or endoscopy may become our ward experts in those areas. Nurses have also gone on to be Staff Training Manager, and to teach on the course.
Research Nurses must have adult nursing registration (RGN) with the NMC, and at least 1 year’s post-registration experience. We don’t require any previous research experience, as we give full training. When recruiting, we give preference to nurses with experience in an acute hospital setting, with hands-on experience of medical emergencies. Research Nurses must have an aptitude for paperwork, so we look carefully at the presentation of applicants’ CVs and their covering e-mails. Nurses also need good word-processing skills, and excellent attention to detail.
We advertise 2 or 3 times a year in the Nursing Times, RCN Bulletin or Nursing Standard.
Research Physicians are, when possible, involved in the whole trial process: discussions with trial sponsor (usually a pharmaceutical company), planning of trial, helping to set up physiological measurements, screening subjects, supervising the dosing sessions, looking after subjects during residence and as outpatients, following up subjects, and writing safety reports. Physicians with good writing skills can draft publications. But the most important requirement of the job is competence in clinical medicine, including standard procedures like IV cannulation. The physician’s work is a mixture of clinical work, science, and administration.
Physicians generally begin at 0730–0800 h and finish at 1600–1700 h, Monday to Friday, but also have to work evenings or weekends from time to time. In addition to the hours above, the job requires them to be resident in HMR for 1 weekday (1800–0800 h) most weeks when volunteers are on the ward. The rota is currently a 1 in 5, so each physician is resident on 1 weekday (Mon–Fri) each week. We have a ‘bank’ of Resident Medical Officers who cover most weekends.
A Research Physician post at HMR leads most naturally towards a career in either clinical pharmacology or pharmaceutical medicine. It’s an excellent introduction for anyone contemplating a career in the pharmaceutical industry, with which we have very close contact. Our physicians get a good overview of the potential new medicines produced by the international pharmaceutical industry. The job would also be very useful experience for someone planning to return to clinical medicine: it gives good experience in clinical pharmacology, and introduces the physician to the discipline of research. HMR offers a great introduction to quality systems and quality-focused activities. There’s a career path within HMR to Senior Research Physician or Clinical Pharmacologist.
Ideally, Research Physician candidates should have MRCP and 4 years’ post-qualification experience, but we also consider less experienced physicians with enthusiasm and willingness to learn. We offer support for study leading to the Diploma in Human Pharmacology, and for Specialty Training in Pharmaceutical Medicine. Suitable candidates receive training to become a Principal Investigator. Physicians must be ALS-registered, hold full GMC registration and a licence to practise, and have at least 3 years’ post-qualification experience.
We advertise in the BMJ.
Some Biomedical Science courses encourage students to do a year’s sandwich placement. Every year we take on 2 students in July and they work, full-time, through to August the following year. Students process samples generated by clinical trials, and help Biomedical Scientists to operate the analysers. They also assist with calibration and maintenance of laboratory equipment and with internal quality control procedures. Students do a project, which is written up as part of their course. Sometimes evening and weekend work is required – students are always supervised on those shifts.
Students must have scored highly in their exams and be able to provide excellent references from university tutors.
The ideal time to apply for Industrial Work Placements is in the spring (around March).
Biomedical Scientists analyse samples generated by clinical trials, and technically validate the results. They liaise with trial teams to determine sample requirements, and help to write trial-specific laboratory instructions. They also validate new methods, maintain and calibrate equipment, ensure strict adherence to internal quality control procedures, and prepare data for submission to external quality assurance programmes. Biomedical Scientists work on a rota of early (0800–1600 h), mid (1030–1800 h), and late (1300–2000 h) shifts. Weekend work is also required. Less frequently, trials might have procedures that require lab procedures during the night.
We have training status from the Institute of Biomedical Scientists (IBMS) to train medical laboratory assistants (MLA) to become HCPC-registered Biomedical Scientists. That route is open only to internal candidates – usually those who’ve worked on our wards as Research Technicians or who’ve done Industrial Work Placements here. Biomedical Scientists with managerial ability have been promoted to Senior Biomedical Scientist or Chief Biomedical Scientist.
We recruit externally for Biomedical Scientists from time to time. We require applicants with multidisciplinary experience, and clinical trials experience is an advantage.
We advertise in the IBMS Gazette.
Pharmacy Assistants and Technicians prepare, dispense, repackage, and relabel IMP (Investigational Medicinal Product) in our GMP-compliant pharmacy and radiopharmacy. Other duties include: receiving trial supplies; cleaning the aseptic unit and pharmacy equipment; ordering supplies; and preparing documents for pharmacy work. Pharmacy Assistants and Technicians work core hours of 0800–1600 h, Monday to Friday, but may be required to work early mornings, evenings or weekends depending on the requirements of specific trials.
The ideal candidate would have a degree in microbiology or pharmaceutical science and/or experience of aseptic handling. Experience of working with radiopharmaceuticals is an advantage. We advertise posts both internally and externally.
We advertise in the PJ.
Pharmacists direct and supervise the Pharmacy Assistants and Pharmacy Technicians responsible for production. They prepare IMP, including sterile dose forms and radiopharmaceuticals, and advise HMR and trial sponsors on pharmacy work. Their core hours are 0800–1600 h, Monday to Friday, but they may be required to work early mornings, evenings or weekends, depending on the requirements of specific trials.
The ideal candidate would have an in-depth understanding of pharmacy quality issues and Good Manufacturing Practice (GMP), and be experienced in preparation of a wide variety of dose forms, including sterile products for parenteral administration. Experience of working with radiopharmaceuticals is an advantage.
Clinical Project Managers are accountable for the management of administrative aspects of trials, from receipt of first enquiry to completion of report. They write and review trial protocols, and advise the sponsor on trial design, logistics and methodology. They write information and consent forms and Ethics Committee submissions, and prepare applications for Clinical Trial Authorisations and for ARSAC approval. They’re key contacts for liaison with pharmaceutical companies, other research laboratories and hospital departments. They also coordinate activities among the various departments at HMR. Clinical Project Managers work office hours, Monday to Friday, but it’s a demanding role which often spills into the evening.
There’s a career path to Senior Clinical Project Manager or Principal Scientist. Those with managerial ability may become Deputy Team Leader or Team Leader.
Competition for Clinical Project Manager posts is fierce. We ask for a PhD or high-calibre graduate, with exceptional writing skills and, ideally, experience of early drug development. However, enthusiasm and commitment are more important than experience. We ask candidates to provide an example of their scientific writing, and those who are shortlisted sit a 2-hour writing test. CVs and covering letters must be close to flawless to stand out.
We advertise in the New Scientist.
Data Managers support trials from CRF design to database lock (including database set-up activities) within agreed project timelines. They’re the point of contact for both internal and external customers for data management, and ensure that trial data are well-protected and reliable. Data Managers work office hours, Monday to Friday. When we’re very busy, evening or weekend work might be required.
Where possible, we recruit Trainee Data Managers from within the company. Experience of working on our wards, eg as a Research Technician, is invaluable for understanding the trial process and resolving data queries. There’s a career path from Trainee Data Manager to Data Manager, and on to Senior Data Manager. Exceptional staff have taken on managerial responsibility as Deputy Team Leader or Team Leader.
Sometimes, owing to short timeframes and expansion of our workload, we take on experienced external candidates. When recruiting Data Managers, we look for: extensive relevant data management experience within the pharmaceutical industry or a clinical research organisation, including experience in Phase I; experience of leading multiple trials at the same time, and acting as point of contact for both internal and external customers; good knowledge of the clinical development process and its critical paths; knowledge of ICH-GCP; strong communication skills; basic knowledge of CDISC and SDTM; basic knowledge of SAS (desirable but not essential); and a BSc or MSc in Computer Science, Statistics, Mathematics, Life Sciences or other subject with high scientific content.
We advertise on Indeed.
SAS Programmers program SDTM datasets and tables, figures and listings for clinical reports, and ad hoc datasets as required. They provide programming support to data management activities, and act as point of contact for both internal and external customers for allocated trials. They ensure that data are well protected and reliable. SAS Programmers work office hours, Monday to Friday. When we’re very busy, evening or weekend work might be required.
As with Data Managers, we recruit Trainee SAS Programmers, when we can, from inside the company. There’s a career path from Trainee SAS Programmer to SAS Programmer, and on to Senior or Principal SAS Programmer.
Sometimes, owing to short timeframes and expansion of our workload, we take on experienced external candidates. When recruiting SAS Programmers we look for: experience of handling multiple requests at the same time and acting as point of contact for both internal and external customers; good knowledge BASE SAS ,and ideally some knowledge of MACRO and STAT; experience of creating complex data sets from various sources, with a careful eye for outliers and errors; strong communication skills; experience of working with CDISC-compliant SDTM datasets; knowledge of ICH-GCP; and a BSc or MSc in Computer Science, Statistics, Mathematics, Life Sciences or other subject with high scientific content.
We are currently recruiting. Click Here
Quality Research Associates assist the Quality Manager in quality assurance activities. They help with internal audits, and control quality system documents and records of non-conformances. They train staff in QC, QA, EU Directive 2001/20/EC, and GCP. Quality Research Associates work office hours, Monday to Friday.
We prefer to recruit internally for these posts. Most Quality Research Associates start on our wards as Research Technicians. An understanding of our procedures and how trials are conducted is invaluable for auditing. There’s a career path to Senior Quality Research Associate. Quality Research Associates have progressed to become Quality Manager or Head of Quality Services.
The ideal candidate would be a life science graduate, with good communication skills and an eye for detail.
Applications aren’t open to external candidates.
Increasingly, we get requests for feedback on rejected applications. Unfortunately we can’t respond to every request. On this page, we’ve grouped information about what we look for, and the mistakes that applicants commonly make.
We receive hundreds of applications each year, so we’re most likely to respond positively to a well presented and well written covering letter, tailored to our vacancy.
Make every approach to a potential employer as professional as possible, including any covering e-mail. Don’t drop capital letters or punctuation. If the job advert doesn’t include a named contact, addressing your letter or e-mail ‘Dear sir or madam’ is fine. Attention to detail is important for most roles here, so re-read your application before sending it, to ensure that there are no mistakes.
We want to hear about your experience and achievements, and how they relate to the job you’ve applied for. We’re less interested in reading claims about personal qualities (‘a self-motivated, innovative thinker, who works as well individually as in a team’) or text cut and pasted from your current job description. Equally, we’d rather not see chunks of text copied from our own websites, telling us what we do.
If your current job is about to end, let us know. If you’re looking for a change in career direction, tell us why, eg if your degree/current job is laboratory-based and you’re applying to work on our wards. If you have ongoing trial commitments, tell us when they’ll finish, and what hours you’re available to work. If you’re working in the UK on a visa or require sponsorship, include details. If you live >1 hour away, tell us if you’re willing to relocate.
A covering letter of 1 page is perfect, with a standard font that’s easily readable (eg Times New Roman, 12). Write in plain English, and avoid embellishments such as ‘I trust that you will view my application favourably and permit me the opportunity of an interview with your esteemed organisation at your earliest convenience’. We prefer a 2- or 3-page CV, without a title page. However, for a professions such as nursing or medicine, or for those with lists of publications, the CV might need to be longer. Be aware that we check formatting of CVs to assess applicants’ word processing skills.
We like to see degree class and A-level grades on your CV – if you don’t include them, we might assume the worst. We search out graduates who’ve done weekend or summer jobs, so if you’ve worked in a shop or fast-food outlet, don’t miss that out of your CV. Account for all gaps in employment, eg tell us if you’ve taken time out to go travelling. If you’ve been president of a university society, arranged a charity event, played a musical instrument to a high grade, or been captain of a football team, let us know.
We prefer a first approach by e-mail rather than by telephone. Send attachments as Word documents or PDFs. Double-check that you’ve attached all your attachments before pressing Send.
E-mail applications to [email protected]
Privacy Overview
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Laboratory Coordinator
- Pathology and Cell Biology
- Columbia University Medical Center
- Opening on: Sep 14 2024
- Job Type: Officer of Administration
- Bargaining Unit:
- Regular/Temporary: Regular
- End Date if Temporary:
- Hours Per Week: 35
- Standard Work Schedule:
- Salary Range: $63,700- $80,000
- Hours Per Week:
Position Summary
The CUIMC Personalized Genomic Medicine has a position opening for a dynamic individual with significant understanding of molecular biology/technology who is able to work in a fast-paced high-volume environment. The individual will perform complex laboratory procedures in order to analyze clinical samples; this will require extensive, accurate, and efficient hands-on work. The Laboratory Coordinator will be operating, maintaining and troubleshooting complex instrumentation. Attention to detail, strict adherence to protocols, maintenance of extensive and accurate documentation is expected.
Duties will include:
1. Perform nucleic acid extraction, amplification, sequencing, Southern Blotting, RT-PCR and other technical tasks as needed. Compile, analyze and document procedures performed.
2. Operate, maintain, calibrate, and troubleshoot complex equipment. Follow-up and troubleshoot un-expected data, investigate non-conformities and notify supervisor/manager of instrument malfunctions or unexpected data.
3. Interact with physicians, nurses, and other healthcare professionals on issues related to testing performed by the laboratory.
4. Maintain accurate and extensive documentation in accordance with internal and external regulatory agencies.
5. Sample receiving, processing, data entry.
6. Maintain inventory, request orders as needed and verify receipt and storage.
7. Develop or assist in the development and validation of clinical assays and generate the corresponding documentation of recommendations and findings.
8. Provide technical guidance and instruction to students, and new personnel, as assigned.
9. Attend continuing education, safety and compliance training as necessary.
10. Maintain continuous and accurate communication with supervisor/manager on all pending and current issues.
Responsibilities
- Perform existing technical assays- 70%
- Validating new assays- 10%
- Troubleshooting technical problems- 10%
- Quality assurance, management, and improvement- 5%
- Perform other duties as assigned- 5%
Minimum Qualifications
- Requires a bachelor's degree or equivalent in education and experience, plus three years of related experience.
Preferred Qualifications
- Requires strong communication skills, both written and oral.
- Should be able to perform efficiently in a fast-paced environment and meet strict deadlines.
- Proficient in Microsoft Office and similar software programs.
- Experience with Southern Blotting, RT-PCR, microarray analysis and DNA sequencing and analysis.
- Master's degree in biology/ related field or a Bachelor's degree in biology/ related field plus 4 years of related experience.
Other Requirements
- The candidate must be Licensed or License eligible to practice as a Clinical Laboratory Technologist/Technician according to New York State licensure requirements. Continued employment will require obtaining a limited permit or a license within 3 months. Experience in morphological identification of tumor, light microscopy, histology and microdissection is preferred. Related experience in a clinical laboratory, or in a laboratory with a molecular or genomics focus is preferred. Should be available to attend out-of-lab discussions and presentations, and must work well in a group environment. Requires strong communication skills, both written and oral.
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Clinical Research Coordinator - Cardiology (CARE)
Research coordinator, clinical research coordinator.
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .

IMAGES
VIDEO
COMMENTS
London's Leading Clinical Research Organisation (CRO). We have carried out over 650 successful trials since 1993. ... Our Quality System is compliant with ISO 9001, our Laboratory with ISO 17025, and our Information Security Management System with ISO 27001. ... HMR is pleased to announce that it will take part in the Medical Research Council ...
The MRC Laboratory of Medical Sciences(LMS) is a biomedical research institute where scientists and clinicians collaborate to advance the understanding of biology and its application to medicine. The LMS is located on the Hammersmith Hospital campus of Imperial College London and is part of the Faculty of Medicine.
An accredited London-based contract laboratory, actively participating in proficiency testing schemes. We were accredited by the College of American Pathologists to their Laboratory Accreditation Program (LAP) from 2002 -2018 and gained UKAS accreditation to ISO17025 in 2018. For a full list of tests accredited by UKAS refer to 8968Testing ...
The Medical Research Council (MRC) Laboratory of Medical Sciences (MRC LMS), one of MRC's institutes, is committed to undertaking high quality fundamental research that underpins human disease that is long-term in scope, multidisciplinary and collaborative in nature, and creative by design. The scientific strategy of the Institute relies on ...
The MRC Laboratory of Molecular Biology (LMB) is a research institute dedicated to the understanding of important biological processes at the levels of atoms, molecules, cells and organisms. In doing so, we provide knowledge needed to solve key problems in human health. Our scientists tackle fundamental, often difficult and long-term research ...
The MRC Laboratory of Medical Sciences (LMS) is a thriving biomedical research environment located within the heart of Hammersmith Hospital in partnership with Imperial College at its White City campus. The LMS forms a key component of the College's Institute of Clinical Sciences, and is a core-funded institute of the Medical Research Council.
Lawson Health Research Institute has been driving innovation and helping to transform health care. Taking a "bench to bedside" approach to medical research, researchers focus their efforts on the development of new knowledge that can be applied directly to patient care. The fast pace of scientific discovery makes medical research an ...
Welcome to London Trials, the premier destination for paid clinical trials in the United Kingdom. At Hammersmith Medicines Research (HMR), we're dedicated to advancing medical research and improving human health. And we need your help to do it. Help shape the future of medicine and earn money at the same time
Services. HMR's medical laboratory in London, UK, offers a full range of tests and services, at competitive prices with fast turnaround times. We have a well established team of Biomedical Scientists who are experienced, have Health & Care Professions Council (HCPC) registration and participate in the Institute of Biomedical Scientists (IBMS ...
Hammersmith Medicines Research. Cumberland Avenue. London. NW10 7EW. Telephone: 020 8961 4130. Fax: 020 8961 8665. Email: [email protected]. Click here to download detailed directions. Hammersmith Medicines Research Ltd, number 02779946, is registered in England and Wales at Cumberland Avenue, London NW10 7EW.
Our Medical Director has nearly 30 years' experience of clinical trials. All our ward staff are enrolled in a unique, university-accredited, training programme, which lasts 18 months, and for which we received a National Training Award. Since 1993, more than 13,000 volunteers have done a trial here. Many come back to repeat the experience.
The London BioScience Innovation Centre, 2 Royal College Street, London, England, NW1 0NH, United Kingdom +44 (0) 20 7691 1122 [email protected] LBIC is owned and operated by the Royal Veterinary College , one of the independent colleges of the University of London and a world-class veterinary and biomedical research institute.
A-Z research labs; Platforms & facilities; A-Z researchers; Research topics and interest groups; ... We're one of Europe's biggest labs and home to more than 1,500 scientists working to understand health, disease and how life works. ... London NW1 1AT +44 (0)20 3796 0000. Send us a message . Quick Links. Media & press;
Wendy joined HMR as a Research Nurse in 1999. She was promoted to Deputy Team Leader in 2001, and to Team Leader in 2002. In 2017, Wendy had her first stint as acting Ward Manager, and in 2018, she became Clinical Nurse Manager. As our lead nurse, she was given special responsibility for patient trials.
The Delas Lab studies cis-regulatory control of development. The LMCB is a hub for cell and tissue biology research in the UK and beyond. Our mission is to develop new understandings of cell and tissue function through interdisciplinary, discovery-based research and technology development. Director | Alison Lloyd.
MRC Human Genetics Unit at the University of Edinburgh. MRC Mitochondrial Biology Unit at the University of Cambridge. MRC Molecular Haematology Unit at the University of Oxford. MRC Prion Unit at University College London. MRC Protein Phosphorylation and Ubiquitylation Unit at the University of Dundee. MRC Toxicology Unit at the University of ...
Global Contract Research Organizations in United Kingdom. 54gene's Clinical Programs Group (CPG) Website: www.54gene.com Email: [email protected] Phone: +971 58 594 9926; +1 310-266-9926. 54gene's Clinical Programs Group (CPG) is a contract research organization that provides innovative, customizable, and cost-effective clinical trials ...
Top 10 Best Diagnostic Services Near London, London - With Real Reviews. 1. Queen Mary BioEnterprise Innovation Centre. "Professional and friendly staff in a new building. They have a lot of volunteers doing what they call "flu camp" which is effectively medical research…" more. 2. InHealth Diagnostic Centre Stratford. 3.
Check our corporate positioning in the medical field, our mission, values and how we deal with privacy and security matters. ... Hammersmith Medicines Research Cumberland Avenue Park Royal London NW10 7EW. [email protected] . ... Our laboratory must, by law, report all the results of COVID-19 tests to Public Health England (PHE), along with ...
Research Excellence Framework 2021 results. The Division of Medicine undertakes world-leading basic and translational research that impacts upon understanding, diagnosis and treatment of human disease. Our aim is to understand the basis of disease, and to develop better diagnostics and treatments for diseases with an emphasis on experimental ...
50-59% of cancers are diagnosed early. We spent over £136m in 2021/22. We receive no government funding for our research. Our life-saving work relies on the money you give us. Last year, Cancer Research UK (CRUK) spent over £136m in London on cancer research to find better ways to prevent, diagnose and treat cancer, where 34,100 people are ...
NMRC Home. The Naval Submarine Medical Research Laboratory (NSMRL) delivers evidence-based solutions that enhance undersea warfighter health and performance. Located at the Submarine Base New London, NSMRL researchers have local access to Submarine Squadrons 4 and 12, the Naval Submarine School, the Naval Submarine Support Center, the Naval ...
London Medical Laboratory is registered with and regulated by the Care Quality Commission. Certificate number: CRT1-4708702735 London Medical Laboratory is accredited in accordance with International Standard ISO 15189:2012 ...
In addition to the hours above, the job requires them to be resident in HMR for 1 weekday (1800-0800 h) most weeks when volunteers are on the ward. The rota is currently a 1 in 5, so each physician is resident on 1 weekday (Mon-Fri) each week. We have a 'bank' of Resident Medical Officers who cover most weekends.
Grade 103 544916 Columbia University Medical Center New York United States Columbia University Medical Center Medicine Research (Lab and Non-Lab) Full Time. The Clinical Research Coordinator provides research coordination support for multiple clinical research projects. The primary focus of this role is to assist with the coordination of ...