- Diabetes & Primary Care
- Vol:25 | No:02

Interactive case study: Gestational diabetes
- 10 May 2023
Share this article + Add to reading list – Remove from reading list ↓ Download pdf

Diabetes & Primary Care ’s series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes.
These two cases provide an overview of gestational diabetes (GDM). The scenarios cover the screening, identification and management of GDM, as well as the steps that should be taken to screen for, and ideally prevent, development of type 2 diabetes in the long term post-pregnancy.
The format uses typical clinical scenarios as tools for learning. Information is provided in short sections, with most ending in a question to answer before moving on to the next section.
Working through the case studies will improve our knowledge and problem-solving skills in diabetes care by encouraging us to make evidence-based decisions in the context of individual cases.
Readers are invited to respond to the questions by typing in their answers. In this way, we are actively involved in the learning process, which is hopefully a much more effective way to learn.
By actively engaging with these case histories, I hope you will feel more confident and empowered to manage such presentations effectively in the future.
Holly is a 31-year-old lady who is now 26 weeks into her first pregnancy. She sees you with a 3-day history of dysuria and frequency of micturition. There is no history of abdominal pain or fever.
A urine dipstick reveals a positive test for nitrites and the presence of white cells. It also shows glycosuria ++.
What is your assessment of Holly’s situation?
Nadia is a 34-year-old lady of Indian ethnic origin who is now 24 weeks into her second pregnancy, her last pregnancy being 7 years ago. Nadia’s BMI is 32.4 kg/m 2 and her father has type 2 diabetes. GDM was not, however, diagnosed during her first pregnancy and her first baby was born at term weighing 3.8 kg.
How would you assess Nadia’s risk of acquiring gestational diabetes?
By working through this interactive case study, we will consider the following issues and more:
- The risk factors for developing gestational diabetes.
- Investigations and how to interpret them.
- Effects of gestational diabetes on outcomes for the mother and offspring.
- Which treatments for diabetes are considered safe and effective in gestational diabetes.
- What arrangements should be set in place for future screening of diabetes post-pregnancy.
Click here to access the case study .
Semaglutide effective treatment for HFpEF in people with type 2 diabetes
Select trial: further analysis shows preventative effects of semaglutide on type 2 diabetes development, pcds national conference 2024: request for poster abstracts, pcds news: obesity survey results, diabetes distilled: keeping kidneys flowing – semaglutide improves renal outcomes, at a glance factsheet: intermittent fasting for the management of weight and diabetes, diabetes distilled: deep dive into diabetes and infection.

ADA 2024: Semaglutide improves HFpEF-related symptoms and physical function in people with type 2 diabetes.
27 Jun 2024
ADA 2024: Semaglutide reduces risk of developing type 2 diabetes and increases likelihood of reverting to normoglycaemia in those with prediabetes.
26 Jun 2024

Poster abstract submissions are invited for the 20th National Conference of the PCDS, which will be held on 6 and 7 November.
25 Jun 2024

Key insights from the PCDS survey on obesity management, conducted in April 2024.
Sign up to all DiabetesontheNet journals
- CPD Learning
- Journal of Diabetes Nursing
- Diabetes Care for Children & Young People
- The Diabetic Foot Journal
- Diabetes Digest
Useful information
- Terms and conditions
- Privacy policy
- Editorial policies and ethics

By clicking ‘Subscribe’, you are agreeing that DiabetesontheNet.com are able to email you periodic newsletters. You may unsubscribe from these at any time. Your info is safe with us and we will never sell or trade your details. For information please review our Privacy Policy .
Are you a healthcare professional? This website is for healthcare professionals only. To continue, please confirm that you are a healthcare professional below.
We use cookies responsibly to ensure that we give you the best experience on our website. If you continue without changing your browser settings, we’ll assume that you are happy to receive all cookies on this website. Read about how we use cookies .

- Previous Article
- Next Article
Case Presentation
Clinical pearls, article information, case report: managing pregnancy with type 1 diabetes using a do-it-yourself artificial pancreas system.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Apoorva Ravindranath Waikar , Tanima Arora , Meagan Haynes , William V. Tamborlane , Laura M. Nally; Case Report: Managing Pregnancy With Type 1 Diabetes Using a Do-It-Yourself Artificial Pancreas System. Clin Diabetes 1 October 2021; 39 (4): 441–444. https://doi.org/10.2337/cd20-0128
Download citation file:
- Ris (Zotero)
- Reference Manager
The stakes are high when pregnancies are complicated by type 1 diabetes. In addition to the maternal risks, there are numerous fetal complications that can result from diabetes, including spontaneous abortion, fetal anomalies, neonatal birth trauma, macrosomia, neonatal hypoglycemia, hyperbilirubinemia, and fetal demise ( 1 ). Consequently, the American Diabetes Association (ADA) recommends stringent glycemic targets in pregnant women with type 1 diabetes to prevent adverse maternal and fetal outcomes.
Recommendations include self-monitoring of both fasting and postprandial blood glucose and a target A1C of <6% without significant hypoglycemia. Preprandial glucose testing is also recommended in women with pregestational diabetes using insulin pumps or basal-bolus therapy so that premeal rapid-acting insulin doses can be adjusted ( 2 ). The following individual glucose targets are recommended for type 1 diabetes: fasting glucose <90 mg/dL, 1-hour postprandial glucose <130–140 mg/dL, and 2-hour postprandial glucose <120 mg/dL ( 2 ).
These strict glycemic targets are difficult to achieve, especially given the insulin resistance commonly observed during the second and third trimesters of pregnancy. Indeed, women with gestational and pregestational diabetes have average A1C levels ranging from 7.25 to 8.15%, values that are considerably higher than ADA targets during pregnancy ( 3 ). Despite the availability of continuous glucose monitoring (CGM) systems since 1999, none have been approved by the U.S. Food and Drug Administration (FDA) for use in pregnancy. Moreover, no insulin pumps or hybrid-closed loop (HCL) automated insulin delivery systems have been approved for use during pregnancy. The lack of FDA-approved diabetes management technologies poses a significant limitation to pregnant women with type 1 diabetes.
Individuals in the type 1 diabetes community have developed open-source software and hardware to create a do-it-yourself artificial pancreas system (DIY APS) that allows for automated insulin dose adjustments. These systems include algorithms that, when coupled with an insulin pump and CGM system, mimic pancreatic β-cell function ( 4 ). A DIY APS requires a processor capable of receiving insulin pump and CGM data and an algorithm to control the rate of insulin delivery using a compatible insulin pump ( 5 ). With these systems, many patients with type 1 diabetes have reported improvements in their quality of life, more time in the target glucose range, better sleep, less frequent and less severe hypoglycemia, lower A1C, greater confidence in diabetes management, more energy, and fewer mood swings ( 3 ).
The use of an innovative and patient-developedDIY APS during pregnancy has not been reported previously. Herein, we present a unique case of type 1 diabetes management during pregnancy in which a DIY APS where the “Loop” algorithm on an iPhone interface was used ( 5 ). The patient provided written informed consent for the publication of this case report.
This case involves a first pregnancy of a 30-year-old woman with type 1 diabetes since the age of 6 years. Before conception, she used a DIY APS with a Medtronic pump and Dexcom CGM system that allowed for her to manage diabetes using the Loop application that she built and installed on her iPhone. Sensor glucose data, insulin doses, and carbohydrate intake were visible through the Loop application and Apple Health app. She had optimized her glycemic control before conception, with an A1C of 6.0%. During the month before conception, her overall mean sensor glucose was 134 mg/dL with a coefficient of variation (CV) of 41%, indicating healthy glycemic control. During this time, her CGM metrics indicated 77% of time in range (TIR; 70–180 mg/dL), 5% of time <70 mg/dL, and 17% of time >180 mg/dL.
During pregnancy, she noted an increase in her insulin requirements. Table 1 lists the insulin-to-carbohydrate ratios and insulin sensitivity factors she used during each trimester of pregnancy. Her A1C levels remained ≤5.7% throughout the pregnancy. She maintained optimal glycemic control, with 87% TIR in the third trimester. Details of her CGM metrics are listed in Table 2 . The patient was managed by the Maternal Fetal Medicine Clinic at Yale New Haven Hospital by a team of physicians, nurses, and diabetes educators knowledgeable in type 1 diabetes management.
A1C Levels and Insulin Doses at the End of Each Trimester of Pregnancy
| Parameters . | 1st Trimester (Weeks 1–12) . | 2nd Trimester (Weeks 13–26) . | 3rd Trimester (Weeks 27–40) . |
|---|---|---|---|
| A1C, % | 5.7 | 5.7 | 5.5 |
| Insulin-to-carbohydrate ratio | 1 unit to 13–14 g | 1 unit to 10–12 g | 1 unit to 8 g |
| Insulin sensitivity factor | 1 unit to 75–80 mg/dL | 1 unit to 65–85 mg/dL | 1 unit to 45–65 mg/dL |
| Total daily bolus insulin, units/day | 8.5 | 11.3 | 15.5 |
| Total daily basal insulin, units/day (%) | 17 (67) | 21 (65) | 32 (67) |
| Total daily insulin, units/day | 25.5 | 32.3 | 47.5 |
| Parameters . | 1st Trimester (Weeks 1–12) . | 2nd Trimester (Weeks 13–26) . | 3rd Trimester (Weeks 27–40) . |
|---|---|---|---|
| A1C, % | 5.7 | 5.7 | 5.5 |
| Insulin-to-carbohydrate ratio | 1 unit to 13–14 g | 1 unit to 10–12 g | 1 unit to 8 g |
| Insulin sensitivity factor | 1 unit to 75–80 mg/dL | 1 unit to 65–85 mg/dL | 1 unit to 45–65 mg/dL |
| Total daily bolus insulin, units/day | 8.5 | 11.3 | 15.5 |
| Total daily basal insulin, units/day (%) | 17 (67) | 21 (65) | 32 (67) |
| Total daily insulin, units/day | 25.5 | 32.3 | 47.5 |
Sensor Glucose Metrics During Each Trimester of Pregnancy Using Recommended Pregnancy-Specific Target Ranges ( 10 )
| Trimester . | Mean Sensor Glucose, mg/dL . | Sensor Glucose CV, % . | % Time <54 mg/dL . | % Time <63 mg/dL . | % Time in Range (64–140 mg/dL) . | % Time 141–250 mg/dL . | % Time >250 mg/dL . | Sensor Usage, % . |
|---|---|---|---|---|---|---|---|---|
| 1 | 127 ± 49 | 38 | 0.9 | 3.7 | 62.8 | 31.6 | 1.9 | 93 |
| 2 | 123 ± 46 | 37 | 2.1 | 4.6 | 66.0 | 27.8 | 1.6 | 96 |
| 3 | 112 ± 37 | 33 | 1.1 | 3.3 | 76.5 | 19.6 | 0.7 | 95 |
| Trimester . | Mean Sensor Glucose, mg/dL . | Sensor Glucose CV, % . | % Time <54 mg/dL . | % Time <63 mg/dL . | % Time in Range (64–140 mg/dL) . | % Time 141–250 mg/dL . | % Time >250 mg/dL . | Sensor Usage, % . |
|---|---|---|---|---|---|---|---|---|
| 1 | 127 ± 49 | 38 | 0.9 | 3.7 | 62.8 | 31.6 | 1.9 | 93 |
| 2 | 123 ± 46 | 37 | 2.1 | 4.6 | 66.0 | 27.8 | 1.6 | 96 |
| 3 | 112 ± 37 | 33 | 1.1 | 3.3 | 76.5 | 19.6 | 0.7 | 95 |
Sensor glucose metrics for each trimester of pregnancy are presented. Excellent sensor usage (>90%) occurred throughout the pregnancy. Of note, while pregnancy-specific targets are presented in the table, the overall time spent in the target range of 70–180 mg/dL through the entire pregnancy was 87%.
The patient presented for induction of labor at 39 weeks’ gestation and delivered a healthy female infant via cesarean section due to arrest of descent. The newborn weighed 2,910 g (16th percentile for age based on Centers for Disease Control and Prevention [CDC] growth charts) and measured 49.53 cm in length (54th percentile for age based on CDC growth charts), and thus her size was appropriate for gestational age.
The infant required temporary monitoring in the Neonatal Intensive Care Unit for hypoglycemia, which resolved within 24 hours of birth with a combination of breast milk and formula feeding. The infant was otherwise healthy and both mother and baby were discharged on the third day after delivery.
What are the challenges of type 1 diabetes management during pregnancy?
How can we incorporate the use of diabetes technology during pregnancy?
What are the advantages of using a DIY APS in pregnancy?
Although the increased risk of adverse pregnancy outcomes in women with type 1 diabetes is well established, FDA approval of the use of diabetes technologies in this population is lacking. The CONCEPTT (Continuous Glucose Monitoring in Women With Type 1 Diabetes in Pregnancy Trial) showed that use of real-time CGM during pregnancy in women with type 1 diabetes was associated with improved neonatal outcomes compared with using multiple fingerstick blood glucose measurements ( 6 ). However, in the United States, only 36% of the 214 pregnant or recently pregnant women who participated in the T1D Exchange clinic registry between 2010 and 2013 reported using CGM ( 7 ). Despite the potential benefits of CGM, the FDA has not approved the use of these devices in pregnancy. Further, newly developed HCL systems that have improved time in healthy glucose ranges in nonpregnant individuals with type 1 diabetes have not been approved for use during pregnancy. These currently available HCL systems do not allow for individuals to set pregnancy-specific targets, thus limiting options for insulin-dependent women who become pregnant. Further, glucose targets in the commercially available HCL systems are not customizable and are typically too high for pregnancy.
Although DIY APS technology has not been formally studied in pregnant women with diabetes, it provides the necessary flexibility in establishing individualized target ranges and automatic adjustments to basal rates when blood glucose levels are anticipated to rise above or fall below prespecified targets, thus helping users improve their TIR with less thought, less effort, and the potential for improved glycemic control and better sleep ( 8 , 9 ). The degree of individualization possible with this type of technology is greater than will be achievable with commercial systems in the near future. The benefits of DIY APS systems should be balanced with risks, including the lack of a formal body to address technical issues that may arise, making DIY APS users largely responsible for finding solutions through the online community, as well as potential issues with lack of support or knowledge from their diabetes provider.
Consensus recommendations state that pregnant women with type 1 diabetes should have >70% TIR (63–140 mg/dL), <25% of time above range (>140 mg/dL), and 5% of time below range (<4% of time <63 mg/dL and <1% of time <54 mg/dL) ( 10 ). Our patient was able to meet these targets during the third trimester and reported improved sleep and less diabetes-related anxiety throughout her pregnancy. Large-for-gestational-age infants are more commonly observed in mothers who have sustained hyperglycemia during pregnancy. Therefore, it is noteworthy that the opposite was true in this patient’s infant, whose weight was at the 16th percentile and length was at the 54th percentile. Normal weight in this baby suggests that maternal blood glucose levels were not substantially increased.
Using a DIY APS requires a high level of motivation and a strong understanding of diabetes technology. Those who choose to use this type of technology tend to be very actively engaged in and adherent to their diabetes management regimen. Hence, a DIY APS may not be appropriate for the general type 1 diabetes population. Nevertheless, our patient’s experience illustrates that these systems can be effectively used to meet and maintain safe glucose levels throughout pregnancy.
Pregnant women have the strictest glycemic targets of any group with diabetes, yet no CGM or insulin pump technology is FDA-approved for this population.
Some women with type 1 diabetes who become pregnant have chosen to use a DIY APS to achieve these targets because of the ability such systems afford to set individualized target glucose ranges and insulin dose settings with more ease and flexibility than commercially available insulin pump and HCL systems.
Enthusiasm for DIY APS technology continues to grow in the diabetes online community, and further studies are needed to support its use and facilitate more widespread acceptance.
Duality of Interest
W.V.T. is a consultant for Medtronic. L.M.N. has received funding from the National Institutes of Health and product support from Dexcom for investigator-initiated research. No other potential conflicts of interest relevant to this article were reported.
This work was supported by National Institutes of Health grant K12 DK094714 (W.V.T. principal investigator).
Author Contributions
A.R.W., T.A., and L.M.N. wrote the manuscript. M.H. cared for the patient during pregnancy and provided data for the case report. L.M.N. conceived the idea for the case report. W.V.T. and M.H. reviewed and edited the manuscript before submission. L.M.N. is the guarantor of this work and, as such, had full access to all the data and takes full responsibility for the integrity of data and the accuracy of data analysis.
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Obstetrics and Gynaecology
At a glance, fourth edition errol r. norwitz, john o. schorge.
- Multiple Choice
- Your feedback
- Become a reviewer
- More student books
- Student Apps
- Join an e-mail list

Find out more
Case Studies
Case 9: gestational diabetes.
A 28-year-old G 4 P 2 presents to your office for a routine prenatal visit at 24 weeks’ gestation. Her physical examination is unremarkable and fetal wellbeing is reassuring. You recommend testing for gestational diabetes mellitus (GDM).
1. What is GDM?
Show Answer
Correct answer: GDM refers to any form of glucose intolerance with the onset of pregnancy or first recognized during pregnancy, and complicates approximately 5% of all pregnancies. It likely includes some women who have undiagnosed pregestational diabetes.
2. Should everyone be screened for GDM? If so, at what gestational age should they be screened?
Correct answer: Patients with GDM are typically asymptomatic. There is a small cohort of pregnant women in whom routine screening for GDM is not cost-effective. These are women under age 25 who have normal body mass index (BMI 2 ), no first-degree relatives with diabetes, no risk factors (such as a history of GDM, insulin resistance/PCOS [polycystic ovarian syndrome], a prior macrosomic infant, a prior unexplained late fetal demise, and women with persistent glycosuria), and who are not members of ethnic or racial groups with a high prevalence of diabetes (such as Hispanic, Native American, Asian, or African–American). As such patients are rare, most experts and organizations recommend screening for GDM in all pregnant women. The ideal time to screen for GDM is 24–28 weeks of gestation. For women at high risk of developing GDM (listed above), early screening for GDM is recommended at the first prenatal visit. If the early screen is negative, it should be repeated at 24–28 weeks.
3. Her 1-hour GLT is 182 mg/dL. Does she have GDM?
Correct answer: The most common screening test for GDM is the glucose load test (GLT) – also known as the glucose challenge test (GCT) – which is a non-fasting 50-g oral glucose challenge followed by a venous plasma glucose measurement at 1 hour. Most authorities consider the GLT to be positive if the 1-hour glucose measurement is >140 mg/dL. Use of a lower cut-off (such as >130 mg/dL) will increase the detection rate of women with GDM, but will result in a substantial increase in the false-positive rate.
There is no GLT cut-off that should be regarded as diagnostic of GDM . A definitive diagnosis of GDM requires a 3-hour glucose tolerance test (GTT). In pregnancy, the GTT involves 3 days of carbohydrate loading followed by a 100-g oral glucose challenge after an overnight fast. Venous plasma glucose is measured fasting and at 1 hour, 2 hours, and 3 hours. Although there is agreement that two or more abnormal values are required to confirm the diagnosis, there is little consensus about the glucose values that define the upper range of normal in pregnancy (see below). Most institutions use the National Diabetes Data Group (NDDG) or Carpenter and Coustan cut-offs. Measurement of glycated hemoglobin (HbA1c) levels is not useful in making the diagnosis of GDM, although it may be useful in the diagnosis of pregestational diabetes.
Plasma glucose values (mg/dL) (mmol/L) *
Sacks et al.
Carpenter and Coustan
* Values in parentheses are mmol/L.
4. All four values of her 3-hour GTT are elevated and her fasting glucose level is 127 mg/dL. How would you manage her GDM? How long would you allow her to try dietary restriction before adding a hypoglycemic agent?
Correct answer: GDM poses little risk to the mother. Such women are not at risk of diabetic ketoacidosis (DKA), which is primarily a disease of absolute insulin deficiency. However, GDM has been associated with an increase in infant birth trauma and perinatal morbidity and mortality. The risk to the fetus/infant is directly related to its size. Fetal macrosomia is defined as an estimated fetal weight (not birthweight) of ≥4,500 g. It is a single cut-off that is unrelated to gestational age, the sex of the baby, or the presence or absence of diabetes, or to the actual birthweight.
The goal of antepartum treatment of GDM is to prevent fetal macrosomia and its resultant complications by maintaining maternal blood glucose at desirable levels throughout gestation, defined as a fasting glucose level 95 mg/dL, treatment can be started immediately because “you can’t diet more than fasting.”
Insulin (which has to be given several times a day by injection) remains the “gold standard” for the medical management of GDM. The use of oral hypoglycemic agents has traditionally been avoided in pregnancy because of concerns over fetal teratogenesis and prolonged neonatal hypoglycemia. However, recent studies suggest that second-generation hypoglycemic agents (glyburide, glipizide) do not cross the placenta, are safe in pregnancy, and can achieve adequate glycemic control in 85% of pregnancies complicated by GDM.
5. The estimated fetal weight at 38 weeks’ gestation is 4,600 g (10 lb 2 oz). She has had six prior uncomplicated vaginal deliveries. How would you counsel her about delivery?
Correct answer: As noted above, the complications of GDM are related primarily to fetal macrosomia, including an increased risk of cesarean section delivery, operative vaginal delivery, and birth injury to both the mother (vaginal, perineal, and rectal trauma) and fetus (including orthopedic and neurologic injury). Shoulder dystocia with resultant brachial plexus injury (Erb’s palsy) is a serious consequence of fetal macrosomia, and further increased in the setting of GDM because the macrosomia of diabetes is associated with increased diameters in the upper thorax of the fetus.
The use of elective cesarean section delivery to reduce the risk of maternal and fetal birth injury in the setting of fetal macrosomia remains controversial. According to current ACOG guidelines, an elective cesarean section delivery at or after 39 weeks’ gestation should be recommended for all non-diabetic women who have a fetus with an estimated fetal weight (EFW) ≥5,000 g (or ≥4,500 g in a diabetic individual) to minimize the risk of birth trauma. Furthermore, it is recommended that a discussion be held about the safest route of delivery with non-diabetic women who have a fetus with an EFW ≥4,500 g (or ≥4,000 g in a diabetic individual) and that this discussion be documented in the medical record.
6. After extensive counseling, the couple decline elective cesarean section delivery. She is now 38 weeks’ gestation. How should she be managed at this point in time?
Correct answer: If the patient declines elective cesarean section delivery, spontaneous labor should be awaited. Induction of labor for so-called “impending macrosomia” does not decrease the risk of cesarean section delivery or intrapartum complications, and is therefore not routinely recommended. If she is still undelivered at 41 weeks’ gestation, she should be counseled again about induction of labor and/or elective cesarean section.
During labor, maternal glucose levels should be maintained at 100–120 mg/dL to minimize the risk of intrapartum fetal hypoxic–ischemic injury. Continuous fetal monitoring is recommended throughout labor and the progress of labor should be carefully charted. Internal monitors such as an intrauterine pressure catheter (IUPC) and/or fetal scalp electrode can be used, if indicated. Neonatal blood glucose levels should be measured within 1 hour of birth and early feeding encouraged.
Delivery of the fetus and placenta effectively removes the source of the anti-insulin (counter-regulatory) hormones that cause GDM. As such, no further management is required in the immediate postpartum period. A 2-hour non-pregnant GTT should be performed at 6–8 weeks postpartum in all women with GDM to exclude pre-gestational diabetes.
See Chapter 45.
Print Answers | « Previous Case | Next Case »

- Advanced Search
Engaging Women with Gestational Diabetes Mellitus in the Design of Self-Management Apps
New citation alert added.
This alert has been successfully added and will be sent to:
You will be notified whenever a record that you have chosen has been cited.
To manage your alert preferences, click on the button below.
New Citation Alert!
Please log in to your account
Information & Contributors
Bibliometrics & citations, view options, index terms.
Applied computing
Life and medical sciences
Health care information systems
Human-centered computing
Interaction design
Interaction design process and methods
Participatory design
User centered design
Social and professional topics
User characteristics
People with disabilities
Recommendations
Incidence of risk factors on the onset of gestational diabetes mellitus: an empirical research in southern italy.
In this study the authors evaluated the prevalence of Gestational Diabetes Mellitus GDM and the incidence in determining the occurrence thereof, given by the following risk factors: age, family history for Diabetes Mellitus DM, Body Mass Index BMI, ...
Self-Management of Diabetes Mellitus with Remote Monitoring: A Retrospective Review of 214 Cases
Purpose: The efficacy of one remote monitoring system was reviewed in order to explore if optimal self-management of diabetes was achieved. Methods: Medical records of 214 patients with diabetes were reviewed from seven diabetes clinics within a single ...
An expert Personal Health System to monitor patients affected by Gestational Diabetes Mellitus: A feasibility study
Gestational Diabetes Mellitus (GDM) is a condition affecting 3–4% of pregnant women due to increased resistance to insulin caused by the growth of the fetus. Such a condition disappears just after delivery, but it is an indicator of the insurgence ...
Information
Published in.

IT University of Copenhagen
Malmö University
LISN-Université Paris Saclay
Northeastern University
- SIGCHI: ACM Special Interest Group on Computer-Human Interaction
Association for Computing Machinery
New York, NY, United States
Publication History
Check for updates, author tags.
- Gestational diabetes mellitus
- self-management
- Research-article
- Refereed limited
Acceptance Rates
Contributors, other metrics, bibliometrics, article metrics.
- 0 Total Citations
- 0 Total Downloads
- Downloads (Last 12 months) 0
- Downloads (Last 6 weeks) 0
View options
Login options.
Check if you have access through your login credentials or your institution to get full access on this article.
Full Access
Share this publication link.
Copying failed.
Share on social media
Affiliations, export citations.
- Please download or close your previous search result export first before starting a new bulk export. Preview is not available. By clicking download, a status dialog will open to start the export process. The process may take a few minutes but once it finishes a file will be downloadable from your browser. You may continue to browse the DL while the export process is in progress. Download
- Download citation
- Copy citation
We are preparing your search results for download ...
We will inform you here when the file is ready.
Your file of search results citations is now ready.
Your search export query has expired. Please try again.
- Open access
- Published: 28 June 2024
The potential causal effect of the pre-pregnancy dietary phytochemical index on gestational diabetes mellitus: a prospective cohort study
- Neda Heidarzadeh-Esfahani 1 ,
- Javad Heshmati 2 ,
- Reihaneh Pirjani 3 ,
- Ashraf Moini 3 , 4 ,
- Mehrnoosh shafaatdoost 5 ,
- Mahnaz Esmaeili 6 ,
- Azar Mardi-Mamaghani 7 ,
- Seyyed Mostafa Nachvak 1 &
- Mahdi Sepidarkish 8
BMC Pregnancy and Childbirth volume 24 , Article number: 447 ( 2024 ) Cite this article
Metrics details
Phytochemicals are non-nutritive bioactive compounds with beneficial effects on the metabolism of glucose. This study aimed to clarify the possible causal effect of the pre-pregnancy dietary phytochemical index (DPI) on gestational diabetes mellitus (GDM).
In this prospective cohort study 1,856 pregnant women aged 18–45 years who were in their first trimester, were recruited and followed up until delivery. The dietary intakes of participants were examined using an interviewer-administered validated 168-item semi-quantitative food frequency questionnaire (FFQ). Inverse probability weighting (IPW) of propensity scores (PS), estimated from the generalized boosted model (GBM) were used to obtain a adjusted risk ratio (aRR) for potential confounders.
During the follow-up period, 369 (19.88%) women were diagnosed with GDM. DPI scores ranged from 6.09 to 89.45. There was no association between DPI scores and GDM (aRR: 1.01, 95% confidence interval [CI]: 0.92, 1.08; p trend = 0.922). When comparing DPI quartile 4 (most pro-phytochemical content) to quartile 1 (few phytochemical contents), there was no significant difference between them (aRR: 0.97; 95% CI: 0.75, 1.25; p = 0.852). Also, there was no significant difference between DPI quartile 3 and quartile 1 (aRR: 1.04; 95% CI: 0.81, 1.34; p = 0.741) as well as DPI quartile 2 and quartile 1 (aRR: 0.92; 95% CI: 0.71, 1.21; p = 0.593).
Conclusions
Although this data did not support the association between pre-pregnancy DPI scores and GDM, further cohort studies to ascertain the causal association between them are warranted.
Peer Review reports
Women with GDM experience glucose intolerance in the second or third trimester of pregnancy without any clear manifest diabetes before pregnancy [ 1 ]. GDM prevalence was reported to range from 9.3 to 25.5% (average 17.8%) and the prevalence has been increasing worldwide [ 2 , 3 ]. GDM is associated with an increased risk for many short-term and long-term consequences for both mother and offspring, including obesity, impaired glucose metabolism, and cardiovascular disease [ 4 , 5 , 6 , 7 , 8 ]. Thus, it is important to come up invaluable approach for GDM prevention and management.
There is now substantial evidence that maternal dietary patterns before and during pregnancy prevent or delay the development of GDM [ 9 , 10 , 11 , 12 ] however, the focus has been on identifying crucial risk factors during pregnancy. Recently, an increasing interest has emerged in the worthwhile effects of plant-based dietary patterns and phytochemical plant-derive bioactive compounds for the management of GDM [ 13 , 14 ].
Phytochemicals are biologically non-nutritive bioactive compounds divided into several classes, including: Alkaloids, Glycosides, organosulfur compounds (thiosulfinate and isothiocyanates) phenolic compounds (flavonoids, phenolic acids, hydroxycinnamic acids, lignans, polyphenols, and stilbenoids), tannins, Terpenes, saponins, Anthraquinones, essential oils, and steroids [ 15 , 16 ].
The DPI, which was proposed and developed for the first time by McCarty, is determined according to the percent of daily energy intake derived from phytochemical-rich foods such as fruits, vegetables, legumes, whole grains, nuts, seeds, soy products, juices (fruit and vegetable), and other plant foods [ 17 ].
DPI have been inversely associated with risk of cardiovascular disease [ 18 , 19 ], insulin resistance [ 20 ], metabolic syndrome [ 21 ], and cancer [ 21 ]. Plausible mechanisms underlying causes of beneficial traits of phytochemicals on non-communicable diseases are antioxidant and anti-inflammatory effects, enhanced glycemic control, regulated body weight, improved insulin sensitivity, and gut microbiota [ 22 , 23 , 24 ].
Limited observational studies have investigated associations between DPI and the improvement of glucose tolerance and insulin sensitivity [ 20 , 25 ]. According to our review, there is no study presenting the association between DPI and GDM. Denoting the association between DPI and glycemic indices among affected women may provide the obvious starting point for GDM prevention and treatment. Hence, in the present study, we aimed to determine the possible causal effect of the pre-pregnancy DPI on GDM.
Study design and participants
We conducted a prospective cohort study - Mothers and their children’s health (MATCH) study at the Arash Women’s Hospital in Tehran, Iran between February 2020 and January 2023. The details of this study and further information on methods have been described previously [ 26 ]. The MATCH protocol was approved by the institutional review boards of the Tehran University of Medical Sciences (Protocol number: IR.TUMS.MEDICINE.REC.1398.576).
Briefly, the pregnant women aged from 18 to 45 years who were at less than 12 weeks of gestation, and attending antenatal care in Arash Women’s Hospital in Tehran were included between February 2020 and August 2021. Furthermore, women who reported a previous diagnosis of metabolic or chronic diseases, following a special diet, using certain food supplements (except for pregnancy supplements such as iron or folate), suffering from physical, mental, cognitive disability, and having an unusual total energy intake (< 800 or > 4200 kcal/day), were excluded from the current study. Total daily energy intake by summing up the calories from all food items were reported in 168-item semi-quantitative food frequency questionnaire (FFQ).
Data collection
Ten trained observers completed a structured questionnaire through face-to-face interviews to obtain sociodemographic, history of underlying disease, and lifestyle variables, including smoking, alcohol, dietary pattern, physical activity, and sleep quality pre-pregnancy and early pregnancy. The quantity and quality of physical activity was assessed by the International Physical Activity Questionnaire (IPAQ) using Metabolic Equivalent of Tasks (METs) [ 26 ]. Also, the anthropometric indices, including weight, height, waist, and hip circumferences were measured by our trained staff accurately.
Dietary intakes assessment
In the first visit, dietary intake was evaluated by using an interviewer-administrated 168-item FFQ that contains questions about the type/brand, cooking methods, frequency, and the amount of all foods and drinks they consumed during the one-year leading up to the pregnancy. The validity and reliability of FFQ were confirmed in the Tehran Lipid and Glucose Study (TLGS) in Iran [ 27 ]. For the FFQ data, portion sizes will be converted to grams per week per food item by two experienced nutritionists.
Exposure assessment
The DPI was determined based on the method developed by McCarty; [PI = (daily energy derived from phytochemical-rich foods (kcal)/total daily energy intake (kcal)) × 100] [ 17 ]. Fruits and vegetables (except potatoes), legumes, whole grains, nuts, soy products, olives, and olive oil were categorized into phytochemical-rich foods. Natural fruit and vegetable juices such as tomato sauces were included in the fruit and vegetable groups due to their high phytochemical content.
Outcome assessment
Screening and diagnosis of GDM were carried out according to the results of the one-step method which includes a fasting glucose test followed by a 75-gram, 2-hour diagnostic oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation. Using the one-step method, women were considered to have screened positive for GDM if they had a serum glucose value fasting ≥ 92 mg/dl, 1-hour ≥ 180 mg/dl, and 2-hour ≥ 153 mg/dl [ 28 ].
Statistical analysis
We presented continuous baseline characteristics as mean (± SD) or median (interquartile range, IQR) and compared using one-way analysis of variance and independent t-test. Also, we expressed categorical variables as numbers (percentages) and compared using Chi-square test. We used multiple imputations based on chained equations, which fill in missing values in multiple variables iteratively using a sequence of univariate imputation models with a fully conditional specification of prediction equations. We used the generalized boosted model (GBM) for the estimation of participants’ propensity scores for DPI, so that covariate imbalance between the exposed (quartiles 1–3 (Q1–Q3) for DPI) and non-exposed groups was minimized. We employed ‘TWANG’ package to estimate propensity scores using an automated, nonparametric machine learning method, and generalized boosted models based on 10,000 regression trees. We selected the minimal sufficient variables using directed acyclic graphs (DAGs), based on the web tool dagitty.net (Fig. 1 ) [ 29 ]. We evaluated the association between DPI and the incidence of GDM by calculating adjusted risk ratios (aRRs) and corresponding 95% confidence intervals based on the weighted modified Poisson regression with the inverse probability weight (IPTW). In addition, we stratified the analysis based on age to determine whether the risk of GDM affected by it. The data processing and statistical analysis were performed using the Stata statistical package version 17 (Stata Corp LP, College Station, TX, USA) and R statistical software (Version 4.2.1; The R Foundation for Statistical Computing, Vienna, Austria).
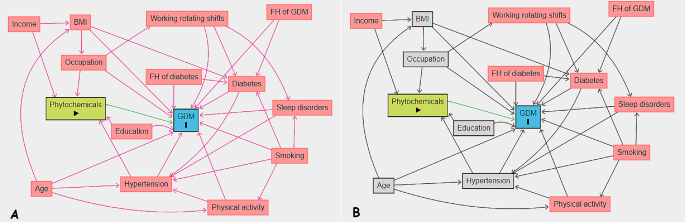
Directed acyclic graphs, A: Unadjusted, B: Adjusted. BMI, Body Mass Index; GDM, Gestational Diabetes Mellitus; FH, Family History
The flow diagram in Fig. 2 depicts the number of pregnant women examined at each time point, as well as those lost to follow-up and the reasons for dropout. A total of 3,285 women were enrolled from 1 February 2020 and January 2023. Based on initial screening, 2,103 women were eligible for inclusion in the study, of whom 1,856 had complete and 247 (11.74%) had incomplete follow-up data. We excluded 1,182 participants for the following reasons: (І) plan to deliver elsewhere ( n = 486, 41.11%); (Π) gestational age > 12 Weeks ( n = 328, 27.74%); (III) multiple pregnancies ( n = 125, 10.57%); (IV) metabolic or chronic diseases ( n = 114; 9.64%); (V) following a special diet ( n = 32; 2.70%); and (VI) declined ( n = 97, 8.20%) (Fig. 2 ).
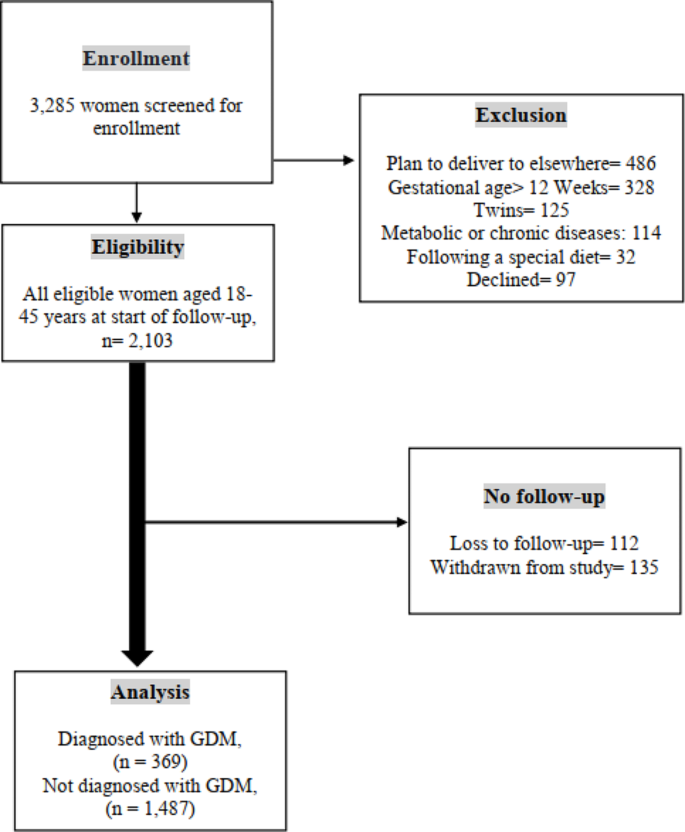
Flow Diagram of Study Participants. † Gestational Diabetes Mellitus; flow diagram showing participant recruitment from enrolment to corresponding numbers of women who were and were not diagnosed with gestational diabetes. Reasons and number of exclusions are stated accordingly
The demographic and clinical characteristics of women with and without GDM as well as across quartiles of DPI are presented in Tables 1 and 2 , respectively. At the study baseline, the mean age and BMI of the included women were 32.9 ± 6.1 years and 25.9 ± 8.3 kg/m2, respectively. 7.5% ( n = 149) of study participants were employed and 45.5% ( n = 845) had an academic education. During the follow-up period, 369 (19.88%) women were diagnosed with GDM. Women with GDM were older (34.8 ± 5.7 vs. 32.4 ± 5.9; p < 0.001), heavier (67.9 ± 13.1 vs. 65.2 ± 12.4; p < 0.001), more likely to be current smokers (90/369, 24.4% vs. 278/1,487, 18.7%; p = 0.140), pre-existing diabetes (83/369, 22.5% vs. 106/1,487, 7.1%; p < 0.001), and had a higher frequency of family history of diabetes than controls (174/369, 47.1% vs. 572/1,487, 38.5%; p = 0.002).
The DPI score of the women’s diet ranged from 6.1 to 89.4 with a median (IQR) of 40.3 (19.8). Also, the DPI score of women across quartile categories in the first, second and third quartiles was 30.9, 40.3, and 50.8, respectively. Pregnant women in the highest quartile had a higher frequency of pre-existing diabetes, GDM, and a family history of diabetes. Also, they had a higher pre-existing BMI and dietary caloric intake (kcal/day). The overall mean DIP in the women with and without GDM were 41.5 ± 13.6 and 41.1 ± 13.8, respectively ( p = 0.614).
We outlined the crude and multivariate-adjusted risk ratios (aRRs) for the association between DPI and GDM in Table 3 . We found no significant association between DPI and GDM in the crude model (crude RR: 1.03, 95% CI: 0.95, 1.12, p = 0.413). This association remained non-significant after adjustment for potential confounders, including body mass index (kg/m2), occupation, age, hypertension, education, and gastrointestinal diseases (aRR: 1.01, 95% CI: 0.92, 1.08, p = 0.922). The crude RR of GDM in a quartile with the highest DPI scores (Q4), compared to that with the lowest scores (Q1), was 1.06 (95% CI: 0.82, 1.38, p = 0.612). After additional adjustment for potential confounders, including body mass index (kg/m2), occupation, age, hypertension, education, and gastrointestinal diseases, associations were attenuated but remained non-significant (aRR: 0.97, 95% CI: 0.75, 1.25, p = 0.852).
In stratified analyses, we studied whether the effects of DFI on GDM could be modified by age. In these analyses, the main analyses showed similar results by age categories. We found no significant association between DPI and GDM in all age categories (Tables 4 , 5 and 6 ).
The purpose of the current study was to evaluate the causal effect of calorie intake from phytochemical-rich foods on GDM, using propensity score to minimize potential confounding factors. In this prospective study, after accounting for non-dietary covariates such as body mass index (kg/m2), occupation, age, hypertension, education, and gastrointestinal diseases, the association between the higher load of calorie intake from phytochemical-rich foods and occurrence of GDM was found to be nonsignificant after a 9-months follow-up in pregnant women.
To our knowledge, this work was the first study observed the association between DPI and GDM. However, some studies have examined the association between DPI and glucose homeostasis disruption which showed controversial results [ 20 , 25 , 30 , 31 , 32 , 33 ]. For instance, the finding of mentioned prospective study are aligned with some observational studies on DPI and hyperglycemia. In a cross-sectional investigation on 2,326 adults aged between 20 and 70 years which aimed to investigate the association between DPI and metabolic syndrome, no significant association was yielded between DPI and the prevalence of high serum FBS in crud and full adjustments model [ 33 ]. Firouzabadi et al. conducted a cross-sectional study reported no significant association between odds of hyperglycemia in men and women across quartiles of DPI in both crude model and after adjusting for age, energy intake, marital status, educational status, occupation physical activity, and smoking status [ 31 ].
In stark contrast, however, in the Tehran Lipid and Glucose cohort study across 1,141 participants with an average of three years of follow-up, showed considerably a reduction risk of insulin insensitivity (OR = 0.11, 95% CI: 0.05, 0.24), insulin resistance (OR = 0.48, 95% CI: 0.25, 0.93) and hyperinsulinmia (OR = 0.14, 95% CI: 0.07, 0.25) in higher quartiles of DPI after adjustment for non-dietary factors [ 20 ]. The potential protective impact of phytochemicals is attributed to their antioxidant properties, enhancement of beta cell function, promotion of insulin response, and reduction of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like polypeptide-1 (GLP-1) levels. These mechanisms are considered key in the pathophysiological effects of phytochemicals [ 20 ]. In agreement with this finding, Delshad Aghdam et al. found that the risk of hyperglycemia significantly decreased by 88% (OR = 0.12, 95% CI: 0.02, 0.82) after adjusted for age, sex, total energy intake (kcal/day), physical activity (MET/min/week), BMI (kg/m2), diabetes duration (year), total insulin dose (unit/day), education and dietary supplement intake in participants with T1DM in the highest tertile of DPI [ 30 ]. Moreover, the case-control study which denoted a high level of DPI score is related to a lower risk of prediabetes (OR = 0.09, 95% CI: 0.03, 0.25). Also this study showed individuals in the higher quartiles of DPI had significantly lower FBG and OGTT (p-trend < 0.001) [ 25 ]. In contrast, we did not observe any statistically significant association between DPI and OGTT in women with GDM.
In addition, in a case-control study with 210 diabetic women, a significant negative association of DPI with FBS ( p = 0.04) was observed in the case group with diabetic nephropathy [ 32 ].
The fact that studies are inconsistent might be due to differences in sample size, methodology, different dietary intake assessment, and eligibility criteria (most of them excluded pregnant women).
It is worth noting that in this study we used DPI which is practically useful to induce synergetic clinical functions of phytochemicals isolated from various types of foods and it can bring in its wake modulating physiologically [ 17 ]. By contrast, the majority of findings from prior studies can be drawn from certain phytochemicals and their effects on GDM.
The results of the present study are in line with the findings of a longitudinal cohort study conducted on pregnant with twins in China which indicated that no significant association was shown between the risk of GDM and vegetables and fruit-based pattern [ 34 ].
Our findings is in accordance with a recent meta-analysis of 12 epidemiological studies revealed that there was not any significant interplay between consuming polyphenol-rich fruits, seeds, and whole grains with GDM, nonetheless, the highest adherence to the Mediterranean diet (MedDiet) associated with lower risk of GDM [ 14 ]. Meanwhile, the inverse association of MedDiet with GDM has been appraised in another systematic review and meta-analysis of observational studies [ 35 ]. MedDiet is associated with better control of lipid and glycemic profiles [ 36 , 37 , 38 ], and eventually lower incident risk in type 2 diabetes [ 39 ]. As a matter of fact, the protection effect of MedDiet was mainly manifested via its content of poly and mono-unsaturated fatty acids by modulating inflammatory processes [ 40 ]. Moreover, the above-mentioned studies were mainly conducted in Western and American countries.
On the other hand, two prospective studies have presented the association between whole grain and the risk of GDM [ 41 , 42 ] but with inconsistent findings. In a prospective cohort study in China, a whole grain-sea food pattern was associated with an increased occurrence of GDM (OR = 1.73, 95% CI: 1.10, 2.74) because of environmental contaminants [ 42 ]. In stark contrast, however, in the PREWICE II cohort study, Tryggvadottir EA et al., reported a higher median concentration of total alkylresorcinols of plasma as a whole-grain consumption biomarker in pregnancies women plasma without GDM rather than women diagnosed with GDM (209 nmol/L vs. 163 nmol/L, respectively; p < 0.001) [ 41 ]. The possible mechanism might be that whole-grain diet contained fiber and phytochemical components increased gut health, and improved glycemic response [ 23 , 43 ].
Meanwhile, one study that has prospectively examined the correlation of fruit intake during pregnancy with GDM incidence, suggested that fresh fruit intake is inversely associated with the risk of GDM [ 44 ]. However, fruits were not been categorized in detail, which might result in misinformation about fruit type.
We are unaware of published prospective studies which assessed the DPI in relation to GDM. Pregnancy outcomes in relation to GDM adversely have imposed an immense burden on the global health system [ 45 ]. Hence, prevention and management of GDM should be getting as a high priority straight. This study is the largest to date to provide data that has investigated the correlation between DPI and GDM risk.
In this study, several strengths and limitations were present. There are no cohort studies have used propensity scores to evaluate the association between DPI and GDM which can preclude bias related to potential confounding variables. The propensity scoring implementation can control confounding by balancing covariates between exposed and non-exposed groups [ 46 ].
Moreover, strong recall bias may present through dietary assessment tool which assessed with FFQ. However, the use of a validated FFQ to collect dietary intake information, and standardized clinical assessments, as well as the prospective large sample size setting in this study can rule out mentioned bias. In this work, Iranian population with the same ethnicity diversity were recruited in order to limiting generalizability.
Inheritance limitation of DPI such as failing to add up non-caloric phytochemical-rich foods like green and black tea and spices should be considered. Furthermore, we failed to conduct dietary questionnaires during the early or pre-pregnancy which can interpret the relationship between DPI and GDM more clearly. Previous data reported that dietary patterns are not, however, varied during pregnancy [ 47 ].
In summary, according to our finding, this prospective cohort study among pregnant women suggest that the DPI has no impact on GDM. More research is needed to determine the exact association between DPI and GDM.
Data availability
The datasets used and analyzed during the present study are available from the corresponding author upon reasonable request.
Abbreviations
Confidence interval
Directed acyclic graphs
- Dietary phytochemical index
Food frequency questionnaires
- Gestational diabetes mellitus
Generalized boosted model
Inverse probability weighting
Inverse probability weight
Interquartile range
Propensity scores
Standard deviation
Tehran Lipid and Glucose Study
Association AD. 2. Classification and diagnosis of diabetes Diabetes care, 2017. 40(Supplement 1): pp. S11-S24.
Sacks DA, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel–recommended criteria: the hyperglycemia and adverse pregnancy outcome (HAPO) study. Diabetes Care. 2012;35(3):526–8.
Article PubMed PubMed Central Google Scholar
Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7.
Kamana K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Annals Nutr Metabolism. 2015;66(Suppl 2):14–20.
Google Scholar
Li J, et al. Increased risk of cardiovascular disease in women with prior gestational diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;140:324–38.
Article PubMed Google Scholar
Boerschmann H, et al. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care. 2010;33(8):1845–9.
Metzger BE, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002.
Bellamy L, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9.
Article CAS PubMed Google Scholar
Tobias DK, et al. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr. 2012;96(2):289–95.
Article CAS PubMed PubMed Central Google Scholar
Bowers K, et al. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr. 2012;95(2):446–53.
Izadi V, et al. Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition. 2016;32(10):1092–6.
He J-R, et al. Maternal dietary patterns and gestational diabetes mellitus: a large prospective cohort study in China. Br J Nutr. 2015;113(8):1292–300.
Chen Z, et al. Prepregnancy plant-based diets and the risk of gestational diabetes mellitus: a prospective cohort study of 14,926 women. Am J Clin Nutr. 2021;114(6):1997–2005.
Pham NM, Do VV, Lee AH. Polyphenol-rich foods and risk of gestational diabetes: a systematic review and meta-analysis. Eur J Clin Nutr. 2019;73(5):647–56.
Rao V. Phytochemicals: a global perspective of their role in nutrition and health. BoD–Books on Demand; 2012.
Patra AK. Dietary phytochemicals and microbes. Springer Science & Business Media; 2012.
McCarty MF. Proposal for a dietary phytochemical index. Med Hypotheses. 2004;63(5):813–7.
Aghdam SD, et al. Dietary phytochemical index associated with cardiovascular risk factor in patients with type 1 diabetes mellitus. BMC Cardiovasc Disord. 2021;21(1):1–11.
Bahadoran Z, et al. The association of dietary phytochemical index and cardiometabolic risk factors in adults: Tehran lipid and glucose study. J Hum Nutr Dietetics. 2013;26:145–53.
Article Google Scholar
Bahadoran Z, et al. Dietary phytochemical index and the risk of insulin resistance and β-cell dysfunction: a prospective approach in Tehran lipid and glucose study. Int J Food Sci Nutr. 2015;66(8):950–5.
ahmadi Vasmehjani A, Darabi Z, Hosseinzadeh M. The relation between dietary phytochemical index and metabolic syndrome and its components in a large sample of Iranian adults: a population-based study. 2020.
González-Castejón M, Rodriguez-Casado A. Dietary phytochemicals and their potential effects on obesity: a review. Pharmacol Res. 2011;64(5):438–55.
Zhao L, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–6.
Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8(9):950–88.
Article CAS PubMed Central Google Scholar
Abshirini M et al. Higher intake of phytochemical-rich foods is inversely related to prediabetes: a case-control study. Int J Prev Med, 2018. 9.
Pirjani R, et al. Mothers and their children’s health (MATCH): a study protocol for a population-based longitudinal cohort. BMC Pregnancy Childbirth. 2021;21(1):1–8.
Esfahani FH, et al. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study. J Epidemiol. 2010;20(2):150–8.
ElSayed NA, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care. 2023;46(Supplement1):S19–40.
Textor J. Drawing and analyzing causal DAGs with DAGitty arXiv preprint arXiv:1508.04633, 2015.
Delshad Aghdam S, et al. Dietary phytochemical index associated with cardiovascular risk factor in patients with type 1 diabetes mellitus. BMC Cardiovasc Disord. 2021;21(1):293.
Firouzabadi FD, et al. The association of dietary phytochemical index with metabolic syndrome in adults. Clin Nutr Res. 2021;10(2):161.
Bahrampour N, et al. High intake of dietary phytochemical index may be related to reducing risk of diabetic nephropathy: a case–control study. BMC Nutr. 2023;9(1):14.
Vasmehjani AA, et al. The relation between dietary phytochemical index and metabolic syndrome and its components in a large sample of Iranian adults: a population-based study. BMC Public Health. 2021;21(1):1–10.
Wen L, et al. Maternal dietary patterns and risk of gestational diabetes mellitus in twin pregnancies: a longitudinal twin pregnancies birth cohort study. Nutr J. 2020;19(1):13.
Zadeh SH, Boffetta P, Hosseinzadeh M. Dietary patterns and risk of gestational diabetes mellitus: a systematic review and meta-analysis of cohort studies. Clin Nutr ESPEN. 2020;36:1–9.
Schwingshackl L, et al. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol. 2018;33(2):157–70.
Neuenschwander M, et al. Impact of different dietary approaches on blood lipid control in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Eur J Epidemiol. 2019;34(9):837–52.
Papamichou D, Panagiotakos D, Itsiopoulos C. Dietary patterns and management of type 2 diabetes: a systematic review of randomised clinical trials. Nutr Metabolism Cardiovasc Dis. 2019;29(6):531–43.
Article CAS Google Scholar
Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr. 2017;147(6):1174–82.
de la García N, et al. Effectiveness of following Mediterranean diet recommendations in the real world in the incidence of gestational diabetes mellitus (GDM) and adverse maternal-foetal outcomes: a prospective, universal, interventional study with a single group. The ST Carlos study. Nutrients. 2019;11(6):1210.
Tryggvadottir EA, et al. Higher alkylresorcinol concentrations, a consequence of whole-grain intake, are inversely Associated with Gestational Diabetes Mellitus in Iceland. J Nutr. 2021;151(5):1159–66.
Hu J, et al. Dietary patterns during pregnancy are associated with the risk of gestational diabetes mellitus: evidence from a Chinese prospective birth cohort study. Nutrients. 2019;11(2):405.
Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23(1):65–134.
Zhou X, et al. Fresh fruit intake in pregnancy and association with gestational diabetes mellitus: a prospective cohort study. Nutrition. 2019;60:129–35.
Hannah W et al. Global burden of early pregnancy gestational diabetes mellitus (eGDM): a systematic review. Acta Diabetol, 2021: p. 1–25.
Ali MS, Groenwold RH, Klungel OH. Best (but oft-forgotten) practices: propensity score methods in clinical nutrition research. Am J Clin Nutr. 2016;104(2):247–58.
Cuco G, et al. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur J Clin Nutr. 2006;60(3):364–71.
Download references
Acknowledgements
The authors are grateful to all the women who consented to participate. We are also grateful to research teams at our participating clinical centers such as Arash Women’s Hospital in Tehran.
This research was supported by the Tehran and Kermanshah University of Medical Sciences.

Author information
Authors and affiliations.
Nutritional Sciences Department, School of Nutrition Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran
Neda Heidarzadeh-Esfahani & Seyyed Mostafa Nachvak
University of Ottawa Heart Institute, University of Ottawa, Ottawa, Canada
Javad Heshmati
Department of Obstetrics and Gynecology, Arash Women’s Hospital, Tehran University of Medical Sciences, Tehran, Iran
Reihaneh Pirjani & Ashraf Moini
Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran
Ashraf Moini
Department of Clinical Nutrition, School of Nutritional Sciences and Dietetic, Tehran University of Medical Sciences, Tehran, Iran
Mehrnoosh shafaatdoost
Department of Genetics, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran
Mahnaz Esmaeili
Department of Andrology, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran
Azar Mardi-Mamaghani
Population, Family and Spiritual Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran
Mahdi Sepidarkish
You can also search for this author in PubMed Google Scholar
Contributions
M.S., M.N., contributed to the study conception and design. Material preparation, data collection, and data analysis were performed by N.H., M.S, J.H., AM, M.Sh., A.M-M, M.E. and R.P. The manuscript was written by N.H, M.S., and M.N. All authors read and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Seyyed Mostafa Nachvak or Mahdi Sepidarkish .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval for this study was provided by ethical committee of Tehran and Kermanshah University of Medical Sciences (Project numbers: IR.TUMS.MEDICINE.REC.1398.576 and IR.KUMS.REC.1399.655). All participants accepted to enroll in this study with written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Heidarzadeh-Esfahani, N., Heshmati, J., Pirjani, R. et al. The potential causal effect of the pre-pregnancy dietary phytochemical index on gestational diabetes mellitus: a prospective cohort study. BMC Pregnancy Childbirth 24 , 447 (2024). https://doi.org/10.1186/s12884-024-06643-4
Download citation
Received : 10 November 2023
Accepted : 14 June 2024
Published : 28 June 2024
DOI : https://doi.org/10.1186/s12884-024-06643-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Gestational Metabolic Risk: A Narrative Review of Pregnancy-Related Complications and of the Effectiveness of Dietary, Exercise and Lifestyle Interventions during Pregnancy on Reducing Gestational Weight Gain and Preventing Gestational Diabetes Mellitus
Affiliations.
- 1 Department of Medicine, University of Ioannina, University Campus, 45110 Ioannina, Greece.
- 2 3rd Department of Cardiology, "Sotiria" Chest Diseases Hospital, Medical School, National and Kapodistrian University of Athens, 11527 Athens, Greece.
- 3 Department of Research for General Medicine and Primary Health Care, Faculty of Medicine, University of Ioannina, University Campus, 45110 Ioannina, Greece.
- 4 Department of Critical Care, University Hospital of Larissa, Faculty of Medicine, University of Thessaly, Mezourlo, 41335 Larissa, Greece.
- 5 Department of Neurosurgery, University Hospitals Sussex NHS Foundation Trust, Brighton BN2 5BE, UK.
- 6 Department of Gastroenterology, University Hospital of Larissa, Faculty of Medicine, University of Thessaly, Mezourlo, 41335 Larissa, Greece.
- 7 Department of Internal Medicine-Endocrinology, University Hospital of Larissa, Faculty of Medicine, University of Thessaly, Mezourlo, 41335 Larissa, Greece.
- PMID: 38929991
- PMCID: PMC11204633
- DOI: 10.3390/jcm13123462
Objective: This study is a Narrative Review that aims at investigating the implications of obesity, excessive gestational weight gain (GWG) and gestational diabetes mellitus (GDM). Additionally, this Review seeks to explore the effectiveness of nutrition, and/or exercise interventions during pregnancy on reducing GWG and preventing GDM. Materials and Methods: The search in literature included studies that identified obesity, GWG, GDM and associated risks during pregnancy. Also, SR and MA focusing on interventions including diet, or physical activity (PA), or combined (i.e., lifestyle interventions) and their impact on metabolic risk during pregnancy, were identified through searches in PubMed, Cochrane Database of Systematic Reviews (CDSRs), and Scopus. Results: The study findings suggest that lifestyle interventions during pregnancy may be effective in reducing excessive GWG. Regarding the prevention of GDM, results from studies evaluating lifestyle interventions vary. However, significant and less controversial results were reported from studies assessing the efficacy of exercise interventions, particularly in high-risk pregnant women. Conclusions: Lifestyle interventions during pregnancy may reduce excessive GWG. Exercise during pregnancy may prevent GDM, especially in high-risk pregnant women. Future research is warranted to tailor lifestyle interventions for optimal effectiveness during pregnancy.
Keywords: GDM; GWG; PA; diet; exercise; nutrition; obesity.
PubMed Disclaimer
Similar articles
- Effectiveness of Lifestyle Interventions during Pregnancy on Preventing Gestational Diabetes Mellitus in High-Risk Women: A Systematic Review and Meta-Analyses of Published RCTs. Tsironikos GI, Potamianos P, Zakynthinos GE, Tsolaki V, Tatsioni A, Bargiota A. Tsironikos GI, et al. J Clin Med. 2023 Nov 10;12(22):7038. doi: 10.3390/jcm12227038. J Clin Med. 2023. PMID: 38002654 Free PMC article. Review.
- App-Supported Lifestyle Interventions in Pregnancy to Manage Gestational Weight Gain and Prevent Gestational Diabetes: Scoping Review. Raab R, Geyer K, Zagar S, Hauner H. Raab R, et al. J Med Internet Res. 2023 Nov 10;25:e48853. doi: 10.2196/48853. J Med Internet Res. 2023. PMID: 37948111 Free PMC article. Review.
- Prevention of Gestational Diabetes Mellitus and Gestational Weight Gain Restriction in Overweight/Obese Pregnant Women: A Systematic Review and Network Meta-Analysis. Wu S, Jin J, Hu KL, Wu Y, Zhang D. Wu S, et al. Nutrients. 2022 Jun 9;14(12):2383. doi: 10.3390/nu14122383. Nutrients. 2022. PMID: 35745114 Free PMC article. Review.
- Effect of Physical Activity and/or Healthy Eating on GDM Risk: The DALI Lifestyle Study. Simmons D, Devlieger R, van Assche A, Jans G, Galjaard S, Corcoy R, Adelantado JM, Dunne F, Desoye G, Harreiter J, Kautzky-Willer A, Damm P, Mathiesen ER, Jensen DM, Andersen L, Lapolla A, Dalfrà MG, Bertolotto A, Wender-Ozegowska E, Zawiejska A, Hill D, Snoek FJ, Jelsma JG, van Poppel MN. Simmons D, et al. J Clin Endocrinol Metab. 2017 Mar 1;102(3):903-913. doi: 10.1210/jc.2016-3455. J Clin Endocrinol Metab. 2017. PMID: 27935767 Free PMC article. Clinical Trial.
- The effect of weight management interventions that include a diet component on weight-related outcomes in pregnant and postpartum women: a systematic review protocol. Spencer L, Rollo M, Hauck Y, MacDonald-Wicks L, Wood L, Hutchesson M, Giglia R, Smith R, Collins C. Spencer L, et al. JBI Database System Rev Implement Rep. 2015 Jan;13(1):88-98. doi: 10.11124/jbisrir-2015-1812. JBI Database System Rev Implement Rep. 2015. PMID: 26447010
- Am J Obstet Gynecol. 2016 Nov;215(5):561-571 - PubMed
- BJOG. 2019 Feb;126(3):311-320 - PubMed
- Nutrients. 2022 Jul 09;14(14): - PubMed
- Br J Sports Med. 2018 Nov;52(21):1376-1385 - PubMed
- BJOG. 2016 May;123(6):965-73 - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources, research materials.
- NCI CPTC Antibody Characterization Program

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Reprod Biomed
- v.19(9); 2021 Sep
Gestational diabetes mellitus: Major risk factors and pregnancy-related outcomes: A cohort study
Azam kouhkan.
1 Reproductive Epidemiology Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
2 Department of Stem Cells and Developmental Biology, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran.
Laily Najafi
3 Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences (IUMS), Tehran, Iran.
Mojtaba Malek
4 Research Center for Prevention of Cardiovascular Disease, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences (IUMS), Tehran, Iran.
Hamid Reza Baradaran
Roya hosseini.
5 Department of Andrology, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
Alireza Khajavi
6 Student Research Committee, Faculty of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Mohammad Ebrahim Khamseh
Gestational diabetes mellitus (GDM) is a major pregnancy endocrine problem that has several confirmed risk factors and is associated with adverse pregnancy-related outcomes (PRO).
To evaluate the relationship between GDM diagnosis and the associated risk factors of PRO (maternal, intrapartum, perinatal, and neonatal) in accordance with International Association of Diabetes and Pregnancy Study Groups criteria.
Materials and Methods
This prospective cohort study was performed with 531 singleton parturient (265 GDM and 266 non-GDM). They were selected consecutively from referral hospitals, and the maternal, intrapartum, perinatal, and neonatal outcomes were assessed.
The major risk factors influencing the GDM diagnosis were maternal age, obesity, family history of diabetes, previous history of GDM, and previous history of macrosomia. In the comparison of PRO between the groups, significant associations were detected for emergency cesarean delivery, preeclampsia, polyhydramnios, premature rupture of membrane, preterm delivery, and neonatal hyperbilirubinemia in the GDM group. In the multivariate logistic regression analysis, a previous history of stillbirth was significantly associated with maternal and perinatal outcomes. The odds ratios (CI 95%) of the PRO in the women with a GDM diagnosis were: maternal = 2.43 (1.51-3.90), intrapartum = 2.05 (1.35-3.11), perinatal = 2.00 (1.29-3.10), and neonatal = 1.68 (1.08-2.62). The PRO was significantly correlated with GDM diagnosis, but not with the risk factors.
The adverse pregnancy outcomes were significantly correlated with GDM diagnosis, and the outcomes were not directly affected by the risk factors. Given the related adverse outcomes for mothers and offspring, early screening and management of GDM is necessary especially in Asians and in low-/middle-income countries.
1. Introduction
Chronic diseases such as diabetes have become one of the major public health problems in recent years. One of the main forms of diabetes is gestational diabetes mellitus (GDM), which is recognized as glucose intolerance, and is diagnosed initially during pregnancy. It could affect between 1.3% and 18.6% of pregnancies in Iran (1), depending on the studied population and the diagnostic criteria used.
The pregnancies complicated by GDM are associated with feto-maternal sequelae. The adverse pregnancy-related outcomes (PRO) are spontaneous abortions, macrosomia, intrauterine growth restriction (IUGR), premature rupture of membranes (PROM), neonatal hypoglycemia, respiratory distress, and the need for neonatal intensive care unit (NICU) admission (2). Maternal poor glycemic control is associated with a high prevalence of adverse perinatal outcomes (3).
The most common risk factors for GDM diagnosis are higher age and body mass index (BMI), previous history of GDM, first-degree relatives with diabetes, and adverse obstetric outcomes (4). Parturients diagnosed with GDM are at an increased risk of obesity, metabolic syndrome, and type-II diabetes mellitus for themselves in the future and their offspring in later life (5). Due to the high incidence of metabolic syndrome and genetic predisposition among Asians, they are more likely to have GDM. Therefore, with an increase in GDM globally, identifying major risk factors and adverse feto-maternal outcomes and providing appropriate care to women developing GDM could substantially impact the health of large numbers of parturients and offspring.
The screening criteria, ideal timing for screening, risk factors, and feto-maternal complications of GDM remain under debate. Considering the importance of early detection and appropriate GDM diagnosis, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) (6) has identified new diagnostic criteria, which distinguish and help manage GDM and the adverse outcomes to prevent further complications.
The current study was undertaken to evaluate the relationship between GDM diagnosis and the associated risk factors of PRO in accordance with the IADPSG criteria.
2. Materials and Methods
This prospective cohort study was carried out between April 2015 and July 2017. The present study was performed on 531 single women, who were selected consecutively from referral hospitals (Kamali, Akbarabadi and Arash); comprising of GDM and non-GDM pregnancies. The sample size was calculated using the G power software (version 3.1), using power = 90%, α = 5%, β = 0.1, d = 0.05, and the prevalence of GDM = 3.41% and 4.9% (1, 7). Considering the probable drop rate of 5%, the sample size calculated each group was 265 parturient. This sample size was confirmed by the prevalence of feto-maternal outcomes (5.1% preterm in Iran (8), 5% large-for-gestational age [GA] (9), 7.9% meconium-stained amniotic fluid (10) and 8% preeclampsia (11)). The study population was comprised of 265 GDM and 266 non-GDM parturient.
The exclusion criteria were parturients who were transferred out at any GA, smoking or substance abuse, abortion, multifetal pregnancy, gross fetal anomalies, overt diabetes, chronic hypertension, systemic disorders, and use of systemic medications.
A complete history was taken and a physical evaluation was performed for all parturients by a trained physician. Maternal baseline demographic characteristics, clinical and obstetrical parameters, laboratory data and anthropometric variables were obtained from existing antenatal records and face-to-face interviews which were conducted in the first and following prenatal visits by trained observers. For height and weight measurement, a calibrated digital scale (Seca gmbh & co. kg., Germany) was used. The BMI measured in the first trimester, was the best predictor of prepregnancy BMI; it is considered by dividing the weight (kilograms) by the square of the height (meters) (12). Standard measurement of blood pressure (BP) was recorded for all the parturients; defined as a seated position, relax after at least 5 min, refrain from talking or moving and cease smoking and drinking tea or coffee, or eating food for at least half an hr.
GDM is diagnosed at 24-28 wk of gestation using a “one-step strategy” (75-gr 2-hr oral glucose tolerance test) which is well-defined according to the American Diabetes Association/IADPSG criteria (6, 13, 14). The test should be done within 8-14 hr of overnight fasting. Based on the aforementioned guidelines criteria, a diagnosis of GDM can be made when one of the following values is met or exceeded in the one-step strategy: 0-hr (fasting) ≥ 92 mg/dL; 1-hr ≥ 180 mg/dL; or 2-hr ≥ 153 mg/dL.
The non-GDM group was defined as those with a normal oral glucose tolerance test. The enzymatic calorimeter method was used for blood glucose measurement by means of a standard kit (EliTech kit, France). The major risk factors which influenced the GDM diagnosis were defined as follows: maternal age > 35 yr, obesity (BMI ≥ 30 kg/m 2 ), family history of diabetes, previous history of GDM, and previous history of macrosomia (neonate weight ≥ 4000 gr) (14).
The following maternal outcomes were included: preeclampsia (as BP ≥ 140/90 mmHg and a positive proteinuria [at least 1+ dipstick, 30 mg/dl] shown by random urine sample or ≥ 300 mg/24 hr, or a urine protein-to-creatinine ratio of ≥ 0.3 after 20 wk of pregnancy (15)), oligohydramnios ( < 5 th percentile of amniotic fluid volume expected for GA), and polyhydramnios (amniotic fluid index > 24 cm expected for GA in the amniotic sac).
The following intrapartum outcomes were included: emergency cesarean section (CS), PROM (rupture of the membrane more than 1 hr before the onset of labor), and preterm delivery (delivery before 37 wk of gestation).
Perinatal outcomes were defined as fetal death, macrosomia (birth weight at delivery > 4,000 gr), IUGR (growth < the third percentile for GA), first- and fifth-min Apgar score > 7, and congenital malformation.
Neonatal outcomes were defined as NICU admission, neonatal hypoglycemia (16), neonatal hyperbilirubinemia (17), and neonatal respiratory distress (18). Neonatal variables were examined by a pediatrician in all cases after delivery.
Ethical considerations
The ethics committee of Iran University of Medical Sciences, Tehran, Iran approved the study protocol (Code: IR.IUMS.REC.1393.24991) and a written informed consent was signed by all parturients and their spouses included in the study. All procedures performed were in accordance with the ethical standards of institute of endocrinology and metabolism, Iran University of Medical Sciences, Tehran, Iran and with the 1964 Helsinki Declaration and its later amendments.
Statistical analysis
The discrete and continuous variables are reported using the number (percent) and mean (standard deviation [SD]), respectively. To compare the continuous variables between GDM and non-GDM groups, t test and Mann-Whitney test were used, depending on the variables' distribution is in accordance with the normal distribution or not, respectively. In addition, the Chi-square test was the tool for comparing the categorical variables between GDM and non-GDM groups. Finally, the responses which owned significant levels between two groups were fitted in the ordinal logistic regression model. Besides, the variables of clinical important but non-significant in group-comparison step were also put in the model.
The analyses were performed using the statistical software Statistical Package for the Social Sciences (SPSS) (version 16, SPSS Inc., Chicago, IL, USA) Stata (ver. 12). The significance level was chosen to be 0.05.
A total of 531 parturients including 265 GDM and 266 non-GDM parturients were included in this study.
The average gravidity in non-GDM and GDM groups was 1.86 ± 0.93 and 2.20 ± 1.00, respectively (p = 0.0001). The parity average in the non-GDM and GDM groups was 0.66 ± 0.78 and 0.90 ± 0.81, respectively (p = 0.0005). The demographic and reproductive characteristics of the parturients are summarized in Table I.
The relationship between GDM and the major risk factors which influenced the GDM diagnosis (maternal age > 35 yr, obesity [BMI ≥ 30 kg/m 2 ], family history of diabetes, previous history of GDM, and previous history of macrosomia [neonate weight ≥ 4000 gr]), were compared between the two groups, using the Chi-square test. The analysis showed significant association between GDM and maternal age > 35 yr (p = 0.0001), obesity [BMI ≥ 30 kg/m 2 ] (p = 0.03), family history of diabetes (p = 0.0001), previous history of GDM (p = 0.0001), and previous history of macrosomia (p = 0.01). Finally, at multivariate logistic regression analysis was used to evaluate the effect of these five risk factors (adjusted for maternal characteristics) on the odds of GDM development (dependent variable), and the same results as above was obtained, with few alterations (findings are not shown).
Table II presents the comparison of the PRO (maternal, intrapartum, perinatal, and neonatal) between the two groups and the association of the aforementioned outcomes with the GDM group.
This study, measured the effect of major risk factors on the PRO (maternal, intrapartum, perinatal, and neonatal) in the GDM group; the results are presented in table III.
Table IV shows the results from the multivariate logistic regression analysis, which was performed on the risk factors for maternal, intrapartum, perinatal, and neonatal outcomes in the study population. The results demonstrated that a previous history of stillbirth was significantly associated with maternal and perinatal outcomes (Table V). The maternal outcomes included preeclampsia, oligohydramnios and polyhydramnios; the intrapartum outcomes were emergency CS, PROM and preterm delivery; the perinatal outcomes were fetal death, macrosomia, IUGR, Apgar 1, 5 and congenital malformation; and the neonatal outcomes were NICU admission, neonatal hypoglycemia, neonatal hyperbilirubinemia, and neonatal respiratory distress.
In an additional analysis, the four aforementioned outcomes were significantly correlated with GDM diagnosis; as the ORs (95% CI, p-value) demonstrate: maternal = 2.43 (1.51-3.90, 0.0001), intrapartum = 2.05 (1.35-3.11, 0.001), perinatal = 2.00 (1.29-3.10, 0.002), and neonatal = 1.68 (1.08-2.62, 0.02).
Demographic and reproductive characteristics of the parturients
| 28.42 5.26 | 31.33 5.41 | 0.0001 | |
| 149 (55.8) | 191 (71.8) | 0.0001 | |
| 135 (50.6) | 90 (33.8) | 0.0001 | |
| ) | 24.72 4.37 | 26.45 4.43 | 0.0001 |
| 38.85 1.30 | 38.07 1.55 | 0.0001 | |
| 108.07 11.21 | 111.97 13.16 | 0.0003 | |
| 70.10 9.23 | 70.83 9.47 | 0.3700 | |
| Data presented as Mean SD, Data presented as n (%). P-values 0.05 were considered significant. test, Chi-square test. GDM: Gestational diabetes mellitus, BMI: Body mass index, BP: Blood pressure | |||
The comparison of pregnancy-related outcomes in GDM and non-GDM groups
| 34 (13.03) | 58 (22.05) | 1.88 (1.18-3.00) | 0.007 | |
| 11 (4.170 | 36 (13.69) | 3.64 (1.81-7.33) | 0.001 | |
| 9 (3.47) | 7 (2.65) | 0.75 (0.27-2.06) | 0.58 | |
| 12 (4.63) | 28 (10.61) | 2.44 (1.21-4.91) | 0.01 | |
| 6 (2.26) | 25 (9.47) | 4.53 (1.82-11.24) | 0.0001 | |
| 2 (0.76) | 2 (0.75) | 0.99 (0.13-7.09) | 0.99 | |
| 12 (5.11) | 29 (11.93) | 2.52 (1.25-5.06) | 0.008 | |
| 7 (2.67) | 15 (5.68) | 2.19 (0.87-5.47) | 0.08 | |
| 8 (3.08) | 16 (6.06) | 2.03 (0.85-4.83) | 0.10 | |
| 7 | 5 (1.87) | 9 (3.38) | 0.98 (0.56-1.73) | 0.27 |
| 7 | 0 (0) | 2 (0.75) | 0.77 (0.42-1.42) | 0.15 |
| 28 (10.69) | 24 (9.13) | 0.83 (0.47-1.49) | 0.54 | |
| 10 (6.21) | 17 (11.18) | 1.90 (0.84-4.29) | 0.11 | |
| 5 (1.89) | 7 (2.65) | 1.41 (0.44-4.52) | 0.55 | |
| 25 (9.51) | 46 (17.42) | 2.00 (1.19-3.38) | 0.008 | |
| 24 (9.02) | 37 (14.02) | 1.64 (0.95-2.83) | 0.07 | |
| *Data presented as n (%), Chi-square test was used. P-value of 0.05 was considered significant. GDM: Gestational diabetes mellitus, CS: Cesarean section, PROM: Premature rupture of membranes, IUGR: Intra-uterine growth restriction, NICU: Neonatal intensive care unit | ||||
The effect of major risk factors on PRO (maternal, intrapartum, perinatal, and neonatal) in the GDM group
| 0.73 (0.38-1.41) | 1.32 (0.73-2.38) | 0.68 (0.29-1.55) | 0.38 (0.13-1.13) | 0.87 (0.23-3.21) | |
| 0.93 (0.55-1.58) | 1.02 (0.62-1.67) | 1.26 (0.66-2.37) | 1.09 (0.54-2.18) | 1.32 (0.46-3.75) | |
| 0.90 (0.17-4.74) | 0.24 (0.02-2.06) | - - | 2.89 (0.32-25.70) | ||
| 0.72 (0.29-1.79) | 1.59 (0.72-3.49) | 1.97 (0.81-4.81) | 2.20 (0.86-5.61) | 2.24 (0.59-8.47) | |
| 1.30 (0.55-3.08) | 1.73 (0.75-3.95) | 0.62 (0.17-2.18) | 0.79 (0.22-2.79) | 1.51 (0.32-7.11) | |
| - - | - 5.94 (0.36-97.12) | - | |||
| 1.69 (0.76-3.75) | 1.94 (0.89-4.25) | 0.53 (0.15-1.84) | 0.94 (0.31-2.90) | 1.69 (0.35-8.22) | |
| 0.54 (0.15-1.99) | 1.01 (0.34-2.92) | 0.74 (0.16-3.44) | 1.52 (0.41-5.69) | 2.79 (0.56-13.69) | |
| 1.38 (0.48-3.94) | 1.19 (0.42-3.30) | 1.14 (0.30-4.21) | 0.84 (0.18-3.85) | 1.11 (0.13-9.05) | |
| 7 | 1.11 (0.27-4.53) | 0.76 (0.19-3.11) | 0.56 (0.07-4.61) | 0.72 (0.09-5.93) | - |
| 1.13 (0.46-2.77) | 1.10 (0.46-2.58) | 0.89 (0.29-2.74) | 0.51 (0.11-2.27) | 0.69 (0.08-5.55) | |
| 2.67 (0.96-7.42) | 1.05 (0.37-2.92) | 0.64 (0.13-3.00) | 1.39 (0.36-5.33) | - | |
| - 2.05 (0.45-9.39) | 0.90 (0.10-7.92) | 0.99 (0.11-8.46) | 2.89 (0.32-25.70) | ||
| 0.86 (0.43-1.75) | 0.96 (0.50-1.85) | 0.49 (0.18-1.34) | 0.87 (0.34-2.22) | 0.32 (0.04-2.52) | |
| 1.45 (0.70-2.99) | 1.73 (0.86-3.47) | 1.56 (0.68-3.58) | 1.17 (0.45-3.05) | 0.94 (0.20-4.34) | |
| Data presented as the OR (95% CI). *A p-value of 0.05 was considered significant. FHDM: Family history of diabetes, BMI: Body mass index, PHGDM: Previous history of GDM, PHMAC: Previous history of macrosomia, C/S: Cesarean section, PROM: Premature rupture of membranes, IUGR: Intra-uterine growth restriction, NICU: Neonatal intensive care unit | |||||
| 0.73 (0.38-1.41) | 1.32 (0.73-2.38) | 0.68 (0.29-1.55) | 0.38 (0.13-1.13) | 0.87 (0.23-3.21) | |
| 0.93 (0.55-1.58) | 1.02 (0.62-1.67) | 1.26 (0.66-2.37) | 1.09 (0.54-2.18) | 1.32 (0.46-3.75) | |
| 0.90 (0.17-4.74) | 0.24 (0.02-2.06) | - - | 2.89 (0.32-25.70) | ||
| 0.72 (0.29-1.79) | 1.59 (0.72-3.49) | 1.97 (0.81-4.81) | 2.20 (0.86-5.61) | 2.24 (0.59-8.47) | |
| 1.30 (0.55-3.08) | 1.73 (0.75-3.95) | 0.62 (0.17-2.18) | 0.79 (0.22-2.79) | 1.51 (0.32-7.11) | |
| - - | - 5.94 (0.36-97.12) | - | |||
| 1.69 (0.76-3.75) | 1.94 (0.89-4.25) | 0.53 (0.15-1.84) | 0.94 (0.31-2.90) | 1.69 (0.35-8.22) | |
| 0.54 (0.15-1.99) | 1.01 (0.34-2.92) | 0.74 (0.16-3.44) | 1.52 (0.41-5.69) | 2.79 (0.56-13.69) | |
| 1.38 (0.48-3.94) | 1.19 (0.42-3.30) | 1.14 (0.30-4.21) | 0.84 (0.18-3.85) | 1.11 (0.13-9.05) | |
| 7 | 1.11 (0.27-4.53) | 0.76 (0.19-3.11) | 0.56 (0.07-4.61) | 0.72 (0.09-5.93) | - |
| 7 | - - | - - | - | ||
| 1.13 (0.46-2.77) | 1.10 (0.46-2.58) | 0.89 (0.29-2.74) | 0.51 (0.11-2.27) | 0.69 (0.08-5.55) | |
| 2.67 (0.96-7.42) | 1.05 (0.37-2.92) | 0.64 (0.13-3.00) | 1.39 (0.36-5.33) | - | |
| - 2.05 (0.45-9.39) | 0.90 (0.10-7.92) | 0.99 (0.11-8.46) | 2.89 (0.32-25.70) | ||
| 0.86 (0.43-1.75) | 0.96 (0.50-1.85) | 0.49 (0.18-1.34) | 0.87 (0.34-2.22) | 0.32 (0.04-2.52) | |
| 1.45 (0.70-2.99) | 1.73 (0.86-3.47) | 1.56 (0.68-3.58) | 1.17 (0.45-3.05) | 0.94 (0.20-4.34) | |
| Data presented as the OR (95% CI). *P-value of 0.05 was considered significant. GDM: Gestational diabetes mellitus, FHDM: Family history of diabetes, BMI: Body mass index, PHGDM: Previous history of GDM, PHMAC: Previous history of macrosomia, CS: Cesarean section, PROM: Premature rupture of membranes, IUGR: Intra-uterine growth restriction, NICU: Neonatal intensive care unit | |||||
The multivariate logistic regression analysis for the risk factors on maternal, intrapartum, perinatal, and neonatal outcomes in the study population
| [1.455in,lr] | ||||
| 35 yr | 0.95 (0.55-1.64) | 0.93 (0.56-1.53) | 1.18 (0.71-1.95) | 0.89 (0.51-1.56) |
| 0.94 (0.57-1.56) | 1.39 (0.89-2.17) | 1.21 (0.76-1.93) | 1.01 (0.62-1.65) | |
| 30 kg/m ) | 1.25 (0.68-2.29) | 0.92 (0.51-1.66) | 1.16 (0.64-2.08) | 1.02 (0.52-1.98) |
| 1.91 (0.94-3.89) | 0.52 (0.23-1.21) | 1.19 (0.58-2.47) | 0.69 (0.28-1.66) | |
| 4000 gr) | 1.31 (0.45-3.80) | 1.41 (0.44-4.45) | 0.85 (0.27-2.66) | 1.16 (0.34-3.96) |
| 0.91 (0.52-1.58) | 0.85 (0.51-1.41) | 1.12 (0.67-1.86) | 1.29 (0.76-2.21) | |
| 3.63 (1.16-11.32)* | 1.56 (0.49-4.96) | 3.65 (1.23-10.79)* | 0.93 (0.15-5.74) | |
| Data presented as the odds ratio (95% CI). *P-values 0.05 were considered significant. BMI: Body mass index, GDM: Gestational diabetes mellitus | ||||
4. Discussion
The aim of the present study was to evaluate the relationship between the GDM diagnosis and the associated risk factors of PRO in accordance with the IADPSG criteria.
The major risk factors that were associated with the GDM diagnosis were maternal age, obesity, family history of diabetes, previous history of GDM, and previous history of macrosomia. In the comparison of PRO between groups, the significant associations were detected for emergency CS delivery, preeclampsia, polyhydramnios, premature rupture of membrane, preterm delivery, and neonatal hyperbilirubinemia in the GDM group. A previous history of stillbirth was significantly associated with maternal and perinatal outcomes. All PRO were significantly correlated with GDM diagnosis, but not with the risk factors.
Early identification of GDM is crucial because it affects clinical decision-making. Optimal GDM management, including lifestyle alterations, medical nutrition therapy, insulin therapy, and antepartum fetal observation, may decrease the perinatal morbidity and mortality associated with GDM (13). However, screening and accurately detecting GDM in asymptomatic pregnant women is controversial. Screening is done using a one-step strategy at least once at or about 24 wk of gestation unless there are suggestions for it to be done earlier (2). Healthcare providers should recognize and screen high-risk groups of pregnant women to detect and manage GDM earlier. Our data showed that major risk factors (such as age, obesity, family history of diabetes, previous history of GDM, and previous history of macrosomia) significantly increased the risk of GDM. In most countries, early screening is performed based on parameters such as belonging to a specific ethnic group related to a high GDM prevalence, advanced maternal age, prepregnancy obesity, history of diabetes in first-degree relatives, previous history of GDM, previous large-for-GA babies, and glucosuria. Early screening using only traditional risk factors may increase the likehood of missing GDM cases (19). Therefore, we suggest that both traditional risk factors and new biomarkers should be researched in large populations and with varied ethnic groups.
In our regional literature review, we detected a varied range of GDM prevalence values from 1.3% to 18.6% in Iranian pregnant women (1). Ethnicity seems to play a principal role as well: the trend of GDM in the Asian population is increasing; also increased insulin resistance is observed at much lower BMI levels in Asian compared with Europeans (20). Although South Asians are a high-risk population, screening, risk factors and complications of GDM are still debated (21). The recognized risk factors (such as age, BMI, family history of diabetes, previous history of macrosomia/congenital malformation, and previous history of GDM) were mainly attained from studies of European populations (22). Furthermore, there was a Malaysian and Iranian study which examined this relationship (2, 23) and found similar results. Keshavarz and colleagues showed that older age, a family history of diabetes, obesity, previous macrosomia, and glycosuria can be risk factors for GDM (23). In another study, the risk of GDM was greater in parturients aged > 35 yr with a fourfold increase that was related to pancreatic β-cell function and insulin-sensitivity falling with age (24). In addition, a 2.45-fold increase of risk has been attributed to obesity (2), which is related to the elevation of insulin resistance that occurs as a result of obesity (19). In the present study, GDM risk was higher by 3.08-fold and 1.7-fold in women > 35 yr and with a BMI ≥ 30 kg/m 2 , respectively. The placenta and adipose tissue that produce a large number of diabetogenic adipokines. Tumor necrosis factor-alpha, which is a as diabetogenic adipokine, plays an important role in insulin-resistance pathways and may induce adverse feto-maternal outcomes (25). In two studies, a family history of DM was presented in 77.7% and 16.3% of GDM cases (26, 27). Our findings showed that a family history of diabetes increased the risk of GDM by 2.90-fold. Keshavarz and colleagues suggested that screening based on risk factors alone could miss 16% of GDM cases (23). A recent systematic review evaluated the cost-effectiveness of identification and/or treatment of GDM. This study found that neither early screening nor treating GDM seems to be convincingly cost-effective in high-income countries, but they suggested that early screening and proper detection of GDM might be worthwhile in low-/middle- incomes countries due to different health systems and other health priorities (28).
The present study revealed that adverse PRO such as emergency CS, preeclampsia, polyhydramnios, PROM, preterm delivery, and neonatal hyperbilirubinemia were significantly associated with GDM diagnosis (OR = 1.88, 3.64, 2.44, 4.53, 2.52, and 2.00, respectively) compared to non-GDM women. All the PROs were significantly correlated with GDM diagnosis. Other studies have shown that the risk of fetal macrosomia (29) may be increased by a diagnosis of GDM, and a greater potential risk may exist for shoulder dystocia in macrosomic infants (30). Additionally, CS delivery, spontaneous miscarriage, preterm delivery, a low Apgar score, the need for NICU admission, hypoglycemia, congenital malformations and respiratory distress syndrome occured more commonly among the infants of GDM parturients (2). Another Iranian study reported a higher risk of pregnancy complications and adverse feto-maternal outcomes with GDM (23). In GDM parturients, a higher risk of having a non-spontaneous vaginal delivery such as operative deliveries was observed in GDM paturients compared with non-GDM women (OR = 1.9) (2). GDM is not only associated with short-term adverse feto-maternal outcomes, such as macrosomia, increased CS rates, hypertensive disorders, and fetal hyperinsulinemia (31), but also significantly increases the risk for long-term adverse events for both mothers and their offspring (19).
Nilofer and co-workers and Opara and colleagues found that hyperbilirubinemia is a common adverse event in GDM, which complicated 20% and 57.4% of the infants they studied, respectively; this resulted from excessive red blood cell breakdown in association with polycythemia because of immature bilirubin conjugation by the liver of the neonate (26, 32). In contrast to our findings, Logakodie and colleagues revealed no significant difference in the fetal outcomes of GDM parturients, which may be due to the tight glycemic control of GDM status (2). In the present study, the major risk factors were not associated with PRO. According to our multivariate logistic regression analysis, after group adjustment, a previous history of stillbirth was an independent risk factor for maternal and perinatal outcomes. Unlike in our study, Jolly and colleagues who observed that maternal obesity was associated with adverse pregnancy outcomes; however, they found similar results to our study in relation to the association of GDM with adverse pregnancy outcomes (24).
5. Limitations
In the present study, we could not evaluate the socioeconomic status or dietary patterns of the parturients.
6. Conclusion
In conclusion, we found that the PRO were significantly correlated with GDM diagnosis, but not with the risk factors. Given the increased rate of GDM worldwide, healthcare providers should attend to risk factors for early diagnosis of GDM. In view of the long- and short-term adverse related outcomes for mothers and offspring, early screening and appropriate management of GDM are necessary, especially in Asians and in low-/middle-income countries. Detection of predictive factors of GDM should be researched in these populations.
Conflict of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgements
This study was registered, funded, and supported by Iran University of Medical Sciences (IUMS), Tehran, Iran at 2015 by Grant No. 93-03-122-24991.
- Jafari-Shobeiri M, Ghojazadeh M, Azami-Aghdash S, Naghavi-Behzad M, Piri R, Pourali-Akbar Y, et al. Prevalence and risk factors of gestational diabetes in Iran: A systematic review and meta-analysis. Iran J Public Health. 2015; 44 :1036–1044. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Logakodie S, Azahadi O, Fuziah P, Norizzati B, Tan SF, Zienna Z, et al. Gestational diabetes mellitus: The prevalence, associated factors and foeto-maternal outcome of women attending antenatal care. Malays Fam Physician. 2017; 12 :9–17. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miao M, Dai M, Zhang Y, Sun F, Guo X, Sun G. Influence of maternal overweight, obesity and gestational weight gain on the perinatal outcomes in women with gestational diabetes mellitus. Sci Rep. 2017; 7 :305. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Erem C, Kuzu UB, Deger O, Can G. Prevalence of gestational diabetes mellitus and associated risk factors in Turkish women: The trabzon GDM study. Arch Med Sci. 2015; 11 :724–735. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lowe Jr WL, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): Maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019; 42 :372–380. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan ThA, Catalano PA, et al. International Association of Diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010; 33 :676–682. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sayehmiri F, Bakhtiyari S, Darvishi P, Sayehmiri K. [Prevalence of gestational diabetes mellitus in Iran: A systematic review and meta-analysis study] The Iranian Journal of Obstetrics, Gynecology and Infertility. 2013; 15 :16–23. [ Google Scholar ]
- Alijahan R, Hazrati S, Mirzarahimi M, Pourfarzi F, Ahmadi Hadi P. Prevalence and risk factors associated with preterm birth in Ardabil, Iran. Iran J Reprod Med. 2014; 12 :47–56. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Henriksen T. The macrosomic fetus: A challenge in current obstetrics. Acta Obstet Gynecol Scand. 2008; 87 :134–145. [ PubMed ] [ Google Scholar ]
- Fischer C, Rybakowski C, Ferdynus C, Sagot P, Gouyon JB. A population-based study of meconium aspiration syndrome in neonates born between 37 and 43 weeks of gestation. Int J Pediatr. 2011; 2012 :321545. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hossein-nezhad A, Mirzaei Kh, Ahmadi S, Maghbooli Zh, Karimi F. Comparison of incidence of pregnancy induced hypertension in gestational diabetes mellitus and healthy pregnant women. Iranian Journal of Diabetes and Lipid Disorders. 2011; 10 :1–9. [ Google Scholar ]
- Krukowski RA, West DS, DiCarlo M, Shankar K, Cleves MA, Saylors ME, et al. Are early first trimester weights valid proxies for preconception weight? BMC Pregnancy Childbirth 2016; 16: 357. [ PMC free article ] [ PubMed ]
- American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care 2015; 38 (Suppl.): S8–S16. [ PubMed ]
- Metzger BE, Buchanan ThA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes Care 2007; 30 (Suppl.): S251–S260. [ PubMed ]
- ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. diagnosis and management of preeclampsia and eclampsia Number 33, January 2002 American College of Obstetricians and Gynecologists Obstet Gynecol. 2002; 99 :159–167. [ PubMed ] [ Google Scholar ]
- Adamkin DH. Neonatal hypoglycemia. Semin Fetal Neonat Med. 2017; 22 :36–41. [ PubMed ] [ Google Scholar ]
- Pichon JBL, Riordan SM, Watchko J, Shapiro SM. The neurological sequelae of neonatal hyperbilirubinemia: Definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDs) Curr Pediatr Rev. 2017; 13 :199–209. [ PubMed ] [ Google Scholar ]
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants-2013 update. Neonatology. 2013; 103 :353–368. [ PubMed ] [ Google Scholar ]
- Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P. Gestational diabetes: A clinical update. World J Diabetes. 2015; 6 :1065–1072. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jenum AK, Sommer Ch, Sletner L, Mørkrid K, Bærug A, Mosdøl A. Adiposity and hyperglycaemia in pregnancy and related health outcomes in European ethnic minorities of Asian and African origin: A review. Food Nutr Res. 2013; 57 :18889. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee KW, Ching SM, Ramachandran V, Yee A, Hoo FK, Chia YCh, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: A systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018; 18 :494. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Avalos GE, Owens LA, Dunne F, Collaborators AD. Applying current screening tools for gestational diabetes mellitus to a European population: Is it time for change? Diabetes Care 2013; 36: 3040–3044. [ PMC free article ] [ PubMed ]
- Keshavarz M, Cheung NW, Babaee GhR, Kalalian Moghadam H, Ajami ME, Shariati M. Gestational diabetes in Iran: Incidence, risk factors and pregnancy outcomes. Diabetes Res Clin Pract. 2005; 69 :279–286. [ PubMed ] [ Google Scholar ]
- Jolly M, Sebire N, Harris J, Robinson S, Regan L. The risks associated with pregnancy in women aged 35 years or older. Hum Reprod. 2000; 15 :2433–2437. [ PubMed ] [ Google Scholar ]
- Korkmazer E, Solak N. Correlation between inflammatory markers and insulin resistance in pregnancy. J Obstet Gynaecol. 2015; 35 :142–145. [ PubMed ] [ Google Scholar ]
- Nilofer AR, Raju VS, Dakshayini BR, Zaki SA. Screening in high-risk group of gestational diabetes mellitus with its maternal and fetal outcomes. Indian J Endocrinol Metab 2012; 16 (Suppl.): S74–S78. [ PMC free article ] [ PubMed ]
- Rajput R, Yadav Y, Nanda S, Rajput M. Prevalence of gestational diabetes mellitus & associated risk factors at a tertiary care hospital in Haryana. Indian J Med Res. 2013; 137 :728–733. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fitria N, van Asselt ADI, Postma MJ. Cost-effectiveness of controlling gestational diabetes mellitus: A systematic review. Eur J Health Econ. 2019; 20 :407–417. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kamana K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: A literature review. Ann Nutr Metab 2015; 66 (Suppl.): 14–20. [ PubMed ]
- Young BC, Ecker JL. Fetal macrosomia and shoulder dystocia in women with gestational diabetes: Risks amenable to treatment? Curr Diab Rep 2013; 13: 12–18. [ PubMed ]
- Metzger BE, Persson B, Lowe LP, Dyer AR, Cruickshank JK, Deerochanawong Ch, et al. Hyperglycemia and adverse pregnancy outcome study: Neonatal glycemia. Pediatrics 2010; 126: e1545–e1552. [ PubMed ]
- Opara PI, Jaja T, Onubogu UC. Morbidity and mortality amongst infants of diabetic mothers admitted into a special care baby unit in Port Harcourt, Nigeria. Ital J Pediatr. 2010; 36 :77. [ PMC free article ] [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Abstract. Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. Screening for GDM is usually done at 24-28 weeks of gestation. In this case, we report a 31-year-old woman who developed gestational diabetes at 6 weeks in two successive pregnancies.
Gestational diabetes is defined as "any degree of carbohydrate intolerance with onset first recognized during pregnancy. This definition applies whether insulin ... is used for treatment and whether or not the condition persists after pregnancy."1 Risk assessment is done early in the pregnancy, with average-risk women being tested at 24-28 weeks' gestation and low-risk women requiring ...
CHARACTERIZING HYPERGLYCEMIA. This patient's hyperglycemia reached a threshold that was diagnostic of diabetes 1 on two occasions: when she was 25 years of age, she had a randomly obtained blood glucose level of 217 mg per deciliter with polyuria (with diabetes defined as a level of ≥200 mg per deciliter [≥11.1 mmol per liter] with symptoms), and when she was 30 years of age, she had on ...
Outputs of the full models for the complete-case, bootstrapped, and MICE datasets are shown in Table S8. Table 2. ... The Treatment Of BOoking Gestational diabetes Mellitus Study: evaluating the ...
Background: The underlying causes of gestational diabetes mellitus (GDM) are important because they are effective for the diagnosis and prevention of this condition.The aim of this study was to identify the risk factors for GDM and the possible etiological agents. Materials and Methods: This case-control study was conducted with 100 women with GDM and 100 healthy pregnant women at a tertiary ...
A total of 145,196 women were delivered during the study period, and 2687 (1.9%) were diagnosed to have diabetes mellitus. Gestational diabetes was diagnosed in 2277 (1.6%) of whom 230 (10%) had ...
Diabetes & Primary Care's series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes.. These two cases provide an overview of gestational diabetes (GDM). The scenarios cover the screening, identification and management of GDM, as well as the steps that should be taken to screen for, and ...
Abstract. Gestational diabetes mellitus, which is defined as the onset or first recognition of carbohydrate intolerance during pregnancy, is estimated to affect between 6 and 9% of pregnant women ...
25 weeks of gestational age, when she weighed 67 Kg of BW and had a BMI of 27.9 Kg.m-2. At the OGTT: The fasting serum glycemia was Abstract Background: How best to define Gestational Diabetes Mellitus (GDM) is the object of debate, with International Association of Diabetes in Pregnancy Study Groups criteria (IADPSGc) differing
Presentation of Case. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia. Eleven years before this presentation, the blood ...
1. Introduction. Gestational diabetes mellitus (GDM), defined as carbohydrate intolerance of variable degree with onset or recognition during pregnancy, has been recently identified as a potential risk factor for Type II Diabetes Mellitus (T2DM). 1 As per International Diabetes Federation (IDF) 2017, one in seven births is affected by GDM. 16.2% (21.3 million) of live births is to women with ...
Summary When adjusted for confounders, gestational diabetes mellitus was signi cantly associated with a range of adverse pregnancy outcomes. Study design Systematic review and meta-analysis. Data sources 156 Overall risk of bias: 7 506 061 studies 19% low, % medium, % high pregnancies were evaluated.
Introduction. Gestational diabetes mellitus (GDM) is classically defined as "Carbohydrate intolerance resulting in hyperglycemia of variable severity with onset or first recognition during pregnancy". 1 It does not rule out a prior unidentified glucose intolerance, and in fact several studies have found 10% to 15% of cases of undiagnosed ...
Gestational diabetes mellitus (GDM) is diagnosed by elevated blood glucose in pregnancy though the definition has changed repeatedly since its first description in the 1960's [1, 2].The most frequently reported perinatal consequence of GDM is macrosomia (usually defined as a neonate weighing over 4 kg) which can increase the risk of caesarean section and shoulder dystocia.
Background: Gestational diabetes mellitus (GDM) deserves proper prevention, diagnosis, and management due to healthcare implications from both maternal and fetal concerns. Objective: To evaluate the rate and investigate the risk factors for developing GDM. Materials and methods: In this case-control, universal screening for GDM between 24 and 28 wk of gestation was performed in 613 pregnant ...
Background: The underlying causes of gestational diabetes mellitus (GDM) are important because they are effective for the diagnosis and prevention of this condition.The aim of this study was to identify the risk factors for GDM and the possible etiological agents. Materials and Methods: This case-control study was conducted with 100 women with GDM and 100 healthy pregnant women at a tertiary ...
Introduction and background. Gestational diabetes mellitus (GDM) is a metabolic condition of pregnancy that presents as newly developing hyperglycemia in pregnant women who did not have diabetes before getting pregnant, and it normally resolves after giving birth [].]. Around 9% of pregnancies around the globe are affected by this prevalent antepartum condition [].
Case Studies | October 01 2021. ... Indeed, women with gestational and pregestational diabetes have average A1C levels ranging from 7.25 to 8.15%, ... Herein, we present a unique case of type 1 diabetes management during pregnancy in which a DIY APS where the "Loop" algorithm on an iPhone interface was used . The patient provided written ...
Background: Gestational diabetes mellitus (GDM) is glucose intolerance first recognized during pregnancy. The objective of this study was to identify the determinant factors of GDM. Methods: An unmatched case-control study was conducted. Descriptive statistics were used to describe the profile of study participants and binary logistic regression was used to identify the determinants of GDM.
Case Studies Case 9: Gestational diabetes. A 28-year-old G 4 P 2 presents to your office for a routine prenatal visit at 24 weeks' gestation. Her physical examination is unremarkable and fetal wellbeing is reassuring. You recommend testing for gestational diabetes mellitus (GDM). 1. What is GDM? Show Answer
Y. is diagnosed with gestational diabetes mellitus (GDM). What is GDM? Glucose intolerance with onset during pregnancy. In true GDM, glucose usually returns to normal by 6 weeks postpartum, although women with GDM have increased risk of developing type 2 diabetes mellitus later in life. List 5 risk factors for GDM.
This question is reflected in the Gestational Diabetes Mellitus Study of Detection Thresholds (GEMS) trial, which evaluated lower (IADPSG) versus higher New Zealand glucose criteria (fasting glucose ≥5·5 mmol/L or a 2-h glucose ≥9·0 mmol/L, or both) for the one-step 75 gm 2-h OGTT among over 4000 New Zealand women. 193 Results of the ...
Magnitude of screening for gestational diabetes mellitus in an urban setting in Tanzania; a cross-sectional analytic study. ... McCowan L ; et al. Gestational diabetes and the risk of late stillbirth: a case-control study from England, UK. ... tested the value of both venous and point-of-care HbA 1c to develop a population-specific strategy for ...
Gestational diabetes mellitus (GDM) is an increasingly prominent health issue in pregnant women. While various technology solutions have been developed to support self-management of women with GDM, usability and functionality limitations have precluded their adoption. ... Case Study. JMIR aging 6, 1 (2023), e41950. Crossref. Google Scholar [24]
A separate search was done to identify any studies or reports associating gestational diabetes mellitus with immunizations and vaccinations, using MEDLINE, Embase, the Cochrane Database of Systematic Reviews, Clinical Key medical reference books, and the Centers for Disease Control and Prevention (CDC) and National Institutes for Health (NIH) websites.
Background. Gestational Diabetes Mellitus (GDM) poses significant risks to maternal and fetal health. Current diagnostic methods based on glucose tolerance tests have limitations for early detection. tRNA-derived small RNAs (tsRNAs) have emerged as potential molecular regulators in various diseases, including metabolic disorders.
During pregnancy, sonographic measurements of subcutaneous adipose tissue may be a reliable diagnostic predictor of gestational diabetes mellitus (GDM) and fetal metabolism. A sonographic view of the fetal abdominal circumference can be used to measure the fetal anterior abdominal wall thickness (AAWT), which is straightforward and simple.
Phytochemicals are non-nutritive bioactive compounds with beneficial effects on the metabolism of glucose. This study aimed to clarify the possible causal effect of the pre-pregnancy dietary phytochemical index (DPI) on gestational diabetes mellitus (GDM). In this prospective cohort study 1,856 pregnant women aged 18-45 years who were in their first trimester, were recruited and followed up ...
Objective: This study is a Narrative Review that aims at investigating the implications of obesity, excessive gestational weight gain (GWG) and gestational diabetes mellitus (GDM). Additionally, this Review seeks to explore the effectiveness of nutrition, and/or exercise interventions during pregnancy on reducing GWG and preventing GDM.
One of the main forms of diabetes is gestational diabetes mellitus (GDM), which is recognized as glucose intolerance, and is diagnosed initially during pregnancy. It could affect between 1.3% and 18.6% of pregnancies in Iran (1), depending on the studied population and the diagnostic criteria used.