Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 06 May 2022

Succession comprises a sequence of threshold-induced community assembly processes towards multidiversity
- Maximilian Hanusch ORCID: orcid.org/0000-0001-7228-1276 1 ,
- Xie He ORCID: orcid.org/0000-0001-6199-0771 1 ,
- Victoria Ruiz-Hernández 1 &
- Robert R. Junker ORCID: orcid.org/0000-0002-7919-9678 1 , 2
Communications Biology volume 5 , Article number: 424 ( 2022 ) Cite this article
2656 Accesses
13 Citations
13 Altmetric
Metrics details
- Biodiversity
- Community ecology
- Microbial ecology
- Plant ecology
Research on successions and community assembly both address the same processes such as dispersal, species sorting, and biotic interactions but lack unifying concepts. Recent theoretical advances integrated both research lines proposing a sequence of stochastic and deterministic processes along successional gradients. Shifts in ecosystem states along successional gradients are predicted to occur abruptly once abiotic and biotic factors dominate over dispersal as main driver. Considering the multidiversity composed of five organismal groups including plants, animals, and microbes, our results imply that stochastic, likely dispersal-dominated, processes are replaced by rather deterministic processes such as environmental filtering and biotic interactions after around 60 years of succession in a glacier forefield. The niche-based character of later successional processes is further supported by a decline in multi-beta-diversity. Our results may update concepts of community assembly by considering multiple taxa, help to bridge the gap between research on successions and community assembly, and provide insights into the emergence of multidiverse and complex ecosystems.
Similar content being viewed by others

Frequent disturbances enhanced the resilience of past human populations

A climate-induced tree species bottleneck for forest management in Europe

Land conversion to agriculture induces taxonomic homogenization of soil microbial communities globally
Introduction.
Succession and community assembly research are neighboring fields, each with a long history of constructive debates and a large body of ecological literature. Both research lines are founded on overlapping concepts considering processes such as dispersal, environmental filtering, biotic interactions, and stochasticity as structuring elements of local communities and ecosystems. Nevertheless, succession and community assembly research focus on different spatial and temporal scales, which may have hindered a mutual exchange of ideas 1 . Succession research relates to the initial development of ecosystems and communities over time. Community assembly studies, on the other hand, try to elucidate the past drivers of local and recent diversity patterns regardless of temporal components. Attempts to synthesize the two fields led to the development of an integrated conceptual framework of succession and community assembly dynamics. Here, drivers of community structure are considered from an explicitly temporal perspective, assuming a temporal sequence of assembly processes shifting from stochastic (likely dispersal) to rather deterministic (niche-based and interaction-mediated) processes along the course of succession 1 . The proposed framework also emphasizes the importance of threshold dynamics, predicting that gradual changes in the abiotic and biotic environment are followed by rapid shifts in the predominant processes that determine community assembly 2 , 3 . These shifts in processes are expected to be associated with various community-level responses such as changes in diversity, species composition, and functionality which ultimately leads to a community state shift 1 , 2 , 4 . Recent research suggests that sudden ecosystem shifts are not restricted to changes in species composition of single organismal groups but that local diversity patterns emerge from on-site interactions between multiple taxa 5 , 6 . Multidiversity, an aggregate measure of biodiversity that integrates the standardized diversities of multiple taxa 7 , has been shown to reflect ecosystem functionality 8 , species composition 9 , and multitrophic interactions 10 more accurately than diversity measures considering only one taxon. Additionally, the responses of different organismal groups to successional gradients may differ either due to stochastic or deterministic drivers 11 , 12 , 13 , 14 , 15 . For instance, the assembly of belowground bacterial and fungal communities follows individual trajectories during primary succession that differ from the assembly of vascular plant communities 16 , 17 . Thus, the concept of multidiversity is a well-suited approach that can reflect various community-level responses and allows more concise conclusions about different ecosystem states along successions, as well as underlying assembly processes than considering a single or few taxa only 16 .
The assumed changes of assembly processes along successions, a decrease in the importance of stochasticity, and a simultaneous increase in niche-based and interaction-based processes, have consequences for the taxonomic, functional, and phylogenetic composition of communities and thus beta diversity 1 , 17 . The framework by Chang and HilleRisLambers (2016) 1 predicts an increase in functional and phylogenetic diversity of local single-taxon communities in later successions due to niche differentiation and intensified biotic interactions. In a multidiversity context where multiple taxa are considered, increased interactions and thus potentially strong dependencies between taxa may result in aggregated co-occurrence patterns 18 , which may have consequences for the beta-diversity between local communities—and not necessarily for the functional and phylogenetic diversity within a community. For instance, interactions between plants and soil-inhabiting microorganisms intensify with successional age 19 and regulate plant-animal interactions 20 , which is reflected in the co-occurrence of the partners involved in these interactions. Such interactions may thus result in reduced species turnover across local assemblages in older successional stages. Yet, it is an ongoing debate whether ecological communities generally converge towards a core set of species over the course of succession 21 , 22 , and studies on successional convergence have mostly been focused on singular taxa neglecting potential interactions and co-occurrences across organismal groups 23 , 24 , 25 . To specifically test the extent of species turnover across several taxa, we introduce multi-betadiversity, an aggregated measure of taxonomic community dissimilarity. This index allows to test whether the expected increase in biotic interactions is reflected in a reduced compositional variation (i.e., reduced multi-betadiversity regarding several taxa) of the multidiverse community, i.e., a community that comprises a number of taxonomic groups, once niche-based and interaction-mediated assembly processes dominate over stochastic dispersal as main mechanisms shaping multidiversity.
Assuming that multidiversity more precisely reflects ecosystem responses and functions, we tested predictions deduced from contemporary succession hypotheses using a dataset on multidiversity comprising inventories of plants, animals, and microorganisms along a successional gradient. Most studies on multidiversity have been undertaken in well-developed ecosystems that have undergone severe anthropogenic alterations in the past and focused on the drivers of biodiversity decline 7 , 9 , 10 . The mechanisms behind the successional emergence of multidiversity and ecosystem complexity under natural conditions, however, are yet poorly understood. Thus, considering multidiversity within a successional framework will help to gain insights into the temporal dynamics of the processes that shape the initial emergence of biodiversity in natural ecosystems that comprise multiple organismal groups with unique and complementary ecological functions. We adopted the concept of multidiversity to empirically test predictions of the integrated framework of succession and community assembly dynamics on an ecological gradient of primary succession following glacial retreat in the Austrian Alps 26 . We assessed the multidiversity and multi-betadiversity of five organismal groups, including vascular plants, bryophytes, invertebrates, and soil-inhabiting fungi and bacteria on 110 plots spanning 170 years of primary succession. We estimated multidiversity by the mean of ranked normalized Shannon-diversities and calculated multi-betadiversity as the mean Bray-Curtis dissimilarities between plots regarding the composition of individual taxa. According to the conceptual framework by Chang and HilleRisLambers (2016) 1 , we predicted that the multidiverse community will undergo a rapid shift that is induced by a sudden change of the biotic and abiotic environment along the successional gradient. We expect this shift to be associated with increased biotic interactions and stronger environmental influences that result in a reduced compositional variation between plots of the multidiverse community.
Accordingly, we identified a threshold in multidiversity development (i.e., a shift from an increase of multidiversity with time to a rather stationary phase) and estimated the relative importance of stochasticity, as well as environmental and biotic drivers on the multidiverse community before and after the threshold using path analysis. We further highlighted the more structured character of community assembly after the shift by comparing multi-betadiversity estimates of the early and late-successional communities.
Breaking point in multidiversity
Multidiversity follows a non-linear trajectory along the successional gradient. We screened the gradient for the predicted shift from a developing (characterized by an increase in multidiversity over time) to a more stationary ecosystem (characterized by non-monotonic variation in multidiversity over time) using breaking point analysis, which detects changes in the slope of associations. According to Groffman (2006) 27 breaking points represent ecological thresholds, which is characterized by a rapid change of the slope in the association between time since deglaciation as explanatory variable and multidiversity as dependent variable in our study. We found such a breaking point by cross-validating a broken-stick model to alternative models that either did not include a breaking point or included a disjunct breaking point using Bayesian inference 28 . We then used Bayes Factors analysis to validate the exact location of the breaking point after about 60 years of succession, i.e., plot index 44 along the gradient (lower-upper bounds: 40–74 years after deglaciation; see “Methods”, Fig. 1 , Supplementary Table 1 ). Resulting piecewise linear models showed a significant linear increase in multidiversity during the early successional stage and a stationary multidiversity (non-significant relationship with time) for the late successional stage (early: t 42 = 5.77, p < 0.001, r 2 = 0.44; late: t 64 = −0.55, p = 0.58, r 2 = 0.004; Fig. 1 ). To assess whether the delineated threshold is also reflected in the composition of the multidiverse community, we calculated the relative abundance of each taxon along the successional gradient and visualized it in a temporally ordered multi-taxa community table of species occurrences (see “Methods”, Fig. 2 ).
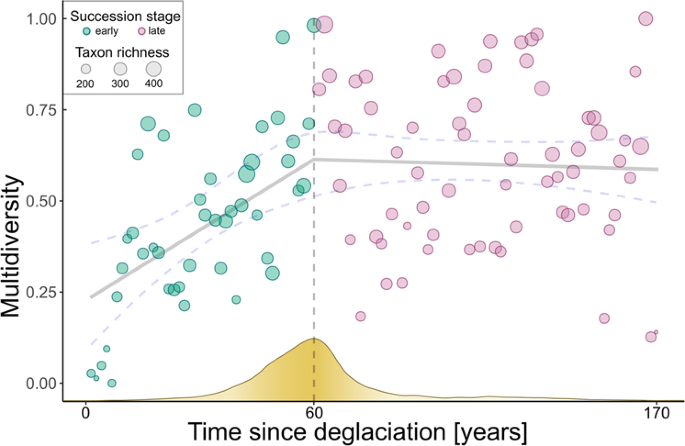
The vertical dashed line indicates the estimated threshold in multidiversity development, which is the threshold in community assembly as predicted by the framework of Chang and HilleRisLambers (2016) 1 . The grey regression line indicates the linear correlation between multidiversity and time since deglaciation using a broken-stick model. Blue dashed lines indicate the 2.5% and 97.5% quantiles of fitted values. The yellow curve on the x-axis resembles the posterior distribution for the estimate of the breaking point, i.e., higher density of the frequency indicates a higher probability for the breaking point to be located at a given value.
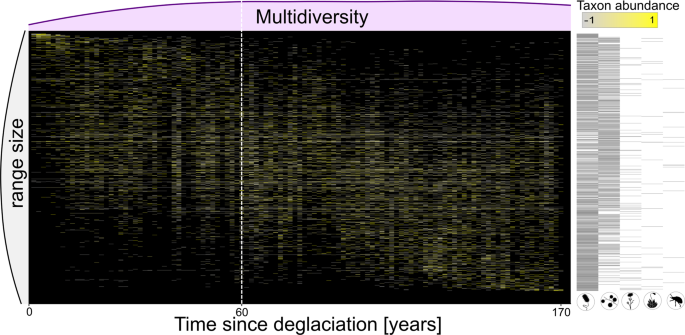
Tiles indicate the relative abundance of each taxon at a given plot relative to the mean abundance of the taxon along the gradient. Taxa are ordered by the weighted average plot of their occurrence, i.e., taxa that reach their abundance optima early in succession appear in the top left and late successional specialist in the bottom right. Relative taxon abundance is color-coded and reflects z-scores with the mean abundance as 0 and one standard deviation as +1 or −1, rescaled between −1 and 1. Multidiversity is indicated as light pink density plot on top of the community composition plot. Grey density plot on the left side indicates the range size of the taxa, i.e., whether their occurrence is restricted to a narrow range of the successional gradient or whether taxa occur along the whole gradient. Black bars on the right represent the organismal group of the taxa in each row, symbols of organismal groups are ordered by decreasing frequency; white dashed horizontal line marks the threshold.
Drivers of multidiversity
To quantify the putative relative importance of stochastic dispersal, species interactions, and environmental drivers before and after the threshold, we specified a path model in which time since deglaciation and mean growth-season soil temperature act as exogenous variables and directly affect diversities of single organismal groups. Soil temperature is an environmental factor that is important for the diversity of several trophic levels in alpine environments 29 , 30 . We also tested the effects of other environmental variables, such as soil nutrient content and soil-pH value but could not detect any significant effects and thus removed these variables from the final model (see “Methods” and Supplementary Data 1 – 4 ). After accounting for environmental variation, we consider the effect of time since deglaciation as a proxy for stochastic events, such as successful dispersal and colonization events 31 , 32 , leading to cumulating multidiversity over time. The total effects of time since deglaciation (proxy for stochastic dispersal) and environmental drivers on multidiversity were estimated as indirect effects mediated through the organismal groups. Biotic interactions were modeled as the residual covariance among the diversity values among all organismal groups that remained after accounting for stochasticity and environmental variation. If corrected for such underlying common causes, strong positive covariances within communities can be interpreted as biotic interactions 33 , 34 . We further tested whether the residuals of the estimated models show significant spatial autocorrelation but could not detect any unexplained variance that could be attributed to geographic variation (see Methods and Supplementary Table 2 ).
The path analysis provided good model fit (Model fit: p chi-square > 0.05; CFI > 0.95; TLI ≥ 0.9; SRMR < 0.09; RMSEA early < 0.05; RMSEA late = 0.066 with lower bound of confidence interval = 0) and revealed different influences of the exogenous variables between the early and late successional environments (Fig. 3 , Supplementary Data 4 ). During early succession, time since deglaciation had a strong positive effect on the diversity of bryophytes ( β = 0.66, p < 0.01), arthropods ( β = 0.62, p < 0.01), and bacteria ( β = 0.32, p = 0.04), whereas there was no significant effect of temperature on any organismal group. Time since deglaciation also had a significant positive indirect effect on total multidiversity ( β = 0.74, p < 0.01) during early succession, whereas temperature did not affect multidiversity ( β = 0.01, p = 0.93, Fig. 3a ). Furthermore, diversities of the five organismal groups varied independently of each other suggesting little or no mutual influences. In late succession, mean growth-season soil temperature had a significant positive direct effect on the diversity of bryophytes ( β = 0.35, p = 0.01) and a clear positive indirect effect on multidiversity mediated through all organismal groups ( β = 0.30, p = 0.01). Plot age had a negative effect on fungal diversity ( β = −0.35, p < 0.01) and a positive effect on bryophyte diversity ( β = 0.30, p = 0.01) but no effect on multidiversity ( β = −0.01, p = 0.89). We further detected positive residual covariances between vascular plant ( β = 0.26, p = 0.04), invertebrate ( β = 0.25, p = 0.05) and bacterial ( β = 0.29, p = 0.03) diversities with fungal diversity, suggesting mutual influences between taxa (Fig. 3b ).
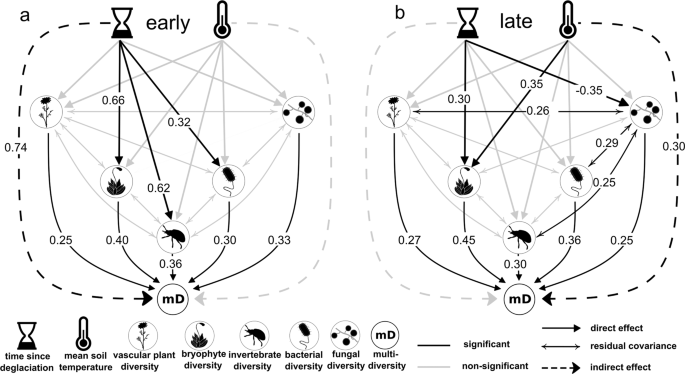
Time since deglaciation and mean growth-season soil temperature act as exogenous variables with directed effects on the diversities of vascular plants, bryophytes, invertebrates, and soil-inhabiting fungi and bacteria. Indirect effects of exogenous variables on multidiversity were calculated by mediation analysis through the direct effects of the organismal groups on multidiversity. Biotic interactions are modeled as covariances between organismal groups. Numbers represent standardized path coefficients and are given for significant paths only. The path model was calculated for ( a ) the early and ( b ) the late successional stage separately. Silhouette images were obtained from PhyloPic ( http://phylopic.org ) under a public domain license (CC0 1.0 license).
Multi-betadiversity
We predicted that the shift from dispersal dominated stochastic events in early successional stages to more deterministic niche-based processes that are induced through environmental filtering and biotic interactions in late successional stages is associated with lower beta-diversity among communities after the threshold. To test this prediction, we calculated a measure of total community dissimilarity, multi-betadiversity (mbD) for both successional stages by the mean Bray-Curtis dissimilarities of the multidiverse community (see “Methods”). Of n = 5 organismal groups surveyed, n = 4 showed a decrease in betadiversity and in total, multi-betadiversity was significantly lower in the late successional stage (mean ± SD; mbD early = 0.75 ± 0.09; mbD late = 0.70 ± 0.08; Fig. 4 ).
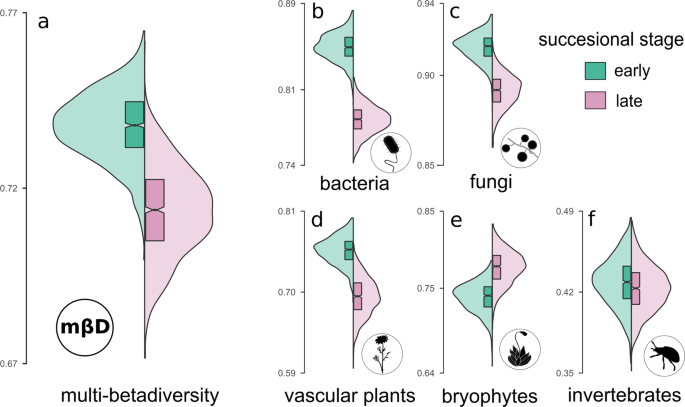
Mean Bray-Curtis dissimilarities were used to calculate betadiversity and multi-betadiversity values. Green violin plots resemble the dissimilarity values of the early successional stage ( n = 44 plots), rose violin plots indicate the late successional stage ( n = 66 plots). Boxplots indicate the upper and lower quartile of the estimates, and the size of the notch represents the confidence interval of the median. a mean of all organismal groups, i.e., multi-betadiversity ( b ) bacteria, c fungi, d vascular plants, e bryopyhtes, and ( f ) invertebrates. Silhouette images were obtained from PhyloPic ( http://phylopic.org ) under a public domain license (CC0 1.0 license).
Our study is an empirical test of the conceptual framework integrating succession and community assembly dynamics proposed by Chang and HilleRisLambers (2016) 1 and modified here in the context of multidiverse communities. We showed that threshold dynamics play an important role in the generation of multidiversity under natural conditions and that succession comprises a sequence of different ecosystem states that can be detected using multidiversity. These threshold-mediated shifts in ecosystem states are associated with substantial changes in the community assembly processes. So far, the importance of threshold dynamics has been recognized for anthropogenically altered ecosystems either as catastrophic shifts with respect to climate change 4 , 6 or for restoration efforts of anthropogenically altered landscapes 2 , 3 . Here, we revealed a threshold after about 60 years of ecosystem development when multidiversity became stationary. Significant thresholds that are marked by a steep increase followed by stationarity after 40–60 years of succession appear to be a generalizable pattern that occurs in various aspects of ecosystem development, such as plant, invertebrate, and microbial diversities and functionality in glacier forefields in Europe and Northern America 11 , 35 , 36 , 37 , 38 . The threshold became also evident in the change of community composition with different sets of taxa before and after the breaking point. Prior to the threshold, communities mainly consist of species that reach their abundance optima early in succession. These pioneering species were soon accompanied by taxa with no clear preference of early or late successional stages leading to an increase in multidiversity over time. After the threshold, specialists for early successions are replaced by specialists for late successional stages, consequently multidiversity remained stationary over time (Fig. 2 ). Thus, acknowledging the effects of threshold dynamics during the development of natural multidiverse ecosystems, and their universality in ecological systems provides valuable insights into the patterns and processes of initial ecosystem development.
Our results indicate an ecosystem state shift associated with different assembly processes: the first 60 years of succession are characterized by stochastic species additions, afterwards rather deterministic processes such as niche-filtering and biotic interactions dominate. Although different organismal groups follow individual trajectories during primary succession, multidiversity is mainly promoted by stochastic drivers during the initial phase of ecosystem development, most assumably by heterogenous dispersal events. Over the course of succession, stochasticity is replaced by environmental filtering and biotic interactions as the structuring mechanism of multidiversity.
Our data do not allow to directly test for mutual influences between organismal groups on the taxon level, but we find a strong pattern of positive covariances of the diversities of various taxonomic groups after accounting for environmental variation that is indicative of biotic dependencies within the community. Community covariances have initially been applied to evaluate the relative importance of biotic interactions in multitrophic communities by estimating synchronized fluctuations in population abundances where positive covariances have been interpreted as a result of facilitative interactions within a community 33 , 34 , 39 . On the diversity level as analyzed in this study, however, positive covariances among organismal groups do not necessarily reflect only facilitative interactions, because diversity in one group is likely to be positively linked to diversity of another trophic group through specialized interactions, either positive or negative 40 . For instance, in host-pathogen relationships, more diverse host communities harbor a higher diversity of obligate pathogens 41 and below-ground fungal diversity is promoted by diverse bacterial assemblages through complex feedback loops 42 . A previous study in the same glacier forefield showed that after more than 50 years of ecosystem development, the soil microbial community is mainly supported by carbon from recent plant production, whereas during the initial stage of ecosystem development, the microbial community is mainly sustained by ancient carbon sources 43 . The time point of the shift in the main carbon source largely coincides with our delineated threshold in multidiversity development. The increased interdependence of the soil microbial and plant community during the later successional stage is also reflected in the positive covariances of vascular plant, fungal and bacterial diversities in our path model. Previous studies have also shown strong mutual dependencies of invertebrate and fungal communities in developing alpine environments 44 , 45 . Invertebrates increase the diversity of soil-inhabiting microbial taxa by propagule dispersal and create a variability of suitable habitats through modifications of the physical environment 46 . Some fungal-feeding taxa show preferences for certain types of fungal hyphae that frequently have distinct associations with selected plant species as pathogens 47 , mycorrhizal partners 48 , or litter decomposers 49 , which well explains the pattern of covariance among these groups in our analysis. Further, the importance of fungi-animal interactions for the development of ecological complexity was predicted in foundational works on succession theory by Connell and Slatyer (1977) 50 , as heterotrophic fungi were expected to feed on the carcasses and dung of animals and in turn, certain arthropods were hypothesized to be reliant on decomposed substrate for the developmental stages of their life cycles. Although bryophytes have been shown to closely interact with epiphytic microorganisms after glacial retreat 51 , potential interactions of bryophytes with microorganisms may not be detectable in our path model as we sampled soil microbial communities and not all substrate types colonized by bryophytes in our study site (e.g., rocks, scree, and litter).
The threshold-mediated character of successional processes is further supported by a pronounced decline in multi-betadiversity that is indicative of an increased dependence structure among the organismal groups. In line with that assumption, we detected a lower beta-diversity in all those organismal groups that also show positive community covariances in the path model. Out of the five organismal groups we studied, only bryophytes were characterized by a higher betadiversity in the late successional stage that might be attributable to increasing variability in microhabitats as the communities mature 52 , 53 . Although co-occurrence patterns must not necessarily reflect biotic interactions 54 , reduced beta-diversity between plots in later successional stages still may be indicative for stronger biotic interactions, which finds support in studies on plant community assembly 55 , 56 . The literature suggests the increasing importance of interactions in community development over time. One explanation put forward is that with increasing niche differentiation, energy and nutrient flows in an ecosystem follow more complex biochemical pathways that necessitate biotic interactions to retain nutrients in the ecosystem through closed mineral cycles and complex food-webs 1 , 17 . Our results support the increasing importance of biotic interactions as in the late successional stage, our path model revealed positive community covariances between the soil microbial, vascular plant, and arthropod communities that may represent such complex nutritional cycles comprising primary producers, heterotrophic organisms, and decomposers For the organismal groups forming these putative cycles, we find a reduced betadiversity between the plots that indicates a strong interrelatedness of individual taxa after the threshold, which may lead to re-occurring core assemblages of species of several taxa. These assumptions and the threshold suggested by our data after 60 years are further supported by the study of Bardgett et al. (2007) 43 that revealed a high dependence of heterotrophic microorganisms on plant communities in the Ödenwinkel forefield after a similar number of years. We thus recommend extending the integrative framework of succession and community assembly for multiple interacting taxa that mutually shape their diversity and composition and thus cause a reduction of betadiversity.
Understanding the relative importance and temporal dynamics of deterministic and stochastic processes is a key challenge in community ecology, especially in natural systems and has been in the center of broad debates among ecologists. Using the multidiversity and multi-betadiversity approach allows us to comprehensively understand the processes leading to stable, resilient, and complex ecosystems, which may remain vague in single-taxon approaches. Thus, our study contributes to a synthesis of community ecological theories into succession research, acknowledging the fundamental importance of abrupt state shifts in natural ecosystems. These results are not only a proof of concept but also (re-)emphasize that succession is a multi-faceted rather than a linear process that comprises a sequence of assembly processes towards multidiversity and ecosystem complexity.
Study design
The study was conducted in the long-term ecological research platform Ödenwinkel which was established in 2019 in the Hohe Tauern National Park, Austria (Dynamic Ecological Information Management System—site and dataset registry: https://deims.org/activity/fefd07db-2f16-46eb-8883-f10fbc9d13a3 , last access: March 2021). A total of n = 135 permanent plots was established within the glacier forefield of the Ödenwinkelkees which was covered by ice at the latest glacial maximum in the Little Ice Age around 1850. For this study, we used a subset of n = 110 plots with complete datasets with all biotic and abiotic variables available. The plots represent a successional gradient spanning over 1.7 km in length and were distributed within the glacier forefield to reflect the gradient of glacial retreat at an altitude ranging from 2070 to 2170 m a.s.l (distance between neighboring plots, median ± SD: 19 m ± 6.3 m). Each plot was given a unique index number according to the location along the gradient: the plot with index 1 is located closest to the glacier, plot index 135 is the farthest away from the glacier. Plot age was estimated based on data of historical glacial extent and as glacial loss did not occur at a constant rate, plot locations were pre-selected to represent a linear gradient of time since deglaciation rather than a spatial gradient with equally spaced intervals between plots. To ensure independence of plots in areas of slow glacial retreat, we chose a minimum distance of 5 m between two neighboring plots. Plots were defined as squares with an area of 1 m² and were all oriented in the same cardinal direction. Further details on the design of the research platform, exact plot positions and details on the surrounding environment, as well as on the historical glacial extent can be found in Junker et al. (2020) 26 .
In 2019, we identified all vascular plant and bryophyte species present on the plots and estimated their cover with a resolution of 0.1%. We sampled above-ground arthropod diversity by installing two pitfall traps on each plot. Traps were set active for a total of n = 7 days. The abundance of all arthropods, excluding Collembola and Acari, larger than 3 mm was counted. The abundance of Collembola and Acari and of animals smaller than 3 mm was estimated based on random samples of aliquots of the total sample. All arthropods and other animals are identified to the order level. Soil-inhabiting bacteria and fungi were sampled from soil cores from an approximate depth of 3 cm, as soil development in the proglacial study area was limited to the top layers of the pedosphere. Soil-microbiome samples were analyzed by next-generation sequencing, and microbiome profiling of isolated DNA was performed by Eurofins Genomics (Ebersberg, Germany). Prior to the statistical analysis of microbial communities, we performed a cumulative sum scaling (CSS) normalization (R-package “metagenomeSeq” v1.28.2) 57 on the count data to account for differences in sequencing depth among samples. Detailed information on the sampling strategies of all organismal groups can be found in Junker et al. (2020) 26 . Soil temperature measurements were done by installing temperature loggers (MF1921G iButton, Fuchs Elektronik, Weinheim, Germany) 10 cm north of each plot center, at the same depth of 3 cm at which the microbial samples were taken. Mean growth-season soil temperature was calculated based on the recordings ranging from 26th of June to 16th of September representing the period in which the plots were free of permanent snow cover before and after the winter 2019/2020. In 2020, soil samples were taken and soil nutrients (Ca, P, K, Mg, and total N 2 ) as well as soil pH were measured on all plots by AGROLAB Agrar und Umwelt GmbH (Sarstedt, Germany). Soil nutrient analysis was performed according to the Ö-Norm Boden: L 1087: 2012-12 (K and P—mg/1000 g), L 1093: 2010-12 (Mg—mg/1000 g), and L 1083: 2006-04 (pH). Total N2 (%) was determined according to the DIN EN 16168: 2012-11.
Statistics and reproducibility
Calculation of multidiversity.
Multidiversity is defined as the cumulative diversity of a number of taxonomic groups 7 . The multidiversity of the n = 110 plots composed of the diversities of vascular plants, bryophytes, invertebrates, fungi, and bacteria present in each plot and was defined as follows: First, we calculated the Shannon diversities of each of the taxonomic groups in each plot. Second, we ranked the plots by increasing diversity for each of the five taxonomic groups individually, i.e., for each plot we received n = 5 ranks. The mean rank of each plot was defined as multidiversity mD. For better interpretability of the index, we then normalized the mean ranks as
to scale them between zero and one. Ranks were used to give the same weighting to each taxonomic group despite deviations in the absolute values of Shannon diversity and to reduce the impact of outliers in mD values. To account for differences in the sequencing depth of the microbial raw dataset and sampling effort of the invertebrate trapping, we performed multiple rarefactioning prior to the calculation of Shannon diversity by averaging the results of n = 999 iterations (R-package “rtk” v0.2.5.7) and used original read numbers instead of using the CSS-normalized dataset for the microbial dataset.
Breaking point analysis
According to the hypothesis of community assembly dynamics 1 and by visual inspection of the relationship between multidiversity and successional age of the plots, we expected two different stages of community establishment along the successional gradient. These stages are separatable by the transition from a developing (characterized by an increase in multidiversity over time) to a more stationary ecosystem (characterized by non-monotonic variation in multidiversity over time). Such non-linear associations are suggestive of regime-shifts of the ecosystem and can be interpreted as ecological thresholds 27 . Piecewise regression models have been shown to be the most suitable tool for the detection of ecological thresholds in natural systems, as they are able to correctly estimate the probability, as well as the number and position of ecological thresholds 58 . We screened the successional gradient for the existence of a threshold by comparing four different models with mD as the dependent and plot age as the independent variable using the R-package “mcp” v0.3.0.9 28 . The mcp-method fits piecewise regression models with a pre-defined number of breaking points and is based on Bayesian inference. We specified a base model m1 (mD-values remain constant along the gradient), and compared it to three alternative models (m2 = constant linear increase of mD-values without a breaking point, m3 = one breaking point in mD-values with a segregated slope (abrupt-threshold model), m4 = one breaking point in mD-values with a joined slope (broken-stick or smooth-threshold model)). For each model, we separately ran three Markov Chain Monte Carlo estimators with a uniform prior for a total of n = 11,000 generations while discarding the first n = 1000 generations as burn-in. Model convergence was estimated by visually inspecting the trace plots and checking that all model parameters reached stationarity. We then compared the predictive performance of the four models using leave-one-out-cross-validation and confirmed the exact position of the breaking point using Bayes Factors. Both validation methods can be applied for a robust and accurate testing of competing hypotheses in ecological datasets 59 , 60 , 61 .
Community taxa occurrence
To visualize the distribution of individual taxa and thus the community-wide taxonomic turnover along the successional gradient, we estimated the abundance optimum of each taxon that occurred on at least three plots. Abundance optima were estimated by calculating the weighted mean plot of occurrence with abundance of each taxon as weighting factor (i.e., cover for vascular plants and bryophytes, individual count for invertebrates, and CSS-normalized read number for microorganisms). We further calculated the relative abundance of each taxon per plot by using z-scores of abundances. The mean abundance was set as 0 with one standard deviation as +1 or −1. To allow a comparison across taxa, z-scores were then rescaled between −1 and 1. Range size was estimated by calculating the variance of occurrence plots (i.e., the span of plots on which a taxon was found along the gradient) weighted by the abundance of the taxon on the respective plots.
Path analysis
We used path analysis (i) to model the influence of the abiotic environment on the diversities of all taxonomic groups individually, (ii) to estimate the strength of covariance between the diversities of those groups, (iii) to infer the effect sizes of the diversity of each group on total mD, and (iv) estimate the strength of indirect effects of the exogenous variables that are mediated through the organismal groups on mD. We built separate models with identical structure for the early ( n = 44 plots) and late ( n = 66 plots) successional stages. The stages were delineated by the threshold identified in the breakpoint analysis. Exogenous variables in the model were time since deglaciation and mean growth-season soil temperature. Time since deglaciation reflects the plot age and can be seen as a proxy for the increasing chance of heterogeneous dispersal events that occur over time. The mean temperature of the growing season is an estimate for environmental heterogeneity and has been shown to affect diversity directly and indirectly on various trophic levels 29 . All variables were scaled by subtracting the mean and dividing through the standard deviation. We first ran a full model that included soil pH and soil nutrient content as additional exogenous variables. None of the two variables showed a significant direct effect on any organismal group or a significant indirect effect on multidiversity during either the young or late successional stage. Accordingly, we stepwise removed the two variables from the model while cross-checking whether the effect strength of one variable became significant in the absence of the other variable. As no significant effects occurred or the model fit significantly decreased, we decided to remove both variables from the final model. Within the final model, the strength of residual covariance between the diversities of all taxonomic groups was estimated while accounting for influences of time since deglaciation and temperature. If corrected for an underlying common cause, such as environmental autocorrelation, strong covariances between members of a community can be interpreted as biotic interactions and especially positive community covariances are indicative of facilitative effects within the community 33 , 34 . All path models were estimated using the R-package “lavaan” v0.6-7 62 .
Test for spatial autocorrelation
Studies taking advantage of space-for-time substitution generally are prone to erroneous conclusions caused by spatial autocorrelation, especially when sampling points are located at varying distances 63 , 64 . We tested for the presence of residual spatial autocorrelation in the estimated path models by calculating spatial neighbor matrices for distance classes of 5 m and 10 m between plots (i.e., the potential to include a maximum of three or five neighboring plots). For both distance classes, we estimated Moran´s I for the residuals of the piece wise linear models of the breaking point analysis and the case-wise residuals of the exogenous variables of the path models using the R-Package “spdep” v1.1-8 65 .
We defined Multi-betadiversity (mbD) as the cumulative averaged pairwise-dissimilarity across a number of organismal groups. First, we split the total community composition data table (sites x species, n = 110 plots) for each organismal group into two tables containing the plots before ( n = 44 plots) and after ( n = 66 plots) the threshold. Second, we calculated pairwise Bray-Curtis dissimilarities for each of the organismal groups individually. Third, we calculated mbD as the mean of the n = 5 dissimilarity estimates of each plot pair.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Raw sequences of next-generation 16 S rRNA gene amplicon sequencing are available at the NCBI Sequence Read Archive (SRA) under the BioProject accession PRJNA701884 and PRJNA701890. Raw floristic, microbial, and zoological community composition data are available in a Mendeley Data repository 66 . https://doi.org/10.17632/xkv89tbftc.1 .
Code availability
R scripts are available in a Mendeley Data repository 67 . https://doi.org/10.17632/dr6d3728xb.1 .
Chang, C. & HilleRisLambers, J. Integrating succession and community assembly perspectives. F1000Research 5 , 1–10 (2016).
Article Google Scholar
Suding, K. N. & Hobbs, R. J. Threshold models in restoration and conservation: a developing framework. Trends Ecol. Evol. 24 , 271–279 (2009).
Article PubMed Google Scholar
Kadowaki, K., Nishijima, S., Kéfi, S., Kameda, K. O. & Sasaki, T. Merging community assembly into the regime-shift approach for informing ecological restoration. Ecol. Indic. 85 , 991–998 (2018).
Scheffer, M., Carpenter, S., Foley, J. A., Folke, C. & Walker, B. Catastrophic shifts in ecosystems. Nature 413 , 591–596 (2001).
Article CAS PubMed Google Scholar
Lu, M. & Hedin, L. O. Global plant–symbiont organization and emergence of biogeochemical cycles resolved by evolution-based trait modelling. Nat. Ecol. Evol. 3 , 239–250 (2019).
Berdugo, M. et al. Global ecosystem thresholds driven by aridity. Science 367 , 787–790 (2020).
Allan, E. et al. Interannual variation in land-use intensity enhances grassland multidiversity. Proc. Natl Acad. Sci. USA 111 , 308–313 (2014).
Delgado-Baquerizo, M. et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 4 , 210–220 (2020).
Felipe-Lucia, M. R. et al. Land-use intensity alters networks between biodiversity, ecosystem functions, and services. Proc. Natl Acad. Sci. USA 117 , 28140–28149 (2020).
Article CAS PubMed PubMed Central Google Scholar
Manning, P. et al. Grassland management intensification weakens the associations among the diversities of multiple plant and animal taxa. Ecology 96 , 1492–1501 (2015).
Kaufmann, R. Invertebrate succession on an alpine glacier foreland. Ecology 82 , 2261–2278 (2001).
Blaalid, R. et al. Changes in the root-associated fungal communities along a primary succession gradient analysed by 454 pyrosequencing. Mol. Ecol. 21 , 1897–1908 (2012).
Fenton, N. J. & Bergeron, Y. Stochastic processes dominate during boreal bryophyte community assembly. Ecology 94 , 1993–2006 (2013).
Dini-Andreote, F., Stegen, J. C., Van Elsas, J. D. & Salles, J. F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl Acad. Sci. USA 112 , E1326–E1332 (2015).
Måren, I. E., Kapfer, J., Aarrestad, P. A., Grytnes, J. A. & Vandvik, V. Changing contributions of stochastic and deterministic processes in community assembly over a successional gradient. Ecology 99 , 148–157 (2018).
Pulsford, S. A., Lindenmayer, D. B. & Driscoll, D. A. A succession of theories: purging redundancy from disturbance theory. Biol. Rev. 91 , 148–167 (2016).
Odum, E. P. The strategy of ecosystem development. Science 164 , 262–270 (1969).
Ohlmann, M. et al. Mapping the imprint of biotic interactions on β-diversity. Ecol. Lett. 21 , 1660–1669 (2018).
Chen, C., Chen, H. Y. H., Chen, X. & Huang, Z. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 10 , 1–10 (2019).
CAS Google Scholar
Howard, M. M., Kao-Kniffin, J. & Kessler, A. Shifts in plant–microbe interactions over community succession and their effects on plant resistance to herbivores. N. Phytol. 226 , 1144–1157 (2020).
Lepš, J., Rejmánek, M., Leps, J. & Rejmanek, M. Convergence or divergence: what should we expect from vegetation succession? Oikos 62 , 261–264 (1991).
Fukami, T., Bezemer, T. M., Mortimer, S. R. & Van Der Putten, W. H. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett. 8 , 1283–1290 (2005).
Brown, S. P. & Jumpponen, A. Contrasting primary successional trajectories of fungi and bacteria in retreating glacier soils. Mol. Ecol. 23 , 481–497 (2014).
Castle, S. C. et al. Biogeochemical drivers of microbial community convergence across actively retreating glaciers. Soil Biol. Biochem. 101 , 74–84 (2016).
Article CAS Google Scholar
Chang, C. C. et al. Testing conceptual models of early plant succession across a disturbance gradient. J. Ecol. 107 , 517–530 (2019).
Junker, R. R. et al. Ödenwinkel: an Alpine platform for observational and experimental research on the emergence of multidiversity and ecosystem complexity. Web Ecol. 20 , 95–106 (2020).
Groffman, P. M. et al. Ecological thresholds: the key to successful environmental management or an important concept with no practical application? Ecosystems 9 , 1–13 (2006).
Lindeløv, J. K. mcp: an R package for regression With multiple change points. J. Stat. Softw. https://doi.org/10.31219/osf.io/fzqxv (2020).
Ohler, L. M., Lechleitner, M. & Junker, R. R. Microclimatic effects on alpine plant communities and flower-visitor interactions. Sci. Rep. 10 , 1–9 (2020).
Cavieres, L. A. et al. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecol. Lett. 17 , 193–202 (2014).
Lowe, W. H. & McPeek, M. A. Is dispersal neutral? Trends Ecol. Evol. 29 , 444–450 (2014).
Legendre, P., Borcard, D. & Peres-Neto, P. R. Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecol. Monogr. 75 , 435–450 (2005).
Ranta, E. et al. Detecting compensatory dynamics in competitive communities under environmental forcing. Oikos 117 , 1907–1911 (2008).
Houlahan, J. E. et al. The utility of covariances: a response to Ranta et al. Oikos 117 , 1912–1913 (2008).
Tscherko, D., Rustemeier, J., Richter, A., Wanek, W. & Kandeler, E. Functional diversity of the soil microflora in primary succession across two glacier forelands in the Central Alps. Eur. J. Soil Sci. 54 , 685–696 (2003).
Raffl, C., Mallaun, M., Mayer, R. & Erschbamer, B. Vegetation succession pattern and diversity changes in a Glacier Valley, Central Alps, Austria. Arct. Antarct. Alp. Res. 38 , 421–428 (2006).
Schütte, U. M. E. et al. Bacterial diversity in a glacier foreland of the high Arctic. Mol. Ecol. 19 , 54–66 (2010).
Dong, K. et al. Soil fungal community development in a high Arctic glacier foreland follows a directional replacement model, with a mid-successional diversity maximum. Sci. Rep. 6 , 1–9 (2016).
Houlahan, J. E. et al. Compensatory dynamics are rare in natural ecological communities. Proc. Natl Acad. Sci. USA 104 , 3273–3277 (2007).
Vellend, M. Effects of diversity on diversity: consequences of competition and facilitation. Oikos 117 , 1075–1085 (2008).
Rottstock, T., Joshi, J., Kummer, V. & Fischer, M. Higher plant diversity promotes higher diversity of fungal pathogens, while it decreases pathogen infection per plant. Ecology 95 , 1907–1917 (2014).
Baudy, P. et al. Fungal–fungal and fungal–bacterial interactions in aquatic decomposer communities: bacteria promote fungal diversity. Ecology 102 , 1–16 (2021).
Bardgett, R. D. et al. Heterotrophic microbial communities use ancient carbon following glacial retreat. Biol. Lett. 3 , 487–490 (2007).
Article PubMed PubMed Central Google Scholar
Bokhorst, S. & Wardle, D. A. Snow fungi as a food source for micro-arthropods. Eur. J. Soil Biol. 60 , 77–80 (2014).
Hågvar, S. et al. Ecosystem birth near melting glaciers: a review on the pioneer role of ground-dwelling arthropods. Insects 11 , 1–35 (2020).
Wardle, D. A. The influence of biotic interactions on soil biodiversity. Ecol. Lett. 9 , 870–886 (2006).
Sabatini, M. A. & Innocenti, G. Functional relationships between Collembola and plant pathogenic fungi of agricultural soils. Pedobiologia (Jena.) 44 , 467–475 (2000).
Klironomos, J. N. & Kendrick, W. B. Palatability of microfungi to soil arthropods in relation to the functioning of arbuscular mycorrhizae. Biol. Fertil. Soils 21 , 43–52 (1996).
Klironomos, J. N., Widden, P. & Deslandes, I. Feeding preferences of the collembolan Folsomia candida in relation to microfungal successions on decaying litter. Soil Biol. Biochem. 24 , 685–692 (1992).
Connell, J. H. & Slatyer, R. O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 111 , 1119–1144 (1977).
Arróniz-Crespo, M. et al. Bryophyte-cyanobacteria associations during primary succession in recently deglaciated areas of Tierra del Fuego (Chile). PLoS One 9 , 15–17 (2014).
Jean, M. et al. Experimental assessment of tree canopy and leaf litter controls on the microbiome and nitrogen fixation rates of two boreal mosses. N. Phytol. 227 , 1335–1349 (2020).
Jean, M., Alexander, H. D., Mack, M. C. & Johnstone, J. F. Patterns of bryophyte succession in a 160-year chronosequence in deciduous and coniferous forests of boreal Alaska. Can. J. Res. 47 , 1021–1032 (2017).
Blanchet, F. G., Cazelles, K. & Gravel, D. Co‐occurrence is not evidence of ecological interactions. Ecol. Lett. 23 , 1050–1063 (2020).
Fukami, T. & Nakajima, M. Complex plant-soil interactions enhance plant species diversity by delaying community convergence. J. Ecol. 101 , 316–324 (2013).
Martínez-García, L. B., Richardson, S. J., Tylianakis, J. M., Peltzer, D. A. & Dickie, I. A. Host identity is a dominant driver of mycorrhizal fungal community composition during ecosystem development. N. Phytol. 205 , 1565–1576 (2015).
Paulson, J. metagenomeSeq: statistical analysis for sparse high-throughput sequencing. Bioconductor.Jp https://github.com/nosson/metagenomeSeq/ (2014).
Francesco Ficetola, G. & Denoël, M. Ecological thresholds: an assessment of methods to identify abrupt changes in species-habitat relationships. Ecography 32 , 1075–1084 (2009).
Aho, K., Derryberry, D. & Peterson, T. Model selection for ecologists: the worldviews of AIC and BIC. Ecology 95 , 631–636 (2014).
Conn, P. B., Johnson, D. S., Williams, P. J., Melin, S. R. & Hooten, M. B. A guide to Bayesian model checking for ecologists. Ecol. Monogr. 88 , 526–542 (2018).
Hanusch, M., Ortiz, E. M., Patiño, J. & Schaefer, H. Biogeography and integrative taxonomy of epipterygium (Mniaceae, Bryophyta). Taxon 69 , 1150–1171 (2020).
Oksanen, J. et al. vegan: community ecology package. Github https://github.com/vegandevs/vegan (2019).
Baker, S. C. & Barmuta, L. A. Evaluating spatial autocorrelation and depletion in pitfall-trap studies of environmental gradients. J. Insect Conserv. 10 , 269–276 (2006).
Pickett, S. T. A. in Long-Term Studies in Ecology (ed. Likens, G. E.) 110–135 (Springer, 1989).
Bivand, R. S. & Wong, D. W. S. Comparing implementations of global and local indicators of spatial association. Test 27 , 716–748 (2018).
Hanusch, M., He, X., Ruiz-Hernández, V. & Junker, R. R. Successional generation of functional multidiversity and ecosystem complexity—a dataset from the Ödenwinkel research platform. Mendeley Data. https://doi.org/10.17632/xkv89tbftc.1 (2022).
Hanusch, M., He, X., Ruiz-Hernández, V. & Junker, R. R. Supporting dataset and code: succession comprises a sequence of threshold-induced community assembly processes towards multidiversity. Mendeley Data. https://doi.org/10.17632/dr6d3728xb.1 (2022).
Download references
Acknowledgements
We thank the Hohe Tauern National Park Salzburg administration and the Rudolfshütte for organizational and logistic support, the governing authority Land Salzburg for the permit to conduct our research (permit no. 20507-96/45/7-2019), Jan-Christoph Otto, Tobias Seifert, and Anna Vojtkó for help in the field. Hamed Azarbad, Lisa-Maria Ohler and Verena Zieschank provided valuable comments to improve the study. This research has been supported by the Austrian Science Fund (FWF), which provided funding to R.R.J. (grant no. Y1102).
Author information
Authors and affiliations.
Department of Environment and Biodiversity, Paris Lodron University Salzburg, 5020, Salzburg, Austria
Maximilian Hanusch, Xie He, Victoria Ruiz-Hernández & Robert R. Junker
Evolutionary Ecology of Plants, Department of Biology, Philipps-University Marburg, 35043, Marburg, Germany
- Robert R. Junker
You can also search for this author in PubMed Google Scholar
Contributions
R.R.J. conceived and initiated the study. M.H., X.H., V.R.-H., and R.R.J. designed the study and conducted fieldwork. M.H. performed the processing and analysis of the data with main inputs from R.R.J. and X.H. M.H. and R.R.J. drafted the initial version of the manuscript. All authors contributed critically to the interpretation of the results, revising, and approving the final version of the manuscript.
Corresponding author
Correspondence to Robert R. Junker .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Primary handling editors: Quan-Xing Liu and George Inglis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, description of additional supplementary files, supplementary data 1, supplementary data 2, supplementary data 3, supplementary data 4, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Hanusch, M., He, X., Ruiz-Hernández, V. et al. Succession comprises a sequence of threshold-induced community assembly processes towards multidiversity. Commun Biol 5 , 424 (2022). https://doi.org/10.1038/s42003-022-03372-2
Download citation
Received : 03 November 2021
Accepted : 14 April 2022
Published : 06 May 2022
DOI : https://doi.org/10.1038/s42003-022-03372-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Key mechanisms shaping plant succession are dynamic over time.
Nature Plants (2024)
Glacier retreat triggers changes in biodiversity and plant–pollinator interaction diversity
- Bao Ngan Tu
- Nora Khelidj
- Gianalberto Losapio
Alpine Botany (2024)
The importance of species addition ‘versus’ replacement varies over succession in plant communities after glacier retreat
- Isabel Cantera
- Alexis Carteron
- Gentile Francesco Ficetola
Exploring the Frequency and Distribution of Ecological Non-monotonicity in Associations among Ecosystem Constituents
- Maximilian Hanusch
Ecosystems (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Advertisement
Ecological succession regulates the relationship between biodiversity and supporting services in arid ecosystems
- Original Paper
- Published: 12 July 2021
- Volume 14 , article number 1370 , ( 2021 )
Cite this article

- Mohsen Sharafatmandrad ORCID: orcid.org/0000-0003-4890-1839 1 &
- Azam Khosravi Mashizi 1
560 Accesses
3 Citations
Explore all metrics
Biodiversity affects the provision of ecosystem services over time and space. This study was done to find how ecological succession regulates the relationship between biodiversity and supporting services in arid ecosystems. So, the effects of biodiversity on ecosystem supporting services, including soil stability, infiltration, and nutrient cycling, were investigated at three successional rangelands (early, mid, and late successional stages) in Iran. Linear and nonlinear modeling and structural equation modeling were used to assess the relationships between species diversity and supportive services at different successional stages. The results showed that the provision of the ecosystem supporting services increased with increasing species diversity along with ecological succession gradients, exponentially at the early and late successional stages and linearly in the mid successional stages. Structural equation modelling showed that succession, the dominance of functional types, species diversity, and vegetation cover were the most critical drivers of supporting services in arid ecosystems. Shrubs were the most critical functional type in providing ecosystem supporting services and should be involved in semi-arid rangeland restoration programs. In general, functional dominance is a good indicator of the health of ecosystems and their potential for providing support services in arid lands. Therefore, preserving plant composition is better than maximizing species richness to maintain semi-arid ecosystem services.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Evaluation of restoration success in arid rangelands of Iran based on the variation of ecosystem services
Aridity-driven shift in biodiversity–soil multifunctionality relationships
Biotic communities cannot mitigate the negative effects of grazing on multiple ecosystem functions and services in an arid shrubland, data availability.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Aghababaei Taqanaki M, Asadi I, Tahmasebi P et al (2014) Determining plant species index at different stages of succession in semi-steppe rangelands of Chaharmahal Bakhtiari province. Plant ecosystem protection 2(4):95–102
Google Scholar
Akaike H (1973) Information theory as an extension of the maximum likelihood principle .pp267-281. In B.N. Petrov and Csaki, Eds. Second International Symposium on Information Theory. Academia Kiado, Budapest and W.G.
Alcamo J et al (2005) Ecosystems and human well being: a framework for assessment. Millennium Ecosystem Assessment Series. Island Press, Washington D.C
Ali HE, Reineking B, Münkemüller T (2016) Effects of plant functional traits on soil stability: intraspecific variability matters. Plant Soil 411:359–375. https://doi.org/10.1007/s11104-016-3036-5
Article Google Scholar
Barrufol M, Schmid B, Bruelheide H, Chi X, Hector A, Ma K, Michalski S, Tang Z, Niklaus PA, Farwig N (2013) Biodiversity promotes tree growth during succession in subtropical forest. PLoS ONE 8 (11):e81246
Belnap J, Phillips SL, Troxler T (2006) Soil lichen and moss cover and species richness can be highly dynamic: the effects of invasion by the annual exotic grass Bromus tectorum, precipitation, and temperature on biological soil crusts in SE Utah. Appl Soil Ecol 32:63–76. https://doi.org/10.1016/j.apsoil.2004.12.010
Bhaskar R, Dawson TE, Balvaner P (2014) Community assembly and functional diversity along succession post-management. Funct Ecol 28:1256–1265
Butterfield BJ, Briggs JM (2009) Patch dynamics of soil biotic feedbacks in the Sonoran Desert. J Arid Environ 73:96–102. https://doi.org/10.1016/j.jaridenv.2008.09.012
Chai Y, Liu X, Yue M, Guo J, Wang M, Wan P, Zhang X, Zhang C (2014) Leaf traits in dominant species from different secondary successional stages of deciduous forest on the Loess Plateau of northern China. Appl Veg Sci 18:50–63. https://doi.org/10.1111/avsc.12123
Chapin FS, Zavaleta ES, Eviner VT et al (2000) Consequences of changing biodiversity. Nature 405:234–242. https://doi.org/10.1038/35012241
Deng L, Wang K-B, Chen M-L, Shangguan ZP, Sweeney S (2013) Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. CATENA 110:1–7. https://doi.org/10.1016/j.catena.2013.06.016
Dettweiler-Robinson E, Bakker JD, Grace JB (2013) Controls of biological soil crust cover and composition shift with succession in sagebrush shrub-steppe. J Arid Environ 94:96–104. https://doi.org/10.1016/j.jaridenv.2013.01.013
Diaz S, Cabido M (1997) Plant functional types and ecosystem function in relation to global change. J Veg Sci 8:463–474. https://doi.org/10.1111/j.1654-1103.1997.tb00842.x
Dunkerley D (2002) Systematic variation of soil infiltration rates within and between the components of the vegetation mosaic in an Australian desert landscape. Hydrol Process 16:119–131. https://doi.org/10.1002/hyp.357
Eisenhauer N, Bowker MA, Grace JB, Powell JR (2015) From patterns to causal understanding: Structural equation modeling (SEM) in soil ecology. Pedobiologia (Jena) 58:65–72. https://doi.org/10.1016/j.pedobi.2015.03.002
Emerson WW (1967) A classification of soil aggregates based on their coherence in water. Soil Res 5:47. https://doi.org/10.1071/sr9670047
Esquivel J, Echeverría C, Saldaña A, Fuentes R (2020) High functional diversity of forest ecosystems is linked to high provision of water flow regulation ecosystem service. Ecol Indic 115:106433. https://doi.org/10.1016/j.ecolind.2020.106433
Fagundes M, Weisser W, Ganade G (2018) The role of nurse successional stages on species-specific facilitation in drylands: Nurse traits and facilitation skills. Ecol Evol 8:5173–5184. https://doi.org/10.1002/ece3.3962
Fan Y, Chen J, Shirkey G, John R, Wu SR, Park H, Shao C (2016) Applications of structural equation modeling (SEM) in ecological studies: an updated review. Ecol Process 5. https://doi.org/10.1186/s13717-016-0063-3
Giller SP, Hillebrand H, Berninger U-G et al (2004) Biodiversity effects on ecosystem functioning: emerging issues and their experimental test in aquatic environments. Oikos 104:423–436. https://doi.org/10.1111/j.0030-1299.2004.13253.x
Grace JB, Anderson TM, Olff H, Scheiner SM (2010) On the specification of structural equation models for ecological systems. Ecol Monogr 80:67–87. https://doi.org/10.1890/09-0464.1
Guo N, Degen AA, Deng B, Shi F, Bai Y, Zhang T, Long R, Shang Z (2019) Changes in vegetation parameters and soil nutrients along degradation and recovery successions on alpine grasslands of the Tibetan plateau. Agric Ecosyst Environ 284:106593. https://doi.org/10.1016/j.agee.2019.106593
Haines-Young R, Potschin M (2010) The links between biodiversity, ecosystem services and human well-being. In: Raffaelli D, Frid C (eds) Ecosystem ecology: a new synthesis (Ecological Reviews, pp. 110-139). Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511750458.007
Hao H-M, Lu R, Liu Y, Fang NF, Wu GL, Shi ZH (2016) Effects of shrub patch size succession on plant diversity and soil water content in the water-wind erosion crisscross region on the Loess Plateau. CATENA 144:177–183. https://doi.org/10.1016/j.catena.2016.05.015
Heydari M, Zeynali N, Bazgir M, Omidipour R, Kohzadian M, Sagar R, Prevosto B (2020) Rapid recovery of the vegetation diversity and soil fertility after cropland abandonment in a semi-arid oak ecosystem: an approach based on plant functional groups. Ecol Eng 155:105963. https://doi.org/10.1016/j.ecoleng.2020.105963
Jun-Hu Y, Guo-Yi Z, De-Qiang Z, Guo-Wei C (2007) Changes of soil water, organic matter, and exchangeable cations along a forest successional gradient in Southern China. Pedosphere 17:397–405
Kinzig AP, Pacala SW, Tilman D, Klaus VH, Whittingham MJ, Báldi A et al (eds) (2020) Do biodiversity-ecosystem functioning experiments inform stakeholders how to simultaneously conserve biodiversity and increase ecosystem service provisioning in grasslands? Biol Conserv 245:108552. https://doi.org/10.1016/j.biocon.2020.108552
Klaus VH, Whittingham MJ, Báldi A, Eggers S, Francksen RM, Hiron M, Lellei-Kovács E, Rhymer CM, Buchmann N (2020) Do biodiversity-ecosystem functioning experiments inform stakeholders how to simultaneously conserve biodiversity and increase ecosystem service provisioning in grasslands?. Biol Conserv 245:108552
Lebrija-Trejos E, Pérez-García EA, Meave JA, Bongers F, Poorter L (2010) Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 91:386–398. https://doi.org/10.1890/08-1449.1
Leger EA, Goergen EM, Forbis de Queiroz T (2014) Can native annual forbs reduce Bromus tectorum biomass and indirectly facilitate establishment of a native perennial grass? J Arid Environ 102:9–16. https://doi.org/10.1016/j.jaridenv.2013.10.015
Lehman CL, Tilman D (2000) Biodiversity, stability, and productivity in competitive communities. Am Nat 156:534–552. https://doi.org/10.1086/303402
Lepš J, Osbornová-Kosinová J, Rejmánek M (1982) Community stability, complexity and species life history strategies. Vegetatio 50:53–63. https://doi.org/10.1007/bf00120678
Levine JM, Vilà M, D’Antonio CM et al (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc Biol Sci 270:775–781. https://doi.org/10.1098/rspb.2003.2327
Li XJ, Li XR, Song WM, Gao YP, Zheng JG, Jia RL (2008) Effects of crust and shrub patches on runoff, sedimentation, and related nutrient (C, N) redistribution in the desertified steppe zone of the Tengger Desert, Northern China. Geomorphology 96:221–232. https://doi.org/10.1016/j.geomorph.2007.08.006
Li J, Shangguan Z, Deng L (2020) Dynamics of soil microbial metabolic activity during grassland succession after farmland abandonment. Geoderma 363:114167. https://doi.org/10.1016/j.geoderma.2019.114167
Liang J, Wang X, Yu Z et al (2010) Effects of vegetation succession on soil fertility within farming-plantation ecotone in Ziwuling mountains of the loess plateau in China. Agric Sci China 9:1481–1491. https://doi.org/10.1016/s1671-2927(09)60241-8
Lohbeck M, Poorter L, Paz H, Pla L, van Breugel M, Martínez-Ramos M, Bongers F (2012) Functional diversity changes during tropical forest succession. Perspect Plant Ecol Evol Syst 14:89–96. https://doi.org/10.1016/j.ppees.2011.10.002
Lohbeck M, Bongers F, Martinez-Ramos M, Poorter L (2016) The importance of biodiversity and dominance for multiple ecosystem functions in a human-modified tropical landscape. Ecology 97:2772–2779. https://doi.org/10.1002/ecy.1499
Loreau M (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808. https://doi.org/10.1126/science.1064088
Ludwig JA, Tongway DJ (1995) Spatial organization of landscapes and its function in semi-arid woodlands, Australia. Landsc Ecol 10:51–63. https://doi.org/10.1007/bf00158553
Ludwig JA, Tongway DJ, Marsden SG (1999) Stripes, strands or stipples: modelling the influence of three landscape banding patterns on resource capture and productivity in semi-arid woodlands, Australia. CATENA 37:257–273. https://doi.org/10.1016/s0341-8162(98)00067-8
Magurran AE (1988) Ecological diversity and its measurement. Princeton Univ. Press, Princeton
Maia GN (2012) Caatinga: árvores e arbustos e suas utilidades (2nded.). Fortaleza, Brazil: Printcolor Gráficae Editora
Martins IS, Navarro LM, Pereira HM, Rosa IMD (2020) Alternative pathways to a sustainable future lead to contrasting biodiversity responses. Glob Ecol Conserv 22:e01028. https://doi.org/10.1016/j.gecco.2020.e01028
Mata-González R, Hunter RG, Coldren CL, McLendon T, Paschke MW (2008) A comparison of modeled and measured impacts of resource manipulations for control of Bromus tectorum in sagebrush steppe. J Arid Environ 72:836–846. https://doi.org/10.1016/j.jaridenv.2007.10.007
Morris RF, Fulton WC (1970) Models for the development and survival of hyphantria cunea in relation to temperature and humidity: Introduction. Mem Entomol Soc Canada 102:1–60. https://doi.org/10.4039/entm10270fv
Naeem S, Li S (1997) Biodiversity enhances ecosystem reliability. Nature 390:507–509. https://doi.org/10.1038/37348
Navas M-L, Roumet C, Bellmann A, Laurent G, Garnier E (2010) Suites of plant traits in species from different stages of a Mediterranean secondary succession. Plant Biol 12:183–196. https://doi.org/10.1111/j.1438-8677.2009.00208.x
Noor Alhamad M (2006) Ecological and species diversity of arid Mediterranean grazing land vegetation. J Arid Environ 66:698–715. https://doi.org/10.1016/j.jaridenv.2006.01.001
Novara A, Gristina L, La Mantia T, Rühl J (2013) Carbon dynamics of soil organic matter in bulk soil and aggregate fraction during secondary succession in a Mediterranean environment. Geoderma 193-194:213–221
Oba G, Vetaas OR, Stenseth NC (2001) Relationships between biomass and plant species richness in arid-zone grazing lands. J Appl Ecol 38:836–845. https://doi.org/10.1046/j.1365-2664.2001.00638.x
Pimentel D, Lach l, Zuniga R, Morrison D (2000) Environmental and economic costs of nonindigenous species in the United States. Bioscience 50:53. https://doi.org/10.1641/0006-3568(2000)050[0053:eaecon]2.3.co;2
Raevel V, Violle C, Munoz F (2012) Mechanisms of ecological succession: insights from plant functional strategies. Oikos 121:1761–1770. https://doi.org/10.1111/j.1600-0706.2012.20261.x
Rusch GM, Oesterheld M, Oesterheld M (1997) Relationship between productivity, and species and functional group diversity in grazed and non-grazed Pampas grassland. Oikos 78:519. https://doi.org/10.2307/3545613
Sakamoto Y, Ishigura M, Kitagawa G (1988) Akaike information criterion statistics. KTK Scientific. Tokyo SAS Institute.1988 SAS/STAT Users Guide. Version 6.03SAS Institute, Cary, NC
Sallaway MM, Waters DK (1994) Spatial variation in runoff generation in granitic grazing lands. Water Down Under 94 , Vol. 3. Institute of Engineers Australia: Adelaide
Sasaki T, Okayasu T, Shirato Y, Jamsran U, Okubo S, Takeuchi K (2007) Can edaphic factors demonstrate landscape-scale differences in vegetation responses to grazing? Plant Ecol 194:51–66. https://doi.org/10.1007/s11258-007-9274-0
Schwartz MW, Brigham CA, Hoeksema JD, Lyons KG, Mills MH, van Mantgem PJ (2000) Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia 122:297–305. https://doi.org/10.1007/s004420050035
Smeti E, von Schiller D, Karaouzas I, Laschou S, Vardakas L, Sabater S, Tornés E, Monllor-Alcaraz LS, Guillem-Argiles N, Martinez E, Barceló D, López de Alda M, Kalogianni E, Elosegi A, Skoulikidis N (2019) Multiple stressor effects on biodiversity and ecosystem functioning in a Mediterranean temporary river. Sci Total Environ 647:1179–1187. https://doi.org/10.1016/j.scitotenv.2018.08.105
Smith TM, Shugart HH, Woodward FI (1997) Plant functional types: their relevance to ecosystem properties and Global Change. Cambridge University Press, Cambridge, p 355
Stachowicz JJ (1999) Species Diversity and invasion resistance in a marine ecosystem. Science (80) 286:1577–1579. https://doi.org/10.1126/science.286.5444.1577
Strawderman RL, Burnham KP, Anderson DR (2000) Model selection and inference: a practical information-theoretic approach. J Am Stat Assoc 95:341. https://doi.org/10.2307/2669574
Su H, Li Y, Liu W, Xu H, Sun OJ (2013) Changes in water use with growth in Ulmus pumila in semi-arid sandy land of northern China. Trees 28:41–52. https://doi.org/10.1007/s00468-013-0928-3
Tongway DJ, Ludwig JA (2002) Reversing desertification. In: Rattan Lal (ed) Encyclopaedia of Soil Science. Marcel Dekker, New York
Walker LR, del Moral R (2009) Lessons from primary succession for restoration of severely damaged habitats. Appl Veg Sci 12:55–67. https://doi.org/10.1111/j.1654-109x.2009.01002.x
Wang Z, Zheng F (2020) Impact of vegetation succession on leaf-litter-soil C:N:P stoichiometry and their intrinsic relationship in the Ziwuling Area of China’s Loess Plateau. J For Res 32:697–711. https://doi.org/10.1007/s11676-020-01149-z
Watson KB, Galford GL, Sonter LJ, Ricketts TH (2020) Conserving ecosystem services and biodiversity: measuring the tradeoffs involved in splitting conservation budgets. Ecosyst Serv 42:101063. https://doi.org/10.1016/j.ecoser.2020.101063
Weber CF (2015) Reduced vertical stratification of soil bacterial community structure and composition is associated with Bromus tectorum invasion of sagebrush steppe. J Arid Environ 115:90–99. https://doi.org/10.1016/j.jaridenv.2015.01.012
Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E (1998) Quantifying threats to imperiled species in the United States. Bioscience 48:607–615. https://doi.org/10.2307/1313420
Wu G-L, Huang Z, Liu Y-F, Cui Z, Liu Y, Chang X, Tian FP, López-Vicente M, Shi ZH (2019) Soil water response of plant functional groups along an artificial legume grassland succession under semi-arid conditions. Agric For Meteorol 278:107670. https://doi.org/10.1016/j.agrformet.2019.107670
Xiao L, Yao K, Li P, Liu Y, Chang E, Zhang Y, Zhu T (2020) Increased soil aggregate stability is strongly correlated with root and soil properties along a gradient of secondary succession on the Loess Plateau. Ecol Eng 143:105671. https://doi.org/10.1016/j.ecoleng.2019.105671
Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci U S A 96:1463–1468. https://doi.org/10.1073/pnas.96.4.1463
Yang J, Tang Y (2019) The increase in ecosystem services values of the sand dune succession in northeastern China. Heliyon 5:e02243–e02243. https://doi.org/10.1016/j.heliyon.2019.e02243
Zeng Q, An S, Liu Y (2017) Soil bacterial community response to vegetation succession after fencing in the grassland of China. Sci Total Environ 609:2–10. https://doi.org/10.1016/j.scitotenv.2017.07.102
Zhang Y, Shangguan Z (2018) Interaction of soil water storage and stoichiometrical characteristics in the long-term natural vegetation restoration on the Loess Plateau. Ecol Eng 116:7–13. https://doi.org/10.1016/j.ecoleng.2018.02.026
Zhao FZ, Bai L, Wang JY, Deng J, Ren CJ, Han XH, Yang GH, Wang J (2019) Change in soil bacterial community during secondary succession depend on plant and soil characteristics. CATENA 173:246–252. https://doi.org/10.1016/j.catena.2018.10.024
Download references
Code availability
Not applicable.
Author information
Authors and affiliations.
Department of Ecological Engineering, University of Jiroft, 8th km of Jiroft - Bandar Abbas Road, P.O. Box: 7867161167, Jiroft, Iran
Mohsen Sharafatmandrad & Azam Khosravi Mashizi
You can also search for this author in PubMed Google Scholar
Contributions
MS involved in the study design, fieldwork, conceptualization, methodology, data collection, data analyses, writing-original draft. AKM involved in conceptualization, methodology, software, writing-original draft. All authors wrote, read, and approved the final manuscript.
Corresponding author
Correspondence to Mohsen Sharafatmandrad .

Ethics declarations
Ethics approval, consent for publication, competing interests.
The authors declare competing interests.
Additional information
Responsible Editor: Haroun Chenchouni
Rights and permissions
Reprints and permissions
About this article
Sharafatmandrad, M., Khosravi Mashizi, A. Ecological succession regulates the relationship between biodiversity and supporting services in arid ecosystems. Arab J Geosci 14 , 1370 (2021). https://doi.org/10.1007/s12517-021-07796-8
Download citation
Received : 04 February 2021
Accepted : 30 June 2021
Published : 12 July 2021
DOI : https://doi.org/10.1007/s12517-021-07796-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Functional types
- Infiltration
- Structural equation modelling
- Find a journal
- Publish with us
- Track your research
Covering a story? Visit our page for journalists or call (773) 702-8360.

New Certificate Program
Top Stories
- UChicago scientist seeks to make plastic more recyclable
- Inside the Lab: A first-hand look at University of Chicago research
- Scav reflections: “for the love of the pursuit and the joy in the attempt”
Ecological succession, explained
Ecological succession is the process by which the mix of species and habitat in an area changes over time. Gradually, these communities replace one another until a “climax community”—like a mature forest—is reached, or until a disturbance, like a fire, occurs.
Ecological succession is a fundamental concept in ecology. The study of succession was pioneered at the University of Chicago by Henry Chandler Cowles , who was also one of the founders of ecology as a discipline, as he studied the plants of the Indiana Dunes.
Jump to a section:
What is ecological succession, what are primary and secondary ecological succession, what is a climax community, what is an example of ecological succession, plant succession at the indiana dunes, how do we understand ecological succession today, henry chandler cowles, ecological succession and the university of chicago.
Ecological succession is the process by which natural communities replace (or “succeed”) one another over time. For example, when an old farm field in the midwestern U.S. is abandoned and left alone for many years, it gradually becomes a meadow, then a few bushes grow, and eventually, trees completely fill in the field, producing a forest.
Each plant community creates conditions that subsequently allow different plant communities to thrive. For example, early colonizers like grasses might add nutrients to the soil, whereas later ones like shrubs and trees might create cover and shade. Succession stops temporarily when a “climax” community forms; such communities remain in relative equilibrium until a disturbance restarts the succession process.
In this video from the National Park Service, Tim Watkins and Robert Boyd explore the Indiana Dunes, learning about its history as an important case study for the development of ecological succession theory.
Understanding how succession happens in a variety of ecosystems—and what kinds of disturbances and time spans lead to the formation of different plant and animal communities—is important for scientists who want to understand ecosystem dynamics and effectively protect or restore natural communities.
For example, many natural communities in North America have adapted to periodic disturbances from wildfires: This can help maintain prairie or savanna communities that depend on open habitat and nutrient cycling that might occur as a result of fire.
There are two major types of ecological succession: primary succession and secondary succession.
Primary succession happens when a new patch of land is created or exposed for the first time. This can happen, for example, when lava cools and creates new rocks, or when a glacier retreats and exposes rocks without any soil. During primary succession, organisms must start from scratch. First, lichens might attach themselves to rocks, and a few small plants able to live without much soil might appear. These are known as “pioneer species.”
Gradually, the decomposition of those plants contributes to soil formation, and more and larger plants begin to colonize the area. Eventually, enough soil forms and enough nutrients become available such that a climax community, like a forest, is formed. If the site is disturbed after this point, secondary succession occurs.
Secondary succession happens when a climax community or intermediate community is impacted by a disturbance. This restarts the cycle of succession, but not back to the beginning—soil and nutrients are still present.
For example, after a forest fire that kills all the mature trees on a particular landscape, grasses might grow, followed by shrubs and a variety of tree species, until eventually the community that existed before the fire is present again.
A climax community is the “endpoint” of succession within the context of a particular climate and geography. In the midwestern U.S., for example, such a community might be a hardwood forest with oaks and hickories as the dominant tree species.
A climax community will persist in a given location until a disturbance occurs. However, in many ecosystems, disturbance occurs frequently enough that a matrix of community types may be consistently present on the landscape.
For example, in an area prone to wildfires like the western U.S., mature forests may exist near grassy meadows with fewer, scattered trees. Consistent disturbance and variation in factors like water and nutrient availability over the course of decades thus allows many plant and animal communities to thrive within a particular climatic and geographic niche—not just those adapted to the absence of disturbance seen in climax communities.
Ecological succession can occur in many contexts and over many time spans.
In Hawaii and Iceland, primary succession occurs on lava flows where new land has formed; in Canada’s Athabasca Dunes, it happens when new sand is deposited along a lakeshore; in the Andes, it occurs when glaciers retreat.
In many regions, secondary succession occurs where wildfires have destroyed conifer forests, or where former agricultural land is reverting to meadow or scrubland.
What these examples have in common is that the climax community is not the first one present on the landscape after succession begins: First, intermediate communities occupy the space, sometimes for many years, creating ideal conditions for the communities that follow.
The Indiana Dunes, 40 miles southeast of Chicago and today the site of both a state and a national park , served as the original field site for research on ecological succession in plant communities, and continue to serve as an ideal case study. (If you visit the dunes, you can walk along the Dune Succession Trail , which highlights the different stages of succession in a real-world context.)
In the 1890s, University of Chicago botanist Henry Chandler Cowles noticed that dunes which were further from Lake Michigan had different plants growing on them than dunes closer to the lake. The lakeside dunes had only beach grass, whereas those further from the shore had other plants like cottonwoods that could grow in sandy soil. Dunes still further back had pines, and finally behind them were mature oak forests that did not resemble the grassy dunes near the lake at all.
Through careful observation and comparisons, Cowles determined that the linear succession of these communities in space also represented a linear progression in time. The dunes farthest from the lake were the oldest and had been stable for longest, since sand shifts frequently in the wind without plants to hold it in place.
From this, he inferred that plant communities trended toward oak forests in northern Indiana over decades and centuries, and that each community created the soil and microclimate conditions required for its successor community to thrive: as grasses and cottonwoods stabilized dunes and added nutrients, they were replaced by later successional communities.
Ecological succession is a foundational concept in ecology, which as a field examines the structure and dynamics of biological communities. Today, the concept of ecological succession continues to be studied from new angles as humans modify the global environment more than ever before. As new nuances have been added to the original theory, insights have emerged that are valuable to humans interested in managing natural resources.
For example, recent studies show that even in “climax” communities, changes in what resources are available may shift the balance of the species composition over time, even without a formal disturbance. Other work has examined the impact of biodiversity loss, invasive species, climate change and other anthropogenic factors in altering the way ecosystems respond to change.
As native species go extinct or become rare, new species enter ecosystems, and climate baselines shift, the communities that once dominated an ecosystem may be less likely to eventually return after a disturbance. However, studying succession can also provide valuable insights for ecologists and wildlife managers interested in restoring those natural systems: through careful management such as controlled burning or invasive species control, people can help ecological communities stay strong.
The University of Chicago played a key role in pioneering the scientific study of ecological succession and ecology as a discipline more broadly. Following his pivotal dissertation work in the Indiana Dunes as a UChicago doctoral student, Henry Chandler Cowles went on to become a professor, remaining at UChicago for more than three decades, until the early 1930s. He taught generations of students about the ecology of North America through course field trips across the continent from Maine to Alaska, California and Texas.
The trips—and Cowles’ fieldwork—are documented in the University’s special collection of American Environmental Photographs , which show Cowles, students, and American landscapes from a century ago in great variety and detail. Cowles’ papers are also housed in the Hanna Holborn Gray Special Collections Research Center, and can be found here . Additionally, Cowles’ student Victor Ernest Shelford became an influential ecologist in his own right and a leader in the founding of the Nature Conservancy , a major conservation nonprofit.
In the early 20th century, the University of Chicago was also home to other noted ecologists and marine biologists, including George Damon Fuller and Warder Clyde Allee , an early expert on social relationships in animals and an important figure in the development of ecology.
Today, the University of Chicago remains a leader in research on ecology and evolutionary biology. The University’s Warren Woods Ecological Field Station in Berrien County, Michigan offers students, faculty and staff the opportunity to study and observe ecosystem dynamics in a landscape that includes both remnant (undisturbed) forest, restored prairie and old fields.
RELATED EXPERTS

Catherine Pfister

Gregory Dwyer
Get more with UChicago News delivered to your inbox.
Related Topics
Latest news, ‘inside the lab’ series provides a unique look at uchicago research.

Mavis Staples, legendary singer and activist, returns to UChicago to inspire next generation

Geophysical Sciences
Scientists find evidence that meltwater is fracturing ice shelves in Antarctica
Pritzker School of Molecular Engineering
UChicago scientists use machine learning to turn cell snapshots dynamic

Where do breakthrough discoveries and ideas come from?
Explore The Day Tomorrow Began

W. Ralph Johnson, pre-eminent UChicago critic of Latin poetry, 1933‒2024

Pulitzer Prize
Trina Reynolds-Tyler, MPP'20, wins Pulitzer Prize in Local Reporting
Around uchicago, uchicago to offer new postbaccalaureate premedical certificate program.
Carnegie Fellow
UChicago political scientist Molly Offer-Westort named Carnegie Fellow
National Academy of Sciences
Five UChicago faculty elected to National Academy of Sciences in 2024
Laing Award
UChicago Press awards top honor to Margareta Ingrid Christian for ‘Objects in A…
Winners of the 2024 UChicago Science as Art competition announced

Six UChicago scholars elected to American Academy of Arts and Sciences in 2024
Biological Sciences Division
“You have to be open minded, planning to reinvent yourself every five to seven years.”

UChicago paleontologist Paul Sereno’s fossil lab moves to Washington Park
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Primary Succession
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Save to Library
- Last »
- Habitats Directive Follow Following
- Natura 2000 Follow Following
- Secondary Succession Follow Following
- Ecological Succession Follow Following
- Plant Conservation Follow Following
- Plant Community Assembly Follow Following
- Fire. The ecological effects of fire in savannas Follow Following
- Vegetation dynamics Follow Following
- Phytosociology Follow Following
- Facilitation Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Publishing
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Commun Integr Biol
- v.6(6); 2013 Nov 1
Primary succession in Mount Pinatubo
Thomas e marler.
1 Western Pacific Tropical Research Center; University of Guam; UOG Station; Mangilao, Guam USA
Roger del Moral
2 Department of Biology; University of Washington; Seattle, WA USA
Vegetation structure on the east flank of Mount Pinatubo was investigated to determine the inventory of species at 15 y post-eruption, then to ascertain environmental variables that have influenced the early patterns of primary succession. Unconstrained and constrained ordination methods were used to determine the influence of spatial, elevation, and substrate patterns on vegetation. Vegetation was assigned to one of 3 habitat types. Scours were eroded flat surfaces, terraces were perched flat surfaces, and talus piles were created along the canyon edges as mass waste events. The influence of habitat type on vegetation was multifaceted because they represent different conditions and different histories. The talus piles have preferential access to colonists from the vegetation on the canyon walls above and a more benign microclimate than the exposed terrace and scour sites. Scoured sites on the valley floor exhibited the least vegetation cover, as these substrates had the least mature surfaces and the most restricted capacity for root exploration. Perched terraces exhibited greater plant dominance than did the other habitats in the early stages of succession because of the ubiquitous appearance of Parasponia rugosa as initial colonists on these relatively flat surfaces. Polynomial canonical correspondence analysis was more closely aligned with the pattern of vegetation than linear canonical correspondence analysis, and therefore more closely approximated accurate descriptions of correlations among site ordination positions and measured variables. These results confirm that a variety of statistical approaches can clarify applications for restoration ecology following landslide and volcanic disturbances or agriculture and forestry anthropogenic disturbances in the lowland tropics.
Introduction
Mount Pinatubo erupted on June 15, 1991, when 5 to 6 km 3 of ejecta were deposited on the upper slopes. 1 Pyroclastic flows covered more than 30% of the watershed for the river systems on the east flanks. 2 Sedimentation and scouring alternated for several years resulting in chronic changes in elevation of the canyon beds. 3
These drainages have been utilized by the Aeta ethnic minority since pre-Hispanic times. Until recently, their way of life depended on extensive use of native vegetation and river resources. 4 The eruption and subsequent lahars have threatened their culture in many ways. 5 - 7 This motivated us to describe patterns of vegetation and growth form structure along 2 river systems on the east flanks of Mount Pinatubo 15 y after the eruption. 8 We elected to focus on the river canyons for this study because ecological recovery in the riparian zones was critical for sustaining traditional knowledge in this people group.
Our earlier work enumerated the flora and used a traditional statistical approach to reveal factors that may have shaped it. 8 However, ecologists have been trying for many years to reach consensus on a comprehensive description of species distribution patterns and the mechanisms explaining these patterns. 9 , 10 Understanding relationships between species and environmental factors is crucial when pursuing this goal. A greater understanding of all factors that have shaped the Mount Pinatubo flora may be more fully realized by use of contemporary statistical approaches. Detrended correspondence analysis (DCA) is highly reliable for data exploration in community ecology. 11 This statistical technique may illuminate the main factors or gradients in large data matrices. Canonical correspondence analysis (CCA) takes advantage of the fact that species abundance or probability of occurrence is often a unimodal function of the environmental variables, and can help ecologists unravel how a many species simultaneously respond to external factors. 12 The relationship between individual species and external factors is rarely linear, so the polynomial of CCA (pCCA) is often employed for improving interpretations. 13 , 14
Here we explore patterns of species and growth form composition using these ordination methods. Understanding vegetation recovery on these new substrates may provide insights for the restoration of other damaged tropical ecosystems, where agriculture, landslides, and forestry 15 have taken a huge toll. The focus of this paper is to: 1) characterize the sites that support succession in the recovering Mount Pinatubo landscape; and 2) determine the plant and environmental variables correlated with vegetation patterns using DCA, CCA, and pCCA. Our outcomes will determine which questions can be tested in subsequent studies of primary succession in tropical volcano systems.
Materials and Methods
Field sampling.
Vegetation was sampled in March and April 2006 along the Pasig-Potrero River (PR) and Sacobia River (SR) systems using a variant of the method described by del Moral and Lacher. 16 Methods of positioning our plots, measuring vegetation cover, and identifying taxa were described by Marler and del Moral. 8 The location of each plot was determined by GPS, from which elevation and distances from the caldera were ascertained. We also measured the lateral distance between the center of each plot and the edge of the canyon or the channel, to the nearest human habitation and to the alluvial fan. Aspect was determined by compass and, for the purposes of direct ordination values were converted to a 5-point scale reflecting insolation. 17 Slope was determined by clinometer and converted to a 5 interval scale: 1 = 0–5°; 2 = 6–10°; 3 = 11–15°; 4 = 16–25° and 5 = > 25°. The percentage of the soil surface covered by rocks, gravel, and sand was estimated visually and converted into 5 categories. Only rocks and sand characteristics were used because also using gravel would lead to strong colinearity. For rocks, 1 = 0 to 9%; 2 = 10 to 19%; 3 = 20 to 29%; 4 = 30 to 39%; and 5 = 40% or more. For sand, 1 = 1 to 25%; 2 = 26 to 40%; 3 = 41 to 50%; 4 = 51 to 80%; and 5 = 81% or more.
For each plot, we calculated the number of species (richness species), total percent cover, Simpson’s diversity index (D = [1 − ΣP i 2 ]), and the Shannon-Weiner index (H' = [−Σ(P i ln P i )]. For both D and H', P i is the proportion of total plot cover of a species. 18
Site characteristics
At the time of our field work, soil surfaces remained unstable in this ecosystem. However, vegetation very rapidly colonized any soil surface that became stable. Our plots were assigned to one of 3 habitat types: 1) perched terraces, formed when torrential rains created lahar or deep aggradation deposits, then subsequent channel incision isolated the new surfaces above the elevation of the channel; terrace surfaces were typically 1–4 m above the adjacent canyon surface; 2) scoured surfaces occurred within the canyons when sheet erosion exposed large rocky surfaces and allowed vegetation to develop after the disturbance ceased; there was little soil remaining to support root development; and 3) the pyroclastic flow substrates on this volcano are vulnerable to mass waste events that ultimately form talus piles. These processes are discussed by Gran. 19 We assessed the influence of habitats on vegetation traits using one-way ANOVA.
We used detrended correspondence analysis (DCA) to analyze the full set of data, 18 in particular species spatial patterns. Species found in fewer than 5 plots were excluded, and those found in fewer than 22 plots were reduced in influence to emphasize larger scale patterns. DCA is robust with low β diversity and is scored in a measure of species turnover. DCA axes were analyzed using stepwise multiple regressions to the measured variables. Sites were treated as a multistate variable.
We used 2 approaches to conduct constrained ordinations of the relationships between vegetation patterns and environmental variables. Canonical correspondence analysis (CCA) 12 was conducted using PC–ORD. 18 Once a canonical analysis of species composition was achieved, the best fit of environmental variables to each ordination axis was calculated. Monte Carlo simulations (n = 1000 trials) were used to assess the validity of each of the first 3 axes. Sites were treated as 3 binary variables; latitude was excluded because it is strongly correlated with elevation. The relationship between environmental factors and plots was visualized in bi-plots. The position of a plot was determined from the linear or polynomial combination of environmental factors determined by the analysis, assessed by the Pearson correlations between the vegetation data and the environmental data.
The relationship between species and environmental variables is rarely linear, which is an assumption of CCA. Therefore, we also used polynomial CCA (pCCA), described by Makarenkov and Legendre, 20 to improve interpretations. 13 , 14 Sites were treated as binary variables while the remaining variables were spatial: coordinates based on GPS position (east–west and north–south); elevation; and distances of the plot from the incised stream channel, the edge of the canyon and the caldera rim. Other distances, e.g., to human habitation or the alluvial fan, were strongly correlated with one or more of the 7 variables used, so these were excluded from pCCA analysis.
Statistical analyses were conducted using Statistix8. 21 Graphs were produced using Axum 7. 22
Percent vegetation cover varied greatly among sites. Cover in terraces and talus piles exceeded cover in scours ( Table 1 ). There were no significant differences in species richness or diversity among types.
Note: Differences among habitat types were determined separately by ANOVA, followed by Bonferroni comparisons when significant.
Species composition—taxonomic and growth form subsets
We found 58 identifiable taxa of vascular plants within our plots. 8 In vegetation with such a large species pool, early patterns are often more clear when growth forms are explored. Therefore, we compared changes in the percent cover of common species arrayed by growth forms in each plot ( Table 2 ).
Trees were dominated by Parasponia rugosa , were abundant throughout the study area, and exhibited greater cover in terraces and talus piles than in scours. Shrubs were rare, although shrub cover in talus piles greatly exceeded that in terraces or scours, while vine cover was less. Large, spreading grasses were abundant. Saccharum spontaneum was the dominant large grass, and was most abundant on terraces. In contrast, Miscanthus floridulus was more common in talus piles. Total large grass cover was greatest in terraces. Small graminoids were also common, and their cover was greatest in talus piles. Forbs were common in aggregate, but no single species dominated. Pityrogramma calomelanos was the most common fern, and was more common in talus piles.
Unconstrained ordination
The general pattern of vegetation, visualized with DCA, employed 33 species after omitting rare ones; of these, 21 were down-weighted. The analysis spread plots well in floristic space. The total variance of the data was 1.384, of which 21.2% was found on the first axis, and 8.7% was on the second. Although there was some overlap between the 2 rivers, plots tended to segregate by their river ( Fig. 1 ). Terrace vegetation of the 2 rivers was similar, and found in the center of the ordination. Talus pile vegetation was more distinct, and found with lower DCA-1 scores. Scoured sites tended to occur with higher DCA-1 scores. The SR scours were extreme on DCA-1, with only one PR scour plot near them in this space.

Figure 1. Detrended correspondence analysis of all 63 plots of vegetation along 2 river systems of Mount Pinatubo, arranged by site and habitat type characteristics. PR, Pasig-Potrero River; SR, Sacobia River.
Individual species patterns did not produce readily interpretable results. The low end of DCA-1 was dominated by species common in talus piles (e.g., Parasponia rugosa , Mikania scandens , Miscanthus floridulus , Pogonatherum crinitum and Pityrogramma calomelanos , while the higher end was dominated by species common on scours (e.g., Calopogonium mucunoides , Saccharum spontaneum , and Mimosa pudica ). Parasponia rugosa and Melinis repens dominated the low end of DCA-2, while Phragmites karka , Centrosema molle and Miscanthus floridulus characterized the higher end. This weaker gradient suggested a shift from PR plots to SR plots.
The environmental interpretation of the first 2 axes was improved by a multiple regression of the DCA scores vs. the environmental variables. DCA-1 was related to east–west position (t = 7.37) and to surface type (t = − 6.55), indicating a strong gradient from talus piles to scours (r 2 = 0.682) that is consistent with the visual analysis. Elevation declines from west to east. DCA-2 was weak (r 2 = 0.228) and related to the north–south coordinates (t = 3.12) and to rock surface (t = 2.85). These provisional interpretations required examination by direct methods.
Constrained ordination
CCA clarified the pattern of species distributions found in the DCA. We excluded highly correlated variables (i.e., longitude is related to elevation on these east-trending valleys) and those with low predictive value in preliminary analyses (aspect, surface composition and distance to the channel). Habitat types again were binary variables. The total variance in this linear analysis (i.e., the eigenvalue) was 1.684 ( Table 3 ). The Pearson correlation between the floristic ordination and the constrained ordination was 0.893 on Axis 1 and 0.786 on Axis 2; both the correlations and the size of the eigenvalues were highly significant (p < 0.0001 by Monte Carlo permutation tests).
CA = the indirect ordination (correspondence analysis)
The strong concentration of variation on Axis 1 was evident when the sites were plotted as linear combinations of the predictive variables with the environmental vectors overlain ( Fig. 2A ). Distance to the caldera (Cald) and elevation (Elev) were nearly mirror images. Elevation was a good surrogate for distances to the alluvial fan (Fan). The degree of slope was inversely correlated with the distance to the caldera, because sites became steeper closer to the caldera. Latitude (N–S) represented a third direction of variation. Terraces and talus pile sites were at opposite sides of the ordination, while scours (not shown) were intermediate. Floristic differences among sites appeared greater than differences among habitat types within a site.

Figure 2. Direct gradient analysis using: 3 habitat types (terrace, scour, talus piles); elevation, latitude (N–S), slope, and aspect; and distance to nearest habitation, edge of river basin, alluvial fan, the caldera. Plots are located as linear combination of predictor variables. ( A ) Linear canonical correspondence analysis (CCA; environmental vectors multiplied by 2.5 to reduce confusion.) ( B ) Polynomial CCA with the same variables (pCCA; environmental vectors multiplied by 3 to reduce confusion.)
Polynomial CCA (pCCA) was applied to these data ( Table 3 ) and appeared to be more closely aligned with the pattern of vegetation than was CCA. In pCCA, the relationship between site positions and the predicted values was substantially larger, more of the floristic variance was explained, and the eigenvalues were larger. The quadratic variables combined to account for 39.4% of the canonical variation in the first 2 axes. The result was highly significant.
Sites were plotted using linear combinations of the predictive variables and overlain with the environmental vectors ( Fig. 2B ). The polynomial result demonstrated significant patterns in each of the first 3 axes. Axis 1 was associated with habitat and to a lesser degree with slope. Elevation, distance to the alluvial fan, distance to the caldera, and latitude all combine the first 2 axes. Plots formed reasonable clusters within a sample site. Pasig-Potrero River sites were well separated from the Sacobia River sites.
While pCCA provided greater confidence in providing provisional explanations for what was controlling vegetation, the pattern of species distribution in the 2 methods was similar. Mann-Whitney U-tests of species order Axis 1 and Axis 2 of the 2 constrained ordinations were similar (0.90 in Axis 1 and 0.60 in Axis 2). Thus, only pCCA will be described.
Centroids are the weighted mean position of each species, such that species concentrated in sites with negative scores occur near the negative end of an axis, while those that tend to occur primarily in sites at the positive extreme have a high value. Widespread species and those found in sites near the middle of an ordination axis have values near zero in this representation. Species such as Pennisetum setaceum , Chromolaena odorata, and Muntingia calabura were more abundant in the PR drainage, while Chloris barbata , Phragmites karka , and Centrosema molle represent species more common on SR. Pityrogramma calomelanos , Pogonatherum crinitum , Mikania scandens, and Miscanthus floridulus were common in talus piles, while Phragmites karka , and Crotalaria pallida represent those absent from talus piles and more common in the other habitats. However, these are merely trends, and no species mentioned was confined to one habitat type (see Table 2 ). Several do, however, fail to occur on talus piles and are found with negative Axis 1 scores.
D iscussion
Spatial effects such as elevation and geographic position as well as habitat type were the main explanatory variables found in our analyses. Greater cover in terraces and talus piles than in scours was a function of at least 2 factors. First, terraces and talus piles were generally more mature than scours in the same location. Second, health and growth of some species were constrained on the scoured surfaces where substrates suitable for root proliferation were limited. In one striking example, an unidentified armored scale exclusively attacked weak P. rugosa trees on scours, while vigorous individuals of this species in adjacent terrace and talus pile surfaces were not attacked. This Homoptera pest severely reduced canopy density of the infested trees. Dominance was stronger on terraces than talus piles or scours. This was due to the dense P. rugosa cover on all terraces. Rarely do the young Mount Pinatubo terrace surfaces initially support a significant number of plants other than P. rugosa .
Trees, shrubs, smaller graminoids, and ferns all increased in cover from scours to talus piles, indicative of the greater fertility and subsequent stability of the talus piles compared with scoured areas. The other growth forms exhibited no significant patterns, though forbs also tended to increase on talus piles. Larger grasses and vines, adept at claiming space and holding soil in unstable surfaces, generally performed better on terraces and scours. Subsequent studies would benefit by spreading sampling along elevational gradients on the major habitat types to capture the existing variation more efficiently.
Gradient analysis
DCA separated the samples well considering the limited β diversity. Both river systems and habitat types exhibited an impact on vegetation. Variation appeared greater on the SR. DCA-1 was defined by SR talus pile and scoured plots. PR tended to occur at low scores of DCA-2, which had substantially less information than DCA-1, but plots of both drainages intermingled.
CCA separated the plots of the 2 river systems very well and a gradient from talus piles to other habitat types was revealed. The pCCA revealed more details in the vegetation pattern. Talus piles were sufficiently different from the other habitats to support distinct vegetation. The analysis preserved the floristic distinction between the river systems and emphasized the importance of spatial variables. The nature of spatial effects remains uncertain because distance could relate to local land use patterns and distance to colonists, or it could be an indicator of subsequent disturbance. While detailed investigation of this question awaits further field studies, it is clear that combined use of traditional statistics, DCA, and pCCA results in a more accurate characterization of the factors that are shaping primary succession.
Applications
As plants become established their roots help stabilize riparian surfaces. 23 Calculations from areas below the alluvial fan of these Mount Pinatubo drainages indicated that vegetation decreased flow velocities by up to 12%, added 8.21–12.31 kPa of cohesion to stream banks, decreased channel width, and created more stable stream banks. 24 Vegetation development has the potential to reduce the extent of rill erosion of the canyon walls and surface erosion of the canyon bottoms in Mount Pinatubo river systems. 3 These habitats exhibit continuing surface scouring and sedimentation at small scale spatial scales, a phenomenon that increases α diversity without affecting β diversity. 25 These issues underscore the fact that our ability to understand what is influencing vegetation recovery will in turn advance our understanding of ecosystem recovery in Mount Pinatubo.
Samples that were at the same elevation and in the same habitat type tended to cluster in the ordinations, but the exceptions point to interesting issues. For example, one SR terrace plot was well isolated from the others, another SR scour plot was scattered, and one set of PR scour plots was not closely clustered. These plots hint that establishment from moderate distances has a distinct stochastic element. 26 Nakashizuka et al., 27 found that dispersal rates onto a small debris avalanche slowed with distance to the edge and were dominated by wind dispersed species. These factors lead to low predictability in species composition. In contrast, talus pile vegetation at any location was much less variable. This may be due to competitive effects of trees, vines, and strongly rhizomatous grasses. Further research is needed to determine if vegetation within terrace and scour habitats will become more predictable with continued vegetation development.
Talus piles result from landslides, which are known to foster heterogeneous edaphic environments for plant and soil development. 28 , 29 The movement of soil, vegetation, and litter varies greatly with each mass waste event, creating patches of heterogeneous fertility. Resource patchiness within a landslide is therefore highly dynamic, both spatially and temporally. 29 Because of this trait, we expected the vegetation in talus piles to be less predictable than in scours and terraces, but our results did not conform to this expectation. Results indicate that the factors driving primary succession in this environment may be more influenced by differences in exposure and microclimate than by traits of the substrates.
Polynomial CCA was more closely aligned with the pattern of vegetation than the CCA, as more of the CA variance was explained and the eigenvalues were larger. These statistics confirm that the polynomial approach more closely approximated an accurate description of the correlations among site ordination positions and measured variables during Mount Pinatubo primary succession. 30 Our results add to previously described lessons that can be learned from Mount Pinatubo 5 , 8 , 25 by showing how these ordinations can disentangle the complex interacting factors that will foster continued primary succession.
The scour habitats were clearly more stressful environments than the talus piles. This disparity may shape future species interactions, as positive interactions among plants are stronger in stressful areas, whereas negative interactions predominate in less stressful environments. 31 Therefore, examples of facilitation may be more prevalent in the scour environments, and examples of competition may shape continued succession in talus piles.
This immature vegetation is developing along several alternative pathways on these 3 habitat types. Terraces and talus piles are relatively stable, permitting rapid species assembly and maturation. Scours are subject to more frequent, unpredictable disturbances that retard further development and even force retrogression. Since these disturbances differ in timing and intensity within and between the 2 river systems, scour vegetation existed in a mosaic of patches at different developmental states. The rate of development on all habitat types was affected by climatic factors that change with elevation. They are also affected by landscape factors that influence colonization in a more random way. Finally, local surface factors influence establishment of some species. This complex example of primary succession on a tropical volcano has disclosed some surprises, and we have no doubt that many more remain to be revealed.
Acknowledgments
Funds were provided by the Western Pacific Tropical Research Center (to TEM) and the US NSF (DEB05–41972 and DEB–1118593 to RdM)
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Previously published online: www.landesbioscience.com/journals/cib/article/25924

Impact of the use of cannabis as a medicine in pregnancy, on the unborn child: a systematic review and meta-analysis protocol
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Info/History
- Preview PDF
Introduction: The use of cannabis for medicinal purposes is on the rise. As more people place their trust in the safety of prescribed alternative plant-based medicine and find it easily accessible, there is a growing concern that pregnant women may be increasingly using cannabis for medicinal purposes to manage their pregnancy symptoms and other health conditions. The aim of this review is to investigate the use of cannabis for medicinal purposes during pregnancy, describe the characteristics of the demographic population, and to measure the impact on the unborn child and up to twelve months postpartum. Methods and analyses: Research on pregnant women who use cannabis for medicinal purposes only and infants up to one year after birth who experienced in utero exposure to cannabis for medicinal purposes will be included in this review. Reviews, randomised controlled trials, case control, cross-sectional and cohort studies, that have been peer reviewed and published between 1996 and April 2024 as a primary research paper that investigates prenatal use of cannabis for medicinal purposes on foetal, perinatal, and neonatal outcomes, will be selected for review. Excluding cover editorials, letters, commentaries, protocols, conference papers and book chapters. Effects of illicit drugs use, alcohol misuse and nicotine exposure on neonate outcome will be controlled by excluding studies reporting on the concomitant use of such substances with cannabis for medicinal purposes during pregnancy. All titles and abstracts will be reviewed independently and in duplicate by at least two researchers. Records will be excluded based on title and abstract screening as well as publication type. Where initial disagreement exists between reviewers regarding the inclusion of a study, team members will review disputed articles status until consensus is gained. Selected studies will then be assessed by at least two independent researchers for risk bias assessment using validated tools. Data will be extracted and analysed following a systematic review and meta-analysis methodology. The statistical analysis will combine three or more outcomes that are reported in a consistent manner. The systematic review and meta-analysis will follow the PRISMA guidelines to facilitate transparent reporting [1].
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This study did not receive any funding.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
The study will use ONLY openly available human data from studies published in biomedical and scientific journals.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
All data produced in the present work are contained in the manuscript.
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
- Addiction Medicine (323)
- Allergy and Immunology (627)
- Anesthesia (163)
- Cardiovascular Medicine (2367)
- Dentistry and Oral Medicine (288)
- Dermatology (206)
- Emergency Medicine (379)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (835)
- Epidemiology (11765)
- Forensic Medicine (10)
- Gastroenterology (702)
- Genetic and Genomic Medicine (3731)
- Geriatric Medicine (348)
- Health Economics (633)
- Health Informatics (2392)
- Health Policy (929)
- Health Systems and Quality Improvement (896)
- Hematology (340)
- HIV/AIDS (780)
- Infectious Diseases (except HIV/AIDS) (13303)
- Intensive Care and Critical Care Medicine (767)
- Medical Education (365)
- Medical Ethics (104)
- Nephrology (398)
- Neurology (3493)
- Nursing (198)
- Nutrition (523)
- Obstetrics and Gynecology (673)
- Occupational and Environmental Health (662)
- Oncology (1819)
- Ophthalmology (535)
- Orthopedics (218)
- Otolaryngology (287)
- Pain Medicine (232)
- Palliative Medicine (66)
- Pathology (446)
- Pediatrics (1032)
- Pharmacology and Therapeutics (426)
- Primary Care Research (420)
- Psychiatry and Clinical Psychology (3172)
- Public and Global Health (6135)
- Radiology and Imaging (1279)
- Rehabilitation Medicine and Physical Therapy (746)
- Respiratory Medicine (825)
- Rheumatology (379)
- Sexual and Reproductive Health (372)
- Sports Medicine (322)
- Surgery (401)
- Toxicology (50)
- Transplantation (172)
- Urology (145)

IMAGES
VIDEO
COMMENTS
In a meta-analysis of primary succession sites across many systems ... This special feature identifies several opportunities for future research that will improve our understanding of the drivers and patterns of succession. First, the papers in this special issue highlight the need for large temporal and spatial scales to best account for ...
Primary succession is the assembly of ecosystems on barren landscapes following severe disturbances that leave little or no biological legacy (lava flows, landslides and mine wastes). The assembly ...
I. INTRODUCTION. Succession is a fundamental concept in ecology because it indicates how populations, species, communities, and ecosystems change over time after disturbance has created a new substrate [i.e. primary succession (Miles & Walton, 1993; Walker & Del Moral, 2003 )] or has removed part of the vegetation (i.e. secondary succession).
Abstract. Ecological succession - how biological communities re‐assemble and change over time following natural or anthropogenic disturbance - has been studied since the birth of ecology ...
Studies from many systems (tropical, temperate, boreal, arctic, alpine, floodplain forest, desert, mines, volcanic eruption and glacier fore-lands) show that dispersal limitation occurs consistently in the early stages of pri-mary succession, suggesting that this is a general pattern. On the other hand, we found strong biases in study sites ...
While little research has focused on this class of successional drivers, site conditions and history have become a primary focus of restoration ecology (e.g. Luken 1990; Bakker & Berendse 1999; Kardol & Wardle 2010). Primary and secondary successional sequences differ widely in this as the starting conditions are dramatically different.
Ecology is a leading journal publishing original research and synthesis papers on all aspects of ecology, with particular emphasis on cutting-edge research and new concepts. ... We test the textbook successional trajectory in Glacier Bay and evaluate long-term plant community development via primary succession through extensive fieldwork ...
Nevertheless, succession and community assembly research focus on different spatial and temporal scales, which may have hindered a mutual exchange of ideas 1. Succession research relates to the ...
Primary succession is the assembly of ecosystems on barren landscapes following severe disturbances that leave little or no biological legacy (lava flows, landslides and mine wastes). The assembly process involves colonisation of newly exposed substrates and subsequent interactions among the colonising plants, animals and soil microbes.
Primary succession describes the establishment of new ecosystems through the colonization of barren substrates (Odum, 1969 ). Bacteria are typically the first colonizers, which initiate key processes that enable ecosystem establishment and provide resources for fungi, plants, and animals to colonize later. The functional roles of these pioneer ...
Eugene Odum's 1969 paper, The Strategy of Ecosystem Development, marks a watershed moment in approaches to the study of succession, ecosystem change caused by discrete disturbances.He argued that succession is unique from other kinds of change with regard to mechanisms (modification of the physical environment by the community), trajectory (orderly, directional and predictable), and endpoint ...
Forest succession is a dynamic process of progressive compositional development of ecological communities of species following natural or anthropogenic disturbance. Despite a rich history of conceptual frameworks, models, and empirical advances, the complex interactions among climatic conditions, disturbances, edaphic factors, and silvicultural treatments still challenge our ability to ...
Primary succession is the assembly of ecosystems on barren landscapes following severe disturbances that leave little or no biological legacy (lava flows, landslides and mine wastes). The assembly process involves colonisation of newly exposed substrates and subsequent interactions among the colonising plants, animals and soil microbes.
succession in the recovering Mount Pinatubo landscape; and 2) determine the plant and environmental variables correlated with vegetation patterns using DCA, CCA, and pCCA. Our outcomes will determine which questions can be tested in subsequent studies of primary succession in tropical volcano systems. Materials and Methods Field sampling
Biodiversity affects the provision of ecosystem services over time and space. This study was done to find how ecological succession regulates the relationship between biodiversity and supporting services in arid ecosystems. So, the effects of biodiversity on ecosystem supporting services, including soil stability, infiltration, and nutrient cycling, were investigated at three successional ...
Research Paper. Primary succession in Mount Pinatubo: Habitat availability and ordination analysis. ... then to ascertain environmental variables that have influenced the early patterns of primary succession. Unconstrained and constrained ordination methods were used to determine the influence of spatial, elevation, and substrate patterns on ...
Abstract During primary succession, the abundance of different species and their associated plant traits change over time. ... although several of the returned papers used Ellenberg indicator values (e.g., Rajandu et al., ... There has been considerable research on primary succession, with many studies undertaken in such environments as ...
The results show that an ecological pattern of spontaneous primary auto-succession is widely recurring in Iberian gypsum quarries, which is capable of regenerating the pre-existing natural vegetation. ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a ...
Succession. Ecological succession is a series of progressive changes in the species that make up a community over time. Ecologists usually identify two types of succession, which differ in their starting points: In primary succession, newly exposed or newly formed rock is colonized by living things for the first time.
Ecological succession is the process by which the mix of species and habitat in an area changes over time. Gradually, these communities replace one another until a "climax community"—like a mature forest—is reached, or until a disturbance, like a fire, occurs. Ecological succession is a fundamental concept in ecology.
View Primary Succession Research Papers on Academia.edu for free.
The focus of this paper is to: 1) characterize the sites that support succession in the recovering Mount Pinatubo landscape; and 2) determine the plant and environmental variables correlated with vegetation patterns using DCA, CCA, and pCCA. Our outcomes will determine which questions can be tested in subsequent studies of primary succession in ...
@article{Geng2024TheRO, title={The roles of rare and abundant microbial species in the primary succession of biological soil crusts are differentiated in metal tailings ponds with different states}, author={Yuchen Geng and Panpan Zhou and Zhicong Wang and Chengrong Peng and Genbao Li and Dun Hai Li}, journal={Journal of Hazardous Materials ...
Lawrence, D.B., Schoenike, R.E., Quispel, A. and Bond, G. 1967: The role of Dryas drummondi in vegetation development following ice recession at Glacier Bay, Alaska ...
Reviews, randomised controlled trials, case control, cross-sectional and cohort studies, that have been peer reviewed and published between 1996 and April 2024 as a primary research paper that investigates prenatal use of cannabis for medicinal purposes on foetal, perinatal, and neonatal outcomes, will be selected for review.
Innovation is the primary driving force for development, and enterprises, as the main drivers of innovation, are an important part of implementing the national innovation strategy. This paper, combining the perspective of the enterprise lifecycle, thoroughly examines the differential impact of the CEO's financial background on green innovation in enterprises at different stages of the lifecycle.