

Pulmonary Embolism Case Study: Diagnosis and Treatment
by John Landry, BS, RRT | Updated: May 23, 2024
A pulmonary embolism is a blockage in the pulmonary artery caused by a blood clot in the lungs. This is a life-threatening condition and results in symptoms that respiratory therapists and medical professionals must be able to identify.
This case study will explore the events leading up to a patient being diagnosed with a pulmonary embolism, as well as the treatment and management of this condition.
25+ RRT Cheat Sheets and Quizzes
Get access to 25+ premium quizzes, mini-courses, and downloadable cheat sheets for FREE.
Pulmonary Embolism Clinical Scenario
You are called to the emergency room to treat a 25-year-old, 67 kg female patient. She is experiencing new onset chest pain and shortness of breath. She describes her chest pain as a stabbing sensation that radiates down to her left arm and gets worse during periods of exertion. She also feels lightheaded and highly anxious. In addition, the patient has a history of allergic asthma. Her only home medications are Microgestin Fe 1/20 (i.e., birth control) and albuterol PRN . She has no history of smoking or vaping.
Patient Assessment
- The patient’s pupils are round and reactive.
- She is mildly diaphoretic.
- She is showing signs of nasal flaring without pursed-lip breathing.
- Her trachea is located in the midline.
- She has no jugular venous distention.
- She has been coughing up small amounts of blood-tinged sputum.
- She has bilateral, decreased chest rise.
- Auscultation reveals crackles and a third heart sound.
- Palpation reveals normal tactile fremitus.
- Her percussion findings are normal at the apexes and decreased at the bases.
- She has a normal anterior-posterior chest diameter.
- Her chest is not tender to the touch.
- Her abdomen is soft and not distended.
Extremities:
- She shows no sign of digital clubbing.
- Her capillary refill time is 4 seconds.
- Her fingertips are slightly cyanotic and cool to the touch.
- She shows no signs of pedal edema.
- She has a moderately sized bruise on her right leg that is tender and warm to the touch.
Vital Signs:
- Respiratory rate: 30 breaths/min
- Heart rate: 120 beats/min
- Blood pressure: 100/75 mmHg
- Chest x-ray: Consolidation in both lung bases
Diagnosis and Treatment
Based on the patient’s assessment , history, and vital signs, what condition does the patient have, and why?
The patient is presenting with a pulmonary embolism (PE).
Key Components:
- The use of oral contraceptives is important for the diagnosis because one common side effect is hyper-coagulation.
- A bruise that is accompanied by tenderness and warmth in her leg is a sign of deep vein thrombosis (i.e., blood clot). This is important because blood clots can travel from the legs to the lungs, resulting in a pulmonary embolism.
- Other important signs include hypoxemia (i.e., low SpO2), increased capillary refill, cyanosis, and coolness to the touch. This could be caused by decreased perfusion and/or atelectasis .
- Diaphoresis and anxiety
- The patient has decreased percussion and crackles in the lung bases, which indicates atelectasis. Atelectasis can occur in patients who experience pulmonary infarction due to a pulmonary embolism.
- A third heart sound is sometimes heard in patients with a pulmonary embolism.
- Another important finding is the patient’s chest x-ray, which only shows atelectasis. A pulmonary embolism will not show up on a chest x-ray, but sometimes a wedge-shaped inflate will appear if pulmonary infarction has occurred as a result.
Bonus Point: You should have been able to recognize that, while the patient had a history of allergic asthma , their current presentation did not align with that of an asthma exacerbation. Remember that additional information may be given to you in scenario-based testing. When this happens, take note of the information in case it becomes important later on, but don’t let it distract you from the task at hand.
What tests can confirm the presence of a pulmonary embolism?
- Computed tomography pulmonary angiogram (CTPA): This is the preferred test for confirming a pulmonary embolism. The presence of a blood clot will show as a darkened area.
- V/Q scan: This is the second most preferred radiological test for a suspected pulmonary embolism. It will show a disturbance in gas distribution in the patient’s lungs when a thrombus is present.
- Pulmonary angiogram: This is the least preferred test because it is the most invasive. It involves the insertion of a catheter while dye is injected into the pulmonary artery, which will reveal the presence of an embolism.
You may also wish to recommend specific blood tests, such as D-dimer and platelet count. These will give you clues about the patient’s clotting status. D-dimer is most often used to look for the presence of a blood clot, as it will be increased if a clot is present.
It is important to remember that other factors can cause a patient’s d-dimer and clotting factors to increase; therefore, you should not rely on this test solely to confirm that a pulmonary embolism is present.
Additional Treatment
Let’s assume that you initiated the patient on oxygen therapy via nasal cannula at 2 L/min to try and correct their hypoxemia. After 20 minutes, you decided to incrementally increase the flow to 5 L/min, but there was no improvement in their oxygenation status.
Why is the patient’s SpO2 and PaO2 unresponsive to receiving supplemental oxygen?
This occurs because blood clots reduce or entirely prevent blood from flowing past a clot. Therefore, any alveoli distal to the clot will receive little to no perfusion. This decrease in perfusion prevents carbon dioxide and oxygen from effectively being exchanged at the alveolar-capillary membrane, even when the patient is ventilating normally.
This prevention of effective gas exchange due to low perfusion is part of what causes patients with a pulmonary embolism to be unresponsive to supplemental oxygen. The development of atelectasis due to pulmonary infarction secondary to a pulmonary embolism can further reduce the patient’s responsiveness to oxygen.
What other treatment methods would you recommend?
- Anticoagulants: The administration of a fast-acting anticoagulant, like heparin, and a slow-acting anticoagulant, like Warfarin should be recommended. This can help stop the existing clot from growing and to prevent new clots from forming. Patients who are prescribed Warfarin will need to have their other medications, dietary supplements, and nutrition plan reviewed. That is because medications, supplements, or food can impact the blood’s ability to clot while potentially negatively impacting the drug.
- Thrombolytic agents: The administration of thrombolytic drugs, such as altepase, streptokinase, or urokinase, can help break down the embolism. Patients who are prescribed a thrombolytic should be monitored for an increased risk of bleeding. This is especially true when prescribed heparin alongside a thrombolytic agent.
- Analgesics: These drugs can be administered for any pain the patient may be experiencing.
- Preventative actions: Ensuring the patient stays active, moves their limbs, is well-hydrated, and wears compression socks can help prevent another clot from forming.
- Pneumatic compression cuffs: These should be placed on the patient’s legs while they’re bedridden to decrease the risk of more blood clots forming.
- Surgical interventions: A pulmonary embolectomy can be performed to remove an existing clot that is not dissolved by medications. The placement of an inferior vena cava filter can also be used to prevent future clots from reaching the patient’s lungs. These filters are usually reserved for patients who are at high risk for developing further embolisms despite receiving pharmaceutical interventions.
Final Thoughts
A pulmonary embolism is a serious medical condition that can be difficult to diagnose. Respiratory therapists must be aware of the risk factors and symptoms to properly assess and treat their patients. A few key things to remember about patients with a pulmonary embolism include:
- They often present with radiating chest pain.
- They need radiological testing that is more extensive than a simple chest x-ray.
- They are often unresponsive to supplemental oxygen.
Treatment for a pulmonary embolism should be aimed at dissolving existing clots while preventing future clots from forming. Thanks for reading, and, as always, breathe easy, my friend.

Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
- Egan’s Fundamentals of Respiratory Care. 12th ed., Mosby, 2020.
- Wilkins’ Clinical Assessment in Respiratory Care. 8th ed., Mosby, 2017.
- Clinical Manifestations and Assessment of Respiratory Disease. 8th ed., Mosby, 2019.
- Tarbox, Abigail K., and Mamta Swaroop. “Pulmonary Embolism.” National Library of Medicine, Int J Crit Illn Inj Sci, Jan. 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3665123 .
- Turetz, Meredith, et al. “Epidemiology, Pathophysiology, and Natural History of Pulmonary Embolism.” National Library of Medicine, Semin Intervent Radiol, Jan. 2018, www.ncbi.nlm.nih.gov/pmc/articles/PMC5986574 .
- Morrone, Doralisa, and Vincenzo Morrone. “Acute Pulmonary Embolism: Focus on the Clinical Picture.” National Library of Medicine, Korean Circ J., May 2018, www.ncbi.nlm.nih.gov/pmc/articles/PMC5940642 .
- Lavorini, Federico, et al. “Diagnosis and Treatment of Pulmonary Embolism: A Multidisciplinary Approach.” National Library of Medicine, Multidiscip Respir Med, 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3878229 .
Recommended Reading
Faqs about the clinical simulation exam (cse), pulmonary embolism: overview and practice questions, copd: overview and practice questions, pulmonary edema: overview and practice questions, pleural effusion: overview and practice questions, myocardial infarction: overview and practice questions, what is the recovery time for blood clots in the lungs, 7+ mistakes to avoid on the clinical simulation exam (cse), the 50+ diseases to learn for the clinical sims exam (cse).
- Search Menu
- Sign in through your institution
- Advance Articles
- Editor's Choice
- Braunwald's Corner
- ESC Guidelines
- EHJ Dialogues
- Issue @ a Glance Podcasts
- CardioPulse
- Weekly Journal Scan
- European Heart Journal Supplements
- Year in Cardiovascular Medicine
- Asia in EHJ
- Most Cited Articles
- ESC Content Collections
- Author Guidelines
- Submission Site
- Why publish with EHJ?
- Open Access Options
- Submit from medRxiv or bioRxiv
- Author Resources
- Self-Archiving Policy
- Read & Publish
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Journals Career Network
- About European Heart Journal
- Editorial Board
- About the European Society of Cardiology
- ESC Publications
- War in Ukraine
- ESC Membership
- ESC Journals App
- Developing Countries Initiative
- Dispatch Dates
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Declarations, contemporary outcomes in patients hospitalized with pulmonary embolism: what can we learn from observational data.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
- Article contents
- Figures & tables
- Supplementary Data
Andrew Sharp, Felix Mahfoud, Contemporary outcomes in patients hospitalized with pulmonary embolism: what can we learn from observational data?, European Heart Journal , 2024;, ehae263, https://doi.org/10.1093/eurheartj/ehae263
- Permissions Icon Permissions

This editorial refers to ‘Catheter-based therapy for intermediate or high-risk pulmonary embolism: death and re-hospitalisation’, by O. Leiva et al ., https://doi.org/10.1093/eurheartj/ehae184 .
Pulmonary embolism (PE) can be an elusive disease. This was well characterized by Cohen et al ., who identified 370 012 venous thromboembolism (VTE)-related deaths within one year from just six EU countries, with 126 145 presenting with sudden death and a further 217 394 diagnosed post-mortem. This left only 27 473 patients in whom the diagnosis was made pre-mortem. 1 It therefore behoves us to do what we can for those in whom the diagnosis can actually be made, in the hope of improving outcomes across the larger group of patients we cannot help.
So how are we doing in terms of PE outcomes, and do we currently have the tools to improve care? In this issue of the journal, Leiva et al. analyse outcomes from a large cohort of patients admitted to US hospitals with a diagnosis of PE and stratify mortality by PE severity, and whether catheter-based therapies (CBT) were used. 2 This database has many strengths, collating data on 50% of all hospital admissions in the USA and tracking them within a calendar year. Looking at a 4-year period between 2017 and 2020, the authors report PE-related mortality in over 400 000 patients, of whom over 10 000 received CBT as part of their care. Mortality is further stratified according to disease severity, into high-risk and intermediate-risk PE, presenting different pictures of two groups with radically differing risk profiles.
Categories of PE were defined by the 2019 ESC Guidelines on Pulmonary Embolism. 3 Low-risk PE describes a patient with no clinical or anatomical markers of risk of early death, 4 and high-risk PE is that causing shock—both readily understandable and well-defined categories. The intermediate-risk category, however, covers a broad swathe of disease, from a mildly elevated troponin in a well elderly patient to a patient on the edge of shock. This category is further subdivided into ‘intermediate-low’ and ‘intermediate-high’ risk according to whether one or two markers of right ventricular (RV) strain (imaging/biomarkers) are present, categorizing a disease with at least four modes of clinical presentation.
Focusing first on high-risk PE, it is fair to say that, with the aid of the US Pulmonary Embolism Response Team (PERT) initiative 5 and a well-resourced/trained hospital system, care of PE in the USA is among the best in the world. The period studied is recent and therefore reasonably represents ‘state-of-the-art’. Despite this, the mortality rate for high-risk PE remains high, with a 30-day mortality rate of over 45%. Using propensity score adjustment to adjust for some (but not all) major differences between cohorts treated with or without catheter assistance, the authors were able to detect a lower rate of mortality among the catheter cohort, with a 45.0% mortality rate for those who received CBT and 49.7% for those who did not, representing a 17% relative reduction in death. Well worth having, but still demonstrating the field has a long way to go.
Why so high a mortality rate? The clue may partly lie in the rate of prior cardiac arrest of over 45%. This was an extremely high-risk, ‘high-risk’ group and one that may struggle to ever be adequately represented within prospective randomized clinical trials (RCTs). We have learned from trials of cardiogenic shock following ST-elevation myocardial infarction that demonstrating the effectiveness of any intervention can be difficult, perhaps because for so many, ‘the die is cast’ by the time any therapy can be delivered within an RCT. These data on high-risk PE may suggest a similar phenomenon, although catheter therapy and its use was at least a marker of, and possibly a contributor to, improved outcomes. Although the outcomes were not as impressive as those described in the FLAME study of CBT, 6 a 4.7% actual risk reduction in mortality is not a trivial reduction for any disease and may more closely describe the status of high-risk PE treatment in the real world and the contribution of CBT to improved outcomes.
How about intermediate risk? One difficulty here is the breadth of the category, which includes patients both ambulatory and near-ventilatory. This makes interpreting coding data fraught with difficulty, as defining the cohort is challenging. Did all patients have standardized risk stratification according to the guidelines? Was a Pulmonary Embolism Severity Index (PESI) score calculated? If so, were repeated measures taken to account for dynamic bedside clinical parameters? Was a troponin measured (if so, which assay)? Were RV to left ventricular (LV) diameter ratio measurements carefully assessed on CT or echocardiography in all patients? If patients were transiently hypotensive and responded to fluids, how were they categorized? And what about patients with raised plasma lactate, and therefore evidence of reduced end-organ perfusion—should they be categorized as high risk or intermediate risk? These represent challenges when we try to retrospectively define intermediate-risk PE, this broadest of clinical groups.
In the current manuscript, the Achilles Heel may be how intermediate risk is defined and captured—‘PE with cor pulmonale, type 2 myocardial infarction (MI), or right heart failure without cardiogenic shock, vasopressor use, or cardiac arrest’. This is not how intermediate risk is defined in the guidelines, whereby measures of RV function with biomarkers are combined with clinical parameters within a PESI score, and so the terminologies and therefore interpretation of results for intermediate-risk PE cannot be considered equivalent.
What is the significance of this difference? Although data sets vary, it is said that low-risk PE represents up to 60% of PE presentations and high-risk PE around 5% of presentations (with some variability in data). 7 The current manuscript is therefore striking for the proportion of patients falling into the relative risk categories. For high-risk PE, we see an incidence of 4.1%, which seems in line with other data sets. For intermediate-risk PE, however, we see an incidence rate of 10.6%. This would mean over 80% of remaining PEs admitted to US hospitals would be low risk, which seems out of kilter with current data. The most likely explanation is that this diverse and dynamic mode of presentation is not fully captured in coding data, perhaps missing up to five times as many patients.
Of the 42 829 patients who presented with intermediate-risk PE, 8824 underwent CBT, using catheter-directed thrombolysis in most cases. Mortality rates for CBT vs. medical management were low, at 2.0% and 2.6%, respectively, which can be described as a 24% relative risk reduction in death, although at 0.6%, a less impressive actual risk reduction. This baseline mortality rate for intermediate risk is relatively low, which raises the possibility that the methodology for capturing data may be distorting mortality rates in both intermediate- and high-risk categories. For example, if a patient arrives in hospital with intermediate-risk PE and deteriorates, requiring vasopressor support, will they be coded as high-risk PE according to study definitions? If they then die, they appear to die from high-risk PE, thus transferring outcomes of some of the sickest patients within the intermediate-risk arm into the high-risk arm. This might partly explain the low event rate within the intermediate-risk category and the high event rate in the high-risk arm. Therefore, we should cautiously interpret hard absolute event rates in both arms of the study.
Patients who received CBT had slightly longer length of stay, which is surprising given the transformative impact of catheter intervention on some PE patients. Alternative contemporary data support the concept of a rapid recovery and early discharge following CBT, 8 although it is not seen here. Nevertheless, the use of CBT was associated with reduced rates of readmission, pointing to additional potential benefits from catheter treatment.
The authors allude to RCTs in this space and we are currently in a golden age for the design and conduct of these essential trials. For catheter-directed lysis vs. anticoagulation in intermediate- to high-risk PE, we have HI-PEITHO, designed to provide clinical outcome data at early follow-up. 9 For longer-term outcomes with CBT vs. anticoagulation in intermediate- to high-risk PE, we have PE-TRACT ( NCT05591118 ). For mechanical thrombectomy, we have the PEERLESS II trial, randomizing 1200 patients with intermediate-risk PE between catheter thrombectomy and anticoagulation alone, utilizing a hierarchal outcome score to quantify clinical benefit ( NCT06055920 ) and PEERLESS I, randomizing thrombectomy against catheter lysis in 550 patients. 10 STORM PE will also capture clinical data in 100 intermediate- to high-risk patients randomized to thrombectomy vs. anticoagulation ( NCT05684796 ); other investigator-led studies are also described on clinicaltrials.gov , suggesting that, although it has taken time for the techniques and technologies to mature, the necessary trials are now underway and will give us the randomized outcomes data we need in this area.
For PE causing shock, myocardial infarction has shown us how difficult these trials are to provide proof-of-benefit in the sickest patients. In this space, where randomization is difficult and current outcomes are poor, we benefit from guidance from high-quality observational data, extrapolation from first principles and clinical consensus. Leiva et al . have provided one important piece of this jigsaw, but have also reminded us that we have much work left to do.
Disclosure of Interest
A.S. reports speaker honoraria/consulting fees from Medtronic, Boston Scientific, Philips, Recor Medical and Penumbra and stock options with Althea Medical. F.M. is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung. His institution (Saarland University) has received scientific support from Ablative Solutions, Medtronic and ReCor Medical. He has received speaker honoraria/consulting fees from Ablative Solutions, Amgen, Astra-Zeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, ReCor Medical, Servier, and Terumo.
Cohen AT , Agnelli G , Anderson FA , Arcelus JI , Bergqvist D , Brecht JG , et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality . Thromb Haemost 2007 ; 98 : 756 – 64 . https://doi.org/10.1160/TH07-03-0212
Google Scholar
Leiva O , Alviar C , Khandhar S , Parikh SA , Toma C , Postelnicu R , et al. Catheter-based therapy for high-risk or intermediate-risk pulmonary embolism: death and re-hospitalization . Eur Heart J 2024 : ehae184 . https://doi.org/10.1093/eurheartj/ehae184
Konstantinides SV , Meyer G . The 2019 ESC guidelines on the diagnosis and management of acute pulmonary embolism . Eur Heart J 2019 ; 40 : 3453 – 5 . https://doi.org/10.1093/eurheartj/ehz726
Aujesky D , Obrosky DS , Stone RA , Auble TE , Perrier A , Cornuz J , et al. Derivation and validation of a prognostic model for pulmonary embolism . Am J Respir Crit Care Med 2005 ; 172 : 1041 – 6 . https://doi.org/10.1164/rccm.200506-862OC
Rosovsky R , Chang Y , Rosenfield K , Channick R , Jaff MR , Weinberg I , et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis . J Thromb Thrombolysis 2019 ; 47 : 31 – 40 . https://doi.org/10.1007/s11239-018-1737-8
Silver MJ , Gibson CM , Giri J , Khandhar S , Jaber W , Toma C , et al. Outcomes in high-risk pulmonary embolism patients undergoing FlowTriever mechanical thrombectomy or other contemporary therapies: results from the FLAME study . Circ Cardiovasc Interv 2023 ; 16 : e013406 . https://doi.org/10.1161/CIRCINTERVENTIONS.123.013406
Gotzinger F , Lauder L , Sharp ASP , Lang IM , Rosenkranz S , Konstantinides S , et al. Interventional therapies for pulmonary embolism . Nat Rev Cardiol 2023 ; 20 : 670 – 84 . https://doi.org/10.1038/s41569-023-00876-0
Monteleone P , Ahern R , Banerjee S , Desai K , Kadian-Dodov D , Webber E , et al. Modern treatment of pulmonary embolism (USCDT versus MT): results from a real-world, big data analysis (REAL-PE) . J Soc Cardiovasc Angiogr Interv 2024 ; 3 : 101192 . https://doi.org/10.1016/j.jscai.2023.101192
Klok FA , Piazza G , Sharp ASP , Ni Ainle F , Jaff MR , Chauhan N , et al. Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate-high-risk pulmonary embolism: rationale and design of the HI-PEITHO study . Am Heart J 2022 ; 251 : 43 – 53 . https://doi.org/10.1016/j.ahj.2022.05.011
Gonsalves CF , Gibson CM , Stortecky S , Alvarez RA , Beam DM , Horowitz JM , et al. Randomized controlled trial of mechanical thrombectomy vs catheter-directed thrombolysis for acute hemodynamically stable pulmonary embolism: rationale and design of the PEERLESS study . Am Heart J 2023 ; 266 : 128 – 37 . https://doi.org/10.1016/j.ahj.2023.09.002
Author notes
Email alerts, companion article.
- Catheter-based therapy for high-risk or intermediate-risk pulmonary embolism: death and re-hospitalization
Citing articles via
Looking for your next opportunity, affiliations.
- Online ISSN 1522-9645
- Print ISSN 0195-668X
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Create Free Account or
- Acute Coronary Syndromes
- Anticoagulation Management
- Arrhythmias and Clinical EP
- Cardiac Surgery
- Cardio-Oncology
- Cardiovascular Care Team
- Congenital Heart Disease and Pediatric Cardiology
- COVID-19 Hub
- Diabetes and Cardiometabolic Disease
- Dyslipidemia
- Geriatric Cardiology
- Heart Failure and Cardiomyopathies
- Invasive Cardiovascular Angiography and Intervention
- Noninvasive Imaging
- Pericardial Disease
- Pulmonary Hypertension and Venous Thromboembolism
- Sports and Exercise Cardiology
- Stable Ischemic Heart Disease
- Valvular Heart Disease
- Vascular Medicine
- Clinical Updates & Discoveries
- Advocacy & Policy
- Perspectives & Analysis
- Meeting Coverage
- ACC Member Publications
- ACC Podcasts
- View All Cardiology Updates
- Earn Credit
- View the Education Catalog
- ACC Anywhere: The Cardiology Video Library
- CardioSource Plus for Institutions and Practices
- ECG Drill and Practice
- Heart Songs
- Nuclear Cardiology
- Online Courses
- Collaborative Maintenance Pathway (CMP)
- Understanding MOC
- Image and Slide Gallery
- Annual Scientific Session and Related Events
- Chapter Meetings
- Live Meetings
- Live Meetings - International
- Webinars - Live
- Webinars - OnDemand
- Certificates and Certifications
- ACC Accreditation Services
- ACC Quality Improvement for Institutions Program
- CardioSmart
- National Cardiovascular Data Registry (NCDR)
- Advocacy at the ACC
- Cardiology as a Career Path
- Cardiology Careers
- Cardiovascular Buyers Guide
- Clinical Solutions
- Clinician Well-Being Portal
- Diversity and Inclusion
- Infographics
- Innovation Program
- Mobile and Web Apps
Pulmonary Embolism: Clinical Case
The following are key points to remember about this clinical case on pulmonary embolism (PE):
- Although approximately 20% of patients who are treated for PE die within 90 days, true short-term mortality attributed to PE is estimated to be <5%. Approximately 50% of the patients who receive a diagnosis of PE have functional and exercise limitations 1 year later (known as post–PE syndrome), and the health-related quality of life for patients with a history of PE is diminished as compared with that of matched controls.
- Newer approaches such as YEARS algorithm and age adjustment for D-dimer thresholds for ruling out PE are recommended.
- Diagnostic chest imaging is reserved for patients in whom PE cannot be ruled out based on clinical decision making.
- After initial diagnosis, clinical risk stratification into high, intermediate high risk, intermediate low risk, and low risk is recommended next. The nomenclature of “massive” and “submassive” in describing PE is confusing, given that clot size does not dictate therapy.
- High risk: Intravenous systemic thrombolysis is the most readily available reperfusion option in high-risk PE patients. Alternative reperfusion approaches include surgical thrombectomy and catheter-directed thrombolysis (with or without thrombectomy). Additional supportive measures include the administration of inotropes and the use of extracorporeal life support.
- Intermediate high risk: When available, catheter-directed thrombus removal remains an option for such. At this time, there is insufficient evidence to support catheter-directed thrombolysis over anticoagulation alone in these patients. Systemic thrombolysis is not typically recommended for these patients.
- Intermediate low risk: Anticoagulation with low molecular weight heparin and close monitoring for 24-48 hours for clinical worsening is recommended.
- Low risk: Outpatient management with direct oral anticoagulants is the preferred strategy.
- All patients with acute PE should receive anticoagulant therapy for ≥3 months. The decision to continue treatment indefinitely depends on whether the associated reduction in the risk of recurrent venous thromboembolism outweighs the increased risk of bleeding and should take into account patient preferences.
- Patients should be followed longitudinally after an acute PE to assess for dyspnea or functional limitation, which may indicate the development of post–PE syndrome or chronic thromboembolic pulmonary hypertension.
Clinical Topics: Anticoagulation Management, Cardiac Surgery, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine, Anticoagulation Management and Venothromboembolism, Cardiac Surgery and Arrhythmias, Cardiac Surgery and Heart Failure, Interventions and Imaging, Interventions and Vascular Medicine
Keywords: Anticoagulants, Diagnostic Imaging, Dyspnea, Extracorporeal Membrane Oxygenation, Heparin, Low-Molecular-Weight, Outpatients, Pulmonary Embolism, Quality of Life, Reperfusion, Risk Assessment, Secondary Prevention, Thrombectomy, Thrombolytic Therapy, Thrombosis, Vascular Diseases, Venous Thromboembolism
You must be logged in to save to your library.
Jacc journals on acc.org.
- JACC: Advances
- JACC: Basic to Translational Science
- JACC: CardioOncology
- JACC: Cardiovascular Imaging
- JACC: Cardiovascular Interventions
- JACC: Case Reports
- JACC: Clinical Electrophysiology
- JACC: Heart Failure
- Current Members
- Campaign for the Future
- Become a Member
- Renew Your Membership
- Member Benefits and Resources
- Member Sections
- ACC Member Directory
- ACC Innovation Program
- Our Strategic Direction
- Our History
- Our Bylaws and Code of Ethics
- Leadership and Governance
- Annual Report
- Industry Relations
- Support the ACC
- Jobs at the ACC
- Press Releases
- Social Media
- Book Our Conference Center
Clinical Topics
- Chronic Angina
- Congenital Heart Disease and Pediatric Cardiology
- Diabetes and Cardiometabolic Disease
- Hypertriglyceridemia
- Invasive Cardiovascular Angiography and Intervention
- Pulmonary Hypertension and Venous Thromboembolism
Latest in Cardiology
Education and meetings.
- Online Learning Catalog
- Products and Resources
- Annual Scientific Session
Tools and Practice Support
- Quality Improvement for Institutions
- Accreditation Services
- Practice Solutions
Heart House
- 2400 N St. NW
- Washington , DC 20037
- Contact Member Care
- Phone: 1-202-375-6000
- Toll Free: 1-800-253-4636
- Fax: 1-202-375-6842
- Media Center
- Advertising & Sponsorship Policy
- Clinical Content Disclaimer
- Editorial Board
- Privacy Policy
- Registered User Agreement
- Terms of Service
- Cookie Policy
© 2024 American College of Cardiology Foundation. All rights reserved.
- Case report
- Open access
- Published: 15 September 2009
Pulmonary embolism presenting as syncope: a case report
- Ahmet Demircan 1 ,
- Gulbin Aygencel 2 ,
- Ayfer Keles 1 ,
- Ozgur Ozsoylar 3 &
- Fikret Bildik 1
Journal of Medical Case Reports volume 3 , Article number: 7440 ( 2009 ) Cite this article
34k Accesses
13 Citations
4 Altmetric
Metrics details
Introduction
Despite the high incidence of pulmonary embolism its diagnosis continues to be difficult, primarily because of the vagaries of symptoms and signs in presentation. Conversely, syncope is a relatively easy clinical symptom to detect, but has varied etiologies that lead to a documented cause in only 58% of syncopal events. Syncope as the presenting symptom of pulmonary embolism has proven to be a difficult clinical correlation to make.
Case presentation
We present the case of a 26-year-old Caucasian man with pulmonary embolism induced-syncope and review the pathophysiology and diagnostic considerations.
Conclusions
Pulmonary embolism should be considered in the differential diagnosis of every syncopal event that presents at an emergency department.
Recognized venous thromboembolism (pulmonary embolism and deep venous thrombosis) is responsible for more than 250,000 hospitalizations and approximately 50,000 deaths per year in the United States. Because it is difficult to diagnose, the true incidence of pulmonary embolism is unknown, but it is estimated that approximately 650,000 cases occur annually [ 1 ].
Despite this high incidence, the diagnosis of pulmonary embolism continues to be difficult primarily because of the notorious vagaries of symptoms and signs in its presentation. Conversely, syncope is a relatively easy clinical symptom to detect, but has varied etiologies that lead to a documented cause in only 58% of syncopal events [ 2 ].
Syncope as the presenting symptom of pulmonary embolism has proven to be a difficult clinical correlation to make. We present the case of a patient with pulmonary embolism-induced syncope and review the pathophysiology and diagnostic considerations.
A 26-year-old Caucasian man with no history of disease was admitted to Gazi University Emergency Department after he had a syncopal episode in his home. The patient was in his usual good state of health until he suddenly collapsed while standing and lost consciousness for approximately five minutes. He recovered spontaneously but was extremely weak and dyspneic. He was also diaphoretic and tachypneic, but denied any associated chest pain or palpitations. No tonic-clonic activity was witnessed, and he experienced no incontinence.
The patient was a computer programmer and he had been working 18 hours a day without rest periods for a month. On admission, physical examination revealed a diaphoretic and dyspneic patient without focal neurologic findings. His heart rate was regular but tachycardic at 128 beats/minute, his blood pressure was 126/72 mmHg without orthostatic changes, and his respiratory rate was 32 breaths/minute. The room air oxygen saturation was 90%, and arterial blood gas analysis in room air revealed hypoxemia (PO 2 = 58 mmHg) with an elevated alveolo-arterial oxygen gradient (A-a O 2 gradient). Examination of his head and neck was normal. The results of chest wall examination revealed reduced breath sounds bilaterally at the lung bases. The findings of heart and abdominal examinations were unremarkable, but on examination of his legs, deep venous thrombosis (DVT) was noted in his left leg, with a positive Homans' sign in the left leg and the left calf measured 3 cm more than the right one.
Levels of serum electrolytes, glucose, blood urea and creatinine, and complete blood counts were normal. Results of a computed tomographic scan of his head were negative for bleeding, aneurysm or an embolic event. Chest X-ray was clear. An electrocardiogram showed a regular rhythm consistent with sinus tachycardia; there were Q and T waves in lead III and an S wave in lead I. A ventilation-perfusion scan demonstrated an unmatched segmental perfusion defect, indicating a high probability of the presence of a pulmonary thromboembolism (PTE) (Figures 1 and 2 ). A transthoracic echocardiogram revealed normal left ventricle function without a patent foramen ovale, an atrial septal defect or a ventricular septal defect, but with mild pulmonary hypertension (42 mmHg). A Doppler scan of the legs revealed an acute DVT in the patient's left leg, in the popliteal vein. Thrombolytic treatment was not given - the patient received standard anticoagulation treatment with unfractionated heparin and an oral anticoagulant. Before treatment, a blood sample was taken to examine the thrombophilia panel. After a 12-day course of hospital treatment, he was discharged on oral warfarin therapy. The patient's long-term follow-up was performed by the Department of Pulmonary Disease, and we learned that the patient was well for four months after that episode without any evidence of recurrent syncope or pulmonary embolism.
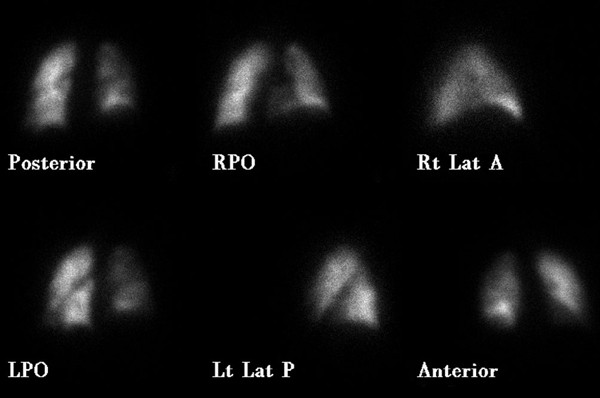
Decreased perfusion is seen to the right lung (particularly evident in the right lower lobe on the RPO image) in our case (perfusion scan was performed with Tc-99m MAA) .
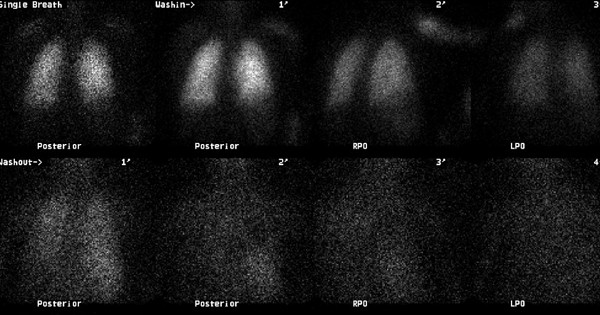
There is no significant ventilation defect in our case (ventilation scan was performed with Xe-133 gas) .
Pulmonary embolism is a frequent cause of death in the United States. Nevertheless, it remains difficult to diagnose. Pulmonary emboli differ considerably in size and number, and the underlying disorders, including malignancy, trauma, and protein C or S deficiency, are numerous [ 1 ]. The classic triad of pleuritic chest pain, dyspnea, and hemoptysis is rare, and clinically apparent DVT is present in only 11% of confirmed cases of pulmonary embolism in patients without underlying cardiopulmonary disease [ 3 ].
However, the clinical picture of pulmonary embolism is variable and most patients suffering from acute pulmonary embolism present with one of three different clinical syndromes. These clinical syndromes are pulmonary infarction, acute unexplained dyspnea, and acute cor pulmonale. The pulmonary infarct syndrome usually occurs with a submassive embolism that completely occludes a distal branch of the pulmonary circulation. Patients with this condition have pleuritic chest pain, hemoptysis, rales, and abnormal findings on chest X-ray. The acute, unexplained dyspnea pattern may also be the result of submassive pulmonary embolism without pulmonary infarction. Results of a chest X-ray and electrocardiogram are usually normal, but pulse oxygen saturation is often depressed. The third pattern, acute cor pulmonale syndrome, is caused by the complete obstruction of 60 to 75% of pulmonary circulation. Patients with this pattern experience shock, syncope, or sudden death [ 4 , 5 ].
Syncope, in contrast to pulmonary embolism, is relatively easy to detect, but can be a difficult symptom from which to determine the etiology. In as many as 50% of patients with syncope, no specific cause is found despite extensive evaluation. Syncope has been classified as cardiovascular (reflex and cardiac syncope), noncardiovascular (including neurologic and metabolic disorders) and unexplained [ 2 , 6 ]. It occurs in approximately 10% of patients with acute pulmonary embolism and is commonly ascribed to a massive, hemodynamically unstable acute pulmonary embolism. Although the prognostic value of syncope has not been specifically addressed, it has generally been considered a poor indicator in diagnosing pulmonary embolism [ 7 ].
Syncope in the setting of pulmonary embolism can be the result of three possible mechanisms. First, greater than 50% occlusion of the pulmonary vascular tree causes right ventricular failure and impaired left ventricular filling, leading to a reduction in cardiac output, arterial hypotension, reduced cerebral blood flow, and ultimately syncope. The second mechanism of syncope associated with pulmonary embolism is the appearance of arrhythmias associated with right ventricular overload. In the third mechanism, the embolism can trigger a vasovagal reflex that leads to neurogenic syncope. However, the contribution of hypoxemia secondary to ventilation or perfusion abnormalities must also be considered and may play an important role in the development of syncope. Moreover, acute pulmonary hypertension may also lead to right-to-left flow across a patent foramen ovale, and thus exacerbate hypoxemia [ 8 , 9 ].
The clinician should seek the following clues to the diagnosis of pulmonary embolism in patients who have had a syncopal episode: (a) hypotension and tachycardia or transient bradyarrhythmia; (b) acute cor pulmonale according to electrocardiogram criteria or physical examination; and (c) other signs and symptoms indicative of pulmonary embolism. The presence of any of these findings without other obvious causes of syncope should lead to further work-up, including arterial blood gas analysis, ventilation-perfusion scanning, lower extremity duplex sonogram, echocardiography, multislice computed tomography and angiography, if necessary. Although oxygen saturation levels are inadequate for screening purposes, respiratory alkalosis with hypoxia and increased A-a O 2 gradient are typically seen. However, results of blood gas analysis are normal in 10% of cases [ 4 , 10 ].
In our case, the patient presented to the emergency department with complaints of dyspnea, tachypnea and tachycardia, following a syncopal episode. He had experienced immobilization for one month, hypoxemia in room air, and DVT according to the ultrasonographic results. PTE was initially considered and all of the diagnostic procedures were carried out to prove this presumptive diagnosis. Because DVT and PTE developed in this young patient with no history of any underlying diseases or disorders, he was referred for thrombophilia panel testing (including protein C or S deficiency and Factor V mutation) before treatment; however, as his long-term follow-up was performed by the Department of Pulmonary Diseases, we do not have any further detailed results from these examinations. This case is interesting because the patient did not experience a massive embolism but did develop syncope.
Pulmonary embolism presenting with syncope is difficult to diagnose. Physicians and other health care professionals must be vigilant with patients who have syncope, because this symptom may be a 'forgotten sign' of life-threatening pulmonary embolism.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Wolfe TR, Allen TL: Syncope as an emergency department presentation of pulmonary embolism. J Emerg Med. 1998, 16: 27-31. 10.1016/S0736-4679(97)00228-X.
Article CAS PubMed Google Scholar
Manolis AS, Linzer M, Estes M: Syncope: current diagnostic evaluation and management. Ann Intern Med. 1990, 112: 850-863.
Koutkia P, Wachtel TJ: Pulmonary embolism presenting as syncope: case report and review of the literature. Heart Lung. 1999, 28: 342-347. 10.1053/hl.1999.v28.a99733.
Varon J, Fromm RE: Syncope: the forgotten sign of pulmonary embolism. J Emerg Med. 1998, 16: 117-118. 10.1016/S0736-4679(98)00061-4.
Bell WR, Simon TL, DeMets DS: The clinical features of submassive and massive pulmonary emboli. Am J Med. 1977, 62: 355-360. 10.1016/0002-9343(77)90832-4.
Kapoor WN: Evaluation and management of patients with syncope. J Am Med Assoc. 1992, 268: 2553-2560. 10.1001/jama.268.18.2553.
Article CAS Google Scholar
Edelson G, Reis ND, Hettinger E: Syncope as a premonitory sign of fatal pulmonary embolism. Acta Orthop Scand. 1988, 59: 71-73. 10.3109/17453678809149349.
Thames MD, Alpert JS, Dalen JE: Syncope in patients with pulmonary embolism. JAMA. 1977, 238: 2509-2511. 10.1001/jama.238.23.2509.
Simpson RJ, Podolak R, Mangano CA, Foster JR, Dalldorf FG: Vagal syncope during recurrent pulmonary embolism. JAMA. 1983, 249: 390-393. 10.1001/jama.249.3.390.
Article PubMed Google Scholar
Soloff LA, Rodman T: Acute pulmonary embolism. Am Heart J. 1967, 74: 629-647.
Article Google Scholar
Download references
Author information
Authors and affiliations.
Department of Emergency Medicine, Gazi University Faculty of Medicine, Ankara, Turkey
Ahmet Demircan, Ayfer Keles & Fikret Bildik
Department of Internal Medicine, Gazi University Faculty of Medicine, Ankara, Turkey
Gulbin Aygencel
Department of Anesthesiology and Reanimation, Gazi University Faculty of Medicine, Ankara, Turkey
Ozgur Ozsoylar
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Gulbin Aygencel .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
AD, AK and FB analyzed and interpreted the patient data regarding the syncope and the pulmonary embolism. GA and OO performed the acute treatment of the patient, and were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Demircan, A., Aygencel, G., Keles, A. et al. Pulmonary embolism presenting as syncope: a case report. J Med Case Reports 3 , 7440 (2009). https://doi.org/10.4076/1752-1947-3-7440
Download citation
Received : 17 January 2008
Accepted : 06 March 2009
Published : 15 September 2009
DOI : https://doi.org/10.4076/1752-1947-3-7440
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pulmonary Embolism
- Deep Venous Thrombosis
- Acute Pulmonary Embolism
- Syncopic Episode
- Pleuritic Chest Pain
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Pulmonary embolism:...
Pulmonary embolism: update on management and controversies
- Related content
- Peer review
- Lisa Duffett , associate scientist , assistant professor 1 2 ,
- Lana A Castellucci , scientist , assistant professor 1 2 ,
- Melissa A Forgie , vice dean of undergraduate medical education and professor of medicine 2
- 1 Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON, Canada
- 2 Department of Medicine, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada
- Correspondence to: L A Castellucci lcastellucci{at}toh.ca
Pulmonary embolism is a common and potentially fatal cardiovascular disorder that must be promptly diagnosed and treated. The diagnosis, risk assessment, and management of pulmonary embolism have evolved with a better understanding of efficient use of diagnostic and therapeutic options. The use of either clinical probability adjusted or age adjusted D-dimer interpretation has led to a reduction in diagnostic imaging to exclude pulmonary embolism. Direct oral anticoagulation therapies are safe, effective, and convenient treatments for most patients with acute venous thromboembolism, with a lower risk of bleeding than vitamin K antagonists. These oral therapeutic options have opened up opportunities for safe outpatient management of pulmonary embolism in selected patients. Recent clinical trials exploring the use of systemic thrombolysis in intermediate to high risk pulmonary embolism suggest that this therapy should be reserved for patients with evidence of hemodynamic compromise. The role of low dose systemic or catheter directed thrombolysis in other patient subgroups is uncertain. After a diagnosis of pulmonary embolism, all patients should be assessed for risk of recurrent venous thromboembolism to guide duration of anticoagulation. Patients with a venous thromboembolism associated with a strong, transient, provoking risk factor can safely discontinue anticoagulation after three months of treatment. Patients with an ongoing strong risk factor, such as cancer, or unprovoked events are at increased risk of recurrent events and should be considered for extended treatment. The use of a risk prediction score can help to identify patients with unprovoked venous thromboembolism who can benefit from extended duration therapy. Despite major advances in the management of pulmonary embolism, up to half of patients report chronic functional limitations. Such patients should be screened for chronic thromboembolic pulmonary hypertension, but only a small proportion will have this as the explanation of their symptoms. In the remaining patients, future studies are needed to understand the pathophysiology and explore interventions to improve quality of life.
Introduction
Venous thromboembolism, which includes deep venous thrombosis (DVT) and pulmonary embolism, is the third most common cardiovascular disorder and affects up to 5% of the population during their lifetime. 1 The increased sensitivity of imaging modalities has more than doubled rates of hospital admission for pulmonary embolism in the past 10 years, although the case fatality rate has remained stable or decreased. 2 3 4 Embolization of a DVT in the lower extremity into the pulmonary arteries is thought to be the most common mechanism for pulmonary embolism. Registry studies found that up to 17% of patients die within three months of diagnosis of venous thromboembolism, 5 although many of these deaths may be due to associated comorbidities rather than direct causation. For those patients included in the more recent large randomized controlled trials (RCTs), the three month all cause mortality has been approximately 2%. 6 7 8 9
Careful clinical assessment is needed for diagnosis of pulmonary embolism, as the presentation can mimic other common medical conditions. Clinical probability scores in combination with D-dimer testing improve the use and interpretation of diagnostic imaging. 10 Important recent advances in diagnosis of pulmonary embolism have been the use of clinical probability adjusted, or age adjusted, D-dimer interpretation. 11 12 13 Only a small proportion of patients with acute pulmonary embolism will have high risk features associated with short term clinical deterioration, but identification of such patients and consideration of therapies in addition to anticoagulation, such as thrombolysis, are important. 14 15 16 Various risk prediction scores, serum biomarkers, and imaging abnormalities such as right ventricular strain can identify patients at higher short term risk for all cause mortality. 10 14 16 What interventions can be made to alter this prognosis remains unclear.
The major advance in management for patients with pulmonary embolism in the past decade has been the introduction of direct oral anticoagulants (DOACs). This class of drugs includes direct Xa inhibitors (apixaban, edoxaban, rivaroxaban) and a direct thrombin inhibitor (dabigatran). Large RCTs have shown these therapies to be non-inferior to vitamin K antagonists (warfarin). 6 7 8 17 Rates of major bleeding seem to be similar or reduced in patients treated with DOACs compared with warfarin, but whether this is a class effect or whether differences exist between drugs is uncertain. Duration of anticoagulation should be determined after weighing the risk of recurrent venous thromboembolism against the risk of bleeding, along with the associated morbidity and mortality of each outcome. In the era of DOAC therapy, weighing the risk of recurrent venous thromboembolism against that of bleeding remains a challenge as data on bleeding risk and direct comparisons between types and doses of DOACs are lacking. This review is aimed at clinicians caring for patients with pulmonary embolism and researchers interested in recent advances in its management.
Epidemiology
The annual incidence of pulmonary embolism in the population is 1 per 1000 people, but this increases sharply with age, from 1.4 per 1000 people aged 40-49 to 11.3 per 1000 aged 80 years or over. 1 18 19 Recurrent venous thromboembolism occurs in 30% of people, making the attack rate (including incident and recurrent venous thromboembolism) higher, estimated as up to 30 per 1000 person years. 19 The influence of race on venous incidence of thromboembolism is uncertain, but incidence may be higher in white and African-American populations and lower in Asians and Native Americans. 19 Overall, the incidence of venous thromboembolism in men is slightly higher than in women, but the balance changes according to age categories. 19 Among women under 45 years or over 80 years, the incidence of venous thromboembolism is higher than in men. This interaction with age and sex is likely related to estrogen and pregnancy related risk factors at a young age and longer life expectancy of women at advanced ages. Vital registration data indicate that women aged 15-55 and over 80 years have an excess pulmonary embolism related mortality compared with men. 20 Although increased incidence of pulmonary embolism in women among both of these age groups may be contributing to this, whether true sex and/or gender differences exist in case fatality rates remains to determined. Data from registry studies have suggested a higher in-hospital and 30 day pulmonary embolism related mortality in women, 21 whereas other studies have not observed a difference. 22 Subgroup analyses of RCTs comparing warfarin and DOAC therapy have not suggested a difference.
Fifty per cent of venous thromboembolism events are associated with a transient risk factor, such as recent surgery or hospital admission for medical illness, 20% are associated with cancer, and the remainder are associated with minor or no risk factors and are thus classified as unprovoked. 23 Box 1 summarizes common risk factors for venous thromboembolism. 19 24 Despite comprehensive literature on the epidemiology of venous thromboembolism and its risk factors, public awareness is poor compared with other health conditions with comparable incidence. This was illustrated in an international survey of more than 7000 people in nine countries. Half of respondents had no awareness of venous thromboembolism conditions and risk factors, and less than 30% knew the signs and symptoms of venous thromboembolism. 25
Transient risk factors for venous thrombosis 16
Strong risk factor (odds ratio >10).
Hip or leg fracture
Hip or leg joint replacement
Major general surgery
Major trauma
Spinal cord injury
Moderate risk factor (odds ratio 2-9)
Arthroscopic knee surgery
Central venous lines
Congestive heart or respiratory failure
Hormone replacement therapy
Oral contraceptive therapy
Paralytic stroke
Previous venous thromboembolism
Thrombophilia
Weak risk factor (odds ratio <2)
Bed rest >3 days
Immobility due to sitting (eg, prolonged road or air travel)
Increasing age
Laparoscopic surgery (eg, cholecystectomy)
Pregnancy (antepartum)
Varicose veins
Sources and selection criteria
We searched Ovid Medline, Cochrane CENTRAL, and other non-indexed citations from 1 January 2010 to 7 August 2019 to find English language systematic reviews, meta-analyses, and RCTs that evaluated management of pulmonary embolism. We included clinical practice guidelines (American College of Chest Physicians, American Society of Hematology, and European Society of Cardiology), as well as screening them to identify additional studies. We used Ovid Medline and PubMed for dedicated search strategies of selected topics thought not to be included in the above search. These topics included inferior vena cava filters, bleeding and anticoagulation, post-thrombotic syndrome, post-pulmonary embolism syndrome, chronic thromboembolic pulmonary hypertension, quality of life and patient experience, cancer, inherited thrombophilia, and antiphospholipid syndrome. A health sciences librarian did all the searches. Additional references were suggested during the peer review process.
Two authors (LD and LAC) independently evaluated the 360 non-duplicate references retrieved and identified 162 articles as potentially related to our overview. We focused our search on systematic reviews and meta-analyses judged to be of medium or high quality by the AMSTAR tool or as of acceptable quality by the SIGN-50 tool. 26 27 When multiple systematic reviews or meta-analyses covered the same topic, we chose the study with the best methodological quality; when studies had similar quality, we chose the most recent. If topic advances were not fully covered by a systematic review, meta-analysis, or RCT, we included observational studies or expert consensus and opinion. In the end, 11 endorsed clinical practice guidelines/consensus statements, 24 systematic reviews/meta-analysis, 25 randomized trials, 39 prospective studies, and 21 retrospective/secondary analysis studies informed our overview ( fig 1 ). We also included six actively recruiting clinical trials, identified using NCT registration numbers (clincaltrials.gov). These registered clinical trials were either selected by the authors or suggested through the peer review process as having the potential to affect the field, and the conclusions of this review, on completion. After this review was accepted for publication, one of these clinical trials, CARAVAGGIO, was completed and its results published; we updated the manuscript to include the details of this trial and its results.

PRISMA flow diagram
- Download figure
- Open in new tab
- Download powerpoint
Prompt recognition of a constellation of nonspecific signs and symptoms is needed for diagnosis of pulmonary embolism. Prompt initiation of anticoagulation while awaiting investigations is prudent because of the high risk of early mortality with untreated pulmonary embolism. 28 29 30 Although this approach for starting anticoagulation in patients in whom a pulmonary embolism is suspected has been shown to be safe in outpatient settings, 31 risks of bleeding and overuse of diagnostic tests remain. Inappropriately proceeding down a diagnostic pathway for pulmonary embolism may also distract clinicians from identifying the alternative causes of the symptoms.
Clinical probability scores
Clinical probability scores can be used to assign a pre-test probability for pulmonary embolism. Consideration of the probability of pulmonary embolism before testing (that is, pre-test probability) avoids unnecessary testing and is critical to the interpretation of results. This was first illustrated in the PIOPED (Prospective Investigation of Pulmonary Embolism Diagnosis) study. A high probability planar ventilation-perfusion lung scan was almost as likely to give a false positive result as a true positive one if the pre-test probability was low, with 44% having no evidence of pulmonary embolism on angiography. Conversely, with a low probability ventilation-perfusion lung scan and a high pre-test probability, 60% had pulmonary embolism by angiography. 32
The Geneva and Wells rules are among the most commonly cited clinical probability scores ( table 1 ). 10 34 37 Both the Geneva rule and the Wells rule have been studied in more than 55 000 patients and have been shown to be reliable, accurate, and superior to a gestalt, non-standardized, clinical assessment. 37 An adaption of the Wells rule, keeping three items only (clinical signs of DVT, hemoptysis, and whether pulmonary embolism is the most likely diagnosis), the YEARS rule, has been evaluated in one observational study of 3465 patients with suspected pulmonary embolism. 13 In this study, pulmonary embolism was excluded if patients had either absence of all three criteria and a D-dimer less than 1000 ng/mL or one or more criteria and a D-dimer less than 500 ng/mL. Of the patients in whom pulmonary embolism was ruled out at baseline and remained untreated, 0.61% (95% confidence interval 0.36% to 0.96%) were diagnosed as having venous thromboembolism during the three month follow-up. Limitations of this study include that investigators were not blinded to the D-dimer results when making the assessment of the most likely diagnosis, small numbers of patients with cancer, and the absence of a control arm.
Comparison of pulmonary embolism clinical probability scores
- View inline
Despite the routine use of clinical probability scores, only 8% of patients in the US and 27% in Europe investigated for pulmonary embolism will have the diagnosis confirmed. 38 To overcome this, the pulmonary embolism rule-out criteria (PERC rule) were studied in a crossover cluster RCT of 1916 patients who were judged by treating physicians to have a gestalt probability of pulmonary embolism of less than 15%. 39 The PERC rule consists of eight clinical variables (hypoxia, unilateral leg swelling, hemoptysis, previous venous thromboembolism, recent surgery or trauma, age >50, hormone use, tachycardia), and further testing (D-dimer and/or imaging) was withheld if all eight variables were absent. This study showed that in patients deemed to be at very low risk of pulmonary embolism by gestalt, the PERC rule was non-inferior to standard of care for the primary outcome of venous thromboembolism rate during three months of follow-up (mean difference 0.2, one sided upper 95% confidence limit 1.6%). The PERC rule should not be applied to patients at higher risk of pulmonary embolism, defined as gestalt pre-test probability of pulmonary embolism higher than 15%.
D-dimer testing
Physiologically, the activation of coagulation and generation of cross linked fibrin simultaneously leads to the activation of fibrinolysis. The D-dimer is a degradation product of fibrinolysis and is increased in patients with acute venous thromboembolism as well other non-thrombotic disorders. 40 D-dimer is a helpful diagnostic tool, and a negative value in combination with a low clinical probability score is useful for excluding a diagnosis of venous thromboembolism. D-dimer should not be used as a screening tool in patients in whom venous thromboembolism is not clinically suspected. Clinicians should assess the clinical pre-test probability of pulmonary embolism before ordering D-dimer testing, as knowledge of D-dimer results can influence the assessment of the clinical probability score. 41
D-dimer is a sensitive but not specific diagnostic test. Improvements to the specificity can be made by using a dichotomized cut-off value according to the pre-test probability. A recent observational study of 2017 patients with suspected pulmonary embolism showed that a cut-off of 1000 ng/mL in patients with a low pre-test clinical probability score (traditional Wells) and 500 ng/mL in patients with a moderate clinical probability score could safely exclude pulmonary embolism without the need for further diagnostic imaging. 11 All other patients (high clinical probability score) underwent diagnostic imaging. In this study, no patients with low or moderate clinical probability score had a recurrent venous thromboembolism event in the three months of study follow-up (0%, 95% confidence interval 0.00% to 0.29%) and the dichotomized D-dimer cut-off strategy reduced the use of diagnostic imaging by 17.6% (15.9% to 19.2%) compared with the reanalysis of results with a single 500 ng/mL cut-off. Alternatively, D-dimer concentrations increase with age, and specificity can be improved with an age adjusted cut-off value. 42 An observational study of 3346 patients evaluated an age adjusted D-dimer (500 µg/L cut-off for patients ≤50 or age×10 µg/L for patients >50 years), whereby patients with a negative D-dimer and an unlikely (Wells) or non-high (revised Geneva) clinical probability did not undergo diagnostic imaging. 12 This age adjusted D-dimer approach increased the number of patients in whom pulmonary embolism could be excluded without diagnostic imaging from 6% to 30% without additional false negative findings. The three month venous thromboembolism rate in patients with a D-dimer concentration higher than 500 μg/L but below the age adjusted cut-off was 1 in 331 patients (0.3%, 0.1% to 1.7%).
Imaging for suspected pulmonary embolism
The gold standard diagnostic test for pulmonary embolism has historically been interventional pulmonary angiography. This invasive procedure has been largely abandoned, and diagnostic management studies have used the clinical safety measurement of frequency of venous thromboembolism events in the three months after evaluation in patients in whom pulmonary embolism is considered ruled out. The target is to match what was historically observed in similar patients after a negative pulmonary angiography—that is, 1.6% (0.3% to 2.9%) venous thromboembolism rate in the three month follow-up period. 43 Planar ventilation-perfusion lung scans and computed tomography pulmonary angiography (CTPA) are validated imaging tests. Both should be used in combination with the probability scores and D-dimer testing to accurately interpret results, as both false negative and false positive results can be observed when test results are discordant with clinical probability scores ( fig 2 ). 44

Diagnostic work-up of patients with suspected pulmonary embolism (PE). CTPA=computed tomography pulmonary angiography; PERC=pulmonary embolism rule-out criteria; V/Q=ventilation-perfusion. Adapted from Wells PS, et al. Ann Intern Med 2018 44
On the basis of a meta-analysis of observational and randomized studies, a normal CTPA is associated with a pooled incidence of venous thromboembolism at three months of 1.2% (0.8% to 1.8%) and negative predictive value of 98.8% (98.2% to 99.2%). 45 A ventilation-perfusion lung scan in a validated diagnostic algorithm performs equally well as CTPA in the diagnosis of pulmonary embolism. 46 47 48 Patients with pulmonary embolism excluded by a diagnostic algorithm combining ventilation-perfusion lung scan, D-dimer, compression ultrasound, and clinical probability score had an incidence of venous thromboembolism at three months of 0.1% (0.0% to 0.7%) with a negative predictive value of 99.5% (99.1% to 100%). 48
An RCT comparing CTPA and ventilation-perfusion lung scanning found that CTPA detected 5% (1.1% to 8.9%) more pulmonary embolisms, but patients in whom pulmonary embolism was excluded by a diagnostic algorithm based on ventilation-perfusion lung scanning did not have a higher three month incidence of venous thromboembolism during follow-up: 2/561 (0.4%) patients randomized to CTPA versus 6/611 (1.0%) patients undergoing ventilation-perfusion lung scan (difference −0.6%, −1.6% to 0.3%). 46 This calls into question the clinical significance of these pulmonary embolisms “missed” by ventilation-perfusion lung scans. Nevertheless, the wide availability, fewer non-diagnostic results, and ability to provide alternative diagnoses have made CTPA the most common diagnostic modality. Important limitations to CTPA, however, should cause clinicians to reassess this shift in choice of tests, including exposure to ionizing radiation and risk of secondary malignancy, 49 renal toxicity with pre-existing renal disease, and risk of over-diagnosis and over-treatment of clinically insignificant pulmonary embolism.
Single photon emission computed tomography (SPECT) ventilation-perfusion scanning is proposed as an alternative to planar ventilation-perfusion scanning, as this technique may reduce the proportion of non-diagnostic results. The technique and diagnostic criteria for reporting SPECT ventilation-perfusion scans are variable and have not been validated sufficently. 16 On this basis, we suggest favoring planar ventilation-perfusion lung scans over SPECT.
Diagnosis of pulmonary embolism in pregnancy
Pregnancy and the postpartum period confer an increased risk of venous thromboembolism, but only 4-7% of women investigated are diagnosed as having pregnancy associated pulmonary embolism. 50 51 Diagnosing pulmonary embolism in pregnancy is challenging, as shortness of breath and lower extremity swelling are common complaints and D-dimer concentration is increased in normal pregnancies. Diagnostic management studies have either excluded or included very few pregnant women, and safe diagnostic strategies were lacking until recently. Two large observational studies specific to pregnant women have recently been published. The first evaluated the use of the modified Geneva score and a high sensitivity D-dimer in 441 pregnant patients. 51 Women with a low or intermediate clinical probability and negative D-dimer (<500 μg/L) had pulmonary embolism excluded; all others underwent bilateral lower limb compression ultrasonography and, if this was negative, CTPA. Although this approach was safe, with no venous thromboembolism events (0.0%, 0.0% to 1.0%), in three months of follow-up among untreated women in whom pulmonary embolism was excluded, the algorithm could avoid diagnostic imaging in only 10% of patients. This was because D-dimer testing was positive in 87% of women who underwent testing and was more likely to be positive with advanced gestation.
A second observational study of 510 pregnant women applied the YEARS probability score and D-dimer with a stratified cut-off (1000 ng/mL if no criteria were met or 500 ng/mL if one or more criteria were met). 50 Compression ultrasonography was performed only in women with symptoms of DVT. Using this approach, 39% of women were able to avoid diagnostic imaging, with an acceptably low three month venous thromboembolism incidence of 0.21% (0.04% to 1.2%). Furthermore, post hoc retrospective application of this pregnancy adapted YEARS algorithm to the cohort of patients in the first study showed similar findings, with 21% of women meeting criteria for exclusion of pulmonary embolism without diagnostic imaging and no venous thromboembolism events during follow-up. 52 Limitations of these studies include relative small sample sizes and possible bias for inclusion of patients at lower risk. Nevertheless, a pregnancy adapted YEARS algorithm seems to be safe and effective at reducing the need for diagnostic imaging in some patients.
Diagnostic imaging choices for suspected pulmonary embolism in pregnancy are similar to those in non-pregnant patients. Pregnancy alone does not increase the occurrence of non-diagnostic imaging results, and both ventilation-perfusion lung scans and CTPA are safe and accurate diagnostic imaging modalities in pregnancy. 53 54 Fetal exposure to radiation is well under acceptable limits for both tests. 53 Given the younger age, and thus longer lifetime risk for secondary malignancies, we favor the use of ventilation-perfusion lung scans in pregnant women, a position similar to the American Society of Hematology guidelines. 53 First investigating for DVT with compression ultrasonography can be considered in patients who have symptoms suggestive of a DVT. The absence of DVT does not exclude the need for chest imaging, but if a proximal DVT is confirmed then a presumptive diagnosis of pulmonary embolism may be made without dedicated imaging.
Thrombophilia testing
Family history of venous thromboembolism portends higher risk, 55 particularly when the venous thromboembolism is unprovoked or the patient is under 50 years of age. 56 Despite this, considerable controversy remains around the value of inherited thrombophilia testing (factor V Leiden mutation, prothrombin gene mutation, protein C deficiency, protein S deficiency, and antithrombin deficiency), as evidence suggests that the presence of thrombophilia does not alter management. 56 Furthermore, thrombophilia testing does not identify all inherited causes of venous thromboembolism. 57 58 This is illustrated by the observation that only 30% of people with a family history of a first degree relative with venous thromboembolism will have a positive thrombophilia screen. 59
Patients who have a venous thromboembolism diagnosed in the context of a strong provoking risk factor, such as major surgery, are at a low risk for recurrence, and this risk is not significantly altered by the presence of an inherited thrombophilia. 56 Patients who have a venous thromboembolism that is classified as unprovoked are at a significant increased risk of recurrence, but testing for inherited thrombophilia has not been shown to alter this risk in a way that might guide decisions about duration of anticoagulation. 60 61 Relatives identified as asymptomatic carriers of thrombophilia are at increased lifetime risk of venous thromboembolism (factor V Leiden mutation: 0.58-0.67% per year; protein C deficiency: 1.0-2.5% per year; protein S deficiency: 0.7-2.2% per year; antithrombin deficiency: 4% per year), but half of all events occur with additional provoking risk factors. 62 The presence of a positive family history remains significant, as such patients are more likely to develop a venous thromboembolism event compared with those with an inherited thrombophilia with no family history. 59 62 How thrombophilia testing informs the care of family members without symptoms beyond consideration of the risk imposed by a positive family history is therefore unclear.
If thrombophilia testing is used, it should be done after completion of treatment for an acute venous thromboembolism event and preferably in the absence of anticoagulation therapy, as false positive results are associated with warfarin (protein C deficiency, protein S deficiency), heparin (lupus anticoagulant), and DOACs (lupus anticoagulant). 56 We suggest that inherited thrombophilia testing should not be done when venous thromboembolism is associated with a strong provoking factor, as such patients have a low risk of recurrent venous thromboembolism, even when an inherited thrombophilia is identified. 60 We also suggest that thrombophilia testing should not be done in patients with unprovoked venous thromboembolism who already have an indication for long term anticoagulation (based on sex or risk predictions scores). In the remaining patients with unprovoked venous thromboembolism and no indication for indefinite anticoagulation, we suggest discussing inherited thrombophilia testing with them. In most cases, testing will not change the decision on duration of anticoagulation, but rare exceptions include high risk inherited thrombophilia such as antithrombin deficiency, or combined thrombophilia. In the absence of high quality evidence, the patient’s preference should be considered in such decisions. Genetic counseling should be offered to patients undergoing testing, with acknowledgment of the psychological effects such results can have. 63 64 65 66
Antiphospholipid syndrome
Antiphospholipid syndrome is a thrombophilia that should be considered separately. It is acquired, so most affected people will not have a family history of venous thromboembolism. Antiphospholipid syndrome is thought to be associated with a high risk for both recurrent venous thromboembolism and arterial thrombosis. 67 The presence of persistently elevated antiphospholipid antibodies with a first venous thromboembolism is an acceptable indication for indefinite duration of anticoagulation. 16 67 A diagnosis of antiphospholipid syndrome is made on the basis of laboratory and clinical criteria. 68 Laboratory criteria include the presence of at least one associated antibody on two or more occasions and at least 12 weeks apart: lupus anticoagulant (detected according to the guidelines of the International Society on Thrombosis and Hemostasis (ISTH)), 69 anti-β2-glycoprotein I (>99th centile of controls), or anti-cardiolipin antibodies (>40 GPL units or >99th centile of controls). Clinical criteria include one or more episodes of arterial, venous, or small vessel thrombosis or one or more defined pregnancy morbidities. In patients presenting with an unprovoked venous thromboembolism event, 6% of patients overall and up to 19% of those under 50 years old will meet the criteria for antiphospholipid syndrome. 70 71
The identification of antiphospholipid syndrome may be important to guide decisions on choice of anticoagulant therapy. A randomized controlled, non-inferiority trial compared rivaroxaban and warfarin in patients with high risk antiphospholipid syndrome, defined as positive for all three laboratory criteria, for the primary outcome of cumulative incidence of thrombotic events, major bleeding, and vascular death. 72 This trial was terminated after 120 patients were enrolled, as interim analyses showed excess events in the rivaroxaban arm (hazard ratio 6.7, 95% confidence interval 1.5 to 30.5). All trial participants discontinued the assigned study drug and switched to a non-study vitamin K antagonist (VKA). Another non-inferiority RCT of 190 patients with thrombotic antiphospholipid syndrome (required one laboratory criterion: lupus anticoagulant, or moderate to high titer IgG anti-cardiolipin or anti-β2-glycoprotein I antibodies), randomized participants to rivaroxaban or warfarin. 73 The primary outcome of proportion of patients with new thrombotic events during three years of follow-up occurred more frequently in the rivaroxaban arm (risk ratio 1.83, 0.71 to 4.76). Most patients (96%) were positive for lupus anticoagulant, and 60% were triple positive. Both trials showed a trend of increased arterial rather than venous thrombotic events.
Given the high prevalence of antiphospholipid syndrome among patients under 50 years old with unprovoked venous thromboembolism, and implications for duration and choice of anticoagulation, screening for antiphospholipid syndrome should be considered in these patients. Further studies are needed to determine the efficacy of DOACs in lower risk antiphospholipid syndrome (for example, non-lupus anticoagulant, IgM class, and low titer antibodies) and to identify subpopulations of patients with antiphospholipid syndrome in whom DOACs might be acceptable (for example, non-arterial thrombotic history). Until such time, we discuss the risk and benefits of therapeutic options with patients with venous thromboembolism associated with antiphospholipid syndrome and suggest the use of VKAs over other therapies in most patients with antiphospholipid syndrome associated with lupus anticoagulant and triple positive serology.
Diagnosis of recurrent pulmonary embolism
Patients who have a history of a previous DVT or pulmonary embolism are at a lifetime increased risk of recurrent events. 29 74 Anticoagulation reduces the incidence of recurrent venous thromboembolism by about 80-85%. 75 Nevertheless, patients often present with symptoms of recurrent DVT and pulmonary embolism, and differentiating symptoms related to chronic complications of venous thromboembolism, such as post-thrombotic syndrome and post-pulmonary embolism syndrome, represents a diagnostic challenge. Because a history of previous venous thromboembolism is a variable in some clinical probability scores ( table 1 ), such patients are often categorized as having a high probability, necessitating further diagnostic imaging. The most commonly used clinical probability scores were derived in, and are therefore generalizable to, cohorts that included patients with previous venous thromboembolism. Additionally, the D-dimer concentration remains elevated in many patients after completion of a standard treatment course for acute venous thromboembolism, limiting its usefulness for excluding recurrent events. 76 77 Nevertheless, in a combined subgroup analysis of observational studies (1721 patients in total), patients with a previous history of venous thromboembolism and clinically suspected pulmonary embolism (306 patients) were safely managed using a clinical probability and D-dimer diagnostic approach (three month venous thromboembolism incidence in patients with pulmonary embolism excluded by negative D-dimer 0%, 0% to 7.9%). However, only 16% (compared with 33% of those without previous venous thromboembolism history) were able to have pulmonary embolism excluded without imaging tests. 78 Another observational study included 516 patients with clinically suspected recurrent pulmonary embolism while not on anticoagulation therapy. 79 This diagnostic strategy excluded pulmonary embolism on the basis of a Wells pulmonary embolism score of 4 or lower (“pulmonary embolism unlikely”) and a negative D-dimer test; all other patients underwent CTPA. The prevalence of pulmonary embolism in the study was 33%, and the primary outcome of three month recurrent venous thromboembolism in patients with pulmonary embolism excluded was 2.8% (1.2% to 5.5%). The strategy was able to exclude pulmonary embolism without imaging tests in only 17% of patients. Additionally, none of the patients was on anticoagulation at the time of D-dimer testing, so whether this strategy can be generalized to patients who are on anticoagulation is unknown. We support the position endorsed by the ISTH that a combination of low clinical probability score and negative D-dimer test can be used to exclude pulmonary embolism in patients with a history of previous venous thromboembolism, but patients with an intermediate or high clinical probability score should undergo diagnostic imaging. 76
As residual defects often persist on CTPA and ventilation-perfusion lung scans six to 12 months after the initial diagnosis, interpretation of diagnostic imaging for suspected recurrent events requires prudent comparison with previous imaging to prevent over-diagnosis. The rate of complete resolution on baseline imaging varies from about 50% to 84%. 80 81 82 83 Differentiating acute pulmonary embolism from residual thrombi is difficult, and inter-observer agreement between radiologists is poor. 82 Characteristics of thrombi such as density, intramural calcification, or eccentric filling defects have been proposed but never validated. 76 We would advise caution in relying on such descriptive features. The availability, and careful review with an experienced radiologist, of previous imaging and ideally baseline imaging performed six to 12 months after an acute pulmonary embolism is advised when evaluating a patient for recurrent pulmonary embolism and has been shown to be a safe and accurate approach. 84 We routinely do a baseline ventilation-perfusion lung scan six to 12 months after an acute pulmonary embolism. Although this may not be a widely adopted approach, the risk of radiation exposure with ventilation-perfusion lung scans is low and the availability of such baseline imaging has been shown to improve the interpretation of diagnostic tests for suspected recurrent venous thromboembolism. 84 85
Initial treatment for pulmonary embolism
Pulmonary embolism risk assessment.
Pulmonary embolism remains a heterogeneous condition, ranging from presentation with sudden death to incidental findings with no symptoms. Initial hemodynamic instability, defined as systolic blood pressure below 90 mm Hg for 15 minutes or more, is an important marker of prognosis. However, this presentation is uncommon, being found in only 5% of cases; the short term mortality exceeds 15%. 14 15 16 86 For the remaining 95% of cases, several risk prediction scores have been proposed to estimate the risk of an adverse outcome ( table 2 ). 33 88 89 90
Comparison of pulmonary embolism risk prediction scores
A systematic review assessing the characteristics and quality of pulmonary embolism risk prediction scores identified 17 models in the literature. 91 Of these, the Pulmonary Embolism Severity Index (PESI) and the simplified-PESI (sPESI) had the most robust evidence and validation. Both risk prediction scores were able to differentiate between low and high risk of 30 day mortality in patients with pulmonary embolism. 91 The PESI and the Hestia criteria have been used in randomized studies to select patients with low risk pulmonary embolism suited to outpatient care (discussed below). 92 93 Biomarkers have also been studied. A systematic review of cardiac troponin as a predictor of early mortality showed that in patients otherwise classified as being at low risk by the PESI or sPESI score, the presence of a positive troponin had a pooled fivefold increased odds of 30 day mortality (odds ratio 4.79, 1.11 to 20.68), although the wide confidence interval casts doubt on the reliability of this estimate. 94
Other prognostic markers have been proposed for risk stratification, including B-type natriuretic peptide and N-terminal pro-b-type natriuretic peptide (NT-proBNP). Evidence of right ventricular dysfunction by echocardiography and CTPA are also indicators of worse prognosis. 16 95 The combination of the prognostic markers of positive cardiac troponin and right ventricular dysfunction was used in an RCT of 1005 patients identified as having “intermediate risk” pulmonary embolism who were candidates for thrombolysis therapy. 96 The results of the thrombolysis arm are discussed below in the section “Thrombolytic therapy for pulmonary embolism.” In the control arm, a 5% rate of hemodynamic decompensation (25/499 patients) was seen within the first seven days; most of these patients (23/499) went on to need rescue thrombolytic therapy. Although this observation might justify the combination of right ventricular dysfunction and cardiac troponin as predicators of early decompensation, whether clinical characteristics alone would have also identified these patients at high risk is unclear. Although opinion on their usefulness diverges, right ventricular imaging and cardiac biomarkers may be considered for selecting patients who need cardiac monitoring, should close follow-up be unavailable.
Outpatient versus inpatient management of acute pulmonary embolism
Risk stratification has been used to identify patients with a low short term mortality risk to select for outpatient management. The availability of DOACs has simplified outpatient management of pulmonary embolism because some DOACs do not require initial self-administration of parenteral therapies. RCTs have compared outpatient versus inpatient management of pulmonary embolism and found no difference in outcomes in selected patients. A randomized controlled non-inferiority trial allocated 344 patients with low risk pulmonary embolism (PESI class I or II; table 2 ) to inpatient or outpatient treatment, with patients in both arms receiving low molecular weight heparin before transition to an oral agent. 92 No significant difference was seen in the primary outcome of three month incidence of recurrent venous thromboembolism in outpatients versus inpatients (difference 0.6%, 95% upper confidence limit 2.7%, meeting non-inferiority margin of 4%). The Hestia criteria ( table 2 ) have been combined with cardiac troponin and NT-proBNP, with no added benefit of either marker seen compared with the Hestia criteria alone. 93 97 An RCT of 114 patients with low risk pulmonary embolism, no Hestia criteria, and a negative troponin reported a reduction in the primary outcome of time spent in the hospital for venous thromboembolism or bleeding events 30 days after randomization (difference 28.8 (95% confidence interval 16.2 to 41.5) hours lower in outpatient arm). No difference was seen in the three month event rate of venous thromboembolism (predefined secondary outcome). 93 A non-inferiority RCT of 550 patients with no Hestia criteria and negative NT-proBNP compared inpatient and outpatient treatment. The composite primary outcome was 30 day pulmonary embolism or bleeding related mortality, cardiopulmonary resuscitation, or intensive care unit admission. 97 Although the lower than expected positive NT-proBNP concentrations (12% v 40% expected) prevented the trial from being powered to conclude non-inferiority, the primary endpoint occurred in none of the 275 patients (0%, 0% to 1.3%) who had NT-proBNP testing, compared with 3/275 patients (1.1%, 0.2% to 3.2%) in the direct discharge group (P=0.25). The authors speculate that the lower than expected positive biomarkers observed could be because the Heista criteria alone identified a low risk population, so lower amounts of NT-proBNP were detected. On the basis of this evidence, we support the recommendations for outpatient management of pulmonary embolism. 14 16 The identification and outpatient management of appropriate pulmonary embolisms will represent a significant cost savings without compromise to patient safety. 98
Subsegmental pulmonary embolism
The increased use and sensitivity of CTPA has seen an increase in single or multiple pulmonary emboli isolated to the smaller, subsegmental pulmonary arteries. 99 Despite this increase, overall pulmonary embolism related mortality has not changed, and this may account for the decrease in case fatality. 100 101 102 The clinical significance of subsegmental pulmonary emboli remains uncertain, and recommendations are extrapolated mainly from historical ventilation-perfusion lung scan trials.
In the PIOPED study, 17% of patients had defects isolated to the subsegmental pulmonary arteries, which corresponds to a “low probability” ventilation-perfusion lung scan. 32 In observational studies, these low probability ventilation-perfusion patients were not treated if bilateral leg compression ultrasonography and serial compression ultrasonography were performed. 48 This was shown to be a safe strategy and remains the current management of such patients. 16 A systematic review and meta-analysis of observational studies and RCTs showed that the rate of subsegmental pulmonary embolism was higher when multi-row detector computed tomography was used compared with single detector computed tomography, but the three month incidence of recurrent venous thromboembolism in patients left untreated was the same in both groups (0.9% (0.4% to 1.4%) and 1.1% (0.7% to 1.4%) for single and multi-row detectors respectively), suggesting that the extra subsegmental pulmonary embolisms detected may not have the same clinical significance. 99 Similarly, another systematic review and meta-analysis of observational studies and RCTs showed no difference between patients with subsegmental pulmonary embolism who were treated with anticoagulation and those not treated for the pooled outcomes of three month incidence of recurrent venous thromboembolism (5.3% (1.6% to 10.9%) treated, 3.9% (4.8% to 13.4%) untreated) and all cause mortality (2.1% (3.4% to 5.2%) treated, 3.0% (2.8% to 8.6%) untreated). 103 The diagnosis of subsegmental pulmonary embolism is complicated by low inter-observer agreement between radiologists and the recognition that many subsegmental pulmonary embolisms are interpreted as false positives by more experienced radiologists. 100 Collectively, this has led to the recommendation that subsegmental pulmonary embolism in the absence of DVT may not need to be treated with anticoagulation. 14 Until further research is completed, we suggest that isolated subsegmental pulmonary embolism on CTPA, in the absence of cancer or high risk features such as poor cardiopulmonary reserve, may be approached as one would a non-diagnostic ventilation-perfusion lung scan: with baseline and serial bilateral leg compression ultrasonography and no anticoagulation treatment unless DVT is found. An ongoing observational study is assessing the safety of such a management strategy (clinicaltrials.gov NCT01455818 ).
Choice of anticoagulation for acute pulmonary embolism
Anticoagulation therapy for confirmed acute pulmonary embolism is the mainstay of treatment and can be divided into three phases: initial phase from zero to seven days, long term therapy from one week to three months, and extended therapy from three months to indefinite. 14 Box 2 shows anticoagulation options and dosing during each phase. Parenteral anticoagulation with low molecular weight heparin (LMWH), fondaparinux, or intravenous unfractionated heparin is typically used in patients admitted to hospital for initial management of pulmonary embolism. Stable patients on discharge from hospital or those patients suitable for outpatient treatment from the time of diagnosis of acute pulmonary embolism may be treated with DOACs. DOACs are given at fixed doses and do not necessitate routine laboratory monitoring ( table 3 ). 105 Each DOAC has been deemed non-inferior to the VKA/LMWH combination in phase III RCTs for the prevention of symptomatic recurrent venous thromboembolism in patients with an acute venous thromboembolism). DOACs also have significantly fewer major bleeding events compared with VKAs ( table 4 ). 6 7 8 17 Limitations of these trials include heterogeneous populations and lack of direct comparisons between DOACs. An RCT comparing rivaroxaban and apixaban for patients with acute venous thromboembolism is ongoing ( NCT03266783 ), evaluating the differences in clinically relevant bleeding with these anticoagulants.
Phases of pulmonary embolism treatment 104
Initial (0-7 days).
Apixaban 10 mg BID for 7 days
Rivaroxaban 15 mg BID for 21 days
LMWH/fondaparinux for minimum 5 days* and INR ≥2 for 2 days
Long term (1 week to 3 months)
Apixaban 5 mg BID
Dabigatran 150 mg BID
Edoxaban 60 mg daily†
Rivaroxaban 20 mg daily
Warfarin for INR 2-3

Extended (3 months to indefinite)
Apixaban 5 mg BID or 2.5 mg BID‡
Acetylsalicylic acid 81-100 mg daily, if anticoagulation not possible
Rivaroxaban 20 mg daily or 10 mg daily‡
BID=twice daily; INR=international normalized ratio; LMWH=low molecular weight heparin
*LMWH is needed for 5-10 days before starting dabigatran or edoxaban
†30 mg daily if creatinine clearance is 30-50 mL/min or weight <60 kg
‡Dose reduction may be considered after 6 months of therapy
Characteristics of direct oral anticoagulant drugs
Phase III randomized controlled trials comparing direct oral anticoagulants and vitamin K antagonists
Until the past decade, VKAs were the only oral anticoagulants available for treatment of venous thromboembolism, used concurrently with parenteral anticoagulation for at least five days and until two consecutive international normalized ratio readings are between 2 and 3. Although VKA use has diminished with the availability and relative simplicity of DOACs, they remain a critical part of pulmonary embolism management in patients with severe renal insufficiency, antiphospholipid syndrome, 72 73 or inability to cover the cost of DOACs.
Treatment of cancer associated pulmonary embolism
Patients with cancer have a sevenfold increased risk for venous thromboembolism, with an overall absolute risk of 7% within the first year of a cancer diagnosis and up to 20% depending on type of cancer and treatments used. 108 109 110 Pulmonary embolism may be symptomatic or found incidentally on imaging to assess response to cancer treatment. Symptomatic or incidental pulmonary embolisms have similar high risk for recurrence. 111 Major bleeding complications are also more common with venous thromboembolism in patients with cancer. 112 113 Treatment of acute symptomatic and incidental pulmonary embolism is individualized according to risk of recurrent pulmonary embolism and bleeding. Prolonged use of LMWH dominated the cancer associated venous thromboembolism field for a long time, on the basis of the results of trials comparing LMWH and VKAs. 114 Since then, four RCTs have compared DOACs and LMWH in patients with cancer associated venous thromboembolism. The HOKUSAI VTE Cancer RCT randomized 1050 patients with cancer and acute venous thromboembolism and showed that edoxaban (after a five day lead-in with LMWH) was non-inferior to LMWH for the primary outcome of recurrent venous thromboembolism or major bleeding during 12 month follow-up (hazard ratio 0.97, 95% confidence interval 0.70 to 1.36; P=0.006 for non-inferiority). 115 A non-significant lower venous thromboembolism rate was seen (difference in risk −3.4 (−7.0 to 0.2) percentage points), but the major bleeding rate was significantly higher (difference in risk 2.9 (0.1 to 5.6) percentage points) in the edoxaban treated patients. Major bleeding events were mostly seen in the subgroup of patients with upper gastrointestinal tract malignancies.
A second RCT, SELECT-D, compared rivaroxaban and LMWH for the acute treatment of cancer associated venous thromboembolism in 406 patients. This pilot trial was originally designed to inform feasibility of recruiting patients to a phase III RCT. It was powered to estimate venous thromboembolism recurrence rates at six months to within an 8% width of the 95% confidence interval within each arm, assuming a recurrent venous thromboembolism rate of 10% at six months. As a result of slow recruitment, it was later modified to within 9% width. The cumulative venous thromboembolism recurrence rate at six months was 11% (7% to 16%) for dalteparin and 4% (2% to 9%) for rivaroxaban, with fewer recurrent venous thromboembolisms in patients treated with rivaroxaban (hazard ratio 0.43, 0.19 to 0.99). A non-significant increase in major bleeding was seen in patients treated with rivaroxaban (hazard ratio 1.83, 0.68 to 4.96) and a significant increase in clinically relevant non-major bleeding with rivaroxaban (3.76, 1.63 to 8.69). 116 A planned interim safety analysis identified a non-significant difference in major bleeding between arms in patients with esophageal cancers, and these cancers were later excluded from the trial. Unfortunately, slow recruitment in the SELECT-D pilot trial resulted in an inability to definitively compare the efficacy and safety of rivaroxaban and LMWH.
Two RCTs have compared apixaban and LMWH for the treatment of cancer associated venous thromboembolism. The ADAM VTE trial randomized 300 patients to either apixaban or LMWH for six months’ treatment of cancer associated venous thromboembolism. 117 Recurrent thrombosis was more common in the LMWH group (hazard ratio 0.099, 0.013 to 0.780). No differences were seen in safety outcomes of major bleeding or clinically relevant non-major bleeding rates at 6% in each group. The CARAVAGGIO trial randomized 1170 patients to apixaban or LMWH for six months’ treatment. 118 Apixaban was non-inferior to LMWH for the primary outcome of recurrent venous thromboembolism during the trial period of six months (hazard ratio 0.63, 0.37 to 1.07; P<0.001 for non-inferiority). No difference in major bleeding, the primary safety outcome, was observed (hazard ratio 0.82, 0.40 to 1.69). 118
Caution should be applied in making indirect comparisons of the major bleeding rate in CARAVAGGIO with those in other trials, as important differences in enrolled patients exist. Notably, CARAVGGIO excluded patients with either primary or metastatic central nervous system disease and acute leukemia. There was also an imbalance with less upper gastrointestinal malignancies in the apixaban arm than in the LMWH arm (4.0% v 5.4%), whereas HOKUSAI VTE had an imbalance in the opposite direction for edoxaban compared with LMWH (6.3% v . 4.0%).
Consensus from Canadian clinical experts provides a treatment algorithm for patients with cancer and acute venous thromboembolism, considering the risk of bleeding, informed patient preferences, and reimbursement of drugs ( fig 3 ). 112 Of note, this consensus statement was made before the publication of the ADAM VTE and CARAVAGGIO trials, the results of which would also support apixaban for the treatment of cancer associated venous thromboembolism. In general, patients with cancer associated pulmonary embolism without contraindication to anticoagulation are assessed for bleeding risk on the basis of a previous history of bleeding, comorbidities, and type of malignancy. Drug-drug interactions are another consideration, particularly for DOACs. All DOACs are substrates of P-glycoprotein; apixaban and rivaroxaban are also substrates of cytochrome P450 (CYP3A4), whereas edoxaban and dabigatran are not. Determination of clinically relevant drug interactions is complex in patients with cancer, as they are often treated with many anticancer therapies that may compete for a common metabolic pathway. The choice of anticoagulant should be made on an individual basis and in consultation with a pharmacist for assessment of drug-drug interactions. 112 A list of common drug-drug interactions for direct Xa inhibitors can be found in the Canadian expert consensus. 112 The initial phase of cancer associated pulmonary embolism treatment requires use of parenteral anticoagulation (LMWH, fondaparinux) or rivaroxaban in patients without significant renal impairment, according to the algorithm proposed. The choice of long term anticoagulant can include LMWH, edoxaban, or rivaroxaban over VKAs, which are inferior to LMWH. VKAs may be used if LMWH or DOACs are unavailable or contraindicated, such as with severe renal impairment or drug-drug interactions. Duration of therapy for acute venous thromboembolism in cancers patients is usually six months, and extended treatment is individualized on the basis of the patient’s cancer status and treatments ( box 3 ). An ongoing RCT is comparing low dose apixaban with standard dose apixaban in cancer patients treated beyond six months ( NCT03692065 ).

Suggested algorithm for management of cancer associated thrombosis. DOAC=direct oral anticoagulant; LMWH=low molecular weight heparin. *Consider risk factors for bleeding including gastrointestinal (GI) toxicity (previous GI bleed, treatment associated with GI toxicity), thrombocytopenia (<50 000 platelets/mL), renal impairment, recent and/or life threatening bleeding, intracranial lesion, and use of antiplatelet agents. Adapted from Carrier M, et al. Curr Oncol 2018 112
Phases of cancer associated pulmonary embolism treatment
LMWH/fondaparinux for minimum 5 days*
Apixaban 10 mg BID for 7 days†
Long term (1 week to 6 months)
Apixaban 5 mg PO BID†
Edoxaban 60 mg daily‡
VKA for INR 2-3
Extended (6 months to indefinite)
BID=twice daily; INR=international normalized ratio; LMWH=low molecular weight heparin; VKA=vitamin K antagonist
*LMWH is needed for 5-10 days before starting edoxaban
†Not included in original Canadian expert consensus recommendations
‡30 mg daily if creatinine clearance 30-50 mL/min or weight <60 kg
Treatment of pregnancy associated pulmonary embolism
DOACs and fondaparinux cross the placenta and should be avoided in pregnancy. Unfractionated heparin and LMWH are safest during pregnancy as they do not cross the placenta; LMWH is the mainstay of treatment owing to its once daily dosing and self-administered subcutaneous route. Management of anticoagulation around the time of delivery requires close coordination with a multidisciplinary team of obstetrics, anesthesia, thrombosis, and maternal fetal medicine. A recent RCT of 3062 low risk pregnancies showed that scheduled induction of labor is safe, does not increase the risk for cesarean section delivery, and had a small benefit on the primary outcome of perinatal death or severe neonatal complications (relative risk 0.80, 0.64 to 1.00). 119 In patients with an acute venous thromboembolism event in the current pregnancy that occurred more than a month before the expected delivery date, we suggest a scheduled induction of labor with the last dose of LMWH administered 24 hours before. Stopping LMWH 24 hours before delivery allows the safe use of neuro-axial anesthesia if needed. 120 121 In the absence of any postpartum hemorrhage, LMWH is restarted six hours after delivery and continued for at least six weeks post partum. In patients who have an acute pulmonary embolism within one month of expected delivery, we also suggest scheduled induction of labor but administration of unfractionated heparin at therapeutic dose until active labor to avoid prolonged interruptions of therapy. If pulmonary embolism occurred less than two weeks from time of delivery, an inferior vena cava (IVC) filter may be considered. 122 Post partum, anticoagulant treatment options for women who are breast feeding include unfractionated heparin, LMWH, VKA, fondaparinux, or danaparoid. DOACs concentrate in breast milk and are contraindicated but can be considered in women who are not breast feeding or after completion of breast feeding in those who have an indication for longer term treatment. Antepartum and postpartum venous thromboembolism prophylaxis with LMWH are recommended for future pregnancies. 53
Thrombolysis for acute pulmonary embolism
Thrombolytic therapy, either systemic (most common) or directed by a catheter into the pulmonary arteries, can be used to accelerate the resolution of acute pulmonary embolism, lower pulmonary artery pressure, and increase arterial oxygenation. 123 Five per cent of patients with acute pulmonary embolism will present with hemodynamic compromise with systolic blood pressure persistently less than 90 mm Hg; they represent the subgroup at the highest risk for early mortality from pulmonary embolism, thus standing to benefit the most from thrombolytic therapy. 124 Bleeding is the major limitation of thrombolytic therapy, with major bleeding rates reported to be 10% or greater. 125 Overall, a systolic blood pressure persistently less than 90 mm Hg for at least 15 minutes and without high risk for bleeding is considered to be an indication for immediate treatment with systemic thrombolytic therapy. 14 15 This recommendation, however, is based on poor quality evidence, likely because of challenges in studying patients presenting with acute instability.
The results of the International Cooperative Pulmonary Embolism Registry (ICOPER), showed no benefit in terms of 90 day mortality with thrombolytic therapy in hemodynamically unstable pulmonary embolism but should be interpreted with caution as only 32% of all such patients received thrombolysis and selection bias is likely present. 124 A systematic review identified 18 randomized trials using thrombolytic therapy for the treatment of pulmonary embolism, including both hemodynamically stable and unstable pulmonary embolism. 123 Overall a reduction in death with thrombolytic therapy was observed (odds ratio 0.51, 0.29 to 0.89; P=0.02; 1898 participants; low quality evidence), but this overall effect was lost when studies with a high risk of bias were excluded (odds ratio 0.66, 0.42 to 1.06; P=0.08; 2054 participants).
The use of thrombolytic therapy in selected hemodynamically stable patients with high risk features has been better studied in clinical trials. The largest RCT to evaluate the benefit of thrombolysis in hemodynamically stable patients was the Pulmonary Embolism Thrombolysis (PEITHO) trial, which randomized 1005 patients with right ventricular dysfunction on either CTPA or echocardiogram or an elevated troponin to receive thrombolysis (tenecteplase) in addition to unfractionated heparin, compared with unfractionated heparin alone. 96 This study showed a benefit in the study’s composite primary outcome of death or hemodynamic decompensation within seven days (odds ratio 0.44, 0.23 to 0.87; P=0.02) but at a significant cost of major bleeding (major extracranial bleeding: odds ratio 5.55, 2.3 to 13.39; P<0.001). The most notable finding of this trial was that no difference in overall death was seen between the two groups, perhaps because patients randomized to the heparin only group successfully received rescue thrombolysis on development of hemodynamic decompensation. This would suggest that a strategy of close observation of such patients with escalation to systemic thrombolysis in those who decompensate is worthy of study. Three year follow-up in PEITHO showed no effect of thrombolysis therapy on residual dyspnea, right ventricular dysfunction, or overall mortality. 126
Catheter directed thrombolysis (CDT) is an alternative method for delivery of thrombolysis with potentially a lower risk of bleeding (one third the dose of thrombolytic drug compared with systemic delivery). This approach has been studied in an RCT of 59 patients with acute pulmonary embolism without evidence of hemodynamic compromise on presentation, and CDT showed a benefit in the primary outcome of improved right ventricular function (right ventricular/left ventricular ratio) at 24 hours (mean difference 0.30 (SD 0.20) versus 0.03 (0.16), heparin and CDT respectively; P<0.001). 127 Cohort and registry studies have shown improvement in surrogate outcomes of right ventricular function but no difference in recurrent pulmonary embolism or mortality. 15 Major bleeding rates are variable across studies but reported by some to be similar to those with systemic thrombolysis. 128 129 The role for CTD remains unclear, and we do not recommend its routine use except in experienced centers when a patient has hemodynamic compromise and a high risk of bleeding and therapy can be started without delay.
A network meta-analysis of all RCTs that compared recanalization procedures for acute pulmonary embolism (full dose systemic thrombolysis, low dose systemic thrombolysis, and catheter directed thrombolysis) found no significant benefit on overall mortality for any thrombolysis methods (full dose systemic thrombolysis: odds ratio 0.60, 0.36 to 1.01; low dose thrombolysis: 0.47, 0.14 to 1.59; catheter directed thrombolysis: 0.31, 0.01 to 7.96) and a significantly increased risk of bleeding, especially with full dose systemic thrombolysis (odds ratio 2.00, 1.06 to 3.78). 125 For patients presenting with persisting hemodynamic instability for at least 15 minutes, in the absence of high quality evidence, but also considering the high short term mortality of this group, we suggest the use of systemic thrombolysis in patients without absolute contraindication. 16 For patients with persisting hemodynamic instability but at high risk or with contraindications to systemic thrombolysis, we suggest that catheter directed thrombolysis may be considered on an individual case basis, where available. For all other patients deemed to be at high risk for short term deterioration (see “Pulmonary embolism risk assessment” above), we suggest observation in a monitored setting with thrombolytic therapy reserved for hemodynamic deterioration.
Surgical embolectomy
Surgical embolectomy with cardiopulmonary bypass can be performed in patients with acute pulmonary embolism associated with hemodynamic instability and contraindication to thrombolytic therapy. 14 16 Published case series have shown variable results, with perioperative mortality ranging from 4% to 59%. 130 131 Advanced age, pre-surgical cardiac arrest, and pre-surgical thrombolytic therapy are associated with worse outcomes. Extracorporeal membrane oxygenation (ECMO) either alone or as a bridge to surgical embolectomy has also shown benefit in case reports and small case series. 130 ECMO requires continuous anticoagulation and can induce a consumptive coagulopathy, resulting in high risk of bleeding. In a patient with significant hemodynamic instability and contraindication to thrombolysis, surgical embolectomy and/or ECMO may be considered.
Vena cava filters
IVC filters were first introduced in 1973 and designed to mechanically trap venous emboli from the lower extremities to prevent pulmonary embolism. 122 Since this time, the use of IVC filters has dramatically increased, despite a lack of evidence for an effect on venous thromboembolism related mortality. 132 Guidelines from major clinical societies differ in their suggested indication for IVC filters but generally agree on their use in patients with an acute proximal DVT or pulmonary embolism and a contraindication to anticoagulation. 122 The use of IVC filters for other indications, such as failure of anticoagulation, massive pulmonary embolism clot burden with residual DVT, severe cardiopulmonary disease, use before thrombolysis, or prophylaxis in patients at high risk, has expanded greatly in recent years but is not driven by evidence. 122 133
Pre-emptive placement of a permanent IVC filter in addition to standard anticoagulation in patients at high risk with acute proximal DVT was investigated in the Prévention du Risque d’Embolie Pulmonaire par Interruption Cave (PREPIC) study, an RCT of 400 patients, which showed a reduction in the primary outcome of early pulmonary embolism diagnosed within the first 12 days (odds ratio 0.22, 0.05 to 0.90) but no difference in mortality (odds ratio 0.99, 0.29 to 3.42). 134 Longer term follow-up data showed similar results, with reduction of pulmonary embolism in the IVC filter arm but a significant increase in recurrent DVT and no difference in overall mortality. 47 A follow-up RCT, PREPIC-2, studied removable IVC filters in 399 patients with high risk pulmonary embolism and showed no benefit in the use of the filter combined with standard anticoagulation compared with anticoagulation alone on the primary outcome of recurrent pulmonary embolism at three months (relative risk 2.00, 0.51 to 7.89; P=0.50). 135 We suggest that IVC filters should be restricted to patients with an acute proximal DVT or pulmonary embolism in whom full dose anticoagulation cannot be given because of uncontrollable active bleeding or a high risk for life threatening bleeding (for example, coagulation defect, severe thrombocytopenia, recent intracerebral hemorrhage, or cerebral lesion at high risk of bleeding) or urgent surgery requiring interruption of anticoagulation. In such patients, the safety of starting or resuming anticoagulation should be assessed frequently. Once full dose anticoagulation can be restarted without recurrence of major bleeding, the IVC filter should be promptly removed to reduce the chance of IVC filter related complications, which are increased over time. 122
Duration of treatment for pulmonary embolism
The duration of treatment depends on the presence or absence of risk factors at the time of diagnosis of the index pulmonary embolism (see box 1 ). The ISTH Scientific Subcommittee suggests evaluating patients’ risk for recurrent venous thromboembolism. 14 In patients with less than 5% risk at one year or less than 15% at five years, the recommendation is to stop anticoagulation. In pulmonary embolism provoked by major transient risk factors such as major surgery, the risk of recurrent pulmonary embolism at one year is less than 1%, favoring discontinuation of anticoagulation after three months. In those with minor transient risk factors such as hormone associated pulmonary embolism, the risk of recurrent venous thromboembolism is approximately 15% at five years and consideration of the risks of anticoagulation related major bleeding is important when recommending extended treatment in this intermediate group.
In patients without an identifiable risk factor (unprovoked pulmonary embolism), a recent systematic review and meta-analysis of 18 studies (RCTs and observational studies) evaluated the risk of recurrent venous thromboembolism in patients with a first unprovoked venous thromboembolism. 74 In total, 7515 patients were included, and all completed at least three months’ anticoagulation before discontinuing therapy. In the first year after stopping anticoagulation, the pooled rate of recurrent venous thromboembolism was 10.3 (95% confidence interval 8.6 to 12.1) events per 100 person years and the rate of recurrent pulmonary embolism was 3.3 (2.4 to 4.2) events per 100 person years. Table 5 shows the cumulative incidence of recurrent venous thromboembolism and recurrent pulmonary embolism. The case fatality rate of recurrent venous thromboembolism was 3.8% (2.0% to 6.1%). These data suggest that patients with a first unprovoked venous thromboembolism are at substantial risk for recurrent thrombosis, and this should guide decisions on extended anticoagulation therapy. Intermediate duration anticoagulation, such as extending the initial treatment period to one or two years before discontinuing therapy, does not reduce the subsequent risk of recurrent venous thromboembolism after anticoagulation is discontinued. 136
Risk of recurrent venous thromboembolism (VTE) and pulmonary embolism (PE) after discontinuing anticoagulation* 74
Risk stratification for patients with unprovoked venous thromboembolism may also help to determine the risk of recurrent thrombosis. Prognostic markers of recurrent venous thromboembolism include male sex, advanced age, 137 138 inherited thrombophilia, 70 obesity, 70 persistently positive D-dimer, 77 139 and residual pulmonary obstruction on ventilation-perfusion lung scan. 140 Individually, these risk factors are insufficient to recommend long term anticoagulation; however, risk prediction models incorporating various combinations have been proposed. 137 138 The largest prospectively validated (2785 patients) clinical decision rule is the “Men Continue and HERDOO-2.” 75 141 In the derivation cohort of this prediction rule, stratifying men into high and low risk categories was not possible; men had an annual risk of recurrent venous thromboembolism of 13.9% (10.8% to 17.0%) while off anticoagulation, so they remained on anticoagulation in the validation cohort. Women, on the other hand, were stratified into risk groups, such that anticoagulation could be discontinued in women with 0 or 1 HERDOO points (hyperpigmentation, edema or redness of either leg, D-dimer >250 μg/L, obesity (body mass index >30), older age (≥65 years)). The annual risk of recurrent venous thromboembolism in women at low risk was 1.6% (0.3% to 4.6%) in the derivation cohort and 3% (1.8% to 4.8%) in the validation cohort. Women with 2 or more HERDOO points were deemed to be at high risk and had an annual recurrent venous thromboembolism rate of 14.1% (10.9% to 17.3%) in the derivation cohort and remained on anticoagulation in the validation study. Limitations to this rule include the misclassification of women at high and low risk of recurrent venous thromboembolism risk with use of non-VIDAS d -Dimer assays (bioMérieux, Marcy L’Etoile, France), 142 and D-dimer testing was done on anticoagulation at six months after the initial venous thromboembolism event. Use of the rule at other time points or off anticoagulation has not been validated. Anticoagulant options for extended venous thromboembolism treatment are shown in box 2 .
Oral anticoagulation reduces the risk of recurrent venous thromboembolism only during therapy. Identifying patients with unprovoked index venous thromboembolism who would benefit from prolonged anticoagulation for extended treatment and secondary prevention needs to be balanced with risk of bleeding while on anticoagulation. Risk factors for bleeding include age over 75 years, history of bleeding, chronic liver disease, chronic renal disease, previous stroke, and use of concurrent antiplatelet agents or non-steroidal anti-inflammatory drugs. 16 As the bleeding risks and associated case fatality rates are lower for DOACs than VKAs, 143 144 when possible, DOACs should be considered over VKAs.
Box 2 shows the DOAC dosing options for extended treatment, including continuation of the same dosing as for long term treatment or reduced dosing for rivaroxaban and apixaban. The EINSTEIN CHOICE RCT compared rivaroxaban 20 mg daily and rivaroxaban 10 mg daily against aspirin 100 mg daily for extended treatment of venous thromboembolism in 3400 participants who completed at least six to 12 months of anticoagulation for acute venous thromboembolism. 145 The trial was not sufficiently powered to compare the different doses of rivaroxaban with each other. For the primary efficacy outcome of recurrent/fatal venous thromboembolism, each dose of rivaroxaban was associated with fewer events compared with aspirin (hazard ratio 0.34 (0.20 to 0.59) for rivaroxaban 20 mg versus aspirin and 0.26 (0.14 to 0.47) for rivaroxaban 10 mg compared with aspirin). The primary safety outcome of major bleeding was not different for either dose of rivaroxaban compared with aspirin (hazard ratio 2.01 (0.50 to 8.04) for rivaroxaban 20 mg compared with aspirin and 1.64 (0.39 to 6.84) for rivaroxaban 10 mg compared with aspirin). Limitations of EINSTEIN CHOICE are centered on the predominantly provoked venous thromboembolism population (60% of participants). The benefit of extended therapy in this population is less clear, as the risk of recurrent venous thromboembolism is lower in patients with provoked index venous thromboembolism. Whether rivaroxaban 10 mg daily is as effective as 20 mg daily in unselected high risk patients with unprovoked venous thromboembolism is also unknown.
The AMPLIFY EXT RCT compared two doses of apixaban, 5 mg twice daily and 2.5 mg twice daily, with placebo for 12 months for prevention of recurrent venous thromboembolism/all cause mortality. 146 Participants were randomized after completing six to12 months of therapy for acute venous thromboembolism and received either dose of apixaban or placebo for 12 months. Apixaban at both doses resulted in fewer recurrent primary outcome events compared with placebo (hazard ratio 0.36 (0.25 to 0.53) for apixaban 5 mg versus placebo and 0.33 (0.22 to 0.48) for apixaban 2.5 mg versus placebo). Major bleeding was the primary safety outcome and occurred with similar frequency in each apixaban group (hazard ratio 0.25 (0.03 to 2.24) for apixaban 5 mg versus placebo and 0.49 (0.09 to 2.64) for apixaban 2.5 mg versus placebo). More than 90% of participants in AMPLIFY EXT had unprovoked index venous thromboembolism, providing reassurance that both doses of apixaban reduce the risk of recurrent venous thromboembolism in this high risk patient population, without increasing bleeding events. Unfortunately, the study was not sufficiently powered to compare the apixaban doses with each other. Ongoing studies such as RENOVE ( NCT03285438 ) are evaluating extended therapy of full dose DOAC compared with reduced dose DOAC for patients with unprovoked index venous thromboembolism. In the meantime, patients’ preferences and regular evaluation of bleeding risks should be incorporated into decisions about extended therapy. We recommend annual reassessment of risks of bleeding and recurrent venous thromboembolism to inform decisions about prolonged anticoagulation.
In cancer associated pulmonary embolism, cancer is a major persistent risk factor and the need for extended anticoagulation therapy, beyond six months, is suggested for patients with active cancer (metastatic disease) or receiving chemotherapy. 112 Box 3 shows the options for extended therapy. To ensure that the benefit of continuing anticoagulation outweighs the potential harm of bleeding, we suggest that the decision to continue anticoagulation should be regularly reassessed. Figure 4 summarizes our suggested approach to duration of anticoagulant treatment. 147

Approach to duration of treatment of venous thromboembolism (VTE). *If transient risk factor is non-surgical (eg, immobilization, pregnancy, or estrogen therapy), extended treatment can be considered given the safety profile of direct oral anticoagulants. †According to “Men continue and HERDOO2” risk prediction score: low=women with 0-1 points; high risk=all men and women with ≥2 points. ‡Bleeding risk according to HAS-BLED score: low risk 0-2 points or high risk ≥3 points. Adapted from Tritschler T, et al. JAMA 2018 147
Long term effect of pulmonary embolism
Post-pulmonary embolism syndrome.
As many as 50% of patients report long term sequelae after pulmonary embolism. 148 149 150 Post-pulmonary embolism syndrome has been defined by suboptimal cardiac function, pulmonary artery flow dynamics, or pulmonary gas exchange at rest or during exercise, in combination with dyspnea, decreased exercise tolerance, or diminished functional status or quality of life, without an alternative explanation. 148 149 At the extreme end, chronic thromboembolic pulmonary hypertension (CTEPH) occurs in an estimated 3% of patients surviving after a six month treatment period for acute pulmonary embolism. 151 The exact pathophysiology of why CTEPH occurs in a minority of patients remains unknown. Risk factors for development of CTEPH after acute pulmonary embolism include diagnostic delay, high thrombus load, recurrent symptomatic pulmonary embolism, pulmonary hypertension or right ventricular dysfunction at baseline, and failure to achieve thrombus resolution. 148 152 153 A diagnosis of CTEPH is confirmed by showing a mean pulmonary artery pressure above 25 mm Hg combined with thrombotic pulmonary vascular obstructions. Planar ventilation-perfusion lung scanning is the preferred imaging modality, with high sensitivity and specificity for CTEPH. 15 Bilateral pulmonary endarterectomy through the medial layer of the pulmonary arteries is a curative treatment for CTEPH, but most patients need lifelong anticoagulation because of the risk of recurrent venous thromboembolism. 15
A second subset of patients is those with evidence of chronic thromboembolic disease without pulmonary hypertension. Cardiopulmonary functional testing suggests that this is an intermediate clinical phenotype in response to exercise. 154 The relation between residual pulmonary obstruction and the patient’s risk of developing CTEPH and how the prognosis differs from those with functional symptoms without evidence of residual pulmonary obstruction remain unclear. An observational study, the Prospective Evaluation of Long-term Outcomes After Pulmonary Embolism (ELOPE), followed 100 unselected patients with an acute pulmonary embolism and did cardiopulmonary exercise testing at one and 12 months. 150 Consistent with self-reported symptoms at one year, almost 50% of these patients had evidence of diminished exercise capacity. The observed reduced cardiopulmonary exercise capacity correlated well with several quality of life measurements and the six minute walk test. Baseline residual pulmonary obstruction was not associated with the exercise limitation, and nor were pulmonary function testing or echocardiographic results. 155 Predictors of exercise limitations were age, body mass index, and smoking history. These observations led the investigators to speculate that general deconditioning may be the cause of the patient’s reported dyspnea and exercise limitation. The absence of association with baseline residual clot burden and cardiopulmonary exercise capacity is also consistent with the long term follow-up study of patients with pulmonary embolism who had systemic thrombolysis, as no benefit was seen on reported dyspnea or exercise capacity. 126
Post-pulmonary embolism syndrome describes a heterogeneous consolidation of symptoms and objective findings that has an important effect on the quality of life of patients with pulmonary embolism. Following patients beyond the acute pulmonary embolism period and screening for persisting dyspnea and functional limitations at three to six months is recommended. An ongoing observational study is evaluating a CTEPH clinical prediction score to select patients for screening with echocardiography ( NCT02555137 ). Until these results are available, we continue to screen all patients reporting persisting dyspnea with a ventilation-perfusion lung scan to evaluate for persistent mismatched defects and transthoracic echocardiogram for pulmonary hypertension. If these are found, these patients are referred to a CTEPH expert center for further diagnostic work-up and treatments. Targeted cardiopulmonary rehabilitation and lifestyle modifications may be offered to the remaining patients, although future research is needed to determine the benefits of such programs.
Psychological impact and quality of life
The diagnosis of a pulmonary embolism has a significant psychological effect on patients, who often refer to such an event as a near-miss death experience. The above described ELOPE study followed a cohort of patients with acute pulmonary embolism over one year and showed an acute decline in both generic and pulmonary embolism specific quality of life scores, but these scores then improved over the one year follow-up. 156 Cancer patients with venous thromboembolism also experience a decline in quality of life scores. 157 Qualitative interviews of patients six to 12 months after a diagnosis of venous thromboembolism reported a major theme of “life changing and forever changed” when describing their lived experience with venous thromboembolism. 158 Some patients also noted a “post-thrombotic panic,” describing feelings of hypervigilance and panic related to fear of illness recurring. A need for greater recognition of patients’ psychological wellness and research into potential targeted supports clearly exists.
Table 6 summarizes the guidelines that seem to be the most relevant, updated, and endorsed by leading international societies concerning management of patients with pulmonary embolism. 14 16 159 Of these, the guidelines from the European Society of Cardiology (ESC) and the American Society of Cardiology (ASH) have been updated within the last one or two years and are thus based on the most recent clinical trials. The completed ASH guidelines are in progress, with six of 10 intended sections published at this time (prophylaxis for medical patients, 160 diagnosis, 161 anticoagulation therapy, 162 pediatrics, 163 heparin induced thrombocytopenia, 164 and pregnancy 53 ). The remaining four sections are expected to be released later in 2020 (treatment, cancer, thrombophilia, prophylaxis in surgical patients). The completed ASH guidelines will represent the most comprehensive and updated guideline set. The guidelines released by the American College of Chest Physicians in 2016 are a partial update of the comprehensive 2012 guidelines. 165 The field of pulmonary embolism has had several important advances in the four years since this release.
Comparison of guideline recommendations from ASH*, CHEST†, and ESC‡ for diagnosis and treatment of pulmonary embolism
Emerging treatments
Anticoagulant therapies targeting coagulation factors IX, XI, and XII are under research and development. 166 167 Of these, factor XIa inhibition is most developed and includes targeted strategies such as antisense oligonucleotide agents to reduce hepatic biosynthesis, aptamers to target DNA or RNA expression, and monoclonal antibodies and small molecules that block activity of factor XIa. 168 169 Two phase II RCTs of novel factor XI inhibitors have been published, both testing various doses after elective knee arthroplasty for the primary outcome of new venous thromboembolism (symptomatic and asymptomatic). Büller et al randomized 300 patients to either 200 mg or 300 mg of FXI-ASO, given as a series of subcutaneous injections staring 36 days preoperatively, or enoxaparin prophylaxis. 170 The 200 mg regimen was non-inferior and the 300 mg regimen superior to enoxaparin (P<0.001). Weitz et al randomized 813 patients post-elective knee arthroplasty to enoxaparin, apixaban, or single intravenous infusions of the factor XIa inhibitor osocimab (BAY1213790) at various dose and schedules (preoperative/postoperative). 171 In this open label dose finding study, osocimab at doses of 0.6 mg/kg, 1.2 mg/kg, and 1.8 mg/kg given postoperatively met criteria for non-inferiority compared with enoxaparin for the primary outcome of new venous thromboembolism (symptomatic or asymptomatic), and the preoperative 1.8 mg/kg dose of osocimab met criteria for superiority compared with enoxaparin (risk difference 10.6, 95% confidence interval –1.2 to 22.4; P=0.07). Further studies are needed to determine the true efficacy and bleeding risk of these novel anticoagulants.
The management of pulmonary embolism has changed considerably over the past decade, most substantially driven by the introduction of direct oral anticoagulation therapies. The convenience of use, lack of routine laboratory monitoring, and lower bleeding rates have allowed a greater acceptance by patients compared with VKAs. Extended treatment duration in selected patients with pulmonary embolism has had a significant effect on risk of recurrent venous thromboembolism. Other important management updates include a recognition of over-investigation and perhaps over-treatment of pulmonary embolism in some patients. The use of clinical probability scores and advances in the interpretation of D-dimer results reduces the use of diagnostic imaging to exclude pulmonary embolism. Recognition of subsegmental pulmonary embolism as a distinct entity and careful evaluation of need for anticoagulation have been important to avoid over-diagnosis and over-treatment. Despite a decade of advances, however, pulmonary embolism continues to have important long term consequences for patients, including chronic dyspnea, diminished exercise capacity, and effects on quality of life. Future research is needed to identify targeted interventions and supports.
Glossary of abbreviations
ASH—American Society of Cardiology
CDT—catheter directed thrombolysis
CTEPH—chronic thromboembolic pulmonary hypertension
CTPA—computed tomography pulmonary angiography
DOAC—direct oral anticoagulant
DVT—deep venous thrombosis
ECMO—extracorporeal membrane oxygenation
ESC—European Society of Cardiology
ISTH—International Society on Thrombosis and Hemostasis
IVC—inferior vena cava
LMWH—low molecular weight heparin
NT-proBNP—N-terminal pro-b-type natriuretic peptide
PERC—pulmonary embolism rule-out criteria
PESI—Pulmonary Embolism Severity Index
pro-BNP—pro-B-type brain natriuretic peptide
RCT—randomized controlled trial
SPECT—Single photon emission computed tomography
sPESI—simplified Pulmonary Embolism Severity Index
VKA—vitamin K antagonist
How patients were involved in the creation of this article
The authors of this clinical review are members of Canadian Venous Thromboembolism Clinical Trials and Outcomes Research (CanVECTOR) network. This network includes patient partner members. Three CanVECTOR patient partners were consulted for the preparation of the manuscript and were asked to review a proposed outline of topics to include and provided their contributions and feedback. Specifically, patients were asked to review the manuscript outline with the following question in mind: “If your clinicians were to read one review paper for the purpose of updating their knowledge of pulmonary embolism management, which topics do you feel are most important to include?” Additions to the manuscript as a direct result of this engagement with patient partners included a discussion of thrombophilia testing, with specific reference to benefits of thrombophilia testing in patients with identified transient provoking risk factors; a discussion of the detailed management of pregnancies in patient with pulmonary embolism; and a discussion of the psychological impact of a diagnosis of pulmonary embolism in survivors. The final manuscript of this article was reviewed and approved by one lead patient partner from this group.
Questions for future research
Can the use of clinical probability score and D-dimer testing be optimized for the diagnosis of pulmonary embolism in subgroups of patients such as those with a previous history of pulmonary embolism and pregnant women?
What is the appropriate management of a patient with pulmonary emboli located to within the subsegmental pulmonary arteries?
How can clinicians recognize and manage the long term sequelae of pulmonary embolism such as chronic thromboembolic pulmonary hypertension and post-pulmonary embolism syndrome?
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: LD and LAC did the primary literature search in collaboration with a health information librarian. LD was the lead author of the manuscript, and LAC wrote the sections on choice of anticoagulation for acute pulmonary embolism, treatment of cancer associated pulmonary embolism, and duration of treatment for pulmonary embolism. MAF guided the writing of the full manuscript. All authors reviewed the full manuscript and contributed to its content and references.
Funding: LAC is supported by Heart and Stroke Foundation of Canada National New Investigator and Ontario Clinician Scientist Phase I award. LD, LAC, and MAF are investigators of the Canadian Venous Thromboembolism Clinical Trials and Outcomes Research (CanVECTOR) Network; the Network receives grant funding from the Canadian Institutes of Health Research (Funding Reference: CDT-142654). CanVECTOR’s Patient Partners platform provided support for patient engagement activities..
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: none.
Provenance and peer review: Commissioned; externally peer reviewed.
- Kathuria P ,
- Alrifai A ,
- Jiménez D ,
- de Miguel-Díez J ,
- Guijarro R ,
- RIETE Investigators
- Goldhaber SZ ,
- Schulman S ,
- Kakkar AK ,
- RE-COVER Study Group
- Bauersachs R ,
- Berkowitz SD ,
- Brenner B ,
- EINSTEIN Investigators
- Agnelli G ,
- Buller HR ,
- AMPLIFY Investigators
- Büller HR ,
- Lensin AW ,
- EINSTEIN–PE Investigators
- Ceriani E ,
- Combescure C ,
- PEGeD Study Investigators
- Righini M ,
- Den Exter PL ,
- van der Hulle T ,
- Cheung WY ,
- YEARS study group
- Ornelas J ,
- Konstantinides SV ,
- Becattini C ,
- ESC Scientific Document Group
- The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC)
- Décousus H ,
- Grosso MA ,
- Hokusai-VTE Investigators
- Alshab AK ,
- Mahmoudpour SH ,
- Valerio L ,
- Yamamoto T ,
- Rappold L ,
- Gerhold-Ay A ,
- Wendelboe AM ,
- Anderson FA Jr . ,
- McCumber M ,
- ISTH Steering Committee for World Thrombosis Day
- Grimshaw JM ,
- ↵ SIGN 50: a guideline developer's handbook. 2019. https://www.sign.ac.uk/sign-50.html .
- Bĕlohlávek J ,
- Dytrych V ,
- Barritt DW ,
- PIOPED Investigators
- Robert-Ebadi H ,
- Mostaguir K ,
- Hovens MM ,
- Anderson DR ,
- Gibson NS ,
- Christopher study investigators
- Lucassen W ,
- Geersing GJ ,
- Erkens PM ,
- Cachanado M ,
- PROPER Investigator Group
- Linkins LA ,
- Takach Lapner S
- Kessels JB ,
- Goehring C ,
- Bounameaux H ,
- Ihaddadene R ,
- DE Roos A ,
- Dekkers OM ,
- Rodger MA ,
- Hayashino Y ,
- Noguchi Y ,
- Brenner DJ ,
- van der Pol LM ,
- Tromeur C ,
- Bistervels IM ,
- Artemis Study Investigators
- CT-PE-Pregnancy Group
- Langlois E ,
- Cusson-Dufour C ,
- Moumneh T ,
- Rajasekhar A ,
- Middeldorp S ,
- van Mens TE ,
- Scheres LJ ,
- de Jong PG ,
- Leeflang MM ,
- Nijkeuter M ,
- Middeldorp S
- Sørensen HT ,
- Andersen EW ,
- Andersen PK
- Couturaud F ,
- Leroyer C ,
- Julian JA ,
- Bezemer ID ,
- van der Meer FJ ,
- Eikenboom JC ,
- Rosendaal FR ,
- Coppens M ,
- Reijnders JH ,
- Doggen CJ ,
- Rosendaal FR
- Greaves M ,
- British Committee for Standards in Haematology
- Langlois NJ ,
- Etchegary H ,
- Brehaut J ,
- Langlois N ,
- Palleschi C ,
- Vansenne F ,
- Kaptein AA ,
- De Borgie CA ,
- Louzada ML ,
- Taljaard M ,
- Miyakis S ,
- Lockshin MD ,
- Brandt JT ,
- Triplett DA ,
- García-Fuster MJ ,
- Forner MJ ,
- Fernández C ,
- Maldonado L
- Zoppellaro G ,
- Ordi-Ros J ,
- Sáez-Comet L ,
- Pérez-Conesa M ,
- Carrier M ,
- MARVELOUS Collaborators
- REVERSE II Study Investigators
- Squizzato A ,
- Douketis J ,
- Tosetto A ,
- Marcucci M ,
- Prometheus Study Group
- Davidson BL ,
- Wartski M ,
- Collignon MA
- Donadini MP ,
- Dentali F ,
- den Exter PL ,
- Prometheus Follow-Up Investigators
- Kovacs MJ ,
- Hamadah A ,
- Alwasaidi T ,
- Aujesky D ,
- Obrosky DS ,
- Crobach MJ ,
- Hestia Study Investigators
- Perrier A ,
- Perneger TV ,
- Mallett S ,
- Daoud-Elias M ,
- Verschuren F ,
- Frank Peacock W ,
- Coleman CI ,
- Diercks DB ,
- Darwish OS ,
- Mahayni A ,
- Coutance G ,
- Cauderlier E ,
- Ehtisham J ,
- PEITHO Investigators
- Vesta Study Investigators *
- Schmidtmann I ,
- HoT-PE Investigators
- Fernandes A ,
- Connors JM ,
- Wiener RS ,
- Schwartz LM ,
- Bariteau A ,
- Stewart LK ,
- Emmett TW ,
- Castellucci LA ,
- Vílchez JA ,
- Gallego P ,
- RE-COVER II Trial Investigators
- Lensing AW ,
- Braekkan SK ,
- Versteeg HH ,
- Cannegieter SC
- Dunkler D ,
- Kraaijpoel N ,
- Bleker SM ,
- UPE investigators
- Crowther M ,
- Mellemkjaer L ,
- Khorana AA ,
- American Society of Clinical Oncology
- Raskob GE ,
- Verhamme P ,
- Hokusai VTE Cancer Investigators
- Marshall A ,
- Thirlwall J ,
- McBane RD 2nd . ,
- Wysokinski WE ,
- Le-Rademacher JG ,
- Caravaggio Investigators
- Grobman WA ,
- Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network
- Gogarten W ,
- Vandermeulen E ,
- Van Aken H ,
- Samama CM ,
- European Scoeity of Anaesthesiology
- Horlocker TT ,
- Vandermeuelen E ,
- Leffert LR ,
- Duffett L ,
- De Rosa M ,
- Goldhaber SZ
- Jimenez D ,
- Martin-Saborido C ,
- Boekstegers P ,
- Müller OJ ,
- Hohlfelder B ,
- SEATTLE II Investigators
- Avgerinos ED ,
- Saadeddin Z ,
- Abou Ali AN ,
- Weinberg A ,
- Tapson VF ,
- O’Malley TJ ,
- Maynes EJ ,
- Decousus H ,
- Leizorovicz A ,
- Mismetti P ,
- Laporte S ,
- Pellerin O ,
- PREPIC2 Study Group
- Eichinger S ,
- Jandeck LM ,
- Palareti G ,
- Legnani C ,
- DULCIS (D-dimer and ULtrasonography in Combination Italian Study) Investigators
- Giustozzi M ,
- Cimini LA ,
- Riera-Mestre A ,
- REVERSE II investigators
- Chai-Adisaksopha C ,
- Isayama T ,
- Lensing AWA ,
- EINSTEIN CHOICE Investigators
- AMPLIFY-EXT Investigators
- Tritschler T ,
- Lankeit M ,
- Huisman MV ,
- Konstantinides S
- Hirsch AM ,
- Akaberi A ,
- Ende-Verhaar YM ,
- Cannegieter SC ,
- Vonk Noordegraaf A ,
- Ribeiro A ,
- Lindmarker P ,
- Johnsson H ,
- Juhlin-Dannfelt A ,
- Fernandes T ,
- Claessen G ,
- La Gerche A ,
- ELOPE Study group
- Granton JT ,
- Dewilde S ,
- ASH Clinical Practice Guidelines on Venous Thromboembolism
- Schünemann HJ ,
- Cushman M ,
- Burnett AE ,
- Nieuwlaat R ,
- Monagle P ,
- Cuello CA ,
- Augustine C ,
- Arepally GM ,
- Guyatt GH ,
- Gutterman DD ,
- Schuünemann HJ ,
- American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel
- DeLoughery EP ,
- McCarty OJT ,
- Balters MW ,
- Al-Horani RA
- Bethune C ,
- FXI-ASO TKA Investigators
- Patient Care & Health Information
- Diseases & Conditions
- Pulmonary embolism
A pulmonary embolism can be difficult to diagnose, especially if you have underlying heart or lung disease. For that reason, your health care provider will likely discuss your medical history, do a physical exam, and order tests that may include one or more of the following.
Blood tests
Your health care provider may order a blood test for the clot-dissolving substance D dimer. High levels may suggest an increased likelihood of blood clots, although many other factors can cause high D dimer levels.
Blood tests also can measure the amount of oxygen and carbon dioxide in your blood. A clot in a blood vessel in your lungs may lower the level of oxygen in your blood.
In addition, blood tests may be done to determine whether you have an inherited clotting disorder.
Chest X-ray
This noninvasive test shows images of your heart and lungs on film. Although X-rays can't diagnose a pulmonary embolism and may even appear fine when a pulmonary embolism exists, they can rule out other conditions with similar symptoms.
A noninvasive test known as duplex ultrasonography, sometimes called a duplex scan or compression ultrasonography, uses sound waves to scan veins to check for deep vein blood clots. This test can look at veins in the thigh, knee and calf, and sometimes the arms.
A wand-shaped device called a transducer is moved over the skin, directing the sound waves to the veins being tested. These waves are then reflected back to the transducer to create a moving image on a computer. The absence of clots reduces the likelihood of deep vein thrombosis. If clots are present, treatment likely will be started immediately.
CT pulmonary angiography
CT scanning generates X-rays to produce cross-sectional images of your body. CT pulmonary angiography — also called a CT pulmonary embolism study — creates 3D images that can find changes such as a pulmonary embolism within the arteries in your lungs. In some cases, contrast material is given through a vein in the hand or arm during the CT scan to outline the pulmonary arteries.
Ventilation-perfusion (V/Q) scan
When there is a need to avoid radiation exposure or contrast from a CT scan due to a medical condition, a V/Q scan may be done. In this test, a small amount of a radioactive substance called a tracer is injected into a vein in your arm. The tracer maps blood flow, called perfusion, and compares it with the airflow to your lungs, called ventilation. This test can be used to see if blood clots are causing symptoms of pulmonary hypertension.
Pulmonary angiogram
This test provides a clear picture of the blood flow in the arteries of your lungs. It's the most accurate way to diagnose a pulmonary embolism. But because it requires a high degree of skill to perform and has potentially serious risks, it's usually done when other tests fail to provide a definite diagnosis.
In a pulmonary angiogram, a thin, flexible tube called a catheter is inserted into a large vein — usually in your groin — and threaded through your heart and into the pulmonary arteries. A special dye is then injected into the catheter. X-rays are taken as the dye travels along the arteries in your lungs.
In some people, this procedure may cause a temporary change in heart rhythm. In addition, the dye may cause increased risk of kidney damage in people with reduced kidney function.
MRI is a medical imaging technique that uses a magnetic field and computer-generated radio waves to create detailed images of the organs and tissues in your body. MRI is usually only done in those who are pregnant — to avoid radiation to the baby — and in people whose kidneys may be harmed by dyes used in other tests.
More Information
- Chest X-rays
Treatment of a pulmonary embolism focuses on keeping the blood clot from getting bigger and preventing new clots from forming. Prompt treatment is essential to prevent serious complications or death.
Treatment can include medicines, surgery and other procedures, and ongoing care.
Medicines include different types of blood thinners and clot dissolvers.
Blood thinners. These blood-thinning medicines called anticoagulants prevent existing clots from getting bigger and new clots from forming while your body works to break up the clots. Heparin is a frequently used anticoagulant that can be given through a vein or injected under the skin. It acts quickly and is often given along with an oral anticoagulant, such as warfarin (Jantovin), until the oral medicine becomes effective. This can take several days.
Newer oral anticoagulants work more quickly and have fewer interactions with other medicines. Some have the advantage of being given by mouth until they're effective, without the need for heparin. However, all anticoagulants have side effects, and bleeding is the most common.
- Clot dissolvers. While clots usually dissolve on their own, sometimes thrombolytics — medicines that dissolve clots — given through a vein can dissolve clots quickly. Because these clot-busting medicines can cause sudden and severe bleeding, they usually are reserved for life-threatening situations.
Surgical and other procedures
- Clot removal. If you have a large, life-threatening clot in your lung, your health care provider may remove it using a thin, flexible catheter threaded through your blood vessels.
- Vein filter. A catheter also can be used to position a filter in the body's main vein, the inferior vena cava, that leads from your legs to the right side of your heart. The filter can help keep clots from going to your lungs. This procedure is usually only used for people who can't take anticoagulant drugs or those who get blood clots even with the use of anticoagulants. Some filters can be removed when no longer needed.
Ongoing care
Because you may be at risk of another deep vein thrombosis or pulmonary embolism, it's important to continue treatment, such as remaining on anticoagulants and being monitored as often as suggested by your health care provider. Also, keep regular visits with your provider to prevent or treat complications.
- Extracorporeal membrane oxygenation (ECMO)
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Preparing for your appointment
A pulmonary embolism is often first evaluated in a hospital, emergency room or urgent care center. If you think you might have a pulmonary embolism, seek medical attention right away.
What you can do
You may want to prepare a list that includes:
- A detailed description of your symptoms
- Information about your past medical problems, especially any recent surgeries, injuries or illnesses that kept you in bed for several days
- Details on any recent trips that involved long car or plane rides
- All medicines you're taking, including vitamins, herbal products and any other supplements, and the doses
- Information about the medical problems of parents or siblings
- Questions you want to ask your health care provider
What to expect from your doctor
During the physical exam, your health care provider will likely examine your legs for evidence of a deep vein clot — an area that's swollen, tender, red and warm. Your provider will also listen to your heart and lungs, check your blood pressure and likely order one or more tests.
Living with pulmonary embolism?
Connect with others like you for support and answers to your questions in the Blood Cancers & Disorders support group on Mayo Clinic Connect, a patient community.
Blood Cancers & Disorders Discussions

387 Replies Sat, Jun 01, 2024

37 Replies Sat, Jun 01, 2024

49 Replies Sat, Jun 01, 2024
- AskMayoExpert. Pulmonary embolism (adult). Mayo Clinic; 2021.
- AskMayoExpert. Deep vein thrombosis (adult). Mayo Clinic; 2021.
- AskMayoExpert. Inferior vena cava (IVC) filters. Mayo Clinic; 2022.
- AskMayoExpert. Health considerations for air travelers: Venous thromboembolism. Mayo Clinic; 2022.
- Grillet F, et al. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. RSNA. 2020; doi:10.1148/radiol.2020201544.
- Stevens SM, et al. Antithrombotic therapy for VTE disease: Second update of the CHEST guideline and expert panel report. Chest. 2021; doi:10.1016/jchest.2021.07.055.
- Ferri FF. Pulmonary embolism. In: Ferri's Clinical Advisor 2023. Elsevier; 2023. https://www.clinicalkey.com. Accessed Aug. 18, 2022.
- Your guide to preventing and treating blood clots. Agency for Healthcare Research and Quality. https://www.ahrq.gov/patients-consumers/prevention/disease/bloodclots.html. Accessed Aug. 18, 2022.
- Essien E-O, et al. Pulmonary embolism. Medical Clinics of North America. 2019; doi:10.1016/j.mcna.2018.12.013.
- Broaddus VC, et al., eds. Pulmonary thromboembolism: Presentation and diagnosis. In: Murray and Nadel's Textbook of Respiratory Medicine. 7th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed Aug. 18, 2022.
- Broaddus VC, et al., eds. Pulmonary thromboembolism: Prophylaxis and treatment. In: Murray and Nadel's Textbook of Respiratory Medicine. 7th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed Aug. 18, 2022.
- Froehling DA (expert opinion). Mayo Clinic. Aug. 29, 2022.
- Blood clot in leg vein
Associated Procedures
Products & services.
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Assortment of Compression Products at Mayo Clinic Store
- Assortment of Health Products from Mayo Clinic Store
- Newsletter: Mayo Clinic Health Letter — Digital Edition
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

- Pulmonary aspergillosis
Citation, DOI, disclosures and case data
At the time the case was submitted for publication AHMED MANSOUR ALANI had no financial relationships to ineligible companies to disclose.
Presentation
Follow-up of pulmonary aspergillosis in a patient with chronic kidney disease.
Patient Data
Two rounded, soft tissue-attenuating masses within air-containing cavities in the right upper zone. Minor linear scarring or atelectasis. Bilateral pleural effusion.
7 years earlier
Two air-containing cavities with fibrotic strands in the right upper zone.
2 case questions available
Q: What are the typical chest X-ray (CXR) findings of a pulmonary aspergilloma? show answer
A: On a chest X-ray (CXR), a pulmonary aspergilloma typically appears as a round or oval mass within a pre-existing lung cavity. The mass may show the characteristic "air crescent sign," which is a crescent-shaped radiolucency indicating air surrounding the fungal ball. This sign suggests that the mass is mobile within the cavity. The surrounding lung tissue may display signs of chronic scarring or fibrosis, and there may be evidence of previous conditions that led to cavity formation, such as tuberculosis or sarcoidosis. The appearance of the aspergilloma may vary depending on the size of the cavity and the position of the patient at the time of the X-ray.
Q: How is pulmonary aspergilloma typically identified and characterized on a chest CT scan? show answer
A: Pulmonary aspergilloma, also known as a fungal ball, is typically identified on a chest CT scan as a solid, round mass within a pre-existing lung cavity. The key radiological feature is the presence of a well-defined, mobile intracavitary mass that often demonstrates a characteristic "air crescent sign". This sign is seen as a crescent of air surrounding the fungal ball within the cavity, indicating the presence of air between the wall of the cavity and the aspergilloma. Additionally, there may be evidence of surrounding lung fibrosis or other changes indicative of previous lung disease. The aspergilloma can move within the cavity when the patient's position changes, which helps differentiate it from other intracavitary lesions.
Case Discussion
All patients with chronic pulmonary aspergillosis (CPA) have a history of structural lung disease, such as residual cavities, bullae, or scarring. Specific risk factors include:
pulmonary tuberculosis (TB)
chronic obstructive pulmonary disease (COPD), especially bullous disease and allergic bronchopulmonary aspergillosis (ABPA)
nontuberculous mycobacteria (NTM) lung infection
thoracic surgery, particularly for lung cancer
sarcoidosis
hyper-IgE syndrome
Other conditions associated with CPA include bronchiectasis, prior pneumothorax, COVID-19 infection, previous severe pneumonia, pneumoconiosis, silicosis, rheumatoid arthritis, ankylosing spondylitis, granulomatosis with polyangiitis, previous pulmonary embolism, and hydatid disease.
- 1. https://www.uptodate.com/contents/chronic-pulmonary-aspergillosis-epidemiology-clinical-manifestations-and-diagnosis/abstract/9.
0 public playlists include this case
Related radiopaedia articles.
- Chronic cavitary pulmonary aspergillosis
Promoted articles (advertising)
How to use cases.
You can use Radiopaedia cases in a variety of ways to help you learn and teach.
- Add cases to playlists
- Share cases with the diagnosis hidden
- Use images in presentations
- Use them in multiple choice question
Creating your own cases is easy.
- Case creation learning pathway
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Thorac Res Pract
- v.25(2); 2024 Mar
- PMC11114191

Assessing the Prevalence of Pulmonary Embolism and the Clot Burden in Hospitalized Patients with Chronic Obstructive Pulmonary Disease Exacerbation
Neda akhoundi.
1 Department of Radiology, Hillcrest Hospital, University of California San Diego, San Diego, CA, USA
Mahlagha Amirbakhtiarvand
2 Islamic Azad University School of Medicine, Shahroud, Iran
Mobina Goli
Zahra naseri.
3 Department of Radiology, Shahid Beheshti University of Medical Science, Tehran, Iran
Alireza Siami
4 Biostatistical Analyzer, Amirkabir University of Technology, Tehran, Iran

This prospective cohort study aimed to assess the pulmonary embolism (PE) rate and clot burden in patients with chronic obstructive pulmonary disease (COPD) exacerbation.
Material and Methods:
A total of 248 patients entered the study, and their clinical probability of PE was assessed using the Geneva score. Patients with high clinical probability underwent computed tomographic pulmonary angiography, while those with low or intermediate probability underwent a d -dimer test.
Among the patients analyzed, 14 individuals (5.6%) were confirmed to have PE using computed tomographic pulmonary angiography. A 3-month follow-up revealed 3 cases of PE out of 232 patients initially deemed PE-free. Mortality rates were higher among patients with venous thromboembolism at admission than those diagnosed with PE during follow-up. Pulmonary embolism (PE) prevalence among patients with COPD exacerbation was 5.6%.
Conclusion:
The results of this study show the importance of screening for PE in patients with COPD presenting with dyspnea. Not all of them are due to COPD exacerbation; a small minority of them can be due to PE, which needs prompt screening, confirmation, and therapy. However, further research with larger cohorts is required to understand better the potential benefits and implications of systematic screening for pulmonary embolism in this specific patient population.
Main Points
- The clinical manifestations of acute pulmonary embolism and chronic obstructive pulmonary disease (COPD) exacerbation exhibit similarities, complicating the decision of whether pulmonary embolism should be considered in this specific clinical scenario.
- A total of 5.6% of the patients with the symptoms of COPD exacerbation were diagnosed with pulmonary embolism.
- Mean main pulmonary artery diameter was higher in patients with COPD and pulmonary embolism in the mortality group compared to the survivors.
Introduction
Chronic obstructive pulmonary disease (COPD) is a debilitating respiratory condition characterized by persistent airflow constriction and gradual lung function deterioration. It leads to substantial sickness and loss of life annually. The fatality linked to COPD is impacted by sudden worsening episodes known as acute exacerbations, necessitating alterations in treatment due to sudden respiratory symptom aggravation. 1
Triggers of these acute COPD exacerbations encompass multiple factors, including bronchial infections and exposure to air pollutants. Intriguingly, several investigations have indicated a notable prevalence of pulmonary embolism occurrences in patients undergoing COPD exacerbations. 2 - 5 A comprehensive analysis found that approximately 16% of hospitalized patients encountering unexplained acute COPD exacerbations simultaneously suffered from pulmonary embolism. Furthermore, a retrospective assessment disclosed that regardless of the initial suspected cause of exacerbation, pulmonary embolism emerged as the primary cause of fatality in 21% of patients admitted for acute COPD exacerbations. 6 , 7 The connection between exacerbations of COPD and the occurrence of pulmonary embolism poses a complex clinical situation, given that the symptoms of both conditions can overlap, leading to challenges in diagnosis and possible delays in appropriate treatment. However, determining the most effective approach for screening and diagnosing pulmonary embolism in hospitalized patients experiencing acute COPD exacerbation remains a formidable task. The current diagnostic methods employed for suspected acute pulmonary embolism in the general population might not yield the same results when applied to COPD exacerbations, particularly in the context of ventilation–perfusion lung scans. Furthermore, the clinical manifestations of acute pulmonary embolism and COPD exacerbation exhibit similarities, complicating the decision of whether pulmonary embolism should be considered in this specific clinical scenario. 8 , 9
It is crucial to establish the precise frequency of pulmonary embolism among patients undergoing COPD exacerbation in order to enhance patient care and outcomes. This study, conducted with prospective follow-up, aimed primarily to determine the prevalence of pulmonary embolism in COPD patients admitted to the hospital due to sudden worsening of respiratory symptoms. Additionally, we aimed to evaluate the extent of clot presence through pulmonary CT angiography. 10 , 11 Our intention is to raise health-care professionals’ awareness about the concealed risk of pulmonary embolism in cases of COPD exacerbations, prompting them to remain vigilant and factor in this potential complication when assessing patients with abruptly deteriorating respiratory symptoms.
Material and Methods
Ethical review and study organization.
The Ethics Committee of IAU (Approval number: 9716, Date: July 25, 2017) approved our prospective cohort study. Informed consent was obtained. This study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki.
Study Participants
From September 2017 to December 2022, a continuous series of adult outpatients (18 years or older) were enrolled to the teaching hospital due to sudden worsening of respiratory symptoms associated with COPD. To confirm the COPD diagnosis, patients were required to show either previous pulmonary function test results indicating a post-bronchodilator forced expiratory volume in the first second of expiration to forced vital capacity ratio of less than 70%, or a COPD diagnosis previously established by a pulmonologist. The criteria for defining acutely worsening respiratory symptoms in COPD encompassed a sustained deterioration in the patient’s typical symptoms (such as dyspnea, cough, and sputum production) beyond the expected day-to-day fluctuations, necessitating an alteration in treatment.
Exclusion criteria consisted of individuals with contraindications for undergoing computed tomographic pulmonary angiography (specifically those with a known allergy to iodine contrast agents), a creatinine clearance level below 30 mL/min/1.73 m² (indicative of severe kidney impairment), pregnancy, hospitalization exceeding 48 hours before inclusion, and anticoagulant therapy administered for reasons unrelated to venous thromboembolism.
Pulmonary Embolism Diagnostic Procedure
Patients were enrolled within 48 hours of being admitted to the hospital and categorized based on whether they displayed clinically suggestive symptoms of pulmonary embolism or not, as well as whether an alternative diagnosis more or less likely than pulmonary embolism was established.
The clinical likelihood of pulmonary embolism was evaluated for all patients using the Geneva score. 12 Patients with a high clinical likelihood (defined as a Geneva score ≥11) underwent computed tomographic pulmonary angiography. For patients with a low or moderate clinical likelihood (Geneva score <11), a d -dimer test was conducted and interpreted using the standard threshold of 500 ng/mL, denoted as Fibrinogen Equivalent Units. In cases where the d -dimer test yielded a negative result, the presence of pulmonary embolism was ruled out, and no further testing was conducted.
Follow-up visits were conducted monthly over a period of 3 months to identify any indications of venous thromboembolism.
Pulmonary Computed Tomography Angiography
Chest CT scans were performed using a multidetector CT scanner (Brilliance 64, Philips Medical Systems, Cleveland, Ohio, USA). The scan slice thickness was configured at 1mm with a 0.5 mm increment. For CT angiography, a total of 50ml of iodinated contrast material (Visipaque 320 mm iodine/mL) was administered intravenously, and scans were captured at the end of an inhalation during a single breath-hold. Two radiologists with 14 and 17 years of experience in interpreting thoracic CT scans evaluated the images. These radiologists evaluated the images without access to the patient's medical history, ensuring impartial assessment.
For patients diagnosed with pulmonary embolism (PE), the pulmonary artery obstructive index (Qanadli index) was calculated based on the degree of involvement of pulmonary arterial branches.
The primary objective of the study was to identify cases of pulmonary embolism within the initial 48 hours of admission. Diagnosis of pulmonary embolism was confirmed by detecting the presence of acute thrombosis within the lumen of the pulmonary arterial tree using computed tomographic pulmonary angiography.
A significant secondary outcome concentrated on observing instances of pulmonary embolism during the 3-month tracking period in patients who were initially determined to be free of venous thromboembolism within the first 48 hours of hospital admission.
Statistical Analysis
Descriptive statistics were presented as mean ± SD for continuous variables and as numbers (percentages) for categorical variables. Comparisons between means of continuous variables were conducted using either an independent group t -test or the Mann–Whitney U-test. The chi-square test was employed to compare the proportions of categorical variables. The association between quantitative variables was examined using either Pearson's or Spearman's correlation tests. Statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS) version 22.0 software (IBM Corp., Armonk, NY, USA), with a significance level set at P < .05.
In the present study, a total of 286 patients with COPD and acutely worsened respiratory symptoms were admitted to the hospital and screened for potential participation. However, 21 patients were excluded from the study due to being on long-term anticoagulation therapy and 17 patients were excluded because of being unable to provide informed consent. Finally, 248 patients entered the study.
The baseline characteristics of the patients are summarized in Table 1 . These patients had a mean age of 67.3 years (SD of 10.4 years), and among them, 93 were women (34.0% of the total sample). Based on the Geneva score, out of the 248 patients analyzed in the study, 130 patients (52%) had a low score. Additionally, 112 patients (45%) had an intermediate score, while 6 patients (2%) had a high score ( Table 1 ).
Basic Characteristics of Patients
Primary Outcome
Among the 248 patients included in the study, 6 patients (2.4%) were classified as having a high pretest clinical probability according to the Geneva score. Among these 6 patients, pulmonary embolism was confirmed in 4 patients through spiral computed tomographic pulmonary angiography.
For the remaining 242 patients with a low or moderate pretest clinical probability, a d -dimer assay was conducted. Among these patients, 173 individuals had d -dimer levels lower than 500 μg/mL, and pulmonary embolism was considered excluded without further diagnostic work-up. Among the 69 patients with positive d -dimer test results, 68 patients underwent spiral computed tomographic pulmonary angiography. Out of these 68 patients, 10 were confirmed to have pulmonary embolism. In total, among the 248 included patients, pulmonary embolism was confirmed in 14 patients, resulting in a prevalence of 5.6% (95% CI, 4.2%-8.1%) ( Figure 1 ).

Flowchart showing the patients’ entry into the study and their investigation results.
Three-Month Follow-up
During the 3-month follow-up period, among the initial 234 patients who were determined to be free of pulmonary embolism, 2 patients were lost to follow-up, leaving a total of 232 patients available for further evaluation. Over the course of the follow-up, 3 out of the remaining 232 patients were diagnosed with pulmonary embolism. The study also disclosed mortality rates for patients with venous thromboembolism (VTE) at admission and those diagnosed with pulmonary embolism during the 3-month follow-up. Among the patients with VTE at admission, the mortality rate was 3 out of 14 patients (25.1%). In contrast, among those patients diagnosed with pulmonary embolism during the follow-up period, 1 out of 3 patients (5.1%) experienced mortality.
Furthermore, the investigation assessed the average pulmonary artery obstruction index (PAOI) based on the Qanadli score in patients with pulmonary embolism. In the patients who suffered mortality, the mean PAOI was 13, while among the survivors, the mean PAOI was 4. This discrepancy was found to have statistical significance, with a P -value of 0.002. Similarly, the mean diameter of the main pulmonary artery (MPA) in the mortality group measured 30.5 ± 3.4 mm, which was higher than that of the survivors (26.6 ± 2.6 mm). This difference also exhibited statistical significance, with a P -value of 0.003, as indicated in Table 2 .
Comparison of Quantitative and Qualitative Characteristics of Patients with Pulmonary Embolism Among Subgroups of Mortality and Survivors in Patients with Chronic Obstructive Pulmonary Disease
COPD, chronic obstructive pulmonary disease; LPA, left pulmonary artery; MPA, main pulmonary artery; N/A, not applicable; PAOI, pulmonary artery obstruction index; PE, pulmonary embolism; RPA, right pulmonary artery.
This cohort study observed 248 patients with COPD who were hospitalized due to COPD exacerbation. Within 48 hours of their admission, an evaluation was conducted to detect the presence of pulmonary embolism. The findings demonstrated that 5.6% of the patients were diagnosed with pulmonary embolism. Among the initial 234 patients identified as not having venous thromboembolism at the outset of the study, 1.2% of them eventually developed pulmonary embolism.
The prevalence of pulmonary embolism in this specific study (5.6%), focusing on COPD patients admitted to the hospital, was observed to be lower in comparison to a meta-analysis that reported a higher prevalence of 16.1%. 13 This discrepancy could be attributed to the diversity observed across various studies. These variations encompass differences in diagnostic approaches for pulmonary embolism, methodologies used for COPD assessment, and disparities within the study populations. 3
The utilization of imaging for the assessment of various medical conditions is widely recognized. 14 - 19 This study stands apart from preceding research due to its distinctive investigation involving pulmonary CT angiography to evaluate clot burden in COPD patients. To the best of our knowledge, this study constitutes the largest cohort analysis aimed at appraising the PAOI in documented COPD patients concurrently affected by PE. A notable strength of this study is its meticulous process for validating instances of pulmonary embolism. 20 The study provides a dependable approximation of pulmonary embolism prevalence within the particular context under scrutiny. The observed prevalence of pulmonary embolism among patients with suspected cases upon admission aligns with rates seen in recent studies encompassing a broader spectrum of outpatients with suspected pulmonary embolism. This concurrence in prevalence implies that the study’s findings harmonize with outcomes from earlier investigations conducted among distinct patient groups, thereby fortifying the credibility of the prevalence estimation rendered by this study. 21 , 22 The study reveals a 4% prevalence of venous thromboembolism (VTE) for patients initially lacking clinical suspicion of pulmonary embolism. This finding underscores the significance of not overlooking VTE as insignificant within this subgroup. As a result, the study prompts a pertinent question concerning the necessity for systematic screening for pulmonary embolism even in patients initially lacking suspicion. 7 , 23 - 25 This study has some limitations. First of all, the study has been conducted in only 1 center. Secondly, while the study comprises a large cohort and holds value, it is important to note that generalizations cannot be made. Therefore, it is necessary to conduct a clinical study that would yield higher levels of evidence.
Funding Statement
This study received no funding.
Ethics Committee Approval: The study was approved by the medical ethics committee of IAU (Approval No: 97169716, Date: July 25, 2017). This study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki.
Informed Consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – N.A., Z.N.; Design – Z.N., M.A.; Supervision – M.A., M.G.; Resources – M.A., Z.N.; Materials – M.A., M.G.; Data Collection and or Processing – M.A., M.G.; Analysis and/or Interpretation – A.S.; Literature Search – M.A., M.G.; Writing – N.A., Z.N.; Critical Review – M.A., M.G.
Declaration of Interests: The authors have no conflict of interest to declare.

COMMENTS
A massive or submassive pulmonary embolism is preferentially managed with mechanical intervention. 20 In this case, the patient's condition was hemodynamically stable; thus, he was assessed as ...
CASE PRESENTATION: A 27-year-old male long-haul truck driver was admitted to our hospital after a 4-day history of severe chest pain and a 1-day history of sputum contained some blood and swollen left lower extremity. Ambulatory pulmonary computed tomography showed a patchy shadow in the lingula of the left lung, and the edges of both lower lobes showed decreased sharpness.
In this case, the diagnosis of HIT was surprising, especially due to only a mild decline in platelet levels that were well within normal range. We also acknowledge the significance of our PERT in the key diagnosis made in this case. Keywords: Pulmonary embolism, Thrombosis, Thrombolysis failure, Catheter-guided thrombolysis, Heparin-induced ...
The "big three" cardiovascular disorders comprise venous thromboembolism (VTE), myocardial infarction and stroke. The incidence of PE ranges from 39 to 115 per 100,000 population annually [ 1 ]. Right ventricular failure due to acute pressure overload is the primary cause of death in severe PE.
Stent placement results have been shown to be excellent in one study (a primary patency rate of 95% with cumulative patency of 90%- 100% over 1 year). 12 In one study, a 2-year follow-up evaluation has suggested primary and secondary post-venous stenting patency rates like 78% and 95%, respectively. 19 Poststent placement, anticoagulation for ...
Acute pulmonary embolism (PE) is a common disease encountered by pulmonologists, cardiologists, and critical care physicians throughout the world. For patients with high risk (defined by systemic hypotension) and intermediate high-risk (defined by the absence of systemic hypotension but the presence of numerous other concerning clinical and imaging features) acute PE, intensive care is often ...
A pulmonary embolism is a blockage in the pulmonary artery caused by a blood clot in the lungs. This is a life-threatening condition and results in symptoms that respiratory therapists and medical professionals must be able to identify. This case study will explore the events leading up to a patient being diagnosed with a pulmonary embolism, as ...
This editorial refers to 'Catheter-based therapy for intermediate or high-risk pulmonary embolism: death and re-hospitalisation', by O. Leiva et al., ... (NCT05684796); other investigator-led studies are also described on clinicaltrials.gov, ... a case report . Chest tube-related complete atrioventricular block
Clinical and Case Study Article. Approach to pulmonary embolism: A clinical care pathway. El Hussein, Mohamed Toufic RN, PhD, NP (Professor, ... Acute pulmonary embolism (PE) is a potentially fatal condition that is often underdiagnosed due to its ambiguous and generalized symptoms. As such, nurse practitioners (NPs) may struggle to respond in ...
The following are key points to remember about this clinical case on pulmonary embolism (PE): Although approximately 20% of patients who are treated for PE die within 90 days, true short-term mortality attributed to PE is estimated to be ; 5%.; Approximately 50% of the patients who receive a diagnosis of PE have functional and exercise limitations 1 year later (known as post-PE syndrome ...
Pre-op CT images. Pulmonary embolism (PE) is the obstruction of a pulmonary artery or one of its branches by thrombus, tumor, air, or fat that originated from other parts of the body. Approximately 600, 000 PE cases per year; 10% will not survive the initial PE event. If a prompt diagnosis is made, the mortality rate will reduce from 30% to 10%.
Accumulating data suggests that COVID-19 is associated with an increased risk of thrombotic events including pulmonary embolism. 3 Retrospective studies of patients with severe SARS-CoV-2 infections identified rates of VTE much higher than that of average ICU patients, 4 , 5 even in populations of patients with high rates of therapeutic ...
Introduction Despite the high incidence of pulmonary embolism its diagnosis continues to be difficult, primarily because of the vagaries of symptoms and signs in presentation. Conversely, syncope is a relatively easy clinical symptom to detect, but has varied etiologies that lead to a documented cause in only 58% of syncopal events. Syncope as the presenting symptom of pulmonary embolism has ...
Introduction. Pulmonary embolism (PE) and deep venous thrombosis (DVT) are leading causes of preventable death and disability in the United States. Acute PE is the third most common acute cardiovascular disease, with about 600,000 cases annually. Untreated PE is fatal in up to 30% of patients. Risk of death from acute PE is greatest within the ...
Pulmonary embolism is a common and potentially fatal cardiovascular disorder that must be promptly diagnosed and treated. The diagnosis, risk assessment, and management of pulmonary embolism have evolved with a better understanding of efficient use of diagnostic and therapeutic options. The use of either clinical probability adjusted or age adjusted D-dimer interpretation has led to a ...
CT pulmonary angiography — also called a CT pulmonary embolism study — creates 3D images that can find changes such as a pulmonary embolism within the arteries in your lungs. In some cases, contrast material is given through a vein in the hand or arm during the CT scan to outline the pulmonary arteries. Ventilation-perfusion (V/Q) scan
Recent years have brought several attempts at improving selection of patients with suspected pulmonary embolism for diagnostic imaging. Notable examples are the ADJUST-PE and PEGeD studies which showed that adjustable D-dimer cut-off levels based on age or pre-test probability reduce referral to diagnostic imaging while maintaining acceptable failure rates.
PDF | On Jan 1, 2016, Samsun Nahar and others published Pulmonary Embolism-A Case Report | Find, read and cite all the research you need on ResearchGate. ... This study was a population-based ...
VTE, which comprises deep vein thrombosis (DVT) and its life-threatening complication, acute pulmonary embolism (PE), represents a significant worldwide health problem which can result in death. The annual incidence of VTE ranges between 75 and 269 cases per 100,000 individuals, as shown by global studies in Western Europe, North America ...
Typical appearances of a large pulmonary emboli. The site and number of pulmonary emboli should be described: central, main, lobar, segmental or subsegmental vessels. Associated signs include: straightening of the intraventricular septum from right heart strain or pulmonary infarction. Signs of chronicity include: main pulmonary artery ...
A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT study. Arch Intern Med . 1991;151(5):933‐938.
Case Study #3: Pulmonary Embolism. Scenario. Stan is a 41-year-old roofer admitted to the hospital via the emergency department yesterday after falling 15 feet from a roof. He sustained bilateral fractured wrists and an open fracture of the left tibia and fibula. He was immediately taken to surgery for open reduction and internal fixation (ORIF ...
Septic pulmonary embolism (SPE) is a non-thrombotic, pathogen-containing thrombus causing bacterial embolism in the pulmonary vessels derived from a primary infection site via the venous circulation . The reported incidence of SPE in patients with KPLA varies from 2.7% to 16.3% [6 - 11]. However, its prevalence remains unclear due to the ...
The first PDE case was reported in 1877 by Cohnheim, a German pathologist, who followed the path of the emboli through the patent foramen ovale during postmortem studies and found the thrombus of venous origin in the left atrium. 4-6 A PDE may pass into the systemic circulation where it can cause stroke or other serious thromboembolic events ...
Case Discussion. All patients with chronic pulmonary aspergillosis (CPA) have a history of structural lung disease, such as residual cavities, bullae, or scarring. Specific risk factors include: pulmonary tuberculosis (TB) chronic obstructive pulmonary disease (COPD), especially bullous disease and allergic bronchopulmonary aspergillosis (ABPA ...
Pulmonary embolism is a life-threatening disease. Its development is generally thought to be due to causes collectively known as the Virchow's triad. ... Sundquist K. Risk of pulmonary embolism and deep venous thrombosis in patients with asthma: A nationwide case−control study from Sweden. Eur. Respir. J. 2017; 49:1601014. doi: 10.1183 ...
This prospective cohort study aimed to assess the pulmonary embolism (PE) rate and clot burden in patients with chronic obstructive pulmonary disease (COPD) exacerbation. ... Jabbarzadeh MJ, Talebi S. Incidental abdominal aortic aneurysm in the psoriasis patient: a case report and review of literature. Galen Med J. 2018; 7:e1168. 10.22086/gmj ...