
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction & Top Questions

Host range and distribution
- The nucleic acid
- The protein capsid
- The lipoprotein envelope
- The cycle of infection
- Malignant transformation
- Infectious patterns
- Chronic and slowly progressive diseases
- Evolutionary origins
- Evolution of new virus strains
- Distinguishing taxonomic features
- Annotated classification
Why are some viruses dangerous?
- Does the virus that causes COVID-19 belong to the coronavirus family?

Our editors will review what you’ve submitted and determine whether to revise the article.
- Biology LibreTexts - Viruses
- Cleveland Clinic - Virus
- University of California Museum of Paleontology - Introduction to the Viruses
- National Center for Biotechnology Information - PubMed Central - What is a Virus?
- Khan Academy - Viruses
- The Nature - The Origins of Viruses
- virus - Children's Encyclopedia (Ages 8-11)
- virus - Student Encyclopedia (Ages 11 and up)
- Table Of Contents
What is a virus?
A virus is an infectious agent of small size and simple composition that can multiply only in living cells of animals, plants, or bacteria.
What are viruses made of?
A virus particle is made up of genetic material housed inside a protein shell, or capsid. The genetic material, or genome, of a virus may consist of single-stranded or double-stranded DNA or RNA and may be linear or circular in form.
What size are viruses?
Most viruses vary in diameter from 20 nanometres (nm; 0.0000008 inch) to 250–400 nm. The largest viruses measure about 500 nm in diameter and are about 700–1,000 nm in length.
Are all viruses spherical in shape?
Shapes of viruses are predominantly of two kinds: rods (or filaments), so called because of the linear array of the nucleic acid and the protein subunits, and spheres, which are actually 20-sided (icosahedral) polygons.
When some disease-causing viruses enter host cells, they start making new copies of themselves very quickly, often outpacing the immune system’s production of protective antibodies. Rapid virus production can result in cell death and spread of the virus to nearby cells. Some viruses replicate themselves by integrating into the host cell genome, which can lead to chronic illness or malignant transformation and cancer.
virus , infectious agent of small size and simple composition that can multiply only in living cells of animals , plants , or bacteria . The name is from a Latin word meaning “slimy liquid” or “poison.”
The earliest indications of the biological nature of viruses came from studies in 1892 by the Russian scientist Dmitry I. Ivanovsky and in 1898 by the Dutch scientist Martinus W. Beijerinck . Beijerinck first surmised that the virus under study was a new kind of infectious agent, which he designated contagium vivum fluidum , meaning that it was a live, reproducing organism that differed from other organisms. Both of these investigators found that a disease of tobacco plants could be transmitted by an agent, later called tobacco mosaic virus , passing through a minute filter that would not allow the passage of bacteria. This virus and those subsequently isolated would not grow on an artificial medium and were not visible under the light microscope. In independent studies in 1915 by the British investigator Frederick W. Twort and in 1917 by the French Canadian scientist Félix H. d’Hérelle , lesions in cultures of bacteria were discovered and attributed to an agent called bacteriophage (“eater of bacteria”), now known to be viruses that specifically infect bacteria.
The unique nature of these agents meant that new methods and alternative models had to be developed to study and classify them. The study of viruses confined exclusively or largely to humans , however, posed the formidable problem of finding a susceptible animal host . In 1933 the British investigators Wilson Smith, Christopher H. Andrewes, and Patrick P. Laidlaw were able to transmit influenza to ferrets, and the influenza virus was subsequently adapted to mice. In 1941 the American scientist George K. Hirst found that influenza virus grown in tissues of the chicken embryo could be detected by its capacity to agglutinate (draw together) red blood cells.
A significant advance was made by the American scientists John Enders , Thomas Weller , and Frederick Robbins , who in 1949 developed the technique of culturing cells on glass surfaces; cells could then be infected with the viruses that cause polio ( poliovirus ) and other diseases. (Until this time, the poliovirus could be grown only in the brains of chimpanzees or the spinal cords of monkeys.) Culturing cells on glass surfaces opened the way for diseases caused by viruses to be identified by their effects on cells ( cytopathogenic effect ) and by the presence of antibodies to them in the blood. Cell culture then led to the development and production of vaccines (preparations used to elicit immunity against a disease) such as the poliovirus vaccine .

Scientists were soon able to detect the number of bacterial viruses in a culture vessel by measuring their ability to break apart (lyse) adjoining bacteria in an area of bacteria (lawn) overlaid with an inert gelatinous substance called agar —viral action that resulted in a clearing, or “ plaque .” The American scientist Renato Dulbecco in 1952 applied this technique to measuring the number of animal viruses that could produce plaques in layers of adjoining animal cells overlaid with agar. In the 1940s the development of the electron microscope permitted individual virus particles to be seen for the first time, leading to the classification of viruses and giving insight into their structure.
Advancements that have been made in chemistry, physics, and molecular biology since the 1960s have revolutionized the study of viruses. For example, electrophoresis on gel substrates gave a deeper understanding of the protein and nucleic acid composition of viruses. More-sophisticated immunologic procedures, including the use of monoclonal antibodies directed to specific antigenic sites on proteins, gave a better insight into the structure and function of viral proteins. The progress made in the physics of crystals that could be studied by X-ray diffraction provided the high resolution required to discover the basic structure of minute viruses. Applications of new knowledge about cell biology and biochemistry helped to determine how viruses use their host cells for synthesizing viral nucleic acids and proteins.

The revolution that took place in the field of molecular biology allowed the genetic information encoded in nucleic acids of viruses—which enables viruses to reproduce, synthesize unique proteins, and alter cellular functions—to be studied. In fact, the chemical and physical simplicity of viruses has made them an incisive experimental tool for probing the molecular events involved in certain life processes. Their potential ecological significance was realized in the early 21st century, following the discovery of giant viruses in aquatic environments in different parts of the world.
This article discusses the fundamental nature of viruses: what they are, how they cause infection, and how they may ultimately cause disease or bring about the death of their host cells. For more-detailed treatment of specific viral diseases, see infection .
General features
Viruses occupy a special taxonomic position: they are not plants, animals, or prokaryotic bacteria (single-cell organisms without defined nuclei), and they are generally placed in their own kingdom. In fact, viruses should not even be considered organisms, in the strictest sense, because they are not free-living—i.e., they cannot reproduce and carry on metabolic processes without a host cell .
All true viruses contain nucleic acid —either DNA (deoxyribonucleic acid) or RNA (ribonucleic acid)—and protein . The nucleic acid encodes the genetic information unique for each virus. The infective, extracellular (outside the cell) form of a virus is called the virion . It contains at least one unique protein synthesized by specific genes in the nucleic acid of that virus. In virtually all viruses, at least one of these proteins forms a shell (called a capsid ) around the nucleic acid. Certain viruses also have other proteins internal to the capsid; some of these proteins act as enzymes , often during the synthesis of viral nucleic acids. Viroids (meaning “viruslike”) are disease-causing organisms that contain only nucleic acid and have no structural proteins. Other viruslike particles called prions are composed primarily of a protein tightly complexed with a small nucleic acid molecule . Prions are very resistant to inactivation and appear to cause degenerative brain disease in mammals, including humans.
Viruses are quintessential parasites ; they depend on the host cell for almost all of their life-sustaining functions. Unlike true organisms, viruses cannot synthesize proteins, because they lack ribosomes (cell organelles) for the translation of viral messenger RNA (mRNA; a complementary copy of the nucleic acid of the nucleus that associates with ribosomes and directs protein synthesis) into proteins. Viruses must use the ribosomes of their host cells to translate viral mRNA into viral proteins.
Viruses are also energy parasites; unlike cells, they cannot generate or store energy in the form of adenosine triphosphate (ATP). The virus derives energy, as well as all other metabolic functions, from the host cell. The invading virus uses the nucleotides and amino acids of the host cell to synthesize its nucleic acids and proteins, respectively. Some viruses use the lipids and sugar chains of the host cell to form their membranes and glycoproteins (proteins linked to short polymers consisting of several sugars ).
The true infectious part of any virus is its nucleic acid, either DNA or RNA but never both. In many viruses, but not all, the nucleic acid alone, stripped of its capsid, can infect (transfect) cells, although considerably less efficiently than can the intact virions .
The virion capsid has three functions: (1) to protect the viral nucleic acid from digestion by certain enzymes ( nucleases ), (2) to furnish sites on its surface that recognize and attach (adsorb) the virion to receptors on the surface of the host cell, and, in some viruses, (3) to provide proteins that form part of a specialized component that enables the virion to penetrate through the cell surface membrane or, in special cases, to inject the infectious nucleic acid into the interior of the host cell.
Logic originally dictated that viruses be identified on the basis of the host they infect. This is justified in many cases but not in others, and the host range and distribution of viruses are only one criterion for their classification. It is still traditional to divide viruses into three categories: those that infect animals, plants, or bacteria.
Virtually all plant viruses are transmitted by insects or other organisms (vectors) that feed on plants. The hosts of animal viruses vary from protozoans (single-celled animal organisms) to humans. Many viruses infect either invertebrate animals or vertebrates, and some infect both. Certain viruses that cause serious diseases of animals and humans are carried by arthropods . These vector -borne viruses multiply in both the invertebrate vector and the vertebrate host.
Certain viruses are limited in their host range to the various orders of vertebrates . Some viruses appear to be adapted for growth only in ectothermic vertebrates (animals commonly referred to as cold-blooded , such as fishes and reptiles ), possibly because they can reproduce only at low temperatures . Other viruses are limited in their host range to endothermic vertebrates (animals commonly referred to as warm-blooded, such as mammals ).
- COVID-19 Tracker
- Biochemistry
- Anatomy & Physiology
- Microbiology
- Neuroscience
- Animal Kingdom
- NGSS High School
- Latest News
- Editors’ Picks
- Weekly Digest
- Quotes about Biology

Reviewed by: BD Editors
Virus Definition
A virus is a chain of nucleic acids (DNA or RNA) which lives in a host cell, uses parts of the cellular machinery to reproduce, and releases the replicated nucleic acid chains to infect more cells. A virus is often housed in a protein coat or protein envelope , a protective covering which allows the virus to survive between hosts.
Virus Structure
A virus can take on a variety of different structures. The smallest virus is only 17 nanometers, barely longer than an average sized protein. The largest virus is nearly a thousand times that size, at 1,500 nanometers. This is really small. A human hair is approximately 20,000 nanometers across. This means that most virus particles are well beyond the capability of a normal light microscope. Below is a scanning electron microscope (SEM) image of the Ebola virus.
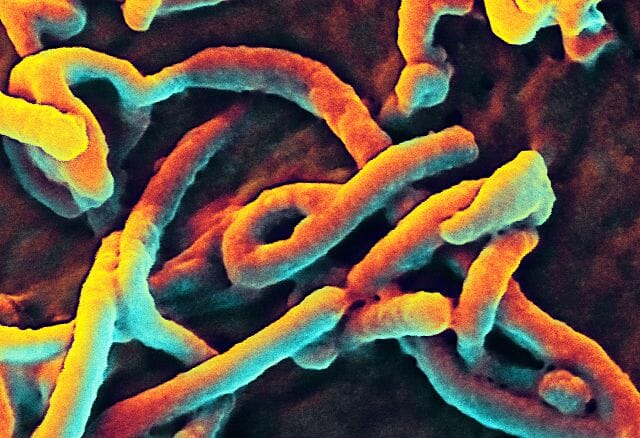
Here, you can only see the protein coat of the Ebola virus. Each virus looks like a little bent worm. However, these are not cells. Inside of the protein coat is a carefully folded RNA molecule, which contains the information necessary to replicate the protein coat, the RNA molecule, and the components necessary to hijack a cell’s natural processes to complete these tasks.
The exact structure of a virus is dependent upon which species serves as its host. A virus which replicates in mammalian cells will have a protein coat which enables it to attach to and infiltrate mammalian cells. The shape, structure, and function of these proteins changes depending on the species of virus. A typical virus can be seen below.
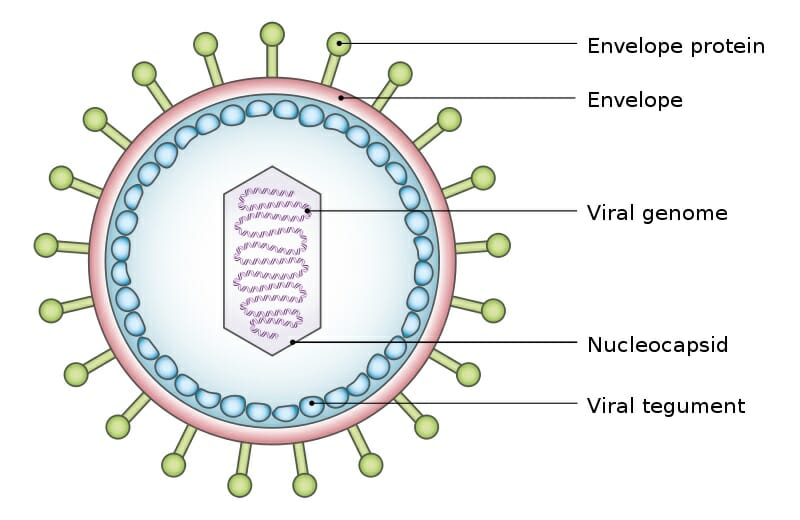
The above virus shows the typical structure a virus takes, a viral genome surrounded by a shield of proteins. The various envelope proteins will enable the virus to interact with the host cell it finds. Part of the protein coat will then open, puncture through the cell membrane, and deposit the viral genome within the cell. The protein coat can then be discarded, as the viral genome will now replicate within the host cell. The replicated virus molecules will be packaged within their own protein coats, and be released into the environment to find another host. While many virus particles take a simple shape like the one above, some are much more complicated.
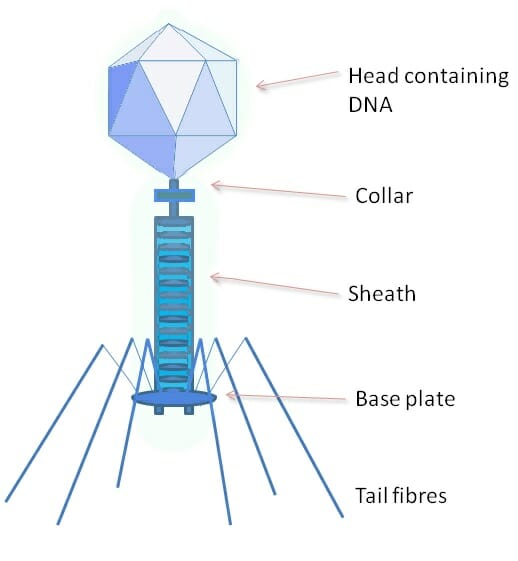
The above image shows a phage , a type of virus which specializes on bacterial cells. The protein coat of a phage is much more complex, and has a variety of specialized parts. The head portion contains the viral genome. The collar, sheath, base plate, and tail fibers are part of an intricate system to attach to and inject the genome into a bacterial cell. The tail fibers grasp the bacterial cell, pulling the base plate up to the cell wall or membrane. The sheath and collar compress, puncture the cell, and deposit the DNA into the bacterial cell.
Some virus molecules have no protein coat whatsoever, or have never been identified making on. In some plant virus species, the virus is passed from cell to cell within the plant. When seeds are created within the plant, the virus spreads to the seeds. In this way the virus can live within cells its entire existence, and never need a protein coat to protect it in the environment. Other virus molecules have even larger and more complex protein coats, and specialize on various hosts.
Is a Virus Living?
This is a complicated question. A cell is considered to be living because it contains all the necessary components to replicate its DNA, grow, and divide into new cells. This is the process all life takes, where it is a single-celled organism or a multi-cellular organism. Some people do not consider a virus living because a virus does not contain all of the mechanisms necessary to replicate itself. They would say that a virus, without a host cell, cannot replicate on its own and is therefore not alive.
Yet, by the definition of life laid out before, it seems that when a virus is inside of a host cell it does have all the machinery it needs to survive. The protein coat it exists in outside of a cell is the equivalent of a bacterial spore , a small capsule bacteria form around themselves to survive harsh conditions. Scientists who support a virus being a living organisms note the similarity between a virus in a protein coat and a bacterial spore. Neither organism is active within their protective coat, they only become active when they reach favorable conditions.
In fact, the only reason a virus affects us at all is because it becomes active within our cells. Further, a virus tends to evolve with its host. Most dangerous viruses have just recently jumped to a new species. The biochemistry they evolved to live within the other species is not compatible with the new species, and cell damage and death occur. This causes a number of reactions, depending on which cells were infected. The HIV virus, for instance, attacks immune cells exclusively. This leads to a total loss of immune function in patients. With the virus causing the common cold, the virus attacks respiratory cells and damages them as it does its work.
Yet, not all virus infections will be detrimental to the host. A virus that kills the host will be less successful over time, compared to a virus which doesn’t harm the host. A healthy host increases the number of virus molecules released into the environment, which is the ultimate goal of the virus. In fact, some virus particles may actually benefit the host. A good example is a form of herpes virus, found in mice. This virus, while it is infecting a mouse, provides the mouse with a good defense against the bacteria which carry the plague. While the mechanism is not clear, the virus somehow prevents the bacteria from taking hold in the mouse’s system.
When viewed in this light, it is easy to see how a virus is very similar to a bacteria. The bacteria creates and maintains the tools needed to reproduce DNA, where the virus steals them. This is the only real difference between a virus and a bacteria. Because of this, many scientists consider a virus a living organism. Scientists who study viruses, virologists , note that virus particles (alive or not) have been evolving with life probably as long as the first cells were present. Because of this, there is a virus which specializes on almost every single species on the planet.
Virus Classification
Scientists classify viruses based on how they replicate their genome. Some virus genomes are made of RNA, others are made of DNA. Some viruses use a single strand, others use a double strand. The complexities involved in replicating and packaging these different molecules places viruses into seven different categories.
Class I virus genomes are made of double stranded DNA, the same as the human genome. This makes it easy for these virus molecules to use the cell’s natural machinery to produce proteins from the virus DNA. However, in order for DNA polymerase (the molecule which copies DNA) to be active the cell must be dividing. Some Class I virus molecules include sections of DNA which make the cell actively start dividing. These virus molecules can lead to cancer. Human papilloma virus is a sexually-transmitted Class I virus, and can cause cervical cancer.
A Class II virus contains only a single strand of DNA. Before it can be read by the host’s DNA polymerase enzymes, it must be converted to double stranded DNA. It does this by hijacking the host cell’s histones (DNA proteins) and DNA polymerase. Instead of waiting for the cell to divide or forcing it to, Class II virus DNA contains coding for a protein called Rep . This replication enzyme replicates the original single-stranded virus genome. Other proteins are created from the DNA and used to create protein coats with the cellular machinery. The single-stranded DNA is then packaged into these protein coats, and new virus packages are created.
Class III virus genomes are created from double-stranded RNA. While this is unusual, these virus packages come with their own protein, RNA polymerase . This protein can create messenger RNA (mRNA) from the double-stranded virus RNA. The virus RNA therefore stays within the virus capsule, and only the mRNA enters the cytoplasm of the host. Here, the mRNA is converted into proteins, some of which include more RNA polymerase. This RNA polymerase creates a new double-stranded RNA, which is encapsulated by the proteins and released from the cell.
Class IV viruses are single-stranded RNA, almost identical to mRNA produced by the host cell. With these viruses the entire protein coat is engulfed by an uninfected host cell. The small RNA genome escapes the protein coat, and makes its way into the cytoplasm. This one mRNA-like strand codes for a large polyprotein , which will be created by the hosts ribosomes . The polyprotein naturally breaks into different parts. Some create protein coats, while others read and replicate the original strand of viral RNA. The virus continues to replicate and create new, fully packed virus particles. When the cell is completely full, it ruptures and releases the virus particles into the blood or environment. Up to 10,000 virus particles can be release from a single cell.
The virus genomes in Class V are also single-stranded RNA. However, they run in the opposite direction from normal mRNA. Therefore, the cell’s machinery cannot read them directly. These virus molecules contain a RNA polymerase molecule which can read in reverse. These virus molecules have large capsules, surrounded by cell membrane and proteins. When the virus approaches a cell, its membrane proteins bind with the cell, and it is drawn into the cytoplasm. Here, it breaks apart, releasing the backwards viral RNA and associated proteins. These small complexes produce regular mRNA, which creates new virus complexes. These unfinished complexes move to the cell surface, where they line the cell membrane with proteins they create. When they are finished, they wrap themselves in this membrane, and tear away from the cell.
Class VI virus genomes are the same as Class V, but they use a different method to replicate. Class VI virus particles are known as retroviruses . Instead of creating mRNA from the viral RNA, these virus molecules work with a different protein. Known as reverse transcriptase , this enzyme is able to create DNA from the virus RNA. In doing so, the viral RNA is converted to double-stranded DNA. This DNA then produces new virus. The DNA can incorporate with the host DNA, and in doing so become endogenized . This means that the DNA will remain in the cell as long as the cell lives. If the cell is found in a germ line , such as a sperm or egg, the virus will permanently become a part of the host’s genome. It is estimated that 5-8% of the human genome is left over retrovirus DNA.
The final class, Class VII, includes the pararetroviruses . Similar to Class VI, these virus genomes use reverse transcriptase. However, these virus genomes are package as DNA, not RNA. These viruses insert themselves directly into the host genome, which begins transposing the viral DNA into RNA. Most of this RNA will be mRNA, used to create a polyprotein. Part of the polyprotein is reverse transcriptase. This reverse transcriptase works on pieces of RNA known as pregenome . It reads these RNA molecules and produces the original virus DNA. This is then packaged into viral protein coats. Class VII viruses are often found in plants, and can travel between cells using the plasmodesmata , or they can be carried by herbivorous insects feeding on the plants. Aphids carry many plant diseases, as their proboscis pierces plant cell walls and they drink the cytoplasm.
Examples of a Virus
Polio virus.
The Polio virus, which crippled President Franklin Roosevelt, is a Class III virus. This double-stranded RNA virus encodes for 12 proteins. Like other Class III virus genomes, it reproduces by releasing mRNA strands into the cytosol of host cells, which code for new virus molecules. Interestingly, the polio virus was not deadly, until people started treating their water. Before chlorinated water, polio survived in most water sources. Thus, most infants were exposed to polio right off the bat.
In infants, there are usually no symptoms of polio, and the immune system responds to the virus. However, after chlorinated water was established, most children did not experience polio. However, the disease was not eradicated. Many people were exposed in adulthood to pockets of polio which still persisted. These people suffered greatly from the disease, as the immune system did not react quickly enough to it. Like FDR, they were usually permanently crippled from the effects of the virus on bone health. Luckily the vaccine for polio, one of the first ever created, is easily made from killing live polio virus with heat. The dead protein coats allow the body to develop an immunity to the virus, without cells being infected.
Rabies Virus
The rabies virus is a Class V virus, with a bullet-shaped protein coat. This virus is made of linear, single-stranded RNA. The rabies virus genome codes for five proteins, from 12,000 nucleotides. Interestingly, the symptoms of rabies in many animals include increased aggression. This trait, caused by where the virus attacks and the damage it does, causes animals to bite other animals more often than they normally would. The assembled rabies virus particles accumulate in the saliva. Thus, when an infected animal bites another one the virus is passed to the new animal.
Rabies virus is almost always fatal in humans, if not treated immediately. Yearly, there are nearly 15 million post-exposure vaccinations given for rabies. The vaccine essentially loads the body with the dead virus, allowing a large immune response against the virus. This can stop the virus before it gets established in the system. If this happens, there is little chance of recovery. Dogs are commonly vaccinated pre-exposure, which provides a general protection to their owners on the chance they are bitten by an animal infected with the virus.
Nelson, D. L., & Cox, M. M. (2008). Principles of Biochemistry. New York: W.H. Freeman and Company. Roossinck, M. J. (2016). Virus. Princeton: Princeton University Press. Widmaier, E. P., Raff, H., & Strang, K. T. (2008). Vander’s Human Physiology: The Mechanisms of Body Function (11th ed.). Boston: McGraw-Hill Higher Education.
Cite This Article
Subscribe to our newsletter, privacy policy, terms of service, scholarship, latest posts, white blood cell, t cell immunity, satellite cells, embryonic stem cells, popular topics, animal cell, scientific method, pituitary gland, hermaphrodite, water cycle.

8 Introduction to Viruses
Viruses are typically described as obligate intracellular parasites , acellular infectious agents that require the presence of a host cell in order to multiply. Viruses that have been found to infect all types of cells – humans, animals, plants, bacteria, yeast, archaea, protozoa…some scientists even claim they have found a virus that infects other viruses! But that is not going to happen without some cellular help.
Virus Characteristics
Viruses can be extremely simple in design, consisting of nucleic acid surrounded by a protein coat known as a capsid . The capsid is composed of smaller protein components referred to as capsomers . The capsid+genome combination is called a nucleocapsid .
Viruses can also possess additional components, with the most common being an additional membranous layer that surrounds the nucleocapsid, called an envelope . The envelope is actually acquired from the nuclear or plasma membrane of the infected host cell, and then modified with viral proteins called peplomers . Some viruses contain viral enzymes that are necessary for infection of a host cell and coded for within the viral genome. A complete virus, with all the components needed for host cell infection, is referred to as a virion .
Virus Genome
While cells contain double-stranded DNA for their genome, viruses are not limited to this form. While there are dsDNA viruses, there are also viruses with single-stranded DNA ( ssDNA ), double-stranded RNA ( dsRNA ), and single-stranded RNA ( ssRNA ). In this last category, the ssRNA can either positive-sense ( +ssRNA , meaning it can transcribe a message, like mRNA) or it can be negative-sense ( -ssRNA , indicating that it is complementary to mRNA). Some viruses even start with one form of nucleic acid in the nucleocapsid and then convert it to a different form during replication.
Virus Structure
Viral nucleocapsids come in two basic shapes, although the overall appearance of a virus can be altered by the presence of an envelope, if present. Helical viruses have an elongated tube-like structure, with the capsomers arranged helically around the coiled genome. Icosahedral viruses have a spherical shape, with icosahedral symmetry consisting of 20 triangular faces. The simplest icosahedral capsid has 3 capsomers per triangular face, resulting in 60 capsomers for the entire virus. Some viruses do not neatly fit into either of the two previous categories because they are so unusual in design or components, so there is a third category known as complex viruses . Examples include the poxvirus with a brick-shaped exterior and a complicated internal structure, as well as bacteriophage with tail fibers attached to an icosahedral head.
Virus Replication Cycle
While the replication cycle of viruses can vary from virus to virus, there is a general pattern that can be described, consisting of five steps:
- Attachment – the virion attaches to the correct host cell.
- Penetration or Viral Entry – the virus or viral nucleic acid gains entrance into the cell.
- Synthesis – the viral proteins and nucleic acid copies are manufactured by the cells’ machinery.
- Assembly – viruses are produced from the viral components.
- Release – newly formed virions are released from the cell.
Outside of their host cell, viruses are inert or metabolically inactive. Therefore, the encounter of a virion to an appropriate host cell is a random event. The attachment itself is highly specific, between molecules on the outside of the virus and receptors on the host cell surface. This accounts for the specificity of viruses to only infect particular cell types or particular hosts.
Penetration or Viral Entry
Many unenveloped (or naked ) viruses inject their nucleic acid into the host cell, leaving an empty capsid on the outside. This process is termed penetration and is common with bacteriophage, the viruses that infect bacteria. With the eukaryotic viruses, it is more likely for the entire capsid to gain entrance into the cell, with the capsid being removed in the cytoplasm. An unenveloped eukaryotic virus often gains entry via endocytosis , where the host cell is compelled to engulf the capsid resulting in an endocytic vesicle, allowing the virus access to the cell contents. An enveloped eukaryotic virus gains entrance for its nucleocapsid through membrane fusion , where the viral envelope fuses with the host cell membrane, pushing the nucleocapsid past the cell membrane. If the entire nucleocapsid is brought into the cell then there is an uncoating process to strip away the capsid and release the viral genome.
The synthesis stage is largely dictated by the type of viral genome, since genomes that differ from the cell’s dsDNA genome can involve intricate viral strategies for genome replication and protein synthesis. Viral specific enzymes, such as RNA-dependent RNA polymerases, might be necessary for the replication process to proceed. Protein production is tightly controlled, to insure that components are made at the right time in viral development.
The complexity of viral assembly depends upon the virus being made. The simplest virus has a capsid composed of 3 different types of proteins, which self-assembles with little difficulty. The most complex virus is composed of over 60 different proteins, which must all come together in a specific order. These viruses often employ multiple assembly lines to create the different viral structures and then utilize scaffolding proteins to put all the viral components together in an organized fashion.
The majority of viruses lyse their host cell at the end of replication, allowing all the newly formed virions to be released to the environment. Another possibility, common for enveloped viruses, is budding , where one virus is released from the cell at a time. The cell membrane is modified by the insertion of viral proteins, with the nucleocapsid pushing out through this modified portion of the membrane, allowing it to acquire an envelope.
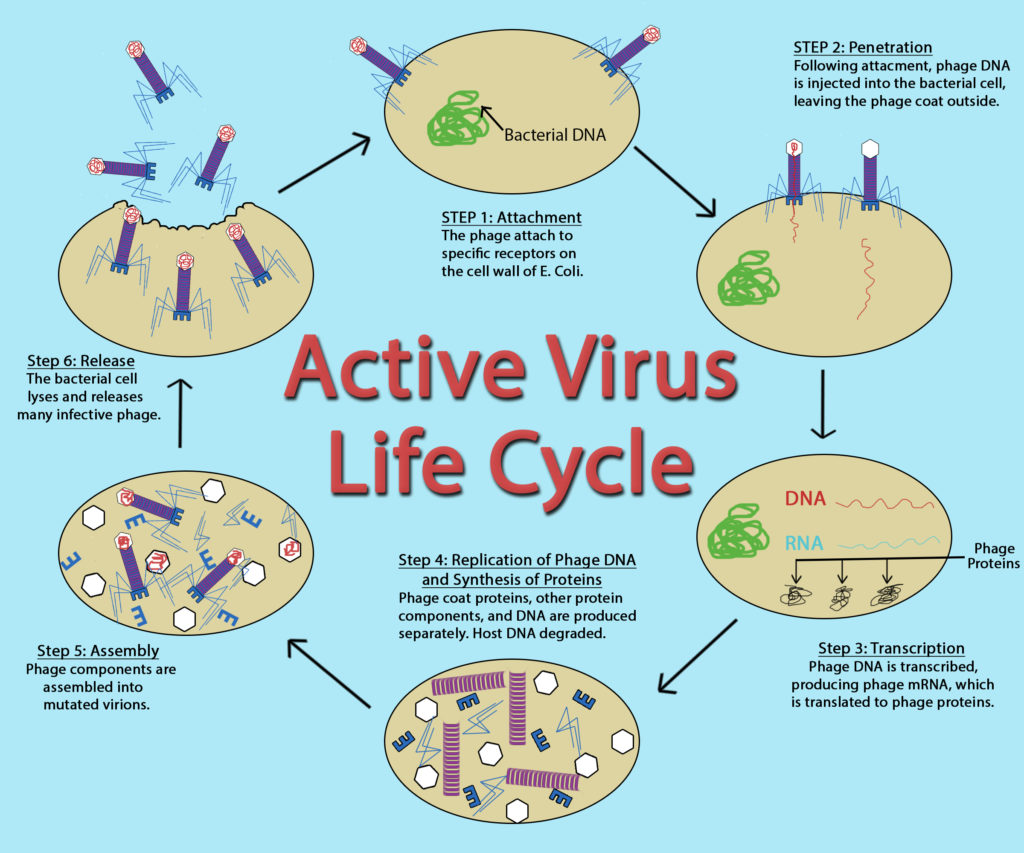
Bacteriophage
Viruses that infect bacteria are known as bacteriophage or phage . A virulent phage is one that always lyses the host cell at the end of replication, after following the five steps of replication described above. This is called the lytic cycle of replication.
There are also temperate phage , viruses that have two options regarding their replication. Option 1 is to mimic a virulent phage, following the five steps of replication and lysing the host cell at the end, referred to as the lytic cycle. But temperate phage differ from virulent phage in that they have another choice: Option 2, where they remain within the host cell without destroying it. This process is known as lysogeny or the lysogenic cycle of replication.
A phage employing lysogeny still undergoes the first two steps of a typical replication cycle, attachment and penetration. Once the viral DNA has been inserted into the cell it integrates with the host DNA, forming a prophage . The infected bacterium is referred to as a lysogen or lysogenic bacterium . In this state, the virus enjoys a stable relationship with its host, where it does not interfere with host cell metabolism or reproduction. The host cell enjoys immunity from reinfection from the same virus.
Exposure of the host cell to stressful conditions (i.e. UV irradiation) causes induction , where the viral DNA excises from the host cell DNA. This event triggers the remaining steps of the lytic cycle, synthesis, maturation, and release, leading to lysis of the host cell and release of newly formed virions.
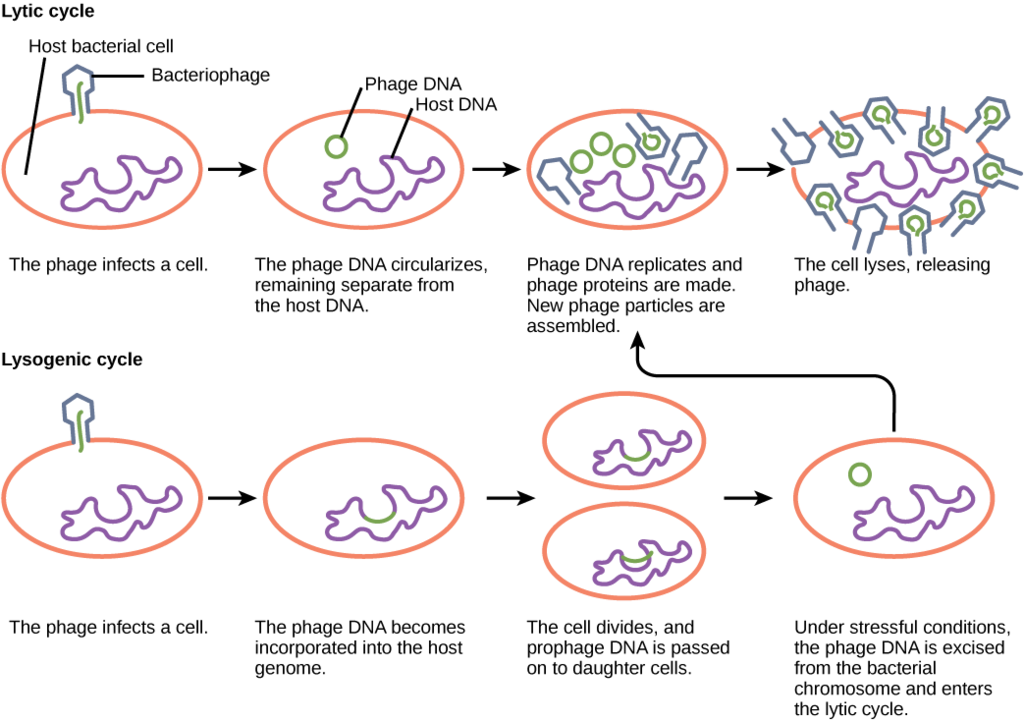
So, what dictates the replication type that will be used by a temperate phage? If there are plenty of host cells around, it is likely that a temperate phage will engage in the lytic cycle of replication, leading to a large increase in viral production. If host cells are scarce, a temperate phage is more likely to enter lysogeny, allowing for viral survival until host cell numbers increase. The same is true if the number of phage in an environment greatly outnumber the host cells, since lysogeny would allow for host cells numbers to rebound, ensuring long term viral survival.
Lysogens can experience a benefit from lysogeny as well, since it can result in lysogenic conversion , a situation where the development of a prophage leads to a change in the host’s phenotype. One of the best examples of this is for the bacterium Corynebacterium diphtheriae , the causative agent of diphtheria. The diphtheria toxin that causes the disease is encoded within the phage genome, so only C. diphtheriae lysogens cause diphtheria.
Eukaryotic Viruses
Eukaryotic viruses can cause one of four different outcomes for their host cell. The most common outcome is host cell lysis, resulting from a virulent infection (essentially the lytic cycle of replication seen in phage). Some viruses can cause a latent infection , inserting their viral DNA into the host cell genome, allowing them to co-exist peacefully with their host cells for long periods of time (much like a temperate phage during lysogeny). Some enveloped eukaryotic viruses can also be released one at a time from an infected host cell, in a type of budding process, causing a persistent infection . Lastly, certain eukaryotic viruses can cause the host cell to transform into a malignant or cancerous cell, a mechanism known as transformation .
Viruses and Cancer
There are many different causes of cancer, or unregulated cell growth and reproduction. Some known causes include exposure to certain chemicals or UV light. There are also certain viruses that have a known associated with the development of cancer. Such viruses are referred to as oncoviruses . Oncoviruses can cause cancer by producing proteins that bind to host proteins known as tumor suppressor proteins , which function to regulate cell growth and to initiate programmed cell death, if needed. If the tumor suppressor proteins are inactivated by viral proteins then cells grow out of control, leading to the development of tumors and metastasis, where the cells spread throughout the body.
virus, obligate intracellular parasite, capsid, bacteriophage, capsomere, nucleocapsid, envelope, peplomer, virion, dsDNA, ssDNA, dsRNA, +ssRNA, -ssRNA, helical viruses, icosahedral viruses, complex viruses, attachment, penetration, viral entry, synthesis, assembly, release, naked virus, endocytosis, membrane fusion, budding, bacteriophage, phage, virulent phage, lytic cycle, temperate phage, lysogeny, lysogenic cycle, prophage, lysogen, lysogenic bacterium, induction, lysogenic conversion, virulent infection, latent infection, persistent infection, transformation, oncovirus, tumor suppressor proteins.
Study Questions
- What are the general properties of a virus?
- What is the size range of viruses? How do they compare, size-wise, to bacteria?
- What is the general structure of viruses? What are the different components?
- What viral shapes exist?
- How do envelopes and enzymes relate to viruses?
- What types of viral genomes exist?
- What are the 5 basic steps of viral replication? What happens at each step? How do bacterial/archaeal viruses differ from eukaryotic viruses, in regards to replication details?
- What are the 2 types of viral infection found in Bacteria/Archaea ? What are the specific terms associated with viral infection of bacterial/archaeal cells?
- What are the 4 types of viral infection found in eukaryotes?
- How do some viruses cause cancer?
Exploratory Questions (OPTIONAL)
- What is the largest bacterium or archaean ever discovered? What is the smallest eukaryote ever discovered?
General Microbiology Copyright © 2019 by Linda Bruslind is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Properties of viruses
- no membranes, cytoplasm, ribosomes, or other cellular components
- they cannot move or grow
- they can only reproduce inside a host cell
- 2 major parts - a protein coat, and DNA or RNA
- they are extremely tiny, smaller than cells and only visible with electron microscopes
Review the structure of DNA
RNA is similar to DNA Instead of thymine, it has uracil It has the sugar ribose, instead of deoxyribose It is single stranded
Shape of a double helix, repeating units of nucleotides
Base pairs held together by hydrogen bonds (weak)
Adenine -----|-----Thymine Guanine -----|---- Cytosine
The sides of the DNA made of alternating deoxyribose (5 ring sugar) and phosphates
Virus Structure
Virus has a covering that has a capsid and sometimes an envelope Inner core contains a nucleic acid molecule (DNA or RNA) and various proteins
Viruses are usually very specific to their host and to the cells they can infect.
Viral Reproduction
See Video on How a Virus Invades Your Body (NPR)
Lytic cycle = reproduction occurs, cells burst Lysogenic cycle = reproduction does not immediately occur (dormancy)
2. Penetration - the virus is engulfed by the cell (Cell can enter Lysogenic or Lytic Cycle)
3 . Biosynthesis - viral components are made (protein coat, capsid, DNA/RNA)
4. Maturation - assembly of viral components
5. Release - viruses leave host cell to infect new cells (often destroys host)
Types of Viruses
Bacteriophage - viruses that infect bacteria.
Retroviruses -- RNA viruses that have a DNA stage
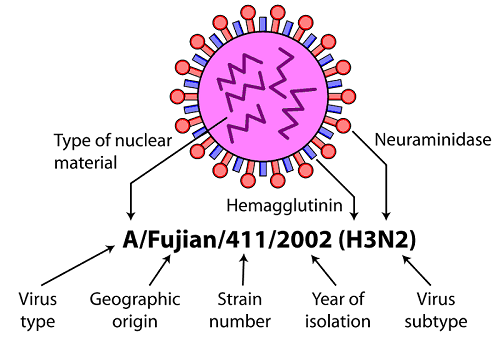
How Are Viruses Named
Historically they have been named for a variety of factors, including
- the associated diseases (poliovirus, rabies) the type of disease caused (murine leukemia virus)
- the sites in the body affected or from which the virus was first isolated (rhinovirus, adenovirus)
- where they were first isolated (Ebola virus, Hantavirus)
- the animal that carries the virus (bird flu, swine flu)
- for the way people imagined they were contracted (dengue = ‘evil spirit’; influenza = ‘influence’ of bad air).
Newer Conventions
Focus on Human Immunodeficiency Virus
- Causes the disease AIDS (Acquired Immune Deficiency Syndrome)
HIV Infection Cycle (animation) | HIV Life Cycle - drugs target specific viral processes HIV Coloring Assignment *Make sure you understand the steps involved in infection and how drugs treat the disease.
Related to Viruses
Viroids - even smaller than viruses, consist of RNA strands that lack a protein coat Prions - "rogue protein", believed to be the cause of Mad Cow Disease, also may cause Kuru in cannibal tribes
Treatment of Viruses
Vaccines Antiviral Drugs
DNA Image by WPClipart
Browse Course Material
Course info, instructors.
- Prof. Barbara Imperiali
- Prof. Adam Martin
- Dr. Diviya Ray
Departments
As taught in.
- Functional Genomics
- Biochemistry
- Cell Biology
- Microbiology
- Molecular Biology

Learning Resource Types
Introductory biology, lecture 32: infectious disease, viruses, and bacteria, description.
This lecture covers microorganisms and some of the threats they pose to human health, such as infectious diseases. Professor Imperiali also discusses antibiotics and the mechanisms by which bacteria become resistant.
Instructor: Barbara Imperiali
- Download video
- Download transcript

Biology of Viruses and Viral Diseases
Viruses exact an enormous toll on the human population and are the single most important cause of infectious disease morbidity and mortality worldwide. Viral diseases in humans were first noted in ancient times and have since shaped our history. Scientific approaches to the study of viruses and viral disease began in the 19th century and led to the identification of specific disease entities caused by viruses. Careful clinical observations enabled the identification of many viral illnesses and allowed several viral diseases to be differentiated (e.g., smallpox vs. chickenpox and measles vs. rubella). Progress in an understanding of disease at the level of cells and tissues, exemplified by the pioneering work of Virchow, allowed the pathology of many viral diseases to be defined. Finally, the work of Pasteur ushered in the systematic use of laboratory animals for studies of the pathogenesis of infectious diseases, including those caused by viruses.
The first viruses were identified as the 19th century ended. Ivanovsky and Beijerinck identified tobacco mosaic virus, and Loeffler and Frosch discovered foot-and-mouth disease virus. These observations were quickly followed by the discovery of yellow fever virus and the seminal research on the pathogenesis of yellow fever by Walter Reed and the U. S. Army Yellow Fever Commission. 1 By the end of the 1930s, tumor viruses, bacteriophages, influenza virus, mumps virus, and many arthropod-borne viruses had been identified. This process of discovery has continued with growing momentum to the present, with recently identified skin cancer–associated Merkel cell polyomavirus, 2 novel Old World arenaviruses causing fatal disease, 3 , 4 bat-related respiratory coronavirus 5 and reoviruses, 6 , 7 and novel swine- and avian-origin influenza viruses 8 , 9 counted among the most recent entries in the catalog of human disease-causing viruses.
In the 1940s, Delbruck, Luria, and others 10 , 11 used bacteriophages as models to establish many basic principles of microbial genetics and molecular biology and identified key steps in viral replication. The pioneering experiments of Avery, MacLeod, and McCarty 12 on the transformation of pneumococci established DNA as the genetic material and set the stage for corroborating experiments by Hershey and Chase using bacteriophages. 13 In the late 1940s, Enders and colleagues 14 cultivated poliovirus in tissue culture. This accomplishment led to the development of both formalin-inactivated (Salk) 15 and live-attenuated (Sabin) 16 vaccines for polio and ushered in the modern era of experimental and clinical virology.
In recent years, x-ray crystallography has allowed visualization of virus structures at an atomic level of resolution. Nucleotide sequences of entire genomes of most human viruses are known, and functional domains of many viral structural and enzymatic proteins have been defined. This information is being applied to the development of new strategies to diagnose viral illnesses and design effective antiviral therapies. Techniques to detect viral genomes, such as the polymerase chain reaction (PCR) and its derivatives, have proven superior to conventional serologic assays and culture techniques for the diagnosis of many viral diseases. Nucleic acid–based strategies are now used routinely in the diagnosis of infections caused by enteroviruses, hepatitis B virus (HBV), hepatitis C virus (HCV), herpesviruses, human immunodeficiency virus (HIV), and, with increasing frequency, respiratory and enteric viral pathogens. Furthermore, rapid developments in mass spectrometry and nucleotide sequencing technology are permitting the application of these tools to highly sensitive and specific virus detection in clinical specimens.
Perhaps an even more exciting development is the means to introduce new genetic material into viral genomes. Strategies now exist whereby specific mutations or even entire genes can be inserted into the genomes of many viruses. Such approaches can be exploited in the rational design of vaccines and the development of viral vectors for use in gene delivery. Furthermore, these powerful new techniques are leading to breakthroughs in foundational problems in viral pathogenesis, such as the nature of virus–cell interactions that produce disease, immunoprotective and immunopathologic host responses to infection, and viral and host determinants of contagion. Improved understanding of these aspects of viral infection will facilitate new approaches to the prevention, diagnosis, and treatment of viral diseases.
Virus Structure and Classification
The first classification of viruses as a group distinct from other microorganisms was based on the capacity to pass through filters of a small pore size (filterable agents). Initial subclassifications were based primarily on pathologic properties such as specific organ tropism (e.g., hepatitis viruses) or common epidemiologic features such as transmission by arthropod vectors (e.g., arboviruses). Current classification systems are based on the following: (1) the type and structure of the viral nucleic acid and the strategy used in its replication; (2) the type of symmetry of the virus capsid (helical vs. icosahedral); and (3) the presence or absence of a lipid envelope ( Table 134-1 ).
TABLE 134-1
Classification of Viruses
| FAMILY | EXAMPLE | TYPE OF NUCLEIC ACID | GENOME SIZE (kb or kb pair) | ENVELOPE | CAPSID SYMMETRY |
|---|---|---|---|---|---|
| Picornaviridae | Poliovirus | SS (+) RNA | 7-9 | No | I |
| Astroviridae | Astrovirus | SS (+) RNA | 6-7 | No | I |
| Caliciviridae | Norwalk virus | SS (+) RNA | 7-8 | No | I |
| Togaviridae | Rubella virus | SS (+) RNA | 10-12 | Yes | I |
| Flaviviridae | Yellow fever virus | SS (+) RNA | 10-12 | Yes | S |
| Coronaviridae | Coronavirus | SS (+) RNA | 28-31 | Yes | H |
| Rhabdoviridae | Rabies virus | SS (−) RNA | 11-15 | Yes | H |
| Paramyxoviridae | Measles virus | SS (−) RNA | 13-18 | Yes | H |
| Filoviridae | Ebola virus | SS (−) RNA | 19 | Yes | H |
| Arenaviridae | Lymphocytic choriomeningitis virus | 2 SS (ambisense) RNA segments | 11 | Yes | S |
| Bunyaviridae | California encephalitis virus | 3 SS (ambisense) RNA segments | 11-19 | Yes | H |
| Orthomyxoviridae | Influenza virus | 6-8 SS (−) RNA segments | 10-15 | Yes | H |
| Reoviridae | Rotavirus | 10-12 DS RNA segments | 19-32 | No | I |
| Retroviridae | HIV | 2 identical SS (+) RNA segments | 7-13 | Yes | S |
| Hepadnaviridae | Hepatitis B virus | Circular DS DNA with SS portions | 3-4 | Yes | I |
| Parvoviridae | Human parvovirus B19 | SS (+) or (−) DNA | 4-6 | No | I |
| Polyomaviridae | JC virus | Circular DS DNA | 5 | No | I |
| Papillomaviridae | Human papillomavirus | Circular DS DNA | 7-8 | No | I |
| Adenoviridae | Adenovirus | Linear DS DNA | 26-45 | No | I |
| Herpesviridae | Herpes simplex virus | Linear DS DNA | 125-240 | Yes | I |
| Poxviridae | Vaccinia virus | Linear DS DNA | 130-375 | Yes | Complex |
(+), message sense; (−), complement of message sense; DS, double-stranded; H, helical; I, icosahedral; S, spherical; SS, single-stranded.
Virus particles—virions—can be functionally conceived as a delivery system that surrounds a payload. The delivery system consists of structural components used by the virus to survive in the environment and bind to host cells. The payload contains the viral genome and often includes enzymes required for the initial steps in viral replication. In almost all cases, the delivery system must be removed from the virion to allow viral replication to commence.
In addition to mediating attachment to host cells, the delivery system also plays a crucial role in determining the mode of transmission between hosts. Viruses containing lipid envelopes are sensitive to desiccation in the environment and, for the most part, are transmitted by the respiratory, parenteral, and sexual routes. Nonenveloped viruses are stable to harsh environmental conditions and are often transmitted by the fecal-oral route.
Viral genomes exist in a variety of forms and sizes and consist of RNA or DNA (see Table 134-1 ). Animal virus genomes range in size from 3 kb, encoding only three or four proteins in small viruses such as the hepadnaviruses, to more than 300 kb, encoding several hundred proteins in large viruses such as the poxviruses. Viral genomes are single- or double-stranded and circular or linear. RNA genomes are composed of a single molecule of nucleic acid or multiple discrete segments, which can vary in number from as few as two in the arenaviruses up to 12 in some members of the Reoviridae. Viral nucleic acid is packaged in a protein coat, or capsid, that consists of multiple protein subunits. The combination of the viral nucleic acid and the surrounding protein capsid is often referred to as the nucleocapsid ( Fig. 134-1 ).

Schematic diagrams illustrating the structure of a nonenveloped icosahedral virus (A) and an enveloped helical virus (B). Nucleocapsid: combination of a viral nucleic acid and surrounding protein capsid.
Structural details of many viruses have now been defined at an atomic level of resolution ( Fig. 134-2 ). General features of virus structure can be gained from examination of electron micrographs of negatively stained virions and thin-section electron micrographs of virus-infected tissues and cultured cells. These techniques allow rapid identification of viral size, shape, symmetry, and surface features, presence or absence of an envelope, and intracellular site of viral assembly. Cryoelectron microscopy and computer image processing techniques are used to determine the three-dimensional structures of spherical viruses at a level of resolution far superior to that of negatively stained electron micrographs. A major advantage of cryoelectron microscopy is that it allows structural studies of viruses to be performed under conditions that do not alter native virion structure. Moreover, recent advances in cryoelectron microscopy have extended the achievable resolution of particle-associated proteins to near-atomic levels, sufficient to recognize characteristic features of secondary structural elements. 17 Image reconstructions of cryoelectron micrographs, sometimes in combination with x-ray crystallography, can also be used to investigate structural aspects of various virus functions, including receptor binding 18 , 19 , 20 and interaction with antibodies. 21 , 22 Identification of key motifs, such as receptor binding sites or immunodominant domains, provides the framework for understanding the structural basis of virus–cell interactions. Electron tomography with image reconstruction has been applied to architectural studies of viruses and intracellular foci of virus replication, rendering exquisite three-dimensional representations of particle organization and revealing the structure and subcellular origins of virus manufacturing centers. 23 , 24

Structural studies of poliovirus.
A, Negative-stained electron micrograph. B, Three-dimensional image reconstruction of cryoelectron micrographs. C, Structure determined by x-ray crystallography.
A number of general principles have emerged from studies of virus structure. In almost all cases, the capsid is composed of a repeating series of structurally similar subunits, each of which in turn is composed of only a few different proteins. The parsimonious use of structural proteins in a repetitive motif minimizes the amount of genetic information required to encode the viral capsid and leads to structural arrangements with symmetrical features. All but the most complex viruses exhibit either helical or icosahedral symmetry (see Table 134-1 ). Viruses with helical symmetry contain repeating protein subunits bound at regular intervals along a spiral formed by the viral nucleic acid. Interestingly, all known animal viruses that show this type of symmetry have RNA genomes. Viruses with icosahedral symmetry display twofold, threefold, and fivefold axes of rotational symmetry, and viral nucleic acid is intimately associated with specific capsid proteins in an ordered packing arrangement.
The use of repeating subunits with symmetrical protein-protein interactions facilitates the assembly of the viral capsid. In most cases, viral assembly appears to be a spontaneous process that occurs under the appropriate physiologic conditions and often can be reproduced when recombinant viral proteins are expressed in the absence of viral replication. 25 , 26 For many viruses, assembly of the capsid proceeds through a series of intermediates, each of which nucleates the addition of subsequent components in the assembly sequence.
One of the most poorly understood aspects of viral assembly is the process that ensures that the viral nucleic acid is correctly packaged into the capsid. In the case of viruses with helical symmetry, there may be an initiation site on the nucleic acid to which the initial capsid protein subunit binds, triggering the addition of subsequent subunits. The genomes of most DNA-containing viruses are inserted into preassembled capsid intermediates (procapsids) through adenosine triphosphate–driven mechanisms. 27 In preparations of many icosahedral viruses, empty capsids (i.e., capsids lacking nucleic acid) are frequently observed, indicating that assembly may proceed to completion without a requirement for the viral genome.
In some viruses, the nucleocapsid is surrounded by a lipid envelope acquired as the virus particle buds from the host cell cytoplasmic, nuclear, or endoplasmic reticular membrane (see Fig. 134-1 ). Inserted into this lipid bilayer are virus-encoded proteins (e.g., the hemagglutinin [HA] and neuraminidase proteins of influenza virus and gp41 and gp120 of HIV), which are exposed on the surface of the virus particle. These viral proteins usually contain a glycosylated hydrophilic external portion and internal hydrophobic domains that span the lipid membrane and anchor the protein into the viral envelope. In some cases, another viral protein, often termed a matrix protein, associates with the internal (cytoplasmic) surface of the lipid envelope, where it can interact with the cytoplasmic domains of the envelope glycoproteins. Matrix proteins may play roles in stabilizing the interaction between viral glycoproteins and the lipid envelope, directing the viral genome to intracellular sites of viral assembly, or facilitating viral budding. Matrix proteins can also influence a diverse set of cellular functions, such as inhibition of host cell transcription 28 , 29 and evasion of the cellular innate antiviral response. 30
Virus–Cell Interactions
Viruses require an intact cell to replicate and can direct the synthesis of hundreds to thousands of progeny viruses during a single cycle of infection. In contrast to other microorganisms, viruses do not replicate by binary fission. Instead, the infecting particle must disassemble in order to direct synthesis of viral progeny.
The interaction between a virus and its host cell begins with attachment of the virus particle to specific receptors on the cell surface. Viral proteins that mediate the attachment function (viral attachment proteins) include the following: single-capsid components that extend from the virion surface, such as the attachment proteins of adenovirus, 31 reovirus, 32 and rotavirus 33 , 34 ; surface glycoproteins of enveloped viruses, such as influenza virus 35 , 36 ( Fig. 134-3 ) and HIV 37 , 38 ; viral capsid proteins that form binding pockets that engage cellular receptors, such as the canyon formed by the capsid proteins of poliovirus 39 and rhinovirus 40 ; and viral capsid proteins that contain extended loops capable of binding receptors, such as foot-and-mouth disease virus. 41 Studies of the attachment of several diverse virus groups, including adenoviruses, coronaviruses, herpesviruses, lentiviruses, and reoviruses, indicate that multiple interactions between virus and cell occur during the attachment step. These observations indicate that a specific sequence of binding events between virus and cell optimizes specificity and contributes significant stability to the association. 42

The folded structure of the influenza virus hemagglutinin (HA) and its rearrangement when exposed to low pH.
A, The HA monomer. HA1 is blue, and HA2 is multicolored. The receptor-binding pocket resides in the virion-distal portion of HA1. The viral membrane would be at the bottom of this figure. B, Conformational change in HA induced by exposure to low pH. Note the dramatic structural rearrangement in HA2, in which amino acid residues 40-105 become a continuous alpha helix. Dashed lines indicate regions of undetermined structure. This model of HA in its fusion conformation is a composite of the HA1 domain structure and the low-pH HA2 structure.
One of the most dynamic areas of virology concerns the identification of virus receptors on host cells. This interest stems in part from the critical importance of the attachment step as a determinant of target cell selection by many viruses. Several virus receptors have now been identified ( Table 134-2 ), and three important principles have emerged from studies of these receptors. First, viruses have adapted to use cell surface molecules designed to facilitate a variety of normal cellular functions. Virus receptors may be highly specialized proteins with limited tissue distribution, such as complement receptors, growth factor receptors, or neurotransmitter receptors, or more ubiquitous components of cellular membranes, such as integrins and other intercellular adhesion molecules, glycosaminoglycans, or sialic acid–containing oligosaccharides. Second, many viruses use more than a single receptor to mediate multistep attachment and internalization. For example, adenovirus binds coxsackievirus and adenovirus receptor (CAR) 43 and the integrins α v β 3 or α v β 5 44 ; herpes simplex virus (HSV) binds heparan sulfate 45 , 46 , 47 and herpesvirus entry mediator (HVEM/HveA), 48 nectin 1 (PRR1/HveC), 49 or nectin 2 (PRR2/HveB) 50 ; HIV binds CD4 51 , 52 and chemokine receptors CXCR4 53 , 54 or CCR5 55 , 56 , 57 ; and reovirus binds sialylated glycans 58 , 59 and JAM-A. 60 , 61 Third, in many cases, receptor expression is not the sole determinant of viral tropism for particular cells and tissues in the host. Therefore, although receptor binding is the first step in the interaction between virus and cell, subsequent events in the viral replication cycle must also be supported for productive viral infection to occur.
TABLE 134-2
Receptors and Entry Mediators Used by Selected Human Viruses
| VIRUS | RECEPTOR |
|---|---|
| Adenovirus | Coxsackievirus and adenovirus receptor (CAR) , |
| CD46 , | |
| Integrins α β , α β | |
| Sialic acid–containing oligosaccharides | |
| Coronavirus | 9- -acetylated sialic acid–containing oligosaccharides (HCoV-OC43) |
| Aminopeptidase N (HCoV-229E) , | |
| Angiotensin-converting enzyme 2 (SARS-CoV and NL63 ) | |
| Dipeptidyl peptidase 4 (MERS-CoV) | |
| Coxsackievirus | Integrin α β |
| Decay-accelerating factor (CD55) , | |
| Coxsackievirus and adenovirus receptor (CAR) | |
| Intercellular adhesion molecule 1 (ICAM-1) | |
| GRP78/BiP | |
| Heparan sulfate | |
| Cytomegalovirus | Heparan sulfate , |
| Integrins α β , α β , α β | |
| Platelet-derived growth factor-α receptor | |
| Echovirus | Integrin α β |
| Decay accelerating factor (CD55) , | |
| Ebola virus | Niemann-Pick C1 cholesterol transporter , |
| Enterovirus 71 | P-selectin glycoprotein ligand-1 (PSGL-1) |
| Scavenger receptor B2 (SR-B2) | |
| Epstein-Barr virus | Complement receptor 2 (CD21) , |
| MHC class II protein | |
| Hantaviruses | β Integrins |
| Henipaviruses | Ephrin-B2 , |
| Hepatitis A virus | Mucin-like protein TIM-1 |
| Hepatitis C virus | CD81 , |
| Scavenger receptor B1 (SRB1) , | |
| Claudin | |
| Occludin | |
| Herpes simplex virus | Heparan sulfate , , |
| Herpesvirus entry mediator (HVEM/HveA) | |
| Nectin 1 (PRR1/HveC) | |
| Nectin 2 (PRR2/HveB) | |
| Human immunodeficiency virus | CD4 , |
| Chemokine receptor CXCR4 , | |
| Chemokine receptor CCR5 , , | |
| Human metapneumovirus | Integrin α β |
| Human T-cell leukemia virus | Glucose transporter GLUT-1 |
| Neuropilin-1 | |
| Influenza virus | Sialic acid–containing oligosaccharides , |
| JC polyomavirus | Serotonin receptor 5HT2A |
| LSTc pentasaccharide | |
| Kaposi sarcoma herpesvirus | Integrin α β |
| Measles virus | CD46 , |
| Signaling lymphocyte-activation molecule (SLAM) | |
| Nectin-4 , | |
| New World hemorrhagic fever arenaviruses (e.g., Junin virus) | Transferrin receptor 1 |
| Norovirus | Histo-blood group antigens , |
| Old World hemorrhagic fever arenaviruses (e.g., Lassa fever virus) | α-Dystroglycan |
| Parvovirus B19 | Erythrocyte P antigen (globoside) |
| Poliovirus | Poliovirus receptor (PVR, CD155) |
| Rabies virus | Neural cell adhesion molecule (CD56) |
| Nerve growth factor receptor (P75NTR) | |
| Reovirus | Sialic acid–containing oligosaccharides , |
| Junctional adhesion molecule-A (JAM-A) | |
| β integrins | |
| Rhinovirus (major group) | Intercellular adhesion molecule 1 (ICAM-1) , , |
| Rhinovirus (minor group) | Low-density lipoprotein receptor |
| Rotavirus | Sialic acid–containing oligosaccharides , |
| Integrins α β , α β , α β , α β , | |
| Rubella virus | Myelin oligodendrocyte glycoprotein (MOG) |
| Sindbis virus | Natural resistance–associated macrophage protein (NRAMP) |
Several viruses bind receptors expressed at regions of cell-cell contact. 62 Junctional adhesion molecule-A (JAM-A), which serves as a receptor for reovirus 60 and feline calicivirus, 63 and CAR, which serves as a receptor for some coxsackieviruses and adenoviruses, 43 are expressed at tight junctions 64 , 65 and adherens junctions. 66 , 67 Junctional regions are sites of enhanced membrane recycling, endocytic uptake, and intracellular signaling. 68 Therefore, it is possible that viruses have selected junction-associated proteins as receptors to usurp the physiologic functions of these molecules. In this regard, interactions of coxsackievirus with decay-accelerating factor elicit a tyrosine kinase–based signaling cascade that mediates subsequent interactions of the virus with CAR in tight junctions. 69 Structures of viral proteins or whole viral particles in complex with sialic acid have been determined for some viruses, including the influenza virus hemagglutinin (HA) 36 , 70 (see Fig. 134-3 ), polyomavirus, 71 , 72 , 73 , 74 foot-and-mouth disease virus, 75 reovirus attachment protein σ1, 58 , 59 and the VP8 domain of rotavirus capsid protein VP4. 34 Sialic acid binding in each of these cases occurs in a shallow groove at the surface of the viral protein. However, the architectures of the binding sites differ. Structures of complexes of viral proteins or viral particles and cell surface protein receptors have also been determined. These include adenovirus fiber knob and CAR, 76 Epstein-Barr virus (EBV) gp42 and major histocompatibility complex (MHC) class II protein, 77 HSV glycoprotein D and HVEM/HveA, 78 HIV gp120 and CD4, 38 measles virus HA and CD46 79 and SLAM (signaling lymphocyte-activation molecule), 80 reovirus σ1 and JAM-A, 61 and rhinovirus and ICAM-1 (intercellular adhesion molecule 1). 81 In several of these cases, the viral attachment proteins engage precisely the same domains used by their cognate receptors to bind natural ligands.
Penetration and Disassembly
Once attachment has occurred, the virus must penetrate the cell membrane, and the capsid must undergo a series of disassembly steps (uncoating) that prepare the virus for the next phases in viral replication. Enveloped viruses such as the paramyxoviruses and retroviruses enter cells by fusion of the viral envelope with the cell membrane ( Fig. 134-4 ). Attachment of these viruses to the cell surface induces changes in viral envelope proteins required for membrane fusion. For example, the binding of CD4 and certain chemokine receptors by HIV envelope glycoprotein gp120 induces a series of conformational changes in gp120 that lead to the exposure of transmembrane protein gp41. 82 , 83 Fusion of viral and cellular membranes proceeds through subsequent interactions of the hydrophobic gp41 fusion peptide with the cell membrane. 84 , 85 , 86 , 87

Mechanisms of viral entry into cells.
Nonenveloped (A) and enveloped (B) virus internalization by receptor-mediated endocytosis.
Other viruses enter cells by some form of receptor-mediated endocytic uptake (see Fig. 134-4 ). For several viruses, virus–receptor complexes induce formation of clathrin-coated pits that invaginate from the cell membrane to form coated vesicles. 88 These vesicles are rapidly uncoated and fuse with early endosomes, which sort internalized proteins for recycling to the cell surface or other cellular compartments, such as late endosomes or lysosomes. For other viruses, virus–receptor complexes are taken into cells by caveolae in lipid rafts. 88 Enveloped viruses such as dengue virus, 89 influenza virus, 90 and Semliki Forest virus 91 exploit the acidic environment of the endocytic compartment to induce conformational changes in surface glycoproteins required for membrane fusion. High-resolution structures of the influenza virus HA at acidic pH illustrate a dramatic conformational alteration leading to the fusion-active state (see Fig. 134-3 ). 90
Endocytic uptake and acidification are also required for entry of some nonenveloped viruses such as adenovirus, 92 , 93 parvovirus, 94 and reovirus. 95 , 96 In these cases, acidic pH may facilitate disassembly of the viral capsid to enable subsequent penetration of endosomal membranes. In addition to acidic pH, endocytic cathepsin proteases are required for disassembly of several viruses, including Ebola virus, 97 Hendra virus, 98 reovirus, 99 and severe acute respiratory syndrome (SARS) coronavirus. 100
In contrast to enveloped viruses, nonenveloped viruses cross cell membranes using mechanisms that do not involve membrane fusion. This group of viruses includes several human pathogens, with adenoviruses, picornaviruses, and rotaviruses serving as prominent examples. Despite differences in genome and capsid composition, each of these viruses must penetrate cell membranes to deliver the genetic payload to the interior of the cell. Capsid rearrangements triggered by receptor binding, 101 , 102 acidic pH, 92 , 93 or proteolysis 103 , 104 serve essential functions in membrane penetration by some nonenveloped viruses. Although a precise understanding of the biochemical mechanisms that underlie viral membrane penetration is incomplete, small capsid proteins of several nonenveloped viruses, such as adenovirus, 105 poliovirus, 106 and reovirus, 107 are required for membrane penetration, perhaps by forming pores in host cell membranes.
Genome Replication
Once a virus has entered a target cell, it must replicate its genome and proteins. Replication strategies used by single-stranded RNA-containing viruses depend on whether the genome can be used as messenger (m)RNA. Translation-competent genomes, which include those of the coronaviruses, flaviviruses, picornaviruses, and togaviruses, are termed plus (+) sense and are translated by cellular ribosomes immediately following entry of the genome into the cytoplasm. For most viruses containing (+) sense RNA genomes, translation results in the synthesis of a large polyprotein that is cleaved into several smaller proteins through the action of viral and sometimes host proteases. One of these proteins is an RNA-dependent RNA polymerase (RdRp), which replicates the viral RNA. Genome replication of (+) sense RNA-containing viruses requires synthesis of a minus (–) sense RNA intermediate, which serves as template for production of (+) sense genomic RNA.
A different strategy is used by viruses containing (−) sense RNA genomes. The genomes of these viruses, which include the filoviruses, orthomyxoviruses, paramyxoviruses, and rhabdoviruses, cannot serve directly as mRNA. Therefore, viral particles must contain a co-packaged RdRp to transcribe (+) sense mRNAs using the (−) sense genomic RNA as template. Genome replication of (−) sense RNA-containing viruses requires synthesis of a (+) sense RNA intermediate, which serves as a template for production of (−) sense genomic RNA. Mechanisms that determine whether (+) sense RNAs are used as templates for translation or genome replication are not well understood.
RNA-containing viruses belonging to the family Reoviridae have segmented double-stranded (ds) RNA genomes. The innermost protein shell of these viruses (termed a single-shelled particle or core ) contains an RdRp that catalyzes the synthesis of (+) sense mRNA using as a template the (−) sense strand of each dsRNA segment. The mRNAs of these viruses are capped at their 5′-termini by virus-encoded enzymes and then extruded into the cytoplasm through channels in the single-shelled particle. 108 The (+) sense mRNAs also serve as a template for replication of dsRNA gene segments. Viral genome replication is thus completely conservative; neither strand of parental dsRNA is present in newly formed genomic segments.
The retroviruses are RNA-containing viruses that replicate using a DNA intermediate. The viral genomic RNA is (+) sense and single stranded; however, it does not serve as mRNA following viral entry. Instead, the retrovirus RNA genome is a template for synthesis of a double-stranded DNA copy, termed the provirus. Synthesis of the provirus is mediated by a virus-encoded RNA-dependent DNA polymerase or reverse transcriptase, so named because of the reversal of genetic information from RNA to DNA. The provirus translocates to the nucleus and integrates into host DNA. Expression of this integrated DNA is regulated for the most part by cellular transcriptional machinery. However, the human retroviruses HIV and human T-cell leukemia virus (HTLV) encode proteins that augment transcription of viral genes. Intracellular signaling pathways are capable of activating retroviral gene expression and play important roles in inducing high levels of viral replication in response to certain stimuli. 109 Transcription of the provirus yields mRNAs that encode viral proteins and genome-length RNAs that are packaged into progeny virions. Such a replication strategy results in persistent infection in the host because the viral genome is maintained in the host cell genome and replicated with each cell division.
With the exception of the poxviruses, viruses containing DNA genomes replicate in the nucleus and for the most part use cellular enzymes for transcription and replication of their genomes. Transcription of most DNA-containing viruses is tightly regulated and results in the synthesis of early and late mRNA transcripts. The early transcripts encode regulatory proteins and proteins required for DNA replication, whereas the late transcripts encode structural proteins. Several DNA-containing viruses, such as adenovirus and human papillomavirus (HPV), induce cells to express host proteins required for viral DNA replication by stimulating cell-cycle progression. For example, the HPV E7 protein binds the retinoblastoma gene product pRB and liberates transcription factor E2F, which induces the cell cycle. 110 , 111 To prevent programmed cell death in response to E7-mediated unscheduled cell cycle progression, the HPV E6 protein mediates the ubiquitylation and degradation of tumor suppressor protein p53. 112 , 113 , 114
Some DNA-containing viruses, such as the herpesviruses, can establish latent infections in the host. Unlike the retroviruses, genomes of the herpesviruses do not integrate into host chromosomes but instead exist as plasmid-like episomes. Mechanisms that govern establishment of latency and subsequent reactivation of replication are not well understood. However, microRNAs encoded by cytomegalovirus (CMV) and perhaps other herpesviruses may promote persistence by targeting viral and cellular mRNAs that control viral gene expression and replication and innate immune responses to viral infection. 115 , 116
A fascinating aspect of virus–cell interactions is the replication microenvironments established in infected cells. Viral replication is a sophisticated interplay of transcription, translation, nucleic acid amplification, and particle assembly. Furthermore, infection must proceed under sensitive pathogen surveillance systems trained on virus-associated molecular patterns (e.g., unmethylated CpG dinucleotides in DNA viral genomes) and replicative intermediates (e.g., dsRNA generated during RNA virus replication) that may impose impassable blocks to infection. 117 Partitioning of the viral replication machinery from the surrounding intracellular milieu satisfies a spatial requirement to concentrate viral proteins and nucleic acid for efficient genome amplification and encapsidation while simultaneously shielding viral products from cellular sensors that provoke antiviral innate immune responses. Hence, as a rule, viral replication is a localized process, occurring within morphologically discrete cytoplasmic or nuclear structures variously termed viral inclusions (or inclusion bodies ), virosomes, viral factories, or viroplasm. These entities are novel, metabolically active organelles formed by contributions from both virus and cell. Many highly recognizable features of viral cytopathic effect observed using light microscopy, such as dense nuclear inclusions or refractile cytoplasmic densities, represent locally concentrated regions of viral nucleic acid and protein.
Membrane-associated replicase complexes appropriated by (+) sense RNA viruses are perhaps the most conspicuous examples of compartmentalized viral replication. In cells infected by these viruses, intracellular membranes originating from the endoplasmic reticulum (ER; e.g., picornaviruses 118 , 119 ), ER-Golgi intermediate compartment and trans -Golgi network (e.g., flaviviruses 120 ), endolysosomal vesicles (e.g., alphaviruses 121 ), and autophagic vacuoles (e.g., poliovirus 122 ) are reduplicated and reorganized by viral proteins into platforms that anchor viral replication complexes consisting of the RdRp and other RNA-modifying enzymes necessary for RNA synthesis. Curiously, dsRNA viruses are thought to generate nonmembranous intracytoplasmic replication factories, even though their life cycles pass through a (+) polarity RNA intermediate. However, in an interesting functional parallel with (+) sense RNA viruses, the assembly pathway of rotavirus, a dsRNA virus, involves budding of immature particles into the ER, where a lipid envelope is transiently acquired and subsequently replaced by the outermost protein shell. 123 Perhaps additional roles for cellular membranes in non–membrane-bound viral replication complexes await discovery.
The tight relationship of RNA virus replication to cellular membranes is less predictable for DNA viruses. For example, in distinction to the supporting role of autophagy in the replication of some RNA viruses, autophagosomes (stress-induced, double-membraned vesicles that remove noxious cytoplasmic materials to lysosomes for degradation) defend against infection by HSV-1, which encodes a protein that inhibits induction of autophagy and accentuates viral virulence. 124 , 125 The replication and assembly complexes of many DNA viruses, including adenoviruses, herpesviruses, papillomaviruses, polyomaviruses, and parvoviruses, are associated with promyelocytic leukemia (PML) nuclear bodies, 126 , 127 which have been ascribed functions in diverse nuclear processes encompassing gene regulation, tumor suppression, apoptosis, and removal of aggregated or foreign proteins. 128 It appears that DNA viruses exploit PML bodies in a variety of ways, which include consolidation and disposal of misfolded viral proteins, sequestration of host-cell stress response factors that block infection, and segregation of interfering cellular DNA repair proteins from sites of viral replication. 129
The life cycles of all viruses that replicate in eukaryotic cells are physically and functionally intertwined with the cytoskeleton. Many viruses with nuclear replication programs, such as adenovirus, HSV, and influenza virus, are transported by motor proteins along microtubules toward the nucleus, resulting ultimately in release of the viral genome into the nucleoplasm through nuclear pores. 130 The microtubule network is also conscripted as an egress pathway by a number of enveloped viruses (e.g., HIV, HSV, vaccinia virus) for conveyance of immature particles to cytolemmal sites of virion budding. 131 Furthermore, microtubules and actin filaments may serve as anchorage points for nucleoprotein complexes that coordinate genome expression or replication with cytoplasmic replication programs, exemplified by parainfluenza virus (PIV), 132 reovirus, 133 and vaccinia virus. 134 Because the cytoskeleton is a decentralized organelle linking cellular structural elements to the metabolic and transport machineries, it is not surprising that viruses capitalize on this highly integrative system, which provides a stable platform for replication and enables purposeful movement of virions or subviral components within cells to facilitate the requisite partitioning of viral assembly and disassembly.
Cell Killing
Viral infection can compromise numerous cellular processes, such as nucleic acid and protein synthesis, maintenance of cytoskeletal architecture, and preservation of membrane integrity. 135 Many viruses are also capable of inducing the genetically programmed mechanism of cell death that leads to apoptosis of host cells. 136 , 137 Apoptotic cell death is characterized by cell shrinkage, membrane blebbing, condensation of nuclear chromatin, and activation of an endogenous endonuclease, which results in cleavage of cellular DNA into oligonucleosome-length DNA fragments. 138 These changes occur according to predetermined developmental programs or in response to certain environmental stimuli. In some cases, apoptosis may serve as an antiviral defense mechanism to limit viral replication by destruction of virus-infected cells or reduction of potentially harmful inflammatory responses elicited by viral infection. 139 In other cases, apoptosis may result from viral induction of cellular factors required for efficient viral replication. 136 , 137 Generally, RNA-containing viruses, including influenza virus, measles virus, poliovirus, reovirus, and Sindbis virus, induce apoptosis of host cells, whereas DNA-containing viruses, including adenovirus, CMV, EBV, HPV, and the poxviruses, encode proteins that block apoptosis. For some viruses, the duration of the viral infectious cycle may determine whether apoptosis is induced or inhibited. Viruses capable of completing an infectious cycle before induction of apoptosis would not require a means to inhibit this cellular response to viral infection. Interestingly, several viruses that cause encephalitis are capable of inducing apoptosis of infected neurons ( Fig. 134-5 ). 140 , 141 , 142

Reovirus-induced apoptosis in the murine central nervous system.
Consecutive sections of the hippocampus prepared from a newborn mouse 10 days following intracranial inoculation with reovirus strain type 3 Dearing. Cells were stained with (A) hematoxylin and eosin, (B) reovirus antigen, and (C) the activated form of apoptosis protease caspase-3. Cells that stain positive for reovirus antigen or activated caspase 3 contain a dark precipitate in the cytoplasm, including neuronal processes. Scale bars, 100 µm.
Antiviral Drugs
(Also see Chapters 43 to 47Chapter 43Chapter 44Chapter 45Chapter 46Chapter 47.)
Knowledge of viral replication strategies has provided insights into critical steps in the viral life cycle that can serve as potential targets for antiviral therapy. For example, drugs can be designed to interfere with virus binding to target cells or prevent penetration and disassembly once receptor engagement has occurred. Steps involved in the replication of the viral genome are also obvious targets for antiviral therapy. A number of antiviral agents inhibit viral polymerases, including those active against herpesviruses (e.g., acyclovir), HIV (e.g., zidovudine), and HBV (e.g., entecavir). Drugs that inhibit viral proteases have been developed; several are used to treat HCV 143 , 144 and HIV 145 infection. These drugs block the proteolytic processing of viral precursor polyproteins and serve as potent inhibitors of replication. Other viral enzymes also serve as targets for antiviral therapy. The influenza virus neuraminidase is required for the release of progeny influenza virus particles from infected cells. Oseltamivir and zanamivir bind the neuraminidase catalytic site and efficiently inhibit the enzyme. 146 These drugs have been used in the prophylaxis and treatment of influenza virus infection. 147
Better understanding of viral replication strategies and mechanisms of virus-induced cell killing is paving the way for the rational design of novel antiviral therapeutics. One of the most exciting approaches to the development of antiviral agents is the use of high-resolution x-ray crystallography and molecular modeling to optimize interactions between these inhibitory molecules and their target viral proteins. Such structure-based drug design has led to the development of synthetic peptides (e.g., enfuvirtide) that inhibit HIV entry by blocking gp41-mediated membrane fusion. 148 Other vulnerable steps in HIV replication are targets of drugs approved for patient treatment, including entry inhibitors that interfere with gp120 binding to CCR5 149 and agents that prevent proviral integration into cellular DNA through inhibition of viral integrase activity 150 (see Chapter 130). Several inhibitors of the HCV protease and polymerase are also in clinical development 151 (see Chapter 46).
Despite promising advances in rational antiviral drug design, current therapeutic approaches to some viral infections rely heavily on compounds with less specific mechanisms of action. One such agent, interferon (IFN)-α, efficiently inhibits a broad spectrum of viruses and is secreted by diverse cell types as part of the host innate immune response. Recombinant IFN-α is presently used to treat HBV and HCV infections. Ribavirin, a synthetic guanosine analogue, inhibits the replication of many RNA- and DNA-containing viruses through complex mechanisms involving inhibition of viral RNA synthesis and disturbances in intracellular pools of guanosine triphosphate. 152 , 153 This drug is routinely used to treat HCV infection and sometimes administered in aerosolized form to treat respiratory syncytial virus (RSV) lower respiratory tract infection in hospitalized children and in severely ill and immunocompromised patients. Ribavirin therapy reduces the mortality associated with certain viral hemorrhagic fevers, such as that caused by Lassa virus. 154 Broader-spectrum therapies exemplified by IFN-α and ribavirin remain part of the first-line defense against emerging pathogens and other susceptible viruses for which biochemical and structural information is insufficient to design high-potency agent-specific drugs.
Virus–Host Interaction
One of the most formidable challenges in virology is to apply knowledge gained from studies of virus–cell interactions in tissue culture systems to an understanding of how viruses interact with host organisms to cause disease. Virus–host interactions are often described in terms of pathogenesis and virulence. Pathogenesis is the process whereby a virus interacts with its host in a discrete series of stages to produce disease ( Table 134-3 ). Virulence is the capacity of a virus to produce disease in a susceptible host. Virulence is often measured in terms of the quantity of virus required to cause illness or death in a predefined fraction of experimental animals infected with the virus. Virulence is dependent on viral and host factors and must be measured using carefully defined conditions (e.g., virus strain, dose, and route of inoculation; host species, age, and immune status). In many cases, it has been possible to identify roles played by individual viral and host proteins at specific stages in viral pathogenesis and to define the importance of these proteins in viral virulence.
TABLE 134-3
Stages in Virus–Host Interaction
The first step in the process of virus–host interaction is the exposure of a susceptible host to viable virus under conditions that promote infection ( Fig. 134-6 ). Infectious virus may be present in respiratory droplets or aerosols, in fecally contaminated food or water, or in a body fluid or tissue (e.g., blood, saliva, urine, semen, or a transplanted organ) to which the susceptible host is exposed. In some cases, the virus is inoculated directly into the host through the bite of an animal vector or through the use of a contaminated needle. Infection can also be transmitted from mother to infant through virus that has infected the placenta or birth canal or by virus in breast milk. In some cases, acute viral infections result from the reactivation of endogenous latent virus (e.g., reactivation of HSV giving rise to herpes labialis) rather than de novo exposure to exogenous virus.

Entry and spread of viruses in human hosts.
Some major steps in viral spread and invasion of target organs are shown. Neural spread is not illustrated. GI, gastrointestinal; HIV, human immunodeficiency virus; HPV, human papillomavirus.
Exposure of respiratory mucosa to virus by direct inoculation or inhalation is an important route of viral entry into the host. A simple cough can generate up to 10,000 small, potentially infectious aerosol particles, and a sneeze can produce nearly 2 million. The distribution of these particles depends on a variety of environmental factors, the most important of which are temperature, humidity, and air currents. In addition to these factors, particle size is an important determinant of particle distribution. In general, smaller particles remain airborne longer than larger ones. Particle size also contributes to particle fate after inhalation. Larger particles (>6 µm) are generally trapped in the nasal turbinates, whereas smaller particles may ultimately travel to the alveolar spaces of the lower respiratory tract.
Fecal-oral transmission represents an additional important route of viral entry into the host. Food, water, or hands contaminated by infected fecal material can facilitate the entry of a virus via the mouth into the gastrointestinal tract, the environment of which requires viruses that infect by this route to have certain physical properties. Viruses capable of enteric transmission must be acid stable and resistant to bile salts. Because conditions in the stomach and intestine are destructive to lipids contained in viral envelopes, most viruses that spread by the fecal-oral route are nonenveloped. Interestingly, many viruses that enter the host via the gastrointestinal tract require proteolysis of certain capsid components to infect intestinal cells productively. Treatment of mice with inhibitors of intestinal proteases blocks infection by reovirus 155 and rotavirus, 156 which demonstrates the critical importance of proteolysis in the initiation of enteric infection by these viruses. The host microbiota is essential for infection by some viruses. 157 , 158
To produce systemic disease, a virus must cross the mucosal barrier that separates the luminal compartments of the respiratory, gastrointestinal, and genitourinary tracts from the host's parenchymal tissues. Studies with reovirus illustrate one strategy used by viruses to cross mucosal surfaces to invade the host after entry into the gastrointestinal tract. 159 , 160 After oral inoculation of mice, reovirus adheres to the surface of intestinal microfold cells (M cells) that overlie collections of intestinal lymphoid tissue (Peyer's patches). In electron micrographs, reovirus virions can be followed sequentially as they are transported within vesicles from the luminal to the subluminal surface of M cells. Virions subsequently appear within Peyer's patches and then spread to regional lymph nodes and extraintestinal lymphoid organs such as the spleen. A similar pathway of spread has been described for poliovirus 161 and HIV, 162 suggesting that M cells represent an important portal for viral invasion of the host after entry into the gastrointestinal tract.
Once a virus has entered the host, it can replicate locally or spread from the site of entry to distant organs to produce systemic disease (see Fig. 134-6 ). Classic examples of localized infections in which viral entry and replication occur at the same anatomic site include respiratory infections caused by influenza virus, RSV, and rhinovirus; enteric infections produced by norovirus and rotavirus; and dermatologic infections caused by HPV (warts) and paravaccinia virus (milker's nodules). Other viruses spread to distant sites in the host after primary replication at sites of entry. For example, poliovirus spreads from the gastrointestinal tract to the central nervous system (CNS) to produce meningitis, encephalitis, or poliomyelitis. Measles virus and varicella-zoster virus (VZV) enter the host through the respiratory tract and then spread to lymph nodes, skin, and viscera. Pathobiologic definitions of viruses based on spread potential have begun to blur amid accumulating evidence that model agents of localized infection may disseminate to distant sites. For example, rotavirus, an important cause of pediatric acute gastroenteritis, replicates vigorously in villous tip epithelial cells of the small intestine but is also frequently associated with viral antigen and RNA in blood, the clinical significance of which is unclear. 163 Influenza virus is another case in point; viral RNA in blood is detected at a substantial frequency in hematopoietic cell transplant recipients and correlates with more severe disease and increased mortality. 164
Release of some viruses occurs preferentially from the apical or basolateral surface of polarized cells, such as epithelial cells. In the case of enveloped viruses, polarized release is frequently determined by preferential sorting of envelope glycoproteins to sites of viral budding. Specific amino-acid sequences in these viral proteins direct their transport to a particular aspect of the cell surface. 165 , 166 Polarized release of virus at apical surfaces may facilitate local spread of infection, whereas release at basolateral surfaces may facilitate systemic invasion by providing virus access to subepithelial lymphoid, neural, or vascular tissues.
Many viruses use the bloodstream to spread in the host from sites of primary replication to distant target tissues (see Fig. 134-6 ). In some cases, viruses may enter the bloodstream directly, such as during a blood transfusion or via an arthropod bite. More commonly, viruses enter the bloodstream after replication at some primary site. Important sites of primary replication preceding hematogenous spread of viruses include Peyer's patches and mesenteric lymph nodes for enteric viruses, bronchoalveolar cells for respiratory viruses, and subcutaneous tissue and skeletal muscle for alphaviruses and flaviviruses. In the case of reovirus, infection of endothelial cells leads to hematogenous dissemination in the host. 167 , 168
Pioneering studies by Fenner with mousepox (ectromelia) virus suggest that an initial low-titer viremia (primary viremia) serves to seed virus to a variety of intermediate organs, where a period of further replication leads to a high-titer viremia (secondary viremia) that disseminates virus to the ultimate target organs ( Fig. 134-7 ). 169 It is often difficult to identify primary and secondary viremias in naturally occurring viral infections. However, replication of many viruses in reticuloendothelial organs (e.g., liver, spleen, lymph nodes, bone marrow), muscle, fat, and even vascular endothelial cells can play an important role in maintaining viremia. 168

Pathogenesis of mousepox virus infection.
Successive waves of viremia are shown to seed the spleen and liver and then the skin.
Viruses that reach the bloodstream may travel free in plasma (e.g., enteroviruses and togaviruses) or in association with specific blood cells. 170 A number of viruses are spread hematogenously by macrophages (e.g., CMV, HIV, measles virus) or lymphocytes (e.g., CMV, EBV, HIV, HTLV, measles virus). Although many viruses have the capacity to agglutinate erythrocytes in vitro (a process called hemagglutination), only in exceptional cases (e.g., Colorado tick fever virus) are erythrocytes used to transport virus in the bloodstream.
The maintenance of viremia depends on the interplay among factors that promote virus production and those that favor viral clearance. A number of variables that affect the efficiency of virus removal from plasma have been identified. In general, the larger the viral particle, the more efficiently it is cleared. Viruses that induce high titers of neutralizing antibodies are more efficiently cleared than those that do not induce humoral immune responses. Finally, phagocytosis of virus by cells in the host reticuloendothelial system can contribute to viral clearance.
A major pathway used by viruses to spread from sites of primary replication to the nervous system is through nerves. Numerous diverse viruses, including Borna disease virus, coronavirus, HSV, poliovirus, rabies virus, reovirus, and Venezuelan equine encephalitis virus (VEE), are capable of neural spread. Several of these viruses accumulate at the neuromuscular junction after primary replication in skeletal muscle. 171 , 172 HSV appears to enter nerve cells via receptors that are located primarily at synaptic endings rather than on the nerve cell body. 173 Spread to the CNS by HSV, 174 rabies virus, 171 , 172 and reovirus 175 , 176 can be interrupted by scission of the appropriate nerves or by chemical agents that inhibit axonal transport. Neural spread of some of these viruses occurs by the microtubule-based system of fast axonal transport. 177
Viruses are not limited to a single route of spread. VZV, for example, enters the host by the respiratory route and then spreads from respiratory epithelium to the reticuloendothelial system and skin via the bloodstream. Infection of the skin produces the characteristic exanthem of chickenpox. The virus subsequently enters distal terminals of sensory neurons and travels to dorsal root ganglia, where it establishes latent infection. Reactivation of VZV from latency results in transport of the virus in sensory nerves to skin, where it gives rise to vesicular lesions in a dermatomal distribution characteristic of zoster or shingles .
Poliovirus is also capable of spreading by hematogenous and neural routes. Poliovirus is generally thought to spread from the gastrointestinal tract to the CNS via the bloodstream, although it has been suggested that the virus may spread via autonomic nerves in the intestine to the brainstem and spinal cord. 178 , 179 This hypothesis is supported by experiments using transgenic mice expressing the human poliovirus receptor. 180 When these mice are inoculated with poliovirus intramuscularly in the hind limb, virus does not reach the CNS if the sciatic nerve ipsilateral to the site of inoculation is transected. 181 Once poliovirus reaches the CNS, axonal transport is the major route of viral dissemination. Similar mechanisms of spread may be used by other enteroviruses.
The capability of a virus to infect a distinct group of cells in the host is referred to as tropism. For many viruses, tropism is determined by the availability of virus receptors on the surface of a host cell. This concept was first appreciated in studies of poliovirus when it was recognized that the capacity of the virus to infect specific tissues paralleled its capacity to bind homogenates of the susceptible tissues in vitro. 182 The importance of receptor expression as a determinant of poliovirus tropism was conclusively demonstrated by showing that cells not susceptible for poliovirus replication could be made susceptible by recombinant expression of the poliovirus receptor. 183 In addition to the availability of virus receptors, tropism can also be determined by postattachment steps in viral replication, such as the regulation of viral gene expression. For example, some viruses contain genetic elements, termed enhancers, that act to stimulate transcription of viral genes. 184 , 185 Some enhancers are active in virtually all types of cells, whereas others show exquisite tissue specificity. The promoter-enhancer region of John Cunningham (JC) polyomavirus is active in cultured human glial cells but not in HeLa cervical epithelial cells. 186 Cell-specific expression of the JC virus genome correlates well with the capacity of this virus in immunocompromised persons to produce progressive multifocal leukoencephalopathy, a disease in which JC virus infection is limited to oligodendroglia in the CNS.
Specific steps in virus–host interaction, such as the route of entry and pathway of spread, also can strongly influence viral tropism. For example, encephalitis viruses such as VEE are transmitted to humans by insect bites. These viruses undergo local primary replication and then spread to the CNS by hematogenous and neural routes. 187 After oral inoculation, VEE is incapable of primary replication and spread to the CNS, illustrating that tropism can be determined by the site of entry into the host. Influenza virus buds exclusively from the apical surface of respiratory epithelial cells, 188 which may limit its capacity to spread within the host and infect cells at distant sites.
A wide variety of host factors can influence viral tropism. These include age, nutritional status, and immune responsiveness, as well as certain genetic polymorphisms that affect susceptibility to viral infection. Age-related susceptibility to infection is observed for many viruses, including reovirus, 189 , 190 RSV, 191 , 192 , 193 and rotavirus. 194 , 195 The increased susceptibility in young children to these viruses may in part be due to immaturity of the immune response but also may be related to intrinsic age-specific factors that enhance host susceptibility to infection. Nutritional status is a critical determinant of the tropism and virulence of many viruses. For example, persons with vitamin A deficiency have enhanced susceptibility to measles virus infection. 196 , 197 Similarly, the outcome of most viral infections is strongly linked to the immune competence of the host.
The genetic basis of host susceptibility to viral infections is complex. Studies with inbred strains of mice indicate that genetic variation can alter susceptibility to viral disease by a variety of mechanisms. 198 These can involve differences in immune responses, variability in the ability to produce antiviral mediators such as IFN, and differential expression of functional virus receptors. Polymorphisms in the expression of chemokine receptor CCR5, which serves as a co-receptor for HIV, 55 , 56 , 57 are associated with alterations in susceptibility to HIV infection. 199 , 200
Persistent Infections
Many viruses are capable of establishing persistent infections, of which two types are recognized: chronic and latent. Chronic viral infections are characterized by continuous shedding of virus for prolonged periods of time. Congenital infections with rubella virus and CMV and persistent infections with HBV and HCV are examples of chronic viral infections. Latent viral infections are characterized by maintenance of the viral genome in host cells in the absence of viral replication. Herpesviruses and retroviruses can establish latent infections. The distinction between chronic and latent infections is not readily apparent for some viruses, such as HIV, which can establish both chronic and latent infections in the host. 201 , 202 , 203 Viruses capable of establishing persistent infections must have a means of evading the host immune response and a mechanism of attenuating their virulence. Lentiviruses such as equine infectious anemia virus 204 and HIV 205 , 206 , 207 are capable of extensive antigenic variation resulting in escape from neutralizing antibody responses by the host.
Several viruses encode proteins that directly attenuate the host immune response (e.g., the adenovirus E3/19K protein 208 and CMV US11 gene product 209 block cell surface expression of MHC class I proteins, resulting in diminished presentation of viral antigens to cytotoxic T lymphocytes [CTLs]). The poxviruses encode a variety of immunomodulatory molecules including CrmA, which blocks T-cell–mediated apoptosis of virus-infected cells. 210 In some cases (e.g., the CNS), preferential sites for persistent viral infections are not readily accessible by the immune system, 211 which may favor establishment of persistence.
Viruses and Cancer
Several viruses produce disease by promoting malignant transformation of host cells. Work by Peyton Rous with an avian retrovirus was the first to demonstrate that viral infections can cause cancer. 212 Rous sarcoma virus encodes an oncogene, v -src, which is a homologue of a cellular proto-oncogene, c -src. 213 , 214 Cells infected with Rous sarcoma virus become transformed. 215 , 216 , 217 , 218 , 219 Several viruses are associated with malignancies in humans. EBV is associated with many neoplasms, including Burkitt's lymphoma, Hodgkin's disease, large B-cell lymphoma, leiomyosarcoma, and nasopharyngeal carcinoma. HBV and HCV are associated with hepatocellular carcinoma. HPV is associated with cervical cancer and a variety of anogenital and esophageal neoplasms. Kaposi sarcoma–associated herpesvirus is associated with Kaposi sarcoma and primary effusion lymphoma in persons with HIV infection.
Often, the linkage of a virus to a particular neoplasm can be attributed to transforming properties of the virus itself. For example, EBV encodes several latency-associated proteins that are responsible for immortalization of B cells; these proteins likely play crucial roles in the pathogenesis of EBV-associated malignancies. 220 Similarly, HPV encodes the E6 and E7 proteins that block apoptosis 112 , 113 , 114 and induce cell cycle progression, 110 , 111 respectively. It is hypothesized that unregulated expression of these proteins induced by the aberrant integration of the HPV genome into host DNA is responsible for malignant transformation. 221 The tumorigenicity of polyomaviruses, which are oncogenic in rodent species, is mediated by a family of viral proteins known as tumor (T) antigens. Reminiscent of the HPV E6 and E7 proteins, T antigens induce cell cycling and block the ensuing cellular apoptotic response to unscheduled cell division. 222 The normally episomal polyomavirus genome becomes integrated into cellular DNA during neoplastic transformation of nonpermissive cells unable to support the entire viral replication program, which would otherwise culminate in cell death. Discovery of a human polyomavirus clonally integrated into cells of an aggressive form of skin cancer, Merkel cell carcinoma, 2 substantiates the long-standing suspicion that polyomaviruses can also promote neoplasia in humans.
In other cases, mechanisms of malignancy triggered by viral infection are less clear. HCV is an RNA-containing virus that lacks reverse transcriptase and a means of viral genome integration. However, chronic infection with HCV is strongly associated with hepatocellular cancer. 223 It is possible that increased cell turnover and inflammatory mediators elicited by chronic HCV infection increase the risk of genetic damage, which results in malignant transformation. Some HCV proteins may also play a contributory role in neoplasia. For example, the HCV core protein can protect cells against apoptosis induced by a variety of stimuli, including tumor necrosis factor-α (TNF-α). 224
Viral Virulence Determinants
Viral surface proteins involved in attachment and entry influence the virulence of diverse groups of viruses. For example, polymorphisms in the attachment proteins of influenza virus, 225 , 226 polyomavirus, 227 reovirus, 228 rotavirus, 229 and VEE 230 are strongly linked to virulence and can be accurately termed virulence determinants. Viral attachment proteins can serve this function by altering the affinity of virus–receptor interactions or modulating the kinetics of viral disassembly. Importantly, sequences in viral genomes that do not encode protein can also influence viral virulence. Mutations that contribute to the attenuated virulence of the Sabin strains of poliovirus are located in the 5′ nontranslated region of the viral genome. 231 These mutations attenuate poliovirus virulence by altering the efficiency of viral protein synthesis.
A number of viruses encode proteins that enhance virulence by modulation of host immune responses. Illustrative examples include the influenza A NS1 protein, which interferes with activation of cellular innate immune responses to viral infection, 232 and translation products of the adenovirus E3 transcriptional unit, which serve to prevent cytotoxic T-cell recognition of virally infected cells and block immunologically activated signaling pathways that lead to infected-cell death. 208 , 233 In many cases, these proteins are dispensable for viral replication in cultured cells. In this way, immunomodulatory viral virulence determinants resemble classic bacterial virulence factors such as various types of secreted toxins.
Host Responses to Infection
The immune response to viral infection involves complex interactions among leukocytes, nonhematopoietic cells, signaling proteins, soluble proinflammatory mediators, antigen-presenting molecules, and antibodies. These cells and molecules collaborate in a highly regulated fashion to limit viral replication and dissemination through recognition of broadly conserved molecular signatures, followed by virus-specific adaptive responses that further control infection and establish antigen-selective immunologic memory. The innate antiviral response is a local, transient, antigen-independent perimeter defense strategically focused at the site of virus incursion into an organ or tissue. Mediated by ancient families of membrane-associated and cytosolic molecules known as pattern recognition receptors (PRRs), the innate immune system detects pathogen-associated molecular patterns (PAMPs), which are fundamental structural components of microbial products including nucleic acids, carbohydrates, and lipids. 234 Viral PAMPs in the form of single-stranded (ss)RNA, dsRNA, and DNA evoke the innate immune response through two groups of PRRs: the transmembrane Toll-like receptors (TLRs) and the cytosolic nucleic acid sensors. The latter include retinoic acid inducible gene-I (RIG-I)-like receptors, nucleotide-binding domain and leucine-rich-repeat containing proteins (NLRs) such as NLRP, and DNA sensors. 235 Nucleic acid binding by PRRs activates signaling pathways leading to the production and extracellular release of IFN-α, IFN-β, and proinflammatory cytokines such as interleukin (IL)-1β and IL-18. IFN-α and IFN-β engage the cell surface IFN-α/β receptor and thereby mediate expression of hundreds of gene products that corporately suppress viral replication and establish an intracellular antiviral state in neighboring uninfected cells. Well-described IFN-inducible gene products include the latent enzymes dsRNA-dependent protein kinase (PKR) and 2′,5′-oligoadenylate synthetase (OAS), both of which are activated by dsRNA. 236 PKR inhibits the initiation of protein synthesis through phosphorylation of translation initiation factor eIF2α. The 2′,5′-oligoandenylates generated by OAS bind and activate endoribonuclease RNAse L, which degrades viral mRNA. In addition to mediating an intracellular antiviral state, IFN-α/β also stimulates the antigen-independent destruction of virus-infected cells by a specialized population of lymphocytes known as natural killer (NK) cells. 237 Importantly, IFNs bridge innate and adaptive antiviral immune responses through multiple modes of action, which include enhancing viral antigen presentation by class I MHC proteins, 238 promoting the proliferation of MHC class I–restricted CD8 + CTLs, 239 and facilitating the functional maturation of dendritic cells. 240 Proinflammatory mediators IL-1β and IL-18 pleiotropically stimulate and amplify the innate immune response through induction of other inflammatory mediators, immune cell activation, and migration of inflammatory cells into sites of infection. 241 These molecules perform essential functions in host antiviral defense. 242
The adaptive immune response confers systemic and enduring pathogen-selective immunity through expansion and functional differentiation of viral antigen-specific T and B lymphocytes. Having both regulatory and effector roles, T lymphocytes are centrally positioned in the scheme of adaptive immunity. The primary cell type involved in the resolution of acute viral infection is the CD8 + CTL, which induces lethal proapoptotic signaling in virus-infected cells upon recognition of endogenously produced viral protein fragments presented by cell surface MHC class I molecules. Less frequently, CD4 + T cells, which recognize MHC class II–associated viral oligopeptides processed from exogenously acquired proteins, also demonstrate cytotoxicity against viral antigen-presenting cells. 243 The usual function of CD4 + T lymphocytes is to orchestrate and balance cell-mediated (CTL) and humoral (B lymphocyte) responses to infection. Classes of CD4 + helper T-cell subsets—Th1, Th2, Th17, Treg (regulatory T), and Tfh (follicular helper T)—have been defined based on characteristic patterns of cytokine secretion and effector activities. 244 , 245 Th1 and Th2 lymphocytes are usually associated with the development of cell-mediated and humoral responses, respectively, to viral infection. Th17 and Treg CD4 + subsets are important for control of immune responses and prevention of autoimmunity, but their precise roles in viral disease and antiviral immunity are not clear. For certain persistent viral infections, such as those caused by HIV and HSV, Treg cells might exacerbate disease through suppression of CTLs or, paradoxically, ameliorate illness by attenuating immune-mediated cell and tissue injury. 246 Tfh cells promote differentiation of antigen-specific memory B lymphocytes and plasma cells within germinal centers. 247 Therefore, Tfh cells likely occupy a central place in the humoral response to viral infection and vaccination. Although Tfh cell functions are not unique to antiviral responses, chronic viral infections including HBV and HIV appear to stimulate proliferation of these cells. 248 , 249 The Tfh phenotype may interconvert with other T-helper lineage profiles and thus represent a differentiation intermediate rather than a unique CD4 + T lymphocyte subset. 245
The primacy of cell-mediated immune responses in combating viral infections is revealed by the extreme vulnerability of individuals to chronic and life-threatening viral diseases when cellular immunity is dysfunctional. Those with acquired immunodeficiency syndrome (AIDS) exemplify the catastrophic consequences of collapsing cell-mediated immunity; progressive multifocal leukoencephalopathy caused by JC polyomavirus, along with severe mucocutaneous and disseminated CMV, HSV, and VZV infections, are frequent complications of vanishing CD4 + T cells. Similarly, iatrogenic cellular immunodeficiency associated with hematopoietic stem cell and solid-organ transplantation or antineoplastic treatment regimens predisposes to severe, potentially fatal infections with herpesviruses and respiratory viral pathogens such as adenovirus, PIV, and RSV, 250 all of which normally produce self-limited illness in immunocompetent hosts. Prevention and management of serious viral respiratory infections are significant challenges in myelosuppression units because of the communicability of respiratory viruses and paucity of effective drugs to combat these ubiquitous agents. Individuals with significantly impaired cell-mediated immunity are also at increased risk for enhanced viral replication and systemic disease following immunization with live, attenuated viral vaccines (e.g., measles-mumps-rubella [MMR] and VZV vaccines). Hence, live viral vaccines are generally contraindicated for immunocompromised persons (see Chapter 321). TNF-α inhibitor therapy, increasingly employed to manage a variety of rheumatologic and inflammatory diseases, enhances the risk of HBV reactivation with potentially life-threatening consequences. 251 Preventive and interventional HBV treatment strategies are necessary to circumvent complications of uncontrolled viral replication in these patients.
In contrast to cell-mediated immune mechanisms, humoral responses are usually not a determinative factor in the resolution of primary viral infections. (One notable exception is a syndrome of chronic enteroviral meningitis in the setting of agammaglobulinemia. 252 ) However, for most human viral pathogens, the presence of antibody is associated with protection against initial infection in vaccinees or reinfection in hosts with a history of natural infection. 253 Longitudinal studies indicate that levels of protective serum antibodies (induced by natural infection or immunization) to common viruses, including EBV, measles, mumps, and rubella, are remarkably stable, with calculated antibody half-lives ranging from several decades to thousands of years. 254 The protective role of antibodies on secondary exposure is frequently explained as interruption of viremic spread where a hematogenous phase is involved, such as occurs with measles, mumps, and rubella viruses, poliovirus, VZV, and most arboviruses. Nevertheless, most human viruses, excluding insect-transmitted agents, enter their hosts by transgression of a mucosal barrier, frequently undergoing primary replication in mucosal epithelium or adjacent lymphoid tissues. Neutralizing IgA exuded onto mucosal epithelial surfaces may protect against primary infection at this portal of viral entry. A classic example is gut mucosal immunity induced by orally administered Sabin poliovirus vaccine containing live-attenuated virus. Secretory IgA against poliovirus blocks infection at the site of primary replication and consequently interrupts the chain of viral transmission, although fully virulent revertant viruses arise at regular frequency in vaccine recipients, who may develop disease and also transmit revertant strains to nonimmune individuals. 255 Clinical and experimental studies of immunity to HIV have led to the recognition that resident immune responses at exposed mucosal surfaces are likely critical components of host resistance to primary HIV infection, and achievement of potent mucosal immunity has emerged as an important consideration for the design of candidate HIV vaccines. 256 Despite the appearance of serum neutralizing antibodies to HIV several weeks after infection, viral eradication is thwarted by selection of neutralization-resistant variant strains from a mutant pool, which is perpetually replenished because of extreme plasticity within neutralization determinants on the viral envelope glycoproteins. 257 Identification of epitopes bound by broadly neutralizing antiviral antibodies has provided potential new targets for structure-based vaccine design. 258
Protection against viral infection by serum immunoglobulins is often correlated with antibody-mediated neutralization of viral infectivity in cultured cells. Antibodies interrupt the viral life cycle at early steps, which may include cross-linking virion particles into noninfectious aggregates, steric hindrance of receptor engagement, and interference with viral disassembly. 259 It is presumed that virus neutralization in cell culture by human serum is reflective of antibody activity in the intact host, but the mechanistic basis of infection blockade and disease prevention by antibodies in vivo is difficult to define precisely. For example, exclusively in vivo functions of the humoral antiviral response include Fc-mediated virion phagocytosis 260 , 261 and antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC responses require effectors from both the innate and adaptive systems, NK cells and antibodies, respectively. 262 The basis of ADCC is FcγRIIIa receptor-dependent recognition by NK cells of virus-specific IgG bound to antigens expressed on the surface of infected cells, leading to release of perforin and granzymes from NK cells that eventuate in target cell apoptosis. Neutrophils, lymphocytes, and macrophages also possess Fc receptors and may participate in ADCC.
Key References
The complete reference list is available online at Expert Consult.
What is a Virus?
- Download PDF Copy

A virus is the smallest type of parasite to exist and is typically within the size range of 0.02 to 0.3 micrometers (μm) in size; however, some viruses can be as large as 1 μm.

Image Credit: Rolling Stones / Shutterstock.com
The contents of a virus
Viruses consist of short sequences of nucleic acid , which can be ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) as their genetic material. Unlike most other organisms, where DNA is always a double-stranded structure, viruses are unique because their DNA or RNA material can be either single-stranded or double-stranded.
The typical structure of a virion, which is the term used to describe a single virus, includes an outer shell that is otherwise referred to as the protein capsid or membrane. The primary function of this outer shell is to protect the virion’s genetic information from physical, chemical, or enzymatic damage.
Classifying human viruses
A virus is often classified according to its physicochemical properties, genome structure, size, morphology, and molecular processes. In terms of their genetic material, viruses are classified according to whether they are RNA or DNA viruses and the strandedness of their genetic material, which can include double-stranded (ds), single-stranded (ss), or partially ds. Furthermore, ss viruses will also be classified as to whether they are positive ss, negative ss, or negative with ambisense viruses.
To date, five dsDNA human virus families have been identified, which include adenoviridae, herpesviridae, papillomaviridae, parvoviridae, and poxiviridae. Picobirnaviridae, picornaviridae, and reoviridae are the three human dsRNA viruses that have been identified.
As compared to DNA viruses, there have been many more RNA viruses that have infected humans. These include nine negative ssRNA and eight positive ssRNA virus families.
Capsid morphology, including icosahedral, helical, or complex shapes, can also be used to classify a given virus. The presence or absence of an envelope on the virus is also incorporated into its classification.

Image Credit: Cartoon Millie_art / Shutterstock.com
How do viruses infect?
The outer surface of the virus is crucial for its ability to recognize and attach to host cells. Typically, the surface of the virus will contain certain proteins that can recognize and bind to cellular receptors to facilitate its attachment to the host cell. Once the virus attaches to the host cell, it is engulfed through the cellular membrane and subsequently enters the cell’s cytoplasm. Inside the cell, the virus will remove its viral coat into smaller cellular vesicles, thereby releasing its genetic material into the cytoplasm for its replication.
Viruses do not have the mechanisms needed to survive independently and seek out plant, animal, or bacterial host cells where they can replicate using those cells' machinery. Therefore, viruses will use one of several different transmission routes to infect host cells, which include direct contact, indirect, common vehicle, and airborne transmission.
Viruses do not have the mechanisms needed to survive independently and seek out plant, animal, or bacterial host cells where they can replicate using those cells' machinery. Therefore, viruses will use one of several different transmission routes to infect host cells, including direct contact, indirect, common vehicle, and airborne transmission.
Direct contact transmission
The direct contact transmission route requires physical contact between an infected and uninfected subject through kissing, biting, or sexual intercourse, for example. Some of the most notable sexually transmitted viruses include the human immunodeficiency virus type 1 (HIV-1), human T-lymphotropic virus type 1 (HTLV-1), hepatitis B virus (HBV), and human papillomavirus types 16 and 18 (HPV-16 and HPV18, respectively).
Indirect transmission
The virus is transmitted indirectly through contact with contaminated objects or materials, such as medical equipment or shared eating utensils.
Common vehicle transmission
Common vehicle transmission refers to when individuals are exposed to the virus from a contaminated source such as food, water, medications, or intravenous fluids. Whereas cytomegalovirus is a urine-associated virus, several viruses are transmitted through the fecal-oral route, including polioviruses, coxsackieviruses, hepatitis A virus, rotavirus, astrovirus, and norovirus.
Airborne transmission
Related stories.
- Bird flu virus detected in Alaskan polar bear
- Intranasal vaccine shows broad SARS-CoV-2 variant protection
- APOE ε4 carrier status increases the long-term cognitive decline risk associated with herpes zoster
Airborne transmission refers to the respiratory route of exposure to viruses that can be in the form of droplets, aerosols, and respiratory secretions on the hands and objects. Some of the most notable viruses that are transmitted through this route include influenza virus, varicella-zoster virus, human rhinovirus, human adenovirus, respiratory syncytial virus, parainfluenza virus, metapneumovirus, and coronaviruses .
Coronaviruses

Image Credit: Design_Cells / Shutterstock.com
Within the coronaviridae family are several coronaviruses that can cause both respiratory and digestive diseases in vertebrates. The most recent coronavirus to alter both society and human health around the world is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for the coronavirus disease 2019 (COVID-19).
The term coronavirus is based on the crown-shaped spike proteins that wrap around the surface of these viruses. Within these spike proteins is the receptor-binding domain (RBD), which plays a crucial role in coronaviruses' entry into host cells. For example, both the RBDs of SARS-CoV and SARS-CoV-2 bind to the angiotensin-converting enzyme 2 (ACE2) receptor present on the surface of host cells to gain entry.
Coronaviruses, which are enveloped, positive-sense ssRNA viruses, can be further categorized into four genera, including alpha-beta-, delta-, and gammacoronaviruses. Whereas bats are often the reservoir for a majority of alpha- and betacoronaviruses, including SARS-CoV-2, wild birds are more likely to be reservoirs for gamma- and deltacoronaviruses.
Coronaviruses can be further split into distinct species due to their rapid evolution capabilities. To this end, they exhibit high mutation rates and homologous RNA recombination, both of which contribute to their extensive diversity and the recurrent emergence of new variants.
Certain alphacoronaviruses like NL63 and 229E, as well as betacoronaviruses like OC43 and HKU1, cause mild symptoms in humans that are associated with the common cold. Comparatively, SARS-CoV-2 infection has the potential to cause extremely severe symptoms that lead to acute respiratory distress syndrome (ARDS) and death. In fact, as of June 26, 2024, SARS-CoV-2 has infected over 775 million people around the world and caused over 7 million deaths.
Continuous Adaptive Evolution in Endemic Human Viruses
Recent research highlights ongoing adaptive evolution in several endemic human viruses, particularly in their surface proteins, which play a critical role in immune evasion. This adaptive evolution is driven by the need for viruses to evade the host's immune system, especially neutralizing antibodies that are produced following infection or vaccination.
SARS-CoV-2 has been noted to accumulate protein-coding changes at a faster rate compared to other endemic viruses, underscoring its rapid adaptive evolution. This phenomenon is particularly evident in the receptor-binding domain (RBD) of the spike protein, which undergoes frequent mutations to escape immune detection.
The study of 28 human endemic viruses found that 10 of these viruses are undergoing significant antigenic evolution. This demonstrates that immune evasion is a common strategy among viruses that persist in human populations. This insight is crucial for informing vaccine development and predicting future viral evolution.
The research also emphasized the importance of monitoring viral evolution to manage and mitigate the impact of viral diseases effectively. Understanding the mechanisms behind antigenic evolution can aid in the design of more effective vaccines and therapeutic strategies, ensuring better preparedness for both existing and emerging viral threats.
- Taylor, M. W. (2014). What Is a Virus? Viruses and Man: A history of Interactions; 23-40. doi:10.1007/978-3-319-00758-1_2, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7122971/
- Woolhouse, M., Scott, F., Hudson, Z., et al. (2012). Human viruses: discovery and emergence. Philosophical Transactions B 367 (1604); 2864-2871. doi:10.1098/rstb.2011.0354, https://royalsocietypublishing.org/doi/10.1098/rstb.2011.0354
- Siegel, R. D. (2018). Classification of Human Viruses. Principles and Practice of Pediatric Infectious Diseases ; 1044-1048. doi:10.1016/B978-0-323-40181-4-00201-2, https://linkinghub.elsevier.com/retrieve/pii/B9780323401814002012
- Nova, N. (2021). Cross-Species Transmission of Coronaviruses in Humans and Domestic Mammals, What Are the Ecological Mechanisms Driving Transmission, Spillover, and Disease Emergence? Frontiers in Public Health. doi:10.3389/fpubh.2021.717941, www.frontiersin.org/.../full
- Kistler, K. E., & Bedford, T. (2023). An atlas of continuous adaptive evolution in endemic human viruses. Cell Host & Microbe, 31(11), 1898-1909.e3. DOI: 10.1016/j.chom.2023.09.012, https://www.sciencedirect.com/science/article/pii/S1931312823003803
- COVID-19 cases | WHO COVID-19 dashboard . (n.d.). Datadot. https://data.who.int/dashboards/covid19
Article Revisions
- Jun 26 2024 - Updated COVID-19 figures to 26th June 2024. Formatting of citation links and grammatical improvements.
Last Updated: Jun 26, 2024

Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Please use one of the following formats to cite this article in your essay, paper or report:
Robertson, Sally. (2024, June 26). What is a Virus?. News-Medical. Retrieved on September 03, 2024 from https://www.news-medical.net/health/What-is-a-Virus.aspx.
Robertson, Sally. "What is a Virus?". News-Medical . 03 September 2024. <https://www.news-medical.net/health/What-is-a-Virus.aspx>.
Robertson, Sally. "What is a Virus?". News-Medical. https://www.news-medical.net/health/What-is-a-Virus.aspx. (accessed September 03, 2024).
Robertson, Sally. 2024. What is a Virus? . News-Medical, viewed 03 September 2024, https://www.news-medical.net/health/What-is-a-Virus.aspx.
Suggested Reading

The article is good. I would like to know more about viruses.
best webside thank you

In this article's 2nd paragraph, headlined "How big are viruses?" are the following words: "The virus particles are 100 times smaller than a single bacteria cell. The bacterial cell alone is more than 10 times smaller than a human cell and a human cell is 10 times smaller than the diameter of a single human hair." May I point out that you really can't get smaller than one time the size of something at which point you disappear. What I assume is meant by the above paragraph is the following re-written set of words: "The virus particles are 1/100 the size of a single bacteria cell. The bacterial cell alone is smaller than 1/10 the size of a human cell and a human cell is 1/10 the diameter of a single human hair." Or "The Virus particle's size is 1% of that of a single bacteria cell. That bacteria cell is less than 10% of the size of a human cell and that human cell's size is 10% of the diameter of a human hair. So that makes the virus particle's size approximately 1/1000th the size of a human hair!" (Hope I got all the math right there! It's 1% of 10% of 10% of the diameter of a human hair) Remember if I subtract one whole you (1 time smaller) there is nothing left. You want to speak about fractions, not whole numbers when speaking about something smaller. Unless of course it somehow is possible for the item being described to actually go into negative numbers. Physical items don't. You can only have one less of a physical item. I know, I am feeling very much like a word Nazi, but I only do it for this particular peeve.....
I wonder if a prolonged burst of low voltage electricity would affect the virus ability to function.
Modern Latin is what the state Universities use. Since the 16th century. Yale would be different using Old Latin or Middle Latin, and is not what anyone has classes for. The Modern Latin meaning for "Virus" is a slimy liquid, or in my opinion, the reason for a pimple, chicken pox, small pox, cow pox. Nitric acid, has been a problem ever since we did what was not allowed and pumped up crude oil. The nitric acid from coal oil is the worst of all the acids we have, without the word cataylst being what we know, and how much it does not dilute, but comes back into its origional form and strength. The experts eliminating this acid from effecting us today is the reason our teeth don't rot, and our children all don't have acne.
virus .. I am looking forward to learn a lot on virus
it's 1/10,000th not 1/1,000
useful for school but the aids news letter isn't aproporiot for young people

Would you rather ‘young people’ learn by getting AIDs instead of a good article? Kids especially need to learn about safety.
A virus is not a parasite.
Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

Global and Local Efforts to Take Action Against Hepatitis
Lindsey Hiebert and James Amugsi
In this interview, we explore global and local efforts to combat viral hepatitis with Lindsey Hiebert, Deputy Director of the Coalition for Global Hepatitis Elimination (CGHE), and James Amugsi, a Mandela Washington Fellow and Physician Assistant at Sandema Hospital in Ghana. Together, they provide valuable insights into the challenges, successes, and the importance of partnerships in the fight against hepatitis.

Addressing Important Cardiac Biology Questions with Shotgun Top-Down Proteomics
In this interview conducted at Pittcon 2024, we spoke to Professor John Yates about capturing cardiomyocyte cell-to-cell heterogeneity via shotgun top-down proteomics.

A Discussion with Hologic’s Tim Simpson on the Future of Cervical Cancer Screening
Tim Simpson
Hologic’s Tim Simpson Discusses the Future of Cervical Cancer Screening.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback


IMAGES
VIDEO
COMMENTS
virus, infectious agent of small size and simple composition that can multiply only in living cells of animals, plants, or bacteria.The name is from a Latin word meaning "slimy liquid" or "poison.". The earliest indications of the biological nature of viruses came from studies in 1892 by the Russian scientist Dmitry I. Ivanovsky and in 1898 by the Dutch scientist Martinus W. Beijerinck.
Virus Definition. A virus is a chain of nucleic acids (DNA or RNA) which lives in a host cell, uses parts of the cellular machinery to reproduce, and releases the replicated nucleic acid chains to infect more cells. A virus is often housed in a protein coat or protein envelope, a protective covering which allows the virus to survive between hosts.. Virus Structure
8. Introduction to Viruses. Viruses are typically described as obligate intracellular parasites, acellular infectious agents that require the presence of a host cell in order to multiply. Viruses that have been found to infect all types of cells - humans, animals, plants, bacteria, yeast, archaea, protozoa…some scientists even claim they ...
In a nutshell, a virus is a non-cellular, infectious entity made up of genetic material and protein that can invade and reproduce only within the living cells of bacteria, plants and animals. For instance, a virus cannot replicate itself outside the host cell. This is because viruses lack the required cellular machinery.
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. [1] Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. [2] [3] Viruses are found in almost every ecosystem on Earth and are the most numerous type of biological entity.[4] [5] Since Dmitri Ivanovsky's 1892 article describing a non ...
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
There are 5 modules in this course. This course describes the basic nature if viruses and basic concepts of how they replicate. It looks at traditional methods for propagating and assaying viruses as well as methods to identify and follow them in individuals and populations. It will also look at how we can use molecular genetics to learn how ...
HIV Coloring Assignment *Make sure you understand the steps involved in infection and how drugs treat the disease. Related to Viruses . Viroids - even smaller than viruses, consist of RNA strands that lack a protein coat Prions - "rogue protein", believed to be the cause of Mad Cow Disease, also may cause Kuru in cannibal tribes. Treatment of ...
Viruses are small obligate intracellular parasites, which by definition contain either a RNA or DNA genome surrounded by a protective, virus-coded protein coat. Viruses may be viewed as mobile genetic elements, most probably of cellular origin and characterized by a long co-evolution of virus and host. For propagation viruses depend on specialized host cells supplying the complex metabolic and ...
Assignments Exams Study Materials Lecture Videos. Lecture 32: Infectious Disease, Viruses, and Bacteria. Viewing videos requires an internet connection Description. This lecture covers microorganisms and some of the threats they pose to human health, such as infectious diseases. Professor Imperiali also discusses antibiotics and the mechanisms ...
A virus is a tiny infectious agent that reproduces inside the cells of living hosts. When infected, the host cell is forced to rapidly produce thousands of identical copies of the original virus. Unlike most living things, viruses do not have cells that divide; new viruses assemble in the infected host cell. But unlike simpler infectious agents ...
The opportunity to safely explore questions about viruses, virus transmission, and related health and safety can help students better understand the current pandemic and the science behind many of the strategies and variables that are often discussed in relation to COVID-19. Teach about Viruses and Healthy Practices with STEM Activities. 1.
Some viruses, for example, HIV and herpes simplex virus (HSV), cause both lytic and latent infections (Chapter 5). Some viruses infect bacteria, and the occurrence of such viral infections has led to considerations of viruses as therapeutic agents. For example, bacteriophages, viruses that speci cally infect bacteria, have
Viruses exact an enormous toll on the human population and are the single most important cause of infectious disease morbidity and mortality worldwide. Viral diseases in humans were first noted in ancient times and have since shaped our history. Scientific approaches to the study of viruses and viral disease began in the 19th century and led to ...
infect a human. The influenza virus is shed by infected birds in their excrement, mucus, and saliva. Humans can contract the virus through direct contact with an infected animal, droplet spread, contact with the environment of a sick animal, inhalation of airborne viruses, or fomite transmission, which is touching infected surfaces. Pigs
What is a Virus? Download PDF Copy. Revised. By Sally Robertson, B.Sc. Reviewed by Benedette Cuffari, M.Sc. A virus is the smallest type of parasite to exist and is typically within the size range ...