Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF

Assignment #4 HACCP Plan – (30

Objective of this Assignment: To design a quality risk management HACCP system and control for quality in food production.
Related papers
analisis puntos criticos de control haccp
Food-borne diseases of multiple aetiologies are a widespread and growing public health problem, both in developed and developing countries. Microbial health hazards are responsible for over 90% of the incidents of global food-borne illenesses. It is estimated that about one-third of people in the developing nations are affected by food-borne pathogens every year. Hazard analysis and critical control point (HACCP) is an internationally agreed approach to food safety management system. It was initially developed for use by food processors to prevent or control hazards, but the application of HACCP system has been evolving and expanding to form a basis for official food control and for establishing food safety standards for the international food trade as well. HACCP is a proactive approach to food safety management and it is flexible, where necessary control measures can be adapted to changes in operations. HACCP helps to target resources to the most critical part of the food operations, and it is applicable to the entire food chain, from the raw material to the end product. In addition, the application of HACCP system can aid inspection by food control regulatory authorities, and promote international trade by increasing buyer confidence in food safety. HACCP overcomes many of the limitations of the traditional approaches to food safety control. HACCP system is comprised of seven principles, and its application is not a stand-alone system, but it should be seen as an element of food safety management. It complements basic good hygienic practices in food safety assurance by targeting product-specific hazards, and devising control measures necessary for managing risks relevant to the product and conditions of operations. HACCP can be a powerful tool for the management of food safety only if it is correctly understood and applied, and if there is adequate commitment by the management for providing necessary resources and expertise. It is recommended that training of personnel in food industry, government, and academia in HACCP principles and applications, and increasing awareness of consumers are pertinent elements for effective implementation of HACCP programme.
Wayamba Journal of Animal Science
With the increasing demand and production of processed meat food, safety for the consumers is an aspect of great importance and it is a challenging task. It is well known that hazards (mainly pathogens) associated with food be the cause of food borne illness. Public health safety is a serious issue and can be prevented by adopting HACCP in food chain. HACCP program has been developed for consumer food safety as well as to protect manufacturer from food safetyrelated issues. The objective of this study is to determine the food safety measures related to the implementation of HACCP in frozen boneless buffalo meat manufacturing industry.
Food safety is a significant quality aspect of any food product. A food safety management system using Hazard Analysis Critical Control Points (HACCP) serves to identify 1) preventive steps to reduce hazards at each critical control point (CCP), 2) corrective responses if control limits are not met, and 3) documentation and verification requirements. This paper presents the implementation of a HACCP system for of a food manufacturer of flaked, ready-to-eat breakfast cereals in Trinidad. The company has implemented HACCP for its own benefit, and has not sought HACCP certification. The implementation has improved personal hygiene and sanitation practices, attained encouraging results of microbiological spot checks, and reduced customer complaints caused by internal infestation and extraneous matter by 71% and 83%, respectively. Improvements in good manufacturing practices were achieved in the areas of plant and equipment, operating procedures and policies. HACCP documents also assisted in...
To guarantee the safety of yoghurt, fermented cream and kariesh cheese production, the Hazard Analysis Critical Control Points (HACCP) system was applied to the production process. The biological, chemical, and physical hazards that may exist in every step of yoghurt, fermented cream and kariesh cheese production were identified. In addition, the critical control points were selected and the critical limits, monitoring, corrective measures, records, and verifications were established. The critical control point, which includes pasteurization, was identified. Implementing the HACCP system in food manufacturing can effectively ensure food safety and quality. The HACCP plan tasks positively influenced the microbiological quality in the assessment of the final products.
Food Control, 2014
Internet Journal of …, 2008
The Medieval Review, 2024
Antiquity left significant fingerprints across the Byzantine Empire. From physical sites such as urban spaces, civic structures, and their corresponding entrance gates, to the imaginative and syllogistic realms of novels and other literary forms, the past was spread ubiquitously across the present. In her second monograph, Between the Pagan Past and Christian Present in Byzantine Culture: Statues in Constantinople, 4th-13th Centuries CE, Paroma Chatterjee sinuously foregrounds how the vestiges of pre-Christian culture--and particularly sculptural representations of mythic heroes and animals, as well as famed monuments from the empire’s polytheistic history--resonated throughout the Constantinopolitan zeitgeist. While scholarship has consistently addressed Byzantium’s unsteady relationship with objects of Christian devotion, and particularly the lives and deaths of icons, Chatterjee takes interest in the under-discussed, but captivating roles that pagan statues occupied in the visual culture of the empire. Substituting a traditional Introduction with Chapter 1 (titled “The Byzantine Statue: Problems and Questions”), Chatterjee begins by outlining that pagan statues exacted “an enduring and crucial effect in the visual discourse” of Constantinople (33). She deftly points out to readers that, despite the sheer weight of research centering holy images, such artworks were not the sole constitutive parts of the empire’s visual identity, and that at certain points in its history, pagan statues were perceived as carrying a cultural importance that was comparable, if not equal, to icons. This is a unique assertion, and it is satisfactorily explicated: the author is quick to clarify that while pagan statues were apprehended with proportional cultural significance, they departed from icons in that they inhabited a category of image that was “resistant to imperial control and interference” (39). She outlines that Christian images were explicitly tied to constructs of power and easily interpreted by imperial authorities, but pagan statues were tethered to more amorphous structures--those of thought. (continued on TMR website.)
It's How You Flip It. Multiple Perspectives on Hip-Hop and Music Education, 2024
Belkıs Dinçol ve Ali Dinçol’a Armağan VITA, ed. . Alparslan, M.D. Alparslan ve H.Peker, ). 106-115, Ege Yayınları, İstanbul,, 2007
La lingua variabile nei testi letterari, artistici e funzionali contemporanei. Analisi, interpretazione, traduzione, a cura di F. P. Macaluso, Palermo, Centro di studi filologici e linguistici siciliani, 2014
Historia, 2023
IRJMETS Publication, 2021
Aurora, 2019
Revista Horizontes de Linguistica Aplicada, 2011
Pakistan Journal of Biological Sciences, 2000
Anais do 47º Congresso Brasileiro de Ciências da Comunicação (Intercom), 2024
Nephrology Dialysis Transplantation, 2002
Entropy, 2014
Geophysical Research Letters, 2011
Journal of Islamic Law Research, 2024
Biomedical microdevices, 2017
Organic letters, 2017
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Health Science
haccp assignment

Related documents
Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer
How to Write a HACCP Plan: Free HACCP Plan Template & eBook
Writing a HACCP plan is a crucial step in keeping control of food safety in the food industry.
Get your HACCP in 1 hour!

Create Your Food Safety System in 15 Minutes

Writing a HACCP plan is a crucial step in keeping control of food safety in the food industry.
If you are looking just for a summary table where the most important information is shown and do not need more comprehensive HACCP documents, then check out our free HACCP plan template below.
You can add the information you have gathered and further customize the HACCP plan anytime you want. It is also a good tool to play with to understand the meaning of the HACCP Plan .
We've got you covered with our HACCP plan example template . All you need to do is click on the editable cells and input your specific information. Customize the entire template according to your business operations .
Company name:
Date: _________.

HACCP Plan Template by www.fooddocs.com

Thank you for downloading our free template!
Want to create a full HACCP plan in 1 hour? How about a digital Food Safety Management System in 15 minutes?
Check our free tool
In this article, we also cover how to make a comprehensive HACCP Plan in just 1 hour or manually write the HACCP Plan with step-by-step instructions .
Or if you're tight for time right now, download our free How to Write a HACCP Plan eBook to read later.
Several hours of thought processes and physical inspections may go into making this food safety management plan. Building a comprehensive and accurate HACCP plan shows your committed approach to food safety for your consumers.
A HACCP plan can help prevent any unacceptable health risks and the occurrence of any severe illness as a result of consuming your finished products.
Key points covered:
- A HACCP plan is an industry standard for food safety that addresses the hazards in your operations by carefully identifying them and assigning preventive measures.
- While HACCP plans are the written and developed documents, a HACCP system is the implementation of said plan .
- Writing a HACCP plan requires a significant amount of time and knowledge of food safety.
- Teams often seek support from food safety management software companies and/or consultants .
- Every HACCP plan first begins with preoperational steps that include establishing a food safety team, reviewing Prerequisite Prorgams, outlining product and market information, and more.
- The steps involved in creating a HACCP plan are based on the seven principles of HACCP .
- FoodDocs equips businesses with a HACCP system that includes Monitoring and Traceability capabilities that make food safety management easy.
WHAT WE'LL COVER:
- Foundations of writing a HACCP plan
- Download the How to Write a HACCP Plan eBook
- Review prerequisite programs
- Perform 5 preliminary tasks
- Manually write the HACCP Plan
- Identify and analyze all hazards
- Establish critical control points
- Set up critical limits
- Build a monitoring procedure system for CCPs
- Identify corrective actions
- Verify the whole HACCP plan
- Establish record-keeping procedures
- How to best implement a HACCP system
In addition to being a mandate in many countries, making a HACCP plan ensures that your business or manufacturing plant only serves and produces safe food items free from hazards. If you are a new food industry player, the biggest question you might have is probably :
The foundation of how to write a HACCP plan
Let's start with a high-level view of what's going to be involved and outlined in detail later in this article. Before writing the HACCP plan itself, the process involves preoperational steps. These preparatory steps include:
- Reviewing Prerequisite Programs (PRPs)
- Establishing an interdisciplinary food safety team
- Laying out product and market information
- Constructing a commodity flow diagram
- Verifying all preoperational steps above
After preliminary tasks, what will the HACCP team need to work on?
Once you've done that, you can move onto the step-by-step process of writing a HACCP plan which are based on the seven principles of HACCP .
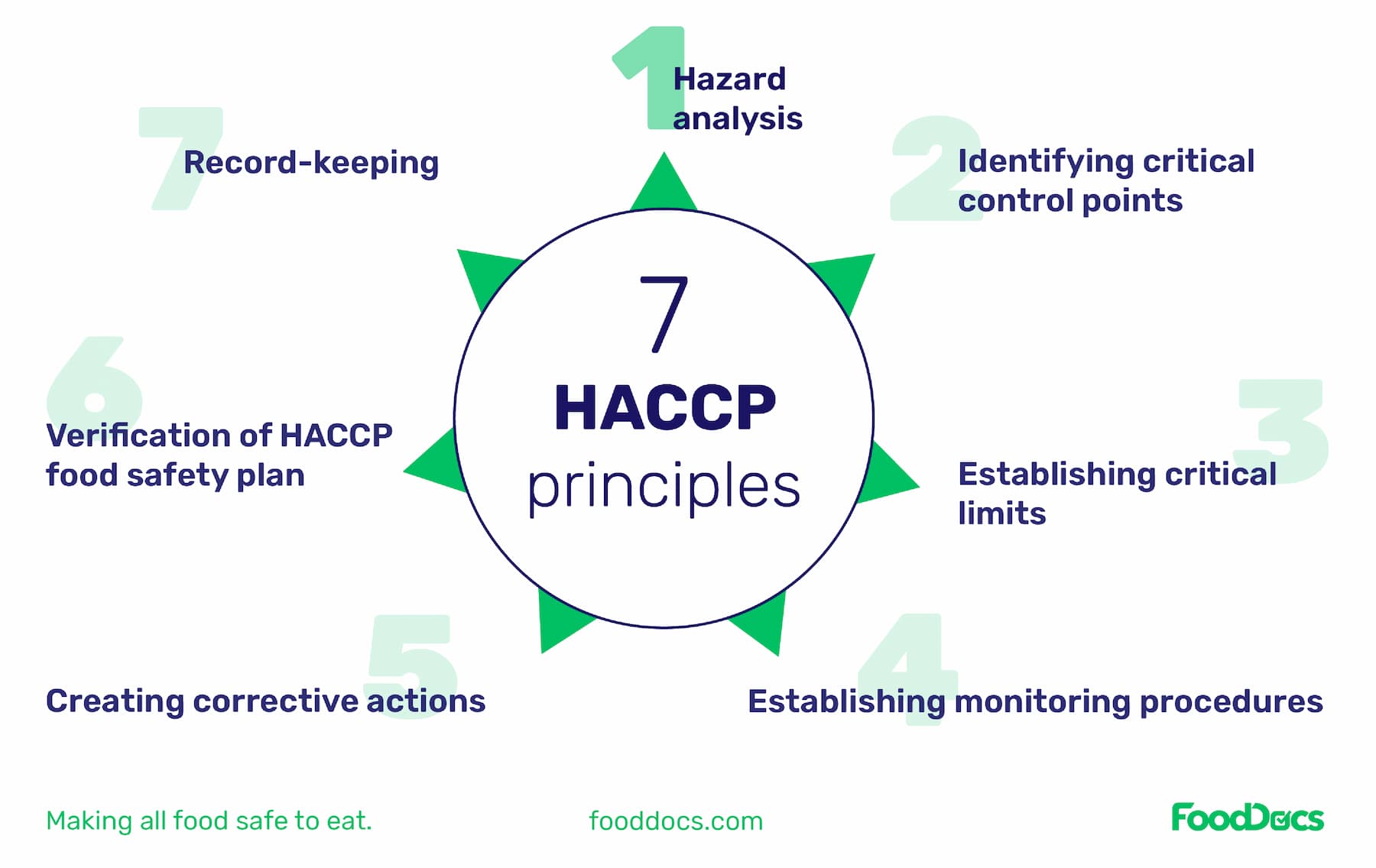
The 7 steps of writing a HACCP Plan are:
- Identify all Critical Control Points (CCPs)
- Establish corrective action procedures
- Establish verification procedures
- Align on record-keeping and documentation processes

A HACCP plan is an internationally recognized food safety management system that addresses the hazards in your food operations by carefully identifying them and assigning preventive measures.
Charles C., an ISFQN Moderator, shared in the International Food Safety and Quality Network forum that a HACCP system is larger in scope than a HACCP plan; the HACCP system is the implementation of the developed plan.
This food safety system aims to prevent any foodborne illness and other adverse health consequences of poor food employee practices from occurring. We also invite you to read more about the HACCP definition . Writing a HACCP plan involves several subsequent steps that you need to follow to achieve the system's best advantages.
How do you create a HACCP plan that complements your HACCP system
Behind every effective HACCP system is a well-designed HACCP plan.
Creating a HACCP plan needs preparation and the correct mindset. It’s a written plan that properly addresses the food safety risks of your food business, which requires focus and adequate knowledge about food safety and related manufacturing operations.
Making your own HACCP plan in-house promotes a sense of ownership and a general understanding of your food operations. In case of problems, you can resolve unexplained system failures if you know your plan in-depth.
While making a HACCP plan from scratch can be tedious, some food companies seek the advice of independent experts such as food safety consultants from regulatory agencies in the food industry.
Download the eBook

Thank you for downloading How to Write a HACCP Plan eBook!
Want to get a customizable HACCP template? Or set up your food safety system in 15 minutes?
Now, let's go into detail about the preparatory and process steps to create your HACCP plan.
HACCP Plan preparation steps
1. review your prerequisite programs.
The first step to creating a HACCP plan is to check your prerequisite programs. These programs are foundational to your eventual HACCP plan and ensure the basic conditions of the manufacturing process and service.
They address minimum quality standards regarding sanitary design principles, general food safety and hygiene practices, employee health, proper environmental conditions, and proper employee training.
Guaranteeing that your food business or manufacturing plant implements and maintains basic prerequisite principles and sanitary conditions ensures that low-risk that low-risk health hazards are controlled to safe levels.
Some basic prerequisite programs that can easily address low-risk food safety concerns may include:
- Sanitation standard operating procedures
- Sanitation design principles
- Proper layout of your food business (e.g., one-direction flow, easy-to-clean walls, and availability of handwashing facilities )
- Food safety posters and reminders
- Air and water controls
- Employee training on food hygiene
- Effective pest control program
- Proper waste management
2. Perform the five preliminary tasks of HACCP plan
Before building a complete HACCP plan, you need to clearly lay out important information about your products and food operations and establish a working team for the task. These preliminary steps are explained below.
1. Build your HACCP team
Who is responsible for creating the HACCP plan?
People who are responsible for creating the HACCP plan are key company employees from each department in your food business. Building a HACCP plan is not the sole responsibility of a food business manager.
Before you can put up a plan, you will need to build your HACCP team. Your team must consist of people who know how to make a HACCP plan or its basic principles.
An efficient HACCP plan team will includes members from any position responsible for your food production process to offer expert advice from their fields.
Having a team of purely quality control personnel or just a food manufacturing plant manager may lack the necessary information regarding equipment's technicalities. Similarly, a team composed entirely of only food engineers may lack expertise in quality control aspects.
Some of the members who will be tasked with establishing your HACCP system may include representatives from the quality assurance , engineering, manufacturing, logistics, and managerial departments.
Each representative must have substantial information about how the business operates. They will be responsible for raising both safety and quality concerns about the HACCP system concerning their departments and contribute to addressing them.
2. Identify your product's intended use and general food group
A HACCP plan is a food safety management system specific to a food business. It sets out food safety standards, rules, and preventative measures for operating to produce safe food products.
As such, it is important to identify the nature of your business, products, and target market to become the basis of your HACCP system. Below are some of the most important information that you need to provide for your HACCP plan:
Describing the product you are analyzing gives your team an idea of what hazards may be involved.
- Complete description of the product
- Ingredient declaration
- Allergen information
- Specifications and general food group
- Physical dimensions and visual appearance
- Method of storage between packaging and the end user
Your product description becomes more useful if the details become very specific.
The sample description of general food groups
Information such as the chemical and physical parameters of the raw materials and the finished product's titratable acidity level, moisture level, water activity, salt concentration, product formulation control, and chemical composition will contribute to hazard identification and their analysis.
This information may also include specifications from suppliers, including minimum enteric pathogen load.
3. Identify target consumers
Your team will also be responsible for identifying which market segment your product is made for. This step is particularly essential if your product is intended for niche consumers, which refers to those who have intolerances or weak immune systems.
These groups include infants, pregnant women, the elderly, and individuals with special diet needs.

Example of the market segment
4. Develop a block-type flow diagram of your food service operations
This commodity flow diagram is a simple outline of the whole production processing method of your food operations , from receiving the raw materials to the distribution procedures of the finished safe products.
Your simple schematic diagram describes the process steps needed to produce a specific product which means all inward and outward flows of processes must be included.
Your process flow diagram must be accurate and comprehensive. Every processing line must have its own flow chart. Differences in equipment design and process steps may render different conclusions in a HACCP plan.
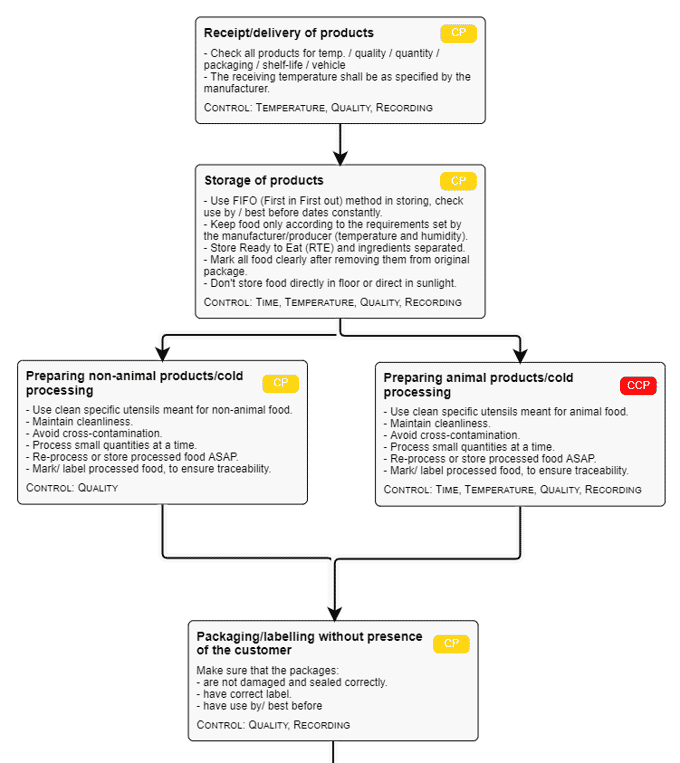
Free flow chart template from FoodDocs
We emphasize this importance because this flow chart will be your basis for later steps in knowing how to write a HACCP plan. Process flow charts differ depending on the type of product you are trying to analyze. The process of making a sandwich is very different from the pasteurization of milk.
Build your own flow chart using our free template tool (click on the link below the image) or save time and generate a flow chart automatically with all other required HACCP Plan parts with our HACCP builder.
5. Verify all preparatory steps
Traditionally, this step calls for an on-site monitoring & verification procedure for your commodity flow chart. We suggest applying the whole comprehensive verification activities to all of the mentioned preparatory steps.
All of these verification steps are vital to preparing your whole food business in making a HACCP plan the traditional way. Check for any spots in the preparatory plan that may have been missed during any activity of verification.
Need help preparing for your HACCP plan-making phase and its verification? The USDA has provided a guidebook for small to very small businesse s that want to comply with HACCP requirements .
3. Manually write the HACCP plan
After completing all mentioned prerequisite steps, you are ready to write a HACCP plan. These steps apply the seven major principles of a HACCP food safety program known throughout the food industry.
In this section, we will apply these principles to restaurant businesses, food processing plants, or retail food stores in order to answer the commonly asked question: "How do I write a HACCP plan?"
The key to making a comprehensive HACCP plan for a restaurant, or any food business for that matter, is the attention to detail and proper execution of each HACCP principle.
7 Steps to write a HACCP Plan
Below are the HACCP writing stages and accompanying HACCP plan examples you can use for inspiration.
So without further ado, what is the first step in developing a HACCP plan?
Step 1. Identify and analyze all hazards
A HACCP plan aims to control potential hazards in food to an acceptable level before they cause significant harm. To do this, you must accurately identify all of the potential food safety hazards in your operations — in other words, conduct a hazard analysis — before proceeding to any of the next operations in a HACCP plan.
Hazards are any material that can contaminate food and harm customers. These potential hazards may be classified as:
- Biological hazards (e.g., enteric pathogens such as cells of Salmonella and E. coli in raw meat)
- Physical hazards (e.g., stones, glass, seeds)
- Chemical hazards (e.g., cleaning solution)
They must be listed for further hazard analysis and identification of the point they would most likely occur.
In hazard identification, list the hazard's likelihood of occurrence, the duration of illness, and the level of risk of damage as an assessment for illness caused in addition to their proper identification.
After identifying and analyzing the hazards, control measures needed to control the hazard must be added. Each preventive and control measure must then be identified, whether it is a CCP with a YES or NO based on hazard risk assessment .
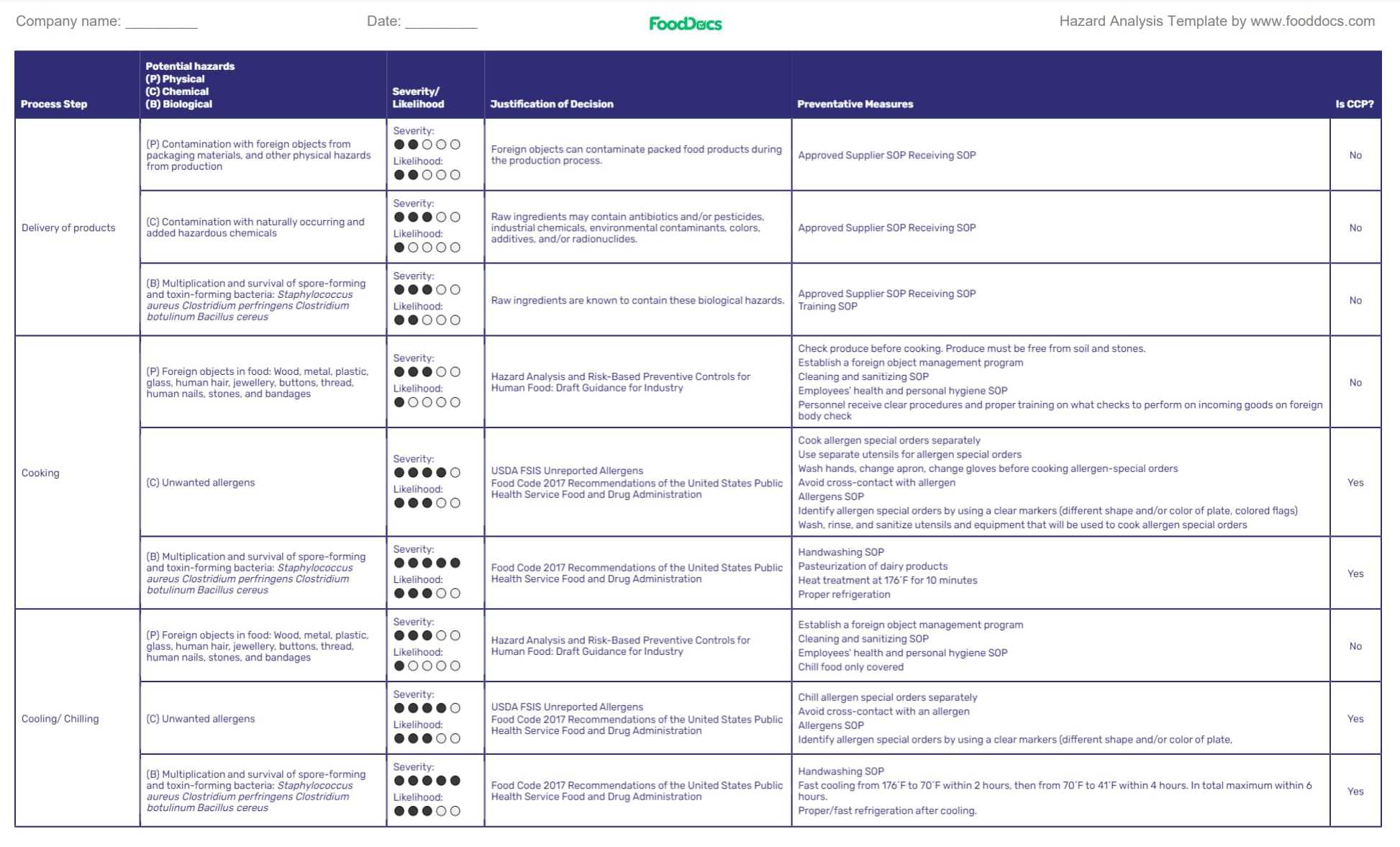
Free Hazard Analysis Template from FoodDocs
Hazards are quite specific to the type of food you are producing and the operations you are using. Some of the most common, unsafe food safety hazards in a restaurant or food service setup that can cause foodborne illness to consumers may come from:
- High level of physical agents in raw material production and receiving
- Biological hazard contamination due to poor employee health and personal hygiene
- Cleaning and sanitation solution
- Cross-contamination
- Food allergens
- Preparation of raw materials
- Inadequate cooking process to the correct internal temperature
- Improper product storage conditions (e.g., food temperature control, sanitation, and ventilation)
- Poor pest control
- Packaging materials (e.g., for takeout)
Safe food products can only be produced if your team properly performs hazard identification and analysis. This step is vital for setting up control measures in your operations.
Use our free Hazard Analysis worksheet template to help you map out and present all identified hazards in your food establishment.
Step 2. Establish critical control points (CCP)
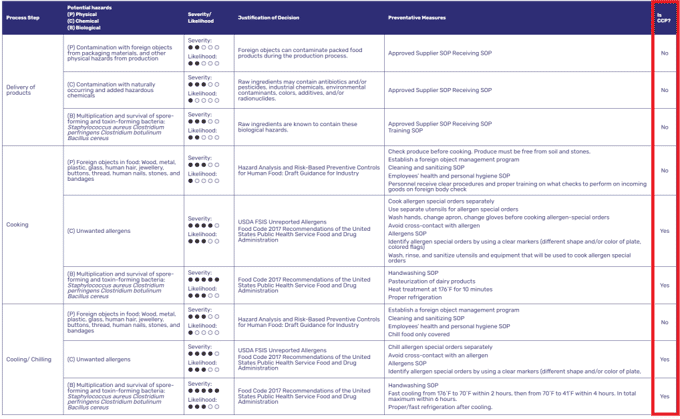
Hazard Analysis Template from FoodDocs (highlighted: CCP decision)
Identifying which among your controllable processing steps is a critical control point is also a mandatory element in knowing how to make a HACCP plan. From your previously analyzed hazards and designated preventive measures, your HACCP team must identify which steps are critical control points.
A critical control point is any step in your operation where measurable controls and critical limits can be applied to control specific hazards to an acceptable level. Learn more from our well-explained article on what is a critical control point .
Plainly, critical control points are your company's main defense against hazards. Evaluation of control measures can be done using established scientific literature surveys or decision tools such as a HACCP decision tree or a food risk assessment matrix.
In each step, one or multiple hazards may be addressed and must be listed under a food operation for evaluation. CCPs must be consistently applied to every batch of food products you serve.
Examples of CCPs may include the following for a restaurant business and other food establishments:
- Receiving and food supply
- Conditions of storage
- Cooking to minimum safe temperature s or other thermal processing
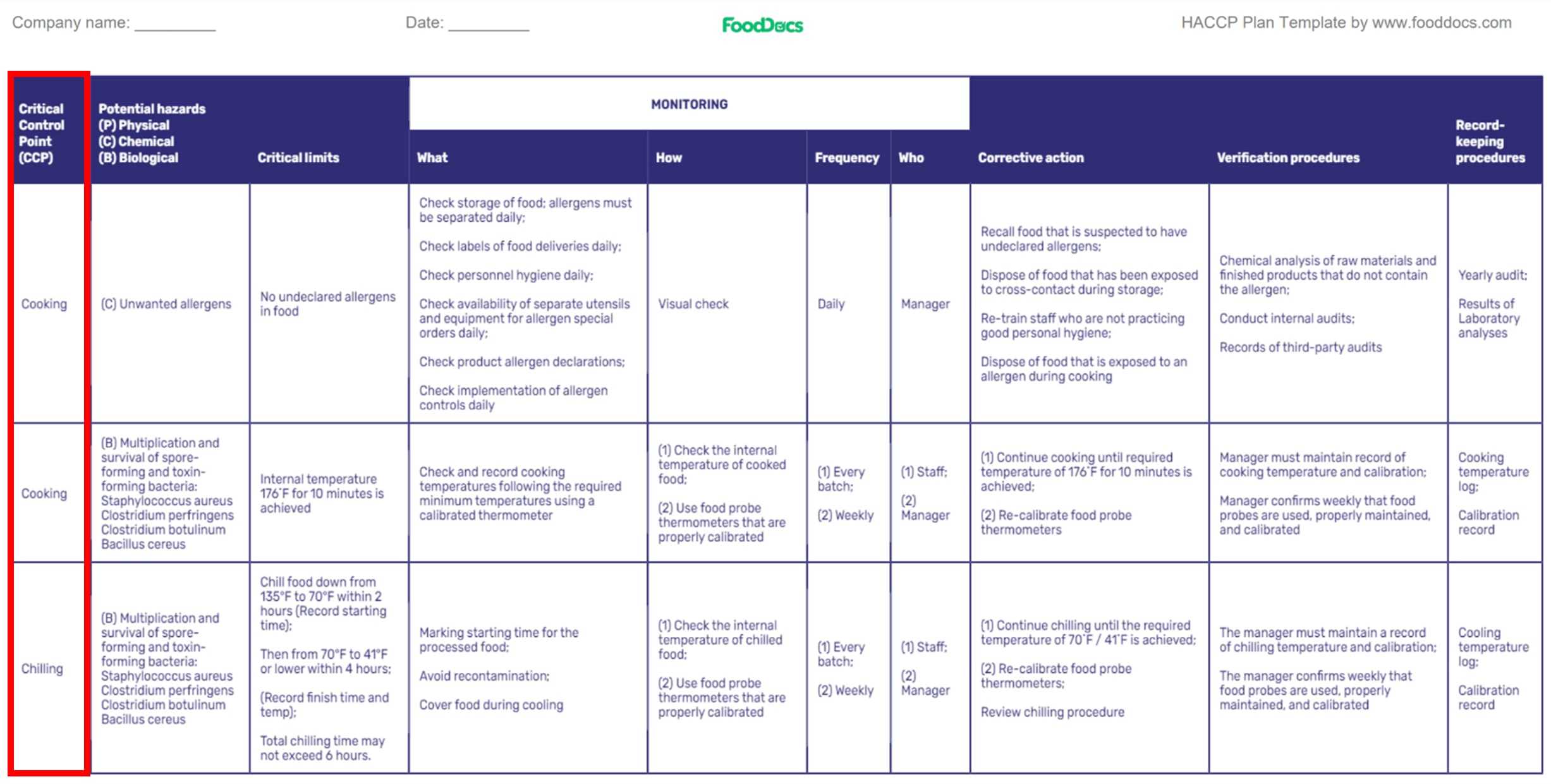
HACCP plan template (Highlighted: Critical Control Points from Hazard Analysis)
These critical control point examples may be more specific for some food businesses. Some operations, such as microbiological testing products, physical and chemical measurements, and analytical testing, may be applied to verify the effectiveness of CCPs in controlling hazards that may cause severe health effects.
A concrete example of a CCP in a restaurant is cooking beef patties. Undercooked beef patties have been related to causing severe illnesses in consumers due to the unwanted multiplication of pathogens.
Unwanted pathogen growth is caused by the prevalence of pathogenic microorganisms in a batch of undercooked beef patties. These biological hazards can be inactivated through adequate cooking of beef patties.
In this scenario, the proper cooking step to the correct internal temperatures can be considered a CCP since there will be no further processing after this step. Food preparations such as assembling the cooked patties and applying packaging materials will not be able to further eliminate pathogen growth.
The CCP ensures that cooked beef patties, including other raw meats, are free of microbiological pathogens and safe for consumption.
Read more about whether to choose a HACCP Decision tree or a risk assessment matrix for the safety of food in your operations.
Step 3. Set up critical limits
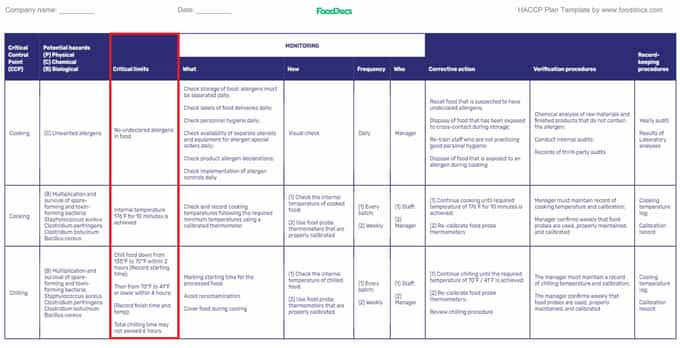
HACCP plan template (Highlighted: Critical Limits)
Identifying the risk level and likelihood of occurrence of a food safety hazard in combination with CCPs leads to establishing critical limits . These acceptable limits are the lowest and highest tolerable values and control factors for your critical control for safety.
Critical limit deviation may lead to the production of a non-compliant product or hazardous foods and cause severe health effects. Staying within the critical limit range is an essential criterion for food safety.
For a restaurant food business HACCP plan, typical critical limits are usually related to the:
- Adequate time-temperature combination for cooking food
- pH of beverage and water
- Minimum chlorine level for sanitizing solution
- Correct hot holding and cold holding temperature for food preparation .
Critical limits can be science-based facts for regulatory standards such as the minimum and maximum limits for raw meat's internal temperature for a thermal process. Breach of critical limits may lead to the production of unsafe food and unacceptable health risks.
A good example of setting critical limits is when storing foods away from ambient temperature or the temperature danger zone . High-risk foods must be stored using hot or cold holding procedures as a control measure.
The general method of storage rule is for any safe food product to not stay for more than two hours in temperature ranges between 41°F to 140°F (5°C to 60°C) or microbial pathogens may spoil the food. For cold holding at 41°F (5°C) or lower, the critical limit would be 41°F (5°C). For improper storage beyond this point, corrective actions for deviations must be applied to the non-compliant product.
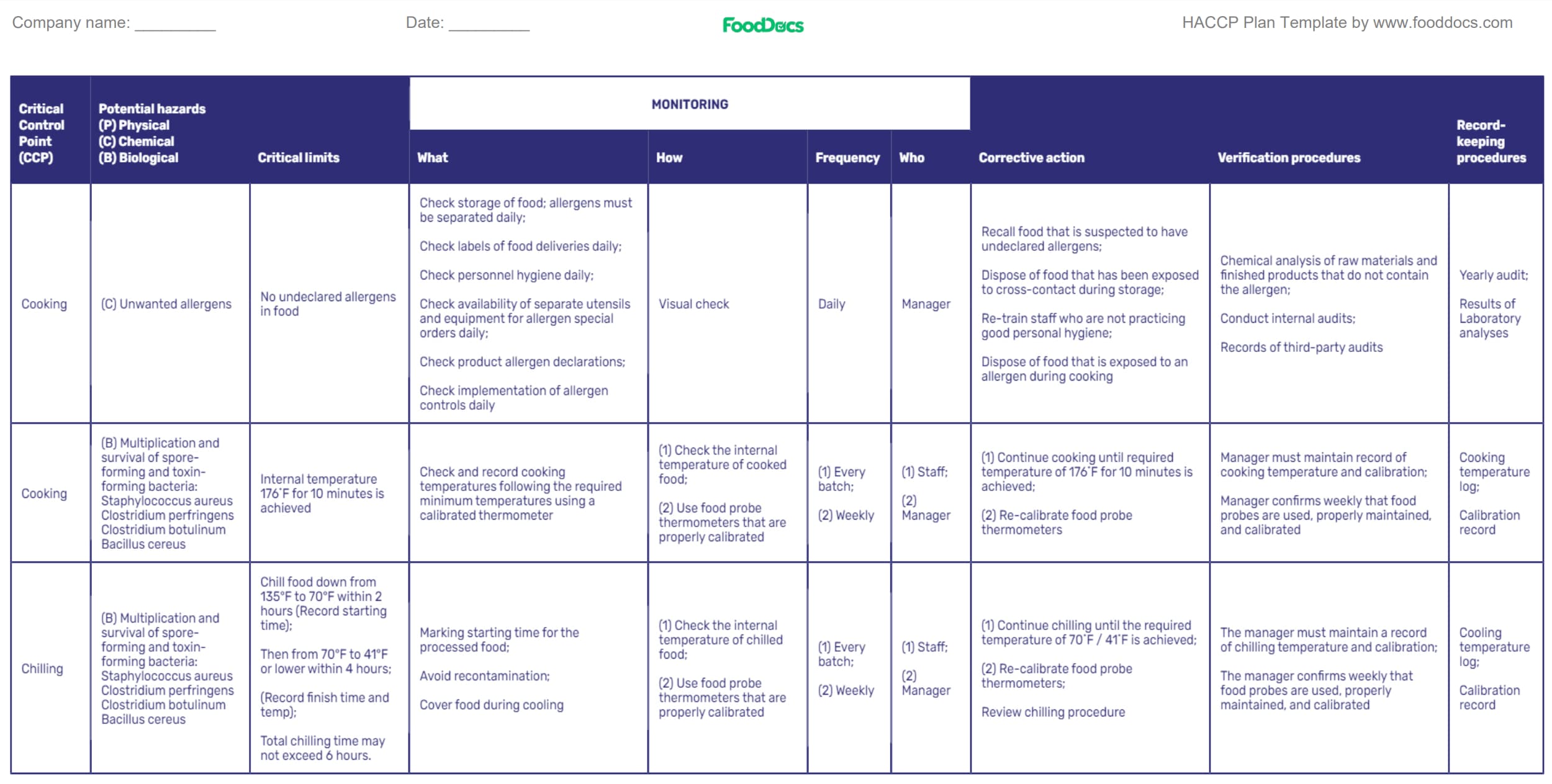
Step 4. Build a monitoring procedure system for CCPs
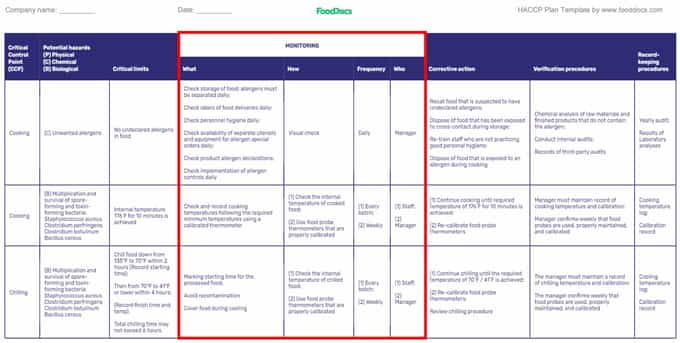
HACCP plan template: (Highlighted Monitoring procedures)
To ensure that the CCPs and other control measures do their intended purpose, your HACCP team is tasked with establishing CCP monitoring procedures and have the responsibility for oversight of production.
These monitoring activities aim to record a sequence of observations for CCPs and will serve as documentation after the food operations in appropriate monitoring logs . They can serve as proof that your HACCP food safety plan is working.
Examples of monitoring activities on essential food preparation steps may include physical and chemical methods with appropriate logs. Monitoring forms or logs are composed of several parameters that need to be filled by the person with the position responsible for daily record review. These parameters vary in frequency of continuous monitoring.
Each CCP will need a corresponding monitoring form to determine any concerns, such as loss of control in their critical limits. A good monitoring form must be comprehensive and flexible. Some components of a monitoring record may include:
- What: Parameters to monitor
- How: Procedures for monitoring
- When: Monitoring frequency
- Who: Individuals responsible for monitoring
- Summary and remarks
The complexity of monitoring procedures may depend on these factors as well.
For a restaurant food business, monitoring techniques will require you to create logs for the following activities:
- Internal temperature logs
- Critical limit monitoring logs
- Corrective action log
- Deviation log
The responsibility for monitoring must be clearly communicated to employees in charge of food safety and quality control. Employees must undergo an effective training program to familiarize themselves with the monitoring procedures. Parameters that need to be included in monitoring records and the appropriate critical limits must be clear to avoid producing unsafe food.
Monitoring records must clearly represent what occurred during food preparation as proof of food safety compliance. Any critical point unaccompanied by a monitoring procedure may introduce a chance for cross-contact product contamination.
Step 5. Identify corrective actions
A HACCP plan is a systematic approach to food safety, but that doesn't mean it's a zero-risk approach. This means there may be lapses in the system, especially if there is due maintenance in the operations or other unforeseen circumstances. Monitoring procedures can reveal a trend towards loss of control, which is when corrective actions are needed.
Corrective actions aim to gain control over the operations again and address the remaining food safety risk with little to no compromise to the food products.
Corrective actions are meant to maintain food safety when a critical control point fails to control a hazard. In establishing corrective actions for deviations, an employee must be assigned to conduct the task and audit why the corrective action was applied.
An example of a corrective action procedure in a restaurant setup would be if a thermometer failed to read the correct temperature of the food for consumption. The inadequate cooking step will encourage the multiplication of pathogens.
The first corrective action may be to prolong the cooking time and use a different thermometer to address this. After the process, ensure the calibration schedules for all thermometers to prevent similar instances. Calibration schedules must always be followed to ensure consistently accurate records.
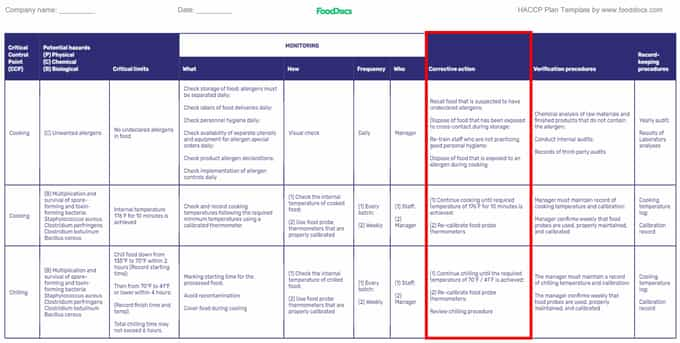
HACCP plan template (Highlighted: Corrective Actions)
Step 6. Verify the whole HACCP plan
Learning how to write a HACCP plan means knowing that this food safety system is based on scientific studies and facts, and that all steps must undergo verification. This step aims to ensure that all procedures work and achieve their targeted functions when needed. The initial validation step can be in the form of internal audits.
Verification and validation of your system is not a one-time thing. An aspect of verification is the commitment to food safety that ensures your HACCP system is still effective and does not need to be revised. External audits, frequent reviews, and health inspections from regulatory agencies are occasionally conducted to validate your system.
Your verification procedures include product testing, CCP evaluation, in-house plant observations, revision of operational limits, and review of monitoring records.
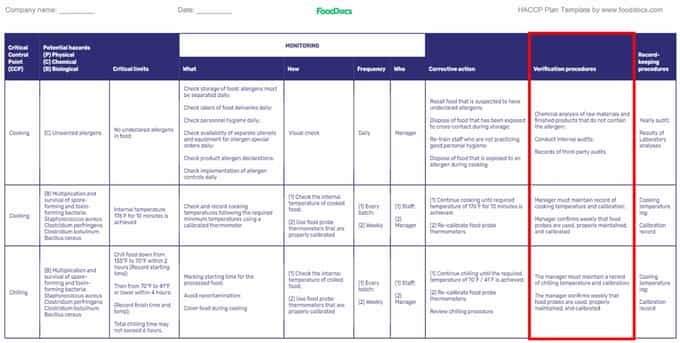
HACCP plan template (Highlighted: Verification Procedures)
Step 7. Record-keeping and documentation
A HACCP plan is a system that requires extensive documentation. Every monitoring, revision, deviation, corrective action record, sampling system, layout, employee training record, certificate, calibration record, and verification report must be properly documented.
Individuals responsible for record-keeping are required to keep all these documents for a minimum of 2 years for future verification and reviews.
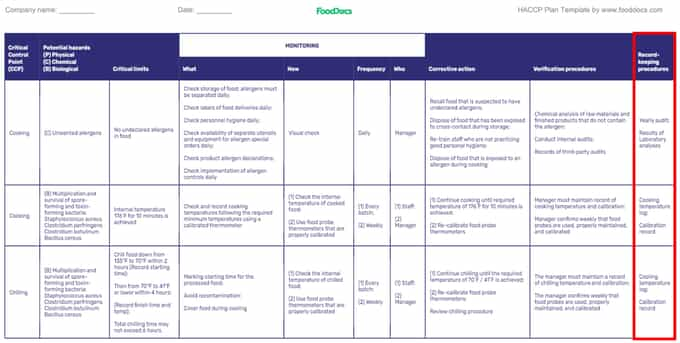
HACCP plan template (Highlight: Determining Record-keeping Procedures)
Proper documentation is key proof that your food business has successfully implemented your HACCP plan and that your team is performing their assigned tasks. If in case a known hazard occurs in your operations, your record will be proof for regulatory agencies that you applied control measures to bring the hazard to an acceptable level.
A great way how to create a HACCP plan accurate record-keeping system is to have sets of templates for all your procedures. This allows you to easily add new information and tailor the forms to your food business.
Use our free monitoring log templates from our food safety template hub .
How can a HACCP plan best be implemented?
FoodDocs is the only digital solution that offers an AI-powered HACCP plan builder. In just one hour, you'll get a comprehensive and working HACCP plan — based on your specific business operations — that you can start using quickly in your HACCP system . No more long hours of meetings and revisions.
And, if needed, the HACCP plan is fully customizable so you can make updates as your operations change or easily tweak sections based on and inspector's or auditor's feedback!

FoodDocs' HACCP system also supports your monitoring and traceability needs
The smart HACCP system includes the most important components of a food safety plan by getting your answers to basic questions during the setup process.
Our solution cross-references the information with our digital food safety knowledge library and uses artificial intelligence to generate the template. Some of the food safety questions include:
What is your business type?
What raw materials are used?
What kind of food do you sell?
How big is the volume?
What duties do you have in your business?
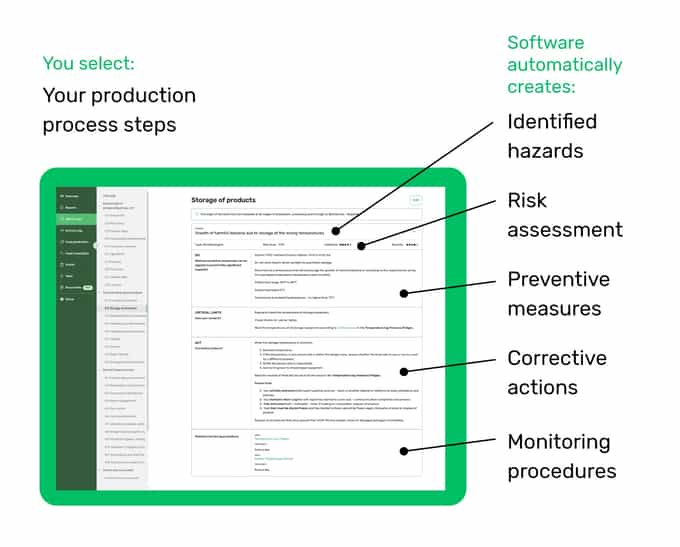
As seen in the example of the HACCP plan above, our digital solution can generate the most important information for you. What's even greater is that you can customize the information to fit your business better.
Our system generates a customizable HACCP plan template based on your answers to a few questions about your business. This helps us identify the necessary procedures and forms related to your food business and tailor the HACCP plan for you.
Here is an example of the contents of a HACCP plan template you will get when you sign up with us at FoodDocs.
- Basic and advanced prerequisite programs
- Flow chart of your food operations
- Identification of hazards and a complete analysis table
- Established critical control points
- Established critical limits
- CCP monitoring procedures
- Verification procedures
- Record-keeping and documentation procedures
In addition to the AI-powered HACCP Plan builder, FoodDocs also offers an all-in-one food safety monitoring and traceability system. Check out the two-minute FSMS explainer video below:
What are some of the advantages you'll get by using FoodDocs' HACCP system?
- All generated information is based on the knowledge of our food safety experts, who have worked in the food chain industry for many years, stored in a machine learning program . This program additionally cross-references your food business with other similarly-natured ones for better suggestions.
- We automatically identify all potential hazards related to your food business.
- Our system evaluates the identified food hazards through an established risk assessment matrix. We identify their level of likelihood of occurrence and potential damage. We then suggest which action level is required for each hazard based on our risk assessment matrix (e.g., critical point, critical control point, prerequisite program , or operational prerequisite program).
- Save time from manually making a HACCP plan. Our system can get you the plan template you need 500x faster than hiring a food safety consultant .
- Our system accounts for major food safety rules and legislation in your area .
- Our HACCP plan template builder is compatible with food service, retail food stores, and productions.
- Ask for third-party comments and apply them immediately to improve your HACCP Plan .
- Share your digital HACCP Plan with local authorities fast via email or download and print your documents.
- All information is securely stored in the cloud and accessible whenever you need it. Allowing you to implement your HACCP food safety system more sustainably.
- You can upgrade to HACCP-based food safety monitoring automatically if needed in future
There is literally no reason for you not to go digital to prevent losing control of food safety. All it takes is about an hour of your time to build your digital HACCP plan with our services, and you can start your 14-day free trial today!

In conclusion, why are HACCP plans and systems important?
A HACCP plan is an important food safety management document to help food business owners prevent loss of control over food safety and protect their consumers from any foodborne illness by establishing basic conditions of safety.
Food safety teams write a HACCP plan to identify and analyze food hazards and establish operations that will control or eliminate these potential hazards.
Every food business needs a safety plan with proper control measures to produce safe food products. The HACCP program is considered one of the standard plans in the food chain and has been adopted by different countries and organizations worldwide for educational programs on food safety. Several HACCP-based food safety plans are present in the food industry.
HACCP ensures that a food business does not have unsafe operating conditions that will affect the production of wholesome foods on a continuous basis. It aims to create conducive and sanitary conditions for food production.
Below is an example HACCP plan to help you visualize what it would look like and how can our team at FoodDocs help you:
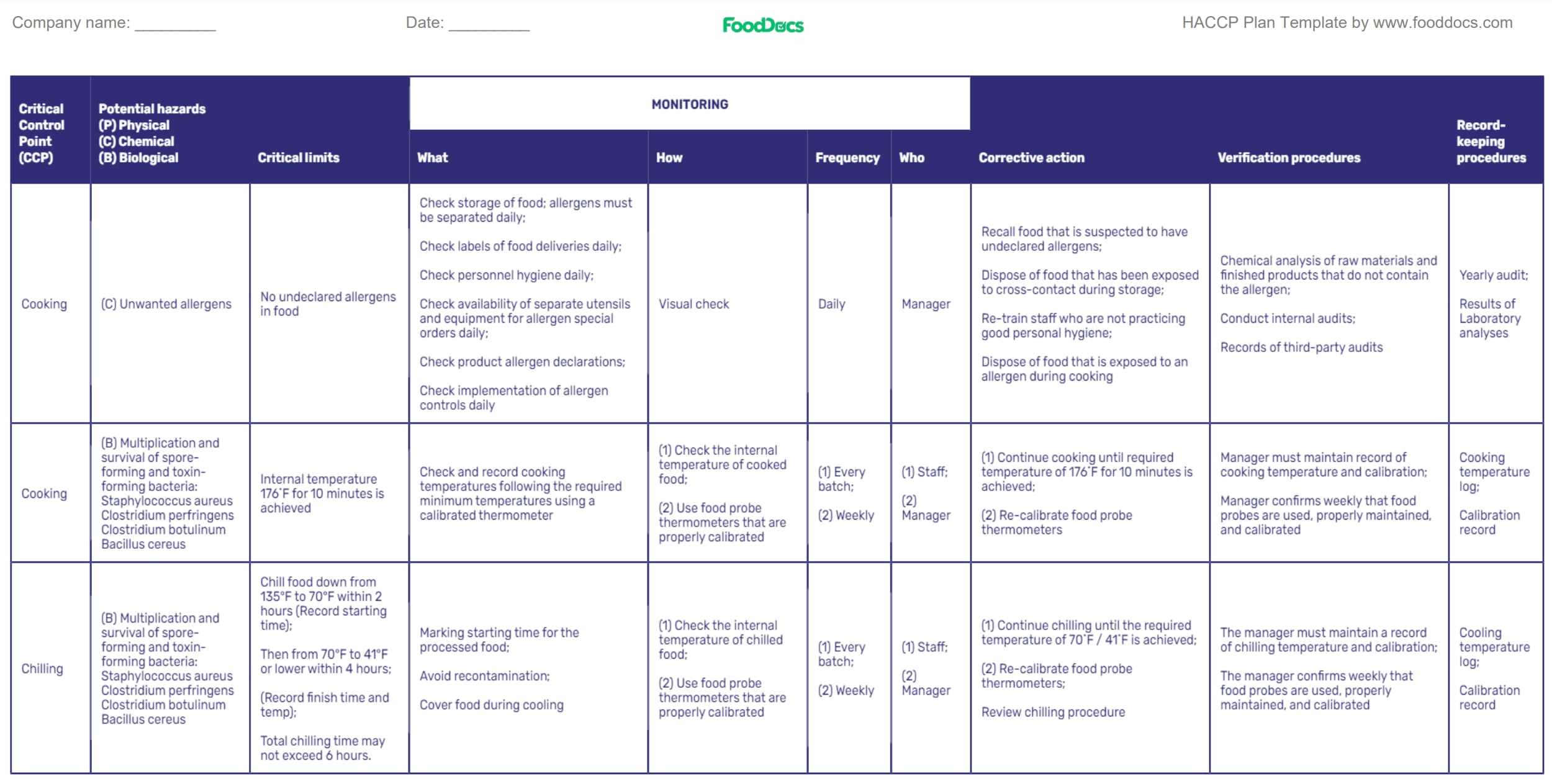
The likelihood of causing any severe illness or a foodborne outbreak is low with a comprehensive HACCP plan. With a working HACCP plan, less end-product testing and post-analyses would be required for the purposes of product safety, and wholesome foods would be ensured.

Similar posts
The haccp system - when should a haccp system be reviewed.
HACCP system refers to the food safety management system. A successful HACCP system requires regular updates for the following reasons. See the...
What is the purpose of a HACCP program? HACCP program example
A HACCP program is a food safety system that controls hazards with critical control measures.
Seafood HACCP - All you need to know
To identify whether your food processing business qualifies for a seafood HACCP plan, take a look at some examples of seafood products.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
On Oct. 1, 2024, the FDA began implementing a reorganization impacting many parts of the agency. We are in the process of updating FDA.gov content to reflect these changes.
U.S. Food and Drug Administration
- Search
- Menu
- Guidance & Regulation (Food and Dietary Supplements)
- Hazard Analysis Critical Control Point (HACCP)
HACCP Principles & Application Guidelines
Adopted August 14, 1997
NATIONAL ADVISORY COMMITTEE ON MICROBIOLOGICAL CRITERIA FOR FOODS
The National Advisory Committee on Microbiological Criteria for Foods (NACMCF) is an advisory committee chartered under the U.S. Department of Agriculture (USDA) and comprised of participants from the USDA (Food Safety and Inspection Service), Department of Health and Human Services (U.S. Food and Drug Administration and the Centers for Disease Control and Prevention) the Department of Commerce (National Marine Fisheries Service), the Department of Defense (Office of the Army Surgeon General), academia, industry and state employees. NACMCF provides guidance and recommendations to the Secretary of Agriculture and the Secretary of Health and Human Services regarding the microbiological safety of foods.
Table of Contents
EXECUTIVE SUMMARY
Definitions, haccp principles, guidelines for application of haccp principles.
Introduction
Prerequisite Programs
Education and Training
Developing a HACCP Plan
- Assemble the HACCP team
Describe the food and its distribution
Describe the intended use and consumers of the food
Develop a flow diagram which describes the process
Verify the flow diagram
Conduct a hazard analysis (Principle 1)
Determine critical control points (CCPs) (Principle 2)
Establish critical limits (Principle 3)
Establish monitoring procedures (Principle 4)
Establish corrective actions (Principle 5)
Establish verification procedures (Principle 6)
Establish record-keeping and documentation procedures (Principle 7)
IMPLEMENTATION AND MAINTENANCE OF THE HACCP PLAN
APPENDIX A - Examples of common prerequisite programs
APPENDIX B - Example of a flow diagram for the production of frozen cooked beef patties.
APPENDIX C - Examples of questions to be considered when conducting a hazard analysis
APPENDIX D - Examples of how the stages of hazard analysis are used to identify and evaluate hazards
APPENDIX E - Example I of a CCP decision tree
APPENDIX F - Example II of a CCP decision tree
APPENDIX G - Examples of verification activities
APPENDIX H - Examples of HACCP records
The National Advisory Committee on Microbiological Criteria for Foods (Committee) reconvened a Hazard Analysis and Critical Control Point (HACCP) Working Group in 1995. The primary goal was to review the Committee's November 1992 HACCP document, comparing it to current HACCP guidance prepared by the Codex Committee on Food Hygiene. Based upon its review, the Committee made the HACCP principles more concise; revised and added definitions; included sections on prerequisite programs, education and training, and implementation and maintenance of the HACCP plan; revised and provided a more detailed explanation of the application of HACCP principles; and provided an additional decision tree for identifying critical control points (CCPs).
The Committee again endorses HACCP as an effective and rational means of assuring food safety from harvest to consumption. Preventing problems from occurring is the paramount goal underlying any HACCP system. Seven basic principles are employed in the development of HACCP plans that meet the stated goal. These principles include hazard analysis, CCP identification, establishing critical limits, monitoring procedures, corrective actions, verification procedures, and record-keeping and documentation. Under such systems, if a deviation occurs indicating that control has been lost, the deviation is detected and appropriate steps are taken to reestablish control in a timely manner to assure that potentially hazardous products do not reach the consumer.
In the application of HACCP, the use of microbiological testing is seldom an effective means of monitoring CCPs because of the time required to obtain results. In most instances, monitoring of CCPs can best be accomplished through the use of physical and chemical tests, and through visual observations. Microbiological criteria do, however, play a role in verifying that the overall HACCP system is working.
The Committee believes that the HACCP principles should be standardized to provide uniformity in training and applying the HACCP system by industry and government. In accordance with the National Academy of Sciences recommendation, the HACCP system must be developed by each food establishment and tailored to its individual product, processing and distribution conditions.
In keeping with the Committee's charge to provide recommendations to its sponsoring agencies regarding microbiological food safety issues, this document focuses on this area. The Committee recognizes that in order to assure food safety, properly designed HACCP systems must also consider chemical and physical hazards in addition to other biological hazards.
For a successful HACCP program to be properly implemented, management must be committed to a HACCP approach. A commitment by management will indicate an awareness of the benefits and costs of HACCP and include education and training of employees. Benefits, in addition to enhanced assurance of food safety, are better use of resources and timely response to problems.
The Committee designed this document to guide the food industry and advise its sponsoring agencies in the implementation of HACCP systems.
CCP Decision Tree: A sequence of questions to assist in determining whether a control point is a CCP.
Control: (a) To manage the conditions of an operation to maintain compliance with established criteria. (b) The state where correct procedures are being followed and criteria are being met.
Control Measure: Any action or activity that can be used to prevent, eliminate or reduce a significant hazard.
Control Point: Any step at which biological, chemical, or physical factors can be controlled.
Corrective Action: Procedures followed when a deviation occurs.
Criterion: A requirement on which a judgement or decision can be based.
Critical Control Point: A step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level.
Critical Limit: A maximum and/or minimum value to which a biological, chemical or physical parameter must be controlled at a CCP to prevent, eliminate or reduce to an acceptable level the occurrence of a food safety hazard.
Deviation: Failure to meet a critical limit.
HACCP: A systematic approach to the identification, evaluation, and control of food safety hazards.
HACCP Plan: The written document which is based upon the principles of HACCP and which delineates the procedures to be followed.
HACCP System: The result of the implementation of the HACCP Plan.
HACCP Team: The group of people who are responsible for developing, implementing and maintaining the HACCP system.
Hazard: A biological, chemical, or physical agent that is reasonably likely to cause illness or injury in the absence of its control.
Hazard Analysis: The process of collecting and evaluating information on hazards associated with the food under consideration to decide which are significant and must be addressed in the HACCP plan.
Monitor: To conduct a planned sequence of observations or measurements to assess whether a CCP is under control and to produce an accurate record for future use in verification.
Prerequisite Programs: Procedures, including Good Manufacturing Practices, that address operational conditions providing the foundation for the HACCP system.
Severity: The seriousness of the effect(s) of a hazard.
Step: A point, procedure, operation or stage in the food system from primary production to final consumption.
Validation: That element of verification focused on collecting and evaluating scientific and technical information to determine if the HACCP plan, when properly implemented, will effectively control the hazards.Verification: Those activities, other than monitoring, that determine the validity of the HACCP plan and that the system is operating according to the plan.
HACCP is a systematic approach to the identification, evaluation, and control of food safety hazards based on the following seven principles:
Principle 1: Conduct a hazard analysis.
Principle 2: Determine the critical control points (CCPs).
Principle 3: Establish critical limits.
Principle 4: Establish monitoring procedures.
Principle 5: Establish corrective actions.
Principle 6: Establish verification procedures.
Principle 7: Establish record-keeping and documentation procedures.
HACCP is a management system in which food safety is addressed through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product. For successful implementation of a HACCP plan, management must be strongly committed to the HACCP concept. A firm commitment to HACCP by top management provides company employees with a sense of the importance of producing safe food.
HACCP is designed for use in all segments of the food industry from growing, harvesting, processing, manufacturing, distributing, and merchandising to preparing food for consumption. Prerequisite programs such as current Good Manufacturing Practices (cGMPs) are an essential foundation for the development and implementation of successful HACCP plans. Food safety systems based on the HACCP principles have been successfully applied in food processing plants, retail food stores, and food service operations. The seven principles of HACCP have been universally accepted by government agencies, trade associations and the food industry around the world.
The following guidelines will facilitate the development and implementation of effective HACCP plans. While the specific application of HACCP to manufacturing facilities is emphasized here, these guidelines should be applied as appropriate to each segment of the food industry under consideration.
The production of safe food products requires that the HACCP system be built upon a solid foundation of prerequisite programs. Examples of common prerequisite programs are listed in Appendix A . Each segment of the food industry must provide the conditions necessary to protect food while it is under their control. This has traditionally been accomplished through the application of cGMPs. These conditions and practices are now considered to be prerequisite to the development and implementation of effective HACCP plans. Prerequisite programs provide the basic environmental and operating conditions that are necessary for the production of safe, wholesome food. Many of the conditions and practices are specified in federal, state and local regulations and guidelines (e.g., cGMPs and Food Code). The Codex Alimentarius General Principles of Food Hygiene describe the basic conditions and practices expected for foods intended for international trade. In addition to the requirements specified in regulations, industry often adopts policies and procedures that are specific to their operations. Many of these are proprietary. While prerequisite programs may impact upon the safety of a food, they also are concerned with ensuring that foods are wholesome and suitable for consumption ( Appendix A ). HACCP plans are narrower in scope, being limited to ensuring food is safe to consume.
The existence and effectiveness of prerequisite programs should be assessed during the design and implementation of each HACCP plan. All prerequisite programs should be documented and regularly audited. Prerequisite programs are established and managed separately from the HACCP plan. Certain aspects, however, of a prerequisite program may be incorporated into a HACCP plan. For example, many establishments have preventive maintenance procedures for processing equipment to avoid unexpected equipment failure and loss of production. During the development of a HACCP plan, the HACCP team may decide that the routine maintenance and calibration of an oven should be included in the plan as an activity of verification. This would further ensure that all the food in the oven is cooked to the minimum internal temperature that is necessary for food safety.
The success of a HACCP system depends on educating and training management and employees in the importance of their role in producing safe foods. This should also include information the control of foodborne hazards related to all stages of the food chain. It is important to recognize that employees must first understand what HACCP is and then learn the skills necessary to make it function properly. Specific training activities should include working instructions and procedures that outline the tasks of employees monitoring each CCP.
Management must provide adequate time for thorough education and training. Personnel must be given the materials and equipment necessary to perform these tasks. Effective training is an important prerequisite to successful implementation of a HACCP plan.
The format of HACCP plans will vary. In many cases the plans will be product and process specific. However, some plans may use a unit operations approach. Generic HACCP plans can serve as useful guides in the development of process and product HACCP plans; however, it is essential that the unique conditions within each facility be considered during the development of all components of the HACCP plan.
In the development of a HACCP plan, five preliminary tasks need to be accomplished before the application of the HACCP principles to a specific product and process. The five preliminary tasks are given in Figure 1.
Figure 1. Preliminary Tasks in the Development of the HACCP Plan
Assemble the HACCP Team
The first task in developing a HACCP plan is to assemble a HACCP team consisting of individuals who have specific knowledge and expertise appropriate to the product and process. It is the team's responsibility to develop the HACCP plan. The team should be multi disciplinary and include individuals from areas such as engineering, production, sanitation, quality assurance, and food microbiology. The team should also include local personnel who are involved in the operation as they are more familiar with the variability and limitations of the operation. In addition, this fosters a sense of ownership among those who must implement the plan. The HACCP team may need assistance from outside experts who are knowledgeable in the potential biological, chemical and/or physical hazards associated with the product and the process. However, a plan which is developed totally by outside sources may be erroneous, incomplete, and lacking in support at the local level.
Due to the technical nature of the information required for hazard analysis, it is recommended that experts who are knowledgeable in the food process should either participate in or verify the completeness of the hazard analysis and the HACCP plan. Such individuals should have the knowledge and experience to correctly: (a) conduct a hazard analysis; (b) identify potential hazards; (c) identify hazards which must be controlled; (d) recommend controls, critical limits, and procedures for monitoring and verification; (e) recommend appropriate corrective actions when a deviation occurs; (f) recommend research related to the HACCP plan if important information is not known; and (g) validate the HACCP plan.
The HACCP team first describes the food. This consists of a general description of the food, ingredients, and processing methods. The method of distribution should be described along with information on whether the food is to be distributed frozen, refrigerated, or at ambient temperature.
Describe the normal expected use of the food. The intended consumers may be the general public or a particular segment of the population (e.g., infants, immunocompromised individuals, the elderly, etc.).
The purpose of a flow diagram is to provide a clear, simple outline of the steps involved in the process. The scope of the flow diagram must cover all the steps in the process which are directly under the control of the establishment. In addition, the flow diagram can include steps in the food chain which are before and after the processing that occurs in the establishment. The flow diagram need not be as complex as engineering drawings. A block type flow diagram is sufficiently descriptive (see Appendix B ). Also, a simple schematic of the facility is often useful in understanding and evaluating product and process flow.
The HACCP team should perform an on-site review of the operation to verify the accuracy and completeness of the flow diagram. Modifications should be made to the flow diagram as necessary and documented.
After these five preliminary tasks have been completed, the seven principles of HACCP are applied.
After addressing the preliminary tasks discussed above, the HACCP team conducts a hazard analysis and identifies appropriate control measures. The purpose of the hazard analysis is to develop a list of hazards which are of such significance that they are reasonably likely to cause injury or illness if not effectively controlled. Hazards that are not reasonably likely to occur would not require further consideration within a HACCP plan. It is important to consider in the hazard analysis the ingredients and raw materials, each step in the process, product storage and distribution, and final preparation and use by the consumer. When conducting a hazard analysis, safety concerns must be differentiated from quality concerns. A hazard is defined as a biological, chemical or physical agent that is reasonably likely to cause illness or injury in the absence of its control. Thus, the word hazard as used in this document is limited to safety.
A thorough hazard analysis is the key to preparing an effective HACCP plan. If the hazard analysis is not done correctly and the hazards warranting control within the HACCP system are not identified, the plan will not be effective regardless of how well it is followed.
The hazard analysis and identification of associated control measures accomplish three objectives: Those hazards and associated control measures are identified. The analysis may identify needed modifications to a process or product so that product safety is further assured or improved. The analysis provides a basis for determining CCPs in Principle 2.
The process of conducting a hazard analysis involves two stages. The first, hazard identification, can be regarded as a brain storming session. During this stage, the HACCP team reviews the ingredients used in the product, the activities conducted at each step in the process and the equipment used, the final product and its method of storage and distribution, and the intended use and consumers of the product. Based on this review, the team develops a list of potential biological, chemical or physical hazards which may be introduced, increased, or controlled at each step in the production process. Appendix C lists examples of questions that may be helpful to consider when identifying potential hazards. Hazard identification focuses on developing a list of potential hazards associated with each process step under direct control of the food operation. A knowledge of any adverse health-related events historically associated with the product will be of value in this exercise.
After the list of potential hazards is assembled, stage two, the hazard evaluation, is conducted. In stage two of the hazard analysis, the HACCP team decides which potential hazards must be addressed in the HACCP plan. During this stage, each potential hazard is evaluated based on the severity of the potential hazard and its likely occurrence. Severity is the seriousness of the consequences of exposure to the hazard. Considerations of severity (e.g., impact of sequelae, and magnitude and duration of illness or injury) can be helpful in understanding the public health impact of the hazard. Consideration of the likely occurrence is usually based upon a combination of experience, epidemiological data, and information in the technical literature. When conducting the hazard evaluation, it is helpful to consider the likelihood of exposure and severity of the potential consequences if the hazard is not properly controlled. In addition, consideration should be given to the effects of short term as well as long term exposure to the potential hazard. Such considerations do not include common dietary choices which lie outside of HACCP. During the evaluation of each potential hazard, the food, its method of preparation, transportation, storage and persons likely to consume the product should be considered to determine how each of these factors may influence the likely occurrence and severity of the hazard being controlled. The team must consider the influence of likely procedures for food preparation and storage and whether the intended consumers are susceptible to a potential hazard. However, there may be differences of opinion, even among experts, as to the likely occurrence and severity of a hazard. The HACCP team may have to rely upon the opinion of experts who assist in the development of the HACCP plan.
Hazards identified in one operation or facility may not be significant in another operation producing the same or a similar product. For example, due to differences in equipment and/or an effective maintenance program, the probability of metal contamination may be significant in one facility but not in another. A summary of the HACCP team deliberations and the rationale developed during the hazard analysis should be kept for future reference. This information will be useful during future reviews and updates of the hazard analysis and the HACCP plan.
Appendix D gives three examples of using a logic sequence in conducting a hazard analysis. While these examples relate to biological hazards, chemical and physical hazards are equally important to consider. Appendix D is for illustration purposes to further explain the stages of hazard analysis for identifying hazards. Hazard identification and evaluation as outlined in Appendix D may eventually be assisted by biological risk assessments as they become available. While the process and output of a risk assessment (NACMCF, 1997) (1) is significantly different from a hazard analysis, the identification of hazards of concern and the hazard evaluation may be facilitated by information from risk assessments. Thus, as risk assessments addressing specific hazards or control factors become available, the HACCP team should take these into consideration.
Upon completion of the hazard analysis, the hazards associated with each step in the production of the food should be listed along with any measure(s) that are used to control the hazard(s). The term control measure is used because not all hazards can be prevented, but virtually all can be controlled. More than one control measure may be required for a specific hazard. On the other hand, more than one hazard may be addressed by a specific control measure (e.g. pasteurization of milk).
For example, if a HACCP team were to conduct a hazard analysis for the production of frozen cooked beef patties ( Appendices B and D) , enteric pathogens (e.g., Salmonella and verotoxin-producing Escherichia coli ) in the raw meat would be identified as hazards. Cooking is a control measure which can be used to eliminate these hazards. The following is an excerpt from a hazard analysis summary table for this product.
The hazard analysis summary could be presented in several different ways. One format is a table such as the one given above. Another could be a narrative summary of the HACCP team's hazard analysis considerations and a summary table listing only the hazards and associated control measures.
A critical control point is defined as a step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level. The potential hazards that are reasonably likely to cause illness or injury in the absence of their control must be addressed in determining CCPs.
Complete and accurate identification of CCPs is fundamental to controlling food safety hazards. The information developed during the hazard analysis is essential for the HACCP team in identifying which steps in the process are CCPs. One strategy to facilitate the identification of each CCP is the use of a CCP decision tree (Examples of decision trees are given in Appendices E and F ). Although application of the CCP decision tree can be useful in determining if a particular step is a CCP for a previously identified hazard, it is merely a tool and not a mandatory element of HACCP. A CCP decision tree is not a substitute for expert knowledge.
Critical control points are located at any step where hazards can be either prevented, eliminated, or reduced to acceptable levels. Examples of CCPs may include: thermal processing, chilling, testing ingredients for chemical residues, product formulation control, and testing product for metal contaminants. CCPs must be carefully developed and documented. In addition, they must be used only for purposes of product safety. For example, a specified heat process, at a given time and temperature designed to destroy a specific microbiological pathogen, could be a CCP. Likewise, refrigeration of a precooked food to prevent hazardous microorganisms from multiplying, or the adjustment of a food to a pH necessary to prevent toxin formation could also be CCPs. Different facilities preparing similar food items can differ in the hazards identified and the steps which are CCPs. This can be due to differences in each facility's layout, equipment, selection of ingredients, processes employed, etc.
A critical limit is a maximum and/or minimum value to which a biological, chemical or physical parameter must be controlled at a CCP to prevent, eliminate or reduce to an acceptable level the occurrence of a food safety hazard. A critical limit is used to distinguish between safe and unsafe operating conditions at a CCP. Critical limits should not be confused with operational limits which are established for reasons other than food safety.
Each CCP will have one or more control measures to assure that the identified hazards are prevented, eliminated or reduced to acceptable levels. Each control measure has one or more associated critical limits. Critical limits may be based upon factors such as: temperature, time, physical dimensions, humidity, moisture level, water activity (a w ), pH, titratable acidity, salt concentration, available chlorine, viscosity, preservatives, or sensory information such as aroma and visual appearance. Critical limits must be scientifically based. For each CCP, there is at least one criterion for food safety that is to be met. An example of a criterion is a specific lethality of a cooking process such as a 5D reduction in Salmonella . The critical limits and criteria for food safety may be derived from sources such as regulatory standards and guidelines, literature surveys, experimental results, and experts.
An example is the cooking of beef patties ( Appendix B ). The process should be designed to ensure the production of a safe product. The hazard analysis for cooked meat patties identified enteric pathogens (e.g., verotoxigenic E. coli such as E. coli O157:H7, and salmonellae) as significant biological hazards. Furthermore, cooking is the step in the process at which control can be applied to reduce the enteric pathogens to an acceptable level. To ensure that an acceptable level is consistently achieved, accurate information is needed on the probable number of the pathogens in the raw patties, their heat resistance, the factors that influence the heating of the patties, and the area of the patty which heats the slowest. Collectively, this information forms the scientific basis for the critical limits that are established. Some of the factors that may affect the thermal destruction of enteric pathogens are listed in the following table. In this example, the HACCP team concluded that a thermal process equivalent to 155° F for 16 seconds would be necessary to assure the safety of this product. To ensure that this time and temperature are attained, the HACCP team for one facility determined that it would be necessary to establish critical limits for the oven temperature and humidity, belt speed (time in oven), patty thickness and composition (e.g., all beef, beef and other ingredients). Control of these factors enables the facility to produce a wide variety of cooked patties, all of which will be processed to a minimum internal temperature of 155° F for 16 seconds. In another facility, the HACCP team may conclude that the best approach is to use the internal patty temperature of 155° F and hold for 16 seconds as critical limits. In this second facility the internal temperature and hold time of the patties are monitored at a frequency to ensure that the critical limits are constantly met as they exit the oven. The example given below applies to the first facility.
Monitoring is a planned sequence of observations or measurements to assess whether a CCP is under control and to produce an accurate record for future use in verification. Monitoring serves three main purposes. First, monitoring is essential to food safety management in that it facilitates tracking of the operation. If monitoring indicates that there is a trend towards loss of control, then action can be taken to bring the process back into control before a deviation from a critical limit occurs. Second, monitoring is used to determine when there is loss of control and a deviation occurs at a CCP, i.e., exceeding or not meeting a critical limit. When a deviation occurs, an appropriate corrective action must be taken. Third, it provides written documentation for use in verification.
An unsafe food may result if a process is not properly controlled and a deviation occurs. Because of the potentially serious consequences of a critical limit deviation, monitoring procedures must be effective. Ideally, monitoring should be continuous, which is possible with many types of physical and chemical methods. For example, the temperature and time for the scheduled thermal process of low-acid canned foods is recorded continuously on temperature recording charts. If the temperature falls below the scheduled temperature or the time is insufficient, as recorded on the chart, the product from the retort is retained and the disposition determined as in Principle 5. Likewise, pH measurement may be performed continually in fluids or by testing each batch before processing. There are many ways to monitor critical limits on a continuous or batch basis and record the data on charts. Continuous monitoring is always preferred when feasible. Monitoring equipment must be carefully calibrated for accuracy.
Assignment of the responsibility for monitoring is an important consideration for each CCP. Specific assignments will depend on the number of CCPs and control measures and the complexity of monitoring. Personnel who monitor CCPs are often associated with production (e.g., line supervisors, selected line workers and maintenance personnel) and, as required, quality control personnel. Those individuals must be trained in the monitoring technique for which they are responsible, fully understand the purpose and importance of monitoring, be unbiased in monitoring and reporting, and accurately report the results of monitoring. In addition, employees should be trained in procedures to follow when there is a trend towards loss of control so that adjustments can be made in a timely manner to assure that the process remains under control. The person responsible for monitoring must also immediately report a process or product that does not meet critical limits.
All records and documents associated with CCP monitoring should be dated and signed or initialed by the person doing the monitoring.
When it is not possible to monitor a CCP on a continuous basis, it is necessary to establish a monitoring frequency and procedure that will be reliable enough to indicate that the CCP is under control. Statistically designed data collection or sampling systems lend themselves to this purpose.
Most monitoring procedures need to be rapid because they relate to on-line, "real-time" processes and there will not be time for lengthy analytical testing. Examples of monitoring activities include: visual observations and measurement of temperature, time, pH, and moisture level.
Microbiological tests are seldom effective for monitoring due to their time-consuming nature and problems with assuring detection of contaminants. Physical and chemical measurements are often preferred because they are rapid and usually more effective for assuring control of microbiological hazards. For example, the safety of pasteurized milk is based upon measurements of time and temperature of heating rather than testing the heated milk to assure the absence of surviving pathogens.
With certain foods, processes, ingredients, or imports, there may be no alternative to microbiological testing. However, it is important to recognize that a sampling protocol that is adequate to reliably detect low levels of pathogens is seldom possible because of the large number of samples needed. This sampling limitation could result in a false sense of security by those who use an inadequate sampling protocol. In addition, there are technical limitations in many laboratory procedures for detecting and quantitating pathogens and/or their toxins.
The HACCP system for food safety management is designed to identify health hazards and to establish strategies to prevent, eliminate, or reduce their occurrence. However, ideal circumstances do not always prevail and deviations from established processes may occur. An important purpose of corrective actions is to prevent foods which may be hazardous from reaching consumers. Where there is a deviation from established critical limits, corrective actions are necessary. Therefore, corrective actions should include the following elements: (a) determine and correct the cause of non-compliance; (b) determine the disposition of non-compliant product and (c) record the corrective actions that have been taken. Specific corrective actions should be developed in advance for each CCP and included in the HACCP plan. As a minimum, the HACCP plan should specify what is done when a deviation occurs, who is responsible for implementing the corrective actions, and that a record will be developed and maintained of the actions taken. Individuals who have a thorough understanding of the process, product and HACCP plan should be assigned the responsibility for oversight of corrective actions. As appropriate, experts may be consulted to review the information available and to assist in determining disposition of non-compliant product.
Verification is defined as those activities, other than monitoring, that determine the validity of the HACCP plan and that the system is operating according to the plan. The NAS (1985) (2) pointed out that the major infusion of science in a HACCP system centers on proper identification of the hazards, critical control points, critical limits, and instituting proper verification procedures. These processes should take place during the development and implementation of the HACCP plans and maintenance of the HACCP system. An example of a verification schedule is given in Figure 2 .
One aspect of verification is evaluating whether the facility's HACCP system is functioning according to the HACCP plan. An effective HACCP system requires little end-product testing, since sufficient validated safeguards are built in early in the process. Therefore, rather than relying on end-product testing, firms should rely on frequent reviews of their HACCP plan, verification that the HACCP plan is being correctly followed, and review of CCP monitoring and corrective action records.
Another important aspect of verification is the initial validation of the HACCP plan to determine that the plan is scientifically and technically sound, that all hazards have been identified and that if the HACCP plan is properly implemented these hazards will be effectively controlled. Information needed to validate the HACCP plan often include (1) expert advice and scientific studies and (2) in-plant observations, measurements, and evaluations. For example, validation of the cooking process for beef patties should include the scientific justification of the heating times and temperatures needed to obtain an appropriate destruction of pathogenic microorganisms (i.e., enteric pathogens) and studies to confirm that the conditions of cooking will deliver the required time and temperature to each beef patty.
Subsequent validations are performed and documented by a HACCP team or an independent expert as needed. For example, validations are conducted when there is an unexplained system failure; a significant product, process or packaging change occurs; or new hazards are recognized.
In addition, a periodic comprehensive verification of the HACCP system should be conducted by an unbiased, independent authority. Such authorities can be internal or external to the food operation. This should include a technical evaluation of the hazard analysis and each element of the HACCP plan as well as on-site review of all flow diagrams and appropriate records from operation of the plan. A comprehensive verification is independent of other verification procedures and must be performed to ensure that the HACCP plan is resulting in the control of the hazards. If the results of the comprehensive verification identifies deficiencies, the HACCP team modifies the HACCP plan as necessary.
Verification activities are carried out by individuals within a company, third party experts, and regulatory agencies. It is important that individuals doing verification have appropriate technical expertise to perform this function. The role of regulatory and industry in HACCP was further described by the NACMCF (1994) (3) .
Examples of verification activities are included as Appendix G .
Figure 2. Example of a Company Established HACCP Verification Schedule
Generally, the records maintained for the HACCP System should include the following:
A summary of the hazard analysis, including the rationale for determining hazards and control measures.
The HACCP Plan
Listing of the HACCP team and assigned responsibilities.
Description of the food, its distribution, intended use, and consumer.
Verified flow diagram.
HACCP Plan Summary Table that includes information for:
Steps in the process that are CCPs
The hazard(s) of concern.
Critical limits
Monitoring*
Corrective actions*
Verification procedures and schedule*
Record-keeping procedures*
* A brief summary of position responsible for performing the activity and the procedures and frequency should be provided
The following is an example of a HACCP plan summary table:
Support documentation such as validation records.
Records that are generated during the operation of the plan.
Examples of HACCP records are given in Appendix H .
The successful implementation of a HACCP plan is facilitated by commitment from top management. The next step is to establish a plan that describes the individuals responsible for developing, implementing and maintaining the HACCP system. Initially, the HACCP coordinator and team are selected and trained as necessary. The team is then responsible for developing the initial plan and coordinating its implementation. Product teams can be appointed to develop HACCP plans for specific products. An important aspect in developing these teams is to assure that they have appropriate training. The workers who will be responsible for monitoring need to be adequately trained. Upon completion of the HACCP plan, operator procedures, forms and procedures for monitoring and corrective action are developed. Often it is a good idea to develop a timeline for the activities involved in the initial implementation of the HACCP plan. Implementation of the HACCP system involves the continual application of the monitoring, record-keeping, corrective action procedures and other activities as described in the HACCP plan.
Maintaining an effective HACCP system depends largely on regularly scheduled verification activities. The HACCP plan should be updated and revised as needed. An important aspect of maintaining the HACCP system is to assure that all individuals involved are properly trained so they understand their role and can effectively fulfill their responsibilities.
(1) National Advisory Committee on Microbiological Criteria for Foods. 1997. The principles of risk assessment for illness caused by foodborne biological agents. Adopted April 4, 1997.
(2) An Evaluation of the Role of Microbiological Criteria for Foods and Food Ingredients . 1985. National Academy of Sciences, National Academy Press, Washington, DC.
(3) National Advisory Committee on Microbiological Criteria for Foods. 1994. The role of regulatory agencies and industry in HACCP. Int. J. Food Microbiol. 21:187-195.
Examples of Common Prerequisite Programs
The production of safe food products requires that the HACCP system be built upon a solid foundation of prerequisite programs. Each segment of the food industry must provide the conditions necessary to protect food while it is under their control. This has traditionally been accomplished through the application of cGMPs. These conditions and practices are now considered to be prerequisite to the development and implementation of effective HACCP plans. Prerequisite programs provide the basic environmental and operating conditions that are necessary for the production of safe, wholesome food. Common prerequisite programs may include, but are not limited to:
Facilities: The establishment should be located, constructed and maintained according to sanitary design principles. There should be linear product flow and traffic control to minimize cross-contamination from raw to cooked materials.
Supplier Control: Each facility should assure that its suppliers have in place effective GMP and food safety programs. These may be the subject of continuing supplier guarantee and supplier HACCP system verification.
Specifications: There should be written specifications for all ingredients, products, and packaging materials.
Production Equipment: All equipment should be constructed and installed according to sanitary design principles. Preventive maintenance and calibration schedules should be established and documented.
Cleaning and Sanitation: All procedures for cleaning and sanitation of the equipment and the facility should be written and followed. A master sanitation schedule should be in place.
Personal Hygiene: All employees and other persons who enter the manufacturing plant should follow the requirements for personal hygiene.
Training: All employees should receive documented training in personal hygiene, GMP, cleaning and sanitation procedures, personal safety, and their role in the HACCP program.
Chemical Control: Documented procedures must be in place to assure the segregation and proper use of non-food chemicals in the plant. These include cleaning chemicals, fumigants, and pesticides or baits used in or around the plant.
Receiving, Storage and Shipping: All raw materials and products should be stored under sanitary conditions and the proper environmental conditions such as temperature and humidity to assure their safety and wholesomeness
Traceability and Recall: All raw materials and products should be lot-coded and a recall system in place so that rapid and complete traces and recalls can be done when a product retrieval is necessary.
Pest Control: Effective pest control programs should be in place.
Other examples of prerequisite programs might include quality assurance procedures; standard operating procedures for sanitation, processes, product formulations and recipes; glass control; procedures for receiving, storage and shipping; labeling; and employee food and ingredient handling practices.
Example of a Flow Diagram for the Production of Frozen Cooked Beef Patties
Examples of Questions to be Considered When Conducting a Hazard Analysis
The hazard analysis consists of asking a series of questions which are appropriate to the process under consideration. The purpose of the questions is to assist in identifying potential hazards.
Ingredients
- Does the food contain any sensitive ingredients that may present microbiological hazards (e.g., Salmonella, Staphylococcus aureus); chemical hazards (e.g., aflatoxin, antibiotic or pesticide residues); or physical hazards (stones, glass, metal)?
- Are potable water, ice and steam used in formulating or in handling the food?
- What are the sources (e.g., geographical region, specific supplier)
Intrinsic Factors - Physical characteristics and composition (e.g., pH, type of acidulants, fermentable carbohydrate, water activity, preservatives) of the food during and after processing.
- What hazards may result if the food composition is not controlled?
- Does the food permit survival or multiplication of pathogens and/or toxin formation in the food during processing?
- Will the food permit survival or multiplication of pathogens and/or toxin formation during subsequent steps in the food chain?
- Are there other similar products in the market place? What has been the safety record for these products? What hazards have been associated with the products?
Procedures used for processing
- Does the process include a controllable processing step that destroys pathogens? If so, which pathogens? Consider both vegetative cells and spores.
- If the product is subject to recontamination between processing (e.g., cooking, pasteurizing) and packaging which biological, chemical or physical hazards are likely to occur?
Microbial content of the food
- What is the normal microbial content of the food?
- Does the microbial population change during the normal time the food is stored prior to consumption?
- Does the subsequent change in microbial population alter the safety of the food?
- Do the answers to the above questions indicate a high likelihood of certain biological hazards?
Facility design
- Does the layout of the facility provide an adequate separation of raw materials from ready-to-eat (RTE) foods if this is important to food safety? If not, what hazards should be considered as possible contaminants of the RTE products?
- Is positive air pressure maintained in product packaging areas? Is this essential for product safety?
- Is the traffic pattern for people and moving equipment a significant source of contamination?
Equipment design and use
- Will the equipment provide the time-temperature control that is necessary for safe food?
- Is the equipment properly sized for the volume of food that will be processed?
- Can the equipment be sufficiently controlled so that the variation in performance will be within the tolerances required to produce a safe food?
- Is the equipment reliable or is it prone to frequent breakdowns?
- Is the equipment designed so that it can be easily cleaned and sanitized?
- Is there a chance for product contamination with hazardous substances; e.g., glass?
- metal detectors
- thermometers
- bone removal devices
- dud detectors
- To what degree will normal equipment wear affect the likely occurrence of a physical hazard (e.g., metal) in the product?
- Are allergen protocols needed in using equipment for different products?
- Does the method of packaging affect the multiplication of microbial pathogens and/or the formation of toxins?
- Is the package clearly labeled "Keep Refrigerated" if this is required for safety?
- Does the package include instructions for the safe handling and preparation of the food by the end user?
- Is the packaging material resistant to damage thereby preventing the entrance of microbial contamination?
- Are tamper-evident packaging features used?
- Is each package and case legibly and accurately coded?
- Does each package contain the proper label?
- Are potential allergens in the ingredients included in the list of ingredients on the label?
- Can sanitation have an impact upon the safety of the food that is being processed?
- Can the facility and equipment be easily cleaned and sanitized to permit the safe handling of food?
- Is it possible to provide sanitary conditions consistently and adequately to assure safe foods?
Employee health, hygiene and education
- Can employee health or personal hygiene practices impact upon the safety of the food being processed?
- Do the employees understand the process and the factors they must control to assure the preparation of safe foods?
- Will the employees inform management of a problem which could impact upon safety of food?
Conditions of storage between packaging and the end user
- What is the likelihood that the food will be improperly stored at the wrong temperature?
- Would an error in improper storage lead to a microbiologically unsafe food?
Intended use
- Will the food be heated by the consumer?
- Will there likely be leftovers?
Intended consumer
- Is the food intended for the general public?
- Is the food intended for consumption by a population with increased susceptibility to illness (e.g., infants, the aged, the infirmed, immunocompromised individuals)?
- Is the food to be used for institutional feeding or the home?
* For illustrative purposes only. The potential hazards identified may not be the only hazards associated with the products listed. The responses may be different for different establishments.
Example I of a CCP Decision Tree
Important considerations when using the decision tree:
The decision tree is used after the hazard analysis.
The decision tree then is used at the steps where a hazard that must be addressed in the HACCP plan has been identified.
A subsequent step in the process may be more effective for controlling a hazard and may be the preferred CCP.
More than one step in a process may be involved in controlling a hazard.
More than one hazard may be controlled by a specific control measure.
* Proceed to next step in the process.
Example II of a CCP Decision Tree
*Proceed to next step in the described process
Examples of Verification Activities
Verification procedures may include:
- Establishment of appropriate verification schedules.
- Review of the HACCP plan for completeness.
- Confirmation of the accuracy of the flow diagram.
- Review of the HACCP system to determine if the facility is operating according to the HACCP plan.
- Review of CCP monitoring records.
- Review of records for deviations and corrective actions.
- Validation of critical limits to confirm that they are adequate to control significant hazards.
- Validation of HACCP plan, including on-site review.
- Review of modifications of the HACCP plan.
- Sampling and testing to verify CCPs.
Verification should be conducted:
- Routinely, or on an unannounced basis, to assure CCPs are under control.
- When there are emerging concerns about the safety of the product.
- When foods have been implicated as a vehicle of foodborne disease.
- To confirm that changes have been implemented correctly after a HACCP plan has been modified.
- To assess whether a HACCP plan should be modified due to a change in the process, equipment, ingredients, etc.
Verification reports may include information on the presence and adequacy of.
- The HACCP plan and the person(s) responsible for administering and updating the HACCP plan.
- The records associated with CCP monitoring.
- Direct recording of monitoring data of the CCP while in operation.
- Certification that monitoring equipment is properly calibrated and in working order.
- Corrective actions for deviations.
- Sampling and testing methods used to verify that CCPs are under control.
- Modifications to the HACCP plan.
- Training and knowledge of individuals responsible for monitoring CCPs.
- Validation activities.
Examples of HACCP Records
Ingredients for which critical limits have been established.
- Supplier certification records documenting compliance of an ingredient with a critical limit.
- Processor audit records verifying supplier compliance.
- Storage records (e.g., time, temperature) for when ingredient storage is a CCP.
Processing, storage and distribution records
- Information that establishes the efficacy of a CCP to maintain product safety.
- Data establishing the safe shelf life of the product; if age of product can affect safety.
- Records indicating compliance with critical limits when packaging materials, labeling or sealing specifications are necessary for food safety.
- Monitoring records.
- Verification records.
Deviation and corrective action records.
Employee training records that are pertinent to CCPs and the HACCP plan.
Documentation of the adequacy of the HACCP plan from a knowledgeable HACCP expert.

- +1 844-596-0962

- Certification
- Plan Guidance
How to Create a HACCP Plan? Step-by-Step Guide with Examples
- HACCP Certified, Trained SQF Practitioner
- October 24, 2024

Are you wondering how to develop or write a HACCP plan? If so, keep reading because this article is for you.
A Hazard Analysis and Critical Control Points (HACCP) is the core element of most food safety systems. Chances are if you are reading this article it’s because you have been told that you need to have a HACCP Plan to make or produce your food in a way that is safe for the public to consume.
FoodReady food safety software has a HACCP builder and customizable HACCP templates that make creating your HACCP plan flow chart and associated logs and documents much simpler and easier than using a paper system.
In this article, we will be discussing the steps of writing a HACCP Plan. The images you see are screenshots of our drag-drop HACCP builder at various stages of HACCP creation.
How to Write an Effective HACCP Plan?
Now you are about to learn how to develop a comprehensive Hazard Analysis Critical Control Points (HACCP) plan with this clear, sequential approach. Ensure food safety by identifying potential hazards, establishing critical control points, and implementing effective monitoring procedures.
Assemble Your HACCP Team
You need staff educated in HACCP principles to build and execute your HACCP plan. The staff you choose for this first HACCP plan step need to have technical knowledge of your food preparation or production and of the chemical, biological, and physical hazards present at your facility or at your restaurant.
With Foodready’s Enterprise solution, we can create your HACCP plan for you and help you set up your HACCP software/system at your business. If you need our HACCP consultant to visit your facility we can also arrange a site visit for an extra charge.
Describe Your Product, Intended Consumer, and Distribution
For demonstration purposes, our product is ready-to-eat salsa.
Product Description – Ready-to-eat unpasteurized tomato-based salsa. The salsa will be stored in cold cases and refrigerated display cases.
Intended Consumer – Our consumer is likely between the ages of 2-70. It is unknown if our consumer is of robust or fragile health. Our product will be sold at convenience stores, delis, and grocery stores.
Distribution – Our salsa will be shipped to retailers in refrigerated trucks.
Describe Your Food Process
- Pre-approve and verify suppliers.
- Receive ingredients from suppliers. Check delivery trucks and supplies for contamination, dirt, insects, spoilage, and general condition.
- Perishable ingredients are put into cold storage. Shelf-stable ingredients are stored in the warehouse.
- Prepare Salsa (chopping, mixing, etc.)
- Package and Label Salsa
- Pack salsa into boxes
- Boxes are put into cold storage.
- Load boxes onto refrigerated trucks. (Most processes will also include various sanitation and standard operating procedures)
Develop a Simple Flow Chart of Your Food Process
This should illustrate a clear and simple outline of every step from receiving the ingredients to distributing the finished product.
FoodReady food safety software has a drag-and-drop HACCP flow chart builder and a library of over seventy Food Safety / HACCP plan examples for GFSI, SQF, GMP, USDA, FDA, Local Health, and Retail compliance.
Simply choose the template that most closely resembles your food product and customize the HACCP steps to your process.
We have a video knowledge base to help you learn this process, or you can hire one of FoodReady’s food safety consultants like SQF Consultant , GMP Consultant , GFSI Consultant , FDA Consultant to build your HACCP plan for you.
Analyze the Flow Chart With Your HACCP Team
Your HACCP team then needs to verify that the HACCP flow chart you created is correct and that there are no missing steps in your food production process. Any corrections to your process are made at this time before implementation.
The 7 Principles of HACCP
These seven HACCP principles are the cornerstone of a HACCP plan and must be completed correctly.
HACCP Principle #1 – Perform a Hazard Analysis
The hazard analysis is the step where the HACCP team scrutinizes the production process and documents where and how any hazards can be introduced to the food process. Food safety hazards are either biological, chemical, or physical. HACCP principle #1 is perhaps the most important step in writing a HACCP plan because this is the step that identifies food hazards that can make your customer sick.
For instance, if you are working with raw meat there are biological hazards like E-coli and Salmonella bacteria that can contaminate the meat. Any surface or equipment that comes into contact with raw meat could have these biological hazards on it and needs to be controlled to keep your customers from getting food poisoning.
Machinery used at certain points of the process could introduce a physical hazard like metal shards which pose a danger to your customer and must be addressed as a hazard in your HACCP plan.
Allergens, such as shellfish, fish, peanuts, tree nuts, soy, sesame, dairy wheat, and eggs are a significant food hazard.
Any food allergen contained in your product must be included on the food label so the consumer can protect themselves if they have an intolerance or allergy to an ingredient.
Steps in your food process that could introduce allergens need to be noted. Allergens need to be carefully stored and used so they do not cause cross-contamination.
Any step in your process that introduces chemicals (such as equipment sanitizer) to the process or machinery needs to be noted.
In the hazard analysis, your entire process must be evaluated from the receiving of the ingredients to the delivery to your customer.
To ensure the safety of the ingredients you receive you need to make sure your suppliers have their own functioning food safety system like a HACCP, PC, or GMP certification.
Having pre-qualified suppliers or ingredients and packaging is a PRP (pre-requisite program) that most manufacturers have in place.
A PRP is any program that you have to help ensure the safety of your product before your process begins.
After the hazards are identified the HACCP team must evaluate the severity of each possible hazard, how much it could harm the consumer, and the likelihood of this hazard occurring.
For instance, if Listeria is found in baby formula it’s an enormous risk because of the fragility of the population that consumes baby formula. This is an example of a food safety problem that causes a very severe hazard to a sensitive population.
As for the likelihood of occurrence, it’s much more likely that a product like packaged lettuce which has a lot of moisture would have more bacterial growth, than a product that is pre-cooked and then frozen.
Once the list of hazards is made you must list the “controls” you can use to eliminate, or reduce the hazards.
For example, if your product is packaged lettuce, the lettuce would be washed in a solution to control the growth of bacteria. If your product is pre-cooked frozen hamburger patties, the patties would be cooked to a specific temperature for a certain amount of time to kill pathogenic bacteria which could be harmful to your customers.
After all the hazards are identified, apply them to your flow chart at the appropriate steps.
HACCP Principle #2 – Determine Critical Control Points
Now that you know which hazards could be the most detrimental to the health of your consumer and which are the most likely to occur, you need to determine at which point in your process you can control the hazard by eliminating it or reducing it to acceptable standards. These are called Critical Control Points .
In our example of ready-to-eat salsa, there is a CCP at the final step “serving at a cold bar”. If the temperature of the salsa rises above a certain level there is potential for biological pathogens to grow.
HACCP Principle #3 – Establish Critical Limits
Critical limits are the maximum and minimum levels needed to achieve control over the hazards at each CCP.
For instance, you may need to cook food to a specific temperature for a certain amount of time to kill biological pathogens. These parameters must be listed at each critical control point.
For example, salsa must be kept at or below a minimum temperature of 40℉ according to the FDA to control bacterial growth.
HACCP Principle #4 – Establish Monitoring Procedures
There must be a way to monitor that any hazards were controlled or stopped at the critical control point. Examples of monitoring could be logging times and temperatures needed to kill pathogens, swabbing for pathogen identification on sanitized equipment, testing the magnets used to detect metal in food to make sure they are working properly, and other measures.
As monitoring measures are performed, the staff responsible for monitoring must document and record their actions in monitoring logs. These monitoring logs must be maintained and available for retrieval for audit and inspection purposes.
HACCP Principle #5 – Establish Corrective Actions
This step is needed when a hazard is not properly controlled at the CCP.
For example, let’s say one of the HACCP team uses their Bluetooth thermometer and discovers that the salsa temperature is at 50℉ which is above the 40℉ maximum temperature. In this case, the corrective action would be to dispose of the salsa to prevent people from eating food that could have biological contamination.
HACCP Principle #6 – Establish Verification Procedures
Firstly, the initial HACCP plan must be verified for validity by HACCP-trained individuals. For example; if cooking at a certain temperature is needed to kill pathogens, the oven temperature must be verified to make sure the proper temperature is obtained.
If the HACCP plan is verified and followed precisely, there is less need for corrective actions later on, resulting in safer food and a reduction in costs.
Verification procedures are in place so that when there is a failure in the HACCP system, compromised food can be targeted and dealt with in a manner that protects the consumer.
Additionally, the HACCP plan needs to be verified occasionally by an unbiased third-party auditor to ensure the HACCP is being followed correctly.
HACCP Principle #7- Establish Record Keeping and Documentation
An important part of a HACCP plan is making sure that all actions associated with the HACCP plan are recorded and these documents are maintained and easily accessible.
This means that the HACCP plan itself, any corrective actions, monitoring, and verification are documented and retrievable for audits, recalls, food traceability, labeling, batch management, training logs, etc.
FDA’s Food Safety Modernization Act rule 204 is legislation that requires food manufacturers to have food recall plans in place, food traceability, and record keeping.
In the past, this meant having to maintain binders of documents to present during inspections and wade through when a recall needed to happen.
FoodReady can build your HACCP plan, create your food safety system, and your process ready for any 3rd-party audit like SQF, BRC, GMP, GFSI, and the Costco Audit .
Table of Contents
- Saro Loucks
More Blogs by FoodReady

- Radojka Barycki
- March 20, 2024
FSMA Rule 204: How Does the New Traceability Rule Affect Distributors?
- FDA & USDA Compliance

- April 9, 2024
What are TCS Foods?
- Hazard Controls & Recall Management

- February 19, 2024
What Is Food Safety Certification?
- Food Safety Certifications & Audits

Speak with our Expert Consultants
Get a Free Consultation With Our Food Safety and Quality Experts Today!
How can we assist you?
Free consultation.
Use the power of software automation with a combination of expert food safety consulting and fast State / FDA / USDA Compliance, and GMP, CGMP, GFSI, SQF, BRCGS Certifications.
- SQF Consultant
- HACCP Consultant
- GMP Consultant
- BRC Consultant
- FDA Consultant
- USDA Consultant
- Food Recall Consultant
- FSSC Consultant
- FSMA 204 Consultant
- Sanitation Consultant
- SQF Certification
- HACCP Certification
- GMP Certification
- cGMP Certification
- BRC Certification
- FDA Compliance
- FSMA Compliance
- HACCP Builder
- Quality Management
- Traceability
- Food Quality Management
- Food Manufacturing
- Food Inventory
- Best Food ERP software
- Best Food Traceability Software
- Best HACCP Software
- Best SQF Software
- Best BRC Software
- Best Quality Management System
©FoodReady 2024, All Rights Reserved.
Terms of Services
Privacy Policy

COMMENTS
Example of Corrective action for CCP’s Receiving: Cold food is rejected if the temperature is above 50°F/10°C; temperatures of 46°F/7.8°C require management intervention. Frozen foods that have started to thaw are …
Learn how to write a HACCP plan with visual examples and then download the free, customizable HACCP Plan template to create your own!
The HACCP is a method used to effectively identify the critical points for which control measures are essential to preventing or limiting identified hazards. It is implemented for a
HACCP is a systematic approach to the identification, evaluation, and control of food safety hazards based on the following seven principles: Principle 1: Conduct a hazard analysis. Principle 2...
By adhering to the guidelines outlined in a HACCP plan, businesses can demonstrate their commitment to food safety and maintain compliance with regulatory standards. Additionally, implementing a HACCP …
This Guidebook for the Preparation of HACCP Plans provides information that may be useful to establishments when developing plans specific to their food production processes and when …
FoodReady food safety software has a drag-and-drop HACCP flow chart builder and a library of over seventy Food Safety / HACCP plan examples for GFSI, SQF, GMP, USDA, FDA, Local Health, and Retail compliance.