
New findings offer potential breakthrough in HIV cure research
The results of a novel study presented by Emory researchers during the International AIDS Society (IAS) Conference in Brisbane, Australia, have revealed exciting findings in the pursuit of an HIV cure. The study, led by Monica Reece, a PhD candidate in Emory's Microbiology and Genetics Program, and directed by Christina Gavegnano, PhD, demonstrates the potential of Jak inhibitors, specifically ruxolitinib, to significantly decay the viral reservoir in people with HIV, offering a novel pathway toward long-term remission or a cure.
The HIV viral reservoir, essentially a small number of immune cells containing dormant virus integrated into the genomes of individuals who have suppressed viral replication with HIV treatment, has posed a major impediment to achieving an HIV cure. These cells are completely undetectable by the immune system because the virus is dormant. But as soon as treatment stops, the virus reactivates.
"The barrier to an HIV cure is that the virus hides inside the DNA of cells," says Gavegnano, director of the Gavegnano Drug Discovery Program and senior author on the study. "The brass ring is an agent that can eliminate these'reservoir cells,' which would ultimately eliminate HIV from a person's body."
While Gavegnano and her Emory colleagues have shown that Jak inhibitors (Janus kinase inhibitors) could reverse the immune dysfunction caused by HIV since their discovery in 2010, questions about their impact on the HIV reservoir and the exact mechanism contributing to the immunologic improvements have remained unanswered, until now.
The data presented at IAS represented secondary results from a Phase 2a clinical trial centered on investigating ruxolitinib's effects on viral reservoirs in people with HIV during a five-week regimen, specifically in a subset of individuals with high viral reservoir levels at baseline.
The study measured integrated proviral DNA, which is the genetic material of a virus as incorporated into, and able to replicate with, the genome of a host cell, and examined changes in total, intact only, and defective proviral DNA copies over time. Based on a linear model of decay, the researchers estimated an astonishing 99.99% clearance of the peripheral HIV-1 reservoir in less than three years. These data provide optimism for the use of Jak inhibitors as a backbone for cure-based eradication strategies in the battle against HIV.
Reece, lead author of the study says, "These data suggest that our Jak inhibitors can not only reverse the immune dysfunction that prevents HIV-1 cure, but also significantly decay the reservoir in people living with HIV. Collectively our trial demonstrates a mechanism by which ruxolitinib, or other Jak inhibitors such as baricitinib, also extensively studied by our group, decay the reservoir, which underscores potential for cure-based therapies."
The profound impact of Ruxolitinib treatment was not limited to reservoir reduction. The study also shed light on several significant biomarkers that were altered by the drug primarily related to:
- Immune activation: Ruxolitinib exhibited the potential to modulate immune activation, which is crucial in controlling viral replication and maintaining immune health in individuals with HIV.
- Cell survival: Ruxolitinib demonstrated the ability to impact cell survival, influencing the lifespan of reservoir cells and potentially limiting viral reservoir longevity.
- Immune dysregulation: The study identified ruxolitinib's impact on immune dysregulation, offering hope for mitigating the chronic inflammation and immune dysfunction often observed in individuals with HIV.
It is important to note that the study focused on the peripheral viral reservoir and may not fully represent the entire viral reservoir within the body, including sanctuary sites where HIV can persist despite treatment.
Regardless, the findings from Emory University's study offer hope and renewed enthusiasm for efforts to unravel the complexities of HIV persistence and ultimately find a cure.
"These data are valuable because they show that Jak inhibitors can contribute to a long-term cure strategy for HIV, but they can also be used to slow the inflammatory process caused by other infectious diseases," says Vincent Marconi, MD, professor of medicine and global health at Emory University School of Medicine.
Marconi, who led the initial phase 2a trial, has already been investigating the efficacy of Jak inhibitors, like ruxolitinib and baricitinib, in patients with acute COVID and now long COVID. He continues, "using an anti-inflammatory drug to treat the effects of a virus could be revolutionary."
In addition to the data presented by Reece and Gavegnano, another presentation at IAS has shown how ruxolitinib administered to a patient following a stem cell transplant led to an undetectable viral load 20 months after stopping antiretroviral therapy, highlighting the different mechanisms in which these class of drugs could be valuable in HIV care and treatment.
Further research and clinical trials will be needed to fully understand the effects of Jak inhibitor use in HIV and other immune-suppressing conditions. Emory researchers have an extensive history of working with Jak inhibitors. Gavegnano and researcher Raymond Schinazi are listed on the issued patents as sole inventors, and they, alongside their co-investigators, have built a roadmap for tackling a variety of immunosuppressive viruses with these drugs.
Gavegnano emphasizes, "The safety and efficacy outcomes we observed in this study provide a strong foundation for further research on cure-based interventions containing a Jak inhibitor, and we hope to bring this therapy one step closer to helping people living with HIV."
- HIV and AIDS
- Infectious Diseases
- Immune System
- Biotechnology
- Encephalopathy
- Antiretroviral drug
- Health science
- Animal cognition
- Mammal classification
- Epinephrine
Story Source:
Materials provided by Emory Health Sciences . Note: Content may be edited for style and length.
Cite This Page :
Explore More
- Future Climate Impacts Put Whale Diet at Risk
- Charge Your Laptop in a Minute?
- Caterpillars Detect Predators by Electricity
- 'Electronic Spider Silk' Printed On Human Skin
- Engineered Surfaces Made to Shed Heat
- Innovative Material for Sustainable Building
- Human Brain: New Gene Transcripts
- Epstein-Barr Virus and Resulting Diseases
- Origins of the Proton's Spin
- Symbiotic Bacteria Communicate With Plants
Trending Topics
Strange & offbeat.
- What Are HIV and AIDS?
- How Is HIV Transmitted?
- Who Is at Risk for HIV?
- Symptoms of HIV
- U.S. Statistics
- Impact on Racial and Ethnic Minorities
- Global Statistics
- HIV and AIDS Timeline
- In Memoriam
- Supporting Someone Living with HIV
- Standing Up to Stigma
- Getting Involved
- HIV Treatment as Prevention
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Preventing Sexual Transmission of HIV
- Alcohol and HIV Risk
- Substance Use and HIV Risk
- Preventing Perinatal Transmission of HIV
- HIV Vaccines
- Long-acting HIV Prevention Tools
- Microbicides
- Who Should Get Tested?
- HIV Testing Locations
- HIV Testing Overview
- Understanding Your HIV Test Results
- Living with HIV
- Talking About Your HIV Status
- Locate an HIV Care Provider
- Types of Providers
- Take Charge of Your Care
- What to Expect at Your First HIV Care Visit
- Making Care Work for You
- Seeing Your Health Care Provider
- HIV Lab Tests and Results
- Returning to Care
- HIV Treatment Overview
- Viral Suppression and Undetectable Viral Load
- Taking Your HIV Medicine as Prescribed
- Tips on Taking Your HIV Medication Every Day
- Paying for HIV Care and Treatment
- Other Health Issues of Special Concern for People Living with HIV
- Alcohol and Drug Use
- Coronavirus (COVID-19) and People with HIV
- Hepatitis B & C
- Vaccines and People with HIV
- Flu and People with HIV
- Mental Health
- Mpox and People with HIV
- Opportunistic Infections
- Sexually Transmitted Infections
- Syphilis and People with HIV
- HIV and Women's Health Issues
- Aging with HIV
- Emergencies and Disasters and HIV
- Employment and Health
- Exercise and Physical Activity
- Food Safety and Nutrition
- Housing and Health
- Traveling Outside the U.S.
- Civil Rights
- Workplace Rights
- Limits on Confidentiality
- National HIV/AIDS Strategy (2022-2025)
- Implementing the National HIV/AIDS Strategy
- Prior National HIV/AIDS Strategies (2010-2021)
- Key Strategies
- Priority Jurisdictions
- HHS Agencies Involved
- Learn More About EHE
- Ready, Set, PrEP
- Ready, Set, PrEP Pharmacies
- Ready, Set, PrEP Resources
- AHEAD: America’s HIV Epidemic Analysis Dashboard
- HIV Prevention Activities
- HIV Testing Activities
- HIV Care and Treatment Activities
- HIV Research Activities
- Activities Combating HIV Stigma and Discrimination
- The Affordable Care Act and HIV/AIDS
- HIV Care Continuum
- Syringe Services Programs
- Finding Federal Funding for HIV Programs
- Fund Activities
- The Fund in Action
- About PACHA
- Members & Staff
- Subcommittees
- Prior PACHA Meetings and Recommendations
- I Am a Work of Art Campaign
- Awareness Campaigns
- Global HIV/AIDS Overview
- U.S. Government Global HIV/AIDS Activities
- U.S. Government Global-Domestic Bidirectional HIV Work
- Global HIV/AIDS Organizations
- National Black HIV/AIDS Awareness Day February 7
- HIV Is Not A Crime Awareness Day February 28
- National Women and Girls HIV/AIDS Awareness Day March 10
- National Native HIV/AIDS Awareness Day March 20
- National Youth HIV & AIDS Awareness Day April 10
- HIV Vaccine Awareness Day May 18
- National Asian & Pacific Islander HIV/AIDS Awareness Day May 19
- HIV Long-Term Survivors Awareness Day June 5
- National HIV Testing Day June 27
- Zero HIV Stigma July 21
- Southern HIV/AIDS Awareness Day August 20
- National Faith HIV/AIDS Awareness Day August 27
- National African Immigrant and Refugee HIV/AIDS and Hepatitis Awareness Day September 9
- National HIV/AIDS and Aging Awareness Day September 18
- National Gay Men's HIV/AIDS Awareness Day September 27
- National Latinx AIDS Awareness Day October 15
- World AIDS Day December 1
- Event Planning Guide
- U.S. Conference on HIV/AIDS (USCHA)
- National Ryan White Conference on HIV Care & Treatment
- AIDS 2020 (23rd International AIDS Conference Virtual)
Want to stay abreast of changes in prevention, care, treatment or research or other public health arenas that affect our collective response to the HIV epidemic? Or are you new to this field?
HIV.gov curates learning opportunities for you, and the people you serve and collaborate with.
Stay up to date with the webinars, Twitter chats, conferences and more in this section.
HIV Treatment Research and Key Takeaways: Dr. Dieffenbach’s Final Update from CROI 2024
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on Email
On Wednesday as the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) was winding down, HIV.gov spoke with NIH’s Dr. Carl Dieffenbach about highlights of long-acting HIV treatment research discussed at the conference. Dr. Dieffenbach is the Director of the Division of AIDS at NIH’s National Institute of Allergy and Infectious Diseases . He spoke with Brian Minalga, MSW, Deputy Director of the NIH-supported Office of HIV/AIDS Network Coordination Exit Disclaimer . Watch our conversation with Dr. Dieffenbach below:
Research Suggests Possible Expanded Options for Long-Acting HIV Treatment
Dr. Dieffenbach highlighted findings from several clinical trials and a plenary session presented at CROI about current and future options for long-acting antiretroviral treatment (ART) for HIV.
First, he discussed a NIAID-supported randomized clinical trial that found that long-acting ART with cabotegravir and rilpivirine was superior in suppressing HIV replication compared to daily oral ART in adults who had been unable to maintain viral suppression through an oral daily regimen. The LATITUDE study Exit Disclaimer enrolled participants in 31 sites in the United States. Last month, the trial’s Data and Safety Monitoring Board conducted a planned review of interim data and recommended halting randomization and offering all eligible study participants long-acting ART based on its observed superior viral suppression of HIV. At CROI, study leaders reported that the interim analysis of data from 294 participants showed that the chance of experiencing unsuppressed HIV was 7% among people taking long-acting ART compared to 25% among those taking daily oral ART . The likelihood of discontinuing the assigned regimen due to adverse events or experiencing unsuppressed HIV was 10% among people taking long-acting ART compared to 26% among those taking daily ART. These findings were statistically significant. Dr. Dieffenbach observed that these results may support expanding the use of long-acting ART among a broader population. Read the study abstract Exit Disclaimer . Read more in this NIAID news release .
Another ongoing clinical trial reported initial findings on the safety of the same long-acting injectable treatment regimen for adolescents with HIV with a suppressed viral load. The NIH-supported MOCHA study Exit Disclaimer enrolled participants aged 12 to 17 who were virally suppressed in Botswana, South Africa, Thailand, Uganda, and the United States. In what he characterized as very encouraging results, Dr. Aditya Gaur of St. Jude Children's Research Hospital, one of the trial’s co-chairs, reported that after the first six months all participants remained virally suppressed, and the level of the ART in their systems was comparable to what has been shown as efficacious in adult studies of the same drug . He also reported that, while about one-third of the participants reported an injection-site reaction, there were no surprising or unanticipated adverse events. These data support the use of cabotegravir and rilpivirine in virally suppressed adolescents, according to Dr. Gaur and colleagues. Dr. Dieffenbach noted that NIH will continue to support safety and dosing studies to determine the proper doses for adolescents and that these studies could eventually expand access to this long-acting HIV treatment to more people. Read the abstract Exit Disclaimer . Read NIAID’s news release about the study .
In addition, Dr. Dieffenbach mentioned an industry-sponsored Phase 2 trial that presented 24-week results of an oral once-weekly investigational combination of two drugs ( islatravir and lenacapavir ). Researchers reported that the investigational combination maintained a high level of viral suppression among study participants and was well tolerated. The study will continue to gather data and suggests that a weekly oral HIV treatment regimen could someday be possible . Read the abstract Exit Disclaimer .
Finally, Dr. Dieffenbach discussed Wednesday’s plenary session by Dr. Charles Flexner of The Johns Hopkins University School of Medicine, which was titled “The End of Oral? How Long-Acting Formulations Are Changing the Management of Infectious Diseases.” In his big picture, future-focused presentation exploring long-acting drug delivery, Dr. Flexner observed that there is a need for HIV products with less frequent dosing, greater convenience, and greater likelihood of viral suppression, as well as for the prevention and treatment of other diseases, including tuberculosis, malaria, and viral hepatitis. He discussed recent advances in formulation science that are going to help make available better replacements for daily oral drugs for HIV and many other infectious diseases . Dr. Dieffenbach underscored Dr. Flexner’s point that these novel products must be developed with access and equity in mind so that people who need them, especially in resource-limited settings, can use them.
Key Takeaways
Reflecting on key takeaways from the entire conference, both Dr. Dieffenbach and Brian pointed to the importance of partnership between the HIV community and scientists in all aspects of HIV research , a theme also discussed in HIV.gov’s conversation with Dr. LaRon Nelson from the conference. In terms of research highlights, Dr. Dieffenbach pointed to the results reported from the IMPAACT P1115 study in which several children who started HIV treatment within hours of birth later surpassed a year of HIV remission after a treatment pause. ( See HIV.gov’s interview with Dr. Deborah Persaud about this study .) He also noted that the additional data accumulating on the effectiveness of Doxy-PEP is encouraging and will hopefully soon be reflected in clinical guidelines that help to reduce the incidence of syphilis, chlamydia, and gonorrhea in men who have sex with men and transgender women.
Catch Up on More HIV Research Updates
HIV.gov has shared other interviews from CROI 2024 with federal HIV leaders, participating researchers, and community members. You can find all of them on HIV.gov’s social media channels and recapped here on the blog this week and next week.
More than 3,600 HIV and infectious disease researchers from 73 countries gathered in Denver and virtually from March 3-6 this year for CROI, an annual scientific meeting on the latest research that can help accelerate global progress in the response to HIV and other infectious diseases, including STIs and viral hepatitis. Over 1,000 summaries of original research were presented. Visit the conference website Exit Disclaimer for more information. Session webcasts and more information will be published there for public access in 30 days.
Related HIV.gov Blogs
- CROI Conference on Retroviruses & Opportunistic Infections
- NIAID National Institute of Allergy & Infectious Diseases
- NIH National Institutes of Health
- Treatment HIV Treatment
- Skip to main content
- Keyboard shortcuts for audio player
The FDA has approved a new drug in the fight against AIDS
Jason Beaubien
The Food and Drug Administration has approved the first injectable medication for HIV prevention. Health advocates say it could be a game changer in protecting people against AIDS
Copyright © 2021 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by an NPR contractor. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
Emily Mullin
There’s New Hope for an HIV Vaccine
Since it was first identified in 1983, HIV has infected more than 85 million people and caused some 40 million deaths worldwide.
While medication known as pre-exposure prophylaxis , or PrEP, can significantly reduce the risk of getting HIV, it has to be taken every day to be effective. A vaccine to provide lasting protection has eluded researchers for decades. Now, there may finally be a viable strategy for making one.
An experimental vaccine developed at Duke University triggered an elusive type of broadly neutralizing antibody in a small group of people enrolled in a 2019 clinical trial. The findings were published today in the scientific journal Cell .
“This is one of the most pivotal studies in the HIV vaccine field to date,” says Glenda Gray, an HIV expert and the president and CEO of the South African Medical Research Council, who was not involved in the study.
A few years ago, a team from Scripps Research and the International AIDS Vaccine Initiative (IAVI) showed that it was possible to stimulate the precursor cells needed to make these rare antibodies in people. The Duke study goes a step further to generate these antibodies, albeit at low levels.
“This is a scientific feat and gives the field great hope that one can construct an HIV vaccine regimen that directs the immune response along a path that is required for protection,” Gray says.
Vaccines work by training the immune system to recognize a virus or other pathogen. They introduce something that looks like the virus—a piece of it, for example, or a weakened version of it—and by doing so, spur the body’s B cells into producing protective antibodies against it. Those antibodies stick around so that when a person later encounters the real virus, the immune system remembers and is poised to attack.
While researchers were able to produce Covid-19 vaccines in a matter of months, creating a vaccine against HIV has proven much more challenging. The problem is the unique nature of the virus. HIV mutates rapidly, meaning it can quickly outmaneuver immune defenses. It also integrates into the human genome within a few days of exposure, hiding out from the immune system.
“Parts of the virus look like our own cells, and we don’t like to make antibodies against our own selves,” says Barton Haynes, director of the Duke Human Vaccine Institute and one of the authors on the paper.
The particular antibodies that researchers are interested in are known as broadly neutralizing antibodies, which can recognize and block different versions of the virus. Because of HIV’s shape-shifting nature, there are two main types of HIV and each has several strains. An effective vaccine will need to target many of them.
Some HIV-infected individuals generate broadly neutralizing antibodies, although it often takes years of living with HIV to do so, Haynes says. Even then, people don’t make enough of them to fight off the virus. These special antibodies are made by unusual B cells that are loaded with mutations they’ve acquired over time in reaction to the virus changing inside the body. “These are weird antibodies,” Haynes says. “The body doesn’t make them easily.”

By Louryn Strampe

By Medea Giordano

By Scott Gilbertson

By Ron Amadeo, Ars Technica
Haynes and his colleagues aimed to speed up that process in healthy, HIV-negative people. Their vaccine uses synthetic molecules that mimic a part of HIV’s outer coat, or envelope, called the membrane proximal external region. This area remains stable even as the virus mutates. Antibodies against this region can block many circulating strains of HIV.
The trial enrolled 20 healthy participants who were HIV-negative. Of those, 15 people received two of four planned doses of the investigational vaccine, and five received three doses. The trial was halted when one participant experienced an allergic reaction that was not life-threatening. The team found that the reaction was likely due to an additive in the vaccine, which they plan to remove in future testing.
Still, they found that two doses of the vaccine were enough to induce low levels of broadly neutralizing antibodies within a few weeks. Notably, B cells seemed to remain in a state of development to allow them to continue acquiring mutations, so they could evolve along with the virus. Researchers tested the antibodies on HIV samples in the lab and found that they were able to neutralize between 15 and 35 percent of them.
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but that challenges remain. “It outlines a path for vaccine development, but there’s a lot of work that needs to be done,” he says.
For one, he says, a vaccine would need to generate antibody levels that are significantly higher and able to neutralize with greater efficacy. He also says a one-dose vaccine would be ideal. “If you’re ever going to have a vaccine that’s helpful to the world, you’re going to need one dose,” he says.
Targeting more regions of the virus envelope could produce a more robust response. Haynes says the next step is designing a vaccine with at least three components, all aimed at distinct regions of the virus. The goal is to guide the B cells to become much stronger neutralizers, Haynes says. “We’re going to move forward and build on what we have learned.”
You Might Also Like …
In your inbox: Will Knight's Fast Forward explores advances in AI
Indian voters are being bombarded with millions of deepfakes
They bought tablets in prison —and found a broken promise
The one thing that’s holding back the heat pump
It's always sunny: Here are the best sunglasses for every adventure

Carl Zimmer
Matt Reynolds
Beth Mole, Ars Technica

Max G. Levy
After decades of failures, researchers have renewed hopes for an effective HIV vaccine
The world needs an HIV vaccine if it ever hopes to beat a virus that still infects over 1 million people a year and contributes to hundreds of thousands of deaths.
Despite 20 years of failures in major HIV vaccine trials — four this decade alone — researchers say recent scientific advances have likely, hopefully, put them on the right track to develop a highly effective vaccine against the insidious virus.
But probably not until the 2030s.
“An effective vaccine is really the only way to provide long-term immunity against HIV, and that’s what we need,” Dr. Julie McElrath, the director of the vaccine and infectious disease division at the Fred Hutchinson Cancer Center in Seattle, said Monday at the Conference on Retroviruses and Opportunistic Infections in Denver.
All current HIV vaccine action is in the laboratory, animal studies or very early human trials.
Researchers at the retrovirus conference presented favorable results from two HIV vaccine studies. One found that a modification to the simian version of HIV spurred production of what are known as broadly neutralizing antibodies against the virus in monkeys. Another showed promise in the effort to coax the immune system’s B cells to make the powerful antibodies in humans.
“These trials illustrate as a proof of concept that we can train the immune system. But we need to further optimize it and test it in clinical trials,” Karlijn van der Straten, a Ph.D. student at the Academic Medical Center at Amsterdam University, who presented the human study, said at a news conference Monday.
Still, the scrappy scientists in this field face a towering challenge. HIV is perhaps the most complex pathogen ever known.
“The whole field has learned from the past,” said William Schief, who leads Moderna’s HIV vaccine efforts. “We’ve learned strategies that don’t work.”
The cost has already been immense. Nearly $17 billion was spent worldwide on HIV -vaccine research from 2000 to 2021. Nearly $1 billion more is spent annually, according to the Joint United Nations Program on HIV/AIDS and the nonprofit HIV group AVAC.
“Maintaining the funding for HIV vaccines right now is really important,” said Dr. Nina Russell, who directs HIV research at the Bill & Melinda Gates Foundation. She pointed to the field’s own “progress and the excitement” and to how “HIV vaccine science and scientists continue to drive innovation and science that benefits other infectious diseases and global health in general.”
Case in point: Covid. Thanks to HIV research, the mRNA vaccine technology was already available in 2020 to speed a coronavirus vaccine to market.
Why the HIV vaccine efficacy trials failed
In strong contrast to Covid, the HIV vaccine endeavor has spanned four decades. Only one of the nine HIV vaccine trials have shown efficacy: a trial conducted in Thailand and published in 2009 that reported a modest 31% reduction in HIV risk.
HIV vaccine researchers subsequently spent years seeking to retool and improve that vaccine strategy, leading to a series of trials that launched in the late 2010s — only to fail.
Researchers have concluded those latest trials were doomed because, aside from prompting an anti-HIV response based in immune cells, they only drove the immune system to produce what are known as non-neutralizing antibodies. Those weapons just weren’t strong enough for such a fearsome foe.
Preventing HIV through vaccination remains a daunting challenge because the immune system doesn’t naturally mount an effective defense against the virus, as it does with so many other vaccine-preventable infections, including Covid. An HIV vaccine must coax from the body a supercharged immune response with no natural equivalent.
That path to victory is based on a crucial caveat: A small proportion of people with HIV do produce what are known as broadly neutralizing antibodies against the virus. They attack HIV in multiple ways and can neutralize a swath of variants of the virus.
Those antibodies don’t do much apparent good for people who develop them naturally, because they typically don’t arise until years into infection. HIV establishes a permanent reservoir in the body within about a week after infection, one that their immune response can’t eliminate. So HIV-positive people with such antibodies still require antiretroviral treatment to remain healthy.
Researchers believe that broadly neutralizing antibodies could prevent HIV from ever seeding an infection, provided the defense was ready in advance of exposure. A pair of major efficacy trials, published in 2021 , demonstrated that infusions of cloned versions of one such antibody did, indeed, protect people who were exposed to certain HIV strains that are susceptible to that antibody.
However, globally, those particular strains of the virus comprise only a small subset of all circulating HIV. That means researchers can’t simply prompt a vaccine to produce that one antibody and expect it to be effective. Importantly, from this study they got a sense of what antibody level would be required to prevent infection.
It’s a high benchmark, but at least investigators now have a clearer sense of the challenge before them.
Also frustrating the HIV vaccine quest is that the virus mutates like mad. Whatever spot on the surface of the virus that antibodies target might be prone to change through mutation, thus allowing the virus to evade their attack. Consequently, researchers search for targets on the virus’ surface that aren’t highly subject to mutation.
Experts also believe warding off the mutation threat will require targeting multiple sites on the virus. So researchers are seeking to develop a portfolio of immune system prompts that would spur production of an array of broadly neutralizing antibodies.
Prompting the development of such antibodies requires a complex, step-by step process of coaxing the infection-fighting B cells, getting them to multiply and then guiding their maturation into potent broadly neutralizing antibody-producing factories.
HIV vaccine development ‘in a better place’
Dr. Carl Dieffenbach, the head of the AIDS division at the National Institute of Allergy and Infectious Diseases, said numerous recent technological advances — including mRNA, better animal models of HIV infection and high-tech imaging technology — have improved researchers’ precision in designing, and speed in producing, new proteins to spur anti-HIV immune responses.
Global collaboration among major players is also flourishing, researchers said. There are several early-stage human clinical trials of HIV-vaccine components underway.
Three mRNA- based early human trials of such components have been launched since 2022. Among them, they have been led or otherwise funded by the global vaccine research nonprofit group IAVI, Fred Hutch, Moderna, Scripps Research, the Gates Foundation, the National Institutes of Health, the U.S. Agency for International Development, and university teams. More such trials are in the works.
On Friday, Science magazine reported concerning recent findings that among the three mRNA trials, a substantial proportion of participants — 7% to 18%, IAVI said in a statement — experienced skin-related symptoms following injections, including hives, itching and welts.
IAVI said in its statement that it and partners are investigating the HIV trials’ skin-related outcomes, most of which were “mild or moderate and managed with simple allergy medications.”
Researchers have shown success in one of those mRNA trials in executing a particular step in the B-cell cultivation process.
That vaccine component also generated “helper” CD4 cells primed to combat HIV. The immune cells are expected to operate like an orchestra conductor for the immune system, coordinating a response by sending instructions to B cells and scaling up other facets of an assault on HIV.
A complementary strategy under investigation seeks to promote the development of “killer” CD8 cells that might be primed to kill off any immune cells that the antibodies failed to save from infection.
Crucially, investigators believe they are now much better able to discern top vaccine component candidates from the duds. They plan to spend the coming years developing such components so that when they do assemble the most promising among them into a multi-pronged vaccine, they can be much more confident of ultimate success in a trial.
“An HIV vaccine could end HIV,” McElrath said at the Denver conference. “So I say, ‘Let’s just get on with it.”
Dr. Mark Feinberg, president and CEO of IAVI, suggested that the first trial to test effectiveness of the vaccine might not launch until 2030 or later.
Even so, he was bullish.
“The field of HIV vaccine development is in a better place now than it’s ever been,” he said.
Benjamin Ryan is independent journalist specializing in science and LGBTQ coverage. He contributes to NBC News, The New York Times, The Guardian and Thomson Reuters Foundation and has also written for The Washington Post, The Nation, The Atlantic and New York.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

HIV/AIDS: Current Updates on the Disease, Treatment and Prevention
Praveen kumar gupta.
Department of Biotechnology, R.V College of Engineering, Bangalore, 560059 India
Apoorva Saxena
CCR5-delta 32 homozygous stem cell transplantation for HIV-infected individuals is being treated as a milestone in the global AIDS epidemic. Since 2008, when the second Berlin patient was cured from HIV after undergoing transplantation from a donor with delta-32 mutation, scientists are aiming for a long-term cure for the wider population. In 2019, a London patient became the second person to be free of HIV and came off the antiretroviral drugs completely. CCR5 gene is now being treated as a viable target for HIV treatment. It can be used in the treatment of HIV either through administration of drugs that bind to CCR5 and stop the receptor from working or through gene therapy to alter the CCR5 gene using CRISPR/Cas9 and prevent protein production. This review article aims to identify the obstacles and the need to overcome them in order to bridge the gap between current research and future potential cures for HIV.
Introduction
Human immunodeficiency virus or HIV is the cause of HIV infection that leads to the autoimmune disorder acquired immune deficiency syndrome (AIDS) [ 1 ] (Fig. 1 ). The major cause of spreading of HIV is through unprotected sex, during pregnancy from mother to foetus, through contaminated hypodermic needles and infected blood transfusions [ 1 ]. In the year 2016, an estimated 37 million people were living with HIV and 1 million deaths were reported. HIV/AIDS is a pandemic condition—an epidemic of diseases that spreads across large areas like multiple continents or even worldwide [ 1 ]. The first time AIDS was recognized was in the year 1981 by the United States Center for Disease Control and Prevention (CDC). Since the reported case of an individual who had successfully undergone a stem cell transplant from a person who showed a homozygous CCR5-delta 32 mutation, after receiving extensive high dose chemotherapy, there has been a greater interest in finding a potential cure.

Human Immunodeficiency Virus [ 5 ]
HIV is a type of retrovirus that adversely infects the immune system of a human, mainly targeting the CD4 + T-helper cells, accessory cells and the macrophages [ 2 ]. When it gains entry into the target cell, the viral genomic RNA undergoes a process of the reverse transcription with the help of reverse transcriptase enzyme and forms double stranded DNA (ds-DNA). This ds-DNA then gets integrated into the target cellular DNA with the help of enzyme integrase and other host co-factors [ 3 ]. The virus now can either become dormant or conceal itself and the target cell detection by the host immune system or it can get transcribed into new viral RNA and proteins that are released from the cell and begin the cycle again. HIV can be characterized into 2 major classes—HIV-1 and HIV-2. HIV-1, which is more virulent, infective and the major cause of HIV in humans, was discovered first and was initially referred to as HTLV-III or LAV [ 4 ] (Fig. 2 ). HIV-2 is less infective and far fewer people exposed to it are infected.

Structure of HIV-1 [ 8 ]
The crucial factor in gaining entry into target cell is through binding of HIV to the CD4 receptor present on the T-helper cells and to one of the chemokine receptors- either CCR5 or CXCR4 [ 6 , 7 ]. Binding to the co-receptor depends on the virus’s tropism which is the ability to bind to a specific receptor. Naturally, there are two types of tropic strains—R5 that bind to CCR5 and X4 which bind to CXCR4. Dual tropic strains are capable of binding to both. Of these two co-receptors, CCR5 is the prime receptor for virus’s entry into the target cell. R5-tropic strains prevail during early stages of infection, whereas the X4-tropic strains emerge later with disease progression. The envelope-like glycoprotein structure of HIV-1 is paramount in ensuring the viral entry into a target host cell [ 7 ]. This glycoprotein has 2 protein subunits: the gp41 (transmembrane) subunit and gp120 (external) subunit, which mimics a chemokine [ 6 , 7 ]. It does not manifest the unique structure of the chemokine but somehow manages to bind to both the co-receptors [ 6 ]. It forms a heterotrimeric complex wherein the gp120 subunit binds to the CD4 protein and specific co-receptor present on the target cell [ 6 ]. When this complex is formed, it triggers the release of a peptide which facilitates cell–cell fusion, that causes the viral membrane to fuse with the target cell membrane [ 6 ]. Binding to CD4 alone is not sufficient as it can result in gp120 shedding. So, it has to bind to the specific co-receptor for the fusion to proceed. The V1–V2 region of gp120 is recognized by the co-receptor, that influences which co-receptor will bind to the protein and is determined by degree of N-linked glycosylation and peptide composition. The highly variable V3 loop is the one that determines co-receptor specificity. The binding of gp120 glycoprotein to the CCR5 co-receptor is determined by two essential factors—the tyrosine-sulphated amino terminus of CCR5 receptor and following which there must be reciprocal action between the transmembrane domains of CCR5 and gp120 protein, i.e., inter-communication and synergy.
Antiretroviral Therapy
The usage of a combination of three or more antiretroviral drugs for suppression of the HIV infection is called antiretroviral therapy. Using multiple drugs in combination to increase the effectivity on various viral targets is called highly active antiretroviral therapy (HAART). It helps in maintaining the immune system to function, preventing HIV from developing resistance and other infections that potentially lead to death. The five classes of drugs used in combination to treat HIV infection are: entry inhibitors, nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, integrase inhibitors and protease inhibitors.
Zidovudine/ZVD (also called azidothymidine) is an extensively used antiretroviral medication [ 9 ]. It is a thymidine analogue and is dosed twice daily in combination with other antiretrovirals. Its function is to particularly inhibit the reverse transcriptase enzyme which is necessary for the production of ds-DNA.
Cellular enzymes are used in converting AZT into the 5′-triphosphate form. Research studies suggest that the termination of forming ds-DNA chains is a crucial factor that leads to an inhibitory effect.
Studies have also shown that at very high dosage of this drug, its triphosphate form may inhibit the DNA polymerase enzyme which is used for cell division by the uninfected cells and mitochondria for replication. It may lead to toxic but reversible effects on certain skeletal and the cardiac muscles, causing the condition of myositis [ 10 ]. However, zidovudine also shows greater affinity for the reverse transcriptase enzyme, which is around 100-fold. This selectivity has been proven by the cell's ability to quickly repair its DNA strands if broken by AZT during its formation, whereas the HIV virus will lack this ability (Fig. 3 ).

Structure of zidovudine [ 11 ]
Zidovudine is commonly used in combination with nucleotide reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, HIV integrase strand transfer inhibitor and protease inhibitor [ 9 ]. The combination of lamivudine and zidovudine is not recommended for non-pregnant HIV-infected adults and adolescents due to greater toxicity but is used as an alternative, though not a preferred one, in antiretroviral-naive pregnant women as an initial treatment [ 9 ]. However, for paediatric patients (neonates, infants and children of age 12 or less), zidovudine with lamivudine/emtricitabine is a preferred option. For adolescents greater than the age of 12, it is an alternative [ 9 ].
Zidovudine Administration and Pharmacokinetics
Administration and dosage.
It is usually administered orally or by continuous IV infusion, although not rapid infusion and IM injection [ 9 ] (Tables (Tables1, 1 , ,2). 2 ). The dosage for paediatric patients and adult patients depends on their body weight (Tables (Tables3, 3 , ,4 4 ).
Oral administration [ 9 ]
IV administration [ 9 ]
Dosage for paediatric patients [ 9 ]
Dosage for adult patients [ 9 ]
Administration
Zidovudine: 1 mg/kg every 4 h [ 9 ].
Pharmacokinetics
Pharmacokinetics gives a detailed view of the fate of drugs in the human system. It includes various components like absorption, distribution, excretion or elimination and metabolism (Tables (Tables5, 5 , ,6, 6 , ,7). 7 ). The stability of such retroviral drugs should also be taken into account for both oral and parenteral dosage forms (Table (Table8 8 ).
Absorption [ 9 ]
Distribution [ 9 ]
Elimination process [ 9 ]
Stability of antiretrovirals [ 9 ]
Contraindications [ 9 ]
- Zidovudine has a history of life-threatening hypersensitivity reactions like Stevens–Johnson syndrome and anaphylaxis to the drug or maybe due to some ingredient in the formulation.
- Lamivudine/zidovudine: hypersensitivity history.
- Abacavir/zidovudine/lamivudine: history of hypersensitivity to abacavir, zidovudine or lamivudine; hepatic impairments may be mild or severe.
CCR5 Gene Structure
C–C chemokine receptor type 5 (also called CCR5 or CD195) is a receptor for chemokines present on the white blood cells. The CCR5 gene in humans is located on the short arm (p) at position 21 on chromosome number 3 (Fig. 4 ). It is mainly expressed cells like T-cells, macrophages, microglia, dendritic cells and eosinophils and is found within a cluster of genes coding for some other receptors like XCR1, CCBP2, etc. [ 12 , 13 ]. The gene has two promoters, three exons and two introns. Pu or PR2, the upstream promoter, has a 1.9 kb region, 57 bp in length and precedes the exon 1 [ 12 ]. Exon 1, which is the start of the coding region, is followed by the first intron, 501 bp in length. The second exon 2 is intron-less. It is found as exon 2a, 235 bp in length, and exon 2b, 54 bp in length. Pd or PR1, the second promoter, accommodates the intron 1 and exon 2 regions [ 12 ]. A 1.9 kb length intron is located between exon 2 and exon 3. Exon 3 is also intron-less and consists of the full ORF of the CCR5 gene, 11 bp of the 5′ untranslated regions and the complete 3′ untranslated regions [ 12 ].

Location of CCR5 gene on chromosome 21 [ 14 ]
These two promoters are devoid of the consensus TATA and CCAAT sequences, although the Pd promoter has a non-consensus TATA sequence and have an unusually high content of pyrimidine in them [ 12 ]. The upstream Pu promoter was found to be weaker than the downstream Pd promoter which had exhibited up to fivefold greater activity. But these results were established as erroneous [ 13 ]. With the help of RT-PCR technique, it was later identified that the Pu promoter was used in stimulated T-cells and the Pd promoter was used in unstimulated primary T-cells [ 13 ]. The error resulted due to the use of transformed T-cells affecting the overall expression of CCR5 protein via the Pu promoter [ 13 ]. Results also showed that transcription of the CCR5 gene when controlled by the Pu promoter containing exon 1 resulted in CCR5A or B and when controlled by the Pd promoter resulted in truncated isoforms [ 13 ].
CCR5 Gene Expression Regulation
The expression of CCR5 gene is regulated at three levels: 1. genetic factors, 2. factors involved in activation, signalling and trafficking of the receptor which includes desensitization, internalization and recycling and 3. environmental triggers [ 13 ].
CCR5 receptor is part of the G-protein coupled receptor family, which binds to its ligand and releases αi and βγ G-protein subunits. This results in a mediated effector response. Such responses stimulate the release of phospholipase Cβ and adenylyl cyclase. This in turn facilitates the release of intracellular calcium and form inositol triphosphate [ 13 ]. This leads to activation of phosphorylation of the CCR5 receptor which occurs at the serine and C-terminal residues via protein kinase C and G-protein coupled receptor kinases [ 13 ]. The regulatory proteins, β-arrestin 1 and 2, bind to the activated serine and the conserved DRY motif in the intracellular loop [ 13 ]. The β-arrestin proteins have functions like desensitizing the receptor to further stimulation and participating in endocytosis. The CCR5 expression level is controlled by the rates of recycling and endocytosis [ 13 ]. In the endocytosis process, β-arrestin protein facilitates the binding process between clathrin-coated pits and the phosphorylated receptor. Infection and entry of HIV into cells do not require CCR5 signalling, but the chemokine-induced endocytosis decreases the available receptor for HIV entry. This is the process of chemokine-mediated anti-HIV activity [ 13 ].
Environmental factors affecting CCR5 expression are infectious pathogenic agents like Mycobacterium tuberculosis , which increases the CCR5 expression. Studies have shown that CCR5 expression is considerably increased in all leukocyte subset cells during tuberculosis and dual infection with HIV [ 13 ]. However, the level of CCR5 expression on CD4 + T-cells was not increased. Conversely, it was also shown that HIV affects the level of expression of CCR5, due to a correlation with HIV disease progression. Individuals with end stage HIV were shown to have the highest percentages of CCR5 expressing CD4 + T-cells [ 13 ].
The regulation of CCR5 is complex. The introns as well as sequences in the 5′ UTR and 3′ UTR affect CCR5 gene regulation [ 13 ]. Therefore, mutations in these regions should be considered critical in the regulation process.
CCR5-Delta 32 Mutation
The discovery of CCR5-delta 32 mutation in the CCR5 gene in 1996 which exhibited some protection against HIV was a ground breaking one. Studies showed that the CD4 + T-cells when expressing this mutation prevented HIV envelope fusion [ 12 ] (Fig. 5 ). The mutant allele has a length of 215 in comparison to the wild type which contains 352 amino acid residues [ 13 ]. This mutation basically results due to the deletion of 32 base pairs from the position of nucleotides starting from 794 till 825, a frameshift mutation, and seven new amino acids are incorporated between amino acid 174 and stop codon at amino acid 182 [ 13 ] (Fig. 6 ). This mutation affects the region of second extracellular loop where the resultant protein lacked the last three transmembrane domains and also some regions necessary for G-protein interaction and signal transduction.

Comparison of HIV infecting cell with CCR5 and without CCR5 [ 15 ]

Difference between wild type CCR5 and CCR5-delta 32 [ 16 ]
This mutation is majorly restricted to people of European descent. The gene frequencies are found to be around 10% and shows a decline from north to south latitude. A 2–5% gene frequency in Europe, the Middle East and parts of the Indian subcontinent was observed in more than 3000 individuals. The highest frequency, at 20.93%, was discovered in the Ashkenazi Jewish population. The mutant allele is absent in Black populations excluding the African American group who may have acquired the mutation through genetic admixture [ 13 ].
The origin of the delta-32 mutant allele has been dated back to the year 275–1875, which increased over a period of time as a result of selective pressure, mainly the Black plague. However, historical data have shown that Black plague may not in fact be the cause [ 13 ]. The distribution of the delta-32 mutant allele in a north to south gradient does not correlate to the casualties of the plague and instead follows a south to the north gradient. The Black plague has shown the greatest casualties in areas like the Mediterranean region and China, with lowest allele frequencies of the mutation [ 13 ].
Studies suggested that delta-32 arose without a selective event. Tandem repeats found in the coding region of the CCR5 gene could cause unequal homologous recombination, which results in the delta-32 allele. The origins of the delta-32 mutation, however, remain a mystery [ 13 ].
The hype about the delta-32 mutation comes from its ability to protect homozygous individuals from HIV. The protective effect of the delta-32 mutation is a result of eliminating the expression of CCR5 protein on the cell surface, which prevents HIV’s entry into the cell. In the year 1997, however, studies showed that some of them having the homozygous delta-32 mutation were HIV-infected [ 13 ]. Further studies revealed the HIV virus was of the X4 type, which led to very rapid CD4 + T cell decline. Hence, this mutation is limited in its function and does not protect against viral strains which utilize other receptors or show dual-tropism [ 13 ].
In contrast, however, the delta-32 protein product which is localized to the endoplasmic reticulum is an important factor. It is shown to exert a trans-dominant negative effect on the wild-type CCR5 protein, which inhibits its transport to the cell surface. Further analysis in vitro showed the reduction of surface expression of wild type CCR5 and CXCR4 through dimerization by this mutant protein product [ 13 ]. This confers an inhibition to R5, X4 and R5X4 HIV infections [ 13 ]. Homozygous delta-32 individuals with this mutant protein were shown to have suppressed CXCR4 surface protein expression and decreased susceptibility to X4 infection. Experimental proofs also suggested that delta-32 heterozygous individuals with HIV infection do not stably express the mutant protein, are devoid of the molecular mechanism of complete protection and only maybe partially protected [ 13 ].
Stem Cell Transplantation
Stem cells are undifferentiated cells that can differentiate into specialized cells and can also undergo mitosis to produce more stem cells. There are mainly two classes—embryonic stem cells (ECS) and adult stem cells. Stem cells are also taken from the umbilical cord blood just after birth. These act as a repair mechanism for the body, such as skin, blood or intestinal tissues. Adult stem cells are majorly used in medical therapies like bone marrow transplantation. Bone marrow is the spongy tissue present inside the bones which serves as a rich source of adult stem cells. Long-term control of HIV is possible with CCR5-delta 32 stem cell transplantation [ 13 ].
Allogeneic transplantation of stem cells with this mutation in patients with HIV infection and malignancy has been considered as an option since the late 1990s (Fig. 7 ). Human leukocyte antigen (HLA) is a critical factor to be considered during the process of transplantation. The HLA should be a proper match; otherwise, it would lead to rejection by the recipient’s immune system. The limited availability of HLA-matched unrelated donors has made it even more difficult. Only about 1% of Caucasians possess this CCR5 null allele [ 13 ].

Allogeneic hematopoietic stem cell transplant [ 17 ]
Gene Therapy
Zinc finger nuclease technology is a popular tool which can be used for targeting specific DNA sequences in the genome. It falls in the class of restriction enzymes and is artificially made by fusing a zinc finger DNA-binding domain and DNA-cleavage domain. This technique is also engineered to eliminate the CCR5 expression over CD4 + T-cells, and the modified cells have shown to have a half-life of 48 weeks [ 13 ]. But it has its own issues. It is difficult to ensure that the desired repair mechanism is one which is used to repair the double stranded break (DBS) [ 13 ]. It is also challenging to scale it upwards and is an expensive technique.
A breakthrough technique, the CRISPR/Cas9 gene-editing system, is also used to eliminate the CCR5 receptor on the blood stem cells which can give rise to differentiated blood cells that are devoid of this receptor [ 18 ] (Fig. 8 ). These gene-edited stem cells can be established into an HIV-infected patient through bone marrow transplantation and give rise to an HIV-resistant immune system [ 18 ]. This technique, however, can also go sideways which leads to unwanted results that can cause ethical issues to rise. As seen in the highly controversial case of the Chinese scientist, He Jiankui, who with the help of this technology deleted the CCR5 gene in the twins, Lulu and Nana, introduced some unintended mutations in their genetic codes. There is still a lot of research needed to make this technology bioethically a safe tool.

CRISPR/Cas9 gene editing [ 19 ]
Researchers have also engineered a molecule called the chimeric antigen receptor (CAR) and introduced a gene for that molecule into blood-forming stem cells [ 18 ]. This molecule has two receptors that will recognize the antigen (HIV) and direct the immune cells to locate and kill the HIV-infected cells [ 18 ]. When transplanted into mice, which would have the CAR-carrying blood stem cells, it would result in reduced levels of HIV by inducing the immune cells to fight effectively against the virus [ 18 ]. An 80% to 95% drop in viral load was observed in the mice [ 18 ]. It was concluded that gene therapy could be a feasible option for treatment in HIV-positive humans.
Immunological Approaches
Studies have shown that vaccine can contribute effectively in viral clearance such as the Rhesus CMV vaccine vector [ 18 ]. A vaccine vector is a kind of vaccine which consists of chemically weakened viruses that are transported in the body to generate an immune response. The genes used in these vaccines are antigen coding surface proteins from that particular pathogen.
SAV001-H is the first and only preventive HIV vaccine which uses killed HIV-1 virus [ 18 ]. It is unique from other vaccines, as it uses genetically engineered whole virus genome, eliminating its pathogenicity and inactivating its virulence through irradiation and chemical treatments, finally approaching to the first “whole-killed virus”-based HIV vaccine [ 18 ]. The results of Phase 1 clinical trial, which were completed in the year 2013, were found to have serious and adverse effects in the 33 participants [ 18 ]. There was also a surprising boost in the antibody production against p24 and gp120. The HIV viral core is mostly made up of the structural protein, p24, which is called the capsid. A crucial factor in the diagnosis of primary HIV-infected individuals is the p24 antigen assay. High levels of p24 are found in the blood serum during the period between infection and seroconversion. The antibody production is found to increase as much as 64-fold [ 18 ]. The antibody production against gp120, which is a glycoprotein, necessary for attachment to a cell receptor and allow HIV entry, is found to increase up to eight-fold [ 18 ].
Another promising vaccine called the Kang's vaccine also uses the “whole-killed HIV-1,” which is similar to vaccines developed for rabies, polio and influenza [ 18 ]. However, HIV-1 is genetically engineered in such vaccines and raises questions about safety and possibility of large quantity production.
Researchers have also tested an immunogen called eOD-GT8 60mer, a protein nanoparticle, which is designed to mimic a crucial part of the HIV envelope protein which will bind to and activate the B cells to produce plasma cells that secrete antibodies needed to fight HIV [ 18 ]. This nanoparticle was developed in the Schief laboratory and tested in mouse models engineered by the Nemazee laboratory [ 18 ]. The researchers showed that immunization with eOD-GT8 60mer produced antibody progenitors with some of the characters crucial to recognize and block the HIV infection, proposing that it could be a promising first step in a series of immunizations against HIV [ 18 ]. The vaccine appears to work well in mouse models. The researchers are now investigating other immunogens that could work in coexistence with eOD-GT8 60mer [ 18 ].
Case Studies
The berlin patient [ 20 ].
The strongest proof available in favour of a HIV cure stems from the case of Timothy Brown who is popularly known as the Berlin patient (Fig. 9 ). He is considered the first person ever to be cured of HIV. The victory was predicated on doctors taking advantage of nature’s own experiment—the genetic mutation of CCR5 gene that produces a protein co-receptor present on the surface of CD4 + T-cells that HIV uses to gain entry. He was attending university in Berlin when was diagnosed HIV positive. His initial treatment include ART, and he was taking low doses of zidovudine and protease inhibitors. He continued to live a normal life for the next 10 years. But one day, he was again feeling extremely exhausted and the doctor had diagnosed it to be anaemia. He had received red blood cell transfusion for nearly a week and was then sent to an oncologist, Dr Huetter, when the previous doctor was unable to resolve the situation. The oncologist performed a painful bone marrow biopsy and after further diagnosis he was informed that he had acute myeloid leukaemia (AML).

Timothy Ray Brown a.k.a. “The Berlin patient” [ 21 ]
He then started receiving treatment at one of the Berlin University hospitals and had to receive four rounds of chemotherapy treatment. During the third round of chemotherapy, he had gotten a fatally dangerous infection and was immediately put into an induced coma. His blood sample was collected and sent to a stem cell donor bank with the German Red Cross to find matches in case he needed transplantation. Luckily, he had 267 matches which sparked an idea to locate donors with a homozygous CCR5 delta-32 mutation on CD4 + T-cells who are almost immune to HIV infection. A donor was found at the 61st attempt and had agreed to donate when necessary (Fig. 10 ).

Adam Castillejo a.k.a “The London patient” [ 23 ]
However, Timothy Brown had been reluctant and had said no to transplantation as the success rate was only 50–50. But at the end of 2006, leukaemia had rebounded and he desperately needed transplantation to survive. He received the stem cell transplant on February 6, 2007 and stopped taking his antiretroviral medication. Nearly 3 months after he underwent transplantation, HIV was no longer found in his body and he had thrived until the end of the year.
Unfortunately, life had other plans for him. After coming back from a trip to the USA, he was diagnosed with pneumonia and the leukaemia was back. The doctors decided to treat him with a second transplantation from the same donor in February 2008. The recovery was a tough one. He was almost paralyzed and went nearly blind. He had, however, eventually learnt to walk again and fully recovered 6 years later. He was continuously tested for HIV with extensive and precise tests. It was finally good news for him! Since 2010, when he decided to go public, he had interviewed for various magazines: POZ Magazine , New York Magazine and Science Magazine among others and decided to devote his life in supporting research for cures against HIV. In July 2012, he started the Timothy Ray Brown Foundation under World AIDS Institute and has worked with many scientists, organizations, research laboratories and universities to work on cures such as vaccination against HIV.
The London Patient [ 22 ]
The London patient may be the second person with HIV to no longer have the virus. In March 2019, in a report published in journal Nature , a group of investigators had announced the cure of a second HIV-positive patient. His success story depicts that CCR5 is a viable target for HIV research and treatment.
The London patient, who had chosen to remain anonymous, came out in public on March 9 th 2020. Adam Castillejo grew up in Caracas, Venezuela, and later shifted to London with his mother, as his parents were divorced. He was first diagnosed with HIV in 2003 and had started taking drugs to control the HIV infection in 2012. He had taken antiretroviral therapy for years before being diagnosed with an advanced form of blood cancer called Hodgkin’s lymphoma. Again, as in the case of the Berlin patient, the cancer was resistant to standard chemotherapy, so his doctors had advised more intensive chemotherapy along with bone marrow stem cell transplant. In 2016, he had agreed to transplantation and received it from a healthy donor who carried the CCR5 mutation. So, when his immune system regrew, it lacked the protein and was impervious to HIV. His virologist, Dr Ravindra Gupta, from the University of Cambridge, thinks it is a cure because a year had passed and they had carried out a few more tests for the viral load. In Adam Castillejo’s own words, “I don’t want people to think, “Oh, you’ve been chosen.” No, it just happened. I was in the right place, probably at the time right time, when it happened.” Adam Castillejo wants to be the “ambassador of hope” for people with this illness.
Although the scientists describe this case as a long-term remission, experts are calling it a potential cure. Such transplants are, however, dangerous and can be fatal. They are also an impractical approach to cure the millions already infected. These are highly risky procedures and can lead to serious complications. There still has to be a lot of research done to extend this type of treatment to a wider population infected with HIV.
A comparative study of the two patients reveals that their cases were in fact quite similar (Table (Table9 9 ).
Summary of the two cases—the Berlin patient and the London patient [ 24 ]
Lifestyle Practices to Prevent HIV Infection
Prevention is better than cure. And with HIV infections, one should practice prevention with utmost care and sincerity. An HIV diagnosis could turn one’s life upside down. So, it’s better to lead a healthy lifestyle by making the correct choices.
Measures for Protection Against HIV Infection
HIV is majorly spread through unprotected vaginal or anal sex. Choose less risky behaviour and be cautious. Not taking medicines to prevent or treat HIV is equally responsible for HIV infection. The number of sexual partners should be limited. One should get tested for sexually transmitted diseases and also know the sexual partner’s status. One can talk about pre-exposure prophylaxis to their respective healthcare provider. It is a preventive option for people who are not infected yet but are exposed to high risks of being HIV positive. HIV is also spread through intravenous injections and blood transfusions. Use of sterile equipment in such cases is a necessity.
Pre-exposure Prophylaxis
This is a preventive method of taking pills by people who are not HIV positive yet but who are at a high risk of getting infected and spreading it to others. A pill, named Truvada, contains two medicinal components, emtricitabine and tenofovir, that are used in combination with other drugs to treat HIV [ 25 ]. These medicines work on keeping the virus from creating a permanent infection.
Post-exposure Prophylaxis
Post-exposure prophylaxis (PEP) is a short course of HIV medicines taken soon after a possible exposure to HIV [ 25 ]. Every hour counts. For the treatment to be effective, the course should begin within 72 h after exposure to HIV; otherwise, it will not have any effect [ 25 ]. This treatment should be used only in cases of emergency. A person prescribed with PEP will need to take the medicines for 28 days at a stretch and then visit their respective healthcare provider for further tests [ 25 ]. Even if taken correctly, it may not be 100% effective. The sooner the medication is started, the better.
Healthy Practices to Follow When Living with HIV
A healthy, well-balanced and nutritious diet can help a person lead a better life by preventing health related issues like malnutrition and stopping the progression from HIV to AIDS. A well-balance diet is rich in whole grains, fresh fruits and vegetables, protein, low fat dairy products and multivitamins like zinc and B12. It also constitutes what should be cut down—fried foods, processed foods and sugary drinks. Smoking should be stopped when diagnosed with HIV. According to CDC, in the USA, the rate of adults with HIV, smoking is two to three times higher in adults infected with HIV than the nearly 18% of uninfected adults who smoke. Researchers at the Syracuse University analysed the data from 212 adults infected with HIV and found that the ones who smoked reported having more symptoms like dizziness and coughing.
Putting a stop to illegal drug use is equally necessary. People should seek treatment for addiction to illegal drugs like heroin, cocaine and methamphetamines. Sharing of needles for drugs can leave one exposed to other infections like hepatitis which might lead to a faster progression from HIV to AIDS. A recent study from the University of Pennsylvania School of Medicine showed a dramatic increase in the ability of HIV to attack healthy cells when methamphetamine is present in the bloodstream. This indicates that illegal drugs are also aiding in the HIV infection.
Being physically fit through a good work-out three to six times a week can help improve a person’s mood, perspective and overall quality of life. A good amount of moderate exercise can help fight HIV symptoms of nerve pain, loss of appetite and reduce the risks of other chronic diseases like heart disease, diabetes and osteoporosis. Taking the prescribed medication on time is known as adherence. This is vital to help reduce the risk of HIV becoming drug resistant and helps the immune system function for a longer time.
Nowadays, with the help of Internet of Things or IoT, patient’s health can be monitored 24/7. The quality of care provided can be increased many-folds with the help of monitoring devices enabled with current technology [ 26 ]. Concept of E-Health and M-Health is currently trending. E-Health makes use of electronic and communication processes with improved cyber security [ 26 ]. Some of the E-Health devices include GPS tracking, pedometer and electronic health records [ 26 ]. M-Health systems provide doctors with the complete medical history of the patient, so the treatment becomes easier and does not delay in case of emergencies. It makes use of mobile phones and other communication systems to help the patients with information about preventive health care services and collects data in real time as well [ 26 ]. The other important applications include chronic disease management, monitoring of diseases and tracking of epidemic outbreaks [ 26 ].
Genomic Diversity and Clinical Implications
Despite billions of dollars being invested, there is currently no HIV vaccine available that can either prevent the disease or treat those who suffer from it. An AIDS patient harbours 100 million genetically distinct variants of HIV [ 27 ]. This high diversity of HIV-1 is due to high replication rates, errors in reverse transcriptase and recombination events that mainly occur during the viral replication process. Reverse transcriptase enzyme has approximately a rate of 10 –4 nucleotide substitutions per replication cycle. Deletions, insertions and duplications are major contributing factors to the genetic variation of the virus [ 27 ]. Genetic recombination also plays an important role in creating genetic diversity. Template switches between two copies of RNA strands occur regularly during reverse transcription [ 27 ]. This generates a lot of mutations with the help of inter- and intra-molecular jumps. These mutations can either be drug resistant or inhibit the viral replication capacity.
HIV-1 can be classified into four main groups: M, N, O and the recently identified P. The M group is further identified into 4 subtypes (A to J). Studies have shown that there is a worldwide spread of non-B subtype viruses, and with the introduction of antiretroviral drugs, more research has to be conducted regarding the responsiveness of the drug resistance in non-B subtypes [ 27 ]. Different types of HIV-1 resistance are observed in different subtypes at varied levels. For example, subtypes B and G have shown to develop resistance against nelfinavir [ 27 ]. Research is also being done in the role of polymorphisms for development of drug resistance, to assess the genotypes before and after the therapy to be able to establish any association between the two [ 27 ].
Variation of Disease Progression Rate
There are 3 phases of the progression of HIV-1 infection- primary infection, chronic asymptomatic phase of infection and finally, AIDS. In the asymptomatic phase, neither signs nor symptoms of the disease are present, and this phase lasts an average of about 10 years. They can be divided as typical progressors, rapid progressors, slow progressors and long-term progressors. Rapid ones (10–20%) develop AIDS within 5 years of infection [ 28 ]. Slow progressors (5–15%) remain free of AIDS 15 years after infection [ 28 ]. Long-term progressors that constitute 1% show no signs and symptoms [ 28 ]. Factors like host genetic make-up, immune responses, co-infection and viral genetics and adaptation are attribute to this huge variation in disease progression [ 28 ]. But there is no solid evidence as such.
Some individuals known as elite controllers are able to manage the viral replication for longer durations, others are shown to rapidly lose CD4 + T-cells after seroconversion in the absence of cART (combination antiretroviral therapy). Scientists have conducted research studies that has led to the conclusion that rapid progression before administration of cART stops the recovery of CD4 + T-cells once the suppressive response to HIV-1 through cART is achieved. These findings have implications in public health policy making, clinical outcomes and science research. Ideally, cART should be initiated as soon the patient is diagnosed with HIV-1 irrespective of the CD4 + T-cell count. However, in clinical settings where cART is not widely available, these results would support strategies that may help in promoting frequent testing to reduce the proportion of patients initiating cART at low CD4 + T-cell counts. For those testing early, frequent CD4 + T-cell count should be monitored close to the time of HIV diagnoses to establish the rapid progressors phenotype in order to avoid unnecessary CD4 + T-cell count decay among rapid progressors. Finally, interpretation of the immunopathological basis of rapid progression can help improve individual clinical outcomes and limit its impact in the global HIV-1 pandemic.
Development of Drug Resistance as a Major Barrier to Treat HIV
HIV-1 has a high mutation rate. An estimated 10 10 virions per day can be produced in untreated patients that may result in variants called quasispecies. The complexity is also increased due to high recombination rate whenever more than one variant infects the same cell. All these are contributing factors that help in invading the host’s immune system and fostering drug resistance. Salvage therapy is also useful in cases when more than one regimen failed or a single regimen failed for a patient. It can be used to suppress the virus levels below the detection level and should have high genetic barrier to resistance to prevent rebound [ 29 ]. Clinicians need to focus on patient’s adherence as well as access to antiretrovirals (ARVs), drug interactions, tolerability, genotypic and phenotypic resistance testing, cross-resistance, genetic barrier and potency of ARVs [ 29 ].
Overcoming Obstacles and Future Prospects
At present, the reason for not being able to achieve a complete cure with the help of ART, in spite of achievement of undetectable viral load, is due to the presence of dormant virus or HIV latency. In a method call shock and kill, immune stimulants shock the latent virus from hidden reservoirs and then attempt to kill reactivated HIV [ 18 ]. An enzyme has been identified which is called histone deacetylase (HDAC) which is responsible for the sustained latency. Some studies show promise but are yet to be confirmed by clinical trials. Flushing these latent CD4 HIV-infected cells from their reservoirs with these HDAC-inhibitors into the blood circulation makes them susceptible to ART. Vorinostat and panobinostat are two such promising drugs [ 18 ].
Histone deacetylase inhibitors seem to have a broad spectrum of epigenetic activities. Vorinostat (also called Zolinza) is a U.S. Food and Drug Administration approved medicine, which has been used for the treatment of cutaneous T-cell lymphoma (CTCL) [ 18 ]. They help in flushing the virus from the reservoirs into the circulation. The dose is 400 mg. Other drugs on the pipeline are Protein kinase C agonist bryostatin-1 and GS-9620—TLR7 agonist [ 18 ].
Romidepsin (also called Istodax) is another HDAC inhibitor drug, which induces HIV-1 transcription to form plasma HIV-1 RNA that can be easily detected with standard assays [ 18 ]. This gives a possibility of reversing the HIV-1 latency in vivo without hindering T cell mediated immune response [ 18 ]. These findings will help the researchers with future clinical trials aiming to eliminate the HIV-1 reservoirs.
Research for curing HIV is at an infant stage but a promising one. Scientists are working on two broad types of HIV cures—a functional cure and a sterilising one.
The approach of the functional cure is to reduce the virus levels in the body to an undetectable stage, where the patient no longer needs to be on HIV medication or has no risk of progression to AIDS nor transferring the virus to others. Unlike the functional cure, however, a sterilising cure aims to get rid of HIV from the body completely by eliminating cells from latent reservoirs. It has proved to be an extremely challenging task for scientists, who believe it may be unachievable in the majority of them living with HIV. However, some findings by researchers at the University of Pittsburgh could lead to a foundation for an HIV vaccine. Clinical trials are in the works.
Abivax, a French company, is developing a drug that binds to some specific sequence of the viral RNA and inhibits its replication. During clinical trials, it has shown that this may have the potential to become a functional cure. The key is that it can target the reservoir of HIV viruses that hide inactive within our cells. It can target the reservoirs where HIV viruses act as inactive, within the infected cells. The result of phase IIa trial was quite promising. Fifteen patients were given the drug in combination with ART, and it was observed after 28 days of treatment that eight patients showed a 25% to almost 50% reduction of their HIV reservoirs compared to those only taking ART. The company is planning a phase IIb clinical trial to confirm the effects of the drug in the long term.
Research and development in HIV and its cure have come a long way since the disease was discovered in the 1980s. ART was a major milestone that has changed the lives of millions for good, but the next ambitious goal is to find an HIV cure before the year 2020. There are several approaches to an HIV cure ranging from shock and kill therapy, immunotherapy, vaccine development to gene editing using zinc finger nucleases and the CRISPR/Cas9 system, but finding the best possible solution is a challenge. One of the biggest challenges around any HIV treatment is the ability of the virus to rapidly mutate and develop resistance. Many of the new approaches do not provide any valuable insights as to whether the virus has the potential to become resistant. As of now, none of these functional cures have reached late-stage clinical trials, and the aim of finding an HIV cure until 2020 seems far-fetched. However, 2020 will likely be marked as an important milestone as the first late-stage trials will be executed. If successful, it could bring the approval of the first functional HIV cure in ten years.
There are two gene therapies undergoing human trials—one is to destroy the CCR5 receptor of the immune cells of people infected with HIV and the other therapy includes the CRISPR technology which is still under early trials. This mutation does not necessarily protect the person against all types of HIV. It was found that in one of the patients who had received the bone marrow treatment, it was found to have the CXCR4-tropic form. It uses a different type of receptor to enter and infect the cells. It was, however, not known whether this virus was acquired after the treatment or if some patients do contract a small amount of CXCR4-tropic virus that starts to multiply when other types are not present.
HIV research continues on many fronts that could provide the same results and only some of which rely on the CCR5 delta 32 mutation, which should be explored extensively. There are many strategies which are in the early stages of development. Scientific process can be slow but if done correctly, advances can be made to find a scalable, cost-effective cure for everyone.
Acknowledgments
The authors listed in this paper wish to express their appreciation to the RSST trust Bangalore for their continuous support and encouragement.
Authors Contribution
All authors have contributed equally with their valuable comments which made the manuscript to this form.
There was no funding provided for the above research and preparation of the manuscript.
Compliance with Ethical Standards
The authors declare that they have no conflict of interest.
All the authors listed hereby confirmed that in the above research, there were no human participants and/or animals involved in any kind of determination, evaluation or research studies.
There is also final confirmation given by all the listed authors for the submission of manuscript in its actual state. The authors listed above also confirm that the above-mentioned manuscript is in its original state and the manuscript is neither submitted anywhere nor in the submission process in any other journals. In addition, all the authors have solely contributed their original work in the preparation of this manuscript. If the copying or similarity have been found, then in all situations the listed authors are solely responsible.
Significance Statement
This article aims to increase awareness among the society about the current scenario of HIV/AIDS. The scientists are working on 2 types of cures—functional and sterilizing. The path to finding a cure is a promising one as late-phase trials begin in 2020.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Praveen Kumar Gupta, Email: ni.ude.ecvr@atpugkneevarp .
Apoorva Saxena, Email: [email protected] .
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Research & training, advances in hiv/aids research.
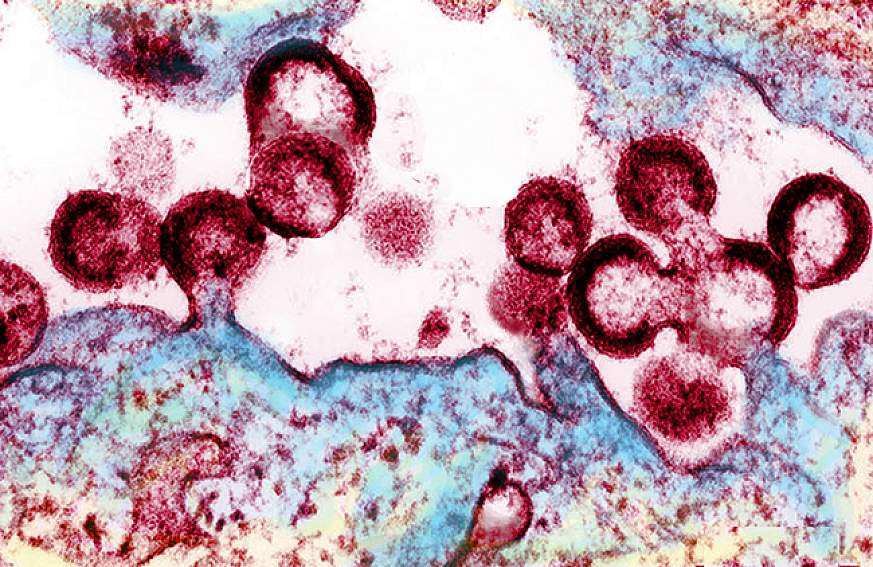
For an update on what medical science is doing to fight the global HIV/AIDS pandemic, read a Parade article by NIH Director Francis S. Collins and NIAID Director Anthony S. Fauci, AIDS in 2010: How We're Living with HIV .
Over the past several decades, researchers have learned a lot about the human immunodeficiency virus (HIV) and the disease it causes, acquired immunodeficiency syndrome (AIDS). But still more research is needed to help the millions of people whose health continues to be threatened by the global HIV/AIDS pandemic.
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as well as to better understand the virus with the goal of finding a cure. For example, in recent months, NIAID and its partners made progress toward finding a vaccine to prevent HIV infection. Check out other promising areas of NIAID-funded research on HIV/AIDS at http://www.niaid.nih.gov/topics/hivaids/Pages/Default.aspx .
Other NIH institutes, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute on Alcohol Abuse and Alcoholism, also support research to better control and ultimately end the HIV/AIDS pandemic. Some of these researchers have found a simple, cost-effective way to cut HIV transmission from infected mothers to their breastfed infants. Others have developed an index to help measure the role of alcohol consumption in illness and death of people with HIV/AIDS.
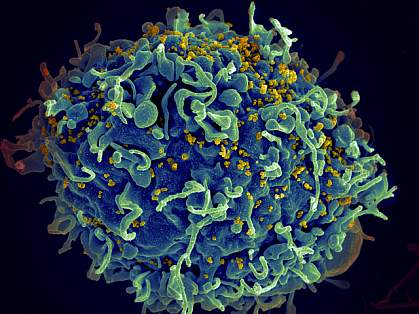
Find out more about these discoveries and what they mean for improving the health of people in the United States and all around the globe.
This page last reviewed on August 20, 2015
Connect with Us
- More Social Media from NIH

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Office of AIDS Research
- HIV Policy and Research
- Research Priorities
- NIH Strategic Plan for HIV and HIV-Related Research
- NIH HIV Research Budget
- NIH HIV Research Priority Areas
- Reduce the Incidence of HIV
- Develop Next-Generation HIV Therapies
Research Toward HIV Cure
- Address HIV-Associated Comorbidities, Coinfections, & Complications
- Cross-Cutting Areas

Viral latency and sanctuaries
Latent HIV reservoirs—small amounts of HIV that persist in people taking ART—present a significant challenge to finding a cure for HIV. Latent reservoirs remain in people with HIV when HIV becomes part of the body’s DNA in infected cells. Additionally, reservoirs of HIV can be found in certain “sanctuary” sites in the body that allow the virus to hide and be protected from both the immune system and ART. To cure HIV, the NIH supports studies to develop novel approaches and treatments that target these HIV reservoirs.
Sustained viral remission and viral eradication

Current science suggests that the path to an HIV cure involves first achieving sustained viral remission without ART. This is called sustained ART-free viral remission or a functional cure. For sustained ART-free viral remission, infectious virus must remain undetectable by sensitive testing methods for a long time without treatment. One research aim will be to prolong the time between treatments to be measured eventually not in weeks, but in months or even years. The NIH supports research into treatments leading to sustained ART-free viral remission . New cure-inducing treatments must be as safe, effective, and available for widespread use as are current-day ART regimens.
Viral eradication—eliminating the virus entirely—is the more challenging, longer-term goal.
Research Strategies
The NIH supports research to better understand how the HIV reservoir forms, persists, and reactivates, as well as investigations to develop new cure treatment strategies targeting HIV reservoirs.
A range of biomarkers and techniques, including single-cell and imaging technologies, are being studied to determine how to identify and describe the HIV reservoir. These techniques also are being used to better understand mechanisms of viral reactivation from latently infected cells.
Experimental treatments in development include therapeutic vaccines, genetically engineered immune cells that are resistant to HIV infection, drugs that reactivate latent HIV to make the virus visible to the immune system, cure-inducing immunotherapies, and interventions to permanently silence HIV in infected cells.
The search for an HIV cure involves important behavioral and social processes that complement the domains of biomedicine. BSSR in HIV cure research is focused on important aspects such as: counseling and support interventions to address the psychosocial needs and concerns of study participants related to analytical treatment interruptions (ATIs); risk reduction in the course of ATI study participation; motivation, acceptability, and decision‐making processes of potential study participants; how cure affects the identity and social position of people with HIV; and the scalability of a proven cure strategy in the context of further advances in HIV prevention and treatment.

The NIH is leveraging resources toward an HIV cure through several public-private partnerships. NIH small business awards enable companies to help foster a diverse pipeline of experimental treatments in development. The combined support of government, industry, and nongovernmental foundations is fostering the expansion of a talented scientific workforce dedicated to advancing HIV cure research.
OAR scientist Dr. Paul Sato coordinates Research Toward an HIV Cure .
This page last reviewed on September 8, 2022

Personalize Your Experience
Log in or create an account for a personalized experience based on your selected interests.
Already have an account? Log In
Free standard shipping is valid on orders of $45 or more (after promotions and discounts are applied, regular shipping rates do not qualify as part of the $45 or more) shipped to US addresses only. Not valid on previous purchases or combined with any other promotional offers.
Register for an enhanced, personalized experience.
Receive free access to exclusive content, a personalized homepage based on your interests, and a weekly newsletter with topics of your choice.
Home / Innovation & Research / The innovative research behind HIV/AIDS treatment
The innovative research behind HIV/AIDS treatment
Please login to bookmark.

It’s been 40 years since the release of the first scientific report describing acquired immune deficiency syndrome (AIDS). Thanks to innovative research, scientists learned how the HIV virus that causes AIDS replicates and how the immune system responds to the virus. Today, many people with HIV take just one pill a day to suppress the virus, and treatment is continuing to evolve.
In this video, Dr. Stacey Rizza , Mayo Clinic infectious disease physician and HIV researcher, explains how dedicated innovative science contributed to where we are today and what scientists are working on for the future.
What did the early research find?
Because of truly dedicated innovative science, within a few years, the scientific community figured out that AIDS was due to HIV. It then took a few years to figure out how to test for that virus. Several years later, the scientific community was able to quantitate how much virus was in a person’s blood. During all this time, truly innovative research into how the virus replicates and how the immune system responds to the virus allowed bio pharmacy companies to develop what we call anti-retroviral drugs or medications to slow down the viral replication. How has medication to treat HIV evolved?
The first drug approved for HIV was in 1987, which was AZT (now known as zidovudine). At that time, it was the fastest drug ever approved by the FDA (Food and Drug Administration) and started the fast-track mechanism through the FDA.
Then several other drugs within that same class were approved in the early 1990s. In late 1995, very early 1996, the first HIV protease inhibitors were approved. At that point, it was possible to combine three different medications from two different classes and completely suppress the HIV replication.
In the last 20 years, we’ve gone from people taking multiple medicines with lots of side effects to many of my patients with HIV now take a single pill a day. That’s a combination of medicines coformulated into one pill a day that’s extremely well-tolerated and completely suppresses their virus. We know it does not eliminate the virus. If they were to stop taking that medicine, the virus would come back. But we now have a handful of people in the world who have been what we called functionally cured of HIV, meaning they’ve gone through some research protocols that eliminated the reservoir of HIV in their body.
The new drugs are so effective in people who have fully suppressed virus that many only need to use two medications to maintain HIV treatment and control. New research is investigating ways to deliver the medications differently, such as a shot that lasts several months, or maybe someday even implantable medication delivery mechanisms so that people don’t have to take the pill every day. It is very exciting that HIV therapy is moving that direction.
Why isn’t there a cure for HIV?
The reason why it is so difficult to cure HIV is that once HIV infects a person’s body, it integrates into the host genome of several cell types. Those cells then hide in any of the lymphoid tissue, such as the lymph nodes, the liver and the spleen. And they lay there as what we call “latent” or “hiding”, as long as the person is on HIV therapy. Anytime a virus does leave a cell, it gets taken care of by HIV therapy. But if the infected individual stops the HIV therapy, that latent virus will come back. To cure HIV, you have to eliminate those hiding viruses in the cells or that latent viral reservoir, which is the term. There are many ways you can approach eliminating the reservoir.
Where is the research now?
One of the more popular ways that have been investigated is something called — and there are many different terms for it — “prime, shock, and kill” or “kick, and kill”, which is essentially giving medications that first wake the virus up from latency and then find ways to make the cells that have the virus susceptible to dying. When the virus is awake, and the cell is susceptible to dying, it kills itself but does not kill any other cells in the body.
Essentially, it specifically targets the HIV-infected cells and eliminates them without hurting anything else. This new science is exciting. It’s getting closer and closer to understanding how to do this effectively. And if you can do that with oral medications rather than fancy therapies like gene therapy or bone marrow transplant, it’s scalable to large parts of the world, and you can touch millions of people that way. That’s where the area of research is on how to make those hiding cells wake up, how to make them sensitive to die, and how to target just the HIV-infected cell.
Will we see a vaccine for HIV?
HIV has been a very hard vaccine to develop. In the world of viruses, vaccines fall into one of three buckets. They fall into the bucket where they respond to antibodies induced by the vaccine, and the vaccines are outstanding. Such viruses include polio, mumps, and lucky for us, SARS-CoV-2. Then we have the second category, like the influenza vaccine, which is about 60% effective. It certainly saves lives and makes a difference, but it’s not perfect. And then we have the third bucket, which quite frankly is the vast majority of viruses that infect humans. And HIV is in that category, where simply forming an antibody to the virus is not adequate to prevent infection. You have to do very sophisticated engineering to induce T cell effects, as well as innate effects and antibody effects. Even then, sometimes it’s very hard to decide what is the part of the virus to target. After decades, and billions of dollars of research, we’re still not there for HIV. There have been many approaches of how to do this science. Many different scientific delivery mechanisms, many different areas of the viruses targeted, many different parts of the immune system targeted, and so far, none of them have been effective at preventing HIV infection.
What needs to happen next?
We still need to slow down the number of people getting infected through good public health measures and good education to stop the HIV epidemic. We still need to get more people who are infected on therapy.
We know we can do it with public health measures. But we also need to find out more about how we eliminate that reservoir and get people cured of the virus in a simple and effective way so that we can cure more people. And the last major hurdle we have is to develop an effective vaccine. We still don’t have a vaccine that can prevent infection, a preventive vaccine, or a therapeutic vaccine where you give it to people who already have the virus that can help them control the infection. A huge amount of research has happened, but we’re still not there yet.
This article originally appeared on Mayo Clinic News Network.

Relevant reading
Living Medicine
A sweeping biography of the visionary behind bone marrow transplantation and the story of the diseases cured by Don Thomas's discovery.

Discover more Innovation & Research content from articles, podcasts, to videos.
You May Also Enjoy

by Victor Montori, M.D.

by Mary Beth Sartor Obermeyer

by Thomas M. Habermann, M.D., Renee Ziemer, Carolyn Stickney Beck, Ph.D.

Privacy Policy
We've made some updates to our Privacy Policy. Please take a moment to review.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 21 June 2023
Prevention, treatment and cure of HIV infection
- Raphael J. Landovitz ORCID: orcid.org/0000-0002-1442-714X 1 ,
- Hyman Scott ORCID: orcid.org/0000-0001-8775-7214 2 , 3 &
- Steven G. Deeks ORCID: orcid.org/0000-0001-6371-747X 3
Nature Reviews Microbiology volume 21 , pages 657–670 ( 2023 ) Cite this article
15 Citations
197 Altmetric
Metrics details
- Antiviral agents
- HIV infections
The development of antiretroviral therapy for the prevention and treatment of HIV infection has been marked by a series of remarkable successes. However, the efforts to develop a vaccine have largely failed, and efforts to discover a cure are only now beginning to gain traction. In this Review, we describe recent progress on all fronts — pre-exposure prophylaxis, vaccines, treatment and cure — and we discuss the unmet needs, both current and in the coming years. We describe the emerging arsenal of drugs, biologics and strategies that will hopefully address these needs. Although HIV research has largely been siloed in the past, this is changing, as the emerging research agenda is marked by multiple cross-discipline synergies and collaborations. As the limitations of antiretroviral drugs as a means to truly end the epidemic are becoming more apparent, there is a great need for continued efforts to develop an effective preventative vaccine and a scalable cure, both of which remain formidable challenges.
This is a preview of subscription content, access via your institution

Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
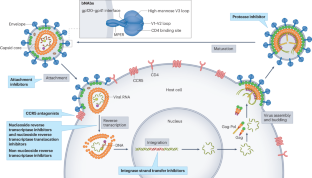
Similar content being viewed by others
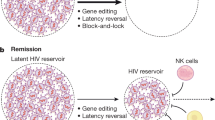
Why and where an HIV cure is needed and how it might be achieved
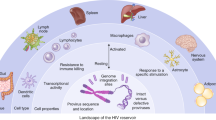
Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021
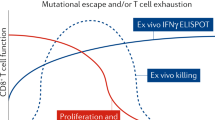
CD8+ T cells in HIV control, cure and prevention
Landovitz, R. J. et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N. Engl. J. Med. 385 , 595–608 (2021).
CAS PubMed PubMed Central Google Scholar
Grant, R. M. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363 , 2587–2599 (2010).
Joint United Nations Programme on HIV/AIDS. Global HIV and AIDS statistics — 2022 fact sheet. UNAIDS https://www.unaids.org/en/resources/fact-sheet (2022).
Cohen, S. E. et al. Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report. Lancet HIV https://doi.org/10.1016/S2352-3018(18)30288-1 (2018).
Article PubMed PubMed Central Google Scholar
Cohen, S. M., Hu, X., Sweeney, P., Johnson, A. S. & Hall, H. I. HIV viral suppression among persons with varying levels of engagement in HIV medical care, 19 US jurisdictions. J. Acquir. Immune Defic. Syndr. 67 , 519–527 (2014).
PubMed Google Scholar
Havlir, D. V. et al. HIV testing and treatment with the use of a community health approach in rural Africa. N. Engl. J. Med. 381 , 219–229 (2019).
PubMed PubMed Central Google Scholar
Hayes, R. J. et al. Effect of universal testing and treatment on HIV incidence — HPTN 071 (PopART). N. Engl. J. Med. 381 , 207–218 (2019).
Makhema, J. et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N. Engl. J. Med. 381 , 230–242 (2019).
Cohen, M. S. et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 365 , 493–505 (2011).
Larmarange, J. et al. The impact of population dynamics on the population HIV care cascade: results from the ANRS 12249 Treatment as Prevention trial in rural KwaZulu-Natal (South Africa). J. Int. AIDS Soc. 21 , e25128 (2018).
Thigpen, M. C. et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med. 367 , 423–434 (2012).
CAS PubMed Google Scholar
Liu, A. Y. et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern. Med. 176 , 75–84 (2016).
Choopanya, K. et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 381 , 2083–2090 (2013).
Mayer, K. H. et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 396 , 239–254 (2020).
Molina, J. M. et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 Infection. N. Engl. J. Med. 373 , 2237–2246 (2015).
World Health Organization. Guidelines on Long-Acting Injectable Cabotegravir for HIV Prevention (WHO, 2022).
Gandhi, R. T. et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA panel. J. Am. Med. Assoc. 329 , 63–84 (2022).
Google Scholar
Van Damme, L. et al. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367 , 411–422 (2012).
Marrazzo, J. M. et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 372 , 509–518 (2015).
Abdool Karim, Q. et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329 , 1168–1174 (2010).
Baeten, J. M. et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367 , 399–410 (2012).
Baeten, J. M. et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N. Engl. J. Med. 375 , 2121–2132 (2016).
World Health Organization. WHO recommends the dapivirine vaginal ring as a new choice for HIV prevention for women at substantial risk of HIV infection. WHO https://www.who.int/news/item/26-01-2021-who-recommends-the-dapivirine-vaginal-ring-as-a-new-choice-for-hiv-prevention-for-women-at-substantial-risk-of-hiv-infection (2021).
Baeten, J. M., Hendrix, C. W. & Hillier, S. L. Topical microbicides in HIV prevention: state of the promise. Annu. Rev. Med. 71 , 361–377 (2020).
Delany-Moretlwe, S. et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet 399 , 1779–1789 (2022).
Marzinke, M. A. et al. Characterization of human immunodeficiency virus (HIV) infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J. Infect. Dis. 224 , 1581–1592 (2021).
Gulick, R. M. et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337 , 734–739 (1997).
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Clinicalinfo.HIV.gov https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new (2023).
Trickey, A. et al. Associations of modern initial antiretroviral drug regimens with all-cause mortality in adults with HIV in Europe and North America: a cohort study. Lancet HIV 9 , e404–e413 (2022).
Venter, W. D. F. et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N. Engl. J. Med. 381 , 803–815 (2019).
Editorial. PEPFAR looks to the future. Lancet HIV 9 , e367 (2022).
Orkin, C. et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 8 , e185–e196 (2021).
Christopoulos, K. A. et al. First demonstration project of long-acting injectable antiretroviral therapy for persons with and without detectable HIV viremia in an urban HIV clinic. Clin. Infect. Dis. 76 , e645–e651 (2022).
PubMed Central Google Scholar
Segal-Maurer, S. et al. Capsid Inhibition with lenacapavir in multidrug-resistant HIV-1 infection. N. Engl. J. Med. 386 , 1793–1803 (2022).
Benhabbour, S. R. et al. Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nat. Commun. 10 , 4324 (2019).
Kovarova, M. et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat. Commun. 9 , 4156 (2018).
Haynes, B. F., Burton, D. R. & Mascola, J. R. Multiple roles for HIV broadly neutralizing antibodies. Sci. Transl Med. 11 , eaaz2686 (2019).
Caskey, M., Klein, F. & Nussenzweig, M. C. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat. Med. 25 , 547–553 (2019).
Gaudinski, M. R. et al. Safety and pharmacokinetics of broadly neutralising human monoclonal antibody VRC07-523LS in healthy adults: a phase 1 dose-escalation clinical trial. Lancet HIV 6 , e667–e679 (2019).
Gaudinski, M. R. et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 15 , e1002493 (2018).
Corey, L. et al. Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition. N. Engl. J. Med. 384 , 1003–1014 (2021).
Sneller, M. C. et al. Combination anti-HIV antibodies provide sustained virological suppression. Nature 606 , 375–381 (2022).
Gaebler, C. et al. Prolonged viral suppression with anti-HIV-1 antibody therapy. Nature 606 , 368–374 (2022).
Pegu, A. et al. Potent anti-viral activity of a trispecific HIV neutralizing antibody in SHIV-infected monkeys. Cell Rep. 38 , 110199 (2022).
Collins, F. et al. The NIH-led research response to COVID-19. Science 379 , 441–444 (2023).
Buchbinder, S. P. et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372 , 1881–1893 (2008).
Rerks-Ngarm, S. et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361 , 2209–2220 (2009).
Haynes, B. F. et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366 , 1275–1286 (2012).
Gray, G. E. et al. Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa. Sci. Transl Med. 11 , eaax1880 (2019).
Moodie, Z. et al. Analysis of the HVTN 702 Phase 2b-3 HIV-1 vaccine trial in South Africa assessing RV144 antibody and T-cell correlates of HIV-1 acquisition risk. J. Infect. Dis. 226 , 246–257 (2022).
Gray, G. E. et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N. Engl. J. Med. 384 , 1089–1100 (2021).
Barouch, D. H. et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 392 , 232–243 (2018).
Hansen, S. G. et al. Immune clearance of highly pathogenic SIV infection. Nature 502 , 100–104 (2013).
Jiang, C. et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 585 , 261–267 (2020).
Turk, G. et al. A possible sterilizing cure of HIV-1 infection without stem cell transplantation. Ann. Intern. Med. 175 , 95–100 (2022).
Jardine, J. G. et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 351 , 1458–1463 (2016).
Haynes, B. F. et al. Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat. Rev. Immunol. 23 , 142–158 (2023).
Leggat, D. J. et al. Vaccination induces HIV broadly neutralizing antibody precursors in humans. Science 378 , eadd6502 (2022).
Sanders, R. W. et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 9 , e1003618 (2013).
Borst, A. J. et al. Germline VRC01 antibody recognition of a modified clade C HIV-1 envelope trimer and a glycosylated HIV-1 gp120 core. eLife 7 , e37688 (2018).
Arunachalam, P. S. et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med. 26 , 932–940 (2020).
McMahan, K. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590 , 630–634 (2021).
Ndung’u, T., McCune, J. M. & Deeks, S. G. Why and where an HIV cure is needed and how it might be achieved. Nature 576 , 397–405 (2019).
Deeks, S. G. et al. Research priorities for an HIV cure: International AIDS Society global scientific strategy 2021. Nat. Med. 27 , 2085–2098 (2021).
Dybul, M. et al. The case for an HIV cure and how to get there. Lancet HIV 8 , e51–e58 (2021).
Ho, Y. C. et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155 , 540–551 (2013).
Siliciano, J. D. et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4 + T cells. Nat. Med. 9 , 727–728 (2003).
Wagner, T. A. et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345 , 570–573 (2014).
Maldarelli, F. et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345 , 179–183 (2014).
Einkauf, K. B. et al. Parallel analysis of transcription, integration, and sequence of single HIV-1 proviruses. Cell 185 , 266–282.e15 (2022).
Cohn, L. B. et al. HIV-1 integration landscape during latent and active infection. Cell 160 , 420–432 (2015).
Nixon, C. C. et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 578 , 160–165 (2020).
Badley, A. D., Sainski, A., Wightman, F. & Lewin, S. R. Altering cell death pathways as an approach to cure HIV infection. Cell Death Dis. 4 , e718 (2013).
Yates, K. B. et al. Epigenetic scars of CD8 + T cell exhaustion persist after cure of chronic infection in humans. Nat. Immunol. 22 , 1020–1029 (2021).
Rutishauser, R. L. et al. TCF-1 regulates HIV-specific CD8 + T cell expansion capacity. JCI Insight 6 , e136648 (2021).
Mancuso, P. et al. CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat. Commun. 11 , 6065 (2020).
Kessing, C. F. et al. In vivo suppression of HIV rebound by didehydro-cortistatin A, a “block-and-lock” strategy for HIV-1 treatment. Cell Rep. 21 , 600–611 (2017).
Borducchi, E. N. et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540 , 284–287 (2016).
Bailon, L. et al. Safety, immunogenicity and effect on viral rebound of HTI vaccines in early treated HIV-1 infection: a randomized, placebo-controlled phase 1 trial. Nat. Med. 28 , 2611–2621 (2022).
Nishimura, Y. et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543 , 559–563 (2017).
Mendoza, P. et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561 , 479–484 (2018).
Borducchi, E. N. et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563 , 360–364 (2018).
Niessl, J. et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat. Med. 26 , 222–227 (2020).
Gunst, J. D. et al. Early intervention with 3BNC117 and romidepsin at antiretroviral treatment initiation in people with HIV-1: a phase 1b/2a, randomized trial. Nat. Med. 28 , 2424–2435 (2022).
Tebas, P. et al. CCR5-edited CD4 + T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. J. Clin. Invest. 131 , e144486 (2021).
Gardner, M. R. et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519 , 87–91 (2015).
Martinez-Navio, J. M. et al. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity 50 , 567–575.e5 (2019).
Casazza, J. P. et al. Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nat. Med. 28 , 1022–1030 (2022).
Hutter, G. et al. Long-term control of HIV by CCR5 delta32/delta32 stem-cell transplantation. N. Engl. J. Med. 360 , 692–698 (2009).
Gupta, R. K. et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 568 , 244–248 (2019).
Okoye, A. A. et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med. 24 , 1430–1440 (2018).
Henrich, T. J. et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: an observational study. PLoS Med. 14 , e1002417 (2017).
Mitchell, J. L. et al. Plasmacytoid dendritic cells sense HIV replication before detectable viremia following treatment interruption. J. Clin. Invest. 130 , 2845–2858 (2020).
Gondim, M. V. P. et al. Heightened resistance to host type 1 interferons characterizes HIV-1 at transmission and after antiretroviral therapy interruption. Sci. Transl Med. 13 , eabd8179 (2021).
Prator, C. A. et al. Circulating CD30 + CD4 + T cells increase before human immunodeficiency virus rebound after analytical antiretroviral treatment interruption. J. Infect. Dis. 221 , 1146–1155 (2020).
Landovitz, R. J. et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV 7 , e472–e481 (2020).
Eshleman, S. H. et al. HIV RNA screening reduces integrase strand transfer inhibitor resistance risk in persons receiving long-acting cabotegravir for HIV prevention. J. Infect. Dis. 226 , 2170–2180 (2022).
Beacroft, L. & Hallett, T. B. The potential impact of a “curative intervention” for HIV: a modelling study. Glob. Health Res. Policy 4 , 2 (2019).
Lehman, D. A. et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J. Infect. Dis. 211 , 1211–1218 (2015).
Dube, K. et al. Participant experiences using novel home-based blood collection device for viral load testing in the HIV cure trials with analytical treatment interruptions. HIV Res. Clin. Pract. 23 , 76–90 (2022).
Deeks, S. G. et al. Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral-treated individuals with drug-resistant human immunodeficiency virus type 1. J. Infect. Dis. 189 , 312–321 (2004).
Bertagnolli, L. N. et al. Autologous IgG antibodies block outgrowth of a substantial but variable fraction of viruses in the latent reservoir for HIV-1. Proc. Natl Acad. Sci. USA 117 , 32066–32077 (2020).
Blazkova, J. et al. Distinct mechanisms of long-term virologic control in two HIV-infected individuals after treatment interruption of anti-retroviral therapy. Nat. Med. 27 , 1893–1898 (2021).
Jones, R. B. & Walker, B. D. HIV-specific CD8 + T cells and HIV eradication. J. Clin. Invest. 126 , 455–463 (2016).
Collins, D. R., Gaiha, G. D. & Walker, B. D. CD8 + T cells in HIV control, cure and prevention. Nat. Rev. Immunol. 20 , 471–482 (2020).
Fukazawa, Y. et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 21 , 132–139 (2015).
Imamichi, H. et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl Acad. Sci. USA 117 , 3704–3710 (2020).
Pollack, R. A. et al. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe 21 , 494–506.e4 (2017).
Gaiha, G. D. et al. Structural topology defines protective CD8 + T cell epitopes in the HIV proteome. Science 364 , 480–484 (2019).
Mothe, B. et al. Therapeutic vaccination refocuses T-cell responses towards conserved regions of HIV-1 in early treated individuals (BCN 01 study). eClinicalMedicine 11 , 65–80 (2019).
Korber, B. & Fischer, W. T cell-based strategies for HIV-1 vaccines. Hum. Vaccin. Immunother. 16 , 713–722 (2020).
Stevenson, E. M. et al. HIV-specific T cell responses reflect substantive in vivo interactions with antigen despite long-term therapy. JCI Insight 6 , e142640 (2021).
Stevenson, E. M. et al. SARS CoV-2 mRNA vaccination exposes latent HIV to Nef-specific CD8 + T-cells. Nat. Commun. 13 , 4888 (2022).
Duette, G. et al. The HIV-1 proviral landscape reveals that Nef contributes to HIV-1 persistence in effector memory CD4 + T cells. J. Clin. Invest. 132 , e154422 (2022).
Collins, D. R. et al. Functional impairment of HIV-specific CD8 + T cells precedes aborted spontaneous control of viremia. Immunity 54 , 2372–2384.e7 (2021).
Lewin, S. R. et al. Multi-stakeholder consensus on a target product profile for an HIV cure. Lancet HIV 8 , e42–e50 (2021).
Colasanti, J. et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin. Infect. Dis. 62 , 648–654 (2016).
Joint United Nations Programme on HIV/AIDS. African leaders unite in pledge to end AIDS in children. UNAIDS https://www.unaids.org/en/keywords/children (2023).
Patel, P. et al. Pregnancy outcomes and pharmacokinetics in pregnant women living with HIV exposed to long-acting cabotegravir and rilpivirine in clinical trials. HIV Med. 24 , 568–579 (2022).
Penazzato, M. et al. Advancing the prevention and treatment of HIV in children: priorities for research and development. Lancet HIV 9 , e658–e666 (2022).
Persaud, D. et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med. 369 , 1828–1835 (2013).
Violari, A. et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat. Commun. 10 , 412 (2019).
Frange, P. et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 3 , e49–54 (2016).
Hartana, C. A. et al. Immune correlates of HIV-1 reservoir cell decline in early-treated infants. Cell Rep. 40 , 111126 (2022).
Uprety, P. et al. Human immunodeficiency virus type 1 DNA decay dynamics with early, long-term virologic control of perinatal infection. Clin. Infect. Dis. 64 , 1471–1478 (2017).
Capparelli, E. V. et al. Safety and pharmacokinetics of intravenous 10-1074 and VRC01LS in young children. J. Acquir. Immune Defic. Syndr. 91 , 182–188 (2022).
Bonacci, R. A., Smith, D. K. & Ojikutu, B. O. Toward greater pre-exposure prophylaxis equity: increasing provision and uptake for black and Hispanic/Latino individuals in the US. Am. J. Prev. Med. 61 , S60–S72 (2021).
Harris, N. S. et al. Vital signs: status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis — United States, 2013–2018. MMWR Morb. Mortal. Wkly. Rep. 68 , 1117–1123 (2019).
Xavier Hall, C. D., Feinstein, B. A., Rusie, L., Phillips Ii, G. & Beach, L. B. Race and sexual identity differences in PrEP continuum outcomes among Latino men in a large Chicago area healthcare network. AIDS Behav. 26 , 1943–1955 (2022).
Monroe, A. K. et al. Integrase inhibitor prescribing disparities in the DC and Johns Hopkins HIV cohorts. Open Forum Infect. Dis. 8 , ofab338 (2021).
Joint United Nations Programme on HIV/AIDS. In danger: UNAIDS global AIDS update 2022 (UNAIDS, 2022).
Yukl, S. A. et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin Patient. PLoS Pathog. 9 , e1003347 (2013).
Jensen, B.-E. O. et al. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat. Med. 29 , 583–587 (2023).
Hsu, J. et al. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 186 , 1115–1126.e8 (2023).
Mendoza, D. et al. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 119 , 4645–4655 (2012).
Saez-Cirion, A. et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9 , e1003211 (2013).
Download references
Author information
Authors and affiliations.
Center for Clinical AIDS Research and Education, David Geffen School of Medicine, University of California, Los Angeles, CA, USA
Raphael J. Landovitz
Bridge HIV, San Francisco Department of Public Health, San Francisco, CA, USA
Hyman Scott
Division of HIV, Infectious Diseases & Global Medicine, Department of Medicine, University of California, San Francisco, CA, USA
Hyman Scott & Steven G. Deeks
You can also search for this author in PubMed Google Scholar
Contributions
S.G.D. researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Steven G. Deeks .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Microbiology thanks Linda-Gail Bekker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Landovitz, R.J., Scott, H. & Deeks, S.G. Prevention, treatment and cure of HIV infection. Nat Rev Microbiol 21 , 657–670 (2023). https://doi.org/10.1038/s41579-023-00914-1
Download citation
Accepted : 12 May 2023
Published : 21 June 2023
Issue Date : October 2023
DOI : https://doi.org/10.1038/s41579-023-00914-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The possible mechanisms of cu and zn in the treatment and prevention of hiv and covid-19 viral infection.
- Shatha A Albalawi
- Raneem A Albalawi
- Mohamed S. Abdel-Maksoud
Biological Trace Element Research (2024)
Making genome editing a success story in Africa
- Hussein M. Abkallo
- Patrick Arbuthnot
- Charles Wondji
Nature Biotechnology (2024)
PrEPping the skin
- Andrea Du Toit
Nature Reviews Microbiology (2023)
AZD5582 plus SIV-specific antibodies reduce lymph node viral reservoirs in antiretroviral therapy-suppressed macaques
- Amir Dashti
- Sophia Sukkestad
- Ann Chahroudi
Nature Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
Last update: just added
Circumcision may reduce the risk of HIV infection
- Date 6 hours 12 hours 1 day 3 days all
- Rank Last day 1 week 1 month all
- LiveRank Last day 1 week 1 month all
- Popular Last day 1 week 1 month all
HIV & AIDS news
A randomized controlled trial comprised of 247 men who have sex with men (MSM) found that voluntary medical male circumcision (VMMC) can prevent incident HIV infection. These findings suggest that MSM should be included in ...
Raising life expectancy for youth with HIV requires more than just adherence to care regimens, researchers say
Life expectancy of youth with HIV is projected to be 10.4 years less in males and 11.8 years less in females compared to individuals without HIV, a study by researchers at Massachusetts General Hospital (MGH), a founding ...
May 22, 2024
A boost for HIV vaccine research: Studies present comprehensive platform for validating next steps
HIV has proven a hard target for vaccine design. The most promising approach, germline-targeting (GT), proposes a series of immunizations: a first shot to activate inexperienced B cells—antibody-producing white blood cells—followed ...
May 21, 2024
US pediatricians reverse decades-old advice against HIV-positive mothers breastfeeding
People with HIV can breastfeed their babies, as long as they are taking medications that effectively suppress the virus that causes AIDS, a top U.S. pediatricians' group said Monday in a sharp policy change.
May 20, 2024

Trial HIV vaccine triggers elusive and essential antibodies, pointing the way toward a successful vaccine
An HIV vaccine candidate developed at the Duke Human Vaccine Institute triggered low levels of an elusive type of broadly neutralizing HIV antibodies among a small group of people enrolled in a 2019 clinical trial.
May 17, 2024

What is PrEP? Will it stop me getting HIV?
HIV prevention was allocated A$43.9 million over three years in this week's federal budget. Some $26m of this is for "PrEP" for people without access to Medicare.
May 16, 2024

Injectable HIV medication is superior to oral medication for patients who frequently miss doses, study finds
When a person is diagnosed with HIV, they are placed on a lifelong HIV treatment regimen, called antiretroviral therapy, to keep the virus under control. But for many people, having to take medicine every day can be a struggle ...
May 14, 2024
Study highlights need for cell-type-specific therapies in treatment of HIV
Researchers from the University of Illinois have demonstrated the importance of cell-type-specific targeting in the treatment of HIV. Their study, published in the Proceedings of the National Academy of Sciences, is one of ...
May 13, 2024
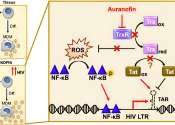
New research traces the spread of HIV in and from Indonesia
The HIV variant dominant in Indonesia was introduced from Thailand over multiple events. A Kobe University study traces where it came from and how it spread from there, offering possible insights into the development of treatments ...
May 10, 2024
GPS-like system shows promise as HIV vaccine strategy to elicit critical antibodies
A team led by the Duke Human Vaccine Institute (DHVI) has developed a vaccine approach that works like a GPS, guiding the immune system through the specific steps to make broadly neutralizing antibodies against HIV.
May 9, 2024
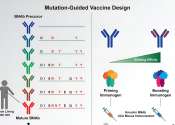
Keto diet boosts lifesaving antifungal drug in mice
For the roughly 150,000 AIDS patients who come down with a life-threatening infection called fungal meningitis each year, there aren't many options.
Social networks provide crucial support for older adults living with HIV, study finds
Having social support and strong social networks is vital to the health and well-being of older adults living with HIV, according to a Rutgers Health study.

US geographic region results in vastly different anal cancer risk for people with HIV
A new study that followed a cohort of more than 110,000 people establishes significant disparities in the risk of anal cancer for people with HIV and for men who have sex with men with HIV, depending on the region of the ...
May 7, 2024

Group-based interventions address HIV stigma
Group-based interventions have the potential to address HIV-related stigma among adolescents living with the virus, finds a recent study from researchers at the Brown School at Washington University in St. Louis and Makerere ...

Study explores coping strategies and self-stigma among people living with HIV in Indonesia
Individuals living with HIV often face significant physical and mental stress, including self-stigma, which can impede their ability to seek treatment and disclose their status.
May 6, 2024

Study shows ChatGPT can be helpful for Black women's self-education about HIV, PrEP
The artificial intelligence (AI) chatbot called ChatGPT is a powerful way for Black women to educate themselves about HIV prevention, as it provides reliable and culturally sensitive information, according to a study in The ...
May 3, 2024

Closing the US/Mexico border during COVID-19 increased HIV transmission, study finds
The border crossing separating San Diego, California, from Tijuana, Mexico, is a dynamic place. When it was closed during the COVID-19 pandemic, drug tourism from San Diego to Tijuana continued. This provided a flow of people ...
May 1, 2024

Study highlights importance of early interventions to combat HIV
A study has compared the development of HIV reservoirs—locations in the body where the virus persists in a latent state—between patients who receive either early or late medical interventions. The findings highlight the ...
Apr 30, 2024
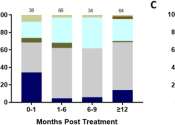
Visa rules jeopardize HIV management, study finds
A Monash University sexual health expert has warned that an unintended consequence of Australia's migration rules could compromise Australia's goal to end the HIV epidemic by 2030.
Semaglutide alleviates metabolic-linked liver disease in people with HIV
For people with HIV (PWH), semaglutide is effective for metabolic dysfunction-associated steatotic liver disease (MASLD), according to a research letter published online April 30 in the Annals of Internal Medicine.
7 minutes ago

45 minutes ago
43 minutes ago
1 minute ago
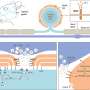
2 hours ago

4 hours ago
21 hours ago

22 hours ago
E-mail newsletter
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- About HIV Surveillance and Monitoring
- National HIV Surveillance System (NHSS)
- National HIV Behavioral Surveillance (NHBS)
- National HIV Prevention Program Monitoring and Evaluation Data
- National HIV Initiatives Data
- Medical Monitoring Project (MMP)
- Surveillance of HIV-Related Service Barriers Among Individuals with Early or Late HIV Diagnoses (SHIELD)
- Tools and Resources
- HIV Public Health Partners
- Ending the HIV Epidemic in the U.S. (EHE)
- Let's Stop HIV Together

Initiatives and Program Monitoring and Evaluation Data

Data Table: Monitoring National HIV Prevention Goals

Select Data Releases

HIV Diagnoses, Deaths, and Prevalence
Estimated HIV Incidence and Prevalence
National HIV Prevention and Care Outcomes
Behavioral and Clinical Characteristics of Persons with Diagnosed HIV
HIV Risk, Prevention, and Testing Behaviors Among Men Who Have Sex with Men
HIV Data Resources
Learn about HIV data systems, various HIV data trends in the United States, as well as how data is used to analyze and prevent further HIV transmission.
- Election 2024
- Entertainment
- Newsletters
- Photography
- Personal Finance
- AP Investigations
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election Results
- Delegate Tracker
- AP & Elections
- Auto Racing
- 2024 Paris Olympic Games
- Movie reviews
- Book reviews
- Personal finance
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
Demi Moore, Cher and more stars raise money for AIDS research at amfAR gala near Cannes
On the red carpet for the annual amfAR gala in Cannes, Cher said the organization is an important one to her. (May 23)
Demi Moore poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Scott A Garfitt/Invision/AP)
- Copy Link copied
Cher poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Vianney Le Caer/Invision/AP)
Tommy Hilfiger, left, and Dee Ocleppo pose for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Vianney Le Caer/Invision/AP)
Leni Olumi Klum, left, and Heidi Klum pose for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Scott A Garfitt/Invision/AP)
Alexander Edwards, from left, Cher, Demi Moore, and Nick Jonas pose for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Vianney Le Caer/Invision/AP)
Demi Moore, left, and Cher pose for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Scott A Garfitt/Invision/AP)
Paris Jackson, center, poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Scott A Garfitt/Invision/AP)
Winnie Harlow poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Vianney Le Caer/Invision/AP)
Alex Scott poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Vianney Le Caer/Invision/AP)
Kelly Rowland poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Vianney Le Caer/Invision/AP)
Nick Jonas poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Vianney Le Caer/Invision/AP)
Maria Borges poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Vianney Le Caer/Invision/AP)
Maria Bakalova poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d’Antibes, southern France, Thursday, May 23, 2024. (Photo by Vianney Le Caer/Invision/AP)
ANTIBES, France (AP) — Some of the biggest stars in the French Rivera for the Cannes Film Festival made appearances at the 30th annual amfAR gala to raise money for AIDS research.
Demi Moore, whose film “The Substance” caused a stir at Cannes, hosted this year’s gala, a role launched by Elizabeth Taylor in 1993.
The red carpet at the exclusive Hôtel du Cap, Eden Roc, was awash with models, actors, singers and fashion designers as well as plenty of festival movers and shakers, who paid thousands of euros for a table at the hottest event in town.
Michelle Yeoh, Heidi Klum, Kelly Rowland, Andie MacDowell, Diane Kruger, Colman Domingo, Michelle Rodriguez, Winnie Harlow, Robin Thicke, Diplo, Paris Jackson, Petra Nemcova, Karolina Kurkova, Natasha Poly and Sarah Ferguson, the Duchess of York, all attended the fundraiser.
Rowland addressed her Cannes controversy from earlier in the week where she appeared to have an altercation with security on the red carpet.
“The woman knows what happened. I know what happened. And, I have a boundary, and I stand by those boundaries, and that is it. And there were other women that attended that carpet who did not quite look like me, and they didn’t get scolded, or pushed off or told to get off,” Rowland told The Associated Press . “And, I stood my ground and she felt like she had to stand hers but I stood my ground and that was it.”
As always the night began with champagne and cocktails under the stars. The sweeping hotel walkway was transformed into a carpet of red glitter down to the ocean, although this year the flashy super yachts were not blocking the horizon.
Guests drank champagne, margaritas, palomas and vodka martinis and posed for multiple selfies and photos at the black tie charity event.
Many of the works of art and sculptures from the auction were on display around the grounds including an iconic hand signed Andy Warhol lithograph of Taylor, which later raised 350,000 euros ($378,289).
A specially commissioned hand drawn picture of the Queen with Swarovski crystals on an aluminum frame, from royal artist Chris Levine, reached 475,000 euros ($513,392) for the charity. Sarah Ferguson took to the podium to help auction this lot with a plea of “this was my mother-in-law” and “stop being so bored, Stop looking at your phone” to the diners.
Guests were served a starter of truffled zucchini flower with cream of mushroom to the live soundtrack of Jess Glynne’s opening performance, followed by beef brulee with a potato emulsion and a strawberry and elderflower profiterole dessert while auctioneers called for bids.
Part way through the dinner guests paused and took to their feet to line the temporary catwalk through the tables for the fashion runway show curated annually by Carine Roitfeld and auctioned off to the highest bidder. This year’s collection, entitled “Fairy Tales” included 26 looks from Chanel, Dior, Louis Vuitton, Saint Laurent, Vivienne Westwood and Prada to name a few. After the models gathered on stage to help the auctioneer up the bids, the collection finally went for 500,000 euros ($540,412).
As part of the experiences on offer, a walk on part in “Emily in Paris“ series 5, plus an invite to the season 4 LA premiere went for 250,000 euros ($270,262).
Nick Jonas brought brother Joe on stage for a medley that got the room dancing and then Cher closed the show with a tribute to Taylor and a high-octane performance of “Believe” complete with gyrating dancers. The afterparty carried on by the hotel’s swimming pool with Diplo on the decks and more drinks as guests danced outdoors until the early hours.
AmfAR, The Foundation for AIDS Research is dedicated to the support of AIDS research, HIV prevention, treatment education and advocacy, raising nearly $900 million in support of it’s programs since 1985.
News and views for the UB community
- Your Colleagues >
UB dental school professor discusses how AI can advance periodontic treatment
By LAURIE KAISER
Published May 28, 2024

When Nathalia P. Andrade joined the faculty of the School of Dental Medicine as a clinical professor of periodontics and endodontics last September, she noticed that her department had impressive technological equipment and software with artificial intelligence capabilities. She also saw an opportunity to leverage the quickly evolving technology into her teaching.
Within a week of being hired, Andrade applied for and was awarded a $5,000 seed grant from UB’s Office of Curriculum, Assessment and Teaching Transformation to create a new course weaving AI into periodontics. This dental specialty focuses on the health of periodontium, the tissues that support teeth.
She taught “Transforming Periodontal and Dental Care Powered by Artificial Intelligence” for the first time this spring. While tailored specifically for periodontal residents, the course was open to all dental school residents and will be offered again in 2026.
Andrade, who started studying AI while completing her residency, published an article in the March 2022 issue of Clinical Advances in Periodontics about successfully using AI in a procedure to lengthen teeth shortened over time or because of teeth malposition, which causes the gums to be more exposed. “Dual Digitally Guided Crown Lengthening in Esthetic Area Compromised by Disharmonic Implant Crown” ranked as a top-cited paper for the publication.
Andrade recently spoke with UBNow about how AI is transforming periodontics and how dental students at UB are using it.
When did you first become interested in AI as it relates to dental care?
I was always passionate about technology of all kinds. I never took a course in using AI, but during my residency, I would sit in front of the computer and figure out how to do it. I saw how it would make periodontic surgery faster and more precise. I started to love using it, and I wanted to teach others how to use it, too.
What is the main focus of your AI research?
AI can be used in three different stages of care: diagnosis, treatment and maintenance. My work is mainly focused on diagnosis and treatment, such as the procedure on lengthening crowns. With AI, we precisely know where to cut the gum and where to remove the bone behind it. When you go freehand, it’s hard to get the measurements right. With AI, you do all the thinking process before the procedure and at the time of the procedure you just need to follow the guide.
How does the guide work?
For the crown-lengthening procedure, for example, we use AI to determine how the smile will look after the procedure. Once the patient signs off, we create a guide for the exact position where we need to cut. We print it and put it in the patient’s mouth. In another example, when doing an implant with a patient, a guide can show us the correct position and angulation of the implant. It’s particular helpful in cases with multiple implants.
What are other ways that AI aids in periodontic care?
It can highlight the treatments that the patient most likely needs by the X-rays, periodontal chart and the information that’s in the patient’s record. It can also tell you if the patient is missing a treatment or is due for a cleaning.
AI can also help diagnose a patient quicker and more accurately. For example, when an X-ray indicates a problem, there are a lot of variables. One clinician can look at the image and say, “There’s 50% bone loss,” while another clinician will say, “No, for me, it looks like 60%.”
I think AI is going to help us create a standard that will be the same for everybody.
What are some of the main points you’re teaching students about using AI?
My main goal is to teach students to use critical thinking while using AI tools. It is a technology in development and can make mistakes. Different kind of procedures have different kind of mistakes; we have to explore them so that we teach the machine how to do the procedures properly in the future.
Talk about the new course and how you used the seed grant to fund it.
The course is designed to supplement the clinical experiences of students in postgraduate programs in 3D surgical digital planning, microscope surgery and AI as applied to periodontics.
With the funding, I brought in experts from other schools who taught different aspects of AI. Four came in person and two presented the lectures virtually. They also worked with me on how to apply AI techniques on a larger scale and how to handle HIPAA (Health Insurance Portability and Accountability Act of 1996) regulations.
How do you think the course went overall?
I was very happy with it. I initially planned for 11 periodontic residents, but in the end, about 25 residents from other dental programs, as well as periodontics faculty, came in to observe the class. I did a survey with students at the end, and they really liked the lectures and the guides because it allowed them to be up to date on new technologies. I can tell that the lessons worked because I see students using AI now. They’re digitally planning the surgical periodontal cases of real patients, using it for crown-lengthening procedures, advanced bone graft cases — like a sinus lift — and implant placement.
When will the AI course be offered going forward?
We plan to offer the course every other spring. The activities and access to new software and intraoral scanners are expensive. So, we will look for solutions and support to have it available to the students more often. The students indicated in the survey that they would like to learn more procedures using AI software. I also would like to introduce the AI-based software to dental students by adding this component to the periodontal course they already take and focus more on the diagnosis of the patient and risk assessment.

unbranded - Lifestyle
New Gel From Whey Protein Aids In Reducing Alcohol In Body
Posted: May 28, 2024 | Last updated: May 28, 2024
Scientists from ETH Zurich have developed a whey protein-based gel that can break down alcohol in the gut, potentially reducing hangovers. The gel, which has been tested on mice, lowers blood alcohol levels by up to 50%. It also prevents harmful effects by converting alcohol into acetic acid before it reaches the bloodstream. This innovation shifts alcohol metabolism from the liver to the digestive tract, avoiding the production of harmful acetaldehyde. While the gel shows promise in animal studies, it must remain in the gut for it to be effective. Future research may develop it into a pill to be taken before or after drinking alcohol. "The gel could be of particular interest to people who don’t want to give up alcohol completely, but don’t want to put a strain on their bodies and aren’t actively seeking the effects of alcohol," explained study co-author Raffaele Mezzenga from ETH Zurich. The gel could be particularly beneficial for people looking to reduce alcohol's harmful impacts without abstaining completely.
More for You
Stephen Hawking once gave a simple answer as to whether there was a God
Is Joe Biden's $320 Million Gaza Pier Sinking? What We Know
Flight attendant explains why you should always throw a bottle of water under hotel beds
40 Years Ago, a Brilliantly Profane Sci-Fi Movie Turned the Genre Upside Down
Scammer Alert: If Someone Calls You Using Any of These 12 Phrases You’re About To Be Scammed
‘Survivor' Winners Through the Years: Where Are They Now?
The 50 best Netflix original series of all time, according to fans
Gavin Newsom Rebuked by California Newspaper: 'Should Be Ashamed'
20 Best Sandwiches in America You Need to Try At Least Once
Lady Gaga rocks car part on red carpet to delight of fans: ‘Weird Gaga is back’
Getting Rid of a Printer? Do This First—or Risk Getting Hacked
Vets say they were sickened at secret base near nuclear test site
Are Retirees Ready for a 21% Cut to Social Security Benefits? Here's Exactly When It Could Happen.
Donald Trump Sells Private Jet Amid Spiraling Legal Costs
“I cried unmercifully for like an hour” - Charles Barkley vowed to get back at Ms. Gomez for making him miss the graduation ceremony
Man’s Hack for Crispy Grilled Cheese Is Nothing Short of Brilliant
Netflix's Most Ambitious Sci-Fi Show Won't Be Coming Back Anytime Soon
10 most common PINs have been revealed – see if yours is on the list
5 Fascinating Rumors About The SR-72 'Darkstar' Jet
Dad Buys Abandoned School and Renovated It For Family Home
- My View My View
- Following Following
- Saved Saved
Cher, Demi Moore entertain guests at glamorous Cannes fundraiser for AIDS
- Medium Text

Sign up here.
Reporting by Miranda Murray; Editing by Stephen Coates
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Thomson Reuters
Speed editor on the Berlin hub who provides general coverage on everything from politics to energy in Germany, Austria and Switzerland, with the goal of getting the news out as quickly as possible. Miranda previously worked at the German press agency dpa and Chicago Tribune

Lifestyle Chevron

Pictures of the Day
Our top photos of the day


IMAGES
VIDEO
COMMENTS
Emory Health Sciences. (2023, July 26). New findings offer potential breakthrough in HIV cure research. ScienceDaily. Retrieved May 27, 2024 from www.sciencedaily.com / releases / 2023 / 07 ...
Future focus. Investing in innovative strategies that meet the needs of individuals won't just be key to ending the HIV/AIDS pandemic by 2030. It will also help to ensure that global health ...
Read about The National HIV/AIDS Strategy, our country's whole-of-society approach to end the HIV epidemic in the United States. ... 73 countries gathered in Denver and virtually from March 3-6 this year for CROI, an annual scientific meeting on the latest research that can help accelerate global progress in the response to HIV and other ...
The edited cells underwent affinity maturation in vivo, improving the potency of HIV-1 and SARS-CoV-2 neutralizing antibodies without loss of bioavailability. Affinity maturation of edited cells ...
The FDA has approved a new drug in the fight against AIDS The Food and Drug Administration has approved the first injectable medication for HIV prevention. Health advocates say it could be a game ...
Interview with Dr. Anthony Fauci on progress made during the past four decades of the HIV/AIDS pandemic and ongoing efforts to end this threat. 18m 44s Download. The dramatic saga of the acquired ...
News releases, fact sheets and other NIAID-related materials are available on the NIAID website. About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and ...
An effective and scalable cure strategy is a top priority for the HIV research field; this Review discusses recent advances, knowledge gaps, and priority research areas for the next 5 years.
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but ...
Nearly $17 billion was spent worldwide on HIV -vaccine research from 2000 to 2021. Nearly $1 billion more is spent annually, according to the Joint United Nations Program on HIV/AIDS and the ...
He was continuously tested for HIV with extensive and precise tests. It was finally good news for him! Since 2010, when he decided to go public, he had interviewed for various magazines: POZ Magazine, New York Magazine and Science Magazine among others and decided to devote his life in supporting research for cures against HIV. In July 2012, he ...
R.T. Gandhi and OthersN Engl J Med 2023;389:2468-2476. A 70-year-old woman with advanced HIV infection was evaluated because of cough, shortness of breath, and malaise. Eleven months earlier, she ...
The research was sponsored by NIAID, co-funded by the Bill & Melinda Gates Foundation, and conducted by the Duke Consortium for HIV/AIDS Vaccine Development (CHAVD), one of two NIAID-supported HIV vaccine consortia, in collaboration with the NIAID-funded HIV Vaccine Trials Network. This study provided the proof of concept that a vaccine can ...
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as ...
At the same time, NIAID continues to support research to develop new drugs with unique mechanisms of action for daily antiretroviral therapy. Such drugs likely would be effective against HIV strains with resistance to other drug types. ... see the AIDSinfo Drug Database. Content last reviewed on August 26, 2019. Website Policies & Notices ...
The Office of AIDS Research (OAR) coordinates HIV/AIDS research across the National Institutes of Health (NIH). The NIH provides the largest public investment in HIV/AIDS research globally. As HIV crosses nearly every area of medicine and scientific investigation, the response to the HIV pandemic requires a multi-Institute, multidisciplinary ...
Investing in research to find a cure for HIV is focused on two broad aims: sustained viral remission and, in the longer term, viral eradication. Viral latency and sanctuaries. Latent HIV reservoirs—small amounts of HIV that persist in people taking ART—present a significant challenge to finding a cure for HIV. Latent reservoirs remain in ...
The new drugs are so effective in people who have fully suppressed virus that many only need to use two medications to maintain HIV treatment and control. New research is investigating ways to deliver the medications differently, such as a shot that lasts several months, or maybe someday even implantable medication delivery mechanisms so that ...
Center for Clinical AIDS Research and Education, David Geffen School of Medicine, University of California, Los Angeles, CA, USA Raphael J. Landovitz Bridge HIV, San Francisco Department of Public ...
HIV & AIDS. Raising life expectancy for youth with HIV requires more than just adherence to care regimens, researchers say. Life expectancy of youth with HIV is projected to be 10.4 years less in ...
Research. CDC provides national leadership for HIV prevention research, including the development and evaluation of HIV biomedical and behavioral interventions to prevent HIV transmission and reduce HIV disease progression in the United States and internationally. CDC's research efforts also include identifying those scientifically proven ...
A vaccine that elicits broadly neutralizing antibodies before infection could be transformative; despite advances in HIV treatment, more than 600,000 people died of AIDS last year.
Human immunodeficiency virus (HIV) is an infection that attacks the body's immune system. Acquired immunodeficiency syndrome (AIDS) is the most advanced stage of the disease. HIV targets the body's white blood cells, weakening the immune system. This makes it easier to get sick with diseases like tuberculosis, infections and some cancers.
Learn about HIV data systems, how data is used in the U.S., and access the latest data releases. Skip directly to site content Skip directly to search An official website of the United States government
Some of the biggest stars in the French Rivera for the Cannes Film Festival made appearances at the 30th annual amfAR gala to raise money for AIDS research. Demi Moore, whose film "The Substance" caused a stir at Cannes, hosted this year's gala, a role launched by Elizabeth Taylor in 1993.
Within a week of being hired, Andrade applied for and was awarded a $5,000 seed grant from UB's Office of Curriculum, Assessment and Teaching Transformation to create a new course weaving AI into periodontics. This dental specialty focuses on the health of periodontium, the tissues that support teeth.
Scientists from ETH Zurich have developed a whey protein-based gel that can break down alcohol in the gut, potentially reducing hangovers. The gel, which has been tested on mice, lowers blood ...
amfAR said that the benefit has to date raised $264 million for its research programs on Acquired Immune Deficiency Syndrome (AIDS), caused by the human immunodeficiency virus (HIV). ($1 = 0.9247 ...