Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines

Understanding Clinical Trials
Clinical research: what is it.

Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
What Are the Types of Clinical Research?
There are two main kinds of clinical research:
Observational Studies
Observational studies are studies that aim to identify and analyze patterns in medical data or in biological samples, such as tissue or blood provided by study participants.
Clinical Trials
Clinical trials, which are also called interventional studies, test the safety and effectiveness of medical interventions — such as medications, procedures and tools — in living people.
Clinical research studies need people of every age, health status, race, gender, ethnicity and cultural background to participate. This will increase the chances that scientists and clinicians will develop treatments and procedures that are likely to be safe and work well in all people. Potential volunteers are carefully screened to ensure that they meet all of the requirements for any study before they begin. Most of the reasons people are not included in studies is because of concerns about safety.
Both healthy people and those with diagnosed medical conditions can take part in clinical research. Participation is always completely voluntary, and participants can leave a study at any time for any reason.
“The only way medical advancements can be made is if people volunteer to participate in clinical research. The research participant is just as necessary as the researcher in this partnership to advance health care.” Liz Martinez, Johns Hopkins Medicine Research Participant Advocate
Types of Research Studies
Within the two main kinds of clinical research, there are many types of studies. They vary based on the study goals, participants and other factors.
Biospecimen studies
Healthy volunteer studies.
Clinical trials study the safety and effectiveness of interventions and procedures on people’s health. Interventions may include medications, radiation, foods or behaviors, such as exercise. Usually, the treatments in clinical trials are studied in a laboratory and sometimes in animals before they are studied in humans. The goal of clinical trials is to find new and better ways of preventing, diagnosing and treating disease. They are used to test:
Drugs or medicines

New types of surgery

Medical devices

New ways of using current treatments

New ways of changing health behaviors

New ways to improve quality of life for sick patients

Goals of Clinical Trials
Because every clinical trial is designed to answer one or more medical questions, different trials have different goals. Those goals include:
Treatment trials
Prevention trials, screening trials, phases of a clinical trial.
In general, a new drug needs to go through a series of four types of clinical trials. This helps researchers show that the medication is safe and effective. As a study moves through each phase, researchers learn more about a medication, including its risks and benefits.
Is the medication safe and what is the right dose? Phase one trials involve small numbers of participants, often normal volunteers.
Does the new medication work and what are the side effects? Phase two trials test the treatment or procedure on a larger number of participants. These participants usually have the condition or disease that the treatment is intended to remedy.
Is the new medication more effective than existing treatments? Phase three trials have even more people enrolled. Some may get a placebo (a substance that has no medical effect) or an already approved treatment, so that the new medication can be compared to that treatment.
Is the new medication effective and safe over the long term? Phase four happens after the treatment or procedure has been approved. Information about patients who are receiving the treatment is gathered and studied to see if any new information is seen when given to a large number of patients.
“Johns Hopkins has a comprehensive system overseeing research that is audited by the FDA and the Association for Accreditation of Human Research Protection Programs to make certain all research participants voluntarily agreed to join a study and their safety was maximized.” Gail Daumit, M.D., M.H.S., Vice Dean for Clinical Investigation, Johns Hopkins University School of Medicine
Is It Safe to Participate in Clinical Research?
There are several steps in place to protect volunteers who take part in clinical research studies. Clinical Research is regulated by the federal government. In addition, the institutional review board (IRB) and Human Subjects Research Protection Program at each study location have many safeguards built in to each study to protect the safety and privacy of participants.
Clinical researchers are required by law to follow the safety rules outlined by each study's protocol. A protocol is a detailed plan of what researchers will do in during the study.
In the U.S., every study site's IRB — which is made up of both medical experts and members of the general public — must approve all clinical research. IRB members also review plans for all clinical studies. And, they make sure that research participants are protected from as much risk as possible.
Earning Your Trust
This was not always the case. Many people of color are wary of joining clinical research because of previous poor treatment of underrepresented minorities throughout the U.S. This includes medical research performed on enslaved people without their consent, or not giving treatment to Black men who participated in the Tuskegee Study of Untreated Syphilis in the Negro Male. Since the 1970s, numerous regulations have been in place to protect the rights of study participants.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial. In addition, Johns Hopkins Medicine’s Research Participant Advocacy Group focuses on improving the experience of people who participate in clinical research.
Clinical research participants with concerns about anything related to the study they are taking part in should contact Johns Hopkins Medicine’s IRB or our Research Participant Advocacy Group .
Learn More About Clinical Research at Johns Hopkins Medicine
For information about clinical trial opportunities at Johns Hopkins Medicine, visit our trials site.
Video Clinical Research for a Healthier Tomorrow: A Family Shares Their Story
Clinical Research for a Healthier Tomorrow: A Family Shares Their Story

Appointments at Mayo Clinic
Laboratory medicine and pathology.
Mayo Clinic staff members are actively engaged in research on several areas within Laboratory Medicine and Pathology. Mayo Clinic researchers continually study new and more efficient medical testing techniques and reflect their advances in tests offered internally at Mayo Clinic as well as externally though Mayo Clinic Laboratories .
Publications
See a list of publications by Mayo Clinic physicians on PubMed, a service of the National Library of Medicine.
Research profiles
Anatomic pathology.
- Keeney, Gary L. M.D. , Chair Minnesota
- Aubry, Marie Christine M.D. Minnesota
- Bell, Debra A. M.D. Minnesota
- Boland Froemming, Jennifer M. M.D. Minnesota
- Chen, Beiyun M.D., Ph.D. Minnesota
- Cheville, John C. M.D. Minnesota
- Cornell, Lynn D. M.D. Minnesota
- Erickson, Lori A. M.D. Minnesota
- Flotte, Thomas J. M.D. Minnesota
- Garcia, Joaquin J. M.D. Minnesota
- Giannini, Caterina M.D., Ph.D. Minnesota
- Grande, Joseph P. M.D., Ph.D. Minnesota
- Kipp, Benjamin R. Ph.D. Minnesota
- Maleszewski, Joseph J. M.D. Minnesota
- Mounajjed, Taofic M.D. Minnesota
- Roden, Anja C. M.D. Minnesota
- Salomao, Diva R. M.D. Minnesota
- Smyrk, Thomas C. M.D. Minnesota
- Visscher, Daniel W. M.D. Minnesota
- Yi, Joanne (Eunhee) E. M.D. Minnesota
- Zhang, Lizhi M.D. Minnesota
Clinical Biochemistry & Immunology
- Algeciras-Schimnich, Alicia Ph.D. , Chair Minnesota
- Baudhuin, Linnea M. Ph.D. Minnesota
- Black, John Logan M.D. Minnesota
- Grebe, Stefan K. M.D., Ph.D. Minnesota
- Klein, Christopher J. M.D. Minnesota
- Lachance, Daniel Honore M.D. Minnesota
- Lennon, Vanda A., M.D., Ph.D. Minnesota
- McKeon, Andrew M.B., B.Ch., M.D. Minnesota
- Pittock, Sean J. M.D. Minnesota
- Singh, Ravinder J. Ph.D. Minnesota
Clinical Core Laboratory Services
- Jaffe, Allan S. M.D. , Chair Minnesota
- Larson, Timothy S. M.D. Minnesota
Clinical Microbiology
- Patel, Robin M.D. , Chair Minnesota
- Pritt, Bobbi S. M.D. Minnesota
- Theel, Elitza S. Ph.D. Minnesota
- Wengenack, Nancy L. Ph.D. Minnesota
- Yao, Joseph D. M.D. Minnesota
Experimental Pathology and Laboratory Medicine
- Couch, Fergus J. Ph.D. , Chair Minnesota
- Cicek, Mine M. Ph.D. Minnesota
- Cunningham, Julie M. Ph.D. Minnesota
- Jen, Jin M.D., Ph.D. Minnesota
- Jenkins, Robert B. M.D., Ph.D. Minnesota
- Klee, George G. M.D., Ph.D. Minnesota
- Shridhar, Vijayalakshmi Ph.D. Minnesota
- Smith, David I. Ph.D. Minnesota
Hematopathology
- Chen, Dong M.D., Ph.D. Minnesota
- Feldman, Andrew L. M.D. Minnesota
- Hanson, Curtis A. M.D. Minnesota
- Heit, John A. M.D. Minnesota
- Kurtin, Paul J. M.D. Minnesota
- Macon, William R. M.D. Minnesota
- McPhail, Ellen D. M.D. Minnesota
- Pruthi, Rajiv K. M.B.B.S. Minnesota
- Rajkumar, S. Vincent M.D. Minnesota
Laboratory Genetics
- Matern, Dietrich M.D., Ph.D. , Chair Minnesota
Biochemical Genetics Laboratory
- Gavrilov, Dimitar K. M.D., Ph.D. Minnesota
- Matern, Dietrich M.D., Ph.D. Minnesota
- Oglesbee, Devin Ph.D. Minnesota
- Rinaldo, Piero M.D., Ph.D. Minnesota
- Tortorelli, Silvia M.D., Ph.D. Minnesota
Cytogenetics Laboratory
- Greipp, Patricia T. D.O. Minnesota
- Ketterling, Rhett P. M.D. Minnesota
- Thorland, Erik C. Ph.D. Minnesota
- Van Dyke, Daniel L. Ph.D. Minnesota
Molecular Genetics Laboratory
- Ferber, Matthew J. Ph.D. Minnesota
- Halling, Kevin C. M.D., Ph.D. Minnesota
- Rumilla, Kandelaria M. M.D. Minnesota
Transfusion Medicine
- Dietz, Allan B. Ph.D. Minnesota
- Gandhi, Manish J. M.D. Minnesota
- Santrach, Paula J. M.D. Minnesota
- Winters, Jeffrey L. M.D. Minnesota
- Medical Departments & Centers
- Laboratory Medicine & Pathology
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- Student/Faculty Portal
- Learning Hub (Brightspace)
- Continuous Professional Development

Medical Laboratory Scientist
What does a medical laboratory scientist do.
A medical laboratory scientist (MLS), also known as a medical technologist or clinical laboratory scientist, works to analyze a variety of biological specimens. They are responsible for performing scientific testing on samples and reporting results to physicians.
Medical laboratory scientists perform complex tests on patient samples using sophisticated equipment like microscopes. The data they find plays an important role in identifying and treating cancer, heart disease, diabetes, and other medical conditions. It is estimated 60 to 70 percent of all decisions regarding a patient's diagnosis, treatment, hospital admission, and discharge are based on the results of the tests medical laboratory scientists perform.
Video: Behind the scenes: Medical Laboratory Scientist
Scope of practice
Medical laboratory scientists collaborate very closely with physicians and medical laboratory technicians in diagnosing and monitoring disease processes, as well as monitoring the effectiveness of therapy. Areas of medical laboratory training include microbiology, chemistry, hematology, immunology, transfusion medicine, toxicology, and molecular diagnostics.
Medical laboratory scientists have a wide variety of responsibilities and duties, including:
- Examining and analyzing blood, body fluids, tissues, and cells
- Relaying test results to physicians
- Utilizing microscopes, cell counters, and other high-precision lab equipment
- Cross-matching blood for transfusion
- Monitoring patient outcomes
- Performing differential cell counts looking for abnormal cells to aid in the diagnosis of anemia and leukemia
- Establishing quality assurance programs to monitor and ensure the accuracy of test results
- Overseeing the work of a medical laboratory technician
Medical laboratory scientist vs. medical laboratory technician
While similar, there are a few key differences between a medical lab scientist and a medical lab technician. They both work in the lab and perform tests on biological samples, however, a medical lab scientist typically has more education and is able to perform more involved lab work. A medical lab technician performs more of the routine lab work and is often supervised by a medical lab scientist.
Medical laboratory scientist vs. medical laboratory assistant
A medical laboratory assistant is a subgroup of medical laboratory technician. They are responsible for preparing biological specimens, recording information, and perform more of the lab maintenance tasks such as cleaning equipment and stocking supplies. A medical laboratory scientist will work with a medical laboratory assistant by analyzing their prepared specimens and relaying information for them to record.
Work environment
Medical lab scientists work in hospitals, clinics, forensic or public health laboratories, as well as pharmaceutical industries, biotechnology companies, veterinary clinics, or research institutions. Depending on the setting, their work hours may vary; but typically labs are run 24 hours a day, seven days a week. This allows for flexibility in scheduling.
Medical laboratory scientists spend the majority of their time on their feet, analyzing test results in the lab.
Becoming a medical laboratory scientist
Successful medical lab scientists are effective communicators with a sound intellect and interest in science and technology. Excellent eye-hand coordination, dexterity, and visual acuity are important to skillfully perform and analyze tests.
Individuals who love science and research, but prefer to have little-to-no interaction with patients, would be a good fit for the medical laboratory scientist career.
Higher education requirements
After obtaining a high school diploma (or the equivalent), most will go on to obtain some level of higher education and training in order to become a medical laboratory scientist.
Common higher education requirements for medical laboratory scientist jobs include:
- Completing a bachelor’s degree in medical technology or clinical laboratory science. A bachelor’s degree in a science or health-related field (e.g. chemistry or microbiology) may also be considered.
- Completing a clinical laboratory program or internship through a hospital-based program or as part of their education
- National certification as a medical technologist (MT), clinical laboratory scientist (CLS), or medical laboratory scientist (MLS)
- Previous experience in a healthcare setting
Certification and licensing
Most employers require medical laboratory scientists to obtain certification through an accrediting body, such as the American Society for Clinical Pathology (ASCP) Board of Certification (BOC) . After passing the credentialing exam, medical laboratory scientists (MLS) can practice under the credentials of MLS(ASCP)CM.
Licensure by state may also be required.
Career opportunities and outlook
Job growth and security are high for medical laboratory technicians and scientists. According to the Bureau of Labor Statistics , there is currently a shortage of medical lab technicians and scientists in many parts of the country which guarantees ample employment opportunities and sometimes higher salaries for graduates. With the volume of laboratory tests continuing to increase due to both population growth and the development of new types of tests, job opportunities are expected to increase faster than average with over 26,000 new positions expected to be available by 2030.
With additional training and experience, a medical lab scientist can become a department lead or lab manager. Others may seek specializations to advance their careers. Typically, a medical lab technician will progress to a medical lab scientist with more training.
Medical laboratory scientist programs at Mayo Clinic
Mayo Clinic offers several programs and rotations to further your education and prepare you for a career as a medical laboratory scientist, medical laboratory assistant, or medical laboratory technician.
- Medical Laboratory Science Clinical Rotation (Arizona)
- Medical Laboratory Science Clinical Rotation (Florida)
- Medical Laboratory Science Program (Florida and Minnesota)
- Medical Laboratory Technician Clinical Rotation (Florida)
Browse similar careers

Cytogenetic technologist

Cytotechnologist

Histology technician
Careers in healthcare: Let us help you find your fit
- UNC Chapel Hill
What is Clinical Laboratory Science?
Clinical Laboratory Science, also called Medical Laboratory Science or Medical Technology, is the health profession that provides laboratory information and services needed for the diagnosis and treatment of disease. Clinical Laboratory Scientists perform a variety of laboratory tests, ensure the quality of the test results, explain the significance of laboratory tests, evaluate new methods and study the effectiveness of laboratory tests. Examples of laboratory tests performed by Clinical Laboratory Scientists include:

- the detection of the abnormal cells that cause leukemia
- the analysis of cardiac enzyme activity released during a heart attack
- the identification of the type of bacteria causing an infection
- the detection of DNA markers for genetic diseases
Molecular Diagnostic Science is a specialized area of Clinical Laboratory Science that uses sensitive and specific techniques to detect and identify biomarkers at the most basic level: that of nucleic acids (DNA and RNA). Common applications of molecular methods include medical diagnosis, establishing prognosis, monitoring the course of disease, and selecting optimal therapies. Molecular methods are also used in both forensic and non-forensic identification. A variety of biological materials can be used for molecular testing including fetal cells from amniotic fluid, dried blood spots from newborn screening programs, blood samples, buccal (mouth) swabs, bone, and hair follicles.
Molecular diagnostic tests are increasingly used in many major areas of medicine including genetic disorders, infectious diseases, cancer, pharmacogenetics and identity testing. Examples include:
- Genetic disorders: Molecular methods are used to detect common inherited diseases such as cystic fibrosis, hemochromatosis, and fragile X syndrome.
- Infectious diseases: Many diseases – including hepatitis, tuberculosis, human immunodeficiency virus (HIV), human papilloma virus (HPV), Chlamydia, Neisseria gonorrhoeae, and methicillin-resistant Staphylococcus aureus (MRSA) – can be identified faster and more accurately using molecular techniques as compared to traditional culture or antibody assays.
- Cancer: Some leukemias and solid tumor cancers can be detected and identified by molecular probes which target the abnormal gene rearrangements occurring in these disorders.
- Pharmacogenetics. Molecular testing can be used to individualize a specific dosing schedule for patients on a common blood thinner, warfarin, and thereby reduce the likelihood of overmedication and potential bleeding problems.
- Identity testing: Molecular diagnostic tests are used in determining the identity of combat casualties, in analyzing crime scene evidence, in determining paternity, and identifying foreign DNA in transplantation medicine.
These examples are only a small sample of the many clinical and other applications of molecular testing methods.
Vignettes of the Profession of Clinical Laboratory Science [ pdf file ] by Barbara Thornton, CLS(NCA), MT(ASCP), M.Ed.
- Laboratories
Narrow your search
- Areas of Research
- active Find a research lab with a name beginning with the letter A A
- Find a research lab with a name beginning with the letter B B
- Find a research lab with a name beginning with the letter C C
- Find a research lab with a name beginning with the letter D D
- Find a research lab with a name beginning with the letter E E
- Find a research lab with a name beginning with the letter F F
- Find a research lab with a name beginning with the letter G G
- Find a research lab with a name beginning with the letter H H
- Find a research lab with a name beginning with the letter I I
- No research labs names begin with the letter J J
- Find a research lab with a name beginning with the letter K K
- Find a research lab with a name beginning with the letter L L
- Find a research lab with a name beginning with the letter M M
- Find a research lab with a name beginning with the letter N N
- Find a research lab with a name beginning with the letter O O
- Find a research lab with a name beginning with the letter P P
- No research labs names begin with the letter Q Q
- Find a research lab with a name beginning with the letter R R
- Find a research lab with a name beginning with the letter S S
- Find a research lab with a name beginning with the letter T T
- No research labs names begin with the letter U U
- Find a research lab with a name beginning with the letter V V
- No research labs names begin with the letter W W
- No research labs names begin with the letter X X
- No research labs names begin with the letter Y Y
- Find a research lab with a name beginning with the letter Z Z
- Advanced Endoscopy Innovation Translation and Clinical Trials Group: Barham Abu Dayyeh
- Advanced Medical Imaging Technology: Richard L. Ehman
- Aerospace Medicine and Vestibular Research: Michael J. Cevette, Jan Stepanek
- Aging and Dementia Imaging Research (ADIR): Clifford R. Jack Jr.; Kejal Kantarci; Prashanthi Vemuri
- Aging and Immune Restoration: Jessica N. Lancaster
- Allergic Diseases: Hirohito Kita
- Anesthesia Outcomes: Juraj Sprung
- Anesthesiology Systematic Review: W. Michael Hooten
- Anticancer Drug Action: Scott H. Kaufmann
- Artificial Liver and Liver Transplantation: Scott L. Nyberg
- Artificial Pancreas: Yogish C. Kudva
- Assistive and Restorative Technology: Kristin D. Zhao
- Atherosclerosis and Lipid Genomics: Iftikhar J. Kullo
More about research at Mayo Clinic
- Research Faculty
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Clinical Trials
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.

Clinical Research vs Lab Research: An In-depth Analysis

Clinical research , a cornerstone in advancing patient care, involves human subjects to test the safety and effectiveness of new treatments , ranging from drugs to diagnostic tools. Unlike clinical research , laboratory research focuses on the foundational science behind medicine without direct human involvement , contributing significantly to medical lab science.
The contrast between clinical research vs lab research highlights the diverse approaches in the scientific pursuit of better healthcare, where every medical advancement once relied on volunteer participation in clinical studies 1 . Bridging these two fields promises to accelerate the translation of lab discoveries into practical medical applications, underscoring the importance of collaboration in future developments in medical lab science 1 2 .
The Evolution of Clinical Research
The evolution of clinical research traces its origins back to ancient times, with the world's first recorded clinical tria l found in the "Book of Daniel" where a dietary intervention was observed to improve health after 10 days. This historical milestone was followed by significant advancements including Avicenna's rules for drug testing in his ‘ Canon of Medicine’ and Ambroise Pare's accidental trial in 1537 , which introduced a novel therapy for wounded soldiers. The modern era of clinical trials was marked by James Lind's controlled trial on scurvy in 1747 , laying the foundational principles for contemporary clinical research methodologies. The progression from these early experiments to the structured, ethical, and scientifically rigorous trials of today highlights the dynamic nature of clinical research. This evolution was further shaped by the introduction of the placebo in the early 1800s and the establishment of ethical frameworks , starting with the Hippocratic Oath and later formalized by the Nuremberg Code in 1947 . The development of clinical research has been instrumental in advancing medical science, with each phase of clinical trials meticulously designed to ensure the safety and efficacy of new treatments for the benefit of patient care.
Key Components of Laboratory Research
Clinical Research Facility Sciences, pivotal in the realm of medical lab science, leverage laboratory data and services extensively for disease diagnosis, monitoring, and treatment 2 4 . These sciences are underpinned by professionals who, after obtaining a Bachelor's degree in fields such as clinical research facility science or biomedical sciences from NAACLS-accredited programs, perform crucial laboratory tests, analyze specimens, and furnish healthcare providers with critical insights into the results' significance and validity 2 . Notably, these activities are conducted in laboratory settings without involving human subjects, emphasizing the distinction between clinical and laboratory research 2 .
The infrastructure of laboratories is meticulously designed to support the complex and sensitive nature of laboratory tests and analyses. This includes sturdy tables and ample counter space for heavy equipment, overhead and adjustable shelving for efficient space utilization, and cabinets and drawers for organized storage. Additionally, the deployment of fume hoods, customized for specific research needs, is essential for the safe handling of chemicals. Compliance with safety regulations and proper storage of flammable items underscore the operational standards necessary for high-quality testing and analysis in medical breakthroughs 6 .
The scientific process in laboratory research unfolds through several key steps: hypothesis formulation, experiment design, data collection, data analysis, and report writing. This structured approach begins with formulating a tentative explanation for a phenomenon, followed by planning and conducting experiments using appropriate methods and tools. The subsequent collection and analysis of data facilitate testing the hypothesis, culminating in the documentation of the entire process and findings in a formal report or paper 7 . This systematic methodology underscores the rigorous and methodical nature of laboratory research, contributing significantly to advancements in medical lab science.
Bridging the Gap: Collaboration between Clinical and Laboratory Research
Bridging the gap between clinical and laboratory research involves fostering collaborative environments that leverage the strengths of both fields to advance medical science. Medical scientific studies bifurcate into clinical laboratory scientists, who interpret critical data for healthcare professionals, and clinical researchers, who lay the groundwork for medical education and understanding 4 . This collaboration is pivotal for both building the future of medicine and administering its current benefits 4 . Enhanced operational efficiency is achieved through cross-departmental synergy, reducing redundancies in resource and personnel utilization, and fostering faster adoption of best practices and innovations across the lab 8 . These collaborations are exemplified by real-world success stories from renowned institutions like Mayo Clinic and Stanford Health Care, which have demonstrated the profound impact of integrated efforts on medical advancements 8 .
Key strategies for effective collaboration include regular meetings to address challenges, the integration of digital communication platforms with lab databases for swift sharing of results, and the establishment of clear guidelines for consistency in sample collection and result dissemination 8 . Unified objectives ensure that despite methodological differences, the end goals of improving patient care and advancing medical knowledge remain aligned 8 . Furthermore, the adoption of cloud-based data systems and AI technologies not only facilitates seamless data sharing but also automates routine tasks, thereby enhancing productivity and enabling the discovery of new insights 9 .
Challenges such as competition, ethics reviews, insufficient research funds, and the recruitment of project managers underscore the complexities of collaborative efforts 9 . However, the benefits, including improved reputation, publication quality, knowledge transfer, and acceleration of the research process, often outweigh the costs and risks associated with collaboration 9 . Collaborative relationships in Translational Medical Research (TMR) among clinicians highlight a strong willingness to collaborate, with preferences varying across different stages of research and between preferring independent and interdependent relationships 9 . This willingness to collaborate is crucial for bridging the gap between clinical and laboratory research, ultimately leading to groundbreaking advancements in medical science.
Future Trends in Clinical and Laboratory Research
The future of clinical and laboratory research is poised for transformative changes, driven by technological advancements and evolving healthcare needs. Notably:
Greater Efficiency through Automation : The integration of automation in research processes promises to streamline workflows, reducing manual labor and enhancing precision 13 .
Collaboration and Capacity Sharing : Partnerships between research institutions will facilitate shared resources and expertise, optimizing research outputs 13 .
Remote Sample Support and Diagnostic Data Interoperability : These advancements will enable more inclusive research and improved patient care by allowing data to flow seamlessly between different healthcare systems 13 .
Artificial Intelligence and Machine Learning : AI and machine learning are set to revolutionize both clinical and laboratory research by providing advanced data analysis, predictive modeling, and personalized medicine approaches 13 14 .
Staffing Solutions and Digital Workflows : Addressing staffing shortages through innovative solutions, alongside the adoption of digital workflows, will be crucial for maintaining research momentum 14 .
New Diagnostic Technologies : The development of novel diagnostic methods and technologies, including next-generation sequencing and biomarker-based screenings, will enhance disease diagnosis and treatment 14 .
Regulatory Changes and Patient-Centric Approaches : Increased FDA oversight of laboratory-developed tests and a shift towards patient-centric research models will ensure safer and more effective healthcare solutions 14 16 .
Precision Medicine and Big Data Analytics : The focus on precision medicine, supported by real-world evidence and big data analytics, will tailor treatments to individual patient needs, improving outcomes 15 .
Decentralized Clinical Trials and Digital Health Technologies : The rise of decentralized trials and digital health tools, including remote monitoring, will make research more accessible and patient-friendly 15 .
Innovation in Testing and Consumer Health : Laboratories will explore new frontiers in diagnostics, such as multi-drug-of-abuse testing and T-cell testing, while also responding to consumer health trends with at-home testing services 14 18 .
These trends underscore a dynamic shift towards more efficient, patient-centered, and technologically advanced clinical and laboratory research, setting the stage for groundbreaking discoveries and innovations in healthcare 13 14 15 16 18 .
Through this detailed exploration, we have seen the distinct yet intertwined roles that clinical and laboratory research play in the advancement of medical science and patient care. By comparing their methodologies, evolution, and collaborative potential, it becomes clear that both domains are crucial for fostering innovations that can bridge the gap between theoretical knowledge and practical healthcare solutions. The synergy between clinical and laboratory research, as highlighted by various examples and future trend predictions, establishes an essential framework for the continual improvement of medical practices and patient outcomes.
As we look toward the future, the significance of embracing technological advancements, enhancing collaboration, and adopting patient-centric approaches cannot be overstressed. These elements are pivotal in navigating the challenges and leveraging the opportunities within clinical and laboratory research landscapes. The potential impacts of such advancements on the field of medicine and on societal health as a whole are immense, underscoring the imperative for ongoing research, dialogue, and innovation in bridging the gap between the laboratory bench and the patient's bedside.
What distinguishes clinical research from laboratory research? Clinical research involves studies that include human participants, aiming to understand health and illness and answer medical questions. Laboratory research, on the other hand, takes place in environments such as chemistry or biology labs, typically at colleges or medical schools, and does not involve human subjects. Instead, it focuses on experiments conducted on non-human samples or models.
How does a clinical laboratory differ from a research laboratory? Clinical laboratories are specialized facilities where laboratory information and services are utilized to diagnose, monitor, and treat diseases. Research laboratories, in contrast, are settings where scientific investigation is conducted to study illness and health in humans to answer medical and behavioral questions.
In what ways do clinical research and scientific research differ? Clinical research is a branch of medical research that directly applies knowledge to improve patient care, often through the study of human subjects. Scientific research, including basic science research, aims to understand the mechanisms of diseases and biological processes, which may not have immediate applications in patient care.
Can you outline the various types of medical research analysis? Medical research can be categorized into three primary types based on the study's nature: basic (experimental) research, clinical research, and epidemiological research. Clinical and epidemiological research can be further divided into interventional studies, which actively involve treating or intervening in the study subjects, and noninterventional studies, which observe outcomes without intervention.

Prevent CRA Fraud: 5 Strategies to Protect Your CRO Team
Everything you need to know about clinical research studies.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Dtsch Arztebl Int
- v.106(15); 2009 Apr
Types of Study in Medical Research
Bernd röhrig.
1 MDK Rheinland-Pfalz, Referat Rehabilitation/Biometrie, Alzey
Jean-Baptist du Prel
2 Zentrum für Präventive Pädiatrie, Zentrum für Kinder- und Jugendmedizin, Mainz
Daniel Wachtlin
3 Interdisziplinäres Zentrum Klinische Studien (IZKS), Fachbereich Medizin der Universität Mainz
Maria Blettner
4 Institut für Medizinische Biometrie, Epidemiologie und Informatik (IMBEI), Johannes Gutenberg Universität Mainz
The choice of study type is an important aspect of the design of medical studies. The study design and consequent study type are major determinants of a study’s scientific quality and clinical value.
This article describes the structured classification of studies into two types, primary and secondary, as well as a further subclassification of studies of primary type. This is done on the basis of a selective literature search concerning study types in medical research, in addition to the authors’ own experience.
Three main areas of medical research can be distinguished by study type: basic (experimental), clinical, and epidemiological research. Furthermore, clinical and epidemiological studies can be further subclassified as either interventional or noninterventional.
Conclusions
The study type that can best answer the particular research question at hand must be determined not only on a purely scientific basis, but also in view of the available financial resources, staffing, and practical feasibility (organization, medical prerequisites, number of patients, etc.).
The quality, reliability and possibility of publishing a study are decisively influenced by the selection of a proper study design. The study type is a component of the study design (see the article "Study Design in Medical Research") and must be specified before the study starts. The study type is determined by the question to be answered and decides how useful a scientific study is and how well it can be interpreted. If the wrong study type has been selected, this cannot be rectified once the study has started.
After an earlier publication dealing with aspects of study design, the present article deals with study types in primary and secondary research. The article focuses on study types in primary research. A special article will be devoted to study types in secondary research, such as meta-analyses and reviews. This article covers the classification of individual study types. The conception, implementation, advantages, disadvantages and possibilities of using the different study types are illustrated by examples. The article is based on a selective literature research on study types in medical research, as well as the authors’ own experience.
Classification of study types
In principle, medical research is classified into primary and secondary research. While secondary research summarizes available studies in the form of reviews and meta-analyses, the actual studies are performed in primary research. Three main areas are distinguished: basic medical research, clinical research, and epidemiological research. In individual cases, it may be difficult to classify individual studies to one of these three main categories or to the subcategories. In the interests of clarity and to avoid excessive length, the authors will dispense with discussing special areas of research, such as health services research, quality assurance, or clinical epidemiology. Figure 1 gives an overview of the different study types in medical research.

Classification of different study types
*1 , sometimes known as experimental research; *2 , analogous term: interventional; *3 , analogous term: noninterventional or nonexperimental
This scheme is intended to classify the study types as clearly as possible. In the interests of clarity, we have excluded clinical epidemiology — a subject which borders on both clinical and epidemiological research ( 3 ). The study types in this area can be found under clinical research and epidemiology.
Basic research
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. In almost all experiments, at least one independent variable is varied and the effects on the dependent variable are investigated. The procedure and the experimental design can be precisely specified and implemented ( 1 ). For example, the population, number of groups, case numbers, treatments and dosages can be exactly specified. It is also important that confounding factors should be specifically controlled or reduced. In experiments, specific hypotheses are investigated and causal statements are made. High internal validity (= unambiguity) is achieved by setting up standardized experimental conditions, with low variability in the units of observation (for example, cells, animals or materials). External validity is a more difficult issue. Laboratory conditions cannot always be directly transferred to normal clinical practice and processes in isolated cells or in animals are not equivalent to those in man (= generalizability) ( 2 ).
Basic research also includes the development and improvement of analytical procedures—such as analytical determination of enzymes, markers or genes—, imaging procedures—such as computed tomography or magnetic resonance imaging—, and gene sequencing—such as the link between eye color and specific gene sequences. The development of biometric procedures—such as statistical test procedures, modeling and statistical evaluation strategies—also belongs here.
Clinical studies
Clinical studies include both interventional (or experimental) studies and noninterventional (or observational) studies. A clinical drug study is an interventional clinical study, defined according to §4 Paragraph 23 of the Medicines Act [Arzneimittelgesetz; AMG] as "any study performed on man with the purpose of studying or demonstrating the clinical or pharmacological effects of drugs, to establish side effects, or to investigate absorption, distribution, metabolism or elimination, with the aim of providing clear evidence of the efficacy or safety of the drug."
Interventional studies also include studies on medical devices and studies in which surgical, physical or psychotherapeutic procedures are examined. In contrast to clinical studies, §4 Paragraph 23 of the AMG describes noninterventional studies as follows: "A noninterventional study is a study in the context of which knowledge from the treatment of persons with drugs in accordance with the instructions for use specified in their registration is analyzed using epidemiological methods. The diagnosis, treatment and monitoring are not performed according to a previously specified study protocol, but exclusively according to medical practice."
The aim of an interventional clinical study is to compare treatment procedures within a patient population, which should exhibit as few as possible internal differences, apart from the treatment ( 4 , e1 ). This is to be achieved by appropriate measures, particularly by random allocation of the patients to the groups, thus avoiding bias in the result. Possible therapies include a drug, an operation, the therapeutic use of a medical device such as a stent, or physiotherapy, acupuncture, psychosocial intervention, rehabilitation measures, training or diet. Vaccine studies also count as interventional studies in Germany and are performed as clinical studies according to the AMG.
Interventional clinical studies are subject to a variety of legal and ethical requirements, including the Medicines Act and the Law on Medical Devices. Studies with medical devices must be registered by the responsible authorities, who must also approve studies with drugs. Drug studies also require a favorable ruling from the responsible ethics committee. A study must be performed in accordance with the binding rules of Good Clinical Practice (GCP) ( 5 , e2 – e4 ). For clinical studies on persons capable of giving consent, it is absolutely essential that the patient should sign a declaration of consent (informed consent) ( e2 ). A control group is included in most clinical studies. This group receives another treatment regimen and/or placebo—a therapy without substantial efficacy. The selection of the control group must not only be ethically defensible, but also be suitable for answering the most important questions in the study ( e5 ).
Clinical studies should ideally include randomization, in which the patients are allocated by chance to the therapy arms. This procedure is performed with random numbers or computer algorithms ( 6 – 8 ). Randomization ensures that the patients will be allocated to the different groups in a balanced manner and that possible confounding factors—such as risk factors, comorbidities and genetic variabilities—will be distributed by chance between the groups (structural equivalence) ( 9 , 10 ). Randomization is intended to maximize homogeneity between the groups and prevent, for example, a specific therapy being reserved for patients with a particularly favorable prognosis (such as young patients in good physical condition) ( 11 ).
Blinding is another suitable method to avoid bias. A distinction is made between single and double blinding. With single blinding, the patient is unaware which treatment he is receiving, while, with double blinding, neither the patient nor the investigator knows which treatment is planned. Blinding the patient and investigator excludes possible subjective (even subconscious) influences on the evaluation of a specific therapy (e.g. drug administration versus placebo). Thus, double blinding ensures that the patient or therapy groups are both handled and observed in the same manner. The highest possible degree of blinding should always be selected. The study statistician should also remain blinded until the details of the evaluation have finally been specified.
A well designed clinical study must also include case number planning. This ensures that the assumed therapeutic effect can be recognized as such, with a previously specified statistical probability (statistical power) ( 4 , 6 , 12 ).
It is important for the performance of a clinical trial that it should be carefully planned and that the exact clinical details and methods should be specified in the study protocol ( 13 ). It is, however, also important that the implementation of the study according to the protocol, as well as data collection, must be monitored. For a first class study, data quality must be ensured by double data entry, programming plausibility tests, and evaluation by a biometrician. International recommendations for the reporting of randomized clinical studies can be found in the CONSORT statement (Consolidated Standards of Reporting Trials, www.consort-statement.org ) ( 14 ). Many journals make this an essential condition for publication.
For all the methodological reasons mentioned above and for ethical reasons, the randomized controlled and blinded clinical trial with case number planning is accepted as the gold standard for testing the efficacy and safety of therapies or drugs ( 4 , e1 , 15 ).
In contrast, noninterventional clinical studies (NIS) are patient-related observational studies, in which patients are given an individually specified therapy. The responsible physician specifies the therapy on the basis of the medical diagnosis and the patient’s wishes. NIS include noninterventional therapeutic studies, prognostic studies, observational drug studies, secondary data analyses, case series and single case analyses ( 13 , 16 ). Similarly to clinical studies, noninterventional therapy studies include comparison between therapies; however, the treatment is exclusively according to the physician’s discretion. The evaluation is often retrospective. Prognostic studies examine the influence of prognostic factors (such as tumor stage, functional state, or body mass index) on the further course of a disease. Diagnostic studies are another class of observational studies, in which either the quality of a diagnostic method is compared to an established method (ideally a gold standard), or an investigator is compared with one or several other investigators (inter-rater comparison) or with himself at different time points (intra-rater comparison) ( e1 ). If an event is very rare (such as a rare disease or an individual course of treatment), a single-case study, or a case series, are possibilities. A case series is a study on a larger patient group with a specific disease. For example, after the discovery of the AIDS virus, the Center for Disease Control (CDC) in the USA collected a case series of 1000 patients, in order to study frequent complications of this infection. The lack of a control group is a disadvantage of case series. For this reason, case series are primarily used for descriptive purposes ( 3 ).

Epidemiological studies
The main point of interest in epidemiological studies is to investigate the distribution and historical changes in the frequency of diseases and the causes for these. Analogously to clinical studies, a distinction is made between experimental and observational epidemiological studies ( 16 , 17 ).
Interventional studies are experimental in character and are further subdivided into field studies (sample from an area, such as a large region or a country) and group studies (sample from a specific group, such as a specific social or ethnic group). One example was the investigation of the iodine supplementation of cooking salt to prevent cretinism in a region with iodine deficiency. On the other hand, many interventions are unsuitable for randomized intervention studies, for ethical, social or political reasons, as the exposure may be harmful to the subjects ( 17 ).
Observational epidemiological studies can be further subdivided into cohort studies (follow-up studies), case control studies, cross-sectional studies (prevalence studies), and ecological studies (correlation studies or studies with aggregated data).
In contrast, studies with only descriptive evaluation are restricted to a simple depiction of the frequency (incidence and prevalence) and distribution of a disease within a population. The objective of the description may also be the regular recording of information (monitoring, surveillance). Registry data are also suited for the description of prevalence and incidence; for example, they are used for national health reports in Germany.
In the simplest case, cohort studies involve the observation of two healthy groups of subjects over time. One group is exposed to a specific substance (for example, workers in a chemical factory) and the other is not exposed. It is recorded prospectively (into the future) how often a specific disease (such as lung cancer) occurs in the two groups ( figure 2a ). The incidence for the occurrence of the disease can be determined for both groups. Moreover, the relative risk (quotient of the incidence rates) is a very important statistical parameter which can be calculated in cohort studies. For rare types of exposure, the general population can be used as controls ( e6 ). All evaluations naturally consider the age and gender distributions in the corresponding cohorts. The objective of cohort studies is to record detailed information on the exposure and on confounding factors, such as the duration of employment, the maximum and the cumulated exposure. One well known cohort study is the British Doctors Study, which prospectively examined the effect of smoking on mortality among British doctors over a period of decades ( e7 ). Cohort studies are well suited for detecting causal connections between exposure and the development of disease. On the other hand, cohort studies often demand a great deal of time, organization, and money. So-called historical cohort studies represent a special case. In this case, all data on exposure and effect (illness) are already available at the start of the study and are analyzed retrospectively. For example, studies of this sort are used to investigate occupational forms of cancer. They are usually cheaper ( 16 ).

Graphical depiction of a prospective cohort study (simplest case [2a]) and a retrospective case control study (2b)
In case control studies, cases are compared with controls. Cases are persons who fall ill from the disease in question. Controls are persons who are not ill, but are otherwise comparable to the cases. A retrospective analysis is performed to establish to what extent persons in the case and control groups were exposed ( figure 2b ). Possible exposure factors include smoking, nutrition and pollutant load. Care should be taken that the intensity and duration of the exposure is analyzed as carefully and in as detailed a manner as possible. If it is observed that ill people are more often exposed than healthy people, it may be concluded that there is a link between the illness and the risk factor. In case control studies, the most important statistical parameter is the odds ratio. Case control studies usually require less time and fewer resources than cohort studies ( 16 ). The disadvantage of case control studies is that the incidence rate (rate of new cases) cannot be calculated. There is also a great risk of bias from the selection of the study population ("selection bias") and from faulty recall ("recall bias") (see too the article "Avoiding Bias in Observational Studies"). Table 1 presents an overview of possible types of epidemiological study ( e8 ). Table 2 summarizes the advantages and disadvantages of observational studies ( 16 ).
1 = slight; 2 = moderate; 3 = high; N/A, not applicable.
*Individual cases may deviate from this pattern.
Selecting the correct study type is an important aspect of study design (see "Study Design in Medical Research" in volume 11/2009). However, the scientific questions can only be correctly answered if the study is planned and performed at a qualitatively high level ( e9 ). It is very important to consider or even eliminate possible interfering factors (or confounders), as otherwise the result cannot be adequately interpreted. Confounders are characteristics which influence the target parameters. Although this influence is not of primary interest, it can interfere with the connection between the target parameter and the factors that are of interest. The influence of confounders can be minimized or eliminated by standardizing the procedure, stratification ( 18 ), or adjustment ( 19 ).
The decision as to which study type is suitable to answer a specific primary research question must be based not only on scientific considerations, but also on issues related to resources (personnel and finances), hospital capacity, and practicability. Many epidemiological studies can only be implemented if there is access to registry data. The demands for planning, implementation, and statistical evaluation for observational studies should be just as high for observational studies as for experimental studies. There are particularly strict requirements, with legally based regulations (such as the Medicines Act and Good Clinical Practice), for the planning, implementation, and evaluation of clinical studies. A study protocol must be prepared for both interventional and noninterventional studies ( 6 , 13 ). The study protocol must contain information on the conditions, question to be answered (objective), the methods of measurement, the implementation, organization, study population, data management, case number planning, the biometric evaluation, and the clinical relevance of the question to be answered ( 13 ).
Important and justified ethical considerations may restrict studies with optimal scientific and statistical features. A randomized intervention study under strictly controlled conditions of the effect of exposure to harmful factors (such as smoking, radiation, or a fatty diet) is not possible and not permissible for ethical reasons. Observational studies are a possible alternative to interventional studies, even though observational studies are less reliable and less easy to control ( 17 ).
A medical study should always be published in a peer reviewed journal. Depending on the study type, there are recommendations and checklists for presenting the results. For example, these may include a description of the population, the procedure for missing values and confounders, and information on statistical parameters. Recommendations and guidelines are available for clinical studies ( 14 , 20 , e10 , e11 ), for diagnostic studies ( 21 , 22 , e12 ), and for epidemiological studies ( 23 , e13 ). Since 2004, the WHO has demanded that studies should be registered in a public registry, such as www.controlled-trials.com or www.clinicaltrials.gov . This demand is supported by the International Committee of Medical Journal Editors (ICMJE) ( 24 ), which specifies that the registration of the study before inclusion of the first subject is an essential condition for the publication of the study results ( e14 ).
When specifying the study type and study design for medical studies, it is essential to collaborate with an experienced biometrician. The quality and reliability of the study can be decisively improved if all important details are planned together ( 12 , 25 ).
Acknowledgments
Translated from the original German by Rodney A. Yeates, M.A., Ph.D.
Conflict of interest statement
The authors declare that there is no conflict of interest in the sense of the International Committee of Medical Journal Editors.
- Find Providers
- Medical Services
- Get Care Now
- Schedule an Appointment
- Find a Career
- Log in to My IU Health
- For Researchers
- Research Services
Medical Research Laboratory
The IU Health Medical Research Laboratory is designed and staffed to provide services to support research, development, education and investigation. These facilities are used by physicians, nursing staff and allied health personnel, as well as external organizations.
The laboratory offers physicians the opportunity to become familiar with state-of-the-art equipment, review surgical techniques, and obtain microsurgical training. Large corporations use the facility for surgical product evaluation.
The laboratory contains the following:
- Four fully equipped surgical suites
- Laparoscopy towers
- Cardiac monitors
- DaVinci robotic training center
- Fluoroscopy
- Microsurgery/neurosurgery microscopes
- Post-op recovery unit
Cadaver Request Form
To request cadaveric materials, please complete the Cadaver Request Form and return to Emma Dossey Curole .
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Institutes at nih, list of institutes and centers, nih institutes.
National Cancer Institute (NCI) — Est. 1937 NCI leads a national effort to eliminate the suffering and death due to cancer. Through basic and clinical biomedical research and training, NCI conducts and supports research that will lead to a future in which we can prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.
National Eye Institute (NEI) — Est. 1968 The National Eye Institute’s mission is to conduct and support research, training, health information dissemination, and other programs with respect to blinding eye diseases, visual disorders, mechanisms of visual function, preservation of sight, and the special health problems and requirements of the blind.
National Heart, Lung, and Blood Institute (NHLBI) — Est. 1948 The National Heart, Lung, and Blood Institute (NHLBI) provides global leadership for a research, training, and education program to promote the prevention and treatment of heart, lung, and blood diseases and enhance the health of all individuals so that they can live longer and more fulfilling lives. The NHLBI stimulates basic discoveries about the causes of disease, enables the translation of basic discoveries into clinical practice, fosters training and mentoring of emerging scientists and physicians, and communicates research advances to the public.
National Human Genome Research Institute (NHGRI) — Est. 1989 NHGRI is devoted to advancing health through genome research. The Institute led NIH’s contribution to the Human Genome Project, which was successfully completed in 2003 ahead of schedule and under budget. Building on the foundation laid by the sequencing of the human genome, NHGRI’s work now encompasses a broad range of research aimed at expanding understanding of human biology and improving human health. In addition, a critical part of NHGRI’s mission continues to be the study of the ethical, legal and social implications of genome research.
National Institute on Aging (NIA) — Est. 1974 NIA leads a national program of research on the biomedical, social, and behavioral aspects of the aging process; the prevention of age-related diseases and disabilities; and the promotion of a better quality of life for all older Americans.
National Institute on Alcohol Abuse and Alcoholism (NIAAA) — Est. 1970 NIAAA conducts research focused on improving the treatment and prevention of alcoholism and alcohol-related problems to reduce the enormous health, social, and economic consequences of this disease.
National Institute of Allergy and Infectious Diseases (NIAID) — Est. 1948 NIAID research strives to understand, treat, and ultimately prevent the myriad infectious, immunologic, and allergic diseases that threaten millions of human lives.
National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) — Est. 1986 NIAMS supports research into the causes, treatment, and prevention of arthritis and musculoskeletal and skin diseases, the training of basic and clinical scientists to carry out this research, and the dissemination of information on research progress in these diseases.
National Institute of Biomedical Imaging and Bioengineering (NIBIB) — Est. 2000 The mission of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) is to transform through engineering the understanding of disease and its prevention, detection, diagnosis, and treatment.
Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) — Est. 1962 NICHD leads research and training to understand human development, improve reproductive health, enhance the lives of children and adolescents, and optimize abilities for all.
National Institute on Deafness and Other Communication Disorders (NIDCD) — Est. 1988 NIDCD conducts and supports biomedical research and research training on normal mechanisms as well as diseases and disorders of hearing, balance, smell, taste, voice, speech, and language that affect 46 million Americans.
National Institute of Dental and Craniofacial Research (NIDCR) — Est. 1948 NIDCR provides leadership for a national research program designed to understand, treat, and ultimately prevent the infectious and inherited craniofacial-oral-dental diseases and disorders that compromise millions of human lives.
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) — Est. 1950 The mission of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) is to conduct and support medical research and research training and to disseminate science-based information on diabetes and other endocrine and metabolic diseases; digestive diseases, nutritional disorders, and obesity; and kidney, urologic, and hematologic diseases, to improve people’s health and quality of life.
National Institute on Drug Abuse (NIDA) — Est. 1974 The mission of the National Institute on Drug Abuse (NIDA) is to advance science on the causes and consequences of drug use and addiction and to apply that knowledge to improve individual and public health.
National Institute of Environmental Health Sciences (NIEHS) — Est. 1969 The mission of the National Institute of Environmental Health Sciences is to discover how the environment affects people in order to promote healthier lives.
National Institute of General Medical Sciences (NIGMS) — Est. 1962 The National Institute of General Medical Sciences (NIGMS) supports basic research that increases understanding of biological processes and lays the foundation for advances in disease diagnosis, treatment and prevention. NIGMS-funded scientists investigate how living systems work at a range of levels, from molecules and cells to tissues, whole organisms and populations. The Institute also supports research in certain clinical areas, primarily those that affect multiple organ systems. To assure the vitality and continued productivity of the research enterprise, NIGMS provides leadership in training the next generation of scientists, in enhancing the diversity of the scientific workforce, and in developing research capacities throughout the country.
National Institute of Mental Health (NIMH) — Est. 1949 NIMH provides national leadership dedicated to understanding, treating, and preventing mental illnesses through basic research on the brain and behavior, and through clinical, epidemiological, and services research.
National Institute on Minority Health and Health Disparities (NIMHD) — Est. 2010 NIMHD has a long history, beginning in 1990 as an Office and later designated a Center in 2000. The mission of NIMHD is to lead scientific research to improve minority health and eliminate health disparities. To accomplish its mission, NIMHD plans, reviews, coordinates, and evaluates all minority health and health disparities research and activities of the National Institutes of Health; conducts and supports research in minority health and health disparities; promotes and supports the training of a diverse research workforce; translates and disseminates research information; and fosters innovative collaborations and partnerships.
National Institute of Neurological Disorders and Stroke (NINDS) — Est. 1950 The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease. To accomplish this goal the NINDS supports and conducts basic, translational, and clinical research on the normal and diseased nervous system. The Institute also fosters the training of investigators in the basic and clinical neurosciences, and seeks better understanding, diagnosis, treatment, and prevention of neurological disorders.
National Institute of Nursing Research (NINR) — Est. 1986 The mission of the National Institute of Nursing Research (NINR) is to lead nursing research to solve pressing health challenges and inform practice and policy—optimizing health and advancing health equity into the future.
National Library of Medicine (NLM) — Est. 1956 NLM collects, organizes, and makes available biomedical science information to scientists, health professionals, and the public. The Library’s Web-based databases, including PubMed/Medline and MedlinePlus, are used extensively around the world. NLM conducts and supports research in biomedical communications; creates information resources for molecular biology, biotechnology, toxicology, and environmental health; and provides grant and contract support for training, medical library resources, and biomedical informatics and communications research.
NIH Centers
NIH Clinical Center (CC) — Est. 1953 The NIH Clinical Center, America’s research hospital, provides a versatile clinical research environment enabling the NIH mission to improve human health by investigating the pathogenesis of disease; conducting first-in-human clinical trials with an emphasis on rare diseases and diseases of high public health impact; developing state-of-the-art diagnostic, preventive, and therapeutic interventions; training the current and next generations of clinical researchers; and, ensuring that clinical research is ethical, efficient, and of high scientific quality.
Center for Information Technology (CIT) — Est. 1964 CIT incorporates the power of modern computers into the biomedical programs and administrative procedures of the NIH by focusing on three primary activities: conducting computational biosciences research, developing computer systems, and providing computer facilities.
Center for Scientific Review (CSR) — Est. 1946 CSR is the portal for NIH grant applications and their review for scientific merit. CSR oversees and implements peer review for over 75% of the more than 88,000 applications submitted to NIH each year, as well as for some other components of HHS. The mission of CSR is to see that NIH grant applications receive fair, independent, expert, and timely scientific reviews — free from inappropriate influences — so NIH can fund the most promising research.
Fogarty International Center (FIC) — Est. 1968 FIC promotes and supports scientific research and training internationally to reduce disparities in global health.
National Center for Advancing Translational Sciences (NCATS) — Est. 2011 The mission of NCATS is to catalyze the generation of innovative methods and technologies that will enhance the development, testing, and implementation of diagnostics and therapeutics across a wide range of human diseases and conditions.
National Center for Complementary and Integrative Health (NCCIH) — Est. 1999 The mission of NCCIH is to define, through rigorous scientific investigation, the usefulness and safety of complementary and integrative health interventions and their roles in improving health and health care.
This page last reviewed on July 12, 2023
Connect with Us
- More Social Media from NIH
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
MRC Laboratory of Molecular Biology
One of the world's leading research institutes, our scientists are working to advance understanding of biological processes at the molecular level - providing the knowledge needed to solve key problems in human health.

The MRC Laboratory of Molecular Biology (LMB) is a research institute dedicated to the understanding of important biological processes at the levels of atoms, molecules, cells and organisms. In doing so, we provide knowledge needed to solve key problems in human health.
Our scientists tackle fundamental, often difficult and long-term research problems. The LMB has made revolutionary contributions to science, such as pioneering X-ray crystallography and electron cryo-microscopy (cryo-EM) to determine protein structures, the sequencing of DNA and the development of monoclonal antibodies. Twelve Nobel Prizes have been awarded for work carried out by LMB scientists.
The LMB also promotes the application and exploitation of our research findings, both by collaboration with existing companies and the founding of new ones, helping to advance medical research and the translation and application of knowledge.
The LMB provides an unsurpassed environment for both young and established researchers, with state-of-the-art facilities and a unique scientific culture. The LMB has always been very diverse, with a truly international outlook. We currently employ men and women from over 50 countries, and LMB alumni work in research organisations across the world.
Insight on Research
First map of the neurotransmitters used in the fruit fly brain.
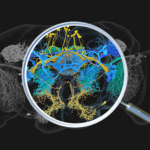
Greg Jefferis’ group in the LMB’s Neurobiology Division, in collaboration with scientists at the Janelia Research Campus, have predicted the sign of each synapsis in the fruit fly brain.
ModelAngelo software expands cryo-EM toolkit with faster atomic model building and identification of novel proteins
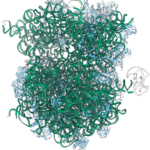
Kiarash Jamali, a Ph.D. student in Sjors Scheres’ group in the LMB’s Structural Studies Division, has designed a new machine learning software capable of fast, accurate model building at atomic levels and identification of new proteins beyond previous capabilities.
Quick Links
- How to Find Us
- Current Vacancies
- Contact Directory
- PhD Programme
- LMB Nobel Prizes
- News & Events
- Useful Contacts
- Goals and Research Focus
- LMB Through the Years
- LMB Exhibitions
Latest News

Christy Russell, Advisor for Fire and Facilities Safety in the LMB’s Health and Safety Team, discusses working towards the NEBOSH qualification. […]

Marta Zlatic, Group Leader in the LMB’s Neurobiology Division, has been elected into the Fellowship of the Academy of Medical Sciences. Joining her is alumna Jane McKeating. […]
LMB in the News

LMB Alumni News

Latest Publications
- How to enjoy and thrive in graduate school. Hegde, RS. Trends Cell Biol 34 (5): 349-351. (23rd May 2024)
- CD8 is down(regulated) for tolerance. Rodríguez-Rodríguez, N., Rosetti, F., Crispín, JC. Trends Immunol [Epub ahead of print]. (22nd May 2024)
- Circadian regulation of macromolecular complex turnover and proteome renewal. Seinkmane, E., et al. EMBO J [Epub ahead of print]. (22nd May 2024)
- Single-Molecule Characterization and Super-Resolution Imaging of Alzheimer's Disease-Relevant Tau Aggregates in Human Samples. Böken, D., et al. Angew Chem Int Ed Engl 63 (21): e202317756. (21st May 2024)
- Quantitative proteomics reveals CLR interactome in primary human cells. Manolis, D., Hasan, S., Maraveyas, A., O'Brien, DP., Kessler, BM., Kramer, H., Nikitenko, LL. J Biol Chem : 107399 [Epub ahead of print]. (20th May 2024)
- Basal delamination during mouse gastrulation primes pluripotent cells for differentiation. Sato, N., et al. Dev Cell 59 (10): 1252-1268.e13. (20th May 2024)
- To slide or not to slide: key role of the hexasome in chromatin remodeling revealed. Rhodes, D. Nat Struct Mol Biol 31 (5): 742-746. (20th May 2024)
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

Research involvement of medical students in a medical school of India: exploring knowledge, attitude, practices, and perceived barriers
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Abhinav Jha
- ORCID record for Deepak Dhamnetiya
- For correspondence: [email protected]
- ORCID record for Ravi Prakash Jha
- Info/History
- Preview PDF
Introduction Research in the medical discipline significantly impacts society by improving the general well-being of the population, through improvements in diagnostic and treatment modalities. However, of 579 Indian medical colleges, 332 (57.3%) did not publish a single paper from the year 2005 to 2014," indicating a limited contribution from medical fraternity In order to probe in to the cause of this a study was conducted to assess the knowledge, attitude, practices (KAP) and perceived barriers to research among students of a medical school in Delhi, India.
Methods A cross-sectional study was conducted among medical students and the data on academic-cum-demographic information, assessment of knowledge, attitude, practices and barriers to research was collected using a pre-tested, semi-structured questionnaire. Chi-square test was used to check the association of various factors with the KAP of research. A p-value less than 0.05 was considered significant.
Results A total of 402 (N) subjects were enrolled in the study. Majority were male (79.6%) and from clinical professional years (57%). Majority (266, 66.2%) of the subjects had adequate knowledge. Of the study subjects (61,15%) having inadequate knowledge of research, sixty percent were from pre- and para-clinical years, while around 70 % of those having good knowledge were from clinical professional years. However, only 16.9% of the participants had participated in a research project, and only 4.72% had authored a publication. Sixty one percent of study subjects having a positive attitude towards research, were from pre- and para-clinical years. Among the study subjects having a positive attitude towards research, over 60% were from pre- and para-clinical years. The barriers for conducting research were mostly; lack of funds/laboratory equipment/infrastructure (85.1%), lack of exposure to opportunities for research in the medical (MBBS) curriculum (83.8%), and lack of time (83.3%). There was a statistically significant association between knowledge and attitude towards research with a professional year of study.
Conclusions The study revealed that while most of the students had a positive attitude towards research as well as an adequate knowledge of research, there was a poor level of participation in research. These challenges can be overcome by incorporating research as a part of the medical school curriculum from early years on, setting aside separate time for research, and establishing student research societies that can actively promote research.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
This study did not receive any funding.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
The ethics committee of Dr Baba Saheb Ambedkar Medical College and Hospital, New Delhi gave ethical approval for this work.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Email id: jhaabhinav677{at}gmail.com , manas.shah1999{at}gmail.com , Ritikgoyal152{at}gmail.com , drdeepakdhamnetiya{at}gmail.com , apoorv1729{at}gmail.com , raviprakashjha{at}gmail.com , dr.prachi.obg{at}bsamch.in
Data Availability
All data produced in the present study are available upon reasonable request to the authors.
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Medical Education
- Addiction Medicine (324)
- Allergy and Immunology (632)
- Anesthesia (168)
- Cardiovascular Medicine (2400)
- Dentistry and Oral Medicine (289)
- Dermatology (207)
- Emergency Medicine (381)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (850)
- Epidemiology (11797)
- Forensic Medicine (10)
- Gastroenterology (705)
- Genetic and Genomic Medicine (3769)
- Geriatric Medicine (350)
- Health Economics (637)
- Health Informatics (2408)
- Health Policy (940)
- Health Systems and Quality Improvement (905)
- Hematology (342)
- HIV/AIDS (787)
- Infectious Diseases (except HIV/AIDS) (13347)
- Intensive Care and Critical Care Medicine (769)
- Medical Education (369)
- Medical Ethics (105)
- Nephrology (401)
- Neurology (3524)
- Nursing (199)
- Nutrition (529)
- Obstetrics and Gynecology (681)
- Occupational and Environmental Health (668)
- Oncology (1835)
- Ophthalmology (539)
- Orthopedics (221)
- Otolaryngology (287)
- Pain Medicine (234)
- Palliative Medicine (66)
- Pathology (447)
- Pediatrics (1037)
- Pharmacology and Therapeutics (426)
- Primary Care Research (424)
- Psychiatry and Clinical Psychology (3190)
- Public and Global Health (6184)
- Radiology and Imaging (1291)
- Rehabilitation Medicine and Physical Therapy (751)
- Respiratory Medicine (832)
- Rheumatology (380)
- Sexual and Reproductive Health (373)
- Sports Medicine (324)
- Surgery (406)
- Toxicology (50)
- Transplantation (172)
- Urology (147)
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Medical Physicist Assistant
- Radiation Oncology
- Columbia University Medical Center
- Opening on: May 23 2024
- Job Type: Officer of Administration
- Bargaining Unit:
- Regular/Temporary: Regular
- End Date if Temporary:
- Hours Per Week: 35
- Standard Work Schedule:
- Building: Medical Center
- Salary Range: $100,000 - $115,000
Position Summary
The Medical Physicist Assistant (MPA) performs tasks in support of faculty medical physicists within the Department of Radiation Oncology’s clinical enterprise. Responsibilities of the MPA include routine clinical quality assurance (patient-specific and subset of linear accelerator performance), assisting treatment platform commissioning, treatment device fabrication, QA equipment management, calibration of research irradiator, support of clinical trial data collection & submission, and associated report and record generation.
The MPA shall function generally as an extender to the medical physics group within the Department, with supervision requirements and a scope of work aligned with guidelines established by the American Association of Physicists in Medicine (AAPM) Medical Physics Practice Guidelines Report 10a and AAPM Policy AP-131-A. All pertinent medical physics tasks performed by the MPA shall be reviewed and co-signed by a qualified faculty medical physicist as their supervisor, who assumes full responsibility for submitted work. The Medical Physics Assistant shall report to the Director of Clinical Medical Physics or their designee. The training and ongoing competency of the MPA shall be the responsibility of the Director of Clinical Medical Physics or their designee.
Responsibilities
- Under the supervision of a qualified medical physicist (QMP), perform routine, non-procedural quality assurance testing on linear accelerators, CT simulators, and ancillary treatment weekly and monthly. Record said measurements using a web-based reporting and analysis system.
- Under the supervision of a qualified medical physicist (QMP), acquire measurements and generate associated documentation to verify the accuracy of the delivery of modulated radiotherapy patient treatments.
- Under the supervision of a qualified medical physicist (QMP), perform limited quality assurance for clinical treatment planning software systems on an annual basis.
- Under the supervision of a qualified medical physicist (QMP), fabricate custom radiation-field shaping apertures for electron radiotherapy treatments and associated quality assurance testing.
- Oversee the inventory and record-keeping of equipment utilized for physics quality assurance procedures. In collaboration with medical physics and radiation safety groups, ensure the timeliness of required equipment calibrations.
- Ensure appropriate calibration of the Department’s research small animal irradiator performing routine quality assurance.
- Collect and aggregate physics-related data for patients enrolled on clinical trials for submission to clinical trial office or trial hosting entity.
- When appropriate, assist the medical physics group in clinical development, research initiatives, and operations optimization projects.
Minimum Qualifications
- A bachelor’s degree in physics, Engineering, or related technical science, or a graduate of a JCERT-accredited program in radiation therapy or suitable equivalent and 4 years of experience.
- Demonstrable skills in technical problem-solving.
- Demonstrate competency in performing requisite tasks via the Department’s continuous credentialing program for MPAs.
Other Requirements
- Standard work week: Monday to Friday, 1:00 pm – 9:00 pm (1hr break)
- Workplace: 90% of the work week will be based at the 168th clinic in Manhattan and 10% at a CU/NYP clinic in Bronxville, NY.
- The proportion of time spent at each site may very infrequently shift due to commissioning project requirements.
- Ability to lift equipment from the floor to a waist height of 35 lbs.
- Flexibility in times of urgent task completion to adapt to clinical needs.
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Senior Admissions Officer
Research assistant.
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .
- Current Employees
- Duke & Durham
- Human Resources
- Connect With Us
- External Applicants
- Current Duke Employees
- Duke Health Careers
Clinical Research Specialist, Sr. (CRS, Sr.) - Psychiatry - Autism Lab/Franz
Durham, NC, US, 27710
School of Medicine
Established in 1930, Duke University School of Medicine is the youngest of the nation's top medical schools. Ranked sixth among medical schools in the nation, the School takes pride in being an inclusive community of outstanding learners, investigators, clinicians, and staff where interdisciplinary collaboration is embraced and great ideas accelerate translation of fundamental scientific discoveries to improve human health locally and around the globe. Composed of more than 2,600 faculty physicians and researchers, nearly 2,000 students, and more than 6,200 staff, the Duke University School of Medicine along with the Duke University School of Nursing, and Duke University Health System comprise Duke Health, a world-class academic medical center. The Health System encompasses Duke University Hospital, Duke Regional Hospital, Duke Raleigh Hospital, Duke Health Integrated Practice, Duke Primary Care, Duke Home Care and Hospice, Duke Health and Wellness, and multiple affiliations.
Operations: Under supervision, assists with managing investigational products including arrival, storage, and handling (requisitions, inventory, and reordering). Under supervision, prepares for study monitoring and audit visits. Maintains participant-level documentation for non-complex (e.g., questionnaire, data registry, scripted) studies outside of the EHR. Follows SOPs and strategies to manage and retain research subjects. Recruits research participants according to study protocol. Screens participants in person or over the phone for non-complex studies (e.g., questionnaire, data registry, scripted) or may collect information from the EHR to assist study team in determining eligibility. Follows SOPs. Independently employs simple procedures for collecting, preparing, processing, shipping, and maintaining inventory of specimens. Assists with establishing and maintaining study level documentation. Schedules participants for research visits (excluding those requiring EHR access). Prepares necessary documents, equipment, supplies, etc. in compliance with the protocol. Conducts and documents non-complex visits and scripted testing or interviews. May manage participant payment. Participates in study team meetings. Ethics: Recognizes known potential adverse events, identified in the protocol or investigator brochure, and reports to study team. Conducts and documents consent for participants in non-complex studies. These are typically repositories, survey studies, simple observational studies and non-patient studies that do not involve investigational products or devices. Cannot consent for any studies that involve investigational products or devices or require clinical research orders in Maestro Care (i.e., electronic health record). Assists with the development of consent plans and documents for participants. Under supervision, for non-complex studies (e.g., survey studies and registries), develops and submits documentation and information for IRB review. Data: Enters and collects basic data for research studies. May score scripted or validated tests and measures. Assists with quality control and data cleaning as directed. Independently responds to queries created by a CRO or the data manager. Independently corrects and documents incomplete, inaccurate or missing data for non-complex studies. Follows SOPs for quality assurance. Runs summaries and reports on existing data. Follows required processes, policies, and systems to ensure data security and provenance. In addition, recognizes and reports security of physical and electronic data vulnerabilities. Documents and maintains documentation to facilitate data sharing during publication or study closeout. For non complex protocols, identifies when various data standards should be used in creating eCRFs and EDCs and integrates as according to best practices. Knowledgeable about the use of data standard policies. Learns and uses new technology when required. Under supervision, assists in preparing tables, data visualizations, and lay summaries to communicate study results to participants. Assists in updating reports on study progress for the PI and other study team members and collaborators. Under supervision, executes testing process after the completion of a build, or following any project changes or system upgrades and may conduct some testing and documentation for Part 11 projects. Science: Assists with simple literature searches. Under guidance, develops elements of research protocols for simple studies (e.g., registries, survey studies). Provides some contribution to scientific publications or presentations (no authorship). Study and Site Management: As directed, attends or schedules site visits. Records participant accrual information and consent documentation for non-complex studies in clinical research management system. Records basic protocol information in clinical research management system. For studies with simple supplies or equipment, ensures that there are ample supplies and that equipment is in good working order. Follows protocol-specific systems and process flows. As directed, assists in preparing studies for closeout, (e.g., packing files, documenting files for storage, shipping extra supplies back to sponsor). Leadership: Works with the manager to understand areas of opportunity and develop a training plan. Takes training courses and applies the knowledge and skills. May also train others in the skills learned. Keeps current with research updates by attending key external departmental meetings (i.e. Research Wednesday, RPN, additional training, etc.). Demonstrates resilience and is adaptive to change. Communicates with other study personnel as required for study implementation and routine problem resolution. Description of Portfolio Responsibilities: (Effort .%): Description of Clinical Responsibilities: Clinical responsibilities: • Type of Research: This position will support the Connect and Grow study, the Clinical Decision Support study, and the Autism Caregiver Coaching in Africa Study within the Duke Center for Autism and Brain Development. Special skills: Preference for skills with data entry and Medical Terminology
Minimum Qualifications
Work requires an Associate's degree.
One year of relevant experience. A Bachelor's degree may substitute for required experience.
Duke is an Affirmative Action/Equal Opportunity Employer committed to providing employment opportunity without regard to an individual's age, color, disability, gender, gender expression, gender identity, genetic information, national origin, race, religion, sex, sexual orientation, or veteran status.
Duke aspires to create a community built on collaboration, innovation, creativity, and belonging. Our collective success depends on the robust exchange of ideas—an exchange that is best when the rich diversity of our perspectives, backgrounds, and experiences flourishes. To achieve this exchange, it is essential that all members of the community feel secure and welcome, that the contributions of all individuals are respected, and that all voices are heard. All members of our community have a responsibility to uphold these values.
Essential Physical Job Functions: Certain jobs at Duke University and Duke University Health System may include essential job functions that require specific physical and/or mental abilities. Additional information and provision for requests for reasonable accommodation will be provided by each hiring department.
Nearest Major Market: Durham Nearest Secondary Market: Raleigh
Duke is an Affirmative Action / Equal Opportunity Employer committed to providing employment opportunity without regard to an individual’s age, color, disability, gender, gender expression, gender identity, genetic information, national origin, race, religion, sex, sexual orientation, or veteran status. Read more about Duke’s commitment to affirmative action and nondiscrimination at hr.duke.edu/eeo.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 20, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Researchers uncover biological trigger of early puberty
by Alena Kuzub, Northeastern University

New research conducted by the Brenhouse Lab reveals how early life adversity triggers early puberty and late-life anxiety, paving the way for potential interventions.
The onset of puberty has been creeping downward for decades.
In the United States, the average age of girls reaching puberty ranges from 8.8 to 10.3 years old . The early start of puberty, which is associated with many health risks , can be triggered by chronic stress in children.
New research by Northeastern scientists published in Hormones and Behavior , has identified for the first time that early life stress affects the part of the brain—specifically, a protein in the membrane of a cell—responsible for preventing premature inception of puberty.
The brain receptor can suppress the release of hormones, or put the "brakes" on early puberty. The receptor malfunctions under chronic stress, unleashing a cascade of messaging that leads to early initiation of puberty, according to Northeastern researchers.
Children with early puberty are at risk of developing cancers of the reproductive tract, metabolic syndromes such as diabetes, cardiovascular disease, emotional and social problems later in adulthood, according to studies.
Researchers hope the findings will lead to medical interventions in the future.
"Early puberty is very important because it seems to be associated with later life psychopathologies like anxiety-related disorders," says Heather Brenhouse, professor of psychology at Northeastern University. "Physiological medical conditions also seem to be potentially linked to early puberty."
The biological mechanism of how early childhood stress leads to early puberty, Brenhouse says, had remained largely unknown.
The new research conducted by the Brenhouse Lab at Northeastern identified a receptor—a part of a brain cell that receives messages from another cell—in the hypothalamus, a region in the brain that controls many body functions via hormones.
The scientists knew from previous studies, Brenhouse says, that premature pubertal development in girls is associated with early life adversity and that early puberty predicts anxiety later in adolescence and adulthood.
They set out to confirm these findings and identify a biological trigger of early puberty in the brain.
Lauren Granata, a Northeastern graduate with a doctoral degree in psychology, co-authored the study and conducted the investigation in animal models. The idea of a stress triggering puberty, she says, seemed counterintuitive to her at first.
"It's pretty well understood nowadays that stress is dampening reproduction," Granata says. "I thought there was a lot of opportunity to find out something new."
First, the scientists confirmed the hypothesis that early childhood adversity indeed triggered early puberty in rats. Working with the animal model, Granata says, allowed them to isolate one specific factor—a disrupted relationship with the mother—aside from other factors such as nutrition, for example.
Of course, Granata says, what's happening in humans does not correlate one-to-one with the animal model, but it is good evidence that dysfunction of maternal care early in life may be one factor that's regulating early puberty.
"The way you can really traumatize a child or a developing rodent is by manipulating and disrupting the caretaker relationship," Brenhouse says.
Other childhood adverse experiences in humans, she says, could be neglect, resource scarcity and maltreatment.
To find a biomarker, a biological molecule in the brain whose state indicates early or normal puberty, Granata looked at the hypothalamus as it is widely known that it controls whether somebody is going to undergo puberty, among other important functions.
"There are cells that are basically activated, and they release certain proteins and peptides [hormones] that initiate puberty," Brenhouse says.
Granata found, she says, that those brain cells actually start expressing and releasing these proteins earlier in female rats that have been exposed to maternal separation. She has identified a specific receptor—CRH-R1—in the hypothalamus that suppresses preliminary puberty and gets affected by chronic stress stimulation.
"You can think of this as there's always a battle between a go signal and a stop signal [in the brain]," Granata says.
Stress hormones usually act as "brakes" on puberty, she says, because they cause the receptor CRH-R1 to suppress the release of hormones essential for puberty. So they hypothesized that it is not one stressful event but chronic stress that wears down puberty brakes, or reduces responsiveness of the receptor to stress hormones.
That unleashes a cascade of signaling in the brain and in the body.
"Now all of the go signals just have free reign to just say go ahead. It's time for puberty," Granata says.
The hypothalamus releases specific hormones that tell the system to release the brakes and produce estrogen and testosterone that are involved in the growth and maintenance of reproductive tissues.
The scientists did not observe accelerated puberty in male rats also exposed to maternal separation.
To study the relationship between adversity and childhood trauma and anxiety in adolescents and adults, the scientists used acoustic startle—startle noise bursts interrupting white noise background—on female rats after puberty. The experiment showed significant negative correlation between the age of puberty and the magnitude of response to the acoustic startle, which is associated with disorders.
A rat that had earlier puberty, Granata says, experienced higher levels of anxiety in adolescence.
She hopes these findings could be used to potentially create interventions and treatments for girls who are at a higher risk of anxiety and depression in adolescence and adulthood because of early puberty .
This story is republished courtesy of Northeastern Global News news.northeastern.edu .
Explore further
Feedback to editors

New technique detects novel biomarkers for kidney diseases with nephrotic syndrome
May 25, 2024

In experiments, mice get ill from raw milk carrying bird flu virus
May 24, 2024
Understanding a broken heart—study finds link between stress and recurrent heart failure

Genetic cause of rare childhood immune disorders discovered

New surgical tool moves tiny bioparticles with robotics and acoustic energy
The link between defective autophagy and pancreatitis could point to new treatments

Possible association between tattoos and lymphoma revealed

Walkability in neighborhoods linked to health, study of siblings shows

Scientists uncover new treatment pathway for rare 'spider web' childhood brain tumors
How COVID-19 'breakthrough' infections alter your immune cells
Related stories.

Study suggests earlier puberty onset may affect adult cardiometabolic health
Mar 27, 2024

Chronic stress during adolescence may reduce fertility in adulthood
May 10, 2024

Study bolsters evidence that effects of puberty blockers are reversible
Apr 8, 2024

Molecular link between body weight, early puberty identified
Oct 11, 2018

Study links blue light from smartphones or tablets to early puberty
Sep 25, 2023

Large-scale German study discovers earlier puberty onset in both girls and boys with diabetes
Sep 22, 2023
Recommended for you

Study suggests psychedelic drug-induced hyperconnectivity in the brain helps clarify altered subjective experiences

Study further illuminates ability of cancer drug to lower blood sugar
May 23, 2024

Two studies offer key insights into the origins and potential treatment of mental health disorders

Researchers unveil shared and unique brain molecular dysregulations in PTSD and depression

Exposure to endocrine-disrupting chemicals in utero associated with higher odds of metabolic syndrome in children

Study uncovers cell type-specific genetic insights underlying schizophrenia
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

An Open Comparative Study of the Effectiveness and Incomparable Study of the Immunogenicity and Safety of the Vaccine (CoviVac) for Adults Aged 60 Years and Older
- Study Details
- Tabular View
- No Results Posted

Inclusion Criteria:
Volunteers must meet the following inclusion criteria:
Type of participants
• Healthy volunteers or volunteers with a history of stable diseases that do not meet any of the criteria for non-inclusion in the study.
Other inclusion criteria
- Written informed consent of volunteers to participate in a clinical trial
- Volunteers who are able to fulfill the Protocol requirements (i.e., fill out a self-observation Diary, come to control visits).
Exclusion Criteria:
SARS-CoV-2 infection • A case of established COVID-19 disease confirmed by PCR and/or ELISA in the last 6 months.
Diseases or medical conditions
- Serious post-vaccination reaction (temperature above 40 C, hyperemia or edema more than 8 cm in diameter) or complication (collapse or shock-like condition that developed within 48 hours after vaccination; convulsions, accompanied or not accompanied by a feverish state) to any previous vaccination.
- Burdened allergic history (anaphylactic shock, Quincke's edema, polymorphic exudative eczema, serum sickness in the anamnesis, hypersensitivity or allergic reactions to the introduction of any vaccines in the anamnesis, known allergic reactions to vaccine components, etc.).
- Guillain-Barre syndrome (acute polyradiculitis) in the anamnesis.
- The axillary temperature at the time of vaccination is more than 37.0 ° C.
- Acute infectious diseases (recovery earlier than 4 weeks before vaccination) according to anamnesis.
- Donation of blood or plasma (in the amount of 450 ml or more) less than 2 months before inclusion in the study.
- Severe and/or uncontrolled diseases of the cardiovascular, bronchopulmonary, neuroendocrine systems, gastrointestinal tract, liver, kidneys, hematopoietic, immune systems.
- Is registered at the dispensary for tuberculosis, leukemia, oncological diseases, autoimmune diseases.
- Any confirmed or suspected immunosuppressive or immunodeficiency condition in the anamnesis.
- Splenectomy in the anamnesis.
- Neutropenia (decrease in the absolute number of neutrophils less than 1000/mm3), agranulocytosis, significant blood loss, severe anemia (hemoglobin less than 80 g/l) according to anamnesis.
- Anorexia according to anamnesis.
Prior or concomitant therapy
- Vaccination with any vaccine carried out within 30 days before vaccination / the first dose of the studied vaccine or planned administration within 30 days after vaccination / the last dose of the studied vaccine.
- Prior vaccination with an experimental or registered vaccine that may affect the interpretation of the study data (any coronavirus or SARS vaccines).
- Long-term use (more than 14 days) of immunosuppressants or other immunomodulatory drugs (immunoregulatory peptides, cytokines, interferons, immune system effector proteins (immunoglobulins), interferon inducers (cycloferon) during the six months preceding the study, according to anamnesis.
- Treatment with systemic glucocorticosteroids (≥ 20 mg of prednisone, or an analog, for more than 15 days during the last month).
- Volunteers who received immunoglobulin preparations or blood transfusion during the last 3 months prior to the start of the study according to anamnesis.
Other non-inclusion criteria
• Participation in any other clinical trial within the last 3 months.
Exclusion criteria:
- Withdrawal of Informed consent by a volunteer;
- The volunteer was included in violation of the inclusion/non-inclusion criteria of the Protocol;
- Any condition of a volunteer that requires, in the reasoned opinion of a medical researcher, the withdrawal of a volunteer from the study;
- Taking unauthorized medications (see section 6.2);
- The volunteer refuses to cooperate or is undisciplined (for example, failure to attend a scheduled visit without warning the researcher and/or loss of communication with the volunteer), or dropped out of observation;
- For administrative reasons (termination of the study by the Sponsor or regulatory authorities), as well as in case of gross violations of the Protocol that may affect the results of the study.
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services
First Deputy Minister --- RYABEV, Lev Dmitriyevich (Izvestiya, 23 May 02). Deputy Minister (Nuclear Weapons Complex) --- KAMENSKIKH, Ivan (Izvestiya, 19 Mar 03). Deputy Minister --- NIGMATULIN, Bulat, ( Interfax 8 Dec 01). Deputy Minister --- VINOGRADOV, V. (Rossiyskaya Gazeta, 4 Apr 00) Deputy Minister --- ANTIPOV, Sergey Viktorovich, appointed (Nuclear.ru, 15 Nov 02).
- ["Antipov was born in 1948, graduated from the Moscow Physical Engineering Institute (1972), and is a physicist and engineer (experimental and theoretical physics, plasma physics). In 1996 he graduated from the Moscow State Law Academy as a lawyer specializing in jurisprudence. Prior to being appointed to the post of deputy minister he headed the Center for Organizational and Legal Issues at the Kurchatovskiy Institut Russian Scientific Center. As director and coordinator of the project on nonproliferation and nuclear weapons control in the sphere of the record keeping and control of nuclear materials, and of systems for the physical protection of nuclear installations, he participated in regular Russian-American working conferences. . . .the main areas of his activity . . . will be the recycling of nuclear submarines, the decommissioning of nuclear reactors, the management of radioactive wastes, and the restoration of land" (Nuclear.ru, 15 Nov 02).]
Deputy Minister (States Secretary) --- LEBEDEV, Valeriy Aleksandrovich. (Atompressa, No 32 Sep 99; Appointed "States Secretary," Rossiyskaya Gazeta, 12 Sep 01).
- Formerly: General Director of the Mining and Chemical Combine, Zheleznogorsk. (Atompressa, No 32 Sep 99)
Accounting and Control of Nuclear Materials and the Provision of Guarantees for their Non-proliferation and Physical Protection, Directorate for the Supervision of
Chief --- Volodin, Yuriy Georgiyevich. Telephone: (095) 9116081.
Central Apparatus
Crisis situation center.
- (Izvestiya 22 May 01).
Department for Liaison with State Power Bodies and Information Policy
- Head -- Nikolay Shingarev (ITAR-TASS, 22 Jan 03).
Environment and Decommissioning of Nuclear Facilities, Administration for
- ["The responsibility for the recycling of nuclear submarines was delegated from the Defense Ministry to the Atomic Energy Ministry only in 1998, and the technical bases of the Northern and Pacific Fleets are currently being handed over to the Atomic Energy Ministry's special enterprises SevRao and DalRao. Notably, the military are quite reluctantly parting with their property because those bases are financed from the state budget. So, Admiral Popov will now be supposed to settle down relations between his former and present co-workers" (Kommersant 05 Dec 01 P2).]
Chief --- POPOV, Vyacheslav, Adm appointed (Kommersant 05 Dec 01 P2). Popov was CINC NORFLT.
Experimental Physics, RF Nuclear Center All-Russian Scientific-Research Institute for (RFYaTs VNIIEF) (Sarov)
- Previously known as Arzamas-16, Nizhegorod Oblast
Director --- Ilkayev, Radiy (Finmarket, 21 Apr 01).
Federal Inspectorate for Nuclear and Radiation Security
- Address: 109147 Moscow, Taganskaya Street, 34.
Director --- Vishnevskiy, Yuriy Georgiyevich , (Chief of Directorate). Telephone: (095) 9116005. Fax: (095) 9124041
Fuel Production Cycle, Directorate for the Supervision of Nuclear and Radiation Security of the Enterprises of the
Chief --- Kislov, Andrey Ivanovich Telephone: (095) 9116061.
Nuclear and Radiation Security for Atomic Stations, Directorate for the Supervision of
Chief --- Adamchik, Sergey Anatolyevich Telephone: (095) 9116061.
Nuclear and Radiation Security in the Economy, Directorate for the Supervision of
Chief --- Mikhaylov, Mikhail Vladimirovich. Telephone: (095) 9116071.
Nuclear and Radiation Security of Research Reactors and Nuclear Power Facilities on Ships, Directorate for the Supervision of
Chief of Directorate --- Nikolskiy, Rostislav Viktorovich Telephone: (095) 9116011
Scientific and Technological Council
Security Section
Chair --- Litvinov, Boris, Academician (ITAR-TASS 1404 GMT 18 Sep 00).
- Litvinov, who chairs the security section under the Atomic Energy Ministry's Scientific and Technological Council, works as deputy head of the Russian Federal Nuclear Centre and was the chief designer of Russian nuclear warheads for many years. (ITAR-TASS 1404 GMT 18 Sep 00).
Structural Directory of Russia's Nuclear Sector
(<Nuclear.ru>, 1 Dec 00, [Source date uncertain. Date given is the date of download from the internet])
[FBIS Translated Text]
The Ministry of Atomic Energy of the Russian Federation. Structure of the Ministry of Atomic Energy.
1. Nuclear Fuel Cycle, Department of the
2. nuclear munitions, department for the development and testing of, 3. nuclear munitions industry, department of the, 4. atomic power engineering, department for, 5. branch economics and planning, department of,, 6. social policy, production relations, and personnel, department of, 7. security and emergency situations, department for, 8. international economic cooperation, department for, 9. atomic science and technology, department for, 10. finance, analysis, and computation department for, 11. information, nuclear materials, and facilities, department for the protection of, 12. atomic facilities construction, department for, 13. normative-legal security and regulation of forms of property, department for, 14. department for the conversion of atomic industry., 15. directorate of bookkeeping and accounts., 16. social-productive directorate., 17. directorate for business and protocol of the ministry., 18. directorate for the optimization of productive-economic relations., 19. directorate for the security of the activities of the scientific and scientific-technological councils., 20. directorate for ecology and the removal of nuclear facilities from operation., 21. directorate for atomic machine-building and instrument-making., scientific-technical directorate. aleksandr anatolyevich.
Chief --- Matveyev, ?? Telephone: (095) 9116411. Office: Telephone (095) 9123911. Fax: (095) 9124041.
Professional emergency-rescue units of the Ministry of Atomic Energy of Russia:
"Eprom", the center for emergency-rescue underwater-technical operations.
Address: 143392 Moscow oblast., Naro-Fominskiy rayon, Selyatino. Director: Mikhail Nikolayevich Gumenok, Chief of Center. Telephone: (812) 2475669. Fax: (812) 2475798. Web: http:
www.atom.nw.ru
The Emergency-Technical Center (Novovoronezh).
Address: 396072 Voronenezh oblast., Novovoronezh. Director: Ivan Ivanovich Burdin, Director of Center. Telephone: (07364) 20268. Fax: (07464) 20268.
Emergency-Technical Center (Sarov).
Address: 607200 Nizhegorodod oblast, Sarov. Director: Vladislav Viktorovich Kuznetsov, Director of Center. Telephone: (83130) 45708. Fax: (83130) 45979.
Emergency-Technical Center (Snezhinsk)
Address: 456770 Chelyabinsk oblast., Snezhinsk. Director: Lev Vladimirovich Borisov, Director of Center. Telephone: (35172) 32424.
Emergency-Technical Center (Seversk).
Address: 636070 Tomsk oblast, Seversk Director[Sic. No director listed].
Telephone: (38242) 62693. Fax: (38242) 21146.
Gas-Rescue Station of the Joint-Stock Company, "Kirovo-Chepetsk Chemical Combine".
Address: 631020 Kirov oblast., Kirovo-Chepetsk, Pozharnyy Lane., 7. Director: Nikolay Ivanovich Aprin. Telephone: (83361) 94204. Fax: (30245) 25121.
Separate Militarized Mine-Rescue Detachment of the Priargunskiy Mining-Chemical Production Association.
Director: Vladimir Georgiyevich Bondarev, Chief of Detachment. Telephone: (30245) 25781. Fax: (30245) 25121.
Engineering-Technical and Training Center for Robotics.
Address: 127410 Moscow, Altufyev Highway, 43. Director: Nikolay Aleksandrovich Sidorkin, Director of Center. Telephone: (095) 4899032. Fax: (095) 4899032. [Sic: Telephone and fax numbers are the same.]
3. Closed Administrative-Territorial Formations.
Zhelznogorsk (Krasnoyarsk-26).
Address: 660026 Krasnoyarsk kray. Zhelznogorsk, XXII Partsyezd Street, 21. Director: Andrey Vasilyevich Katargin, Head of Administration. Telephone: (39197) 26048. Web: http:
www.adm26.krasnoyarsk.su Population: About 105,000.
The Mining-Chemical Combine.
Address: 660033 Krasnoyarsk kray, Zheleznogorsk, Lenin Street, 53. Director: Vasiliy Vasilyevich Zhitkov, General Director. Telephone: (39197) 32001, 32290. Fax: (39197) 320374
Science and Production Association of Applied Mechanics.
Address: 660033 Krasnoyarsk kray, Zheleznogorsk, Lenin Street, 52. Director: Mikhail Fedorovich Reshetnev. Telephone: (39197) 21759, 32032 Fax: (39197) 22635
Construction-Industrial Joint-Stock Company, "Sibkhimstroy".
Address: 660033 Krasnoyarsk kray, Zheleznogorsk, Shtefan Street, 1. Director: Vladimir Mikaylovich Kiyayev. Telephone: (39197) 22076. Fax: (39197) 29839.
"Sibkhimmontazh" Trust.
Address: 660033 Krasnoyarsk kray, Zheleznogorsk, Severnaya Street, 9. Director: Viktor Chukhno Mikhaylovich. Telephone: (39197) 26802.
Krasnoyarskiy State Design and Research Institute. Address: 660026 Krasnoyarsk kray. Zheleznogorsk, Lenin Street, 39. Director: Yuriy Nikolayevich Baskakov. Telephone: (39197) 25625. Fax: (39197) 25625.
Krasnoyarsk Industrial College. Address: 662990 Krasnoyarsk kray, Zheleznogorsk, Sverdlov Street, 5. Director: Yevgeniy Borisovich Vasilyev. Telephone: (39197) 23944.
Zarechnyy (Penza-16). Address: 440901 Penza oblast, Zarechnyy, 30-th Anniversary of Pobeda [Victory] Street, 27. Director: Vyacheslav Vasilyevich Sergeyev, Head of Administration. Telephone: (8412) 664988. Fax: (8412) 664979. Web: http:
zarechnyy.penza.ru Population: About 65,000.
Production Association, "Start", a State enterprise. Address: 440901 Penza oblast, Zarechnyy, Mir Avenue, 1. Director: Anatoliy Andreyevich Yesin, General Director. Telephone: (8412) 550907. Fax: (8412) 665 887.
Open-Type Joint-Stock Company, "Penza Directorate of Construction". Address: 440019 Penza oblast, Zarechnyy, Komsomol Street, 41. Director: Nikolay Semenovich Konolenko. Telephone: (8412) 550907. Fax: (8412) 550973.
State Unitary Branch SNPO [Special Science and Production Association], "Yeleron". (Scientific- Research and Design Institute of Radio- Electronic Engineering.) Address: 440901 Penza oblast, Zarechnyy, Mir Avenue, 1. Director: Yuriy Aleksandrovich Olenin. Telephone: (8412) 692474. Fax: (8412) 552528.
Central Scientific Research Laboratory. Address: 440901 Penza oblast, Zarechnyy, Mir Avenue, 1. Director: Vladimir Mikhaylovich Sorokin. Telephone: (8412) 692706.
Zarechnyy Industrial College (a municipal educational establishment). Address: 440901 Penza oblast, Zarechnyy, Lenin Street, 10. Director: Vasiliy Georgiyevich Zelenskiy. Telephone: (8412) 692182.
Zelenogorsk (Krasnoyarsk-45). Address: 663690 Krasnoyarsk kray, Zelenogorsk, Mir street, 15. Director: Valentin Grigoryevich Kazachenko, Head of Administration. Telephone: (39169) 35532. Fax: (39169) 35640, 35993. Population: About 65,000.
Electro-Chemical Plant. Address: 663690 Krasnoyarsk kray, Zelenogorsk. Director: Anatoliy Nikolayevich Shubin, General Director. Telephone: (39169) 33350, 33321. Fax: (39169) 24225, 21262. Teletype: 288 845 "Tayfun".
Joint-Stock Company, "Sibvolonko". Address: 663960 Krasnoyarsk kray, Zelenogorsk, Joint-Stock Company, "Sibvolonko". Director: Petr Pavlovich Kostyushko. Telephone: (39169) 23020, 24405. Fax: (39169) 28122, 24087. Telex: 288 145 SV SU.
Contruction-Industrial Joint-Stock Company, "Directorate of Construction No. 604". Address: 663960 Krasnoyarsk kray, Zelenogorsk, Kalinin Street, 25. Director: Anatoliy Yakovlevich Kurdyukov. Telephone: (39169) 35722. Fax: (39169) 24940.
Krasnoyarsk Electro-Mechanical Engineering College. Address: 663690 Krasnoyarsk kray, Zelenogorsk, Bortnikov Street, 13. Director: Grigoriy Antipyevich Porsyev. Telephone: (39169) 34433.
Lesnoy (Sverdlovsk-45). Address: 624200 Sverdlovsk oblast, city of Lesnoy, Karl Marks [Marx] Street, 8. Director: Aleksandr Ivanovich Ivannikov, Head of Administration. Telephone: (34342) 57509. Fax: (34342) 24402. Population: 55,000.
Fiftieth Anniversary of the USSR Combine, "Elektrokhimpribor". Director: Leonid Aleksyevich Polyakov, General Director. Telephone: (34342) 24373. Teletype: 721 549 "Kedr".
Construction-Industrial Joint-Stock Company, "Severouralskoye Directorate of Construction". Address: 620045 Sverdslovsk oblast, city of Lesnoy, Lenin Street, 76. Director: Vladimir Timofeyevich Nesterenko. Telephone: (34342) 69271. Fax: (34342) 24054, 24681.
Polytechnic Institute of Moscow State Engineering-Physical Institute. Address: 620045 Sverdlovsk oblast, city of Lesnoy, Kommunist Avenue, 36. Director: Vyacheslav Mikhaylovich Krapal, Rector. Telephone: (34342) 60693. Fax: (34342) 55621.
Novouralsk (Sverdlovsk-44). Address: 624130 Sverdlovsk oblast, city of Novouralsk, Michurin Street, 33. Director: Valentin Yegorovich Feldman, Head of Administration. Telephone: (34370) 23260. Web: http:
www.novouralsk.ru Population: 95,000.
Ural Electro-Chemical Combine. Address: 624130 Sverdlovsk oblast, city of Novouralsk, Dzerzhinskiy Street, 2. Director: Anatoliy Petrovich Knutarev, General Director. Telephone: (34370) 92424. Fax: (34370) 94141. Telex: 721 742 RIKON SU. Web: http:
www.ricon.e-burg.ru Teletype: 348823, 348813 "Tiris".
Construction-Industrial Joint-Stock Company, "Sredneural Directorate of Construction". Address: 620114 Sverdlovsk oblast, city of Novouralsk, Dzerzhinskiy Street, 13. Director: Petr Ivanovich Bokov. Telephone: (34370) 24961. Fax: (34370) 40977.
Novouralsk Polytechnic Institute of Moscow State Engineering-Physics Institute. Address: 624130 Sverdlovsk oblast, city of Novouralsk-3, Lenin Street, 85. Director: Aleksey Petrovich Dyagilev, Rector. Telephone: (34370) 23580.
Ural Institute for the Advancement of Qualification, "Progress". Address: 624133 Sverdlovsk oblast, city of Novouralsk, Pervomayskaya Street, 107. Director: Valeriy Ivanovich Makarov. Telephone: (34370) 91601. Fax: (34370) 91354. Web: http:
www.aib.ru/~uipk
/ Ural Polytechnic College. Address: 624130 Severdlovsk oblast, Novouralsk-3, Lenin Street, 85. Director: Leonid Nikolayevich Mochalov. Telephone: (34370) 22497.
Sarov (Arzamas-16). Address: 607200 Nizhegorod oblast, Sarov. Director: Gennadiy Zakirovich Karatayev. Telephone: (83130) 11303. Fax: (83130) 58789. Population: 85,000.
The All-Russian Scientific-Research Institute of Experimental Physics of the Russian Federal Nuclear Center. Address: 607190 Nizhegorod oblast, Sarov, Mir Avenue, 37. Director: Radiy Ivanovich Ilkayev. Telephone: (83130) 56951, 11264, 11803. Fax: (83130) 53808, 54565. Telex: 151 109 APCA Web: http:
www.vniief.ru
Electro-Mechanical Plant, "Avangard". Address: 607200 Nizhegorod oblast, City of Sarov, Yuzhnoye, area 6. Director: Yuriy Kuzmich Zavlishin. Telephone: (83130) 11077.
Sarov Construction-Industrial Joint-Stock Company. Address: 607200 Nizhegorod oblast, Sarov, Silkin Street, 13. Director: Georgiy Georgiyevich Bulgakov. Telephone: (83130) 45994.
State Unitary Scientific-Technical Enterprise, "Efkon". Address: 601190 Nizhegorod oblast, Sarov, Yunost Street, 22. Director: Anatoliy Petrovich Inozemtsev. Telephone: (83130) 45705. Fax: (83130) 45994..
Central Laboratory for the Protection of the Environment. Address: 607190 Nizhegorod oblast, Sarov, P. O. Box [Russian: a/ya--abonenenitnyy yashchik] 423. Director: Vasiliy Dmitriyevich Davidyuk. Telephone: (83130) 45288. Fax: (83130) 45288. [Sic. Fax no. is same as tel. no.].
Sarov Physics-Technical Institute of Moscow State Engineering Physics Institute. Address: 607200 Nizhegorod oblast, Sarov, Dukhov Street, 6. Director: Yuriy Petrovich Shcherbak, Rector. Telephone: (83130) 59809.
Seversk (Tomsk-7). Address: 636070 Tomsk oblast, city of Seversk, Kommunist Street, 51. Director: Nikolay Ivanovich Kuzmenko, Head of Administration. Telephone: (3822) 222159, 774760. Fax: (3822) 776728. Web: http:
www.seversk.ru Population: About 110,000.
Siberian Chemical Combine. Address: 636070 Tomsk oblast, city of Seversk, Kurchatov Street, 1. Director: Valeriy Konstantinovich Larin, General Director. Telephone: (3822) 771798. Fax: (3822) 772528. Web: http:
www.shk.tsk.ru
Construction-Industrial Joint-Stock Company, "Khimstroy". Address: 636070 Tomsk oblast, city of Seversk, Transport Street, 32. Director: Gennadiy Sergeyevich Molokanov, General Director. Telephone: (3822) 776460. Fax: (3822) 241520.
Open-Type Joint-Stock Company, "Promekhanomontazh". Address: 636070 Tomsk oblast, city of Seversk, P. O. Box 494. Director: Anatoliy Vladimirovich Maksimenko, General Director. Telephone: (3822) 784900. Fax: (3822) 241520.
Joint-Stock Company, "Spetskhimmontazh". Address: 636070 Tomsk oblast, city of Seversk, P. O. Box 563. Director: Boris Alekseyevich Kormashov. Telephone: (3822) 777932. Fax: (3822) 776939.
Tomsk State Design-Research Institute, VNIPIET [All-Russian Design and Scientific-Research Institute of Power Technology] Address: 634039 Tomsk oblast, Seversk, Kurchatov Street, 2. Director: Mishin Vitaliy Alekseyevich. Telephone: (3822) 760913. Fax: (3822) 760913 [Sic. Fax no. is same as tel. no.].
Siberian Branch of "GNTs RF VNIINM" [State Science Center of the Russian federation, All- Russian Scientific-Research Institute of Inorganic Materials]. Address: 634018 Tomsk oblast, Seversk, Lermontov Street, 13. Director: Aleksandr Yakovlevich Svarovskiy. Telephone: (38242) 61880. Fax: (3822) 776739.
Seversk Technological Institute of Tomsk Polytechnic University. Address: 634036 Tomsk-36, Kommunist Avenue, 65. Director: Aleksandr Nikolayevich Zhiganov, Rector/ Telephone: (3822) 779529.
Seversk Industrial College (a municipal educational establishment). Address: 636070 Tomsk oblast, Seversk, Kommunist Avenue, 65. Director: Aleksandr Nikolayevich Zhiganov. Telephone: (3822) 779529.
Snezhinsk (Chelyabinsk-70). Address: 454070 Chelyabinsk oblast, city of Snezhinsk, Sverdlov Street, 24. Director: Anatoliy Vladimirovich Oplanchuk, Head of Administration. Telephone: (35172) 32573. Fax: (35172) 32385. Population: 50,000.
The All-Union Scientific Research Institute of Technical Physics--the Russian Federal Nuclear Center. Address: 456770 Chelyabinsk oblast, city of Snezhinsk, P. O. Box 245. Director: Georgiy Nikolayevich Rykovanov. Telephone: (35172) 32028. Fax: (35172) 32351. Telex: 124 846 SNOW SU. Web: http:
www.vniitf.ru
Snezhinsk Physics-Technical Institute--a branch of Moscow State Engineering-Physics Institute. Address: 456776 Chelyabinsk oblast, city of Snezhinsk, Mir Street, 6/8. Director: Vladimir Makarovich Skovpen, Rector. Telephone: (35172) 32422. Fax: (35172) 32526.
Trekhgornyy (Zlatoust-36). Address: 456080 Chelyabinsk oblast, city of Trekhgornyy, Mir Street, 6. Director: Nikolay Andreyevich Lubenets. Telephone: (351112) 7001. Fax: (351112) 7042. Population: About 40,000.
Instrument-Making Plant. Address: 456080 Chelyabinsk oblast, city of Trekhgornyy, Instrument-Making Plant. Director: Aleksandr Vasilyevich Dolinin, General Director. Telephone: (351112) 5121, 5123, 5210. Fax: (351112) 1622.
Design-Construction-Industrial Joint-Stock Company, "Ural". Address: 456080 Chelyabinsk oblast, city of Trekhgornyy, Street Stroiteley [Street of the Builders], 6. Director: Galina Ivanovna Morozova. Telephone (35111) 67061. Fax: (35111) 651120.
Trekhgornyy Polytechnic Institute of Moscow State Engineering-Physics Institute. Address: 456080 Chelyabinsk oblast, city of Trekhgornyy, Mir Street, 17. Director: Fedor Ivanovich Dolinin. Telephone: (35111) 67067.
Ozersk (Chelyabinsk-65). Address: 456784 Chelyabinsk oblast, city of Ozersk, Lenin Avenue, 30a. Director: Sergey Georgiyevich Chernyshev. Head of Administration. Telephone: (35171) 78959. Population: About 100,000.
Production Association, "Mayak". Address: 456065 Chelyabinsk oblast, city of Ozersk, Lenin Avenue, 31. Director: Vitaliy Ivanovich Sadovnikov, General Director. Telephone: (35151) 31659. Fax: (35171) 73911. Telex: 124 864 ATOM SU.
Closed-Type Joint-Stock Company, "Yuzhno-Ural Directorate of Construction". Address: 456064 Chelyabinsk oblast, city of Ozersk, Oktyabr Street, 7. Director: Aleksey Georgiyevich Belotnitskiy. Telephone: (35171) 45253. Fax: (35171) 73911.
Open-Type Joint-Stock Company, "Uralprommontazh", an installation-industrial company. Address: 456065 Chelyabinsk oblast, city of Ozersk, Semenov Street, 22. Director: Sergey Vladimirovich Voloshin. Telephone: (35171) 79250. Fax: (35171) 22214.
Joint-Stock Company, "Uralgidromontazh" Address: 456065 Chelyabinsk oblast, city of Ozersk, Semenov street, 22. Director: Anatoliy Ivanovich Loboda. Telephone: (35171) 79303. Fax: (35171) 73041.
Ural State Design-Research Institute, "VNIPIET" [see expansion of acronym above]. Address: 454065 Chelyabinsk oblast, Ozersk, Oktyabr Street, 11. Director: Leonid Vladimirovich Vaganov. Telephone: (35171) 79550. Fax: (35171) 73822.
Ozersk Technological Institute of Moscow State Engineering-Physics Institute. Address: 456783 Chelyabinsk oblast, city of Ozersk, Pobeda Avenue, 48. Director: Yuriy Nikolayevich Stepanov. Telephone: (35171) 446646
Yuzhnoural Polytechnic College. Address: 456783 Chelyabinsk oblast, Ozersk, Pobeda Avenue, 48. Director: Angelina Valentinovna Tomanovna. Telephone: (35171) 44623.
4. The "Rosenergoatom" Concern, the atomic electrical power stations and "Rosenergoatom" Concern enterprises.
State Concern, "Rosenergoatom". Address: 101000 Moscow, P. O. Box 912. Director: Erik Nikolayevich Pozdyshev. Telephone: Chancellery: (095) 239740. Fax: Chancellery: (095) 2392724. Web: http:
www.rosatom.ru
Structure of "Rosenergoatom" Concern. Department for the Utilization of Atomic Power Stations and Water-Cooled Reactors. Department for the Utilization of Atomic Power Stations with Fuel-Channel-Type and Fast Reactors. Department for Technical Servicing and Repair of Atomic Power Stations. Department for Technical Inspection and Control and Monitoring of Security. Department for the Licensing of the Utilization of Atomic power Stations. Management for Protection of Nuclear Facilities and Materials. Department for Emergency Control and Prevention of Accidents. Department for Scientific-Technical Support. Structural Design Department. Management for Expertise and Prospective Technologies. Management for Major Construction. Management for Provision of Utilization and Repair. Management for Provision of Nuclear Fuel. Management for Investment Resources. Department of Economics. Department of Finances. Management for Property and Capital. Department for Book-Keeping and Accounting. Department for Auditing Activities. Management for Inspection Work. Legal Department. Department for International Activities. Management of Export. Management of FOREM [Federal Wholesale Market for Electricity and Generating Capacity].. Department for Sales. Directorate of Business Affairs.
Atomic power stations:
Balakov Atomic Power Station. Beloyarsk Atomic Power Station. Bilibin Atomic Power Station. Kalinin Atomic Power Station. Kolsk Atomic Power Station. Kursk Atomic Power Station. Leningrad Atomic Power Station. Novovoronezh Atomic Power Station. Smolensk Atomic Power Station.
Enterprises included in the structure of the "Rosenergoatom" Concern. Branch State Unitary Enterprise, "Energoatomfinans". Address: 123298 Moscow, Narodnoye Opolcheniye Street, 40, building 2. Director: Mikhail Vasilyevich Komissarov. Telephone: (095) 1926174. Fax: (095) 1926174. [Sic: Fax no. is same as tel. no.].
Branch State Unitary Enterprise, "Atomstroyinvest". Address: 123298 Moscow, Narodnoye Opolcheniye Street, 40, building 2. Director: Vitaliy Vladimirovich Veselov. Telephone: (095) 1926029. Fax: (095) 1926029.[Sic: Fax no. is same as tel. no.].
Branch State Unitary Enterprise "Kontrolno-Priemochnaya Inspektsiya". Address: 101000 Moscow, P. O. Box 912. Director: Vladimir Ilich Nikitenko. Telephone: (095) 2392436. Fax: (095) 2392436. [Sic. Fax no. is same as tel. no.].
Branch State Unitary Enterprise, "Press-Tsentr Kontserna". Address: 109507 Moscow, Ferganskaya Street, 25. Director: Andrey Grigoryevich Polous. Telephone: (095) 2384894. Fax: (095) 2392016.
Branch State Unitary Enterprise, "Atom-Servis". Address: 123298 Moscow, Narodnoye Opolcheniye Street, building 2. Director: Grigoriy Alekseyevich Serafimov. Telephone: (095) 1927674.
Branch State Unitary Enterprise, "Kursk Specialized Installment Directorate".". Address: 307239 Kursk oblast, Kurchatov. Director: Nikolay Maksimovich Kovalev. Telephone: (07131) 46395. Fax: (07131) 46395. [Sic: Fax no. is same as tel. no.].
(5). "TVEL" [Fuel Element] Concern. Open Joint-Stock Company, "TVEL".. Address: 101000 Moscow, B. Ordynka Street, 24/26. Director: Vitaliy Fedorovich Konovalov, President. Petr Ivanovich Lavrenyuk, Vice President. Leonid Dmitriyevich Proskuryakov, Vice President. Vladimir Alekseyevich Zubakov, Vice President. Vyacheslav Mikhaylovich Kozhin, Vice President. Telephone: (095) 2394440. Fax: (095) 2394404. Web: http:
www.tvel.ru
/ Structure:
Management for Commerce. Management for Productive-Technical Issues and Security. Management for Scientific-Technical Development. Management for Economics and Corporative Planning. Management for Economic Strategy and Control of Property. Management for International and External Economic Activities. Management for Financial Accounts and Analysis. Management for General Issues and Social Development. Legal Section.
Open Joint-Stock Company, "TVEL Concern". Address: 101000 Moscow, B. Ordynka Street, 24/26. Director: Vladimir Vladimirovich Karetnikov, General Director. Telephone: (095) 2394788.
Trans-Baykal Mining-Concentrating Combine (ZabGOK).
Address: 673382 Chitin oblast, Pervomayskiy Square, Mir Street,18. Director: Gennadiy Mikhaylovich Adosik. Telephone: (302) 42302. Fax: (30262) 41010.
Krasnoyarsk Chemical-Metallurgical Plant (KKhMZ). Address: 660079 Krasnoyarsk, Matrosov Street, 30. Director: Dmitriy Mikhaylovich Kovyadin. Telephone: (3912) 660185. Fax: (3912) 340939.
Chepetsk Mechanical plant (ChMZ). Address: 427000 Respublika Udmurtiya, city of Glazov, Belov Street, 7. Telephone: (34141) 72415. Fax: (34141) 72994. Web: http:
www.chmz.udm.net
/ Moscow Plant of Polymetals (MZP).
Address: 115409 Moscow, Kashirskoye Highway, 49. Director: Valeriy Viktorovich Kryukov, General Director. Telephone: (095) 3247234. Fax: (095) 7428298.
Novosibirsk Plant of Chemical Concentrates (NZKhK). Address: 638038 Novosibirsk, B. Khmelnitskiy Street, 94. Director: Vladimir Leonidovich Afanasyev. Telephone: (3832) 748454. Fax: (3832) 743071.
Machine-Building Plant (MSZ). Address: 144001 Moscow oblast, city of Elektrostal, K. Marks [Marx] Street, 12. Director: Valeriy Alekseyevich Mezhuyev, General Director. Telephone: (095) 7029901. Fax: (095) 7029221.
Production Association, "Ulbinskiy Metallurgical Plant". Address: 492026 Respublika Kazakhstan, city of Ust-Kamenogorsk, Shkolnoye Highway, 102. Director: Vitaliy Grogoryevich Khadeyev, General Director. Telephone: (3232) 475043. Fax: (3232) 640683.
Joint-Stock Company "Commercial Center, 100". Address: 127253 Moscow, Dmitrovskoye Highway, 116, building 3. Director: Aleksey Vasilyevich Goncharov. Telephone: (095) 4007474. Fax: (095) 4007277.
(6). Joint-Stock Company "Atomredmetzoloto" (enterprise). Address: 109017 Moscow, B. Ordynka Street, building 24/26. Director: Vyacheslav Vladimirovich Krotkov, General Director. Telephone: 2394668 (reception room). Fax: 2394679. Director Vitaliy Vasilyevich Shatalov--2392174. Director Yuriy Vasilyevich Nesterov--2394775. Director: Boris Fedorovich Shevchenko--2392430.
Open-Type Joint-Stock Company, "Priargunskoye Proizvodstvennoye Gorno-Khimicheskoye Obyedineniye" [Priargunskiy Mining-Chemical Production association] (AOOT "PPGKhO"). Address: 674665 Chitin oblast, city of Krasnokamensk. Director: Valeriy Konstantinovich Larin, General Director. Telephone: (30245) 46911. Fax: (86535) 50131.
Lermontov State Enterprise, "Almaz" Address: 357340 Stavropol kray, city of Lermontov, Promyshlennaya Street, building 7. Director: Nikolay Nikolayevich Gridin, General Director. Telephone: (86535) 50131. Fax: (86535) 52313.
State Unitary Enterprise, "Gidrometallurgical Zavod" [Hydro-Metallurgical Plant] (GUP "GMZ"). Address: 357340 Stavropol kray, city of Lermontov, Promyshlennaya Street, building 7. Director: Sergey Vasilyevich Pashkov, General Director. Telephone: (86535) 52313. Fax: (86535) 52313 [Sic. Fax no. is same as telephone number].
State Unitary Enterprise, "Electromekhanicheskiy Zavod" [Electro-Mechanical Plant]. Address: 357340 Stavropol kray, city of Lermontov, Promyshlennaya Street, building 7. Director: Valeriy Ivanovich Minenkov. Telephone: (86535) 50350. Fax: (34365) 52077.
Rodniki Directorate of Enterprises. Address: 155240 Ivanov oblast, city of Rodniki. Director: Nikolay Petrovich Vorobyev. Telephone: (09336) 23464. Fax: (09336) 23464 [Sic. Fax no. is same as telephone no.].
Yekaterinburg Plant, "Kauchuk". Address: 620023 city of Yekaterinburg, Garshin Street, building 7. Director: Aleksandr Vasilyevich Svytko. Telephone: (3432) 254905. Fax: (3432) 250262.
Joint-Stock company "Nauchno-Issledovatelskoye Eksperimentalnoye Predpriyatiye" [Scientific- Research Experimental Enterprise] (AO "NIEP"). Address: 143392 Moscow oblast, Narofominskiy rayon, settlement of Selyatino. Director: Vladimir Nikolayevich Kuznetsov. Telephone: (095) 4365697. Fax: (095) 4365697 [Sic. Fax no. and tel. no. are the same].
Federal State Enterprise, "Ekspeditsiya No. 2" [Expedition No. 2]. Address: 163056 Arkhangelsk-56. Director: Anatoliy Andreyevich Gerasimov.
Joint-Stock Company, "Severalmaz". Address: 163061 city of Arkhangelsk, Sadovaya Street, building 2 Director: Vitaliy Sergeyevich Fertygin, General Director. Telephone: (8182) 496112. Fax: (8182) 496112. [Sic. Fax no. is same as tel. No.].
Production Association, "Vostokredmet". Address: 735730 Respublika Tadzhikistan, Leninabad oblast, city of Chkalovsk, Oplanchuk Street, 10. Director: Zafar Abdukkakhorovich Razykov, General Director. Telephone: (37771) 54434. Fax: (37771) 50945.
Navon Mining-Metallurgical Combine. Address: 706800 Respublika Uzbekistan, city of Navon, Navon street, building 27. Director: Nikolay Ivanovich Kucherskiy, General Director. Telephone: (43622) 32928. Fax: (31745) 91456.
Holding Company, "Tselinnoye Proizvodstvennoye Gorno-Khimicheskoye Obedineniye" [Virgin Land Mining-Chemical Production Association]. Address: 474456 Respublika Kazakhstan, Akhmalin oblast, city of Stepnogorsk. Director: Yuriy Nikolayevich Filtsev, General Director. Telephone: (31745) 92464. Fax: (31745) 91456.
Kara-Balta Mining Combine. Address: 720398 Respublika Kirgizstan, city of Kara-Balta, Trud Street, building 1. Director: Zhaman Iymanbekovich Kazakbayev. Telephone: (33133) 23018. Fax: (33133) 23018. [Sic. Fax no. is same as tel. no.].
Science and Production Association, "Vostochnyy Gorno-Obogatelnyy Kombinat" [Eastern Mining Concentrating Combine]. Address: 322530 Respublika Ukraina, Dnepropetrov oblast, city of Zheltyye Vody, Gorkiy street, building 2. Director: Mikhail Ivanovich Babak, General Director. Telephone: (05652) 55309. Fax: (05652) 33005.
(7). Machine-Building and Instrument-Making Plants of the Ministry of Atomic Energy of the Russian Federation.
Angarsk Electrolysis Chemical Combine (AEKhK). Address: 665804 Irkutsk oblast, city of Angarsk. Director: Viktor Panteleymonovich Shopen, General Director. Telephone: (39518) 40710. Fax: (39518) 66715.
B. P. Konstantinov Kirovo-Chepeteskiy Chemical Combine. Address: 613020 Kirov oblast, city of Kirovo-Chepetsk, Pozharnyy Lane., 7. Director: Boris Ivanovich Drozhdin, General director. Telephone: (8332) 624829, 624205. Fax: (8332) 627921.
Experimental Chemical-Technological Plant. Address: 125239 Moscow, Likhoborskaya Embankment, 11. Director: Anatoliy Aleksandrovich Matveyev. Telephone: (095) 9132289.
Scientific Design-Technological Firm of Non-traditional Methods for the Processing of Different Materials ("Netram" firm). Address: 123060 Moscow, P. O. Box 155. Director: Boris Vladimirovich Safronov. Telephone: (095) 1908089. Fax: (095) 1960038.
Joint-Stock Company, "Mashinostroitelnyy Zavod Invis" (Machine-Building Plant "Invis"]. Address: 427600, Respublika Udmurtiya, city of Glazov Director: Vladimir Anatolyevich Yekhlakov. Telephone: (34141) 73784. Fax: (34141) 36440.
Production Association, "Mashinostroyitelnyy Zavod, Molniya" [Machine-Building Plant, "Lightning"] (PO MSZ "Molniya"). Address: 109391 Moscow, Ryazanskiy Avenue, 6a. Director: Vladimir Ivanovich Nikolaichev. Telephone: (095) 1713460. Fax: (095) 1716761.
Electro-Mechanical Plant, "Avangard" (EMZ "Avangard"). Address: Nizhegorod oblast, city of Sarov, Nizhnyeye Highway, 6. Director: Yuriy Kuzmich Zavalishin. Telephone: (83130) 45881. Fax: (83130) 45090.
Nizhnyaya Tura Machine-Building plant, "Venta". Address: 624350 Sverdlovsk oblast, city of Nizhnyaya Tura, Malyshev Street, 2a. Director: Sergey Vladimirovich Nastin. Telephone: (34342) 23020. Fax: (34342) 20733.
Ural Electro-Mechanical plant (UMZ). Address: 620151 Yekaterinburg, Studentskaya Street, 9. Director: Leonid Mikhaylovich Kuznetsov. Telephone: (3432) 741281. Fax: (3432) 413370.
"Elvaks" Plant. Address: 141420 Moscow oblast, Skhodnya, Pervomayskaya Street, 54. Director: Yuriy Georgiyevich Kolmogorov. Telephone: (095) 5742400. Fax: (095) 5740162.
State Enterprise, "Krasnaya Zvezda" [Red Star]. Address: 115230 Moscow, Electrolytic Avenue, 1a. Director: Vladimir Sergeyevich Vasilkovskiy. Telephone: (095) 1132309. Fax: (095) 1133488.
Production Association, "Sever" [North]. Address: 630075 Novosibirsk, Obyedineniye Street, 3. Director: Aleksey Nikolayevich Gorb. Telephone (3832) 255573. Fax: (3832) 741465.
Instrument Plant, "Kristall" [Crystal]. Address: 613020 Kirov oblast, city of Kirovo-Chepetsk, Kirov Avenue, 16. Director: Aleksandr Mikhaylovich Fadeichev. Telephone: (83361) 14913. Fax: (83361) 12150.
Joint-Stock Company, "Priborstroitelnyy Zavod, Signal" [Instrument-Making Plant, "Signal"]. Address: 249020 Kaluga oblast, city of Obninsk, Lenin Street, 121. Director: Vyacheslav Aleksandrovich Anisimov. Telephone: (08439) 79195. Fax: (08439) 40314.
Joint-Stock Company, "Pribornyy Zavod, Tenzor" [Instrument Plant, "Tensor"]. Address: 141980 Moscow oblast, city of Dubna, Street Priborstroiltelney [Street of the Instrument- Makers], 2. Director: Igor Borisovich Barsukov. Telephone: (221) 45524. Fax: (221) 46124.
Joint-Stock Company, "Pyatigorsk Zavod, Impuls" [Pyatigorsk Plant, Impulse]. Address: 357500, Stavropol kray, city of Pyatigorsk, Malgyn street, 5. Director: Sergey Ivanovich Kuzmenko. Telephone: (87933) 54554. Fax: (87933) 78936.
TOO [Limited Partnership], "Konsit-A". Address: 109180 Moscow, P. O. Box 29. Director: Yuriy Arkadyevich Brodskiy. Telephone: (095) 2360416. Fax: (095) 2394054.
Joint-Stock Company, "Gorkiy Kimry Fabrika" [Kimry Factory in the name Gorky]. Address: 171510 Tver oblast, Kimry, Pushkin Street, 72a. Director: Lev Nikolayevich Bocharov. Telephone: (08236) 32156. Fax: (08326) 31027.
Joint-Stock Company, "Energomashinostroitelnaya Korporatsiya, Atommash" [Power-Machine Building Corporation, "Atommash"]. Address: 347340 Rostov-on-the-Don, Volgodonsk-13. Director: Aleksey Ivanovich Golovin, General Director. Telephone: (86392) 20745. Fax: (86392) 21358.
Joint-Stock Company, "Karimos", affiliated to the Ministry of Atomic Energy of Russia. Address: 101000 Moscow, B. Ordynka Street, 24/26. Director: Aleksandra Nikolayevna Strepikheyevna, Director for Development. Telephone: (095) 2392440. Fax: (095) 2316860.
Production-Technical Center, "Komito". Address: 107113 Moscow, Verkhne-Krasnoselskaya Street, 16. Director: Vasiliy Matveyevich Monakov. Telephone: (095) 2642995. Fax: (095) 264258.
Kanash Plant for Technological Equipping. Address: 429300 Respublika Chuvashiya, city of Kanash, Svoboda Street, 36. Director: Vyacheslav Aleksandrovich Kuznetsov. Telephone: (83533) 31994. Fax: (83533) 31994. [Sic. Fax no. is same as tel. no.].
Joint-Stock Company, "Moskovskiy Tekhnicheskiy Tsentr, TESMO" [Moscow Technical Center, "TESMO"]. Address: 144001 Moscow oblast, city of Elektrostal, avenue 48. Director: Vyacheslav Mikhaylovich Pukhov. Telephone: (257) 59218. Fax: (095) 7029122.
Joint-Stock Company, "Zavod Start" ["Start" Plant]. Address: 641730 Kurgan oblast, city of Dolmatovo, Rukmanis Street, 31. Director: Aleksandr Ivanovich Kolmogortsev. Telephone: (35252) 92163. Fax: (35252) 92175.
Astrakhan Experimental Machine-Building Plant, "Sirius". Address: 414000 Astrakhan, Gilyanskaya Street, 94. Director: Aleksandr Vasilyevich Rogov. Telephone: (8512) 220416. Fax: (8512) 254220.
Joint-Stock Company, "Vologoda Mashinostroitelnyy Zavod" [Vologda Machine-Building Plant]. Address: 160604 Vologda, Klubov Street, 5. Director: Leonid Borisovich Fedonov. Telephone: (8172) 257072. Fax: (8172) 257783.
Makhachkala Machine-Building Plant of Separators. Address: 367014 Respublika Dagestan, city of Makhachkala, K. Marks [Marx] Avenue, 9. Director: Igor Gafurovich Gafurov. Telephone: (8722) 643193.
Joint-Stock Company "Plavsk Mashinostroitelnyy Zavod" [Plavsk Machine-Building Plant]. Address: 301050 Tula oblast, city of Plavsk, Kommunarov Street, 25. Director: Viktor Georgiyevich Lifanov. Telephone: (08752) 21065. Fax: (08752) 22132.
Joint-Stock Company, "Opytnyy Zavod, Luch" [Experimental Plant, "Light"]. Address: 142100 Moscow oblast, Podolsk, Zheleznodorozhnaya Street, 22. Director: Viktor Arsenyevich Petrov. Telephone: (095) 9564867. Fax: (095) 9561057.
SKTB [Special Design and Technology Bureau] "UPMASH". Address: 144001 Moscow oblast, Elektrostal, P. O. Box 48. Director: Aleksandr Sergeyevich Igolkin. Telephone: (095) 7029150. Fax: (095) 7029150. [Sic. Fax no. is same as tel. no.].
Production Organizations in the Complement of the Ministry of Atomic Energy of the Russian Federation:
State Joint-Stock Company, "Oboronpromkompleks". Address: 109180 Moscow, Staromonetnyy Lane, 26. Director: Vladimir Mikhaylovich Bednyakov, General Director. Telephone: (095) 2392102. Fax: (095) 9533051.
Joint-Stock Company, "Volgo-Vyatka Proizvodstvenno-Komplektovochnoye Predpriyatiye, Oboronpromkompleks" [Volgo-Vyatka Production Enterprise Unit of "Oboronpromkompleks"]. Address: 603124 Nizhniy Novgorod, Ayvazoskiy Street, 10a. Director: Yevgeniy Vasilyevich Selikhov, General Director. Telephone: (8312) 244398. Fax: (8312) 465470.
Joint-Stock Company, "Yuzhno-Uralskoye Proizvodstvenno-Komplektochnoye Predpriyatiye, Oboronpromkompleks" [South Ural Production Enterprise Unit of "Oboronpromkompleks"]. Address: 454087 Chelyabinsk, 2-nd Potrebitelskaya Street, 2. Director: Viktor Grigoryevich Braslavskiy, General Director. Telephone: (3512) 621198. Fax: (3512) 621196.
Western Siberia Joint-Stock Company, "Oboronpromkompleks". Address: 630075 Novosibirsk, B. Khmelnitskiy Street, 84a. Director: Mikhail Ivanovich Kulagin, General Director. Telephone: (3832) 763973. Fax: (3832) 763551.
Joint-Stock Company, "Vostochno-Sibirskoye Proizvodstvennoye-Komplektovochnoye Predpriyatiye Oboronpromkompleks" [Eastern Siberia Production Enterprise Unit of "Oboronpromkompleks"]. Address: 664053 Irkutsk, P. O. Box 2497. Director: Georgiy Ivanovich Tarusin, General Director. Telephone: (3952) 453625. Fax: (3952) 456341.
Northern Production Enterprise Unit of "Oboronpromkompleks". Address: 150000 Yaroslavl, General Post Office, P. O. Box 76. Director: Oleg Georgiyevich Pozdnyakov, General Director. Telephone: (0852) 231557. Fax: (0852) 558524.
Joint-Stock Company, North-Western Production Enterprise Unit of "Oboronpromkompleks". Address: 196143 St. Petersburg, Predportovaya Street, 7-th Thoroughfare, 1. Director: Anatoliy Artemovich Kurlyandchik. Telephone: (812) 1223715. Fax: (812) 1226790.
Joint-Stock Company, Tver Production Enterprise Unit of "Oboronpromkompleks". Address: 117000 Tver, General Post Office, P. O. Box 379. Director: Vladimir Genrikhovich Moryev, General Director. Telephone: (0822) 332697. Fax: (0822) 426388.
Joint-Stock Company, North Caucasus Production Enterprise Unit of "Oboronpromkompleks". Address: 344104 Rostov-on-the-Don, Dovator Street, 154/1. Director: Anatoliy Ivanovich Pravdyuk, General Director. Telephone: (8632) 240322. Fax: (8632) 220914.
Joint-Stock Company, Production Firm Unit of "Atompromresursy". Address: 101000 Moscow, B. Ordynka Street, 24/26. Director: Ivan Petrovich Guzhov. Telephone: (095) 2362325. Fax: (095) 9556068.
Joint-Stock Company, "Atomprom". Address: 101000 Moscow, B. Ordynok Street, 24/26. Director: Roman Konstantinovich Rusalkin, General Director. Telephone: (095) 1119512. Fax: (095) 1119512. [Sic. Fax no. is same as tel. no.].
Joint-Stock Company, "Kontrakt". Address: 101000 Moscow, Meshchanskaya Street, 7/21. Director: Andrey Grigoryevich Andrukh. Telephone: (095) 2841935. Fax: (095) 9710845.
Joint-Stock Company, "Atomenergozapchast". Address: 396072 Voronezh oblast, Novovoronezh. Director: Vladimir Grigoryevich Churin. Telephone: (073674) 25567. Fax: (073674) 21898.
Joint-Stock Company, Kursk Plant, "Atomremmash". Address: 307720 Kursk oblast, Kurchatov rayon, sub-division [Russian: p/o] Lukashovka. Director: Viktor Semenovich Kurilenko. Telephone: (07131) 21433. Fax: (07131) 61261.
State Enterprise for Repair and Servicing of Atomic Power Stations, Production Association "Atomenergoremont". Address: 141011 Moscow oblast, Mytishci, Kommunist Street, 23. Director: Stanislav Stepanovich Chertov. Telephone: (095) 5821603. Fax: (095) 5819034.
State Enterprise, "Kurskturboatomenergoremont". Address: 307239 Kursk oblast, Kurchatov. Director: Vladimir Prokopyevich Fedorenko. Telephone: (07131) 46258. Fax: (07131) 46258 [Sic. Fax no. is same as tel .no.].
Joint-Stock Company, Perlov Plant of Power Equipment. Address: 141011 Moscow oblast, Mytishchi, Kommunist Street, 23. Director: Valeriy Ivanovich Zabrodin. Telephone: (095) 5813144 Fax: (095) 5813144 [Sic. Fax no. is same as tel. no.].
State Production Enterprise, "Sevatomremont". Address: 184151 Murmanks oblast, Polyarnyye Zori. Director: Inarik Gayazovich Mukhametshin. Telephone: (81532) 65667. Fax: (095) 5813144
"Atomtekhenergo", a firm for the setting up and improvement of the operation and organization of the management of atomic power stations. Address: 141011 Moscow oblast, Mytishchi, Kommunist Street, 23. Director: Anatoliy Grigoryevich Ivannikov. Telephone: (095) 5820454. Fax: (095) 5818011.
Joint-Stock Company, Machine-Building Corporation, "SPLAV". Address: 173021 Velikiy Novgorod, Nezhinskaya Street, 61. Director: Yevgeniy Izyaslavovich Shulman, General Director. Telephone: (8162) 113003. Fax: (8162) 113002.
Construction-Industrial Joint-Stock Companies and Plants for Construction Materials of the Ministry of Atomic Energy of the Russian Federation:
Holding Company, Joint-Stock company "Progress", affiliated to the Ministry of Atomic Energy of Russia. Address: 101000 Moscow, B. Ordynka street, 24/26. Director: Ivan Yegorovich Deryabin. Telephone: (095) 9534553, 2394308. Fax: (095) 2394800.
Construction-Industrial Joint-Stock Company, "Kirovo-Chepetsk Directorate of Construction". Address: 613020 Kirov oblast, Kirovo-Chepets, Shkolnaya Street, 2. Director: Aleksandr Romanovich Verba. Telephone: (83361) 10314. Fax: (83361) 19118.
Construction-Industrial Joint-Stock Company, "Chepetsk Directorate of Construction". Address: 427600 Respublika Udmurtiya, city of Glazov, Belova Street, 7. Director: Vladimir Yuryevich Pereshein. Telephone: (34141) 72206. Fax: (34141) 34304.
Construction-Industrial Joint-Stock Company, "North Ural Directorate of Construction". Address: 620045 Sverdlovsk oblast, city of Lesnoy, Lenin Street, 76. Director: Vladimir Timofeyevich Nesterenko. Telephone: (34342) 69271. Fax: (34342) 24054, 24681.
Design-Construction-Industrial Joint-Stock company, "Ural". Address: 456236 Chelyabinsk oblast, Trekhgornyy, Street Stroiteley [Street of the Builders], 6. Director: Galina Ivanovna Morozova. Telephone: (35111) 67061. Fax: (35111) 65120.
Construction-Industrial Joint-Stock Company, "Sibakademstroy". Address: 630055 Novosibirsk, M. Dzhalil Street, 11. Director: Gennadiy Dmitriyevich Lykov. Telephone: (3832) 322050. Fax: (3832) 322032.
Construction-Industrial Joint-Stock Company, "Khimstroy". Address: 636070 Tomsk oblast, city of Seversk, Transport Street, 32. Director: Gennadiy Sergeyevich Molokanov. Telephone: (3822) 776460. Fax: (3822) 776463, 764754.
Construction-Industrial Joint-Stock Company, "Sibkhimstroy". Address: 660033 Krasnoyarsk kray, city of Zheleznogorsk, Shtefan Street, 1. Director: Vladimir Mikaylovich Kiyayev. Telephone: (39197) 22076, 29812. Fax: (39197) 29839.
Construction-Industrial Joint-Stock Compnay, "Directorate of Construction No. 604". Address: 663960 Krasnoyarsk kray, city of Zelenogorsk, Kalinin Street, 25. Director: Anatoliy Yakovlevich Kurdyukov. Telephone: (39169) 35722. Fax: (39169) 24940, 44094.
Construction-Industrial Joint-Stock Company, "Vostok" [East]. Address: 665358 Irkutsk oblast, city of Sayansk-3, P. O. Box 238. Director: Aleksandr Petrovich Sigal. Telephone: (39513) 32449. Fax: (39513) 32916.
Construction-Industrial Joint-Stock Company, Priargunskiy Directorate of Construction". Address: 674665 Chitin oblast, city of Krasnokamensk, Avenue Stroiteley [Avenue of the Builders], 7. Director: Aleksandr Ivanovich Filonich. Telephone: (30245) 25784. Fax: (30245) 46246.
Construction-Industrial Joint-Stock Company, "Angarsk Directorate of Construction". Address: 665835 Irkutsk oblast, Angarsk, P. O. Box 2060. Director: Viktor Leonidovich Seredkin. Telephone: (39518) 95062. Fax: (39518) 66856.
AOZT {Closed-Type Joint-Stock Company], "South Ural Directorate of Construction".
Construction-Industrial Joint-Stock Company, "Mid-Ural Directorate of Construction". Address: 620114 Sverdlovsk oblast, city of Novouralsk, Dzerzhinskiy Street, 13. Director: Petr Ivanovich Bokov. Telephone: (34370) 24961. Fax: (34370) 40977.
Joint-Stock Construction Firm, "Aviastroy". Address: 432010 city of Ulyanovsk, Engineer Avenue, 24. Director: Yuriy Porfiryevich Grokhotov. Telephone: (8422) 200228. Fax: (8422) 200289.
SPAO [Special Production Joint-Stock Company], "Elektrostal Directorate of Construction". Address: 144000 Moscow oblast, city of Electrostal, Karl Marks [Marx] Street, 18. Director: Sergey Alekseyevich Novgorodov. Telephone: (095) 7024794. Fax: (095) 7024794.
Construction-Industrial Joint-Stock Company, "Atomstroy", affiliated to the Ministry of Atomic Energy of Russia. Address: 1011000 Moscow, B. Ordynka street, 24/26. Director: Konstantin Nikolayevich Moskvin. Telephone: (095) 2394369. Fax: (095) 9535361.
Joint-Stock Company Construction-Industrial Company, "Dimitrovgradstroy". Address: 433510 Ulayanovsk oblast, Dimitrovgrad, Dachnaya Street, 2. Director: Vladimir Sergeyevich Pisarchuk. Telephone: (84235) 32128. Fax: (84235) 54083.
Open-Type Joint-stock company, "Donatostroy". Address: 396072 Voronezh oblast, city of Novo-Voronezh. Director: Ivan Pavlovich Mikhalev. Telephone: (07364) 29646. Fax: (07364) 21802.
Open-Type Joint-Stock Company, "Northern Directorate of Construction". Address: 188537 Leningrad oblast, city of Sosnovyy Bor-537, Leningrad Street, 7. Director: Igor Vladislavovich Ustinov. Telephone: (81269) 62774. Fax: (81269) 62415.
Obninsk Construction-Industrial Joint-Stock Company. Address: 249020 Kaluga oblast, city of Obninsk, Kurchatov Street, 41. Director: Valeriy Ivanovich Chekmazov. Telephone: (08439) 49250. Fax: (08439) 49248.
Open-Type Joint-Stock Company, "Penza Directorate of Construction". Address: 440019 Penza oblast, city of Zarechnyy, Komsomol Street, 41. Director: Nikolay Semenovich Kononenko. Telephone: (8412) 550907. Fax: (8412) 5500973.
Sarov Construction-Industrial Joint-Stock Company. Address: 607200 Nizhegorod oblast, city of Sarov, Silkin street, 13. Director: Georgiy Georgiyevich Bulgakov. Telephone: (83130) 11077.
Directorate of Construction No. 620. Address: 142284 Moscow oblast, Protvino. Director: Aleksandr Ivanovich Syatotskiy. Telephone: (277) 46991.
First Construction-Installation Trust. Address: 113191 Moscow, First Lyusinovskiy Lane., 36. Director: Yuriy Aleksandrovich Shilobreyev. Telephone: (095) 2376660. Fax: (0950 2374726.
Kaluga Construction-Installation Trust of Obninsk Directorate of Construction. Address: 248024 Kaluga oblast, city of Obninsk, K. Libknekht Street, 18. Director: Valeriy Fedorovich Desyatnikov. Telephone: (08422) 27162. Fax: (08422) 27878.
Open-Type Joint-Stock Company, "Directorate of Industrial Enterprises". Address: 188537 Leningrad oblast, city of Sosnovyy Bor, Leningrad Highway, P. O. Box 32. Director: Vladimir Aleksandrovich Shegalo. Telephone: (81269) 62457.
Open-Type Joint-Stock Company, "SMU-15". Address: 142234 Moscow oblast, city of Protvino, Obolensk Highway, 5. Director: Aley Safrovich Nekhay. Telephone: (277) 42517. Fax: (277) 42886.
Joint-Stock Company, "Spetsatommontazh", affiliated to the Ministry of Atomic Energy of Russia. Address: 101000 Moscow, B. Ordynka Street, 24/26. Director: Valeriy Nikolayevich Karmachev, President. Telephone: (095) 2394500. Fax: (095) 2394567.
Joint-Stock Company PMSP "Elektron". Address: 630065 Novosibirsk-65, Tank Street, 72. Director: Valeriy Nikolayevich Karmachev. Telephone: (3832) 761331. Fax: (3832) 760712, 762076.
Joint-Stock Company, "Gidromontazh". Address: 143392 Moscow oblast, Naro-Fominskiy rayon, settlement of Selyatino. Director: Gennadiy Pavlovich Kryuchkov. Telephone: (095) 4365510. Fax: (095) 7204960.
Joint-Stock Company, Energospetsmontazh". Address: 107150 Moscow, Boytsovaya Street, 27. Director: Anatoliy Vasilyevich Shevchenko. Telephone: (0950 1608903. Fax: (095) 1694225.
Joint-Stock Company, "Promelektromontazh". Address: 107150 Moscow, Boytsovaya Street, 27. Director: Vladimir Grigoryevich Dedlovskiy. Telephone: (0950 1602710, 1695275. Fax: (095) 1601313.
Joint-Stock Company, "Mospromtekhmontazh". Address: 103473 Moscow, Third Samotechnyy Lane, 11. Director: Yuriy Leonyevich Ilin. Telephone: (095) 2889221, 2844200. Fax: (095) 2844348.
Joint-Stock Company, "Angarskteplokhimmontazh". Address: 665801 Irkutsk oblast, Angarsk. Director: Vladimir Mikhaylovich Varga. Telephone: (39515) 42658. Fax: (39515) 42678. [Sic. Fax no. is same as tel. no.].
Open-Type Joint-Stock Company "Promekhanomontazh". Address: 636070 Tomsk oblast, city of Seversk, P. O. Box 494. Director: Anatoliy Vladimirovich Maksimenko. Telephone: (3822) 784900. Fax: (3822) 241520.
Open-Type Joint-Stock Company MPK "Uralpromontazh". Address: 454065 Chelyabinsk oblast, city of Ozersk, Semenov Street, 22. Director: Sergey Vladimirovich Voloshin. Telephone: (35171) 79250. Fax: (35117) 122214.
OAO "Spetsmontazhmekhanizatsiya". Address: 115230 Moscow, Nagitinskaya Street, 2 building 1. Director: Aleksandr Mikhaylovich Tarasov. Telephone: (095) 1168791. Fax: (095) 1168863.
OAO "Spetskhimmontazh". Address: 121069 Moscow, Khlebnyy Lane, 2/3. Director: Vladimir Mikhaylovich Tsitlenko. Telephone: (095) 2917136. Fax: (095) 2900830.
Joint-Stock Company, "Atomspetskonstruktsiya" Experimental Plant. Address: 144001 Moscow oblast, Elektrostal, Stroitelnyy Lane, 10. Director: Vladimir Alekseyevich Gurov. Telephone: (095) 7029784. Fax: (095) 7029738.
Open-Type Joint-Stock Company, "Installation-Construction Trust No. 3". Address: 396072 Voronezh oblast, city of Novovoronezh. Director: Anatoliy Nikiforovich Myshko. Telephone: (07364) 20631. Fax: (07364) 20082.
Joint-Stock Company, "Spetsteplokhimmontazh". Address: 636070 Tomsk oblast, city of Seversk, Semenov Street, 22. Director: Boris Alekseyevich Kormashov. Telephone: (3822) 777932. Fax: (3822) 776939.
Joint-Stock Company, "Uralgidromontazh". Address: 456780 Chelyabinsk oblast, city of Ozersk, Semenov Street, 22. Director: Anatoliy Ivanovich Loboda. Telephone: (35171) 79303. Fax: (35171) 73041.
Joint-Stock Company, "Spetskhimmontazh". Address: 188537 Leningrad oblast, city of Sosnovyy Bor, P. O. Box 47. Director: Nikolay Nikolayevich Kiselev. Telephone: (81269) 64310. Fax: (81269) 64846.
Joint-Stock Company, "Orgmontazhproyekt". Address: 119146 Moscow, First Frunze Street, 3a. Director: Igor Sergeyevich Ivashkin. Telephone: (095) 2428692. Fax: (095) 2460191.
Joint-Stock Company, "KONATEM Concern". Address: 144001 Moscow oblast, city of Electrostal, Stroitelnyy Lane, 8. Director: Aleksandr Nikolayevich. Telephone: (095) 70207725. Fax: (095) 7029070.
Novosibirsk Plant, "Promstalkonstruktsia". Address: 630075 city of Novosibirsk, Tayginskaya Street, 11. Director: Vladimir Ivanovich Berezikov. Telephone: (3832) 765797. Fax: (3832) 743221.
Open Joint-Stock Company (Holding), "Spetsstroymaterialy". Address: 113191 Moscow, Kholodilnyy Lane, 3a. Director: Vladimir Aleksandrovich Barkov. Telephone: (095) 2353165. Fax. (095) 2353809.
Open Joint-Stock Company, "Kamishlov Plant of Building Materials". Address: 623530 Sverdlovsk oblast, City of Kamyshlov, Street Stroiteley [Street of the Bulders], 1. Director: Oleg Timofeyevich Boyarnikov. Telephone: (34375) 93205. Fax: (34375) 24547.
Open Joint-Stock Company, "Vikhorevka Timber-Cutting Combine". Address: 665737 Irkutsk oblast, city of Vikhorevka. Director: Vladimir Grigoryevich Serov. Telephone: (39531) 53548.
Open Joint-Stock Company, "Gisopolimer". Address: 614043 Perm, Vasilyev Street, 1. Director: Maksim Veniaminovich Kirichenko, General Director. Telephone: (3422) 257315. Fax: (3422) 250743.
Open Joint-Stock Company, "NIKBOOR". Address: 144001 Moscow oblast, city of Electrostal, Stroitelnyy Lane, 5. Director: Vasiliy Nikolayevich Gulko. Telephone: (095) 7029713. Fax: (095) 7029713. [Sic. Fax no. is same as tel. no.].
Open Joint-Stock Company, "Stroyplastpolimer". Address: 620024 Yekaterinburg-24, Bisertskaya Street, 1. Director: Aleksandr Ivanovich Melnik, General Director. Telephone: (3432) 258811. Fax: (3432) 255233.
Open Joint-Stock Company, "Udmurtiya Plant of Building Materials". Address: 427600 Respublika Udmurtiya, Glazov, Sovetskaya Street, 49. Director: Anatoliy Aleksandrovich Fedorovskiy. Telephone: (34141) 72564. Fax: (34141) 76482.
Open Joint-Stock Company, "Lesstrom". Address: 618500 Perm oblast, city of Solikamsk, Kommunist Street, 44. Director: Aleksandr Nikolayevich Zinovyev. Telephone: (34253) 30107. Fax: (34253) 30100.
Open Joint-Stock Company, "Iskitimramorgranit". Address: 633210 Novosibirsk oblast, Iskitim-5, Tsentralnaya Street, 24. Director: Aleksandr Semenovich Chirkov. Telephone: (38343) 42753, 44745.
Open Joint-Stock Company, "Chuna Timber-Cutting Combine". Address: 665540 Irkutsk oblast, Chuna post office. Director: Vladimir Serafimovich Ilinskiy, General Director. Telephone: (39567) 91930. Fax: (39567) 91162.
Open Joint-Stock Company, "Polistrom". Address: 456616 Chelyabinsk oblast, city of Kopeyk, Tomsk Street, 2. Director: Vladimir Gustavovich Adayev, General Director. Telephone: (35126) 10373. Fax: (35126) 10373. [Sic. Fax no. is same as tel. no.].
Open Joint-Stock Company, "Krasnoyarskpolimerkeramika". Address: 663010 Krasnoyarsk kray, Berezovskiy rayon, Zykovo, Lineynaya Street, 31. Director: Aleksandr Vladimirovich Bevza, General Director. Telephone: (39175) 92510. Fax: (39175) 564089.
Open Joint-Stock Company, "Tomsk Plant of Construction Materials and Products". Address: 634049 Tomsk, Irkutsk Road, 65. Director: Anatoliy Vasilyevich Valov, General Director. Telephone: (3822) 561793. Fax: (3822) 564089.
Open Joint-Stock Company, Stroypolimerkeramika". Address: 249200 Kaluga oblast, Babinskiy rayon, settlement of Vorotynsk. Director: Said Vaitovich Mambetshayev, General Director. Telephone: (08425) 81401. Fax: (08425) 82271.
Open Joint-Stock Company, "Stromashpolimer". Address: 249855 Kaluga oblast, Dzerzhinskiy rayon, settlement of Tovarkovo. Director: Sergey Vasilyevich Kondratyev. Telephone: (08434) 23315. Fax: (08434) 26419.
Open Joint-Stock Company, "Tizol". Address: 624350 Sverdlovsk oblast, city of Nizhnyaya Tura-7. Director: Mikhail Grigoryevich Mansurov, General Director. Telephone: (34342) 23442. Fax: (34342) 211034.
Open Joint-Stock Company, "Silikatstroymaterialy". Address: 636137 Tomsk oblast, settlement of Kopylovo. Director: Aleksey Alekseyevich Shachnev, General Director. Telephone: (38229) 82440. Fax: (38229) 82440.
Open Joint-Stock Company, "Sortavala Crushing-Grading Plant". Address: 186750 Respublika Kareliya, city of Sortavala, Lesnaya Street, 2. Director: Yuriy Borisovich Yudin, General Director. Telephone: (81430) 42969.
Closed Joint-Stock Company, "Filter". Address: 249855 Kaluga oblast, Dzerzhinskiy rayon, settlement of Tovarkovo. Director: Gennadiy Mikhaylovich Kadomtsev, General Director. Telephone: (08434) 23985.
GUP [State Unitary Enterprise], "Volga Testing and Experimental Combine". Address: 171510 Tver oblast, Kimry rayon, settlement of Savelovo. Director: Yuriy Aleksandrovich Matlakhov. Telephone: (095) 2760272. Fax: (095) 5877770.
Scientific-Research and Design Institutes; Scientific and Scientific-Technical Centers and Organizations of the Ministry of Atomic Energy of the Russian Federation; Physical Profile on Research and Servicing of the Nuclear Fuel Cycle and the Servicing of the Atomic Power Plants.
All-Russian Scientific-Research Institute of Experimental Physics of the Russian Federal Nuclear Center (VNIIEF-RFYaTs). Address: 607190 Sarov, Nizhegorodsk oblast, Mir Avenue, 37. Director: Radiy Ivanovich Ilkayev. Telephone: (83130) 56951, 11264, 11803. Fax: (83130) 53808, 54565. Telex: 151 109 APCA. Web: http:
www.vniief.ru.
All-Russian Scientific-Research Institute of Technical Physics--Russian Federal Nuclear Center (VNIITF-RFYaTs). Address: 456770 Snezhinsk, Chelyabinsk oblast, P. O. Box 245. Director: Georgiy Nikolayevich Rykovanov. Telephone: (35172) 32028. Fax: (35172) 32351. Telex: 124 846 SNOW SU. Web: http:
/ Russian Scientific Center, "Kurchatov Institute". Address: 123182 Moscow, Kurchatov Square, 1. Director: Yevgeniy Pavlovich Velikhov, President. Telephone: (095) 9430074. Web: http:
www.kiae.ru
State Scientific Center of the Russian Federation, Troitsk Institute of Innovative and Thermo- Nuclear Research (GNTs RF TRINITI). Address: 142092 Moscow oblast, Troitsk. Director: Vyacheslav Dmitriyevich Pismennyy. Telephone: (095) 3345041. Fax: (095) 3345776. Web: http:
www.triniti.troitsk.ru.
State Scientific Center of the Russian federation, " A. I. Leypunskiy Physics-Energy Institute" (GNTs RF FEI). Address: 249020 Kaluga oblast, city of Obninsk, Bondarenko Square, 1. Director: Anatoliy Vasilyevich Zrodnikov. Telephone: (095) 9530017, extension 8231. Fax: (09439) 48225. Web: http:
www.ippe.obninsk.ru.
State Unitary Enterprise, "Scientific-Research and Design Institute of Power Engineering" (NIKIET). Address: 101000 Moscow, General Post Office, P. O. Box 788. Director: Boris Arsentyevich Gabarayev. Telephone: (095) 9752017. Fax: (095) 9752019. Web: http:
www.entek.ru
Sverdlovsk Branch of the Scientific-Research and Design Institute of Power Engineering (NIKIET). Address: 624051 Yekaterinburg oblast, Beloyarsk rayon, Zarechnyy. Director: Viktor Ivanovich Perekhozhev. Telephone: (34377) 35162. Fax: (34377) 33396.
State Scientific Center, Scientific-Research Institute of Atomic Reactors (GNTs RF NIIAR). Address: 433510 Ulyanovsk oblast, Dimitrovgrad-10. Director: Aleksey Frolovich Grachev. Telephone: (84235) 35648. Web: http:
www.niiar.simbirsk.su
State Scientific Center of the Russian Federation, "Institute of Theoretical and Experimental Physics" (GNTs RF ITEF). Address: 117259 Moscow, Bolshaya Cheremushkinskaya Street, 25. Director: Mikhail Vladimirovich Danilov. Telephone: (095) 1250292. Fax: (095) 1270833. Web: http:
www.itep.ru
State Scientific Center of the Russian Federation, "Institute of High Energy Physics" (GNTs RF IFVE). Address: 142284 Moscow oblast, Serpukhov rayon, Protvino, Pobeda street, 1. Director: Anatoliy Alekseyevich Logunov. Telephone: (095) 2175857. Fax: (095) 9246752. Web: http:
www.ihep.su.
Joint Institute of Nuclear Research (OIYaI). Address: 141980 Moscow oblast, Dubna. Director: Vladimir Georgyevich Kadyshevskiy. Telephone: (095) 2002283. Fax: (095) 9752381. Web: http:
www.jinr.ru
Scientific-Research Institute of Pulse Engineering (NIIIT). Address: 115304 Moscow, Luganskaya Street, 9. Director: Konstantin Nikolayevich Danilenko. Telephone: (095) 3213501. Fax: (095) 3214855.
Yu. S. Sedkov Scientific-Research Institute of Measuring Systems (NIIIS). Address: 603600 N. Novgorod, GSP. Director: Valentin Yefimovich Kostyukov. Telephone: (8312) 654990. Fax: (8312) 668752.
State Unitary Scientific-Technical Enterprise, "EFKON" (GNTP "EFKON"). Address: 601190 Nizhegorod oblast, Sarov, Yunost Street, 22. Director: Anatoliy Petrovich Inozemtsev. Telephone: (83130) 45705. Fax: (83130) 45994.
Design Bureau of Auto-Transport Equipment (KB ATO). Address: 141007 Moscow oblast, city of Mytishchi, Khlebozavodskaya Street, 2. Director: Ernest Pavlovich Kornilovich. Telephone: (095) 5832303. Fax: (095) 5839334.
State Unitary Enterprise, "A. P. Aleksandrov Scientific-Research and Technological Institute" (NITI). Address: 188537 Leningrad oblast, Sosnovyy Bor. Director: Vyacheslav Andreyevich Vasilenko. Telephone: (81269) 62667. Fax: (81269) 63672.
All-Russian Scientific-research Institute of Technical Physics and Automation (VNIITFA). Address: 115230 Moscow, Varshavskoye Highway, 46. Director: Nikolay Revokatovich. Telephone: (095) 1119496. Fax: (095) 1115434. Web: http:
www.vniitfa.ru
/ Saransk Branch of the All-Russian Scientific-Research Institute of Technical Physics And Automation (VNIITFA). Address: 430003 Respublika Mordoviya, city of Saransk, Rabochaya Street, 82. Director: Vladimir Ivanovich Piskunov. Telephone: (8342) 171155. Fax: (8342) 171019.
State Unitary Enterprise, "Special Science and Production Association 'Eleron' (SNPO "Eleron"). Address: 115563 Moscow, General Belov Street, 14. Director: Yevgeniy Trofimovich Mishin. Telephone: (095) 3939072. Fax: (095) 3939163.
Branch State Unitary Enterprise, Special Science and Production Association "Eleron" Scientific-Research and Design Institute of Radio-Electronic Engineering (NIKIRET). Address: 440901 Penza oblast, Zarechnyy, Mir Avenue, 1. Director: Yuriy Aleksandrovich Olenin. Telephone: (8412) 629474. Fax: (8412) 552528.
Branch Enterprise, Special Science and Production Association "Eleron" State Unitary Enterprise "Dedal". Address: 141930 Moscow oblast, Dubna, P.O. Box 89. Director: Sergey Leonidovich Fedyayev. Telephone: (221) 62120. Fax: (221) 40469.
Branch Special Science and Production Association Eleron" "Lepton". Address: 353340 Stavropol kray, Lermontov, Komsomol Street, 24. Director: Anatoliy Vladimirovich Podshibyakin. Telephone: (86535) 22530.
N. L. Dukhov All-Russian Scientific Research Institute of Automation (VNIIA). Address: 101000 Moscow, P. O. Box 918. Director: Yuriy Nikolayevich Barmakov. Telephone: (095) 9787803. Fax: (095) 9780903.
State Unitary Enterprise, "Scientific-Research Institute of Instruments" (NIIP). Address: 140061 Moscow oblast, Lytkarino, settlement of Turayevo. Director: Vladimir Ivanovich Rogov. Telephone: (095) 5523939. Fax: (095) 5523911.
State Enterprise, "D. V. Yeremov Scientific-Research Institute of Electro-Physics Equipment" (NIIEFA). Address: 189631 St. Petersburg, Metallostroy, Sovetskiy Avenue, 1. Director: Vasiliy Andreyevich. Telephone: (812) 4648963 Fax: (812) 4647979.
Institute of Physics-Technical Problems (IFTP). Address: 141980 Moscow oblast, Dubna, GUS, P. O. Box 39. Director: Vladimir Pavlovich Plotnikov. Telephone: (095) 9262209. Fax: (221) 65523.
Research Center of Applied Nuclear Physics (ITsPYaF). Address: 141980 Moscow oblast, Dubna, Zh. Kyuri Street, 6. Director: Vladimir Dmitriyevich Shestakov. Telephone: (221) 40665. Fax: (221) 65523.
Moscow Radio-Engineering Institute (MRTI). Address: 113519 Moscow, Varshavskoye Highway, 132. Director: Gennadyy Ivanovich Batskikh. Telephone: (095) 3153111. Fax: (095) 3141053.
State Center, "Physics of Concentrated Media" Address: 123060 Moscow, VNIINM [All-Russian Scientific-Research Institute of Inorganic Materials]. Director: Aleksandr Zinovyevich Solontsov. Telephone: (095) 1966389. Fax: (095) 1966389. [Sic. Fax no. is same as tel. no.]
Joint-Stock Company, "Scientific-Production Center, "Rosna". Address: 620151 Yekaterinburg, General Post Office, P. O. Box 74. Director: Grigoriy Yemelyanovich Vedernikov. Telephone: (3432) 413228. Fax: (3432) 413370.
Scientific-Engineering Center, "SNIIP" (NITs "SNIIP"). Address: 123060 Moscow, Raspletin street, 5. Director: Sergey Borisovich Chebyshov. Telephone: (095) 1987947. Fax: (095) 9430063. Web: http:
www.sniip.ntl.ru
Specialized Design-Structure Bureau, "Automation" (SPKB "Avtomatika"). Address: 153428 Ivanovo, 11-th Sosnevskaya, 72. Director: Yevgeniy Borisovich Butnikov. Telephone: (0932) 304275. Fax: (0932) 350548.
State Scientific-Research and Design Enterprise, "Vibrotekhnika". (GNIKP "Vibrotekhnika"). Address: 109017 Moscow, B. Ordynka Street, 29. Director: Vladimir Petrovich Savchenko. Telephone: (095) 9516750. Fax: (095) 2394609.
All-Russian Design-Research and Scientific-Research Institute of Industrial Technology (VNIPI Promtekhnolgii). Address: 115409 Moscow, Kashirskoye Highway, 33. Director: Vladimir Viktorovich Lopatin. Telephone: (095) 3247945. Fax: (095) 3245025.
Siberian Branch of the All-Russian Design-Research and Scientific-Research Institute of Industrial Technology (SibNIIPromtekhnologii). Address: 674665 Chitin oblast, Krasnokamensk, P. O. Box 3. Director: Nikolay Matveyevich Zemskov. Telephone: (30245) 26147. Fax: (30245) 43670.
All-Russian Scientific-Research Institute of Chemical Technology (VNIIKhT). Address: 115230 Moscow, Kashirskoye Highway, 3. Director: Viktor Vasilyevich Shatalov. Telephone: (095) 3247584. Fax: (095) 3245441.
State Scientific Center of the Russian Federation, "A. A. Bochvar All-Russian Scientific-Research Institute of Inorganic Materials" (VNIINM). Address: 123060 Moscow, Rogov Street, 5. Director: Mikhail Ivanovich Solonin. Telephone: (095) 1904993. Fax: (095) 1964168. Web: http:
www.bochvar.ru
Siberian Branch of State scientific Center of the Russian Federation, "A. A. Bochvar All- Russian Scientific Research Institute of Inorganic Materials". Address: 634018 Tomsk oblast, Seversk, Lermontov Street, 13. Director: Aleksandr Yakovlevich Svarovskiy. Telephone: (38242) 61880. Fax: (3822) 776739.
State Unitary Enterprise, Scientific-Research Institute, "Luch" (NII "Luch"). Address: 142100 Moscow oblast, city of Podolsk, Zheleznodorozhnaya Street, 24.
Science and Production Association, "V. G. Khlopin Radium Institute". Address: 194021 St. Petersburg, 2-nd Murinskiy Thoroughfare, 28. Director: Aleksandr Andreyevich Rimskiy-Korsakov. Telephone: (812) 2475641. Fax: (812) 2475781. Web: http:
Scientific-Research Institutes and Design Institutes, Scientific Centers, and Scientific- Technical Centers of the Ministry of Atomic Energy of the Russian Federation for Research and Servicing of the Nuclear Fuel Cycle:
Moscow Science and Production Association, "Radon" (NPO "Radon"). Address: 141335 Moscow oblast, Sergiyevo-Posadskiy rayon, sub-division Shemetovo. Director: Igor Andreyevich Sobolev. Telephone: (095) 9289069. Fax: (095) 9289916. Web: http:
www.radon.ru
Central Laboratory for the Protection of the Environment. Address: 607190 Nizhegorod oblast, Sarov, P. O. Box 423. Director: Vasiliy Dmitriyevich Davydok. Telephone: (83130) 45288. Fax: (86130) 45288.
Central Scientific-Research Laboratory. Address: 440901 Penza oblast, Zarechnyy, Mir Avenue, 1. Director: Vladimir Mikhaylovich Sorokin. Telephone: (8412) 692706.
Scientific-Production Center for Conversion. Address: 101000 Moscow, B. Ordynka Street, 24/26. Director: Aleksandr Dmitriyevich Tsisarskiy. Telephone: (095) 2392205. Fax: (095) 2392711.
All-Russian Scientific-Research Institute for Utilization of Atomic Power Stations (VNII AES). Address: 105507 Moscow, Ferganskaya Street, 25. Director: Armen Artvazdovich Abagyan. Telephone: (095) 3761550. Fax: (095) 3768333. Web: http:
www.vniiaes.ru.
Scientific-Technical Center for Emergency-Technical Work at Atomic Power Stations (NTTs ATR). Address: 109507 Moscow, Ferganskaya Street, 25. Director: Eduard Saakovich Saakov. Telephone: (095) 5819223. Fax: (095) 5818011.
Technological Branch of the Scientific-Technical Center for Emergency Work at Atomic Power Stations. Address: 142530 Moscow oblast, Pavlo-Posadskiy rayon, Elektrogorsk, Bezymyannaya Street, 6. Director: Anatoliy Yuryevich Likhachev. Telephone: (095) 3760069. Fax: (095) 3760069 [Sic. Fax no. is same as tel. no.].
Structural Design Branch of the Scientific-Technical Center for Emergency Work at Atomic Power Stations. Address: 107818 Moscow, Bakunin Street, 7, building 1. Director: Mikhail Falevich Rogov. Telephone: (095) 3891355. Fax: (095) 3891355. [Sic. Fax no. is same as tel. no.].
Dimitrovgrad Branch of the Scientific-Technical Center for Emergency Work at Atomic Power Stations. Address: 433510 Ulyanovsk oblast, Dimitrovgrad. Director: Vasiliy Ivanovich Shepilov. Telephone: (84235) 34063. Fax: (84235) 35648.
Elektrogorsk Scientific-Research Center for Security of Atomic Power Stations. Address: 142530 Moscow oblast, Pavlo-Posadskiy rayon, Elektrogorsk, Bezymyannaya Street, 6. Director: Vladimir Nikolayevich Blinkov. Telephone: (243) 33074. Fax: (243) 31235.
Joint-Stock Company, "Small Power Engineering" [Malaya Energetika]". Address: 105318 Moscow, Tkatskaya Street, Building 1, P. O. Box No. 75. Director: Yevgeniy Alekseyevich Kuzin, General Director. Telephone: (095) 9629269. Fax: (095) 9641900. Web: http:
www.glasnet.ru/~merev Design [Institutes]:
State Specialized Design Institute (GSPI). Address: 107014 Moscow, Novoryazan Street, building 8a. Director: Vladimir Lvovich Rozhkov. Telephone: (095) 2611259. Fax: (095) 2617264.
State Unitary Enterprise, Leading Institute "All-Russian Design and Scientific- Research Institute of Integrated Power Engineering technology (GI VNIPIET). Address: 197228 St. Petersburg, Savushkin Street, 82. Director: Valeriy Dmitriyevich Safutin. Telephone: (812) 4301491. Fax: (812) 4300393.
Krasnoyarsk State Design-Research Institute of VNIPIET [see acronym expansion above]. Address: 660026 Krasnoyarsk kray, Zheleznogorsk, Lenin Street, 39. Director: Yuriy Nikolayevich Baskakov. Telephone: (39197) 22087. Fax: (39197) 25625.
Tomsk State Design-Research Institute of VNIPIET. Address: 634039 Tomsk oblast, Seversk, Kurchatov Street, 2. Director: Vitaliy Alekseyevich Mishin. Telephone: (35171) 79550. Fax: (35171) 73822.
Novosibirsk State Design-Research Institute of VNIPIET. Address: 630075 Novosibirsk-75, B. Khmelnitskiy Street, 2. Director: Anatoliy Vladimirovich Volushchuk. Telephone: (3832) 761315. Fax: (3832) 769613.
Sosnovyy Bor State Design-Research Institute of VNIPIET. Address: 188537 Leningrad oblast, Sosnovyy Bor, 50-th October Anniversary Street, 1, P. O. Box 115.
Sosnovyy Bor State Scientific-Research Institute of VNIPIET. Address: 188537 Leningrad oblast, Sosnovyy Bor, P. O. Box 49. Telephone: (81269) 79452. Fax: (81269) 63480.
Sosnovyy Bor State Scientific-Research Institute of VNIPIET. Address: 188537 Leningrad oblast, Sosnovyy Bor, P. O. Box 49. Director: Leonid Vasilyevich Kizhnerov. Telephone: (81269) 64373. Fax: (81269) 61932.
State Unitary Enterprise, Scientific-Research and Structural Design Institute, "Atomenergoproekt". Address: 107815 Moscow, Bakunin Street, block 7, building. 1. Director: Andrey Borisovich Malyshev. Telephone: (095) 2614187. Fax: (095) 2650974.
State Unitary Enterprise, St. Petersburg Scientific-Research and Structural Design Institute, "Atomenergoproekt". Address: 19306 St. Petersburg, Suvorov Avenue, 2a. Director: Vladislav Nikolayevich Korkunov. Telephone: (812) 2772196. Fax: (812) 2770703.
State Unitary Enterprise, Nizhegorod Scientific-Research and Structural Design Institute, "Atomenergoproekt". Address: 603006 N. Novgorod, GSP-54, Svoboda Square, 3. Director: Yevgeniy Mikhaylovich Koroloev. Telephone: (8312) 333424. Fax: (8312) 358490.
Joint-Stock Company, "Sverdlovsk Scientific-Research Institute of Chemical Machine-Building (SverdNIIkhimmash). Address: 620010 Yekaterinburg, Groboyedov Street, 32. Director: Boris Pimanovich Shevelin. Telephone: (3432) 274310. Fax: (3432) 275505.
State Unitary Enterprise, Experimental Design Bureau, "Gidropress" (OKB "GIDROPRESS"). Address: 142103 Moscow oblast, city of Podolsk, Ordzhonikidze Street, 21. Director: Yuriy Grigoryevich Dragunov. Telephone: (275) 42576. Fax: (275) 42516.
State Unitary Enterprise, "I. I. Afrikantov Experimental Design Bureau of Machine- Building". Address: 603074 N. Novgorod, Burnakov Avenue, 15. Director: Aleksandr Ivanovich Kiryushin. Telephone: (8312) 418772. Fax: (8312) 418772. [Sic. Fax no. is same as tel. no.]
State Unitary Enterprise, Central Design Bureau of Machine-Building" (TsKBM). Address: 195272 St. Petersburg, Krasnoyarsk Square, 3. Director: Yevgeniy Nikolayevich Sokolov. Telephone: (812) 2242075. Fax: (812) 2243257.
Science and Production Association, "Scientific-Research and Design Institute of Installation Technology" (NPO NIKIMT). Address: 127410 Moscow Altufyevskoye Highway, 43. Director: Leonid Nikolayevich Shchavelev. Telephone: (095) 4899095. Fax: (095) 903100.
Joint-Stock company, Design-Research and Scientific-Research Institute, "OrgstroyNIIproekt". Address: 113191 Moscow, Kholodilnyy Lane, 3a. Director: Nikolay Nikolayevich Yegorov. Telephone: (095) 2353841. Fax: (095) 2351941.
Joint-Stock Company, Lermontov Design-Research Institute, "OrgstroyNIIproekt". Address: 357340 Stavropol kray, city of Lermontov, Lermontov Avenue, 1. Director: Vladimir Stepenovich Sorokin. Telephone: (86535) 31604. Fax: (86535) 22191.
State Unitary Enterprise, Siberian Design Research Institute, "OrgstroyNIIproekt". Address: 665830 Irkutsk oblast, city of Angarsk, Vostochnaya Street, 14. Director: Viktor Ivanovich Shkaptsov. Telephone: (39518) 95945. Fax: (39518) 526745.
Joint-Stock Company, "Siberian Structural-Design Technological Institute". Address: 630055 Novosibirsk, M. Dzhalil Street, 21. Director: Aleksandr Vladimirovich Glinskiy. Telephone: (3832) 321330. Fax: (3832) 323245.
State Enterprise, Design-Technological Trust, "Orgstroy-11". Address: 113191 Moscow, Danilov Bank, 10/12. Director: Yuriy Aleksandrovich Pokrovskiy. Telephone: (095) 2376400. Fax: (095) 2376407.
Informational and analytical [facilities].
The Situation-Crisis Center. Address: 101100 Moscow, B. Ordynka Street, 24/26. Director: Venedikt Petrovich Berchik. Telephone: (095) 2392875. Fax: (095) 2382890.
Central Scientific-Research Institute of Control, Economics, and Information of The Ministry of Atomic Energy of Russia (TsNIIatominform). Address: 127434 Moscow, Dmirovskoye Highway, 2. Director: Nikolay Yegorovich Yakovlev. Telephone: (095) 9767272. Fax: (095) 9767203.
Novosibirsk Branch of TsNIIATOMINFORM (see acronym expansion above)--Branch Scientific-Technical Center of Information Science in Construction (ONTTs "Informstroy"). Address: 630055 Novosibirsk, M. Dzhalil Street, 23. Director: Anatoliy Nikolayevich Tseba. Telephone: (3832) 321747. Fax: (3832) 325853.
Angarsk Scientific-Research Center of Control, Economics, and Information Science (ATOMINFORM-A). Address: 665816 Irkutsk oblast, Angarsk, P. O. Box 289. Director: Vitaliy Valentinovich Denisenko. Telephone: (39518) 43621. Fax: (39518) 40262.
State Enterprise, "Chernobyl Archive". Address: 109017 Moscow, B. Ordynka Street, 24/26. Director: Yevgeniy Viktorovich Postnikov. Telephone: (095) 2394687. Fax: (095) 2392237.
Firm for Commercial Advertisement and Scientific-Technical Propagation. Address: 113105 Moscow, Varshavskoye Highway, 3. Director: Stanislav Mikhaylovich Tsvetayev. Telephone: (095) 9541082. Fax: (095) 9525963.
Inter-Branch Coordination Center, "Nuklid" Address: 194100 St. Petersburg, Lesnoy Avenue, 64. Director: Nina Simonovna Yanovskaya. Telephone: (812) 5429342. Fax: (812) 5426228.
Informational and Analytical Scientific-Research Institutes, Science Centers, and Scientific-Technical Centers of the Ministry of Atomic Energy of the Russian Federation:
State Unitary Enterprise, "Exhibition and Marketing Center." Address: 109017 Moscow, B. Ordynka Street, 24/26. Director: Galina Viktorovna Gorshteyn. Telephone: (095) 2392853. Fax: (095) 2392690.
Press-Service of the Ministry of Atomic Energy of the Russian Federation. Address: 109017 Moscow, B. Ordynka Street, 24/26. Director: Yuriy Grigoryevich Bespalko. Telephone: (095) 2394650. Fax: (095) 2392535.
Others:
All-Russian Scientific-Research Institute for the Comprehensive Use of Milk Raw Materials (VNIKIM). Address: 355040 Stavropol-40, Dovatortsev Street, 52a. Director: Veterinary Surgeon, Director of Milkmen. Telephone: (8652) 97592. Fax: (8652) 73615.
State Establishment, Institute for Problems of Secure Development of Atomic Power, Russian Academy of Sciences ( IBRAE RAN). Address: 113191 Moscow, B. Tulskaya Street, 52. Director: Leonid Aleksandrovich Bolshov, Corresponding Member of the Russian Academy of Sciences. Telephone: (095) 9522421. Fax: (095) 9581151. Web: http:
www.ibrae.ac.ru
Institute of Nuclear Physics of Budker (IyaF). Address: 630090 Novosibirsk, Lavrentyev Avenue, 11. Director: A. N. Skrynskiy. Telephone: (3832) 356031. Fax: (3832) 352163. Web: http:
www.inp.nsk.su.
D. V. Skobeltsyn Scientific-Research Institute of Nuclear Physics (NIIYaF MGU). Address: 119899 Moscow, Vorobyevy gory, NIIYaF MGU. Director: Mikhail Igoryevich Panasyuk. Telephone: (095) 9391818. Fax: (095) 9390896. Web: http:
www.npi.msu.su.
St. Petersburg Institute of Nuclear Physics. Address: 188350 Leningrad oblast, Gatchina. Director: V. A. Nazarenko. Telephone: (812) 7137196. Fax: (812) 7137196.
11. Educational Establishments.
Moscow State Engineering-Physics Institute (Technical University) (MIFI). Address: 115409 Moscow, Kashirovskoye Highway, 31. Director: Boris Nikolayevich Onykiy. Telephone: (095) 324 3384. Web: http:
www.mifi.ru
Ozersk Technological Institute of Moscow State Engineering-Physics Institute. Address: 456783 Chelyabinsk oblast, city of Ozersk, Pobeda Avenue, 48. Director: Yuriy Nikolayevich Stepanov, Rector. Telephone: (35171) 44646.
Novouralsk Polytechnic Institute of Moscow State Engineering-Physics Institute. Address: 624130 Sverdlovsk oblast, city of Novouralsk-3, Lenin street, 85. Director: Aleksey Petrovich Dyagilev. Telephone: (34370) 23580.
Polytechnic Institute of Moscow State Engineering-Physics Institute. Address: 620045 Sverdlovsk oblast, city of Lesnoy, Kommunist Avenue, 36. Director: Vyacheslav Mikhaylovich Khrapal. Telephone: (34342) 60963. Fax: (34342) 55621.
Sarov Physics-Technical Institute of Moscow State Engineering-Physics Institute. Address: 607200 Nizhegorod oblast, Sarov, Dukhov Street, 6. Director: Yuriy Petrovich Shcherbak. Telephone: (83130) 59809.
Trekhgornyy Polytechnic Institute of Moscow State Engineering-Physics Institute. Address: 456080 Chelyabinsk oblast, city of Trekhgornyy, Mir Street, 17. Director: Fedor Ivanovich Dolinin, Rector. Telephone: (35111) 67067.
Snezhinsk Physics-Technical Institute--Branch of Moscow State Engineering-Physics Institute. Address: 456776 Chelyabinsk oblast, city of Snezhinsk, Mir Street, 6/8. Director: Vladimir Makarovich Skovpen. Telephone: (35172) 32422. Fax: (35172) 32256.
Northern Technological Institute of Tomsk Polytechnic University. Address: 634036 Tomsk-36, Kommunist Avenue, 65. Director: Aleksandr Nikolayevich Zhiganov. Telephone: (3822) 779529.
St. Petersburg State Technical University. Address: 195251 St. Petersburg, Politekhnicheskaya Street, 29. Director: Yuriy Sergeyevich Vasilyev, President. Telephone: (812) 2471616. Fax: (812) 5527882.
St. Petersburg State Technological Institute (Technical University), Engineering Physics- Chemistry Faculty. Address: 198013 St. Petersburg, Moscow Avenue, 26. Director: Anatoliy Sergeyevich Dudyrev. Telephone: (812) 2596500. Fax: (812) 1127791.
Moscow Power Institute (MEI). Address: 105835 Moscow, Krasnokazarmennaya Street, 14. Director: Yevgennyy Viktorovich Ametistov, Rector. Telephone: (095) 3627088. Web: http:
www.mpei.ac.ru
Inter-Branch Special Training Center affiliated to the Ministry of Atomic Energy of Russia. Address: 249020 Kaluga oblast, Obninsk, Kurchatov Street, 21. Director: Vladimir Nikolayevich Serikov. Telephone: (08439) 25344. Fax: (08439) 48510.
State Central Institute for Qualification Enhancement. Address: 249020 Kaluga oblast, Obninsk, Kurchatov Street, 21. Director: Yuriy Petrovich Rydnev. Telephone: (08439) 48833. Fax: (08439) 48011.
Moscow Institute for Qualification Enhancement, "Atomenergo". Address: 125413 Moscow, Senezhskaya Street, 1/9. Director: Nikolay Ivanovich Ishchenko, Rector. Telephone: (095) 453 0277. Fax: (095) 453 8559.
State Regional Educational Center of the Ministry of Atomic Energy of Russia. Address: 197348 St. Petersburg, Aerodromnaya Street, 4. Director: Yuriy Petrovich Lisnenko, Rector. Telephone: (812) 3945005.
Ural Institute for Qualification Enhancement, "Progress". Address: 624133 Sverdlovsk oblast, city of Novouralsk, Pervomayskaya Street, 107. Director: Valeriy Ivanovich Makarov. Telephone: (34370) 91601. Fax: (34370) 91354. Web: http:
/ Siberian Institute for Qualification Enhancement, "Spetsmontazh". Address: 630075 Novosibirsk-75, Narodnaya Street, 7/1. Director: Oleg Igorevich Sidorov. Telephone: (3832) 760564. Fax: (3832) 760117.
Central Scientific-Research Institute of Control, Economics, and Information of the Ministry of Atomic energy of Russia (TsNIIatominform). Address: 127434 Moscow, Dmitrovskoye Highway, 2. Director: Nikolay Yegorovich Yakovlev. Telephone: (095) 9767272. Fax: (095) 9767203.
Novosibirsk Branch of TsNIATOMINFORM [see acronym expansion above]--Branch Scientific-Technical Center of Information Science in Construction (ONTTs "Informstroy). Address: 630055 Novosibirsk, M. Dzhalil Street, 23. Director: Anatoliy Nikolayevich Tseba. Telephone: (3832) 321747. Fax: (3832) 325853.
Angarsk Scientific-Research Center of Control, Economics, and Information (ATOMINFORM-A). Address: 665816 Irkutsk oblast, Angarsk, P. O. Box 289. Director: Vitaliy Valentinovich Denisenko. Telephone: (39518) 43621. Fax: (39518) 40262.
State Enterprise, "Chernobyl Archive". Address: 109017 Moscow, B. Ordynka Street, 24/26. Director: Yevgenyy Viktorovich Postnikov. Telephone: (095) 2394687. Fax: (095) 2392237.
Firm for Commercial Advertisement and Scientific-Technical Propagation. Address: 103105 Moscow, Varshavskoye Highway, 3. Director: Stanislav Mikhaylovich Tsvetayev. Telephone: (095) 9541082. Fax: (095) 9525963.
Northern Indusrial College (municipal educational establishment). Address: 636070 Tomsk obalst, Seversk, Kommunist Avenue, 65. Director: Aleksandr Nikolayevich Zhiganov. Telephone: (3822) 779529.
Beloyarsk Polytechnic College Address: 624051 Sverdlovsk oblast, Zarechnyy, Lenin Street, 27. Director: Oleg Nikolayevich Arefyev. Telephone: (34377) 32004.
Balakhna Polytechnic College. Address: 606400 Nizhegorod oblast, Balakhna, Dzerzhinskiy Street, 21. Director: Aleksandr Aleksandrovich Checherin. Telephone: (83144) 20781.
Moscow Industrial College. Address: 113191 Moscow, Kholodilnyy Lane, 7. Director: Viktor Sergeyevich Geraskin. Telephone: (095) 9522621.
Moscow Oblast Polytechnic College. Address: 144000 Mocow oblast, Electrostal, Lenin Street, 41. Director: Nikolay Stepanovich. Telephone: (095) 7029028.
Siberian Polytechnic College. Address: 630075 Novosibirsk-75, B. Khmelnitskiy Street, 9. Director: Pavel Andreyevich Tereshchenko. Telephone: (3832) 760239.
Novovoronezh Polytechnic College. Address: 396072 Voronezh oblast, Novovoronezh, Oktyabrskaya Street. Director: Mikhail Alekseyevich Dukhanin. Telephone: (07364) 28096.
Yuzhnouralsk Polytechnic College. Address: 456783 Chelyabinsk oblast, Ozersk, Pobeda Street, 48. Director: Angelina Valentinovna Romanovna. Telephone: (35171) 44623.
Uralsk Polytechnic College. Address: 624130 Sverdlovsk oblast, Novouralsk-3, Lenin street, 85. Director: Leonid Nikolayevich Mochalov. Telephone: (34370) 22497.
Volgodonsk Engineering College of Power-Machine Building. Address: 347340 Rostov oblast, Volgodonsk, Lenin street, 27. Director: Tamara Vailyevna Bazavova. Telephone: (86392) 25673.
Zarechnyy Industrial College (municipal educational establishment). Address: 249020 Kaluga oblast, Obninsk, Lenin Avenue, 71. Director: Vladimir Petrovich Petrov. Telephone: (08439) 61209.
Obninsk Poly-Engineering College. Address: 249020 Kaluga oblast, Obninsk, Lenin Avenue, 71. Director: Vladimir Petrovich Petrov. Telephone: (08439) 61209.
Krasnoyarsk Electro-Mechanical Engineering College. Address: 663690 Krasnoyarsk kray, Zelenogorsk, Bortnikov Street, 13. Director: Grigoriy Antipyevich Porsev. Telephone: (39169) 34433.
Angarsk Polytechnic College. Address: 665030 Irkutsk oblast, Angarsk, P. O. Box 60. Director: Yuriy Vasilyevich Dragunov. Telephone: (3951) 999362.
Sosnovyy Bor Branch of Moscow Obalst Polytechnic College. Address: 188537 Leningrad oblast, Sosnovyy Bor, Mir Street, 5. Director: Tatyana Nikolayevna Keller. Telephone: (812) 69662224.
Protvino Branch of Moscow Oblast Polytechnic College. Address: 142284 Moscow Oblast, Serpukhov rayon, P. O. Box 66. Director: Nadezhna Pavlovna Sholokhova, Branch Head. Telephone: (09677) 41362.
Kirovo-Chepetsk Branch of Moscow Oblast Polytechnic College. Address: 613020 Kirov oblast, Kirovo-Chepetsk, P. O. Box 32. Director: Lidiya Aleksandrovna Malykh, Branch Head. Telephone: (83361) 31296.
Dubna Branch of Moscow Oblast Polytechnic College. Address: 141980 Moscow oblast, Dubna, Priborstroitelnaya Street, 2. Director: Galina Nikolayevna Lepunova, Head of Branch. Telephone: (09621) 40523.
Glazov Branch of Moscow Oblast Polytechnic College. Address: 427600 Udmurt Republic, Glazov, General Post Office, P. O. Box 238. Director: Olga Andreyevna Trushkina, Head of Branch. Telephone: (34141) 30476.
Professional-Technical School No. 1. Address: 171850 Tver Oblast, Udomlya, Kurchatov Avenue, 8. Director: Viktor Mikhaylovich Bezverkhov. Telephone: (08255) 43075.
12. Export Services.
Joint-Stock Company, "Tekhsnabeksport". Address: 109180 Moscow, Staromonetnyy Lane, 26. Director: Revmir Georgiyevich Frayshtut. Telephone: (095) 9533864. Fax: (095) 2302638.
Firm, "Uranservis"--Director Aleksy Antonovich Grigoryev. Firm, "TVELy"--Director Oleg Valeriyevich Bondarenko. Firm, "Atomimpeks"--Director Viktor Mikhaylovich Rodin. Currency and Finance Section. Section for State of Market and Prices. Section for Foreign Relations and Protocol. Section for Informational Provision. Section for Processing and Protection of Information.
Separation Plants.
Ural Electro-Chemical Combine. Separation Plant. Address: 624130 Novouralsk, Sverdlovsk oblast, Dzerzhinskiy Street, 2. Director: Anatoliy Petrovich Knutarev, General Director. Telephone: (34370) 92424. Fax: (34370) 94141. Web: http:
www.ricon.e-burg.ru
Electro-Chemical Plant (EkhZ). Separation Plant. Address: 663690 Zelenogorsk, Krasnoyarsk kray. Director: Anatoliy Nikolayevich Shubin, General Director. Telephone: (39169) 33350, 33321.
Siberian Chemical Combine. Separation Plant. Address: 636070 Seversk, Tomsk oblast, Kurchatov oblast, 1. Director: Valeriy Konstantinovich Larin, General Director. Telephone: (3822) 771798. Fax: (3822) 772528. Web: http:
Angarsk Electrolytic Chemical Combine. Separation Plant. Address: 665804 Irkutsk oblast, city of Angarsk. Director: Viktor Panteleymonovich Shopen. Telephone: (39518) 40710. Fax: 39518) 66715.
Joint-Stock Company, "Atomstroyeksport". Address: 113184 Moscow, Malaya Ordynka Street, 35, building 3. Director: Viktor Vasilyevich Kozlov. Telephone: (095) 7379037.
All-Regional Association, "Izotop" [Isotope].
State Unitary Enterprise, All-Regional Association, "Izotop" (V/O "Izotop"). Address: 119435 Moscow, Pogodinskaya Street, 22. Director: Boris Viktorovich Akakiyev. Telephone: (095) 2450118. Fax: (095) 2452492.
Yekaterinburg Enterprise, "Izotop". Address: 620142 Yekaterinburg, Belinskiy Street, 143. Director: Lilian Akhramovich Khamitov. Telephone: (3432) 223149. Fax: (3432) 227473.
Khabarovsk Enterprise, "Izotop". Address: 680020 Khabarovsk, Volchayevskaya Street, 83. Director: Vladimir Vladimirovich Fedorov. Telephone: (4212) 222025. Fax: (4212) 222025.
13. Business Partners of Enterprises of Ministry of Atomic Energy (none listed).
14. Banks, Insurance Organizations, and Investment Companies (none listed).
15. Ecological Organizations.
Bellona. Web: http:
www.bellona.no.
Green World. Web: http:
www.spb.org.ru/greenworld
Social-Ecological Union. Web: http:
www.cci.glasnet.ru/seu
Contructive-Ecological Movement, "Kedr". (no listing)
Ecoline. Web: http:
www.cci.glasnet.ru
World-Wide Informational Service for Problems of Energy. Web: http:
www.antenna.nl
E-tip. Web: http:
www.ecologia.nier.org
EcoNet. Web: http:
www.igs.org/igs/econet
Grinpis [Greenpeace] Russia. Web: http:
www.greenpeace.ru
Grinpis Interneshnl [Greenpeace International]. Web: http:
www.greenpeace.org
Prima-M. Web: http:
www.glasnet.ru/aoprima
Center for Disarmament, Energy, and Ecology MFTI [Moscow Institute of Physics and Technology]. Web: http:
www.armscontrol.ru
16. Social and Non-Commercial Organizations. (none listed)
17. Strategic Nuclear Forces of Russia. (none listed)

COMMENTS
Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people. Every drug, device, tool, diagnostic test, technique and technology used in medicine today was ...
Medical and clinical research can be classified in many different ways. Probably, most people are familiar with basic (laboratory) research, clinical research, healthcare (services) research, health systems (policy) research, and educational research. Clinical research in this review refers to scientific research related to clinical practices.
Research. Mayo Clinic staff members are actively engaged in research on several areas within Laboratory Medicine and Pathology. Mayo Clinic researchers continually study new and more efficient medical testing techniques and reflect their advances in tests offered internally at Mayo Clinic as well as externally though Mayo Clinic Laboratories.
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness and safety of medications, devices, ... Once the promising candidate or the molecule is identified in the lab, it is subjected to pre-clinical studies or animal studies where different aspects of the test article ...
Medical lab scientists work in hospitals, clinics, forensic or public health laboratories, as well as pharmaceutical industries, biotechnology companies, veterinary clinics, or research institutions. Depending on the setting, their work hours may vary; but typically labs are run 24 hours a day, seven days a week.
Clinical Laboratory Science, also called Medical Laboratory Science or Medical Technology, is the health profession that provides laboratory information and services needed for the diagnosis and treatment of disease. Clinical Laboratory Scientists perform a variety of laboratory tests, ensure the quality of the test results, explain the significance of laboratory tests, evaluate new methods ...
The University of Florida Cancer and Genetics Research Complex is an integrated medical research facility. Medical research (or biomedical research ), also known as health research, refers to the process of using scientific methods with the aim to produce knowledge about human diseases, the prevention and treatment of illness, and the promotion ...
What is Clinical Research? Clinical research occurs in many formats and can involve anyone. Learn how you can participate and contribute to medical advances. This page last reviewed on September 29, 2016.
Clinical laboratories are healthcare facilities providing a wide range of laboratory procedures that aid clinicians in diagnosing, treating, and managing patients.[1] These laboratories are manned by scientists trained to perform and analyze tests on samples of biological specimens collected from patients.
A. Advanced Endoscopy Innovation Translation and Clinical Trials Group: Barham Abu Dayyeh. Advanced Medical Imaging Technology: Richard L. Ehman. Aerospace Medicine and Vestibular Research: Michael J. Cevette, Jan Stepanek. Aging and Dementia Imaging Research (ADIR): Clifford R. Jack Jr.; Kejal Kantarci; Prashanthi Vemuri.
A medical laboratory or clinical laboratory is a laboratory where tests are conducted out on clinical specimens to obtain information about the health of a patient to aid in diagnosis, treatment, and prevention of disease. [1] Clinical medical laboratories are an example of applied science, as opposed to research laboratories that focus on ...
Clinical research involves studies that include human participants, aiming to understand health and illness and answer medical questions. Laboratory research, on the other hand, takes place in environments such as chemistry or biology labs, typically at colleges or medical schools, and does not involve human subjects.
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. ... Laboratory conditions cannot always be directly transferred to normal clinical practice and processes in isolated cells or ...
The IU Health Medical Research Laboratory is designed and staffed to provide services to support research, development, education and investigation. These facilities are used by physicians, nursing staff and allied health personnel, as well as external organizations. The laboratory offers physicians the opportunity to become familiar with state ...
Nadine Lerret, PhD, MLS (ASCP) CM Director of Research (312) 942-2780. At Rush University, we believe that basic, clinical and translational research activities serve as the foundation for advances in patient care. Our graduate students are introduced to research related to medical laboratory science early on — in the first academic quarter ...
NIH Institutes. National Cancer Institute (NCI) — Est. 1937 NCI leads a national effort to eliminate the suffering and death due to cancer. Through basic and clinical biomedical research and training, NCI conducts and supports research that will lead to a future in which we can prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through ...
The MRC Laboratory of Molecular Biology (LMB) is a research institute dedicated to the understanding of important biological processes at the levels of atoms, molecules, cells and organisms. In doing so, we provide knowledge needed to solve key problems in human health. Our scientists tackle fundamental, often difficult and long-term research ...
The medical laboratory science profession involves the analysis of blood, body fluids, tissues, and cells to aid in the diagnosis, treatment, and prevention of disease. A Medical Laboratory Scientist (MLS) is a vital member of the healthcare team with employment opportunities in hospital, clinic, research, and pharmaceutical laboratories.
The Laboratory of Investigative Medicine (LIM) is dedicated to analyzing biological specimens for biochemical and pharmacological endpoints to support clinical studies at Thomas Jefferson University. The laboratory participates in the processing and/or analysis of about 5,000 to 10,000 specimens each year. The laboratory has three components ...
The Department of Veterans Affairs (VA) gives notice under the Federal Advisory Committee Act, 5 U.S.C. Ch.10, that a meeting of the Joint Biomedical Laboratory Research and Development and Clinical Science Research and Development Services Scientific Merit Review Board will be held July 9, 2024, via Webex. The meeting will be held between 3 p ...
The Department of Internal Medicine/Center for Hypothalamic Research is recruiting for a Research Associate position in the lab of Dr. Chen Liu. The Liu lab is a multi-disciplinary lab studying the central regulation of metabolism and glucose homeostasis. We use animal models along with cutting edge molecular techniques in our studies.
Among the study subjects having a positive attitude towards research, over 60% were from pre- and para- clinical years. The barriers for conducting research were mostly; lack of funds/laboratory equipment/infrastructure (85.1%), lack of exposure to opportunities for research in the medical (MBBS) curriculum (83.8%), and lack of time (83.3%).
When appropriate, assist the medical physics group in clinical development, research initiatives, and operations optimization projects. Minimum Qualifications. A bachelor's degree in physics, ... research assistant (RA)/Technician to assist in the day-to-day technical tasks of the laboratory. RA will be responsible for maintaining breeding ...
School of Medicine . Established in 1930, Duke University School of Medicine is the youngest of the nation's top medical schools. Ranked sixth among medical schools in the nation, the School takes pride in being an inclusive community of outstanding learners, investigators, clinicians, and staff where interdisciplinary collaboration is embraced and great ideas accelerate translation of ...
New research conducted by the Brenhouse Lab reveals how early life adversity triggers early puberty and late-life anxiety, paving the way for potential interventions. The onset of puberty has been ...
Elektrostal. Elektrostal ( Russian: Электроста́ль) is a city in Moscow Oblast, Russia. It is 58 kilometers (36 mi) east of Moscow. As of 2010, 155,196 people lived there.
Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies.
State Unitary Branch SNPO [Special Science and Production Association], "Yeleron". (Scientific- Research and Design Institute of Radio- Electronic Engineering.) Address: 440901 Penza oblast, Zarechnyy, Mir Avenue, 1. Director: Yuriy Aleksandrovich Olenin. Telephone: (8412) 692474. Fax: (8412) 552528. Central Scientific Research Laboratory.