Pilot Study in Research: Definition & Examples
Julia Simkus
Editor at Simply Psychology
BA (Hons) Psychology, Princeton University
Julia Simkus is a graduate of Princeton University with a Bachelor of Arts in Psychology. She is currently studying for a Master's Degree in Counseling for Mental Health and Wellness in September 2023. Julia's research has been published in peer reviewed journals.
Learn about our Editorial Process
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
A pilot study, also known as a feasibility study, is a small-scale preliminary study conducted before the main research to check the feasibility or improve the research design.
Pilot studies can be very important before conducting a full-scale research project, helping design the research methods and protocol.

How Does it Work?
Pilot studies are a fundamental stage of the research process. They can help identify design issues and evaluate a study’s feasibility, practicality, resources, time, and cost before the main research is conducted.
It involves selecting a few people and trying out the study on them. It is possible to save time and, in some cases, money by identifying any flaws in the procedures designed by the researcher.
A pilot study can help the researcher spot any ambiguities (i.e., unusual things), confusion in the information given to participants, or problems with the task devised.
Sometimes the task is too hard, and the researcher may get a floor effect because none of the participants can score at all or can complete the task – all performances are low.
The opposite effect is a ceiling effect, when the task is so easy that all achieve virtually full marks or top performances and are “hitting the ceiling.”
This enables researchers to predict an appropriate sample size, budget accordingly, and improve the study design before performing a full-scale project.
Pilot studies also provide researchers with preliminary data to gain insight into the potential results of their proposed experiment.
However, pilot studies should not be used to test hypotheses since the appropriate power and sample size are not calculated. Rather, pilot studies should be used to assess the feasibility of participant recruitment or study design.
By conducting a pilot study, researchers will be better prepared to face the challenges that might arise in the larger study. They will be more confident with the instruments they will use for data collection.
Multiple pilot studies may be needed in some studies, and qualitative and/or quantitative methods may be used.
To avoid bias, pilot studies are usually carried out on individuals who are as similar as possible to the target population but not on those who will be a part of the final sample.
Feedback from participants in the pilot study can be used to improve the experience for participants in the main study. This might include reducing the burden on participants, improving instructions, or identifying potential ethical issues.
Experiment Pilot Study
In a pilot study with an experimental design , you would want to ensure that your measures of these variables are reliable and valid.
You would also want to check that you can effectively manipulate your independent variables and that you can control for potential confounding variables.
A pilot study allows the research team to gain experience and training, which can be particularly beneficial if new experimental techniques or procedures are used.
Questionnaire Pilot Study
It is important to conduct a questionnaire pilot study for the following reasons:
- Check that respondents understand the terminology used in the questionnaire.
- Check that emotive questions are not used, as they make people defensive and could invalidate their answers.
- Check that leading questions have not been used as they could bias the respondent’s answer.
- Ensure that the questionnaire can be completed in a reasonable amount of time. If it’s too long, respondents may lose interest or not have enough time to complete it, which could affect the response rate and the data quality.
By identifying and addressing issues in the pilot study, researchers can reduce errors and risks in the main study. This increases the reliability and validity of the main study’s results.
Assessing the practicality and feasibility of the main study
Testing the efficacy of research instruments
Identifying and addressing any weaknesses or logistical problems
Collecting preliminary data
Estimating the time and costs required for the project
Determining what resources are needed for the study
Identifying the necessity to modify procedures that do not elicit useful data
Adding credibility and dependability to the study
Pretesting the interview format
Enabling researchers to develop consistent practices and familiarize themselves with the procedures in the protocol
Addressing safety issues and management problems
Limitations
Require extra costs, time, and resources.
Do not guarantee the success of the main study.
Contamination (ie: if data from the pilot study or pilot participants are included in the main study results).
Funding bodies may be reluctant to fund a further study if the pilot study results are published.
Do not have the power to assess treatment effects due to small sample size.
- Viscocanalostomy: A Pilot Study (Carassa, Bettin, Fiori, & Brancato, 1998)
- WHO International Pilot Study of Schizophrenia (Sartorius, Shapiro, Kimura, & Barrett, 1972)
- Stephen LaBerge of Stanford University ran a series of experiments in the 80s that investigated lucid dreaming. In 1985, he performed a pilot study that demonstrated that time perception is the same as during wakefulness. Specifically, he had participants go into a state of lucid dreaming and count out ten seconds, signaling the start and end with pre-determined eye movements measured with the EOG.
- Negative Word-of-Mouth by Dissatisfied Consumers: A Pilot Study (Richins, 1983)
- A pilot study and randomized controlled trial of the mindful self‐compassion program (Neff & Germer, 2013)
- Pilot study of secondary prevention of posttraumatic stress disorder with propranolol (Pitman et al., 2002)
- In unstructured observations, the researcher records all relevant behavior without a system. There may be too much to record, and the behaviors recorded may not necessarily be the most important, so the approach is usually used as a pilot study to see what type of behaviors would be recorded.
- Perspectives of the use of smartphones in travel behavior studies: Findings from a literature review and a pilot study (Gadziński, 2018)
Further Information
- Lancaster, G. A., Dodd, S., & Williamson, P. R. (2004). Design and analysis of pilot studies: recommendations for good practice. Journal of evaluation in clinical practice, 10 (2), 307-312.
- Thabane, L., Ma, J., Chu, R., Cheng, J., Ismaila, A., Rios, L. P., … & Goldsmith, C. H. (2010). A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology, 10 (1), 1-10.
- Moore, C. G., Carter, R. E., Nietert, P. J., & Stewart, P. W. (2011). Recommendations for planning pilot studies in clinical and translational research. Clinical and translational science, 4 (5), 332-337.
Carassa, R. G., Bettin, P., Fiori, M., & Brancato, R. (1998). Viscocanalostomy: a pilot study. European journal of ophthalmology, 8 (2), 57-61.
Gadziński, J. (2018). Perspectives of the use of smartphones in travel behaviour studies: Findings from a literature review and a pilot study. Transportation Research Part C: Emerging Technologies, 88 , 74-86.
In J. (2017). Introduction of a pilot study. Korean Journal of Anesthesiology, 70 (6), 601–605. https://doi.org/10.4097/kjae.2017.70.6.601
LaBerge, S., LaMarca, K., & Baird, B. (2018). Pre-sleep treatment with galantamine stimulates lucid dreaming: A double-blind, placebo-controlled, crossover study. PLoS One, 13 (8), e0201246.
Leon, A. C., Davis, L. L., & Kraemer, H. C. (2011). The role and interpretation of pilot studies in clinical research. Journal of psychiatric research, 45 (5), 626–629. https://doi.org/10.1016/j.jpsychires.2010.10.008
Malmqvist, J., Hellberg, K., Möllås, G., Rose, R., & Shevlin, M. (2019). Conducting the Pilot Study: A Neglected Part of the Research Process? Methodological Findings Supporting the Importance of Piloting in Qualitative Research Studies. International Journal of Qualitative Methods. https://doi.org/10.1177/1609406919878341
Neff, K. D., & Germer, C. K. (2013). A pilot study and randomized controlled trial of the mindful self‐compassion program. Journal of Clinical Psychology, 69 (1), 28-44.
Pitman, R. K., Sanders, K. M., Zusman, R. M., Healy, A. R., Cheema, F., Lasko, N. B., … & Orr, S. P. (2002). Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological psychiatry, 51 (2), 189-192.
Richins, M. L. (1983). Negative word-of-mouth by dissatisfied consumers: A pilot study. Journal of Marketing, 47 (1), 68-78.
Sartorius, N., Shapiro, R., Kimura, M., & Barrett, K. (1972). WHO International Pilot Study of Schizophrenia1. Psychological medicine, 2 (4), 422-425.
Teijlingen, E. R; V. Hundley (2001). The importance of pilot studies, Social research UPDATE, (35)
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

A tutorial on pilot studies: the what, why and how
Lehana thabane, afisi ismaila, lorena p rios, reid robson, marroon thabane, lora giangregorio, charles h goldsmith.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2009 Aug 9; Accepted 2010 Jan 6; Collection date 2010.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Pilot studies for phase III trials - which are comparative randomized trials designed to provide preliminary evidence on the clinical efficacy of a drug or intervention - are routinely performed in many clinical areas. Also commonly know as "feasibility" or "vanguard" studies, they are designed to assess the safety of treatment or interventions; to assess recruitment potential; to assess the feasibility of international collaboration or coordination for multicentre trials; to increase clinical experience with the study medication or intervention for the phase III trials. They are the best way to assess feasibility of a large, expensive full-scale study, and in fact are an almost essential pre-requisite. Conducting a pilot prior to the main study can enhance the likelihood of success of the main study and potentially help to avoid doomed main studies. The objective of this paper is to provide a detailed examination of the key aspects of pilot studies for phase III trials including: 1) the general reasons for conducting a pilot study; 2) the relationships between pilot studies, proof-of-concept studies, and adaptive designs; 3) the challenges of and misconceptions about pilot studies; 4) the criteria for evaluating the success of a pilot study; 5) frequently asked questions about pilot studies; 7) some ethical aspects related to pilot studies; and 8) some suggestions on how to report the results of pilot investigations using the CONSORT format.
1. Introduction
The Concise Oxford Thesaurus [ 1 ] defines a pilot project or study as an experimental, exploratory, test, preliminary, trial or try out investigation. Epidemiology and statistics dictionaries provide similar definitions of a pilot study as a small scale
• " ... test of the methods and procedures to be used on a larger scale if the pilot study demonstrates that the methods and procedures can work" [ 2 ];
• "...investigation designed to test the feasibility of methods and procedures for later use on a large scale or to search for possible effects and associations that may be worth following up in a subsequent larger study" [ 3 ].
Table 1 provides a summary of definitions found on the Internet. A closer look at these definitions reveals that they are similar to the ones above in that a pilot study is synonymous with a feasibility study intended to guide the planning of a large-scale investigation. Pilot studies are sometimes referred to as "vanguard trials" (i.e. pre-studies) intended to assess the safety of treatment or interventions; to assess recruitment potential; to assess the feasibility of international collaboration or coordination for multicentre trials; to evaluate surrogate marker data in diverse patient cohorts; to increase clinical experience with the study medication or intervention, and identify the optimal dose of treatments for the phase III trials [ 4 ]. As suggested by an African proverb from the Ashanti people in Ghana " You never test the depth of a river with both feet ", the main goal of pilot studies is to assess feasibility so as to avoid potentially disastrous consequences of embarking on a large study - which could potentially "drown" the whole research effort.
Some Adapted Definitions of Pilot Studies on the Web (Date of last access: December 22, 2009)
| Definition* | Source |
|---|---|
| A trial study carried out before a research design is finalised or | |
| A smaller version of a study is carried out before the actual investigation is done. Researchers use information gathered in pilot studies | |
| A small scale study conducted to test the plan and method of a research study. | |
| A small study | |
| An experimental use of a treatment in a small group of patients | |
| The or treatment | |
| A small study often done . | |
| Small, preliminary test or trial run of an intervention, or of an evaluation activity such as an instrument or sampling procedure. The results of the pilot are . | |
*Emphasis is ours
Feasibility studies are routinely performed in many clinical areas. It is fair to say that every major clinical trial had to start with some piloting or a small scale investigation to assess the feasibility of conducting a larger scale study: critical care [ 5 ], diabetes management intervention trials [ 6 ], cardiovascular trials [ 7 ], primary healthcare [ 8 ], to mention a few.
Despite their noted importance, the reality is that pilot studies receive little or no attention in scientific research training. Few epidemiology or research textbooks cover the topic with the necessary detail. In fact, we are not aware of any textbook that dedicates a chapter on this issue - many just mention it in passing or provide a cursory coverage of the topic. The objective of this paper is to provide a detailed examination of the key aspects of pilot studies. In the next section, we narrow the focus of our definition of a pilot to phase III trials. Section 3 covers the general reasons for conducting a pilot study. Section 4 deals with the relationships between pilot studies, proof-of-concept studies, and adaptive designs, while section 5 addresses the challenges of pilot studies. Evaluation of a pilot study (i.e. how to determine if a pilot study was successful) is covered in Section 6. We deal with several frequently asked questions about pilot studies in Section 7 using a "question-and-answer" approach. Section 8 covers some ethical aspects related to pilot studies; and in Section 9, we follow the CONSORT format [ 9 ] to offer some suggestions on how to report the results of pilot investigations.
2. Narrowing the focus: Pilot studies for randomized studies
Pilot studies can be conducted in both quantitative and qualitative studies. Adopting a similar approach to Lancaster et al . [ 10 ], we focus on quantitative pilot studies - particularly those done prior to full-scale phase III trials. Phase I trials are non-randomized studies designed to investigate the pharmacokinetics of a drug (i.e. how a drug is distributed and metabolized in the body) including finding a dose that can be tolerated with minimal toxicity. Phase II trials provide preliminary evidence on the clinical efficacy of a drug or intervention. They may or may not be randomized. Phase III trials are randomized studies comparing two or more drugs or intervention strategies to assess efficacy and safety. Phase IV trials, usually done after registration or marketing of a drug, are non-randomized surveillance studies to document experiences (e.g. side-effects, interactions with other drugs, etc) with using the drug in practice.
For the purposes of this paper, our approach to utilizing pilot studies relies on the model for complex interventions advocated by the British Medical Research Council - which explicitly recommends the use of feasibility studies prior to Phase III clinical trials, but stresses the iterative nature of the processes of development, feasibility and piloting, evaluation and implementation [ 11 ].
3. Reasons for Conducting Pilot Studies
Van Teijlingen et al . [ 12 ] and van Teijlingen and Hundley [ 13 ] provide a summary of the reasons for performing a pilot study. In general, the rationale for a pilot study can be grouped under several broad classifications - process, resources, management and scientific (see also http://www.childrens-mercy.org/stats/plan/pilot.asp for a different classification):
• Process: This assesses the feasibility of the steps that need to take place as part of the main study. Examples include determining recruitment rates, retention rates, etc.
• Resources: This deals with assessing time and budget problems that can occur during the main study. The idea is to collect some pilot data on such things as the length of time to mail or fill out all the survey forms.
• Management: This covers potential human and data optimization problems such as personnel and data management issues at participating centres.
• Scientific: This deals with the assessment of treatment safety, determination of dose levels and response, and estimation of treatment effect and its variance.
Table 2 summarizes this classification with specific examples.
Reasons for conducting pilot studies
| Main Reason | Examples |
|---|---|
| This assesses the feasibility of the processes that are key to the success of the main study | • Recruitment rates |
| • Retention rates | |
| • Refusal rates | |
| • Failure/success rates | |
| • (Non)compliance or adherence rates | |
| • eligibility criteria | |
| - Is it obvious who meets and who does not meet the eligibility requirements? | |
| - Are the eligibility criteria sufficient or too restrictive? | |
| • Understanding of study questionnaires or data collection tools: | |
| - Do subjects provide no answer, multiple answers, qualified answers, or unanticipated answers to study questions? | |
| This deals with assessing time and resource problems that can occur during the main study | • Length of time to fill out all the study forms |
| • Determining capacity: | |
| - Will the study participants overload your phone lines or overflow your waiting room? | |
| • Determining process time | |
| - How much time does it take to mail out a thousand surveys? | |
| • Is the equipment readily available when and where it is needed? | |
| • What happens when it breaks down or gets stolen? | |
| • Can the software used for capturing data read and understand the data? | |
| • Determining centre willingness and capacity | |
| - Do the centres do what they committed to doing? | |
| - Do investigators have the time to Perform the tasks they committed to doing? | |
| - Are there any capacity issues at each participating centre? | |
| This covers potential human and data management problems | • What are the challenges that participating centres have with managing the study? |
| • What challenges do study personnel have? | |
| • Is there enough room on the data collection form for all of the data you receive? | |
| • Are there any problems entering data into the computer? | |
| • Can data coming from different sources be matched? | |
| • Were any important data values forgotten about? | |
| • Do data show too much or too little variability? | |
| This deals with the assessment of treatment safety, dose, response, effect and variance of the effect | • Is it safe to use the study drug/intervention? |
| • What is the safe dose level? | |
| • Do patients respond to the drug? | |
| • What is the estimate of the treatment effect? | |
| • What is the estimate of the variance of the treatment effect? | |
4. Relationships between Pilot Studies, Proof-of-Concept Studies, and Adaptive Designs
A proof-of-concept (PoC) study is defined as a clinical trial carried out to determine if a treatment (drug) is biologically active or inactive [ 14 ]. PoC studies usually use surrogate markers as endpoints. In general, they are phase I/II studies - which, as noted above, investigate the safety profile, dose level and response to new drugs [ 15 ]. Thus, although designed to inform the planning of phase III trials for registration or licensing of new drugs, PoC studies may not necessarily fit our restricted definition of pilot studies aimed at assessing feasibility of phase III trials as outlined in Section 2.
An adaptive trial design refers to a design that allows modifications to be made to a trial's design or statistical procedures during its conduct, with the purpose of efficiently identifying clinical benefits/risks of new drugs or to increase the probability of success of clinical development [ 16 ]. The adaptations can be prospective (e.g. stopping a trial early due to safety or futility or efficacy at interim analysis); concurrent (e.g. changes in eligibility criteria, hypotheses or study endpoints) or retrospective (e.g. changes to statistical analysis plan prior to locking database or revealing treatment codes to trial investigators or patients). Piloting is normally built into adaptive trial designs by determining a priori decision rules to guide the adaptations based on cumulative data. For example, data from interim analyses could be used to refine sample size calculations [ 17 , 18 ]. This approach is routinely used in internal pilot studies - which are primarily designed to inform sample size calculation for the main study, with recalculation of the sample size as the key adaptation. Unlike other phase III pilots, an internal pilot investigation does not usually address any other feasibility aspects - because it is essentially part of the main study [ 10 , 19 , 20 ]..
Nonetheless, we need to emphasize that whether or not a study is a pilot, depends on its objectives. An adaptive method is used as a strategy to reach that objective. Both a pilot and a non-pilot could be adaptive.
5. Challenges of and Common Misconceptions about Pilot Studies
Pilot studies can be very informative, not only to the researchers conducting them but also to others doing similar work. However, many of them never get published, often because of the way the results are presented [ 13 ]. Quite often the emphasis is wrongly placed on statistical significance, not on feasibility - which is the main focus of the pilot study. Our experience in reviewing submissions to a research ethics board also shows that most of the pilot projects are not well designed: i.e. there are no clear feasibility objectives; no clear analytic plans; and certainly no clear criteria for success of feasibility.
In many cases, pilot studies are conducted to generate data for sample size calculations. This seems especially sensible in situations where there are no data from previous studies to inform this process. However, it can be dangerous to use pilot studies to estimate treatment effects, as such estimates may be unrealistic/biased because of the limited sample sizes. Therefore if not used cautiously, results of pilot studies can potentially mislead sample size or power calculations [ 21 ] -- particularly if the pilot study was done to see if there is likely to be a treatment effect in the main study. In section 6, we provide guidance on how to proceed with caution in this regard.
There are also several misconceptions about pilot studies. Below are some of the common reasons that researchers have put forth for calling their study a pilot.
The first common reason is that a pilot study is a small single-centre study. For example, researchers often state lack of resources for a large multi-centre study as a reason for doing a pilot. The second common reason is that a pilot investigation is a small study that is similar in size to someone else's published study. In reviewing submissions to a research ethics board, we have come across sentiments such as
• So-and-so did a similar study with 6 patients and got statistical significance - ours uses 12 patients (double the size)!
• We did a similar pilot before (and it was published!)
The third most common reason is that a pilot is a small study done by a student or an intern - which can be completed quickly and does not require funding. Specific arguments include
• I have funding for 10 patients only;
• I have limited seed (start-up) funding;
• This is just a student project!
• My supervisor (boss) told me to do it as a pilot .
None of the above arguments qualifies as sound reasons for calling a study a pilot. A study should only be conducted if the results will be informative; studies conducted for the reasons above may result in findings of limited utility, which would be a waste of the researchers' and participants' efforts. The focus of a pilot study should be on assessment of feasibility, unless it was powered appropriately to assess statistical significance. Further, there is a vast number of poorly designed and reported studies. Assessment of the quality of a published report may be helpful to guide decisions of whether the report should be used to guide planning or designing of new studies. Finally, if a trainee or researcher is assigned a project as a pilot it is important to discuss how the results will inform the planning of the main study. In addition, clearly defined feasibility objectives and rationale to justify piloting should be provided.
Sample Size for Pilot Studies
In general, sample size calculations may not be required for some pilot studies. It is important that the sample for a pilot be representative of the target study population. It should also be based on the same inclusion/exclusion criteria as the main study. As a rule of thumb, a pilot study should be large enough to provide useful information about the aspects that are being assessed for feasibility. Note that PoC studies require sample size estimation based on surrogate markers [ 22 ], but they are usually not powered to detect meaningful differences in clinically important endpoints. The sample used in the pilot may be included in the main study, but caution is needed to ensure the key features of the main study are preserved in the pilot (e.g. blinding in randomized controlled trials). We recommend if any pooling of pilot and main study data is considered, this should be planned beforehand, described clearly in the protocol with clear discussion of the statistical consequences and methods. The goal is to avoid or minimize the potential bias that may occur due to multiple testing issues or any other opportunistic actions by investigators. In general, pooling when done appropriately can increase the efficiency of the main study [ 23 ].
As noted earlier, a carefully designed pilot study may be used to generate information for sample size calculations. Two approaches may be helpful to optimize information from a pilot study in this context: First, consider eliciting qualitative data to supplement the quantitative information obtained in the pilot. For example, consider having some discussions with clinicians using the approach suggested by Lenth [ 24 ] to illicit additional information on possible effect size and variance estimates. Second, consider creating a sample size table for various values of the effect or variance estimates to acknowledge the uncertainty surrounding the pilot estimates.
In some cases, one could use a confidence interval [CI] approach to estimate the sample size required to establish feasibility. For example, suppose we had a pilot trial designed primarily to determine adherence rates to the standardized risk assessment form to enhance venous thromboprophylaxis in hospitalized patients. Suppose it was also decided a priori that the criterion for success would be: the main trial would be ' feasibl e' if the risk assessment form is completed for ≥ 70% of eligible hospitalized patients.
6. How to Interpret the Results of a Pilot Study: Criteria for Success
It is always important to state the criteria for success of a pilot study. The criteria should be based on the primary feasibility objectives. These provide the basis for interpreting the results of the pilot study and determining whether it is feasible to proceed to the main study. In general, the outcome of a pilot study can be one of the following: (i) Stop - main study not feasible; (ii) Continue, but modify protocol - feasible with modifications; (iii) Continue without modifications, but monitor closely - feasible with close monitoring and (iv) Continue without modifications - feasible as is.
For example, the Prophylaxis of Thromboembolism in Critical Care Trial (PROTECT) was designed to assess the feasibility of a large-scale trial with the following criteria for determining success [ 25 ]:
• 98.5% of patients had to receive study drug within 12 hours of randomization;
• 91.7% of patients had to receive every scheduled dose of the study drug in a blinded manner;
• 90% or more of patients had to have lower limb compression ultrasounds performed at the specified times; and
• > 90% of necessary dose adjustments had to have been made appropriately in response to pre-defined laboratory criteria .
In a second example, the PeriOperative Epidural Trial (POET) Pilot Study was designed to assess the feasibility of a large, multicentre trial with the following criteria for determining success [ 26 ]:
• one subject per centre per week (i.e., 200 subjects from four centres over 50 weeks) can be recruited ;
• at least 70% of all eligible patients can be recruited ;
• no more than 5% of all recruited subjects crossed over from one modality to the other; and
• complete follow-up in at least 95% of all recruited subjects .
7. Frequently asked questions about pilot studies
In this Section, we offer our thoughts on some of the frequently asked questions about pilot studies. These could be helpful to not only clinicians and trainees, but to anyone who is interested in health research.
• Can I publish the results of a pilot study?
- Yes, every attempt should be made to publish.
• Why is it important to publish the results of pilot studies?
- To provide information about feasibility to the research community to save resources being unnecessarily spent on studies that may not be feasible. Further, having such information can help researchers to avoid duplication of efforts in assessing feasibility.
- Finally, researchers have an ethical and scientific obligation to attempt publishing the results of every research endeavor. However, our focus should be on feasibility goals. Emphasis should not be placed on statistical significance when pilot studies are not powered to detect minimal clinically important differences. Such studies typically do not show statistically significant results - remember that underpowered studies (with no statistically significant results) are inconclusive, not negative since "no evidence of effect" is not "evidence of no effect" [ 27 ].
• Can I combine data from a pilot with data from the main study?
- Yes, provided the sampling frame and methodologies are the same. This can increase the efficiency of the main study - see Section 5.
• Can I combine the results of a pilot with the results of another study or in a meta-analysis?
- Yes, provided the sampling frame and methodologies are the same.
- No, if the main study is reported and it includes the pilot study.
• Can the results of the pilot study be valid on their own, without existence of the main study
- Yes, if the results show that it is not feasible to proceed to the main study or there is insufficient funding.
• Can I apply for funding for a pilot study?
- Yes. Like any grant, it is important to justify the need for piloting.
- The pilot has to be placed in the context of the main study.
• Can I randomize patients in a pilot study?
- Yes. For a phase III pilot study, one of the goals could be to assess how a randomization procedure might work in the main study or whether the idea of randomization might be acceptable to patients [ 10 ]. In general, it is always best for a pilot to maintain the same design as the main study.
• How can I use the information from a pilot to estimate the sample size?
- Use with caution, as results from pilot studies can potentially mislead sample size calculations.
- Consider supplementing the information with qualitative discussions with clinicians - see section 5; and
- Create a sample size table to acknowledge the uncertainty of the pilot information - see section 5.
• Can I use the results of a pilot study to treat my patients?
- Not a good idea!
- Pilot studies are primarily for assessing feasibility.
• What can I do with a failed or bad pilot study?
- No study is a complete failure; it can always be used as bad example! However, it is worth making clear that a pilot study that shows the main study is not likely to be feasible is not a failed (pilot) study. In fact, it is a success - because you avoided wasting scarce resources on a study destined for failure!
8. Ethical Aspects of Pilot Studies
Halpern et al . [ 28 ] stated that conducting underpowered trials is unethical. However, they proposed that underpowered trials are ethical in two situations: (i) small trials of interventions for rare diseases -- which require documenting explicit plans for including results with those of similar trials in a prospective meta-analysis; (ii) early-phase trials in the development of drugs or devices - provided they are adequately powered for defined purposes other than randomized treatment comparisons. Pilot studies of phase III trials (dealing with common diseases) are not addressed in their proposal. It is therefore prudent to ask: Is it ethical to conduct a study whose feasibility can not be guaranteed (i.e. with a high probability of success)?
It seems unethical to consider running a phase III study without having sufficient data or information about the feasibility. In fact, most granting agencies often require data on feasibility as part of their assessment of the scientific validity for funding decisions.
There is however one important ethical aspect about pilot studies that has received little or no attention from researchers, research ethics boards and ethicists alike. This pertains to the issue of the obligation that researchers have to patients or participants in a trial to disclose the feasibility nature of pilot studies. This is essential given that some pilot studies may not lead to further studies. A review of the commonly cited research ethics guidelines - the Nuremburg Code [ 29 ], Helsinki Declaration [ 30 ], the Belmont Report [ 31 ], ICH Good Clinical Practice [ 32 ], and the International Ethical Guidelines for Biomedical Research Involving Human Subjects [ 33 ] - shows that pilot studies are not addressed in any of these guidelines. Canadian researchers are also encouraged to follow the Tri-Council Policy Statement (TCPS) [ 34 ] - it too does not address how pilot studies need to be approached. It seems to us that given the special nature of feasibility or pilot studies, the disclosure of their purpose to study participants requires special wording - that informs them of the definition of a pilot study, the feasibility objectives of the study, and also clearly defines the criteria for success of feasibility. To fully inform participants, we suggest using the following wording in the consent form:
" The overall purpose of this pilot study is to assess the feasibility of conducting a large study to [state primary objective of the main study]. A feasibility or pilot study is a study that... [state a general definition of a feasibility study]. The specific feasibility objectives of this study are ... [state the specific feasibility objectives of the pilot study]. We will determine that it is feasible to carry on the main study if ... [state the criteria for success of feasibility] ."
9. Recommendation for Reporting the Results of Pilot Studies
Adopted from the CONSORT Statement [ 9 ], Table 3 provides a checklist of items to consider including in a report of a pilot study.
Pilot Study - Checklist: Items to include when reporting a pilot study
| PAPER SECTION | Item | Descriptor | Reported on Page # |
|---|---|---|---|
| ABSTRACT | 1 | Does the title or abstract indicate that the study is a "pilot"? | |
| Background | 2 | Scientific background for the main study and explanation of rationale for assessing feasibility through piloting | |
| Participants and setting | 3 | • Eligibility criteria for participants in the pilot study (these should be the same as in the main study -- if different, state the differences) | |
| • The settings and locations where the data were collected | |||
| Interventions | 4 | Provide precise details of the interventions intended for each group and how and when they were actually administered (if applicable) -- state clearly if any aspects of the intervention are assessed for feasibility | |
| Objectives | 5 | • Specific scientific objectives and hypotheses for the main study | |
| • Specific feasibility objectives | |||
| Outcomes | 6 | • Clearly defined primary and secondary outcome measures for the main study | |
| • Clearly define the feasibility outcomes and how they were operationalized -- these should include key elements such as recruitment rates, consent rates, completion rates, variance estimates, etc | |||
| Sample size | 7 | Describe how sample size was determined | |
| • In general for a pilot of a phase III trial, there is no need for a formal sample size calculation. However, confidence interval approach may be used to calculate and justify the sample size based on key feasibility objective(s). | |||
| Feasibility Criteria | 8 | Clearly describe the criteria for assessing success of feasibility -- these should be based on the feasibility objectives | |
| Statistical Methods | 9 | Describe the statistical methods for the analysis of primary and secondary feasibility outcomes | |
| Ethical Aspects | 10 | • State whether the study received research ethics approval | |
| • State how informed consent was handled -- given the feasibility nature of the study | |||
| Participant flow | 11 | Flow of participants through each stage (a flow-chart is strongly recommended). | |
| • Describe protocol deviations from pilot study as planned, together with reasons | |||
| • State the number of exclusions at each stage and reasons for exclusions | |||
| Recruitment | 12 | Report the dates defining the periods of recruitment and follow-up | |
| Baseline data | 13 | Report the baseline demographic and clinical characteristics of the participants | |
| Outcomes and estimation | 14 | For each primary and secondary feasibility outcome, report the point estimate of effect and its precision ( ., 95% confidence interval [CI]) -- if applicable | |
| Interpretation | 15 | Interpretation of the results should focus on feasibility, taking into account | |
| • the stated criteria for success of feasibility; | |||
| • study hypotheses, sources of potential bias or imprecision -- given the feasibility nature of the study | |||
| • the dangers associated with multiplicity of analyses and outcomes | |||
| Generalizability | 16 | Generalizability (external validity) of the feasibility. State clearly what modifications in the design of the main study (if any) would be necessary to make it feasible | |
| Overall evidence of feasibility | 17 | General interpretation of the results in the context of current evidence of feasibility | |
| • Focus should be on feasibility | |||
Title and abstract
Item #1: the title or abstract should indicate that the study is a "pilot" or "feasibility".
As a number one summary of the contents of any report, it is important for the title to clearly indicate that the report is for a pilot or feasibility study. This would also be helpful to other researchers during electronic information search about feasibility issues. Our quick search of PUBMED [on July 13, 2009], using the terms "pilot" OR "feasibility" OR "proof-of-concept" for revealed 24423 (16%) hits of studies that had these terms in the title or abstract compared with 149365 hits that had these terms anywhere in the text.
Item #2: Scientific background for the main study and explanation of rationale for assessing feasibility through piloting
The rationale for initiating a pilot should be based on the need to assess feasibility for the main study. Thus, the background of the main study should clearly describe what is known or not known about important feasibility aspects to provide context for piloting.
Item #3: Participants and setting of the study
The description of the inclusion-exclusion or eligibility criteria for participants should be the same as in the main study. The settings and locations where the data were collected should also be clearly described.
Item #4: Interventions
Precise details of the interventions intended for each group and how and when they were actually administered (if applicable) - state clearly if any aspects of the intervention are assessed for feasibility.
Item #5: Objectives
State the specific scientific primary and secondary objectives and hypotheses for the main study and the specific feasibility objectives. It is important to clearly indicate the feasibility objectives as the primary focus for the pilot.
Item #6: Outcomes
Clearly define primary and secondary outcome measures for the main study. Then, clearly define the feasibility outcomes and how they were operationalized - these should include key elements such as recruitment rates, consent rates, completion rates, variance estimates, etc. In some cases, a pilot study may be conducted with the aim to determine a suitable (clinical or surrogate) endpoint for the main study. In such a case, one may not be able to define the primary outcome of the main study until the pilot is finished. However, it is important that determining the primary outcome of the main study be clearly stated as part of feasibility outcomes.
Item #7: Sample Size
Describe how sample size was determined. If the pilot is a proof-of-concept study, is the sample size calculated based on primary/key surrogate marker(s)? In general if the pilot is for a phase III study, there may be no need for a formal sample size calculation. However, the confidence interval approach may be used to calculate and justify the sample size based on key feasibility objective(s).
Item #8: Feasibility criteria
Clearly describe the criteria for assessing success of feasibility - these should be based on the feasibility objectives.
Item #9: Statistical Analysis
Describe the statistical methods for the analysis of primary and secondary feasibility outcomes.
Item #10: Ethical Aspects
State whether the study received research ethics approval. Describe how informed consent was handled - given the feasibility nature of the study.
Item #11: Participant Flow
Describe the flow of participants through each stage of the study (use of a flow-diagram is strongly recommended -- see CONSORT [ 9 ] for a template). Describe protocol deviations from pilot study as planned with reasons for deviations. State the number of exclusions at each stage and corresponding reasons for exclusions.
Item #12: Recruitment
Report the dates defining the periods of recruitment and follow-up.
Item #13: Baseline Data
Report the baseline demographic and clinical characteristics of the participants.
Item #14: Outcomes and Estimation
For each primary and secondary feasibility outcomes, report the point estimate of effect and its precision ( e.g ., 95% CI) - if applicable.
Item # 15: Interpretation
Interpretation of the results should focus on feasibility, taking into account the stated criteria for success of feasibility, study hypotheses, sources of potential bias or imprecision (given the feasibility nature of the study) and the dangers associated with multiplicity - repeated testing on multiple outcomes.
Item #16: Generalizability
Discuss the generalizability (external validity) of the feasibility aspects observed in the study. State clearly what modifications in the design of the main study (if any) would be necessary to make it feasible.
Item #17: Overall evidence of feasibility
Discuss the general results in the context of overall evidence of feasibility. It is important that the focus be on feasibility.
9. Conclusions
Pilot or vanguard studies provide a good opportunity to assess feasibility of large full-scale studies. Pilot studies are the best way to assess feasibility of a large expensive full-scale study, and in fact are an almost essential pre-requisite. Conducting a pilot prior to the main study can enhance the likelihood of success of the main study and potentially help to avoid doomed main studies. Pilot studies should be well designed with clear feasibility objectives, clear analytic plans, and explicit criteria for determining success of feasibility. They should be used cautiously for determining treatment effects and variance estimates for power or sample size calculations. Finally, they should be scrutinized the same way as full scale studies, and every attempt should be taken to publish the results in peer-reviewed journals.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LT drafted the manuscript. All authors reviewed several versions of the manuscript, read and approved the final version.
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/1471-2288/10/1/prepub
Contributor Information
Lehana Thabane, Email: [email protected].
Jinhui Ma, Email: [email protected].
Rong Chu, Email: [email protected].
Ji Cheng, Email: [email protected].
Afisi Ismaila, Email: [email protected].
Lorena P Rios, Email: [email protected].
Reid Robson, Email: [email protected].
Marroon Thabane, Email: [email protected].
Lora Giangregorio, Email: [email protected].
Charles H Goldsmith, Email: [email protected].
Acknowledgements
Dr Lehana Thabane is clinical trials mentor for the Canadian Institutes of Health Research. We thank the reviewers for insightful comments and suggestions which led to improvements in the manuscript.
- Waite M. Concise Oxford Thesaurus. 2. Oxford, England: Oxford University Press; 2002. [ Google Scholar ]
- Last JM, editor. A Dictionary of Epidemiology. 4. Oxford University Press; 2001. [ Google Scholar ]
- Everitt B. Medical Statistics from A to Z: A Guide for Clinicians and Medical Students. 2. Cambridge University Press: Cambridge; 2006. [ Google Scholar ]
- Tavel JA, Fosdick L. ESPRIT Vanguard Group. ESPRIT Executive Committee. Closeout of four phase II Vanguard trials and patient rollover into a large international phase III HIV clinical endpoint trial. Control Clin Trials. 2001;22:42–48. doi: 10.1016/S0197-2456(00)00114-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- Arnold DM, Burns KE, Adhikari NK, Kho ME, Meade MO, Cook DJ. The design and interpretation of pilot trials in clinical research in critical care. Crit Care Med. 2009;37(Suppl 1):69–74. doi: 10.1097/CCM.0b013e3181920e33. [ DOI ] [ PubMed ] [ Google Scholar ]
- Computerization of Medical Practice for the Enhancement of Therapeutic Effectiveness. http://www.compete-study.com/index.htm Last accessed August 8, 2009.
- Heart Outcomes Prevention Evaluation Study. http://www.ccc.mcmaster.ca/hope.htm Last accessed August 8, 2009.
- Cardiovascular Health Awareness Program. http://www.chapprogram.ca/resources.html Last accessed August 8, 2009.
- Moher D, Schulz KF, Altman DG. CONSORT Group (Consolidated Standards of Reporting Trials) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. J Am Podiatr Med Assoc. 2001;91:437–442. doi: 10.7547/87507315-91-8-437. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–12. doi: 10.1111/j..2002.384.doc.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Craig N, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Van Teijlingen ER, Rennie AM, Hundley V, Graham W. The importance of conducting and reporting pilot studies: the example of the Scottish Births Survey. J Adv Nurs. 2001;34:289–295. doi: 10.1046/j.1365-2648.2001.01757.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Van Teijlingen ER, Hundley V. The Importance of Pilot Studies. Social Research Update. 2001. p. 35. http://sru.soc.surrey.ac.uk/SRU35.html
- Lawrence Gould A. Timing of futility analyses for 'proof of concept' trials. Stat Med. 2005;24:1815–1835. doi: 10.1002/sim.2087. [ DOI ] [ PubMed ] [ Google Scholar ]
- Fardon T, Haggart K, Lee DK, Lipworth BJ. A proof of concept study to evaluate stepping down the dose of fluticasone in combination with salmeterol and tiotropium in severe persistent asthma. Respir Med. 2007;101:1218–1228. doi: 10.1016/j.rmed.2006.11.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- Chow SC, Chang M. Adaptive design methods in clinical trials - a review. Orphanet J Rare Dis. 2008;3:11. doi: 10.1186/1750-1172-3-11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gould AL. Planning and revising the sample size for a trial. Stat Med. 1995;14:1039–1051. doi: 10.1002/sim.4780140922. [ DOI ] [ PubMed ] [ Google Scholar ]
- Coffey CS, Muller KE. Properties of internal pilots with the univariate approach to repeated measures. Stat Med. 2003;22:2469–2485. doi: 10.1002/sim.1466. [ DOI ] [ PubMed ] [ Google Scholar ]
- Zucker DM, Wittes JT, Schabenberger O, Brittain E. Internal pilot studies II: comparison of various procedures. Statistics in Medicine. 1999;18:3493–3509. doi: 10.1002/(SICI)1097-0258(19991230)18:24<3493::AID-SIM302>3.0.CO;2-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kieser M, Friede T. Re-calculating the sample size in internal pilot designs with control of the type I error rate. Statistics in Medicine. 2000;19:901–911. doi: 10.1002/(SICI)1097-0258(20000415)19:7<901::AID-SIM405>3.0.CO;2-L. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. [ DOI ] [ PubMed ] [ Google Scholar ]
- Yin Y. Sample size calculation for a proof of concept study. J Biopharm Stat. 2002;12:267–276. doi: 10.1081/BIP-120015748. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wittes J, Brittain E. The role of internal pilot studies in increasing the efficiency of clinical trials. Stat Med. 1990;9:65–71. doi: 10.1002/sim.4780090113. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lenth R. Some Practical Guidelines for Effective Sample Size Determination. The American Statistician. 2001;55:187–193. doi: 10.1198/000313001317098149. [ DOI ] [ Google Scholar ]
- Cook DJ, Rocker G, Meade M, Guyatt G, Geerts W, Anderson D, Skrobik Y, Hebert P, Albert M, Cooper J, Bates S, Caco C, Finfer S, Fowler R, Freitag A, Granton J, Jones G, Langevin S, Mehta S, Pagliarello G, Poirier G, Rabbat C, Schiff D, Griffith L, Crowther M. PROTECT Investigators. Canadian Critical Care Trials Group. Prophylaxis of Thromboembolism in Critical Care (PROTECT) Trial: a pilot study. J Crit Care. 2005;20:364–372. doi: 10.1016/j.jcrc.2005.09.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- Choi PT, Beattie WS, Bryson GL, Paul JE, Yang H. Effects of neuraxial blockade may be difficult to study using large randomized controlled trials: the PeriOperative Epidural Trial (POET) Pilot Study. PLoS One. 2009;4(2):e4644. doi: 10.1371/journal.pone.0004644. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Altman DG, Bland JM. Absence of evidence is not evidence of absence. BMJ. 1995;311:485. doi: 10.1136/bmj.311.7003.485. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Halpern SD, Karlawish JH, Berlin JA. The continuing unethical conduct of underpowered clinical trials. JAMA. 2002;288:358–362. doi: 10.1001/jama.288.3.358. [ DOI ] [ PubMed ] [ Google Scholar ]
- The Nuremberg Code, Research ethics guideline 2005. http://www.hhs.gov/ohrp/references/nurcode.htm Last accessed August 8, 2009.
- The Declaration of Helsinki, Research ethics guideline. http://www.wma.net/en/30publications/10policies/b3/index.html Last accessed December 22, 2009.
- The Belmont Report, Research ethics guideline. http://ohsr.od.nih.gov/guidelines/belmont.html Last accessed August 8, 2009.
- The ICH Harmonized Tripartite Guideline-Guideline for Good Clinical Practice. http://www.gcppl.org.pl/ma_struktura/docs/ich_gcp.pdf Last accessed August 8, 2009. [ PubMed ]
- The International Ethical Guidelines for Biomedical Research Involving Human Subjects. http://www.fhi.org/training/fr/Retc/pdf_files/cioms.pdf Last accessed August 8, 2009. [ PubMed ]
- Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans, Government of Canada. http://www.pre.ethics.gc.ca/english/policystatement/policystatement.cfm Last accessed August 8, 2009.
- View on publisher site
- PDF (207.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- En español – ExME
- Em português – EME
What is a pilot study?
Posted on 31st July 2017 by Luiz Cadete

Pilot studies can play a very important role prior to conducting a full-scale research project
Pilot studies are small-scale, preliminary studies which aim to investigate whether crucial components of a main study – usually a randomized controlled trial (RCT) – will be feasible. For example, they may be used in attempt to predict an appropriate sample size for the full-scale project and/or to improve upon various aspects of the study design. Often RCTs require a lot of time and money to be carried out, so it is crucial that the researchers have confidence in the key steps they will take when conducting this type of study to avoid wasting time and resources.
Thus, a pilot study must answer a simple question: “Can the full-scale study be conducted in the way that has been planned or should some component(s) be altered?”
The reporting of pilot studies must be of high quality to allow readers to interpret the results and implications correctly. This blog will highlight some key things for readers to consider when they are appraising a pilot study.
What are the main reasons to conduct a pilot study?
Pilot studies are conducted to evaluate the feasibility of some crucial component(s) of the full-scale study. Typically, these can be divided into 4 main aspects:
- Process : where the feasibility of the key steps in the main study is assessed (e.g. recruitment rate; retention levels and eligibility criteria)
- Resources: assessing problems with time and resources that may occur during the main study (e.g. how much time the main study will take to be completed; whether use of some equipment will be feasible or whether the form(s) of evaluation selected for the main study are as good as possible)
- Management: problems with data management and with the team involved in the study (e.g. whether there were problems with collecting all the data needed for future analysis; whether the collected data are highly variable and whether data from different institutions can be analyzed together).
Reasons for not conducting a pilot study
A study should not simply be labelled a ‘pilot study’ by researchers hoping to justify a small sample size. Pilot studies should always have their objectives linked with feasibility and should inform researchers about the best way to conduct the future, full-scale project.
How to interpret a pilot study
Readers must interpret pilot studies carefully. Below are some key things to consider when assessing a pilot study:
- The objectives of pilot studies must always be linked with feasibility and the crucial component that will be tested must always be stated.
- The method section must present the criteria for success. For example: “the main study will be feasible if the retention rate of the pilot study exceeds 90%”. Sample size may vary in pilot studies (different articles present different sample size calculations) but the pilot study population, from which the sample is formed, must be the same as the main study. However, the participants in the pilot study should not be entered into the full-scale study. This is because participants may change their later behaviour if they had previously been involved in the research.
- The pilot study may or may not be a randomized trial (depending on the nature of the study). If the researchers do randomize the sample in the pilot study, it is important that the process for randomization is kept the same in the full-scale project. If the authors decide to test the randomization feasibility through a pilot study, different kinds of randomization procedures could be used.
- As well as the method section, the results of the pilot studies should be read carefully. Although pilot studies often present results related to the effectiveness of the interventions, these results should be interpreted as “potential effectiveness”. The focus in the results of pilot studies should always be on feasibility, rather than statistical significance. However, results of the pilot studies should nonetheless be provided with measures of variability (such as confidence intervals), particularly as the sample size of these studies is usually relatively small, and this might produce biased results.
After an interpretation of results, pilot studies should conclude with one of the following:
(1) the main study is not feasible;
(2) the main study is feasible, with changes to the protocol;
(3) the main study is feasible without changes to the protocol OR
(4) the main study is feasible with close monitoring.
Any recommended changes to the protocol should be clearly outlined.

Take home message
- A pilot study must provide information about whether a full-scale study is feasible and list any recommended amendments to the design of the future study.
Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: what, why and how? BMC Med Res Methodol. 2010; 10: 1.
Cocks K and Torgerson DJ. Sample Size Calculations for Randomized Pilot Trials: A Confidence Interval approach . Journal of Clinical Epidemiology. 2013.
Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004; 10 (2): 307-12.
Moore et al. Recommendations for Planning Pilot Studies in Clinical and Translational Research. Clin Transl Sci. 2011 October ; 4(5): 332–337.
Luiz Cadete
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on What is a pilot study?
I want to do pilot study what can I do. My age is 30 .
Dear Khushbu, were you wanting to get involved in research? If so, what type of research were you interested in. There are lots of resources we can point you towards.
very intersesting
How can I study pilot and how can I start at first step? What should I do at the first time.
Informative.Thank you
If i am conducting a RCT then is it necessary to give interventions before conducting pilot study???
This fantastic. I am a doctoral student preparing do a pilot study on my main study.
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Popular Articles

A 20 Minute Introduction to Cluster Randomised Trials
Another 20 minute tutorial from Tim.

A beginner’s guide to interpreting odds ratios, confidence intervals and p-values
The nuts and bolts 20 minute tutorial from Tim.

Free information and resources for pupils and students interested in evidence-based health care
This new webpage from Cochrane UK is aimed at students of all ages. What is evidence-based practice? What is ‘best available research evidence’? Which resources will help you understand evidence and evidence-based practice, and search for evidence?
- Open access
- Published: 31 October 2020
Guidance for conducting feasibility and pilot studies for implementation trials
- Nicole Pearson ORCID: orcid.org/0000-0003-2677-2327 1 , 2 ,
- Patti-Jean Naylor 3 ,
- Maureen C. Ashe 5 ,
- Maria Fernandez 4 ,
- Sze Lin Yoong 1 , 2 &
- Luke Wolfenden 1 , 2
Pilot and Feasibility Studies volume 6 , Article number: 167 ( 2020 ) Cite this article
99k Accesses
159 Citations
24 Altmetric
Metrics details
Implementation trials aim to test the effects of implementation strategies on the adoption, integration or uptake of an evidence-based intervention within organisations or settings. Feasibility and pilot studies can assist with building and testing effective implementation strategies by helping to address uncertainties around design and methods, assessing potential implementation strategy effects and identifying potential causal mechanisms. This paper aims to provide broad guidance for the conduct of feasibility and pilot studies for implementation trials.
We convened a group with a mutual interest in the use of feasibility and pilot trials in implementation science including implementation and behavioural science experts and public health researchers. We conducted a literature review to identify existing recommendations for feasibility and pilot studies, as well as publications describing formative processes for implementation trials. In the absence of previous explicit guidance for the conduct of feasibility or pilot implementation trials specifically, we used the effectiveness-implementation hybrid trial design typology proposed by Curran and colleagues as a framework for conceptualising the application of feasibility and pilot testing of implementation interventions. We discuss and offer guidance regarding the aims, methods, design, measures, progression criteria and reporting for implementation feasibility and pilot studies.
Conclusions
This paper provides a resource for those undertaking preliminary work to enrich and inform larger scale implementation trials.
Peer Review reports
The failure to translate effective interventions for improving population and patient outcomes into policy and routine health service practice denies the community the benefits of investment in such research [ 1 ]. Improving the implementation of effective interventions has therefore been identified as a priority of health systems and research agencies internationally [ 2 , 3 , 4 , 5 , 6 ]. The increased emphasis on research translation has resulted in the rapid emergence of implementation science as a scientific discipline, with the goal of integrating effective medical and public health interventions into health care systems, policies and practice [ 1 ]. Implementation research aims to do this via the generation of new knowledge, including the evaluation of the effectiveness of implementation strategies [ 7 ]. The term “implementation strategies” is used to describe the methods or techniques (e.g. training, performance feedback, communities of practice) used to enhance the adoption, implementation and/or sustainability of evidence-based interventions (Fig. 1 ) [ 8 , 9 ].
| |
Feasibility studies: an umbrella term used to describe any type of study relating to the preparation for a main study | |
: a subset of feasibility studies that specifically look at a design feature proposed for the main trial, whether in part or in full, conducted on a smaller scale [ ] |
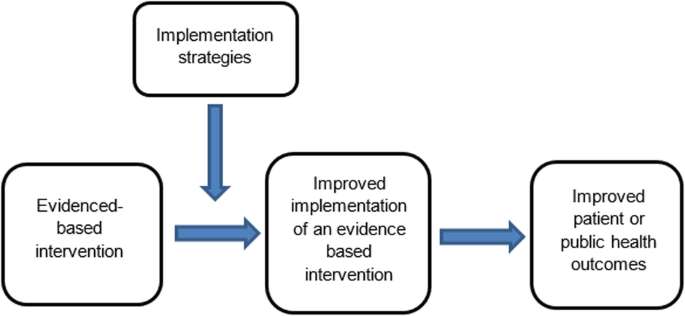
Conceptual role of implementation strategies in improving intervention implementation and patient and public health outcomes
While there has been a rapid increase in the number of implementation trials over the past decade, the quality of trials has been criticised, and the effects of the strategies for such trials on implementation, patient or public health outcomes have been modest [ 11 , 12 , 13 ]. To improve the likelihood of impact, factors that may impede intervention implementation should be considered during intervention development and across each phase of the research translation process [ 2 ]. Feasibility and pilot studies play an important role in improving the conduct and quality of a definitive randomised controlled trial (RCT) for both intervention and implementation trials [ 10 ]. For clinical or public health interventions, pilot and feasibility studies may serve to identify potential refinements to the intervention, address uncertainties around the feasibility of intervention trial methods, or test preliminary effects of the intervention [ 10 ]. In implementation research, feasibility and pilot studies perform the same functions as those for intervention trials, however with a focus on developing or refining implementation strategies, refining research methods for an implementation intervention trial, or undertake preliminary testing of implementation strategies [ 14 , 15 ]. Despite this, reviews of implementation studies appear to suggest that few full implementation randomised controlled trials have undertaken feasibility and pilot work in advance of a larger trial [ 16 ].
A range of publications provides guidance for the conduct of feasibility and pilot studies for conventional clinical or public health efficacy trials including Guidance for Exploratory Studies of complex public health interventions [ 17 ] and the Consolidated Standards of Reporting Trials (CONSORT 2010) for Pilot and Feasibility trials [ 18 ]. However, given the differences between implementation trials and conventional clinical or public health efficacy trials, the field of implementation science has identified the need for nuanced guidance [ 14 , 15 , 16 , 19 , 20 ]. Specifically, unlike traditional feasibility and pilot studies that may include the preliminary testing of interventions on individual clinical or public health outcomes, implementation feasibility and pilot studies that explore strategies to improve intervention implementation often require assessing changes across multiple levels including individuals (e.g. service providers or clinicians) and organisational systems [ 21 ]. Due to the complexity of influencing behaviour change, the role of feasibility and pilot studies of implementation may also extend to identifying potential causal mechanisms of change and facilitate an iterative process of refining intervention strategies and optimising their impact [ 16 , 17 ]. In addition, where conventional clinical or public health efficacy trials are typically conducted under controlled conditions and directed mostly by researchers, implementation trials are more pragmatic [ 15 ]. As is the case for well conducted effectiveness trials, implementation trials often require partnerships with end-users and at times, the prioritisation of end-user needs over methods (e.g. random assignment) that seek to maximise internal validity [ 15 , 22 ]. These factors pose additional challenges for implementation researchers and underscore the need for guidance on conducting feasibility and pilot implementation studies.
Given the importance of feasibility and pilot studies in improving implementation strategies and the quality of full-scale trials of those implementation strategies, our aim is to provide practice guidance for those undertaking formative feasibility or pilot studies in the field of implementation science. Specifically, we seek to provide guidance pertaining to the three possible purposes of undertaking pilot and feasibility studies, namely (i) to inform implementation strategy development, (ii) to assess potential implementation strategy effects and (iii) to assess the feasibility of study methods.
A series of three facilitated group discussions were conducted with a group comprising of the 6 members from Canada, the U.S. and Australia (authors of the manuscript) that were mutually interested in the use of feasibility and pilot trials in implementation science. Members included international experts in implementation and behavioural science, public health and trial methods, and had considerable experience in conducting feasibility, pilot and/ or implementation trials. The group was responsible for developing the guidance document, including identification and synthesis of pertinent literature, and approving the final guidance.
To inform guidance development, a literature review was undertaken in electronic bibliographic databases and google, to identify and compile existing recommendations and guidelines for feasibility and pilot studies broadly. Through this process, we identified 30 such guidelines and recommendations relevant to our aim [ 2 , 10 , 14 , 15 , 17 , 18 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. In addition, seminal methods and implementation science texts recommended by the group were examined. These included the CONSORT 2010 Statement: extension to randomised pilot and feasibility trials [ 18 ], the Medical Research Council’s framework for development and evaluation of randomised controlled trials for complex interventions to improve health [ 2 ], the National Institute of Health Research (NIHR) definitions [ 39 ] and the Quality Enhancement Research Initiative (QUERI) Implementation Guide [ 4 ]. A summary of feasibility and pilot study guidelines and recommendations, and that of seminal methods and implementation science texts, was compiled by two authors. This document served as the primary discussion document in meetings of the group. Additional targeted searches of the literature were undertaken in circumstances where the identified literature did not provide sufficient guidance. The manuscript was developed iteratively over 9 months via electronic circulation and comment by the group. Any differences in views between reviewers was discussed and resolved via consensus during scheduled international video-conference calls. All members of the group supported and approved the content of the final document.
The broad guidance provided is intended to be used as supplementary resources to existing seminal feasibility and pilot study resources. We used the definitions of feasibility and pilot studies as proposed by Eldridge and colleagues [ 10 ]. These definitions propose that any type of study relating to the preparation for a main study may be classified as a “feasibility study”, and that the term “pilot” study represents a subset of feasibility studies that specifically look at a design feature proposed for the main trial, whether in part of in full, that is being conducted on a smaller scale [ 10 ]. In addition, when referring to pilot studies, unless explicitly stated otherwise, we will primarily focus on pilot trials using a randomised design. We focus on randomised trials as such designs are the most common trial design in implementation research, and randomised designs may provide the most robust estimates of the potential effect of implementation strategies [ 46 ]. Those undertaking pilot studies that employ non-randomised designs need to interpret the guidance provided in this context. We acknowledge, however, that using randomised designs can prove particularly challenging in the field of implementation science, where research is often undertaken in real-world contexts with pragmatic constraints.
We used the effectiveness-implementation hybrid trial design typology proposed by Curran and colleagues as the framework for conceptualising the application of feasibility testing of implementation interventions [ 47 ]. The typology makes an explicit distinction between the purpose and methods of implementation and conventional clinical (or public health efficacy) trials. Specifically, the first two of the three hybrid designs may be relevant for implementation feasibility or pilot studies. Hybrid Type 1 trials are those designed to test the effectiveness of an intervention on clinical or public health outcomes (primary aim) while conducting a feasibility or pilot study for future implementation via observing and gathering information regarding implementation in a real-world setting/situation (secondary aim) [ 47 ]. Hybrid Type 2 trials involve the simultaneous testing of both the clinical intervention and the testing or feasibility of a formed implementation intervention/strategy as co-primary aims. For this design, “testing” is inclusive of pilot studies with an outcome measure and related hypothesis [ 47 ]. Hybrid Type 3 trials are definitive implementation trials designed to test the effectiveness of an implementation strategy whilst also collecting secondary outcome data on clinical or public health outcomes on a population of interest [ 47 ]. As the implementation aim of the trial is a definitively powered trial, it was not considered relevant to the conduct of feasibility and pilot studies in the field and will not be discussed.
Embedding of feasibility and pilot studies within Type 1 and Type 2 effectiveness-implementation hybrid trials has been recommended as an efficient way to increase the availability of information and evidence to accelerate the field of implementation science and the development and testing of implementation strategies [ 4 ]. However, implementation feasibility and pilot studies are also undertaken as stand-alone exploratory studies and do not include effectiveness measures in terms of the patient or public health outcomes. As such, in addition to discussing feasibility and pilot trials embedded in hybrid trial designs, we will also refer to stand-alone implementation feasibility and pilot studies.
An overview of guidance (aims, design, measures, sample size and power, progression criteria and reporting) for feasibility and pilot implementation studies can be found in Table 1 .
Purpose (aims)
The primary objective of hybrid type 1 trial is to assess the effectiveness of a clinical or public health intervention (rather than an implementation strategy) on the patient or population health outcomes [ 47 ]. Implementation strategies employed in these trials are often designed to maximise the likelihood of an intervention effect [ 51 ], and may not be intended to represent the strategy that would (or could feasibly), be used to support implementation in more “real world” contexts. Specific aims of implementation feasibility or pilot studies undertaken as part of Hybrid Type 1 trials are therefore formative and descriptive as the implementation strategy has not been fully formed nor will be tested. Thus, the purpose of a Hybrid Type 1 feasibility study is generally to inform the development or refinement of the implementation strategy rather than to test potential effects or mechanisms [ 22 , 47 ]. An example of a Hybrid Type 1 trial by Cabassa and colleagues is provided in Additional file 1 [ 52 ].
In Hybrid Type 2 trial designs, there is a dual purpose to test: (i) the clinical or public health effectiveness of the intervention on clinical or public health outcomes (e.g. measure of disease or health behaviour) and (ii) test or measure the impact of the implementation strategy on implementation outcomes (e.g. adoption of health policy in a community setting) [ 53 ]. However, testing the implementation strategy on implementation outcomes may be a secondary aim in these trials and positioned as a pilot [ 22 ]. In Hybrid Type 2 trial designs, the implementation strategy is more developed than in Hybrid Type 1 trials, resembling that intended for future testing in a definitive implementation randomised controlled trial. The dual testing of the evidence-based intervention and implementation interventions or strategies in Hybrid Type 2 trial designs allows for direct assessment of potential effects of an implementation strategy and exploration of components of the strategy to further refine logic models. Additionally, such trials allow for assessments of the feasibility, utility, acceptability or quality of research methods for use in a planned definitive trial. An example of a Hybrid Type 2 trial design by Barnes and colleagues [ 54 ] is included in Additional file 2 .
Non-hybrid pilot implementation studies are undertaken in the absence of a broader effectiveness trial. Such studies typically occur when the effectiveness of a clinical or public health intervention is well established, but robust strategies to promote its broader uptake and integration into clinical or public health services remain untested [ 15 ]. In these situations, implementation pilot studies may test or explore specific trial methods for a future definitive randomised implementation trial. Similarly, a pilot implementation study may also be undertaken in a way that provides a more rigorous formative evaluation of hypothesised implementation strategy mechanisms [ 55 ], or potential impact of implementation strategies [ 56 ], using similar approaches to that employed in Hybrid Type 2 trials. Examples of potential aims for feasibility and pilot studies are outlined in Table 2 .
For implementation feasibility or pilot studies, as is the case for these types of studies in general, the selection of research design should be guided by the specific research question that the study is seeking to address [ 57 ]. Although almost any study design may be used, researchers should review the merits and potential threats to internal and external validity to help guide the selection of research design for feasibility/pilot testing [ 15 ].
As Hybrid Type 1 trials are primarily concerned with testing the effectiveness of an intervention (rather than implementation strategy), the research design will typically employ power calculations and randomisation procedures at the health outcome level to measure the effect on behaviour, symptoms, functional and/or other clinical or public health outcomes. Hybrid Type 1 feasibility studies may employ a variety of designs usually nested within the experimental group (those receiving the intervention and any form of an implementation support strategy) of the broader efficacy trial [ 47 ]. Consistent with the aims of Hybrid Type 1 feasibility and pilot studies, the research designs employed are likely to be non-comparative. Cross-sectional surveys, interviews or document review, qualitative research or mix methods approaches may be used to assess implementation contextual factors, such as barriers and enablers to implementation and/or the acceptability, perceived feasibility or utility of implementation strategies or research methods [ 47 ].
Pilot implementation studies as part of Hybrid Type 2 designs can make use of the comparative design of the broader effectiveness trial to examine the potential effects of the implementation strategy [ 47 ] and more robustly assess the implementation mechanisms, determinants and influence of broader contextual factors [ 53 ]. In this trial type, mixed method and qualitative methods may complement the findings of between group (implementation strategy arm versus comparison) quantitative comparisons, enable triangulation and provide more comprehensive evidence to inform implementation strategy development and assessment. Stand-alone implementation feasibility and pilot implementation studies are free from the constraints and opportunities of research embedded in broader effectiveness trials. As such, research can be designed in a way that best addresses the explicit implementation objectives of the study. Specifically, non-hybrid pilot studies can maximise the applicability of study findings for future definitive trials by employing methods to directly test trial methods such as recruitment or retention strategies [ 17 ], enabling estimates of implementation strategies effects [ 56 ] or capturing data to explicitly test logic models or strategy mechanisms.
The selection of outcome measures should be linked directly to the objectives of the feasibility or pilot study. Where appropriate, measures should be objective or have suitable psychometric properties, such as evidence of reliability and validity [ 58 , 59 ]. Public health evaluation frameworks often guide the choice of outcome measure in feasibility and pilot implementation work and include RE_AIM [ 60 ], PRECEDE_PROCEED [ 61 ], Proctor and colleagues framework on outcomes for implementation research [ 62 ] and more recently, the “Implementation Mapping” framework [ 63 ]. Recent work by McKay and colleagues suggests a minimum data set of implementation outcomes that includes measures of adoption, reach, dose, fidelity and sustainability [ 46 ]. We discuss selected measures below and provide a summary in Table 3 [ 46 ]. Such measures could be assessed using quantitative or qualitative or mixed methods [ 46 ].
Measures to assess potential implementation strategy effects
In addition to assessing the effects of an intervention on individual clinical or public health outcomes, Hybrid Type 2 trials (and some non-hybrid pilot studies) are interested in measures of the potential effects of an implementation strategy on desired organisational or clinician practice change such as adherence to a guideline, process, clinical standard or delivery of a program [ 62 ]. A range of potential outcomes that could be used to assess implementation strategy effects has been identified, including measures of adoption, reach, fidelity and sustainability [ 46 ]. These outcomes are described in Table 2 , including definitions and examples of how they may be applied to the implementation component of innovation being piloted. Standardised tools to assess these outcomes are often unavailable due to the unique nature of interventions being implemented and the variable (and changing) implementation context in which the research is undertaken [ 64 ]. Researchers may collect outcome data for these measures as part of environmental observations, self-completed checklists or administrative records, audio recording of client sessions or other methods suited to their study and context [ 62 ]. The limitations of such methods, however, need to be considered.
Measures to inform the design or development of the implementation strategy
Measures informing the design or development of the implementation strategy are potentially part of all types of feasibility and pilot implementation studies. An understanding of the determinants of implementation is critical to implementation strategy development. A range of theoretical determinant frameworks have been published which describe factors that may influence intervention implementation [ 65 ], and systematic reviews have been undertaken describing the psychometric properties of many of these measures [ 64 , 66 ]. McKay and colleagues have also identified a priority set of determinants for implementation trials that could be considered for use in implementation feasibility and pilot studies, including measures of context, acceptability, adaptability, feasibility, compatibility, cost, culture, dose, complexity and self-efficacy [ 46 ]. These determinants are described in Table 3 , including definitions and how such measures may be applied to an implementation feasibility or pilot study. Researchers should consider, however, the application of such measures to assess both the intervention that is being implemented (as in a conventional intervention feasibility and pilot study) and the strategy that is being employed to facilitate its implementation, given the importance of the interaction between these factors and implementation success [ 46 ]. Examples of the potential application of measures to both the intervention and its implementation strategies have been outlined elsewhere [ 46 ]. Although a range of quantitative tools could be used to measure such determinants [ 58 , 66 ], qualitative or mixed methods are generally recommended given the capacity of qualitative measures to provide depth to the interpretation of such evaluations [ 40 ].
Measures of potential implementation determinants may be included to build or enhance logic models (Hybrid Type 1 and 2 feasibility and pilot studies) and explore implementation strategy mechanisms (Hybrid Type 2 pilot studies and non-hybrid pilot studies) [ 67 ]. If exploring strategy mechanisms, a hypothesized logic model underpinning the implementation strategy should be articulated including strategy-mechanism linkages, which are required to guide the measurement of key determinants [ 55 , 63 ]. An important determinant which can complicate logic model specification and measurement is the process of adaptation—modifications to the intervention or its delivery (implementation), through the input of service providers or implementers [ 68 ]. Logic models should specify components of implementation strategies thought to be “core” to their effects and those which are thought to be “non-core” where adaptation may occur without adversely impacting on effects. Stirman and colleagues propose a method for assessing adaptations that could be considered for use in pilot and feasibility studies of implementation trials [ 69 ]. Figure 2 provides an example of some of the implementation logic model components that may be developed or refined as part of feasibility or pilot studies of implementation [ 15 , 63 ].
Example of components of an Implementation logic model
Measures to assess the feasibility of study methods
Measures of implementation feasibility and pilot study methods are similar to those of conventional studies for clinical or public health interventions. For example, standard measures of study participation and thresholds for study attrition (e.g. >20%) rates [ 73 ] can be employed in implementation studies [ 67 ]. Previous studies have also surveyed study data collectors to assess the success of blinding strategies [ 74 ]. Researchers may also consider assessing participation or adherence to implementation data collection procedures, the comprehension of survey items, data management strategies or other measures of feasibility of study methods [ 15 ].
Pilot study sample size and power
In effectiveness trials, power calculations and sample size decisions are primarily based on the detection of a clinically meaningful difference in measures of the effects of the intervention on the patient or public health outcomes such as behaviour, disease, symptomatology or functional outcomes [ 24 ]. In this context, the available study sample for implementation measures included in Hybrid Type 1 or 2 feasibility and pilot studies may be constrained by the sample and power calculations of the broader effectiveness trial in which they are embedded [ 47 ]. Nonetheless, a justification for the anticipated sample size for all implementation feasibility or pilot studies (hybrid or stand-alone) is recommended [ 18 ], to ensure that implementation measures and outcomes achieve sufficient estimates of precision to be useful. For Hybrid type 2 and relevant stand-alone implementation pilot studies, sample size calculations for implementation outcomes should seek to achieve adequate estimates of precision deemed sufficient to inform progression to a fully powered trial [ 18 ].
Progression criteria
Stating progression criteria when reporting feasibility and pilot studies is recommended as part of the CONSORT 2010 extension to randomised pilot and feasibility trials guidelines [ 18 ]. Generally, it is recommended that progression criteria should be set a priori and be specific to the feasibility measures, components and/or outcomes assessed in the study [ 18 ]. While little guidance is available, ideas around suitable progression criteria include assessment of uncertainties around feasibility, meeting recruitment targets, cost-effectiveness and refining causal hypotheses to be tested in future trials [ 17 ]. When developing progression criteria, the use of guidelines is suggested rather than strict thresholds [ 18 ], in order to allow for appropriate interpretation and exploration of potential solutions, for example, the use of a traffic light system with varying levels of acceptability [ 17 , 24 ]. For example, Thabane and colleagues recommend that, in general, the outcome of a pilot study can be one of the following: (i) stop—main study not feasible (red); (ii) continue, but modify protocol—feasible with modifications (yellow); (iii) continue without modifications, but monitor closely—feasible with close monitoring and (iv) continue without modifications (green) (44)p5.
As the goal of Hybrid Type 1 implementation component is usually formative, it may not be necessary to set additional progression criteria in terms of the implementation outcomes and measures examined. As Hybrid Type 2 trials test an intervention and can pilot an implementation strategy, criteria for these and non-hybrid pilot studies may set progression criteria based on evidence of potential effects but may also consider the feasibility of trial methods, service provider, organisational or patient (or community) acceptability, fit with organisational systems and cost-effectiveness [ 17 ]. In many instances, the progression of implementation pilot studies will often require the input and agreement of stakeholders [ 27 ]. As such, the establishment of progression criteria and the interpretation of pilot and feasibility study findings in the context of such criteria require stakeholder input [ 27 ].
Reporting suggestions
As formal reporting guidelines do not exist for hybrid trial designs, we would recommend that feasibility and pilot studies as part of hybrid designs draw upon best practice recommendations from relevant reporting standards such as the CONSORT extension for randomised pilot and feasibility trials, the Standards for Reporting Implementation Studies (STaRI) guidelines and the Template for Intervention Description and Replication (TIDieR) guide as well as any other design relevant reporting standards [ 48 , 50 , 75 ]. These, and further reporting guidelines, specific to the particular research design chosen, can be accessed as part of the EQUATOR (Enhancing the QUAility and Transparency Of health Research) network—a repository for reporting guidance [ 76 ]. In addition, researchers should specify the type of implementation feasibility or pilot study being undertaken using accepted definitions. If applicable, specification and justification behind the choice of hybrid trial design should also be stated. In line with existing recommendations for reporting of implementation trials generally, reporting on the referent of outcomes (e.g. specifying if the measure in relation to the specific intervention or the implementation strategy) [ 62 ], is also particularly pertinent when reporting hybrid trial designs.
Concerns are often raised regarding the quality of implementation trials and their capacity to contribute to the collective evidence base [ 3 ]. Although there have been many recent developments in the standardisation of guidance for implementation trials, information on the conduct of feasibility and pilot studies for implementation interventions remains limited, potentially contributing to a lack of exploratory work in this area and a limited evidence base to inform effective implementation intervention design and conduct [ 15 ]. To address this, we synthesised the existing literature and provide commentary and guidance for the conduct of implementation feasibility and pilot studies. To our knowledge, this work is the first to do so and is an important first step to the development of standardised guidelines for implementation-related feasibility and pilot studies.
Availability of data and materials
Not applicable.
Abbreviations
Randomised controlled trial
Consolidated Standards of Reporting Trials
Enhancing the QUAility and Transparency Of health Research
Standards for Reporting Implementation Studies
Strengthening the Reporting of Observational Studies in Epidemiology
Template for Intervention Description and Replication
National Institute of Health Research
Quality Enhancement Research Initiative
Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3:32.
Article Google Scholar
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655.
Eccles MP, Armstrong D, Baker R, Cleary K, Davies H, Davies S, et al. An implementation research agenda. Implement Sci. 2009;4:18.
Department of Veterans Health Administration. Implementation Guide. Health Services Research & Development, Quality Enhancement Research Initiative. Updated 2013.
Peters DH, Nhan TT, Adam T. Implementation research: a practical guide; 2013.
Google Scholar
Neta G, Sanchez MA, Chambers DA, Phillips SM, Leyva B, Cynkin L, et al. Implementation science in cancer prevention and control: a decade of grant funding by the National Cancer Institute and future directions. Implement Sci. 2015;10:4.
Foy R, Sales A, Wensing M, Aarons GA, Flottorp S, Kent B, et al. Implementation science: a reappraisal of our journal mission and scope. Implement Sci. 2015;10:51.
Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:139.
Leeman J, Birken SA, Powell BJ, Rohweder C, Shea CM. Beyond "implementation strategies": classifying the full range of strategies used in implementation science and practice. Implement Sci. 2017;12(1):125.
Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One. 2016;11(3):e0150205.
Article CAS Google Scholar
Powell BJ, McMillen JC, Proctor EK, Carpenter CR, Griffey RT, Bunger AC, et al. A compilation of strategies for implementing clinical innovations in health and mental health. Med Care Res Rev. 2012;69(2):123–57.
Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci. 2015;10:21.
Lewis CC, Stanick C, Lyon A, Darnell D, Locke J, Puspitasari A, et al. Proceedings of the fourth biennial conference of the Society for Implementation Research Collaboration (SIRC) 2017: implementation mechanisms: what makes implementation work and why? Part 1. Implement Sci. 2018;13(Suppl 2):30.
Levati S, Campbell P, Frost R, Dougall N, Wells M, Donaldson C, et al. Optimisation of complex health interventions prior to a randomised controlled trial: a scoping review of strategies used. Pilot Feasibility Stud. 2016;2:17.
Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, et al. How we design feasibility studies. Am J Prev Med. 2009;36(5):452–7.
Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N. Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005;58(2):107–12.
Hallingberg B, Turley R, Segrott J, Wight D, Craig P, Moore L, et al. Exploratory studies to decide whether and how to proceed with full-scale evaluations of public health interventions: a systematic review of guidance. Pilot Feasibility Stud. 2018;4:104.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64.
Proctor EK, Powell BJ, Baumann AA, Hamilton AM, Santens RL. Writing implementation research grant proposals: ten key ingredients. Implement Sci. 2012;7:96.
Stetler CB, Legro MW, Wallace CM, Bowman C, Guihan M, Hagedorn H, et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med. 2006;21(Suppl 2):S1–8.
Aarons GA, Hurlburt M, Horwitz SM. Advancing a conceptual model of evidence-based practice implementation in public service sectors. Admin Pol Ment Health. 2011;38(1):4–23.
Johnson AL, Ecker AH, Fletcher TL, Hundt N, Kauth MR, Martin LA, et al. Increasing the impact of randomized controlled trials: an example of a hybrid effectiveness-implementation design in psychotherapy research. Transl Behav Med. 2018.
Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10(1):67.
Avery KN, Williamson PR, Gamble C, O’Connell Francischetto E, Metcalfe C, Davidson P, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open. 2017;7(2):e013537.
Bell ML, Whitehead AL, Julious SA. Guidance for using pilot studies to inform the design of intervention trials with continuous outcomes. J Clin Epidemiol. 2018;10:153–7.
Billingham SAM, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom clinical research Network database. BMC Med Res Methodol. 2013;13(1):104.
Bugge C, Williams B, Hagen S, Logan J, Glazener C, Pringle S, et al. A process for decision-making after pilot and feasibility trials (ADePT): development following a feasibility study of a complex intervention for pelvic organ prolapse. Trials. 2013;14:353.
Charlesworth G, Burnell K, Hoe J, Orrell M, Russell I. Acceptance checklist for clinical effectiveness pilot trials: a systematic approach. BMC Med Res Methodol. 2013;13(1):78.
Eldridge SM, Costelloe CE, Kahan BC, Lancaster GA, Kerry SM. How big should the pilot study for my cluster randomised trial be? Stat Methods Med Res. 2016;25(3):1039–56.
Fletcher A, Jamal F, Moore G, Evans RE, Murphy S, Bonell C. Realist complex intervention science: applying realist principles across all phases of the Medical Research Council framework for developing and evaluating complex interventions. Evaluation (Lond). 2016;22(3):286–303.
Hampson LV, Williamson PR, Wilby MJ, Jaki T. A framework for prospectively defining progression rules for internal pilot studies monitoring recruitment. Stat Methods Med Res. 2018;27(12):3612–27.
Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63(5):484–9.
Smith LJ, Harrison MB. Framework for planning and conducting pilot studies. Ostomy Wound Manage. 2009;55(12):34–48.
Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–12.
Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–9.
Medical Research Council. A framework for development and evaluation of RCTs for complex interventions to improve health. London: Medical Research Council; 2000.
Möhler R, Bartoszek G, Meyer G. Quality of reporting of complex healthcare interventions and applicability of the CReDECI list - a survey of publications indexed in PubMed. BMC Med Res Methodol. 2013;13(1):125.
Möhler R, Köpke S, Meyer G. Criteria for reporting the development and evaluation of complex interventions in healthcare: revised guideline (CReDECI 2). Trials. 2015;16(1):204.
National Institute for Health Research. Definitions of feasibility vs pilot stuides [Available from: https://www.nihr.ac.uk/documents/guidance-on-applying-for-feasibility-studies/20474 ].
O'Cathain A, Hoddinott P, Lewin S, Thomas KJ, Young B, Adamson J, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud. 2015;1:32.
Shanyinde M, Pickering RM, Weatherall M. Questions asked and answered in pilot and feasibility randomized controlled trials. BMC Med Res Methodol. 2011;11(1):117.
Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15(1):264.
Thabane L, Lancaster G. Improving the efficiency of trials using innovative pilot designs: the next phase in the conduct and reporting of pilot and feasibility studies. Pilot Feasibility Stud. 2017;4(1):14.
Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1.
Westlund E. E.a. S. The nonuse, misuse, and proper use of pilot studies in experimental evaluation research. Am J Eval. 2016;38(2):246–61.
McKay H, Naylor PJ, Lau E, Gray SM, Wolfenden L, Milat A, et al. Implementation and scale-up of physical activity and behavioural nutrition interventions: an evaluation roadmap. Int J Behav Nutr Phys Act. 2019;16(1):102.
Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26.
Equator Network. Standards for reporting implementation studies (StaRI) statement 2017 [Available from: http://www.equator-network.org/reporting-guidelines/stari-statement/ ].
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297–e.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
Schliep ME, Alonzo CN, Morris MA. Beyond RCTs: innovations in research design and methods to advance implementation science. Evid Based Commun Assess Inter. 2017;11(3-4):82–98.
Cabassa LJ, Stefancic A, O'Hara K, El-Bassel N, Lewis-Fernández R, Luchsinger JA, et al. Peer-led healthy lifestyle program in supportive housing: study protocol for a randomized controlled trial. Trials. 2015;16:388.
Landes SJ, McBain SA, Curran GM. Reprint of: An introduction to effectiveness-implementation hybrid designs. J Psychiatr Res. 2020;283:112630.
Barnes C, Grady A, Nathan N, Wolfenden L, Pond N, McFayden T, Ward DS, Vaughn AE, Yoong SL. A pilot randomised controlled trial of a web-based implementation intervention to increase child intake of fruit and vegetables within childcare centres. Pilot and Feasibility Studies. 2020. https://doi.org/10.1186/s40814-020-00707-w .
Lewis CC, Klasnja P, Powell BJ, Lyon AR, Tuzzio L, Jones S, et al. From classification to causality: advancing understanding of mechanisms of change in implementation science. Front Public Health. 2018;6:136.
Department of Veterans Health Affairs. Implementation Guide. Health Services Research & Development, Quality Enhancement Research Initiative. 2013.
Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350:h1258.
Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108.
Lewis CC, Mettert KD, Dorsey CN, Martinez RG, Weiner BJ, Nolen E, et al. An updated protocol for a systematic review of implementation-related measures. Syst Rev. 2018;7(1):66.
Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ Res. 2006;21(5):688–94.
Green L, Kreuter M. Health promotion planning: an educational and ecological approach. Mountain View: Mayfield Publishing; 1999.
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Admin Pol Ment Health. 2011;38(2):65–76.
Fernandez ME, Ten Hoor GA, van Lieshout S, Rodriguez SA, Beidas RS, Parcel G, et al. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health. 2019;7:158.
Lewis CC, Weiner BJ, Stanick C, Fischer SM. Advancing implementation science through measure development and evaluation: a study protocol. Implement Sci. 2015;10:102.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
Clinton-McHarg T, Yoong SL, Tzelepis F, Regan T, Fielding A, Skelton E, et al. Psychometric properties of implementation measures for public health and community settings and mapping of constructs against the consolidated framework for implementation research: a systematic review. Implement Sci. 2016;11(1):148.
Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci. 2011;4(5):332–7.
Pérez D, Van der Stuyft P, Zabala MC, Castro M, Lefèvre P. A modified theoretical framework to assess implementation fidelity of adaptive public health interventions. Implement Sci. 2016;11(1):91.
Stirman SW, Miller CJ, Toder K, Calloway A. Development of a framework and coding system for modifications and adaptations of evidence-based interventions. Implement Sci. 2013;8:65.
Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci. 2007;2:40.
Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol. 2008;41(3-4):327–50.
Saunders RP, Evans MH, Joshi P. Developing a process-evaluation plan for assessing health promotion program implementation: a how-to guide. Health Promot Pract. 2005;6(2):134–47.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Wyse RJ, Wolfenden L, Campbell E, Brennan L, Campbell KJ, Fletcher A, et al. A cluster randomised trial of a telephone-based intervention for parents to increase fruit and vegetable consumption in their 3- to 5-year-old children: study protocol. BMC Public Health. 2010;10:216.
Consort Transparent Reporting of Trials. Pilot and Feasibility Trials 2016 [Available from: http://www.consort-statement.org/extensions/overview/pilotandfeasibility ].
Equator Network. Ehancing the QUAlity and Transparency Of health Research. [Avaliable from: https://www.equator-network.org/ ].
Download references
Acknowledgements
Associate Professor Luke Wolfenden receives salary support from a NHMRC Career Development Fellowship (grant ID: APP1128348) and Heart Foundation Future Leader Fellowship (grant ID: 101175). Dr Sze Lin Yoong is a postdoctoral research fellow funded by the National Heart Foundation. A/Prof Maureen C. Ashe is supported by the Canada Research Chairs program.
Author information
Authors and affiliations.
School of Medicine and Public Health, University of Newcastle, University Drive, Callaghan, NSW 2308, Australia
Nicole Pearson, Sze Lin Yoong & Luke Wolfenden
Hunter New England Population Health, Locked Bag 10, Wallsend, NSW 2287, Australia
School of Exercise Science, Physical and Health Education, Faculty of Education, University of Victoria, PO Box 3015 STN CSC, Victoria, BC, V8W 3P1, Canada
Patti-Jean Naylor
Center for Health Promotion and Prevention Research, University of Texas Health Science Center at Houston School of Public Health, Houston, TX, 77204, USA
Maria Fernandez
Department of Family Practice, University of British Columbia (UBC) and Centre for Hip Health and Mobility, University Boulevard, Vancouver, BC, V6T 1Z3, Canada
Maureen C. Ashe
You can also search for this author in PubMed Google Scholar
Contributions
NP and LW led the development of the manuscript. NP, LW, NP, MCA, PN, MF and SY contributed to the drafting and final approval of the manuscript.
Corresponding author
Correspondence to Nicole Pearson .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors have no financial or non-financial interests to declare .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Example of a Hybrid Type 1 trial. Summary of publication by Cabassa et al.
Additional file 2.
Example of a Hybrid Type 2 trial. Summary of publication by Barnes et al.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Pearson, N., Naylor, PJ., Ashe, M.C. et al. Guidance for conducting feasibility and pilot studies for implementation trials. Pilot Feasibility Stud 6 , 167 (2020). https://doi.org/10.1186/s40814-020-00634-w
Download citation
Received : 08 January 2020
Accepted : 18 June 2020
Published : 31 October 2020
DOI : https://doi.org/10.1186/s40814-020-00634-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Feasibility
- Hybrid trial designs
- Implementation science
Pilot and Feasibility Studies
ISSN: 2055-5784
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Correspondence
- Open access
- Published: 16 July 2010
What is a pilot or feasibility study? A review of current practice and editorial policy
- Mubashir Arain 1 ,
- Michael J Campbell 1 ,
- Cindy L Cooper 1 &
- Gillian A Lancaster 2
BMC Medical Research Methodology volume 10 , Article number: 67 ( 2010 ) Cite this article
193k Accesses
865 Citations
29 Altmetric
Metrics details
In 2004, a review of pilot studies published in seven major medical journals during 2000-01 recommended that the statistical analysis of such studies should be either mainly descriptive or focus on sample size estimation, while results from hypothesis testing must be interpreted with caution. We revisited these journals to see whether the subsequent recommendations have changed the practice of reporting pilot studies. We also conducted a survey to identify the methodological components in registered research studies which are described as 'pilot' or 'feasibility' studies. We extended this survey to grant-awarding bodies and editors of medical journals to discover their policies regarding the function and reporting of pilot studies.
Papers from 2007-08 in seven medical journals were screened to retrieve published pilot studies. Reports of registered and completed studies on the UK Clinical Research Network (UKCRN) Portfolio database were retrieved and scrutinized. Guidance on the conduct and reporting of pilot studies was retrieved from the websites of three grant giving bodies and seven journal editors were canvassed.
54 pilot or feasibility studies published in 2007-8 were found, of which 26 (48%) were pilot studies of interventions and the remainder feasibility studies. The majority incorporated hypothesis-testing (81%), a control arm (69%) and a randomization procedure (62%). Most (81%) pointed towards the need for further research. Only 8 out of 90 pilot studies identified by the earlier review led to subsequent main studies. Twelve studies which were interventional pilot/feasibility studies and which included testing of some component of the research process were identified through the UKCRN Portfolio database. There was no clear distinction in use of the terms 'pilot' and 'feasibility'. Five journal editors replied to our entreaty. In general they were loathe to publish studies described as 'pilot'.
Pilot studies are still poorly reported, with inappropriate emphasis on hypothesis-testing. Authors should be aware of the different requirements of pilot studies, feasibility studies and main studies and report them appropriately. Authors should be explicit as to the purpose of a pilot study. The definitions of feasibility and pilot studies vary and we make proposals here to clarify terminology.
Peer Review reports
A brief definition is that a pilot study is a 'small study for helping to design a further confirmatory study'[ 1 ]. A very useful discussion of exactly what is a pilot study has been given by Thabane et al. [ 2 ] Such kinds of study may have various purposes such as testing study procedures, validity of tools, estimation of the recruitment rate, and estimation of parameters such as the variance of the outcome variable to calculate sample size etc. In pharmacological trials they may be referred to as 'proof of concept' or Phase I or Phase II studies. It has become apparent to us when reviewing research proposals that small studies with all the trappings of a major study, such as randomization and hypothesis testing may be labeled a 'pilot' because they do not have the power to test clinically meaningful hypotheses. The authors of such studies perhaps hope that reviewers will regard a 'pilot' more favourably than a small clinical trial. This lead us to ask when it is legitimate to label a study as a 'pilot' or 'feasibility' study, and what features should be included in these types of studies.
Lancaster et al [ 3 ] conducted a review of seven major medical journals in 2000-1 to produce evidence regarding the components of pilot studies for randomized controlled trials. Their search included both 'pilot' and 'feasibility' studies as keywords. They reported certain recommendations: having clear objectives in a pilot study, inappropriateness of mixing pilot data with main research study, using mainly descriptive statistics obtained and caution regarding the use of hypothesis testing for conclusions. Arnold et al [ 1 ] recently reviewed pilot studies particularly related to critical care medicine by searching the literature from 1997 to 2007. They provided narrative descriptions of some pilot papers particularly those describing critical care medicine procedures. They pointed out that few pilot trials later evolved into subsequent published major trials. They made useful distinctions between: pilot work which is any background research to inform a future study, a pilot study which has specific hypotheses, objectives and methodology and a pilot trial which is a stand-alone pilot study and includes a randomization procedure. They excluded feasibility studies from their consideration.
Thabane et al [ 2 ] gave a checklist of what they think should be included in a pilot study. They included 'feasibility' or 'vanguard' studies but did not distinguish them from pilot studies. They provided a good discussion on how to interpret a pilot study. They stress that not only the outcome or surrogate outcome for the subsequent main study should be described but also that a pilot study should have feasibility outcomes which should be clearly defined and described. Their article was opinion based and not supported by a review of current practice.
The objective of this paper is to provide writers and reviewers of research proposals with evidence from a variety of sources for which components they should expect, and which are unnecessary or unhelpful, in a study which is labeled as a pilot or feasibility study. To do this we repeated Lancaster et al's [ 3 ] review for current papers see if there has been any change in how pilot studies were reported since their study. As many pilot studies are never published we also identified pilot studies which were registered with the UK Clinical Research Network (UKCRN) Portfolio Database. This aims to be a "complete picture of the clinical research which is currently taking place across the UK". All studies included have to have been peer reviewed through a formal independent process. We examined the websites of some grant giving bodies to find their definition of a pilot study and their funding policy toward them. Finally we contacted editors of leading medical journals to discover their policy of accepting studies described as 'pilot' or 'feasibility'.
Literature survey
MEDLINE, Web of Science and university library data bases were searched for the years 2007-8 using the same key words "Pilot" or "Feasibility" as used by Lancaster et al. [ 3 ]. We reviewed the same four general medicine journals: the British Medical Journal (BMJ), Lancet, the New England Journal of Medicine (NEJM) and the Journal of American Medical Association (JAMA) and the same three specialist journals: British Journal of Surgery (BJS), British Journal of Cancer (BJC), British Journal of Obstetrics and Gynecology (BJOG). We excluded review papers. The full text of the relevant papers was obtained. GL reviewed 20 papers and classified them into groups as described in her original paper [ 3 ]. Subsequently MA, in discussion with MC, designed a data extraction form to classify the papers. We changed one category from GL's original paper. We separated the category 'Phase I/II trials' from the 'Piloting new treatment, technique, combination of treatments' category. We then classified the remaining paper into the categories described in Table 1 . The total number of research papers by journal was obtained by searching journal article with abstracts (excluding reviews) using Pubmed. We searched citations to see whether the pilot studies identified by Lancaster et al [ 3 ] eventually led to main trials.
Portfolio database review
The (UKCRN) Portfolio Database was searched for the terms 'feasibility' or 'pilot' in the title or research summary. Duplicate cases and studies classified as 'observational' were omitted. From the remaining studies those classified as 'closed' were selected to exclude studies which may not have started or progressed. Data were extracted directly from the research summary of the database or where that was insufficient the principle investigator was contacted for related publications or study protocols.
Editor and funding agency survey
We wrote to the seven medical journal editors of the same journals used by Lancaster et al. [ 3 ], (BMJ, Lancet, NEJM, JAMA. BJS, BJC and BJOG) and looked at the policies of three funding agencies (British Medical Research Council, Research for Patient Benefit and NETSCC (National Institute for Health Research Trials and Studies Coordinating Centre). We wished to explore whether there was any specified policy of the journal for publishing pilot trials and how the editors defined a pilot study. We also wished to see if there was funding for pilot studies.
Initially 77 papers were found in the target journals for 2007-8 but 23 were review papers or commentaries or indirectly referred to the word "pilot" or "feasibility" and were not actually pilot studies leaving a total of 54 papers. Table 1 shows the results by journal and by type of study and also shows the numbers reported by Lancaster et al. [ 3 ] for 2000-01 in the same medical journals. There was a decrease in the proportion of pilot studies published over the period of time, however the difference was not statistically significant (2.0% vs 1.6%; X 2 = 1.6, P = 0.2). It is noticeable that the Phase I or Phase II studies are largely confined to the cancer journals.
Lancaster et al [ 3 ] found that 50% of pilot studies reported the intention of further work yet we identified only 8 (8.8%) which were followed up by a major study. Of these 2 (25%) were published in the same journal as the pilot.
Twenty-six of the studies found in 2007-8 were described as pilot or feasibility studies for randomized clinical trials (RCTs) including Phase II studies. Table 2 gives the numbers of studies which describe specific components of RCTs. Sample size calculations were performed and reported in 9 (36%) of the studies. Hypothesis testing and performing inferential statistics to report significant results was observed in 21 (81%) of pilot studies. The processes of blinding was observed in only 5 (20%) although the randomization procedure was applied or tested in 16 (62%) studies. Similarly a control group was assigned in most of the studies (n = 18; 69%). As many as 21 (81%) of pilot studies suggested the need for further investigation of the tested drug or procedure and did not report conclusive results on the basis of their pilot data. The median number of participants was 76, inter-quartile range (42, 216).
Of the 54 studies in 2007-8, a total of 20 were described as 'pilot' and 34 were described as 'feasibility' studies. Table 3 contrasts those which were identified by the keyword 'pilot' with those identified by 'feasibility'. Those using 'pilot' were more likely to have a pre-study sample size estimate, to use randomization and to use a control group. In the 'pilot' group 16(80%) suggested further study, in contrast to 15 (44%) in the 'feasibility' group.
A total of 34 studies were identified using the term 'feasibility' or 'pilot' in the title or research summary which were prospective interventional studies and were closed, i.e. not currently running and available for analysis. Only 12 studies were interventional pilot/feasibility studies which included testing of some component of the research process. Of these 5 were referred to as 'feasibility', 6 as 'pilot' and 1 as both 'feasibility' and 'pilot' (Table 4 ).
The methodological components tested within these studies were: estimation of sample size; number of subjects eligible; resources (e.g. cost), time scale; population-related (e.g. exclusion criteria), randomisation process/acceptability; data collection systems/forms; outcome measures; follow-up (response rates, adherence); overall design; whole trial feasibility. In addition to one or more of these, some studies also looked at clinical outcomes including: feasibility/acceptability of intervention; dose, efficacy and safety of intervention.
The results are shown in Table 4 . Pilot studies alone included estimation of sample size for a future bigger study and tested a greater number of components in each study. The majority of the pilots and the feasibility studies ran the whole study 'in miniature' as it would be in the full study, with or without randomization.
As an example of a pilot study consider 'CHOICES: A pilot patient preference randomised controlled trial of admission to a Women's Crisis House compared with psychiatric hospital admissions' http://www.iop.kcl.ac.uk/projects/default.aspx?id=10290 . This study looked at multiple components of a potential bigger study. It aimed to determine the proportion of women unwilling to be randomised, the feasibility of a patient preference RCT design, the outcome and cost measures to determine which outcome measures to use, the recruitment and drop out rates; and to estimate the levels of outcome variability to calculate sample sizes for the main study. It also intended to develop a user focused and designed instrument which is the outcome from the study. The sample size was 70.
The editors of five (out of seven) medical journals responded to our request for information regarding publishing policy for pilot studies. Four of the journals did not have a specified policy about publishing pilot studies and mostly reported that pilot trials cannot be published if the standard is lower than a full clinical trial requirement. The Lancet has started creating space for preliminary phase I trials and set a different standard for preliminary studies. Most of the other journals do not encourage the publication of pilot studies because they consider them less rigorous than main studies. Nevertheless some editors accepted pilot studies for publication by compromising only on the requirement for a pre-study sample size calculation. All other methodological issued were considered as important as for the full trials, such as trial registration, randomization, hypothesis testing, statistical analysis and reporting according to the CONSORT guidelines.
All three funding bodies made a point to note that pilot and feasibility studies would be considered for funding. Thabane et al [ 2 ] provided a list of websites which define pilot or feasibility studies. We considered the NETSCC definition to be most helpful and to most closely mirror what investigators are doing and it is given below.
NETSCC definition of pilot and feasibility studies http://www.netscc.ac.uk/glossary/
Feasibility Studies
Feasibility Studies are pieces of research done before a main study. They are used to estimate important parameters that are needed to design the main study. For instance:
standard deviation of the outcome measure, which is needed in some cases to estimate sample size,
willingness of participants to be randomised,
willingness of clinicians to recruit participants,
number of eligible patients,
characteristics of the proposed outcome measure and in some cases feasibility studies might involve designing a suitable outcome measure,
follow-up rates, response rates to questionnaires, adherence/compliance rates, ICCs in cluster trials, etc.
Feasibility studies for randomised controlled trials may not themselves be randomised. Crucially, feasibility studies do not evaluate the outcome of interest; that is left to the main study.
If a feasibility study is a small randomised controlled trial, it need not have a primary outcome and the usual sort of power calculation is not normally undertaken. Instead the sample size should be adequate to estimate the critical parameters (e.g. recruitment rate) to the necessary degree of precision.
Pilot studies
A Pilot Study is a version of the main study that is run in miniature to test whether the components of the main study can all work together. It is focused on the processes of the main study, for example to ensure recruitment, randomisation, treatment, and follow-up assessments all run smoothly. It will therefore resemble the main study in many respects. In some cases this will be the first phase of the substantive study and data from the pilot phase may contribute to the final analysis; this can be referred to as an internal pilot. Alternatively at the end of the pilot study the data may be analysed and set aside, a so-called external pilot.
In our repeat of Lancaster et al's study [ 3 ] we found that the reporting of pilot studies was still poor. It is generally accepted that small, underpowered clinical trials are unethical [ 4 ]. Thus it is not an excuse to label such a study as a pilot and hope to make it ethical. We have shown that pilot studies have different objectives to RCTs and these should be clearly described. Participants in such studies should be informed that they are in a pilot study and that there may not be a further larger study.
It is helpful to make a more formal distinction between a 'pilot' and a 'feasibility' study. We found that studies labeled 'feasibility' were conducted with more flexible methodology compared to those labeled 'pilot'. For example the term 'feasibility' has been used for large scale studies such as a screening programme applied at a population level to determine the initial feasibility of the programme. On the other hand 'pilot' studies were reported with more rigorous methodological components like sample size estimation, randomization and control group selection than studies labeled 'feasibility'. We found the NETSCC definition to be the most helpful since it distinguishes between these types of study.
In addition it was observed that most of the pilot studies report their results as inconclusive, with the intention of conducting a further, larger study. In contrast, several of the feasibility studies did not admit such an intention. On the basis of their intention one would have expected about 45 of the studies identified by Lancaster et al in 2000/1 to have been followed by a bigger study whereas we only found 8. This would reflect the opinion of most of the journal editors and experts who responded to our survey, who felt that pilot studies rarely act as a precursor for a bigger study. The main reason given was that if the pilot shows significant results then researchers may not find it necessary to conduct the main trial. In addition if the results are unfavorable or the authors find an unfeasible procedure, the main study is less likely to be considered useful. Our limited review of funding bodies was encouraging. Certainly when reviewing grant applications, we have found it helpful to have the results of a pilot study included in the bid. We think that authors of pilots studies should be explicit as to their purpose, e.g. to test a new procedure in preparation for a clinical trial. We also think that authors of proposals for pilot studies should be more explicit as to the criteria which lead to further studies being abandoned, and that this should be an important part of the proposal.
In the Portfolio Database review, only pilot studies cited an intention to estimate sample size calculations for future studies and the majority of pilot studies were full studies run with smaller sample sizes to test out a number of methodological components and clinical outcomes simultaneously. In comparison the feasibility studies tended to focus on fewer methodological components within individual studies. For example, the 6 pilot studies reported the intention to evaluate a total of 17 methodological components whereas in the 5 feasibility studies a total of only 6 methodological components were specifically identified as being under investigation (Table 4 ). However, both pilot and feasibility studies included trials run as complete studies, including randomization, but with sample sizes smaller than would be intended in the full study and the distinction between the two terms was not clear-cut.
Another reason for conducting a pilot study is to provide information to enable a sample size calculation in a subsequent main study. However since pilot studies tend to be small, the results should be interpreted with caution [ 5 ]. Only a small proportion of published pilot studies reported pre-study sample size calculations. Most journal editors reported that a sample size calculation is not a mandatory criterion for publishing pilot studies and suggested that it should not be done.
Some authors suggest that analysis of pilot studies should mainly be descriptive,[ 3 , 6 ] as hypothesis testing requires a powered sample size which is usually not available in pilot studies. In addition, inferential statistics and testing hypothesis for effectiveness require a control arm which may not be present in all pilot studies. However most of the pilot interventional studies in this review contained a control group and the authors performed and reported hypothesis testing for one or more variables. Some tested the effectiveness of an intervention and others just performed statistical testing to discover any important associations in the study variables. Observed practice is not necessarily good practice and we concur with Thabane et al [ 2 ] that any testing of an intervention needs to be reported cautiously.
The views of the journal editors, albeit from a small sample, were not particularly encouraging and reflected the experience of Lancaster et al [ 3 ]. Pilot studies, by their nature, will not produce 'significant' (i.e P < 0.05) results. We believe that publishing the results of well conducted pilot or feasibility studies is important for research, irrespective of outcome.. There is an increasing awareness that publishing only 'significant' results can lead to considerably error [ 7 ]. The journals we considered were all established, paper journals and perhaps the newer electronic journals will be more willing to consider the publication of the results from these types of studies.
We may expect that trials will increasingly be used to evaluate 'complex interventions'[ 8 , 9 ]. The MRC guidelines [ 8 ] explicitly suggest that preliminary studies, including pilots, be used prior to any major trial which seeks to evaluate a package of interventions (such as an educational course), rather than a single intervention (such as a drug). Thus it is likely that reviewers will be increasingly asked to pronounce on these and will require guidance as to how to review them.
Conclusions
We conclude that pilot studies are still poorly reported, with inappropriate emphasis on hypothesis-testing. We believe authors should be aware of the different requirements of pilot studies and feasibility studies and report them appropriately. We found that in practice the definitions of feasibility and pilot studies are not distinct and vary between health research funding bodies and we suggest use of the NETSCC definition to clarify terminology.
Arnold DM, Burns KE, Adhikari NK, Kho ME, Meade MO, Cook DJ: McMaster Critical Care Interest Group. The design and interpretation of pilot trials in clinical research in critical care. Crit Care Med. 2009, 37 (Suppl 1): S69-74. 10.1097/CCM.0b013e3181920e33.
Article PubMed Google Scholar
Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Goldsmith CH: A tutorial on pilot studies: The what, why and How. BMC Medical Research Methodology. 2010, 10: 1-10.1186/1471-2288-10-1.
Article PubMed PubMed Central Google Scholar
Lancaster GA, Dodd S, Williamson PR: Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004, 10: 307-12. 10.1111/j..2002.384.doc.x.
Halpern SD, Karlawish JH, Berlin JA: The continuing unethical conduct of underpowered clinical trials. JAMA. 2002, 288: 358-62. 10.1001/jama.288.3.358.
Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA: Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006, 63: 484-9. 10.1001/archpsyc.63.5.484.
Grimes DA, Schulz KF: Descriptive studies: what they can and cannot do. Lancet. 2002, 359: 145-9. 10.1016/S0140-6736(02)07373-7.
Ioannidis JPA: Why Most Published Research Findings Are False. PLoS Med. 2005, 2 (8): e124-10.1371/journal.pmed.0020124.
Lancaster GA, Campbell MJ, Eldridge S, Farrin A, Marchant M, Muller S, Perera R, Peters TJ, Prevost AT, Rait G: Trials in Primary Care: issues in the design of complex intervention. Statistical Methods in Medical Research. 2010, to appear
Google Scholar
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M: Developing and evaluating complex interventions: new guidance. 2008, Medical Research Council, [ http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC004871 ]
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/10/67/prepub
Download references
Author information
Authors and affiliations.
Health Services Research, ScHARR, University of Sheffield, Regent Court, Regent St Sheffield, S1 4DA, UK
Mubashir Arain, Michael J Campbell & Cindy L Cooper
Department of Mathematics and Statistics, University of Lancaster, LA1 4YF, UK
Gillian A Lancaster
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Michael J Campbell .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
MA reviewed the papers of 2000/1 and those of 2007/8 under the supervision of MC and helped to draft the manuscript. MC conceived of the study, and participated in its design and coordination and drafted the manuscript. CC conducted the portfolio database study and commented on the manuscript. GA conducted the original study, reviewed 20 papers and commented on the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Arain, M., Campbell, M.J., Cooper, C.L. et al. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 10 , 67 (2010). https://doi.org/10.1186/1471-2288-10-67
Download citation
Received : 20 May 2010
Accepted : 16 July 2010
Published : 16 July 2010
DOI : https://doi.org/10.1186/1471-2288-10-67
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pilot Study
- Feasibility Study
- Journal Editor
- Methodological Component
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
The pilot study we have undertaken and presented in this article comprises several approaches which will influence the main study, to pretest the interview format, and to interrogate the way to conduct the pilot study.
Pilot studies are a fundamental stage of the research process. They can help identify design issues and evaluate a study’s feasibility, practicality, resources, time, and cost before the main research is conducted. It involves selecting a few people and trying out the study on them.
Although lack of funding is definitely an issue, a proper pilot study still needs to have clear goals, details of how these goals will be assessed, justification for the sample size given the goals, and proper methodological rigor for how the pilot study will be conducted.
The objective of this paper is to provide a detailed examination of the key aspects of pilot studies for phase III trials including: 1) the general reasons for conducting a pilot study; 2) the relationships between pilot studies, proof-of-concept studies, and adaptive designs; 3) the challenges of and misconceptions about pilot studies; 4) the c...
A pilot study can be used to evaluate the feasibility of recruitment, randomization, retention, assessment procedures, new methods, and implementation of the novel intervention. A pilot study is not a hypothesis testing study. Safety, efficacy and effectiveness are not evaluated in a pilot.
The objective of this paper is to provide a detailed examination of the key aspects of pilot studies for phase III trials including: 1) the general reasons for conducting a pilot study; 2) the relationships between pilot studies, proof-of-concept studies, and adaptive designs; 3) the challenges of and misconceptions about pilot studies; 4) the ...
A pilot study is a small scale preliminary study conducted in order to evaluate feasibility of the key steps in a future, full-scale project.
This paper discusses literature review as a methodology for conducting research and offers an overview of different types of reviews, as well as some guidelines to how to both conduct and evaluate a literature review paper.
We conducted a literature review to identify existing recommendations for feasibility and pilot studies, as well as publications describing formative processes for implementation trials.
A Pilot Study is a version of the main study that is run in miniature to test whether the components of the main study can all work together. It is focused on the processes of the main study, for example to ensure recruitment, randomisation, treatment, and follow-up assessments all run smoothly.