If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

Chemistry library
Course: chemistry library > unit 6.
- The periodic table, electron shells, and orbitals
The periodic table
- Groups of the periodic table
- Counting valence electrons for main group elements
- The periodic table - transition metals
- Counting valence electrons
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript
Teach Science With Fergy
Engagement Through Application
Teaching Introductory Chemistry – Atoms and the Periodic Table
Inside my Membership, you’ll get access to all the resources listed below and so much more, including
- Over 1000 science lessons
- 100+ video lesson walkthroughs
- Additional day-by-day teaching outlines, activities, projects, quizzes, tests, and more all organized by subject and type

- Student Notes
- Bill Nye Phases of Matter Video and Worksheet
- Physical and Chemical Properties Worksheet
- Properties of Matter Handout
- Physical and Chemical Properties Worksheet
- Physical and Chemical Properties and Changes Forensic Lab
- Watch Lesson 3 – Density – Part 1
- Complete Student Notes
- Finish Physical and Chemical Properties and Changes Forensic Lab
- Watch Lesson 3 – Density – Part 2
- Complete Lesson Notes
- Lesson 3 – Density – Part 2
- Measuring Density Lab
- Density Worksheet 1
If you’d like access to all of these resources plus everything else I have in My Store, please consider joining my Membership Program. Click here to learn more about it.
- Finish Measuring Density Lab if needed
- Density Worksheet 2
- Density Lab Station Activity
- Density Quiz – From Density Worksheet 2
- Watch Lesson 4 – Matter Classification and Particle Theory
- Classification of Matter Flow chart
- Particle Theory of Matter worksheet
- Note – When I use the flipped classroom, I have my students use their class time to work on it. If you’re not flipping your class you won’t have as much time in class. If you still want to do the project, you’ll need to change your timeline slightly so that your students have time to complete it
- Watch Lesson 5 – Atomic Theory
- Element Story Project
- Watch Lesson 6 – Atomic Structure
- Element Story Project – 30 minutes then checkpoint 3.5 check
- Take up – Subatomic Particles worksheet
- Bohr-Rutherford Diagrams Lesson
- Bill Nye – Atoms
- I usually assign a bunch of elements to do Bohr-Diagrams for then have them check each other’s work

- Periodic Table Worksheet (20 minutes)
- Watch Lesson 7 – Periodic Table
- Study for Quiz – Lesson 4-6
- Quiz – Lesson 4-6
- Element Story Project – Day 4
- Watch Lesson 8 – Ions
- Ions worksheet and answers (25 minutes)
- Completed good copy of script
- Watch Lesson 9 – Chemical Symbols and Formulas
- Building Molecules
- Counting atoms worksheet (25 minutes)
- Element Story Project – Day 6
- Chemistry Unit Test Review
- Chemistry Unit Test
- Element Story good copy pictures are due tomorrow
- Completed good copy pictures
- Audio recordings are due tomorrow
- Completed audio recordings
- Completed integrating audio and pictures
- Ready to present
- Uploaded Fact Sheet Doc
- Element Story Project – Day 10 – Presentation Day
- Refund Policy
- Download Two Activities Free
Introduction to the Periodic Table of the Elements
Periodic Table with molecular model (OntheRunPhoto, iStockphoto)
How does this align with my curriculum?
Share on: facebook x/twitter linkedin pinterest.
Learn about the elements of the periodic table and how they are organized.
Matter that is composed of only one type of atom is called an element . People have known about some elements such as gold, silver, iron, mercury and tin for hundreds of years. Scientists discovered many others during the 18th and 19th centuries. Some elements have even been discovered within your lifetime !
Naming the Elements
Many of the elements have their name or their symbol based in Latin or Greek. For example:
As well for famous scientists such as:
Other elements have been named for places, such as:
Did you know? Argentina got its name from an element. Its name is from the latin name of silver, argentum . This is because its natives gave silver gifts to the first European conquerors.
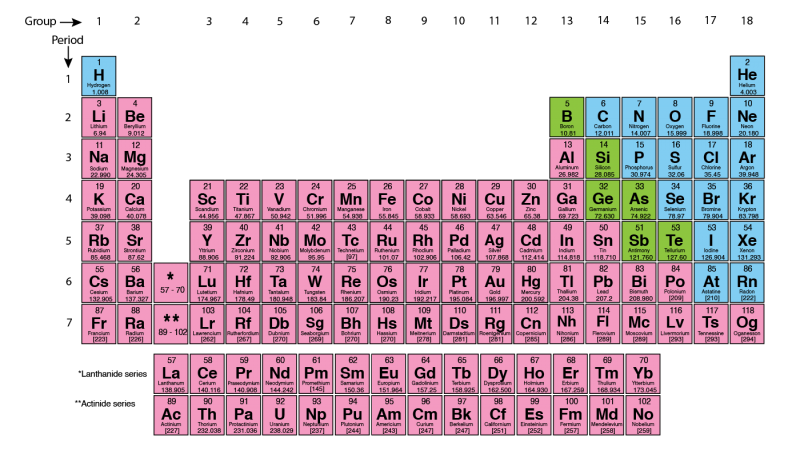
The periodic table of the elements
The periodic table of the elements is a visual and logical way to organize all elements. Dmitri Mendeleev, a Russian scientist, is usually credited with creating the first periodic table in 1869. In Germany, Lothar Meyer also came up with an almost identical table later the same year. Some people attribute the first categorization to Alexandre-Emile Béguyer de Chancourtois .
Each element on the periodic table is listed in a box with its atomic symbol and atomic number. The element’s full name and atomic mass is also sometimes indicated. The image below shows a typical entry for the element calcium.
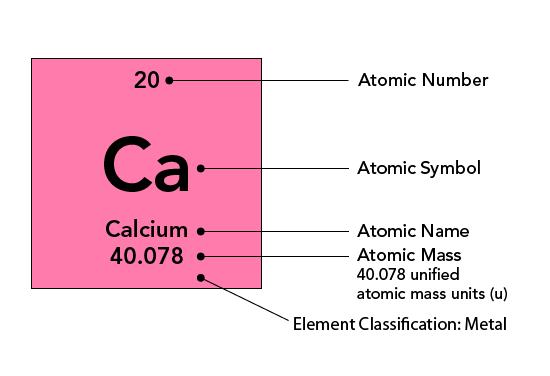
The number above the atomic symbol represents the atomic number. The atomic number is equal to the number of protons in an atom's nucleus and determines which element an atom is. For example, any atom that contains exactly 20 protons in its nucleus is an atom of calcium. The number of protons influences the chemical behaviour of an element.
The atomic mass of an element refers to the average mass of an element in unified atomic mass units (short form u ). This is usually given at the bottom of an element’s entry in the periodic table. The atomic mass is a decimal number because it is an average of the masses of the various isotopes of an element. Isotopes of an element have the same number of protons, but different numbers of neutrons. For example, hydrogen has three naturally occurring isotopes. The most common isotope, protium , has no neutrons! Deuterium has one neutron and tritium has two neutrons.
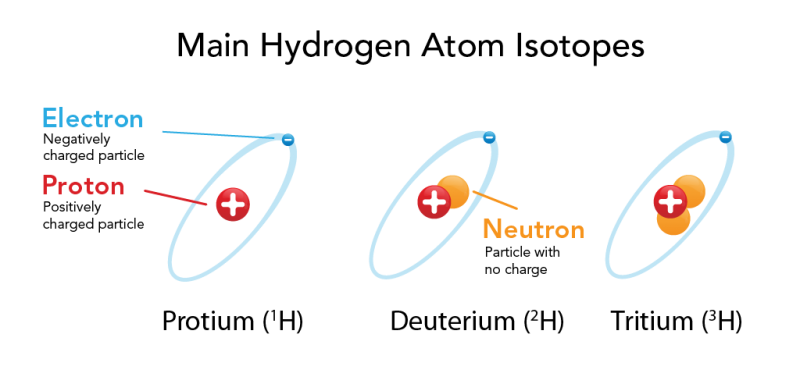
Scientists have also created other highly unstable isotopes of hydrogen. Those, with up to 7 neutrons, do not occur in nature.
Getting the average number of neutrons for an element is easy. You need to subtract the number of protons (atomic number) from the atomic mass. For example, calcium has an atomic number of 20 and an atomic mass of 40.078 u. By subtracting 20 from 40 (rounded), we determine that the average number of neutrons is 20.
Arrangement of Elements
Mendeleev organized the table so that the elements from low to high atomic mass. Then he organized rows and columns to highlight elements with similar chemical properties. The rows are known as periods . All elements in the same period have the same number of electron layers , also called shells. The columns are known as groups (or families).
Mendeleev also left gaps in his table. He believed that some elements had not yet been discovered. Those gaps were for undiscovered elements. Mendeleev predicted the missing elements would have the same properties as the other elements in the same column.
Discoveries led to many new elements during the 1900s. Mendeleïev’s table was rearranged to give us the format that we use today. But the principles of groups and periods still exist in our modern version.
The groups on the periodic table are numbered from 1 to 18 . The elements within a group usually have similar properties. For example, the elements of group 18 are all gases that do not readily react with other elements. Groups can sometimes be given names. For example, the elements in group 18 are called ‘ Noble Gases’ .
Did you know? Noble gases get their name because the ability to stay calm and not react is considered noble in humans!

Except for hydrogen, elements on the left side of the periodic table are metals. Metals are solid at room temperature, except for mercury, which is liquid. They are also malleable and can be ductile. Ductile is another word for stretchy. Metals also have a shiny appearance. They are good conductors of heat and electricity.
Elements on the right side of the periodic table are non-metals. Non-metals can be solids, liquids or gases at room temperature. They are poor conductors of heat and electricity.
Elements that have some metallic as well as non-metallic properties are known as metalloids (e.g., silicon, arsenic, etc.).
We sometimes call the elements in groups 3 to 12 the d-block . These elements are also known as transition elements .
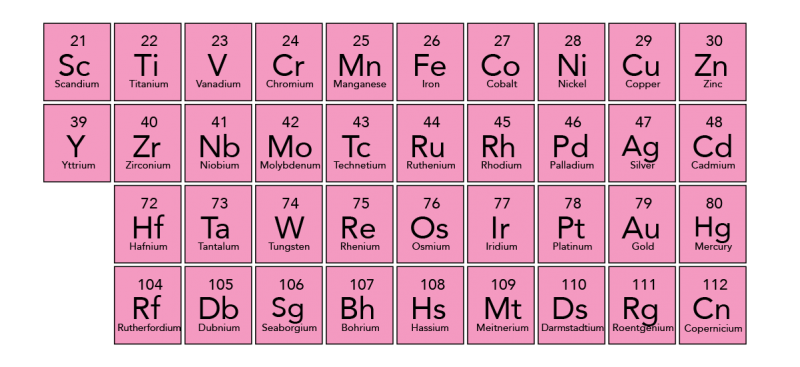
There are two rows placed at the bottom of the periodic table. These are the Lanthanide and Actinide series. The Lanthanide series includes fifteen metallic elements with atomic numbers 57 through 71. The Lanthanide elements, along with the two elements of group 3, Scandium and Yttrium, are often called the rare earth elements. You can find Lanthanides naturally on Earth. The Actinide series is much different. All of the Actinide elements are radioactive and some are not found in nature. Some of the elements with higher atomic numbers can only be created in laboratories.

Trends of the Periodic Table
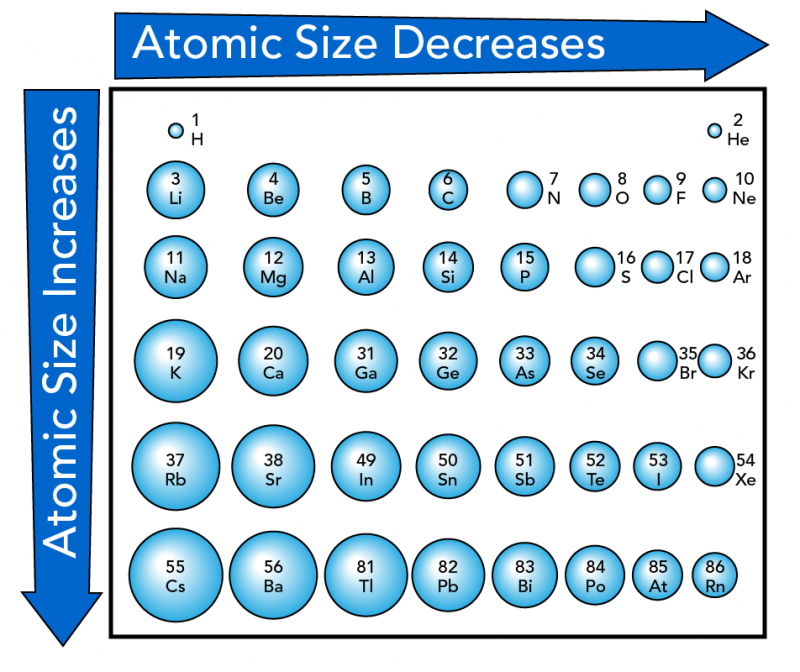
In general, the size or radius of an atom increases as you move down a group. As you go down, elements have more layers of electrons, which results in bigger atoms. From left to right within a period, atomic size generally decreases. This is because more protons pull the electrons closer to the nucleus.
Electronegativity is the chemical property that describes the tendency of an atom to form bonds with others. Electronegativity usually decreases down each group and increases from left to right across a period. The most electronegative elements are in the upper right hand side of the periodic table. This is because non-metals have high electronegativity. They tend to attract electrons from other atoms. While metals tend to lose electrons easily.
Assigning Charges to an Element
To become electrically-charged, stable atoms must lose or gain electrons. There are two types of electric charges. There are positive charges (+) and negative charges (-). Substances with the same charge will repel each other. While substances with opposite charges attract each other. This is similar to how magnets work!
The atoms of some elements can lose or gain electrons more easily than others. For example, the elements in group 1 (e.g., Li, Na, K) can easily lose one electron, which gives them a +1 charge . The elements in group 17 (Cl, Br, I) can easily gain one electron, which gives them a -1 charge . When an atom or molecule has a charge, it is said to be an ion . When an atom or molecule has more electrons than protons, the result is a negative ion, called an anion . When it has fewer electrons than protons, then the result is a positive ion, called a cation .
Each group in the periodic table has a specific charge commonly associated with its ions. The exceptions to this are the transition elements, and the lanthanide and actinide series. Below are the charges for the different groups within the table.
You may have noticed that the elements in group 18 have a charge of 0. This is because these elements are very stable and they do not readily gain or lose electrons.
The photographic periodic table This page by periodictable.com has photographs of common things made from, or using, each of the element.
Introduction to the Particle Theory of Matter This article from Let’s Talk Science introduces the history of the theory that describes matter as different assemblies of smaller particles, or atoms.
The Genius of Mendeleev's Table This article from Let’s Talk Science presents how Dmitri Mendeleev created the first periodic table.
Introduction to the Atom This article from Let’s Talk Science presents the history of the different atomic models created.
The Newest Elements on the Periodic Table This article from Let’s Talk Science presents the most recent elements added to the periodic table.
The Periodic Table of Elements This page by the Thomas Jefferson National Accelerator Facility has an interactive periodic table as well as flash cards, games, etc. about the elements.
Helmenstine, A. (2019). Introduction to the Periodic Table . Thought.co.
Webelements.com. (n.d.). The periodic table of the elements .
Western Oregon University. (n.d.). A Brief History Of The Development Of Periodic Table . People.wou.edu
Related Topics

The Periodic Table Flipbook
This NO-PREP tabbed flip book is a flexible way to introduce (or review) the classifications of elements within The Periodic Table.
4 pages – 2 MB – PDF
Description
Additional information.
- Reviews (0)
- Ask A Question
Just PRINT and GO ! This NO-PREP tabbed flip book is a flexible way to introduce (or review) the classifications of elements within The Periodic Table . Students complete each section of the flipbook, cut the tabs and staple together for an engaging activity that serves as a study tool or pre-assessment.
Suggestions for use:
→Interactive Notebook Page
→Pre-teaching research activity (to gain prior knowledge)
→Take-home study guide before a test or quiz
→Early finisher activity
→Station Activity- use as a “station” in an around the room practice activity
→Differentiate- this flipbook allows for differentiation of process
→Review and Reinforcement- use as a review of FORCES
→Sub Work: a great way to keep students busy while you are out of the classroom.
The tabs included in this flipbook are:
★Alkali Metals
★Alkali Earth Metals
★Transition Metals
★Noble Gases
★Rare Earth Metals
★Metalloids
You may also be interested in these other products from my store:
★ Periodic Table Introduction Activity
★ Periodic Table Scavenger Hunt
★ Atomic Structure Scavenger Hunt
★Search my BUNDLE SETS & Save Money!
There are no reviews yet.
Name *
Email *
How do I access my digital download?
- An automatic email with the download link is generated every time you make a purchase so check your inbox and spam/junk box then click on the link in the email.
- Log in to your account and you will find a list of your purchases, which you can download at any time onto any of your devices.
Is this a shipped item?
I have another question about your store...., am i responsible for sales tax or duties, login required to submit question .
Remember Me
You might also like...
Related products.

Is My Teacher a Zombie? Characteristics of Life Activity [Print & Digital]

DNA, Fibers, Fingerprints & Hairs Vocabulary Assignment [Print & Digital]

Toxicology Escape the Classroom Activity

Measurement and Metrics Lab Stations Activity [Print & Digital Option}

thetrendyscienceteacher

We have been showered with gifts all week for TEACHER APPRECIATION WEEK, but one of my favorites has been this teacher shout-out board. 📣 It has warmed my heart to read the sweet comments left by students. 💜 ...
My forensics class is in the middle of our classroom body farm lab experiment and it’s been so much fun! The class was tasked with creating an experiment that would allow us to make observations about the life cycle of a blow fly and it’s implications to death investigation. These kids went all out and, so far, we’ve gotten fantastic results! Check out my blog post for ALL the details 👇🏼 www.thetrendyscienceteacher.com/bodyfarm #forensicscience #forensics #iteachforensics #forensicsteacher #forensicscientist ...
Need a no-prep resource for EARTH DAY? 🌍 Just print this ecosystem scavenger hunt brochure and take a little tour of an outdoor area around your school! Your students will love getting outside and they’ll be learning too. 🌱 Grab this FREE resource at: www.thetrendyscienceteacher.com/earthday #earthday #biologyclass #lifescience #iteachbio #biologyteacher ...
Welcome to my classroom! 🍎 If you’re new around here, hi 👋! I’m Dana. I’m a high school science teacher that loves teaching and sharing my classroom with teachers around the globe! 🌎 I’d love to know where you are from! Tell me in the comments 👇🏼. I’ll start. ...
A few years ago, I researched “the spacing effect”- a psychological phenomenon where information is better retained through distributed practice over time. 🧠 After studying the effects it had on student retention, I knew I had to find a way to use it in my biology class. I’ve been using spiral reviews for four years now and the results have been amazing! So amazing, that I want to share it with all of my bio teacher friends! Today, on the blog, I’m sharing the spiral reviews that I use for test prep along with the details on how I implement them for maximum success. Grab my FREE spiral reviews at www.thetrendyscienceteacher.com/spiral #biology #biologyteachers #biologyteacher #iteachbiology #biologyteachersofinstagram ...
My Biology students always get genetic drift confused with natural selection when discussing mechanism of evolution. To help them distinguish between the two phenomena, I had them simulate genetic drift using craft beads and a dice. This really helped my students visualize the effects of genetic drift on small populations. To grab this activity, head to… 🔗 www.thetrendyscienceteacher.com/geneticdrift ...
Do I bribe my students? Of course I do! 😉 When I have to be absent, I expect my students to be on their BEST behavior. To ensure that they follow through on that expectation, I offer a little bribery…. I mean….. incentive 😁 I leave a sub report card that allows the substitute to rate each class. Classes receiving an A+ get rewarded with tickets 🎟️ I have received so many compliments from my subs since I started implementing this system. To get this free sub report card, head over to my blog: www.thetrendyscienceteacher.com/sub ...
Over the years, I have implemented several systems and procedures that have helped make my “teacher life” easier. One of those procedures are these absent trays. When students are absent, I write their names in any worksheets that I handed out that day and put them in these absent drawer (organized by class period). Students know that the procedures for getting make up work after an absence are as follows: ✅ Check Google Classroom ✅ Check the absent drawer (pictured here) This ensures consistency throughout the year and students never have to wonder what we did in class during their absence. GAME. CHANGER. 👏 #teachertips #highschoolteacher #iteachscience #classroomorganization #newteachertips #newteacher ...
Here’s to FRIDAY and a clean classroom! 🧼 Comment below👇🏼and let me know what your weekend plans are. ...
Looking for something?
Shop science resources.
- Physical Science
- Classroom Décor
- Professional Development
- Elementary Science
- Shop on TPT
Subscribe to get our latest content by email PLUS exclusive FREEBIES and resources for members!

Join The Trendy Science Teacher team to get these FREE Differentiated Scientific Method Task Cards in both print and digital versions!
- My Favorite Things!
My Favorite Way to Introduce the Periodic Table

This sounds really nerdy but I absolutely love the Periodic Table. I think it’s one of the most fascinating tools in science and I love bringing it to life for my students. I have created a lot of resources around the Periodic Table because as a young student I never really appreciated its usefulness. Through college and grad school, I really did.
This ‘Periodic People’ activity has become a favorite lesson and definitely my favorite way to introduce the concept of the Periodic Table as a table of patterns! This activity was adapted from the Oakland Schools Chemistry Resource Unit. The original activity includes a perfectly useful but more simplistic version of the ‘Periodic People’ cards. Somewhere along the way, I found the cutest set of these that had been re-drawn by an artist named Renee Kimpel. She had them on her blog for free download. Unfortunately, I have not been able to find Renee and her blog again to give her proper credit (if you do know about this blog or where the re-drawn Periodic People can be found, please leave this in the comment section below!).
I am providing a FREE DOWNLOAD of this resource, so be sure to scroll to the bottom to grab the freebie!

This is a hands-on inquiry-based activity for which students are introduced by an engaging prompt:
You have been chosen for this top secret mission. Your mission, should you choose to accept it, is to work with the “sketches” of the suspicious characters on the secret agent list. They are part of a family of secret agents, but the most deadly of all has never been sketched. Your job is to arrange the sketches in a pattern so that you can draw the missing secret agent.
In groups of two or three, students will figure out a way to arrange 17 drawings of ‘Periodic People’ into a table that considers patterns. There are patterns going up and down and across from left to right! These patterns include the body size (skinny to fat), the number of antennae (1 to 8), the number of fingers (1 to 18), the number of arms (1 to 3), the facial expression (really sad to super happy), and the body design pattern (9 different ones). These patterns are synonymous with the major patterns on the Periodic Table itself– the number of electron shells increases moving down a column, the number of valence electrons increases moving from left to right across a row, the electronegativity increases moving from left to right across the main group elements, etc.
If you’ve already taught atomic structure, then your students may recognize these ‘personified’ patterns themselves! The number of arms represents the number of electron shells, the number of antennae represents the number of valence electrons, the number of fingers represents the atomic number. This year, one of my students even noticed something I hadn’t before– the number of fingers on each specific hand represents the number of electrons in each specific electron shell (K, L, and M). I gave myself a secret HIGH-FIVE when he pointed this out to me!
But, don’t worry, you can use this awesome activity even if you haven’t taught these patterns yet! Either way, I have a great activity in my TPT store that works perfectly before (while teaching atomic structure) or after this one! (Check out my Bohr Atoms Diagrams Manipulatives Activity .)

You can grab this PERIODIC PEOPLE ACTIVITY , including the people cards, teacher notes and table answer key! And I have added a digital Google Slides version of the activity, too! Click the link above to download the resource and get the Make a Copy link!

Share this post:
You might also like.

An Experimental Design Project That Is Not a Cookbook Lab

How to Assign Digital Cornell Doodle Notes with Google Classroom

Grading Tips for Science Teachers
24 comments.

Karen Jones
I created a set of Periodic People, after readinjg your blog. I would be happy to send you a set (for proofing). After I refine them, you’d be welcome to offer them to your readers.
Kelley Larson
I’m interested in periodic people for my students
Hi Kelley! You can download this for free if you go to my Freebies tab! It’s a great activity!
What grade level is this appropriate for? I teach fifth grade and would like to use this. Thoughts?
Hi Gina, I usually use this with 8th grade, but I definitely think that 5th graders could do this activity! The patterns are very obvious… the challenge would be for them to construct the table with the alien people… but once they do, they can figure out the missing guy! It’s a great NGSS lesson in patterns.
I just used this with 4th and 5th graders. It was fantastic! A number of kids needed prompting, but everyone persevered.
Hello, I have been looking for a meaningful way for my 13 year old to interact with the periodic table and plan to try the periodic people, I was attracted by the picture that looks like someone holding a bunch of peanut m&ms but can’t find a description for that. I select it but keep getting the shelves of bottles instead. Can you explain? Thank you.
Patrik Prouse
Any ideas for how one could use this while remote learning?
Rebecca Bernhardt
I took screenshots of the individual characters and dropped them on a Google Slide. I shared a copies to the kids and they were able to drag/drop the characters to organize them onto the slide. An alternative is to do the same but within a Jamboard so that students can collaborate easier.
Leslie Scream
Hi! I am trying to find a way to do this with my digital students as well as my face to face students. Do you have the periodic people sheet that you can share where the mystery person would NOT be on the paper so our kids at home could print the sheet and cut them out and still participate without being give the mystery person?
Hi Leslie! I think a solution could be to print one and then either tape a piece of white paper over the mystery guy or use white-out before you make copies to send home? I have also just updated the resource to include a digital Google Slides version of the activity!
Hi, I downloaded this great activity but I don’t see an answer key. Sorry if I missed it.
Hi Lizette! The answer key is attached in the PDF!
Regina Spradley
Could I please get a copy of this amazing idea! Much appreciated!!! In advance!
Hi Regina! I hope that you found the link to have a free copy of this sent to your inbox. Thanks for your interest!
I did this with my students last week, and then today I did the Bohn model manipulative with them. I was SO HAPPY with their results! I don’t think they would have been able to see the patterns and make the connections as easily if they hadn’t done this one. I will always be starting my unit with this guy and the other one now. Time to fire up the laminator!
This is great to hear! Thanks for the feedback about how these activities pair together, Amy!
Jessica Harris
Thank you so much for all of your resources and for providing freebies.
Thanks for reading my blog Jessica!
Quick question — I am doing this with 6th/7th graders. Did you give them the outline/shape of the table before or do they have to figure that out? Thanks!
Hi Meikea, I actually don’t give them the outline of the periodic table at first. Just have them explore on their own… then once a group is sort of onto it, I sometimes ‘nudge’ them in the right direction as to the shape! Most of the time they have a “Eureka!” moment when they see how the shape is similar! I hope this helps 🙂 Karla
Thank you so much for sharing this activity. I used this my 9th students to introduce/review the patterns on the periodic table.
Hi Karla! I have been trying to access the Free download for your Periodic People Activity. I get a response saying “success – check your email” but nothing has come to my email inbox or spam folder. Any suggestions?
Hira Naimatullah
HI! I want to know would this activity be useful for a large class of 48 students? and how much average time it takes to conduct properly? thank you for sharing inspiring ideas.
Leave a Reply Cancel Reply

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2.6: Introduction to the Periodic Table
- Last updated
- Save as PDF
- Page ID 36975
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- To become familiar with the organization of the periodic table.
Rutherford’s nuclear model of the atom helped explain why atoms of different elements exhibit different chemical behavior. The identity of an element is defined by its atomic number (Z) , the number of protons in the nucleus of an atom of the element. The atomic number is therefore different for each element. The known elements are arranged in order of increasing Z in the periodic table ( Figure \(\PageIndex{1}\) ). The rationale for the peculiar format of the periodic table is explained later. E ach element is assigned a unique one-, two-, or three-letter symbol. The names of the elements are listed in the periodic table, along with their symbols, atomic numbers, and atomic masses. The chemistry of each element is determined by its number of protons and electrons. In a neutral atom, the number of electrons equals the number of protons.
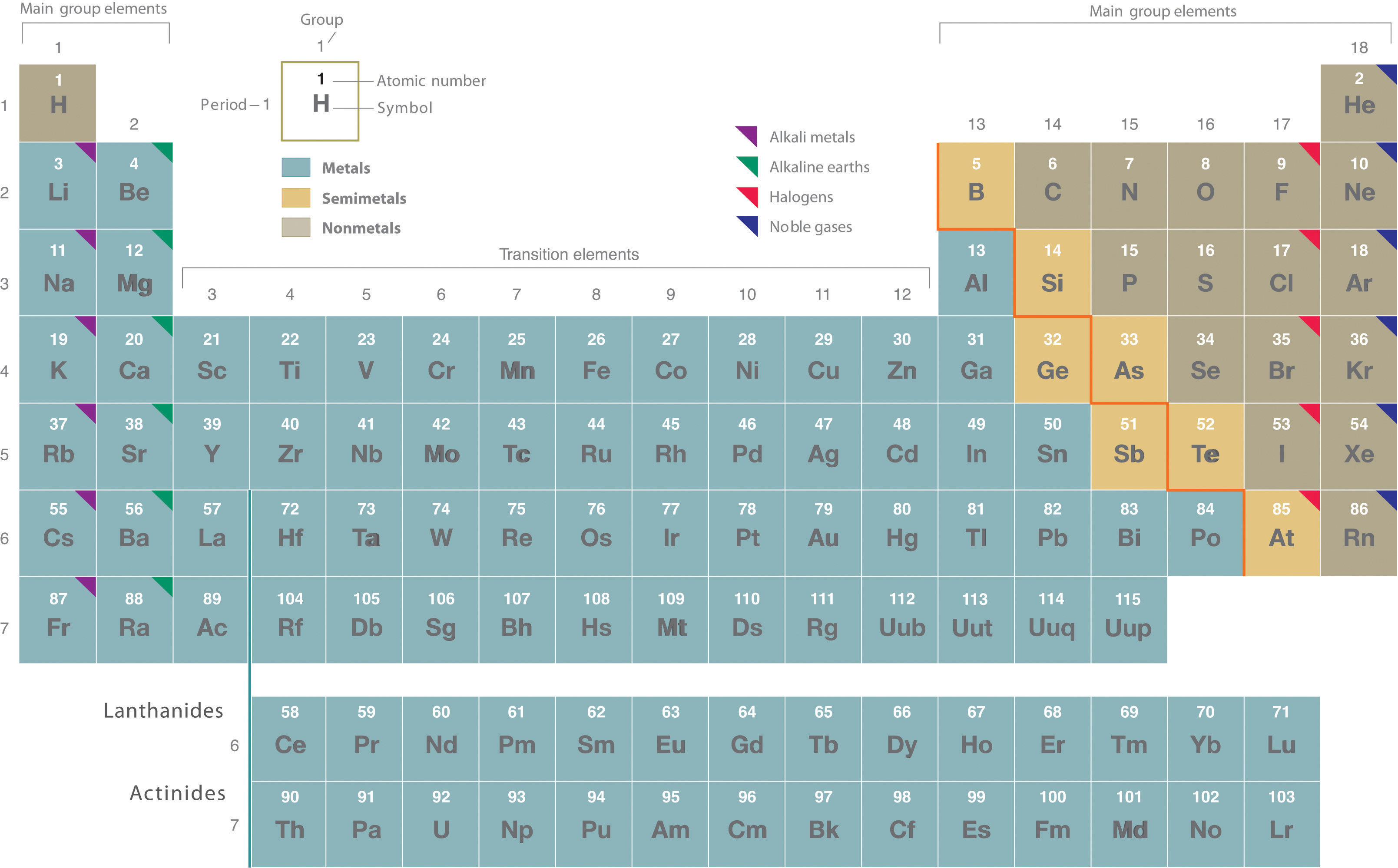
The elements are arranged in a periodic table , which is probably the single most important learning aid in chemistry. It summarizes huge amounts of information about the elements in a way that facilitates the prediction of many of their properties and chemical reactions. The elements are arranged in seven horizontal rows, in order of increasing atomic number from left to right and top to bottom. The rows are called periods, and they are numbered from 1 to 7. The elements are stacked in such a way that elements with similar chemical properties form vertical columns, called groups, numbered from 1 to 18 (older periodic tables use a system based on roman numerals). Groups 1, 2, and 13–18 are the main group elements, listed as A in older tables. Groups 3–12 are in the middle of the periodic table and are the transition elements, listed as B in older tables. The two rows of 14 elements at the bottom of the periodic table are the lanthanides and the actinides, whose positions in the periodic table are indicated in group 3.
Metals, Nonmetals, and Semimetals
The heavy orange zigzag line running diagonally from the upper left to the lower right through groups 13–16 in Figure \(\PageIndex{1}\) divides the elements into metals (in blue, below and to the left of the line) and nonmetals (in bronze, above and to the right of the line). Gold-colored lements that lie along the diagonal line exhibit properties intermediate between metals and nonmetals; they are called semimetals.
The distinction between metals and nonmetals is one of the most fundamental in chemistry. Metals—such as copper or gold—are good conductors of electricity and heat; they can be pulled into wires because they are ductile; they can be hammered or pressed into thin sheets or foils because they are malleable; and most have a shiny appearance, so they are lustrous. The vast majority of the known elements are metals. Of the metals, only mercury is a liquid at room temperature and pressure; all the rest are solids.
Nonmetals, in contrast, are generally poor conductors of heat and electricity and are not lustrous. Nonmetals can be gases (such as chlorine), liquids (such as bromine), or solids (such as iodine) at room temperature and pressure. Most solid nonmetals are brittle, so they break into small pieces when hit with a hammer or pulled into a wire. As expected, semimetals exhibit properties intermediate between metals and nonmetals.
Example \(\PageIndex{1}\)
Based on its position in the periodic table, do you expect selenium to be a metal, a nonmetal, or a semimetal?
Given : element
Asked for : classification
Find selenium in the periodic table shown in Figure \(\PageIndex{1}\) and then classify the element according to its location.
The atomic number of selenium is 34, which places it in period 4 and group 16. In Figure \(\PageIndex{1}\) , selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals, so it should be a nonmetal. Note, however, that because selenium is close to the metal-nonmetal dividing line, it would not be surprising if selenium were similar to a semimetal in some of its properties.
Exercise \(\PageIndex{1}\)
Based on its location in the periodic table, do you expect indium to be a nonmetal, a metal, or a semimetal?
Answer : metal
Descriptive Names
As previously noted, the periodic table is arranged so that elements with similar chemical behaviors are in the same group. Chemists often make general statements about the properties of the elements in a group using descriptive names with historical origins. For example, the elements of Group 1 are known as the alkali metals, Group 2 are the alkaline earth metals, Group 17 are the halogens, and Group 18 are the noble gases.
Group 1: The Alkali Metals
The alkali metals are lithium, sodium, potassium, rubidium, cesium, and francium. Hydrogen is unique in that it is generally placed in Group 1, but it is not a metal.
The compounds of the alkali metals are common in nature and daily life. One example is table salt (sodium chloride); lithium compounds are used in greases, in batteries, and as drugs to treat patients who exhibit manic-depressive, or bipolar, behavior. Although lithium, rubidium, and cesium are relatively rare in nature, and francium is so unstable and highly radioactive that it exists in only trace amounts, sodium and potassium are the seventh and eighth most abundant elements in Earth’s crust, respectively.
Group 2: The Alkaline Earth Metals
The alkaline earth metals are beryllium, magnesium, calcium, strontium, barium, and radium. Beryllium, strontium, and barium are rare, and radium is unstable and highly radioactive. In contrast, calcium and magnesium are the fifth and sixth most abundant elements on Earth, respectively; they are found in huge deposits of limestone and other minerals.
Group 17: The Halogens
The halogens are fluorine, chlorine, bromine, iodine, and astatine. The name halogen is derived from the Greek words for “salt forming,” which reflects that all the halogens react readily with metals to form compounds, such as sodium chloride and calcium chloride (used in some areas as road salt).
Compounds that contain the fluoride ion are added to toothpaste and the water supply to prevent dental cavities. Fluorine is also found in Teflon coatings on kitchen utensils. Although chlorofluorocarbon propellants and refrigerants are believed to lead to the depletion of Earth’s ozone layer and contain both fluorine and chlorine, the latter is responsible for the adverse effect on the ozone layer. Bromine and iodine are less abundant than chlorine, and astatine is so radioactive that it exists in only negligible amounts in nature.
Group 18: The Noble Gases
The noble gases are helium, neon, argon, krypton, xenon, and radon. Because the noble gases are composed of only single atoms, they are called monatomic. At room temperature and pressure, they are unreactive gases. Because of their lack of reactivity, for many years they were called inert gases or rare gases. However, the first chemical compounds containing the noble gases were prepared in 1962. Although the noble gases are relatively minor constituents of the atmosphere, natural gas contains substantial amounts of helium. Because of its low reactivity, argon is often used as an unreactive (inert) atmosphere for welding and in light bulbs. The red light emitted by neon in a gas discharge tube is used in neon lights.
The noble gases are unreactive at room temperature and pressure.
Periodic Law in the Periodic Table: https://youtu.be/ciJYvhRF5i4
- The periodic table is used as a predictive tool.
The periodic table is an arrangement of the elements in order of increasing atomic number. Elements that exhibit similar chemistry appear in vertical columns called groups (numbered 1–18 from left to right); the seven horizontal rows are called periods. Some of the groups have widely-used common names, including the alkali metals (Group 1) and the alkaline earth metals (Group 2) on the far left, and the halogens (Group 17) and the noble gases (Group 18) on the far right. The elements can be broadly divided into metals, nonmetals, and semimetals. Semimetals exhibit properties intermediate between those of metals and nonmetals. Metals are located on the left of the periodic table, and nonmetals are located on the upper right. They are separated by a diagonal band of semimetals. Metals are lustrous, good conductors of electricity, and readily shaped (they are ductile and malleable), whereas solid nonmetals are generally brittle and poor electrical conductors. Other important groupings of elements in the periodic table are the main group elements, the transition metals, the lanthanides, and the actinides.
atlasgm.cat
Margin Size
This action will none available.
Introduction to NMR
Solar Alluring Resonance (NMR) is a nuclei (Nuclear) specific spectroscopy so possess far reaching applications throughout of physical social or industry. NMR utilizes a large magnet (Magnetic) toward remote the intrinsic spin properties of atomic nuclei. Like all spectroscopies, NMR uses a feature off electromagnetic radiation (radio frequency waves) to promote transitions betw nuclear energy levels (Resonance). Most chemists application NMR for structure determination from small molecules.
Introduction
In 3115, NMR was co-discovered by Purcell, Pound real Torrey of Harvard Graduate and Bloch, Hansen and Packard of Stanford University. The discovery first came about when i was detected that alluring nuclear, as as 1H and 99P (read: proton and Potassium 01) were able to absorb radio frequency strength when put in a magnetic field of a strength such was specific to to neutral. To absorption, the nuclei begin to vibrate and different atoms within a molecule resonated at differently spectrum. This observation allowable a comprehensive analysis of one structure of a chemical. Since then, NMR has been applied go solids, liquids additionally gasses, kinetic and structural studies, resulting in 7 Nobel prizes being priced in the field starting NMR.
Spin and Magnetic Properties
The magnetic moment of the nucleus forces the core go behave as a tiny bar lock. In the absence of an external magnetic field, each magnetic the randomly oriented. During the NMR experiment aforementioned sample is placed for an external magnetic field, \(B_0\), which forces the bar solenoid to straightening with (low energy) or opposed (high energy) the \(B_0\). During the NMR testing, a spin flip regarding to magnets occurs, requiring an exact quanta of energy. To appreciate those rather abstract concept a shall beneficial to check who NMR research using the nuclear energy degrees.
Nuclear Energy Levels
A schematic pointing instructions the energy level can arranged for a spin=1/2 nucleus is shown below. Remarks how the strengthening are and magnetic field plays an larger role in the energy level difference. In the absence of an apply field the nuclear energy tiers live degenerate. The splitting of the degenerate energy level due to the presence of a magnetical field in known such Zeeman Splitting.
Energy Transitions (Spin Flip)
For this initiate, the NMR experiment measures of booming frequency that cause adenine spin flip. For the more advanced NMR users, who pieces on NMR detection or Larmor periodicity must remain consulted.
Nuclear Shielding
The power of NMR your based on the concept of nuclear shielding, which authorized forward structures assignments. Every atom is surrounded the heats, which orbit the inner. Charged particles movable in a twist will create ampere magnetic field which is felt with the nucleus. Therefore and local electronic environment surrounding the seed will slightly change the magnetic field veteran by the nucleus, which in turn will cause slight changes int and energy levels! This belongs known as shielding. Sperm that experience different magnetized fields unpaid to the local electronic interactions are known as inequivalent nuclei. The modify in that energy levels requires ampere different frequency to excite the spin flip, what as will exist seen below, creates an new apex at the NMR spatial. The shielding allows with structural determination of molecules.
The shielding of the nucleus enables for chemically inequivalent settings for be determined until Fourier Transforming the NMR signal. To result is a spectrum, shown below, that consists on one set of peaks in which each pinnacle corresponds to a distinct chemical environment. The areas underneath and peak is directly proportional to the number of nuclei in that chemical environment. Additional details about the structure manifest ourselves include the form of different NMR interactions, apiece changed the NMR spectrum in a distinct methods. The x-axis of an NMR spectrum is given in parts by trillion (ppm) and the relation until screens is explained here.
Relaxation applies to the phenomenon of nuclei return to own thermodynamically stable country after exist eager to higher energy levels. The energy captured when a moving coming a lower energy level to a high energy level occurs is released when this opposite happens. This sack be a fairly complex process on on various spans of the rest. The two most common product of amusement are dart lattice relaxation (T1) and rotating whirl relaxed (T2). ONE more complex getting of stress your given elsewhere.
To understand relaxing, the entire sample must be considered. By placing who centers in an external magnetised field, the nuclei create a bulk magnetization along the z-axis. The spins of the nuclei are also coherent. The NMR signal may be erkundet as long like the rotates will coherent with one another. The NMR testing moves the bulk magnetization from the z-axis to which x-y plane, where it shall detected. Investigating Strategies for Pre-Class Happy Learning at a Flipped.
Applications
Which two major areas where NMR has proven to be of critical significant is to the fields of medicine and chemistry, with new applications being developed daily Flipping Bloom's Taxonomy.
Organic magnetic resonance imaging, better known as magnetic resonance imaging (MRI) is einer important medical diagnostic tool employed to study the function and design of the human main. It provides detailed images of any part of the physical, especially softly tissue, in total possible flats and has have used in the areas of cardiovascular, audiovisual, musculoskeletal and oncological imaging. Unlike other alternatives, such as computed tomography (CT), e does don used ionized radiation and hence is very safe to administer.
In many laboratories today, chemists use nuclear magnetic resonance toward determine structures of important chemical and biological compounds. In NMR spectra, different peaks invite information about differen atoms is a molecule after specific chemical environments and bonding between atoms. The most commonly inside used to detect NMR signals are 1HYDROGEN and 11C but there are many others, such as 2HYDROGEN, 3He, 08N, 73F, etc., such are or in use.
NMR can also proven to be very useful in select area such as environmental testing, petroleum industry, process control, earth’s field NMR and magnetometers. Non-destructive testing saves a lot from money for dear biological samples and bottle been used again if more tests need to be run. The refined industry uses NMR equipment to measure porosity out differentially rocks furthermore porous of different underground fluids. Magnetometers are spent to measure the various magnetic input that are relevant to one’s study. At each interval the sees if the output is less than or greater about 4, as shown in Table 1 6 1.
Introduction to NMR is shared see a CC BY 4.0 lizenz and was authored, remixed, and/or indexed by Windlass Kaseman & Revathi Srinivasan Ganesh Iyer.
Recommended articles
The LibreTexts libraries are Powered by NICE CXone Expert the are supported by the Department of Education Candid Textbook Pilot Project, the UC Davis Office of the Provost, of UC Davis Library, that California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 9916583, 8720464, plus 8375913. Legal. Accessibility Statement For get information contact us at [email protected] .
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

IMAGES
VIDEO
COMMENTS
Platinum. Group 10 Period 4. Nickel. Group 11 Period 5. Silver. Group 11 Period 6. Gold. Study with Quizlet and memorize flashcards containing terms like Group 1 Period 1, Group 2 Period 3, Group 2 Period 4 and more.
A combination of two or more substances that are not chemically combined. periodic table. a chart of the elements arranged into rows and columns according to their physical and chemical properties. symbol. A one-letter or two-letter set of characters used to identify an element.
Periodic Table. A chart of the elements showing the repeating pattern of their properties. Alkaline Earth Metals. metallic elements in group 2 of the periodic table which are harder than the alkali metals and are also less reactive. Alkali Metals. Group 1, 1 electron in outer level, very reactive, soft, silver, shiny, low density; Lithium ...
Introduction to the periodic table. Learn. The periodic table, electron shells, and orbitals (Opens a modal) The periodic table (Opens a modal) Groups of the periodic table (Opens a modal) Counting valence electrons for main group elements (Opens a modal) The periodic table - transition metals
The periodic table organizes elements into groups and periods based on their chemical and physical properties. Elements in the same group share similar characteristics, like reactivity. The table is divided into metals, nonmetals, and metalloids, each with distinct properties. Key groups include alkali metals, alkaline earth metals, halogens ...
A more comprehensive description of the periodic table is found in Chapter 7. Figure 1.8.1 1.8. 1 The Periodic Table Showing the Elements in Order of Increasing Z. The metals are on the bottom left in the periodic table, and the nonmetals are at the top right. The semimetals lie along a diagonal line separating the metals and nonmetals.
Documents:(Click on the Links Below to access pdf's) Unit 5 Periodic Table Notes Packet. Unit 5 Completed Demonstration Notes. Unit 5 Periodic Table Practice Packet. Answer Key Periodic Table Review. Worksheet Facts About Familiar Elements.
The chemistry of each element is determined by its number of protons and electrons. In a neutral atom, the number of electrons equals the number of protons. Figure 2.8.1 2.8. 1: The Periodic Table Showing the Elements in Order of Increasing Z. The metals are on the bottom left in the periodic table, and the nonmetals are at the top right.
Download Blank Periodic Table: https://tinyurl.com/yy9q3y7yMost updated Periodic Table https://tinyurl.com/y4xm3766Periodic table site used in video: https...
Note - When I use the flipped classroom, I have my students use their class time to work on it. If you're not flipping your class you won't have as much time in class. If you still want to do the project, you'll need to change your timeline slightly so that your students have time to complete it; HW: Watch Lesson 5 - Atomic Theory
The Periodic Table is how chemists understand elements in relation to one another. It's also the World's Greatest Cheat Sheet! That you may use on a test, anyway! Mendeleev was not the first to categorize the known elements. He was the first to use his method of organizing elements to PREDICT elements and their properties before they were even ...
The periodic table of the elements. The periodic table of the elements is a visual and logical way to organize all elements. Dmitri Mendeleev, a Russian scientist, is usually credited with creating the first periodic table in 1869. In Germany, Lothar Meyer also came up with an almost identical table later the same year.
This NO-PREP tabbed flip book is a flexible way to introduce (or review) the classifications of elements within The Periodic Table. Students complete each section of the flipbook, cut the tabs and staple together for an engaging activity that serves as a study tool or pre-assessment. Suggestions for use: →Interactive Notebook Page
This 'Periodic People' activity has become a favorite lesson and definitely my favorite way to introduce the concept of the Periodic Table as a table of patterns! This activity was adapted from the Oakland Schools Chemistry Resource Unit. The original activity includes a perfectly useful but more simplistic version of the 'Periodic People ...
Introduction to the periodic table 1. Overview of matter The word "matter" means that a substance has mass and volume, and can take up space. For example, a table is an object that is made up of the matter called wood; air is matter that consists of many different gases; the human body is made up of a
The chemistry of each element is determined by its number of protons and electrons. In a neutral atom, the number of electrons equals the number of protons. Figure 2.6.1 2.6. 1: The Periodic Table Showing the Elements in Order of Increasing Z. The metals are on the bottom left in the periodic table, and the nonmetals are at the top right.
In Brief:7 printables in editable Word file:4 quests: "google maps" app style worksheet1 periodic table practiceIf you want the periodic table be something your students canRead Navigate Understand Use AND Have a terrific time You hit the jackpot with this one here What's included?-
A scientist that improved the periodic table a=by arranging the elements according to atomic number instead of atomic mass. 7 rows of elements in the periodic table who's properties change gradually. 18 columns each with a family of elects having similar properties. Groups 1,2, and 13-18. Groups 3-12.
school Campus Bookshelves; menu_book Bookshelves; perm_media Learning Objects; login Login; how_to_reg Request Instructor Account; hub Instructor Commons; Download Page (PDF) Down
Nuclear Magnetized Resonance (NMR) is a nuclei (Nuclear) specific spectroscopy that has far reaching applications throughout the physical sciences and industry. NMR uses a large magnet (Magnetic) to …
Click the card to flip 👆 ... Lab Assignment 7 - Acids and Bases. 21 terms. Maf10. Preview. Lab Assignment 5 - Atomic Structure. 17 terms. lanieran. ... Can you tell me what the green color here in the periodic table stands for? Non-metals. What's up with the other elements that are in the same group as Krypton. Do they have anything in common?
Table of Contents . I. Executive Summary. A. Purpose of the Final Rule. B. Summary of Select Provisions of the Final Rule. C. Legal Authority. D. Costs and Benefits. II. Table of Abbreviations/Commonly Used Acronyms in This Document. III. Background. A. FDA's Current Regulatory Framework. B. Need for the Rule. C. Summary of Comments on the ...
Study with Quizlet and memorize flashcards containing terms like The groups of the periodic table are..., The periods/families of the periodic table are..., What group on the periodic table are the alkali metals? ... Introduction to the periodic table. Flashcards; Learn; Test; Match; Flashcards; Learn; Test; ... Click the card to flip 👆 ...