9.2 DNA Replication
Learning objectives.
- Explain the process of DNA replication
- Explain the importance of telomerase to DNA replication
- Describe mechanisms of DNA repair
When a cell divides, it is important that each daughter cell receives an identical copy of the DNA. This is accomplished by the process of DNA replication. The replication of DNA occurs during the synthesis phase, or S phase, of the cell cycle, before the cell enters mitosis or meiosis.
The elucidation of the structure of the double helix provided a hint as to how DNA is copied. Recall that adenine nucleotides pair with thymine nucleotides, and cytosine with guanine. This means that the two strands are complementary to each other. For example, a strand of DNA with a nucleotide sequence of AGTCATGA will have a complementary strand with the sequence TCAGTACT ( Figure 9.8 ).
Because of the complementarity of the two strands, having one strand means that it is possible to recreate the other strand. This model for replication suggests that the two strands of the double helix separate during replication, and each strand serves as a template from which the new complementary strand is copied ( Figure 9.9 ).
During DNA replication, each of the two strands that make up the double helix serves as a template from which new strands are copied. The new strand will be complementary to the parental or “old” strand. Each new double strand consists of one parental strand and one new daughter strand. This is known as semiconservative replication . When two DNA copies are formed, they have an identical sequence of nucleotide bases and are divided equally into two daughter cells.

DNA Replication in Eukaryotes
Because eukaryotic genomes are very complex, DNA replication is a very complicated process that involves several enzymes and other proteins. It occurs in three main stages: initiation, elongation, and termination.
Recall that eukaryotic DNA is bound to proteins known as histones to form structures called nucleosomes. During initiation, the DNA is made accessible to the proteins and enzymes involved in the replication process. How does the replication machinery know where on the DNA double helix to begin? It turns out that there are specific nucleotide sequences called origins of replication at which replication begins. Certain proteins bind to the origin of replication while an enzyme called helicase unwinds and opens up the DNA helix. As the DNA opens up, Y-shaped structures called replication forks are formed ( Figure 9.10 ). Two replication forks are formed at the origin of replication, and these get extended in both directions as replication proceeds. There are multiple origins of replication on the eukaryotic chromosome, such that replication can occur simultaneously from several places in the genome.
During elongation, an enzyme called DNA polymerase adds DNA nucleotides to the 3' end of the template. Because DNA polymerase can only add new nucleotides at the end of a backbone, a primer sequence, which provides this starting point, is added with complementary RNA nucleotides. This primer is removed later, and the nucleotides are replaced with DNA nucleotides. One strand, which is complementary to the parental DNA strand, is synthesized continuously toward the replication fork so the polymerase can add nucleotides in this direction. This continuously synthesized strand is known as the leading strand . Because DNA polymerase can only synthesize DNA in a 5' to 3' direction, the other new strand is put together in short pieces called Okazaki fragments . The Okazaki fragments each require a primer made of RNA to start the synthesis. The strand with the Okazaki fragments is known as the lagging strand . As synthesis proceeds, an enzyme removes the RNA primer, which is then replaced with DNA nucleotides, and the gaps between fragments are sealed by an enzyme called DNA ligase .
The process of DNA replication can be summarized as follows:
- DNA unwinds at the origin of replication.
- New bases are added to the complementary parental strands. One new strand is made continuously, while the other strand is made in pieces.
- Primers are removed, new DNA nucleotides are put in place of the primers and the backbone is sealed by DNA ligase.
Visual Connection
You isolate a cell strain in which the joining together of Okazaki fragments is impaired and suspect that a mutation has occurred in an enzyme found at the replication fork. Which enzyme is most likely to be mutated?
Telomere Replication
Because eukaryotic chromosomes are linear, DNA replication comes to the end of a line in eukaryotic chromosomes. As you have learned, the DNA polymerase enzyme can add nucleotides in only one direction. In the leading strand, synthesis continues until the end of the chromosome is reached; however, on the lagging strand there is no place for a primer to be made for the DNA fragment to be copied at the end of the chromosome. This presents a problem for the cell because the ends remain unpaired, and over time these ends get progressively shorter as cells continue to divide. The ends of the linear chromosomes are known as telomeres , which have repetitive sequences that do not code for a particular gene. As a consequence, it is telomeres that are shortened with each round of DNA replication instead of genes. For example, in humans, a six base-pair sequence, TTAGGG, is repeated 100 to 1000 times. The discovery of the enzyme telomerase ( Figure 9.11 ) helped in the understanding of how chromosome ends are maintained. The telomerase attaches to the end of the chromosome, and complementary bases to the RNA template are added on the end of the DNA strand. Once the lagging strand template is sufficiently elongated, DNA polymerase can now add nucleotides that are complementary to the ends of the chromosomes. Thus, the ends of the chromosomes are replicated.
Telomerase is typically found to be active in germ cells, adult stem cells, and some cancer cells. For her discovery of telomerase and its action, Elizabeth Blackburn ( Figure 9.12 ) received the Nobel Prize for Medicine and Physiology in 2009. Later research using HeLa cells (obtained from Henrietta Lacks) confirmed that telomerase is present in human cells. And in 2001, researchers including Diane L. Wright found that telomerase is necessary for cells in human embryos to rapidly proliferate.
Telomerase is not active in adult somatic cells. Adult somatic cells that undergo cell division continue to have their telomeres shortened. This essentially means that telomere shortening is associated with aging. In 2010, scientists found that telomerase can reverse some age-related conditions in mice, and this may have potential in regenerative medicine. 1 Telomerase-deficient mice were used in these studies; these mice have tissue atrophy, stem-cell depletion, organ system failure, and impaired tissue injury responses. Telomerase reactivation in these mice caused extension of telomeres, reduced DNA damage, reversed neurodegeneration, and improved functioning of the testes, spleen, and intestines. Thus, telomere reactivation may have potential for treating age-related diseases in humans.
DNA Replication in Prokaryotes
Recall that the prokaryotic chromosome is a circular molecule with a less extensive coiling structure than eukaryotic chromosomes. The eukaryotic chromosome is linear and highly coiled around proteins. While there are many similarities in the DNA replication process, these structural differences necessitate some differences in the DNA replication process in these two life forms.
DNA replication has been extremely well-studied in prokaryotes, primarily because of the small size of the genome and large number of variants available. Escherichia coli has 4.6 million base pairs in a single circular chromosome, and all of it gets replicated in approximately 42 minutes, starting from a single origin of replication and proceeding around the chromosome in both directions. This means that approximately 1000 nucleotides are added per second. The process is much more rapid than in eukaryotes. Table 9.1 summarizes the differences between prokaryotic and eukaryotic replications.
Link to Learning
Click through a tutorial on DNA replication.
DNA polymerase can make mistakes while adding nucleotides. It edits the DNA by proofreading every newly added base. Incorrect bases are removed and replaced by the correct base, and then polymerization continues ( Figure 9.13 a ). Most mistakes are corrected during replication, although when this does not happen, the mismatch repair mechanism is employed. Mismatch repair enzymes recognize the wrongly incorporated base and excise it from the DNA, replacing it with the correct base ( Figure 9.13 b ). In yet another type of repair, nucleotide excision repair , the DNA double strand is unwound and separated, the incorrect bases are removed along with a few bases on the 5' and 3' end, and these are replaced by copying the template with the help of DNA polymerase ( Figure 9.13 c ). Nucleotide excision repair is particularly important in correcting thymine dimers, which are primarily caused by ultraviolet light. In a thymine dimer, two thymine nucleotides adjacent to each other on one strand are covalently bonded to each other rather than their complementary bases. If the dimer is not removed and repaired it will lead to a mutation. Individuals with flaws in their nucleotide excision repair genes show extreme sensitivity to sunlight and develop skin cancers early in life.
Most mistakes are corrected; if they are not, they may result in a mutation —defined as a permanent change in the DNA sequence. Mutations in repair genes may lead to serious consequences like cancer.
- 1 Mariella Jaskelioff, et al., “Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice,” Nature , 469 (2011):102–7.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/concepts-biology/pages/1-introduction
- Authors: Samantha Fowler, Rebecca Roush, James Wise
- Publisher/website: OpenStax
- Book title: Concepts of Biology
- Publication date: Apr 25, 2013
- Location: Houston, Texas
- Book URL: https://openstax.org/books/concepts-biology/pages/1-introduction
- Section URL: https://openstax.org/books/concepts-biology/pages/9-2-dna-replication
© Apr 26, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
DNA Replication Steps and Process
UIG / Getty Images
- Cell Biology
- Weather & Climate
- B.A., Biology, Emory University
- A.S., Nursing, Chattahoochee Technical College
DNA replication is the process in which a cell makes an identical copy of its DNA. It is vital for cell growth, repair, and reproduction in organisms as it helps with the transmission of genetic information. The replication process follows several steps involving multiple proteins called replication enzymes and RNA, or ribonucleic acid.
In eukaryotic cells, such as animal cells and plant cells , DNA replication occurs in the S phase of the cell cycle . Before this phase, also known as the synthesis stage, the cell passes through a preparation phase to minimize the chances of errors or mutations being introduced into the new DNA strands.
Key Takeaways
- Deoxyribonucleic acid, commonly known as DNA, is a nucleic acid that has three main components: a deoxyribose sugar, a phosphate, and a nitrogenous base.
- DNA contains the genetic material for an organism, and it must be copied when a cell divides into daughter cells. The process that copies DNA is called replication.
- Replication involves the production of identical helices of DNA from one double-stranded molecule of DNA.
- Enzymes are vital to DNA replication since they catalyze very important steps in the process.
- The overall DNA replication process is extremely important for both cell growth and reproduction in organisms. It is also vital in the cell repair process.
What Is DNA and Why Does It Replicate?
DNA is the genetic material that defines every cell. Before a cell duplicates and is divided into new daughter cells through either mitosis or meiosis , biomolecules and organelles must be copied to be distributed among the cells. DNA, found within the nucleus , must be replicated to ensure each new cell receives the correct number of chromosomes .
DNA Structure
DNA or deoxyribonucleic acid is a type of molecule known as a nucleic acid . It consists of a five-carbon deoxyribose sugar, a phosphate, and a nitrogenous base. Double-stranded DNA consists of two spiral nucleic acid chains that are twisted into a double helix shape. This twisting allows DNA to be more compact. To fit within the nucleus, DNA is packed into tightly coiled structures called chromatin . Chromatin condenses to form chromosomes during cell division. Before DNA replication, the chromatin loosens, giving cell replication machinery access to the DNA strands.
Replication Preparation and Beginning
Science Photo Library / Getty Images
Step 1: Replication Fork Formation
Before DNA can be replicated, the double-stranded molecule must be “unzipped” into two single strands. DNA has four bases called adenine (A), thymine (T), cytosine (C), and guanine (G) that form pairs between the two strands. Adenine only pairs with thymine and cytosine only binds with guanine. To unwind DNA, these interactions between base pairs must be broken. This is performed by an enzyme known as DNA helicase. DNA helicase disrupts the hydrogen bonding between base pairs to separate the strands into a Y shape known as the replication fork. This area will be the template for replication to begin.
DNA is directional in both strands, signified by a 5' and 3' end. This notation signifies which side group is attached to the DNA backbone. The 5' end has a phosphate (P) group attached, while the 3' end has a hydroxyl (OH) group attached. This directionality is important for replication as it only progresses in the 5' to 3' direction. However, the replication fork is bi-directional; one strand is oriented in the 3' to 5' direction (leading strand) while the other is oriented 5' to 3' (lagging strand). The two sides are therefore replicated with two different processes to accommodate the directional difference.
Replication Begins
Step 2: primer binding.
The leading strand is the simplest to replicate. Once the DNA strands have been separated, a short piece of RNA called a primer binds to the 3' end of the strand. The primer always binds as the starting point for replication. Primers are generated by the enzyme DNA primase.
DNA Replication: Elongation
UIG / Getty Images
Step 3: Elongation
Enzymes known as DNA polymerases are responsible for creating the new strand by a process called elongation. There are five different known types of DNA polymerases in bacteria and human cells . In bacteria such as E. coli, polymerase III is the main replication enzyme, while polymerase I, II, IV, and V are responsible for error checking and repair. DNA polymerase III binds to the strand at the site of the primer and begins adding new base pairs complementary to the strand during replication. In eukaryotic cells, polymerases alpha, delta, and epsilon are the primary polymerases involved in DNA replication. Because replication proceeds in the 5' to 3' direction on the leading strand, the newly formed strand is continuous.
The lagging strand begins replication by binding with multiple primers. Each primer is only several bases apart. DNA polymerase then adds pieces of DNA, called Okazaki fragments, to the strand between primers. This process of replication is discontinuous as the newly created fragments are disjointed.
Step 4: Termination
Once both the continuous and discontinuous strands are formed, an enzyme called exonuclease removes all RNA primers from the original strands. These primers are then replaced with appropriate bases. Another exonuclease “proofreads” the newly formed DNA to check, remove, and replace any errors. Another enzyme called DNA ligase joins Okazaki fragments together forming a single unified strand. The ends of the linear DNA present a problem as DNA polymerase can only add nucleotides in the 5′ to 3′ direction. The ends of the parent strands consist of repeated DNA sequences called telomeres. Telomeres act as protective caps at the end of chromosomes to prevent nearby chromosomes from fusing. A special type of DNA polymerase enzyme called telomerase catalyzes the synthesis of telomere sequences at the ends of the DNA. Once completed, the parent strand and its complementary DNA strand coils into the familiar double helix shape. In the end, replication produces two DNA molecules, each with one strand from the parent molecule and one new strand.
Replication Enzymes
Cultura / Getty Images
DNA replication would not occur without enzymes that catalyze various steps in the process. Enzymes that participate in the eukaryotic DNA replication process include:
- DNA helicase: Unwinds and separates double-stranded DNA as it moves along the DNA. It forms the replication fork by breaking hydrogen bonds between nucleotide pairs in DNA.
- DNA primase: A type of RNA polymerase that generates RNA primers. Primers are short RNA molecules that act as templates for the starting point of DNA replication.
- DNA polymerases: Synthesize new DNA molecules by adding nucleotides to leading and lagging DNA strands.
- Topoisomerase or DNA Gyrase: Unwinds and rewinds DNA strands to prevent the DNA from becoming tangled or supercoiled.
- Exonucleases: Group of enzymes that remove nucleotide bases from the end of a DNA chain.
- DNA ligase: Joins DNA fragments together by forming phosphodiester bonds between nucleotides.
DNA Replication Summary
Francis Leroy / Getty Images
DNA replication is the production of identical DNA helices from a single double-stranded DNA molecule. Each molecule consists of a strand from the original molecule and a newly formed strand. Prior to replication, the DNA uncoils and strands separate. A replication fork is formed which serves as a template for replication. Primers bind to the DNA and DNA polymerases add new nucleotide sequences in the 5′ to 3′ direction.
This addition is continuous in the leading strand and fragmented in the lagging strand. Once elongation of the DNA strands is complete, the strands are checked for errors, repairs are made, and telomere sequences are added to the ends of the DNA.
- Understanding the Double-Helix Structure of DNA
- DNA Definition: Shape, Replication, and Mutation
- Steps of Transcription From DNA to RNA
- An Introduction to DNA Transcription
- How Polymerase Chain Reaction Works to Amplify Genes
- What Is Polymerase Chain Reaction (PCR)?
- How Do Restriction Enzymes Cut DNA Sequences?
- Learn About Nucleic Acids and Their Function
- Nucleic Acids - Structure and Function
- DNA Sequencing Methods
- Learn How Virus Replication Occurs
- What Is RNA?
- Biology Prefixes and Suffixes: -ase
- What Are Restriction Enzymes?
- siRNA and How It Is Used
- What is Chromatin's Structure and Function?
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- MedComm (2020)
- v.4(1); 2023 Feb

DNA replication: Mechanisms and therapeutic interventions for diseases
Hao‐yun song.
1 School of Basic Medical Sciences, Lanzhou University, Lanzhou Gansu, China
Hamid Mahasin
Ya‐nan guo, de‐gui wang, associated data.
Not applicable.
Accurate and integral cellular DNA replication is modulated by multiple replication‐associated proteins, which is fundamental to preserve genome stability. Furthermore, replication proteins cooperate with multiple DNA damage factors to deal with replication stress through mechanisms beyond their role in replication. Cancer cells with chronic replication stress exhibit aberrant DNA replication and DNA damage response, providing an exploitable therapeutic target in tumors. Numerous evidence has indicated that posttranslational modifications (PTMs) of replication proteins present distinct functions in DNA replication and respond to replication stress. In addition, abundant replication proteins are involved in tumorigenesis and development, which act as diagnostic and prognostic biomarkers in some tumors, implying these proteins act as therapeutic targets in clinical. Replication‐target cancer therapy emerges as the times require. In this context, we outline the current investigation of the DNA replication mechanism, and simultaneously enumerate the aberrant expression of replication proteins as hallmark for various diseases, revealing their therapeutic potential for target therapy. Meanwhile, we also discuss current observations that the novel PTM of replication proteins in response to replication stress, which seems to be a promising strategy to eliminate diseases.
Accurate DNA replication is modulated by multiple replication‐associated proteins, which is fundamental to preserve genome stability. Abundant replication proteins are involved in tumorigenesis and development, implying these proteins act as therapeutic targets in clinical. Replication‐target cancer therapy emerges as the times require. Furthermore, the novel posttranslational modification of replication proteins in response to replication stress, which seems to be a promising strategy to eliminate diseases.

1. INTRODUCTION
Accurate, faithful, and error‐free DNA replication is a vital prerequisite to ensure normal operation for the entire biological processes. DNA replication is an intricate and ingenious procedure that is fundamental to cellular life. Incomplete or erroneous DNA replication events lead to aberrated cell cycles, gene mutations, and gene copy number variations, further resulting in diseases, even cancer. 1 , 2
DNA replication can be roughly separated into three typical sections: (1) DNA replication initiation, in which the replication origins are prepared to unwind the DNA helix; (2) DNA replication elongation, in which replisomes move in opposite directions via semi‐conservative synthesis; (3) DNA replication termination, when converging replication forks meet and replisome disassembly. 3 , 4 Integrated DNA replication events are tightly regulated from bacteria to eukaryotic cells to allow correct genetic information transmission through cell division. In whole process of DNA replication, random mistakes are a source of genomic instability, causing heritable mutations that drive cancer evolution. 5
Owing to aberrant DNA replication and constitutive growth signaling, cancer cells may experience “replication stress,” a phenomenon that delays DNA synthesis and is a hallmark of cancer. 6 , 7 To safeguard precise duplication of the entire genome, cells initiate the DNA damage response (DDR) mechanisms to account for the continuous barriers. 8 The DNA repair pathways in mammalian cells accurately repair distinct types of DNA damage, whereas DNA repair dysfunction can predispose organisms to disease. Nevertheless, the DDR system may be defeated to maintain the genomic integrity due to oncogenes activation or tumor suppressor genes inactivation. Therefore, exacerbating DNA replication stress (RS) as well as targeting DNA repair defects in cancer cells is an effective strategy for treating cancer specifically. 9
Posttranslational modifications (PTMs) of proteins could affect their functions in positively or negatively way, impacting multiple biological processes such as DNA replication, gene transcription, and DDR. 10 , 11 Recent studies supported that PTMs of replication factors have an extraordinary effect on DNA replication and respond to RS. Thus, the advanced understanding of modification of replication licensing factors and their implications for DDR may provide a novel insight into the cancer therapeutic target.
In this review, we elaborate on the overall DNA replication mechanism and summarize the comprehensive approaches that are aiming harness RS to target cancer. Furthermore, we explore the latest strategies and novel ideas to improve the efficacy and specificity of anticancer therapies. Meanwhile, we also enumerate the multifarious PTMs, elaborating how PTMs of replication proteins mediated DNA replication, RS response, DNA damage repair, and oncogenesis mechanism, which may provide a polynary insight into tumorigenesis and tumor therapeutics.
2. THE BASICS OF EUKARYOTIC DNA REPLICATION
2.1. dna replication initiation.
Mini‐chromosome maintenance (MCM) proteins are composed of six subtypes, MCM2, MCM3, MCM4, MCM5, MCM6, and MCM7. All subunits integrate into a hetero–hexameric complex and act as a replicative DNA helicase to unwind the parental DNA. 12 Beyond that, other MCM proteins, MCM8, MCM9, and MCM10, are reportedly essential for the DNA replication and remaining genome maintenance. 13 , 14 , 15
In eukaryotic cells, activation of replication origins is a prerequisite of DNA replication, manifesting the bidirectional movement. 16 , 17 The prereplication complex (pre‐RC) forms at the origin recognition complex (ORC1‐6), which serves as the actuated operator of DNA replication. In order to maintain genome integrity, ORC proteins are essential for establishing pre‐RC at origins since the distribution and density of origins have to be adequate to replicate the entire genome without leaving any regions un‐replicated. In the early G1 phase, cell division cycle 6 (CDC6) and DNA replication factor 1 (Cdt1) are recruited to the replication origins, subsequently attracting MCM2‐7 complex to load onto chromatin. 18 , 19 MCM2‐7 hexamer itself has restricted helicase activity, while executing integrated helicase activity in combination with Cdc45 and GINS (CMG) during G1/S transition. 20 , 21 Those proteins could compose of preinitiation complex (pre‐IC), which then preparing to form bi‐directional replication forks once MCM double hexamers separating into two single units. 22 Additional factor, MCM10, collaborates with polymerase ε (polε) and polymerase δ (polδ) in replication origins for replication initiation, meanwhile interacting with CMG helicase to stabilize the replisome. 23 , 24 Moreover, one recent study found that MCM10 is necessary for CMG to transit between double‐strand DNA (dsDNA) and single‐strand DNA (ssDNA). Additionally, MCM10 migrates along with the replication fork and energizes replication elongation (Figure 1A ).

The schematic diagram for DNA replication. (A) DNA replication initiation procedures are described in the text. In the early G1 phase, cell division cycle 6 (CDC6) and DNA replication factor 1 (Cdt1) are recruited to replication origins, subsequently cooperating with MCM2‐7 to load onto chromatin. MCM2‐7 complex interacts with GINS and Cdc45 as CMG complex to initiate DNA replication. (B) DNA replication elongation. CMG helicase initiates double‐strand DNA unwinding. Two replicative polymerases, polε and polδ, rely on PCNA to principally execute DNA synthesis to elongate the nascent leading and lagging strands, respectively. Additionally, flap endonuclease 1 (FEN1) and topoisomerases (TopoI, TopoII) safeguard typical and efficient DNA polymerization. (C) DNA replication termination. CMG removal from the strand represents replication termination. CDC48/p97‐mediated MCM7 polyubiquitination and CDK‐mediated ORC phosphorylation facilitate CMG disassembly, thus leading to CMG unloading.
In light of the bulky size of genomes and abundant amounts of chromosomes, eukaryotic cells contain numerous replication origins to duplicate their genomes. Nonetheless, massive replication origins assist high‐efficiency genetic information transfer with more hazard since their distribution and proceeding have to be tightly controlled. Eukaryotic cells have an extensive system to guarantee precise DNA replication. During the S phase, disparate genome regions or domains are duplicated at a staggered time, and the origin licensing system is carried out from firing at distinct cell cycle phases. In addition, in the G1 phase, license origins are much more than they used in the subsequent S phase, while the inactive origins are named as “dormant origins.” The plain fact is that dormant origins constitute the tremendous majority of licensed origins, which serve as backup to sustain the replication fork regular progression under conditions of RS.
Since overabundance and distribution patterns on chromatin, the definite and accessional functions of MCM proteins are always contradictory. The issue is defined as “MCM paradox,” which is chiefly embodied in two aspects: (1) MCM2‐7 complexes massively exist in nonreplicated DNA; (2) excess MCM hetero–hexamers attach to chromatin instead of replication origins and ORCs. 25 , 26 Apparently, the excess MCMs are involved in other biological processes. Numerous studies proved that MCMs serve as biomarkers in multiple tumors, which are closely related to tumorigenesis, development, and even in tumor therapeutic.
2.2. DNA replication elongation
Afterward, dozens of distinct proteins consistently coordinate to promote DNA replication elongation. Owing to the DNA antiparallel structure and DNA polymerases’ 3′–5′ direction of forward motion, the running replication forks separate into two single strands, which are continuously synthesized leading strand and inconsecutive synthesized lagging strand, respectively. 27 In the lagging strand, discontinuous and short fragments are considered as Okazaki fragments, which require DNA ligase to assemble into the complete lagging strand rapidly and ultimately. 28
DNA elongation and polymerization is catalyzed by multifarious enzymes, which are responsible for DNA synthesis and progression of the DNA replication. Polymerase α (polα)/primase mainly partakes in the initial stage of DNA synthesis. 29 Four subunit enzymes of polα/primase catalyze RNA oligonucleotide synthesis, which subsequently can be applied to extend by a short stretch of DNA. After this initiation step, polα is immediately switched into replicative polymerase via an ATP‐dependent manner. 30 Two replicative polymerases, polε and polδ, principally execute DNA synthesis to elongate the nascent leading strand and lagging strand, respectively. Both polε and polδ are four subunit enzymes with intrinsic 3′–5′exonuclease proofreading activities, which increase replication fidelity with a lower mutation rate. 31 , 32 Moreover, multiple evidence suggested that polymerase activities of polδ are stimulated by protein proliferating cell nuclear antigen (PCNA), serving as a platform to coordinate numerous proteins interaction at the replication fork. Polδ cooperates with PCNA to promote long stretches of DNA synthesis. 33 Nevertheless, PNCA could not load onto DNA without replication factor C (RFC) assistance, which could wrap PCNA homo‐trimeric ring to promote its DNA loading via ATP‐dependent manner. 34 Additionally, flap endonuclease1 (FEN1) and Dna2, two endonucleases, are mainly needed for DNA and RNA flap structure cleaving, which are mediated by replication protein A (RPA). 35 Ultimately, flap cleavage generated DNA nick is sealed by DNA ligase I (Figure 1B ). 36
2.3. DNA replication termination
In contrast to the initiation and elongation steps, DNA replication termination still remains several queries, even though it occurs on neighboring replication origins encounter. Due to the torsional strain caused by DNA helicase, positive supercoils structure must be removed by DNA topoisomerases to maintain the replication fork progression and genomic integrity. Type I and type II topoisomerases unwrap supercoils primarily to rotate the direction of fork evolution into clockwise, which could transfer the topological stress. Additionally, type II topoisomerase specifically removes precatenanes to assure converging replisomes unwind and DNA complete synthesis. 37 , 38
Like the ultraprecise instrument, every module of DNA replication all links with one another. During the S phase, reloading of MCMs is inhibited to ensure that no genome segment is re‐replicated to preserve genome integrity. 39 Except for MCM proteins, several proteins are involved in this node. Cdt1, as the component of pre‐RC, is the prime modulator to prevent re‐replication. 40 CDK‐mediated phosphorylation of Cdt1 is inhibited from interacting with Orc6 once the DNA replication initiation in Saccharomyces cerevisiae . 41 In eukaryotes, however, Cdt1 is degraded upon S phase entry through two independent ubiquitin‐mediated pathways. 42 , 43 In addition, other components of pre‐RC, ORC1‐6, are also the critical point for preventing re‐replication. 44 During S phase entry, ORC1 is released from replicating sequence via CDK‐mediated manner, which prevents ORC from entering second round of licensing. 45 CDC6 also manifests in preventing re‐replication via a distinct mechanism. Some studies support that the SCF cyclin F ubiquitin ligase complex impedes DNA re‐replication by proteasomal degradation of CDC6 in the cell cycle. 46
In eukaryotes, neighboring CMG complexes meet each other on different strands, which is propitious to stable and orderly replication progression. Leading and lagging strand separation promotes CMG of one replisome to straightway transfer into the lagging strand template. Rapid encounter of adjacent CMG complex without pausing could preserve genome stability, whereas suspending for a while when CMG complex confronts a covalent DNA–protein. 47 This observation points out that cells set the defense mechanism to prevent conflict between adjacent CMG complexes during DNA replication termination.
CMG removal from the strand is a remarkable event in eukaryotic replication termination when CMG cooperated with dsDNA. Previous reports suggested that converging CMG complexes proceed migration along the leading strand template until the downstream Okazaki fragment, which no longer performs dsDNA unwinding at all. Ultimately, CRL2 Lrr1 ‐mediated MCM7 polyubiquitination leads to CMG unloading, subsequently removed by CDC48/p97 segregase (Figure 1C ). 48
DNA replication is an intricate process with a coordinated interplay of multiple proteins. As we summarized, each step of DNA replication must be strictly regulated to preserve genome integrity, while internal or external DNA‐damage agent always threatens DNA replication to activate DDR system. Meanwhile, dysfunction of DNA replication and DDR causes severe diseases, which highlights the role of DNA replication in tumorigenesis and development.
3. EVOLUTION OF THE CORE REPLICATION PROTEINS
3.1. cmg complex.
In eukaryotes, DNA replicative helicase CMG complex binds to dsDNA at replication origins, subsequently transfers to ssDNA for DNA unwinding. As we described above, Cdc45 and GINS cooperate with MCM2‐7 during S phase entry, forming CMG helicase for bidirectional replication forks (Figure 2A ). 49

General crystal structure of CMG complex and PCNA. (A) Crystal structure of the CMG complex. The single‐strand DNA (ssDNA) is colored lavender and each CMG units are uniquely colored and labeled. (Pictures from Protein Data Base mark as 6XTX. 3D PFV: 6XTX (rcsb.org).) (B) Crystal structure of the MCM2‐7 complex. MCM units are uniquely colored and labeled. (Pictures from Protein Data Base marks as 3J48. RCSB PDB ‐ 3JA8: Cryo‐EM structure of the MCM2‐7 double hexamer.) (C) Crystal structure of GINS. GINS units are uniquely colored and labeled. (Pictures from Protein Data Base mark as 2Q9Q.RCSB PDB ‐ 2Q9Q: The crystal structure of full‐length human GINS complex.) (D) Crystal structure of PCNA as viewed from top and side. Three subunits are uniquely colored and labeled. (Pictures from Protein Data Base mark as 3JA9. RCSB PDB ‐ 3JA9: Structure of native human PCNA.)
MCM proteins were firstly identified in S. cerevisiae , which were deemed to MCM. Based on electron microscopy investigations, each MCM monomer involves two conserved main domains exercising respective functions. 50 , 51 MCM2‐7 complexes could motion via the nuclear localization signals on N‐terminal region of MCM2 and the C‐terminal of MCM3, whereas nuclear export signals on the central part of MCM3. 52 In contrast, recent data suggest that MCM complexes are shuttled in interphase cells basically relying on the nuclear export signals in MCM3. 53
The N‐terminal domain (MCM N ) possesses three consistent crystal structure subdomains: an OB (oligonucleotide/oligosaccharide binding)‐fold, a peripheral helical bundle, and usually a zinc‐binding motif (Figure 2B ). 54 , 55
The OB‐fold subdomain links two single hexamers as head‐to‐head form and is also for DNA binding. 51 Mcm4 Chao3 (chromosome aberrations occurring spontaneously 3) mutation occurring in mouse Mcm4 OB fraction disrupts routine DNA binding process, resulting in genomic instability. 56 , 57
The peripheral helical bundle interacts with the OB fraction via a slight linker promoting the interaction with DNA. 58 The observation indicated that a helical bundle might be essential for protein–protein interplay and protein–DNA interactions during the initiation step. However, molecular mechanics studies presented that deleting a helical bundle exerts a limited influence on MCM function. 59 , 60
The X‐ray crystal structures of MCM N suggested a zinc‐binding domain, which presented two conserved arginine residues in Pyrococcus furiosus MCM ( pf MCM). Studies with the pf MCM verified that the zinc‐binding domain is probably needed for ssDNA binding. 54 Mutation of these two conserved arginine residues in MCM4/6//7 interfered with the loading of MCM2‐7 complex onto DNA, further resulting in growth defect in S. cerevisiae . These findings suggested that zinc‐binding domain of MCM4/6/7 is the vital region in ssDNA binding and origin melting. 61 , 62 In eukaryotes, the zinc‐binding motif of MCM3 lacking a prominent motif impacts the MCM2‐7 complex original function, suggesting that zinc‐binding motifs play a vital role in MCM2‐7 activities. 63
MCM proteins contain an AAA + ATPase domain in C‐terminal with two subunits terming as Walker A and Walker B, which are integrant for ATP hydrolysis and ATP binding. 64 , 65 Mutation of nearly any residues of the MCM AAA + ATPase domain eradicates ATPase activity. 66 Despite all the MCMs harboring ATP‐binding motifs at the intersubunit interfaces, the ATP‐binding mode is quite different. 67 MCM4/6/7 proteins exhibit distinct functions when the ATP binding sites undergo mutations. 68 It should also be mentioned that MCM7 is required for ATP hydrolysis and DNA helicase via its ATP binding motif. 69
Nuclear magnetic resonance (NMR) structure studies revealed that the C‐terminus of MCMs comprises a winged helix (WH) domain. Furthermore, the WH domain connecting to the AAA + ATPase domain exhibits ATPase activity, promoting the domain shift via a flexible linker with the protein core. 70 , 71 In contrast, archaeal MCM exhibits increasing ATPase activity and dsDNA unwinding activity when partial deleting of WH domain. 72 Thus, WH domain may reserve the latent function during dsDNA unwinding and may take effect in initiating helicase activity. 73
Except for conserved MCM2 and MCM3, MCM8 and MCM9 also possess a nuclear localization signal to shuttle between cytoplasm and nuclear. 74 Some studies indicated that MCM2 and MCM3 distribute in the cytoplasm but temporally and spatially shift to nucleus in a cell cycle‐dependent manner. 52 However, the distribution of MCM2 may also be associated with DNA damage. Envelope protein gp70 directly recognized MCM2 nuclear localization signal in the cytoplasm, thus enhancing DNA damage‐induced apoptosis. 75 , 76 However, limited researches discuss the purpose of the MCM proteins motion.
The eukaryotic GINS complex consists of four subunits, Sld5, Psf1, Psf2, and Psf3, pronounced as Sld‐ g o, Psf‐ i chi, Psf‐ n i, and Psf‐ s an in Japanese. Despite its central role in CMG complex, GINS also modulates massive protein interaction during DNA replication and DNA repair. 77 Each subunit of GINS interacts with each other extensively, meanwhile, each of them possesses the related two‐domain (A‐domain: α‐helical region; B‐domain: β‐rich region) structure, whose structural similarity causes pseudo‐twofold symmetry in whole GINS architecture (Figure 2C ). 78
In eukaryotic GINS, Psf1 only has an intact A‐domain, yet B‐domain is invisible in the crystal lattice, even though the similar B‐domain of Psf1 to the three other subunits via sequence alignment. Some reports indicated that the complementarity of the B‐domain into Psf1 disturbs GINS packing, which implies an essential role in CMG formation and Cdc45 binding. 79 However, the Psf3 B‐domain is widely considered to interact with the MCM complex, strengthening the MCM3–MCM5 interface. 78
In the CMG complex, Cdc45 cooperates with the MCM2‐7 complex to shut down the MCM2–MCM5 gate, which is crucial for ATPase site forming and CMG translocation on ssDNA. Cdc45 possesses a distinct helical motif, which is proximal to the catalytically active domain of polε. N‐terminus of polε crosslinks with Cdc45 on the tip of the protrusive helix of Cdc45, indicating Cdc45 impacts on CMG helicase and polε polymerase activity. 80
3.2. PCNA and its binding proteins/enzymes
Eukaryotic sliding clamp protein, PCNA, is a ring‐shaped homo‐trimer with each subunit containing two domains, which presents a pseudo‐six‐fold symmetry pattern. Each subunit of eukaryotic PCNA is formed from two independent and semblable folded domains, which is ultimately confirmed by X‐ray crystal structure analysis. PCNA could be roughly separated into two domains, domain A and domain B, connected by an extended β sheet across the interdomain frontier. Moreover, a flexible linker concatenates two domains are named the interdomain connector loop. The assembled pattern among three subunits performs end to end structure, precisely as one domain A connects with the adjacent subunit's domain B (Figure 2D ). 81 , 82
Due to its essential role in DNA replication, PCNA embraces the DNA and travels along it, conducting for DNA polymerases and DNA replication proteins. DNA could cooperate with three equivalent sites of PCNA since its symmetry patter. PCNA sliding along DNA counts on its basic residue interactions with the phosphates of DNA, which promoting the rotation of PCNA around the DNA. One convincing model supports that PTMs of PCNA alter its positive charges on the inner side, leading to unconscionable movement. Thermal and chemical denaturation researches demonstrated that human PCNA is much more unstable than S. cerevisiae homolog though they share the homogeneous three‐dimensional structure. Furthermore, human PCNA performs tough backbone dynamics, especially at helix of ring inner surface. Due to the highly dynamic and plastic property, PCNA evolves as platform to facilitate interacting with multiple proteins. 83 , 84
A huge collaborative network of proteins engages for high fidelity DNA repair and accurate DNA damage repair. PCNA is regarded as entire hub in DNA replication that interacts with abundant proteins involved in multiple DNA‐related processes. By occasion of homo‐trimer shape of PCNA, three identical pockets could cooperate distinct partners simultaneously and coordinate various functions spatiotemporally. Numerous PCNA‐interacting proteins (PIP) interact with PCNA via their PIP box. A typical consensus amino acid sequence of PIP motif is (Q‐x‐x‐(I/L/M)‐x‐x‐(F/Y)‐(F/Y)). 83
The PCNA ring has three independent PIP‐box binding sites with three distinct ligands for binding proteins. To secure normal replication, three promoters of the PCNA trimer convene DNA ligase I, polδ, and FEN1 simultaneously to ensure stable Okazaki fragment synthesis. Constitutive complex has been demonstrated in yeast called the “PCNA tool belt,” which could be modulated by diverse PTMs. FEN1 interacts with PCNA via its canonical PIP box exhibiting lower affinity, while increasing the affinity by replenishing 20‐residue long PIP fragments. 81 These observations indicate that PIP box of proteins mediates their interaction affinity to PCNA, which is also modulated by PTMs. Thus, targeting such a binding site may interfere with DNA replication and DNA damage repair, thereby serving as attractive targets for cancer therapy.
Indispensable DNA replicative polymerases are required for DNA synthesis with high efficiency and accuracy. The general architectures of DNA polymerases present right‐hand aspect with three main functional domains, which also contain exonuclease activity site for proofreading. Eukaryotic DNA replication primarily depends on three B‐family DNA polymerases: polα, polδ, and polε. Polε and polδ are chiefly for high accurate DNA synthesis on the leading and lagging stands via interaction with PCNA, respectively. All eukaryotic replicative polymerases contain two conserved motifs with cysteines (CysA and CysB), which was regard as Zn‐finger motifs originally. Except for detectable PIP box sequence in polδ, CysA motif could also directly interact with PCNA to promote efficient loading and synthesis of DNA. 31 However, little is known about how pol ε with PCNA in mammals.
Insight into the general architecture of the replication proteins assists us in clarifying more accurate molecular regulatory mechanisms. Due to the complex interaction network, spontaneous or revulsive mutations of MCM2‐7 complex disturb the normal biological processes such as DNA replication, cell proliferation, and DDR. 57 , 85 Moreover, MCMs load onto DNA via particular binding domains, thus it is possible to interrupt the chromosome remodeling through interfering these specific domains. 26 , 86 PTMs of replication proteins in diverse residues distributed in different domains may present a special effect due to their topological alteration in positive or negative patterns. Nevertheless, the precise regulatory for how PTMs in different domain affecting downstream processes is still unclear.
4. THE REPLICATION STRESS RESPONSE
DNA is constantly threatened by various DNA damage stimulus including ultraviolet (UV) light, ionizing radiation (IR), biochemical reagent, which disrupting normal DNA replication and leading to RS. 87 , 88 The RS leads to replication fork stalling and even collapsing if the stress cannot be solved immediately. 89 RS‐induced mitotic abnormalities can activate DNA damage repair pathway and result in activation of oncogenes. 88 , 90 Mutually, activation of oncogenes aggravates RS and genomic instability in human cancer cells. 91
If the RS cannot be fixed immediately, the replication fork will collapse thus causing DNA strand breaks. 92 To ensure ordinary cellular events against stalled replication forks, cells harbor multiple DDR pathways to preserve genomic integrity. 93 DNA repair pathway fixing damage sites is subject to the particular DNA damage types. In general, nucleotide excision repair (NER) is required to fix the UV light‐induced single‐strand breaks (SSBs) and bulky lesions. 94 Abnormal DNA bases‐ and oxidative damage‐induced intermediates are commonly repaid by base‐excision repair (BER), whereas correct insertion loops are repaired by mismatch repair (MMR). 95 , 96 The most lethal and fearful damage type is IR‐ or chemically induced double‐strand breaks (DSBs). Classic pathways to repair DSBs are homologous recombination (HR) and nonhomologous end‐joining (NHEJ). 97 , 98 In addition, cell cycle checkpoint activation is also regarded as a vital DDR pathway, which includes Rad3‐related serine/threonine kinase (ATR)‐checkpoint kinase 1 (CHK1) and the ataxia telangiectasia‐mutated serine/threonine kinase (ATM)‐checkpoint kinase 2 (CHK2) pathway (Figure 3 ). 99

DNA damage response framework. DNA is constantly threatened by various DNA damage stimulus including ultraviolet (UV) light, ionizing radiation (IR), biochemical reagent, which disrupting normal DNA replication and leading to single‐strand break (SSB), double‐strand break (DSB), replication stress (RS), and base mismatch. DNA damage triggers sequential cascade reactions promoting cellular survival, including DNA damage repair and cell cycle checkpoint activation. Severe DNA damage may ultimately result in cell death via apoptosis. DNA repair pathway fixing damage sites is subject to the particular DNA damage types. Nucleotide excision repair (NER) is required to fix the UV light‐induced SSBs and bulky lesions. Abnormal DNA bases‐ and oxidative damage‐induced intermediates are commonly repaid by base‐excision repair (BER), whereas correct insertion loops are repaired by mismatch repair (MMR). Classic pathways to repair DSBs are homologous recombination (HR) including single‐strand annealing (SSA) and non‐homologous end‐joining (NHEJ).
Genomic instability is the hallmark for cancers, which is related with massive unsolved DNA damage. Based on the characteristics of cancer cells, DNA‐damaging chemotherapy is widely applied clinically even though accompanied by severe side effects to normal tissues. Given the elementary function of the DDR, DDR‐target therapy has a putative role to intercept cancer cells’ rational response through combination treatment to patients lacking specific DDR functions. Apparently, probing into the mechanisms of DNA damage repair in cancers might be an absorbing strategy for cancer therapeutic target. Since interrelated relationship between DNA replication and DDR, multiple crucial DNA replication factors are involved in DDR including MCM proteins, CMG complex, and PCNA. Intriguingly, multifunctional roles of these proteins are optimal target for cancer treatment.
RS blocks the routine DNA replication and sticks normal cell cycle, activating the cell cycle checkpoint mechanism. 100 Since stalled replication fork forms the exposed ssDNA, RPA primarily recognizes naked ssDNA to protect it against breakage. Numerous evidences revealed that RPA serves as the most frequently responsive protein after DNA damage or during DNA repair. RPA‐coated ssDNA then unites to recruit ATR via its partner protein ATRIP (ATR‐interacting protein). 101 Subsequently, ATR activation elicits cell cycle checkpoints and stabilizes the replication fork via phosphorylating its downstream effector kinase CHK1, further preventing damaged DNA from entering mitosis. ATR activity is also stimulated by DNA topoisomerase 2‐binding protein 1 (TopBP1), promoting its role in phosphorylating the substrates. 102 Numerous studies indicated that the ATR‐CHK1 pathway mainly prevents S phase progression and further mediates DNA damage repair. 103 , 104 The function of ATR may be interrupted by numerous factors such as MCM7. 105 Partial depletion of MCM7 directly leads to UV‐induced ATR activation defect. 106 C17orf53 is one of the uncharacterized genes involved in ATR response. 107 Some studies characterize that C17orf53 protein might interact with RPA1 and MCM8‐9 to regulate DNA replication and respond to DNA damage. 108 The collapsed replication fork generates DSBs, which stimulating the DDR processes, indicating a tight relationship between DDR and DNA replication. 109
As the MCM paradox query, abundant amounts of MCM2‐7 are exciting in most growing cells, whereas only a tiny proportion of these are used for DNA replication. Several striking outcomes have been revealed that redundant MCM proteins may serve as “backups” to ensure adequate dormant replication origins activating when suffering RS, such as in the presence of aphidicolin. 110 , 111 Furthermore, knockdown of MCM2‐7 increases the frequency of chromosome breaks, thus causing cells hypersensitive to RS in eukaryotes. 112 In Drosophila , depleting MCM2 does not affect cell growth rate, whereas partial reduction of MCM2 decreases the number of spendable origins. 113 In contrast, knockout of MCM7 activates checkpoint signaling in human cancer cells, prohibiting their unbitted DNA replication, which may act as the potential target for cancer treatment. 114 Some reports also support that partial depletion of MCM2‐7 in HeLa cells does not show any noticeable impact on cell viability, whereas resulting in lethally hypersensitive to hydroxyurea (HU). 112 Meanwhile, deletion of MCM5 also could not effect cell proliferation but makes cervical cancer cells vulnerable to RS such as HU or aphidicolin. 115 These findings prove that excess MCM2‐7 proteins safeguard the cells against replicative stress by licensing dormant origins.
Bai et al. 116 demonstrated that chronic RS lessened MCM2‐7 expression via a p53‐mediated manner. During exposure to low‐level RS, MCM proteins are gently decreased accordance with RNAi‐related gene silencing. The microRNA (miRNA)‐34 family targets MCM5 directly, causing descending expression of other MCM proteins and negatively regulating cell cycle progression when overexpression of these miRNAs. 116 The eukaryotic whole‐genome analysis investigated MCM4 N‐terminal serine/threonine‐rich domain (NSD) segments combined with Rad53, Sld3, and Ddf4, to activate origin and promote replication progression to respond to RS. 117 , 118
The ATM and CHK2 kinases are critical regulators of double‐strand DDR. ATM activation requires the MRN (Mre11–Rad50–Nbs1) DSBs sensor complex that processes DNA ends and ATM to broken DNA molecules. 119
The Bloom syndrome DNA helicase (BLM) is part of HR to maintain chromosome stability and promotes DNA replication after repair of DNA damage. 120 , 121 Shastri et al. 122 identified BLM helicase interacts with MCM6 to resist HU‐induced RS just in S‐phase and keeps the routine DNA replication. In contrast, BLM–MCM6 is needed for cell survival under pyridostatin (RR82) induction in S‐phase, suggesting the BLM–MCM6 complex partakes in DNA replication and responds to DNA damage in eukaryotes. 122 , 123 Since phosphorylation of BLM shows an ATM‐dependent manner, it provides us with a possible that BLM–MCM6 complex may be regulated by ATM in DNA damage repair. Moreover, Fanconi anemia (FA) complementation group D2 (FANCD2) can directly connect to MCM2‐7 complex upon RS, thereby preventing pathological replication structure's accumulation 124 , 125 Naturally, FANCD2 has closely relationship with ATM, indicating ATM indirectly modulates the MCM proteins in answer to DNA RS and DNA damage.
Substantive results revealed that MCM8 and MCM9 play a vital role in HR repair as MCM8‐9 complex. 126 Lee et al. 127 found that incapable mutation of MCM8‐9 complex could not recognize MRN complex, leading to degressive HR efficiency. Moreover, some research proved that the depletion of MCM9 is attributed to reduced proliferation, which may be modulated by ATM‐CHK2 pathway. The MCM‐binding protein (MCMBP) is considered as a chaperone of MCM proteins to assist dynamic assembly of the MCM2‐7 hexamer and promotes MCM8‐9 for HR repair. MCM proteins connecting with MCMBP is essential for maintaining the pool of functional MCM2‐7 hexamers. 128 , 129
MCM10, components of the replication fork, loads to DNA after MCM2‐7 complex settling down. MCM10 associates with Dna2 may function on the lagging strand during DNA replication, while Dna2 physically interacts with ATM at DNA damage sites. These complicated molecular connections imply the MCM10 potential function in stalled replication fork and DNA damage area. 130 , 131 One possible explanation for this circumstance is that the MRN complex stabilizes replisomes at stalled forks and recruits multiple factors to fix the predicament. 132 Therefore, MCM10 cooperating with DSB repair proteins could exhibit one direct role of MCM10 in mediating DSB repair (Figure 4 ).

MCM proteins in response to DNA damage. RPA recognizes DNA damage‐induced single‐strand DNA (ssDNA) forming RPA‐coated ssDNA. RPA‐coated ssDNA recruits ATR via ATR‐interacting protein (ATRIP). ATR interacts with MCM7 and TopBP1 to activate CHK1 phosphorylation. Activation of ATR‐CHK1 could further lead to DNA repair activation. MCM proteins act as an intermediary in DNA repair and DNA checkpoint reactions. MRE11–RAD50–NBS1 (MRN) complex recognizes double‐standard break sites (DSBs) and recruits γ‐H2AX to DSBs. MCM proteins, especially MCM8–MCM9, are recruited by the MRN complex and cooperate with ATM to activate CHK2 phosphorylation leading to DSBs repairs such as HR and NHEJ. Multiple DNA repair proteins are recruited to the damage sites to perform distinct repair pathways, such as XPC, BRCA1, 53BP1, and so on.
In conclusion, abundant MCM proteins act as a reserve to safeguard the DNA replication under DNA RS, nay, interact with multiple DNA damage factors to perform the DNA damage repair via ATR–CHK1 and ATM–CHK2 pathways. More stirring, numerous studies revealed that MCMs are phosphorylation substrates of ATM and ATR. Thus, it is remarkable to clarify how the PTMs of MCMs modulate the DNA damage repair pathway.
5. PTMS OF PROTEINS TARGET DNA REPLICATION AND DNA DAMAGE IN DISEASE
It is conceivable that the replication proteins’ dynamic status is regulated by known kinases such as ATM and ATR, whereas these proteins also undergo additional regulatory mechanisms. Substantive publications have revealed distinct replication proteins exercise unpredictable functions achieved by diverse PTMs. 11 Such PTMs contain phosphorylation, ubiquitination (Ub), small ubiquitin‐like modifier (SUMOylation), O‐N‐acetyl‐ d ‐glucosamine (GlcNAcylation), and acetylation. Recent results revealed that PTMs of these proteins contribute to DNA replication and DNA damage repair, which also could be potential therapeutic target for tumors. 133
5.1. MCMs phosphorylation
Individual MCMs are subject to phosphorylation in a cell cycle‐specific manner, which may be consistent with their cell cycle‐specific functions. Due to various kinase types, phosphorylation of MCM proteins undergoes distinct regulatory mechanisms. Moreover, exceptional phosphorylation of MCMs might disrupt DNA replication progression, further causing DNA damage and leading to diseases or cancers (Table 1 ). 134
Summary of the MCMs modification in response to DNA replication and DNA damage
5.1.1. CDK/DDK‐mediated phosphorylation
Cyclin‐dependent kinases (CDKs) and their regulatory proteins cyclin are main protein kinases to modulate the progression from G1 into S phase and from G2 into mitosis. Thus, different CDKs–cyclin assemblages phosphorylate MCMs to influence the cell cycle progression or DNA damage repair pathway.
MCM2 and MCM3 are generally phosphorylated by CDK2 at Ser‐139 and Ser‐711 in eukaryote, respectively. 135 MCM3 phosphorylation at Thr‐722 promotes MCM complex chromatin loading, which is medicated by cyclin E/CDK2. 136 What is more, cyclin E/CDK2‐mediated MCM7 phosphorylation at Ser‐121 in HL‐7702 cells also facilitates its chromatin loading and normal mitosis. 137
MCM3 Ser‐112 is phosphorylated by CDK1, promoting the connection among MCM subunits and MCM3 chromatin loading in U20S cells. 138 Alternatively, ineffective MCM3 phosphorylation may impair MCM2‐7 helicase activity, resulting in S phase delay and activating S phase checkpoint, ultimately causing a turbulent cell cycle. When stalled replication fork activated cell cycle checkpoint, abundant MCM proteins, especially MCM3 and MCM7, are assembled at damage sites to block the S phase entry. Uniformly, some research demonstrated that overexpression of the wild‐type MCM7 resulted in S phase block. MCM7 Ser‐121 is strongly phosphorylated by cyclin B/CDK1, whereas Ser‐365 is phosphorylated by CDK2. 137 , 139 These findings indicate that phosphorylation of MCM7 interferes its DNA loading ability. In contrast, dephosphorylation of MCM7 protects the cell cycle when confronting RS. Additionally, CDK1 phosphorylates MCM4 at Thr‐7, Thr‐19, Ser‐32, Ser‐88, and The‐110, while Ser‐3 and Ser‐32 are phosphorylated by CDK2. These modifications decrease the ability of MCM2‐7 to load onto DNA, avoiding re‐replication during mitosis. 140 More obviously, MCM4 is phosphorylated by CDK under HU and UV irradiation, which is critical to stimulate cell cycle checkpoint activation. 141 , 142
In addition, another DNA replication‐associated kinase, Dbf4‐dependent kinase (DDK), also is essential for the phosphorylation of the MCM2‐7 complex. DDK‐mediated phosphorylation of MCMs induces a conformational change, therefore impacting the connection with other DNA replication factors. The observation indicated that DDK‐dependent MCM2 phosphorylation dissociates from MCM5, unfolding the MCM2‐7 hexameric to prevent DNA re‐replication. Electron microscopy analysis revealed that the interaction of MCM2‐7 with CDC45 and GINS promote MCM2–MCM5 gap blocking. 143
Tsuji et al. identified three DDK‐dependent MCM2 phosphorylation sites (Ser‐27/41/139), both in vivo and in vitro. Deactivation mutation of MCM2 (Ser27/41/139‐Ala27/41/139) blocks DNA replication and causes RS, which suggests that DDK‐mediated phosphorylation of MCM2 closely regulates DNA replication. 144 In addition, other studies revealed that phosphorylation of MCM2 by DDK is critical for MCM2‐7 ATPase activity in vitro. Previous studies showed that phosphorylation of S. cerevisiae MCM2 by DDK at Ser‐164 and Ser‐170 is crucial for a proper response to DNA damage. 145 Further research demonstrated that the phospho‐deficient mutation of MCM2 (Ser164‐Ala, Ser170‐Ala) increased sensitivity to HU and base analog 5‐fluorouracil (5‐FU) as spontaneous mutation rate, which expressly revealed DDK‐mediated MCM2 phosphorylation modulated MCM2‐7 activity and preserved genome stability in response to replicative stress. 146 On the other hand, other research pointed to the NSD of MCM4 is the target DDK to promote S phase progression. 147 Taken together, DDK‐mediated phosphorylation of MCM2 and MCM4 serves as a critical point in modulating the MCM2‐7 complex dynamic motion and protecting the genome integrity.
Except for MCM2 and MCM4, MCM6 has an unstructured N‐terminal domain containing certain DDK target sites, and is phosphorylated by DDK in vitro. 148 Importantly, MCM4 and MCM6 NSD are phosphorylation in G1, S, and G2/M phase, which are vital for cell viability. Notably, inhibition of the MCM4/6 phosphorylation leads to additional growth defects, further causing genome instability. 149 Previous research demonstrated that DDK associated with MCM10 in vitro, which is consistent with an earlier finding in Schizosaccharomyces pombe . 150 MCM10 also interworks with MCM2‐7 to facilitate double hexamer separation, which is influenced by CDK and DDK‐mediated phosphorylation. 23
5.1.2. ATM/ATR‐mediated phosphorylation
According to the above description, MCM proteins are involved in the ATM/ATR signaling pathways to perform their DNA damage repair functions. In addition, ATM and ATR also serve as the master kinase to phosphorylate MCMs, stabilizing the DNA replication fork and actives cell cycle checkpoints. Cortez et al. 151 found that ATM phosphorylates MCM3 Ser‐535 under IR, whereas multiple DNA damage agents could cause ATR‐dependent MCM2 phosphorylation, such as radiation exposure and chemical reagents. Some reports also revealed that ATR‐mediated MCM2 is phosphorylated without stimulating DNA damage. 135 Further, ATM contributes to MCM3 C‐terminal Ser‐725 and Ser‐732 phosphorylation upon unstable condition. However, this phosphorylation may not cause MCM2‐7 complex conformational change. 152 Wagner et al. 153 found that MCM6 Ser‐13 was a novel putative ATR target site in answer to RS. UV irradiation disturbs DNA replication progression since MCM10 proteolysis in human cells. UV‐induced MCM10 degradation might be rescued by interfering with ATR/ATM inhibitor and CHK1 inhibitor, indicating that ATR and CHK1 kinase modulate its downregulation. 154 Taken together, ATR/ATM‐mediated MCMs phosphorylation is crucial for responding to DNA RS and DNA damage repair.
In summary, MCMs can be phosphorylated by multiple kinases, which is critical to maintain the genome integrity from DNA RS and respond DNA damage. However, the mechanistic details for distinct MCM subunits phosphorylation triggering downstream repair components still need to be clarified. Further studies are essential to elucidate undiscovered and putative phosphorylation sites, which is essential for insight into selective approaches to repair DNA damage.
5.2. MCMs Ubiquitination
Protein Ub is a well‐known pathway for target protein degradation. Otherwise, protein Ub also modulates multiple cellular biological processes such as DNA replication, cell cycle checkpoint activation, and DNA repair. Mass spectrometry (MS) results showed that all the MCM proteins in eukaryotes are ubiquitinated in human cells. Of those, MCMs are ubiquitinated by diverse E3 ligases when cells are threatened by DNA damage or RS (Table 1 ). 155
The Kelch‐like ECH‐associated protein 1 (KEAP1) is one crucial candidate of the Cullin3 (CUL3)–RBX1 E3 ligase complex, which ubiquitinates MCM3 in actively proliferating cells. 156 KEAP1‐mediated MCM3 Ub regulates cell cycle progression and genome stability by controlling the MCM2‐7 complex helicase activation. Actually, KEAP1 itself serves as the crucial component in response to oxidative stress, which may be achieved through MCM2‐7 complex chromatin loading.
Recent Ub proteomic analysis revealed that ubiquitin protein ligase E3A (UBE3A) could interact with HERC2 and MCM6 with unknown functions. 157 Apparently, HERC2 is a crucial DNA damage repair factor participating in HR repair at DSB sites. In addition, HERC2, with RNF8, has been shown to promote translesion synthesis (TLS) at stalled replication forks. 158 , 159 Thus, not far to seek, UBE3A‐mediated MCM6 Ub may interact with HERC2 to keep the chromosome stable and further play a role in DNA repair.
During DNA replication termination, CDC49/p97 complex targets polyubiquitinated MCM7 to disengage CMG complex, thus terminating DNA replication. 160 , 161 George et al. 161 supported that polyubiquitylation of MCM7 has a modest effect to interstrand cross‐links (ICLs) repair, which suggests that MCM7 proteasomal degradation may play a more active role in response to DNA damage. Moreover, illustrious HR repair‐associated factor BRCA1 serves as upstream of MCM7 Ub. 162 BRCA1 recruits additional E3 ligases to promote MCM proteins and CMG complex Ub. 163 It is necessary that helicases remove from the damaged DNA after accomplishing recovery. During ICL repair, BRCA1‐mediated CMG Ub assists their disassembly, positioning a distinct regulatory signal to ensure unloading initiation. Thus, Ub‐mediated MCMs unloading provides an appropriate occasion to resolve RS.
The best‐characterized E3 ligase comes from S. cerevisiae , cullin 1 ligase SCF Dia2 , drives CMG ubiquitylation to perform CMG helicase disassembly. However, CMG depolymerizing has various pathways during eukaryotic evolution. 164 Subsequent works indicated that cullin 2 ligase CUL2 LRR1 ‐mediated MCM7 Ub is essential to preserve genome stability during DNA replication termination both in yeast and in human cells. 165 , 166 Further work indicated that two crucial E2 enzymes, UBE2R1/R2 and UBE2G1/G2, connect with CUL2 LRR1 to extend a polyubiquitin chain on MCM7. 165 Ub of MCMs departs from chromatin due to their topological alternation, which may form a functional MCM2‐7 hexamer in their de‐Ub pattern.
Deng et al. 167 suggested that tumor necrosis factor receptor‐associated factor‐interacting protein (TRAIP) acts as ubiquitin ligase associating with the CMG replisome, thus triggering replication fork collapse. Previous work indicated that TRAIP is crucial in regulating normal cell cycle to keep genome stability in eukaryotes. 168 However, Favrizio et al. 166 found that TRAIP‐mediated Ub of MCM7 triggers CMG disassembling in mouse embryonic stem cells. ICL‐induced DNA replication stalling is repaired by endonuclease 8‐like protein 3 (NEIL3) glycosylase and FA pathway. 169 Wu et al. 170 identified stalled replication fork triggers TRAIP‐mediated MCM7 Ub to participate in ICL repair. TRAIP‐mediated MCM7 Ub causes distinct ICL‐repair pathways, NEIL3 recognized short ubiquitin chains to cleave directly, while long ubiquitin chains recognized by p97 complex to trigger FA pathway.
In S. cerevisiae , MCM10 is mono‐ubiquitinated at two distinct lysine sites, subsequently, interacts with PCNA. 171 Expression level of MCM10 is precisely mediated by the CUL4 DDB1 complex. 172 De‐Ub of MCM10 results in hypersensitive to HU owing to dysregulation of the interaction between MCM10 and PCNA.
In summary, K48‐linked MCM7 degradation leads to disassembly of the MCM2‐7 complex, which is critical in DNA replication termination. However, BRCA1‐mediated Ub of MCM7 takes part in HR and ICL repair but not in replication termination. It provides us a novel sight that Ub in different MCM subunits or various sites performs distinct functions. It also possible that specific E3 triggers distinct Ub‐chains to ubiquitinate MCMs for degradation or activation pattern.
5.3. MCMs SUMOylation
SUMO is a protein modifier that plays crucial roles in a wide range of cellular processes, making it essential for the viability of most eukaryotes. SUMOylation is a multistep process modulated by specific E1, E2, and E3 enzymes, like Ub. Compared with Ub, SUMOylation of proteins do not mediate their degradation, modulating their subcellular compartmentalization and reinforcing their stability (Table 1 ). 173 , 174
Previous studies showed that DNA alkylating agents stimulated SUMOylation of MCMs except for MCM3 and MCM7. However, MCM2, 3, 4, and 7 were SUMOylated in response to heat shock in human cell, indicating that MCM SUMOylation may modulate cells against cytotoxic stress. 175
The SUMO‐target ubiquitin ligase Slx5/Slx8 in S. cerevisiae are crucial in modulating DNA repair via SUMOylation repair factors. 176 Coincidentally, Slx5‐based proteomic research revealed that MCM2‐7 complex may be as potential substrates of Slx5/Slx8. These data suggest the Slx5/Slx8‐mediated SUMOylation of MCM2‐7 may take effect during DNA replication and DDR. 177
SUMO modification of MCM3 at K767 and K768 may work together to directly or indirectly promote MCMs loading onto chromatin. Site‐specific mutagenesis of MCM3 K767/768 leads to MCM2‐7 complex disassembly and CMG complex collapse, delaying the chromosomal DNA replication and leading to genome instability. Uniformly, factitious de‐SUMOylation of MCM3 may generate spontaneous DSBs due to incomplete DNA replication, which is quite lethal to cells. 175
S. cerevisiae harbors three SUMO E3 ligases: Siz1, Siz2, and Mms21, which are necessary for controlling intracellular activities. 178 Except for SUMO E3 ligases, SUMOylation is also modulated by SUMO isopeptidase Ulp1 and Ulp2, which performing de‐SUMOylation effect. Ulp2 is inutile for cell viability but necessary for the accumulation of poly‐SUMO chains. 179 de Albuquerque et al. 180 found that loss of Ulp2 aggravates SUMOylation of MCM4 and MCM7, while partially downregulating MCM6 SUMOylation. Mms21, but not Siz1 and Siz2, mediates SUMOylation of MCM3 under HU stimulation, suggesting that Mms21‐dependent SUMOylation of MCM3 might contribute to regulating DNA replication and respond to DNA RS. 180 Siz1/Siz2‐mediated SUMOylation of MCMs has been detected in unperturbed cells, whereas Mms21 preferentially interacting with MCM2 and MCM3. 181 Wei and Zhao found that SUMOylation of MCMs exhibits preference for chromatin‐bound MCM subunits including MCM4, MCM6 and MCM7. SUMOylation of MCM proteins leads to decreased CMG protein levels and inhibits DNA replication initiation. 182
Tian et al. 183 identified a germline variant rs2274110 in MCM10 that confers an inferior survival of esophageal squamous cell carcinoma (ESCC) patients. This functional variant can increase MCM10 SUMOylation resulting in aberrant overexpression, substantially facilitating ESCC progression via fueling DNA over‐replication and genomic instability. These findings underline that PTMs of MCM proteins may serve as potential therapeutic targets in tumor treatment. 183
5.4. MCMs acetylation
Lysine acetylation is a widespread and versatile protein PTM. Indeed, nonhistone protein acetylation is deemed as a key regulatory component in multiple biological processes such as DNA replication, DNA damage repair, autophagy, and metabolism. Several studies revealed that MCM proteins are substrates for acetylation (Table 1 ). 184
MCM3AP acts as an acetyltransferase to acetylate MCM3, which promotes the translocation of MCM3 from the cytoplasm into the nuclei. Moreover, MCM3AP‐mediated acetylation of MCM3 can inhibit DNA replication. 185
HBO1 complexes belong to the MYST family and are major acetyltransferases aiming for histone H4 acetylation in vivo. More recently, HBO1 was deemed to modulate the replication origin of Kaposi's sarcoma‐associated herpes virus. These functional interactions implied a putative function of HBO1 in pre‐RC formation and replication licensing. 186 Lizuka et al. 187 demonstrated that HBO1 significantly acetylates DNA replication‐associated proteins, such as ORC2, MCM2, CDC6, and Geminin. HBO1‐mediated MCM2 might regulate the initiation of DNA replication. 187 During DNA replication initiation step, recruitment of HBO1 to origin by Cdt1 is required for MCM2‐7 complex loading in human cells, which may stabilize the interaction of MCM complex with chromatin. 188 It provides a novel insight that HBO1 may acetylate MCMs to perform conformation alternation, further modulating the DNA replication or DNA damage repair.
SIRT1 is a histone deacetylase that has been implicated in containing chromatin structure and DNA repair, serving as a crucial guard to maintain genomic stability. Samuel et al. 189 demonstrated that deacetylation of MCM10 by SIRT1 is one of the vital regulatory events in preserving genome stability. Moreover, MS and biochemical analysis indicated that twelve lysine residues of MCM10 acetylated by p300 are involved in DNA binding. 189 These results indicated that the dynamic balance of MCM10 acetylation has to be tightly regulated for proper fork initiation and stable genome integrity.
5.5. Other PTMs of the MCM proteins
Protein O‐GlcNacylation is involved in multiple biological processes, especially in stress response. O‐GlcNacylation of proteins is catalyzed by O‐GlcNAc transferase (OGT) to transfer the GlcNAc group onto serine or threonine residues of proteins. Reciprocally, O‐GlcNAcase (OGA) reverses these PTMs by removing the GlcNAc residue. 190 In mammalian cells, O‐GlcNAcylation levels are dynamic alternation during the cell cycle, thus abnormal O‐GlyNAc dynamic cycling disrupts the cell cycle and causes RS. Using a mass‐tagging strategy, Leturcq et al. 191 identified that MCM2‐7 all subunits are O‐GlcNAcylated by OGT in human cells, especially in MCM3, MCM6, and MCM7. Each subunit of MCM2‐7 complex gradually disperses in knockout OGT cells, subsequently departing from chromatin. Thus, it is tempting to speculate that O‐GlcNAcylation of MCMs assists MCM assembly and regulates dynamic balance during DNA damage and DNA replication. 191
Lysine methylation usually occurs in histones, whereas in nonhistones in recent decades. Methylation can alter the conformation of proteins thus changing their function. Xia et al. 192 identified that recombinant Sulfolobus MCM ( sis MCM), an archaeal homolog of MCM2‐7 eukaryotic replicative helicase, is mono‐methylation by aKMT4 in vitro, which is characterized as the first archaeal lysine methyltransferase. Interestingly, MCM methylation (me‐MCM) upregulates MCM complex DNA unwinding ability, modulating their helicase activity. More intriguingly, me‐MCM also enhances heat resistance, which supports that methylation of MCM proteins also impacts protein thermal properties (Table 1 ).
5.6. PCNA Ubiquitination and SUMOylation
During DNA replication, PCNA serves as the pivot to recruit replicative polymerases polε and polδ to perform high‐fidelity DNA synthesis. 193 When replicative DNA polymerase encounters damaged DNA, the progression of the polymerases is blocked, and the replication fork is stalled. If this problem were not be resolved, the replication fork would be collapsed, resulting in cell death. As we summarized above, the blooey replication fork activates a unique DNA repair pathway called postreplication repair (PRR), such as error‐prone translesion DNA synthesis (TLS) and error‐free template switching (TS). 194 Specialized DNA TLS polymerases have been identified in yeast and mammalian, including polymerase η (polη), polymerase ι (polι), and polymerase κ (polκ). For instance, polη mediates efficient and precise TLS past UV‐induced thymine‐thymine CPD (T‐T CPD), whereas resulting in the high frequency of mutations in routine replication progression. 195 Low‐fidelity TLS polymerase causes incorrect nucleotide insertion in normal replication, subsequently stimulating NER, BER, or HR pathways to fix the errors. 196
PCNA is mono‐ubiquitinated at K164 by RAD6–RAD18 E2–E3 complex in response to replication fork stalling. 197 Mono‐ubiquitinated PCNA increases affinity with polη, thus further promoting efficient TLS. 198 Hence, dysregulation of replication and TLS progression cause severe genomic instability and tumorigenesis. Except for the Ub of PCNA, multiple modifications are involved in regulating PCNA functions, thereby impacting DNA replication, DNA repair, and even carcinogenesis.
Ub of PCNA undergoes regulation and control from the chromatin microenvironment by various factors. The chromatin structure could be regulated by PTMs of histone and nucleosome remodeling, which is vital for DNA replication, cell cycle, and DNA damage repair. Mutation of histone H3 and H4 disrupts DNA packaging, resulting in reduced expression of PCNA Ub under methyl methanesulfonate (MMS) or UV irradiation. 199
RAD6–RAD18‐mediated PCNA–K164 mono‐Ub is widely known in a variety of organisms. Meanwhile, polyubiquitination of PCNA has also been observed at K164 residue in yeast and mammal cells, which is essential for inducing error‐free pathways upon damage response. 200 , 201 K63‐linked polyubiquitination of PCNA requires a ternary complex composed of RING‐finger E3 ligase RAD5 and Mms2–Ubc13 complex, protecting cellular DNA against genomic mutations via a TS pathway. 200 , 202 , 203
RNF8 is a crucial E3 ligase in regulating histone Ub during DNA damage repair, while it is also identified as a novel E3 ligase for PCNA. Nevertheless, RNF8 cooperates with distinct E2 such as Ubc15 and Mms2 to activate mono‐Ub and polyubiquitination, respectively. 204 Depletion of RNF8 in medulloblastoma cells significantly suppresses PCNA mono‐Ub under UV damage, which is associated with UV‐induced p53 targeting and checkpoint activation.
In response to stalling DNA replication fork, PCNA could also be modified by SUMOylation at K164 residue, which is commonly observed in yeast and mammals. Furthermore, some reports also supported that PCNA might be SUMOylated at K127 residue and K164 in response to DNA damage. 205 SUMOylation is closely related to Ub that modulates protein coordinated interaction, whereas possibly antagonizes Ub via competing the same residue in substates. 206 In contrast, forceful evidence supported that SUMOylation of PCNA prohibits its Ub and DNA damage repair progression, implying SUMO of PCNA functions on regulating normal DNA replication.
In S. cerevisiae , SUMOylation of PCNA recruit helicase Srs2 via its C‐terminus SUMO‐interaction motif (SIM), which disrupt RAD51 single‐stranded presynaptic filaments, ultimately interrupt HR progression. 207 Furthermore, RFC is required for PCNA SUMOylation, which facilitating PCNA loading onto chromatin. SUMOylation of PCNA also cooperates with PIP, suppressing inappropriate HR. 208 Moreover, Gali et al. 209 revealed that SUMOylation of PCNA could decrease DSBs formation using neutral comets assay, even lower spontaneous recombination frequencies, and enhance damage resistance. Thus, impaired PCNA SUMOylation facilitates DSB formation at stalled replication fork, which highlight its role in preserving genome integrity.
One intriguing perspective supports that SUMOylation could conduct for a signal for Ub. SUMO‐targeting ubiquitin ligases (STUbLs) intervenes protein SUMOylation and Ub of SUMO segments via its SIMs. Mutation of PCNA SUMOylation site K164/K127 and RAD18 SIMs residues directly decrease PCNA Ub level, implying SUMOylation of PCNA facilitates RAD18 to target PCNA. 210 Therefore, it points out that the SUMO and Ub crosstalk may be essential for DNA replication and DNA damage repair (Table 2 ).
Summary of the PCNA modification in response to DNA replication and DNA damage
6. DNA REPLICATION, DISEASE, AND THERAPY
Dysregulation of DNA replication is one remarkable feature of cancer cells associated with tumor progression. 6 Since DNA replication is strictly regulated by CMG complex and multiple polymerases, dysregulation of such replication factors may contribute to abnormal cell cycles causing severe consequences. 211 Accumulating evidence suggests that aberrant expression of CMG serves as reliable diagnostic markers among some cancers. 212 Consistently, MCMs are involved in multiple DDR pathways, such as activation of cell cycle checkpoint, which are also considered as crucial cancer therapeutic strategies.
6.1. MCMs in tumorigenesis and development
Genome instability is a hallmark of cancer. Conventional expression of the MCM2‐7 complex ensures routine DNA replication progression, which is a prerequisite for genome stability. 213 Nevertheless, numerous studies have indicated that both deficiency and overexpression of MCMs are associated with cancer development. Mcm3‐deficient mouse model was used to determine the impact on gene function in hematopoietic stem cells. The results indicated that downregulation of MCM3 results in RS, further leading to fetal anemia during embryonic development. 214 In contrast, MCM6 was overexpressed in clear‐cell renal cell carcinoma. 215 Upregulation of MCM6 also exists in non‐small cell lung cancer and breast cancer with worse survival and higher histological grade. 216 , 217 , 218 The reasons for abnormal MCMs expression remain unclear. There are two possible speculations: (a) CDK‐mediated MCM complex dissociating prevents DNA re‐replication. However, dysregulation of the cell cycle‐dependent kinase CDK permits MCMs constantly binding to each other, resulting in continuous cell division and high expression. 219 , 220 (b) Aberrant DNA replication license system induces abnormal DNA replication, increasing genomic instability, and carcinogenesis. 221 , 222 In addition, spontaneous mutation of MCMs increases chromosome elimination and DNA damage, 223 whereas a more than twofold reduction of MCM protein expression could lead to genomic instability in S. cerevisiae . These findings demonstrated that MCMs are indeed involved in tumorigenesis, but the detailed mechanism is still unclear. 212
In lung cancer, omics data of MCM2 overexpression is analyzed using the Gene Expression Omnibus database, which is associated with large tumor size, different malign degrees, and clinical stages. 224 Using RT‐PCR analysis, except for MCM3 and MCM5, MCMs are upregulated in cervical cancer in vitro and in vivo, which are critical in tumor progression. 225 Spontaneous dominant leukemia ( Sdl ) mice model is a murine model for heritable T cell lymphoblastic leukemia/lymphoma, which harbors a spontaneous mutation in Mcm4 (Mcm4 D573H ). Mcm4 D573H could not alter the total expression of MCM2‐7 complex, whereas it significantly promotes tumor formation. 226 In laryngeal squamous cell carcinoma cells, knockout of MCM4 using siRNA also suppresses cell proliferation and inhibits of tumor progression. 227
MCM7 is regarded as an extensive mark for cancer development. 228 Some studies indicated that MCM7 genome sequence embodies a cluster of miRNAs (miR‐106b, miR‐93, miR‐25), which can downregulate the expression of oncogenes, including p21, E2F1, BIM, and PTEN. 229 PRMT5 acts as one methyltransferase to methylate multiple proteins in histones, 230 which is also deemed a potential target in colorectal cancer development and progression. 231 Recent studies revealed that PRMT5 physically interacts with MCM7 in HCT8 cells, while MCM7 depletion impairs cancer cell migration and invasion. 232 Using TCGA analysis, the expression of MCM7 enhanced by approximately 12% in ESCC. Silencing of MCM7 via siRNA significantly impaired KYSE510 cell proliferation and migration in vitro. 233 Furthermore, miR‐214 targets overexpression of MCM5 and MCM7 in hepatocellular carcinoma (HCC) cells to inhibit cell replication and colony formation. 234
Massive rearrangements are one of the characteristics of aggressive cancer genomes. MCM8, unclassical MCM proteins, is deemed to interrelate with chromosome rearrangement. 235 Knockdown of MCM8 in mice diminished xenografted tumor volume, which implied the critical role of MCM8 in tumor metastasis in vivo. 236
Despite novel and striking findings, additional investigations are still needed to be addressed. Since closely associated with tumorigenesis, MCMs can be used as precise cancer therapy via molecular targets.
6.2. MCMs as diagnostic and prognostic biomarkers
As mentioned above, aberrant expression of MCMs is closely related to tumorigenesis and development. Therefore, MCMs are also used as tumor biomarkers and indicators of prognosis. 237 For example, MCM2 is regarded as a novel proliferation biomarker for oligodendroglioma, 238 ESCC, 239 and breast cancer. 240 , 241 However, the detailed interventional mechanism is different due to MCM2's diversiform role in the distinct process. Knockdown of MCM2 abolishes DNA damage in ESCC cells, interfering with DNA replication in breast cancer cells. Meanwhile, MCM2 is also suggested to be a prognostic marker for some tumors such as renal cell carcinoma, 242 laryngeal carcinoma, 243 , 244 and gastric cancer. 245 , 246 In oral squamous cell carcinoma 247 , 248 and large B‐cell lymphoma, 249 , 250 MCM2 also nominates as a prognostic marker significantly related to malignant progression and the 2‐year survival rate of patients, respectively. In addition, abnormal expression of MCM3 reflects advanced tumor stage and metastatic status in cervical cancer, 251 , 252 breast cancer, 253 , 254 oral squamous cell carcinoma, 255 malignant salivary gland tumors, 256 , 257 and HCC. 258 , 259 Simultaneously, based on the TCGA and GEO analysis, some reports persist that MCM4 mainly serves as a prognostic indicator for HCC, 260 , 261 which is relevant to poor prognosis with MCM4 overexpression pattern. Furthermore, overexpression of MCM4 may also be a diagnostic signal in esophageal cancer, 262 , 263 colorectal cancer, 264 , 265 cervical cancer, 225 , 266 , 267 ovarian cancer, 268 , 269 , 270 , breast cancer, 56 and gastric cancer. 271 , 272 In addition, upregulated expression of MCM5 is mainly related to poor prognosis and malignant status. 115 , 225 , 273 , 274 , 275 Consistent with this notion, overexpression of MCM5 also associates with tumor stages, which appears to be potential diagnostic and prognostic markers in thyroid cancer, 276 , 277 ovarian cancer, 269 , 278 , 279 , 280 bladder cancer, 281 , 282 , 283 and renal cell carcinoma. 284
On the contrary, WGCNA combining with TCGA and GEO analysis identified that exceptional expression of MCM6 reflects pathologic stage of multiple tumors, serving as putative biomarkers for breast cancer, 285 , 286 , 287 gastric cancer, 288 , 289 renal cell carcinoma, 215 , 290 , 291 HCC, 292 , 293 , 294 , 295 and non‐small cell lung carcinoma. 217 , 296 , 297 Meanwhile, MCM7 is widely regarded as an extensive biomarker in multiple tumor types since its overexpression pattern. 233 , 234 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 , 310 Except for conventional MCM proteins, MCM8, MCM9, and MCM10 are also novel prognostic markers in multiple tumors. In tissues, high expression of MCM8 may act as a valuable prognostic indicator for different cancer therapy, consistent with gastric and cervical cancer. 311 , 312 , 313 MCM9 and MCM10 are associated with additional tumor types such as colorectal cancer, breast cancer, ovarian cancer, and HCC (Table 3 ). 314 , 315 , 316 , 317 , 318 , 319 , 320 , 321 , 322
MCM proteins as diagnostic and prognostic biomarker
Compared with the common‐used proliferation biomarker, MCM proteins are more sensitive and particular than PCNA and Ki‐67, 323 accurately reflecting cell proliferation status and predicting prognostic tumor patient's survival rate.
6.3. MCMs as therapeutic target
Since increased DDR preventing cancer cells from effective therapy, MCM2‐7 proteins as intermediates are involved in intricate progression, which may serve as a pivotal role in influencing therapy response.
Conventional chemotherapy is one of the staple cancer treatment strategies, with emerging resistance causing the limited anticancer effects. 324 Previous reviews highlighted that chemotherapy uniting with MCMs knockout approach inhibits tumor cell proliferation. 325 Recently, combination chemotherapies have been widely used in cancer treatment, especially with the knockdown of MCM proteins. Carboplatin‐based chemotherapy is for the initial treatment of ovarian cancer, while carboplatin resistance results in treatment failure. 326 Deng et al. 327 found that knockdown of MCM2 could enhance the carboplatin sensitivity of A2780 cells and increase cells’ UV sensitivity, which may owe to accumulation of damaged DNA and activation of the p53‐dependent apoptotic response. Oxaliplatin or etoposide‐mediated chemotherapy combined with knockdown of MCM7 could reduce the proliferation of colorectal carcinoma cells and induce tumor apoptosis in vitro. 328 In pancreatic ductal adenocarcinoma cells, reduction of MCM4 or MCM7 clearly exhibits more sensitive to gemcitabine and 5‐FU exposure, which may be caused by MCM suppression‐induced RS. 328 Classic hypercholesterolemia curative simvastatin was demonstrated that reduces the expression of MCM7. 329 Thus, it implied that simvastatin combining with chemotherapeutic drugs may be the putative cancer therapy for some chemo‐resistance cancer treatment. Liang et al. 330 demonstrated that simvastatin combines with tamoxifen impaired breast cancer cell proliferation and resulted in apoptosis in vivo and in vitro. Knockout of MCM8 or MCM9 selectively increases cisplatin sensitivity in specific cancer cells such as HCT116 and HeLa cells. 331 , 332 However, siRNA‐mediated MCM8 silencing could not alter the cisplatin sensitivity of normal HFF2/T fibroblasts, indicating MCM8 may act as a molecular target just in cancer cells. Consistent with cisplatin, silencing MCM8 and MCM9 selectively hypersensitizes cancer cells to Olaparib, which may rely on MCM8‐9's role in resolving RS. 333 , 334
Radiotherapy is an alternative cancer therapy known to induce cancer cells’ DNA damage and autophagy to reach the treatment goals, while acquired radio‐resistance disturbs the effectiveness. 335 Next‐generation sequencing of mRNA (RNA‐seq) results revealed that MCM7 is significantly upregulated in radio‐resistant PC‐3 cells after 2Gy IR treatment. Diminishing expression of MCM7 might increase radiotherapy response in prostate cancer. 336 Consistent with this notion that upregulated expression of MCM2 is involved in the radio‐resistant cervical cancer cell, indicating that MCM2 is one potential regulatory factor in increasing radio‐sensitivity in cancer treatment. 337 As mentioned above, MCM3 is one prognosis marker for HCC with high expression in vitro and in vivo. Using MTT and TUNEL methods, low MCM3 expression HCC cell line performs low growth, whereas high MCM3 expression induces lower apoptosis under radiotherapy. In addition, overexpression of MCM3 further promotes HCC cell radio‐resistance, revealing MCM3 prevents HCC radiotherapy efficiency via activating NF‐κB pathway. 259
Specific small molecule inhibition for MCM proteins is an additional invaluable approach for various cancer treatments. 338 There are three typical MCMs‐based small molecule inhibitors with potential chemotherapeutic effects: (a) Enzyme inhibitors such as DNA helicase‐targeting small molecule inhibitors. 339 Ciprofloxacin targets MCM2‐7 complex to block the helicase activity, further inhibiting cell proliferation. 340 , 341 (b) The inhibitors prevent interaction among MCM subunits. 112 , 342 , 343 (c) The inhibitors regulate the expression of MCM proteins. 344 Widdrol could downregulate the expression of MCM proteins to inhibit cancer cell proliferation in G1 phase. 345 , 346 In addition, trichostatin A targets MCM2 to inhibit its expression. 347 , 348 Recent studies revealed that Breviscapine downregulates the expression of MCM7 and impairs tumor progression in prostate cancer via activating DNA damage‐induced apoptosis (Table 4 ). 349
MCM proteins as therapeutic target in different therapeutic scheme
6.4. GINS and Cdc45 as prognostic markers and the target for therapy
Except for MCMs, concurrent overexpression of GINS and Cdc45 are also observed in various cancers. Since Psf1 promoter activity is related with 17β‐estradiol (E2)‐based estrogen receptor pathway, aberrant expression of Psf1 might be a signal in breast cancer. Nakahara et al. 350 revealed that expression of Psf1 is remarkably increased in breast cancer both in vivo and in vitro, whereas siRNA‐mediated depletion of Psf1 inhibits DNA replication and cell growth. This evidence provides that Psf1 could be deemed as a novel breast cancer biomarker and therapeutic benefit for breast cancers with overexpression of Psf1. 350 Moreover, uprelated expression of Psf1 is also detected in non‐small cell lung cancer, which implies Psf1 as a prognostic biomarker and potential target for lung cancer therapy. 351 , 352 Using the tumor cell xenograft model, Nakahama et al. found higher expression of Psf1 is correlative with higher proliferative ability and metastatic capability, implicating Psf1 in tumorigenesis and its conceivable role as a therapeutic target. Furthermore, anomalous expression of Psf1 also exists in prostate cancer and HCC, 353 , 354 significantly correlated with tumor grade and clinical stage.
Using cDNA microarray analysis, Psf2 is frequently upregulated in cholangiocarcinoma, while knockdown of Psf2 drastically reduces cell proliferation and inhibits cell growth. 355 Furthermore, obvious upregulation of Psf2 is detectible in cervical cancer cell, whereas downregulation of Psf2 inhibits cell multiplication and tumorigenic ability. 356 Combined with the observations, Psf2 might be a novel and valuable prognostic biomarker for ovarian cancer and leukemia, 357 , 358 subsequently an underlying molecular target for cancer diagnosis and treatment. In triple negative breast cancer (TNBC) cell lines, similar with Psf1, the expression of Psf2 is also enriched correlated with the advanced stages of tumor. Intriguingly, silencing of Psf2 decreases the expression of matrix metallopeptidase 9, which is necessary for tumor invasion, hence suppress tumor cell migration and invasion. 359
Aberrant expression of Psf3 in colon carcinoma cell line associates with tumor cell proliferation and tumor progression, simultaneously, the similar results have also been found in non‐small cell lung cancer, lung adenocarcinoma, and colorectal cancer patients. 360 , 361 , 362 , 363 Finally, abundant Sld5 expression is closely related to bladder cancer and gastric cancer, since defect of Sld5 causes severe tumor cell proliferation disorder and inhibits cell growth. 364 , 365
Upregulated expression of Cdc45 is also a predictor for multiple cancers, including breast cancer, leukemia, lung cancer and osteosarcoma. 366 , 367 Using the Cancer Genome Atlas, prominent overexpression of Cdc45 is discovered in papillary thyroid cancer tissue, which impacts tumor sizes and cancer stages. 368 In tongue squamous cell carcinomas (SCC), higher expression of Cdc45 with severe lymph node status is observed in malignant tumor than mild precancerous epithelial dysplasia, which implies its role in distinguish precancerous dysplasia from SCC. 369 Myc is one crucial factor in regulating cell growth and tumorigenesis, overexpression of Myc enhances the Cdc45 DNA binding ability and replication fork stalling, which indicates Myc‐induced RS collaborates with Cdc45 in tumor development. 370 , 371
In summary, excessive expression of CMG is generally related with tumor size and malignancy but to varying extents, depending on the type of tumor. Thus, except for MCMs, GINS and Cdc45 could also serve as diagnostic and prognostic biomarkers for multiple tumors, which also as a druggable target to treat cancers (Table 5 ).
GINS, Cdc45, and PCNA as prognostic biomarker
Cancer immunotherapy is a novel biological treatment for malignant tumors, which could be collaborated with traditional radiotherapy and chemotherapy to improve the curative rate. Cancer immunotherapy elicits the immune system by identifying specifically expressed molecules on the tumor surface, thereby eliminating the cancer cells. 372 Cytotoxic T lymphocytes (CTLs) are specific T cells with potent nocuity to cancer cells, which recognizing cancer‐specific antigenic peptides human leukocyte antigen (HLA). Though mass spectrometric analyses and bioinformatic analysis, Yoshida et al. 373 isolates a Psf1‐derived peptide presented by HLA. Moreover, they found no other cancer vaccine target proteins except for Psf1. Detailed peptide is verified using the mouse model, suggesting Psf1 79–87 peptide induces CTL response in vitro and in vivo, which provides a novel cancer immunotherapy for targeting cancer stem cells. Previously, the similar approach was utilized in Cdc45, demonstrating that strongly immunogenic Cdc45‐derived peptides stimulated CTLs to be reactive to lung cancer cells. 373
6.5. PCNA as prognostic markers and the target for therapy
Due to its role in cell proliferation, PCNA is deemed as the tumor marker for diagnosis and patient prognosis. Overexpression of PCNA is observed in lung cancer in vitro and in vivo, while silencing of PCNA reduces cell invasion ability and 95D cells proliferations. 374 Identical results also occur in prostate carcinoma and breast cancer that PCNA connects with pathological stage and cellular grade, suggesting PCNA might be a crucial prognostic indicator of malignant tumors. 375 , 376 Furthermore, upregulated PCNA is associated with various digestive system tumors including colorectal carcinoma, ESCC, and HCC (Table 5 ). 377 , 378 , 379
PCNA is a critical factor in DNA replication and DNA damage repair. Hence, PCNA is a target for designing antiproliferation and anticancer drugs. Since multiple PTMs of PCNA interrupt its chromatin binding ability, developing therapeutics of modified PCNA will be conducive to target cancer cells. Previous research found that Y211 phosphorylation of PCNA is a crucial event to preserve the PCNA stability, promoting DNA damage repair and DNA synthesis. Therefore, mutant of Y211 phosphorylation inhibits tumor cells proliferation including prostate cancer and breast cancer. 380 , 381 In addition, specific small molecular inhibitors targeting PTMs of PCNA are also identified to disrupt cell proliferation and enhance chemosensitivity or radiosensitivity. RAD6 selective small molecular inhibitor SMI#9 leads to PCNA mono‐Ub defect and mitochondrial function reduction, suggesting RAD6 inhibitor serves as a promising strategy for TNBC treatment. 382
Although PTMs of PCNA have several implications in carcinogenesis; however, the detailed antitumor mechanism needs further investigation. Moreover, novel inhibitors or strategies inhibiting tumor development or proliferation via targeting PTMs of PCNA require identification.
7. CONCLUSION AND PERSPECTIVES
Numerous proteins perform DNA replication to maintain genetic information transmission from the parental generation to the next generation. In line with this, DNA replication factors are strongly associated with DDR to ensure genome integrity. Abnormal expression of DNA replication proteins results in genomic instability and performs a surprising diversity of symptoms. In this review, we outline the DNA replication mechanism and review the distinct roles of replication factors in DNA replication, DDR, and tumorigenesis.
Increasing publications revealed abundant replication factors are involved in DDR upon RS. Dynamic status of these proteins is regulated by some kinases, such as ATR and ATM, while multiple PTMs also modulate their functions and architecture. However, PTM‐mediated structure alternation may also contribute to DNA topological change to assist chromosomal rearrangement. 383 As we summarized, PTMs of MCMs and PCNA impact critical integration of DNA replication, DDR, and even cancer therapy. Nevertheless, the temporospatial modification crosstalk and definite modification sites still need deep investigation. It will be fascinating to clarify the crosstalk among distinct PTMs and map a trenchant signal network of DNA replication licensing and DNA damage repair.
Nevertheless, loss and mutation of the DNA replication factors is also the source of various disorders. In mammals, loss of function of polη leads to a hereditary disease xeroderma pigmentosum variant (XPV), which is characterized by a high frequency of skin cancer. 384 In addition, mutations in ORC complex cause the developmental disorder Meier‐Gorlin syndrome and Wolf‐Hirschhorn syndrome. 385 These atypical diseases with defects in DNA replication are still a tough job to explore in the future.
Dysregulation of DNA replication in cancer cells causes carcinogenesis with aberrant expression of replication proteins. Thus, such replication factors are closely related to tumorigenesis and development, acting as diagnostic and prognostic biomarkers among multiple tumors. Based on numerous biological research, overexpression of CMG and PCNA disturbs routine cell proliferation and regular DNA damage repair, promoting tumor development. Therefore, target therapy may be a potential approach to cancer treatment. In this review, we also arranged the current understanding of combination anticancer strategies with knockdown of replication factors. With the rapid evolution of pharmaceutics, small molecule inhibitions targeting these proteins are also designed for clinical application. Considering the PTMs of these proteins are also involved in DNA replication and their activity, PTMs of replication proteins may be a putative field to design associate small molecule inhibitors.
AUTHOR CONTRIBUTIONS
Hao‐yun Song was responsible for the manuscript. Hao‐yun Song designed the project in collaboration with De‐gui Wang and Rong Shen. Hao‐yun Song wrote the manuscript. Ya‐nan Guo, Hamid Mahasin, Rong Shen, and De‐gui Wang revised the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICS STATEMENT
Acknowledgments.
This work was financially supported by National Natural Science Foundation of China (No. 82071695 and 82060535), Natural Science Foundation of Gansu Province (No. 21JR7RA450), Non‐profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019PT320005), and Fundamental Research Funds for the Central Universities (lzujbky‐2022‐it14).
Song H‐Y, Shen R, Mahasin H, Guo Y‐N, Wang D‐G. DNA replication: Mechanisms and therapeutic interventions for diseases . MedComm . 2023; 4 :e210. 10.1002/mco2.210 [ CrossRef ] [ Google Scholar ]
Contributor Information
Hao‐Yun Song, Email: nc.ude.uzl@02yhgnos .
Rong Shen, Email: nc.ude.uzl@rnehs .
Hamid Mahasin, Email: nc.ude.uzl@9102dimah .
Ya‐Nan Guo, Email: nc.ude.uzl@1202nyoug .
De‐Gui Wang, Email: nc.ude.uzl@iugedgnaw .
DATA AVAILABILITY STATEMENTS

Book a free consultation now
100+ Video Tutorials, Flashcards and Weekly Seminars
- Revision notes >
- A-Level Biology Revision Notes >
- CIE A-level Biology Revision Notes
DNA Replication (A-level Biology)
Why does dna replicate.
Most organisms produce new cells every day through a process called cell division which occurs continuously.
DNA replication occurs before the cell divides. DNA replicates itself during the S phase of the cell cycle so that each daughter cells has a copy of the DNA after cell division.
DNA replication mean that parents can pass their DNA to their offspring. This passing of DNA and the genetic information stored in DNA is known as “ Genetic Continuity ”. The replication of DNA is crucial to ensuring genetic continuity both during cell division and between parents and offspring during reproduction.
The Process of DNA Replication
1) double helix unwinding.
- The first step of DNA replication is unwinding of the DNA double helix . Because DNA is a base-paired double helix, it replicates itself by unwinding and using each of its strands as a template to form a new strand.
- Hydrogen bonds are broken during unwinding . There is breakage of hydrogen bonds between complementary base pairs on the two polynucleotide chains.
- An enzyme called DNA helicase is involved . DNA helicase unwinds the DNA by breaking the hydrogen bonds between complementary base pairs on the two strands of DNA.
- It is important to understand that the entire DNA does not unwind simultaneously . DNA replication occurs along an entire molecule of DNA and the unwinding happens in one region of the molecule at a time. This is done to ensure stability of the molecule.
- The unwound region of the DNA is called a “ replication fork ” . DNA gets unwound in one direction only, meaning the replication fork moves continuously in a unilateral direction.
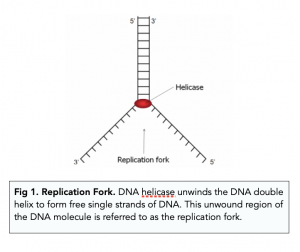
2) Semi-Conservative Replication
- DNA replication is semiconservative . The original strands of DNA act as a template for the synthesis of new strands of DNA. So each new DNA molecule is made up of one parent strand (see next point) from the original DNA molecule, and one new, daughter strand.
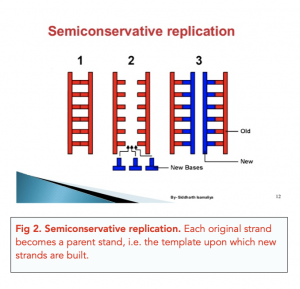
- The unwound strands of DNA are referred to as the parental strands . Free floating nucleotides in the nucleus are attracted to these parental strands of DNA.
3) DNA Polymerase (Condensation Reactions)
- Condensation reactions occur to complete DNA replication . The newly attracted nucleotides are only hydrogen bonded with the parental strand. To create a new strand of DNA, condensation reactions between these nucleotides need to occur in order to synthesise the daughter polynucleotide chain in order to complete DNA replication.
- DNA polymerase is the key enzyme. These condensation reactions are catalysed by the enzyme DNA polymerase , which reads the nucleotides and enables them to join. DNA ligase is responsible for the actual condensation reaction.
Mechanism of DNA Polymerase
- In a DNA double helix, the two strands are antiparallel. We previously established how in DNA, one strands goes from 3’ to 5’, and the opposite strand goes from 5’ to 3’.
DNA polymerase Works in the 5′ to 3′ direction
- DNA polymerase catalyses addition of free nucleotides . DNA polymerase “reads” the parental strand, and catalyses the addition of the free-floating nucleotides.
- DNA polymerase starts building at the 5’ end of the daughter strand . DNA polymerase can only bind to the 3′ end of a parental strand and work in one direction. This means they build the new strand in the 5′ to 3′ direction only.
- One of the daughter strands will be the leading strand. Since DNA strands are antiparallel but DNA polymerase can only work in one direction, replication has to occur in opposite directions on the two strands. Remember that DNA is also being unwound in one direction only too. The daughter strand which will go in the 5′ to 3′ direction towards the replication fork can be made continuously because the DNA polymerase can move continuously in this direction and follow the replication fork. This strand is called the leading strand .
- The other daughter strand will be the lagging strand. The other daughter strand will run 5′ to 3′ away from the replication fork. This strand cannot be made continuously as DNA polymerase can only build the new strand in opposite direction. Thus, DNA polymerase will need to keep detaching and reattaching to this strand, and this results in the new strand being built in short segments. This strand is called the lagging strand .
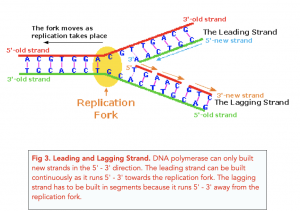
DNA polymerase reads and DNA ligase catalyses
- DNA polymerase reads the nucleotide sequence . When DNA polymerase binds to the parental DNA it reads the nucleotide sequence and recruits complementary nucleotides to form a hydrogen bond with the parental nucleotide. In doing so, DNA polymerase carries out a “proofreading” activity. It makes sure that only complementary nucleotides are pairing in order to prevent mutations from happening.
- DNA ligase catalyses condensation reactions . As the DNA polymerase recruits new nucleotides, DNA ligase catalyses condensation reactions between the new nucleotides to create a polynucleotide chain.
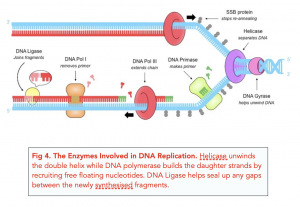
DNA Replication is the process of making a copy of the genetic information contained in DNA. This process is necessary for cell division and the transfer of genetic information from one generation to the next.
DNA Replication occurs through the semi-conservative mechanism, where each strand of the DNA double helix acts as a template for the synthesis of a new complementary strand. The DNA strands separate, and each strand is used as a template to build a new complementary strand by the addition of nucleotides.
The main enzymes involved in DNA Replication are helicase, primase, DNA polymerase, and ligase. helicase unwinds the double helix, primase synthesizes RNA primers, DNA polymerase adds nucleotides to the template strand, and ligase seals the gaps between the nucleotides.
RNA primers are short stretches of RNA that are synthesized by primase and are used to initiate DNA Replication. The primers provide a starting point for the addition of nucleotides by DNA polymerase. Once the primer is in place, the DNA polymerase can start adding nucleotides to the template strand, building the new complementary strand.
DNA Replication ensures the accuracy of the copied genetic information through the proofreading function of DNA polymerase. DNA polymerase checks each nucleotide before adding it to the new strand and corrects any mistakes. Additionally, there are enzymes, such as exonucleases, that can remove incorrect nucleotides from the new strand before the replication process is complete.
If DNA Replication goes wrong, it can result in mutations in the genetic information. Mutations can have a variety of effects on an organism, ranging from no effect at all to serious health problems. Some mutations can lead to the development of diseases, such as cancer, while others can result in changes in physical characteristics or behavior.
DNA Replication is important because it allows for the transfer of genetic information from one generation to the next. It is also necessary for cell division, allowing cells to divide and form new cells. Additionally, DNA Replication is essential for the repair of damaged DNA, helping to maintain the stability and integrity of the genetic information.
Still got a question? Leave a comment
Leave a comment, cancel reply.
Save my name, email, and website in this browser for the next time I comment.
CIE 1 Cell structure
Roles of atp (a-level biology), atp as an energy source (a-level biology), the synthesis and hydrolysis of atp (a-level biology), the structure of atp (a-level biology), magnification and resolution (a-level biology), calculating cell size (a-level biology), studying cells: confocal microscopes (a-level biology), studying cells: electron microscopes (a-level biology), studying cells: light microscopes (a-level biology), life cycle and replication of viruses (a-level biology), cie 10 infectious disease, bacteria, antibiotics, and other medicines (a-level biology), pathogens and infectious diseases (a-level biology), cie 11 immunity, types of immunity and vaccinations (a-level biology), structure and function of antibodies (a-level biology), the adaptive immune response (a-level biology), introduction to the immune system (a-level biology), primary defences against pathogens (a-level biology), cie 12 energy and respiration, anaerobic respiration in mammals, plants and fungi (a-level biology), anaerobic respiration (a-level biology), oxidative phosphorylation and chemiosmosis (a-level biology), oxidative phosphorylation and the electron transport chain (a-level biology), the krebs cycle (a-level biology), the link reaction (a-level biology), the stages and products of glycolysis (a-level biology), glycolysis (a-level biology), the structure of mitochondria (a-level biology), the need for cellular respiration (a-level biology), cie 13 photosynthesis, limiting factors of photosynthesis (a-level biology), cyclic and non-cyclic phosphorylation (a-level biology), the 2 stages of photosynthesis (a-level biology), photosystems and photosynthetic pigments (a-level biology), site of photosynthesis, overview of photosynthesis (a-level biology), cie 14 homeostasis, ectotherms and endotherms (a-level biology), thermoregulation (a-level biology), plant responses to changes in the environment (a-level biology), cie 15 control and co-ordination, the nervous system (a-level biology), sources of atp during contraction (a-level biology), the ultrastructure of the sarcomere during contraction (a-level biology), the role of troponin and tropomyosin (a-level biology), the structure of myofibrils (a-level biology), slow and fast twitch muscles (a-level biology), the structure of mammalian muscles (a-level biology), how muscles allow movement (a-level biology), the neuromuscular junction (a-level biology), features of synapses (a-level biology), cie 16 inherited change, calculating genetic diversity (a-level biology), how meiosis produces variation (a-level biology), cell division by meiosis (a-level biology), importance of meiosis (a-level biology), cie 17 selection and evolution, types of selection (a-level biology), mechanism of natural selection (a-level biology), types of variation (a-level biology), cie 18 biodiversity, classification and conservation, biodiversity and gene technology (a-level biology), factors affecting biodiversity (a-level biology), biodiversity calculations (a-level biology), introducing biodiversity (a-level biology), the three domain system (a-level biology), phylogeny and classification (a-level biology), classifying organisms (a-level biology), cie 19 genetic technology, cie 2 biological molecules, properties of water (a-level biology), structure of water (a-level biology), test for lipids and proteins (a-level biology), tests for carbohydrates (a-level biology), protein structures: globular and fibrous proteins (a-level biology), protein structures: tertiary and quaternary structures (a-level biology), protein structures: primary and secondary structures (a-level biology), protein formation (a-level biology), proteins and amino acids: an introduction (a-level biology), phospholipid bilayer (a-level biology), cie 3 enzymes, enzymes: inhibitors (a-level biology), enzymes: rates of reaction (a-level biology), enzymes: intracellular and extracellular forms (a-level biology), enzymes: mechanism of action (a-level biology), enzymes: key concepts (a-level biology), enzymes: introduction (a-level biology), cie 4 cell membranes and transport, transport across membranes: active transport (a-level biology), investigating transport across membranes (a-level biology), transport across membranes: osmosis (a-level biology), transport across membranes: diffusion (a-level biology), signalling across cell membranes (a-level biology), function of cell membrane (a-level biology), factors affecting cell membrane structure (a-level biology), structure of cell membranes (a-level biology), cie 5 the mitotic cell cycle, chromosome mutations (a-level biology), cell division: checkpoints and mutations (a-level biology), cell division: phases of mitosis (a-level biology), cell division: the cell cycle (a-level biology), cell division: chromosomes (a-level biology), cie 6 nucleic acids and protein synthesis, transfer rna (a-level biology), transcription (a-level biology), messenger rna (a-level biology), introducing the genetic code (a-level biology), genes and protein synthesis (a-level biology), synthesising proteins from dna (a-level biology), structure of rna (a-level biology), dna structure and the double helix (a-level biology), polynucleotides (a-level biology), cie 7 transport in plants, translocation and evidence of the mass flow hypothesis (a-level biology), the phloem (a-level biology), importance of and evidence for transpiration (a-level biology), introduction to transpiration (a-level biology), the pathway and movement of water into the roots and xylem (a-level biology), the xylem (a-level biology), cie 8 transport in mammals, controlling heart rate (a-level biology), structure of the heart (a-level biology), transport of carbon dioxide (a-level biology), transport of oxygen (a-level biology), exchange in capillaries (a-level biology), structure and function of blood vessels (a-level biology), cie 9 gas exchange and smoking, lung disease (a-level biology), pulmonary ventilation rate (a-level biology), ventilation (a-level biology), structure of the lungs (a-level biology), general features of exchange surfaces (a-level biology), understanding surface area to volume ratio (a-level biology), the need for exchange surfaces (a-level biology), edexcel a 1: lifestyle, health and risk, phospholipids – introduction (a-level biology), edexcel a 2: genes and health, features of the genetic code (a-level biology), gas exchange in plants (a-level biology), gas exchange in insects (a-level biology), edexcel a 3: voice of the genome, edexcel a 4: biodiversity and natural resources, edexcel a 5: on the wild side, reducing biomass loss (a-level biology), sources of biomass loss (a-level biology), transfer of biomass (a-level biology), measuring biomass (a-level biology), net primary production (a-level biology), gross primary production (a-level biology), trophic levels (a-level biology), edexcel a 6: immunity, infection & forensics, microbial techniques (a-level biology), the innate immune response (a-level biology), edexcel a 7: run for your life, edexcel a 8: grey matter, inhibitory synapses (a-level biology), synaptic transmission (a-level biology), the structure of the synapse (a-level biology), factors affecting the speed of transmission (a-level biology), myelination (a-level biology), the refractory period (a-level biology), all or nothing principle (a-level biology), edexcel b 1: biological molecules, inorganic ions (a-level biology), edexcel b 10: ecosystems, nitrogen cycle: nitrification and denitrification (a-level biology), the phosphorus cycle (a-level biology), nitrogen cycle: fixation and ammonification (a-level biology), introduction to nutrient cycles (a-level biology), edexcel b 2: cells, viruses, reproduction, edexcel b 3: classification & biodiversity, edexcel b 4: exchange and transport, edexcel b 5: energy for biological processes, edexcel b 6: microbiology and pathogens, edexcel b 7: modern genetics, edexcel b 8: origins of genetic variation, edexcel b 9: control systems, ocr 2.1.1 cell structure, structure of prokaryotic cells (a-level biology), eukaryotic cells: comparing plant and animal cells (a-level biology), eukaryotic cells: plant cell organelles (a-level biology), eukaryotic cells: the endoplasmic reticulum (a-level biology), eukaryotic cells: the golgi apparatus and lysosomes (a-level biology), ocr 2.1.2 biological molecules, introduction to eukaryotic cells and organelles (a-level biology), ocr 2.1.3 nucleotides and nucleic acids, ocr 2.1.4 enzymes, ocr 2.1.5 biological membranes, ocr 2.1.6 cell division, diversity & organisation, ocr 3.1.1 exchange surfaces, ocr 3.1.2 transport in animals, ocr 3.1.3 transport in plants, examples of xerophytes (a-level biology), introduction to xerophytes (a-level biology), ocr 4.1.1 communicable diseases, structure of viruses (a-level biology), ocr 4.2.1 biodiversity, ocr 4.2.2 classification and evolution, ocr 5.1.1 communication and homeostasis, the resting potential (a-level biology), ocr 5.1.2 excretion, ocr 5.1.3 neuronal communication, hyperpolarisation and transmission of the action potential (a-level biology), depolarisation and repolarisation in the action potential (a-level biology), ocr 5.1.4 hormonal communication, ocr 5.1.5 plant and animal responses, ocr 5.2.1 photosynthesis, ocr 5.2.2 respiration, ocr 6.1.1 cellular control, ocr 6.1.2 patterns of inheritance, ocr 6.1.3 manipulating genomes, ocr 6.2.1 cloning and biotechnology, ocr 6.3.1 ecosystems, ocr 6.3.2 populations and sustainability.

Let's get acquainted ? What is your name?
Nice to meet you, {{name}} what is your preferred e-mail address, nice to meet you, {{name}} what is your preferred phone number, what is your preferred phone number, just to check, what are you interested in, when should we call you.
It would be great to have a 15m chat to discuss a personalised plan and answer any questions

What time works best for you? (UK Time)
Pick a time-slot that works best for you ?
How many hours of 1-1 tutoring are you looking for?
My whatsapp number is..., for our safeguarding policy, please confirm....
Please provide the mobile number of a guardian/parent
Which online course are you interested in?
What is your query, you can apply for a bursary by clicking this link, sure, what is your query, thank you for your response. we will aim to get back to you within 12-24 hours., lock in a 2 hour 1-1 tutoring lesson now.
If you're ready and keen to get started click the button below to book your first 2 hour 1-1 tutoring lesson with us. Connect with a tutor from a university of your choice in minutes. (Use FAST5 to get 5% Off!)
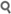
- Submit Content
- Business And Management
- Social Anthropology
- World Religions
- Biology 2016
- Design Technology
- Environmental Systems And Societies
- Sports Exercise And Health Science
- Mathematics Studies
- Mathematics SL
- Mathematics HL
- Computer Science
- Visual Arts
- Theory Of Knowledge
- Extended Essay
- Creativity Activity Service
- Video guides
- External links
1 Statistical Analysis
- Working with data
- Cell theory
- Prokaryotic cells
- Eukaryotic cells
- Cell division
3 Chemical elements and water
- Chemical elements & water
- Carbohydrates, lipids & proteins
- Dna structure
- Dna replication
- Transcription & translation
- Cell respiration
- Photosynthesis
- Chromosomes, genes, alleles & mutations
- Theoretical genetics
- Genetic engineering & biotechnology
5 Ecology and evolution
- Communities & ecosystems
- The greenhouse effect
- Populations
- Classification
6 Human health and physiology
- The transport system
- Defence against infectious disease
- Gas exchange
- Nerves, hormones & homeostasis
- Reproduction
7 Nucleic acids and proteins
- Transcription
- Translation
8 Cell respiration and photosynthesis
9 plant science.
- Plant structure & growth
- Transport in angiospermophytes
- Reproduction in angiospermophytes
10 Genetics
- Dihybrid crosses & gene linkage
- Polygenic inheritance

DNA replication
7.2.1 state that dna replication occurs in a 5 → 3 direction..
DNA replication occurs in a 5'→3' direction.
7.2.2 Explain the process of DNA replication in prokaryotes, including the role of enzymes (helicase, DNA polymerase, RNA primase and DNA ligase), Okazaki fragments and deoxynucleoside triphosphates.
The first stage of DNA replication in prokaryotes is the uncoiling of the DNA double helix by the enzyme helicase. Helicase separates the DNA into two template strands. RNA primase then adds a short sequence of RNA to the template strands. This short sequence of RNA is a primer which allows DNA polymerase III to bind to the strands and start the replication process. Once this is done, DNA polymerase III adds nucleotides to each template strand in a 5'→3' direction. The nucleotides have 3 phosphate groups and are called deoxyribonucleoside triphosphates. Two of these phosphate groups break off during the replication process to release energy. Since the strands are anti-parallel (the two strands have their 5' end and 3' end in opposite sides) and replication can only occur in a 5'→3' direction, one of the strands will be replicated in the same direction as the replication fork and the other will be replicated in the opposite direction of the replication fork. This means that one of the strands is synthesised in a continuous manner (named the leading strand) while the other one is synthesised in fragments (named the lagging strand). The leading strand only needs one primer while the lagging strand needs quite a few as it is formed in fragments. These fragments are called Okazaki fragments. DNA polymerase I will remove the RNA primers and replace these with DNA. The enzyme DNA ligase then joins the Okazaki fragments together to form a continuous strand.
Summary:
Helicase uncoils the DNA
RNA primase adds short sequences of RNA to both strands (the primer)
The primer allows DNA polymerase III to bind and start replication
DNA polymerase III adds nucleotides to each template strand in a 5'→3' direction
These nucleotides are initially deoxyribonucleoside triphosphates but they lose two phosphate groups during the replication process to release energy
One strand is replicated in a continuous manner in the same direction as the replication fork (leading strand)
The other strand is replicated in fragments (Okazaki fragments) in the opposite direction (lagging strand)
DNA polymerase I removes the RNA primers and replaces them with DNA
DNA ligase then joins the Okazaki fragments together to form a continuous strand
7.2.3 State that DNA replication is initiated at many points in eukaryotic chromosomes.
DNA replication is initiated at many points in eukaryotic chromosomes.
DNA Replication as a Semiconservative Process Essay
The process of DNA replication has been studied extensively as the pathway to understanding the processes of inheritance and the possible platform for addressing a range of health issues occurring as a result of DNA mutations.
However, the subject matter is still plagued by grey areas that require further analysis, the very properties of the process being one of the core issues of debating. Specifically, whether DNA replication can be deemed as semiconservative remains largely an unanswered question (Georgia Highlands College, n.d.). I believe that, despite the lack of certainty regarding the problem under analysis, it would be reasonable to believe that DNA replication is semiconservative since it is consistent with the fact that, during the reproduction process, DNS is separated into two bands.
The statement concerning DNA replication being a semiconservative process that leads to the development of two separate strands of DNA material has been supported by a vast range of evidence. Recent experiments point to the correctness of the semiconservative framework as the most legitimate theory that allows describing the process of DNA replication in the greatest detail possible (Georgia Highlands College, n.d.). In order to concede that the process of DNA replication is semiconservative, one should take a closer look at the outcomes of the experiment performed.
Since the test performed by Meselson and Stahl showed that the amount of the DNA material was equal in two daughter cells, yet the density thereof was different, the presence of semiconservative properties in DNA replication can be regarded as proven. By asserting that the observed tendency could be found in not only the strands of E.coli but also in other species, Meselson and Stahl made it evident that the DNA replication did, in fact, show semiconservative properties (Georgia Highlands College, n.d.). Thus, I insist that the existing evidence points to the DNA replication process being semiconservative.
The described outcomes of the experiment also lead to a vast range of conclusions concerning the nature and outcomes of DNA replications in different species. By defining DNA replication as semiconservative, the researchers made it evident that every double helix axis in the DNA structure built with the help of DNA polymerases leads to the creation of an entirely new strand that acts as complementary (Georgia Highlands College, n.d.).
It should also be borne in mind that the specified characteristic of the DNA structure makes it possible for the new strand, which is also known as the leading one, to emerge as a continuous piece, whereas the complementary one, or the lagging strand, occurs as a combination of smaller pieces (Georgia Highlands College, n.d.). By applying the notion of the DNA replication process being semiconservative, one can explain the observed changes within the DNA framework and provide the foundation for the further analysis of the subject matter.
Indeed, the results of the experiment described above cannot be deemed as consistent with the theory of dispersive replication, which has been offered as the alternative to the semiconservative framework. Using isotopes of nitrogen as the tools for labeling the DNA of the studied bacteria, Meselson and Stahl staged an experiment in the course of which the nature of the DNA replication process and the basis for its implementation were studied (Georgia Highlands College, n.d.). The semiconservative assumption made by the scientists implied that, in the process of replication, entirely new strands of DNA were produced with the help of the ones that were already present in the cells of the bacteria under analysis.
During the experiment, it was discovered that each of the nitrogenous bases presented in the DNA structure is only capable of connecting to its corresponding complementary partner. For adenine, the process of pairing occurs with thymine, whereas cytosine is connected to guanine in the process (Georgia Highlands College, n.d.). The resulting replication process, thus, takes place due to the combination of the helicase and DNA polymerase procedures (Georgia Highlands College, n.d.). Therefore, I strongly believe that the principle of DNA replication as the notion based on the semiconservative framework seems to be quite valid, given the vast amount of supportive evidence that has been collected.
Based on the outcomes of the Meselson–Stahl experiment, during which the DNA showed a strong propensity toward splitting into two distinct brands, the assumption that the DNA process is semiconservative can be regarded as confirmed. Even though it could be alleged that the current research has been erroneous and that the process of DNA replication may involve different processes and be based on an entirely different principle, the veracity of the identified statement is quite feeble. Thus, the stages of DNA replication can be seen as the semiconservative process. The presence of a synthesized strand along with the preexisting template one has proven to be the most sensible way of looking at the DNA replication stage.
Georgia Highlands College. (n.d.). Chapter 14 – DNA structure and function . Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2021, June 2). DNA Replication as a Semiconservative Process. https://ivypanda.com/essays/dna-replication-as-a-semiconservative-process/
"DNA Replication as a Semiconservative Process." IvyPanda , 2 June 2021, ivypanda.com/essays/dna-replication-as-a-semiconservative-process/.
IvyPanda . (2021) 'DNA Replication as a Semiconservative Process'. 2 June.
IvyPanda . 2021. "DNA Replication as a Semiconservative Process." June 2, 2021. https://ivypanda.com/essays/dna-replication-as-a-semiconservative-process/.
1. IvyPanda . "DNA Replication as a Semiconservative Process." June 2, 2021. https://ivypanda.com/essays/dna-replication-as-a-semiconservative-process/.
Bibliography
IvyPanda . "DNA Replication as a Semiconservative Process." June 2, 2021. https://ivypanda.com/essays/dna-replication-as-a-semiconservative-process/.
- Interesting and Relevant Applications of DNA Technology
- Infectious Bacterial Identification From DNA Sequencing
- Database Replication Among Geographically Remote Sites
- Plasmids, Their Characteristics and Role in Genetics
- The Dangers of Genetic Engineering and the Issue of Human Genes’ Modification
- "How One Cell Gives Rise to an Entire Body" by Pennisi
- Sickle Cell Disease and Scientific Inventions
- Gene Therapy: Risks and Benefits

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.2: Semi-Conservative DNA Replication
- Last updated
- Save as PDF
- Page ID 16129

- Axolotl Academica Publishing
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
DNA replication is similar to transcription in its most general idea: a polymerase enzyme reads a strand of DNA one nucleotide at a time, it takes a random nucleotide from the nucleoplasm, and if it is complementary to the nucleotide in the DNA, the polymerase adds it to the new strand it is creating. Of course, there are significant differences between replication and transcription too, not the least of which is that both strands of DNA are being read simultaneously in order to create two new complementary strands that will eventually result in a complete and nearly perfect copy of an entire organismal genome.

One of the most important concepts of DNA replication is that it is a semi-conservative process (Figure \(\PageIndex{7}\)). This means that every double helix in the new generation of an organism consists of one complete “old” strand and one complete “new” strand wrapped around each other. This is in contrast to the two other possible models of DNA replication, the conservative model, and the dispersive model. A conservative mechanism of replication proposes that the old DNA is used as a template only and is not incorporated into the new double-helix. Thus the new cell has one completely new double-helix and one completely old double-helix. The dispersive model of replication posits a final product in which each double helix of DNA is a mixture of fragments of old and new DNA. In light of current knowledge, it is difficult to imagine a dispersive mechanism, but at the time, there were no mechanistic models at all. The Meselson-Stahl experiments (1958) clearly demonstrated that the mechanism must be semi-conservative, and this was confirmed once the key enzymes were discovered and their mechanisms elucidated.
In the Meselson-Stahl experiments, E. coli were first incubated with 15 N, a heavy isotope of nitrogen. Although it is only a difference in mass of one neutron per atom, there is a great enough difference in mass between heavy nitrogen-containing DNA (in the purine and pyrimidine bases) and light/normal nitrogen-containing DNA that they can be separated from one another by ultracentrifugation through a CsCl concentration gradient (Figure \(\PageIndex{7}\)).
Over 14 generations, this led to a population of E. coli that had heavy nitrogen incorporated into all of the DNA (shown in blue). Then, the bacteria are grown for one or two divisions in “light” nitrogen, 14 N. When the DNA from the bacterial populations was examined by centrifugation, it was found that instead of light DNA and heavy DNA, as would be expected if DNA replications was conservative, there was a single band in and intermediate position on the gradient. This supports a semi-conservative model in which each strand of original DNA not only acts as a template for making new DNA, it is itself incorporated into the new double-helix.
- Open access
- Published: 16 May 2024
DNMT1-targeting remodeling global DNA hypomethylation for enhanced tumor suppression and circumvented toxicity in oral squamous cell carcinoma
- Yangfan Liu 1 na1 ,
- Yu Sun 1 , 2 na1 ,
- Jin Yang 1 na1 ,
- Deyang Wu 1 ,
- Shuang Yu 1 ,
- Junjiang Liu 1 ,
- Jingjing Luo 1 &
- Hongmei Zhou 1
Molecular Cancer volume 23 , Article number: 104 ( 2024 ) Cite this article
231 Accesses
Metrics details
The faithful maintenance of DNA methylation homeostasis indispensably requires DNA methyltransferase 1 (DNMT1) in cancer progression. We previously identified DNMT1 as a potential candidate target for oral squamous cell carcinoma (OSCC). However, how the DNMT1- associated global DNA methylation is exploited to regulate OSCC remains unclear.
The shRNA-specific DNMT1 knockdown was employed to target DNMT1 on oral cancer cells in vitro, as was the use of DNMT1 inhibitors. A xenografted OSCC mouse model was established to determine the effect on tumor suppression. High-throughput microarrays of DNA methylation, bulk and single-cell RNA sequencing analysis, multiplex immunohistochemistry, functional sphere formation and protein immunoblotting were utilized to explore the molecular mechanism involved. Analysis of human samples revealed associations between DNMT1 expression, global DNA methylation and collaborative molecular signaling with oral malignant transformation.
We investigated DNMT1 expression boosted steadily during oral malignant transformation in human samples, and its inhibition considerably minimized the tumorigenicity in vitro and in a xenografted OSCC model. DNMT1 overexpression was accompanied by the accumulation of cancer-specific DNA hypomethylation during oral carcinogenesis; conversely, DNMT1 knockdown caused atypically extensive genome-wide DNA hypomethylation in cancer cells and xenografted tumors. This novel DNMT1-remodeled DNA hypomethylation pattern hampered the dual activation of PI3K-AKT and CDK2-Rb and inactivated GSK3β collaboratively. When treating OSCC mice, targeting DNMT1 achieved greater anticancer efficacy than the PI3K inhibitor, and reduced the toxicity of blood glucose changes caused by the PI3K inhibitor or combination of PI3K and CDK inhibitors as well as adverse insulin feedback.
Conclusions
Targeting DNMT1 remodels a novel global DNA hypomethylation pattern to facilitate anticancer efficacy and minimize potential toxic effects via balanced signaling synergia. Our study suggests DNMT1 is a crucial gatekeeper regarding OSCC destiny and treatment outcome.
Oral squamous cell carcinoma (OSCC), a heterogeneous malignant tumor originating from oral epithelial cells [ 1 ], represents a prototypical form of cancer that undergoes an intricate process of multistage carcinogenesis, encompassing epithelial hyperplasia, dysplasia and carcinoma in situ [ 2 ]. Due to its tendency for recurrence and metastasis following treatment, OSCC patients, especially those in advanced stages, exhibit a low survival rate [ 3 ]. To improve the survival outcome of OSCC patients, novel therapeutic approaches have been devised to increase the survival time of OSCC patients by selectively targeting aberrant signaling molecules or proteins in cancerous cells, such as EGFR and PD-L1 [ 4 , 5 , 6 ]. Nevertheless, the efficacy of these targeted therapies is often hindered by the emergence of agent resistance in cancer cells, which is attributed to various factors including genomic instability, dysregulation of signal cascades or pharmacological toxicity [ 7 , 8 ]. Thus, it’s imperative to gain a comprehensive understanding of the underlying mechanism that governs epithelial carcinogenesis and tumor growth, with the aim of identifying potential therapeutic targets for the prevention and treatment of OSCC.
DNA methylation, a prevalent epigenetic modification that governs gene expression patterns, cell type-specific genome stability and embryonic development in eukaryotes [ 9 ], has been closely linked to oral cancer progression [ 10 , 11 ]. Changes in DNA methylation patterns associated with carcinogenesis progress gradually with cell proliferation. Notably, cancer cells possess a genome-wide DNA hypomethylation landscape, contributing to cancer cell instability and tumor heterogeneity [ 12 ]. DNA methyltransferase 1 (DNMT1), the most prevalent DNA methyltransferase, is crucial for maintaining DNA methylation homeostasis during DNA replication [ 13 ]. Any alteration in DNMT1 stability and activity can result in extensive changes of DNA methylation [ 14 , 15 , 16 , 17 ]. Our earlier study predicted DNMT1 as a potential target associated with OSCC progression [ 18 ]. Previous studies have shown that DNMT1 inhibition has positive anticancer effects on various types of squamous cancers [ 19 , 20 , 21 ]. However, the mechanism by which DNMT1 regulates OSCC initiation and progression via DNA methylation and signal transduction remains largely unknown.
Several DNA demethylating agents have undergone clinical trials to treat malignant tumors including head and neck cancers, yet individual treatment outcomes are variable and are being investigated further [ 22 ]. Cancers harbor intricate signaling networks that regulate numerous cellular processes, including proliferation, cell death and metabolism; these internal factors influence targeted agents. This aforementioned interactive network is of paramount importance in facilitating the indefinite proliferation and sustained viability of cancer cells [ 23 , 24 , 25 ]. Thus, deliberate interference with essential signaling pathways that result in cell growth arrest or death, such as PI3K and CDK inhibitors, is widely regarded as a significant approach for preventing neoplastic transformation and eradicating cancer cells [ 26 , 27 , 28 ], although some of the aforementioned limitations remain. Considering that DNA methylation is viewed as an upstream regulator of signal transduction modifications [ 29 ], DNMT1, which plays an indispensable role in maintaining genome-wide DNA methylation status, could act as a gatekeeper to maintain the equilibrium of dynamic signal transduction.
In this study, our objective was to elucidate the regulatory role of the DNMT1- dependent global DNA hypomethylation pattern in controlling OSCC development. To achieve this goal, we integrated multilayered experimental data from clinical human samples, xenograft mouse models, and independent high-throughput microarray analysis of DNA methylation alongside bulk and single-cell transcriptome analysis. We demonstrated that DNMT1-knockdown remodels sheer genome-wide DNA hypomethylation in OSCC cells. This novel DNMTI-specific DNA hypomethylation pattern triggers dual inhibition of PI3K-AKT and CDK2-Rb and inactivation of GSK3β, leading to enhanced tumor regression in comparison to that of a PI3K inhibitor. GSK3β deactivation also counteracts the adverse pharmacological toxicity of hyperglycemia and insulin feedback caused by PI3K inhibition. These combined effects create a synergistic function of signal transduction, ultimately resulting in enhanced efficacy and reduced toxicity in treating OSCC. Our research suggested that DNMT1 can serve as an essential gatekeeper of multiple signals, rendering it a promising target for controlling oral neoplastic transformation and improving OSCC treatment.
Materials and methods
Patient tissue samples.
Human OSCC tissues ( n = 22) were obtained from resected primary tumors without recurrence, and without prior chemotherapy or radiotherapy. Human hyperplasia tissues ( n = 6) and dysplasia tissues ( n = 7) were collected from bioptic oral leukoplakia lesions without any prior treatment. Normal human tissues ( n = 15) were procured from healthy oral mucosa during orthognathic surgery or wisdom tooth extraction. All tissue harvesting procedures were performed at West China Hospital of Stomatology, Sichuan University, and approved by the Human Research Ethics Committee of West China Hospital of Stomatology, Sichuan University (No. WCHSIRB-D-2021–548). The tissue samples were fixed in 10% neutral formalin for 24 h, transferred to 75% alcohol and embedded in paraffin. Pathological examination confirmed the identity of all the samples.
Cell culture and cell lines
The Cal27 and FaDu cell lines were obtained from the ATCC (United States); the HSC-3 cell line was obtained from the JCRB Cell Bank (Japanese); the SCC4 cell line was purchased from the BeNa Culture Collection (BNCC, China); and the UM-SCC1 and human normal oral keratinocyte (NOK) cell lines were obtained from the State Key Laboratory of Oral Diseases. All cancer cell lines were authenticated by STR analysis. Cal27, FaDu, SCC4, UM-SCC1 and HSC-3 cells were cultured in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, while NOK cells were cultured in defined keratinocyte SFM (Gibco, Grand Island, NY, USA) with corresponding growth supplement. All cells were maintained at 37 °C in a humidified atmosphere with 5% CO 2 . For cellular intervention involving inhibitors or activators, all reagents were applied at specific final concentrations, which were diluted with dimethyl sulfoxide (DMSO). An equivalent volume of DMSO was added as a negative control. The final concentrations are indicated as described in the figure legends.
Antibodies and reagents
Antibodies against DNMT1 (EM1901-83), BrdU (RT1081), mTOR (ET1608-5), GSK3β (ET1607-71), p-GSK3β (Ser9, ET1607-60) and PKM2 (ER1802-70) were obtained from HuaBio. Antibodies against Ki67 (9449 T, for immunostaining), PI3K (4249 T), p-mTOR (Ser2448, 5536 T), AKT (4691 T), p-AKT (Ser473, 4060 T), cleaved caspase-3 (CC3, 9664S) and p-Rb (Ser807/811, 8516S) were obtained from Cell Signaling Technology. Anti-Ki67 antibody (ab16667, for immunoblotting), anti-CDK1 (phospho T161) + CDK2/CDK3 (phospho T160) antibody (ab201008) and anti-phosphofructokinase antibody (PFK, ab154804) were obtained from Abcam, while an antibody against 5-mC (A-1014) from Epigentek. All the inhibitors and activators used were from MCE, including BEZ235 (HY-50673) for PI3K inhibition, 740Y-P (HY-P0175) for PI3K activation, AT7519 (HY-50943) for CDK2 inhibition, and GSK-3484862 (HY-135146) and GSK-3685032 (HY-139664) for DNMT1 inhibition.
Immunoblotting
Total cell proteins were extracted in lysis buffer (SAB signalway antibody, USA). After gel electrophoresis, the proteins were transferred to PVDF membranes (BioRad, Hercules, CA, USA) and blocked with 5% BSA solution. The blots were incubated with primary antibodies (as described above) at 4℃ overnight, with GAPDH or β-Tubulin serving as the control. Then, the membranes were incubated with the appropriate secondary antibodies (SAB signalway antibody, USA) for 1 h at room temperature, and signal detection was then performed using an Easy ECL protein blotting reagent kit (TransGen Biotech, China), and image scanning was performed using a Chemidoc XRS imaging system (BioRad, Hercules, CA, USA). Blotting images are representative from 3 repeats at least.
Lentiviral construction and cell transfection
DNMT1 was silenced by short-hairpin RNA using the targeting sequences 5'ACTACATCAAAGGCAGCAA-3' (sh-DNMT1-1) and 5'GGATGAGTCCATCAAGGAAGA-3' (sh-DNMT1-2). A scrambled control sequence was used as a control (sh-NC). All recombinant lentiviral viruses were purchased from Shanghai Genechem Co., Ltd. ( https://www.genechem.com.cn/ ), and generated using the hU6-MCS-CBh-gcGFP-IRES-puromycin vector and GV493 packaging cells. Then, Cal27 and FaDu cells were transfected following the manufacturer's instructions, and harvested after 72 h of transfection after selection with puromycin.
Cellular immunofluorescence
Immunofluorescence staining of cells was performed as previously described [ 30 ]. Briefly, cells were fixed with 10% neutralized formalin followed by permeabilization with 0.3% Triton X-100. For anti-BrdU staining, cells were first incubated with 10 µM BrdU-labeled culture medium for 6 h before cell fixation, followed by antigen retrieval with 1 M HCl. After washing with PBS, the cells were blocked with 10% goat serum for 30 min. Then, primary antibodies (as deacribed above) were applied, followed by incubation with the appropriate secondary antibodies and nuclear DAPI staining. Images were captured using an inverted fluorescence microscope (Leica, Germany).
Sphere formation assay
The medium for the sphere formation assay was prepared by adding 1 × B27 (Corning), 20 ng/ml EGF (PeproTech) and 10 ng/ml bFGF (PeproTech) to DMEM/F12. The cancer cells were resuspended in the above medium at an adjusted density of 2000 cells/ml and then inoculated on ultralow adhesion 24-well plates with 500 μl of cell suspension per well. A volume of 200 μl of fresh medium was added to each well every 2 days. Images were captured 10 days after cell seeding using an inverted fluorescence microscope (Leica, Germany).
OSCC xenograft model and animal administration
All animal experiments were reviewed and approved by the Animal Care and Use Ethics Committee of West China Hospital of Stomatology, Sichuan University (No. WCHSIRB-D-2021–628). Six- to eight-week-old female BALB/c nude mice (Beijing Vital River Laboratory Animal Technology Co., Ltd., China) were used as tumor recipients. As described previously [ 31 ], a total of 3 ~ 5 × 10 6 viable Cal27 cells WT, sh-NC and sh-DNMT1 cells with 50% Matrigel (Corning, USA) were subcutaneously transplanted into the right flank of anesthetized mice. All viable cells were confirmed and quantified using an automatic Cell Counter (Countess3, Invitrogen) with Trypan blue staining. When the tumor started to grow, the tumor volume was measured every other day and calculated using the formula 0.50 × long axis × short axis 2 . Beginning at the second or third week (depending on the cell density of the primary injection), after transplantation, when the xenografted tumor reached to at least 200 mm 3 , the mice were rearranged according to body weight and tumor volume, and the intervention started.
For PI3K inhibitor treatment, mice were treated with BEZ235 (Dactolisib, 10 mg/kg/day) by oral gavage or an equal volume of vehicle (10% 1-Methyl-2-pyrrolidinone and 90% Poly) daily for 7–14 consecutive days before being sacrificed, after which the tumors were harvested. For PI3K activator or CDK inhibitor administration, mice were treated with 740Y-P (20 mg/kg/day) or AT7519 (15 mg/kg/day) by intraperitoneal injection (i.p.) or an equal volume of vehicle (2% DMSO + 30% PEG 300 + 2% Tween 80 + ddH 2 O) daily for 7 or 14 days respectively according to the same treatment cycle of PI3K inhibitor treatment. For combined intervention, a CDK inhibitor was applied to mice 30 min after PI3K inhibition. BrdU (100 mg/kg) was administered i.p. 2 h before the tumors were harvested.
Histopathology, immunohistochemistry (IHC), immunofluorescence (IF), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, and periodic acid-Schiff (PAS) staining
Human tissues, and subcutaneous tumors harvested at the endpoint of the study were paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E) for histopathological diagnosis. Then, IHC and IF staining were performed as previously described [ 32 ]. Briefly, after dehydration, rehydration and antigen retrieval, paraffin-embedded sections (pretreated with 3% H 2 O 2 for IHC) were blocked with 15% normal goat serum (NGS) at room temperature for 1 h and then incubated with primary antibodies at 4 °C overnight. For IHC, secondary antibodies conjugated to HPR were used following the addition of brown DAB substrate for target staining and hematoxylin for nuclear staining. For IF, secondary antibodies conjugated to Alexa Fluor 594 (red) or 488 (green) were used (1:500 for all; Cell Signaling Technology), followed by DAPI (Beyotime, China) for nuclear staining. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed with a TMR (red) TUNEL Cell Apoptosis Detection Kit (Solarbio, China) according to the manufacturer's directions to detect apoptotic cells. PAS staining was performed with an AB-PAS Stain Kit with hematoxylin (Solarbio, China) to detect glycogen according to the manufacturer's directions. All slides were mounted with coverslips using Fluoromount-G (Southern Biotech).
Bioinformatics analysis
The differential pancancer mRNA expression of DNMT1 was analyzed by the Sangerbox platform [ 33 ]. The bulk analysis was performed with the GSE30784 dataset, which includes 45 normal tissue, 17 dysplasia, and 167 OSCC tissues, as well as the DNMT1 RNA-seq datasets of OSCC, which include OSCC tissues ( n = 350) and normal tissues ( n = 15) adjacent to cancer, and pancancer analysis in The Cancer Genome Atlas (TCGA) database.
The global DNA methylation data, normalized as total β values log2, were downloaded from GSE204943. This dataset comprised samples from normal tissues ( n = 22), oral leukoplakia (OLK) tissues ( n = 22), typical oral precancerous lesions with hyperplasia or dysplasia, and OSCC tissues ( n = 74), detected using 850 k Infinium Methylation EPIC BeadChips (850 k chip). Additionally, OSCC data from the TCGA database were utilized. For the GSE204943 dataset, we applied the ChAMP package to, filter the data, perform quality control, normalize the data and perform differential methylation site (DMS) analysis. We depicted the distribution of normalized β values and conducted principal component analysis (PCA) to visualize the sample distribution across the three groups. To determine the quality of the methylation data among the three groups, we sorted the variance of each row in the matrix from smallest to largest, and selected the top 1000 rows. The multidimensional scaling (MDS) graph and heatmap were then used to display these 1000 most variable positions.
For both DNMT1 RNA-seq data and global DNA methylation data (normalized as total β values log2) downloaded from the TCGA database, by using Empower (R) software, a smooth curve fitting was carried out respectively based on the clinicopathological data in TCGA database, omitting patients who had radiotherapy and chemotherapy, and adding the follow-up time. Then, data processing and analysis of Kaplan–Meier and univariate/multivariable Cox-regression were performed using R version 4.3.0 (2023–04-21), along with Storm Statistical Platform ( www.medsta.cn/software ) and GraphPad Prism 9.4. The R4.2.2 package was used for restricted cubic spline analysis of hazard ratio in overall survival and ggplot2 for visualization.
High-throughput DNA methylation microarray
To detect the global DNA methylation level of cells, we used an 850 k chip by Shanghai Biotechnology Corporation. Cell pellets of NOK and Cal27 cells, including nontransfected (referred to as WT), sh-NC, and sh-DNMT1 cells, were collected at a density of 1 × 10 7 cells per group with at least three independent replicates for each cell line. After DNA extraction, the Qubit Fluorometer method was used to quantify the DNA samples, while agarose gel electrophoresis and bisulfite conversion were used for quality inspection. Then, whole-genome amplification (WGA), fragmentation, alkaline denaturation, chip hybridization, washing, extension, imaging, and scanning were conducted to obtain the raw data of the chip. All the raw Illumina methylation microarray data were converted to methylation values (beta values, β) and then normalized within-array using the subset-quantile within array normalization (SWAN) algorithm. DMSs between groups were calculated based on β values, using the pool.t-test method, setting the threshold as P < 0.05, |beta. difference|> 0.1. Information on the differentially methylated sites included the P value, adjusted P value (FDR), and beta value. Differences and annotation information. A clustering heatmap of the differentially methylated sites was generated with Sangerbox ( http://vip.sangerbox.com/login.html ). ChAMP was used for the DMS analysis of key genes (R package ChAMP v2.30.0 and DMP.GUI function). All the original EPIC microarray data have been deposited in GEO with the accession ID GSE262310.
RNA extraction and sequencing
Total RNA was extracted using TRIzol reagent (R411-C3, Vazyme, China) following the manufacturer’s protocol. Three replicates of RNA samples from each cell type were used for cDNA library construction. RNA purity and quantification were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, USA), while RNA integrity was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Transcriptome libraries were then constructed following the instructions provided with the VAHTS Universal V5 RNA-seq Library Prep Kit. Finally, the RNA libraries were sequenced with an Illumina NovaSeq 6000 system by OE Biotech Co., Ltd. (Shanghai, China). All the original RNA-seq data have been deposited in GEO with the accession ID GSE262505.
Screening of key functional genes downstream of DNMT1-DNA methylation
Four available DNA methylation datasets (GSE123781, GSE87053, GSE75537, and GSE136704) were ultimately obtained from the Gene Expression Omnibus (GEO). These datasets met specific criteria including pathological confirmation for OSCC, DNA methylation data from the same detection platform, normal oral tissues as controls, and patients who had not received chemo-/radiotherapy or medical therapy. The four available methylation GEO datasets and the TCGA methylation dataset above were combined. The combined datasets were standardized using the minfi package. Then, differentially expressed CpG sites were screened using the R packet ChAMP. To identify consistency across datasets, the Meta R package was further used for the aforementioned differential CpGs. With a single CpG |logFC|> 0.1 and a false discovery rate (FDR) < 0.05 as the thresholds, all the meta- P values were further corrected by the Benjamini–Hochberg method to identify the final differential CpGs and their differential DNA methylation genes (DMGs).
Moreover, after normalizing the original TCGA RNA-seq data, the genes coexpressed with DNMT1 were obtained by Pearson correlation analysis (r), with correlation coefficients of r ≥ 0.3 and P < 0.05 as filtering conditions. Next, using Cor ≥ 0.4 and P < 0.05 as the threshold, differential expression genes (DEGs) that interact with DNMT1 were identified. Subsequently, we overlapped these DEGs with the identified DMGs above, and the DMGs associated with the top 20,000 differential CpG sites from sh-DNMT1 vs sh-NC OSCC cells, obtained from our DNA methylation high-throughput microarray analysis. This intersection yielded 152 genes that exhibited dual correlations with both DNMT1 and DNA methylation. Next, we conducted PPI network analysis using the String website, and visualized the network using Cytoscape 3.6.0. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed to identify potential functional pathways.
Moreover, FunRich3.1.3 was used to construct a PPI network filter for these 152 screened genes. With Nodes ≥ 100 as the standard, the 5 key node genes closely associated with DNMT1-DNA methylation were screened. Finally, based on the TCGA data and considering that the RNA expression of these genes was significantly different from that in normal tissues, we identified the key functional genes downstream of DNMT1-DNA methylation ( P < 0.05).
Gene function enrichment analysis
All the differentially methylated sites located in CpG islands were sorted in descending order based on |beta. Difference|, and the corresponding genes were extracted. After removing all blank values and duplicates, the top 3000 genes were selected for subsequent gene function enrichment analysis.
For Gene Ontology (GO) biological process enrichment analysis, the subset (c5.go.bp.v7.4.symbols.gmt) was downloaded from the Molecular Signatures Database ( http://www.gsea-msigdb.org/gsea/downloads.jsp ) [ 34 ], as the background gene set to map genes. For KEGG enrichment analysis, the most recent gene annotations were obtained from the KEGG REST API ( https://www.kegg.jp/kegg/rest/keggapi.html ), as the background gene set to map genes. Next, the R software package clusterProfiler (v3.14.3) was used for both GO and KEGG enrichment analysis, with a threshold setting of 5 to 5000 for the gene set, P < 0.05 and FDR < 0.1.
Multiplex immunohistochemistry (mIHC) staining
For the mIHC staining in this study, a multicolor-kit (Absin, China) was used as previously described [ 35 ]. We used the Opal 520 channel [fluorescein isothiocyanate (FITC), a green fluorescence stain], the Opal 570 channel [cyanine 3 (Cy3), an orange fluorescence stain], and the Opal 670 channel [cyanine 5 (Cy5), a red fluorescence stain] to locate different proteins (as detailed in the figure legends). DAPI was used for nuclear staining. All the slides were observed and imaged with an Olympus FV1200 confocal microscope (Tokyo, Japan).
Glucose and insulin measurement
For xenografted mice treated with vehicle, BEZ235 and/or AT7519, peripheral blood was collected from the tail of mice every 60 min and subjected to glucose measurement with a Rapid Blood Glucose Monitor (Sinocare, China). Blood insulin was measured 5 h after mouse administration with an Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem).
Single-cell transcriptome analysis
The single-cell RNA sequencing (scRNA-seq) dataset GSE181919 was downloaded [ 36 ], and included 9 normal tissue samples, 4 precancerous leukoplakia samples and 20 primary HNSCC samples. The QC process was performed using Seurat (v4.3.0.1) in R version 4.3.1. The Seurat anchor-based integration method was used to correct the batch and merge 33 samples. Low-quality cells with fewer than 200 or more than 7000 unique molecular identifiers (UMIs) or more than 30% mitochondrion-derived UMI counts were filtered out. From these, we obtained a final dataset of 45,754 single-cell transcriptomes, following normalization using the NormalizeData and ScaleData functions. The top 20 principal components along with the top 2000 variable genes were used in this process. Dimensionality reduction through principal component analysis reduced variables and finally clustered the cells. The main cell clusters were identified using the FindNeighbors and FindClusters functions (dims = 40, resolutio n = 0.1) of Seurat and visualized using t-distributed stochastic neighbor embedding (tSNE). Cell-type annotation was performed by the R package SingleR (v2.3.5), alongside manual comparison of marker gene expression across different clusters by the FindAllMarkers function. A particular set of marker genes was projected into dot plots for cell type identification. To accurately distinguish cancer cells from epithelial cell clusters, the R package CopyKAT v1.1.0 was used to classify epithelial cells as either aneuploid (to represent malignant cells) or diploid [ 37 ]. Finally, 5258 single epithelial cells, including 1562 aneuploid cells and 3696 diploid cells, were subjected to pseudotime trajectory analysis.
To reveal the changes in epithelial cells during the multistage carcinogenesis process, we used Monocle 2 (v2.29.0) [ 38 ]. The following parameters were set as mean expression ≥ 0.1 and qval < 0.01 via the differential GeneTest function. The trajectories were visualized by the plot_pseudotime_heatmap and plot_complex_cell_trajectory functions, as well as the geom_density function in the R package ggplot2 v3.4.2. To calculate pathway activity for each epithelial cell, the R package AUCell (v1.23.0) and AddModuleScore_UCell function were utilized to calculate the hallmark gene set score, and ggplot2 was subsequently used for visualization.
Statistical analyses
The normality of the group datasets was assessed using the Shapiro–Wilk normality test. Statistical differences between two groups were analyzed using either an unpaired Student’s t test or a nonparametric Mann–Whitney exact test, depending on the data distribution. For comparisons involving more than two groups, one-way ANOVA with Tukey’s multiple comparison test was used. All the statistical analyses were conducted using GraphPad Prism version 9 (GraphPad Software, San Diego, CA, United States). Individual data points represent values obtained from technical or biological replicates.
DNMT1 overexpression gradually increases during oral neoplastic transformation and is linked to tumor growth in OSCC
To evaluate the expression of DNMT1 during OSCC development, we examined human samples ranging from oral normal tissue to hyperplastic and dysplastic lesions, and ultimately to OSCC tissues. The results revealed a progressive increase in DNMT1 expression with the process of oral neoplastic transformation, peaking in OSCC tissues (Fig. 1 A). A series of OSCC cell lines were utilized and initially confirmed to have markedly higher DNMT1 expression than NOK cells (Fig. S1 A). Representative Cal27 and FaDu cells were then used to generate DNMT1-knockdown cell lines (sh-DNMT1-1 and sh-DNMT1-2) (Fig. S1 B and C). Given the more pronounced gene silencing effect of sh-DNMT1-1, it was selected for subsequent investigations. DNMT1 silencing led to a considerable reduction in cell proliferation, as evidenced by decreases in BrdU and Ki67 expression (Fig. 1 B) and in the sphere-forming capacity of OSCC cells (Fig. 1 C) in vitro. Furthermore, an OSCC xenograft mouse model was generated (Fig. 1 D). sh-DNMT1-transfected cancer cells exhibited a notably reduced tumorigenic ability, as indicated by decreased tumor growth, reduced cell proliferation and augmented cell apoptosis (Fig. 1 , E–G; Fig. S1 D).
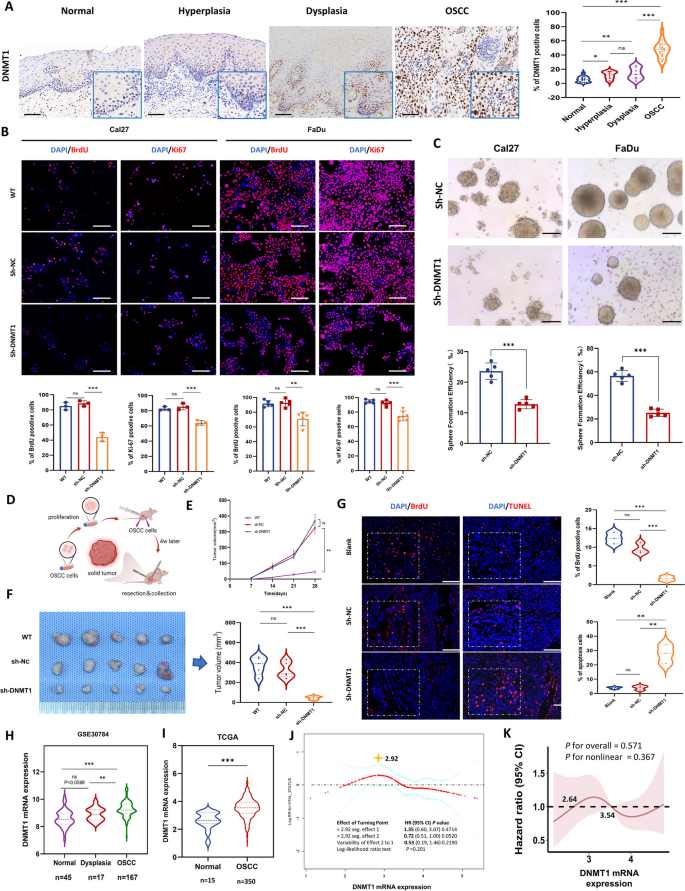
DNMT1 expression increases along with oral neoplastic transformation and its overexpression is correlated with tumor growth. A Representative IHC images and analysis of DNMT1 in oral human samples including normal ( n = 15), hyperplastic ( n = 6), dysplastic ( n = 7) and OSCC tissues ( n = 22). Scale bars, 100 μm. B Immunofluorescence of BrdU and Ki67 in OSCC cell lines. The data are presented as the means ± SDs of three independent experiments. Scale bars, 100 μm. C Sphere formation assay and statistical quantification of OSCC cell lines. Scale bars, 100 μm. D Schematic showing the xenografted OSCC mouse model. n = 5 mice in each group. E Tumor growth curve. F Presentation of OSCC xenografted tumors and tumor volume statistics at the endpoint of the study. G Immunofluorescence and analysis of BrdU and TUNEL in OSCC xenografted tumors. Scale bars, 100 μm. H The mRNA expression of DNMT1 in multiple stages of carcinogenesis, including normal ( n = 45), oral dysplasia ( n = 17), and OSCC ( n = 167) (data provided by GSE30784). I The mRNA expression of DNMT1 in OSCC ( n = 350) compared to that in oral normal tissues ( n = 15) (data from TCGA). J Smooth curve fitting showing the correlation between DNMT1 expression and mortality risk in OSCC patients. K Restricted cubic spline analysis indicating the correlation of DNMT1 expression with the hazard ratio of overall survival in OSCC patients. The values of DNMT1 RNA expression were 2.64 and 3.54, respectively, when HR = 1. * P < 0.05, ** P < 0.01, and *** P < 0.001 according to unpaired Student’s t test or one-way ANOVA with Tukey’s multiple comparison test
Utilizing the RNA-seq data of the GSE30784 dataset (Fig. S2 , A-C), we observed a gradual increase in DNMT1 expression during oral neoplastic transformation (Fig. 1 H). Subsequently, we procured primary OSCC and normal tissues from the TCGA database, and conducted further analysis on a larger patient cohort with matched clinicopathological and follow-up records. DNMT1 was overexpressed in OSCC tissues compared to adjacent normal tissues (Fig. 1 I). Smoothing curve fitting using clinicopathological and follow-up data from patients revealed a nonlinear and dynamic association between DNMT1 expression and the risk of OSCC mortality, although the difference was not significant (Fig. 1 J). Briefly, there was a general upward trend in mortality risk with increasing DNMT1 expression before the infection point at 2.92; thereafter, the potential relationship reversed. In addition to linear analyses such as multivariate Cox regression or Kaplan ‒ Meier analysis (Fig. S1 E and F), we opted to assess their nonlinear relationship using restricted cubic spline analysis. This analysis demonstrated a fluctuating relationship between DNMT1 expression and the hazard ratio of overall survival (Fig. 1 K). Notably, a significant decrease in DNMT1 expression in cancer correlated with a notable reduction in mortality and the hazard ratio for overall survival. Pancancer analysis confirmed DNMT1 overexpression in various cancer types, including head and neck cancers (Fig. S2 D), indirectly suggesting that DNMT1 is a potential target. Our findings indicate that DNMT1 overexpression contributes to the initiation of oral malignant transformation and facilitates the malignant behavior of cancer cells, resulting in the growth of OSCC tumors.
To further validate the potential of DNMT1 to target OSCC, we selected established DNMT1 inhibitors, namely, GSK-3484862 and GSK-3685032 [ 39 , 40 ], for parallel experiments to determine the role of DNMT1 inhibition in the biological behaviors of OSCC cells, primarily proliferation and apoptosis. After one week of uninterrupted intervention, both inhibitors consistently reduced DNMT1 expression in OSCC cells (Fig. 2 A). At various concentrations, both inhibitors decreased the expression of the proliferation marker Ki67 and increased the expression of the apoptosis marker cleaved caspase 3 (CC3) in OSCC cells, consistent with the effects of DNMT1 silencing (Fig. 2 A-C). Functionally, both inhibitors effectively suppressed the self-renewal capacity of OSCC cells (Fig. 2 D and E). These results further reinforce the notion that DNMT1 is a promising target for inhibiting the malignant behaviors of OSCC cells.
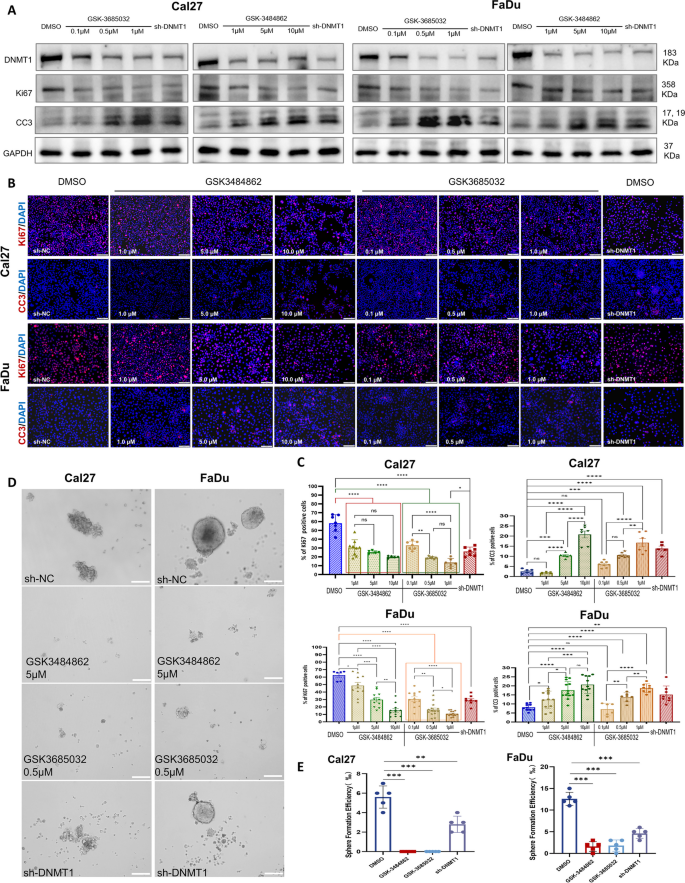
DNMT1 inhibitors consistently significantly inhibited proliferation and promoted of apoptosis in OSCC cells. A Western blot analysis of DNMT1, Ki67, and CC3 in OSCC cells. GSK-3484862 and GSK-3685032 were diluted in DMSO at different concentrations. The data are shown as the mean ± SEM. B and C Immunofluorescence and statistical quantification of Ki67 and CC3 in OSCC cells. Scale bars, 100 μm. D and E Sphere formation assay and statistical quantification of OSCC cells. Scale bars, 100 μm. * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001 by one-way ANOVA with Tukey’s multiple comparison test
Genome-wide DNA hypomethylation occurs during oral carcinogenesis and is stably maintained as a cancer-specific homeostasis in OSCC
DNMT1 alteration directly induces changes in genome-wide DNA methylation [ 41 ]. Thus, we examined DNA methylation in a set of freshly collected human samples comprising oral normal tissue, hyperplastic and dysplastic lesions, and OSCC tissues, by detecting 5-methylcytosine (5-mC), a covalent methylation at the fifth carbon atom of cytosine that is acknowledged as a specific marker of global DNA methylation [ 42 ]. Notably, both dysplastic and OSCC tissues showed significantly lower 5-mC expression than normal tissues, while there was no significant difference between normal and hyperplastic tissues (Fig. 3 A).
Global DNA hypomethylation occurs during oral carcinogenesis and is relatively stable in cancer cells and is associated with OSCC prognosis. A Representative IHC images and analysis of 5-mC in oral human samples, including normal ( n = 15), hyperplastic ( n = 6), dysplastic ( n = 7) and OSCC ( n = 22) tissue samples. Scale bars, 100 μm. B Volcano plots from the DNA methylation 850 k chip showing significantly differential CpG sites in Cal27 cells compared to NOK cells. C and D Nightingale rose chart showing the number of all significant DMSs with a CpG island probe distribution (C) and a gene probe distribution (D), respectively. E Ridge plot showing the β value distribution of the top 3000 significant differential sites in Cal27 cells and NOK cells. F Heatmap showing the top 1000 differential CpG sites with the absolute differences in β values. The class of CpGs (in relation to CpG islands) is shown on the right of the heatmap. G Bubble chart showing GO biological process enrichment of genes related to differential DNA methylation sites. The differential DNA methylation sites distributed on CpG islands were sorted by β value, and the top 3000 related genes were selected for enrichment analysis. H Heatmap showing the top 500 upregulated genes and 500 downregulated genes in Cal27 and NOK cells according to RNA-seq. UpSetR visualized overlapping genes among the DMGs and DEGs of Cal27 and NOK, as well as DEGs from the TCGA database. The bottom circle graph shows the hallmark gene set enrichment of the 175 overlapping genes. I Global DNA methylation (normalized as total β values log2) in OSCC ( n = 74) compared with that in OLK tissues (typical oral precancerous lesion, n = 22) and normal oral tissues ( n = 22) based on GSE204943 dataset. J Global DNA methylation (normalized as total β values log2) in OSCC ( n = 350) compared with that in normal oral tissues ( n = 15) based on TCGA data. K Smooth curve fitting showing the correlation between global DNA methylation and mortality risk in OSCC patients. L Restricted cubic spline analysis indicating the correlation of global DNA methylation with the hazard ratio of overall survival in OSCC patients. * P < 0.05, ** P < 0.01, and *** P < 0.001 by unpaired Student’s t test or one-way ANOVA with Tukey’s multiple comparison test
To delve deeper into genome-wide DNA hypomethylation, we employed 850 k chip to determine the global DNA methylation status of OSCC cells (Fig. S 3 , A and B). Compared to normal cells, OSCC cells exhibited prominently differential DNA hypomethylation sites, with an approximately 2.16-fold count of hypermethylated sites (Fig. 3 B). The distribution of differential CpG sites between these two cell types mirrored that of the Infinium probe distribution on the 850 k microarray (Fig. 3 , C and D; Fig. S 3 , C and D). Most DMSs were located within CpG islands rather than in the adjacent island shores, the region within 2 kb of the islands (Fig. 3 C; Fig. S 3 D). When employing gene region probes, the majority of DMSs were placed within the gene body region rather than the promoter (Fig. 3 D; Fig. S 3 D). Analysis of the β values of the top 3000 significant DMSs revealed greater hypomethylation than in NOK cells (Fig. 3 E). Analysis of the top 1000 significant DMSs indicated that a small portion of significant hypermethylation sites occurred in OSCC cells, with most of them located within CpG islands (Fig. 3 F). Together, these DNA methylation microarray data indicated a cancer- specific hypomethylation status in OSCC cells. This internal hypomethylated homeostasis may be related to the maintenance of cancer cell survival, as evidenced by the enrichment of biological processes, including cell–cell signaling, cell population proliferation and regulation of transport and cell differentiation through GO analysis (Fig. 3 G; Fig. S 3 E), and the involvement of signal transduction pathways, such as the PI3K-AKT, MAKP, and Rap1 signaling pathways (Fig. S 3 E), according to KEGG classification.
To better figure out the potential functions of these DMGs, additional RNA-seq of Cal27 and NOK cells was performed (Fig. S 3 F), revealing 979 upregulated genes and 629 downregulated genes with |logFC|≥ 1 and FDR < 0.05 (Fig. S 3 G). When overlapping these DMGs from 850k chip, DEGs from both cell RNA-seq and TCGA database, the hallmark gene set enrichment analysis identified pathways closely linked to epithelial malignancy and cancer progression, including Hedgehog, Hypoxia, PI3K-AKT-mTOR, P53 and epithelial- mesenchymal- transition (Fig. 3 H).
In addition, we analyzed global DNA methylation using the GSE204943 dataset, encompassing data from normal tissues, OLK lesions and OSCC tissues (Fig. S 4 ). While DNA methylation levels in OLK lesions did not decrease compared to those in normal samples, a significant reduction was observed in OSCC tissues (Fig. 3 I). This could be because the OLK lesions in this dataset were not pathologically subclassified as hyperplasia or dysplasia. Further analysis of the TCGA database confirmed lower global DNA methylation in OSCC tissues than in adjacent normal tissues (Fig. 3 J). Also, smoothing curve fitting revealed a nonlinear and dynamic association between global DNA methylation and mortality risk, with a single inflection point at 17.56 (normalized by log2), corresponding to a total β value of 193,145.44. A higher level of DNA methylation was associated with a lower mortality risk in OSCC patients (Fig. 3 K). A nonlinear association between the hazard ratio and global DNA methylation was observed, with a decrease in the hazard ratio with a substantial alteration in overall DNA methylation, either through a significant decrease or increase, leading to an improved survival rate (Fig. 3 L).
Taken together, these findings strongly suggest that the progressive genome-wide hypomethylation observed during oral malignant transformation represents a departure from the typical methylation patterns observed in healthy cells. However, cancer cells seem capable of maintaining unique methylation homeostasis specific to their malignant phenotype, which could contribute to their unchecked growth. Targeting this cancer-specific methylation homeostasis may represent a potential intervention to alter the proliferation of cancer cells.
DNMT1 knockdown remodels a highly disrupted and sheer genome-wide DNA hypomethylation pattern in OSCC
As described above, OSCC tissues and cell lines exhibit DNMT1 overexpression alongside global DNA hypomethylation. Furthermore, silencing DNMT1 effectively inhibited tumor growth. Subsequent 5-mC detection in the shrunken OSCC tumors derived from DNMT1 knockdown mice revealed a substantial decrease in 5-mC expression (Fig. 4 A). This finding suggested that the hypomethylated status, contingent on DNMT1, is associated with tumor suppression.
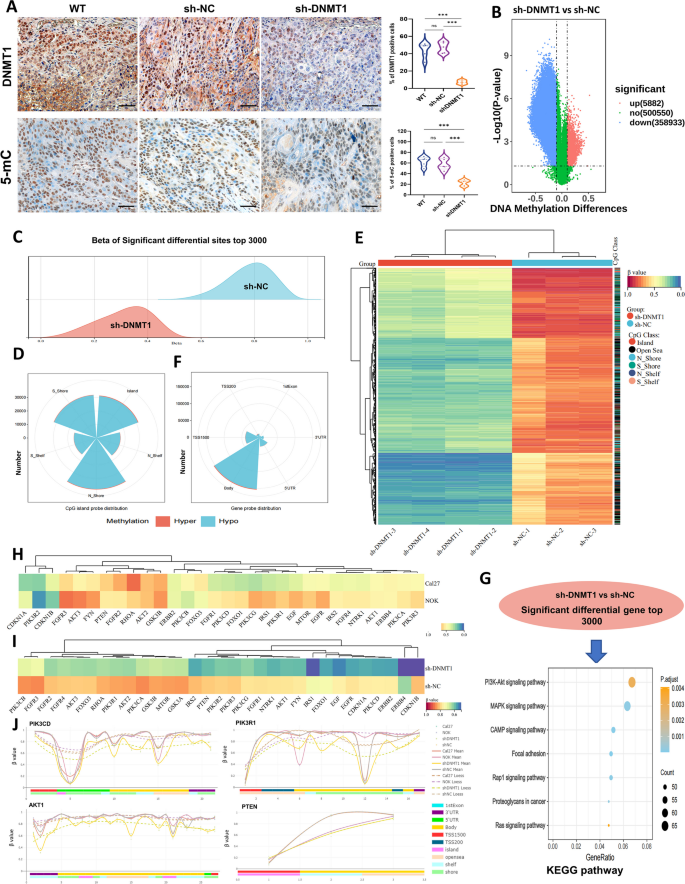
DNMT1 targeting remodeled an extensive and sheer genome-wide DNA hypomethylation pattern in OSCC. A Representative IHC images and analysis of DNMT1 and 5-mC in xenografted OSCC tumors. n = 5 mice in each group. Scale bars, 50 μm. B Volcano plots from DNA methylation 850 k chip showing significantly differential DNA methylation sites in sh-DNMT1 cells compared to sh-NC Cal27 cells. C Ridge plot showing the β value distribution of the top 3000 significantly differential sites in sh-DNMT1 and sh-NC Cal27 cells. D Nightingale rose chart showing the distribution of all DMSs with CpG island probes. E Heatmap showing the top 1000 differential CpG sites with the absolute differences in β values. The class of CpGs (in relation to CpG islands) is shown on the right of the heatmap. F Nightingale rose chart showing the proportion of all significant DMSs with gene probe distribution. G Bubble chart showing KEGG enrichment of DEGs related to DNA methylation sites. The differentially methylated sites distributed on CpG islands were sorted by β value difference, and the top 3000 related genes were selected for enrichment analysis. H and I Heatmap showing the mean methylation levels at differentially methylated sites of genes in the PI3K-AKT pathway between Cal27 and NOK cells, and between sh-DNMT1 and sh-NC cancer cells. J All significantly DMSs were enriched in the genes PIK3CD , PIK3R1 , AKT1 and PTEN , which are representative key genes in the PI3K-AKT pathway. Solid lines, the mean β values of each cell line; dotted lines, the β values loess of each cell lines. * P < 0.05, ** P < 0.01, and *** P < 0.001 by unpaired Student’s t test or one-way ANOVA with Tukey’s multiple comparison test
To further figure out how DNMT1 remodeled global DNA hypomethylation in OSCC, we compared sh-DNMT1 cancer cells to sh-NC cells using a DNA methylation 850 k chip. Strikingly, sh-DNMT1 cancer cells exhibited extensive and nearly complete genome-wide DNA hypomethylation. This was evident from the identification of 358,933 differentially hypomethylated sites, accounting for approximately 61.02 times of differentially hypermethylated sites in comparison to sh-NC cancer cells (Fig. 4 B). The distribution of β values for the top 3000 significant DMSs resulted in the near-complete elimination of DNA methylation in OSCC cells (Fig. 4 C). These findings provide further evidence that DNMT1 is an essential enzyme that regulates DNA methylation; any alteration or interruption in DNMT1 activity is strongly associated with changes in DNA methylation [ 14 , 42 ]. However, the significant DNA hypomethylation observed in cancer cells following DNMT1 knockdown seems to contradict the prevailing notion that DNA hypomethylation may be linked to cancer initiation [ 43 ]. Actually, when revisiting the previously mentioned correlation analysis between DNA methylation and patient survival, it was observed that either a substantial decrease or increase in overall DNA methylation leads to an improved survival rate in the context of global DNA hypomethylation among OSCC patients. Our observation somewhat aligns with the hypothesis that extensive alterations in the DNMT1-mediated DNA hypomethylation pattern are linked to a tumor-suppressive effect.
To ascertain the potential underlying cause in greater detail, we proceeded to deduce the blueprint of the global DNA hypomethylation pattern of DNMT1 remodeling. The majority of differential hypomethylation sites caused by DNMT1 targeting were located at CpG island shores rather than CpG islands (Fig. 4 D; Fig. S 5 A). The observed differences in the distributions of the primary probes (Fig. S 3 C) and the DMSs between OSCC cells and NOK cells were notable (Fig. S 5 B). When the top 1000 DMSs were assessed, sh-DNMT1 cancer cells exhibited complete DNA hypomethylation, and a few DMSs were distributed at CpG islands but at island shores (Fig. 4 E). Analysis of the distribution of functional gene regions revealed that DMSs were predominantly situated within the gene body (Fig. 4 F; Fig. S 5 A), whereas the top 3000 DMSs were primarily found within the gene body, followed by the transcription start site 1500 (TSS1500) region (Fig. S 5 B). Compared to NOK cells, sh-DNMT1 cancer cells also exhibited more pronounced hypomethylation than sh-NC cells; the majority of these differential hypomethylation sites were at CpG island shores and gene bodies (Fig. S 5 B).
Based on these results presented, it can be concluded that the downregulation of DNMT1 widely disrupted the homeostasis of global DNA methylation and led to a direct and persistent decrease in OSCC cells; this hypomethylation was characterized by a highly dispersed pattern of distribution transformation. Due to these extraordinary alterations, cancer cells may struggle to maintain proliferation. KEGG enrichment analysis and GO biological process analysis provided additional support for this finding. Both sets of DMGs were associated with various biological processes, including cell population proliferation, apoptotic processes, and specific signal transduction, with PI3K-AKT being the most prominent (Fig. 4 G; Fig. S 5 C). We then examined the methylation alterations in the PI3K-AKT pathway, and found that while the associated DMSs were slightly hypomethylated in cancer cells compared with those in NOK cells (Fig. 4 H), DNMT1 knockdown resulted in extensive hypomethylation of critical genes (Fig. 4 I). Key genes implicated in the activation of the PI3K-AKT signaling pathway, such as PIK3CD , PIK3R1 and AKT1 , showed dynamic hypomethylation; conversely, the inhibitory gene PTEN exhibited a pattern consistent with that observed in NOK cells (Fig. 4 J; Fig. S 6 A). Collectively, the aforementioned findings suggest that DNMT1 knockdown can directly remodel the global DNA hypomethylation in OSCC cells, leading to a pervasive disorder but sheer genome-wide DNA hypomethylation pattern subsequent to PI3K-AKT signaling alteration.
DNMT1-remodeled global DNA hypomethylation mediates PI3K-AKT inhibition and enhances tumor growth suppression
Through KEGG classification analysis, we identified the PI3K-AKT signaling pathway as an essential pathway associated with the DNMT1-specific DNA methylation pattern. This finding was further supported by the overlap of the top 3000 DMGs between Cal27 cells and NOK cells, and between sh-DNMT1 cells and sh-NC Cal27 cells (Fig. 5 A; Fig. S5D). Considering the DMSs associated with the PI3K-AKT signaling pathway, all of these DMSs were hypomethylated following DNMT1 knockdown, and the majority were located at CpG island shores and within gene bodies, especially AKT-related DMSs (Fig. S 6 B). This finding suggested that the activation of PI3K-AKT may be inhibited, as the methylation levels within gene bodies and methylations specific to tumors that occur at CpG island shores are often positively correlated with gene expression [ 44 , 45 ].
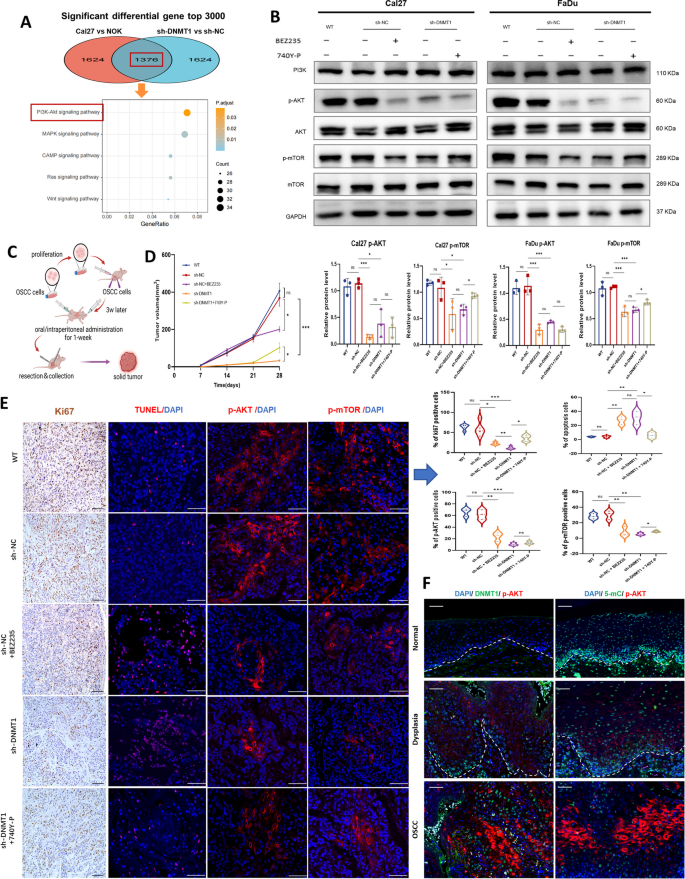
The PI3K-AKT pathway is involved in DNMT1-remodeled DNA hypomethylation pattern to regulate oral neoplastic transformation and tumor growth. A Bubble chart showing KEGG enrichment of the top 3000 overlapping genes related to differential DNA methylation sites between Cal27 and NOK and between sh-DNMT1 and sh-NC cells. B Western blot analysis of the indicated proteins in Cal27 and FaDu cells under the different conditions shown in the graph. Cells were treated with 1 µM BEZ235 or 25 µg/ml 740 Y-P for 24 h. GAPDH was used as an internal control. Upper panel: representative blots; lower panel: The densitometry quantification of the means ± SDs of three independent experiments. C Schematic showing the xenografted OSCC model. n = 5 mice in the WT, sh-NC, sh-DNMT1 and sh-DNMT1 + 740 Y-P groups, and n = 3 mice in the sh-NC + BEZ235 group. D Tumor growth curve. E Representative staining images and quantification of Ki67, TUNEL, p-AKT and p-mTOR staining in xenografted OSCC tumors. Scale bars, 100 μm. F Representative mIHC images of three channels, namely, DAPI, DNMT1 or 5-mC, and p-AKT in oral human samples including normal, dysplastic and OSCC tissues. Scale bars, 50 μm. * P < 0.05, ** P < 0.01, and *** P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test
The expression levels of total and phosphorylated proteins were then assessed, revealing a significant reduction in phosphorylated AKT and mTOR in sh-DNMT1 cancer cells, although the overall protein expression levels of PI3K and AKT did not exhibit substantial alterations (Fig. 5 B). This implies that the activation of PI3K-AKT is significantly hindered due to DNMT1-remodeled DNA hypomethylation. A rescue experiment was performed by using a PI3K agonist (740 Y-P) in sh-DNMT1 cells. These results indicated that 740 Y-P was able to partially restore the phosphorylation levels of mTOR in sh-DNMT1 cells (Fig. 5 B). This finding provided evidence that DNMT1 regulates PI3K-AKT signal transduction and suggested the possible involvement of other mechanisms modulated by DNMT1.
PI3K inhibitors have been verified to restrain tumor growth in several SCCs [ 46 , 47 ]. Then, we performed in vivo investigations utilizing a xenografted OSCC mouse model in which PI3K was inhibited with BEZ235 (Fig. 5 C). A reduction in tumor growth was observed after both DNMT1 and PI3K inhibition (Fig. 5 D). Intriguingly, the suppressive effect on tumor shrinkage was more prominent in cases of DNMT1 knockdown compared to PI3K inhibitor treatment. When PI3K agonists were applied to DNMT1-silenced mice, only a modest increase in tumor growth was observed (Fig. 5 D). The observed reduction in the number of proliferative cells and increase in the number of apoptotic cells within the sh-DNMT1 group provided evidence of a significant tumor-suppressing effect (Fig. 5 E). Moreover, DNMT1 knockdown effectively blocked the PI3K-AKT signaling pathway in subcutaneous tumors, as indicated by the reduced phosphorylation of AKT and mTOR (p-AKT and p-mTOR). PI3K-AKT signal transduction was partially restored upon treatment with PI3K agonists, but was suppressed compared to that in the sh-NC group (Fig. 5 E). This discovery provides additional evidence for the existence of other mechanisms that inhibit tumor growth as a result of DNMT1-specific DNA hypomethylation. Additionally, we examined the colocalization of DNMT1 or 5-mC and p-AKT in human samples, encompassing normal, dysplastic and OSCC tissues. During oral carcinogenesis, an increase in p-AKT was observed, followed by the overexpression of DNMT1 and the loss of 5-mC expression (Fig. 5 F).
DNMT1 targeting results in greater tumor suppression through the additional inhibition of the CDK2-Rb pathway
To determine the additional molecular mechanisms that underlie DNMT1 inhibition and resulting alterations in DNA methylation, we conducted an intersectional meta-analysis using the TCGA database and 4 GEO datasets, and then overlapped with the DEGs linked to DNMT1 from our 850 k chip profile. This analysis identified a total of 152 DEGs connected to both DNMT1 and global DNA methylation (Fig. S 7 A). These DEGs were subsequently validated to be associated with DNA replication, cell cycle, base excision repair and apoptosis pathways by KEGG enrichment analysis (Fig. S 7 B). By employing PPI analysis and implementing a network filter, we identified a set of five distinct node molecules, namely, CDK2, GSK3B, ABL1, NFKB1, and CREBBP (Fig. S 7 C). Only the mRNA expression of CDK2 and GSK3B was significantly greater in the OSCC samples from the TCGA database, as compared to the normal tissues (Fig. S 7 D). Both exhibited a positive correlation with DNMT1 expression in OSCC (Fig. S 7 E).
CDK2 plays a critical role in cancer progression by regulating the cell cycle and phosphorylating retinoblastoma protein (Rb), particularly during the G1–S phase transition [ 48 ]. A reduction in the phosphorylation levels of CDK1/2/3 and Rb was observed in sh-DNMT1 cancer cells compared to sh-NC cells (Fig. 6 A), confirming the additional inhibition of CDK2-Rb signal transduction. Furthermore, a PI3K inhibitor was shown to function in the deactivation of CDK2-Rb, and the combined use of PI3K and CDK2 inhibitors (BEZ235 and AT7519) had the most pronounced inhibitory effect, indicating a potential interplay between PI3K-AKT and CDK2-Rb signaling pathways [ 49 ]. However, the PI3K agonist did not fully restore the activation of CDK2-Rb, implying that DNMT1-mediated repression of CDK2-Rb and PI3K-AKT may occur in parallel. Also, we observed a consistent increase in the p-Rb level during neoplastic transformation, along with elevated DNMT1 expression and attenuated 5-mC (Fig. 6 B), confirming the regulatory effect of the DNMT1-DNA methylation pattern on CDK2-Rb pathway.
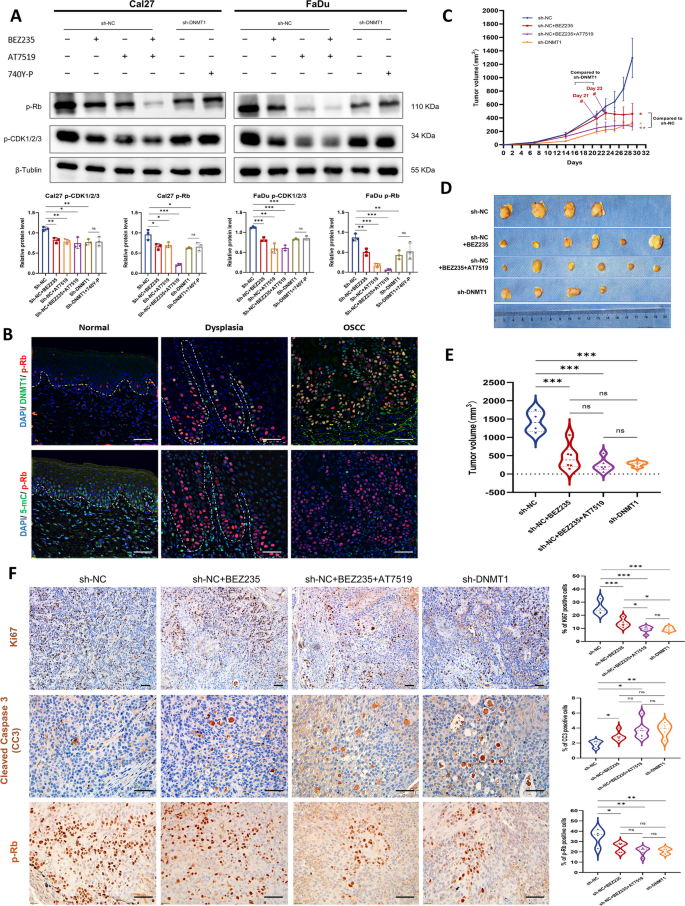
Restraining the CDK2-Rb signaling pathway contributed to the enhanced tumor-suppression caused by DNMT1-remodeled global DNA hypomethylation. A Western blot analysis of the indicated proteins in Cal27 and FaDu cells under different conditions is shown in the graph. Cells were treated with 1 µM BEZ235 or 25 µg/ml 740 Y-P for 24 h or with 1 µM AT7519 for 8 h. β-Tubulin was used as an internal control. Upper panel:: representative blots; lower panel: The densitometry quantification of the means ± SDs of three independent experiments. B Representative mIHC images of three channels, namely, DAPI, DNMT1 or 5-mC and p-Rb in oral human samples including normal, dysplastic and OSCC tissues. Scale bars, 50 μm. C Tumor growth curve. n = 5 mice for the sh-NC + BEZ235 and sh-NC BEZ235 + 740 Y-P groups, and n = 4 mice for the sh-NC and sh-DNMT1 groups. # P < 0.05 by unpaired Student’s t test. D and E Presentation of gross xenograft tumors (d) and tumor volume statistics (e) at the endpoint of the study. F Representative IHC images and analysis of Ki67, cleaved caspase 3 (CC3) and p-Rb in xenograft OSCC tumors. Scale bars, 50 μm. * P < 0.05, ** P < 0.01, and *** P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test
We further conducted concurrent treatment in xenografted mice with BEZ235 and AT7519, targeting the dual suppression of the PI3K-AKT and CDK2-Rb pathways, as a positive control. Mice treated with combination inhibitors exhibited comparable suppression of tumor growth to mice bearing sh-DNMT1 tumors. Compared to mice that received a single PI3K inhibitor, mice in which DNMT1 was silenced also exhibited the slowest tumorigenesis and superior control of tumor growth in the earlier intervention (Fig. 6 C). At the endpoint of the study, all three groups of tumor-bearing mice subjected to the intervention exhibited significant shrinkage in tumor volume compared to those bearing sh-NC tumors. However, the sh-DNMT1 group did not exhibit superior tumor suppression compared to the other two groups treated with inhibitors (Fig. 6 , D and E). This lack of superiority may be attributed to the reversible nature of DNMT1, which could counteract the effects of DNMT1 gene silencing. Nevertheless, we did observe a decrease in cell proliferation and an increase in cell apoptosis among these three experimental groups, providing compelling evidence for the anticancer effectiveness of these interventions. The proportion of Ki67-positive cells in sh-DNMT1 tumor-bearing mice was found to be mitigated, offering further evidence for the enhanced anticancer efficacy of DNMT1 silencing (Fig. 6 F). Moreover, the phosphorylation of the Rb protein decreased in the sh-DNMT1 group (Fig. 6 F), further supporting our discovery that DNMT1-remodeled DNA hypomethylation can inhibit tumor growth by concurrently suppressing the PI3K-AKT and CDK2-Rb signaling pathways.
DNMT1 knockdown further induces GSK3β inactivation to facilitate cell apoptosis and to antagonize PI3K inhibition-induced insulin feedback
Regarding GSK3B, the other gene candidate strongly associated with DNMT1-mediated DNA hypomethylation, our observations revealed significant changes in GSK3β phosphorylation at Ser9 (p-GSK3β) in xenografted tumors. When either DNMT1 was knocked down or a combination of PI3K and CDK2 inhibitors was applied, there was a notable increase in p-GSK3β. Conversely, PI3K inhibition resulted in a noticeable decrease in p-GSK3β (Fig. 7 A). GSK3β serves as an important regulatory enzyme in maintaining cell metabolic balance, especially in processes such as glycogen synthesis and glycolysis in cancer cells [ 50 , 51 ]. Its inactivation occurs through the phosphorylation of Ser9, facilitating glucose synthesis [ 52 ]. We subsequently examined increased glycogen clustering in sh-DNMT1 tumor tissues, similar to those treated with combined inhibitors, but notably more than in tumors treated in solely with PI3K inhibitors or vehicle. Besides, PI3K inhibitor-treated tumors exhibited very little glycogen accumulation (Fig. 7 A). Both DNMT1 knockdown and combined PI3K and CDK inhibition led to increased levels of glycolysis in tumor tissues, as indicated by increased numbers of PFK- and PKM2- positive cells. These cells were highly located in regions with large glycogen deposits (Fig. 7 , A and B). Interestingly, these cells showing glycogen clustering and heightened glycolysis were predominantly found near the apoptotic tumor area and far from the proliferative region (Fig. 7 B). Taken together, these results implied that the DNMT1-induced GSK3β inactivation promotes cancer cell death through abnormal glycogen metabolism.
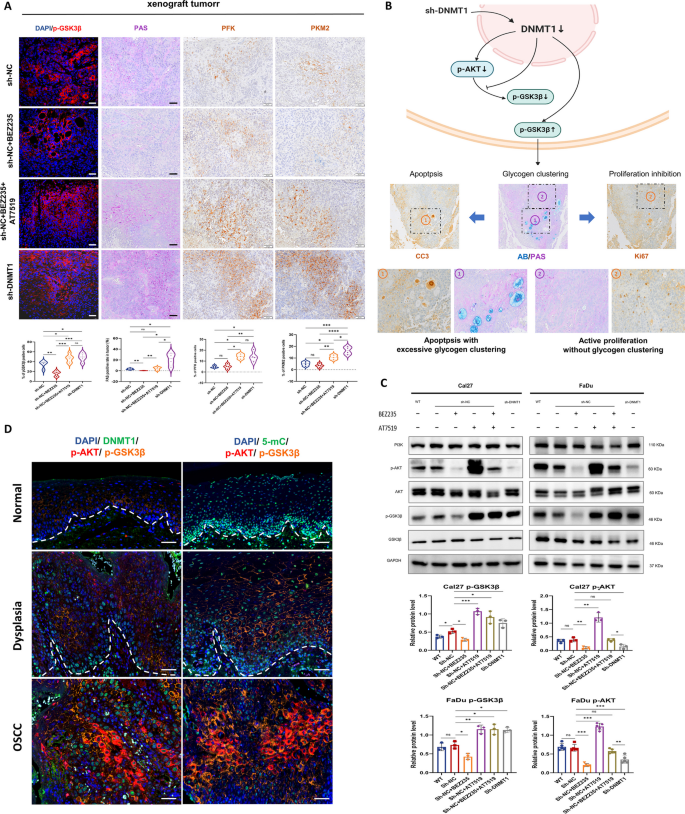
The GSK3β inactivation by DNMT1 knockdown leads to excessive glycogen clustering and apoptosis in tumors. A Representative IF images for p-GSK3β and PAS/IHC images for glycogen, PFK and PKM2 in xenografted OSCC tumors. Below are the corresponding statistical analysis results. Scale bars, 50 μm. B As shown in the schematic, DNMT1 knockdown prevents GSK3β activation downstream of PI3K inhibition by upregulating p-GSK3β, leading to excessive glycogen clustering in the tumor (AB-PAS staining), which is located around apoptosis and far from proliferation (IHC staining). C Western blot analysis of the indicated proteins in Cal27 and FaDu cells under the different conditions shown in the graph. Cells were treated with 1 µM BEZ235 for 24 h and 1 µM AT7519 for 8 h. GAPDH was used as an internal control. Upper panel: representative blots; lower panel: densitometry quantification of the means ± SDs of at least three independent experiments. D Representative mIHC images showing DAPI, DNMT1 or 5-mC, p-AKT and p-GSK3β in oral human samples, including normal, dysplastic and OSCC tissues. Scale bars, 50 μm. * P < 0.05, ** P < 0.01, and *** P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test
While GSK3β is typically regulated by AKT signaling [ 53 ], our in vitro experiments showed that both targeting DNMT1 and combined inhibition of PI3K and CDK2 reversed the increase in p-GSK3β expression in OSCC cells when compared to that resulting from single PI3K inhibition. Interestingly, only DNMT1 knockdown continuously suppressed PI3K-AKT activation (Fig. 7 C). In contrast, a single CDK2 inhibitor had a stimulating effect on AKT phosphorylation, and combined CDK2 inhibitors showed no effect on PI3K-AKT activation. These findings additionally imply that DNMT1 silencing could restore the signaling equilibrium by simultaneously inactivating PI3K-AKT, CDK2-Rb and GSK3β. Moreover, in human oral multicarcinogenesis samples, we observed the extensive changes in the expression of p-GSK3β along with changes in p-AKT, and DNMT1 expression and DNA methylation changing (Fig. 7 D). This finding provides further evidence supporting the involvement of the internal PI3K-AKT-GSK3β signaling pathway of in OSCC.
Systemic glucose disruption secondary to PI3K inhibitors has been evident from their use in animal cancer models and clinical trials. This disruption can limit the effectiveness of anticancer therapies [ 54 , 55 ]. In our OSCC mouse model, we carried out experiments to observe alterations in blood glucose levels (Fig. 8 A). The administration of a PI3K inhibitor gradually induced hyperglycemia. Conversely, when PI3K inhibitors were used in combination with CDK2 inhibitors, there was a rapid and notable decrease in blood glucose levels (Fig. 8 , B-D). At the 5-h time point after treatment, the serum insulin level in mice treated with BEZ235 remained consistently elevated, while a dramatic fall was observed in mice treated with combined inhibitors (Fig. 8 E). Moreover, tumor-bearing mice in which DNMT1 was targeted exhibited a notable stability in both blood glucose and serum insulin levels.
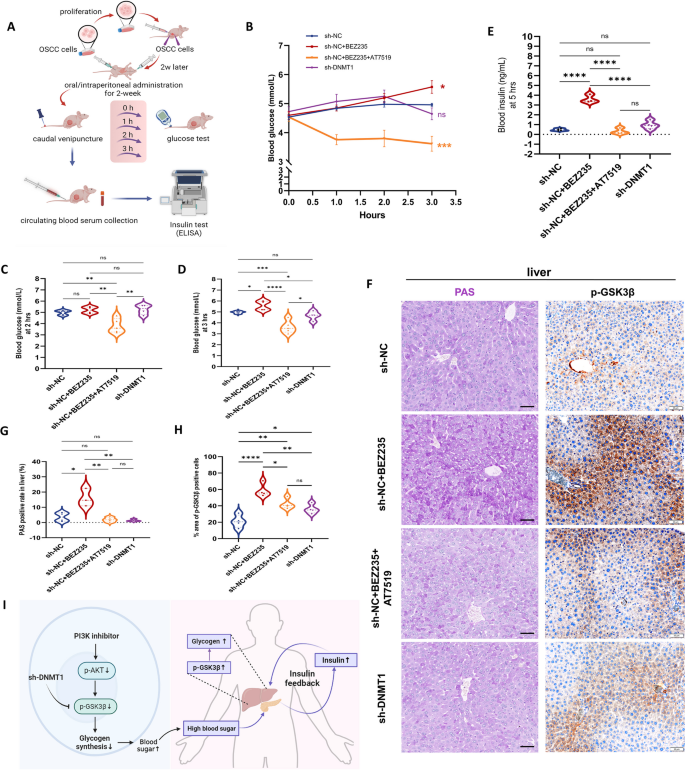
The GSK3β inactivation by DNMT1 knockdown antagonizes the insulin feedback resulting from PI3K inhibition. A Experimental schematic of blood glucose and serum insulin detection in tumor-bearing mice. n = 4 mice for each group. B Line chart of blood glucose within 3 h after administration. C and D Blood glucose of mice at 2 h (C) and 3 h (D) after administration respectively. e Serum insulin levels of the mice at 5 h after administration. F–H Representative PAS/IHC images (f) and analysis (g, h) of glycogen/p-GSK3β in mouse livers 5 h after administration. Scale bars, 50 μm. I As shown in the schematic, a PI3K inhibitor activates GSK3β through dephosphorylation, hindering glycogen synthesis and causing detrimental hyperglycemic effects. Elevated blood glucose levels can induce insulin feedback, culminating in hepatic glycogen accumulation. DNMT1 silencing can block this process by promoting GSK3β phosphorylation. * P < 0.05, ** P < 0.01, and *** P < 0.001 by a one-way ANOVA with Tukey’s multiple comparison test
As evidenced in prior research, the transient hyperglycemia caused by PI3K inhibition often remains within a few hours, as insulin feedback mechanisms kick in to restore normal glucose homeostasis [ 54 ]. Thus, we examined the glycogen synthesis conditions in the liver and found that only tumor-bearing mice treated with a PI3K inhibitor exhibited significantly high glycogen levels (Fig. 8 , F and G), accompanied by elevated expression of p-GSK3β (Fig. 8 , F and H). Conversely, mice experiencing hypoglycemia due to combined inhibitor treatment exhibited reduced liver glycogen storage and lower levels of p-GSK3β, further suggesting that liver glycogenolysis contributed to the recovery of normal blood sugar. However, sh-DNMT1 tumor-bearing mice exhibited a slightly greater level of p-GSK3β than nontreated xenografted mice, suggesting that glycogen depredation in sh-DNMT1 tumors themselves may also trigger compensatory liver glycogen metabolism to a certain extent. Together, these findings suggest that a suppressive intervention targeting DNMT1 is most likely to reduce the adverse toxicity to maintain normal glucose balance in the context of PI3K inhibition-induced hyperglycemia, further enhancing the effectiveness of anticancer treatments.
The DNMT1-mediated signaling synergia pattern and mechanism schematic in oral carcinogenesis and anti-cancer efficacy
Based on the results above, it appears that there are synergetic signal transduction pathways involving PI3K-AKT, CDK2-Rb, and GSK3β during oral neoplastic transformation and in treating OSCC, influenced by the DNMT1-DNA methylation pattern. OSCC, a heterogeneous solid tumor, progresses through multiple steps of carcinogenesis from normal to precancerous to cancerous lesions. To further validate this synergistic signaling synergy pattern in oral malignant transformation, we performed the single-cell transcriptome analysis using the GSE181919 dataset, to better understand the genetic variation of tumor heterogeneity at the single-cell level.
We first separated epithelial cells from normal, OLK and HNSCC tissues (Fig. S 8 A-E), respectively. Considering the presence of cell heterogeneity in the epithelial cell population, we reclassified diploid and aneuploid cells (Fig. S 8 F) since the latter can completely represent the malignant populations. Pseudotime trajectory analysis revealed two progressive branches from normal epithelial cells, one leading to a precancerous state and the other to a cancerous state, with aneuploid cells prominently located in the latter (Fig. 9 A; Fig. S 8 , G-I). Collaborative signaling pathways, including PI3K-AKT, mTOR, CDK-Rb-E2F and Glycolysis were activated as the epithelial cells progressed toward a cancerous state (Fig. 9 B, Top). Comparing the trend towards the precancerous state, all involved signal transduction pathways involved in the malignant process showed persistent activation (Fig. 9 B, bottom). Furthermore, pseudotime trajectory analysis of individual key genes regulating this signaling synergia pattern confirmed increased expression of DNMT1, AKT1, CDK2 and GSK3B during epithelial carcinogenesis, while the expression of the negative regulatory gene PTEN decreased (Fig. 9 D). Thus, the results from single-cell transcriptome analysis further confirmed the critical role of signal synergistic function pattern in oral carcinogenesis.
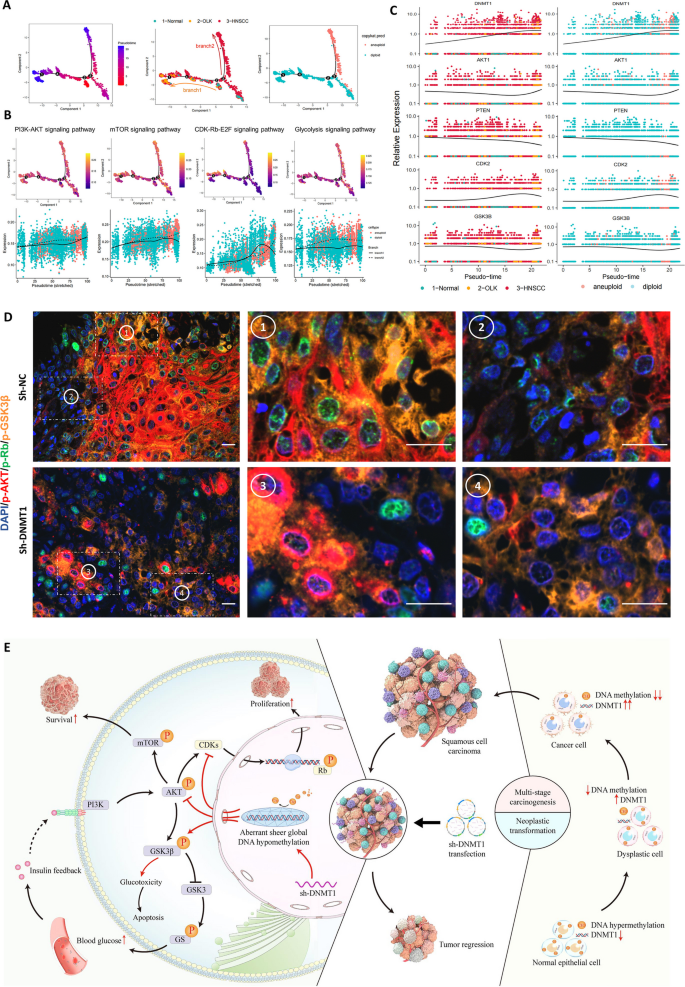
The DNMT1-mediated signaling synergy pattern and schematic in oral carcinogenesis and anticancer efficacy. A Pseudotime trajectory of epithelial cells, with each color coded for pseudotime (left), groups (middle), and copykat.pred (right) B Pseudotime trajectory analysis of the AUCell score of PI3K-AKT, mTOR, CDK-Rb-E2F and glycolysis signaling pathways. C Pseudotime trajectory analysis of the expression of DNMT1, PTEN, AKT1, CDK2 and GSK3B. D Representative mIHC staining images of DAPI, p-AKT, p-Rb and p-GSK3β in xenografted OSCC tumors. Scale bars, 20 μm. E Schematic indicating that the DNMT1- dependent global DNA methylation pattern functions in oral carcinogenesis and treatment of OSCC, as well as the collaborative signal transduction involved. Red arrows represent the effects of sh-DNMT1; black arrows represent natural biological processes. This schematic image was created by BioRender
In the xenografted tumors, we also utilized multiplex immunohistochemical technique to determine the colocalization of the core regulatory markers at the protein functional level (Fig. 9 D). In actively proliferative OSCC tumors, cancer cells exhibit high levels of p-AKT, p-Rb, and p-GSK3β. Most cancer cells showed frequent colocalization of these three markers, regardless of whether they were either highly or weakly expressed (Fig. 9 D, top). Upon DNMT1 targeting, the xenografted tumors bared smaller cancerous lesions scattered, with reduced expression of p-AKT and p-Rb but still extensive expression of p-GSK3β. In particular, the alterations in both p-Rb and p-GSK3β were independent of the changes in AKT phosphorylation (Fig. 9 D, bottom). These findings reaffirmed the potent regulatory role of DNMT1 in coordinating the multiple signals transduction.
After compiling the aforementioned findings, we present a schematic elucidating the role of DNMT1 in oral malignant transformation and in controlling tumor growth. As illustrated (Fig. 9 E), DNMT1 expression gradually rises increases in tandem with genome-wide DNA hypomethylation, which triggers the multistage carcinogenesis of OSCC. Targeting DNMT1 leads to the formation of an aberrant and widespread DNA hypomethylation state, inducing a specific signaling synergia. These pathways involve the dual inhibition of PI3K-AKT and CDK2-Rb, along with the inactivation of GSK3β, which regulates reduced cell proliferation and increased cell apoptosis, thus impeding tumor growth. Additionally, targeting DNMT1 potentially counteracts the pharmacological toxicity of hyperglycemia and insulin feedback stemming from PI3K inhibition. This process is activated by inducing supererogatory GSK3β inactivation, leading to sustained blockade of PI3K-AKT activation. All these processes represent a signaling synergy orchestrated by DNMT1, acting as a gatekeeper to effectively restrain tumor growth through enhancing efficiency and reducing toxicity.
Oral squamous cell carcinoma (OSCC) represents a highly heterogeneous and aggressive cancer type that undergoes a multistage neoplastic transformation process. This process encompasses genomic instability and epigenetic aberrations, leading to intricate alterations in gene expression, anomalies in signaling pathways, and changes in physiological functions [ 2 , 56 ]. Global DNA hypomethylation, a prominent feature of malignancies, is largely dependent on the stable maintenance by DNMT1 [ 17 , 57 , 58 ]. Building upon our precious prediction of DNMT1 as a potential marker of OSCC progression, this present study proposed that DNMT1 regulates oral carcinogenesis and OSCC growth through a novel mechanism involving the remodeling of specific global DNA methylation patterns to initiate multiple signaling collaborations. Our findings revealed that targeting DNMT1 in oral cancer cells resulted in near-complete genome-wide DNA hypomethylation, triggering the dual attenuation of PI3K-AKT and CDK2-Rb and collaborative phosphorylation of GSK3β. This cascade eventually produces a remarkable anticancer effect-enhancing and toxicity-reducing.
DNMT1 is commonly overexpressed in various cancers and is often linked to poor patient prognosis, as indicated by previous studies [ 59 , 60 , 61 , 62 ]. In our study, we have demonstrated a new discovery regarding DNMT1 expression, which progressively increased throughout epithelial multistage carcinogenesis. While OSCC tissues exhibited significantly increased DNMT1 expression, we observed a dynamic correlation between DNMT1 expression, mortality risk, and survival hazard ratio, taking into account complex clinical variables. Notably, relatively lower DNMT1 expression was associated with a more favorable prognosis in OSCC individuals. When DNMT1 was silenced or targeted with inhibitors, OSCC cells displayed quite hysteretic tumorigenic capacity and restricted tumor growth. These results provide compelling evidence that DNMT1 represents a potent target for controlling OSCC progression.
Host cells possess a specific mechanism known as global DNA methylation homeostasis to maintain the stability of their cellular genome, a process heavily dependent on the accurate maintenance carried out by DNMT1 [ 63 ]. In cancer cells, the phenomenon of genome-wide hypomethylation generally persists throughout biological processes but has a limited efficacy in maintenance, leading to a selective and imbalanced DNA methylation status during cell division [ 9 , 63 ]. Our findings confirmed that the progression of oral epithelial carcinogenesis is accompanied by a gradual decrease in DNA methylation, ultimately resulting in the genome-wide hypomethylation pattern observed in OSCC. Additionally, we investigated a undulatory link between global DNA hypomethylation and OSCC prognosis. A significant alteration in overall DNA methylation, either a substantial decrease or increase, may correlate with an improved survival rate. Unexpectedly, we observed a nearly complete hypomethylation pattern, remodeled by DNMT1 silencing, alongside effective suppression of cancer. This observation underscores the crucial role of DNMT1 in preserving the preexisting methylation pattern [ 15 , 63 ] and highlights that its inactivation can directly trigger dysregulation of global DNA methylation in OSCC. We also observed that the genome-wide methylation remodeling pattern specific to DNMT1 may not follow a restorative trajectory towards normal cells. Instead, it has the potential to significantly disrupt DNA methylation homeostasis during DNA replication, ultimately impeding the proliferation of cancer cells or inducing cell death. Besides, the coexistence of DNMT1 overexpression and genome-wide hypomethylation in oral cancer cells may reflect that, given the low maintenance efficacy of DNA methylation [ 63 ], the upregulation of DNMT1 functions as a self-compensatory mechanism to maintain DNA methylation equilibrium.
In addition to a sheer decline in genome-wide DNA methylation level, we observed a striking expansion in the distribution of hypomethylated CpG sites, particularly at CpG island shores and within gene body regions. These regions are crucial functional components involved in gene expression regulation [ 44 ]. Some scholars have noted that most tissue-specific or cancer-associated DNA methylation changes tend to occur at CpG island shores [ 64 ], although the precise mechanism underlying these changes has yet to be fully elucidated. On the other hand, the disruption of global DNA methylation caused by DNMT1 alteration triggered a series of biological processes and changes in signal transduction, which were sufficient to change the malignant behavior of OSCC cells. In this scenario, the PI3K-AKT signaling pathway is strongly activated. Our study confirmed the promotion of its activation in conjunction with epithelial carcinogenesis, as well as its inhibition following DNMT1 knockdown, resulting in decreased phosphorylation of AKT and mTOR. This finding aligns with reports suggesting that DNMT1 overexpression could activate the PI3K-AKT signaling pathway to promote melanoma development [ 65 , 66 ]. However, it's noteworthy that the total protein expression of PI3K, AKT and mTOR remained unaltered despite the reconstructed DNA methylation pattern. This observation is reminiscent of findings in Arabidopsis , where ectopic DNA methylation mediated by the bacterial SssI methyltransferase had little effect on transcription, despite the hypothesized link between modified DNA methylation at the gene body and gene expression [ 67 ].
The current study unveils an intriguing discovery indicating that DNMT1 knockdown more strongly inhibits tumor growth than does inhibition of the PI3K-AKT pathway alone. This finding implies that DNMT1 play a role in regulating other signaling pathways associated with oncogenes or cancer suppressor genes. CDK2, known for its crucial involvement in the cell cycle process, has emerged as a promising therapeutic target for treating cancer [ 68 ]. In this research, the concurrent suppression of the CDK2-Rb signaling pathway was confirmed following DNMT1 knockdown. This was evidenced by the reduction in phosphorylation levels of CDK1/2/3 and Rb proteins in sh-DNMT1 cancer cells. Activation of this pathway, indicated by increased p-Rb, was observed as tissues progressed from normal to dysplastic and OSCC tissues, highlighting its function in oral carcinogenesis. Furthermore, our research elucidated the tumor-promoting function of PI3K-AKT and CDK2-Rb activation, both of which can be suppressed by DNMT1-specific DNA hypomethylation. The interaction between PI3K-AKT and CDK2-Rb is indisputably advantageous for cancer cell growth. For instance, activated AKT can phosphorylate CDK inhibitors such as p21 [ 69 ] and p27 [ 70 ] to further activate Rb transcription, thus facilitating cell proliferation. In the context of DNMT1-mediated global DNA hypomethylation, our study put forth a proposal that the concurrent inhibition of these two pathways is partially independent. This is supported by the finding that the administration of a PI3K agonist did not fully restore CDK2-Rb activation. The observation of nonoverlapping patterns for immunofluorescent p-AKT and p-Rb in DNMT1-knockdown tissues provides additional evidence supporting this perspective.
Moreover, in xenografted OSCC tumors, both dual inhibition of PI3K and CDK2, as well as DNMT1 knockdown, resulted in an extra elevation of p-GSK3β ser9, leading to the blockade of GSK3β activation and promoting more glycogen storage [ 51 , 52 ]. While increased glycogen synthesis and glycogenolysis in cancer cells are typically associated with cancer progression [ 71 ], this study revealed unique findings regarding DNMT1-mediated glycogen deposition. Interestingly, DNMT1-mediated glycogen deposition was abnormally located in apoptotic cells around necrotic tumor areas, accompanied by enhanced glycolysis. These results demonstrate an exceptional mechanism by which glucotoxicity that promotes apoptosis, which is closely linked to DNMT1-induced GSK3β inactivation. Notably, under certain tumor-treating conditions, inhibited glycogen clustering has been shown to attenuate the anticancer effect [ 72 ]. Additionally, proliferating cancer cells generally do not exhibit PKM2-mediated glycolysis [ 73 ]. These findings provide some indirect support for the results obtained in this study but remain to be explored further.
PI3K inhibitors used in cancer treatment often induce hyperglycemia, triggering insulin feedback mechanisms that diminish their efficacy in treating cancer [ 54 , 55 ]. GSK3β, a key regulator in the insulin receptor signaling pathway for the regulation of blood glucose levels, plays a crucial role in this process [ 74 ]. We observed that mice orally administered with a PI3K inhibitor exhibited elevated blood glucose levels accompanied by a corresponding increase in serum insulin levels as a feedback response. This finding is likely due to a biochemical mechanism whereby the inactivation of AKT inhibits the phosphorylation of GSK3β ser9 [ 75 ]. In the context of biological insulin feedback derived from hyperglycemia, liver glycogen synthesis increases through elevated phosphorylation of GSK3β, contributing to the recovery of normal glycemic homeostasis. Remarkably, we found that DNMT1 silencing led to a compensatory increase in p-GSK3β levels, as did the sustained suppression of PI3K-AKT activation. This combination may contribute to the maintenance of stable blood glucose levels. Conversely, additional inhibition of CDK2-Rb partially reactivated PI3K-AKT signaling, leading to an excessive reduction in blood glucose. These cumulative results suggest that DNMT1 has the capacity to concurrently and accurately modulate the signal transduction of PI3K-AKT, CDK2-Rb, and GSK3β- mediated glycogen metabolism. This mechanism contributes to the establishment of signaling synergia and an inherent balance governing cancer behavior, thereby improving anticancer effects and preventing adverse effects resulting from intercommunication between these pathways.
In summary, our study provides comprehensive data demonstrating that precise DNMT1-targeting disrupts global DNA methylation, forming as a vital approach to facilitate anticancer efficacy while minimizing potential toxic effects arising from signal crosstalk in targeted therapy. Mechanistically, we propose a functional model wherein DNMT1-remodeled genome-wide DNA hypomethylation patterns regulate oral malignant transformation and tumor growth, through signal collaborations involving PI3K-AKT, CDK2-Rb and GSK3β-mediated glycogen metabolism. Our findings suggest that targeted intervention against DNMT1 using compounds, biomaterials or nanomedicines holds promise as an alternative approach for OSCC therapy, regarding its pivotal role as a signal gatekeeper. Further investigations are warranted to discover how DNMT1 remodels specific DNA hypomethylation patterns, which will contribute to a deeper understanding of the underlying epigenetic mechanism driving OSCC progression. This research direction holds potential for identifying novel therapeutic targets and improving treatment outcomes in OSCC patients.
In this study, we precisely simulated DNMT1-targeted interventions in cancer cells and validated their potent efficacy in suppressing tumor growth in an OSCC mouse model. Mechanistically, DNMT1 inhibition led to a reshaping of the genome-wide DNA hypomethylation pattern, which hindered the dual activation of PI3K-AKT and CDK2-Rb while inducing GSK3β inactivation. Compared to PI3K inhibitors, DNMT1 targeting demonstrated superior in vivo tumor suppression, mitigating the toxic effects of blood glucose variation caused by PI3K and PI3K-CDK inhibitor combinations. Analysis of human samples revealed a correlation between oral malignant transformation, elevated DNMT1 expression, and accumulating cancer-specific DNA hypomethylation. This was associated with collaborative signal transduction involving the PI3K-AKT, CDK2-Rb, and GSK3β pathways. DNMT1 targeting not only remodels the genome-wide DNA hypomethylation pattern, but also achieves enhanced anticancer efficacy and reduced toxicity by equilibrating signaling synergia. Our research highlights DNMT1 as a gatekeeper in determining OSCC destiny and treatment outcome, confirming its potential as a useful therapeutic target for OSCC. These findings contribute to a deeper understanding of the molecular mechanisms underlying OSCC progression and provide a basis for the development of more effective and targeted therapeutic strategies against this malignancy.
Availability of data and materials
All the data needed to evaluate the conclusions in the article are presented in the article and/or the Supplementary Materials. The original data and materials used in the current study are available from the corresponding authors upon reasonable request.
Abbreviations
- Oral squamous cell carcinoma
DNA methyltransferase 1
Cleaved Caspase-3
Phospho fructo kinase
Immunohistochemistry
Immunofluorescence
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Periodic acid-schiff
The Cancer Genome Atlas
Oral leukoplakia
Principal component analysis
Multidimensional scaling
Whole-genome amplification
Subset-quantile Within Array Normalization
Differential DNA methylation sites
False discovery rate/ Adjusted-P value
Gene Expression Omnibus
Differential DNA methylation genes
Differential expression genes
Kyoto Encyclopedia of Genes and Genomes
Gene Ontology
Fluorescein isothiocyanate
4′ 6-Diamidino-2-phenylindole
5-Methylcytosine
Retinoblastoma protein
RNA sequencing
Single-cell RNA sequencing
Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021;398:2289–99.
Article PubMed Google Scholar
Luo JJ, Young CD, Zhou HM, Wang XJ. Mouse models for studying oral cancer: impact in the era of cancer immunotherapy. J Dent Res. 2018;97:683–90.
Article CAS PubMed PubMed Central Google Scholar
Chang MS, Azin M, Demehri S. Cutaneous squamous cell carcinoma: the frontier of cancer immunoprevention. Annu Rev Pathol. 2022;17:101–19.
Article CAS PubMed Google Scholar
Kaidar-Person O, Gil Z, Billan S. Precision medicine in head and neck cancer. Drug Resist Updat. 2018;40:13–6.
Qi Z, Qiu Y, Wang Z, Zhang H, Lu L, Liu Y, Mathes D, Pomfret EA, Gao D, Lu SL, Wang Z. A novel diphtheria toxin-based bivalent human EGF fusion toxin for treatment of head and neck squamous cell carcinoma. Mol Oncol. 2021;15:1054–68.
Redman JM, Friedman J, Robbins Y, Sievers C, Yang X, Lassoued W, et al. Enhanced neoepitope-specific immunity following neoadjuvant PD-L1 and TGF-beta blockade in HPV-unrelated head and neck cancer. J Clin Invest. 2022;132(18):e161400.
Willey CD, Anderson JC, Trummell HQ, Naji F, de Wijn R, Yang ES, Bredel M, Thudi NK, Bonner JA. Differential escape mechanisms in cetuximab-resistant head and neck cancer cells. Biochem Biophys Res Commun. 2019;517:36–42.
Jiang Z, Lim SO, Yan M, Hsu JL, Yao J, Wei Y, et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest. 2021;131(8):e139434.
Endicott JL, Nolte PA, Shen H, Laird PW. Cell division drives DNA methylation loss in late-replicating domains in primary human cells. Nat Commun. 2022;13:6659.
Mori K, Hamada T, Beppu M, Tsuchihashi H, Goto Y, Kume K, et al. Detecting early-stage oral cancer from clinically diagnosed oral potentially malignant disorders by DNA methylation profile. Cancers (Basel). 2022;14(11):2646.
Calanca N, Francisco ALN, Bizinelli D, Kuasne H, Barros Filho MC, Flores BCT, Pinto CAL, Rainho CA, Soares MBP, Marchi FA, et al. DNA methylation-based depiction of the immune microenvironment and immune-associated long non-coding RNAs in oral cavity squamous cell carcinomas. Biomed Pharmacother. 2023;167:115559.
Carter B, Zhao K. The epigenetic basis of cellular heterogeneity. Nat Rev Genet. 2021;22:235–50.
Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat Rev Genet. 2018;19:81–92.
Zhang H, Gao Q, Tan S, You J, Lyu C, Zhang Y, Han M, Chen Z, Li J, Wang H, et al. SET8 prevents excessive DNA methylation by methylation-mediated degradation of UHRF1 and DNMT1. Nucleic Acids Res. 2019;47:9053–68.
CAS PubMed PubMed Central Google Scholar
Stankevicius V, Gibas P, Masiulionyte B, Gasiule L, Masevicius V, Klimasauskas S, Vilkaitis G. Selective chemical tracking of Dnmt1 catalytic activity in live cells. Mol Cell. 2022;82:1053–65. e1058.
Estève PO, Chang Y, Samaranayake M, Upadhyay AK, Horton JR, Feehery GR, Cheng X, Pradhan S. A methylation and phosphorylation switch between an adjacent lysine and serine determines human DNMT1 stability. Nat Struct Mol Biol. 2011;18:42–8.
Di Ruscio A, Ebralidze AK, Benoukraf T, Amabile G, Goff LA, Terragni J, Figueroa ME, De Figueiredo Pontes LL, Alberich-Jorda M, Zhang P, et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature. 2013;503:371–6.
Article PubMed PubMed Central Google Scholar
Meng W, Wu Y, He X, Liu C, Gao Q, Ge L, Wu L, Liu Y, Guo Y, Li X, et al. A systems biology approach identifies effective tumor-stroma common targets for oral squamous cell carcinoma. Cancer Res. 2014;74:2306–15.
Liu YY, Ding CZ, Chen JL, Wang ZS, Yang B, Wu XM. A novel small molecular inhibitor of DNMT1 enhances the antitumor effect of radiofrequency ablation in lung squamous cell carcinoma cells. Front Pharmacol. 2022;13:863339.
Dongoran RA, Wang KH, Lin TJ, Yuan TC, Liu CH. Anti-proliferative effect of statins is mediated by DNMT1 inhibition and p21 expression in OSCC cells. Cancers (Basel). 2020;12(8):2084.
Yang SC, Wang WY, Zhou JJ, Wu L, Zhang MJ, Yang QC, Deng WW, Sun ZJ. Inhibition of DNMT1 potentiates antitumor immunity in oral squamous cell carcinoma. Int Immunopharmacol. 2022;111:109113.
Babar Q, Saeed A, Tabish TA, Pricl S, Townley H, Thorat N. Novel epigenetic therapeutic strategies and targets in cancer. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166552.
Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22:138.
Wright NE, Mandal M, Clark MR. Molecular mechanisms insulating proliferation from genotoxic stress in B lymphocytes. Trends Immunol. 2023;44:668–77.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541.
Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395–417.
He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW, Li B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6:425.
Bury M, Le Calve B, Ferbeyre G, Blank V, Lessard F. New Insights into CDK regulators: novel opportunities for cancer therapy. Trends Cell Biol. 2021;31:331–44.
Mattei AL, Bailly N, Meissner A. DNA methylation: a historical perspective. Trends Genet. 2022;38:676–707.
Liu Y, Wu Y, Yang M, Yang J, Tong R, Zhao W, Wu F, Tian Y, Li X, Luo J, Zhou H. Ionizing radiation-induced “zombie” carcinoma-associated fibroblasts with suppressed pro-radioresistance on OSCC cells. Oral Dis. 2023;29:563–73.
Shi X, Luo J, Weigel KJ, Hall SC, Du D, Wu F, Rudolph MC, Zhou H, Young CD, Wang XJ. Cancer-associated fibroblasts facilitate squamous cell carcinoma lung metastasis in mice by providing TGFbeta-mediated cancer stem cell niche. Front Cell Dev Biol. 2021;9:668164.
Luo J, Bian L, Blevins MA, Wang D, Liang C, Du D, Wu F, Holwerda B, Zhao R, Raben D, et al. Smad7 promotes healing of radiotherapy-induced oral mucositis without compromising oral cancer therapy in a xenograft mouse model. Clin Cancer Res. 2019;25:808–18.
Wang Y, Bai X, Guo X, Gao X, Chen Y, Li H, Fan W, Han C. Bioinformatics analysis combined with clinical sample screening reveals that leptin may be a biomarker of preeclampsia. Front Physiol. 2022;13:1031950.
Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–40.
Wang S, Wang X, Sun J, Yang J, Wu D, Wu F, Zhou H. Down-regulation of DNA key protein-FEN1 inhibits OSCC growth by affecting immunosuppressive phenotypes via IFN-gamma/JAK/STAT-1. Int J Oral Sci. 2023;15:17.
Choi JH, Lee BS, Jang JY, Lee YS, Kim HJ, Roh J, Shin YS, Woo HG, Kim CH. Single-cell transcriptome profiling of the stepwise progression of head and neck cancer. Nat Commun. 2023;14:1055.
Gao R, Bai S, Henderson YC, Lin Y, Schalck A, Yan Y, Kumar T, Hu M, Sei E, Davis A, et al. Delineating copy number and clonal substructure in human tumors from single-cell transcriptomes. Nat Biotechnol. 2021;39:599–608.
Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14:979–82.
Pappalardi MB, Keenan K, Cockerill M, Kellner WA, Stowell A, Sherk C, Wong K, Pathuri S, Briand J, Steidel M, et al. Discovery of a first-in-class reversible DNMT1-selective inhibitor with improved tolerability and efficacy in acute myeloid leukemia. Nat Cancer. 2021;2:1002–17.
Azevedo Portilho N, Saini D, Hossain I, Sirois J, Moraes C, Pastor WA. The DNMT1 inhibitor GSK-3484862 mediates global demethylation in murine embryonic stem cells. Epigenetics Chromatin. 2021;14:56.
Van Tongelen A, Loriot A, De Smet C. Oncogenic roles of DNA hypomethylation through the activation of cancer-germline genes. Cancer Lett. 2017;396:130–7.
Chen Z, Zhang Y. Role of Mammalian DNA Methyltransferases in Development. Annu Rev Biochem. 2020;89:135–58.
Nishiyama A, Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021;37:1012–27.
Arechederra M, Daian F, Yim A, Bazai SK, Richelme S, Dono R, Saurin AJ, Habermann BH, Maina F. Hypermethylation of gene body CpG islands predicts high dosage of functional oncogenes in liver cancer. Nat Commun. 2018;9:3164.
Skvortsova K, Masle-Farquhar E, Luu PL, Song JZ, Qu W, Zotenko E, Gould CM, Du Q, Peters TJ, Colino-Sanguino Y, et al. DNA hypermethylation encroachment at CpG island borders in cancer is predisposed by H3K4 monomethylation patterns. Cancer Cell. 2019;35:297–314. e298.
Soulieres D, Faivre S, Mesia R, Remenar E, Li SH, Karpenko A, Dechaphunkul A, Ochsenreither S, Kiss LA, Lin JC, et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18:323–35.
Jimeno A, Shirai K, Choi M, Laskin J, Kochenderfer M, Spira A, Cline-Burkhardt V, Winquist E, Hausman D, Walker L, Cohen RB. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann Oncol. 2015;26:556–61.
Tadesse S, Anshabo AT, Portman N, Lim E, Tilley W, Caldon CE, Wang S. Targeting CDK2 in cancer: challenges and opportunities for therapy. Drug Discov Today. 2020;25:406–13.
Yang C, Wang M, Gong Y, Deng M, Ling Y, Li Q, Wang J, Zhou Y. Discovery and identification of a novel PI3K inhibitor with enhanced CDK2 inhibition for the treatment of triple negative breast cancer. Bioorg Chem. 2023;140:106779.
Fang G, Zhang P, Liu J, Zhang X, Zhu X, Li R, Wang H. Inhibition of GSK-3β activity suppresses HCC malignant phenotype by inhibiting glycolysis via activating AMPK/mTOR signaling. Cancer Lett. 2019;463:11–26.
Elgendy M, Cirò M, Hosseini A, Weiszmann J, Mazzarella L, Ferrari E, Cazzoli R, Curigliano G, DeCensi A, Bonanni B, et al. Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3β-MCL-1 axis. Cancer Cell. 2019;35:798–815.e795.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9.
Jere SW, Houreld NN, Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3beta) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019;50:52–9.
Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, Amadiume SC, Goncalves MD, Hodakoski C, Lundquist MR, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560:499–503.
Kishikawa T, Higuchi H, Wang L, Panch N, Maymi V, Best S, et al. WWP1 inactivation enhances efficacy of PI3K inhibitors while suppressing their toxicities in breast cancer models. J Clin Invest. 2021;131(24):e140436.
Chadwick JW, Macdonald R, Ali AA, Glogauer M, Magalhaes MA. TNFalpha Signaling is increased in progressing oral potentially malignant disorders and regulates malignant transformation in an oral carcinogenesis model. Front Oncol. 2021;11:741013.
Chattopadhyaya S, Ghosal S. DNA methylation: a saga of genome maintenance in hematological perspective. Hum Cell. 2022;35:448–61.
Hoang NM, Rui L. DNA methyltransferases in hematological malignancies. J Genet Genomics. 2020;47:361–72.
Barcena-Varela M, Caruso S, Llerena S, Alvarez-Sola G, Uriarte I, Latasa MU, Urtasun R, Rebouissou S, Alvarez L, Jimenez M, et al. Dual Targeting of histone methyltransferase G9a and DNA-Methyltransferase 1 for the treatment of experimental hepatocellular carcinoma. Hepatology. 2019;69:587–603.
Li Z, Li B, Yu H, Wang P, Wang W, Hou P, Li M, Chu S, Zheng J, Mao L, Bai J. DNMT1-mediated epigenetic silencing of TRAF6 promotes prostate cancer tumorigenesis and metastasis by enhancing EZH2 stability. Oncogene. 2022;41:3991–4002.
Liu H, Song Y, Qiu H, Liu Y, Luo K, Yi Y, Jiang G, Lu M, Zhang Z, Yin J, et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020;27:966–83.
Xing J, Stewart DJ, Gu J, Lu C, Spitz MR, Wu X. Expression of methylation-related genes is associated with overall survival in patients with non-small cell lung cancer. Br J Cancer. 2008;98:1716–22.
Ming X, Zhang Z, Zou Z, Lv C, Dong Q, He Q, Yi Y, Li Y, Wang H, Zhu B. Kinetics and mechanisms of mitotic inheritance of DNA methylation and their roles in aging-associated methylome deterioration. Cell Res. 2020;30:980–96.
Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–92.
Yang Y, Ma S, Ye Z, Zheng Y, Zheng Z, Liu X, Zhou X. Oncogenic DNA methyltransferase 1 activates the PI3K/AKT/mTOR signalling by blocking the binding of HSPB8 and BAG3 in melanoma. Epigenetics. 2023;18:2239607.
Sun L, Zhao H, Xu Z, Liu Q, Liang Y, Wang L, Cai X, Zhang L, Hu L, Wang G, Zha X. Phosphatidylinositol 3-kinase/protein kinase B pathway stabilizes DNA methyltransferase I protein and maintains DNA methylation. Cell Signal. 2007;19:2255–63.
Liu W, Gallego-Bartolome J, Zhou Y, Zhong Z, Wang M, Wongpalee SP, Gardiner J, Feng S, Kuo PH, Jacobsen SE. Ectopic targeting of CG DNA methylation in Arabidopsis with the bacterial SssI methyltransferase. Nat Commun. 2021;12:3130.
Zhang J, Gan Y, Li H, Yin J, He X, Lin L, Xu S, Fang Z, Kim BW, Gao L, et al. Inhibition of the CDK2 and Cyclin A complex leads to autophagic degradation of CDK2 in cancer cells. Nat Commun. 2022;13:2835.
Gesbert F, Sellers WR, Signoretti S, Loda M, Griffin JD. BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-Kinase/AKT pathway. J Biol Chem. 2000;275:39223–30.
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–52.
Curtis M, Kenny HA, Ashcroft B, Mukherjee A, Johnson A, Zhang Y, Helou Y, Batlle R, Liu X, Gutierrez N, et al. Fibroblasts mobilize tumor cell glycogen to promote proliferation and metastasis. Cell Metab. 2019;29:141–155.e149.
Fan H, He Y, Xiang J, Zhou J, Wan X, You J, Du K, Li Y, Cui L, Wang Y, et al. ROS generation attenuates the anti-cancer effect of CPX on cervical cancer cells by inducing autophagy and inhibiting glycophagy. Redox Biol. 2022;53:102339.
Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397–409.
Pecoraro C, Faggion B, Balboni B, Carbone D, Peters GJ, Diana P, Assaraf YG, Giovannetti E. GSK3beta as a novel promising target to overcome chemoresistance in pancreatic cancer. Drug Resist Updat. 2021;58:100779.
Mulholland DJ, Dedhar S, Wu H, Nelson CC. PTEN and GSK3beta: key regulators of progression to androgen-independent prostate cancer. Oncogene. 2006;25:329–37.
Download references
Acknowledgements
We wish to extend our appreciation to LetPub for its language editing service. We would also like to express our gratitude to Curie, an AI-driven writing assistant specializing in academic papers, endorsed by the official website of Molecular Cancer for its meticulous language editing of our article. Moreover, we are thankful to Mr. Fred Richardson, a trusted American friend and skilled writer with a profound understanding of medical terminology, whose contributions have greatly improved the language quality of our article. Finally, we thank for AJE Grammar Check to evaluate and ensure the quality of our language usage.
This work was supported by the National Natural Science Foundation of China (82071124, 82001061, 82101028 and U20A20365), the Natural Science Foundation of Sichuan Province (2022NSFSC1377), and Research Funding from West China Hospital of Stomatology, Sichuan University (RD-02-202208, RD-03-202410 and RCDWJS2024-9).
Author information
Yangfan Liu, Yu Sun and Jin Yang contributed equally to this work.
Authors and Affiliations
State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases & Frontier Innovation Center for Dental Medicine Plus, West China Hospital of Stomatology, Sichuan University, Chengdu, 610041, Sichuan, China
Yangfan Liu, Yu Sun, Jin Yang, Deyang Wu, Shuang Yu, Junjiang Liu, Tao Hu, Jingjing Luo & Hongmei Zhou
School of Stomatology, Hainan Medical University, Haikou, 571199, Hainan, China
You can also search for this author in PubMed Google Scholar
Contributions
H.Z., T.H. and J.Luo put forward ideas and designed the research. Y.L., Y.S., and J.Luo conducted the research and analyzed the data. Y.L. and J.Luo wrote the first draft. Y.L., J.Y., D.W. and S.Y. compiled the data and figures. J.Y. and J.Liu provided method and technical support. Y.L., J.Luo, J.Y. and Y.S. reviewed and revised all versions of the draft. H.Z., J.Luo and J.Y. edited and revised the final manuscript. H.Z., T.H., J.Luo and J.Y. provided essential reagents and materials. All the authors have read and approved the final manuscript.
Corresponding authors
Correspondence to Jingjing Luo or Hongmei Zhou .
Ethics declarations
Ethics approval and consent to participate.
All harvesting of human tissues was conducted with informed consent from the patients at West China Hospital of Stomatology, Sichuan University, and was approved by the Human Research Ethics Committee of West China Hospital of Stomatology, Sichuan University (No. WCHSIRB-D-2021–548). All animal experiments and procedures were approved and performed in accordance with the Animal Care and Use Ethics Committee of West China Hospital of Stomatology, Sichuan University (No. WCHSIRB-D-2021–628).
Consent for publication
All the authors provided consent for the publication of the manuscript in the journal Molecular Cancer .
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, Y., Sun, Y., Yang, J. et al. DNMT1-targeting remodeling global DNA hypomethylation for enhanced tumor suppression and circumvented toxicity in oral squamous cell carcinoma. Mol Cancer 23 , 104 (2024). https://doi.org/10.1186/s12943-024-01993-1
Download citation
Received : 19 December 2023
Accepted : 03 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1186/s12943-024-01993-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- DNA methylation
- Tumor growth
- Neoplastic transformation
- Pharmacological toxicity
- Insulin feedback
Molecular Cancer
ISSN: 1476-4598
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Figure 9.2.1 9.2. 1: The two strands of DNA are complementary, meaning the sequence of bases in one strand can be used to create the correct sequence of bases in the other strand. Because of the complementarity of the two strands, having one strand means that it is possible to recreate the other strand.
DNA replication has been extremely well-studied in prokaryotes, primarily because of the small size of the genome and large number of variants available. Escherichia coli has 4.6 million base pairs in a single circular chromosome, and all of it gets replicated in approximately 42 minutes, starting from a single origin of replication and ...
DNA replication is semiconservative, meaning that each strand in the DNA double helix acts as a template for the synthesis of a new, complementary strand. This process takes us from one starting molecule to two "daughter" molecules, with each newly formed double helix containing one new and one old strand.
ADVERTISEMENTS: In this essay we will discuss about:- 1. Definition of DNA Replication 2. Mechanism of DNA Replication 3. Evidences for Semi-Conservative DNA Replication 4. Models for Replication of Prokaryotic DNA. Essay # Definition of DNA Replication: DNA replicates by "unzipping" along the two strands, breaking the hydrogen bonds which link the pairs of nucleotides. […]
Figure 14.3C. 1 14.3 C. 1: Replication Fork Formation: A replication fork is formed by the opening of the origin of replication; helicase separates the DNA strands. An RNA primer is synthesized by primase and is elongated by the DNA polymerase. On the leading strand, only a single RNA primer is needed, and DNA is synthesized continuously ...
which replication generates a double helix in which both strands are newly synthesized. Ergo, DNA replication is semi-conservative. DNA is synthesized by the repetitive addition of nucleotides to the 3' end of the growing polynucleotide chain DNA is synthesized by an iterative process in which nucleotides are added
The replication process follows several steps involving multiple proteins called replication enzymes and RNA, or ribonucleic acid. In eukaryotic cells, such as animal cells and plant cells, DNA replication occurs in the S phase of the cell cycle. Before this phase, also known as the synthesis stage, the cell passes through a preparation phase ...
Introduction. DNA synthesis occurs during the S phase of the cell cycle and is ensured by the replisome, a molecular machine made of a large number of proteins acting in a coordinated manner to synthesize DNA at many genomic locations, the replication origins 1.Replication origin activation in space and time (or replication program) is set by a sequence of events, starting already at the end ...
replication. DNA replication is the process by which a double-stranded DNA molecule is copied to produce two identical DNA molecules. Replication is an essential process because, whenever a cell ...
Now imagine copying something 1000x larger! With that in mind, it is worth noting that a human cell can take about 24 hours to divide (DNA replication must therefore be a little faster). A healthy E. coli cell may take only 20 minutes to divide (including replicating its ~4.5 million base pair genome).
The existence of cell division implies that there is a mechanism that replicates DNA and supplies identical copies for the daughter cells while still maintaining an accurate representation of the genome. This mechanism, known as DNA replication, occurs in all organisms and allows for genetic inheritance. It can occur in a short period, copying up to approximately ten to the 11th power (10^11 ...
Errors Are a Natural Part of DNA Replication. After James Watson and Francis Crick published their model of the double-helix structure of DNA in 1953, biologists initially speculated that most ...
Summary: DNA replication takes place in three major steps. Opening of the double-stranded helical structure of DNA and separation of the strands. Priming of the template strands. Assembly of the newly formed DNA segments. During the separation of DNA, the two strands uncoil at a specific site known as the origin.
Accurate DNA replication is modulated by multiple replication‐associated proteins, which is fundamental to preserve genome stability. Abundant replication proteins are involved in tumorigenesis and development, implying these proteins act as therapeutic targets in clinical. Replication‐target cancer therapy emerges as the times require.
DNA replication occurs before the cell divides. DNA replicates itself during the S phase of the cell cycle so that each daughter cells has a copy of the DNA after cell division. DNA replication mean that parents can pass their DNA to their offspring. This passing of DNA and the genetic information stored in DNA is known as " Genetic ...
The configuration of the DNA molecule is highly stable, allowing it to act as a template for the replication of new DNA molecules, as well as for the production (transcription) of the related RNA (ribonucleic acid) molecule.A segment of DNA that codes for the cell's synthesis of a specific protein is called a gene.. DNA replicates by separating into two single strands, each of which serves ...
DNA replication is the biological process by which an exact copy of a deoxyribonucleic acid (DNA) molecule is created and it is the basis for biological inheritance. Each of the two strands of the ...
2) coat the single strands of DNA near the replication fork to prevent the single-stranded DNA from winding back into a double helix. Figure 13.1.1 13.1. 1: DNA replication in prokaryotes, which have one circular chromosome. The next important enzyme is DNA polymerase III, also known as DNA pol III, which adds nucleotides one by one to the ...
Working with Molecular Genetics Chapter 6, DNA Replication_2, Control analogy. Imagine that 20 students are writing essays using word processors set to use black letters. They are all at different stages of completing their papers, and they are not revising or editing their essays - just writing them from start to finish.
The first stage of DNA replication in prokaryotes is the uncoiling of the DNA double helix by the enzyme helicase. Helicase separates the DNA into two template strands. RNA primase then adds a short sequence of RNA to the template strands. This short sequence of RNA is a primer which allows DNA polymerase III to bind to the strands and start ...
DNA replication is conservative, because one resulting molecule is identical to the original and the other consists of two new strands. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class ...
The statement concerning DNA replication being a semiconservative process that leads to the development of two separate strands of DNA material has been supported by a vast range of evidence. Recent experiments point to the correctness of the semiconservative framework as the most legitimate theory that allows describing the process of DNA ...
One of the most important concepts of DNA replication is that it is a semi-conservative process (Figure 7.2.7 7.2. 7 ). This means that every double helix in the new generation of an organism consists of one complete "old" strand and one complete "new" strand wrapped around each other. This is in contrast to the two other possible ...
The faithful maintenance of DNA methylation homeostasis indispensably requires DNA methyltransferase 1 (DNMT1) in cancer progression. We previously identified DNMT1 as a potential candidate target for oral squamous cell carcinoma (OSCC). However, how the DNMT1- associated global DNA methylation is exploited to regulate OSCC remains unclear. The shRNA-specific DNMT1 knockdown was employed to ...