- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- State-of-the-Art Reviews
- Voices of ID
- Author Guidelines
- Open Access
- Why Publish
- IDSA Journals Calls for Papers
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Acknowledgments.
- < Previous
Clinical Presentation of Nipah Virus Infection in Bangladesh
- Article contents
- Figures & tables
- Supplementary Data
M. Jahangir Hossain, Emily S. Gurley, Joel M. Montgomery, Michael Bell, Darin S. Carroll, Vincent P. Hsu, P. Formenty, A. Croisier, E. Bertherat, M. A. Faiz, Abul Kalam Azad, Rafiqul Islam, M. Abdur Rahim Molla, Thomas G. Ksiazek, Paul A. Rota, James A. Comer, Pierre E. Rollin, Stephen P. Luby, Robert F. Breiman, Clinical Presentation of Nipah Virus Infection in Bangladesh, Clinical Infectious Diseases , Volume 46, Issue 7, 1 April 2008, Pages 977–984, https://doi.org/10.1086/529147
- Permissions Icon Permissions
Background . In Bangladesh, 4 outbreaks of Nipah virus infection were identified during the period 2001–2004.
Methods . We characterized the clinical features of Nipah virus-infected individuals affected by these outbreaks. We classified patients as having confirmed cases of Nipah virus infection if they had antibodies reactive with Nipah virus antigen. Patients were considered to have probable cases of Nipah virus infection if they had symptoms consistent with Nipah virus infection during the same time and in the same community as patients with confirmed cases.
Results . We identified 92 patients with Nipah virus infection, 67 (73%) of whom died. Although all age groups were affected, 2 outbreaks principally affected young persons (median age, 12 years); 62% of the affected persons were male. Fever, altered mental status, headache, cough, respiratory difficulty, vomiting, and convulsions were the most common signs and symptoms; clinical and radiographic features of acute respiratory distress syndrome of Nipah illness were identified during the fourth outbreak. Among those who died, death occurred a median of 6 days (range, 2–36 days) after the onset of illness. Patients who died were more likely than survivors to have a temperature >37.8eg;C, altered mental status, difficulty breathing, and abnormal plantar reflexes. Among patients with Nipah virus infection who had well-defined exposure to another patient infected with Nipah virus, the median incubation period was 9 days (range, 6–11 days).
Conclusions . Nipah virus infection produced rapidly progressive severe illness affecting the central nervous and respiratory systems. Clinical characteristics of Nipah virus infection in Bangladesh, including a severe respiratory component, appear distinct from clinical characteristics reported during earlier outbreaks in other countries.
Nipah virus is a recently identified paramyxovirus that is closely related to Hendra virus [ 1 ]. The first recognized outbreaks of Nipah virus illness in humans occurred in Malaysia and Singapore from September 1998 through June 1999; 283 persons, mostly pig farm and abattoir workers were infected through contact with sick pigs [ 2–6 ]. A case-fatality rate of 40% was observed in Malaysia and Singapore; patients presented primarily with CNS symptoms [ 2 , 7 , 8 ]. A second outbreak of Nipah virus infection, with a case-fatality rate of 68%, occurred from January through February 2001 in Siliguri, India, a town close to the northern border of Bangladesh. Patients affected by this outbreak presented with both encephalitis and respiratory symptoms [ 9 ].
In Bangladesh, 7 outbreaks of Nipah virus infection were identified during the period 2001–2007. In Bangladesh, Nipah virus infection was associated with contact with a sick cow, consumption of fresh date palm sap (potentially contaminated with pteropid bat saliva), and person-to-person transmission [ 10–12 ]. The Malaysian outbreak was associated with a single strain [ 13 ]. By contrast, viruses isolated in Bangladesh represent diverse strains [ 14 ]. Hypothetically, a single strain of Nipah virus could result in a narrower range of clinical presentations than those found during epidemics associated with genetically diverse strains. Thus, Nipah virus illnesses occurring in Bangladesh potentially provide insight into broader clinical manifestations of Nipah virus infection. We describe the clinical presentation of 92 Nipah virus-infected patients identified during the first 4 outbreaks in Bangladesh during the period 2001–2004.
We investigated cases of Nipah virus infection from 4 outbreaks in the following regions: Meherpur District (from April through May 2001), Naogaon District (in January 2003), Rajbari (Goalanda subdistrict) and 7 other northwestern districts (from January through April 2004), and Faridpur District (from February through April 2004) [ 10 , 11 , 15–20 ]. The investigations for the first and second outbreaks, in Mehepur and Naogaon, took place after the outbreaks occurred. Because of increased awareness of Nipah virus among health officials, the third and fourth outbreaks, in Rajbari and Faridpur, were reported to authorities and investigated while the outbreaks were ongoing. Therefore, the case definitions and case detection methods differed slightly because of differences in the timing of investigations.
Outbreaks of Nipah virus infection in Meherpur and Naogaon . The investigation of the Meherpur outbreak was conducted 2 years after the outbreak, and the investigation of the Naogaon outbreak was performed 2 months after the outbreak. Patients with suspected cases of Nipah virus infection were persons residing in the outbreak areas who experienced fever with either headache or altered mental status during the time of the outbreak [ 11 ]. Field research assistants identified patients with suspected cases during house-to-house case-finding efforts. Study physicians collected illness histories from either patients with suspected cases or their caregivers (when the patients were minors or decedents) using a standardized case report form. We also collected information from hospital records, if available. A 5-mL blood specimen was collected from each living patient with a suspected case, and the serum was transported to the International Centre for Diarrhoeal Disease Research, Bangladesh (Dhaka), on ice for storage at inus;70eg;C. Serum samples were then shipped to the Centers for Disease Control and Prevention (Atlanta, GA) for Nipah virus serologic testing. Patients with suspected cases of Nipah virus infection who survived and had evidence of the infection, demonstrated by the presence of either IgM or IgG antibodies, were considered to have laboratory-confirmed cases. Patients with suspected cases who died during the outbreak, experienced fever with altered mental status, and were linked to patients with laboratory-confirmed cases by place of residence and timing of symptom onset were considered to have had probable cases of Nipah virus infection.
Outbreaks of Nipah virus infection in Rajbari and Faridpur . The Rajbari and Faridpur cases were investigated while the outbreaks were ongoing. A similar definition for a suspected case (fever and headache or altered mental status) was used during these investigations. However, because many severely ill patients with suspected cases also presented with respiratory symptoms, patients with a history of cough and fever were also considered to have suspected cases. Patients with suspected cases of Nipah virus infection were identified by house-to-house and hospital visits in the affected area. Physicians working with the investigation team collected illness histories from patients with suspected cases or their caregivers (when the patients were minors, decedents, or unable to provide personal histories because of altered mental status) using a standardized case report form. They verified clinical information by physical examination of the surviving patients (when possible) and by reviewing hospital records (when available).
Case-finding efforts were expanded to 7 northwestern districts from January through April 2004. Tertiary care hospitals in the region were visited, and all patients hospitalized with fever and altered mental status during this period were investigated. We identified 19 additional cases of Nipah virus infection during this effort; some were clustered, and some were isolated. We included these 19 cases with the cases identified in Rajbari District for this report, because the cases were identified beginning with the Rajbari outbreak in January.
We obtained acute serum samples from all living patients with suspected Nipah virus infection and convalescent serum samples at least 10 days after onset of illness from patients surviving acute illness. In addition to blood samples, we obtained throat swab, urine, and when possible, CSF samples from hospitalized patients with altered mental status. Serum and CSF samples were assayed for the presence or absence of Nipah virus-specific IgM and IgG antibodies, as described elsewhere [ 21 ]. CSF and throat swab specimens were tested for presence of Nipah virus RNA by RT-PCR using a primer set to detect the nucleocapsid gene, as described elsewhere [ 7 ]. Attempts were also made to isolate virus from CSF, throat swab, and urine specimens by placing 100 uL of specimen in cell culture, according to methods described elsewhere [ 7 , 22 ]. The samples were transported and stored in the same manner as in the first 2 outbreaks.
Patients with suspected cases who had evidence of Nipah virus infection, demonstrated by the presence of Nipah virus IgM antibodies or by isolation of Nipah virus, were considered to have laboratory-confirmed cases [ 21 ]. Although other laboratory tests were performed, they were not considered to be reliable enough to use for the case definition. Patients with probable cases were those who had fever with altered mental status or breathing difficulty and whose cases were part of a cluster of laboratory-confirmed cases in the outbreak region; adequate specimens (including convalescent-phase specimens) for detection of Nipah virus antibodies could not be obtained from these patients because of fatal outcomes. Those with specimens (including convalescent-phase serum specimens) shown to be Nipah virus negative using all Nipah virus tests were considered to not be infected with Nipah virus.
Statistical analysis . Clinical findings were contrasted between patients with fatal outcomes and patients who survived. Pearson's hi; 2 test or Fisher's exact test and univariate logistic regression were performed to determine ORs. Normally distributed continuous variables were analyzed using Student's t test, and nonnormally distributed continuous variables were analyzed using the Mann-Whitney U test. Associations were considered to be statistically significant at Pt;.05. Stata, version 8.0 (Stata), was used for statistical analysis.
We identified 92 patients with confirmed and probable cases of Nipah virus infection, including 13 patients in Meherpur, 12 in Naogaon, 31 in Rajbari (and other districts), and 36 in Faridpur. Forty-seven patients (51%) had laboratory-confirmed cases, 5 (5%; 4 from Meherpur and 1 from Naogaon) of whom were IgG positive only; all other patients with laboratory-confirmed cases (n=42) were IgM positive. Nipah virus was isolated from 4 patients (all of whom had detectable IgM antibodies to Nipah virus), and each isolate was identified by indirect fluorescent study test with Nipah virus antibodies and by RT-PCR [ 14 ]. Nipah virus was isolated from 2 patients associated with the third outbreak in Rajbari and 1 patient infected during the fourth outbreak in Faridpur; the fourth isolate was from a patient with an isolated case identified from Rajshahi District. Among 15 patients, all of whom had IgM antibodies, PCR results were positive for 8 throat swab, 6 urine, 5 CSF, and 4 saliva samples.
Twenty-two (47%) of 47 laboratory confirmed-cases resulted in death. Of the 45 patients (49%) with probable cases, 41 (91%) died before collection of any specimens. Four patients had 1 specimen obtained during the early phase of illness (median time after onset of illness, 3.5 days; range, 2–5 days), and each patient had negative results of tests for Nipah virus and died before additional, potentially confirmatory specimens could be obtained.
The age distribution of patients varied between outbreaks. The outbreaks in Naogaon (median patient age, 12 years; range, 4–42 years) and Rajbari (median patient age, 12 years; range, 2–50 years) occurred primarily among younger patients, compared with the outbreaks in Meherpur (median patient age, 38 years; range, 4–60 years) and Faridpur (median patient age, 35 years; range, 5–60 years). Fifty-seven (62%) patients were male; however, the proportion of male patients varied from 46% to 74% during the various outbreaks.
Fever, required as part of the probable case definition, was universally (100%) present, followed by altered mental status (90%), headache (73%), severe weakness (67%), cough (62%), difficult breathing (69%), diarrhea (29%), and seizures (23%) ( table 1 ). The clinical presentation started with mild-to-severe fever, followed by altered mental status (median time after onset of illness, 4 days; range, 0–10 days) or cough with respiratory difficulty (median time after onset of illness, 4 days; range, 0–13 days). Compared with the other outbreaks, during the Faridpur outbreak, respiratory difficulty was more common (75%) and seizures were less common (3%). The case-fatality rate was high (73%; range, 69%–75%). Most patients who died did so shortly after onset of illness (mean duration±SD from onset of illness to death, 7±4.6 days; median, 6 days; range, 2–36 days).
Clinical characteristics of Nipah virus infection during different outbreaks in Bangladesh.
In the Faridpur outbreak, during which most persons became infected through person-to-person transmission [ 19 ], a short, specific period of exposure could be determined for 11 patients who were exposed to only 1 infected patient for defined periods. Seven patients had a single close exposure (touched or were in the same room with an infected patient for at least 10 min) over the course of 1 day, and 4 had close exposures with a patient with Nipah virus infection during 2 consecutive days. On the basis of the exposure histories of these 11 patients, the median incubation period was 9 days (range, 6–11 days).
During all 4 outbreaks, a total of 64 serum specimens were obtained from 51 patients. Two specimens were obtained from 13 patients; 23 patients died before a second sample could be obtained, and the remaining patients had only 1 specimen obtained after illness. We used antibody test results from all patients to assess the relationship between onset of symptoms and seroconversion ( table 2 ). During the first 5 days of illness, 6 (66%) of 9 patients were IgM positive, and only 2 were IgG positive. By 2 weeks after symptom onset, all patients were IgM positive, and after 2 weeks, all patients were still IgG positive. Between 2 and 3 months after symptom onset, IgM antibody levels began to decrease, and after 2 years, no survivors were IgM positive, but 100% remained IgG positive. In total, 5 patients who were Nipah antibody negative when the first specimens were tested had detectable IgM antibodies in specimens obtained at least 2 weeks after the onset of illness.
Relationship between onset of Nipah virus illness and seroconversion in patients with acute Nipah virus infection.
CSF specimens from 6 patients with confirmed Nipah virus infection were analyzed; 3 (50%) had a WBC count t;5 cells/mm 3 . CSF glucose levels were within normal limits, and CSF protein levels were elevated in all patients except 1, who had normal levels. There was no bacterial growth yielded by any of the CSF cultures ( table 3 ).
Analysis of CSF specimens from 6 patients with laboratory-confirmed Nipah virus infection in 2004.
Patients who died were significantly older (median age, 28 years; range, 2–60 years) than those who survived (median age, 15 years; range, 4–50 years; P=.018). Patients who died were more likely than survivors to have altered mental status (OR, 12.4; 95% CI, 2.1–128.3), difficulty breathing (OR, 6.0; 95% CI, 2.0–18.5), documented temperature >37.8eg;C (OR, 11.2; 95% CI, 1.4–96.0), and abnormal (diminished or extensor) planter reflexes (undefined OR; P=.004) ( table 4 ). All surviving patients had laboratory-confirmed cases by definition, compared with 22 (33%) of 67 patients who died (Pt;.001).
Clinical features of Nipah virus infection in patients who died, compared with those who survived.
Chest radiographs were performed for 5 patients from the Faridpur outbreak; all had a history of cough and difficulty breathing, and radiographic findings for all 5 were consistent with acute respiratory distress syndrome ( figure 1 ). In patients with chest radiograph-confirmed acute respiratory distress syndrome, respiratory symptoms (cough and difficulty breathing) started within 2–5 days after the onset of fever. All 5 patients with acute respiratory distress syndrome died. Only 1 patient who had severe respiratory difficulty received mechanical ventilation; a chest radiograph was not performed for this patient, and the patient died on day 17 of his illness.
Chest radiographs for 4 patients, showing diffuse bilateral opacities covering majority of the lung fields, consistent with acute respiratory distress syndrome.
There are important differences between the clinical descriptions of Nipah virus illness in Bangladesh and those from Malaysia. Cough or breathing difficulties were common during all of the first 4 outbreaks in Bangladesh and were particularly pronounced during the Faridpur outbreak. In the Faridpur outbreak, additional patients had respiratory symptoms similar to those experienced by the patients with documented acute respiratory distress syndrome, but chest radiographs were not performed because of the lack of facilities at treatment centers. In Malaysia and Singapore, respiratory symptoms were reported less frequently. Only 14% of Malaysian patients had a history of nonproductive cough [ 8 ], and 3 (27%) of 11 patients in Singapore had atypical pneumonia, along with abnormal chest radiographic findings [ 3 , 4 , 6 , 23 ]. Respiratory symptoms were also common in patients during the outbreak in Siliguri, India in 2001, providing further evidence of a clinical presentation of Nipah virus infection in the southern Asian region that was different from the clinical presentation described in Malaysia [ 9 ].
Person-to-person transmission was documented during 1 outbreak in Faridpur [ 19 ] and was suggested in the Meherpur outbreak in Bangladesh [ 11 ]. Health care workers and hospital visitors were infected after exposure to hospitalized patients during the outbreak of Nipah virus infection in Siliguri, India, also suggesting person-to-person transmission [ 9 ]. The high prevalence of respiratory symptoms, especially cough, may have contributed to the transmissibility of Nipah virus infection from person to person that was observed in Bangladesh and Siliguri but not in Malaysia [ 5 , 24 , 25 ]. Coinfections with other respiratory pathogens have not been documented and were not systematically investigated.
Genetic differences in Nipah virus strains between those isolated in Malaysia and those isolated in Bangladesh could explain the differences in clinical presentation observed, specifically the importance of respiratory symptoms in Bangladeshi patients [ 13 , 26 ]. Because Nipah virus has been isolated from respiratory secretions of humans [ 27 ] and commonly causes pulmonary symptoms in other animals [ 28–30 ], it is plausible that some strains cause pulmonary symptoms in humans. Comparing sequence differences between patients with and without severe respiratory manifestations would help to evaluate this hypothesis.
The case-fatality rate was higher in Bangladesh and Siliguri than in Malaysia. We found that clinical features, including respiratory symptoms, fever, and absence of plantar reflexes, were associated with death. In Bangladesh, the higher case-fatality rate could be related to suboptimal health care. Diagnostic and supportive care facilities were limited in the areas affected by the outbreaks in Bangladesh. Other factors contributing to a higher case-fatality rate in Bangladesh may include any or a combination of the following: a more virulent Nipah virus genotype, exposure to secretions and/or excretions of patients with extremely high viral loads, and suppressed immunity, perhaps because of malnutrition (commonly seen in Bangladesh).
WBC counts in CSF specimens were not elevated in 3 of 6 patients with Nipah virus infection, a finding similar to that for the Malaysian patients [ 8 ]. These findings suggest that normal WBC count and normal chemical parameters in CSF do not rule out Nipah virus infection in patients with encephalitis.
There are several limitations to our study. The context of an investigation of an outbreak of Nipah virus infection in Bangladesh is challenging because of the quick progression from symptom onset to death, coupled with reluctance of sick patients to seek care and inadequate diagnostic infrastructure in health care facilities. For these reasons, many infected patients in Bangladesh were not hospitalized, and clinical evaluations were not performed. Even when hospital records were available, they were often incomplete. These challenges also produce delays in identification and investigation of outbreaks, which contributes to incomplete recall or recall bias by patients and/or relatives. This might have resulted in underreporting of some symptoms, especially when the recall time was long, as was the case for the Meherpur outbreak.
In addition, because of delays in outbreak detection, the confirmed case definitions from the first and second outbreaks in Meherpur and Naogaon were based on the presence of IgG (rather than IgM) antibody to Nipah virus antigen. A case definition based on the presence of IgG antibody is reasonable, even if suboptimal, because all patients from these 2 outbreaks experienced encephalitis during a very specific period and in a specific geographic location. Although our case definition was based on the presence of IgG antibody, 3 of 4 patients with laboratory-confirmed cases who lived in Naogaon also had IgM antibodies, providing further evidence that presence of IgG antibodies is a reasonable indicator of past infection. In addition, there has been no evidence of another outbreak with similar impact in the area in the recent or more remote past; thus, previous exposure was unlikely.
A third limitation is that our series may be biased toward more-severe cases, considering our reliance on hospital surveillance for case detection in many areas. However, door-to-door visits in areas where outbreaks occurred limited the effect of this bias. Asymptomatic infection was reported in 8% of patients with laboratory-confirmed cases in Malaysia [ 25 ]; however, there is no evidence of asymptomatic Nipah virus infection in Bangladesh during outbreaks, although cases of mild illness were identified.
The findings of our investigation suggest that Nipah virus infections occurred in all age groups and that fever, altered mental status, cough, and respiratory symptoms were the most common symptoms among those infected in Bangladesh. Severe neurologic manifestations are consistently the most substantial and severe components of Nipah virus infection. Severe chronic sequelae occur in many survivors, adding to the significant public health burden of Nipah virus-associated disease [ 31 ]. Future priorities should include ongoing surveillance and investigation of outbreaks of Nipah virus infection, identification and evaluation of strategies to prevent Nipah virus transmission, and improvement of clinical management of cases in resource-poor settings.
We thank the Centers for Disease Control and Prevention; World Health Organization; Institute of Epidemiology Disease Control and Research; Ministry of Health and Family Welfare, Bangladesh; Dr. Abu Taher Azad, Dr. Sultana Monira Hossain, and Dr. A. K. M. Saifuddin Ekram, for assistance in data collection; the study participants and their relatives; Mr. Milton Quiah, for administrative support; the Director and staff of the Dhaka, Faridpur, and Rajshahi Medical College Hospitals; and Dr. M. A. Salam, for his critical review of the manuscript.
Financial support . Centers for Disease Control and Prevention, World Health Organization, and Ministry of Health and Family Welfare, Bangladesh.
Potential conflicts of interest . All authors: no conflicts.
Google Scholar
- disease outbreaks
- mental state abnormal
- nipah virus
- symptom onset
| Month: | Total Views: |
|---|---|
| December 2016 | 7 |
| January 2017 | 11 |
| February 2017 | 18 |
| March 2017 | 29 |
| April 2017 | 13 |
| May 2017 | 20 |
| June 2017 | 17 |
| July 2017 | 28 |
| August 2017 | 22 |
| September 2017 | 18 |
| October 2017 | 21 |
| November 2017 | 30 |
| December 2017 | 91 |
| January 2018 | 121 |
| February 2018 | 121 |
| March 2018 | 132 |
| April 2018 | 146 |
| May 2018 | 2,597 |
| June 2018 | 1,307 |
| July 2018 | 477 |
| August 2018 | 298 |
| September 2018 | 354 |
| October 2018 | 405 |
| November 2018 | 518 |
| December 2018 | 345 |
| January 2019 | 230 |
| February 2019 | 303 |
| March 2019 | 300 |
| April 2019 | 286 |
| May 2019 | 306 |
| June 2019 | 470 |
| July 2019 | 315 |
| August 2019 | 260 |
| September 2019 | 227 |
| October 2019 | 114 |
| November 2019 | 104 |
| December 2019 | 67 |
| January 2020 | 62 |
| February 2020 | 94 |
| March 2020 | 79 |
| April 2020 | 122 |
| May 2020 | 67 |
| June 2020 | 42 |
| July 2020 | 80 |
| August 2020 | 50 |
| September 2020 | 62 |
| October 2020 | 47 |
| November 2020 | 77 |
| December 2020 | 48 |
| January 2021 | 64 |
| February 2021 | 76 |
| March 2021 | 131 |
| April 2021 | 111 |
| May 2021 | 78 |
| June 2021 | 90 |
| July 2021 | 63 |
| August 2021 | 60 |
| September 2021 | 203 |
| October 2021 | 134 |
| November 2021 | 195 |
| December 2021 | 194 |
| January 2022 | 94 |
| February 2022 | 88 |
| March 2022 | 141 |
| April 2022 | 132 |
| May 2022 | 117 |
| June 2022 | 84 |
| July 2022 | 103 |
| August 2022 | 99 |
| September 2022 | 136 |
| October 2022 | 137 |
| November 2022 | 112 |
| December 2022 | 106 |
| January 2023 | 127 |
| February 2023 | 177 |
| March 2023 | 160 |
| April 2023 | 150 |
| May 2023 | 127 |
| June 2023 | 100 |
| July 2023 | 90 |
| August 2023 | 109 |
| September 2023 | 413 |
| October 2023 | 303 |
| November 2023 | 207 |
| December 2023 | 183 |
| January 2024 | 194 |
| February 2024 | 160 |
| March 2024 | 228 |
| April 2024 | 224 |
| May 2024 | 138 |
| June 2024 | 110 |
| July 2024 | 130 |
| August 2024 | 116 |
| September 2024 | 53 |
Email alerts
More on this topic, related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Clinical presentation of Nipah virus infection in Bangladesh
- Montgomery J
- et al. See more
This article is free to access.
Background. In Bangladesh, 4 outbreaks of Nipah virus infection were identified during the period 2001-2004. Methods. We characterized the clinical features of Nipah virus-infected individuals affected by these outbreaks. We classified patients as having confirmed cases of Nipah virus infection if they had antibodies reactive with Nipah virus antigen. Patients were considered to have probable cases of Nipah virus infection if they had symptoms consistent with Nipah virus infection during the same time and in the same community as patients with confirmed cases. Results. We identified 92 patients with Nipah virus infection, 67 (73%) of whom died. Although all age groups were affected, 2 outbreaks principally affected young persons (median age, 12 years); 62% of the affected persons were male. Fever, altered mental status, headache, cough, respiratory difficulty, vomiting, and convulsions were the most common signs and symptoms; clinical and radiographic features of acute respiratory distress syndrome of Nipah illness were identified during the fourth outbreak. Among those who died, death occurred a median of 6 days (range, 2-36 days) after the onset of illness. Patients who died were more likely than survivors to have a temperature >37.8°C, altered mental status, difficulty breathing, and abnormal plantar reflexes. Among patients with Nipah virus infection who had well-defined exposure to another patient infected with Nipah virus, the median incubation period was 9 days (range, 6-11 days). Conclusions. Nipah virus infection produced rapidly progressive severe illness affecting the central nervous and respiratory systems. Clinical characteristics of Nipah virus infection in Bangladesh, including a severe respiratory component, appear distinct from clinical characteristics reported during earlier outbreaks in other countries. © 2008 by the Infectious Diseases Society of America. All rights reserved.
Register to see more suggestions
Mendeley helps you to discover research relevant for your work.
CITATION STYLE
Hossain, M. J., Gurley, E. S., Montgomery, J. M., Bell, M., Carroll, D. S., Hsu, V. P., … Breiman, R. F. (2008). Clinical presentation of Nipah virus infection in Bangladesh. Clinical Infectious Diseases , 46 (7), 977–984. https://doi.org/10.1086/529147
Readers' Seniority
PhD / Post grad / Masters / Doc 48
Researcher 30
Professor / Associate Prof. 9
Lecturer / Post doc 1
Readers' Discipline
Medicine and Dentistry 33
Agricultural and Biological Sciences 32
Biochemistry, Genetics and Molecular Bi... 24
Immunology and Microbiology 18
Save time finding and organizing research with Mendeley
- Help & FAQ
Clinical presentation of Nipah virus infection in Bangladesh
Research output : Contribution to journal › Article › peer-review
Background. In Bangladesh, 4 outbreaks of Nipah virus infection were identified during the period 2001-2004. Methods. We characterized the clinical features of Nipah virus-infected individuals affected by these outbreaks. We classified patients as having confirmed cases of Nipah virus infection if they had antibodies reactive with Nipah virus antigen. Patients were considered to have probable cases of Nipah virus infection if they had symptoms consistent with Nipah virus infection during the same time and in the same community as patients with confirmed cases. Results. We identified 92 patients with Nipah virus infection, 67 (73%) of whom died. Although all age groups were affected, 2 outbreaks principally affected young persons (median age, 12 years); 62% of the affected persons were male. Fever, altered mental status, headache, cough, respiratory difficulty, vomiting, and convulsions were the most common signs and symptoms; clinical and radiographic features of acute respiratory distress syndrome of Nipah illness were identified during the fourth outbreak. Among those who died, death occurred a median of 6 days (range, 2-36 days) after the onset of illness. Patients who died were more likely than survivors to have a temperature >37.8°C, altered mental status, difficulty breathing, and abnormal plantar reflexes. Among patients with Nipah virus infection who had well-defined exposure to another patient infected with Nipah virus, the median incubation period was 9 days (range, 6-11 days). Conclusions. Nipah virus infection produced rapidly progressive severe illness affecting the central nervous and respiratory systems. Clinical characteristics of Nipah virus infection in Bangladesh, including a severe respiratory component, appear distinct from clinical characteristics reported during earlier outbreaks in other countries.
| Original language | English (US) |
|---|---|
| Pages (from-to) | 977-984 |
| Number of pages | 8 |
| Journal | |
| Volume | 46 |
| Issue number | 7 |
| DOIs | |
| State | Published - Apr 1 2008 |
| Externally published | Yes |
ASJC Scopus subject areas
- Microbiology (medical)
- Infectious Diseases
Access to Document
- 10.1086/529147
Other files and links
- Link to publication in Scopus
- Link to the citations in Scopus
Fingerprint
- Nipah Virus Infection Keyphrases 100%
- Bangladesh Keyphrases 100%
- Clinical Presentation Keyphrases 100%
- Viral Disease Immunology and Microbiology 100%
- Nipah Virus Immunology and Microbiology 100%
- Disease Pharmacology, Toxicology and Pharmaceutical Science 33%
- Illness Keyphrases 22%
- Clinical Features Keyphrases 22%
T1 - Clinical presentation of Nipah virus infection in Bangladesh
AU - Hossain, M. Jahangir
AU - Gurley, Emily S.
AU - Montgomery, Joel M.
AU - Bell, Michael
AU - Carroll, Darin S.
AU - Hsu, Vincent P.
AU - Formenty, P.
AU - Croisier, A.
AU - Bertherat, E.
AU - Faiz, M. A.
AU - Azad, Abul Kalam
AU - Islam, Rafiqul
AU - Molla, M. Abdur Rahim
AU - Ksiazek, Thomas G.
AU - Rota, Paul A.
AU - Comer, James A.
AU - Rollin, Pierre E.
AU - Luby, Stephen P.
AU - Breiman, Robert F.
PY - 2008/4/1
Y1 - 2008/4/1
N2 - Background. In Bangladesh, 4 outbreaks of Nipah virus infection were identified during the period 2001-2004. Methods. We characterized the clinical features of Nipah virus-infected individuals affected by these outbreaks. We classified patients as having confirmed cases of Nipah virus infection if they had antibodies reactive with Nipah virus antigen. Patients were considered to have probable cases of Nipah virus infection if they had symptoms consistent with Nipah virus infection during the same time and in the same community as patients with confirmed cases. Results. We identified 92 patients with Nipah virus infection, 67 (73%) of whom died. Although all age groups were affected, 2 outbreaks principally affected young persons (median age, 12 years); 62% of the affected persons were male. Fever, altered mental status, headache, cough, respiratory difficulty, vomiting, and convulsions were the most common signs and symptoms; clinical and radiographic features of acute respiratory distress syndrome of Nipah illness were identified during the fourth outbreak. Among those who died, death occurred a median of 6 days (range, 2-36 days) after the onset of illness. Patients who died were more likely than survivors to have a temperature >37.8°C, altered mental status, difficulty breathing, and abnormal plantar reflexes. Among patients with Nipah virus infection who had well-defined exposure to another patient infected with Nipah virus, the median incubation period was 9 days (range, 6-11 days). Conclusions. Nipah virus infection produced rapidly progressive severe illness affecting the central nervous and respiratory systems. Clinical characteristics of Nipah virus infection in Bangladesh, including a severe respiratory component, appear distinct from clinical characteristics reported during earlier outbreaks in other countries.
AB - Background. In Bangladesh, 4 outbreaks of Nipah virus infection were identified during the period 2001-2004. Methods. We characterized the clinical features of Nipah virus-infected individuals affected by these outbreaks. We classified patients as having confirmed cases of Nipah virus infection if they had antibodies reactive with Nipah virus antigen. Patients were considered to have probable cases of Nipah virus infection if they had symptoms consistent with Nipah virus infection during the same time and in the same community as patients with confirmed cases. Results. We identified 92 patients with Nipah virus infection, 67 (73%) of whom died. Although all age groups were affected, 2 outbreaks principally affected young persons (median age, 12 years); 62% of the affected persons were male. Fever, altered mental status, headache, cough, respiratory difficulty, vomiting, and convulsions were the most common signs and symptoms; clinical and radiographic features of acute respiratory distress syndrome of Nipah illness were identified during the fourth outbreak. Among those who died, death occurred a median of 6 days (range, 2-36 days) after the onset of illness. Patients who died were more likely than survivors to have a temperature >37.8°C, altered mental status, difficulty breathing, and abnormal plantar reflexes. Among patients with Nipah virus infection who had well-defined exposure to another patient infected with Nipah virus, the median incubation period was 9 days (range, 6-11 days). Conclusions. Nipah virus infection produced rapidly progressive severe illness affecting the central nervous and respiratory systems. Clinical characteristics of Nipah virus infection in Bangladesh, including a severe respiratory component, appear distinct from clinical characteristics reported during earlier outbreaks in other countries.
UR - http://www.scopus.com/inward/record.url?scp=42549092343&partnerID=8YFLogxK
UR - http://www.scopus.com/inward/citedby.url?scp=42549092343&partnerID=8YFLogxK
U2 - 10.1086/529147
DO - 10.1086/529147
M3 - Article
C2 - 18444812
AN - SCOPUS:42549092343
SN - 1058-4838
JO - Clinical Infectious Diseases
JF - Clinical Infectious Diseases
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Transmission of human infection with Nipah virus
Affiliation.
- 1 International Centre for Diarrheal Diseases Research, Bangladesh, Dhaka, Bangladesh. [email protected]
- PMID: 19886791
- PMCID: PMC2784122
- DOI: 10.1086/647951
Nipah virus (NiV) is a paramyxovirus whose reservoir host is fruit bats of the genus Pteropus. Occasionally the virus is introduced into human populations and causes severe illness characterized by encephalitis or respiratory disease. The first outbreak of NiV was recognized in Malaysia, but 8 outbreaks have been reported from Bangladesh since 2001. The primary pathways of transmission from bats to people in Bangladesh are through contamination of raw date palm sap by bats with subsequent consumption by humans and through infection of domestic animals (cattle, pigs, and goats), presumably from consumption of food contaminated with bat saliva or urine with subsequent transmission to people. Approximately one-half of recognized Nipah case patients in Bangladesh developed their disease following person-to-person transmission of the virus. Efforts to prevent transmission should focus on decreasing bat access to date palm sap and reducing family members' and friends' exposure to infected patients' saliva.
PubMed Disclaimer
Range of Pterpous bats based…
Range of Pterpous bats based on RM Nowak [16]
Chain of person to person…
Chain of person to person transmission in Nipah outbreak, Faridpur, Bangladesh, 2004.
Similar articles
- Nipah virus dynamics in bats and implications for spillover to humans. Epstein JH, Anthony SJ, Islam A, Kilpatrick AM, Ali Khan S, Balkey MD, Ross N, Smith I, Zambrana-Torrelio C, Tao Y, Islam A, Quan PL, Olival KJ, Khan MSU, Gurley ES, Hossein MJ, Field HE, Fielder MD, Briese T, Rahman M, Broder CC, Crameri G, Wang LF, Luby SP, Lipkin WI, Daszak P. Epstein JH, et al. Proc Natl Acad Sci U S A. 2020 Nov 17;117(46):29190-29201. doi: 10.1073/pnas.2000429117. Epub 2020 Nov 2. Proc Natl Acad Sci U S A. 2020. PMID: 33139552 Free PMC article.
- Nipah Virus Transmission from Bats to Humans Associated with Drinking Traditional Liquor Made from Date Palm Sap, Bangladesh, 2011-2014. Islam MS, Sazzad HM, Satter SM, Sultana S, Hossain MJ, Hasan M, Rahman M, Campbell S, Cannon DL, Ströher U, Daszak P, Luby SP, Gurley ES. Islam MS, et al. Emerg Infect Dis. 2016 Apr;22(4):664-70. doi: 10.3201/eid2204.151747. Emerg Infect Dis. 2016. PMID: 26981928 Free PMC article.
- Epidemiology of henipavirus disease in humans. Luby SP, Gurley ES. Luby SP, et al. Curr Top Microbiol Immunol. 2012;359:25-40. doi: 10.1007/82_2012_207. Curr Top Microbiol Immunol. 2012. PMID: 22752412 Review.
- Use of infrared camera to understand bats' access to date palm sap: implications for preventing Nipah virus transmission. Khan MS, Hossain J, Gurley ES, Nahar N, Sultana R, Luby SP. Khan MS, et al. Ecohealth. 2010 Dec;7(4):517-25. doi: 10.1007/s10393-010-0366-2. Epub 2011 Jan 5. Ecohealth. 2010. PMID: 21207105
- Nipah Virus Infection. Ang BSP, Lim TCC, Wang L. Ang BSP, et al. J Clin Microbiol. 2018 May 25;56(6):e01875-17. doi: 10.1128/JCM.01875-17. Print 2018 Jun. J Clin Microbiol. 2018. PMID: 29643201 Free PMC article. Review.
- A Comparative Assessment of the Pathogenic Potential of Newly Discovered Henipaviruses. Meier K, Olejnik J, Hume AJ, Mühlberger E. Meier K, et al. Pathogens. 2024 Jul 16;13(7):587. doi: 10.3390/pathogens13070587. Pathogens. 2024. PMID: 39057814 Free PMC article. Review.
- A vaccine targeting antigen-presenting cells through CD40 induces protective immunity against Nipah disease. Pastor Y, Reynard O, Iampietro M, Surenaud M, Picard F, El Jahrani N, Lefebvre C, Hammoudi A, Dupaty L, Brisebard É, Reynard S, Moureaux É, Moroso M, Durand S, Gonzalez C, Amurri L, Gallouët AS, Marlin R, Baize S, Chevillard E, Raoul H, Hocini H, Centlivre M, Thiébaut R, Horvat B, Godot V, Lévy Y, Cardinaud S. Pastor Y, et al. Cell Rep Med. 2024 Mar 19;5(3):101467. doi: 10.1016/j.xcrm.2024.101467. Epub 2024 Mar 11. Cell Rep Med. 2024. PMID: 38471503 Free PMC article.
- Nipah Virus: A Multidimensional Update. Faus-Cotino J, Reina G, Pueyo J. Faus-Cotino J, et al. Viruses. 2024 Jan 25;16(2):179. doi: 10.3390/v16020179. Viruses. 2024. PMID: 38399954 Free PMC article. Review.
- Updated Insights into the Phylogenetics, Phylodynamics, and Genetic Diversity of Nipah Virus (NiV). de Campos GM, Cella E, Kashima S, Alcântara LCJ, Sampaio SC, Elias MC, Giovanetti M, Slavov SN. de Campos GM, et al. Viruses. 2024 Jan 24;16(2):171. doi: 10.3390/v16020171. Viruses. 2024. PMID: 38399947 Free PMC article.
- Prefusion stabilization of the Hendra and Langya virus F proteins. Byrne PO, Blade EG, Fisher BE, Ambrozak DR, Ramamohan AR, Graham BS, Loomis RJ, McLellan JS. Byrne PO, et al. J Virol. 2024 Feb 20;98(2):e0137223. doi: 10.1128/jvi.01372-23. Epub 2024 Jan 12. J Virol. 2024. PMID: 38214525 Free PMC article.
- Chua KB, Bellini WJ, Rota PA, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000 May 26;288(5470):1432–5. - PubMed
- Paton NI, Leo YS, Zaki SR, et al. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. 1999 Oct 9;354(9186):1253–6. - PubMed
- Chua KB. Nipah virus outbreak in Malaysia. J Clin Virol. 2003 Apr;26(3):265–75. - PubMed
- Parashar UD, Sunn LM, Ong F, et al. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998-1999 outbreak of severe encephalitis in Malaysia. The Journal of Infectious Diseases. 2000 May;181(5):1755–9. - PubMed
- Goh KJ, Tan CT, Chew NK, et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. The New England Journal of Medicine. 2000 Apr 27;342(17):1229–35. - PubMed
Publication types
- Search in MeSH
Related information
- Cited in Books
Grants and funding
- R01 TW005869/TW/FIC NIH HHS/United States
- R01 TW005869-05/TW/FIC NIH HHS/United States
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- Ovid Technologies, Inc.
- PubMed Central
- Silverchair Information Systems
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Volume 28, Number 7—July 2022
Nipah Virus Detection at Bat Roosts after Spillover Events, Bangladesh, 2012–2019
Cite This Article
Knowledge of the dynamics and genetic diversity of Nipah virus circulating in bats and at the human-animal interface is limited by current sampling efforts, which produce few detections of viral RNA. We report a series of investigations at Pteropus medius bat roosts identified near the locations of human Nipah cases in Bangladesh during 2012–2019. Pooled bat urine was collected from 23 roosts; 7 roosts (30%) had > 1 sample in which Nipah RNA was detected from the first visit. In subsequent visits to these 7 roosts, RNA was detected in bat urine up to 52 days after the presumed exposure of the human case-patient, although the probability of detection declined rapidly with time. These results suggest that rapidly deployed investigations of Nipah virus shedding from bat roosts near human cases could increase the success of viral sequencing compared with background surveillance and could enhance understanding of Nipah virus ecology and evolution.
Nipah virus is a paramyxovirus (genus Henipavirus ) that has caused outbreaks of neurologic and respiratory disease in humans and livestock in Bangladesh, India, Malaysia, Singapore, and the Philippines ( 1 – 4 ). The primary hosts of henipaviruses are fruit bats (family Pteropodidae) in Africa, Asia, and Oceania ( 5 ). Although Nipah virus causes no apparent disease in bats ( 6 , 7 ), the case-fatality rate in humans can be 40%–70% ( 2 , 8 , 9 ). In addition, Nipah virus has characteristics that enable repeated human outbreaks. Its bat hosts are widespread in South Asia and Southeast Asia, regions with dense human and livestock populations ( 10 ), which could lead to virus spillover and spread ( 11 ). Nipah virus can transmit directly from bats when humans consume date palm sap that is contaminated with bat saliva, urine, or feces or can transmit indirectly through spillover to domesticated animals ( 12 – 14 ).
Since 2001, Bangladesh has experienced multiple Nipah virus outbreaks with confirmed person-to-person transmission, albeit below the threshold necessary for sustained epidemics ( 8 ); however, the virus transmitted rapidly among pig populations in Malaysia, producing infection rates of 100% on some farms, and spread between farms through shipments of infected animals ( 15 , 16 ). No commercially available vaccines or therapeutics for Nipah virus exist to prevent or mitigate disease in case of an epidemic, although these interventions are areas of active research ( 17 , 18 ). Finally, RNA viruses such as Nipah have high mutation rates, which are a predictor of zoonotic potential ( 19 ). Although documented genetic diversity within Nipah viruses is limited ( 20 – 24 ), high mutation rates could potentially produce variants with sufficient transmissibility in humans to cause a sustained epidemic ( 25 , 26 ). Given the wide geographic range and unsampled diversity of Nipah viruses, variants that are more transmissible among humans might exist and circulate in bats, and each spillover event could be an opportunity for such variants to emerge ( 27 ).
Genetic and phenotypic diversity among Nipah viruses exists, but the human health implications are unclear. Nipah virus genotypes from Bangladesh and India are genetically distinct from genotypes from Malaysia ( 22 – 24 ). Although Malaysia genotypes are less diverse than those from Bangladesh and India ( 24 ), genotypes from Malaysia derive solely from pigs, humans, and bats during the 1998–1999 outbreak, whereas genotypes from Bangladesh and India derive from multiple human outbreaks and surveys of bats since 2004. Another difference is that person-to-person transmission of Nipah virus has rarely been observed in Malaysia ( 28 – 30 ) but accounted for one third of reported cases in Bangladesh ( 8 ) and >75% of cases in India ( 1 , 9 , 31 ). However, person-to-person transmission in Malaysia was not investigated beyond healthcare workers, and <10% of persons with Nipah virus transmit it to another person, usually a family caregiver ( 8 , 28 ). Some of this variation in transmission mode and severity could reflect differences in exposure, sampling, infrastructure, and culture between countries, but differences between viral strains might explain additional variation. Case-patients in Malaysia were less likely to experience cough, difficulty breathing, or abnormal chest radiography than case-patients in Bangladesh ( 29 , 32 , 33 ). These differences in transmissibility and pathogenicity between Nipah virus strains from Malaysia and Bangladesh have been observed in some animal experiments, although with conflicting results ( 34 – 36 ). The reviewed evidence suggests that genetic variation in Nipah virus might produce differences in pathogenicity or transmissibility, so more transmissible strains of Nipah virus could be circulating undetected in bat populations.
Knowledge of Nipah virus diversity is limited to the few virus sequences obtained to date. Available sequences from GenBank and recent studies ( 20 , 24 ) include only 76 Nipah virus genomes, 51 of which derive from human patients, and 153 nucleocapsid protein genes, 37 of which derive from humans. Previous studies have not been optimized to characterize Nipah virus genotypes circulating in bats.
The Indian flying fox ( Pteropus medius ) is the major reservoir of Nipah virus in Bangladesh and India ( 37 , 38 ). Longitudinal surveys indicate that exposure to Nipah virus is high (≈40%) in some P. medius populations in Bangladesh on the basis of serologic tests, but the prevalence of detectable Nipah virus RNA is low (<5%) at any given time ( 37 ). In addition, viral loads in collected bat samples are often low ( 24 ), limiting the success of virus sequencing or isolation necessary for describing viral diversity. Sampling methods that increase the success of detecting Nipah virus in bats and increase yield so that sequencing is possible would be useful for monitoring genetic changes in this virus. In this study, we focused Nipah virus detection to P. medius bat roosts near human cases identified in Bangladesh during outbreak investigations during 2012–2019. We aimed to identify whether bat roosts were actively shedding Nipah virus RNA in urine and how long shedding continued after initial detection. In addition, we sought to identify characteristics of bat roosts potentially associated with higher likelihood of testing positive.
Materials and Methods
Nipah virus case investigations.
Human case-patients with suspected Nipah virus infection with a history of consuming date palm sap were identified at 3 surveillance hospitals in the Faridpur, Rajshahi, and Rangpur Districts of Bangladesh ( 39 ). Additional suspected cases in other regions were identified from media reports ( 40 ). A total of 47 primary cases of Nipah virus representing spillover from bats were identified in 2012–2018; we investigated 17 in this study. Four additional spillover cases were investigated in 2019, but the total number of spillover cases from that year is unclear because of a lack of reporting. Case exposure to Nipah virus was evaluated with ELISA or PCR ( 41 ). Investigation teams visited the suspected case villages to gather evidence of case clusters and identify the exposure route ( 42 ). In some cases, teams were deployed before human cases were confirmed by ELISA or PCR.
Teams searched for P. medius bat roosts within a 20 km radius of the human case-patient’s residence by asking community members about known roost sites and by scouting. Some identified roosts were located on burial grounds or over water and could not be sampled ( Appendix 1 Table 1). During 12:00–4:00 AM, teams placed 4–20 polyethylene tarps under each roost, depending on the available area and size of the roost, to collect urine. Tarps were concentrated under branches with denser aggregations of bats. Tarps were ≈6 feet × 4 feet in size before 2019 and 3 feet × 2 feet in 2019; we made this change so that fewer bats contributed to urine pools to improve estimates of prevalence ( 43 ). During 5:00–6:00 AM, teams returned to the roosts and collected bat urine from the tarps with a sterile syringe. Urine collected from tarps was either pooled by individual tarp or mixed together from multiple tarps and then divided into aliquots. We found no significant difference in Nipah detection between the 2 strategies ( Appendix 1 ). We tested aliquots for Nipah virus RNA at icddr,b (Dhaka, Bangladesh) or National Institutes of Health (Hamilton, MT, USA) laboratories by using quantitative real-time reverse transcription PCR (qRT-PCR) targeting the nucleoprotein gene ( 44 ). Roosts with Nipah virus RNA detected in any aliquots at the first sampling event were revisited (3–16 days between sampling events) until all aliquots from a roost tested negative. Attempts to culture Nipah virus from positive samples at National Institutes of Health yielded no virus isolates; viral culture was not attempted at icddr,b because of the absence of BioSafety Level 4 facilities.
Statistical Analysis
For each laboratory-confirmed spillover case of Nipah virus in a human, we recorded the symptom onset date and the coordinates of the case-patient’s residence. Teams identified the probable date of patient exposure to Nipah virus by the date of palm sap consumption for some cases; otherwise, the exposure date was assumed to be 7 days before symptom onset on the basis of the mean incubation period of Nipah virus for primary cases linked to spillover ( 45 ).
We used logistic regression to assess features of the roost sites associated with a roost testing positive for Nipah virus at the first sampling visit. Covariates in the model included the number of days between the first case-patient exposure to date palm sap and roost sampling, the number of bats in the roost, the distance between the case-patient’s home and the roost site, and the number of human spillover cases associated with each nearby roost. We then performed model selection to choose important features using Akaike corrected information criterion ( 46 ).
For all roost sites that tested positive for Nipah virus at first sampling, we recorded the number of tested urine aliquots that were positive for Nipah virus at each visit. Because cycle threshold (Ct) values from qRT-PCR were not reported for all tests, we used the proportion of positive aliquots as a proxy for the intensity of virus shedding in bats, assuming that roosts with higher virus concentrations in urine would produce more positive aliquots. We then analyzed changes in the proportion of positive aliquots across roosts along 2 time axes. We aligned dates to the number of days since the presumed exposure date of the first human spillover Nipah case associated with each roost site. We then aligned roost-sampling dates to the number of days since the start of the calendar year for comparison. We fit binomial linear models to estimate the probability of detecting a Nipah virus–positive aliquot at each roost along each time axis.
To evaluate the utility of sampling bat roosts near human Nipah virus cases as a surveillance approach, we compared the rate of successful Nipah virus detections from this study to data reported by Epstein et al. ( 37 ). Samples from that study were collected quarterly from a P. medius bat roost in Faridpur District during 2007–2012 as part of a longitudinal study; from visits to different roosts throughout Bangladesh during 2006–2011 as part of a cross-sectional spatial analysis; or as part of Nipah virus outbreak investigations in 2009, 2010, and 2012. Urine samples were either collected from individual bats or from underneath roosts. For these comparisons, we considered each roost visit as a discrete sampling event, including repeat visits to the same roost. Ignoring the initial visits to 7 roosts near 5 suspected human cases that were Nipah virus–negative, the 23 roosts in our study were sampled across 47 visits. We made comparisons between studies for the number of sampling visits with positive Nipah detections and the number of positive urine samples (individual or pooled aliquots from roosts) across all sampling visits or during the first visit to each roost. We evaluated comparisons by using a χ 2 test of proportions or Fisher exact test. We considered statistical tests significant if p values were <0.05.
All study participants or proxies provided informed consent before participation and personally identifiable information from patients was delinked from the data before use. Written permission was obtained from the Bangladesh Forest Department for sampling the bats, and team members obtained permission from landowners before sampling roosts. Protocols for case investigations and roost sampling were reviewed and approved by the Institutional Review Board at icddr,b.
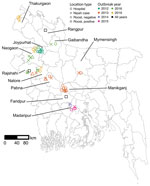
- Figure 1 . Locations of human Nipah cases (n = 21) and Pteropus medius bat roosts (n = 30) investigated in Bangladesh, 2012–2019. Roosts with urine aliquots that tested positive for Nipah...
Teams investigated roosts near homes of 21 suspected human cases of Nipah virus infection during 2012–2019 ( Appendix 1 Table 1). The cases were clustered in the central and northwest districts of Bangladesh, close to the 3 surveillance hospitals ( Figure 1 ). Symptom onset for patients occurred in winter (December–February), with the exception of 1 case-patient in Manikganj District whose symptoms began in March 2013. No roost investigations were performed in 2017 and 2018 because of funding constraints.
For each case-patient, we identified 1–3 P. medius bat roosts within 0–17.9 km of the patient’s home ( Appendix 2 Table 1). An additional 5 identified roosts were not sampled because they were located on burial grounds or over water ( Appendix 1 Table 1). We sampled a total of 30 roosts. The first sampling visits occurred 17–62 days after the case-patients’ exposure to date palm sap, either reported from the case investigation or back-calculated as 7 days before the onset of symptoms ( Appendix 2 Table 1). Five of the suspected patients tested negative for Nipah virus by ELISA or PCR, and the 7 roosts identified near the patients’ homes yielded no Nipah virus RNA. Because our interest was in whether sampling near human Nipah virus cases would help to identify roosts with active Nipah virus shedding, we excluded suspected but Nipah virus–negative case-patients and associated bat roosts from statistical analyses. Sensitivity analyses that included these samples produced statistically similar results. Testing by qRT-PCR of pooled urine aliquots detected 7/23 (30%) roosts as positive for Nipah virus RNA in > 1 aliquots at the first sampling visit.

- Figure 2 . Descriptive variables for 23 Pteropus medius bat roosts sampled near confirmed human Nipah virus cases, Bangladesh, 2012–2019. Open circles show the values associated with the first human case associated...
We performed Logistic regression on the presence of Nipah virus RNA in roost urine at the first sampling event on 22 distinct roosts using 4 explanatory variables; 1 roost was omitted because of missing data on the number of bats. Roosts with positive urine aliquots tended to have more associated human Nipah spillover cases, were sampled sooner after patient exposure, were more distant from patients’ homes, and had a smaller number of bats, but none of these variables were significantly associated with roost positivity in univariate or multiple regression analyses ( Figure 2 ; Appendix 1 Table 2), and Akaike corrected information criterion identified the intercept-only model as the best model ( Appendix 1 Table 3).
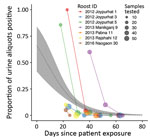
- Figure 3 . Results of screening of Pteropus medius bat roost urine aliquots for Nipah virus RNA, Bangladesh, 2012–2019. For each roost, the proportion of urine aliquots out of the total tested...
For the 7 roosts where Nipah virus RNA was detected > 1 time, data were compiled on the number of urine aliquots that tested positive at each repeated sampling visit. Of these 7 roosts, 4 were positive at the first visit only and were revisited only once. The other 3 roosts remained positive at 1–2 additional sampling visits, although the proportion of aliquots that tested positive declined rapidly with the time since exposure of the first associated human case ( Figure 3 ). For the 2 roosts with reported Ct values from qRT-PCR, the proportion of positive aliquots decreased over the repeated sampling visits while Ct values increased, indicating a decline in viral load ( Appendix 1 Table 4).
Fitting a binomial model to the PCR data predicted that the probability of detecting at least 1 urine aliquot from under-roost sampling as positive for Nipah virus RNA at the time the associated case-patient was presumably exposed (day 0) was 0.66 (95% CI 0.42–0.84) ( Figure 3 ). This probability declined to 0.02 (95% CI 0.01–0.04) by day 52, when the last positive roost aliquots were detected, and to 0.01 (95% CI 0–0.02) by day 65, when the last roost was sampled. We also fit a binomial model by using the days elapsed since the start of the calendar year ( Appendix 1 Figure), but alignment of the virus detections among the roosts was less clustered on that time axis than the days-since-patient-exposure time axis, and the binomial model did not show a significant trend in detection over time.
Roost urine samples from our study and individual urine samples from longitudinally sampled roosts in Epstein et al. ( 37 ) produced similar proportions of positive sampling visits (comparison A in Table ); the detection rate was also similar if only the first visit to each roost in our study was considered (7/23, 30%). In contrast, the proportion of positive aliquots from all sampling visits was significantly higher in our investigations than in the individual urine samples from longitudinal roosts in Epstein et al. ( 37 ) (comparison B in Table ). The detection rate from our study for positive urine aliquots at the first sampling visit was also higher than the detection rate for individual urine samples collected from 8 roosts from a cross-sectional study by Epstein et al. ( 37 ) (comparison C in Table ). The detection rate for positive urine aliquots from our study was substantially higher than the detection rate from similarly pooled urine aliquots from underneath longitudinal and cross-sectional roosts in Epstein et al. ( 37 ) (comparison D in Table ). Last, outbreak investigations of roosts performed by Epstein et al. ( 37 ) produced a higher detection rate than our own roost investigations (comparison E in Table ), although only 4 roosts were visited by Epstein et al. ( 37 ), and the same roosts were not repeatedly visited as we did in our study.
Nipah virus spillover from bats occurs sporadically in Bangladesh, so surveillance that optimizes viral detection in bats is a challenge. In contrast with cross-sectional or longitudinal bat roost surveillance used previously ( 37 ), the roost sampling in this study was triggered by Nipah virus outbreaks in nearby villages. Our approach identified roosts with active Nipah virus shedding at an equivalent rate to background surveillance ( 37 ) but had a higher detection rate in roost urine on a per sample basis. These results indicate that investigating roosts near spillover cases is more efficient than cross-sectional or longitudinal surveillance for obtaining samples with detectable viral RNA ( Table ). Repeated visits to positive roosts also demonstrated that viral RNA was detectable for weeks after the purported exposure date of human cases, although the proportion of positive urine aliquots declined sharply with time. Detections by PCR do not always produce sequences or genomes, so surveillance approaches that increase the number or quality of detections (e.g., higher viral loads) could maximize opportunities to collect samples with sufficient viral RNA for sequencing. These data suggest that rapid investigations to sample urine from bat roosts could increase the probability of detecting and sequencing Nipah virus. Used in combination with longitudinal sampling of roosts and surveillance of human or domesticated animal cases, this method could enhance our understanding of Nipah virus dynamics and genetic diversity in bats.
This study also provides critical information about the timing of Nipah virus shedding in bats in Bangladesh. Longitudinal surveys have shown that Nipah virus shedding from bats is sporadic throughout the year ( 37 ), so the peaks in viral detection in roost urine from our study likely coincided with shedding events. However, because these shedding events occurred during winter (when date palm sap is harvested for human consumption), bat visits to date palm trees might be more likely to contaminate sap with virus and lead to human infections ( 47 ). This factor suggests that the intensity of shedding events in bats occurring in winter could help to explain some of the spatiotemporal variation in the number of human spillovers that occur in Bangladesh annually ( 42 ), although more data on the frequency and timing of shedding events and human sap consumption will be needed to fully understand the dynamics of Nipah virus spillover.
Our findings come with several caveats because of limitations in our sample size and study design. Our analysis of factors associated with a roost testing positive at first sampling was unable to pinpoint significant relationships, likely because of low statistical power. We also did not systematically attempt virus isolation or sequencing in all positive samples, so we cannot estimate the probability of successful isolation or sequencing. However, Nipah virus isolates and sequences have been obtained from some of the roost urine samples included in this study. One of the positive roosts in Joypurhat from 2012 produced 9 nucleocapsid sequences (GenBank accession nos. MT890702–10) ( 24 ), and the positive roost in Manikganj from 2013 produced 10 virus isolates with full-genome sequences (GenBank accession nos. MK575060–9) ( 21 ). In fact, of the 39 Nipah virus sequences from bats in Bangladesh, 28 (72%) came from under-roost urine samples and 24 (86%) came from roost investigations near human cases ( Appendix 2 Table 2). These patterns suggest that roost urine, especially from roosts near human spillover cases, might contain sufficient Nipah virus for sequencing or culture. Furthermore, in several human case-patients in Joypurhat in 2012 who drank date palm sap, we identified Nipah virus sequences that were genetically similar (>99.6% sequence identity) to sequences from the Joypurhat bat roost (roost 1 in Figure 3 ), providing additional evidence that connects virus shedding in local bat populations with human cases ( Appendix 1 ). Future investigations could track how viral load in roost urine varies during viral shedding events, which could improve sequencing and isolation success and shed light on the ecologic conditions that lead to Nipah shedding from bats ( 48 ).
Our case investigations were also limited to the catchment area of 3 surveillance hospitals and the winter seasonality of Nipah virus spillover surveillance. This design systematically misses virus shedding events at bat roosts outside the surveillance area or during seasons when humans are not drinking fresh date palm sap ( 13 ). The logistical constraints of our surveillance approach cannot capture all Nipah virus genotypes circulating in P. medius across Bangladesh, but increasing the number of detections is still crucial, especially given the few Nipah virus isolates currently available (n = 11). Reactive roost investigations could be complemented with additional roost surveys outside of surveillance areas to learn more about Nipah virus transmission and genetic diversity in bat populations across Bangladesh.
This study provides proof of concept that reactive investigations of bat roosts near human Nipah virus cases can complement ongoing surveillance efforts and could increase the likelihood of viral detection and sequencing. Improvements in virus detection would aid in characterizing the genetic diversity of Nipah viruses circulating in bats and identify novel genotypes that might pose pandemic threats. Furthermore, these data provide evidence that viral shedding can continue for weeks after an initial spillover event, posing a hazard for additional contamination. Precise knowledge of when bats are shedding Nipah virus could be used to deploy public health campaigns more efficiently, such as by using barriers to prevent bat access to date palm sap ( 49 ).
Dr. McKee is a postdoctoral fellow in the Department of Epidemiology, John Hopkins Bloomberg School of Public Health. His primary research interests include microbiology, epidemiology, and wildlife disease ecology. Dr. Ausraful Islam is an assistant scientist in the Infectious Diseases Division at icddr,b. His primary research interests include zoonotic disease ecology and epidemiology.
This article was preprinted at https://www.biorxiv.org/content/10.1101/2021.12.29.474445v1 .

Acknowledgments
We thank the Bangladesh Forest Department, the Ministry of Environment and Forest for their permission to conduct these investigations. We thank Robert Fischer and Trenton Bushmaker for technical assistance with bat sample screening.
This work was funded by the DARPA PREEMPT program Cooperative Agreement (D18AC00031). Additional funds came from the National Institutes of Health (NIH) grant number 00991, National Academy of Science (NAS) grant number PGA-2000002048, and the US Agency for International Development (USAID) Emerging Pandemic Threats PREDICT Project Awards GHN-A-00-09-00010-00 and AID-OAA-A-14-00102. C.K.Y. and V.J.M. are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (1ZIAAI001179-01). R.K.P. was supported by the US National Science Foundation (DEB-1716698) and the USDA National Institute of Food and Agriculture (Hatch project 1015891). J.H.E., Ariful Islam, and P.D. were supported by USAID and NIH NIAID (AI153420). icddr,b acknowledges with gratitude the commitment of NIH, NAS, and DARPA to its research efforts. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden, and the United Kingdom for providing core/unrestricted support.
- Chadha MS , Comer JA , Lowe L , Rota PA , Rollin PE , Bellini WJ , et al. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis . 2006 ; 12 : 235 – 40 . DOI PubMed Google Scholar
- Chua KB , Bellini WJ , Rota PA , Harcourt BH , Tamin A , Lam SK , et al. Nipah virus: a recently emergent deadly paramyxovirus. Science . 2000 ; 288 : 1432 – 5 . DOI PubMed Google Scholar
- Hsu VP , Hossain MJ , Parashar UD , Ali MM , Ksiazek TG , Kuzmin I , et al. Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis . 2004 ; 10 : 2082 – 7 . DOI PubMed Google Scholar
- Ching PKG , de los Reyes VC , Sucaldito MN , Tayag E , Columna-Vingno AB , Malbas FF Jr , et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg Infect Dis . 2015 ; 21 : 328 – 31 . DOI PubMed Google Scholar
- Kessler MK , Becker DJ , Peel AJ , Justice NV , Lunn T , Crowley DE , et al. Changing resource landscapes and spillover of henipaviruses. Ann N Y Acad Sci . 2018 ; 1429 : 78 – 99 . DOI PubMed Google Scholar
- Williamson MM , Hooper PT , Selleck PW , Westbury HA , Slocombe RF . Experimental hendra virus infectionin pregnant guinea-pigs and fruit Bats ( Pteropus poliocephalus ). J Comp Pathol . 2000 ; 122 : 201 – 7 . DOI PubMed Google Scholar
- Halpin K , Hyatt AD , Fogarty R , Middleton D , Bingham J , Epstein JH , et al. ; Henipavirus Ecology Research Group . Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am J Trop Med Hyg . 2011 ; 85 : 946 – 51 . DOI PubMed Google Scholar
- Nikolay B , Salje H , Hossain MJ , Khan AKMD , Sazzad HMS , Rahman M , et al. Transmission of Nipah virus—14 years of investigations in Bangladesh. N Engl J Med . 2019 ; 380 : 1804 – 14 . DOI PubMed Google Scholar
- Arunkumar G , Chandni R , Mourya DT , Singh SK , Sadanandan R , Sudan P , et al. ; Nipah Investigators People and Health Study Group . Outbreak investigation of Nipah virus disease in Kerala, India, 2018. J Infect Dis . 2019 ; 219 : 1867 – 78 . DOI PubMed Google Scholar
- Robinson TP , Wint GRW , Conchedda G , Van Boeckel TP , Ercoli V , Palamara E , et al. Mapping the global distribution of livestock. PLoS One . 2014 ; 9 : e96084 . DOI PubMed Google Scholar
- Walsh MG , Sawleshwarkar S , Hossain S , Mor SM . Whence the next pandemic? The intersecting global geography of the animal-human interface, poor health systems and air transit centrality reveals conduits for high-impact spillover. One Health . 2020 ; 11 : 100177 . DOI PubMed Google Scholar
- Luby SP , Gurley ES , Hossain MJ . Transmission of human infection with Nipah virus. Clin Infect Dis . 2009 ; 49 : 1743 – 8 . DOI PubMed Google Scholar
- Gurley ES , Hegde ST , Hossain K , Sazzad HMS , Hossain MJ , Rahman M , et al. Convergence of humans, bats, trees, and culture in Nipah virus transmission, Bangladesh. Emerg Infect Dis . 2017 ; 23 : 1446 – 53 . DOI PubMed Google Scholar
- Pulliam JRC , Epstein JH , Dushoff J , Rahman SA , Bunning M , Jamaluddin AA , et al. ; Henipavirus Ecology Research Group (HERG) . Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J R Soc Interface . 2012 ; 9 : 89 – 101 . DOI PubMed Google Scholar
- Mohd Nor MN , Gan CH , Ong BL . Nipah virus infection of pigs in peninsular Malaysia. Rev Sci Tech . 2000 ; 19 : 160 – 5 . DOI PubMed Google Scholar
- Chua KB . Nipah virus outbreak in Malaysia. J Clin Virol . 2003 ; 26 : 265 – 75 . DOI PubMed Google Scholar
- Mehand MS , Al-Shorbaji F , Millett P , Murgue B . The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res . 2018 ; 159 : 63 – 7 . DOI PubMed Google Scholar
- Geisbert TW , Bobb K , Borisevich V , Geisbert JB , Agans KN , Cross RW , et al. A single dose investigational subunit vaccine for human use against Nipah virus and Hendra virus. NPJ Vaccines . 2021 ; 6 : 23 . DOI PubMed Google Scholar
- Olival KJ , Hosseini PR , Zambrana-Torrelio C , Ross N , Bogich TL , Daszak P . Host and viral traits predict zoonotic spillover from mammals. [Erratum in: Nature. 2017;548:612] . Nature . 2017 ; 546 : 646 – 50 . DOI PubMed Google Scholar
- Whitmer SLM , Lo MK , Sazzad HMS , Zufan S , Gurley ES , Sultana S , et al. Inference of Nipah virus evolution, 1999–2015. Virus Evol. 2021 ;7:veaa062.
- Anderson DE , Islam A , Crameri G , Todd S , Islam A , Khan SU , et al. Isolation and full-genome characterization of Nipah viruses from bats, Bangladesh. Emerg Infect Dis . 2019 ; 25 : 166 – 70 . DOI PubMed Google Scholar
- Olival KJ , Latinne A , Islam A , Epstein JH , Hersch R , Engstrand RC , et al. Population genetics of fruit bat reservoir informs the dynamics, distribution and diversity of Nipah virus. Mol Ecol . 2020 ; 29 : 970 – 85 . DOI PubMed Google Scholar
- Harcourt BH , Lowe L , Tamin A , Liu X , Bankamp B , Bowden N , et al. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg Infect Dis . 2005 ; 11 : 1594 – 7 . DOI PubMed Google Scholar
- Rahman MZ , Islam MM , Hossain ME , Rahman MM , Islam A , Siddika A , et al. Genetic diversity of Nipah virus in Bangladesh. Int J Infect Dis . 2021 ; 102 : 144 – 51 . DOI PubMed Google Scholar
- Makeyev EV , Bamford DH . Evolutionary potential of an RNA virus. J Virol . 2004 ; 78 : 2114 – 20 . DOI PubMed Google Scholar
- Drake JW , Holland JJ . Mutation rates among RNA viruses. Proc Natl Acad Sci U S A . 1999 ; 96 : 13910 – 3 . DOI PubMed Google Scholar
- Luby SP . The pandemic potential of Nipah virus. Antiviral Res . 2013 ; 100 : 38 – 43 . DOI PubMed Google Scholar
- Mounts AW , Kaur H , Parashar UD , Ksiazek TG , Cannon D , Arokiasamy JT , et al. ; Nipah Virus Nosocomial Study Group . A cohort study of health care workers to assess nosocomial transmissibility of Nipah virus, Malaysia, 1999. J Infect Dis . 2001 ; 183 : 810 – 3 . DOI PubMed Google Scholar
- Goh KJ , Tan CT , Chew NK , Tan PSK , Kamarulzaman A , Sarji SA , et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med . 2000 ; 342 : 1229 – 35 . DOI PubMed Google Scholar
- Parashar UD , Sunn LM , Ong F , Mounts AW , Arif MT , Ksiazek TG , et al. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998-1999 outbreak of severe encephalitis in Malaysia. J Infect Dis . 2000 ; 181 : 1755 – 9 . DOI PubMed Google Scholar
- Arankalle VA , Bandyopadhyay BT , Ramdasi AY , Jadi R , Patil DR , Rahman M , et al. Genomic characterization of Nipah virus, West Bengal, India. Emerg Infect Dis . 2011 ; 17 : 907 – 9 . DOI PubMed Google Scholar
- Chong HT , Hossain MJ , Tan CT . Differences in epidemiologic and clinical features of Nipah virus encephalitis between the Malaysian and Bangladesh outbreaks. Neurol Asia . 2008 ; 13 : 23 – 6 .
- Hossain MJ , Gurley ES , Montgomery JM , Bell M , Carroll DS , Hsu VP , et al. Clinical presentation of nipah virus infection in Bangladesh. Clin Infect Dis . 2008 ; 46 : 977 – 84 . DOI PubMed Google Scholar
- Mire CE , Satterfield BA , Geisbert JB , Agans KN , Borisevich V , Yan L , et al. Pathogenic differences between Nipah virus Bangladesh and Malaysia strains in primates: implications for antibody therapy. Sci Rep . 2016 ; 6 : 30916 . DOI PubMed Google Scholar
- Clayton BA , Middleton D , Bergfeld J , Haining J , Arkinstall R , Wang L , et al. Transmission routes for nipah virus from Malaysia and Bangladesh. Emerg Infect Dis . 2012 ; 18 : 1983 – 93 . DOI PubMed Google Scholar
- DeBuysscher BL , de Wit E , Munster VJ , Scott D , Feldmann H , Prescott J . Comparison of the pathogenicity of Nipah virus isolates from Bangladesh and Malaysia in the Syrian hamster. PLoS Negl Trop Dis . 2013 ; 7 : e2024 . DOI PubMed Google Scholar
- Epstein JH , Anthony SJ , Islam A , Kilpatrick AM , Ali Khan S , Balkey MD , et al. Nipah virus dynamics in bats and implications for spillover to humans. Proc Natl Acad Sci U S A . 2020 ; 117 : 29190 – 201 . DOI PubMed Google Scholar
- Yadav PD , Shete AM , Kumar GA , Sarkale P , Sahay RR , Radhakrishnan C , et al. Nipah virus sequences from humans and bats during Nipah outbreak, Kerala, India, 2018. Emerg Infect Dis . 2019 ; 25 : 1003 – 6 . DOI PubMed Google Scholar
- Naser AM , Hossain MJ , Sazzad HMS , Homaira N , Gurley ES , Podder G , et al. Integrated cluster- and case-based surveillance for detecting stage III zoonotic pathogens: an example of Nipah virus surveillance in Bangladesh. Epidemiol Infect . 2015 ; 143 : 1922 – 30 . DOI PubMed Google Scholar
- Ao TT , Rahman M , Haque F , Chakraborty A , Hossain MJ , Haider S , et al. Low-cost national media-based surveillance system for public health events, Bangladesh. Emerg Infect Dis . 2016 ; 22 : 720 – 2 . DOI PubMed Google Scholar
- Daniels P , Ksiazek T , Eaton BT . Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect . 2001 ; 3 : 289 – 95 . DOI PubMed Google Scholar
- Nikolay B , Salje H , Khan AKMD , Sazzad HMS , Satter SM , Rahman M , et al. A framework to monitor changes in transmission and epidemiology of emerging pathogens: lessons from Nipah virus. J Infect Dis . 2020 ; 221 ( Suppl 4 ): S363 – 9 . DOI PubMed Google Scholar
- Giles JR , Peel AJ , Wells K , Plowright RK , McCallum H , Restif O . Optimizing noninvasive sampling of a zoonotic bat virus. Ecol Evol . 2021 ; 11 : 12307 – 21 . DOI PubMed Google Scholar
- Lo MK , Lowe L , Hummel KB , Sazzad HMS , Gurley ES , Hossain MJ , et al. Characterization of Nipah virus from outbreaks in Bangladesh, 2008-2010. Emerg Infect Dis . 2012 ; 18 : 248 – 55 . DOI PubMed Google Scholar
- Rahman MA , Hossain MJ , Sultana S , Homaira N , Khan SU , Rahman M , et al. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis . 2012 ; 12 : 65 – 72 . DOI PubMed Google Scholar
- Burnham KP , Anderson DR . Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res . 2004 ; 33 : 261 – 304 . DOI Google Scholar
- Khan MSU , Hossain J , Gurley ES , Nahar N , Sultana R , Luby SP . Use of infrared camera to understand bats’ access to date palm sap: implications for preventing Nipah virus transmission. EcoHealth . 2010 ; 7 : 517 – 25 . DOI PubMed Google Scholar
- Plowright RK , Peel AJ , Streicker DG , Gilbert AT , McCallum H , Wood J , et al. Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir–host populations. PLoS Negl Trop Dis . 2016 ; 10 : e0004796 . DOI PubMed Google Scholar
- Khan SU , Gurley ES , Hossain MJ , Nahar N , Sharker MAY , Luby SP . A randomized controlled trial of interventions to impede date palm sap contamination by bats to prevent nipah virus transmission in Bangladesh. PLoS One . 2012 ; 7 : e42689 . DOI PubMed Google Scholar
- Table . Nipah virus detection success from study of bat roosts after spillover events, Bangladesh, 2012–2019, compared with results from previous study
DOI: 10.3201/eid2807.212614
Original Publication Date: June 06, 2022
1 These first authors contributed equally to this article.
Table of Contents – Volume 28, Number 7—July 2022
| EID Search Options |
|---|
| – Search articles by author and/or keyword. |
| – Search articles by the topic country. |
| – Search articles by article type and issue. |
Please use the form below to submit correspondence to the authors or contact them at the following address:
Clifton D. McKee, Department of Epidemiology, John Hopkins Bloomberg School of Public Health, 615 N Wolfe St, Baltimore, MD 21205, USA
Comment submitted successfully, thank you for your feedback.
There was an unexpected error. Message not sent.
Article Citations
Highlight and copy the desired format.
| EID | McKee CD, Islam A, Rahman M, Khan S, Rahman M, Satter SM, et al. Nipah Virus Detection at Bat Roosts after Spillover Events, Bangladesh, 2012–2019. Emerg Infect Dis. 2022;28(7):1384-1392. https://doi.org/10.3201/eid2807.212614 |
|---|---|
| AMA | McKee CD, Islam A, Rahman M, et al. Nipah Virus Detection at Bat Roosts after Spillover Events, Bangladesh, 2012–2019. . 2022;28(7):1384-1392. doi:10.3201/eid2807.212614. |
| APA | McKee, C. D., Islam, A., Rahman, M., Khan, S., Rahman, M., Satter, S. M....Gurley, E. S. (2022). Nipah Virus Detection at Bat Roosts after Spillover Events, Bangladesh, 2012–2019. , (7), 1384-1392. https://doi.org/10.3201/eid2807.212614. |
Metric Details
Article views: 1691.
Data is collected weekly and does not include downloads and attachments. View data is from .
What is the Altmetric Attention Score?
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Health Sci Rep
- v.6(7); 2023 Jul
- PMC10336337
The recent Nipah virus outbreak in Bangladesh could be a threat for global public health: A brief report
Nazmunnahar.
1 Department of Sociology, Eden Women's College, National University Bangladesh, Gazipur Bangladesh
Iftekhar Ahmed
2 Department of Pharmacy, University of Asia Pacific, Dhaka Bangladesh
A. S. M. Roknuzzaman
Md. rabiul islam, associated data.
Data available on request from the authors.
The Nipah virus is a zoonotic infection that can potentially be transmitted from person to person as well as through ingesting contaminated food. It has a high fatality rate, and no treatment or cure at present. Several nations in South Asia have reported Nipah virus outbreaks occurred during a particular season of the year. Since it was first found in Bangladesh in 2001, there have been a total of 335 people infected with it, and 237 of those people have passed away as a result of their infection. With increased public awareness, community engagement, and preventative measures, this potentially fatal virus has been suppressed. Yet, following a pandemic and a considerable increase in the health burden, the transmission rate continuously increased over a few years, indicating that there is a growing possibility to become a global public health concern. Without effective vaccines and reliable treatment options, its capacity for human‐to‐human transmission and potential to spread throughout the area could result in a disastrous public health emergency worldwide.
1. BACKGROUND
The recent outbreak of deadly Nipah virus (NiV) infection has raised concern and fear among the general population, who are still fighting the COVID‐19. 1 According to the World Health Organization (WHO), NiV infection is a zoonotic infection that typically transmits from animal to human. But it may also get transmitted through food contaminated with the virus. Even human transmission of this deadly virus is possible. According to WHO, the mortality rate of infected population by the NiV is extremely high. 2 Fruit bats of the Pteropus genus are a natural reservoir host of the NiV. 3 But there have been several reported cases of human‐to‐human transmission. 4 This deadly virus has been found in urine and respiratory secretions of infected individuals. 5 Besides, the NiV is an RNA virus that is prone to mutation. 6 This virus may mutate suddenly and increase its power of transmission and fatality. Similar to COVID‐19, the NiV is a potential virus that may cause a pandemic on a global scale. 7 There are no established treatments against the NiV infection. The care for infected patients primarily consists of symptomatic treatments and supportive. 8 Its high mortality rate is a matter of concern. Moreover, the absence of efficacious treatment measures makes this virus a significant threat to the global public health. 9 The outbreak in Bangladesh may be a wake‐up call to the rest of the world. Hence, scientists, the government, and epidemiologists should be aware and take precautionary steps as quickly as possible.
2. HISTORY OF NIPAH VIRUS INFECTION
From 1998 to 1999, NiV infection among humans was first identified. 10 At that time, the pig was associated with the NiV outbreak. Malaysia launched a pig culling operation to curb the epidemic. This outbreak killed 105 people in Malaysia. 11 By this time, the outbreak had spread to Singapore. The country used to import pigs from Malaysia. The virus entered Singapore through the pig slaughterhouse. It could only kill one person due to the prompt action of the government of Singapore. The Singaporean authorities prohibited the export of pigs from Malaysia to prevent a possible epidemic. 12 In early 2001, an encephalitis outbreak due to the NiV was recorded in Siliguri, India. It killed 45 infected individuals. The series of events suggest the association between the outbreaks. 13 Another outbreak in the Nadia district in India killed five people with a 100% mortality rate. 14 In 2018, the NiV outbreak in Kerala caused 21 deaths. 15 In 2014, the NiV was found in people with neurological diseases like encephalitis, meningitis, and so forth, in the Philippines. Several horses died during that period as well. Therefore, the horse was thought to act as an intermediate host in this outbreak. 16 At first, researchers identified the virus causing the outbreak as the Japanese encephalitis virus. Scientists later identified the virus and its reservoir host as bats. 3 The epidemics in Malaysia and Singapore happened because of pigs, as the pigs may have been the intermediate hosts. The pigs may have consumed bat‐contaminated fruits.
3. EPIDEMIOLOGY OF NIPAH VIRUS INFECTION
Although the NiV is zoonotic disease but it can be transmitted through contaminated food and date palm sap (DPS). NiV is found in the secretions of bats. In addition, it can be transmitted through body secretions such as saliva and respiratory droplets of an infected individual. A survey in Goalondo suggests that people who climb trees are more susceptible to infection. 17 , 18 According to the WHO, the fatality rate due to NiV infection ranges between 40% and 75%. 2 Institute of Epidemiology, Disease Control and Research (IEEDCR), Bangladesh estimated that the local death rate due to NiV infection is approximately 73%. Notwithstanding the fact that the fatality rate is contingent on the clinical and epidemiological knowledge of the outbreak region, it is sufficient to cause global alarm. NiV infection causes a severe inflammation in the brain called encephalitis. The high mortality rate of NiV infection associated with encephalitis is a common feature. 19 There are currently no specific treatments for this viral infection. The majority of treatments for those infected with the NiV are symptomatic and supportive. Anticonvulsants are used for seizure prevention. Treatment is given against secondary infections. Those suffering from respiratory distress are administered ventilation and intensive care unit treatments. Ribavirin was administered as a broad‐spectrum antiviral during the outbreak in Malaysia, which lowered mortality somewhat. 20 In Singapore, Acyclovir was utilized. But, its effectiveness against the infection is inexplicable. In India, anti‐G and anti‐F monoclonal antibodies were utilized in emergency situations. 21
4. RECENT NIPAH VIRUS OUTBREAK IN BANGLADESH
We have seen several outbreaks of NiV infection in India and some other South Asian countries. Bangladesh is three‐sided bordered by India. Therefore, the country recorded its very first outbreak of NiV infection in 2001 along with India. NiV infection outbreaks are seasonal in Bangladesh. People suffered from encephalitis in the Meherpur district of Bangladesh. But the virus was not identified there. A similar event was seen in parts of the Naogaon district that killed eight people. Concerns were raised regarding the similarities between the events and their causative agent. After that, samples were sent to CDC. CDC reported that the disease was caused by the NiV. 22 After 2001, NiV outbreaks were reported annually in India and Bangladesh. 23 Since 2001, seasonal outbreaks of NiV infection between December and May, consistent with the DPS harvesting season in the country from November to March. Reported cases ranged from 0 to 67 from 2001 to 2016. The country observed a reduced number of reported cases from 2016 due to the mass awareness campaign against the consumption of raw DPS.
However, between January 4, 2013, to February 13, 2023, 11 cases (10 laboratories confirmed and one probable) of NiV infection cases and eight associated deaths were reported from seven districts of two divisions in Bangladesh. It is the highest number of reported NiV cases and deaths after 2015 when the country recorded 15 NiV cases and 11 associated deaths (WHO). Dhaka division reported six NiV cases and four associated deaths (6/4) from the districts of Narsingdi (1/1), Rajbari (4/3), and Shariatpur (1/0). Rajshahi Division reported five cases and four related deaths (5/4) from the districts of Naogaon (2/1), Natore (1/1), Pabna (1/1), and Rajshahi (1/1). The average case fatality rate is 73% which is very alarming. Among the 11 cases, 4 were females, and 7 were males. The median age of the case was 16 years ranging from 15 to 50. Ten NiV cases had a raw DPS drinking history out of 11. The median incubation period was 14 days ranging from 3 to 15. After the onset of symptoms, all 11 cases were hospitalized. 24 Year wise number of reported NiV cases and deaths in Bangladesh from 2001 to 2013 are presented in Figure 1 . 24 , 25 A geographical distribution‐comparison of so far and this year's outbreak in Bangladesh is shown in Figure 2 . 24 , 25

Number of reported Nipah virus cases and deaths from 2001 to 2023, Bangladesh. This figure has been adopted from the original work “Nipah virus disease–Bangladesh. Geneva: World Health Organization (WHO); 2023. Licence: CC BY‐NC‐SA 3.0 IGO.” This adoptation was not created by WHO. WHO is not responsible for the content or accuracy of this adoptation. The original edition shall be the binding and authentic edition. Source : Bangladesh Ministry of Health and Family Welfare. *As of 16 February, 2013.

Comparison of Nipah virus distribution in Bangladesh. Source : Bangladesh Ministry of Health and Family Welfare. *As of February 16, 2023.
5. A POTENTIAL THREAT TO GLOBAL PUBLIC HEALTH
Current circumstances indicate that the NiV has the potential to cause global public health emergency. We have previously observed that the viral outbreak in Bangladesh extended to India. 13 Hence, the virus can circumvent regional borders and spread to adjacent nations. The proven instances of human‐to‐human transmission are a grave warning to the entire globe. 18 It can spread through saliva and respiratory droplets. Hence, there will inevitably be a case of secondary transmission. Past outbreaks indicate that domestic animals are responsible for the virus's propagation. Thus, domestic animals were slaughtered to combat the epidemic. Further outbreaks may result in the death of the cattle, and people may be forced to kill the animals as well. This will exacerbate the persistent global food shortage and developing agricultural issues. Infectious disease has a significant negative impact on society's socioeconomic balance as well. 26 The NiV is a related RNA virus to the coronavirus. Mutations of the RNA virus are a regular occurrence. Similar to the coronavirus, the NiV can undergo rapid mutations that increase its virulence, transmission, mortality, and morbidity. 27 Globally, infectious diseases have a negative impact on people's mental health. For example, the COVID‐19 pandemic has significantly affected the mental equilibrium of people of all ages. 28 Infected individuals with the NiV also exhibit depressive symptoms and other mental disorders. So, it can be hypothesized that the NiV is capable of causing a pandemic with catastrophic results.
6. RISK COMMUNICATION AND PREVENTATIVE MEASURES
As there are no effective treatments against the NiV infection, preventive measures should be strictly followed. Reports suggest that the raw DPS contaminated by a bat is responsible for the NiV infection in Bangladesh. Therefore, people should not be allowed to drink raw date palm juice. Besides, fruits partially eaten by bats should not be consumed as well. Seminars and workshops should be arranged for rural people not accustomed to following health care measures. Pigs and horses were responsible for the outbreaks in Malaysia and the Philippines, respectively. 11 , 16 So, the infected animals should be identified as quickly as possible and should be isolated. The infected animals might be killed by the government if needed. Supportive treatments like mechanical ventilation must be ensured throughout the country, specifically in the areas prone to outbreaks. Adequate supportive care will reduce the mortality rate as well. 2 Bats are the natural reservoir host of the NiV. So, areas inhabited by bats should be appropriately monitored. Human‐to‐human transmission is possible, so anyone who comes into direct contact with an infected person or eats contaminated fruit or raw DPS and develops symptoms like fever, headache, myalgia, vomiting, neurological issues, encephalitis, and respiratory distress should be isolated immediately to prevent secondary transmission. They should be kept inside a ward dedicated to NiV infection. They can only be discharged from there after getting a negative RT‐PCR result. As the incubation period is not fully elucidated, the discharged patients are also advised to get isolated for 21 days from the day of infection detection. 29 Unfortunately, no currently established therapeutics or vaccines are available against this virus. So, we have to emphasize prevention rather than treatment. The Old people is typically thought to be the most reluctant to alter their lifelong health behaviors. 30 Seminars targeting the old people should be arranged to raise awareness against this deadly virus. Healthcare professionals should wear PPE while providing health services. Early detection is the key to preventing any outbreaks caused by infectious diseases. Hence, the suspected person should be tested quickly. The government needs to ensure diagnostic facilities in the affected areas. BSL‐4 must be maintained in the lab to prevent further infection while handling. Antiviral drugs may be prescribed for infected people. WHO has identified NiV infection as a priority disease for research. Other concerned authorities should also come forward to raise awareness among the general population regarding this new enemy. Currently, there are no vaccines or therapeutics available against the NiV. Scientists must take prompt action to develop an effective vaccine against the NiV, as they have already developed corona vaccines very quickly. 31 Besides, effective antiviral drugs must be discovered and formulated to treat this disease. Affected people show signs of depression and other symptoms of neurological disorders in the long run. These need to be correctly addressed and effectively treated. People of all classes have been severely affected by COVID‐19. 32 The newer variants of COVID‐19 are always a headache to the world. 33 , 34 Among the existing pandemic, the world and its inhabitants are not ready for another pandemic.
7. COMMUNITY INVOLVEMENT IN EFFECTIVE RESPONSE
Moreover, the mental health of people worldwide has been affected by quarantine and isolation. This emerging virus may also cause people to follow the similar preventive measures, which will be very hard for the general population to deal with. Hence, healthcare professionals, government, and respective authorities should take necessary steps to prevent the outbreak and raise awareness among the general population. Authorities concerned with the public health should always keep monitoring the emergence of infectious diseases to take prompt action. 35 Lessons learnt from COVID‐19 pandemic may help to direct health policies to combat this new emerging infectious disease. 36 Furthermore, the concerned health authorities should create comprehensive preventive and therapeutic strategies to counteract the recent rise of this viral infection. They should develop evidence‐based treatment and management guidelines. 37 Researchers should also conduct studies to develop vaccines and therapeutics. Considering the recent outbreaks of COVID‐19 and monkeypox, the researchers and respective authorities should be aware of the NiV outbreak. More research should be conducted to elaborate on the nature, symptoms, and treatments of NiV infection.
8. CONCLUSION
The latest outbreak of NiV in Bangladesh, which resulted in an extremely high case fatality rate, will create pressure on public health systems already struggling to cope with the COVID‐19. Due to the high mortality rate associated with this virus, it has the potential to cause death and is even more dangerous than coronavirus disease. In addition, it can be transmitted from human to human makes it a possible risk for another pandemic. Therefore, the respective authorities should take the necessary actions to stop the current spread of the virus.
AUTHOR CONTRIBUTIONS
Nazmunnahar : Conceptualization; data curation; writing—original draft. Iftekhar Ahmed : Conceptualization; data curation; writing—original draft. A. S. M. Roknuzzaman : Conceptualization; supervision; writing—review and editing. Md. Rabiul Islam : Conceptualization; supervision; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Md. Rabiul Islam affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Nazmunnahar, Ahmed I, Roknuzzaman ASM, Islam MR. Recent Nipah virus outbreak in Bangladesh could be a threat for global public health: a brief report . Health Sci Rep . 2023; 6 :e1423. 10.1002/hsr2.1423 [ CrossRef ] [ Google Scholar ]
DATA AVAILABILITY STATEMENT
Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Observatory
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /
Dengue and severe dengue
- Dengue is a viral infection transmitted to humans through the bite of infected mosquitoes.
- About half of the world's population is now at risk of dengue with an estimated 100–400 million infections occurring each year.
- Dengue is found in tropical and sub-tropical climates worldwide, mostly in urban and semi-urban areas.
- While many dengue infections are asymptomatic or produce only mild illness, the virus can occasionally cause more severe cases, and even death.
- Prevention and control of dengue depend on vector control. There is no specific treatment for dengue/severe dengue, and early detection and access to proper medical care greatly lower fatality rates of severe dengue.
Dengue (break-bone fever) is a viral infection that spreads from mosquitoes to people. It is more common in tropical and subtropical climates.
Most people who get dengue will not have symptoms. But for those who do, the most common symptoms are high fever, headache, body aches, nausea, and rash. Most will get better in 1–2 weeks. Some people develop severe dengue and need care in a hospital.
In severe cases, dengue can be fatal.
You can lower your risk of dengue by avoiding mosquito bites especially during the day.
Dengue is treated with pain medicine as there is no specific treatment currently.
Most people with dengue have mild or no symptoms and will get better in 1–2 weeks. Rarely, dengue can be severe and lead to death.
If symptoms occur, they usually begin 4–10 days after infection and last for 2–7 days. Symptoms may include:
- high fever (40°C/104°F)
- severe headache
- pain behind the eyes
- muscle and joint pains
- swollen glands
- rash.
Individuals who are infected for the second time are at greater risk of severe dengue.
Severe dengue symptoms often come after the fever has gone away:
- severe abdominal pain
- persistent vomiting
- rapid breathing
- bleeding gums or nose
- restlessness
- blood in vomit or stool
- being very thirsty
- pale and cold skin
- feeling weak.
People with these severe symptoms should get care right away.
After recovery, people who have had dengue may feel tired for several weeks.
Diagnostics and treatment
There is no specific treatment for dengue. The focus is on treating pain symptoms. Most cases of dengue fever can be treated at home with pain medicine.
Acetaminophen (paracetamol) is often used to control pain. Non-steroidal anti-inflammatory drugs like ibuprofen and aspirin are avoided as they can increase the risk of bleeding.
For people with severe dengue, hospitalization is often needed.
Global burden
The incidence of dengue has grown dramatically around the world in recent decades, with cases reported to WHO increasing from 505 430 cases in 2000 to 5.2 million in 2019. A vast majority of cases are asymptomatic or mild and self-managed, and hence the actual numbers of dengue cases are under-reported. Many cases are also misdiagnosed as other febrile illnesses (1) .
The highest number of dengue cases was recorded in 2023, affecting over 80 countries in all regions of WHO. Since the beginning of 2023 ongoing transmission, combined with an unexpected spike in dengue cases, resulted in a historic high of over 6.5 million cases and more than 7300 dengue-related deaths reported.
Several factors are associated with the increasing risk of spread of the dengue epidemic: the changing distribution of the vectors (chiefly Aedes aegypti and Aedes albopictus mosquitoes), especially in previously dengue naïve countries; the consequences of El Niño phenomena in 2023 and climate change leading to increasing temperatures and high rainfall and humidity; fragile health systems in the midst of the COVID-19 pandemic; and political and financial instabilities in countries facing complex humanitarian crises and high population movements.
One modelling estimate indicates 390 million dengue virus infections per year of which 96 million manifest clinically (2) . Another study on the prevalence of dengue estimates that 3.9 billion people are at risk of infection with dengue viruses (3).
The disease is now endemic in more than 100 countries in the WHO Regions of Africa, the Americas, the Eastern Mediterranean, South-East Asia and the Western Pacific. The Americas, South-East Asia and Western Pacific regions are the most seriously affected, with Asia representing around 70% of the global disease burden.
Dengue is spreading to new areas in Europe, the Eastern Mediterranean and South America.
The largest number of dengue cases reported was in 2023. The WHO Region of the Americas reported 4.5 million cases, with 2300 deaths. A high number of cases were reported in Asia: Bangladesh (321 000), Malaysia (111 400), Thailand (150 000), and Viet Nam (369 000).
Transmission
Transmission through the mosquito bite
The dengue virus is transmitted to humans through the bites of infected female mosquitoes, primarily the Aedes aegypti mosquito. Other species within the Aedes genus can also act as vectors, but their contribution is normally secondary to Aedes aegypti . However, in 2023, a surge in local transmission of dengue by Aedes albopictus (tiger mosquito) has been seen in Europe.
After feeding on a infected person, the virus replicates in the mosquito midgut before disseminating to secondary tissues, including the salivary glands. The time it takes from ingesting the virus to actual transmission to a new host is termed the extrinsic incubation period (EIP). The EIP takes about 8–12 days when the ambient temperature is between 25–28°C. Variations in the extrinsic incubation period are not only influenced by ambient temperature; several factors such as the magnitude of daily temperature fluctuations, virus genotype, and initial viral concentration can also alter the time it takes for a mosquito to transmit the virus. Once infectious, the mosquito can transmit the virus for the rest of its life .
Human-to-mosquito transmission
Mosquitoes can become infected by people who are viremic with the dengue virus. This can be someone who has a symptomatic dengue infection, someone who is yet to have a symptomatic infection (they are pre-symptomatic), and also someone who shows no signs of illness (they are asymptomatic).
Human-to-mosquito transmission can occur up to 2 days before someone shows symptoms of the illness, and up to 2 days after the fever has resolved.
The risk of mosquito infection is positively associated with high viremia and high fever in the patient; conversely, high levels of DENV-specific antibodies are associated with a decreased risk of mosquito infection. Most people are viremic for about 4–5 days, but viremia can last as long as 12 days.
Maternal transmission
The primary mode of transmission of the dengue virus between humans involves mosquito vectors. There is evidence however, of the possibility of maternal transmission (from a pregnant mother to her baby). At the same time, vertical transmission rates appear low, with the risk of vertical transmission seemingly linked to the timing of the dengue infection during the pregnancy. When a mother does have a dengue infection when she is pregnant, babies may suffer from pre-term birth, low birthweight, and fetal distress.
Other transmission modes
Rare cases of transmission via blood products, organ donation and transfusions have been recorded. Similarly, transovarial transmission of the virus within mosquitoes have also been recorded.
Risk factors
Previous infection with DENV increases the risk of the individual developing severe dengue.
Urbanization (especially unplanned), is associated with dengue transmission through multiple social and environmental factors: population density, human mobility, access to reliable water source, water storage practice etc.
Community risks to dengue also depend on a population’s knowledge, attitude and practice towards dengue, as the exposure is closely related to behaviours such as water storage, plant keeping, and self-protection against mosquito bites. Routine vector surveillance and control activities engaging community greatly enhances a community’s resilience.
Vectors might adapt to new environments and climate. The interaction between dengue virus, the host and the environment is dynamic. Consequently, disease risks may change and shift with climate change in tropical and subtropical areas, in combination with increased urbanization and movement of populations.
Prevention and control
The mosquitoes that spread dengue are active during the day.
Lower the risk of getting dengue by protecting yourself from mosquito bites by using:
- clothes that cover as much of your body as possible;
- mosquito nets if sleeping during the day, ideally nets sprayed with insect repellent;
- window screens;
- mosquito repellents (containing DEET, Picaridin or IR3535); and
- coils and vaporizers.
Mosquito breeding can be prevented by:
- preventing mosquitoes from accessing egg-laying habitats by environmental management and modification;
- disposing of solid waste properly and removing artificial man-made habitats that can hold water;
- covering, emptying and cleaning domestic water storage containers on a weekly basis;
- applying appropriate insecticides to outdoor water storage containers.
If you get dengue, it’s important to:
- drink plenty of liquids;
- use acetaminophen (paracetamol) for pain;
- avoid non-steroidal anti-inflammatory drugs, like ibuprofen and aspirin; and
- watch for severe symptoms and contact your doctor as soon as possible if you notice any.
So far one vaccine (QDenga) has been approved and licensed in some countries. However, it is recommended only for the age group of 6 to 16 years in high transmission settings. Several additional vaccines are under evaluation.
WHO response
WHO responds to dengue in the following ways:
- supports countries in the confirmation of outbreaks through its collaborating network of laboratories;
- provides technical support and guidance to countries for the effective management of dengue outbreaks;
- supports countries in improving their reporting systems and capture the true burden of the disease;
- provides training on clinical management, diagnosis and vector control at the country and regional level with some of its collaborating centres;
- formulates evidence-based strategies and policies;
- support countries in the development of dengue prevention and control strategies and adopting the Global Vector Control Response (2017–2030) and the Global Arbovirus Initiative (2022–2025).
- reviews and recommends the development of new tools, including insecticide products and application technologies;
- gathers official records of dengue and severe dengue from over 100 Member States; and
- publishes guidelines and handbooks for surveillance, case management, diagnosis, dengue prevention and control for Member States.
- Waggoner, J.J., et al., Viremia and Clinical Presentation in Nicaraguan Patients Infected Wi1. Waggoner, J.J., et al., Viremia and Clinical Presentation in Nicaraguan Patients Infected With Zika Virus, Chikungunya Virus, and Dengue Virus. Clinical Infectious Diseases, 2016. 63(12): p. 1584-1590.
- Bhatt, S., et al., The global distribution and burden of dengue. Nature , 2013. 496(7446): p. 504–507.
- Brady, O.J., et al., Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLOS Neglected Tropical Diseases , 2012. 6(8): p. e1760.

COMMENTS
Background: In Bangladesh, 4 outbreaks of Nipah virus infection were identified during the period 2001-2004. Methods: We characterized the clinical features of Nipah virus-infected individuals affected by these outbreaks. We classified patients as having confirmed cases of Nipah virus infection if they had antibodies reactive with Nipah virus antigen.
Nipah virus is a recently identified paramyxovirus that is closely related to Hendra virus [].The first recognized outbreaks of Nipah virus illness in humans occurred in Malaysia and Singapore from September 1998 through June 1999; 283 persons, mostly pig farm and abattoir workers were infected through contact with sick pigs [].A case-fatality rate of 40% was observed in Malaysia and Singapore ...
Clinical characteristics of Nipah virus infection in Bangladesh, including a. Nipah virus is a recently identified paramyxovirus Nipah virus that infection, with a case-fatality rate of 68%, is closely related to Hendra virus [1]. The occurred first recog- from January through February 2001 in Sil-.
The overall epidemiology of Nipah virus infection in Bangladesh has remained consistent throughout the years. National Nipah surveillance of Bangladesh is the only global, systematic surveillance to detect human NiV infection. ... clinical presentation, and fatality [5,11,17]. Despite having such a robust surveillance platform, most of the ...
Nipah virus infection in 82 of the 248 case patients was suspected to be the result of person-to-person transmission, which corresponded to a reproduction number of 0.33 (95% confidence interval ...
In Bangladesh, 4 outbreaks of Nipah virus infection were identified during the period 2001-2004. Methods We characterized the clinical features of Nipah virus-infected individuals affected by these outbreaks. We classified patients as having confirmed cases of Nipah virus infection if they had antibodies reactive with Nipah virus antigen.
(2008) Hossain et al. Clinical Infectious Diseases. Background. In Bangladesh, 4 outbreaks of Nipah virus infection were identified during the period 2001-2004. Methods. We characterized the clinical features of Nipah virus-infected individuals affected by these outbreaks. We classified patients ...
Abstract. Nipah virus (NiV) is an emerging bat-borne pathogen. It was first identified 20 years ago in Malaysia and has since caused outbreaks in other parts of South and Southeast Asia. It causes severe neurological and respiratory disease which is highly lethal.
M. Jahangir Hossain, Emily S. Gurley, Joel M. Montgomery, Michael Bell, Darin S. Carroll, Vincent P. Hsu, P. Formenty, A. Croisier, E. Bertherat, M. A. Faiz, Abul ...
Nipah virus therapeutics development is not motivated by commercial interest. Therefore, we propose a regionally led, patient-centred, and public health-centred, end-to-end framework that articulates a public health vision and a roadmap for research, development, manufacturing, and access towards the goal of improving patient outcomes.
The Nipah cases were mostly distributed in the northwestern and central part of Bangladesh. Outbreaks occurred during December to May, which coincides with the winter season in Bangladesh. Cases were distributed in all age groups. Median age was 25 years (range: 0.5-75 years) and 124 (63%) cases were males.
This activity reviews the clinical evaluation of Nipah virus infection and explains the role of an inter-professional team in coordinating the care of this disease. ... are the reservoirs of the Nipah virus in Bangladesh and ... Clinical presentation of nipah virus infection in Bangladesh. Clin Infect Dis. 2008 Apr 01; 46 (7):977-84. [PubMed ...
Nipah virus (NiV) is a paramyxovirus whose reservoir host is fruit bats of the genus Pteropus. Occasionally the virus is introduced into human populations and causes severe illness characterized by encephalitis or respiratory disease. The first outbreak of NiV was recognized in Malaysia, but 8 outbreaks have been reported from Bangladesh since ...
Nipah virus infection outbreaks are seasonal in Bangladesh, with cases usually occurring annually between December and May. Since the report of the first case in 2001, the number of yearly cases has ranged from zero to 67, though in the last five years, reported cases have been comparatively lower ranging from zero in 2016 to eight in 2019. However, since 4 January 2023 and as of 13 February ...
Outbreaks of Nipah virus (NiV) infection are seasonal in Bangladesh, with cases usually occurring annually between December and April corresponding with the harvesting and consumption of date palm sap. Since 1 January and as of 9 February 2024, two laboratory-confirmed cases of NiV have been reported from the Dhaka division of Bangladesh. Both cases have died. WHO assesses the overall risk at ...
Europe PMC is an archive of life sciences journal literature. Search life-sciences literature (43,198,198 articles, preprints and more)
Nipah virus infection in 82 of the 248 case patients was suspected to be the result of person-to-person transmission, which corresponded to a reproduction number of 0.33 (95% confidence interval [CI], 0.19 to 0.59). We identified 17 transmission trees; the largest extended over five generations and included 32 cases 5 ( Fig. 1A and.
Introduction. Nipah virus (NiV) is a highly pathogenic zoonotic paramyxovirus causing severe respiratory disease and encephalitis in Southeast Asia with high mortality (Luby and Gurley, 2012, Satterfield et al., 2016).It is a negative-stranded RNA virus with a large genome of 18 kb and is classified in the Henipaviruses genus of Paramyxoviridae family along with its close relatives: Hendra ...
Nipah Virus Case Investigations. Human case-patients with suspected Nipah virus infection with a history of consuming date palm sap were identified at 3 surveillance hospitals in the Faridpur, Rajshahi, and Rangpur Districts of Bangladesh ().Additional suspected cases in other regions were identified from media reports ().A total of 47 primary cases of Nipah virus representing spillover from ...
All 248 cases of Nipah virus represented in our analysis have been re-ported previously,1,2,4-7,10-19 but we have now ana-lyzed these cases together with information on case contacts to gain new ...
1. BACKGROUND. The recent outbreak of deadly Nipah virus (NiV) infection has raised concern and fear among the general population, who are still fighting the COVID‐19. 1 According to the World Health Organization (WHO), NiV infection is a zoonotic infection that typically transmits from animal to human. But it may also get transmitted through food contaminated with the virus.
One modelling estimate indicates 390 million dengue virus infections per year of which 96 million ... with 2300 deaths. A high number of cases were reported in Asia: Bangladesh (321 000), Malaysia (111 400), Thailand (150 000), and Viet Nam (369 000). ... J.J., et al., Viremia and Clinical Presentation in Nicaraguan Patients Infected Wi1 ...